User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
Will Compounding ‘Best Practices’ Guide Reassure Clinicians?
A new “best practices” guide released by the Alliance for Pharmacy Compounding (APC) aims to educate compounding pharmacists and reassure prescribers about the ethical, legal, and practical considerations that must be addressed to ensure quality standards and protect patients’ health.
Endocrinologists have expressed skepticism about the quality of compounded drugs, particularly the popular glucagon-like peptide 1 (GLP-1) semaglutide. The Food and Drug Administration (FDA) recently issued an alert linking hospitalizations to overdoses of compounded semaglutide.
“This document goes beyond today’s media-grabbing shortages,” APC Board Chair-Elect Gina Besteman, RPh, of Belmar Pharma Solutions told this news organization. “We developed these best practices to apply to all shortage drug compounding, and especially in this moment when so many are compounding GLP-1s. These serve as a reminder about what compliance and care look like.”
Prescribers determine whether a patient needs a compounded medication, not pharmacists, Ms. Besteman noted. “A patient-specific prescription order must be authorized for a compounded medication to be dispensed. Prescribers should ensure pharmacies they work with regularly check the FDA Drug Shortage List, as compounding of ‘essential copies’ of FDA-approved drugs is only allowed when a drug is listed as ‘currently in shortage.’ ”
Framework for Compounding
“With fake and illegal online stores popping up, it’s critical for legitimate, state-licensed compounding pharmacies to maintain the profession’s high standards,” the APC said in a media communication.
Highlights of its best practices, which are directed toward 503A state-licensed compounding pharmacies, include the following, among others:
- Pharmacies should check the FDA drug shortage list prior to preparing a copy of an FDA-approved drug and maintain documentation to demonstrate to regulators that the drug was in shortage at the time it was compounded.
- Pharmacies may only source active pharmaceutical ingredients (APIs) from state-licensed wholesalers who purchase from FDA-registered manufacturers or order directly from FDA-registered manufacturers.
- Verify from the wholesaler that the manufacturer is registered with the FDA and the API meets all the requirements of section 503A, and that both hold the appropriate permits or licenses in their home state and the shipped to state.
- Adhere to USP Chapter <797> testing requirements for sterility, endotoxin, stability, particulate, antimicrobial effectiveness, and container closure integrity studies.
- Counseling must be offered to the patient or the patient’s agent/caregiver. Providing written information that assists in the understanding of how to properly use the compounded medication is advised.
- Instructions should be written in a way that a layperson can understand (especially directions including dosage titrations and conversions between milligrams and milliliters or units).
- Like all medications, compounded drugs can only be prescribed in the presence of a valid patient-practitioner relationship and can only be dispensed by a pharmacy after receipt of a valid patient-specific prescription order.
- When marketing, never make claims of safety or efficacy of the compounded product.
- Advertising that patients will/may save money using compounded medications, compared with manufactured products is not allowed.
“Compounding FDA-approved drugs during shortages is nothing new — pharmacies have been doing it well before GLP-1s came on the scene, and they’ll continue long after this current shortage ends,” Ms. Besteman said. “Prescribers should be aware of APC’s guidelines because they provide a framework for ethically and legally compounding medications during drug shortages.
“To paraphrase The Police,” she concluded, “every move you make, every step you take, they’ll be watching you. Make sure they see those best practices in action.”
‘Reduces the Risks’
Commenting on the best practices guidance, Ivania Rizo, MD, director of Obesity Medicine and Diabetes and clinical colead at Boston Medical Center’s Health Equity Accelerator in Massachusetts, said: “These best practices will hopefully make a difference in the quality of compounded drugs.”
“The emphasis on rigorous testing of APIs and adherence to USP standards is particularly important for maintaining drug quality,” she noted. “This structured approach reduces the risk of variability and ensures that compounded drugs meet high-quality standards, thus enhancing their reliability.”
“Knowing that compounding pharmacies are adhering to rigorous standards for sourcing, testing, and compounding can at least reassure clinicians that specific steps are being taken for the safety and efficacy of these medications,” she said. “The transparency in documenting compliance with FDA guidelines and maintaining high-quality control measures can enhance trust among healthcare providers.”
Although clinicians are likely to have more confidence in compounded drugs when these best practices are followed, she said, “overall, we all hope that the shortages of medications such as tirzepatide are resolved promptly, allowing patients to access FDA-approved drugs without the need for compounding.”
“While the implementation of best practices for compounding during shortages is a positive and necessary step, our ultimate goal remains to address and resolve these shortages in the near future,” she concluded.
Dr. Rizo declared no competing interests.
A version of this article first appeared on Medscape.com.
A new “best practices” guide released by the Alliance for Pharmacy Compounding (APC) aims to educate compounding pharmacists and reassure prescribers about the ethical, legal, and practical considerations that must be addressed to ensure quality standards and protect patients’ health.
Endocrinologists have expressed skepticism about the quality of compounded drugs, particularly the popular glucagon-like peptide 1 (GLP-1) semaglutide. The Food and Drug Administration (FDA) recently issued an alert linking hospitalizations to overdoses of compounded semaglutide.
“This document goes beyond today’s media-grabbing shortages,” APC Board Chair-Elect Gina Besteman, RPh, of Belmar Pharma Solutions told this news organization. “We developed these best practices to apply to all shortage drug compounding, and especially in this moment when so many are compounding GLP-1s. These serve as a reminder about what compliance and care look like.”
Prescribers determine whether a patient needs a compounded medication, not pharmacists, Ms. Besteman noted. “A patient-specific prescription order must be authorized for a compounded medication to be dispensed. Prescribers should ensure pharmacies they work with regularly check the FDA Drug Shortage List, as compounding of ‘essential copies’ of FDA-approved drugs is only allowed when a drug is listed as ‘currently in shortage.’ ”
Framework for Compounding
“With fake and illegal online stores popping up, it’s critical for legitimate, state-licensed compounding pharmacies to maintain the profession’s high standards,” the APC said in a media communication.
Highlights of its best practices, which are directed toward 503A state-licensed compounding pharmacies, include the following, among others:
- Pharmacies should check the FDA drug shortage list prior to preparing a copy of an FDA-approved drug and maintain documentation to demonstrate to regulators that the drug was in shortage at the time it was compounded.
- Pharmacies may only source active pharmaceutical ingredients (APIs) from state-licensed wholesalers who purchase from FDA-registered manufacturers or order directly from FDA-registered manufacturers.
- Verify from the wholesaler that the manufacturer is registered with the FDA and the API meets all the requirements of section 503A, and that both hold the appropriate permits or licenses in their home state and the shipped to state.
- Adhere to USP Chapter <797> testing requirements for sterility, endotoxin, stability, particulate, antimicrobial effectiveness, and container closure integrity studies.
- Counseling must be offered to the patient or the patient’s agent/caregiver. Providing written information that assists in the understanding of how to properly use the compounded medication is advised.
- Instructions should be written in a way that a layperson can understand (especially directions including dosage titrations and conversions between milligrams and milliliters or units).
- Like all medications, compounded drugs can only be prescribed in the presence of a valid patient-practitioner relationship and can only be dispensed by a pharmacy after receipt of a valid patient-specific prescription order.
- When marketing, never make claims of safety or efficacy of the compounded product.
- Advertising that patients will/may save money using compounded medications, compared with manufactured products is not allowed.
“Compounding FDA-approved drugs during shortages is nothing new — pharmacies have been doing it well before GLP-1s came on the scene, and they’ll continue long after this current shortage ends,” Ms. Besteman said. “Prescribers should be aware of APC’s guidelines because they provide a framework for ethically and legally compounding medications during drug shortages.
“To paraphrase The Police,” she concluded, “every move you make, every step you take, they’ll be watching you. Make sure they see those best practices in action.”
‘Reduces the Risks’
Commenting on the best practices guidance, Ivania Rizo, MD, director of Obesity Medicine and Diabetes and clinical colead at Boston Medical Center’s Health Equity Accelerator in Massachusetts, said: “These best practices will hopefully make a difference in the quality of compounded drugs.”
“The emphasis on rigorous testing of APIs and adherence to USP standards is particularly important for maintaining drug quality,” she noted. “This structured approach reduces the risk of variability and ensures that compounded drugs meet high-quality standards, thus enhancing their reliability.”
“Knowing that compounding pharmacies are adhering to rigorous standards for sourcing, testing, and compounding can at least reassure clinicians that specific steps are being taken for the safety and efficacy of these medications,” she said. “The transparency in documenting compliance with FDA guidelines and maintaining high-quality control measures can enhance trust among healthcare providers.”
Although clinicians are likely to have more confidence in compounded drugs when these best practices are followed, she said, “overall, we all hope that the shortages of medications such as tirzepatide are resolved promptly, allowing patients to access FDA-approved drugs without the need for compounding.”
“While the implementation of best practices for compounding during shortages is a positive and necessary step, our ultimate goal remains to address and resolve these shortages in the near future,” she concluded.
Dr. Rizo declared no competing interests.
A version of this article first appeared on Medscape.com.
A new “best practices” guide released by the Alliance for Pharmacy Compounding (APC) aims to educate compounding pharmacists and reassure prescribers about the ethical, legal, and practical considerations that must be addressed to ensure quality standards and protect patients’ health.
Endocrinologists have expressed skepticism about the quality of compounded drugs, particularly the popular glucagon-like peptide 1 (GLP-1) semaglutide. The Food and Drug Administration (FDA) recently issued an alert linking hospitalizations to overdoses of compounded semaglutide.
“This document goes beyond today’s media-grabbing shortages,” APC Board Chair-Elect Gina Besteman, RPh, of Belmar Pharma Solutions told this news organization. “We developed these best practices to apply to all shortage drug compounding, and especially in this moment when so many are compounding GLP-1s. These serve as a reminder about what compliance and care look like.”
Prescribers determine whether a patient needs a compounded medication, not pharmacists, Ms. Besteman noted. “A patient-specific prescription order must be authorized for a compounded medication to be dispensed. Prescribers should ensure pharmacies they work with regularly check the FDA Drug Shortage List, as compounding of ‘essential copies’ of FDA-approved drugs is only allowed when a drug is listed as ‘currently in shortage.’ ”
Framework for Compounding
“With fake and illegal online stores popping up, it’s critical for legitimate, state-licensed compounding pharmacies to maintain the profession’s high standards,” the APC said in a media communication.
Highlights of its best practices, which are directed toward 503A state-licensed compounding pharmacies, include the following, among others:
- Pharmacies should check the FDA drug shortage list prior to preparing a copy of an FDA-approved drug and maintain documentation to demonstrate to regulators that the drug was in shortage at the time it was compounded.
- Pharmacies may only source active pharmaceutical ingredients (APIs) from state-licensed wholesalers who purchase from FDA-registered manufacturers or order directly from FDA-registered manufacturers.
- Verify from the wholesaler that the manufacturer is registered with the FDA and the API meets all the requirements of section 503A, and that both hold the appropriate permits or licenses in their home state and the shipped to state.
- Adhere to USP Chapter <797> testing requirements for sterility, endotoxin, stability, particulate, antimicrobial effectiveness, and container closure integrity studies.
- Counseling must be offered to the patient or the patient’s agent/caregiver. Providing written information that assists in the understanding of how to properly use the compounded medication is advised.
- Instructions should be written in a way that a layperson can understand (especially directions including dosage titrations and conversions between milligrams and milliliters or units).
- Like all medications, compounded drugs can only be prescribed in the presence of a valid patient-practitioner relationship and can only be dispensed by a pharmacy after receipt of a valid patient-specific prescription order.
- When marketing, never make claims of safety or efficacy of the compounded product.
- Advertising that patients will/may save money using compounded medications, compared with manufactured products is not allowed.
“Compounding FDA-approved drugs during shortages is nothing new — pharmacies have been doing it well before GLP-1s came on the scene, and they’ll continue long after this current shortage ends,” Ms. Besteman said. “Prescribers should be aware of APC’s guidelines because they provide a framework for ethically and legally compounding medications during drug shortages.
“To paraphrase The Police,” she concluded, “every move you make, every step you take, they’ll be watching you. Make sure they see those best practices in action.”
‘Reduces the Risks’
Commenting on the best practices guidance, Ivania Rizo, MD, director of Obesity Medicine and Diabetes and clinical colead at Boston Medical Center’s Health Equity Accelerator in Massachusetts, said: “These best practices will hopefully make a difference in the quality of compounded drugs.”
“The emphasis on rigorous testing of APIs and adherence to USP standards is particularly important for maintaining drug quality,” she noted. “This structured approach reduces the risk of variability and ensures that compounded drugs meet high-quality standards, thus enhancing their reliability.”
“Knowing that compounding pharmacies are adhering to rigorous standards for sourcing, testing, and compounding can at least reassure clinicians that specific steps are being taken for the safety and efficacy of these medications,” she said. “The transparency in documenting compliance with FDA guidelines and maintaining high-quality control measures can enhance trust among healthcare providers.”
Although clinicians are likely to have more confidence in compounded drugs when these best practices are followed, she said, “overall, we all hope that the shortages of medications such as tirzepatide are resolved promptly, allowing patients to access FDA-approved drugs without the need for compounding.”
“While the implementation of best practices for compounding during shortages is a positive and necessary step, our ultimate goal remains to address and resolve these shortages in the near future,” she concluded.
Dr. Rizo declared no competing interests.
A version of this article first appeared on Medscape.com.
Do You Have Patients With JAKne — JAK Inhibitor–Associated Acne? Here’s What to Know
Since the first Food and Drug Administration approval of a Janus kinase (JAK) inhibitor in 2011, the number of these medications available — and their treatment indications — have continued to grow. Prescribing physicians are familiar with the benefits and risks for these drugs, including higher risk for cardiac events and malignancy; however, one adverse effect may be overlooked, especially by specialties outside of dermatology: acne. Though less serious than some other side effects, JAK inhibitor–associated acne — JAKne, for short — can be a concern for patients.
“Your physical appearance and how you present yourself to the world is an important part of your self-confidence and living life on your own terms,” said Arash Mostaghimi, MD, the director of inpatient dermatology at Brigham and Women’s Hospital in Boston, Massachusetts. “I think letting people know about [JAKne] and then addressing it when it occurs should be a normal part of managing these medications.”
What Is JAKne?
JAKne generally looks like other kinds of acne, explained Janelle Nassim, MD, director of laser and cosmetic dermatology at the Indiana University School of Medicine, Indianapolis. “It can affect the same areas that typical acne affects, including the face, chest, back, neck, and upper shoulders.”
Though it appears like typical forms of acne, it is not clear what drives these skin eruptions in patients taking JAK inhibitors.
“We don’t understand the underlying pathophysiology,” Dr. Mostaghimi said. “It looks like acne, but we don’t know if the exact underlying inflammatory process is the same or if it’s different.”
In a 2023 systematic review of clinical studies, Dr. Mostaghimi and colleagues found that patients on any JAK inhibitor were nearly four times more likely to experience acne than patients who received placebo, but risk varied between medications. Patients taking JAK inhibitors for skin conditions had higher risk for acne than those given the medications for other indications. However, Dr. Mostaghimi thinks this finding is the result of selection bias.
Participants may not mention side effects like acne in trials for rheumatologic or gastrointestinal conditions, he said, unlike in trials for skin conditions. “Clinically, I’ve seen it in patients across every indication.”
Patients with a history of acne seem to be more likely to develop this side effect, though formal studies looking into risk factors are lacking. In Dr. Mostaghimi’s own clinical experience, JAKne is also more common in younger patients, but it can happen to anyone. “I’ve seen 70-year-olds develop acne — patients who’ve never had an issue their whole life — when they’re taking a JAK inhibitor.”
This issue also appears to be more common earlier in treatment, he added, and may improve over time as a patient continues with the medication.
How Do You Treat It?
“I think in other specialties, you will often feel awkward addressing skin conditions or pointing out acne,” Dr. Mostaghimi said. The most important steps are being aware of this potential side effect, and if you see it practice, to bring it up.
“Say: I’m noticing there’s some changes in your skin. Some patients on JAK inhibitors develop more acne. Have you noticed this? And if so, is this bothering you?”
Generally, JAKne is mild to moderate, explained Dr. Nassim, and if non-dermatologists are comfortable, they can prescribe a first-line topical regimen for patients. Dr. Mostaghimi recommends prescribing a clindamycin 1% lotion or gel. In addition, patients can use a benzoyl peroxide wash (4% or 10%) combined with a gentle retinoid, such as adapalene. (Both of these treatments are now available over the counter.)
In patients with scalp or hairline involvement, he often prescribes a ketoconazole 2% shampoo, which patients can use to wash their scalp, face, chest, and back in the shower.
If they aren’t responding to these initial treatments, then refer to a dermatologist for further assessment.
“Ultimately, referring to a dermatologist is the best course of action,” Dr. Nassim said. “I have had patients on JAK inhibitors who improved with topical acne treatments, and some that required more aggressive treatment with oral medications.”
Dr. Mostaghimi reported consulting fees from AbbVie, Concert Pharmaceuticals, Pfizer, and 3Derm Systems; research funding from Incyte, Aclaris Therapeutics, Eli Lilly, and Concert Pharmaceuticals; personal fees from Equillium, ASLAN Pharmaceuticals, ACOM, and Boehringer Ingelheim; and advisory board fees from Fig.1 Beauty, Eli Lilly, Pfizer, and Hims & Hers Health. Dr. Nassim had no relevant disclosures.
A version of this article first appeared on Medscape.com.
Since the first Food and Drug Administration approval of a Janus kinase (JAK) inhibitor in 2011, the number of these medications available — and their treatment indications — have continued to grow. Prescribing physicians are familiar with the benefits and risks for these drugs, including higher risk for cardiac events and malignancy; however, one adverse effect may be overlooked, especially by specialties outside of dermatology: acne. Though less serious than some other side effects, JAK inhibitor–associated acne — JAKne, for short — can be a concern for patients.
“Your physical appearance and how you present yourself to the world is an important part of your self-confidence and living life on your own terms,” said Arash Mostaghimi, MD, the director of inpatient dermatology at Brigham and Women’s Hospital in Boston, Massachusetts. “I think letting people know about [JAKne] and then addressing it when it occurs should be a normal part of managing these medications.”
What Is JAKne?
JAKne generally looks like other kinds of acne, explained Janelle Nassim, MD, director of laser and cosmetic dermatology at the Indiana University School of Medicine, Indianapolis. “It can affect the same areas that typical acne affects, including the face, chest, back, neck, and upper shoulders.”
Though it appears like typical forms of acne, it is not clear what drives these skin eruptions in patients taking JAK inhibitors.
“We don’t understand the underlying pathophysiology,” Dr. Mostaghimi said. “It looks like acne, but we don’t know if the exact underlying inflammatory process is the same or if it’s different.”
In a 2023 systematic review of clinical studies, Dr. Mostaghimi and colleagues found that patients on any JAK inhibitor were nearly four times more likely to experience acne than patients who received placebo, but risk varied between medications. Patients taking JAK inhibitors for skin conditions had higher risk for acne than those given the medications for other indications. However, Dr. Mostaghimi thinks this finding is the result of selection bias.
Participants may not mention side effects like acne in trials for rheumatologic or gastrointestinal conditions, he said, unlike in trials for skin conditions. “Clinically, I’ve seen it in patients across every indication.”
Patients with a history of acne seem to be more likely to develop this side effect, though formal studies looking into risk factors are lacking. In Dr. Mostaghimi’s own clinical experience, JAKne is also more common in younger patients, but it can happen to anyone. “I’ve seen 70-year-olds develop acne — patients who’ve never had an issue their whole life — when they’re taking a JAK inhibitor.”
This issue also appears to be more common earlier in treatment, he added, and may improve over time as a patient continues with the medication.
How Do You Treat It?
“I think in other specialties, you will often feel awkward addressing skin conditions or pointing out acne,” Dr. Mostaghimi said. The most important steps are being aware of this potential side effect, and if you see it practice, to bring it up.
“Say: I’m noticing there’s some changes in your skin. Some patients on JAK inhibitors develop more acne. Have you noticed this? And if so, is this bothering you?”
Generally, JAKne is mild to moderate, explained Dr. Nassim, and if non-dermatologists are comfortable, they can prescribe a first-line topical regimen for patients. Dr. Mostaghimi recommends prescribing a clindamycin 1% lotion or gel. In addition, patients can use a benzoyl peroxide wash (4% or 10%) combined with a gentle retinoid, such as adapalene. (Both of these treatments are now available over the counter.)
In patients with scalp or hairline involvement, he often prescribes a ketoconazole 2% shampoo, which patients can use to wash their scalp, face, chest, and back in the shower.
If they aren’t responding to these initial treatments, then refer to a dermatologist for further assessment.
“Ultimately, referring to a dermatologist is the best course of action,” Dr. Nassim said. “I have had patients on JAK inhibitors who improved with topical acne treatments, and some that required more aggressive treatment with oral medications.”
Dr. Mostaghimi reported consulting fees from AbbVie, Concert Pharmaceuticals, Pfizer, and 3Derm Systems; research funding from Incyte, Aclaris Therapeutics, Eli Lilly, and Concert Pharmaceuticals; personal fees from Equillium, ASLAN Pharmaceuticals, ACOM, and Boehringer Ingelheim; and advisory board fees from Fig.1 Beauty, Eli Lilly, Pfizer, and Hims & Hers Health. Dr. Nassim had no relevant disclosures.
A version of this article first appeared on Medscape.com.
Since the first Food and Drug Administration approval of a Janus kinase (JAK) inhibitor in 2011, the number of these medications available — and their treatment indications — have continued to grow. Prescribing physicians are familiar with the benefits and risks for these drugs, including higher risk for cardiac events and malignancy; however, one adverse effect may be overlooked, especially by specialties outside of dermatology: acne. Though less serious than some other side effects, JAK inhibitor–associated acne — JAKne, for short — can be a concern for patients.
“Your physical appearance and how you present yourself to the world is an important part of your self-confidence and living life on your own terms,” said Arash Mostaghimi, MD, the director of inpatient dermatology at Brigham and Women’s Hospital in Boston, Massachusetts. “I think letting people know about [JAKne] and then addressing it when it occurs should be a normal part of managing these medications.”
What Is JAKne?
JAKne generally looks like other kinds of acne, explained Janelle Nassim, MD, director of laser and cosmetic dermatology at the Indiana University School of Medicine, Indianapolis. “It can affect the same areas that typical acne affects, including the face, chest, back, neck, and upper shoulders.”
Though it appears like typical forms of acne, it is not clear what drives these skin eruptions in patients taking JAK inhibitors.
“We don’t understand the underlying pathophysiology,” Dr. Mostaghimi said. “It looks like acne, but we don’t know if the exact underlying inflammatory process is the same or if it’s different.”
In a 2023 systematic review of clinical studies, Dr. Mostaghimi and colleagues found that patients on any JAK inhibitor were nearly four times more likely to experience acne than patients who received placebo, but risk varied between medications. Patients taking JAK inhibitors for skin conditions had higher risk for acne than those given the medications for other indications. However, Dr. Mostaghimi thinks this finding is the result of selection bias.
Participants may not mention side effects like acne in trials for rheumatologic or gastrointestinal conditions, he said, unlike in trials for skin conditions. “Clinically, I’ve seen it in patients across every indication.”
Patients with a history of acne seem to be more likely to develop this side effect, though formal studies looking into risk factors are lacking. In Dr. Mostaghimi’s own clinical experience, JAKne is also more common in younger patients, but it can happen to anyone. “I’ve seen 70-year-olds develop acne — patients who’ve never had an issue their whole life — when they’re taking a JAK inhibitor.”
This issue also appears to be more common earlier in treatment, he added, and may improve over time as a patient continues with the medication.
How Do You Treat It?
“I think in other specialties, you will often feel awkward addressing skin conditions or pointing out acne,” Dr. Mostaghimi said. The most important steps are being aware of this potential side effect, and if you see it practice, to bring it up.
“Say: I’m noticing there’s some changes in your skin. Some patients on JAK inhibitors develop more acne. Have you noticed this? And if so, is this bothering you?”
Generally, JAKne is mild to moderate, explained Dr. Nassim, and if non-dermatologists are comfortable, they can prescribe a first-line topical regimen for patients. Dr. Mostaghimi recommends prescribing a clindamycin 1% lotion or gel. In addition, patients can use a benzoyl peroxide wash (4% or 10%) combined with a gentle retinoid, such as adapalene. (Both of these treatments are now available over the counter.)
In patients with scalp or hairline involvement, he often prescribes a ketoconazole 2% shampoo, which patients can use to wash their scalp, face, chest, and back in the shower.
If they aren’t responding to these initial treatments, then refer to a dermatologist for further assessment.
“Ultimately, referring to a dermatologist is the best course of action,” Dr. Nassim said. “I have had patients on JAK inhibitors who improved with topical acne treatments, and some that required more aggressive treatment with oral medications.”
Dr. Mostaghimi reported consulting fees from AbbVie, Concert Pharmaceuticals, Pfizer, and 3Derm Systems; research funding from Incyte, Aclaris Therapeutics, Eli Lilly, and Concert Pharmaceuticals; personal fees from Equillium, ASLAN Pharmaceuticals, ACOM, and Boehringer Ingelheim; and advisory board fees from Fig.1 Beauty, Eli Lilly, Pfizer, and Hims & Hers Health. Dr. Nassim had no relevant disclosures.
A version of this article first appeared on Medscape.com.
A 62-year-old Black female presented with an epidermal inclusion cyst on her left upper back
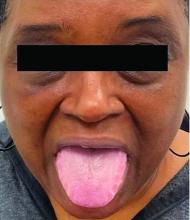
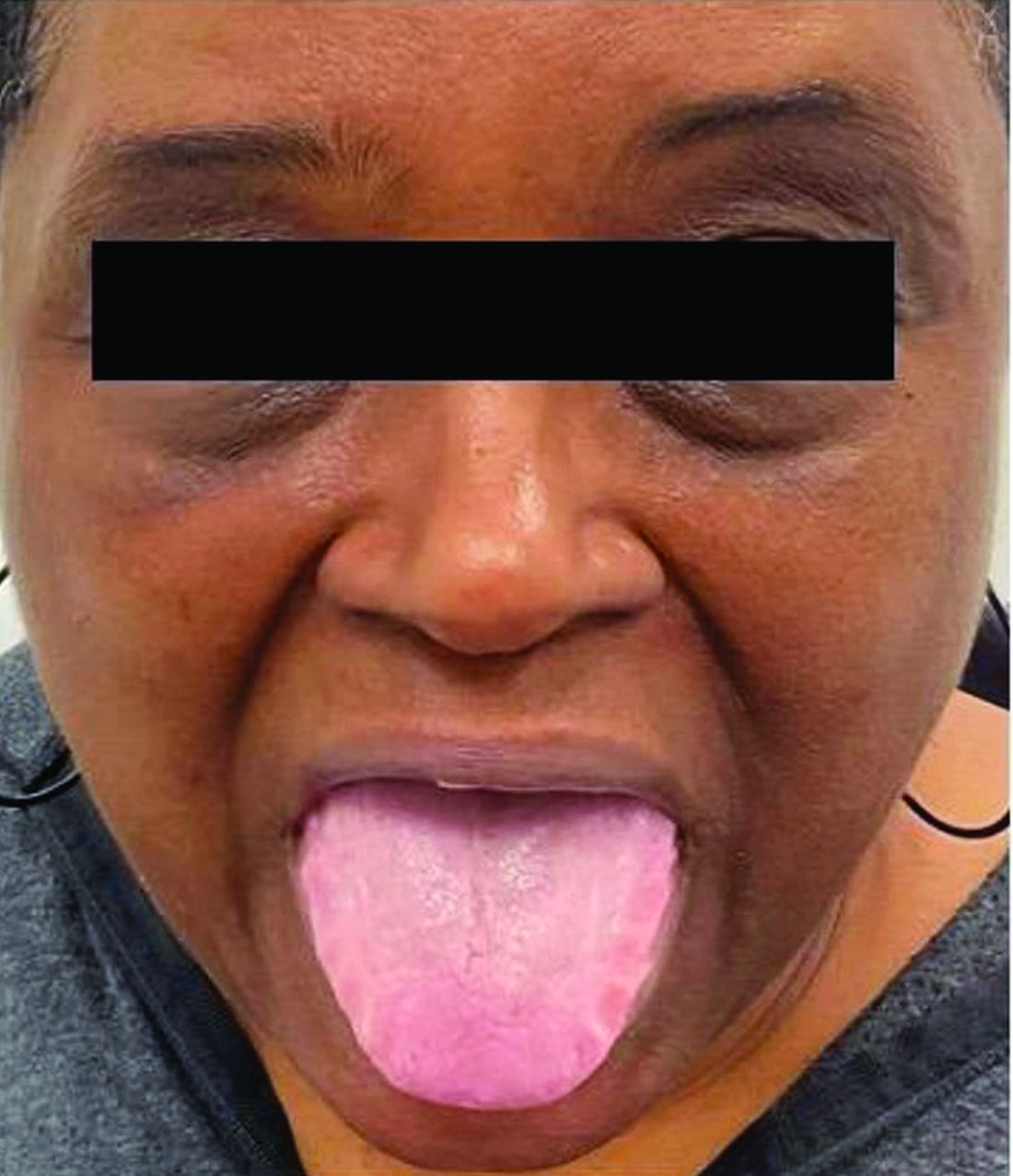
This heterogeneous disorder can present with a wide range of clinical manifestations, including dermatological symptoms that may be the first or predominant feature. Systemic amyloidosis is characterized by macroglossia, periorbital purpura, and waxy skin plaques. Lateral scalloping of the tongue may be seen due to impingement of the teeth. Cutaneous amyloidosis occurs when amyloid is deposited in the skin, without internal organ involvement. Variants of cutaneous amyloidosis include macular, lichen, nodular and biphasic.
This condition requires a thorough diagnostic workup, including serum and urine protein electrophoresis and biopsy of the affected tissue. Biopsy of a cutaneous amyloidosis lesion will show fractured, amorphous, eosinophilic material in the dermis. Pigment and epidermal changes are often found with cutaneous amyloidosis, including hyperkeratosis, acanthosis, hypergranulosis, parakeratosis, and epidermal atrophy. Stains that may be used include Congo red showing apple-green birefringence, thioflavin T, and crystal violet.
If untreated, the prognosis is generally poor, related to the extent of organ involvement. Cardiac involvement, a common feature of systemic amyloidosis, can lead to restrictive cardiomyopathy, heart failure, and arrhythmias. Management strategies include steroids, chemotherapy, and stem cell transplantation. Medications include dexamethasone, cyclophosphamide, bortezomib, and melphalan.
This patient went undiagnosed for several years until she began experiencing cardiac issues, including syncope, angina, and restrictive cardiomyopathy with heart failure. A cardiac biopsy confirmed the diagnosis of systemic amyloidosis. This patient is currently awaiting a heart transplant. Early diagnosis of amyloidosis is vital, as it can help prevent severe complications such as heart involvement, significantly impacting the patient’s prognosis and quality of life. When amyloidosis is suspected based on dermatological findings, it is essential to distinguish it from other conditions, such as chronic cutaneous lupus erythematosus, dermatomyositis, scleromyxedema, and lipoid proteinosis. Early identification of characteristic skin lesions and systemic features can lead to timely interventions, more favorable outcomes, and reduction in the risk of advanced organ damage.
The case and photo were submitted by Ms. Cael Aoki and Mr. Shapiro of Nova Southeastern University College of Osteopathic Medicine, Davie, Florida, and Dr. Bartos, of Imperial Dermatology, Hollywood, Florida. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Brunt EM, Tiniakos DG. Clin Liver Dis. 2004 Nov;8(4):915-30, x. doi: 10.1016/j.cld.2004.06.009.
2. Bolognia JL et al. (2017). Dermatology E-Book. Elsevier Health Sciences.
3. Mehrotra K et al. J Clin Diagn Res. 2017 Aug;11(8):WC01-WC05. doi: 10.7860/JCDR/2017/24273.10334.
4. Banypersad SM et al. J Am Heart Assoc. 2012 Apr;1(2):e000364. doi: 10.1161/JAHA.111.000364.
5. Bustamante JG, Zaidi SRH. Amyloidosis. [Updated 2023 Jul 31]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
This heterogeneous disorder can present with a wide range of clinical manifestations, including dermatological symptoms that may be the first or predominant feature. Systemic amyloidosis is characterized by macroglossia, periorbital purpura, and waxy skin plaques. Lateral scalloping of the tongue may be seen due to impingement of the teeth. Cutaneous amyloidosis occurs when amyloid is deposited in the skin, without internal organ involvement. Variants of cutaneous amyloidosis include macular, lichen, nodular and biphasic.
This condition requires a thorough diagnostic workup, including serum and urine protein electrophoresis and biopsy of the affected tissue. Biopsy of a cutaneous amyloidosis lesion will show fractured, amorphous, eosinophilic material in the dermis. Pigment and epidermal changes are often found with cutaneous amyloidosis, including hyperkeratosis, acanthosis, hypergranulosis, parakeratosis, and epidermal atrophy. Stains that may be used include Congo red showing apple-green birefringence, thioflavin T, and crystal violet.
If untreated, the prognosis is generally poor, related to the extent of organ involvement. Cardiac involvement, a common feature of systemic amyloidosis, can lead to restrictive cardiomyopathy, heart failure, and arrhythmias. Management strategies include steroids, chemotherapy, and stem cell transplantation. Medications include dexamethasone, cyclophosphamide, bortezomib, and melphalan.
This patient went undiagnosed for several years until she began experiencing cardiac issues, including syncope, angina, and restrictive cardiomyopathy with heart failure. A cardiac biopsy confirmed the diagnosis of systemic amyloidosis. This patient is currently awaiting a heart transplant. Early diagnosis of amyloidosis is vital, as it can help prevent severe complications such as heart involvement, significantly impacting the patient’s prognosis and quality of life. When amyloidosis is suspected based on dermatological findings, it is essential to distinguish it from other conditions, such as chronic cutaneous lupus erythematosus, dermatomyositis, scleromyxedema, and lipoid proteinosis. Early identification of characteristic skin lesions and systemic features can lead to timely interventions, more favorable outcomes, and reduction in the risk of advanced organ damage.
The case and photo were submitted by Ms. Cael Aoki and Mr. Shapiro of Nova Southeastern University College of Osteopathic Medicine, Davie, Florida, and Dr. Bartos, of Imperial Dermatology, Hollywood, Florida. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Brunt EM, Tiniakos DG. Clin Liver Dis. 2004 Nov;8(4):915-30, x. doi: 10.1016/j.cld.2004.06.009.
2. Bolognia JL et al. (2017). Dermatology E-Book. Elsevier Health Sciences.
3. Mehrotra K et al. J Clin Diagn Res. 2017 Aug;11(8):WC01-WC05. doi: 10.7860/JCDR/2017/24273.10334.
4. Banypersad SM et al. J Am Heart Assoc. 2012 Apr;1(2):e000364. doi: 10.1161/JAHA.111.000364.
5. Bustamante JG, Zaidi SRH. Amyloidosis. [Updated 2023 Jul 31]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
This heterogeneous disorder can present with a wide range of clinical manifestations, including dermatological symptoms that may be the first or predominant feature. Systemic amyloidosis is characterized by macroglossia, periorbital purpura, and waxy skin plaques. Lateral scalloping of the tongue may be seen due to impingement of the teeth. Cutaneous amyloidosis occurs when amyloid is deposited in the skin, without internal organ involvement. Variants of cutaneous amyloidosis include macular, lichen, nodular and biphasic.
This condition requires a thorough diagnostic workup, including serum and urine protein electrophoresis and biopsy of the affected tissue. Biopsy of a cutaneous amyloidosis lesion will show fractured, amorphous, eosinophilic material in the dermis. Pigment and epidermal changes are often found with cutaneous amyloidosis, including hyperkeratosis, acanthosis, hypergranulosis, parakeratosis, and epidermal atrophy. Stains that may be used include Congo red showing apple-green birefringence, thioflavin T, and crystal violet.
If untreated, the prognosis is generally poor, related to the extent of organ involvement. Cardiac involvement, a common feature of systemic amyloidosis, can lead to restrictive cardiomyopathy, heart failure, and arrhythmias. Management strategies include steroids, chemotherapy, and stem cell transplantation. Medications include dexamethasone, cyclophosphamide, bortezomib, and melphalan.
This patient went undiagnosed for several years until she began experiencing cardiac issues, including syncope, angina, and restrictive cardiomyopathy with heart failure. A cardiac biopsy confirmed the diagnosis of systemic amyloidosis. This patient is currently awaiting a heart transplant. Early diagnosis of amyloidosis is vital, as it can help prevent severe complications such as heart involvement, significantly impacting the patient’s prognosis and quality of life. When amyloidosis is suspected based on dermatological findings, it is essential to distinguish it from other conditions, such as chronic cutaneous lupus erythematosus, dermatomyositis, scleromyxedema, and lipoid proteinosis. Early identification of characteristic skin lesions and systemic features can lead to timely interventions, more favorable outcomes, and reduction in the risk of advanced organ damage.
The case and photo were submitted by Ms. Cael Aoki and Mr. Shapiro of Nova Southeastern University College of Osteopathic Medicine, Davie, Florida, and Dr. Bartos, of Imperial Dermatology, Hollywood, Florida. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Brunt EM, Tiniakos DG. Clin Liver Dis. 2004 Nov;8(4):915-30, x. doi: 10.1016/j.cld.2004.06.009.
2. Bolognia JL et al. (2017). Dermatology E-Book. Elsevier Health Sciences.
3. Mehrotra K et al. J Clin Diagn Res. 2017 Aug;11(8):WC01-WC05. doi: 10.7860/JCDR/2017/24273.10334.
4. Banypersad SM et al. J Am Heart Assoc. 2012 Apr;1(2):e000364. doi: 10.1161/JAHA.111.000364.
5. Bustamante JG, Zaidi SRH. Amyloidosis. [Updated 2023 Jul 31]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
Diagnosing, Treating Rashes In Patients on Immune Checkpoint Inhibitors
WASHINGTON, DC — and with judicious usage and dosing of prednisone when deemed necessary, Blair Allais, MD, said during a session on supportive oncodermatology at the ElderDerm conference on dermatology in the older patient hosted by the George Washington University School of Medicine and Health Sciences, Washington, DC.
“It’s important when you see these patients to be as specific as possible” based on morphology and histopathology, and to treat the rashes in a similar way as in the non-ICI setting,” said Dr. Allais, a dermato-oncologist at the Inova Schar Cancer Institute, Fairfax, Virginia.
cirAEs are the most frequently reported and most visible adverse effects of checkpoint inhibition — a treatment that has emerged as a standard therapy for many malignancies since the first ICI was approved in 2011 for metastatic melanoma.
And contrary to what the phenomenon of immunosenescence might suggest, older patients are no less prone to cirAEs than younger patients. “You’d think you’d have fewer rashes and side effects as you age, but that’s not true,” said Dr. Allais, who completed a fellowship in cutaneous oncology after her dermatology residency.
A 2021 multicenter international cohort study of over 900 patients aged ≥ 80 years treated with single-agent ICIs for cancer did not find any significant differences in the development of immune-related adverse events among those younger than 85, those aged 85-89 years, and those 90 and older. Neither did the ELDERS study in the United Kingdom; this prospective observational study found similar rates of high-grade and low-grade immune toxicity in its two cohorts of patients ≥ 70 and < 70 years of age.
At the meeting, Dr. Allais, who coauthored a 2023 review of cirAEs from ICIs, reviewed recent developments and provided the following advice:
New diagnostic criteria: “Really exciting” news for more precise diagnosis and optimal therapy of cirAEs, Dr. Allais said, is a position paper published in the Journal for ImmunoTherapy of Cancer that offers consensus-based diagnostic criteria for the 10 most common types of dermatologic immune-related adverse events and an overall diagnostic framework. “Luckily, through the work of a Delphi consensus group, we can now have [more diagnostic specificity],” which is important for both clinical care and research, she said.
Most cirAEs have typically been reported nonspecifically as “rash,” but diagnosing a rash subtype is “critical in tailoring appropriate therapy that it is both effective and the least detrimental to the oncology treatment plan for patients with cancer,” the group’s coauthors wrote.
The 10 core diagnoses include psoriasis, eczematous dermatitis, vitiligo, Grover disease, eruptive atypical squamous proliferation, and bullous pemphigoid. Outside of the core diagnoses are other nonspecific presentations that require evaluation to arrive at a diagnosis, if possible, or to reveal data that can allow for targeted therapy and severity grading, the group explains in its paper.
“To prednisone or not to prednisone”: The development of cirAEs is associated with reduced mortality and improved cancer outcomes, making the use of immunosuppressants such as corticosteroids a therapeutic dilemma. “Patients who get these rashes usually do better with respect to their cancer, so the concern has been, if we affect how they respond to their immunotherapy, we may minimize that improvement in mortality,” said Dr. Allais, also assistant professor at the University of Virginia, Charlottesville, and clinical assistant professor of dermatology at George Washington University.
A widely discussed study published in 2015 reported on 254 patients with melanoma who developed an immune-related adverse event during treatment with ipilimumab — approximately one third of whom required systemic corticosteroids — and concluded that systemic corticosteroids did not affect overall survival or time to (cancer) treatment failure. This study from Memorial Sloan Kettering Cancer Center, New York City, “was the first large study looking at this question,” she said, and the subsequent message for several years in conferences and the literature was that steroids do not affect the efficacy of checkpoint inhibitors.
“But the study was not without limitations,” Dr. Allais said, “because the patients who got prednisone were mainly those with higher-grade toxicities,” while those not treated with corticosteroids had either no toxicities or low-grade toxicities. “If higher-grade toxicities were associated with better (antitumor) response, the steroids may have just [blunted] that benefit.”
The current totality of data available in the literature suggests that corticosteroids may indeed have an impact on the efficacy of ICI therapy. “Subsequent studies have come out in the community that have shown that we should probably think twice about giving prednisone to some patients, particularly within the first 50 days of ICI treatment, and that we should be mindful of the dose,” Dr. Allais said.
The takeaways from these studies — all published in the past few years — are to use prednisone early and liberally for life-threatening toxicity, to use it at the lowest dose and for the shortest course when there is not an appropriate alternative, to avoid it for diagnoses that are not treated with prednisone outside the ICI setting, and to “have a plan” for a steroid-sparing agent to use after prednisone, she said.
Dr. Allais recommends heightened consideration during the first 50 days of ICI treatment based on a multicenter retrospective study that found a significant association between use of high-dose glucocorticoids (≥ 60 mg prednisone equivalent once a day) within 8 weeks of anti–programmed cell death protein 1 (PD-1) monotherapy initiation and poorer progression-free and overall survival. The study covered a cohort of 947 patients with advanced melanoma treated with anti–PD-1 monotherapy between 2009 and 2019, 54% of whom developed immune-related adverse events.
This study and other recent studies addressing the association between steroids and survival outcomes in patients with immune-related adverse events during ICI therapy are described in Dr. Allais’ 2023 review of cirAEs from ICIs.
Approach to morbilliform eruptions: This rash is “super common” in patients on ICIs, occurring generally within 2-3 weeks of starting treatment. “It tends to be self-limited and can recur with future infusions,” Dr. Allais said.
Systemic steroids should be reserved for severe or refractory eruptions. “Usually, I treat the patients with topical steroids, and I manage their expectations (that the rash may recur with subsequent infusions), but I closely follow them up” within 2-3 weeks, she said. It’s important to rule out a severe cutaneous adverse drug eruption, of course, and to start high-dose systemic steroids immediately if necessary. “Antibiotics are a big culprit” and often can be discontinued.
Soak and smear: “I’m obsessed” with this technique of a 20-minute soak in plain water followed by application of steroid ointment, said Dr. Allais, referring to a small study published in 2005 that reported a complete response after 2 weeks in 60% of patients with psoriasis, atopic dermatitis, and other inflammatory skin conditions (none had cancer), who had failed prior systemic therapy. All patients had at least a 75% response.
The method offers a way to “avoid the systemic immunosuppression we’d get with prednisone,” she said. One just needs to make sure the older patient can get in and out of their tub safely.
ICI-induced bullous pemphigoid (BP): BP occurs more frequently in the ICI setting, compared with the general population, with a median time to development of 8.5 months after ICI initiation. It is associated in this setting with improved tumor response, but “many oncologists stop anticancer treatment because of this diagnosis,” she said.
In the supportive oncodermatology space, however, ICI-induced BP exemplifies the value of tailored treatment regimens, she said. A small multi-institutional retrospective cohort study published in 2023 identified 35 cases of ICI-BP among 5636 ICI-treated patients and found that 8 out of 11 patients who received biologic therapy (rituximab, omalizumab, or dupilumab) had a complete response to ICI-BP without flares following subsequent ICI cycles. And while statistical significance was not reached, the study showed that no cancer-related outcomes were worsened.
“If you see someone with ICI-induced BP and they have a lot of involvement, you could start them on steroids and get that steroid-sparing agent initiated for approval. ... And if IgE is elevated, you might reach for omalizumab,” said Dr. Allais, noting that her favored treatment overall is dupilumab.
Risk factors for the development of ICI-induced BP include age > 70, skin cancer, and having an initial response to ICI on first imaging, the latter of which “I find fascinating ... because imaging occurs within the first 12 weeks of treatment, but we don’t see BP popping up until 8.5 months into treatment,” she noted. “So maybe there’s a baseline risk factor that could predispose them.”
Caution with antibiotics: “I try to avoid antibiotics in the ICI setting,” Dr. Allais said, in deference to the “ever-important microbiome.” Studies have demonstrated that the microbiomes of responders to ICI treatment are different from those of nonresponders, she said.
And a “fascinating” study of patients with melanoma undergoing ICI therapy showed not only a higher abundance of Ruminococcaceae bacteria in responders vs nonresponders but a significant impact of dietary fiber. High dietary fiber was associated with significantly improved overall survival in the patients on ICI, with the most pronounced benefit in patients with good fiber intake and no probiotic use. “Even wilder, their T cells changed,” she said. “They had a high expression of genes related to T-cell activation ... so more tumor-infiltrating lymphocytes.”
A retrospective study of 568 patients with stages III and IV melanoma treated with ICI showed that those exposed to antibiotics prior to ICI had significantly worse overall survival than those not exposed to antibiotics. “Think before you give them,” Dr. Allais said. “And try to tell your older patients to eat beans and greens.”
Dr. Allais reported having no relevant disclosures.
A version of this article first appeared on Medscape.com.
WASHINGTON, DC — and with judicious usage and dosing of prednisone when deemed necessary, Blair Allais, MD, said during a session on supportive oncodermatology at the ElderDerm conference on dermatology in the older patient hosted by the George Washington University School of Medicine and Health Sciences, Washington, DC.
“It’s important when you see these patients to be as specific as possible” based on morphology and histopathology, and to treat the rashes in a similar way as in the non-ICI setting,” said Dr. Allais, a dermato-oncologist at the Inova Schar Cancer Institute, Fairfax, Virginia.
cirAEs are the most frequently reported and most visible adverse effects of checkpoint inhibition — a treatment that has emerged as a standard therapy for many malignancies since the first ICI was approved in 2011 for metastatic melanoma.
And contrary to what the phenomenon of immunosenescence might suggest, older patients are no less prone to cirAEs than younger patients. “You’d think you’d have fewer rashes and side effects as you age, but that’s not true,” said Dr. Allais, who completed a fellowship in cutaneous oncology after her dermatology residency.
A 2021 multicenter international cohort study of over 900 patients aged ≥ 80 years treated with single-agent ICIs for cancer did not find any significant differences in the development of immune-related adverse events among those younger than 85, those aged 85-89 years, and those 90 and older. Neither did the ELDERS study in the United Kingdom; this prospective observational study found similar rates of high-grade and low-grade immune toxicity in its two cohorts of patients ≥ 70 and < 70 years of age.
At the meeting, Dr. Allais, who coauthored a 2023 review of cirAEs from ICIs, reviewed recent developments and provided the following advice:
New diagnostic criteria: “Really exciting” news for more precise diagnosis and optimal therapy of cirAEs, Dr. Allais said, is a position paper published in the Journal for ImmunoTherapy of Cancer that offers consensus-based diagnostic criteria for the 10 most common types of dermatologic immune-related adverse events and an overall diagnostic framework. “Luckily, through the work of a Delphi consensus group, we can now have [more diagnostic specificity],” which is important for both clinical care and research, she said.
Most cirAEs have typically been reported nonspecifically as “rash,” but diagnosing a rash subtype is “critical in tailoring appropriate therapy that it is both effective and the least detrimental to the oncology treatment plan for patients with cancer,” the group’s coauthors wrote.
The 10 core diagnoses include psoriasis, eczematous dermatitis, vitiligo, Grover disease, eruptive atypical squamous proliferation, and bullous pemphigoid. Outside of the core diagnoses are other nonspecific presentations that require evaluation to arrive at a diagnosis, if possible, or to reveal data that can allow for targeted therapy and severity grading, the group explains in its paper.
“To prednisone or not to prednisone”: The development of cirAEs is associated with reduced mortality and improved cancer outcomes, making the use of immunosuppressants such as corticosteroids a therapeutic dilemma. “Patients who get these rashes usually do better with respect to their cancer, so the concern has been, if we affect how they respond to their immunotherapy, we may minimize that improvement in mortality,” said Dr. Allais, also assistant professor at the University of Virginia, Charlottesville, and clinical assistant professor of dermatology at George Washington University.
A widely discussed study published in 2015 reported on 254 patients with melanoma who developed an immune-related adverse event during treatment with ipilimumab — approximately one third of whom required systemic corticosteroids — and concluded that systemic corticosteroids did not affect overall survival or time to (cancer) treatment failure. This study from Memorial Sloan Kettering Cancer Center, New York City, “was the first large study looking at this question,” she said, and the subsequent message for several years in conferences and the literature was that steroids do not affect the efficacy of checkpoint inhibitors.
“But the study was not without limitations,” Dr. Allais said, “because the patients who got prednisone were mainly those with higher-grade toxicities,” while those not treated with corticosteroids had either no toxicities or low-grade toxicities. “If higher-grade toxicities were associated with better (antitumor) response, the steroids may have just [blunted] that benefit.”
The current totality of data available in the literature suggests that corticosteroids may indeed have an impact on the efficacy of ICI therapy. “Subsequent studies have come out in the community that have shown that we should probably think twice about giving prednisone to some patients, particularly within the first 50 days of ICI treatment, and that we should be mindful of the dose,” Dr. Allais said.
The takeaways from these studies — all published in the past few years — are to use prednisone early and liberally for life-threatening toxicity, to use it at the lowest dose and for the shortest course when there is not an appropriate alternative, to avoid it for diagnoses that are not treated with prednisone outside the ICI setting, and to “have a plan” for a steroid-sparing agent to use after prednisone, she said.
Dr. Allais recommends heightened consideration during the first 50 days of ICI treatment based on a multicenter retrospective study that found a significant association between use of high-dose glucocorticoids (≥ 60 mg prednisone equivalent once a day) within 8 weeks of anti–programmed cell death protein 1 (PD-1) monotherapy initiation and poorer progression-free and overall survival. The study covered a cohort of 947 patients with advanced melanoma treated with anti–PD-1 monotherapy between 2009 and 2019, 54% of whom developed immune-related adverse events.
This study and other recent studies addressing the association between steroids and survival outcomes in patients with immune-related adverse events during ICI therapy are described in Dr. Allais’ 2023 review of cirAEs from ICIs.
Approach to morbilliform eruptions: This rash is “super common” in patients on ICIs, occurring generally within 2-3 weeks of starting treatment. “It tends to be self-limited and can recur with future infusions,” Dr. Allais said.
Systemic steroids should be reserved for severe or refractory eruptions. “Usually, I treat the patients with topical steroids, and I manage their expectations (that the rash may recur with subsequent infusions), but I closely follow them up” within 2-3 weeks, she said. It’s important to rule out a severe cutaneous adverse drug eruption, of course, and to start high-dose systemic steroids immediately if necessary. “Antibiotics are a big culprit” and often can be discontinued.
Soak and smear: “I’m obsessed” with this technique of a 20-minute soak in plain water followed by application of steroid ointment, said Dr. Allais, referring to a small study published in 2005 that reported a complete response after 2 weeks in 60% of patients with psoriasis, atopic dermatitis, and other inflammatory skin conditions (none had cancer), who had failed prior systemic therapy. All patients had at least a 75% response.
The method offers a way to “avoid the systemic immunosuppression we’d get with prednisone,” she said. One just needs to make sure the older patient can get in and out of their tub safely.
ICI-induced bullous pemphigoid (BP): BP occurs more frequently in the ICI setting, compared with the general population, with a median time to development of 8.5 months after ICI initiation. It is associated in this setting with improved tumor response, but “many oncologists stop anticancer treatment because of this diagnosis,” she said.
In the supportive oncodermatology space, however, ICI-induced BP exemplifies the value of tailored treatment regimens, she said. A small multi-institutional retrospective cohort study published in 2023 identified 35 cases of ICI-BP among 5636 ICI-treated patients and found that 8 out of 11 patients who received biologic therapy (rituximab, omalizumab, or dupilumab) had a complete response to ICI-BP without flares following subsequent ICI cycles. And while statistical significance was not reached, the study showed that no cancer-related outcomes were worsened.
“If you see someone with ICI-induced BP and they have a lot of involvement, you could start them on steroids and get that steroid-sparing agent initiated for approval. ... And if IgE is elevated, you might reach for omalizumab,” said Dr. Allais, noting that her favored treatment overall is dupilumab.
Risk factors for the development of ICI-induced BP include age > 70, skin cancer, and having an initial response to ICI on first imaging, the latter of which “I find fascinating ... because imaging occurs within the first 12 weeks of treatment, but we don’t see BP popping up until 8.5 months into treatment,” she noted. “So maybe there’s a baseline risk factor that could predispose them.”
Caution with antibiotics: “I try to avoid antibiotics in the ICI setting,” Dr. Allais said, in deference to the “ever-important microbiome.” Studies have demonstrated that the microbiomes of responders to ICI treatment are different from those of nonresponders, she said.
And a “fascinating” study of patients with melanoma undergoing ICI therapy showed not only a higher abundance of Ruminococcaceae bacteria in responders vs nonresponders but a significant impact of dietary fiber. High dietary fiber was associated with significantly improved overall survival in the patients on ICI, with the most pronounced benefit in patients with good fiber intake and no probiotic use. “Even wilder, their T cells changed,” she said. “They had a high expression of genes related to T-cell activation ... so more tumor-infiltrating lymphocytes.”
A retrospective study of 568 patients with stages III and IV melanoma treated with ICI showed that those exposed to antibiotics prior to ICI had significantly worse overall survival than those not exposed to antibiotics. “Think before you give them,” Dr. Allais said. “And try to tell your older patients to eat beans and greens.”
Dr. Allais reported having no relevant disclosures.
A version of this article first appeared on Medscape.com.
WASHINGTON, DC — and with judicious usage and dosing of prednisone when deemed necessary, Blair Allais, MD, said during a session on supportive oncodermatology at the ElderDerm conference on dermatology in the older patient hosted by the George Washington University School of Medicine and Health Sciences, Washington, DC.
“It’s important when you see these patients to be as specific as possible” based on morphology and histopathology, and to treat the rashes in a similar way as in the non-ICI setting,” said Dr. Allais, a dermato-oncologist at the Inova Schar Cancer Institute, Fairfax, Virginia.
cirAEs are the most frequently reported and most visible adverse effects of checkpoint inhibition — a treatment that has emerged as a standard therapy for many malignancies since the first ICI was approved in 2011 for metastatic melanoma.
And contrary to what the phenomenon of immunosenescence might suggest, older patients are no less prone to cirAEs than younger patients. “You’d think you’d have fewer rashes and side effects as you age, but that’s not true,” said Dr. Allais, who completed a fellowship in cutaneous oncology after her dermatology residency.
A 2021 multicenter international cohort study of over 900 patients aged ≥ 80 years treated with single-agent ICIs for cancer did not find any significant differences in the development of immune-related adverse events among those younger than 85, those aged 85-89 years, and those 90 and older. Neither did the ELDERS study in the United Kingdom; this prospective observational study found similar rates of high-grade and low-grade immune toxicity in its two cohorts of patients ≥ 70 and < 70 years of age.
At the meeting, Dr. Allais, who coauthored a 2023 review of cirAEs from ICIs, reviewed recent developments and provided the following advice:
New diagnostic criteria: “Really exciting” news for more precise diagnosis and optimal therapy of cirAEs, Dr. Allais said, is a position paper published in the Journal for ImmunoTherapy of Cancer that offers consensus-based diagnostic criteria for the 10 most common types of dermatologic immune-related adverse events and an overall diagnostic framework. “Luckily, through the work of a Delphi consensus group, we can now have [more diagnostic specificity],” which is important for both clinical care and research, she said.
Most cirAEs have typically been reported nonspecifically as “rash,” but diagnosing a rash subtype is “critical in tailoring appropriate therapy that it is both effective and the least detrimental to the oncology treatment plan for patients with cancer,” the group’s coauthors wrote.
The 10 core diagnoses include psoriasis, eczematous dermatitis, vitiligo, Grover disease, eruptive atypical squamous proliferation, and bullous pemphigoid. Outside of the core diagnoses are other nonspecific presentations that require evaluation to arrive at a diagnosis, if possible, or to reveal data that can allow for targeted therapy and severity grading, the group explains in its paper.
“To prednisone or not to prednisone”: The development of cirAEs is associated with reduced mortality and improved cancer outcomes, making the use of immunosuppressants such as corticosteroids a therapeutic dilemma. “Patients who get these rashes usually do better with respect to their cancer, so the concern has been, if we affect how they respond to their immunotherapy, we may minimize that improvement in mortality,” said Dr. Allais, also assistant professor at the University of Virginia, Charlottesville, and clinical assistant professor of dermatology at George Washington University.
A widely discussed study published in 2015 reported on 254 patients with melanoma who developed an immune-related adverse event during treatment with ipilimumab — approximately one third of whom required systemic corticosteroids — and concluded that systemic corticosteroids did not affect overall survival or time to (cancer) treatment failure. This study from Memorial Sloan Kettering Cancer Center, New York City, “was the first large study looking at this question,” she said, and the subsequent message for several years in conferences and the literature was that steroids do not affect the efficacy of checkpoint inhibitors.
“But the study was not without limitations,” Dr. Allais said, “because the patients who got prednisone were mainly those with higher-grade toxicities,” while those not treated with corticosteroids had either no toxicities or low-grade toxicities. “If higher-grade toxicities were associated with better (antitumor) response, the steroids may have just [blunted] that benefit.”
The current totality of data available in the literature suggests that corticosteroids may indeed have an impact on the efficacy of ICI therapy. “Subsequent studies have come out in the community that have shown that we should probably think twice about giving prednisone to some patients, particularly within the first 50 days of ICI treatment, and that we should be mindful of the dose,” Dr. Allais said.
The takeaways from these studies — all published in the past few years — are to use prednisone early and liberally for life-threatening toxicity, to use it at the lowest dose and for the shortest course when there is not an appropriate alternative, to avoid it for diagnoses that are not treated with prednisone outside the ICI setting, and to “have a plan” for a steroid-sparing agent to use after prednisone, she said.
Dr. Allais recommends heightened consideration during the first 50 days of ICI treatment based on a multicenter retrospective study that found a significant association between use of high-dose glucocorticoids (≥ 60 mg prednisone equivalent once a day) within 8 weeks of anti–programmed cell death protein 1 (PD-1) monotherapy initiation and poorer progression-free and overall survival. The study covered a cohort of 947 patients with advanced melanoma treated with anti–PD-1 monotherapy between 2009 and 2019, 54% of whom developed immune-related adverse events.
This study and other recent studies addressing the association between steroids and survival outcomes in patients with immune-related adverse events during ICI therapy are described in Dr. Allais’ 2023 review of cirAEs from ICIs.
Approach to morbilliform eruptions: This rash is “super common” in patients on ICIs, occurring generally within 2-3 weeks of starting treatment. “It tends to be self-limited and can recur with future infusions,” Dr. Allais said.
Systemic steroids should be reserved for severe or refractory eruptions. “Usually, I treat the patients with topical steroids, and I manage their expectations (that the rash may recur with subsequent infusions), but I closely follow them up” within 2-3 weeks, she said. It’s important to rule out a severe cutaneous adverse drug eruption, of course, and to start high-dose systemic steroids immediately if necessary. “Antibiotics are a big culprit” and often can be discontinued.
Soak and smear: “I’m obsessed” with this technique of a 20-minute soak in plain water followed by application of steroid ointment, said Dr. Allais, referring to a small study published in 2005 that reported a complete response after 2 weeks in 60% of patients with psoriasis, atopic dermatitis, and other inflammatory skin conditions (none had cancer), who had failed prior systemic therapy. All patients had at least a 75% response.
The method offers a way to “avoid the systemic immunosuppression we’d get with prednisone,” she said. One just needs to make sure the older patient can get in and out of their tub safely.
ICI-induced bullous pemphigoid (BP): BP occurs more frequently in the ICI setting, compared with the general population, with a median time to development of 8.5 months after ICI initiation. It is associated in this setting with improved tumor response, but “many oncologists stop anticancer treatment because of this diagnosis,” she said.
In the supportive oncodermatology space, however, ICI-induced BP exemplifies the value of tailored treatment regimens, she said. A small multi-institutional retrospective cohort study published in 2023 identified 35 cases of ICI-BP among 5636 ICI-treated patients and found that 8 out of 11 patients who received biologic therapy (rituximab, omalizumab, or dupilumab) had a complete response to ICI-BP without flares following subsequent ICI cycles. And while statistical significance was not reached, the study showed that no cancer-related outcomes were worsened.
“If you see someone with ICI-induced BP and they have a lot of involvement, you could start them on steroids and get that steroid-sparing agent initiated for approval. ... And if IgE is elevated, you might reach for omalizumab,” said Dr. Allais, noting that her favored treatment overall is dupilumab.
Risk factors for the development of ICI-induced BP include age > 70, skin cancer, and having an initial response to ICI on first imaging, the latter of which “I find fascinating ... because imaging occurs within the first 12 weeks of treatment, but we don’t see BP popping up until 8.5 months into treatment,” she noted. “So maybe there’s a baseline risk factor that could predispose them.”
Caution with antibiotics: “I try to avoid antibiotics in the ICI setting,” Dr. Allais said, in deference to the “ever-important microbiome.” Studies have demonstrated that the microbiomes of responders to ICI treatment are different from those of nonresponders, she said.
And a “fascinating” study of patients with melanoma undergoing ICI therapy showed not only a higher abundance of Ruminococcaceae bacteria in responders vs nonresponders but a significant impact of dietary fiber. High dietary fiber was associated with significantly improved overall survival in the patients on ICI, with the most pronounced benefit in patients with good fiber intake and no probiotic use. “Even wilder, their T cells changed,” she said. “They had a high expression of genes related to T-cell activation ... so more tumor-infiltrating lymphocytes.”
A retrospective study of 568 patients with stages III and IV melanoma treated with ICI showed that those exposed to antibiotics prior to ICI had significantly worse overall survival than those not exposed to antibiotics. “Think before you give them,” Dr. Allais said. “And try to tell your older patients to eat beans and greens.”
Dr. Allais reported having no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM ELDERDERM 2024
Jeffrey Weber, MD, PhD, Giant of Cancer Care, Dies
Dr. Weber, a melanoma and cancer immunotherapy specialist, served as deputy director of the Laura and Isaac Perlmutter Cancer Center at New York University (NYU) Langone Medical Center in New York City. He also held positions as the Laura and Isaac Perlmutter Professor of Oncology in the Department of Medicine at the NYU Grossman School of Medicine, director of the Experimental Therapeutics Program, and co-leader of the Clinical Melanoma Program Board at NYU Langone Health.
Dr. Weber was a principal investigator on many studies, including pivotal clinical drug trials in melanoma and trials focused on managing autoimmune side effects from immunotherapy. He published more than 150 articles in top peer-reviewed journals.
For many years, Dr. Weber hosted the popular “Weber on Oncology” series of video contributions for Medscape Oncology, sharing updates and insights on noteworthy research and breakthroughs in melanoma.
“The Melanoma Research Alliance mourns the passing of Dr. Jeffrey S. Weber, a true pioneer in the field of cancer immunotherapy and an extraordinary leader in melanoma research. His contributions have forever changed the landscape of melanoma treatment, bringing groundbreaking advances from the lab into clinical practice and offering hope to countless patients,” the Melanoma Research Alliance posted on LinkedIn.
Many X users also shared condolences and memories of Dr. Weber, praising his numerous contributions and accomplishments.
“[Cancer Research Institute] mourns the loss of Dr. Jeffrey S. Weber ... [a]s an accomplished physician scientist, Dr. Weber drove advances in melanoma research, and played an active role in educating patients about the lifesaving power of immunotherapy,” the Cancer Research Institute posted.
A colleague noted that “[h]e was involved in the early days of cytokine and cell therapy and most recently led studies of personalized vaccines for melanoma patients. ... He was a great friend and colleague to many of us in the melanoma and immunotherapy field and we will remember him as a pioneer, thought leader and compassionate physician.”
A version of this article first appeared on Medscape.com.
Dr. Weber, a melanoma and cancer immunotherapy specialist, served as deputy director of the Laura and Isaac Perlmutter Cancer Center at New York University (NYU) Langone Medical Center in New York City. He also held positions as the Laura and Isaac Perlmutter Professor of Oncology in the Department of Medicine at the NYU Grossman School of Medicine, director of the Experimental Therapeutics Program, and co-leader of the Clinical Melanoma Program Board at NYU Langone Health.
Dr. Weber was a principal investigator on many studies, including pivotal clinical drug trials in melanoma and trials focused on managing autoimmune side effects from immunotherapy. He published more than 150 articles in top peer-reviewed journals.
For many years, Dr. Weber hosted the popular “Weber on Oncology” series of video contributions for Medscape Oncology, sharing updates and insights on noteworthy research and breakthroughs in melanoma.
“The Melanoma Research Alliance mourns the passing of Dr. Jeffrey S. Weber, a true pioneer in the field of cancer immunotherapy and an extraordinary leader in melanoma research. His contributions have forever changed the landscape of melanoma treatment, bringing groundbreaking advances from the lab into clinical practice and offering hope to countless patients,” the Melanoma Research Alliance posted on LinkedIn.
Many X users also shared condolences and memories of Dr. Weber, praising his numerous contributions and accomplishments.
“[Cancer Research Institute] mourns the loss of Dr. Jeffrey S. Weber ... [a]s an accomplished physician scientist, Dr. Weber drove advances in melanoma research, and played an active role in educating patients about the lifesaving power of immunotherapy,” the Cancer Research Institute posted.
A colleague noted that “[h]e was involved in the early days of cytokine and cell therapy and most recently led studies of personalized vaccines for melanoma patients. ... He was a great friend and colleague to many of us in the melanoma and immunotherapy field and we will remember him as a pioneer, thought leader and compassionate physician.”
A version of this article first appeared on Medscape.com.
Dr. Weber, a melanoma and cancer immunotherapy specialist, served as deputy director of the Laura and Isaac Perlmutter Cancer Center at New York University (NYU) Langone Medical Center in New York City. He also held positions as the Laura and Isaac Perlmutter Professor of Oncology in the Department of Medicine at the NYU Grossman School of Medicine, director of the Experimental Therapeutics Program, and co-leader of the Clinical Melanoma Program Board at NYU Langone Health.
Dr. Weber was a principal investigator on many studies, including pivotal clinical drug trials in melanoma and trials focused on managing autoimmune side effects from immunotherapy. He published more than 150 articles in top peer-reviewed journals.
For many years, Dr. Weber hosted the popular “Weber on Oncology” series of video contributions for Medscape Oncology, sharing updates and insights on noteworthy research and breakthroughs in melanoma.
“The Melanoma Research Alliance mourns the passing of Dr. Jeffrey S. Weber, a true pioneer in the field of cancer immunotherapy and an extraordinary leader in melanoma research. His contributions have forever changed the landscape of melanoma treatment, bringing groundbreaking advances from the lab into clinical practice and offering hope to countless patients,” the Melanoma Research Alliance posted on LinkedIn.
Many X users also shared condolences and memories of Dr. Weber, praising his numerous contributions and accomplishments.
“[Cancer Research Institute] mourns the loss of Dr. Jeffrey S. Weber ... [a]s an accomplished physician scientist, Dr. Weber drove advances in melanoma research, and played an active role in educating patients about the lifesaving power of immunotherapy,” the Cancer Research Institute posted.
A colleague noted that “[h]e was involved in the early days of cytokine and cell therapy and most recently led studies of personalized vaccines for melanoma patients. ... He was a great friend and colleague to many of us in the melanoma and immunotherapy field and we will remember him as a pioneer, thought leader and compassionate physician.”
A version of this article first appeared on Medscape.com.
Second Treatment for Prurigo Nodularis Approved by FDA
On August 13, 2024, the
A first-in-class monoclonal antibody specifically designed to inhibit interleukin (IL)–31 signaling, nemolizumab, will be available in a prefilled pen for subcutaneous injection and will be marketed as Nemluvio. It is currently under FDA review for treating atopic dermatitis in adolescents and adults.
Approval for PN is based on data from the phase 3 OLYMPIA clinical trial program, which evaluated the efficacy and safety of nemolizumab administered subcutaneously every 4 weeks in 560 patients with PN, according to a press release from Galderma, the manufacturer.
According to the press release, in OLYMPIA 1 and OLYMPIA 2, 58% and 56% of patients, respectively, achieved at least a 4-point reduction in itch intensity at week 16 as measured by the Peak Pruritus Numerical Rating Scale, compared with 16% in both placebo groups (P < .0001). At the same time, 26% and 38% of nemolizumab-treated patients reached clearance or almost-clearance of skin lesions on the Investigator Global Assessment score at week 16, compared with 7% and 11% in the placebo groups (P < .0001).
According to the company press release, the most common side effects of nemolizumab are headache and rashes in the form of eczema, atopic dermatitis, and nummular eczema.
“By inhibiting the signaling of IL-31, Nemluvio addresses a key driver of prurigo nodularis, safely and effectively improving itch as well as skin nodules,” Shawn G. Kwatra, MD, PhD, professor and chair of dermatology at the University of Maryland School of Medicine, Baltimore, and lead investigator of the OLYMPIA program, stated in the press release.
The regulatory submission of nemolizumab in atopic dermatitis is based on data from the phase 3 ARCADIA clinical trial program, which evaluated the efficacy and safety of nemolizumab administered subcutaneously every 4 weeks in adolescents and adults with moderate to severe atopic dermatitis. A decision on approval for this indication from the FDA is expected in December 2024.
In September 2022, dupilumab became the first FDA-approved treatment for PN in the United States.
A version of this article first appeared on Medscape.com.
On August 13, 2024, the
A first-in-class monoclonal antibody specifically designed to inhibit interleukin (IL)–31 signaling, nemolizumab, will be available in a prefilled pen for subcutaneous injection and will be marketed as Nemluvio. It is currently under FDA review for treating atopic dermatitis in adolescents and adults.
Approval for PN is based on data from the phase 3 OLYMPIA clinical trial program, which evaluated the efficacy and safety of nemolizumab administered subcutaneously every 4 weeks in 560 patients with PN, according to a press release from Galderma, the manufacturer.
According to the press release, in OLYMPIA 1 and OLYMPIA 2, 58% and 56% of patients, respectively, achieved at least a 4-point reduction in itch intensity at week 16 as measured by the Peak Pruritus Numerical Rating Scale, compared with 16% in both placebo groups (P < .0001). At the same time, 26% and 38% of nemolizumab-treated patients reached clearance or almost-clearance of skin lesions on the Investigator Global Assessment score at week 16, compared with 7% and 11% in the placebo groups (P < .0001).
According to the company press release, the most common side effects of nemolizumab are headache and rashes in the form of eczema, atopic dermatitis, and nummular eczema.
“By inhibiting the signaling of IL-31, Nemluvio addresses a key driver of prurigo nodularis, safely and effectively improving itch as well as skin nodules,” Shawn G. Kwatra, MD, PhD, professor and chair of dermatology at the University of Maryland School of Medicine, Baltimore, and lead investigator of the OLYMPIA program, stated in the press release.
The regulatory submission of nemolizumab in atopic dermatitis is based on data from the phase 3 ARCADIA clinical trial program, which evaluated the efficacy and safety of nemolizumab administered subcutaneously every 4 weeks in adolescents and adults with moderate to severe atopic dermatitis. A decision on approval for this indication from the FDA is expected in December 2024.
In September 2022, dupilumab became the first FDA-approved treatment for PN in the United States.
A version of this article first appeared on Medscape.com.
On August 13, 2024, the
A first-in-class monoclonal antibody specifically designed to inhibit interleukin (IL)–31 signaling, nemolizumab, will be available in a prefilled pen for subcutaneous injection and will be marketed as Nemluvio. It is currently under FDA review for treating atopic dermatitis in adolescents and adults.
Approval for PN is based on data from the phase 3 OLYMPIA clinical trial program, which evaluated the efficacy and safety of nemolizumab administered subcutaneously every 4 weeks in 560 patients with PN, according to a press release from Galderma, the manufacturer.
According to the press release, in OLYMPIA 1 and OLYMPIA 2, 58% and 56% of patients, respectively, achieved at least a 4-point reduction in itch intensity at week 16 as measured by the Peak Pruritus Numerical Rating Scale, compared with 16% in both placebo groups (P < .0001). At the same time, 26% and 38% of nemolizumab-treated patients reached clearance or almost-clearance of skin lesions on the Investigator Global Assessment score at week 16, compared with 7% and 11% in the placebo groups (P < .0001).
According to the company press release, the most common side effects of nemolizumab are headache and rashes in the form of eczema, atopic dermatitis, and nummular eczema.
“By inhibiting the signaling of IL-31, Nemluvio addresses a key driver of prurigo nodularis, safely and effectively improving itch as well as skin nodules,” Shawn G. Kwatra, MD, PhD, professor and chair of dermatology at the University of Maryland School of Medicine, Baltimore, and lead investigator of the OLYMPIA program, stated in the press release.
The regulatory submission of nemolizumab in atopic dermatitis is based on data from the phase 3 ARCADIA clinical trial program, which evaluated the efficacy and safety of nemolizumab administered subcutaneously every 4 weeks in adolescents and adults with moderate to severe atopic dermatitis. A decision on approval for this indication from the FDA is expected in December 2024.
In September 2022, dupilumab became the first FDA-approved treatment for PN in the United States.
A version of this article first appeared on Medscape.com.
PrEP Prescription Pickups Vary With Prescriber Specialty
Preexposure prophylaxis prescription reversals and abandonments were lower for patients seen by primary care clinicians than by other non–infectious disease clinicians, based on data from approximately 37,000 individuals.
Although preexposure prophylaxis (PrEP) has been associated with a reduced risk of HIV (human immunodeficiency virus) infection when used as prescribed, the association between PrEP prescription pickup and specialty of the prescribing clinician has not been examined, wrote Lorraine T. Dean, ScD, an epidemiologist at Johns Hopkins University, Baltimore, Maryland, and colleagues.
“HIV PrEP is highly effective at preventing new HIV cases, and while use is on the rise, is still used much less than it should be by people who are at risk of HIV,” Dr. Dean said in an interview. “This study is helpful in pinpointing who is at risk for not picking up PrEP and in helping us think through how to reach them so that they can be better positioned to get PrEP,” she said.
In a study published in JAMA Internal Medicine, the researchers reviewed data for PrEP care. The study population included 37,003 patients aged 18 years and older who received new insurer-approved PrEP prescriptions between 2015 and 2019. Most of the patients (77%) ranged in age from 25 to 64 years; 88% were male.
Pharmacy claims data were matched with clinician data from the US National Plan and Provider Enumeration System.
Clinicians were divided into three groups: primary care providers (PCPs), infectious disease specialists (IDs), and other specialists (defined as any clinician prescribing PrEP but not classified as a PCP or an ID specialist). The main binary outcomes were prescription reversal (defined as when a patient failed to retrieve a prescription) and abandonment (defined as when a patient neglected to pick up a prescription for 1 year).
Overall, of 24,604 patients 67% received prescriptions from PCPs, 3,571 (10%) received prescriptions from ID specialists, and 8828 (24%) received prescriptions from other specialty clinicians.
The prevalence of reversals for patients seen by PCPs, ID specialists, and other specialty clinicians was 18%, 18%, and 25%, respectively. The prevalence of abandonments by clinician group was 12%, 12%, and 20%, respectively.
In a regression analysis, patients prescribed PrEP by ID specialists had 10% lower odds of reversals and 12% lower odds of abandonments compared to those seen by PCPs (odds ratio 0.90 and 0.88, respectively). However, patients seen by other clinicians (not primary care or ID) were 33% and 54% more likely to have reversals and abandonments, respectively, compared with those seen by PCPs.
Many patients at risk for HIV first see a PCP and then are referred to a specialist, such as an ID physician, Dr. Dean said. “The patients who take the time to then follow up with a specialist may be most motivated and able to follow through with the specialist’s request, in this case, accessing their PrEP prescription,” she said. In the current study, the researchers were most surprised by how many other specialty providers are involved in PrEP care, which is very positive given the effectiveness of the medication, she noted.
“Our results suggest that a wide range of prescribers, regardless of specialty, should be equipped to prescribe PrEP as well as offer PrEP counseling,” Dr. Dean said. A key takeaway for clinicians is that PrEP should have no cost for the majority of patients in the United States, she emphasized. The absence of cost expands the population who should be interested and able to access PrEP, she said. Therefore, providers should be prepared to recommend PrEP to eligible patients, and seek training or continuing medical education for themselves so they feel equipped to prescribe and counsel patients on PrEP, she said.
“One limitation of this work is that, while it can point to what is happening, it cannot tell us why the reversals are happening; what is the reason patients prescribed by certain providers are more or less likely to get their PrEP,” Dr. Dean explained. “We have tried to do interviews with patients to understand why this might be happening, but it’s hard to find people who aren’t showing up to do something, compared to finding people who are showing up to do something,” she said. Alternatively, researchers could interview providers to understand their perspective on why differences in prescription pickups occur across specialties, she said.
Looking ahead, “a national PrEP program that includes elements of required clinician training could be beneficial, and research on how a national PrEP program could be implemented and impact HIV rates would be helpful in considering this strategy of prevention,” said Dr. Dean.
Support All Prescribers to Increase PrEP Adherence
Differences in uptake of PrEP prescriptions may be explained by the different populations seen by various specialties, Meredith Green, MD, of Indiana University School of Medicine, Indianapolis, and Lona Mody, MD, of the University of Michigan, Ann Arbor, wrote in an accompanying editorial. However, the key question is how to support all prescribers and promote initiation and adherence to PrEP, they said.
Considerations include whether people at risk for HIV prefer to discuss PrEP with a clinician they already know, vs. a new specialist, but many PCPs are not familiar with the latest PrEP guidelines, they said.
“Interventions that support PrEP provision by PCPs, especially since they prescribed the largest proportion of PrEP prescriptions, can accelerate the uptake of PrEP,” the editorialists wrote.
“Supporting a diverse clinician workforce reflective of communities most impacted by HIV will remain critical, as will acknowledging and addressing HIV stigma,” they said. Educational interventions, including online programs and specialist access for complex cases, would help as well, they said. The approval of additional PrEP agents since the current study was conducted make it even more important to support PrEP prescribers and promote treatment adherence for those at risk for HIV, Dr. Green and Dr. Mody emphasized.
The study was funded by the National Institutes of Health. Dr. Dean had no financial conflicts to disclose. Dr. Green disclosed grants from Gilead and royalties from Wolters Kluwer unrelated to the current study; she also disclosed serving on the Centers for Disease Control and Prevention/Health Resources and Services Administration advisory committee on HIV, viral hepatitis, and sexually transmitted infection prevention and treatment. Dr. Mody disclosed grants from the US National Institute on Aging, Veterans Affairs, Centers for Disease Control and Prevention, NanoVibronix, and UpToDate unrelated to the current study.
Preexposure prophylaxis prescription reversals and abandonments were lower for patients seen by primary care clinicians than by other non–infectious disease clinicians, based on data from approximately 37,000 individuals.
Although preexposure prophylaxis (PrEP) has been associated with a reduced risk of HIV (human immunodeficiency virus) infection when used as prescribed, the association between PrEP prescription pickup and specialty of the prescribing clinician has not been examined, wrote Lorraine T. Dean, ScD, an epidemiologist at Johns Hopkins University, Baltimore, Maryland, and colleagues.
“HIV PrEP is highly effective at preventing new HIV cases, and while use is on the rise, is still used much less than it should be by people who are at risk of HIV,” Dr. Dean said in an interview. “This study is helpful in pinpointing who is at risk for not picking up PrEP and in helping us think through how to reach them so that they can be better positioned to get PrEP,” she said.
In a study published in JAMA Internal Medicine, the researchers reviewed data for PrEP care. The study population included 37,003 patients aged 18 years and older who received new insurer-approved PrEP prescriptions between 2015 and 2019. Most of the patients (77%) ranged in age from 25 to 64 years; 88% were male.
Pharmacy claims data were matched with clinician data from the US National Plan and Provider Enumeration System.
Clinicians were divided into three groups: primary care providers (PCPs), infectious disease specialists (IDs), and other specialists (defined as any clinician prescribing PrEP but not classified as a PCP or an ID specialist). The main binary outcomes were prescription reversal (defined as when a patient failed to retrieve a prescription) and abandonment (defined as when a patient neglected to pick up a prescription for 1 year).
Overall, of 24,604 patients 67% received prescriptions from PCPs, 3,571 (10%) received prescriptions from ID specialists, and 8828 (24%) received prescriptions from other specialty clinicians.
The prevalence of reversals for patients seen by PCPs, ID specialists, and other specialty clinicians was 18%, 18%, and 25%, respectively. The prevalence of abandonments by clinician group was 12%, 12%, and 20%, respectively.
In a regression analysis, patients prescribed PrEP by ID specialists had 10% lower odds of reversals and 12% lower odds of abandonments compared to those seen by PCPs (odds ratio 0.90 and 0.88, respectively). However, patients seen by other clinicians (not primary care or ID) were 33% and 54% more likely to have reversals and abandonments, respectively, compared with those seen by PCPs.
Many patients at risk for HIV first see a PCP and then are referred to a specialist, such as an ID physician, Dr. Dean said. “The patients who take the time to then follow up with a specialist may be most motivated and able to follow through with the specialist’s request, in this case, accessing their PrEP prescription,” she said. In the current study, the researchers were most surprised by how many other specialty providers are involved in PrEP care, which is very positive given the effectiveness of the medication, she noted.
“Our results suggest that a wide range of prescribers, regardless of specialty, should be equipped to prescribe PrEP as well as offer PrEP counseling,” Dr. Dean said. A key takeaway for clinicians is that PrEP should have no cost for the majority of patients in the United States, she emphasized. The absence of cost expands the population who should be interested and able to access PrEP, she said. Therefore, providers should be prepared to recommend PrEP to eligible patients, and seek training or continuing medical education for themselves so they feel equipped to prescribe and counsel patients on PrEP, she said.
“One limitation of this work is that, while it can point to what is happening, it cannot tell us why the reversals are happening; what is the reason patients prescribed by certain providers are more or less likely to get their PrEP,” Dr. Dean explained. “We have tried to do interviews with patients to understand why this might be happening, but it’s hard to find people who aren’t showing up to do something, compared to finding people who are showing up to do something,” she said. Alternatively, researchers could interview providers to understand their perspective on why differences in prescription pickups occur across specialties, she said.
Looking ahead, “a national PrEP program that includes elements of required clinician training could be beneficial, and research on how a national PrEP program could be implemented and impact HIV rates would be helpful in considering this strategy of prevention,” said Dr. Dean.
Support All Prescribers to Increase PrEP Adherence
Differences in uptake of PrEP prescriptions may be explained by the different populations seen by various specialties, Meredith Green, MD, of Indiana University School of Medicine, Indianapolis, and Lona Mody, MD, of the University of Michigan, Ann Arbor, wrote in an accompanying editorial. However, the key question is how to support all prescribers and promote initiation and adherence to PrEP, they said.
Considerations include whether people at risk for HIV prefer to discuss PrEP with a clinician they already know, vs. a new specialist, but many PCPs are not familiar with the latest PrEP guidelines, they said.
“Interventions that support PrEP provision by PCPs, especially since they prescribed the largest proportion of PrEP prescriptions, can accelerate the uptake of PrEP,” the editorialists wrote.
“Supporting a diverse clinician workforce reflective of communities most impacted by HIV will remain critical, as will acknowledging and addressing HIV stigma,” they said. Educational interventions, including online programs and specialist access for complex cases, would help as well, they said. The approval of additional PrEP agents since the current study was conducted make it even more important to support PrEP prescribers and promote treatment adherence for those at risk for HIV, Dr. Green and Dr. Mody emphasized.
The study was funded by the National Institutes of Health. Dr. Dean had no financial conflicts to disclose. Dr. Green disclosed grants from Gilead and royalties from Wolters Kluwer unrelated to the current study; she also disclosed serving on the Centers for Disease Control and Prevention/Health Resources and Services Administration advisory committee on HIV, viral hepatitis, and sexually transmitted infection prevention and treatment. Dr. Mody disclosed grants from the US National Institute on Aging, Veterans Affairs, Centers for Disease Control and Prevention, NanoVibronix, and UpToDate unrelated to the current study.
Preexposure prophylaxis prescription reversals and abandonments were lower for patients seen by primary care clinicians than by other non–infectious disease clinicians, based on data from approximately 37,000 individuals.
Although preexposure prophylaxis (PrEP) has been associated with a reduced risk of HIV (human immunodeficiency virus) infection when used as prescribed, the association between PrEP prescription pickup and specialty of the prescribing clinician has not been examined, wrote Lorraine T. Dean, ScD, an epidemiologist at Johns Hopkins University, Baltimore, Maryland, and colleagues.
“HIV PrEP is highly effective at preventing new HIV cases, and while use is on the rise, is still used much less than it should be by people who are at risk of HIV,” Dr. Dean said in an interview. “This study is helpful in pinpointing who is at risk for not picking up PrEP and in helping us think through how to reach them so that they can be better positioned to get PrEP,” she said.
In a study published in JAMA Internal Medicine, the researchers reviewed data for PrEP care. The study population included 37,003 patients aged 18 years and older who received new insurer-approved PrEP prescriptions between 2015 and 2019. Most of the patients (77%) ranged in age from 25 to 64 years; 88% were male.
Pharmacy claims data were matched with clinician data from the US National Plan and Provider Enumeration System.
Clinicians were divided into three groups: primary care providers (PCPs), infectious disease specialists (IDs), and other specialists (defined as any clinician prescribing PrEP but not classified as a PCP or an ID specialist). The main binary outcomes were prescription reversal (defined as when a patient failed to retrieve a prescription) and abandonment (defined as when a patient neglected to pick up a prescription for 1 year).
Overall, of 24,604 patients 67% received prescriptions from PCPs, 3,571 (10%) received prescriptions from ID specialists, and 8828 (24%) received prescriptions from other specialty clinicians.
The prevalence of reversals for patients seen by PCPs, ID specialists, and other specialty clinicians was 18%, 18%, and 25%, respectively. The prevalence of abandonments by clinician group was 12%, 12%, and 20%, respectively.
In a regression analysis, patients prescribed PrEP by ID specialists had 10% lower odds of reversals and 12% lower odds of abandonments compared to those seen by PCPs (odds ratio 0.90 and 0.88, respectively). However, patients seen by other clinicians (not primary care or ID) were 33% and 54% more likely to have reversals and abandonments, respectively, compared with those seen by PCPs.
Many patients at risk for HIV first see a PCP and then are referred to a specialist, such as an ID physician, Dr. Dean said. “The patients who take the time to then follow up with a specialist may be most motivated and able to follow through with the specialist’s request, in this case, accessing their PrEP prescription,” she said. In the current study, the researchers were most surprised by how many other specialty providers are involved in PrEP care, which is very positive given the effectiveness of the medication, she noted.
“Our results suggest that a wide range of prescribers, regardless of specialty, should be equipped to prescribe PrEP as well as offer PrEP counseling,” Dr. Dean said. A key takeaway for clinicians is that PrEP should have no cost for the majority of patients in the United States, she emphasized. The absence of cost expands the population who should be interested and able to access PrEP, she said. Therefore, providers should be prepared to recommend PrEP to eligible patients, and seek training or continuing medical education for themselves so they feel equipped to prescribe and counsel patients on PrEP, she said.
“One limitation of this work is that, while it can point to what is happening, it cannot tell us why the reversals are happening; what is the reason patients prescribed by certain providers are more or less likely to get their PrEP,” Dr. Dean explained. “We have tried to do interviews with patients to understand why this might be happening, but it’s hard to find people who aren’t showing up to do something, compared to finding people who are showing up to do something,” she said. Alternatively, researchers could interview providers to understand their perspective on why differences in prescription pickups occur across specialties, she said.
Looking ahead, “a national PrEP program that includes elements of required clinician training could be beneficial, and research on how a national PrEP program could be implemented and impact HIV rates would be helpful in considering this strategy of prevention,” said Dr. Dean.
Support All Prescribers to Increase PrEP Adherence
Differences in uptake of PrEP prescriptions may be explained by the different populations seen by various specialties, Meredith Green, MD, of Indiana University School of Medicine, Indianapolis, and Lona Mody, MD, of the University of Michigan, Ann Arbor, wrote in an accompanying editorial. However, the key question is how to support all prescribers and promote initiation and adherence to PrEP, they said.
Considerations include whether people at risk for HIV prefer to discuss PrEP with a clinician they already know, vs. a new specialist, but many PCPs are not familiar with the latest PrEP guidelines, they said.
“Interventions that support PrEP provision by PCPs, especially since they prescribed the largest proportion of PrEP prescriptions, can accelerate the uptake of PrEP,” the editorialists wrote.
“Supporting a diverse clinician workforce reflective of communities most impacted by HIV will remain critical, as will acknowledging and addressing HIV stigma,” they said. Educational interventions, including online programs and specialist access for complex cases, would help as well, they said. The approval of additional PrEP agents since the current study was conducted make it even more important to support PrEP prescribers and promote treatment adherence for those at risk for HIV, Dr. Green and Dr. Mody emphasized.
The study was funded by the National Institutes of Health. Dr. Dean had no financial conflicts to disclose. Dr. Green disclosed grants from Gilead and royalties from Wolters Kluwer unrelated to the current study; she also disclosed serving on the Centers for Disease Control and Prevention/Health Resources and Services Administration advisory committee on HIV, viral hepatitis, and sexually transmitted infection prevention and treatment. Dr. Mody disclosed grants from the US National Institute on Aging, Veterans Affairs, Centers for Disease Control and Prevention, NanoVibronix, and UpToDate unrelated to the current study.
FROM JAMA INTERNAL MEDICINE
Low HPV Vaccination in the United States Is a Public Health ‘Failure’
This transcript has been edited for clarity.
I would like to briefly discuss what I consider to be a very discouraging report and one that I believe we as an oncology society and, quite frankly, as a medical community need to deal with.
The manuscript I’m referring to is from the United States Department of Health and Human Services, titled, “Human Papillomavirus Vaccination Coverage in Children Ages 9-17 Years: United States, 2022.” This particular analysis looked at the coverage of both men and women — young boys and young girls, I would say — receiving at least one dose of the recommended human papillomavirus (HPV) vaccination.
Since 2006, girls have been recommended to receive HPV vaccination; for boys, it’s been since 2011. Certainly, the time period that we’re considering falls within the recommendations based on overwhelmingly positive data. Now, today, still, the recommendation is for more than one vaccine. Obviously, there may be evidence in the future that a single vaccination may be acceptable or appropriate. But today, it’s more than one.
In this particular analysis, they were looking at just a single vaccination. The vaccines have targeted young individuals, both male and female children aged 11-12 years, but it’s certainly acceptable to look starting at age 9.
What is the bottom line? At least one dose of the HPV vaccination was given to 38.6% of children aged 9-17 years in 2022. We are talking about a cancer-preventive vaccine, which on the basis of population-based data in the United States, but also in other countries, is incredibly effective in preventing HPV-associated cancers. This not only includes cervical cancer, but also a large percentage of head and neck cancers.
For this vaccine, which is incredibly safe and incredibly effective, in this country, only 38.6% have received even a single dose. It is noted that the individuals with private insurance had a higher rate, at 41.5%, than individuals with no insurance, at only 20.7%.
In my opinion, this is clearly a failure of our public health establishment at all levels. My own focus has been in gynecologic cancers. I’ve seen young women with advanced cervical cancer, and this is a disease we can prevent. Yet, this is where we are.
For those of you who are interested in cancer prevention or public health, I think this is a very sobering statistic. It’s my plea and my hope that we can, as a society, somehow do something about it.
I thank you for listening. I would encourage you to think about this question if you’re in this area.
Dr. Markman, professor, Department of Medical Oncology and Therapeutics Research, City of Hope, Duarte, California, and president of Medicine & Science, City of Hope Atlanta, Chicago, and Phoenix, disclosed ties with GlaxoSmithKline and AstraZeneca.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I would like to briefly discuss what I consider to be a very discouraging report and one that I believe we as an oncology society and, quite frankly, as a medical community need to deal with.
The manuscript I’m referring to is from the United States Department of Health and Human Services, titled, “Human Papillomavirus Vaccination Coverage in Children Ages 9-17 Years: United States, 2022.” This particular analysis looked at the coverage of both men and women — young boys and young girls, I would say — receiving at least one dose of the recommended human papillomavirus (HPV) vaccination.
Since 2006, girls have been recommended to receive HPV vaccination; for boys, it’s been since 2011. Certainly, the time period that we’re considering falls within the recommendations based on overwhelmingly positive data. Now, today, still, the recommendation is for more than one vaccine. Obviously, there may be evidence in the future that a single vaccination may be acceptable or appropriate. But today, it’s more than one.
In this particular analysis, they were looking at just a single vaccination. The vaccines have targeted young individuals, both male and female children aged 11-12 years, but it’s certainly acceptable to look starting at age 9.
What is the bottom line? At least one dose of the HPV vaccination was given to 38.6% of children aged 9-17 years in 2022. We are talking about a cancer-preventive vaccine, which on the basis of population-based data in the United States, but also in other countries, is incredibly effective in preventing HPV-associated cancers. This not only includes cervical cancer, but also a large percentage of head and neck cancers.
For this vaccine, which is incredibly safe and incredibly effective, in this country, only 38.6% have received even a single dose. It is noted that the individuals with private insurance had a higher rate, at 41.5%, than individuals with no insurance, at only 20.7%.
In my opinion, this is clearly a failure of our public health establishment at all levels. My own focus has been in gynecologic cancers. I’ve seen young women with advanced cervical cancer, and this is a disease we can prevent. Yet, this is where we are.
For those of you who are interested in cancer prevention or public health, I think this is a very sobering statistic. It’s my plea and my hope that we can, as a society, somehow do something about it.
I thank you for listening. I would encourage you to think about this question if you’re in this area.
Dr. Markman, professor, Department of Medical Oncology and Therapeutics Research, City of Hope, Duarte, California, and president of Medicine & Science, City of Hope Atlanta, Chicago, and Phoenix, disclosed ties with GlaxoSmithKline and AstraZeneca.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I would like to briefly discuss what I consider to be a very discouraging report and one that I believe we as an oncology society and, quite frankly, as a medical community need to deal with.
The manuscript I’m referring to is from the United States Department of Health and Human Services, titled, “Human Papillomavirus Vaccination Coverage in Children Ages 9-17 Years: United States, 2022.” This particular analysis looked at the coverage of both men and women — young boys and young girls, I would say — receiving at least one dose of the recommended human papillomavirus (HPV) vaccination.
Since 2006, girls have been recommended to receive HPV vaccination; for boys, it’s been since 2011. Certainly, the time period that we’re considering falls within the recommendations based on overwhelmingly positive data. Now, today, still, the recommendation is for more than one vaccine. Obviously, there may be evidence in the future that a single vaccination may be acceptable or appropriate. But today, it’s more than one.
In this particular analysis, they were looking at just a single vaccination. The vaccines have targeted young individuals, both male and female children aged 11-12 years, but it’s certainly acceptable to look starting at age 9.
What is the bottom line? At least one dose of the HPV vaccination was given to 38.6% of children aged 9-17 years in 2022. We are talking about a cancer-preventive vaccine, which on the basis of population-based data in the United States, but also in other countries, is incredibly effective in preventing HPV-associated cancers. This not only includes cervical cancer, but also a large percentage of head and neck cancers.
For this vaccine, which is incredibly safe and incredibly effective, in this country, only 38.6% have received even a single dose. It is noted that the individuals with private insurance had a higher rate, at 41.5%, than individuals with no insurance, at only 20.7%.
In my opinion, this is clearly a failure of our public health establishment at all levels. My own focus has been in gynecologic cancers. I’ve seen young women with advanced cervical cancer, and this is a disease we can prevent. Yet, this is where we are.
For those of you who are interested in cancer prevention or public health, I think this is a very sobering statistic. It’s my plea and my hope that we can, as a society, somehow do something about it.
I thank you for listening. I would encourage you to think about this question if you’re in this area.
Dr. Markman, professor, Department of Medical Oncology and Therapeutics Research, City of Hope, Duarte, California, and president of Medicine & Science, City of Hope Atlanta, Chicago, and Phoenix, disclosed ties with GlaxoSmithKline and AstraZeneca.
A version of this article appeared on Medscape.com.
Storybooks Can Help Children Deal with Skin Conditions
TORONTO —
So far, “the study demonstrates that these books have value to patients and families,” one of the study authors, Sonia Havele, MD, a pediatrician and dermatology resident at Children’s Mercy Hospital Kansas City, Kansas City, Missouri, said in an interview.
“There are tools to help kids cope with their skin conditions, but we’re underutilizing them,” she added. “And part of the reason we’re underutilizing storybooks is that we just don’t know what’s out there.” For the study, the researchers received funding to purchase 18 “creative and thoughtful” storybooks related to pediatric skin conditions, reviewed by at least two pediatric dermatologists before being selected, which are just a sample of related books that are available.
The study results were presented as a poster at the annual meeting of the Society for Pediatric Dermatology.
Children with visible skin conditions, which can include port-wine stains, capillary malformations, and congenital moles, may be subjected to teasing or bullying at school, and the conditions can also affect their quality of life.
Beauty and the Birthmark
The books include one titled “Beauty with a Birthmark” and another, “My Hair Went on Vacation.” An illustrated book, “Just Ask: Be Different, Be Brave, Be You,” by US Supreme Court Justice Sonia Sotomayor, offers tips on how to answer common questions about someone’s appearance.
Dr. Havele said that Justice Sotomayor’s book “empowers kids, their siblings, their classmates ... to ask questions, and it teaches patients not to be afraid of those questions, and to really lean into educating their peers, and their family members.”
“Kids are really just curious,” she added. “They’ll make comments like: ‘Hey, what’s that spot on your face?’ Or, they’ll ask about vitiligo because they’ve never seen somebody with it before.”
To evaluate the psychosocial impact of these types of books for children with visible skin conditions, Dr. Havele and colleagues designed a study that includes patients aged 2-12 years dealing with issues related to self-esteem, acceptance, coping, or bullying. Parents are provided with a relevant storybook to read at home with their child in a “safe and comfortable space” and “at their own pace and their own time,” said Dr. Havele.
Inside the book is a QR code to access the validated Children’s Dermatology Life Quality Index (CDLQI). Families complete the survey at baseline and provide feedback after reading the book. Researchers collect information about demographics, age, gender, and skin conditions, which included atopic dermatitis, alopecia areata, vitiligo, hemangioma, and port-wine stain.
The response rate so far is 34%, and close to 80 parents have completed the survey with their child, Dr. Havele said.
At baseline, many of the children were either moderately or severely affected in terms of their quality of life (45% scored ≥ 6 on the CDLQI).
After reading the book, about 80% of parents reported it had a positive impact, and about 20% said it had a somewhat positive impact on their child’s self-image or confidence. Almost 80% agreed, and the remainder somewhat agreed it encouraged their child to embrace differences.
Most respondents also said the book helped the parent and child cope with the child’s condition. “So really, it was overall a positive response,” said Dr. Havele. “We are able to demonstrate that these books have value in a more scientific or objective way.”
This may not be surprising. Dr. Havele referred to more formal bibliotherapy (book therapy), which has been studied in other pediatric populations, including patients with cancer and those who have experienced trauma.
Awesome Space
Pediatric dermatologists are perfectly positioned to play a role in improving the lives of their patients with skin issues. “We see the impact of visible skin disease on children all the time,” said Dr. Havele. “The dermatology visit is an awesome space and opportunity to introduce these books to families and potentially help them talk about the skin condition with their child.”
In addition to prescribing therapies, “we’re also with these kids through an emotional journey, and I think giving them tools for that emotional journey is very helpful,” she added.
Such books would have been a great help to Dr. Havele herself. Growing up, she had severe atopic dermatitis covering much of her body. “Having such a resource would have helped me better cope with my reality of being different than everyone else.”
She hopes a database will be established to house these resources so other providers can refer patients to the list of books. Other books include “The Itchy-saurus: The Dino with an itch that can’t be scratched,” “Hair in My Brush,” and “I am Unique!”
Dr. Havele had no relevant disclosures.
A version of this article first appeared on Medscape.com.
TORONTO —
So far, “the study demonstrates that these books have value to patients and families,” one of the study authors, Sonia Havele, MD, a pediatrician and dermatology resident at Children’s Mercy Hospital Kansas City, Kansas City, Missouri, said in an interview.
“There are tools to help kids cope with their skin conditions, but we’re underutilizing them,” she added. “And part of the reason we’re underutilizing storybooks is that we just don’t know what’s out there.” For the study, the researchers received funding to purchase 18 “creative and thoughtful” storybooks related to pediatric skin conditions, reviewed by at least two pediatric dermatologists before being selected, which are just a sample of related books that are available.
The study results were presented as a poster at the annual meeting of the Society for Pediatric Dermatology.
Children with visible skin conditions, which can include port-wine stains, capillary malformations, and congenital moles, may be subjected to teasing or bullying at school, and the conditions can also affect their quality of life.
Beauty and the Birthmark
The books include one titled “Beauty with a Birthmark” and another, “My Hair Went on Vacation.” An illustrated book, “Just Ask: Be Different, Be Brave, Be You,” by US Supreme Court Justice Sonia Sotomayor, offers tips on how to answer common questions about someone’s appearance.
Dr. Havele said that Justice Sotomayor’s book “empowers kids, their siblings, their classmates ... to ask questions, and it teaches patients not to be afraid of those questions, and to really lean into educating their peers, and their family members.”
“Kids are really just curious,” she added. “They’ll make comments like: ‘Hey, what’s that spot on your face?’ Or, they’ll ask about vitiligo because they’ve never seen somebody with it before.”
To evaluate the psychosocial impact of these types of books for children with visible skin conditions, Dr. Havele and colleagues designed a study that includes patients aged 2-12 years dealing with issues related to self-esteem, acceptance, coping, or bullying. Parents are provided with a relevant storybook to read at home with their child in a “safe and comfortable space” and “at their own pace and their own time,” said Dr. Havele.
Inside the book is a QR code to access the validated Children’s Dermatology Life Quality Index (CDLQI). Families complete the survey at baseline and provide feedback after reading the book. Researchers collect information about demographics, age, gender, and skin conditions, which included atopic dermatitis, alopecia areata, vitiligo, hemangioma, and port-wine stain.
The response rate so far is 34%, and close to 80 parents have completed the survey with their child, Dr. Havele said.
At baseline, many of the children were either moderately or severely affected in terms of their quality of life (45% scored ≥ 6 on the CDLQI).
After reading the book, about 80% of parents reported it had a positive impact, and about 20% said it had a somewhat positive impact on their child’s self-image or confidence. Almost 80% agreed, and the remainder somewhat agreed it encouraged their child to embrace differences.
Most respondents also said the book helped the parent and child cope with the child’s condition. “So really, it was overall a positive response,” said Dr. Havele. “We are able to demonstrate that these books have value in a more scientific or objective way.”
This may not be surprising. Dr. Havele referred to more formal bibliotherapy (book therapy), which has been studied in other pediatric populations, including patients with cancer and those who have experienced trauma.
Awesome Space
Pediatric dermatologists are perfectly positioned to play a role in improving the lives of their patients with skin issues. “We see the impact of visible skin disease on children all the time,” said Dr. Havele. “The dermatology visit is an awesome space and opportunity to introduce these books to families and potentially help them talk about the skin condition with their child.”
In addition to prescribing therapies, “we’re also with these kids through an emotional journey, and I think giving them tools for that emotional journey is very helpful,” she added.
Such books would have been a great help to Dr. Havele herself. Growing up, she had severe atopic dermatitis covering much of her body. “Having such a resource would have helped me better cope with my reality of being different than everyone else.”
She hopes a database will be established to house these resources so other providers can refer patients to the list of books. Other books include “The Itchy-saurus: The Dino with an itch that can’t be scratched,” “Hair in My Brush,” and “I am Unique!”
Dr. Havele had no relevant disclosures.
A version of this article first appeared on Medscape.com.
TORONTO —
So far, “the study demonstrates that these books have value to patients and families,” one of the study authors, Sonia Havele, MD, a pediatrician and dermatology resident at Children’s Mercy Hospital Kansas City, Kansas City, Missouri, said in an interview.
“There are tools to help kids cope with their skin conditions, but we’re underutilizing them,” she added. “And part of the reason we’re underutilizing storybooks is that we just don’t know what’s out there.” For the study, the researchers received funding to purchase 18 “creative and thoughtful” storybooks related to pediatric skin conditions, reviewed by at least two pediatric dermatologists before being selected, which are just a sample of related books that are available.
The study results were presented as a poster at the annual meeting of the Society for Pediatric Dermatology.
Children with visible skin conditions, which can include port-wine stains, capillary malformations, and congenital moles, may be subjected to teasing or bullying at school, and the conditions can also affect their quality of life.
Beauty and the Birthmark
The books include one titled “Beauty with a Birthmark” and another, “My Hair Went on Vacation.” An illustrated book, “Just Ask: Be Different, Be Brave, Be You,” by US Supreme Court Justice Sonia Sotomayor, offers tips on how to answer common questions about someone’s appearance.
Dr. Havele said that Justice Sotomayor’s book “empowers kids, their siblings, their classmates ... to ask questions, and it teaches patients not to be afraid of those questions, and to really lean into educating their peers, and their family members.”
“Kids are really just curious,” she added. “They’ll make comments like: ‘Hey, what’s that spot on your face?’ Or, they’ll ask about vitiligo because they’ve never seen somebody with it before.”
To evaluate the psychosocial impact of these types of books for children with visible skin conditions, Dr. Havele and colleagues designed a study that includes patients aged 2-12 years dealing with issues related to self-esteem, acceptance, coping, or bullying. Parents are provided with a relevant storybook to read at home with their child in a “safe and comfortable space” and “at their own pace and their own time,” said Dr. Havele.
Inside the book is a QR code to access the validated Children’s Dermatology Life Quality Index (CDLQI). Families complete the survey at baseline and provide feedback after reading the book. Researchers collect information about demographics, age, gender, and skin conditions, which included atopic dermatitis, alopecia areata, vitiligo, hemangioma, and port-wine stain.
The response rate so far is 34%, and close to 80 parents have completed the survey with their child, Dr. Havele said.
At baseline, many of the children were either moderately or severely affected in terms of their quality of life (45% scored ≥ 6 on the CDLQI).
After reading the book, about 80% of parents reported it had a positive impact, and about 20% said it had a somewhat positive impact on their child’s self-image or confidence. Almost 80% agreed, and the remainder somewhat agreed it encouraged their child to embrace differences.
Most respondents also said the book helped the parent and child cope with the child’s condition. “So really, it was overall a positive response,” said Dr. Havele. “We are able to demonstrate that these books have value in a more scientific or objective way.”
This may not be surprising. Dr. Havele referred to more formal bibliotherapy (book therapy), which has been studied in other pediatric populations, including patients with cancer and those who have experienced trauma.
Awesome Space
Pediatric dermatologists are perfectly positioned to play a role in improving the lives of their patients with skin issues. “We see the impact of visible skin disease on children all the time,” said Dr. Havele. “The dermatology visit is an awesome space and opportunity to introduce these books to families and potentially help them talk about the skin condition with their child.”
In addition to prescribing therapies, “we’re also with these kids through an emotional journey, and I think giving them tools for that emotional journey is very helpful,” she added.
Such books would have been a great help to Dr. Havele herself. Growing up, she had severe atopic dermatitis covering much of her body. “Having such a resource would have helped me better cope with my reality of being different than everyone else.”
She hopes a database will be established to house these resources so other providers can refer patients to the list of books. Other books include “The Itchy-saurus: The Dino with an itch that can’t be scratched,” “Hair in My Brush,” and “I am Unique!”
Dr. Havele had no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM SPD 2024
Experts Highlight Challenges That Remain for AI Devices in Triaging Skin Cancer
Emerging according to researchers and dermatologists investigating AI.
While some AI-integrated devices designed to triage skin lesions have emerged, including one that received Food and Drug Administration (FDA) clearance earlier in 2024, it may be some time before AI has a meaningful clinical impact in dermatology and, more specifically, the diagnosis of skin cancer, Ivy Lee, MD, a dermatologist in Pasadena, California, and chair of the American Academy of Dermatology’s augmented intelligence committee, told this news organization.
“It hasn’t really translated into clinical practice yet,” Dr. Lee said of AI in dermatology. “There have been significant advances in terms of the technical possibility and feasibility of these tools, but the translation and integration of AI into actual clinical work flows to benefit patients beyond academic research studies has been limited.” More studies and more “easily accessible and digestible information” are needed to evaluate AI tools in dermatologic practice.
“In dermatology, we’re on a cusp with AI,” said Rebecca Hartman, MD, MPH, chief of dermatology at the VA Boston Healthcare System and director of melanoma epidemiology at Brigham and Women’s Hospital, Boston, Massachusetts. “I think it’s going to come and change what we do,” which is especially true for any image-based specialty,” including radiology and pathology, in addition to dermatology.
Dr. Hartman led a study of one of these emerging technologies, the handheld elastic scattering spectroscopy device DermaSensor, which was cleared by the FDA in January for evaluating skin lesions suggestive of skin cancer.
Early AI Devices for Skin Cancer Detection
At the American Society for Laser Medicine and Surgery (ASLMS) meeting in April, a panel explored a number of algorithms with dermatologic applications that use AI to triage skin lesions, including DermaSensor.
Raman spectroscopy, which contains a handheld Raman probe, a diode laser, and a detecting spectrograph. A laser beam — which at 1.56 W/cm2 is below the maximum permissible exposure — focuses on the skin target with a 3.5-mm spot, gathers data on the target, and feeds it back into the unit that houses the algorithm that evaluates the spot analysis. It’s still in the investigative phase. A clinical trial, published almost 5 years ago, demonstrated a sensitivity of 90%-99% and a specificity of 24%-66% for skin cancer.
A dermatoscope called Sklip clips onto a smartphone and performs what company cofounder Alexander Witkowski, MD, PhD, described as an “optical painless virtual biopsy” for at-home use. The device uploads the captured image to an AI platform for analysis. It received FDA breakthrough device designation in 2022. At the ASLMS meeting, Dr. Witkowski said that clinical performance showed the device had a 97% sensitivity and 30% specificity for skin cancer.
DermaSensor, described in the study conducted by Dr. Hartman and others as a noninvasive, point-and-click spectrometer, is a wireless handheld piece that weighs about 10 ounces. The unit captures five recordings to generate a spectral reading, which an algorithm in the software unit analyzes. The study found a sensitivity of 95.5% and specificity of 32.5% for melanoma detection with the device.
The target market for DermaSensor is primary care physicians, and, according to the FDA announcement in January, it is indicated for evaluating skin lesions “suggestive” of melanoma, basal cell carcinoma (BCC), and/or squamous cell carcinoma (SCC) in patients aged 40 and older to “assist healthcare providers in determining whether to refer a patient to a dermatologist.”
So Many Cases, So Few Dermatologists
In dermatology, AI devices have the potential to streamline the crushing burden of diagnosing skin cancer, said Yun Liu, PhD, a senior staff scientist at Google Research, Mountain View, California, who’s worked on developing machine-learning tools in dermatology among other medical fields. “Many people cannot access dermatology expertise when they most need it, ie, without waiting a long time. This causes substantial morbidity for patients,” Dr. Liu said in an interview.
His own research of an AI-based tool to help primary care physicians and nurse practitioners in teledermatology practices diagnose skin conditions documented the shortage of dermatologists to triage lesions, including a finding that only about one quarter of skin conditions are seen by a specialist and that nonspecialists play a pivotal role in the management of skin lesions.
The Centers for Disease Control and Prevention reports that about 6.1 million adults are treated for BCC and SCCs each year. The American Medical Association estimates that 13,200 active dermatologists practice in the United States.
Overcoming Barriers to AI in Dermatology
Before AI makes significant inroads in dermatology, clinicians need to see more verifiable data, said Roxana Daneshjou, MD, PhD, assistant professor of biomedical data science and dermatology at Stanford University, Stanford, California. “One of the challenges is having the availability of models that actually improve clinical care because we have some very early prospective trials on different devices, but we don’t have large-scale randomized clinical trials of AI devices showing definitive behaviors such as improved patient outcomes, that it helps curb skin cancer, or it catches it like dermatologists but helps reduce the biopsy load,” she said. “You need good data.”
Another challenge she noted was overcoming biases built into medicine. “A lot of the image-based models are built on datasets depicting skin disease on White skin, and those models don’t work so well on people with brown and black skin, who have historically had worse outcomes and also have been underrepresented in dermatology,” said Dr. Daneshjou, an associate editor of NEJM AI.
There’s also the challenge of getting verified AI models into the clinic. “Similar to many medical AI endeavors, developing a proof-of-concept or research prototype is far easier and faster than bringing the development to real users,” Dr. Liu said. “In particular, it is important to conduct thorough validation studies on various patient populations and settings and understand how these AI tools can best fit into the workflow or patient journey.”
A study published in 2023 documented progress Google made in deploying AI models in retina specialty clinics in India and Thailand, Dr. Liu noted.
Another challenge is to avoid overdiagnosis with these new technologies, Dr. Hartman said. Her group’s study showed the DermaSensor had a positive predictive value of 16% and a negative predictive value of 98.5%. “I think there’s some question about how this will factor into overdiagnosis. Could this actually bombard dermatologists more if the positive predictive value’s only 16%?”
One key to dermatologists accepting AI tools is having a transparent process for validating them, Dr. Lee said. “Even with FDA clearance, we don’t have the transparency we need as clinicians, researchers, and advocates of machine learning and AI in healthcare.”
But, Dr. Lee noted, the FDA in June took a step toward illuminating its validation process when it adopted guiding principles for transparency for machine learning–enabled devices. “Once we can get more access to this information and have more transparency, that’s where we can think about actually about making the decision to implement or not implement into local healthcare settings,” she said. The process was further enabled by a White House executive order in October 2023 on the safe, secure, and trustworthy development and use of AI.
The experience with telehealth during the COVID-19 pandemic, when patients and providers quickly embraced the technology to stay connected, serves as a potential template for AI, Dr. Lee noted. “As we’d seen with telehealth through the pandemic, you also need the cultural evolution and the development of the infrastructure around it to actually make sure this is a sustainable implementation and a scalable implementation in healthcare.”
Dr. Lee had no relevant relationships to disclose. Dr. Hartman received funding from DermaSensor for a study. Dr. Witkowski is a cofounder of Sklip. Dr. Liu is an employee of Google Research. Dr. Daneshjou reported financial relationships with MD Algorithms, Revea, and L’Oreal.
A version of this article first appeared on Medscape.com.
Emerging according to researchers and dermatologists investigating AI.
While some AI-integrated devices designed to triage skin lesions have emerged, including one that received Food and Drug Administration (FDA) clearance earlier in 2024, it may be some time before AI has a meaningful clinical impact in dermatology and, more specifically, the diagnosis of skin cancer, Ivy Lee, MD, a dermatologist in Pasadena, California, and chair of the American Academy of Dermatology’s augmented intelligence committee, told this news organization.
“It hasn’t really translated into clinical practice yet,” Dr. Lee said of AI in dermatology. “There have been significant advances in terms of the technical possibility and feasibility of these tools, but the translation and integration of AI into actual clinical work flows to benefit patients beyond academic research studies has been limited.” More studies and more “easily accessible and digestible information” are needed to evaluate AI tools in dermatologic practice.
“In dermatology, we’re on a cusp with AI,” said Rebecca Hartman, MD, MPH, chief of dermatology at the VA Boston Healthcare System and director of melanoma epidemiology at Brigham and Women’s Hospital, Boston, Massachusetts. “I think it’s going to come and change what we do,” which is especially true for any image-based specialty,” including radiology and pathology, in addition to dermatology.
Dr. Hartman led a study of one of these emerging technologies, the handheld elastic scattering spectroscopy device DermaSensor, which was cleared by the FDA in January for evaluating skin lesions suggestive of skin cancer.
Early AI Devices for Skin Cancer Detection
At the American Society for Laser Medicine and Surgery (ASLMS) meeting in April, a panel explored a number of algorithms with dermatologic applications that use AI to triage skin lesions, including DermaSensor.
Raman spectroscopy, which contains a handheld Raman probe, a diode laser, and a detecting spectrograph. A laser beam — which at 1.56 W/cm2 is below the maximum permissible exposure — focuses on the skin target with a 3.5-mm spot, gathers data on the target, and feeds it back into the unit that houses the algorithm that evaluates the spot analysis. It’s still in the investigative phase. A clinical trial, published almost 5 years ago, demonstrated a sensitivity of 90%-99% and a specificity of 24%-66% for skin cancer.
A dermatoscope called Sklip clips onto a smartphone and performs what company cofounder Alexander Witkowski, MD, PhD, described as an “optical painless virtual biopsy” for at-home use. The device uploads the captured image to an AI platform for analysis. It received FDA breakthrough device designation in 2022. At the ASLMS meeting, Dr. Witkowski said that clinical performance showed the device had a 97% sensitivity and 30% specificity for skin cancer.
DermaSensor, described in the study conducted by Dr. Hartman and others as a noninvasive, point-and-click spectrometer, is a wireless handheld piece that weighs about 10 ounces. The unit captures five recordings to generate a spectral reading, which an algorithm in the software unit analyzes. The study found a sensitivity of 95.5% and specificity of 32.5% for melanoma detection with the device.
The target market for DermaSensor is primary care physicians, and, according to the FDA announcement in January, it is indicated for evaluating skin lesions “suggestive” of melanoma, basal cell carcinoma (BCC), and/or squamous cell carcinoma (SCC) in patients aged 40 and older to “assist healthcare providers in determining whether to refer a patient to a dermatologist.”
So Many Cases, So Few Dermatologists
In dermatology, AI devices have the potential to streamline the crushing burden of diagnosing skin cancer, said Yun Liu, PhD, a senior staff scientist at Google Research, Mountain View, California, who’s worked on developing machine-learning tools in dermatology among other medical fields. “Many people cannot access dermatology expertise when they most need it, ie, without waiting a long time. This causes substantial morbidity for patients,” Dr. Liu said in an interview.
His own research of an AI-based tool to help primary care physicians and nurse practitioners in teledermatology practices diagnose skin conditions documented the shortage of dermatologists to triage lesions, including a finding that only about one quarter of skin conditions are seen by a specialist and that nonspecialists play a pivotal role in the management of skin lesions.
The Centers for Disease Control and Prevention reports that about 6.1 million adults are treated for BCC and SCCs each year. The American Medical Association estimates that 13,200 active dermatologists practice in the United States.
Overcoming Barriers to AI in Dermatology
Before AI makes significant inroads in dermatology, clinicians need to see more verifiable data, said Roxana Daneshjou, MD, PhD, assistant professor of biomedical data science and dermatology at Stanford University, Stanford, California. “One of the challenges is having the availability of models that actually improve clinical care because we have some very early prospective trials on different devices, but we don’t have large-scale randomized clinical trials of AI devices showing definitive behaviors such as improved patient outcomes, that it helps curb skin cancer, or it catches it like dermatologists but helps reduce the biopsy load,” she said. “You need good data.”
Another challenge she noted was overcoming biases built into medicine. “A lot of the image-based models are built on datasets depicting skin disease on White skin, and those models don’t work so well on people with brown and black skin, who have historically had worse outcomes and also have been underrepresented in dermatology,” said Dr. Daneshjou, an associate editor of NEJM AI.
There’s also the challenge of getting verified AI models into the clinic. “Similar to many medical AI endeavors, developing a proof-of-concept or research prototype is far easier and faster than bringing the development to real users,” Dr. Liu said. “In particular, it is important to conduct thorough validation studies on various patient populations and settings and understand how these AI tools can best fit into the workflow or patient journey.”
A study published in 2023 documented progress Google made in deploying AI models in retina specialty clinics in India and Thailand, Dr. Liu noted.
Another challenge is to avoid overdiagnosis with these new technologies, Dr. Hartman said. Her group’s study showed the DermaSensor had a positive predictive value of 16% and a negative predictive value of 98.5%. “I think there’s some question about how this will factor into overdiagnosis. Could this actually bombard dermatologists more if the positive predictive value’s only 16%?”
One key to dermatologists accepting AI tools is having a transparent process for validating them, Dr. Lee said. “Even with FDA clearance, we don’t have the transparency we need as clinicians, researchers, and advocates of machine learning and AI in healthcare.”
But, Dr. Lee noted, the FDA in June took a step toward illuminating its validation process when it adopted guiding principles for transparency for machine learning–enabled devices. “Once we can get more access to this information and have more transparency, that’s where we can think about actually about making the decision to implement or not implement into local healthcare settings,” she said. The process was further enabled by a White House executive order in October 2023 on the safe, secure, and trustworthy development and use of AI.
The experience with telehealth during the COVID-19 pandemic, when patients and providers quickly embraced the technology to stay connected, serves as a potential template for AI, Dr. Lee noted. “As we’d seen with telehealth through the pandemic, you also need the cultural evolution and the development of the infrastructure around it to actually make sure this is a sustainable implementation and a scalable implementation in healthcare.”
Dr. Lee had no relevant relationships to disclose. Dr. Hartman received funding from DermaSensor for a study. Dr. Witkowski is a cofounder of Sklip. Dr. Liu is an employee of Google Research. Dr. Daneshjou reported financial relationships with MD Algorithms, Revea, and L’Oreal.
A version of this article first appeared on Medscape.com.
Emerging according to researchers and dermatologists investigating AI.
While some AI-integrated devices designed to triage skin lesions have emerged, including one that received Food and Drug Administration (FDA) clearance earlier in 2024, it may be some time before AI has a meaningful clinical impact in dermatology and, more specifically, the diagnosis of skin cancer, Ivy Lee, MD, a dermatologist in Pasadena, California, and chair of the American Academy of Dermatology’s augmented intelligence committee, told this news organization.
“It hasn’t really translated into clinical practice yet,” Dr. Lee said of AI in dermatology. “There have been significant advances in terms of the technical possibility and feasibility of these tools, but the translation and integration of AI into actual clinical work flows to benefit patients beyond academic research studies has been limited.” More studies and more “easily accessible and digestible information” are needed to evaluate AI tools in dermatologic practice.
“In dermatology, we’re on a cusp with AI,” said Rebecca Hartman, MD, MPH, chief of dermatology at the VA Boston Healthcare System and director of melanoma epidemiology at Brigham and Women’s Hospital, Boston, Massachusetts. “I think it’s going to come and change what we do,” which is especially true for any image-based specialty,” including radiology and pathology, in addition to dermatology.
Dr. Hartman led a study of one of these emerging technologies, the handheld elastic scattering spectroscopy device DermaSensor, which was cleared by the FDA in January for evaluating skin lesions suggestive of skin cancer.
Early AI Devices for Skin Cancer Detection
At the American Society for Laser Medicine and Surgery (ASLMS) meeting in April, a panel explored a number of algorithms with dermatologic applications that use AI to triage skin lesions, including DermaSensor.
Raman spectroscopy, which contains a handheld Raman probe, a diode laser, and a detecting spectrograph. A laser beam — which at 1.56 W/cm2 is below the maximum permissible exposure — focuses on the skin target with a 3.5-mm spot, gathers data on the target, and feeds it back into the unit that houses the algorithm that evaluates the spot analysis. It’s still in the investigative phase. A clinical trial, published almost 5 years ago, demonstrated a sensitivity of 90%-99% and a specificity of 24%-66% for skin cancer.
A dermatoscope called Sklip clips onto a smartphone and performs what company cofounder Alexander Witkowski, MD, PhD, described as an “optical painless virtual biopsy” for at-home use. The device uploads the captured image to an AI platform for analysis. It received FDA breakthrough device designation in 2022. At the ASLMS meeting, Dr. Witkowski said that clinical performance showed the device had a 97% sensitivity and 30% specificity for skin cancer.
DermaSensor, described in the study conducted by Dr. Hartman and others as a noninvasive, point-and-click spectrometer, is a wireless handheld piece that weighs about 10 ounces. The unit captures five recordings to generate a spectral reading, which an algorithm in the software unit analyzes. The study found a sensitivity of 95.5% and specificity of 32.5% for melanoma detection with the device.
The target market for DermaSensor is primary care physicians, and, according to the FDA announcement in January, it is indicated for evaluating skin lesions “suggestive” of melanoma, basal cell carcinoma (BCC), and/or squamous cell carcinoma (SCC) in patients aged 40 and older to “assist healthcare providers in determining whether to refer a patient to a dermatologist.”
So Many Cases, So Few Dermatologists
In dermatology, AI devices have the potential to streamline the crushing burden of diagnosing skin cancer, said Yun Liu, PhD, a senior staff scientist at Google Research, Mountain View, California, who’s worked on developing machine-learning tools in dermatology among other medical fields. “Many people cannot access dermatology expertise when they most need it, ie, without waiting a long time. This causes substantial morbidity for patients,” Dr. Liu said in an interview.
His own research of an AI-based tool to help primary care physicians and nurse practitioners in teledermatology practices diagnose skin conditions documented the shortage of dermatologists to triage lesions, including a finding that only about one quarter of skin conditions are seen by a specialist and that nonspecialists play a pivotal role in the management of skin lesions.
The Centers for Disease Control and Prevention reports that about 6.1 million adults are treated for BCC and SCCs each year. The American Medical Association estimates that 13,200 active dermatologists practice in the United States.
Overcoming Barriers to AI in Dermatology
Before AI makes significant inroads in dermatology, clinicians need to see more verifiable data, said Roxana Daneshjou, MD, PhD, assistant professor of biomedical data science and dermatology at Stanford University, Stanford, California. “One of the challenges is having the availability of models that actually improve clinical care because we have some very early prospective trials on different devices, but we don’t have large-scale randomized clinical trials of AI devices showing definitive behaviors such as improved patient outcomes, that it helps curb skin cancer, or it catches it like dermatologists but helps reduce the biopsy load,” she said. “You need good data.”
Another challenge she noted was overcoming biases built into medicine. “A lot of the image-based models are built on datasets depicting skin disease on White skin, and those models don’t work so well on people with brown and black skin, who have historically had worse outcomes and also have been underrepresented in dermatology,” said Dr. Daneshjou, an associate editor of NEJM AI.
There’s also the challenge of getting verified AI models into the clinic. “Similar to many medical AI endeavors, developing a proof-of-concept or research prototype is far easier and faster than bringing the development to real users,” Dr. Liu said. “In particular, it is important to conduct thorough validation studies on various patient populations and settings and understand how these AI tools can best fit into the workflow or patient journey.”
A study published in 2023 documented progress Google made in deploying AI models in retina specialty clinics in India and Thailand, Dr. Liu noted.
Another challenge is to avoid overdiagnosis with these new technologies, Dr. Hartman said. Her group’s study showed the DermaSensor had a positive predictive value of 16% and a negative predictive value of 98.5%. “I think there’s some question about how this will factor into overdiagnosis. Could this actually bombard dermatologists more if the positive predictive value’s only 16%?”
One key to dermatologists accepting AI tools is having a transparent process for validating them, Dr. Lee said. “Even with FDA clearance, we don’t have the transparency we need as clinicians, researchers, and advocates of machine learning and AI in healthcare.”
But, Dr. Lee noted, the FDA in June took a step toward illuminating its validation process when it adopted guiding principles for transparency for machine learning–enabled devices. “Once we can get more access to this information and have more transparency, that’s where we can think about actually about making the decision to implement or not implement into local healthcare settings,” she said. The process was further enabled by a White House executive order in October 2023 on the safe, secure, and trustworthy development and use of AI.
The experience with telehealth during the COVID-19 pandemic, when patients and providers quickly embraced the technology to stay connected, serves as a potential template for AI, Dr. Lee noted. “As we’d seen with telehealth through the pandemic, you also need the cultural evolution and the development of the infrastructure around it to actually make sure this is a sustainable implementation and a scalable implementation in healthcare.”
Dr. Lee had no relevant relationships to disclose. Dr. Hartman received funding from DermaSensor for a study. Dr. Witkowski is a cofounder of Sklip. Dr. Liu is an employee of Google Research. Dr. Daneshjou reported financial relationships with MD Algorithms, Revea, and L’Oreal.
A version of this article first appeared on Medscape.com.