User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
FDA Approves First Engineered Cell Therapy for a Solid Tumor
Afami-cel — the first engineered cell therapy for a solid tumor — is indicated specifically for adults with unresectable or metastatic synovial sarcoma who have received prior chemotherapy, are positive for several human leukocyte antigens (HLAs), and whose tumors express melanoma-associated antigen A4, as determined by FDA-authorized companion diagnostic devices.
The single-dose treatment targets solid tumors expressing melanoma-associated antigen A4, a protein highly expressed in synovial sarcoma.
Synovial sarcoma is a rare form of cancer, which affects about 1000 people in the US each year. Malignant cells develop and form a tumor in soft tissues, often in the extremities.
“Adults with metastatic synovial sarcoma, a life-threatening form of cancer, often face limited treatment options in addition to the risk of cancer spread or recurrence,” Nicole Verdun, MD, director of the Office of Therapeutic Products in the FDA’s Center for Biologics Evaluation and Research, said in the agency press release announcing the approval. “Today’s approval represents a significant milestone in the development of an innovative, safe and effective therapy for patients with this rare but potentially fatal disease.”
T-cell receptor therapy, like chimeric antigen receptor (CAR) T-cell (CAR-T) therapy, involves altering patient T cells to fight cancer. While CAR-T therapy inserts an artificial receptor to target a specific surface protein on cancer cells, the T-cell receptor therapy modifies existing receptors to recognize an array of antigens on the surface of cancer cells — a promising strategy for targeting solid tumors.
The accelerated approval of afami-cel was based on the phase 2 SPEARHEAD-1 trial in 44 patients with synovial sarcoma who received a single infusion of the therapy. The trial had enrolled 52 patients, but 8 did not receive afami-cel, including 3 who died and 1 who withdrew.
According to the FDA announcement, the overall response rate was 43.2%, with a median time to response of 4.9 weeks. The median duration of response was 6 months (95% CI, 4.6 months to not reached). Among patients who responded, 39% had a duration of response of 12 months or longer.
“These results suggest that a one-time treatment with afami-cel has the potential to extend life while allowing responders to go off chemotherapy,” said lead investigator Sandra D’Angelo, MD, a sarcoma specialist at Memorial Sloan Kettering Cancer Center in New York City, in a company press release.
The prescribing information includes a boxed warning for serious or fatal cytokine release syndrome.
The most common nonlaboratory adverse reactions, occurring in at least 20% of patients, included cytokine release syndrome, nausea, vomiting, fatigue, infections, pyrexia, constipation, dyspnea, tachycardia, hypotension, diarrhea, and edema. The most common grade 3 or 4 laboratory abnormalities, occurring in at least 20% of patients, included decreased lymphocyte count, neutrophil count, white cell blood count, red blood cell, and platelet count.
The recommended dose is between 2.68x109 to 10x109 MAGE-A4 T-cell receptor–positive T-cells. The FDA notice specifies not using a leukodepleting filter or prophylactic systemic corticosteroids.
The list price for the one-time therapy is $727,000, according to Fierce Pharma.
A version of this article first appeared on Medscape.com.
Afami-cel — the first engineered cell therapy for a solid tumor — is indicated specifically for adults with unresectable or metastatic synovial sarcoma who have received prior chemotherapy, are positive for several human leukocyte antigens (HLAs), and whose tumors express melanoma-associated antigen A4, as determined by FDA-authorized companion diagnostic devices.
The single-dose treatment targets solid tumors expressing melanoma-associated antigen A4, a protein highly expressed in synovial sarcoma.
Synovial sarcoma is a rare form of cancer, which affects about 1000 people in the US each year. Malignant cells develop and form a tumor in soft tissues, often in the extremities.
“Adults with metastatic synovial sarcoma, a life-threatening form of cancer, often face limited treatment options in addition to the risk of cancer spread or recurrence,” Nicole Verdun, MD, director of the Office of Therapeutic Products in the FDA’s Center for Biologics Evaluation and Research, said in the agency press release announcing the approval. “Today’s approval represents a significant milestone in the development of an innovative, safe and effective therapy for patients with this rare but potentially fatal disease.”
T-cell receptor therapy, like chimeric antigen receptor (CAR) T-cell (CAR-T) therapy, involves altering patient T cells to fight cancer. While CAR-T therapy inserts an artificial receptor to target a specific surface protein on cancer cells, the T-cell receptor therapy modifies existing receptors to recognize an array of antigens on the surface of cancer cells — a promising strategy for targeting solid tumors.
The accelerated approval of afami-cel was based on the phase 2 SPEARHEAD-1 trial in 44 patients with synovial sarcoma who received a single infusion of the therapy. The trial had enrolled 52 patients, but 8 did not receive afami-cel, including 3 who died and 1 who withdrew.
According to the FDA announcement, the overall response rate was 43.2%, with a median time to response of 4.9 weeks. The median duration of response was 6 months (95% CI, 4.6 months to not reached). Among patients who responded, 39% had a duration of response of 12 months or longer.
“These results suggest that a one-time treatment with afami-cel has the potential to extend life while allowing responders to go off chemotherapy,” said lead investigator Sandra D’Angelo, MD, a sarcoma specialist at Memorial Sloan Kettering Cancer Center in New York City, in a company press release.
The prescribing information includes a boxed warning for serious or fatal cytokine release syndrome.
The most common nonlaboratory adverse reactions, occurring in at least 20% of patients, included cytokine release syndrome, nausea, vomiting, fatigue, infections, pyrexia, constipation, dyspnea, tachycardia, hypotension, diarrhea, and edema. The most common grade 3 or 4 laboratory abnormalities, occurring in at least 20% of patients, included decreased lymphocyte count, neutrophil count, white cell blood count, red blood cell, and platelet count.
The recommended dose is between 2.68x109 to 10x109 MAGE-A4 T-cell receptor–positive T-cells. The FDA notice specifies not using a leukodepleting filter or prophylactic systemic corticosteroids.
The list price for the one-time therapy is $727,000, according to Fierce Pharma.
A version of this article first appeared on Medscape.com.
Afami-cel — the first engineered cell therapy for a solid tumor — is indicated specifically for adults with unresectable or metastatic synovial sarcoma who have received prior chemotherapy, are positive for several human leukocyte antigens (HLAs), and whose tumors express melanoma-associated antigen A4, as determined by FDA-authorized companion diagnostic devices.
The single-dose treatment targets solid tumors expressing melanoma-associated antigen A4, a protein highly expressed in synovial sarcoma.
Synovial sarcoma is a rare form of cancer, which affects about 1000 people in the US each year. Malignant cells develop and form a tumor in soft tissues, often in the extremities.
“Adults with metastatic synovial sarcoma, a life-threatening form of cancer, often face limited treatment options in addition to the risk of cancer spread or recurrence,” Nicole Verdun, MD, director of the Office of Therapeutic Products in the FDA’s Center for Biologics Evaluation and Research, said in the agency press release announcing the approval. “Today’s approval represents a significant milestone in the development of an innovative, safe and effective therapy for patients with this rare but potentially fatal disease.”
T-cell receptor therapy, like chimeric antigen receptor (CAR) T-cell (CAR-T) therapy, involves altering patient T cells to fight cancer. While CAR-T therapy inserts an artificial receptor to target a specific surface protein on cancer cells, the T-cell receptor therapy modifies existing receptors to recognize an array of antigens on the surface of cancer cells — a promising strategy for targeting solid tumors.
The accelerated approval of afami-cel was based on the phase 2 SPEARHEAD-1 trial in 44 patients with synovial sarcoma who received a single infusion of the therapy. The trial had enrolled 52 patients, but 8 did not receive afami-cel, including 3 who died and 1 who withdrew.
According to the FDA announcement, the overall response rate was 43.2%, with a median time to response of 4.9 weeks. The median duration of response was 6 months (95% CI, 4.6 months to not reached). Among patients who responded, 39% had a duration of response of 12 months or longer.
“These results suggest that a one-time treatment with afami-cel has the potential to extend life while allowing responders to go off chemotherapy,” said lead investigator Sandra D’Angelo, MD, a sarcoma specialist at Memorial Sloan Kettering Cancer Center in New York City, in a company press release.
The prescribing information includes a boxed warning for serious or fatal cytokine release syndrome.
The most common nonlaboratory adverse reactions, occurring in at least 20% of patients, included cytokine release syndrome, nausea, vomiting, fatigue, infections, pyrexia, constipation, dyspnea, tachycardia, hypotension, diarrhea, and edema. The most common grade 3 or 4 laboratory abnormalities, occurring in at least 20% of patients, included decreased lymphocyte count, neutrophil count, white cell blood count, red blood cell, and platelet count.
The recommended dose is between 2.68x109 to 10x109 MAGE-A4 T-cell receptor–positive T-cells. The FDA notice specifies not using a leukodepleting filter or prophylactic systemic corticosteroids.
The list price for the one-time therapy is $727,000, according to Fierce Pharma.
A version of this article first appeared on Medscape.com.
Future of Lupus Treatments Looks Brighter With Multiple Promising Therapeutic Approaches
VIENNA — It may have been a while since there have been any major breakthroughs in the treatment of systemic lupus erythematosus (SLE), but there are high hopes that this is a situation that may be about to change, experts agreed at the annual European Congress of Rheumatology.
“It’s an incredibly vivid area of development,” Laurent Arnaud, MD, PhD, professor of rheumatology at the University of Strasbourg in Strasbourg, France, said during one of the first sessions of the meeting. He reported that there were at least 17 phase 2 and 14 phase 3 trials that were expected to start within the next few years, all with investigational agents that target different immune cells or pathways that have been implicated in the pathogenesis of SLE.
In a systematic review published last year, Dr. Arnaud and coauthors found that there were 92 investigational biologic or novel targeted agents in various phases of clinical testing. This included B-cell–targeting agents such as ianalumab, plasma cell-targeting agents such as daratumumab, and drugs with novel mechanisms of action such as KPG-818, which targets the CRL4-Cereblon (CRBN) E3 ubiquitin ligase complex. Phase 2 data on all three of these investigational agents were presented during various sessions at EULAR 2024, all with positive results, suggesting that their further development in SLE is worth pursuing.
There are of course “many more candidates in the pipeline,” Dr. Arnaud said. “I’m very happy that I think we are going to see great days for lupus right in front of our eyes.”
Targeting B Cells
Drugs that target B cells have been at the forefront of lupus treatment for several years, as David Isenberg, MD, professor of rheumatology at University College London, pointed out during an interview for EULAR TV.
“It’s clearly important to target the cells which are likely to be causing the problem in lupus, and in the main, that tends to be B cells,” he said.
Dr. Isenberg, who is renowned for his work with the B-cell–targeting agent rituximab, added: “But we know that obviously T cells integrate with B cells, so anything which interrupts the link between the T cell and the B cell is likely to be important.”
Chimeric Antigen Receptor (CAR) T-Cell Therapy ‘Revolution’
One new way of targeting B cells is with CAR T-cell therapy, which David D’Cruz , MD, PhD, a consultant rheumatologist for Guy’s and St. Thomas’ Hospital NHS Foundation Trust in London, picked as one of the “most striking” topics highlighted at EULAR 2024.
This is “truly personalized medicine,” Dr. D’Cruz said. This is an autologous therapy because a patient’s T cells are removed by leukapheresis, transfected with a CAR T vector directed against a component of the B cell, and then returned to them.
“I do feel that we’re on the cusp of a major revolution,” Dr. D’Cruz told this news organization. Not only in lupus but also in other rheumatic conditions that have proved really difficult to treat, such as systemic sclerosis and myositis, he said.
“Basically, it’s a very powerful B-cell–depleting tool, but it’s much more profound B-cell–depleting tool than, for example, rituximab or belimumab,” explained Dr. D’Cruz. “What you’re doing is reprogramming T cells to attack the B cells.”
Although rituximab and belimumab clear all the B cells in the circulation, there are still some cells left behind in the bone marrow, “and it’s very difficult to get rid of those,” Dr. D’Cruz said. “What CAR T-cell therapy appears to do is wipe out all the CD19-positive B cells everywhere, in the blood and the tissue. So you get a really profound B-cell depletion.”
Eric Morand, MBBS, PhD, head of rheumatology at Monash Health in Melbourne, Australia, told this news organization that there was obviously “a lot of buzz” about CAR T-cell therapy.
“We’re waiting to see if the exciting data from Erlangen can be reproduced in other centers with other CAR T products to show that it is a universal effect. We haven’t seen that yet, but I think we will by next year.”
Cost and expertise are two major considerations and potential limiting factors, however, as Dr. D’Cruz and Dr. Isenberg both pointed out in separate interviews with this news organization.
Dr. D’Cruz said: “It’s very expensive, it takes a while, and it doesn’t always work is what I’m hearing. It’s usually successful, but again, a little bit depends on the technique and the people doing the process.”
Dr. Isenberg said: “CAR T-cell therapy is, I think, very exciting because it does look to be quite promising. But as it costs 350,000 euros per patient, I don’t think that it is going to be widely adopted.”
Even if it could be afforded by certain centers in the West, he added, this just would not be feasible in poorer nations. “So, we’ve got to find other effective, cheaper ways to go,” Dr. Isenberg said.
“I think there are some very interesting ideas with monoclonal antibodies which target at least two different targets — one on the B cell, one on the T cell — and that could well be the way to take this forward,” he suggested.
Ianalumab ‘Double Blocking’ B Cells
Another way could be to develop more potent B-cell–depleting drugs, as Nancy Agmon-Levin , MD, head of the Clinical Immunology, Angioedema and Allergy Unit, Lupus and Autoimmune Diseases Clinic, at Sheba Medical Center, Tel Aviv University in Tel Aviv, Israel, reported during one of the clinical abstract sessions at EULAR 2024.
Dr. Agmon-Levin presented data on 67 individuals with SLE who had participated in a multicenter phase 2 study of ianalumab, a fully human immunoglobulin (Ig) G1 monoclonal antibody that results in a “double blocking of the B-cell lineage.”
Ianalumab targets the B-cell–activating factor receptor (BAFFR), but what makes it distinct from other BAFF-targeting drugs is that it has had a fructose molecule removed from its Fc portion, which renders it more likely to trigger antibody-dependent cellular cytotoxicity.
“This is a B-cell depletion therapy,” Agmon-Levin said, but it also blocks BAFFR-mediated survival of B cells, so the subsequent recuperation process of BAFFR-expressing B cells is affected, leading to continued B-cell depletion.
The phase 2 study she presented consisted of an initial 28-week, double-blind period, during which time participants had been randomly allocated to receive either subcutaneous injections of ianalumab 300 mg or a matching placebo every 4 weeks. This was followed by a 24-week, open-label period where all participants were treated with ianalumab, and then an off-treatment, minimal follow-up period that lasted up to 68 weeks, with continued data collection for safety.
The primary outcome measure was a composite of meeting criteria for the SLE Responder Index 4 and a sustained reduction in corticosteroid use at 28 weeks. This was achieved in 15 of the 34 (44.1%) people treated with ianalumab vs only 3 (9.1%) of the 33 people who had been given a placebo.
Dr. Agmon-Levin reported that the effect on this outcome was sustained to the end of the open-label period, at 1 year, in 15 (45.5%) of 33 participants who had continued treatment with ianalumab and achieved in 13 (40.6%) of 32 participants who had switched from placebo to ianalumab treatment.
Moreover, longer durations of treatment were associated with a host of improved outcomes, Dr. Agmon-Levin said: “Treatment was improved along the 52 weeks, and we can see from the LLDAS [Lupus Low Disease Activity State], DORIS [Definition Of Remission In SLE], and SRI-6 and -8 that as you continue the therapy, you improve the outcomes.”
The potential benefits of ianalumab in the treatment of SLE and lupus nephritis will now be further examined in the phase 3 SIRIUS-SLE1 , SIRIUS-SLE2, and SIRIUS-LN trials, which are estimated to provide initial results in 2027 and complete in early 2029 or 2030.
Targeting Plasma Cells With Daratumumab
Another drug showing signs that it might be useful as a treatment for SLE is daratumumab, as Tobias Alexander, MD, of Charité — Universitätsmedizin Berlin, reported during one of the late-breaking abstract sessions at EULAR 2024.
“Daratumumab is a human, first-in-class anti-CD38 antibody that efficiently depletes plasma cells,” Dr. Alexander said. CD38 is both a receptor and an enzyme, and while it is found on the surface of most immune cells, it’s particularly expressed by plasma cells, he added.
Daratumumab is not a total newcomer, however, as it’s already approved for the treatment of multiple myeloma under the trade name Darzalex. The rationale for using it in SLE comes from two case reports, Dr. Alexander explained. The first, published in 2020 in The New England Journal of Medicine, involved two patients with severe and life-threatening lupus who were given off-label treatment for a period of 4 weeks and experienced good clinical and serologic responses. The second, published last year in Nature Medicine, involved six patients with refractory lupus nephritis, five of whom had a clinical response at 6 months.
“On this background, we conducted an investigator-initiated trial, which was an open-label, single-center, proof-of-concept study,” Dr. Alexander said. A total of 10 female patients whose ages ranged from 24 to 43 years were included in the phase 2 trial that was dubbed DARALUP. For inclusion, all had to have a Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) of four or more for clinical manifestations, have been treated with at least two prior disease-modifying drugs to no avail, and be anti–double-stranded DNA (anti-dsDNA) antibody positive. Dr. Alexander reported that the median baseline SLEDAI-2K score was 12 and ranged from 8 to 20, with the number of prior therapies ranging from two to nine.
Daratumumab was given at a dose of 1800 mg via subcutaneous injection every week for 8 weeks. This is the same dose that is used to treat multiple myeloma, Dr. Alexander explained, although the dosing is not stopped. The reason for stopping after 8 weeks in the current trial was to be able to see what happened once the treatment was stopped. The follow-up was for 36 weeks.
Dr. Alexander reported that there was a “very dramatic and significant” effect on the primary endpoint of a reduction in anti-dsDNA antibody levels, decreasing from a median of 166.3 U/mL at baseline to 61.1 U/mL at week 12 (P = .002). Alongside, there was a reduction in the SLEDAI-2K score from 12 to 4 within 12 weeks, which was sustained at the 36-week follow-up assessment. Improvements in skin, joint, kidney, and level of proteinuria were also seen.
Although all patients experienced adverse events, none were serious. Infections and infestations (mostly nasopharyngitis, COVID-19, and gastroenteritis) were the most common, experienced by 80% of the participants; 70% had injection site reactions or fatigue, 60% had gastrointestinal symptoms, 50% had a fall of IgG < 5 g/L, 40% had headache, and 20% had back pain.
“This is a positive trial. I think we could demonstrate that [daratumumab] produced very strong, rapid, and durable clinical improvements,” Dr. Alexander said. “We think that targeting CD38 is relevant; plasma cells had been depleted based on the reduction of anti-dsDNA antibodies,” he added.
From the audience, however, Peter Nash, MBBS, of Griffith University in Brisbane, Australia, questioned whether the results could be attributed to “a steroid effect” because patients had been treated with oral dexamethasone throughout the study.
Dr. Alexander noted that steroid use had been part of the treatment schedule but acknowledged it was a possible confounder.
“I think we can be confident that [daratumumab] had a major effect on plasma cells decreasing…because we see that also the vaccine titers decreased,” Dr. Alexander said. “Time will tell, but even more important is the durability of the responses over time, which you don’t achieve under steroids.”
KPG-818’s Novel Mechanism of Action
Elsewhere at EULAR 2024, positive results of another phase 2 study involving a drug with an entirely different mechanism of action, KPG-818, were reported in a poster presentation. KPG-818 modulates CRBN, which results in the degradation of two transcription factors (Aiolos and Ikaros) that are involved in the development, maturation, and proliferation of innate and adaptive immune cells and have been linked to genetic risk in SLE, according to the poster’s authors. It is currently in development for the treatment of SLE, Behçet disease, inflammatory bowel disease, multiple myeloma, and non-Hodgkin lymphoma.
Yao Wang, MD, chief medical officer of KPG-818’s developer Kangpu Biopharmaceuticals, Hefei, China, and associates found that oral doses of 0.15 or 0.6 mg KPG-818 were “generally well-tolerated” and produced immunomodulatory changes that could be beneficial in people with SLE over a 12-week treatment period.
“Only two new agents have been approved for the treatment of SLE in the past five decades in USA and Europe,” Dr. Wang and team wrote, which highlights “a significant unmet need for more effective and safe treatment options.”
They believe that KPG-818 might well fit the bill based on the results of their study, in which 35 of 37 recruited patients completed the treatment. Compared with placebo, they observed reduced numbers of total B cells, Aiolos+ T and B cells, and increased Treg cells.
SLEDAI-2K and Cutaneous Lupus Erythematosus Disease Area and Severity Index activity scores in the 0.15-mg group were improved relative to baseline and placebo.
“The proof-of-concept findings suggest a favorable benefit/risk ratio in SLE for KPG-818,” Dr. Wang and coauthors said, supporting its further development in SLE.
Need for Treatments
Dr. Isenberg told this news organization that both daratumumab and KPG-818 would be welcome additions as treatment options if further trials proved their worth.
“The great frustration about lupus is that, compared to patients with rheumatoid arthritis, the choice has been so limited,” Dr. Isenberg said. Aside from rituximab (Rituxan) and belimumab (Benlysta), which are used with certain restrictions, there are no other biologic targeted treatments available in the United Kingdom. Anifrolumab (Saphnelo) has a license in the United States and some European countries but is not yet available for him to use in his practice.
Daratumumab and KPG-818 are “different types of molecules, and if they work that will be great. It would be nice to have the choice,” Dr. Isenberg said. “Whether they will be as effective as I think rituximab is, I don’t know, but these are some very encouraging results.”
Of course, these are all phase 2 trials, and the “big problem” is that such positive results do not always translate when it comes to phase 3, as Dr. D’Cruz told this news organization.
“Until a few years ago, there had been about 25 or 30 industry-led trails, and they’d all failed, except for belimumab and anifrolumab,” Dr. D’Cruz said. These drugs were found to work and be generally safe in phase 1 and 2 trials, but “when they come to phase 3, they all seem to fail, and we don’t know why.”
These are large global studies, D’Cruz added, observing that problems with patient selection, steroid use, and choice of outcome measures were possible factors for why the EXPLORER and LUNAR studies had shown no benefit for rituximab despite the drug being widely used to treat SLE.
Dr. Isenberg, who has coauthored an article on the topic of why drugs seem to fail at the final hurdle, noted: “I think it has a lot to do with the nature of the disease. It’s a complicated disease.” From having “savvy physicians doing the trials for you” to the placebo response, there are “a whole bunch or reasons why these things haven’t worked in lupus.”
Dr. Morand commented: “We’ve got many programs in phase 2 and 3, and because there’s so many, they’re all facing recruitment challenges, and as a consequence of so much activity, every program is going a little slower than hoped for.”
As for other drugs on the horizon, Dr. Morand noted: “We’re very optimistic about things like litifilimab and deucravacitinib; that’s two examples that are in phase 3. Earlier in the program of development, [there are] a huge range of targets being addressed. The future looks bright. But we might have to wait a while.”
Dr. Arnaud has consulted for AstraZeneca, AbbVie, Alpine Immune Sciences, Biogen, Bristol Myers Squibb, Boehringer Ingelheim, Chugai Pharmaceutical, GlaxoSmithKline, Grifols, Janssen, Kezar Life Sciences, LFB, Lilly, Medac, Merck, Novartis, Pfizer, Roche, and UCB. Dr. Isenberg has served as an adviser to Merck Serono, AstraZeneca, Eli Lilly, Servier, and ImmuPharma. Any honoraria received is passed on to a local arthritis charity connected to his hospital. Dr. D’Cruz has served as a consultant and advisory board member for GlaxoSmithKline and CSL Vifor. Dr. Morand has received research support, consultancy fees, or both from multiple pharmaceutical companies paid to his institution including AbbVie, Amgen, AstraZeneca, Biogen, Bristol Myers Squibb, Eli Lilly, EMD Serono, Dragonfly, Genentech, GlaxoSmithKline, Janssen, Novartis, RemeGen, Takeda, UCB, and Zenas. The ianalumab trial presented by Dr. Agmon-Levin was sponsored by Novartis Pharma; however, she reported having no conflicts of interest. The DARALUP study was an investigator-initiated trial supported by Janssen. Dr. Alexander has received consulting fees, study support, honoraria, and travel grants from various pharmaceutical companies including AbbVie, Amgen, AstraZeneca, Bayer, GlaxoSmithKline, Janssen, and Lilly. Dr. Nash has consulted for The Rheumatology Education Group Consultants. The KPG-818 study reported by Dr. Wang was sponsored by Kangpu Biopharmaceuticals.
A version of this article first appeared on Medscape.com.
VIENNA — It may have been a while since there have been any major breakthroughs in the treatment of systemic lupus erythematosus (SLE), but there are high hopes that this is a situation that may be about to change, experts agreed at the annual European Congress of Rheumatology.
“It’s an incredibly vivid area of development,” Laurent Arnaud, MD, PhD, professor of rheumatology at the University of Strasbourg in Strasbourg, France, said during one of the first sessions of the meeting. He reported that there were at least 17 phase 2 and 14 phase 3 trials that were expected to start within the next few years, all with investigational agents that target different immune cells or pathways that have been implicated in the pathogenesis of SLE.
In a systematic review published last year, Dr. Arnaud and coauthors found that there were 92 investigational biologic or novel targeted agents in various phases of clinical testing. This included B-cell–targeting agents such as ianalumab, plasma cell-targeting agents such as daratumumab, and drugs with novel mechanisms of action such as KPG-818, which targets the CRL4-Cereblon (CRBN) E3 ubiquitin ligase complex. Phase 2 data on all three of these investigational agents were presented during various sessions at EULAR 2024, all with positive results, suggesting that their further development in SLE is worth pursuing.
There are of course “many more candidates in the pipeline,” Dr. Arnaud said. “I’m very happy that I think we are going to see great days for lupus right in front of our eyes.”
Targeting B Cells
Drugs that target B cells have been at the forefront of lupus treatment for several years, as David Isenberg, MD, professor of rheumatology at University College London, pointed out during an interview for EULAR TV.
“It’s clearly important to target the cells which are likely to be causing the problem in lupus, and in the main, that tends to be B cells,” he said.
Dr. Isenberg, who is renowned for his work with the B-cell–targeting agent rituximab, added: “But we know that obviously T cells integrate with B cells, so anything which interrupts the link between the T cell and the B cell is likely to be important.”
Chimeric Antigen Receptor (CAR) T-Cell Therapy ‘Revolution’
One new way of targeting B cells is with CAR T-cell therapy, which David D’Cruz , MD, PhD, a consultant rheumatologist for Guy’s and St. Thomas’ Hospital NHS Foundation Trust in London, picked as one of the “most striking” topics highlighted at EULAR 2024.
This is “truly personalized medicine,” Dr. D’Cruz said. This is an autologous therapy because a patient’s T cells are removed by leukapheresis, transfected with a CAR T vector directed against a component of the B cell, and then returned to them.
“I do feel that we’re on the cusp of a major revolution,” Dr. D’Cruz told this news organization. Not only in lupus but also in other rheumatic conditions that have proved really difficult to treat, such as systemic sclerosis and myositis, he said.
“Basically, it’s a very powerful B-cell–depleting tool, but it’s much more profound B-cell–depleting tool than, for example, rituximab or belimumab,” explained Dr. D’Cruz. “What you’re doing is reprogramming T cells to attack the B cells.”
Although rituximab and belimumab clear all the B cells in the circulation, there are still some cells left behind in the bone marrow, “and it’s very difficult to get rid of those,” Dr. D’Cruz said. “What CAR T-cell therapy appears to do is wipe out all the CD19-positive B cells everywhere, in the blood and the tissue. So you get a really profound B-cell depletion.”
Eric Morand, MBBS, PhD, head of rheumatology at Monash Health in Melbourne, Australia, told this news organization that there was obviously “a lot of buzz” about CAR T-cell therapy.
“We’re waiting to see if the exciting data from Erlangen can be reproduced in other centers with other CAR T products to show that it is a universal effect. We haven’t seen that yet, but I think we will by next year.”
Cost and expertise are two major considerations and potential limiting factors, however, as Dr. D’Cruz and Dr. Isenberg both pointed out in separate interviews with this news organization.
Dr. D’Cruz said: “It’s very expensive, it takes a while, and it doesn’t always work is what I’m hearing. It’s usually successful, but again, a little bit depends on the technique and the people doing the process.”
Dr. Isenberg said: “CAR T-cell therapy is, I think, very exciting because it does look to be quite promising. But as it costs 350,000 euros per patient, I don’t think that it is going to be widely adopted.”
Even if it could be afforded by certain centers in the West, he added, this just would not be feasible in poorer nations. “So, we’ve got to find other effective, cheaper ways to go,” Dr. Isenberg said.
“I think there are some very interesting ideas with monoclonal antibodies which target at least two different targets — one on the B cell, one on the T cell — and that could well be the way to take this forward,” he suggested.
Ianalumab ‘Double Blocking’ B Cells
Another way could be to develop more potent B-cell–depleting drugs, as Nancy Agmon-Levin , MD, head of the Clinical Immunology, Angioedema and Allergy Unit, Lupus and Autoimmune Diseases Clinic, at Sheba Medical Center, Tel Aviv University in Tel Aviv, Israel, reported during one of the clinical abstract sessions at EULAR 2024.
Dr. Agmon-Levin presented data on 67 individuals with SLE who had participated in a multicenter phase 2 study of ianalumab, a fully human immunoglobulin (Ig) G1 monoclonal antibody that results in a “double blocking of the B-cell lineage.”
Ianalumab targets the B-cell–activating factor receptor (BAFFR), but what makes it distinct from other BAFF-targeting drugs is that it has had a fructose molecule removed from its Fc portion, which renders it more likely to trigger antibody-dependent cellular cytotoxicity.
“This is a B-cell depletion therapy,” Agmon-Levin said, but it also blocks BAFFR-mediated survival of B cells, so the subsequent recuperation process of BAFFR-expressing B cells is affected, leading to continued B-cell depletion.
The phase 2 study she presented consisted of an initial 28-week, double-blind period, during which time participants had been randomly allocated to receive either subcutaneous injections of ianalumab 300 mg or a matching placebo every 4 weeks. This was followed by a 24-week, open-label period where all participants were treated with ianalumab, and then an off-treatment, minimal follow-up period that lasted up to 68 weeks, with continued data collection for safety.
The primary outcome measure was a composite of meeting criteria for the SLE Responder Index 4 and a sustained reduction in corticosteroid use at 28 weeks. This was achieved in 15 of the 34 (44.1%) people treated with ianalumab vs only 3 (9.1%) of the 33 people who had been given a placebo.
Dr. Agmon-Levin reported that the effect on this outcome was sustained to the end of the open-label period, at 1 year, in 15 (45.5%) of 33 participants who had continued treatment with ianalumab and achieved in 13 (40.6%) of 32 participants who had switched from placebo to ianalumab treatment.
Moreover, longer durations of treatment were associated with a host of improved outcomes, Dr. Agmon-Levin said: “Treatment was improved along the 52 weeks, and we can see from the LLDAS [Lupus Low Disease Activity State], DORIS [Definition Of Remission In SLE], and SRI-6 and -8 that as you continue the therapy, you improve the outcomes.”
The potential benefits of ianalumab in the treatment of SLE and lupus nephritis will now be further examined in the phase 3 SIRIUS-SLE1 , SIRIUS-SLE2, and SIRIUS-LN trials, which are estimated to provide initial results in 2027 and complete in early 2029 or 2030.
Targeting Plasma Cells With Daratumumab
Another drug showing signs that it might be useful as a treatment for SLE is daratumumab, as Tobias Alexander, MD, of Charité — Universitätsmedizin Berlin, reported during one of the late-breaking abstract sessions at EULAR 2024.
“Daratumumab is a human, first-in-class anti-CD38 antibody that efficiently depletes plasma cells,” Dr. Alexander said. CD38 is both a receptor and an enzyme, and while it is found on the surface of most immune cells, it’s particularly expressed by plasma cells, he added.
Daratumumab is not a total newcomer, however, as it’s already approved for the treatment of multiple myeloma under the trade name Darzalex. The rationale for using it in SLE comes from two case reports, Dr. Alexander explained. The first, published in 2020 in The New England Journal of Medicine, involved two patients with severe and life-threatening lupus who were given off-label treatment for a period of 4 weeks and experienced good clinical and serologic responses. The second, published last year in Nature Medicine, involved six patients with refractory lupus nephritis, five of whom had a clinical response at 6 months.
“On this background, we conducted an investigator-initiated trial, which was an open-label, single-center, proof-of-concept study,” Dr. Alexander said. A total of 10 female patients whose ages ranged from 24 to 43 years were included in the phase 2 trial that was dubbed DARALUP. For inclusion, all had to have a Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) of four or more for clinical manifestations, have been treated with at least two prior disease-modifying drugs to no avail, and be anti–double-stranded DNA (anti-dsDNA) antibody positive. Dr. Alexander reported that the median baseline SLEDAI-2K score was 12 and ranged from 8 to 20, with the number of prior therapies ranging from two to nine.
Daratumumab was given at a dose of 1800 mg via subcutaneous injection every week for 8 weeks. This is the same dose that is used to treat multiple myeloma, Dr. Alexander explained, although the dosing is not stopped. The reason for stopping after 8 weeks in the current trial was to be able to see what happened once the treatment was stopped. The follow-up was for 36 weeks.
Dr. Alexander reported that there was a “very dramatic and significant” effect on the primary endpoint of a reduction in anti-dsDNA antibody levels, decreasing from a median of 166.3 U/mL at baseline to 61.1 U/mL at week 12 (P = .002). Alongside, there was a reduction in the SLEDAI-2K score from 12 to 4 within 12 weeks, which was sustained at the 36-week follow-up assessment. Improvements in skin, joint, kidney, and level of proteinuria were also seen.
Although all patients experienced adverse events, none were serious. Infections and infestations (mostly nasopharyngitis, COVID-19, and gastroenteritis) were the most common, experienced by 80% of the participants; 70% had injection site reactions or fatigue, 60% had gastrointestinal symptoms, 50% had a fall of IgG < 5 g/L, 40% had headache, and 20% had back pain.
“This is a positive trial. I think we could demonstrate that [daratumumab] produced very strong, rapid, and durable clinical improvements,” Dr. Alexander said. “We think that targeting CD38 is relevant; plasma cells had been depleted based on the reduction of anti-dsDNA antibodies,” he added.
From the audience, however, Peter Nash, MBBS, of Griffith University in Brisbane, Australia, questioned whether the results could be attributed to “a steroid effect” because patients had been treated with oral dexamethasone throughout the study.
Dr. Alexander noted that steroid use had been part of the treatment schedule but acknowledged it was a possible confounder.
“I think we can be confident that [daratumumab] had a major effect on plasma cells decreasing…because we see that also the vaccine titers decreased,” Dr. Alexander said. “Time will tell, but even more important is the durability of the responses over time, which you don’t achieve under steroids.”
KPG-818’s Novel Mechanism of Action
Elsewhere at EULAR 2024, positive results of another phase 2 study involving a drug with an entirely different mechanism of action, KPG-818, were reported in a poster presentation. KPG-818 modulates CRBN, which results in the degradation of two transcription factors (Aiolos and Ikaros) that are involved in the development, maturation, and proliferation of innate and adaptive immune cells and have been linked to genetic risk in SLE, according to the poster’s authors. It is currently in development for the treatment of SLE, Behçet disease, inflammatory bowel disease, multiple myeloma, and non-Hodgkin lymphoma.
Yao Wang, MD, chief medical officer of KPG-818’s developer Kangpu Biopharmaceuticals, Hefei, China, and associates found that oral doses of 0.15 or 0.6 mg KPG-818 were “generally well-tolerated” and produced immunomodulatory changes that could be beneficial in people with SLE over a 12-week treatment period.
“Only two new agents have been approved for the treatment of SLE in the past five decades in USA and Europe,” Dr. Wang and team wrote, which highlights “a significant unmet need for more effective and safe treatment options.”
They believe that KPG-818 might well fit the bill based on the results of their study, in which 35 of 37 recruited patients completed the treatment. Compared with placebo, they observed reduced numbers of total B cells, Aiolos+ T and B cells, and increased Treg cells.
SLEDAI-2K and Cutaneous Lupus Erythematosus Disease Area and Severity Index activity scores in the 0.15-mg group were improved relative to baseline and placebo.
“The proof-of-concept findings suggest a favorable benefit/risk ratio in SLE for KPG-818,” Dr. Wang and coauthors said, supporting its further development in SLE.
Need for Treatments
Dr. Isenberg told this news organization that both daratumumab and KPG-818 would be welcome additions as treatment options if further trials proved their worth.
“The great frustration about lupus is that, compared to patients with rheumatoid arthritis, the choice has been so limited,” Dr. Isenberg said. Aside from rituximab (Rituxan) and belimumab (Benlysta), which are used with certain restrictions, there are no other biologic targeted treatments available in the United Kingdom. Anifrolumab (Saphnelo) has a license in the United States and some European countries but is not yet available for him to use in his practice.
Daratumumab and KPG-818 are “different types of molecules, and if they work that will be great. It would be nice to have the choice,” Dr. Isenberg said. “Whether they will be as effective as I think rituximab is, I don’t know, but these are some very encouraging results.”
Of course, these are all phase 2 trials, and the “big problem” is that such positive results do not always translate when it comes to phase 3, as Dr. D’Cruz told this news organization.
“Until a few years ago, there had been about 25 or 30 industry-led trails, and they’d all failed, except for belimumab and anifrolumab,” Dr. D’Cruz said. These drugs were found to work and be generally safe in phase 1 and 2 trials, but “when they come to phase 3, they all seem to fail, and we don’t know why.”
These are large global studies, D’Cruz added, observing that problems with patient selection, steroid use, and choice of outcome measures were possible factors for why the EXPLORER and LUNAR studies had shown no benefit for rituximab despite the drug being widely used to treat SLE.
Dr. Isenberg, who has coauthored an article on the topic of why drugs seem to fail at the final hurdle, noted: “I think it has a lot to do with the nature of the disease. It’s a complicated disease.” From having “savvy physicians doing the trials for you” to the placebo response, there are “a whole bunch or reasons why these things haven’t worked in lupus.”
Dr. Morand commented: “We’ve got many programs in phase 2 and 3, and because there’s so many, they’re all facing recruitment challenges, and as a consequence of so much activity, every program is going a little slower than hoped for.”
As for other drugs on the horizon, Dr. Morand noted: “We’re very optimistic about things like litifilimab and deucravacitinib; that’s two examples that are in phase 3. Earlier in the program of development, [there are] a huge range of targets being addressed. The future looks bright. But we might have to wait a while.”
Dr. Arnaud has consulted for AstraZeneca, AbbVie, Alpine Immune Sciences, Biogen, Bristol Myers Squibb, Boehringer Ingelheim, Chugai Pharmaceutical, GlaxoSmithKline, Grifols, Janssen, Kezar Life Sciences, LFB, Lilly, Medac, Merck, Novartis, Pfizer, Roche, and UCB. Dr. Isenberg has served as an adviser to Merck Serono, AstraZeneca, Eli Lilly, Servier, and ImmuPharma. Any honoraria received is passed on to a local arthritis charity connected to his hospital. Dr. D’Cruz has served as a consultant and advisory board member for GlaxoSmithKline and CSL Vifor. Dr. Morand has received research support, consultancy fees, or both from multiple pharmaceutical companies paid to his institution including AbbVie, Amgen, AstraZeneca, Biogen, Bristol Myers Squibb, Eli Lilly, EMD Serono, Dragonfly, Genentech, GlaxoSmithKline, Janssen, Novartis, RemeGen, Takeda, UCB, and Zenas. The ianalumab trial presented by Dr. Agmon-Levin was sponsored by Novartis Pharma; however, she reported having no conflicts of interest. The DARALUP study was an investigator-initiated trial supported by Janssen. Dr. Alexander has received consulting fees, study support, honoraria, and travel grants from various pharmaceutical companies including AbbVie, Amgen, AstraZeneca, Bayer, GlaxoSmithKline, Janssen, and Lilly. Dr. Nash has consulted for The Rheumatology Education Group Consultants. The KPG-818 study reported by Dr. Wang was sponsored by Kangpu Biopharmaceuticals.
A version of this article first appeared on Medscape.com.
VIENNA — It may have been a while since there have been any major breakthroughs in the treatment of systemic lupus erythematosus (SLE), but there are high hopes that this is a situation that may be about to change, experts agreed at the annual European Congress of Rheumatology.
“It’s an incredibly vivid area of development,” Laurent Arnaud, MD, PhD, professor of rheumatology at the University of Strasbourg in Strasbourg, France, said during one of the first sessions of the meeting. He reported that there were at least 17 phase 2 and 14 phase 3 trials that were expected to start within the next few years, all with investigational agents that target different immune cells or pathways that have been implicated in the pathogenesis of SLE.
In a systematic review published last year, Dr. Arnaud and coauthors found that there were 92 investigational biologic or novel targeted agents in various phases of clinical testing. This included B-cell–targeting agents such as ianalumab, plasma cell-targeting agents such as daratumumab, and drugs with novel mechanisms of action such as KPG-818, which targets the CRL4-Cereblon (CRBN) E3 ubiquitin ligase complex. Phase 2 data on all three of these investigational agents were presented during various sessions at EULAR 2024, all with positive results, suggesting that their further development in SLE is worth pursuing.
There are of course “many more candidates in the pipeline,” Dr. Arnaud said. “I’m very happy that I think we are going to see great days for lupus right in front of our eyes.”
Targeting B Cells
Drugs that target B cells have been at the forefront of lupus treatment for several years, as David Isenberg, MD, professor of rheumatology at University College London, pointed out during an interview for EULAR TV.
“It’s clearly important to target the cells which are likely to be causing the problem in lupus, and in the main, that tends to be B cells,” he said.
Dr. Isenberg, who is renowned for his work with the B-cell–targeting agent rituximab, added: “But we know that obviously T cells integrate with B cells, so anything which interrupts the link between the T cell and the B cell is likely to be important.”
Chimeric Antigen Receptor (CAR) T-Cell Therapy ‘Revolution’
One new way of targeting B cells is with CAR T-cell therapy, which David D’Cruz , MD, PhD, a consultant rheumatologist for Guy’s and St. Thomas’ Hospital NHS Foundation Trust in London, picked as one of the “most striking” topics highlighted at EULAR 2024.
This is “truly personalized medicine,” Dr. D’Cruz said. This is an autologous therapy because a patient’s T cells are removed by leukapheresis, transfected with a CAR T vector directed against a component of the B cell, and then returned to them.
“I do feel that we’re on the cusp of a major revolution,” Dr. D’Cruz told this news organization. Not only in lupus but also in other rheumatic conditions that have proved really difficult to treat, such as systemic sclerosis and myositis, he said.
“Basically, it’s a very powerful B-cell–depleting tool, but it’s much more profound B-cell–depleting tool than, for example, rituximab or belimumab,” explained Dr. D’Cruz. “What you’re doing is reprogramming T cells to attack the B cells.”
Although rituximab and belimumab clear all the B cells in the circulation, there are still some cells left behind in the bone marrow, “and it’s very difficult to get rid of those,” Dr. D’Cruz said. “What CAR T-cell therapy appears to do is wipe out all the CD19-positive B cells everywhere, in the blood and the tissue. So you get a really profound B-cell depletion.”
Eric Morand, MBBS, PhD, head of rheumatology at Monash Health in Melbourne, Australia, told this news organization that there was obviously “a lot of buzz” about CAR T-cell therapy.
“We’re waiting to see if the exciting data from Erlangen can be reproduced in other centers with other CAR T products to show that it is a universal effect. We haven’t seen that yet, but I think we will by next year.”
Cost and expertise are two major considerations and potential limiting factors, however, as Dr. D’Cruz and Dr. Isenberg both pointed out in separate interviews with this news organization.
Dr. D’Cruz said: “It’s very expensive, it takes a while, and it doesn’t always work is what I’m hearing. It’s usually successful, but again, a little bit depends on the technique and the people doing the process.”
Dr. Isenberg said: “CAR T-cell therapy is, I think, very exciting because it does look to be quite promising. But as it costs 350,000 euros per patient, I don’t think that it is going to be widely adopted.”
Even if it could be afforded by certain centers in the West, he added, this just would not be feasible in poorer nations. “So, we’ve got to find other effective, cheaper ways to go,” Dr. Isenberg said.
“I think there are some very interesting ideas with monoclonal antibodies which target at least two different targets — one on the B cell, one on the T cell — and that could well be the way to take this forward,” he suggested.
Ianalumab ‘Double Blocking’ B Cells
Another way could be to develop more potent B-cell–depleting drugs, as Nancy Agmon-Levin , MD, head of the Clinical Immunology, Angioedema and Allergy Unit, Lupus and Autoimmune Diseases Clinic, at Sheba Medical Center, Tel Aviv University in Tel Aviv, Israel, reported during one of the clinical abstract sessions at EULAR 2024.
Dr. Agmon-Levin presented data on 67 individuals with SLE who had participated in a multicenter phase 2 study of ianalumab, a fully human immunoglobulin (Ig) G1 monoclonal antibody that results in a “double blocking of the B-cell lineage.”
Ianalumab targets the B-cell–activating factor receptor (BAFFR), but what makes it distinct from other BAFF-targeting drugs is that it has had a fructose molecule removed from its Fc portion, which renders it more likely to trigger antibody-dependent cellular cytotoxicity.
“This is a B-cell depletion therapy,” Agmon-Levin said, but it also blocks BAFFR-mediated survival of B cells, so the subsequent recuperation process of BAFFR-expressing B cells is affected, leading to continued B-cell depletion.
The phase 2 study she presented consisted of an initial 28-week, double-blind period, during which time participants had been randomly allocated to receive either subcutaneous injections of ianalumab 300 mg or a matching placebo every 4 weeks. This was followed by a 24-week, open-label period where all participants were treated with ianalumab, and then an off-treatment, minimal follow-up period that lasted up to 68 weeks, with continued data collection for safety.
The primary outcome measure was a composite of meeting criteria for the SLE Responder Index 4 and a sustained reduction in corticosteroid use at 28 weeks. This was achieved in 15 of the 34 (44.1%) people treated with ianalumab vs only 3 (9.1%) of the 33 people who had been given a placebo.
Dr. Agmon-Levin reported that the effect on this outcome was sustained to the end of the open-label period, at 1 year, in 15 (45.5%) of 33 participants who had continued treatment with ianalumab and achieved in 13 (40.6%) of 32 participants who had switched from placebo to ianalumab treatment.
Moreover, longer durations of treatment were associated with a host of improved outcomes, Dr. Agmon-Levin said: “Treatment was improved along the 52 weeks, and we can see from the LLDAS [Lupus Low Disease Activity State], DORIS [Definition Of Remission In SLE], and SRI-6 and -8 that as you continue the therapy, you improve the outcomes.”
The potential benefits of ianalumab in the treatment of SLE and lupus nephritis will now be further examined in the phase 3 SIRIUS-SLE1 , SIRIUS-SLE2, and SIRIUS-LN trials, which are estimated to provide initial results in 2027 and complete in early 2029 or 2030.
Targeting Plasma Cells With Daratumumab
Another drug showing signs that it might be useful as a treatment for SLE is daratumumab, as Tobias Alexander, MD, of Charité — Universitätsmedizin Berlin, reported during one of the late-breaking abstract sessions at EULAR 2024.
“Daratumumab is a human, first-in-class anti-CD38 antibody that efficiently depletes plasma cells,” Dr. Alexander said. CD38 is both a receptor and an enzyme, and while it is found on the surface of most immune cells, it’s particularly expressed by plasma cells, he added.
Daratumumab is not a total newcomer, however, as it’s already approved for the treatment of multiple myeloma under the trade name Darzalex. The rationale for using it in SLE comes from two case reports, Dr. Alexander explained. The first, published in 2020 in The New England Journal of Medicine, involved two patients with severe and life-threatening lupus who were given off-label treatment for a period of 4 weeks and experienced good clinical and serologic responses. The second, published last year in Nature Medicine, involved six patients with refractory lupus nephritis, five of whom had a clinical response at 6 months.
“On this background, we conducted an investigator-initiated trial, which was an open-label, single-center, proof-of-concept study,” Dr. Alexander said. A total of 10 female patients whose ages ranged from 24 to 43 years were included in the phase 2 trial that was dubbed DARALUP. For inclusion, all had to have a Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) of four or more for clinical manifestations, have been treated with at least two prior disease-modifying drugs to no avail, and be anti–double-stranded DNA (anti-dsDNA) antibody positive. Dr. Alexander reported that the median baseline SLEDAI-2K score was 12 and ranged from 8 to 20, with the number of prior therapies ranging from two to nine.
Daratumumab was given at a dose of 1800 mg via subcutaneous injection every week for 8 weeks. This is the same dose that is used to treat multiple myeloma, Dr. Alexander explained, although the dosing is not stopped. The reason for stopping after 8 weeks in the current trial was to be able to see what happened once the treatment was stopped. The follow-up was for 36 weeks.
Dr. Alexander reported that there was a “very dramatic and significant” effect on the primary endpoint of a reduction in anti-dsDNA antibody levels, decreasing from a median of 166.3 U/mL at baseline to 61.1 U/mL at week 12 (P = .002). Alongside, there was a reduction in the SLEDAI-2K score from 12 to 4 within 12 weeks, which was sustained at the 36-week follow-up assessment. Improvements in skin, joint, kidney, and level of proteinuria were also seen.
Although all patients experienced adverse events, none were serious. Infections and infestations (mostly nasopharyngitis, COVID-19, and gastroenteritis) were the most common, experienced by 80% of the participants; 70% had injection site reactions or fatigue, 60% had gastrointestinal symptoms, 50% had a fall of IgG < 5 g/L, 40% had headache, and 20% had back pain.
“This is a positive trial. I think we could demonstrate that [daratumumab] produced very strong, rapid, and durable clinical improvements,” Dr. Alexander said. “We think that targeting CD38 is relevant; plasma cells had been depleted based on the reduction of anti-dsDNA antibodies,” he added.
From the audience, however, Peter Nash, MBBS, of Griffith University in Brisbane, Australia, questioned whether the results could be attributed to “a steroid effect” because patients had been treated with oral dexamethasone throughout the study.
Dr. Alexander noted that steroid use had been part of the treatment schedule but acknowledged it was a possible confounder.
“I think we can be confident that [daratumumab] had a major effect on plasma cells decreasing…because we see that also the vaccine titers decreased,” Dr. Alexander said. “Time will tell, but even more important is the durability of the responses over time, which you don’t achieve under steroids.”
KPG-818’s Novel Mechanism of Action
Elsewhere at EULAR 2024, positive results of another phase 2 study involving a drug with an entirely different mechanism of action, KPG-818, were reported in a poster presentation. KPG-818 modulates CRBN, which results in the degradation of two transcription factors (Aiolos and Ikaros) that are involved in the development, maturation, and proliferation of innate and adaptive immune cells and have been linked to genetic risk in SLE, according to the poster’s authors. It is currently in development for the treatment of SLE, Behçet disease, inflammatory bowel disease, multiple myeloma, and non-Hodgkin lymphoma.
Yao Wang, MD, chief medical officer of KPG-818’s developer Kangpu Biopharmaceuticals, Hefei, China, and associates found that oral doses of 0.15 or 0.6 mg KPG-818 were “generally well-tolerated” and produced immunomodulatory changes that could be beneficial in people with SLE over a 12-week treatment period.
“Only two new agents have been approved for the treatment of SLE in the past five decades in USA and Europe,” Dr. Wang and team wrote, which highlights “a significant unmet need for more effective and safe treatment options.”
They believe that KPG-818 might well fit the bill based on the results of their study, in which 35 of 37 recruited patients completed the treatment. Compared with placebo, they observed reduced numbers of total B cells, Aiolos+ T and B cells, and increased Treg cells.
SLEDAI-2K and Cutaneous Lupus Erythematosus Disease Area and Severity Index activity scores in the 0.15-mg group were improved relative to baseline and placebo.
“The proof-of-concept findings suggest a favorable benefit/risk ratio in SLE for KPG-818,” Dr. Wang and coauthors said, supporting its further development in SLE.
Need for Treatments
Dr. Isenberg told this news organization that both daratumumab and KPG-818 would be welcome additions as treatment options if further trials proved their worth.
“The great frustration about lupus is that, compared to patients with rheumatoid arthritis, the choice has been so limited,” Dr. Isenberg said. Aside from rituximab (Rituxan) and belimumab (Benlysta), which are used with certain restrictions, there are no other biologic targeted treatments available in the United Kingdom. Anifrolumab (Saphnelo) has a license in the United States and some European countries but is not yet available for him to use in his practice.
Daratumumab and KPG-818 are “different types of molecules, and if they work that will be great. It would be nice to have the choice,” Dr. Isenberg said. “Whether they will be as effective as I think rituximab is, I don’t know, but these are some very encouraging results.”
Of course, these are all phase 2 trials, and the “big problem” is that such positive results do not always translate when it comes to phase 3, as Dr. D’Cruz told this news organization.
“Until a few years ago, there had been about 25 or 30 industry-led trails, and they’d all failed, except for belimumab and anifrolumab,” Dr. D’Cruz said. These drugs were found to work and be generally safe in phase 1 and 2 trials, but “when they come to phase 3, they all seem to fail, and we don’t know why.”
These are large global studies, D’Cruz added, observing that problems with patient selection, steroid use, and choice of outcome measures were possible factors for why the EXPLORER and LUNAR studies had shown no benefit for rituximab despite the drug being widely used to treat SLE.
Dr. Isenberg, who has coauthored an article on the topic of why drugs seem to fail at the final hurdle, noted: “I think it has a lot to do with the nature of the disease. It’s a complicated disease.” From having “savvy physicians doing the trials for you” to the placebo response, there are “a whole bunch or reasons why these things haven’t worked in lupus.”
Dr. Morand commented: “We’ve got many programs in phase 2 and 3, and because there’s so many, they’re all facing recruitment challenges, and as a consequence of so much activity, every program is going a little slower than hoped for.”
As for other drugs on the horizon, Dr. Morand noted: “We’re very optimistic about things like litifilimab and deucravacitinib; that’s two examples that are in phase 3. Earlier in the program of development, [there are] a huge range of targets being addressed. The future looks bright. But we might have to wait a while.”
Dr. Arnaud has consulted for AstraZeneca, AbbVie, Alpine Immune Sciences, Biogen, Bristol Myers Squibb, Boehringer Ingelheim, Chugai Pharmaceutical, GlaxoSmithKline, Grifols, Janssen, Kezar Life Sciences, LFB, Lilly, Medac, Merck, Novartis, Pfizer, Roche, and UCB. Dr. Isenberg has served as an adviser to Merck Serono, AstraZeneca, Eli Lilly, Servier, and ImmuPharma. Any honoraria received is passed on to a local arthritis charity connected to his hospital. Dr. D’Cruz has served as a consultant and advisory board member for GlaxoSmithKline and CSL Vifor. Dr. Morand has received research support, consultancy fees, or both from multiple pharmaceutical companies paid to his institution including AbbVie, Amgen, AstraZeneca, Biogen, Bristol Myers Squibb, Eli Lilly, EMD Serono, Dragonfly, Genentech, GlaxoSmithKline, Janssen, Novartis, RemeGen, Takeda, UCB, and Zenas. The ianalumab trial presented by Dr. Agmon-Levin was sponsored by Novartis Pharma; however, she reported having no conflicts of interest. The DARALUP study was an investigator-initiated trial supported by Janssen. Dr. Alexander has received consulting fees, study support, honoraria, and travel grants from various pharmaceutical companies including AbbVie, Amgen, AstraZeneca, Bayer, GlaxoSmithKline, Janssen, and Lilly. Dr. Nash has consulted for The Rheumatology Education Group Consultants. The KPG-818 study reported by Dr. Wang was sponsored by Kangpu Biopharmaceuticals.
A version of this article first appeared on Medscape.com.
FROM EULAR 2024
The Last 30 Days: How Oncologists’ Choices Affect End-of-Life Cancer Care
TOPLINE:
Patients treated by oncologists in the top quartile for end-of-life prescribing behavior were almost four and a half times more likely to receive end-of-life therapy than those treated by these specialists in the bottom quartile.
METHODOLOGY:
- Researchers analyzed data from the Surveillance, Epidemiology, and End Results (SEER)-Medicare database, focusing on patients who died of cancer between 2012 and 2017.
- A total of 17,609 patients with breast, lung, colorectal, or prostate cancer were included, treated by 960 oncologists across 388 practices.
- Patients were required to have had at least one systemic cancer therapy claim in the last 180 days of life, with the treating oncologist identified on the basis of the therapy claim closest to the time of death.
- The study used multilevel models to estimate oncologists’ rates of providing cancer therapy in the last 30 days of life, adjusting for patient characteristics and practice variation.
- Functional status was assessed on the basis of paid claims for durable medical equipment in the last 60 months of life, with scores categorized as 0, 1, ≥ 2, or unknown.
TAKEAWAY:
- Oncologists in the 95th percentile for high end-of-life prescribing behavior had a 45% adjusted rate of treating patients in the last 30 days of life, compared with 17% among those in the 5th percentile.
- Patients treated by high end-of-life prescribing oncologists had over four times higher odds of receiving systemic therapy in the last 30 days of life (odds ratio [OR], 4.42; 95% CI, 4.00-4.89).
- Higher end-of-life prescribing oncologists also had a higher proportion of patients hospitalized in the last 30 days of life than low prescribers (58% vs 51.9%).
- No significant association was found between oncologist prescribing behavior and patient race or ethnicity, except for Black patients who had lower odds of receiving treatment (OR, 0.77; P < .001).
IN PRACTICE:
“Given calls to rein in overutilization of end-of-life six to eight cancer therapies, our findings highlight an underappreciated area for further research: How treatment discontinuation before death is shaped by oncologists’ unique treatment propensities. Elucidating the reasons for this remarkable variability in oncologist treatment behavior could inform efforts to reduce end-of-life cancer treatment overutilization,” wrote the authors of the study.
SOURCE:
The study was led by Login S. George, PhD, Institute for Health, Health Care Policy and Aging Research, Rutgers University in New Brunswick, New Jersey. It was published online in Cancer.
LIMITATIONS:
The study’s reliance on SEER-Medicare data may limit the generalizability of the findings to patients with Medicare Advantage, private insurance, or Medicaid, as well as younger patients. The lack of data on patient preferences and other health characteristics could confound the results. The study focused on systemic therapies and may not be generalizable to other treatments such as clinical trial drugs, oral therapies, surgery, or radiation. The data from 2012 to 2017 may not reflect more recent trends in cancer treatment.
DISCLOSURES:
The study was supported by grants from the National Cancer Institute and the Rutgers Cancer Institute of New Jersey. George disclosed receiving grants from these organizations. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Patients treated by oncologists in the top quartile for end-of-life prescribing behavior were almost four and a half times more likely to receive end-of-life therapy than those treated by these specialists in the bottom quartile.
METHODOLOGY:
- Researchers analyzed data from the Surveillance, Epidemiology, and End Results (SEER)-Medicare database, focusing on patients who died of cancer between 2012 and 2017.
- A total of 17,609 patients with breast, lung, colorectal, or prostate cancer were included, treated by 960 oncologists across 388 practices.
- Patients were required to have had at least one systemic cancer therapy claim in the last 180 days of life, with the treating oncologist identified on the basis of the therapy claim closest to the time of death.
- The study used multilevel models to estimate oncologists’ rates of providing cancer therapy in the last 30 days of life, adjusting for patient characteristics and practice variation.
- Functional status was assessed on the basis of paid claims for durable medical equipment in the last 60 months of life, with scores categorized as 0, 1, ≥ 2, or unknown.
TAKEAWAY:
- Oncologists in the 95th percentile for high end-of-life prescribing behavior had a 45% adjusted rate of treating patients in the last 30 days of life, compared with 17% among those in the 5th percentile.
- Patients treated by high end-of-life prescribing oncologists had over four times higher odds of receiving systemic therapy in the last 30 days of life (odds ratio [OR], 4.42; 95% CI, 4.00-4.89).
- Higher end-of-life prescribing oncologists also had a higher proportion of patients hospitalized in the last 30 days of life than low prescribers (58% vs 51.9%).
- No significant association was found between oncologist prescribing behavior and patient race or ethnicity, except for Black patients who had lower odds of receiving treatment (OR, 0.77; P < .001).
IN PRACTICE:
“Given calls to rein in overutilization of end-of-life six to eight cancer therapies, our findings highlight an underappreciated area for further research: How treatment discontinuation before death is shaped by oncologists’ unique treatment propensities. Elucidating the reasons for this remarkable variability in oncologist treatment behavior could inform efforts to reduce end-of-life cancer treatment overutilization,” wrote the authors of the study.
SOURCE:
The study was led by Login S. George, PhD, Institute for Health, Health Care Policy and Aging Research, Rutgers University in New Brunswick, New Jersey. It was published online in Cancer.
LIMITATIONS:
The study’s reliance on SEER-Medicare data may limit the generalizability of the findings to patients with Medicare Advantage, private insurance, or Medicaid, as well as younger patients. The lack of data on patient preferences and other health characteristics could confound the results. The study focused on systemic therapies and may not be generalizable to other treatments such as clinical trial drugs, oral therapies, surgery, or radiation. The data from 2012 to 2017 may not reflect more recent trends in cancer treatment.
DISCLOSURES:
The study was supported by grants from the National Cancer Institute and the Rutgers Cancer Institute of New Jersey. George disclosed receiving grants from these organizations. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Patients treated by oncologists in the top quartile for end-of-life prescribing behavior were almost four and a half times more likely to receive end-of-life therapy than those treated by these specialists in the bottom quartile.
METHODOLOGY:
- Researchers analyzed data from the Surveillance, Epidemiology, and End Results (SEER)-Medicare database, focusing on patients who died of cancer between 2012 and 2017.
- A total of 17,609 patients with breast, lung, colorectal, or prostate cancer were included, treated by 960 oncologists across 388 practices.
- Patients were required to have had at least one systemic cancer therapy claim in the last 180 days of life, with the treating oncologist identified on the basis of the therapy claim closest to the time of death.
- The study used multilevel models to estimate oncologists’ rates of providing cancer therapy in the last 30 days of life, adjusting for patient characteristics and practice variation.
- Functional status was assessed on the basis of paid claims for durable medical equipment in the last 60 months of life, with scores categorized as 0, 1, ≥ 2, or unknown.
TAKEAWAY:
- Oncologists in the 95th percentile for high end-of-life prescribing behavior had a 45% adjusted rate of treating patients in the last 30 days of life, compared with 17% among those in the 5th percentile.
- Patients treated by high end-of-life prescribing oncologists had over four times higher odds of receiving systemic therapy in the last 30 days of life (odds ratio [OR], 4.42; 95% CI, 4.00-4.89).
- Higher end-of-life prescribing oncologists also had a higher proportion of patients hospitalized in the last 30 days of life than low prescribers (58% vs 51.9%).
- No significant association was found between oncologist prescribing behavior and patient race or ethnicity, except for Black patients who had lower odds of receiving treatment (OR, 0.77; P < .001).
IN PRACTICE:
“Given calls to rein in overutilization of end-of-life six to eight cancer therapies, our findings highlight an underappreciated area for further research: How treatment discontinuation before death is shaped by oncologists’ unique treatment propensities. Elucidating the reasons for this remarkable variability in oncologist treatment behavior could inform efforts to reduce end-of-life cancer treatment overutilization,” wrote the authors of the study.
SOURCE:
The study was led by Login S. George, PhD, Institute for Health, Health Care Policy and Aging Research, Rutgers University in New Brunswick, New Jersey. It was published online in Cancer.
LIMITATIONS:
The study’s reliance on SEER-Medicare data may limit the generalizability of the findings to patients with Medicare Advantage, private insurance, or Medicaid, as well as younger patients. The lack of data on patient preferences and other health characteristics could confound the results. The study focused on systemic therapies and may not be generalizable to other treatments such as clinical trial drugs, oral therapies, surgery, or radiation. The data from 2012 to 2017 may not reflect more recent trends in cancer treatment.
DISCLOSURES:
The study was supported by grants from the National Cancer Institute and the Rutgers Cancer Institute of New Jersey. George disclosed receiving grants from these organizations. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Ancient Viruses in Our DNA Hold Clues to Cancer Treatment
according to a fascinating new study in Science Advances. Targeting these viral remnants still lingering in our DNA could lead to more effective cancer treatment with fewer side effects, the researchers said.
The study “gives a better understanding of how gene regulation can be impacted by these ancient retroviral sequences,” said Dixie Mager, PhD, scientist emeritus at the Terry Fox Laboratory at the British Columbia Cancer Research Institute, Vancouver, British Columbia, Canada. (Mager was not involved in the study.)
Long thought to be “junk” DNA with no biologic function, “endogenous retroviruses,” which have mutated over time and lost their ability to create the virus, are now known to regulate genes — allowing some genes to turn on and off. Research in recent years suggests they may play a role in diseases like cancer.
But scientists weren’t exactly sure what that role was, said senior study author Edward Chuong, PhD, a genome biologist at the University of Colorado Boulder.
Most studies have looked at whether endogenous retroviruses code for proteins that influence cancer. But these ancient viral strands usually don’t code for proteins at all.
Dr. Chuong took a different approach. Inspired by scientists who’ve studied how viral remnants regulate positive processes (immunity, brain development, or placenta development), he and his team explored whether some might regulate genes that, once activated, help cancer thrive.
Borrowing from epigenomic analysis data (data on molecules that alter gene expression) for 21 cancers mapped by the Cancer Genome Atlas, the researchers identified 19 virus-derived DNA sequences that bind to regulatory proteins more in cancer cells than in healthy cells. All of these could potentially act as gene regulators that promote cancer.
The researchers homed in on one sequence, called LTR10, because it showed especially high activity in several cancers, including lung and colorectal cancer. This DNA segment comes from a virus that entered our ancestors’ genome 30 million years ago, and it’s activated in a third of colorectal cancers.
Using the gene editing technology clustered regularly interspaced short palindromic repeats (CRISPR), Dr. Chuong’s team silenced LTR10 in colorectal cancer cells, altering the gene sequence so it couldn’t bind to regulatory proteins. Doing so dampened the activity of nearby cancer-promoting genes.
“They still behaved like cancer cells,” Dr. Chuong said. But “it made the cancer cells more susceptible to radiation. That would imply that the presence of that viral ‘switch’ actually helped those cancer cells survive radiation therapy.”
Previously, two studies had found that viral regulators play a role in promoting two types of cancer: Leukemia and prostate cancer. The new study shows these two cases weren’t flukes. All 21 cancers they looked at had at least one of those 19 viral elements, presumably working as cancer enhancers.
The study also identified what activates LTR10 to make it promote cancer. The culprit is a regulator protein called mitogen-activated protein (MAP) kinase, which is overactivated in about 40% of all human cancers.
Some cancer drugs — MAP kinase inhibitors — already target MAP kinase, and they’re often the first ones prescribed when a patient is diagnosed with cancer, Dr. Chuong said. As with many cancer treatments, doctors don’t know why they work, just that they do.
“By understanding the mechanisms in the cell, we might be able to make them work better or further optimize their treatment,” he said.
“MAP kinase inhibitors are really like a sledgehammer to the cell,” Dr. Chuong said — meaning they affect many cellular processes, not just those related to cancer.
“If we’re able to say that these viral switches are what’s important, then that could potentially help us develop a more targeted therapy that uses something like CRISPR to silence these viral elements,” he said. Or it could help providers choose a MAP kinase inhibitor from among the dozens available best suited to treat an individual patient and avoid side effects.
Still, whether the findings translate to real cancer patients remains to be seen. “It’s very, very hard to go the final step of showing in a patient that these actually make a difference in the cancer,” Dr. Mager said.
More lab research, human trials, and at least a few years will be needed before this discovery could help treat cancer. “Directly targeting these elements as a therapy would be at least 5 years out,” Dr. Chuong said, “partly because that application would rely on CRISPR epigenome editing technology that is still being developed for clinical use.”
A version of this article first appeared on Medscape.com.
according to a fascinating new study in Science Advances. Targeting these viral remnants still lingering in our DNA could lead to more effective cancer treatment with fewer side effects, the researchers said.
The study “gives a better understanding of how gene regulation can be impacted by these ancient retroviral sequences,” said Dixie Mager, PhD, scientist emeritus at the Terry Fox Laboratory at the British Columbia Cancer Research Institute, Vancouver, British Columbia, Canada. (Mager was not involved in the study.)
Long thought to be “junk” DNA with no biologic function, “endogenous retroviruses,” which have mutated over time and lost their ability to create the virus, are now known to regulate genes — allowing some genes to turn on and off. Research in recent years suggests they may play a role in diseases like cancer.
But scientists weren’t exactly sure what that role was, said senior study author Edward Chuong, PhD, a genome biologist at the University of Colorado Boulder.
Most studies have looked at whether endogenous retroviruses code for proteins that influence cancer. But these ancient viral strands usually don’t code for proteins at all.
Dr. Chuong took a different approach. Inspired by scientists who’ve studied how viral remnants regulate positive processes (immunity, brain development, or placenta development), he and his team explored whether some might regulate genes that, once activated, help cancer thrive.
Borrowing from epigenomic analysis data (data on molecules that alter gene expression) for 21 cancers mapped by the Cancer Genome Atlas, the researchers identified 19 virus-derived DNA sequences that bind to regulatory proteins more in cancer cells than in healthy cells. All of these could potentially act as gene regulators that promote cancer.
The researchers homed in on one sequence, called LTR10, because it showed especially high activity in several cancers, including lung and colorectal cancer. This DNA segment comes from a virus that entered our ancestors’ genome 30 million years ago, and it’s activated in a third of colorectal cancers.
Using the gene editing technology clustered regularly interspaced short palindromic repeats (CRISPR), Dr. Chuong’s team silenced LTR10 in colorectal cancer cells, altering the gene sequence so it couldn’t bind to regulatory proteins. Doing so dampened the activity of nearby cancer-promoting genes.
“They still behaved like cancer cells,” Dr. Chuong said. But “it made the cancer cells more susceptible to radiation. That would imply that the presence of that viral ‘switch’ actually helped those cancer cells survive radiation therapy.”
Previously, two studies had found that viral regulators play a role in promoting two types of cancer: Leukemia and prostate cancer. The new study shows these two cases weren’t flukes. All 21 cancers they looked at had at least one of those 19 viral elements, presumably working as cancer enhancers.
The study also identified what activates LTR10 to make it promote cancer. The culprit is a regulator protein called mitogen-activated protein (MAP) kinase, which is overactivated in about 40% of all human cancers.
Some cancer drugs — MAP kinase inhibitors — already target MAP kinase, and they’re often the first ones prescribed when a patient is diagnosed with cancer, Dr. Chuong said. As with many cancer treatments, doctors don’t know why they work, just that they do.
“By understanding the mechanisms in the cell, we might be able to make them work better or further optimize their treatment,” he said.
“MAP kinase inhibitors are really like a sledgehammer to the cell,” Dr. Chuong said — meaning they affect many cellular processes, not just those related to cancer.
“If we’re able to say that these viral switches are what’s important, then that could potentially help us develop a more targeted therapy that uses something like CRISPR to silence these viral elements,” he said. Or it could help providers choose a MAP kinase inhibitor from among the dozens available best suited to treat an individual patient and avoid side effects.
Still, whether the findings translate to real cancer patients remains to be seen. “It’s very, very hard to go the final step of showing in a patient that these actually make a difference in the cancer,” Dr. Mager said.
More lab research, human trials, and at least a few years will be needed before this discovery could help treat cancer. “Directly targeting these elements as a therapy would be at least 5 years out,” Dr. Chuong said, “partly because that application would rely on CRISPR epigenome editing technology that is still being developed for clinical use.”
A version of this article first appeared on Medscape.com.
according to a fascinating new study in Science Advances. Targeting these viral remnants still lingering in our DNA could lead to more effective cancer treatment with fewer side effects, the researchers said.
The study “gives a better understanding of how gene regulation can be impacted by these ancient retroviral sequences,” said Dixie Mager, PhD, scientist emeritus at the Terry Fox Laboratory at the British Columbia Cancer Research Institute, Vancouver, British Columbia, Canada. (Mager was not involved in the study.)
Long thought to be “junk” DNA with no biologic function, “endogenous retroviruses,” which have mutated over time and lost their ability to create the virus, are now known to regulate genes — allowing some genes to turn on and off. Research in recent years suggests they may play a role in diseases like cancer.
But scientists weren’t exactly sure what that role was, said senior study author Edward Chuong, PhD, a genome biologist at the University of Colorado Boulder.
Most studies have looked at whether endogenous retroviruses code for proteins that influence cancer. But these ancient viral strands usually don’t code for proteins at all.
Dr. Chuong took a different approach. Inspired by scientists who’ve studied how viral remnants regulate positive processes (immunity, brain development, or placenta development), he and his team explored whether some might regulate genes that, once activated, help cancer thrive.
Borrowing from epigenomic analysis data (data on molecules that alter gene expression) for 21 cancers mapped by the Cancer Genome Atlas, the researchers identified 19 virus-derived DNA sequences that bind to regulatory proteins more in cancer cells than in healthy cells. All of these could potentially act as gene regulators that promote cancer.
The researchers homed in on one sequence, called LTR10, because it showed especially high activity in several cancers, including lung and colorectal cancer. This DNA segment comes from a virus that entered our ancestors’ genome 30 million years ago, and it’s activated in a third of colorectal cancers.
Using the gene editing technology clustered regularly interspaced short palindromic repeats (CRISPR), Dr. Chuong’s team silenced LTR10 in colorectal cancer cells, altering the gene sequence so it couldn’t bind to regulatory proteins. Doing so dampened the activity of nearby cancer-promoting genes.
“They still behaved like cancer cells,” Dr. Chuong said. But “it made the cancer cells more susceptible to radiation. That would imply that the presence of that viral ‘switch’ actually helped those cancer cells survive radiation therapy.”
Previously, two studies had found that viral regulators play a role in promoting two types of cancer: Leukemia and prostate cancer. The new study shows these two cases weren’t flukes. All 21 cancers they looked at had at least one of those 19 viral elements, presumably working as cancer enhancers.
The study also identified what activates LTR10 to make it promote cancer. The culprit is a regulator protein called mitogen-activated protein (MAP) kinase, which is overactivated in about 40% of all human cancers.
Some cancer drugs — MAP kinase inhibitors — already target MAP kinase, and they’re often the first ones prescribed when a patient is diagnosed with cancer, Dr. Chuong said. As with many cancer treatments, doctors don’t know why they work, just that they do.
“By understanding the mechanisms in the cell, we might be able to make them work better or further optimize their treatment,” he said.
“MAP kinase inhibitors are really like a sledgehammer to the cell,” Dr. Chuong said — meaning they affect many cellular processes, not just those related to cancer.
“If we’re able to say that these viral switches are what’s important, then that could potentially help us develop a more targeted therapy that uses something like CRISPR to silence these viral elements,” he said. Or it could help providers choose a MAP kinase inhibitor from among the dozens available best suited to treat an individual patient and avoid side effects.
Still, whether the findings translate to real cancer patients remains to be seen. “It’s very, very hard to go the final step of showing in a patient that these actually make a difference in the cancer,” Dr. Mager said.
More lab research, human trials, and at least a few years will be needed before this discovery could help treat cancer. “Directly targeting these elements as a therapy would be at least 5 years out,” Dr. Chuong said, “partly because that application would rely on CRISPR epigenome editing technology that is still being developed for clinical use.”
A version of this article first appeared on Medscape.com.
FROM SCIENCE ADVANCES
Anti-Smith and Anti–Double-Stranded DNA Antibodies in a Patient With Henoch-Schönlein Purpura Following COVID-19 Vaccination
To the Editor:
Henoch-Schönlein purpura (HSP)(also known as IgA vasculitis) is a small vessel vasculitis characterized by deposition of IgA in small vessels, resulting in the development of purpura on the legs. Based on the European Alliance of Associations for Rheumatology criteria,1 the patient also must have at least 1 of the following: arthritis, arthralgia, abdominal pain, leukocytoclastic vasculitis with IgA deposition, or kidney involvement. The disease can be triggered by infection—with more than 75% of patients reporting an antecedent upper respiratory tract infection2—as well as medications, circulating immune complexes, certain foods, vaccines, and rarely cancer.3,4 The disease more commonly occurs in children but also can affect adults.
Several cases of HSP have been reported following COVID-19 vaccination.5 We report a case of HSP developing days after the messenger RNA Pfizer-BioNTech COVID-19 vaccine booster that was associated with anti-Smith and anti–double-stranded DNA (dsDNA) antibodies as well as antineutrophil cytoplasmic antibodies (ANCAs).
A 24-year-old man presented to dermatology with a rash of 3 weeks’ duration that first appeared 1 week after receiving his second booster of the messenger RNA Pfizer-BioNTech COVID-19 vaccine. Physical examination revealed petechiae with nonblanching erythematous macules and papules covering the legs below the knees (Figure 1) as well as the back of the right arm. A few days later, he developed arthralgia in the knees, hands, and feet. The patient denied any recent infections as well as respiratory and urinary tract symptoms. Approximately 10 days after the rash appeared, he developed epigastric abdominal pain that gradually worsened and sought care from his primary care physician, who ordered computed tomography and referred him for endoscopy. Computed tomography with and without contrast was suspicious for colitis. Colonoscopy and endoscopy were unremarkable. Laboratory tests were notable for elevated white blood cell count (17.08×103/µL [reference range, 3.66–10.60×103/µL]), serum IgA (437 mg/dL [reference range, 70–400 mg/dL]), C-reactive protein (1.5 mg/dL [reference range, <0.5 mg/dL]), anti-Smith antibody (28.1 CU [reference range, <20 CU), positive antinuclear antibody with titer (1:160 [reference range, <1:80]), anti-dsDNA (40.4 IU/mL [reference range, <27 IU/mL]), and cytoplasmic ANCA (c-ANCA) titer (1:320 [reference range, <1:20]). Blood urea nitrogen, creatinine, and estimated glomerular filtration rate were all within reference range. Urinalysis with microscopic examination was notable for 2 to 5 red blood cells per high-power field (reference range, 0) and proteinuria of 1+ (reference range, negative for protein).
The patient’s rash progressively worsened over the next few weeks, spreading proximally on the legs to the buttocks and the back of both elbows. A repeat complete blood cell count showed resolution of the leukocytosis. Two biopsies were taken from a lesion on the left proximal thigh: 1 for hematoxylin and eosin stain for histopathologic examination and 1 for direct immunofluorescence examination.
The patient was preliminarily diagnosed with HSP, and dermatology prescribed oral tofacitinib 5 mg twice daily for 5 days, which was supposed to be increased to 10 mg twice daily on the sixth day of treatment; however, the patient discontinued the medication after 4 days based on his primary care physician’s recommendation due to clotting concerns. The rash and arthralgia temporarily improved for 1 week, then relapsed.
Histopathology revealed neutrophils surrounding and infiltrating small dermal blood vessel walls as well as associated neutrophilic debris and erythrocytes, consistent with leukocytoclastic vasculitis (Figure 2). Direct immunofluorescence was negative for IgA antibodies. His primary care physician, in consultation with his dermatologist, then started the patient on oral prednisone 70 mg once daily for 7 days with a plan to taper. Three days after prednisone was started, the arthralgia and abdominal pain resolved, and the rash became lighter in color. After 1 week, the rash resolved completely.
Due to the unusual antibodies, the patient was referred to a rheumatologist, who repeated the blood tests approximately 1 week after the patient started prednisone. The tests were negative for anti-Smith, anti-dsDNA, and c-ANCA but showed an elevated atypical perinuclear ANCA (p-ANCA) titer of 1:80 (reference range [negative], <1:20). A repeat urinalysis was unremarkable. The patient slowly tapered the prednisone over the course of 3 months and was subsequently lost to follow-up. The rash and other symptoms had not recurred as of the patient’s last physician contact. The most recent laboratory results showed a white blood cell count of 14.0×103/µL (reference range, 3.4–10.8×103/µL), likely due to the prednisone; blood urea nitrogen, creatinine, and estimated glomerular filtration rate were within reference range. The urinalysis was notable for occult blood and was negative for protein. C-reactive protein was 1 mg/dL (reference range, 0–10 mg/dL); p-ANCA, c-ANCA, and atypical p-ANCA, as well as antinuclear antibody, were negative. As of his last follow-up, the patient felt well.
The major differential diagnoses for our patient included HSP, ANCA vasculitis, and systemic lupus erythematosus. Although ANCA vasculitis has been reported after SARS-CoV-2 infection,6 the lack of pulmonary symptoms made this diagnosis unlikely.7 Although our patient initially had elevated anti-Smith and anti-dsDNA antibodies as well as mild renal involvement, he fulfilled at most only 2 of the 11 criteria necessary for diagnosing lupus: malar rash, discoid rash (includes alopecia), photosensitivity, ocular ulcers, nonerosive arthritis, serositis, renal disorder (protein >500 mg/24 h, red blood cells, casts), neurologic disorder (seizures, psychosis), hematologic disorders (hemolytic anemia, leukopenia), ANA, and immunologic disorder (anti-Smith). Four of the 11 criteria are necessary for the diagnosis of lupus.8
Torraca et al7 reported a case of HSP with positive c-ANCA (1:640) in a patient lacking pulmonary symptoms who was diagnosed with HSP. Cytoplasmic ANCA is not a typical finding in HSP. However, the additional findings of anti-Smith, anti-dsDNA, and mildly elevated atypical p-ANCA antibodies in our patient were unexpected and could be explained by the proposed pathogenesis of HSP—an overzealous immune response resulting in aberrant antibody complex deposition with ensuing complement activation.5,9 Production of these additional antibodies could be part of the overzealous response to COVID-19 vaccination.
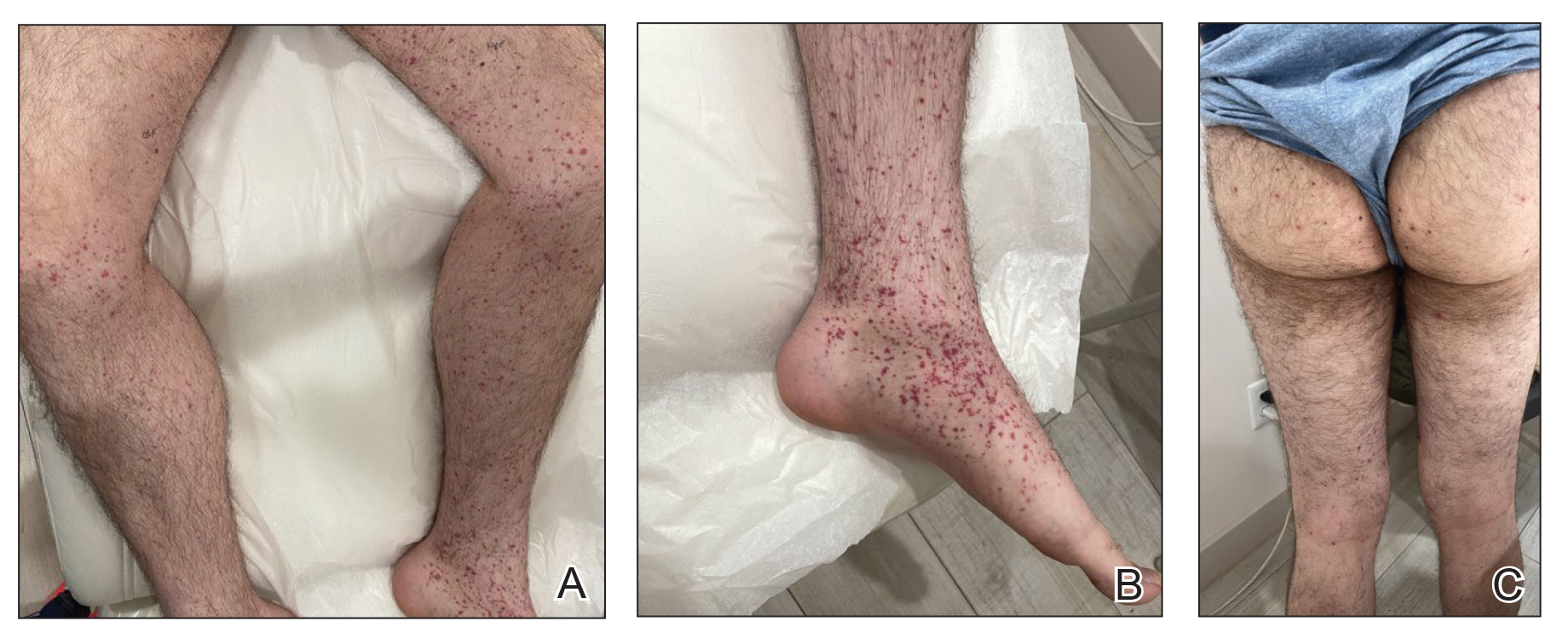
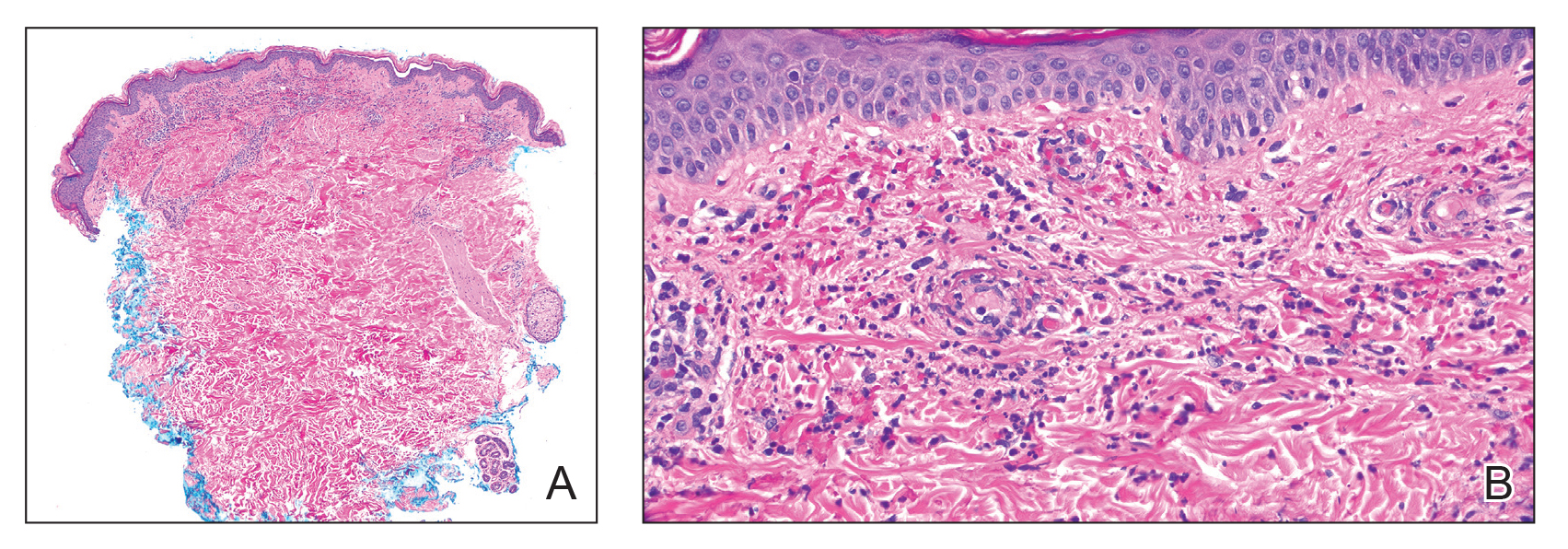
Of all the COVID-19 vaccines, messenger RNA–based vaccines have been associated with the majority of cutaneous reactions, including local injection-site reactions (most common), delayed local reactions, urticaria, angioedema, morbilliform eruption, herpes zoster eruption, bullous eruptions, dermal filler reactions, chilblains, and pityriasis rosea. Less common reactions have included acute generalized exanthematous pustulosis, Stevens-Johnson syndrome, erythema multiforme, Sweet Syndrome, lichen planus, papulovesicular eruptions, pityriasis rosea–like eruptions, generalized annular lesions, facial pustular neutrophilic eruptions, and flares of underlying autoimmune skin conditions.10 Multiple cases of HSP have been reported following COVID-19 vaccination from all the major vaccine companies.5
In our patient, laboratory tests were repeated by a rheumatologist and were negative for anti-Smith and anti-dsDNA antibodies as well as c-ANCA, most likely because he started taking prednisone approximately 1 week prior, which may have resulted in decreased antibodies. Also, the patient’s symptoms resolved after 1 week of steroid therapy. Therefore, the diagnosis is most consistent with HSP associated with COVID-19 vaccination. The clinical presentation, microscopic hematuria and proteinuria, and histopathology were consistent with the European Alliance of Associations for Rheumatology criteria for HSP.1
Although direct immunofluorescence typically is positive for IgA deposition on biopsies, it can be negative for IgA, especially in lesions that are biopsied more than 7 days after their appearance, as shown in our case; a negative IgA on immunofluorescence does not rule out HSP.4 Elevated serum IgA is seen in more than 50% of cases of HSP.11 Although the disease typically is self-limited, glucocorticoids are used if the disease course is prolonged or if there is evidence of kidney involvement.9 The unique combination of anti-Smith and anti-dsDNA antibodies as well as ANCAs associated with HSP with negative IgA on direct immunofluorescence has been reported with lupus.12 Clinicians should be aware of COVID-19 vaccine–associated HSP that is negative for IgA deposition and positive for anti-Smith and anti-dsDNA antibodies as well as ANCAs.
Acknowledgment—We thank our patient for granting permission to publish this information.
- Ozen S, Ruperto N, Dillon MJ, et al. EULAR/PReS endorsed consensus criteria for the classification of childhood vasculitides. Ann Rheum Dis. 2006;65:936-941. doi:10.1136/ard.2005.046300
- Rai A, Nast C, Adler S. Henoch–Schönlein purpura nephritis. J Am Soc Nephrol. 1999;10:2637-2644.
- Casini F, Magenes VC, De Sanctis M, et al. Henoch-Schönlein purpura following COVID-19 vaccine in a child: a case report. Ital J Pediatr. 2022;48:158. doi:10.1186/s13052-022-01351-1
- Poudel P, Adams SH, Mirchia K, et al. IgA negative immunofluorescence in diagnoses of adult-onset Henoch-Schönlein purpura. Proc (Bayl Univ Med Cent). 2020;33:436-437. doi:10.1080/08998280.2020.1770526
- Maronese CA, Zelin E, Avallone G, et al. Cutaneous vasculitis and vasculopathy in the era of COVID-19 pandemic. Front Med (Lausanne). 2022;9:996288. doi:10.3389/fmed.2022.996288
- Bryant MC, Spencer LT, Yalcindag A. A case of ANCA-associated vasculitis in a 16-year-old female following SARS-COV-2 infection and a systematic review of the literature. Pediatr Rheumatol Online J. 2022;20:65. doi:10.1186/s12969-022-00727-1
- Torraca PFS, Castro BC, Hans Filho G. Henoch-Schönlein purpura with c-ANCA antibody in adult. An Bras Dermatol. 2016;91:667-669. doi:10.1590/abd1806-4841.20164181
- Agabegi SS, Agabegi ED. Step-Up to Medicine. 4th ed. Wolters Kluwer; 2015.
- Ball-Burack MR, Kosowsky JM. A Case of leukocytoclastic vasculitis following SARS-CoV-2 vaccination. J Emerg Med. 2022;63:E62-E65. doi:10.1016/j.jemermed.2021.10.005
- Tan SW, Tam YC, Pang SM. Cutaneous reactions to COVID-19 vaccines: a review. JAAD Int. 2022;7:178-186. doi:10.1016/j.jdin.2022.01.011
- Calviño MC, Llorca J, García-Porrúa C, et al. Henoch-Schönlein purpura in children from northwestern Spain: a 20-year epidemiologic and clinical study. Medicine (Baltimore). 2001;80:279-290.
- Hu P, Huang BY, Zhang DD, et al. Henoch-Schönlein purpura in a pediatric patient with lupus. Arch Med Sci. 2017;13:689-690. doi:10.5114/aoms.2017.67288
To the Editor:
Henoch-Schönlein purpura (HSP)(also known as IgA vasculitis) is a small vessel vasculitis characterized by deposition of IgA in small vessels, resulting in the development of purpura on the legs. Based on the European Alliance of Associations for Rheumatology criteria,1 the patient also must have at least 1 of the following: arthritis, arthralgia, abdominal pain, leukocytoclastic vasculitis with IgA deposition, or kidney involvement. The disease can be triggered by infection—with more than 75% of patients reporting an antecedent upper respiratory tract infection2—as well as medications, circulating immune complexes, certain foods, vaccines, and rarely cancer.3,4 The disease more commonly occurs in children but also can affect adults.
Several cases of HSP have been reported following COVID-19 vaccination.5 We report a case of HSP developing days after the messenger RNA Pfizer-BioNTech COVID-19 vaccine booster that was associated with anti-Smith and anti–double-stranded DNA (dsDNA) antibodies as well as antineutrophil cytoplasmic antibodies (ANCAs).
A 24-year-old man presented to dermatology with a rash of 3 weeks’ duration that first appeared 1 week after receiving his second booster of the messenger RNA Pfizer-BioNTech COVID-19 vaccine. Physical examination revealed petechiae with nonblanching erythematous macules and papules covering the legs below the knees (Figure 1) as well as the back of the right arm. A few days later, he developed arthralgia in the knees, hands, and feet. The patient denied any recent infections as well as respiratory and urinary tract symptoms. Approximately 10 days after the rash appeared, he developed epigastric abdominal pain that gradually worsened and sought care from his primary care physician, who ordered computed tomography and referred him for endoscopy. Computed tomography with and without contrast was suspicious for colitis. Colonoscopy and endoscopy were unremarkable. Laboratory tests were notable for elevated white blood cell count (17.08×103/µL [reference range, 3.66–10.60×103/µL]), serum IgA (437 mg/dL [reference range, 70–400 mg/dL]), C-reactive protein (1.5 mg/dL [reference range, <0.5 mg/dL]), anti-Smith antibody (28.1 CU [reference range, <20 CU), positive antinuclear antibody with titer (1:160 [reference range, <1:80]), anti-dsDNA (40.4 IU/mL [reference range, <27 IU/mL]), and cytoplasmic ANCA (c-ANCA) titer (1:320 [reference range, <1:20]). Blood urea nitrogen, creatinine, and estimated glomerular filtration rate were all within reference range. Urinalysis with microscopic examination was notable for 2 to 5 red blood cells per high-power field (reference range, 0) and proteinuria of 1+ (reference range, negative for protein).
The patient’s rash progressively worsened over the next few weeks, spreading proximally on the legs to the buttocks and the back of both elbows. A repeat complete blood cell count showed resolution of the leukocytosis. Two biopsies were taken from a lesion on the left proximal thigh: 1 for hematoxylin and eosin stain for histopathologic examination and 1 for direct immunofluorescence examination.
The patient was preliminarily diagnosed with HSP, and dermatology prescribed oral tofacitinib 5 mg twice daily for 5 days, which was supposed to be increased to 10 mg twice daily on the sixth day of treatment; however, the patient discontinued the medication after 4 days based on his primary care physician’s recommendation due to clotting concerns. The rash and arthralgia temporarily improved for 1 week, then relapsed.
Histopathology revealed neutrophils surrounding and infiltrating small dermal blood vessel walls as well as associated neutrophilic debris and erythrocytes, consistent with leukocytoclastic vasculitis (Figure 2). Direct immunofluorescence was negative for IgA antibodies. His primary care physician, in consultation with his dermatologist, then started the patient on oral prednisone 70 mg once daily for 7 days with a plan to taper. Three days after prednisone was started, the arthralgia and abdominal pain resolved, and the rash became lighter in color. After 1 week, the rash resolved completely.
Due to the unusual antibodies, the patient was referred to a rheumatologist, who repeated the blood tests approximately 1 week after the patient started prednisone. The tests were negative for anti-Smith, anti-dsDNA, and c-ANCA but showed an elevated atypical perinuclear ANCA (p-ANCA) titer of 1:80 (reference range [negative], <1:20). A repeat urinalysis was unremarkable. The patient slowly tapered the prednisone over the course of 3 months and was subsequently lost to follow-up. The rash and other symptoms had not recurred as of the patient’s last physician contact. The most recent laboratory results showed a white blood cell count of 14.0×103/µL (reference range, 3.4–10.8×103/µL), likely due to the prednisone; blood urea nitrogen, creatinine, and estimated glomerular filtration rate were within reference range. The urinalysis was notable for occult blood and was negative for protein. C-reactive protein was 1 mg/dL (reference range, 0–10 mg/dL); p-ANCA, c-ANCA, and atypical p-ANCA, as well as antinuclear antibody, were negative. As of his last follow-up, the patient felt well.
The major differential diagnoses for our patient included HSP, ANCA vasculitis, and systemic lupus erythematosus. Although ANCA vasculitis has been reported after SARS-CoV-2 infection,6 the lack of pulmonary symptoms made this diagnosis unlikely.7 Although our patient initially had elevated anti-Smith and anti-dsDNA antibodies as well as mild renal involvement, he fulfilled at most only 2 of the 11 criteria necessary for diagnosing lupus: malar rash, discoid rash (includes alopecia), photosensitivity, ocular ulcers, nonerosive arthritis, serositis, renal disorder (protein >500 mg/24 h, red blood cells, casts), neurologic disorder (seizures, psychosis), hematologic disorders (hemolytic anemia, leukopenia), ANA, and immunologic disorder (anti-Smith). Four of the 11 criteria are necessary for the diagnosis of lupus.8
Torraca et al7 reported a case of HSP with positive c-ANCA (1:640) in a patient lacking pulmonary symptoms who was diagnosed with HSP. Cytoplasmic ANCA is not a typical finding in HSP. However, the additional findings of anti-Smith, anti-dsDNA, and mildly elevated atypical p-ANCA antibodies in our patient were unexpected and could be explained by the proposed pathogenesis of HSP—an overzealous immune response resulting in aberrant antibody complex deposition with ensuing complement activation.5,9 Production of these additional antibodies could be part of the overzealous response to COVID-19 vaccination.


Of all the COVID-19 vaccines, messenger RNA–based vaccines have been associated with the majority of cutaneous reactions, including local injection-site reactions (most common), delayed local reactions, urticaria, angioedema, morbilliform eruption, herpes zoster eruption, bullous eruptions, dermal filler reactions, chilblains, and pityriasis rosea. Less common reactions have included acute generalized exanthematous pustulosis, Stevens-Johnson syndrome, erythema multiforme, Sweet Syndrome, lichen planus, papulovesicular eruptions, pityriasis rosea–like eruptions, generalized annular lesions, facial pustular neutrophilic eruptions, and flares of underlying autoimmune skin conditions.10 Multiple cases of HSP have been reported following COVID-19 vaccination from all the major vaccine companies.5
In our patient, laboratory tests were repeated by a rheumatologist and were negative for anti-Smith and anti-dsDNA antibodies as well as c-ANCA, most likely because he started taking prednisone approximately 1 week prior, which may have resulted in decreased antibodies. Also, the patient’s symptoms resolved after 1 week of steroid therapy. Therefore, the diagnosis is most consistent with HSP associated with COVID-19 vaccination. The clinical presentation, microscopic hematuria and proteinuria, and histopathology were consistent with the European Alliance of Associations for Rheumatology criteria for HSP.1
Although direct immunofluorescence typically is positive for IgA deposition on biopsies, it can be negative for IgA, especially in lesions that are biopsied more than 7 days after their appearance, as shown in our case; a negative IgA on immunofluorescence does not rule out HSP.4 Elevated serum IgA is seen in more than 50% of cases of HSP.11 Although the disease typically is self-limited, glucocorticoids are used if the disease course is prolonged or if there is evidence of kidney involvement.9 The unique combination of anti-Smith and anti-dsDNA antibodies as well as ANCAs associated with HSP with negative IgA on direct immunofluorescence has been reported with lupus.12 Clinicians should be aware of COVID-19 vaccine–associated HSP that is negative for IgA deposition and positive for anti-Smith and anti-dsDNA antibodies as well as ANCAs.
Acknowledgment—We thank our patient for granting permission to publish this information.
To the Editor:
Henoch-Schönlein purpura (HSP)(also known as IgA vasculitis) is a small vessel vasculitis characterized by deposition of IgA in small vessels, resulting in the development of purpura on the legs. Based on the European Alliance of Associations for Rheumatology criteria,1 the patient also must have at least 1 of the following: arthritis, arthralgia, abdominal pain, leukocytoclastic vasculitis with IgA deposition, or kidney involvement. The disease can be triggered by infection—with more than 75% of patients reporting an antecedent upper respiratory tract infection2—as well as medications, circulating immune complexes, certain foods, vaccines, and rarely cancer.3,4 The disease more commonly occurs in children but also can affect adults.
Several cases of HSP have been reported following COVID-19 vaccination.5 We report a case of HSP developing days after the messenger RNA Pfizer-BioNTech COVID-19 vaccine booster that was associated with anti-Smith and anti–double-stranded DNA (dsDNA) antibodies as well as antineutrophil cytoplasmic antibodies (ANCAs).
A 24-year-old man presented to dermatology with a rash of 3 weeks’ duration that first appeared 1 week after receiving his second booster of the messenger RNA Pfizer-BioNTech COVID-19 vaccine. Physical examination revealed petechiae with nonblanching erythematous macules and papules covering the legs below the knees (Figure 1) as well as the back of the right arm. A few days later, he developed arthralgia in the knees, hands, and feet. The patient denied any recent infections as well as respiratory and urinary tract symptoms. Approximately 10 days after the rash appeared, he developed epigastric abdominal pain that gradually worsened and sought care from his primary care physician, who ordered computed tomography and referred him for endoscopy. Computed tomography with and without contrast was suspicious for colitis. Colonoscopy and endoscopy were unremarkable. Laboratory tests were notable for elevated white blood cell count (17.08×103/µL [reference range, 3.66–10.60×103/µL]), serum IgA (437 mg/dL [reference range, 70–400 mg/dL]), C-reactive protein (1.5 mg/dL [reference range, <0.5 mg/dL]), anti-Smith antibody (28.1 CU [reference range, <20 CU), positive antinuclear antibody with titer (1:160 [reference range, <1:80]), anti-dsDNA (40.4 IU/mL [reference range, <27 IU/mL]), and cytoplasmic ANCA (c-ANCA) titer (1:320 [reference range, <1:20]). Blood urea nitrogen, creatinine, and estimated glomerular filtration rate were all within reference range. Urinalysis with microscopic examination was notable for 2 to 5 red blood cells per high-power field (reference range, 0) and proteinuria of 1+ (reference range, negative for protein).
The patient’s rash progressively worsened over the next few weeks, spreading proximally on the legs to the buttocks and the back of both elbows. A repeat complete blood cell count showed resolution of the leukocytosis. Two biopsies were taken from a lesion on the left proximal thigh: 1 for hematoxylin and eosin stain for histopathologic examination and 1 for direct immunofluorescence examination.
The patient was preliminarily diagnosed with HSP, and dermatology prescribed oral tofacitinib 5 mg twice daily for 5 days, which was supposed to be increased to 10 mg twice daily on the sixth day of treatment; however, the patient discontinued the medication after 4 days based on his primary care physician’s recommendation due to clotting concerns. The rash and arthralgia temporarily improved for 1 week, then relapsed.
Histopathology revealed neutrophils surrounding and infiltrating small dermal blood vessel walls as well as associated neutrophilic debris and erythrocytes, consistent with leukocytoclastic vasculitis (Figure 2). Direct immunofluorescence was negative for IgA antibodies. His primary care physician, in consultation with his dermatologist, then started the patient on oral prednisone 70 mg once daily for 7 days with a plan to taper. Three days after prednisone was started, the arthralgia and abdominal pain resolved, and the rash became lighter in color. After 1 week, the rash resolved completely.
Due to the unusual antibodies, the patient was referred to a rheumatologist, who repeated the blood tests approximately 1 week after the patient started prednisone. The tests were negative for anti-Smith, anti-dsDNA, and c-ANCA but showed an elevated atypical perinuclear ANCA (p-ANCA) titer of 1:80 (reference range [negative], <1:20). A repeat urinalysis was unremarkable. The patient slowly tapered the prednisone over the course of 3 months and was subsequently lost to follow-up. The rash and other symptoms had not recurred as of the patient’s last physician contact. The most recent laboratory results showed a white blood cell count of 14.0×103/µL (reference range, 3.4–10.8×103/µL), likely due to the prednisone; blood urea nitrogen, creatinine, and estimated glomerular filtration rate were within reference range. The urinalysis was notable for occult blood and was negative for protein. C-reactive protein was 1 mg/dL (reference range, 0–10 mg/dL); p-ANCA, c-ANCA, and atypical p-ANCA, as well as antinuclear antibody, were negative. As of his last follow-up, the patient felt well.
The major differential diagnoses for our patient included HSP, ANCA vasculitis, and systemic lupus erythematosus. Although ANCA vasculitis has been reported after SARS-CoV-2 infection,6 the lack of pulmonary symptoms made this diagnosis unlikely.7 Although our patient initially had elevated anti-Smith and anti-dsDNA antibodies as well as mild renal involvement, he fulfilled at most only 2 of the 11 criteria necessary for diagnosing lupus: malar rash, discoid rash (includes alopecia), photosensitivity, ocular ulcers, nonerosive arthritis, serositis, renal disorder (protein >500 mg/24 h, red blood cells, casts), neurologic disorder (seizures, psychosis), hematologic disorders (hemolytic anemia, leukopenia), ANA, and immunologic disorder (anti-Smith). Four of the 11 criteria are necessary for the diagnosis of lupus.8
Torraca et al7 reported a case of HSP with positive c-ANCA (1:640) in a patient lacking pulmonary symptoms who was diagnosed with HSP. Cytoplasmic ANCA is not a typical finding in HSP. However, the additional findings of anti-Smith, anti-dsDNA, and mildly elevated atypical p-ANCA antibodies in our patient were unexpected and could be explained by the proposed pathogenesis of HSP—an overzealous immune response resulting in aberrant antibody complex deposition with ensuing complement activation.5,9 Production of these additional antibodies could be part of the overzealous response to COVID-19 vaccination.


Of all the COVID-19 vaccines, messenger RNA–based vaccines have been associated with the majority of cutaneous reactions, including local injection-site reactions (most common), delayed local reactions, urticaria, angioedema, morbilliform eruption, herpes zoster eruption, bullous eruptions, dermal filler reactions, chilblains, and pityriasis rosea. Less common reactions have included acute generalized exanthematous pustulosis, Stevens-Johnson syndrome, erythema multiforme, Sweet Syndrome, lichen planus, papulovesicular eruptions, pityriasis rosea–like eruptions, generalized annular lesions, facial pustular neutrophilic eruptions, and flares of underlying autoimmune skin conditions.10 Multiple cases of HSP have been reported following COVID-19 vaccination from all the major vaccine companies.5
In our patient, laboratory tests were repeated by a rheumatologist and were negative for anti-Smith and anti-dsDNA antibodies as well as c-ANCA, most likely because he started taking prednisone approximately 1 week prior, which may have resulted in decreased antibodies. Also, the patient’s symptoms resolved after 1 week of steroid therapy. Therefore, the diagnosis is most consistent with HSP associated with COVID-19 vaccination. The clinical presentation, microscopic hematuria and proteinuria, and histopathology were consistent with the European Alliance of Associations for Rheumatology criteria for HSP.1
Although direct immunofluorescence typically is positive for IgA deposition on biopsies, it can be negative for IgA, especially in lesions that are biopsied more than 7 days after their appearance, as shown in our case; a negative IgA on immunofluorescence does not rule out HSP.4 Elevated serum IgA is seen in more than 50% of cases of HSP.11 Although the disease typically is self-limited, glucocorticoids are used if the disease course is prolonged or if there is evidence of kidney involvement.9 The unique combination of anti-Smith and anti-dsDNA antibodies as well as ANCAs associated with HSP with negative IgA on direct immunofluorescence has been reported with lupus.12 Clinicians should be aware of COVID-19 vaccine–associated HSP that is negative for IgA deposition and positive for anti-Smith and anti-dsDNA antibodies as well as ANCAs.
Acknowledgment—We thank our patient for granting permission to publish this information.
- Ozen S, Ruperto N, Dillon MJ, et al. EULAR/PReS endorsed consensus criteria for the classification of childhood vasculitides. Ann Rheum Dis. 2006;65:936-941. doi:10.1136/ard.2005.046300
- Rai A, Nast C, Adler S. Henoch–Schönlein purpura nephritis. J Am Soc Nephrol. 1999;10:2637-2644.
- Casini F, Magenes VC, De Sanctis M, et al. Henoch-Schönlein purpura following COVID-19 vaccine in a child: a case report. Ital J Pediatr. 2022;48:158. doi:10.1186/s13052-022-01351-1
- Poudel P, Adams SH, Mirchia K, et al. IgA negative immunofluorescence in diagnoses of adult-onset Henoch-Schönlein purpura. Proc (Bayl Univ Med Cent). 2020;33:436-437. doi:10.1080/08998280.2020.1770526
- Maronese CA, Zelin E, Avallone G, et al. Cutaneous vasculitis and vasculopathy in the era of COVID-19 pandemic. Front Med (Lausanne). 2022;9:996288. doi:10.3389/fmed.2022.996288
- Bryant MC, Spencer LT, Yalcindag A. A case of ANCA-associated vasculitis in a 16-year-old female following SARS-COV-2 infection and a systematic review of the literature. Pediatr Rheumatol Online J. 2022;20:65. doi:10.1186/s12969-022-00727-1
- Torraca PFS, Castro BC, Hans Filho G. Henoch-Schönlein purpura with c-ANCA antibody in adult. An Bras Dermatol. 2016;91:667-669. doi:10.1590/abd1806-4841.20164181
- Agabegi SS, Agabegi ED. Step-Up to Medicine. 4th ed. Wolters Kluwer; 2015.
- Ball-Burack MR, Kosowsky JM. A Case of leukocytoclastic vasculitis following SARS-CoV-2 vaccination. J Emerg Med. 2022;63:E62-E65. doi:10.1016/j.jemermed.2021.10.005
- Tan SW, Tam YC, Pang SM. Cutaneous reactions to COVID-19 vaccines: a review. JAAD Int. 2022;7:178-186. doi:10.1016/j.jdin.2022.01.011
- Calviño MC, Llorca J, García-Porrúa C, et al. Henoch-Schönlein purpura in children from northwestern Spain: a 20-year epidemiologic and clinical study. Medicine (Baltimore). 2001;80:279-290.
- Hu P, Huang BY, Zhang DD, et al. Henoch-Schönlein purpura in a pediatric patient with lupus. Arch Med Sci. 2017;13:689-690. doi:10.5114/aoms.2017.67288
- Ozen S, Ruperto N, Dillon MJ, et al. EULAR/PReS endorsed consensus criteria for the classification of childhood vasculitides. Ann Rheum Dis. 2006;65:936-941. doi:10.1136/ard.2005.046300
- Rai A, Nast C, Adler S. Henoch–Schönlein purpura nephritis. J Am Soc Nephrol. 1999;10:2637-2644.
- Casini F, Magenes VC, De Sanctis M, et al. Henoch-Schönlein purpura following COVID-19 vaccine in a child: a case report. Ital J Pediatr. 2022;48:158. doi:10.1186/s13052-022-01351-1
- Poudel P, Adams SH, Mirchia K, et al. IgA negative immunofluorescence in diagnoses of adult-onset Henoch-Schönlein purpura. Proc (Bayl Univ Med Cent). 2020;33:436-437. doi:10.1080/08998280.2020.1770526
- Maronese CA, Zelin E, Avallone G, et al. Cutaneous vasculitis and vasculopathy in the era of COVID-19 pandemic. Front Med (Lausanne). 2022;9:996288. doi:10.3389/fmed.2022.996288
- Bryant MC, Spencer LT, Yalcindag A. A case of ANCA-associated vasculitis in a 16-year-old female following SARS-COV-2 infection and a systematic review of the literature. Pediatr Rheumatol Online J. 2022;20:65. doi:10.1186/s12969-022-00727-1
- Torraca PFS, Castro BC, Hans Filho G. Henoch-Schönlein purpura with c-ANCA antibody in adult. An Bras Dermatol. 2016;91:667-669. doi:10.1590/abd1806-4841.20164181
- Agabegi SS, Agabegi ED. Step-Up to Medicine. 4th ed. Wolters Kluwer; 2015.
- Ball-Burack MR, Kosowsky JM. A Case of leukocytoclastic vasculitis following SARS-CoV-2 vaccination. J Emerg Med. 2022;63:E62-E65. doi:10.1016/j.jemermed.2021.10.005
- Tan SW, Tam YC, Pang SM. Cutaneous reactions to COVID-19 vaccines: a review. JAAD Int. 2022;7:178-186. doi:10.1016/j.jdin.2022.01.011
- Calviño MC, Llorca J, García-Porrúa C, et al. Henoch-Schönlein purpura in children from northwestern Spain: a 20-year epidemiologic and clinical study. Medicine (Baltimore). 2001;80:279-290.
- Hu P, Huang BY, Zhang DD, et al. Henoch-Schönlein purpura in a pediatric patient with lupus. Arch Med Sci. 2017;13:689-690. doi:10.5114/aoms.2017.67288
Practice Points
- Dermatologists should be vigilant for Henoch-Schönlein purpura (HSP) despite negative direct immunofluorescence of IgA deposition and unusual antibodies.
- Messenger RNA–based COVID-19 vaccines are associated with various cutaneous reactions, including HSP.
- Anti-Smith and anti–double-stranded DNA antibodies typically are not associated with HSP but may be seen in patients with coexisting systemic lupus erythematosus.
Consider Risks, Toxicity of Some Topical Ingredients in Infants, Young Children
TORONTO — Lawrence A. Schachner, MD, would like pediatric dermatologists to adopt a “toxic agent of the year” to raise awareness about the potential harm related to certain topical treatments in babies and young children.
Dr. Schachner, director of the Division of Pediatric Dermatology in the Department of Dermatology & Cutaneous Surgery at the University of Miami, Coral Gables, Florida, said he got the idea from the American Contact Dermatitis Society, which annually names the “Allergen of the Year.”
, said Dr. Schachner, professor of pediatrics and dermatology at the University of Miami.
“Any one of those would be excellent toxic substances of the year” that could be the focus of an educational campaign, he told this news organization following his presentation on “Toxicology of Topical Ingredients in Pediatric Dermatology” at the annual meeting of the Society for Pediatric Dermatology on July 14.
Benzene might also be a good candidate for the list, although the jury seems to be still out on its toxicity, said Dr. Schachner.
He talked about the “four Ps” of poisoning — the physician, pharmacy, parents, and pharmaceutical manufacturing — which all have some responsibility for errors that lead to adverse outcomes but can also take steps to prevent them.
During his presentation, Dr. Schachner discussed how babies are especially sensitive to topical therapies, noting that a baby’s skin is thinner and more permeable than that of an adult. And children have a greater body surface-to-weight ratio, so they absorb more substances through their skin.
He also noted that babies lack natural moisturizing factors, and their skin barrier isn’t mature until about age 3-5 years, stressing the need for extreme care when applying a topical agent to a baby’s skin.
Tragic Stories
Dr. Schachner pointed to some instances of mishaps related to toxic topical substances in children. There was the outbreak in the early 1980s of accidental hexachlorophene poisoning among children in France exposed to talc “baby powder.” Of the 204 affected children, 36 died.
The cause was a manufacturing error; the product contained 6.3% hexachlorophene, as opposed to the 0.1% limit recommended by the US Food and Drug Administration (FDA).
Local anesthetics, including lidocaine, dibucaine, and prilocaine, can cause local anesthetic systemic toxicity, a syndrome with symptoms that include central nervous system depression, seizures, and cardiotoxicity. Dr. Schachner described the case of a 3-year-old who developed methemoglobinemia, with seizures, after treatment with an excessive amount of eutectic mixture of local anesthetics (EMLA) cream, which contains both lidocaine and prilocaine.
EMLA shouldn’t be used with methemoglobinemia-inducing agents, such as some antimalarials, analgesics, anesthetics, and antineoplastic agents. It’s not recommended in neonates or for those under 12 months if receiving methemoglobinemia-inducing agents, “and I would keep an eye on it after 12 months of age,” said Dr. Schachner.
He cited a retrospective review of topical lidocaine toxicity in pediatric patients reported to the National Poison Data System from 2000 to 2020. It found 37 cases of toxicity, the most common from application prior to dermatologic procedures (37.5%), which led to two deaths.
Not Benign Agents
“These are not benign agents; we have to use them correctly,” Dr. Schachner stressed. When discussing alcohols and antiseptics, he noted that phenol is found in a variety of household disinfectants, gargling products, ointments, and lip balms. Phenol can be used as a chemical peel and is the antiseptic component of Castellani paint. He also referred to cases of alcohol intoxication linked to umbilical care in newborns.
Benzene at elevated levels has been found in some topical benzoyl peroxide acne products and in some sunscreens. There have been suggestions, not strongly substantiated, that benzene may increase the risk for cancer, especially leukemias.
But there is sparse data on the absorption and toxicity of benzene exposure with sunscreen use. The data, he said, include an analysis of National Health and Nutrition Examination Survey data, which found that people who regularly used sunscreens were less likely to have elevated benzene levels compared with those who didn’t use sunscreens.
Turning to insecticides, Dr. Schachner discussed N,N-diethyl-m-toluamide (DEET), the active ingredient in many insect repellents. It helps avoid “some terrible diseases,” including mosquito-borne illnesses such as malaria and tick-borne conditions such as Lyme disease, and is available in several convenient formulations, he said.
When used on children, the American Academy of Pediatrics (AAP) recommends products with no more than 30% DEET. And insect repellents are not recommended for children younger than 2 months, or under clothing or damaged skin, he said.
Dr. Schachner referred to a case series of 18 children who developed DEET-induced encephalopathy; 13 (72%) involved dermal exposure. Three of those with cutaneous exposure died, mostly from neurologic, respiratory, and cardiac issues. “What’s very striking is that 55% of the kids were exposed to DEET of 20% or less, even though the AAP approves DEET at 30%, so maybe that’s something we have to look at,” he said.
Medication Patches
With medication patches, especially fentanyl transdermal patches, much can go wrong when it comes to children. This was highlighted by the cases Schachner cited, including an infant who developed acute cytotoxic cerebellar edema from fentanyl patch intoxication.
In another case, emergency room staff found a fentanyl patch stuck to the back of a 3-year-old girl. A CT scan showed global cerebral edema, and the patient progressed to brain death. “This is not a unique case; there have been over 10 such cases in the United States,” said Dr. Schachner. “We should be doing better with fentanyl.”
Nicotine patches can also be dangerous to children, he added. As for other topical agents, there have been reports of toxicity and deaths linked to salicylic acid, commonly used by dermatologists because of its bacteriostatic, fungicidal, keratolytic, and photoprotective properties.
Dr. Schachner cited the case of a 2-month-old where the pediatrician prescribed 50% salicylic acid for seborrheic dermatitis of the scalp, under occlusion. “It’s amazing this child survived; that’s clearly a physician error,” he said.
Henna, a reddish-brown dye derived from the crushed leaves of Lawsonia alba, is used cosmetically for the hair, skin, and nails. Many henna products are mixed with additives, including para-phenylenediamine, which has been associated with dermatitis, asthma, renal failure, and permanent vision loss.
Asked to comment on the presentation, Sheilagh Maguiness, MD, professor of dermatology and pediatrics and chair of pediatric dermatology at the University of Minnesota, Minneapolis, recalled a particularly concerning story in 2008, when the FDA issued a warning about Mommy’s Bliss, a cream containing chlorphenesin and phenoxyethanol as preservatives, promoted to nursing mothers for soothing cracked nipples. There were reports of the cream causing respiratory distress, vomiting, and diarrhea in nursing infants.
Dr. Schachner is chair of Stiefel Laboratories and is an investigator with: Astellas, Berg Pharma, Celgene, Ferndale Labs, Lilly, Medimetriks Pharmaceuticals, Novartis, Organogenesis, Pfizer, Sciton; is a consultant for: Alphyn, Amryt Pharma, Beiersdorf, Brickell, Cutanea, Hoth, Lexington, Mustela, TopMD, Noble Pharma; a speaker for: Novartis, Sanofi-Regeneron, CeraVe; is on the advisory boards of: Almirall, Alphyn, Apogee, Aslan, Biofrontera, CeraVe, Krystal Biotech, Mustela, Noble Pharma, Pfizer, Pierre Fabre, Sanofi-Regeneron; and owns stocks in: TopMD and Alphyn. Dr. Maguiness had no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
TORONTO — Lawrence A. Schachner, MD, would like pediatric dermatologists to adopt a “toxic agent of the year” to raise awareness about the potential harm related to certain topical treatments in babies and young children.
Dr. Schachner, director of the Division of Pediatric Dermatology in the Department of Dermatology & Cutaneous Surgery at the University of Miami, Coral Gables, Florida, said he got the idea from the American Contact Dermatitis Society, which annually names the “Allergen of the Year.”
, said Dr. Schachner, professor of pediatrics and dermatology at the University of Miami.
“Any one of those would be excellent toxic substances of the year” that could be the focus of an educational campaign, he told this news organization following his presentation on “Toxicology of Topical Ingredients in Pediatric Dermatology” at the annual meeting of the Society for Pediatric Dermatology on July 14.
Benzene might also be a good candidate for the list, although the jury seems to be still out on its toxicity, said Dr. Schachner.
He talked about the “four Ps” of poisoning — the physician, pharmacy, parents, and pharmaceutical manufacturing — which all have some responsibility for errors that lead to adverse outcomes but can also take steps to prevent them.
During his presentation, Dr. Schachner discussed how babies are especially sensitive to topical therapies, noting that a baby’s skin is thinner and more permeable than that of an adult. And children have a greater body surface-to-weight ratio, so they absorb more substances through their skin.
He also noted that babies lack natural moisturizing factors, and their skin barrier isn’t mature until about age 3-5 years, stressing the need for extreme care when applying a topical agent to a baby’s skin.
Tragic Stories
Dr. Schachner pointed to some instances of mishaps related to toxic topical substances in children. There was the outbreak in the early 1980s of accidental hexachlorophene poisoning among children in France exposed to talc “baby powder.” Of the 204 affected children, 36 died.
The cause was a manufacturing error; the product contained 6.3% hexachlorophene, as opposed to the 0.1% limit recommended by the US Food and Drug Administration (FDA).
Local anesthetics, including lidocaine, dibucaine, and prilocaine, can cause local anesthetic systemic toxicity, a syndrome with symptoms that include central nervous system depression, seizures, and cardiotoxicity. Dr. Schachner described the case of a 3-year-old who developed methemoglobinemia, with seizures, after treatment with an excessive amount of eutectic mixture of local anesthetics (EMLA) cream, which contains both lidocaine and prilocaine.
EMLA shouldn’t be used with methemoglobinemia-inducing agents, such as some antimalarials, analgesics, anesthetics, and antineoplastic agents. It’s not recommended in neonates or for those under 12 months if receiving methemoglobinemia-inducing agents, “and I would keep an eye on it after 12 months of age,” said Dr. Schachner.
He cited a retrospective review of topical lidocaine toxicity in pediatric patients reported to the National Poison Data System from 2000 to 2020. It found 37 cases of toxicity, the most common from application prior to dermatologic procedures (37.5%), which led to two deaths.
Not Benign Agents
“These are not benign agents; we have to use them correctly,” Dr. Schachner stressed. When discussing alcohols and antiseptics, he noted that phenol is found in a variety of household disinfectants, gargling products, ointments, and lip balms. Phenol can be used as a chemical peel and is the antiseptic component of Castellani paint. He also referred to cases of alcohol intoxication linked to umbilical care in newborns.
Benzene at elevated levels has been found in some topical benzoyl peroxide acne products and in some sunscreens. There have been suggestions, not strongly substantiated, that benzene may increase the risk for cancer, especially leukemias.
But there is sparse data on the absorption and toxicity of benzene exposure with sunscreen use. The data, he said, include an analysis of National Health and Nutrition Examination Survey data, which found that people who regularly used sunscreens were less likely to have elevated benzene levels compared with those who didn’t use sunscreens.
Turning to insecticides, Dr. Schachner discussed N,N-diethyl-m-toluamide (DEET), the active ingredient in many insect repellents. It helps avoid “some terrible diseases,” including mosquito-borne illnesses such as malaria and tick-borne conditions such as Lyme disease, and is available in several convenient formulations, he said.
When used on children, the American Academy of Pediatrics (AAP) recommends products with no more than 30% DEET. And insect repellents are not recommended for children younger than 2 months, or under clothing or damaged skin, he said.
Dr. Schachner referred to a case series of 18 children who developed DEET-induced encephalopathy; 13 (72%) involved dermal exposure. Three of those with cutaneous exposure died, mostly from neurologic, respiratory, and cardiac issues. “What’s very striking is that 55% of the kids were exposed to DEET of 20% or less, even though the AAP approves DEET at 30%, so maybe that’s something we have to look at,” he said.
Medication Patches
With medication patches, especially fentanyl transdermal patches, much can go wrong when it comes to children. This was highlighted by the cases Schachner cited, including an infant who developed acute cytotoxic cerebellar edema from fentanyl patch intoxication.
In another case, emergency room staff found a fentanyl patch stuck to the back of a 3-year-old girl. A CT scan showed global cerebral edema, and the patient progressed to brain death. “This is not a unique case; there have been over 10 such cases in the United States,” said Dr. Schachner. “We should be doing better with fentanyl.”
Nicotine patches can also be dangerous to children, he added. As for other topical agents, there have been reports of toxicity and deaths linked to salicylic acid, commonly used by dermatologists because of its bacteriostatic, fungicidal, keratolytic, and photoprotective properties.
Dr. Schachner cited the case of a 2-month-old where the pediatrician prescribed 50% salicylic acid for seborrheic dermatitis of the scalp, under occlusion. “It’s amazing this child survived; that’s clearly a physician error,” he said.
Henna, a reddish-brown dye derived from the crushed leaves of Lawsonia alba, is used cosmetically for the hair, skin, and nails. Many henna products are mixed with additives, including para-phenylenediamine, which has been associated with dermatitis, asthma, renal failure, and permanent vision loss.
Asked to comment on the presentation, Sheilagh Maguiness, MD, professor of dermatology and pediatrics and chair of pediatric dermatology at the University of Minnesota, Minneapolis, recalled a particularly concerning story in 2008, when the FDA issued a warning about Mommy’s Bliss, a cream containing chlorphenesin and phenoxyethanol as preservatives, promoted to nursing mothers for soothing cracked nipples. There were reports of the cream causing respiratory distress, vomiting, and diarrhea in nursing infants.
Dr. Schachner is chair of Stiefel Laboratories and is an investigator with: Astellas, Berg Pharma, Celgene, Ferndale Labs, Lilly, Medimetriks Pharmaceuticals, Novartis, Organogenesis, Pfizer, Sciton; is a consultant for: Alphyn, Amryt Pharma, Beiersdorf, Brickell, Cutanea, Hoth, Lexington, Mustela, TopMD, Noble Pharma; a speaker for: Novartis, Sanofi-Regeneron, CeraVe; is on the advisory boards of: Almirall, Alphyn, Apogee, Aslan, Biofrontera, CeraVe, Krystal Biotech, Mustela, Noble Pharma, Pfizer, Pierre Fabre, Sanofi-Regeneron; and owns stocks in: TopMD and Alphyn. Dr. Maguiness had no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
TORONTO — Lawrence A. Schachner, MD, would like pediatric dermatologists to adopt a “toxic agent of the year” to raise awareness about the potential harm related to certain topical treatments in babies and young children.
Dr. Schachner, director of the Division of Pediatric Dermatology in the Department of Dermatology & Cutaneous Surgery at the University of Miami, Coral Gables, Florida, said he got the idea from the American Contact Dermatitis Society, which annually names the “Allergen of the Year.”
, said Dr. Schachner, professor of pediatrics and dermatology at the University of Miami.
“Any one of those would be excellent toxic substances of the year” that could be the focus of an educational campaign, he told this news organization following his presentation on “Toxicology of Topical Ingredients in Pediatric Dermatology” at the annual meeting of the Society for Pediatric Dermatology on July 14.
Benzene might also be a good candidate for the list, although the jury seems to be still out on its toxicity, said Dr. Schachner.
He talked about the “four Ps” of poisoning — the physician, pharmacy, parents, and pharmaceutical manufacturing — which all have some responsibility for errors that lead to adverse outcomes but can also take steps to prevent them.
During his presentation, Dr. Schachner discussed how babies are especially sensitive to topical therapies, noting that a baby’s skin is thinner and more permeable than that of an adult. And children have a greater body surface-to-weight ratio, so they absorb more substances through their skin.
He also noted that babies lack natural moisturizing factors, and their skin barrier isn’t mature until about age 3-5 years, stressing the need for extreme care when applying a topical agent to a baby’s skin.
Tragic Stories
Dr. Schachner pointed to some instances of mishaps related to toxic topical substances in children. There was the outbreak in the early 1980s of accidental hexachlorophene poisoning among children in France exposed to talc “baby powder.” Of the 204 affected children, 36 died.
The cause was a manufacturing error; the product contained 6.3% hexachlorophene, as opposed to the 0.1% limit recommended by the US Food and Drug Administration (FDA).
Local anesthetics, including lidocaine, dibucaine, and prilocaine, can cause local anesthetic systemic toxicity, a syndrome with symptoms that include central nervous system depression, seizures, and cardiotoxicity. Dr. Schachner described the case of a 3-year-old who developed methemoglobinemia, with seizures, after treatment with an excessive amount of eutectic mixture of local anesthetics (EMLA) cream, which contains both lidocaine and prilocaine.
EMLA shouldn’t be used with methemoglobinemia-inducing agents, such as some antimalarials, analgesics, anesthetics, and antineoplastic agents. It’s not recommended in neonates or for those under 12 months if receiving methemoglobinemia-inducing agents, “and I would keep an eye on it after 12 months of age,” said Dr. Schachner.
He cited a retrospective review of topical lidocaine toxicity in pediatric patients reported to the National Poison Data System from 2000 to 2020. It found 37 cases of toxicity, the most common from application prior to dermatologic procedures (37.5%), which led to two deaths.
Not Benign Agents
“These are not benign agents; we have to use them correctly,” Dr. Schachner stressed. When discussing alcohols and antiseptics, he noted that phenol is found in a variety of household disinfectants, gargling products, ointments, and lip balms. Phenol can be used as a chemical peel and is the antiseptic component of Castellani paint. He also referred to cases of alcohol intoxication linked to umbilical care in newborns.
Benzene at elevated levels has been found in some topical benzoyl peroxide acne products and in some sunscreens. There have been suggestions, not strongly substantiated, that benzene may increase the risk for cancer, especially leukemias.
But there is sparse data on the absorption and toxicity of benzene exposure with sunscreen use. The data, he said, include an analysis of National Health and Nutrition Examination Survey data, which found that people who regularly used sunscreens were less likely to have elevated benzene levels compared with those who didn’t use sunscreens.
Turning to insecticides, Dr. Schachner discussed N,N-diethyl-m-toluamide (DEET), the active ingredient in many insect repellents. It helps avoid “some terrible diseases,” including mosquito-borne illnesses such as malaria and tick-borne conditions such as Lyme disease, and is available in several convenient formulations, he said.
When used on children, the American Academy of Pediatrics (AAP) recommends products with no more than 30% DEET. And insect repellents are not recommended for children younger than 2 months, or under clothing or damaged skin, he said.
Dr. Schachner referred to a case series of 18 children who developed DEET-induced encephalopathy; 13 (72%) involved dermal exposure. Three of those with cutaneous exposure died, mostly from neurologic, respiratory, and cardiac issues. “What’s very striking is that 55% of the kids were exposed to DEET of 20% or less, even though the AAP approves DEET at 30%, so maybe that’s something we have to look at,” he said.
Medication Patches
With medication patches, especially fentanyl transdermal patches, much can go wrong when it comes to children. This was highlighted by the cases Schachner cited, including an infant who developed acute cytotoxic cerebellar edema from fentanyl patch intoxication.
In another case, emergency room staff found a fentanyl patch stuck to the back of a 3-year-old girl. A CT scan showed global cerebral edema, and the patient progressed to brain death. “This is not a unique case; there have been over 10 such cases in the United States,” said Dr. Schachner. “We should be doing better with fentanyl.”
Nicotine patches can also be dangerous to children, he added. As for other topical agents, there have been reports of toxicity and deaths linked to salicylic acid, commonly used by dermatologists because of its bacteriostatic, fungicidal, keratolytic, and photoprotective properties.
Dr. Schachner cited the case of a 2-month-old where the pediatrician prescribed 50% salicylic acid for seborrheic dermatitis of the scalp, under occlusion. “It’s amazing this child survived; that’s clearly a physician error,” he said.
Henna, a reddish-brown dye derived from the crushed leaves of Lawsonia alba, is used cosmetically for the hair, skin, and nails. Many henna products are mixed with additives, including para-phenylenediamine, which has been associated with dermatitis, asthma, renal failure, and permanent vision loss.
Asked to comment on the presentation, Sheilagh Maguiness, MD, professor of dermatology and pediatrics and chair of pediatric dermatology at the University of Minnesota, Minneapolis, recalled a particularly concerning story in 2008, when the FDA issued a warning about Mommy’s Bliss, a cream containing chlorphenesin and phenoxyethanol as preservatives, promoted to nursing mothers for soothing cracked nipples. There were reports of the cream causing respiratory distress, vomiting, and diarrhea in nursing infants.
Dr. Schachner is chair of Stiefel Laboratories and is an investigator with: Astellas, Berg Pharma, Celgene, Ferndale Labs, Lilly, Medimetriks Pharmaceuticals, Novartis, Organogenesis, Pfizer, Sciton; is a consultant for: Alphyn, Amryt Pharma, Beiersdorf, Brickell, Cutanea, Hoth, Lexington, Mustela, TopMD, Noble Pharma; a speaker for: Novartis, Sanofi-Regeneron, CeraVe; is on the advisory boards of: Almirall, Alphyn, Apogee, Aslan, Biofrontera, CeraVe, Krystal Biotech, Mustela, Noble Pharma, Pfizer, Pierre Fabre, Sanofi-Regeneron; and owns stocks in: TopMD and Alphyn. Dr. Maguiness had no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
FROM SPD 2024
Insurers’ Rules and AI for Preauthorization: ‘Ethically Nuts,’ Says Ethicist
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I’m at the Division of Medical Ethics at New York University Grossman School of Medicine in New York City.
There are many things screwy with our healthcare system. Many of you [reading] this are dealing with bureaucracy, paperwork, all sorts of constraints, restraints, and requirements that sometimes make the practice of medicine, or even nursing, difficult.
I don’t think I’ve seen anything screwier, from a moral point of view, than the system we have that allows for preauthorization by third-party payers, or insurers, in order to give care to patients. It’s pretty clear that a third-party payer has a conflict of interest. It’s simple: They don’t want to spend money.
Their goal as profit-making companies is to reduce what it is that they’re going to authorize. That clearly is driving how the preauthorization process works. or somebody saying, this is the standard of care and this is what ought to happen.
We’re letting the people who have the pocketbooks and the wallets have prior approval of what the doctor thinks is correct. That is really not the way to practice medicine.
We now have more evidence about what really is going on. A doctor was recently interviewed by ProPublica and said that she had worked for Cigna as a reviewer. Basically, the message she got from that insurer was to speed it up, go fast, and basically “deny, deny, deny” when she got requests. Those are her words, not mine.
We get a peek under the tent of how this works, and Dr. Day is basically saying she had to leave because she just didn’t feel that it was evidence-driven. It was driven by concerns about who’s going to lose money or make money.
If you want to check to see whether something is appropriate, the question becomes, who ought to do prior review?
Who does it now? Sometimes doctors. Sometimes nurses who aren’t in the specialty where the request is coming in for preapproval. I’ve even seen situations where some companies use nurses in other countries, such as the Philippines, to do preapproval. They send them information, like a clip, to use to deny things that basically is boilerplate language, whatever the request is.
Looming up now, some insurers are starting to think, well, maybe artificial intelligence could do it. Just review the written request, trigger certain responses on the part of the artificial intelligence — it can deny the claims just as well as a human — and maybe it’s even cheaper to set up that system for the insurer.
This is ethically nuts. We need to have a system where doctors’ judgments drive what patients get. You listen to doctors, as I do, about preapproval access and they say patients sometimes give up trying to get what they think is needed. Continuity of care is interrupted if they have to keep making requests all the time.
There are adverse events when the thing that the doctor thought was most appropriate isn’t approved and something else is used that is less safe or less efficacious. It isn’t in patient interest to have the person with the wallet saying, this is what we think you need, and then having unqualified people or even automated intelligence with no accountability and no transparency get involved in preauthorization.
This system costs us money because middlemen are doing all this work. It basically becomes one of the huge scandals, in my view, of our health system, that doctors don’t ultimately decide what the patient needs. A preauthorizing third party or robot, without transparency, without accountability, and behind closed doors second-guesses what’s going on.
I’m Art Caplan at the Division of Medical Ethics at the New York University Grossman School of Medicine.
Arthur L. Caplan, Director, Division of Medical Ethics, New York University Langone Medical Center, New York, New York, has disclosed the following relevant financial relationships: Served as a director, officer, partner, employee, advisor, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position). Serves as a contributing author and advisor for Medscape.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I’m at the Division of Medical Ethics at New York University Grossman School of Medicine in New York City.
There are many things screwy with our healthcare system. Many of you [reading] this are dealing with bureaucracy, paperwork, all sorts of constraints, restraints, and requirements that sometimes make the practice of medicine, or even nursing, difficult.
I don’t think I’ve seen anything screwier, from a moral point of view, than the system we have that allows for preauthorization by third-party payers, or insurers, in order to give care to patients. It’s pretty clear that a third-party payer has a conflict of interest. It’s simple: They don’t want to spend money.
Their goal as profit-making companies is to reduce what it is that they’re going to authorize. That clearly is driving how the preauthorization process works. or somebody saying, this is the standard of care and this is what ought to happen.
We’re letting the people who have the pocketbooks and the wallets have prior approval of what the doctor thinks is correct. That is really not the way to practice medicine.
We now have more evidence about what really is going on. A doctor was recently interviewed by ProPublica and said that she had worked for Cigna as a reviewer. Basically, the message she got from that insurer was to speed it up, go fast, and basically “deny, deny, deny” when she got requests. Those are her words, not mine.
We get a peek under the tent of how this works, and Dr. Day is basically saying she had to leave because she just didn’t feel that it was evidence-driven. It was driven by concerns about who’s going to lose money or make money.
If you want to check to see whether something is appropriate, the question becomes, who ought to do prior review?
Who does it now? Sometimes doctors. Sometimes nurses who aren’t in the specialty where the request is coming in for preapproval. I’ve even seen situations where some companies use nurses in other countries, such as the Philippines, to do preapproval. They send them information, like a clip, to use to deny things that basically is boilerplate language, whatever the request is.
Looming up now, some insurers are starting to think, well, maybe artificial intelligence could do it. Just review the written request, trigger certain responses on the part of the artificial intelligence — it can deny the claims just as well as a human — and maybe it’s even cheaper to set up that system for the insurer.
This is ethically nuts. We need to have a system where doctors’ judgments drive what patients get. You listen to doctors, as I do, about preapproval access and they say patients sometimes give up trying to get what they think is needed. Continuity of care is interrupted if they have to keep making requests all the time.
There are adverse events when the thing that the doctor thought was most appropriate isn’t approved and something else is used that is less safe or less efficacious. It isn’t in patient interest to have the person with the wallet saying, this is what we think you need, and then having unqualified people or even automated intelligence with no accountability and no transparency get involved in preauthorization.
This system costs us money because middlemen are doing all this work. It basically becomes one of the huge scandals, in my view, of our health system, that doctors don’t ultimately decide what the patient needs. A preauthorizing third party or robot, without transparency, without accountability, and behind closed doors second-guesses what’s going on.
I’m Art Caplan at the Division of Medical Ethics at the New York University Grossman School of Medicine.
Arthur L. Caplan, Director, Division of Medical Ethics, New York University Langone Medical Center, New York, New York, has disclosed the following relevant financial relationships: Served as a director, officer, partner, employee, advisor, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position). Serves as a contributing author and advisor for Medscape.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I’m at the Division of Medical Ethics at New York University Grossman School of Medicine in New York City.
There are many things screwy with our healthcare system. Many of you [reading] this are dealing with bureaucracy, paperwork, all sorts of constraints, restraints, and requirements that sometimes make the practice of medicine, or even nursing, difficult.
I don’t think I’ve seen anything screwier, from a moral point of view, than the system we have that allows for preauthorization by third-party payers, or insurers, in order to give care to patients. It’s pretty clear that a third-party payer has a conflict of interest. It’s simple: They don’t want to spend money.
Their goal as profit-making companies is to reduce what it is that they’re going to authorize. That clearly is driving how the preauthorization process works. or somebody saying, this is the standard of care and this is what ought to happen.
We’re letting the people who have the pocketbooks and the wallets have prior approval of what the doctor thinks is correct. That is really not the way to practice medicine.
We now have more evidence about what really is going on. A doctor was recently interviewed by ProPublica and said that she had worked for Cigna as a reviewer. Basically, the message she got from that insurer was to speed it up, go fast, and basically “deny, deny, deny” when she got requests. Those are her words, not mine.
We get a peek under the tent of how this works, and Dr. Day is basically saying she had to leave because she just didn’t feel that it was evidence-driven. It was driven by concerns about who’s going to lose money or make money.
If you want to check to see whether something is appropriate, the question becomes, who ought to do prior review?
Who does it now? Sometimes doctors. Sometimes nurses who aren’t in the specialty where the request is coming in for preapproval. I’ve even seen situations where some companies use nurses in other countries, such as the Philippines, to do preapproval. They send them information, like a clip, to use to deny things that basically is boilerplate language, whatever the request is.
Looming up now, some insurers are starting to think, well, maybe artificial intelligence could do it. Just review the written request, trigger certain responses on the part of the artificial intelligence — it can deny the claims just as well as a human — and maybe it’s even cheaper to set up that system for the insurer.
This is ethically nuts. We need to have a system where doctors’ judgments drive what patients get. You listen to doctors, as I do, about preapproval access and they say patients sometimes give up trying to get what they think is needed. Continuity of care is interrupted if they have to keep making requests all the time.
There are adverse events when the thing that the doctor thought was most appropriate isn’t approved and something else is used that is less safe or less efficacious. It isn’t in patient interest to have the person with the wallet saying, this is what we think you need, and then having unqualified people or even automated intelligence with no accountability and no transparency get involved in preauthorization.
This system costs us money because middlemen are doing all this work. It basically becomes one of the huge scandals, in my view, of our health system, that doctors don’t ultimately decide what the patient needs. A preauthorizing third party or robot, without transparency, without accountability, and behind closed doors second-guesses what’s going on.
I’m Art Caplan at the Division of Medical Ethics at the New York University Grossman School of Medicine.
Arthur L. Caplan, Director, Division of Medical Ethics, New York University Langone Medical Center, New York, New York, has disclosed the following relevant financial relationships: Served as a director, officer, partner, employee, advisor, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position). Serves as a contributing author and advisor for Medscape.
A version of this article first appeared on Medscape.com.
Lipedema: Current Diagnostic and Treatment Evidence
Lipedema affects about 11% of cisgender women, according to the Brazilian Society of Angiology and Vascular Surgery. Yet the condition remains wrapped in uncertainties. Despite significant advancements in understanding its physiology, diagnosis, and treatment, more clarity is needed as awareness and diagnoses increase.
At the latest International Congress on Obesity (ICO) in São Paulo, Brazil, Philipp Scherer, PhD, director of the Touchstone Diabetes Center, discussed the complexities of lipedema. “It is an extremely frustrating condition for someone like me, who has spent a lifetime studying functional and dysfunctional adipose tissue. We are trying to understand the physiology of this pathology, but it is challenging, and so far, we have not been able to find a concrete answer,” he noted.
Lipedema is characterized by the abnormal accumulation of subcutaneous adipose tissue, especially in the lower limbs, and almost exclusively affects cisgender women. The reason for this gender disparity is unclear. It could be an intrinsic characteristic of the disease or a result from clinicians’ lack of familiarity with lipedema, which often leads to misdiagnosis as obesity. This misdiagnosis results in fewer men seeking treatment.
Research has predominantly focused on women, and evidence suggests that hormones play a crucial role in the disease’s pathophysiology. Lipedema typically manifests during periods of hormonal changes, such as puberty, pregnancy, menopause, and hormone replacement therapies, reinforcing the idea that hormones significantly influence the condition’s development and progression.
Main Symptoms
Jonathan Kartt, CEO of the Lipedema Foundation, emphasized that intense pain in the areas of adipose tissue accumulation is a hallmark symptom of lipedema, setting it apart from obesity. Pain levels can vary widely among patients, ranging from moderate to severe, with unbearable peaks on certain days. Mr. Kartt stressed the importance of recognizing and addressing this often underestimated symptom.
Lipedema is characterized by a bilateral, symmetrical increase in mass compared with the rest of the body. This is commonly distinguished by the “cuff sign,” a separation between normal tissue in the feet and abnormal tissue from the ankle upward. Other frequent symptoms include a feeling of heaviness, discomfort, fatigue, frequent bruising, and tiredness. A notable sign is the presence of subcutaneous nodules with a texture similar to that of rice grains, which are crucial for differentiating lipedema from other conditions. Palpation during anamnesis is essential to identify these nodules and confirm the diagnosis.
“It is crucial to investigate the family history for genetic predisposition. Additionally, it is fundamental to ask whether, even with weight loss, the affected areas retain accumulated fat. Hormonal changes, pain symptoms, and impact on quality of life should also be carefully evaluated,” advised Mr. Kartt.
Diagnostic Tools
André Murad, MD, a clinical consultant at the Instituto Lipedema Brazil, has been exploring new diagnostic approaches for lipedema beyond traditional anamnesis. During his presentation at the ICO, he shared studies on the efficacy of imaging exams such as ultrasound, tomography, and MRI in diagnosing the characteristic lipedema-associated increase in subcutaneous tissue.
He also discussed lymphangiography and lymphoscintigraphy, highlighting the use of magnetic resonance lymphangiography to evaluate dilated lymphatic vessels often observed in patients with lipedema. “By injecting contrast into the feet, this technique allows the evaluation of vessels, which are usually dilated, indicating characteristic lymphatic system overload in lipedema. Lymphoscintigraphy is crucial for detecting associated lymphedema, revealing delayed lymphatic flow and asymmetry between limbs in cases of lipedema without lymphedema,” he explained.
Despite the various diagnostic options, Dr. Murad highlighted two highly effective studies. A Brazilian study used ultrasound to establish a cutoff point of 11.7 mm in the pretibial subcutaneous tissue thickness, achieving 96% specificity for diagnosis. Another study emphasized the value of dual-energy x-ray absorptiometry (DXA), which demonstrated 95% sensitivity. This method assesses fat distribution by correlating the amount present in the legs with the total body, providing a cost-effective and accessible option for specialists.
“DXA allows for a precise mathematical evaluation of fat distribution relative to the total body. A ratio of 0.38 in the leg-to-body relationship is a significant indicator of high suspicion of lipedema,” highlighted Dr. Murad. “In clinical practice, many patients self-diagnose with lipedema, but the clinical exam often reveals no disproportion, with the leg-to-body ratio below 0.38 being common in these cases,” he added.
Treatment Approaches
Treatments for lipedema are still evolving, with considerable debate about the best approach. While some specialists advocate exclusively for conservative treatment, others recommend combining these methods with surgical interventions, depending on the stage of the disease. The relative novelty of lipedema and the scarcity of robust, long-term studies contribute to the uncertainty around treatment efficacy.
Conservative treatment typically includes compression, lymphatic drainage techniques, and pressure therapy. An active lifestyle and a healthy diet are also recommended. Although these measures do not prevent the accumulation of adipose tissue, they help reduce inflammation and improve quality of life. “Even though the causes of lipedema are not fully known, lifestyle management is essential for controlling symptoms, starting with an anti-inflammatory diet,” emphasized Dr. Murad.
Because insulin promotes lipogenesis, a diet that avoids spikes in glycemic and insulin levels is advisable. Insulin resistance can exacerbate edema formation, so a Mediterranean diet may be beneficial. This diet limits fast-absorbing carbohydrates, such as added sugar, refined grains, and ultraprocessed foods, while promoting complex carbohydrates from whole grains and legumes.
Dr. Murad also presented a study evaluating the potential benefits of a low-carbohydrate, high-fat diet for patients with lipedema. The study demonstrated weight loss, reduced body fat, controlled leg volume, and, notably, pain relief.
For more advanced stages of lipedema, plastic surgery is often considered when conservative approaches do not yield satisfactory results. Some specialists advocate for surgery as an effective way to remove diseased adipose cells and reduce excess fat accumulation, which can improve physical appearance and associated pain. There is a growing consensus that surgical intervention should be performed early, ideally in stage I of IV, to maximize efficacy and prevent disease progression.
Fábio Masato Kamamoto, MD, a plastic surgeon and director of the Instituto Lipedema Brazil, shared insights into surgical treatments for lipedema. He discussed techniques from liposuction to advanced skin retraction and dermolipectomy, crucial for addressing more advanced stages of the condition. “It’s a complex process that demands precision to protect the lymphatic system, especially considering the characteristic nodules of lipedema,” he noted.
Dr. Kamamoto discussed a former patient with stage III lipedema. In the initial stage, he performed liposuction, removing 8 L of fat and 3.4 kg of skin. After 6 months, a follow-up procedure resulted in a total removal of 15 kg. Complementary procedures, such as microneedling, were performed to stimulate collagen production and reduce skin sagging. In addition to cosmetic improvements, the procedure also removed the distinctive lipedema nodules, which Mr. Kartt described as feeling like “rice grains.” Removing these nodules significantly alleviates pain, according to Dr. Kamamoto.
The benefits of surgical treatment for lipedema can be long lasting. Dr. Kamamoto noted that fat tends not to reaccumulate in treated areas, with patients often experiencing lower weight, reduced edema, and decreased pain over time. “While we hope that patients do not regain weight, the benefits of surgery persist even if weight is regained. Therefore, combining conservative and surgical treatments remains a valid and effective approach,” he concluded.
Dr. Scherer highlighted that despite various approaches, there is still no definitive “magic signature” that fully explains lipedema. This lack of clarity directly affects the effectiveness of diagnoses and treatments. He expressed hope that future integration of data from different studies and approaches will lead to the identification of a clinically useful molecular signature. “The true cause of lipedema remains unknown, requiring more speculation, hypothesis formulation, and testing for significant discoveries. This situation is frustrating, as the disease affects many women who lack a clear diagnosis that differentiates them from patients with obesity, as well as evidence-based recommendations,” he concluded.
This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Lipedema affects about 11% of cisgender women, according to the Brazilian Society of Angiology and Vascular Surgery. Yet the condition remains wrapped in uncertainties. Despite significant advancements in understanding its physiology, diagnosis, and treatment, more clarity is needed as awareness and diagnoses increase.
At the latest International Congress on Obesity (ICO) in São Paulo, Brazil, Philipp Scherer, PhD, director of the Touchstone Diabetes Center, discussed the complexities of lipedema. “It is an extremely frustrating condition for someone like me, who has spent a lifetime studying functional and dysfunctional adipose tissue. We are trying to understand the physiology of this pathology, but it is challenging, and so far, we have not been able to find a concrete answer,” he noted.
Lipedema is characterized by the abnormal accumulation of subcutaneous adipose tissue, especially in the lower limbs, and almost exclusively affects cisgender women. The reason for this gender disparity is unclear. It could be an intrinsic characteristic of the disease or a result from clinicians’ lack of familiarity with lipedema, which often leads to misdiagnosis as obesity. This misdiagnosis results in fewer men seeking treatment.
Research has predominantly focused on women, and evidence suggests that hormones play a crucial role in the disease’s pathophysiology. Lipedema typically manifests during periods of hormonal changes, such as puberty, pregnancy, menopause, and hormone replacement therapies, reinforcing the idea that hormones significantly influence the condition’s development and progression.
Main Symptoms
Jonathan Kartt, CEO of the Lipedema Foundation, emphasized that intense pain in the areas of adipose tissue accumulation is a hallmark symptom of lipedema, setting it apart from obesity. Pain levels can vary widely among patients, ranging from moderate to severe, with unbearable peaks on certain days. Mr. Kartt stressed the importance of recognizing and addressing this often underestimated symptom.
Lipedema is characterized by a bilateral, symmetrical increase in mass compared with the rest of the body. This is commonly distinguished by the “cuff sign,” a separation between normal tissue in the feet and abnormal tissue from the ankle upward. Other frequent symptoms include a feeling of heaviness, discomfort, fatigue, frequent bruising, and tiredness. A notable sign is the presence of subcutaneous nodules with a texture similar to that of rice grains, which are crucial for differentiating lipedema from other conditions. Palpation during anamnesis is essential to identify these nodules and confirm the diagnosis.
“It is crucial to investigate the family history for genetic predisposition. Additionally, it is fundamental to ask whether, even with weight loss, the affected areas retain accumulated fat. Hormonal changes, pain symptoms, and impact on quality of life should also be carefully evaluated,” advised Mr. Kartt.
Diagnostic Tools
André Murad, MD, a clinical consultant at the Instituto Lipedema Brazil, has been exploring new diagnostic approaches for lipedema beyond traditional anamnesis. During his presentation at the ICO, he shared studies on the efficacy of imaging exams such as ultrasound, tomography, and MRI in diagnosing the characteristic lipedema-associated increase in subcutaneous tissue.
He also discussed lymphangiography and lymphoscintigraphy, highlighting the use of magnetic resonance lymphangiography to evaluate dilated lymphatic vessels often observed in patients with lipedema. “By injecting contrast into the feet, this technique allows the evaluation of vessels, which are usually dilated, indicating characteristic lymphatic system overload in lipedema. Lymphoscintigraphy is crucial for detecting associated lymphedema, revealing delayed lymphatic flow and asymmetry between limbs in cases of lipedema without lymphedema,” he explained.
Despite the various diagnostic options, Dr. Murad highlighted two highly effective studies. A Brazilian study used ultrasound to establish a cutoff point of 11.7 mm in the pretibial subcutaneous tissue thickness, achieving 96% specificity for diagnosis. Another study emphasized the value of dual-energy x-ray absorptiometry (DXA), which demonstrated 95% sensitivity. This method assesses fat distribution by correlating the amount present in the legs with the total body, providing a cost-effective and accessible option for specialists.
“DXA allows for a precise mathematical evaluation of fat distribution relative to the total body. A ratio of 0.38 in the leg-to-body relationship is a significant indicator of high suspicion of lipedema,” highlighted Dr. Murad. “In clinical practice, many patients self-diagnose with lipedema, but the clinical exam often reveals no disproportion, with the leg-to-body ratio below 0.38 being common in these cases,” he added.
Treatment Approaches
Treatments for lipedema are still evolving, with considerable debate about the best approach. While some specialists advocate exclusively for conservative treatment, others recommend combining these methods with surgical interventions, depending on the stage of the disease. The relative novelty of lipedema and the scarcity of robust, long-term studies contribute to the uncertainty around treatment efficacy.
Conservative treatment typically includes compression, lymphatic drainage techniques, and pressure therapy. An active lifestyle and a healthy diet are also recommended. Although these measures do not prevent the accumulation of adipose tissue, they help reduce inflammation and improve quality of life. “Even though the causes of lipedema are not fully known, lifestyle management is essential for controlling symptoms, starting with an anti-inflammatory diet,” emphasized Dr. Murad.
Because insulin promotes lipogenesis, a diet that avoids spikes in glycemic and insulin levels is advisable. Insulin resistance can exacerbate edema formation, so a Mediterranean diet may be beneficial. This diet limits fast-absorbing carbohydrates, such as added sugar, refined grains, and ultraprocessed foods, while promoting complex carbohydrates from whole grains and legumes.
Dr. Murad also presented a study evaluating the potential benefits of a low-carbohydrate, high-fat diet for patients with lipedema. The study demonstrated weight loss, reduced body fat, controlled leg volume, and, notably, pain relief.
For more advanced stages of lipedema, plastic surgery is often considered when conservative approaches do not yield satisfactory results. Some specialists advocate for surgery as an effective way to remove diseased adipose cells and reduce excess fat accumulation, which can improve physical appearance and associated pain. There is a growing consensus that surgical intervention should be performed early, ideally in stage I of IV, to maximize efficacy and prevent disease progression.
Fábio Masato Kamamoto, MD, a plastic surgeon and director of the Instituto Lipedema Brazil, shared insights into surgical treatments for lipedema. He discussed techniques from liposuction to advanced skin retraction and dermolipectomy, crucial for addressing more advanced stages of the condition. “It’s a complex process that demands precision to protect the lymphatic system, especially considering the characteristic nodules of lipedema,” he noted.
Dr. Kamamoto discussed a former patient with stage III lipedema. In the initial stage, he performed liposuction, removing 8 L of fat and 3.4 kg of skin. After 6 months, a follow-up procedure resulted in a total removal of 15 kg. Complementary procedures, such as microneedling, were performed to stimulate collagen production and reduce skin sagging. In addition to cosmetic improvements, the procedure also removed the distinctive lipedema nodules, which Mr. Kartt described as feeling like “rice grains.” Removing these nodules significantly alleviates pain, according to Dr. Kamamoto.
The benefits of surgical treatment for lipedema can be long lasting. Dr. Kamamoto noted that fat tends not to reaccumulate in treated areas, with patients often experiencing lower weight, reduced edema, and decreased pain over time. “While we hope that patients do not regain weight, the benefits of surgery persist even if weight is regained. Therefore, combining conservative and surgical treatments remains a valid and effective approach,” he concluded.
Dr. Scherer highlighted that despite various approaches, there is still no definitive “magic signature” that fully explains lipedema. This lack of clarity directly affects the effectiveness of diagnoses and treatments. He expressed hope that future integration of data from different studies and approaches will lead to the identification of a clinically useful molecular signature. “The true cause of lipedema remains unknown, requiring more speculation, hypothesis formulation, and testing for significant discoveries. This situation is frustrating, as the disease affects many women who lack a clear diagnosis that differentiates them from patients with obesity, as well as evidence-based recommendations,” he concluded.
This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Lipedema affects about 11% of cisgender women, according to the Brazilian Society of Angiology and Vascular Surgery. Yet the condition remains wrapped in uncertainties. Despite significant advancements in understanding its physiology, diagnosis, and treatment, more clarity is needed as awareness and diagnoses increase.
At the latest International Congress on Obesity (ICO) in São Paulo, Brazil, Philipp Scherer, PhD, director of the Touchstone Diabetes Center, discussed the complexities of lipedema. “It is an extremely frustrating condition for someone like me, who has spent a lifetime studying functional and dysfunctional adipose tissue. We are trying to understand the physiology of this pathology, but it is challenging, and so far, we have not been able to find a concrete answer,” he noted.
Lipedema is characterized by the abnormal accumulation of subcutaneous adipose tissue, especially in the lower limbs, and almost exclusively affects cisgender women. The reason for this gender disparity is unclear. It could be an intrinsic characteristic of the disease or a result from clinicians’ lack of familiarity with lipedema, which often leads to misdiagnosis as obesity. This misdiagnosis results in fewer men seeking treatment.
Research has predominantly focused on women, and evidence suggests that hormones play a crucial role in the disease’s pathophysiology. Lipedema typically manifests during periods of hormonal changes, such as puberty, pregnancy, menopause, and hormone replacement therapies, reinforcing the idea that hormones significantly influence the condition’s development and progression.
Main Symptoms
Jonathan Kartt, CEO of the Lipedema Foundation, emphasized that intense pain in the areas of adipose tissue accumulation is a hallmark symptom of lipedema, setting it apart from obesity. Pain levels can vary widely among patients, ranging from moderate to severe, with unbearable peaks on certain days. Mr. Kartt stressed the importance of recognizing and addressing this often underestimated symptom.
Lipedema is characterized by a bilateral, symmetrical increase in mass compared with the rest of the body. This is commonly distinguished by the “cuff sign,” a separation between normal tissue in the feet and abnormal tissue from the ankle upward. Other frequent symptoms include a feeling of heaviness, discomfort, fatigue, frequent bruising, and tiredness. A notable sign is the presence of subcutaneous nodules with a texture similar to that of rice grains, which are crucial for differentiating lipedema from other conditions. Palpation during anamnesis is essential to identify these nodules and confirm the diagnosis.
“It is crucial to investigate the family history for genetic predisposition. Additionally, it is fundamental to ask whether, even with weight loss, the affected areas retain accumulated fat. Hormonal changes, pain symptoms, and impact on quality of life should also be carefully evaluated,” advised Mr. Kartt.
Diagnostic Tools
André Murad, MD, a clinical consultant at the Instituto Lipedema Brazil, has been exploring new diagnostic approaches for lipedema beyond traditional anamnesis. During his presentation at the ICO, he shared studies on the efficacy of imaging exams such as ultrasound, tomography, and MRI in diagnosing the characteristic lipedema-associated increase in subcutaneous tissue.
He also discussed lymphangiography and lymphoscintigraphy, highlighting the use of magnetic resonance lymphangiography to evaluate dilated lymphatic vessels often observed in patients with lipedema. “By injecting contrast into the feet, this technique allows the evaluation of vessels, which are usually dilated, indicating characteristic lymphatic system overload in lipedema. Lymphoscintigraphy is crucial for detecting associated lymphedema, revealing delayed lymphatic flow and asymmetry between limbs in cases of lipedema without lymphedema,” he explained.
Despite the various diagnostic options, Dr. Murad highlighted two highly effective studies. A Brazilian study used ultrasound to establish a cutoff point of 11.7 mm in the pretibial subcutaneous tissue thickness, achieving 96% specificity for diagnosis. Another study emphasized the value of dual-energy x-ray absorptiometry (DXA), which demonstrated 95% sensitivity. This method assesses fat distribution by correlating the amount present in the legs with the total body, providing a cost-effective and accessible option for specialists.
“DXA allows for a precise mathematical evaluation of fat distribution relative to the total body. A ratio of 0.38 in the leg-to-body relationship is a significant indicator of high suspicion of lipedema,” highlighted Dr. Murad. “In clinical practice, many patients self-diagnose with lipedema, but the clinical exam often reveals no disproportion, with the leg-to-body ratio below 0.38 being common in these cases,” he added.
Treatment Approaches
Treatments for lipedema are still evolving, with considerable debate about the best approach. While some specialists advocate exclusively for conservative treatment, others recommend combining these methods with surgical interventions, depending on the stage of the disease. The relative novelty of lipedema and the scarcity of robust, long-term studies contribute to the uncertainty around treatment efficacy.
Conservative treatment typically includes compression, lymphatic drainage techniques, and pressure therapy. An active lifestyle and a healthy diet are also recommended. Although these measures do not prevent the accumulation of adipose tissue, they help reduce inflammation and improve quality of life. “Even though the causes of lipedema are not fully known, lifestyle management is essential for controlling symptoms, starting with an anti-inflammatory diet,” emphasized Dr. Murad.
Because insulin promotes lipogenesis, a diet that avoids spikes in glycemic and insulin levels is advisable. Insulin resistance can exacerbate edema formation, so a Mediterranean diet may be beneficial. This diet limits fast-absorbing carbohydrates, such as added sugar, refined grains, and ultraprocessed foods, while promoting complex carbohydrates from whole grains and legumes.
Dr. Murad also presented a study evaluating the potential benefits of a low-carbohydrate, high-fat diet for patients with lipedema. The study demonstrated weight loss, reduced body fat, controlled leg volume, and, notably, pain relief.
For more advanced stages of lipedema, plastic surgery is often considered when conservative approaches do not yield satisfactory results. Some specialists advocate for surgery as an effective way to remove diseased adipose cells and reduce excess fat accumulation, which can improve physical appearance and associated pain. There is a growing consensus that surgical intervention should be performed early, ideally in stage I of IV, to maximize efficacy and prevent disease progression.
Fábio Masato Kamamoto, MD, a plastic surgeon and director of the Instituto Lipedema Brazil, shared insights into surgical treatments for lipedema. He discussed techniques from liposuction to advanced skin retraction and dermolipectomy, crucial for addressing more advanced stages of the condition. “It’s a complex process that demands precision to protect the lymphatic system, especially considering the characteristic nodules of lipedema,” he noted.
Dr. Kamamoto discussed a former patient with stage III lipedema. In the initial stage, he performed liposuction, removing 8 L of fat and 3.4 kg of skin. After 6 months, a follow-up procedure resulted in a total removal of 15 kg. Complementary procedures, such as microneedling, were performed to stimulate collagen production and reduce skin sagging. In addition to cosmetic improvements, the procedure also removed the distinctive lipedema nodules, which Mr. Kartt described as feeling like “rice grains.” Removing these nodules significantly alleviates pain, according to Dr. Kamamoto.
The benefits of surgical treatment for lipedema can be long lasting. Dr. Kamamoto noted that fat tends not to reaccumulate in treated areas, with patients often experiencing lower weight, reduced edema, and decreased pain over time. “While we hope that patients do not regain weight, the benefits of surgery persist even if weight is regained. Therefore, combining conservative and surgical treatments remains a valid and effective approach,” he concluded.
Dr. Scherer highlighted that despite various approaches, there is still no definitive “magic signature” that fully explains lipedema. This lack of clarity directly affects the effectiveness of diagnoses and treatments. He expressed hope that future integration of data from different studies and approaches will lead to the identification of a clinically useful molecular signature. “The true cause of lipedema remains unknown, requiring more speculation, hypothesis formulation, and testing for significant discoveries. This situation is frustrating, as the disease affects many women who lack a clear diagnosis that differentiates them from patients with obesity, as well as evidence-based recommendations,” he concluded.
This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Painful Anal Lesions in a Patient With HIV
The Diagnosis: Condyloma Latum
Laboratory test results were notable for a rapid plasma reagin titer of 1:512, a positive Treponema pallidum particle agglutination test, negative rectal nucleic acid amplification tests for gonorrhea and chlamydia, and a negative herpes simplex virus polymerase chain reaction. A VDRL test of cerebrospinal fluid from a lumbar puncture was negative. Histopathology of the punch biopsy sample revealed marked verrucous epidermal hyperplasia without keratinocytic atypia and with mixed inflammation (Figure 1), while immunohistochemical staining showed numerus T pallidum organisms (Figure 2). A diagnosis of condyloma latum was made based on the laboratory, lumbar puncture, and punch biopsy results. Due to a penicillin allergy, the patient was treated with oral doxycycline for 14 days. On follow-up at day 12 of therapy, he reported cessation of rectal pain, and resolution of anal lesions was noted on physical examination.
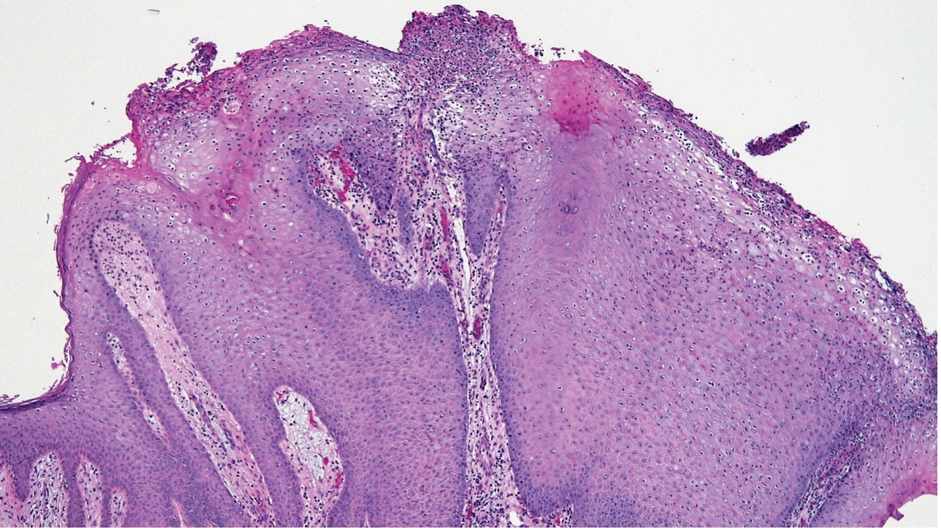
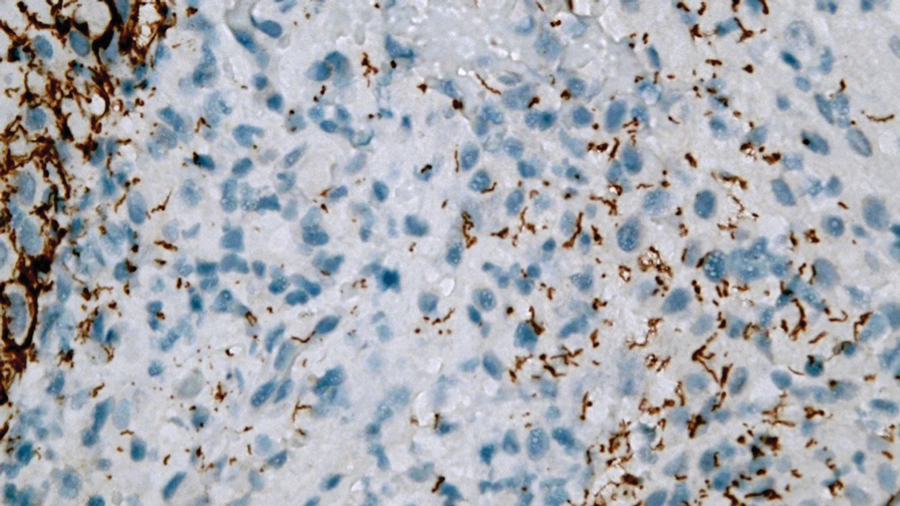
Condylomata lata are highly infectious cutaneous lesions that can manifest during secondary syphilis.1 They typically are described as white or gray, raised, flatappearing plaques and occur in moist areas or skin folds including the anus, scrotum, and vulva. However, these lesions also have been reported in the axillae, umbilicus, nasolabial folds, and other anatomic areas.1,2 The lesions can be painful and often manifest in multiples, especially in patients living with HIV.3
Condylomata lata can have a verrucous appearance and may mimic other anogenital lesions, such as condylomata acuminata, genital herpes, and malignant tumors, leading to an initial misdiagnosis.1,2 Condylomata lata should always be included in the differential when evaluating anogenital lesions. Other conditions in the differential diagnosis include psoriasis, typically manifesting as erythematous plaques with silver scale, and molluscum contagiosum, appearing as small umbilicated papules on physical examination.
Condylomata lata have been reported to occur in 6% to 23% of patients with secondary syphilis.1 Although secondary syphilis more typically manifests with a diffuse maculopapular rash, condylomata lata may be the sole dermatologic manifestation.4
Histopathology of condylomata lata consists of epithelial hyperplasia as well as lymphocytic and plasma cell infiltrates. It is diagnosed by serologic testing as well as immunohistochemical staining or dark-field microscopy.
First-line treatment of secondary syphilis is a single dose of benzathine penicillin G administered intramuscularly.5 However, a 14-day course of oral doxycycline can be used in patients with a penicillin allergy. When compliance and follow-up cannot be guaranteed, penicillin desensitization and treatment with benzathine penicillin G is recommended. Clinical evaluation and repeat serologic testing should be performed at 6 and 12 months follow-up, or more frequently if clinically indicated.5
- Pourang A, Fung MA, Tartar D, et al. Condyloma lata in secondary syphilis. JAAD Case Rep. 2021;10:18-21. doi:10.1016/j.jdcr.2021.01.025
- Liu Z, Wang L, Zhang G, et al. Warty mucosal lesions: oral condyloma lata of secondary syphilis. Indian J Dermatol Venereol Leprol. 2017;83:277. doi:10.4103/0378-6323.191129
- Rompalo AM, Joesoef MR, O’Donnell JA, et al; Syphilis and HIV Study Group. Clinical manifestations of early syphilis by HIV status and gender: results of the syphilis and HIV study. Sex Transm Dis.2001;28:158-165.
- Kumar P, Das A, Mondal A. Secondary syphilis: an unusual presentation. Indian J Sex Transm Dis AIDS. 2017;38:98-99. doi:10.4103/0253-7184.194318
- Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187. doi:10.15585/mmwr.rr7004a1
The Diagnosis: Condyloma Latum
Laboratory test results were notable for a rapid plasma reagin titer of 1:512, a positive Treponema pallidum particle agglutination test, negative rectal nucleic acid amplification tests for gonorrhea and chlamydia, and a negative herpes simplex virus polymerase chain reaction. A VDRL test of cerebrospinal fluid from a lumbar puncture was negative. Histopathology of the punch biopsy sample revealed marked verrucous epidermal hyperplasia without keratinocytic atypia and with mixed inflammation (Figure 1), while immunohistochemical staining showed numerus T pallidum organisms (Figure 2). A diagnosis of condyloma latum was made based on the laboratory, lumbar puncture, and punch biopsy results. Due to a penicillin allergy, the patient was treated with oral doxycycline for 14 days. On follow-up at day 12 of therapy, he reported cessation of rectal pain, and resolution of anal lesions was noted on physical examination.


Condylomata lata are highly infectious cutaneous lesions that can manifest during secondary syphilis.1 They typically are described as white or gray, raised, flatappearing plaques and occur in moist areas or skin folds including the anus, scrotum, and vulva. However, these lesions also have been reported in the axillae, umbilicus, nasolabial folds, and other anatomic areas.1,2 The lesions can be painful and often manifest in multiples, especially in patients living with HIV.3
Condylomata lata can have a verrucous appearance and may mimic other anogenital lesions, such as condylomata acuminata, genital herpes, and malignant tumors, leading to an initial misdiagnosis.1,2 Condylomata lata should always be included in the differential when evaluating anogenital lesions. Other conditions in the differential diagnosis include psoriasis, typically manifesting as erythematous plaques with silver scale, and molluscum contagiosum, appearing as small umbilicated papules on physical examination.
Condylomata lata have been reported to occur in 6% to 23% of patients with secondary syphilis.1 Although secondary syphilis more typically manifests with a diffuse maculopapular rash, condylomata lata may be the sole dermatologic manifestation.4
Histopathology of condylomata lata consists of epithelial hyperplasia as well as lymphocytic and plasma cell infiltrates. It is diagnosed by serologic testing as well as immunohistochemical staining or dark-field microscopy.
First-line treatment of secondary syphilis is a single dose of benzathine penicillin G administered intramuscularly.5 However, a 14-day course of oral doxycycline can be used in patients with a penicillin allergy. When compliance and follow-up cannot be guaranteed, penicillin desensitization and treatment with benzathine penicillin G is recommended. Clinical evaluation and repeat serologic testing should be performed at 6 and 12 months follow-up, or more frequently if clinically indicated.5
The Diagnosis: Condyloma Latum
Laboratory test results were notable for a rapid plasma reagin titer of 1:512, a positive Treponema pallidum particle agglutination test, negative rectal nucleic acid amplification tests for gonorrhea and chlamydia, and a negative herpes simplex virus polymerase chain reaction. A VDRL test of cerebrospinal fluid from a lumbar puncture was negative. Histopathology of the punch biopsy sample revealed marked verrucous epidermal hyperplasia without keratinocytic atypia and with mixed inflammation (Figure 1), while immunohistochemical staining showed numerus T pallidum organisms (Figure 2). A diagnosis of condyloma latum was made based on the laboratory, lumbar puncture, and punch biopsy results. Due to a penicillin allergy, the patient was treated with oral doxycycline for 14 days. On follow-up at day 12 of therapy, he reported cessation of rectal pain, and resolution of anal lesions was noted on physical examination.


Condylomata lata are highly infectious cutaneous lesions that can manifest during secondary syphilis.1 They typically are described as white or gray, raised, flatappearing plaques and occur in moist areas or skin folds including the anus, scrotum, and vulva. However, these lesions also have been reported in the axillae, umbilicus, nasolabial folds, and other anatomic areas.1,2 The lesions can be painful and often manifest in multiples, especially in patients living with HIV.3
Condylomata lata can have a verrucous appearance and may mimic other anogenital lesions, such as condylomata acuminata, genital herpes, and malignant tumors, leading to an initial misdiagnosis.1,2 Condylomata lata should always be included in the differential when evaluating anogenital lesions. Other conditions in the differential diagnosis include psoriasis, typically manifesting as erythematous plaques with silver scale, and molluscum contagiosum, appearing as small umbilicated papules on physical examination.
Condylomata lata have been reported to occur in 6% to 23% of patients with secondary syphilis.1 Although secondary syphilis more typically manifests with a diffuse maculopapular rash, condylomata lata may be the sole dermatologic manifestation.4
Histopathology of condylomata lata consists of epithelial hyperplasia as well as lymphocytic and plasma cell infiltrates. It is diagnosed by serologic testing as well as immunohistochemical staining or dark-field microscopy.
First-line treatment of secondary syphilis is a single dose of benzathine penicillin G administered intramuscularly.5 However, a 14-day course of oral doxycycline can be used in patients with a penicillin allergy. When compliance and follow-up cannot be guaranteed, penicillin desensitization and treatment with benzathine penicillin G is recommended. Clinical evaluation and repeat serologic testing should be performed at 6 and 12 months follow-up, or more frequently if clinically indicated.5
- Pourang A, Fung MA, Tartar D, et al. Condyloma lata in secondary syphilis. JAAD Case Rep. 2021;10:18-21. doi:10.1016/j.jdcr.2021.01.025
- Liu Z, Wang L, Zhang G, et al. Warty mucosal lesions: oral condyloma lata of secondary syphilis. Indian J Dermatol Venereol Leprol. 2017;83:277. doi:10.4103/0378-6323.191129
- Rompalo AM, Joesoef MR, O’Donnell JA, et al; Syphilis and HIV Study Group. Clinical manifestations of early syphilis by HIV status and gender: results of the syphilis and HIV study. Sex Transm Dis.2001;28:158-165.
- Kumar P, Das A, Mondal A. Secondary syphilis: an unusual presentation. Indian J Sex Transm Dis AIDS. 2017;38:98-99. doi:10.4103/0253-7184.194318
- Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187. doi:10.15585/mmwr.rr7004a1
- Pourang A, Fung MA, Tartar D, et al. Condyloma lata in secondary syphilis. JAAD Case Rep. 2021;10:18-21. doi:10.1016/j.jdcr.2021.01.025
- Liu Z, Wang L, Zhang G, et al. Warty mucosal lesions: oral condyloma lata of secondary syphilis. Indian J Dermatol Venereol Leprol. 2017;83:277. doi:10.4103/0378-6323.191129
- Rompalo AM, Joesoef MR, O’Donnell JA, et al; Syphilis and HIV Study Group. Clinical manifestations of early syphilis by HIV status and gender: results of the syphilis and HIV study. Sex Transm Dis.2001;28:158-165.
- Kumar P, Das A, Mondal A. Secondary syphilis: an unusual presentation. Indian J Sex Transm Dis AIDS. 2017;38:98-99. doi:10.4103/0253-7184.194318
- Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187. doi:10.15585/mmwr.rr7004a1
A 24-year-old man presented to the emergency department with rectal pain and lesions of 3 weeks’ duration that were progressively worsening. He had a medical history of poorly controlled HIV, cerebral toxoplasmosis, and genital herpes, as well as a social history of sexual activity with other men.
He had been diagnosed with HIV 7 years prior and had been off therapy until 1 year prior to the current presentation, when he was hospitalized with encephalopathy (CD4 count, <50 cells/mm3). A diagnosis of cerebral toxoplasmosis was made, and he began a treatment regimen of sulfadiazine, pyrimethamine, and leucovorin, as well as bictegravir, emtricitabine, and tenofovir alafenamide. Since then, the patient admitted to difficulty with medication adherence.
Rapid plasma reagin, gonorrhea, and chlamydia testing were negative during a routine workup 6 months prior to the current presentation. He initially presented to an urgent care clinic for evaluation of the rectal pain and lesions and was treated empirically with topical podofilox. He presented to the emergency department 1 week later (3 weeks after symptom onset) with anal warts and apparent vesicular lesions. Empiric treatment with oral valacyclovir was prescribed.
Despite these treatments, the rectal pain became severe—especially upon sitting, defecation, and physical exertion—prompting further evaluation. Physical examination revealed soft, flat-topped, moist-appearing, gray plaques with minimal surrounding erythema at the anus. Laboratory test results demonstrated a CD4 count of 161 cells/mm3 and an HIV viral load of 137 copies/mL.
The Shield Sign of Cutaneous Metastases Is Associated With Carcinoma Hemorrhagiectoides
To the Editor:
We read with interest the Case Letter from Wang et al1 (Cutis. 2023;112:E13-E15) of a 60-year-old man whose metastatic salivary duct adenocarcinoma manifested with the shield sign as well as carcinoma hemorrhagiectoides. Cutaneous metastases have seldom been described in association with salivary duct carcinoma.2-7 In addition, carcinoma hemorrhagiectoides–associated shield sign has not been commonly reported.5,8-12
Salivary duct carcinoma—an uncommon head and neck malignancy characterized by androgen receptor expression—rarely is associated with cutaneous metastases. Based on a PubMed search of articles indexed for MEDLINE using the terms cutaneous, metastatic, salivary duct carcinoma, and/or skin, including the patient described by Wang et al,1 there have been 8 individuals with cutaneous metastases from this cancer. The morphology of the cutaneous metastases has varied from angiomatous to angiokeratomalike (black and keratotic) papules, bullae, macules (red), papules and nodules (erythematous and scaly), plaques (cellulitislike and confluent that were purpuric, hemorrhagic, and violaceous), pseudovesicles, purpuric papules, subcutaneous nodules, and an ulcer (superficial and mimicked a basal cell carcinoma).1-7 Remarkably, 4 of 8 patients (50%) with salivary duct carcinoma cutaneous metastases presented with a shield sign,5,7 including the case reported by Wang et al.1
The shield sign is a distinctive clinical manifestation of cutaneous metastasis.10 It was named to describe the skin metastases located predominantly on the chest area that would be covered by a medieval knight’s shield5,10,12; metastatic lesions also have been noted on the proximal arm and/or the upper back in a similar distribution.8,9 To date, based on a PubMed search of articles indexed for MEDLINE using the search terms breast cancer, carcinoma, hemorrhagiectoides, metastases, salivary duct carcinoma, shield, and/or sign, the shield sign has been described in 6 patients with cutaneous metastases either from salivary duct carcinoma (4 patients)1,5,7 or breast cancer (2 patients).8,9 The shield sign pathologically corresponds to carcinoma hemorrhagiectoides, an inflammatory pattern of cutaneous metastases.5,11
Inflammatory cutaneous metastatic carcinoma has 3 distinctive clinical and pathologic manifestations.11 Carcinoma erysipelatoides and carcinoma telangiectoides were the earlier described variants.11 In 2012, carcinoma hemorrhagiectoides was described as the third pattern of inflammatory cutaneous metastasis.5
Carcinoma erysipelatoides, which clinically mimics cutaneous streptococcal cellulitis, appears as a well-defined erythematous patch or plaque; the tumor cells can be found in the lymphatic vessels and either are absent or minimally present in the dermis. Carcinoma telangiectoides, which clinically mimics idiopathic telangiectases, appears as an erythematous patch with prominent telangiectases; the tumor cells can be found in the blood vessels and are either absent or minimally present in the dermis. Carcinoma hemorrhagiectoides appears as purpuric or violaceous indurated plaques; the tumor cells are not only found in the blood vessels, in the lymphatic vessels, or both, but also can be mildly to extensively present in the dermis.5,10,11
In conclusion, the shield sign is a unique presentation of inflammatory cutaneous metastatic carcinoma, which is associated with carcinoma hemorrhagiectoides. The clinical features of the infiltrated plaques correspond to the presence of tumor cells in the blood vessels, lymphatic vessels, and the dermis; in addition, the purpuric and violaceous appearance correlates with the presence of extravasated erythrocytes or hemorrhage in the dermis. To date, half of the patients with skin metastases from salivary duct carcinoma have presented with carcinoma hemorrhagiectoides–associated shield sign.
Authors’ Response
We appreciate and welcome the comments provided by the authors. Drawing attention to unusual pathologic manifestations of cutaneous metastatic salivary duct carcinoma manifesting with the shield sign, the authors present a comprehensive review of 3 distinctive presentations: carcinoma erysipelatoides, carcinoma telangiectoides, and carcinoma hemorrhagiectoides. The inclusion of these variants enriches the discussion and makes this letter a valuable addition to the literature on cutaneous metastatic carcinoma, particularly metastatic salivary duct carcinoma.
Xintong Wang, MD; William H. Westra, MD
From the Department of Pathology, Icahn School of Medicine at Mount Sinai, New York, New York.
The authors report no conflict of interest.
- Wang X, Vyas NS, Alghamdi AA, et al. Cutaneous presentation of metastatic salivary duct carcinoma. Cutis. 2023;112:E13-E15.
- Pollock JL, Catalano E. Metastatic ductal carcinoma of the parotid gland in a patient with sarcoidosis. Arch Dermatol. 1979;115:1098-1099.
- Pollock JL. Metastatic carcinoma of the parotid gland resembling carcinoma of the breast. J Am Acad Dermatol. 1996;34:1093.
- Aygit AC, Top H, Cakir B, et al. Salivary duct carcinoma of the parotid gland metastasizing to the skin: a case report and review of the literature. Am J Dermatopathol. 2005;27:48-50.
- Cohen PR, Prieto VG, Piha-Paul SA, et al. The “shield sign” in two men with metastatic salivary duct carcinoma to the skin: cutaneous metastases presenting as carcinoma hemorrhagiectoides. J Clin Aesthet Dermatol. 2012;5:27-36.
- Chakari W, Andersen L, Anderson JL. Cutaneous metastases from salivary duct carcinoma of the submandibular gland. Case Rep Dermatol. 2017;9:254-258.
- Shin JY, Eun DH, Lee JY, et al. A case of cutaneous metastases of salivary duct carcinoma mimicking radiation recall dermatitis. Ann Dermatol. 2020;32:436-438.
- Aravena RC, Aravena DC, Velasco MJ, et al. Carcinoma hemorrhagiectoides: case report of an uncommon presentation of cutaneous metastatic breast carcinoma. Dermatol Online J. 2017;23:13030/qt3hn3z850.
- Smith KA, Basko-Plluska J, Kothari AD, et al. Cutaneous metastatic breast adenocarcinoma. Cutis. 2020;105:E20-E22.
- Cohen PR, Kurzrock R. Cutaneous metastatic cancer: carcinoma hemorrhagiectoides presenting as the shield sign. Cureus. 2021;13:e12627.
- Cohen PR. Pleomorphic appearance of breast cancer cutaneous metastases. Cureus. 2021;13:e20301.
- Cohen PR, Prieto VG, Kurzrock R. Tumor lysis syndrome: introduction of a cutaneous variant and a new classification system. Cureus. 2021;13:e13816.
To the Editor:
We read with interest the Case Letter from Wang et al1 (Cutis. 2023;112:E13-E15) of a 60-year-old man whose metastatic salivary duct adenocarcinoma manifested with the shield sign as well as carcinoma hemorrhagiectoides. Cutaneous metastases have seldom been described in association with salivary duct carcinoma.2-7 In addition, carcinoma hemorrhagiectoides–associated shield sign has not been commonly reported.5,8-12
Salivary duct carcinoma—an uncommon head and neck malignancy characterized by androgen receptor expression—rarely is associated with cutaneous metastases. Based on a PubMed search of articles indexed for MEDLINE using the terms cutaneous, metastatic, salivary duct carcinoma, and/or skin, including the patient described by Wang et al,1 there have been 8 individuals with cutaneous metastases from this cancer. The morphology of the cutaneous metastases has varied from angiomatous to angiokeratomalike (black and keratotic) papules, bullae, macules (red), papules and nodules (erythematous and scaly), plaques (cellulitislike and confluent that were purpuric, hemorrhagic, and violaceous), pseudovesicles, purpuric papules, subcutaneous nodules, and an ulcer (superficial and mimicked a basal cell carcinoma).1-7 Remarkably, 4 of 8 patients (50%) with salivary duct carcinoma cutaneous metastases presented with a shield sign,5,7 including the case reported by Wang et al.1
The shield sign is a distinctive clinical manifestation of cutaneous metastasis.10 It was named to describe the skin metastases located predominantly on the chest area that would be covered by a medieval knight’s shield5,10,12; metastatic lesions also have been noted on the proximal arm and/or the upper back in a similar distribution.8,9 To date, based on a PubMed search of articles indexed for MEDLINE using the search terms breast cancer, carcinoma, hemorrhagiectoides, metastases, salivary duct carcinoma, shield, and/or sign, the shield sign has been described in 6 patients with cutaneous metastases either from salivary duct carcinoma (4 patients)1,5,7 or breast cancer (2 patients).8,9 The shield sign pathologically corresponds to carcinoma hemorrhagiectoides, an inflammatory pattern of cutaneous metastases.5,11
Inflammatory cutaneous metastatic carcinoma has 3 distinctive clinical and pathologic manifestations.11 Carcinoma erysipelatoides and carcinoma telangiectoides were the earlier described variants.11 In 2012, carcinoma hemorrhagiectoides was described as the third pattern of inflammatory cutaneous metastasis.5
Carcinoma erysipelatoides, which clinically mimics cutaneous streptococcal cellulitis, appears as a well-defined erythematous patch or plaque; the tumor cells can be found in the lymphatic vessels and either are absent or minimally present in the dermis. Carcinoma telangiectoides, which clinically mimics idiopathic telangiectases, appears as an erythematous patch with prominent telangiectases; the tumor cells can be found in the blood vessels and are either absent or minimally present in the dermis. Carcinoma hemorrhagiectoides appears as purpuric or violaceous indurated plaques; the tumor cells are not only found in the blood vessels, in the lymphatic vessels, or both, but also can be mildly to extensively present in the dermis.5,10,11
In conclusion, the shield sign is a unique presentation of inflammatory cutaneous metastatic carcinoma, which is associated with carcinoma hemorrhagiectoides. The clinical features of the infiltrated plaques correspond to the presence of tumor cells in the blood vessels, lymphatic vessels, and the dermis; in addition, the purpuric and violaceous appearance correlates with the presence of extravasated erythrocytes or hemorrhage in the dermis. To date, half of the patients with skin metastases from salivary duct carcinoma have presented with carcinoma hemorrhagiectoides–associated shield sign.
Authors’ Response
We appreciate and welcome the comments provided by the authors. Drawing attention to unusual pathologic manifestations of cutaneous metastatic salivary duct carcinoma manifesting with the shield sign, the authors present a comprehensive review of 3 distinctive presentations: carcinoma erysipelatoides, carcinoma telangiectoides, and carcinoma hemorrhagiectoides. The inclusion of these variants enriches the discussion and makes this letter a valuable addition to the literature on cutaneous metastatic carcinoma, particularly metastatic salivary duct carcinoma.
Xintong Wang, MD; William H. Westra, MD
From the Department of Pathology, Icahn School of Medicine at Mount Sinai, New York, New York.
The authors report no conflict of interest.
To the Editor:
We read with interest the Case Letter from Wang et al1 (Cutis. 2023;112:E13-E15) of a 60-year-old man whose metastatic salivary duct adenocarcinoma manifested with the shield sign as well as carcinoma hemorrhagiectoides. Cutaneous metastases have seldom been described in association with salivary duct carcinoma.2-7 In addition, carcinoma hemorrhagiectoides–associated shield sign has not been commonly reported.5,8-12
Salivary duct carcinoma—an uncommon head and neck malignancy characterized by androgen receptor expression—rarely is associated with cutaneous metastases. Based on a PubMed search of articles indexed for MEDLINE using the terms cutaneous, metastatic, salivary duct carcinoma, and/or skin, including the patient described by Wang et al,1 there have been 8 individuals with cutaneous metastases from this cancer. The morphology of the cutaneous metastases has varied from angiomatous to angiokeratomalike (black and keratotic) papules, bullae, macules (red), papules and nodules (erythematous and scaly), plaques (cellulitislike and confluent that were purpuric, hemorrhagic, and violaceous), pseudovesicles, purpuric papules, subcutaneous nodules, and an ulcer (superficial and mimicked a basal cell carcinoma).1-7 Remarkably, 4 of 8 patients (50%) with salivary duct carcinoma cutaneous metastases presented with a shield sign,5,7 including the case reported by Wang et al.1
The shield sign is a distinctive clinical manifestation of cutaneous metastasis.10 It was named to describe the skin metastases located predominantly on the chest area that would be covered by a medieval knight’s shield5,10,12; metastatic lesions also have been noted on the proximal arm and/or the upper back in a similar distribution.8,9 To date, based on a PubMed search of articles indexed for MEDLINE using the search terms breast cancer, carcinoma, hemorrhagiectoides, metastases, salivary duct carcinoma, shield, and/or sign, the shield sign has been described in 6 patients with cutaneous metastases either from salivary duct carcinoma (4 patients)1,5,7 or breast cancer (2 patients).8,9 The shield sign pathologically corresponds to carcinoma hemorrhagiectoides, an inflammatory pattern of cutaneous metastases.5,11
Inflammatory cutaneous metastatic carcinoma has 3 distinctive clinical and pathologic manifestations.11 Carcinoma erysipelatoides and carcinoma telangiectoides were the earlier described variants.11 In 2012, carcinoma hemorrhagiectoides was described as the third pattern of inflammatory cutaneous metastasis.5
Carcinoma erysipelatoides, which clinically mimics cutaneous streptococcal cellulitis, appears as a well-defined erythematous patch or plaque; the tumor cells can be found in the lymphatic vessels and either are absent or minimally present in the dermis. Carcinoma telangiectoides, which clinically mimics idiopathic telangiectases, appears as an erythematous patch with prominent telangiectases; the tumor cells can be found in the blood vessels and are either absent or minimally present in the dermis. Carcinoma hemorrhagiectoides appears as purpuric or violaceous indurated plaques; the tumor cells are not only found in the blood vessels, in the lymphatic vessels, or both, but also can be mildly to extensively present in the dermis.5,10,11
In conclusion, the shield sign is a unique presentation of inflammatory cutaneous metastatic carcinoma, which is associated with carcinoma hemorrhagiectoides. The clinical features of the infiltrated plaques correspond to the presence of tumor cells in the blood vessels, lymphatic vessels, and the dermis; in addition, the purpuric and violaceous appearance correlates with the presence of extravasated erythrocytes or hemorrhage in the dermis. To date, half of the patients with skin metastases from salivary duct carcinoma have presented with carcinoma hemorrhagiectoides–associated shield sign.
Authors’ Response
We appreciate and welcome the comments provided by the authors. Drawing attention to unusual pathologic manifestations of cutaneous metastatic salivary duct carcinoma manifesting with the shield sign, the authors present a comprehensive review of 3 distinctive presentations: carcinoma erysipelatoides, carcinoma telangiectoides, and carcinoma hemorrhagiectoides. The inclusion of these variants enriches the discussion and makes this letter a valuable addition to the literature on cutaneous metastatic carcinoma, particularly metastatic salivary duct carcinoma.
Xintong Wang, MD; William H. Westra, MD
From the Department of Pathology, Icahn School of Medicine at Mount Sinai, New York, New York.
The authors report no conflict of interest.
- Wang X, Vyas NS, Alghamdi AA, et al. Cutaneous presentation of metastatic salivary duct carcinoma. Cutis. 2023;112:E13-E15.
- Pollock JL, Catalano E. Metastatic ductal carcinoma of the parotid gland in a patient with sarcoidosis. Arch Dermatol. 1979;115:1098-1099.
- Pollock JL. Metastatic carcinoma of the parotid gland resembling carcinoma of the breast. J Am Acad Dermatol. 1996;34:1093.
- Aygit AC, Top H, Cakir B, et al. Salivary duct carcinoma of the parotid gland metastasizing to the skin: a case report and review of the literature. Am J Dermatopathol. 2005;27:48-50.
- Cohen PR, Prieto VG, Piha-Paul SA, et al. The “shield sign” in two men with metastatic salivary duct carcinoma to the skin: cutaneous metastases presenting as carcinoma hemorrhagiectoides. J Clin Aesthet Dermatol. 2012;5:27-36.
- Chakari W, Andersen L, Anderson JL. Cutaneous metastases from salivary duct carcinoma of the submandibular gland. Case Rep Dermatol. 2017;9:254-258.
- Shin JY, Eun DH, Lee JY, et al. A case of cutaneous metastases of salivary duct carcinoma mimicking radiation recall dermatitis. Ann Dermatol. 2020;32:436-438.
- Aravena RC, Aravena DC, Velasco MJ, et al. Carcinoma hemorrhagiectoides: case report of an uncommon presentation of cutaneous metastatic breast carcinoma. Dermatol Online J. 2017;23:13030/qt3hn3z850.
- Smith KA, Basko-Plluska J, Kothari AD, et al. Cutaneous metastatic breast adenocarcinoma. Cutis. 2020;105:E20-E22.
- Cohen PR, Kurzrock R. Cutaneous metastatic cancer: carcinoma hemorrhagiectoides presenting as the shield sign. Cureus. 2021;13:e12627.
- Cohen PR. Pleomorphic appearance of breast cancer cutaneous metastases. Cureus. 2021;13:e20301.
- Cohen PR, Prieto VG, Kurzrock R. Tumor lysis syndrome: introduction of a cutaneous variant and a new classification system. Cureus. 2021;13:e13816.
- Wang X, Vyas NS, Alghamdi AA, et al. Cutaneous presentation of metastatic salivary duct carcinoma. Cutis. 2023;112:E13-E15.
- Pollock JL, Catalano E. Metastatic ductal carcinoma of the parotid gland in a patient with sarcoidosis. Arch Dermatol. 1979;115:1098-1099.
- Pollock JL. Metastatic carcinoma of the parotid gland resembling carcinoma of the breast. J Am Acad Dermatol. 1996;34:1093.
- Aygit AC, Top H, Cakir B, et al. Salivary duct carcinoma of the parotid gland metastasizing to the skin: a case report and review of the literature. Am J Dermatopathol. 2005;27:48-50.
- Cohen PR, Prieto VG, Piha-Paul SA, et al. The “shield sign” in two men with metastatic salivary duct carcinoma to the skin: cutaneous metastases presenting as carcinoma hemorrhagiectoides. J Clin Aesthet Dermatol. 2012;5:27-36.
- Chakari W, Andersen L, Anderson JL. Cutaneous metastases from salivary duct carcinoma of the submandibular gland. Case Rep Dermatol. 2017;9:254-258.
- Shin JY, Eun DH, Lee JY, et al. A case of cutaneous metastases of salivary duct carcinoma mimicking radiation recall dermatitis. Ann Dermatol. 2020;32:436-438.
- Aravena RC, Aravena DC, Velasco MJ, et al. Carcinoma hemorrhagiectoides: case report of an uncommon presentation of cutaneous metastatic breast carcinoma. Dermatol Online J. 2017;23:13030/qt3hn3z850.
- Smith KA, Basko-Plluska J, Kothari AD, et al. Cutaneous metastatic breast adenocarcinoma. Cutis. 2020;105:E20-E22.
- Cohen PR, Kurzrock R. Cutaneous metastatic cancer: carcinoma hemorrhagiectoides presenting as the shield sign. Cureus. 2021;13:e12627.
- Cohen PR. Pleomorphic appearance of breast cancer cutaneous metastases. Cureus. 2021;13:e20301.
- Cohen PR, Prieto VG, Kurzrock R. Tumor lysis syndrome: introduction of a cutaneous variant and a new classification system. Cureus. 2021;13:e13816.