User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
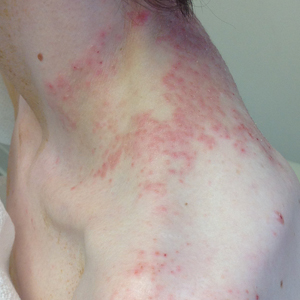
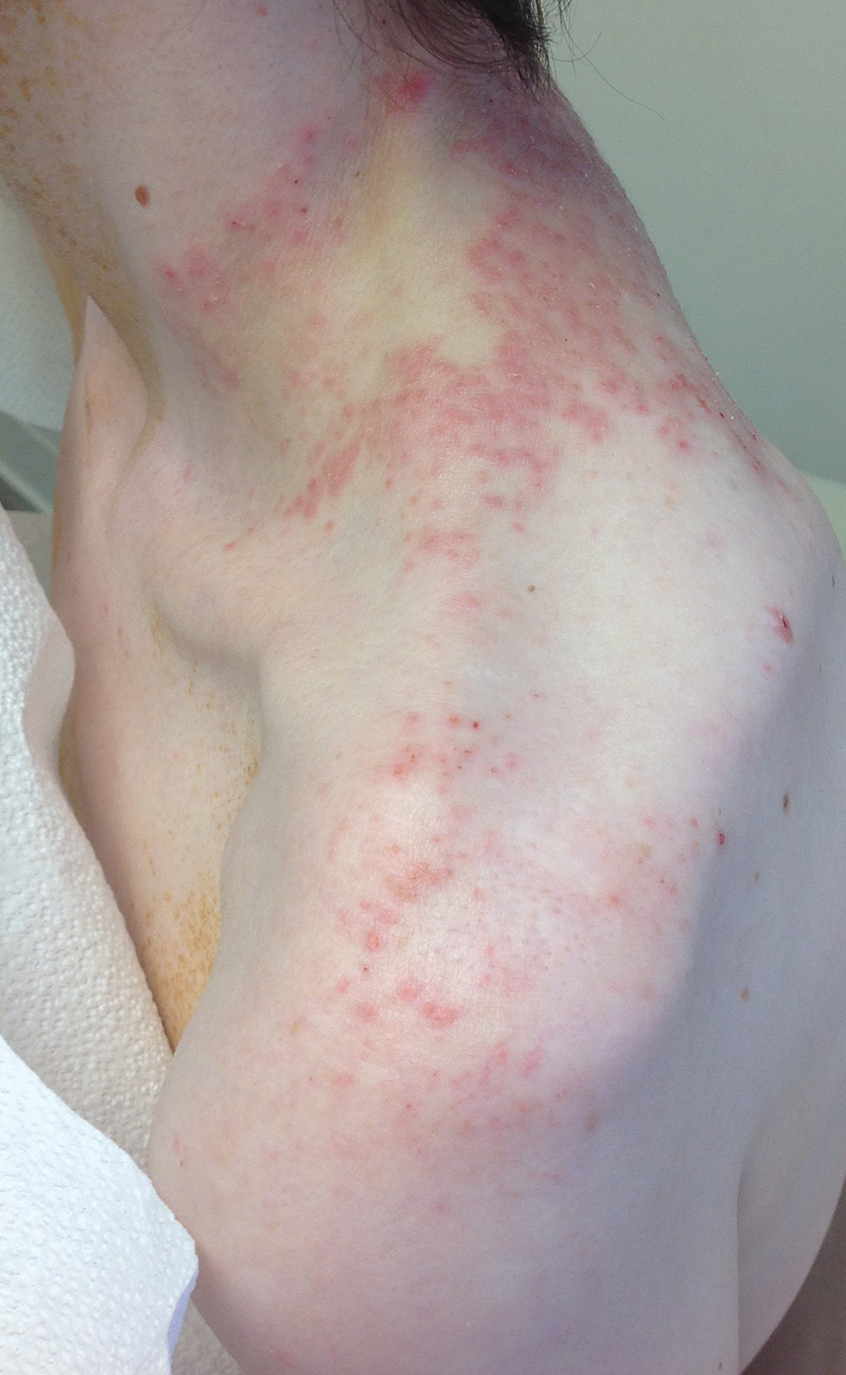
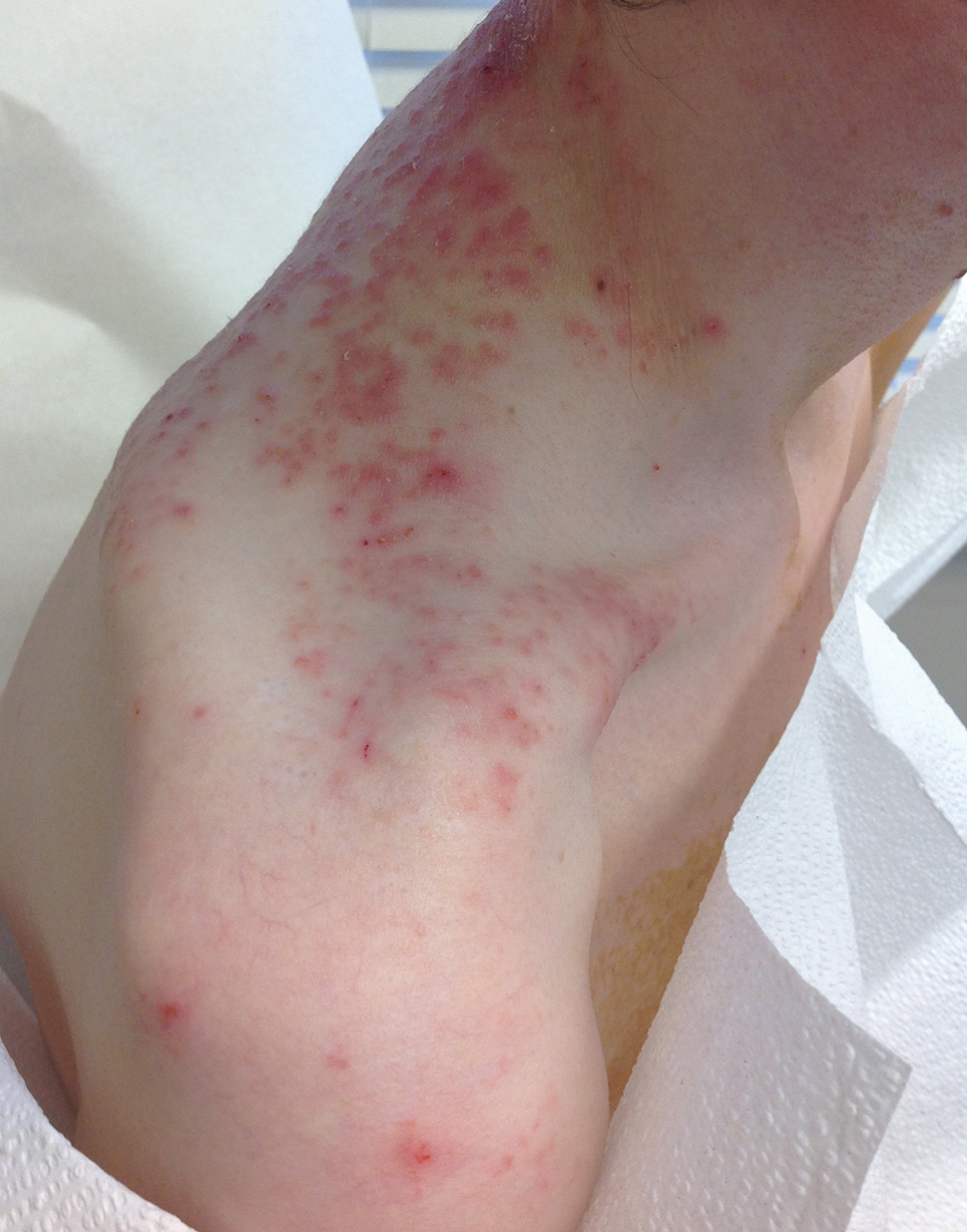
Pruritic Rash on the Neck and Back
The Diagnosis: Prurigo Pigmentosa
A comprehensive metabolic panel collected from our patient 1 month earlier did not reveal any abnormalities. Serum methylmalonic acid and homocysteine were both elevated at 417 nmol/L (reference range [for those aged 2–59 years], 55–335 nmol/L) and 23 μmol/L (reference range, 5–15 μmol/L), respectively. Serum folate and 25-hydroxyvitamin D were low at 3.1 ng/mL (reference range, >4.8 ng/mL) and 5 ng/mL (reference range, 30–80 ng/mL), respectively. Vitamin B12 was within reference range. Two 4-mm punch biopsies collected from the upper back showed spongiotic dermatitis.
Our patient’s histopathology results along with the rash distribution and medical history of anorexia increased suspicion for prurigo pigmentosa. A trial of oral doxycycline 100 mg twice daily for 2 weeks was prescribed. At 2-week follow-up, the patient’s mother revealed a history of ketosis in her daughter, solidifying the diagnosis. The patient was counseled on maintaining a healthy diet to prevent future breakouts. The patient’s rash resolved with diet modification and doxycycline; however, it recurred upon relapse of anorexia 4 months later.
Prurigo pigmentosa, originally identified in Japan by Nagashima et al,1 is an uncommon recurrent inflammatory disorder predominantly observed in young adults of Asian descent. Subsequently, it was reported to occur among individuals from different ethnic backgrounds, indicating potential underdiagnosis or misdiagnosis in Western countries.2 Although a direct pathogenic cause for prurigo pigmentosa has not been identified, a strong association has been linked to diet, specifically when ketosis is induced, such as in ketogenic diets and anorexia nervosa.3-5 Other possible causes include sunlight exposure, clothing friction, and sweating.1,5 The disease course is characterized by intermittent flares and spontaneous resolution, with recurrence in most cases. During the active phase, intensely pruritic, papulovesicular or urticarial papules are predominant and most often are localized to the upper body and torso, including the back, shoulders, neck, and chest.5 These flares can persist for several days but eventually subside, leaving behind a characteristic reticular pigmentation that can persist for months.5 First-line treatment often involves the use of tetracycline antibiotics, such as minocycline or doxycycline. 2,4,5 Dapsone often is used with successful resolution. 6 Dietary modifications also have been found to be effective in treating prurigo pigmentosa, particularly in patients presenting with dietary insufficiency.6,7 Increased carbohydrate intake has been shown to promote resolution. 6 Topical corticosteroids demonstrate limited efficacy in controlling flares.6,8
Histopathology has been variably described, with initial findings reported as nonspecific.1 However, it was later described as a distinct inflammatory disease of the skin with histologically distinct stages.2,9 Early stages reveal scattered dermal, dermal papillary, and perivascular neutrophilic infiltration.9 The lesions then progress and become fully developed, at which point neutrophilic infiltration becomes more prominent, accompanied by the presence of intraepidermal neutrophils and spongiosis. As the lesions resolve, the infiltration transitions to lymphocytic, and lichenoid changes can sometimes be appreciated along with epidermal hyperplasia, hyperpigmentation, and dermal melanophages.9 Although these findings aid in the diagnosis of prurigo pigmentosa, a clinicopathologic correlation is necessary to establish a definitive diagnosis.
Because prurigo pigmentosa is rare, it often is misdiagnosed as another condition with a similar presentation and nonspecific biopsy findings.6 Allergic contact dermatitis is a common type IV delayed hypersensitivity reaction that manifests similar to prurigo pigmentosa with pruritus and a well-demarcated distribution10 that is related to the pattern of allergen exposure; in the case of allergic contact dermatitis related to textiles, a well-demarcated rash will appear in the distribution area of the associated clothing (eg, shirt, pants, shorts).11 Development of allergy involves exposure and sensitization to an allergen, followed by subsequent re-exposure that results in cutaneous T-cell activation and inflammation. 10 Histopathology shows nonspecific spongiotic inflammation, and the gold standard for diagnosis is patch testing to identify the causative substance(s). Definitive treatment includes avoidance of identified allergies; however, if patients are unable to avoid the allergen or the cause is unknown, then corticosteroids, antihistamines, and/or calcineurin inhibitors are beneficial in controlling symptoms and flares.10
Pityrosporum folliculitis (also known as Malassezia folliculitis) is a fungal acneform condition that arises from overgrowth of normal skin flora Malassezia yeast,12 which may be due to occlusion of follicles or disruption of the normal flora composition. Clinically, the manifestation may resemble prurigo pigmentosa in distribution and presence of intense pruritus. However, pustular lesions and involvement of the face can aid in differentiating Pityrosporum from prurigo pigmentosa, which can be confirmed via periodic acid–Schiff staining with numerous round yeasts within affected follicles. Oral antifungal therapy typically yields rapid improvement and resolution of symptoms.12
Urticaria and prurigo pigmentosa share similar clinical characteristics, with symptoms of intense pruritus and urticarial lesions on the trunk.2,13 Urticaria is an IgEmediated type I hypersensitivity reaction characterized by wheals (ie, edematous red or pink lesions of variable size and shape that typically resolve spontaneously within 24–48 hours).13 Notably, urticaria will improve and in some cases completely resolve with antihistamines or anti-IgE antibody treatment, which may aid in distinguishing it from prurigo pigmentosa, as the latter typically exhibits limited response to such treatment.2 Histopathology also can assist in the diagnosis by ruling out other causes of similar rash; however, biopsies are not routinely done unless other inflammatory conditions are of high suspicion.13
Bullous pemphigoid is an autoimmune, subepidermal, blistering dermatosis that is most common among the elderly.14 It is characterized by the presence of IgG antibodies that target BP180 and BP230, which initiate inflammatory cascades that lead to tissue damage and blister formation. It typically manifests as pruritic blistering eruptions, primarily on the limbs and trunk, but may involve the head, neck, or palmoplantar regions.14 Although blistering eruptions are the prodrome of the disease, some cases may present with nonspecific urticarial or eczematous lesions14,15 that may resemble prurigo pigmentosa. The diagnosis is confirmed through direct immunofluorescence microscopy of biopsied lesions, which reveals IgG and/or C3 deposits along the dermoepidermal junction.14 Management of bullous pemphigoid involves timely initiation of dapsone or systemic corticosteroids, which have demonstrated high efficacy in controlling the disease and its associated symptoms.15
Our patient achieved a favorable response to diet modification and doxycycline therapy consistent with the diagnosis of prurigo pigmentosa. Unfortunately, the condition recurred following a relapse of anorexia. Management of prurigo pigmentosa necessitates not only accurate diagnosis but also addressing any underlying factors that may contribute to disease exacerbation. We anticipate the eating disorder will pose a major challenge in achieving long-term control of prurigo pigmentosa.
- Nagashima M, Ohshiro A, Shimizu N. A peculiar pruriginous dermatosis with gross reticular pigmentation. Jpn J Dermatol. 1971;81:38-39.
- Boer A, Asgari M. Prurigo pigmentosa: an underdiagnosed disease? Indian J Dermatol Venereol Leprol. 2006;72:405-409. doi:10.4103/0378-6323.29334
- Michaels JD, Hoss E, DiCaudo DJ, et al. Prurigo pigmentosa after a strict ketogenic diet. Pediatr Dermatol. 2013;32:248-251. doi:10.1111/pde.12275
- Teraki Y, Teraki E, Kawashima M, et al. Ketosis is involved in the origin of prurigo pigmentosa. J Am Acad Dermatol. 1996;34:509-511. doi:10.1016/s0190-9622(96)90460-0
- Böer A, Misago N, Wolter M, et al. Prurigo pigmentosa: a distinctive inflammatory disease of the skin. Am J Dermatopathol. 2003;25:117-129. doi:10.1097/00000372-200304000-00005
- Mufti A, Mirali S, Abduelmula A, et al. Clinical manifestations and treatment outcomes in prurigo pigmentosa (Nagashima disease): a systematic review of the literature. JAAD Int. 2021;3:79-87. doi:10.1016/j.jdin.2021.03.003
- Wong M, Lee E, Wu Y, et al. Treatment of prurigo pigmentosa with diet modification: a medical case study. Hawaii J Med Public Health. 2018;77:114-117.
- Almaani N, Al-Tarawneh AH, Msallam H. Prurigo pigmentosa: a clinicopathological report of three Middle Eastern patients. Case Rep Dermatol Med. 2018;2018:9406797. doi:10.1155/2018/9406797
- Kim JK, Chung WK, Chang SE, et al. Prurigo pigmentosa: clinicopathological study and analysis of 50 cases in Korea. J Dermatol. 2012;39:891-897. doi:10.1111/j.1346-8138.2012.01640.x
- Mowad CM, Anderson B, Scheinman P, et al. Allergic contact dermatitis: patient diagnosis and evaluation. J Am Acad Dermatol. 2016;74:1029-1040. doi:10.1016/j.jaad.2015.02.1139
- Lazarov A, Cordoba M, Plosk N, et al. Atypical and unusual clinical manifestations of contact dermatitis to clothing (textile contact dermatitis)—case presentation and review of the literature. Dermatol Online J. 2003;9. doi:10.5070/d30kd1d259
- Rubenstein RM, Malerich SA. Malassezia (Pityrosporum) folliculitis. J Clin Aesthet Dermatol. 2014;7:37-41.
- Bernstein JA, Lang DM, Khan DA, et al. The diagnosis and management of acute and chronic urticaria: 2014 update. J Allergy Clin Immunol. 2014;133:1270-1277. doi:10.1016/j.jaci.2014.02.036
- della Torre R, Combescure C, Cortés B, et al. Clinical presentation and diagnostic delay in bullous pemphigoid: a prospective nationwide cohort. Br J Dermatol. 2012;167:1111-1117. doi:10.1111/j.1365-2133.2012.11108.x
- Alonso-Llamazares J, Rogers RS 3rd, Oursler JR, et al. Bullous pemphigoid presenting as generalized pruritus: observations in six patients. Int J Dermatol. 1998;37:508-514.
The Diagnosis: Prurigo Pigmentosa
A comprehensive metabolic panel collected from our patient 1 month earlier did not reveal any abnormalities. Serum methylmalonic acid and homocysteine were both elevated at 417 nmol/L (reference range [for those aged 2–59 years], 55–335 nmol/L) and 23 μmol/L (reference range, 5–15 μmol/L), respectively. Serum folate and 25-hydroxyvitamin D were low at 3.1 ng/mL (reference range, >4.8 ng/mL) and 5 ng/mL (reference range, 30–80 ng/mL), respectively. Vitamin B12 was within reference range. Two 4-mm punch biopsies collected from the upper back showed spongiotic dermatitis.
Our patient’s histopathology results along with the rash distribution and medical history of anorexia increased suspicion for prurigo pigmentosa. A trial of oral doxycycline 100 mg twice daily for 2 weeks was prescribed. At 2-week follow-up, the patient’s mother revealed a history of ketosis in her daughter, solidifying the diagnosis. The patient was counseled on maintaining a healthy diet to prevent future breakouts. The patient’s rash resolved with diet modification and doxycycline; however, it recurred upon relapse of anorexia 4 months later.
Prurigo pigmentosa, originally identified in Japan by Nagashima et al,1 is an uncommon recurrent inflammatory disorder predominantly observed in young adults of Asian descent. Subsequently, it was reported to occur among individuals from different ethnic backgrounds, indicating potential underdiagnosis or misdiagnosis in Western countries.2 Although a direct pathogenic cause for prurigo pigmentosa has not been identified, a strong association has been linked to diet, specifically when ketosis is induced, such as in ketogenic diets and anorexia nervosa.3-5 Other possible causes include sunlight exposure, clothing friction, and sweating.1,5 The disease course is characterized by intermittent flares and spontaneous resolution, with recurrence in most cases. During the active phase, intensely pruritic, papulovesicular or urticarial papules are predominant and most often are localized to the upper body and torso, including the back, shoulders, neck, and chest.5 These flares can persist for several days but eventually subside, leaving behind a characteristic reticular pigmentation that can persist for months.5 First-line treatment often involves the use of tetracycline antibiotics, such as minocycline or doxycycline. 2,4,5 Dapsone often is used with successful resolution. 6 Dietary modifications also have been found to be effective in treating prurigo pigmentosa, particularly in patients presenting with dietary insufficiency.6,7 Increased carbohydrate intake has been shown to promote resolution. 6 Topical corticosteroids demonstrate limited efficacy in controlling flares.6,8
Histopathology has been variably described, with initial findings reported as nonspecific.1 However, it was later described as a distinct inflammatory disease of the skin with histologically distinct stages.2,9 Early stages reveal scattered dermal, dermal papillary, and perivascular neutrophilic infiltration.9 The lesions then progress and become fully developed, at which point neutrophilic infiltration becomes more prominent, accompanied by the presence of intraepidermal neutrophils and spongiosis. As the lesions resolve, the infiltration transitions to lymphocytic, and lichenoid changes can sometimes be appreciated along with epidermal hyperplasia, hyperpigmentation, and dermal melanophages.9 Although these findings aid in the diagnosis of prurigo pigmentosa, a clinicopathologic correlation is necessary to establish a definitive diagnosis.
Because prurigo pigmentosa is rare, it often is misdiagnosed as another condition with a similar presentation and nonspecific biopsy findings.6 Allergic contact dermatitis is a common type IV delayed hypersensitivity reaction that manifests similar to prurigo pigmentosa with pruritus and a well-demarcated distribution10 that is related to the pattern of allergen exposure; in the case of allergic contact dermatitis related to textiles, a well-demarcated rash will appear in the distribution area of the associated clothing (eg, shirt, pants, shorts).11 Development of allergy involves exposure and sensitization to an allergen, followed by subsequent re-exposure that results in cutaneous T-cell activation and inflammation. 10 Histopathology shows nonspecific spongiotic inflammation, and the gold standard for diagnosis is patch testing to identify the causative substance(s). Definitive treatment includes avoidance of identified allergies; however, if patients are unable to avoid the allergen or the cause is unknown, then corticosteroids, antihistamines, and/or calcineurin inhibitors are beneficial in controlling symptoms and flares.10
Pityrosporum folliculitis (also known as Malassezia folliculitis) is a fungal acneform condition that arises from overgrowth of normal skin flora Malassezia yeast,12 which may be due to occlusion of follicles or disruption of the normal flora composition. Clinically, the manifestation may resemble prurigo pigmentosa in distribution and presence of intense pruritus. However, pustular lesions and involvement of the face can aid in differentiating Pityrosporum from prurigo pigmentosa, which can be confirmed via periodic acid–Schiff staining with numerous round yeasts within affected follicles. Oral antifungal therapy typically yields rapid improvement and resolution of symptoms.12
Urticaria and prurigo pigmentosa share similar clinical characteristics, with symptoms of intense pruritus and urticarial lesions on the trunk.2,13 Urticaria is an IgEmediated type I hypersensitivity reaction characterized by wheals (ie, edematous red or pink lesions of variable size and shape that typically resolve spontaneously within 24–48 hours).13 Notably, urticaria will improve and in some cases completely resolve with antihistamines or anti-IgE antibody treatment, which may aid in distinguishing it from prurigo pigmentosa, as the latter typically exhibits limited response to such treatment.2 Histopathology also can assist in the diagnosis by ruling out other causes of similar rash; however, biopsies are not routinely done unless other inflammatory conditions are of high suspicion.13
Bullous pemphigoid is an autoimmune, subepidermal, blistering dermatosis that is most common among the elderly.14 It is characterized by the presence of IgG antibodies that target BP180 and BP230, which initiate inflammatory cascades that lead to tissue damage and blister formation. It typically manifests as pruritic blistering eruptions, primarily on the limbs and trunk, but may involve the head, neck, or palmoplantar regions.14 Although blistering eruptions are the prodrome of the disease, some cases may present with nonspecific urticarial or eczematous lesions14,15 that may resemble prurigo pigmentosa. The diagnosis is confirmed through direct immunofluorescence microscopy of biopsied lesions, which reveals IgG and/or C3 deposits along the dermoepidermal junction.14 Management of bullous pemphigoid involves timely initiation of dapsone or systemic corticosteroids, which have demonstrated high efficacy in controlling the disease and its associated symptoms.15
Our patient achieved a favorable response to diet modification and doxycycline therapy consistent with the diagnosis of prurigo pigmentosa. Unfortunately, the condition recurred following a relapse of anorexia. Management of prurigo pigmentosa necessitates not only accurate diagnosis but also addressing any underlying factors that may contribute to disease exacerbation. We anticipate the eating disorder will pose a major challenge in achieving long-term control of prurigo pigmentosa.
The Diagnosis: Prurigo Pigmentosa
A comprehensive metabolic panel collected from our patient 1 month earlier did not reveal any abnormalities. Serum methylmalonic acid and homocysteine were both elevated at 417 nmol/L (reference range [for those aged 2–59 years], 55–335 nmol/L) and 23 μmol/L (reference range, 5–15 μmol/L), respectively. Serum folate and 25-hydroxyvitamin D were low at 3.1 ng/mL (reference range, >4.8 ng/mL) and 5 ng/mL (reference range, 30–80 ng/mL), respectively. Vitamin B12 was within reference range. Two 4-mm punch biopsies collected from the upper back showed spongiotic dermatitis.
Our patient’s histopathology results along with the rash distribution and medical history of anorexia increased suspicion for prurigo pigmentosa. A trial of oral doxycycline 100 mg twice daily for 2 weeks was prescribed. At 2-week follow-up, the patient’s mother revealed a history of ketosis in her daughter, solidifying the diagnosis. The patient was counseled on maintaining a healthy diet to prevent future breakouts. The patient’s rash resolved with diet modification and doxycycline; however, it recurred upon relapse of anorexia 4 months later.
Prurigo pigmentosa, originally identified in Japan by Nagashima et al,1 is an uncommon recurrent inflammatory disorder predominantly observed in young adults of Asian descent. Subsequently, it was reported to occur among individuals from different ethnic backgrounds, indicating potential underdiagnosis or misdiagnosis in Western countries.2 Although a direct pathogenic cause for prurigo pigmentosa has not been identified, a strong association has been linked to diet, specifically when ketosis is induced, such as in ketogenic diets and anorexia nervosa.3-5 Other possible causes include sunlight exposure, clothing friction, and sweating.1,5 The disease course is characterized by intermittent flares and spontaneous resolution, with recurrence in most cases. During the active phase, intensely pruritic, papulovesicular or urticarial papules are predominant and most often are localized to the upper body and torso, including the back, shoulders, neck, and chest.5 These flares can persist for several days but eventually subside, leaving behind a characteristic reticular pigmentation that can persist for months.5 First-line treatment often involves the use of tetracycline antibiotics, such as minocycline or doxycycline. 2,4,5 Dapsone often is used with successful resolution. 6 Dietary modifications also have been found to be effective in treating prurigo pigmentosa, particularly in patients presenting with dietary insufficiency.6,7 Increased carbohydrate intake has been shown to promote resolution. 6 Topical corticosteroids demonstrate limited efficacy in controlling flares.6,8
Histopathology has been variably described, with initial findings reported as nonspecific.1 However, it was later described as a distinct inflammatory disease of the skin with histologically distinct stages.2,9 Early stages reveal scattered dermal, dermal papillary, and perivascular neutrophilic infiltration.9 The lesions then progress and become fully developed, at which point neutrophilic infiltration becomes more prominent, accompanied by the presence of intraepidermal neutrophils and spongiosis. As the lesions resolve, the infiltration transitions to lymphocytic, and lichenoid changes can sometimes be appreciated along with epidermal hyperplasia, hyperpigmentation, and dermal melanophages.9 Although these findings aid in the diagnosis of prurigo pigmentosa, a clinicopathologic correlation is necessary to establish a definitive diagnosis.
Because prurigo pigmentosa is rare, it often is misdiagnosed as another condition with a similar presentation and nonspecific biopsy findings.6 Allergic contact dermatitis is a common type IV delayed hypersensitivity reaction that manifests similar to prurigo pigmentosa with pruritus and a well-demarcated distribution10 that is related to the pattern of allergen exposure; in the case of allergic contact dermatitis related to textiles, a well-demarcated rash will appear in the distribution area of the associated clothing (eg, shirt, pants, shorts).11 Development of allergy involves exposure and sensitization to an allergen, followed by subsequent re-exposure that results in cutaneous T-cell activation and inflammation. 10 Histopathology shows nonspecific spongiotic inflammation, and the gold standard for diagnosis is patch testing to identify the causative substance(s). Definitive treatment includes avoidance of identified allergies; however, if patients are unable to avoid the allergen or the cause is unknown, then corticosteroids, antihistamines, and/or calcineurin inhibitors are beneficial in controlling symptoms and flares.10
Pityrosporum folliculitis (also known as Malassezia folliculitis) is a fungal acneform condition that arises from overgrowth of normal skin flora Malassezia yeast,12 which may be due to occlusion of follicles or disruption of the normal flora composition. Clinically, the manifestation may resemble prurigo pigmentosa in distribution and presence of intense pruritus. However, pustular lesions and involvement of the face can aid in differentiating Pityrosporum from prurigo pigmentosa, which can be confirmed via periodic acid–Schiff staining with numerous round yeasts within affected follicles. Oral antifungal therapy typically yields rapid improvement and resolution of symptoms.12
Urticaria and prurigo pigmentosa share similar clinical characteristics, with symptoms of intense pruritus and urticarial lesions on the trunk.2,13 Urticaria is an IgEmediated type I hypersensitivity reaction characterized by wheals (ie, edematous red or pink lesions of variable size and shape that typically resolve spontaneously within 24–48 hours).13 Notably, urticaria will improve and in some cases completely resolve with antihistamines or anti-IgE antibody treatment, which may aid in distinguishing it from prurigo pigmentosa, as the latter typically exhibits limited response to such treatment.2 Histopathology also can assist in the diagnosis by ruling out other causes of similar rash; however, biopsies are not routinely done unless other inflammatory conditions are of high suspicion.13
Bullous pemphigoid is an autoimmune, subepidermal, blistering dermatosis that is most common among the elderly.14 It is characterized by the presence of IgG antibodies that target BP180 and BP230, which initiate inflammatory cascades that lead to tissue damage and blister formation. It typically manifests as pruritic blistering eruptions, primarily on the limbs and trunk, but may involve the head, neck, or palmoplantar regions.14 Although blistering eruptions are the prodrome of the disease, some cases may present with nonspecific urticarial or eczematous lesions14,15 that may resemble prurigo pigmentosa. The diagnosis is confirmed through direct immunofluorescence microscopy of biopsied lesions, which reveals IgG and/or C3 deposits along the dermoepidermal junction.14 Management of bullous pemphigoid involves timely initiation of dapsone or systemic corticosteroids, which have demonstrated high efficacy in controlling the disease and its associated symptoms.15
Our patient achieved a favorable response to diet modification and doxycycline therapy consistent with the diagnosis of prurigo pigmentosa. Unfortunately, the condition recurred following a relapse of anorexia. Management of prurigo pigmentosa necessitates not only accurate diagnosis but also addressing any underlying factors that may contribute to disease exacerbation. We anticipate the eating disorder will pose a major challenge in achieving long-term control of prurigo pigmentosa.
- Nagashima M, Ohshiro A, Shimizu N. A peculiar pruriginous dermatosis with gross reticular pigmentation. Jpn J Dermatol. 1971;81:38-39.
- Boer A, Asgari M. Prurigo pigmentosa: an underdiagnosed disease? Indian J Dermatol Venereol Leprol. 2006;72:405-409. doi:10.4103/0378-6323.29334
- Michaels JD, Hoss E, DiCaudo DJ, et al. Prurigo pigmentosa after a strict ketogenic diet. Pediatr Dermatol. 2013;32:248-251. doi:10.1111/pde.12275
- Teraki Y, Teraki E, Kawashima M, et al. Ketosis is involved in the origin of prurigo pigmentosa. J Am Acad Dermatol. 1996;34:509-511. doi:10.1016/s0190-9622(96)90460-0
- Böer A, Misago N, Wolter M, et al. Prurigo pigmentosa: a distinctive inflammatory disease of the skin. Am J Dermatopathol. 2003;25:117-129. doi:10.1097/00000372-200304000-00005
- Mufti A, Mirali S, Abduelmula A, et al. Clinical manifestations and treatment outcomes in prurigo pigmentosa (Nagashima disease): a systematic review of the literature. JAAD Int. 2021;3:79-87. doi:10.1016/j.jdin.2021.03.003
- Wong M, Lee E, Wu Y, et al. Treatment of prurigo pigmentosa with diet modification: a medical case study. Hawaii J Med Public Health. 2018;77:114-117.
- Almaani N, Al-Tarawneh AH, Msallam H. Prurigo pigmentosa: a clinicopathological report of three Middle Eastern patients. Case Rep Dermatol Med. 2018;2018:9406797. doi:10.1155/2018/9406797
- Kim JK, Chung WK, Chang SE, et al. Prurigo pigmentosa: clinicopathological study and analysis of 50 cases in Korea. J Dermatol. 2012;39:891-897. doi:10.1111/j.1346-8138.2012.01640.x
- Mowad CM, Anderson B, Scheinman P, et al. Allergic contact dermatitis: patient diagnosis and evaluation. J Am Acad Dermatol. 2016;74:1029-1040. doi:10.1016/j.jaad.2015.02.1139
- Lazarov A, Cordoba M, Plosk N, et al. Atypical and unusual clinical manifestations of contact dermatitis to clothing (textile contact dermatitis)—case presentation and review of the literature. Dermatol Online J. 2003;9. doi:10.5070/d30kd1d259
- Rubenstein RM, Malerich SA. Malassezia (Pityrosporum) folliculitis. J Clin Aesthet Dermatol. 2014;7:37-41.
- Bernstein JA, Lang DM, Khan DA, et al. The diagnosis and management of acute and chronic urticaria: 2014 update. J Allergy Clin Immunol. 2014;133:1270-1277. doi:10.1016/j.jaci.2014.02.036
- della Torre R, Combescure C, Cortés B, et al. Clinical presentation and diagnostic delay in bullous pemphigoid: a prospective nationwide cohort. Br J Dermatol. 2012;167:1111-1117. doi:10.1111/j.1365-2133.2012.11108.x
- Alonso-Llamazares J, Rogers RS 3rd, Oursler JR, et al. Bullous pemphigoid presenting as generalized pruritus: observations in six patients. Int J Dermatol. 1998;37:508-514.
- Nagashima M, Ohshiro A, Shimizu N. A peculiar pruriginous dermatosis with gross reticular pigmentation. Jpn J Dermatol. 1971;81:38-39.
- Boer A, Asgari M. Prurigo pigmentosa: an underdiagnosed disease? Indian J Dermatol Venereol Leprol. 2006;72:405-409. doi:10.4103/0378-6323.29334
- Michaels JD, Hoss E, DiCaudo DJ, et al. Prurigo pigmentosa after a strict ketogenic diet. Pediatr Dermatol. 2013;32:248-251. doi:10.1111/pde.12275
- Teraki Y, Teraki E, Kawashima M, et al. Ketosis is involved in the origin of prurigo pigmentosa. J Am Acad Dermatol. 1996;34:509-511. doi:10.1016/s0190-9622(96)90460-0
- Böer A, Misago N, Wolter M, et al. Prurigo pigmentosa: a distinctive inflammatory disease of the skin. Am J Dermatopathol. 2003;25:117-129. doi:10.1097/00000372-200304000-00005
- Mufti A, Mirali S, Abduelmula A, et al. Clinical manifestations and treatment outcomes in prurigo pigmentosa (Nagashima disease): a systematic review of the literature. JAAD Int. 2021;3:79-87. doi:10.1016/j.jdin.2021.03.003
- Wong M, Lee E, Wu Y, et al. Treatment of prurigo pigmentosa with diet modification: a medical case study. Hawaii J Med Public Health. 2018;77:114-117.
- Almaani N, Al-Tarawneh AH, Msallam H. Prurigo pigmentosa: a clinicopathological report of three Middle Eastern patients. Case Rep Dermatol Med. 2018;2018:9406797. doi:10.1155/2018/9406797
- Kim JK, Chung WK, Chang SE, et al. Prurigo pigmentosa: clinicopathological study and analysis of 50 cases in Korea. J Dermatol. 2012;39:891-897. doi:10.1111/j.1346-8138.2012.01640.x
- Mowad CM, Anderson B, Scheinman P, et al. Allergic contact dermatitis: patient diagnosis and evaluation. J Am Acad Dermatol. 2016;74:1029-1040. doi:10.1016/j.jaad.2015.02.1139
- Lazarov A, Cordoba M, Plosk N, et al. Atypical and unusual clinical manifestations of contact dermatitis to clothing (textile contact dermatitis)—case presentation and review of the literature. Dermatol Online J. 2003;9. doi:10.5070/d30kd1d259
- Rubenstein RM, Malerich SA. Malassezia (Pityrosporum) folliculitis. J Clin Aesthet Dermatol. 2014;7:37-41.
- Bernstein JA, Lang DM, Khan DA, et al. The diagnosis and management of acute and chronic urticaria: 2014 update. J Allergy Clin Immunol. 2014;133:1270-1277. doi:10.1016/j.jaci.2014.02.036
- della Torre R, Combescure C, Cortés B, et al. Clinical presentation and diagnostic delay in bullous pemphigoid: a prospective nationwide cohort. Br J Dermatol. 2012;167:1111-1117. doi:10.1111/j.1365-2133.2012.11108.x
- Alonso-Llamazares J, Rogers RS 3rd, Oursler JR, et al. Bullous pemphigoid presenting as generalized pruritus: observations in six patients. Int J Dermatol. 1998;37:508-514.
A 43-year-old woman presented with a pruritic rash across the neck and back of 6 months’ duration that progressively worsened. She had a medical history of anorexia nervosa, herpes zoster with a recent flare, and peripheral neuropathy. Physical examination showed numerous red scaly papules across the upper back and shoulders that coalesced in a reticular pattern. No similar papules were seen elsewhere on the body.
Advantages of a Pediatric Rheumatology/Dermatology Clinic Evaluated
results from a retrospective cohort study showed.
“This finding highlights the complexity of patients referred to this clinic,” the study’s first author, Jessica Crockett, a fourth-year medical student at UCSF, told this news organization following the annual meeting of the Society for Pediatric Dermatology, where the study was presented during a poster session. “Integrated care models such as rheumatology/dermatology clinics (RDCs) have been shown to facilitate complete clinical evaluations, establish new or revised diagnoses, and streamline care for adult patients with complex autoimmune skin diseases. However, few pediatric RDCs exist nationwide, and data therefore is quite limited.”
To advance the understanding of pediatric RDC practice patterns, the influence of the care model on patient care, and professional development for trainees and clinicians, Ms. Crockett collaborated with senior author Kelly Cordoro, MD, professor of dermatology and pediatrics at UCSF, and colleagues to evaluate a cohort of 71 patients who received care at the UCSF pediatric RDC. The clinic, which was launched in 2017, includes two dermatologists, two rheumatologists, trainees, a social worker, and a nurse. Team members participate in a preclinic conference to review patient data and images, discuss relevant literature, and develop an approach to each patient.
In a separate part of the study, the researchers distributed a survey to 17 pediatric dermatologists who participate in unique RDCs in North America. Respondents were asked to describe the variability of clinical operations, participants, administrative/clinical support, and educational value for participating physicians and trainees.
Of the 71 patients cared for at the UCSF pediatric RDC, 69% were female, 44% were White, 51% were aged 13-21 years, 42% were aged 3-12 years, and 7% were aged 0-11 years at their first clinic visit. The top four primary RDC diagnoses were linear morphea (33%), lupus (23%), psoriasis (13%), and juvenile dermatomyositis (10%).
Nearly one in four patients (17, or 24%) presented to the RDC without a confirmed diagnosis. A diagnosis was established at the first RDC visit for 7 of these 17 patients (41%). Among 54 patients who presented with an established diagnosis, the first RDC visit confirmed the diagnosis for 52 (96%) and revised it for 2 (4%). “Initial pediatric RDC evaluation significantly influenced patient care by confirming or revising preexisting diagnoses, rendering new diagnoses, and streamlining additional laboratory and imaging recommendations,” the researchers wrote in their poster.
The evaluation also resulted in modified disease management in the form of systemic medication changes or dosage adjustments as well as the initiation of novel therapies. For example, systemic medication changes were made during the first RDC visit in 34 of the 46 patients (74%) who were on systemic medication at presentation.
“Seeing complex patients together in real time allows specialists and other team members (social work, nursing, PT/OT, for example) to share ideas, communicate clearly to families, and efficiently develop recommendations,” Ms. Crockett said of the UCSF pediatric RDC. “Exposure to other specialists while caring for patients enhances medical knowledge, communication skills, and professional competency of faculty and trainees alike.”
In the survey portion of the study, each of the 17 dermatologists reported that the pediatric RDC is valuable for patient care, and 88% believed the RDC was a valuable use of their time. However, only 59% of respondents reported having administrative support, and only 29% had a dedicated clinic coordinator or navigator.
“We were surprised to find that only a quarter of pediatric RDCs incorporate an educational conference,” Dr. Cordoro told this news organization. “We have found that assembling the care team prior to seeing patients to review clinical data, discuss relevant literature, and define the clinical questions for each patient is an integral part of the clinical operation. The trainees are involved in these conference presentations, and it really enhances their understanding of the complex diagnoses we manage in this clinic and the issues faced by affected children and families. The preclinical conference increases efficiency, positively influences patient care, and supports professional development for all participants.”
The study was indirectly supported by a fellowship grant awarded to Ms. Crockett from the Pediatric Dermatology Research Alliance. The researchers reported having no relevant disclosures.
A version of this article appeared on Medscape.com.
results from a retrospective cohort study showed.
“This finding highlights the complexity of patients referred to this clinic,” the study’s first author, Jessica Crockett, a fourth-year medical student at UCSF, told this news organization following the annual meeting of the Society for Pediatric Dermatology, where the study was presented during a poster session. “Integrated care models such as rheumatology/dermatology clinics (RDCs) have been shown to facilitate complete clinical evaluations, establish new or revised diagnoses, and streamline care for adult patients with complex autoimmune skin diseases. However, few pediatric RDCs exist nationwide, and data therefore is quite limited.”
To advance the understanding of pediatric RDC practice patterns, the influence of the care model on patient care, and professional development for trainees and clinicians, Ms. Crockett collaborated with senior author Kelly Cordoro, MD, professor of dermatology and pediatrics at UCSF, and colleagues to evaluate a cohort of 71 patients who received care at the UCSF pediatric RDC. The clinic, which was launched in 2017, includes two dermatologists, two rheumatologists, trainees, a social worker, and a nurse. Team members participate in a preclinic conference to review patient data and images, discuss relevant literature, and develop an approach to each patient.
In a separate part of the study, the researchers distributed a survey to 17 pediatric dermatologists who participate in unique RDCs in North America. Respondents were asked to describe the variability of clinical operations, participants, administrative/clinical support, and educational value for participating physicians and trainees.
Of the 71 patients cared for at the UCSF pediatric RDC, 69% were female, 44% were White, 51% were aged 13-21 years, 42% were aged 3-12 years, and 7% were aged 0-11 years at their first clinic visit. The top four primary RDC diagnoses were linear morphea (33%), lupus (23%), psoriasis (13%), and juvenile dermatomyositis (10%).
Nearly one in four patients (17, or 24%) presented to the RDC without a confirmed diagnosis. A diagnosis was established at the first RDC visit for 7 of these 17 patients (41%). Among 54 patients who presented with an established diagnosis, the first RDC visit confirmed the diagnosis for 52 (96%) and revised it for 2 (4%). “Initial pediatric RDC evaluation significantly influenced patient care by confirming or revising preexisting diagnoses, rendering new diagnoses, and streamlining additional laboratory and imaging recommendations,” the researchers wrote in their poster.
The evaluation also resulted in modified disease management in the form of systemic medication changes or dosage adjustments as well as the initiation of novel therapies. For example, systemic medication changes were made during the first RDC visit in 34 of the 46 patients (74%) who were on systemic medication at presentation.
“Seeing complex patients together in real time allows specialists and other team members (social work, nursing, PT/OT, for example) to share ideas, communicate clearly to families, and efficiently develop recommendations,” Ms. Crockett said of the UCSF pediatric RDC. “Exposure to other specialists while caring for patients enhances medical knowledge, communication skills, and professional competency of faculty and trainees alike.”
In the survey portion of the study, each of the 17 dermatologists reported that the pediatric RDC is valuable for patient care, and 88% believed the RDC was a valuable use of their time. However, only 59% of respondents reported having administrative support, and only 29% had a dedicated clinic coordinator or navigator.
“We were surprised to find that only a quarter of pediatric RDCs incorporate an educational conference,” Dr. Cordoro told this news organization. “We have found that assembling the care team prior to seeing patients to review clinical data, discuss relevant literature, and define the clinical questions for each patient is an integral part of the clinical operation. The trainees are involved in these conference presentations, and it really enhances their understanding of the complex diagnoses we manage in this clinic and the issues faced by affected children and families. The preclinical conference increases efficiency, positively influences patient care, and supports professional development for all participants.”
The study was indirectly supported by a fellowship grant awarded to Ms. Crockett from the Pediatric Dermatology Research Alliance. The researchers reported having no relevant disclosures.
A version of this article appeared on Medscape.com.
results from a retrospective cohort study showed.
“This finding highlights the complexity of patients referred to this clinic,” the study’s first author, Jessica Crockett, a fourth-year medical student at UCSF, told this news organization following the annual meeting of the Society for Pediatric Dermatology, where the study was presented during a poster session. “Integrated care models such as rheumatology/dermatology clinics (RDCs) have been shown to facilitate complete clinical evaluations, establish new or revised diagnoses, and streamline care for adult patients with complex autoimmune skin diseases. However, few pediatric RDCs exist nationwide, and data therefore is quite limited.”
To advance the understanding of pediatric RDC practice patterns, the influence of the care model on patient care, and professional development for trainees and clinicians, Ms. Crockett collaborated with senior author Kelly Cordoro, MD, professor of dermatology and pediatrics at UCSF, and colleagues to evaluate a cohort of 71 patients who received care at the UCSF pediatric RDC. The clinic, which was launched in 2017, includes two dermatologists, two rheumatologists, trainees, a social worker, and a nurse. Team members participate in a preclinic conference to review patient data and images, discuss relevant literature, and develop an approach to each patient.
In a separate part of the study, the researchers distributed a survey to 17 pediatric dermatologists who participate in unique RDCs in North America. Respondents were asked to describe the variability of clinical operations, participants, administrative/clinical support, and educational value for participating physicians and trainees.
Of the 71 patients cared for at the UCSF pediatric RDC, 69% were female, 44% were White, 51% were aged 13-21 years, 42% were aged 3-12 years, and 7% were aged 0-11 years at their first clinic visit. The top four primary RDC diagnoses were linear morphea (33%), lupus (23%), psoriasis (13%), and juvenile dermatomyositis (10%).
Nearly one in four patients (17, or 24%) presented to the RDC without a confirmed diagnosis. A diagnosis was established at the first RDC visit for 7 of these 17 patients (41%). Among 54 patients who presented with an established diagnosis, the first RDC visit confirmed the diagnosis for 52 (96%) and revised it for 2 (4%). “Initial pediatric RDC evaluation significantly influenced patient care by confirming or revising preexisting diagnoses, rendering new diagnoses, and streamlining additional laboratory and imaging recommendations,” the researchers wrote in their poster.
The evaluation also resulted in modified disease management in the form of systemic medication changes or dosage adjustments as well as the initiation of novel therapies. For example, systemic medication changes were made during the first RDC visit in 34 of the 46 patients (74%) who were on systemic medication at presentation.
“Seeing complex patients together in real time allows specialists and other team members (social work, nursing, PT/OT, for example) to share ideas, communicate clearly to families, and efficiently develop recommendations,” Ms. Crockett said of the UCSF pediatric RDC. “Exposure to other specialists while caring for patients enhances medical knowledge, communication skills, and professional competency of faculty and trainees alike.”
In the survey portion of the study, each of the 17 dermatologists reported that the pediatric RDC is valuable for patient care, and 88% believed the RDC was a valuable use of their time. However, only 59% of respondents reported having administrative support, and only 29% had a dedicated clinic coordinator or navigator.
“We were surprised to find that only a quarter of pediatric RDCs incorporate an educational conference,” Dr. Cordoro told this news organization. “We have found that assembling the care team prior to seeing patients to review clinical data, discuss relevant literature, and define the clinical questions for each patient is an integral part of the clinical operation. The trainees are involved in these conference presentations, and it really enhances their understanding of the complex diagnoses we manage in this clinic and the issues faced by affected children and families. The preclinical conference increases efficiency, positively influences patient care, and supports professional development for all participants.”
The study was indirectly supported by a fellowship grant awarded to Ms. Crockett from the Pediatric Dermatology Research Alliance. The researchers reported having no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM SPD 2024
Underserved Families Share Ways to Improve Access to Pediatric Dermatologists
, a theme emerged that surprised lead study author Lucinda L. Kohn, MD, MHS.
“Most families said that racial concordance didn’t matter that much, but they did place high value on being heard,” Dr. Kohn, of the Department of Dermatology at the University of Colorado, Aurora, told this news organization following the Society for Pediatric Dermatology annual meeting, where the study was presented during a poster session. “Being heard means that their experience was respected; that their questions and worries were anticipated, addressed, and answered; and that their feelings were acknowledged.”
As a way to understand these families’ knowledge, attitudes, and beliefs about access to pediatric dermatology care and how the hospital system and medical team could better support them, Dr. Kohn and colleagues conducted in-depth, semi-structured interviews with 32 English-speaking parents and/or guardians of children who received care at the Children’s Hospital Colorado Anschutz Medical Campus pediatric dermatology clinic. The researchers conducted and recorded the 30- to 60-minute interviews via Zoom or phone call from October 17, 2023, to January 23, 2024. Domains of interest included participant background and experiences, communication preferences, and experience accessing pediatric dermatology care. Next, Dr. Kohn and colleagues used a reflexive, team-based inductive approach to carry out a thematic analysis from the interviews.
The mean age of the 32 study participants was 38.9 years; 14 (43.75%) identified as Hispanic, 11 (34.38%) as Black, and 12 (37.50%) as American Indian/Alaska Native (response categories were not mutually exclusive). Several themes emerged from analysis of the interviews. Barriers to receiving pediatric dermatology care included distrust of the healthcare system, generational and community lack of awareness about dermatology, distance to the hospital, and household income.
“One family mentioned that they needed to save up for 3 months to be able to afford the drive, hotel, and food needed for their child to attend their pediatric dermatology visit,” Dr. Kohn said. “As we know, most pediatric dermatology visits are 10-15 minutes long, so that they needed to cut groceries for 3 months to be able to see a pediatric dermatologist for 10-15 minutes is just heart wrenching. Families also didn’t understand the large teams that we have in medicine: The medical students, residents, nurses, medical assistants, attendings, and physician extenders.”
One key facilitator to receiving pediatric dermatology care was the family’s perception that the provider shares their minoritized experience because of similarities in skin tone. “When it’s your own race, whether it’s Black, Hispanic, or you know, we feel like when it’s someone like me, they will look out for me more,” one study participant said. Other facilitators expressed by the study participants included increased representation from the family’s community at all levels of healthcare (“the more you see providers and people in a space that look like you, I think the more welcoming it will feel,” one said) and normalizing dermatology care (“letting it be known that going to the dermatologist is just like going to a regular doctor,” another said).
Dr. Kohn acknowledged certain limitations of the study, including its single-center qualitative design. “Qualitative studies are not generalizable, but they do dive into the lived experiences of a few,” she said. “There aren’t a lot of qualitative studies in derm, so even though this was a very simple study, we hope the findings will help us to support our most diverse and underserved families access the pediatric dermatology care that they need.”
The researchers reported having no relevant financial disclosures. The study was recognized as an award-winning poster at the meeting.
A version of this article appeared on Medscape.com.
, a theme emerged that surprised lead study author Lucinda L. Kohn, MD, MHS.
“Most families said that racial concordance didn’t matter that much, but they did place high value on being heard,” Dr. Kohn, of the Department of Dermatology at the University of Colorado, Aurora, told this news organization following the Society for Pediatric Dermatology annual meeting, where the study was presented during a poster session. “Being heard means that their experience was respected; that their questions and worries were anticipated, addressed, and answered; and that their feelings were acknowledged.”
As a way to understand these families’ knowledge, attitudes, and beliefs about access to pediatric dermatology care and how the hospital system and medical team could better support them, Dr. Kohn and colleagues conducted in-depth, semi-structured interviews with 32 English-speaking parents and/or guardians of children who received care at the Children’s Hospital Colorado Anschutz Medical Campus pediatric dermatology clinic. The researchers conducted and recorded the 30- to 60-minute interviews via Zoom or phone call from October 17, 2023, to January 23, 2024. Domains of interest included participant background and experiences, communication preferences, and experience accessing pediatric dermatology care. Next, Dr. Kohn and colleagues used a reflexive, team-based inductive approach to carry out a thematic analysis from the interviews.
The mean age of the 32 study participants was 38.9 years; 14 (43.75%) identified as Hispanic, 11 (34.38%) as Black, and 12 (37.50%) as American Indian/Alaska Native (response categories were not mutually exclusive). Several themes emerged from analysis of the interviews. Barriers to receiving pediatric dermatology care included distrust of the healthcare system, generational and community lack of awareness about dermatology, distance to the hospital, and household income.
“One family mentioned that they needed to save up for 3 months to be able to afford the drive, hotel, and food needed for their child to attend their pediatric dermatology visit,” Dr. Kohn said. “As we know, most pediatric dermatology visits are 10-15 minutes long, so that they needed to cut groceries for 3 months to be able to see a pediatric dermatologist for 10-15 minutes is just heart wrenching. Families also didn’t understand the large teams that we have in medicine: The medical students, residents, nurses, medical assistants, attendings, and physician extenders.”
One key facilitator to receiving pediatric dermatology care was the family’s perception that the provider shares their minoritized experience because of similarities in skin tone. “When it’s your own race, whether it’s Black, Hispanic, or you know, we feel like when it’s someone like me, they will look out for me more,” one study participant said. Other facilitators expressed by the study participants included increased representation from the family’s community at all levels of healthcare (“the more you see providers and people in a space that look like you, I think the more welcoming it will feel,” one said) and normalizing dermatology care (“letting it be known that going to the dermatologist is just like going to a regular doctor,” another said).
Dr. Kohn acknowledged certain limitations of the study, including its single-center qualitative design. “Qualitative studies are not generalizable, but they do dive into the lived experiences of a few,” she said. “There aren’t a lot of qualitative studies in derm, so even though this was a very simple study, we hope the findings will help us to support our most diverse and underserved families access the pediatric dermatology care that they need.”
The researchers reported having no relevant financial disclosures. The study was recognized as an award-winning poster at the meeting.
A version of this article appeared on Medscape.com.
, a theme emerged that surprised lead study author Lucinda L. Kohn, MD, MHS.
“Most families said that racial concordance didn’t matter that much, but they did place high value on being heard,” Dr. Kohn, of the Department of Dermatology at the University of Colorado, Aurora, told this news organization following the Society for Pediatric Dermatology annual meeting, where the study was presented during a poster session. “Being heard means that their experience was respected; that their questions and worries were anticipated, addressed, and answered; and that their feelings were acknowledged.”
As a way to understand these families’ knowledge, attitudes, and beliefs about access to pediatric dermatology care and how the hospital system and medical team could better support them, Dr. Kohn and colleagues conducted in-depth, semi-structured interviews with 32 English-speaking parents and/or guardians of children who received care at the Children’s Hospital Colorado Anschutz Medical Campus pediatric dermatology clinic. The researchers conducted and recorded the 30- to 60-minute interviews via Zoom or phone call from October 17, 2023, to January 23, 2024. Domains of interest included participant background and experiences, communication preferences, and experience accessing pediatric dermatology care. Next, Dr. Kohn and colleagues used a reflexive, team-based inductive approach to carry out a thematic analysis from the interviews.
The mean age of the 32 study participants was 38.9 years; 14 (43.75%) identified as Hispanic, 11 (34.38%) as Black, and 12 (37.50%) as American Indian/Alaska Native (response categories were not mutually exclusive). Several themes emerged from analysis of the interviews. Barriers to receiving pediatric dermatology care included distrust of the healthcare system, generational and community lack of awareness about dermatology, distance to the hospital, and household income.
“One family mentioned that they needed to save up for 3 months to be able to afford the drive, hotel, and food needed for their child to attend their pediatric dermatology visit,” Dr. Kohn said. “As we know, most pediatric dermatology visits are 10-15 minutes long, so that they needed to cut groceries for 3 months to be able to see a pediatric dermatologist for 10-15 minutes is just heart wrenching. Families also didn’t understand the large teams that we have in medicine: The medical students, residents, nurses, medical assistants, attendings, and physician extenders.”
One key facilitator to receiving pediatric dermatology care was the family’s perception that the provider shares their minoritized experience because of similarities in skin tone. “When it’s your own race, whether it’s Black, Hispanic, or you know, we feel like when it’s someone like me, they will look out for me more,” one study participant said. Other facilitators expressed by the study participants included increased representation from the family’s community at all levels of healthcare (“the more you see providers and people in a space that look like you, I think the more welcoming it will feel,” one said) and normalizing dermatology care (“letting it be known that going to the dermatologist is just like going to a regular doctor,” another said).
Dr. Kohn acknowledged certain limitations of the study, including its single-center qualitative design. “Qualitative studies are not generalizable, but they do dive into the lived experiences of a few,” she said. “There aren’t a lot of qualitative studies in derm, so even though this was a very simple study, we hope the findings will help us to support our most diverse and underserved families access the pediatric dermatology care that they need.”
The researchers reported having no relevant financial disclosures. The study was recognized as an award-winning poster at the meeting.
A version of this article appeared on Medscape.com.
FROM SPD 2024
Study Finds Gout Drug Effective for Aphthous Ulcers in Children
“Complex aphthous stomatitis in children is typically treated with topical supportive care, which is often not effective,” one of the study investigators, Ananya Shah, a third-year medical student at the University of Rochester School of Medicine & Dentistry, Rochester, New York, told this news organization following the Society for Pediatric Dermatology annual meeting, where the study was presented during a poster session. “There is limited research on CAS and its treatment in children. Colchicine is often used for treatment of CAS in adults, but its use in children has not been studied.”
Ms. Shah, in collaboration with Hilary Kunkel, MD, Nessa Aghazadeh, MD, and Megha Tollefson, MD, of the Department of Dermatology, Mayo Clinic, Rochester, Minnesota, retrospectively reviewed the charts of 20 children diagnosed with CAS who were treated with colchicine, an anti-inflammatory drug often used to treat gout, at the clinic between 2000 and 2023. Treatment responses were defined as no response, partial response, and complete response. Half of the patients were girls, and their median age at presentation was 5 years.
Ulcers were most commonly located in the buccal mucosa (80%), followed by the gingiva (50%), the mucosal lip (50%), and the palate (40%). Nearly all patients (95%) reported that the CAS caused difficulties with eating or drinking. Other effects on their quality of life included weight loss (35%), bleeding (30%), and difficulty brushing teeth (25%). “I was surprised by how much CAS impacts pediatric patients’ quality of life,” Ms. Shah said. “Almost all of the patients experienced trouble with basic activities of daily living, including eating and drinking. In addition, CAS negatively impacted mental health and led to missed school for patients.”
The researchers had follow-up data on responses to colchicine for 14 of the 20 patients. Of these, 12 (86%) had symptom improvement, 5 (36%) had a complete response, 8 (57%) had a partial response, and 1 (7%) did not respond. Nine patients (64%) experienced side effects. Of these, six had diarrhea, two had nausea, and one had constipation.
“Colchicine should be considered as a treatment in pediatric patients who have refractory complex aphthous stomatitis as it is generally well tolerated with minimal side effects,” Ms. Shah said. She acknowledged certain limitations of the study, including its single-center, retrospective design.
The researchers reported having no relevant disclosures.
A version of this article first appeared on Medscape.com.
“Complex aphthous stomatitis in children is typically treated with topical supportive care, which is often not effective,” one of the study investigators, Ananya Shah, a third-year medical student at the University of Rochester School of Medicine & Dentistry, Rochester, New York, told this news organization following the Society for Pediatric Dermatology annual meeting, where the study was presented during a poster session. “There is limited research on CAS and its treatment in children. Colchicine is often used for treatment of CAS in adults, but its use in children has not been studied.”
Ms. Shah, in collaboration with Hilary Kunkel, MD, Nessa Aghazadeh, MD, and Megha Tollefson, MD, of the Department of Dermatology, Mayo Clinic, Rochester, Minnesota, retrospectively reviewed the charts of 20 children diagnosed with CAS who were treated with colchicine, an anti-inflammatory drug often used to treat gout, at the clinic between 2000 and 2023. Treatment responses were defined as no response, partial response, and complete response. Half of the patients were girls, and their median age at presentation was 5 years.
Ulcers were most commonly located in the buccal mucosa (80%), followed by the gingiva (50%), the mucosal lip (50%), and the palate (40%). Nearly all patients (95%) reported that the CAS caused difficulties with eating or drinking. Other effects on their quality of life included weight loss (35%), bleeding (30%), and difficulty brushing teeth (25%). “I was surprised by how much CAS impacts pediatric patients’ quality of life,” Ms. Shah said. “Almost all of the patients experienced trouble with basic activities of daily living, including eating and drinking. In addition, CAS negatively impacted mental health and led to missed school for patients.”
The researchers had follow-up data on responses to colchicine for 14 of the 20 patients. Of these, 12 (86%) had symptom improvement, 5 (36%) had a complete response, 8 (57%) had a partial response, and 1 (7%) did not respond. Nine patients (64%) experienced side effects. Of these, six had diarrhea, two had nausea, and one had constipation.
“Colchicine should be considered as a treatment in pediatric patients who have refractory complex aphthous stomatitis as it is generally well tolerated with minimal side effects,” Ms. Shah said. She acknowledged certain limitations of the study, including its single-center, retrospective design.
The researchers reported having no relevant disclosures.
A version of this article first appeared on Medscape.com.
“Complex aphthous stomatitis in children is typically treated with topical supportive care, which is often not effective,” one of the study investigators, Ananya Shah, a third-year medical student at the University of Rochester School of Medicine & Dentistry, Rochester, New York, told this news organization following the Society for Pediatric Dermatology annual meeting, where the study was presented during a poster session. “There is limited research on CAS and its treatment in children. Colchicine is often used for treatment of CAS in adults, but its use in children has not been studied.”
Ms. Shah, in collaboration with Hilary Kunkel, MD, Nessa Aghazadeh, MD, and Megha Tollefson, MD, of the Department of Dermatology, Mayo Clinic, Rochester, Minnesota, retrospectively reviewed the charts of 20 children diagnosed with CAS who were treated with colchicine, an anti-inflammatory drug often used to treat gout, at the clinic between 2000 and 2023. Treatment responses were defined as no response, partial response, and complete response. Half of the patients were girls, and their median age at presentation was 5 years.
Ulcers were most commonly located in the buccal mucosa (80%), followed by the gingiva (50%), the mucosal lip (50%), and the palate (40%). Nearly all patients (95%) reported that the CAS caused difficulties with eating or drinking. Other effects on their quality of life included weight loss (35%), bleeding (30%), and difficulty brushing teeth (25%). “I was surprised by how much CAS impacts pediatric patients’ quality of life,” Ms. Shah said. “Almost all of the patients experienced trouble with basic activities of daily living, including eating and drinking. In addition, CAS negatively impacted mental health and led to missed school for patients.”
The researchers had follow-up data on responses to colchicine for 14 of the 20 patients. Of these, 12 (86%) had symptom improvement, 5 (36%) had a complete response, 8 (57%) had a partial response, and 1 (7%) did not respond. Nine patients (64%) experienced side effects. Of these, six had diarrhea, two had nausea, and one had constipation.
“Colchicine should be considered as a treatment in pediatric patients who have refractory complex aphthous stomatitis as it is generally well tolerated with minimal side effects,” Ms. Shah said. She acknowledged certain limitations of the study, including its single-center, retrospective design.
The researchers reported having no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM SPD 2024
Safety Standards a Top Priority for ASLMS President
Arisa E. Ortiz, MD, began her term as president of the American Society for Laser Medicine and Surgery (ASLMS) during the organization’s annual meeting in April 2024.
After earning her medical degree from Albany Medical College, Albany, New York, Dr. Ortiz, a native of Los Angeles, completed her dermatology residency training at the University of California, Irvine, and the university’s Beckman Laser Institute. Next, she completed a laser and cosmetic dermatology fellowship at Massachusetts General Hospital, Harvard Medical School, and the Wellman Center for Photomedicine, all in Boston, and acquired additional fellowship training in Mohs micrographic surgery at the University of California, San Diego (UCSD). Dr. Ortiz is currently director of laser and cosmetic dermatology and a clinical professor of dermatology at UCSD.
She has authored more than 60 publications on new innovations in cutaneous surgery and is a frequent speaker at meetings of the American Academy of Dermatology, the American Society for Dermatologic Surgery (ASDS), and ASLMS, and she cochairs the annual Masters of Aesthetics Symposium in San Diego. Dr. Ortiz has received several awards, including the 2024 Castle Connolly Top Doctor Award and the Exceptional Women in Medicine Award; Newsweek America’s Best Dermatologists; the ASLMS Dr. Horace Furumoto Young Investigator Award, the ASLMS Best of Session Award for Cutaneous Applications, and the ASDS President’s Outstanding Service Award. Her primary research focuses on the laser treatment of nonmelanoma skin cancer.
In an interview, Dr. Ortiz spoke about her goals as ASLMS president and other topics related to dermatology.
Who inspired you most to become a doctor?
I’ve wanted to become a doctor for as long as I can remember. My fascination with science and the idea of helping people improve their health were driving forces. However, my biggest influence early on was my uncle, who was a pediatrician. His dedication and passion for medicine deeply inspired me and solidified my desire to pursue a career in healthcare.
I understand that a bout with chickenpox as a teenager influenced your decision to specialize in dermatology.
It’s an interesting and somewhat humorous story. When I was 18, I contracted chickenpox and ended up with scars on my face. It was a tough experience as a teenager, but it’s fascinating how such events can shape your life. In my quest for help, I opened the Yellow Pages and randomly chose a dermatologist nearby, who turned out to be Gary Lask, MD, director of lasers at UCLA [University of California, Los Angeles]. During our visit, I mentioned that I was premed, and he encouraged me to consider dermatology. About 6 years later, as a second-year medical student, I realized my passion for dermatology. I reached out to Dr. Lask and told him: “You were right. I want to be a dermatologist. Now, you have to help me get in!” Today, he remains my mentor, and I am deeply grateful for his guidance and support on this journey.
One of the initiatives for your term as ASLMS president includes a focus on safety standards for lasers and energy-based devices. Why is this important now?
Working at the university, I frequently encounter severe complications arising from the improper use of lasers and energy-based devices. As these procedures gain popularity, more providers are offering them, yet often without adequate training. As the world’s premier laser society, it is our duty to ensure patient safety. In the ever-evolving field of laser medicine, it is crucial that we continually strive to enhance the regulation of laser usage, ensuring that patients receive the highest standard of care with minimal risk.
One of the suggestions you have for the safety initiative is to offer a rigorous laser safety certification course with continuing education opportunities as a way foster a culture of heightened safety standards. Please explain what would be included in such a course and how it would align with current efforts to report adverse events such as the ASDS-Northwestern University Cutaneous Procedures Adverse Events Reporting (CAPER) registry and the Food and Drug Administration’s MedWatch Program.
A laser safety certification task force has been established to determine the best approach for developing a comprehensive course. The task force aims to assess the necessity of a formal safety certification in our industry, identify the resources needed to support such a certification, establish general safety protocols to form the content foundation, address potential legal concerns, and outline the process for formal certification program recognition. This exploratory work is expected to conclude by the end of the year. The proposed course may include modules on the fundamentals of laser physics, safe operation techniques, patient selection and management, and emergency protocols. Continuing education opportunities would be considered to keep practitioners updated on the latest advancements and safety protocols in laser medicine, thereby fostering a culture of heightened safety standards.
Another initiative for your term is the rollout of a tattoo removal program for former gang members based on the UCSD Clean Slate Tattoo Removal Program. Please tell us more about your vision for this national program.
UCSD Dermatology, in collaboration with UCSD Global Health, has been involved in the Clean Slate Tattoo Removal Program for the past decade. This initiative supports and rehabilitates former gang members by offering laser tattoo removal, helping them reintegrate into society. My vision is to equip our members with the necessary protocols to implement this outreach initiative in their own communities. By providing opportunities for reform and growth, we aim to foster safer and more inclusive communities nationwide.
You were one of the first clinicians to use a laser to treat basal cell carcinoma (BCC). Who are the ideal candidates for this procedure? Is the technique ready for wide clinical adoption? If not, what kind of studies are needed to make it so?
My research passion lies in optimizing laser treatments for BCC. During my fellowship with R. Rox Anderson, MD, and Mathew Avram, MD, at the MGH Wellman Center for Photomedicine, we conducted a pilot study using the 1064-nm Nd:YAG laser, achieving a 92% clearance rate after one treatment. Inspired by these results, we conducted a larger multicenter study, which demonstrated a 90% clearance rate after a single treatment. I now incorporate this technique into my daily practice. The ideal candidates for this procedure are patients with BCC that do not meet the Mohs Appropriate Use Criteria, such as those with nodular or superficial BCC subtypes on the body, individuals who are poor surgical candidates, or those who are surgically exhausted. However, I do not recommend this treatment for patients who are primarily concerned about facial scarring, particularly younger individuals; in such cases, Mohs surgery still remains the preferred option. While I believe this technique is ready for broader clinical adoption, it requires an understanding of laser endpoints. We are also exploring antibody-targeted gold nanorods to enhance the selectivity and standardization of the treatment.
Who inspires you most in your work today?
My patients are my greatest inspiration. Their trust and dedication motivate me to stay at the forefront of dermatologic advancements, ensuring I provide the most cutting-edge and safe treatments possible. Their commitment drives my relentless pursuit of continuous learning and innovation in the field.
What’s the best advice you can give to female dermatologists seeking leadership positions at the local, state, or national level?
My best advice is to have the courage to ask for what you seek. Societies are always looking for members who are eager to participate and contribute. If you express your interest in becoming more involved, there is likely a position available for you. The more you are willing to contribute to a society, the more likely you will be noticed and excel into higher leadership positions. Take initiative, show your commitment, and don’t hesitate to step forward when opportunities arise.
What’s the one tried-and-true laser- or energy-based procedure that you consider a “must” for your dermatology practice? And why?
Determining a single “must-have” laser- or energy-based procedure is a challenging question as it greatly depends on the specific needs of your patient population. However, one of the most common concerns among patients involves issues like redness and pigmentation. Therefore, having a versatile laser or an intense pulsed light device that effectively targets both red and brown pigmentation is indispensable for most practices.
In your view, what are the top three trends in aesthetic dermatology?
Over the years, I have observed several key trends in aesthetic dermatology:
- Minimally invasive procedures. There is a growing preference for less invasive treatments. Patients increasingly desire minimal downtime while still achieving significant results.
- Advancements in laser and energy-based devices for darker skin. There have been substantial advancements in technologies that are safer and more effective for darker skin tones. These developments play a crucial role in addressing diverse patient needs and providing inclusive dermatologic care.
- Natural aesthetic. I am hopeful that the trend toward an overdone appearance is fading. There seems to be a shift back towards a more natural and conservative aesthetic, emphasizing subtle enhancements over dramatic changes.
What development in dermatology are you most excited about in the next 5 years?
I am most excited to see how artificial intelligence and robotics play a role in energy-based devices.
Dr. Ortiz disclosed having financial relationships with several pharmaceutical and device companies. She is also cochair of the MOAS.
Arisa E. Ortiz, MD, began her term as president of the American Society for Laser Medicine and Surgery (ASLMS) during the organization’s annual meeting in April 2024.
After earning her medical degree from Albany Medical College, Albany, New York, Dr. Ortiz, a native of Los Angeles, completed her dermatology residency training at the University of California, Irvine, and the university’s Beckman Laser Institute. Next, she completed a laser and cosmetic dermatology fellowship at Massachusetts General Hospital, Harvard Medical School, and the Wellman Center for Photomedicine, all in Boston, and acquired additional fellowship training in Mohs micrographic surgery at the University of California, San Diego (UCSD). Dr. Ortiz is currently director of laser and cosmetic dermatology and a clinical professor of dermatology at UCSD.
She has authored more than 60 publications on new innovations in cutaneous surgery and is a frequent speaker at meetings of the American Academy of Dermatology, the American Society for Dermatologic Surgery (ASDS), and ASLMS, and she cochairs the annual Masters of Aesthetics Symposium in San Diego. Dr. Ortiz has received several awards, including the 2024 Castle Connolly Top Doctor Award and the Exceptional Women in Medicine Award; Newsweek America’s Best Dermatologists; the ASLMS Dr. Horace Furumoto Young Investigator Award, the ASLMS Best of Session Award for Cutaneous Applications, and the ASDS President’s Outstanding Service Award. Her primary research focuses on the laser treatment of nonmelanoma skin cancer.
In an interview, Dr. Ortiz spoke about her goals as ASLMS president and other topics related to dermatology.
Who inspired you most to become a doctor?
I’ve wanted to become a doctor for as long as I can remember. My fascination with science and the idea of helping people improve their health were driving forces. However, my biggest influence early on was my uncle, who was a pediatrician. His dedication and passion for medicine deeply inspired me and solidified my desire to pursue a career in healthcare.
I understand that a bout with chickenpox as a teenager influenced your decision to specialize in dermatology.
It’s an interesting and somewhat humorous story. When I was 18, I contracted chickenpox and ended up with scars on my face. It was a tough experience as a teenager, but it’s fascinating how such events can shape your life. In my quest for help, I opened the Yellow Pages and randomly chose a dermatologist nearby, who turned out to be Gary Lask, MD, director of lasers at UCLA [University of California, Los Angeles]. During our visit, I mentioned that I was premed, and he encouraged me to consider dermatology. About 6 years later, as a second-year medical student, I realized my passion for dermatology. I reached out to Dr. Lask and told him: “You were right. I want to be a dermatologist. Now, you have to help me get in!” Today, he remains my mentor, and I am deeply grateful for his guidance and support on this journey.
One of the initiatives for your term as ASLMS president includes a focus on safety standards for lasers and energy-based devices. Why is this important now?
Working at the university, I frequently encounter severe complications arising from the improper use of lasers and energy-based devices. As these procedures gain popularity, more providers are offering them, yet often without adequate training. As the world’s premier laser society, it is our duty to ensure patient safety. In the ever-evolving field of laser medicine, it is crucial that we continually strive to enhance the regulation of laser usage, ensuring that patients receive the highest standard of care with minimal risk.
One of the suggestions you have for the safety initiative is to offer a rigorous laser safety certification course with continuing education opportunities as a way foster a culture of heightened safety standards. Please explain what would be included in such a course and how it would align with current efforts to report adverse events such as the ASDS-Northwestern University Cutaneous Procedures Adverse Events Reporting (CAPER) registry and the Food and Drug Administration’s MedWatch Program.
A laser safety certification task force has been established to determine the best approach for developing a comprehensive course. The task force aims to assess the necessity of a formal safety certification in our industry, identify the resources needed to support such a certification, establish general safety protocols to form the content foundation, address potential legal concerns, and outline the process for formal certification program recognition. This exploratory work is expected to conclude by the end of the year. The proposed course may include modules on the fundamentals of laser physics, safe operation techniques, patient selection and management, and emergency protocols. Continuing education opportunities would be considered to keep practitioners updated on the latest advancements and safety protocols in laser medicine, thereby fostering a culture of heightened safety standards.
Another initiative for your term is the rollout of a tattoo removal program for former gang members based on the UCSD Clean Slate Tattoo Removal Program. Please tell us more about your vision for this national program.
UCSD Dermatology, in collaboration with UCSD Global Health, has been involved in the Clean Slate Tattoo Removal Program for the past decade. This initiative supports and rehabilitates former gang members by offering laser tattoo removal, helping them reintegrate into society. My vision is to equip our members with the necessary protocols to implement this outreach initiative in their own communities. By providing opportunities for reform and growth, we aim to foster safer and more inclusive communities nationwide.
You were one of the first clinicians to use a laser to treat basal cell carcinoma (BCC). Who are the ideal candidates for this procedure? Is the technique ready for wide clinical adoption? If not, what kind of studies are needed to make it so?
My research passion lies in optimizing laser treatments for BCC. During my fellowship with R. Rox Anderson, MD, and Mathew Avram, MD, at the MGH Wellman Center for Photomedicine, we conducted a pilot study using the 1064-nm Nd:YAG laser, achieving a 92% clearance rate after one treatment. Inspired by these results, we conducted a larger multicenter study, which demonstrated a 90% clearance rate after a single treatment. I now incorporate this technique into my daily practice. The ideal candidates for this procedure are patients with BCC that do not meet the Mohs Appropriate Use Criteria, such as those with nodular or superficial BCC subtypes on the body, individuals who are poor surgical candidates, or those who are surgically exhausted. However, I do not recommend this treatment for patients who are primarily concerned about facial scarring, particularly younger individuals; in such cases, Mohs surgery still remains the preferred option. While I believe this technique is ready for broader clinical adoption, it requires an understanding of laser endpoints. We are also exploring antibody-targeted gold nanorods to enhance the selectivity and standardization of the treatment.
Who inspires you most in your work today?
My patients are my greatest inspiration. Their trust and dedication motivate me to stay at the forefront of dermatologic advancements, ensuring I provide the most cutting-edge and safe treatments possible. Their commitment drives my relentless pursuit of continuous learning and innovation in the field.
What’s the best advice you can give to female dermatologists seeking leadership positions at the local, state, or national level?
My best advice is to have the courage to ask for what you seek. Societies are always looking for members who are eager to participate and contribute. If you express your interest in becoming more involved, there is likely a position available for you. The more you are willing to contribute to a society, the more likely you will be noticed and excel into higher leadership positions. Take initiative, show your commitment, and don’t hesitate to step forward when opportunities arise.
What’s the one tried-and-true laser- or energy-based procedure that you consider a “must” for your dermatology practice? And why?
Determining a single “must-have” laser- or energy-based procedure is a challenging question as it greatly depends on the specific needs of your patient population. However, one of the most common concerns among patients involves issues like redness and pigmentation. Therefore, having a versatile laser or an intense pulsed light device that effectively targets both red and brown pigmentation is indispensable for most practices.
In your view, what are the top three trends in aesthetic dermatology?
Over the years, I have observed several key trends in aesthetic dermatology:
- Minimally invasive procedures. There is a growing preference for less invasive treatments. Patients increasingly desire minimal downtime while still achieving significant results.
- Advancements in laser and energy-based devices for darker skin. There have been substantial advancements in technologies that are safer and more effective for darker skin tones. These developments play a crucial role in addressing diverse patient needs and providing inclusive dermatologic care.
- Natural aesthetic. I am hopeful that the trend toward an overdone appearance is fading. There seems to be a shift back towards a more natural and conservative aesthetic, emphasizing subtle enhancements over dramatic changes.
What development in dermatology are you most excited about in the next 5 years?
I am most excited to see how artificial intelligence and robotics play a role in energy-based devices.
Dr. Ortiz disclosed having financial relationships with several pharmaceutical and device companies. She is also cochair of the MOAS.
Arisa E. Ortiz, MD, began her term as president of the American Society for Laser Medicine and Surgery (ASLMS) during the organization’s annual meeting in April 2024.
After earning her medical degree from Albany Medical College, Albany, New York, Dr. Ortiz, a native of Los Angeles, completed her dermatology residency training at the University of California, Irvine, and the university’s Beckman Laser Institute. Next, she completed a laser and cosmetic dermatology fellowship at Massachusetts General Hospital, Harvard Medical School, and the Wellman Center for Photomedicine, all in Boston, and acquired additional fellowship training in Mohs micrographic surgery at the University of California, San Diego (UCSD). Dr. Ortiz is currently director of laser and cosmetic dermatology and a clinical professor of dermatology at UCSD.
She has authored more than 60 publications on new innovations in cutaneous surgery and is a frequent speaker at meetings of the American Academy of Dermatology, the American Society for Dermatologic Surgery (ASDS), and ASLMS, and she cochairs the annual Masters of Aesthetics Symposium in San Diego. Dr. Ortiz has received several awards, including the 2024 Castle Connolly Top Doctor Award and the Exceptional Women in Medicine Award; Newsweek America’s Best Dermatologists; the ASLMS Dr. Horace Furumoto Young Investigator Award, the ASLMS Best of Session Award for Cutaneous Applications, and the ASDS President’s Outstanding Service Award. Her primary research focuses on the laser treatment of nonmelanoma skin cancer.
In an interview, Dr. Ortiz spoke about her goals as ASLMS president and other topics related to dermatology.
Who inspired you most to become a doctor?
I’ve wanted to become a doctor for as long as I can remember. My fascination with science and the idea of helping people improve their health were driving forces. However, my biggest influence early on was my uncle, who was a pediatrician. His dedication and passion for medicine deeply inspired me and solidified my desire to pursue a career in healthcare.
I understand that a bout with chickenpox as a teenager influenced your decision to specialize in dermatology.
It’s an interesting and somewhat humorous story. When I was 18, I contracted chickenpox and ended up with scars on my face. It was a tough experience as a teenager, but it’s fascinating how such events can shape your life. In my quest for help, I opened the Yellow Pages and randomly chose a dermatologist nearby, who turned out to be Gary Lask, MD, director of lasers at UCLA [University of California, Los Angeles]. During our visit, I mentioned that I was premed, and he encouraged me to consider dermatology. About 6 years later, as a second-year medical student, I realized my passion for dermatology. I reached out to Dr. Lask and told him: “You were right. I want to be a dermatologist. Now, you have to help me get in!” Today, he remains my mentor, and I am deeply grateful for his guidance and support on this journey.
One of the initiatives for your term as ASLMS president includes a focus on safety standards for lasers and energy-based devices. Why is this important now?
Working at the university, I frequently encounter severe complications arising from the improper use of lasers and energy-based devices. As these procedures gain popularity, more providers are offering them, yet often without adequate training. As the world’s premier laser society, it is our duty to ensure patient safety. In the ever-evolving field of laser medicine, it is crucial that we continually strive to enhance the regulation of laser usage, ensuring that patients receive the highest standard of care with minimal risk.
One of the suggestions you have for the safety initiative is to offer a rigorous laser safety certification course with continuing education opportunities as a way foster a culture of heightened safety standards. Please explain what would be included in such a course and how it would align with current efforts to report adverse events such as the ASDS-Northwestern University Cutaneous Procedures Adverse Events Reporting (CAPER) registry and the Food and Drug Administration’s MedWatch Program.
A laser safety certification task force has been established to determine the best approach for developing a comprehensive course. The task force aims to assess the necessity of a formal safety certification in our industry, identify the resources needed to support such a certification, establish general safety protocols to form the content foundation, address potential legal concerns, and outline the process for formal certification program recognition. This exploratory work is expected to conclude by the end of the year. The proposed course may include modules on the fundamentals of laser physics, safe operation techniques, patient selection and management, and emergency protocols. Continuing education opportunities would be considered to keep practitioners updated on the latest advancements and safety protocols in laser medicine, thereby fostering a culture of heightened safety standards.
Another initiative for your term is the rollout of a tattoo removal program for former gang members based on the UCSD Clean Slate Tattoo Removal Program. Please tell us more about your vision for this national program.
UCSD Dermatology, in collaboration with UCSD Global Health, has been involved in the Clean Slate Tattoo Removal Program for the past decade. This initiative supports and rehabilitates former gang members by offering laser tattoo removal, helping them reintegrate into society. My vision is to equip our members with the necessary protocols to implement this outreach initiative in their own communities. By providing opportunities for reform and growth, we aim to foster safer and more inclusive communities nationwide.
You were one of the first clinicians to use a laser to treat basal cell carcinoma (BCC). Who are the ideal candidates for this procedure? Is the technique ready for wide clinical adoption? If not, what kind of studies are needed to make it so?
My research passion lies in optimizing laser treatments for BCC. During my fellowship with R. Rox Anderson, MD, and Mathew Avram, MD, at the MGH Wellman Center for Photomedicine, we conducted a pilot study using the 1064-nm Nd:YAG laser, achieving a 92% clearance rate after one treatment. Inspired by these results, we conducted a larger multicenter study, which demonstrated a 90% clearance rate after a single treatment. I now incorporate this technique into my daily practice. The ideal candidates for this procedure are patients with BCC that do not meet the Mohs Appropriate Use Criteria, such as those with nodular or superficial BCC subtypes on the body, individuals who are poor surgical candidates, or those who are surgically exhausted. However, I do not recommend this treatment for patients who are primarily concerned about facial scarring, particularly younger individuals; in such cases, Mohs surgery still remains the preferred option. While I believe this technique is ready for broader clinical adoption, it requires an understanding of laser endpoints. We are also exploring antibody-targeted gold nanorods to enhance the selectivity and standardization of the treatment.
Who inspires you most in your work today?
My patients are my greatest inspiration. Their trust and dedication motivate me to stay at the forefront of dermatologic advancements, ensuring I provide the most cutting-edge and safe treatments possible. Their commitment drives my relentless pursuit of continuous learning and innovation in the field.
What’s the best advice you can give to female dermatologists seeking leadership positions at the local, state, or national level?
My best advice is to have the courage to ask for what you seek. Societies are always looking for members who are eager to participate and contribute. If you express your interest in becoming more involved, there is likely a position available for you. The more you are willing to contribute to a society, the more likely you will be noticed and excel into higher leadership positions. Take initiative, show your commitment, and don’t hesitate to step forward when opportunities arise.
What’s the one tried-and-true laser- or energy-based procedure that you consider a “must” for your dermatology practice? And why?
Determining a single “must-have” laser- or energy-based procedure is a challenging question as it greatly depends on the specific needs of your patient population. However, one of the most common concerns among patients involves issues like redness and pigmentation. Therefore, having a versatile laser or an intense pulsed light device that effectively targets both red and brown pigmentation is indispensable for most practices.
In your view, what are the top three trends in aesthetic dermatology?
Over the years, I have observed several key trends in aesthetic dermatology:
- Minimally invasive procedures. There is a growing preference for less invasive treatments. Patients increasingly desire minimal downtime while still achieving significant results.
- Advancements in laser and energy-based devices for darker skin. There have been substantial advancements in technologies that are safer and more effective for darker skin tones. These developments play a crucial role in addressing diverse patient needs and providing inclusive dermatologic care.
- Natural aesthetic. I am hopeful that the trend toward an overdone appearance is fading. There seems to be a shift back towards a more natural and conservative aesthetic, emphasizing subtle enhancements over dramatic changes.
What development in dermatology are you most excited about in the next 5 years?
I am most excited to see how artificial intelligence and robotics play a role in energy-based devices.
Dr. Ortiz disclosed having financial relationships with several pharmaceutical and device companies. She is also cochair of the MOAS.
FDA Approves Deuruxolitinib for Severe Alopecia Areata in Adults
The
The development, which was announced in a July 25, 2024, news release from the drug’s manufacturer Sun Pharma, is based on data from two pivotal randomized, double-blind, placebo-controlled phase 3 clinical trials: THRIVE-AA1 and THRIVE-AA2, which included 1220 adults with severe alopecia areata enrolled at sites in the United States, Canada, and Europe. Study participants had at least 50% scalp hair loss as measured by Severity of Alopecia Tool (SALT) for more than 6 months. Data were also collected from two open-label, long-term extension trials in which patients were eligible to enroll upon completion of the 24-week trials.
Deuruxolitinib, which comes in 8-mg tablets, is an oral selective inhibitor of JAK1 and JAK2 and is administered twice a day. According to the company press release, the average patient enrolled in the clinical trials had only 13% of their scalp hair coverage at baseline. At week 24, more than 30% of patients taking deuruxolitinib experiencing 80% or more scalp hair coverage (SALT score ≤ 20). Also, up to 25% of patients had almost all of their scalp hair back at 24 weeks (≥ 90% coverage).
In terms of safety, the data showed that 3.1% of patients who received deuruxolitinib 8 mg twice daily in the phase 2 dose-ranging study and phase 3 randomized placebo-controlled trials discontinued treatment owing to adverse reactions. The three most common adverse events in placebo-controlled trials were headache (12.4% vs 9.4% with placebo), acne (10% vs 4.3% with placebo), and nasopharyngitis (8.1% vs 6.7% with placebo). More than 100 people continued taking deuruxolitinib for more than 3 years.
Deuruxolitinib is the third treatment and third JAK inhibitor approved by the FDA for severe alopecia areata. Baricitinib (Olumiant) was approved in June 2022 for adults with alopecia areata, followed by ritlecitinib (Litfulo) approved in June 2023 for patients aged 12 years and older.
In a statement from the National Alopecia Areata Foundation (NAAF), Nicole Friedland, NAAF’s president and CEO, said that “it is with tremendous excitement that we welcome the FDA’s approval of a third treatment for severe alopecia areata in as many years.”
A version of this article first appeared on Medscape.com.
The
The development, which was announced in a July 25, 2024, news release from the drug’s manufacturer Sun Pharma, is based on data from two pivotal randomized, double-blind, placebo-controlled phase 3 clinical trials: THRIVE-AA1 and THRIVE-AA2, which included 1220 adults with severe alopecia areata enrolled at sites in the United States, Canada, and Europe. Study participants had at least 50% scalp hair loss as measured by Severity of Alopecia Tool (SALT) for more than 6 months. Data were also collected from two open-label, long-term extension trials in which patients were eligible to enroll upon completion of the 24-week trials.
Deuruxolitinib, which comes in 8-mg tablets, is an oral selective inhibitor of JAK1 and JAK2 and is administered twice a day. According to the company press release, the average patient enrolled in the clinical trials had only 13% of their scalp hair coverage at baseline. At week 24, more than 30% of patients taking deuruxolitinib experiencing 80% or more scalp hair coverage (SALT score ≤ 20). Also, up to 25% of patients had almost all of their scalp hair back at 24 weeks (≥ 90% coverage).
In terms of safety, the data showed that 3.1% of patients who received deuruxolitinib 8 mg twice daily in the phase 2 dose-ranging study and phase 3 randomized placebo-controlled trials discontinued treatment owing to adverse reactions. The three most common adverse events in placebo-controlled trials were headache (12.4% vs 9.4% with placebo), acne (10% vs 4.3% with placebo), and nasopharyngitis (8.1% vs 6.7% with placebo). More than 100 people continued taking deuruxolitinib for more than 3 years.
Deuruxolitinib is the third treatment and third JAK inhibitor approved by the FDA for severe alopecia areata. Baricitinib (Olumiant) was approved in June 2022 for adults with alopecia areata, followed by ritlecitinib (Litfulo) approved in June 2023 for patients aged 12 years and older.
In a statement from the National Alopecia Areata Foundation (NAAF), Nicole Friedland, NAAF’s president and CEO, said that “it is with tremendous excitement that we welcome the FDA’s approval of a third treatment for severe alopecia areata in as many years.”
A version of this article first appeared on Medscape.com.
The
The development, which was announced in a July 25, 2024, news release from the drug’s manufacturer Sun Pharma, is based on data from two pivotal randomized, double-blind, placebo-controlled phase 3 clinical trials: THRIVE-AA1 and THRIVE-AA2, which included 1220 adults with severe alopecia areata enrolled at sites in the United States, Canada, and Europe. Study participants had at least 50% scalp hair loss as measured by Severity of Alopecia Tool (SALT) for more than 6 months. Data were also collected from two open-label, long-term extension trials in which patients were eligible to enroll upon completion of the 24-week trials.
Deuruxolitinib, which comes in 8-mg tablets, is an oral selective inhibitor of JAK1 and JAK2 and is administered twice a day. According to the company press release, the average patient enrolled in the clinical trials had only 13% of their scalp hair coverage at baseline. At week 24, more than 30% of patients taking deuruxolitinib experiencing 80% or more scalp hair coverage (SALT score ≤ 20). Also, up to 25% of patients had almost all of their scalp hair back at 24 weeks (≥ 90% coverage).
In terms of safety, the data showed that 3.1% of patients who received deuruxolitinib 8 mg twice daily in the phase 2 dose-ranging study and phase 3 randomized placebo-controlled trials discontinued treatment owing to adverse reactions. The three most common adverse events in placebo-controlled trials were headache (12.4% vs 9.4% with placebo), acne (10% vs 4.3% with placebo), and nasopharyngitis (8.1% vs 6.7% with placebo). More than 100 people continued taking deuruxolitinib for more than 3 years.
Deuruxolitinib is the third treatment and third JAK inhibitor approved by the FDA for severe alopecia areata. Baricitinib (Olumiant) was approved in June 2022 for adults with alopecia areata, followed by ritlecitinib (Litfulo) approved in June 2023 for patients aged 12 years and older.
In a statement from the National Alopecia Areata Foundation (NAAF), Nicole Friedland, NAAF’s president and CEO, said that “it is with tremendous excitement that we welcome the FDA’s approval of a third treatment for severe alopecia areata in as many years.”
A version of this article first appeared on Medscape.com.
Pilot Study Finds Experimental CBD Cream Decreases UVA Skin Damage
, results from a small prospective pilot study showed.
“This study hopefully reinvigorates interest in the utilization of whether it be plant-based, human-derived, or synthetic cannabinoids in the management of dermatologic disease,” one of the study investigators, Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, DC, told this news organization. The study was published in the Journal of the American Academy of Dermatology.
For the prospective, single-center, pilot trial, which is believed to be the first of its kind, 19 volunteers aged 22-65 with Fitzpatrick skin types I-III applied either a nano-encapsulated CBD cream or a vehicle cream to blind spots on the skin of the buttocks twice daily for 14 days. Next, researchers applied a minimal erythema dose of UV radiation to the treated skin areas for 30 minutes. After 24 hours, they visually inspected the treated areas to clinically compare the erythema. They also performed five 4-mm punch biopsies from UVA- and non-UVA–exposed treatment sites on each buttock, as well as from an untreated control site that was at least 5 cm away from the treated left buttock.
At 24 hours, 21% of study participants showed less redness on CBD-treated skin compared with control-treated skin, while histology showed that CBD-treated skin demonstrated reduced UVA-induced epidermal hyperplasia compared with control-treated skin (a mean 11.3% change from baseline vs 28.7%, respectively; P = .01). In other findings, application of CBD cream reduced DNA damage and DNA mutations associated with UVA-induced skin aging/damage and ultimately skin cancer.
In addition, the CBD-treated skin samples had a reduction in the UVA-associated increase in the premutagenic marker 8-oxoguanine DNA glycosylase 1 and a reduction of two major UVA-induced mitochondrial DNA deletions associated with skin photoaging.
The research, Dr. Friedman noted, “took a village of collaborators and almost 3 years to pull together,” including collaborating with his long-standing mentor, Brian Berman, MD, PhD, professor emeritus of dermatology and dermatologic surgery at the University of Miami, Coral Gables, Florida, and a study coauthor. The study “demonstrated that purposeful delivery of CBD using an established nanoparticle platform ... can have a quantifiable impact on preventing the expected DNA damage and cellular injury one should see from UVA exposure,” said Dr. Friedman, who codeveloped the nanoparticle platform with his father, Joel M. Friedman, MD, PhD, professor of microbiology and immunology at Albert Einstein College of Medicine, New York City.
“Never before has a dermatologic study on topical cannabinoids dove so deeply into the biological impact of this natural ingredient to highlight its potential, here, as a mitigation strategy for unprotected exposure to prevent the downstream sequelae of UV radiation,” Dr. Friedman said.
In the paper, he and his coauthors acknowledged certain limitations of their study, including its small sample size and the single-center design.
Dr. Friedman disclosed that he coinvented the nanoparticle technology used in the trial. Dr. Berman is a consultant at MINO Labs, which funded the study. The remaining authors had no disclosures. The study was done in collaboration with the Center for Clinical and Cosmetic Research in Aventura, Florida.
A version of this article first appeared on Medscape.com.
, results from a small prospective pilot study showed.
“This study hopefully reinvigorates interest in the utilization of whether it be plant-based, human-derived, or synthetic cannabinoids in the management of dermatologic disease,” one of the study investigators, Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, DC, told this news organization. The study was published in the Journal of the American Academy of Dermatology.
For the prospective, single-center, pilot trial, which is believed to be the first of its kind, 19 volunteers aged 22-65 with Fitzpatrick skin types I-III applied either a nano-encapsulated CBD cream or a vehicle cream to blind spots on the skin of the buttocks twice daily for 14 days. Next, researchers applied a minimal erythema dose of UV radiation to the treated skin areas for 30 minutes. After 24 hours, they visually inspected the treated areas to clinically compare the erythema. They also performed five 4-mm punch biopsies from UVA- and non-UVA–exposed treatment sites on each buttock, as well as from an untreated control site that was at least 5 cm away from the treated left buttock.
At 24 hours, 21% of study participants showed less redness on CBD-treated skin compared with control-treated skin, while histology showed that CBD-treated skin demonstrated reduced UVA-induced epidermal hyperplasia compared with control-treated skin (a mean 11.3% change from baseline vs 28.7%, respectively; P = .01). In other findings, application of CBD cream reduced DNA damage and DNA mutations associated with UVA-induced skin aging/damage and ultimately skin cancer.
In addition, the CBD-treated skin samples had a reduction in the UVA-associated increase in the premutagenic marker 8-oxoguanine DNA glycosylase 1 and a reduction of two major UVA-induced mitochondrial DNA deletions associated with skin photoaging.
The research, Dr. Friedman noted, “took a village of collaborators and almost 3 years to pull together,” including collaborating with his long-standing mentor, Brian Berman, MD, PhD, professor emeritus of dermatology and dermatologic surgery at the University of Miami, Coral Gables, Florida, and a study coauthor. The study “demonstrated that purposeful delivery of CBD using an established nanoparticle platform ... can have a quantifiable impact on preventing the expected DNA damage and cellular injury one should see from UVA exposure,” said Dr. Friedman, who codeveloped the nanoparticle platform with his father, Joel M. Friedman, MD, PhD, professor of microbiology and immunology at Albert Einstein College of Medicine, New York City.
“Never before has a dermatologic study on topical cannabinoids dove so deeply into the biological impact of this natural ingredient to highlight its potential, here, as a mitigation strategy for unprotected exposure to prevent the downstream sequelae of UV radiation,” Dr. Friedman said.
In the paper, he and his coauthors acknowledged certain limitations of their study, including its small sample size and the single-center design.
Dr. Friedman disclosed that he coinvented the nanoparticle technology used in the trial. Dr. Berman is a consultant at MINO Labs, which funded the study. The remaining authors had no disclosures. The study was done in collaboration with the Center for Clinical and Cosmetic Research in Aventura, Florida.
A version of this article first appeared on Medscape.com.
, results from a small prospective pilot study showed.
“This study hopefully reinvigorates interest in the utilization of whether it be plant-based, human-derived, or synthetic cannabinoids in the management of dermatologic disease,” one of the study investigators, Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, DC, told this news organization. The study was published in the Journal of the American Academy of Dermatology.
For the prospective, single-center, pilot trial, which is believed to be the first of its kind, 19 volunteers aged 22-65 with Fitzpatrick skin types I-III applied either a nano-encapsulated CBD cream or a vehicle cream to blind spots on the skin of the buttocks twice daily for 14 days. Next, researchers applied a minimal erythema dose of UV radiation to the treated skin areas for 30 minutes. After 24 hours, they visually inspected the treated areas to clinically compare the erythema. They also performed five 4-mm punch biopsies from UVA- and non-UVA–exposed treatment sites on each buttock, as well as from an untreated control site that was at least 5 cm away from the treated left buttock.
At 24 hours, 21% of study participants showed less redness on CBD-treated skin compared with control-treated skin, while histology showed that CBD-treated skin demonstrated reduced UVA-induced epidermal hyperplasia compared with control-treated skin (a mean 11.3% change from baseline vs 28.7%, respectively; P = .01). In other findings, application of CBD cream reduced DNA damage and DNA mutations associated with UVA-induced skin aging/damage and ultimately skin cancer.
In addition, the CBD-treated skin samples had a reduction in the UVA-associated increase in the premutagenic marker 8-oxoguanine DNA glycosylase 1 and a reduction of two major UVA-induced mitochondrial DNA deletions associated with skin photoaging.
The research, Dr. Friedman noted, “took a village of collaborators and almost 3 years to pull together,” including collaborating with his long-standing mentor, Brian Berman, MD, PhD, professor emeritus of dermatology and dermatologic surgery at the University of Miami, Coral Gables, Florida, and a study coauthor. The study “demonstrated that purposeful delivery of CBD using an established nanoparticle platform ... can have a quantifiable impact on preventing the expected DNA damage and cellular injury one should see from UVA exposure,” said Dr. Friedman, who codeveloped the nanoparticle platform with his father, Joel M. Friedman, MD, PhD, professor of microbiology and immunology at Albert Einstein College of Medicine, New York City.
“Never before has a dermatologic study on topical cannabinoids dove so deeply into the biological impact of this natural ingredient to highlight its potential, here, as a mitigation strategy for unprotected exposure to prevent the downstream sequelae of UV radiation,” Dr. Friedman said.
In the paper, he and his coauthors acknowledged certain limitations of their study, including its small sample size and the single-center design.
Dr. Friedman disclosed that he coinvented the nanoparticle technology used in the trial. Dr. Berman is a consultant at MINO Labs, which funded the study. The remaining authors had no disclosures. The study was done in collaboration with the Center for Clinical and Cosmetic Research in Aventura, Florida.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Gluconolactone
This derivative of oxidized glucose lactone is present naturally in bread, cheese, fruit juices, honey, tofu, and wine, and is used as a food additive in Europe.1,2 In dermatology, it is most often used in chemical peels.
Polyhydroxy acids (PHAs) were discovered about 3 decades ago to exert similar functions as alpha hydroxy acids without provoking sensory irritation reactions. Gluconolactone along with lactobionic acid were the identified PHAs and further characterized as delivering more humectant and moisturizing activity than alpha hydroxy acids and effective in combination with retinoic acid to treat adult acne and with retinyl acetate to confer antiaging benefits.3 It is typically added to products for its skin-conditioning qualities, resulting in smoother, brighter, more toned skin.4 This column focuses on recent studies using this bioactive agent for dermatologic purposes.
Split-Face Studies Show Various Benefits
In 2023, Jarząbek-Perz and colleagues conducted a split-face evaluation to assess the effects on various skin parameters (ie, hydration, pH, sebum, and transepidermal water loss [TEWL]) of gluconolactone and oxybrasion, compared with gluconolactone and microneedling. Twenty-one White women underwent a series of three split-face treatments at 1-week intervals. Chemical peels with 10% gluconolactone were performed on the whole face. The right side of the face was also treated with oxybrasion and the left with microneedle mesotherapy. Skin parameters were measured before the first and third treatments and 2 weeks following the final treatment. Photos were taken before and after the study. Both treatments resulted in improved hydration and reductions in sebum, pH, and TEWL. No statistically significant differences were noted between the treatment protocols. The researchers concluded that gluconolactone peels can be effectively combined with oxybrasion or microneedle mesotherapy to enhance skin hydration and to secure the hydrolipid barrier.5
Later that year, the same team evaluated pH, sebum levels, and TEWL before, during, and after several applications of 10% and 30% gluconolactone chemical peels in a split-face model in 16 female participants. The investigators conducted three procedures on both sides of the face, taking measurements on the forehead, periorbital area, on the cheek, and on the nose wing before, during, and 7 days after the final treatment. They found statistically significant improvements in sebum levels in the cheeks after the treatment series. Also, pH values were lower at each measurement site after each procedure. TEWL levels were significantly diminished around the eyes, as well as the left forehead and right cheek, with no significant discrepancy between gluconolactone concentrations. The researchers concluded that gluconolactone plays a major role in reducing cutaneous pH and TEWL and imparts a regulatory effect on sebum.1
Two years earlier, Jarząbek-Perz and colleagues assessed skin moisture in a split-face model in 16 healthy women after the application of 10% and 30% gluconolactone. Investigators measured skin moisture before and after each of three treatments and a week after the final treatment from the forehead, periorbital area, and on the cheek. They observed no significant discrepancies between the 10% and 30% formulations, but a significant elevation in facial skin hydration was found to be promoted by gluconolactone. The investigators concluded that gluconolactone is an effective moisturizer for care of dry skin.6
Topical Formulation
In 2023, Zerbinati and colleagues determined that a gluconolactone-based lotion that they had begun testing 2 years earlier was safe and effective for dermatologic applications, with the noncomedogenic formulation found suitable as an antiaging agent, particularly as it treats aging-related pore dilatation.7,8
Acne Treatment
In 2019, Kantikosum and colleagues conducted a double-blind, within-person comparative study to assess the efficacy of various cosmeceutical ingredients, including gluconolactone, glycolic acid, licochalcone A, and salicylic acid, combined with the acne treatment adapalene vs adapalene monotherapy for mild to moderate acne. Each of 25 subjects over 28 days applied a product mixed with 0.1% adapalene on one side of the face, and 0.1% adapalene alone on the other side of the face once nightly. The VISIA camera system spot score pointed to a statistically significant improvement on the combination sides. Differences in lesion reduction and severity were within acceptable margins, the authors reported. They concluded that the cosmeceutical combinations yielded similar benefits as adapalene alone, with the combination formulations decreasing acne complications.9
Potential Use as an Antifibrotic Agent
In 2018, Jayamani and colleagues investigated the antifibrotic characteristics of glucono-delta-lactone, a known acidifier, to ascertain if it could directly suppress collagen fibrils or even cause them to disintegrate. The researchers noted that collagen fibrillation is pH dependent, and that glucono-delta-lactone was found to exert a concentration-dependent suppression of fibrils and disintegration of preformed collagen fibrils with the antifibrotic function of the compound ascribed to its capacity to decrease pH. Further, glucono-delta-lactone appeared to emerge as an ideal antifibrotic agent as it left intact the triple helical structure of collagen after treatment. The investigators concluded that glucono-delta-lactone provides the foundation for developing antifibrotic agents intended to treat disorders characterized by collagen deposition.10
Conclusion
Gluconolactone emerged in the 1990s as a PHA useful in skin peels as an alternative to alpha hydroxy acids because of its nonirritating qualities. Since then, its soothing, hydrating, and, in particular, antiacne and antiaging qualities have become established. Wider applications of this versatile agent for dermatologic purposes are likely to be further investigated.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, a SaaS company used to generate skin care routines in office and as a ecommerce solution. Write to her at [email protected].
References
1. Jarząbek-Perz S et al. J Cosmet Dermatol. 2023 Dec;22(12):3305-3312..
2. Qin X et al. Front Physiol. 2022 Mar 14;13:856699.
3. Grimes PE et al. Cutis. 2004 Feb;73(2 Suppl):3-13.
4. Glaser DA. Facial Plast Surg Clin North Am. 2003 May;11(2):219-227.
5. Jarząbek-Perz S et al. Skin Res Technol. 2023 Jun;29(6):e13353.
6. Jarząbek-Perz S et al. Skin Res Technol. 2021 Sep;27(5):925-930.
7. Zerbinati N et al. Molecules. 2021 Dec 15;26(24):7592.
8. Zerbinati Net al. Pharmaceuticals (Basel). 2023 Apr 27;16(5):655.
9. Kantikosum K et al. Clin Cosmet Investig Dermatol. 2019 Feb 19;12:151-161.
10. Jayamani J et al. Int J Biol Macromol. 2018 Feb;107(Pt A):175-185.
This derivative of oxidized glucose lactone is present naturally in bread, cheese, fruit juices, honey, tofu, and wine, and is used as a food additive in Europe.1,2 In dermatology, it is most often used in chemical peels.
Polyhydroxy acids (PHAs) were discovered about 3 decades ago to exert similar functions as alpha hydroxy acids without provoking sensory irritation reactions. Gluconolactone along with lactobionic acid were the identified PHAs and further characterized as delivering more humectant and moisturizing activity than alpha hydroxy acids and effective in combination with retinoic acid to treat adult acne and with retinyl acetate to confer antiaging benefits.3 It is typically added to products for its skin-conditioning qualities, resulting in smoother, brighter, more toned skin.4 This column focuses on recent studies using this bioactive agent for dermatologic purposes.
Split-Face Studies Show Various Benefits
In 2023, Jarząbek-Perz and colleagues conducted a split-face evaluation to assess the effects on various skin parameters (ie, hydration, pH, sebum, and transepidermal water loss [TEWL]) of gluconolactone and oxybrasion, compared with gluconolactone and microneedling. Twenty-one White women underwent a series of three split-face treatments at 1-week intervals. Chemical peels with 10% gluconolactone were performed on the whole face. The right side of the face was also treated with oxybrasion and the left with microneedle mesotherapy. Skin parameters were measured before the first and third treatments and 2 weeks following the final treatment. Photos were taken before and after the study. Both treatments resulted in improved hydration and reductions in sebum, pH, and TEWL. No statistically significant differences were noted between the treatment protocols. The researchers concluded that gluconolactone peels can be effectively combined with oxybrasion or microneedle mesotherapy to enhance skin hydration and to secure the hydrolipid barrier.5
Later that year, the same team evaluated pH, sebum levels, and TEWL before, during, and after several applications of 10% and 30% gluconolactone chemical peels in a split-face model in 16 female participants. The investigators conducted three procedures on both sides of the face, taking measurements on the forehead, periorbital area, on the cheek, and on the nose wing before, during, and 7 days after the final treatment. They found statistically significant improvements in sebum levels in the cheeks after the treatment series. Also, pH values were lower at each measurement site after each procedure. TEWL levels were significantly diminished around the eyes, as well as the left forehead and right cheek, with no significant discrepancy between gluconolactone concentrations. The researchers concluded that gluconolactone plays a major role in reducing cutaneous pH and TEWL and imparts a regulatory effect on sebum.1
Two years earlier, Jarząbek-Perz and colleagues assessed skin moisture in a split-face model in 16 healthy women after the application of 10% and 30% gluconolactone. Investigators measured skin moisture before and after each of three treatments and a week after the final treatment from the forehead, periorbital area, and on the cheek. They observed no significant discrepancies between the 10% and 30% formulations, but a significant elevation in facial skin hydration was found to be promoted by gluconolactone. The investigators concluded that gluconolactone is an effective moisturizer for care of dry skin.6
Topical Formulation
In 2023, Zerbinati and colleagues determined that a gluconolactone-based lotion that they had begun testing 2 years earlier was safe and effective for dermatologic applications, with the noncomedogenic formulation found suitable as an antiaging agent, particularly as it treats aging-related pore dilatation.7,8
Acne Treatment
In 2019, Kantikosum and colleagues conducted a double-blind, within-person comparative study to assess the efficacy of various cosmeceutical ingredients, including gluconolactone, glycolic acid, licochalcone A, and salicylic acid, combined with the acne treatment adapalene vs adapalene monotherapy for mild to moderate acne. Each of 25 subjects over 28 days applied a product mixed with 0.1% adapalene on one side of the face, and 0.1% adapalene alone on the other side of the face once nightly. The VISIA camera system spot score pointed to a statistically significant improvement on the combination sides. Differences in lesion reduction and severity were within acceptable margins, the authors reported. They concluded that the cosmeceutical combinations yielded similar benefits as adapalene alone, with the combination formulations decreasing acne complications.9
Potential Use as an Antifibrotic Agent
In 2018, Jayamani and colleagues investigated the antifibrotic characteristics of glucono-delta-lactone, a known acidifier, to ascertain if it could directly suppress collagen fibrils or even cause them to disintegrate. The researchers noted that collagen fibrillation is pH dependent, and that glucono-delta-lactone was found to exert a concentration-dependent suppression of fibrils and disintegration of preformed collagen fibrils with the antifibrotic function of the compound ascribed to its capacity to decrease pH. Further, glucono-delta-lactone appeared to emerge as an ideal antifibrotic agent as it left intact the triple helical structure of collagen after treatment. The investigators concluded that glucono-delta-lactone provides the foundation for developing antifibrotic agents intended to treat disorders characterized by collagen deposition.10
Conclusion
Gluconolactone emerged in the 1990s as a PHA useful in skin peels as an alternative to alpha hydroxy acids because of its nonirritating qualities. Since then, its soothing, hydrating, and, in particular, antiacne and antiaging qualities have become established. Wider applications of this versatile agent for dermatologic purposes are likely to be further investigated.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, a SaaS company used to generate skin care routines in office and as a ecommerce solution. Write to her at [email protected].
References
1. Jarząbek-Perz S et al. J Cosmet Dermatol. 2023 Dec;22(12):3305-3312..
2. Qin X et al. Front Physiol. 2022 Mar 14;13:856699.
3. Grimes PE et al. Cutis. 2004 Feb;73(2 Suppl):3-13.
4. Glaser DA. Facial Plast Surg Clin North Am. 2003 May;11(2):219-227.
5. Jarząbek-Perz S et al. Skin Res Technol. 2023 Jun;29(6):e13353.
6. Jarząbek-Perz S et al. Skin Res Technol. 2021 Sep;27(5):925-930.
7. Zerbinati N et al. Molecules. 2021 Dec 15;26(24):7592.
8. Zerbinati Net al. Pharmaceuticals (Basel). 2023 Apr 27;16(5):655.
9. Kantikosum K et al. Clin Cosmet Investig Dermatol. 2019 Feb 19;12:151-161.
10. Jayamani J et al. Int J Biol Macromol. 2018 Feb;107(Pt A):175-185.
This derivative of oxidized glucose lactone is present naturally in bread, cheese, fruit juices, honey, tofu, and wine, and is used as a food additive in Europe.1,2 In dermatology, it is most often used in chemical peels.
Polyhydroxy acids (PHAs) were discovered about 3 decades ago to exert similar functions as alpha hydroxy acids without provoking sensory irritation reactions. Gluconolactone along with lactobionic acid were the identified PHAs and further characterized as delivering more humectant and moisturizing activity than alpha hydroxy acids and effective in combination with retinoic acid to treat adult acne and with retinyl acetate to confer antiaging benefits.3 It is typically added to products for its skin-conditioning qualities, resulting in smoother, brighter, more toned skin.4 This column focuses on recent studies using this bioactive agent for dermatologic purposes.
Split-Face Studies Show Various Benefits
In 2023, Jarząbek-Perz and colleagues conducted a split-face evaluation to assess the effects on various skin parameters (ie, hydration, pH, sebum, and transepidermal water loss [TEWL]) of gluconolactone and oxybrasion, compared with gluconolactone and microneedling. Twenty-one White women underwent a series of three split-face treatments at 1-week intervals. Chemical peels with 10% gluconolactone were performed on the whole face. The right side of the face was also treated with oxybrasion and the left with microneedle mesotherapy. Skin parameters were measured before the first and third treatments and 2 weeks following the final treatment. Photos were taken before and after the study. Both treatments resulted in improved hydration and reductions in sebum, pH, and TEWL. No statistically significant differences were noted between the treatment protocols. The researchers concluded that gluconolactone peels can be effectively combined with oxybrasion or microneedle mesotherapy to enhance skin hydration and to secure the hydrolipid barrier.5
Later that year, the same team evaluated pH, sebum levels, and TEWL before, during, and after several applications of 10% and 30% gluconolactone chemical peels in a split-face model in 16 female participants. The investigators conducted three procedures on both sides of the face, taking measurements on the forehead, periorbital area, on the cheek, and on the nose wing before, during, and 7 days after the final treatment. They found statistically significant improvements in sebum levels in the cheeks after the treatment series. Also, pH values were lower at each measurement site after each procedure. TEWL levels were significantly diminished around the eyes, as well as the left forehead and right cheek, with no significant discrepancy between gluconolactone concentrations. The researchers concluded that gluconolactone plays a major role in reducing cutaneous pH and TEWL and imparts a regulatory effect on sebum.1
Two years earlier, Jarząbek-Perz and colleagues assessed skin moisture in a split-face model in 16 healthy women after the application of 10% and 30% gluconolactone. Investigators measured skin moisture before and after each of three treatments and a week after the final treatment from the forehead, periorbital area, and on the cheek. They observed no significant discrepancies between the 10% and 30% formulations, but a significant elevation in facial skin hydration was found to be promoted by gluconolactone. The investigators concluded that gluconolactone is an effective moisturizer for care of dry skin.6
Topical Formulation
In 2023, Zerbinati and colleagues determined that a gluconolactone-based lotion that they had begun testing 2 years earlier was safe and effective for dermatologic applications, with the noncomedogenic formulation found suitable as an antiaging agent, particularly as it treats aging-related pore dilatation.7,8
Acne Treatment
In 2019, Kantikosum and colleagues conducted a double-blind, within-person comparative study to assess the efficacy of various cosmeceutical ingredients, including gluconolactone, glycolic acid, licochalcone A, and salicylic acid, combined with the acne treatment adapalene vs adapalene monotherapy for mild to moderate acne. Each of 25 subjects over 28 days applied a product mixed with 0.1% adapalene on one side of the face, and 0.1% adapalene alone on the other side of the face once nightly. The VISIA camera system spot score pointed to a statistically significant improvement on the combination sides. Differences in lesion reduction and severity were within acceptable margins, the authors reported. They concluded that the cosmeceutical combinations yielded similar benefits as adapalene alone, with the combination formulations decreasing acne complications.9
Potential Use as an Antifibrotic Agent
In 2018, Jayamani and colleagues investigated the antifibrotic characteristics of glucono-delta-lactone, a known acidifier, to ascertain if it could directly suppress collagen fibrils or even cause them to disintegrate. The researchers noted that collagen fibrillation is pH dependent, and that glucono-delta-lactone was found to exert a concentration-dependent suppression of fibrils and disintegration of preformed collagen fibrils with the antifibrotic function of the compound ascribed to its capacity to decrease pH. Further, glucono-delta-lactone appeared to emerge as an ideal antifibrotic agent as it left intact the triple helical structure of collagen after treatment. The investigators concluded that glucono-delta-lactone provides the foundation for developing antifibrotic agents intended to treat disorders characterized by collagen deposition.10
Conclusion
Gluconolactone emerged in the 1990s as a PHA useful in skin peels as an alternative to alpha hydroxy acids because of its nonirritating qualities. Since then, its soothing, hydrating, and, in particular, antiacne and antiaging qualities have become established. Wider applications of this versatile agent for dermatologic purposes are likely to be further investigated.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, a SaaS company used to generate skin care routines in office and as a ecommerce solution. Write to her at [email protected].
References
1. Jarząbek-Perz S et al. J Cosmet Dermatol. 2023 Dec;22(12):3305-3312..
2. Qin X et al. Front Physiol. 2022 Mar 14;13:856699.
3. Grimes PE et al. Cutis. 2004 Feb;73(2 Suppl):3-13.
4. Glaser DA. Facial Plast Surg Clin North Am. 2003 May;11(2):219-227.
5. Jarząbek-Perz S et al. Skin Res Technol. 2023 Jun;29(6):e13353.
6. Jarząbek-Perz S et al. Skin Res Technol. 2021 Sep;27(5):925-930.
7. Zerbinati N et al. Molecules. 2021 Dec 15;26(24):7592.
8. Zerbinati Net al. Pharmaceuticals (Basel). 2023 Apr 27;16(5):655.
9. Kantikosum K et al. Clin Cosmet Investig Dermatol. 2019 Feb 19;12:151-161.
10. Jayamani J et al. Int J Biol Macromol. 2018 Feb;107(Pt A):175-185.
Study Finds Differences in Side Effect Profiles With Two Oral Psoriasis Therapies
TOPLINE:
, according to a retrospective comparison using US Food and Drug Administration (FDA) data.
METHODOLOGY:
- To evaluate the adverse events associated with apremilast, an oral phosphodiesterase-4 (PDE4) inhibitor, and deucravacitinib, an oral tyrosine kinase 2 (TYK2) inhibitor, data were drawn from the FDA’s Adverse Event Reporting System database.
- The Medex_UIMA_1.8.3 system was used to standardize drug names, and MedDRA terminology was used to encode, categorize, and localize signals.
- AE event signals were grouped by skin and subcutaneous tissue disorders, gastrointestinal disorders, infections and infestations, and nervous system disorders.
TAKEAWAY:
- There were 95,734 AE reports for apremilast and 760 AE reports for deucravacitinib, and AEs were found to be significant over time.
- The more common cutaneous AEs were psoriasis recurrence and acne (associated with apremilast) and skin burning and erythema (associated with deucravacitinib).
- The more common gastrointestinal AEs were diarrhea and nausea (apremilast) and mouth ulceration (deucravacitinib).
- Deucravacitinib-related pruritus and rash, as well as apremilast-related tension headache, were more common in women than men; deucravacitinib-related skin burning was more common in men.
IN PRACTICE:
The results “can help the doctors to choose the right treatment options based on the baseline characteristics of different patients,” said Yuanyuan Xu, a graduate student in the Department of Dermatology, Sichuan University, Chengdu, China.
SOURCE:
Mr. Xu presented the study as a poster at the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis 2024 annual meeting.
LIMITATIONS:
The study was retrospective and cannot prove causality, and there were far fewer AE reports related to deucravacitinib, likely because the drug was introduced more recently.
DISCLOSURES:
The study received no funding, and the authors had no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
TOPLINE:
, according to a retrospective comparison using US Food and Drug Administration (FDA) data.
METHODOLOGY:
- To evaluate the adverse events associated with apremilast, an oral phosphodiesterase-4 (PDE4) inhibitor, and deucravacitinib, an oral tyrosine kinase 2 (TYK2) inhibitor, data were drawn from the FDA’s Adverse Event Reporting System database.
- The Medex_UIMA_1.8.3 system was used to standardize drug names, and MedDRA terminology was used to encode, categorize, and localize signals.
- AE event signals were grouped by skin and subcutaneous tissue disorders, gastrointestinal disorders, infections and infestations, and nervous system disorders.
TAKEAWAY:
- There were 95,734 AE reports for apremilast and 760 AE reports for deucravacitinib, and AEs were found to be significant over time.
- The more common cutaneous AEs were psoriasis recurrence and acne (associated with apremilast) and skin burning and erythema (associated with deucravacitinib).
- The more common gastrointestinal AEs were diarrhea and nausea (apremilast) and mouth ulceration (deucravacitinib).
- Deucravacitinib-related pruritus and rash, as well as apremilast-related tension headache, were more common in women than men; deucravacitinib-related skin burning was more common in men.
IN PRACTICE:
The results “can help the doctors to choose the right treatment options based on the baseline characteristics of different patients,” said Yuanyuan Xu, a graduate student in the Department of Dermatology, Sichuan University, Chengdu, China.
SOURCE:
Mr. Xu presented the study as a poster at the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis 2024 annual meeting.
LIMITATIONS:
The study was retrospective and cannot prove causality, and there were far fewer AE reports related to deucravacitinib, likely because the drug was introduced more recently.
DISCLOSURES:
The study received no funding, and the authors had no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
TOPLINE:
, according to a retrospective comparison using US Food and Drug Administration (FDA) data.
METHODOLOGY:
- To evaluate the adverse events associated with apremilast, an oral phosphodiesterase-4 (PDE4) inhibitor, and deucravacitinib, an oral tyrosine kinase 2 (TYK2) inhibitor, data were drawn from the FDA’s Adverse Event Reporting System database.
- The Medex_UIMA_1.8.3 system was used to standardize drug names, and MedDRA terminology was used to encode, categorize, and localize signals.
- AE event signals were grouped by skin and subcutaneous tissue disorders, gastrointestinal disorders, infections and infestations, and nervous system disorders.
TAKEAWAY:
- There were 95,734 AE reports for apremilast and 760 AE reports for deucravacitinib, and AEs were found to be significant over time.
- The more common cutaneous AEs were psoriasis recurrence and acne (associated with apremilast) and skin burning and erythema (associated with deucravacitinib).
- The more common gastrointestinal AEs were diarrhea and nausea (apremilast) and mouth ulceration (deucravacitinib).
- Deucravacitinib-related pruritus and rash, as well as apremilast-related tension headache, were more common in women than men; deucravacitinib-related skin burning was more common in men.
IN PRACTICE:
The results “can help the doctors to choose the right treatment options based on the baseline characteristics of different patients,” said Yuanyuan Xu, a graduate student in the Department of Dermatology, Sichuan University, Chengdu, China.
SOURCE:
Mr. Xu presented the study as a poster at the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis 2024 annual meeting.
LIMITATIONS:
The study was retrospective and cannot prove causality, and there were far fewer AE reports related to deucravacitinib, likely because the drug was introduced more recently.
DISCLOSURES:
The study received no funding, and the authors had no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Risk of MACE Comparable Among Biologic Classes for Psoriasis, PsA
TOPLINE:
a database analysis finds.
METHODOLOGY:
- Data from the TriNetX health records database included 32,758 patients treated with TNF inhibitors (TNFi, 62.9%), interleukin-17 inhibitors (IL-17i, 15.4%), IL-23i (10.7%), and IL-12i/IL-23i (10.7%).
- The researchers calculated time-dependent risk for MACE using multinomial Cox proportional hazard ratios. The reference was TNFi exposure.
- Subset analyses compared MACE in patients with and without existing cardiovascular disease.
TAKEAWAY:
- Compared with TNFi use, there was no difference in the incidence of MACE events in the IL-17i, IL-23i, or IL-12i/IL-23i group.
- There were also no significant differences between biologic groups in the incidence of congestive heart failure, myocardial infarction, or cerebral vascular accident/stroke.
IN PRACTICE:
Despite some concern about increased risk for MACE with TNFi use, this study suggests no special risk for patients with psoriasis or PsA associated with TNFi vs other biologics. “Given our results, as it pertains to MACE, prescribers shouldn’t favor any one biologic class over another,” said lead investigator Shikha Singla, MD, medical director of the Psoriatic Arthritis Program at Medical College of Wisconsin in Milwaukee, Wisconsin.
SOURCE:
Bonit Gill, MD, a second-year fellow at Medical College of Wisconsin, presented the study as a poster at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
LIMITATIONS:
The study’s retrospective nature makes it impossible to prove causation and the patients included in the study were from Wisconsin, which may limit generalizability.
DISCLOSURES:
Dr. Gill had no relevant financial disclosures. Other study authors participated in trials or consulted for AbbVie, AstraZeneca, Novartis, Eli Lilly, Janssen, and UCB.
A version of this article first appeared on Medscape.com.
TOPLINE:
a database analysis finds.
METHODOLOGY:
- Data from the TriNetX health records database included 32,758 patients treated with TNF inhibitors (TNFi, 62.9%), interleukin-17 inhibitors (IL-17i, 15.4%), IL-23i (10.7%), and IL-12i/IL-23i (10.7%).
- The researchers calculated time-dependent risk for MACE using multinomial Cox proportional hazard ratios. The reference was TNFi exposure.
- Subset analyses compared MACE in patients with and without existing cardiovascular disease.
TAKEAWAY:
- Compared with TNFi use, there was no difference in the incidence of MACE events in the IL-17i, IL-23i, or IL-12i/IL-23i group.
- There were also no significant differences between biologic groups in the incidence of congestive heart failure, myocardial infarction, or cerebral vascular accident/stroke.
IN PRACTICE:
Despite some concern about increased risk for MACE with TNFi use, this study suggests no special risk for patients with psoriasis or PsA associated with TNFi vs other biologics. “Given our results, as it pertains to MACE, prescribers shouldn’t favor any one biologic class over another,” said lead investigator Shikha Singla, MD, medical director of the Psoriatic Arthritis Program at Medical College of Wisconsin in Milwaukee, Wisconsin.
SOURCE:
Bonit Gill, MD, a second-year fellow at Medical College of Wisconsin, presented the study as a poster at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
LIMITATIONS:
The study’s retrospective nature makes it impossible to prove causation and the patients included in the study were from Wisconsin, which may limit generalizability.
DISCLOSURES:
Dr. Gill had no relevant financial disclosures. Other study authors participated in trials or consulted for AbbVie, AstraZeneca, Novartis, Eli Lilly, Janssen, and UCB.
A version of this article first appeared on Medscape.com.
TOPLINE:
a database analysis finds.
METHODOLOGY:
- Data from the TriNetX health records database included 32,758 patients treated with TNF inhibitors (TNFi, 62.9%), interleukin-17 inhibitors (IL-17i, 15.4%), IL-23i (10.7%), and IL-12i/IL-23i (10.7%).
- The researchers calculated time-dependent risk for MACE using multinomial Cox proportional hazard ratios. The reference was TNFi exposure.
- Subset analyses compared MACE in patients with and without existing cardiovascular disease.
TAKEAWAY:
- Compared with TNFi use, there was no difference in the incidence of MACE events in the IL-17i, IL-23i, or IL-12i/IL-23i group.
- There were also no significant differences between biologic groups in the incidence of congestive heart failure, myocardial infarction, or cerebral vascular accident/stroke.
IN PRACTICE:
Despite some concern about increased risk for MACE with TNFi use, this study suggests no special risk for patients with psoriasis or PsA associated with TNFi vs other biologics. “Given our results, as it pertains to MACE, prescribers shouldn’t favor any one biologic class over another,” said lead investigator Shikha Singla, MD, medical director of the Psoriatic Arthritis Program at Medical College of Wisconsin in Milwaukee, Wisconsin.
SOURCE:
Bonit Gill, MD, a second-year fellow at Medical College of Wisconsin, presented the study as a poster at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
LIMITATIONS:
The study’s retrospective nature makes it impossible to prove causation and the patients included in the study were from Wisconsin, which may limit generalizability.
DISCLOSURES:
Dr. Gill had no relevant financial disclosures. Other study authors participated in trials or consulted for AbbVie, AstraZeneca, Novartis, Eli Lilly, Janssen, and UCB.
A version of this article first appeared on Medscape.com.