User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
Avoid ‘mutant selection window’ when prescribing antibiotics for acne
LAHAINA, HAWAII – Consider the “mutant selection window” to reduce antibiotic resistance when treating acne, Hilary E. Baldwin, MD, advised at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
Dermatologists continue to write a disproportionate number of prescriptions for antibiotics, particularly tetracyclines, noted Dr. Baldwin, medical director of the Acne Treatment and Research Center in New York. In addition to limiting unnecessary use of antimicrobials, strategies for slowing antimicrobial resistance include using anti-inflammatory doses of doxycycline; using more retinoids, isotretinoin, spironolactone, and oral contraceptives; and improving patient compliance with treatment.
Dermatologists can also “pay attention to the bug we are treating and ... make sure the concentration of the drug that we are using is appropriate to the bug we’re trying to kill,” while also targeting resistant organisms. Dr. Baldwin referred to a paper in the infectious disease literature titled: “The mutant selection window and antimicrobial resistance,” which points out that a drug concentration range exists for which mutant strains of bacteria are selected most frequently (J Antimicrob Chemother. 2003 Jul;52[1]:11-7). The dimensions of this range, or “window,” are characteristic of each pathogen-antimicrobial combination. A high enough drug concentration will eliminate both resistant and sensitive strains of the pathogen.
The paper notes that the minimum inhibitory concentration (MIC) is the lowest concentration that will inhibit the visible growth of a microorganism. The mutant prevention concentration (MPC) is the minimum drug concentration needed to prevent the growth of resistant strains, Dr. Baldwin said. The mutant selection window is the concentration range that extends from the MIC up to the MPC, the range “within which resistant mutants are likely to emerge.” If the antimicrobial concentration falls within this window, a mutant strain is likely to develop and “you’re going to add to the problem of antibiotic resistance,” she explained. “So the goal is to treat low or to treat high, but not right in the middle.”
“This is not theoretical,” and has been shown over and over again, with, for example, Streptococcus pneumonia and moxifloxacin, she said (J Antimicrob Chemother. 2003 Oct;52[4]:616-22.).
When the therapeutic window does not extend all the way to the MPC, “toxicity starts to kick in before you can get high enough to kill off the whole group of organisms,” in which case a low-dose strategy would reduce the development of resistant organisms, she noted.
“We’re doing this already,” with topical antifungals, Dr. Baldwin pointed out, asking when the last time anyone heard that a fungus developed resistance to topical antifungal therapy. “Never, because we use our antifungals in such a high dose, that we’re 500 times the MPC.”
Using an anti-inflammatory dose of doxycycline for treating acne or rosacea is a low-dose strategy, and the 40-mg delayed-release dose stays “way below” the antimicrobial threshold, she said, but the 50-mg dose falls “right in the middle of that mutant selection window.”
As more treatments become available, it will be important to determine how to dose topical antibiotics so that they do not fall within the mutant selection window and avoid what happened with clindamycin and erythromycin, “where the topical use of these medications led to the development of resistance such that they no longer work for the treatment” of Cutibacterium acnes.
Dr. Baldwin disclosures included being on the speakers bureau, serving as an advisor, and/or an investigator for companies that include Almirall, BioPharmx, Foamix, Galderma, Ortho Dermatologics, Sun Pharmaceuticals, Johnson & Johnson, and La Roche–Posay.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – Consider the “mutant selection window” to reduce antibiotic resistance when treating acne, Hilary E. Baldwin, MD, advised at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
Dermatologists continue to write a disproportionate number of prescriptions for antibiotics, particularly tetracyclines, noted Dr. Baldwin, medical director of the Acne Treatment and Research Center in New York. In addition to limiting unnecessary use of antimicrobials, strategies for slowing antimicrobial resistance include using anti-inflammatory doses of doxycycline; using more retinoids, isotretinoin, spironolactone, and oral contraceptives; and improving patient compliance with treatment.
Dermatologists can also “pay attention to the bug we are treating and ... make sure the concentration of the drug that we are using is appropriate to the bug we’re trying to kill,” while also targeting resistant organisms. Dr. Baldwin referred to a paper in the infectious disease literature titled: “The mutant selection window and antimicrobial resistance,” which points out that a drug concentration range exists for which mutant strains of bacteria are selected most frequently (J Antimicrob Chemother. 2003 Jul;52[1]:11-7). The dimensions of this range, or “window,” are characteristic of each pathogen-antimicrobial combination. A high enough drug concentration will eliminate both resistant and sensitive strains of the pathogen.
The paper notes that the minimum inhibitory concentration (MIC) is the lowest concentration that will inhibit the visible growth of a microorganism. The mutant prevention concentration (MPC) is the minimum drug concentration needed to prevent the growth of resistant strains, Dr. Baldwin said. The mutant selection window is the concentration range that extends from the MIC up to the MPC, the range “within which resistant mutants are likely to emerge.” If the antimicrobial concentration falls within this window, a mutant strain is likely to develop and “you’re going to add to the problem of antibiotic resistance,” she explained. “So the goal is to treat low or to treat high, but not right in the middle.”
“This is not theoretical,” and has been shown over and over again, with, for example, Streptococcus pneumonia and moxifloxacin, she said (J Antimicrob Chemother. 2003 Oct;52[4]:616-22.).
When the therapeutic window does not extend all the way to the MPC, “toxicity starts to kick in before you can get high enough to kill off the whole group of organisms,” in which case a low-dose strategy would reduce the development of resistant organisms, she noted.
“We’re doing this already,” with topical antifungals, Dr. Baldwin pointed out, asking when the last time anyone heard that a fungus developed resistance to topical antifungal therapy. “Never, because we use our antifungals in such a high dose, that we’re 500 times the MPC.”
Using an anti-inflammatory dose of doxycycline for treating acne or rosacea is a low-dose strategy, and the 40-mg delayed-release dose stays “way below” the antimicrobial threshold, she said, but the 50-mg dose falls “right in the middle of that mutant selection window.”
As more treatments become available, it will be important to determine how to dose topical antibiotics so that they do not fall within the mutant selection window and avoid what happened with clindamycin and erythromycin, “where the topical use of these medications led to the development of resistance such that they no longer work for the treatment” of Cutibacterium acnes.
Dr. Baldwin disclosures included being on the speakers bureau, serving as an advisor, and/or an investigator for companies that include Almirall, BioPharmx, Foamix, Galderma, Ortho Dermatologics, Sun Pharmaceuticals, Johnson & Johnson, and La Roche–Posay.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – Consider the “mutant selection window” to reduce antibiotic resistance when treating acne, Hilary E. Baldwin, MD, advised at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
Dermatologists continue to write a disproportionate number of prescriptions for antibiotics, particularly tetracyclines, noted Dr. Baldwin, medical director of the Acne Treatment and Research Center in New York. In addition to limiting unnecessary use of antimicrobials, strategies for slowing antimicrobial resistance include using anti-inflammatory doses of doxycycline; using more retinoids, isotretinoin, spironolactone, and oral contraceptives; and improving patient compliance with treatment.
Dermatologists can also “pay attention to the bug we are treating and ... make sure the concentration of the drug that we are using is appropriate to the bug we’re trying to kill,” while also targeting resistant organisms. Dr. Baldwin referred to a paper in the infectious disease literature titled: “The mutant selection window and antimicrobial resistance,” which points out that a drug concentration range exists for which mutant strains of bacteria are selected most frequently (J Antimicrob Chemother. 2003 Jul;52[1]:11-7). The dimensions of this range, or “window,” are characteristic of each pathogen-antimicrobial combination. A high enough drug concentration will eliminate both resistant and sensitive strains of the pathogen.
The paper notes that the minimum inhibitory concentration (MIC) is the lowest concentration that will inhibit the visible growth of a microorganism. The mutant prevention concentration (MPC) is the minimum drug concentration needed to prevent the growth of resistant strains, Dr. Baldwin said. The mutant selection window is the concentration range that extends from the MIC up to the MPC, the range “within which resistant mutants are likely to emerge.” If the antimicrobial concentration falls within this window, a mutant strain is likely to develop and “you’re going to add to the problem of antibiotic resistance,” she explained. “So the goal is to treat low or to treat high, but not right in the middle.”
“This is not theoretical,” and has been shown over and over again, with, for example, Streptococcus pneumonia and moxifloxacin, she said (J Antimicrob Chemother. 2003 Oct;52[4]:616-22.).
When the therapeutic window does not extend all the way to the MPC, “toxicity starts to kick in before you can get high enough to kill off the whole group of organisms,” in which case a low-dose strategy would reduce the development of resistant organisms, she noted.
“We’re doing this already,” with topical antifungals, Dr. Baldwin pointed out, asking when the last time anyone heard that a fungus developed resistance to topical antifungal therapy. “Never, because we use our antifungals in such a high dose, that we’re 500 times the MPC.”
Using an anti-inflammatory dose of doxycycline for treating acne or rosacea is a low-dose strategy, and the 40-mg delayed-release dose stays “way below” the antimicrobial threshold, she said, but the 50-mg dose falls “right in the middle of that mutant selection window.”
As more treatments become available, it will be important to determine how to dose topical antibiotics so that they do not fall within the mutant selection window and avoid what happened with clindamycin and erythromycin, “where the topical use of these medications led to the development of resistance such that they no longer work for the treatment” of Cutibacterium acnes.
Dr. Baldwin disclosures included being on the speakers bureau, serving as an advisor, and/or an investigator for companies that include Almirall, BioPharmx, Foamix, Galderma, Ortho Dermatologics, Sun Pharmaceuticals, Johnson & Johnson, and La Roche–Posay.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM SDEF HAWAII DERMATOLOGY SEMINAR
Vascular occlusion management
The time course and proper management of vascular occlusion attributable to interarterial hyaluronic acid fillers is critical. Albeit a rare complication, off-label uses of HA fillers, lack of proper training of injectors, and lack of clear appropriate guidelines in the management of these complications are some of the causes of delayed treatment and necrotic complications.
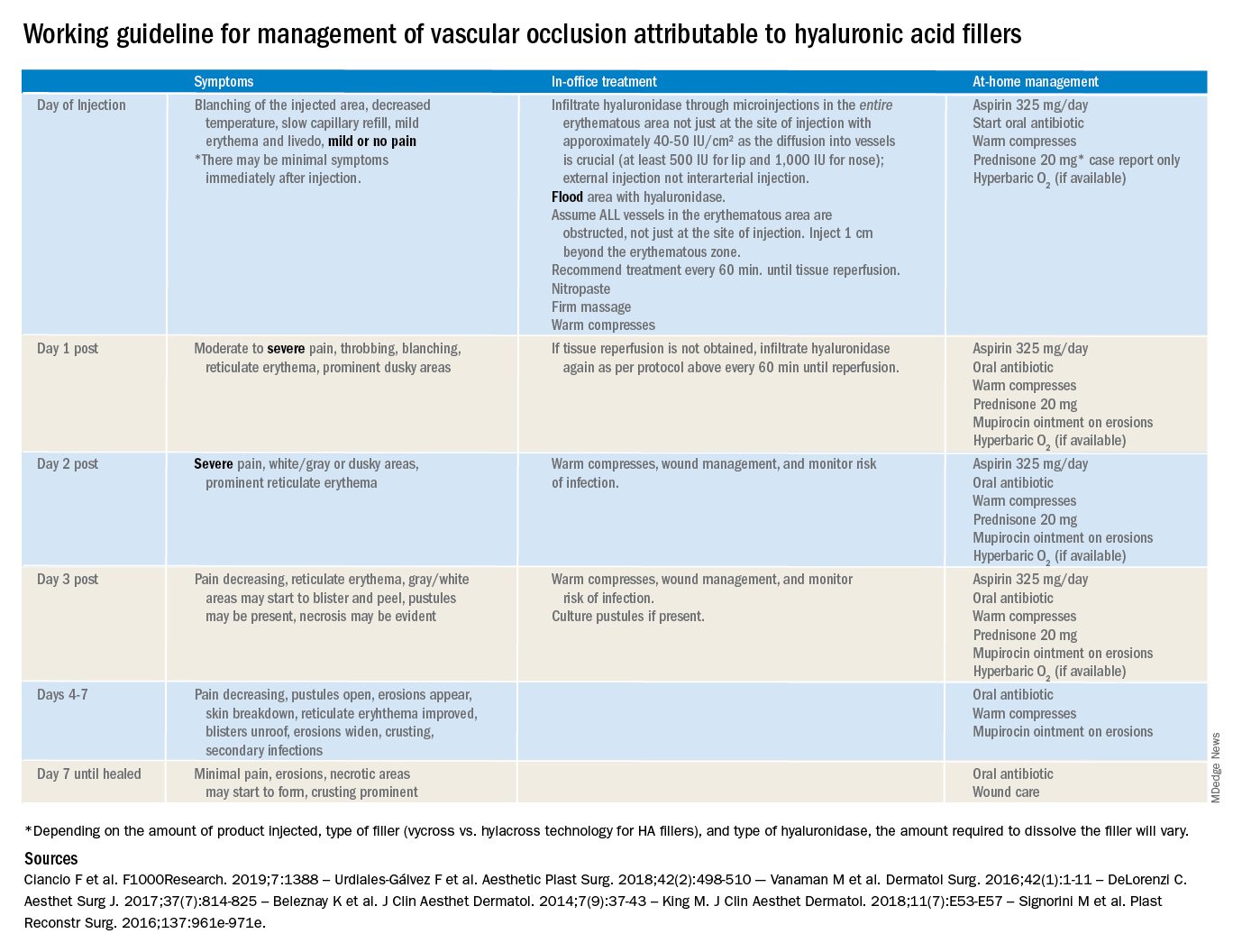
There are currently no definitive guidelines for the management of filler-associated cutaneous necrosis as experience with its treatment continues to evolve and be reported. In an attempt to consolidate the published data, as well as to give somewhat of a clear guideline of expectations, a time course and treatment guide has been outlined. The following is a working guideline for management of vascular occlusion attributable to HA fillers based on reports in the literature. This is not a consensus statement, rather it is a consolidation of the anecdotal reports and case studies outlined to help practitioners. It is also not inclusive of all the presentations of vascular occlusion. There are delayed cases of vascular occlusion beginning several days after injection, as well as alternative treatment options that may be considered.
These guidelines also are not for the devastating complication of blindness because of vascular occlusion secondary to fillers. Blindness is beyond the scope of the current article; however, we believe all experienced injectors should have emergency preparations in place and a relationship with an ophthalmologist or other trained surgeons experienced in performing retrobulbar hyaluronidase injections who can be reached in the event of a suspected occlusion. Any symptoms of eye pain, headache, or visual changes need to be immediately treated. Vascular occlusion is an emergency and timing is critical to prevent permanent blindness and facial deformities.
As with all filler injections, risks and complications can happen, and we cannot stress enough the appropriate level of training, as well as expert understanding of anatomy and injection technique, in minimizing potential risks. We encourage regulations and a required level of training to perform these procedures.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
The time course and proper management of vascular occlusion attributable to interarterial hyaluronic acid fillers is critical. Albeit a rare complication, off-label uses of HA fillers, lack of proper training of injectors, and lack of clear appropriate guidelines in the management of these complications are some of the causes of delayed treatment and necrotic complications.

There are currently no definitive guidelines for the management of filler-associated cutaneous necrosis as experience with its treatment continues to evolve and be reported. In an attempt to consolidate the published data, as well as to give somewhat of a clear guideline of expectations, a time course and treatment guide has been outlined. The following is a working guideline for management of vascular occlusion attributable to HA fillers based on reports in the literature. This is not a consensus statement, rather it is a consolidation of the anecdotal reports and case studies outlined to help practitioners. It is also not inclusive of all the presentations of vascular occlusion. There are delayed cases of vascular occlusion beginning several days after injection, as well as alternative treatment options that may be considered.
These guidelines also are not for the devastating complication of blindness because of vascular occlusion secondary to fillers. Blindness is beyond the scope of the current article; however, we believe all experienced injectors should have emergency preparations in place and a relationship with an ophthalmologist or other trained surgeons experienced in performing retrobulbar hyaluronidase injections who can be reached in the event of a suspected occlusion. Any symptoms of eye pain, headache, or visual changes need to be immediately treated. Vascular occlusion is an emergency and timing is critical to prevent permanent blindness and facial deformities.
As with all filler injections, risks and complications can happen, and we cannot stress enough the appropriate level of training, as well as expert understanding of anatomy and injection technique, in minimizing potential risks. We encourage regulations and a required level of training to perform these procedures.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
The time course and proper management of vascular occlusion attributable to interarterial hyaluronic acid fillers is critical. Albeit a rare complication, off-label uses of HA fillers, lack of proper training of injectors, and lack of clear appropriate guidelines in the management of these complications are some of the causes of delayed treatment and necrotic complications.

There are currently no definitive guidelines for the management of filler-associated cutaneous necrosis as experience with its treatment continues to evolve and be reported. In an attempt to consolidate the published data, as well as to give somewhat of a clear guideline of expectations, a time course and treatment guide has been outlined. The following is a working guideline for management of vascular occlusion attributable to HA fillers based on reports in the literature. This is not a consensus statement, rather it is a consolidation of the anecdotal reports and case studies outlined to help practitioners. It is also not inclusive of all the presentations of vascular occlusion. There are delayed cases of vascular occlusion beginning several days after injection, as well as alternative treatment options that may be considered.
These guidelines also are not for the devastating complication of blindness because of vascular occlusion secondary to fillers. Blindness is beyond the scope of the current article; however, we believe all experienced injectors should have emergency preparations in place and a relationship with an ophthalmologist or other trained surgeons experienced in performing retrobulbar hyaluronidase injections who can be reached in the event of a suspected occlusion. Any symptoms of eye pain, headache, or visual changes need to be immediately treated. Vascular occlusion is an emergency and timing is critical to prevent permanent blindness and facial deformities.
As with all filler injections, risks and complications can happen, and we cannot stress enough the appropriate level of training, as well as expert understanding of anatomy and injection technique, in minimizing potential risks. We encourage regulations and a required level of training to perform these procedures.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
Vitiligo tied to lower risk of internal malignancies
LAHAINA, HAWAII – from South Korea, Iltefat Hamzavi, MD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
Previous studies by Dr. Hamzavi and others have established that vitiligo patients have a reduced risk of melanoma and perhaps nonmelanoma skin cancers as well. But the South Korean national study of 101,078 vitiligo patients matched by age and sex to twice as many vitiligo-free controls was the first large examination of the association between vitiligo and internal malignancies. The findings suggest that immunosurveillance in patients with the disease is not merely a skin-deep phenomenon, noted Dr. Hamzavi, of the MultiCultural Dermatology Center at Henry Ford Hospital in Detroit.
“Vitiligo is probably a systemic disease in which there may be increased immunosurveillance. The point here is that as we suppress the disease, we have to be careful that we’re not going to increase cancer rates,” the dermatologist explained in an interview. “This is big data, and something to be aware of, but don’t act on it yet in clinical practice. I just want people to be aware that all of these autoimmune diseases are there for a reason. There are lower rates of melanoma and internal cancers in patients who have vitiligo, but what that means for our new therapies that are coming up we don’t know yet.”
He predicted that the study will open up an active new research domain, but it will take time to find definitive answers as to whether emerging immunomodulatory therapies for patients with vitiligo might, in some instances, increase their current favorably lower risk of internal malignancies. In the meantime, physicians interested in treating vitiligo off label with, for example, Janus kinase (JAK) inhibitors will want to be particularly cautious in patients with a strong history of skin cancer or internal malignancies.
The retrospective, population-based study utilized data from the Korean National Health Insurance claims database. The investigators found that the incidence rate of internal malignancies was 612.9 per 100,000 person-years in the vitiligo group and 708.9 per 100,000 person-years in controls, for a statistically significant and clinically meaningful 14% relative risk reduction after adjustment for age, sex, and comorbid conditions.
Among the most striking organ-specific findings: the vitiligo group had a 38% relative risk reduction in colorectal cancer, a 25% reduction in the risk of lung cancer, and a 38% decrease in ovarian cancer. In contrast, they had a 20% increase in the risk of thyroid cancer (J Clin Oncol. 2019 Apr 10;37[11]:903-11).
Despite the fact that vitiligo is a common disease that affects 0.5%-1% of the population worldwide, for decades it has been something of a pharmacotherapeutic backwater. That’s changed recently and in dramatic fashion as a result of new understanding of the disease pathogenesis. The JAK inhibitors are now under active investigation for the treatment of vitiligo. Indeed, ruxolitinib cream, a potent JAK-1 and -2 inhibitor, is now in phase 3 investigation following a highly successful phase 2 trial. Interleukin-15 blockade is another promising avenue.
Dr. Hamzavi reported serving as a consultant to AbbVie, Aclaris, Novartis, and Pfizer, and receiving research funding from Estee Lauder, Clinuvel Pharmaceuticals, Incyte, and Pfizer. SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – from South Korea, Iltefat Hamzavi, MD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
Previous studies by Dr. Hamzavi and others have established that vitiligo patients have a reduced risk of melanoma and perhaps nonmelanoma skin cancers as well. But the South Korean national study of 101,078 vitiligo patients matched by age and sex to twice as many vitiligo-free controls was the first large examination of the association between vitiligo and internal malignancies. The findings suggest that immunosurveillance in patients with the disease is not merely a skin-deep phenomenon, noted Dr. Hamzavi, of the MultiCultural Dermatology Center at Henry Ford Hospital in Detroit.
“Vitiligo is probably a systemic disease in which there may be increased immunosurveillance. The point here is that as we suppress the disease, we have to be careful that we’re not going to increase cancer rates,” the dermatologist explained in an interview. “This is big data, and something to be aware of, but don’t act on it yet in clinical practice. I just want people to be aware that all of these autoimmune diseases are there for a reason. There are lower rates of melanoma and internal cancers in patients who have vitiligo, but what that means for our new therapies that are coming up we don’t know yet.”
He predicted that the study will open up an active new research domain, but it will take time to find definitive answers as to whether emerging immunomodulatory therapies for patients with vitiligo might, in some instances, increase their current favorably lower risk of internal malignancies. In the meantime, physicians interested in treating vitiligo off label with, for example, Janus kinase (JAK) inhibitors will want to be particularly cautious in patients with a strong history of skin cancer or internal malignancies.
The retrospective, population-based study utilized data from the Korean National Health Insurance claims database. The investigators found that the incidence rate of internal malignancies was 612.9 per 100,000 person-years in the vitiligo group and 708.9 per 100,000 person-years in controls, for a statistically significant and clinically meaningful 14% relative risk reduction after adjustment for age, sex, and comorbid conditions.
Among the most striking organ-specific findings: the vitiligo group had a 38% relative risk reduction in colorectal cancer, a 25% reduction in the risk of lung cancer, and a 38% decrease in ovarian cancer. In contrast, they had a 20% increase in the risk of thyroid cancer (J Clin Oncol. 2019 Apr 10;37[11]:903-11).
Despite the fact that vitiligo is a common disease that affects 0.5%-1% of the population worldwide, for decades it has been something of a pharmacotherapeutic backwater. That’s changed recently and in dramatic fashion as a result of new understanding of the disease pathogenesis. The JAK inhibitors are now under active investigation for the treatment of vitiligo. Indeed, ruxolitinib cream, a potent JAK-1 and -2 inhibitor, is now in phase 3 investigation following a highly successful phase 2 trial. Interleukin-15 blockade is another promising avenue.
Dr. Hamzavi reported serving as a consultant to AbbVie, Aclaris, Novartis, and Pfizer, and receiving research funding from Estee Lauder, Clinuvel Pharmaceuticals, Incyte, and Pfizer. SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – from South Korea, Iltefat Hamzavi, MD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
Previous studies by Dr. Hamzavi and others have established that vitiligo patients have a reduced risk of melanoma and perhaps nonmelanoma skin cancers as well. But the South Korean national study of 101,078 vitiligo patients matched by age and sex to twice as many vitiligo-free controls was the first large examination of the association between vitiligo and internal malignancies. The findings suggest that immunosurveillance in patients with the disease is not merely a skin-deep phenomenon, noted Dr. Hamzavi, of the MultiCultural Dermatology Center at Henry Ford Hospital in Detroit.
“Vitiligo is probably a systemic disease in which there may be increased immunosurveillance. The point here is that as we suppress the disease, we have to be careful that we’re not going to increase cancer rates,” the dermatologist explained in an interview. “This is big data, and something to be aware of, but don’t act on it yet in clinical practice. I just want people to be aware that all of these autoimmune diseases are there for a reason. There are lower rates of melanoma and internal cancers in patients who have vitiligo, but what that means for our new therapies that are coming up we don’t know yet.”
He predicted that the study will open up an active new research domain, but it will take time to find definitive answers as to whether emerging immunomodulatory therapies for patients with vitiligo might, in some instances, increase their current favorably lower risk of internal malignancies. In the meantime, physicians interested in treating vitiligo off label with, for example, Janus kinase (JAK) inhibitors will want to be particularly cautious in patients with a strong history of skin cancer or internal malignancies.
The retrospective, population-based study utilized data from the Korean National Health Insurance claims database. The investigators found that the incidence rate of internal malignancies was 612.9 per 100,000 person-years in the vitiligo group and 708.9 per 100,000 person-years in controls, for a statistically significant and clinically meaningful 14% relative risk reduction after adjustment for age, sex, and comorbid conditions.
Among the most striking organ-specific findings: the vitiligo group had a 38% relative risk reduction in colorectal cancer, a 25% reduction in the risk of lung cancer, and a 38% decrease in ovarian cancer. In contrast, they had a 20% increase in the risk of thyroid cancer (J Clin Oncol. 2019 Apr 10;37[11]:903-11).
Despite the fact that vitiligo is a common disease that affects 0.5%-1% of the population worldwide, for decades it has been something of a pharmacotherapeutic backwater. That’s changed recently and in dramatic fashion as a result of new understanding of the disease pathogenesis. The JAK inhibitors are now under active investigation for the treatment of vitiligo. Indeed, ruxolitinib cream, a potent JAK-1 and -2 inhibitor, is now in phase 3 investigation following a highly successful phase 2 trial. Interleukin-15 blockade is another promising avenue.
Dr. Hamzavi reported serving as a consultant to AbbVie, Aclaris, Novartis, and Pfizer, and receiving research funding from Estee Lauder, Clinuvel Pharmaceuticals, Incyte, and Pfizer. SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM SDEF HAWAII DERMATOLOGY SEMINAR
My inspiration
Kobe Bryant knew me. Not personally, of course. I never received an autograph or shook his hand. But once in a while if I was up early enough, I’d run into Kobe at the gym in Newport Beach where he and I both worked out. As he did for all his fans at the gym, he’d make eye contact with me and nod hello. He was always focused on his workout – working with a trainer, never with headphones on. In person, he appeared enormous. Unlike most retired professional athletes, he still was in great shape. No doubt he could have suited up in purple and gold, and played against the Clippers that night if needed.
Being from New England, I never was a Laker fan. But I thought, if Kobe can head to the gym after midnight and take a 1,000 shots to prepare for a game, then I could set my alarm for 4 a.m. and take a few dozen more questions from my First Aid books. Head down, “Kryptonite” cranked on my iPod, I wasn’t going to let anyone in that test room outwork me. Neither did he. I put in the time and, like Kobe in the 2002 conference finals against Sacramento, I crushed it.*
When we moved to California, I followed Kobe and the Lakers until he retired. To be clear, I didn’t aspire to be like him, firstly because I’m slightly shorter than Michael Bloomberg, but also because although accomplished, Kobe made some poor choices at times. Indeed, it seems he might have been kinder and more considerate when he was at the top. But in his retirement he looked to be toiling to make reparations, refocusing his prodigious energy and talent for the benefit of others rather than for just for scoring 81 points. His Rolls Royce was there before mine at the gym, and I was there early. He was still getting up early and now preparing to be a great venture capitalist, podcaster, author, and father to his girls.
Watching him carry kettle bells across the floor one morning, I wondered, do people like Kobe Bryant look to others for inspiration? Or are they are born with an endless supply of it? For me, I seemed to push harder and faster when watching idols pass by. Whether it was Kobe or Clayton Christensen (author of “The Innovator’s Dilemma”), Joe Jorizzo, or Barack Obama, I found I could do just a bit more if I had them in mind.
On game days, Kobe spoke of arriving at the arena early, long before anyone. He would use the silent, solo time to reflect on what he needed to do perform that night. I tried this last week, arriving at our clinic early, before any patients or staff. I turned the lights on and took a few minutes to think about what we needed to accomplish that day. I previewed patients on my schedule, searched Up to Date for the latest recommendations on a difficult case. I didn’t know Kobe, but I felt like I did.
When I received the text that Kobe Bryant had died, I was actually working on this column. So I decided to change the topic to write about people who inspire me, ironically inspired by him again. May he rest in peace.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
*This article was updated 2/19/2020.
Kobe Bryant knew me. Not personally, of course. I never received an autograph or shook his hand. But once in a while if I was up early enough, I’d run into Kobe at the gym in Newport Beach where he and I both worked out. As he did for all his fans at the gym, he’d make eye contact with me and nod hello. He was always focused on his workout – working with a trainer, never with headphones on. In person, he appeared enormous. Unlike most retired professional athletes, he still was in great shape. No doubt he could have suited up in purple and gold, and played against the Clippers that night if needed.
Being from New England, I never was a Laker fan. But I thought, if Kobe can head to the gym after midnight and take a 1,000 shots to prepare for a game, then I could set my alarm for 4 a.m. and take a few dozen more questions from my First Aid books. Head down, “Kryptonite” cranked on my iPod, I wasn’t going to let anyone in that test room outwork me. Neither did he. I put in the time and, like Kobe in the 2002 conference finals against Sacramento, I crushed it.*
When we moved to California, I followed Kobe and the Lakers until he retired. To be clear, I didn’t aspire to be like him, firstly because I’m slightly shorter than Michael Bloomberg, but also because although accomplished, Kobe made some poor choices at times. Indeed, it seems he might have been kinder and more considerate when he was at the top. But in his retirement he looked to be toiling to make reparations, refocusing his prodigious energy and talent for the benefit of others rather than for just for scoring 81 points. His Rolls Royce was there before mine at the gym, and I was there early. He was still getting up early and now preparing to be a great venture capitalist, podcaster, author, and father to his girls.
Watching him carry kettle bells across the floor one morning, I wondered, do people like Kobe Bryant look to others for inspiration? Or are they are born with an endless supply of it? For me, I seemed to push harder and faster when watching idols pass by. Whether it was Kobe or Clayton Christensen (author of “The Innovator’s Dilemma”), Joe Jorizzo, or Barack Obama, I found I could do just a bit more if I had them in mind.
On game days, Kobe spoke of arriving at the arena early, long before anyone. He would use the silent, solo time to reflect on what he needed to do perform that night. I tried this last week, arriving at our clinic early, before any patients or staff. I turned the lights on and took a few minutes to think about what we needed to accomplish that day. I previewed patients on my schedule, searched Up to Date for the latest recommendations on a difficult case. I didn’t know Kobe, but I felt like I did.
When I received the text that Kobe Bryant had died, I was actually working on this column. So I decided to change the topic to write about people who inspire me, ironically inspired by him again. May he rest in peace.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
*This article was updated 2/19/2020.
Kobe Bryant knew me. Not personally, of course. I never received an autograph or shook his hand. But once in a while if I was up early enough, I’d run into Kobe at the gym in Newport Beach where he and I both worked out. As he did for all his fans at the gym, he’d make eye contact with me and nod hello. He was always focused on his workout – working with a trainer, never with headphones on. In person, he appeared enormous. Unlike most retired professional athletes, he still was in great shape. No doubt he could have suited up in purple and gold, and played against the Clippers that night if needed.
Being from New England, I never was a Laker fan. But I thought, if Kobe can head to the gym after midnight and take a 1,000 shots to prepare for a game, then I could set my alarm for 4 a.m. and take a few dozen more questions from my First Aid books. Head down, “Kryptonite” cranked on my iPod, I wasn’t going to let anyone in that test room outwork me. Neither did he. I put in the time and, like Kobe in the 2002 conference finals against Sacramento, I crushed it.*
When we moved to California, I followed Kobe and the Lakers until he retired. To be clear, I didn’t aspire to be like him, firstly because I’m slightly shorter than Michael Bloomberg, but also because although accomplished, Kobe made some poor choices at times. Indeed, it seems he might have been kinder and more considerate when he was at the top. But in his retirement he looked to be toiling to make reparations, refocusing his prodigious energy and talent for the benefit of others rather than for just for scoring 81 points. His Rolls Royce was there before mine at the gym, and I was there early. He was still getting up early and now preparing to be a great venture capitalist, podcaster, author, and father to his girls.
Watching him carry kettle bells across the floor one morning, I wondered, do people like Kobe Bryant look to others for inspiration? Or are they are born with an endless supply of it? For me, I seemed to push harder and faster when watching idols pass by. Whether it was Kobe or Clayton Christensen (author of “The Innovator’s Dilemma”), Joe Jorizzo, or Barack Obama, I found I could do just a bit more if I had them in mind.
On game days, Kobe spoke of arriving at the arena early, long before anyone. He would use the silent, solo time to reflect on what he needed to do perform that night. I tried this last week, arriving at our clinic early, before any patients or staff. I turned the lights on and took a few minutes to think about what we needed to accomplish that day. I previewed patients on my schedule, searched Up to Date for the latest recommendations on a difficult case. I didn’t know Kobe, but I felt like I did.
When I received the text that Kobe Bryant had died, I was actually working on this column. So I decided to change the topic to write about people who inspire me, ironically inspired by him again. May he rest in peace.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
*This article was updated 2/19/2020.
Hyperhidrosis treatment options include glycopyrrolate
LAHAINA, HAWAII – Hyperhidrosis affects nearly 5% of the U.S. population, and in a survey of U.S. teenagers, about 17% reported excessive sweating, Jashin Wu, MD, said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
In an interview with MDedge reporter Bruce Jancin, Dr. Wu, founder of the Dermatology Research and Education Foundation, Irvine, Calif., discussed the off-label use of oral agents to treat hyperhidrosis. Dr. Wu said he is a fan of oral glycopyrrolate in particular, which he tends to use even earlier than suggested in the International Hyperhidrosis Society guidelines.
Glycopyrrolate is available in 1 mg and 2 mg tablets; Dr. Wu starts patients at a dose of 1 mg twice a day, escalating by 1 mg per week until the “desired effects occur” or the patient has problems tolerating treatment because of side effects.
Other oral options include oxybutynin and propranolol. Sofpironium bromide, an analog of glycopyrrolate, is in the pipeline, he said.
During the interview, Dr. Wu discussed mydriasis, an adverse effect associated with both topical and systemic anticholinergic treatment. In the two pivotal phase 3 randomized trials of prescription glycopyrronium cloth (Qbrexza) for axillary hyperhidrosis, the incidence of mydriasis was 6.8% in 463 patients on active treatment for 4 weeks. Three-quarters of cases were unilateral. The mydriasis resolved without permanent treatment discontinuation in 27 of the 31 patients (J Am Acad Dermatol. 2019 Jan;80[1]:128-138.e2).
“The most important point is that patients need to be educated that they need to wash their hands very well after they apply it to the affected areas” to prevent accidental medication contact with the eyes, he advised.
Alarm bells can go off when a patient with anticholinergic therapy–induced mydriasis presents to an ED without mentioning their treatment status, Dr. Wu observed.
Dr. Wu had no relevant disclosures. SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
To listen to the interview, click the play button below.
LAHAINA, HAWAII – Hyperhidrosis affects nearly 5% of the U.S. population, and in a survey of U.S. teenagers, about 17% reported excessive sweating, Jashin Wu, MD, said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
In an interview with MDedge reporter Bruce Jancin, Dr. Wu, founder of the Dermatology Research and Education Foundation, Irvine, Calif., discussed the off-label use of oral agents to treat hyperhidrosis. Dr. Wu said he is a fan of oral glycopyrrolate in particular, which he tends to use even earlier than suggested in the International Hyperhidrosis Society guidelines.
Glycopyrrolate is available in 1 mg and 2 mg tablets; Dr. Wu starts patients at a dose of 1 mg twice a day, escalating by 1 mg per week until the “desired effects occur” or the patient has problems tolerating treatment because of side effects.
Other oral options include oxybutynin and propranolol. Sofpironium bromide, an analog of glycopyrrolate, is in the pipeline, he said.
During the interview, Dr. Wu discussed mydriasis, an adverse effect associated with both topical and systemic anticholinergic treatment. In the two pivotal phase 3 randomized trials of prescription glycopyrronium cloth (Qbrexza) for axillary hyperhidrosis, the incidence of mydriasis was 6.8% in 463 patients on active treatment for 4 weeks. Three-quarters of cases were unilateral. The mydriasis resolved without permanent treatment discontinuation in 27 of the 31 patients (J Am Acad Dermatol. 2019 Jan;80[1]:128-138.e2).
“The most important point is that patients need to be educated that they need to wash their hands very well after they apply it to the affected areas” to prevent accidental medication contact with the eyes, he advised.
Alarm bells can go off when a patient with anticholinergic therapy–induced mydriasis presents to an ED without mentioning their treatment status, Dr. Wu observed.
Dr. Wu had no relevant disclosures. SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
To listen to the interview, click the play button below.
LAHAINA, HAWAII – Hyperhidrosis affects nearly 5% of the U.S. population, and in a survey of U.S. teenagers, about 17% reported excessive sweating, Jashin Wu, MD, said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
In an interview with MDedge reporter Bruce Jancin, Dr. Wu, founder of the Dermatology Research and Education Foundation, Irvine, Calif., discussed the off-label use of oral agents to treat hyperhidrosis. Dr. Wu said he is a fan of oral glycopyrrolate in particular, which he tends to use even earlier than suggested in the International Hyperhidrosis Society guidelines.
Glycopyrrolate is available in 1 mg and 2 mg tablets; Dr. Wu starts patients at a dose of 1 mg twice a day, escalating by 1 mg per week until the “desired effects occur” or the patient has problems tolerating treatment because of side effects.
Other oral options include oxybutynin and propranolol. Sofpironium bromide, an analog of glycopyrrolate, is in the pipeline, he said.
During the interview, Dr. Wu discussed mydriasis, an adverse effect associated with both topical and systemic anticholinergic treatment. In the two pivotal phase 3 randomized trials of prescription glycopyrronium cloth (Qbrexza) for axillary hyperhidrosis, the incidence of mydriasis was 6.8% in 463 patients on active treatment for 4 weeks. Three-quarters of cases were unilateral. The mydriasis resolved without permanent treatment discontinuation in 27 of the 31 patients (J Am Acad Dermatol. 2019 Jan;80[1]:128-138.e2).
“The most important point is that patients need to be educated that they need to wash their hands very well after they apply it to the affected areas” to prevent accidental medication contact with the eyes, he advised.
Alarm bells can go off when a patient with anticholinergic therapy–induced mydriasis presents to an ED without mentioning their treatment status, Dr. Wu observed.
Dr. Wu had no relevant disclosures. SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
To listen to the interview, click the play button below.
REPORTING FROM THE HAWAII DERMATOLOGY SEMINAR
Private equity firms acquiring more physician group practices
Lead author Jane M. Zhu, MD, of Oregon Health & Science University, Portland, and colleagues examined physician group practice acquisitions by private equity firms using the Irving Levin Associates Health Care M&A data set, which includes manually collected and verified transactional information on health care mergers and acquisitions. Investigators linked acquisitions to the SK&A data set, a commercial data set of verified physicians and practice-level characteristics of U.S. office-based practices.
Of about 18,000 unique group medical practices, private equity firms acquired 355 physician practice acquisitions from 2013 to 2016, a trend that rose from 59 practices in 2013 to 136 practices in 2016, Dr. Zhu and colleagues reported on Feb. 18 , 2020, in a research letter published in JAMA.
Acquired practices had a mean of four sites, 16 physicians in each practice, and 6 physicians affiliated with each site, the data found. Overall, 81% of these medical practices reported accepting new patients, 83% accepted Medicare, and 60% accepted Medicaid. The majority of acquired practices were in the South (44%).
Anesthesiology (19%) and multispecialty (19%) were the most commonly represented medical groups in the acquisitions, followed by emergency medicine (12%), family practice (11%), and dermatology (10%). In addition, from 2015 to 2016, the number of acquired cardiology, ophthalmology, radiology, and ob.gyn. practices increased. Within acquired practices, anesthesiologists represented the majority of all physicians, followed by emergency medicine specialists, family physicians, and dermatologists.
Dr. Zhu and colleagues cited a key limitation: Because the data are based on transactions that have been publicly announced, the acquisition of smaller practices might have been underestimated.
Still, the findings demonstrate that private equity acquisitions of physician medical groups are accelerating across multiple specialties, Dr. Zhu said in an interview.
“From our data, acquired medical groups seem to have relatively large footprints with multiple office sites and multiple physicians, which mirrors a typical investment strategy for these firms,” she said.
Dr. Zhu said that more research is needed about how these purchases affect practice patterns, delivery of care, and clinician behavior. Private equity firms expect greater than 20% annual returns, and such financial incentives may conflict with the need for longer-term investments in practice stability, physician recruitment, quality, and safety, according to the study.
“In theory, there may be greater efficiencies introduced from private equity investment – for example, through administrative and billing efficiencies, reorganizing practice structures, or strengthening technology supports,” Dr. Zhu said. “But because of private equity firms’ emphasis on return on investment, there may be unintended consequences of these purchases on practice stability and patient care. We don’t yet know what these effects will be, and we need robust, longitudinal data to investigate this question.”
Dr. Zhu and colleagues reported that they had no disclosures.
SOURCE: Zhu JM et al. JAMA. 2020 Feb 18;323(17):663-5.
Lead author Jane M. Zhu, MD, of Oregon Health & Science University, Portland, and colleagues examined physician group practice acquisitions by private equity firms using the Irving Levin Associates Health Care M&A data set, which includes manually collected and verified transactional information on health care mergers and acquisitions. Investigators linked acquisitions to the SK&A data set, a commercial data set of verified physicians and practice-level characteristics of U.S. office-based practices.
Of about 18,000 unique group medical practices, private equity firms acquired 355 physician practice acquisitions from 2013 to 2016, a trend that rose from 59 practices in 2013 to 136 practices in 2016, Dr. Zhu and colleagues reported on Feb. 18 , 2020, in a research letter published in JAMA.
Acquired practices had a mean of four sites, 16 physicians in each practice, and 6 physicians affiliated with each site, the data found. Overall, 81% of these medical practices reported accepting new patients, 83% accepted Medicare, and 60% accepted Medicaid. The majority of acquired practices were in the South (44%).
Anesthesiology (19%) and multispecialty (19%) were the most commonly represented medical groups in the acquisitions, followed by emergency medicine (12%), family practice (11%), and dermatology (10%). In addition, from 2015 to 2016, the number of acquired cardiology, ophthalmology, radiology, and ob.gyn. practices increased. Within acquired practices, anesthesiologists represented the majority of all physicians, followed by emergency medicine specialists, family physicians, and dermatologists.
Dr. Zhu and colleagues cited a key limitation: Because the data are based on transactions that have been publicly announced, the acquisition of smaller practices might have been underestimated.
Still, the findings demonstrate that private equity acquisitions of physician medical groups are accelerating across multiple specialties, Dr. Zhu said in an interview.
“From our data, acquired medical groups seem to have relatively large footprints with multiple office sites and multiple physicians, which mirrors a typical investment strategy for these firms,” she said.
Dr. Zhu said that more research is needed about how these purchases affect practice patterns, delivery of care, and clinician behavior. Private equity firms expect greater than 20% annual returns, and such financial incentives may conflict with the need for longer-term investments in practice stability, physician recruitment, quality, and safety, according to the study.
“In theory, there may be greater efficiencies introduced from private equity investment – for example, through administrative and billing efficiencies, reorganizing practice structures, or strengthening technology supports,” Dr. Zhu said. “But because of private equity firms’ emphasis on return on investment, there may be unintended consequences of these purchases on practice stability and patient care. We don’t yet know what these effects will be, and we need robust, longitudinal data to investigate this question.”
Dr. Zhu and colleagues reported that they had no disclosures.
SOURCE: Zhu JM et al. JAMA. 2020 Feb 18;323(17):663-5.
Lead author Jane M. Zhu, MD, of Oregon Health & Science University, Portland, and colleagues examined physician group practice acquisitions by private equity firms using the Irving Levin Associates Health Care M&A data set, which includes manually collected and verified transactional information on health care mergers and acquisitions. Investigators linked acquisitions to the SK&A data set, a commercial data set of verified physicians and practice-level characteristics of U.S. office-based practices.
Of about 18,000 unique group medical practices, private equity firms acquired 355 physician practice acquisitions from 2013 to 2016, a trend that rose from 59 practices in 2013 to 136 practices in 2016, Dr. Zhu and colleagues reported on Feb. 18 , 2020, in a research letter published in JAMA.
Acquired practices had a mean of four sites, 16 physicians in each practice, and 6 physicians affiliated with each site, the data found. Overall, 81% of these medical practices reported accepting new patients, 83% accepted Medicare, and 60% accepted Medicaid. The majority of acquired practices were in the South (44%).
Anesthesiology (19%) and multispecialty (19%) were the most commonly represented medical groups in the acquisitions, followed by emergency medicine (12%), family practice (11%), and dermatology (10%). In addition, from 2015 to 2016, the number of acquired cardiology, ophthalmology, radiology, and ob.gyn. practices increased. Within acquired practices, anesthesiologists represented the majority of all physicians, followed by emergency medicine specialists, family physicians, and dermatologists.
Dr. Zhu and colleagues cited a key limitation: Because the data are based on transactions that have been publicly announced, the acquisition of smaller practices might have been underestimated.
Still, the findings demonstrate that private equity acquisitions of physician medical groups are accelerating across multiple specialties, Dr. Zhu said in an interview.
“From our data, acquired medical groups seem to have relatively large footprints with multiple office sites and multiple physicians, which mirrors a typical investment strategy for these firms,” she said.
Dr. Zhu said that more research is needed about how these purchases affect practice patterns, delivery of care, and clinician behavior. Private equity firms expect greater than 20% annual returns, and such financial incentives may conflict with the need for longer-term investments in practice stability, physician recruitment, quality, and safety, according to the study.
“In theory, there may be greater efficiencies introduced from private equity investment – for example, through administrative and billing efficiencies, reorganizing practice structures, or strengthening technology supports,” Dr. Zhu said. “But because of private equity firms’ emphasis on return on investment, there may be unintended consequences of these purchases on practice stability and patient care. We don’t yet know what these effects will be, and we need robust, longitudinal data to investigate this question.”
Dr. Zhu and colleagues reported that they had no disclosures.
SOURCE: Zhu JM et al. JAMA. 2020 Feb 18;323(17):663-5.
FROM JAMA
Rosacea: Target treatment to pathogenic pathway
LAHAINA, HAWAII – The pathophysiology of rosacea is complicated, which is why “we try to target our treatments to various areas in this pathogenic pathway” to achieve optimal results, according to Linda Stein Gold, MD, director of dermatology research at the Henry Ford Health System in Detroit.
For example, in a patient with papules and pustules, a topical or oral anti-inflammatory agent is needed “to calm that down.” If background erythema is present, separate from papules and pustules, use a topical alpha-adrenergic agonist, she advised. For telangiectasias, consider a device-based treatment, and for a phyma, a surgical approach, she recommended at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
For the background erythema of rosacea, which she described as “that pink face that’s always there,” the two alpha-adrenergic receptor agonists available, brimonidine gel 0.33%, approved by the Food and Drug Administration in 2013, and oxymetazoline cream 1%, approved in 2017, both work on the neurovascular junction, but on different receptors.
Brimonidine “kicks in very, very rapidly,” with a significant decrease in background erythema evident within 30 minutes and improvements that last over a 12-hour day, she said. It is effective over a year, but in longterm and postmarketing studies, about 20% of patients experienced exacerbation of erythema, with two peaks of redness. “One occurs at 3-6 hours,” and the other peak occurs when the drug is wearing off later in the day, Dr. Stein Gold said.
A study that sought to identify factors that might make patients more prone to this adverse effect found that “less is better” regarding brimonidine application, with an optimal application of one to three pea-sized dollops on the face, not five as instructed in the package insert. In addition, patients with more than five flushing episodes a week, particularly women, “tend to have more labile disease and [are] more likely to get that rebound erythema,” the study found.
Oxymetazoline 1% in a cream formulation has a “slightly more gentle onset of action and a more gentle offset of action,” without exacerbation of erythema and has been shown to have sustained efficacy over 52 weeks. In a yearlong safety study, there were “no new red flags and we weren’t seeing that redness at hours 3 to 6, or even when you take the patient off the drug,” she noted.
Dr. Stein Gold reported that she has served as a consultant, investigator, or speaker for Galderma, Dermira, Foamix Pharmaceuticals, Valeant, Allergan, Actavis, and Roche.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – The pathophysiology of rosacea is complicated, which is why “we try to target our treatments to various areas in this pathogenic pathway” to achieve optimal results, according to Linda Stein Gold, MD, director of dermatology research at the Henry Ford Health System in Detroit.
For example, in a patient with papules and pustules, a topical or oral anti-inflammatory agent is needed “to calm that down.” If background erythema is present, separate from papules and pustules, use a topical alpha-adrenergic agonist, she advised. For telangiectasias, consider a device-based treatment, and for a phyma, a surgical approach, she recommended at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
For the background erythema of rosacea, which she described as “that pink face that’s always there,” the two alpha-adrenergic receptor agonists available, brimonidine gel 0.33%, approved by the Food and Drug Administration in 2013, and oxymetazoline cream 1%, approved in 2017, both work on the neurovascular junction, but on different receptors.
Brimonidine “kicks in very, very rapidly,” with a significant decrease in background erythema evident within 30 minutes and improvements that last over a 12-hour day, she said. It is effective over a year, but in longterm and postmarketing studies, about 20% of patients experienced exacerbation of erythema, with two peaks of redness. “One occurs at 3-6 hours,” and the other peak occurs when the drug is wearing off later in the day, Dr. Stein Gold said.
A study that sought to identify factors that might make patients more prone to this adverse effect found that “less is better” regarding brimonidine application, with an optimal application of one to three pea-sized dollops on the face, not five as instructed in the package insert. In addition, patients with more than five flushing episodes a week, particularly women, “tend to have more labile disease and [are] more likely to get that rebound erythema,” the study found.
Oxymetazoline 1% in a cream formulation has a “slightly more gentle onset of action and a more gentle offset of action,” without exacerbation of erythema and has been shown to have sustained efficacy over 52 weeks. In a yearlong safety study, there were “no new red flags and we weren’t seeing that redness at hours 3 to 6, or even when you take the patient off the drug,” she noted.
Dr. Stein Gold reported that she has served as a consultant, investigator, or speaker for Galderma, Dermira, Foamix Pharmaceuticals, Valeant, Allergan, Actavis, and Roche.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – The pathophysiology of rosacea is complicated, which is why “we try to target our treatments to various areas in this pathogenic pathway” to achieve optimal results, according to Linda Stein Gold, MD, director of dermatology research at the Henry Ford Health System in Detroit.
For example, in a patient with papules and pustules, a topical or oral anti-inflammatory agent is needed “to calm that down.” If background erythema is present, separate from papules and pustules, use a topical alpha-adrenergic agonist, she advised. For telangiectasias, consider a device-based treatment, and for a phyma, a surgical approach, she recommended at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
For the background erythema of rosacea, which she described as “that pink face that’s always there,” the two alpha-adrenergic receptor agonists available, brimonidine gel 0.33%, approved by the Food and Drug Administration in 2013, and oxymetazoline cream 1%, approved in 2017, both work on the neurovascular junction, but on different receptors.
Brimonidine “kicks in very, very rapidly,” with a significant decrease in background erythema evident within 30 minutes and improvements that last over a 12-hour day, she said. It is effective over a year, but in longterm and postmarketing studies, about 20% of patients experienced exacerbation of erythema, with two peaks of redness. “One occurs at 3-6 hours,” and the other peak occurs when the drug is wearing off later in the day, Dr. Stein Gold said.
A study that sought to identify factors that might make patients more prone to this adverse effect found that “less is better” regarding brimonidine application, with an optimal application of one to three pea-sized dollops on the face, not five as instructed in the package insert. In addition, patients with more than five flushing episodes a week, particularly women, “tend to have more labile disease and [are] more likely to get that rebound erythema,” the study found.
Oxymetazoline 1% in a cream formulation has a “slightly more gentle onset of action and a more gentle offset of action,” without exacerbation of erythema and has been shown to have sustained efficacy over 52 weeks. In a yearlong safety study, there were “no new red flags and we weren’t seeing that redness at hours 3 to 6, or even when you take the patient off the drug,” she noted.
Dr. Stein Gold reported that she has served as a consultant, investigator, or speaker for Galderma, Dermira, Foamix Pharmaceuticals, Valeant, Allergan, Actavis, and Roche.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM SDEF HAWAII DERMATOLOGY SEMINAR
Dupilumab for severe AD: Expert advocates continuous treatment
LAHAINA, HAWAII – rather than treatment on an as-needed basis, Andrew Blauvelt, MD, advised at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“I view atopic dermatitis as a chronic disease requiring chronic treatment. So be very careful about stopping. We know that if you start and stop biologics you’re going to be far more prone to develop antidrug antibodies resulting in drug resistance than with continual dosing,” said Dr. Blauvelt, a dermatologist and clinical trialist who is president of the Oregon Medical Research Center, Portland.
He said dupilumab (Dupixent) seldom induces disease remission as defined by clear skin while off all drugs for at least 1 year, although he has a few patients who seem to be exceptions. Yet clearly dupilumab doesn’t change an individual’s predisposing genetics or environmental allergen exposure pattern, so it’s best to think of it as a treatment for the long haul.
Dr. Blauvelt considers dupilumab far and away the best medication for treatment of adults and teenagers whose atopic dermatitis (AD) is uncontrolled with topical therapy. Payers often balk at authorizing dupilumab unless a patient has first undergone an unsuccessful trial of cyclosporine or methotrexate, which are far less expensive. But that’s not what the expert consensus guidelines recommend (Ann Allergy Asthma Immunol. 2018 Jan;120[1]:10-22.e2).
“The guidelines don’t suggest that failure on methotrexate or cyclosporine should be a prerequisite for dupilumab. So if you’re having problems with an insurance company and you really want to use dupilumab, you can point to this paper and say, ‘Look, the experts do not recommend step therapy, we can go directly to dupilumab.’ And the dupilumab label says simply that failure of topical therapy is required before being allowed to use dupilumab. So both the label and the experts say you don’t have to go through a bunch of steps in order to get to what I consider the very best drug for our patients,” he explained.
Both cyclosporine and methotrexate are far more broadly immunosuppressive and hence less safe than dupilumab. Both require laboratory monitoring. In contrast, blood work isn’t required in patients on dupilumab; in fact, Dr. Blauvelt considers it an unwise use of resources. Nor is tuberculosis testing advised prior to starting dupilumab.
When he can’t get authorization for dupilumab, Dr. Blauvelt’s go-to drug is methotrexate at 15-25 mg/week. It’s not as effective as cyclosporine for rapid clearing, but it’s safer for long-term use.
“Methotrexate is the devil we know – we know how to use it, and we know how to monitor for it,” he commented, adding that he reserves cyclosporine for a maximum of a month or 2 of acute crisis management, or as a bridge in getting patients off of systemic corticosteroids.
Set realistic efficacy expectations
Dermatologists who prescribe the newest biologics for psoriasis are accustomed to routinely seeing PASI 90 responses and even complete disease clearing. However, AD is a more challenging disease. In the landmark dupilumab phase 3 randomized trials, roughly two-thirds of patients achieved an Eczema Area and Severity Index (EASI) 75 response, with a mean 80% improvement in EASI symptom scores over baseline. Roughly 20% of dupilumab-treated adults with AD achieve disease clearance, and a similar percentage become almost clear. The improvements are durable in long-term follow-up studies.
“Dupilumab doesn’t get a lot of people to zero. They’re not going to be completely clearing their eczema. So they shouldn’t be freaking out if they still have eczema. What they can expect is diminution of the disease to much lower levels,” Dr. Blauvelt said.
The marked improvement in quality of life that occurs with dupilumab therapy isn’t adequately captured by EASI scores. “In my experience, more than 80%-90% of patients are happy on this drug,” Dr. Blauvelt said.
Conference codirector Linda Stein Gold, MD, agreed, commenting that she has found dupilumab to be “absolutely life altering” for her patients with severe AD.
“They know they still have AD, but now they can go whole days without thinking about it,” said Dr. Stein Gold, director of dermatology research and head of the division of dermatology at the Henry Ford Health System in Detroit.
Dr. Blauvelt noted that most of his patients on dupilumab remain on topical therapy, typically with triamcinolone on the body and hydrocortisone on the face. What he terms “miniflares” in patients on dupilumab are not at all unusual, but they’re readily manageable.
“Flares that used to last for weeks now last for a day or 2, maybe 3, and then it’s back to normal in patients on dupilumab,” Dr. Blauvelt said.
Safety
Dupilumab is a targeted inhibitor of interleukins-4 and -13, cytokines involved in allergy-mediated inflammation and the control of parasitic infections, but which have no bearing on control of bacterial or viral infections or malignancies. Indeed, the randomized trials have demonstrated that the incidence of skin infections is actually lower with dupilumab than with placebo.
“You’re improving the skin barrier so much that they’re not going to be getting staph or herpes simplex,” he explained.
The main side effect consists of dupilumab-associated eye issues. These occur in up to 20% of treated patients and encompass a spectrum ranging from dry eye to nonallergic conjunctivitis, inflammation of the eyelid, and keratitis. The mechanism is unknown. The condition is not infectious and doesn’t affect vision. Intriguingly, it doesn’t occur in patients with asthma, a disease for which dupilumab is also approved.
“Ask about eye issues at every office visit,” the dermatologist urged.
He sends all of his AD patients with dupilumab-associated eye issues to a single trusted local ophthalmologist and lets him manage the condition, which is generally mild to moderate. Eye issues have resulted in discontinuation of dupilumab in only 2 of the roughly 150 AD patients Dr. Blauvelt has placed on the biologic. The ophthalmologist generally relies upon lubricating eye drops and a couple of weeks of steroid eye drops or, in some cases, topical cyclosporine 0.05% ophthalmic emulsion, followed by episodic use of the steroid eye drops on an as-needed basis.
Residual facial disease in AD patients on dupilumab can be caused by a variety of causes, including breakthrough AD, rosacea, allergic contact dermatitis, steroid withdrawal, or photosensitivity, with Demodex thought to play a role in some cases.
Dr. Blauvelt reported serving as a scientific adviser to and paid clinical trial investigator for several dozen pharmaceutical companies. SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – rather than treatment on an as-needed basis, Andrew Blauvelt, MD, advised at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“I view atopic dermatitis as a chronic disease requiring chronic treatment. So be very careful about stopping. We know that if you start and stop biologics you’re going to be far more prone to develop antidrug antibodies resulting in drug resistance than with continual dosing,” said Dr. Blauvelt, a dermatologist and clinical trialist who is president of the Oregon Medical Research Center, Portland.
He said dupilumab (Dupixent) seldom induces disease remission as defined by clear skin while off all drugs for at least 1 year, although he has a few patients who seem to be exceptions. Yet clearly dupilumab doesn’t change an individual’s predisposing genetics or environmental allergen exposure pattern, so it’s best to think of it as a treatment for the long haul.
Dr. Blauvelt considers dupilumab far and away the best medication for treatment of adults and teenagers whose atopic dermatitis (AD) is uncontrolled with topical therapy. Payers often balk at authorizing dupilumab unless a patient has first undergone an unsuccessful trial of cyclosporine or methotrexate, which are far less expensive. But that’s not what the expert consensus guidelines recommend (Ann Allergy Asthma Immunol. 2018 Jan;120[1]:10-22.e2).
“The guidelines don’t suggest that failure on methotrexate or cyclosporine should be a prerequisite for dupilumab. So if you’re having problems with an insurance company and you really want to use dupilumab, you can point to this paper and say, ‘Look, the experts do not recommend step therapy, we can go directly to dupilumab.’ And the dupilumab label says simply that failure of topical therapy is required before being allowed to use dupilumab. So both the label and the experts say you don’t have to go through a bunch of steps in order to get to what I consider the very best drug for our patients,” he explained.
Both cyclosporine and methotrexate are far more broadly immunosuppressive and hence less safe than dupilumab. Both require laboratory monitoring. In contrast, blood work isn’t required in patients on dupilumab; in fact, Dr. Blauvelt considers it an unwise use of resources. Nor is tuberculosis testing advised prior to starting dupilumab.
When he can’t get authorization for dupilumab, Dr. Blauvelt’s go-to drug is methotrexate at 15-25 mg/week. It’s not as effective as cyclosporine for rapid clearing, but it’s safer for long-term use.
“Methotrexate is the devil we know – we know how to use it, and we know how to monitor for it,” he commented, adding that he reserves cyclosporine for a maximum of a month or 2 of acute crisis management, or as a bridge in getting patients off of systemic corticosteroids.
Set realistic efficacy expectations
Dermatologists who prescribe the newest biologics for psoriasis are accustomed to routinely seeing PASI 90 responses and even complete disease clearing. However, AD is a more challenging disease. In the landmark dupilumab phase 3 randomized trials, roughly two-thirds of patients achieved an Eczema Area and Severity Index (EASI) 75 response, with a mean 80% improvement in EASI symptom scores over baseline. Roughly 20% of dupilumab-treated adults with AD achieve disease clearance, and a similar percentage become almost clear. The improvements are durable in long-term follow-up studies.
“Dupilumab doesn’t get a lot of people to zero. They’re not going to be completely clearing their eczema. So they shouldn’t be freaking out if they still have eczema. What they can expect is diminution of the disease to much lower levels,” Dr. Blauvelt said.
The marked improvement in quality of life that occurs with dupilumab therapy isn’t adequately captured by EASI scores. “In my experience, more than 80%-90% of patients are happy on this drug,” Dr. Blauvelt said.
Conference codirector Linda Stein Gold, MD, agreed, commenting that she has found dupilumab to be “absolutely life altering” for her patients with severe AD.
“They know they still have AD, but now they can go whole days without thinking about it,” said Dr. Stein Gold, director of dermatology research and head of the division of dermatology at the Henry Ford Health System in Detroit.
Dr. Blauvelt noted that most of his patients on dupilumab remain on topical therapy, typically with triamcinolone on the body and hydrocortisone on the face. What he terms “miniflares” in patients on dupilumab are not at all unusual, but they’re readily manageable.
“Flares that used to last for weeks now last for a day or 2, maybe 3, and then it’s back to normal in patients on dupilumab,” Dr. Blauvelt said.
Safety
Dupilumab is a targeted inhibitor of interleukins-4 and -13, cytokines involved in allergy-mediated inflammation and the control of parasitic infections, but which have no bearing on control of bacterial or viral infections or malignancies. Indeed, the randomized trials have demonstrated that the incidence of skin infections is actually lower with dupilumab than with placebo.
“You’re improving the skin barrier so much that they’re not going to be getting staph or herpes simplex,” he explained.
The main side effect consists of dupilumab-associated eye issues. These occur in up to 20% of treated patients and encompass a spectrum ranging from dry eye to nonallergic conjunctivitis, inflammation of the eyelid, and keratitis. The mechanism is unknown. The condition is not infectious and doesn’t affect vision. Intriguingly, it doesn’t occur in patients with asthma, a disease for which dupilumab is also approved.
“Ask about eye issues at every office visit,” the dermatologist urged.
He sends all of his AD patients with dupilumab-associated eye issues to a single trusted local ophthalmologist and lets him manage the condition, which is generally mild to moderate. Eye issues have resulted in discontinuation of dupilumab in only 2 of the roughly 150 AD patients Dr. Blauvelt has placed on the biologic. The ophthalmologist generally relies upon lubricating eye drops and a couple of weeks of steroid eye drops or, in some cases, topical cyclosporine 0.05% ophthalmic emulsion, followed by episodic use of the steroid eye drops on an as-needed basis.
Residual facial disease in AD patients on dupilumab can be caused by a variety of causes, including breakthrough AD, rosacea, allergic contact dermatitis, steroid withdrawal, or photosensitivity, with Demodex thought to play a role in some cases.
Dr. Blauvelt reported serving as a scientific adviser to and paid clinical trial investigator for several dozen pharmaceutical companies. SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – rather than treatment on an as-needed basis, Andrew Blauvelt, MD, advised at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“I view atopic dermatitis as a chronic disease requiring chronic treatment. So be very careful about stopping. We know that if you start and stop biologics you’re going to be far more prone to develop antidrug antibodies resulting in drug resistance than with continual dosing,” said Dr. Blauvelt, a dermatologist and clinical trialist who is president of the Oregon Medical Research Center, Portland.
He said dupilumab (Dupixent) seldom induces disease remission as defined by clear skin while off all drugs for at least 1 year, although he has a few patients who seem to be exceptions. Yet clearly dupilumab doesn’t change an individual’s predisposing genetics or environmental allergen exposure pattern, so it’s best to think of it as a treatment for the long haul.
Dr. Blauvelt considers dupilumab far and away the best medication for treatment of adults and teenagers whose atopic dermatitis (AD) is uncontrolled with topical therapy. Payers often balk at authorizing dupilumab unless a patient has first undergone an unsuccessful trial of cyclosporine or methotrexate, which are far less expensive. But that’s not what the expert consensus guidelines recommend (Ann Allergy Asthma Immunol. 2018 Jan;120[1]:10-22.e2).
“The guidelines don’t suggest that failure on methotrexate or cyclosporine should be a prerequisite for dupilumab. So if you’re having problems with an insurance company and you really want to use dupilumab, you can point to this paper and say, ‘Look, the experts do not recommend step therapy, we can go directly to dupilumab.’ And the dupilumab label says simply that failure of topical therapy is required before being allowed to use dupilumab. So both the label and the experts say you don’t have to go through a bunch of steps in order to get to what I consider the very best drug for our patients,” he explained.
Both cyclosporine and methotrexate are far more broadly immunosuppressive and hence less safe than dupilumab. Both require laboratory monitoring. In contrast, blood work isn’t required in patients on dupilumab; in fact, Dr. Blauvelt considers it an unwise use of resources. Nor is tuberculosis testing advised prior to starting dupilumab.
When he can’t get authorization for dupilumab, Dr. Blauvelt’s go-to drug is methotrexate at 15-25 mg/week. It’s not as effective as cyclosporine for rapid clearing, but it’s safer for long-term use.
“Methotrexate is the devil we know – we know how to use it, and we know how to monitor for it,” he commented, adding that he reserves cyclosporine for a maximum of a month or 2 of acute crisis management, or as a bridge in getting patients off of systemic corticosteroids.
Set realistic efficacy expectations
Dermatologists who prescribe the newest biologics for psoriasis are accustomed to routinely seeing PASI 90 responses and even complete disease clearing. However, AD is a more challenging disease. In the landmark dupilumab phase 3 randomized trials, roughly two-thirds of patients achieved an Eczema Area and Severity Index (EASI) 75 response, with a mean 80% improvement in EASI symptom scores over baseline. Roughly 20% of dupilumab-treated adults with AD achieve disease clearance, and a similar percentage become almost clear. The improvements are durable in long-term follow-up studies.
“Dupilumab doesn’t get a lot of people to zero. They’re not going to be completely clearing their eczema. So they shouldn’t be freaking out if they still have eczema. What they can expect is diminution of the disease to much lower levels,” Dr. Blauvelt said.
The marked improvement in quality of life that occurs with dupilumab therapy isn’t adequately captured by EASI scores. “In my experience, more than 80%-90% of patients are happy on this drug,” Dr. Blauvelt said.
Conference codirector Linda Stein Gold, MD, agreed, commenting that she has found dupilumab to be “absolutely life altering” for her patients with severe AD.
“They know they still have AD, but now they can go whole days without thinking about it,” said Dr. Stein Gold, director of dermatology research and head of the division of dermatology at the Henry Ford Health System in Detroit.
Dr. Blauvelt noted that most of his patients on dupilumab remain on topical therapy, typically with triamcinolone on the body and hydrocortisone on the face. What he terms “miniflares” in patients on dupilumab are not at all unusual, but they’re readily manageable.
“Flares that used to last for weeks now last for a day or 2, maybe 3, and then it’s back to normal in patients on dupilumab,” Dr. Blauvelt said.
Safety
Dupilumab is a targeted inhibitor of interleukins-4 and -13, cytokines involved in allergy-mediated inflammation and the control of parasitic infections, but which have no bearing on control of bacterial or viral infections or malignancies. Indeed, the randomized trials have demonstrated that the incidence of skin infections is actually lower with dupilumab than with placebo.
“You’re improving the skin barrier so much that they’re not going to be getting staph or herpes simplex,” he explained.
The main side effect consists of dupilumab-associated eye issues. These occur in up to 20% of treated patients and encompass a spectrum ranging from dry eye to nonallergic conjunctivitis, inflammation of the eyelid, and keratitis. The mechanism is unknown. The condition is not infectious and doesn’t affect vision. Intriguingly, it doesn’t occur in patients with asthma, a disease for which dupilumab is also approved.
“Ask about eye issues at every office visit,” the dermatologist urged.
He sends all of his AD patients with dupilumab-associated eye issues to a single trusted local ophthalmologist and lets him manage the condition, which is generally mild to moderate. Eye issues have resulted in discontinuation of dupilumab in only 2 of the roughly 150 AD patients Dr. Blauvelt has placed on the biologic. The ophthalmologist generally relies upon lubricating eye drops and a couple of weeks of steroid eye drops or, in some cases, topical cyclosporine 0.05% ophthalmic emulsion, followed by episodic use of the steroid eye drops on an as-needed basis.
Residual facial disease in AD patients on dupilumab can be caused by a variety of causes, including breakthrough AD, rosacea, allergic contact dermatitis, steroid withdrawal, or photosensitivity, with Demodex thought to play a role in some cases.
Dr. Blauvelt reported serving as a scientific adviser to and paid clinical trial investigator for several dozen pharmaceutical companies. SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT OPINION FROM SDEF HAWAII DERMATOLOGY SEMINAR
50 years of growth: More dermatologists, more demand
A dive into the dermatology data pool reveals a great deal of change in the 50 years that Dermatology News and Skin & Allergy News have been covering the specialty.
For one thing, there are a lot more dermatologists now. The American Academy of Dermatology puts its 2020 membership at a somewhat lower 18,898, noting that not all dermatologists are AAD members.
Much of that 50-year increase comes from the larger proportion of women entering the specialty. In 1970, only 7% of dermatologists were women, but by 2019 they represented over 37% of the dermatology workforce, according to the AMA numbers. The AAD, however, reports more women than the AMA (8,940 vs. 7,482), so the proportion of female academy members in 2020 is about 47%.
The population of dermatologists has increased faster than the general population since 1970, leading to a rising density of providers. A report from 1973 put the national figure at 1.7 dermatologists per 100,000 population in 1970, and two more recent studies in JAMA Dermatology reported density levels of 3.65 per 100,000 in 2013 and 3.36 per 100,000 in 2016.
In that 1973 report, the authors said that there was “no evidence to suggest that there will not be sufficient dermatologists in the future, if training centers are maintained and adequately financed,” noting that “the current rate of filling of dermatologic residency positions should not diminish and may continue to increase.”
In 2017, the conclusion was quite different: “Dermatologists alone have been unable to meet increasing patient demand for dermatologic services. The number of dermatology residency training positions has been relatively stagnant, suggesting that the current supply of dermatologists in training will be insufficient to fully meet growing future demand.”
The current state of strong demand for dermatologists does come with some benefits. In a 2019 survey by physician recruitment firm Merritt Hawkins, dermatologists had the 6th-highest starting salary at $420,000 a year. An article in the July 1971 issue of Skin & Allergy News offered a somewhat different perspective on compensation for first-year dermatologists: Recommendations offered by those already in practice ranged from $12,000 to $24,000.
Those first-year physicians are coming from residencies that, not too surprisingly, are now producing more new dermatologists than they did in 1970, although perhaps not as many more as might be expected.
There were an estimated 250 individuals completing dermatology residencies annually in 1976 (J Am Acad Dermatol. 1981;4[3]:344-5), which suggests a total of approximately 750 active residents at a time when there were about 5,000 practicing dermatologists in the country.
For the 2018-2019 academic year, there were 1,439 active residents in U.S. training programs, according to the Association of American Medical Colleges, so there were twice the number of residents but four times as many dermatologists in practice, compared with 1976.
The next link in the dermatology supply chain would be the medical schools, and there the numbers of full-time faculty have more than kept up. For the 1969-1970 academic year, there were 202 full-time positions in dermatology (JAMA. 1970;214:1483-581). By 2019, the number had risen to 1,519, according to the Association of American Medical Colleges.
A dive into the dermatology data pool reveals a great deal of change in the 50 years that Dermatology News and Skin & Allergy News have been covering the specialty.

For one thing, there are a lot more dermatologists now. The American Academy of Dermatology puts its 2020 membership at a somewhat lower 18,898, noting that not all dermatologists are AAD members.
Much of that 50-year increase comes from the larger proportion of women entering the specialty. In 1970, only 7% of dermatologists were women, but by 2019 they represented over 37% of the dermatology workforce, according to the AMA numbers. The AAD, however, reports more women than the AMA (8,940 vs. 7,482), so the proportion of female academy members in 2020 is about 47%.
The population of dermatologists has increased faster than the general population since 1970, leading to a rising density of providers. A report from 1973 put the national figure at 1.7 dermatologists per 100,000 population in 1970, and two more recent studies in JAMA Dermatology reported density levels of 3.65 per 100,000 in 2013 and 3.36 per 100,000 in 2016.
In that 1973 report, the authors said that there was “no evidence to suggest that there will not be sufficient dermatologists in the future, if training centers are maintained and adequately financed,” noting that “the current rate of filling of dermatologic residency positions should not diminish and may continue to increase.”
In 2017, the conclusion was quite different: “Dermatologists alone have been unable to meet increasing patient demand for dermatologic services. The number of dermatology residency training positions has been relatively stagnant, suggesting that the current supply of dermatologists in training will be insufficient to fully meet growing future demand.”
The current state of strong demand for dermatologists does come with some benefits. In a 2019 survey by physician recruitment firm Merritt Hawkins, dermatologists had the 6th-highest starting salary at $420,000 a year. An article in the July 1971 issue of Skin & Allergy News offered a somewhat different perspective on compensation for first-year dermatologists: Recommendations offered by those already in practice ranged from $12,000 to $24,000.
Those first-year physicians are coming from residencies that, not too surprisingly, are now producing more new dermatologists than they did in 1970, although perhaps not as many more as might be expected.
There were an estimated 250 individuals completing dermatology residencies annually in 1976 (J Am Acad Dermatol. 1981;4[3]:344-5), which suggests a total of approximately 750 active residents at a time when there were about 5,000 practicing dermatologists in the country.
For the 2018-2019 academic year, there were 1,439 active residents in U.S. training programs, according to the Association of American Medical Colleges, so there were twice the number of residents but four times as many dermatologists in practice, compared with 1976.
The next link in the dermatology supply chain would be the medical schools, and there the numbers of full-time faculty have more than kept up. For the 1969-1970 academic year, there were 202 full-time positions in dermatology (JAMA. 1970;214:1483-581). By 2019, the number had risen to 1,519, according to the Association of American Medical Colleges.
A dive into the dermatology data pool reveals a great deal of change in the 50 years that Dermatology News and Skin & Allergy News have been covering the specialty.

For one thing, there are a lot more dermatologists now. The American Academy of Dermatology puts its 2020 membership at a somewhat lower 18,898, noting that not all dermatologists are AAD members.
Much of that 50-year increase comes from the larger proportion of women entering the specialty. In 1970, only 7% of dermatologists were women, but by 2019 they represented over 37% of the dermatology workforce, according to the AMA numbers. The AAD, however, reports more women than the AMA (8,940 vs. 7,482), so the proportion of female academy members in 2020 is about 47%.
The population of dermatologists has increased faster than the general population since 1970, leading to a rising density of providers. A report from 1973 put the national figure at 1.7 dermatologists per 100,000 population in 1970, and two more recent studies in JAMA Dermatology reported density levels of 3.65 per 100,000 in 2013 and 3.36 per 100,000 in 2016.
In that 1973 report, the authors said that there was “no evidence to suggest that there will not be sufficient dermatologists in the future, if training centers are maintained and adequately financed,” noting that “the current rate of filling of dermatologic residency positions should not diminish and may continue to increase.”
In 2017, the conclusion was quite different: “Dermatologists alone have been unable to meet increasing patient demand for dermatologic services. The number of dermatology residency training positions has been relatively stagnant, suggesting that the current supply of dermatologists in training will be insufficient to fully meet growing future demand.”
The current state of strong demand for dermatologists does come with some benefits. In a 2019 survey by physician recruitment firm Merritt Hawkins, dermatologists had the 6th-highest starting salary at $420,000 a year. An article in the July 1971 issue of Skin & Allergy News offered a somewhat different perspective on compensation for first-year dermatologists: Recommendations offered by those already in practice ranged from $12,000 to $24,000.
Those first-year physicians are coming from residencies that, not too surprisingly, are now producing more new dermatologists than they did in 1970, although perhaps not as many more as might be expected.
There were an estimated 250 individuals completing dermatology residencies annually in 1976 (J Am Acad Dermatol. 1981;4[3]:344-5), which suggests a total of approximately 750 active residents at a time when there were about 5,000 practicing dermatologists in the country.
For the 2018-2019 academic year, there were 1,439 active residents in U.S. training programs, according to the Association of American Medical Colleges, so there were twice the number of residents but four times as many dermatologists in practice, compared with 1976.
The next link in the dermatology supply chain would be the medical schools, and there the numbers of full-time faculty have more than kept up. For the 1969-1970 academic year, there were 202 full-time positions in dermatology (JAMA. 1970;214:1483-581). By 2019, the number had risen to 1,519, according to the Association of American Medical Colleges.
Two new Novel Coronavirus cases confirmed among quarantined U.S. patients
The Centers for Disease Control and Prevention announced two new patients now have the 2019 Novel Coronavirus (2019-nCoV), bringing the case total in the United States to 15.

The 14th case was discovered in California among a group of people under federal quarantine after returning from the Hubei Province in China. That patient was on a U.S. State Department–chartered flight that arrived in the United States on Feb. 7.
The 15th case was discovered in Texas among a group of people who also are under federal quarantine. That patient arrived on a State Department–chartered flight that arrived on Feb. 7. It is the first person in Texas that has tested positive for 2019-nCoV.
CDC said in a statement announcing the Texas case that there “will likely be additional cases in the coming days and weeks, including among other people recently returned from Wuhan.” Officials noted that more than 600 people who have returned as part of State Department–chartered flights are currently under that 14-day quarantine.
The agency is preparing for more widespread cases of 2019-nCoV.
Nancy Messonnier, MD, director of the CDC National Center for Immunization and Respiratory Diseases, said that containment has been the early focus for the agency.
“The goal of the measures we have taken to date are to slow the introduction and impact of this disease in the United States, but at some point, we are likely to see community spread in the U.S.,” Dr. Messonnier said during a Feb. 12 teleconference with reporters. She added that the federal response will change over time as the virus spreads.
Dr. Messonnier noted that public health officials are planning for the increased demands that a wider outbreak of 2019-nCov would place on the health care delivery system, including ensuring an adequate supply of medical equipment.
The Centers for Disease Control and Prevention announced two new patients now have the 2019 Novel Coronavirus (2019-nCoV), bringing the case total in the United States to 15.

The 14th case was discovered in California among a group of people under federal quarantine after returning from the Hubei Province in China. That patient was on a U.S. State Department–chartered flight that arrived in the United States on Feb. 7.
The 15th case was discovered in Texas among a group of people who also are under federal quarantine. That patient arrived on a State Department–chartered flight that arrived on Feb. 7. It is the first person in Texas that has tested positive for 2019-nCoV.
CDC said in a statement announcing the Texas case that there “will likely be additional cases in the coming days and weeks, including among other people recently returned from Wuhan.” Officials noted that more than 600 people who have returned as part of State Department–chartered flights are currently under that 14-day quarantine.
The agency is preparing for more widespread cases of 2019-nCoV.
Nancy Messonnier, MD, director of the CDC National Center for Immunization and Respiratory Diseases, said that containment has been the early focus for the agency.
“The goal of the measures we have taken to date are to slow the introduction and impact of this disease in the United States, but at some point, we are likely to see community spread in the U.S.,” Dr. Messonnier said during a Feb. 12 teleconference with reporters. She added that the federal response will change over time as the virus spreads.
Dr. Messonnier noted that public health officials are planning for the increased demands that a wider outbreak of 2019-nCov would place on the health care delivery system, including ensuring an adequate supply of medical equipment.
The Centers for Disease Control and Prevention announced two new patients now have the 2019 Novel Coronavirus (2019-nCoV), bringing the case total in the United States to 15.

The 14th case was discovered in California among a group of people under federal quarantine after returning from the Hubei Province in China. That patient was on a U.S. State Department–chartered flight that arrived in the United States on Feb. 7.
The 15th case was discovered in Texas among a group of people who also are under federal quarantine. That patient arrived on a State Department–chartered flight that arrived on Feb. 7. It is the first person in Texas that has tested positive for 2019-nCoV.
CDC said in a statement announcing the Texas case that there “will likely be additional cases in the coming days and weeks, including among other people recently returned from Wuhan.” Officials noted that more than 600 people who have returned as part of State Department–chartered flights are currently under that 14-day quarantine.
The agency is preparing for more widespread cases of 2019-nCoV.
Nancy Messonnier, MD, director of the CDC National Center for Immunization and Respiratory Diseases, said that containment has been the early focus for the agency.
“The goal of the measures we have taken to date are to slow the introduction and impact of this disease in the United States, but at some point, we are likely to see community spread in the U.S.,” Dr. Messonnier said during a Feb. 12 teleconference with reporters. She added that the federal response will change over time as the virus spreads.
Dr. Messonnier noted that public health officials are planning for the increased demands that a wider outbreak of 2019-nCov would place on the health care delivery system, including ensuring an adequate supply of medical equipment.














