User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Cutaneous Signs of Malnutrition Secondary to Eating Disorders
Eating disorders (EDs) and feeding disorders refer to a wide spectrum of complex biopsychosocial illnesses. The spectrum of EDs encompasses anorexia nervosa (AN), bulimia nervosa (BN), binge eating disorder, and other specified feeding or eating disorders. Feeding disorders, distinguished from EDs based on the absence of body image disturbance, include pica, rumination syndrome, and avoidant/restrictive food intake disorder (ARFID).1
This spectrum of illnesses predominantly affect young females aged 15 to 45 years, with recent increases in the rates of EDs among males, patients with skin of color, and adolescent females.2-5 Patients with EDs are at an elevated lifetime risk of suicidal ideation, suicide attempts, and other psychiatric comorbidities compared to the general population.6 Specifically, AN and BN are associated with high psychiatric morbidity and mortality. A meta-analysis by Arcelus et al7 demonstrated the weighted annual mortality for AN was 5.10 deaths per 1000 person-years (95% CI, 3.57-7.59) among patients with EDs and 4.55 deaths for studies that selected inpatients (95% CI, 3.09-6.28); for BN, the weighted mortality was 1.74 deaths per 1000 person-years (95% CI, 1.09-2.44). Unfortunately, ED diagnoses often are delayed or missed in clinical settings. Patients may lack insight into the severity of their illness, experience embarrassment about their eating behaviors, or actively avoid treatment for their ED.8
Pica—compulsive eating of nonnutritive substances outside the cultural norm—and rumination syndrome—regurgitation of undigested food—are feeding disorders more commonly recognized in childhood.9-11 Pregnancy, intellectual disability, iron deficiency, and lead poisoning are other conditions associated with pica.6,9,10 Avoidant/restrictive food intake disorder, a new diagnosis added to the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5)1 in 2013, is an eating or feeding disturbance resulting in persistent failure to meet nutritional or energy needs. Etiologies of ARFID may include sensory sensitivities and/or a traumatic event related to eating, leading to avoidance of associated foods.12
Patients with an ED or a feeding disorder frequently experience malnutrition, including deficiencies, excesses, or imbalances in nutritional intake, which may lead to nutritional dermatoses.13 As a result, the skin may present the first visible clues to an ED diagnosis.8,14-19 Gupta et al18 organized the skin signs of EDs into 4 categories: (1) those secondary to starvation or malnutrition; (2) cutaneous injury related to self-induced vomiting; (3) dermatoses due to laxative, diuretic, or emetic use; and (4) other concomitant psychiatric illnesses (eg, hand dermatitis from compulsive handwashing, dermatodaxia, onychophagia, trichotillomania). This review will focus on the effects of malnutrition and starvation on the skin.
Skin findings in patients with EDs offer the treating dermatologist a special opportunity for early diagnosis and appropriate consultation with specialists trained in ED treatment. It is important for dermatologists to be vigilant in looking for skin findings of nutritional dermatoses, especially in populations at an increased risk for developing an ED, such as young female patients. The approach to therapy and treatment must occur through a collaborative multidisciplinary effort in a thoughtful and nonjudgmental environment.
Xerosis
Xerosis, or dry skin, is the most common dermatologic finding in both adult and pediatric patients with AN and BN.14,19 It presents as skin roughness, tightness, flaking, and scaling, which may be complicated by fissuring, itching, and bleeding.20 In healthy skin, moisture is maintained by the stratum corneum and its lipids such as ceramides, cholesterol, and free fatty acids.21 Natural moisturizing factor (NMF) within the skin is composed of amino acids, ammonia, urea, uric acid, inorganic salts, lactic acid derivatives, and pyrrolidine-3-carboxylic acid.20-22 Disruptions to this system result in increased transepidermal water loss and impaired barrier function.23
In patients with ED, xerosis arises through several mechanisms. Chronic illness or starvation can lead to euthyroid sick syndrome with decreased peripheral conversion of thyroxine (T4) to triiodothyronine (T3).24,25 In the context of functional hypothyroidism, xerosis can arise from decreased eccrine gland secretion.26 Secretions of water, lactate, urea, sodium, and potassium from eccrine glands help to maintain NMF for skin hydration.27 Persistent laxative or diuretic abuse and fluid intake restriction, which are common behaviors across the spectrum of EDs, lead to dehydration and electrolyte imbalances that can manifest as skin dryness.20 Disrupted keratinocyte differentiation due to insufficient stores of vitamins and minerals involved in keratinocyte differentiation, such as vitamins A and C, selenium, and zinc, also may contribute to xerosis.25,28,29
Severely restrictive eating patterns may lead to development of protein energy malnutrition (PEM). Cutaneous findings in PEM occur due to dysmaturation of epidermal keratinocytes and epidermal atrophy.30 Patients with severe persistent depletion of macronutrients—carbohydrates, fat, and protein—may experience marasmus, resulting in loss of subcutaneous fat that causes the appearance of dry loose skin.29,31
Xerosis is exceedingly common in the general population and has no predictive value in ED diagnosis; however, this finding should be noted in the context of other signs suggestive of an ED. Treatment of xerosis in the setting of an ED should focus on correction of the underlying malnutrition. Symptomatic alleviation requires improving skin hydration and repairing barrier function. Mild xerosis may not need treatment or can be ameliorated with over-the-counter moisturizers and emollients. Scaling secondary to dry skin can be improved by ingredients such as glycerol, urea, lactic acid, and dexpanthenol.20,32 Glycerol and urea are small hydrophilic molecules that penetrate the stratum corneum and help to bind moisture within the skin to reduce transepidermal water loss. Urea and lactic acid are keratolytics of NMF commonly found in moisturizers and emollients.33,34 Dexpanthenol may be used for soothing fissures and pruritus; in vitro and in vivo studies have demonstrated its ability to upregulate dermal fibroblast proliferation and epidermal re-epithelization to promote faster wound healing.35
Lanugo
Lanugo is clinically apparent as a layer of fine, minimally pigmented hair. It is physiologically present on the skin surface of fetuses and newborns. In utero, lanugo plays an essential role in fetal skin protection from amniotic fluid, as well as promotion of proper hydration, thermoregulation, and innate immune development.36-38 Although it may be found on approximately 30% of newborns as normal variation, its presence beyond the neonatal period signals underlying systemic disease and severe undernutrition.16,36,39 Rarely, hypertrichosis lanuginosa acquisita has been reported in association with malignancy.40,41 The finding of lanugo beyond the neonatal period should prompt exclusion of other medical disorders, including neoplasms, chronic infections, hyperthyroidism, malabsorption syndromes, and inflammatory bowel disease.41-47
There is a limited understanding of the pathomechanism behind lanugo development in the context of malnutrition. Intentional starvation leads to loss of subcutaneous fat and a state of functional hypothyroidism.48 Studies hypothesize that lanugo develops as a response to hypothermia, regulated by dermal papillae cell–derived exosomes that may stimulate hair growth via paracrine signaling to outer root sheath cells.36,49 Molecular studies have found that T3 impacts skin and hair differentiation and proliferation by modulating thyroid hormone receptor regulation of keratin expression in epithelial cells.50,51 Lanugo may be a clinical indicator of severe malnutrition among ED patients, especially children and adolescents. A study of 30 patients aged 8 to 17 years with AN and BN who underwent a standard dermatologic examination found significant positive correlation between the presence of lanugo hair growth and concomitant amenorrhea (P<.01) as well as between lanugo hair and body mass index lower than 16 kg/m2 (P<.05).19 Discovery of lanugo in the dermatology clinical setting should prompt a thorough history, including screening questions about eating patterns; attitudes on eating, exercise, and appearance; personal and family history of EDs or other psychiatric disorders; and screening for depression and anxiety. Given its association with other signs of severe malnutrition, a clinical finding of lanugo should prompt close physical examination for other potential signs of an ED and laboratory evaluation for electrolyte levels and blood counts.52 Resolution of lanugo secondary to an ED is achieved with restoration of normal total body fat.18 Treatment should be focused on appropriate weight gain with the guidance of an ED specialist.
Pruritus
The prevalence and pathomechanism of pruritus secondary to EDs remains unclear.16,53,54 There have been limited reports of pruritus secondary to ED, with Gupta et al53 providing a case series of 6 patients with generalized pruritus in association with starvation and/or rapid weight loss. The study reported remission of pruritus with nutritional rehabilitation and/or weight gain of 5 to 10 pounds. Laboratory evaluation ruled out other causes of pruritus such as cholestasis and uremia.53 Other case reports have associated pruritus with iron deficiency, with anecdotal evidence of pruritus resolution following iron supplementation.55-59 Although we found no studies specifically relating iron deficiency, EDs, and pruritus, iron deficiency routinely is seen in ED patients and has a known association with pica.9,10,60 As such, iron deficiency may be a contributing factor in pruritus in ED patients. A UK study of 19 women with AN and a body mass index lower than 16 kg/m2 found that more than half of the patients (11/19 [57.9%]) described pruritus on the St. Thomas’ Itch Questionnaire, postulating that pruritus may be a clinical feature of AN.61 Limited studies with small samples make it difficult to conclude whether pruritus arises as a direct consequence of malnutrition.
Treatment of pruritus should address the underlying ED, as the pathophysiology of itch as it relates to malnutrition is poorly understood. Correction of existing nutritional imbalances by iron supplementation and appropriate weight gain may lead to symptom resolution. Because xerosis may be a contributing factor to pruritus, correction of the xerosis also may be therapeutic. More studies are needed on the connection between pruritus and the nutritional imbalances encountered in patients with EDs.
Acrocyanosis
Acrocyanosis is clinically seen as bluish-dusky discoloration most commonly affecting the hands and feet but also may affect the nose, ears, and nipples. Acrocyanosis typically is a sign of cold intolerance, hypothesized to occur in the context of AN due to shunting of blood centrally in response to hypothermia.39,62 The diminished oxyhemoglobin delivery to extremity sites leads to the characteristic blue color.63 In a study of 211 adolescent females (age range, 13–17 years) with AN, physical examination revealed peripheral hypothermia and peripheral cyanosis in 80% and 43% of patients, respectively.48 Cold intolerance seen in EDs may be secondary to a functional hypothyroid state similar to euthyroid sick syndrome seen in conditions of severe caloric deficit.25
It is possible that anemia and dehydration can worsen acrocyanosis due to impaired delivery of oxyhemoglobin to the body’s periphery.63 In a study of 14 ED patients requiring inpatient care, 6 were found to have underlying anemia following intravenous fluid supplementation.64 On admission, the mean (SD) hemoglobin and hematocrit across 14 patients was 12.74 (2.19) and 37.42 (5.99), respectively. Following intravenous fluid supplementation, the mean (SD) hemoglobin and hematocrit decreased to 9.88 (1.79)(P<.001) and 29.56 (4.91)(P=.008), respectively. Most cases reported intentional restriction of dietary sodium and fluid intake, with 2 patients reporting a history of diuretic misuse.64 These findings demonstrate that hemoglobin and hematocrit may be falsely normal in patients with AN due to hemoconcentration, suggesting that anemia may be underdiagnosed in inpatients with AN.
Beyond treatment of the underlying ED, acrocyanosis therapy is focused on improvement of circulation and avoidance of exacerbating factors. Pharmacologic intervention rarely is needed. Patients should be reassured that acrocyanosis is a benign condition and often can be improved by dressing warmly and avoiding exposure to cold. Severe cases may warrant trial treatment with nicotinic acid derivatives, α-adrenergic blockade, and topical minoxidil, which have demonstrated limited benefit in treating primary idiopathic acrocyanosis.63
Carotenoderma
Carotenoderma—the presence of a yellow discoloration to skin secondary to hypercarotenemia—has been described in patients with EDs since the 1960s.65,66 Beyond its clinical appearance, carotenoderma is asymptomatic. Carotenoids are lipid-soluble compounds present in the diet that are metabolized by the intestinal mucosa and liver to the primary conversion product, retinaldehyde, which is further converted to retinol, retinyl esters, and other retinoid metabolites.67,68 Retinol is bound by lipoproteins and transported in the plasma, then deposited in peripheral tissues,69 including in intercellular lipids in the stratum corneum, resulting in an orange hue that is most apparent in sites of increased skin thickness and sweating (eg, palms, soles, nasolabial folds).70 In an observational study of ED patients, Glorio et al14 found that carotenoderma was present in 23.77% (29/122) and 25% (4/16) of patients with BN and other specified feeding or eating disorder, respectively; it was not noted among patients with AN. Prior case reports have provided anecdotal evidence of carotenoderma in AN patients.66,71 In the setting of an ED, increased serum carotenoids likely are due to increased ingestion of carotene-rich foods, leading to increased levels of carotenoid-bound lipoproteins in the serum.70 Resolution of xanthoderma requires restriction of carotenoid intake and may take 2 to 3 months to be clinically apparent. The lipophilic nature of carotenoids allows storage in body fat, prolonging resolution.71
Hair Changes
Telogen effluvium (TE) and hair pigmentary changes are clinical findings that have been reported in association with EDs.14,16,19,72 Telogen effluvium occurs when physiologic stress causes a large portion of hairs in the anagen phase of growth to prematurely shift into the catagen then telogen phase. Approximately 2 to 3 months following the initial insult, there is clinically apparent excessive hair shedding compared to baseline.73 Studies have demonstrated that patients with EDs commonly have psychiatric comorbidities such as mood and anxiety disorders, obsessive compulsive disorder, posttraumatic stress disorder, and panic disorder compared to the general population.6,74-76 As such, stress experienced by ED patients may contribute to TE. Despite TE being commonly reported in ED patients,16-18 there is a lack of controlled studies of TE in human subjects with ED. An animal model for TE demonstrated that stressed mice exhibited further progression in the hair cycle compared with nonstressed mice (P<.01); the majority of hair follicles in stressed mice were in the catagen phase, while the majority of hair follicles in nonstressed mice were in the anagen phase.77 Stressed mice demonstrated an increased number of major histocompatibility complex class II+ cell clusters, composed mostly of activated macrophages, per 12.5-mm epidermal length compared to nonstressed mice (mean [SEM], 7.0 [1.1] vs 2.0 [0.3][P<.05]). This study illustrated that stress can lead to inflammatory cell recruitment and activation in the hair follicle microenvironment with growth-inhibitory effects.77
The flag sign, or alternating bands of lesser and greater pigmentation in the hair, has been reported in cases of severe PEM.31 In addition, PEM may lead to scalp alopecia, dry and brittle hair, and/or hypopigmentation with periods of inadequate nutrition.29,78 Scalp hair hypopigmentation, brittleness, and alopecia have been reported in pediatric patients with highly selective eating and/or ARFID.79,80 Maruo et al80 described a 3-year-old boy with ASD who consumed only potato chips for more than a year. Physical examination revealed reduced skin turgor overall and sparse red-brown hair on the scalp; laboratory testing showed deficiencies of protein, vitamin A, vitamin D, copper, and zinc. The patient was admitted for nutritional rehabilitation via nasogastric tube feeding, leading to resolution of laboratory abnormalities and growth of thicker black scalp hair over the course of several months.80
Neuroendocrine control of keratin expression by thyroid-stimulating hormone (TSH) and thyroid hormones likely plays a role in the regulation of hair follicle activities, including hair growth, structure, and stem cell differentiation.81,82 Altered thyroid hormone activity, which commonly is seen in patients with EDs,24,25 may contribute to impaired hair growth and pigmentation.26,51,83-85 Using tissue cultures of human anagen hair follicles, van Beek et al85 provided in vitro evidence that T3 and T4 modulate scalp hair follicle growth and pigmentation. Both T3- and T4-treated tissue exhibited increased numbers of anagen and decreased numbers of catagen hair follicles in organ cultures compared with control (P<.01); on quantitative Fontana-Masson histochemistry, T3 and T4 significantly stimulated hair follicle melanin synthesis compared with control (P<.001 and P<.01, respectively).85 Molecular studies by Bodó et al83 have shown that the human scalp epidermis expresses TSH at the messenger RNA and protein levels. Both studies showed that intraepidermal TSH expression is downregulated by thyroid hormones.83,85 Further studies are needed to examine the impact of malnutrition on local thyroid hormone signaling and action at the level of the dermis, epidermis, and hair follicle.
Discovery of TE, hair loss, and/or hair hypopigmentation should prompt close investigation for other signs of thyroid dysfunction, specifically secondary to malnutrition. Imbalances in TSH, T3, and T4 should be corrected. Nutritional deficiencies and dietary habits should be addressed through careful nutritional rehabilitation and targeted ED treatment.
Oral and Mucosal Symptoms
Symptoms of the oral cavity that may arise secondary to EDs and feeding disorders include glossitis, stomatitis, cheilitis, and dental erosions. Mucosal symptoms have been observed in patients with vitamin B deficiencies, inflammatory bowel disease, and other malabsorptive disorders, including patients with EDs.86-88 Patients following restrictive diets, specifically strict vegan diets, without additional supplementation are at risk for developing vitamin B12 deficiency. Because vitamin B12 is stored in the liver, symptoms of deficiency appear when hepatic stores are depleted over the course of several years.89 Insufficient vitamin B12 prevents the proper functioning of methionine synthase, which is required for the conversion of homocysteine to methionine and for the conversion of methyl-tetrahydrofolate to tetrahydrofolate.89 Impairment of this process impedes the synthesis of pyrimidine bases of DNA, disrupting the production of rapidly proliferating cells such as myeloid cells or mucosal lining cells. In cases of glossitis and/or stomatitis due to vitamin B12 deficiency, resolution of lesions was achieved within 4 weeks of daily oral supplementation with vitamin B12 at 2 μg daily.90,91 Iron deficiency, a common finding in EDs, also may contribute to glossitis and angular cheilitis.29 If uncovered, iron deficiency should be corrected by supplementation based on total deficit, age, and sex. Oral supplementation may be done with oral ferrous sulfate (325 mg provides 65 mg elemental iron) or with other iron salts such as ferrous gluconate (325 mg provides 38 mg elemental iron).29 Mucosal symptoms of cheilitis and labial erythema may arise from irritation due to self-induced vomiting.88
Dental erosion refers to loss of tooth structure via a chemical process that does not involve bacteria; in contrast, dental caries refer to tooth damage secondary to bacterial acid production. Patients with EDs who repeatedly self-induce vomiting have persistent introduction of gastric acids into the oral cavity, resulting in dissolution of the tooth enamel, which occurs when teeth are persistently exposed to a pH less than 5.5.92 Feeding disorders also may predispose patients to dental pathology. In a study of 60 pediatric patients, those with rumination syndrome were significantly more likely to have dental erosions than age- and sex-matched healthy controls (23/30 [77%] vs 4/30 [13%][P<.001]). The same study found no difference in the frequency of dental caries between children with and without rumination syndrome.92 These findings suggest that rumination syndrome increases the risk for dental erosions but not dental caries. The distribution of teeth affected by dental erosions may differ between EDs and feeding disorders. Patients with BN are more likely to experience involvement of the palatal surfaces of maxillary teeth, while patients with rumination syndrome had equal involvement of maxillary and mandibular teeth.92
There is limited literature on the role of dentists in the care of patients with EDs and feeding disorders, though existing studies suggest inclusion of a dental care professional in multidisciplinary treatment along with emphasis on education around a home dental care regimen and frequent dental follow-up.76,93,94 Prevention of further damage requires correction of the underlying behaviors and ED.
Other Dermatologic Findings
Russell sign refers to the development of calluses on the dorsal metacarpophalangeal joints of the dominant hand due to self-induced vomiting. Due to its specificity in purging-type EDs, the discovery of Russell sign should greatly increase suspicion for an ED.17 Patients with EDs also are at an increased risk for self-harming and body-focused repetitive behaviors, including skin cutting, superficial burning, onychophagia, and trichotillomania.19 It is important to recognize these signs in patients for whom an ED is suspected. The role of the dermatologist should include careful examination of the skin and documentation of findings that may aid in the diagnosis of an underlying ED.
Final Thoughts
A major limitation of this review is the reliance on small case reports and case series reporting cutaneous manifestations of ED. Controlled studies with larger cohorts are challenging in this population but are needed to substantiate the dermatologic signs commonly associated with EDs. Translational studies may help elucidate the pathomechanisms underlying dermatologic diseases such as lanugo, pruritus, and alopecia in the context of EDs and malnutrition. The known association between thyroid dysfunction and skin disease has been substantiated by clinical and basic science investigation, suggesting a notable role of thyroid hormone and TSH signaling in the skin local environment. Further investigation into nutritional and neuroendocrine regulation of skin health will aid in the diagnosis and treatment of patients impacted by EDs.
The treatment of the underlying ED is key in correcting associated skin disease, which requires interdisciplinary collaboration that addresses the psychological, behavioral, and social components of the condition. Following a diagnosis of ED, assessment should be made of the nutritional rehabilitation required to restore weight and nutritional status. Inpatient treatment may be indicated for patients requiring close monitoring to avoid refeeding syndrome, or those who meet the criteria for extreme AN in the DSM-5 (ie, body mass index <15 kg/m2),1 or demonstrate signs of medical instability or organ failure secondary to malnutrition.62 Long-term recovery for ED patients should focus on behavioral therapy with a multidisciplinary team consisting of a psychiatrist, therapist, dietitian, and primary care provider. Comparative studies in large-scale trials of cognitive behavioral therapy, focal psychodynamic psychotherapy, and specialist supportive clinical management have shown little to no difference in efficacy in treating EDs.75,95,96
Dermatologists may be the first providers to observe sequelae of nutritional and behavioral derangement in patients with EDs. Existing literature on the dermatologic findings of EDs report great heterogeneity of skin signs, with a very limited number of controlled studies available. Each cutaneous symptom described in this review should not be interpreted as an isolated pathology but should be placed in the context of patient predisposing risk factors and the constellation of other skin findings that may be suggestive of disordered eating behavior or other psychiatric illness. The observation of multiple signs and symptoms at the same time, especially of symptoms uncommonly encountered or suggestive of a severe and prolonged imbalance (eg, xanthoderma with vitamin A excess, aphthous stomatitis with vitamin B deficiency), should heighten clinical suspicion for an underlying ED. A clinician’s highest priority should be to resolve life-threatening medical emergencies and address nutritional derangements with the assistance of experts who are well versed in EDs. The patient should undergo workup to rule out organic causes of their nutritional dermatoses. Given the high psychiatric morbidity and mortality of patients with an ED and the demonstrated benefit of early intervention, recognition of cutaneous manifestations of malnutrition and EDs may be paramount to improving outcomes.
- Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
- Siddiqui A, Ramsay B, Leonard J. The cutaneous signs of eating disorders. Acta Derm Venereol. 1994;74:68-69. doi:10.2340/00015555746869
- Cheng ZH, Perko VL, Fuller-Marashi L, et al. Ethnic differences in eating disorder prevalence, risk factors, and predictive effects of risk factors among young women. Eat Behav. 2019;32:23-30. doi:10.1016/j. eatbeh.2018.11.004
- Smink FR, van Hoeken D, Hoek HW. Epidemiology of eating disorders: incidence, prevalence and mortality rates. Curr Psychiatry Rep. 2012;14:406-414. doi:10.1007/s11920-012-0282-y
- Campbell K, Peebles R. Eating disorders in children and adolescents: state of the art review. Pediatrics. 2014;134:582-592. doi:10.1542/peds.2014-0194
- Herpertz-Dahlmann B. Adolescent eating disorders: definitions, symptomatology, epidemiology and comorbidity. Child Adolesc Psychiatr Clin N Am. 2009;18:31-47. doi:10.1016/j.chc.2008.07.005
- Arcelus J, Mitchell AJ, Wales J, et al. Mortality rates in patients with anorexia nervosa and other eating disorders: a meta-analysis of 3 6 studies. Arch General Psychiatry. 2011;68:724-731. doi:10.1001 /archgenpsychiatry.2011.74
- Tyler I, Wiseman MC, Crawford RI, et al. Cutaneous manifestations of eating disorders. J Cutan Med Surg. 2002;6:345-353. doi:10.1177/120347540200600407
- Al Nasser Y, Muco E, Alsaad AJ. Pica. StatPearls. StatPearls Publishing; 2023.
- Borgna-Pignatti C, Zanella S. Pica as a manifestation of iron deficiency. Expert Rev Hematol. 2016;9:1075-1080. doi:10.1080/1747408 6.2016.1245136
- Talley NJ. Rumination syndrome. Gastroenterol Hepatol (N Y). 2011;7:117- 118.
- Sanchez-Cerezo J, Nagularaj L, Gledhill J, et al. What do we know about the epidemiology of avoidant/restrictive food intake disorder in children and adolescents? a systematic review of the literature. Eur Eat Disord Rev. 2023;31:226-246. doi:10.1002/erv.2964
- World Health Organization. Malnutrition. Published June 9, 2021. Accessed April 20, 2023. https://www.who.int/news-room/fact-sheets/detail/malnutrition
- Glorio R, Allevato M, De Pablo A, et al. Prevalence of cutaneous manifestations in 200 patients with eating disorders. Int J Dermatol. 2000;39:348-353. doi:10.1046/j.1365-4362.2000.00924.x
- Strumia R, Manzato E, Gualandi M. Is there a role for dermatologists in eating disorders? Expert Rev Dermatol. 2007;2:109-112. doi:10.1586/17469872.2.2.109
- Strumia R. Skin signs in anorexia nervosa. Dermatoendocrinol. 2009;1:268-270. doi:10.4161/derm.1.5.10193
- Strumia R. Eating disorders and the skin. Clin Dermatol. 2013;31:80-85. doi:http://doi.org/10.1016/j.clindermatol.2011.11.011
- Gupta MA, Gupta AK, Haberman HF. Dermatologic signs in anorexia nervosa and bulimia nervosa. Arch Dermatol. 1987;123:1386-1390. doi:10.1001/archderm.1987.01660340159040
- Schulze UM, Pettke-Rank CV, Kreienkamp M, et al. Dermatologic findings in anorexia and bulimia nervosa of childhood and adolescence. Pediatr Dermatol. 1999;16:90-94. doi:10.1046/j.1525-1470.1999.00022.x
- Augustin M, Wilsmann-Theis D, Körber A, et al. Diagnosis and treatment of xerosis cutis—a position paper. J Dtsch Dermatol Ges. 2019;17(suppl 7):3-33. doi:10.1111/ddg.13906
- Grubauer G, Feingold KR, Harris RM, et al. Lipid content and lipid type as determinants of the epidermal permeability barrier. J Lipid Res. 1989;30:89-96.
- Feingold KR, Man MQ, Menon GK, et al. Cholesterol synthesis is required for cutaneous barrier function in mice. J Clin Invest. 1990;86:1738-1745. doi:10.1172/jci114899
- Madison KC. Barrier function of the skin: “la raison d’être” of the epidermis. J Invest Dermatol. 2003;121:231-241. doi:10.106 /j.1523-1747.2003.12359.x
- Usdan LS, Khaodhiar L, Apovian CM. The endocrinopathies of anorexia nervosa. Endocr Pract. 2008;14:1055-1063. doi:10.4158/ep.14.8.1055
- Warren MP. Endocrine manifestations of eating disorders. J Clin Endocrinol Metabol. 2011;96:333-343. doi:10.1210/jc.2009-2304
- Safer JD. Thyroid hormone action on skin. Dermatoendocrinol. 2011;3:211-215. doi:10.4161/derm.3.3.17027
- Cui CY, Schlessinger D. Eccrine sweat gland development and sweat secretion. Exp Dermatol. 2015;24:644-650. doi:10.1111/exd.12773
- Nosewicz J, Spaccarelli N, Roberts KM, et al. The epidemiology, impact, and diagnosis of micronutrient nutritional dermatoses part 1: zinc, selenium, copper, vitamin A, and vitamin C. J Am Acad Dermatol. 2022;86:267-278. doi:10.1016/j.jaad.2021.07.079
- Hoffman M, Micheletti RG, Shields BE. Nutritional dermatoses in the hospitalized patient. Cutis. 2020;105:296;302-308, E1-E5.
- Cox JA, Beachkofsky T, Dominguez A. Flaky paint dermatosis. kwashiorkor. JAMA Dermatol. 2014;150:85-86. doi:10.1001 /jamadermatol.2013.5520
- Bradfield RB. Hair tissue as a medium for the differential diagnosis of protein-calorie malnutrition: a commentary. J Pediatr. 1974;84:294-296.
- Proksch E, Lachapelle J-M. The management of dry skin with topical emollients—recent perspectives. J Dtsch Dermatol Ges. 2005;3:768-774. doi:10.1111/j.1610-0387.2005.05068.x
- Watabe A, Sugawara T, Kikuchi K, et al. Sweat constitutes several natural moisturizing factors, lactate, urea, sodium, and potassium. J Dermatol Sci. 2013;72:177-182. doi:10.1016/j.jdermsci.2013.06.005
- Sugawara T, Kikuchi K, Tagami H, et al. Decreased lactate and potassium levels in natural moisturizing factor from the stratum corneum of mild atopic dermatitis patients are involved with the reduced hydration state. J Dermatol Sci. 2012;66:154-159. doi:10.1016/j .jdermsci.2012.02.011
- Gorski J, Proksch E, Baron JM, et al. Dexpanthenol in wound healing after medical and cosmetic interventions (postprocedure wound healing). Pharmaceuticals (Basel). 2020;13:138. doi:10.3390 /ph13070138
- Verhave BL, Nassereddin A, Lappin SL. Embryology, lanugo. StatPearls. StatPearls Publishing; 2022.
- Faist T. Vernix caseoza—composition and function. Ceska Gynekol. 2020;85:263-267.
- Bystrova K. Novel mechanism of human fetal growth regulation: a potential role of lanugo, vernix caseosa and a second tactile system of unmyelinated low-threshold C-afferents. Med Hypotheses. 2009;72:143-146. doi:10.1016/j.mehy.2008.09.033
- Mitchell JE, Crow S. Medical complications of anorexia nervosa and bulimia nervosa. Curr Opin Psychiatry. 2006;19:438-443. doi:10.1097/01.yco.0000228768.79097.3e
- Dalcin D, Manser C, Mahler R. Malignant down: hypertrichosis lanuginosa acquisita associated with endometrial adenocarcinoma. J Cutan Med Surg. 2015;19:507-510. doi:10.1177/1203475415582319
- Slee PH, van der Waal RI, Schagen van Leeuwen JH, et al. Paraneoplastic hypertrichosis lanuginosa acquisita: uncommon or overlooked? Br J Dermatol. 2007;157:1087-1092. doi:10.1111/j.1365-2133.2007.08253.x
- Lause M, Kamboj A, Fernandez Faith E. Dermatologic manifestations of endocrine disorders. Transl Pediatr. 2017;6:300-312. doi:10.21037 /tp.2017.09.08
- Vulink AJ, ten Bokkel Huinink D. Acquired hypertrichosis lanuginosa: a rare cutaneous paraneoplastic syndrome. J Clin Oncol. 2007;25:1625-1626. doi:10.1200/jco.2007.10.6963
- Wyatt JP, Anderson HF, Greer KE, et al. Acquired hypertrichosis lanuginosa as a presenting sign of metastatic prostate cancer with rapid resolution after treatment. J Am Acad Dermatol. 2007;56 (2 suppl):S45-S47. doi:10.1016/j.jaad.2006.07.011
- Saad N, Hot A, Ninet J, et al. Acquired hypertrichosis lanuginosa and gastric adenocarcinoma [in French]. Ann Dermatol Venereol. 2007;134:55-58. doi:10.1016/s0151-9638(07)88991-5
- Pruijm MC, van Houtum WH. An unusual cause of hypertrichosis. Neth J Med. 2007;65:42, 45.
- Lorette G, Maruani A. Images in clinical medicine. acquired hypertrichosis lanuginosa. N Engl J Med. 2006;354:2696. doi:10.1056 /NEJMicm050344
- Swenne I, Engström I. Medical assessment of adolescent girls with eating disorders: an evaluation of symptoms and signs of starvation. Acta Paediatr. 2005;94:1363-1371. doi:10.1111/j.1651-2227.2005.tb01805.x
- Zhou L, Wang H, Jing J, et al. Regulation of hair follicle development by exosomes derived from dermal papilla cells. Biochem Biophys Res Comm. 2018;500:325-332. doi:10.1016/j.bbrc.2018.04.067
- Tomic-Canic M, Day D, Samuels HH, et al. Novel regulation of keratin gene expression by thyroid hormone and retinoid receptors. J Biol Chem. 1996;271:1416-1423. doi:10.1074/jbc.271.3.1416
- Contreras-Jurado C, Lorz C, García-Serrano L, et al. Thyroid hormone signaling controls hair follicle stem cell function. Mol Biol Cell. 2015;26:1263-1272. doi:10.1091/mbc.E14-07-1251
- Hornberger LL, Lane MA. Identification and management of eating disorders in children and adolescents [published online December 20, 2021]. Pediatrics. doi:10.1542/peds.2020-040279
- Gupta MA, Gupta AK, Voorhees JJ. Starvation-associated pruritus: a clinical feature of eating disorders. J Am Acad Dermatol. 1992; 27:118-120. doi:10.1016/s0190-9622(08)80824-9
- Cevikbas F, Lerner EA. Physiology and pathophysiology of itch. Physiol Rev. 2020;100:945-982. doi:10.1152/physrev.00017.2019
- Stäubli M. Pruritus—a little known iron-deficiency symptom [in German]. Schweiz Med Wochenschr. 1981;111:1394-1398.
- Saini S, Jain AK, Agarwal S, et al. Iron deficiency and pruritus: a cross-sectional analysis to assess its association and relationship. Indian J Dermatol. 2021;66:705. doi:10.4103/ijd.ijd_326_21
- Tammaro A, Chello C, Di Fraia M, et al. Iron-deficiency and pruritus: a possible explanation of their relationship. Int J Research Dermatol. 2018;4:605. doi:10.18203/issn.2455-4529.IntJResDermatol20184470
- Takkunen H. Iron-deficiency pruritus. JAMA. 1978;239:1394.
- Lewiecki EM, Rahman F. Pruritus. a manifestation of iron deficiency. JAMA. 1976;236:2319-2320. doi:10.1001/jama.236.20.2319
- Kennedy A, Kohn M, Lammi A, et al. Iron status and haematological changes in adolescent female inpatients with anorexia nervosa. J Paediatr Child Health. 2004;40:430-432. doi:10.1111/j.1440-1754.2004.00432.x
- Morgan JF, Lacey JH. Scratching and fasting: a study of pruritus and anorexia nervosa. Br J Dermatol. 1999;140:453-456. doi:10.1046/j.1365- 2133.1999.02708.x
- Mehler PS. Anorexia nervosa in adults: evaluation for medical complications and criteria for hospitalization to manage these complications. UpToDate. Updated August 3, 2022. Accessed April 20, 2023. https://www.uptodate.com/contents/anorexia-nervosa-in-adults-evaluation-for-medical-complications-and-criteria-for -hospitalization-to-manage-these-complications
- Das S, Maiti A. Acrocyanosis: an overview. Indian J Dermatol. 2013;58:417-420. doi:10.4103/0019-5154.119946
- Caregaro L, Di Pascoli L, Favaro A, et al. Sodium depletion and hemoconcentration: overlooked complications in patients with anorexia nervosa? Nutrition. 2005;21:438-445. doi:10.1016/j.nut.2004.08.022
- Crisp AH, Stonehill E. Hypercarotenaemia as a symptom of weight phobia. Postgrad Med J. 1967;43:721. doi:10.1136/pgmj.43.505.721
- Pops MA, Schwabe AD. Hypercarotenemia in anorexia nervosa. JAMA. 1968;205:533-534. doi:10.1001/jama.1968.03140330075020.
- Bohn T, Desmarchelier C, El SN, et al. β-Carotene in the human body: metabolic bioactivation pathways—from digestion to tissue distribution and excretion. Proc Nutr Soc. 2019;78:68-87. doi:10.1017/S0029665118002641
- von Lintig J, Moon J, Lee J, et al. Carotenoid metabolism at the intestinal barrier. Biochim Biophys Acta Mol Cell Biol Lipids. 2020;1865:158580. doi:10.1016/j.bbalip.2019.158580
- Kanai M, Raz A, Goodman DS. Retinol-binding protein: the transport protein for vitamin A in human plasma. J Clin Invest. 1968;47:2025-2044. doi:10.1172/jci105889
- Haught JM, Patel S, English JC. Xanthoderma: a clinical review. J Am Acad Dermatol. 2007;57:1051-1058. doi:10.1016/j.jaad.2007.06.011
- Tung EE, Drage LA, Ghosh AK. Carotenoderma and hypercarotenemia: markers for disordered eating habits. J Eur Acad Dermatol Venereol. 2006;20:1147-1148. doi:10.1111/j.1468-3083.2006.01643.x
- Heilskov S, Vestergaard C, Babirekere E, et al. Characterization and scoring of skin changes in severe acute malnutrition in children between 6 months and 5 years of age. J Eur Acad Dermatol Venereol. 2015;29:2463-2469. doi:10.1111/jdv.13328
- Malkud S. Telogen effluvium: a review. J Clin Diagn Res. 2015;9:We01-3. doi:10.7860/jcdr/2015/15219.6492
- Filipponi C, Visentini C, Filippini T, et al. The follow-up of eating disorders from adolescence to early adulthood: a systematic review. Int J Environ Res Public Health. 2022;19:16237. doi:10.3390/ijerph192316237
- Byrne S, Wade T, Hay P, et al. A randomised controlled trial of three psychological treatments for anorexia nervosa. Psychol Med. 2017;47:2823-2833. doi:10.1017/s0033291717001349
- Ranalli DN, Studen-Pavlovich D. Eating disorders in the adolescent patient. Dent Clin North Am. 2021;65:689-703. doi:10.1016/j. cden.2021.06.009
- Arck PC, Handjiski B, Peters EM, et al. Stress inhibits hair growth in mice by induction of premature catagen development and deleterious perifollicular inflammatory events via neuropeptide substance P-dependent pathways. Am J Pathol. 2003;162:803-814. doi:10.1016/s0002-9440(10)63877-1
- Roy SK. Achromotrichia in tropical malnutrition. Br Med J. 1947;1:392. doi:10.1136/bmj.1.4498.392-c
- Swed-Tobia R, Haj A, Militianu D, et al. Highly selective eating in autism spectrum disorder leading to scurvy: a series of three patients. Pediatr Neurol. 2019;94:61-63. doi:10.1016/j.pediatrneurol.2018.12.011
- Maruo Y, Uetake K, Egawa K, et al. Selective eating in autism spectrum disorder leading to hair color change. Pediatr Neurol. 2021;120:1-2. doi:10.1016/j.pediatrneurol.2021.03.001
- Paus R, Langan EA, Vidali S, et al. Neuroendocrinology of the hair follicle: principles and clinical perspectives. Trends Mol Med. 2014;20:559-570. doi:10.1016/j.molmed.2014.06.002
- Antonini D, Sibilio A, Dentice M, et al. An intimate relationship between thyroid hormone and skin: regulation of gene expression. Front Endocrinol (Lausanne). 2013;4:104. doi: 10.3389/fendo.2013.00104
- Bodó E, Kany B, Gáspár E, et al. Thyroid-stimulating hormone, a novel, locally produced modulator of human epidermal functions, is regulated by thyrotropin-releasing hormone and thyroid hormones. Endocrinology. 2010;151:1633-1642. doi:10.1210/en.2009-0306
- Taguchi T. Brittle nails and hair loss in hypothyroidism. N Engl J Med. 2018;379:1363-1363. doi:10.1056/NEJMicm1801633
- van Beek N, Bodó E, Kromminga A, et al. Thyroid hormones directly alter human hair follicle functions: anagen prolongation and stimulation of both hair matrix keratinocyte proliferation and hair pigmentation. J Clin Endocrinol Metab. 2008;93:4381-4388. doi:10.1210/jc.2008-0283
- Zippi M, Corrado C, Pica R, et al. Extraintestinal manifestations in a large series of Italian inflammatory bowel disease patients. World J Gastroenterol. 2014;20:17463-7467. doi:10.3748/wjg.v20.i46.17463.
- Gutierrez Gossweiler A, Martinez-Mier EA. Chapter 6: vitamins and oral health. Monogr Oral Sci. 2020;28:59-67. doi:10.1159/000455372
- Monda M, Costacurta M, Maffei L, et al. Oral manifestations of eating disorders in adolescent patients. a review. Eur J Paediatr Dent. 2021;22:155-158. doi:10.23804/ejpd.2021.22.02.13
- Ankar A, Kumar A. Vitamin B12 deficiency. StatPearls. StatPearls Publishing; 2022.
- Graells J, Ojeda RM, Muniesa C, et al. Glossitis with linear lesions: an early sign of vitamin B12 deficiency. J Am Acad Dermatol. 2009;60:498- 500. doi:10.1016/j.jaad.2008.09.011
- Pétavy-Catala C, Fontès V, Gironet N, et al. Clinical manifestations of the mouth revealing vitamin B12 deficiency before the onset of anemia [in French]. Ann Dermatol Venereol. 2003;130(2 pt 1):191-194.
- Monagas J, Ritwik P, Kolomensky A, et al. Rumination syndrome and dental erosions in children. J Pediatr Gastroenterol Nutr. 2017; 64:930-932. doi:10.1097/mpg.0000000000001395
- Silverstein LS, Haggerty C, Sams L, et al. Impact of an oral health education intervention among a group of patients with eating disorders (anorexia nervosa and bulimia nervosa). J Eat Disord. 2019;7:29. doi:10.1186/s40337-019-0259-x
- Rangé H, Colon P, Godart N, et al. Eating disorders through the periodontal lens. Periodontol 2000. 2021;87:17-31. doi:10.1111 /prd.12391
- Zipfel S, Wild B, Groß G, et al. Focal psychodynamic therapy, cognitive behaviour therapy, and optimised treatment as usual in outpatients with anorexia nervosa (ANTOP study): randomised controlled trial. Lancet Psychiatry. 2014;383:127-137. doi:10.1016 /S2215-0366(22)00028-1
- Schmidt U, Ryan EG, Bartholdy S, et al. Two-year follow-up of the MOSAIC trial: a multicenter randomized controlled trial comparing two psychological treatments in adult outpatients with broadly defined anorexia nervosa. Int J Eat Disord. 2016;49:793-800. doi:10.1002/eat.22523
Eating disorders (EDs) and feeding disorders refer to a wide spectrum of complex biopsychosocial illnesses. The spectrum of EDs encompasses anorexia nervosa (AN), bulimia nervosa (BN), binge eating disorder, and other specified feeding or eating disorders. Feeding disorders, distinguished from EDs based on the absence of body image disturbance, include pica, rumination syndrome, and avoidant/restrictive food intake disorder (ARFID).1
This spectrum of illnesses predominantly affect young females aged 15 to 45 years, with recent increases in the rates of EDs among males, patients with skin of color, and adolescent females.2-5 Patients with EDs are at an elevated lifetime risk of suicidal ideation, suicide attempts, and other psychiatric comorbidities compared to the general population.6 Specifically, AN and BN are associated with high psychiatric morbidity and mortality. A meta-analysis by Arcelus et al7 demonstrated the weighted annual mortality for AN was 5.10 deaths per 1000 person-years (95% CI, 3.57-7.59) among patients with EDs and 4.55 deaths for studies that selected inpatients (95% CI, 3.09-6.28); for BN, the weighted mortality was 1.74 deaths per 1000 person-years (95% CI, 1.09-2.44). Unfortunately, ED diagnoses often are delayed or missed in clinical settings. Patients may lack insight into the severity of their illness, experience embarrassment about their eating behaviors, or actively avoid treatment for their ED.8
Pica—compulsive eating of nonnutritive substances outside the cultural norm—and rumination syndrome—regurgitation of undigested food—are feeding disorders more commonly recognized in childhood.9-11 Pregnancy, intellectual disability, iron deficiency, and lead poisoning are other conditions associated with pica.6,9,10 Avoidant/restrictive food intake disorder, a new diagnosis added to the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5)1 in 2013, is an eating or feeding disturbance resulting in persistent failure to meet nutritional or energy needs. Etiologies of ARFID may include sensory sensitivities and/or a traumatic event related to eating, leading to avoidance of associated foods.12
Patients with an ED or a feeding disorder frequently experience malnutrition, including deficiencies, excesses, or imbalances in nutritional intake, which may lead to nutritional dermatoses.13 As a result, the skin may present the first visible clues to an ED diagnosis.8,14-19 Gupta et al18 organized the skin signs of EDs into 4 categories: (1) those secondary to starvation or malnutrition; (2) cutaneous injury related to self-induced vomiting; (3) dermatoses due to laxative, diuretic, or emetic use; and (4) other concomitant psychiatric illnesses (eg, hand dermatitis from compulsive handwashing, dermatodaxia, onychophagia, trichotillomania). This review will focus on the effects of malnutrition and starvation on the skin.
Skin findings in patients with EDs offer the treating dermatologist a special opportunity for early diagnosis and appropriate consultation with specialists trained in ED treatment. It is important for dermatologists to be vigilant in looking for skin findings of nutritional dermatoses, especially in populations at an increased risk for developing an ED, such as young female patients. The approach to therapy and treatment must occur through a collaborative multidisciplinary effort in a thoughtful and nonjudgmental environment.
Xerosis
Xerosis, or dry skin, is the most common dermatologic finding in both adult and pediatric patients with AN and BN.14,19 It presents as skin roughness, tightness, flaking, and scaling, which may be complicated by fissuring, itching, and bleeding.20 In healthy skin, moisture is maintained by the stratum corneum and its lipids such as ceramides, cholesterol, and free fatty acids.21 Natural moisturizing factor (NMF) within the skin is composed of amino acids, ammonia, urea, uric acid, inorganic salts, lactic acid derivatives, and pyrrolidine-3-carboxylic acid.20-22 Disruptions to this system result in increased transepidermal water loss and impaired barrier function.23
In patients with ED, xerosis arises through several mechanisms. Chronic illness or starvation can lead to euthyroid sick syndrome with decreased peripheral conversion of thyroxine (T4) to triiodothyronine (T3).24,25 In the context of functional hypothyroidism, xerosis can arise from decreased eccrine gland secretion.26 Secretions of water, lactate, urea, sodium, and potassium from eccrine glands help to maintain NMF for skin hydration.27 Persistent laxative or diuretic abuse and fluid intake restriction, which are common behaviors across the spectrum of EDs, lead to dehydration and electrolyte imbalances that can manifest as skin dryness.20 Disrupted keratinocyte differentiation due to insufficient stores of vitamins and minerals involved in keratinocyte differentiation, such as vitamins A and C, selenium, and zinc, also may contribute to xerosis.25,28,29
Severely restrictive eating patterns may lead to development of protein energy malnutrition (PEM). Cutaneous findings in PEM occur due to dysmaturation of epidermal keratinocytes and epidermal atrophy.30 Patients with severe persistent depletion of macronutrients—carbohydrates, fat, and protein—may experience marasmus, resulting in loss of subcutaneous fat that causes the appearance of dry loose skin.29,31
Xerosis is exceedingly common in the general population and has no predictive value in ED diagnosis; however, this finding should be noted in the context of other signs suggestive of an ED. Treatment of xerosis in the setting of an ED should focus on correction of the underlying malnutrition. Symptomatic alleviation requires improving skin hydration and repairing barrier function. Mild xerosis may not need treatment or can be ameliorated with over-the-counter moisturizers and emollients. Scaling secondary to dry skin can be improved by ingredients such as glycerol, urea, lactic acid, and dexpanthenol.20,32 Glycerol and urea are small hydrophilic molecules that penetrate the stratum corneum and help to bind moisture within the skin to reduce transepidermal water loss. Urea and lactic acid are keratolytics of NMF commonly found in moisturizers and emollients.33,34 Dexpanthenol may be used for soothing fissures and pruritus; in vitro and in vivo studies have demonstrated its ability to upregulate dermal fibroblast proliferation and epidermal re-epithelization to promote faster wound healing.35
Lanugo
Lanugo is clinically apparent as a layer of fine, minimally pigmented hair. It is physiologically present on the skin surface of fetuses and newborns. In utero, lanugo plays an essential role in fetal skin protection from amniotic fluid, as well as promotion of proper hydration, thermoregulation, and innate immune development.36-38 Although it may be found on approximately 30% of newborns as normal variation, its presence beyond the neonatal period signals underlying systemic disease and severe undernutrition.16,36,39 Rarely, hypertrichosis lanuginosa acquisita has been reported in association with malignancy.40,41 The finding of lanugo beyond the neonatal period should prompt exclusion of other medical disorders, including neoplasms, chronic infections, hyperthyroidism, malabsorption syndromes, and inflammatory bowel disease.41-47
There is a limited understanding of the pathomechanism behind lanugo development in the context of malnutrition. Intentional starvation leads to loss of subcutaneous fat and a state of functional hypothyroidism.48 Studies hypothesize that lanugo develops as a response to hypothermia, regulated by dermal papillae cell–derived exosomes that may stimulate hair growth via paracrine signaling to outer root sheath cells.36,49 Molecular studies have found that T3 impacts skin and hair differentiation and proliferation by modulating thyroid hormone receptor regulation of keratin expression in epithelial cells.50,51 Lanugo may be a clinical indicator of severe malnutrition among ED patients, especially children and adolescents. A study of 30 patients aged 8 to 17 years with AN and BN who underwent a standard dermatologic examination found significant positive correlation between the presence of lanugo hair growth and concomitant amenorrhea (P<.01) as well as between lanugo hair and body mass index lower than 16 kg/m2 (P<.05).19 Discovery of lanugo in the dermatology clinical setting should prompt a thorough history, including screening questions about eating patterns; attitudes on eating, exercise, and appearance; personal and family history of EDs or other psychiatric disorders; and screening for depression and anxiety. Given its association with other signs of severe malnutrition, a clinical finding of lanugo should prompt close physical examination for other potential signs of an ED and laboratory evaluation for electrolyte levels and blood counts.52 Resolution of lanugo secondary to an ED is achieved with restoration of normal total body fat.18 Treatment should be focused on appropriate weight gain with the guidance of an ED specialist.
Pruritus
The prevalence and pathomechanism of pruritus secondary to EDs remains unclear.16,53,54 There have been limited reports of pruritus secondary to ED, with Gupta et al53 providing a case series of 6 patients with generalized pruritus in association with starvation and/or rapid weight loss. The study reported remission of pruritus with nutritional rehabilitation and/or weight gain of 5 to 10 pounds. Laboratory evaluation ruled out other causes of pruritus such as cholestasis and uremia.53 Other case reports have associated pruritus with iron deficiency, with anecdotal evidence of pruritus resolution following iron supplementation.55-59 Although we found no studies specifically relating iron deficiency, EDs, and pruritus, iron deficiency routinely is seen in ED patients and has a known association with pica.9,10,60 As such, iron deficiency may be a contributing factor in pruritus in ED patients. A UK study of 19 women with AN and a body mass index lower than 16 kg/m2 found that more than half of the patients (11/19 [57.9%]) described pruritus on the St. Thomas’ Itch Questionnaire, postulating that pruritus may be a clinical feature of AN.61 Limited studies with small samples make it difficult to conclude whether pruritus arises as a direct consequence of malnutrition.
Treatment of pruritus should address the underlying ED, as the pathophysiology of itch as it relates to malnutrition is poorly understood. Correction of existing nutritional imbalances by iron supplementation and appropriate weight gain may lead to symptom resolution. Because xerosis may be a contributing factor to pruritus, correction of the xerosis also may be therapeutic. More studies are needed on the connection between pruritus and the nutritional imbalances encountered in patients with EDs.
Acrocyanosis
Acrocyanosis is clinically seen as bluish-dusky discoloration most commonly affecting the hands and feet but also may affect the nose, ears, and nipples. Acrocyanosis typically is a sign of cold intolerance, hypothesized to occur in the context of AN due to shunting of blood centrally in response to hypothermia.39,62 The diminished oxyhemoglobin delivery to extremity sites leads to the characteristic blue color.63 In a study of 211 adolescent females (age range, 13–17 years) with AN, physical examination revealed peripheral hypothermia and peripheral cyanosis in 80% and 43% of patients, respectively.48 Cold intolerance seen in EDs may be secondary to a functional hypothyroid state similar to euthyroid sick syndrome seen in conditions of severe caloric deficit.25
It is possible that anemia and dehydration can worsen acrocyanosis due to impaired delivery of oxyhemoglobin to the body’s periphery.63 In a study of 14 ED patients requiring inpatient care, 6 were found to have underlying anemia following intravenous fluid supplementation.64 On admission, the mean (SD) hemoglobin and hematocrit across 14 patients was 12.74 (2.19) and 37.42 (5.99), respectively. Following intravenous fluid supplementation, the mean (SD) hemoglobin and hematocrit decreased to 9.88 (1.79)(P<.001) and 29.56 (4.91)(P=.008), respectively. Most cases reported intentional restriction of dietary sodium and fluid intake, with 2 patients reporting a history of diuretic misuse.64 These findings demonstrate that hemoglobin and hematocrit may be falsely normal in patients with AN due to hemoconcentration, suggesting that anemia may be underdiagnosed in inpatients with AN.
Beyond treatment of the underlying ED, acrocyanosis therapy is focused on improvement of circulation and avoidance of exacerbating factors. Pharmacologic intervention rarely is needed. Patients should be reassured that acrocyanosis is a benign condition and often can be improved by dressing warmly and avoiding exposure to cold. Severe cases may warrant trial treatment with nicotinic acid derivatives, α-adrenergic blockade, and topical minoxidil, which have demonstrated limited benefit in treating primary idiopathic acrocyanosis.63
Carotenoderma
Carotenoderma—the presence of a yellow discoloration to skin secondary to hypercarotenemia—has been described in patients with EDs since the 1960s.65,66 Beyond its clinical appearance, carotenoderma is asymptomatic. Carotenoids are lipid-soluble compounds present in the diet that are metabolized by the intestinal mucosa and liver to the primary conversion product, retinaldehyde, which is further converted to retinol, retinyl esters, and other retinoid metabolites.67,68 Retinol is bound by lipoproteins and transported in the plasma, then deposited in peripheral tissues,69 including in intercellular lipids in the stratum corneum, resulting in an orange hue that is most apparent in sites of increased skin thickness and sweating (eg, palms, soles, nasolabial folds).70 In an observational study of ED patients, Glorio et al14 found that carotenoderma was present in 23.77% (29/122) and 25% (4/16) of patients with BN and other specified feeding or eating disorder, respectively; it was not noted among patients with AN. Prior case reports have provided anecdotal evidence of carotenoderma in AN patients.66,71 In the setting of an ED, increased serum carotenoids likely are due to increased ingestion of carotene-rich foods, leading to increased levels of carotenoid-bound lipoproteins in the serum.70 Resolution of xanthoderma requires restriction of carotenoid intake and may take 2 to 3 months to be clinically apparent. The lipophilic nature of carotenoids allows storage in body fat, prolonging resolution.71
Hair Changes
Telogen effluvium (TE) and hair pigmentary changes are clinical findings that have been reported in association with EDs.14,16,19,72 Telogen effluvium occurs when physiologic stress causes a large portion of hairs in the anagen phase of growth to prematurely shift into the catagen then telogen phase. Approximately 2 to 3 months following the initial insult, there is clinically apparent excessive hair shedding compared to baseline.73 Studies have demonstrated that patients with EDs commonly have psychiatric comorbidities such as mood and anxiety disorders, obsessive compulsive disorder, posttraumatic stress disorder, and panic disorder compared to the general population.6,74-76 As such, stress experienced by ED patients may contribute to TE. Despite TE being commonly reported in ED patients,16-18 there is a lack of controlled studies of TE in human subjects with ED. An animal model for TE demonstrated that stressed mice exhibited further progression in the hair cycle compared with nonstressed mice (P<.01); the majority of hair follicles in stressed mice were in the catagen phase, while the majority of hair follicles in nonstressed mice were in the anagen phase.77 Stressed mice demonstrated an increased number of major histocompatibility complex class II+ cell clusters, composed mostly of activated macrophages, per 12.5-mm epidermal length compared to nonstressed mice (mean [SEM], 7.0 [1.1] vs 2.0 [0.3][P<.05]). This study illustrated that stress can lead to inflammatory cell recruitment and activation in the hair follicle microenvironment with growth-inhibitory effects.77
The flag sign, or alternating bands of lesser and greater pigmentation in the hair, has been reported in cases of severe PEM.31 In addition, PEM may lead to scalp alopecia, dry and brittle hair, and/or hypopigmentation with periods of inadequate nutrition.29,78 Scalp hair hypopigmentation, brittleness, and alopecia have been reported in pediatric patients with highly selective eating and/or ARFID.79,80 Maruo et al80 described a 3-year-old boy with ASD who consumed only potato chips for more than a year. Physical examination revealed reduced skin turgor overall and sparse red-brown hair on the scalp; laboratory testing showed deficiencies of protein, vitamin A, vitamin D, copper, and zinc. The patient was admitted for nutritional rehabilitation via nasogastric tube feeding, leading to resolution of laboratory abnormalities and growth of thicker black scalp hair over the course of several months.80
Neuroendocrine control of keratin expression by thyroid-stimulating hormone (TSH) and thyroid hormones likely plays a role in the regulation of hair follicle activities, including hair growth, structure, and stem cell differentiation.81,82 Altered thyroid hormone activity, which commonly is seen in patients with EDs,24,25 may contribute to impaired hair growth and pigmentation.26,51,83-85 Using tissue cultures of human anagen hair follicles, van Beek et al85 provided in vitro evidence that T3 and T4 modulate scalp hair follicle growth and pigmentation. Both T3- and T4-treated tissue exhibited increased numbers of anagen and decreased numbers of catagen hair follicles in organ cultures compared with control (P<.01); on quantitative Fontana-Masson histochemistry, T3 and T4 significantly stimulated hair follicle melanin synthesis compared with control (P<.001 and P<.01, respectively).85 Molecular studies by Bodó et al83 have shown that the human scalp epidermis expresses TSH at the messenger RNA and protein levels. Both studies showed that intraepidermal TSH expression is downregulated by thyroid hormones.83,85 Further studies are needed to examine the impact of malnutrition on local thyroid hormone signaling and action at the level of the dermis, epidermis, and hair follicle.
Discovery of TE, hair loss, and/or hair hypopigmentation should prompt close investigation for other signs of thyroid dysfunction, specifically secondary to malnutrition. Imbalances in TSH, T3, and T4 should be corrected. Nutritional deficiencies and dietary habits should be addressed through careful nutritional rehabilitation and targeted ED treatment.
Oral and Mucosal Symptoms
Symptoms of the oral cavity that may arise secondary to EDs and feeding disorders include glossitis, stomatitis, cheilitis, and dental erosions. Mucosal symptoms have been observed in patients with vitamin B deficiencies, inflammatory bowel disease, and other malabsorptive disorders, including patients with EDs.86-88 Patients following restrictive diets, specifically strict vegan diets, without additional supplementation are at risk for developing vitamin B12 deficiency. Because vitamin B12 is stored in the liver, symptoms of deficiency appear when hepatic stores are depleted over the course of several years.89 Insufficient vitamin B12 prevents the proper functioning of methionine synthase, which is required for the conversion of homocysteine to methionine and for the conversion of methyl-tetrahydrofolate to tetrahydrofolate.89 Impairment of this process impedes the synthesis of pyrimidine bases of DNA, disrupting the production of rapidly proliferating cells such as myeloid cells or mucosal lining cells. In cases of glossitis and/or stomatitis due to vitamin B12 deficiency, resolution of lesions was achieved within 4 weeks of daily oral supplementation with vitamin B12 at 2 μg daily.90,91 Iron deficiency, a common finding in EDs, also may contribute to glossitis and angular cheilitis.29 If uncovered, iron deficiency should be corrected by supplementation based on total deficit, age, and sex. Oral supplementation may be done with oral ferrous sulfate (325 mg provides 65 mg elemental iron) or with other iron salts such as ferrous gluconate (325 mg provides 38 mg elemental iron).29 Mucosal symptoms of cheilitis and labial erythema may arise from irritation due to self-induced vomiting.88
Dental erosion refers to loss of tooth structure via a chemical process that does not involve bacteria; in contrast, dental caries refer to tooth damage secondary to bacterial acid production. Patients with EDs who repeatedly self-induce vomiting have persistent introduction of gastric acids into the oral cavity, resulting in dissolution of the tooth enamel, which occurs when teeth are persistently exposed to a pH less than 5.5.92 Feeding disorders also may predispose patients to dental pathology. In a study of 60 pediatric patients, those with rumination syndrome were significantly more likely to have dental erosions than age- and sex-matched healthy controls (23/30 [77%] vs 4/30 [13%][P<.001]). The same study found no difference in the frequency of dental caries between children with and without rumination syndrome.92 These findings suggest that rumination syndrome increases the risk for dental erosions but not dental caries. The distribution of teeth affected by dental erosions may differ between EDs and feeding disorders. Patients with BN are more likely to experience involvement of the palatal surfaces of maxillary teeth, while patients with rumination syndrome had equal involvement of maxillary and mandibular teeth.92
There is limited literature on the role of dentists in the care of patients with EDs and feeding disorders, though existing studies suggest inclusion of a dental care professional in multidisciplinary treatment along with emphasis on education around a home dental care regimen and frequent dental follow-up.76,93,94 Prevention of further damage requires correction of the underlying behaviors and ED.
Other Dermatologic Findings
Russell sign refers to the development of calluses on the dorsal metacarpophalangeal joints of the dominant hand due to self-induced vomiting. Due to its specificity in purging-type EDs, the discovery of Russell sign should greatly increase suspicion for an ED.17 Patients with EDs also are at an increased risk for self-harming and body-focused repetitive behaviors, including skin cutting, superficial burning, onychophagia, and trichotillomania.19 It is important to recognize these signs in patients for whom an ED is suspected. The role of the dermatologist should include careful examination of the skin and documentation of findings that may aid in the diagnosis of an underlying ED.
Final Thoughts
A major limitation of this review is the reliance on small case reports and case series reporting cutaneous manifestations of ED. Controlled studies with larger cohorts are challenging in this population but are needed to substantiate the dermatologic signs commonly associated with EDs. Translational studies may help elucidate the pathomechanisms underlying dermatologic diseases such as lanugo, pruritus, and alopecia in the context of EDs and malnutrition. The known association between thyroid dysfunction and skin disease has been substantiated by clinical and basic science investigation, suggesting a notable role of thyroid hormone and TSH signaling in the skin local environment. Further investigation into nutritional and neuroendocrine regulation of skin health will aid in the diagnosis and treatment of patients impacted by EDs.
The treatment of the underlying ED is key in correcting associated skin disease, which requires interdisciplinary collaboration that addresses the psychological, behavioral, and social components of the condition. Following a diagnosis of ED, assessment should be made of the nutritional rehabilitation required to restore weight and nutritional status. Inpatient treatment may be indicated for patients requiring close monitoring to avoid refeeding syndrome, or those who meet the criteria for extreme AN in the DSM-5 (ie, body mass index <15 kg/m2),1 or demonstrate signs of medical instability or organ failure secondary to malnutrition.62 Long-term recovery for ED patients should focus on behavioral therapy with a multidisciplinary team consisting of a psychiatrist, therapist, dietitian, and primary care provider. Comparative studies in large-scale trials of cognitive behavioral therapy, focal psychodynamic psychotherapy, and specialist supportive clinical management have shown little to no difference in efficacy in treating EDs.75,95,96
Dermatologists may be the first providers to observe sequelae of nutritional and behavioral derangement in patients with EDs. Existing literature on the dermatologic findings of EDs report great heterogeneity of skin signs, with a very limited number of controlled studies available. Each cutaneous symptom described in this review should not be interpreted as an isolated pathology but should be placed in the context of patient predisposing risk factors and the constellation of other skin findings that may be suggestive of disordered eating behavior or other psychiatric illness. The observation of multiple signs and symptoms at the same time, especially of symptoms uncommonly encountered or suggestive of a severe and prolonged imbalance (eg, xanthoderma with vitamin A excess, aphthous stomatitis with vitamin B deficiency), should heighten clinical suspicion for an underlying ED. A clinician’s highest priority should be to resolve life-threatening medical emergencies and address nutritional derangements with the assistance of experts who are well versed in EDs. The patient should undergo workup to rule out organic causes of their nutritional dermatoses. Given the high psychiatric morbidity and mortality of patients with an ED and the demonstrated benefit of early intervention, recognition of cutaneous manifestations of malnutrition and EDs may be paramount to improving outcomes.
Eating disorders (EDs) and feeding disorders refer to a wide spectrum of complex biopsychosocial illnesses. The spectrum of EDs encompasses anorexia nervosa (AN), bulimia nervosa (BN), binge eating disorder, and other specified feeding or eating disorders. Feeding disorders, distinguished from EDs based on the absence of body image disturbance, include pica, rumination syndrome, and avoidant/restrictive food intake disorder (ARFID).1
This spectrum of illnesses predominantly affect young females aged 15 to 45 years, with recent increases in the rates of EDs among males, patients with skin of color, and adolescent females.2-5 Patients with EDs are at an elevated lifetime risk of suicidal ideation, suicide attempts, and other psychiatric comorbidities compared to the general population.6 Specifically, AN and BN are associated with high psychiatric morbidity and mortality. A meta-analysis by Arcelus et al7 demonstrated the weighted annual mortality for AN was 5.10 deaths per 1000 person-years (95% CI, 3.57-7.59) among patients with EDs and 4.55 deaths for studies that selected inpatients (95% CI, 3.09-6.28); for BN, the weighted mortality was 1.74 deaths per 1000 person-years (95% CI, 1.09-2.44). Unfortunately, ED diagnoses often are delayed or missed in clinical settings. Patients may lack insight into the severity of their illness, experience embarrassment about their eating behaviors, or actively avoid treatment for their ED.8
Pica—compulsive eating of nonnutritive substances outside the cultural norm—and rumination syndrome—regurgitation of undigested food—are feeding disorders more commonly recognized in childhood.9-11 Pregnancy, intellectual disability, iron deficiency, and lead poisoning are other conditions associated with pica.6,9,10 Avoidant/restrictive food intake disorder, a new diagnosis added to the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5)1 in 2013, is an eating or feeding disturbance resulting in persistent failure to meet nutritional or energy needs. Etiologies of ARFID may include sensory sensitivities and/or a traumatic event related to eating, leading to avoidance of associated foods.12
Patients with an ED or a feeding disorder frequently experience malnutrition, including deficiencies, excesses, or imbalances in nutritional intake, which may lead to nutritional dermatoses.13 As a result, the skin may present the first visible clues to an ED diagnosis.8,14-19 Gupta et al18 organized the skin signs of EDs into 4 categories: (1) those secondary to starvation or malnutrition; (2) cutaneous injury related to self-induced vomiting; (3) dermatoses due to laxative, diuretic, or emetic use; and (4) other concomitant psychiatric illnesses (eg, hand dermatitis from compulsive handwashing, dermatodaxia, onychophagia, trichotillomania). This review will focus on the effects of malnutrition and starvation on the skin.
Skin findings in patients with EDs offer the treating dermatologist a special opportunity for early diagnosis and appropriate consultation with specialists trained in ED treatment. It is important for dermatologists to be vigilant in looking for skin findings of nutritional dermatoses, especially in populations at an increased risk for developing an ED, such as young female patients. The approach to therapy and treatment must occur through a collaborative multidisciplinary effort in a thoughtful and nonjudgmental environment.
Xerosis
Xerosis, or dry skin, is the most common dermatologic finding in both adult and pediatric patients with AN and BN.14,19 It presents as skin roughness, tightness, flaking, and scaling, which may be complicated by fissuring, itching, and bleeding.20 In healthy skin, moisture is maintained by the stratum corneum and its lipids such as ceramides, cholesterol, and free fatty acids.21 Natural moisturizing factor (NMF) within the skin is composed of amino acids, ammonia, urea, uric acid, inorganic salts, lactic acid derivatives, and pyrrolidine-3-carboxylic acid.20-22 Disruptions to this system result in increased transepidermal water loss and impaired barrier function.23
In patients with ED, xerosis arises through several mechanisms. Chronic illness or starvation can lead to euthyroid sick syndrome with decreased peripheral conversion of thyroxine (T4) to triiodothyronine (T3).24,25 In the context of functional hypothyroidism, xerosis can arise from decreased eccrine gland secretion.26 Secretions of water, lactate, urea, sodium, and potassium from eccrine glands help to maintain NMF for skin hydration.27 Persistent laxative or diuretic abuse and fluid intake restriction, which are common behaviors across the spectrum of EDs, lead to dehydration and electrolyte imbalances that can manifest as skin dryness.20 Disrupted keratinocyte differentiation due to insufficient stores of vitamins and minerals involved in keratinocyte differentiation, such as vitamins A and C, selenium, and zinc, also may contribute to xerosis.25,28,29
Severely restrictive eating patterns may lead to development of protein energy malnutrition (PEM). Cutaneous findings in PEM occur due to dysmaturation of epidermal keratinocytes and epidermal atrophy.30 Patients with severe persistent depletion of macronutrients—carbohydrates, fat, and protein—may experience marasmus, resulting in loss of subcutaneous fat that causes the appearance of dry loose skin.29,31
Xerosis is exceedingly common in the general population and has no predictive value in ED diagnosis; however, this finding should be noted in the context of other signs suggestive of an ED. Treatment of xerosis in the setting of an ED should focus on correction of the underlying malnutrition. Symptomatic alleviation requires improving skin hydration and repairing barrier function. Mild xerosis may not need treatment or can be ameliorated with over-the-counter moisturizers and emollients. Scaling secondary to dry skin can be improved by ingredients such as glycerol, urea, lactic acid, and dexpanthenol.20,32 Glycerol and urea are small hydrophilic molecules that penetrate the stratum corneum and help to bind moisture within the skin to reduce transepidermal water loss. Urea and lactic acid are keratolytics of NMF commonly found in moisturizers and emollients.33,34 Dexpanthenol may be used for soothing fissures and pruritus; in vitro and in vivo studies have demonstrated its ability to upregulate dermal fibroblast proliferation and epidermal re-epithelization to promote faster wound healing.35
Lanugo
Lanugo is clinically apparent as a layer of fine, minimally pigmented hair. It is physiologically present on the skin surface of fetuses and newborns. In utero, lanugo plays an essential role in fetal skin protection from amniotic fluid, as well as promotion of proper hydration, thermoregulation, and innate immune development.36-38 Although it may be found on approximately 30% of newborns as normal variation, its presence beyond the neonatal period signals underlying systemic disease and severe undernutrition.16,36,39 Rarely, hypertrichosis lanuginosa acquisita has been reported in association with malignancy.40,41 The finding of lanugo beyond the neonatal period should prompt exclusion of other medical disorders, including neoplasms, chronic infections, hyperthyroidism, malabsorption syndromes, and inflammatory bowel disease.41-47
There is a limited understanding of the pathomechanism behind lanugo development in the context of malnutrition. Intentional starvation leads to loss of subcutaneous fat and a state of functional hypothyroidism.48 Studies hypothesize that lanugo develops as a response to hypothermia, regulated by dermal papillae cell–derived exosomes that may stimulate hair growth via paracrine signaling to outer root sheath cells.36,49 Molecular studies have found that T3 impacts skin and hair differentiation and proliferation by modulating thyroid hormone receptor regulation of keratin expression in epithelial cells.50,51 Lanugo may be a clinical indicator of severe malnutrition among ED patients, especially children and adolescents. A study of 30 patients aged 8 to 17 years with AN and BN who underwent a standard dermatologic examination found significant positive correlation between the presence of lanugo hair growth and concomitant amenorrhea (P<.01) as well as between lanugo hair and body mass index lower than 16 kg/m2 (P<.05).19 Discovery of lanugo in the dermatology clinical setting should prompt a thorough history, including screening questions about eating patterns; attitudes on eating, exercise, and appearance; personal and family history of EDs or other psychiatric disorders; and screening for depression and anxiety. Given its association with other signs of severe malnutrition, a clinical finding of lanugo should prompt close physical examination for other potential signs of an ED and laboratory evaluation for electrolyte levels and blood counts.52 Resolution of lanugo secondary to an ED is achieved with restoration of normal total body fat.18 Treatment should be focused on appropriate weight gain with the guidance of an ED specialist.
Pruritus
The prevalence and pathomechanism of pruritus secondary to EDs remains unclear.16,53,54 There have been limited reports of pruritus secondary to ED, with Gupta et al53 providing a case series of 6 patients with generalized pruritus in association with starvation and/or rapid weight loss. The study reported remission of pruritus with nutritional rehabilitation and/or weight gain of 5 to 10 pounds. Laboratory evaluation ruled out other causes of pruritus such as cholestasis and uremia.53 Other case reports have associated pruritus with iron deficiency, with anecdotal evidence of pruritus resolution following iron supplementation.55-59 Although we found no studies specifically relating iron deficiency, EDs, and pruritus, iron deficiency routinely is seen in ED patients and has a known association with pica.9,10,60 As such, iron deficiency may be a contributing factor in pruritus in ED patients. A UK study of 19 women with AN and a body mass index lower than 16 kg/m2 found that more than half of the patients (11/19 [57.9%]) described pruritus on the St. Thomas’ Itch Questionnaire, postulating that pruritus may be a clinical feature of AN.61 Limited studies with small samples make it difficult to conclude whether pruritus arises as a direct consequence of malnutrition.
Treatment of pruritus should address the underlying ED, as the pathophysiology of itch as it relates to malnutrition is poorly understood. Correction of existing nutritional imbalances by iron supplementation and appropriate weight gain may lead to symptom resolution. Because xerosis may be a contributing factor to pruritus, correction of the xerosis also may be therapeutic. More studies are needed on the connection between pruritus and the nutritional imbalances encountered in patients with EDs.
Acrocyanosis
Acrocyanosis is clinically seen as bluish-dusky discoloration most commonly affecting the hands and feet but also may affect the nose, ears, and nipples. Acrocyanosis typically is a sign of cold intolerance, hypothesized to occur in the context of AN due to shunting of blood centrally in response to hypothermia.39,62 The diminished oxyhemoglobin delivery to extremity sites leads to the characteristic blue color.63 In a study of 211 adolescent females (age range, 13–17 years) with AN, physical examination revealed peripheral hypothermia and peripheral cyanosis in 80% and 43% of patients, respectively.48 Cold intolerance seen in EDs may be secondary to a functional hypothyroid state similar to euthyroid sick syndrome seen in conditions of severe caloric deficit.25
It is possible that anemia and dehydration can worsen acrocyanosis due to impaired delivery of oxyhemoglobin to the body’s periphery.63 In a study of 14 ED patients requiring inpatient care, 6 were found to have underlying anemia following intravenous fluid supplementation.64 On admission, the mean (SD) hemoglobin and hematocrit across 14 patients was 12.74 (2.19) and 37.42 (5.99), respectively. Following intravenous fluid supplementation, the mean (SD) hemoglobin and hematocrit decreased to 9.88 (1.79)(P<.001) and 29.56 (4.91)(P=.008), respectively. Most cases reported intentional restriction of dietary sodium and fluid intake, with 2 patients reporting a history of diuretic misuse.64 These findings demonstrate that hemoglobin and hematocrit may be falsely normal in patients with AN due to hemoconcentration, suggesting that anemia may be underdiagnosed in inpatients with AN.
Beyond treatment of the underlying ED, acrocyanosis therapy is focused on improvement of circulation and avoidance of exacerbating factors. Pharmacologic intervention rarely is needed. Patients should be reassured that acrocyanosis is a benign condition and often can be improved by dressing warmly and avoiding exposure to cold. Severe cases may warrant trial treatment with nicotinic acid derivatives, α-adrenergic blockade, and topical minoxidil, which have demonstrated limited benefit in treating primary idiopathic acrocyanosis.63
Carotenoderma
Carotenoderma—the presence of a yellow discoloration to skin secondary to hypercarotenemia—has been described in patients with EDs since the 1960s.65,66 Beyond its clinical appearance, carotenoderma is asymptomatic. Carotenoids are lipid-soluble compounds present in the diet that are metabolized by the intestinal mucosa and liver to the primary conversion product, retinaldehyde, which is further converted to retinol, retinyl esters, and other retinoid metabolites.67,68 Retinol is bound by lipoproteins and transported in the plasma, then deposited in peripheral tissues,69 including in intercellular lipids in the stratum corneum, resulting in an orange hue that is most apparent in sites of increased skin thickness and sweating (eg, palms, soles, nasolabial folds).70 In an observational study of ED patients, Glorio et al14 found that carotenoderma was present in 23.77% (29/122) and 25% (4/16) of patients with BN and other specified feeding or eating disorder, respectively; it was not noted among patients with AN. Prior case reports have provided anecdotal evidence of carotenoderma in AN patients.66,71 In the setting of an ED, increased serum carotenoids likely are due to increased ingestion of carotene-rich foods, leading to increased levels of carotenoid-bound lipoproteins in the serum.70 Resolution of xanthoderma requires restriction of carotenoid intake and may take 2 to 3 months to be clinically apparent. The lipophilic nature of carotenoids allows storage in body fat, prolonging resolution.71
Hair Changes
Telogen effluvium (TE) and hair pigmentary changes are clinical findings that have been reported in association with EDs.14,16,19,72 Telogen effluvium occurs when physiologic stress causes a large portion of hairs in the anagen phase of growth to prematurely shift into the catagen then telogen phase. Approximately 2 to 3 months following the initial insult, there is clinically apparent excessive hair shedding compared to baseline.73 Studies have demonstrated that patients with EDs commonly have psychiatric comorbidities such as mood and anxiety disorders, obsessive compulsive disorder, posttraumatic stress disorder, and panic disorder compared to the general population.6,74-76 As such, stress experienced by ED patients may contribute to TE. Despite TE being commonly reported in ED patients,16-18 there is a lack of controlled studies of TE in human subjects with ED. An animal model for TE demonstrated that stressed mice exhibited further progression in the hair cycle compared with nonstressed mice (P<.01); the majority of hair follicles in stressed mice were in the catagen phase, while the majority of hair follicles in nonstressed mice were in the anagen phase.77 Stressed mice demonstrated an increased number of major histocompatibility complex class II+ cell clusters, composed mostly of activated macrophages, per 12.5-mm epidermal length compared to nonstressed mice (mean [SEM], 7.0 [1.1] vs 2.0 [0.3][P<.05]). This study illustrated that stress can lead to inflammatory cell recruitment and activation in the hair follicle microenvironment with growth-inhibitory effects.77
The flag sign, or alternating bands of lesser and greater pigmentation in the hair, has been reported in cases of severe PEM.31 In addition, PEM may lead to scalp alopecia, dry and brittle hair, and/or hypopigmentation with periods of inadequate nutrition.29,78 Scalp hair hypopigmentation, brittleness, and alopecia have been reported in pediatric patients with highly selective eating and/or ARFID.79,80 Maruo et al80 described a 3-year-old boy with ASD who consumed only potato chips for more than a year. Physical examination revealed reduced skin turgor overall and sparse red-brown hair on the scalp; laboratory testing showed deficiencies of protein, vitamin A, vitamin D, copper, and zinc. The patient was admitted for nutritional rehabilitation via nasogastric tube feeding, leading to resolution of laboratory abnormalities and growth of thicker black scalp hair over the course of several months.80
Neuroendocrine control of keratin expression by thyroid-stimulating hormone (TSH) and thyroid hormones likely plays a role in the regulation of hair follicle activities, including hair growth, structure, and stem cell differentiation.81,82 Altered thyroid hormone activity, which commonly is seen in patients with EDs,24,25 may contribute to impaired hair growth and pigmentation.26,51,83-85 Using tissue cultures of human anagen hair follicles, van Beek et al85 provided in vitro evidence that T3 and T4 modulate scalp hair follicle growth and pigmentation. Both T3- and T4-treated tissue exhibited increased numbers of anagen and decreased numbers of catagen hair follicles in organ cultures compared with control (P<.01); on quantitative Fontana-Masson histochemistry, T3 and T4 significantly stimulated hair follicle melanin synthesis compared with control (P<.001 and P<.01, respectively).85 Molecular studies by Bodó et al83 have shown that the human scalp epidermis expresses TSH at the messenger RNA and protein levels. Both studies showed that intraepidermal TSH expression is downregulated by thyroid hormones.83,85 Further studies are needed to examine the impact of malnutrition on local thyroid hormone signaling and action at the level of the dermis, epidermis, and hair follicle.
Discovery of TE, hair loss, and/or hair hypopigmentation should prompt close investigation for other signs of thyroid dysfunction, specifically secondary to malnutrition. Imbalances in TSH, T3, and T4 should be corrected. Nutritional deficiencies and dietary habits should be addressed through careful nutritional rehabilitation and targeted ED treatment.
Oral and Mucosal Symptoms
Symptoms of the oral cavity that may arise secondary to EDs and feeding disorders include glossitis, stomatitis, cheilitis, and dental erosions. Mucosal symptoms have been observed in patients with vitamin B deficiencies, inflammatory bowel disease, and other malabsorptive disorders, including patients with EDs.86-88 Patients following restrictive diets, specifically strict vegan diets, without additional supplementation are at risk for developing vitamin B12 deficiency. Because vitamin B12 is stored in the liver, symptoms of deficiency appear when hepatic stores are depleted over the course of several years.89 Insufficient vitamin B12 prevents the proper functioning of methionine synthase, which is required for the conversion of homocysteine to methionine and for the conversion of methyl-tetrahydrofolate to tetrahydrofolate.89 Impairment of this process impedes the synthesis of pyrimidine bases of DNA, disrupting the production of rapidly proliferating cells such as myeloid cells or mucosal lining cells. In cases of glossitis and/or stomatitis due to vitamin B12 deficiency, resolution of lesions was achieved within 4 weeks of daily oral supplementation with vitamin B12 at 2 μg daily.90,91 Iron deficiency, a common finding in EDs, also may contribute to glossitis and angular cheilitis.29 If uncovered, iron deficiency should be corrected by supplementation based on total deficit, age, and sex. Oral supplementation may be done with oral ferrous sulfate (325 mg provides 65 mg elemental iron) or with other iron salts such as ferrous gluconate (325 mg provides 38 mg elemental iron).29 Mucosal symptoms of cheilitis and labial erythema may arise from irritation due to self-induced vomiting.88
Dental erosion refers to loss of tooth structure via a chemical process that does not involve bacteria; in contrast, dental caries refer to tooth damage secondary to bacterial acid production. Patients with EDs who repeatedly self-induce vomiting have persistent introduction of gastric acids into the oral cavity, resulting in dissolution of the tooth enamel, which occurs when teeth are persistently exposed to a pH less than 5.5.92 Feeding disorders also may predispose patients to dental pathology. In a study of 60 pediatric patients, those with rumination syndrome were significantly more likely to have dental erosions than age- and sex-matched healthy controls (23/30 [77%] vs 4/30 [13%][P<.001]). The same study found no difference in the frequency of dental caries between children with and without rumination syndrome.92 These findings suggest that rumination syndrome increases the risk for dental erosions but not dental caries. The distribution of teeth affected by dental erosions may differ between EDs and feeding disorders. Patients with BN are more likely to experience involvement of the palatal surfaces of maxillary teeth, while patients with rumination syndrome had equal involvement of maxillary and mandibular teeth.92
There is limited literature on the role of dentists in the care of patients with EDs and feeding disorders, though existing studies suggest inclusion of a dental care professional in multidisciplinary treatment along with emphasis on education around a home dental care regimen and frequent dental follow-up.76,93,94 Prevention of further damage requires correction of the underlying behaviors and ED.
Other Dermatologic Findings
Russell sign refers to the development of calluses on the dorsal metacarpophalangeal joints of the dominant hand due to self-induced vomiting. Due to its specificity in purging-type EDs, the discovery of Russell sign should greatly increase suspicion for an ED.17 Patients with EDs also are at an increased risk for self-harming and body-focused repetitive behaviors, including skin cutting, superficial burning, onychophagia, and trichotillomania.19 It is important to recognize these signs in patients for whom an ED is suspected. The role of the dermatologist should include careful examination of the skin and documentation of findings that may aid in the diagnosis of an underlying ED.
Final Thoughts
A major limitation of this review is the reliance on small case reports and case series reporting cutaneous manifestations of ED. Controlled studies with larger cohorts are challenging in this population but are needed to substantiate the dermatologic signs commonly associated with EDs. Translational studies may help elucidate the pathomechanisms underlying dermatologic diseases such as lanugo, pruritus, and alopecia in the context of EDs and malnutrition. The known association between thyroid dysfunction and skin disease has been substantiated by clinical and basic science investigation, suggesting a notable role of thyroid hormone and TSH signaling in the skin local environment. Further investigation into nutritional and neuroendocrine regulation of skin health will aid in the diagnosis and treatment of patients impacted by EDs.
The treatment of the underlying ED is key in correcting associated skin disease, which requires interdisciplinary collaboration that addresses the psychological, behavioral, and social components of the condition. Following a diagnosis of ED, assessment should be made of the nutritional rehabilitation required to restore weight and nutritional status. Inpatient treatment may be indicated for patients requiring close monitoring to avoid refeeding syndrome, or those who meet the criteria for extreme AN in the DSM-5 (ie, body mass index <15 kg/m2),1 or demonstrate signs of medical instability or organ failure secondary to malnutrition.62 Long-term recovery for ED patients should focus on behavioral therapy with a multidisciplinary team consisting of a psychiatrist, therapist, dietitian, and primary care provider. Comparative studies in large-scale trials of cognitive behavioral therapy, focal psychodynamic psychotherapy, and specialist supportive clinical management have shown little to no difference in efficacy in treating EDs.75,95,96
Dermatologists may be the first providers to observe sequelae of nutritional and behavioral derangement in patients with EDs. Existing literature on the dermatologic findings of EDs report great heterogeneity of skin signs, with a very limited number of controlled studies available. Each cutaneous symptom described in this review should not be interpreted as an isolated pathology but should be placed in the context of patient predisposing risk factors and the constellation of other skin findings that may be suggestive of disordered eating behavior or other psychiatric illness. The observation of multiple signs and symptoms at the same time, especially of symptoms uncommonly encountered or suggestive of a severe and prolonged imbalance (eg, xanthoderma with vitamin A excess, aphthous stomatitis with vitamin B deficiency), should heighten clinical suspicion for an underlying ED. A clinician’s highest priority should be to resolve life-threatening medical emergencies and address nutritional derangements with the assistance of experts who are well versed in EDs. The patient should undergo workup to rule out organic causes of their nutritional dermatoses. Given the high psychiatric morbidity and mortality of patients with an ED and the demonstrated benefit of early intervention, recognition of cutaneous manifestations of malnutrition and EDs may be paramount to improving outcomes.
- Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
- Siddiqui A, Ramsay B, Leonard J. The cutaneous signs of eating disorders. Acta Derm Venereol. 1994;74:68-69. doi:10.2340/00015555746869
- Cheng ZH, Perko VL, Fuller-Marashi L, et al. Ethnic differences in eating disorder prevalence, risk factors, and predictive effects of risk factors among young women. Eat Behav. 2019;32:23-30. doi:10.1016/j. eatbeh.2018.11.004
- Smink FR, van Hoeken D, Hoek HW. Epidemiology of eating disorders: incidence, prevalence and mortality rates. Curr Psychiatry Rep. 2012;14:406-414. doi:10.1007/s11920-012-0282-y
- Campbell K, Peebles R. Eating disorders in children and adolescents: state of the art review. Pediatrics. 2014;134:582-592. doi:10.1542/peds.2014-0194
- Herpertz-Dahlmann B. Adolescent eating disorders: definitions, symptomatology, epidemiology and comorbidity. Child Adolesc Psychiatr Clin N Am. 2009;18:31-47. doi:10.1016/j.chc.2008.07.005
- Arcelus J, Mitchell AJ, Wales J, et al. Mortality rates in patients with anorexia nervosa and other eating disorders: a meta-analysis of 3 6 studies. Arch General Psychiatry. 2011;68:724-731. doi:10.1001 /archgenpsychiatry.2011.74
- Tyler I, Wiseman MC, Crawford RI, et al. Cutaneous manifestations of eating disorders. J Cutan Med Surg. 2002;6:345-353. doi:10.1177/120347540200600407
- Al Nasser Y, Muco E, Alsaad AJ. Pica. StatPearls. StatPearls Publishing; 2023.
- Borgna-Pignatti C, Zanella S. Pica as a manifestation of iron deficiency. Expert Rev Hematol. 2016;9:1075-1080. doi:10.1080/1747408 6.2016.1245136
- Talley NJ. Rumination syndrome. Gastroenterol Hepatol (N Y). 2011;7:117- 118.
- Sanchez-Cerezo J, Nagularaj L, Gledhill J, et al. What do we know about the epidemiology of avoidant/restrictive food intake disorder in children and adolescents? a systematic review of the literature. Eur Eat Disord Rev. 2023;31:226-246. doi:10.1002/erv.2964
- World Health Organization. Malnutrition. Published June 9, 2021. Accessed April 20, 2023. https://www.who.int/news-room/fact-sheets/detail/malnutrition
- Glorio R, Allevato M, De Pablo A, et al. Prevalence of cutaneous manifestations in 200 patients with eating disorders. Int J Dermatol. 2000;39:348-353. doi:10.1046/j.1365-4362.2000.00924.x
- Strumia R, Manzato E, Gualandi M. Is there a role for dermatologists in eating disorders? Expert Rev Dermatol. 2007;2:109-112. doi:10.1586/17469872.2.2.109
- Strumia R. Skin signs in anorexia nervosa. Dermatoendocrinol. 2009;1:268-270. doi:10.4161/derm.1.5.10193
- Strumia R. Eating disorders and the skin. Clin Dermatol. 2013;31:80-85. doi:http://doi.org/10.1016/j.clindermatol.2011.11.011
- Gupta MA, Gupta AK, Haberman HF. Dermatologic signs in anorexia nervosa and bulimia nervosa. Arch Dermatol. 1987;123:1386-1390. doi:10.1001/archderm.1987.01660340159040
- Schulze UM, Pettke-Rank CV, Kreienkamp M, et al. Dermatologic findings in anorexia and bulimia nervosa of childhood and adolescence. Pediatr Dermatol. 1999;16:90-94. doi:10.1046/j.1525-1470.1999.00022.x
- Augustin M, Wilsmann-Theis D, Körber A, et al. Diagnosis and treatment of xerosis cutis—a position paper. J Dtsch Dermatol Ges. 2019;17(suppl 7):3-33. doi:10.1111/ddg.13906
- Grubauer G, Feingold KR, Harris RM, et al. Lipid content and lipid type as determinants of the epidermal permeability barrier. J Lipid Res. 1989;30:89-96.
- Feingold KR, Man MQ, Menon GK, et al. Cholesterol synthesis is required for cutaneous barrier function in mice. J Clin Invest. 1990;86:1738-1745. doi:10.1172/jci114899
- Madison KC. Barrier function of the skin: “la raison d’être” of the epidermis. J Invest Dermatol. 2003;121:231-241. doi:10.106 /j.1523-1747.2003.12359.x
- Usdan LS, Khaodhiar L, Apovian CM. The endocrinopathies of anorexia nervosa. Endocr Pract. 2008;14:1055-1063. doi:10.4158/ep.14.8.1055
- Warren MP. Endocrine manifestations of eating disorders. J Clin Endocrinol Metabol. 2011;96:333-343. doi:10.1210/jc.2009-2304
- Safer JD. Thyroid hormone action on skin. Dermatoendocrinol. 2011;3:211-215. doi:10.4161/derm.3.3.17027
- Cui CY, Schlessinger D. Eccrine sweat gland development and sweat secretion. Exp Dermatol. 2015;24:644-650. doi:10.1111/exd.12773
- Nosewicz J, Spaccarelli N, Roberts KM, et al. The epidemiology, impact, and diagnosis of micronutrient nutritional dermatoses part 1: zinc, selenium, copper, vitamin A, and vitamin C. J Am Acad Dermatol. 2022;86:267-278. doi:10.1016/j.jaad.2021.07.079
- Hoffman M, Micheletti RG, Shields BE. Nutritional dermatoses in the hospitalized patient. Cutis. 2020;105:296;302-308, E1-E5.
- Cox JA, Beachkofsky T, Dominguez A. Flaky paint dermatosis. kwashiorkor. JAMA Dermatol. 2014;150:85-86. doi:10.1001 /jamadermatol.2013.5520
- Bradfield RB. Hair tissue as a medium for the differential diagnosis of protein-calorie malnutrition: a commentary. J Pediatr. 1974;84:294-296.
- Proksch E, Lachapelle J-M. The management of dry skin with topical emollients—recent perspectives. J Dtsch Dermatol Ges. 2005;3:768-774. doi:10.1111/j.1610-0387.2005.05068.x
- Watabe A, Sugawara T, Kikuchi K, et al. Sweat constitutes several natural moisturizing factors, lactate, urea, sodium, and potassium. J Dermatol Sci. 2013;72:177-182. doi:10.1016/j.jdermsci.2013.06.005
- Sugawara T, Kikuchi K, Tagami H, et al. Decreased lactate and potassium levels in natural moisturizing factor from the stratum corneum of mild atopic dermatitis patients are involved with the reduced hydration state. J Dermatol Sci. 2012;66:154-159. doi:10.1016/j .jdermsci.2012.02.011
- Gorski J, Proksch E, Baron JM, et al. Dexpanthenol in wound healing after medical and cosmetic interventions (postprocedure wound healing). Pharmaceuticals (Basel). 2020;13:138. doi:10.3390 /ph13070138
- Verhave BL, Nassereddin A, Lappin SL. Embryology, lanugo. StatPearls. StatPearls Publishing; 2022.
- Faist T. Vernix caseoza—composition and function. Ceska Gynekol. 2020;85:263-267.
- Bystrova K. Novel mechanism of human fetal growth regulation: a potential role of lanugo, vernix caseosa and a second tactile system of unmyelinated low-threshold C-afferents. Med Hypotheses. 2009;72:143-146. doi:10.1016/j.mehy.2008.09.033
- Mitchell JE, Crow S. Medical complications of anorexia nervosa and bulimia nervosa. Curr Opin Psychiatry. 2006;19:438-443. doi:10.1097/01.yco.0000228768.79097.3e
- Dalcin D, Manser C, Mahler R. Malignant down: hypertrichosis lanuginosa acquisita associated with endometrial adenocarcinoma. J Cutan Med Surg. 2015;19:507-510. doi:10.1177/1203475415582319
- Slee PH, van der Waal RI, Schagen van Leeuwen JH, et al. Paraneoplastic hypertrichosis lanuginosa acquisita: uncommon or overlooked? Br J Dermatol. 2007;157:1087-1092. doi:10.1111/j.1365-2133.2007.08253.x
- Lause M, Kamboj A, Fernandez Faith E. Dermatologic manifestations of endocrine disorders. Transl Pediatr. 2017;6:300-312. doi:10.21037 /tp.2017.09.08
- Vulink AJ, ten Bokkel Huinink D. Acquired hypertrichosis lanuginosa: a rare cutaneous paraneoplastic syndrome. J Clin Oncol. 2007;25:1625-1626. doi:10.1200/jco.2007.10.6963
- Wyatt JP, Anderson HF, Greer KE, et al. Acquired hypertrichosis lanuginosa as a presenting sign of metastatic prostate cancer with rapid resolution after treatment. J Am Acad Dermatol. 2007;56 (2 suppl):S45-S47. doi:10.1016/j.jaad.2006.07.011
- Saad N, Hot A, Ninet J, et al. Acquired hypertrichosis lanuginosa and gastric adenocarcinoma [in French]. Ann Dermatol Venereol. 2007;134:55-58. doi:10.1016/s0151-9638(07)88991-5
- Pruijm MC, van Houtum WH. An unusual cause of hypertrichosis. Neth J Med. 2007;65:42, 45.
- Lorette G, Maruani A. Images in clinical medicine. acquired hypertrichosis lanuginosa. N Engl J Med. 2006;354:2696. doi:10.1056 /NEJMicm050344
- Swenne I, Engström I. Medical assessment of adolescent girls with eating disorders: an evaluation of symptoms and signs of starvation. Acta Paediatr. 2005;94:1363-1371. doi:10.1111/j.1651-2227.2005.tb01805.x
- Zhou L, Wang H, Jing J, et al. Regulation of hair follicle development by exosomes derived from dermal papilla cells. Biochem Biophys Res Comm. 2018;500:325-332. doi:10.1016/j.bbrc.2018.04.067
- Tomic-Canic M, Day D, Samuels HH, et al. Novel regulation of keratin gene expression by thyroid hormone and retinoid receptors. J Biol Chem. 1996;271:1416-1423. doi:10.1074/jbc.271.3.1416
- Contreras-Jurado C, Lorz C, García-Serrano L, et al. Thyroid hormone signaling controls hair follicle stem cell function. Mol Biol Cell. 2015;26:1263-1272. doi:10.1091/mbc.E14-07-1251
- Hornberger LL, Lane MA. Identification and management of eating disorders in children and adolescents [published online December 20, 2021]. Pediatrics. doi:10.1542/peds.2020-040279
- Gupta MA, Gupta AK, Voorhees JJ. Starvation-associated pruritus: a clinical feature of eating disorders. J Am Acad Dermatol. 1992; 27:118-120. doi:10.1016/s0190-9622(08)80824-9
- Cevikbas F, Lerner EA. Physiology and pathophysiology of itch. Physiol Rev. 2020;100:945-982. doi:10.1152/physrev.00017.2019
- Stäubli M. Pruritus—a little known iron-deficiency symptom [in German]. Schweiz Med Wochenschr. 1981;111:1394-1398.
- Saini S, Jain AK, Agarwal S, et al. Iron deficiency and pruritus: a cross-sectional analysis to assess its association and relationship. Indian J Dermatol. 2021;66:705. doi:10.4103/ijd.ijd_326_21
- Tammaro A, Chello C, Di Fraia M, et al. Iron-deficiency and pruritus: a possible explanation of their relationship. Int J Research Dermatol. 2018;4:605. doi:10.18203/issn.2455-4529.IntJResDermatol20184470
- Takkunen H. Iron-deficiency pruritus. JAMA. 1978;239:1394.
- Lewiecki EM, Rahman F. Pruritus. a manifestation of iron deficiency. JAMA. 1976;236:2319-2320. doi:10.1001/jama.236.20.2319
- Kennedy A, Kohn M, Lammi A, et al. Iron status and haematological changes in adolescent female inpatients with anorexia nervosa. J Paediatr Child Health. 2004;40:430-432. doi:10.1111/j.1440-1754.2004.00432.x
- Morgan JF, Lacey JH. Scratching and fasting: a study of pruritus and anorexia nervosa. Br J Dermatol. 1999;140:453-456. doi:10.1046/j.1365- 2133.1999.02708.x
- Mehler PS. Anorexia nervosa in adults: evaluation for medical complications and criteria for hospitalization to manage these complications. UpToDate. Updated August 3, 2022. Accessed April 20, 2023. https://www.uptodate.com/contents/anorexia-nervosa-in-adults-evaluation-for-medical-complications-and-criteria-for -hospitalization-to-manage-these-complications
- Das S, Maiti A. Acrocyanosis: an overview. Indian J Dermatol. 2013;58:417-420. doi:10.4103/0019-5154.119946
- Caregaro L, Di Pascoli L, Favaro A, et al. Sodium depletion and hemoconcentration: overlooked complications in patients with anorexia nervosa? Nutrition. 2005;21:438-445. doi:10.1016/j.nut.2004.08.022
- Crisp AH, Stonehill E. Hypercarotenaemia as a symptom of weight phobia. Postgrad Med J. 1967;43:721. doi:10.1136/pgmj.43.505.721
- Pops MA, Schwabe AD. Hypercarotenemia in anorexia nervosa. JAMA. 1968;205:533-534. doi:10.1001/jama.1968.03140330075020.
- Bohn T, Desmarchelier C, El SN, et al. β-Carotene in the human body: metabolic bioactivation pathways—from digestion to tissue distribution and excretion. Proc Nutr Soc. 2019;78:68-87. doi:10.1017/S0029665118002641
- von Lintig J, Moon J, Lee J, et al. Carotenoid metabolism at the intestinal barrier. Biochim Biophys Acta Mol Cell Biol Lipids. 2020;1865:158580. doi:10.1016/j.bbalip.2019.158580
- Kanai M, Raz A, Goodman DS. Retinol-binding protein: the transport protein for vitamin A in human plasma. J Clin Invest. 1968;47:2025-2044. doi:10.1172/jci105889
- Haught JM, Patel S, English JC. Xanthoderma: a clinical review. J Am Acad Dermatol. 2007;57:1051-1058. doi:10.1016/j.jaad.2007.06.011
- Tung EE, Drage LA, Ghosh AK. Carotenoderma and hypercarotenemia: markers for disordered eating habits. J Eur Acad Dermatol Venereol. 2006;20:1147-1148. doi:10.1111/j.1468-3083.2006.01643.x
- Heilskov S, Vestergaard C, Babirekere E, et al. Characterization and scoring of skin changes in severe acute malnutrition in children between 6 months and 5 years of age. J Eur Acad Dermatol Venereol. 2015;29:2463-2469. doi:10.1111/jdv.13328
- Malkud S. Telogen effluvium: a review. J Clin Diagn Res. 2015;9:We01-3. doi:10.7860/jcdr/2015/15219.6492
- Filipponi C, Visentini C, Filippini T, et al. The follow-up of eating disorders from adolescence to early adulthood: a systematic review. Int J Environ Res Public Health. 2022;19:16237. doi:10.3390/ijerph192316237
- Byrne S, Wade T, Hay P, et al. A randomised controlled trial of three psychological treatments for anorexia nervosa. Psychol Med. 2017;47:2823-2833. doi:10.1017/s0033291717001349
- Ranalli DN, Studen-Pavlovich D. Eating disorders in the adolescent patient. Dent Clin North Am. 2021;65:689-703. doi:10.1016/j. cden.2021.06.009
- Arck PC, Handjiski B, Peters EM, et al. Stress inhibits hair growth in mice by induction of premature catagen development and deleterious perifollicular inflammatory events via neuropeptide substance P-dependent pathways. Am J Pathol. 2003;162:803-814. doi:10.1016/s0002-9440(10)63877-1
- Roy SK. Achromotrichia in tropical malnutrition. Br Med J. 1947;1:392. doi:10.1136/bmj.1.4498.392-c
- Swed-Tobia R, Haj A, Militianu D, et al. Highly selective eating in autism spectrum disorder leading to scurvy: a series of three patients. Pediatr Neurol. 2019;94:61-63. doi:10.1016/j.pediatrneurol.2018.12.011
- Maruo Y, Uetake K, Egawa K, et al. Selective eating in autism spectrum disorder leading to hair color change. Pediatr Neurol. 2021;120:1-2. doi:10.1016/j.pediatrneurol.2021.03.001
- Paus R, Langan EA, Vidali S, et al. Neuroendocrinology of the hair follicle: principles and clinical perspectives. Trends Mol Med. 2014;20:559-570. doi:10.1016/j.molmed.2014.06.002
- Antonini D, Sibilio A, Dentice M, et al. An intimate relationship between thyroid hormone and skin: regulation of gene expression. Front Endocrinol (Lausanne). 2013;4:104. doi: 10.3389/fendo.2013.00104
- Bodó E, Kany B, Gáspár E, et al. Thyroid-stimulating hormone, a novel, locally produced modulator of human epidermal functions, is regulated by thyrotropin-releasing hormone and thyroid hormones. Endocrinology. 2010;151:1633-1642. doi:10.1210/en.2009-0306
- Taguchi T. Brittle nails and hair loss in hypothyroidism. N Engl J Med. 2018;379:1363-1363. doi:10.1056/NEJMicm1801633
- van Beek N, Bodó E, Kromminga A, et al. Thyroid hormones directly alter human hair follicle functions: anagen prolongation and stimulation of both hair matrix keratinocyte proliferation and hair pigmentation. J Clin Endocrinol Metab. 2008;93:4381-4388. doi:10.1210/jc.2008-0283
- Zippi M, Corrado C, Pica R, et al. Extraintestinal manifestations in a large series of Italian inflammatory bowel disease patients. World J Gastroenterol. 2014;20:17463-7467. doi:10.3748/wjg.v20.i46.17463.
- Gutierrez Gossweiler A, Martinez-Mier EA. Chapter 6: vitamins and oral health. Monogr Oral Sci. 2020;28:59-67. doi:10.1159/000455372
- Monda M, Costacurta M, Maffei L, et al. Oral manifestations of eating disorders in adolescent patients. a review. Eur J Paediatr Dent. 2021;22:155-158. doi:10.23804/ejpd.2021.22.02.13
- Ankar A, Kumar A. Vitamin B12 deficiency. StatPearls. StatPearls Publishing; 2022.
- Graells J, Ojeda RM, Muniesa C, et al. Glossitis with linear lesions: an early sign of vitamin B12 deficiency. J Am Acad Dermatol. 2009;60:498- 500. doi:10.1016/j.jaad.2008.09.011
- Pétavy-Catala C, Fontès V, Gironet N, et al. Clinical manifestations of the mouth revealing vitamin B12 deficiency before the onset of anemia [in French]. Ann Dermatol Venereol. 2003;130(2 pt 1):191-194.
- Monagas J, Ritwik P, Kolomensky A, et al. Rumination syndrome and dental erosions in children. J Pediatr Gastroenterol Nutr. 2017; 64:930-932. doi:10.1097/mpg.0000000000001395
- Silverstein LS, Haggerty C, Sams L, et al. Impact of an oral health education intervention among a group of patients with eating disorders (anorexia nervosa and bulimia nervosa). J Eat Disord. 2019;7:29. doi:10.1186/s40337-019-0259-x
- Rangé H, Colon P, Godart N, et al. Eating disorders through the periodontal lens. Periodontol 2000. 2021;87:17-31. doi:10.1111 /prd.12391
- Zipfel S, Wild B, Groß G, et al. Focal psychodynamic therapy, cognitive behaviour therapy, and optimised treatment as usual in outpatients with anorexia nervosa (ANTOP study): randomised controlled trial. Lancet Psychiatry. 2014;383:127-137. doi:10.1016 /S2215-0366(22)00028-1
- Schmidt U, Ryan EG, Bartholdy S, et al. Two-year follow-up of the MOSAIC trial: a multicenter randomized controlled trial comparing two psychological treatments in adult outpatients with broadly defined anorexia nervosa. Int J Eat Disord. 2016;49:793-800. doi:10.1002/eat.22523
- Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
- Siddiqui A, Ramsay B, Leonard J. The cutaneous signs of eating disorders. Acta Derm Venereol. 1994;74:68-69. doi:10.2340/00015555746869
- Cheng ZH, Perko VL, Fuller-Marashi L, et al. Ethnic differences in eating disorder prevalence, risk factors, and predictive effects of risk factors among young women. Eat Behav. 2019;32:23-30. doi:10.1016/j. eatbeh.2018.11.004
- Smink FR, van Hoeken D, Hoek HW. Epidemiology of eating disorders: incidence, prevalence and mortality rates. Curr Psychiatry Rep. 2012;14:406-414. doi:10.1007/s11920-012-0282-y
- Campbell K, Peebles R. Eating disorders in children and adolescents: state of the art review. Pediatrics. 2014;134:582-592. doi:10.1542/peds.2014-0194
- Herpertz-Dahlmann B. Adolescent eating disorders: definitions, symptomatology, epidemiology and comorbidity. Child Adolesc Psychiatr Clin N Am. 2009;18:31-47. doi:10.1016/j.chc.2008.07.005
- Arcelus J, Mitchell AJ, Wales J, et al. Mortality rates in patients with anorexia nervosa and other eating disorders: a meta-analysis of 3 6 studies. Arch General Psychiatry. 2011;68:724-731. doi:10.1001 /archgenpsychiatry.2011.74
- Tyler I, Wiseman MC, Crawford RI, et al. Cutaneous manifestations of eating disorders. J Cutan Med Surg. 2002;6:345-353. doi:10.1177/120347540200600407
- Al Nasser Y, Muco E, Alsaad AJ. Pica. StatPearls. StatPearls Publishing; 2023.
- Borgna-Pignatti C, Zanella S. Pica as a manifestation of iron deficiency. Expert Rev Hematol. 2016;9:1075-1080. doi:10.1080/1747408 6.2016.1245136
- Talley NJ. Rumination syndrome. Gastroenterol Hepatol (N Y). 2011;7:117- 118.
- Sanchez-Cerezo J, Nagularaj L, Gledhill J, et al. What do we know about the epidemiology of avoidant/restrictive food intake disorder in children and adolescents? a systematic review of the literature. Eur Eat Disord Rev. 2023;31:226-246. doi:10.1002/erv.2964
- World Health Organization. Malnutrition. Published June 9, 2021. Accessed April 20, 2023. https://www.who.int/news-room/fact-sheets/detail/malnutrition
- Glorio R, Allevato M, De Pablo A, et al. Prevalence of cutaneous manifestations in 200 patients with eating disorders. Int J Dermatol. 2000;39:348-353. doi:10.1046/j.1365-4362.2000.00924.x
- Strumia R, Manzato E, Gualandi M. Is there a role for dermatologists in eating disorders? Expert Rev Dermatol. 2007;2:109-112. doi:10.1586/17469872.2.2.109
- Strumia R. Skin signs in anorexia nervosa. Dermatoendocrinol. 2009;1:268-270. doi:10.4161/derm.1.5.10193
- Strumia R. Eating disorders and the skin. Clin Dermatol. 2013;31:80-85. doi:http://doi.org/10.1016/j.clindermatol.2011.11.011
- Gupta MA, Gupta AK, Haberman HF. Dermatologic signs in anorexia nervosa and bulimia nervosa. Arch Dermatol. 1987;123:1386-1390. doi:10.1001/archderm.1987.01660340159040
- Schulze UM, Pettke-Rank CV, Kreienkamp M, et al. Dermatologic findings in anorexia and bulimia nervosa of childhood and adolescence. Pediatr Dermatol. 1999;16:90-94. doi:10.1046/j.1525-1470.1999.00022.x
- Augustin M, Wilsmann-Theis D, Körber A, et al. Diagnosis and treatment of xerosis cutis—a position paper. J Dtsch Dermatol Ges. 2019;17(suppl 7):3-33. doi:10.1111/ddg.13906
- Grubauer G, Feingold KR, Harris RM, et al. Lipid content and lipid type as determinants of the epidermal permeability barrier. J Lipid Res. 1989;30:89-96.
- Feingold KR, Man MQ, Menon GK, et al. Cholesterol synthesis is required for cutaneous barrier function in mice. J Clin Invest. 1990;86:1738-1745. doi:10.1172/jci114899
- Madison KC. Barrier function of the skin: “la raison d’être” of the epidermis. J Invest Dermatol. 2003;121:231-241. doi:10.106 /j.1523-1747.2003.12359.x
- Usdan LS, Khaodhiar L, Apovian CM. The endocrinopathies of anorexia nervosa. Endocr Pract. 2008;14:1055-1063. doi:10.4158/ep.14.8.1055
- Warren MP. Endocrine manifestations of eating disorders. J Clin Endocrinol Metabol. 2011;96:333-343. doi:10.1210/jc.2009-2304
- Safer JD. Thyroid hormone action on skin. Dermatoendocrinol. 2011;3:211-215. doi:10.4161/derm.3.3.17027
- Cui CY, Schlessinger D. Eccrine sweat gland development and sweat secretion. Exp Dermatol. 2015;24:644-650. doi:10.1111/exd.12773
- Nosewicz J, Spaccarelli N, Roberts KM, et al. The epidemiology, impact, and diagnosis of micronutrient nutritional dermatoses part 1: zinc, selenium, copper, vitamin A, and vitamin C. J Am Acad Dermatol. 2022;86:267-278. doi:10.1016/j.jaad.2021.07.079
- Hoffman M, Micheletti RG, Shields BE. Nutritional dermatoses in the hospitalized patient. Cutis. 2020;105:296;302-308, E1-E5.
- Cox JA, Beachkofsky T, Dominguez A. Flaky paint dermatosis. kwashiorkor. JAMA Dermatol. 2014;150:85-86. doi:10.1001 /jamadermatol.2013.5520
- Bradfield RB. Hair tissue as a medium for the differential diagnosis of protein-calorie malnutrition: a commentary. J Pediatr. 1974;84:294-296.
- Proksch E, Lachapelle J-M. The management of dry skin with topical emollients—recent perspectives. J Dtsch Dermatol Ges. 2005;3:768-774. doi:10.1111/j.1610-0387.2005.05068.x
- Watabe A, Sugawara T, Kikuchi K, et al. Sweat constitutes several natural moisturizing factors, lactate, urea, sodium, and potassium. J Dermatol Sci. 2013;72:177-182. doi:10.1016/j.jdermsci.2013.06.005
- Sugawara T, Kikuchi K, Tagami H, et al. Decreased lactate and potassium levels in natural moisturizing factor from the stratum corneum of mild atopic dermatitis patients are involved with the reduced hydration state. J Dermatol Sci. 2012;66:154-159. doi:10.1016/j .jdermsci.2012.02.011
- Gorski J, Proksch E, Baron JM, et al. Dexpanthenol in wound healing after medical and cosmetic interventions (postprocedure wound healing). Pharmaceuticals (Basel). 2020;13:138. doi:10.3390 /ph13070138
- Verhave BL, Nassereddin A, Lappin SL. Embryology, lanugo. StatPearls. StatPearls Publishing; 2022.
- Faist T. Vernix caseoza—composition and function. Ceska Gynekol. 2020;85:263-267.
- Bystrova K. Novel mechanism of human fetal growth regulation: a potential role of lanugo, vernix caseosa and a second tactile system of unmyelinated low-threshold C-afferents. Med Hypotheses. 2009;72:143-146. doi:10.1016/j.mehy.2008.09.033
- Mitchell JE, Crow S. Medical complications of anorexia nervosa and bulimia nervosa. Curr Opin Psychiatry. 2006;19:438-443. doi:10.1097/01.yco.0000228768.79097.3e
- Dalcin D, Manser C, Mahler R. Malignant down: hypertrichosis lanuginosa acquisita associated with endometrial adenocarcinoma. J Cutan Med Surg. 2015;19:507-510. doi:10.1177/1203475415582319
- Slee PH, van der Waal RI, Schagen van Leeuwen JH, et al. Paraneoplastic hypertrichosis lanuginosa acquisita: uncommon or overlooked? Br J Dermatol. 2007;157:1087-1092. doi:10.1111/j.1365-2133.2007.08253.x
- Lause M, Kamboj A, Fernandez Faith E. Dermatologic manifestations of endocrine disorders. Transl Pediatr. 2017;6:300-312. doi:10.21037 /tp.2017.09.08
- Vulink AJ, ten Bokkel Huinink D. Acquired hypertrichosis lanuginosa: a rare cutaneous paraneoplastic syndrome. J Clin Oncol. 2007;25:1625-1626. doi:10.1200/jco.2007.10.6963
- Wyatt JP, Anderson HF, Greer KE, et al. Acquired hypertrichosis lanuginosa as a presenting sign of metastatic prostate cancer with rapid resolution after treatment. J Am Acad Dermatol. 2007;56 (2 suppl):S45-S47. doi:10.1016/j.jaad.2006.07.011
- Saad N, Hot A, Ninet J, et al. Acquired hypertrichosis lanuginosa and gastric adenocarcinoma [in French]. Ann Dermatol Venereol. 2007;134:55-58. doi:10.1016/s0151-9638(07)88991-5
- Pruijm MC, van Houtum WH. An unusual cause of hypertrichosis. Neth J Med. 2007;65:42, 45.
- Lorette G, Maruani A. Images in clinical medicine. acquired hypertrichosis lanuginosa. N Engl J Med. 2006;354:2696. doi:10.1056 /NEJMicm050344
- Swenne I, Engström I. Medical assessment of adolescent girls with eating disorders: an evaluation of symptoms and signs of starvation. Acta Paediatr. 2005;94:1363-1371. doi:10.1111/j.1651-2227.2005.tb01805.x
- Zhou L, Wang H, Jing J, et al. Regulation of hair follicle development by exosomes derived from dermal papilla cells. Biochem Biophys Res Comm. 2018;500:325-332. doi:10.1016/j.bbrc.2018.04.067
- Tomic-Canic M, Day D, Samuels HH, et al. Novel regulation of keratin gene expression by thyroid hormone and retinoid receptors. J Biol Chem. 1996;271:1416-1423. doi:10.1074/jbc.271.3.1416
- Contreras-Jurado C, Lorz C, García-Serrano L, et al. Thyroid hormone signaling controls hair follicle stem cell function. Mol Biol Cell. 2015;26:1263-1272. doi:10.1091/mbc.E14-07-1251
- Hornberger LL, Lane MA. Identification and management of eating disorders in children and adolescents [published online December 20, 2021]. Pediatrics. doi:10.1542/peds.2020-040279
- Gupta MA, Gupta AK, Voorhees JJ. Starvation-associated pruritus: a clinical feature of eating disorders. J Am Acad Dermatol. 1992; 27:118-120. doi:10.1016/s0190-9622(08)80824-9
- Cevikbas F, Lerner EA. Physiology and pathophysiology of itch. Physiol Rev. 2020;100:945-982. doi:10.1152/physrev.00017.2019
- Stäubli M. Pruritus—a little known iron-deficiency symptom [in German]. Schweiz Med Wochenschr. 1981;111:1394-1398.
- Saini S, Jain AK, Agarwal S, et al. Iron deficiency and pruritus: a cross-sectional analysis to assess its association and relationship. Indian J Dermatol. 2021;66:705. doi:10.4103/ijd.ijd_326_21
- Tammaro A, Chello C, Di Fraia M, et al. Iron-deficiency and pruritus: a possible explanation of their relationship. Int J Research Dermatol. 2018;4:605. doi:10.18203/issn.2455-4529.IntJResDermatol20184470
- Takkunen H. Iron-deficiency pruritus. JAMA. 1978;239:1394.
- Lewiecki EM, Rahman F. Pruritus. a manifestation of iron deficiency. JAMA. 1976;236:2319-2320. doi:10.1001/jama.236.20.2319
- Kennedy A, Kohn M, Lammi A, et al. Iron status and haematological changes in adolescent female inpatients with anorexia nervosa. J Paediatr Child Health. 2004;40:430-432. doi:10.1111/j.1440-1754.2004.00432.x
- Morgan JF, Lacey JH. Scratching and fasting: a study of pruritus and anorexia nervosa. Br J Dermatol. 1999;140:453-456. doi:10.1046/j.1365- 2133.1999.02708.x
- Mehler PS. Anorexia nervosa in adults: evaluation for medical complications and criteria for hospitalization to manage these complications. UpToDate. Updated August 3, 2022. Accessed April 20, 2023. https://www.uptodate.com/contents/anorexia-nervosa-in-adults-evaluation-for-medical-complications-and-criteria-for -hospitalization-to-manage-these-complications
- Das S, Maiti A. Acrocyanosis: an overview. Indian J Dermatol. 2013;58:417-420. doi:10.4103/0019-5154.119946
- Caregaro L, Di Pascoli L, Favaro A, et al. Sodium depletion and hemoconcentration: overlooked complications in patients with anorexia nervosa? Nutrition. 2005;21:438-445. doi:10.1016/j.nut.2004.08.022
- Crisp AH, Stonehill E. Hypercarotenaemia as a symptom of weight phobia. Postgrad Med J. 1967;43:721. doi:10.1136/pgmj.43.505.721
- Pops MA, Schwabe AD. Hypercarotenemia in anorexia nervosa. JAMA. 1968;205:533-534. doi:10.1001/jama.1968.03140330075020.
- Bohn T, Desmarchelier C, El SN, et al. β-Carotene in the human body: metabolic bioactivation pathways—from digestion to tissue distribution and excretion. Proc Nutr Soc. 2019;78:68-87. doi:10.1017/S0029665118002641
- von Lintig J, Moon J, Lee J, et al. Carotenoid metabolism at the intestinal barrier. Biochim Biophys Acta Mol Cell Biol Lipids. 2020;1865:158580. doi:10.1016/j.bbalip.2019.158580
- Kanai M, Raz A, Goodman DS. Retinol-binding protein: the transport protein for vitamin A in human plasma. J Clin Invest. 1968;47:2025-2044. doi:10.1172/jci105889
- Haught JM, Patel S, English JC. Xanthoderma: a clinical review. J Am Acad Dermatol. 2007;57:1051-1058. doi:10.1016/j.jaad.2007.06.011
- Tung EE, Drage LA, Ghosh AK. Carotenoderma and hypercarotenemia: markers for disordered eating habits. J Eur Acad Dermatol Venereol. 2006;20:1147-1148. doi:10.1111/j.1468-3083.2006.01643.x
- Heilskov S, Vestergaard C, Babirekere E, et al. Characterization and scoring of skin changes in severe acute malnutrition in children between 6 months and 5 years of age. J Eur Acad Dermatol Venereol. 2015;29:2463-2469. doi:10.1111/jdv.13328
- Malkud S. Telogen effluvium: a review. J Clin Diagn Res. 2015;9:We01-3. doi:10.7860/jcdr/2015/15219.6492
- Filipponi C, Visentini C, Filippini T, et al. The follow-up of eating disorders from adolescence to early adulthood: a systematic review. Int J Environ Res Public Health. 2022;19:16237. doi:10.3390/ijerph192316237
- Byrne S, Wade T, Hay P, et al. A randomised controlled trial of three psychological treatments for anorexia nervosa. Psychol Med. 2017;47:2823-2833. doi:10.1017/s0033291717001349
- Ranalli DN, Studen-Pavlovich D. Eating disorders in the adolescent patient. Dent Clin North Am. 2021;65:689-703. doi:10.1016/j. cden.2021.06.009
- Arck PC, Handjiski B, Peters EM, et al. Stress inhibits hair growth in mice by induction of premature catagen development and deleterious perifollicular inflammatory events via neuropeptide substance P-dependent pathways. Am J Pathol. 2003;162:803-814. doi:10.1016/s0002-9440(10)63877-1
- Roy SK. Achromotrichia in tropical malnutrition. Br Med J. 1947;1:392. doi:10.1136/bmj.1.4498.392-c
- Swed-Tobia R, Haj A, Militianu D, et al. Highly selective eating in autism spectrum disorder leading to scurvy: a series of three patients. Pediatr Neurol. 2019;94:61-63. doi:10.1016/j.pediatrneurol.2018.12.011
- Maruo Y, Uetake K, Egawa K, et al. Selective eating in autism spectrum disorder leading to hair color change. Pediatr Neurol. 2021;120:1-2. doi:10.1016/j.pediatrneurol.2021.03.001
- Paus R, Langan EA, Vidali S, et al. Neuroendocrinology of the hair follicle: principles and clinical perspectives. Trends Mol Med. 2014;20:559-570. doi:10.1016/j.molmed.2014.06.002
- Antonini D, Sibilio A, Dentice M, et al. An intimate relationship between thyroid hormone and skin: regulation of gene expression. Front Endocrinol (Lausanne). 2013;4:104. doi: 10.3389/fendo.2013.00104
- Bodó E, Kany B, Gáspár E, et al. Thyroid-stimulating hormone, a novel, locally produced modulator of human epidermal functions, is regulated by thyrotropin-releasing hormone and thyroid hormones. Endocrinology. 2010;151:1633-1642. doi:10.1210/en.2009-0306
- Taguchi T. Brittle nails and hair loss in hypothyroidism. N Engl J Med. 2018;379:1363-1363. doi:10.1056/NEJMicm1801633
- van Beek N, Bodó E, Kromminga A, et al. Thyroid hormones directly alter human hair follicle functions: anagen prolongation and stimulation of both hair matrix keratinocyte proliferation and hair pigmentation. J Clin Endocrinol Metab. 2008;93:4381-4388. doi:10.1210/jc.2008-0283
- Zippi M, Corrado C, Pica R, et al. Extraintestinal manifestations in a large series of Italian inflammatory bowel disease patients. World J Gastroenterol. 2014;20:17463-7467. doi:10.3748/wjg.v20.i46.17463.
- Gutierrez Gossweiler A, Martinez-Mier EA. Chapter 6: vitamins and oral health. Monogr Oral Sci. 2020;28:59-67. doi:10.1159/000455372
- Monda M, Costacurta M, Maffei L, et al. Oral manifestations of eating disorders in adolescent patients. a review. Eur J Paediatr Dent. 2021;22:155-158. doi:10.23804/ejpd.2021.22.02.13
- Ankar A, Kumar A. Vitamin B12 deficiency. StatPearls. StatPearls Publishing; 2022.
- Graells J, Ojeda RM, Muniesa C, et al. Glossitis with linear lesions: an early sign of vitamin B12 deficiency. J Am Acad Dermatol. 2009;60:498- 500. doi:10.1016/j.jaad.2008.09.011
- Pétavy-Catala C, Fontès V, Gironet N, et al. Clinical manifestations of the mouth revealing vitamin B12 deficiency before the onset of anemia [in French]. Ann Dermatol Venereol. 2003;130(2 pt 1):191-194.
- Monagas J, Ritwik P, Kolomensky A, et al. Rumination syndrome and dental erosions in children. J Pediatr Gastroenterol Nutr. 2017; 64:930-932. doi:10.1097/mpg.0000000000001395
- Silverstein LS, Haggerty C, Sams L, et al. Impact of an oral health education intervention among a group of patients with eating disorders (anorexia nervosa and bulimia nervosa). J Eat Disord. 2019;7:29. doi:10.1186/s40337-019-0259-x
- Rangé H, Colon P, Godart N, et al. Eating disorders through the periodontal lens. Periodontol 2000. 2021;87:17-31. doi:10.1111 /prd.12391
- Zipfel S, Wild B, Groß G, et al. Focal psychodynamic therapy, cognitive behaviour therapy, and optimised treatment as usual in outpatients with anorexia nervosa (ANTOP study): randomised controlled trial. Lancet Psychiatry. 2014;383:127-137. doi:10.1016 /S2215-0366(22)00028-1
- Schmidt U, Ryan EG, Bartholdy S, et al. Two-year follow-up of the MOSAIC trial: a multicenter randomized controlled trial comparing two psychological treatments in adult outpatients with broadly defined anorexia nervosa. Int J Eat Disord. 2016;49:793-800. doi:10.1002/eat.22523
Practice Points
- Cutaneous manifestations of malnutrition may be the presenting sign of disordered eating.
- Dermatologists have a unique opportunity for early recognition and intervention in patients with eating disorders (EDs).
- Rapid identification and multidisciplinary management of EDs may improve patient outcomes and potentially attenuate the risk of irreversible damage from malnutrition.
Coding the “Spot Check”: Part 1
On January 1, 2021, the Current Procedural Terminology (CPT) evaluation and management (E/M) reporting rules changed dramatically, with “bullet counting” no longer necessary and the coding level now based on either the new medical decision making (MDM) table or time spent on all activities relating to the care of the patient on the day of the encounter.1 This is described in the CPT Professional Edition 2023, a book every practitioner should review annually.2 In particular, every provider should read and reread pages 1 to 14—and beyond if you provide services beyond standard office visits. These changes were made with the intent to simplify the process of documentation and allow a provider to spend more time with patients, though there is still a paucity of data related to whether the new system achieves these aims.
The general rule of reporting work with CPT codes can be simply stated—“Document what you did, do what you documented, and report that which is medically necessary” (David McCafferey, MD, personal communication)—and you should never have any difficulty with audits. Unfortunately, the new system does not let an auditor, who typically lacks a medical degree, audit effectively unless they have a clear understanding of diseases and their stages. Many medical societies, including the American Medical Association3 and American Academy of Dermatology,4 have provided education that focuses on how to report a given vignette, but specific examples of documentation with commentary are uncommon.
To make your documentation more likely to pass audits, explicitly link parts of your documentation to CPT MDM descriptors. We offer scenarios and tips. In part 1 of this series, we discuss how to approach the “spot check,” a commonly encountered chief concern (CC) within dermatology.
A 34-year-old presents with a new spot on the left cheek that seems to be growing and changing shape rapidly. You examine the patient and discuss treatment options. The documentation reads as follows:
• CC: New spot on left cheek that seems to be growing and changing shape rapidly
• History: No family history of skin cancer; concerned about scarring, no blood thinner
• Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy
• Impression: rule out melanoma.
• Plan:
As was the case before 2021, you still need a CC, along with a medically (and medicolegally) appropriate history and physical examination. A diagnostic impression and treatment plan also should be included.
In this situation, reporting is straightforward. There is no separate E/M visit; only the CPT code 11102 for tangential biopsy is reported. An International Classification of Diseases, Tenth Revision code of D48.5 (neoplasm of uncertain behavior of skin) will be included.
Why no E/M code? This is because the biopsy includes preservice and postservice time and work that would be double reported with the E/M. Remember that the preservice work would include any history and physical examination related to the area to be biopsied.
Specifically, preservice work includes:
Inspect and palpate lesion to assess surface size, subcutaneous depth and extension, and whether fixed to underlying structures. Select the most representative and appropriate site to obtain specimen. Examine draining lymph node basins. Discuss need for skin biopsy and biopsy technique options. Describe the tangential biopsy procedure method and expected result and the potential for inconclusive pathology result. Review procedural risks, including bleeding, pain, edema, infection, delayed healing, scarring, and hyper- or hypopigmentation.5
Postservice work includes:
Instruct patient and family on postoperative wound care and dressing changes, as well as problems such as bleeding or pain and restrictions on activities, and follow-up care. Provide prescriptions for pain and antibiotics as necessary. Advise patient and family when results will be available and how they will be communicated. The pathology request form is filled out and signed by the physician. Complete medical record and communicate procedure/results to referring physician as appropriate.5
The Takeaway—Procedure codes include preservice and postservice work. If additional work for the procedure is not documented beyond that, an E/M cannot be included in the encounter.
Scenario 2: What If We Don’t Biopsy?
• CC: New spot on left cheek that seems to be growing and changing shape rapidly.
• History: No family history of skin cancer; concerned about scarring, no blood thinner.
• Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy.
• Impression: rule out melanoma.
• Plan: Review risk, benefits, and alternative options. Schedule biopsy. Discuss unique risk factor of sebaceous peau d’orange skin more prone to contour defects after biopsy.
When determining the coding level for this scenario by MDM, 3 components must be considered: number and complexity of problems addressed at the encounter (column 1), amount and/or complexity of data to be reviewed and analyzed (column 2), and risk of complications and/or morbidity or mortality of patient management (column 3).1 There are no data that are reviewed, so the auditor will assume minimal data to be reviewed and/or analyzed (level 2, row 2 in the MDM table). However, there may be a lot of variation in how an auditor would address the number and complexity of problems (level 1). Consider that you must explicitly state what you are thinking, as an auditor may not know melanoma is a life-threatening diagnosis. From the perspective of the auditor, could this be a:
• Self-limited or minor problem (level 2, or minimal problem in the MDM table)?1
• Stable chronic illness (level 3, or low-level problem)?1
• Undiagnosed new problem with uncertain prognosis (level 4, or moderate level problem)?1
• Acute illness with systemic symptoms (level 4, or moderate level problem)?1
• Acute or chronic illness or injury that poses a threat to life or bodily function (level 5, or high-level problem)?1
• All of the above?
Similarly, there may be variation in how the risk (column 3) would be interpreted in this scenario. The treatment gives no guidance, so the auditor may assume this has a minimal risk of morbidity (level 2) or possibly a low risk of morbidity from additional diagnostic testing or treatment (level 3), as opposed to a moderate risk of morbidity (level 4).1The Takeaway—In the auditor’s mind, this could be a straightforward (CPT codes 99202/99212) or lowlevel (99203/99213) visit as opposed to a moderate-level (99204/99214) visit. From the above documentation, an auditor would not be able to tell what you are thinking, and you can be assured they will not look further into the diagnosis or treatment to learn. That is not their job. So, let us clarify by explicitly stating what you are thinking in the context of the MDM grid.
Modified Scenario 2: A Funny-Looking New Spot With MDM Descriptors to Guide an Auditor
Below are modifications to the documentation for scenario 2 to guide an auditor:
• CC: New spot on left cheek that seems to be growing and changing shape rapidly.
• History: No family history of skin cancer; concerned about scarring, no blood thinner.
• Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy.
• Impression: rule out melanoma
• Plan: Discuss risks, benefits, and alternatives, including biopsy (
In this scenario, the level of MDM is much more clearly documented (as bolded above).
The number and complexity of problems would be an undiagnosed new problem with uncertain prognosis, which would be moderate complexity (column 1, level 4).1 There are no data that are reviewed or analyzed, which would be straightforward (column 2, level 2). For risk, the discussion of the biopsy as part of the diagnostic choices should include discussion of possible scarring, bleeding, pain, and infection, which would be considered best described as a decision regarding minor surgery with identified patient or procedure risk factors, which would make this of moderate complexity (column 3, level 4).1
Importantly, even if the procedure is not chosen as the final treatment plan, the discussion regarding the surgery, including the risks, benefits, and alternatives, can still count toward this category in the MDM table. Therefore, in this scenario with the updated and clarified documentation, this would be reported as CPT code 99204 for a new patient, while an established patient would be 99214.
Scenario 1 Revisited: A Funny-Looking New Spot
Below is scenario 1 with enhanced documentation, now applied to our procedure-only visit.
• CC: New spot on left cheek that seems to be growing and changing shape rapidly.
• History: No family history of skin cancer; concerned about scarring, no blood thinner.
• Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy.
• Impression: rule out melanoma (undiagnosed new problem with uncertain prognosis).
• Plan: Discuss risks, benefits, and alternatives, including biopsy (decision regarding minor surgery with identified patient or procedure risk factors) vs a noninvasive 2 gene expression profiling melanoma rule-out test. Patient wants biopsy. Consent, biopsy via shave technique. Lidocaine hydrochloride 1% with epinephrine, 1 cc, prepare and drape, hemostasis obtained, ointment and bandage applied, and care instructions provided.
This documentation would only allow reporting the biopsy as in Scenario 1, as the decision to perform a 0- or 10-day global procedure is bundled with the procedure if performed on the same date of service.
Final Thoughts
Spot checks are commonly encountered dermatologic visits. With the updated E/M guidelines, clarifying and streamlining your documentation is crucial. In particular, utilizing language that clearly defines number and complexity of problems, amount and/or complexity of data to be reviewed and analyzed, and appropriate risk stratification is crucial to ensuring appropriate reimbursement and minimizing your pain with audits.
- American Medical Association. CPT evaluation and management (E/M) code and guideline changes; 2023. Accessed April 13, 2023. https://www.ama-assn.org/system/files/2023-e-m-descriptors-guidelines.pdf
- American Medical Association. CPT Professional Edition 2023. American Medical Association; 2022.
- American Medical Association. Evaluation and management (E/M) coding. Accessed April 25, 2023. https://www.ama-assn.org/topics/evaluation-and-management-em-coding
- American Academy of Dermatology Association. Coding resource center. Accessed April 13, 2023. https://www.aad.org/member/practice/coding
- American Medical Association. RBVS DataManager Online. Accessed April 13, 2023. https://commerce.ama-assn.org/store/ui/catalog/productDetail?product_id=prod280002&navAction=push
On January 1, 2021, the Current Procedural Terminology (CPT) evaluation and management (E/M) reporting rules changed dramatically, with “bullet counting” no longer necessary and the coding level now based on either the new medical decision making (MDM) table or time spent on all activities relating to the care of the patient on the day of the encounter.1 This is described in the CPT Professional Edition 2023, a book every practitioner should review annually.2 In particular, every provider should read and reread pages 1 to 14—and beyond if you provide services beyond standard office visits. These changes were made with the intent to simplify the process of documentation and allow a provider to spend more time with patients, though there is still a paucity of data related to whether the new system achieves these aims.
The general rule of reporting work with CPT codes can be simply stated—“Document what you did, do what you documented, and report that which is medically necessary” (David McCafferey, MD, personal communication)—and you should never have any difficulty with audits. Unfortunately, the new system does not let an auditor, who typically lacks a medical degree, audit effectively unless they have a clear understanding of diseases and their stages. Many medical societies, including the American Medical Association3 and American Academy of Dermatology,4 have provided education that focuses on how to report a given vignette, but specific examples of documentation with commentary are uncommon.
To make your documentation more likely to pass audits, explicitly link parts of your documentation to CPT MDM descriptors. We offer scenarios and tips. In part 1 of this series, we discuss how to approach the “spot check,” a commonly encountered chief concern (CC) within dermatology.
A 34-year-old presents with a new spot on the left cheek that seems to be growing and changing shape rapidly. You examine the patient and discuss treatment options. The documentation reads as follows:
• CC: New spot on left cheek that seems to be growing and changing shape rapidly
• History: No family history of skin cancer; concerned about scarring, no blood thinner
• Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy
• Impression: rule out melanoma.
• Plan:
As was the case before 2021, you still need a CC, along with a medically (and medicolegally) appropriate history and physical examination. A diagnostic impression and treatment plan also should be included.
In this situation, reporting is straightforward. There is no separate E/M visit; only the CPT code 11102 for tangential biopsy is reported. An International Classification of Diseases, Tenth Revision code of D48.5 (neoplasm of uncertain behavior of skin) will be included.
Why no E/M code? This is because the biopsy includes preservice and postservice time and work that would be double reported with the E/M. Remember that the preservice work would include any history and physical examination related to the area to be biopsied.
Specifically, preservice work includes:
Inspect and palpate lesion to assess surface size, subcutaneous depth and extension, and whether fixed to underlying structures. Select the most representative and appropriate site to obtain specimen. Examine draining lymph node basins. Discuss need for skin biopsy and biopsy technique options. Describe the tangential biopsy procedure method and expected result and the potential for inconclusive pathology result. Review procedural risks, including bleeding, pain, edema, infection, delayed healing, scarring, and hyper- or hypopigmentation.5
Postservice work includes:
Instruct patient and family on postoperative wound care and dressing changes, as well as problems such as bleeding or pain and restrictions on activities, and follow-up care. Provide prescriptions for pain and antibiotics as necessary. Advise patient and family when results will be available and how they will be communicated. The pathology request form is filled out and signed by the physician. Complete medical record and communicate procedure/results to referring physician as appropriate.5
The Takeaway—Procedure codes include preservice and postservice work. If additional work for the procedure is not documented beyond that, an E/M cannot be included in the encounter.
Scenario 2: What If We Don’t Biopsy?
• CC: New spot on left cheek that seems to be growing and changing shape rapidly.
• History: No family history of skin cancer; concerned about scarring, no blood thinner.
• Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy.
• Impression: rule out melanoma.
• Plan: Review risk, benefits, and alternative options. Schedule biopsy. Discuss unique risk factor of sebaceous peau d’orange skin more prone to contour defects after biopsy.
When determining the coding level for this scenario by MDM, 3 components must be considered: number and complexity of problems addressed at the encounter (column 1), amount and/or complexity of data to be reviewed and analyzed (column 2), and risk of complications and/or morbidity or mortality of patient management (column 3).1 There are no data that are reviewed, so the auditor will assume minimal data to be reviewed and/or analyzed (level 2, row 2 in the MDM table). However, there may be a lot of variation in how an auditor would address the number and complexity of problems (level 1). Consider that you must explicitly state what you are thinking, as an auditor may not know melanoma is a life-threatening diagnosis. From the perspective of the auditor, could this be a:
• Self-limited or minor problem (level 2, or minimal problem in the MDM table)?1
• Stable chronic illness (level 3, or low-level problem)?1
• Undiagnosed new problem with uncertain prognosis (level 4, or moderate level problem)?1
• Acute illness with systemic symptoms (level 4, or moderate level problem)?1
• Acute or chronic illness or injury that poses a threat to life or bodily function (level 5, or high-level problem)?1
• All of the above?
Similarly, there may be variation in how the risk (column 3) would be interpreted in this scenario. The treatment gives no guidance, so the auditor may assume this has a minimal risk of morbidity (level 2) or possibly a low risk of morbidity from additional diagnostic testing or treatment (level 3), as opposed to a moderate risk of morbidity (level 4).1The Takeaway—In the auditor’s mind, this could be a straightforward (CPT codes 99202/99212) or lowlevel (99203/99213) visit as opposed to a moderate-level (99204/99214) visit. From the above documentation, an auditor would not be able to tell what you are thinking, and you can be assured they will not look further into the diagnosis or treatment to learn. That is not their job. So, let us clarify by explicitly stating what you are thinking in the context of the MDM grid.
Modified Scenario 2: A Funny-Looking New Spot With MDM Descriptors to Guide an Auditor
Below are modifications to the documentation for scenario 2 to guide an auditor:
• CC: New spot on left cheek that seems to be growing and changing shape rapidly.
• History: No family history of skin cancer; concerned about scarring, no blood thinner.
• Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy.
• Impression: rule out melanoma
• Plan: Discuss risks, benefits, and alternatives, including biopsy (
In this scenario, the level of MDM is much more clearly documented (as bolded above).
The number and complexity of problems would be an undiagnosed new problem with uncertain prognosis, which would be moderate complexity (column 1, level 4).1 There are no data that are reviewed or analyzed, which would be straightforward (column 2, level 2). For risk, the discussion of the biopsy as part of the diagnostic choices should include discussion of possible scarring, bleeding, pain, and infection, which would be considered best described as a decision regarding minor surgery with identified patient or procedure risk factors, which would make this of moderate complexity (column 3, level 4).1
Importantly, even if the procedure is not chosen as the final treatment plan, the discussion regarding the surgery, including the risks, benefits, and alternatives, can still count toward this category in the MDM table. Therefore, in this scenario with the updated and clarified documentation, this would be reported as CPT code 99204 for a new patient, while an established patient would be 99214.
Scenario 1 Revisited: A Funny-Looking New Spot
Below is scenario 1 with enhanced documentation, now applied to our procedure-only visit.
• CC: New spot on left cheek that seems to be growing and changing shape rapidly.
• History: No family history of skin cancer; concerned about scarring, no blood thinner.
• Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy.
• Impression: rule out melanoma (undiagnosed new problem with uncertain prognosis).
• Plan: Discuss risks, benefits, and alternatives, including biopsy (decision regarding minor surgery with identified patient or procedure risk factors) vs a noninvasive 2 gene expression profiling melanoma rule-out test. Patient wants biopsy. Consent, biopsy via shave technique. Lidocaine hydrochloride 1% with epinephrine, 1 cc, prepare and drape, hemostasis obtained, ointment and bandage applied, and care instructions provided.
This documentation would only allow reporting the biopsy as in Scenario 1, as the decision to perform a 0- or 10-day global procedure is bundled with the procedure if performed on the same date of service.
Final Thoughts
Spot checks are commonly encountered dermatologic visits. With the updated E/M guidelines, clarifying and streamlining your documentation is crucial. In particular, utilizing language that clearly defines number and complexity of problems, amount and/or complexity of data to be reviewed and analyzed, and appropriate risk stratification is crucial to ensuring appropriate reimbursement and minimizing your pain with audits.
On January 1, 2021, the Current Procedural Terminology (CPT) evaluation and management (E/M) reporting rules changed dramatically, with “bullet counting” no longer necessary and the coding level now based on either the new medical decision making (MDM) table or time spent on all activities relating to the care of the patient on the day of the encounter.1 This is described in the CPT Professional Edition 2023, a book every practitioner should review annually.2 In particular, every provider should read and reread pages 1 to 14—and beyond if you provide services beyond standard office visits. These changes were made with the intent to simplify the process of documentation and allow a provider to spend more time with patients, though there is still a paucity of data related to whether the new system achieves these aims.
The general rule of reporting work with CPT codes can be simply stated—“Document what you did, do what you documented, and report that which is medically necessary” (David McCafferey, MD, personal communication)—and you should never have any difficulty with audits. Unfortunately, the new system does not let an auditor, who typically lacks a medical degree, audit effectively unless they have a clear understanding of diseases and their stages. Many medical societies, including the American Medical Association3 and American Academy of Dermatology,4 have provided education that focuses on how to report a given vignette, but specific examples of documentation with commentary are uncommon.
To make your documentation more likely to pass audits, explicitly link parts of your documentation to CPT MDM descriptors. We offer scenarios and tips. In part 1 of this series, we discuss how to approach the “spot check,” a commonly encountered chief concern (CC) within dermatology.
A 34-year-old presents with a new spot on the left cheek that seems to be growing and changing shape rapidly. You examine the patient and discuss treatment options. The documentation reads as follows:
• CC: New spot on left cheek that seems to be growing and changing shape rapidly
• History: No family history of skin cancer; concerned about scarring, no blood thinner
• Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy
• Impression: rule out melanoma.
• Plan:
As was the case before 2021, you still need a CC, along with a medically (and medicolegally) appropriate history and physical examination. A diagnostic impression and treatment plan also should be included.
In this situation, reporting is straightforward. There is no separate E/M visit; only the CPT code 11102 for tangential biopsy is reported. An International Classification of Diseases, Tenth Revision code of D48.5 (neoplasm of uncertain behavior of skin) will be included.
Why no E/M code? This is because the biopsy includes preservice and postservice time and work that would be double reported with the E/M. Remember that the preservice work would include any history and physical examination related to the area to be biopsied.
Specifically, preservice work includes:
Inspect and palpate lesion to assess surface size, subcutaneous depth and extension, and whether fixed to underlying structures. Select the most representative and appropriate site to obtain specimen. Examine draining lymph node basins. Discuss need for skin biopsy and biopsy technique options. Describe the tangential biopsy procedure method and expected result and the potential for inconclusive pathology result. Review procedural risks, including bleeding, pain, edema, infection, delayed healing, scarring, and hyper- or hypopigmentation.5
Postservice work includes:
Instruct patient and family on postoperative wound care and dressing changes, as well as problems such as bleeding or pain and restrictions on activities, and follow-up care. Provide prescriptions for pain and antibiotics as necessary. Advise patient and family when results will be available and how they will be communicated. The pathology request form is filled out and signed by the physician. Complete medical record and communicate procedure/results to referring physician as appropriate.5
The Takeaway—Procedure codes include preservice and postservice work. If additional work for the procedure is not documented beyond that, an E/M cannot be included in the encounter.
Scenario 2: What If We Don’t Biopsy?
• CC: New spot on left cheek that seems to be growing and changing shape rapidly.
• History: No family history of skin cancer; concerned about scarring, no blood thinner.
• Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy.
• Impression: rule out melanoma.
• Plan: Review risk, benefits, and alternative options. Schedule biopsy. Discuss unique risk factor of sebaceous peau d’orange skin more prone to contour defects after biopsy.
When determining the coding level for this scenario by MDM, 3 components must be considered: number and complexity of problems addressed at the encounter (column 1), amount and/or complexity of data to be reviewed and analyzed (column 2), and risk of complications and/or morbidity or mortality of patient management (column 3).1 There are no data that are reviewed, so the auditor will assume minimal data to be reviewed and/or analyzed (level 2, row 2 in the MDM table). However, there may be a lot of variation in how an auditor would address the number and complexity of problems (level 1). Consider that you must explicitly state what you are thinking, as an auditor may not know melanoma is a life-threatening diagnosis. From the perspective of the auditor, could this be a:
• Self-limited or minor problem (level 2, or minimal problem in the MDM table)?1
• Stable chronic illness (level 3, or low-level problem)?1
• Undiagnosed new problem with uncertain prognosis (level 4, or moderate level problem)?1
• Acute illness with systemic symptoms (level 4, or moderate level problem)?1
• Acute or chronic illness or injury that poses a threat to life or bodily function (level 5, or high-level problem)?1
• All of the above?
Similarly, there may be variation in how the risk (column 3) would be interpreted in this scenario. The treatment gives no guidance, so the auditor may assume this has a minimal risk of morbidity (level 2) or possibly a low risk of morbidity from additional diagnostic testing or treatment (level 3), as opposed to a moderate risk of morbidity (level 4).1The Takeaway—In the auditor’s mind, this could be a straightforward (CPT codes 99202/99212) or lowlevel (99203/99213) visit as opposed to a moderate-level (99204/99214) visit. From the above documentation, an auditor would not be able to tell what you are thinking, and you can be assured they will not look further into the diagnosis or treatment to learn. That is not their job. So, let us clarify by explicitly stating what you are thinking in the context of the MDM grid.
Modified Scenario 2: A Funny-Looking New Spot With MDM Descriptors to Guide an Auditor
Below are modifications to the documentation for scenario 2 to guide an auditor:
• CC: New spot on left cheek that seems to be growing and changing shape rapidly.
• History: No family history of skin cancer; concerned about scarring, no blood thinner.
• Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy.
• Impression: rule out melanoma
• Plan: Discuss risks, benefits, and alternatives, including biopsy (
In this scenario, the level of MDM is much more clearly documented (as bolded above).
The number and complexity of problems would be an undiagnosed new problem with uncertain prognosis, which would be moderate complexity (column 1, level 4).1 There are no data that are reviewed or analyzed, which would be straightforward (column 2, level 2). For risk, the discussion of the biopsy as part of the diagnostic choices should include discussion of possible scarring, bleeding, pain, and infection, which would be considered best described as a decision regarding minor surgery with identified patient or procedure risk factors, which would make this of moderate complexity (column 3, level 4).1
Importantly, even if the procedure is not chosen as the final treatment plan, the discussion regarding the surgery, including the risks, benefits, and alternatives, can still count toward this category in the MDM table. Therefore, in this scenario with the updated and clarified documentation, this would be reported as CPT code 99204 for a new patient, while an established patient would be 99214.
Scenario 1 Revisited: A Funny-Looking New Spot
Below is scenario 1 with enhanced documentation, now applied to our procedure-only visit.
• CC: New spot on left cheek that seems to be growing and changing shape rapidly.
• History: No family history of skin cancer; concerned about scarring, no blood thinner.
• Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy.
• Impression: rule out melanoma (undiagnosed new problem with uncertain prognosis).
• Plan: Discuss risks, benefits, and alternatives, including biopsy (decision regarding minor surgery with identified patient or procedure risk factors) vs a noninvasive 2 gene expression profiling melanoma rule-out test. Patient wants biopsy. Consent, biopsy via shave technique. Lidocaine hydrochloride 1% with epinephrine, 1 cc, prepare and drape, hemostasis obtained, ointment and bandage applied, and care instructions provided.
This documentation would only allow reporting the biopsy as in Scenario 1, as the decision to perform a 0- or 10-day global procedure is bundled with the procedure if performed on the same date of service.
Final Thoughts
Spot checks are commonly encountered dermatologic visits. With the updated E/M guidelines, clarifying and streamlining your documentation is crucial. In particular, utilizing language that clearly defines number and complexity of problems, amount and/or complexity of data to be reviewed and analyzed, and appropriate risk stratification is crucial to ensuring appropriate reimbursement and minimizing your pain with audits.
- American Medical Association. CPT evaluation and management (E/M) code and guideline changes; 2023. Accessed April 13, 2023. https://www.ama-assn.org/system/files/2023-e-m-descriptors-guidelines.pdf
- American Medical Association. CPT Professional Edition 2023. American Medical Association; 2022.
- American Medical Association. Evaluation and management (E/M) coding. Accessed April 25, 2023. https://www.ama-assn.org/topics/evaluation-and-management-em-coding
- American Academy of Dermatology Association. Coding resource center. Accessed April 13, 2023. https://www.aad.org/member/practice/coding
- American Medical Association. RBVS DataManager Online. Accessed April 13, 2023. https://commerce.ama-assn.org/store/ui/catalog/productDetail?product_id=prod280002&navAction=push
- American Medical Association. CPT evaluation and management (E/M) code and guideline changes; 2023. Accessed April 13, 2023. https://www.ama-assn.org/system/files/2023-e-m-descriptors-guidelines.pdf
- American Medical Association. CPT Professional Edition 2023. American Medical Association; 2022.
- American Medical Association. Evaluation and management (E/M) coding. Accessed April 25, 2023. https://www.ama-assn.org/topics/evaluation-and-management-em-coding
- American Academy of Dermatology Association. Coding resource center. Accessed April 13, 2023. https://www.aad.org/member/practice/coding
- American Medical Association. RBVS DataManager Online. Accessed April 13, 2023. https://commerce.ama-assn.org/store/ui/catalog/productDetail?product_id=prod280002&navAction=push
Practice Points
- Clear documentation that reflects your thought process is an important component of effective coding and billing.
- Include Current Procedural Terminology–defined language within documentation to help ensure appropriate reimbursement and decrease the risk of audits.
Gene Expression Profiling for Melanoma Prognosis: Going Beyond What We See With Our Eyes
Dermatology certainly is the most visual medical specialty. In the current era of powerful electronic imaging and laboratory techniques, the skills of physical diagnosis seem to have become less important in medicine—not so in dermatology, in which the experienced clinician is able to identify many conditions by simply looking at the skin. Of course, dermatologists do heavily rely on dermatopathologists to microscopically visualize biopsies to distinguish diseases. Even as we acknowledge the dominant role of visual recognition, there is increasing progress in making clinical determinations based on molecular events. The era of genomic dermatology is here.
The Genodermatoses
There are more than 500 dermatologic conditions resulting from heritable mutational events.1 The rarity of most of these diseases and variability in phenotypic manifestations presents considerable diagnostic challenges, typically the province of a select group of clinical pediatric dermatologists whose abilities have been developed by experience.2 However, the addition of genomic analysis has now made reliable identification more accessible to a wider group of clinicians.3 The Human Genome Project was arguably the most successful health policy endeavor in human history, promoting the development of massive automated, information theory–driven applications to analyze DNA sequences.4 We all think of DNA analysis as the ultimate means to detect mutations by sequencing whole exomes—and in fact the entire genome of affected individuals searching for mutations—but DNA sequencing often is insufficient to detect mutations in noncoding regions of genes and to identify abnormalities of gene expression (eg, splice variants). Building on the advances in high-throughput nucleic acid sequencing and massive computerized analysis, the field has now taken a quantum leap further to sequence transcribed RNA to detect abnormalities.5
The techniques are straightforward: RNA is isolated and reverse transcribed to complementary DNA. The complementary DNA is amplified and then processed by high-throughput sequencers. The sequences are then identified by computer algorithms. It is possible to fully define the transcriptomes of multiple genes, even reaching the threshold of resolution of gene expression emanating from a single cell.6
Studying Gene Expression for Malignant Melanoma
As much as we rely on visual interpretations, we acknowledge that many conditions look very similar, whether to the naked eye or under the microscope. This is true for rare diseases but also for the rashes we routinely see. A group of investigators recently used RNA transcriptome sequencing to analyze differences between atopic dermatitis and psoriasis, permitting better differentiation of these 2 common conditions.7
One of the greatest challenges confronting dermatologists and their dermatopathologist partners is to distinguish malignant melanoma from benign nevi.8 Despite staining for a number of molecular markers, some lesions defy histopathology, such as distinguishing benign and malignant Spitz nevi; however, recent work on RNA transcriptomes suggests that gene expression may increase confidence in assessing atypical Spitz nevi.9 A 23-gene expression panel has yielded a sensitivity of 91.5% and a specificity of 92.5% in differentiating benign nevi from malignant melanoma.10
From the Research Laboratory to Routine Clinical Use
Undoubtedly, it is a large step from proof-of-concept studies to accepted clinical use. The ultimate achievement for a laboratory technique is to enter approved clinical use. Gene expression panels have now been approved by numerous third-party insurers to help predict future clinical evolution of biopsied melanomas. Although early in situ melanomas are eminently curable by wide excision, lesions that have more concerning characteristics (eg, depth >0.8 mm, ulceration) may progress to metastatic disease. The gratifying success of checkpoint inhibitor therapy has improved the previously dismal outlook for advanced melanomas.11 Dermatologists search for clues to suggest which patients may benefit from adjuvant therapy. Sentinel lymph node biopsy (SLNB) has been a standard-of-care technique to help make this determination.12
It has now been demonstrated that gene expression array analysis can provide evidence complementing SLNB results or even independent of SLNB results. In extensive validation studies, a 31-gene expression panel analyzing initial melanoma biopsy specimens showed predictive value for later recurrence and development of metastatic disease.13,14 The gene expression studies have identified patients with negative SLNBs who have gone on to develop metastatic melanomas.15 It has been suggested that gene expression panel diagnosis may reduce the need for invasive SLNBs in patients in whom the surgical procedure may involve risk.16
Looking to the Future
The progress of science is the result of many small steps building on prior work. The terms breakthrough and game changer in medicine have been popularized by the media and rarely are valid. On the contrary, sequential development of methods over many years has preceded the acclaimed successes of medical research; for example, the best-known medical breakthrough—that of Salk’s inactivated polio vaccine—was preceded by the use of an inactivated polio vaccine by Brodie and Park17 in 1935. However, it was the development of tissue culture of poliomyelitis virus by Enders et al18 that provided the methodology to Salk’s group to produce their inactivated polio vaccine.
The ability to go beyond our visual senses will be of great importance in characterizing the variability of skin diseases, especially in skin of color patients; for example, acral melanoma is perhaps the primary melanocytic malignancy in darker-skinned patients and is the target of RNA transcriptomic research.19 Progress is continuing on gene therapy for a growing number of skin conditions.20,21 In vivo correction of abnormal genes is being attempted for a number of inherited cutaneous diseases,22 notably for disorders of skin fragility.23 For now, we welcome the addition of genomic capabilities to the visual practice of dermatology and the capability to go beyond that which we can see with our eyes.
- Feramisco JD, Sadreyev RI, Murray ML, et al. Phenotypic and enotypic analyses of genetic skin disease through the Online Mendelian Inheritance in Man (OMIM) database. J Investig Derm. 2009;129:2628-2636.
- Parker JC, Rangu S, Grand KL, et al. Genetic skin disorders: the value of a multidisciplinary clinic. Am J Med Genet A. 2021;185:1159-1167.
- Richert B, Smits G. Clinical and molecular diagnosis of genodermatoses: review and perspectives. J Eur Acad Dermatol Venereol. 2023;37:488-500.
- Green ED, Watson JD, Collins FS. Human genome project: twenty-five years of big biology. Nature. 2015;526:29-31.
- Saeidian AH, Youssefian L, Vahidnezhad H, et al. Research techniques made simple: whole-transcriptome sequencing by RNA-seq for diagnosis of monogenic disorders. J Invest Dermatol. 2020;140:1117-1126.e1.
- Deutsch A, McLellan BN, Shinoda K. Single-cell transcriptomics in dermatology. JAAD Int. 2020;1:182-188.
- Liu Y, Wang H, Taylor M, et al. Classification of human chronic inflammatory skin disease based on single-cell immune profiling [published online April 15, 2022]. Sci Immunol. doi:10.1126/sciimmunol.abl9165
- Reimann JDR, Salim S, Velazquez EF, et al. Comparison of melanoma gene expression score with histopathology, fluorescence in situ hybridization, and SNP array for the classification of melanocytic neoplasms. Mod Pathol. 2018;31:1733-1743.
- Hillen LM, Geybels MS, Spassova I, et al. A digital mRNA expression signature to classify challenging spitzoid melanocytic neoplasms. FEBS Open Bio. 2020;10:1326-1341.
- Clarke LE, Flake DD 2nd, Busam K, et al. An independent validation of a gene expression signature to differentiate malignant melanoma from benign melanocytic nevi. Cancer. 2017;123:617-628.
- Stege H, Haist M, Nikfarjam U, et al. The status of adjuvant and neoadjuvant melanoma therapy, new developments and upcoming challenges. Target Oncol. 2021;16:537-552.
- Morrison S, Han D. Re-evaluation of sentinel lymph node biopsy for melanoma. Curr Treat Options Oncol. 2021;22:22.
- Gerami P, Cook RW, Russell MC, et al. Gene expression profiling for molecular staging of cutaneous melanoma in patients with sentinel lymph node biopsy. J Am Acad Dermatol. 2015;72:780-785.e3.
- Keller J, Schwartz TL, Lizalek JM, et al. Prospective validation of the prognostic 31-gene expression profiling test in primary cutaneous melanoma. Cancer Med. 2019;8:2205-2212.
- Gastman BR, Gerami P, Kurley SJ, et al. Identification of patients at risk for metastasis using a prognostic 31-gene expression profile in subpopulations of melanoma patients with favorable outcomes by standard criteria. J Am Acad Dermatol. 2019;80:149-157.
- Vetto JT, Hsueh EC, Gastman BR, et al. Guidance of sentinel lymph node biopsy decisions in patients with T1-T2 melanoma using gene expression profiling. Future Oncol. 2019;15:1207-1217.
- Brodie M, Park W. Active immunization against poliomyelitis. JAMA. 1935;105:1089-1093.
- Enders JF, Weller TH, Robbins FC. Cultivation of the Lansing strain of poliomyelitis virus in cultures of various human embryonic tissues. Science. 1949;109:85-87.
- Li J, Smalley I, Chen Z, et al. Single-cell characterization of the cellular landscape of acral melanoma identifies novel targets for immunotherapy. Clin Cancer Res. 2022;28:2131-2146.
- Gorell E, Nguyen N, Lane A, et al. Gene therapy for skin diseases. Cold Spring Harb Perspect Med. 2014;4:A015149.
- Cavazza A, Mavilio F. Gene therapy of skin adhesion disorders (mini review). Curr Pharm Biotechnol. 2012;13:1868-1876.
- Abdul-Wahab A, Qasim W, McGrath JA. Gene therapies for inherited skin disorders. Semin Cutan Med Surg. 2014;33:83-90.
- Bilousova G. Gene therapy for skin fragility diseases: the new generation. J Invest Dermatol. 2019;139:1634-1637.
Dermatology certainly is the most visual medical specialty. In the current era of powerful electronic imaging and laboratory techniques, the skills of physical diagnosis seem to have become less important in medicine—not so in dermatology, in which the experienced clinician is able to identify many conditions by simply looking at the skin. Of course, dermatologists do heavily rely on dermatopathologists to microscopically visualize biopsies to distinguish diseases. Even as we acknowledge the dominant role of visual recognition, there is increasing progress in making clinical determinations based on molecular events. The era of genomic dermatology is here.
The Genodermatoses
There are more than 500 dermatologic conditions resulting from heritable mutational events.1 The rarity of most of these diseases and variability in phenotypic manifestations presents considerable diagnostic challenges, typically the province of a select group of clinical pediatric dermatologists whose abilities have been developed by experience.2 However, the addition of genomic analysis has now made reliable identification more accessible to a wider group of clinicians.3 The Human Genome Project was arguably the most successful health policy endeavor in human history, promoting the development of massive automated, information theory–driven applications to analyze DNA sequences.4 We all think of DNA analysis as the ultimate means to detect mutations by sequencing whole exomes—and in fact the entire genome of affected individuals searching for mutations—but DNA sequencing often is insufficient to detect mutations in noncoding regions of genes and to identify abnormalities of gene expression (eg, splice variants). Building on the advances in high-throughput nucleic acid sequencing and massive computerized analysis, the field has now taken a quantum leap further to sequence transcribed RNA to detect abnormalities.5
The techniques are straightforward: RNA is isolated and reverse transcribed to complementary DNA. The complementary DNA is amplified and then processed by high-throughput sequencers. The sequences are then identified by computer algorithms. It is possible to fully define the transcriptomes of multiple genes, even reaching the threshold of resolution of gene expression emanating from a single cell.6
Studying Gene Expression for Malignant Melanoma
As much as we rely on visual interpretations, we acknowledge that many conditions look very similar, whether to the naked eye or under the microscope. This is true for rare diseases but also for the rashes we routinely see. A group of investigators recently used RNA transcriptome sequencing to analyze differences between atopic dermatitis and psoriasis, permitting better differentiation of these 2 common conditions.7
One of the greatest challenges confronting dermatologists and their dermatopathologist partners is to distinguish malignant melanoma from benign nevi.8 Despite staining for a number of molecular markers, some lesions defy histopathology, such as distinguishing benign and malignant Spitz nevi; however, recent work on RNA transcriptomes suggests that gene expression may increase confidence in assessing atypical Spitz nevi.9 A 23-gene expression panel has yielded a sensitivity of 91.5% and a specificity of 92.5% in differentiating benign nevi from malignant melanoma.10
From the Research Laboratory to Routine Clinical Use
Undoubtedly, it is a large step from proof-of-concept studies to accepted clinical use. The ultimate achievement for a laboratory technique is to enter approved clinical use. Gene expression panels have now been approved by numerous third-party insurers to help predict future clinical evolution of biopsied melanomas. Although early in situ melanomas are eminently curable by wide excision, lesions that have more concerning characteristics (eg, depth >0.8 mm, ulceration) may progress to metastatic disease. The gratifying success of checkpoint inhibitor therapy has improved the previously dismal outlook for advanced melanomas.11 Dermatologists search for clues to suggest which patients may benefit from adjuvant therapy. Sentinel lymph node biopsy (SLNB) has been a standard-of-care technique to help make this determination.12
It has now been demonstrated that gene expression array analysis can provide evidence complementing SLNB results or even independent of SLNB results. In extensive validation studies, a 31-gene expression panel analyzing initial melanoma biopsy specimens showed predictive value for later recurrence and development of metastatic disease.13,14 The gene expression studies have identified patients with negative SLNBs who have gone on to develop metastatic melanomas.15 It has been suggested that gene expression panel diagnosis may reduce the need for invasive SLNBs in patients in whom the surgical procedure may involve risk.16
Looking to the Future
The progress of science is the result of many small steps building on prior work. The terms breakthrough and game changer in medicine have been popularized by the media and rarely are valid. On the contrary, sequential development of methods over many years has preceded the acclaimed successes of medical research; for example, the best-known medical breakthrough—that of Salk’s inactivated polio vaccine—was preceded by the use of an inactivated polio vaccine by Brodie and Park17 in 1935. However, it was the development of tissue culture of poliomyelitis virus by Enders et al18 that provided the methodology to Salk’s group to produce their inactivated polio vaccine.
The ability to go beyond our visual senses will be of great importance in characterizing the variability of skin diseases, especially in skin of color patients; for example, acral melanoma is perhaps the primary melanocytic malignancy in darker-skinned patients and is the target of RNA transcriptomic research.19 Progress is continuing on gene therapy for a growing number of skin conditions.20,21 In vivo correction of abnormal genes is being attempted for a number of inherited cutaneous diseases,22 notably for disorders of skin fragility.23 For now, we welcome the addition of genomic capabilities to the visual practice of dermatology and the capability to go beyond that which we can see with our eyes.
Dermatology certainly is the most visual medical specialty. In the current era of powerful electronic imaging and laboratory techniques, the skills of physical diagnosis seem to have become less important in medicine—not so in dermatology, in which the experienced clinician is able to identify many conditions by simply looking at the skin. Of course, dermatologists do heavily rely on dermatopathologists to microscopically visualize biopsies to distinguish diseases. Even as we acknowledge the dominant role of visual recognition, there is increasing progress in making clinical determinations based on molecular events. The era of genomic dermatology is here.
The Genodermatoses
There are more than 500 dermatologic conditions resulting from heritable mutational events.1 The rarity of most of these diseases and variability in phenotypic manifestations presents considerable diagnostic challenges, typically the province of a select group of clinical pediatric dermatologists whose abilities have been developed by experience.2 However, the addition of genomic analysis has now made reliable identification more accessible to a wider group of clinicians.3 The Human Genome Project was arguably the most successful health policy endeavor in human history, promoting the development of massive automated, information theory–driven applications to analyze DNA sequences.4 We all think of DNA analysis as the ultimate means to detect mutations by sequencing whole exomes—and in fact the entire genome of affected individuals searching for mutations—but DNA sequencing often is insufficient to detect mutations in noncoding regions of genes and to identify abnormalities of gene expression (eg, splice variants). Building on the advances in high-throughput nucleic acid sequencing and massive computerized analysis, the field has now taken a quantum leap further to sequence transcribed RNA to detect abnormalities.5
The techniques are straightforward: RNA is isolated and reverse transcribed to complementary DNA. The complementary DNA is amplified and then processed by high-throughput sequencers. The sequences are then identified by computer algorithms. It is possible to fully define the transcriptomes of multiple genes, even reaching the threshold of resolution of gene expression emanating from a single cell.6
Studying Gene Expression for Malignant Melanoma
As much as we rely on visual interpretations, we acknowledge that many conditions look very similar, whether to the naked eye or under the microscope. This is true for rare diseases but also for the rashes we routinely see. A group of investigators recently used RNA transcriptome sequencing to analyze differences between atopic dermatitis and psoriasis, permitting better differentiation of these 2 common conditions.7
One of the greatest challenges confronting dermatologists and their dermatopathologist partners is to distinguish malignant melanoma from benign nevi.8 Despite staining for a number of molecular markers, some lesions defy histopathology, such as distinguishing benign and malignant Spitz nevi; however, recent work on RNA transcriptomes suggests that gene expression may increase confidence in assessing atypical Spitz nevi.9 A 23-gene expression panel has yielded a sensitivity of 91.5% and a specificity of 92.5% in differentiating benign nevi from malignant melanoma.10
From the Research Laboratory to Routine Clinical Use
Undoubtedly, it is a large step from proof-of-concept studies to accepted clinical use. The ultimate achievement for a laboratory technique is to enter approved clinical use. Gene expression panels have now been approved by numerous third-party insurers to help predict future clinical evolution of biopsied melanomas. Although early in situ melanomas are eminently curable by wide excision, lesions that have more concerning characteristics (eg, depth >0.8 mm, ulceration) may progress to metastatic disease. The gratifying success of checkpoint inhibitor therapy has improved the previously dismal outlook for advanced melanomas.11 Dermatologists search for clues to suggest which patients may benefit from adjuvant therapy. Sentinel lymph node biopsy (SLNB) has been a standard-of-care technique to help make this determination.12
It has now been demonstrated that gene expression array analysis can provide evidence complementing SLNB results or even independent of SLNB results. In extensive validation studies, a 31-gene expression panel analyzing initial melanoma biopsy specimens showed predictive value for later recurrence and development of metastatic disease.13,14 The gene expression studies have identified patients with negative SLNBs who have gone on to develop metastatic melanomas.15 It has been suggested that gene expression panel diagnosis may reduce the need for invasive SLNBs in patients in whom the surgical procedure may involve risk.16
Looking to the Future
The progress of science is the result of many small steps building on prior work. The terms breakthrough and game changer in medicine have been popularized by the media and rarely are valid. On the contrary, sequential development of methods over many years has preceded the acclaimed successes of medical research; for example, the best-known medical breakthrough—that of Salk’s inactivated polio vaccine—was preceded by the use of an inactivated polio vaccine by Brodie and Park17 in 1935. However, it was the development of tissue culture of poliomyelitis virus by Enders et al18 that provided the methodology to Salk’s group to produce their inactivated polio vaccine.
The ability to go beyond our visual senses will be of great importance in characterizing the variability of skin diseases, especially in skin of color patients; for example, acral melanoma is perhaps the primary melanocytic malignancy in darker-skinned patients and is the target of RNA transcriptomic research.19 Progress is continuing on gene therapy for a growing number of skin conditions.20,21 In vivo correction of abnormal genes is being attempted for a number of inherited cutaneous diseases,22 notably for disorders of skin fragility.23 For now, we welcome the addition of genomic capabilities to the visual practice of dermatology and the capability to go beyond that which we can see with our eyes.
- Feramisco JD, Sadreyev RI, Murray ML, et al. Phenotypic and enotypic analyses of genetic skin disease through the Online Mendelian Inheritance in Man (OMIM) database. J Investig Derm. 2009;129:2628-2636.
- Parker JC, Rangu S, Grand KL, et al. Genetic skin disorders: the value of a multidisciplinary clinic. Am J Med Genet A. 2021;185:1159-1167.
- Richert B, Smits G. Clinical and molecular diagnosis of genodermatoses: review and perspectives. J Eur Acad Dermatol Venereol. 2023;37:488-500.
- Green ED, Watson JD, Collins FS. Human genome project: twenty-five years of big biology. Nature. 2015;526:29-31.
- Saeidian AH, Youssefian L, Vahidnezhad H, et al. Research techniques made simple: whole-transcriptome sequencing by RNA-seq for diagnosis of monogenic disorders. J Invest Dermatol. 2020;140:1117-1126.e1.
- Deutsch A, McLellan BN, Shinoda K. Single-cell transcriptomics in dermatology. JAAD Int. 2020;1:182-188.
- Liu Y, Wang H, Taylor M, et al. Classification of human chronic inflammatory skin disease based on single-cell immune profiling [published online April 15, 2022]. Sci Immunol. doi:10.1126/sciimmunol.abl9165
- Reimann JDR, Salim S, Velazquez EF, et al. Comparison of melanoma gene expression score with histopathology, fluorescence in situ hybridization, and SNP array for the classification of melanocytic neoplasms. Mod Pathol. 2018;31:1733-1743.
- Hillen LM, Geybels MS, Spassova I, et al. A digital mRNA expression signature to classify challenging spitzoid melanocytic neoplasms. FEBS Open Bio. 2020;10:1326-1341.
- Clarke LE, Flake DD 2nd, Busam K, et al. An independent validation of a gene expression signature to differentiate malignant melanoma from benign melanocytic nevi. Cancer. 2017;123:617-628.
- Stege H, Haist M, Nikfarjam U, et al. The status of adjuvant and neoadjuvant melanoma therapy, new developments and upcoming challenges. Target Oncol. 2021;16:537-552.
- Morrison S, Han D. Re-evaluation of sentinel lymph node biopsy for melanoma. Curr Treat Options Oncol. 2021;22:22.
- Gerami P, Cook RW, Russell MC, et al. Gene expression profiling for molecular staging of cutaneous melanoma in patients with sentinel lymph node biopsy. J Am Acad Dermatol. 2015;72:780-785.e3.
- Keller J, Schwartz TL, Lizalek JM, et al. Prospective validation of the prognostic 31-gene expression profiling test in primary cutaneous melanoma. Cancer Med. 2019;8:2205-2212.
- Gastman BR, Gerami P, Kurley SJ, et al. Identification of patients at risk for metastasis using a prognostic 31-gene expression profile in subpopulations of melanoma patients with favorable outcomes by standard criteria. J Am Acad Dermatol. 2019;80:149-157.
- Vetto JT, Hsueh EC, Gastman BR, et al. Guidance of sentinel lymph node biopsy decisions in patients with T1-T2 melanoma using gene expression profiling. Future Oncol. 2019;15:1207-1217.
- Brodie M, Park W. Active immunization against poliomyelitis. JAMA. 1935;105:1089-1093.
- Enders JF, Weller TH, Robbins FC. Cultivation of the Lansing strain of poliomyelitis virus in cultures of various human embryonic tissues. Science. 1949;109:85-87.
- Li J, Smalley I, Chen Z, et al. Single-cell characterization of the cellular landscape of acral melanoma identifies novel targets for immunotherapy. Clin Cancer Res. 2022;28:2131-2146.
- Gorell E, Nguyen N, Lane A, et al. Gene therapy for skin diseases. Cold Spring Harb Perspect Med. 2014;4:A015149.
- Cavazza A, Mavilio F. Gene therapy of skin adhesion disorders (mini review). Curr Pharm Biotechnol. 2012;13:1868-1876.
- Abdul-Wahab A, Qasim W, McGrath JA. Gene therapies for inherited skin disorders. Semin Cutan Med Surg. 2014;33:83-90.
- Bilousova G. Gene therapy for skin fragility diseases: the new generation. J Invest Dermatol. 2019;139:1634-1637.
- Feramisco JD, Sadreyev RI, Murray ML, et al. Phenotypic and enotypic analyses of genetic skin disease through the Online Mendelian Inheritance in Man (OMIM) database. J Investig Derm. 2009;129:2628-2636.
- Parker JC, Rangu S, Grand KL, et al. Genetic skin disorders: the value of a multidisciplinary clinic. Am J Med Genet A. 2021;185:1159-1167.
- Richert B, Smits G. Clinical and molecular diagnosis of genodermatoses: review and perspectives. J Eur Acad Dermatol Venereol. 2023;37:488-500.
- Green ED, Watson JD, Collins FS. Human genome project: twenty-five years of big biology. Nature. 2015;526:29-31.
- Saeidian AH, Youssefian L, Vahidnezhad H, et al. Research techniques made simple: whole-transcriptome sequencing by RNA-seq for diagnosis of monogenic disorders. J Invest Dermatol. 2020;140:1117-1126.e1.
- Deutsch A, McLellan BN, Shinoda K. Single-cell transcriptomics in dermatology. JAAD Int. 2020;1:182-188.
- Liu Y, Wang H, Taylor M, et al. Classification of human chronic inflammatory skin disease based on single-cell immune profiling [published online April 15, 2022]. Sci Immunol. doi:10.1126/sciimmunol.abl9165
- Reimann JDR, Salim S, Velazquez EF, et al. Comparison of melanoma gene expression score with histopathology, fluorescence in situ hybridization, and SNP array for the classification of melanocytic neoplasms. Mod Pathol. 2018;31:1733-1743.
- Hillen LM, Geybels MS, Spassova I, et al. A digital mRNA expression signature to classify challenging spitzoid melanocytic neoplasms. FEBS Open Bio. 2020;10:1326-1341.
- Clarke LE, Flake DD 2nd, Busam K, et al. An independent validation of a gene expression signature to differentiate malignant melanoma from benign melanocytic nevi. Cancer. 2017;123:617-628.
- Stege H, Haist M, Nikfarjam U, et al. The status of adjuvant and neoadjuvant melanoma therapy, new developments and upcoming challenges. Target Oncol. 2021;16:537-552.
- Morrison S, Han D. Re-evaluation of sentinel lymph node biopsy for melanoma. Curr Treat Options Oncol. 2021;22:22.
- Gerami P, Cook RW, Russell MC, et al. Gene expression profiling for molecular staging of cutaneous melanoma in patients with sentinel lymph node biopsy. J Am Acad Dermatol. 2015;72:780-785.e3.
- Keller J, Schwartz TL, Lizalek JM, et al. Prospective validation of the prognostic 31-gene expression profiling test in primary cutaneous melanoma. Cancer Med. 2019;8:2205-2212.
- Gastman BR, Gerami P, Kurley SJ, et al. Identification of patients at risk for metastasis using a prognostic 31-gene expression profile in subpopulations of melanoma patients with favorable outcomes by standard criteria. J Am Acad Dermatol. 2019;80:149-157.
- Vetto JT, Hsueh EC, Gastman BR, et al. Guidance of sentinel lymph node biopsy decisions in patients with T1-T2 melanoma using gene expression profiling. Future Oncol. 2019;15:1207-1217.
- Brodie M, Park W. Active immunization against poliomyelitis. JAMA. 1935;105:1089-1093.
- Enders JF, Weller TH, Robbins FC. Cultivation of the Lansing strain of poliomyelitis virus in cultures of various human embryonic tissues. Science. 1949;109:85-87.
- Li J, Smalley I, Chen Z, et al. Single-cell characterization of the cellular landscape of acral melanoma identifies novel targets for immunotherapy. Clin Cancer Res. 2022;28:2131-2146.
- Gorell E, Nguyen N, Lane A, et al. Gene therapy for skin diseases. Cold Spring Harb Perspect Med. 2014;4:A015149.
- Cavazza A, Mavilio F. Gene therapy of skin adhesion disorders (mini review). Curr Pharm Biotechnol. 2012;13:1868-1876.
- Abdul-Wahab A, Qasim W, McGrath JA. Gene therapies for inherited skin disorders. Semin Cutan Med Surg. 2014;33:83-90.
- Bilousova G. Gene therapy for skin fragility diseases: the new generation. J Invest Dermatol. 2019;139:1634-1637.
Polyurethane Tubing to Minimize Pain During Nail Injections
Practice Gap
Nail matrix and nail bed injections with triamcinolone acetonide are used to treat trachyonychia and inflammatory nail conditions, including nail psoriasis and nail lichen planus. The procedure should be quick in well-trained hands, with each nail injection taking only seconds to perform. Typically, patients have multiple nails involved, requiring at least 1 injection into the nail matrix or the nail bed (or both) in each nail at each visit. Patients often are anxious when undergoing nail injections; the nail unit is highly innervated and vascular, which can cause notable transient discomfort during the procedure1,2 as well as postoperative pain.3
Nail injections must be repeated every 4 to 6 weeks to sustain clinical benefit and maximize outcomes, which can lead to heightened anxiety and apprehension before and during the visit. Furthermore, pain and anxiety associated with the procedure may deter patients from returning for follow-up injections, which can impact treatment adherence and clinical outcomes.
Dermatologists should implement strategies to decrease periprocedural anxiety to improve the nail injection experience. In our practice, we routinely incorporate stress-reducing techniques—music, talkesthesia, a sleep mask, cool air, ethyl chloride, and squeezing a stress ball—into the clinical workflow of the procedure. The goal of these techniques is to divert attention away from painful stimuli. Most patients, however, receive injections in both hands, making it impractical to employ some of these techniques, particularly squeezing a stress ball. We employed a unique method involving polyurethane tubing to reduce stress and anxiety during nail procedures.
The Technique
A patient was receiving treatment with intralesional triamcinolone injections to the nail matrix for trachyonychia involving all of the fingernails. He worked as an equipment and facilities manager, giving him access to polyurethane tubing, which is routinely used in the manufacture of some medical devices that require gas or liquid to operate. He found the nail injections to be painful but was motivated to proceed with treatment. He brought in a piece of polyurethane tubing to a subsequent visit to bite on during the injections (Figure) and reported considerable relief of pain.
What you were not taught in United States history class was that this method—clenching an object orally—dates to the era before the Civil War, before appropriate anesthetics and analgesics were developed, when patients and soldiers bit on a bullet or leather strap during surgical procedures.4 Clenching and chewing have been shown to promote relaxation and reduce acute pain and stress.5
Practical Implications
Polyurethane tubing can be purchased in bulk, is inexpensive ($0.30/foot on Amazon), and unlikely to damage teeth due to its flexibility. It can be cut into 6-inch pieces and given to the patient at their first nail injection appointment. The patient can then bring the tubing to subsequent appointments to use as a mastication tool during nail injections.
We instruct the patient to disinfect the dedicated piece of tubing after the initial visit and each subsequent visit by soaking it for 15 minutes in either a 3% hydrogen peroxide solution, antibacterial mouthwash, a solution of baking soda (bicarbonate of soda) and water (1 cup of water to 2 teaspoons of baking soda), or white vinegar. We instruct them to thoroughly dry the disinfected polyurethane tube and store it in a clean, reusable, resealable zipper storage bag between appointments.
In addition to reducing anxiety and pain, this method also distracts the patient and therefore promotes patient and physician safety. Patients are less likely to jump or startle during the injection, thereby reducing the risk of physically interfering with the nail surgeon or making an unanticipated advance into the surgical field.
Although frustrated patients with nail disease may need to “bite the bullet” when they accept treatment with nail injections, lessons from our patient and from United States history offer a safe and cost-effective pain management strategy. Minimizing discomfort and anxiety during the first nail injection is crucial because doing so is likely to promote adherence with follow-up injections and therefore improve clinical outcomes.
Future clinical studies should validate the clinical utility of oral mastication and clenching during nail procedures compared to other perioperative stress- and anxiety-reducing techniques.
- Ricardo JW, Lipner SR. Utilization of a stress ball to diminish anxiety during nail surgery. Cutis. 2020;105:294. doi:10.12788/cutis.0013
- Ricardo JW, Lipner SR. Utilizing a sleep mask to reduce patient anxiety during nail surgery. Cutis. 2021;108:36. doi:10.12788/cutis.0285
- Ip HYV, Abrishami A, Peng PW, et al. Predictors of postoperative pain and analgesic consumption: a qualitative systematic review. Anesthesiology. 2009;111:657-677. doi:10.1097/ALN.0b013e3181aae87a
- Albin MS. The use of anesthetics during the Civil War, 1861-1865. Pharm Hist. 2000;42:99-114.
- Tahara Y, Sakurai K, Ando T. Influence of chewing and clenching on salivary cortisol levels as an indicator of stress. J Prosthodont. 2007;16:129-135. doi:10.1111/j.1532-849X.2007.00178.x
Practice Gap
Nail matrix and nail bed injections with triamcinolone acetonide are used to treat trachyonychia and inflammatory nail conditions, including nail psoriasis and nail lichen planus. The procedure should be quick in well-trained hands, with each nail injection taking only seconds to perform. Typically, patients have multiple nails involved, requiring at least 1 injection into the nail matrix or the nail bed (or both) in each nail at each visit. Patients often are anxious when undergoing nail injections; the nail unit is highly innervated and vascular, which can cause notable transient discomfort during the procedure1,2 as well as postoperative pain.3
Nail injections must be repeated every 4 to 6 weeks to sustain clinical benefit and maximize outcomes, which can lead to heightened anxiety and apprehension before and during the visit. Furthermore, pain and anxiety associated with the procedure may deter patients from returning for follow-up injections, which can impact treatment adherence and clinical outcomes.
Dermatologists should implement strategies to decrease periprocedural anxiety to improve the nail injection experience. In our practice, we routinely incorporate stress-reducing techniques—music, talkesthesia, a sleep mask, cool air, ethyl chloride, and squeezing a stress ball—into the clinical workflow of the procedure. The goal of these techniques is to divert attention away from painful stimuli. Most patients, however, receive injections in both hands, making it impractical to employ some of these techniques, particularly squeezing a stress ball. We employed a unique method involving polyurethane tubing to reduce stress and anxiety during nail procedures.
The Technique
A patient was receiving treatment with intralesional triamcinolone injections to the nail matrix for trachyonychia involving all of the fingernails. He worked as an equipment and facilities manager, giving him access to polyurethane tubing, which is routinely used in the manufacture of some medical devices that require gas or liquid to operate. He found the nail injections to be painful but was motivated to proceed with treatment. He brought in a piece of polyurethane tubing to a subsequent visit to bite on during the injections (Figure) and reported considerable relief of pain.

What you were not taught in United States history class was that this method—clenching an object orally—dates to the era before the Civil War, before appropriate anesthetics and analgesics were developed, when patients and soldiers bit on a bullet or leather strap during surgical procedures.4 Clenching and chewing have been shown to promote relaxation and reduce acute pain and stress.5
Practical Implications
Polyurethane tubing can be purchased in bulk, is inexpensive ($0.30/foot on Amazon), and unlikely to damage teeth due to its flexibility. It can be cut into 6-inch pieces and given to the patient at their first nail injection appointment. The patient can then bring the tubing to subsequent appointments to use as a mastication tool during nail injections.
We instruct the patient to disinfect the dedicated piece of tubing after the initial visit and each subsequent visit by soaking it for 15 minutes in either a 3% hydrogen peroxide solution, antibacterial mouthwash, a solution of baking soda (bicarbonate of soda) and water (1 cup of water to 2 teaspoons of baking soda), or white vinegar. We instruct them to thoroughly dry the disinfected polyurethane tube and store it in a clean, reusable, resealable zipper storage bag between appointments.
In addition to reducing anxiety and pain, this method also distracts the patient and therefore promotes patient and physician safety. Patients are less likely to jump or startle during the injection, thereby reducing the risk of physically interfering with the nail surgeon or making an unanticipated advance into the surgical field.
Although frustrated patients with nail disease may need to “bite the bullet” when they accept treatment with nail injections, lessons from our patient and from United States history offer a safe and cost-effective pain management strategy. Minimizing discomfort and anxiety during the first nail injection is crucial because doing so is likely to promote adherence with follow-up injections and therefore improve clinical outcomes.
Future clinical studies should validate the clinical utility of oral mastication and clenching during nail procedures compared to other perioperative stress- and anxiety-reducing techniques.
Practice Gap
Nail matrix and nail bed injections with triamcinolone acetonide are used to treat trachyonychia and inflammatory nail conditions, including nail psoriasis and nail lichen planus. The procedure should be quick in well-trained hands, with each nail injection taking only seconds to perform. Typically, patients have multiple nails involved, requiring at least 1 injection into the nail matrix or the nail bed (or both) in each nail at each visit. Patients often are anxious when undergoing nail injections; the nail unit is highly innervated and vascular, which can cause notable transient discomfort during the procedure1,2 as well as postoperative pain.3
Nail injections must be repeated every 4 to 6 weeks to sustain clinical benefit and maximize outcomes, which can lead to heightened anxiety and apprehension before and during the visit. Furthermore, pain and anxiety associated with the procedure may deter patients from returning for follow-up injections, which can impact treatment adherence and clinical outcomes.
Dermatologists should implement strategies to decrease periprocedural anxiety to improve the nail injection experience. In our practice, we routinely incorporate stress-reducing techniques—music, talkesthesia, a sleep mask, cool air, ethyl chloride, and squeezing a stress ball—into the clinical workflow of the procedure. The goal of these techniques is to divert attention away from painful stimuli. Most patients, however, receive injections in both hands, making it impractical to employ some of these techniques, particularly squeezing a stress ball. We employed a unique method involving polyurethane tubing to reduce stress and anxiety during nail procedures.
The Technique
A patient was receiving treatment with intralesional triamcinolone injections to the nail matrix for trachyonychia involving all of the fingernails. He worked as an equipment and facilities manager, giving him access to polyurethane tubing, which is routinely used in the manufacture of some medical devices that require gas or liquid to operate. He found the nail injections to be painful but was motivated to proceed with treatment. He brought in a piece of polyurethane tubing to a subsequent visit to bite on during the injections (Figure) and reported considerable relief of pain.

What you were not taught in United States history class was that this method—clenching an object orally—dates to the era before the Civil War, before appropriate anesthetics and analgesics were developed, when patients and soldiers bit on a bullet or leather strap during surgical procedures.4 Clenching and chewing have been shown to promote relaxation and reduce acute pain and stress.5
Practical Implications
Polyurethane tubing can be purchased in bulk, is inexpensive ($0.30/foot on Amazon), and unlikely to damage teeth due to its flexibility. It can be cut into 6-inch pieces and given to the patient at their first nail injection appointment. The patient can then bring the tubing to subsequent appointments to use as a mastication tool during nail injections.
We instruct the patient to disinfect the dedicated piece of tubing after the initial visit and each subsequent visit by soaking it for 15 minutes in either a 3% hydrogen peroxide solution, antibacterial mouthwash, a solution of baking soda (bicarbonate of soda) and water (1 cup of water to 2 teaspoons of baking soda), or white vinegar. We instruct them to thoroughly dry the disinfected polyurethane tube and store it in a clean, reusable, resealable zipper storage bag between appointments.
In addition to reducing anxiety and pain, this method also distracts the patient and therefore promotes patient and physician safety. Patients are less likely to jump or startle during the injection, thereby reducing the risk of physically interfering with the nail surgeon or making an unanticipated advance into the surgical field.
Although frustrated patients with nail disease may need to “bite the bullet” when they accept treatment with nail injections, lessons from our patient and from United States history offer a safe and cost-effective pain management strategy. Minimizing discomfort and anxiety during the first nail injection is crucial because doing so is likely to promote adherence with follow-up injections and therefore improve clinical outcomes.
Future clinical studies should validate the clinical utility of oral mastication and clenching during nail procedures compared to other perioperative stress- and anxiety-reducing techniques.
- Ricardo JW, Lipner SR. Utilization of a stress ball to diminish anxiety during nail surgery. Cutis. 2020;105:294. doi:10.12788/cutis.0013
- Ricardo JW, Lipner SR. Utilizing a sleep mask to reduce patient anxiety during nail surgery. Cutis. 2021;108:36. doi:10.12788/cutis.0285
- Ip HYV, Abrishami A, Peng PW, et al. Predictors of postoperative pain and analgesic consumption: a qualitative systematic review. Anesthesiology. 2009;111:657-677. doi:10.1097/ALN.0b013e3181aae87a
- Albin MS. The use of anesthetics during the Civil War, 1861-1865. Pharm Hist. 2000;42:99-114.
- Tahara Y, Sakurai K, Ando T. Influence of chewing and clenching on salivary cortisol levels as an indicator of stress. J Prosthodont. 2007;16:129-135. doi:10.1111/j.1532-849X.2007.00178.x
- Ricardo JW, Lipner SR. Utilization of a stress ball to diminish anxiety during nail surgery. Cutis. 2020;105:294. doi:10.12788/cutis.0013
- Ricardo JW, Lipner SR. Utilizing a sleep mask to reduce patient anxiety during nail surgery. Cutis. 2021;108:36. doi:10.12788/cutis.0285
- Ip HYV, Abrishami A, Peng PW, et al. Predictors of postoperative pain and analgesic consumption: a qualitative systematic review. Anesthesiology. 2009;111:657-677. doi:10.1097/ALN.0b013e3181aae87a
- Albin MS. The use of anesthetics during the Civil War, 1861-1865. Pharm Hist. 2000;42:99-114.
- Tahara Y, Sakurai K, Ando T. Influence of chewing and clenching on salivary cortisol levels as an indicator of stress. J Prosthodont. 2007;16:129-135. doi:10.1111/j.1532-849X.2007.00178.x
Artificial Intelligence vs Medical Providers in the Dermoscopic Diagnosis of Melanoma
The incidence of skin cancer continues to increase, and it is by far the most common malignancy in the United States. Based on the sheer incidence and prevalence of skin cancer, early detection and treatment are critical. Looking at melanoma alone, the 5-year survival rate is greater than 99% when detected early but falls to 71% when the disease reaches the lymph nodes and 32% with metastasis to distant organs.1 Furthermore, a 2018 study found stage I melanoma patients who were treated 4 months after biopsy had a 41% increased risk of death compared with those treated within the first month.2 However, many patients are not seen by a dermatologist first for examination of suspicious skin lesions and instead are referred by a general practitioner or primary care mid-level provider. Therefore, many patients experience a longer time to diagnosis or treatment, which directly correlates with survival rate.
Dermoscopy is a noninvasive diagnostic tool for skin lesions, including melanoma. Using a handheld dermoscope (or dermatoscope), a transilluminating light source magnifies skin lesions and allows for the visualization of subsurface skin structures within the epidermis, dermoepidermal junction, and papillary dermis.3 Dermoscopy has been shown to improve a dermatologist’s accuracy in diagnosing malignant melanoma vs clinical evaluation with the unaided eye.4,5 More recently, dermoscopy has been digitized, allowing for the collection and documentation of case photographs. Dermoscopy also has expanded past the scope of dermatologists and has become increasingly useful in primary care.6 Among family physicians, dermoscopy also has been shown to have a higher sensitivity for melanoma detection compared to gross examination.7 Therefore, both the increased diagnostic performance of malignant melanoma using a dermoscope and the expanded use of dermoscopy in medical care validate the evaluation of an artificial intelligence (AI) algorithm in diagnosing malignant melanoma using dermoscopic images.
Triage (Triage Technologies Inc) is an AI application that uses a web interface and combines a pretrained convolutional neural network (CNN) with a reinforcement learning agent as a question-answering model. The CNN algorithm can classify 133 different skin diseases, 7 of which it is able to classify using dermoscopic images. This study sought to evaluate the performance of Triage’s dermoscopic classifier in identifying lesions as benign or malignant to determine whether AI could assist in the triage of skin cancer cases to shorten time to diagnosis.
Materials and Methods
The MClass-D test set from the International Skin Imaging Collaboration was assessed by both AI and practicing medical providers. The set was composed of 80 benign nevi and 20 biopsy-verified malignant melanomas. Board-certified US dermatologists (n=23), family physicians (n=7), and primary care mid-level providers (n=12)(ie, nurse practitioners, physician assistants) were asked to label the images as benign or malignant. The results from the medical providers were then compared to the performance of the AI application by looking at the sensitivity, specificity, accuracy, positive predictive value (PPV), and negative predictive value (NPV). Statistical significance was determined with a 1 sample t test run through RStudio (Posit Software, PBC), and P<.05 was considered significant.
Results
The AI application performed extremely well in differentiating between benign nevi and malignant melanomas, with a sensitivity of 80%, specificity of 95%, accuracy of 92%, PPV of 80%, and NPV of 95% (Table 1). When compared with practicing medical providers, the AI performed significantly better in almost all categories (P<.05)(Figure 1). With all medical providers combined, the AI had significantly higher accuracy, sensitivity, and specificity (P<.05). The accuracy of the individual medical providers ranged from 32% to 78%.
Compared with dermatologists, the AI was significantly more specific and accurate and demonstrated a higher PPV and NPV (P<.05). There was no significant difference between the AI and dermatologists in sensitivity or labeling the true malignant lesions as malignant. The dermatologists who participated had been practicing from 1.5 years to 44 years, with an average of 16 years of dermatologic experience. There was no correlation between years practicing and performance in determining the malignancy of lesions. Of 14 dermatologists, dermoscopy was used daily by 10 and occasionally by 3, but only 6 dermatologists had any formal training. Dermatologists who used dermoscopy averaged 11 years of use.
The AI also performed significantly better than the primary care providers, including both family physicians and mid-level providers (P<.05). With the family physicians and mid-level provider scores combined, the AI showed a statistically significantly better performance in all categories examined, including sensitivity, specificity, accuracy, PPV, and NPV (P<.05). However, when compared with family physicians alone, the AI did not demonstrate a statistically significant difference in sensitivity.
Comment
Automatic Visual Recognition Development—The AI application we studied was developed by dermatologists as a tool to assist in the screening of skin lesions suspicious for melanoma or a benign neoplasm.8 Developing AI applications that can reliably recognize objects in photographs has been the subject of considerable research. Notable progress in automatic visual recognition was shown in 2012 when a deep learning model won the ImageNet object recognition challenge and outperformed competing approaches by a large margin.9,10 The ImageNet competition, which has been held annually since 2010, required participants to build a visual classification system that distinguished among 1000 object categories using 1.2 million labeled images as training data. In 2017, participants developed automated visual systems that surpassed the estimated human performance.11 Given this success, the organization decided to deliver a more challenging competition involving 3D imaging—Medical ImageNet, a petabyte-scale, cloud-based, open repository project—with goals including image classification and annotation.12
Convolutional Neural Networks—Convolutional neural networks are computer system architectures commonly employed for making predictions from images.13 Convolutional neural networks are based on a set of layers of learned filters that perform convolution, a mathematical operation that reflects the relationship between the 2 functions. The main algorithm that makes the learning possible is called backpropagation, wherein an error is computed at the output and distributed backward through the neural network’s layers.14 Although CNNs and backpropagation methods have existed since 1989, recent technologic advances have allowed for deep learning–based algorithms to be widely integrated with everyday applications.15 Advances in computational power in the form of graphics processing units and parallelization, the existence of large data sets such as the ImageNet database, and the rise of software frameworks have allowed for quick prototyping and deployment of deep learning models.16,17
Convolutional neural networks have demonstrated potential to excel at a wide range of visual tasks. In dermatology, visual recognition methods often rely on using either a pretrained CNN as a feature extractor for further classification or fine-tuning a pretrained network on dermoscopic images.18-20 In 2017, a model was trained on 130,000 clinical images of benign and malignant skin lesions. Its performance was found to be in line with that of 21 US board-certified dermatology experts when diagnosing skin cancers from clinical images confirmed by biopsy.21
Triage—The AI application Triage is composed of several components contained in a web interface (Figure 2). To use the interface, the user must sign up and upload a photograph to the website. The image first passes through a gated-logic visual classifier that rejects any images that do not contain a visible skin condition. If the image contains a skin condition, the image is passed to a skin classifier that predicts the probability of the image containing 1 of 133 classes of skin conditions, 7 of which the application can diagnose with a dermoscopic image.
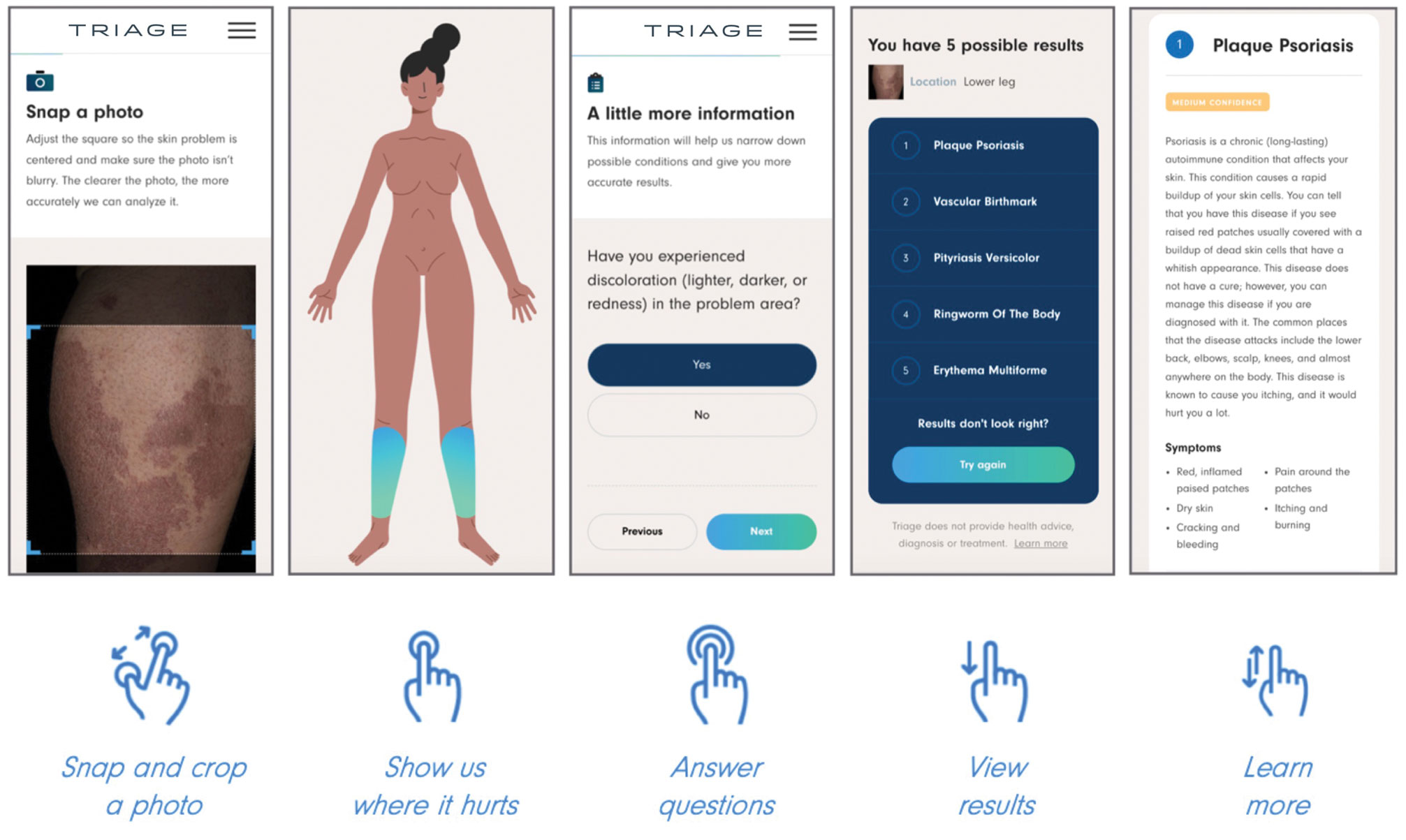
The AI application uses several techniques when training a CNN model. To address skin condition class imbalances (when more examples exist for 1 class than the others) in the training data, additional weights are applied to mistakes made on underrepresented classes, which encourages the model to better detect cases with low prevalence in the data set. Data augmentation techniques such as rotating, zooming, and flipping the training images are applied to allow the model to become more familiar with variability in the input images. Convolutional neural networks are trained using a well-known neural network optimization method called Stochastic gradient descent with momentum.22
The final predictions are refined by a question-and-answer system that encodes dermatology knowledge and is currently under active development. Finally, the top k most probable conditions are displayed to the user, where k≤5. An initial prototype of the system was described in a published research paper in the 2019 medical imaging workshop of the Neural Information Systems conference.23
The prototype demonstrated that combining a pretrained CNN with a reinforcement learning agent as a question-answering model increased the classification confidence and accuracy of its visual symptom checker and decreased the average number of questions asked to narrow down the differential diagnosis. The reinforcement learning approach increases the accuracy more than 20% compared with the CNN-only approach, which only uses visual information to predict the condition.23
This application’s current visual question-answering system is trained on a diverse set of data that includes more than 20 years of clinical encounters and user-uploaded cases submitted by more than 150,000 patients and 10,000 clinicians in more than 150 countries. All crowdsourced images used for training the dermoscopy classifier are biopsy-verified images contributed by dermatologists. These data are made up of case photographs that are tagged with metadata around the patient’s age, sex, symptoms, and diagnoses. The CNN algorithm used covers 133 skin disease classes, representing 588 clinical conditions. It also can automatically detect 7 malignant, premalignant, and benign dermoscopic categories, which is the focus of this study (Table 2). Diagnoses are verified by patient response to treatment, biopsy results, and dermatologist consensus.
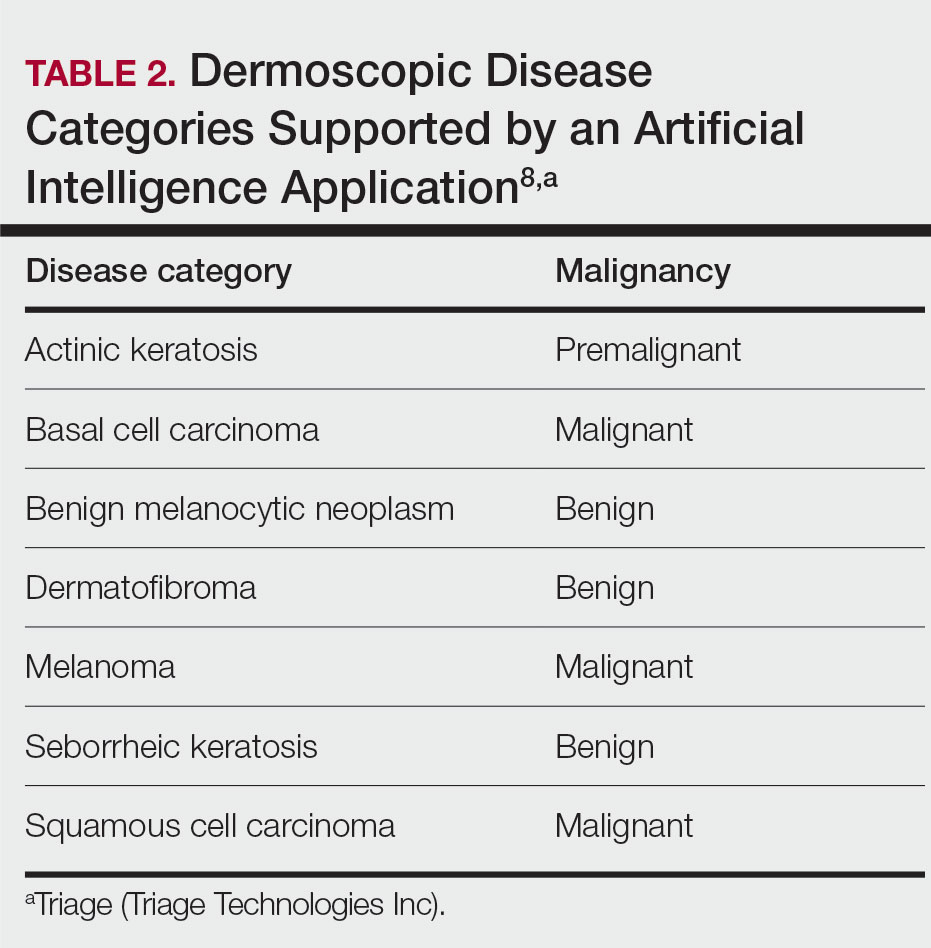
In addition to having improved performance, supporting more than 130 disease classes, and having a diverse data set, the application used has beat competing technologies.20,24 The application currently is available on the internet in more than 30 countries after it received Health Canada Class I medical device approval and the CE mark in Europe.
Can AI Reliably Detect Melanoma?—In our study, of the lesions labeled benign, the higher PPV and NPV of the AI algorithm means that the lesions were more reliably true benign lesions, and the lesions labeled as malignant were more likely to be true malignant lesions. Therefore, the diagnosis given by the AI compared with the medical provider was significantly more likely to be correct. These findings demonstrate that this AI application can reliably detect malignant melanoma using dermoscopic images. However, this study was limited by the small sample size of medical providers. Further studies are necessary to assess whether the high diagnostic accuracy of the application translates to expedited referrals and a decrease in unnecessary biopsies.
Dermoscopy Training—This study looked at dermoscopic images instead of gross examination, as is often done in clinic, which draws into question the dermoscopic training dermatologists receive. The diagnostic accuracy using dermoscopic images has been shown to be higher than evaluation with the naked eye.5,6 However, there currently is no standard for dermoscopic training in dermatology residencies, and education varies widely.25 These data suggest that there may be a lack of dermoscopic training among dermatologists, which could accentuate the difference in performance between dermatologists and AI. Most primary care providers also lack formal dermoscopy training. Although dermoscopy has been shown to increase the diagnostic efficacy of primary care providers, this increase does not become apparent until the medical provider has had years of formal training in addition to clinical experience, which is not commonly provided in the medical training that primary care providers receive.8,26
Conclusion
It is anticipated that AI will shape the future of medicine and become incorporated into daily practice.27 Artificial intelligence will not replace physicians but rather assist clinicians and help to streamline medical care. Clinicians will take on the role of interpreting AI output and integrate it into patient care. With this advancement, it is important to highlight that for AI to improve the quality, efficiency, and accessibility of health care, clinicians must be equipped with the right training.27-29
- Cancer facts & figures 2023. American Cancer Society. Accessed April 20, 2023. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2023/2023-cancer-facts-and-figures.pdf
- Conic RZ, Cabrera CI, Khorana AA, et al. Determination of the impact of melanoma surgical timing on survival using the National Cancer Database. J Am Acad Dermatol. 2018;78:40-46.e7. doi:10.1016/j.jaad.2017.08.039
- Lallas A, Zalaudek I, Argenziano G, et al. Dermoscopy in general dermatology. Dermatol Clin. 2013;31:679-694, x. doi:10.1016/j.det.2013.06.008
- Bafounta M-L, Beauchet A, Aegerter P, et al. Is dermoscopy (epiluminescence microscopy) useful for the diagnosis of melanoma?: results of a meta-analysis using techniques adapted to the evaluation of diagnostic tests. Arch Dermatol. 2001;137:1343-1350. doi:10.1001/archderm.137.10.1343
- Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676. doi:10.1111/j.1365-2133.2008.08713.x
- Marghoob AA, Usatine RP, Jaimes N. Dermoscopy for the family physician. Am Fam Physician. 2013;88:441-450.
- Herschorn A. Dermoscopy for melanoma detection in family practice. Can Fam Physician. 2012;58:740-745, e372-8.
- Instructions for use for the Triage app. Triage website. Accessed April 20, 2023. https://www.triage.com/pdf/en/Instructions%20for%20Use.pdf
- Krizhevsky A, Sutskever I, Hinton GE. ImageNet classification with deep convolutional neural networks. In: Pereira F, Burges CJC, Bottou L, et al, eds. Advances in Neural Information Processing Systems. Vol 25. Curran Associates, Inc; 2012. Accessed April 17, 2023. https://proceedings.neurips.cc/paper/2012/file/c399862d3b9d6b76c8436e924a68c45b-Paper.pdf
- Russakovsky O, Deng J, Su H, et al. ImageNet large scale visualrecognition challenge. Int J Comput Vis. 2015;115:211-252. doi:10.1007/s11263-015-0816-y
- Hu J, Shen L, Albanie S, et al. Squeeze-and-excitation networks. IEEE Trans Patt Anal Mach Intell. 2020;42:2011-2023. doi:10.1109/TPAMI.2019.2913372
- Medical image net-radiology informatics. Stanford University Center for Artificial Intelligence in Medicine & Imaging website. Accessed April 20, 2023. https://aimi.stanford.edu/medical-imagenet
- LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521:436-444. doi:10.1038/nature14539
- Le Cun Yet al. A theoretical framework for back-propagation. In:Touretzky D, Honton G, Sejnowski T, eds. Proceedings of the 1988 Connect Models Summer School. Morgan Kaufmann; 1988:21-28.
- Lecun Y, Bottou L, Bengio Y, et al. Gradient-based learning applied to document recognition. Proc IEEE. 1998;86:2278-2324. doi:10.1109/5.726791
- Chollet E. About Keras. Keras website. Accessed April 21, 2023. https://keras.io/about/
- Introduction to TensorFlow. TensorFlow website. Accessed April 21, 2023. https://www.tensorflow.org/learn
- Kawahara J, BenTaieb A, Hamarneh G. Deep features to classify skin lesions. 2016 IEEE 13th International Symposium on Biomedical Imaging. 2016. doi:10.1109/ISBI.2016.7493528
- Lopez AR, Giro-i-Nieto X, Burdick J, et al. Skin lesion classification from dermoscopic images using deep learning techniques. doi:10.2316/P.2017.852-053
- Codella NCF, Nguyen QB, Pankanti S, et al. Deep learning ensembles for melanoma recognition in dermoscopy images. IBM J Res Dev. 2017;61:1-28. doi:10.1147/JRD.2017.2708299
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115-118. doi:10.1038/nature21056
- Sutskever I, Martens J, Dahl G, et al. On the importance of initialization and momentum in deep learning. ICML’13: Proceedings of the 30th International Conference on International Conference on Machine Learning. 2013;28:1139-1147.
- Akrout M, Farahmand AM, Jarmain T, et al. Improving skin condition classification with a visual symptom checker trained using reinforcement learning. In: Medical Image Computing and Computer Assisted Intervention – MICCAI 2019: 22nd International Conference. October 13-17, 2019. Shenzhen, China. Proceedings, Part IV. Springer-Verlag; 549-557. doi:10.1007/978-3-030-32251-9_60
- Liu Y, Jain A, Eng C, et al. A deep learning system for differential diagnosis of skin diseases. Nat Med. 2020;26:900-908. doi:10.1038/s41591-020-0842-3
- Fried LJ, Tan A, Berry EG, et al. Dermoscopy proficiency expectations for US dermatology resident physicians: results of a modified delphi survey of pigmented lesion experts. JAMA Dermatol. 2021;157:189-197. doi:10.1001/jamadermatol.2020.5213
- Fee JA, McGrady FP, Rosendahl C, et al. Training primary care physicians in dermoscopy for skin cancer detection: a scoping review. J Cancer Educ. 2020;35:643-650. doi:10.1007/s13187-019-01647-7
- James CA, Wachter RM, Woolliscroft JO. Preparing clinicians for a clinical world influenced by artificial intelligence. JAMA. 2022;327:1333-1334. doi:10.1001/jama.2022.3580
- Yu K-H, Beam AL, Kohane IS. Artificial intelligence in healthcare. Nat Biomed Eng. 2018;2:719-731. doi:10.1038/s41551-018-0305-z
- Chen M, Decary M. Artificial intelligence in healthcare: an essential guide for health leaders. Healthc Manag Forum. 2020;33:10-18. doi:10.1177/0840470419873123
The incidence of skin cancer continues to increase, and it is by far the most common malignancy in the United States. Based on the sheer incidence and prevalence of skin cancer, early detection and treatment are critical. Looking at melanoma alone, the 5-year survival rate is greater than 99% when detected early but falls to 71% when the disease reaches the lymph nodes and 32% with metastasis to distant organs.1 Furthermore, a 2018 study found stage I melanoma patients who were treated 4 months after biopsy had a 41% increased risk of death compared with those treated within the first month.2 However, many patients are not seen by a dermatologist first for examination of suspicious skin lesions and instead are referred by a general practitioner or primary care mid-level provider. Therefore, many patients experience a longer time to diagnosis or treatment, which directly correlates with survival rate.
Dermoscopy is a noninvasive diagnostic tool for skin lesions, including melanoma. Using a handheld dermoscope (or dermatoscope), a transilluminating light source magnifies skin lesions and allows for the visualization of subsurface skin structures within the epidermis, dermoepidermal junction, and papillary dermis.3 Dermoscopy has been shown to improve a dermatologist’s accuracy in diagnosing malignant melanoma vs clinical evaluation with the unaided eye.4,5 More recently, dermoscopy has been digitized, allowing for the collection and documentation of case photographs. Dermoscopy also has expanded past the scope of dermatologists and has become increasingly useful in primary care.6 Among family physicians, dermoscopy also has been shown to have a higher sensitivity for melanoma detection compared to gross examination.7 Therefore, both the increased diagnostic performance of malignant melanoma using a dermoscope and the expanded use of dermoscopy in medical care validate the evaluation of an artificial intelligence (AI) algorithm in diagnosing malignant melanoma using dermoscopic images.
Triage (Triage Technologies Inc) is an AI application that uses a web interface and combines a pretrained convolutional neural network (CNN) with a reinforcement learning agent as a question-answering model. The CNN algorithm can classify 133 different skin diseases, 7 of which it is able to classify using dermoscopic images. This study sought to evaluate the performance of Triage’s dermoscopic classifier in identifying lesions as benign or malignant to determine whether AI could assist in the triage of skin cancer cases to shorten time to diagnosis.
Materials and Methods
The MClass-D test set from the International Skin Imaging Collaboration was assessed by both AI and practicing medical providers. The set was composed of 80 benign nevi and 20 biopsy-verified malignant melanomas. Board-certified US dermatologists (n=23), family physicians (n=7), and primary care mid-level providers (n=12)(ie, nurse practitioners, physician assistants) were asked to label the images as benign or malignant. The results from the medical providers were then compared to the performance of the AI application by looking at the sensitivity, specificity, accuracy, positive predictive value (PPV), and negative predictive value (NPV). Statistical significance was determined with a 1 sample t test run through RStudio (Posit Software, PBC), and P<.05 was considered significant.

Results
The AI application performed extremely well in differentiating between benign nevi and malignant melanomas, with a sensitivity of 80%, specificity of 95%, accuracy of 92%, PPV of 80%, and NPV of 95% (Table 1). When compared with practicing medical providers, the AI performed significantly better in almost all categories (P<.05)(Figure 1). With all medical providers combined, the AI had significantly higher accuracy, sensitivity, and specificity (P<.05). The accuracy of the individual medical providers ranged from 32% to 78%.

Compared with dermatologists, the AI was significantly more specific and accurate and demonstrated a higher PPV and NPV (P<.05). There was no significant difference between the AI and dermatologists in sensitivity or labeling the true malignant lesions as malignant. The dermatologists who participated had been practicing from 1.5 years to 44 years, with an average of 16 years of dermatologic experience. There was no correlation between years practicing and performance in determining the malignancy of lesions. Of 14 dermatologists, dermoscopy was used daily by 10 and occasionally by 3, but only 6 dermatologists had any formal training. Dermatologists who used dermoscopy averaged 11 years of use.
The AI also performed significantly better than the primary care providers, including both family physicians and mid-level providers (P<.05). With the family physicians and mid-level provider scores combined, the AI showed a statistically significantly better performance in all categories examined, including sensitivity, specificity, accuracy, PPV, and NPV (P<.05). However, when compared with family physicians alone, the AI did not demonstrate a statistically significant difference in sensitivity.
Comment
Automatic Visual Recognition Development—The AI application we studied was developed by dermatologists as a tool to assist in the screening of skin lesions suspicious for melanoma or a benign neoplasm.8 Developing AI applications that can reliably recognize objects in photographs has been the subject of considerable research. Notable progress in automatic visual recognition was shown in 2012 when a deep learning model won the ImageNet object recognition challenge and outperformed competing approaches by a large margin.9,10 The ImageNet competition, which has been held annually since 2010, required participants to build a visual classification system that distinguished among 1000 object categories using 1.2 million labeled images as training data. In 2017, participants developed automated visual systems that surpassed the estimated human performance.11 Given this success, the organization decided to deliver a more challenging competition involving 3D imaging—Medical ImageNet, a petabyte-scale, cloud-based, open repository project—with goals including image classification and annotation.12
Convolutional Neural Networks—Convolutional neural networks are computer system architectures commonly employed for making predictions from images.13 Convolutional neural networks are based on a set of layers of learned filters that perform convolution, a mathematical operation that reflects the relationship between the 2 functions. The main algorithm that makes the learning possible is called backpropagation, wherein an error is computed at the output and distributed backward through the neural network’s layers.14 Although CNNs and backpropagation methods have existed since 1989, recent technologic advances have allowed for deep learning–based algorithms to be widely integrated with everyday applications.15 Advances in computational power in the form of graphics processing units and parallelization, the existence of large data sets such as the ImageNet database, and the rise of software frameworks have allowed for quick prototyping and deployment of deep learning models.16,17
Convolutional neural networks have demonstrated potential to excel at a wide range of visual tasks. In dermatology, visual recognition methods often rely on using either a pretrained CNN as a feature extractor for further classification or fine-tuning a pretrained network on dermoscopic images.18-20 In 2017, a model was trained on 130,000 clinical images of benign and malignant skin lesions. Its performance was found to be in line with that of 21 US board-certified dermatology experts when diagnosing skin cancers from clinical images confirmed by biopsy.21
Triage—The AI application Triage is composed of several components contained in a web interface (Figure 2). To use the interface, the user must sign up and upload a photograph to the website. The image first passes through a gated-logic visual classifier that rejects any images that do not contain a visible skin condition. If the image contains a skin condition, the image is passed to a skin classifier that predicts the probability of the image containing 1 of 133 classes of skin conditions, 7 of which the application can diagnose with a dermoscopic image.

The AI application uses several techniques when training a CNN model. To address skin condition class imbalances (when more examples exist for 1 class than the others) in the training data, additional weights are applied to mistakes made on underrepresented classes, which encourages the model to better detect cases with low prevalence in the data set. Data augmentation techniques such as rotating, zooming, and flipping the training images are applied to allow the model to become more familiar with variability in the input images. Convolutional neural networks are trained using a well-known neural network optimization method called Stochastic gradient descent with momentum.22
The final predictions are refined by a question-and-answer system that encodes dermatology knowledge and is currently under active development. Finally, the top k most probable conditions are displayed to the user, where k≤5. An initial prototype of the system was described in a published research paper in the 2019 medical imaging workshop of the Neural Information Systems conference.23
The prototype demonstrated that combining a pretrained CNN with a reinforcement learning agent as a question-answering model increased the classification confidence and accuracy of its visual symptom checker and decreased the average number of questions asked to narrow down the differential diagnosis. The reinforcement learning approach increases the accuracy more than 20% compared with the CNN-only approach, which only uses visual information to predict the condition.23
This application’s current visual question-answering system is trained on a diverse set of data that includes more than 20 years of clinical encounters and user-uploaded cases submitted by more than 150,000 patients and 10,000 clinicians in more than 150 countries. All crowdsourced images used for training the dermoscopy classifier are biopsy-verified images contributed by dermatologists. These data are made up of case photographs that are tagged with metadata around the patient’s age, sex, symptoms, and diagnoses. The CNN algorithm used covers 133 skin disease classes, representing 588 clinical conditions. It also can automatically detect 7 malignant, premalignant, and benign dermoscopic categories, which is the focus of this study (Table 2). Diagnoses are verified by patient response to treatment, biopsy results, and dermatologist consensus.

In addition to having improved performance, supporting more than 130 disease classes, and having a diverse data set, the application used has beat competing technologies.20,24 The application currently is available on the internet in more than 30 countries after it received Health Canada Class I medical device approval and the CE mark in Europe.
Can AI Reliably Detect Melanoma?—In our study, of the lesions labeled benign, the higher PPV and NPV of the AI algorithm means that the lesions were more reliably true benign lesions, and the lesions labeled as malignant were more likely to be true malignant lesions. Therefore, the diagnosis given by the AI compared with the medical provider was significantly more likely to be correct. These findings demonstrate that this AI application can reliably detect malignant melanoma using dermoscopic images. However, this study was limited by the small sample size of medical providers. Further studies are necessary to assess whether the high diagnostic accuracy of the application translates to expedited referrals and a decrease in unnecessary biopsies.
Dermoscopy Training—This study looked at dermoscopic images instead of gross examination, as is often done in clinic, which draws into question the dermoscopic training dermatologists receive. The diagnostic accuracy using dermoscopic images has been shown to be higher than evaluation with the naked eye.5,6 However, there currently is no standard for dermoscopic training in dermatology residencies, and education varies widely.25 These data suggest that there may be a lack of dermoscopic training among dermatologists, which could accentuate the difference in performance between dermatologists and AI. Most primary care providers also lack formal dermoscopy training. Although dermoscopy has been shown to increase the diagnostic efficacy of primary care providers, this increase does not become apparent until the medical provider has had years of formal training in addition to clinical experience, which is not commonly provided in the medical training that primary care providers receive.8,26
Conclusion
It is anticipated that AI will shape the future of medicine and become incorporated into daily practice.27 Artificial intelligence will not replace physicians but rather assist clinicians and help to streamline medical care. Clinicians will take on the role of interpreting AI output and integrate it into patient care. With this advancement, it is important to highlight that for AI to improve the quality, efficiency, and accessibility of health care, clinicians must be equipped with the right training.27-29
The incidence of skin cancer continues to increase, and it is by far the most common malignancy in the United States. Based on the sheer incidence and prevalence of skin cancer, early detection and treatment are critical. Looking at melanoma alone, the 5-year survival rate is greater than 99% when detected early but falls to 71% when the disease reaches the lymph nodes and 32% with metastasis to distant organs.1 Furthermore, a 2018 study found stage I melanoma patients who were treated 4 months after biopsy had a 41% increased risk of death compared with those treated within the first month.2 However, many patients are not seen by a dermatologist first for examination of suspicious skin lesions and instead are referred by a general practitioner or primary care mid-level provider. Therefore, many patients experience a longer time to diagnosis or treatment, which directly correlates with survival rate.
Dermoscopy is a noninvasive diagnostic tool for skin lesions, including melanoma. Using a handheld dermoscope (or dermatoscope), a transilluminating light source magnifies skin lesions and allows for the visualization of subsurface skin structures within the epidermis, dermoepidermal junction, and papillary dermis.3 Dermoscopy has been shown to improve a dermatologist’s accuracy in diagnosing malignant melanoma vs clinical evaluation with the unaided eye.4,5 More recently, dermoscopy has been digitized, allowing for the collection and documentation of case photographs. Dermoscopy also has expanded past the scope of dermatologists and has become increasingly useful in primary care.6 Among family physicians, dermoscopy also has been shown to have a higher sensitivity for melanoma detection compared to gross examination.7 Therefore, both the increased diagnostic performance of malignant melanoma using a dermoscope and the expanded use of dermoscopy in medical care validate the evaluation of an artificial intelligence (AI) algorithm in diagnosing malignant melanoma using dermoscopic images.
Triage (Triage Technologies Inc) is an AI application that uses a web interface and combines a pretrained convolutional neural network (CNN) with a reinforcement learning agent as a question-answering model. The CNN algorithm can classify 133 different skin diseases, 7 of which it is able to classify using dermoscopic images. This study sought to evaluate the performance of Triage’s dermoscopic classifier in identifying lesions as benign or malignant to determine whether AI could assist in the triage of skin cancer cases to shorten time to diagnosis.
Materials and Methods
The MClass-D test set from the International Skin Imaging Collaboration was assessed by both AI and practicing medical providers. The set was composed of 80 benign nevi and 20 biopsy-verified malignant melanomas. Board-certified US dermatologists (n=23), family physicians (n=7), and primary care mid-level providers (n=12)(ie, nurse practitioners, physician assistants) were asked to label the images as benign or malignant. The results from the medical providers were then compared to the performance of the AI application by looking at the sensitivity, specificity, accuracy, positive predictive value (PPV), and negative predictive value (NPV). Statistical significance was determined with a 1 sample t test run through RStudio (Posit Software, PBC), and P<.05 was considered significant.

Results
The AI application performed extremely well in differentiating between benign nevi and malignant melanomas, with a sensitivity of 80%, specificity of 95%, accuracy of 92%, PPV of 80%, and NPV of 95% (Table 1). When compared with practicing medical providers, the AI performed significantly better in almost all categories (P<.05)(Figure 1). With all medical providers combined, the AI had significantly higher accuracy, sensitivity, and specificity (P<.05). The accuracy of the individual medical providers ranged from 32% to 78%.

Compared with dermatologists, the AI was significantly more specific and accurate and demonstrated a higher PPV and NPV (P<.05). There was no significant difference between the AI and dermatologists in sensitivity or labeling the true malignant lesions as malignant. The dermatologists who participated had been practicing from 1.5 years to 44 years, with an average of 16 years of dermatologic experience. There was no correlation between years practicing and performance in determining the malignancy of lesions. Of 14 dermatologists, dermoscopy was used daily by 10 and occasionally by 3, but only 6 dermatologists had any formal training. Dermatologists who used dermoscopy averaged 11 years of use.
The AI also performed significantly better than the primary care providers, including both family physicians and mid-level providers (P<.05). With the family physicians and mid-level provider scores combined, the AI showed a statistically significantly better performance in all categories examined, including sensitivity, specificity, accuracy, PPV, and NPV (P<.05). However, when compared with family physicians alone, the AI did not demonstrate a statistically significant difference in sensitivity.
Comment
Automatic Visual Recognition Development—The AI application we studied was developed by dermatologists as a tool to assist in the screening of skin lesions suspicious for melanoma or a benign neoplasm.8 Developing AI applications that can reliably recognize objects in photographs has been the subject of considerable research. Notable progress in automatic visual recognition was shown in 2012 when a deep learning model won the ImageNet object recognition challenge and outperformed competing approaches by a large margin.9,10 The ImageNet competition, which has been held annually since 2010, required participants to build a visual classification system that distinguished among 1000 object categories using 1.2 million labeled images as training data. In 2017, participants developed automated visual systems that surpassed the estimated human performance.11 Given this success, the organization decided to deliver a more challenging competition involving 3D imaging—Medical ImageNet, a petabyte-scale, cloud-based, open repository project—with goals including image classification and annotation.12
Convolutional Neural Networks—Convolutional neural networks are computer system architectures commonly employed for making predictions from images.13 Convolutional neural networks are based on a set of layers of learned filters that perform convolution, a mathematical operation that reflects the relationship between the 2 functions. The main algorithm that makes the learning possible is called backpropagation, wherein an error is computed at the output and distributed backward through the neural network’s layers.14 Although CNNs and backpropagation methods have existed since 1989, recent technologic advances have allowed for deep learning–based algorithms to be widely integrated with everyday applications.15 Advances in computational power in the form of graphics processing units and parallelization, the existence of large data sets such as the ImageNet database, and the rise of software frameworks have allowed for quick prototyping and deployment of deep learning models.16,17
Convolutional neural networks have demonstrated potential to excel at a wide range of visual tasks. In dermatology, visual recognition methods often rely on using either a pretrained CNN as a feature extractor for further classification or fine-tuning a pretrained network on dermoscopic images.18-20 In 2017, a model was trained on 130,000 clinical images of benign and malignant skin lesions. Its performance was found to be in line with that of 21 US board-certified dermatology experts when diagnosing skin cancers from clinical images confirmed by biopsy.21
Triage—The AI application Triage is composed of several components contained in a web interface (Figure 2). To use the interface, the user must sign up and upload a photograph to the website. The image first passes through a gated-logic visual classifier that rejects any images that do not contain a visible skin condition. If the image contains a skin condition, the image is passed to a skin classifier that predicts the probability of the image containing 1 of 133 classes of skin conditions, 7 of which the application can diagnose with a dermoscopic image.

The AI application uses several techniques when training a CNN model. To address skin condition class imbalances (when more examples exist for 1 class than the others) in the training data, additional weights are applied to mistakes made on underrepresented classes, which encourages the model to better detect cases with low prevalence in the data set. Data augmentation techniques such as rotating, zooming, and flipping the training images are applied to allow the model to become more familiar with variability in the input images. Convolutional neural networks are trained using a well-known neural network optimization method called Stochastic gradient descent with momentum.22
The final predictions are refined by a question-and-answer system that encodes dermatology knowledge and is currently under active development. Finally, the top k most probable conditions are displayed to the user, where k≤5. An initial prototype of the system was described in a published research paper in the 2019 medical imaging workshop of the Neural Information Systems conference.23
The prototype demonstrated that combining a pretrained CNN with a reinforcement learning agent as a question-answering model increased the classification confidence and accuracy of its visual symptom checker and decreased the average number of questions asked to narrow down the differential diagnosis. The reinforcement learning approach increases the accuracy more than 20% compared with the CNN-only approach, which only uses visual information to predict the condition.23
This application’s current visual question-answering system is trained on a diverse set of data that includes more than 20 years of clinical encounters and user-uploaded cases submitted by more than 150,000 patients and 10,000 clinicians in more than 150 countries. All crowdsourced images used for training the dermoscopy classifier are biopsy-verified images contributed by dermatologists. These data are made up of case photographs that are tagged with metadata around the patient’s age, sex, symptoms, and diagnoses. The CNN algorithm used covers 133 skin disease classes, representing 588 clinical conditions. It also can automatically detect 7 malignant, premalignant, and benign dermoscopic categories, which is the focus of this study (Table 2). Diagnoses are verified by patient response to treatment, biopsy results, and dermatologist consensus.

In addition to having improved performance, supporting more than 130 disease classes, and having a diverse data set, the application used has beat competing technologies.20,24 The application currently is available on the internet in more than 30 countries after it received Health Canada Class I medical device approval and the CE mark in Europe.
Can AI Reliably Detect Melanoma?—In our study, of the lesions labeled benign, the higher PPV and NPV of the AI algorithm means that the lesions were more reliably true benign lesions, and the lesions labeled as malignant were more likely to be true malignant lesions. Therefore, the diagnosis given by the AI compared with the medical provider was significantly more likely to be correct. These findings demonstrate that this AI application can reliably detect malignant melanoma using dermoscopic images. However, this study was limited by the small sample size of medical providers. Further studies are necessary to assess whether the high diagnostic accuracy of the application translates to expedited referrals and a decrease in unnecessary biopsies.
Dermoscopy Training—This study looked at dermoscopic images instead of gross examination, as is often done in clinic, which draws into question the dermoscopic training dermatologists receive. The diagnostic accuracy using dermoscopic images has been shown to be higher than evaluation with the naked eye.5,6 However, there currently is no standard for dermoscopic training in dermatology residencies, and education varies widely.25 These data suggest that there may be a lack of dermoscopic training among dermatologists, which could accentuate the difference in performance between dermatologists and AI. Most primary care providers also lack formal dermoscopy training. Although dermoscopy has been shown to increase the diagnostic efficacy of primary care providers, this increase does not become apparent until the medical provider has had years of formal training in addition to clinical experience, which is not commonly provided in the medical training that primary care providers receive.8,26
Conclusion
It is anticipated that AI will shape the future of medicine and become incorporated into daily practice.27 Artificial intelligence will not replace physicians but rather assist clinicians and help to streamline medical care. Clinicians will take on the role of interpreting AI output and integrate it into patient care. With this advancement, it is important to highlight that for AI to improve the quality, efficiency, and accessibility of health care, clinicians must be equipped with the right training.27-29
- Cancer facts & figures 2023. American Cancer Society. Accessed April 20, 2023. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2023/2023-cancer-facts-and-figures.pdf
- Conic RZ, Cabrera CI, Khorana AA, et al. Determination of the impact of melanoma surgical timing on survival using the National Cancer Database. J Am Acad Dermatol. 2018;78:40-46.e7. doi:10.1016/j.jaad.2017.08.039
- Lallas A, Zalaudek I, Argenziano G, et al. Dermoscopy in general dermatology. Dermatol Clin. 2013;31:679-694, x. doi:10.1016/j.det.2013.06.008
- Bafounta M-L, Beauchet A, Aegerter P, et al. Is dermoscopy (epiluminescence microscopy) useful for the diagnosis of melanoma?: results of a meta-analysis using techniques adapted to the evaluation of diagnostic tests. Arch Dermatol. 2001;137:1343-1350. doi:10.1001/archderm.137.10.1343
- Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676. doi:10.1111/j.1365-2133.2008.08713.x
- Marghoob AA, Usatine RP, Jaimes N. Dermoscopy for the family physician. Am Fam Physician. 2013;88:441-450.
- Herschorn A. Dermoscopy for melanoma detection in family practice. Can Fam Physician. 2012;58:740-745, e372-8.
- Instructions for use for the Triage app. Triage website. Accessed April 20, 2023. https://www.triage.com/pdf/en/Instructions%20for%20Use.pdf
- Krizhevsky A, Sutskever I, Hinton GE. ImageNet classification with deep convolutional neural networks. In: Pereira F, Burges CJC, Bottou L, et al, eds. Advances in Neural Information Processing Systems. Vol 25. Curran Associates, Inc; 2012. Accessed April 17, 2023. https://proceedings.neurips.cc/paper/2012/file/c399862d3b9d6b76c8436e924a68c45b-Paper.pdf
- Russakovsky O, Deng J, Su H, et al. ImageNet large scale visualrecognition challenge. Int J Comput Vis. 2015;115:211-252. doi:10.1007/s11263-015-0816-y
- Hu J, Shen L, Albanie S, et al. Squeeze-and-excitation networks. IEEE Trans Patt Anal Mach Intell. 2020;42:2011-2023. doi:10.1109/TPAMI.2019.2913372
- Medical image net-radiology informatics. Stanford University Center for Artificial Intelligence in Medicine & Imaging website. Accessed April 20, 2023. https://aimi.stanford.edu/medical-imagenet
- LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521:436-444. doi:10.1038/nature14539
- Le Cun Yet al. A theoretical framework for back-propagation. In:Touretzky D, Honton G, Sejnowski T, eds. Proceedings of the 1988 Connect Models Summer School. Morgan Kaufmann; 1988:21-28.
- Lecun Y, Bottou L, Bengio Y, et al. Gradient-based learning applied to document recognition. Proc IEEE. 1998;86:2278-2324. doi:10.1109/5.726791
- Chollet E. About Keras. Keras website. Accessed April 21, 2023. https://keras.io/about/
- Introduction to TensorFlow. TensorFlow website. Accessed April 21, 2023. https://www.tensorflow.org/learn
- Kawahara J, BenTaieb A, Hamarneh G. Deep features to classify skin lesions. 2016 IEEE 13th International Symposium on Biomedical Imaging. 2016. doi:10.1109/ISBI.2016.7493528
- Lopez AR, Giro-i-Nieto X, Burdick J, et al. Skin lesion classification from dermoscopic images using deep learning techniques. doi:10.2316/P.2017.852-053
- Codella NCF, Nguyen QB, Pankanti S, et al. Deep learning ensembles for melanoma recognition in dermoscopy images. IBM J Res Dev. 2017;61:1-28. doi:10.1147/JRD.2017.2708299
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115-118. doi:10.1038/nature21056
- Sutskever I, Martens J, Dahl G, et al. On the importance of initialization and momentum in deep learning. ICML’13: Proceedings of the 30th International Conference on International Conference on Machine Learning. 2013;28:1139-1147.
- Akrout M, Farahmand AM, Jarmain T, et al. Improving skin condition classification with a visual symptom checker trained using reinforcement learning. In: Medical Image Computing and Computer Assisted Intervention – MICCAI 2019: 22nd International Conference. October 13-17, 2019. Shenzhen, China. Proceedings, Part IV. Springer-Verlag; 549-557. doi:10.1007/978-3-030-32251-9_60
- Liu Y, Jain A, Eng C, et al. A deep learning system for differential diagnosis of skin diseases. Nat Med. 2020;26:900-908. doi:10.1038/s41591-020-0842-3
- Fried LJ, Tan A, Berry EG, et al. Dermoscopy proficiency expectations for US dermatology resident physicians: results of a modified delphi survey of pigmented lesion experts. JAMA Dermatol. 2021;157:189-197. doi:10.1001/jamadermatol.2020.5213
- Fee JA, McGrady FP, Rosendahl C, et al. Training primary care physicians in dermoscopy for skin cancer detection: a scoping review. J Cancer Educ. 2020;35:643-650. doi:10.1007/s13187-019-01647-7
- James CA, Wachter RM, Woolliscroft JO. Preparing clinicians for a clinical world influenced by artificial intelligence. JAMA. 2022;327:1333-1334. doi:10.1001/jama.2022.3580
- Yu K-H, Beam AL, Kohane IS. Artificial intelligence in healthcare. Nat Biomed Eng. 2018;2:719-731. doi:10.1038/s41551-018-0305-z
- Chen M, Decary M. Artificial intelligence in healthcare: an essential guide for health leaders. Healthc Manag Forum. 2020;33:10-18. doi:10.1177/0840470419873123
- Cancer facts & figures 2023. American Cancer Society. Accessed April 20, 2023. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2023/2023-cancer-facts-and-figures.pdf
- Conic RZ, Cabrera CI, Khorana AA, et al. Determination of the impact of melanoma surgical timing on survival using the National Cancer Database. J Am Acad Dermatol. 2018;78:40-46.e7. doi:10.1016/j.jaad.2017.08.039
- Lallas A, Zalaudek I, Argenziano G, et al. Dermoscopy in general dermatology. Dermatol Clin. 2013;31:679-694, x. doi:10.1016/j.det.2013.06.008
- Bafounta M-L, Beauchet A, Aegerter P, et al. Is dermoscopy (epiluminescence microscopy) useful for the diagnosis of melanoma?: results of a meta-analysis using techniques adapted to the evaluation of diagnostic tests. Arch Dermatol. 2001;137:1343-1350. doi:10.1001/archderm.137.10.1343
- Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676. doi:10.1111/j.1365-2133.2008.08713.x
- Marghoob AA, Usatine RP, Jaimes N. Dermoscopy for the family physician. Am Fam Physician. 2013;88:441-450.
- Herschorn A. Dermoscopy for melanoma detection in family practice. Can Fam Physician. 2012;58:740-745, e372-8.
- Instructions for use for the Triage app. Triage website. Accessed April 20, 2023. https://www.triage.com/pdf/en/Instructions%20for%20Use.pdf
- Krizhevsky A, Sutskever I, Hinton GE. ImageNet classification with deep convolutional neural networks. In: Pereira F, Burges CJC, Bottou L, et al, eds. Advances in Neural Information Processing Systems. Vol 25. Curran Associates, Inc; 2012. Accessed April 17, 2023. https://proceedings.neurips.cc/paper/2012/file/c399862d3b9d6b76c8436e924a68c45b-Paper.pdf
- Russakovsky O, Deng J, Su H, et al. ImageNet large scale visualrecognition challenge. Int J Comput Vis. 2015;115:211-252. doi:10.1007/s11263-015-0816-y
- Hu J, Shen L, Albanie S, et al. Squeeze-and-excitation networks. IEEE Trans Patt Anal Mach Intell. 2020;42:2011-2023. doi:10.1109/TPAMI.2019.2913372
- Medical image net-radiology informatics. Stanford University Center for Artificial Intelligence in Medicine & Imaging website. Accessed April 20, 2023. https://aimi.stanford.edu/medical-imagenet
- LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521:436-444. doi:10.1038/nature14539
- Le Cun Yet al. A theoretical framework for back-propagation. In:Touretzky D, Honton G, Sejnowski T, eds. Proceedings of the 1988 Connect Models Summer School. Morgan Kaufmann; 1988:21-28.
- Lecun Y, Bottou L, Bengio Y, et al. Gradient-based learning applied to document recognition. Proc IEEE. 1998;86:2278-2324. doi:10.1109/5.726791
- Chollet E. About Keras. Keras website. Accessed April 21, 2023. https://keras.io/about/
- Introduction to TensorFlow. TensorFlow website. Accessed April 21, 2023. https://www.tensorflow.org/learn
- Kawahara J, BenTaieb A, Hamarneh G. Deep features to classify skin lesions. 2016 IEEE 13th International Symposium on Biomedical Imaging. 2016. doi:10.1109/ISBI.2016.7493528
- Lopez AR, Giro-i-Nieto X, Burdick J, et al. Skin lesion classification from dermoscopic images using deep learning techniques. doi:10.2316/P.2017.852-053
- Codella NCF, Nguyen QB, Pankanti S, et al. Deep learning ensembles for melanoma recognition in dermoscopy images. IBM J Res Dev. 2017;61:1-28. doi:10.1147/JRD.2017.2708299
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115-118. doi:10.1038/nature21056
- Sutskever I, Martens J, Dahl G, et al. On the importance of initialization and momentum in deep learning. ICML’13: Proceedings of the 30th International Conference on International Conference on Machine Learning. 2013;28:1139-1147.
- Akrout M, Farahmand AM, Jarmain T, et al. Improving skin condition classification with a visual symptom checker trained using reinforcement learning. In: Medical Image Computing and Computer Assisted Intervention – MICCAI 2019: 22nd International Conference. October 13-17, 2019. Shenzhen, China. Proceedings, Part IV. Springer-Verlag; 549-557. doi:10.1007/978-3-030-32251-9_60
- Liu Y, Jain A, Eng C, et al. A deep learning system for differential diagnosis of skin diseases. Nat Med. 2020;26:900-908. doi:10.1038/s41591-020-0842-3
- Fried LJ, Tan A, Berry EG, et al. Dermoscopy proficiency expectations for US dermatology resident physicians: results of a modified delphi survey of pigmented lesion experts. JAMA Dermatol. 2021;157:189-197. doi:10.1001/jamadermatol.2020.5213
- Fee JA, McGrady FP, Rosendahl C, et al. Training primary care physicians in dermoscopy for skin cancer detection: a scoping review. J Cancer Educ. 2020;35:643-650. doi:10.1007/s13187-019-01647-7
- James CA, Wachter RM, Woolliscroft JO. Preparing clinicians for a clinical world influenced by artificial intelligence. JAMA. 2022;327:1333-1334. doi:10.1001/jama.2022.3580
- Yu K-H, Beam AL, Kohane IS. Artificial intelligence in healthcare. Nat Biomed Eng. 2018;2:719-731. doi:10.1038/s41551-018-0305-z
- Chen M, Decary M. Artificial intelligence in healthcare: an essential guide for health leaders. Healthc Manag Forum. 2020;33:10-18. doi:10.1177/0840470419873123
Practice Points
- Artificial intelligence (AI) has the potential to facilitate the diagnosis of pigmented lesions and expedite the management of malignant melanoma.
- Further studies should be done to see if the high diagnostic accuracy of the AI application we studied translates to a decrease in unnecessary biopsies or expedited referral for pigmented lesions.
- The large variability of formal dermoscopy training among board-certified dermatologists may contribute to the decreased ability to identify pigmented lesions with dermoscopic imaging compared to AI.
Treatment of Angiosarcoma of the Head and Neck: A Systematic Review
Cutaneous angiosarcoma (cAS) is a rare malignancy arising from vascular or lymphatic tissue. It classically presents during the sixth or seventh decades of life as a raised purple papule or plaque on the head and neck areas.1 Primary cAS frequently mimics benign conditions, leading to delays in care. Such delays coupled with the aggressive nature of angiosarcomas leads to a poor prognosis. Five-year survival rates range from 11% to 50%, and more than half of patients die within 1 year of diagnosis.2-7
Currently, there is no consensus on the most effective treatments, as the rare nature of cAS has made the development of controlled clinical trials difficult. Wide local excision (WLE) is most frequently employed; however, the tumor’s infiltrative growth makes complete resection and negative surgical margins difficult to achieve.8 Recently, Mohs micrographic surgery (MMS) has been postulated as a treatment option. The tissue-sparing nature and intraoperative margin control of MMS may provide tumor eradication and cosmesis benefits reported with other cutaneous malignancies.9
Nearly all localized cASs are treated with surgical excision with or without adjuvant treatment modalities; however, it is unclear which of these modalities provide a survival benefit. We conducted a systematic review of the literature to compare treatment modalities for localized cAS of the head and neck regions and to compare treatments based on tumor stage.
METHODS
A literature search was performed to identify published studies indexed by MEDLINE, Cochrane Central Register of Controlled Trials (CENTRAL), Embase, and PubMed from January 1, 1977, to May 8, 2020, reporting on cAS and treatment modalities used. The search was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines.5 Data extracted included patient demographics, tumor characteristics (including T1 [≤5 cm] and T2 [>5 cm and ≤10 cm] based on the American Joint Committee on Cancer soft tissue sarcoma staging criteria), treatments used, follow-up time, overall survival (OS) rates, and complications.10,11
Studies were required to (1) include participants with head and neck cAS; (2) report original patient data following cAS treatment with surgical (WLE or MMS) and/or nonsurgical modalities (chemotherapy [CT], radiotherapy [RT], immunotherapy [IT]); (3) report outcome data related to OS rates following treatment; and (4) have articles published in English. Given the rare nature of cAS, there was no limitation on the number of participants needed.
The Newcastle-Ottawa scale for observational studies was used to assess the quality of studies.12 Higher scores indicate low risk of bias, while lower scores represent high risk of bias.
Continuous data were reported with means and SDs, while categorical variables were reported as percentages. Overall survival means and SDs were compared between treatment modalities using an independent sample t test with P<.05 considered statistically significant. Due to the heterogeneity of the data, a meta-analysis was not reported.
RESULTS
Literature Search and Risk of Bias Assessment
There were 283 manuscripts identified, 56 articles read in full, and 40 articles included in the review (Figure). Among the 16 studies not meeting inclusion criteria, 7 did not provide enough data to isolate head and neck cAS cases,1,13-18 6 did not report outcomes related to the current review,19-24 and 3 did not provide enough data to isolate different treatment outcomes.25-27 Among the included studies, 32 reported use of WLE: WLE alone (n=21)2,7,11,28-45; WLE with RT (n=24)2,3,11,28-31,33-36,38-41,43-51; WLE with CT (n=7)2,31,35,39,41,48,52; WLE with RT and CT (n=11)2,29,31,33-35,39,40,48,52,53; WLE with RT and IT (n=3)35,54,55; and WLE with RT, CT, and IT (n=1).53 Nine studies reported MMS: MMS alone (n=5)39,56-59; MMS with RT (n=3)32,50,60,61; and MMS with RT and CT (n=1).51
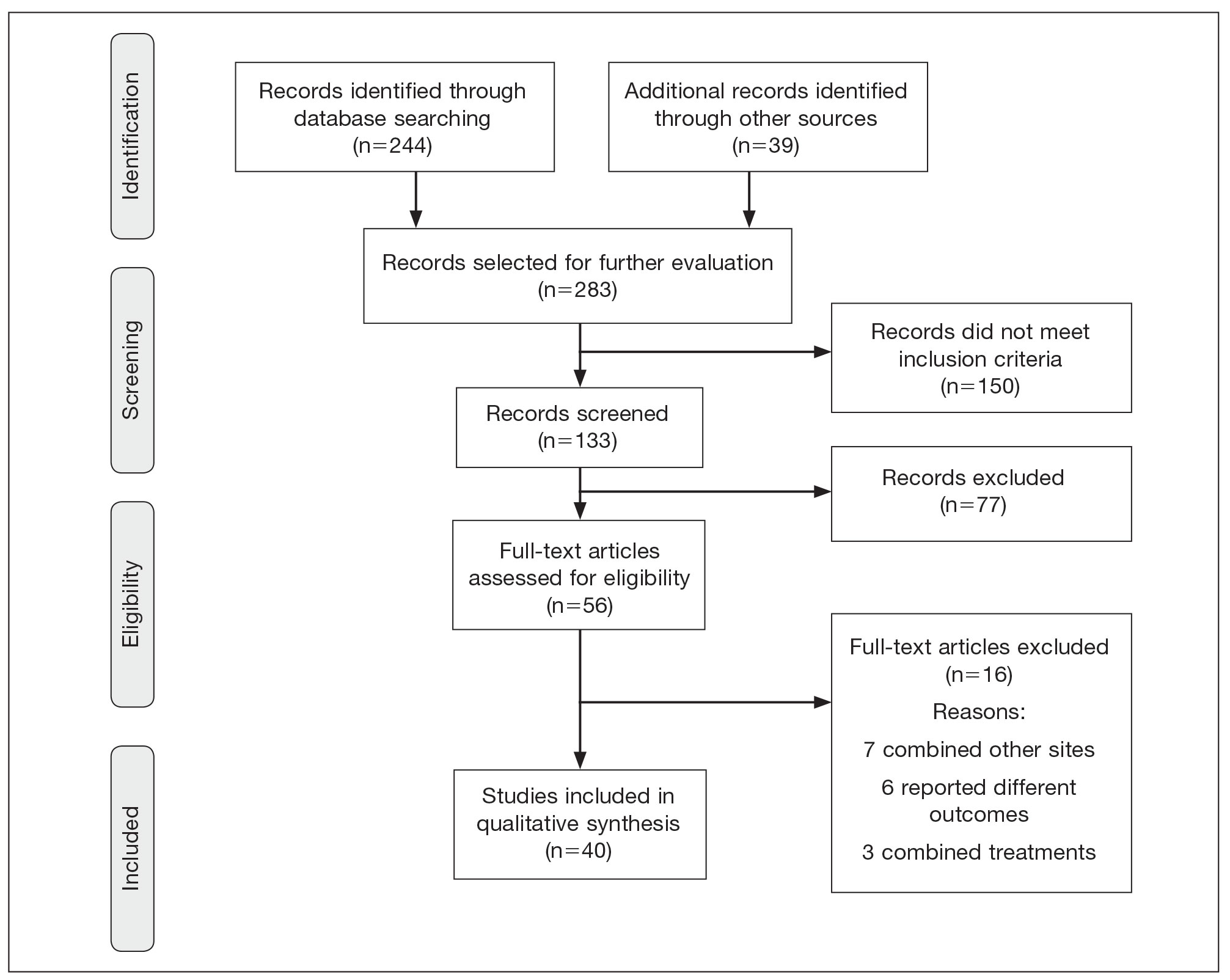
Risk of bias assessment identified low risk in 3 articles. High risk was identified in 5 case reports,57-61 and 1 study did not describe patient selection.43 Clayton et al56 showed intermediate risk, given the study controlled for 1 factor.
Patient Demographics
A total of 1295 patients were included. The pooled mean age of the patients was 67.5 years (range, 3–88 years), and 64.7% were male. There were 79 cases identified as T1 and 105 as T2. A total of 825 cases were treated using WLE with or without adjuvant therapy, while a total of 9 cases were treated using MMS with and without adjuvant therapies (Table). There were 461 cases treated without surgical excision: RT alone (n=261), CT alone (n=38), IT alone (n=35), RT with CT (n=81), RT with IT (n=34), and RT with CT and IT (n=12)(Table). The median follow-up period across all studies was 23.5 months (range, 1–228 months).
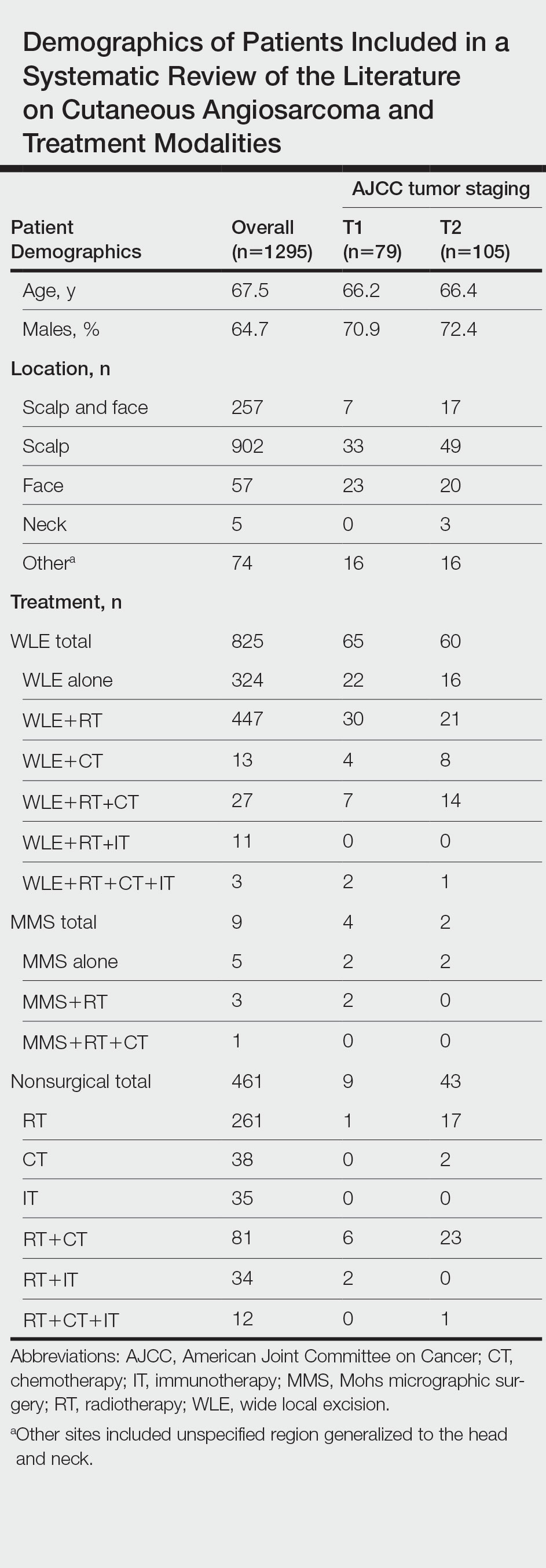
Comparison Between Surgical and Nonsurgical Modalities
Wide Local Excision—Wide local excision (n=825; 63.7%) alone or in combination with other therapies was the most frequently used treatment modality. The mean (SD) OS was longest for WLE with RT, CT, and IT (n=3; 39.3 [24.1]), followed by WLE with RT (n=447; 35.9 [34.3] months), WLE with CT (n=13; 32.4 [30.2] months), WLE alone (n=324; 29.6 [34.1] months), WLE with RT and IT (n=11; 23.5 [4.9] months), and WLE with RT and CT (n=27; 20.7 [13.1] months).
Nonsurgical Modalities—Nonsurgical methods were used less frequently than surgical methods (n=461; 35.6%). The mean (SD) OS time in descending order was as follows: RT with CT and IT (n=12; 34.9 [1.2] months), RT with CT (n=81; 30.4 [37.8] months), IT alone (n=35; 25.7 [no SD reported] months), RT with IT (n=34; 20.5 [8.6] months), CT alone (n=38; 20.1 [15.9] months), and RT alone (n=261; 12.8 [8.3] months).
When comparing mean (SD) OS outcomes between surgical and nonsurgical treatment modalities, only the addition of WLE to RT significantly increased OS when compared with RT alone (WLE, 35.9 [34.3] months; RT alone, 12.8 [8.3] months; P=.001). When WLE was added to CT or both RT and CT, there was no significant difference with OS when compared with CT alone (WLE with CT, 32.4 [30.2] months; CT alone, 20.1 [15.9] months; P=.065); or both RT and CT in combination (WLE with RT and CT, 20.7 [13.1] months; RT and CT, 30.4 [37.8] months; P=.204).
Comparison Between T1 and T2 cAS
T1 Angiosarcoma—There were 79 patients identified as having T1 tumors across 16 studies.2,31,32,34,39-41,46,48-50,53,58-60,62 The mean (SD) OS was longest for WLE with RT, CT, and IT (n=2; 56.0 [6.0] months), followed by WLE with CT (n=4; 54.5 [41.0] months); WLE with RT (n=30; 39.7 [41.2] months); WLE alone (n=22; 37.2 [37.3] months); WLE with both RT and CT (n=7; 25.5 [18.7] months); RT with IT (n=2; 20.0 [11.0] months); RT with CT (n=6; 15.7 [6.8] months); and RT alone (n=1; 13 [no SD]) months)(eTable).
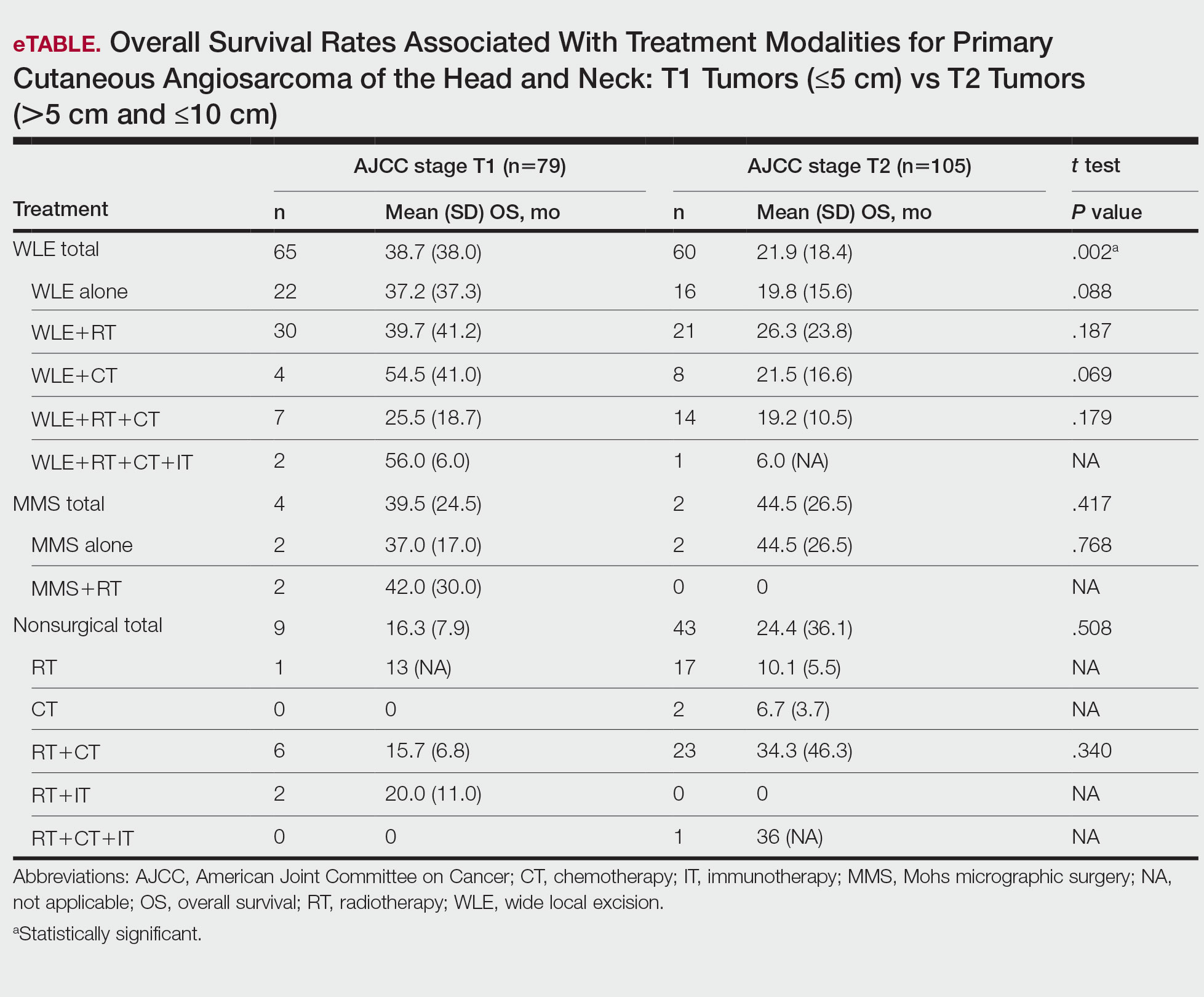
T2 Angiosarcoma—There were 105 patients with T2 tumors in 15 studies.2,31,32,34,39-41,46,48-50,52,53,57,62 The mean (SD) OS for each treatment modality in descending order was as follows: RT with CT and IT (n=1; 36 [no SD reported] months); RT with CT (n=23; 34.3 [46.3] months); WLE with RT (n=21; 26.3 [23.8] months); WLE with CT (n=8; 21.5 [16.6] months); WLE alone (n=16; 19.8 [15.6] months); WLE with RT and CT (n=14; 19.2 [10.5] months); RT alone (n=17; 10.1 [5.5] months); CT alone (n=2; 6.7 [3.7] months); and WLE with RT, CT, and IT (n=1; 6.0 [no SD] months)(eTable).
Mohs Micrographic Surgery—The use of MMS was only identified in case reports or small observational studies for a total of 9 patients. Five cASs were treated with MMS alone for a mean (SD) OS of 37 (21.5) months, with 4 reporting cAS staging: 2 were T158,59 (mean [SD] OS, 37.0 [17.0] months) and 2 were T2 tumors39,57 (mean [SD] OS, 44.5 [26.5] months). Mohs micrographic surgery with RT was used for 3 tumors (mean [SD] OS, 34.0 [26.9] months); 2 were T150,60 (mean [SD] OS, 42.0 [30.0] months) and 1 unreported staging (eTable).56 Mohs micrographic surgery with both RT and CT was used in 1 patient (unreported staging; OS, 82 months).51
Complications
Complications were rare and mainly associated with CT and RT. Four studies reported radiation dermatitis with RT.53,55,62,63 Two studies reported peripheral neuropathy and myelotoxicity with CT.35,51 Only 1 study reported poor wound healing due to surgical complications.29
COMMENT
Cutaneous angiosarcomas are rare and have limited treatment guidelines. Surgical excision does appear to be an effective adjunct to nonsurgical treatments, particularly WLE combined with RT, CT, and IT. Although MMS ultimately may be useful for cAS, the limited number and substantial heterogeneity of reported cases precludes definitive conclusions at this time.
Achieving margin control during WLE is associated with higher OS when treating angiosarcoma,36,46 which is particularly true for T1 tumors where margin control is imperative, and many cases are treated with a combination of WLE and RT. Overall survival times are lower for T2 tumors, as these tumors are larger and most likely have spread; therefore, more aggressive combination treatments were more prevalent. In these cases, complete margin control may be difficult to achieve and may not be as critical to the outcome if another form of adjuvant therapy can be administered promptly.24,64
When surgery is contraindicated, RT with or without CT was the most commonly reported treatment modality. However, these treatments were notably less effective than when used in combination with surgical resection. The use of RT alone has a recurrence rate reported up to 100% in certain studies, suggesting the need to utilize RT in combination with other modalities.23,39 It is important to note that RT often is used as monotherapy in palliative treatment, which may indirectly skew survival rates.2
Limitations of the study include a lack of randomized controlled trials. Most reports were retrospective reviews or case series, and tumor staging was sparsely reported. Finally, although MMS may provide utility in the treatment of cAS, the sample size of 9 precluded definitive conclusions from being formed about its efficacy.
CONCLUSION
Cutaneous angiosarcoma is rare and has limited data comparing different treatment modalities. The paucity of data currently limits definitive recommendations; however, both surgical and nonsurgical modalities have demonstrated potential efficacy in the treatment of cAS and may benefit from additional research. Clinicians should consider a multidisciplinary approach for patients with a diagnosis of cAS to tailor treatments on a case-by-case basis.
- Rodríguez-Jiménez P, Jimenez YD, Reolid A, et al. State of the art of Mohs surgery for rare cutaneous tumors in the Spanish Registry of Mohs Surgery (REGESMOHS). Int J Dermatol. 2020;59:321-325.
- Alqumber NA, Choi JW, Kang MK. The management and prognosis of facial and scalp angiosarcoma: a retrospective analysis of 15 patients. Ann Plast Surg. 2019;83:55-62.
- Pawlik TM, Paulino AF, McGinn CJ, et al. Cutaneous angiosarcoma of the scalp: a multidisciplinary approach. Cancer. 2003;98:1716-1726.
- Deyrup AT, McKenney JK, Tighiouart M, et al. Sporadic cutaneous angiosarcomas: a proposal for risk stratification based on 69 cases. Am J Surg Pathol. 2008;32:72-77.
- Meis-Kindblom JM, Kindblom LG. Angiosarcoma of soft tissue: a study of 80 cases. Am J Surg Pathol. 1998;22:683-697.
- Harbour P, Song DH. The skin and subcutaneous tissue. In: Brunicardi FC, Andersen DK, Billiar TR, et al, eds. Schwartz’s Principles of Surgery. 11th ed. McGraw-Hill Education; 2019. Accessed April 24, 2023. https://accesssurgery.mhmedical.com/content.aspx?bookid=2576§ionid=216206374
- Oashi K, Namikawa K, Tsutsumida A, et al. Surgery with curative intent is associated with prolonged survival in patients with cutaneous angiosarcoma of the scalp and face—a retrospective study of 38 untreated cases in the Japanese population. Eur J Surg Oncol. 2018;44:823-829.
- Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol. 2010;11:983-991.
- Tolkachjov SN, Brodland DG, Coldiron BM, et al. Understanding Mohs micrographic surgery: a review and practical guide for the nondermatologist. Mayo Clin Proc. 2017;92:1261-1271.
- Amin M, Edge SB, Greene FL, et al, eds. AJCC Cancer Staging Manual. 8th ed. Springer; 2017.
- Holden CA, Spittle MF, Jones EW. Angiosarcoma of the face and scalp, prognosis and treatment. Cancer. 1987;59:1046-1057.
- Cook DA, Reed DA. Appraising the quality of medical education research methods: the Medical Education Research Study Quality Instrument and the Newcastle-Ottawa Scale-Education. Acad Med. 2015;90:1067-1076.
- Lee BL, Chen CF, Chen PC, et al. Investigation of prognostic features in primary cutaneous and soft tissue angiosarcoma after surgical resection: a retrospective study. Ann Plast Surg. 2017;78(3 suppl 2):S41-S46.
- Shen CJ, Parzuchowski AS, Kummerlowe MN, et al. Combined modality therapy improves overall survival for angiosarcoma. Acta Oncol. 2017;56:1235-1238.
- Breakey RW, Crowley TP, Anderson IB, et al. The surgical management of head and neck sarcoma: the Newcastle experience. J Plast Reconstr Aesthet Surg. 2017;70:78-84.
- Singla S, Papavasiliou P, Powers B, et al. Challenges in the treatment of angiosarcoma: a single institution experience. Am J Surg. 2014;208:254-259.
- Sasaki R, Soejima T, Kishi K, et al. Angiosarcoma treated with radiotherapy: impact of tumor type and size on outcome. Int J Radiat Oncol Biol Phys. 2002;52:1032-1040.
- Naka N, Ohsawa M, Tomita Y, et al. Angiosarcoma in Japan. A review of 99 cases. Cancer. 1995;75:989-996.
- DeMartelaere SL, Roberts D, Burgess MA, et al. Neoadjuvant chemotherapy-specific and overall treatment outcomes in patients with cutaneous angiosarcoma of the face with periorbital involvement. Head Neck. 2008;30:639-646.
- Ward JR, Feigenberg SJ, Mendenhall NP, et al. Radiation therapy for angiosarcoma. Head Neck. 2003;25:873-878.
- Letsa I, Benson C, Al-Muderis O, et al. Angiosarcoma of the face and scalp: effective systemic treatment in the older patient. J Geriatr Oncol. 2014;5:276-280.
- Buehler D, Rice SR, Moody JS, et al. Angiosarcoma outcomes and prognostic factors: a 25-year single institution experience. Am J Clin Oncol. 2014;37:473-479.
- Patel SH, Hayden RE, Hinni ML, et al. Angiosarcoma of the scalp and face: the Mayo Clinic experience. JAMA Otolaryngol Head Neck Surg. 2015;141:335-340.
- Guadagnolo BA, Zagars GK, Araujo D, et al. Outcomes after definitive treatment for cutaneous angiosarcoma of the face and scalp. Head Neck. 2011;33:661-667.
- Zhang Y, Yan Y, Zhu M, et al. Clinical outcomes in primary scalp angiosarcoma. Oncol Lett. 2019;18:5091-5096.
- Kamo R, Ishii M. Histological differentiation, histogenesis and prognosis of cutaneous angiosarcoma. Osaka City Med J. 2011;57:31-44.
- Ito T, Uchi H, Nakahara T, et al. Cutaneous angiosarcoma of the head and face: a single-center analysis of treatment outcomes in 43 patients in Japan. J Cancer Res Clin Oncol. 2016;142:1387-1394.
- Aust MR, Olsen KD, Lewis JE, et al. Angiosarcomas of the head and neck: clinical and pathologic characteristics. Ann Otol Rhinol Laryngol. 1997;106:943-951.
- Buschmann A, Lehnhardt M, Toman N, et al. Surgical treatment of angiosarcoma of the scalp: less is more. Ann Plast Surg. 2008;61:399-403.
- Cassidy RJ, Switchenko JM, Yushak ML, et al. The importance of surgery in scalp angiosarcomas. Surg Oncol. 2018;27:A3-A8.
- Choi JH, Ahn KC, Chang H, et al. Surgical treatment and prognosis of angiosarcoma of the scalp: a retrospective analysis of 14 patients in a single institution. Biomed Res Int. 2015;2015:321896.
- Chow TL, Kwan WW, Kwan CK. Treatment of cutaneous angiosarcoma of the scalp and face in Chinese patients: local experience at a regional hospital in Hong Kong. Hong Kong Med J. 2018;24:25-31.
- Donghi D, Kerl K, Dummer R, et al. Cutaneous angiosarcoma: own experience over 13 years. clinical features, disease course and immunohistochemical profile. J Eur Acad Dermatol Venereol. 2010;24:1230-1234.
- Ferrari A, Casanova M, Bisogno G, et al. Malignant vascular tumors in children and adolescents: a report from the Italian and German Soft Tissue Sarcoma Cooperative Group. Med Pediatr Oncol. 2002;39:109-114.
- Fujisawa Y, Nakamura Y, Kawachi Y, et al. Comparison between taxane-based chemotherapy with conventional surgery-based therapy for cutaneous angiosarcoma: a single-center experience. J Dermatolog Treat. 2014;25:419-423.
- Hodgkinson DJ, Soule EH, Woods JE. Cutaneous angiosarcoma of the head and neck. Cancer. 1979;44:1106-1113.
- Lim SY, Pyon JK, Mun GH, et al. Surgical treatment of angiosarcoma of the scalp with superficial parotidectomy. Ann Plast Surg. 2010;64:180-182.
- Maddox JC, Evans HL. Angiosarcoma of skin and soft tissue: a study of forty-four cases. Cancer. 1981;48:1907-1921.
- Mark RJ, Tran LM, Sercarz J, et al. Angiosarcoma of the head and neck. The UCLA experience 1955 through 1990. Arch Otolaryngol Head Neck Surg. 1993;119:973-978.
- Morgan MB, Swann M, Somach S, et al. Cutaneous angiosarcoma: a case series with prognostic correlation. J Am Acad Dermatol. 2004;50:867-874.
- Mullins B, Hackman T. Angiosarcoma of the head and neck. Int Arch Otorhinolaryngol. 2015;19:191-195.
- Ogawa K, Takahashi K, Asato Y, et al. Treatment and prognosis of angiosarcoma of the scalp and face: a retrospective analysis of 48 patients. Br J Radiol. 2012;85:E1127-E1133.
- Panje WR, Moran WJ, Bostwick DG, et al. Angiosarcoma of the head and neck: review of 11 cases. Laryngoscope. 1986;96:1381-1384.
- Perez MC, Padhya TA, Messina JL, et al. Cutaneous angiosarcoma: a single-institution experience. Ann Surg Oncol. 2013;20:3391-3397.
- Veness M, Cooper S. Treatment of cutaneous angiosarcomas of the head and neck. Australas Radiol. 1995;39:277-281.
- Barttelbort SW, Stahl R, Ariyan S. Cutaneous angiosarcoma of the face and scalp. Plast Reconstr Surg. 1989;84:55-59.
- Bernstein JM, Irish JC, Brown DH, et al. Survival outcomes for cutaneous angiosarcoma of the scalp versus face. Head Neck. 2017;39:1205-1211.
- Köhler HF, Neves RI, Brechtbühl ER, et al. Cutaneous angiosarcoma of the head and neck: report of 23 cases from a single institution. Otolaryngol Head Neck Surg. 2008;139:519-524.
- Morales PH, Lindberg RD, Barkley HT Jr. Soft tissue angiosarcomas. Int J Radiat Oncol Biol Phys. 1981;7:1655-1659.
- Wollina U, Hansel G, Schönlebe J, et al. Cutaneous angiosarcoma is a rare aggressive malignant vascular tumour of the skin. J Eur Acad Dermatol Venereol. 2011;25:964-968.
- Wollina U, Koch A, Hansel G, et al. A 10-year analysis of cutaneous mesenchymal tumors (sarcomas and related entities) in a skin cancer center. Int J Dermatol. 2013;52:1189-1197.
- Bien E, Stachowicz-Stencel T, Balcerska A, et al. Angiosarcoma in children - still uncontrollable oncological problem. The report of the Polish Paediatric Rare Tumours Study. Eur J Cancer Care (Engl). 2009;18:411-420.
- Suzuki G, Yamazaki H, Takenaka H, et al. Definitive radiation therapy for angiosarcoma of the face and scalp. In Vivo. 2016;30:921-926.
- Miki Y, Tada T, Kamo R, et al. Single institutional experience of the treatment of angiosarcoma of the face and scalp. Br J Radiol. 2013;86:20130439.
- Ohguri T, Imada H, Nomoto S, et al. Angiosarcoma of the scalp treated with curative radiotherapy plus recombinant interleukin-2 immunotherapy. Int J Radiat Oncol Biol Phys. 2005;61:1446-1453.
- Clayton BD, Leshin B, Hitchcock MG, et al. Utility of rush paraffin-embedded tangential sections in the management of cutaneous neoplasms. Dermatol Surg. 2000;26:671-678.
- Goldberg DJ, Kim YA. Angiosarcoma of the scalp treated with Mohs micrographic surgery. J Dermatol Surg Oncol. 1993;19:156-158.
- Mikhail GR, Kelly AP Jr. Malignant angioendothelioma of the face. J Dermatol Surg Oncol. 1977;3:181-183.
- Muscarella VA. Angiosarcoma treated by Mohs micrographic surgery. J Dermatol Surg Oncol. 1993;19:1132-1133.
- Bullen R, Larson PO, Landeck AE, et al. Angiosarcoma of the head and neck managed by a combination of multiple biopsies to determine tumor margin and radiation therapy. report of three cases and review of the literature. Dermatol Surg. 1998;24:1105-1110.
- Wiwatwongwana D, White VA, Dolman PJ. Two cases of periocular cutaneous angiosarcoma. Ophthalmic Plast Reconstr Surg. 2010;26:365-366.
- Morrison WH, Byers RM, Garden AS, et al. Cutaneous angiosarcoma of the head and neck. A therapeutic dilemma. Cancer. 1995;76:319-327.
- Hata M, Wada H, Ogino I, et al. Radiation therapy for angiosarcoma of the scalp: treatment outcomes of total scalp irradiation with X-rays and electrons. Strahlenther Onkol. 2014;190:899-904.
- Hwang K, Kim MY, Lee SH. Recommendations for therapeutic decisions of angiosarcoma of the scalp and face. J Craniofac Surg. 2015;26:E253-E256.
Cutaneous angiosarcoma (cAS) is a rare malignancy arising from vascular or lymphatic tissue. It classically presents during the sixth or seventh decades of life as a raised purple papule or plaque on the head and neck areas.1 Primary cAS frequently mimics benign conditions, leading to delays in care. Such delays coupled with the aggressive nature of angiosarcomas leads to a poor prognosis. Five-year survival rates range from 11% to 50%, and more than half of patients die within 1 year of diagnosis.2-7
Currently, there is no consensus on the most effective treatments, as the rare nature of cAS has made the development of controlled clinical trials difficult. Wide local excision (WLE) is most frequently employed; however, the tumor’s infiltrative growth makes complete resection and negative surgical margins difficult to achieve.8 Recently, Mohs micrographic surgery (MMS) has been postulated as a treatment option. The tissue-sparing nature and intraoperative margin control of MMS may provide tumor eradication and cosmesis benefits reported with other cutaneous malignancies.9
Nearly all localized cASs are treated with surgical excision with or without adjuvant treatment modalities; however, it is unclear which of these modalities provide a survival benefit. We conducted a systematic review of the literature to compare treatment modalities for localized cAS of the head and neck regions and to compare treatments based on tumor stage.
METHODS
A literature search was performed to identify published studies indexed by MEDLINE, Cochrane Central Register of Controlled Trials (CENTRAL), Embase, and PubMed from January 1, 1977, to May 8, 2020, reporting on cAS and treatment modalities used. The search was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines.5 Data extracted included patient demographics, tumor characteristics (including T1 [≤5 cm] and T2 [>5 cm and ≤10 cm] based on the American Joint Committee on Cancer soft tissue sarcoma staging criteria), treatments used, follow-up time, overall survival (OS) rates, and complications.10,11
Studies were required to (1) include participants with head and neck cAS; (2) report original patient data following cAS treatment with surgical (WLE or MMS) and/or nonsurgical modalities (chemotherapy [CT], radiotherapy [RT], immunotherapy [IT]); (3) report outcome data related to OS rates following treatment; and (4) have articles published in English. Given the rare nature of cAS, there was no limitation on the number of participants needed.
The Newcastle-Ottawa scale for observational studies was used to assess the quality of studies.12 Higher scores indicate low risk of bias, while lower scores represent high risk of bias.
Continuous data were reported with means and SDs, while categorical variables were reported as percentages. Overall survival means and SDs were compared between treatment modalities using an independent sample t test with P<.05 considered statistically significant. Due to the heterogeneity of the data, a meta-analysis was not reported.
RESULTS
Literature Search and Risk of Bias Assessment
There were 283 manuscripts identified, 56 articles read in full, and 40 articles included in the review (Figure). Among the 16 studies not meeting inclusion criteria, 7 did not provide enough data to isolate head and neck cAS cases,1,13-18 6 did not report outcomes related to the current review,19-24 and 3 did not provide enough data to isolate different treatment outcomes.25-27 Among the included studies, 32 reported use of WLE: WLE alone (n=21)2,7,11,28-45; WLE with RT (n=24)2,3,11,28-31,33-36,38-41,43-51; WLE with CT (n=7)2,31,35,39,41,48,52; WLE with RT and CT (n=11)2,29,31,33-35,39,40,48,52,53; WLE with RT and IT (n=3)35,54,55; and WLE with RT, CT, and IT (n=1).53 Nine studies reported MMS: MMS alone (n=5)39,56-59; MMS with RT (n=3)32,50,60,61; and MMS with RT and CT (n=1).51

Risk of bias assessment identified low risk in 3 articles. High risk was identified in 5 case reports,57-61 and 1 study did not describe patient selection.43 Clayton et al56 showed intermediate risk, given the study controlled for 1 factor.
Patient Demographics
A total of 1295 patients were included. The pooled mean age of the patients was 67.5 years (range, 3–88 years), and 64.7% were male. There were 79 cases identified as T1 and 105 as T2. A total of 825 cases were treated using WLE with or without adjuvant therapy, while a total of 9 cases were treated using MMS with and without adjuvant therapies (Table). There were 461 cases treated without surgical excision: RT alone (n=261), CT alone (n=38), IT alone (n=35), RT with CT (n=81), RT with IT (n=34), and RT with CT and IT (n=12)(Table). The median follow-up period across all studies was 23.5 months (range, 1–228 months).

Comparison Between Surgical and Nonsurgical Modalities
Wide Local Excision—Wide local excision (n=825; 63.7%) alone or in combination with other therapies was the most frequently used treatment modality. The mean (SD) OS was longest for WLE with RT, CT, and IT (n=3; 39.3 [24.1]), followed by WLE with RT (n=447; 35.9 [34.3] months), WLE with CT (n=13; 32.4 [30.2] months), WLE alone (n=324; 29.6 [34.1] months), WLE with RT and IT (n=11; 23.5 [4.9] months), and WLE with RT and CT (n=27; 20.7 [13.1] months).
Nonsurgical Modalities—Nonsurgical methods were used less frequently than surgical methods (n=461; 35.6%). The mean (SD) OS time in descending order was as follows: RT with CT and IT (n=12; 34.9 [1.2] months), RT with CT (n=81; 30.4 [37.8] months), IT alone (n=35; 25.7 [no SD reported] months), RT with IT (n=34; 20.5 [8.6] months), CT alone (n=38; 20.1 [15.9] months), and RT alone (n=261; 12.8 [8.3] months).
When comparing mean (SD) OS outcomes between surgical and nonsurgical treatment modalities, only the addition of WLE to RT significantly increased OS when compared with RT alone (WLE, 35.9 [34.3] months; RT alone, 12.8 [8.3] months; P=.001). When WLE was added to CT or both RT and CT, there was no significant difference with OS when compared with CT alone (WLE with CT, 32.4 [30.2] months; CT alone, 20.1 [15.9] months; P=.065); or both RT and CT in combination (WLE with RT and CT, 20.7 [13.1] months; RT and CT, 30.4 [37.8] months; P=.204).
Comparison Between T1 and T2 cAS
T1 Angiosarcoma—There were 79 patients identified as having T1 tumors across 16 studies.2,31,32,34,39-41,46,48-50,53,58-60,62 The mean (SD) OS was longest for WLE with RT, CT, and IT (n=2; 56.0 [6.0] months), followed by WLE with CT (n=4; 54.5 [41.0] months); WLE with RT (n=30; 39.7 [41.2] months); WLE alone (n=22; 37.2 [37.3] months); WLE with both RT and CT (n=7; 25.5 [18.7] months); RT with IT (n=2; 20.0 [11.0] months); RT with CT (n=6; 15.7 [6.8] months); and RT alone (n=1; 13 [no SD]) months)(eTable).

T2 Angiosarcoma—There were 105 patients with T2 tumors in 15 studies.2,31,32,34,39-41,46,48-50,52,53,57,62 The mean (SD) OS for each treatment modality in descending order was as follows: RT with CT and IT (n=1; 36 [no SD reported] months); RT with CT (n=23; 34.3 [46.3] months); WLE with RT (n=21; 26.3 [23.8] months); WLE with CT (n=8; 21.5 [16.6] months); WLE alone (n=16; 19.8 [15.6] months); WLE with RT and CT (n=14; 19.2 [10.5] months); RT alone (n=17; 10.1 [5.5] months); CT alone (n=2; 6.7 [3.7] months); and WLE with RT, CT, and IT (n=1; 6.0 [no SD] months)(eTable).
Mohs Micrographic Surgery—The use of MMS was only identified in case reports or small observational studies for a total of 9 patients. Five cASs were treated with MMS alone for a mean (SD) OS of 37 (21.5) months, with 4 reporting cAS staging: 2 were T158,59 (mean [SD] OS, 37.0 [17.0] months) and 2 were T2 tumors39,57 (mean [SD] OS, 44.5 [26.5] months). Mohs micrographic surgery with RT was used for 3 tumors (mean [SD] OS, 34.0 [26.9] months); 2 were T150,60 (mean [SD] OS, 42.0 [30.0] months) and 1 unreported staging (eTable).56 Mohs micrographic surgery with both RT and CT was used in 1 patient (unreported staging; OS, 82 months).51
Complications
Complications were rare and mainly associated with CT and RT. Four studies reported radiation dermatitis with RT.53,55,62,63 Two studies reported peripheral neuropathy and myelotoxicity with CT.35,51 Only 1 study reported poor wound healing due to surgical complications.29
COMMENT
Cutaneous angiosarcomas are rare and have limited treatment guidelines. Surgical excision does appear to be an effective adjunct to nonsurgical treatments, particularly WLE combined with RT, CT, and IT. Although MMS ultimately may be useful for cAS, the limited number and substantial heterogeneity of reported cases precludes definitive conclusions at this time.
Achieving margin control during WLE is associated with higher OS when treating angiosarcoma,36,46 which is particularly true for T1 tumors where margin control is imperative, and many cases are treated with a combination of WLE and RT. Overall survival times are lower for T2 tumors, as these tumors are larger and most likely have spread; therefore, more aggressive combination treatments were more prevalent. In these cases, complete margin control may be difficult to achieve and may not be as critical to the outcome if another form of adjuvant therapy can be administered promptly.24,64
When surgery is contraindicated, RT with or without CT was the most commonly reported treatment modality. However, these treatments were notably less effective than when used in combination with surgical resection. The use of RT alone has a recurrence rate reported up to 100% in certain studies, suggesting the need to utilize RT in combination with other modalities.23,39 It is important to note that RT often is used as monotherapy in palliative treatment, which may indirectly skew survival rates.2
Limitations of the study include a lack of randomized controlled trials. Most reports were retrospective reviews or case series, and tumor staging was sparsely reported. Finally, although MMS may provide utility in the treatment of cAS, the sample size of 9 precluded definitive conclusions from being formed about its efficacy.
CONCLUSION
Cutaneous angiosarcoma is rare and has limited data comparing different treatment modalities. The paucity of data currently limits definitive recommendations; however, both surgical and nonsurgical modalities have demonstrated potential efficacy in the treatment of cAS and may benefit from additional research. Clinicians should consider a multidisciplinary approach for patients with a diagnosis of cAS to tailor treatments on a case-by-case basis.
Cutaneous angiosarcoma (cAS) is a rare malignancy arising from vascular or lymphatic tissue. It classically presents during the sixth or seventh decades of life as a raised purple papule or plaque on the head and neck areas.1 Primary cAS frequently mimics benign conditions, leading to delays in care. Such delays coupled with the aggressive nature of angiosarcomas leads to a poor prognosis. Five-year survival rates range from 11% to 50%, and more than half of patients die within 1 year of diagnosis.2-7
Currently, there is no consensus on the most effective treatments, as the rare nature of cAS has made the development of controlled clinical trials difficult. Wide local excision (WLE) is most frequently employed; however, the tumor’s infiltrative growth makes complete resection and negative surgical margins difficult to achieve.8 Recently, Mohs micrographic surgery (MMS) has been postulated as a treatment option. The tissue-sparing nature and intraoperative margin control of MMS may provide tumor eradication and cosmesis benefits reported with other cutaneous malignancies.9
Nearly all localized cASs are treated with surgical excision with or without adjuvant treatment modalities; however, it is unclear which of these modalities provide a survival benefit. We conducted a systematic review of the literature to compare treatment modalities for localized cAS of the head and neck regions and to compare treatments based on tumor stage.
METHODS
A literature search was performed to identify published studies indexed by MEDLINE, Cochrane Central Register of Controlled Trials (CENTRAL), Embase, and PubMed from January 1, 1977, to May 8, 2020, reporting on cAS and treatment modalities used. The search was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines.5 Data extracted included patient demographics, tumor characteristics (including T1 [≤5 cm] and T2 [>5 cm and ≤10 cm] based on the American Joint Committee on Cancer soft tissue sarcoma staging criteria), treatments used, follow-up time, overall survival (OS) rates, and complications.10,11
Studies were required to (1) include participants with head and neck cAS; (2) report original patient data following cAS treatment with surgical (WLE or MMS) and/or nonsurgical modalities (chemotherapy [CT], radiotherapy [RT], immunotherapy [IT]); (3) report outcome data related to OS rates following treatment; and (4) have articles published in English. Given the rare nature of cAS, there was no limitation on the number of participants needed.
The Newcastle-Ottawa scale for observational studies was used to assess the quality of studies.12 Higher scores indicate low risk of bias, while lower scores represent high risk of bias.
Continuous data were reported with means and SDs, while categorical variables were reported as percentages. Overall survival means and SDs were compared between treatment modalities using an independent sample t test with P<.05 considered statistically significant. Due to the heterogeneity of the data, a meta-analysis was not reported.
RESULTS
Literature Search and Risk of Bias Assessment
There were 283 manuscripts identified, 56 articles read in full, and 40 articles included in the review (Figure). Among the 16 studies not meeting inclusion criteria, 7 did not provide enough data to isolate head and neck cAS cases,1,13-18 6 did not report outcomes related to the current review,19-24 and 3 did not provide enough data to isolate different treatment outcomes.25-27 Among the included studies, 32 reported use of WLE: WLE alone (n=21)2,7,11,28-45; WLE with RT (n=24)2,3,11,28-31,33-36,38-41,43-51; WLE with CT (n=7)2,31,35,39,41,48,52; WLE with RT and CT (n=11)2,29,31,33-35,39,40,48,52,53; WLE with RT and IT (n=3)35,54,55; and WLE with RT, CT, and IT (n=1).53 Nine studies reported MMS: MMS alone (n=5)39,56-59; MMS with RT (n=3)32,50,60,61; and MMS with RT and CT (n=1).51

Risk of bias assessment identified low risk in 3 articles. High risk was identified in 5 case reports,57-61 and 1 study did not describe patient selection.43 Clayton et al56 showed intermediate risk, given the study controlled for 1 factor.
Patient Demographics
A total of 1295 patients were included. The pooled mean age of the patients was 67.5 years (range, 3–88 years), and 64.7% were male. There were 79 cases identified as T1 and 105 as T2. A total of 825 cases were treated using WLE with or without adjuvant therapy, while a total of 9 cases were treated using MMS with and without adjuvant therapies (Table). There were 461 cases treated without surgical excision: RT alone (n=261), CT alone (n=38), IT alone (n=35), RT with CT (n=81), RT with IT (n=34), and RT with CT and IT (n=12)(Table). The median follow-up period across all studies was 23.5 months (range, 1–228 months).

Comparison Between Surgical and Nonsurgical Modalities
Wide Local Excision—Wide local excision (n=825; 63.7%) alone or in combination with other therapies was the most frequently used treatment modality. The mean (SD) OS was longest for WLE with RT, CT, and IT (n=3; 39.3 [24.1]), followed by WLE with RT (n=447; 35.9 [34.3] months), WLE with CT (n=13; 32.4 [30.2] months), WLE alone (n=324; 29.6 [34.1] months), WLE with RT and IT (n=11; 23.5 [4.9] months), and WLE with RT and CT (n=27; 20.7 [13.1] months).
Nonsurgical Modalities—Nonsurgical methods were used less frequently than surgical methods (n=461; 35.6%). The mean (SD) OS time in descending order was as follows: RT with CT and IT (n=12; 34.9 [1.2] months), RT with CT (n=81; 30.4 [37.8] months), IT alone (n=35; 25.7 [no SD reported] months), RT with IT (n=34; 20.5 [8.6] months), CT alone (n=38; 20.1 [15.9] months), and RT alone (n=261; 12.8 [8.3] months).
When comparing mean (SD) OS outcomes between surgical and nonsurgical treatment modalities, only the addition of WLE to RT significantly increased OS when compared with RT alone (WLE, 35.9 [34.3] months; RT alone, 12.8 [8.3] months; P=.001). When WLE was added to CT or both RT and CT, there was no significant difference with OS when compared with CT alone (WLE with CT, 32.4 [30.2] months; CT alone, 20.1 [15.9] months; P=.065); or both RT and CT in combination (WLE with RT and CT, 20.7 [13.1] months; RT and CT, 30.4 [37.8] months; P=.204).
Comparison Between T1 and T2 cAS
T1 Angiosarcoma—There were 79 patients identified as having T1 tumors across 16 studies.2,31,32,34,39-41,46,48-50,53,58-60,62 The mean (SD) OS was longest for WLE with RT, CT, and IT (n=2; 56.0 [6.0] months), followed by WLE with CT (n=4; 54.5 [41.0] months); WLE with RT (n=30; 39.7 [41.2] months); WLE alone (n=22; 37.2 [37.3] months); WLE with both RT and CT (n=7; 25.5 [18.7] months); RT with IT (n=2; 20.0 [11.0] months); RT with CT (n=6; 15.7 [6.8] months); and RT alone (n=1; 13 [no SD]) months)(eTable).

T2 Angiosarcoma—There were 105 patients with T2 tumors in 15 studies.2,31,32,34,39-41,46,48-50,52,53,57,62 The mean (SD) OS for each treatment modality in descending order was as follows: RT with CT and IT (n=1; 36 [no SD reported] months); RT with CT (n=23; 34.3 [46.3] months); WLE with RT (n=21; 26.3 [23.8] months); WLE with CT (n=8; 21.5 [16.6] months); WLE alone (n=16; 19.8 [15.6] months); WLE with RT and CT (n=14; 19.2 [10.5] months); RT alone (n=17; 10.1 [5.5] months); CT alone (n=2; 6.7 [3.7] months); and WLE with RT, CT, and IT (n=1; 6.0 [no SD] months)(eTable).
Mohs Micrographic Surgery—The use of MMS was only identified in case reports or small observational studies for a total of 9 patients. Five cASs were treated with MMS alone for a mean (SD) OS of 37 (21.5) months, with 4 reporting cAS staging: 2 were T158,59 (mean [SD] OS, 37.0 [17.0] months) and 2 were T2 tumors39,57 (mean [SD] OS, 44.5 [26.5] months). Mohs micrographic surgery with RT was used for 3 tumors (mean [SD] OS, 34.0 [26.9] months); 2 were T150,60 (mean [SD] OS, 42.0 [30.0] months) and 1 unreported staging (eTable).56 Mohs micrographic surgery with both RT and CT was used in 1 patient (unreported staging; OS, 82 months).51
Complications
Complications were rare and mainly associated with CT and RT. Four studies reported radiation dermatitis with RT.53,55,62,63 Two studies reported peripheral neuropathy and myelotoxicity with CT.35,51 Only 1 study reported poor wound healing due to surgical complications.29
COMMENT
Cutaneous angiosarcomas are rare and have limited treatment guidelines. Surgical excision does appear to be an effective adjunct to nonsurgical treatments, particularly WLE combined with RT, CT, and IT. Although MMS ultimately may be useful for cAS, the limited number and substantial heterogeneity of reported cases precludes definitive conclusions at this time.
Achieving margin control during WLE is associated with higher OS when treating angiosarcoma,36,46 which is particularly true for T1 tumors where margin control is imperative, and many cases are treated with a combination of WLE and RT. Overall survival times are lower for T2 tumors, as these tumors are larger and most likely have spread; therefore, more aggressive combination treatments were more prevalent. In these cases, complete margin control may be difficult to achieve and may not be as critical to the outcome if another form of adjuvant therapy can be administered promptly.24,64
When surgery is contraindicated, RT with or without CT was the most commonly reported treatment modality. However, these treatments were notably less effective than when used in combination with surgical resection. The use of RT alone has a recurrence rate reported up to 100% in certain studies, suggesting the need to utilize RT in combination with other modalities.23,39 It is important to note that RT often is used as monotherapy in palliative treatment, which may indirectly skew survival rates.2
Limitations of the study include a lack of randomized controlled trials. Most reports were retrospective reviews or case series, and tumor staging was sparsely reported. Finally, although MMS may provide utility in the treatment of cAS, the sample size of 9 precluded definitive conclusions from being formed about its efficacy.
CONCLUSION
Cutaneous angiosarcoma is rare and has limited data comparing different treatment modalities. The paucity of data currently limits definitive recommendations; however, both surgical and nonsurgical modalities have demonstrated potential efficacy in the treatment of cAS and may benefit from additional research. Clinicians should consider a multidisciplinary approach for patients with a diagnosis of cAS to tailor treatments on a case-by-case basis.
- Rodríguez-Jiménez P, Jimenez YD, Reolid A, et al. State of the art of Mohs surgery for rare cutaneous tumors in the Spanish Registry of Mohs Surgery (REGESMOHS). Int J Dermatol. 2020;59:321-325.
- Alqumber NA, Choi JW, Kang MK. The management and prognosis of facial and scalp angiosarcoma: a retrospective analysis of 15 patients. Ann Plast Surg. 2019;83:55-62.
- Pawlik TM, Paulino AF, McGinn CJ, et al. Cutaneous angiosarcoma of the scalp: a multidisciplinary approach. Cancer. 2003;98:1716-1726.
- Deyrup AT, McKenney JK, Tighiouart M, et al. Sporadic cutaneous angiosarcomas: a proposal for risk stratification based on 69 cases. Am J Surg Pathol. 2008;32:72-77.
- Meis-Kindblom JM, Kindblom LG. Angiosarcoma of soft tissue: a study of 80 cases. Am J Surg Pathol. 1998;22:683-697.
- Harbour P, Song DH. The skin and subcutaneous tissue. In: Brunicardi FC, Andersen DK, Billiar TR, et al, eds. Schwartz’s Principles of Surgery. 11th ed. McGraw-Hill Education; 2019. Accessed April 24, 2023. https://accesssurgery.mhmedical.com/content.aspx?bookid=2576§ionid=216206374
- Oashi K, Namikawa K, Tsutsumida A, et al. Surgery with curative intent is associated with prolonged survival in patients with cutaneous angiosarcoma of the scalp and face—a retrospective study of 38 untreated cases in the Japanese population. Eur J Surg Oncol. 2018;44:823-829.
- Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol. 2010;11:983-991.
- Tolkachjov SN, Brodland DG, Coldiron BM, et al. Understanding Mohs micrographic surgery: a review and practical guide for the nondermatologist. Mayo Clin Proc. 2017;92:1261-1271.
- Amin M, Edge SB, Greene FL, et al, eds. AJCC Cancer Staging Manual. 8th ed. Springer; 2017.
- Holden CA, Spittle MF, Jones EW. Angiosarcoma of the face and scalp, prognosis and treatment. Cancer. 1987;59:1046-1057.
- Cook DA, Reed DA. Appraising the quality of medical education research methods: the Medical Education Research Study Quality Instrument and the Newcastle-Ottawa Scale-Education. Acad Med. 2015;90:1067-1076.
- Lee BL, Chen CF, Chen PC, et al. Investigation of prognostic features in primary cutaneous and soft tissue angiosarcoma after surgical resection: a retrospective study. Ann Plast Surg. 2017;78(3 suppl 2):S41-S46.
- Shen CJ, Parzuchowski AS, Kummerlowe MN, et al. Combined modality therapy improves overall survival for angiosarcoma. Acta Oncol. 2017;56:1235-1238.
- Breakey RW, Crowley TP, Anderson IB, et al. The surgical management of head and neck sarcoma: the Newcastle experience. J Plast Reconstr Aesthet Surg. 2017;70:78-84.
- Singla S, Papavasiliou P, Powers B, et al. Challenges in the treatment of angiosarcoma: a single institution experience. Am J Surg. 2014;208:254-259.
- Sasaki R, Soejima T, Kishi K, et al. Angiosarcoma treated with radiotherapy: impact of tumor type and size on outcome. Int J Radiat Oncol Biol Phys. 2002;52:1032-1040.
- Naka N, Ohsawa M, Tomita Y, et al. Angiosarcoma in Japan. A review of 99 cases. Cancer. 1995;75:989-996.
- DeMartelaere SL, Roberts D, Burgess MA, et al. Neoadjuvant chemotherapy-specific and overall treatment outcomes in patients with cutaneous angiosarcoma of the face with periorbital involvement. Head Neck. 2008;30:639-646.
- Ward JR, Feigenberg SJ, Mendenhall NP, et al. Radiation therapy for angiosarcoma. Head Neck. 2003;25:873-878.
- Letsa I, Benson C, Al-Muderis O, et al. Angiosarcoma of the face and scalp: effective systemic treatment in the older patient. J Geriatr Oncol. 2014;5:276-280.
- Buehler D, Rice SR, Moody JS, et al. Angiosarcoma outcomes and prognostic factors: a 25-year single institution experience. Am J Clin Oncol. 2014;37:473-479.
- Patel SH, Hayden RE, Hinni ML, et al. Angiosarcoma of the scalp and face: the Mayo Clinic experience. JAMA Otolaryngol Head Neck Surg. 2015;141:335-340.
- Guadagnolo BA, Zagars GK, Araujo D, et al. Outcomes after definitive treatment for cutaneous angiosarcoma of the face and scalp. Head Neck. 2011;33:661-667.
- Zhang Y, Yan Y, Zhu M, et al. Clinical outcomes in primary scalp angiosarcoma. Oncol Lett. 2019;18:5091-5096.
- Kamo R, Ishii M. Histological differentiation, histogenesis and prognosis of cutaneous angiosarcoma. Osaka City Med J. 2011;57:31-44.
- Ito T, Uchi H, Nakahara T, et al. Cutaneous angiosarcoma of the head and face: a single-center analysis of treatment outcomes in 43 patients in Japan. J Cancer Res Clin Oncol. 2016;142:1387-1394.
- Aust MR, Olsen KD, Lewis JE, et al. Angiosarcomas of the head and neck: clinical and pathologic characteristics. Ann Otol Rhinol Laryngol. 1997;106:943-951.
- Buschmann A, Lehnhardt M, Toman N, et al. Surgical treatment of angiosarcoma of the scalp: less is more. Ann Plast Surg. 2008;61:399-403.
- Cassidy RJ, Switchenko JM, Yushak ML, et al. The importance of surgery in scalp angiosarcomas. Surg Oncol. 2018;27:A3-A8.
- Choi JH, Ahn KC, Chang H, et al. Surgical treatment and prognosis of angiosarcoma of the scalp: a retrospective analysis of 14 patients in a single institution. Biomed Res Int. 2015;2015:321896.
- Chow TL, Kwan WW, Kwan CK. Treatment of cutaneous angiosarcoma of the scalp and face in Chinese patients: local experience at a regional hospital in Hong Kong. Hong Kong Med J. 2018;24:25-31.
- Donghi D, Kerl K, Dummer R, et al. Cutaneous angiosarcoma: own experience over 13 years. clinical features, disease course and immunohistochemical profile. J Eur Acad Dermatol Venereol. 2010;24:1230-1234.
- Ferrari A, Casanova M, Bisogno G, et al. Malignant vascular tumors in children and adolescents: a report from the Italian and German Soft Tissue Sarcoma Cooperative Group. Med Pediatr Oncol. 2002;39:109-114.
- Fujisawa Y, Nakamura Y, Kawachi Y, et al. Comparison between taxane-based chemotherapy with conventional surgery-based therapy for cutaneous angiosarcoma: a single-center experience. J Dermatolog Treat. 2014;25:419-423.
- Hodgkinson DJ, Soule EH, Woods JE. Cutaneous angiosarcoma of the head and neck. Cancer. 1979;44:1106-1113.
- Lim SY, Pyon JK, Mun GH, et al. Surgical treatment of angiosarcoma of the scalp with superficial parotidectomy. Ann Plast Surg. 2010;64:180-182.
- Maddox JC, Evans HL. Angiosarcoma of skin and soft tissue: a study of forty-four cases. Cancer. 1981;48:1907-1921.
- Mark RJ, Tran LM, Sercarz J, et al. Angiosarcoma of the head and neck. The UCLA experience 1955 through 1990. Arch Otolaryngol Head Neck Surg. 1993;119:973-978.
- Morgan MB, Swann M, Somach S, et al. Cutaneous angiosarcoma: a case series with prognostic correlation. J Am Acad Dermatol. 2004;50:867-874.
- Mullins B, Hackman T. Angiosarcoma of the head and neck. Int Arch Otorhinolaryngol. 2015;19:191-195.
- Ogawa K, Takahashi K, Asato Y, et al. Treatment and prognosis of angiosarcoma of the scalp and face: a retrospective analysis of 48 patients. Br J Radiol. 2012;85:E1127-E1133.
- Panje WR, Moran WJ, Bostwick DG, et al. Angiosarcoma of the head and neck: review of 11 cases. Laryngoscope. 1986;96:1381-1384.
- Perez MC, Padhya TA, Messina JL, et al. Cutaneous angiosarcoma: a single-institution experience. Ann Surg Oncol. 2013;20:3391-3397.
- Veness M, Cooper S. Treatment of cutaneous angiosarcomas of the head and neck. Australas Radiol. 1995;39:277-281.
- Barttelbort SW, Stahl R, Ariyan S. Cutaneous angiosarcoma of the face and scalp. Plast Reconstr Surg. 1989;84:55-59.
- Bernstein JM, Irish JC, Brown DH, et al. Survival outcomes for cutaneous angiosarcoma of the scalp versus face. Head Neck. 2017;39:1205-1211.
- Köhler HF, Neves RI, Brechtbühl ER, et al. Cutaneous angiosarcoma of the head and neck: report of 23 cases from a single institution. Otolaryngol Head Neck Surg. 2008;139:519-524.
- Morales PH, Lindberg RD, Barkley HT Jr. Soft tissue angiosarcomas. Int J Radiat Oncol Biol Phys. 1981;7:1655-1659.
- Wollina U, Hansel G, Schönlebe J, et al. Cutaneous angiosarcoma is a rare aggressive malignant vascular tumour of the skin. J Eur Acad Dermatol Venereol. 2011;25:964-968.
- Wollina U, Koch A, Hansel G, et al. A 10-year analysis of cutaneous mesenchymal tumors (sarcomas and related entities) in a skin cancer center. Int J Dermatol. 2013;52:1189-1197.
- Bien E, Stachowicz-Stencel T, Balcerska A, et al. Angiosarcoma in children - still uncontrollable oncological problem. The report of the Polish Paediatric Rare Tumours Study. Eur J Cancer Care (Engl). 2009;18:411-420.
- Suzuki G, Yamazaki H, Takenaka H, et al. Definitive radiation therapy for angiosarcoma of the face and scalp. In Vivo. 2016;30:921-926.
- Miki Y, Tada T, Kamo R, et al. Single institutional experience of the treatment of angiosarcoma of the face and scalp. Br J Radiol. 2013;86:20130439.
- Ohguri T, Imada H, Nomoto S, et al. Angiosarcoma of the scalp treated with curative radiotherapy plus recombinant interleukin-2 immunotherapy. Int J Radiat Oncol Biol Phys. 2005;61:1446-1453.
- Clayton BD, Leshin B, Hitchcock MG, et al. Utility of rush paraffin-embedded tangential sections in the management of cutaneous neoplasms. Dermatol Surg. 2000;26:671-678.
- Goldberg DJ, Kim YA. Angiosarcoma of the scalp treated with Mohs micrographic surgery. J Dermatol Surg Oncol. 1993;19:156-158.
- Mikhail GR, Kelly AP Jr. Malignant angioendothelioma of the face. J Dermatol Surg Oncol. 1977;3:181-183.
- Muscarella VA. Angiosarcoma treated by Mohs micrographic surgery. J Dermatol Surg Oncol. 1993;19:1132-1133.
- Bullen R, Larson PO, Landeck AE, et al. Angiosarcoma of the head and neck managed by a combination of multiple biopsies to determine tumor margin and radiation therapy. report of three cases and review of the literature. Dermatol Surg. 1998;24:1105-1110.
- Wiwatwongwana D, White VA, Dolman PJ. Two cases of periocular cutaneous angiosarcoma. Ophthalmic Plast Reconstr Surg. 2010;26:365-366.
- Morrison WH, Byers RM, Garden AS, et al. Cutaneous angiosarcoma of the head and neck. A therapeutic dilemma. Cancer. 1995;76:319-327.
- Hata M, Wada H, Ogino I, et al. Radiation therapy for angiosarcoma of the scalp: treatment outcomes of total scalp irradiation with X-rays and electrons. Strahlenther Onkol. 2014;190:899-904.
- Hwang K, Kim MY, Lee SH. Recommendations for therapeutic decisions of angiosarcoma of the scalp and face. J Craniofac Surg. 2015;26:E253-E256.
- Rodríguez-Jiménez P, Jimenez YD, Reolid A, et al. State of the art of Mohs surgery for rare cutaneous tumors in the Spanish Registry of Mohs Surgery (REGESMOHS). Int J Dermatol. 2020;59:321-325.
- Alqumber NA, Choi JW, Kang MK. The management and prognosis of facial and scalp angiosarcoma: a retrospective analysis of 15 patients. Ann Plast Surg. 2019;83:55-62.
- Pawlik TM, Paulino AF, McGinn CJ, et al. Cutaneous angiosarcoma of the scalp: a multidisciplinary approach. Cancer. 2003;98:1716-1726.
- Deyrup AT, McKenney JK, Tighiouart M, et al. Sporadic cutaneous angiosarcomas: a proposal for risk stratification based on 69 cases. Am J Surg Pathol. 2008;32:72-77.
- Meis-Kindblom JM, Kindblom LG. Angiosarcoma of soft tissue: a study of 80 cases. Am J Surg Pathol. 1998;22:683-697.
- Harbour P, Song DH. The skin and subcutaneous tissue. In: Brunicardi FC, Andersen DK, Billiar TR, et al, eds. Schwartz’s Principles of Surgery. 11th ed. McGraw-Hill Education; 2019. Accessed April 24, 2023. https://accesssurgery.mhmedical.com/content.aspx?bookid=2576§ionid=216206374
- Oashi K, Namikawa K, Tsutsumida A, et al. Surgery with curative intent is associated with prolonged survival in patients with cutaneous angiosarcoma of the scalp and face—a retrospective study of 38 untreated cases in the Japanese population. Eur J Surg Oncol. 2018;44:823-829.
- Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol. 2010;11:983-991.
- Tolkachjov SN, Brodland DG, Coldiron BM, et al. Understanding Mohs micrographic surgery: a review and practical guide for the nondermatologist. Mayo Clin Proc. 2017;92:1261-1271.
- Amin M, Edge SB, Greene FL, et al, eds. AJCC Cancer Staging Manual. 8th ed. Springer; 2017.
- Holden CA, Spittle MF, Jones EW. Angiosarcoma of the face and scalp, prognosis and treatment. Cancer. 1987;59:1046-1057.
- Cook DA, Reed DA. Appraising the quality of medical education research methods: the Medical Education Research Study Quality Instrument and the Newcastle-Ottawa Scale-Education. Acad Med. 2015;90:1067-1076.
- Lee BL, Chen CF, Chen PC, et al. Investigation of prognostic features in primary cutaneous and soft tissue angiosarcoma after surgical resection: a retrospective study. Ann Plast Surg. 2017;78(3 suppl 2):S41-S46.
- Shen CJ, Parzuchowski AS, Kummerlowe MN, et al. Combined modality therapy improves overall survival for angiosarcoma. Acta Oncol. 2017;56:1235-1238.
- Breakey RW, Crowley TP, Anderson IB, et al. The surgical management of head and neck sarcoma: the Newcastle experience. J Plast Reconstr Aesthet Surg. 2017;70:78-84.
- Singla S, Papavasiliou P, Powers B, et al. Challenges in the treatment of angiosarcoma: a single institution experience. Am J Surg. 2014;208:254-259.
- Sasaki R, Soejima T, Kishi K, et al. Angiosarcoma treated with radiotherapy: impact of tumor type and size on outcome. Int J Radiat Oncol Biol Phys. 2002;52:1032-1040.
- Naka N, Ohsawa M, Tomita Y, et al. Angiosarcoma in Japan. A review of 99 cases. Cancer. 1995;75:989-996.
- DeMartelaere SL, Roberts D, Burgess MA, et al. Neoadjuvant chemotherapy-specific and overall treatment outcomes in patients with cutaneous angiosarcoma of the face with periorbital involvement. Head Neck. 2008;30:639-646.
- Ward JR, Feigenberg SJ, Mendenhall NP, et al. Radiation therapy for angiosarcoma. Head Neck. 2003;25:873-878.
- Letsa I, Benson C, Al-Muderis O, et al. Angiosarcoma of the face and scalp: effective systemic treatment in the older patient. J Geriatr Oncol. 2014;5:276-280.
- Buehler D, Rice SR, Moody JS, et al. Angiosarcoma outcomes and prognostic factors: a 25-year single institution experience. Am J Clin Oncol. 2014;37:473-479.
- Patel SH, Hayden RE, Hinni ML, et al. Angiosarcoma of the scalp and face: the Mayo Clinic experience. JAMA Otolaryngol Head Neck Surg. 2015;141:335-340.
- Guadagnolo BA, Zagars GK, Araujo D, et al. Outcomes after definitive treatment for cutaneous angiosarcoma of the face and scalp. Head Neck. 2011;33:661-667.
- Zhang Y, Yan Y, Zhu M, et al. Clinical outcomes in primary scalp angiosarcoma. Oncol Lett. 2019;18:5091-5096.
- Kamo R, Ishii M. Histological differentiation, histogenesis and prognosis of cutaneous angiosarcoma. Osaka City Med J. 2011;57:31-44.
- Ito T, Uchi H, Nakahara T, et al. Cutaneous angiosarcoma of the head and face: a single-center analysis of treatment outcomes in 43 patients in Japan. J Cancer Res Clin Oncol. 2016;142:1387-1394.
- Aust MR, Olsen KD, Lewis JE, et al. Angiosarcomas of the head and neck: clinical and pathologic characteristics. Ann Otol Rhinol Laryngol. 1997;106:943-951.
- Buschmann A, Lehnhardt M, Toman N, et al. Surgical treatment of angiosarcoma of the scalp: less is more. Ann Plast Surg. 2008;61:399-403.
- Cassidy RJ, Switchenko JM, Yushak ML, et al. The importance of surgery in scalp angiosarcomas. Surg Oncol. 2018;27:A3-A8.
- Choi JH, Ahn KC, Chang H, et al. Surgical treatment and prognosis of angiosarcoma of the scalp: a retrospective analysis of 14 patients in a single institution. Biomed Res Int. 2015;2015:321896.
- Chow TL, Kwan WW, Kwan CK. Treatment of cutaneous angiosarcoma of the scalp and face in Chinese patients: local experience at a regional hospital in Hong Kong. Hong Kong Med J. 2018;24:25-31.
- Donghi D, Kerl K, Dummer R, et al. Cutaneous angiosarcoma: own experience over 13 years. clinical features, disease course and immunohistochemical profile. J Eur Acad Dermatol Venereol. 2010;24:1230-1234.
- Ferrari A, Casanova M, Bisogno G, et al. Malignant vascular tumors in children and adolescents: a report from the Italian and German Soft Tissue Sarcoma Cooperative Group. Med Pediatr Oncol. 2002;39:109-114.
- Fujisawa Y, Nakamura Y, Kawachi Y, et al. Comparison between taxane-based chemotherapy with conventional surgery-based therapy for cutaneous angiosarcoma: a single-center experience. J Dermatolog Treat. 2014;25:419-423.
- Hodgkinson DJ, Soule EH, Woods JE. Cutaneous angiosarcoma of the head and neck. Cancer. 1979;44:1106-1113.
- Lim SY, Pyon JK, Mun GH, et al. Surgical treatment of angiosarcoma of the scalp with superficial parotidectomy. Ann Plast Surg. 2010;64:180-182.
- Maddox JC, Evans HL. Angiosarcoma of skin and soft tissue: a study of forty-four cases. Cancer. 1981;48:1907-1921.
- Mark RJ, Tran LM, Sercarz J, et al. Angiosarcoma of the head and neck. The UCLA experience 1955 through 1990. Arch Otolaryngol Head Neck Surg. 1993;119:973-978.
- Morgan MB, Swann M, Somach S, et al. Cutaneous angiosarcoma: a case series with prognostic correlation. J Am Acad Dermatol. 2004;50:867-874.
- Mullins B, Hackman T. Angiosarcoma of the head and neck. Int Arch Otorhinolaryngol. 2015;19:191-195.
- Ogawa K, Takahashi K, Asato Y, et al. Treatment and prognosis of angiosarcoma of the scalp and face: a retrospective analysis of 48 patients. Br J Radiol. 2012;85:E1127-E1133.
- Panje WR, Moran WJ, Bostwick DG, et al. Angiosarcoma of the head and neck: review of 11 cases. Laryngoscope. 1986;96:1381-1384.
- Perez MC, Padhya TA, Messina JL, et al. Cutaneous angiosarcoma: a single-institution experience. Ann Surg Oncol. 2013;20:3391-3397.
- Veness M, Cooper S. Treatment of cutaneous angiosarcomas of the head and neck. Australas Radiol. 1995;39:277-281.
- Barttelbort SW, Stahl R, Ariyan S. Cutaneous angiosarcoma of the face and scalp. Plast Reconstr Surg. 1989;84:55-59.
- Bernstein JM, Irish JC, Brown DH, et al. Survival outcomes for cutaneous angiosarcoma of the scalp versus face. Head Neck. 2017;39:1205-1211.
- Köhler HF, Neves RI, Brechtbühl ER, et al. Cutaneous angiosarcoma of the head and neck: report of 23 cases from a single institution. Otolaryngol Head Neck Surg. 2008;139:519-524.
- Morales PH, Lindberg RD, Barkley HT Jr. Soft tissue angiosarcomas. Int J Radiat Oncol Biol Phys. 1981;7:1655-1659.
- Wollina U, Hansel G, Schönlebe J, et al. Cutaneous angiosarcoma is a rare aggressive malignant vascular tumour of the skin. J Eur Acad Dermatol Venereol. 2011;25:964-968.
- Wollina U, Koch A, Hansel G, et al. A 10-year analysis of cutaneous mesenchymal tumors (sarcomas and related entities) in a skin cancer center. Int J Dermatol. 2013;52:1189-1197.
- Bien E, Stachowicz-Stencel T, Balcerska A, et al. Angiosarcoma in children - still uncontrollable oncological problem. The report of the Polish Paediatric Rare Tumours Study. Eur J Cancer Care (Engl). 2009;18:411-420.
- Suzuki G, Yamazaki H, Takenaka H, et al. Definitive radiation therapy for angiosarcoma of the face and scalp. In Vivo. 2016;30:921-926.
- Miki Y, Tada T, Kamo R, et al. Single institutional experience of the treatment of angiosarcoma of the face and scalp. Br J Radiol. 2013;86:20130439.
- Ohguri T, Imada H, Nomoto S, et al. Angiosarcoma of the scalp treated with curative radiotherapy plus recombinant interleukin-2 immunotherapy. Int J Radiat Oncol Biol Phys. 2005;61:1446-1453.
- Clayton BD, Leshin B, Hitchcock MG, et al. Utility of rush paraffin-embedded tangential sections in the management of cutaneous neoplasms. Dermatol Surg. 2000;26:671-678.
- Goldberg DJ, Kim YA. Angiosarcoma of the scalp treated with Mohs micrographic surgery. J Dermatol Surg Oncol. 1993;19:156-158.
- Mikhail GR, Kelly AP Jr. Malignant angioendothelioma of the face. J Dermatol Surg Oncol. 1977;3:181-183.
- Muscarella VA. Angiosarcoma treated by Mohs micrographic surgery. J Dermatol Surg Oncol. 1993;19:1132-1133.
- Bullen R, Larson PO, Landeck AE, et al. Angiosarcoma of the head and neck managed by a combination of multiple biopsies to determine tumor margin and radiation therapy. report of three cases and review of the literature. Dermatol Surg. 1998;24:1105-1110.
- Wiwatwongwana D, White VA, Dolman PJ. Two cases of periocular cutaneous angiosarcoma. Ophthalmic Plast Reconstr Surg. 2010;26:365-366.
- Morrison WH, Byers RM, Garden AS, et al. Cutaneous angiosarcoma of the head and neck. A therapeutic dilemma. Cancer. 1995;76:319-327.
- Hata M, Wada H, Ogino I, et al. Radiation therapy for angiosarcoma of the scalp: treatment outcomes of total scalp irradiation with X-rays and electrons. Strahlenther Onkol. 2014;190:899-904.
- Hwang K, Kim MY, Lee SH. Recommendations for therapeutic decisions of angiosarcoma of the scalp and face. J Craniofac Surg. 2015;26:E253-E256.
Practice Points
- Angiosarcoma is a rare tumor that is difficult to treat, with multiple treatment options being utilized.
- Within this systematic review, wide local excision (WLE) combined with radiotherapy (RT), chemotherapy, and immunotherapy, as well as Mohs micrographic surgery (MMS), offered the longest mean (SD) overall survival time.
- When clinicians are tasked with treating primary cutaneous angiosarcoma of the head and neck, they should consider MMS or WLE combined with RT.
Scalp Nodule Associated With Hair Loss
The Diagnosis: Alopecic and Aseptic Nodule of the Scalp
Alopecic and aseptic nodule of the scalp (AANS) is an underdiagnosed condition presenting with one or few inflammatory nodules on the scalp with overlying nonscarring alopecia. The nodules can be soft, fluctuant, or firm and are characterized by negative fungal and bacterial stains as well as cultures.1 Trichoscopic features such as black or yellow dots, fine vellus hairs, and broken hairs have been reported.1-3 Dilated follicular openings may be seen and are termed the Eastern pancake sign, as they resemble the bubble cavities formed during the cooking of atayef.2 The histologic features of AANS often are nonspecific but show a nodular or pseudocystic, lymphohistiocytic to acute inflammatory component centered in the dermis.1 Granulomatous inflammation or isolated giant cells have been reported within the deep dermis.1,4 In our patient, histopathology revealed admixed acute and granulomatous inflammation within the deep dermis (Figure). Treatment of AANS includes oral antibiotics such as doxycycline, intralesional corticosteroids, or excision.1
Although the etiology of AANS currently is unclear, a process of follicular plugging or a deep folliculitis sparing the bulge stem cells has been theorized. Young males are disproportionately affected.1 It is uncertain how much overlap there is, if any, between AANS and pseudocyst of the scalp, the latter of which primarily is reported in the Japanese literature and demonstrates alopecic nodules between the forehead and vertex of the scalp with pseudocystic architecture and granulomatous infiltration on histopathology.4-7
There are several clinical and histologic differences between AANS and other diagnoses in the differential. Dermoid cysts tend to present at birth, with 70% of cases presenting before the age of 6 years, and without overlying skin changes.8 They represent a benign entrapment of ectoderm along embryonic closure lines during development.9 Histologic examination typically will show a squamous-lined cyst within the dermis with associated adnexal structures.10 Cylindromas are benign neoplasms of eccrine sweat glands named after the histologic presentation of cylinder-shaped basaloid cell populations when cross-sectioned.11,12 When cylindromas coalesce on the scalp, they form a distinctive morphology sometimes loosely resembling a turban, giving them the previously more common name turban tumors.11,13 Cylindromas appear as slow-growing protuberant tumors that are erythematous or flesh colored. Cylindromas are 9 times more common in females.13 Pilar cysts have a stratified squamous epithelium lining with a palisaded outer layer and are derived from the outer root sheath of hair follicles.14 Clinically, pilar cysts are smooth mobile cysts that favor skin with a dense concentration of hair follicles.14,15 On palpation, pilar cysts are firm due to their keratinous contents and typically are nontender unless inflamed.15 Lipomas are benign mesenchymal tumors with mature adipocytes that often appear as subcutaneous nodules without overlying skin changes, though they can involve deep fascia. On palpation, lipomas generally are soft, mobile, and nontender.16
- Bellinato F, Maurelli M, Colato C, et al. Alopecic and aseptic nodules of the scalp: a new case with a systematic review of the literature [published online May 1, 2021]. Clin Case Rep. 2021;9:E04153. doi:10.1002/ccr3.4153
- Lázaro-Simó AI, Sancho MI, Quintana-Codina M, et al. Alopecic and aseptic nodules of the scalp with trichoscopic and ultrasonographic findings. Indian J Dermatol. 2017;62:515-518.
- Garrido-Colmenero C, Arias-Santiago S, Aneiros Fernández J, et al. Trichoscopy and ultrasonography features of aseptic and alopecic nodules of the scalp. J Eur Acad Dermatol Venereol. 2016;30:507-509. doi:10.1111/jdv.12903
- Seol JE, Park IH, Kim DH, et al. Alopecic and aseptic nodules of the scalp/pseudocyst of the scalp: clinicopathological and therapeutic analyses in 11 Korean patients. Dermatology. 2016;232:165-170.
- Lee SS, Kim SY, Im M, et al. Pseudocyst of the scalp. Ann Dermatol. 2011;23(suppl 2):S267-S269.
- Eisenberg EL. Alopecia-associated pseudocyst of the scalp. J Am Acad Dermatol. 2012;67:E114-E116.
- Tsuruta D, Hayashi A, Kobayashi H, et al. Pseudocyst of the scalp. Dermatology. 2005;210:333-335.
- Orozco-Covarrubias L, Lara-Carpio R, Saez-De-Ocariz M, et al. Dermoid cysts: a report of 75 pediatric patients. Pediatr Dermatol. 2013;30:706-711.
- Julapalli MR, Cohen BA, Hollier LH, et al. Congenital, ill-defined, yellowish plaque: the nasal dermoid. Pediatr Dermatol. 2006;23:556-559.
- Reissis D, Pfaff MJ, Patel A, et al. Craniofacial dermoid cysts: histological analysis and inter-site comparison. Yale J Biol Med. 2014;87:349-357.
- Chauhan DS, Guruprasad Y. Dermal cylindroma of the scalp. Natl J Maxillofac Surg. 2012;3:59-61.
- Albores-Saavedra J, Heard SC, McLaren B, et al. Cylindroma (dermal analog tumor) of the breast: a comparison with cylindroma of the skin and adenoid cystic carcinoma of the breast. Am J Clin Pathol. 2005;123:866-873.
- Myers DJ, Fillman EP. Cylindroma. StatPearls. StatPearls Publishing; 2022.
- Ramaswamy AS, Manjunatha HK, Sunilkumar B, et al. Morphological spectrum of pilar cysts. N Am J Med Sci. 2013;5:124-128. doi:10.4103/1947-2714.107532
- Al Aboud DM, Yarrarapu SNS, Patel BC. Pilar cyst. StatPearls. StatPearls Publishing; 2022. 16. Kolb L, Yarrarapu SNS, Ameer MA, et al. Lipoma. StatPearls. StatPearls Publishing; 2022.
The Diagnosis: Alopecic and Aseptic Nodule of the Scalp
Alopecic and aseptic nodule of the scalp (AANS) is an underdiagnosed condition presenting with one or few inflammatory nodules on the scalp with overlying nonscarring alopecia. The nodules can be soft, fluctuant, or firm and are characterized by negative fungal and bacterial stains as well as cultures.1 Trichoscopic features such as black or yellow dots, fine vellus hairs, and broken hairs have been reported.1-3 Dilated follicular openings may be seen and are termed the Eastern pancake sign, as they resemble the bubble cavities formed during the cooking of atayef.2 The histologic features of AANS often are nonspecific but show a nodular or pseudocystic, lymphohistiocytic to acute inflammatory component centered in the dermis.1 Granulomatous inflammation or isolated giant cells have been reported within the deep dermis.1,4 In our patient, histopathology revealed admixed acute and granulomatous inflammation within the deep dermis (Figure). Treatment of AANS includes oral antibiotics such as doxycycline, intralesional corticosteroids, or excision.1

Although the etiology of AANS currently is unclear, a process of follicular plugging or a deep folliculitis sparing the bulge stem cells has been theorized. Young males are disproportionately affected.1 It is uncertain how much overlap there is, if any, between AANS and pseudocyst of the scalp, the latter of which primarily is reported in the Japanese literature and demonstrates alopecic nodules between the forehead and vertex of the scalp with pseudocystic architecture and granulomatous infiltration on histopathology.4-7
There are several clinical and histologic differences between AANS and other diagnoses in the differential. Dermoid cysts tend to present at birth, with 70% of cases presenting before the age of 6 years, and without overlying skin changes.8 They represent a benign entrapment of ectoderm along embryonic closure lines during development.9 Histologic examination typically will show a squamous-lined cyst within the dermis with associated adnexal structures.10 Cylindromas are benign neoplasms of eccrine sweat glands named after the histologic presentation of cylinder-shaped basaloid cell populations when cross-sectioned.11,12 When cylindromas coalesce on the scalp, they form a distinctive morphology sometimes loosely resembling a turban, giving them the previously more common name turban tumors.11,13 Cylindromas appear as slow-growing protuberant tumors that are erythematous or flesh colored. Cylindromas are 9 times more common in females.13 Pilar cysts have a stratified squamous epithelium lining with a palisaded outer layer and are derived from the outer root sheath of hair follicles.14 Clinically, pilar cysts are smooth mobile cysts that favor skin with a dense concentration of hair follicles.14,15 On palpation, pilar cysts are firm due to their keratinous contents and typically are nontender unless inflamed.15 Lipomas are benign mesenchymal tumors with mature adipocytes that often appear as subcutaneous nodules without overlying skin changes, though they can involve deep fascia. On palpation, lipomas generally are soft, mobile, and nontender.16
The Diagnosis: Alopecic and Aseptic Nodule of the Scalp
Alopecic and aseptic nodule of the scalp (AANS) is an underdiagnosed condition presenting with one or few inflammatory nodules on the scalp with overlying nonscarring alopecia. The nodules can be soft, fluctuant, or firm and are characterized by negative fungal and bacterial stains as well as cultures.1 Trichoscopic features such as black or yellow dots, fine vellus hairs, and broken hairs have been reported.1-3 Dilated follicular openings may be seen and are termed the Eastern pancake sign, as they resemble the bubble cavities formed during the cooking of atayef.2 The histologic features of AANS often are nonspecific but show a nodular or pseudocystic, lymphohistiocytic to acute inflammatory component centered in the dermis.1 Granulomatous inflammation or isolated giant cells have been reported within the deep dermis.1,4 In our patient, histopathology revealed admixed acute and granulomatous inflammation within the deep dermis (Figure). Treatment of AANS includes oral antibiotics such as doxycycline, intralesional corticosteroids, or excision.1

Although the etiology of AANS currently is unclear, a process of follicular plugging or a deep folliculitis sparing the bulge stem cells has been theorized. Young males are disproportionately affected.1 It is uncertain how much overlap there is, if any, between AANS and pseudocyst of the scalp, the latter of which primarily is reported in the Japanese literature and demonstrates alopecic nodules between the forehead and vertex of the scalp with pseudocystic architecture and granulomatous infiltration on histopathology.4-7
There are several clinical and histologic differences between AANS and other diagnoses in the differential. Dermoid cysts tend to present at birth, with 70% of cases presenting before the age of 6 years, and without overlying skin changes.8 They represent a benign entrapment of ectoderm along embryonic closure lines during development.9 Histologic examination typically will show a squamous-lined cyst within the dermis with associated adnexal structures.10 Cylindromas are benign neoplasms of eccrine sweat glands named after the histologic presentation of cylinder-shaped basaloid cell populations when cross-sectioned.11,12 When cylindromas coalesce on the scalp, they form a distinctive morphology sometimes loosely resembling a turban, giving them the previously more common name turban tumors.11,13 Cylindromas appear as slow-growing protuberant tumors that are erythematous or flesh colored. Cylindromas are 9 times more common in females.13 Pilar cysts have a stratified squamous epithelium lining with a palisaded outer layer and are derived from the outer root sheath of hair follicles.14 Clinically, pilar cysts are smooth mobile cysts that favor skin with a dense concentration of hair follicles.14,15 On palpation, pilar cysts are firm due to their keratinous contents and typically are nontender unless inflamed.15 Lipomas are benign mesenchymal tumors with mature adipocytes that often appear as subcutaneous nodules without overlying skin changes, though they can involve deep fascia. On palpation, lipomas generally are soft, mobile, and nontender.16
- Bellinato F, Maurelli M, Colato C, et al. Alopecic and aseptic nodules of the scalp: a new case with a systematic review of the literature [published online May 1, 2021]. Clin Case Rep. 2021;9:E04153. doi:10.1002/ccr3.4153
- Lázaro-Simó AI, Sancho MI, Quintana-Codina M, et al. Alopecic and aseptic nodules of the scalp with trichoscopic and ultrasonographic findings. Indian J Dermatol. 2017;62:515-518.
- Garrido-Colmenero C, Arias-Santiago S, Aneiros Fernández J, et al. Trichoscopy and ultrasonography features of aseptic and alopecic nodules of the scalp. J Eur Acad Dermatol Venereol. 2016;30:507-509. doi:10.1111/jdv.12903
- Seol JE, Park IH, Kim DH, et al. Alopecic and aseptic nodules of the scalp/pseudocyst of the scalp: clinicopathological and therapeutic analyses in 11 Korean patients. Dermatology. 2016;232:165-170.
- Lee SS, Kim SY, Im M, et al. Pseudocyst of the scalp. Ann Dermatol. 2011;23(suppl 2):S267-S269.
- Eisenberg EL. Alopecia-associated pseudocyst of the scalp. J Am Acad Dermatol. 2012;67:E114-E116.
- Tsuruta D, Hayashi A, Kobayashi H, et al. Pseudocyst of the scalp. Dermatology. 2005;210:333-335.
- Orozco-Covarrubias L, Lara-Carpio R, Saez-De-Ocariz M, et al. Dermoid cysts: a report of 75 pediatric patients. Pediatr Dermatol. 2013;30:706-711.
- Julapalli MR, Cohen BA, Hollier LH, et al. Congenital, ill-defined, yellowish plaque: the nasal dermoid. Pediatr Dermatol. 2006;23:556-559.
- Reissis D, Pfaff MJ, Patel A, et al. Craniofacial dermoid cysts: histological analysis and inter-site comparison. Yale J Biol Med. 2014;87:349-357.
- Chauhan DS, Guruprasad Y. Dermal cylindroma of the scalp. Natl J Maxillofac Surg. 2012;3:59-61.
- Albores-Saavedra J, Heard SC, McLaren B, et al. Cylindroma (dermal analog tumor) of the breast: a comparison with cylindroma of the skin and adenoid cystic carcinoma of the breast. Am J Clin Pathol. 2005;123:866-873.
- Myers DJ, Fillman EP. Cylindroma. StatPearls. StatPearls Publishing; 2022.
- Ramaswamy AS, Manjunatha HK, Sunilkumar B, et al. Morphological spectrum of pilar cysts. N Am J Med Sci. 2013;5:124-128. doi:10.4103/1947-2714.107532
- Al Aboud DM, Yarrarapu SNS, Patel BC. Pilar cyst. StatPearls. StatPearls Publishing; 2022. 16. Kolb L, Yarrarapu SNS, Ameer MA, et al. Lipoma. StatPearls. StatPearls Publishing; 2022.
- Bellinato F, Maurelli M, Colato C, et al. Alopecic and aseptic nodules of the scalp: a new case with a systematic review of the literature [published online May 1, 2021]. Clin Case Rep. 2021;9:E04153. doi:10.1002/ccr3.4153
- Lázaro-Simó AI, Sancho MI, Quintana-Codina M, et al. Alopecic and aseptic nodules of the scalp with trichoscopic and ultrasonographic findings. Indian J Dermatol. 2017;62:515-518.
- Garrido-Colmenero C, Arias-Santiago S, Aneiros Fernández J, et al. Trichoscopy and ultrasonography features of aseptic and alopecic nodules of the scalp. J Eur Acad Dermatol Venereol. 2016;30:507-509. doi:10.1111/jdv.12903
- Seol JE, Park IH, Kim DH, et al. Alopecic and aseptic nodules of the scalp/pseudocyst of the scalp: clinicopathological and therapeutic analyses in 11 Korean patients. Dermatology. 2016;232:165-170.
- Lee SS, Kim SY, Im M, et al. Pseudocyst of the scalp. Ann Dermatol. 2011;23(suppl 2):S267-S269.
- Eisenberg EL. Alopecia-associated pseudocyst of the scalp. J Am Acad Dermatol. 2012;67:E114-E116.
- Tsuruta D, Hayashi A, Kobayashi H, et al. Pseudocyst of the scalp. Dermatology. 2005;210:333-335.
- Orozco-Covarrubias L, Lara-Carpio R, Saez-De-Ocariz M, et al. Dermoid cysts: a report of 75 pediatric patients. Pediatr Dermatol. 2013;30:706-711.
- Julapalli MR, Cohen BA, Hollier LH, et al. Congenital, ill-defined, yellowish plaque: the nasal dermoid. Pediatr Dermatol. 2006;23:556-559.
- Reissis D, Pfaff MJ, Patel A, et al. Craniofacial dermoid cysts: histological analysis and inter-site comparison. Yale J Biol Med. 2014;87:349-357.
- Chauhan DS, Guruprasad Y. Dermal cylindroma of the scalp. Natl J Maxillofac Surg. 2012;3:59-61.
- Albores-Saavedra J, Heard SC, McLaren B, et al. Cylindroma (dermal analog tumor) of the breast: a comparison with cylindroma of the skin and adenoid cystic carcinoma of the breast. Am J Clin Pathol. 2005;123:866-873.
- Myers DJ, Fillman EP. Cylindroma. StatPearls. StatPearls Publishing; 2022.
- Ramaswamy AS, Manjunatha HK, Sunilkumar B, et al. Morphological spectrum of pilar cysts. N Am J Med Sci. 2013;5:124-128. doi:10.4103/1947-2714.107532
- Al Aboud DM, Yarrarapu SNS, Patel BC. Pilar cyst. StatPearls. StatPearls Publishing; 2022. 16. Kolb L, Yarrarapu SNS, Ameer MA, et al. Lipoma. StatPearls. StatPearls Publishing; 2022.
A 9-year-old boy presented with a soft subcutaneous nodule with overlying alopecia on the right parietal scalp of 5 months’ duration that had grown in size, became increasingly alopecic, and was complicated by intermittent pain. An excisional biopsy of the nodule revealed deep dermal mixed inflammation with scattered granulomas. No foreign material, definitive cystic spaces, or cyst wall lining was identified. Special stains including periodic acid– Schiff, Fite acid-fast, and Twort Gram were negative for infectious organisms. His postoperative course was uneventful, and no recurrence of the nodule was reported.

Two Canadian provinces lift licensing barriers for U.S. doctors
They’ll no longer have to start with a limited license and take additional exams or be supervised for up to a year to become fully licensed.
Canada is experiencing an acute shortage of licensed physicians that’s expected to intensify over the next decade. The shortfall is estimated to be about 44,000 physicians by 2028, with family doctors accounting for 72% of the deficit.
“Reducing licensing barriers should make Canada a more attractive option for U.S. doctors who may be considering a move north,” said Tom Florence, president of AMN Healthcare’s Physician Solutions division, which recruits American physicians to work in Canada.
“Canada also has a truly expedited work visa process for qualifying physicians who have a job offer and wish to practice there,” said Mr. Florence. It usually takes about 6 months compared with at least 18 months for Canadian physicians who want to work in the United States, he said.
Few U.S.-trained physicians work in Canada, which has a population of nearly 39 million. Just 812 of them practiced in Canada in 2019, the last year data was collected, according to the Canadian Medical Association.
But Canada may attract American physicians who find U.S. medicine to be fraught with ethical dilemmas and restrictions from insurance companies and elected officials, said Theresa Rohr-Kirchgraber, MD, an internist and immediate past president of the American Medical Women’s Association.
“Rather than give up practicing medicine, a move to Canada may be a welcome respite for some U.S. physicians,” she said.
Physician recruiters in Ontario and Nova Scotia welcomed the news. About 13% of the population is without a family doctor, according to news reports.
A number of U.S. physicians have started practices in Nova Scotia in recent years, said Katrina Philopoulos, Nova Scotia Health’s director of physician recruitment. “I think this momentum will help us,” she said.
Other Canadian provinces with physician shortages are also considering making similar changes. Alberta recently announced a 5-year pilot project to waive some licensing requirements for family doctors and general practitioners trained in Australia, Ireland, United Kingdom, and the United States.
What are the pros and cons of working in Canada?
“Some U.S. physicians may be attracted by a single-payer system in which all patients have access to coverage, but there are a range of drawbacks and benefits to consider in both systems,” said Mr. Florence.
U.S. physicians generally earn more than their Canadian counterparts, so income is not likely to be a draw, he said.
That appears to be the case for both family medicine physicians and specialists when comparing average net annual salaries. (To obtain Canadian salaries, 2021 gross income data from the Canadian Institute for Health Information were used; 20% was deducted for operation costs; and Canadian dollars were converted into U.S. dollars based on the current exchange rate.)
A family medicine doctor in Canada will earn an annual average salary of $195,853 USD compared with $236,000 in the United States. A cardiologist in Canada will earn $314,051 USD annually compared with $459,000 in the United States. A dermatologist in Canada will earn $270,018 annually compared with $394,000 in the United States.
Everett Fuller, MD, an emergency medicine physician who moved from Texas to Nova Scotia in 2015 for his Canadian wife, recently wrote about the pros and cons of working there compared with the United States. For him, it was a worthwhile move.
“It’s getting back to making medicine and patient care the priority instead of the business of medicine,” Dr. Fuller wrote.
“I have the comfort of knowing that a patient and their family will not go bankrupt trying to pay medical bills if I make a catastrophic diagnosis. There’s no out-of-pocket cost, other than prescriptions (depending on their drug plan).”
Dr. Fuller also doesn’t have to fight insurers for reimbursement or preapprovals, and he pays much less for medical malpractice premiums in a less litigious environment, he said.
But he mentioned a few negatives. Some treatment is rationed, which can lead to long wait times for patients to get appointments. Also, “hospitals aren’t in it for the profit, so you’re not going to get a CT, MRI, and cath lab in every hospital,” he noted.
Mr. Florence doesn’t think either system “offers a panacea for many of the challenges physicians face today. Even with reduced barriers to licensure, we do not anticipate an exodus to U.S. physicians to the north.”
A version of this article first appeared on Medscape.com.
They’ll no longer have to start with a limited license and take additional exams or be supervised for up to a year to become fully licensed.
Canada is experiencing an acute shortage of licensed physicians that’s expected to intensify over the next decade. The shortfall is estimated to be about 44,000 physicians by 2028, with family doctors accounting for 72% of the deficit.
“Reducing licensing barriers should make Canada a more attractive option for U.S. doctors who may be considering a move north,” said Tom Florence, president of AMN Healthcare’s Physician Solutions division, which recruits American physicians to work in Canada.
“Canada also has a truly expedited work visa process for qualifying physicians who have a job offer and wish to practice there,” said Mr. Florence. It usually takes about 6 months compared with at least 18 months for Canadian physicians who want to work in the United States, he said.
Few U.S.-trained physicians work in Canada, which has a population of nearly 39 million. Just 812 of them practiced in Canada in 2019, the last year data was collected, according to the Canadian Medical Association.
But Canada may attract American physicians who find U.S. medicine to be fraught with ethical dilemmas and restrictions from insurance companies and elected officials, said Theresa Rohr-Kirchgraber, MD, an internist and immediate past president of the American Medical Women’s Association.
“Rather than give up practicing medicine, a move to Canada may be a welcome respite for some U.S. physicians,” she said.
Physician recruiters in Ontario and Nova Scotia welcomed the news. About 13% of the population is without a family doctor, according to news reports.
A number of U.S. physicians have started practices in Nova Scotia in recent years, said Katrina Philopoulos, Nova Scotia Health’s director of physician recruitment. “I think this momentum will help us,” she said.
Other Canadian provinces with physician shortages are also considering making similar changes. Alberta recently announced a 5-year pilot project to waive some licensing requirements for family doctors and general practitioners trained in Australia, Ireland, United Kingdom, and the United States.
What are the pros and cons of working in Canada?
“Some U.S. physicians may be attracted by a single-payer system in which all patients have access to coverage, but there are a range of drawbacks and benefits to consider in both systems,” said Mr. Florence.
U.S. physicians generally earn more than their Canadian counterparts, so income is not likely to be a draw, he said.
That appears to be the case for both family medicine physicians and specialists when comparing average net annual salaries. (To obtain Canadian salaries, 2021 gross income data from the Canadian Institute for Health Information were used; 20% was deducted for operation costs; and Canadian dollars were converted into U.S. dollars based on the current exchange rate.)
A family medicine doctor in Canada will earn an annual average salary of $195,853 USD compared with $236,000 in the United States. A cardiologist in Canada will earn $314,051 USD annually compared with $459,000 in the United States. A dermatologist in Canada will earn $270,018 annually compared with $394,000 in the United States.
Everett Fuller, MD, an emergency medicine physician who moved from Texas to Nova Scotia in 2015 for his Canadian wife, recently wrote about the pros and cons of working there compared with the United States. For him, it was a worthwhile move.
“It’s getting back to making medicine and patient care the priority instead of the business of medicine,” Dr. Fuller wrote.
“I have the comfort of knowing that a patient and their family will not go bankrupt trying to pay medical bills if I make a catastrophic diagnosis. There’s no out-of-pocket cost, other than prescriptions (depending on their drug plan).”
Dr. Fuller also doesn’t have to fight insurers for reimbursement or preapprovals, and he pays much less for medical malpractice premiums in a less litigious environment, he said.
But he mentioned a few negatives. Some treatment is rationed, which can lead to long wait times for patients to get appointments. Also, “hospitals aren’t in it for the profit, so you’re not going to get a CT, MRI, and cath lab in every hospital,” he noted.
Mr. Florence doesn’t think either system “offers a panacea for many of the challenges physicians face today. Even with reduced barriers to licensure, we do not anticipate an exodus to U.S. physicians to the north.”
A version of this article first appeared on Medscape.com.
They’ll no longer have to start with a limited license and take additional exams or be supervised for up to a year to become fully licensed.
Canada is experiencing an acute shortage of licensed physicians that’s expected to intensify over the next decade. The shortfall is estimated to be about 44,000 physicians by 2028, with family doctors accounting for 72% of the deficit.
“Reducing licensing barriers should make Canada a more attractive option for U.S. doctors who may be considering a move north,” said Tom Florence, president of AMN Healthcare’s Physician Solutions division, which recruits American physicians to work in Canada.
“Canada also has a truly expedited work visa process for qualifying physicians who have a job offer and wish to practice there,” said Mr. Florence. It usually takes about 6 months compared with at least 18 months for Canadian physicians who want to work in the United States, he said.
Few U.S.-trained physicians work in Canada, which has a population of nearly 39 million. Just 812 of them practiced in Canada in 2019, the last year data was collected, according to the Canadian Medical Association.
But Canada may attract American physicians who find U.S. medicine to be fraught with ethical dilemmas and restrictions from insurance companies and elected officials, said Theresa Rohr-Kirchgraber, MD, an internist and immediate past president of the American Medical Women’s Association.
“Rather than give up practicing medicine, a move to Canada may be a welcome respite for some U.S. physicians,” she said.
Physician recruiters in Ontario and Nova Scotia welcomed the news. About 13% of the population is without a family doctor, according to news reports.
A number of U.S. physicians have started practices in Nova Scotia in recent years, said Katrina Philopoulos, Nova Scotia Health’s director of physician recruitment. “I think this momentum will help us,” she said.
Other Canadian provinces with physician shortages are also considering making similar changes. Alberta recently announced a 5-year pilot project to waive some licensing requirements for family doctors and general practitioners trained in Australia, Ireland, United Kingdom, and the United States.
What are the pros and cons of working in Canada?
“Some U.S. physicians may be attracted by a single-payer system in which all patients have access to coverage, but there are a range of drawbacks and benefits to consider in both systems,” said Mr. Florence.
U.S. physicians generally earn more than their Canadian counterparts, so income is not likely to be a draw, he said.
That appears to be the case for both family medicine physicians and specialists when comparing average net annual salaries. (To obtain Canadian salaries, 2021 gross income data from the Canadian Institute for Health Information were used; 20% was deducted for operation costs; and Canadian dollars were converted into U.S. dollars based on the current exchange rate.)
A family medicine doctor in Canada will earn an annual average salary of $195,853 USD compared with $236,000 in the United States. A cardiologist in Canada will earn $314,051 USD annually compared with $459,000 in the United States. A dermatologist in Canada will earn $270,018 annually compared with $394,000 in the United States.
Everett Fuller, MD, an emergency medicine physician who moved from Texas to Nova Scotia in 2015 for his Canadian wife, recently wrote about the pros and cons of working there compared with the United States. For him, it was a worthwhile move.
“It’s getting back to making medicine and patient care the priority instead of the business of medicine,” Dr. Fuller wrote.
“I have the comfort of knowing that a patient and their family will not go bankrupt trying to pay medical bills if I make a catastrophic diagnosis. There’s no out-of-pocket cost, other than prescriptions (depending on their drug plan).”
Dr. Fuller also doesn’t have to fight insurers for reimbursement or preapprovals, and he pays much less for medical malpractice premiums in a less litigious environment, he said.
But he mentioned a few negatives. Some treatment is rationed, which can lead to long wait times for patients to get appointments. Also, “hospitals aren’t in it for the profit, so you’re not going to get a CT, MRI, and cath lab in every hospital,” he noted.
Mr. Florence doesn’t think either system “offers a panacea for many of the challenges physicians face today. Even with reduced barriers to licensure, we do not anticipate an exodus to U.S. physicians to the north.”
A version of this article first appeared on Medscape.com.
Researchers seek to understand post-COVID autoimmune disease risk
Since the COVID-19 pandemic started more than 3 years ago, the longer-lasting effects of SARS-CoV-2 infection have continued to reveal themselves. Approximately 28% of Americans report having ever experienced post-COVID conditions, such as brain fog, postexertional malaise, and joint pain, and 11% say they are still experiencing these long-term effects. Now, new research is showing that people who have had COVID are more likely to newly develop an autoimmune disease. Exactly why this is happening is less clear, experts say.
Two preprint studies and one study published in a peer-reviewed journal provide strong evidence that patients who have been infected with SARS-CoV-2 are at elevated risk of developing an autoimmune disease. The studies retrospectively reviewed medical records from three countries and compared the incidence of new-onset autoimmune disease among patients who had polymerase chain reaction–confirmed COVID-19 and those who had never been diagnosed with the virus.
A study analyzing the health records of 3.8 million U.S. patients – more than 888,460 with confirmed COVID-19 – found that the COVID-19 group was two to three times as likely to develop various autoimmune diseases, such as rheumatoid arthritis, systemic lupus erythematosus, and systemic sclerosis. A U.K. preprint study that included more than 458,000 people with confirmed COVID found that those who had previously been infected with SARS-CoV-2 were 22% more likely to develop an autoimmune disease compared with the control group. In this cohort, the diseases most strongly associated with COVID-19 were type 1 diabetes, inflammatory bowel disease, and psoriasis. A preprint study from German researchers found that COVID-19 patients were almost 43% more likely to develop an autoimmune disease, compared with those who had never been infected. COVID-19 was most strongly linked to vasculitis.
These large studies are telling us, “Yes, this link is there, so we have to accept it,” Sonia Sharma, PhD, of the Center for Autoimmunity and Inflammation at the La Jolla (Calif.) Institute for Immunology, told this news organization. But this is not the first time that autoimmune diseases have been linked to previous infections.
Researchers have known for decades that Epstein-Barr virus infection is linked to several autoimmune diseases, including systemic lupus erythematosus, multiple sclerosis, and rheumatoid arthritis. More recent research suggests the virus may activate certain genes associated with these immune disorders. Hepatitis C virus can induce cryoglobulinemia, and infection with cytomegalovirus has been implicated in several autoimmune diseases. Bacterial infections have also been linked to autoimmunity, such as group A streptococcus and rheumatic fever, as well as salmonella and reactive arthritis, to name only a few.
“In a way, this isn’t necessarily a new concept to physicians, particularly rheumatologists,” said Jeffrey A. Sparks, MD, a rheumatologist at Brigham and Women’s Hospital in Boston. “There’s a fine line between appropriately clearing an infection and the body overreacting and setting off a cascade where the immune system is chronically overactive that can manifest as an autoimmune disease,” he told this news organization.
A dysregulated response to infection
It takes the immune system a week or two to develop antigen-specific antibodies to a new pathogen. But for patients with serious infections – in this instance, COVID-19 – that’s time they don’t have. Therefore, the immune system has an alternative pathway, called extrafollicular activation, that creates fast-acting antibodies, explained Matthew Woodruff, PhD, an instructor of immunology and rheumatology at Emory University, Atlanta.
The trade-off is that these antibodies are not as specific and can target the body’s own tissues. This dysregulation of antibody selection is generally short lived and fades when more targeted antibodies are produced and take over, but in some cases, this process can lead to high levels of self-targeting antibodies that can harm the body’s organs and tissues. Research also suggests that for patients who experience long COVID, the same autoantibodies that drive the initial immune response are detectable in the body months after infection, though it is not known whether these lingering immune cells cause these longer-lasting symptoms.
“If you have a virus that causes hyperinflammation plus organ damage, that is a recipe for disaster,” Dr. Sharma said. “It’s a recipe for autoantibodies and autoreactive T cells that down the road can attack the body’s own tissues, especially in people whose immune system is trained in such a way to cause self-reactivity,” she added.
This hyperinflammation can result in rare but serious complications, such as multisystem inflammatory syndrome in children and adults, which can occur 2-6 weeks after SARS-CoV-2 infection. But even in these patients with severe illness, organ-specific complications tend to resolve in 6 months with “no significant sequelae 1 year after diagnosis,” according to the Centers for Disease Control and Prevention. And while long COVID can last for a year or longer, data suggest that symptoms do eventually resolve for most people. What is not clear is why acute autoimmunity triggered by COVID-19 can become a chronic condition in certain patients.
Predisposition to autoimmunity
P. J. Utz, MD, PhD, professor of immunology and rheumatology at Stanford (Calif.) University, said that people who develop autoimmune disease after SARS-CoV-2 infection may have already been predisposed toward autoimmunity. Especially for autoimmune diseases such as type 1 diabetes and lupus, autoantibodies can appear and circulate in the body for more than a decade in some people before they present with any clinical symptoms. “Their immune system is primed such that if they get infected with something – or they have some other environmental trigger that maybe we don’t know about yet – that is enough to then push them over the edge so that they get full-blown autoimmunity,” he said. What is not known is whether these patients’ conditions would have advanced to true clinical disease had they not been infected, he said.
He also noted that the presence of autoantibodies does not necessarily mean someone has autoimmune disease; healthy people can also have autoantibodies, and everyone develops them with age. “My advice would be, ‘Don’t lose sleep over this,’ “ he said.
Dr. Sparks agreed that while these retrospective studies did show an elevated risk of autoimmune disease after COVID-19, that risk appears to be relatively small. “As a practicing rheumatologist, we aren’t seeing a stampede of patients with new-onset rheumatic diseases,” he said. “It’s not like we’re overwhelmed with autoimmune patients, even though almost everyone’s had COVID. So, if there is a risk, it’s very modest.”
Dr. Sparks is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the R. Bruce and Joan M. Mickey Research Scholar Fund, and the Llura Gund Award for Rheumatoid Arthritis Research and Care. Dr. Utz receives research funding from Pfizer. Dr. Sharma and Dr. Woodruff have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Since the COVID-19 pandemic started more than 3 years ago, the longer-lasting effects of SARS-CoV-2 infection have continued to reveal themselves. Approximately 28% of Americans report having ever experienced post-COVID conditions, such as brain fog, postexertional malaise, and joint pain, and 11% say they are still experiencing these long-term effects. Now, new research is showing that people who have had COVID are more likely to newly develop an autoimmune disease. Exactly why this is happening is less clear, experts say.
Two preprint studies and one study published in a peer-reviewed journal provide strong evidence that patients who have been infected with SARS-CoV-2 are at elevated risk of developing an autoimmune disease. The studies retrospectively reviewed medical records from three countries and compared the incidence of new-onset autoimmune disease among patients who had polymerase chain reaction–confirmed COVID-19 and those who had never been diagnosed with the virus.
A study analyzing the health records of 3.8 million U.S. patients – more than 888,460 with confirmed COVID-19 – found that the COVID-19 group was two to three times as likely to develop various autoimmune diseases, such as rheumatoid arthritis, systemic lupus erythematosus, and systemic sclerosis. A U.K. preprint study that included more than 458,000 people with confirmed COVID found that those who had previously been infected with SARS-CoV-2 were 22% more likely to develop an autoimmune disease compared with the control group. In this cohort, the diseases most strongly associated with COVID-19 were type 1 diabetes, inflammatory bowel disease, and psoriasis. A preprint study from German researchers found that COVID-19 patients were almost 43% more likely to develop an autoimmune disease, compared with those who had never been infected. COVID-19 was most strongly linked to vasculitis.
These large studies are telling us, “Yes, this link is there, so we have to accept it,” Sonia Sharma, PhD, of the Center for Autoimmunity and Inflammation at the La Jolla (Calif.) Institute for Immunology, told this news organization. But this is not the first time that autoimmune diseases have been linked to previous infections.
Researchers have known for decades that Epstein-Barr virus infection is linked to several autoimmune diseases, including systemic lupus erythematosus, multiple sclerosis, and rheumatoid arthritis. More recent research suggests the virus may activate certain genes associated with these immune disorders. Hepatitis C virus can induce cryoglobulinemia, and infection with cytomegalovirus has been implicated in several autoimmune diseases. Bacterial infections have also been linked to autoimmunity, such as group A streptococcus and rheumatic fever, as well as salmonella and reactive arthritis, to name only a few.
“In a way, this isn’t necessarily a new concept to physicians, particularly rheumatologists,” said Jeffrey A. Sparks, MD, a rheumatologist at Brigham and Women’s Hospital in Boston. “There’s a fine line between appropriately clearing an infection and the body overreacting and setting off a cascade where the immune system is chronically overactive that can manifest as an autoimmune disease,” he told this news organization.
A dysregulated response to infection
It takes the immune system a week or two to develop antigen-specific antibodies to a new pathogen. But for patients with serious infections – in this instance, COVID-19 – that’s time they don’t have. Therefore, the immune system has an alternative pathway, called extrafollicular activation, that creates fast-acting antibodies, explained Matthew Woodruff, PhD, an instructor of immunology and rheumatology at Emory University, Atlanta.
The trade-off is that these antibodies are not as specific and can target the body’s own tissues. This dysregulation of antibody selection is generally short lived and fades when more targeted antibodies are produced and take over, but in some cases, this process can lead to high levels of self-targeting antibodies that can harm the body’s organs and tissues. Research also suggests that for patients who experience long COVID, the same autoantibodies that drive the initial immune response are detectable in the body months after infection, though it is not known whether these lingering immune cells cause these longer-lasting symptoms.
“If you have a virus that causes hyperinflammation plus organ damage, that is a recipe for disaster,” Dr. Sharma said. “It’s a recipe for autoantibodies and autoreactive T cells that down the road can attack the body’s own tissues, especially in people whose immune system is trained in such a way to cause self-reactivity,” she added.
This hyperinflammation can result in rare but serious complications, such as multisystem inflammatory syndrome in children and adults, which can occur 2-6 weeks after SARS-CoV-2 infection. But even in these patients with severe illness, organ-specific complications tend to resolve in 6 months with “no significant sequelae 1 year after diagnosis,” according to the Centers for Disease Control and Prevention. And while long COVID can last for a year or longer, data suggest that symptoms do eventually resolve for most people. What is not clear is why acute autoimmunity triggered by COVID-19 can become a chronic condition in certain patients.
Predisposition to autoimmunity
P. J. Utz, MD, PhD, professor of immunology and rheumatology at Stanford (Calif.) University, said that people who develop autoimmune disease after SARS-CoV-2 infection may have already been predisposed toward autoimmunity. Especially for autoimmune diseases such as type 1 diabetes and lupus, autoantibodies can appear and circulate in the body for more than a decade in some people before they present with any clinical symptoms. “Their immune system is primed such that if they get infected with something – or they have some other environmental trigger that maybe we don’t know about yet – that is enough to then push them over the edge so that they get full-blown autoimmunity,” he said. What is not known is whether these patients’ conditions would have advanced to true clinical disease had they not been infected, he said.
He also noted that the presence of autoantibodies does not necessarily mean someone has autoimmune disease; healthy people can also have autoantibodies, and everyone develops them with age. “My advice would be, ‘Don’t lose sleep over this,’ “ he said.
Dr. Sparks agreed that while these retrospective studies did show an elevated risk of autoimmune disease after COVID-19, that risk appears to be relatively small. “As a practicing rheumatologist, we aren’t seeing a stampede of patients with new-onset rheumatic diseases,” he said. “It’s not like we’re overwhelmed with autoimmune patients, even though almost everyone’s had COVID. So, if there is a risk, it’s very modest.”
Dr. Sparks is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the R. Bruce and Joan M. Mickey Research Scholar Fund, and the Llura Gund Award for Rheumatoid Arthritis Research and Care. Dr. Utz receives research funding from Pfizer. Dr. Sharma and Dr. Woodruff have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Since the COVID-19 pandemic started more than 3 years ago, the longer-lasting effects of SARS-CoV-2 infection have continued to reveal themselves. Approximately 28% of Americans report having ever experienced post-COVID conditions, such as brain fog, postexertional malaise, and joint pain, and 11% say they are still experiencing these long-term effects. Now, new research is showing that people who have had COVID are more likely to newly develop an autoimmune disease. Exactly why this is happening is less clear, experts say.
Two preprint studies and one study published in a peer-reviewed journal provide strong evidence that patients who have been infected with SARS-CoV-2 are at elevated risk of developing an autoimmune disease. The studies retrospectively reviewed medical records from three countries and compared the incidence of new-onset autoimmune disease among patients who had polymerase chain reaction–confirmed COVID-19 and those who had never been diagnosed with the virus.
A study analyzing the health records of 3.8 million U.S. patients – more than 888,460 with confirmed COVID-19 – found that the COVID-19 group was two to three times as likely to develop various autoimmune diseases, such as rheumatoid arthritis, systemic lupus erythematosus, and systemic sclerosis. A U.K. preprint study that included more than 458,000 people with confirmed COVID found that those who had previously been infected with SARS-CoV-2 were 22% more likely to develop an autoimmune disease compared with the control group. In this cohort, the diseases most strongly associated with COVID-19 were type 1 diabetes, inflammatory bowel disease, and psoriasis. A preprint study from German researchers found that COVID-19 patients were almost 43% more likely to develop an autoimmune disease, compared with those who had never been infected. COVID-19 was most strongly linked to vasculitis.
These large studies are telling us, “Yes, this link is there, so we have to accept it,” Sonia Sharma, PhD, of the Center for Autoimmunity and Inflammation at the La Jolla (Calif.) Institute for Immunology, told this news organization. But this is not the first time that autoimmune diseases have been linked to previous infections.
Researchers have known for decades that Epstein-Barr virus infection is linked to several autoimmune diseases, including systemic lupus erythematosus, multiple sclerosis, and rheumatoid arthritis. More recent research suggests the virus may activate certain genes associated with these immune disorders. Hepatitis C virus can induce cryoglobulinemia, and infection with cytomegalovirus has been implicated in several autoimmune diseases. Bacterial infections have also been linked to autoimmunity, such as group A streptococcus and rheumatic fever, as well as salmonella and reactive arthritis, to name only a few.
“In a way, this isn’t necessarily a new concept to physicians, particularly rheumatologists,” said Jeffrey A. Sparks, MD, a rheumatologist at Brigham and Women’s Hospital in Boston. “There’s a fine line between appropriately clearing an infection and the body overreacting and setting off a cascade where the immune system is chronically overactive that can manifest as an autoimmune disease,” he told this news organization.
A dysregulated response to infection
It takes the immune system a week or two to develop antigen-specific antibodies to a new pathogen. But for patients with serious infections – in this instance, COVID-19 – that’s time they don’t have. Therefore, the immune system has an alternative pathway, called extrafollicular activation, that creates fast-acting antibodies, explained Matthew Woodruff, PhD, an instructor of immunology and rheumatology at Emory University, Atlanta.
The trade-off is that these antibodies are not as specific and can target the body’s own tissues. This dysregulation of antibody selection is generally short lived and fades when more targeted antibodies are produced and take over, but in some cases, this process can lead to high levels of self-targeting antibodies that can harm the body’s organs and tissues. Research also suggests that for patients who experience long COVID, the same autoantibodies that drive the initial immune response are detectable in the body months after infection, though it is not known whether these lingering immune cells cause these longer-lasting symptoms.
“If you have a virus that causes hyperinflammation plus organ damage, that is a recipe for disaster,” Dr. Sharma said. “It’s a recipe for autoantibodies and autoreactive T cells that down the road can attack the body’s own tissues, especially in people whose immune system is trained in such a way to cause self-reactivity,” she added.
This hyperinflammation can result in rare but serious complications, such as multisystem inflammatory syndrome in children and adults, which can occur 2-6 weeks after SARS-CoV-2 infection. But even in these patients with severe illness, organ-specific complications tend to resolve in 6 months with “no significant sequelae 1 year after diagnosis,” according to the Centers for Disease Control and Prevention. And while long COVID can last for a year or longer, data suggest that symptoms do eventually resolve for most people. What is not clear is why acute autoimmunity triggered by COVID-19 can become a chronic condition in certain patients.
Predisposition to autoimmunity
P. J. Utz, MD, PhD, professor of immunology and rheumatology at Stanford (Calif.) University, said that people who develop autoimmune disease after SARS-CoV-2 infection may have already been predisposed toward autoimmunity. Especially for autoimmune diseases such as type 1 diabetes and lupus, autoantibodies can appear and circulate in the body for more than a decade in some people before they present with any clinical symptoms. “Their immune system is primed such that if they get infected with something – or they have some other environmental trigger that maybe we don’t know about yet – that is enough to then push them over the edge so that they get full-blown autoimmunity,” he said. What is not known is whether these patients’ conditions would have advanced to true clinical disease had they not been infected, he said.
He also noted that the presence of autoantibodies does not necessarily mean someone has autoimmune disease; healthy people can also have autoantibodies, and everyone develops them with age. “My advice would be, ‘Don’t lose sleep over this,’ “ he said.
Dr. Sparks agreed that while these retrospective studies did show an elevated risk of autoimmune disease after COVID-19, that risk appears to be relatively small. “As a practicing rheumatologist, we aren’t seeing a stampede of patients with new-onset rheumatic diseases,” he said. “It’s not like we’re overwhelmed with autoimmune patients, even though almost everyone’s had COVID. So, if there is a risk, it’s very modest.”
Dr. Sparks is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the R. Bruce and Joan M. Mickey Research Scholar Fund, and the Llura Gund Award for Rheumatoid Arthritis Research and Care. Dr. Utz receives research funding from Pfizer. Dr. Sharma and Dr. Woodruff have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FMT in a pill: FDA approves second product to prevent C. diff recurrence
The recent approval of the first oral fecal-derived microbiota therapy to prevent the recurrence of Clostridioides difficile (C. diff) infection in patients was welcome news for physicians who’ve struggled under the weight of having too few treatment options for the prevention of C. diff recurrence.
The product, developed by Massachusetts-based Seres Therepeutics and marketed as Vowst, was approved by the U.S. Food and Drug Administration on April 26. It is approved for use in adults who have already been treated with antibiotics for a recurrent infection with C. diff bacteria.
and is designed to be delivered in four capsules taken daily for 3 days.
Gastroenterologist Phillip I. Tarr, MD, division chief of gastroenterology at Washington University, St. Louis, and chair of the American Gastroenterological Association Center for Gut Microbiome Research and Education, said that prevention of recurrent C. diff infection “remains challenging,” and that Vowst “provides the first FDA-approved, orally administered microbiome therapeutic with which to achieve this goal. This advance also makes us optimistic we might soon be able to prevent other disorders by managing gut microbial communities.”
Vowst is the second therapy derived from human stool to be approved for the indication in less than 6 months. In December, the FDA approved Rebyota (Ferring), a rectally delivered treatment that also uses microbes from donor feces. Both products were given priority review, orphan drug, and breakthrough therapy designations by the agency.
C. diff infection can be aggravated by an alteration of normal gut flora associated with antibiotics treatment, leading to cycles of repeated infections. Infection can produce diarrhea, abdominal pain, fever, and severe morbidity. In the United States, an estimated 15,000 to 30,000 deaths per year are linked to C. diff. Risk factors for recurrent infection include being 65 or older, hospitalization, being in a nursing home, a weakened immune system, and previous infection with C. diff.
Therapies transplanting fecal microbiota from donors have been used since the 1950s as treatments for recurrent C. diff infection, and in the past decade, as stool banks recruiting screened donors have made fecal microbiota transplants, or FMT, standard of care. However, only in recent years have fecal-derived therapies become subject to standardized safety and efficacy testing.
Both the current FDA-approved products, Rebyota and Vowst, were shown in randomized controlled trials to reduce recurrence of C. diff infection, compared with placebo. In a phase 3 clinical trial of Rebyota (n = 262) in antibiotic-treated patients, one rectally administered dose reduced recurrence of C. diff infection by 70.6% at 8 weeks, compared with 57.5% for placebo. A phase 3 study of Vowst (n = 281) showed recurrence in treated subjects to be 12.4% at 8 weeks, compared with nearly 40% of those receiving placebo (relative risk, 0.32; 95% confidence interval, 0.18-0.58; P less than .001).
Despite screening protocols that have become increasingly homogenized and rigorous, FMT is associated with the risk of introducing pathogens. Vowst is manufactured with purified bacterial spores derived from donor feces, not whole stool. Nonetheless, FDA noted in its statement that Vowst could still potentially introduce infectious agents or allergens.
Antibiotics are still first-line treatment
In an interview, Jessica Allegretti, MD, MPH, AGAF, medical director of the Crohn’s and Colitis Center at Brigham & Women’s Hospital, Boston, said that having two FDA-approved therapies with different means of administration “is great for the field and great for patients. These are both meant to be used after a course of antibiotics, so antibiotics are still the mainstay of treatment for C. diff and recurrent C. diff, but we now have more options to prevent recurrence.”
The convenience of an oral therapy that can be taken at home is “very attractive,” Dr. Allegretti added, noting that there will also be patients “who either don’t want to or can’t take capsules, for whom a rectal administration [in a health care setting] may be preferred.”
Dr. Allegretti, who has used FMT to treat recurrent C. difficile for more than a decade, said that she expected traditional FMT using screened donor stool to remain available even as the new products are adopted by clinicians. FMT centers like OpenBiome “will continue to provide access for patients who either don’t have the ability to get the FDA-approved products because of insurance coverage, or for financial reasons, or maybe neither of the new products is appropriate for them,” she said. “I do think there will always be a need for the traditional option. The more options that we have available the better.”
TD Cowen analyst Joseph Thome told Reuters that the drug could be priced close to $20,000 per course, expecting peak sales of $750 million in the U.S. in 2033.
Dr. Allegretti disclosed consulting work for Seres Therapeutics, Ferring, and other manufacturers. She is a member of OpenBiome’s clinical advisory board.
The recent approval of the first oral fecal-derived microbiota therapy to prevent the recurrence of Clostridioides difficile (C. diff) infection in patients was welcome news for physicians who’ve struggled under the weight of having too few treatment options for the prevention of C. diff recurrence.
The product, developed by Massachusetts-based Seres Therepeutics and marketed as Vowst, was approved by the U.S. Food and Drug Administration on April 26. It is approved for use in adults who have already been treated with antibiotics for a recurrent infection with C. diff bacteria.
and is designed to be delivered in four capsules taken daily for 3 days.
Gastroenterologist Phillip I. Tarr, MD, division chief of gastroenterology at Washington University, St. Louis, and chair of the American Gastroenterological Association Center for Gut Microbiome Research and Education, said that prevention of recurrent C. diff infection “remains challenging,” and that Vowst “provides the first FDA-approved, orally administered microbiome therapeutic with which to achieve this goal. This advance also makes us optimistic we might soon be able to prevent other disorders by managing gut microbial communities.”
Vowst is the second therapy derived from human stool to be approved for the indication in less than 6 months. In December, the FDA approved Rebyota (Ferring), a rectally delivered treatment that also uses microbes from donor feces. Both products were given priority review, orphan drug, and breakthrough therapy designations by the agency.
C. diff infection can be aggravated by an alteration of normal gut flora associated with antibiotics treatment, leading to cycles of repeated infections. Infection can produce diarrhea, abdominal pain, fever, and severe morbidity. In the United States, an estimated 15,000 to 30,000 deaths per year are linked to C. diff. Risk factors for recurrent infection include being 65 or older, hospitalization, being in a nursing home, a weakened immune system, and previous infection with C. diff.
Therapies transplanting fecal microbiota from donors have been used since the 1950s as treatments for recurrent C. diff infection, and in the past decade, as stool banks recruiting screened donors have made fecal microbiota transplants, or FMT, standard of care. However, only in recent years have fecal-derived therapies become subject to standardized safety and efficacy testing.
Both the current FDA-approved products, Rebyota and Vowst, were shown in randomized controlled trials to reduce recurrence of C. diff infection, compared with placebo. In a phase 3 clinical trial of Rebyota (n = 262) in antibiotic-treated patients, one rectally administered dose reduced recurrence of C. diff infection by 70.6% at 8 weeks, compared with 57.5% for placebo. A phase 3 study of Vowst (n = 281) showed recurrence in treated subjects to be 12.4% at 8 weeks, compared with nearly 40% of those receiving placebo (relative risk, 0.32; 95% confidence interval, 0.18-0.58; P less than .001).
Despite screening protocols that have become increasingly homogenized and rigorous, FMT is associated with the risk of introducing pathogens. Vowst is manufactured with purified bacterial spores derived from donor feces, not whole stool. Nonetheless, FDA noted in its statement that Vowst could still potentially introduce infectious agents or allergens.
Antibiotics are still first-line treatment
In an interview, Jessica Allegretti, MD, MPH, AGAF, medical director of the Crohn’s and Colitis Center at Brigham & Women’s Hospital, Boston, said that having two FDA-approved therapies with different means of administration “is great for the field and great for patients. These are both meant to be used after a course of antibiotics, so antibiotics are still the mainstay of treatment for C. diff and recurrent C. diff, but we now have more options to prevent recurrence.”
The convenience of an oral therapy that can be taken at home is “very attractive,” Dr. Allegretti added, noting that there will also be patients “who either don’t want to or can’t take capsules, for whom a rectal administration [in a health care setting] may be preferred.”
Dr. Allegretti, who has used FMT to treat recurrent C. difficile for more than a decade, said that she expected traditional FMT using screened donor stool to remain available even as the new products are adopted by clinicians. FMT centers like OpenBiome “will continue to provide access for patients who either don’t have the ability to get the FDA-approved products because of insurance coverage, or for financial reasons, or maybe neither of the new products is appropriate for them,” she said. “I do think there will always be a need for the traditional option. The more options that we have available the better.”
TD Cowen analyst Joseph Thome told Reuters that the drug could be priced close to $20,000 per course, expecting peak sales of $750 million in the U.S. in 2033.
Dr. Allegretti disclosed consulting work for Seres Therapeutics, Ferring, and other manufacturers. She is a member of OpenBiome’s clinical advisory board.
The recent approval of the first oral fecal-derived microbiota therapy to prevent the recurrence of Clostridioides difficile (C. diff) infection in patients was welcome news for physicians who’ve struggled under the weight of having too few treatment options for the prevention of C. diff recurrence.
The product, developed by Massachusetts-based Seres Therepeutics and marketed as Vowst, was approved by the U.S. Food and Drug Administration on April 26. It is approved for use in adults who have already been treated with antibiotics for a recurrent infection with C. diff bacteria.
and is designed to be delivered in four capsules taken daily for 3 days.
Gastroenterologist Phillip I. Tarr, MD, division chief of gastroenterology at Washington University, St. Louis, and chair of the American Gastroenterological Association Center for Gut Microbiome Research and Education, said that prevention of recurrent C. diff infection “remains challenging,” and that Vowst “provides the first FDA-approved, orally administered microbiome therapeutic with which to achieve this goal. This advance also makes us optimistic we might soon be able to prevent other disorders by managing gut microbial communities.”
Vowst is the second therapy derived from human stool to be approved for the indication in less than 6 months. In December, the FDA approved Rebyota (Ferring), a rectally delivered treatment that also uses microbes from donor feces. Both products were given priority review, orphan drug, and breakthrough therapy designations by the agency.
C. diff infection can be aggravated by an alteration of normal gut flora associated with antibiotics treatment, leading to cycles of repeated infections. Infection can produce diarrhea, abdominal pain, fever, and severe morbidity. In the United States, an estimated 15,000 to 30,000 deaths per year are linked to C. diff. Risk factors for recurrent infection include being 65 or older, hospitalization, being in a nursing home, a weakened immune system, and previous infection with C. diff.
Therapies transplanting fecal microbiota from donors have been used since the 1950s as treatments for recurrent C. diff infection, and in the past decade, as stool banks recruiting screened donors have made fecal microbiota transplants, or FMT, standard of care. However, only in recent years have fecal-derived therapies become subject to standardized safety and efficacy testing.
Both the current FDA-approved products, Rebyota and Vowst, were shown in randomized controlled trials to reduce recurrence of C. diff infection, compared with placebo. In a phase 3 clinical trial of Rebyota (n = 262) in antibiotic-treated patients, one rectally administered dose reduced recurrence of C. diff infection by 70.6% at 8 weeks, compared with 57.5% for placebo. A phase 3 study of Vowst (n = 281) showed recurrence in treated subjects to be 12.4% at 8 weeks, compared with nearly 40% of those receiving placebo (relative risk, 0.32; 95% confidence interval, 0.18-0.58; P less than .001).
Despite screening protocols that have become increasingly homogenized and rigorous, FMT is associated with the risk of introducing pathogens. Vowst is manufactured with purified bacterial spores derived from donor feces, not whole stool. Nonetheless, FDA noted in its statement that Vowst could still potentially introduce infectious agents or allergens.
Antibiotics are still first-line treatment
In an interview, Jessica Allegretti, MD, MPH, AGAF, medical director of the Crohn’s and Colitis Center at Brigham & Women’s Hospital, Boston, said that having two FDA-approved therapies with different means of administration “is great for the field and great for patients. These are both meant to be used after a course of antibiotics, so antibiotics are still the mainstay of treatment for C. diff and recurrent C. diff, but we now have more options to prevent recurrence.”
The convenience of an oral therapy that can be taken at home is “very attractive,” Dr. Allegretti added, noting that there will also be patients “who either don’t want to or can’t take capsules, for whom a rectal administration [in a health care setting] may be preferred.”
Dr. Allegretti, who has used FMT to treat recurrent C. difficile for more than a decade, said that she expected traditional FMT using screened donor stool to remain available even as the new products are adopted by clinicians. FMT centers like OpenBiome “will continue to provide access for patients who either don’t have the ability to get the FDA-approved products because of insurance coverage, or for financial reasons, or maybe neither of the new products is appropriate for them,” she said. “I do think there will always be a need for the traditional option. The more options that we have available the better.”
TD Cowen analyst Joseph Thome told Reuters that the drug could be priced close to $20,000 per course, expecting peak sales of $750 million in the U.S. in 2033.
Dr. Allegretti disclosed consulting work for Seres Therapeutics, Ferring, and other manufacturers. She is a member of OpenBiome’s clinical advisory board.