User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Controlled hyperthermia: Novel treatment of BCCs without surgery continues to be refined
PHOENIX – .
“For 2,000 years, it’s been known that heat can kill cancers,” an apoptotic reaction “rather than a destructive reaction coming from excessive heat,” Christopher B. Zachary, MD, said at the annual conference of the American Society for Laser Medicine and Surgery, where the study was presented during an abstract session.
Dr. Zachary, professor and chair emeritus of the department of dermatology at the University of California, Irvine, and colleagues, evaluated a novel, noninvasive technique of controlled hyperthermia and mapping protocol (CHAMP) designed to help clinicians with margin assessment and treatment of superficial and nodular BCCs. For this prospective study, which was first described at the 2022 ASLMS annual conference and is being conducted at three centers, 73 patients with biopsy-proven superficial and nodular BCCs have been scanned with the VivoSight Dx optical coherence tomography (OCT) device to map BCC tumor margins.
The BCCs were treated with the Sciton 1,064-nm Er:YAG laser equipped with a 4-mm beam diameter scan pattern with no overlap and an 8-millisecond pulse duration, randomized to either standard 120-140 J/cm2 pulses until tissue graying and contraction was observed, or the CHAMP controlled hyperthermia technique using repeated 25 J/cm2 pulses under thermal camera imaging to maintain a consistent temperature of 55º C for 60 seconds. Patients were rescanned by OCT at 3 to 12 months for any signs of residual tumor and if positive, were retreated. Finally, lesions were excised for evidence of histological clearance.
To date, 48 patients have completed the study. Among the 26 patients treated with the CHAMP method, 22 (84.6%) were histologically clear, as were 19 of the 22 (86.4%) in the standard treatment group. Ulceration was uncommon with the CHAMP method, and patients healed with modest erythema, Dr. Zachary said.
Pretreatment OCT mapping of BCCs indicated that tumors extended beyond their 5-mm clinical margins in 11 cases (15%). “This will be of interest to those who treat BCCs by Mohs or standard excision,” he said. Increased vascularity measured by dynamic OCT was noted in most CHAMP patients immediately after irradiation, which suggests that apoptosis was the primary mechanism of tumor response instead of vascular destruction.
“The traditional technique for using the long pulsed 1,064-nm Er:YAG laser to cause damage and destruction of BCC is 120-140 J/cm2 at one or two passes until you get to an endpoint of graying and contraction of tissue,” Dr. Zachary said. “That’s opposed to the ‘Low and Slow’ approach [where you use] multiple pulses at 25 J/cm2 until you achieve an optimal time and temperature. If you treat above 60º C, you tend to get epidermal blistering, prolonged healing, and interestingly, absence of pain. I think that’s because you kill off the nerve fibers. With the low fluence multiple scan technique, you’re going for an even flat-top heating.”
Currently, he and his colleagues consider 55 degrees at 60 seconds as “the optimal parameters,” he said, but “it could be 45 degrees at 90 seconds or two minutes. We don’t know yet.”
In an interview at the meeting, one of the abstract session moderators, Mathew M. Avram, MD, JD, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital, Boston, said that he was encouraged by the study results as investigations into effective, noninvasive treatment of BCC continue to move forward. “Details matter such as the temperature [of energy delivery] and noninvasive imaging to delineate the appropriate margins,” said Dr. Avram, who has conducted research on the 1,064-nm long-pulsed Nd:YAG laser as an alternative treatment for nonfacial BCCs in patients who are poor surgical candidates.
“Hopefully, at some point,” he said, such approaches will “become the standard of care for many BCCs that we are now treating surgically. I don’t think this will happen in the next 3 years, but I think in the long term, it will emerge as the treatment of choice.”
The study is being funded by Michelson Diagnostics. Sciton provided the long-pulsed 1,064-nm lasers devices being used in the trial. Dr. Zachary reported having no relevant disclosures. Dr. Avram disclosed that he has received consulting fees from Sciton.
PHOENIX – .
“For 2,000 years, it’s been known that heat can kill cancers,” an apoptotic reaction “rather than a destructive reaction coming from excessive heat,” Christopher B. Zachary, MD, said at the annual conference of the American Society for Laser Medicine and Surgery, where the study was presented during an abstract session.
Dr. Zachary, professor and chair emeritus of the department of dermatology at the University of California, Irvine, and colleagues, evaluated a novel, noninvasive technique of controlled hyperthermia and mapping protocol (CHAMP) designed to help clinicians with margin assessment and treatment of superficial and nodular BCCs. For this prospective study, which was first described at the 2022 ASLMS annual conference and is being conducted at three centers, 73 patients with biopsy-proven superficial and nodular BCCs have been scanned with the VivoSight Dx optical coherence tomography (OCT) device to map BCC tumor margins.
The BCCs were treated with the Sciton 1,064-nm Er:YAG laser equipped with a 4-mm beam diameter scan pattern with no overlap and an 8-millisecond pulse duration, randomized to either standard 120-140 J/cm2 pulses until tissue graying and contraction was observed, or the CHAMP controlled hyperthermia technique using repeated 25 J/cm2 pulses under thermal camera imaging to maintain a consistent temperature of 55º C for 60 seconds. Patients were rescanned by OCT at 3 to 12 months for any signs of residual tumor and if positive, were retreated. Finally, lesions were excised for evidence of histological clearance.
To date, 48 patients have completed the study. Among the 26 patients treated with the CHAMP method, 22 (84.6%) were histologically clear, as were 19 of the 22 (86.4%) in the standard treatment group. Ulceration was uncommon with the CHAMP method, and patients healed with modest erythema, Dr. Zachary said.
Pretreatment OCT mapping of BCCs indicated that tumors extended beyond their 5-mm clinical margins in 11 cases (15%). “This will be of interest to those who treat BCCs by Mohs or standard excision,” he said. Increased vascularity measured by dynamic OCT was noted in most CHAMP patients immediately after irradiation, which suggests that apoptosis was the primary mechanism of tumor response instead of vascular destruction.
“The traditional technique for using the long pulsed 1,064-nm Er:YAG laser to cause damage and destruction of BCC is 120-140 J/cm2 at one or two passes until you get to an endpoint of graying and contraction of tissue,” Dr. Zachary said. “That’s opposed to the ‘Low and Slow’ approach [where you use] multiple pulses at 25 J/cm2 until you achieve an optimal time and temperature. If you treat above 60º C, you tend to get epidermal blistering, prolonged healing, and interestingly, absence of pain. I think that’s because you kill off the nerve fibers. With the low fluence multiple scan technique, you’re going for an even flat-top heating.”
Currently, he and his colleagues consider 55 degrees at 60 seconds as “the optimal parameters,” he said, but “it could be 45 degrees at 90 seconds or two minutes. We don’t know yet.”
In an interview at the meeting, one of the abstract session moderators, Mathew M. Avram, MD, JD, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital, Boston, said that he was encouraged by the study results as investigations into effective, noninvasive treatment of BCC continue to move forward. “Details matter such as the temperature [of energy delivery] and noninvasive imaging to delineate the appropriate margins,” said Dr. Avram, who has conducted research on the 1,064-nm long-pulsed Nd:YAG laser as an alternative treatment for nonfacial BCCs in patients who are poor surgical candidates.
“Hopefully, at some point,” he said, such approaches will “become the standard of care for many BCCs that we are now treating surgically. I don’t think this will happen in the next 3 years, but I think in the long term, it will emerge as the treatment of choice.”
The study is being funded by Michelson Diagnostics. Sciton provided the long-pulsed 1,064-nm lasers devices being used in the trial. Dr. Zachary reported having no relevant disclosures. Dr. Avram disclosed that he has received consulting fees from Sciton.
PHOENIX – .
“For 2,000 years, it’s been known that heat can kill cancers,” an apoptotic reaction “rather than a destructive reaction coming from excessive heat,” Christopher B. Zachary, MD, said at the annual conference of the American Society for Laser Medicine and Surgery, where the study was presented during an abstract session.
Dr. Zachary, professor and chair emeritus of the department of dermatology at the University of California, Irvine, and colleagues, evaluated a novel, noninvasive technique of controlled hyperthermia and mapping protocol (CHAMP) designed to help clinicians with margin assessment and treatment of superficial and nodular BCCs. For this prospective study, which was first described at the 2022 ASLMS annual conference and is being conducted at three centers, 73 patients with biopsy-proven superficial and nodular BCCs have been scanned with the VivoSight Dx optical coherence tomography (OCT) device to map BCC tumor margins.
The BCCs were treated with the Sciton 1,064-nm Er:YAG laser equipped with a 4-mm beam diameter scan pattern with no overlap and an 8-millisecond pulse duration, randomized to either standard 120-140 J/cm2 pulses until tissue graying and contraction was observed, or the CHAMP controlled hyperthermia technique using repeated 25 J/cm2 pulses under thermal camera imaging to maintain a consistent temperature of 55º C for 60 seconds. Patients were rescanned by OCT at 3 to 12 months for any signs of residual tumor and if positive, were retreated. Finally, lesions were excised for evidence of histological clearance.
To date, 48 patients have completed the study. Among the 26 patients treated with the CHAMP method, 22 (84.6%) were histologically clear, as were 19 of the 22 (86.4%) in the standard treatment group. Ulceration was uncommon with the CHAMP method, and patients healed with modest erythema, Dr. Zachary said.
Pretreatment OCT mapping of BCCs indicated that tumors extended beyond their 5-mm clinical margins in 11 cases (15%). “This will be of interest to those who treat BCCs by Mohs or standard excision,” he said. Increased vascularity measured by dynamic OCT was noted in most CHAMP patients immediately after irradiation, which suggests that apoptosis was the primary mechanism of tumor response instead of vascular destruction.
“The traditional technique for using the long pulsed 1,064-nm Er:YAG laser to cause damage and destruction of BCC is 120-140 J/cm2 at one or two passes until you get to an endpoint of graying and contraction of tissue,” Dr. Zachary said. “That’s opposed to the ‘Low and Slow’ approach [where you use] multiple pulses at 25 J/cm2 until you achieve an optimal time and temperature. If you treat above 60º C, you tend to get epidermal blistering, prolonged healing, and interestingly, absence of pain. I think that’s because you kill off the nerve fibers. With the low fluence multiple scan technique, you’re going for an even flat-top heating.”
Currently, he and his colleagues consider 55 degrees at 60 seconds as “the optimal parameters,” he said, but “it could be 45 degrees at 90 seconds or two minutes. We don’t know yet.”
In an interview at the meeting, one of the abstract session moderators, Mathew M. Avram, MD, JD, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital, Boston, said that he was encouraged by the study results as investigations into effective, noninvasive treatment of BCC continue to move forward. “Details matter such as the temperature [of energy delivery] and noninvasive imaging to delineate the appropriate margins,” said Dr. Avram, who has conducted research on the 1,064-nm long-pulsed Nd:YAG laser as an alternative treatment for nonfacial BCCs in patients who are poor surgical candidates.
“Hopefully, at some point,” he said, such approaches will “become the standard of care for many BCCs that we are now treating surgically. I don’t think this will happen in the next 3 years, but I think in the long term, it will emerge as the treatment of choice.”
The study is being funded by Michelson Diagnostics. Sciton provided the long-pulsed 1,064-nm lasers devices being used in the trial. Dr. Zachary reported having no relevant disclosures. Dr. Avram disclosed that he has received consulting fees from Sciton.
AT ASLMS 2023
Miliarial Gout in an Immunocompromised Patient
To the Editor:
Miliarial gout is a rare intradermal manifestation of tophaceous gout. It was first described in 2007 when a patient presented with multiple small papules with a red base containing a white- to cream-colored substance,1 which has rarely been reported,1-6 according to a PubMed search of articles indexed for MEDLINE from 2007 to 2023 using the term miliarial gout. We describe a case of miliarial gout in a patient with a history of gout, uric acid levels within reference range, and immunocompromised status due to a prior orthotopic heart transplant.
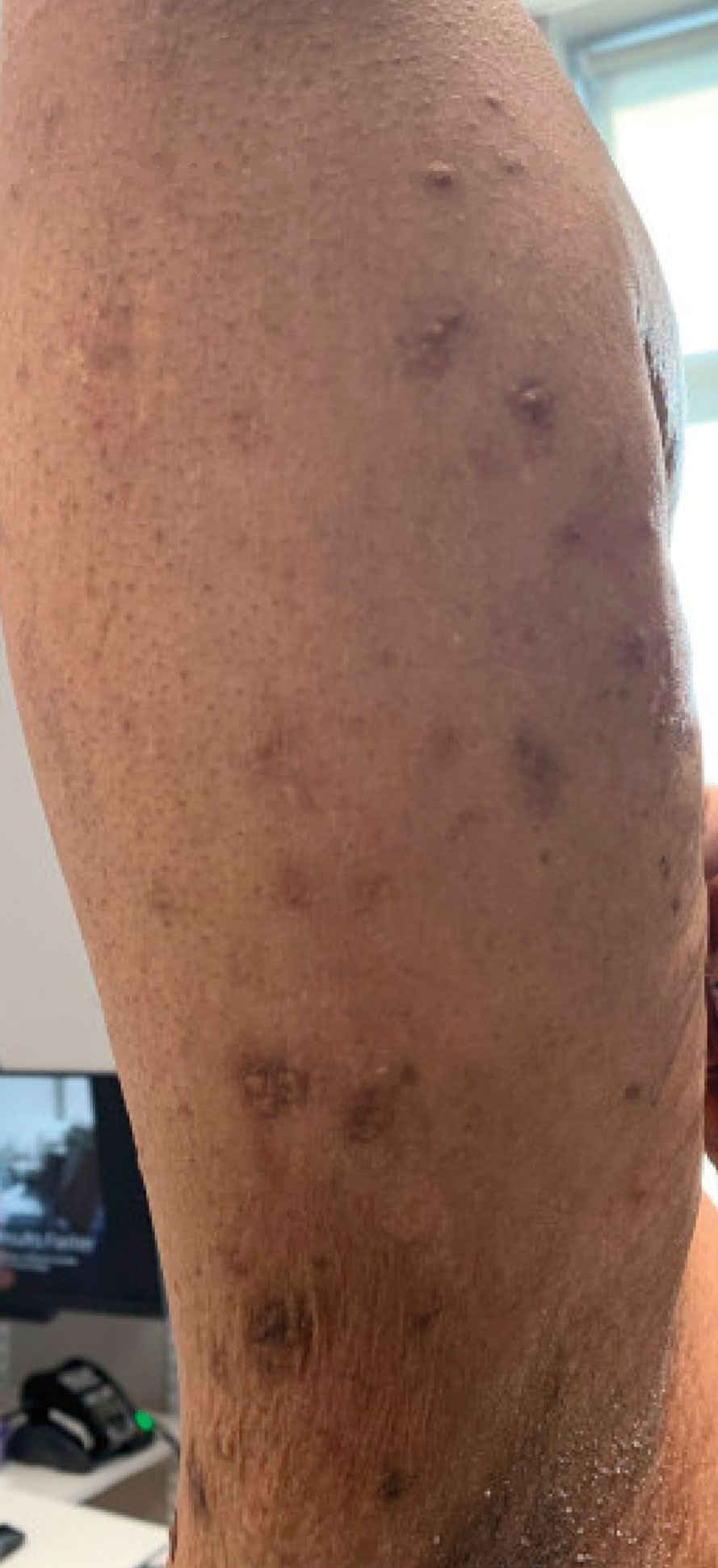
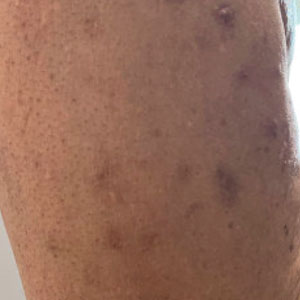
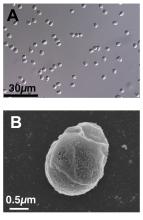
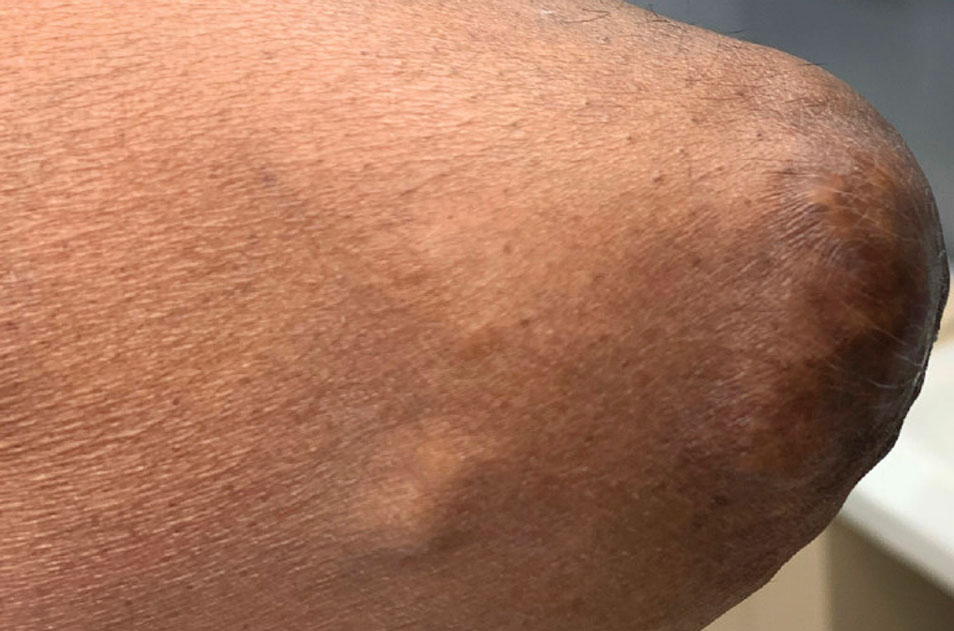
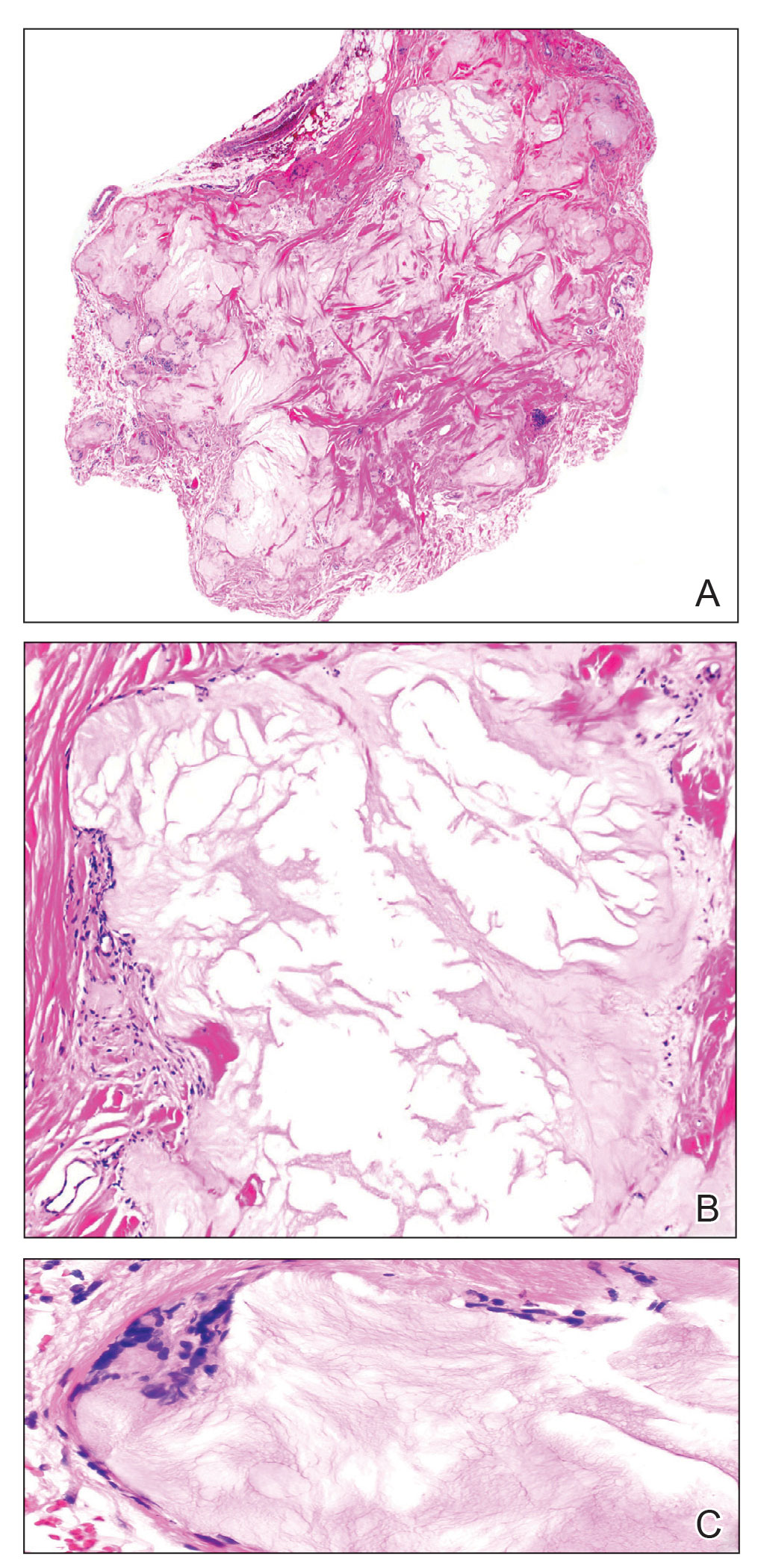
A 59-year-old man presented with innumerable subcutaneous, firm, popcornlike clustered papules on the posterior surfaces of the upper arms and thighs of 5 years’ duration (Figure 1). The involved areas were sometimes painful on manipulation, but the patient was otherwise asymptomatic. His medical history was notable for tophaceous gout of more than 10 years’ duration, calcinosis cutis, adrenal insufficiency, essential hypertension, and an orthotopic heart transplant 2 years prior to the current presentation. At the current presentation he was taking tacrolimus, colchicine, febuxostat, and low-dose prednisone. The patient denied any other skin changes such as ulceration or bullae. In addition to the innumerable subcutaneous papules, he had much larger firm deep nodules bilaterally on the elbow (Figure 2). A complete blood cell count with differential and comprehensive metabolic panel results were within reference range. A 4-mm punch biopsy of the right posterior arm revealed dermal deposits consistent with gout on hematoxylin and eosin staining (Figure 3) but no calcium deposits on von Kossa staining, consistent with miliarial gout.
He was treated with 0.6 mg of colchicine daily, 80 mg of febuxostat twice daily, and 2.5 mg of prednisone daily. Unfortunately, the patient had difficulty affording his medications and therefore experienced frequent flares.
Gout is caused by inflammation that occurs from deposition of monosodium urate crystals in tissues, most commonly occurring in the skin and joints. Gout affects8.3 million individuals and is one of the most common rheumatic diseases of adulthood. The classic presentation of the acute form is monoarticular with associated swelling, erythema, and pain. The chronic form (also known as tophaceous gout) affects soft tissue and presents with smooth or multilobulated nodules.2 Miliarial gout is a rare variant of chronic tophaceous gout, and the diagnosis is based on atypical location, size, and distribution of tophi deposition.
In the updated American College of Rheumatology criteria for gout published in 2020, tophi are defined as draining or chalklike subcutaneous nodules that typically are located in joints, ears, olecranon bursae, finger pads, and tendons.3 The term miliarial gout, which is not universally defined, is used to describe the morphology and distribution of tophi deposition in areas outside of the typical locations defined by the American College of Rheumatology criteria. Miliarial refers to the small, multilobulated, and disseminated presentation of tophi. The involvement of atypical locations distinguishes miliarial gout from chronic tophaceous gout.
The cause of tophi deposition in atypical locations is unknown. It is thought that patients with a history of sustained hyperuricemia have a much greater burden of urate crystal deposition, which can lead to involvement of atypical locations. Our patient had innumerable, discrete, 1- to 5-mm, multilobulated tophi located on the posterior upper arms and thighs even though his uric acid levels were within reference range over the last 5 years.
Miliarial gout is a rare entity.1 In 2007, Shukla et al1 coined the term miliarial gout when reporting the first known presentation of a patient with multiple tiny papules containing a white or creamlike substance scattered on an erythematous base. Other cases of miliarial gout have commonly involved the metacarpophalangeal joints of the hands, knees, abdomen, extensor forearms, and thighs.5 Similarly, our patient had disease involvement of the posterior upper arms and thighs. Furthermore, miliarial gout has been associated with carpal tunnel syndrome; monosodium urate crystal deposition in this space can lead to a clinical diagnosis of this condition.6
With a history of orthotopic heart transplant, it is possible that our patient’s immunocompromised status could have increased his susceptibility for the miliarial form of chronic tophaceous gout. Gout reportedly is the most common inflammatory arthritis in transplant recipients, with the highest prevalence following renal and heart transplantation.7 Pretransplant hyperuricemia is correlated with higher probabilities of posttransplant gout.8 In patients with a heart transplant, hyperuricemia may be due to diuretic use. Additionally, the presence of a gout diagnosis before transplant nearly triples the likelihood of posttransplant gout, which often is more severe than de novo gout, as seen in our patient. Calcineurin inhibitors, including tacrolimus, also can predispose patients to hyperuricemia and more severe forms of gout in the posttransplant phase by limiting fractional urate excretion within the first 3 months of therapy.7 Treatment with oral steroids, as in our patient, also has been identified as a potential inciting factor for the development of cutaneous tophaceous gout.9
Treatment with allopurinol and colchicine has been effective in patients with miliarial gout. Obesity and long-term treatment with furosemide (which our patient was not taking) are considered risk factors for the deposition of dermal and hypodermal urates.9 Our patient had a body mass index of 35 (≥30 indicates obesity); therefore, he also should be counseled on lifestyle modifications for optimal disease control.
- Shukla R, Vender RB, Alhabeeb A, et al. Miliarial gout (a new entity). J Cutan Med Surg. 2007;11:31-34.
- Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum. 2011;63:3136-3141.
- Neogi T, Jansen, TL, Dalbeth N, et al. 2015 gout classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheumatol. 2015;67:2557-2568.
- Hung TL, Wang WM, Chiang CP. Miliarial gout: a rare presentation of extensive cutaneous tophi. QJM. 2016;109:811-812.
- Mireku KA, Burgy JR, Davis LS. Miliarial gout: a rare clinical presentation. J Am Acad Dermatol. 2014;71:E17-E18.
- Sadovici-Bobeica V, Mazur-Nicorici L, Nicorici A, et al. Chronic miliarial gout associated with carpal tunnel syndrome: a very rare clinical presentation. Eur J Case Rep Intern Med. 2018;5:000926.
- Schwab P, Lipton S, Kerr GS. Rheumatologic sequelae and challenges in organ transplantation. Best Pract Res Clin Rheumatol. 2010;24:329-340.
- Hernández-Molina G, Cachafeiro-Vilar A, Villa AR, et al. Gout in renal allograft recipients according to the pretransplant hyperuricemic status. Transplantation. 2008;86:1543-1547.
- Aguayo RS, Baradad M, Soria X, et al. Unilateral milia‐type intradermal tophi associated with underlying urate subcutaneous deposition: an uncommon cutaneous presentation of gout. Clin Exp Dermatol. 2013;38:622-625.
To the Editor:
Miliarial gout is a rare intradermal manifestation of tophaceous gout. It was first described in 2007 when a patient presented with multiple small papules with a red base containing a white- to cream-colored substance,1 which has rarely been reported,1-6 according to a PubMed search of articles indexed for MEDLINE from 2007 to 2023 using the term miliarial gout. We describe a case of miliarial gout in a patient with a history of gout, uric acid levels within reference range, and immunocompromised status due to a prior orthotopic heart transplant.

A 59-year-old man presented with innumerable subcutaneous, firm, popcornlike clustered papules on the posterior surfaces of the upper arms and thighs of 5 years’ duration (Figure 1). The involved areas were sometimes painful on manipulation, but the patient was otherwise asymptomatic. His medical history was notable for tophaceous gout of more than 10 years’ duration, calcinosis cutis, adrenal insufficiency, essential hypertension, and an orthotopic heart transplant 2 years prior to the current presentation. At the current presentation he was taking tacrolimus, colchicine, febuxostat, and low-dose prednisone. The patient denied any other skin changes such as ulceration or bullae. In addition to the innumerable subcutaneous papules, he had much larger firm deep nodules bilaterally on the elbow (Figure 2). A complete blood cell count with differential and comprehensive metabolic panel results were within reference range. A 4-mm punch biopsy of the right posterior arm revealed dermal deposits consistent with gout on hematoxylin and eosin staining (Figure 3) but no calcium deposits on von Kossa staining, consistent with miliarial gout.

He was treated with 0.6 mg of colchicine daily, 80 mg of febuxostat twice daily, and 2.5 mg of prednisone daily. Unfortunately, the patient had difficulty affording his medications and therefore experienced frequent flares.

Gout is caused by inflammation that occurs from deposition of monosodium urate crystals in tissues, most commonly occurring in the skin and joints. Gout affects8.3 million individuals and is one of the most common rheumatic diseases of adulthood. The classic presentation of the acute form is monoarticular with associated swelling, erythema, and pain. The chronic form (also known as tophaceous gout) affects soft tissue and presents with smooth or multilobulated nodules.2 Miliarial gout is a rare variant of chronic tophaceous gout, and the diagnosis is based on atypical location, size, and distribution of tophi deposition.
In the updated American College of Rheumatology criteria for gout published in 2020, tophi are defined as draining or chalklike subcutaneous nodules that typically are located in joints, ears, olecranon bursae, finger pads, and tendons.3 The term miliarial gout, which is not universally defined, is used to describe the morphology and distribution of tophi deposition in areas outside of the typical locations defined by the American College of Rheumatology criteria. Miliarial refers to the small, multilobulated, and disseminated presentation of tophi. The involvement of atypical locations distinguishes miliarial gout from chronic tophaceous gout.
The cause of tophi deposition in atypical locations is unknown. It is thought that patients with a history of sustained hyperuricemia have a much greater burden of urate crystal deposition, which can lead to involvement of atypical locations. Our patient had innumerable, discrete, 1- to 5-mm, multilobulated tophi located on the posterior upper arms and thighs even though his uric acid levels were within reference range over the last 5 years.
Miliarial gout is a rare entity.1 In 2007, Shukla et al1 coined the term miliarial gout when reporting the first known presentation of a patient with multiple tiny papules containing a white or creamlike substance scattered on an erythematous base. Other cases of miliarial gout have commonly involved the metacarpophalangeal joints of the hands, knees, abdomen, extensor forearms, and thighs.5 Similarly, our patient had disease involvement of the posterior upper arms and thighs. Furthermore, miliarial gout has been associated with carpal tunnel syndrome; monosodium urate crystal deposition in this space can lead to a clinical diagnosis of this condition.6
With a history of orthotopic heart transplant, it is possible that our patient’s immunocompromised status could have increased his susceptibility for the miliarial form of chronic tophaceous gout. Gout reportedly is the most common inflammatory arthritis in transplant recipients, with the highest prevalence following renal and heart transplantation.7 Pretransplant hyperuricemia is correlated with higher probabilities of posttransplant gout.8 In patients with a heart transplant, hyperuricemia may be due to diuretic use. Additionally, the presence of a gout diagnosis before transplant nearly triples the likelihood of posttransplant gout, which often is more severe than de novo gout, as seen in our patient. Calcineurin inhibitors, including tacrolimus, also can predispose patients to hyperuricemia and more severe forms of gout in the posttransplant phase by limiting fractional urate excretion within the first 3 months of therapy.7 Treatment with oral steroids, as in our patient, also has been identified as a potential inciting factor for the development of cutaneous tophaceous gout.9
Treatment with allopurinol and colchicine has been effective in patients with miliarial gout. Obesity and long-term treatment with furosemide (which our patient was not taking) are considered risk factors for the deposition of dermal and hypodermal urates.9 Our patient had a body mass index of 35 (≥30 indicates obesity); therefore, he also should be counseled on lifestyle modifications for optimal disease control.
To the Editor:
Miliarial gout is a rare intradermal manifestation of tophaceous gout. It was first described in 2007 when a patient presented with multiple small papules with a red base containing a white- to cream-colored substance,1 which has rarely been reported,1-6 according to a PubMed search of articles indexed for MEDLINE from 2007 to 2023 using the term miliarial gout. We describe a case of miliarial gout in a patient with a history of gout, uric acid levels within reference range, and immunocompromised status due to a prior orthotopic heart transplant.

A 59-year-old man presented with innumerable subcutaneous, firm, popcornlike clustered papules on the posterior surfaces of the upper arms and thighs of 5 years’ duration (Figure 1). The involved areas were sometimes painful on manipulation, but the patient was otherwise asymptomatic. His medical history was notable for tophaceous gout of more than 10 years’ duration, calcinosis cutis, adrenal insufficiency, essential hypertension, and an orthotopic heart transplant 2 years prior to the current presentation. At the current presentation he was taking tacrolimus, colchicine, febuxostat, and low-dose prednisone. The patient denied any other skin changes such as ulceration or bullae. In addition to the innumerable subcutaneous papules, he had much larger firm deep nodules bilaterally on the elbow (Figure 2). A complete blood cell count with differential and comprehensive metabolic panel results were within reference range. A 4-mm punch biopsy of the right posterior arm revealed dermal deposits consistent with gout on hematoxylin and eosin staining (Figure 3) but no calcium deposits on von Kossa staining, consistent with miliarial gout.

He was treated with 0.6 mg of colchicine daily, 80 mg of febuxostat twice daily, and 2.5 mg of prednisone daily. Unfortunately, the patient had difficulty affording his medications and therefore experienced frequent flares.

Gout is caused by inflammation that occurs from deposition of monosodium urate crystals in tissues, most commonly occurring in the skin and joints. Gout affects8.3 million individuals and is one of the most common rheumatic diseases of adulthood. The classic presentation of the acute form is monoarticular with associated swelling, erythema, and pain. The chronic form (also known as tophaceous gout) affects soft tissue and presents with smooth or multilobulated nodules.2 Miliarial gout is a rare variant of chronic tophaceous gout, and the diagnosis is based on atypical location, size, and distribution of tophi deposition.
In the updated American College of Rheumatology criteria for gout published in 2020, tophi are defined as draining or chalklike subcutaneous nodules that typically are located in joints, ears, olecranon bursae, finger pads, and tendons.3 The term miliarial gout, which is not universally defined, is used to describe the morphology and distribution of tophi deposition in areas outside of the typical locations defined by the American College of Rheumatology criteria. Miliarial refers to the small, multilobulated, and disseminated presentation of tophi. The involvement of atypical locations distinguishes miliarial gout from chronic tophaceous gout.
The cause of tophi deposition in atypical locations is unknown. It is thought that patients with a history of sustained hyperuricemia have a much greater burden of urate crystal deposition, which can lead to involvement of atypical locations. Our patient had innumerable, discrete, 1- to 5-mm, multilobulated tophi located on the posterior upper arms and thighs even though his uric acid levels were within reference range over the last 5 years.
Miliarial gout is a rare entity.1 In 2007, Shukla et al1 coined the term miliarial gout when reporting the first known presentation of a patient with multiple tiny papules containing a white or creamlike substance scattered on an erythematous base. Other cases of miliarial gout have commonly involved the metacarpophalangeal joints of the hands, knees, abdomen, extensor forearms, and thighs.5 Similarly, our patient had disease involvement of the posterior upper arms and thighs. Furthermore, miliarial gout has been associated with carpal tunnel syndrome; monosodium urate crystal deposition in this space can lead to a clinical diagnosis of this condition.6
With a history of orthotopic heart transplant, it is possible that our patient’s immunocompromised status could have increased his susceptibility for the miliarial form of chronic tophaceous gout. Gout reportedly is the most common inflammatory arthritis in transplant recipients, with the highest prevalence following renal and heart transplantation.7 Pretransplant hyperuricemia is correlated with higher probabilities of posttransplant gout.8 In patients with a heart transplant, hyperuricemia may be due to diuretic use. Additionally, the presence of a gout diagnosis before transplant nearly triples the likelihood of posttransplant gout, which often is more severe than de novo gout, as seen in our patient. Calcineurin inhibitors, including tacrolimus, also can predispose patients to hyperuricemia and more severe forms of gout in the posttransplant phase by limiting fractional urate excretion within the first 3 months of therapy.7 Treatment with oral steroids, as in our patient, also has been identified as a potential inciting factor for the development of cutaneous tophaceous gout.9
Treatment with allopurinol and colchicine has been effective in patients with miliarial gout. Obesity and long-term treatment with furosemide (which our patient was not taking) are considered risk factors for the deposition of dermal and hypodermal urates.9 Our patient had a body mass index of 35 (≥30 indicates obesity); therefore, he also should be counseled on lifestyle modifications for optimal disease control.
- Shukla R, Vender RB, Alhabeeb A, et al. Miliarial gout (a new entity). J Cutan Med Surg. 2007;11:31-34.
- Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum. 2011;63:3136-3141.
- Neogi T, Jansen, TL, Dalbeth N, et al. 2015 gout classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheumatol. 2015;67:2557-2568.
- Hung TL, Wang WM, Chiang CP. Miliarial gout: a rare presentation of extensive cutaneous tophi. QJM. 2016;109:811-812.
- Mireku KA, Burgy JR, Davis LS. Miliarial gout: a rare clinical presentation. J Am Acad Dermatol. 2014;71:E17-E18.
- Sadovici-Bobeica V, Mazur-Nicorici L, Nicorici A, et al. Chronic miliarial gout associated with carpal tunnel syndrome: a very rare clinical presentation. Eur J Case Rep Intern Med. 2018;5:000926.
- Schwab P, Lipton S, Kerr GS. Rheumatologic sequelae and challenges in organ transplantation. Best Pract Res Clin Rheumatol. 2010;24:329-340.
- Hernández-Molina G, Cachafeiro-Vilar A, Villa AR, et al. Gout in renal allograft recipients according to the pretransplant hyperuricemic status. Transplantation. 2008;86:1543-1547.
- Aguayo RS, Baradad M, Soria X, et al. Unilateral milia‐type intradermal tophi associated with underlying urate subcutaneous deposition: an uncommon cutaneous presentation of gout. Clin Exp Dermatol. 2013;38:622-625.
- Shukla R, Vender RB, Alhabeeb A, et al. Miliarial gout (a new entity). J Cutan Med Surg. 2007;11:31-34.
- Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum. 2011;63:3136-3141.
- Neogi T, Jansen, TL, Dalbeth N, et al. 2015 gout classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheumatol. 2015;67:2557-2568.
- Hung TL, Wang WM, Chiang CP. Miliarial gout: a rare presentation of extensive cutaneous tophi. QJM. 2016;109:811-812.
- Mireku KA, Burgy JR, Davis LS. Miliarial gout: a rare clinical presentation. J Am Acad Dermatol. 2014;71:E17-E18.
- Sadovici-Bobeica V, Mazur-Nicorici L, Nicorici A, et al. Chronic miliarial gout associated with carpal tunnel syndrome: a very rare clinical presentation. Eur J Case Rep Intern Med. 2018;5:000926.
- Schwab P, Lipton S, Kerr GS. Rheumatologic sequelae and challenges in organ transplantation. Best Pract Res Clin Rheumatol. 2010;24:329-340.
- Hernández-Molina G, Cachafeiro-Vilar A, Villa AR, et al. Gout in renal allograft recipients according to the pretransplant hyperuricemic status. Transplantation. 2008;86:1543-1547.
- Aguayo RS, Baradad M, Soria X, et al. Unilateral milia‐type intradermal tophi associated with underlying urate subcutaneous deposition: an uncommon cutaneous presentation of gout. Clin Exp Dermatol. 2013;38:622-625.
Practice Points
- Miliarial gout is a rare intradermal manifestation of tophaceous gout and often presents as multiple small papules containing a white- to cream-colored substance.
- Immunocompromised status may be a risk factor for miliarial gout, especially in patients with a history of gout or hyperuricemia.
- Effective treatments for miliarial gout include allopurinol and colchicine.
COVID-19 and psoriasis: Is there a link?
.
Psoriasis has several well-established triggers, including stress, skin injury, cold or warm air, and allergies. Illnesses like strep throat can also cause a psoriasis flare in some people – and it appears COVID may also do so. “Psoriasis flares have long been associated with bacterial and viral infections, particularly a form of psoriasis called guttate, which is characterized by tons of tiny red scaly bumps all over the body,” said Joel M. Gelfand, MD, a professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia. “Infection with COVID-19 has been associated with flares of guttate and pustular psoriasis, and even psoriasis that affects 100% of the skin ... in many published case reports.”
Israeli researchers recently found that psoriasis patients have a slightly higher risk of getting COVID, although they are not at higher risk of hospitalization or death, which could be related to treatment with immune-modulating therapy, which can increase their risk of infections.
How could COVID cause psoriasis to flare?
Psoriasis is an autoimmune condition, and inflammation can cause symptoms.
Investigators for a study from Albany (N.Y.) Medical College and Weirton (Pa.) Medical Center found that people in the study who were already diagnosed with the skin condition had an unexpected flare within a week to a month after testing positive for COVID. New psoriasis after a COVID infection was also found. The researchers think this could be because COVID causes inflammation in the body, which negatively affects previously well-controlled psoriasis. They also think it’s possible that COVID-related inflammation could trigger a genetic tendency to have psoriasis, which may explain why it can appear for the first time after a positive test.
“A viral infection like COVID-19 can signal the release of proinflammatory factors that can appear as rashes, such as with psoriasis.” said Robert O. Carpenter, MD, director of wellness at Texas A&M University in Bryan.
What are the symptoms of COVID-related psoriasis?
The signs are the same as those of any form of psoriasis.
For a patient with psoriasis, will COVID automatically make it worse?
Not necessarily.
“Psoriasis is a common condition, so people should be aware that new psoriasis that develops may not be related to COVID-19,” said Esther Freeman MD, PhD, director of global health dermatology at Massachusetts General Hospital in Boston.
As with every aspect of COVID, doctors and scientists are still learning about how serious and widespread a problem psoriasis after COVID-19 may be. “We have seen case reports that psoriasis can flare after COVID-19,” said Dr. Freeman, who is also an associate professor of dermatology at Harvard Medical School. “I will say, this has not been a tidal wave – more like sporadic cases here and there. So I do not think psoriasis flares are a major post-COVID finding, nor do they necessarily mean you have long COVID. That being said, we know that many different infections can cause psoriasis flares, and so, in that respect, it’s not that surprising that SARS-CoV-2, like other infections, could trigger a psoriasis flare.”
Could getting COVID more than once cause psoriasis to flare? It’s possible.
“Your body can change after having COVID-19,” said Dr. Carpenter. “We don’t know the long-term implications, but having COVID-19 repeatedly can increase the risk of long COVID, which can cause many systemic changes in your body.”
Another important point: For patients who take biologics for treating psoriasis, getting vaccinated and boosted for COVID is an important step to take to help protect themselves.
Is psoriasis itself a potential symptom of COVID?
“Yes, but we don’t know the frequency at which this may occur, and a causal relationship is difficult to establish from just case reports,” said Dr. Gelfand, who’s also medical director of the clinical studies unit in the department of dermatology at his university. “Typically, if a patient presents with a flare of psoriasis, particularly guttate, pustular, or erythrodermic forms, an infectious trigger should be considered, and testing for strep and possibly COVID-19 may be appropriate.”
A version of this article first appeared on Medscape.com.
.
Psoriasis has several well-established triggers, including stress, skin injury, cold or warm air, and allergies. Illnesses like strep throat can also cause a psoriasis flare in some people – and it appears COVID may also do so. “Psoriasis flares have long been associated with bacterial and viral infections, particularly a form of psoriasis called guttate, which is characterized by tons of tiny red scaly bumps all over the body,” said Joel M. Gelfand, MD, a professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia. “Infection with COVID-19 has been associated with flares of guttate and pustular psoriasis, and even psoriasis that affects 100% of the skin ... in many published case reports.”
Israeli researchers recently found that psoriasis patients have a slightly higher risk of getting COVID, although they are not at higher risk of hospitalization or death, which could be related to treatment with immune-modulating therapy, which can increase their risk of infections.
How could COVID cause psoriasis to flare?
Psoriasis is an autoimmune condition, and inflammation can cause symptoms.
Investigators for a study from Albany (N.Y.) Medical College and Weirton (Pa.) Medical Center found that people in the study who were already diagnosed with the skin condition had an unexpected flare within a week to a month after testing positive for COVID. New psoriasis after a COVID infection was also found. The researchers think this could be because COVID causes inflammation in the body, which negatively affects previously well-controlled psoriasis. They also think it’s possible that COVID-related inflammation could trigger a genetic tendency to have psoriasis, which may explain why it can appear for the first time after a positive test.
“A viral infection like COVID-19 can signal the release of proinflammatory factors that can appear as rashes, such as with psoriasis.” said Robert O. Carpenter, MD, director of wellness at Texas A&M University in Bryan.
What are the symptoms of COVID-related psoriasis?
The signs are the same as those of any form of psoriasis.
For a patient with psoriasis, will COVID automatically make it worse?
Not necessarily.
“Psoriasis is a common condition, so people should be aware that new psoriasis that develops may not be related to COVID-19,” said Esther Freeman MD, PhD, director of global health dermatology at Massachusetts General Hospital in Boston.
As with every aspect of COVID, doctors and scientists are still learning about how serious and widespread a problem psoriasis after COVID-19 may be. “We have seen case reports that psoriasis can flare after COVID-19,” said Dr. Freeman, who is also an associate professor of dermatology at Harvard Medical School. “I will say, this has not been a tidal wave – more like sporadic cases here and there. So I do not think psoriasis flares are a major post-COVID finding, nor do they necessarily mean you have long COVID. That being said, we know that many different infections can cause psoriasis flares, and so, in that respect, it’s not that surprising that SARS-CoV-2, like other infections, could trigger a psoriasis flare.”
Could getting COVID more than once cause psoriasis to flare? It’s possible.
“Your body can change after having COVID-19,” said Dr. Carpenter. “We don’t know the long-term implications, but having COVID-19 repeatedly can increase the risk of long COVID, which can cause many systemic changes in your body.”
Another important point: For patients who take biologics for treating psoriasis, getting vaccinated and boosted for COVID is an important step to take to help protect themselves.
Is psoriasis itself a potential symptom of COVID?
“Yes, but we don’t know the frequency at which this may occur, and a causal relationship is difficult to establish from just case reports,” said Dr. Gelfand, who’s also medical director of the clinical studies unit in the department of dermatology at his university. “Typically, if a patient presents with a flare of psoriasis, particularly guttate, pustular, or erythrodermic forms, an infectious trigger should be considered, and testing for strep and possibly COVID-19 may be appropriate.”
A version of this article first appeared on Medscape.com.
.
Psoriasis has several well-established triggers, including stress, skin injury, cold or warm air, and allergies. Illnesses like strep throat can also cause a psoriasis flare in some people – and it appears COVID may also do so. “Psoriasis flares have long been associated with bacterial and viral infections, particularly a form of psoriasis called guttate, which is characterized by tons of tiny red scaly bumps all over the body,” said Joel M. Gelfand, MD, a professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia. “Infection with COVID-19 has been associated with flares of guttate and pustular psoriasis, and even psoriasis that affects 100% of the skin ... in many published case reports.”
Israeli researchers recently found that psoriasis patients have a slightly higher risk of getting COVID, although they are not at higher risk of hospitalization or death, which could be related to treatment with immune-modulating therapy, which can increase their risk of infections.
How could COVID cause psoriasis to flare?
Psoriasis is an autoimmune condition, and inflammation can cause symptoms.
Investigators for a study from Albany (N.Y.) Medical College and Weirton (Pa.) Medical Center found that people in the study who were already diagnosed with the skin condition had an unexpected flare within a week to a month after testing positive for COVID. New psoriasis after a COVID infection was also found. The researchers think this could be because COVID causes inflammation in the body, which negatively affects previously well-controlled psoriasis. They also think it’s possible that COVID-related inflammation could trigger a genetic tendency to have psoriasis, which may explain why it can appear for the first time after a positive test.
“A viral infection like COVID-19 can signal the release of proinflammatory factors that can appear as rashes, such as with psoriasis.” said Robert O. Carpenter, MD, director of wellness at Texas A&M University in Bryan.
What are the symptoms of COVID-related psoriasis?
The signs are the same as those of any form of psoriasis.
For a patient with psoriasis, will COVID automatically make it worse?
Not necessarily.
“Psoriasis is a common condition, so people should be aware that new psoriasis that develops may not be related to COVID-19,” said Esther Freeman MD, PhD, director of global health dermatology at Massachusetts General Hospital in Boston.
As with every aspect of COVID, doctors and scientists are still learning about how serious and widespread a problem psoriasis after COVID-19 may be. “We have seen case reports that psoriasis can flare after COVID-19,” said Dr. Freeman, who is also an associate professor of dermatology at Harvard Medical School. “I will say, this has not been a tidal wave – more like sporadic cases here and there. So I do not think psoriasis flares are a major post-COVID finding, nor do they necessarily mean you have long COVID. That being said, we know that many different infections can cause psoriasis flares, and so, in that respect, it’s not that surprising that SARS-CoV-2, like other infections, could trigger a psoriasis flare.”
Could getting COVID more than once cause psoriasis to flare? It’s possible.
“Your body can change after having COVID-19,” said Dr. Carpenter. “We don’t know the long-term implications, but having COVID-19 repeatedly can increase the risk of long COVID, which can cause many systemic changes in your body.”
Another important point: For patients who take biologics for treating psoriasis, getting vaccinated and boosted for COVID is an important step to take to help protect themselves.
Is psoriasis itself a potential symptom of COVID?
“Yes, but we don’t know the frequency at which this may occur, and a causal relationship is difficult to establish from just case reports,” said Dr. Gelfand, who’s also medical director of the clinical studies unit in the department of dermatology at his university. “Typically, if a patient presents with a flare of psoriasis, particularly guttate, pustular, or erythrodermic forms, an infectious trigger should be considered, and testing for strep and possibly COVID-19 may be appropriate.”
A version of this article first appeared on Medscape.com.
FDA puts partial hold on investigational alopecia areata drug deuruxolitinib
The in a press release on May 2.
The announcement came after a pulmonary embolism occurred with the 12-mg twice-daily dose in one of the long-term open-label extension (OLE) studies, the company, Sun Pharmaceutical Industries, said.
The company stated that the FDA has placed the Investigational New Drug testing for deuruxolitinib on partial clinical hold, and the agency is requiring that study participants who are currently on the 12-mg twice-daily dose in the OLE studies stop taking that dose. The hold covers only the 12-mg dose.
No hold on 8-mg dose
“There have been no thrombotic events reported to date for the 8-mg b.i.d. dose and U.S. FDA has not placed the 8-mg b.i.d. dose on hold,” the company said in the statement.
The statement added, “We are taking immediate steps to transition the patients in the OLE studies to the 8-mg b.i.d. dose arm in the ongoing studies.”
The company said that no thromboembolic events were observed in the phase 2 or phase 3 trials and said that it will work closely with the FDA to address its concerns. A formal letter detailing the FDA’s concerns is expected within 30 days.
Deuruxolitinib is an investigational oral selective inhibitor of Janus kinase 1 (JAK1) and JAK2 enzymes.
The FDA has granted deuruxolitinib breakthrough therapy designation for the treatment of adult patients with moderate to severe alopecia areata as well as fast-track designation for the treatment of alopecia areata.
In March, this news organization reported from the annual meeting of the American Academy of Dermatology that, based on phase 3 studies that demonstrate robust hair growth in about one-third of patients, deuruxolitinib has the potential to become the second JAK inhibitor available for the treatment of alopecia areata. If approved, it will join baricitinib (Olumiant), which received FDA approval almost 1 year ago.
Also at the AAD annual meeting, this news organization reported that principal investigator Brett A. King, MD, PhD, associate professor of dermatology, Yale University, New Haven, Conn., in his presentation on the results of THRIVE-AA2, one of the two phase 3 trials of deuruxolitinib, displayed several before-and-after photos and said, “The photos tell the whole story. This is why there is so much excitement about these drugs.” Dr King also was a principal investigator in studies of baricitinib.
With one exception, labeling for baricitinib and other JAK inhibitors with dermatologic indications includes a boxed warning listing serious adverse events including the risk for major adverse cardiac events and thrombosis, including pulmonary embolism, based on the risks in a rheumatoid arthritis study.
A version of this article first appeared on Medscape.com.
The in a press release on May 2.
The announcement came after a pulmonary embolism occurred with the 12-mg twice-daily dose in one of the long-term open-label extension (OLE) studies, the company, Sun Pharmaceutical Industries, said.
The company stated that the FDA has placed the Investigational New Drug testing for deuruxolitinib on partial clinical hold, and the agency is requiring that study participants who are currently on the 12-mg twice-daily dose in the OLE studies stop taking that dose. The hold covers only the 12-mg dose.
No hold on 8-mg dose
“There have been no thrombotic events reported to date for the 8-mg b.i.d. dose and U.S. FDA has not placed the 8-mg b.i.d. dose on hold,” the company said in the statement.
The statement added, “We are taking immediate steps to transition the patients in the OLE studies to the 8-mg b.i.d. dose arm in the ongoing studies.”
The company said that no thromboembolic events were observed in the phase 2 or phase 3 trials and said that it will work closely with the FDA to address its concerns. A formal letter detailing the FDA’s concerns is expected within 30 days.
Deuruxolitinib is an investigational oral selective inhibitor of Janus kinase 1 (JAK1) and JAK2 enzymes.
The FDA has granted deuruxolitinib breakthrough therapy designation for the treatment of adult patients with moderate to severe alopecia areata as well as fast-track designation for the treatment of alopecia areata.
In March, this news organization reported from the annual meeting of the American Academy of Dermatology that, based on phase 3 studies that demonstrate robust hair growth in about one-third of patients, deuruxolitinib has the potential to become the second JAK inhibitor available for the treatment of alopecia areata. If approved, it will join baricitinib (Olumiant), which received FDA approval almost 1 year ago.
Also at the AAD annual meeting, this news organization reported that principal investigator Brett A. King, MD, PhD, associate professor of dermatology, Yale University, New Haven, Conn., in his presentation on the results of THRIVE-AA2, one of the two phase 3 trials of deuruxolitinib, displayed several before-and-after photos and said, “The photos tell the whole story. This is why there is so much excitement about these drugs.” Dr King also was a principal investigator in studies of baricitinib.
With one exception, labeling for baricitinib and other JAK inhibitors with dermatologic indications includes a boxed warning listing serious adverse events including the risk for major adverse cardiac events and thrombosis, including pulmonary embolism, based on the risks in a rheumatoid arthritis study.
A version of this article first appeared on Medscape.com.
The in a press release on May 2.
The announcement came after a pulmonary embolism occurred with the 12-mg twice-daily dose in one of the long-term open-label extension (OLE) studies, the company, Sun Pharmaceutical Industries, said.
The company stated that the FDA has placed the Investigational New Drug testing for deuruxolitinib on partial clinical hold, and the agency is requiring that study participants who are currently on the 12-mg twice-daily dose in the OLE studies stop taking that dose. The hold covers only the 12-mg dose.
No hold on 8-mg dose
“There have been no thrombotic events reported to date for the 8-mg b.i.d. dose and U.S. FDA has not placed the 8-mg b.i.d. dose on hold,” the company said in the statement.
The statement added, “We are taking immediate steps to transition the patients in the OLE studies to the 8-mg b.i.d. dose arm in the ongoing studies.”
The company said that no thromboembolic events were observed in the phase 2 or phase 3 trials and said that it will work closely with the FDA to address its concerns. A formal letter detailing the FDA’s concerns is expected within 30 days.
Deuruxolitinib is an investigational oral selective inhibitor of Janus kinase 1 (JAK1) and JAK2 enzymes.
The FDA has granted deuruxolitinib breakthrough therapy designation for the treatment of adult patients with moderate to severe alopecia areata as well as fast-track designation for the treatment of alopecia areata.
In March, this news organization reported from the annual meeting of the American Academy of Dermatology that, based on phase 3 studies that demonstrate robust hair growth in about one-third of patients, deuruxolitinib has the potential to become the second JAK inhibitor available for the treatment of alopecia areata. If approved, it will join baricitinib (Olumiant), which received FDA approval almost 1 year ago.
Also at the AAD annual meeting, this news organization reported that principal investigator Brett A. King, MD, PhD, associate professor of dermatology, Yale University, New Haven, Conn., in his presentation on the results of THRIVE-AA2, one of the two phase 3 trials of deuruxolitinib, displayed several before-and-after photos and said, “The photos tell the whole story. This is why there is so much excitement about these drugs.” Dr King also was a principal investigator in studies of baricitinib.
With one exception, labeling for baricitinib and other JAK inhibitors with dermatologic indications includes a boxed warning listing serious adverse events including the risk for major adverse cardiac events and thrombosis, including pulmonary embolism, based on the risks in a rheumatoid arthritis study.
A version of this article first appeared on Medscape.com.
Medical-level empathy? Yup, ChatGPT can fake that
Caution: Robotic uprisings in the rearview mirror are closer than they appear
ChatGPT. If you’ve been even in the proximity of the Internet lately, you may have heard of it. It’s quite an incredible piece of technology, an artificial intelligence that really could up-end a lot of industries. And lest doctors believe they’re safe from robotic replacement, consider this: ChatGPT took a test commonly used as a study resource by ophthalmologists and scored a 46%. Obviously, that’s not a passing grade. Job safe, right?
A month later, the researchers tried again. This time, ChatGPT got a 58%. Still not passing, and ChatGPT did especially poorly on ophthalmology specialty questions (it got 80% of general medicine questions right), but still, the jump in quality after just a month is ... concerning. It’s not like an AI will forget things. That score can only go up, and it’ll go up faster than you think.
“Sure, the robot is smart,” the doctors out there are thinking, “but how can an AI compete with human compassion, understanding, and bedside manner?”
And they’d be right. When it comes to bedside manner, there’s no competition between man and bot. ChatGPT is already winning.
In another study, researchers sampled nearly 200 questions from the subreddit r/AskDocs, which received verified physician responses. The researchers fed ChatGPT the questions – without the doctor’s answer – and a panel of health care professionals evaluated both the human doctor and ChatGPT in terms of quality and empathy.
Perhaps not surprisingly, the robot did better when it came to quality, providing a high-quality response 79% of the time, versus 22% for the human. But empathy? It was a bloodbath. ChatGPT provided an empathetic or very empathetic response 45% of the time, while humans could only do so 4.6% of the time. So much for bedside manner.
The researchers were suspiciously quick to note that ChatGPT isn’t a legitimate replacement for physicians, but could represent a tool to better provide care for patients. But let’s be honest, given ChatGPT’s quick advancement, how long before some intrepid stockholder says: “Hey, instead of paying doctors, why don’t we just use the free robot instead?” We give it a week. Or 11 minutes.
This week, on ‘As the sperm turns’
We’ve got a lot of spermy ground to cover, so let’s get right to it, starting with the small and working our way up.
We’re all pretty familiar with the basic structure of a sperm cell, yes? Bulbous head that contains all the important genetic information and a tail-like flagellum to propel it to its ultimate destination. Not much to work with there, you’d think, but what if Mother Nature, who clearly has a robust sense of humor, had something else in mind?
We present exhibit A, Paramormyorps kingsleyae, also known as the electric elephantfish, which happens to be the only known vertebrate species with tailless sperm. Sounds crazy to us, too, but Jason Gallant, PhD, of
Michigan State University, Lansing, has a theory: “A general notion in biology is that sperm are cheap, and eggs are expensive – but these fish may be telling us that sperm are more expensive than we might think. They could be saving energy by cutting back on sperm tails.”
He and his team think that finding the gene that turns off development of the flagellum in the elephant fish could benefit humans, specifically those with a genetic disorder called primary ciliary dyskinesia, whose lack of normally functioning cilia and flagella leads to chronic respiratory infection, abnormally positioned organs, fluid on the brain, and infertility.
And that – with “that” being infertility – brings us to exhibit B, a 41-year-old Dutch man named Jonathan Meijer who clearly has too much time on his hands.
A court in the Netherlands recently ordered him, and not for the first time, to stop donating sperm to fertility clinics after it was discovered that he had fathered between 500 and 600 children around the world. He had been banned from donating to Dutch clinics in 2017, at which point he had already fathered 100 children, but managed a workaround by donating internationally and online, sometimes using another name.
The judge ordered Mr. Meijer to contact all of the clinics abroad and ask them to destroy any of his sperm they still had in stock and threatened to fine him over $100,000 for each future violation.
Okay, so here’s the thing. We have been, um, let’s call it ... warned, about the evils of tastelessness in journalism, so we’re going to do what Mr. Meijer should have done and abstain. And we can last for longer than 11 minutes.
The realm of lost luggage and lost sleep
It may be convenient to live near an airport if you’re a frequent flyer, but it really doesn’t help your sleep numbers.
The first look at how such a common sound affects sleep duration showed that people exposed to even 45 decibels of airplane noise were less likely to get the 7-9 hours of sleep needed for healthy functioning, investigators said in Environmental Health Perspectives.
How loud is 45 dB exactly? A normal conversation is about 50 dB, while a whisper is 30 dB, to give you an idea. Airplane noise at 45 dB? You might not even notice it amongst the other noises in daily life.
The researchers looked at data from about 35,000 participants in the Nurses’ Health Study who live around 90 major U.S. airports. They examined plane noise every 5 years between 1995 and 2005, focusing on estimates of nighttime and daytime levels. Short sleep was most common among the nurses who lived on the West Coast, near major cargo airports or large bodies of water, and also among those who reported no hearing loss.
The investigators noted, however, that there was no consistent association between airplane noise and quality of sleep and stopped short of making any policy recommendations. Still, sleep is a very important, yet slept-on (pun intended) factor for our overall health, so it’s good to know if anything has the potential to cause disruption.
Caution: Robotic uprisings in the rearview mirror are closer than they appear
ChatGPT. If you’ve been even in the proximity of the Internet lately, you may have heard of it. It’s quite an incredible piece of technology, an artificial intelligence that really could up-end a lot of industries. And lest doctors believe they’re safe from robotic replacement, consider this: ChatGPT took a test commonly used as a study resource by ophthalmologists and scored a 46%. Obviously, that’s not a passing grade. Job safe, right?
A month later, the researchers tried again. This time, ChatGPT got a 58%. Still not passing, and ChatGPT did especially poorly on ophthalmology specialty questions (it got 80% of general medicine questions right), but still, the jump in quality after just a month is ... concerning. It’s not like an AI will forget things. That score can only go up, and it’ll go up faster than you think.
“Sure, the robot is smart,” the doctors out there are thinking, “but how can an AI compete with human compassion, understanding, and bedside manner?”
And they’d be right. When it comes to bedside manner, there’s no competition between man and bot. ChatGPT is already winning.
In another study, researchers sampled nearly 200 questions from the subreddit r/AskDocs, which received verified physician responses. The researchers fed ChatGPT the questions – without the doctor’s answer – and a panel of health care professionals evaluated both the human doctor and ChatGPT in terms of quality and empathy.
Perhaps not surprisingly, the robot did better when it came to quality, providing a high-quality response 79% of the time, versus 22% for the human. But empathy? It was a bloodbath. ChatGPT provided an empathetic or very empathetic response 45% of the time, while humans could only do so 4.6% of the time. So much for bedside manner.
The researchers were suspiciously quick to note that ChatGPT isn’t a legitimate replacement for physicians, but could represent a tool to better provide care for patients. But let’s be honest, given ChatGPT’s quick advancement, how long before some intrepid stockholder says: “Hey, instead of paying doctors, why don’t we just use the free robot instead?” We give it a week. Or 11 minutes.
This week, on ‘As the sperm turns’
We’ve got a lot of spermy ground to cover, so let’s get right to it, starting with the small and working our way up.
We’re all pretty familiar with the basic structure of a sperm cell, yes? Bulbous head that contains all the important genetic information and a tail-like flagellum to propel it to its ultimate destination. Not much to work with there, you’d think, but what if Mother Nature, who clearly has a robust sense of humor, had something else in mind?
We present exhibit A, Paramormyorps kingsleyae, also known as the electric elephantfish, which happens to be the only known vertebrate species with tailless sperm. Sounds crazy to us, too, but Jason Gallant, PhD, of
Michigan State University, Lansing, has a theory: “A general notion in biology is that sperm are cheap, and eggs are expensive – but these fish may be telling us that sperm are more expensive than we might think. They could be saving energy by cutting back on sperm tails.”
He and his team think that finding the gene that turns off development of the flagellum in the elephant fish could benefit humans, specifically those with a genetic disorder called primary ciliary dyskinesia, whose lack of normally functioning cilia and flagella leads to chronic respiratory infection, abnormally positioned organs, fluid on the brain, and infertility.
And that – with “that” being infertility – brings us to exhibit B, a 41-year-old Dutch man named Jonathan Meijer who clearly has too much time on his hands.
A court in the Netherlands recently ordered him, and not for the first time, to stop donating sperm to fertility clinics after it was discovered that he had fathered between 500 and 600 children around the world. He had been banned from donating to Dutch clinics in 2017, at which point he had already fathered 100 children, but managed a workaround by donating internationally and online, sometimes using another name.
The judge ordered Mr. Meijer to contact all of the clinics abroad and ask them to destroy any of his sperm they still had in stock and threatened to fine him over $100,000 for each future violation.
Okay, so here’s the thing. We have been, um, let’s call it ... warned, about the evils of tastelessness in journalism, so we’re going to do what Mr. Meijer should have done and abstain. And we can last for longer than 11 minutes.
The realm of lost luggage and lost sleep
It may be convenient to live near an airport if you’re a frequent flyer, but it really doesn’t help your sleep numbers.
The first look at how such a common sound affects sleep duration showed that people exposed to even 45 decibels of airplane noise were less likely to get the 7-9 hours of sleep needed for healthy functioning, investigators said in Environmental Health Perspectives.
How loud is 45 dB exactly? A normal conversation is about 50 dB, while a whisper is 30 dB, to give you an idea. Airplane noise at 45 dB? You might not even notice it amongst the other noises in daily life.
The researchers looked at data from about 35,000 participants in the Nurses’ Health Study who live around 90 major U.S. airports. They examined plane noise every 5 years between 1995 and 2005, focusing on estimates of nighttime and daytime levels. Short sleep was most common among the nurses who lived on the West Coast, near major cargo airports or large bodies of water, and also among those who reported no hearing loss.
The investigators noted, however, that there was no consistent association between airplane noise and quality of sleep and stopped short of making any policy recommendations. Still, sleep is a very important, yet slept-on (pun intended) factor for our overall health, so it’s good to know if anything has the potential to cause disruption.
Caution: Robotic uprisings in the rearview mirror are closer than they appear
ChatGPT. If you’ve been even in the proximity of the Internet lately, you may have heard of it. It’s quite an incredible piece of technology, an artificial intelligence that really could up-end a lot of industries. And lest doctors believe they’re safe from robotic replacement, consider this: ChatGPT took a test commonly used as a study resource by ophthalmologists and scored a 46%. Obviously, that’s not a passing grade. Job safe, right?
A month later, the researchers tried again. This time, ChatGPT got a 58%. Still not passing, and ChatGPT did especially poorly on ophthalmology specialty questions (it got 80% of general medicine questions right), but still, the jump in quality after just a month is ... concerning. It’s not like an AI will forget things. That score can only go up, and it’ll go up faster than you think.
“Sure, the robot is smart,” the doctors out there are thinking, “but how can an AI compete with human compassion, understanding, and bedside manner?”
And they’d be right. When it comes to bedside manner, there’s no competition between man and bot. ChatGPT is already winning.
In another study, researchers sampled nearly 200 questions from the subreddit r/AskDocs, which received verified physician responses. The researchers fed ChatGPT the questions – without the doctor’s answer – and a panel of health care professionals evaluated both the human doctor and ChatGPT in terms of quality and empathy.
Perhaps not surprisingly, the robot did better when it came to quality, providing a high-quality response 79% of the time, versus 22% for the human. But empathy? It was a bloodbath. ChatGPT provided an empathetic or very empathetic response 45% of the time, while humans could only do so 4.6% of the time. So much for bedside manner.
The researchers were suspiciously quick to note that ChatGPT isn’t a legitimate replacement for physicians, but could represent a tool to better provide care for patients. But let’s be honest, given ChatGPT’s quick advancement, how long before some intrepid stockholder says: “Hey, instead of paying doctors, why don’t we just use the free robot instead?” We give it a week. Or 11 minutes.
This week, on ‘As the sperm turns’
We’ve got a lot of spermy ground to cover, so let’s get right to it, starting with the small and working our way up.
We’re all pretty familiar with the basic structure of a sperm cell, yes? Bulbous head that contains all the important genetic information and a tail-like flagellum to propel it to its ultimate destination. Not much to work with there, you’d think, but what if Mother Nature, who clearly has a robust sense of humor, had something else in mind?
We present exhibit A, Paramormyorps kingsleyae, also known as the electric elephantfish, which happens to be the only known vertebrate species with tailless sperm. Sounds crazy to us, too, but Jason Gallant, PhD, of
Michigan State University, Lansing, has a theory: “A general notion in biology is that sperm are cheap, and eggs are expensive – but these fish may be telling us that sperm are more expensive than we might think. They could be saving energy by cutting back on sperm tails.”
He and his team think that finding the gene that turns off development of the flagellum in the elephant fish could benefit humans, specifically those with a genetic disorder called primary ciliary dyskinesia, whose lack of normally functioning cilia and flagella leads to chronic respiratory infection, abnormally positioned organs, fluid on the brain, and infertility.
And that – with “that” being infertility – brings us to exhibit B, a 41-year-old Dutch man named Jonathan Meijer who clearly has too much time on his hands.
A court in the Netherlands recently ordered him, and not for the first time, to stop donating sperm to fertility clinics after it was discovered that he had fathered between 500 and 600 children around the world. He had been banned from donating to Dutch clinics in 2017, at which point he had already fathered 100 children, but managed a workaround by donating internationally and online, sometimes using another name.
The judge ordered Mr. Meijer to contact all of the clinics abroad and ask them to destroy any of his sperm they still had in stock and threatened to fine him over $100,000 for each future violation.
Okay, so here’s the thing. We have been, um, let’s call it ... warned, about the evils of tastelessness in journalism, so we’re going to do what Mr. Meijer should have done and abstain. And we can last for longer than 11 minutes.
The realm of lost luggage and lost sleep
It may be convenient to live near an airport if you’re a frequent flyer, but it really doesn’t help your sleep numbers.
The first look at how such a common sound affects sleep duration showed that people exposed to even 45 decibels of airplane noise were less likely to get the 7-9 hours of sleep needed for healthy functioning, investigators said in Environmental Health Perspectives.
How loud is 45 dB exactly? A normal conversation is about 50 dB, while a whisper is 30 dB, to give you an idea. Airplane noise at 45 dB? You might not even notice it amongst the other noises in daily life.
The researchers looked at data from about 35,000 participants in the Nurses’ Health Study who live around 90 major U.S. airports. They examined plane noise every 5 years between 1995 and 2005, focusing on estimates of nighttime and daytime levels. Short sleep was most common among the nurses who lived on the West Coast, near major cargo airports or large bodies of water, and also among those who reported no hearing loss.
The investigators noted, however, that there was no consistent association between airplane noise and quality of sleep and stopped short of making any policy recommendations. Still, sleep is a very important, yet slept-on (pun intended) factor for our overall health, so it’s good to know if anything has the potential to cause disruption.
Gray hair and aging: Could ‘stuck’ stem cells be to blame?
New evidence points more to a cycle wherein undifferentiated stem cells mature to perform their hair-coloring duties and then transform back to their primitive form. To accomplish this, they need to stay on the move.
When these special stem cells get “stuck” in the follicle, gray hair is the result, according to a new study reported online in Nature.
The regeneration cycle of melanocyte stem cells (McSCs) to melanocytes and back again can last for years. However, McSCs die sooner than do other cells nearby, such as hair follicle stem cells. This difference can explain why people go gray but still grow hair.
“It was thought that melanocyte stem cells are maintained in an undifferentiated state, instead of repeating differentiation and de-differentiation,” said the study’s senior investigator Mayumi Ito, PhD, professor in the departments of dermatology and cell biology at NYU Langone Health, New York.
The process involves different compartments in the hair follicle – the germ area is where the stem cells regenerate; the follicle bulge is where they get stuck. A different microenvironment in each location dictates how they change. This “chameleon-like” property surprised researchers.
Now that investigators figured out how gray hair might get started, a next step will be to search for a way to stop it.
The research has been performed in mice to date but could translate to humans. “Because the structure of the hair follicle is similar between mice and humans, we speculate that human melanocytes may also demonstrate the plasticity during hair regeneration,” Dr. Ito told this news organization.
Future findings could also lead to new therapies. “Our study suggests that moving melanocytes to a proper location within the hair follicle may help prevent gray hair,” Dr. Ito said.
Given the known effects of ultraviolet B (UVB) radiation on melanocytes, Dr. Ito and colleagues wanted to see what effect it might have on this cycle. So in the study, they exposed hair follicles of mice to UVB radiation and report it speeds up the process for McSCs to transform to color-producing melanocytes. They found that these McSCs can regenerate or change back to undifferentiated stem cells, so UVB radiation does not interrupt the process.
A melanoma clue?
The study also could have implications for melanoma. Unlike other tumors, melanocytes that cause cancer can self-renew even from a fully differentiated, pigmented form, the researchers note.
This makes melanomas more difficult to eliminate.
“Our study suggests normal melanocytes are very plastic and can reverse a differentiation state. Melanoma cells are known to be very plastic,” Dr. Ito said. “We consider this feature of melanoma may be related to the high plasticity of original melanocytes.”
The finding that melanocyte stem cells “are more plastic than maybe previously given credit for … certainly has implications in melanoma,” agreed Melissa Harris, PhD, associate professor, department of biology at the University of Alabama, Birmingham, when asked to comment on the study.
Small technology, big insights?
The advanced technology used by Dr. Ito and colleagues in the study included 3D-intravital imaging and single-cell RNA sequencing to track the stem cells in almost real time as they aged and moved within each hair follicle.
“This paper uses a nice mix of classic and modern techniques to help answer a question that many in the field of pigmentation biology have suspected for a long time. Not all dormant melanocyte stem cells are created equal,” Dr. Harris said.
“The one question not answered in this paper is how to reverse the dysfunction of the melanocyte stem cell ‘stuck’ in the hair bulge,” Dr. Harris added. “There are numerous clinical case studies in humans showing medicine-induced hair repigmentation, and perhaps these cases are examples of dysfunctional melanocyte stem cells becoming ‘unstuck.’ ”
‘Very interesting’ findings
The study and its results “are very interesting from a mechanistic perspective and basic science view,” said Anthony M. Rossi, MD, a private practice dermatologist and assistant attending dermatologist at Memorial Sloan Kettering Cancer Center in New York, when asked to comment on the results.
The research provides another view of how melanocyte stem cells can pigment the hair shaft, Dr. Rossi added. “It gives insight into the behavior of stem cells and how they can travel and change state, something not well-known before.”
Dr. Rossi cautioned that other mechanisms are likely taking place. He pointed out that graying of hair can actually occur after a sudden stress event, as well as with vitamin B12 deficiency, thyroid disease, vitiligo-related autoimmune destruction, neurofibromatosis, tuberous sclerosis, and alopecia areata.
The “standout concept” in this paper is that the melanocyte stem cells are stranded and are not getting the right signal from the microenvironment to amplify and appropriately migrate to provide pigment to the hair shaft, said Paradi Mirmirani, MD, a private practice dermatologist in Vallejo, Calif.
It could be challenging to find the right signaling to reverse the graying process, Dr. Mirmirani added. “But the first step is always to understand the underlying basic mechanism. It would be interesting to see if other factors such as smoking, stress … influence the melanocyte stem cells in the same way.”
Grants from the National Institutes of Health and the Department of Defense supported the study. Dr. Ito, Dr. Harris, Dr. Mirmirani, and Dr. Rossi had no relevant disclosures.
A version of this article first appeared on Medscape.com.
New evidence points more to a cycle wherein undifferentiated stem cells mature to perform their hair-coloring duties and then transform back to their primitive form. To accomplish this, they need to stay on the move.
When these special stem cells get “stuck” in the follicle, gray hair is the result, according to a new study reported online in Nature.
The regeneration cycle of melanocyte stem cells (McSCs) to melanocytes and back again can last for years. However, McSCs die sooner than do other cells nearby, such as hair follicle stem cells. This difference can explain why people go gray but still grow hair.
“It was thought that melanocyte stem cells are maintained in an undifferentiated state, instead of repeating differentiation and de-differentiation,” said the study’s senior investigator Mayumi Ito, PhD, professor in the departments of dermatology and cell biology at NYU Langone Health, New York.
The process involves different compartments in the hair follicle – the germ area is where the stem cells regenerate; the follicle bulge is where they get stuck. A different microenvironment in each location dictates how they change. This “chameleon-like” property surprised researchers.
Now that investigators figured out how gray hair might get started, a next step will be to search for a way to stop it.
The research has been performed in mice to date but could translate to humans. “Because the structure of the hair follicle is similar between mice and humans, we speculate that human melanocytes may also demonstrate the plasticity during hair regeneration,” Dr. Ito told this news organization.
Future findings could also lead to new therapies. “Our study suggests that moving melanocytes to a proper location within the hair follicle may help prevent gray hair,” Dr. Ito said.
Given the known effects of ultraviolet B (UVB) radiation on melanocytes, Dr. Ito and colleagues wanted to see what effect it might have on this cycle. So in the study, they exposed hair follicles of mice to UVB radiation and report it speeds up the process for McSCs to transform to color-producing melanocytes. They found that these McSCs can regenerate or change back to undifferentiated stem cells, so UVB radiation does not interrupt the process.
A melanoma clue?
The study also could have implications for melanoma. Unlike other tumors, melanocytes that cause cancer can self-renew even from a fully differentiated, pigmented form, the researchers note.
This makes melanomas more difficult to eliminate.
“Our study suggests normal melanocytes are very plastic and can reverse a differentiation state. Melanoma cells are known to be very plastic,” Dr. Ito said. “We consider this feature of melanoma may be related to the high plasticity of original melanocytes.”
The finding that melanocyte stem cells “are more plastic than maybe previously given credit for … certainly has implications in melanoma,” agreed Melissa Harris, PhD, associate professor, department of biology at the University of Alabama, Birmingham, when asked to comment on the study.
Small technology, big insights?
The advanced technology used by Dr. Ito and colleagues in the study included 3D-intravital imaging and single-cell RNA sequencing to track the stem cells in almost real time as they aged and moved within each hair follicle.
“This paper uses a nice mix of classic and modern techniques to help answer a question that many in the field of pigmentation biology have suspected for a long time. Not all dormant melanocyte stem cells are created equal,” Dr. Harris said.
“The one question not answered in this paper is how to reverse the dysfunction of the melanocyte stem cell ‘stuck’ in the hair bulge,” Dr. Harris added. “There are numerous clinical case studies in humans showing medicine-induced hair repigmentation, and perhaps these cases are examples of dysfunctional melanocyte stem cells becoming ‘unstuck.’ ”
‘Very interesting’ findings
The study and its results “are very interesting from a mechanistic perspective and basic science view,” said Anthony M. Rossi, MD, a private practice dermatologist and assistant attending dermatologist at Memorial Sloan Kettering Cancer Center in New York, when asked to comment on the results.
The research provides another view of how melanocyte stem cells can pigment the hair shaft, Dr. Rossi added. “It gives insight into the behavior of stem cells and how they can travel and change state, something not well-known before.”
Dr. Rossi cautioned that other mechanisms are likely taking place. He pointed out that graying of hair can actually occur after a sudden stress event, as well as with vitamin B12 deficiency, thyroid disease, vitiligo-related autoimmune destruction, neurofibromatosis, tuberous sclerosis, and alopecia areata.
The “standout concept” in this paper is that the melanocyte stem cells are stranded and are not getting the right signal from the microenvironment to amplify and appropriately migrate to provide pigment to the hair shaft, said Paradi Mirmirani, MD, a private practice dermatologist in Vallejo, Calif.
It could be challenging to find the right signaling to reverse the graying process, Dr. Mirmirani added. “But the first step is always to understand the underlying basic mechanism. It would be interesting to see if other factors such as smoking, stress … influence the melanocyte stem cells in the same way.”
Grants from the National Institutes of Health and the Department of Defense supported the study. Dr. Ito, Dr. Harris, Dr. Mirmirani, and Dr. Rossi had no relevant disclosures.
A version of this article first appeared on Medscape.com.
New evidence points more to a cycle wherein undifferentiated stem cells mature to perform their hair-coloring duties and then transform back to their primitive form. To accomplish this, they need to stay on the move.
When these special stem cells get “stuck” in the follicle, gray hair is the result, according to a new study reported online in Nature.
The regeneration cycle of melanocyte stem cells (McSCs) to melanocytes and back again can last for years. However, McSCs die sooner than do other cells nearby, such as hair follicle stem cells. This difference can explain why people go gray but still grow hair.
“It was thought that melanocyte stem cells are maintained in an undifferentiated state, instead of repeating differentiation and de-differentiation,” said the study’s senior investigator Mayumi Ito, PhD, professor in the departments of dermatology and cell biology at NYU Langone Health, New York.
The process involves different compartments in the hair follicle – the germ area is where the stem cells regenerate; the follicle bulge is where they get stuck. A different microenvironment in each location dictates how they change. This “chameleon-like” property surprised researchers.
Now that investigators figured out how gray hair might get started, a next step will be to search for a way to stop it.
The research has been performed in mice to date but could translate to humans. “Because the structure of the hair follicle is similar between mice and humans, we speculate that human melanocytes may also demonstrate the plasticity during hair regeneration,” Dr. Ito told this news organization.
Future findings could also lead to new therapies. “Our study suggests that moving melanocytes to a proper location within the hair follicle may help prevent gray hair,” Dr. Ito said.
Given the known effects of ultraviolet B (UVB) radiation on melanocytes, Dr. Ito and colleagues wanted to see what effect it might have on this cycle. So in the study, they exposed hair follicles of mice to UVB radiation and report it speeds up the process for McSCs to transform to color-producing melanocytes. They found that these McSCs can regenerate or change back to undifferentiated stem cells, so UVB radiation does not interrupt the process.
A melanoma clue?
The study also could have implications for melanoma. Unlike other tumors, melanocytes that cause cancer can self-renew even from a fully differentiated, pigmented form, the researchers note.
This makes melanomas more difficult to eliminate.
“Our study suggests normal melanocytes are very plastic and can reverse a differentiation state. Melanoma cells are known to be very plastic,” Dr. Ito said. “We consider this feature of melanoma may be related to the high plasticity of original melanocytes.”
The finding that melanocyte stem cells “are more plastic than maybe previously given credit for … certainly has implications in melanoma,” agreed Melissa Harris, PhD, associate professor, department of biology at the University of Alabama, Birmingham, when asked to comment on the study.
Small technology, big insights?
The advanced technology used by Dr. Ito and colleagues in the study included 3D-intravital imaging and single-cell RNA sequencing to track the stem cells in almost real time as they aged and moved within each hair follicle.
“This paper uses a nice mix of classic and modern techniques to help answer a question that many in the field of pigmentation biology have suspected for a long time. Not all dormant melanocyte stem cells are created equal,” Dr. Harris said.
“The one question not answered in this paper is how to reverse the dysfunction of the melanocyte stem cell ‘stuck’ in the hair bulge,” Dr. Harris added. “There are numerous clinical case studies in humans showing medicine-induced hair repigmentation, and perhaps these cases are examples of dysfunctional melanocyte stem cells becoming ‘unstuck.’ ”
‘Very interesting’ findings
The study and its results “are very interesting from a mechanistic perspective and basic science view,” said Anthony M. Rossi, MD, a private practice dermatologist and assistant attending dermatologist at Memorial Sloan Kettering Cancer Center in New York, when asked to comment on the results.
The research provides another view of how melanocyte stem cells can pigment the hair shaft, Dr. Rossi added. “It gives insight into the behavior of stem cells and how they can travel and change state, something not well-known before.”
Dr. Rossi cautioned that other mechanisms are likely taking place. He pointed out that graying of hair can actually occur after a sudden stress event, as well as with vitamin B12 deficiency, thyroid disease, vitiligo-related autoimmune destruction, neurofibromatosis, tuberous sclerosis, and alopecia areata.
The “standout concept” in this paper is that the melanocyte stem cells are stranded and are not getting the right signal from the microenvironment to amplify and appropriately migrate to provide pigment to the hair shaft, said Paradi Mirmirani, MD, a private practice dermatologist in Vallejo, Calif.
It could be challenging to find the right signaling to reverse the graying process, Dr. Mirmirani added. “But the first step is always to understand the underlying basic mechanism. It would be interesting to see if other factors such as smoking, stress … influence the melanocyte stem cells in the same way.”
Grants from the National Institutes of Health and the Department of Defense supported the study. Dr. Ito, Dr. Harris, Dr. Mirmirani, and Dr. Rossi had no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM NATURE
FDA fast tracks potential CAR T-cell therapy for lupus
The U.S. Food and Drug Administration has granted Fast Track designation for Cabaletta Bio’s cell therapy CABA-201 for the treatment of systemic lupus erythematosus (SLE) and lupus nephritis (LN), the company announced May 1.
The FDA cleared Cabaletta to begin a phase 1/2 clinical trial of CABA-201, the statement says, which will be the first trial accessing Cabaletta’s Chimeric Antigen Receptor T cells for Autoimmunity (CARTA) approach. CABA-201, a 4-1BB–containing fully human CD19-CAR T-cell investigational therapy, is designed to target and deplete CD19-positive B cells, “enabling an ‘immune system reset’ with durable remission in patients with SLE,” according to the press release. This news organization previously reported on a small study in Germany, published in Nature Medicine, that also used anti-CD19 CAR T cells to treat five patients with SLE.
This upcoming open-label study will enroll two cohorts containing six patients each. One cohort will be patients with SLE and active LN, and the other will be patients with SLE without renal involvement. The therapy is designed as a one-time infusion and will be administered at a dose of 1.0 x 106 cells/kg.
“We believe the FDA’s decision to grant Fast Track Designation for CABA-201 underscores the unmet need for a treatment that has the potential to provide deep and durable responses for people living with lupus and potentially other autoimmune diseases where B cells contribute to disease,” David J. Chang, MD, chief medical officer of Cabaletta, said in the press release.
FDA Fast Track is a process designed to expedite the development and review of drugs and other therapeutics that treat serious conditions and address unmet medical needs. Companies that receive Fast Track designation for a drug have the opportunity for more frequent meetings and written communication with the FDA about the drug’s development plan and design of clinical trials. The fast-tracked drug can also be eligible for accelerated approval and priority review if relevant criteria are met.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has granted Fast Track designation for Cabaletta Bio’s cell therapy CABA-201 for the treatment of systemic lupus erythematosus (SLE) and lupus nephritis (LN), the company announced May 1.
The FDA cleared Cabaletta to begin a phase 1/2 clinical trial of CABA-201, the statement says, which will be the first trial accessing Cabaletta’s Chimeric Antigen Receptor T cells for Autoimmunity (CARTA) approach. CABA-201, a 4-1BB–containing fully human CD19-CAR T-cell investigational therapy, is designed to target and deplete CD19-positive B cells, “enabling an ‘immune system reset’ with durable remission in patients with SLE,” according to the press release. This news organization previously reported on a small study in Germany, published in Nature Medicine, that also used anti-CD19 CAR T cells to treat five patients with SLE.
This upcoming open-label study will enroll two cohorts containing six patients each. One cohort will be patients with SLE and active LN, and the other will be patients with SLE without renal involvement. The therapy is designed as a one-time infusion and will be administered at a dose of 1.0 x 106 cells/kg.
“We believe the FDA’s decision to grant Fast Track Designation for CABA-201 underscores the unmet need for a treatment that has the potential to provide deep and durable responses for people living with lupus and potentially other autoimmune diseases where B cells contribute to disease,” David J. Chang, MD, chief medical officer of Cabaletta, said in the press release.
FDA Fast Track is a process designed to expedite the development and review of drugs and other therapeutics that treat serious conditions and address unmet medical needs. Companies that receive Fast Track designation for a drug have the opportunity for more frequent meetings and written communication with the FDA about the drug’s development plan and design of clinical trials. The fast-tracked drug can also be eligible for accelerated approval and priority review if relevant criteria are met.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has granted Fast Track designation for Cabaletta Bio’s cell therapy CABA-201 for the treatment of systemic lupus erythematosus (SLE) and lupus nephritis (LN), the company announced May 1.
The FDA cleared Cabaletta to begin a phase 1/2 clinical trial of CABA-201, the statement says, which will be the first trial accessing Cabaletta’s Chimeric Antigen Receptor T cells for Autoimmunity (CARTA) approach. CABA-201, a 4-1BB–containing fully human CD19-CAR T-cell investigational therapy, is designed to target and deplete CD19-positive B cells, “enabling an ‘immune system reset’ with durable remission in patients with SLE,” according to the press release. This news organization previously reported on a small study in Germany, published in Nature Medicine, that also used anti-CD19 CAR T cells to treat five patients with SLE.
This upcoming open-label study will enroll two cohorts containing six patients each. One cohort will be patients with SLE and active LN, and the other will be patients with SLE without renal involvement. The therapy is designed as a one-time infusion and will be administered at a dose of 1.0 x 106 cells/kg.
“We believe the FDA’s decision to grant Fast Track Designation for CABA-201 underscores the unmet need for a treatment that has the potential to provide deep and durable responses for people living with lupus and potentially other autoimmune diseases where B cells contribute to disease,” David J. Chang, MD, chief medical officer of Cabaletta, said in the press release.
FDA Fast Track is a process designed to expedite the development and review of drugs and other therapeutics that treat serious conditions and address unmet medical needs. Companies that receive Fast Track designation for a drug have the opportunity for more frequent meetings and written communication with the FDA about the drug’s development plan and design of clinical trials. The fast-tracked drug can also be eligible for accelerated approval and priority review if relevant criteria are met.
A version of this article first appeared on Medscape.com.
Best practices document outlines genitourinary applications of lasers and energy-based devices
PHOENIX –
“Even a cursory review of PubMed today yields over 100,000 results” on this topic, Macrene R. Alexiades, MD, PhD, associate clinical professor of dermatology at Yale University, New Haven, Conn., said at the annual conference of the American Society for Laser Medicine and Surgery. “Add to that radiofrequency and various diagnoses, the number of publications has skyrocketed, particularly over the last 10 years.”
What has been missing from this hot research topic all these years, she continued, is that no one has distilled this pile of data into a practical guide for office-based clinicians who use lasers and energy-based devices for genitourinary conditions – until now. Working with experts in gynecology and urogynecology, Dr. Alexiades spearheaded a 2-year-long effort to assemble a document on optimal protocols and best practices for genitourinary application of lasers and energy-based devices. The document, published soon after the ASLMS meeting in Lasers in Medicine and Surgery, includes a table that lists the current Food and Drug Administration approval status of devices in genitourinary applications, as well as individual sections dedicated to fractional lasers, radiofrequency (RF) devices, and high-intensity focused electromagnetic technology. It concludes with a section on the current status of clearances and future pathways.
“The work we did was exhaustive,” said Dr. Alexiades, who is also founder and director of Dermatology & Laser Surgery Center of New York. “We went through all the clinical trial data and compiled the parameters that, as a consensus, we agree are best practices for each technology for which we had rigorous published data.”
The document contains a brief background on the history of the devices used for genitourinary issues and it addresses core topics for each technology, such as conditions treated, contraindications, preoperative physical assessment and preparation, perioperative protocols, and postoperative care.
Contraindications to the genitourinary use of lasers and energy-based devices are numerous and include use of an intrauterine device, active urinary tract or genital infection, vaginal bleeding, current pregnancy, active or recent malignancy, having an electrical implant anywhere in the body, significant concurrent illness, and an anticoagulative or thromboembolic condition or taking anticoagulant medications 1 week prior to the procedure. Another condition to screen for is advanced prolapse, which was considered a contraindication in all clinical trials, she added. “It’s important that you’re able to do the speculum exam and stage the prolapse” so that a patient with this contraindication is not treated.
Dr. Alexiades shared the following highlights from the document’s section related to the use of fractional CO2 lasers:
Preoperative management. Schedule the treatment one week after the patient’s menstrual period. Patients should avoid blood thinners for 7 days and avoid intercourse the night before the procedure. Reschedule in the case of fever, chills, or vaginal bleeding or discharge.
Preoperative physical exam and testing. A normal speculum exam and a recent negative PAP smear are required. For those of child-bearing potential, a pregnancy test is warranted. Obtain written and verbal consent, including discussion of all treatment options, risks, and benefits. No topical or local anesthesia is necessary internally. “Externally, we sometimes apply topical lidocaine gel, but I have found that’s not necessary in most cases,” Dr. Alexiades said. “The treatment is so quick.”
Peri-operative management. In general, device settings are provided by the manufacturer. “For most of the studies that had successful outcomes and no adverse events, researchers adhered to the mild or moderate settings on the technology,” she said. Energy settings were between 15 and 30 watts, delivered at a laser fluence of about 250-300 mJ/cm2 with a spacing of microbeams 1 mm apart. Typically, three treatments are done at 1-month intervals and maintenance treatments are recommended at 6 and 12 months based on duration of the outcomes.
Vulvovaginal postoperative management. A 3-day recovery time is recommended with avoidance of intercourse during this period, because “re-epithelialization is usually complete in 3 days, so we want to give the opportunity for the lining to heal prior to introducing any friction, Dr. Alexiades said.” Rarely, spotting or discharge may occur and there should be no discomfort. “Any severe discomfort or burning may potentially signify infection and should prompt evaluation and possibly vaginal cultures. The patient can shower, but we recommend avoiding seated baths to decrease any introduction of infectious agents.”
Patients should be followed up monthly until three treatments are completed, and a maintenance treatment is considered appropriate between 6 and 12 months. “I do recommend doing a 1-month follow-up following the final treatment, unless it’s a patient who has already had a series of three treatments and is coming in for maintenance,” she said.
In a study from her own practice, Dr. Alexiades evaluated a series of three fractional CO2 laser treatments to the vulva and vagina with a 1-year follow-up in postmenopausal patients. She used the Vaginal Health Index (VHI) to assess changes in vaginal elasticity, fluid volume, vaginal pH, epithelial integrity, and moisture. She and her colleagues discovered that there was improvement in every VHI category after treatment and during the follow-up interval up to 6 months.
“Between 6 and 12 months, we started to see a return a bit toward baseline on all of these parameters,” she said. “The serendipitous discovery that I made during the course of that study was that early intervention improves outcomes. I observed that the younger, most recently postmenopausal cohort seemed to attain normal or near normal VHI quicker than the more extended postmenopausal cohorts.”
In an editorial published in 2020, Dr. Alexiades reviewed the effects of fractional CO2 laser treatment of vulvar skin on vaginal pH and referred to a study she conducted that found that the mean baseline pH pretreatment was 6.32 in the cohort of postmenopausal patients, and was reduced after 3 treatments. “Postmenopausally, the normal acidic pH becomes alkaline,” she said. But she did not expect to see an additional reduction in pH following the treatment out to 6 months. “This indicates that, whatever the wound healing and other restorative effects of these devices are, they seem to continue out to 6 months, at which point it turns around and moves toward baseline [levels].”
Dr. Alexiades highlighted two published meta-analyses of studies related to the genitourinary use of lasers and energy-based devices. One included 59 studies of 3,609 women treated for vaginal rejuvenation using either radiofrequency or fractional ablative laser therapy. The studies reported improvements in symptoms of GSM/VVA and sexual function, high patient satisfaction, with minor adverse events, including treatment-associated vaginal swelling or vaginal discharge.
“Further research needs to be completed to determine which specific pathologies can be treated, if maintenance treatment is necessary, and long-term safety concerns,” the authors concluded.
In another review, researchers analyzed 64 studies related to vaginal laser therapy for GSM. Of these, 47 were before and after studies without a control group, 10 were controlled intervention studies, and 7 were observational cohort and cross-sectional studies.
Vaginal laser treatment “seems to improve scores on the visual analogue scale, Female Sexual Function Index, and the Vaginal Health Index over the short term,” the authors wrote. “Safety outcomes are underreported and short term. Further well-designed clinical trials with sham-laser control groups and evaluating objective variables are needed to provide the best evidence on efficacy.”
“Lasers and energy-based devices are now considered alternative therapeutic modalities for genitourinary conditions,” Dr. Alexiades concluded. “The shortcomings in the literature with respect to lasers and device treatments demonstrate the need for the consensus on best practices and protocols.”
During a separate presentation at the meeting, Michael Gold, MD, highlighted data from Grand View Research, a market research database, which estimated that the global women’s health and wellness market is valued at more than $31 billion globally and is expected to grow at a compound annual growth rate of 4.8% from 2022 to 2030.
“Sales of women’s health energy-based devices continue to grow as new technologies are developed,” said Dr. Gold, a Nashville, Tenn.–based dermatologist and cosmetic surgeon who is also editor-in-chief of the Journal of Cosmetic Dermatology. “Evolving societal norms have made discussions about feminine health issues acceptable. Suffering in silence is no longer necessary or advocated.”
Dr. Alexiades disclosed that she has conducted research for Candela Lasers, Lumenis, Allergan/AbbVie, InMode, and Endymed. She is also the founder and CEO of Macrene Actives. Dr. Gold disclosed that he is a consultant to and/or an investigator and a speaker for Joylux, InMode, and Alma Lasers.
PHOENIX –
“Even a cursory review of PubMed today yields over 100,000 results” on this topic, Macrene R. Alexiades, MD, PhD, associate clinical professor of dermatology at Yale University, New Haven, Conn., said at the annual conference of the American Society for Laser Medicine and Surgery. “Add to that radiofrequency and various diagnoses, the number of publications has skyrocketed, particularly over the last 10 years.”
What has been missing from this hot research topic all these years, she continued, is that no one has distilled this pile of data into a practical guide for office-based clinicians who use lasers and energy-based devices for genitourinary conditions – until now. Working with experts in gynecology and urogynecology, Dr. Alexiades spearheaded a 2-year-long effort to assemble a document on optimal protocols and best practices for genitourinary application of lasers and energy-based devices. The document, published soon after the ASLMS meeting in Lasers in Medicine and Surgery, includes a table that lists the current Food and Drug Administration approval status of devices in genitourinary applications, as well as individual sections dedicated to fractional lasers, radiofrequency (RF) devices, and high-intensity focused electromagnetic technology. It concludes with a section on the current status of clearances and future pathways.
“The work we did was exhaustive,” said Dr. Alexiades, who is also founder and director of Dermatology & Laser Surgery Center of New York. “We went through all the clinical trial data and compiled the parameters that, as a consensus, we agree are best practices for each technology for which we had rigorous published data.”
The document contains a brief background on the history of the devices used for genitourinary issues and it addresses core topics for each technology, such as conditions treated, contraindications, preoperative physical assessment and preparation, perioperative protocols, and postoperative care.
Contraindications to the genitourinary use of lasers and energy-based devices are numerous and include use of an intrauterine device, active urinary tract or genital infection, vaginal bleeding, current pregnancy, active or recent malignancy, having an electrical implant anywhere in the body, significant concurrent illness, and an anticoagulative or thromboembolic condition or taking anticoagulant medications 1 week prior to the procedure. Another condition to screen for is advanced prolapse, which was considered a contraindication in all clinical trials, she added. “It’s important that you’re able to do the speculum exam and stage the prolapse” so that a patient with this contraindication is not treated.
Dr. Alexiades shared the following highlights from the document’s section related to the use of fractional CO2 lasers:
Preoperative management. Schedule the treatment one week after the patient’s menstrual period. Patients should avoid blood thinners for 7 days and avoid intercourse the night before the procedure. Reschedule in the case of fever, chills, or vaginal bleeding or discharge.
Preoperative physical exam and testing. A normal speculum exam and a recent negative PAP smear are required. For those of child-bearing potential, a pregnancy test is warranted. Obtain written and verbal consent, including discussion of all treatment options, risks, and benefits. No topical or local anesthesia is necessary internally. “Externally, we sometimes apply topical lidocaine gel, but I have found that’s not necessary in most cases,” Dr. Alexiades said. “The treatment is so quick.”
Peri-operative management. In general, device settings are provided by the manufacturer. “For most of the studies that had successful outcomes and no adverse events, researchers adhered to the mild or moderate settings on the technology,” she said. Energy settings were between 15 and 30 watts, delivered at a laser fluence of about 250-300 mJ/cm2 with a spacing of microbeams 1 mm apart. Typically, three treatments are done at 1-month intervals and maintenance treatments are recommended at 6 and 12 months based on duration of the outcomes.
Vulvovaginal postoperative management. A 3-day recovery time is recommended with avoidance of intercourse during this period, because “re-epithelialization is usually complete in 3 days, so we want to give the opportunity for the lining to heal prior to introducing any friction, Dr. Alexiades said.” Rarely, spotting or discharge may occur and there should be no discomfort. “Any severe discomfort or burning may potentially signify infection and should prompt evaluation and possibly vaginal cultures. The patient can shower, but we recommend avoiding seated baths to decrease any introduction of infectious agents.”
Patients should be followed up monthly until three treatments are completed, and a maintenance treatment is considered appropriate between 6 and 12 months. “I do recommend doing a 1-month follow-up following the final treatment, unless it’s a patient who has already had a series of three treatments and is coming in for maintenance,” she said.
In a study from her own practice, Dr. Alexiades evaluated a series of three fractional CO2 laser treatments to the vulva and vagina with a 1-year follow-up in postmenopausal patients. She used the Vaginal Health Index (VHI) to assess changes in vaginal elasticity, fluid volume, vaginal pH, epithelial integrity, and moisture. She and her colleagues discovered that there was improvement in every VHI category after treatment and during the follow-up interval up to 6 months.
“Between 6 and 12 months, we started to see a return a bit toward baseline on all of these parameters,” she said. “The serendipitous discovery that I made during the course of that study was that early intervention improves outcomes. I observed that the younger, most recently postmenopausal cohort seemed to attain normal or near normal VHI quicker than the more extended postmenopausal cohorts.”
In an editorial published in 2020, Dr. Alexiades reviewed the effects of fractional CO2 laser treatment of vulvar skin on vaginal pH and referred to a study she conducted that found that the mean baseline pH pretreatment was 6.32 in the cohort of postmenopausal patients, and was reduced after 3 treatments. “Postmenopausally, the normal acidic pH becomes alkaline,” she said. But she did not expect to see an additional reduction in pH following the treatment out to 6 months. “This indicates that, whatever the wound healing and other restorative effects of these devices are, they seem to continue out to 6 months, at which point it turns around and moves toward baseline [levels].”
Dr. Alexiades highlighted two published meta-analyses of studies related to the genitourinary use of lasers and energy-based devices. One included 59 studies of 3,609 women treated for vaginal rejuvenation using either radiofrequency or fractional ablative laser therapy. The studies reported improvements in symptoms of GSM/VVA and sexual function, high patient satisfaction, with minor adverse events, including treatment-associated vaginal swelling or vaginal discharge.
“Further research needs to be completed to determine which specific pathologies can be treated, if maintenance treatment is necessary, and long-term safety concerns,” the authors concluded.
In another review, researchers analyzed 64 studies related to vaginal laser therapy for GSM. Of these, 47 were before and after studies without a control group, 10 were controlled intervention studies, and 7 were observational cohort and cross-sectional studies.
Vaginal laser treatment “seems to improve scores on the visual analogue scale, Female Sexual Function Index, and the Vaginal Health Index over the short term,” the authors wrote. “Safety outcomes are underreported and short term. Further well-designed clinical trials with sham-laser control groups and evaluating objective variables are needed to provide the best evidence on efficacy.”
“Lasers and energy-based devices are now considered alternative therapeutic modalities for genitourinary conditions,” Dr. Alexiades concluded. “The shortcomings in the literature with respect to lasers and device treatments demonstrate the need for the consensus on best practices and protocols.”
During a separate presentation at the meeting, Michael Gold, MD, highlighted data from Grand View Research, a market research database, which estimated that the global women’s health and wellness market is valued at more than $31 billion globally and is expected to grow at a compound annual growth rate of 4.8% from 2022 to 2030.
“Sales of women’s health energy-based devices continue to grow as new technologies are developed,” said Dr. Gold, a Nashville, Tenn.–based dermatologist and cosmetic surgeon who is also editor-in-chief of the Journal of Cosmetic Dermatology. “Evolving societal norms have made discussions about feminine health issues acceptable. Suffering in silence is no longer necessary or advocated.”
Dr. Alexiades disclosed that she has conducted research for Candela Lasers, Lumenis, Allergan/AbbVie, InMode, and Endymed. She is also the founder and CEO of Macrene Actives. Dr. Gold disclosed that he is a consultant to and/or an investigator and a speaker for Joylux, InMode, and Alma Lasers.
PHOENIX –
“Even a cursory review of PubMed today yields over 100,000 results” on this topic, Macrene R. Alexiades, MD, PhD, associate clinical professor of dermatology at Yale University, New Haven, Conn., said at the annual conference of the American Society for Laser Medicine and Surgery. “Add to that radiofrequency and various diagnoses, the number of publications has skyrocketed, particularly over the last 10 years.”
What has been missing from this hot research topic all these years, she continued, is that no one has distilled this pile of data into a practical guide for office-based clinicians who use lasers and energy-based devices for genitourinary conditions – until now. Working with experts in gynecology and urogynecology, Dr. Alexiades spearheaded a 2-year-long effort to assemble a document on optimal protocols and best practices for genitourinary application of lasers and energy-based devices. The document, published soon after the ASLMS meeting in Lasers in Medicine and Surgery, includes a table that lists the current Food and Drug Administration approval status of devices in genitourinary applications, as well as individual sections dedicated to fractional lasers, radiofrequency (RF) devices, and high-intensity focused electromagnetic technology. It concludes with a section on the current status of clearances and future pathways.
“The work we did was exhaustive,” said Dr. Alexiades, who is also founder and director of Dermatology & Laser Surgery Center of New York. “We went through all the clinical trial data and compiled the parameters that, as a consensus, we agree are best practices for each technology for which we had rigorous published data.”
The document contains a brief background on the history of the devices used for genitourinary issues and it addresses core topics for each technology, such as conditions treated, contraindications, preoperative physical assessment and preparation, perioperative protocols, and postoperative care.
Contraindications to the genitourinary use of lasers and energy-based devices are numerous and include use of an intrauterine device, active urinary tract or genital infection, vaginal bleeding, current pregnancy, active or recent malignancy, having an electrical implant anywhere in the body, significant concurrent illness, and an anticoagulative or thromboembolic condition or taking anticoagulant medications 1 week prior to the procedure. Another condition to screen for is advanced prolapse, which was considered a contraindication in all clinical trials, she added. “It’s important that you’re able to do the speculum exam and stage the prolapse” so that a patient with this contraindication is not treated.
Dr. Alexiades shared the following highlights from the document’s section related to the use of fractional CO2 lasers:
Preoperative management. Schedule the treatment one week after the patient’s menstrual period. Patients should avoid blood thinners for 7 days and avoid intercourse the night before the procedure. Reschedule in the case of fever, chills, or vaginal bleeding or discharge.
Preoperative physical exam and testing. A normal speculum exam and a recent negative PAP smear are required. For those of child-bearing potential, a pregnancy test is warranted. Obtain written and verbal consent, including discussion of all treatment options, risks, and benefits. No topical or local anesthesia is necessary internally. “Externally, we sometimes apply topical lidocaine gel, but I have found that’s not necessary in most cases,” Dr. Alexiades said. “The treatment is so quick.”
Peri-operative management. In general, device settings are provided by the manufacturer. “For most of the studies that had successful outcomes and no adverse events, researchers adhered to the mild or moderate settings on the technology,” she said. Energy settings were between 15 and 30 watts, delivered at a laser fluence of about 250-300 mJ/cm2 with a spacing of microbeams 1 mm apart. Typically, three treatments are done at 1-month intervals and maintenance treatments are recommended at 6 and 12 months based on duration of the outcomes.
Vulvovaginal postoperative management. A 3-day recovery time is recommended with avoidance of intercourse during this period, because “re-epithelialization is usually complete in 3 days, so we want to give the opportunity for the lining to heal prior to introducing any friction, Dr. Alexiades said.” Rarely, spotting or discharge may occur and there should be no discomfort. “Any severe discomfort or burning may potentially signify infection and should prompt evaluation and possibly vaginal cultures. The patient can shower, but we recommend avoiding seated baths to decrease any introduction of infectious agents.”
Patients should be followed up monthly until three treatments are completed, and a maintenance treatment is considered appropriate between 6 and 12 months. “I do recommend doing a 1-month follow-up following the final treatment, unless it’s a patient who has already had a series of three treatments and is coming in for maintenance,” she said.
In a study from her own practice, Dr. Alexiades evaluated a series of three fractional CO2 laser treatments to the vulva and vagina with a 1-year follow-up in postmenopausal patients. She used the Vaginal Health Index (VHI) to assess changes in vaginal elasticity, fluid volume, vaginal pH, epithelial integrity, and moisture. She and her colleagues discovered that there was improvement in every VHI category after treatment and during the follow-up interval up to 6 months.
“Between 6 and 12 months, we started to see a return a bit toward baseline on all of these parameters,” she said. “The serendipitous discovery that I made during the course of that study was that early intervention improves outcomes. I observed that the younger, most recently postmenopausal cohort seemed to attain normal or near normal VHI quicker than the more extended postmenopausal cohorts.”
In an editorial published in 2020, Dr. Alexiades reviewed the effects of fractional CO2 laser treatment of vulvar skin on vaginal pH and referred to a study she conducted that found that the mean baseline pH pretreatment was 6.32 in the cohort of postmenopausal patients, and was reduced after 3 treatments. “Postmenopausally, the normal acidic pH becomes alkaline,” she said. But she did not expect to see an additional reduction in pH following the treatment out to 6 months. “This indicates that, whatever the wound healing and other restorative effects of these devices are, they seem to continue out to 6 months, at which point it turns around and moves toward baseline [levels].”
Dr. Alexiades highlighted two published meta-analyses of studies related to the genitourinary use of lasers and energy-based devices. One included 59 studies of 3,609 women treated for vaginal rejuvenation using either radiofrequency or fractional ablative laser therapy. The studies reported improvements in symptoms of GSM/VVA and sexual function, high patient satisfaction, with minor adverse events, including treatment-associated vaginal swelling or vaginal discharge.
“Further research needs to be completed to determine which specific pathologies can be treated, if maintenance treatment is necessary, and long-term safety concerns,” the authors concluded.
In another review, researchers analyzed 64 studies related to vaginal laser therapy for GSM. Of these, 47 were before and after studies without a control group, 10 were controlled intervention studies, and 7 were observational cohort and cross-sectional studies.
Vaginal laser treatment “seems to improve scores on the visual analogue scale, Female Sexual Function Index, and the Vaginal Health Index over the short term,” the authors wrote. “Safety outcomes are underreported and short term. Further well-designed clinical trials with sham-laser control groups and evaluating objective variables are needed to provide the best evidence on efficacy.”
“Lasers and energy-based devices are now considered alternative therapeutic modalities for genitourinary conditions,” Dr. Alexiades concluded. “The shortcomings in the literature with respect to lasers and device treatments demonstrate the need for the consensus on best practices and protocols.”
During a separate presentation at the meeting, Michael Gold, MD, highlighted data from Grand View Research, a market research database, which estimated that the global women’s health and wellness market is valued at more than $31 billion globally and is expected to grow at a compound annual growth rate of 4.8% from 2022 to 2030.
“Sales of women’s health energy-based devices continue to grow as new technologies are developed,” said Dr. Gold, a Nashville, Tenn.–based dermatologist and cosmetic surgeon who is also editor-in-chief of the Journal of Cosmetic Dermatology. “Evolving societal norms have made discussions about feminine health issues acceptable. Suffering in silence is no longer necessary or advocated.”
Dr. Alexiades disclosed that she has conducted research for Candela Lasers, Lumenis, Allergan/AbbVie, InMode, and Endymed. She is also the founder and CEO of Macrene Actives. Dr. Gold disclosed that he is a consultant to and/or an investigator and a speaker for Joylux, InMode, and Alma Lasers.
AT ASLMS 2023
Subcutaneous Nodule on the Postauricular Neck
The Diagnosis: Pleomorphic Lipoma
Pleomorphic lipoma is a rare, benign, adipocytic neoplasm that presents in the subcutaneous tissues of the upper shoulder, back, or neck. It predominantly affects men aged 50 to 70 years. Most lesions are situated in the subcutaneous tissues; few cases of intramuscular and retroperitoneal tumors have been reported.1 Clinically, pleomorphic lipomas present as painless, well-circumscribed lesions of the subcutaneous tissue that often resemble a lipoma or occasionally may be mistaken for liposarcoma. Histopathologic examination of ordinary lipomas reveals uniform mature adipocytes. However, pleomorphic lipomas consist of a mixture of multinucleated floretlike giant cells, variable-sized adipocytes, and fibrous tissue (ropy collagen bundles) with some myxoid and spindled areas.1,2 The most characteristic histologic feature of pleomorphic lipoma is multinucleated floretlike giant cells. The nuclei of these giant cells appear hyperchromatic, enlarged, and disposed to the periphery of the cell in a circular pattern. Additionally, tumors frequently contain excess mature dense collagen bundles that are strongly refractile in polarized light. Numerous mast cells are present. Atypical lipoblasts and capillary networks commonly are not visible in pleomorphic lipoma.3 The spindle cells express CD34 on immunohistochemistry. Loss of Rb-1 expression is typical.4
Dermatofibrosarcoma protuberans is a slow-growing soft tissue sarcoma that commonly begins as a pink or violet plaque on the trunk or upper limbs. Involvement of the head or neck accounts for only 10% to 15% of cases.5 This tumor has low metastatic potential but is highly infiltrative of surrounding tissues. It is associated with a translocation between chromosomes 22 and 17, leading to the fusion of the platelet-derived growth factor subunit β, PDGFB, and collagen type 1α1, COL1A1, genes.5 Clinically, patients often report that the lesion was present for several years prior to presentation with general stability in size and shape. Eventually, untreated lesions progress to become nodules or tumors and may even bleed or ulcerate. Histology reveals a storiform spindle cell proliferation throughout the dermis with infiltration into subcutaneous fat, commonly appearing in a honeycomblike pattern (Figure 1). Numerous histologic variants exist, including myxoid, sclerosing, pigmented (Bednar tumor), myoid, atrophic, or fibrosarcomatous dermatofibrosarcoma protuberans, as well as a giant cell fibroblastoma variant.6 These tumor subtypes can exist independently or in association with one another, creating hybrid lesions that can closely mimic other entities such as pleomorphic lipoma. The spindle cells stain positively for CD34. Treatment of these tumors involves complete surgical excision or Mohs micrographic surgery; however, recurrence is common for tumors involving the head or neck.5
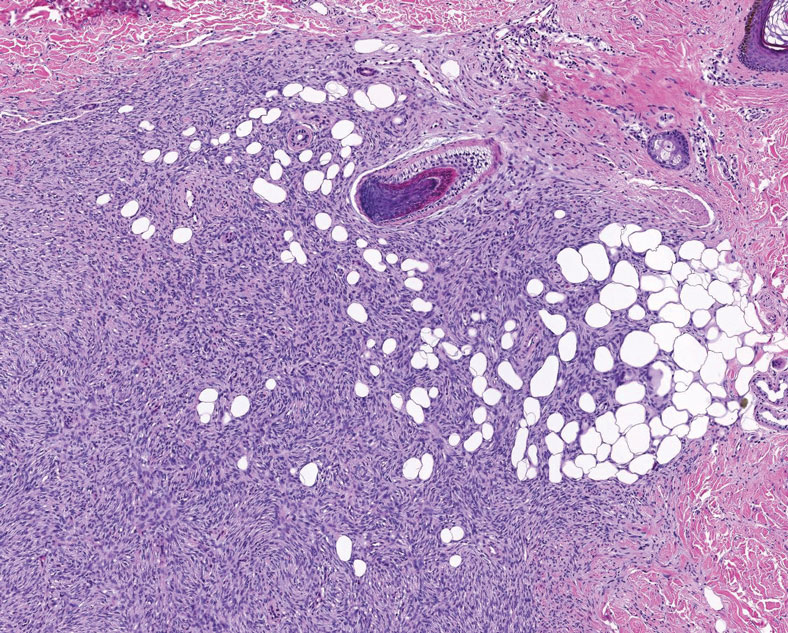
Superficial angiomyxoma is a slow-growing papule that most commonly appears on the trunk, head, or neck in middle-aged adults. Occasionally, patients with Carney complex also can develop lesions on the external ear or breast.7 Histologically, superficial angiomyxoma is a hypocellular tumor characterized by abundant myxoid stroma, thin blood vessels, and small spindled and stellate cells with minimal cytoplasm (Figure 2).8 Superficial angiomyxoma and pleomorphic lipoma present differently on histology; superficial angiomyxoma is not associated with nuclear atypia or pleomorphism, whereas pleomorphic lipoma characteristically contains multinucleated floretlike giant cells and pleomorphism. Frequently, there also is loss of normal PRKAR1A gene expression, which is responsible for protein kinase A regulatory subunit 1-alpha expression.8
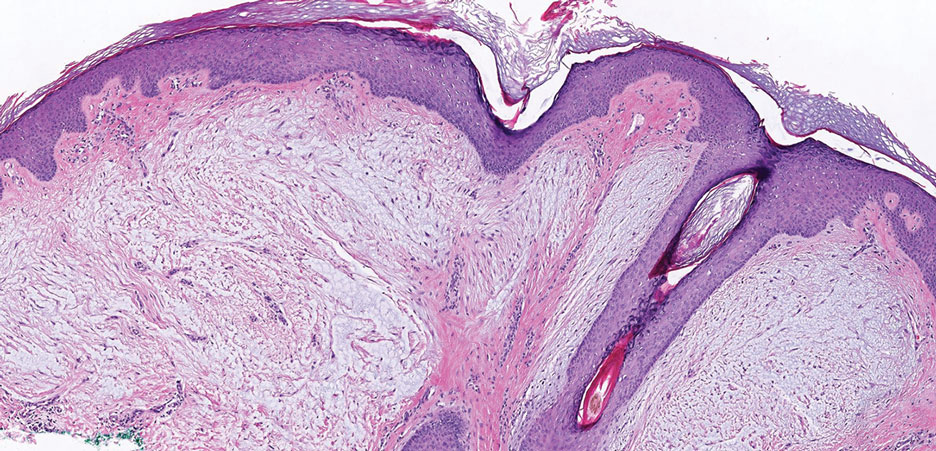
Multinucleate cell angiohistiocytoma is a rare benign proliferation that presents with numerous red-violet asymptomatic papules that commonly appear on the upper and lower extremities of women aged 40 to 70 years. Lesions feature both a fibrohistiocytic and vascular component.9 Histologic examination commonly shows multinucleated cells with angular outlining in the superficial dermis accompanied by fibrosis and ectatic small-caliber vessels (Figure 3). Although both pleomorphic lipoma and multinucleate cell angiohistiocytoma have similar-appearing multinucleated giant cells, the latter has a proliferation of narrow vessels in thick collagen bundles and lacks an adipocytic component, which distinguishes it from the former.10 Multinucleate cell angiohistiocytoma also is characterized by a substantial number of factor XIIIa–positive fibrohistiocytic interstitial cells and vascular hyperplasia.9
Nodular fasciitis is a benign lesion involving the rapid proliferation of myofibroblasts and fibroblasts in the subcutaneous tissue and most commonly is encountered on the extremities or head and neck regions. Many cases appear at sites of prior trauma, especially in patients aged 20 to 40 years. However, in infants and children the lesions typically are found in the head and neck regions.11 Clinically, lesions present as subcutaneous nodules. Histology reveals an infiltrative and poorly circumscribed proliferation of spindled myofibroblasts associated with myxoid stroma and dense collagen depositions. The spindled cells are loosely associated, rendering a tissue culture–like appearance (Figure 4). It also is common to see erythrocyte extravasation adjacent to myxoid stroma.11 Positive stains include vimentin, smooth muscle actin, and CD68, though immunohistochemistry often is not necessary for diagnosis.12 There often is abundant mitotic activity in nodular fasciitis, especially in early lesions, and the differential diagnosis includes sarcoma. Although nodular fasciitis is mitotically active, it does not show atypical mitotic figures. Nodular fasciitis commonly harbors a gene translocation of the MYH9 gene’s promoter region to the USP6 gene’s coding region.13
- Sakhadeo U, Mundhe R, DeSouza MA, et al. Pleomorphic lipoma: a gentle giant of pathology. J Cytol. 2015;32:201-203. doi:10.4103 /0970-9371.168904
- Shmookler BM, Enzinger FM. Pleomorphic lipoma: a benign tumor simulating liposarcoma. a clinicopathologic analysis of 48 cases. Cancer. 1981;47:126-133.
- Azzopardi JG, Iocco J, Salm R. Pleomorphic lipoma: a tumour simulating liposarcoma. Histopathology. 1983;7:511-523. doi:10.1111/j.1365-2559.1983.tb02264.x
- Jäger M, Winkelmann R, Eichler K, et al. Pleomorphic lipoma. J Dtsch Dermatol Ges. 2018;16:208-210. doi:10.1111/ddg.13422
- Allen A, Ahn C, Sangüeza OP. Dermatofibrosarcoma protuberans. Dermatol Clin. 2019;37:483-488. doi:10.1016/j.det.2019.05.006
- Socoliuc C, Zurac S, Andrei R, et al. Multiple histological subtypes of dermatofibrosarcoma protuberans occurring in the same tumor. Rom J Intern Med. 2015;53:79-88. doi:10.1515/rjim-2015-0011
- Abarzúa-Araya A, Lallas A, Piana S, et al. Superficial angiomyxoma of the skin. Dermatol Pract Concept. 2016;6:47-49. doi:10.5826 /dpc.0603a09
- Hornick J. Practical Soft Tissue Pathology A Diagnostic Approach. 2nd ed. Elsevier Health Sciences; 2017.
- Rato M, Monteiro AF, Parente J, et al. Case for diagnosis. multinucleated cell angiohistiocytoma. An Bras Dermatol. 2018;93:291-293. doi:10.1590 /abd1806-4841.20186821
- Grgurich E, Quinn K, Oram C, et al. Multinucleate cell angiohistiocytoma: case report and literature review. J Cutan Pathol. 2019;46:59-61. doi:10.1111/cup.13361
- Zuber TJ, Finley JL. Nodular fasciitis. South Med J. 1994;87:842-844. doi:10.1097/00007611-199408000-00020
- Yver CM, Husson MA, Friedman O. Pathology clinic: nodular fasciitis involving the external ear [published online March 18, 2021]. Ear Nose Throat J. doi:10.1177/01455613211001958
- Erickson-Johnson M, Chou M, Evers B, et al. Nodular fasciitis: a novel model of transient neoplasia induced by MYH9-USP6 gene fusion. Lab Invest. 2011;91:1427-1433. https://doi.org/10.1038 /labinvest.2011.118
The Diagnosis: Pleomorphic Lipoma
Pleomorphic lipoma is a rare, benign, adipocytic neoplasm that presents in the subcutaneous tissues of the upper shoulder, back, or neck. It predominantly affects men aged 50 to 70 years. Most lesions are situated in the subcutaneous tissues; few cases of intramuscular and retroperitoneal tumors have been reported.1 Clinically, pleomorphic lipomas present as painless, well-circumscribed lesions of the subcutaneous tissue that often resemble a lipoma or occasionally may be mistaken for liposarcoma. Histopathologic examination of ordinary lipomas reveals uniform mature adipocytes. However, pleomorphic lipomas consist of a mixture of multinucleated floretlike giant cells, variable-sized adipocytes, and fibrous tissue (ropy collagen bundles) with some myxoid and spindled areas.1,2 The most characteristic histologic feature of pleomorphic lipoma is multinucleated floretlike giant cells. The nuclei of these giant cells appear hyperchromatic, enlarged, and disposed to the periphery of the cell in a circular pattern. Additionally, tumors frequently contain excess mature dense collagen bundles that are strongly refractile in polarized light. Numerous mast cells are present. Atypical lipoblasts and capillary networks commonly are not visible in pleomorphic lipoma.3 The spindle cells express CD34 on immunohistochemistry. Loss of Rb-1 expression is typical.4
Dermatofibrosarcoma protuberans is a slow-growing soft tissue sarcoma that commonly begins as a pink or violet plaque on the trunk or upper limbs. Involvement of the head or neck accounts for only 10% to 15% of cases.5 This tumor has low metastatic potential but is highly infiltrative of surrounding tissues. It is associated with a translocation between chromosomes 22 and 17, leading to the fusion of the platelet-derived growth factor subunit β, PDGFB, and collagen type 1α1, COL1A1, genes.5 Clinically, patients often report that the lesion was present for several years prior to presentation with general stability in size and shape. Eventually, untreated lesions progress to become nodules or tumors and may even bleed or ulcerate. Histology reveals a storiform spindle cell proliferation throughout the dermis with infiltration into subcutaneous fat, commonly appearing in a honeycomblike pattern (Figure 1). Numerous histologic variants exist, including myxoid, sclerosing, pigmented (Bednar tumor), myoid, atrophic, or fibrosarcomatous dermatofibrosarcoma protuberans, as well as a giant cell fibroblastoma variant.6 These tumor subtypes can exist independently or in association with one another, creating hybrid lesions that can closely mimic other entities such as pleomorphic lipoma. The spindle cells stain positively for CD34. Treatment of these tumors involves complete surgical excision or Mohs micrographic surgery; however, recurrence is common for tumors involving the head or neck.5

Superficial angiomyxoma is a slow-growing papule that most commonly appears on the trunk, head, or neck in middle-aged adults. Occasionally, patients with Carney complex also can develop lesions on the external ear or breast.7 Histologically, superficial angiomyxoma is a hypocellular tumor characterized by abundant myxoid stroma, thin blood vessels, and small spindled and stellate cells with minimal cytoplasm (Figure 2).8 Superficial angiomyxoma and pleomorphic lipoma present differently on histology; superficial angiomyxoma is not associated with nuclear atypia or pleomorphism, whereas pleomorphic lipoma characteristically contains multinucleated floretlike giant cells and pleomorphism. Frequently, there also is loss of normal PRKAR1A gene expression, which is responsible for protein kinase A regulatory subunit 1-alpha expression.8

Multinucleate cell angiohistiocytoma is a rare benign proliferation that presents with numerous red-violet asymptomatic papules that commonly appear on the upper and lower extremities of women aged 40 to 70 years. Lesions feature both a fibrohistiocytic and vascular component.9 Histologic examination commonly shows multinucleated cells with angular outlining in the superficial dermis accompanied by fibrosis and ectatic small-caliber vessels (Figure 3). Although both pleomorphic lipoma and multinucleate cell angiohistiocytoma have similar-appearing multinucleated giant cells, the latter has a proliferation of narrow vessels in thick collagen bundles and lacks an adipocytic component, which distinguishes it from the former.10 Multinucleate cell angiohistiocytoma also is characterized by a substantial number of factor XIIIa–positive fibrohistiocytic interstitial cells and vascular hyperplasia.9

Nodular fasciitis is a benign lesion involving the rapid proliferation of myofibroblasts and fibroblasts in the subcutaneous tissue and most commonly is encountered on the extremities or head and neck regions. Many cases appear at sites of prior trauma, especially in patients aged 20 to 40 years. However, in infants and children the lesions typically are found in the head and neck regions.11 Clinically, lesions present as subcutaneous nodules. Histology reveals an infiltrative and poorly circumscribed proliferation of spindled myofibroblasts associated with myxoid stroma and dense collagen depositions. The spindled cells are loosely associated, rendering a tissue culture–like appearance (Figure 4). It also is common to see erythrocyte extravasation adjacent to myxoid stroma.11 Positive stains include vimentin, smooth muscle actin, and CD68, though immunohistochemistry often is not necessary for diagnosis.12 There often is abundant mitotic activity in nodular fasciitis, especially in early lesions, and the differential diagnosis includes sarcoma. Although nodular fasciitis is mitotically active, it does not show atypical mitotic figures. Nodular fasciitis commonly harbors a gene translocation of the MYH9 gene’s promoter region to the USP6 gene’s coding region.13

The Diagnosis: Pleomorphic Lipoma
Pleomorphic lipoma is a rare, benign, adipocytic neoplasm that presents in the subcutaneous tissues of the upper shoulder, back, or neck. It predominantly affects men aged 50 to 70 years. Most lesions are situated in the subcutaneous tissues; few cases of intramuscular and retroperitoneal tumors have been reported.1 Clinically, pleomorphic lipomas present as painless, well-circumscribed lesions of the subcutaneous tissue that often resemble a lipoma or occasionally may be mistaken for liposarcoma. Histopathologic examination of ordinary lipomas reveals uniform mature adipocytes. However, pleomorphic lipomas consist of a mixture of multinucleated floretlike giant cells, variable-sized adipocytes, and fibrous tissue (ropy collagen bundles) with some myxoid and spindled areas.1,2 The most characteristic histologic feature of pleomorphic lipoma is multinucleated floretlike giant cells. The nuclei of these giant cells appear hyperchromatic, enlarged, and disposed to the periphery of the cell in a circular pattern. Additionally, tumors frequently contain excess mature dense collagen bundles that are strongly refractile in polarized light. Numerous mast cells are present. Atypical lipoblasts and capillary networks commonly are not visible in pleomorphic lipoma.3 The spindle cells express CD34 on immunohistochemistry. Loss of Rb-1 expression is typical.4
Dermatofibrosarcoma protuberans is a slow-growing soft tissue sarcoma that commonly begins as a pink or violet plaque on the trunk or upper limbs. Involvement of the head or neck accounts for only 10% to 15% of cases.5 This tumor has low metastatic potential but is highly infiltrative of surrounding tissues. It is associated with a translocation between chromosomes 22 and 17, leading to the fusion of the platelet-derived growth factor subunit β, PDGFB, and collagen type 1α1, COL1A1, genes.5 Clinically, patients often report that the lesion was present for several years prior to presentation with general stability in size and shape. Eventually, untreated lesions progress to become nodules or tumors and may even bleed or ulcerate. Histology reveals a storiform spindle cell proliferation throughout the dermis with infiltration into subcutaneous fat, commonly appearing in a honeycomblike pattern (Figure 1). Numerous histologic variants exist, including myxoid, sclerosing, pigmented (Bednar tumor), myoid, atrophic, or fibrosarcomatous dermatofibrosarcoma protuberans, as well as a giant cell fibroblastoma variant.6 These tumor subtypes can exist independently or in association with one another, creating hybrid lesions that can closely mimic other entities such as pleomorphic lipoma. The spindle cells stain positively for CD34. Treatment of these tumors involves complete surgical excision or Mohs micrographic surgery; however, recurrence is common for tumors involving the head or neck.5

Superficial angiomyxoma is a slow-growing papule that most commonly appears on the trunk, head, or neck in middle-aged adults. Occasionally, patients with Carney complex also can develop lesions on the external ear or breast.7 Histologically, superficial angiomyxoma is a hypocellular tumor characterized by abundant myxoid stroma, thin blood vessels, and small spindled and stellate cells with minimal cytoplasm (Figure 2).8 Superficial angiomyxoma and pleomorphic lipoma present differently on histology; superficial angiomyxoma is not associated with nuclear atypia or pleomorphism, whereas pleomorphic lipoma characteristically contains multinucleated floretlike giant cells and pleomorphism. Frequently, there also is loss of normal PRKAR1A gene expression, which is responsible for protein kinase A regulatory subunit 1-alpha expression.8

Multinucleate cell angiohistiocytoma is a rare benign proliferation that presents with numerous red-violet asymptomatic papules that commonly appear on the upper and lower extremities of women aged 40 to 70 years. Lesions feature both a fibrohistiocytic and vascular component.9 Histologic examination commonly shows multinucleated cells with angular outlining in the superficial dermis accompanied by fibrosis and ectatic small-caliber vessels (Figure 3). Although both pleomorphic lipoma and multinucleate cell angiohistiocytoma have similar-appearing multinucleated giant cells, the latter has a proliferation of narrow vessels in thick collagen bundles and lacks an adipocytic component, which distinguishes it from the former.10 Multinucleate cell angiohistiocytoma also is characterized by a substantial number of factor XIIIa–positive fibrohistiocytic interstitial cells and vascular hyperplasia.9

Nodular fasciitis is a benign lesion involving the rapid proliferation of myofibroblasts and fibroblasts in the subcutaneous tissue and most commonly is encountered on the extremities or head and neck regions. Many cases appear at sites of prior trauma, especially in patients aged 20 to 40 years. However, in infants and children the lesions typically are found in the head and neck regions.11 Clinically, lesions present as subcutaneous nodules. Histology reveals an infiltrative and poorly circumscribed proliferation of spindled myofibroblasts associated with myxoid stroma and dense collagen depositions. The spindled cells are loosely associated, rendering a tissue culture–like appearance (Figure 4). It also is common to see erythrocyte extravasation adjacent to myxoid stroma.11 Positive stains include vimentin, smooth muscle actin, and CD68, though immunohistochemistry often is not necessary for diagnosis.12 There often is abundant mitotic activity in nodular fasciitis, especially in early lesions, and the differential diagnosis includes sarcoma. Although nodular fasciitis is mitotically active, it does not show atypical mitotic figures. Nodular fasciitis commonly harbors a gene translocation of the MYH9 gene’s promoter region to the USP6 gene’s coding region.13

- Sakhadeo U, Mundhe R, DeSouza MA, et al. Pleomorphic lipoma: a gentle giant of pathology. J Cytol. 2015;32:201-203. doi:10.4103 /0970-9371.168904
- Shmookler BM, Enzinger FM. Pleomorphic lipoma: a benign tumor simulating liposarcoma. a clinicopathologic analysis of 48 cases. Cancer. 1981;47:126-133.
- Azzopardi JG, Iocco J, Salm R. Pleomorphic lipoma: a tumour simulating liposarcoma. Histopathology. 1983;7:511-523. doi:10.1111/j.1365-2559.1983.tb02264.x
- Jäger M, Winkelmann R, Eichler K, et al. Pleomorphic lipoma. J Dtsch Dermatol Ges. 2018;16:208-210. doi:10.1111/ddg.13422
- Allen A, Ahn C, Sangüeza OP. Dermatofibrosarcoma protuberans. Dermatol Clin. 2019;37:483-488. doi:10.1016/j.det.2019.05.006
- Socoliuc C, Zurac S, Andrei R, et al. Multiple histological subtypes of dermatofibrosarcoma protuberans occurring in the same tumor. Rom J Intern Med. 2015;53:79-88. doi:10.1515/rjim-2015-0011
- Abarzúa-Araya A, Lallas A, Piana S, et al. Superficial angiomyxoma of the skin. Dermatol Pract Concept. 2016;6:47-49. doi:10.5826 /dpc.0603a09
- Hornick J. Practical Soft Tissue Pathology A Diagnostic Approach. 2nd ed. Elsevier Health Sciences; 2017.
- Rato M, Monteiro AF, Parente J, et al. Case for diagnosis. multinucleated cell angiohistiocytoma. An Bras Dermatol. 2018;93:291-293. doi:10.1590 /abd1806-4841.20186821
- Grgurich E, Quinn K, Oram C, et al. Multinucleate cell angiohistiocytoma: case report and literature review. J Cutan Pathol. 2019;46:59-61. doi:10.1111/cup.13361
- Zuber TJ, Finley JL. Nodular fasciitis. South Med J. 1994;87:842-844. doi:10.1097/00007611-199408000-00020
- Yver CM, Husson MA, Friedman O. Pathology clinic: nodular fasciitis involving the external ear [published online March 18, 2021]. Ear Nose Throat J. doi:10.1177/01455613211001958
- Erickson-Johnson M, Chou M, Evers B, et al. Nodular fasciitis: a novel model of transient neoplasia induced by MYH9-USP6 gene fusion. Lab Invest. 2011;91:1427-1433. https://doi.org/10.1038 /labinvest.2011.118
- Sakhadeo U, Mundhe R, DeSouza MA, et al. Pleomorphic lipoma: a gentle giant of pathology. J Cytol. 2015;32:201-203. doi:10.4103 /0970-9371.168904
- Shmookler BM, Enzinger FM. Pleomorphic lipoma: a benign tumor simulating liposarcoma. a clinicopathologic analysis of 48 cases. Cancer. 1981;47:126-133.
- Azzopardi JG, Iocco J, Salm R. Pleomorphic lipoma: a tumour simulating liposarcoma. Histopathology. 1983;7:511-523. doi:10.1111/j.1365-2559.1983.tb02264.x
- Jäger M, Winkelmann R, Eichler K, et al. Pleomorphic lipoma. J Dtsch Dermatol Ges. 2018;16:208-210. doi:10.1111/ddg.13422
- Allen A, Ahn C, Sangüeza OP. Dermatofibrosarcoma protuberans. Dermatol Clin. 2019;37:483-488. doi:10.1016/j.det.2019.05.006
- Socoliuc C, Zurac S, Andrei R, et al. Multiple histological subtypes of dermatofibrosarcoma protuberans occurring in the same tumor. Rom J Intern Med. 2015;53:79-88. doi:10.1515/rjim-2015-0011
- Abarzúa-Araya A, Lallas A, Piana S, et al. Superficial angiomyxoma of the skin. Dermatol Pract Concept. 2016;6:47-49. doi:10.5826 /dpc.0603a09
- Hornick J. Practical Soft Tissue Pathology A Diagnostic Approach. 2nd ed. Elsevier Health Sciences; 2017.
- Rato M, Monteiro AF, Parente J, et al. Case for diagnosis. multinucleated cell angiohistiocytoma. An Bras Dermatol. 2018;93:291-293. doi:10.1590 /abd1806-4841.20186821
- Grgurich E, Quinn K, Oram C, et al. Multinucleate cell angiohistiocytoma: case report and literature review. J Cutan Pathol. 2019;46:59-61. doi:10.1111/cup.13361
- Zuber TJ, Finley JL. Nodular fasciitis. South Med J. 1994;87:842-844. doi:10.1097/00007611-199408000-00020
- Yver CM, Husson MA, Friedman O. Pathology clinic: nodular fasciitis involving the external ear [published online March 18, 2021]. Ear Nose Throat J. doi:10.1177/01455613211001958
- Erickson-Johnson M, Chou M, Evers B, et al. Nodular fasciitis: a novel model of transient neoplasia induced by MYH9-USP6 gene fusion. Lab Invest. 2011;91:1427-1433. https://doi.org/10.1038 /labinvest.2011.118
An otherwise healthy 56-year-old man with a family history of lymphoma presented with a raised lesion on the postauricular neck. He first noticed the nodule 3 months prior and was unsure if it was still getting larger. It was predominantly asymptomatic. Physical examination revealed a 1.5×1.5-cm, mobile, subcutaneous nodule. An incisional biopsy was performed and submitted for histologic evaluation.
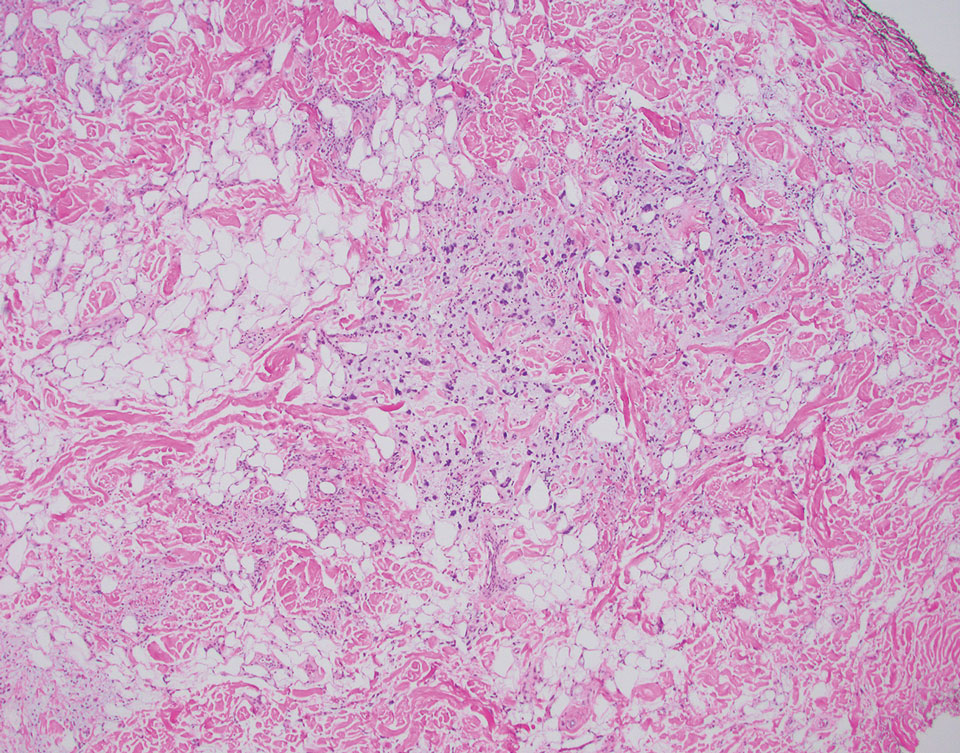
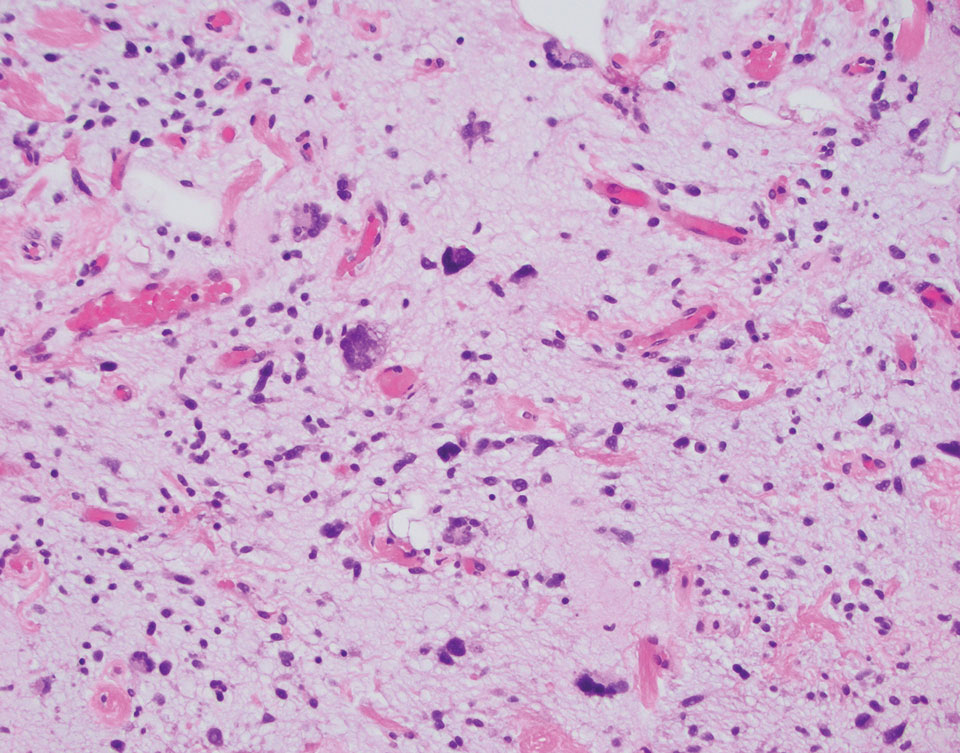
Disparities in Melanoma Demographics, Tumor Stage, and Metastases in Hispanic and Latino Patients: A Retrospective Study
To the Editor:
Melanoma is an aggressive form of skin cancer with a high rate of metastasis and poor prognosis.1 Historically, Hispanic and/or Latino patients have presented with more advanced-stage melanomas and have lower survival rates compared with non-Hispanic and/or non-Latino White patients.2 In this study, we evaluated recent data from the last decade to investigate if disparities in melanoma tumor stage at diagnosis and risk for metastases continue to exist in the Hispanic and/or Latino population.
We conducted a retrospective review of melanoma patients at 2 major medical centers in Los Angeles, California—Keck Medicine of USC and Los Angeles County-USC Medical Center—from January 2010 to January 2020. The data collected from electronic medical records included age at melanoma diagnosis, sex, race and ethnicity, insurance type, Breslow depth of lesion, presence of ulceration, and presence of lymph node or distant metastases. Melanoma tumor stage was determined using the American Joint Committee on Cancer classification. Patients who self-reported their ethnicity as not Hispanic and/or Latino were designated to this group regardless of their reported race. Those patients who reported their ethnicity as not Hispanic and/or Latino and reported their race as White were designated as non-Hispanic and/or non-Latino White. This study was approved by the institutional review board of the University of Southern California (Los Angeles). Data analysis was performed using the Pearson χ2 test, Fisher exact test, and Wilcoxon rank sum test. Statistical significance was determined at P<.05.
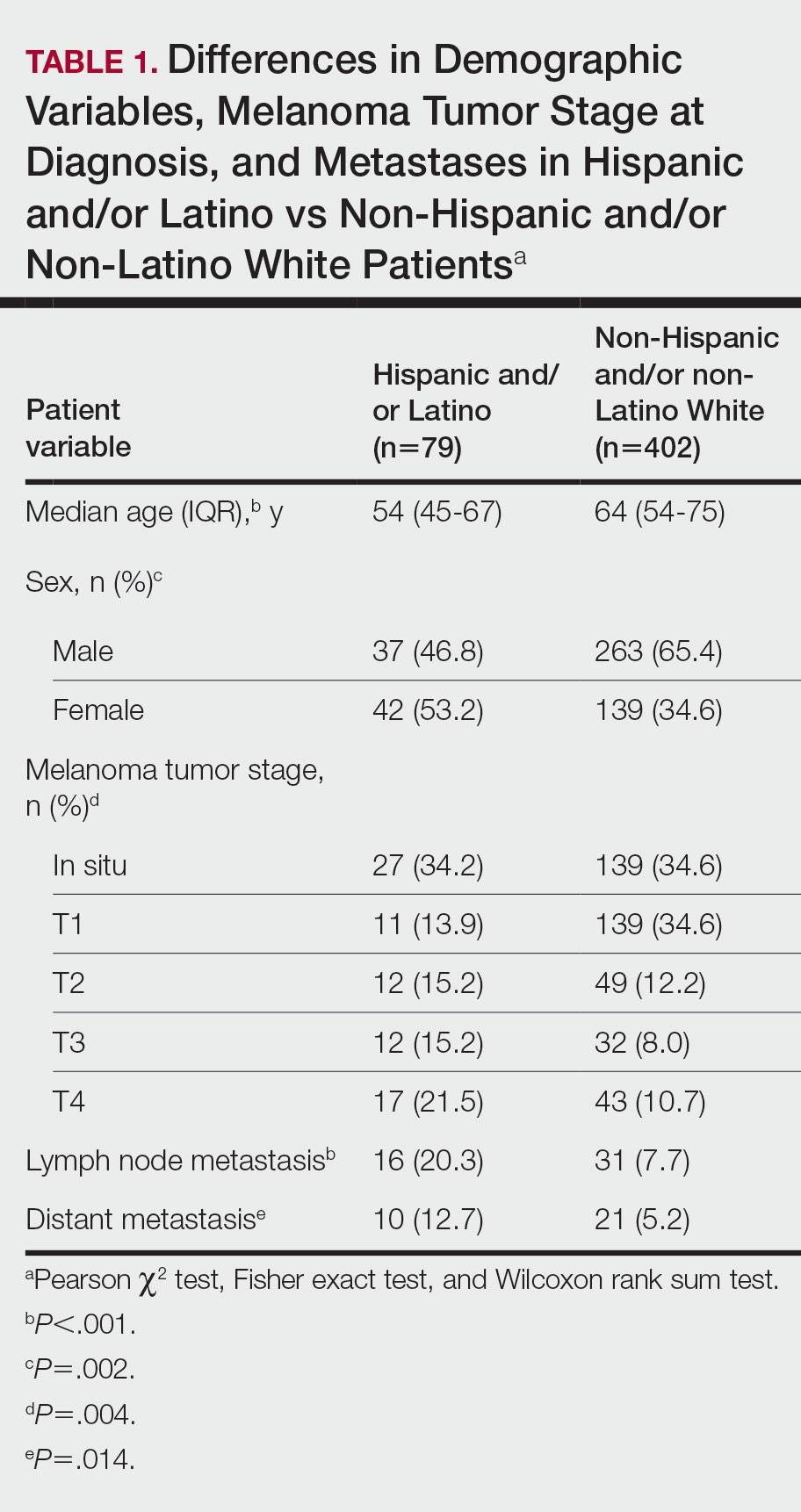
The final cohort of patients included 79 Hispanic and/or Latino patients and 402 non-Hispanic and/or non-Latino White patients. The median age for the Hispanic and/or Latino group was 54 years and 64 years for the non-Hispanic and/or non-Latino White group (P<.001). There was a greater percentage of females in the Hispanic and/or Latino group compared with the non-Hispanic and/or non-Latino White group (53.2% vs 34.6%)(P=.002). Hispanic and/or Latino patients presented with more advanced tumor stage melanomas (T3: 15.2%; T4: 21.5%) compared with non-Hispanic and/or non-Latino White patients (T3: 8.0%; T4: 10.7%)(P=.004). Furthermore, Hispanic and/or Latino patients had higher rates of lymph node metastases compared with non-Hispanic and/or non-Latino White patients (20.3% vs 7.7% [P<.001]) and higher rates of distant metastases (12.7% vs 5.2% [P=.014])(Table 1). The majority of Hispanic and/or Latino patients had Medicaid (39.2%), while most non-Hispanic and/or non-Latino White patients had a preferred provider organization insurance plan (37.3%) or Medicare (34.3%)(P<.001)(Table 2).
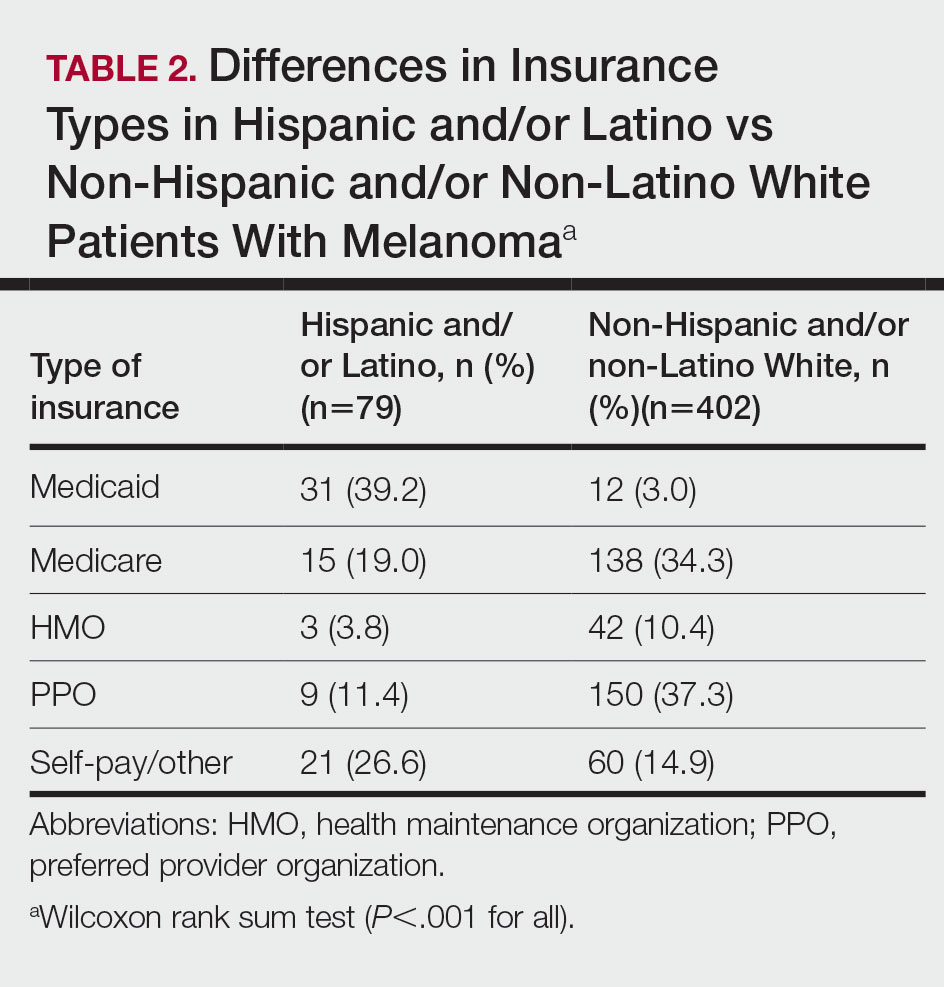
This retrospective study analyzing nearly 10 years of recent melanoma data found that disparities in melanoma diagnosis and treatment continue to exist among Hispanic and/or Latino patients. Compared to non-Hispanic and/or non-Latino White patients, Hispanic and/or Latino patients were diagnosed with melanoma at a younger age and the proportion of females with melanoma was higher. Cormier et al2 also reported that Hispanic patients were younger at melanoma diagnosis, and females represented a larger majority of patients in the Hispanic population compared with the White population. Hispanic and/or Latino patients in our study had more advanced melanoma tumor stage at diagnosis and a higher risk of lymph node and distant metastases, similar to findings reported by Koblinksi et al.3
Our retrospective cohort study demonstrated that the demographics of Hispanic and/or Latino patients with melanoma differ from non-Hispanic and/or non-Latino White patients, specifically with a greater proportion of younger and female patients in the Hispanic and/or Latino population. We also found that Hispanic and/or Latino patients continue to experience worse melanoma outcomes compared with non-Hispanic and/or non-Latino White patients. Further studies are needed to investigate the etiologies behind these health care disparities and potential interventions to address them. In addition, there needs to be increased awareness of the risk for melanoma in Hispanic and/or Latino patients among both health care providers and patients.
Limitations of this study included a smaller sample size of patients from one geographic region. The retrospective design of this study also increased the risk for selection bias, as some of the patients may have had incomplete records or were lost to follow-up. Therefore, the study cohort may not be representative of the general population. Additionally, patients’ skin types could not be determined using standardized tools such as the Fitzpatrick scale, thus we could not assess how patient skin type may have affected melanoma outcomes.
- Aggarwal P, Knabel P, Fleischer AB. United States burden of melanoma and non-melanoma skin cancer from 1990 to 2019. J Am Acad Dermatol. 2021;85:388-395. doi:10.1016/j.jaad.2021.03.109
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907. doi:10.1001/archinte.166.17.1907
- Koblinski JE, Maykowski P, Zeitouni NC. Disparities in melanoma stage at diagnosis in Arizona: a 10-year Arizona Cancer Registry study. J Am Acad Dermatol. 2021;84:1776-1779. doi:10.1016/j.jaad.2021.02.045
To the Editor:
Melanoma is an aggressive form of skin cancer with a high rate of metastasis and poor prognosis.1 Historically, Hispanic and/or Latino patients have presented with more advanced-stage melanomas and have lower survival rates compared with non-Hispanic and/or non-Latino White patients.2 In this study, we evaluated recent data from the last decade to investigate if disparities in melanoma tumor stage at diagnosis and risk for metastases continue to exist in the Hispanic and/or Latino population.
We conducted a retrospective review of melanoma patients at 2 major medical centers in Los Angeles, California—Keck Medicine of USC and Los Angeles County-USC Medical Center—from January 2010 to January 2020. The data collected from electronic medical records included age at melanoma diagnosis, sex, race and ethnicity, insurance type, Breslow depth of lesion, presence of ulceration, and presence of lymph node or distant metastases. Melanoma tumor stage was determined using the American Joint Committee on Cancer classification. Patients who self-reported their ethnicity as not Hispanic and/or Latino were designated to this group regardless of their reported race. Those patients who reported their ethnicity as not Hispanic and/or Latino and reported their race as White were designated as non-Hispanic and/or non-Latino White. This study was approved by the institutional review board of the University of Southern California (Los Angeles). Data analysis was performed using the Pearson χ2 test, Fisher exact test, and Wilcoxon rank sum test. Statistical significance was determined at P<.05.

The final cohort of patients included 79 Hispanic and/or Latino patients and 402 non-Hispanic and/or non-Latino White patients. The median age for the Hispanic and/or Latino group was 54 years and 64 years for the non-Hispanic and/or non-Latino White group (P<.001). There was a greater percentage of females in the Hispanic and/or Latino group compared with the non-Hispanic and/or non-Latino White group (53.2% vs 34.6%)(P=.002). Hispanic and/or Latino patients presented with more advanced tumor stage melanomas (T3: 15.2%; T4: 21.5%) compared with non-Hispanic and/or non-Latino White patients (T3: 8.0%; T4: 10.7%)(P=.004). Furthermore, Hispanic and/or Latino patients had higher rates of lymph node metastases compared with non-Hispanic and/or non-Latino White patients (20.3% vs 7.7% [P<.001]) and higher rates of distant metastases (12.7% vs 5.2% [P=.014])(Table 1). The majority of Hispanic and/or Latino patients had Medicaid (39.2%), while most non-Hispanic and/or non-Latino White patients had a preferred provider organization insurance plan (37.3%) or Medicare (34.3%)(P<.001)(Table 2).

This retrospective study analyzing nearly 10 years of recent melanoma data found that disparities in melanoma diagnosis and treatment continue to exist among Hispanic and/or Latino patients. Compared to non-Hispanic and/or non-Latino White patients, Hispanic and/or Latino patients were diagnosed with melanoma at a younger age and the proportion of females with melanoma was higher. Cormier et al2 also reported that Hispanic patients were younger at melanoma diagnosis, and females represented a larger majority of patients in the Hispanic population compared with the White population. Hispanic and/or Latino patients in our study had more advanced melanoma tumor stage at diagnosis and a higher risk of lymph node and distant metastases, similar to findings reported by Koblinksi et al.3
Our retrospective cohort study demonstrated that the demographics of Hispanic and/or Latino patients with melanoma differ from non-Hispanic and/or non-Latino White patients, specifically with a greater proportion of younger and female patients in the Hispanic and/or Latino population. We also found that Hispanic and/or Latino patients continue to experience worse melanoma outcomes compared with non-Hispanic and/or non-Latino White patients. Further studies are needed to investigate the etiologies behind these health care disparities and potential interventions to address them. In addition, there needs to be increased awareness of the risk for melanoma in Hispanic and/or Latino patients among both health care providers and patients.
Limitations of this study included a smaller sample size of patients from one geographic region. The retrospective design of this study also increased the risk for selection bias, as some of the patients may have had incomplete records or were lost to follow-up. Therefore, the study cohort may not be representative of the general population. Additionally, patients’ skin types could not be determined using standardized tools such as the Fitzpatrick scale, thus we could not assess how patient skin type may have affected melanoma outcomes.
To the Editor:
Melanoma is an aggressive form of skin cancer with a high rate of metastasis and poor prognosis.1 Historically, Hispanic and/or Latino patients have presented with more advanced-stage melanomas and have lower survival rates compared with non-Hispanic and/or non-Latino White patients.2 In this study, we evaluated recent data from the last decade to investigate if disparities in melanoma tumor stage at diagnosis and risk for metastases continue to exist in the Hispanic and/or Latino population.
We conducted a retrospective review of melanoma patients at 2 major medical centers in Los Angeles, California—Keck Medicine of USC and Los Angeles County-USC Medical Center—from January 2010 to January 2020. The data collected from electronic medical records included age at melanoma diagnosis, sex, race and ethnicity, insurance type, Breslow depth of lesion, presence of ulceration, and presence of lymph node or distant metastases. Melanoma tumor stage was determined using the American Joint Committee on Cancer classification. Patients who self-reported their ethnicity as not Hispanic and/or Latino were designated to this group regardless of their reported race. Those patients who reported their ethnicity as not Hispanic and/or Latino and reported their race as White were designated as non-Hispanic and/or non-Latino White. This study was approved by the institutional review board of the University of Southern California (Los Angeles). Data analysis was performed using the Pearson χ2 test, Fisher exact test, and Wilcoxon rank sum test. Statistical significance was determined at P<.05.

The final cohort of patients included 79 Hispanic and/or Latino patients and 402 non-Hispanic and/or non-Latino White patients. The median age for the Hispanic and/or Latino group was 54 years and 64 years for the non-Hispanic and/or non-Latino White group (P<.001). There was a greater percentage of females in the Hispanic and/or Latino group compared with the non-Hispanic and/or non-Latino White group (53.2% vs 34.6%)(P=.002). Hispanic and/or Latino patients presented with more advanced tumor stage melanomas (T3: 15.2%; T4: 21.5%) compared with non-Hispanic and/or non-Latino White patients (T3: 8.0%; T4: 10.7%)(P=.004). Furthermore, Hispanic and/or Latino patients had higher rates of lymph node metastases compared with non-Hispanic and/or non-Latino White patients (20.3% vs 7.7% [P<.001]) and higher rates of distant metastases (12.7% vs 5.2% [P=.014])(Table 1). The majority of Hispanic and/or Latino patients had Medicaid (39.2%), while most non-Hispanic and/or non-Latino White patients had a preferred provider organization insurance plan (37.3%) or Medicare (34.3%)(P<.001)(Table 2).

This retrospective study analyzing nearly 10 years of recent melanoma data found that disparities in melanoma diagnosis and treatment continue to exist among Hispanic and/or Latino patients. Compared to non-Hispanic and/or non-Latino White patients, Hispanic and/or Latino patients were diagnosed with melanoma at a younger age and the proportion of females with melanoma was higher. Cormier et al2 also reported that Hispanic patients were younger at melanoma diagnosis, and females represented a larger majority of patients in the Hispanic population compared with the White population. Hispanic and/or Latino patients in our study had more advanced melanoma tumor stage at diagnosis and a higher risk of lymph node and distant metastases, similar to findings reported by Koblinksi et al.3
Our retrospective cohort study demonstrated that the demographics of Hispanic and/or Latino patients with melanoma differ from non-Hispanic and/or non-Latino White patients, specifically with a greater proportion of younger and female patients in the Hispanic and/or Latino population. We also found that Hispanic and/or Latino patients continue to experience worse melanoma outcomes compared with non-Hispanic and/or non-Latino White patients. Further studies are needed to investigate the etiologies behind these health care disparities and potential interventions to address them. In addition, there needs to be increased awareness of the risk for melanoma in Hispanic and/or Latino patients among both health care providers and patients.
Limitations of this study included a smaller sample size of patients from one geographic region. The retrospective design of this study also increased the risk for selection bias, as some of the patients may have had incomplete records or were lost to follow-up. Therefore, the study cohort may not be representative of the general population. Additionally, patients’ skin types could not be determined using standardized tools such as the Fitzpatrick scale, thus we could not assess how patient skin type may have affected melanoma outcomes.
- Aggarwal P, Knabel P, Fleischer AB. United States burden of melanoma and non-melanoma skin cancer from 1990 to 2019. J Am Acad Dermatol. 2021;85:388-395. doi:10.1016/j.jaad.2021.03.109
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907. doi:10.1001/archinte.166.17.1907
- Koblinski JE, Maykowski P, Zeitouni NC. Disparities in melanoma stage at diagnosis in Arizona: a 10-year Arizona Cancer Registry study. J Am Acad Dermatol. 2021;84:1776-1779. doi:10.1016/j.jaad.2021.02.045
- Aggarwal P, Knabel P, Fleischer AB. United States burden of melanoma and non-melanoma skin cancer from 1990 to 2019. J Am Acad Dermatol. 2021;85:388-395. doi:10.1016/j.jaad.2021.03.109
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907. doi:10.1001/archinte.166.17.1907
- Koblinski JE, Maykowski P, Zeitouni NC. Disparities in melanoma stage at diagnosis in Arizona: a 10-year Arizona Cancer Registry study. J Am Acad Dermatol. 2021;84:1776-1779. doi:10.1016/j.jaad.2021.02.045
Practice Points
- Hispanic and/or Latino patients often present with more advanced-stage melanomas and have decreased survival rates compared with non-Hispanic and/or non-Latino White patients.
- More education and awareness on the risk for melanoma as well as sun-protective behaviors in the Hispanic and/or Latino population is needed among both health care providers and patients to prevent diagnosis of melanoma in later stages and improve outcomes.