User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
NP-PA turf fights: Where the relationship can improve
– The U.S. Bureau of Labor Statistics forecasts a 40% increase in the NP workforce by 2031, coupled with a 28% rise in PAs.
In recent reports on the quality of the relationships involving these health care professions, survey respondents mostly gave positive accounts of collaboration, using words such as like “comradery,” “teamwork,” “congenial,” and “cohesion.” But all was not perfect. Where and how could these important health care provider relationships improve?
PAs: “Competition and collaboration’ with RNs
In a Medscape survey of more than 770 PAs about their working relationships with other health care professionals; 83% of them supported the idea of PAs and NPs practicing more independently from physicians, but sometimes it’s not easy to stay in their individual lanes.
One PA respondent complained that NPs get “more opportunities and preference,” another pointed to PA-NP “turf issues,” and a third griped about NPs’ “strong unions,” which have stoked more fighting about practice abilities and available settings.
Robert Blumm, MA, PA-C, a retired surgical and emergency medicine PA who regards himself as an advocate for both PAs and NPs, describes their interaction as a “mixture of competition and collaboration.”
On one hand, the two groups typically “cooperate and do an excellent job, incurring patient errors similar to or less than physician colleagues or senior residents.” On the other hand, Mr. Blumm conceded, there is some jealousy among PAs over NPs’ advantage in staffing and hiring decisions, “since they don’t need [direct physician] supervision ... and there are limits on how many PAs can be supervised by one physician.”
Most PA-NP interactions are collaborative, although many people emphasize the relatively few conflicts, said Jennifer Orozco, DMSc, PA-C, president and chair of the American Academy of PAs.
“We see that a lot in this country,” she said. “People try to drive a wedge, but it’s often a misnomer that there’s a lot of arguing and infighting.”
NPs: Different backgrounds, same goal
The Medscape survey also included information from 750 NPs on working relationships; 93% of them favored nurses and PAs working more independently from doctors.
April Kapu, DNP, ARPN, has worked closely with PAs for more than 20 years. “In my experience ... they complement one another as health team members, although the education and training are somewhat different,” said Ms. Kapu, , president of the American Association of Nurse Practitioners.
Some respondents noted the different educational trajectories for NPs and PAs. “Doctors and PAs are taught using the same model, but NPs are taught under the nursing model,” wrote a family medicine PA.
In emergency departments where Mr. Blumm has worked, ICU NPs have an edge over PAs in terms of preparation, organization, and the tabulation of formulas. On the other hand, some of Mr. Blumm’s fellow PAs were also emergency medicine technicians or respiratory therapists, who had “2 years of classroom training, on par with that of medical students.”
Must these differences in training and education foment conflict between NPs and PAs? “We all bring something different to the table,” said Ms. Kapu, who also is associate dean for clinical and community partnerships at Vanderbilt University, Nashville, Tenn. “It is important to respect each person’s entry point, education, and training.”
Differing personalities and environments
Numerous PA respondents said that individual personalities and work environments are more likely to trigger issues with NPs than are differences in training.
“It depends on the team and situation and who the people are, not the letters behind their names,” an emergency medicine PA wrote. A surgical PA noted that “group dynamics and work culture differ from place to place,” while a third PA agreed that “it’s personality dependent, not title dependent.”
No single formula will resolve areas of NP-PA conflict, Ms. Orozco said. “What works in Chicago might not work in rural Colorado or Texas or California, but we do have to come together. The overall focus should be on greater flexibility for PAs and NPs. Patients will fare better.”
Joint research, publishing could help
About a decade ago, Mr. Blumm joined with another PA and an NP to form the American College of Clinicians, the first joint PA-NP national professional organization. Although it disbanded after 6 years, owing to low membership, he hopes a similar collaboration will take off in the future.
“I also recommend that PAs and NPs publish articles together, with research as an excellent place to start,” he added. “PAs and NPs should stand together and be a source of healing for all our patients. Regardless of our titles, our responsibility is to bring healing together.”
A version of this article first appeared on Medscape.com.
– The U.S. Bureau of Labor Statistics forecasts a 40% increase in the NP workforce by 2031, coupled with a 28% rise in PAs.
In recent reports on the quality of the relationships involving these health care professions, survey respondents mostly gave positive accounts of collaboration, using words such as like “comradery,” “teamwork,” “congenial,” and “cohesion.” But all was not perfect. Where and how could these important health care provider relationships improve?
PAs: “Competition and collaboration’ with RNs
In a Medscape survey of more than 770 PAs about their working relationships with other health care professionals; 83% of them supported the idea of PAs and NPs practicing more independently from physicians, but sometimes it’s not easy to stay in their individual lanes.
One PA respondent complained that NPs get “more opportunities and preference,” another pointed to PA-NP “turf issues,” and a third griped about NPs’ “strong unions,” which have stoked more fighting about practice abilities and available settings.
Robert Blumm, MA, PA-C, a retired surgical and emergency medicine PA who regards himself as an advocate for both PAs and NPs, describes their interaction as a “mixture of competition and collaboration.”
On one hand, the two groups typically “cooperate and do an excellent job, incurring patient errors similar to or less than physician colleagues or senior residents.” On the other hand, Mr. Blumm conceded, there is some jealousy among PAs over NPs’ advantage in staffing and hiring decisions, “since they don’t need [direct physician] supervision ... and there are limits on how many PAs can be supervised by one physician.”
Most PA-NP interactions are collaborative, although many people emphasize the relatively few conflicts, said Jennifer Orozco, DMSc, PA-C, president and chair of the American Academy of PAs.
“We see that a lot in this country,” she said. “People try to drive a wedge, but it’s often a misnomer that there’s a lot of arguing and infighting.”
NPs: Different backgrounds, same goal
The Medscape survey also included information from 750 NPs on working relationships; 93% of them favored nurses and PAs working more independently from doctors.
April Kapu, DNP, ARPN, has worked closely with PAs for more than 20 years. “In my experience ... they complement one another as health team members, although the education and training are somewhat different,” said Ms. Kapu, , president of the American Association of Nurse Practitioners.
Some respondents noted the different educational trajectories for NPs and PAs. “Doctors and PAs are taught using the same model, but NPs are taught under the nursing model,” wrote a family medicine PA.
In emergency departments where Mr. Blumm has worked, ICU NPs have an edge over PAs in terms of preparation, organization, and the tabulation of formulas. On the other hand, some of Mr. Blumm’s fellow PAs were also emergency medicine technicians or respiratory therapists, who had “2 years of classroom training, on par with that of medical students.”
Must these differences in training and education foment conflict between NPs and PAs? “We all bring something different to the table,” said Ms. Kapu, who also is associate dean for clinical and community partnerships at Vanderbilt University, Nashville, Tenn. “It is important to respect each person’s entry point, education, and training.”
Differing personalities and environments
Numerous PA respondents said that individual personalities and work environments are more likely to trigger issues with NPs than are differences in training.
“It depends on the team and situation and who the people are, not the letters behind their names,” an emergency medicine PA wrote. A surgical PA noted that “group dynamics and work culture differ from place to place,” while a third PA agreed that “it’s personality dependent, not title dependent.”
No single formula will resolve areas of NP-PA conflict, Ms. Orozco said. “What works in Chicago might not work in rural Colorado or Texas or California, but we do have to come together. The overall focus should be on greater flexibility for PAs and NPs. Patients will fare better.”
Joint research, publishing could help
About a decade ago, Mr. Blumm joined with another PA and an NP to form the American College of Clinicians, the first joint PA-NP national professional organization. Although it disbanded after 6 years, owing to low membership, he hopes a similar collaboration will take off in the future.
“I also recommend that PAs and NPs publish articles together, with research as an excellent place to start,” he added. “PAs and NPs should stand together and be a source of healing for all our patients. Regardless of our titles, our responsibility is to bring healing together.”
A version of this article first appeared on Medscape.com.
– The U.S. Bureau of Labor Statistics forecasts a 40% increase in the NP workforce by 2031, coupled with a 28% rise in PAs.
In recent reports on the quality of the relationships involving these health care professions, survey respondents mostly gave positive accounts of collaboration, using words such as like “comradery,” “teamwork,” “congenial,” and “cohesion.” But all was not perfect. Where and how could these important health care provider relationships improve?
PAs: “Competition and collaboration’ with RNs
In a Medscape survey of more than 770 PAs about their working relationships with other health care professionals; 83% of them supported the idea of PAs and NPs practicing more independently from physicians, but sometimes it’s not easy to stay in their individual lanes.
One PA respondent complained that NPs get “more opportunities and preference,” another pointed to PA-NP “turf issues,” and a third griped about NPs’ “strong unions,” which have stoked more fighting about practice abilities and available settings.
Robert Blumm, MA, PA-C, a retired surgical and emergency medicine PA who regards himself as an advocate for both PAs and NPs, describes their interaction as a “mixture of competition and collaboration.”
On one hand, the two groups typically “cooperate and do an excellent job, incurring patient errors similar to or less than physician colleagues or senior residents.” On the other hand, Mr. Blumm conceded, there is some jealousy among PAs over NPs’ advantage in staffing and hiring decisions, “since they don’t need [direct physician] supervision ... and there are limits on how many PAs can be supervised by one physician.”
Most PA-NP interactions are collaborative, although many people emphasize the relatively few conflicts, said Jennifer Orozco, DMSc, PA-C, president and chair of the American Academy of PAs.
“We see that a lot in this country,” she said. “People try to drive a wedge, but it’s often a misnomer that there’s a lot of arguing and infighting.”
NPs: Different backgrounds, same goal
The Medscape survey also included information from 750 NPs on working relationships; 93% of them favored nurses and PAs working more independently from doctors.
April Kapu, DNP, ARPN, has worked closely with PAs for more than 20 years. “In my experience ... they complement one another as health team members, although the education and training are somewhat different,” said Ms. Kapu, , president of the American Association of Nurse Practitioners.
Some respondents noted the different educational trajectories for NPs and PAs. “Doctors and PAs are taught using the same model, but NPs are taught under the nursing model,” wrote a family medicine PA.
In emergency departments where Mr. Blumm has worked, ICU NPs have an edge over PAs in terms of preparation, organization, and the tabulation of formulas. On the other hand, some of Mr. Blumm’s fellow PAs were also emergency medicine technicians or respiratory therapists, who had “2 years of classroom training, on par with that of medical students.”
Must these differences in training and education foment conflict between NPs and PAs? “We all bring something different to the table,” said Ms. Kapu, who also is associate dean for clinical and community partnerships at Vanderbilt University, Nashville, Tenn. “It is important to respect each person’s entry point, education, and training.”
Differing personalities and environments
Numerous PA respondents said that individual personalities and work environments are more likely to trigger issues with NPs than are differences in training.
“It depends on the team and situation and who the people are, not the letters behind their names,” an emergency medicine PA wrote. A surgical PA noted that “group dynamics and work culture differ from place to place,” while a third PA agreed that “it’s personality dependent, not title dependent.”
No single formula will resolve areas of NP-PA conflict, Ms. Orozco said. “What works in Chicago might not work in rural Colorado or Texas or California, but we do have to come together. The overall focus should be on greater flexibility for PAs and NPs. Patients will fare better.”
Joint research, publishing could help
About a decade ago, Mr. Blumm joined with another PA and an NP to form the American College of Clinicians, the first joint PA-NP national professional organization. Although it disbanded after 6 years, owing to low membership, he hopes a similar collaboration will take off in the future.
“I also recommend that PAs and NPs publish articles together, with research as an excellent place to start,” he added. “PAs and NPs should stand together and be a source of healing for all our patients. Regardless of our titles, our responsibility is to bring healing together.”
A version of this article first appeared on Medscape.com.
Docs struggle to keep up with the flood of new medical knowledge. Here’s advice
making it much tougher for physicians to identify innovative findings and newer guidelines for helping patients. Yet not keeping up with the latest information can put doctors at risk.
“Most doctors are feeling lost about keeping up to date,” said John P.A. Ioannidis, MD, professor of medicine at Stanford (Calif.) University School of Medicine. “The vast majority of new studies are either wrong or not useful, but physicians cannot sort out which are those studies.”
The sheer number of new studies may even force some doctors to retreat from areas where they have not kept up, said Stephen A. Martin, MD, professor of family medicine and community health at the University of Massachusetts, Worcester. “When doctors don’t feel they can stay current, they may refer more cases to specialists or narrow their focus,” he said.
Some specialties have a greater challenge than others
Dr. Martin said the deluge of studies heavily impacts generalists because they have a wider field of information to keep up with. However, certain specialties like oncology are particularly flooded with new findings.
Specialties with the greatest number of published studies are reportedly oncology, cardiology, and neurology. A 2021 study found that the number of articles with the word “stroke” in them increased five times from 2000 to 2020. And investigative treatments targeting cancer nearly quadrupled just between 2010 and 2020.
What’s more, physicians spend a great deal of time sifting through studies that are ultimately useless. In a survey of internists by Univadis, which is part of WebMD/Medscape, 82% said that fewer than half of the studies they read actually had an impact on how they practice medicine.
“You often have to dig into an article and learn more about a finding before you now whether it’s useful,” Dr. Martin said. “And in the end, relatively few new findings are truly novel ones that are useful for patient care.”
So what can a physician do? First, find out what you don’t know
Looking for new findings needs to be carried out systematically, according to William B. Cutrer, MD, MEd, a pediatric intensivist who is associate dean for undergraduate medical education at Vanderbilt University School of Medicine, Nashville, Tenn.
“Before you start, you have to know what you don’t know, and that’s often not so easy,” he said. “You may get a spark about what you don’t know in an encounter with a patient or colleague or through patient outcomes data,” he said.
Dr. Martin, on the other hand, advocates a broad approach that involves finding out at least a little about everything in one’s field. “If you have a good base, you’re not starting from zero when you encounter a new clinical situation,” he said.
“The idea is that you don’t need to memorize most things, but you do need to know how to access them,” Dr. Martin said. “I memorize the things I do all the time, such as dosing or indicated testing, but I look up things that I don’t see that often and ones that have some complexity.”
Updating the old ways
For generations, doctors have stayed current by going to meetings, conversing with colleagues, and reading journals, but many physicians have updated these methods through various resources on the internet.
For example, meetings went virtual during the pandemic, and now that face-to-face meetings are back, many of them retain a virtual option, said Kevin Campbell, MD, a cardiologist at Health First Medical Group, Melbourne, Fla. “I typically go to one or two conferences a year, but I also learn a lot digitally,” he said.
As to journal reading, “assessing an article is an essential skill,” Dr. Cutrer said. “It’s important to quickly decide whether a journal article is worth reading or not. One answer to this problem is to consult summaries of important articles. But summaries are sometimes unhelpful, and it is hard to know which articles are significant. Therefore, doctors have been reaching out to others who can research the articles for them.”
For many years, some physicians have pooled their resources in journal clubs. “You get a chance to cross-cultivate your skills with others,” Dr. Ioannidis said. “But you need someone who is well informed and dedicated to run the journal club, using evidence-based principles.”
Dr. Cutrer said physicians like to cast their net wide because they are understandably wary of changing their practice based on one study. “Unless there is one large study that is really well designed, doctors will need two or more findings to be convinced,” he said. This requires having the ability to match studies across many journals.
Using research summaries
In the past two decades, physicians have gained access to countless summaries of journal articles prepared by armies of clinical experts working for review services such as the New England Journal of Medicine’s “Journal Watch,” Annals of Internal Medicine’s “In the Clinic,” and BMJ’s “State of the Arts.”
In addition to summarizing findings from a wide variety of journals in plain language, reviewers may compare them to similar studies and assess the validity of the finding by assigning a level of evidence.
Some commercial ventures provide similar services. Betsy Jones, executive vice president of clinical decisions at EBSCO, said the DynaMed service is now available through an app on the physician’s smartphone or through the electronic health record.
Physicians like this approach. Many specialists have noted that reading full-length articles was not an efficient use of their time, while even more said that reviews are efficient.
Exchanging information online
Physicians are increasingly keeping current by using the internet, especially on social media, Dr. Cutrer said. “Young doctors in particular are more likely to keep up digitally,” he said.
Internet-based information has become so widespread that disparities in health care from region to region have somewhat abated, according to Stuart J. Fischer, MD, an orthopedic surgeon at Summit Orthopaedics and Sports Medicine, New Jersey. “One positive outcome of this plethora of information today is that geographic disparities in clinical practice are not as great as they used to be,” he said.
Rather than chatting up colleagues in the hallway, many physicians have come to rely on internet-based discussion boards.
Blogs, podcasts, and Twitter
Blogs and podcasts, often focused on a specialty, can be a great way for physicians to keep up, said UMass Chan professor Dr. Martin. “Podcasts in particular have enhanced the ability to stay current,” he said. “You want to find someone you trust.”
Internal medicine podcasts include Annals on Call, where doctors discuss articles in the Annals of Internal Medicine, and the Curbsiders, where two internists interview a guest expert.
Orthopedic surgeons can visit podcasts like Nailed it, Orthobullets, the Ortho Show, and Inside Orthopedics. Neurologists can consult Brainwaves, Neurology Podcast, Practical Neurology Podcast, and Clinical Neurology with KD. And pediatricians can drop in on Talking Pediatrics, The Cribsiders, and PedsCases.
Meanwhile, Twitter has become a particularly effective way to broadcast new findings, speeding up the transition from the bench to the bedside, said Dr. Campbell, the Florida cardiologist.
“I visit cardio-specific resources on Twitter,” he said. “They can be real-time video chats or posted messages. They spur discussion like a journal club. Colleagues present cases and drop in and out of the discussion.”
Others are not as enthusiastic. Although Stanford’s Dr. Ioannidis is in the heart of the Silicon Valley, he is leery of some of the new digital methods. “I don’t use Twitter,” he says. “You just add more people to the process, which could only make things more confusing. I want to be able to think a lot about it.”
Cutting-edge knowledge at the point of care
Consulting the literature often takes place at the point of care, when a particular patient requires treatment. This can be done by using clinical decision support (CDS) and by using clinical practice guidelines (CPGs), which are typically developed by panels of doctors at specialty societies.
“It used to be that the doctor was expected to know everything,” said Ms. Jones at DynaMed. “Today there is no way to keep up with it all. Doctors often need a quick memory jog.”
Ms. Jones said the CDS result always requires the doctor’s interpretation. “It is up to the doctor to decide whether a new finding is the best choice for his or her patient,” she said.
Dr. Martin recommends going easy on point-of-care resources. “They can be used for showing a patient a differential diagnosis list or checking the cost of a procedure, but they are harder to use for novel developments that require time and context to evaluate their impact,” he said.
CPGs, meanwhile, have a high profile in the research world. In a 2018 study, Dr. Ioannidis found that 8 of the 15 most-cited articles were CPGs, disease definitions, or disease statistics.
Dr. Fischer said CPGs are typically based on thorough reviews of the literature, but they do involve experts’ interpretation of the science. “It can be difficult to obtain specific answers to some medical questions, especially for problems with complex treatments or variations,” he said.
As a result, Dr. Fischer said doctors have to use their judgment in applying CPGs to a specific patient. “For example, the orthopedic surgeon would normally recommend a total hip replacement for patients with a bad hip, but it might not be appropriate for an overweight patient.”
Stay skeptical
There are many novel ways for physicians to keep current, including summaries of articles, discussion boards, blogs, podcasts, Twitter, clinical decision support, and clinical practice guidelines.
Even with all these new services, though, doctors need to retain a healthy amount of skepticism about new research findings, Dr. Ioannidis said. “Ask yourself questions such as: Does it deal with a real problem? Am I getting the real information? Is it relevant to real patients? Is it offering good value for money?”
A version of this article first appeared on Medscape.com.
making it much tougher for physicians to identify innovative findings and newer guidelines for helping patients. Yet not keeping up with the latest information can put doctors at risk.
“Most doctors are feeling lost about keeping up to date,” said John P.A. Ioannidis, MD, professor of medicine at Stanford (Calif.) University School of Medicine. “The vast majority of new studies are either wrong or not useful, but physicians cannot sort out which are those studies.”
The sheer number of new studies may even force some doctors to retreat from areas where they have not kept up, said Stephen A. Martin, MD, professor of family medicine and community health at the University of Massachusetts, Worcester. “When doctors don’t feel they can stay current, they may refer more cases to specialists or narrow their focus,” he said.
Some specialties have a greater challenge than others
Dr. Martin said the deluge of studies heavily impacts generalists because they have a wider field of information to keep up with. However, certain specialties like oncology are particularly flooded with new findings.
Specialties with the greatest number of published studies are reportedly oncology, cardiology, and neurology. A 2021 study found that the number of articles with the word “stroke” in them increased five times from 2000 to 2020. And investigative treatments targeting cancer nearly quadrupled just between 2010 and 2020.
What’s more, physicians spend a great deal of time sifting through studies that are ultimately useless. In a survey of internists by Univadis, which is part of WebMD/Medscape, 82% said that fewer than half of the studies they read actually had an impact on how they practice medicine.
“You often have to dig into an article and learn more about a finding before you now whether it’s useful,” Dr. Martin said. “And in the end, relatively few new findings are truly novel ones that are useful for patient care.”
So what can a physician do? First, find out what you don’t know
Looking for new findings needs to be carried out systematically, according to William B. Cutrer, MD, MEd, a pediatric intensivist who is associate dean for undergraduate medical education at Vanderbilt University School of Medicine, Nashville, Tenn.
“Before you start, you have to know what you don’t know, and that’s often not so easy,” he said. “You may get a spark about what you don’t know in an encounter with a patient or colleague or through patient outcomes data,” he said.
Dr. Martin, on the other hand, advocates a broad approach that involves finding out at least a little about everything in one’s field. “If you have a good base, you’re not starting from zero when you encounter a new clinical situation,” he said.
“The idea is that you don’t need to memorize most things, but you do need to know how to access them,” Dr. Martin said. “I memorize the things I do all the time, such as dosing or indicated testing, but I look up things that I don’t see that often and ones that have some complexity.”
Updating the old ways
For generations, doctors have stayed current by going to meetings, conversing with colleagues, and reading journals, but many physicians have updated these methods through various resources on the internet.
For example, meetings went virtual during the pandemic, and now that face-to-face meetings are back, many of them retain a virtual option, said Kevin Campbell, MD, a cardiologist at Health First Medical Group, Melbourne, Fla. “I typically go to one or two conferences a year, but I also learn a lot digitally,” he said.
As to journal reading, “assessing an article is an essential skill,” Dr. Cutrer said. “It’s important to quickly decide whether a journal article is worth reading or not. One answer to this problem is to consult summaries of important articles. But summaries are sometimes unhelpful, and it is hard to know which articles are significant. Therefore, doctors have been reaching out to others who can research the articles for them.”
For many years, some physicians have pooled their resources in journal clubs. “You get a chance to cross-cultivate your skills with others,” Dr. Ioannidis said. “But you need someone who is well informed and dedicated to run the journal club, using evidence-based principles.”
Dr. Cutrer said physicians like to cast their net wide because they are understandably wary of changing their practice based on one study. “Unless there is one large study that is really well designed, doctors will need two or more findings to be convinced,” he said. This requires having the ability to match studies across many journals.
Using research summaries
In the past two decades, physicians have gained access to countless summaries of journal articles prepared by armies of clinical experts working for review services such as the New England Journal of Medicine’s “Journal Watch,” Annals of Internal Medicine’s “In the Clinic,” and BMJ’s “State of the Arts.”
In addition to summarizing findings from a wide variety of journals in plain language, reviewers may compare them to similar studies and assess the validity of the finding by assigning a level of evidence.
Some commercial ventures provide similar services. Betsy Jones, executive vice president of clinical decisions at EBSCO, said the DynaMed service is now available through an app on the physician’s smartphone or through the electronic health record.
Physicians like this approach. Many specialists have noted that reading full-length articles was not an efficient use of their time, while even more said that reviews are efficient.
Exchanging information online
Physicians are increasingly keeping current by using the internet, especially on social media, Dr. Cutrer said. “Young doctors in particular are more likely to keep up digitally,” he said.
Internet-based information has become so widespread that disparities in health care from region to region have somewhat abated, according to Stuart J. Fischer, MD, an orthopedic surgeon at Summit Orthopaedics and Sports Medicine, New Jersey. “One positive outcome of this plethora of information today is that geographic disparities in clinical practice are not as great as they used to be,” he said.
Rather than chatting up colleagues in the hallway, many physicians have come to rely on internet-based discussion boards.
Blogs, podcasts, and Twitter
Blogs and podcasts, often focused on a specialty, can be a great way for physicians to keep up, said UMass Chan professor Dr. Martin. “Podcasts in particular have enhanced the ability to stay current,” he said. “You want to find someone you trust.”
Internal medicine podcasts include Annals on Call, where doctors discuss articles in the Annals of Internal Medicine, and the Curbsiders, where two internists interview a guest expert.
Orthopedic surgeons can visit podcasts like Nailed it, Orthobullets, the Ortho Show, and Inside Orthopedics. Neurologists can consult Brainwaves, Neurology Podcast, Practical Neurology Podcast, and Clinical Neurology with KD. And pediatricians can drop in on Talking Pediatrics, The Cribsiders, and PedsCases.
Meanwhile, Twitter has become a particularly effective way to broadcast new findings, speeding up the transition from the bench to the bedside, said Dr. Campbell, the Florida cardiologist.
“I visit cardio-specific resources on Twitter,” he said. “They can be real-time video chats or posted messages. They spur discussion like a journal club. Colleagues present cases and drop in and out of the discussion.”
Others are not as enthusiastic. Although Stanford’s Dr. Ioannidis is in the heart of the Silicon Valley, he is leery of some of the new digital methods. “I don’t use Twitter,” he says. “You just add more people to the process, which could only make things more confusing. I want to be able to think a lot about it.”
Cutting-edge knowledge at the point of care
Consulting the literature often takes place at the point of care, when a particular patient requires treatment. This can be done by using clinical decision support (CDS) and by using clinical practice guidelines (CPGs), which are typically developed by panels of doctors at specialty societies.
“It used to be that the doctor was expected to know everything,” said Ms. Jones at DynaMed. “Today there is no way to keep up with it all. Doctors often need a quick memory jog.”
Ms. Jones said the CDS result always requires the doctor’s interpretation. “It is up to the doctor to decide whether a new finding is the best choice for his or her patient,” she said.
Dr. Martin recommends going easy on point-of-care resources. “They can be used for showing a patient a differential diagnosis list or checking the cost of a procedure, but they are harder to use for novel developments that require time and context to evaluate their impact,” he said.
CPGs, meanwhile, have a high profile in the research world. In a 2018 study, Dr. Ioannidis found that 8 of the 15 most-cited articles were CPGs, disease definitions, or disease statistics.
Dr. Fischer said CPGs are typically based on thorough reviews of the literature, but they do involve experts’ interpretation of the science. “It can be difficult to obtain specific answers to some medical questions, especially for problems with complex treatments or variations,” he said.
As a result, Dr. Fischer said doctors have to use their judgment in applying CPGs to a specific patient. “For example, the orthopedic surgeon would normally recommend a total hip replacement for patients with a bad hip, but it might not be appropriate for an overweight patient.”
Stay skeptical
There are many novel ways for physicians to keep current, including summaries of articles, discussion boards, blogs, podcasts, Twitter, clinical decision support, and clinical practice guidelines.
Even with all these new services, though, doctors need to retain a healthy amount of skepticism about new research findings, Dr. Ioannidis said. “Ask yourself questions such as: Does it deal with a real problem? Am I getting the real information? Is it relevant to real patients? Is it offering good value for money?”
A version of this article first appeared on Medscape.com.
making it much tougher for physicians to identify innovative findings and newer guidelines for helping patients. Yet not keeping up with the latest information can put doctors at risk.
“Most doctors are feeling lost about keeping up to date,” said John P.A. Ioannidis, MD, professor of medicine at Stanford (Calif.) University School of Medicine. “The vast majority of new studies are either wrong or not useful, but physicians cannot sort out which are those studies.”
The sheer number of new studies may even force some doctors to retreat from areas where they have not kept up, said Stephen A. Martin, MD, professor of family medicine and community health at the University of Massachusetts, Worcester. “When doctors don’t feel they can stay current, they may refer more cases to specialists or narrow their focus,” he said.
Some specialties have a greater challenge than others
Dr. Martin said the deluge of studies heavily impacts generalists because they have a wider field of information to keep up with. However, certain specialties like oncology are particularly flooded with new findings.
Specialties with the greatest number of published studies are reportedly oncology, cardiology, and neurology. A 2021 study found that the number of articles with the word “stroke” in them increased five times from 2000 to 2020. And investigative treatments targeting cancer nearly quadrupled just between 2010 and 2020.
What’s more, physicians spend a great deal of time sifting through studies that are ultimately useless. In a survey of internists by Univadis, which is part of WebMD/Medscape, 82% said that fewer than half of the studies they read actually had an impact on how they practice medicine.
“You often have to dig into an article and learn more about a finding before you now whether it’s useful,” Dr. Martin said. “And in the end, relatively few new findings are truly novel ones that are useful for patient care.”
So what can a physician do? First, find out what you don’t know
Looking for new findings needs to be carried out systematically, according to William B. Cutrer, MD, MEd, a pediatric intensivist who is associate dean for undergraduate medical education at Vanderbilt University School of Medicine, Nashville, Tenn.
“Before you start, you have to know what you don’t know, and that’s often not so easy,” he said. “You may get a spark about what you don’t know in an encounter with a patient or colleague or through patient outcomes data,” he said.
Dr. Martin, on the other hand, advocates a broad approach that involves finding out at least a little about everything in one’s field. “If you have a good base, you’re not starting from zero when you encounter a new clinical situation,” he said.
“The idea is that you don’t need to memorize most things, but you do need to know how to access them,” Dr. Martin said. “I memorize the things I do all the time, such as dosing or indicated testing, but I look up things that I don’t see that often and ones that have some complexity.”
Updating the old ways
For generations, doctors have stayed current by going to meetings, conversing with colleagues, and reading journals, but many physicians have updated these methods through various resources on the internet.
For example, meetings went virtual during the pandemic, and now that face-to-face meetings are back, many of them retain a virtual option, said Kevin Campbell, MD, a cardiologist at Health First Medical Group, Melbourne, Fla. “I typically go to one or two conferences a year, but I also learn a lot digitally,” he said.
As to journal reading, “assessing an article is an essential skill,” Dr. Cutrer said. “It’s important to quickly decide whether a journal article is worth reading or not. One answer to this problem is to consult summaries of important articles. But summaries are sometimes unhelpful, and it is hard to know which articles are significant. Therefore, doctors have been reaching out to others who can research the articles for them.”
For many years, some physicians have pooled their resources in journal clubs. “You get a chance to cross-cultivate your skills with others,” Dr. Ioannidis said. “But you need someone who is well informed and dedicated to run the journal club, using evidence-based principles.”
Dr. Cutrer said physicians like to cast their net wide because they are understandably wary of changing their practice based on one study. “Unless there is one large study that is really well designed, doctors will need two or more findings to be convinced,” he said. This requires having the ability to match studies across many journals.
Using research summaries
In the past two decades, physicians have gained access to countless summaries of journal articles prepared by armies of clinical experts working for review services such as the New England Journal of Medicine’s “Journal Watch,” Annals of Internal Medicine’s “In the Clinic,” and BMJ’s “State of the Arts.”
In addition to summarizing findings from a wide variety of journals in plain language, reviewers may compare them to similar studies and assess the validity of the finding by assigning a level of evidence.
Some commercial ventures provide similar services. Betsy Jones, executive vice president of clinical decisions at EBSCO, said the DynaMed service is now available through an app on the physician’s smartphone or through the electronic health record.
Physicians like this approach. Many specialists have noted that reading full-length articles was not an efficient use of their time, while even more said that reviews are efficient.
Exchanging information online
Physicians are increasingly keeping current by using the internet, especially on social media, Dr. Cutrer said. “Young doctors in particular are more likely to keep up digitally,” he said.
Internet-based information has become so widespread that disparities in health care from region to region have somewhat abated, according to Stuart J. Fischer, MD, an orthopedic surgeon at Summit Orthopaedics and Sports Medicine, New Jersey. “One positive outcome of this plethora of information today is that geographic disparities in clinical practice are not as great as they used to be,” he said.
Rather than chatting up colleagues in the hallway, many physicians have come to rely on internet-based discussion boards.
Blogs, podcasts, and Twitter
Blogs and podcasts, often focused on a specialty, can be a great way for physicians to keep up, said UMass Chan professor Dr. Martin. “Podcasts in particular have enhanced the ability to stay current,” he said. “You want to find someone you trust.”
Internal medicine podcasts include Annals on Call, where doctors discuss articles in the Annals of Internal Medicine, and the Curbsiders, where two internists interview a guest expert.
Orthopedic surgeons can visit podcasts like Nailed it, Orthobullets, the Ortho Show, and Inside Orthopedics. Neurologists can consult Brainwaves, Neurology Podcast, Practical Neurology Podcast, and Clinical Neurology with KD. And pediatricians can drop in on Talking Pediatrics, The Cribsiders, and PedsCases.
Meanwhile, Twitter has become a particularly effective way to broadcast new findings, speeding up the transition from the bench to the bedside, said Dr. Campbell, the Florida cardiologist.
“I visit cardio-specific resources on Twitter,” he said. “They can be real-time video chats or posted messages. They spur discussion like a journal club. Colleagues present cases and drop in and out of the discussion.”
Others are not as enthusiastic. Although Stanford’s Dr. Ioannidis is in the heart of the Silicon Valley, he is leery of some of the new digital methods. “I don’t use Twitter,” he says. “You just add more people to the process, which could only make things more confusing. I want to be able to think a lot about it.”
Cutting-edge knowledge at the point of care
Consulting the literature often takes place at the point of care, when a particular patient requires treatment. This can be done by using clinical decision support (CDS) and by using clinical practice guidelines (CPGs), which are typically developed by panels of doctors at specialty societies.
“It used to be that the doctor was expected to know everything,” said Ms. Jones at DynaMed. “Today there is no way to keep up with it all. Doctors often need a quick memory jog.”
Ms. Jones said the CDS result always requires the doctor’s interpretation. “It is up to the doctor to decide whether a new finding is the best choice for his or her patient,” she said.
Dr. Martin recommends going easy on point-of-care resources. “They can be used for showing a patient a differential diagnosis list or checking the cost of a procedure, but they are harder to use for novel developments that require time and context to evaluate their impact,” he said.
CPGs, meanwhile, have a high profile in the research world. In a 2018 study, Dr. Ioannidis found that 8 of the 15 most-cited articles were CPGs, disease definitions, or disease statistics.
Dr. Fischer said CPGs are typically based on thorough reviews of the literature, but they do involve experts’ interpretation of the science. “It can be difficult to obtain specific answers to some medical questions, especially for problems with complex treatments or variations,” he said.
As a result, Dr. Fischer said doctors have to use their judgment in applying CPGs to a specific patient. “For example, the orthopedic surgeon would normally recommend a total hip replacement for patients with a bad hip, but it might not be appropriate for an overweight patient.”
Stay skeptical
There are many novel ways for physicians to keep current, including summaries of articles, discussion boards, blogs, podcasts, Twitter, clinical decision support, and clinical practice guidelines.
Even with all these new services, though, doctors need to retain a healthy amount of skepticism about new research findings, Dr. Ioannidis said. “Ask yourself questions such as: Does it deal with a real problem? Am I getting the real information? Is it relevant to real patients? Is it offering good value for money?”
A version of this article first appeared on Medscape.com.
Epithelioma Cuniculatum (Plantar Verrucous Carcinoma): A Systematic Review of Treatment Options
Verrucous carcinoma (VC) is an uncommon type of well-differentiated squamous cell carcinoma (SCC) that most commonly affects men in the fifth to sixth decades of life. 1 The tumor grows slowly over a decade or more and does not frequently metastasize but has a high propensity for recurrence and local invasion. 2 There are 3 main subtypes of VC classified by anatomic site: oral florid papillomatosis (oral cavity), Buschke-Lowenstein tumor (anogenital region), and epithelioma cuniculatum (EC)(feet). 3 Epithelioma cuniculatum, also known as carcinoma cuniculatum or papillomatosis cutis carcinoides, most commonly presents as a solitary, warty or cauliflowerlike, exophytic mass with keratin-filled sinus tracts and malodorous discharge. 4 Diabetic foot ulcers and chronic inflammatory conditions are predisposing risk factors for EC, and it can result in difficulty walking/immobility, pain, and bleeding depending on anatomic involvement. 5-9
The differential diagnosis for VC includes refractory verruca vulgaris, clavus, SCC, keratoacanthoma, deep fungal or mycobacterial infection, eccrine poroma or porocarcinoma, amelanotic melanoma, and sarcoma.10-13 The slow-growing nature of VC, sampling error of superficial biopsies, and minimal cytological atypia on histologic examination can contribute to delayed diagnosis and appropriate treatment.14 Characteristic histologic features include hyperkeratosis, papillomatosis, marked acanthosis, broad blunt-ended rete ridges with a “bulldozing” architecture, and minimal cytologic atypia and mitoses.5,6 In some cases, pleomorphism and glassy eosinophilic cytoplasmic changes may be more pronounced than that of a common wart though less dramatic than that of conventional SCCs.15 Antigen Ki-67 and tumor protein p53 have been proposed to help differentiate between common plantar verruca, VC, and SCC, but the histologic diagnosis remains challenging, and repeat histopathologic examination often is required.16-19 Following diagnosis, computed tomography or magnetic resonance imaging may be necessary to determine tumor extension and assess for deep tissue and bony involvement.20-22
Treatment of EC is particularly challenging because of the anatomic location and need for margin control while maintaining adequate function, preserving healthy tissue, and providing coverage of defects. Surgical excision of EC is the first-line treatment, most commonly by wide local excision (WLE) or amputation. Mohs micrographic surgery (MMS) also has been utilized. One review found no recurrences in 5 cases of EC treated with MMS.23 As MMS is a tissue-sparing technique, this is a valuable modality for sites of functional importance such as the feet. Herein, we review various reported EC treatment modalities and outcomes, with an emphasis on recurrence rates for WLE and MMS.
METHODS
A systematic literature review of PubMed articles indexed for MEDLINE, as well as databases including the Cochrane Library, Web of Science, and Cumulative Index to Nursing and Allied Health Literature (CINAHL), was performed on January 14, 2020. Two authors (S.S.D. and S.V.C.) independently screened results using the search terms (plantar OR foot) AND (verrucous carcinoma OR epithelioma cuniculatum OR carcinoma cuniculatum). The search terms were chosen according to MeSH subject headings. All articles from the start date of the databases through the search date were screened, and articles pertaining to VC, EC, or carcinoma cuniculatum located on the foot were included. Of these, non–English-language articles were translated and included. Articles reporting VC on a site other than the foot (eg, the oral cavity) or benign verrucous skin lesions were excluded. The reference lists for all articles also were reviewed for additional reports that were absent from the initial search using both included and excluded articles. A full-text review was performed on 221 articles published between 1954 and 2019 per the PRISMA guidelines (Figure).
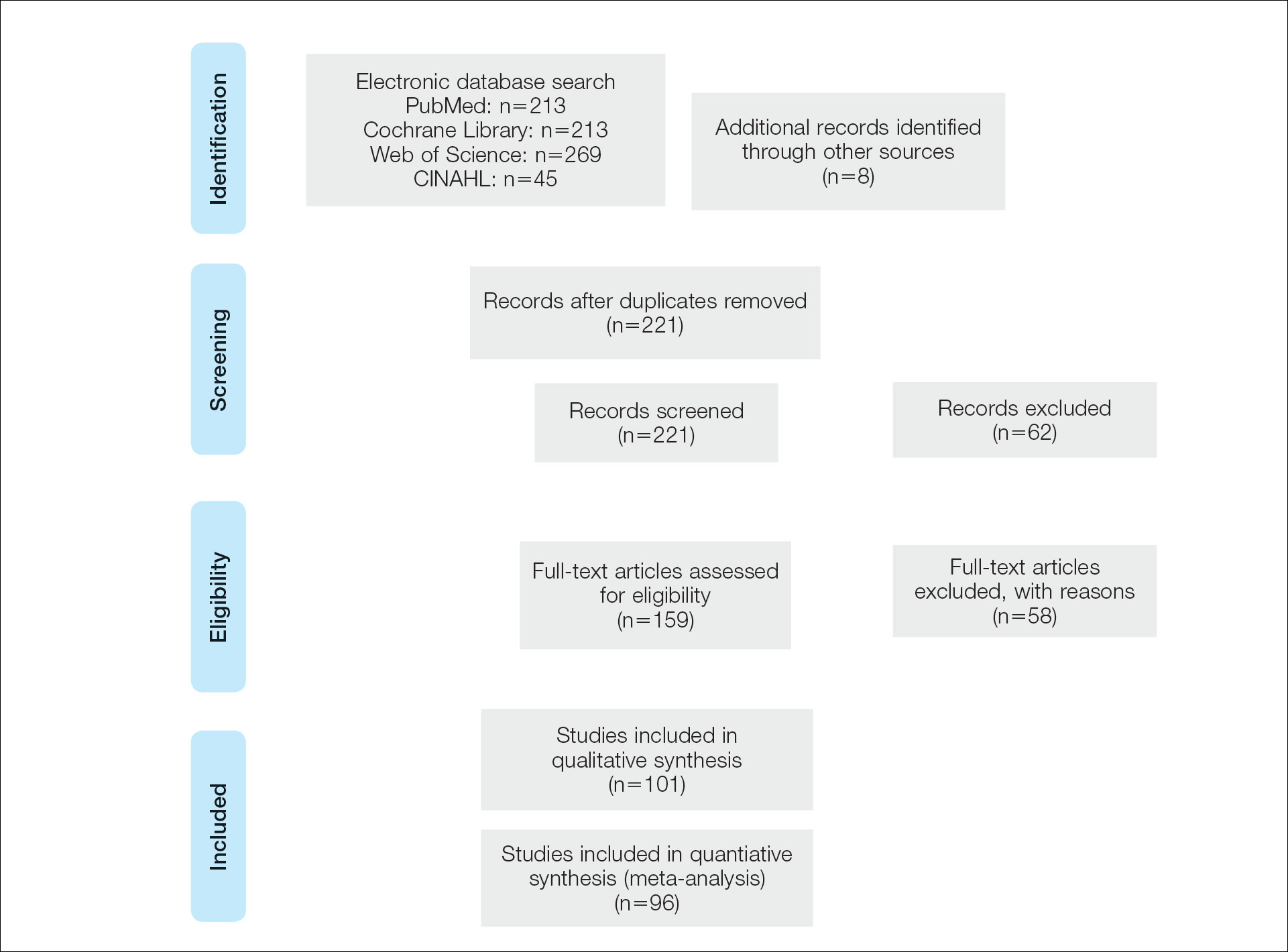
A total of 101 articles were included in the study for qualitative analysis. Nearly all articles identified were case reports, giving an evidence level of 5 by the Centre for Evidence-Based Medicine rating scale. Five articles reported data on multiple patients without individual demographic or clinical details and were excluded from analysis. Of the remaining 96 articles, information about patient characteristics, tumor size, treatment modality, and recurrence were extracted for 115 cases.
RESULTS
Of the 115 cases that were reviewed, 81 (70%) were male and 33 (29%) were female with a male-to-female ratio of 2.4:1. Ages of the patients ranged from 18 to 88 years; the mean and median age was 56 years. Nearly all reported cases of EC affected the plantar surface of one foot, with 4 reports of tumors affecting both feet.24-27 One case affecting both feet reported known exposure to lead arsenate pesticides27; all others were associated with a clinical history of chronic ulcers or warts persisting for several years to decades. Other less common sites of EC included the dorsal foot, interdigital web space, and subungual digit.28-30 The most common location reported was the anterior ball of the foot. Tumors were reported to arise within pre-existing lesions, such as hypertrophic lichen planus or chronic foot wounds associated with diabetes mellitus or leprosy.31-35 Tumor size ranged from 1 to 22 cm with a median of 4.5 cm.
Eight cases were reported to be associated with human papillomavirus; low-risk types 6 and 11 and high-risk types 16 and 18 were found in 6 cases.36-41 Two cases reported association with human papillomavirus type 2.7,42
Metastases to dermal and subdermal lymphatics, regional lymph nodes, and the lungs were reported in 3 cases, repectively.43-45 Of these, one primary tumor had received low-dose irradiation in the form of X-ray therapy.45
Treatment Modalities
The cases of EC that we reviewed included treatment with surgical and systemic therapies as well as other modalities such as acitretin, interferon alfa, topical imiquimod, curettage, debridement, electrodesiccation, and radiation. The Table includes a complete summary of the treatments we analyzed.
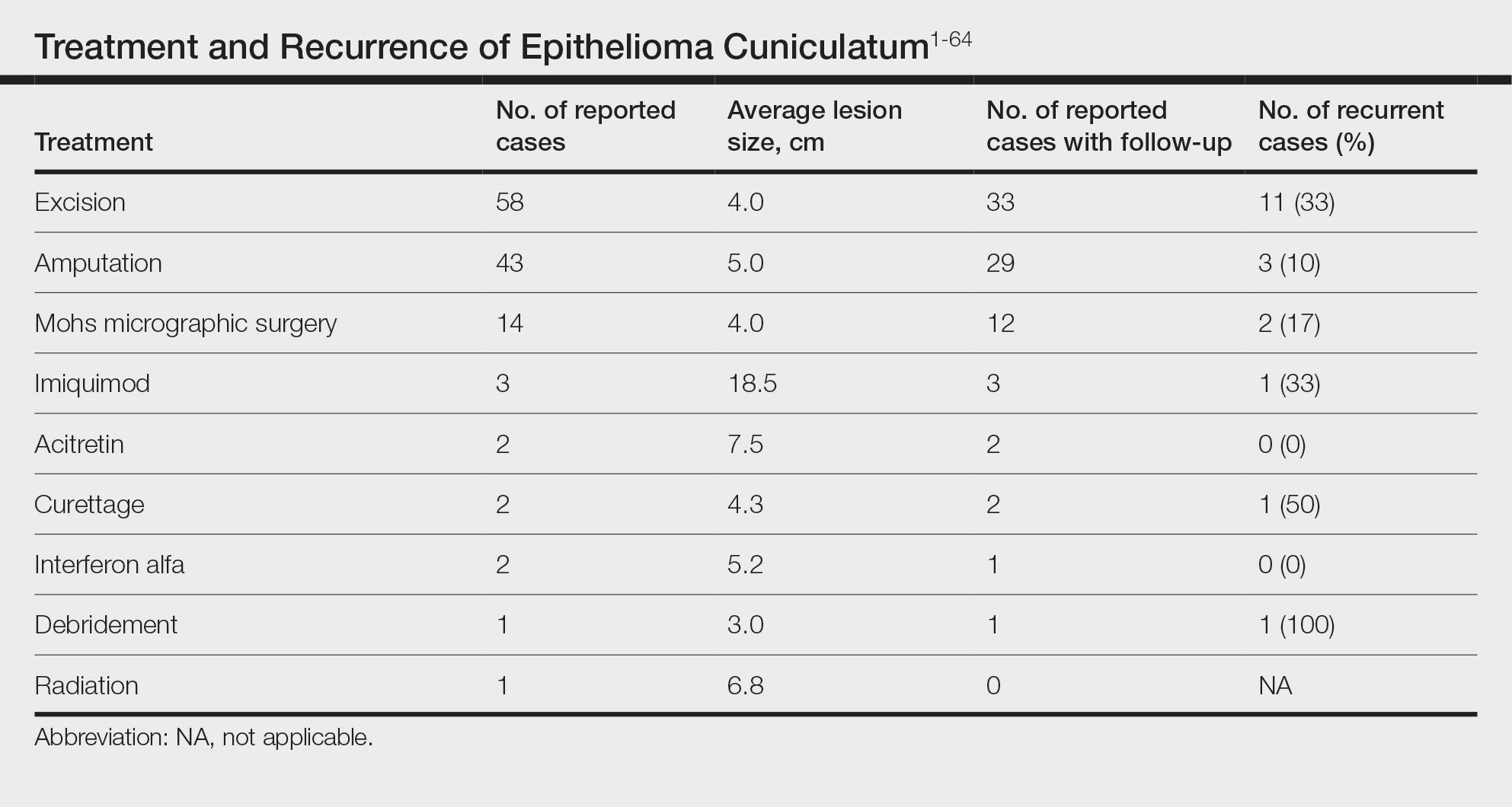
Surgical Therapy—The majority (91% [105/115]) of cases were treated surgically. The most common treatment modality was WLE (50% [58/115]), followed by amputation (37% [43/115]) and MMS (12% [14/115]).
Wide local excision was the most frequently reported treatment, with excision margins of at least 5 mm to 1 cm.48 Incidence of recurrence was reported for 57% (33/58) of cases treated with WLE; of these, the recurrence rate was 33% (11/33). For patients with EC recurrence, the most common secondary treatment was repeat excision with wider margins (1–2 cm) or amputation (5/11).49-52 Few postoperative complications were reported but included pain, infection, and difficulty walking, which were mostly associated with repair modality (eg, split-thickness skin grafts, rotational flaps).53 Amputation was the second most common treatment modality, with a 67% (29/43) incidence of recurrence. Types of amputation included transmetatarsal ray amputation (7/43 [16%]), foot or forefoot amputation (2/43 [5%]), above-the-knee amputation (1/43 [2%]), and below-the-knee amputation (1/43 [2%]). Complications associated with amputation included infection and requirement of prosthetics for ambulation. Split-thickness skin grafts and rotational flaps were the most common surgical repairs performed.52,53
Mohs micrographic surgery was the least frequently reported surgical treatment modality. Both traditional MMS on fresh tissue and “slow Mohs,” with formalin-fixed paraffin embedded tissue examination over several days, were performed for EC with horizontal en face sectioning.54-56 Incidence of recurrence was reported for 86% (12/14) of MMS cases. Of these, recurrence was seen in 17% (2/12) that utilized a flat horizontal processing of tissue sections coupled with saucerlike excisions to enable examination of the entire undersurface and margins. In one case, the patient was treated with MMS with recurrence noted 1 month later; thus, repeat MMS was performed, and the tumor was found to be entwined around the flexor tendon.57 The tendon was removed, and clear margins were obtained. Follow-up 3 years after the second MMS revealed no signs of recurrence.57 In the other case, the patient had a particularly aggressive course with bilateral VC in the setting of diabetic ulcers that was treated with WLE prior to MMS and recurrence still noted after MMS.26 No complications were reported with MMS.
Overall, recurrence was most frequently reported with WLE (11/33 [33%]), followed by MMS (2/12 [17%]) and amputation (3/29 [10%]). When comparing WLE and amputation, the relationship between treatment modality and recurrence was statistically significant using a χ2 test of independence (χ2=4.7; P=.03). However, results were not significant with Yates correction for continuity (χ2=3.4; P=.06). The χ2 test of independence showed no significant association between treatment method and recurrence when comparing WLE with MMS (χ2=1.2; P=.28). Reported follow-up times varied greatly from a few months to 10 years.
Systemic Therapy—Of the total cases, only 2 cases reported treatment with acitretin and 2 utilized interferon alfa.58,59 In one case, treatment of EC with interferon alfa alone required more aggressive therapy (ie, amputation).58 Neither of the 2 cases using acitretin reported recurrence.59,60 Complications of acitretin therapy included cheilitis and transaminitis.60
Other Treatment Modalities—Three cases utilized imiquimod, with 2 cases of imiquimod monotherapy and 1 case of imiquimod in combination with electrodesiccation and WLE.37 One of the cases of EC treated with imiquimod monotherapy recurred and required WLE.61
There were reports of other treatments including curettage alone (2% [2/115]),40,62 debridement alone (1% [1/115]),40 electrodesiccation (1% [1/115]),37 and radiation (1% [1/115]).43 Recurrence was found with curettage alone and debridement alone. Electrodesiccation was reported in conjunction with WLE without recurrence. Radiation was used to treat a case of VC that had metastasized to the lymph nodes; no follow-up was described.43
COMMENT
Epithelioma cuniculatum is an indolent malignancy of the plantar foot that likely is frequently underdiagnosed or misdiagnosed because of location, sampling error, and challenges in histopathologic diagnosis. Once diagnosed, surgical removal with margin control is the first-line therapy for EC. Our review found a number of surgical, systemic, and other treatment modalities that have been used to treat EC, but there remains a lack of evidence to provide clear guidelines as to which therapies are most effective. Current data on the treatment of EC largely are limited to case reports and case series. To date, there are no reports of higher-quality studies or randomized controlled trials to assess the efficacy of various treatment modalities.
Our review found that WLE is the most common treatment modality for EC, followed by amputation and MMS. Three cases43-45 that reported metastasis to lymph nodes also were treated with fine-needle aspiration or biopsy, and it is recommended that sentinel lymph node biopsy be performed when there is a history of radiation exposure or clinically and sonographically unsuspicious lymph nodes, while dissection of regional nodes should be performed if lymph node metastasis is suspected.53 Additional treatments reported included acitretin, interferon alfa, topical imiquimod, curettage, debridement, and electrodesiccation, but because of the limited number of cases and variable efficacy, no conclusions can be made on the utility of these alternative modalities.
The lowest rate of reported recurrence was found with amputation, followed by MMS and WLE. Amputation is the most aggressive treatment option, but its superiority in lower recurrence rates was not statistically significant when compared with either WLE or MMS after Yates correction. Despite treatment with radical surgery, recurrence is still possible and may be associated with factors including greater size (>2 cm) and depth (>4 mm), poor histologic differentiation, perineural involvement, failure of previous treatments, and immunosuppression.63 No statistically significant difference in recurrence rates was found among surgical methods, though data trended toward lower rates of recurrence with MMS compared with WLE, as recurrence with MMS was only reported in 2 cases.25,56
The efficacy of MMS is well documented for tumors with contiguous growth and enables maximum preservation of normal tissue structure and function with complete margin visualization. Thus, our results are in agreement with those of prior studies,54-56,64 suggesting that MMS is associated with lower recurrence rates for EC than WLE. Future studies and reporting of MMS for EC are particularly important because of the functional importance of the plantar foot.
It is important to note that there are local and systemic risk factors that increase the likelihood of developing EC and facilitate tumor growth, including antecedent trauma to the lesion site, chronic irritation or infection, and immunosuppression (HIV related or iatrogenic medication induced). These risk factors may play a role in the treatment modality utilized (eg, more aggressive EC may be treated with amputation instead of WLE). Underlying patient comorbidities could potentially affect recurrence rates, which is a variable we could not control for in our analysis.
Our findings are limited by study design, with supporting evidence consisting of case reports and series. The review is limited by interstudy variability and heterogeneity of results. Additionally, recurrence is not reported in all cases and may be a source of sampling bias. Further complicating the generalizability of these results is the lack of follow-up to evaluate morbidity and quality of life after treatment.
CONCLUSION
This review suggests that MMS is associated with lower recurrence rates than WLE for the treatment of EC. Further investigation of MMS for EC with appropriate follow-up is necessary to identify whether MMS is associated with lower recurrence and less functional impairment. Nonsurgical treatments, including topical imiquimod, interferon alfa, and acitretin, may be useful in cases where surgical therapies are contraindicated, but there is little evidence to support these treatment modalities. Treatment guidelines for EC are not established, and appropriate treatment guidelines should be developed in the future.
- McKee PH, Wilkinson JD, Black MM, et al. Carcinoma (epithelioma) cuniculatum: a clinicopathological study of nineteen cases and review of the literature. Histopathology. 1981;5:425-436.
- Aird I, Johnson HD, Lennox B, et al. Epithelioma cuniculatum: a variety of squamous carcinoma peculiar to the foot. Br J Surg. 1954;42:245-250.
- Seremet S, Erdemir AT, Kiremitci U, et al. Unusually early-onset plantar verrucous carcinoma. Cutis. 2019;104:34-36.
- Spyriounis PK, Tentis D, Sparveri IF, et al. Plantar epithelioma cuniculatum. a case report with review of the literature. Eur J Plast Surg. 2004;27:253-256.
- Ho J, Diven G, Bu J, et al. An ulcerating verrucous plaque on the foot. verrucous carcinoma (epithelioma cuniculatum). Arch Dermatol. 2000;136:547-548, 550-551.
- Kao GF, Graham JH, Helwig EB. Carcinoma cuniculatum (verrucous carcinoma of the skin): a clinicopathologic study of 46 cases with ultrastructural observations. Cancer. 1982;49:2395-2403.
- Zielonka E, Goldschmidt D, de Fontaine S. Verrucous carcinoma or epithelioma cuniculatum plantare. Eur J Surg Oncol. 1997;23:86-87.
- Dogan G, Oram Y, Hazneci E, et al. Three cases of verrucous carcinoma. Australas J Dermatol. 1998;39:251-254.
- Schwartz RA, Burgess GH. Verrucous carcinoma of the foot. J Surg Oncol. 1980;14:333-339.
- McKay C, McBride P, Muir J. Plantar verrucous carcinoma masquerading as toe web intertrigo. Australas J Dermatol. 2012;53:2010-2012.
- Shenoy AS, Waghmare RS, Kavishwar VS, et al. Carcinoma cuniculatum of foot. Foot. 2011;21:207-208.
- Lozzi G, Perris K. Carcinoma cuniculatum. CMAJ. 2007;177:249-251.
- Schein O, Orenstein A, Bar-Meir E. Plantar verrucous carcicoma (epithelioma cuniculatum): rare form of the common wart. Isr Med Assoc J. 2006;8:885.
- Rheingold LM, Roth LM. Carcinoma of the skin of the foot exhibiting some verrucous features. Plast Reconstr Surg. 1978;61:605-609.
- Klima M, Kurtis B, Jordan PH. Verrucous carcinoma of skin. J Cutan Pathol. 1980;7:88-98.
- Nakamura Y, Kashiwagi K, Nakamura A, et al. Verrucous carcinoma of the foot diagnosed using p53 and Ki-67 immunostaining in a patient with diabetic neuropathy. Am J Dermatopathol. 2015;37:257-259.
- Costache M, Desa LT, Mitrache LE, et al. Cutaneous verrucous carcinoma—report of three cases with review of literature. Rom J Morphol Embryol. 2014;55:383-388.
- Terada T. Verrucous carcinoma of the skin: a report on 5 Japanese cases. Ann Diagn Pathol. 2011;15:175-180.
- Noel JC, Heenen M, Peny MO, et al. Proliferating cell nuclear antigen distribution in verrucous carcinoma of the skin. Br J Dermatol. 1995;133:868-873.
- García-Gavín J, González-Vilas D, Rodríguez-Pazos L, et al. Verrucous carcinoma of the foot affecting the bone: utility of the computed tomography scanner. Dermatol Online J. 2010;16:3-5.
- Wasserman PL, Taylor RC, Pinillia J, et al. Verrucous carcinoma of the foot and enhancement assessment by MRI. Skeletal Radiol. 2009;38:393-395.
- Bhushan MH, Ferguson JE, Hutchinson CE. Carcinoma cuniculatum of the foot assessed by magnetic resonance scanning. Clin Exp Dermatol. 2001;26:419-422.
- Penera KE, Manji KA, Craig AB, et al. Atypical presentation of verrucous carcinoma: a case study and review of the literature. Foot Ankle Spec. 2013;6:318-322.
- Suen K, Wijeratne S, Patrikios J. An unusual case of bilateral verrucous carcinoma of the foot (epithelioma cuniculatum). J Surg Case Rep. 2012;2012:rjs020.
- Riccio C, King K, Elston JB, et al. Bilateral plantar verrucous carcinoma. Eplasty. 2016;16:ic46.
- Di Palma V, Stone JP, Schell A, et al. Mistaken diabetic ulcers: a case of bilateral foot verrucous carcinoma. Case Rep Dermatol Med. 2018;2018:4192657.
- Seehafer JR, Muller SA, Dicken CH. Bilateral verrucous carcinoma of the feet. Orthop Surv. 1979;3:205.
- Tosti A, Morelli R, Fanti PA, et al. Carcinoma cuniculatum of the nail apparatus: report of three cases. Dermatology. 1993;186:217-221.
- Melo CR, Melo IS, Souza LP. Epithelioma cuniculatum, a verrucous carcinoma of the foot. report of 2 cases. Dermatologica. 1981;163:338-342.
- Van Geertruyden JP, Olemans C, Laporte M, et al. Verrucous carcinoma of the nail bed. Foot Ankle Int. 1998;19:327-328.
- Thakur BK, Verma S, Raphael V. Verrucous carcinoma developing in a long standing case of ulcerative lichen planus of sole: a rare case report. J Eur Acad Dermatol Venereol. 2015;29:399-401.
- Mayron R, Grimwood RE, Siegle RJ, et al. Verrucous carcinoma arising in ulcerative lichen planus of the soles. J Dermatol Surg Oncol. 1988;14:547-551.
- Boussofara L, Belajouza-Noueiri C, Ghariani N, et al. Verrucous epidermoid carcinoma as a complication in cutaneous lichen planus [article in French]. Ann Dermatol Venereol. 2006;133:404-405.
- Khullar G, Mittal S, Sharma S. Verrucous carcinoma on the foot arising in a chronic neuropathic ulcer of leprosy. Australas J Dermatol. 2019;60:245-246.
- Ochsner PE, Hausman R, Olsthoorn PGM. Epithelioma cunicalutum developing in a neuropathic ulcer of leprous etiology. Arch Orthop Trauma Surg. 1979;94:227-231.
- Ray R, Bhagat A, Vasudevan B, et al. A rare case of plantar epithelioma cuniculatum arising from a wart. Indian J Dermatol. 2015;60:485-487.
- Imko-Walczuk B, Cegielska A, Placek W, et al. Human papillomavirus-related verrucous carcinoma in a renal transplant patient after long-term immunosuppression: a case report. Transplant Proc. 2014;46:2916-2919.
- Floristán MU, Feltes RA, Sáenz JC, et al. Verrucous carcinoma of the foot associated with human papillomavirus type 18. Actas Dermosifiliogr. 2009;100:433-435.
- Sasaoka R, Morimura T, Mihara M, et al. Detection of human pupillomavirus type 16 DNA in two cases of verriicous carcinoma of the foot. Br J Dermatol. 1996;134:983984.
- Schell BJ, Rosen T, Rády P, et al. Verrucous carcinoma of the foot associated with human papillomavirus type 16. J Am Acad Dermatol. 2001;45:49-55.
- Knobler RM, Schneider S, Neumann RA, et al. DNA dot‐blot hybridization implicates human papillomavirus type 11‐DNA in epithelioma cuniculatum. J Med Virol. 1989;29:33-37.
- Noel JC, Peny MO, Detremmerie O, et al. Demonstration of human papillomavirus type 2 in a verrucous carcinoma of the foot. Dermatology. 1993;187:58-61.
- Jungmann J, Vogt T, Müller CSL. Giant verrucous carcinoma of the lower extremity in women with dementia. BMJ Case Rep. 2012;2012:bcr2012006357.
- McKee PH, Wilkinson JD, Corbett MF, et al. Carcinoma cuniculatum: a case metastasizing to skin and lymph nodes. Clin Exp Dermatol. 1981;6:613-618.
- Owen WR, Wolfe ID, Burnett JW, et al. Epithelioma cuniculatum. South Med J. 1978;71:477-479.
- Patel AN, Bedforth N, Varma S. Pain-free treatment of carcinoma cuniculatum on the heel using Mohs micrographic surgery and ultrasonography-guided sciatic nerve block. Clin Exp Dermatol. 2013;38:569-571.
- Padilla RS, Bailin PL, Howard WR, et al. Verrucous carcinoma of the skin and its management by Mohs’ surgery. Plast Reconstr Surg. 1984;73:442-447.
- Kotwal M, Poflee S, Bobhate S. Carcinoma cuniculatum at various anatomical sites. Indian J Dermatol. 2005;50:216-220.
- Arefi M, Philipone E, Caprioli R, et al. A case of verrucous carcinoma (epithelioma cuniculatum) of the heel mimicking infected epidermal cyst and gout. Foot Ankle Spec. 2008;1:297-299.
- Trebing D, Brunner M, Kröning Y, et al. Young man with verrucous heel tumor [article in German]. J Dtsch Dermatol Ges. 2003;9:739-741.
- Thompson SG. Epithelioma cuniculatum: an unusual tumour of the foot. Br J Plast Surg. 1965;18:214-217.
- Thomas EJ, Graves NC, Meritt SM. Carcinoma cuniculatum: an atypical presentation in the foot. J Foot Ankle Surg. 2014;53:356-359.
- Koch H, Kowatsch E, Hödl S, et al. Verrucous carcinoma of the skin: long-term follow-up results following surgical therapy. Dermatol Surg. 2004;30:1124-1130.
- Mallatt BD, Ceilley RI, Dryer RF. Management of verrucous carcinoma on a foot by a combination of chemosurgery and plastic repair: report of a case. J Dermatol Surg Oncol. 1980;6:532-534.
- Mohs FE, Sahl WJ. Chemosurgery for verrucous carcinoma. J Dermatol Surg Oncol. 1979;5:302-306.
- Alkalay R, Alcalay J, Shiri J. Plantar verrucous carcinoma treated with Mohs micrographic surgery: a case report and literature review. J Drugs Dermatol. 2006;5:68-73.
- Mora RG. Microscopically controlled surgery (Mohs’ chemosurgery) for treatment of verrucous squamous cell carcinoma of the foot (epithelioma cuniculatum). J Am Acad Dermatol. 1983;8:354-362.
- Risse L, Negrier P, Dang PM, et al. Treatment of verrucous carcinoma with recombinant alfa-interferon. Dermatology. 1995;190:142-144.
- Rogozin´ski TT, Schwartz RA, Towpik E. Verrucous carcinoma in Unna-Thost hyperkeratosis of the palms and soles. J Am Acad Dermatol. 1994;31:1061-1062.
- Kuan YZ, Hsu HC, Kuo TT, et al. Multiple verrucous carcinomas treated with acitretin. J Am Acad Dermatol. 2007;56(2 suppl):S29-S32.
- Schalock PC, Kornik RI, Baughman RD, et al. Treatment of verrucous carcinoma with topical imiquimod. J Am Acad Dermatol. 2006;54:233-234.
- Brown SM, Freeman RG. Epithelioma cuniculatum. Arch Dermatol. 1976;112:1295-1296.
- Rowe DE, Carroll RJ, Day CL, et al. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. J Am Acad Dermatol. 1992;26:976-990.
- Swanson NA, Taylor WB. Plantar verrucous carcinoma: literature review and treatment by the Mohs’ chemosurgery technique. Arch Dermatol. 1980;116:794-797.
Verrucous carcinoma (VC) is an uncommon type of well-differentiated squamous cell carcinoma (SCC) that most commonly affects men in the fifth to sixth decades of life. 1 The tumor grows slowly over a decade or more and does not frequently metastasize but has a high propensity for recurrence and local invasion. 2 There are 3 main subtypes of VC classified by anatomic site: oral florid papillomatosis (oral cavity), Buschke-Lowenstein tumor (anogenital region), and epithelioma cuniculatum (EC)(feet). 3 Epithelioma cuniculatum, also known as carcinoma cuniculatum or papillomatosis cutis carcinoides, most commonly presents as a solitary, warty or cauliflowerlike, exophytic mass with keratin-filled sinus tracts and malodorous discharge. 4 Diabetic foot ulcers and chronic inflammatory conditions are predisposing risk factors for EC, and it can result in difficulty walking/immobility, pain, and bleeding depending on anatomic involvement. 5-9
The differential diagnosis for VC includes refractory verruca vulgaris, clavus, SCC, keratoacanthoma, deep fungal or mycobacterial infection, eccrine poroma or porocarcinoma, amelanotic melanoma, and sarcoma.10-13 The slow-growing nature of VC, sampling error of superficial biopsies, and minimal cytological atypia on histologic examination can contribute to delayed diagnosis and appropriate treatment.14 Characteristic histologic features include hyperkeratosis, papillomatosis, marked acanthosis, broad blunt-ended rete ridges with a “bulldozing” architecture, and minimal cytologic atypia and mitoses.5,6 In some cases, pleomorphism and glassy eosinophilic cytoplasmic changes may be more pronounced than that of a common wart though less dramatic than that of conventional SCCs.15 Antigen Ki-67 and tumor protein p53 have been proposed to help differentiate between common plantar verruca, VC, and SCC, but the histologic diagnosis remains challenging, and repeat histopathologic examination often is required.16-19 Following diagnosis, computed tomography or magnetic resonance imaging may be necessary to determine tumor extension and assess for deep tissue and bony involvement.20-22
Treatment of EC is particularly challenging because of the anatomic location and need for margin control while maintaining adequate function, preserving healthy tissue, and providing coverage of defects. Surgical excision of EC is the first-line treatment, most commonly by wide local excision (WLE) or amputation. Mohs micrographic surgery (MMS) also has been utilized. One review found no recurrences in 5 cases of EC treated with MMS.23 As MMS is a tissue-sparing technique, this is a valuable modality for sites of functional importance such as the feet. Herein, we review various reported EC treatment modalities and outcomes, with an emphasis on recurrence rates for WLE and MMS.
METHODS
A systematic literature review of PubMed articles indexed for MEDLINE, as well as databases including the Cochrane Library, Web of Science, and Cumulative Index to Nursing and Allied Health Literature (CINAHL), was performed on January 14, 2020. Two authors (S.S.D. and S.V.C.) independently screened results using the search terms (plantar OR foot) AND (verrucous carcinoma OR epithelioma cuniculatum OR carcinoma cuniculatum). The search terms were chosen according to MeSH subject headings. All articles from the start date of the databases through the search date were screened, and articles pertaining to VC, EC, or carcinoma cuniculatum located on the foot were included. Of these, non–English-language articles were translated and included. Articles reporting VC on a site other than the foot (eg, the oral cavity) or benign verrucous skin lesions were excluded. The reference lists for all articles also were reviewed for additional reports that were absent from the initial search using both included and excluded articles. A full-text review was performed on 221 articles published between 1954 and 2019 per the PRISMA guidelines (Figure).

A total of 101 articles were included in the study for qualitative analysis. Nearly all articles identified were case reports, giving an evidence level of 5 by the Centre for Evidence-Based Medicine rating scale. Five articles reported data on multiple patients without individual demographic or clinical details and were excluded from analysis. Of the remaining 96 articles, information about patient characteristics, tumor size, treatment modality, and recurrence were extracted for 115 cases.
RESULTS
Of the 115 cases that were reviewed, 81 (70%) were male and 33 (29%) were female with a male-to-female ratio of 2.4:1. Ages of the patients ranged from 18 to 88 years; the mean and median age was 56 years. Nearly all reported cases of EC affected the plantar surface of one foot, with 4 reports of tumors affecting both feet.24-27 One case affecting both feet reported known exposure to lead arsenate pesticides27; all others were associated with a clinical history of chronic ulcers or warts persisting for several years to decades. Other less common sites of EC included the dorsal foot, interdigital web space, and subungual digit.28-30 The most common location reported was the anterior ball of the foot. Tumors were reported to arise within pre-existing lesions, such as hypertrophic lichen planus or chronic foot wounds associated with diabetes mellitus or leprosy.31-35 Tumor size ranged from 1 to 22 cm with a median of 4.5 cm.
Eight cases were reported to be associated with human papillomavirus; low-risk types 6 and 11 and high-risk types 16 and 18 were found in 6 cases.36-41 Two cases reported association with human papillomavirus type 2.7,42
Metastases to dermal and subdermal lymphatics, regional lymph nodes, and the lungs were reported in 3 cases, repectively.43-45 Of these, one primary tumor had received low-dose irradiation in the form of X-ray therapy.45
Treatment Modalities
The cases of EC that we reviewed included treatment with surgical and systemic therapies as well as other modalities such as acitretin, interferon alfa, topical imiquimod, curettage, debridement, electrodesiccation, and radiation. The Table includes a complete summary of the treatments we analyzed.

Surgical Therapy—The majority (91% [105/115]) of cases were treated surgically. The most common treatment modality was WLE (50% [58/115]), followed by amputation (37% [43/115]) and MMS (12% [14/115]).
Wide local excision was the most frequently reported treatment, with excision margins of at least 5 mm to 1 cm.48 Incidence of recurrence was reported for 57% (33/58) of cases treated with WLE; of these, the recurrence rate was 33% (11/33). For patients with EC recurrence, the most common secondary treatment was repeat excision with wider margins (1–2 cm) or amputation (5/11).49-52 Few postoperative complications were reported but included pain, infection, and difficulty walking, which were mostly associated with repair modality (eg, split-thickness skin grafts, rotational flaps).53 Amputation was the second most common treatment modality, with a 67% (29/43) incidence of recurrence. Types of amputation included transmetatarsal ray amputation (7/43 [16%]), foot or forefoot amputation (2/43 [5%]), above-the-knee amputation (1/43 [2%]), and below-the-knee amputation (1/43 [2%]). Complications associated with amputation included infection and requirement of prosthetics for ambulation. Split-thickness skin grafts and rotational flaps were the most common surgical repairs performed.52,53
Mohs micrographic surgery was the least frequently reported surgical treatment modality. Both traditional MMS on fresh tissue and “slow Mohs,” with formalin-fixed paraffin embedded tissue examination over several days, were performed for EC with horizontal en face sectioning.54-56 Incidence of recurrence was reported for 86% (12/14) of MMS cases. Of these, recurrence was seen in 17% (2/12) that utilized a flat horizontal processing of tissue sections coupled with saucerlike excisions to enable examination of the entire undersurface and margins. In one case, the patient was treated with MMS with recurrence noted 1 month later; thus, repeat MMS was performed, and the tumor was found to be entwined around the flexor tendon.57 The tendon was removed, and clear margins were obtained. Follow-up 3 years after the second MMS revealed no signs of recurrence.57 In the other case, the patient had a particularly aggressive course with bilateral VC in the setting of diabetic ulcers that was treated with WLE prior to MMS and recurrence still noted after MMS.26 No complications were reported with MMS.
Overall, recurrence was most frequently reported with WLE (11/33 [33%]), followed by MMS (2/12 [17%]) and amputation (3/29 [10%]). When comparing WLE and amputation, the relationship between treatment modality and recurrence was statistically significant using a χ2 test of independence (χ2=4.7; P=.03). However, results were not significant with Yates correction for continuity (χ2=3.4; P=.06). The χ2 test of independence showed no significant association between treatment method and recurrence when comparing WLE with MMS (χ2=1.2; P=.28). Reported follow-up times varied greatly from a few months to 10 years.
Systemic Therapy—Of the total cases, only 2 cases reported treatment with acitretin and 2 utilized interferon alfa.58,59 In one case, treatment of EC with interferon alfa alone required more aggressive therapy (ie, amputation).58 Neither of the 2 cases using acitretin reported recurrence.59,60 Complications of acitretin therapy included cheilitis and transaminitis.60
Other Treatment Modalities—Three cases utilized imiquimod, with 2 cases of imiquimod monotherapy and 1 case of imiquimod in combination with electrodesiccation and WLE.37 One of the cases of EC treated with imiquimod monotherapy recurred and required WLE.61
There were reports of other treatments including curettage alone (2% [2/115]),40,62 debridement alone (1% [1/115]),40 electrodesiccation (1% [1/115]),37 and radiation (1% [1/115]).43 Recurrence was found with curettage alone and debridement alone. Electrodesiccation was reported in conjunction with WLE without recurrence. Radiation was used to treat a case of VC that had metastasized to the lymph nodes; no follow-up was described.43
COMMENT
Epithelioma cuniculatum is an indolent malignancy of the plantar foot that likely is frequently underdiagnosed or misdiagnosed because of location, sampling error, and challenges in histopathologic diagnosis. Once diagnosed, surgical removal with margin control is the first-line therapy for EC. Our review found a number of surgical, systemic, and other treatment modalities that have been used to treat EC, but there remains a lack of evidence to provide clear guidelines as to which therapies are most effective. Current data on the treatment of EC largely are limited to case reports and case series. To date, there are no reports of higher-quality studies or randomized controlled trials to assess the efficacy of various treatment modalities.
Our review found that WLE is the most common treatment modality for EC, followed by amputation and MMS. Three cases43-45 that reported metastasis to lymph nodes also were treated with fine-needle aspiration or biopsy, and it is recommended that sentinel lymph node biopsy be performed when there is a history of radiation exposure or clinically and sonographically unsuspicious lymph nodes, while dissection of regional nodes should be performed if lymph node metastasis is suspected.53 Additional treatments reported included acitretin, interferon alfa, topical imiquimod, curettage, debridement, and electrodesiccation, but because of the limited number of cases and variable efficacy, no conclusions can be made on the utility of these alternative modalities.
The lowest rate of reported recurrence was found with amputation, followed by MMS and WLE. Amputation is the most aggressive treatment option, but its superiority in lower recurrence rates was not statistically significant when compared with either WLE or MMS after Yates correction. Despite treatment with radical surgery, recurrence is still possible and may be associated with factors including greater size (>2 cm) and depth (>4 mm), poor histologic differentiation, perineural involvement, failure of previous treatments, and immunosuppression.63 No statistically significant difference in recurrence rates was found among surgical methods, though data trended toward lower rates of recurrence with MMS compared with WLE, as recurrence with MMS was only reported in 2 cases.25,56
The efficacy of MMS is well documented for tumors with contiguous growth and enables maximum preservation of normal tissue structure and function with complete margin visualization. Thus, our results are in agreement with those of prior studies,54-56,64 suggesting that MMS is associated with lower recurrence rates for EC than WLE. Future studies and reporting of MMS for EC are particularly important because of the functional importance of the plantar foot.
It is important to note that there are local and systemic risk factors that increase the likelihood of developing EC and facilitate tumor growth, including antecedent trauma to the lesion site, chronic irritation or infection, and immunosuppression (HIV related or iatrogenic medication induced). These risk factors may play a role in the treatment modality utilized (eg, more aggressive EC may be treated with amputation instead of WLE). Underlying patient comorbidities could potentially affect recurrence rates, which is a variable we could not control for in our analysis.
Our findings are limited by study design, with supporting evidence consisting of case reports and series. The review is limited by interstudy variability and heterogeneity of results. Additionally, recurrence is not reported in all cases and may be a source of sampling bias. Further complicating the generalizability of these results is the lack of follow-up to evaluate morbidity and quality of life after treatment.
CONCLUSION
This review suggests that MMS is associated with lower recurrence rates than WLE for the treatment of EC. Further investigation of MMS for EC with appropriate follow-up is necessary to identify whether MMS is associated with lower recurrence and less functional impairment. Nonsurgical treatments, including topical imiquimod, interferon alfa, and acitretin, may be useful in cases where surgical therapies are contraindicated, but there is little evidence to support these treatment modalities. Treatment guidelines for EC are not established, and appropriate treatment guidelines should be developed in the future.
Verrucous carcinoma (VC) is an uncommon type of well-differentiated squamous cell carcinoma (SCC) that most commonly affects men in the fifth to sixth decades of life. 1 The tumor grows slowly over a decade or more and does not frequently metastasize but has a high propensity for recurrence and local invasion. 2 There are 3 main subtypes of VC classified by anatomic site: oral florid papillomatosis (oral cavity), Buschke-Lowenstein tumor (anogenital region), and epithelioma cuniculatum (EC)(feet). 3 Epithelioma cuniculatum, also known as carcinoma cuniculatum or papillomatosis cutis carcinoides, most commonly presents as a solitary, warty or cauliflowerlike, exophytic mass with keratin-filled sinus tracts and malodorous discharge. 4 Diabetic foot ulcers and chronic inflammatory conditions are predisposing risk factors for EC, and it can result in difficulty walking/immobility, pain, and bleeding depending on anatomic involvement. 5-9
The differential diagnosis for VC includes refractory verruca vulgaris, clavus, SCC, keratoacanthoma, deep fungal or mycobacterial infection, eccrine poroma or porocarcinoma, amelanotic melanoma, and sarcoma.10-13 The slow-growing nature of VC, sampling error of superficial biopsies, and minimal cytological atypia on histologic examination can contribute to delayed diagnosis and appropriate treatment.14 Characteristic histologic features include hyperkeratosis, papillomatosis, marked acanthosis, broad blunt-ended rete ridges with a “bulldozing” architecture, and minimal cytologic atypia and mitoses.5,6 In some cases, pleomorphism and glassy eosinophilic cytoplasmic changes may be more pronounced than that of a common wart though less dramatic than that of conventional SCCs.15 Antigen Ki-67 and tumor protein p53 have been proposed to help differentiate between common plantar verruca, VC, and SCC, but the histologic diagnosis remains challenging, and repeat histopathologic examination often is required.16-19 Following diagnosis, computed tomography or magnetic resonance imaging may be necessary to determine tumor extension and assess for deep tissue and bony involvement.20-22
Treatment of EC is particularly challenging because of the anatomic location and need for margin control while maintaining adequate function, preserving healthy tissue, and providing coverage of defects. Surgical excision of EC is the first-line treatment, most commonly by wide local excision (WLE) or amputation. Mohs micrographic surgery (MMS) also has been utilized. One review found no recurrences in 5 cases of EC treated with MMS.23 As MMS is a tissue-sparing technique, this is a valuable modality for sites of functional importance such as the feet. Herein, we review various reported EC treatment modalities and outcomes, with an emphasis on recurrence rates for WLE and MMS.
METHODS
A systematic literature review of PubMed articles indexed for MEDLINE, as well as databases including the Cochrane Library, Web of Science, and Cumulative Index to Nursing and Allied Health Literature (CINAHL), was performed on January 14, 2020. Two authors (S.S.D. and S.V.C.) independently screened results using the search terms (plantar OR foot) AND (verrucous carcinoma OR epithelioma cuniculatum OR carcinoma cuniculatum). The search terms were chosen according to MeSH subject headings. All articles from the start date of the databases through the search date were screened, and articles pertaining to VC, EC, or carcinoma cuniculatum located on the foot were included. Of these, non–English-language articles were translated and included. Articles reporting VC on a site other than the foot (eg, the oral cavity) or benign verrucous skin lesions were excluded. The reference lists for all articles also were reviewed for additional reports that were absent from the initial search using both included and excluded articles. A full-text review was performed on 221 articles published between 1954 and 2019 per the PRISMA guidelines (Figure).

A total of 101 articles were included in the study for qualitative analysis. Nearly all articles identified were case reports, giving an evidence level of 5 by the Centre for Evidence-Based Medicine rating scale. Five articles reported data on multiple patients without individual demographic or clinical details and were excluded from analysis. Of the remaining 96 articles, information about patient characteristics, tumor size, treatment modality, and recurrence were extracted for 115 cases.
RESULTS
Of the 115 cases that were reviewed, 81 (70%) were male and 33 (29%) were female with a male-to-female ratio of 2.4:1. Ages of the patients ranged from 18 to 88 years; the mean and median age was 56 years. Nearly all reported cases of EC affected the plantar surface of one foot, with 4 reports of tumors affecting both feet.24-27 One case affecting both feet reported known exposure to lead arsenate pesticides27; all others were associated with a clinical history of chronic ulcers or warts persisting for several years to decades. Other less common sites of EC included the dorsal foot, interdigital web space, and subungual digit.28-30 The most common location reported was the anterior ball of the foot. Tumors were reported to arise within pre-existing lesions, such as hypertrophic lichen planus or chronic foot wounds associated with diabetes mellitus or leprosy.31-35 Tumor size ranged from 1 to 22 cm with a median of 4.5 cm.
Eight cases were reported to be associated with human papillomavirus; low-risk types 6 and 11 and high-risk types 16 and 18 were found in 6 cases.36-41 Two cases reported association with human papillomavirus type 2.7,42
Metastases to dermal and subdermal lymphatics, regional lymph nodes, and the lungs were reported in 3 cases, repectively.43-45 Of these, one primary tumor had received low-dose irradiation in the form of X-ray therapy.45
Treatment Modalities
The cases of EC that we reviewed included treatment with surgical and systemic therapies as well as other modalities such as acitretin, interferon alfa, topical imiquimod, curettage, debridement, electrodesiccation, and radiation. The Table includes a complete summary of the treatments we analyzed.

Surgical Therapy—The majority (91% [105/115]) of cases were treated surgically. The most common treatment modality was WLE (50% [58/115]), followed by amputation (37% [43/115]) and MMS (12% [14/115]).
Wide local excision was the most frequently reported treatment, with excision margins of at least 5 mm to 1 cm.48 Incidence of recurrence was reported for 57% (33/58) of cases treated with WLE; of these, the recurrence rate was 33% (11/33). For patients with EC recurrence, the most common secondary treatment was repeat excision with wider margins (1–2 cm) or amputation (5/11).49-52 Few postoperative complications were reported but included pain, infection, and difficulty walking, which were mostly associated with repair modality (eg, split-thickness skin grafts, rotational flaps).53 Amputation was the second most common treatment modality, with a 67% (29/43) incidence of recurrence. Types of amputation included transmetatarsal ray amputation (7/43 [16%]), foot or forefoot amputation (2/43 [5%]), above-the-knee amputation (1/43 [2%]), and below-the-knee amputation (1/43 [2%]). Complications associated with amputation included infection and requirement of prosthetics for ambulation. Split-thickness skin grafts and rotational flaps were the most common surgical repairs performed.52,53
Mohs micrographic surgery was the least frequently reported surgical treatment modality. Both traditional MMS on fresh tissue and “slow Mohs,” with formalin-fixed paraffin embedded tissue examination over several days, were performed for EC with horizontal en face sectioning.54-56 Incidence of recurrence was reported for 86% (12/14) of MMS cases. Of these, recurrence was seen in 17% (2/12) that utilized a flat horizontal processing of tissue sections coupled with saucerlike excisions to enable examination of the entire undersurface and margins. In one case, the patient was treated with MMS with recurrence noted 1 month later; thus, repeat MMS was performed, and the tumor was found to be entwined around the flexor tendon.57 The tendon was removed, and clear margins were obtained. Follow-up 3 years after the second MMS revealed no signs of recurrence.57 In the other case, the patient had a particularly aggressive course with bilateral VC in the setting of diabetic ulcers that was treated with WLE prior to MMS and recurrence still noted after MMS.26 No complications were reported with MMS.
Overall, recurrence was most frequently reported with WLE (11/33 [33%]), followed by MMS (2/12 [17%]) and amputation (3/29 [10%]). When comparing WLE and amputation, the relationship between treatment modality and recurrence was statistically significant using a χ2 test of independence (χ2=4.7; P=.03). However, results were not significant with Yates correction for continuity (χ2=3.4; P=.06). The χ2 test of independence showed no significant association between treatment method and recurrence when comparing WLE with MMS (χ2=1.2; P=.28). Reported follow-up times varied greatly from a few months to 10 years.
Systemic Therapy—Of the total cases, only 2 cases reported treatment with acitretin and 2 utilized interferon alfa.58,59 In one case, treatment of EC with interferon alfa alone required more aggressive therapy (ie, amputation).58 Neither of the 2 cases using acitretin reported recurrence.59,60 Complications of acitretin therapy included cheilitis and transaminitis.60
Other Treatment Modalities—Three cases utilized imiquimod, with 2 cases of imiquimod monotherapy and 1 case of imiquimod in combination with electrodesiccation and WLE.37 One of the cases of EC treated with imiquimod monotherapy recurred and required WLE.61
There were reports of other treatments including curettage alone (2% [2/115]),40,62 debridement alone (1% [1/115]),40 electrodesiccation (1% [1/115]),37 and radiation (1% [1/115]).43 Recurrence was found with curettage alone and debridement alone. Electrodesiccation was reported in conjunction with WLE without recurrence. Radiation was used to treat a case of VC that had metastasized to the lymph nodes; no follow-up was described.43
COMMENT
Epithelioma cuniculatum is an indolent malignancy of the plantar foot that likely is frequently underdiagnosed or misdiagnosed because of location, sampling error, and challenges in histopathologic diagnosis. Once diagnosed, surgical removal with margin control is the first-line therapy for EC. Our review found a number of surgical, systemic, and other treatment modalities that have been used to treat EC, but there remains a lack of evidence to provide clear guidelines as to which therapies are most effective. Current data on the treatment of EC largely are limited to case reports and case series. To date, there are no reports of higher-quality studies or randomized controlled trials to assess the efficacy of various treatment modalities.
Our review found that WLE is the most common treatment modality for EC, followed by amputation and MMS. Three cases43-45 that reported metastasis to lymph nodes also were treated with fine-needle aspiration or biopsy, and it is recommended that sentinel lymph node biopsy be performed when there is a history of radiation exposure or clinically and sonographically unsuspicious lymph nodes, while dissection of regional nodes should be performed if lymph node metastasis is suspected.53 Additional treatments reported included acitretin, interferon alfa, topical imiquimod, curettage, debridement, and electrodesiccation, but because of the limited number of cases and variable efficacy, no conclusions can be made on the utility of these alternative modalities.
The lowest rate of reported recurrence was found with amputation, followed by MMS and WLE. Amputation is the most aggressive treatment option, but its superiority in lower recurrence rates was not statistically significant when compared with either WLE or MMS after Yates correction. Despite treatment with radical surgery, recurrence is still possible and may be associated with factors including greater size (>2 cm) and depth (>4 mm), poor histologic differentiation, perineural involvement, failure of previous treatments, and immunosuppression.63 No statistically significant difference in recurrence rates was found among surgical methods, though data trended toward lower rates of recurrence with MMS compared with WLE, as recurrence with MMS was only reported in 2 cases.25,56
The efficacy of MMS is well documented for tumors with contiguous growth and enables maximum preservation of normal tissue structure and function with complete margin visualization. Thus, our results are in agreement with those of prior studies,54-56,64 suggesting that MMS is associated with lower recurrence rates for EC than WLE. Future studies and reporting of MMS for EC are particularly important because of the functional importance of the plantar foot.
It is important to note that there are local and systemic risk factors that increase the likelihood of developing EC and facilitate tumor growth, including antecedent trauma to the lesion site, chronic irritation or infection, and immunosuppression (HIV related or iatrogenic medication induced). These risk factors may play a role in the treatment modality utilized (eg, more aggressive EC may be treated with amputation instead of WLE). Underlying patient comorbidities could potentially affect recurrence rates, which is a variable we could not control for in our analysis.
Our findings are limited by study design, with supporting evidence consisting of case reports and series. The review is limited by interstudy variability and heterogeneity of results. Additionally, recurrence is not reported in all cases and may be a source of sampling bias. Further complicating the generalizability of these results is the lack of follow-up to evaluate morbidity and quality of life after treatment.
CONCLUSION
This review suggests that MMS is associated with lower recurrence rates than WLE for the treatment of EC. Further investigation of MMS for EC with appropriate follow-up is necessary to identify whether MMS is associated with lower recurrence and less functional impairment. Nonsurgical treatments, including topical imiquimod, interferon alfa, and acitretin, may be useful in cases where surgical therapies are contraindicated, but there is little evidence to support these treatment modalities. Treatment guidelines for EC are not established, and appropriate treatment guidelines should be developed in the future.
- McKee PH, Wilkinson JD, Black MM, et al. Carcinoma (epithelioma) cuniculatum: a clinicopathological study of nineteen cases and review of the literature. Histopathology. 1981;5:425-436.
- Aird I, Johnson HD, Lennox B, et al. Epithelioma cuniculatum: a variety of squamous carcinoma peculiar to the foot. Br J Surg. 1954;42:245-250.
- Seremet S, Erdemir AT, Kiremitci U, et al. Unusually early-onset plantar verrucous carcinoma. Cutis. 2019;104:34-36.
- Spyriounis PK, Tentis D, Sparveri IF, et al. Plantar epithelioma cuniculatum. a case report with review of the literature. Eur J Plast Surg. 2004;27:253-256.
- Ho J, Diven G, Bu J, et al. An ulcerating verrucous plaque on the foot. verrucous carcinoma (epithelioma cuniculatum). Arch Dermatol. 2000;136:547-548, 550-551.
- Kao GF, Graham JH, Helwig EB. Carcinoma cuniculatum (verrucous carcinoma of the skin): a clinicopathologic study of 46 cases with ultrastructural observations. Cancer. 1982;49:2395-2403.
- Zielonka E, Goldschmidt D, de Fontaine S. Verrucous carcinoma or epithelioma cuniculatum plantare. Eur J Surg Oncol. 1997;23:86-87.
- Dogan G, Oram Y, Hazneci E, et al. Three cases of verrucous carcinoma. Australas J Dermatol. 1998;39:251-254.
- Schwartz RA, Burgess GH. Verrucous carcinoma of the foot. J Surg Oncol. 1980;14:333-339.
- McKay C, McBride P, Muir J. Plantar verrucous carcinoma masquerading as toe web intertrigo. Australas J Dermatol. 2012;53:2010-2012.
- Shenoy AS, Waghmare RS, Kavishwar VS, et al. Carcinoma cuniculatum of foot. Foot. 2011;21:207-208.
- Lozzi G, Perris K. Carcinoma cuniculatum. CMAJ. 2007;177:249-251.
- Schein O, Orenstein A, Bar-Meir E. Plantar verrucous carcicoma (epithelioma cuniculatum): rare form of the common wart. Isr Med Assoc J. 2006;8:885.
- Rheingold LM, Roth LM. Carcinoma of the skin of the foot exhibiting some verrucous features. Plast Reconstr Surg. 1978;61:605-609.
- Klima M, Kurtis B, Jordan PH. Verrucous carcinoma of skin. J Cutan Pathol. 1980;7:88-98.
- Nakamura Y, Kashiwagi K, Nakamura A, et al. Verrucous carcinoma of the foot diagnosed using p53 and Ki-67 immunostaining in a patient with diabetic neuropathy. Am J Dermatopathol. 2015;37:257-259.
- Costache M, Desa LT, Mitrache LE, et al. Cutaneous verrucous carcinoma—report of three cases with review of literature. Rom J Morphol Embryol. 2014;55:383-388.
- Terada T. Verrucous carcinoma of the skin: a report on 5 Japanese cases. Ann Diagn Pathol. 2011;15:175-180.
- Noel JC, Heenen M, Peny MO, et al. Proliferating cell nuclear antigen distribution in verrucous carcinoma of the skin. Br J Dermatol. 1995;133:868-873.
- García-Gavín J, González-Vilas D, Rodríguez-Pazos L, et al. Verrucous carcinoma of the foot affecting the bone: utility of the computed tomography scanner. Dermatol Online J. 2010;16:3-5.
- Wasserman PL, Taylor RC, Pinillia J, et al. Verrucous carcinoma of the foot and enhancement assessment by MRI. Skeletal Radiol. 2009;38:393-395.
- Bhushan MH, Ferguson JE, Hutchinson CE. Carcinoma cuniculatum of the foot assessed by magnetic resonance scanning. Clin Exp Dermatol. 2001;26:419-422.
- Penera KE, Manji KA, Craig AB, et al. Atypical presentation of verrucous carcinoma: a case study and review of the literature. Foot Ankle Spec. 2013;6:318-322.
- Suen K, Wijeratne S, Patrikios J. An unusual case of bilateral verrucous carcinoma of the foot (epithelioma cuniculatum). J Surg Case Rep. 2012;2012:rjs020.
- Riccio C, King K, Elston JB, et al. Bilateral plantar verrucous carcinoma. Eplasty. 2016;16:ic46.
- Di Palma V, Stone JP, Schell A, et al. Mistaken diabetic ulcers: a case of bilateral foot verrucous carcinoma. Case Rep Dermatol Med. 2018;2018:4192657.
- Seehafer JR, Muller SA, Dicken CH. Bilateral verrucous carcinoma of the feet. Orthop Surv. 1979;3:205.
- Tosti A, Morelli R, Fanti PA, et al. Carcinoma cuniculatum of the nail apparatus: report of three cases. Dermatology. 1993;186:217-221.
- Melo CR, Melo IS, Souza LP. Epithelioma cuniculatum, a verrucous carcinoma of the foot. report of 2 cases. Dermatologica. 1981;163:338-342.
- Van Geertruyden JP, Olemans C, Laporte M, et al. Verrucous carcinoma of the nail bed. Foot Ankle Int. 1998;19:327-328.
- Thakur BK, Verma S, Raphael V. Verrucous carcinoma developing in a long standing case of ulcerative lichen planus of sole: a rare case report. J Eur Acad Dermatol Venereol. 2015;29:399-401.
- Mayron R, Grimwood RE, Siegle RJ, et al. Verrucous carcinoma arising in ulcerative lichen planus of the soles. J Dermatol Surg Oncol. 1988;14:547-551.
- Boussofara L, Belajouza-Noueiri C, Ghariani N, et al. Verrucous epidermoid carcinoma as a complication in cutaneous lichen planus [article in French]. Ann Dermatol Venereol. 2006;133:404-405.
- Khullar G, Mittal S, Sharma S. Verrucous carcinoma on the foot arising in a chronic neuropathic ulcer of leprosy. Australas J Dermatol. 2019;60:245-246.
- Ochsner PE, Hausman R, Olsthoorn PGM. Epithelioma cunicalutum developing in a neuropathic ulcer of leprous etiology. Arch Orthop Trauma Surg. 1979;94:227-231.
- Ray R, Bhagat A, Vasudevan B, et al. A rare case of plantar epithelioma cuniculatum arising from a wart. Indian J Dermatol. 2015;60:485-487.
- Imko-Walczuk B, Cegielska A, Placek W, et al. Human papillomavirus-related verrucous carcinoma in a renal transplant patient after long-term immunosuppression: a case report. Transplant Proc. 2014;46:2916-2919.
- Floristán MU, Feltes RA, Sáenz JC, et al. Verrucous carcinoma of the foot associated with human papillomavirus type 18. Actas Dermosifiliogr. 2009;100:433-435.
- Sasaoka R, Morimura T, Mihara M, et al. Detection of human pupillomavirus type 16 DNA in two cases of verriicous carcinoma of the foot. Br J Dermatol. 1996;134:983984.
- Schell BJ, Rosen T, Rády P, et al. Verrucous carcinoma of the foot associated with human papillomavirus type 16. J Am Acad Dermatol. 2001;45:49-55.
- Knobler RM, Schneider S, Neumann RA, et al. DNA dot‐blot hybridization implicates human papillomavirus type 11‐DNA in epithelioma cuniculatum. J Med Virol. 1989;29:33-37.
- Noel JC, Peny MO, Detremmerie O, et al. Demonstration of human papillomavirus type 2 in a verrucous carcinoma of the foot. Dermatology. 1993;187:58-61.
- Jungmann J, Vogt T, Müller CSL. Giant verrucous carcinoma of the lower extremity in women with dementia. BMJ Case Rep. 2012;2012:bcr2012006357.
- McKee PH, Wilkinson JD, Corbett MF, et al. Carcinoma cuniculatum: a case metastasizing to skin and lymph nodes. Clin Exp Dermatol. 1981;6:613-618.
- Owen WR, Wolfe ID, Burnett JW, et al. Epithelioma cuniculatum. South Med J. 1978;71:477-479.
- Patel AN, Bedforth N, Varma S. Pain-free treatment of carcinoma cuniculatum on the heel using Mohs micrographic surgery and ultrasonography-guided sciatic nerve block. Clin Exp Dermatol. 2013;38:569-571.
- Padilla RS, Bailin PL, Howard WR, et al. Verrucous carcinoma of the skin and its management by Mohs’ surgery. Plast Reconstr Surg. 1984;73:442-447.
- Kotwal M, Poflee S, Bobhate S. Carcinoma cuniculatum at various anatomical sites. Indian J Dermatol. 2005;50:216-220.
- Arefi M, Philipone E, Caprioli R, et al. A case of verrucous carcinoma (epithelioma cuniculatum) of the heel mimicking infected epidermal cyst and gout. Foot Ankle Spec. 2008;1:297-299.
- Trebing D, Brunner M, Kröning Y, et al. Young man with verrucous heel tumor [article in German]. J Dtsch Dermatol Ges. 2003;9:739-741.
- Thompson SG. Epithelioma cuniculatum: an unusual tumour of the foot. Br J Plast Surg. 1965;18:214-217.
- Thomas EJ, Graves NC, Meritt SM. Carcinoma cuniculatum: an atypical presentation in the foot. J Foot Ankle Surg. 2014;53:356-359.
- Koch H, Kowatsch E, Hödl S, et al. Verrucous carcinoma of the skin: long-term follow-up results following surgical therapy. Dermatol Surg. 2004;30:1124-1130.
- Mallatt BD, Ceilley RI, Dryer RF. Management of verrucous carcinoma on a foot by a combination of chemosurgery and plastic repair: report of a case. J Dermatol Surg Oncol. 1980;6:532-534.
- Mohs FE, Sahl WJ. Chemosurgery for verrucous carcinoma. J Dermatol Surg Oncol. 1979;5:302-306.
- Alkalay R, Alcalay J, Shiri J. Plantar verrucous carcinoma treated with Mohs micrographic surgery: a case report and literature review. J Drugs Dermatol. 2006;5:68-73.
- Mora RG. Microscopically controlled surgery (Mohs’ chemosurgery) for treatment of verrucous squamous cell carcinoma of the foot (epithelioma cuniculatum). J Am Acad Dermatol. 1983;8:354-362.
- Risse L, Negrier P, Dang PM, et al. Treatment of verrucous carcinoma with recombinant alfa-interferon. Dermatology. 1995;190:142-144.
- Rogozin´ski TT, Schwartz RA, Towpik E. Verrucous carcinoma in Unna-Thost hyperkeratosis of the palms and soles. J Am Acad Dermatol. 1994;31:1061-1062.
- Kuan YZ, Hsu HC, Kuo TT, et al. Multiple verrucous carcinomas treated with acitretin. J Am Acad Dermatol. 2007;56(2 suppl):S29-S32.
- Schalock PC, Kornik RI, Baughman RD, et al. Treatment of verrucous carcinoma with topical imiquimod. J Am Acad Dermatol. 2006;54:233-234.
- Brown SM, Freeman RG. Epithelioma cuniculatum. Arch Dermatol. 1976;112:1295-1296.
- Rowe DE, Carroll RJ, Day CL, et al. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. J Am Acad Dermatol. 1992;26:976-990.
- Swanson NA, Taylor WB. Plantar verrucous carcinoma: literature review and treatment by the Mohs’ chemosurgery technique. Arch Dermatol. 1980;116:794-797.
- McKee PH, Wilkinson JD, Black MM, et al. Carcinoma (epithelioma) cuniculatum: a clinicopathological study of nineteen cases and review of the literature. Histopathology. 1981;5:425-436.
- Aird I, Johnson HD, Lennox B, et al. Epithelioma cuniculatum: a variety of squamous carcinoma peculiar to the foot. Br J Surg. 1954;42:245-250.
- Seremet S, Erdemir AT, Kiremitci U, et al. Unusually early-onset plantar verrucous carcinoma. Cutis. 2019;104:34-36.
- Spyriounis PK, Tentis D, Sparveri IF, et al. Plantar epithelioma cuniculatum. a case report with review of the literature. Eur J Plast Surg. 2004;27:253-256.
- Ho J, Diven G, Bu J, et al. An ulcerating verrucous plaque on the foot. verrucous carcinoma (epithelioma cuniculatum). Arch Dermatol. 2000;136:547-548, 550-551.
- Kao GF, Graham JH, Helwig EB. Carcinoma cuniculatum (verrucous carcinoma of the skin): a clinicopathologic study of 46 cases with ultrastructural observations. Cancer. 1982;49:2395-2403.
- Zielonka E, Goldschmidt D, de Fontaine S. Verrucous carcinoma or epithelioma cuniculatum plantare. Eur J Surg Oncol. 1997;23:86-87.
- Dogan G, Oram Y, Hazneci E, et al. Three cases of verrucous carcinoma. Australas J Dermatol. 1998;39:251-254.
- Schwartz RA, Burgess GH. Verrucous carcinoma of the foot. J Surg Oncol. 1980;14:333-339.
- McKay C, McBride P, Muir J. Plantar verrucous carcinoma masquerading as toe web intertrigo. Australas J Dermatol. 2012;53:2010-2012.
- Shenoy AS, Waghmare RS, Kavishwar VS, et al. Carcinoma cuniculatum of foot. Foot. 2011;21:207-208.
- Lozzi G, Perris K. Carcinoma cuniculatum. CMAJ. 2007;177:249-251.
- Schein O, Orenstein A, Bar-Meir E. Plantar verrucous carcicoma (epithelioma cuniculatum): rare form of the common wart. Isr Med Assoc J. 2006;8:885.
- Rheingold LM, Roth LM. Carcinoma of the skin of the foot exhibiting some verrucous features. Plast Reconstr Surg. 1978;61:605-609.
- Klima M, Kurtis B, Jordan PH. Verrucous carcinoma of skin. J Cutan Pathol. 1980;7:88-98.
- Nakamura Y, Kashiwagi K, Nakamura A, et al. Verrucous carcinoma of the foot diagnosed using p53 and Ki-67 immunostaining in a patient with diabetic neuropathy. Am J Dermatopathol. 2015;37:257-259.
- Costache M, Desa LT, Mitrache LE, et al. Cutaneous verrucous carcinoma—report of three cases with review of literature. Rom J Morphol Embryol. 2014;55:383-388.
- Terada T. Verrucous carcinoma of the skin: a report on 5 Japanese cases. Ann Diagn Pathol. 2011;15:175-180.
- Noel JC, Heenen M, Peny MO, et al. Proliferating cell nuclear antigen distribution in verrucous carcinoma of the skin. Br J Dermatol. 1995;133:868-873.
- García-Gavín J, González-Vilas D, Rodríguez-Pazos L, et al. Verrucous carcinoma of the foot affecting the bone: utility of the computed tomography scanner. Dermatol Online J. 2010;16:3-5.
- Wasserman PL, Taylor RC, Pinillia J, et al. Verrucous carcinoma of the foot and enhancement assessment by MRI. Skeletal Radiol. 2009;38:393-395.
- Bhushan MH, Ferguson JE, Hutchinson CE. Carcinoma cuniculatum of the foot assessed by magnetic resonance scanning. Clin Exp Dermatol. 2001;26:419-422.
- Penera KE, Manji KA, Craig AB, et al. Atypical presentation of verrucous carcinoma: a case study and review of the literature. Foot Ankle Spec. 2013;6:318-322.
- Suen K, Wijeratne S, Patrikios J. An unusual case of bilateral verrucous carcinoma of the foot (epithelioma cuniculatum). J Surg Case Rep. 2012;2012:rjs020.
- Riccio C, King K, Elston JB, et al. Bilateral plantar verrucous carcinoma. Eplasty. 2016;16:ic46.
- Di Palma V, Stone JP, Schell A, et al. Mistaken diabetic ulcers: a case of bilateral foot verrucous carcinoma. Case Rep Dermatol Med. 2018;2018:4192657.
- Seehafer JR, Muller SA, Dicken CH. Bilateral verrucous carcinoma of the feet. Orthop Surv. 1979;3:205.
- Tosti A, Morelli R, Fanti PA, et al. Carcinoma cuniculatum of the nail apparatus: report of three cases. Dermatology. 1993;186:217-221.
- Melo CR, Melo IS, Souza LP. Epithelioma cuniculatum, a verrucous carcinoma of the foot. report of 2 cases. Dermatologica. 1981;163:338-342.
- Van Geertruyden JP, Olemans C, Laporte M, et al. Verrucous carcinoma of the nail bed. Foot Ankle Int. 1998;19:327-328.
- Thakur BK, Verma S, Raphael V. Verrucous carcinoma developing in a long standing case of ulcerative lichen planus of sole: a rare case report. J Eur Acad Dermatol Venereol. 2015;29:399-401.
- Mayron R, Grimwood RE, Siegle RJ, et al. Verrucous carcinoma arising in ulcerative lichen planus of the soles. J Dermatol Surg Oncol. 1988;14:547-551.
- Boussofara L, Belajouza-Noueiri C, Ghariani N, et al. Verrucous epidermoid carcinoma as a complication in cutaneous lichen planus [article in French]. Ann Dermatol Venereol. 2006;133:404-405.
- Khullar G, Mittal S, Sharma S. Verrucous carcinoma on the foot arising in a chronic neuropathic ulcer of leprosy. Australas J Dermatol. 2019;60:245-246.
- Ochsner PE, Hausman R, Olsthoorn PGM. Epithelioma cunicalutum developing in a neuropathic ulcer of leprous etiology. Arch Orthop Trauma Surg. 1979;94:227-231.
- Ray R, Bhagat A, Vasudevan B, et al. A rare case of plantar epithelioma cuniculatum arising from a wart. Indian J Dermatol. 2015;60:485-487.
- Imko-Walczuk B, Cegielska A, Placek W, et al. Human papillomavirus-related verrucous carcinoma in a renal transplant patient after long-term immunosuppression: a case report. Transplant Proc. 2014;46:2916-2919.
- Floristán MU, Feltes RA, Sáenz JC, et al. Verrucous carcinoma of the foot associated with human papillomavirus type 18. Actas Dermosifiliogr. 2009;100:433-435.
- Sasaoka R, Morimura T, Mihara M, et al. Detection of human pupillomavirus type 16 DNA in two cases of verriicous carcinoma of the foot. Br J Dermatol. 1996;134:983984.
- Schell BJ, Rosen T, Rády P, et al. Verrucous carcinoma of the foot associated with human papillomavirus type 16. J Am Acad Dermatol. 2001;45:49-55.
- Knobler RM, Schneider S, Neumann RA, et al. DNA dot‐blot hybridization implicates human papillomavirus type 11‐DNA in epithelioma cuniculatum. J Med Virol. 1989;29:33-37.
- Noel JC, Peny MO, Detremmerie O, et al. Demonstration of human papillomavirus type 2 in a verrucous carcinoma of the foot. Dermatology. 1993;187:58-61.
- Jungmann J, Vogt T, Müller CSL. Giant verrucous carcinoma of the lower extremity in women with dementia. BMJ Case Rep. 2012;2012:bcr2012006357.
- McKee PH, Wilkinson JD, Corbett MF, et al. Carcinoma cuniculatum: a case metastasizing to skin and lymph nodes. Clin Exp Dermatol. 1981;6:613-618.
- Owen WR, Wolfe ID, Burnett JW, et al. Epithelioma cuniculatum. South Med J. 1978;71:477-479.
- Patel AN, Bedforth N, Varma S. Pain-free treatment of carcinoma cuniculatum on the heel using Mohs micrographic surgery and ultrasonography-guided sciatic nerve block. Clin Exp Dermatol. 2013;38:569-571.
- Padilla RS, Bailin PL, Howard WR, et al. Verrucous carcinoma of the skin and its management by Mohs’ surgery. Plast Reconstr Surg. 1984;73:442-447.
- Kotwal M, Poflee S, Bobhate S. Carcinoma cuniculatum at various anatomical sites. Indian J Dermatol. 2005;50:216-220.
- Arefi M, Philipone E, Caprioli R, et al. A case of verrucous carcinoma (epithelioma cuniculatum) of the heel mimicking infected epidermal cyst and gout. Foot Ankle Spec. 2008;1:297-299.
- Trebing D, Brunner M, Kröning Y, et al. Young man with verrucous heel tumor [article in German]. J Dtsch Dermatol Ges. 2003;9:739-741.
- Thompson SG. Epithelioma cuniculatum: an unusual tumour of the foot. Br J Plast Surg. 1965;18:214-217.
- Thomas EJ, Graves NC, Meritt SM. Carcinoma cuniculatum: an atypical presentation in the foot. J Foot Ankle Surg. 2014;53:356-359.
- Koch H, Kowatsch E, Hödl S, et al. Verrucous carcinoma of the skin: long-term follow-up results following surgical therapy. Dermatol Surg. 2004;30:1124-1130.
- Mallatt BD, Ceilley RI, Dryer RF. Management of verrucous carcinoma on a foot by a combination of chemosurgery and plastic repair: report of a case. J Dermatol Surg Oncol. 1980;6:532-534.
- Mohs FE, Sahl WJ. Chemosurgery for verrucous carcinoma. J Dermatol Surg Oncol. 1979;5:302-306.
- Alkalay R, Alcalay J, Shiri J. Plantar verrucous carcinoma treated with Mohs micrographic surgery: a case report and literature review. J Drugs Dermatol. 2006;5:68-73.
- Mora RG. Microscopically controlled surgery (Mohs’ chemosurgery) for treatment of verrucous squamous cell carcinoma of the foot (epithelioma cuniculatum). J Am Acad Dermatol. 1983;8:354-362.
- Risse L, Negrier P, Dang PM, et al. Treatment of verrucous carcinoma with recombinant alfa-interferon. Dermatology. 1995;190:142-144.
- Rogozin´ski TT, Schwartz RA, Towpik E. Verrucous carcinoma in Unna-Thost hyperkeratosis of the palms and soles. J Am Acad Dermatol. 1994;31:1061-1062.
- Kuan YZ, Hsu HC, Kuo TT, et al. Multiple verrucous carcinomas treated with acitretin. J Am Acad Dermatol. 2007;56(2 suppl):S29-S32.
- Schalock PC, Kornik RI, Baughman RD, et al. Treatment of verrucous carcinoma with topical imiquimod. J Am Acad Dermatol. 2006;54:233-234.
- Brown SM, Freeman RG. Epithelioma cuniculatum. Arch Dermatol. 1976;112:1295-1296.
- Rowe DE, Carroll RJ, Day CL, et al. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. J Am Acad Dermatol. 1992;26:976-990.
- Swanson NA, Taylor WB. Plantar verrucous carcinoma: literature review and treatment by the Mohs’ chemosurgery technique. Arch Dermatol. 1980;116:794-797.
Practice Points
- Because of its slow-growing nature and propensity for local invasion and recurrence, diagnosis of epithelioma cuniculatum (EC) often is delayed and therefore can be associated with notable morbidity.
- Wide local excision with 5-mm to 1-cm margins is considered standard of care and is the most commonly reported treatment of EC. Amputation may be required in cases with extensive local destruction.
- Mohs micrographic surgery is a viable option for treatment of EC, with more recent cases suggesting favorable outcomes regarding recurrence rates.
Can skin care aid use of diabetes devices?
Technologies that allow people to monitor blood sugar and automate the administration of insulin have radically transformed the lives of patients – and children in particular – with type 1 diabetes. But the devices often come with a cost: Insulin pumps and continuous glucose monitors can irritate the skin at the points of contact, causing some people to stop using their pumps or monitors altogether.
Regular use of lipid-rich skin creams can reduce eczema in children who use insulin pumps and continuous glucose monitors to manage type 1 diabetes, Danish researchers reported last month. The article is currently undergoing peer review at The Lancet Diabetes and Endocrinology, and the authors said they hope their approach will deter more children from abandoning diabetes technology.
“A simple thing can actually change a lot,” said Anna Korsgaard Berg, MD, a pediatrician who specializes in diabetes care at Copenhagen University Hospital’s Steno Diabetes Center in Herlev, Denmark, and a coauthor of the new study. “Not all skin reactions can be solved by the skin care program, but it can help improve the issue.”
More than 1.5 million children and adolescents worldwide live with type 1 diabetes, a condition that requires continuous insulin infusion. Insulin pumps meet this need in many wealthier countries, and are often used in combination with sensors that measure a child’s glucose level. Both the American Diabetes Association and the International Society for Adolescent and Pediatric Diabetes recommend insulin pumps and continuous glucose monitors as core treatment tools.
Dr. Berg and colleagues, who have previously shown that as many as 90% of children who use these devices experience some kind of skin reaction, want to minimize the rate of such discomfort in hopes that fewer children stop using the devices. According to a 2014 study, 18% of people with type 1 diabetes who stopped using continuous glucose monitors did so because of skin irritation.
Lather on that lipid-rich lotion
Dr. Berg and colleagues studied 170 children and adolescents with type 1 diabetes (average age, 11 years) who use insulin pumps, continuous glucose monitors, or both. From March 2020 to July 2021, 112 children (55 girls) employed a skin care program developed for the study, while the other 58 (34 girls) did not receive any skin care advice.
The skin care group received instructions about how to gently insert and remove their insulin pumps or glucose monitors, to minimize skin damage. They also were told to avoid disinfectants such as alcohol, which can irritate skin. The children in this group used a cream containing 70% lipids to help rehydrate their skin, applying the salve each day a device was not inserted into their skin.
Eczema can be a real problem for kids who use insulin pumps and continuous glucose monitors to manage type 1 diabetes. Researchers found that regular use of lipid-rich skin creams can reduce its incidence.
Although insulin pumps and glucose monitors are kept in place for longer periods of time than they once were, Dr. Berg and colleagues noted, users do periodically remove them when bathing or when undergoing medical tests that involve x-rays. On days when the devices were not in place for a period of time, children in the skin care group were encouraged to follow the protocol.
Study results
One-third of children in the skin care group developed eczema or experienced a wound, compared with almost half of the children in the control group, according to the researchers. The absolute difference in developing eczema or wounds between the two groups was 12.9 % (95% confidence interval, –28.7% to 2.9%).
Children in the skin care group were much less likely to develop wounds, the researchers found, when they focused only on wounds and not eczema (odds ratio, 0.29, 95% CI, 0.12-0.68).
Dr. Berg said she would like to explore whether other techniques, such as a combination of patches, adhesives, or other lotions, yield even better results.
“Anything that can help people use technology more consistently is better for both quality of life and diabetes outcomes,” said Priya Prahalad, MD, a specialist in pediatric endocrinology and diabetes at Stanford Medicine Children’s Health in Palo Alto and Sunnyvale, Calif.
Dr. Prahalad, who was not involved in the Danish study, said that although the sample sizes in the trial were relatively small, the data are “headed in the right direction.”
Pediatricians already recommend using moisturizing creams at the sites where pumps or glucose monitors are inserted into the skin, she noted. But the new study simply employed an especially moisturizing cream to mitigate skin damage.
Although one reason for skin irritation may be the repeated insertion and removal of devices, Dr. Berg and Dr. Prahalad stressed that the medical devices themselves may contain allergy-causing components. Device makers are not required to disclose what’s inside the boxes.
“I do not understand why the full content of a device is not by law mandatory to declare, when declaration by law is mandatory for many other products and drugs but not for medical devices,” Dr. Berg said.
Dr. Berg reports receiving lipid cream from Teva Pharmaceuticals and research support from Medtronic. Dr. Prahalad reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Technologies that allow people to monitor blood sugar and automate the administration of insulin have radically transformed the lives of patients – and children in particular – with type 1 diabetes. But the devices often come with a cost: Insulin pumps and continuous glucose monitors can irritate the skin at the points of contact, causing some people to stop using their pumps or monitors altogether.
Regular use of lipid-rich skin creams can reduce eczema in children who use insulin pumps and continuous glucose monitors to manage type 1 diabetes, Danish researchers reported last month. The article is currently undergoing peer review at The Lancet Diabetes and Endocrinology, and the authors said they hope their approach will deter more children from abandoning diabetes technology.
“A simple thing can actually change a lot,” said Anna Korsgaard Berg, MD, a pediatrician who specializes in diabetes care at Copenhagen University Hospital’s Steno Diabetes Center in Herlev, Denmark, and a coauthor of the new study. “Not all skin reactions can be solved by the skin care program, but it can help improve the issue.”
More than 1.5 million children and adolescents worldwide live with type 1 diabetes, a condition that requires continuous insulin infusion. Insulin pumps meet this need in many wealthier countries, and are often used in combination with sensors that measure a child’s glucose level. Both the American Diabetes Association and the International Society for Adolescent and Pediatric Diabetes recommend insulin pumps and continuous glucose monitors as core treatment tools.
Dr. Berg and colleagues, who have previously shown that as many as 90% of children who use these devices experience some kind of skin reaction, want to minimize the rate of such discomfort in hopes that fewer children stop using the devices. According to a 2014 study, 18% of people with type 1 diabetes who stopped using continuous glucose monitors did so because of skin irritation.
Lather on that lipid-rich lotion
Dr. Berg and colleagues studied 170 children and adolescents with type 1 diabetes (average age, 11 years) who use insulin pumps, continuous glucose monitors, or both. From March 2020 to July 2021, 112 children (55 girls) employed a skin care program developed for the study, while the other 58 (34 girls) did not receive any skin care advice.
The skin care group received instructions about how to gently insert and remove their insulin pumps or glucose monitors, to minimize skin damage. They also were told to avoid disinfectants such as alcohol, which can irritate skin. The children in this group used a cream containing 70% lipids to help rehydrate their skin, applying the salve each day a device was not inserted into their skin.
Eczema can be a real problem for kids who use insulin pumps and continuous glucose monitors to manage type 1 diabetes. Researchers found that regular use of lipid-rich skin creams can reduce its incidence.
Although insulin pumps and glucose monitors are kept in place for longer periods of time than they once were, Dr. Berg and colleagues noted, users do periodically remove them when bathing or when undergoing medical tests that involve x-rays. On days when the devices were not in place for a period of time, children in the skin care group were encouraged to follow the protocol.
Study results
One-third of children in the skin care group developed eczema or experienced a wound, compared with almost half of the children in the control group, according to the researchers. The absolute difference in developing eczema or wounds between the two groups was 12.9 % (95% confidence interval, –28.7% to 2.9%).
Children in the skin care group were much less likely to develop wounds, the researchers found, when they focused only on wounds and not eczema (odds ratio, 0.29, 95% CI, 0.12-0.68).
Dr. Berg said she would like to explore whether other techniques, such as a combination of patches, adhesives, or other lotions, yield even better results.
“Anything that can help people use technology more consistently is better for both quality of life and diabetes outcomes,” said Priya Prahalad, MD, a specialist in pediatric endocrinology and diabetes at Stanford Medicine Children’s Health in Palo Alto and Sunnyvale, Calif.
Dr. Prahalad, who was not involved in the Danish study, said that although the sample sizes in the trial were relatively small, the data are “headed in the right direction.”
Pediatricians already recommend using moisturizing creams at the sites where pumps or glucose monitors are inserted into the skin, she noted. But the new study simply employed an especially moisturizing cream to mitigate skin damage.
Although one reason for skin irritation may be the repeated insertion and removal of devices, Dr. Berg and Dr. Prahalad stressed that the medical devices themselves may contain allergy-causing components. Device makers are not required to disclose what’s inside the boxes.
“I do not understand why the full content of a device is not by law mandatory to declare, when declaration by law is mandatory for many other products and drugs but not for medical devices,” Dr. Berg said.
Dr. Berg reports receiving lipid cream from Teva Pharmaceuticals and research support from Medtronic. Dr. Prahalad reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Technologies that allow people to monitor blood sugar and automate the administration of insulin have radically transformed the lives of patients – and children in particular – with type 1 diabetes. But the devices often come with a cost: Insulin pumps and continuous glucose monitors can irritate the skin at the points of contact, causing some people to stop using their pumps or monitors altogether.
Regular use of lipid-rich skin creams can reduce eczema in children who use insulin pumps and continuous glucose monitors to manage type 1 diabetes, Danish researchers reported last month. The article is currently undergoing peer review at The Lancet Diabetes and Endocrinology, and the authors said they hope their approach will deter more children from abandoning diabetes technology.
“A simple thing can actually change a lot,” said Anna Korsgaard Berg, MD, a pediatrician who specializes in diabetes care at Copenhagen University Hospital’s Steno Diabetes Center in Herlev, Denmark, and a coauthor of the new study. “Not all skin reactions can be solved by the skin care program, but it can help improve the issue.”
More than 1.5 million children and adolescents worldwide live with type 1 diabetes, a condition that requires continuous insulin infusion. Insulin pumps meet this need in many wealthier countries, and are often used in combination with sensors that measure a child’s glucose level. Both the American Diabetes Association and the International Society for Adolescent and Pediatric Diabetes recommend insulin pumps and continuous glucose monitors as core treatment tools.
Dr. Berg and colleagues, who have previously shown that as many as 90% of children who use these devices experience some kind of skin reaction, want to minimize the rate of such discomfort in hopes that fewer children stop using the devices. According to a 2014 study, 18% of people with type 1 diabetes who stopped using continuous glucose monitors did so because of skin irritation.
Lather on that lipid-rich lotion
Dr. Berg and colleagues studied 170 children and adolescents with type 1 diabetes (average age, 11 years) who use insulin pumps, continuous glucose monitors, or both. From March 2020 to July 2021, 112 children (55 girls) employed a skin care program developed for the study, while the other 58 (34 girls) did not receive any skin care advice.
The skin care group received instructions about how to gently insert and remove their insulin pumps or glucose monitors, to minimize skin damage. They also were told to avoid disinfectants such as alcohol, which can irritate skin. The children in this group used a cream containing 70% lipids to help rehydrate their skin, applying the salve each day a device was not inserted into their skin.
Eczema can be a real problem for kids who use insulin pumps and continuous glucose monitors to manage type 1 diabetes. Researchers found that regular use of lipid-rich skin creams can reduce its incidence.
Although insulin pumps and glucose monitors are kept in place for longer periods of time than they once were, Dr. Berg and colleagues noted, users do periodically remove them when bathing or when undergoing medical tests that involve x-rays. On days when the devices were not in place for a period of time, children in the skin care group were encouraged to follow the protocol.
Study results
One-third of children in the skin care group developed eczema or experienced a wound, compared with almost half of the children in the control group, according to the researchers. The absolute difference in developing eczema or wounds between the two groups was 12.9 % (95% confidence interval, –28.7% to 2.9%).
Children in the skin care group were much less likely to develop wounds, the researchers found, when they focused only on wounds and not eczema (odds ratio, 0.29, 95% CI, 0.12-0.68).
Dr. Berg said she would like to explore whether other techniques, such as a combination of patches, adhesives, or other lotions, yield even better results.
“Anything that can help people use technology more consistently is better for both quality of life and diabetes outcomes,” said Priya Prahalad, MD, a specialist in pediatric endocrinology and diabetes at Stanford Medicine Children’s Health in Palo Alto and Sunnyvale, Calif.
Dr. Prahalad, who was not involved in the Danish study, said that although the sample sizes in the trial were relatively small, the data are “headed in the right direction.”
Pediatricians already recommend using moisturizing creams at the sites where pumps or glucose monitors are inserted into the skin, she noted. But the new study simply employed an especially moisturizing cream to mitigate skin damage.
Although one reason for skin irritation may be the repeated insertion and removal of devices, Dr. Berg and Dr. Prahalad stressed that the medical devices themselves may contain allergy-causing components. Device makers are not required to disclose what’s inside the boxes.
“I do not understand why the full content of a device is not by law mandatory to declare, when declaration by law is mandatory for many other products and drugs but not for medical devices,” Dr. Berg said.
Dr. Berg reports receiving lipid cream from Teva Pharmaceuticals and research support from Medtronic. Dr. Prahalad reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The Evidence Behind Topical Hair Loss Remedies on TikTok
Hair loss is an exceedingly common chief concern in outpatient dermatology clinics. An estimated 50% of males and females will experience androgenetic alopecia.1 Approximately 2% of new dermatology outpatient visits in the United States and the United Kingdom are for alopecia areata, the second most common type of hair loss.2 As access to dermatology appointments remains an issue with some studies citing wait times ranging from 2 to 25 days for a dermatologic consultation, the ease of accessibility of medical information on social media continues to grow,3 which leaves many of our patients turning to social media as a first-line source of information. As dermatology resident physicians, it is essential to be aware of popular dermatologic therapies on social media so that we may provide evidence-based opinions to our patients.
Remedies for Hair Loss on Social Media
Many trends on hair loss therapies found on TikTok focus on natural remedies that are produced by ingredients accessible to patients at home and over the counter, which may increase the appeal due to ease of treatment.
Rosemary Oil—The top trends in hair loss remedies I have come across are rosemary oil and rosemary water. Rosemary (Rosmarinus officinalis) has been known to possess antimicrobial and antioxidant properties but also has shown enhancement of microcapillary perfusion, which could explain its role in the prevention of hair loss and aiding hair growth in a similar mechanism to minoxidil.4,5 Unlike many other natural hair loss remedies, there are randomized controlled trials that assess the efficacy of rosemary oil for the treatment of hair loss. In a 2015 study of 100 patients with androgenetic alopecia,there was no statistically significant difference in mean hair count measured by microphotographic assessment after 6 months of treatment in 2 groups treated with either minoxidil solution 2% or rosemary oil, and both groups experienced a significant increase in hair count at 6 months (P<.05) compared with baseline and 3 months.6 Additionally, essential oils, including a mixture of thyme, rosemary, lavender, and cedarwood oils for alopecia were superior to placebo carrier oils in a posttreatment photographic assessment of their efficacy.7
Rice Water—The use of rice water and rice bran extract is a common hair care practice in Asia. Rice bran extract preparations have been shown in vivo to increase the number of anagen hair follicles as well as the number of anagen-related molecules in the dermal papillae.8,9 However, there are limited clinical data to support the use of rice water for hair growth.10
Onion Juice—Sharquie and Al-Obaidi11 conducted a study comparing crude onion juice to tap water in 38 patients with alopecia areata. They found that onion juice produced hair regrowth in significantly more patients than tap water (P<.0001).11 The mechanism of crude onion juice in hair growth is unknown; however, the induction of irritant or allergic contact dermatitis to components in crude onion juice may stimulate antigenic competition.12
Garlic Gel—Garlic gel, which is in the genus Allium, produces organosulfur compounds that provide antimicrobial and anti-inflammatory benefits.12 Additionally, in a double-blind randomized controlled trial, garlic powder was shown to increase cutaneous capillary perfusion.5 One study in 40 patients with alopecia areata demonstrated garlic gel 5% added to betamethasone valerate cream 0.1% was statistically superior to betamethasone alone in stimulating terminal hair growth (P=.001).13
Limitations and Downsides to Hair Loss Remedies on Social Media
Social media continues to be a prominent source of medical information for our patients, but most sources of hair content on social media are not board-certified dermatologists. A recent review of alopecia-related content found only 4% and 10% of posts were created by medical professionals on Instagram and TikTok, respectively, making misinformation extremely likely.14 Natural hair loss remedies contrived by TikTok have little clinical evidence to support their claims. Few data are available that compare these treatments to gold-standard hair loss therapies. Additionally, while some of these agents may be beneficial, the lack of standardized dosing may counteract these benefits. For example, videos on rosemary water advise the viewer to boil fresh rosemary sprigs in water and apply the solution to the hair daily with a spray bottle or apply cloves of garlic directly to the scalp, as opposed to a measured and standardized percentage. Some preparations may even induce harm to patients. Over-the-counter oils with added fragrances and natural compounds in onion and garlic may cause contact dermatitis. Finally, by using these products, patients may delay consultation with a board-certified dermatologist, leading to delays in applying evidence-based therapies targeted to specific hair loss subtypes while also incurring unnecessary expenses for these preparations.
Final Thoughts
Hair loss affects a notable portion of the population and is a common chief concern in dermatology clinics. Misinformation on social media continues to grow in prevalence. It is important to be aware of the hair loss remedies that are commonly touted to patients online and the evidence behind them.
- Ho CH, Sood T, Zito PM. Androgenetic alopecia. StatPearls. StatPearls Publishing; 2022.
- McMichael AJ, Pearce DJ, Wasserman D, et al. Alopecia in the United States: outpatient utilization and common prescribing patterns. J Am Acad Dermatol. 2007;57(2 suppl):S49-S51.
- Creadore A, Desai S, Li SJ, et al. Insurance acceptance, appointment wait time, and dermatologist access across practice types in the US. JAMA Dermatol. 2021;157:181-188. doi:10.1001/jamadermatol.2020.5173
- Bassino E, Gasparri F, Munaron L. Protective role of nutritional plants containing flavonoids in hair follicle disruption: a review. Int J Mol Sci. 2020;21:523. doi:10.3390/ijms21020523
- Ezekwe N, King M, Hollinger JC. The use of natural ingredients in the treatment of alopecias with an emphasis on central centrifugal cicatricial alopecia: a systematic review [published online August 1, 2020]. J Clin Aesthet Dermatol. 2020;13:23-27.
- Panahi Y, Taghizadeh M, Marzony ET, et al. Rosemary oil vs minoxidil 2% for the treatment of androgenetic alopecia: a randomized comparative trial. Skinmed. 2015;13:15-21.
- Hay IC, Jamieson M, Ormerod AD. Randomized trial of aromatherapy. successful treatment for alopecia areata. Arch Dermatol. 1998;134:1349-1352. doi:10.1001/archderm.134.11.1349
- Choi JS, Jeon MH, Moon WS, et al. In vivo hair growth-promoting effect of rice bran extract prepared by supercritical carbon dioxide fluid. Biol Pharm Bull. 2014;37:44-53. doi:10.1248/bpb.b13-00528
- Kim YM, Kwon SJ, Jang HJ, et al. Rice bran mineral extract increases the expression of anagen-related molecules in human dermal papilla through wnt/catenin pathway. Food Nutr Res. 2017;61:1412792. doi:10.1080/16546628.2017.1412792
- Hashemi K, Pham C, Sung C, et al. A systematic review: application of rice products for hair growth. J Drugs Dermatol. 2022;21:177-185. doi:10.36849/jdd.6345
- Sharquie KE, Al-Obaidi HK. Onion juice (Allium cepa L.), a new topical treatment for alopecia areata. J Dermatol. 2002;29:343-346. doi:10.1111/j.1346-8138.2002.tb00277.x
- Hosking AM, Juhasz M, Atanaskova Mesinkovska N. Complementary and alternative treatments for alopecia: a comprehensive review. Skin Appendage Disord. 2019;5:72-89. doi:10.1159/000492035
- Hajheydari Z, Jamshidi M, Akbari J, et al. Combination of topical garlic gel and betamethasone valerate cream in the treatment of localized alopecia areata: a double-blind randomized controlled study. Indian J Dermatol Venereol Leprol. 2007;73:29-32. doi:10.4103/0378-6323.30648
- Laughter M, Anderson J, Kolla A, et al. An analysis of alopecia related content on Instagram and TikTok. J Drugs Dermatol. 2022;21:1316-1321. doi:10.36849/JDD.6707
Hair loss is an exceedingly common chief concern in outpatient dermatology clinics. An estimated 50% of males and females will experience androgenetic alopecia.1 Approximately 2% of new dermatology outpatient visits in the United States and the United Kingdom are for alopecia areata, the second most common type of hair loss.2 As access to dermatology appointments remains an issue with some studies citing wait times ranging from 2 to 25 days for a dermatologic consultation, the ease of accessibility of medical information on social media continues to grow,3 which leaves many of our patients turning to social media as a first-line source of information. As dermatology resident physicians, it is essential to be aware of popular dermatologic therapies on social media so that we may provide evidence-based opinions to our patients.
Remedies for Hair Loss on Social Media
Many trends on hair loss therapies found on TikTok focus on natural remedies that are produced by ingredients accessible to patients at home and over the counter, which may increase the appeal due to ease of treatment.
Rosemary Oil—The top trends in hair loss remedies I have come across are rosemary oil and rosemary water. Rosemary (Rosmarinus officinalis) has been known to possess antimicrobial and antioxidant properties but also has shown enhancement of microcapillary perfusion, which could explain its role in the prevention of hair loss and aiding hair growth in a similar mechanism to minoxidil.4,5 Unlike many other natural hair loss remedies, there are randomized controlled trials that assess the efficacy of rosemary oil for the treatment of hair loss. In a 2015 study of 100 patients with androgenetic alopecia,there was no statistically significant difference in mean hair count measured by microphotographic assessment after 6 months of treatment in 2 groups treated with either minoxidil solution 2% or rosemary oil, and both groups experienced a significant increase in hair count at 6 months (P<.05) compared with baseline and 3 months.6 Additionally, essential oils, including a mixture of thyme, rosemary, lavender, and cedarwood oils for alopecia were superior to placebo carrier oils in a posttreatment photographic assessment of their efficacy.7
Rice Water—The use of rice water and rice bran extract is a common hair care practice in Asia. Rice bran extract preparations have been shown in vivo to increase the number of anagen hair follicles as well as the number of anagen-related molecules in the dermal papillae.8,9 However, there are limited clinical data to support the use of rice water for hair growth.10
Onion Juice—Sharquie and Al-Obaidi11 conducted a study comparing crude onion juice to tap water in 38 patients with alopecia areata. They found that onion juice produced hair regrowth in significantly more patients than tap water (P<.0001).11 The mechanism of crude onion juice in hair growth is unknown; however, the induction of irritant or allergic contact dermatitis to components in crude onion juice may stimulate antigenic competition.12
Garlic Gel—Garlic gel, which is in the genus Allium, produces organosulfur compounds that provide antimicrobial and anti-inflammatory benefits.12 Additionally, in a double-blind randomized controlled trial, garlic powder was shown to increase cutaneous capillary perfusion.5 One study in 40 patients with alopecia areata demonstrated garlic gel 5% added to betamethasone valerate cream 0.1% was statistically superior to betamethasone alone in stimulating terminal hair growth (P=.001).13
Limitations and Downsides to Hair Loss Remedies on Social Media
Social media continues to be a prominent source of medical information for our patients, but most sources of hair content on social media are not board-certified dermatologists. A recent review of alopecia-related content found only 4% and 10% of posts were created by medical professionals on Instagram and TikTok, respectively, making misinformation extremely likely.14 Natural hair loss remedies contrived by TikTok have little clinical evidence to support their claims. Few data are available that compare these treatments to gold-standard hair loss therapies. Additionally, while some of these agents may be beneficial, the lack of standardized dosing may counteract these benefits. For example, videos on rosemary water advise the viewer to boil fresh rosemary sprigs in water and apply the solution to the hair daily with a spray bottle or apply cloves of garlic directly to the scalp, as opposed to a measured and standardized percentage. Some preparations may even induce harm to patients. Over-the-counter oils with added fragrances and natural compounds in onion and garlic may cause contact dermatitis. Finally, by using these products, patients may delay consultation with a board-certified dermatologist, leading to delays in applying evidence-based therapies targeted to specific hair loss subtypes while also incurring unnecessary expenses for these preparations.
Final Thoughts
Hair loss affects a notable portion of the population and is a common chief concern in dermatology clinics. Misinformation on social media continues to grow in prevalence. It is important to be aware of the hair loss remedies that are commonly touted to patients online and the evidence behind them.
Hair loss is an exceedingly common chief concern in outpatient dermatology clinics. An estimated 50% of males and females will experience androgenetic alopecia.1 Approximately 2% of new dermatology outpatient visits in the United States and the United Kingdom are for alopecia areata, the second most common type of hair loss.2 As access to dermatology appointments remains an issue with some studies citing wait times ranging from 2 to 25 days for a dermatologic consultation, the ease of accessibility of medical information on social media continues to grow,3 which leaves many of our patients turning to social media as a first-line source of information. As dermatology resident physicians, it is essential to be aware of popular dermatologic therapies on social media so that we may provide evidence-based opinions to our patients.
Remedies for Hair Loss on Social Media
Many trends on hair loss therapies found on TikTok focus on natural remedies that are produced by ingredients accessible to patients at home and over the counter, which may increase the appeal due to ease of treatment.
Rosemary Oil—The top trends in hair loss remedies I have come across are rosemary oil and rosemary water. Rosemary (Rosmarinus officinalis) has been known to possess antimicrobial and antioxidant properties but also has shown enhancement of microcapillary perfusion, which could explain its role in the prevention of hair loss and aiding hair growth in a similar mechanism to minoxidil.4,5 Unlike many other natural hair loss remedies, there are randomized controlled trials that assess the efficacy of rosemary oil for the treatment of hair loss. In a 2015 study of 100 patients with androgenetic alopecia,there was no statistically significant difference in mean hair count measured by microphotographic assessment after 6 months of treatment in 2 groups treated with either minoxidil solution 2% or rosemary oil, and both groups experienced a significant increase in hair count at 6 months (P<.05) compared with baseline and 3 months.6 Additionally, essential oils, including a mixture of thyme, rosemary, lavender, and cedarwood oils for alopecia were superior to placebo carrier oils in a posttreatment photographic assessment of their efficacy.7
Rice Water—The use of rice water and rice bran extract is a common hair care practice in Asia. Rice bran extract preparations have been shown in vivo to increase the number of anagen hair follicles as well as the number of anagen-related molecules in the dermal papillae.8,9 However, there are limited clinical data to support the use of rice water for hair growth.10
Onion Juice—Sharquie and Al-Obaidi11 conducted a study comparing crude onion juice to tap water in 38 patients with alopecia areata. They found that onion juice produced hair regrowth in significantly more patients than tap water (P<.0001).11 The mechanism of crude onion juice in hair growth is unknown; however, the induction of irritant or allergic contact dermatitis to components in crude onion juice may stimulate antigenic competition.12
Garlic Gel—Garlic gel, which is in the genus Allium, produces organosulfur compounds that provide antimicrobial and anti-inflammatory benefits.12 Additionally, in a double-blind randomized controlled trial, garlic powder was shown to increase cutaneous capillary perfusion.5 One study in 40 patients with alopecia areata demonstrated garlic gel 5% added to betamethasone valerate cream 0.1% was statistically superior to betamethasone alone in stimulating terminal hair growth (P=.001).13
Limitations and Downsides to Hair Loss Remedies on Social Media
Social media continues to be a prominent source of medical information for our patients, but most sources of hair content on social media are not board-certified dermatologists. A recent review of alopecia-related content found only 4% and 10% of posts were created by medical professionals on Instagram and TikTok, respectively, making misinformation extremely likely.14 Natural hair loss remedies contrived by TikTok have little clinical evidence to support their claims. Few data are available that compare these treatments to gold-standard hair loss therapies. Additionally, while some of these agents may be beneficial, the lack of standardized dosing may counteract these benefits. For example, videos on rosemary water advise the viewer to boil fresh rosemary sprigs in water and apply the solution to the hair daily with a spray bottle or apply cloves of garlic directly to the scalp, as opposed to a measured and standardized percentage. Some preparations may even induce harm to patients. Over-the-counter oils with added fragrances and natural compounds in onion and garlic may cause contact dermatitis. Finally, by using these products, patients may delay consultation with a board-certified dermatologist, leading to delays in applying evidence-based therapies targeted to specific hair loss subtypes while also incurring unnecessary expenses for these preparations.
Final Thoughts
Hair loss affects a notable portion of the population and is a common chief concern in dermatology clinics. Misinformation on social media continues to grow in prevalence. It is important to be aware of the hair loss remedies that are commonly touted to patients online and the evidence behind them.
- Ho CH, Sood T, Zito PM. Androgenetic alopecia. StatPearls. StatPearls Publishing; 2022.
- McMichael AJ, Pearce DJ, Wasserman D, et al. Alopecia in the United States: outpatient utilization and common prescribing patterns. J Am Acad Dermatol. 2007;57(2 suppl):S49-S51.
- Creadore A, Desai S, Li SJ, et al. Insurance acceptance, appointment wait time, and dermatologist access across practice types in the US. JAMA Dermatol. 2021;157:181-188. doi:10.1001/jamadermatol.2020.5173
- Bassino E, Gasparri F, Munaron L. Protective role of nutritional plants containing flavonoids in hair follicle disruption: a review. Int J Mol Sci. 2020;21:523. doi:10.3390/ijms21020523
- Ezekwe N, King M, Hollinger JC. The use of natural ingredients in the treatment of alopecias with an emphasis on central centrifugal cicatricial alopecia: a systematic review [published online August 1, 2020]. J Clin Aesthet Dermatol. 2020;13:23-27.
- Panahi Y, Taghizadeh M, Marzony ET, et al. Rosemary oil vs minoxidil 2% for the treatment of androgenetic alopecia: a randomized comparative trial. Skinmed. 2015;13:15-21.
- Hay IC, Jamieson M, Ormerod AD. Randomized trial of aromatherapy. successful treatment for alopecia areata. Arch Dermatol. 1998;134:1349-1352. doi:10.1001/archderm.134.11.1349
- Choi JS, Jeon MH, Moon WS, et al. In vivo hair growth-promoting effect of rice bran extract prepared by supercritical carbon dioxide fluid. Biol Pharm Bull. 2014;37:44-53. doi:10.1248/bpb.b13-00528
- Kim YM, Kwon SJ, Jang HJ, et al. Rice bran mineral extract increases the expression of anagen-related molecules in human dermal papilla through wnt/catenin pathway. Food Nutr Res. 2017;61:1412792. doi:10.1080/16546628.2017.1412792
- Hashemi K, Pham C, Sung C, et al. A systematic review: application of rice products for hair growth. J Drugs Dermatol. 2022;21:177-185. doi:10.36849/jdd.6345
- Sharquie KE, Al-Obaidi HK. Onion juice (Allium cepa L.), a new topical treatment for alopecia areata. J Dermatol. 2002;29:343-346. doi:10.1111/j.1346-8138.2002.tb00277.x
- Hosking AM, Juhasz M, Atanaskova Mesinkovska N. Complementary and alternative treatments for alopecia: a comprehensive review. Skin Appendage Disord. 2019;5:72-89. doi:10.1159/000492035
- Hajheydari Z, Jamshidi M, Akbari J, et al. Combination of topical garlic gel and betamethasone valerate cream in the treatment of localized alopecia areata: a double-blind randomized controlled study. Indian J Dermatol Venereol Leprol. 2007;73:29-32. doi:10.4103/0378-6323.30648
- Laughter M, Anderson J, Kolla A, et al. An analysis of alopecia related content on Instagram and TikTok. J Drugs Dermatol. 2022;21:1316-1321. doi:10.36849/JDD.6707
- Ho CH, Sood T, Zito PM. Androgenetic alopecia. StatPearls. StatPearls Publishing; 2022.
- McMichael AJ, Pearce DJ, Wasserman D, et al. Alopecia in the United States: outpatient utilization and common prescribing patterns. J Am Acad Dermatol. 2007;57(2 suppl):S49-S51.
- Creadore A, Desai S, Li SJ, et al. Insurance acceptance, appointment wait time, and dermatologist access across practice types in the US. JAMA Dermatol. 2021;157:181-188. doi:10.1001/jamadermatol.2020.5173
- Bassino E, Gasparri F, Munaron L. Protective role of nutritional plants containing flavonoids in hair follicle disruption: a review. Int J Mol Sci. 2020;21:523. doi:10.3390/ijms21020523
- Ezekwe N, King M, Hollinger JC. The use of natural ingredients in the treatment of alopecias with an emphasis on central centrifugal cicatricial alopecia: a systematic review [published online August 1, 2020]. J Clin Aesthet Dermatol. 2020;13:23-27.
- Panahi Y, Taghizadeh M, Marzony ET, et al. Rosemary oil vs minoxidil 2% for the treatment of androgenetic alopecia: a randomized comparative trial. Skinmed. 2015;13:15-21.
- Hay IC, Jamieson M, Ormerod AD. Randomized trial of aromatherapy. successful treatment for alopecia areata. Arch Dermatol. 1998;134:1349-1352. doi:10.1001/archderm.134.11.1349
- Choi JS, Jeon MH, Moon WS, et al. In vivo hair growth-promoting effect of rice bran extract prepared by supercritical carbon dioxide fluid. Biol Pharm Bull. 2014;37:44-53. doi:10.1248/bpb.b13-00528
- Kim YM, Kwon SJ, Jang HJ, et al. Rice bran mineral extract increases the expression of anagen-related molecules in human dermal papilla through wnt/catenin pathway. Food Nutr Res. 2017;61:1412792. doi:10.1080/16546628.2017.1412792
- Hashemi K, Pham C, Sung C, et al. A systematic review: application of rice products for hair growth. J Drugs Dermatol. 2022;21:177-185. doi:10.36849/jdd.6345
- Sharquie KE, Al-Obaidi HK. Onion juice (Allium cepa L.), a new topical treatment for alopecia areata. J Dermatol. 2002;29:343-346. doi:10.1111/j.1346-8138.2002.tb00277.x
- Hosking AM, Juhasz M, Atanaskova Mesinkovska N. Complementary and alternative treatments for alopecia: a comprehensive review. Skin Appendage Disord. 2019;5:72-89. doi:10.1159/000492035
- Hajheydari Z, Jamshidi M, Akbari J, et al. Combination of topical garlic gel and betamethasone valerate cream in the treatment of localized alopecia areata: a double-blind randomized controlled study. Indian J Dermatol Venereol Leprol. 2007;73:29-32. doi:10.4103/0378-6323.30648
- Laughter M, Anderson J, Kolla A, et al. An analysis of alopecia related content on Instagram and TikTok. J Drugs Dermatol. 2022;21:1316-1321. doi:10.36849/JDD.6707
Resident Pearl
- With terabytes of information at their fingertips, patients often turn to social media for hair loss advice. Many recommended therapies lack evidence-based research, and some may even be harmful to patients or delay time to efficacious treatments.
Multimodal Treatment of Epidermodysplasia Verruciformis in an HIV-Positive Man
To the Editor:
Epidermodysplasia verruciformis (EDV) is a rare generalized form of epidermal dysplasia that is linked to certain subtypes of human papillomavirus (HPV) infection and inherited or acquired states of immunodeficiency.1-3 The inherited form most commonly manifests via autosomal-recessive inactivation of the EVER1 and EVER2 genes that encode integral membrane proteins in the endoplasmic reticulum, though cases of autosomal-dominant and X-linked inheritance have been reported.1-3 Acquired cases have been reported in patients lacking immunocompetency, including transplant recipients and patients living with HIV.4-11 We present the case of a patient with HIV-associated EDV who was treated successfully with intralesional Candida albicans antigen, oral acitretin, and cryotherapy.
A 56-year-old man presented for evaluation of several cutaneous lesions that had developed over several months on the neck and over many years on the hands and feet. He had a 16-year history of HIV, Castleman disease, and primary effusion lymphoma in remission that was treated with rituximab, etoposide phosphate, prednisone, vincristine sulfate, cyclophosphamide, and doxorubicin hydrochloride 10 or more years ago. The patient denied pruritus or pain associated with the skin lesions. He was intermittently taking immunosuppressants and antiretrovirals including dolutegravir and emtricitabine-tenofovir for 3 years. Prior treatments of the lesions included cryotherapy and over-the-counter 17% salicylic acid. Physical examination revealed the presence of innumerable, clustered, verrucous, scaly papules on the dorsal and palmoplantar regions of the hands (Figure 1), as well as hypopigmented macules clustered on the neck that morphologically resembled tinea versicolor (Figure 2). The physical examination was otherwise unremarkable.
Complete blood cell counts as well as lipid, liver, and renal function panel results were unremarkable. Laboratory examination also revealed a CD4 cell count of 373/µL (reference range, 320–1900/µL) and an undetectable HIV copy number (<40 copies/mL). A punch biopsy of a hypopigmented macule on the left side of the neck revealed epidermal acanthosis, hypergranulosis, and hyperkeratosis, with blue-gray cytoplasm observed in the keratinocytes (Figure 3). Koilocytes with perinuclear clearing associated with keratinocytes in the upper epidermis were noted. Based on the clinical and histopathologic correlation, acquired EDV was diagnosed.
Given that HIV-associated EDV often is recalcitrant and there is a lack of consistent and effective treatment, the patient initially was prescribed oral acitretin 25 mg/d with intralesional C albicans antigen injected once per month into the lesions along with concurrent cryotherapy. At subsequent monthly follow-ups, the involved areas were notably thinner and flat. The patient reported no remarkable side effects from the systemic retinoid treatment such as abdominal pain, photosensitivity, or headaches, though he did experience mild xerosis. Complete resolution of EDV occurred with multimodal therapy—acitretin, cryotherapy, and intralesional Candida antigen. Palmar verrucae were much improved, and he is currently continuing therapy.
Epidermodysplasia verruciformis is a rare genodermatosis associated with an abnormal susceptibility to cutaneous HPV and can be acquired in immunocompromised patients. Patients with EDV present with a clinically heterogeneous disease that can manifest as hypopigmented, red-brown macules with scaling on the trunk, neck, and extremities, which are morphologically similar to tinea versicolor, or patients can present with flat wartlike papules that are most commonly found on the face, hands, and feet.2,3 Epidermodysplasia verruciformis can be distinguished from EDV-like eruptions and other generalized verrucoses by its characteristic histologic appearance and by the demonstration of HPV within the lesions, typically subtypes HPV-5 and HPV-8.1-3 Classic EDV histopathologic findings include mild to moderate acanthosis and hyperkeratosis with enlarged keratinocytes featuring blue-gray cytoplasm and perinuclear halos.1
The histologic differential diagnosis of EDV is quite broad and includes common verrucae, which may be distinguished by the absence of blue-gray discoloration of the cytoplasm among the individual keratinocytes.1 Verruca plana and condylomata also may mimic EDV, and patients may present with minimal papillomatosis of the surface epidermis.2 Squamous cell carcinoma in situ (SCC-IS) and particularly bowenoid papulosis also may share similar histologic features.2 However, in SCC-IS, there typically is full-thickness dysplasia of the epidermis, which is not present in EDV. Nonetheless, EDV is equivalent to SCC-IS in its clinical behavior. Bowenoid papulosis shares similar findings, but lesions generally are located in the genital areas and linked to HPV-16 and HPV-18.2 Additional histologic features of EDV have been described in the entity of EDV acanthoma, specifically incidental findings present in association with other cutaneous neoplasms including acantholytic acanthomas, condylomas, intradermal nevi, and seborrheic keratoses.12
The pathophysiology of EDV is thought to be specifically associated with patients with immunocompromised conditions. Particular attention has been paid to the association between EDV and HIV. Anselmo et al13 reported a case of HIV-associated acquired EDV with preexisting lesions that were spread along the distribution of the patient’s tattoo, suggesting potential autoinoculation. In individuals living with HIV, the cutaneous features of EDV are not associated with immune status.14
Acquired EDV also may be associated with other conditions including renal transplantation, IgM deficiency, severe combined immunodeficiency, common variable immunodeficiency, systemic lupus erythematosus, and myasthenia gravis.2 Hematologic malignancies such as Hodgkin disease,4 natural killer/T-cell lymphoma,5 cutaneous T-cell lymphoma,6 adult T-cell leukemia,7 intestinal diffuse large B-cell lymphoma,8,9 transformed acute myelogenous lymphoma,10 and chronic myelogenous leukemia11 also may be associated with EDV. In the inherited form, integral membrane proteins of the endoplasmic reticulum encoded by the genes EVER1 and EVER2 on chromosome 17 are thought to act as restriction factors for certain types of HPV.2,3 Inactivating mutations in EVER1 and EVER2 result in defects in cell-mediated immunity, rendering patients susceptible to both benign and oncogenic verrucous infections.2,3 Currently, it is believed that immunosuppressed states may result in defects in cell-mediated immunity that make patients similarly susceptible to these virulent strains of HPV, resulting in an acquired form of EDV.3 Interestingly, the clinical and histologic presentation is identical for acquired EDV and genetic EDV.
Due to the general resistance of EDV to treatment, a variety of options for acquired EDV have been explored including topical and systemic retinoids, cryotherapy, interferon alfa‐2a, zidovudine, ketoconazole, corticosteroids, podophyllotoxin, imiquimod, cidofovir, electrosurgery, 5‐fluorouracil, glycolic acid, temporized diathermy, and methyl aminolevulinate photodynamic therapy.3 Highly active antiretroviral therapy has been proposed as a potential treatment modality for HIV-associated cases; however, acquired EDV has been reported to develop as an immune reconstitution inflammatory syndrome after the initiation of highly active antiretroviral therapy.15
Combination therapy consisting of a systemic retinoid, immunotherapy, and cryotherapy was initiated for our patient. Human papillomavirus infection is marked by epithelial hyperplasia, and retinoids induce antiproliferation through the control of epithelial cell differentiation.16 The specific mechanism of action of retinoids in EDV treatment is unknown; however, the beneficial effects may result from the modification of terminal differentiation, a direct antiviral action, or the enhancement of killer T cells.17 Immunotherapy with C albicans antigen initiates an inflammatory reaction that leads to an immune response directed against the virus, thus reducing the number of warts.2 Cryotherapy aims to destroy the lesion but not the virus.2 The combination of systemic retinoids, immunotherapy, and destruction may target EDV via multiple potentially synergistic mechanisms. Thus, a multimodal approach can be beneficial in patients with recalcitrant acquired EDV.
The occurrence of EDV is rare, and data on treatment are limited in number resulting in general uncertainty about the efficacy of therapies. Elucidation of the specific mechanism of immunosuppression and its effects on T lymphocytes in acquired EDV may shed light on the most effective treatments. We present this novel case of a patient with HIV-associated acquired EDV who responded favorably to a combination treatment of acitretin, intralesional C albicans antigen, and cryotherapy.
- Nuovo GJ, Ishag M. The histologic spectrum of epidermodysplasia verruciformis. Am J Surg Pathol. 2000;24:1400-1406.
- Sri JC, Dubina MI, Kao GF, et al. Generalized verrucosis: a review of the associated diseases, evaluation, and treatments. J Am Acad Dermatol. 2012;66:292-311.
- Zampetti A, Giurdanella F, Manco S, et al. Acquired epidermodysplasia verruciformis: a comprehensive review and a proposal for treatment. Dermatol Surg. 2013;39:974-980.
- Gross G, Ellinger K, Roussaki A, et al. Epidermodysplasia verruciformis in a patient with Hodgkin’s disease: characterization of a new papillomavirus type and interferon treatment. J Invest Dermatol. 1988;91:43-48.
- Boran P, Tokuc G, Ozberk M, et al. Epidermodysplasia verruciformis associated with natural killer/T cell lymphoma. J Pediatr. 2010;156:340-340.e1.
- Cutlan JE, Rashid RM, Torres-Cabala C, et al. Epidermodysplasia verruciformis after cutaneous T-cell lymphoma: periungual presentation. Dermatol Online J. 2010;16:12.
- Kawai K, Egawa N, Kiyono T, et al. Epidermodysplasia-verruciformis-like eruption associated with gamma-papillomavirus infection in a patient with adult T-cell leukemia. Dermatology. 2009;219:274-278.
- Slawsky LD, Gilson RT, Hockley AJ, et al. Epidermodysplasia verruciformis associated with severe immunodeficiency, lymphoma, and disseminated molluscum contagiosum. J Am Acad Dermatol. 1992;27:448-450.
- Youssef M, Denguezli M, Ghariani N, et al. Epidermodysplasia verruciformis associated with intestinal lymphoma: a model of viral oncogenicity. Pediatr Dermatol. 2007;24:511-513.
- Kunishige JH, Hymes SR, Madkan V, et al. Epidermodysplasia verruciformis in the setting of graft-versus-host disease. J Am Acad Dermatol. 2007;57(5 suppl):S78-S80.
- Binkley GW. A case for diagnosis (epidermodysplasia verruciformis?) chronic myeloid leukemia. Arch Derm Syphilol. 1947;55:280-282.
- Ko CJ, Iftner T, Barr RJ, et al. Changes of epidermodysplasia verruciformis in benign skin lesions: the EV acanthoma. J Cutan Pathol. 2007;34:44-48.
- Anselmo F, Ansari U, Gagnier JM, et al. Verrucous lesions in an HIV-positive man. JAAD Case Reports. 2019;5:825-827.
- Huang S, Wu JH, Lewis DJ, et al. A novel approach to the classification of epidermodysplasia verruciformis. Int J Dermatol. 2018;57:1344-1350.
- Jacobelli S, Laude H, Carlotti A, et al. Epidermodysplasia verruciformis in human immunodeficiency virus-infected patients: a marker of human papillomavirus-related disorders not affected by antiretroviral therapy. Arch Dermatol. 2011;147:590-596.
- Limmer AL, Wu JH, Doan HQ, et al. Acquired epidermodysplasia verruciformis: a 10-year anniversary update. Br J Dermatol. 2020;182:790-792.
- Anadolu R, Oskay T, Erdem C, et al. Treatment of epidermodysplasia verruciformis with a combination of acitretin and interferon alfa-2a.J Am Acad Dermatol. 2001;45:296-299.
To the Editor:
Epidermodysplasia verruciformis (EDV) is a rare generalized form of epidermal dysplasia that is linked to certain subtypes of human papillomavirus (HPV) infection and inherited or acquired states of immunodeficiency.1-3 The inherited form most commonly manifests via autosomal-recessive inactivation of the EVER1 and EVER2 genes that encode integral membrane proteins in the endoplasmic reticulum, though cases of autosomal-dominant and X-linked inheritance have been reported.1-3 Acquired cases have been reported in patients lacking immunocompetency, including transplant recipients and patients living with HIV.4-11 We present the case of a patient with HIV-associated EDV who was treated successfully with intralesional Candida albicans antigen, oral acitretin, and cryotherapy.

A 56-year-old man presented for evaluation of several cutaneous lesions that had developed over several months on the neck and over many years on the hands and feet. He had a 16-year history of HIV, Castleman disease, and primary effusion lymphoma in remission that was treated with rituximab, etoposide phosphate, prednisone, vincristine sulfate, cyclophosphamide, and doxorubicin hydrochloride 10 or more years ago. The patient denied pruritus or pain associated with the skin lesions. He was intermittently taking immunosuppressants and antiretrovirals including dolutegravir and emtricitabine-tenofovir for 3 years. Prior treatments of the lesions included cryotherapy and over-the-counter 17% salicylic acid. Physical examination revealed the presence of innumerable, clustered, verrucous, scaly papules on the dorsal and palmoplantar regions of the hands (Figure 1), as well as hypopigmented macules clustered on the neck that morphologically resembled tinea versicolor (Figure 2). The physical examination was otherwise unremarkable.

Complete blood cell counts as well as lipid, liver, and renal function panel results were unremarkable. Laboratory examination also revealed a CD4 cell count of 373/µL (reference range, 320–1900/µL) and an undetectable HIV copy number (<40 copies/mL). A punch biopsy of a hypopigmented macule on the left side of the neck revealed epidermal acanthosis, hypergranulosis, and hyperkeratosis, with blue-gray cytoplasm observed in the keratinocytes (Figure 3). Koilocytes with perinuclear clearing associated with keratinocytes in the upper epidermis were noted. Based on the clinical and histopathologic correlation, acquired EDV was diagnosed.

Given that HIV-associated EDV often is recalcitrant and there is a lack of consistent and effective treatment, the patient initially was prescribed oral acitretin 25 mg/d with intralesional C albicans antigen injected once per month into the lesions along with concurrent cryotherapy. At subsequent monthly follow-ups, the involved areas were notably thinner and flat. The patient reported no remarkable side effects from the systemic retinoid treatment such as abdominal pain, photosensitivity, or headaches, though he did experience mild xerosis. Complete resolution of EDV occurred with multimodal therapy—acitretin, cryotherapy, and intralesional Candida antigen. Palmar verrucae were much improved, and he is currently continuing therapy.
Epidermodysplasia verruciformis is a rare genodermatosis associated with an abnormal susceptibility to cutaneous HPV and can be acquired in immunocompromised patients. Patients with EDV present with a clinically heterogeneous disease that can manifest as hypopigmented, red-brown macules with scaling on the trunk, neck, and extremities, which are morphologically similar to tinea versicolor, or patients can present with flat wartlike papules that are most commonly found on the face, hands, and feet.2,3 Epidermodysplasia verruciformis can be distinguished from EDV-like eruptions and other generalized verrucoses by its characteristic histologic appearance and by the demonstration of HPV within the lesions, typically subtypes HPV-5 and HPV-8.1-3 Classic EDV histopathologic findings include mild to moderate acanthosis and hyperkeratosis with enlarged keratinocytes featuring blue-gray cytoplasm and perinuclear halos.1
The histologic differential diagnosis of EDV is quite broad and includes common verrucae, which may be distinguished by the absence of blue-gray discoloration of the cytoplasm among the individual keratinocytes.1 Verruca plana and condylomata also may mimic EDV, and patients may present with minimal papillomatosis of the surface epidermis.2 Squamous cell carcinoma in situ (SCC-IS) and particularly bowenoid papulosis also may share similar histologic features.2 However, in SCC-IS, there typically is full-thickness dysplasia of the epidermis, which is not present in EDV. Nonetheless, EDV is equivalent to SCC-IS in its clinical behavior. Bowenoid papulosis shares similar findings, but lesions generally are located in the genital areas and linked to HPV-16 and HPV-18.2 Additional histologic features of EDV have been described in the entity of EDV acanthoma, specifically incidental findings present in association with other cutaneous neoplasms including acantholytic acanthomas, condylomas, intradermal nevi, and seborrheic keratoses.12
The pathophysiology of EDV is thought to be specifically associated with patients with immunocompromised conditions. Particular attention has been paid to the association between EDV and HIV. Anselmo et al13 reported a case of HIV-associated acquired EDV with preexisting lesions that were spread along the distribution of the patient’s tattoo, suggesting potential autoinoculation. In individuals living with HIV, the cutaneous features of EDV are not associated with immune status.14
Acquired EDV also may be associated with other conditions including renal transplantation, IgM deficiency, severe combined immunodeficiency, common variable immunodeficiency, systemic lupus erythematosus, and myasthenia gravis.2 Hematologic malignancies such as Hodgkin disease,4 natural killer/T-cell lymphoma,5 cutaneous T-cell lymphoma,6 adult T-cell leukemia,7 intestinal diffuse large B-cell lymphoma,8,9 transformed acute myelogenous lymphoma,10 and chronic myelogenous leukemia11 also may be associated with EDV. In the inherited form, integral membrane proteins of the endoplasmic reticulum encoded by the genes EVER1 and EVER2 on chromosome 17 are thought to act as restriction factors for certain types of HPV.2,3 Inactivating mutations in EVER1 and EVER2 result in defects in cell-mediated immunity, rendering patients susceptible to both benign and oncogenic verrucous infections.2,3 Currently, it is believed that immunosuppressed states may result in defects in cell-mediated immunity that make patients similarly susceptible to these virulent strains of HPV, resulting in an acquired form of EDV.3 Interestingly, the clinical and histologic presentation is identical for acquired EDV and genetic EDV.
Due to the general resistance of EDV to treatment, a variety of options for acquired EDV have been explored including topical and systemic retinoids, cryotherapy, interferon alfa‐2a, zidovudine, ketoconazole, corticosteroids, podophyllotoxin, imiquimod, cidofovir, electrosurgery, 5‐fluorouracil, glycolic acid, temporized diathermy, and methyl aminolevulinate photodynamic therapy.3 Highly active antiretroviral therapy has been proposed as a potential treatment modality for HIV-associated cases; however, acquired EDV has been reported to develop as an immune reconstitution inflammatory syndrome after the initiation of highly active antiretroviral therapy.15
Combination therapy consisting of a systemic retinoid, immunotherapy, and cryotherapy was initiated for our patient. Human papillomavirus infection is marked by epithelial hyperplasia, and retinoids induce antiproliferation through the control of epithelial cell differentiation.16 The specific mechanism of action of retinoids in EDV treatment is unknown; however, the beneficial effects may result from the modification of terminal differentiation, a direct antiviral action, or the enhancement of killer T cells.17 Immunotherapy with C albicans antigen initiates an inflammatory reaction that leads to an immune response directed against the virus, thus reducing the number of warts.2 Cryotherapy aims to destroy the lesion but not the virus.2 The combination of systemic retinoids, immunotherapy, and destruction may target EDV via multiple potentially synergistic mechanisms. Thus, a multimodal approach can be beneficial in patients with recalcitrant acquired EDV.
The occurrence of EDV is rare, and data on treatment are limited in number resulting in general uncertainty about the efficacy of therapies. Elucidation of the specific mechanism of immunosuppression and its effects on T lymphocytes in acquired EDV may shed light on the most effective treatments. We present this novel case of a patient with HIV-associated acquired EDV who responded favorably to a combination treatment of acitretin, intralesional C albicans antigen, and cryotherapy.
To the Editor:
Epidermodysplasia verruciformis (EDV) is a rare generalized form of epidermal dysplasia that is linked to certain subtypes of human papillomavirus (HPV) infection and inherited or acquired states of immunodeficiency.1-3 The inherited form most commonly manifests via autosomal-recessive inactivation of the EVER1 and EVER2 genes that encode integral membrane proteins in the endoplasmic reticulum, though cases of autosomal-dominant and X-linked inheritance have been reported.1-3 Acquired cases have been reported in patients lacking immunocompetency, including transplant recipients and patients living with HIV.4-11 We present the case of a patient with HIV-associated EDV who was treated successfully with intralesional Candida albicans antigen, oral acitretin, and cryotherapy.

A 56-year-old man presented for evaluation of several cutaneous lesions that had developed over several months on the neck and over many years on the hands and feet. He had a 16-year history of HIV, Castleman disease, and primary effusion lymphoma in remission that was treated with rituximab, etoposide phosphate, prednisone, vincristine sulfate, cyclophosphamide, and doxorubicin hydrochloride 10 or more years ago. The patient denied pruritus or pain associated with the skin lesions. He was intermittently taking immunosuppressants and antiretrovirals including dolutegravir and emtricitabine-tenofovir for 3 years. Prior treatments of the lesions included cryotherapy and over-the-counter 17% salicylic acid. Physical examination revealed the presence of innumerable, clustered, verrucous, scaly papules on the dorsal and palmoplantar regions of the hands (Figure 1), as well as hypopigmented macules clustered on the neck that morphologically resembled tinea versicolor (Figure 2). The physical examination was otherwise unremarkable.

Complete blood cell counts as well as lipid, liver, and renal function panel results were unremarkable. Laboratory examination also revealed a CD4 cell count of 373/µL (reference range, 320–1900/µL) and an undetectable HIV copy number (<40 copies/mL). A punch biopsy of a hypopigmented macule on the left side of the neck revealed epidermal acanthosis, hypergranulosis, and hyperkeratosis, with blue-gray cytoplasm observed in the keratinocytes (Figure 3). Koilocytes with perinuclear clearing associated with keratinocytes in the upper epidermis were noted. Based on the clinical and histopathologic correlation, acquired EDV was diagnosed.

Given that HIV-associated EDV often is recalcitrant and there is a lack of consistent and effective treatment, the patient initially was prescribed oral acitretin 25 mg/d with intralesional C albicans antigen injected once per month into the lesions along with concurrent cryotherapy. At subsequent monthly follow-ups, the involved areas were notably thinner and flat. The patient reported no remarkable side effects from the systemic retinoid treatment such as abdominal pain, photosensitivity, or headaches, though he did experience mild xerosis. Complete resolution of EDV occurred with multimodal therapy—acitretin, cryotherapy, and intralesional Candida antigen. Palmar verrucae were much improved, and he is currently continuing therapy.
Epidermodysplasia verruciformis is a rare genodermatosis associated with an abnormal susceptibility to cutaneous HPV and can be acquired in immunocompromised patients. Patients with EDV present with a clinically heterogeneous disease that can manifest as hypopigmented, red-brown macules with scaling on the trunk, neck, and extremities, which are morphologically similar to tinea versicolor, or patients can present with flat wartlike papules that are most commonly found on the face, hands, and feet.2,3 Epidermodysplasia verruciformis can be distinguished from EDV-like eruptions and other generalized verrucoses by its characteristic histologic appearance and by the demonstration of HPV within the lesions, typically subtypes HPV-5 and HPV-8.1-3 Classic EDV histopathologic findings include mild to moderate acanthosis and hyperkeratosis with enlarged keratinocytes featuring blue-gray cytoplasm and perinuclear halos.1
The histologic differential diagnosis of EDV is quite broad and includes common verrucae, which may be distinguished by the absence of blue-gray discoloration of the cytoplasm among the individual keratinocytes.1 Verruca plana and condylomata also may mimic EDV, and patients may present with minimal papillomatosis of the surface epidermis.2 Squamous cell carcinoma in situ (SCC-IS) and particularly bowenoid papulosis also may share similar histologic features.2 However, in SCC-IS, there typically is full-thickness dysplasia of the epidermis, which is not present in EDV. Nonetheless, EDV is equivalent to SCC-IS in its clinical behavior. Bowenoid papulosis shares similar findings, but lesions generally are located in the genital areas and linked to HPV-16 and HPV-18.2 Additional histologic features of EDV have been described in the entity of EDV acanthoma, specifically incidental findings present in association with other cutaneous neoplasms including acantholytic acanthomas, condylomas, intradermal nevi, and seborrheic keratoses.12
The pathophysiology of EDV is thought to be specifically associated with patients with immunocompromised conditions. Particular attention has been paid to the association between EDV and HIV. Anselmo et al13 reported a case of HIV-associated acquired EDV with preexisting lesions that were spread along the distribution of the patient’s tattoo, suggesting potential autoinoculation. In individuals living with HIV, the cutaneous features of EDV are not associated with immune status.14
Acquired EDV also may be associated with other conditions including renal transplantation, IgM deficiency, severe combined immunodeficiency, common variable immunodeficiency, systemic lupus erythematosus, and myasthenia gravis.2 Hematologic malignancies such as Hodgkin disease,4 natural killer/T-cell lymphoma,5 cutaneous T-cell lymphoma,6 adult T-cell leukemia,7 intestinal diffuse large B-cell lymphoma,8,9 transformed acute myelogenous lymphoma,10 and chronic myelogenous leukemia11 also may be associated with EDV. In the inherited form, integral membrane proteins of the endoplasmic reticulum encoded by the genes EVER1 and EVER2 on chromosome 17 are thought to act as restriction factors for certain types of HPV.2,3 Inactivating mutations in EVER1 and EVER2 result in defects in cell-mediated immunity, rendering patients susceptible to both benign and oncogenic verrucous infections.2,3 Currently, it is believed that immunosuppressed states may result in defects in cell-mediated immunity that make patients similarly susceptible to these virulent strains of HPV, resulting in an acquired form of EDV.3 Interestingly, the clinical and histologic presentation is identical for acquired EDV and genetic EDV.
Due to the general resistance of EDV to treatment, a variety of options for acquired EDV have been explored including topical and systemic retinoids, cryotherapy, interferon alfa‐2a, zidovudine, ketoconazole, corticosteroids, podophyllotoxin, imiquimod, cidofovir, electrosurgery, 5‐fluorouracil, glycolic acid, temporized diathermy, and methyl aminolevulinate photodynamic therapy.3 Highly active antiretroviral therapy has been proposed as a potential treatment modality for HIV-associated cases; however, acquired EDV has been reported to develop as an immune reconstitution inflammatory syndrome after the initiation of highly active antiretroviral therapy.15
Combination therapy consisting of a systemic retinoid, immunotherapy, and cryotherapy was initiated for our patient. Human papillomavirus infection is marked by epithelial hyperplasia, and retinoids induce antiproliferation through the control of epithelial cell differentiation.16 The specific mechanism of action of retinoids in EDV treatment is unknown; however, the beneficial effects may result from the modification of terminal differentiation, a direct antiviral action, or the enhancement of killer T cells.17 Immunotherapy with C albicans antigen initiates an inflammatory reaction that leads to an immune response directed against the virus, thus reducing the number of warts.2 Cryotherapy aims to destroy the lesion but not the virus.2 The combination of systemic retinoids, immunotherapy, and destruction may target EDV via multiple potentially synergistic mechanisms. Thus, a multimodal approach can be beneficial in patients with recalcitrant acquired EDV.
The occurrence of EDV is rare, and data on treatment are limited in number resulting in general uncertainty about the efficacy of therapies. Elucidation of the specific mechanism of immunosuppression and its effects on T lymphocytes in acquired EDV may shed light on the most effective treatments. We present this novel case of a patient with HIV-associated acquired EDV who responded favorably to a combination treatment of acitretin, intralesional C albicans antigen, and cryotherapy.
- Nuovo GJ, Ishag M. The histologic spectrum of epidermodysplasia verruciformis. Am J Surg Pathol. 2000;24:1400-1406.
- Sri JC, Dubina MI, Kao GF, et al. Generalized verrucosis: a review of the associated diseases, evaluation, and treatments. J Am Acad Dermatol. 2012;66:292-311.
- Zampetti A, Giurdanella F, Manco S, et al. Acquired epidermodysplasia verruciformis: a comprehensive review and a proposal for treatment. Dermatol Surg. 2013;39:974-980.
- Gross G, Ellinger K, Roussaki A, et al. Epidermodysplasia verruciformis in a patient with Hodgkin’s disease: characterization of a new papillomavirus type and interferon treatment. J Invest Dermatol. 1988;91:43-48.
- Boran P, Tokuc G, Ozberk M, et al. Epidermodysplasia verruciformis associated with natural killer/T cell lymphoma. J Pediatr. 2010;156:340-340.e1.
- Cutlan JE, Rashid RM, Torres-Cabala C, et al. Epidermodysplasia verruciformis after cutaneous T-cell lymphoma: periungual presentation. Dermatol Online J. 2010;16:12.
- Kawai K, Egawa N, Kiyono T, et al. Epidermodysplasia-verruciformis-like eruption associated with gamma-papillomavirus infection in a patient with adult T-cell leukemia. Dermatology. 2009;219:274-278.
- Slawsky LD, Gilson RT, Hockley AJ, et al. Epidermodysplasia verruciformis associated with severe immunodeficiency, lymphoma, and disseminated molluscum contagiosum. J Am Acad Dermatol. 1992;27:448-450.
- Youssef M, Denguezli M, Ghariani N, et al. Epidermodysplasia verruciformis associated with intestinal lymphoma: a model of viral oncogenicity. Pediatr Dermatol. 2007;24:511-513.
- Kunishige JH, Hymes SR, Madkan V, et al. Epidermodysplasia verruciformis in the setting of graft-versus-host disease. J Am Acad Dermatol. 2007;57(5 suppl):S78-S80.
- Binkley GW. A case for diagnosis (epidermodysplasia verruciformis?) chronic myeloid leukemia. Arch Derm Syphilol. 1947;55:280-282.
- Ko CJ, Iftner T, Barr RJ, et al. Changes of epidermodysplasia verruciformis in benign skin lesions: the EV acanthoma. J Cutan Pathol. 2007;34:44-48.
- Anselmo F, Ansari U, Gagnier JM, et al. Verrucous lesions in an HIV-positive man. JAAD Case Reports. 2019;5:825-827.
- Huang S, Wu JH, Lewis DJ, et al. A novel approach to the classification of epidermodysplasia verruciformis. Int J Dermatol. 2018;57:1344-1350.
- Jacobelli S, Laude H, Carlotti A, et al. Epidermodysplasia verruciformis in human immunodeficiency virus-infected patients: a marker of human papillomavirus-related disorders not affected by antiretroviral therapy. Arch Dermatol. 2011;147:590-596.
- Limmer AL, Wu JH, Doan HQ, et al. Acquired epidermodysplasia verruciformis: a 10-year anniversary update. Br J Dermatol. 2020;182:790-792.
- Anadolu R, Oskay T, Erdem C, et al. Treatment of epidermodysplasia verruciformis with a combination of acitretin and interferon alfa-2a.J Am Acad Dermatol. 2001;45:296-299.
- Nuovo GJ, Ishag M. The histologic spectrum of epidermodysplasia verruciformis. Am J Surg Pathol. 2000;24:1400-1406.
- Sri JC, Dubina MI, Kao GF, et al. Generalized verrucosis: a review of the associated diseases, evaluation, and treatments. J Am Acad Dermatol. 2012;66:292-311.
- Zampetti A, Giurdanella F, Manco S, et al. Acquired epidermodysplasia verruciformis: a comprehensive review and a proposal for treatment. Dermatol Surg. 2013;39:974-980.
- Gross G, Ellinger K, Roussaki A, et al. Epidermodysplasia verruciformis in a patient with Hodgkin’s disease: characterization of a new papillomavirus type and interferon treatment. J Invest Dermatol. 1988;91:43-48.
- Boran P, Tokuc G, Ozberk M, et al. Epidermodysplasia verruciformis associated with natural killer/T cell lymphoma. J Pediatr. 2010;156:340-340.e1.
- Cutlan JE, Rashid RM, Torres-Cabala C, et al. Epidermodysplasia verruciformis after cutaneous T-cell lymphoma: periungual presentation. Dermatol Online J. 2010;16:12.
- Kawai K, Egawa N, Kiyono T, et al. Epidermodysplasia-verruciformis-like eruption associated with gamma-papillomavirus infection in a patient with adult T-cell leukemia. Dermatology. 2009;219:274-278.
- Slawsky LD, Gilson RT, Hockley AJ, et al. Epidermodysplasia verruciformis associated with severe immunodeficiency, lymphoma, and disseminated molluscum contagiosum. J Am Acad Dermatol. 1992;27:448-450.
- Youssef M, Denguezli M, Ghariani N, et al. Epidermodysplasia verruciformis associated with intestinal lymphoma: a model of viral oncogenicity. Pediatr Dermatol. 2007;24:511-513.
- Kunishige JH, Hymes SR, Madkan V, et al. Epidermodysplasia verruciformis in the setting of graft-versus-host disease. J Am Acad Dermatol. 2007;57(5 suppl):S78-S80.
- Binkley GW. A case for diagnosis (epidermodysplasia verruciformis?) chronic myeloid leukemia. Arch Derm Syphilol. 1947;55:280-282.
- Ko CJ, Iftner T, Barr RJ, et al. Changes of epidermodysplasia verruciformis in benign skin lesions: the EV acanthoma. J Cutan Pathol. 2007;34:44-48.
- Anselmo F, Ansari U, Gagnier JM, et al. Verrucous lesions in an HIV-positive man. JAAD Case Reports. 2019;5:825-827.
- Huang S, Wu JH, Lewis DJ, et al. A novel approach to the classification of epidermodysplasia verruciformis. Int J Dermatol. 2018;57:1344-1350.
- Jacobelli S, Laude H, Carlotti A, et al. Epidermodysplasia verruciformis in human immunodeficiency virus-infected patients: a marker of human papillomavirus-related disorders not affected by antiretroviral therapy. Arch Dermatol. 2011;147:590-596.
- Limmer AL, Wu JH, Doan HQ, et al. Acquired epidermodysplasia verruciformis: a 10-year anniversary update. Br J Dermatol. 2020;182:790-792.
- Anadolu R, Oskay T, Erdem C, et al. Treatment of epidermodysplasia verruciformis with a combination of acitretin and interferon alfa-2a.J Am Acad Dermatol. 2001;45:296-299.
Practice Points
- Acquired epidermodysplasia verruciformis (EDV) is associated with immunocompromised patients with conditions such as HIV.
- Multimodal treatment of HIV-associated acquired EDV with acitretin, intralesional Candida albicans antigen, and cryotherapy may be efficacious for patients with recalcitrant disease.
Transplant surgeon to 30,000 marathoners: Give me that liver
Surgeon goes the extra half mile for his patient
Sorry medical profession, but it’s Adam Bodzin’s world now. When a donor liver got stuck in the middle of the Philadelphia Half Marathon’s 30,000 participants, Dr. Bodzin, the transplant team’s lead surgeon, took matters into his own hands. And by hands, of course, we mean feet.
Still wearing his hospital scrubs, Dr. Bodzin ran more than half a mile to where the van carrying the liver was stranded, according to the Philadelphia Inquirer. Fortunately, he was able to hitch a ride in a police car for the return trip and didn’t have to run back through the crowd carrying his somewhat unusual package. By package, of course, we mean human liver.
It’s been 3 months since the surgery/marathon and it’s still not clear why the driver had such trouble getting through – he had been trying for more than an hour and half by the time Dr. Bodzin reached him – but the surgery half of the big event was deemed a success and the patient has recovered.
Rick Hasz, president and chief executive officer of the Gift of Life Donor Program, which coordinates organ donation for transplants in the Philadelphia region, told the newspaper that “Dr. Bodzin’s quick action demonstrated his commitment to honoring the selfless generosity of all donors and their families and gives hope to everyone waiting for a second chance at life.”
Should Dr. Bodzin consider a step up from the transplant team to another group that’s fighting for the common good? The recipient of the liver in question seems to think so. “I guess he has a cape on under that white jacket,” 66-year-old Charles Rowe told Fox29. You already know where we’re going with this, right?
Avengers … Assemble.
Your spleen’s due for its 5,000-mile oil change
The human body is an incredible biological machine, capable of performing a countless array of tasks automatically and essentially without flaw, but there’s always room for improvement. After all, there are animals that can regrow entire missing limbs or live for up to 500 years. It would be nice if we could get some of that going.
Rather than any of that cool stuff, a recent survey of 2,000 average Americans revealed that our ambitions for improving the human body are a bit more mundane. The big thing that would make our lives better and easier, according to three-fourths of Americans, would be a built-in “check engine” light in our bodies. Come on guys, starfish can literally be cut in half and not only survive, but become two starfish. Mantis shrimp can punch with a force thousands of times their own weight. If we could punch like they could, we could literally break steel with our fists. Wouldn’t we rather have that?
Apparently not. Fine, we’ll stick with the check engine light.
Maybe it isn’t a huge surprise that we’d like the extra help in figuring out what our body needs. According to the survey, more than 60% of Americans struggle to identify when their body is trying to tell them something important, and only one-third actively checked in with their health every day. Considering about 40% said they feel tired for much of the day and nearly half reported not having a meal with fruits or vegetables in the past 3 days, perhaps a gentle reminder wouldn’t be the worst thing in the world.
So, if we did have a built-in check engine light, what would we use it for? A majority said they’d like to be reminded to drink a glass of water, with 45% saying they wanted to know when to take a nap. Feeling thirsty or tired isn’t quite enough, it seems.
Of course, the technology certainly exists to make the human check engine light a reality. An implanted microchip could absolutely tell us to drink a glass of water, but that would put our health in the hands of tech companies, and you just know Meta and Elon Muskrat wouldn’t pass up the chance for monetization. “Oh, sorry, we could have notified the hospital that you were about to have a heart attack, but you didn’t pay your life subscription this month.”
Sext offenders show more than their, well, you know
As we have become more and more attached to our phones, especially post pandemic, it’s no surprise that sexting – sending sexually explicit images and messages with those phones – has become a fairly common way for people to sexually communicate. And with dating apps just another venture in the dating landscape, regardless of age, sexting is an easy avenue to incite a mood without being physically present.
A recent study, though, has linked sexting with anxiety, sleep issues, depression, and compulsive sexual behaviors. Yikes.
Although the researchers noted that sexting was primarily reciprocal (sending and receiving), “over 50% of adults report sending a sext, while women are up to four times more likely than men to report having received nonconsensual sexts,” said Brenda K. Wiederhold, PhD, editor-in-chief of Cyberpsychology, Behavior, and Social Networking, which published the study, in which Dr. Wiederhold was not involved.
Among the 2,160 U.S. college students who were involved, participants who had only sent sexts reported more anxiety, depression, and sleep problems than other groups (no sexting, received only, reciprocal). There was also a possible connection between sexting, marijuana use, and compulsive sexual behavior, the investigators said in a written statement.
Considering the study population, these data are perhaps not that surprising. For young adults, to receive or send an elusive nude is as common as it once was to give someone flowers. Not that the two things elicit the same reactions. “Many individuals reveal they enjoy consensual sexting and feel it empowers them and builds self-confidence,” Dr. Wiederhold added.
Receiving a nonconsensual sext, though, is definitely going to result in feeling violated and super awkward. Senders beware: Don’t be surprised if you’re ghosted after that.
Surgeon goes the extra half mile for his patient
Sorry medical profession, but it’s Adam Bodzin’s world now. When a donor liver got stuck in the middle of the Philadelphia Half Marathon’s 30,000 participants, Dr. Bodzin, the transplant team’s lead surgeon, took matters into his own hands. And by hands, of course, we mean feet.
Still wearing his hospital scrubs, Dr. Bodzin ran more than half a mile to where the van carrying the liver was stranded, according to the Philadelphia Inquirer. Fortunately, he was able to hitch a ride in a police car for the return trip and didn’t have to run back through the crowd carrying his somewhat unusual package. By package, of course, we mean human liver.
It’s been 3 months since the surgery/marathon and it’s still not clear why the driver had such trouble getting through – he had been trying for more than an hour and half by the time Dr. Bodzin reached him – but the surgery half of the big event was deemed a success and the patient has recovered.
Rick Hasz, president and chief executive officer of the Gift of Life Donor Program, which coordinates organ donation for transplants in the Philadelphia region, told the newspaper that “Dr. Bodzin’s quick action demonstrated his commitment to honoring the selfless generosity of all donors and their families and gives hope to everyone waiting for a second chance at life.”
Should Dr. Bodzin consider a step up from the transplant team to another group that’s fighting for the common good? The recipient of the liver in question seems to think so. “I guess he has a cape on under that white jacket,” 66-year-old Charles Rowe told Fox29. You already know where we’re going with this, right?
Avengers … Assemble.
Your spleen’s due for its 5,000-mile oil change
The human body is an incredible biological machine, capable of performing a countless array of tasks automatically and essentially without flaw, but there’s always room for improvement. After all, there are animals that can regrow entire missing limbs or live for up to 500 years. It would be nice if we could get some of that going.
Rather than any of that cool stuff, a recent survey of 2,000 average Americans revealed that our ambitions for improving the human body are a bit more mundane. The big thing that would make our lives better and easier, according to three-fourths of Americans, would be a built-in “check engine” light in our bodies. Come on guys, starfish can literally be cut in half and not only survive, but become two starfish. Mantis shrimp can punch with a force thousands of times their own weight. If we could punch like they could, we could literally break steel with our fists. Wouldn’t we rather have that?
Apparently not. Fine, we’ll stick with the check engine light.
Maybe it isn’t a huge surprise that we’d like the extra help in figuring out what our body needs. According to the survey, more than 60% of Americans struggle to identify when their body is trying to tell them something important, and only one-third actively checked in with their health every day. Considering about 40% said they feel tired for much of the day and nearly half reported not having a meal with fruits or vegetables in the past 3 days, perhaps a gentle reminder wouldn’t be the worst thing in the world.
So, if we did have a built-in check engine light, what would we use it for? A majority said they’d like to be reminded to drink a glass of water, with 45% saying they wanted to know when to take a nap. Feeling thirsty or tired isn’t quite enough, it seems.
Of course, the technology certainly exists to make the human check engine light a reality. An implanted microchip could absolutely tell us to drink a glass of water, but that would put our health in the hands of tech companies, and you just know Meta and Elon Muskrat wouldn’t pass up the chance for monetization. “Oh, sorry, we could have notified the hospital that you were about to have a heart attack, but you didn’t pay your life subscription this month.”
Sext offenders show more than their, well, you know
As we have become more and more attached to our phones, especially post pandemic, it’s no surprise that sexting – sending sexually explicit images and messages with those phones – has become a fairly common way for people to sexually communicate. And with dating apps just another venture in the dating landscape, regardless of age, sexting is an easy avenue to incite a mood without being physically present.
A recent study, though, has linked sexting with anxiety, sleep issues, depression, and compulsive sexual behaviors. Yikes.
Although the researchers noted that sexting was primarily reciprocal (sending and receiving), “over 50% of adults report sending a sext, while women are up to four times more likely than men to report having received nonconsensual sexts,” said Brenda K. Wiederhold, PhD, editor-in-chief of Cyberpsychology, Behavior, and Social Networking, which published the study, in which Dr. Wiederhold was not involved.
Among the 2,160 U.S. college students who were involved, participants who had only sent sexts reported more anxiety, depression, and sleep problems than other groups (no sexting, received only, reciprocal). There was also a possible connection between sexting, marijuana use, and compulsive sexual behavior, the investigators said in a written statement.
Considering the study population, these data are perhaps not that surprising. For young adults, to receive or send an elusive nude is as common as it once was to give someone flowers. Not that the two things elicit the same reactions. “Many individuals reveal they enjoy consensual sexting and feel it empowers them and builds self-confidence,” Dr. Wiederhold added.
Receiving a nonconsensual sext, though, is definitely going to result in feeling violated and super awkward. Senders beware: Don’t be surprised if you’re ghosted after that.
Surgeon goes the extra half mile for his patient
Sorry medical profession, but it’s Adam Bodzin’s world now. When a donor liver got stuck in the middle of the Philadelphia Half Marathon’s 30,000 participants, Dr. Bodzin, the transplant team’s lead surgeon, took matters into his own hands. And by hands, of course, we mean feet.
Still wearing his hospital scrubs, Dr. Bodzin ran more than half a mile to where the van carrying the liver was stranded, according to the Philadelphia Inquirer. Fortunately, he was able to hitch a ride in a police car for the return trip and didn’t have to run back through the crowd carrying his somewhat unusual package. By package, of course, we mean human liver.
It’s been 3 months since the surgery/marathon and it’s still not clear why the driver had such trouble getting through – he had been trying for more than an hour and half by the time Dr. Bodzin reached him – but the surgery half of the big event was deemed a success and the patient has recovered.
Rick Hasz, president and chief executive officer of the Gift of Life Donor Program, which coordinates organ donation for transplants in the Philadelphia region, told the newspaper that “Dr. Bodzin’s quick action demonstrated his commitment to honoring the selfless generosity of all donors and their families and gives hope to everyone waiting for a second chance at life.”
Should Dr. Bodzin consider a step up from the transplant team to another group that’s fighting for the common good? The recipient of the liver in question seems to think so. “I guess he has a cape on under that white jacket,” 66-year-old Charles Rowe told Fox29. You already know where we’re going with this, right?
Avengers … Assemble.
Your spleen’s due for its 5,000-mile oil change
The human body is an incredible biological machine, capable of performing a countless array of tasks automatically and essentially without flaw, but there’s always room for improvement. After all, there are animals that can regrow entire missing limbs or live for up to 500 years. It would be nice if we could get some of that going.
Rather than any of that cool stuff, a recent survey of 2,000 average Americans revealed that our ambitions for improving the human body are a bit more mundane. The big thing that would make our lives better and easier, according to three-fourths of Americans, would be a built-in “check engine” light in our bodies. Come on guys, starfish can literally be cut in half and not only survive, but become two starfish. Mantis shrimp can punch with a force thousands of times their own weight. If we could punch like they could, we could literally break steel with our fists. Wouldn’t we rather have that?
Apparently not. Fine, we’ll stick with the check engine light.
Maybe it isn’t a huge surprise that we’d like the extra help in figuring out what our body needs. According to the survey, more than 60% of Americans struggle to identify when their body is trying to tell them something important, and only one-third actively checked in with their health every day. Considering about 40% said they feel tired for much of the day and nearly half reported not having a meal with fruits or vegetables in the past 3 days, perhaps a gentle reminder wouldn’t be the worst thing in the world.
So, if we did have a built-in check engine light, what would we use it for? A majority said they’d like to be reminded to drink a glass of water, with 45% saying they wanted to know when to take a nap. Feeling thirsty or tired isn’t quite enough, it seems.
Of course, the technology certainly exists to make the human check engine light a reality. An implanted microchip could absolutely tell us to drink a glass of water, but that would put our health in the hands of tech companies, and you just know Meta and Elon Muskrat wouldn’t pass up the chance for monetization. “Oh, sorry, we could have notified the hospital that you were about to have a heart attack, but you didn’t pay your life subscription this month.”
Sext offenders show more than their, well, you know
As we have become more and more attached to our phones, especially post pandemic, it’s no surprise that sexting – sending sexually explicit images and messages with those phones – has become a fairly common way for people to sexually communicate. And with dating apps just another venture in the dating landscape, regardless of age, sexting is an easy avenue to incite a mood without being physically present.
A recent study, though, has linked sexting with anxiety, sleep issues, depression, and compulsive sexual behaviors. Yikes.
Although the researchers noted that sexting was primarily reciprocal (sending and receiving), “over 50% of adults report sending a sext, while women are up to four times more likely than men to report having received nonconsensual sexts,” said Brenda K. Wiederhold, PhD, editor-in-chief of Cyberpsychology, Behavior, and Social Networking, which published the study, in which Dr. Wiederhold was not involved.
Among the 2,160 U.S. college students who were involved, participants who had only sent sexts reported more anxiety, depression, and sleep problems than other groups (no sexting, received only, reciprocal). There was also a possible connection between sexting, marijuana use, and compulsive sexual behavior, the investigators said in a written statement.
Considering the study population, these data are perhaps not that surprising. For young adults, to receive or send an elusive nude is as common as it once was to give someone flowers. Not that the two things elicit the same reactions. “Many individuals reveal they enjoy consensual sexting and feel it empowers them and builds self-confidence,” Dr. Wiederhold added.
Receiving a nonconsensual sext, though, is definitely going to result in feeling violated and super awkward. Senders beware: Don’t be surprised if you’re ghosted after that.
Is cellular senescence related to post–COVID-19 syndrome?
Proinflammatory elements mediated through metabolic pathways related to obesity and increased cellular senescence in CD57 expression in CD8+ T cells are associated with postacute sequelae of COVID-19 (PASC), according to a Mexican study. The researchers followed a Mexican cohort of 102 patients 3 months and 6 months after acute SARS-CoV-2 infection.
The study’s principal investigator was Diana Gómez-Martín, MD, PhD, of the department of immunology and rheumatology at the Salvador Zubirán National Institute of Medical Sciences and Nutrition, Mexico City. She told this news organization that follow-up of the patients began with the objective of understanding the determinative clinical, genetic, metabolic, and immunological factors in the progression of the acute disease. However, clinical aspects associated with PASC developed in the selected cohort. As a result, the study was extended, and the clinical, metabolic, and immunologic conditions in this single-center Mexican cohort were evaluated 3 months 6 months after the onset of infection.
Dr. Gómez-Martín explained that the immune senescence in CD57 of CD8+ T cells is one of the best-known findings of the present study. If it is confirmed in future studies, it could have important implications. “Its main implication is the possibility of better understanding the physiopathology of the clinical aspects associated with postacute sequelae of COVID-19, potentially being used for early detection and to provide follow-up aimed at patients, in addition to eventually developing targeted therapeutic strategies, such as immunometabolism regulation, in certain populations.”
Patients with PASC
The study was conducted from August 2020 to August 2021. Investigators recruited 102 patients (median age, 50.5 years; 55% were women) at the Mexico City Temporary Unit with a confirmed diagnosis of SARS-CoV-2. Of the patients, 44% had mild or moderate COVID-19, 30% had severe cases, and 26% of patients had critical cases. The most frequent comorbidities were obesity (44%), hypertension (24%), and type 2 diabetes (24%). The authors used a questionnaire to assess the presence of symptoms during follow-up. They analyzed immunologic variables at the time of recruitment, as well as levels of cytokines, immunoglobulin G against SARS-CoV-2, and neutrophil extracellular traps (NETs) at 1, 3, and 6 months. At 6 months’ follow-up, 12.7% of the cohort had symptoms compatible with PASC, which was defined for the study as the presence and report of three or more symptoms at 6 months’ follow-up.
As in similar studies, the authors found that female gender, remaining in intensive care, and having had more symptoms and greater titers of anti-SARS-CoV-2 antibodies during the acute infection were associated with the development of clinical aspects associated with PASC. Patients who had the disease at 6 months had increased serum levels of interleukin-1 alpha (6.21 pg/mL vs. 2.21 pg/mL), granulocyte colony-stimulating factor (55.08 pg/mL vs. 14.68 pg/mL), and interferon gamma-induced protein 10 (2,309.40 pg/mL vs. 780 pg/mL). Also, there was a trend toward an increase in serum concentration of interleukin-1 beta, interleukin-6, and interferon-gamma.
Patients whose condition met the definition of persistent PASC had increased expression of CD57 in CD8+ T cells (42,714 arbitrary units vs. 28,506) 6 months after the acute infection. The authors reported that there was no association between the persistence of PASC and the baseline amount of NETs, TRIM63, and anticellular antibodies. Nor was there an association between PASC and the titers of anti-SARS-CoV-2 antibodies at baseline and 1 month after COVID-19 diagnosis. Nonetheless, patients with persistent PASC had higher titers of anti-SARS-CoV-2 IgGs 3 months after the onset of COVID-19.
On the basis of previous data, the researchers aimed to construct a preliminary explanatory model to address the clinical and immunologic features associated with persistent PASC 6 months after SARS-CoV-2 infection. In the univariate analysis, the variables associated with the diagnosis of persistent PASC were the serum levels of granulocyte colony-stimulating factor (odds ratio, 1.01), macrophage inflammatory protein-1 alpha (OR, 1.13), interferon gamma-induced protein 10 (OR, 1.00), interleukin-6 (OR, 1.03), the expression of CD57 in CD8+ T cells (OR, 1.00), and the titers of anti-SARS-CoV-2 IgG at 1 month (OR, 1.45).
, such as obesity, greater levels of macrophage inflammatory protein-1 alpha and interferon gamma-induced protein 10 in peripheral blood, greater expression of the senescence CD57 marker in CD8+ T lymphocytes, and persistent symptoms at 3 months.
Using these parameters to construct a predictive model after 3 months, the authors found a sensitivity of 97.7%, specificity of 53.8%, positive predictive value of 93.5%, and a negative predictive value of 77.7% for the diagnosis of clinical aspects associated with PASC at 6 months.
Interpreting CD57
One of the researchers who participated in the study was Luis Martínez-Juárez, MD, MPH, DrPH. He is on the operative solutions team at the Carlos Slim Foundation. Dr. Martínez-Juárez pointed out that one of the contributions of this study was that it specifically examined the Mexican population. He noted that “according to the findings, obesity is not only a comorbidity associated with more severe progressions during acute COVID-19 disease, but also, through inflammation parameters, such as interleukin-6, interferon gamma-induced protein 10, and macrophage inflammatory protein-1 alpha, it’s involved in the development of clinical aspects related to postacute sequelae of COVID-19.”
Dr. Gómez-Martín added that finding proinflammatory and obesity parameters in the patients could potentially support the hypothesis of the persistence of virus fragments in adipose tissue as possibly involved in clinical aspects associated with PASC, as some groups have reported in the medical literature.
Angélica Cuapio, MD, DrMed, an immunologist and senior investigator at the Karolinska Institute, Stockholm, who did not participate in the study, said in an interview that the authors’ findings on the sustained increase of the CD57 marker in CD8+ lymphocytes are of notable interest. They may be associated with senescence states or cellular aging or with a stage of chronic viral infections. Therefore, Dr. Cuapio argued, it would have been valuable to include cellular markers of the innate system, such as natural killer cells, since in various infections, an increase in CD57 in lymphocytes is accompanied by an almost proportional increase of this marker in natural killer cells.
“This information would help to determine more accurately if we are talking about a cellular senescence or more about a chronic infection in persistent COVID-19.” The finding is important, but future research is needed in this developing field.
Dr. Cuapio pointed out that the authors found an interesting elevation in interleukin-1 alpha in patients with clinical aspects associated with PASC in a clinically well-characterized population in Mexico. “It is possible that this is a specific marker either of a specific population or location, or this could be an association with a humoral response. Despite the fact that this finding is new and unclear, it is worth investigating. This study is of great value for the scientific community because it’s one more piece in the complex puzzle of clinical aspects associated with postacute sequelae of COVID-19.”
Dr. Gómez-Martín noted that the main limitations of the study consist of its single-center design and the small patient sample. Dr. Martínez-Juárez added that the study did not consider reinfections. In future studies, it would be ideal to integrate other molecular assessments associated with various hypotheses of the physiopathology of clinical aspects associated with PASC, such as microbiota alteration, coagulation anomalies, endothelial damage, and dysfunctional neurologic signaling.
The study was supported and funded by the Carlos Slim Foundation. Dr. Gómez-Martín, Dr. Martínez-Juárez, and Dr. Cuapio have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Proinflammatory elements mediated through metabolic pathways related to obesity and increased cellular senescence in CD57 expression in CD8+ T cells are associated with postacute sequelae of COVID-19 (PASC), according to a Mexican study. The researchers followed a Mexican cohort of 102 patients 3 months and 6 months after acute SARS-CoV-2 infection.
The study’s principal investigator was Diana Gómez-Martín, MD, PhD, of the department of immunology and rheumatology at the Salvador Zubirán National Institute of Medical Sciences and Nutrition, Mexico City. She told this news organization that follow-up of the patients began with the objective of understanding the determinative clinical, genetic, metabolic, and immunological factors in the progression of the acute disease. However, clinical aspects associated with PASC developed in the selected cohort. As a result, the study was extended, and the clinical, metabolic, and immunologic conditions in this single-center Mexican cohort were evaluated 3 months 6 months after the onset of infection.
Dr. Gómez-Martín explained that the immune senescence in CD57 of CD8+ T cells is one of the best-known findings of the present study. If it is confirmed in future studies, it could have important implications. “Its main implication is the possibility of better understanding the physiopathology of the clinical aspects associated with postacute sequelae of COVID-19, potentially being used for early detection and to provide follow-up aimed at patients, in addition to eventually developing targeted therapeutic strategies, such as immunometabolism regulation, in certain populations.”
Patients with PASC
The study was conducted from August 2020 to August 2021. Investigators recruited 102 patients (median age, 50.5 years; 55% were women) at the Mexico City Temporary Unit with a confirmed diagnosis of SARS-CoV-2. Of the patients, 44% had mild or moderate COVID-19, 30% had severe cases, and 26% of patients had critical cases. The most frequent comorbidities were obesity (44%), hypertension (24%), and type 2 diabetes (24%). The authors used a questionnaire to assess the presence of symptoms during follow-up. They analyzed immunologic variables at the time of recruitment, as well as levels of cytokines, immunoglobulin G against SARS-CoV-2, and neutrophil extracellular traps (NETs) at 1, 3, and 6 months. At 6 months’ follow-up, 12.7% of the cohort had symptoms compatible with PASC, which was defined for the study as the presence and report of three or more symptoms at 6 months’ follow-up.
As in similar studies, the authors found that female gender, remaining in intensive care, and having had more symptoms and greater titers of anti-SARS-CoV-2 antibodies during the acute infection were associated with the development of clinical aspects associated with PASC. Patients who had the disease at 6 months had increased serum levels of interleukin-1 alpha (6.21 pg/mL vs. 2.21 pg/mL), granulocyte colony-stimulating factor (55.08 pg/mL vs. 14.68 pg/mL), and interferon gamma-induced protein 10 (2,309.40 pg/mL vs. 780 pg/mL). Also, there was a trend toward an increase in serum concentration of interleukin-1 beta, interleukin-6, and interferon-gamma.
Patients whose condition met the definition of persistent PASC had increased expression of CD57 in CD8+ T cells (42,714 arbitrary units vs. 28,506) 6 months after the acute infection. The authors reported that there was no association between the persistence of PASC and the baseline amount of NETs, TRIM63, and anticellular antibodies. Nor was there an association between PASC and the titers of anti-SARS-CoV-2 antibodies at baseline and 1 month after COVID-19 diagnosis. Nonetheless, patients with persistent PASC had higher titers of anti-SARS-CoV-2 IgGs 3 months after the onset of COVID-19.
On the basis of previous data, the researchers aimed to construct a preliminary explanatory model to address the clinical and immunologic features associated with persistent PASC 6 months after SARS-CoV-2 infection. In the univariate analysis, the variables associated with the diagnosis of persistent PASC were the serum levels of granulocyte colony-stimulating factor (odds ratio, 1.01), macrophage inflammatory protein-1 alpha (OR, 1.13), interferon gamma-induced protein 10 (OR, 1.00), interleukin-6 (OR, 1.03), the expression of CD57 in CD8+ T cells (OR, 1.00), and the titers of anti-SARS-CoV-2 IgG at 1 month (OR, 1.45).
, such as obesity, greater levels of macrophage inflammatory protein-1 alpha and interferon gamma-induced protein 10 in peripheral blood, greater expression of the senescence CD57 marker in CD8+ T lymphocytes, and persistent symptoms at 3 months.
Using these parameters to construct a predictive model after 3 months, the authors found a sensitivity of 97.7%, specificity of 53.8%, positive predictive value of 93.5%, and a negative predictive value of 77.7% for the diagnosis of clinical aspects associated with PASC at 6 months.
Interpreting CD57
One of the researchers who participated in the study was Luis Martínez-Juárez, MD, MPH, DrPH. He is on the operative solutions team at the Carlos Slim Foundation. Dr. Martínez-Juárez pointed out that one of the contributions of this study was that it specifically examined the Mexican population. He noted that “according to the findings, obesity is not only a comorbidity associated with more severe progressions during acute COVID-19 disease, but also, through inflammation parameters, such as interleukin-6, interferon gamma-induced protein 10, and macrophage inflammatory protein-1 alpha, it’s involved in the development of clinical aspects related to postacute sequelae of COVID-19.”
Dr. Gómez-Martín added that finding proinflammatory and obesity parameters in the patients could potentially support the hypothesis of the persistence of virus fragments in adipose tissue as possibly involved in clinical aspects associated with PASC, as some groups have reported in the medical literature.
Angélica Cuapio, MD, DrMed, an immunologist and senior investigator at the Karolinska Institute, Stockholm, who did not participate in the study, said in an interview that the authors’ findings on the sustained increase of the CD57 marker in CD8+ lymphocytes are of notable interest. They may be associated with senescence states or cellular aging or with a stage of chronic viral infections. Therefore, Dr. Cuapio argued, it would have been valuable to include cellular markers of the innate system, such as natural killer cells, since in various infections, an increase in CD57 in lymphocytes is accompanied by an almost proportional increase of this marker in natural killer cells.
“This information would help to determine more accurately if we are talking about a cellular senescence or more about a chronic infection in persistent COVID-19.” The finding is important, but future research is needed in this developing field.
Dr. Cuapio pointed out that the authors found an interesting elevation in interleukin-1 alpha in patients with clinical aspects associated with PASC in a clinically well-characterized population in Mexico. “It is possible that this is a specific marker either of a specific population or location, or this could be an association with a humoral response. Despite the fact that this finding is new and unclear, it is worth investigating. This study is of great value for the scientific community because it’s one more piece in the complex puzzle of clinical aspects associated with postacute sequelae of COVID-19.”
Dr. Gómez-Martín noted that the main limitations of the study consist of its single-center design and the small patient sample. Dr. Martínez-Juárez added that the study did not consider reinfections. In future studies, it would be ideal to integrate other molecular assessments associated with various hypotheses of the physiopathology of clinical aspects associated with PASC, such as microbiota alteration, coagulation anomalies, endothelial damage, and dysfunctional neurologic signaling.
The study was supported and funded by the Carlos Slim Foundation. Dr. Gómez-Martín, Dr. Martínez-Juárez, and Dr. Cuapio have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Proinflammatory elements mediated through metabolic pathways related to obesity and increased cellular senescence in CD57 expression in CD8+ T cells are associated with postacute sequelae of COVID-19 (PASC), according to a Mexican study. The researchers followed a Mexican cohort of 102 patients 3 months and 6 months after acute SARS-CoV-2 infection.
The study’s principal investigator was Diana Gómez-Martín, MD, PhD, of the department of immunology and rheumatology at the Salvador Zubirán National Institute of Medical Sciences and Nutrition, Mexico City. She told this news organization that follow-up of the patients began with the objective of understanding the determinative clinical, genetic, metabolic, and immunological factors in the progression of the acute disease. However, clinical aspects associated with PASC developed in the selected cohort. As a result, the study was extended, and the clinical, metabolic, and immunologic conditions in this single-center Mexican cohort were evaluated 3 months 6 months after the onset of infection.
Dr. Gómez-Martín explained that the immune senescence in CD57 of CD8+ T cells is one of the best-known findings of the present study. If it is confirmed in future studies, it could have important implications. “Its main implication is the possibility of better understanding the physiopathology of the clinical aspects associated with postacute sequelae of COVID-19, potentially being used for early detection and to provide follow-up aimed at patients, in addition to eventually developing targeted therapeutic strategies, such as immunometabolism regulation, in certain populations.”
Patients with PASC
The study was conducted from August 2020 to August 2021. Investigators recruited 102 patients (median age, 50.5 years; 55% were women) at the Mexico City Temporary Unit with a confirmed diagnosis of SARS-CoV-2. Of the patients, 44% had mild or moderate COVID-19, 30% had severe cases, and 26% of patients had critical cases. The most frequent comorbidities were obesity (44%), hypertension (24%), and type 2 diabetes (24%). The authors used a questionnaire to assess the presence of symptoms during follow-up. They analyzed immunologic variables at the time of recruitment, as well as levels of cytokines, immunoglobulin G against SARS-CoV-2, and neutrophil extracellular traps (NETs) at 1, 3, and 6 months. At 6 months’ follow-up, 12.7% of the cohort had symptoms compatible with PASC, which was defined for the study as the presence and report of three or more symptoms at 6 months’ follow-up.
As in similar studies, the authors found that female gender, remaining in intensive care, and having had more symptoms and greater titers of anti-SARS-CoV-2 antibodies during the acute infection were associated with the development of clinical aspects associated with PASC. Patients who had the disease at 6 months had increased serum levels of interleukin-1 alpha (6.21 pg/mL vs. 2.21 pg/mL), granulocyte colony-stimulating factor (55.08 pg/mL vs. 14.68 pg/mL), and interferon gamma-induced protein 10 (2,309.40 pg/mL vs. 780 pg/mL). Also, there was a trend toward an increase in serum concentration of interleukin-1 beta, interleukin-6, and interferon-gamma.
Patients whose condition met the definition of persistent PASC had increased expression of CD57 in CD8+ T cells (42,714 arbitrary units vs. 28,506) 6 months after the acute infection. The authors reported that there was no association between the persistence of PASC and the baseline amount of NETs, TRIM63, and anticellular antibodies. Nor was there an association between PASC and the titers of anti-SARS-CoV-2 antibodies at baseline and 1 month after COVID-19 diagnosis. Nonetheless, patients with persistent PASC had higher titers of anti-SARS-CoV-2 IgGs 3 months after the onset of COVID-19.
On the basis of previous data, the researchers aimed to construct a preliminary explanatory model to address the clinical and immunologic features associated with persistent PASC 6 months after SARS-CoV-2 infection. In the univariate analysis, the variables associated with the diagnosis of persistent PASC were the serum levels of granulocyte colony-stimulating factor (odds ratio, 1.01), macrophage inflammatory protein-1 alpha (OR, 1.13), interferon gamma-induced protein 10 (OR, 1.00), interleukin-6 (OR, 1.03), the expression of CD57 in CD8+ T cells (OR, 1.00), and the titers of anti-SARS-CoV-2 IgG at 1 month (OR, 1.45).
, such as obesity, greater levels of macrophage inflammatory protein-1 alpha and interferon gamma-induced protein 10 in peripheral blood, greater expression of the senescence CD57 marker in CD8+ T lymphocytes, and persistent symptoms at 3 months.
Using these parameters to construct a predictive model after 3 months, the authors found a sensitivity of 97.7%, specificity of 53.8%, positive predictive value of 93.5%, and a negative predictive value of 77.7% for the diagnosis of clinical aspects associated with PASC at 6 months.
Interpreting CD57
One of the researchers who participated in the study was Luis Martínez-Juárez, MD, MPH, DrPH. He is on the operative solutions team at the Carlos Slim Foundation. Dr. Martínez-Juárez pointed out that one of the contributions of this study was that it specifically examined the Mexican population. He noted that “according to the findings, obesity is not only a comorbidity associated with more severe progressions during acute COVID-19 disease, but also, through inflammation parameters, such as interleukin-6, interferon gamma-induced protein 10, and macrophage inflammatory protein-1 alpha, it’s involved in the development of clinical aspects related to postacute sequelae of COVID-19.”
Dr. Gómez-Martín added that finding proinflammatory and obesity parameters in the patients could potentially support the hypothesis of the persistence of virus fragments in adipose tissue as possibly involved in clinical aspects associated with PASC, as some groups have reported in the medical literature.
Angélica Cuapio, MD, DrMed, an immunologist and senior investigator at the Karolinska Institute, Stockholm, who did not participate in the study, said in an interview that the authors’ findings on the sustained increase of the CD57 marker in CD8+ lymphocytes are of notable interest. They may be associated with senescence states or cellular aging or with a stage of chronic viral infections. Therefore, Dr. Cuapio argued, it would have been valuable to include cellular markers of the innate system, such as natural killer cells, since in various infections, an increase in CD57 in lymphocytes is accompanied by an almost proportional increase of this marker in natural killer cells.
“This information would help to determine more accurately if we are talking about a cellular senescence or more about a chronic infection in persistent COVID-19.” The finding is important, but future research is needed in this developing field.
Dr. Cuapio pointed out that the authors found an interesting elevation in interleukin-1 alpha in patients with clinical aspects associated with PASC in a clinically well-characterized population in Mexico. “It is possible that this is a specific marker either of a specific population or location, or this could be an association with a humoral response. Despite the fact that this finding is new and unclear, it is worth investigating. This study is of great value for the scientific community because it’s one more piece in the complex puzzle of clinical aspects associated with postacute sequelae of COVID-19.”
Dr. Gómez-Martín noted that the main limitations of the study consist of its single-center design and the small patient sample. Dr. Martínez-Juárez added that the study did not consider reinfections. In future studies, it would be ideal to integrate other molecular assessments associated with various hypotheses of the physiopathology of clinical aspects associated with PASC, such as microbiota alteration, coagulation anomalies, endothelial damage, and dysfunctional neurologic signaling.
The study was supported and funded by the Carlos Slim Foundation. Dr. Gómez-Martín, Dr. Martínez-Juárez, and Dr. Cuapio have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Expert dispels myths about hair care in patients with skin of color
HONOLULU –
“This is false,” Dr. Heath, director of pediatric dermatology at Temple University, Philadelphia, said at the Hawaii Dermatology Seminar provided by MedscapeLIVE! With little manipulation, length may be retained, since tightly coiled hair has a higher likelihood of breakage, she said. “But washing the scalp and hair is recommended for tightly coiled hair weekly or every other week. Exclusively co-washing – a technique where hair conditioner is used instead of shampooing – is also not advised due to scalp build-up.”
Other myths she addressed include the following:
“I have a weak spot (or stress spot) on the top of my scalp.” These terms may be used to describe hair on a spot that goes through cycles of breaking off and re-growing. This is false. “If someone were to say that, and we see short hairs on the top of a patient’s scalp, with or without tenderness, pruritus, or pain, we want to recognize that as possibly an early sign of central centrifugal cicatricial alopecia [CCCA],” she said. “We want to pick up cases of CCCA forme fruste [central hair breakage] early.”
Medicated shampoos are helpful for all patients with seborrheic dermatitis. This notion is more complicated. “In theory, medicated shampoos like ketoconazole should be helpful, but if the shampoos are too drying for the hair and they cause further hair breakage, that’s going to be a problem as well,” explained Dr. Heath, who was the senior author of an article on how to address common conditions affecting pediatric and adolescent patients with skin of color. For patients with tightly coiled hair, she recommends applying antifungal shampoos to the scalp only, waiting 5-10 minutes, rinsing, and shampooing the scalp and hair with a moisturizing shampoo and rinsing. They can then condition with a moisturizing conditioner and style their hair as desired.
Don’t touch a Black woman’s hair. That unwritten rule may apply to a woman you pass on the street, she said, but not during clinical exams in cases where clinicians and patients seeking hair loss treatment have different hair types. “Touch the hair; don’t do a lean-in exam,” emphasized Dr. Heath, who is the inaugural faculty scholar at Temple University Lewis Katz School of Medicine’s Office of Health Equity Diversity, and Inclusion. “You want to perform the scalp and hair exam with cultural humility.” Understanding the patient’s hair care goals and perspective allows dermatologists to take a more individualized approach to their concerns, especially in race-discordant patient-physician interactions.
Going natural (chemical-free) will solve scarring hair loss problems. This is false. “Genetic defects in the hair shaft have been described as the cause of some CCCA cases, so we need to stop solely blaming the patient for that condition,” she said. Dr. Heath noted that the transition point between natural hair and relaxed hair is highly prone to breakage. She suggests low or lower tension options such as knotless braids, and crochet hairstyles, and when patients have locs, they should be shoulder length or higher to reduce tension.
Dr. Heath disclosed that she has served as a consultant or adviser for Arcutis, CeraVe, Janssen Pharmaceuticals, Johnson & Johnson Pharmaceutical Research & Development, Leo, Lilly, Pfizer, and Regeneron Pharmaceuticals.
Medscape and this news organization are owned by the same parent company.
HONOLULU –
“This is false,” Dr. Heath, director of pediatric dermatology at Temple University, Philadelphia, said at the Hawaii Dermatology Seminar provided by MedscapeLIVE! With little manipulation, length may be retained, since tightly coiled hair has a higher likelihood of breakage, she said. “But washing the scalp and hair is recommended for tightly coiled hair weekly or every other week. Exclusively co-washing – a technique where hair conditioner is used instead of shampooing – is also not advised due to scalp build-up.”
Other myths she addressed include the following:
“I have a weak spot (or stress spot) on the top of my scalp.” These terms may be used to describe hair on a spot that goes through cycles of breaking off and re-growing. This is false. “If someone were to say that, and we see short hairs on the top of a patient’s scalp, with or without tenderness, pruritus, or pain, we want to recognize that as possibly an early sign of central centrifugal cicatricial alopecia [CCCA],” she said. “We want to pick up cases of CCCA forme fruste [central hair breakage] early.”
Medicated shampoos are helpful for all patients with seborrheic dermatitis. This notion is more complicated. “In theory, medicated shampoos like ketoconazole should be helpful, but if the shampoos are too drying for the hair and they cause further hair breakage, that’s going to be a problem as well,” explained Dr. Heath, who was the senior author of an article on how to address common conditions affecting pediatric and adolescent patients with skin of color. For patients with tightly coiled hair, she recommends applying antifungal shampoos to the scalp only, waiting 5-10 minutes, rinsing, and shampooing the scalp and hair with a moisturizing shampoo and rinsing. They can then condition with a moisturizing conditioner and style their hair as desired.
Don’t touch a Black woman’s hair. That unwritten rule may apply to a woman you pass on the street, she said, but not during clinical exams in cases where clinicians and patients seeking hair loss treatment have different hair types. “Touch the hair; don’t do a lean-in exam,” emphasized Dr. Heath, who is the inaugural faculty scholar at Temple University Lewis Katz School of Medicine’s Office of Health Equity Diversity, and Inclusion. “You want to perform the scalp and hair exam with cultural humility.” Understanding the patient’s hair care goals and perspective allows dermatologists to take a more individualized approach to their concerns, especially in race-discordant patient-physician interactions.
Going natural (chemical-free) will solve scarring hair loss problems. This is false. “Genetic defects in the hair shaft have been described as the cause of some CCCA cases, so we need to stop solely blaming the patient for that condition,” she said. Dr. Heath noted that the transition point between natural hair and relaxed hair is highly prone to breakage. She suggests low or lower tension options such as knotless braids, and crochet hairstyles, and when patients have locs, they should be shoulder length or higher to reduce tension.
Dr. Heath disclosed that she has served as a consultant or adviser for Arcutis, CeraVe, Janssen Pharmaceuticals, Johnson & Johnson Pharmaceutical Research & Development, Leo, Lilly, Pfizer, and Regeneron Pharmaceuticals.
Medscape and this news organization are owned by the same parent company.
HONOLULU –
“This is false,” Dr. Heath, director of pediatric dermatology at Temple University, Philadelphia, said at the Hawaii Dermatology Seminar provided by MedscapeLIVE! With little manipulation, length may be retained, since tightly coiled hair has a higher likelihood of breakage, she said. “But washing the scalp and hair is recommended for tightly coiled hair weekly or every other week. Exclusively co-washing – a technique where hair conditioner is used instead of shampooing – is also not advised due to scalp build-up.”
Other myths she addressed include the following:
“I have a weak spot (or stress spot) on the top of my scalp.” These terms may be used to describe hair on a spot that goes through cycles of breaking off and re-growing. This is false. “If someone were to say that, and we see short hairs on the top of a patient’s scalp, with or without tenderness, pruritus, or pain, we want to recognize that as possibly an early sign of central centrifugal cicatricial alopecia [CCCA],” she said. “We want to pick up cases of CCCA forme fruste [central hair breakage] early.”
Medicated shampoos are helpful for all patients with seborrheic dermatitis. This notion is more complicated. “In theory, medicated shampoos like ketoconazole should be helpful, but if the shampoos are too drying for the hair and they cause further hair breakage, that’s going to be a problem as well,” explained Dr. Heath, who was the senior author of an article on how to address common conditions affecting pediatric and adolescent patients with skin of color. For patients with tightly coiled hair, she recommends applying antifungal shampoos to the scalp only, waiting 5-10 minutes, rinsing, and shampooing the scalp and hair with a moisturizing shampoo and rinsing. They can then condition with a moisturizing conditioner and style their hair as desired.
Don’t touch a Black woman’s hair. That unwritten rule may apply to a woman you pass on the street, she said, but not during clinical exams in cases where clinicians and patients seeking hair loss treatment have different hair types. “Touch the hair; don’t do a lean-in exam,” emphasized Dr. Heath, who is the inaugural faculty scholar at Temple University Lewis Katz School of Medicine’s Office of Health Equity Diversity, and Inclusion. “You want to perform the scalp and hair exam with cultural humility.” Understanding the patient’s hair care goals and perspective allows dermatologists to take a more individualized approach to their concerns, especially in race-discordant patient-physician interactions.
Going natural (chemical-free) will solve scarring hair loss problems. This is false. “Genetic defects in the hair shaft have been described as the cause of some CCCA cases, so we need to stop solely blaming the patient for that condition,” she said. Dr. Heath noted that the transition point between natural hair and relaxed hair is highly prone to breakage. She suggests low or lower tension options such as knotless braids, and crochet hairstyles, and when patients have locs, they should be shoulder length or higher to reduce tension.
Dr. Heath disclosed that she has served as a consultant or adviser for Arcutis, CeraVe, Janssen Pharmaceuticals, Johnson & Johnson Pharmaceutical Research & Development, Leo, Lilly, Pfizer, and Regeneron Pharmaceuticals.
Medscape and this news organization are owned by the same parent company.
AT THE MEDSCAPE LIVE! HAWAII DERMATOLOGY SEMINAR
Commentary: Sorting out useful atopic dermatitis research from filler, March 2023
Another study caught my attention this month for having presented a lot of information with no clinically important conclusions. In "Mode of Delivery and Offspring Atopic Dermatitis in a Swedish Nationwide Study," Mubanga and colleagues studied 1.4 million children! With that many participants, they were almost certain to find associations that were statistically significant and clinically irrelevant. They reported that children born by instrumental vaginal delivery, emergency caesarean section, and elective caesarean section were at a higher risk for AD compared with those born by uncomplicated vaginal delivery. They failed to report the absolute magnitude of the associations, which were undoubtedly so small as to be clinically meaningless. Even if the observed association were not due to some hidden bias, the association is not anything that would change treatment in any way.
On the other hand, the small, open label registry analysis, "Experiences From Daily Practice of Upadacitinib Treatment on Atopic Dermatitis With a Focus on Hand Eczema: Results From the BioDay Registry," published by Kamphuis and colleagues, is of much greater value, reporting the effectiveness and safety of upadacitinib on hand eczema. Not surprisingly, there were large improvements in the investigators' assessments of the dermatitis and in patients' quality of life. This small study is informative about efficacy; it is too small, though, to evaluate how frequently rare severe adverse events occur.
The use of probiotics to safely improve skin disease is such an appealing concept, yet it sounds a lot like hocus-pocus to me. Feíto-Rodríguez and colleagues report in the journal Clinical and Experimental Dermatology that a probiotic mixture of Bifidobacterium lactis, Bifidobacterium longum, and Lactobacillus casei improved atopic dermatitis more than did placebo. The findings are not compelling. Differences were small. Rates of being clear or almost clear weren't reported. We can get atopic dermatitis to clear up in a few days with topical triamcinolone (if we can get patients to use it); so far, the effects of probiotics on the presumed gut-immune system-skin axis seem very much underwhelming.
Another study caught my attention this month for having presented a lot of information with no clinically important conclusions. In "Mode of Delivery and Offspring Atopic Dermatitis in a Swedish Nationwide Study," Mubanga and colleagues studied 1.4 million children! With that many participants, they were almost certain to find associations that were statistically significant and clinically irrelevant. They reported that children born by instrumental vaginal delivery, emergency caesarean section, and elective caesarean section were at a higher risk for AD compared with those born by uncomplicated vaginal delivery. They failed to report the absolute magnitude of the associations, which were undoubtedly so small as to be clinically meaningless. Even if the observed association were not due to some hidden bias, the association is not anything that would change treatment in any way.
On the other hand, the small, open label registry analysis, "Experiences From Daily Practice of Upadacitinib Treatment on Atopic Dermatitis With a Focus on Hand Eczema: Results From the BioDay Registry," published by Kamphuis and colleagues, is of much greater value, reporting the effectiveness and safety of upadacitinib on hand eczema. Not surprisingly, there were large improvements in the investigators' assessments of the dermatitis and in patients' quality of life. This small study is informative about efficacy; it is too small, though, to evaluate how frequently rare severe adverse events occur.
The use of probiotics to safely improve skin disease is such an appealing concept, yet it sounds a lot like hocus-pocus to me. Feíto-Rodríguez and colleagues report in the journal Clinical and Experimental Dermatology that a probiotic mixture of Bifidobacterium lactis, Bifidobacterium longum, and Lactobacillus casei improved atopic dermatitis more than did placebo. The findings are not compelling. Differences were small. Rates of being clear or almost clear weren't reported. We can get atopic dermatitis to clear up in a few days with topical triamcinolone (if we can get patients to use it); so far, the effects of probiotics on the presumed gut-immune system-skin axis seem very much underwhelming.
Another study caught my attention this month for having presented a lot of information with no clinically important conclusions. In "Mode of Delivery and Offspring Atopic Dermatitis in a Swedish Nationwide Study," Mubanga and colleagues studied 1.4 million children! With that many participants, they were almost certain to find associations that were statistically significant and clinically irrelevant. They reported that children born by instrumental vaginal delivery, emergency caesarean section, and elective caesarean section were at a higher risk for AD compared with those born by uncomplicated vaginal delivery. They failed to report the absolute magnitude of the associations, which were undoubtedly so small as to be clinically meaningless. Even if the observed association were not due to some hidden bias, the association is not anything that would change treatment in any way.
On the other hand, the small, open label registry analysis, "Experiences From Daily Practice of Upadacitinib Treatment on Atopic Dermatitis With a Focus on Hand Eczema: Results From the BioDay Registry," published by Kamphuis and colleagues, is of much greater value, reporting the effectiveness and safety of upadacitinib on hand eczema. Not surprisingly, there were large improvements in the investigators' assessments of the dermatitis and in patients' quality of life. This small study is informative about efficacy; it is too small, though, to evaluate how frequently rare severe adverse events occur.
The use of probiotics to safely improve skin disease is such an appealing concept, yet it sounds a lot like hocus-pocus to me. Feíto-Rodríguez and colleagues report in the journal Clinical and Experimental Dermatology that a probiotic mixture of Bifidobacterium lactis, Bifidobacterium longum, and Lactobacillus casei improved atopic dermatitis more than did placebo. The findings are not compelling. Differences were small. Rates of being clear or almost clear weren't reported. We can get atopic dermatitis to clear up in a few days with topical triamcinolone (if we can get patients to use it); so far, the effects of probiotics on the presumed gut-immune system-skin axis seem very much underwhelming.