User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
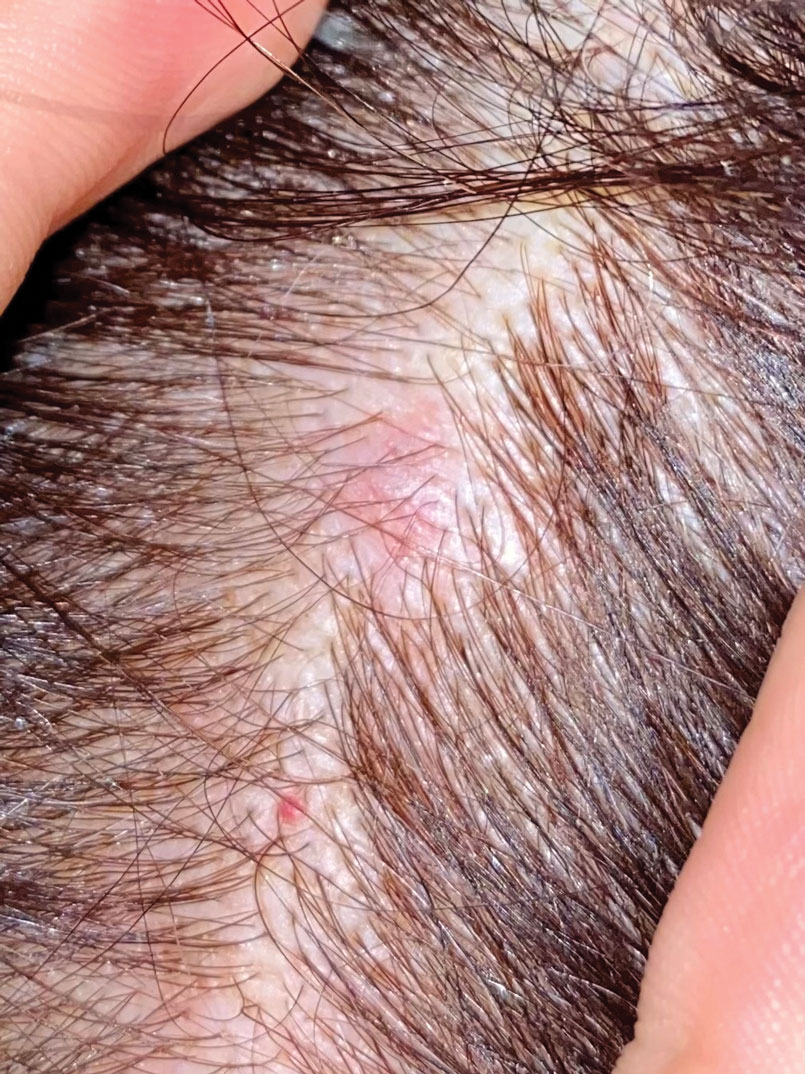
Firm Mobile Nodule on the Scalp
The Diagnosis: Metastatic Carcinoid Tumor
Carcinoid tumors are derived from neuroendocrine cell compartments and generally arise in the gastrointestinal tract, with a quarter of carcinoids arising in the small bowel.1 Carcinoid tumors have an incidence of approximately 2 to 5 per 100,000 patients.2 Metastasis of carcinoids is approximately 31.2% to 46.7%.1 Metastasis to the skin is uncommon; we present a rare case of a carcinoid tumor of the terminal ileum with metastasis to the scalp.
Unlike our patient, most patients with carcinoid tumors have an indolent clinical course. The most common cutaneous symptom is flushing, which occurs in 75% of patients.3 Secreted vasoactive peptides such as serotonin may cause other symptoms such as tachycardia, diarrhea, and bronchospasm; together, these symptoms comprise carcinoid syndrome. Carcinoid syndrome requires metastasis of the tumor to the liver or a site outside of the gastrointestinal tract because the liver will metabolize the secreted serotonin. However, even in patients with liver metastasis, carcinoid syndrome only occurs in approximately 10% of patients.4 Common skin findings of carcinoid syndrome include pellagralike dermatitis, flushing, and sclerodermalike changes.5 Our patient experienced several episodes of presyncope with symptoms of dyspnea, lightheadedness, and flushing but did not have bronchospasm or recurrent diarrhea. Intramuscular octreotide improved some symptoms.
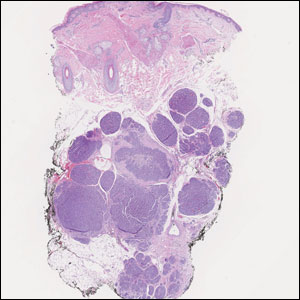
The scalp accounts for approximately 15% of cutaneous metastases, the most common being from the lung, renal, and breast cancers.6 Cutaneous metastases of carcinoid tumors are rare. A PubMed search of articles indexed for MEDLINE using the terms metastatic AND [carcinoid OR neuroendocrine] tumors AND [skin OR cutaneous] revealed 47 cases.7-11 Similar to other skin metastases, cutaneous metastases of carcinoid tumors commonly present as firm erythematous nodules of varying sizes that may be asymptomatic, tender, or pruritic (Figure 1). Cases of carcinoid tumors with cutaneous metastasis as the initial and only presenting sign are exceedingly rare.12
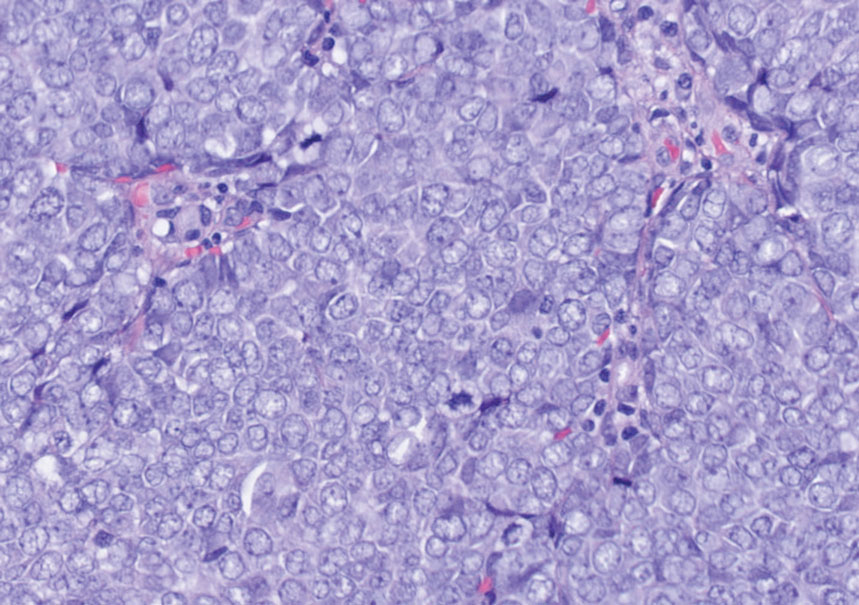
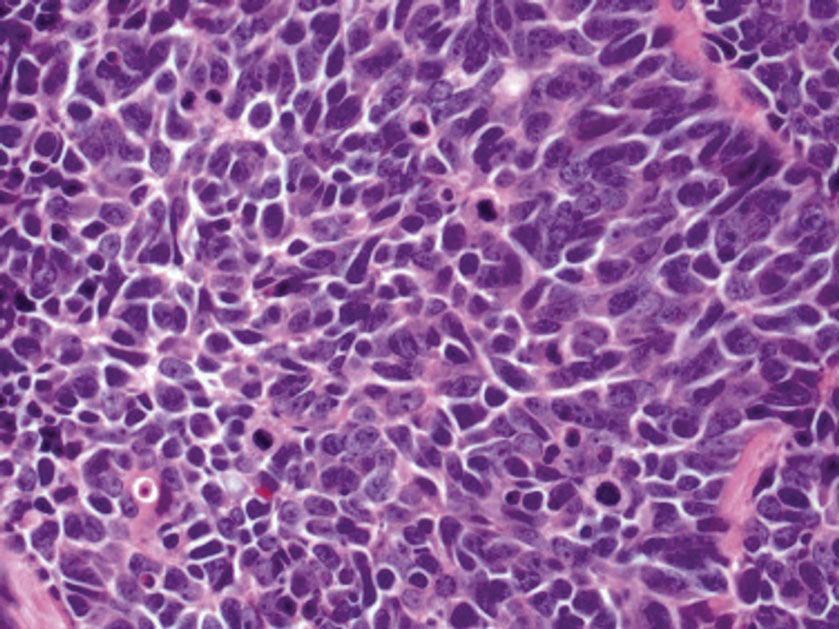
Histology of carcinoid tumors reveals a dermal neoplasm composed of loosely cohesive, mildly atypical, polygonal cells with salt-and-pepper chromatin and eosinophilic cytoplasm, which are similar findings to the primary tumor. The cells may grow in the typical trabecular or organoid neuroendocrine pattern or exhibit a pseudoglandular growth pattern with prominent vessels (quiz image, top).12 Positive chromogranin and synaptophysin immunostaining are the most common and reliable markers used for the diagnosis of carcinoid tumors.
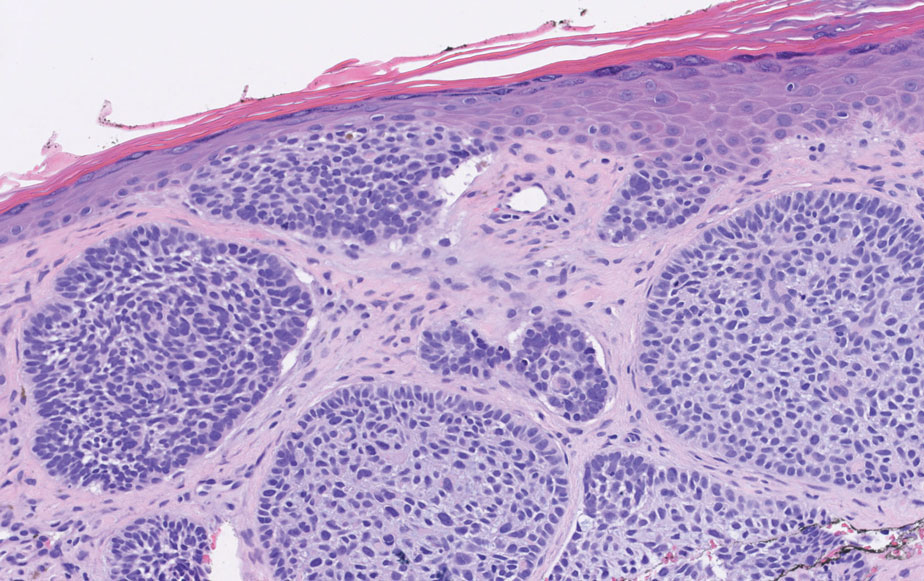
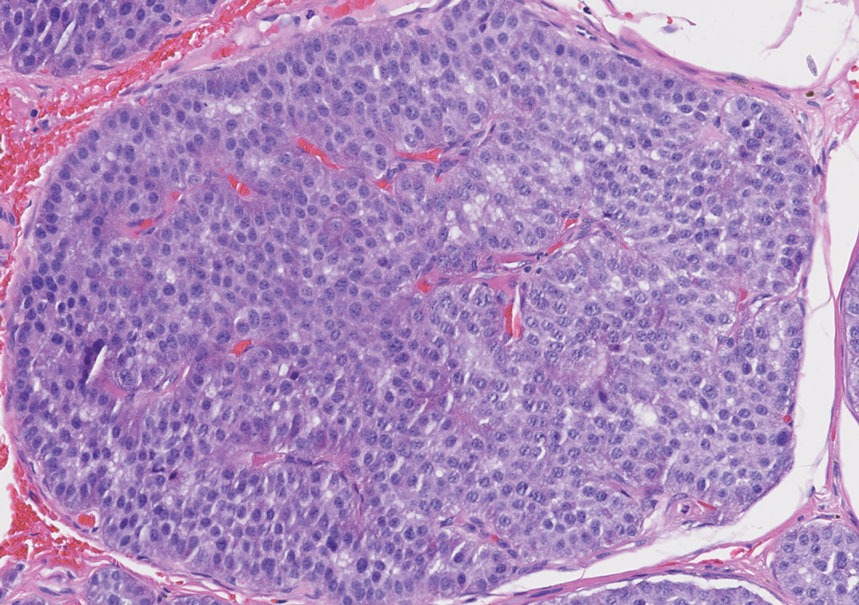
An important histopathologic differential diagnosis is the aggressive Merkel cell carcinoma, which also demonstrates homogenous salt-and-pepper chromatin but exhibits a higher mitotic rate and positive cytokeratin 20 staining (Figure 2).13 Basal cell carcinoma (BCC) also may display similar features, including a blue tumor at scanning magnification and nodular or infiltrative growth patterns. The cell morphology of BCC is characterized by islands of basaloid cells with minimal cytoplasm and frequent apoptosis, connecting to the epidermis with peripheral palisading, retraction artifact, and a myxoid stroma; BCC lacks the salt-and-pepper chromatin commonly seen in carcinoid tumors (Figure 3). Basal cell carcinoma is characterized by positive BerEP4 (epithelial cell adhesion molecule immunostain), cytokeratin 5/6, and cytokeratin 14 uptake. Cytokeratin 20, often used to diagnose Merkel cell carcinoma, is negative in BCC. Chromogranin and synaptophysin occasionally may be positive in BCC.14
The superficial Ewing sarcoma family of tumors also may be included in the differential diagnosis of small round cell tumors of the skin, but they are very rare. These tumors possess strong positive membranous staining of cytokeratin 99 and also can stain positively for synaptophysin and chromogranin.15 Epithelial membrane antigen, which is negative in Ewing sarcomas, is positive in carcinoid tumors.16 Neuroendocrine tumors of all sites share similar basic morphologic patterns, and multiple primary tumors should be considered, including small cell lung carcinoma (Figure 4).17,18 Red granulations and true glandular lumina typically are not seen in the lungs but are common in gastrointestinal carcinoids.18 Regarding immunohistochemistry, TTF-1 is negative and CDX2 is positive in gastroenteropancreatic carcinoids, suggesting that these 2 markers can help distinguish carcinoids of unknown primary origin.19
Metastases in carcinoid tumors are common, with one study noting that the highest frequency of small intestinal metastases was from the ileal subset.20 At the time of diagnosis, 58% to 64% of patients with small intestine carcinoid tumors already had nonlocalized disease, with frequent sites being the lymph nodes (89.8%), liver (44.1%), lungs (13.6%), and peritoneum (13.6%). Regional and distant metastases are associated with substantially worse prognoses, with survival rates of 71.7% and 38.5%, respectively.1 Treatment of symptomatic unresectable disease focuses on symptomatic management with somatostatin analogs that also control tumor growth.21
We present a rare case of scalp metastasis of a carcinoid tumor of the terminal ileum. Distant metastasis is associated with poorer prognosis and should be considered in patients with a known history of a carcinoid tumor.
Acknowledgment—We would like to acknowledge the Research Histology and Tissue Imaging Core at University of Illinois Chicago Research Resources Center for the immunohistochemistry studies.
- Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934-959.
- Lawrence B, Gustafsson BI, Chan A, et al. The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am. 2011;40:1-18, vii.
- Sabir S, James WD, Schuchter LM. Cutaneous manifestations of cancer. Curr Opin Oncol. 1999;11:139-144.
- Tomassetti P. Clinical aspects of carcinoid tumours. Italian J Gastroenterol Hepatol. 1999;31(suppl 2):S143-S146.
- Bell HK, Poston GJ, Vora J, et al. Cutaneous manifestations of the malignant carcinoid syndrome. Br J Dermatol. 2005;152:71-75.
- Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29(2 pt 1):228-236.
- Garcia A, Mays S, Silapunt S. Metastatic neuroendocrine carcinoma in the skin. Dermatol Online J. 2017;23:13030/qt9052w9x1.
- Ciliberti MP, Carbonara R, Grillo A, et al. Unexpected response to palliative radiotherapy for subcutaneous metastases of an advanced small cell pancreatic neuroendocrine carcinoma: a case report of two different radiation schedules. BMC Cancer. 2020;20:311.
- Devnani B, Kumar R, Pathy S, et al. Cutaneous metastases from neuroendocrine carcinoma of the cervix: an unusual metastatic lesion from an uncommon malignancy. Curr Probl Cancer. 2018; 42:527-533.
- Falto-Aizpurua L, Seyfer S, Krishnan B, et al. Cutaneous metastasis of a pulmonary carcinoid tumor. Cutis. 2017;99:E13-E15.
- Dhingra R, Tse JY, Saif MW. Cutaneous metastasis of gastroenteropancreatic neuroendocrine tumors (GEP-Nets)[published online September 8, 2018]. JOP. 2018;19.
- Jedrych J, Busam K, Klimstra DS, et al. Cutaneous metastases as an initial manifestation of visceral well-differentiated neuroendocrine tumor: a report of four cases and a review of literature. J Cutan Pathol. 2014;41:113-122.
- Lloyd RV. Practical markers used in the diagnosis of neuroendocrine tumors. Endocr Pathol. 2003;14:293-301.
- Stanoszek LM, Wang GY, Harms PW. Histologic mimics of basal cell carcinoma. Arch Pathol Lab Med. 2017;141:1490-1502.
- Machado I, Llombart B, Calabuig-Fariñas S, et al. Superficial Ewing’s sarcoma family of tumors: a clinicopathological study with differential diagnoses. J Cutan Pathol. 2011;38:636-643.
- D’Cruze L, Dutta R, Rao S, et al. The role of immunohistochemistry in the analysis of the spectrum of small round cell tumours at a tertiary care centre. J Clin Diagn Res. 2013;7:1377-1382.
- Chirila DN, Turdeanu NA, Constantea NA, et al. Multiple malignant tumors. Chirurgia (Bucur). 2013;108:498-502.
- Rekhtman N. Neuroendocrine tumors of the lung: an update. Arch Pathol Lab Med. 2010;134:1628-1638.
- Lin X, Saad RS, Luckasevic TM, et al. Diagnostic value of CDX-2 and TTF-1 expressions in separating metastatic neuroendocrine neoplasms of unknown origin. Appl Immunohistochem Mol Morphol. 2007;15:407-414.
- Olney JR, Urdaneta LF, Al-Jurf AS, et al. Carcinoid tumors of the gastrointestinal tract. Am Surg. 1985;51:37-41.
- Strosberg JR, Halfdanarson TR, Bellizzi AM, et al. The North American Neuroendocrine Tumor Society consensus guidelines for surveillance and medical management of midgut neuroendocrine tumors. Pancreas. 2017;46:707-714.
The Diagnosis: Metastatic Carcinoid Tumor
Carcinoid tumors are derived from neuroendocrine cell compartments and generally arise in the gastrointestinal tract, with a quarter of carcinoids arising in the small bowel.1 Carcinoid tumors have an incidence of approximately 2 to 5 per 100,000 patients.2 Metastasis of carcinoids is approximately 31.2% to 46.7%.1 Metastasis to the skin is uncommon; we present a rare case of a carcinoid tumor of the terminal ileum with metastasis to the scalp.
Unlike our patient, most patients with carcinoid tumors have an indolent clinical course. The most common cutaneous symptom is flushing, which occurs in 75% of patients.3 Secreted vasoactive peptides such as serotonin may cause other symptoms such as tachycardia, diarrhea, and bronchospasm; together, these symptoms comprise carcinoid syndrome. Carcinoid syndrome requires metastasis of the tumor to the liver or a site outside of the gastrointestinal tract because the liver will metabolize the secreted serotonin. However, even in patients with liver metastasis, carcinoid syndrome only occurs in approximately 10% of patients.4 Common skin findings of carcinoid syndrome include pellagralike dermatitis, flushing, and sclerodermalike changes.5 Our patient experienced several episodes of presyncope with symptoms of dyspnea, lightheadedness, and flushing but did not have bronchospasm or recurrent diarrhea. Intramuscular octreotide improved some symptoms.
The scalp accounts for approximately 15% of cutaneous metastases, the most common being from the lung, renal, and breast cancers.6 Cutaneous metastases of carcinoid tumors are rare. A PubMed search of articles indexed for MEDLINE using the terms metastatic AND [carcinoid OR neuroendocrine] tumors AND [skin OR cutaneous] revealed 47 cases.7-11 Similar to other skin metastases, cutaneous metastases of carcinoid tumors commonly present as firm erythematous nodules of varying sizes that may be asymptomatic, tender, or pruritic (Figure 1). Cases of carcinoid tumors with cutaneous metastasis as the initial and only presenting sign are exceedingly rare.12

Histology of carcinoid tumors reveals a dermal neoplasm composed of loosely cohesive, mildly atypical, polygonal cells with salt-and-pepper chromatin and eosinophilic cytoplasm, which are similar findings to the primary tumor. The cells may grow in the typical trabecular or organoid neuroendocrine pattern or exhibit a pseudoglandular growth pattern with prominent vessels (quiz image, top).12 Positive chromogranin and synaptophysin immunostaining are the most common and reliable markers used for the diagnosis of carcinoid tumors.

An important histopathologic differential diagnosis is the aggressive Merkel cell carcinoma, which also demonstrates homogenous salt-and-pepper chromatin but exhibits a higher mitotic rate and positive cytokeratin 20 staining (Figure 2).13 Basal cell carcinoma (BCC) also may display similar features, including a blue tumor at scanning magnification and nodular or infiltrative growth patterns. The cell morphology of BCC is characterized by islands of basaloid cells with minimal cytoplasm and frequent apoptosis, connecting to the epidermis with peripheral palisading, retraction artifact, and a myxoid stroma; BCC lacks the salt-and-pepper chromatin commonly seen in carcinoid tumors (Figure 3). Basal cell carcinoma is characterized by positive BerEP4 (epithelial cell adhesion molecule immunostain), cytokeratin 5/6, and cytokeratin 14 uptake. Cytokeratin 20, often used to diagnose Merkel cell carcinoma, is negative in BCC. Chromogranin and synaptophysin occasionally may be positive in BCC.14

The superficial Ewing sarcoma family of tumors also may be included in the differential diagnosis of small round cell tumors of the skin, but they are very rare. These tumors possess strong positive membranous staining of cytokeratin 99 and also can stain positively for synaptophysin and chromogranin.15 Epithelial membrane antigen, which is negative in Ewing sarcomas, is positive in carcinoid tumors.16 Neuroendocrine tumors of all sites share similar basic morphologic patterns, and multiple primary tumors should be considered, including small cell lung carcinoma (Figure 4).17,18 Red granulations and true glandular lumina typically are not seen in the lungs but are common in gastrointestinal carcinoids.18 Regarding immunohistochemistry, TTF-1 is negative and CDX2 is positive in gastroenteropancreatic carcinoids, suggesting that these 2 markers can help distinguish carcinoids of unknown primary origin.19

Metastases in carcinoid tumors are common, with one study noting that the highest frequency of small intestinal metastases was from the ileal subset.20 At the time of diagnosis, 58% to 64% of patients with small intestine carcinoid tumors already had nonlocalized disease, with frequent sites being the lymph nodes (89.8%), liver (44.1%), lungs (13.6%), and peritoneum (13.6%). Regional and distant metastases are associated with substantially worse prognoses, with survival rates of 71.7% and 38.5%, respectively.1 Treatment of symptomatic unresectable disease focuses on symptomatic management with somatostatin analogs that also control tumor growth.21
We present a rare case of scalp metastasis of a carcinoid tumor of the terminal ileum. Distant metastasis is associated with poorer prognosis and should be considered in patients with a known history of a carcinoid tumor.
Acknowledgment—We would like to acknowledge the Research Histology and Tissue Imaging Core at University of Illinois Chicago Research Resources Center for the immunohistochemistry studies.
The Diagnosis: Metastatic Carcinoid Tumor
Carcinoid tumors are derived from neuroendocrine cell compartments and generally arise in the gastrointestinal tract, with a quarter of carcinoids arising in the small bowel.1 Carcinoid tumors have an incidence of approximately 2 to 5 per 100,000 patients.2 Metastasis of carcinoids is approximately 31.2% to 46.7%.1 Metastasis to the skin is uncommon; we present a rare case of a carcinoid tumor of the terminal ileum with metastasis to the scalp.
Unlike our patient, most patients with carcinoid tumors have an indolent clinical course. The most common cutaneous symptom is flushing, which occurs in 75% of patients.3 Secreted vasoactive peptides such as serotonin may cause other symptoms such as tachycardia, diarrhea, and bronchospasm; together, these symptoms comprise carcinoid syndrome. Carcinoid syndrome requires metastasis of the tumor to the liver or a site outside of the gastrointestinal tract because the liver will metabolize the secreted serotonin. However, even in patients with liver metastasis, carcinoid syndrome only occurs in approximately 10% of patients.4 Common skin findings of carcinoid syndrome include pellagralike dermatitis, flushing, and sclerodermalike changes.5 Our patient experienced several episodes of presyncope with symptoms of dyspnea, lightheadedness, and flushing but did not have bronchospasm or recurrent diarrhea. Intramuscular octreotide improved some symptoms.
The scalp accounts for approximately 15% of cutaneous metastases, the most common being from the lung, renal, and breast cancers.6 Cutaneous metastases of carcinoid tumors are rare. A PubMed search of articles indexed for MEDLINE using the terms metastatic AND [carcinoid OR neuroendocrine] tumors AND [skin OR cutaneous] revealed 47 cases.7-11 Similar to other skin metastases, cutaneous metastases of carcinoid tumors commonly present as firm erythematous nodules of varying sizes that may be asymptomatic, tender, or pruritic (Figure 1). Cases of carcinoid tumors with cutaneous metastasis as the initial and only presenting sign are exceedingly rare.12

Histology of carcinoid tumors reveals a dermal neoplasm composed of loosely cohesive, mildly atypical, polygonal cells with salt-and-pepper chromatin and eosinophilic cytoplasm, which are similar findings to the primary tumor. The cells may grow in the typical trabecular or organoid neuroendocrine pattern or exhibit a pseudoglandular growth pattern with prominent vessels (quiz image, top).12 Positive chromogranin and synaptophysin immunostaining are the most common and reliable markers used for the diagnosis of carcinoid tumors.

An important histopathologic differential diagnosis is the aggressive Merkel cell carcinoma, which also demonstrates homogenous salt-and-pepper chromatin but exhibits a higher mitotic rate and positive cytokeratin 20 staining (Figure 2).13 Basal cell carcinoma (BCC) also may display similar features, including a blue tumor at scanning magnification and nodular or infiltrative growth patterns. The cell morphology of BCC is characterized by islands of basaloid cells with minimal cytoplasm and frequent apoptosis, connecting to the epidermis with peripheral palisading, retraction artifact, and a myxoid stroma; BCC lacks the salt-and-pepper chromatin commonly seen in carcinoid tumors (Figure 3). Basal cell carcinoma is characterized by positive BerEP4 (epithelial cell adhesion molecule immunostain), cytokeratin 5/6, and cytokeratin 14 uptake. Cytokeratin 20, often used to diagnose Merkel cell carcinoma, is negative in BCC. Chromogranin and synaptophysin occasionally may be positive in BCC.14

The superficial Ewing sarcoma family of tumors also may be included in the differential diagnosis of small round cell tumors of the skin, but they are very rare. These tumors possess strong positive membranous staining of cytokeratin 99 and also can stain positively for synaptophysin and chromogranin.15 Epithelial membrane antigen, which is negative in Ewing sarcomas, is positive in carcinoid tumors.16 Neuroendocrine tumors of all sites share similar basic morphologic patterns, and multiple primary tumors should be considered, including small cell lung carcinoma (Figure 4).17,18 Red granulations and true glandular lumina typically are not seen in the lungs but are common in gastrointestinal carcinoids.18 Regarding immunohistochemistry, TTF-1 is negative and CDX2 is positive in gastroenteropancreatic carcinoids, suggesting that these 2 markers can help distinguish carcinoids of unknown primary origin.19

Metastases in carcinoid tumors are common, with one study noting that the highest frequency of small intestinal metastases was from the ileal subset.20 At the time of diagnosis, 58% to 64% of patients with small intestine carcinoid tumors already had nonlocalized disease, with frequent sites being the lymph nodes (89.8%), liver (44.1%), lungs (13.6%), and peritoneum (13.6%). Regional and distant metastases are associated with substantially worse prognoses, with survival rates of 71.7% and 38.5%, respectively.1 Treatment of symptomatic unresectable disease focuses on symptomatic management with somatostatin analogs that also control tumor growth.21
We present a rare case of scalp metastasis of a carcinoid tumor of the terminal ileum. Distant metastasis is associated with poorer prognosis and should be considered in patients with a known history of a carcinoid tumor.
Acknowledgment—We would like to acknowledge the Research Histology and Tissue Imaging Core at University of Illinois Chicago Research Resources Center for the immunohistochemistry studies.
- Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934-959.
- Lawrence B, Gustafsson BI, Chan A, et al. The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am. 2011;40:1-18, vii.
- Sabir S, James WD, Schuchter LM. Cutaneous manifestations of cancer. Curr Opin Oncol. 1999;11:139-144.
- Tomassetti P. Clinical aspects of carcinoid tumours. Italian J Gastroenterol Hepatol. 1999;31(suppl 2):S143-S146.
- Bell HK, Poston GJ, Vora J, et al. Cutaneous manifestations of the malignant carcinoid syndrome. Br J Dermatol. 2005;152:71-75.
- Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29(2 pt 1):228-236.
- Garcia A, Mays S, Silapunt S. Metastatic neuroendocrine carcinoma in the skin. Dermatol Online J. 2017;23:13030/qt9052w9x1.
- Ciliberti MP, Carbonara R, Grillo A, et al. Unexpected response to palliative radiotherapy for subcutaneous metastases of an advanced small cell pancreatic neuroendocrine carcinoma: a case report of two different radiation schedules. BMC Cancer. 2020;20:311.
- Devnani B, Kumar R, Pathy S, et al. Cutaneous metastases from neuroendocrine carcinoma of the cervix: an unusual metastatic lesion from an uncommon malignancy. Curr Probl Cancer. 2018; 42:527-533.
- Falto-Aizpurua L, Seyfer S, Krishnan B, et al. Cutaneous metastasis of a pulmonary carcinoid tumor. Cutis. 2017;99:E13-E15.
- Dhingra R, Tse JY, Saif MW. Cutaneous metastasis of gastroenteropancreatic neuroendocrine tumors (GEP-Nets)[published online September 8, 2018]. JOP. 2018;19.
- Jedrych J, Busam K, Klimstra DS, et al. Cutaneous metastases as an initial manifestation of visceral well-differentiated neuroendocrine tumor: a report of four cases and a review of literature. J Cutan Pathol. 2014;41:113-122.
- Lloyd RV. Practical markers used in the diagnosis of neuroendocrine tumors. Endocr Pathol. 2003;14:293-301.
- Stanoszek LM, Wang GY, Harms PW. Histologic mimics of basal cell carcinoma. Arch Pathol Lab Med. 2017;141:1490-1502.
- Machado I, Llombart B, Calabuig-Fariñas S, et al. Superficial Ewing’s sarcoma family of tumors: a clinicopathological study with differential diagnoses. J Cutan Pathol. 2011;38:636-643.
- D’Cruze L, Dutta R, Rao S, et al. The role of immunohistochemistry in the analysis of the spectrum of small round cell tumours at a tertiary care centre. J Clin Diagn Res. 2013;7:1377-1382.
- Chirila DN, Turdeanu NA, Constantea NA, et al. Multiple malignant tumors. Chirurgia (Bucur). 2013;108:498-502.
- Rekhtman N. Neuroendocrine tumors of the lung: an update. Arch Pathol Lab Med. 2010;134:1628-1638.
- Lin X, Saad RS, Luckasevic TM, et al. Diagnostic value of CDX-2 and TTF-1 expressions in separating metastatic neuroendocrine neoplasms of unknown origin. Appl Immunohistochem Mol Morphol. 2007;15:407-414.
- Olney JR, Urdaneta LF, Al-Jurf AS, et al. Carcinoid tumors of the gastrointestinal tract. Am Surg. 1985;51:37-41.
- Strosberg JR, Halfdanarson TR, Bellizzi AM, et al. The North American Neuroendocrine Tumor Society consensus guidelines for surveillance and medical management of midgut neuroendocrine tumors. Pancreas. 2017;46:707-714.
- Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934-959.
- Lawrence B, Gustafsson BI, Chan A, et al. The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am. 2011;40:1-18, vii.
- Sabir S, James WD, Schuchter LM. Cutaneous manifestations of cancer. Curr Opin Oncol. 1999;11:139-144.
- Tomassetti P. Clinical aspects of carcinoid tumours. Italian J Gastroenterol Hepatol. 1999;31(suppl 2):S143-S146.
- Bell HK, Poston GJ, Vora J, et al. Cutaneous manifestations of the malignant carcinoid syndrome. Br J Dermatol. 2005;152:71-75.
- Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29(2 pt 1):228-236.
- Garcia A, Mays S, Silapunt S. Metastatic neuroendocrine carcinoma in the skin. Dermatol Online J. 2017;23:13030/qt9052w9x1.
- Ciliberti MP, Carbonara R, Grillo A, et al. Unexpected response to palliative radiotherapy for subcutaneous metastases of an advanced small cell pancreatic neuroendocrine carcinoma: a case report of two different radiation schedules. BMC Cancer. 2020;20:311.
- Devnani B, Kumar R, Pathy S, et al. Cutaneous metastases from neuroendocrine carcinoma of the cervix: an unusual metastatic lesion from an uncommon malignancy. Curr Probl Cancer. 2018; 42:527-533.
- Falto-Aizpurua L, Seyfer S, Krishnan B, et al. Cutaneous metastasis of a pulmonary carcinoid tumor. Cutis. 2017;99:E13-E15.
- Dhingra R, Tse JY, Saif MW. Cutaneous metastasis of gastroenteropancreatic neuroendocrine tumors (GEP-Nets)[published online September 8, 2018]. JOP. 2018;19.
- Jedrych J, Busam K, Klimstra DS, et al. Cutaneous metastases as an initial manifestation of visceral well-differentiated neuroendocrine tumor: a report of four cases and a review of literature. J Cutan Pathol. 2014;41:113-122.
- Lloyd RV. Practical markers used in the diagnosis of neuroendocrine tumors. Endocr Pathol. 2003;14:293-301.
- Stanoszek LM, Wang GY, Harms PW. Histologic mimics of basal cell carcinoma. Arch Pathol Lab Med. 2017;141:1490-1502.
- Machado I, Llombart B, Calabuig-Fariñas S, et al. Superficial Ewing’s sarcoma family of tumors: a clinicopathological study with differential diagnoses. J Cutan Pathol. 2011;38:636-643.
- D’Cruze L, Dutta R, Rao S, et al. The role of immunohistochemistry in the analysis of the spectrum of small round cell tumours at a tertiary care centre. J Clin Diagn Res. 2013;7:1377-1382.
- Chirila DN, Turdeanu NA, Constantea NA, et al. Multiple malignant tumors. Chirurgia (Bucur). 2013;108:498-502.
- Rekhtman N. Neuroendocrine tumors of the lung: an update. Arch Pathol Lab Med. 2010;134:1628-1638.
- Lin X, Saad RS, Luckasevic TM, et al. Diagnostic value of CDX-2 and TTF-1 expressions in separating metastatic neuroendocrine neoplasms of unknown origin. Appl Immunohistochem Mol Morphol. 2007;15:407-414.
- Olney JR, Urdaneta LF, Al-Jurf AS, et al. Carcinoid tumors of the gastrointestinal tract. Am Surg. 1985;51:37-41.
- Strosberg JR, Halfdanarson TR, Bellizzi AM, et al. The North American Neuroendocrine Tumor Society consensus guidelines for surveillance and medical management of midgut neuroendocrine tumors. Pancreas. 2017;46:707-714.
A 47-year-old woman was admitted to the hospital with abdominal pain and flushing. She had a history of a midgut carcinoid that originated in the ileum with metastasis to the colon, liver, and pancreas. Dermatologic examination revealed a firm, nontender, mobile, 7-mm scalp nodule with a pink-purple overlying epidermis. The lesion was associated with a slight decrease in hair density. A 4-mm punch biopsy was performed.

Physicians speak out: Why they love or hate incentive bonuses
Incentive bonuses have long been part and parcel of many physicians’ compensation packages. They allow doctors in some specialties to boost their compensation by tens of thousands of dollars.
Often tied to metrics that doctors must hit,
A recent Medscape poll asked what physicians think about incentive bonuses and whether or not tying metrics to salary is an outdated practice that interferes with the integrity of a physician’s job or contributes to excellence in patient care and increased productivity.
Here is what 406 physicians who answered the poll, which ran from Aug. 17 to Sept. 1, had to say about incentive bonuses:
More than half the physicians polled (58%) received an incentive bonus in 2021. Of those who received a bonus, 44% received up to $25,000. Almost 30% received $25,001-$50,000 in incentive bonus money. Only 14% received more than $100,000.
When we asked physicians which metrics they prefer their bonus to be based on, a large majority (64%) agreed quality of care was most relevant. Other metrics that respondents think appropriate included professionalism (40%), patient outcomes (40%), patient satisfaction (34%), patient volume (26%), market expansion (7%), and other (3%).
The problem with bonuses
Once thought to improve quality and consistency of care, incentive bonuses may be falling out of favor. Developing, administrating, and tracking them may be cumbersome for the institutions that advocate for them. For instance, determining who gave quality care and how to measure that care can be difficult.
What’s more, some top health care employers, Mayo Clinic and Kaiser Permanente, have switched from the incentive bonus model to straight salaries. Data show that the number of tests patients have and the number of treatments they try decreases when doctors receive straight salaries.
In fact, 74% of the polled physicians think that bonuses can result in consequences like unnecessary tests and higher patient costs. Three-fourths of respondents don’t think incentives improve patient care either.
Physicians have long thought incentive bonuses can also have unintended consequences. For example, tying a physician’s monetary reward to metrics such as patient outcomes, like adherence to treatment protocols, may mean that noncompliant patients can jeopardize your metrics and prevent physicians from getting bonuses.
A Merritt Hawkins’ 2019 Review of Physician and Advanced Practitioner Recruiting Incentives found that 56% of bonuses are based in whole or in part on metrics like a patient’s adherence.
Additionally, tying monetary rewards to patient volume encourages some physicians to overbook patients, work more and longer hours, and risk burnout to meet their bonus criteria.
When we asked how hard it was to meet metrics in the Medscape poll, 45% of respondents who receive incentive bonuses said it was somewhat or very difficult. Only 9% consider it very easy. And 71% of physicians say their bonus is at risk because of not meeting their metrics.
Not surprisingly, large pay-for-performance bonuses are only offered to certain specialists and physician specialties in high demand. An orthopedist, for example, can earn up to an average of $126,000 in incentive bonuses, while a pediatrician brings in an average of $28,000, according to the Medscape Physician Compensation Report 2022.
Yet physicians are still torn
Despite these negatives, physicians are split about whether bonuses are good for doctors. The poll shows 51% said no, and 49% said yes. Further, physicians were split 50-50 on whether the bonus makes physicians more productive. Interestingly though, 76% think the bonus compensation method should be phased out in favor of straight salaries.
But many physicians may welcome the “lump sum” nature of receiving large bonuses at certain times of the year to help pay off student loan debt or other expenses, or are just comfortable having a bonus.
Financially speaking
If you have the choice, you may fare better by taking a higher salary and eliminating a bonus. Receiving your pay throughout the year may be preferable to receiving large lump sums only at certain times. Another thing to remember about your incentive bonus is that they are sometimes taxed more heavily based on “supplemental income.” The IRS considers bonuses supplemental to your income, so they may have a higher withholding rate, which can feel penalizing. You may have noticed the extra withholding in your last bonus check.
A version of this article first appeared on Medscape.com.
Incentive bonuses have long been part and parcel of many physicians’ compensation packages. They allow doctors in some specialties to boost their compensation by tens of thousands of dollars.
Often tied to metrics that doctors must hit,
A recent Medscape poll asked what physicians think about incentive bonuses and whether or not tying metrics to salary is an outdated practice that interferes with the integrity of a physician’s job or contributes to excellence in patient care and increased productivity.
Here is what 406 physicians who answered the poll, which ran from Aug. 17 to Sept. 1, had to say about incentive bonuses:
More than half the physicians polled (58%) received an incentive bonus in 2021. Of those who received a bonus, 44% received up to $25,000. Almost 30% received $25,001-$50,000 in incentive bonus money. Only 14% received more than $100,000.
When we asked physicians which metrics they prefer their bonus to be based on, a large majority (64%) agreed quality of care was most relevant. Other metrics that respondents think appropriate included professionalism (40%), patient outcomes (40%), patient satisfaction (34%), patient volume (26%), market expansion (7%), and other (3%).
The problem with bonuses
Once thought to improve quality and consistency of care, incentive bonuses may be falling out of favor. Developing, administrating, and tracking them may be cumbersome for the institutions that advocate for them. For instance, determining who gave quality care and how to measure that care can be difficult.
What’s more, some top health care employers, Mayo Clinic and Kaiser Permanente, have switched from the incentive bonus model to straight salaries. Data show that the number of tests patients have and the number of treatments they try decreases when doctors receive straight salaries.
In fact, 74% of the polled physicians think that bonuses can result in consequences like unnecessary tests and higher patient costs. Three-fourths of respondents don’t think incentives improve patient care either.
Physicians have long thought incentive bonuses can also have unintended consequences. For example, tying a physician’s monetary reward to metrics such as patient outcomes, like adherence to treatment protocols, may mean that noncompliant patients can jeopardize your metrics and prevent physicians from getting bonuses.
A Merritt Hawkins’ 2019 Review of Physician and Advanced Practitioner Recruiting Incentives found that 56% of bonuses are based in whole or in part on metrics like a patient’s adherence.
Additionally, tying monetary rewards to patient volume encourages some physicians to overbook patients, work more and longer hours, and risk burnout to meet their bonus criteria.
When we asked how hard it was to meet metrics in the Medscape poll, 45% of respondents who receive incentive bonuses said it was somewhat or very difficult. Only 9% consider it very easy. And 71% of physicians say their bonus is at risk because of not meeting their metrics.
Not surprisingly, large pay-for-performance bonuses are only offered to certain specialists and physician specialties in high demand. An orthopedist, for example, can earn up to an average of $126,000 in incentive bonuses, while a pediatrician brings in an average of $28,000, according to the Medscape Physician Compensation Report 2022.
Yet physicians are still torn
Despite these negatives, physicians are split about whether bonuses are good for doctors. The poll shows 51% said no, and 49% said yes. Further, physicians were split 50-50 on whether the bonus makes physicians more productive. Interestingly though, 76% think the bonus compensation method should be phased out in favor of straight salaries.
But many physicians may welcome the “lump sum” nature of receiving large bonuses at certain times of the year to help pay off student loan debt or other expenses, or are just comfortable having a bonus.
Financially speaking
If you have the choice, you may fare better by taking a higher salary and eliminating a bonus. Receiving your pay throughout the year may be preferable to receiving large lump sums only at certain times. Another thing to remember about your incentive bonus is that they are sometimes taxed more heavily based on “supplemental income.” The IRS considers bonuses supplemental to your income, so they may have a higher withholding rate, which can feel penalizing. You may have noticed the extra withholding in your last bonus check.
A version of this article first appeared on Medscape.com.
Incentive bonuses have long been part and parcel of many physicians’ compensation packages. They allow doctors in some specialties to boost their compensation by tens of thousands of dollars.
Often tied to metrics that doctors must hit,
A recent Medscape poll asked what physicians think about incentive bonuses and whether or not tying metrics to salary is an outdated practice that interferes with the integrity of a physician’s job or contributes to excellence in patient care and increased productivity.
Here is what 406 physicians who answered the poll, which ran from Aug. 17 to Sept. 1, had to say about incentive bonuses:
More than half the physicians polled (58%) received an incentive bonus in 2021. Of those who received a bonus, 44% received up to $25,000. Almost 30% received $25,001-$50,000 in incentive bonus money. Only 14% received more than $100,000.
When we asked physicians which metrics they prefer their bonus to be based on, a large majority (64%) agreed quality of care was most relevant. Other metrics that respondents think appropriate included professionalism (40%), patient outcomes (40%), patient satisfaction (34%), patient volume (26%), market expansion (7%), and other (3%).
The problem with bonuses
Once thought to improve quality and consistency of care, incentive bonuses may be falling out of favor. Developing, administrating, and tracking them may be cumbersome for the institutions that advocate for them. For instance, determining who gave quality care and how to measure that care can be difficult.
What’s more, some top health care employers, Mayo Clinic and Kaiser Permanente, have switched from the incentive bonus model to straight salaries. Data show that the number of tests patients have and the number of treatments they try decreases when doctors receive straight salaries.
In fact, 74% of the polled physicians think that bonuses can result in consequences like unnecessary tests and higher patient costs. Three-fourths of respondents don’t think incentives improve patient care either.
Physicians have long thought incentive bonuses can also have unintended consequences. For example, tying a physician’s monetary reward to metrics such as patient outcomes, like adherence to treatment protocols, may mean that noncompliant patients can jeopardize your metrics and prevent physicians from getting bonuses.
A Merritt Hawkins’ 2019 Review of Physician and Advanced Practitioner Recruiting Incentives found that 56% of bonuses are based in whole or in part on metrics like a patient’s adherence.
Additionally, tying monetary rewards to patient volume encourages some physicians to overbook patients, work more and longer hours, and risk burnout to meet their bonus criteria.
When we asked how hard it was to meet metrics in the Medscape poll, 45% of respondents who receive incentive bonuses said it was somewhat or very difficult. Only 9% consider it very easy. And 71% of physicians say their bonus is at risk because of not meeting their metrics.
Not surprisingly, large pay-for-performance bonuses are only offered to certain specialists and physician specialties in high demand. An orthopedist, for example, can earn up to an average of $126,000 in incentive bonuses, while a pediatrician brings in an average of $28,000, according to the Medscape Physician Compensation Report 2022.
Yet physicians are still torn
Despite these negatives, physicians are split about whether bonuses are good for doctors. The poll shows 51% said no, and 49% said yes. Further, physicians were split 50-50 on whether the bonus makes physicians more productive. Interestingly though, 76% think the bonus compensation method should be phased out in favor of straight salaries.
But many physicians may welcome the “lump sum” nature of receiving large bonuses at certain times of the year to help pay off student loan debt or other expenses, or are just comfortable having a bonus.
Financially speaking
If you have the choice, you may fare better by taking a higher salary and eliminating a bonus. Receiving your pay throughout the year may be preferable to receiving large lump sums only at certain times. Another thing to remember about your incentive bonus is that they are sometimes taxed more heavily based on “supplemental income.” The IRS considers bonuses supplemental to your income, so they may have a higher withholding rate, which can feel penalizing. You may have noticed the extra withholding in your last bonus check.
A version of this article first appeared on Medscape.com.
Three COVID scenarios that could spell trouble for the fall
As the United States enters a third fall with COVID-19, the virus for many is seemingly gone – or at least out of mind. But for those keeping watch, it is far from forgotten as deaths and infections continue to mount at a lower but steady pace.
What does that mean for the upcoming months? Experts predict different scenarios, some more dire than others – with one more encouraging.
In the United States, more than 300 people still die every day from COVID and more than 44,000 new daily cases are reported, according to the Centers for Disease Control and Prevention.
But progress is undeniable. The stark daily death tolls of 2020 have plummeted. Vaccines and treatments have dramatically reduced severe illness, and mask requirements have mostly turned to personal preference.
among them more-resistant variants coupled with waning immunity, the potential for a “twindemic” with a flu/COVID onslaught, and underuse of lifesaving vaccines and treatments.
Variants loom/waning immunity
Omicron variant BA.5 still makes up about 80% of infections in the United States, followed by BA4.6, according to the CDC, but other subvariants are emerging and showing signs of resistance to current antiviral treatments.
Eric Topol, MD, founder and director of the Scripps Research Translational Institute in San Diego, said about COVID this fall: “There will be another wave, magnitude unknown.”
He said subvariants XBB and BQ.1.1 “have extreme levels of immune evasion and both could pose a challenge,” explaining that XBB is more likely to cause trouble than BQ.1.1 because it is even more resistant to natural or vaccine-induced immunity.
Dr. Topol pointed to new research on those variants in a preprint posted on bioRxiv. The authors’ conclusion: “These results suggest that current herd immunity and BA.5 vaccine boosters may not provide sufficiently broad protection against infection.”
Another variant to watch, some experts say, is Omicron subvariant BA.2.75.2, which has shown resistance to antiviral treatments. It is also growing at a rather alarming rate, says Michael Sweat, PhD, director of the Medical University of South Carolina Center for Global Health in Charleston. That subvariant currently makes up under 2% of U.S. cases but has spread to at least 55 countries and 43 U.S. states after first appearing at the end of last year globally and in mid-June in the United States.
A non–peer-reviewed preprint study from Sweden found that the variant in blood samples was neutralized on average “at titers approximately 6.5 times lower than BA.5, making BA.2.75.2 the most [neutralization-resistant] variant evaluated to date.”
Katelyn Jetelina, PhD, assistant professor in the department of epidemiology at University of Texas Health Science Center, Houston, said in an interview the U.S. waves often follow Europe’s, and Europe has seen a recent spike in cases and hospitalizations not related to Omicron subvariants, but to weather changes, waning immunity, and changes in behavior.
The World Health Organization reported on Oct. 5 that, while cases were down in every other region of the world, Europe’s numbers stand out, with an 8% increase in cases from the week before.
Dr. Jetelina cited events such as Oktoberfest in Germany, which ended in the first week of October after drawing nearly 6 million people over 2 weeks, as a potential contributor, and people heading indoors as weather patterns change in Europe.
Ali Mokdad, PhD, chief strategy officer for population health at the University of Washington, Seattle, said in an interview he is less worried about the documented variants we know about than he is about the potential for a new immune-escape variety yet to emerge.
“Right now we know the Chinese are gearing up to open up the country, and because they have low immunity and little infection, we expect in China there will be a lot of spread of Omicron,” he said. “It’s possible because of the number of infections we could see a new variant.”
Dr. Mokdad said waning immunity could also leave populations vulnerable to variants.
“Even if you get infected, after about 5 months, you’re susceptible again. Remember, most of the infections from Omicron happened in January or February 2022, and we had two waves after that,” he said.
The new bivalent vaccines tweaked to target some Omicron variants will help, Dr. Mokdad said, but he noted, “people are very reluctant to take it.”
Jennifer Nuzzo, DrPH, professor of epidemiology and director of the Pandemic Center at Brown University, Providence, R.I., worries that in the United States we have less ability this year to track variants as funding has receded for testing kits and testing sites. Most people are testing at home – which doesn’t show up in the numbers – and the United States is relying more on other countries’ data to spot trends.
“I think we’re just going to have less visibility into the circulation of this virus,” she said in an interview.
‘Twindemic’: COVID and flu
Dr. Jetelina noted Australia and New Zealand just wrapped up a flu season that saw flu numbers returning to normal after a sharp drop in the last 2 years, and North America typically follows suit.
“We do expect flu will be here in the United States and probably at levels that we saw prepandemic. We’re all holding our breath to see how our health systems hold up with COVID-19 and flu. We haven’t really experienced that yet,” she said.
There is some disagreement, however, about the possibility of a so-called “twindemic” of influenza and COVID.
Richard Webby, PhD, an infectious disease specialist at St. Jude Children’s Research Hospital in Memphis, said in an interview he thinks the possibility of both viruses spiking at the same time is unlikely.
“That’s not to say we won’t get flu and COVID activity in the same winter,” he explained, “but I think both roaring at the same time is unlikely.”
As an indicator, he said, at the beginning of the flu season last year in the Northern Hemisphere, flu activity started to pick up, but when the Omicron variant came along, “flu just wasn’t able to compete in that same environment and flu numbers dropped right off.” Previous literature suggests that when one virus is spiking it’s hard for another respiratory virus to take hold.
Vaccine, treatment underuse
Another threat is vaccines, boosters, and treatments sitting on shelves.
Dr. Sweat referred to frustration with vaccine uptake that seems to be “frozen in amber.”
As of Oct. 4, only 5.3% of people in the United States who were eligible had received the updated booster launched in early September.
Dr. Nuzzo said boosters for people at least 65 years old will be key to severity of COVID this season.
“I think that’s probably the biggest factor going into the fall and winter,” she said.
Only 38% of people at least 50 years old and 45% of those at least 65 years old had gotten a second booster as of early October.
“If we do nothing else, we have to increase booster uptake in that group,” Dr. Nuzzo said.
She said the treatment nirmatrelvir/ritonavir (Paxlovid, Pfizer) for treating mild to moderate COVID-19 in patients at high risk for severe disease is greatly underused, often because providers aren’t prescribing it because they don’t think it helps, are worried about drug interactions, or are worried about its “rebound” effect.
Dr. Nuzzo urged greater use of the drug and education on how to manage drug interactions.
“We have very strong data that it does help keep people out of hospital. Sure, there may be a rebound, but that pales in comparison to the risk of being hospitalized,” she said.
Calm COVID season?
Not all predictions are dire. There is another little-talked-about scenario, Dr. Sweat said – that we could be in for a calm COVID season, and those who seem to be only mildly concerned about COVID may find those thoughts justified in the numbers.
Omicron blew through with such strength, he noted, that it may have left wide immunity in its wake. Because variants seem to be staying in the Omicron family, that may signal optimism.
“If the next variant is a descendant of the Omicron lineage, I would suspect that all these people who just got infected will have some protection, not perfect, but quite a bit of protection,” Dr. Sweat said.
Dr. Topol, Dr. Nuzzo, Dr. Sweat, Dr. Webby, Dr. Mokdad, and Dr. Jetelina reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
As the United States enters a third fall with COVID-19, the virus for many is seemingly gone – or at least out of mind. But for those keeping watch, it is far from forgotten as deaths and infections continue to mount at a lower but steady pace.
What does that mean for the upcoming months? Experts predict different scenarios, some more dire than others – with one more encouraging.
In the United States, more than 300 people still die every day from COVID and more than 44,000 new daily cases are reported, according to the Centers for Disease Control and Prevention.
But progress is undeniable. The stark daily death tolls of 2020 have plummeted. Vaccines and treatments have dramatically reduced severe illness, and mask requirements have mostly turned to personal preference.
among them more-resistant variants coupled with waning immunity, the potential for a “twindemic” with a flu/COVID onslaught, and underuse of lifesaving vaccines and treatments.
Variants loom/waning immunity
Omicron variant BA.5 still makes up about 80% of infections in the United States, followed by BA4.6, according to the CDC, but other subvariants are emerging and showing signs of resistance to current antiviral treatments.
Eric Topol, MD, founder and director of the Scripps Research Translational Institute in San Diego, said about COVID this fall: “There will be another wave, magnitude unknown.”
He said subvariants XBB and BQ.1.1 “have extreme levels of immune evasion and both could pose a challenge,” explaining that XBB is more likely to cause trouble than BQ.1.1 because it is even more resistant to natural or vaccine-induced immunity.
Dr. Topol pointed to new research on those variants in a preprint posted on bioRxiv. The authors’ conclusion: “These results suggest that current herd immunity and BA.5 vaccine boosters may not provide sufficiently broad protection against infection.”
Another variant to watch, some experts say, is Omicron subvariant BA.2.75.2, which has shown resistance to antiviral treatments. It is also growing at a rather alarming rate, says Michael Sweat, PhD, director of the Medical University of South Carolina Center for Global Health in Charleston. That subvariant currently makes up under 2% of U.S. cases but has spread to at least 55 countries and 43 U.S. states after first appearing at the end of last year globally and in mid-June in the United States.
A non–peer-reviewed preprint study from Sweden found that the variant in blood samples was neutralized on average “at titers approximately 6.5 times lower than BA.5, making BA.2.75.2 the most [neutralization-resistant] variant evaluated to date.”
Katelyn Jetelina, PhD, assistant professor in the department of epidemiology at University of Texas Health Science Center, Houston, said in an interview the U.S. waves often follow Europe’s, and Europe has seen a recent spike in cases and hospitalizations not related to Omicron subvariants, but to weather changes, waning immunity, and changes in behavior.
The World Health Organization reported on Oct. 5 that, while cases were down in every other region of the world, Europe’s numbers stand out, with an 8% increase in cases from the week before.
Dr. Jetelina cited events such as Oktoberfest in Germany, which ended in the first week of October after drawing nearly 6 million people over 2 weeks, as a potential contributor, and people heading indoors as weather patterns change in Europe.
Ali Mokdad, PhD, chief strategy officer for population health at the University of Washington, Seattle, said in an interview he is less worried about the documented variants we know about than he is about the potential for a new immune-escape variety yet to emerge.
“Right now we know the Chinese are gearing up to open up the country, and because they have low immunity and little infection, we expect in China there will be a lot of spread of Omicron,” he said. “It’s possible because of the number of infections we could see a new variant.”
Dr. Mokdad said waning immunity could also leave populations vulnerable to variants.
“Even if you get infected, after about 5 months, you’re susceptible again. Remember, most of the infections from Omicron happened in January or February 2022, and we had two waves after that,” he said.
The new bivalent vaccines tweaked to target some Omicron variants will help, Dr. Mokdad said, but he noted, “people are very reluctant to take it.”
Jennifer Nuzzo, DrPH, professor of epidemiology and director of the Pandemic Center at Brown University, Providence, R.I., worries that in the United States we have less ability this year to track variants as funding has receded for testing kits and testing sites. Most people are testing at home – which doesn’t show up in the numbers – and the United States is relying more on other countries’ data to spot trends.
“I think we’re just going to have less visibility into the circulation of this virus,” she said in an interview.
‘Twindemic’: COVID and flu
Dr. Jetelina noted Australia and New Zealand just wrapped up a flu season that saw flu numbers returning to normal after a sharp drop in the last 2 years, and North America typically follows suit.
“We do expect flu will be here in the United States and probably at levels that we saw prepandemic. We’re all holding our breath to see how our health systems hold up with COVID-19 and flu. We haven’t really experienced that yet,” she said.
There is some disagreement, however, about the possibility of a so-called “twindemic” of influenza and COVID.
Richard Webby, PhD, an infectious disease specialist at St. Jude Children’s Research Hospital in Memphis, said in an interview he thinks the possibility of both viruses spiking at the same time is unlikely.
“That’s not to say we won’t get flu and COVID activity in the same winter,” he explained, “but I think both roaring at the same time is unlikely.”
As an indicator, he said, at the beginning of the flu season last year in the Northern Hemisphere, flu activity started to pick up, but when the Omicron variant came along, “flu just wasn’t able to compete in that same environment and flu numbers dropped right off.” Previous literature suggests that when one virus is spiking it’s hard for another respiratory virus to take hold.
Vaccine, treatment underuse
Another threat is vaccines, boosters, and treatments sitting on shelves.
Dr. Sweat referred to frustration with vaccine uptake that seems to be “frozen in amber.”
As of Oct. 4, only 5.3% of people in the United States who were eligible had received the updated booster launched in early September.
Dr. Nuzzo said boosters for people at least 65 years old will be key to severity of COVID this season.
“I think that’s probably the biggest factor going into the fall and winter,” she said.
Only 38% of people at least 50 years old and 45% of those at least 65 years old had gotten a second booster as of early October.
“If we do nothing else, we have to increase booster uptake in that group,” Dr. Nuzzo said.
She said the treatment nirmatrelvir/ritonavir (Paxlovid, Pfizer) for treating mild to moderate COVID-19 in patients at high risk for severe disease is greatly underused, often because providers aren’t prescribing it because they don’t think it helps, are worried about drug interactions, or are worried about its “rebound” effect.
Dr. Nuzzo urged greater use of the drug and education on how to manage drug interactions.
“We have very strong data that it does help keep people out of hospital. Sure, there may be a rebound, but that pales in comparison to the risk of being hospitalized,” she said.
Calm COVID season?
Not all predictions are dire. There is another little-talked-about scenario, Dr. Sweat said – that we could be in for a calm COVID season, and those who seem to be only mildly concerned about COVID may find those thoughts justified in the numbers.
Omicron blew through with such strength, he noted, that it may have left wide immunity in its wake. Because variants seem to be staying in the Omicron family, that may signal optimism.
“If the next variant is a descendant of the Omicron lineage, I would suspect that all these people who just got infected will have some protection, not perfect, but quite a bit of protection,” Dr. Sweat said.
Dr. Topol, Dr. Nuzzo, Dr. Sweat, Dr. Webby, Dr. Mokdad, and Dr. Jetelina reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
As the United States enters a third fall with COVID-19, the virus for many is seemingly gone – or at least out of mind. But for those keeping watch, it is far from forgotten as deaths and infections continue to mount at a lower but steady pace.
What does that mean for the upcoming months? Experts predict different scenarios, some more dire than others – with one more encouraging.
In the United States, more than 300 people still die every day from COVID and more than 44,000 new daily cases are reported, according to the Centers for Disease Control and Prevention.
But progress is undeniable. The stark daily death tolls of 2020 have plummeted. Vaccines and treatments have dramatically reduced severe illness, and mask requirements have mostly turned to personal preference.
among them more-resistant variants coupled with waning immunity, the potential for a “twindemic” with a flu/COVID onslaught, and underuse of lifesaving vaccines and treatments.
Variants loom/waning immunity
Omicron variant BA.5 still makes up about 80% of infections in the United States, followed by BA4.6, according to the CDC, but other subvariants are emerging and showing signs of resistance to current antiviral treatments.
Eric Topol, MD, founder and director of the Scripps Research Translational Institute in San Diego, said about COVID this fall: “There will be another wave, magnitude unknown.”
He said subvariants XBB and BQ.1.1 “have extreme levels of immune evasion and both could pose a challenge,” explaining that XBB is more likely to cause trouble than BQ.1.1 because it is even more resistant to natural or vaccine-induced immunity.
Dr. Topol pointed to new research on those variants in a preprint posted on bioRxiv. The authors’ conclusion: “These results suggest that current herd immunity and BA.5 vaccine boosters may not provide sufficiently broad protection against infection.”
Another variant to watch, some experts say, is Omicron subvariant BA.2.75.2, which has shown resistance to antiviral treatments. It is also growing at a rather alarming rate, says Michael Sweat, PhD, director of the Medical University of South Carolina Center for Global Health in Charleston. That subvariant currently makes up under 2% of U.S. cases but has spread to at least 55 countries and 43 U.S. states after first appearing at the end of last year globally and in mid-June in the United States.
A non–peer-reviewed preprint study from Sweden found that the variant in blood samples was neutralized on average “at titers approximately 6.5 times lower than BA.5, making BA.2.75.2 the most [neutralization-resistant] variant evaluated to date.”
Katelyn Jetelina, PhD, assistant professor in the department of epidemiology at University of Texas Health Science Center, Houston, said in an interview the U.S. waves often follow Europe’s, and Europe has seen a recent spike in cases and hospitalizations not related to Omicron subvariants, but to weather changes, waning immunity, and changes in behavior.
The World Health Organization reported on Oct. 5 that, while cases were down in every other region of the world, Europe’s numbers stand out, with an 8% increase in cases from the week before.
Dr. Jetelina cited events such as Oktoberfest in Germany, which ended in the first week of October after drawing nearly 6 million people over 2 weeks, as a potential contributor, and people heading indoors as weather patterns change in Europe.
Ali Mokdad, PhD, chief strategy officer for population health at the University of Washington, Seattle, said in an interview he is less worried about the documented variants we know about than he is about the potential for a new immune-escape variety yet to emerge.
“Right now we know the Chinese are gearing up to open up the country, and because they have low immunity and little infection, we expect in China there will be a lot of spread of Omicron,” he said. “It’s possible because of the number of infections we could see a new variant.”
Dr. Mokdad said waning immunity could also leave populations vulnerable to variants.
“Even if you get infected, after about 5 months, you’re susceptible again. Remember, most of the infections from Omicron happened in January or February 2022, and we had two waves after that,” he said.
The new bivalent vaccines tweaked to target some Omicron variants will help, Dr. Mokdad said, but he noted, “people are very reluctant to take it.”
Jennifer Nuzzo, DrPH, professor of epidemiology and director of the Pandemic Center at Brown University, Providence, R.I., worries that in the United States we have less ability this year to track variants as funding has receded for testing kits and testing sites. Most people are testing at home – which doesn’t show up in the numbers – and the United States is relying more on other countries’ data to spot trends.
“I think we’re just going to have less visibility into the circulation of this virus,” she said in an interview.
‘Twindemic’: COVID and flu
Dr. Jetelina noted Australia and New Zealand just wrapped up a flu season that saw flu numbers returning to normal after a sharp drop in the last 2 years, and North America typically follows suit.
“We do expect flu will be here in the United States and probably at levels that we saw prepandemic. We’re all holding our breath to see how our health systems hold up with COVID-19 and flu. We haven’t really experienced that yet,” she said.
There is some disagreement, however, about the possibility of a so-called “twindemic” of influenza and COVID.
Richard Webby, PhD, an infectious disease specialist at St. Jude Children’s Research Hospital in Memphis, said in an interview he thinks the possibility of both viruses spiking at the same time is unlikely.
“That’s not to say we won’t get flu and COVID activity in the same winter,” he explained, “but I think both roaring at the same time is unlikely.”
As an indicator, he said, at the beginning of the flu season last year in the Northern Hemisphere, flu activity started to pick up, but when the Omicron variant came along, “flu just wasn’t able to compete in that same environment and flu numbers dropped right off.” Previous literature suggests that when one virus is spiking it’s hard for another respiratory virus to take hold.
Vaccine, treatment underuse
Another threat is vaccines, boosters, and treatments sitting on shelves.
Dr. Sweat referred to frustration with vaccine uptake that seems to be “frozen in amber.”
As of Oct. 4, only 5.3% of people in the United States who were eligible had received the updated booster launched in early September.
Dr. Nuzzo said boosters for people at least 65 years old will be key to severity of COVID this season.
“I think that’s probably the biggest factor going into the fall and winter,” she said.
Only 38% of people at least 50 years old and 45% of those at least 65 years old had gotten a second booster as of early October.
“If we do nothing else, we have to increase booster uptake in that group,” Dr. Nuzzo said.
She said the treatment nirmatrelvir/ritonavir (Paxlovid, Pfizer) for treating mild to moderate COVID-19 in patients at high risk for severe disease is greatly underused, often because providers aren’t prescribing it because they don’t think it helps, are worried about drug interactions, or are worried about its “rebound” effect.
Dr. Nuzzo urged greater use of the drug and education on how to manage drug interactions.
“We have very strong data that it does help keep people out of hospital. Sure, there may be a rebound, but that pales in comparison to the risk of being hospitalized,” she said.
Calm COVID season?
Not all predictions are dire. There is another little-talked-about scenario, Dr. Sweat said – that we could be in for a calm COVID season, and those who seem to be only mildly concerned about COVID may find those thoughts justified in the numbers.
Omicron blew through with such strength, he noted, that it may have left wide immunity in its wake. Because variants seem to be staying in the Omicron family, that may signal optimism.
“If the next variant is a descendant of the Omicron lineage, I would suspect that all these people who just got infected will have some protection, not perfect, but quite a bit of protection,” Dr. Sweat said.
Dr. Topol, Dr. Nuzzo, Dr. Sweat, Dr. Webby, Dr. Mokdad, and Dr. Jetelina reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Dupilumab study outlines benefits, safety profile in infants, preschoolers with atopic dermatitis
at 31 treatment centers in North America and Europe.
Children younger than 6 years with moderate to severe AD have few options if their symptoms are uncontrolled with topical therapies, and persistent itchiness has a negative impact on quality of life for patients and families, Amy S. Paller, MD, professor and chair of dermatology, and professor of pediatrics at Northwestern University, Chicago, and colleagues wrote in the study, published in the Lancet.
The study was the basis of the Food and Drug Administration expanded approval of dupilumab in June 2022, to include children aged 6 months to 5 years with moderate to severe AD, whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable. Regulatory submission for this age group is under review by the European Medicines Agency, and by regulatory authorities in other countries, according to the manufacturers.
Dupilumab (Dupixent), which inhibits the signaling of the interleukin-4 and IL-13 pathways, was first approved in 2017 for treating adults with moderate to severe AD.
“There has not been a biologic approved before at such a young age, and for such a common disease,” Dr. Paller said in an interview. “This is the drug that has revolutionized care of the most common inflammatory skin disease in children, and this is the pivotal study that brought it to market for the youngest children who suffer from the severe forms.”
The study also sets a precedent for a lower threshold for starting systemic medication in young children for treating moderate to severe disease given the absence of severe side effects and no need for lab monitoring, Dr. Paller noted. However, dupilumab will also be closely watched “for both impact on the developing immune system and the possibility that it will alter the long-term course of the eczema and the development of allergic comorbidities, such as lowering the risk of developing asthma, GI, allergy, and possibly other conditions.”
In the study, the researchers randomized 83 children aged 6 months up to 6 years to treatment with dupilumab, administered subcutaneously, and 79 to placebo every 4 weeks for 16 weeks; both groups also received topical corticosteroids. Dosage of dupilumab was based on body weight; those with a body weight of 5-15 kg received 200 mg, while those with a body weight of 15-30 kg received 300 mg. The primary endpoint was the proportion of patients with clear or almost clear skin at 16 weeks, defined as scores of 0 or 1 on the Investigator’s Global Assessment.
After 16 weeks, 28% of dupilumab patients met the primary endpoint versus 4% of those on placebo (P < .0001). In addition, 53% of dupilumab patients met the key secondary endpoint of a 75% improvement from baseline in Eczema Area and Severity Index, compared with 11% of patients on placebo (P < .0001). Treatment with dupilumab also resulted in significantly greater improvements in pruritus and skin pain, and sleep quality, as well as improved quality of life for patients and their caregivers, the authors reported.
Overall, adverse event rates were slightly lower in the dupilumab-treated patients, compared with patients on placebo (64% vs. 74%); there were no adverse events related to dupilumab that were serious or resulted in treatment discontinuation. Treatment-emergent adverse effects that were reported in 3% or more of patients and affected more of those on dupilumab than those on placebo included molluscum contagiosum (5% vs. 3%), viral gastroenteritis (4% vs. 0), rhinorrhea (5% vs. 1%), dental caries (5% vs. 0), and conjunctivitis (4% vs 0).
The rate of skin infections among the children on dupilumab was 12% vs. 24% among those on placebo.
Severe and treatment-related adverse events also were similar in both subgroups of body weight.
The findings were limited by the small number of patients younger than 2 years and the lack of study sites outside of North America and Europe, the researchers noted. However, the results were strengthened by the randomized, double-blind design and use of background topical therapy to provide a real-world safety and efficacy assessment in a very young population.
Overcoming injection issues
The safety profile for dupilumab, which is of the highest importance, “did not surprise me at all,” Dr. Paller said in an interview. “My only surprise is that the placebo injections actually led to more injection site reactions than [with] dupilumab, but numbers were quite low in both groups.” (Rates were 2% among those on dupilumab and 3% among those on placebo.)
The major barrier to the use of dupilumab in clinical practice is the requirement for injection, which, she explained, can be “unbearable for some young children, and thus becomes impossible for parents because of lack of cooperation and their intensified concern about giving the injection,” because of their child’s response.
“We like to administer the first dose in the office, allowing us to teach parents a few tricks related to proper technique,” including audio and visual distraction, tactile stimulation before and during the injection, use of topical anesthetic if helpful, “and making sure that the medication is at room temperature before administration,” she said. Cost is another potential barrier; however, even public insurance has been covering the medication, often after optimized use of topical medications has been unsuccessful.
Future research questions
As for additional research, the current study had a relatively small number of patients younger than 2 years, and more data are needed for this age group, said Dr. Paller. “We also need better understanding of the safety of dupilumab administration when live vaccines are administered. Finally, we certainly want to know what additional effects dupilumab may have beyond just the efficacy for treating eczema.”
In particular, these questions include whether dupilumab modifies the long-term course of the disease, possibly reducing the risk of persistence of disease with advancing age, or even cures the disease if started at a young age, she said. In addition, research has yet to show whether dupilumab might reduce the risk of other atopic disorders, such as asthma, food allergy, and allergic rhinitis.
“Ongoing studies and real-life experiences in the next several years will help us to answer these questions,” Dr. Paller said.
Data support safety, efficacy, quality of life
AD is associated with immense quality of life impairment, Raj Chovatiya, MD, of Northwestern University, Chicago, said in an interview. Most AD is initially diagnosed in early childhood, but previous treatment options for those with moderate to severe disease have been limited by safety concerns, which adds to the burden on infants and young children, and their parents and caregivers, said Dr. Chovatiya, who was not involved in the study.
“This phase 3 study showed that dupilumab, a fully human monoclonal antibody that selectively inhibits IL-4 and IL-13 mediated type 2 inflammatory signaling, provided both meaningful and statistically significant improvement in AD severity, extent of disease, and itch in patients,” he said. Dupilumab also improved children’s sleep quality and the overall quality of life in both patients and caregivers.
“These findings were quite similar to those described in older children and adults, where dupilumab is already approved for the treatment of moderate-severe AD and has demonstrated real-world safety and efficacy,” said Dr. Chovatiya. However, “the current study was limited to only a short-term analysis of 16 weeks, an ongoing open-label study should further address long-term treatment responses.”
The study was supported by Sanofi and Regeneron Pharmaceuticals. In addition to being an investigator for Regeneron, and several other pharmaceutical companies, Dr. Paller has been a consultant with honorarium for Regeneron, Sanofi, and multiple other companies. Dr. Chovatiya disclosed serving as a consultant and speaker for Regeneron and Sanofi, but was not involved in the current study.
at 31 treatment centers in North America and Europe.
Children younger than 6 years with moderate to severe AD have few options if their symptoms are uncontrolled with topical therapies, and persistent itchiness has a negative impact on quality of life for patients and families, Amy S. Paller, MD, professor and chair of dermatology, and professor of pediatrics at Northwestern University, Chicago, and colleagues wrote in the study, published in the Lancet.
The study was the basis of the Food and Drug Administration expanded approval of dupilumab in June 2022, to include children aged 6 months to 5 years with moderate to severe AD, whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable. Regulatory submission for this age group is under review by the European Medicines Agency, and by regulatory authorities in other countries, according to the manufacturers.
Dupilumab (Dupixent), which inhibits the signaling of the interleukin-4 and IL-13 pathways, was first approved in 2017 for treating adults with moderate to severe AD.
“There has not been a biologic approved before at such a young age, and for such a common disease,” Dr. Paller said in an interview. “This is the drug that has revolutionized care of the most common inflammatory skin disease in children, and this is the pivotal study that brought it to market for the youngest children who suffer from the severe forms.”
The study also sets a precedent for a lower threshold for starting systemic medication in young children for treating moderate to severe disease given the absence of severe side effects and no need for lab monitoring, Dr. Paller noted. However, dupilumab will also be closely watched “for both impact on the developing immune system and the possibility that it will alter the long-term course of the eczema and the development of allergic comorbidities, such as lowering the risk of developing asthma, GI, allergy, and possibly other conditions.”
In the study, the researchers randomized 83 children aged 6 months up to 6 years to treatment with dupilumab, administered subcutaneously, and 79 to placebo every 4 weeks for 16 weeks; both groups also received topical corticosteroids. Dosage of dupilumab was based on body weight; those with a body weight of 5-15 kg received 200 mg, while those with a body weight of 15-30 kg received 300 mg. The primary endpoint was the proportion of patients with clear or almost clear skin at 16 weeks, defined as scores of 0 or 1 on the Investigator’s Global Assessment.
After 16 weeks, 28% of dupilumab patients met the primary endpoint versus 4% of those on placebo (P < .0001). In addition, 53% of dupilumab patients met the key secondary endpoint of a 75% improvement from baseline in Eczema Area and Severity Index, compared with 11% of patients on placebo (P < .0001). Treatment with dupilumab also resulted in significantly greater improvements in pruritus and skin pain, and sleep quality, as well as improved quality of life for patients and their caregivers, the authors reported.
Overall, adverse event rates were slightly lower in the dupilumab-treated patients, compared with patients on placebo (64% vs. 74%); there were no adverse events related to dupilumab that were serious or resulted in treatment discontinuation. Treatment-emergent adverse effects that were reported in 3% or more of patients and affected more of those on dupilumab than those on placebo included molluscum contagiosum (5% vs. 3%), viral gastroenteritis (4% vs. 0), rhinorrhea (5% vs. 1%), dental caries (5% vs. 0), and conjunctivitis (4% vs 0).
The rate of skin infections among the children on dupilumab was 12% vs. 24% among those on placebo.
Severe and treatment-related adverse events also were similar in both subgroups of body weight.
The findings were limited by the small number of patients younger than 2 years and the lack of study sites outside of North America and Europe, the researchers noted. However, the results were strengthened by the randomized, double-blind design and use of background topical therapy to provide a real-world safety and efficacy assessment in a very young population.
Overcoming injection issues
The safety profile for dupilumab, which is of the highest importance, “did not surprise me at all,” Dr. Paller said in an interview. “My only surprise is that the placebo injections actually led to more injection site reactions than [with] dupilumab, but numbers were quite low in both groups.” (Rates were 2% among those on dupilumab and 3% among those on placebo.)
The major barrier to the use of dupilumab in clinical practice is the requirement for injection, which, she explained, can be “unbearable for some young children, and thus becomes impossible for parents because of lack of cooperation and their intensified concern about giving the injection,” because of their child’s response.
“We like to administer the first dose in the office, allowing us to teach parents a few tricks related to proper technique,” including audio and visual distraction, tactile stimulation before and during the injection, use of topical anesthetic if helpful, “and making sure that the medication is at room temperature before administration,” she said. Cost is another potential barrier; however, even public insurance has been covering the medication, often after optimized use of topical medications has been unsuccessful.
Future research questions
As for additional research, the current study had a relatively small number of patients younger than 2 years, and more data are needed for this age group, said Dr. Paller. “We also need better understanding of the safety of dupilumab administration when live vaccines are administered. Finally, we certainly want to know what additional effects dupilumab may have beyond just the efficacy for treating eczema.”
In particular, these questions include whether dupilumab modifies the long-term course of the disease, possibly reducing the risk of persistence of disease with advancing age, or even cures the disease if started at a young age, she said. In addition, research has yet to show whether dupilumab might reduce the risk of other atopic disorders, such as asthma, food allergy, and allergic rhinitis.
“Ongoing studies and real-life experiences in the next several years will help us to answer these questions,” Dr. Paller said.
Data support safety, efficacy, quality of life
AD is associated with immense quality of life impairment, Raj Chovatiya, MD, of Northwestern University, Chicago, said in an interview. Most AD is initially diagnosed in early childhood, but previous treatment options for those with moderate to severe disease have been limited by safety concerns, which adds to the burden on infants and young children, and their parents and caregivers, said Dr. Chovatiya, who was not involved in the study.
“This phase 3 study showed that dupilumab, a fully human monoclonal antibody that selectively inhibits IL-4 and IL-13 mediated type 2 inflammatory signaling, provided both meaningful and statistically significant improvement in AD severity, extent of disease, and itch in patients,” he said. Dupilumab also improved children’s sleep quality and the overall quality of life in both patients and caregivers.
“These findings were quite similar to those described in older children and adults, where dupilumab is already approved for the treatment of moderate-severe AD and has demonstrated real-world safety and efficacy,” said Dr. Chovatiya. However, “the current study was limited to only a short-term analysis of 16 weeks, an ongoing open-label study should further address long-term treatment responses.”
The study was supported by Sanofi and Regeneron Pharmaceuticals. In addition to being an investigator for Regeneron, and several other pharmaceutical companies, Dr. Paller has been a consultant with honorarium for Regeneron, Sanofi, and multiple other companies. Dr. Chovatiya disclosed serving as a consultant and speaker for Regeneron and Sanofi, but was not involved in the current study.
at 31 treatment centers in North America and Europe.
Children younger than 6 years with moderate to severe AD have few options if their symptoms are uncontrolled with topical therapies, and persistent itchiness has a negative impact on quality of life for patients and families, Amy S. Paller, MD, professor and chair of dermatology, and professor of pediatrics at Northwestern University, Chicago, and colleagues wrote in the study, published in the Lancet.
The study was the basis of the Food and Drug Administration expanded approval of dupilumab in June 2022, to include children aged 6 months to 5 years with moderate to severe AD, whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable. Regulatory submission for this age group is under review by the European Medicines Agency, and by regulatory authorities in other countries, according to the manufacturers.
Dupilumab (Dupixent), which inhibits the signaling of the interleukin-4 and IL-13 pathways, was first approved in 2017 for treating adults with moderate to severe AD.
“There has not been a biologic approved before at such a young age, and for such a common disease,” Dr. Paller said in an interview. “This is the drug that has revolutionized care of the most common inflammatory skin disease in children, and this is the pivotal study that brought it to market for the youngest children who suffer from the severe forms.”
The study also sets a precedent for a lower threshold for starting systemic medication in young children for treating moderate to severe disease given the absence of severe side effects and no need for lab monitoring, Dr. Paller noted. However, dupilumab will also be closely watched “for both impact on the developing immune system and the possibility that it will alter the long-term course of the eczema and the development of allergic comorbidities, such as lowering the risk of developing asthma, GI, allergy, and possibly other conditions.”
In the study, the researchers randomized 83 children aged 6 months up to 6 years to treatment with dupilumab, administered subcutaneously, and 79 to placebo every 4 weeks for 16 weeks; both groups also received topical corticosteroids. Dosage of dupilumab was based on body weight; those with a body weight of 5-15 kg received 200 mg, while those with a body weight of 15-30 kg received 300 mg. The primary endpoint was the proportion of patients with clear or almost clear skin at 16 weeks, defined as scores of 0 or 1 on the Investigator’s Global Assessment.
After 16 weeks, 28% of dupilumab patients met the primary endpoint versus 4% of those on placebo (P < .0001). In addition, 53% of dupilumab patients met the key secondary endpoint of a 75% improvement from baseline in Eczema Area and Severity Index, compared with 11% of patients on placebo (P < .0001). Treatment with dupilumab also resulted in significantly greater improvements in pruritus and skin pain, and sleep quality, as well as improved quality of life for patients and their caregivers, the authors reported.
Overall, adverse event rates were slightly lower in the dupilumab-treated patients, compared with patients on placebo (64% vs. 74%); there were no adverse events related to dupilumab that were serious or resulted in treatment discontinuation. Treatment-emergent adverse effects that were reported in 3% or more of patients and affected more of those on dupilumab than those on placebo included molluscum contagiosum (5% vs. 3%), viral gastroenteritis (4% vs. 0), rhinorrhea (5% vs. 1%), dental caries (5% vs. 0), and conjunctivitis (4% vs 0).
The rate of skin infections among the children on dupilumab was 12% vs. 24% among those on placebo.
Severe and treatment-related adverse events also were similar in both subgroups of body weight.
The findings were limited by the small number of patients younger than 2 years and the lack of study sites outside of North America and Europe, the researchers noted. However, the results were strengthened by the randomized, double-blind design and use of background topical therapy to provide a real-world safety and efficacy assessment in a very young population.
Overcoming injection issues
The safety profile for dupilumab, which is of the highest importance, “did not surprise me at all,” Dr. Paller said in an interview. “My only surprise is that the placebo injections actually led to more injection site reactions than [with] dupilumab, but numbers were quite low in both groups.” (Rates were 2% among those on dupilumab and 3% among those on placebo.)
The major barrier to the use of dupilumab in clinical practice is the requirement for injection, which, she explained, can be “unbearable for some young children, and thus becomes impossible for parents because of lack of cooperation and their intensified concern about giving the injection,” because of their child’s response.
“We like to administer the first dose in the office, allowing us to teach parents a few tricks related to proper technique,” including audio and visual distraction, tactile stimulation before and during the injection, use of topical anesthetic if helpful, “and making sure that the medication is at room temperature before administration,” she said. Cost is another potential barrier; however, even public insurance has been covering the medication, often after optimized use of topical medications has been unsuccessful.
Future research questions
As for additional research, the current study had a relatively small number of patients younger than 2 years, and more data are needed for this age group, said Dr. Paller. “We also need better understanding of the safety of dupilumab administration when live vaccines are administered. Finally, we certainly want to know what additional effects dupilumab may have beyond just the efficacy for treating eczema.”
In particular, these questions include whether dupilumab modifies the long-term course of the disease, possibly reducing the risk of persistence of disease with advancing age, or even cures the disease if started at a young age, she said. In addition, research has yet to show whether dupilumab might reduce the risk of other atopic disorders, such as asthma, food allergy, and allergic rhinitis.
“Ongoing studies and real-life experiences in the next several years will help us to answer these questions,” Dr. Paller said.
Data support safety, efficacy, quality of life
AD is associated with immense quality of life impairment, Raj Chovatiya, MD, of Northwestern University, Chicago, said in an interview. Most AD is initially diagnosed in early childhood, but previous treatment options for those with moderate to severe disease have been limited by safety concerns, which adds to the burden on infants and young children, and their parents and caregivers, said Dr. Chovatiya, who was not involved in the study.
“This phase 3 study showed that dupilumab, a fully human monoclonal antibody that selectively inhibits IL-4 and IL-13 mediated type 2 inflammatory signaling, provided both meaningful and statistically significant improvement in AD severity, extent of disease, and itch in patients,” he said. Dupilumab also improved children’s sleep quality and the overall quality of life in both patients and caregivers.
“These findings were quite similar to those described in older children and adults, where dupilumab is already approved for the treatment of moderate-severe AD and has demonstrated real-world safety and efficacy,” said Dr. Chovatiya. However, “the current study was limited to only a short-term analysis of 16 weeks, an ongoing open-label study should further address long-term treatment responses.”
The study was supported by Sanofi and Regeneron Pharmaceuticals. In addition to being an investigator for Regeneron, and several other pharmaceutical companies, Dr. Paller has been a consultant with honorarium for Regeneron, Sanofi, and multiple other companies. Dr. Chovatiya disclosed serving as a consultant and speaker for Regeneron and Sanofi, but was not involved in the current study.
FROM THE LANCET
Evusheld PrEP may protect immunocompromised patients from severe COVID-19
Tixagevimab copackaged with cilgavimab (Evusheld) is a safe and effective preexposure prophylaxis (PrEP) in patients undergoing B-cell-depleting therapies who have poor immune response to COVID-19 vaccination and are at high risk for serious COVID-19 illness, a small, single-site study suggests.
Evusheld, the only COVID-19 PrEP option available, has Emergency Use Authorization (EUA) from the Food and Drug Administration for treatment of immunocompromised patients who may not respond sufficiently to COVID-19 vaccination and patients who’ve had a severe adverse reaction to COVID-19 vaccination.
“We report the largest real-world experience of Evusheld in this population, and our findings are encouraging,” lead study author Cassandra Calabrese, DO, rheumatologist and infectious disease specialist at Cleveland Clinic, said in an interview.
“Of 412 patients who received Evusheld, 12 [2.9%] developed breakthrough COVID-19, with 11 having mild courses and 1 who required hospitalization but recovered,” she added.
“Our data suggest that Evusheld PrEP, in combination with aggressive outpatient treatment of COVID-19, is likely effective in lowering risk of severe COVID in this vulnerable group.
“Practitioners who care for patients with immune-mediated inflammatory diseases should triage high-risk patients for Evusheld as well as rapid diagnosis and aggressive outpatient therapy if infected,” Dr. Calabrese advised.
For the study, Dr. Calabrese and colleagues at Cleveland Clinic searched the health care system pharmacy records for patients with immune‐mediated inflammatory diseases (IMIDs) or inborn errors of humoral immunity (IEI) who met the criteria to receive Evusheld. The researchers included patients on B-cell-depleting therapies or with humoral IEI who had received at least one dose of Evusheld and were later diagnosed with COVID-19, and they excluded those treated with B-cell-depleting therapies for cancer.
EVUSHELD was well tolerated
After extracting data on COVID-19 infection, vaccination status, and outcomes, they found that, between Jan. 18 and May 28, 2022, 412 patients with IMIDs or humoral IEI received Evusheld. No deaths occurred among these patients and, overall, they tolerated the medication well.
All 12 patients who experienced breakthrough COVID-19 infection were treated with B-cell-depleting therapies. Among the 12 patients:
- Six patients developed infection 13-84 (median 19) days after receiving 150 mg/150 mg tixagevimab/cilgavimab.
- Six patients developed infection 19-72 (median of 38.5) days after either a single dose of 300 mg/300 mg or a second dose of 150 mg/150 mg.
- Eleven patients had mild illness and recovered at home; one patient was hospitalized and treated with high-flow oxygen. All cases had been vaccinated against COVID-19 (five received two vaccinations, six received three, and one received four).
- One possible serious adverse event involved a patient with COVID-19 and immune-mediated thrombocytopenia (ITP) who was hospitalized soon after receiving Evusheld with ITP flare that resolved with intravenous immunoglobulin.
Dr. Calabrese acknowledged limitations to the study, including few patients, lack of a comparator group, and the study period falling during the Omicron wave.
“Also, nine of the breakthrough cases received additional COVID-19 therapy (oral antiviral or monoclonal antibody), which falls within standard of care for this high-risk group but prevents ascribing effectiveness to individual components of the regimen,” she added.
“Evusheld is authorized for PrEP against COVID-19 in patients at high risk for severe COVID due to suboptimal vaccine responses. This includes patients receiving B-cell-depleting drugs like rituximab, and patients with inborn errors of humoral immunity,” Dr. Calabrese explained.
“It is well known that this group of patients is at very high risk for severe COVID and death, even when fully vaccinated, and it has become clear that more strategies are needed to protect this vulnerable group, including use of Evusheld as well as aggressive treatment if infected,” she added.
Evusheld not always easy to obtain
Although the medication has been available in the United States since January 2022, Dr. Calabrese said, patients may not receive it because of barriers including lack of both awareness and access.
Davey Smith, MD, professor of medicine and head of infectious diseases and global public health at the University of California San Diego, in La Jolla, said in an interview that he was not surprised by the results, but added that the study was conducted in too few patients to draw any strong conclusions or affect patient care.
“This small study that showed that breakthrough infections occurred but were generally mild, provides a small glimpse of real-world use of tixagevimab/cilgavimab as PrEP for immunocompromised persons,” said Dr. Smith, who was not involved in the study.
“In the setting of Omicron and vaccination, I would expect the same outcomes reported even without the treatment,” he added.
Dr. Smith recommends larger related randomized, controlled trials to provide clinicians with sufficient data to guide them in their patient care.
Graham Snyder, MD, associate professor in the division of infectious diseases at the University of Pittsburgh and medical director of infection prevention and hospital epidemiology at the University of Pittsburgh Medical Center, noted that the study “adds to a quickly growing literature on the real-world benefits of tixagevimab/cilgavimab to protect vulnerable individuals with weakened immune systems from the complications of COVID-19.
“This study provides a modest addition to our understanding of the role and benefit of Evusheld,” Dr. Snyder said in an interview. “By characterizing only patients who have received Evusheld without an untreated comparison group, we can’t draw any inference about the extent of benefit the agent provided to these patients.
“Substantial data already show that this agent is effective in preventing complications of COVID-19 infection in immunocompromised individuals,” added Dr. Snyder, who was not involved in the study.
“ ‘Immunocompromised’ represents a very diverse set of clinical conditions,” he said. “The research agenda should therefore focus on a more refined description of the effect in specific populations and a continued understanding of the effect of Evusheld in the context of updated vaccination strategies and changing virus ecology.”
Dr. Calabrese and her colleagues wrote that larger, controlled trials are underway.
FDA: Evusheld may not neutralize certain SARS-CoV-2 variants
“The biggest unanswered question is how Evusheld will hold up against new variants,” Dr. Calabrese said.
In an Oct. 3, 2022, update, the Food and Drug Administration released a statement about the risk of developing COVID-19 from SARS-CoV-2 variants that are not neutralized by Evusheld. The statement mentions an updated fact sheet that describes reduced protection from Evusheld against the Omicron subvariant BA.4.6, which accounted for nearly 13% of all new COVID-19 cases in the United States in the week ending Oct. 1.
There was no outside funding for the study. Dr. Smith reported no relevant financial conflicts of interest. Dr. Snyder said he is an unpaid adviser to an AstraZeneca observational study that’s assessing the real-world effectiveness of Evusheld.
Tixagevimab copackaged with cilgavimab (Evusheld) is a safe and effective preexposure prophylaxis (PrEP) in patients undergoing B-cell-depleting therapies who have poor immune response to COVID-19 vaccination and are at high risk for serious COVID-19 illness, a small, single-site study suggests.
Evusheld, the only COVID-19 PrEP option available, has Emergency Use Authorization (EUA) from the Food and Drug Administration for treatment of immunocompromised patients who may not respond sufficiently to COVID-19 vaccination and patients who’ve had a severe adverse reaction to COVID-19 vaccination.
“We report the largest real-world experience of Evusheld in this population, and our findings are encouraging,” lead study author Cassandra Calabrese, DO, rheumatologist and infectious disease specialist at Cleveland Clinic, said in an interview.
“Of 412 patients who received Evusheld, 12 [2.9%] developed breakthrough COVID-19, with 11 having mild courses and 1 who required hospitalization but recovered,” she added.
“Our data suggest that Evusheld PrEP, in combination with aggressive outpatient treatment of COVID-19, is likely effective in lowering risk of severe COVID in this vulnerable group.
“Practitioners who care for patients with immune-mediated inflammatory diseases should triage high-risk patients for Evusheld as well as rapid diagnosis and aggressive outpatient therapy if infected,” Dr. Calabrese advised.
For the study, Dr. Calabrese and colleagues at Cleveland Clinic searched the health care system pharmacy records for patients with immune‐mediated inflammatory diseases (IMIDs) or inborn errors of humoral immunity (IEI) who met the criteria to receive Evusheld. The researchers included patients on B-cell-depleting therapies or with humoral IEI who had received at least one dose of Evusheld and were later diagnosed with COVID-19, and they excluded those treated with B-cell-depleting therapies for cancer.
EVUSHELD was well tolerated
After extracting data on COVID-19 infection, vaccination status, and outcomes, they found that, between Jan. 18 and May 28, 2022, 412 patients with IMIDs or humoral IEI received Evusheld. No deaths occurred among these patients and, overall, they tolerated the medication well.
All 12 patients who experienced breakthrough COVID-19 infection were treated with B-cell-depleting therapies. Among the 12 patients:
- Six patients developed infection 13-84 (median 19) days after receiving 150 mg/150 mg tixagevimab/cilgavimab.
- Six patients developed infection 19-72 (median of 38.5) days after either a single dose of 300 mg/300 mg or a second dose of 150 mg/150 mg.
- Eleven patients had mild illness and recovered at home; one patient was hospitalized and treated with high-flow oxygen. All cases had been vaccinated against COVID-19 (five received two vaccinations, six received three, and one received four).
- One possible serious adverse event involved a patient with COVID-19 and immune-mediated thrombocytopenia (ITP) who was hospitalized soon after receiving Evusheld with ITP flare that resolved with intravenous immunoglobulin.
Dr. Calabrese acknowledged limitations to the study, including few patients, lack of a comparator group, and the study period falling during the Omicron wave.
“Also, nine of the breakthrough cases received additional COVID-19 therapy (oral antiviral or monoclonal antibody), which falls within standard of care for this high-risk group but prevents ascribing effectiveness to individual components of the regimen,” she added.
“Evusheld is authorized for PrEP against COVID-19 in patients at high risk for severe COVID due to suboptimal vaccine responses. This includes patients receiving B-cell-depleting drugs like rituximab, and patients with inborn errors of humoral immunity,” Dr. Calabrese explained.
“It is well known that this group of patients is at very high risk for severe COVID and death, even when fully vaccinated, and it has become clear that more strategies are needed to protect this vulnerable group, including use of Evusheld as well as aggressive treatment if infected,” she added.
Evusheld not always easy to obtain
Although the medication has been available in the United States since January 2022, Dr. Calabrese said, patients may not receive it because of barriers including lack of both awareness and access.
Davey Smith, MD, professor of medicine and head of infectious diseases and global public health at the University of California San Diego, in La Jolla, said in an interview that he was not surprised by the results, but added that the study was conducted in too few patients to draw any strong conclusions or affect patient care.
“This small study that showed that breakthrough infections occurred but were generally mild, provides a small glimpse of real-world use of tixagevimab/cilgavimab as PrEP for immunocompromised persons,” said Dr. Smith, who was not involved in the study.
“In the setting of Omicron and vaccination, I would expect the same outcomes reported even without the treatment,” he added.
Dr. Smith recommends larger related randomized, controlled trials to provide clinicians with sufficient data to guide them in their patient care.
Graham Snyder, MD, associate professor in the division of infectious diseases at the University of Pittsburgh and medical director of infection prevention and hospital epidemiology at the University of Pittsburgh Medical Center, noted that the study “adds to a quickly growing literature on the real-world benefits of tixagevimab/cilgavimab to protect vulnerable individuals with weakened immune systems from the complications of COVID-19.
“This study provides a modest addition to our understanding of the role and benefit of Evusheld,” Dr. Snyder said in an interview. “By characterizing only patients who have received Evusheld without an untreated comparison group, we can’t draw any inference about the extent of benefit the agent provided to these patients.
“Substantial data already show that this agent is effective in preventing complications of COVID-19 infection in immunocompromised individuals,” added Dr. Snyder, who was not involved in the study.
“ ‘Immunocompromised’ represents a very diverse set of clinical conditions,” he said. “The research agenda should therefore focus on a more refined description of the effect in specific populations and a continued understanding of the effect of Evusheld in the context of updated vaccination strategies and changing virus ecology.”
Dr. Calabrese and her colleagues wrote that larger, controlled trials are underway.
FDA: Evusheld may not neutralize certain SARS-CoV-2 variants
“The biggest unanswered question is how Evusheld will hold up against new variants,” Dr. Calabrese said.
In an Oct. 3, 2022, update, the Food and Drug Administration released a statement about the risk of developing COVID-19 from SARS-CoV-2 variants that are not neutralized by Evusheld. The statement mentions an updated fact sheet that describes reduced protection from Evusheld against the Omicron subvariant BA.4.6, which accounted for nearly 13% of all new COVID-19 cases in the United States in the week ending Oct. 1.
There was no outside funding for the study. Dr. Smith reported no relevant financial conflicts of interest. Dr. Snyder said he is an unpaid adviser to an AstraZeneca observational study that’s assessing the real-world effectiveness of Evusheld.
Tixagevimab copackaged with cilgavimab (Evusheld) is a safe and effective preexposure prophylaxis (PrEP) in patients undergoing B-cell-depleting therapies who have poor immune response to COVID-19 vaccination and are at high risk for serious COVID-19 illness, a small, single-site study suggests.
Evusheld, the only COVID-19 PrEP option available, has Emergency Use Authorization (EUA) from the Food and Drug Administration for treatment of immunocompromised patients who may not respond sufficiently to COVID-19 vaccination and patients who’ve had a severe adverse reaction to COVID-19 vaccination.
“We report the largest real-world experience of Evusheld in this population, and our findings are encouraging,” lead study author Cassandra Calabrese, DO, rheumatologist and infectious disease specialist at Cleveland Clinic, said in an interview.
“Of 412 patients who received Evusheld, 12 [2.9%] developed breakthrough COVID-19, with 11 having mild courses and 1 who required hospitalization but recovered,” she added.
“Our data suggest that Evusheld PrEP, in combination with aggressive outpatient treatment of COVID-19, is likely effective in lowering risk of severe COVID in this vulnerable group.
“Practitioners who care for patients with immune-mediated inflammatory diseases should triage high-risk patients for Evusheld as well as rapid diagnosis and aggressive outpatient therapy if infected,” Dr. Calabrese advised.
For the study, Dr. Calabrese and colleagues at Cleveland Clinic searched the health care system pharmacy records for patients with immune‐mediated inflammatory diseases (IMIDs) or inborn errors of humoral immunity (IEI) who met the criteria to receive Evusheld. The researchers included patients on B-cell-depleting therapies or with humoral IEI who had received at least one dose of Evusheld and were later diagnosed with COVID-19, and they excluded those treated with B-cell-depleting therapies for cancer.
EVUSHELD was well tolerated
After extracting data on COVID-19 infection, vaccination status, and outcomes, they found that, between Jan. 18 and May 28, 2022, 412 patients with IMIDs or humoral IEI received Evusheld. No deaths occurred among these patients and, overall, they tolerated the medication well.
All 12 patients who experienced breakthrough COVID-19 infection were treated with B-cell-depleting therapies. Among the 12 patients:
- Six patients developed infection 13-84 (median 19) days after receiving 150 mg/150 mg tixagevimab/cilgavimab.
- Six patients developed infection 19-72 (median of 38.5) days after either a single dose of 300 mg/300 mg or a second dose of 150 mg/150 mg.
- Eleven patients had mild illness and recovered at home; one patient was hospitalized and treated with high-flow oxygen. All cases had been vaccinated against COVID-19 (five received two vaccinations, six received three, and one received four).
- One possible serious adverse event involved a patient with COVID-19 and immune-mediated thrombocytopenia (ITP) who was hospitalized soon after receiving Evusheld with ITP flare that resolved with intravenous immunoglobulin.
Dr. Calabrese acknowledged limitations to the study, including few patients, lack of a comparator group, and the study period falling during the Omicron wave.
“Also, nine of the breakthrough cases received additional COVID-19 therapy (oral antiviral or monoclonal antibody), which falls within standard of care for this high-risk group but prevents ascribing effectiveness to individual components of the regimen,” she added.
“Evusheld is authorized for PrEP against COVID-19 in patients at high risk for severe COVID due to suboptimal vaccine responses. This includes patients receiving B-cell-depleting drugs like rituximab, and patients with inborn errors of humoral immunity,” Dr. Calabrese explained.
“It is well known that this group of patients is at very high risk for severe COVID and death, even when fully vaccinated, and it has become clear that more strategies are needed to protect this vulnerable group, including use of Evusheld as well as aggressive treatment if infected,” she added.
Evusheld not always easy to obtain
Although the medication has been available in the United States since January 2022, Dr. Calabrese said, patients may not receive it because of barriers including lack of both awareness and access.
Davey Smith, MD, professor of medicine and head of infectious diseases and global public health at the University of California San Diego, in La Jolla, said in an interview that he was not surprised by the results, but added that the study was conducted in too few patients to draw any strong conclusions or affect patient care.
“This small study that showed that breakthrough infections occurred but were generally mild, provides a small glimpse of real-world use of tixagevimab/cilgavimab as PrEP for immunocompromised persons,” said Dr. Smith, who was not involved in the study.
“In the setting of Omicron and vaccination, I would expect the same outcomes reported even without the treatment,” he added.
Dr. Smith recommends larger related randomized, controlled trials to provide clinicians with sufficient data to guide them in their patient care.
Graham Snyder, MD, associate professor in the division of infectious diseases at the University of Pittsburgh and medical director of infection prevention and hospital epidemiology at the University of Pittsburgh Medical Center, noted that the study “adds to a quickly growing literature on the real-world benefits of tixagevimab/cilgavimab to protect vulnerable individuals with weakened immune systems from the complications of COVID-19.
“This study provides a modest addition to our understanding of the role and benefit of Evusheld,” Dr. Snyder said in an interview. “By characterizing only patients who have received Evusheld without an untreated comparison group, we can’t draw any inference about the extent of benefit the agent provided to these patients.
“Substantial data already show that this agent is effective in preventing complications of COVID-19 infection in immunocompromised individuals,” added Dr. Snyder, who was not involved in the study.
“ ‘Immunocompromised’ represents a very diverse set of clinical conditions,” he said. “The research agenda should therefore focus on a more refined description of the effect in specific populations and a continued understanding of the effect of Evusheld in the context of updated vaccination strategies and changing virus ecology.”
Dr. Calabrese and her colleagues wrote that larger, controlled trials are underway.
FDA: Evusheld may not neutralize certain SARS-CoV-2 variants
“The biggest unanswered question is how Evusheld will hold up against new variants,” Dr. Calabrese said.
In an Oct. 3, 2022, update, the Food and Drug Administration released a statement about the risk of developing COVID-19 from SARS-CoV-2 variants that are not neutralized by Evusheld. The statement mentions an updated fact sheet that describes reduced protection from Evusheld against the Omicron subvariant BA.4.6, which accounted for nearly 13% of all new COVID-19 cases in the United States in the week ending Oct. 1.
There was no outside funding for the study. Dr. Smith reported no relevant financial conflicts of interest. Dr. Snyder said he is an unpaid adviser to an AstraZeneca observational study that’s assessing the real-world effectiveness of Evusheld.
FROM RMD OPEN
Noninvasive combination procedure effective for upper arm fat reduction, muscle toning
DENVER – , according to results from a study that analyzed results with MRI and other measures at two dermatology practices.
Simultaneous use of HIFEM and RF has been shown to be safe and effective “for fat reduction and muscle toning in various body parts,” lead study author Carolyn Jacob, MD, founder and director of Chicago Cosmetic Surgery and Dermatology, wrote in an abstract presented at the annual meeting of the American Society for Dermatologic Surgery. This study investigated the effect of the HIFEM and RF procedure on muscle toning and adipose tissue in the upper arms.
In what Dr. Jacob described as the first study of its kind because magnetic resonance imaging (MRI) was used to evaluate results, she and her coauthors enrolled 34 patients aged 23-72 years at two centers who had a BMI in the range of 18.5-33.9 kg/m2. The patients underwent four 30-minute bilateral procedures over the upper arms spaced 1 week apart with the Emsculpt NEO (BTL Aesthetics), which simultaneously delivers HIFEM and RF therapy.
NEO small sized applicators were used, which at the time of the study were under investigation but have since been cleared for use with the device. According to the manufacturer’s website, Emsculpt NEO is indicated for noninvasive lipolysis of the abdomen and thighs and reduction in the circumference of the abdomen and thighs in patients with skin types I-VI; and for noninvasive lipolysis of the upper arms “limited to skin types II and III and BMI 30 or under.”
The investigators measured changes in fat and triceps muscle tissue via MRI at baseline, 1-month, and 3-month follow-up visits. They also obtained digital photographs, administered patient questionnaires regarding comfort and satisfaction, and monitored safety of the treatments.
Of the 28 patients who completed their 1-month follow-up visit, analysis of MRI images showed a 22.3% average decrease in fat tissue from baseline MRIs (a decrease of 4.0 ± 1.2 mm; P < .01) and a 21.5% average increase in muscle mass (an increase of 8.2 ± 2.3 mm; P < .001). For the 25 patients who completed their 3-month follow-up visit, analysis of MRI images showed a 25.5% average decrease in fat tissue (a decrease of 4.9 ± 1.5 mm; P < .01) and a 23.9% average increase in muscle mass (an increase of 8.9 ± 2.0 mm; P < .001).
The analysis of questionnaires revealed high patient satisfaction with the results (87.1%), high comfort during the treatment (91.2%), and a low Visual Analogue Scale (VAS) score (1.6 ± 2.0) used to evaluate pain.
“This study shows that HIFEM and RF consistently increases muscle and decreases fat,” Dr. Jacob said in an interview. “It’s the only study on the triceps showing MRI evidence of fat loss with a nonsurgical body shaping device.”
She characterized the learning curve for the Emsculpt NEO as “small, as the previous Emsculpt small applicators have a similar fit.”
Pooja Sodha, MD, director of the center for laser and cosmetic dermatology at George Washington University, Washington, who was asked to comment on the study, said that the combination of radiofrequency energy and high-intensity focused electromagnetic technology triggers heat-induced damage of adipose tissue and muscle strengthening, respectively, to improve overall appearance and tone.
“Simultaneous delivery is the key here, and the real technological superhero, allowing us to take advantage of the synergistic effects of the muscle contractions and the tissue heating,” Dr. Sodha told this news organization. “Earlier this year, we saw published data on success with abdominal contouring with similar fat reduction and muscle enhancement as reported in this study, and these results persisted at 6 months,” with some declines noted at that time, she said.
“It is very encouraging and exciting to have similar effectiveness and safety for the arms, with such high satisfaction and comfort,” she added.
Dr. Jacob disclosed that she has conducted research studies for BTL Aesthetics since 2017 and is a member of the company’s advisory board. Dr. Sodha reported having no financial disclosures.
DENVER – , according to results from a study that analyzed results with MRI and other measures at two dermatology practices.
Simultaneous use of HIFEM and RF has been shown to be safe and effective “for fat reduction and muscle toning in various body parts,” lead study author Carolyn Jacob, MD, founder and director of Chicago Cosmetic Surgery and Dermatology, wrote in an abstract presented at the annual meeting of the American Society for Dermatologic Surgery. This study investigated the effect of the HIFEM and RF procedure on muscle toning and adipose tissue in the upper arms.
In what Dr. Jacob described as the first study of its kind because magnetic resonance imaging (MRI) was used to evaluate results, she and her coauthors enrolled 34 patients aged 23-72 years at two centers who had a BMI in the range of 18.5-33.9 kg/m2. The patients underwent four 30-minute bilateral procedures over the upper arms spaced 1 week apart with the Emsculpt NEO (BTL Aesthetics), which simultaneously delivers HIFEM and RF therapy.
NEO small sized applicators were used, which at the time of the study were under investigation but have since been cleared for use with the device. According to the manufacturer’s website, Emsculpt NEO is indicated for noninvasive lipolysis of the abdomen and thighs and reduction in the circumference of the abdomen and thighs in patients with skin types I-VI; and for noninvasive lipolysis of the upper arms “limited to skin types II and III and BMI 30 or under.”
The investigators measured changes in fat and triceps muscle tissue via MRI at baseline, 1-month, and 3-month follow-up visits. They also obtained digital photographs, administered patient questionnaires regarding comfort and satisfaction, and monitored safety of the treatments.
Of the 28 patients who completed their 1-month follow-up visit, analysis of MRI images showed a 22.3% average decrease in fat tissue from baseline MRIs (a decrease of 4.0 ± 1.2 mm; P < .01) and a 21.5% average increase in muscle mass (an increase of 8.2 ± 2.3 mm; P < .001). For the 25 patients who completed their 3-month follow-up visit, analysis of MRI images showed a 25.5% average decrease in fat tissue (a decrease of 4.9 ± 1.5 mm; P < .01) and a 23.9% average increase in muscle mass (an increase of 8.9 ± 2.0 mm; P < .001).
The analysis of questionnaires revealed high patient satisfaction with the results (87.1%), high comfort during the treatment (91.2%), and a low Visual Analogue Scale (VAS) score (1.6 ± 2.0) used to evaluate pain.
“This study shows that HIFEM and RF consistently increases muscle and decreases fat,” Dr. Jacob said in an interview. “It’s the only study on the triceps showing MRI evidence of fat loss with a nonsurgical body shaping device.”
She characterized the learning curve for the Emsculpt NEO as “small, as the previous Emsculpt small applicators have a similar fit.”
Pooja Sodha, MD, director of the center for laser and cosmetic dermatology at George Washington University, Washington, who was asked to comment on the study, said that the combination of radiofrequency energy and high-intensity focused electromagnetic technology triggers heat-induced damage of adipose tissue and muscle strengthening, respectively, to improve overall appearance and tone.
“Simultaneous delivery is the key here, and the real technological superhero, allowing us to take advantage of the synergistic effects of the muscle contractions and the tissue heating,” Dr. Sodha told this news organization. “Earlier this year, we saw published data on success with abdominal contouring with similar fat reduction and muscle enhancement as reported in this study, and these results persisted at 6 months,” with some declines noted at that time, she said.
“It is very encouraging and exciting to have similar effectiveness and safety for the arms, with such high satisfaction and comfort,” she added.
Dr. Jacob disclosed that she has conducted research studies for BTL Aesthetics since 2017 and is a member of the company’s advisory board. Dr. Sodha reported having no financial disclosures.
DENVER – , according to results from a study that analyzed results with MRI and other measures at two dermatology practices.
Simultaneous use of HIFEM and RF has been shown to be safe and effective “for fat reduction and muscle toning in various body parts,” lead study author Carolyn Jacob, MD, founder and director of Chicago Cosmetic Surgery and Dermatology, wrote in an abstract presented at the annual meeting of the American Society for Dermatologic Surgery. This study investigated the effect of the HIFEM and RF procedure on muscle toning and adipose tissue in the upper arms.
In what Dr. Jacob described as the first study of its kind because magnetic resonance imaging (MRI) was used to evaluate results, she and her coauthors enrolled 34 patients aged 23-72 years at two centers who had a BMI in the range of 18.5-33.9 kg/m2. The patients underwent four 30-minute bilateral procedures over the upper arms spaced 1 week apart with the Emsculpt NEO (BTL Aesthetics), which simultaneously delivers HIFEM and RF therapy.
NEO small sized applicators were used, which at the time of the study were under investigation but have since been cleared for use with the device. According to the manufacturer’s website, Emsculpt NEO is indicated for noninvasive lipolysis of the abdomen and thighs and reduction in the circumference of the abdomen and thighs in patients with skin types I-VI; and for noninvasive lipolysis of the upper arms “limited to skin types II and III and BMI 30 or under.”
The investigators measured changes in fat and triceps muscle tissue via MRI at baseline, 1-month, and 3-month follow-up visits. They also obtained digital photographs, administered patient questionnaires regarding comfort and satisfaction, and monitored safety of the treatments.
Of the 28 patients who completed their 1-month follow-up visit, analysis of MRI images showed a 22.3% average decrease in fat tissue from baseline MRIs (a decrease of 4.0 ± 1.2 mm; P < .01) and a 21.5% average increase in muscle mass (an increase of 8.2 ± 2.3 mm; P < .001). For the 25 patients who completed their 3-month follow-up visit, analysis of MRI images showed a 25.5% average decrease in fat tissue (a decrease of 4.9 ± 1.5 mm; P < .01) and a 23.9% average increase in muscle mass (an increase of 8.9 ± 2.0 mm; P < .001).
The analysis of questionnaires revealed high patient satisfaction with the results (87.1%), high comfort during the treatment (91.2%), and a low Visual Analogue Scale (VAS) score (1.6 ± 2.0) used to evaluate pain.
“This study shows that HIFEM and RF consistently increases muscle and decreases fat,” Dr. Jacob said in an interview. “It’s the only study on the triceps showing MRI evidence of fat loss with a nonsurgical body shaping device.”
She characterized the learning curve for the Emsculpt NEO as “small, as the previous Emsculpt small applicators have a similar fit.”
Pooja Sodha, MD, director of the center for laser and cosmetic dermatology at George Washington University, Washington, who was asked to comment on the study, said that the combination of radiofrequency energy and high-intensity focused electromagnetic technology triggers heat-induced damage of adipose tissue and muscle strengthening, respectively, to improve overall appearance and tone.
“Simultaneous delivery is the key here, and the real technological superhero, allowing us to take advantage of the synergistic effects of the muscle contractions and the tissue heating,” Dr. Sodha told this news organization. “Earlier this year, we saw published data on success with abdominal contouring with similar fat reduction and muscle enhancement as reported in this study, and these results persisted at 6 months,” with some declines noted at that time, she said.
“It is very encouraging and exciting to have similar effectiveness and safety for the arms, with such high satisfaction and comfort,” she added.
Dr. Jacob disclosed that she has conducted research studies for BTL Aesthetics since 2017 and is a member of the company’s advisory board. Dr. Sodha reported having no financial disclosures.
AT ASDS 2022
‘Low and Slow’ hyperthermic treatment being evaluated for superficial and nodular BCCs
DENVER –
At the annual meeting of the American Society for Dermatologic Surgery, Christopher Zachary, MD, and colleagues described a novel, noninvasive standardized controlled hyperthermia and mapping protocol (CHAMP) designed to help clinicians with margin assessment and treatment of superficial and nodular basal cell cancers (BCCs). “There’s considerable interest on the part of the public in having CHAMP treatment for their BCCs,” Dr. Zachary, professor and chair emeritus, University of California, Irvine, told this news organization in advance of the meeting.
In the study, which is being conducted at three centers and plans to enroll 100 patients, more than 70 patients with biopsy-proven superficial and nodular BCCs have been scanned with the VivoSight Dx optical coherence tomography (OCT) device to map BCC tumor margins. Next, they were treated with the Sciton 1,064-nm Er:YAG laser equipped with a 4-mm beam diameter scan pattern with no overlap and an 8-millisecond pulse duration, randomized to either 120 J/cm2 pulses, until tissue graying and contraction was observed, or a novel controlled hyperthermia technique known as “Low and Slow” using repeated 25 J/cm2 pulses under thermal camera imaging to maintain a consistent temperature of 55º C for 60 seconds.
The researchers reassessed the tissue response both clinically and by OCT at 3 months and the patients were retreated with the same method if residual BCC was demonstrated. At 3-12 months post treatment, the lesion sites were saucerized and examined histologically by step sections to confirm clearance.
“In contrast to the more commonly performed ‘standard’ long-pulse 1,064-nm laser tumor coagulation, where the end point is graying and contraction of tissue, the new controlled ‘Low and Slow’ technique heats the tissue to 55º C for 60 seconds, avoids ulceration, and induces apoptotic tumor disappearance by a caspase-3 and -7 mechanism,” Dr. Zachary explained in an interview. “It’s a gentler process that allows patients an alternative to second intention wounds that occur after electrodessication and curettage or Mohs,” he added, noting that CHAMP is not intended for the treatment of more complex, large, recurrent, or infiltrative BCCs.
In both study arms, the majority of patients enrolled to date have been found to be free of tumor at 3 months by clinical and OCT examination. “The study is ongoing, but the current numbers indicate that 9 out of 10 superficial and nodular BCCs are free of tumor at 3-12 months after the last treatment,” Dr. Zachary said. The standard-treatment arm, where tissue was treated to a gray color with tissue contraction, generally resulted in more blistering and tissue necrosis with prolonged healing, compared with the Low and Slow–controlled hyperthermia arm. BCC lesions treated in the controlled hyperthermia arm had a lilac gray color with “a surprising increase” in the Doppler blood flow rate, compared with those in the standard-treatment arm, he noted.
“Blood flow following the standard technique is dramatically reduced immediately post treatment, which accounts in part for the frequent ulceration and slow healing in that group,” Dr. Zachary said.
He acknowledged certain limitations of the study, including its relatively small sample size and the fact that the optimal treatment parameters of the Low and Slow technique have yet to be realized. “It could be that we will achieve better results at 50º C for 70 seconds or similar,” he said. “While this technique will not in any way reduce the great benefits of Mohs surgery for complex BCCs, it will benefit those with simpler superficial and nodular BCCs, particularly in those who are not good surgical candidates.”
As an aside, Dr. Zachary supports the increased use of OCT scanners to improve the ability to diagnose and assess the lateral and deep margins of skin cancers. “I think that all dermatology residents should understand how to use these devices,” he said. “I’m convinced they are going to be useful in their clinical practice in the future.”
Keith L. Duffy, MD, who was asked to comment on the work, said that the study demonstrates novel ways to use existing and developing technologies in dermatology and highlights the intersection of aesthetic, surgical, and medical dermatology. “CHAMP is promising as shown by the data in the abstract and I am eager to see the final results of the study with an eye toward final cure rate and cosmesis,” said Dr. Duffy, associate professor of dermatology at the University of Utah, Salt Lake City.
“In my estimation, this technology will need to prove to be superior in one or both of these parameters in order to be considered a first- or second-line therapy,” he added. “My practice for these types of basal cell carcinomas is a simple one pass of curettage with aluminum chloride or pressure for hemostasis. The healing is fast, the cosmesis is excellent, and the cure rate is more than 90% for this simple in-office destruction. However, for those with access to this technology and proficiency with its use, CHAMP may become a viable alternative to our existing destructive methods. I look forward to seeing the published results of this multicenter trial.”
This study is being funded by Michelson Diagnostics. Sciton provided the long-pulsed 1,064-nm lasers devices being used in the trial. Neither Dr. Zachary nor Dr. Duffy reported having relevant disclosures.
DENVER –
At the annual meeting of the American Society for Dermatologic Surgery, Christopher Zachary, MD, and colleagues described a novel, noninvasive standardized controlled hyperthermia and mapping protocol (CHAMP) designed to help clinicians with margin assessment and treatment of superficial and nodular basal cell cancers (BCCs). “There’s considerable interest on the part of the public in having CHAMP treatment for their BCCs,” Dr. Zachary, professor and chair emeritus, University of California, Irvine, told this news organization in advance of the meeting.

In the study, which is being conducted at three centers and plans to enroll 100 patients, more than 70 patients with biopsy-proven superficial and nodular BCCs have been scanned with the VivoSight Dx optical coherence tomography (OCT) device to map BCC tumor margins. Next, they were treated with the Sciton 1,064-nm Er:YAG laser equipped with a 4-mm beam diameter scan pattern with no overlap and an 8-millisecond pulse duration, randomized to either 120 J/cm2 pulses, until tissue graying and contraction was observed, or a novel controlled hyperthermia technique known as “Low and Slow” using repeated 25 J/cm2 pulses under thermal camera imaging to maintain a consistent temperature of 55º C for 60 seconds.
The researchers reassessed the tissue response both clinically and by OCT at 3 months and the patients were retreated with the same method if residual BCC was demonstrated. At 3-12 months post treatment, the lesion sites were saucerized and examined histologically by step sections to confirm clearance.
“In contrast to the more commonly performed ‘standard’ long-pulse 1,064-nm laser tumor coagulation, where the end point is graying and contraction of tissue, the new controlled ‘Low and Slow’ technique heats the tissue to 55º C for 60 seconds, avoids ulceration, and induces apoptotic tumor disappearance by a caspase-3 and -7 mechanism,” Dr. Zachary explained in an interview. “It’s a gentler process that allows patients an alternative to second intention wounds that occur after electrodessication and curettage or Mohs,” he added, noting that CHAMP is not intended for the treatment of more complex, large, recurrent, or infiltrative BCCs.
In both study arms, the majority of patients enrolled to date have been found to be free of tumor at 3 months by clinical and OCT examination. “The study is ongoing, but the current numbers indicate that 9 out of 10 superficial and nodular BCCs are free of tumor at 3-12 months after the last treatment,” Dr. Zachary said. The standard-treatment arm, where tissue was treated to a gray color with tissue contraction, generally resulted in more blistering and tissue necrosis with prolonged healing, compared with the Low and Slow–controlled hyperthermia arm. BCC lesions treated in the controlled hyperthermia arm had a lilac gray color with “a surprising increase” in the Doppler blood flow rate, compared with those in the standard-treatment arm, he noted.
“Blood flow following the standard technique is dramatically reduced immediately post treatment, which accounts in part for the frequent ulceration and slow healing in that group,” Dr. Zachary said.
He acknowledged certain limitations of the study, including its relatively small sample size and the fact that the optimal treatment parameters of the Low and Slow technique have yet to be realized. “It could be that we will achieve better results at 50º C for 70 seconds or similar,” he said. “While this technique will not in any way reduce the great benefits of Mohs surgery for complex BCCs, it will benefit those with simpler superficial and nodular BCCs, particularly in those who are not good surgical candidates.”
As an aside, Dr. Zachary supports the increased use of OCT scanners to improve the ability to diagnose and assess the lateral and deep margins of skin cancers. “I think that all dermatology residents should understand how to use these devices,” he said. “I’m convinced they are going to be useful in their clinical practice in the future.”
Keith L. Duffy, MD, who was asked to comment on the work, said that the study demonstrates novel ways to use existing and developing technologies in dermatology and highlights the intersection of aesthetic, surgical, and medical dermatology. “CHAMP is promising as shown by the data in the abstract and I am eager to see the final results of the study with an eye toward final cure rate and cosmesis,” said Dr. Duffy, associate professor of dermatology at the University of Utah, Salt Lake City.
“In my estimation, this technology will need to prove to be superior in one or both of these parameters in order to be considered a first- or second-line therapy,” he added. “My practice for these types of basal cell carcinomas is a simple one pass of curettage with aluminum chloride or pressure for hemostasis. The healing is fast, the cosmesis is excellent, and the cure rate is more than 90% for this simple in-office destruction. However, for those with access to this technology and proficiency with its use, CHAMP may become a viable alternative to our existing destructive methods. I look forward to seeing the published results of this multicenter trial.”
This study is being funded by Michelson Diagnostics. Sciton provided the long-pulsed 1,064-nm lasers devices being used in the trial. Neither Dr. Zachary nor Dr. Duffy reported having relevant disclosures.
DENVER –
At the annual meeting of the American Society for Dermatologic Surgery, Christopher Zachary, MD, and colleagues described a novel, noninvasive standardized controlled hyperthermia and mapping protocol (CHAMP) designed to help clinicians with margin assessment and treatment of superficial and nodular basal cell cancers (BCCs). “There’s considerable interest on the part of the public in having CHAMP treatment for their BCCs,” Dr. Zachary, professor and chair emeritus, University of California, Irvine, told this news organization in advance of the meeting.

In the study, which is being conducted at three centers and plans to enroll 100 patients, more than 70 patients with biopsy-proven superficial and nodular BCCs have been scanned with the VivoSight Dx optical coherence tomography (OCT) device to map BCC tumor margins. Next, they were treated with the Sciton 1,064-nm Er:YAG laser equipped with a 4-mm beam diameter scan pattern with no overlap and an 8-millisecond pulse duration, randomized to either 120 J/cm2 pulses, until tissue graying and contraction was observed, or a novel controlled hyperthermia technique known as “Low and Slow” using repeated 25 J/cm2 pulses under thermal camera imaging to maintain a consistent temperature of 55º C for 60 seconds.
The researchers reassessed the tissue response both clinically and by OCT at 3 months and the patients were retreated with the same method if residual BCC was demonstrated. At 3-12 months post treatment, the lesion sites were saucerized and examined histologically by step sections to confirm clearance.
“In contrast to the more commonly performed ‘standard’ long-pulse 1,064-nm laser tumor coagulation, where the end point is graying and contraction of tissue, the new controlled ‘Low and Slow’ technique heats the tissue to 55º C for 60 seconds, avoids ulceration, and induces apoptotic tumor disappearance by a caspase-3 and -7 mechanism,” Dr. Zachary explained in an interview. “It’s a gentler process that allows patients an alternative to second intention wounds that occur after electrodessication and curettage or Mohs,” he added, noting that CHAMP is not intended for the treatment of more complex, large, recurrent, or infiltrative BCCs.
In both study arms, the majority of patients enrolled to date have been found to be free of tumor at 3 months by clinical and OCT examination. “The study is ongoing, but the current numbers indicate that 9 out of 10 superficial and nodular BCCs are free of tumor at 3-12 months after the last treatment,” Dr. Zachary said. The standard-treatment arm, where tissue was treated to a gray color with tissue contraction, generally resulted in more blistering and tissue necrosis with prolonged healing, compared with the Low and Slow–controlled hyperthermia arm. BCC lesions treated in the controlled hyperthermia arm had a lilac gray color with “a surprising increase” in the Doppler blood flow rate, compared with those in the standard-treatment arm, he noted.
“Blood flow following the standard technique is dramatically reduced immediately post treatment, which accounts in part for the frequent ulceration and slow healing in that group,” Dr. Zachary said.
He acknowledged certain limitations of the study, including its relatively small sample size and the fact that the optimal treatment parameters of the Low and Slow technique have yet to be realized. “It could be that we will achieve better results at 50º C for 70 seconds or similar,” he said. “While this technique will not in any way reduce the great benefits of Mohs surgery for complex BCCs, it will benefit those with simpler superficial and nodular BCCs, particularly in those who are not good surgical candidates.”
As an aside, Dr. Zachary supports the increased use of OCT scanners to improve the ability to diagnose and assess the lateral and deep margins of skin cancers. “I think that all dermatology residents should understand how to use these devices,” he said. “I’m convinced they are going to be useful in their clinical practice in the future.”
Keith L. Duffy, MD, who was asked to comment on the work, said that the study demonstrates novel ways to use existing and developing technologies in dermatology and highlights the intersection of aesthetic, surgical, and medical dermatology. “CHAMP is promising as shown by the data in the abstract and I am eager to see the final results of the study with an eye toward final cure rate and cosmesis,” said Dr. Duffy, associate professor of dermatology at the University of Utah, Salt Lake City.
“In my estimation, this technology will need to prove to be superior in one or both of these parameters in order to be considered a first- or second-line therapy,” he added. “My practice for these types of basal cell carcinomas is a simple one pass of curettage with aluminum chloride or pressure for hemostasis. The healing is fast, the cosmesis is excellent, and the cure rate is more than 90% for this simple in-office destruction. However, for those with access to this technology and proficiency with its use, CHAMP may become a viable alternative to our existing destructive methods. I look forward to seeing the published results of this multicenter trial.”
This study is being funded by Michelson Diagnostics. Sciton provided the long-pulsed 1,064-nm lasers devices being used in the trial. Neither Dr. Zachary nor Dr. Duffy reported having relevant disclosures.
AT ASDS 2022
Liquid injectable silicone safe for acne scarring in dark-skinned patients, study finds
DENVER – Highly , results from a recent study showed.
“Acne is pervasive, and acne scarring disproportionately affects darker skin types,” lead study author Nicole Salame, MD, told this news organization in advance of the annual meeting of the American Society for Dermatologic Surgery, where she presented the results of the study. “Treatment of acne scarring in darker skin is also particularly challenging since resurfacing can be problematic. Numerous treatment options exist but vary in effectiveness, sustainability, and side-effect profile, especially for patients with darker skin.”
Highly purified liquid injectable silicone (also known as LIS) is approved by the Food and Drug Administration for treating intraocular tamponade of retinal detachment, and has been used off label for skin augmentation. A 2005 study of LIS for five patients with acne scarring, with up to 30 years of follow-up, showed efficacy and preservation of product without complications for depressed, broad-based acne scars .
“Use of LIS as a permanent treatment for acne scarring in darker skin types has yet to be evaluated,” said Dr. Salame, a 4th-year dermatology resident at Emory University, Atlanta. “Our study is the first to retrospectively evaluate the safety and efficacy of highly purified LIS for the treatment of acne scars in all skin types.”
Dr. Salame and coauthor Harold J. Brody, MD, evaluated the charts of 96 patients with a mean age of 51 years who received highly purified LIS for the treatment of acne scars at Dr. Brody’s Atlanta-based private dermatology practice between July 2010 and March 2021. Of the 96 patients, 31 had darker skin types (20 were Fitzpatrick skin type IV and 11 were Fitzpatrick skin type V). Dr. Brody performed all treatments: a total of 206 in the 96 patients.
The average time of follow-up was 6.31 years; 19 patients had a follow-up of 1-3 years, 25 had a follow-up of 3-5 years, and 52 had a follow-up of greater than 5 years. The researchers did not observe any complications along the course of the patients’ treatments, and no patients reported complications or dissatisfaction with treatment.
“Among the most impressive findings of our study was the permanence of effectiveness of LIS for acne scarring in patients who had treatment over a decade before,” Dr. Salame said. “Our longest follow up was 12 years. These patients continued to show improvement in their acne scarring years after treatment with LIS, even as they lost collagen and volume in their face with advancing age.”
In addition, she said, none of the patients experienced complications of granulomatous reactions, migration, or extrusion of product, which were previously documented with the use of macrodroplet injectable silicone techniques. “This is likely due to the consistent use of the microdroplet injection technique in our study – less than 0.01 cc per injection at minimum 6- to 8-week intervals or more,” Dr. Salame said.
Lawrence J. Green, MD, of the department of dermatology at George Washington University, Washington, who was asked to comment on the study, said that the findings “show safety and durability of highly purified microdroplet liquid silicone to treat acne scars. The numbers of patients reviewed are small and selective (one highly skilled dermatologist), but with the right material (highly purified liquid silicone) and in a qualified and experienced physician’s hand, this treatment seems like a great option.”
Dr. Salame acknowledged certain limitations of the study, including its single-center, retrospective design. “Future prospective studies with larger patient populations of all skin types recruited from multiple centers may be needed,” she said.
The researchers reported having no relevant conflicts of interest or funding sources to disclose. Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies.
DENVER – Highly , results from a recent study showed.
“Acne is pervasive, and acne scarring disproportionately affects darker skin types,” lead study author Nicole Salame, MD, told this news organization in advance of the annual meeting of the American Society for Dermatologic Surgery, where she presented the results of the study. “Treatment of acne scarring in darker skin is also particularly challenging since resurfacing can be problematic. Numerous treatment options exist but vary in effectiveness, sustainability, and side-effect profile, especially for patients with darker skin.”
Highly purified liquid injectable silicone (also known as LIS) is approved by the Food and Drug Administration for treating intraocular tamponade of retinal detachment, and has been used off label for skin augmentation. A 2005 study of LIS for five patients with acne scarring, with up to 30 years of follow-up, showed efficacy and preservation of product without complications for depressed, broad-based acne scars .
“Use of LIS as a permanent treatment for acne scarring in darker skin types has yet to be evaluated,” said Dr. Salame, a 4th-year dermatology resident at Emory University, Atlanta. “Our study is the first to retrospectively evaluate the safety and efficacy of highly purified LIS for the treatment of acne scars in all skin types.”
Dr. Salame and coauthor Harold J. Brody, MD, evaluated the charts of 96 patients with a mean age of 51 years who received highly purified LIS for the treatment of acne scars at Dr. Brody’s Atlanta-based private dermatology practice between July 2010 and March 2021. Of the 96 patients, 31 had darker skin types (20 were Fitzpatrick skin type IV and 11 were Fitzpatrick skin type V). Dr. Brody performed all treatments: a total of 206 in the 96 patients.
The average time of follow-up was 6.31 years; 19 patients had a follow-up of 1-3 years, 25 had a follow-up of 3-5 years, and 52 had a follow-up of greater than 5 years. The researchers did not observe any complications along the course of the patients’ treatments, and no patients reported complications or dissatisfaction with treatment.
“Among the most impressive findings of our study was the permanence of effectiveness of LIS for acne scarring in patients who had treatment over a decade before,” Dr. Salame said. “Our longest follow up was 12 years. These patients continued to show improvement in their acne scarring years after treatment with LIS, even as they lost collagen and volume in their face with advancing age.”
In addition, she said, none of the patients experienced complications of granulomatous reactions, migration, or extrusion of product, which were previously documented with the use of macrodroplet injectable silicone techniques. “This is likely due to the consistent use of the microdroplet injection technique in our study – less than 0.01 cc per injection at minimum 6- to 8-week intervals or more,” Dr. Salame said.
Lawrence J. Green, MD, of the department of dermatology at George Washington University, Washington, who was asked to comment on the study, said that the findings “show safety and durability of highly purified microdroplet liquid silicone to treat acne scars. The numbers of patients reviewed are small and selective (one highly skilled dermatologist), but with the right material (highly purified liquid silicone) and in a qualified and experienced physician’s hand, this treatment seems like a great option.”
Dr. Salame acknowledged certain limitations of the study, including its single-center, retrospective design. “Future prospective studies with larger patient populations of all skin types recruited from multiple centers may be needed,” she said.
The researchers reported having no relevant conflicts of interest or funding sources to disclose. Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies.
DENVER – Highly , results from a recent study showed.
“Acne is pervasive, and acne scarring disproportionately affects darker skin types,” lead study author Nicole Salame, MD, told this news organization in advance of the annual meeting of the American Society for Dermatologic Surgery, where she presented the results of the study. “Treatment of acne scarring in darker skin is also particularly challenging since resurfacing can be problematic. Numerous treatment options exist but vary in effectiveness, sustainability, and side-effect profile, especially for patients with darker skin.”
Highly purified liquid injectable silicone (also known as LIS) is approved by the Food and Drug Administration for treating intraocular tamponade of retinal detachment, and has been used off label for skin augmentation. A 2005 study of LIS for five patients with acne scarring, with up to 30 years of follow-up, showed efficacy and preservation of product without complications for depressed, broad-based acne scars .
“Use of LIS as a permanent treatment for acne scarring in darker skin types has yet to be evaluated,” said Dr. Salame, a 4th-year dermatology resident at Emory University, Atlanta. “Our study is the first to retrospectively evaluate the safety and efficacy of highly purified LIS for the treatment of acne scars in all skin types.”
Dr. Salame and coauthor Harold J. Brody, MD, evaluated the charts of 96 patients with a mean age of 51 years who received highly purified LIS for the treatment of acne scars at Dr. Brody’s Atlanta-based private dermatology practice between July 2010 and March 2021. Of the 96 patients, 31 had darker skin types (20 were Fitzpatrick skin type IV and 11 were Fitzpatrick skin type V). Dr. Brody performed all treatments: a total of 206 in the 96 patients.
The average time of follow-up was 6.31 years; 19 patients had a follow-up of 1-3 years, 25 had a follow-up of 3-5 years, and 52 had a follow-up of greater than 5 years. The researchers did not observe any complications along the course of the patients’ treatments, and no patients reported complications or dissatisfaction with treatment.
“Among the most impressive findings of our study was the permanence of effectiveness of LIS for acne scarring in patients who had treatment over a decade before,” Dr. Salame said. “Our longest follow up was 12 years. These patients continued to show improvement in their acne scarring years after treatment with LIS, even as they lost collagen and volume in their face with advancing age.”
In addition, she said, none of the patients experienced complications of granulomatous reactions, migration, or extrusion of product, which were previously documented with the use of macrodroplet injectable silicone techniques. “This is likely due to the consistent use of the microdroplet injection technique in our study – less than 0.01 cc per injection at minimum 6- to 8-week intervals or more,” Dr. Salame said.
Lawrence J. Green, MD, of the department of dermatology at George Washington University, Washington, who was asked to comment on the study, said that the findings “show safety and durability of highly purified microdroplet liquid silicone to treat acne scars. The numbers of patients reviewed are small and selective (one highly skilled dermatologist), but with the right material (highly purified liquid silicone) and in a qualified and experienced physician’s hand, this treatment seems like a great option.”
Dr. Salame acknowledged certain limitations of the study, including its single-center, retrospective design. “Future prospective studies with larger patient populations of all skin types recruited from multiple centers may be needed,” she said.
The researchers reported having no relevant conflicts of interest or funding sources to disclose. Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies.
AT ASDS 2022
Blindness from PRP injections a rare but potentially devastating side effect
DENVER – None of the cases involved scalp injections.
“Both soft tissue fillers and [PRP] are common injection-type treatments that dermatologists perform on the head and neck area,” lead study author Sean Wu, MD, said in an interview in advance of the annual meeting of the American Society for Dermatologic Surgery, where he presented the results during an oral abstract session. “Fillers are usually used to replace volume and fill in lines while PRP is usually used for skin rejuvenation and certain forms of hair loss. We know that fillers may rarely cause blindness if accidentally injected into a facial artery.”
Certain facial areas such as the glabella, nose, and forehead are considered high risk for blindness with filler injections. But whether PRP injections in those areas may also result in blindness is not yet known, so Dr. Wu and his colleagues, Xu He, MD, and Robert Weiss, MD, at the Maryland Laser, Skin, and Vein Institute in Hunt Valley, Md., performed what is believed to be the first systematic review of the topic. In January 2022 they searched the PubMed database, which yielded 224 articles from which they selected four for full review. The results were recently published in Dermatologic Surgery.
Collectively, the four articles reported a total of seven patients with unilateral vision loss or impairment following PRP injection. They ranged in age from 41 to 63 years. Skin rejuvenation was the indication for PRP injection in six patients and temporomandibular joint (TMJ) disorder in one. Three of the cases occurred in Venezuela while one each occurred in the United States, the United Kingdom, and Malaysia. All patients had signs of arterial occlusion or ischemia on retinal examination or imaging.
Dr. Wu and colleagues found that the glabella was the most common site of injection associated with vision loss (five cases), followed by the forehead (two cases), and one case each in the lateral canthus, nasolabial fold, and the TMJ. In all but two cases, vision loss occurred immediately after injection. (The number of injections exceeded seven because two patients received PRP in more than one site.)
Associated symptoms included ocular pain, fullness, eyelid ptosis, headache, nausea, vomiting, dizziness, tinnitus, and urinary urgency. At their initial ophthalmology evaluation, six patients had no light perception in the affected eye. Only one patient reported recovery of visual acuity at 3 months but with residual deficits on eye exam. This person had been evaluated and treated by an ophthalmologist within 3 hours of symptom onset.
“The other cases reported complete blindness in one eye,” Dr. Wu said. “There is no reversing agent for PRP, unlike for many fillers, so there is no clear-cut solution for this issue.”
Based on the results of the systematic review, Dr. Wu concluded that blindness is a rare complication of PRP. “We should take the same precautions when injecting PRP on the face as we do when injecting fillers,” he advised. “This may include not injecting in high-risk areas and aspirating prior to injection to make sure we are not accidentally injecting into an artery.”
It was “notable,” he added, that no cases of blindness occurred following scalp injections of PRP for hair loss, indicating “that this use of PRP is likely very safe from a vision loss standpoint.”
Dr. Wu acknowledged certain imitations of the analysis, including the low quality of some case reports/series. “There is a notable lack of detail on the PRP injection technique, as the authors of the case reports were generally not the PRP injectors themselves,” he said. “There was also no attempt at treatment in a series of four cases.”
Asked to comment on the review, Terrence Keaney, MD, founder and director of SkinDC, in Arlington, Va., said that the analysis underscores the importance of considering blindness as a possible side effect when injecting PRP into the face. “Using techniques that can minimize intravascular injections including the use of cannulas, aspiration, and larger needle size may help reduce this rare side effect,” said Dr. Keaney, a clinical associate professor of dermatology at George Washington University, Washington.
“It is important to recognize the lack of cases of blindness when injecting the scalp, one of the most popular PRP injection locations. This reduced risk may be due to the reduced communication between the scalp vasculature and the ophthalmic vasculature,” he added.
The study authors reported having no financial disclosures. Dr. Keaney disclosed that he is a member of the advisory board for Crown Aesthetics.
DENVER – None of the cases involved scalp injections.
“Both soft tissue fillers and [PRP] are common injection-type treatments that dermatologists perform on the head and neck area,” lead study author Sean Wu, MD, said in an interview in advance of the annual meeting of the American Society for Dermatologic Surgery, where he presented the results during an oral abstract session. “Fillers are usually used to replace volume and fill in lines while PRP is usually used for skin rejuvenation and certain forms of hair loss. We know that fillers may rarely cause blindness if accidentally injected into a facial artery.”
Certain facial areas such as the glabella, nose, and forehead are considered high risk for blindness with filler injections. But whether PRP injections in those areas may also result in blindness is not yet known, so Dr. Wu and his colleagues, Xu He, MD, and Robert Weiss, MD, at the Maryland Laser, Skin, and Vein Institute in Hunt Valley, Md., performed what is believed to be the first systematic review of the topic. In January 2022 they searched the PubMed database, which yielded 224 articles from which they selected four for full review. The results were recently published in Dermatologic Surgery.
Collectively, the four articles reported a total of seven patients with unilateral vision loss or impairment following PRP injection. They ranged in age from 41 to 63 years. Skin rejuvenation was the indication for PRP injection in six patients and temporomandibular joint (TMJ) disorder in one. Three of the cases occurred in Venezuela while one each occurred in the United States, the United Kingdom, and Malaysia. All patients had signs of arterial occlusion or ischemia on retinal examination or imaging.
Dr. Wu and colleagues found that the glabella was the most common site of injection associated with vision loss (five cases), followed by the forehead (two cases), and one case each in the lateral canthus, nasolabial fold, and the TMJ. In all but two cases, vision loss occurred immediately after injection. (The number of injections exceeded seven because two patients received PRP in more than one site.)
Associated symptoms included ocular pain, fullness, eyelid ptosis, headache, nausea, vomiting, dizziness, tinnitus, and urinary urgency. At their initial ophthalmology evaluation, six patients had no light perception in the affected eye. Only one patient reported recovery of visual acuity at 3 months but with residual deficits on eye exam. This person had been evaluated and treated by an ophthalmologist within 3 hours of symptom onset.
“The other cases reported complete blindness in one eye,” Dr. Wu said. “There is no reversing agent for PRP, unlike for many fillers, so there is no clear-cut solution for this issue.”
Based on the results of the systematic review, Dr. Wu concluded that blindness is a rare complication of PRP. “We should take the same precautions when injecting PRP on the face as we do when injecting fillers,” he advised. “This may include not injecting in high-risk areas and aspirating prior to injection to make sure we are not accidentally injecting into an artery.”
It was “notable,” he added, that no cases of blindness occurred following scalp injections of PRP for hair loss, indicating “that this use of PRP is likely very safe from a vision loss standpoint.”
Dr. Wu acknowledged certain imitations of the analysis, including the low quality of some case reports/series. “There is a notable lack of detail on the PRP injection technique, as the authors of the case reports were generally not the PRP injectors themselves,” he said. “There was also no attempt at treatment in a series of four cases.”
Asked to comment on the review, Terrence Keaney, MD, founder and director of SkinDC, in Arlington, Va., said that the analysis underscores the importance of considering blindness as a possible side effect when injecting PRP into the face. “Using techniques that can minimize intravascular injections including the use of cannulas, aspiration, and larger needle size may help reduce this rare side effect,” said Dr. Keaney, a clinical associate professor of dermatology at George Washington University, Washington.
“It is important to recognize the lack of cases of blindness when injecting the scalp, one of the most popular PRP injection locations. This reduced risk may be due to the reduced communication between the scalp vasculature and the ophthalmic vasculature,” he added.
The study authors reported having no financial disclosures. Dr. Keaney disclosed that he is a member of the advisory board for Crown Aesthetics.
DENVER – None of the cases involved scalp injections.
“Both soft tissue fillers and [PRP] are common injection-type treatments that dermatologists perform on the head and neck area,” lead study author Sean Wu, MD, said in an interview in advance of the annual meeting of the American Society for Dermatologic Surgery, where he presented the results during an oral abstract session. “Fillers are usually used to replace volume and fill in lines while PRP is usually used for skin rejuvenation and certain forms of hair loss. We know that fillers may rarely cause blindness if accidentally injected into a facial artery.”
Certain facial areas such as the glabella, nose, and forehead are considered high risk for blindness with filler injections. But whether PRP injections in those areas may also result in blindness is not yet known, so Dr. Wu and his colleagues, Xu He, MD, and Robert Weiss, MD, at the Maryland Laser, Skin, and Vein Institute in Hunt Valley, Md., performed what is believed to be the first systematic review of the topic. In January 2022 they searched the PubMed database, which yielded 224 articles from which they selected four for full review. The results were recently published in Dermatologic Surgery.
Collectively, the four articles reported a total of seven patients with unilateral vision loss or impairment following PRP injection. They ranged in age from 41 to 63 years. Skin rejuvenation was the indication for PRP injection in six patients and temporomandibular joint (TMJ) disorder in one. Three of the cases occurred in Venezuela while one each occurred in the United States, the United Kingdom, and Malaysia. All patients had signs of arterial occlusion or ischemia on retinal examination or imaging.
Dr. Wu and colleagues found that the glabella was the most common site of injection associated with vision loss (five cases), followed by the forehead (two cases), and one case each in the lateral canthus, nasolabial fold, and the TMJ. In all but two cases, vision loss occurred immediately after injection. (The number of injections exceeded seven because two patients received PRP in more than one site.)
Associated symptoms included ocular pain, fullness, eyelid ptosis, headache, nausea, vomiting, dizziness, tinnitus, and urinary urgency. At their initial ophthalmology evaluation, six patients had no light perception in the affected eye. Only one patient reported recovery of visual acuity at 3 months but with residual deficits on eye exam. This person had been evaluated and treated by an ophthalmologist within 3 hours of symptom onset.
“The other cases reported complete blindness in one eye,” Dr. Wu said. “There is no reversing agent for PRP, unlike for many fillers, so there is no clear-cut solution for this issue.”
Based on the results of the systematic review, Dr. Wu concluded that blindness is a rare complication of PRP. “We should take the same precautions when injecting PRP on the face as we do when injecting fillers,” he advised. “This may include not injecting in high-risk areas and aspirating prior to injection to make sure we are not accidentally injecting into an artery.”
It was “notable,” he added, that no cases of blindness occurred following scalp injections of PRP for hair loss, indicating “that this use of PRP is likely very safe from a vision loss standpoint.”
Dr. Wu acknowledged certain imitations of the analysis, including the low quality of some case reports/series. “There is a notable lack of detail on the PRP injection technique, as the authors of the case reports were generally not the PRP injectors themselves,” he said. “There was also no attempt at treatment in a series of four cases.”
Asked to comment on the review, Terrence Keaney, MD, founder and director of SkinDC, in Arlington, Va., said that the analysis underscores the importance of considering blindness as a possible side effect when injecting PRP into the face. “Using techniques that can minimize intravascular injections including the use of cannulas, aspiration, and larger needle size may help reduce this rare side effect,” said Dr. Keaney, a clinical associate professor of dermatology at George Washington University, Washington.
“It is important to recognize the lack of cases of blindness when injecting the scalp, one of the most popular PRP injection locations. This reduced risk may be due to the reduced communication between the scalp vasculature and the ophthalmic vasculature,” he added.
The study authors reported having no financial disclosures. Dr. Keaney disclosed that he is a member of the advisory board for Crown Aesthetics.
AT ASDS 2022
Expert makes the case for not subtyping patients with rosacea
. At least they should be, according to Julie C. Harper, MD.
“How many people with papules and pustules don’t also have redness?” Dr. Harper, who practices in Birmingham, Ala., said at Medscape Live’s annual Coastal Dermatology Symposium. “If we’re not careful, and we try to classify a person into a subtype of rosacea, we end up treating only part of their rosacea; we don’t treat all of it. We have seen this in the literature,” she added.
“The idea now is to take a phenotypic approach to rosacea. What we mean by that is that you look at the patient, you document every part of rosacea that you see, and you treat according to that,” she continued. “That person with papules and pustules may also have phyma and ocular disease. They may have telangiectasia and persistent background erythema. They may also have flushing.”
Dr. Harper incorporates the mnemonic “STOP” to her visits with rosacea patients.
S stands for: Identify signs and symptoms of the condition. “Listen to the patient for symptoms,” she advised. “We’ve learned to listen to darker skinned patients for what they tell us about erythema, for example, because we may not be able to see it, yet they are experiencing it. They may also have symptomatic burning, itching, and stinging.”
T stands for: Discuss triggers. “Ask patients, ‘what is it that makes your rosacea worse?’ That’s different for everyone,” she said.
O stands for: Agree on a treatment outcome. “Ask, ‘what is it that really bothers you? Are you bothered by the bumps? The redness?’ ” she said.
“The P stands for: Develop a plan that addresses all of that,” she said.
Different treatments for different rosacea symptoms
No one-size-fits-all treatment exists for rosacea. Options that work well for papules and pustules aren’t effective for redness. Similarly, products that work for redness don’t work for telangiectasia.
“Different lesions and signs of rosacea will likely require multiple modes of treatment,” Dr. Harper said. “So, when you evaluate your rosacea patients, if they’re doing great, don’t change their regimen. But if you see somebody who is not well controlled, is there an opportunity for you to come in and add something to that regimen that may make them better? Maybe so.”
Treatment options indicated for papules and pustules include ivermectin, metronidazole, azelaic acid, sodium sulfacetamide/sulfur, modified release doxycycline, minocycline foam, and encapsulated benzoyl peroxide.
Options indicated for persistent background erythema include brimonidine and oxymetazoline, while device-based treatments include the pulsed dye laser, the KTP laser, intense pulsed light, and electrosurgery.
Anti-inflammatory action for pustules and papules
A relatively new product indicated for pustules and papules is minocycline 1.5% foam, the only minocycline that is FDA approved to treat rosacea.
“There is no oral minocycline product approved for rosacea yet,” Dr. Harper said. “There is not a known bacterial pathogen in rosacea. Tetracyclines likely work in rosacea by inhibiting neutrophil chemotaxis, inhibiting MMP and thus KLK-5 and LL-37, inhibiting pro-inflammatory cytokines, downregulating reactive oxygen species, and inhibiting angiogenesis.”
In two 12-week, phase 3 randomized studies of 1,522 patients with moderate to severe rosacea, participants were assigned to receive minocycline 5% foam or a vehicle that contained mineral oil and coconut oil.
At week 12, about 50% of patients who received minocycline 5% foam were clear, compared with about 40% of those in the vehicle arm. Also, the reduction of lesion count was about 63% for patients in the treatment group, compared with a reduction of about 54% in the vehicle arm.
Dr. Harper characterized the 63% reduction as “pretty good, but is it good enough or fast enough? I don’t think so, so even with a great drug like this, I would use something else. You can use two medications sometimes to get people better faster. There’s room to bring in something for that background erythema.”
Minocycline 1.5% foam is colored yellow and may stain fabric. “It contains coconut oil, soybean oil, and light mineral oil,” she said. “Most people prefer to use this at bedtime, but you don’t have to.”
Another treatment option is 5% microencapsulated benzoyl peroxide cream, which is FDA approved for inflammatory lesions of rosacea.
“What’s the mechanism of action? Probably not being antimicrobial,” Dr. Harper said. “I think it’s probably at least in part anti-inflammatory, because we have some data to show that it’s killing Demodex [mites]. If Demodex [are] a trigger of inflammation, and we can lessen Demodex, then we could lessen the inflammatory response after that.”
The drug’s approval was based on data from two positive, identical phase 3 randomized, double-blind, multicenter, 12-week clinical trials that evaluated its safety compared with vehicle in 733 people with inflammatory lesions of rosacea (NCT03564119 and NCT03448939).
At week 12, inflammatory lesions of rosacea were reduced by nearly 70% in both trials among those who received 5% microencapsulated benzoyl peroxide cream, compared with 38%-46% among those who received the vehicle. Also, nearly 50% of subjects in the treatment groups were clear or almost clear at 12 weeks, compared with 38%-46% of those who received the vehicle.
Dr. Harper added that about one-quarter of patients in the treatment group of the trials were clear or almost clear by week 4. “That’s pretty fast,” she said, noting that the product’s microencapsulated shell acts as a fenestrated barrier. “It has little openings, which means that it takes a while for the drug to work itself out,” she said. “I think of it as being like a speed bump for benzoyl peroxide delivery. It has to get through this little maze before it lands on the skin. We think that is what has helped with tolerability.”
Oral sarecycline, a narrow spectrum tetracycline that was FDA approved for acne in 2018, may also benefit rosacea patients. In a 12-week, investigator-blinded pilot study, 72 patients with papulopustular rosacea were assigned to receive sarecycline, while 25 received a multivitamin.
By week 12, 75% of patients in the sarecycline group were clear, compared with 16% of those in the multivitamin group, while the inflammatory lesion counts dropped from baseline by 80% and 60%, respectively. Studies of sarecycline for acne have demonstrated similar rates of vertigo, dizziness, and sunburn to those of placebo.
“There were also low rates of gastrointestinal disturbances,” Dr. Harper said. “That’s important in rosacea, because there is no bacterial pathogen.”
Dr. Harper disclosed that she serves as an advisor or consultant for Almirall, BioPharmX, Cassiopeia, Cutanea, Cutera, Dermira, EPI, Galderma, LaRoche-Posay, Ortho, Vyne, Sol Gel, and Sun. She also serves as a speaker or member of a speakers bureau for Almirall, EPI, Galderma, Ortho, and Vyne.
Medscape Live and this news organization are owned by the same parent company.
. At least they should be, according to Julie C. Harper, MD.
“How many people with papules and pustules don’t also have redness?” Dr. Harper, who practices in Birmingham, Ala., said at Medscape Live’s annual Coastal Dermatology Symposium. “If we’re not careful, and we try to classify a person into a subtype of rosacea, we end up treating only part of their rosacea; we don’t treat all of it. We have seen this in the literature,” she added.
“The idea now is to take a phenotypic approach to rosacea. What we mean by that is that you look at the patient, you document every part of rosacea that you see, and you treat according to that,” she continued. “That person with papules and pustules may also have phyma and ocular disease. They may have telangiectasia and persistent background erythema. They may also have flushing.”
Dr. Harper incorporates the mnemonic “STOP” to her visits with rosacea patients.
S stands for: Identify signs and symptoms of the condition. “Listen to the patient for symptoms,” she advised. “We’ve learned to listen to darker skinned patients for what they tell us about erythema, for example, because we may not be able to see it, yet they are experiencing it. They may also have symptomatic burning, itching, and stinging.”
T stands for: Discuss triggers. “Ask patients, ‘what is it that makes your rosacea worse?’ That’s different for everyone,” she said.
O stands for: Agree on a treatment outcome. “Ask, ‘what is it that really bothers you? Are you bothered by the bumps? The redness?’ ” she said.
“The P stands for: Develop a plan that addresses all of that,” she said.
Different treatments for different rosacea symptoms
No one-size-fits-all treatment exists for rosacea. Options that work well for papules and pustules aren’t effective for redness. Similarly, products that work for redness don’t work for telangiectasia.
“Different lesions and signs of rosacea will likely require multiple modes of treatment,” Dr. Harper said. “So, when you evaluate your rosacea patients, if they’re doing great, don’t change their regimen. But if you see somebody who is not well controlled, is there an opportunity for you to come in and add something to that regimen that may make them better? Maybe so.”
Treatment options indicated for papules and pustules include ivermectin, metronidazole, azelaic acid, sodium sulfacetamide/sulfur, modified release doxycycline, minocycline foam, and encapsulated benzoyl peroxide.
Options indicated for persistent background erythema include brimonidine and oxymetazoline, while device-based treatments include the pulsed dye laser, the KTP laser, intense pulsed light, and electrosurgery.
Anti-inflammatory action for pustules and papules
A relatively new product indicated for pustules and papules is minocycline 1.5% foam, the only minocycline that is FDA approved to treat rosacea.
“There is no oral minocycline product approved for rosacea yet,” Dr. Harper said. “There is not a known bacterial pathogen in rosacea. Tetracyclines likely work in rosacea by inhibiting neutrophil chemotaxis, inhibiting MMP and thus KLK-5 and LL-37, inhibiting pro-inflammatory cytokines, downregulating reactive oxygen species, and inhibiting angiogenesis.”
In two 12-week, phase 3 randomized studies of 1,522 patients with moderate to severe rosacea, participants were assigned to receive minocycline 5% foam or a vehicle that contained mineral oil and coconut oil.
At week 12, about 50% of patients who received minocycline 5% foam were clear, compared with about 40% of those in the vehicle arm. Also, the reduction of lesion count was about 63% for patients in the treatment group, compared with a reduction of about 54% in the vehicle arm.
Dr. Harper characterized the 63% reduction as “pretty good, but is it good enough or fast enough? I don’t think so, so even with a great drug like this, I would use something else. You can use two medications sometimes to get people better faster. There’s room to bring in something for that background erythema.”
Minocycline 1.5% foam is colored yellow and may stain fabric. “It contains coconut oil, soybean oil, and light mineral oil,” she said. “Most people prefer to use this at bedtime, but you don’t have to.”
Another treatment option is 5% microencapsulated benzoyl peroxide cream, which is FDA approved for inflammatory lesions of rosacea.
“What’s the mechanism of action? Probably not being antimicrobial,” Dr. Harper said. “I think it’s probably at least in part anti-inflammatory, because we have some data to show that it’s killing Demodex [mites]. If Demodex [are] a trigger of inflammation, and we can lessen Demodex, then we could lessen the inflammatory response after that.”
The drug’s approval was based on data from two positive, identical phase 3 randomized, double-blind, multicenter, 12-week clinical trials that evaluated its safety compared with vehicle in 733 people with inflammatory lesions of rosacea (NCT03564119 and NCT03448939).
At week 12, inflammatory lesions of rosacea were reduced by nearly 70% in both trials among those who received 5% microencapsulated benzoyl peroxide cream, compared with 38%-46% among those who received the vehicle. Also, nearly 50% of subjects in the treatment groups were clear or almost clear at 12 weeks, compared with 38%-46% of those who received the vehicle.
Dr. Harper added that about one-quarter of patients in the treatment group of the trials were clear or almost clear by week 4. “That’s pretty fast,” she said, noting that the product’s microencapsulated shell acts as a fenestrated barrier. “It has little openings, which means that it takes a while for the drug to work itself out,” she said. “I think of it as being like a speed bump for benzoyl peroxide delivery. It has to get through this little maze before it lands on the skin. We think that is what has helped with tolerability.”
Oral sarecycline, a narrow spectrum tetracycline that was FDA approved for acne in 2018, may also benefit rosacea patients. In a 12-week, investigator-blinded pilot study, 72 patients with papulopustular rosacea were assigned to receive sarecycline, while 25 received a multivitamin.
By week 12, 75% of patients in the sarecycline group were clear, compared with 16% of those in the multivitamin group, while the inflammatory lesion counts dropped from baseline by 80% and 60%, respectively. Studies of sarecycline for acne have demonstrated similar rates of vertigo, dizziness, and sunburn to those of placebo.
“There were also low rates of gastrointestinal disturbances,” Dr. Harper said. “That’s important in rosacea, because there is no bacterial pathogen.”
Dr. Harper disclosed that she serves as an advisor or consultant for Almirall, BioPharmX, Cassiopeia, Cutanea, Cutera, Dermira, EPI, Galderma, LaRoche-Posay, Ortho, Vyne, Sol Gel, and Sun. She also serves as a speaker or member of a speakers bureau for Almirall, EPI, Galderma, Ortho, and Vyne.
Medscape Live and this news organization are owned by the same parent company.
. At least they should be, according to Julie C. Harper, MD.
“How many people with papules and pustules don’t also have redness?” Dr. Harper, who practices in Birmingham, Ala., said at Medscape Live’s annual Coastal Dermatology Symposium. “If we’re not careful, and we try to classify a person into a subtype of rosacea, we end up treating only part of their rosacea; we don’t treat all of it. We have seen this in the literature,” she added.
“The idea now is to take a phenotypic approach to rosacea. What we mean by that is that you look at the patient, you document every part of rosacea that you see, and you treat according to that,” she continued. “That person with papules and pustules may also have phyma and ocular disease. They may have telangiectasia and persistent background erythema. They may also have flushing.”
Dr. Harper incorporates the mnemonic “STOP” to her visits with rosacea patients.
S stands for: Identify signs and symptoms of the condition. “Listen to the patient for symptoms,” she advised. “We’ve learned to listen to darker skinned patients for what they tell us about erythema, for example, because we may not be able to see it, yet they are experiencing it. They may also have symptomatic burning, itching, and stinging.”
T stands for: Discuss triggers. “Ask patients, ‘what is it that makes your rosacea worse?’ That’s different for everyone,” she said.
O stands for: Agree on a treatment outcome. “Ask, ‘what is it that really bothers you? Are you bothered by the bumps? The redness?’ ” she said.
“The P stands for: Develop a plan that addresses all of that,” she said.
Different treatments for different rosacea symptoms
No one-size-fits-all treatment exists for rosacea. Options that work well for papules and pustules aren’t effective for redness. Similarly, products that work for redness don’t work for telangiectasia.
“Different lesions and signs of rosacea will likely require multiple modes of treatment,” Dr. Harper said. “So, when you evaluate your rosacea patients, if they’re doing great, don’t change their regimen. But if you see somebody who is not well controlled, is there an opportunity for you to come in and add something to that regimen that may make them better? Maybe so.”
Treatment options indicated for papules and pustules include ivermectin, metronidazole, azelaic acid, sodium sulfacetamide/sulfur, modified release doxycycline, minocycline foam, and encapsulated benzoyl peroxide.
Options indicated for persistent background erythema include brimonidine and oxymetazoline, while device-based treatments include the pulsed dye laser, the KTP laser, intense pulsed light, and electrosurgery.
Anti-inflammatory action for pustules and papules
A relatively new product indicated for pustules and papules is minocycline 1.5% foam, the only minocycline that is FDA approved to treat rosacea.
“There is no oral minocycline product approved for rosacea yet,” Dr. Harper said. “There is not a known bacterial pathogen in rosacea. Tetracyclines likely work in rosacea by inhibiting neutrophil chemotaxis, inhibiting MMP and thus KLK-5 and LL-37, inhibiting pro-inflammatory cytokines, downregulating reactive oxygen species, and inhibiting angiogenesis.”
In two 12-week, phase 3 randomized studies of 1,522 patients with moderate to severe rosacea, participants were assigned to receive minocycline 5% foam or a vehicle that contained mineral oil and coconut oil.
At week 12, about 50% of patients who received minocycline 5% foam were clear, compared with about 40% of those in the vehicle arm. Also, the reduction of lesion count was about 63% for patients in the treatment group, compared with a reduction of about 54% in the vehicle arm.
Dr. Harper characterized the 63% reduction as “pretty good, but is it good enough or fast enough? I don’t think so, so even with a great drug like this, I would use something else. You can use two medications sometimes to get people better faster. There’s room to bring in something for that background erythema.”
Minocycline 1.5% foam is colored yellow and may stain fabric. “It contains coconut oil, soybean oil, and light mineral oil,” she said. “Most people prefer to use this at bedtime, but you don’t have to.”
Another treatment option is 5% microencapsulated benzoyl peroxide cream, which is FDA approved for inflammatory lesions of rosacea.
“What’s the mechanism of action? Probably not being antimicrobial,” Dr. Harper said. “I think it’s probably at least in part anti-inflammatory, because we have some data to show that it’s killing Demodex [mites]. If Demodex [are] a trigger of inflammation, and we can lessen Demodex, then we could lessen the inflammatory response after that.”
The drug’s approval was based on data from two positive, identical phase 3 randomized, double-blind, multicenter, 12-week clinical trials that evaluated its safety compared with vehicle in 733 people with inflammatory lesions of rosacea (NCT03564119 and NCT03448939).
At week 12, inflammatory lesions of rosacea were reduced by nearly 70% in both trials among those who received 5% microencapsulated benzoyl peroxide cream, compared with 38%-46% among those who received the vehicle. Also, nearly 50% of subjects in the treatment groups were clear or almost clear at 12 weeks, compared with 38%-46% of those who received the vehicle.
Dr. Harper added that about one-quarter of patients in the treatment group of the trials were clear or almost clear by week 4. “That’s pretty fast,” she said, noting that the product’s microencapsulated shell acts as a fenestrated barrier. “It has little openings, which means that it takes a while for the drug to work itself out,” she said. “I think of it as being like a speed bump for benzoyl peroxide delivery. It has to get through this little maze before it lands on the skin. We think that is what has helped with tolerability.”
Oral sarecycline, a narrow spectrum tetracycline that was FDA approved for acne in 2018, may also benefit rosacea patients. In a 12-week, investigator-blinded pilot study, 72 patients with papulopustular rosacea were assigned to receive sarecycline, while 25 received a multivitamin.
By week 12, 75% of patients in the sarecycline group were clear, compared with 16% of those in the multivitamin group, while the inflammatory lesion counts dropped from baseline by 80% and 60%, respectively. Studies of sarecycline for acne have demonstrated similar rates of vertigo, dizziness, and sunburn to those of placebo.
“There were also low rates of gastrointestinal disturbances,” Dr. Harper said. “That’s important in rosacea, because there is no bacterial pathogen.”
Dr. Harper disclosed that she serves as an advisor or consultant for Almirall, BioPharmX, Cassiopeia, Cutanea, Cutera, Dermira, EPI, Galderma, LaRoche-Posay, Ortho, Vyne, Sol Gel, and Sun. She also serves as a speaker or member of a speakers bureau for Almirall, EPI, Galderma, Ortho, and Vyne.
Medscape Live and this news organization are owned by the same parent company.
FROM MEDSCAPE LIVE COASTAL DERM