User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Vision loss may be a risk with PRP facial injections
A systematic review was recently conducted by Wu and colleagues examining the risk of blindness associated with platelet-rich plasma (PRP) injection. In dermatology, PRP is used more commonly now than 5 years ago to promote hair growth with injections on the scalp, as an adjunct to microneedling procedures, and sometimes – in a similar way to facial fillers – to improve volume loss, and skin tone and texture (particularly to the tear trough region).
Total unilateral blindness occurred in all cases. In one of the seven reported cases, the patient experienced recovery of vision after 3 months, but with some residual deficits noted on the ophthalmologist examination. In this case, the patient was evaluated and treated by an ophthalmologist within 3 hours of symptom onset.
In addition, four cases were reported from Venezuela, one from the United States, one from the United Kingdom, and one from Malaysia. Similar to reports of blindness with facial fillers, the most common injection site reported with this adverse effect was the glabella (five cases);
Other reports involved injections of the forehead (two), followed by the nasolabial fold (one), lateral canthus (one), and temporomandibular joint (one). Two of the seven patients received injections at more than one site, resulting in the total number of injections reported (10) being higher than the number of patients.
The risk of blindness is inherent with deep injection into a vessel that anastomoses with the blood supply to the eye. No mention was made as to whether PRP or platelet-rich fibrin was used. Other details are lacking from the original articles as to injection technique and whether or not cannula injection was used. No treatment was attempted in four of seven cases.
As plasma is native to the arteries and dissolves in the blood stream naturally, the mechanism as to why retinal artery occlusion or blindness would occur is not completely clear. One theory is that it is volume related and results from the speed of injection, causing a large rapid bolus that temporarily occludes or compresses an involved vessel.
Another theory is that damage to the vessel results from the injection itself or injection technique, leading to a clotting cascade and clot of the involved vessel with subsequent retrograde flow or blockade of the retinal artery. But if this were the case, we would expect to hear about more cases of clots leading to vascular occlusion or skin necrosis, which does not typically occur or we do not hear about.
Details about proper collection materials and technique or mixing with some other materials are also unknown in these cases, thus leaving the possibility that a more occlusive material may have been injected, as opposed to the fluid-like composition of the typical PRP preparation.With regards to risk with scalp PRP injection, the frontal scalp does receive blood supply from the supratrochlear artery that anastomoses with the angular artery of the face – both of which anastomose with the retinal artery (where occlusion would occur via back flow). The scalp tributaries are small and far enough away from the retina at that point that risk of back flow the to retinal artery should be minimal. Additionally, no reports of vascular occlusion from PRP scalp injection leading to skin necrosis have ever been reported. Of note, this is also not a risk that has been reported with the use of PRP with microneedling procedures, where PRP is placed on top of the skin before, during and after microneedling.
Anything that occludes the blood supply to the eye, whether it be fat, filler, or PRP, has an inherent risk of blindness. As there is no reversal agent or designated treatment for PRP occlusion, care must be taken to minimize risk, including awareness of anatomy and avoidance of injection into high risk areas, and cannula use where appropriate. Gentle, slow, low-volume administration, and when possible, use of a retrograde injection technique, may also be helpful.
Dr. Wesley and Lily Talakoub, MD, are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
A systematic review was recently conducted by Wu and colleagues examining the risk of blindness associated with platelet-rich plasma (PRP) injection. In dermatology, PRP is used more commonly now than 5 years ago to promote hair growth with injections on the scalp, as an adjunct to microneedling procedures, and sometimes – in a similar way to facial fillers – to improve volume loss, and skin tone and texture (particularly to the tear trough region).
Total unilateral blindness occurred in all cases. In one of the seven reported cases, the patient experienced recovery of vision after 3 months, but with some residual deficits noted on the ophthalmologist examination. In this case, the patient was evaluated and treated by an ophthalmologist within 3 hours of symptom onset.
In addition, four cases were reported from Venezuela, one from the United States, one from the United Kingdom, and one from Malaysia. Similar to reports of blindness with facial fillers, the most common injection site reported with this adverse effect was the glabella (five cases);
Other reports involved injections of the forehead (two), followed by the nasolabial fold (one), lateral canthus (one), and temporomandibular joint (one). Two of the seven patients received injections at more than one site, resulting in the total number of injections reported (10) being higher than the number of patients.
The risk of blindness is inherent with deep injection into a vessel that anastomoses with the blood supply to the eye. No mention was made as to whether PRP or platelet-rich fibrin was used. Other details are lacking from the original articles as to injection technique and whether or not cannula injection was used. No treatment was attempted in four of seven cases.
As plasma is native to the arteries and dissolves in the blood stream naturally, the mechanism as to why retinal artery occlusion or blindness would occur is not completely clear. One theory is that it is volume related and results from the speed of injection, causing a large rapid bolus that temporarily occludes or compresses an involved vessel.
Another theory is that damage to the vessel results from the injection itself or injection technique, leading to a clotting cascade and clot of the involved vessel with subsequent retrograde flow or blockade of the retinal artery. But if this were the case, we would expect to hear about more cases of clots leading to vascular occlusion or skin necrosis, which does not typically occur or we do not hear about.
Details about proper collection materials and technique or mixing with some other materials are also unknown in these cases, thus leaving the possibility that a more occlusive material may have been injected, as opposed to the fluid-like composition of the typical PRP preparation.With regards to risk with scalp PRP injection, the frontal scalp does receive blood supply from the supratrochlear artery that anastomoses with the angular artery of the face – both of which anastomose with the retinal artery (where occlusion would occur via back flow). The scalp tributaries are small and far enough away from the retina at that point that risk of back flow the to retinal artery should be minimal. Additionally, no reports of vascular occlusion from PRP scalp injection leading to skin necrosis have ever been reported. Of note, this is also not a risk that has been reported with the use of PRP with microneedling procedures, where PRP is placed on top of the skin before, during and after microneedling.
Anything that occludes the blood supply to the eye, whether it be fat, filler, or PRP, has an inherent risk of blindness. As there is no reversal agent or designated treatment for PRP occlusion, care must be taken to minimize risk, including awareness of anatomy and avoidance of injection into high risk areas, and cannula use where appropriate. Gentle, slow, low-volume administration, and when possible, use of a retrograde injection technique, may also be helpful.
Dr. Wesley and Lily Talakoub, MD, are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
A systematic review was recently conducted by Wu and colleagues examining the risk of blindness associated with platelet-rich plasma (PRP) injection. In dermatology, PRP is used more commonly now than 5 years ago to promote hair growth with injections on the scalp, as an adjunct to microneedling procedures, and sometimes – in a similar way to facial fillers – to improve volume loss, and skin tone and texture (particularly to the tear trough region).
Total unilateral blindness occurred in all cases. In one of the seven reported cases, the patient experienced recovery of vision after 3 months, but with some residual deficits noted on the ophthalmologist examination. In this case, the patient was evaluated and treated by an ophthalmologist within 3 hours of symptom onset.
In addition, four cases were reported from Venezuela, one from the United States, one from the United Kingdom, and one from Malaysia. Similar to reports of blindness with facial fillers, the most common injection site reported with this adverse effect was the glabella (five cases);
Other reports involved injections of the forehead (two), followed by the nasolabial fold (one), lateral canthus (one), and temporomandibular joint (one). Two of the seven patients received injections at more than one site, resulting in the total number of injections reported (10) being higher than the number of patients.
The risk of blindness is inherent with deep injection into a vessel that anastomoses with the blood supply to the eye. No mention was made as to whether PRP or platelet-rich fibrin was used. Other details are lacking from the original articles as to injection technique and whether or not cannula injection was used. No treatment was attempted in four of seven cases.
As plasma is native to the arteries and dissolves in the blood stream naturally, the mechanism as to why retinal artery occlusion or blindness would occur is not completely clear. One theory is that it is volume related and results from the speed of injection, causing a large rapid bolus that temporarily occludes or compresses an involved vessel.
Another theory is that damage to the vessel results from the injection itself or injection technique, leading to a clotting cascade and clot of the involved vessel with subsequent retrograde flow or blockade of the retinal artery. But if this were the case, we would expect to hear about more cases of clots leading to vascular occlusion or skin necrosis, which does not typically occur or we do not hear about.
Details about proper collection materials and technique or mixing with some other materials are also unknown in these cases, thus leaving the possibility that a more occlusive material may have been injected, as opposed to the fluid-like composition of the typical PRP preparation.With regards to risk with scalp PRP injection, the frontal scalp does receive blood supply from the supratrochlear artery that anastomoses with the angular artery of the face – both of which anastomose with the retinal artery (where occlusion would occur via back flow). The scalp tributaries are small and far enough away from the retina at that point that risk of back flow the to retinal artery should be minimal. Additionally, no reports of vascular occlusion from PRP scalp injection leading to skin necrosis have ever been reported. Of note, this is also not a risk that has been reported with the use of PRP with microneedling procedures, where PRP is placed on top of the skin before, during and after microneedling.
Anything that occludes the blood supply to the eye, whether it be fat, filler, or PRP, has an inherent risk of blindness. As there is no reversal agent or designated treatment for PRP occlusion, care must be taken to minimize risk, including awareness of anatomy and avoidance of injection into high risk areas, and cannula use where appropriate. Gentle, slow, low-volume administration, and when possible, use of a retrograde injection technique, may also be helpful.
Dr. Wesley and Lily Talakoub, MD, are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
Atypical Localized Scleroderma Development During Nivolumab Therapy for Metastatic Lung Adenocarcinoma
To the Editor:
Immune checkpoint inhibitors such as anti–programmed cell death protein 1 (anti–PD-1) and anticytotoxic T lymphocyte–associated protein 4 therapies are a promising class of cancer therapeutics. However, they are associated with a variety of immune-related adverse events (irAEs), including cutaneous toxicity.1 The PD-1/programmed death ligand 1 (PD-L1) pathway is important for the maintenance of immune tolerance, and a blockade has been shown to lead to development of various autoimmune diseases.2 We present the case of a patient who developed new-onset localized scleroderma during treatment with the PD-1 inhibitor nivolumab.
A 65-year-old woman presented with a rash on the left thigh that was associated with pruritus, pain, and a pulling sensation. She had a history of stage IV lung adenocarcinoma, with a mass in the right upper lobe with metastatic foci to the left femur, right humerus, right hilar, and pretracheal lymph nodes. She received palliative radiation to the left femur and was started on carboplatin and pemetrexed. Metastasis to the liver was noted after completion of 6 cycles of therapy, and the patient’s treatment was changed to nivolumab. After 17 months on nivolumab therapy (2 years after initial diagnosis and 20 months after radiation therapy), she presented to our dermatology clinic with a cutaneous eruption on the buttocks that spread to the left thigh. The rash failed to improve after 1 month of treatment with emollients and triamcinolone cream 0.1%.
At the current presentation, which was 2 months after she initially presented to our clinic, dermatologic examination revealed erythematous and sclerotic plaques on the left lateral thigh (Figure 1A). Betamethasone cream 0.05% was prescribed, and nivolumab was discontinued due to progression of cutaneous symptoms. A punch biopsy from the left thigh demonstrated superficial dermal sclerosis that was suggestive of chronic radiation dermatitis; direct immunofluorescence testing was negative. The patient was started on prednisone 50 mg daily, which resulted in mild improvement in symptoms.
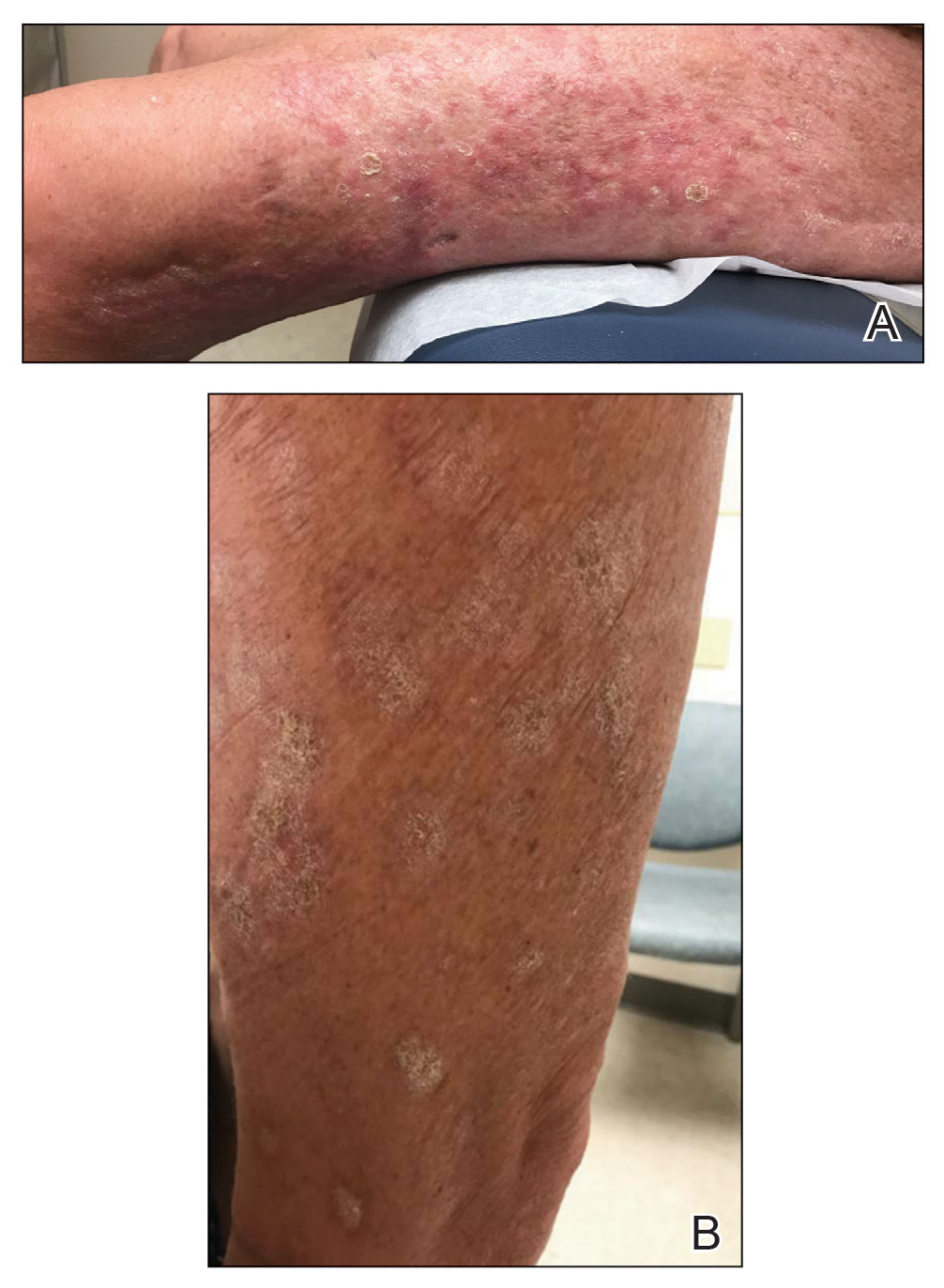
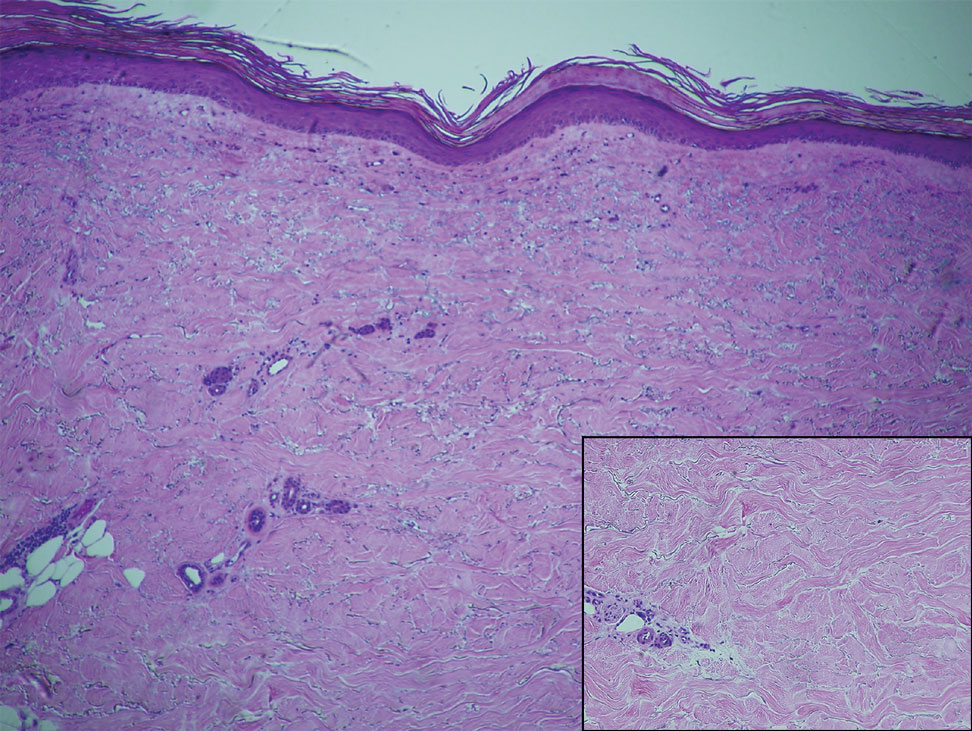
Within 6 months, new sclerotic plaques developed on the patient’s back and right thigh (Figure 1B). Because the lesions were located outside the radiation field of the left femur, a second biopsy was obtained from the right thigh. Histopathology revealed extensive dermal sclerosis and a perivascular lymphoplasmacytic infiltrate (Figure 2). An antinuclear antibody test was weakly positive (1:40, nucleolar pattern) with a negative extractable nuclear antigen panel result. Anti–double-stranded DNA, anti–topoisomerase 1, anti-Smith, antiribonucleoprotein, anti–Sjögren syndrome type A, anti–Sjögren syndrome type B, and anticentromere serology test results were negative. The patient denied decreased oral aperture, difficulty swallowing, or Raynaud phenomenon. Due to the atypical clinical presentation in the setting of PD-1 inhibitor therapy, the etiology of the eruption was potentially attributable to nivolumab. She was started on treatment with methotrexate 20 mg weekly and clobetasol cream 0.05% twice daily; she continued taking prednisone 5 mg daily. The cutaneous manifestations on the patient’s back completely resolved, and the legs continued to gradually improve on this regimen. Immunotherapy continued to be held due to skin toxicity.
Localized scleroderma is an autoimmune disorder characterized by inflammation and skin thickening. Overactive fibroblasts produce excess collagen, leading to the clinical symptoms of skin thickening, hardening, and discoloration.3 Lesions frequently develop on the arms, face, or legs and can present as patches or linear bands. Unlike systemic sclerosis, the internal organs typically are uninvolved; however, sclerotic lesions can be disfiguring and cause notable disability if they impede joint movement.
The PD-1/PD-L1 pathway is a negative regulator of the immune response that inactivates T cells and helps maintain self-tolerance. Modulation of the PD-1/PD-L1 pathway and overexpression of PD-L1 are seen in various cancers as a mechanism to help malignant cells avoid immune destruction.4 Conversely, inhibition of this pathway can be used to stimulate an antitumor immune response. This checkpoint inhibition strategy has been highly successful for the treatment of various cancers including melanoma and non–small cell lung carcinoma. There are several checkpoint inhibitors approved in the United States that are used for cancer therapy and target the PD-1/PD-L1 pathway, such as nivolumab, pembrolizumab, atezolizumab, durvalumab, and avelumab.4 A downside of checkpoint inhibitor treatment is that uncontrolled T-cell activation can lead to irAEs, including cutaneous eruptions, pruritus, diarrhea, colitis, hepatitis, endocrinopathies, pneumonitis, and renal insufficiency.5 These toxicities are reversible if treated appropriately but can cause notable morbidity and mortality if left unrecognized. Cutaneous eruption is one of the most common irAEs associated with anti–PD-1 and anti–PD-L1 therapies and can limit therapeutic efficacy, as the drug may need to be held or discontinued due to the severity of the eruption.6 Mid-potency to high-potency topical corticosteroids and systemic antihistamines are first-line treatments of grades 1 and 2 skin toxicities associated with PD-1 inhibitor therapy. For eruptions classified as grades 3 or 4 or refractory grade 2, discontinuation of the drug and systemic corticosteroids is recommended.7
The cutaneous eruption in immunotherapy-mediated dermatitis is thought to be largely mediated by activated T cells infiltrating the dermis.8 In localized scleroderma, increased tumor necrosis factor α, IFN-γ, IFN-γ–induced protein 10, and granulocyte macrophage colony stimulating factor activity have been shown to correlate with disease activity.9,10 Interestingly, increased tumor necrosis factor α and IFN-γ correlate with better response and increased overall survival in PD-1 inhibition therapy, suggesting a correlation between PD-1 inhibition and T helper activation as noted by the etiology of sclerosis in our patient.11 Additionally, history of radiation was a confounding factor in the diagnosis of our patient, as both sclerodermoid reactions and chronic radiation dermatitis can present with dermal sclerosis. However, the progression of disease outside of the radiation field excluded this etiology. Although new-onset sclerodermoid reactions have been reported with PD-1 inhibitors, they have been described secondary to sclerodermoid reactions from treatment with pembrolizumab.12,13 One case series reported a case of diffuse sclerodermoid reaction and a limited reaction in response to pembrolizumab treatment, while another case report described a relapse of generalized morphea in response to pembrolizumab treatment.12,13 One case of relapsing morphea in response to nivolumab treatment for stage IV lung adenocarcinoma also has been reported.14
Cutaneous toxicities are one of the most common irAEs associated with checkpoint inhibitors and are seen in more than one-third of treated patients. Most frequently, these irAEs manifest as spongiotic dermatitis on histopathology, but a broad spectrum of cutaneous reactions have been observed.15 Although sclerodermoid reactions have been reported with PD-1 inhibitors, most are described secondary to sclerodermoid reactions with pembrolizumab and involve relapse of previously diagnosed morphea rather than new-onset disease.12-14
Our case highlights new-onset localized scleroderma in the setting of nivolumab therapy that showed clinical improvement with methotrexate and topical and systemic steroids. This reaction pattern should be considered in all patients who develop cutaneous eruptions when treated with a PD-1 inhibitor. There should be a high index of suspicion for the potential occurrence of irAEs to ensure early recognition and treatment to minimize morbidity and maximize adherence to therapy for the underlying malignancy.
- Baxi S, Yang A, Gennarelli RL, et al. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: systematic review and meta-analysis. BMJ. 2018;360:k793.
- Dai S, Jia R, Zhang X, et al. The PD-1/PD-Ls pathway and autoimmune diseases. Cell Immunol. 2014;290:72-79.
- Badea I, Taylor M, Rosenberg A, et al. Pathogenesis and therapeutic approaches for improved topical treatment in localized scleroderma and systemic sclerosis. Rheumatology (Oxford). 2009;48:213-221.
- Constantinidou A, Alifieris C, Trafalis DT. Targeting programmed cell death-1 (PD-1) and ligand (PD-L1): a new era in cancer active immunotherapy. Pharmacol Ther. 2019;194:84-106.
- Villadolid J, Asim A. Immune checkpoint inhibitors in clinical practice: update on management of immune-related toxicities. Transl Lung Cancer Res. 2015;4:560-575.
- Naidoo J, Page DB, Li BT, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2016;27:1362.
- O’Kane GM, Labbé C, Doherty MK, et al. Monitoring and management of immune-related adverse events associated with programmed cell death protein-1 axis inhibitors in lung cancer. Oncologist. 2017;22:70-80.
- Shi VJ, Rodic N, Gettinger S, et al. Clinical and histologic features of lichenoid mucocutaneous eruptions due to anti-programmed celldeath 1 and anti-programmed cell death ligand 1 immunotherapy. JAMA Dermatol. 2016;152:1128-1136.
- Torok KS, Kurzinski K, Kelsey C, et al. Peripheral blood cytokine and chemokine profiles in juvenile localized scleroderma: T-helper cell-associated cytokine profiles. Semin Arthritis Rheum. 2015;45:284-293.
- Guo X, Higgs BW, Bay-Jensen AC, et al. Suppression of T cell activation and collagen accumulation by an anti-IFNAR1 mAb, anifrolumab, in adult patients with systemic sclerosis. J Invest Dermatol. 2015;135:2402-2409.
- Boutsikou E, Domvri K, Hardavella G, et al. Tumor necrosis factor, interferon-gamma and interleukins as predictive markers of antiprogrammed cell-death protein-1 treatment in advanced non-small cell lung cancer: a pragmatic approach in clinical practice. Ther Adv Med Oncol. 2018;10:1758835918768238.
- Barbosa NS, Wetter DA, Wieland CN, et al. Scleroderma induced by pembrolizumab: a case series. Mayo Clin Proc. 2017;92:1158-1163.
- Cheng MW, Hisaw LD, Bernet L. Generalized morphea in the setting of pembrolizumab. Int J Dermatol. 2019;58:736-738.
- Alegre-Sánchez A, Fonda-Pascual P, Saceda-Corralo D, et al. Relapse of morphea during nivolumab therapy for lung adenocarcinoma. Actas Dermosifiliogr. 2017;108:69-70.
- Sibaud V. Dermatologic reactions to immune checkpoint inhibitors: skin toxicities and immunotherapy. Am J Clin Dermatol. 2018;19:345-361.
To the Editor:
Immune checkpoint inhibitors such as anti–programmed cell death protein 1 (anti–PD-1) and anticytotoxic T lymphocyte–associated protein 4 therapies are a promising class of cancer therapeutics. However, they are associated with a variety of immune-related adverse events (irAEs), including cutaneous toxicity.1 The PD-1/programmed death ligand 1 (PD-L1) pathway is important for the maintenance of immune tolerance, and a blockade has been shown to lead to development of various autoimmune diseases.2 We present the case of a patient who developed new-onset localized scleroderma during treatment with the PD-1 inhibitor nivolumab.
A 65-year-old woman presented with a rash on the left thigh that was associated with pruritus, pain, and a pulling sensation. She had a history of stage IV lung adenocarcinoma, with a mass in the right upper lobe with metastatic foci to the left femur, right humerus, right hilar, and pretracheal lymph nodes. She received palliative radiation to the left femur and was started on carboplatin and pemetrexed. Metastasis to the liver was noted after completion of 6 cycles of therapy, and the patient’s treatment was changed to nivolumab. After 17 months on nivolumab therapy (2 years after initial diagnosis and 20 months after radiation therapy), she presented to our dermatology clinic with a cutaneous eruption on the buttocks that spread to the left thigh. The rash failed to improve after 1 month of treatment with emollients and triamcinolone cream 0.1%.
At the current presentation, which was 2 months after she initially presented to our clinic, dermatologic examination revealed erythematous and sclerotic plaques on the left lateral thigh (Figure 1A). Betamethasone cream 0.05% was prescribed, and nivolumab was discontinued due to progression of cutaneous symptoms. A punch biopsy from the left thigh demonstrated superficial dermal sclerosis that was suggestive of chronic radiation dermatitis; direct immunofluorescence testing was negative. The patient was started on prednisone 50 mg daily, which resulted in mild improvement in symptoms.

Within 6 months, new sclerotic plaques developed on the patient’s back and right thigh (Figure 1B). Because the lesions were located outside the radiation field of the left femur, a second biopsy was obtained from the right thigh. Histopathology revealed extensive dermal sclerosis and a perivascular lymphoplasmacytic infiltrate (Figure 2). An antinuclear antibody test was weakly positive (1:40, nucleolar pattern) with a negative extractable nuclear antigen panel result. Anti–double-stranded DNA, anti–topoisomerase 1, anti-Smith, antiribonucleoprotein, anti–Sjögren syndrome type A, anti–Sjögren syndrome type B, and anticentromere serology test results were negative. The patient denied decreased oral aperture, difficulty swallowing, or Raynaud phenomenon. Due to the atypical clinical presentation in the setting of PD-1 inhibitor therapy, the etiology of the eruption was potentially attributable to nivolumab. She was started on treatment with methotrexate 20 mg weekly and clobetasol cream 0.05% twice daily; she continued taking prednisone 5 mg daily. The cutaneous manifestations on the patient’s back completely resolved, and the legs continued to gradually improve on this regimen. Immunotherapy continued to be held due to skin toxicity.

Localized scleroderma is an autoimmune disorder characterized by inflammation and skin thickening. Overactive fibroblasts produce excess collagen, leading to the clinical symptoms of skin thickening, hardening, and discoloration.3 Lesions frequently develop on the arms, face, or legs and can present as patches or linear bands. Unlike systemic sclerosis, the internal organs typically are uninvolved; however, sclerotic lesions can be disfiguring and cause notable disability if they impede joint movement.
The PD-1/PD-L1 pathway is a negative regulator of the immune response that inactivates T cells and helps maintain self-tolerance. Modulation of the PD-1/PD-L1 pathway and overexpression of PD-L1 are seen in various cancers as a mechanism to help malignant cells avoid immune destruction.4 Conversely, inhibition of this pathway can be used to stimulate an antitumor immune response. This checkpoint inhibition strategy has been highly successful for the treatment of various cancers including melanoma and non–small cell lung carcinoma. There are several checkpoint inhibitors approved in the United States that are used for cancer therapy and target the PD-1/PD-L1 pathway, such as nivolumab, pembrolizumab, atezolizumab, durvalumab, and avelumab.4 A downside of checkpoint inhibitor treatment is that uncontrolled T-cell activation can lead to irAEs, including cutaneous eruptions, pruritus, diarrhea, colitis, hepatitis, endocrinopathies, pneumonitis, and renal insufficiency.5 These toxicities are reversible if treated appropriately but can cause notable morbidity and mortality if left unrecognized. Cutaneous eruption is one of the most common irAEs associated with anti–PD-1 and anti–PD-L1 therapies and can limit therapeutic efficacy, as the drug may need to be held or discontinued due to the severity of the eruption.6 Mid-potency to high-potency topical corticosteroids and systemic antihistamines are first-line treatments of grades 1 and 2 skin toxicities associated with PD-1 inhibitor therapy. For eruptions classified as grades 3 or 4 or refractory grade 2, discontinuation of the drug and systemic corticosteroids is recommended.7
The cutaneous eruption in immunotherapy-mediated dermatitis is thought to be largely mediated by activated T cells infiltrating the dermis.8 In localized scleroderma, increased tumor necrosis factor α, IFN-γ, IFN-γ–induced protein 10, and granulocyte macrophage colony stimulating factor activity have been shown to correlate with disease activity.9,10 Interestingly, increased tumor necrosis factor α and IFN-γ correlate with better response and increased overall survival in PD-1 inhibition therapy, suggesting a correlation between PD-1 inhibition and T helper activation as noted by the etiology of sclerosis in our patient.11 Additionally, history of radiation was a confounding factor in the diagnosis of our patient, as both sclerodermoid reactions and chronic radiation dermatitis can present with dermal sclerosis. However, the progression of disease outside of the radiation field excluded this etiology. Although new-onset sclerodermoid reactions have been reported with PD-1 inhibitors, they have been described secondary to sclerodermoid reactions from treatment with pembrolizumab.12,13 One case series reported a case of diffuse sclerodermoid reaction and a limited reaction in response to pembrolizumab treatment, while another case report described a relapse of generalized morphea in response to pembrolizumab treatment.12,13 One case of relapsing morphea in response to nivolumab treatment for stage IV lung adenocarcinoma also has been reported.14
Cutaneous toxicities are one of the most common irAEs associated with checkpoint inhibitors and are seen in more than one-third of treated patients. Most frequently, these irAEs manifest as spongiotic dermatitis on histopathology, but a broad spectrum of cutaneous reactions have been observed.15 Although sclerodermoid reactions have been reported with PD-1 inhibitors, most are described secondary to sclerodermoid reactions with pembrolizumab and involve relapse of previously diagnosed morphea rather than new-onset disease.12-14
Our case highlights new-onset localized scleroderma in the setting of nivolumab therapy that showed clinical improvement with methotrexate and topical and systemic steroids. This reaction pattern should be considered in all patients who develop cutaneous eruptions when treated with a PD-1 inhibitor. There should be a high index of suspicion for the potential occurrence of irAEs to ensure early recognition and treatment to minimize morbidity and maximize adherence to therapy for the underlying malignancy.
To the Editor:
Immune checkpoint inhibitors such as anti–programmed cell death protein 1 (anti–PD-1) and anticytotoxic T lymphocyte–associated protein 4 therapies are a promising class of cancer therapeutics. However, they are associated with a variety of immune-related adverse events (irAEs), including cutaneous toxicity.1 The PD-1/programmed death ligand 1 (PD-L1) pathway is important for the maintenance of immune tolerance, and a blockade has been shown to lead to development of various autoimmune diseases.2 We present the case of a patient who developed new-onset localized scleroderma during treatment with the PD-1 inhibitor nivolumab.
A 65-year-old woman presented with a rash on the left thigh that was associated with pruritus, pain, and a pulling sensation. She had a history of stage IV lung adenocarcinoma, with a mass in the right upper lobe with metastatic foci to the left femur, right humerus, right hilar, and pretracheal lymph nodes. She received palliative radiation to the left femur and was started on carboplatin and pemetrexed. Metastasis to the liver was noted after completion of 6 cycles of therapy, and the patient’s treatment was changed to nivolumab. After 17 months on nivolumab therapy (2 years after initial diagnosis and 20 months after radiation therapy), she presented to our dermatology clinic with a cutaneous eruption on the buttocks that spread to the left thigh. The rash failed to improve after 1 month of treatment with emollients and triamcinolone cream 0.1%.
At the current presentation, which was 2 months after she initially presented to our clinic, dermatologic examination revealed erythematous and sclerotic plaques on the left lateral thigh (Figure 1A). Betamethasone cream 0.05% was prescribed, and nivolumab was discontinued due to progression of cutaneous symptoms. A punch biopsy from the left thigh demonstrated superficial dermal sclerosis that was suggestive of chronic radiation dermatitis; direct immunofluorescence testing was negative. The patient was started on prednisone 50 mg daily, which resulted in mild improvement in symptoms.

Within 6 months, new sclerotic plaques developed on the patient’s back and right thigh (Figure 1B). Because the lesions were located outside the radiation field of the left femur, a second biopsy was obtained from the right thigh. Histopathology revealed extensive dermal sclerosis and a perivascular lymphoplasmacytic infiltrate (Figure 2). An antinuclear antibody test was weakly positive (1:40, nucleolar pattern) with a negative extractable nuclear antigen panel result. Anti–double-stranded DNA, anti–topoisomerase 1, anti-Smith, antiribonucleoprotein, anti–Sjögren syndrome type A, anti–Sjögren syndrome type B, and anticentromere serology test results were negative. The patient denied decreased oral aperture, difficulty swallowing, or Raynaud phenomenon. Due to the atypical clinical presentation in the setting of PD-1 inhibitor therapy, the etiology of the eruption was potentially attributable to nivolumab. She was started on treatment with methotrexate 20 mg weekly and clobetasol cream 0.05% twice daily; she continued taking prednisone 5 mg daily. The cutaneous manifestations on the patient’s back completely resolved, and the legs continued to gradually improve on this regimen. Immunotherapy continued to be held due to skin toxicity.

Localized scleroderma is an autoimmune disorder characterized by inflammation and skin thickening. Overactive fibroblasts produce excess collagen, leading to the clinical symptoms of skin thickening, hardening, and discoloration.3 Lesions frequently develop on the arms, face, or legs and can present as patches or linear bands. Unlike systemic sclerosis, the internal organs typically are uninvolved; however, sclerotic lesions can be disfiguring and cause notable disability if they impede joint movement.
The PD-1/PD-L1 pathway is a negative regulator of the immune response that inactivates T cells and helps maintain self-tolerance. Modulation of the PD-1/PD-L1 pathway and overexpression of PD-L1 are seen in various cancers as a mechanism to help malignant cells avoid immune destruction.4 Conversely, inhibition of this pathway can be used to stimulate an antitumor immune response. This checkpoint inhibition strategy has been highly successful for the treatment of various cancers including melanoma and non–small cell lung carcinoma. There are several checkpoint inhibitors approved in the United States that are used for cancer therapy and target the PD-1/PD-L1 pathway, such as nivolumab, pembrolizumab, atezolizumab, durvalumab, and avelumab.4 A downside of checkpoint inhibitor treatment is that uncontrolled T-cell activation can lead to irAEs, including cutaneous eruptions, pruritus, diarrhea, colitis, hepatitis, endocrinopathies, pneumonitis, and renal insufficiency.5 These toxicities are reversible if treated appropriately but can cause notable morbidity and mortality if left unrecognized. Cutaneous eruption is one of the most common irAEs associated with anti–PD-1 and anti–PD-L1 therapies and can limit therapeutic efficacy, as the drug may need to be held or discontinued due to the severity of the eruption.6 Mid-potency to high-potency topical corticosteroids and systemic antihistamines are first-line treatments of grades 1 and 2 skin toxicities associated with PD-1 inhibitor therapy. For eruptions classified as grades 3 or 4 or refractory grade 2, discontinuation of the drug and systemic corticosteroids is recommended.7
The cutaneous eruption in immunotherapy-mediated dermatitis is thought to be largely mediated by activated T cells infiltrating the dermis.8 In localized scleroderma, increased tumor necrosis factor α, IFN-γ, IFN-γ–induced protein 10, and granulocyte macrophage colony stimulating factor activity have been shown to correlate with disease activity.9,10 Interestingly, increased tumor necrosis factor α and IFN-γ correlate with better response and increased overall survival in PD-1 inhibition therapy, suggesting a correlation between PD-1 inhibition and T helper activation as noted by the etiology of sclerosis in our patient.11 Additionally, history of radiation was a confounding factor in the diagnosis of our patient, as both sclerodermoid reactions and chronic radiation dermatitis can present with dermal sclerosis. However, the progression of disease outside of the radiation field excluded this etiology. Although new-onset sclerodermoid reactions have been reported with PD-1 inhibitors, they have been described secondary to sclerodermoid reactions from treatment with pembrolizumab.12,13 One case series reported a case of diffuse sclerodermoid reaction and a limited reaction in response to pembrolizumab treatment, while another case report described a relapse of generalized morphea in response to pembrolizumab treatment.12,13 One case of relapsing morphea in response to nivolumab treatment for stage IV lung adenocarcinoma also has been reported.14
Cutaneous toxicities are one of the most common irAEs associated with checkpoint inhibitors and are seen in more than one-third of treated patients. Most frequently, these irAEs manifest as spongiotic dermatitis on histopathology, but a broad spectrum of cutaneous reactions have been observed.15 Although sclerodermoid reactions have been reported with PD-1 inhibitors, most are described secondary to sclerodermoid reactions with pembrolizumab and involve relapse of previously diagnosed morphea rather than new-onset disease.12-14
Our case highlights new-onset localized scleroderma in the setting of nivolumab therapy that showed clinical improvement with methotrexate and topical and systemic steroids. This reaction pattern should be considered in all patients who develop cutaneous eruptions when treated with a PD-1 inhibitor. There should be a high index of suspicion for the potential occurrence of irAEs to ensure early recognition and treatment to minimize morbidity and maximize adherence to therapy for the underlying malignancy.
- Baxi S, Yang A, Gennarelli RL, et al. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: systematic review and meta-analysis. BMJ. 2018;360:k793.
- Dai S, Jia R, Zhang X, et al. The PD-1/PD-Ls pathway and autoimmune diseases. Cell Immunol. 2014;290:72-79.
- Badea I, Taylor M, Rosenberg A, et al. Pathogenesis and therapeutic approaches for improved topical treatment in localized scleroderma and systemic sclerosis. Rheumatology (Oxford). 2009;48:213-221.
- Constantinidou A, Alifieris C, Trafalis DT. Targeting programmed cell death-1 (PD-1) and ligand (PD-L1): a new era in cancer active immunotherapy. Pharmacol Ther. 2019;194:84-106.
- Villadolid J, Asim A. Immune checkpoint inhibitors in clinical practice: update on management of immune-related toxicities. Transl Lung Cancer Res. 2015;4:560-575.
- Naidoo J, Page DB, Li BT, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2016;27:1362.
- O’Kane GM, Labbé C, Doherty MK, et al. Monitoring and management of immune-related adverse events associated with programmed cell death protein-1 axis inhibitors in lung cancer. Oncologist. 2017;22:70-80.
- Shi VJ, Rodic N, Gettinger S, et al. Clinical and histologic features of lichenoid mucocutaneous eruptions due to anti-programmed celldeath 1 and anti-programmed cell death ligand 1 immunotherapy. JAMA Dermatol. 2016;152:1128-1136.
- Torok KS, Kurzinski K, Kelsey C, et al. Peripheral blood cytokine and chemokine profiles in juvenile localized scleroderma: T-helper cell-associated cytokine profiles. Semin Arthritis Rheum. 2015;45:284-293.
- Guo X, Higgs BW, Bay-Jensen AC, et al. Suppression of T cell activation and collagen accumulation by an anti-IFNAR1 mAb, anifrolumab, in adult patients with systemic sclerosis. J Invest Dermatol. 2015;135:2402-2409.
- Boutsikou E, Domvri K, Hardavella G, et al. Tumor necrosis factor, interferon-gamma and interleukins as predictive markers of antiprogrammed cell-death protein-1 treatment in advanced non-small cell lung cancer: a pragmatic approach in clinical practice. Ther Adv Med Oncol. 2018;10:1758835918768238.
- Barbosa NS, Wetter DA, Wieland CN, et al. Scleroderma induced by pembrolizumab: a case series. Mayo Clin Proc. 2017;92:1158-1163.
- Cheng MW, Hisaw LD, Bernet L. Generalized morphea in the setting of pembrolizumab. Int J Dermatol. 2019;58:736-738.
- Alegre-Sánchez A, Fonda-Pascual P, Saceda-Corralo D, et al. Relapse of morphea during nivolumab therapy for lung adenocarcinoma. Actas Dermosifiliogr. 2017;108:69-70.
- Sibaud V. Dermatologic reactions to immune checkpoint inhibitors: skin toxicities and immunotherapy. Am J Clin Dermatol. 2018;19:345-361.
- Baxi S, Yang A, Gennarelli RL, et al. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: systematic review and meta-analysis. BMJ. 2018;360:k793.
- Dai S, Jia R, Zhang X, et al. The PD-1/PD-Ls pathway and autoimmune diseases. Cell Immunol. 2014;290:72-79.
- Badea I, Taylor M, Rosenberg A, et al. Pathogenesis and therapeutic approaches for improved topical treatment in localized scleroderma and systemic sclerosis. Rheumatology (Oxford). 2009;48:213-221.
- Constantinidou A, Alifieris C, Trafalis DT. Targeting programmed cell death-1 (PD-1) and ligand (PD-L1): a new era in cancer active immunotherapy. Pharmacol Ther. 2019;194:84-106.
- Villadolid J, Asim A. Immune checkpoint inhibitors in clinical practice: update on management of immune-related toxicities. Transl Lung Cancer Res. 2015;4:560-575.
- Naidoo J, Page DB, Li BT, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2016;27:1362.
- O’Kane GM, Labbé C, Doherty MK, et al. Monitoring and management of immune-related adverse events associated with programmed cell death protein-1 axis inhibitors in lung cancer. Oncologist. 2017;22:70-80.
- Shi VJ, Rodic N, Gettinger S, et al. Clinical and histologic features of lichenoid mucocutaneous eruptions due to anti-programmed celldeath 1 and anti-programmed cell death ligand 1 immunotherapy. JAMA Dermatol. 2016;152:1128-1136.
- Torok KS, Kurzinski K, Kelsey C, et al. Peripheral blood cytokine and chemokine profiles in juvenile localized scleroderma: T-helper cell-associated cytokine profiles. Semin Arthritis Rheum. 2015;45:284-293.
- Guo X, Higgs BW, Bay-Jensen AC, et al. Suppression of T cell activation and collagen accumulation by an anti-IFNAR1 mAb, anifrolumab, in adult patients with systemic sclerosis. J Invest Dermatol. 2015;135:2402-2409.
- Boutsikou E, Domvri K, Hardavella G, et al. Tumor necrosis factor, interferon-gamma and interleukins as predictive markers of antiprogrammed cell-death protein-1 treatment in advanced non-small cell lung cancer: a pragmatic approach in clinical practice. Ther Adv Med Oncol. 2018;10:1758835918768238.
- Barbosa NS, Wetter DA, Wieland CN, et al. Scleroderma induced by pembrolizumab: a case series. Mayo Clin Proc. 2017;92:1158-1163.
- Cheng MW, Hisaw LD, Bernet L. Generalized morphea in the setting of pembrolizumab. Int J Dermatol. 2019;58:736-738.
- Alegre-Sánchez A, Fonda-Pascual P, Saceda-Corralo D, et al. Relapse of morphea during nivolumab therapy for lung adenocarcinoma. Actas Dermosifiliogr. 2017;108:69-70.
- Sibaud V. Dermatologic reactions to immune checkpoint inhibitors: skin toxicities and immunotherapy. Am J Clin Dermatol. 2018;19:345-361.
Practice Points
- Immune checkpoint inhibitors such as nivolumab, a programmed cell death protein 1 (PD-1) inhibitor, are associated with immune-related adverse events (irAEs) such as skin toxicity.
- Scleroderma should be considered in the differential diagnosis of patients who develop cutaneous eruptions during treatment with PD-1 inhibitors.
- To ensure prompt recognition and treatment, health care providers should maintain a high index of suspicion for development of cutaneous irAEs in patients using checkpoint inhibitors.
Transitioning From an Intern to a Dermatology Resident
The transition from medical school to residency is a rewarding milestone but involves a steep learning curve wrought with new responsibilities, new colleagues, and a new schedule, often all within a new setting. This transition period has been a longstanding focus of graduate medical education research, and a recent study identified 6 key areas that residency programs need to address to better facilitate this transition: (1) a sense of community within the residency program, (2) relocation resources, (3) residency preparation courses in medical school, (4) readiness to address racism and bias, (5) connecting with peers, and (6) open communication with program leadership.1 There is considerable interest in ensuring that this transition is smooth for all graduates, as nearly all US medical schools feature some variety of a residency preparation course during the fourth year of medical school, which, alongside the subinternships, serves to better prepare their graduates for the healthcare workforce.2
What about the transition from intern to dermatology resident? Near the end of intern year, my categorical medicine colleagues experienced a crescendo of responsibilities, all in preparation for junior year. The senior medicine residents, themselves having previously experienced the graduated responsibilities, knew to ease their grip on the reins and provide the late spring interns an opportunity to lead rounds or run a code. This was not the case for the preliminary interns for whom there was no preview available for what was to come; little guidance exists on how to best transform from a preliminary or transitional postgraduate year (PGY) 1 to a dermatology PGY-2. A survey of 44 dermatology residents and 33 dermatology program directors found electives such as rheumatology, infectious diseases, and allergy and immunology to be helpful for this transition, and residents most often cited friendly and supportive senior and fellow residents as the factor that eased their transition to PGY-2.3 Notably, less than half of the residents (40%) surveyed stated that team-building exercises and dedicated time to meet colleagues were helpful for this transition. They identified studying principles of dermatologic disease, learning new clinical duties, and adjusting to new coworkers and supervisors as the greatest work-related stressors during entry to PGY-2.3
My transition from intern year to dermatology was shrouded in uncertainty, and I was fortunate to have supportive seniors and co-residents to ease the process. There is much about starting dermatology residency that cannot be prepared for by reading a book, and a natural metamorphosis into the new role is hard to articulate. Still, the following are pieces of information I wish I knew as a graduating intern, which I hope will prove useful for those graduating to their PGY-2 dermatology year.
The Pace of Outpatient Dermatology
If the preliminary or transitional year did not have an ambulatory component, the switch from wards to clinic can be jarring. An outpatient encounter can be as short as 10 to 15 minutes, necessitating an efficient interview and examination to avoid a backup of patients. Unlike a hospital admission where the history of present illness can expound on multiple concerns and organ systems, the general dermatology visit must focus on the chief concern, with priority given to the clinical examination of the skin. For total-body skin examinations, a formulaic approach to assessing all areas of the body, with fluent transitions and minimal repositioning of the patient, is critical for patient comfort and to save time. Of course, accuracy and thoroughness are paramount, but the constant mindfulness of time and efficiency is uniquely emphasized in the outpatient setting.
Continuity of Care
On the wards, patients are admitted with an acute problem and discharged with the aim to prevent re-admission. However, in the dermatology clinic, the conditions encountered often are chronic, requiring repeated follow-ups that involve dosage tapers, laboratory monitoring, and trial and error. Unlike the rigid algorithm-based treatments utilized in the inpatient setting, the management of the same chronic disease can vary, as it is tailored to the patient based on their comorbidities and response. This longitudinal relationship with patients, whereby many disorders are managed rather than treated, stands in stark contrast to inpatient medicine, and learning to value symptom management rather than focusing on a cure is critical in a largely outpatient specialty such as dermatology.
Consulter to Consultant
Calling a consultation as an intern is challenging and requires succinct delivery of pertinent information while fearing pushback from the consultant. In a survey of 50 hospitalist attendings, only 11% responded that interns could be entrusted to call an effective consultation without supervision.4 When undertaking the role of a consultant, the goals should be to identify the team’s main question and to obtain key information necessary to formulate a differential diagnosis. The quality of the consultation will inevitably fluctuate; try to remember what it was like for you as a member of the primary team and remain patient and courteous during the exchange.5 In 1983, Goldman et al6 published a guideline on effective consultations that often is cited to this day, dubbed the “Ten Commandments for Effective Consultations,” which consists of the following: (1) determine the question that is being asked, (2) establish the urgency of the consultation, (3) gather primary data, (4) communicate as briefly as appropriate, (5) make specific recommendations, (6) provide contingency plans, (7) understand your own role in the process, (8) offer educational information, (9) communicate recommendations directly to the requesting physician, and (10) provide appropriate follow-up.
Consider Your Future
Frequently reflect on what you most enjoy about your job. Although it can be easy to passively engage with intern year as a mere stepping-stone to dermatology residency, the years in PGY-2 and onward require active introspection to find a future niche. What made you gravitate to the specialty of dermatology? Try to identify your predilections for dermatopathology, pediatric dermatology, dermatologic surgery, cosmetic dermatology, and academia. Be consistently cognizant of your life after residency, as some fellowships such as dermatopathology require applications to be submitted at the conclusion of the PGY-2 year. Seek out faculty mentors or alumni who are walking a path similar to the one you want to embark on, as the next stop after graduation may be your forever job.
Depth, Not Breadth
The practice of medicine changes when narrowing the focus to one organ system. In both medical school and intern year, my study habits and history-taking of patients cast a wide net across multiple organ systems, aiming to know just enough about any one specialty to address all chief concerns and to know when it was appropriate to consult a specialist. This paradigm inevitably shifts in dermatology residency, as residents are tasked with memorizing the endless number of diagnoses of the skin alone, comprehending the many shades of “erythematous,” including pink, salmon, red, and purple. Both on the wards and in clinics, I had to grow comfortable with telling patients that I did not have an answer for many of their nondermatologic concerns and directing them to the right specialist. As medicine continues trending to specialization, subspecialization, and sub-subspecialization, the scope of any given physician likely will continue to narrow,7 as evidenced by specialty clinics within dermatology such as those focusing on hair loss or immunobullous disease. In this health care system, it is imperative to remember that you are only one physician within a team of care providers—understand your own role in the process and become comfortable with not having the answer to all the questions.
Final Thoughts
In a study of 44 dermatology residents, 35 (83%) indicated zero to less than 1 hour per week of independent preparation for dermatology residency during PGY-1.3 Although the usefulness of preparing is debatable, this figure likely reflects the absence of any insight on how to best prepare for the transition. Recognizing the many contrasts between internal medicine and dermatology and embracing the changes will enable a seamless promotion from a medicine PGY-1 to a dermatology PGY-2.
- Staples H, Frank S, Mullen M, et al. Improving the medical school to residency transition: narrative experiences from first-year residents.J Surg Educ. 2022;S1931-7204(22)00146-5. doi:10.1016/j.jsurg.2022.06.001
- Heidemann LA, Walford E, Mack J, et al. Is there a role for internal medicine residency preparation courses in the fourth year curriculum? a single-center experience. J Gen Intern Med. 2018;33:2048-2050.
- Hopkins C, Jalali O, Guffey D, et al. A survey of dermatology residents and program directors assessing the transition to dermatology residency. Proc (Bayl Univ Med Cent). 2020;34:59-62.
- Marcus CH, Winn AS, Sectish TC, et al. How much supervision is required is the beginning of intern year? Acad Pediatr. 2016;16:E3-E4.
- Bly RA, Bly EG. Consult courtesy. J Grad Med Educ. 2013;5:533-534.
- Goldman L, Lee T, Rudd P. Ten commandments for effective consultations. Arch Intern Med. 1983;143:1753-1755.
- Oren O, Gersh BJ, Bhatt DL. On the pearls and perils of sub-subspecialization. Am J Med. 2020;133:158-159.
The transition from medical school to residency is a rewarding milestone but involves a steep learning curve wrought with new responsibilities, new colleagues, and a new schedule, often all within a new setting. This transition period has been a longstanding focus of graduate medical education research, and a recent study identified 6 key areas that residency programs need to address to better facilitate this transition: (1) a sense of community within the residency program, (2) relocation resources, (3) residency preparation courses in medical school, (4) readiness to address racism and bias, (5) connecting with peers, and (6) open communication with program leadership.1 There is considerable interest in ensuring that this transition is smooth for all graduates, as nearly all US medical schools feature some variety of a residency preparation course during the fourth year of medical school, which, alongside the subinternships, serves to better prepare their graduates for the healthcare workforce.2
What about the transition from intern to dermatology resident? Near the end of intern year, my categorical medicine colleagues experienced a crescendo of responsibilities, all in preparation for junior year. The senior medicine residents, themselves having previously experienced the graduated responsibilities, knew to ease their grip on the reins and provide the late spring interns an opportunity to lead rounds or run a code. This was not the case for the preliminary interns for whom there was no preview available for what was to come; little guidance exists on how to best transform from a preliminary or transitional postgraduate year (PGY) 1 to a dermatology PGY-2. A survey of 44 dermatology residents and 33 dermatology program directors found electives such as rheumatology, infectious diseases, and allergy and immunology to be helpful for this transition, and residents most often cited friendly and supportive senior and fellow residents as the factor that eased their transition to PGY-2.3 Notably, less than half of the residents (40%) surveyed stated that team-building exercises and dedicated time to meet colleagues were helpful for this transition. They identified studying principles of dermatologic disease, learning new clinical duties, and adjusting to new coworkers and supervisors as the greatest work-related stressors during entry to PGY-2.3
My transition from intern year to dermatology was shrouded in uncertainty, and I was fortunate to have supportive seniors and co-residents to ease the process. There is much about starting dermatology residency that cannot be prepared for by reading a book, and a natural metamorphosis into the new role is hard to articulate. Still, the following are pieces of information I wish I knew as a graduating intern, which I hope will prove useful for those graduating to their PGY-2 dermatology year.
The Pace of Outpatient Dermatology
If the preliminary or transitional year did not have an ambulatory component, the switch from wards to clinic can be jarring. An outpatient encounter can be as short as 10 to 15 minutes, necessitating an efficient interview and examination to avoid a backup of patients. Unlike a hospital admission where the history of present illness can expound on multiple concerns and organ systems, the general dermatology visit must focus on the chief concern, with priority given to the clinical examination of the skin. For total-body skin examinations, a formulaic approach to assessing all areas of the body, with fluent transitions and minimal repositioning of the patient, is critical for patient comfort and to save time. Of course, accuracy and thoroughness are paramount, but the constant mindfulness of time and efficiency is uniquely emphasized in the outpatient setting.
Continuity of Care
On the wards, patients are admitted with an acute problem and discharged with the aim to prevent re-admission. However, in the dermatology clinic, the conditions encountered often are chronic, requiring repeated follow-ups that involve dosage tapers, laboratory monitoring, and trial and error. Unlike the rigid algorithm-based treatments utilized in the inpatient setting, the management of the same chronic disease can vary, as it is tailored to the patient based on their comorbidities and response. This longitudinal relationship with patients, whereby many disorders are managed rather than treated, stands in stark contrast to inpatient medicine, and learning to value symptom management rather than focusing on a cure is critical in a largely outpatient specialty such as dermatology.
Consulter to Consultant
Calling a consultation as an intern is challenging and requires succinct delivery of pertinent information while fearing pushback from the consultant. In a survey of 50 hospitalist attendings, only 11% responded that interns could be entrusted to call an effective consultation without supervision.4 When undertaking the role of a consultant, the goals should be to identify the team’s main question and to obtain key information necessary to formulate a differential diagnosis. The quality of the consultation will inevitably fluctuate; try to remember what it was like for you as a member of the primary team and remain patient and courteous during the exchange.5 In 1983, Goldman et al6 published a guideline on effective consultations that often is cited to this day, dubbed the “Ten Commandments for Effective Consultations,” which consists of the following: (1) determine the question that is being asked, (2) establish the urgency of the consultation, (3) gather primary data, (4) communicate as briefly as appropriate, (5) make specific recommendations, (6) provide contingency plans, (7) understand your own role in the process, (8) offer educational information, (9) communicate recommendations directly to the requesting physician, and (10) provide appropriate follow-up.
Consider Your Future
Frequently reflect on what you most enjoy about your job. Although it can be easy to passively engage with intern year as a mere stepping-stone to dermatology residency, the years in PGY-2 and onward require active introspection to find a future niche. What made you gravitate to the specialty of dermatology? Try to identify your predilections for dermatopathology, pediatric dermatology, dermatologic surgery, cosmetic dermatology, and academia. Be consistently cognizant of your life after residency, as some fellowships such as dermatopathology require applications to be submitted at the conclusion of the PGY-2 year. Seek out faculty mentors or alumni who are walking a path similar to the one you want to embark on, as the next stop after graduation may be your forever job.
Depth, Not Breadth
The practice of medicine changes when narrowing the focus to one organ system. In both medical school and intern year, my study habits and history-taking of patients cast a wide net across multiple organ systems, aiming to know just enough about any one specialty to address all chief concerns and to know when it was appropriate to consult a specialist. This paradigm inevitably shifts in dermatology residency, as residents are tasked with memorizing the endless number of diagnoses of the skin alone, comprehending the many shades of “erythematous,” including pink, salmon, red, and purple. Both on the wards and in clinics, I had to grow comfortable with telling patients that I did not have an answer for many of their nondermatologic concerns and directing them to the right specialist. As medicine continues trending to specialization, subspecialization, and sub-subspecialization, the scope of any given physician likely will continue to narrow,7 as evidenced by specialty clinics within dermatology such as those focusing on hair loss or immunobullous disease. In this health care system, it is imperative to remember that you are only one physician within a team of care providers—understand your own role in the process and become comfortable with not having the answer to all the questions.
Final Thoughts
In a study of 44 dermatology residents, 35 (83%) indicated zero to less than 1 hour per week of independent preparation for dermatology residency during PGY-1.3 Although the usefulness of preparing is debatable, this figure likely reflects the absence of any insight on how to best prepare for the transition. Recognizing the many contrasts between internal medicine and dermatology and embracing the changes will enable a seamless promotion from a medicine PGY-1 to a dermatology PGY-2.
The transition from medical school to residency is a rewarding milestone but involves a steep learning curve wrought with new responsibilities, new colleagues, and a new schedule, often all within a new setting. This transition period has been a longstanding focus of graduate medical education research, and a recent study identified 6 key areas that residency programs need to address to better facilitate this transition: (1) a sense of community within the residency program, (2) relocation resources, (3) residency preparation courses in medical school, (4) readiness to address racism and bias, (5) connecting with peers, and (6) open communication with program leadership.1 There is considerable interest in ensuring that this transition is smooth for all graduates, as nearly all US medical schools feature some variety of a residency preparation course during the fourth year of medical school, which, alongside the subinternships, serves to better prepare their graduates for the healthcare workforce.2
What about the transition from intern to dermatology resident? Near the end of intern year, my categorical medicine colleagues experienced a crescendo of responsibilities, all in preparation for junior year. The senior medicine residents, themselves having previously experienced the graduated responsibilities, knew to ease their grip on the reins and provide the late spring interns an opportunity to lead rounds or run a code. This was not the case for the preliminary interns for whom there was no preview available for what was to come; little guidance exists on how to best transform from a preliminary or transitional postgraduate year (PGY) 1 to a dermatology PGY-2. A survey of 44 dermatology residents and 33 dermatology program directors found electives such as rheumatology, infectious diseases, and allergy and immunology to be helpful for this transition, and residents most often cited friendly and supportive senior and fellow residents as the factor that eased their transition to PGY-2.3 Notably, less than half of the residents (40%) surveyed stated that team-building exercises and dedicated time to meet colleagues were helpful for this transition. They identified studying principles of dermatologic disease, learning new clinical duties, and adjusting to new coworkers and supervisors as the greatest work-related stressors during entry to PGY-2.3
My transition from intern year to dermatology was shrouded in uncertainty, and I was fortunate to have supportive seniors and co-residents to ease the process. There is much about starting dermatology residency that cannot be prepared for by reading a book, and a natural metamorphosis into the new role is hard to articulate. Still, the following are pieces of information I wish I knew as a graduating intern, which I hope will prove useful for those graduating to their PGY-2 dermatology year.
The Pace of Outpatient Dermatology
If the preliminary or transitional year did not have an ambulatory component, the switch from wards to clinic can be jarring. An outpatient encounter can be as short as 10 to 15 minutes, necessitating an efficient interview and examination to avoid a backup of patients. Unlike a hospital admission where the history of present illness can expound on multiple concerns and organ systems, the general dermatology visit must focus on the chief concern, with priority given to the clinical examination of the skin. For total-body skin examinations, a formulaic approach to assessing all areas of the body, with fluent transitions and minimal repositioning of the patient, is critical for patient comfort and to save time. Of course, accuracy and thoroughness are paramount, but the constant mindfulness of time and efficiency is uniquely emphasized in the outpatient setting.
Continuity of Care
On the wards, patients are admitted with an acute problem and discharged with the aim to prevent re-admission. However, in the dermatology clinic, the conditions encountered often are chronic, requiring repeated follow-ups that involve dosage tapers, laboratory monitoring, and trial and error. Unlike the rigid algorithm-based treatments utilized in the inpatient setting, the management of the same chronic disease can vary, as it is tailored to the patient based on their comorbidities and response. This longitudinal relationship with patients, whereby many disorders are managed rather than treated, stands in stark contrast to inpatient medicine, and learning to value symptom management rather than focusing on a cure is critical in a largely outpatient specialty such as dermatology.
Consulter to Consultant
Calling a consultation as an intern is challenging and requires succinct delivery of pertinent information while fearing pushback from the consultant. In a survey of 50 hospitalist attendings, only 11% responded that interns could be entrusted to call an effective consultation without supervision.4 When undertaking the role of a consultant, the goals should be to identify the team’s main question and to obtain key information necessary to formulate a differential diagnosis. The quality of the consultation will inevitably fluctuate; try to remember what it was like for you as a member of the primary team and remain patient and courteous during the exchange.5 In 1983, Goldman et al6 published a guideline on effective consultations that often is cited to this day, dubbed the “Ten Commandments for Effective Consultations,” which consists of the following: (1) determine the question that is being asked, (2) establish the urgency of the consultation, (3) gather primary data, (4) communicate as briefly as appropriate, (5) make specific recommendations, (6) provide contingency plans, (7) understand your own role in the process, (8) offer educational information, (9) communicate recommendations directly to the requesting physician, and (10) provide appropriate follow-up.
Consider Your Future
Frequently reflect on what you most enjoy about your job. Although it can be easy to passively engage with intern year as a mere stepping-stone to dermatology residency, the years in PGY-2 and onward require active introspection to find a future niche. What made you gravitate to the specialty of dermatology? Try to identify your predilections for dermatopathology, pediatric dermatology, dermatologic surgery, cosmetic dermatology, and academia. Be consistently cognizant of your life after residency, as some fellowships such as dermatopathology require applications to be submitted at the conclusion of the PGY-2 year. Seek out faculty mentors or alumni who are walking a path similar to the one you want to embark on, as the next stop after graduation may be your forever job.
Depth, Not Breadth
The practice of medicine changes when narrowing the focus to one organ system. In both medical school and intern year, my study habits and history-taking of patients cast a wide net across multiple organ systems, aiming to know just enough about any one specialty to address all chief concerns and to know when it was appropriate to consult a specialist. This paradigm inevitably shifts in dermatology residency, as residents are tasked with memorizing the endless number of diagnoses of the skin alone, comprehending the many shades of “erythematous,” including pink, salmon, red, and purple. Both on the wards and in clinics, I had to grow comfortable with telling patients that I did not have an answer for many of their nondermatologic concerns and directing them to the right specialist. As medicine continues trending to specialization, subspecialization, and sub-subspecialization, the scope of any given physician likely will continue to narrow,7 as evidenced by specialty clinics within dermatology such as those focusing on hair loss or immunobullous disease. In this health care system, it is imperative to remember that you are only one physician within a team of care providers—understand your own role in the process and become comfortable with not having the answer to all the questions.
Final Thoughts
In a study of 44 dermatology residents, 35 (83%) indicated zero to less than 1 hour per week of independent preparation for dermatology residency during PGY-1.3 Although the usefulness of preparing is debatable, this figure likely reflects the absence of any insight on how to best prepare for the transition. Recognizing the many contrasts between internal medicine and dermatology and embracing the changes will enable a seamless promotion from a medicine PGY-1 to a dermatology PGY-2.
- Staples H, Frank S, Mullen M, et al. Improving the medical school to residency transition: narrative experiences from first-year residents.J Surg Educ. 2022;S1931-7204(22)00146-5. doi:10.1016/j.jsurg.2022.06.001
- Heidemann LA, Walford E, Mack J, et al. Is there a role for internal medicine residency preparation courses in the fourth year curriculum? a single-center experience. J Gen Intern Med. 2018;33:2048-2050.
- Hopkins C, Jalali O, Guffey D, et al. A survey of dermatology residents and program directors assessing the transition to dermatology residency. Proc (Bayl Univ Med Cent). 2020;34:59-62.
- Marcus CH, Winn AS, Sectish TC, et al. How much supervision is required is the beginning of intern year? Acad Pediatr. 2016;16:E3-E4.
- Bly RA, Bly EG. Consult courtesy. J Grad Med Educ. 2013;5:533-534.
- Goldman L, Lee T, Rudd P. Ten commandments for effective consultations. Arch Intern Med. 1983;143:1753-1755.
- Oren O, Gersh BJ, Bhatt DL. On the pearls and perils of sub-subspecialization. Am J Med. 2020;133:158-159.
- Staples H, Frank S, Mullen M, et al. Improving the medical school to residency transition: narrative experiences from first-year residents.J Surg Educ. 2022;S1931-7204(22)00146-5. doi:10.1016/j.jsurg.2022.06.001
- Heidemann LA, Walford E, Mack J, et al. Is there a role for internal medicine residency preparation courses in the fourth year curriculum? a single-center experience. J Gen Intern Med. 2018;33:2048-2050.
- Hopkins C, Jalali O, Guffey D, et al. A survey of dermatology residents and program directors assessing the transition to dermatology residency. Proc (Bayl Univ Med Cent). 2020;34:59-62.
- Marcus CH, Winn AS, Sectish TC, et al. How much supervision is required is the beginning of intern year? Acad Pediatr. 2016;16:E3-E4.
- Bly RA, Bly EG. Consult courtesy. J Grad Med Educ. 2013;5:533-534.
- Goldman L, Lee T, Rudd P. Ten commandments for effective consultations. Arch Intern Med. 1983;143:1753-1755.
- Oren O, Gersh BJ, Bhatt DL. On the pearls and perils of sub-subspecialization. Am J Med. 2020;133:158-159.
Resident Pearl
- There is surprisingly little information on what to expect when transitioning from intern year to dermatology residency. Recognizing the unique aspects of a largely outpatient specialty and embracing the role of a specialist will help facilitate this transition.
Ossification and Migration of a Nodule Following Calcium Hydroxylapatite Injection
To the Editor:
Calcium hydroxylapatite is an injectable filler approved by the US Food and Drug Administration for moderate to severe rhytides of the face and the treatment of facial lipodystrophy in patients with HIV.1 This long-lasting filler generally is well tolerated with minimal side effects; however, there have been reports of nodules or granulomatous formation following injection.2 We present a case of a migrating nodule following injection of a calcium hydroxylapatite filler that appeared ossified on radiographic imaging. We highlight this rarely reported phenomenon to increase awareness of this complication.
A 72-year-old woman presented to our clinic with a mass on the left cheek. The patient had a history of treatment with facial fillers but no notable medical conditions. She initially received hyaluronic acid injectable gel dermal filler twice—3 years apart—before switching to calcium hydroxylapatite injections twice—4 months apart—from an outside provider. One month after the second treatment, she noticed a mass on the left cheek and promptly returned to the provider who performed the calcium hydroxylapatite injections. The provider, who had originally injected in the infraorbital area, stated it was unlikely that the filler would have migrated to the mid cheek and referred the patient to a general dentist who suspected salivary gland pathology. The patient was referred to an oral and maxillofacial surgeon who suspected the mass was related to the parotid gland. Maxillofacial computed tomography (CT) revealed heterotopic ossification vs myositis ossificans, possibly related to the recent injection. The patient was eventually referred to the Division of Plastic Surgery, Department of Surgery, at the University of Texas Medical Branch (Galveston, Texas) for further evaluation. Physical examination revealed a 2×1-cm firm, mobile, nontender mass in the left cheek in the area of the buccinator muscles. The mass did not express any fluid and was most easily palpable from the oral cavity. Radiography findings showed that the calcium hydroxylapatite filler had migrated to this location and formed a nodule (Figure). Because calcium hydroxylapatite fillers generally last 12 to 18 months, we opted to observe the lesion for spontaneous resolution. Four months later, the patient presented to our clinic for follow-up and the mass had reduced in size and appeared to be spontaneously resolving.
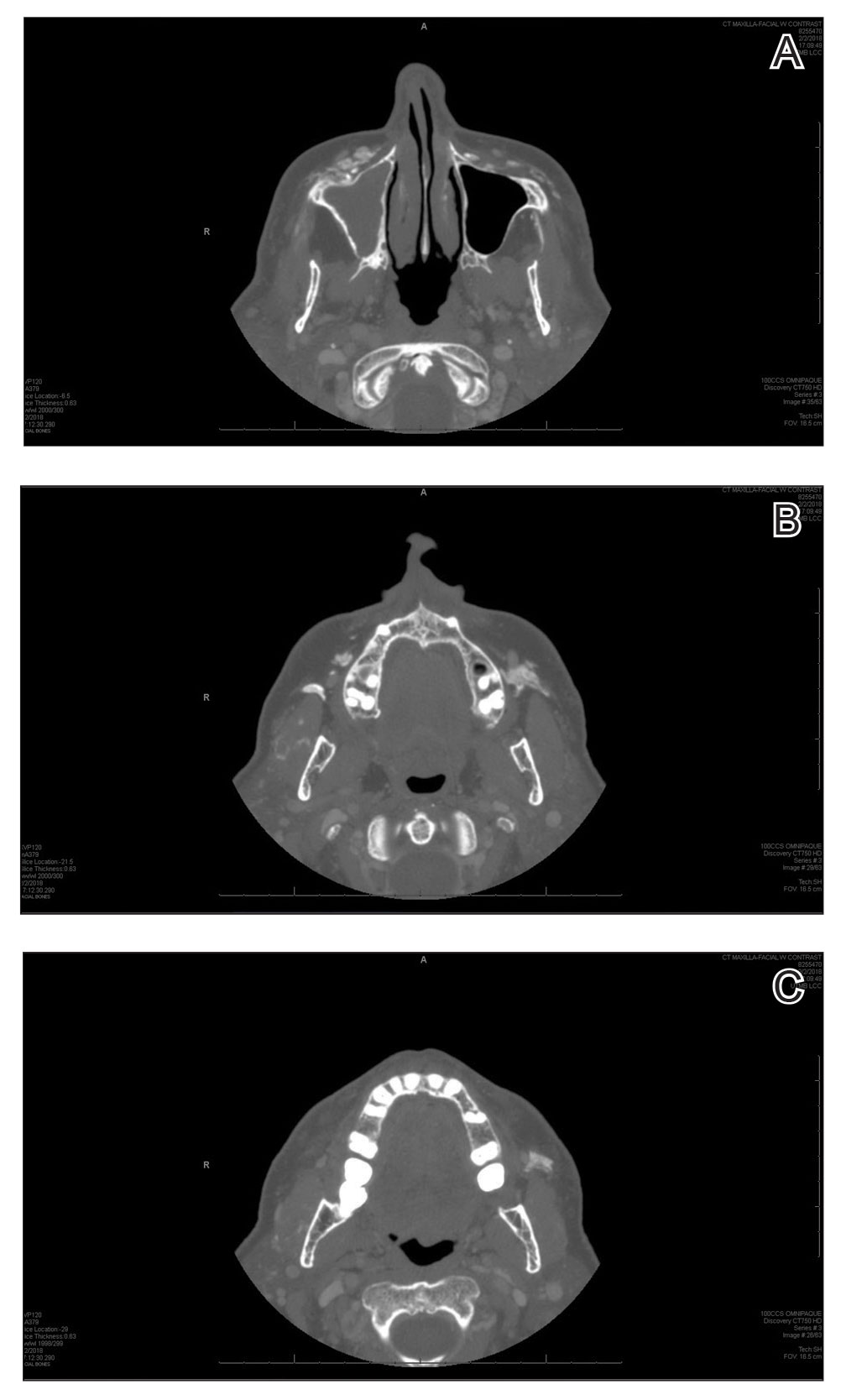
We present a unique case of a migrating nodule that occurred after injection with calcium hydroxylapatite, which led to concern for neoplastic tumor formation. This complication is rare, and it is important for practitioners who inject calcium hydroxylapatite as well as those who these patients may be referred to for evaluation to be aware that migrating nodules can occur. This awareness can help reduce unnecessary referrals, medical procedures, and anxiety.
Calcium hydroxylapatite filler is composed of 30% calcium hydroxylapatite microspheres suspended in a 70% sodium carboxymethylcellulose gel. The water-soluble gel rapidly becomes absorbed upon injection; however, the microspheres form a scaffold for the production of newly synthesized collagen. The filling effect generally lasts 12 to 18 months.1
Calcium hydroxylapatite, similar to most fillers, generally is well tolerated with a low complication rate of 3%.1 Although nodule formation with calcium hydroxylapatite is rare, it is the most common adverse event and encompasses 96% of complications. The remaining 4% of complications include persistent inflammation, swelling, erythema, and technical mistakes leading to overcorrection.1 Migrating nodules are rare; however, Beer3 reported a similar case.
Treatment of calcium hydroxylapatite nodules depends on differentiating a cause based on the time of onset. Early nodules that occur within 1 to 2 weeks of the injection usually represent incorrect positioning of the filler and can be treated by massaging the nodule. Other more invasive techniques involve aspiration or injection of sterile water. Late-onset nodules have shown response to corticosteroid injections. For inflammatory nodules of infectious origin, antibiotics can be useful. Surgical excision of the nodule rarely is required, as most nodules will resolve spontaneously, even without intervention.1,2
Radiologic findings of calcium hydroxylapatite appear as high-attenuation linear streaks or masses on CT (280–700 HU) and as low to intermediate signal intensity on T1- or T2-weighted sequences on magnetic resonance imaging. Oftentimes, calcium hydroxylapatite has a similar radiographic appearance to bone and can persist for 2 years or more on radiographic imaging, longer than they are clinically visible.4 The nodule formation from injection with calcium hydroxylapatite can mimic pathologic conditions such as miliary osteomas, myositis ossificans, heterotrophic/dystrophic calcifications, and foreign bodies on CT. Our patient’s CT findings of high attenuation linear streaks and nodules of similar signal intensity to bone were consistent with those previously described in the radiographic literature.
Calcium hydroxylapatite fillers have a good safety profile, but it is important to recognize that nodule formation is a common adverse event and that migration of nodules can occur. Practitioners should recognize this possibility in patients presenting with new masses after filler injection before advocating for potentially invasive and costly procedures and diagnostic modalities.
- Kadouch JA. Calcium hydroxylapatite: a review on safety and complications. J Cosmet Dermatol. 2017;16:152-161.
- Moulinets I, Arnaud E, Bui P, et al. Foreign body reaction to Radiesse: 2 cases. Am J Dermatopathol. 2013;35:e37-40.
- Beer KR. Radiesse nodule of the lips from a distant injection site: report of a case and consideration of etiology and management. J Drugs Dermatol. 2007;6:846-847.
- Ginat DT, Schatz CJ. Imaging features of midface injectable fillers and associated complications. AJNR Am J Neuroradiol. 2013;34:1488-1495.
To the Editor:
Calcium hydroxylapatite is an injectable filler approved by the US Food and Drug Administration for moderate to severe rhytides of the face and the treatment of facial lipodystrophy in patients with HIV.1 This long-lasting filler generally is well tolerated with minimal side effects; however, there have been reports of nodules or granulomatous formation following injection.2 We present a case of a migrating nodule following injection of a calcium hydroxylapatite filler that appeared ossified on radiographic imaging. We highlight this rarely reported phenomenon to increase awareness of this complication.
A 72-year-old woman presented to our clinic with a mass on the left cheek. The patient had a history of treatment with facial fillers but no notable medical conditions. She initially received hyaluronic acid injectable gel dermal filler twice—3 years apart—before switching to calcium hydroxylapatite injections twice—4 months apart—from an outside provider. One month after the second treatment, she noticed a mass on the left cheek and promptly returned to the provider who performed the calcium hydroxylapatite injections. The provider, who had originally injected in the infraorbital area, stated it was unlikely that the filler would have migrated to the mid cheek and referred the patient to a general dentist who suspected salivary gland pathology. The patient was referred to an oral and maxillofacial surgeon who suspected the mass was related to the parotid gland. Maxillofacial computed tomography (CT) revealed heterotopic ossification vs myositis ossificans, possibly related to the recent injection. The patient was eventually referred to the Division of Plastic Surgery, Department of Surgery, at the University of Texas Medical Branch (Galveston, Texas) for further evaluation. Physical examination revealed a 2×1-cm firm, mobile, nontender mass in the left cheek in the area of the buccinator muscles. The mass did not express any fluid and was most easily palpable from the oral cavity. Radiography findings showed that the calcium hydroxylapatite filler had migrated to this location and formed a nodule (Figure). Because calcium hydroxylapatite fillers generally last 12 to 18 months, we opted to observe the lesion for spontaneous resolution. Four months later, the patient presented to our clinic for follow-up and the mass had reduced in size and appeared to be spontaneously resolving.

We present a unique case of a migrating nodule that occurred after injection with calcium hydroxylapatite, which led to concern for neoplastic tumor formation. This complication is rare, and it is important for practitioners who inject calcium hydroxylapatite as well as those who these patients may be referred to for evaluation to be aware that migrating nodules can occur. This awareness can help reduce unnecessary referrals, medical procedures, and anxiety.
Calcium hydroxylapatite filler is composed of 30% calcium hydroxylapatite microspheres suspended in a 70% sodium carboxymethylcellulose gel. The water-soluble gel rapidly becomes absorbed upon injection; however, the microspheres form a scaffold for the production of newly synthesized collagen. The filling effect generally lasts 12 to 18 months.1
Calcium hydroxylapatite, similar to most fillers, generally is well tolerated with a low complication rate of 3%.1 Although nodule formation with calcium hydroxylapatite is rare, it is the most common adverse event and encompasses 96% of complications. The remaining 4% of complications include persistent inflammation, swelling, erythema, and technical mistakes leading to overcorrection.1 Migrating nodules are rare; however, Beer3 reported a similar case.
Treatment of calcium hydroxylapatite nodules depends on differentiating a cause based on the time of onset. Early nodules that occur within 1 to 2 weeks of the injection usually represent incorrect positioning of the filler and can be treated by massaging the nodule. Other more invasive techniques involve aspiration or injection of sterile water. Late-onset nodules have shown response to corticosteroid injections. For inflammatory nodules of infectious origin, antibiotics can be useful. Surgical excision of the nodule rarely is required, as most nodules will resolve spontaneously, even without intervention.1,2
Radiologic findings of calcium hydroxylapatite appear as high-attenuation linear streaks or masses on CT (280–700 HU) and as low to intermediate signal intensity on T1- or T2-weighted sequences on magnetic resonance imaging. Oftentimes, calcium hydroxylapatite has a similar radiographic appearance to bone and can persist for 2 years or more on radiographic imaging, longer than they are clinically visible.4 The nodule formation from injection with calcium hydroxylapatite can mimic pathologic conditions such as miliary osteomas, myositis ossificans, heterotrophic/dystrophic calcifications, and foreign bodies on CT. Our patient’s CT findings of high attenuation linear streaks and nodules of similar signal intensity to bone were consistent with those previously described in the radiographic literature.
Calcium hydroxylapatite fillers have a good safety profile, but it is important to recognize that nodule formation is a common adverse event and that migration of nodules can occur. Practitioners should recognize this possibility in patients presenting with new masses after filler injection before advocating for potentially invasive and costly procedures and diagnostic modalities.
To the Editor:
Calcium hydroxylapatite is an injectable filler approved by the US Food and Drug Administration for moderate to severe rhytides of the face and the treatment of facial lipodystrophy in patients with HIV.1 This long-lasting filler generally is well tolerated with minimal side effects; however, there have been reports of nodules or granulomatous formation following injection.2 We present a case of a migrating nodule following injection of a calcium hydroxylapatite filler that appeared ossified on radiographic imaging. We highlight this rarely reported phenomenon to increase awareness of this complication.
A 72-year-old woman presented to our clinic with a mass on the left cheek. The patient had a history of treatment with facial fillers but no notable medical conditions. She initially received hyaluronic acid injectable gel dermal filler twice—3 years apart—before switching to calcium hydroxylapatite injections twice—4 months apart—from an outside provider. One month after the second treatment, she noticed a mass on the left cheek and promptly returned to the provider who performed the calcium hydroxylapatite injections. The provider, who had originally injected in the infraorbital area, stated it was unlikely that the filler would have migrated to the mid cheek and referred the patient to a general dentist who suspected salivary gland pathology. The patient was referred to an oral and maxillofacial surgeon who suspected the mass was related to the parotid gland. Maxillofacial computed tomography (CT) revealed heterotopic ossification vs myositis ossificans, possibly related to the recent injection. The patient was eventually referred to the Division of Plastic Surgery, Department of Surgery, at the University of Texas Medical Branch (Galveston, Texas) for further evaluation. Physical examination revealed a 2×1-cm firm, mobile, nontender mass in the left cheek in the area of the buccinator muscles. The mass did not express any fluid and was most easily palpable from the oral cavity. Radiography findings showed that the calcium hydroxylapatite filler had migrated to this location and formed a nodule (Figure). Because calcium hydroxylapatite fillers generally last 12 to 18 months, we opted to observe the lesion for spontaneous resolution. Four months later, the patient presented to our clinic for follow-up and the mass had reduced in size and appeared to be spontaneously resolving.

We present a unique case of a migrating nodule that occurred after injection with calcium hydroxylapatite, which led to concern for neoplastic tumor formation. This complication is rare, and it is important for practitioners who inject calcium hydroxylapatite as well as those who these patients may be referred to for evaluation to be aware that migrating nodules can occur. This awareness can help reduce unnecessary referrals, medical procedures, and anxiety.
Calcium hydroxylapatite filler is composed of 30% calcium hydroxylapatite microspheres suspended in a 70% sodium carboxymethylcellulose gel. The water-soluble gel rapidly becomes absorbed upon injection; however, the microspheres form a scaffold for the production of newly synthesized collagen. The filling effect generally lasts 12 to 18 months.1
Calcium hydroxylapatite, similar to most fillers, generally is well tolerated with a low complication rate of 3%.1 Although nodule formation with calcium hydroxylapatite is rare, it is the most common adverse event and encompasses 96% of complications. The remaining 4% of complications include persistent inflammation, swelling, erythema, and technical mistakes leading to overcorrection.1 Migrating nodules are rare; however, Beer3 reported a similar case.
Treatment of calcium hydroxylapatite nodules depends on differentiating a cause based on the time of onset. Early nodules that occur within 1 to 2 weeks of the injection usually represent incorrect positioning of the filler and can be treated by massaging the nodule. Other more invasive techniques involve aspiration or injection of sterile water. Late-onset nodules have shown response to corticosteroid injections. For inflammatory nodules of infectious origin, antibiotics can be useful. Surgical excision of the nodule rarely is required, as most nodules will resolve spontaneously, even without intervention.1,2
Radiologic findings of calcium hydroxylapatite appear as high-attenuation linear streaks or masses on CT (280–700 HU) and as low to intermediate signal intensity on T1- or T2-weighted sequences on magnetic resonance imaging. Oftentimes, calcium hydroxylapatite has a similar radiographic appearance to bone and can persist for 2 years or more on radiographic imaging, longer than they are clinically visible.4 The nodule formation from injection with calcium hydroxylapatite can mimic pathologic conditions such as miliary osteomas, myositis ossificans, heterotrophic/dystrophic calcifications, and foreign bodies on CT. Our patient’s CT findings of high attenuation linear streaks and nodules of similar signal intensity to bone were consistent with those previously described in the radiographic literature.
Calcium hydroxylapatite fillers have a good safety profile, but it is important to recognize that nodule formation is a common adverse event and that migration of nodules can occur. Practitioners should recognize this possibility in patients presenting with new masses after filler injection before advocating for potentially invasive and costly procedures and diagnostic modalities.
- Kadouch JA. Calcium hydroxylapatite: a review on safety and complications. J Cosmet Dermatol. 2017;16:152-161.
- Moulinets I, Arnaud E, Bui P, et al. Foreign body reaction to Radiesse: 2 cases. Am J Dermatopathol. 2013;35:e37-40.
- Beer KR. Radiesse nodule of the lips from a distant injection site: report of a case and consideration of etiology and management. J Drugs Dermatol. 2007;6:846-847.
- Ginat DT, Schatz CJ. Imaging features of midface injectable fillers and associated complications. AJNR Am J Neuroradiol. 2013;34:1488-1495.
- Kadouch JA. Calcium hydroxylapatite: a review on safety and complications. J Cosmet Dermatol. 2017;16:152-161.
- Moulinets I, Arnaud E, Bui P, et al. Foreign body reaction to Radiesse: 2 cases. Am J Dermatopathol. 2013;35:e37-40.
- Beer KR. Radiesse nodule of the lips from a distant injection site: report of a case and consideration of etiology and management. J Drugs Dermatol. 2007;6:846-847.
- Ginat DT, Schatz CJ. Imaging features of midface injectable fillers and associated complications. AJNR Am J Neuroradiol. 2013;34:1488-1495.
Practice Points
- Calcium hydroxylapatite filler can migrate and form nodules in distant locations from the original injection site.
- Practitioners of calcium hydroxylapatite fillers should be aware of the potential for nodule migration to avoid costly, time-consuming, and invasive referrals and procedures.
An infant with a tender bump on her ear
A biopsy of the lesion was performed that showed a well-defined nodulocystic tumor composed of nests of basaloid cells that are undergoing trichilemmal keratinization. Shadow cells are seen as well as small areas of calcification. There is also a histiocytic infiltrate with multinucleated giant cells. The histologic diagnosis is of a pilomatrixoma.
Pilomatrixoma, also known as calcifying epithelioma of Malherbe, was first described in 1880, as a tumor of sebaceous gland origin. Later, in 1961, Robert Forbis Jr, MD, and Elson B. Helwig, MD, coined the term pilomatrixoma to describe the hair follicle matrix as the source of the tumor. Pilomatrixomas are commonly seen in the pediatric population, usually in children between 8 and 13 years of age. Our patient is one of the youngest described. The lesions are commonly seen on the face and neck in about 70% of the cases followed by the upper extremities, back, and legs. Clinically, the lesions appear as a firm dermal papule or nodule, which moves freely and may have associated erythema on the skin surface or a blueish gray hue on the underlying skin.
Most pilomatrixomas that have been studied have shown a mutation in Exon 3 of the beta-catenin gene (CTNNB1). The beta-catenin molecule is a subunit of the cadherin protein, which is part of an important pathway in the terminal hair follicle differentiation. Beta-catenin also plays an important role in the Wnt pathway, which regulates cell fate as well as early embryonic patterning. Beta-catenin is responsible for forming adhesion junctions among cells. There have also been immunohistochemical studies that have shown a BCL2 proto-oncogene overexpression to pilomatrixoma.
There are several genetic syndromes that have been associated with the presence of pilomatrixomas: Turner syndrome (XO chromosome abnormality associated with short stature and cardiac defects), Gardner syndrome (polyposis coli and colon and rectal cancer), myotonic dystrophy, Rubinstein-Taybi syndrome (characterized by broad thumbs and toes, short stature, distinctive facial features, and varying degrees of intellectual disability), and trisomy 9. On physical examination our patient didn’t present with any of the typical features or history that could suggest any of these syndromes. A close follow-up and evaluation by a geneticist was recommended because after the initial visit she developed a second lesion on the forehead.
The differential diagnosis for this lesion includes other cysts that may occur on the ear such as epidermal inclusion cyst or dermoid cysts, though these lesions do not tend to be as firm as pilomatrixomas are, which can help with the diagnosis. Dermoid cysts are made of dermal and epidermal components. They are usually present at birth and are commonly seen on the scalp and the periorbital face.
Keloids are rubbery nodules of scar tissue that can form on sites of trauma, and although the lesion occurred after she had her ears pierced, the consistency and rapid growth of the lesion as well as the pathological description made this benign fibrous growth less likely.
When pilomatrixomas are inflamed they can be confused with vascular growths: in this particular case, a hemangioma or another vascular tumor such as a tufted angioma or kaposiform hemangioendothelioma. An ultrasound of the lesion could have helped in the differential diagnosis of the lesion.
Pilomatrixomas can grow significantly and in some cases get inflamed or infected. Surgical management of pilomatrixomas is often required because the lesions do not regress spontaneously.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
Forbis R Jr and Helwig EB. Arch Dermatol 1961;83:606-18.
Schwarz Y et al. Int J Pediatr Otorhinolaryngol. 2016 Jun;85:148-53.
A biopsy of the lesion was performed that showed a well-defined nodulocystic tumor composed of nests of basaloid cells that are undergoing trichilemmal keratinization. Shadow cells are seen as well as small areas of calcification. There is also a histiocytic infiltrate with multinucleated giant cells. The histologic diagnosis is of a pilomatrixoma.
Pilomatrixoma, also known as calcifying epithelioma of Malherbe, was first described in 1880, as a tumor of sebaceous gland origin. Later, in 1961, Robert Forbis Jr, MD, and Elson B. Helwig, MD, coined the term pilomatrixoma to describe the hair follicle matrix as the source of the tumor. Pilomatrixomas are commonly seen in the pediatric population, usually in children between 8 and 13 years of age. Our patient is one of the youngest described. The lesions are commonly seen on the face and neck in about 70% of the cases followed by the upper extremities, back, and legs. Clinically, the lesions appear as a firm dermal papule or nodule, which moves freely and may have associated erythema on the skin surface or a blueish gray hue on the underlying skin.
Most pilomatrixomas that have been studied have shown a mutation in Exon 3 of the beta-catenin gene (CTNNB1). The beta-catenin molecule is a subunit of the cadherin protein, which is part of an important pathway in the terminal hair follicle differentiation. Beta-catenin also plays an important role in the Wnt pathway, which regulates cell fate as well as early embryonic patterning. Beta-catenin is responsible for forming adhesion junctions among cells. There have also been immunohistochemical studies that have shown a BCL2 proto-oncogene overexpression to pilomatrixoma.
There are several genetic syndromes that have been associated with the presence of pilomatrixomas: Turner syndrome (XO chromosome abnormality associated with short stature and cardiac defects), Gardner syndrome (polyposis coli and colon and rectal cancer), myotonic dystrophy, Rubinstein-Taybi syndrome (characterized by broad thumbs and toes, short stature, distinctive facial features, and varying degrees of intellectual disability), and trisomy 9. On physical examination our patient didn’t present with any of the typical features or history that could suggest any of these syndromes. A close follow-up and evaluation by a geneticist was recommended because after the initial visit she developed a second lesion on the forehead.
The differential diagnosis for this lesion includes other cysts that may occur on the ear such as epidermal inclusion cyst or dermoid cysts, though these lesions do not tend to be as firm as pilomatrixomas are, which can help with the diagnosis. Dermoid cysts are made of dermal and epidermal components. They are usually present at birth and are commonly seen on the scalp and the periorbital face.
Keloids are rubbery nodules of scar tissue that can form on sites of trauma, and although the lesion occurred after she had her ears pierced, the consistency and rapid growth of the lesion as well as the pathological description made this benign fibrous growth less likely.
When pilomatrixomas are inflamed they can be confused with vascular growths: in this particular case, a hemangioma or another vascular tumor such as a tufted angioma or kaposiform hemangioendothelioma. An ultrasound of the lesion could have helped in the differential diagnosis of the lesion.
Pilomatrixomas can grow significantly and in some cases get inflamed or infected. Surgical management of pilomatrixomas is often required because the lesions do not regress spontaneously.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
Forbis R Jr and Helwig EB. Arch Dermatol 1961;83:606-18.
Schwarz Y et al. Int J Pediatr Otorhinolaryngol. 2016 Jun;85:148-53.
A biopsy of the lesion was performed that showed a well-defined nodulocystic tumor composed of nests of basaloid cells that are undergoing trichilemmal keratinization. Shadow cells are seen as well as small areas of calcification. There is also a histiocytic infiltrate with multinucleated giant cells. The histologic diagnosis is of a pilomatrixoma.
Pilomatrixoma, also known as calcifying epithelioma of Malherbe, was first described in 1880, as a tumor of sebaceous gland origin. Later, in 1961, Robert Forbis Jr, MD, and Elson B. Helwig, MD, coined the term pilomatrixoma to describe the hair follicle matrix as the source of the tumor. Pilomatrixomas are commonly seen in the pediatric population, usually in children between 8 and 13 years of age. Our patient is one of the youngest described. The lesions are commonly seen on the face and neck in about 70% of the cases followed by the upper extremities, back, and legs. Clinically, the lesions appear as a firm dermal papule or nodule, which moves freely and may have associated erythema on the skin surface or a blueish gray hue on the underlying skin.
Most pilomatrixomas that have been studied have shown a mutation in Exon 3 of the beta-catenin gene (CTNNB1). The beta-catenin molecule is a subunit of the cadherin protein, which is part of an important pathway in the terminal hair follicle differentiation. Beta-catenin also plays an important role in the Wnt pathway, which regulates cell fate as well as early embryonic patterning. Beta-catenin is responsible for forming adhesion junctions among cells. There have also been immunohistochemical studies that have shown a BCL2 proto-oncogene overexpression to pilomatrixoma.
There are several genetic syndromes that have been associated with the presence of pilomatrixomas: Turner syndrome (XO chromosome abnormality associated with short stature and cardiac defects), Gardner syndrome (polyposis coli and colon and rectal cancer), myotonic dystrophy, Rubinstein-Taybi syndrome (characterized by broad thumbs and toes, short stature, distinctive facial features, and varying degrees of intellectual disability), and trisomy 9. On physical examination our patient didn’t present with any of the typical features or history that could suggest any of these syndromes. A close follow-up and evaluation by a geneticist was recommended because after the initial visit she developed a second lesion on the forehead.
The differential diagnosis for this lesion includes other cysts that may occur on the ear such as epidermal inclusion cyst or dermoid cysts, though these lesions do not tend to be as firm as pilomatrixomas are, which can help with the diagnosis. Dermoid cysts are made of dermal and epidermal components. They are usually present at birth and are commonly seen on the scalp and the periorbital face.
Keloids are rubbery nodules of scar tissue that can form on sites of trauma, and although the lesion occurred after she had her ears pierced, the consistency and rapid growth of the lesion as well as the pathological description made this benign fibrous growth less likely.
When pilomatrixomas are inflamed they can be confused with vascular growths: in this particular case, a hemangioma or another vascular tumor such as a tufted angioma or kaposiform hemangioendothelioma. An ultrasound of the lesion could have helped in the differential diagnosis of the lesion.
Pilomatrixomas can grow significantly and in some cases get inflamed or infected. Surgical management of pilomatrixomas is often required because the lesions do not regress spontaneously.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
Forbis R Jr and Helwig EB. Arch Dermatol 1961;83:606-18.
Schwarz Y et al. Int J Pediatr Otorhinolaryngol. 2016 Jun;85:148-53.
A 4-month-old female was referred to our clinic for evaluation of a bump on the right ear. The lesion was first noted at 2 months of age as a little pimple. She was evaluated by her pediatrician and was treated with topical and oral antibiotics without resolution of the lesion. The bump continued to grow and seemed tender to palpation, so she was referred to dermatology for evaluation.
She was born via normal vaginal delivery at 40 weeks. Her mother has no medical conditions and the pregnancy was uneventful. She has been growing and developing well. She takes vitamin D and is currently breast fed.
There have been no other family members with similar lesions. She had her ears pierced at a month of age without any complications.
On skin examination she has a firm red nodule on the right ear that appears slightly tender to touch. She has no other skin lesions of concern. She has normal muscle tone and there are no other abnormalities noted on the physical exam. She has no hepatomegaly, splenomegaly, or lymphadenopathy.
Poor evidence for vaginal laser therapy
Despite a lack of evidence and high cost, laser therapy continues to attract many women seeking “vaginal rejuvenation” to help reverse the physical symptoms of menopause.
Recent reviews of the medical literature continue to show that laser treatment appears to be less effective than estrogen at improving vaginal dryness and pain during sex, according to Cheryl B. Iglesia, MD, a professor of ob.gyn. and urology at Georgetown University, Washington.
“Laser for GSM [genitourinary syndrome of menopause] is showing some promise, but patients need to be offered [Food and Drug Administration]–approved treatments prior to considering laser, and users need to know how to do speculum and pelvic exams and understand vulvovaginal anatomy and pathology,” Dr. Iglesia, who directs the section of female pelvic medicine and reconstructive surgery at MedStar Washington Hospital Center, said in an interview, adding that patients should avoid “vaginal rejuvenation” treatments offered at med-spas.
Dr. Iglesia reviewed how these lasers work and then discussed the controversy over their marketing and the evidence for their use at the annual meeting of the North American Menopause Society.
By 3 years after menopause, more than half of women experience atrophy in their vagina resulting from a lack of estrogen. Marked by a thinning of the epithelium, reduced blood supply, and loss of glycogen, vulvovaginal atrophy is to blame for GSM.
Vaginal laser therapy has been a popular option for women for the last decade, despite a lack of evidence supporting its use or approval from regulators.
The FDA has issued broad clearance for laser therapy for incision, ablation, vaporization, and coagulation of body soft tissues, such as dysplasia, vulvar or anal neoplasia, endometriosis, condylomas, and other disorders. However, the agency has not approved the use of laser therapy for vulvovaginal atrophy, GSM, vaginal dryness, or dyspareunia.
Evidence regarding vaginal laser therapy
According to Dr. Iglesia, the evidence for vaginal laser therapy is mixed and of generally low quality. A systematic review published in the Journal of Sexual Medicine (2022 Jan 29. doi: 10.1016/j.jsxm.2021.12.010) presented mostly low-quality evidence from 25 studies and found promising data for genitourinary symptoms but not enough to justify its use for genitourinary symptoms just yet. Dr. Iglesia discussed her own small, multisite study of 62 participants, which compared vaginal laser with vaginal estrogen and found no differences between the two for multiple outcomes. (The study would have been larger if not for interruption from an FDA warning for an Investigational Device Exemption.)
A JAMA study from Australia found no difference between laser therapy and sham laser therapy, but the most recent systematic review, from JAMA Network Open, found no significant difference between vaginal laser and vaginal estrogen for vaginal and sexual function symptoms. This review, however, covered only the six existing randomized controlled trials, including Dr. Iglesia’s, which were small and had a follow-up period of only 3-6 months.
“There have only been a few randomized controlled trials comparing laser to vaginal estrogen therapy, and most of those did not include a placebo or sham arm,” Monica Christmas, MD, director of the Center for Women’s Integrated Health at the University of Chicago Medicine, said in an interview. “This is extremely important, as most of the trials that did include a sham arm did not find that laser was better than the sham.” Dr. Christmas was not a part of the presentation but attended it at NAMS.
The bottom line, she said, is that “current evidence is not sufficient to make conclusions on long-term safety or sustainability, nor is there compelling evidence to make claims on equivalence to vaginal estrogen therapy.” Currently, committee opinions from a half-dozen medical societies, including NAMS, oppose using vaginal laser therapy until rigorous, robust trials on long-term safety and efficacy have been conducted. The International Continence Society and International Society for the Study of Vulvovaginal Disease issued a joint statement in 2018 that emphasized that histologic changes from lasers do not necessarily equate with changes in function. The statement noted the lack of evidence for laser treatment of incontinence and prolapse and stated that it should not be used for vulvodynia or lichen sclerosus.
A 2020 statement from NAMS found “insufficient placebo-controlled trials of energy-based therapies, including laser, to draw conclusions of efficacy or safety or to make treatment recommendations.” A slightly more optimistic statement from the American Urogynecologic Society concluded that energy-based devices have shown short-term efficacy for menopause-related vaginal atrophy and dyspareunia, including effects lasting up to 1 year from fractionated laser for treat dyspareunia, but also noted that studies up to that time were small and measure various outcomes.
Recommendations on vaginal laser therapy
Given this landscape of uneven and poor-quality evidence, Dr. Iglesia provided several “common sense” recommendations for energy-based therapies, starting with the need for any practitioner to have working knowledge of vulvovaginal anatomy. Contraindications for laser therapy include any malignancy – especially gynecologic – undiagnosed bleeding, active herpes or other infections, radiation, and vaginal mesh, particularly transvaginal mesh. The provider also must discuss the limited data on long-term function and treatment alternatives, including FDA-approved therapies like topical estrogen, dehydroepiandrosterone sulfate (DHEA-S), ospemifene, and moisturizers, Dr. Iglesia said.
Adverse events associated with laser therapy, such as scarring or burning, are rare but do occur, and cost remains an issue, Dr. Iglesia said.
“Vaginal estrogen therapy is well established as a safe and effective treatment option based on high quality evidence,” Dr. Christmas said. “This is not the case for laser therapy. Rare, but serious harms are reported with vaginal laser, including burns, scarring, dyspareunia, pain, and potential irreversible damage.”
Dr. Iglesia also cautioned that clinicians should take extra care with vulnerable populations, particularly cancer patients and others with contraindications for estrogen treatment.
For those in whom vaginal estrogen is contraindicated, Dr. Christmas recommended vaginal moisturizers, lubricants, dilators, and physical therapy for the pelvic floor.
“In patients who fail those nonhormonal approaches, short courses of vaginal estrogen therapy or DHEA-S suppository may be employed with approval from their oncologist,” Dr. Christmas said.
Dr. Iglesia finally reviewed the major research questions that remain with laser therapy:
- What are outcomes for laser versus sham studies?
- What are long-term outcomes (beyond 6 months)
- What pretreatment is necessary?
- Could laser be used as a drug delivery mechanism for estrogen, and could this provide a synergistic effect?
- What is the optimal number and interval for laser treatments?
Dr. Iglesia had no industry disclosures but received honoraria for consulting at UpToDate. Dr. Christmas is a consultant for Materna. The presentation did not rely on any external funding.
Despite a lack of evidence and high cost, laser therapy continues to attract many women seeking “vaginal rejuvenation” to help reverse the physical symptoms of menopause.
Recent reviews of the medical literature continue to show that laser treatment appears to be less effective than estrogen at improving vaginal dryness and pain during sex, according to Cheryl B. Iglesia, MD, a professor of ob.gyn. and urology at Georgetown University, Washington.
“Laser for GSM [genitourinary syndrome of menopause] is showing some promise, but patients need to be offered [Food and Drug Administration]–approved treatments prior to considering laser, and users need to know how to do speculum and pelvic exams and understand vulvovaginal anatomy and pathology,” Dr. Iglesia, who directs the section of female pelvic medicine and reconstructive surgery at MedStar Washington Hospital Center, said in an interview, adding that patients should avoid “vaginal rejuvenation” treatments offered at med-spas.
Dr. Iglesia reviewed how these lasers work and then discussed the controversy over their marketing and the evidence for their use at the annual meeting of the North American Menopause Society.
By 3 years after menopause, more than half of women experience atrophy in their vagina resulting from a lack of estrogen. Marked by a thinning of the epithelium, reduced blood supply, and loss of glycogen, vulvovaginal atrophy is to blame for GSM.
Vaginal laser therapy has been a popular option for women for the last decade, despite a lack of evidence supporting its use or approval from regulators.
The FDA has issued broad clearance for laser therapy for incision, ablation, vaporization, and coagulation of body soft tissues, such as dysplasia, vulvar or anal neoplasia, endometriosis, condylomas, and other disorders. However, the agency has not approved the use of laser therapy for vulvovaginal atrophy, GSM, vaginal dryness, or dyspareunia.
Evidence regarding vaginal laser therapy
According to Dr. Iglesia, the evidence for vaginal laser therapy is mixed and of generally low quality. A systematic review published in the Journal of Sexual Medicine (2022 Jan 29. doi: 10.1016/j.jsxm.2021.12.010) presented mostly low-quality evidence from 25 studies and found promising data for genitourinary symptoms but not enough to justify its use for genitourinary symptoms just yet. Dr. Iglesia discussed her own small, multisite study of 62 participants, which compared vaginal laser with vaginal estrogen and found no differences between the two for multiple outcomes. (The study would have been larger if not for interruption from an FDA warning for an Investigational Device Exemption.)
A JAMA study from Australia found no difference between laser therapy and sham laser therapy, but the most recent systematic review, from JAMA Network Open, found no significant difference between vaginal laser and vaginal estrogen for vaginal and sexual function symptoms. This review, however, covered only the six existing randomized controlled trials, including Dr. Iglesia’s, which were small and had a follow-up period of only 3-6 months.
“There have only been a few randomized controlled trials comparing laser to vaginal estrogen therapy, and most of those did not include a placebo or sham arm,” Monica Christmas, MD, director of the Center for Women’s Integrated Health at the University of Chicago Medicine, said in an interview. “This is extremely important, as most of the trials that did include a sham arm did not find that laser was better than the sham.” Dr. Christmas was not a part of the presentation but attended it at NAMS.
The bottom line, she said, is that “current evidence is not sufficient to make conclusions on long-term safety or sustainability, nor is there compelling evidence to make claims on equivalence to vaginal estrogen therapy.” Currently, committee opinions from a half-dozen medical societies, including NAMS, oppose using vaginal laser therapy until rigorous, robust trials on long-term safety and efficacy have been conducted. The International Continence Society and International Society for the Study of Vulvovaginal Disease issued a joint statement in 2018 that emphasized that histologic changes from lasers do not necessarily equate with changes in function. The statement noted the lack of evidence for laser treatment of incontinence and prolapse and stated that it should not be used for vulvodynia or lichen sclerosus.
A 2020 statement from NAMS found “insufficient placebo-controlled trials of energy-based therapies, including laser, to draw conclusions of efficacy or safety or to make treatment recommendations.” A slightly more optimistic statement from the American Urogynecologic Society concluded that energy-based devices have shown short-term efficacy for menopause-related vaginal atrophy and dyspareunia, including effects lasting up to 1 year from fractionated laser for treat dyspareunia, but also noted that studies up to that time were small and measure various outcomes.
Recommendations on vaginal laser therapy
Given this landscape of uneven and poor-quality evidence, Dr. Iglesia provided several “common sense” recommendations for energy-based therapies, starting with the need for any practitioner to have working knowledge of vulvovaginal anatomy. Contraindications for laser therapy include any malignancy – especially gynecologic – undiagnosed bleeding, active herpes or other infections, radiation, and vaginal mesh, particularly transvaginal mesh. The provider also must discuss the limited data on long-term function and treatment alternatives, including FDA-approved therapies like topical estrogen, dehydroepiandrosterone sulfate (DHEA-S), ospemifene, and moisturizers, Dr. Iglesia said.
Adverse events associated with laser therapy, such as scarring or burning, are rare but do occur, and cost remains an issue, Dr. Iglesia said.
“Vaginal estrogen therapy is well established as a safe and effective treatment option based on high quality evidence,” Dr. Christmas said. “This is not the case for laser therapy. Rare, but serious harms are reported with vaginal laser, including burns, scarring, dyspareunia, pain, and potential irreversible damage.”
Dr. Iglesia also cautioned that clinicians should take extra care with vulnerable populations, particularly cancer patients and others with contraindications for estrogen treatment.
For those in whom vaginal estrogen is contraindicated, Dr. Christmas recommended vaginal moisturizers, lubricants, dilators, and physical therapy for the pelvic floor.
“In patients who fail those nonhormonal approaches, short courses of vaginal estrogen therapy or DHEA-S suppository may be employed with approval from their oncologist,” Dr. Christmas said.
Dr. Iglesia finally reviewed the major research questions that remain with laser therapy:
- What are outcomes for laser versus sham studies?
- What are long-term outcomes (beyond 6 months)
- What pretreatment is necessary?
- Could laser be used as a drug delivery mechanism for estrogen, and could this provide a synergistic effect?
- What is the optimal number and interval for laser treatments?
Dr. Iglesia had no industry disclosures but received honoraria for consulting at UpToDate. Dr. Christmas is a consultant for Materna. The presentation did not rely on any external funding.
Despite a lack of evidence and high cost, laser therapy continues to attract many women seeking “vaginal rejuvenation” to help reverse the physical symptoms of menopause.
Recent reviews of the medical literature continue to show that laser treatment appears to be less effective than estrogen at improving vaginal dryness and pain during sex, according to Cheryl B. Iglesia, MD, a professor of ob.gyn. and urology at Georgetown University, Washington.
“Laser for GSM [genitourinary syndrome of menopause] is showing some promise, but patients need to be offered [Food and Drug Administration]–approved treatments prior to considering laser, and users need to know how to do speculum and pelvic exams and understand vulvovaginal anatomy and pathology,” Dr. Iglesia, who directs the section of female pelvic medicine and reconstructive surgery at MedStar Washington Hospital Center, said in an interview, adding that patients should avoid “vaginal rejuvenation” treatments offered at med-spas.
Dr. Iglesia reviewed how these lasers work and then discussed the controversy over their marketing and the evidence for their use at the annual meeting of the North American Menopause Society.
By 3 years after menopause, more than half of women experience atrophy in their vagina resulting from a lack of estrogen. Marked by a thinning of the epithelium, reduced blood supply, and loss of glycogen, vulvovaginal atrophy is to blame for GSM.
Vaginal laser therapy has been a popular option for women for the last decade, despite a lack of evidence supporting its use or approval from regulators.
The FDA has issued broad clearance for laser therapy for incision, ablation, vaporization, and coagulation of body soft tissues, such as dysplasia, vulvar or anal neoplasia, endometriosis, condylomas, and other disorders. However, the agency has not approved the use of laser therapy for vulvovaginal atrophy, GSM, vaginal dryness, or dyspareunia.
Evidence regarding vaginal laser therapy
According to Dr. Iglesia, the evidence for vaginal laser therapy is mixed and of generally low quality. A systematic review published in the Journal of Sexual Medicine (2022 Jan 29. doi: 10.1016/j.jsxm.2021.12.010) presented mostly low-quality evidence from 25 studies and found promising data for genitourinary symptoms but not enough to justify its use for genitourinary symptoms just yet. Dr. Iglesia discussed her own small, multisite study of 62 participants, which compared vaginal laser with vaginal estrogen and found no differences between the two for multiple outcomes. (The study would have been larger if not for interruption from an FDA warning for an Investigational Device Exemption.)
A JAMA study from Australia found no difference between laser therapy and sham laser therapy, but the most recent systematic review, from JAMA Network Open, found no significant difference between vaginal laser and vaginal estrogen for vaginal and sexual function symptoms. This review, however, covered only the six existing randomized controlled trials, including Dr. Iglesia’s, which were small and had a follow-up period of only 3-6 months.
“There have only been a few randomized controlled trials comparing laser to vaginal estrogen therapy, and most of those did not include a placebo or sham arm,” Monica Christmas, MD, director of the Center for Women’s Integrated Health at the University of Chicago Medicine, said in an interview. “This is extremely important, as most of the trials that did include a sham arm did not find that laser was better than the sham.” Dr. Christmas was not a part of the presentation but attended it at NAMS.
The bottom line, she said, is that “current evidence is not sufficient to make conclusions on long-term safety or sustainability, nor is there compelling evidence to make claims on equivalence to vaginal estrogen therapy.” Currently, committee opinions from a half-dozen medical societies, including NAMS, oppose using vaginal laser therapy until rigorous, robust trials on long-term safety and efficacy have been conducted. The International Continence Society and International Society for the Study of Vulvovaginal Disease issued a joint statement in 2018 that emphasized that histologic changes from lasers do not necessarily equate with changes in function. The statement noted the lack of evidence for laser treatment of incontinence and prolapse and stated that it should not be used for vulvodynia or lichen sclerosus.
A 2020 statement from NAMS found “insufficient placebo-controlled trials of energy-based therapies, including laser, to draw conclusions of efficacy or safety or to make treatment recommendations.” A slightly more optimistic statement from the American Urogynecologic Society concluded that energy-based devices have shown short-term efficacy for menopause-related vaginal atrophy and dyspareunia, including effects lasting up to 1 year from fractionated laser for treat dyspareunia, but also noted that studies up to that time were small and measure various outcomes.
Recommendations on vaginal laser therapy
Given this landscape of uneven and poor-quality evidence, Dr. Iglesia provided several “common sense” recommendations for energy-based therapies, starting with the need for any practitioner to have working knowledge of vulvovaginal anatomy. Contraindications for laser therapy include any malignancy – especially gynecologic – undiagnosed bleeding, active herpes or other infections, radiation, and vaginal mesh, particularly transvaginal mesh. The provider also must discuss the limited data on long-term function and treatment alternatives, including FDA-approved therapies like topical estrogen, dehydroepiandrosterone sulfate (DHEA-S), ospemifene, and moisturizers, Dr. Iglesia said.
Adverse events associated with laser therapy, such as scarring or burning, are rare but do occur, and cost remains an issue, Dr. Iglesia said.
“Vaginal estrogen therapy is well established as a safe and effective treatment option based on high quality evidence,” Dr. Christmas said. “This is not the case for laser therapy. Rare, but serious harms are reported with vaginal laser, including burns, scarring, dyspareunia, pain, and potential irreversible damage.”
Dr. Iglesia also cautioned that clinicians should take extra care with vulnerable populations, particularly cancer patients and others with contraindications for estrogen treatment.
For those in whom vaginal estrogen is contraindicated, Dr. Christmas recommended vaginal moisturizers, lubricants, dilators, and physical therapy for the pelvic floor.
“In patients who fail those nonhormonal approaches, short courses of vaginal estrogen therapy or DHEA-S suppository may be employed with approval from their oncologist,” Dr. Christmas said.
Dr. Iglesia finally reviewed the major research questions that remain with laser therapy:
- What are outcomes for laser versus sham studies?
- What are long-term outcomes (beyond 6 months)
- What pretreatment is necessary?
- Could laser be used as a drug delivery mechanism for estrogen, and could this provide a synergistic effect?
- What is the optimal number and interval for laser treatments?
Dr. Iglesia had no industry disclosures but received honoraria for consulting at UpToDate. Dr. Christmas is a consultant for Materna. The presentation did not rely on any external funding.
FROM NAMS 2022
Nonblanching Rash on the Legs and Chest
The Diagnosis: Leukemia Cutis
Hematoxylin and eosin staining revealed an infiltration of monomorphic atypical myeloid cells with cleaved nuclei within the dermis, with a relatively uninvolved epidermis (Figure, A). The cells formed aggregates in single-file lines along dermal collagen bundles. Occasional Auer rods, which are crystal aggregates of the enzyme myeloperoxidase, a marker unique to cells of the myeloid lineage (Figure, B) were appreciated.
Immunohistochemical staining for myeloperoxidase was weakly positive; however, flow cytometric evaluation of the bone marrow aspirate revealed that approximately 20% of all CD45+ cells were myeloid blasts. These findings confirmed the diagnosis of recurrent acute myeloid leukemia (AML). The diagnosis of AML can be confirmed with a bone marrow biopsy demonstrating more than 20% of the total cells in blast form as well as evidence that the cells are of myeloid origin, which can be inferred by the presence of Auer rods, positive myeloperoxidase staining, or immunophenotyping. In our patient, the Auer rods, myeloperoxidase staining, and atypical myeloid cells on skin biopsy, in conjunction with the bone marrow biopsy results, confirmed leukemia cutis.
Leukemia cutis is the infiltration of neoplastic proliferating leukocytes in the epidermis, dermis, or subcutis from a primary or more commonly metastatic malignancy. Leukemic cutaneous involvement is seen in up to 13% of leukemia patients and most commonly is seen in monocytic or myelomonocytic forms of AML.1 It may present anywhere on the body but mostly is found on the back, trunk, and head. It also may have a predilection for areas with a history of trauma or inflammation. The lesions most often are firm, erythematous to violaceous papules and nodules, though leukemia cutis can present with hemorrhagic ulcers, purpura, or other cutaneous manifestations of concomitant thrombocytopenia such as petechiae and ecchymoses.2 Involvement of the lower extremities mimicking venous stasis dermatitis has been described.3,4
Treatment of leukemia cutis requires targeting the underlying leukemia2 under the guidance of hematology and oncology as well as the use of chemotherapeutic agents.5 The presence of leukemia cutis is a poor prognostic sign, and a discussion regarding goals of care often is appropriate. Our patient initially responded to FLAG (fludarabine, cytarabine, filgrastim) chemotherapy induction and consolidation, which was followed by midostaurin maintenance. However, she ultimately regressed, requiring decitabine and gilteritinib treatment, and died 9 months later from the course of the disease.
Although typically asymptomatic and presenting on the lower limbs, capillaritis (also known as the pigmented purpuric dermatoses) consists of a set of cutaneous conditions that often are chronic and relapsing in nature, as opposed to our patient’s subacute presentation. These benign conditions have several distinct morphologies; some are characterized by pigmented macules or pinpoint red-brown petechiae that most often are found on the legs but also are seen on the trunk and upper extremities.6 Of the various clinical presentations of capillaritis, our patient’s skin findings may be most consistent with pigmented purpuric lichenoid dermatitis of Gougerot and Blum, in which purpuric red-brown papules coalesce into plaques, though her lesions were not raised. The other pigmented purpuric dermatoses can present with cayenne pepper–colored petechiae, golden-brown macules, pruritic purpuric patches, or red-brown annular patches,6 which were not seen in our patient.
Venous stasis dermatitis also favors the lower extremities7; however, it classically includes the medial malleolus and often presents with scaling and hyperpigmentation from hemosiderin deposition.8 It often is associated with pruritus, as opposed to the nonpruritic nonpainful lesions in leukemia cutis. Other signs of venous insufficiency also may be appreciated, including edema or varicose veins,7 which were not evident in our patient.
Leukocytoclastic vasculitis, a small vessel vasculitis, also appears as palpable or macular purpura, which classically is asymptomatic and erupts on the shins approximately 1 week after an inciting exposure,9 such as medications, pathogens, or autoimmune diseases. One of the least distinctive vasculitides is polyarteritis nodosa, a form of medium vessel vasculitis, which presents most often with palpable purpura or painful nodules on the lower extremities and may be accompanied by livedo reticularis or digital necrosis.9 Acute leukemia may be accompanied by inflammatory paraneoplastic conditions including vasculitis, which is thought to be due to leukemic cells infiltrating and damaging blood vessels.10
Pretibial myxedema is closely associated with Graves disease and shares some features seen in the presentation of our patient’s leukemia cutis. It is asymptomatic, classically affects the pretibial regions, and most commonly affects older adults and women.11,12 Pretibial myxedema presents with thick indurated plaques rather than patches. Our patient did not demonstrate ophthalmopathy, which nearly always precedes pretibial myxedema.12 The most common form of pretibial myxedema is nonpitting, though nodular, plaquelike, polypoid, and elephantiasic forms also exist.11 Pretibial myxedema classically favors the shins; however, it also can affect the ankles, dorsal aspects of the feet, and toes. The characteristic induration of the skin is believed to be the result of excess fibroblast production of glycosaminoglycans in the dermis and subcutis likely triggered by stimulation of fibroblast thyroid stimulating hormone receptors.11
- Bakst RL, Tallman MS, Douer D, et al. How I treat extramedullary acute myeloid leukemia. Blood. 2011;118:3785-3793.
- Bolognia JL, Schaffer JV, Duncan KO, et al. Other lymphoproliferative and myeloproliferative diseases. In: Bolognia JL, Schaffer JV, Duncan KO, et al, eds. Dermatology Essentials. 2nd ed. Elsevier; 2014:973-977.
- Papadavid E, Panayiotides I, Katoulis A, et al. Stasis dermatitis-like leukaemic infiltration in a patient with myelodysplastic syndrome. Clin Exp Dermatol. 2008;33:298-300.
- Chang HY, Wong KM, Bosenberg M, et al. Myelogenous leukemia cutis resembling stasis dermatitis. J Am Acad Dermatol. 2003;49:128-129.
- Aguilera SB, Zarraga M, Rosen L. Leukemia cutis in a patient with acute myelogenous leukemia: a case report and review of the literature. Cutis. 2010;85:31-36.
- Kim DH, Seo SH, Ahn HH, et al. Characteristics and clinical manifestations of pigmented purpuric dermatosis. Ann Dermatol. 2015;27:404-410.
- Bolognia JL, Schaffer JV, Duncan KO, et al. Other eczematous eruptions. In: Bolognia JL, Schaffer JV, Duncan KO, et al, eds. Dermatology Essentials. 2nd ed. Elsevier; 2014:103-108.
- Krooks JA, Weatherall AG. Leukemia cutis in acute myeloid leukemia signifies a poor prognosis. Cutis. 2018;102:266, 271-272.
- Wetter DA, Dutz JP, Shinkai K, et al. Cutaneous vasculitis. In: Bolognia JL, Schaffer JV, Lorenzo C, eds. Dermatology. 4th ed. Elsevier; 2018:409-439.
- Jones D, Dorfman DM, Barnhill RL, et al. Leukemic vasculitis: a feature of leukemia cutis in some patients. Am J Clin Pathol. 1997;107:637-642.
- Fatourechi V. Pretibial myxedema: pathophysiology and treatment options. Am J Clin Dermatol. 2005;6:295-309.
- Fatourechi V, Pajouhi M, Fransway AF. Dermopathy of Graves disease (pretibial myxedema). review of 150 cases. Medicine (Baltimore). 1994;73:1-7.
The Diagnosis: Leukemia Cutis
Hematoxylin and eosin staining revealed an infiltration of monomorphic atypical myeloid cells with cleaved nuclei within the dermis, with a relatively uninvolved epidermis (Figure, A). The cells formed aggregates in single-file lines along dermal collagen bundles. Occasional Auer rods, which are crystal aggregates of the enzyme myeloperoxidase, a marker unique to cells of the myeloid lineage (Figure, B) were appreciated.

Immunohistochemical staining for myeloperoxidase was weakly positive; however, flow cytometric evaluation of the bone marrow aspirate revealed that approximately 20% of all CD45+ cells were myeloid blasts. These findings confirmed the diagnosis of recurrent acute myeloid leukemia (AML). The diagnosis of AML can be confirmed with a bone marrow biopsy demonstrating more than 20% of the total cells in blast form as well as evidence that the cells are of myeloid origin, which can be inferred by the presence of Auer rods, positive myeloperoxidase staining, or immunophenotyping. In our patient, the Auer rods, myeloperoxidase staining, and atypical myeloid cells on skin biopsy, in conjunction with the bone marrow biopsy results, confirmed leukemia cutis.
Leukemia cutis is the infiltration of neoplastic proliferating leukocytes in the epidermis, dermis, or subcutis from a primary or more commonly metastatic malignancy. Leukemic cutaneous involvement is seen in up to 13% of leukemia patients and most commonly is seen in monocytic or myelomonocytic forms of AML.1 It may present anywhere on the body but mostly is found on the back, trunk, and head. It also may have a predilection for areas with a history of trauma or inflammation. The lesions most often are firm, erythematous to violaceous papules and nodules, though leukemia cutis can present with hemorrhagic ulcers, purpura, or other cutaneous manifestations of concomitant thrombocytopenia such as petechiae and ecchymoses.2 Involvement of the lower extremities mimicking venous stasis dermatitis has been described.3,4
Treatment of leukemia cutis requires targeting the underlying leukemia2 under the guidance of hematology and oncology as well as the use of chemotherapeutic agents.5 The presence of leukemia cutis is a poor prognostic sign, and a discussion regarding goals of care often is appropriate. Our patient initially responded to FLAG (fludarabine, cytarabine, filgrastim) chemotherapy induction and consolidation, which was followed by midostaurin maintenance. However, she ultimately regressed, requiring decitabine and gilteritinib treatment, and died 9 months later from the course of the disease.
Although typically asymptomatic and presenting on the lower limbs, capillaritis (also known as the pigmented purpuric dermatoses) consists of a set of cutaneous conditions that often are chronic and relapsing in nature, as opposed to our patient’s subacute presentation. These benign conditions have several distinct morphologies; some are characterized by pigmented macules or pinpoint red-brown petechiae that most often are found on the legs but also are seen on the trunk and upper extremities.6 Of the various clinical presentations of capillaritis, our patient’s skin findings may be most consistent with pigmented purpuric lichenoid dermatitis of Gougerot and Blum, in which purpuric red-brown papules coalesce into plaques, though her lesions were not raised. The other pigmented purpuric dermatoses can present with cayenne pepper–colored petechiae, golden-brown macules, pruritic purpuric patches, or red-brown annular patches,6 which were not seen in our patient.
Venous stasis dermatitis also favors the lower extremities7; however, it classically includes the medial malleolus and often presents with scaling and hyperpigmentation from hemosiderin deposition.8 It often is associated with pruritus, as opposed to the nonpruritic nonpainful lesions in leukemia cutis. Other signs of venous insufficiency also may be appreciated, including edema or varicose veins,7 which were not evident in our patient.
Leukocytoclastic vasculitis, a small vessel vasculitis, also appears as palpable or macular purpura, which classically is asymptomatic and erupts on the shins approximately 1 week after an inciting exposure,9 such as medications, pathogens, or autoimmune diseases. One of the least distinctive vasculitides is polyarteritis nodosa, a form of medium vessel vasculitis, which presents most often with palpable purpura or painful nodules on the lower extremities and may be accompanied by livedo reticularis or digital necrosis.9 Acute leukemia may be accompanied by inflammatory paraneoplastic conditions including vasculitis, which is thought to be due to leukemic cells infiltrating and damaging blood vessels.10
Pretibial myxedema is closely associated with Graves disease and shares some features seen in the presentation of our patient’s leukemia cutis. It is asymptomatic, classically affects the pretibial regions, and most commonly affects older adults and women.11,12 Pretibial myxedema presents with thick indurated plaques rather than patches. Our patient did not demonstrate ophthalmopathy, which nearly always precedes pretibial myxedema.12 The most common form of pretibial myxedema is nonpitting, though nodular, plaquelike, polypoid, and elephantiasic forms also exist.11 Pretibial myxedema classically favors the shins; however, it also can affect the ankles, dorsal aspects of the feet, and toes. The characteristic induration of the skin is believed to be the result of excess fibroblast production of glycosaminoglycans in the dermis and subcutis likely triggered by stimulation of fibroblast thyroid stimulating hormone receptors.11
The Diagnosis: Leukemia Cutis
Hematoxylin and eosin staining revealed an infiltration of monomorphic atypical myeloid cells with cleaved nuclei within the dermis, with a relatively uninvolved epidermis (Figure, A). The cells formed aggregates in single-file lines along dermal collagen bundles. Occasional Auer rods, which are crystal aggregates of the enzyme myeloperoxidase, a marker unique to cells of the myeloid lineage (Figure, B) were appreciated.

Immunohistochemical staining for myeloperoxidase was weakly positive; however, flow cytometric evaluation of the bone marrow aspirate revealed that approximately 20% of all CD45+ cells were myeloid blasts. These findings confirmed the diagnosis of recurrent acute myeloid leukemia (AML). The diagnosis of AML can be confirmed with a bone marrow biopsy demonstrating more than 20% of the total cells in blast form as well as evidence that the cells are of myeloid origin, which can be inferred by the presence of Auer rods, positive myeloperoxidase staining, or immunophenotyping. In our patient, the Auer rods, myeloperoxidase staining, and atypical myeloid cells on skin biopsy, in conjunction with the bone marrow biopsy results, confirmed leukemia cutis.
Leukemia cutis is the infiltration of neoplastic proliferating leukocytes in the epidermis, dermis, or subcutis from a primary or more commonly metastatic malignancy. Leukemic cutaneous involvement is seen in up to 13% of leukemia patients and most commonly is seen in monocytic or myelomonocytic forms of AML.1 It may present anywhere on the body but mostly is found on the back, trunk, and head. It also may have a predilection for areas with a history of trauma or inflammation. The lesions most often are firm, erythematous to violaceous papules and nodules, though leukemia cutis can present with hemorrhagic ulcers, purpura, or other cutaneous manifestations of concomitant thrombocytopenia such as petechiae and ecchymoses.2 Involvement of the lower extremities mimicking venous stasis dermatitis has been described.3,4
Treatment of leukemia cutis requires targeting the underlying leukemia2 under the guidance of hematology and oncology as well as the use of chemotherapeutic agents.5 The presence of leukemia cutis is a poor prognostic sign, and a discussion regarding goals of care often is appropriate. Our patient initially responded to FLAG (fludarabine, cytarabine, filgrastim) chemotherapy induction and consolidation, which was followed by midostaurin maintenance. However, she ultimately regressed, requiring decitabine and gilteritinib treatment, and died 9 months later from the course of the disease.
Although typically asymptomatic and presenting on the lower limbs, capillaritis (also known as the pigmented purpuric dermatoses) consists of a set of cutaneous conditions that often are chronic and relapsing in nature, as opposed to our patient’s subacute presentation. These benign conditions have several distinct morphologies; some are characterized by pigmented macules or pinpoint red-brown petechiae that most often are found on the legs but also are seen on the trunk and upper extremities.6 Of the various clinical presentations of capillaritis, our patient’s skin findings may be most consistent with pigmented purpuric lichenoid dermatitis of Gougerot and Blum, in which purpuric red-brown papules coalesce into plaques, though her lesions were not raised. The other pigmented purpuric dermatoses can present with cayenne pepper–colored petechiae, golden-brown macules, pruritic purpuric patches, or red-brown annular patches,6 which were not seen in our patient.
Venous stasis dermatitis also favors the lower extremities7; however, it classically includes the medial malleolus and often presents with scaling and hyperpigmentation from hemosiderin deposition.8 It often is associated with pruritus, as opposed to the nonpruritic nonpainful lesions in leukemia cutis. Other signs of venous insufficiency also may be appreciated, including edema or varicose veins,7 which were not evident in our patient.
Leukocytoclastic vasculitis, a small vessel vasculitis, also appears as palpable or macular purpura, which classically is asymptomatic and erupts on the shins approximately 1 week after an inciting exposure,9 such as medications, pathogens, or autoimmune diseases. One of the least distinctive vasculitides is polyarteritis nodosa, a form of medium vessel vasculitis, which presents most often with palpable purpura or painful nodules on the lower extremities and may be accompanied by livedo reticularis or digital necrosis.9 Acute leukemia may be accompanied by inflammatory paraneoplastic conditions including vasculitis, which is thought to be due to leukemic cells infiltrating and damaging blood vessels.10
Pretibial myxedema is closely associated with Graves disease and shares some features seen in the presentation of our patient’s leukemia cutis. It is asymptomatic, classically affects the pretibial regions, and most commonly affects older adults and women.11,12 Pretibial myxedema presents with thick indurated plaques rather than patches. Our patient did not demonstrate ophthalmopathy, which nearly always precedes pretibial myxedema.12 The most common form of pretibial myxedema is nonpitting, though nodular, plaquelike, polypoid, and elephantiasic forms also exist.11 Pretibial myxedema classically favors the shins; however, it also can affect the ankles, dorsal aspects of the feet, and toes. The characteristic induration of the skin is believed to be the result of excess fibroblast production of glycosaminoglycans in the dermis and subcutis likely triggered by stimulation of fibroblast thyroid stimulating hormone receptors.11
- Bakst RL, Tallman MS, Douer D, et al. How I treat extramedullary acute myeloid leukemia. Blood. 2011;118:3785-3793.
- Bolognia JL, Schaffer JV, Duncan KO, et al. Other lymphoproliferative and myeloproliferative diseases. In: Bolognia JL, Schaffer JV, Duncan KO, et al, eds. Dermatology Essentials. 2nd ed. Elsevier; 2014:973-977.
- Papadavid E, Panayiotides I, Katoulis A, et al. Stasis dermatitis-like leukaemic infiltration in a patient with myelodysplastic syndrome. Clin Exp Dermatol. 2008;33:298-300.
- Chang HY, Wong KM, Bosenberg M, et al. Myelogenous leukemia cutis resembling stasis dermatitis. J Am Acad Dermatol. 2003;49:128-129.
- Aguilera SB, Zarraga M, Rosen L. Leukemia cutis in a patient with acute myelogenous leukemia: a case report and review of the literature. Cutis. 2010;85:31-36.
- Kim DH, Seo SH, Ahn HH, et al. Characteristics and clinical manifestations of pigmented purpuric dermatosis. Ann Dermatol. 2015;27:404-410.
- Bolognia JL, Schaffer JV, Duncan KO, et al. Other eczematous eruptions. In: Bolognia JL, Schaffer JV, Duncan KO, et al, eds. Dermatology Essentials. 2nd ed. Elsevier; 2014:103-108.
- Krooks JA, Weatherall AG. Leukemia cutis in acute myeloid leukemia signifies a poor prognosis. Cutis. 2018;102:266, 271-272.
- Wetter DA, Dutz JP, Shinkai K, et al. Cutaneous vasculitis. In: Bolognia JL, Schaffer JV, Lorenzo C, eds. Dermatology. 4th ed. Elsevier; 2018:409-439.
- Jones D, Dorfman DM, Barnhill RL, et al. Leukemic vasculitis: a feature of leukemia cutis in some patients. Am J Clin Pathol. 1997;107:637-642.
- Fatourechi V. Pretibial myxedema: pathophysiology and treatment options. Am J Clin Dermatol. 2005;6:295-309.
- Fatourechi V, Pajouhi M, Fransway AF. Dermopathy of Graves disease (pretibial myxedema). review of 150 cases. Medicine (Baltimore). 1994;73:1-7.
- Bakst RL, Tallman MS, Douer D, et al. How I treat extramedullary acute myeloid leukemia. Blood. 2011;118:3785-3793.
- Bolognia JL, Schaffer JV, Duncan KO, et al. Other lymphoproliferative and myeloproliferative diseases. In: Bolognia JL, Schaffer JV, Duncan KO, et al, eds. Dermatology Essentials. 2nd ed. Elsevier; 2014:973-977.
- Papadavid E, Panayiotides I, Katoulis A, et al. Stasis dermatitis-like leukaemic infiltration in a patient with myelodysplastic syndrome. Clin Exp Dermatol. 2008;33:298-300.
- Chang HY, Wong KM, Bosenberg M, et al. Myelogenous leukemia cutis resembling stasis dermatitis. J Am Acad Dermatol. 2003;49:128-129.
- Aguilera SB, Zarraga M, Rosen L. Leukemia cutis in a patient with acute myelogenous leukemia: a case report and review of the literature. Cutis. 2010;85:31-36.
- Kim DH, Seo SH, Ahn HH, et al. Characteristics and clinical manifestations of pigmented purpuric dermatosis. Ann Dermatol. 2015;27:404-410.
- Bolognia JL, Schaffer JV, Duncan KO, et al. Other eczematous eruptions. In: Bolognia JL, Schaffer JV, Duncan KO, et al, eds. Dermatology Essentials. 2nd ed. Elsevier; 2014:103-108.
- Krooks JA, Weatherall AG. Leukemia cutis in acute myeloid leukemia signifies a poor prognosis. Cutis. 2018;102:266, 271-272.
- Wetter DA, Dutz JP, Shinkai K, et al. Cutaneous vasculitis. In: Bolognia JL, Schaffer JV, Lorenzo C, eds. Dermatology. 4th ed. Elsevier; 2018:409-439.
- Jones D, Dorfman DM, Barnhill RL, et al. Leukemic vasculitis: a feature of leukemia cutis in some patients. Am J Clin Pathol. 1997;107:637-642.
- Fatourechi V. Pretibial myxedema: pathophysiology and treatment options. Am J Clin Dermatol. 2005;6:295-309.
- Fatourechi V, Pajouhi M, Fransway AF. Dermopathy of Graves disease (pretibial myxedema). review of 150 cases. Medicine (Baltimore). 1994;73:1-7.
A 67-year-old woman with history of atrial fibrillation and leukemia presented with a nonpruritic nonpainful rash of 10 days' duration that began on the distal lower extremities (top) and then spread superiorly. She reported having a sore throat and mouth, cough, night sweats, unintentional weight loss, and lymphadenopathy. Physical examination revealed pink-purple nonblanching macules and patches on the lower extremities extending from the ankles to the knees. She also had firm pink papules on the chest (bottom) and back. Punch biopsies of the skin on the chest and leg were obtained for histologic examination and immunohistochemical staining.
Vaccinium myrtillus (bilberry seed oil) extract
A member of the Ericaceae family, bilberry (Vaccinium myrtillus) is native to northern Europe and North America, and its fruit is known to contain myriad polyphenols that display potent antioxidant and anti-inflammatory activity.1,2 Also known as European blueberry or whortleberry, this perennial deciduous shrub is also one of the richest sources of the polyphenolic pigments anthocyanins.3-5 Indeed, anthocyanins impart the blue/black color to bilberries and other berries and are thought to be the primary bioactive constituents of berries associated with numerous health benefits.3,6 They are also known to confer anti-allergic, anticancer, and wound healing activity.4 Overall, bilberry has also been reported to exert anti-inflammatory, lipid-lowering, and antimicrobial activity.3 In this column, the focus will be on the chemical constituents and properties of V. myrtillus that indicate potential or applicability for skin care.
Active ingredients of bilberry
Bilberry seed oil contains unsaturated fatty acids such as linoleic acid and alpha-linolenic acid, which exhibit anti-inflammatory activity and contribute to the suppression of tyrosinase. For instance, Ando et al. showed, in 1998, that linoleic and alpha-linolenic acids lighten UV-induced skin hyperpigmentation. Their in vitro experiments using cultured murine melanoma cells and in vivo study of the topical application of either acid to the UV-induced hyperpigmented dorsal skin of guinea pigs revealed pigment-lightening effects that they partly ascribed to inhibited melanin synthesis by active melanocytes and accelerated desquamation of epidermal melanin pigment.7
A 2009 comparative study of the anthocyanin composition as well as antimicrobial and antioxidant activities delivered by bilberry and blueberry fruits and their skins by Burdulis et al. revealed robust functions in both fruits. Cyanidin was found to be an active anthocyanidin in bilberry. Cultivars of both fruits demonstrated antimicrobial and antioxidant activity, with bilberry fruit skin demonstrating potent antiradical activity.8
The anthocyanins of V. myrtillus are reputed to impart protection against cardiovascular disorders, age-induced oxidative stress, inflammatory responses, and various degenerative conditions, as well ameliorate neuronal and cognitive brain functions and ocular health.6
In 2012, Bornsek et al. demonstrated that bilberry (and blueberry) anthocyanins function as potent intracellular antioxidants, which may account for their noted health benefits despite relatively low bioavailability.9
Six years later, a chemical composition study of wild bilberry found in Montenegro, Brasanac-Vukanovic et al. determined that chlorogenic acid was the most prevalent phenolic constituent, followed by protocatechuic acid, with resveratrol, isoquercetin, quercetin, and hyperoside also found to be abundant. In vitro assays indicated significant antioxidant activity exhibited by these compounds.10
Activity against allergic contact dermatitis
Yamaura et al. used a mouse model, in 2011, to determine that the anthocyanins from a bilberry extract attenuated various symptoms of chronic allergic contact dermatitis, particularly alleviating pruritus.8 A year later, Yamaura et al. used a BALB/c mouse model of allergic contact dermatitis to compare the antipruritic effect of anthocyanin-rich quality-controlled bilberry extract and anthocyanidin-rich degraded extract. The investigators found that anthocyanins, but not anthocyanidins, derived from bilberry exert an antipruritic effect, likely through their inhibitory action on mast cell degranulation. They concluded that anthocyanin-rich bilberry extract could act as an effective oral supplement to treat pruritic symptoms of skin disorders such as chronic allergic contact dermatitis and atopic dermatitis.11
Antioxidant and anti-inflammatory activity
Bilberries, consumed since ancient times, are reputed to function as potent antioxidants because of a wide array of phenolic constituents, and this fruit is gaining interest for use in pharmaceuticals.12
In 2008, Svobodová et al. assessed possible UVA preventive properties of V. myrtillus fruit extract in a human keratinocyte cell line (HaCaT), finding that pre- or posttreatment mitigated UVA-induced harm. They also observed a significant decrease in UVA-caused reactive oxygen species (ROS) formation and the prevention or attenuation of UVA-stimulated peroxidation of membrane lipids. Intracellular glutathione was also protected. The investigators attributed the array of cytoprotective effects conferred by V. myrtillus extract primarily to its constituent anthocyanins.2 A year later, they found that the phenolic fraction of V. myrtillus fruits inhibited UVB-induced damage to HaCaT keratinocytes in vitro.13
In 2014, Calò and Marabini used HaCaT keratinocytes to ascertain whether a water-soluble V. myrtillus extract could mitigate UVA- and UVB-induced damage. They found that the extract diminished UVB-induced cytotoxicity and genotoxicity at lower doses, decreasing lipid peroxidation but exerting no effect on reactive oxygen species generated by UVB. The extract attenuated genotoxicity induced by UVA as well as ROS and apoptosis. Overall, the investigators concluded that V. myrtillus extract demonstrated antioxidant activity, particularly against UVA exposure.14
Four years later, Bucci et al. developed nanoberries, an ultradeformable liposome carrying V. myrtillus ethanolic extract, and determined that the preparation could penetrate the stratum corneum safely and suggested potential for yielding protection against photodamage.15
Skin preparations
In 2021, Tadic et al. developed an oil-in-water (O/W) cream containing wild bilberry leaf extracts and seed oil. The leaves contained copious phenolic acids (particularly chlorogenic acid), flavonoids (especially isoquercetin), and resveratrol. The seed oil was rife with alpha-linolenic, linoleic, and oleic acids. The investigators conducted an in vivo study over 30 days in 25 healthy volunteers (20 women, 5 men; mean age 23.36 ± 0.64 years). They found that the O/W cream successfully increased stratum corneum hydration, enhanced skin barrier function, and maintained skin pH after topical application. The cream was also well tolerated. In vitro assays also indicated that the bilberry isolates displayed notable antioxidant capacity (stronger in the case of the leaves). Tadic et al. suggested that skin disorders characterized by oxidative stress and/or xerosis may be appropriate targets for topically applied bilberry cream.1
Early in 2022, Ruscinc et al. reported on their efforts to incorporate V. myrtillus extract into a multifunctional sunscreen. In vitro and in vivo tests revealed that while sun protection factor was lowered in the presence of the extract, the samples were safe and photostable. The researchers concluded that further study is necessary to elucidate the effect of V. myrtillus extract on photoprotection.16
V. myrtillus has been consumed by human beings for many generations. Skin care formulations based on this ingredient have not been associated with adverse events. Notably, the Environmental Working Group has rated V. myrtillus (bilberry seed) oil as very safe.17
Summary
While research, particularly in the form of randomized controlled trials, is called for, because the fatty acids it contains have been shown to suppress tyrosinase. Currently, this botanical agent seems to be most suited for sensitive, aging skin and for skin with an uneven tone, particularly postinflammatory pigmentation and melasma.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, an SaaS company used to generate skin care routines in office and as an ecommerce solution. Write to her at [email protected].
References
1. Tadic VM et al. Antioxidants (Basel). 2021 Mar 16;10(3):465.
2. Svobodová A et al. Biofactors. 2008;33(4):249-66.
3. Chu WK et al. Bilberry (Vaccinium myrtillus L.), in Benzie IFF, Wachtel-Galor S, eds., “Herbal Medicine: Biomolecular and Clinical Aspects,” 2nd ed. (Boca Raton, Fla.: CRC Press/Taylor & Francis, 2011, Chapter 4).
4. Yamaura K et al. Pharmacognosy Res. 2011 Jul;3(3):173-7.
5. Stefanescu BE et al. Molecules. 2019 May 29;24(11):2046.
6. Smeriglio A et al. Mini Rev Med Chem. 2014;14(7):567-84.
7. Ando H et al. Arch Dermatol Res. 1998 Jul;290(7):375-81.
8. Burdulis D et al. Acta Pol Pharm. 2009 Jul-Aug;66(4):399-408.
9. Bornsek SM et al. Food Chem. 2012 Oct 15;134(4):1878-84.
10. Brasanac-Vukanovic S et al. Molecules. 2018 Jul 26;23(8):1864.
11. Yamaura K et al. J Food Sci. 2012 Dec;77(12):H262-7.
12. Pires TCSP et al. Curr Pharm Des. 2020;26(16):1917-28.
13. Svobodová A et al. J Dermatol Sci. 2009 Dec;56(3):196-204.
14. Calò R, Marabini L. J Photochem Photobiol B. 2014 Mar 5;132:27-35.
15. Bucci P et al. J Cosmet Dermatol. 2018 Oct;17(5):889-99.
16. Ruscinc N et al. J Cosmet Dermatol. 2022 Jan 13.
17. Environmental Working Group’s Skin Deep website. Vaccinium Myrtillus Bilberry Seed Oil. Accessed October 18, 2022.
A member of the Ericaceae family, bilberry (Vaccinium myrtillus) is native to northern Europe and North America, and its fruit is known to contain myriad polyphenols that display potent antioxidant and anti-inflammatory activity.1,2 Also known as European blueberry or whortleberry, this perennial deciduous shrub is also one of the richest sources of the polyphenolic pigments anthocyanins.3-5 Indeed, anthocyanins impart the blue/black color to bilberries and other berries and are thought to be the primary bioactive constituents of berries associated with numerous health benefits.3,6 They are also known to confer anti-allergic, anticancer, and wound healing activity.4 Overall, bilberry has also been reported to exert anti-inflammatory, lipid-lowering, and antimicrobial activity.3 In this column, the focus will be on the chemical constituents and properties of V. myrtillus that indicate potential or applicability for skin care.
Active ingredients of bilberry
Bilberry seed oil contains unsaturated fatty acids such as linoleic acid and alpha-linolenic acid, which exhibit anti-inflammatory activity and contribute to the suppression of tyrosinase. For instance, Ando et al. showed, in 1998, that linoleic and alpha-linolenic acids lighten UV-induced skin hyperpigmentation. Their in vitro experiments using cultured murine melanoma cells and in vivo study of the topical application of either acid to the UV-induced hyperpigmented dorsal skin of guinea pigs revealed pigment-lightening effects that they partly ascribed to inhibited melanin synthesis by active melanocytes and accelerated desquamation of epidermal melanin pigment.7
A 2009 comparative study of the anthocyanin composition as well as antimicrobial and antioxidant activities delivered by bilberry and blueberry fruits and their skins by Burdulis et al. revealed robust functions in both fruits. Cyanidin was found to be an active anthocyanidin in bilberry. Cultivars of both fruits demonstrated antimicrobial and antioxidant activity, with bilberry fruit skin demonstrating potent antiradical activity.8
The anthocyanins of V. myrtillus are reputed to impart protection against cardiovascular disorders, age-induced oxidative stress, inflammatory responses, and various degenerative conditions, as well ameliorate neuronal and cognitive brain functions and ocular health.6
In 2012, Bornsek et al. demonstrated that bilberry (and blueberry) anthocyanins function as potent intracellular antioxidants, which may account for their noted health benefits despite relatively low bioavailability.9
Six years later, a chemical composition study of wild bilberry found in Montenegro, Brasanac-Vukanovic et al. determined that chlorogenic acid was the most prevalent phenolic constituent, followed by protocatechuic acid, with resveratrol, isoquercetin, quercetin, and hyperoside also found to be abundant. In vitro assays indicated significant antioxidant activity exhibited by these compounds.10
Activity against allergic contact dermatitis
Yamaura et al. used a mouse model, in 2011, to determine that the anthocyanins from a bilberry extract attenuated various symptoms of chronic allergic contact dermatitis, particularly alleviating pruritus.8 A year later, Yamaura et al. used a BALB/c mouse model of allergic contact dermatitis to compare the antipruritic effect of anthocyanin-rich quality-controlled bilberry extract and anthocyanidin-rich degraded extract. The investigators found that anthocyanins, but not anthocyanidins, derived from bilberry exert an antipruritic effect, likely through their inhibitory action on mast cell degranulation. They concluded that anthocyanin-rich bilberry extract could act as an effective oral supplement to treat pruritic symptoms of skin disorders such as chronic allergic contact dermatitis and atopic dermatitis.11
Antioxidant and anti-inflammatory activity
Bilberries, consumed since ancient times, are reputed to function as potent antioxidants because of a wide array of phenolic constituents, and this fruit is gaining interest for use in pharmaceuticals.12
In 2008, Svobodová et al. assessed possible UVA preventive properties of V. myrtillus fruit extract in a human keratinocyte cell line (HaCaT), finding that pre- or posttreatment mitigated UVA-induced harm. They also observed a significant decrease in UVA-caused reactive oxygen species (ROS) formation and the prevention or attenuation of UVA-stimulated peroxidation of membrane lipids. Intracellular glutathione was also protected. The investigators attributed the array of cytoprotective effects conferred by V. myrtillus extract primarily to its constituent anthocyanins.2 A year later, they found that the phenolic fraction of V. myrtillus fruits inhibited UVB-induced damage to HaCaT keratinocytes in vitro.13
In 2014, Calò and Marabini used HaCaT keratinocytes to ascertain whether a water-soluble V. myrtillus extract could mitigate UVA- and UVB-induced damage. They found that the extract diminished UVB-induced cytotoxicity and genotoxicity at lower doses, decreasing lipid peroxidation but exerting no effect on reactive oxygen species generated by UVB. The extract attenuated genotoxicity induced by UVA as well as ROS and apoptosis. Overall, the investigators concluded that V. myrtillus extract demonstrated antioxidant activity, particularly against UVA exposure.14
Four years later, Bucci et al. developed nanoberries, an ultradeformable liposome carrying V. myrtillus ethanolic extract, and determined that the preparation could penetrate the stratum corneum safely and suggested potential for yielding protection against photodamage.15
Skin preparations
In 2021, Tadic et al. developed an oil-in-water (O/W) cream containing wild bilberry leaf extracts and seed oil. The leaves contained copious phenolic acids (particularly chlorogenic acid), flavonoids (especially isoquercetin), and resveratrol. The seed oil was rife with alpha-linolenic, linoleic, and oleic acids. The investigators conducted an in vivo study over 30 days in 25 healthy volunteers (20 women, 5 men; mean age 23.36 ± 0.64 years). They found that the O/W cream successfully increased stratum corneum hydration, enhanced skin barrier function, and maintained skin pH after topical application. The cream was also well tolerated. In vitro assays also indicated that the bilberry isolates displayed notable antioxidant capacity (stronger in the case of the leaves). Tadic et al. suggested that skin disorders characterized by oxidative stress and/or xerosis may be appropriate targets for topically applied bilberry cream.1
Early in 2022, Ruscinc et al. reported on their efforts to incorporate V. myrtillus extract into a multifunctional sunscreen. In vitro and in vivo tests revealed that while sun protection factor was lowered in the presence of the extract, the samples were safe and photostable. The researchers concluded that further study is necessary to elucidate the effect of V. myrtillus extract on photoprotection.16
V. myrtillus has been consumed by human beings for many generations. Skin care formulations based on this ingredient have not been associated with adverse events. Notably, the Environmental Working Group has rated V. myrtillus (bilberry seed) oil as very safe.17
Summary
While research, particularly in the form of randomized controlled trials, is called for, because the fatty acids it contains have been shown to suppress tyrosinase. Currently, this botanical agent seems to be most suited for sensitive, aging skin and for skin with an uneven tone, particularly postinflammatory pigmentation and melasma.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, an SaaS company used to generate skin care routines in office and as an ecommerce solution. Write to her at [email protected].
References
1. Tadic VM et al. Antioxidants (Basel). 2021 Mar 16;10(3):465.
2. Svobodová A et al. Biofactors. 2008;33(4):249-66.
3. Chu WK et al. Bilberry (Vaccinium myrtillus L.), in Benzie IFF, Wachtel-Galor S, eds., “Herbal Medicine: Biomolecular and Clinical Aspects,” 2nd ed. (Boca Raton, Fla.: CRC Press/Taylor & Francis, 2011, Chapter 4).
4. Yamaura K et al. Pharmacognosy Res. 2011 Jul;3(3):173-7.
5. Stefanescu BE et al. Molecules. 2019 May 29;24(11):2046.
6. Smeriglio A et al. Mini Rev Med Chem. 2014;14(7):567-84.
7. Ando H et al. Arch Dermatol Res. 1998 Jul;290(7):375-81.
8. Burdulis D et al. Acta Pol Pharm. 2009 Jul-Aug;66(4):399-408.
9. Bornsek SM et al. Food Chem. 2012 Oct 15;134(4):1878-84.
10. Brasanac-Vukanovic S et al. Molecules. 2018 Jul 26;23(8):1864.
11. Yamaura K et al. J Food Sci. 2012 Dec;77(12):H262-7.
12. Pires TCSP et al. Curr Pharm Des. 2020;26(16):1917-28.
13. Svobodová A et al. J Dermatol Sci. 2009 Dec;56(3):196-204.
14. Calò R, Marabini L. J Photochem Photobiol B. 2014 Mar 5;132:27-35.
15. Bucci P et al. J Cosmet Dermatol. 2018 Oct;17(5):889-99.
16. Ruscinc N et al. J Cosmet Dermatol. 2022 Jan 13.
17. Environmental Working Group’s Skin Deep website. Vaccinium Myrtillus Bilberry Seed Oil. Accessed October 18, 2022.
A member of the Ericaceae family, bilberry (Vaccinium myrtillus) is native to northern Europe and North America, and its fruit is known to contain myriad polyphenols that display potent antioxidant and anti-inflammatory activity.1,2 Also known as European blueberry or whortleberry, this perennial deciduous shrub is also one of the richest sources of the polyphenolic pigments anthocyanins.3-5 Indeed, anthocyanins impart the blue/black color to bilberries and other berries and are thought to be the primary bioactive constituents of berries associated with numerous health benefits.3,6 They are also known to confer anti-allergic, anticancer, and wound healing activity.4 Overall, bilberry has also been reported to exert anti-inflammatory, lipid-lowering, and antimicrobial activity.3 In this column, the focus will be on the chemical constituents and properties of V. myrtillus that indicate potential or applicability for skin care.
Active ingredients of bilberry
Bilberry seed oil contains unsaturated fatty acids such as linoleic acid and alpha-linolenic acid, which exhibit anti-inflammatory activity and contribute to the suppression of tyrosinase. For instance, Ando et al. showed, in 1998, that linoleic and alpha-linolenic acids lighten UV-induced skin hyperpigmentation. Their in vitro experiments using cultured murine melanoma cells and in vivo study of the topical application of either acid to the UV-induced hyperpigmented dorsal skin of guinea pigs revealed pigment-lightening effects that they partly ascribed to inhibited melanin synthesis by active melanocytes and accelerated desquamation of epidermal melanin pigment.7
A 2009 comparative study of the anthocyanin composition as well as antimicrobial and antioxidant activities delivered by bilberry and blueberry fruits and their skins by Burdulis et al. revealed robust functions in both fruits. Cyanidin was found to be an active anthocyanidin in bilberry. Cultivars of both fruits demonstrated antimicrobial and antioxidant activity, with bilberry fruit skin demonstrating potent antiradical activity.8
The anthocyanins of V. myrtillus are reputed to impart protection against cardiovascular disorders, age-induced oxidative stress, inflammatory responses, and various degenerative conditions, as well ameliorate neuronal and cognitive brain functions and ocular health.6
In 2012, Bornsek et al. demonstrated that bilberry (and blueberry) anthocyanins function as potent intracellular antioxidants, which may account for their noted health benefits despite relatively low bioavailability.9
Six years later, a chemical composition study of wild bilberry found in Montenegro, Brasanac-Vukanovic et al. determined that chlorogenic acid was the most prevalent phenolic constituent, followed by protocatechuic acid, with resveratrol, isoquercetin, quercetin, and hyperoside also found to be abundant. In vitro assays indicated significant antioxidant activity exhibited by these compounds.10
Activity against allergic contact dermatitis
Yamaura et al. used a mouse model, in 2011, to determine that the anthocyanins from a bilberry extract attenuated various symptoms of chronic allergic contact dermatitis, particularly alleviating pruritus.8 A year later, Yamaura et al. used a BALB/c mouse model of allergic contact dermatitis to compare the antipruritic effect of anthocyanin-rich quality-controlled bilberry extract and anthocyanidin-rich degraded extract. The investigators found that anthocyanins, but not anthocyanidins, derived from bilberry exert an antipruritic effect, likely through their inhibitory action on mast cell degranulation. They concluded that anthocyanin-rich bilberry extract could act as an effective oral supplement to treat pruritic symptoms of skin disorders such as chronic allergic contact dermatitis and atopic dermatitis.11
Antioxidant and anti-inflammatory activity
Bilberries, consumed since ancient times, are reputed to function as potent antioxidants because of a wide array of phenolic constituents, and this fruit is gaining interest for use in pharmaceuticals.12
In 2008, Svobodová et al. assessed possible UVA preventive properties of V. myrtillus fruit extract in a human keratinocyte cell line (HaCaT), finding that pre- or posttreatment mitigated UVA-induced harm. They also observed a significant decrease in UVA-caused reactive oxygen species (ROS) formation and the prevention or attenuation of UVA-stimulated peroxidation of membrane lipids. Intracellular glutathione was also protected. The investigators attributed the array of cytoprotective effects conferred by V. myrtillus extract primarily to its constituent anthocyanins.2 A year later, they found that the phenolic fraction of V. myrtillus fruits inhibited UVB-induced damage to HaCaT keratinocytes in vitro.13
In 2014, Calò and Marabini used HaCaT keratinocytes to ascertain whether a water-soluble V. myrtillus extract could mitigate UVA- and UVB-induced damage. They found that the extract diminished UVB-induced cytotoxicity and genotoxicity at lower doses, decreasing lipid peroxidation but exerting no effect on reactive oxygen species generated by UVB. The extract attenuated genotoxicity induced by UVA as well as ROS and apoptosis. Overall, the investigators concluded that V. myrtillus extract demonstrated antioxidant activity, particularly against UVA exposure.14
Four years later, Bucci et al. developed nanoberries, an ultradeformable liposome carrying V. myrtillus ethanolic extract, and determined that the preparation could penetrate the stratum corneum safely and suggested potential for yielding protection against photodamage.15
Skin preparations
In 2021, Tadic et al. developed an oil-in-water (O/W) cream containing wild bilberry leaf extracts and seed oil. The leaves contained copious phenolic acids (particularly chlorogenic acid), flavonoids (especially isoquercetin), and resveratrol. The seed oil was rife with alpha-linolenic, linoleic, and oleic acids. The investigators conducted an in vivo study over 30 days in 25 healthy volunteers (20 women, 5 men; mean age 23.36 ± 0.64 years). They found that the O/W cream successfully increased stratum corneum hydration, enhanced skin barrier function, and maintained skin pH after topical application. The cream was also well tolerated. In vitro assays also indicated that the bilberry isolates displayed notable antioxidant capacity (stronger in the case of the leaves). Tadic et al. suggested that skin disorders characterized by oxidative stress and/or xerosis may be appropriate targets for topically applied bilberry cream.1
Early in 2022, Ruscinc et al. reported on their efforts to incorporate V. myrtillus extract into a multifunctional sunscreen. In vitro and in vivo tests revealed that while sun protection factor was lowered in the presence of the extract, the samples were safe and photostable. The researchers concluded that further study is necessary to elucidate the effect of V. myrtillus extract on photoprotection.16
V. myrtillus has been consumed by human beings for many generations. Skin care formulations based on this ingredient have not been associated with adverse events. Notably, the Environmental Working Group has rated V. myrtillus (bilberry seed) oil as very safe.17
Summary
While research, particularly in the form of randomized controlled trials, is called for, because the fatty acids it contains have been shown to suppress tyrosinase. Currently, this botanical agent seems to be most suited for sensitive, aging skin and for skin with an uneven tone, particularly postinflammatory pigmentation and melasma.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, an SaaS company used to generate skin care routines in office and as an ecommerce solution. Write to her at [email protected].
References
1. Tadic VM et al. Antioxidants (Basel). 2021 Mar 16;10(3):465.
2. Svobodová A et al. Biofactors. 2008;33(4):249-66.
3. Chu WK et al. Bilberry (Vaccinium myrtillus L.), in Benzie IFF, Wachtel-Galor S, eds., “Herbal Medicine: Biomolecular and Clinical Aspects,” 2nd ed. (Boca Raton, Fla.: CRC Press/Taylor & Francis, 2011, Chapter 4).
4. Yamaura K et al. Pharmacognosy Res. 2011 Jul;3(3):173-7.
5. Stefanescu BE et al. Molecules. 2019 May 29;24(11):2046.
6. Smeriglio A et al. Mini Rev Med Chem. 2014;14(7):567-84.
7. Ando H et al. Arch Dermatol Res. 1998 Jul;290(7):375-81.
8. Burdulis D et al. Acta Pol Pharm. 2009 Jul-Aug;66(4):399-408.
9. Bornsek SM et al. Food Chem. 2012 Oct 15;134(4):1878-84.
10. Brasanac-Vukanovic S et al. Molecules. 2018 Jul 26;23(8):1864.
11. Yamaura K et al. J Food Sci. 2012 Dec;77(12):H262-7.
12. Pires TCSP et al. Curr Pharm Des. 2020;26(16):1917-28.
13. Svobodová A et al. J Dermatol Sci. 2009 Dec;56(3):196-204.
14. Calò R, Marabini L. J Photochem Photobiol B. 2014 Mar 5;132:27-35.
15. Bucci P et al. J Cosmet Dermatol. 2018 Oct;17(5):889-99.
16. Ruscinc N et al. J Cosmet Dermatol. 2022 Jan 13.
17. Environmental Working Group’s Skin Deep website. Vaccinium Myrtillus Bilberry Seed Oil. Accessed October 18, 2022.
The ‘root cause’ visit
“How did we miss out on that?” “What?” my physician friend replied as we stood in line at the coffee cart. “Root cause. I mean, we invented this idea and now all these naturopaths and functional medicine quacks are gettin’ rich off it.” “Take it easy,” he says. “Just order a coffee.”
It’s hard not to be indignant. I had a morning clinic with three patients insisting I find the “root cause” of their problem. Now, if one had flagellate dermatitis after eating Asian mushroom soup, I’d have said “Root cause? Shiitake mushrooms!” and walked out like Costanza in Seinfeld, “All right, that’s it for me! Be good everybody!”
Alas no. They had perioral dermatitis, alopecia areata, eczema – no satisfying “roots” for walk-off answers.
There is a universal desire to find the proximal cause for problems. Patients often want to know it so that we address the root of their trouble and not just cut off the branches. This is deeply gratifying for those who want not only to know why, but also to have agency in how to control their disease. For example, if they believe the root cause of perioral dermatitis was excess yeast, then eating a “candida diet’’ should do the trick! Food sensitivities, hormones, and heavy metals round out the top suspects that root cause patients want to talk about.
Of course, patients have been asking about this for a long time, but lately, the root cause visit seems to be on trend. Check out any hip primary care start-up such as One Medical or any hot direct-to-consumer virtual offering such as ParsleyHealth and you will see root-cause everywhere. Our patients are expecting us to address it, or it seems they will find someone cooler who will.
Yet, it wasn’t the slick marketing team at ParsleyHeath who invented the “root cause doctor visit.” We did. It’s an idea that started with our Greek physician ancestors. Breaking from the diviners and priests, we were the first “naturalists” positing that there was a natural, not a divine cause for illness. The cardinal concept in the Hippocratic Corpus was that health was an equilibrium and illness an imbalance. They didn’t have dehydroepiandrosterone tests or mercury levels, but did have bodily fluids. Yellow bile, black bile, blood, and phlegm, were the root of all root causes. A physician simply had to identify which was in excess or deficient and fix that to cure the disease. Interestingly, the word “diagnosis” appears only once in the Corpus. The word “Diagignoskein” appears occasionally but this describes studying thoroughly, not naming a diagnosis as we understand it.
Advances in chemistry in the 17th century meant physicians could add new theories, and new root causes. Now alkaline or other chemical elixirs were added to cure at the source. Since there was no verifiable evidence to prove causes, theories were adopted to provide some rational direction to treatment. In the 18th century, physicians such as Dr. Benjamin Rush, one of the original faculty at the University of Pennsylvania school of medicine, taught that spasms of the arteries were the root cause of illnesses. “Heroic” treatments such as extreme bloodletting were the cure. (Note, those patients who survived us kept coming back to us for more).
Scientific knowledge and diagnostic technologies led to more and more complex and abstruse causes. Yet, as we became more precise and effective, our explanations became less satisfying to our patients. I can diagnose and readily treat perioral dermatitis, yet I’m hard pressed to give an answer to its root cause. “Root cause? Yes. Just apply this pimecrolimus cream for a couple of weeks and it’ll be better! All right, that’s it for me! Be good everybody!”
You’ll have to do better, George.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected]
“How did we miss out on that?” “What?” my physician friend replied as we stood in line at the coffee cart. “Root cause. I mean, we invented this idea and now all these naturopaths and functional medicine quacks are gettin’ rich off it.” “Take it easy,” he says. “Just order a coffee.”
It’s hard not to be indignant. I had a morning clinic with three patients insisting I find the “root cause” of their problem. Now, if one had flagellate dermatitis after eating Asian mushroom soup, I’d have said “Root cause? Shiitake mushrooms!” and walked out like Costanza in Seinfeld, “All right, that’s it for me! Be good everybody!”
Alas no. They had perioral dermatitis, alopecia areata, eczema – no satisfying “roots” for walk-off answers.
There is a universal desire to find the proximal cause for problems. Patients often want to know it so that we address the root of their trouble and not just cut off the branches. This is deeply gratifying for those who want not only to know why, but also to have agency in how to control their disease. For example, if they believe the root cause of perioral dermatitis was excess yeast, then eating a “candida diet’’ should do the trick! Food sensitivities, hormones, and heavy metals round out the top suspects that root cause patients want to talk about.
Of course, patients have been asking about this for a long time, but lately, the root cause visit seems to be on trend. Check out any hip primary care start-up such as One Medical or any hot direct-to-consumer virtual offering such as ParsleyHealth and you will see root-cause everywhere. Our patients are expecting us to address it, or it seems they will find someone cooler who will.
Yet, it wasn’t the slick marketing team at ParsleyHeath who invented the “root cause doctor visit.” We did. It’s an idea that started with our Greek physician ancestors. Breaking from the diviners and priests, we were the first “naturalists” positing that there was a natural, not a divine cause for illness. The cardinal concept in the Hippocratic Corpus was that health was an equilibrium and illness an imbalance. They didn’t have dehydroepiandrosterone tests or mercury levels, but did have bodily fluids. Yellow bile, black bile, blood, and phlegm, were the root of all root causes. A physician simply had to identify which was in excess or deficient and fix that to cure the disease. Interestingly, the word “diagnosis” appears only once in the Corpus. The word “Diagignoskein” appears occasionally but this describes studying thoroughly, not naming a diagnosis as we understand it.
Advances in chemistry in the 17th century meant physicians could add new theories, and new root causes. Now alkaline or other chemical elixirs were added to cure at the source. Since there was no verifiable evidence to prove causes, theories were adopted to provide some rational direction to treatment. In the 18th century, physicians such as Dr. Benjamin Rush, one of the original faculty at the University of Pennsylvania school of medicine, taught that spasms of the arteries were the root cause of illnesses. “Heroic” treatments such as extreme bloodletting were the cure. (Note, those patients who survived us kept coming back to us for more).
Scientific knowledge and diagnostic technologies led to more and more complex and abstruse causes. Yet, as we became more precise and effective, our explanations became less satisfying to our patients. I can diagnose and readily treat perioral dermatitis, yet I’m hard pressed to give an answer to its root cause. “Root cause? Yes. Just apply this pimecrolimus cream for a couple of weeks and it’ll be better! All right, that’s it for me! Be good everybody!”
You’ll have to do better, George.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected]
“How did we miss out on that?” “What?” my physician friend replied as we stood in line at the coffee cart. “Root cause. I mean, we invented this idea and now all these naturopaths and functional medicine quacks are gettin’ rich off it.” “Take it easy,” he says. “Just order a coffee.”
It’s hard not to be indignant. I had a morning clinic with three patients insisting I find the “root cause” of their problem. Now, if one had flagellate dermatitis after eating Asian mushroom soup, I’d have said “Root cause? Shiitake mushrooms!” and walked out like Costanza in Seinfeld, “All right, that’s it for me! Be good everybody!”
Alas no. They had perioral dermatitis, alopecia areata, eczema – no satisfying “roots” for walk-off answers.
There is a universal desire to find the proximal cause for problems. Patients often want to know it so that we address the root of their trouble and not just cut off the branches. This is deeply gratifying for those who want not only to know why, but also to have agency in how to control their disease. For example, if they believe the root cause of perioral dermatitis was excess yeast, then eating a “candida diet’’ should do the trick! Food sensitivities, hormones, and heavy metals round out the top suspects that root cause patients want to talk about.
Of course, patients have been asking about this for a long time, but lately, the root cause visit seems to be on trend. Check out any hip primary care start-up such as One Medical or any hot direct-to-consumer virtual offering such as ParsleyHealth and you will see root-cause everywhere. Our patients are expecting us to address it, or it seems they will find someone cooler who will.
Yet, it wasn’t the slick marketing team at ParsleyHeath who invented the “root cause doctor visit.” We did. It’s an idea that started with our Greek physician ancestors. Breaking from the diviners and priests, we were the first “naturalists” positing that there was a natural, not a divine cause for illness. The cardinal concept in the Hippocratic Corpus was that health was an equilibrium and illness an imbalance. They didn’t have dehydroepiandrosterone tests or mercury levels, but did have bodily fluids. Yellow bile, black bile, blood, and phlegm, were the root of all root causes. A physician simply had to identify which was in excess or deficient and fix that to cure the disease. Interestingly, the word “diagnosis” appears only once in the Corpus. The word “Diagignoskein” appears occasionally but this describes studying thoroughly, not naming a diagnosis as we understand it.
Advances in chemistry in the 17th century meant physicians could add new theories, and new root causes. Now alkaline or other chemical elixirs were added to cure at the source. Since there was no verifiable evidence to prove causes, theories were adopted to provide some rational direction to treatment. In the 18th century, physicians such as Dr. Benjamin Rush, one of the original faculty at the University of Pennsylvania school of medicine, taught that spasms of the arteries were the root cause of illnesses. “Heroic” treatments such as extreme bloodletting were the cure. (Note, those patients who survived us kept coming back to us for more).
Scientific knowledge and diagnostic technologies led to more and more complex and abstruse causes. Yet, as we became more precise and effective, our explanations became less satisfying to our patients. I can diagnose and readily treat perioral dermatitis, yet I’m hard pressed to give an answer to its root cause. “Root cause? Yes. Just apply this pimecrolimus cream for a couple of weeks and it’ll be better! All right, that’s it for me! Be good everybody!”
You’ll have to do better, George.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected]
Are doctors savers or spenders?
In a poll that ran from August 30 to Sept. 21, conducted by Medscape, physicians were asked if they lived within their means. They were asked whether they pay their bills on time, save at least 20% of their monthly income toward retirement, pay down student loan debt, and contribute to their kids’ college savings or a rainy-day emergency fund.
Medscape polled 468 U.S. physicians and 159 living outside of the United States. Eighty-nine percent of U.S. respondents report living within their means, while only 11% said they don’t.
Medscape’s Physician Wealth & Debt Report 2022 similarly reported that of 13,000 physicians in more than 29 specialties, 94% said they live at or below their means.
For example, over half of physicians have a net worth above $1 million. In contrast, according to Credit Suisse’s Global Wealth Report, less than 7% of the general population has a seven-figure net worth.
So just how do physicians stack up financially?
Habits of physician super savers
Physicians who consider themselves savers likely have money habits that correlate. They buy things on sale, are DIYers for home projects and maintenance, and wait to buy luxury or large expenses when the timing is right, an item is on sale, or they’ve saved for it.
For example, when it comes to life’s luxuries like buying a new car or dining out, overall, physicians seem to be more frugal, as 43% of those who buy cars said they only buy a new car every 10 years; 30% said they buy a new vehicle every 6-7 years, and 22% said every 4-5 years.
When asked about weekly dine-out or delivery habits, 82% of those polled who said they dine out, or order takeout, do so a nominal 1-2 times per week. That’s on par with the Centers for Disease Control, which reports that 3 in 5 Americans eat out once weekly. Another 14% of polled physicians said they dine out 3-5 nights per week. Only 4% revealed they eat out or grab to-go food more than 5 nights a week.
When hiring for essential home maintenance, like house cleaning and pool or lawn service, almost a third of physicians we polled who require such maintenance employ a service for these tasks, and 23% hire out often while 21% hire out only sometimes. However, 14% say they rarely hire out for home maintenance, and 11% never do.
Since physicians are typically tight on time, they tend to favor outsourcing things like housecleaning, lawn service, landscaping, maintenance, and even cooking. So, the fact that a quarter of physicians polled rarely or never hire out for household help is somewhat surprising.
Most physicians also prioritize saving. When asked how important it is to save money consistently, 93% think it’s either extremely or very important, while only 6% think it’s somewhat important.
Barriers to wealth
When asked what barriers prevent them from saving at least 20% of their monthly income, physician respondents who said they live within their means and encountered barriers reported that family necessities (35%), student loan debt (19%), and mortgage sizes (18%) were the top reasons. The average doctor earns five times as much as the average American, according to the Global Wealth Report.
“What prevents me from saving is holding too much debt, responsibilities at home, bills, being unprepared for what is coming, and making excuses to spend even when it’s not necessary,” says Sean Ormond, MD, a dual board-certified physician in Anesthesiology and Pain Management in Phoenix.
When physician respondents who said they didn’t live within their means were asked about the barriers preventing them from saving at least 20% of their monthly income, they cited the cost of family necessities (49%), the size of their mortgage (47%), credit card debt (30%), student loan debt (21%), other loans (15%), and car lease/loan (13%).
“My most significant financial splurge is vacation, since I always choose the best, and the best comes at an extra cost,” says Dr. Ormond.
What’s your financial grade?
Finally, physicians were asked who they considered better at saving money, themselves or their spouse/domestic partner. Forty-four percent think they are the better saver, whereas 41% said that both they and their partner were equally good at saving. Thirteen percent credited their partner with better saving habits, and 2% said neither themselves nor their partner were good at saving money.
More than half (63%) of physicians polled pay off their credit card balance monthly, but 18% carry a $1,000-$5,000 balance, 10% have $5,000-$10,000 in credit card debt, and 6% hold more than $10,000 of credit card debt.
“I would grade myself with a B, because however much I love having the best, I still have a budget, and I always ensure that I follow it to the dot,” says Dr. Ormond.
A version of this article first appeared on Medscape.com.
In a poll that ran from August 30 to Sept. 21, conducted by Medscape, physicians were asked if they lived within their means. They were asked whether they pay their bills on time, save at least 20% of their monthly income toward retirement, pay down student loan debt, and contribute to their kids’ college savings or a rainy-day emergency fund.
Medscape polled 468 U.S. physicians and 159 living outside of the United States. Eighty-nine percent of U.S. respondents report living within their means, while only 11% said they don’t.
Medscape’s Physician Wealth & Debt Report 2022 similarly reported that of 13,000 physicians in more than 29 specialties, 94% said they live at or below their means.
For example, over half of physicians have a net worth above $1 million. In contrast, according to Credit Suisse’s Global Wealth Report, less than 7% of the general population has a seven-figure net worth.
So just how do physicians stack up financially?
Habits of physician super savers
Physicians who consider themselves savers likely have money habits that correlate. They buy things on sale, are DIYers for home projects and maintenance, and wait to buy luxury or large expenses when the timing is right, an item is on sale, or they’ve saved for it.
For example, when it comes to life’s luxuries like buying a new car or dining out, overall, physicians seem to be more frugal, as 43% of those who buy cars said they only buy a new car every 10 years; 30% said they buy a new vehicle every 6-7 years, and 22% said every 4-5 years.
When asked about weekly dine-out or delivery habits, 82% of those polled who said they dine out, or order takeout, do so a nominal 1-2 times per week. That’s on par with the Centers for Disease Control, which reports that 3 in 5 Americans eat out once weekly. Another 14% of polled physicians said they dine out 3-5 nights per week. Only 4% revealed they eat out or grab to-go food more than 5 nights a week.
When hiring for essential home maintenance, like house cleaning and pool or lawn service, almost a third of physicians we polled who require such maintenance employ a service for these tasks, and 23% hire out often while 21% hire out only sometimes. However, 14% say they rarely hire out for home maintenance, and 11% never do.
Since physicians are typically tight on time, they tend to favor outsourcing things like housecleaning, lawn service, landscaping, maintenance, and even cooking. So, the fact that a quarter of physicians polled rarely or never hire out for household help is somewhat surprising.
Most physicians also prioritize saving. When asked how important it is to save money consistently, 93% think it’s either extremely or very important, while only 6% think it’s somewhat important.
Barriers to wealth
When asked what barriers prevent them from saving at least 20% of their monthly income, physician respondents who said they live within their means and encountered barriers reported that family necessities (35%), student loan debt (19%), and mortgage sizes (18%) were the top reasons. The average doctor earns five times as much as the average American, according to the Global Wealth Report.
“What prevents me from saving is holding too much debt, responsibilities at home, bills, being unprepared for what is coming, and making excuses to spend even when it’s not necessary,” says Sean Ormond, MD, a dual board-certified physician in Anesthesiology and Pain Management in Phoenix.
When physician respondents who said they didn’t live within their means were asked about the barriers preventing them from saving at least 20% of their monthly income, they cited the cost of family necessities (49%), the size of their mortgage (47%), credit card debt (30%), student loan debt (21%), other loans (15%), and car lease/loan (13%).
“My most significant financial splurge is vacation, since I always choose the best, and the best comes at an extra cost,” says Dr. Ormond.
What’s your financial grade?
Finally, physicians were asked who they considered better at saving money, themselves or their spouse/domestic partner. Forty-four percent think they are the better saver, whereas 41% said that both they and their partner were equally good at saving. Thirteen percent credited their partner with better saving habits, and 2% said neither themselves nor their partner were good at saving money.
More than half (63%) of physicians polled pay off their credit card balance monthly, but 18% carry a $1,000-$5,000 balance, 10% have $5,000-$10,000 in credit card debt, and 6% hold more than $10,000 of credit card debt.
“I would grade myself with a B, because however much I love having the best, I still have a budget, and I always ensure that I follow it to the dot,” says Dr. Ormond.
A version of this article first appeared on Medscape.com.
In a poll that ran from August 30 to Sept. 21, conducted by Medscape, physicians were asked if they lived within their means. They were asked whether they pay their bills on time, save at least 20% of their monthly income toward retirement, pay down student loan debt, and contribute to their kids’ college savings or a rainy-day emergency fund.
Medscape polled 468 U.S. physicians and 159 living outside of the United States. Eighty-nine percent of U.S. respondents report living within their means, while only 11% said they don’t.
Medscape’s Physician Wealth & Debt Report 2022 similarly reported that of 13,000 physicians in more than 29 specialties, 94% said they live at or below their means.
For example, over half of physicians have a net worth above $1 million. In contrast, according to Credit Suisse’s Global Wealth Report, less than 7% of the general population has a seven-figure net worth.
So just how do physicians stack up financially?
Habits of physician super savers
Physicians who consider themselves savers likely have money habits that correlate. They buy things on sale, are DIYers for home projects and maintenance, and wait to buy luxury or large expenses when the timing is right, an item is on sale, or they’ve saved for it.
For example, when it comes to life’s luxuries like buying a new car or dining out, overall, physicians seem to be more frugal, as 43% of those who buy cars said they only buy a new car every 10 years; 30% said they buy a new vehicle every 6-7 years, and 22% said every 4-5 years.
When asked about weekly dine-out or delivery habits, 82% of those polled who said they dine out, or order takeout, do so a nominal 1-2 times per week. That’s on par with the Centers for Disease Control, which reports that 3 in 5 Americans eat out once weekly. Another 14% of polled physicians said they dine out 3-5 nights per week. Only 4% revealed they eat out or grab to-go food more than 5 nights a week.
When hiring for essential home maintenance, like house cleaning and pool or lawn service, almost a third of physicians we polled who require such maintenance employ a service for these tasks, and 23% hire out often while 21% hire out only sometimes. However, 14% say they rarely hire out for home maintenance, and 11% never do.
Since physicians are typically tight on time, they tend to favor outsourcing things like housecleaning, lawn service, landscaping, maintenance, and even cooking. So, the fact that a quarter of physicians polled rarely or never hire out for household help is somewhat surprising.
Most physicians also prioritize saving. When asked how important it is to save money consistently, 93% think it’s either extremely or very important, while only 6% think it’s somewhat important.
Barriers to wealth
When asked what barriers prevent them from saving at least 20% of their monthly income, physician respondents who said they live within their means and encountered barriers reported that family necessities (35%), student loan debt (19%), and mortgage sizes (18%) were the top reasons. The average doctor earns five times as much as the average American, according to the Global Wealth Report.
“What prevents me from saving is holding too much debt, responsibilities at home, bills, being unprepared for what is coming, and making excuses to spend even when it’s not necessary,” says Sean Ormond, MD, a dual board-certified physician in Anesthesiology and Pain Management in Phoenix.
When physician respondents who said they didn’t live within their means were asked about the barriers preventing them from saving at least 20% of their monthly income, they cited the cost of family necessities (49%), the size of their mortgage (47%), credit card debt (30%), student loan debt (21%), other loans (15%), and car lease/loan (13%).
“My most significant financial splurge is vacation, since I always choose the best, and the best comes at an extra cost,” says Dr. Ormond.
What’s your financial grade?
Finally, physicians were asked who they considered better at saving money, themselves or their spouse/domestic partner. Forty-four percent think they are the better saver, whereas 41% said that both they and their partner were equally good at saving. Thirteen percent credited their partner with better saving habits, and 2% said neither themselves nor their partner were good at saving money.
More than half (63%) of physicians polled pay off their credit card balance monthly, but 18% carry a $1,000-$5,000 balance, 10% have $5,000-$10,000 in credit card debt, and 6% hold more than $10,000 of credit card debt.
“I would grade myself with a B, because however much I love having the best, I still have a budget, and I always ensure that I follow it to the dot,” says Dr. Ormond.
A version of this article first appeared on Medscape.com.