User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
Make the Diagnosis - March 2020
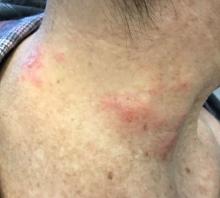
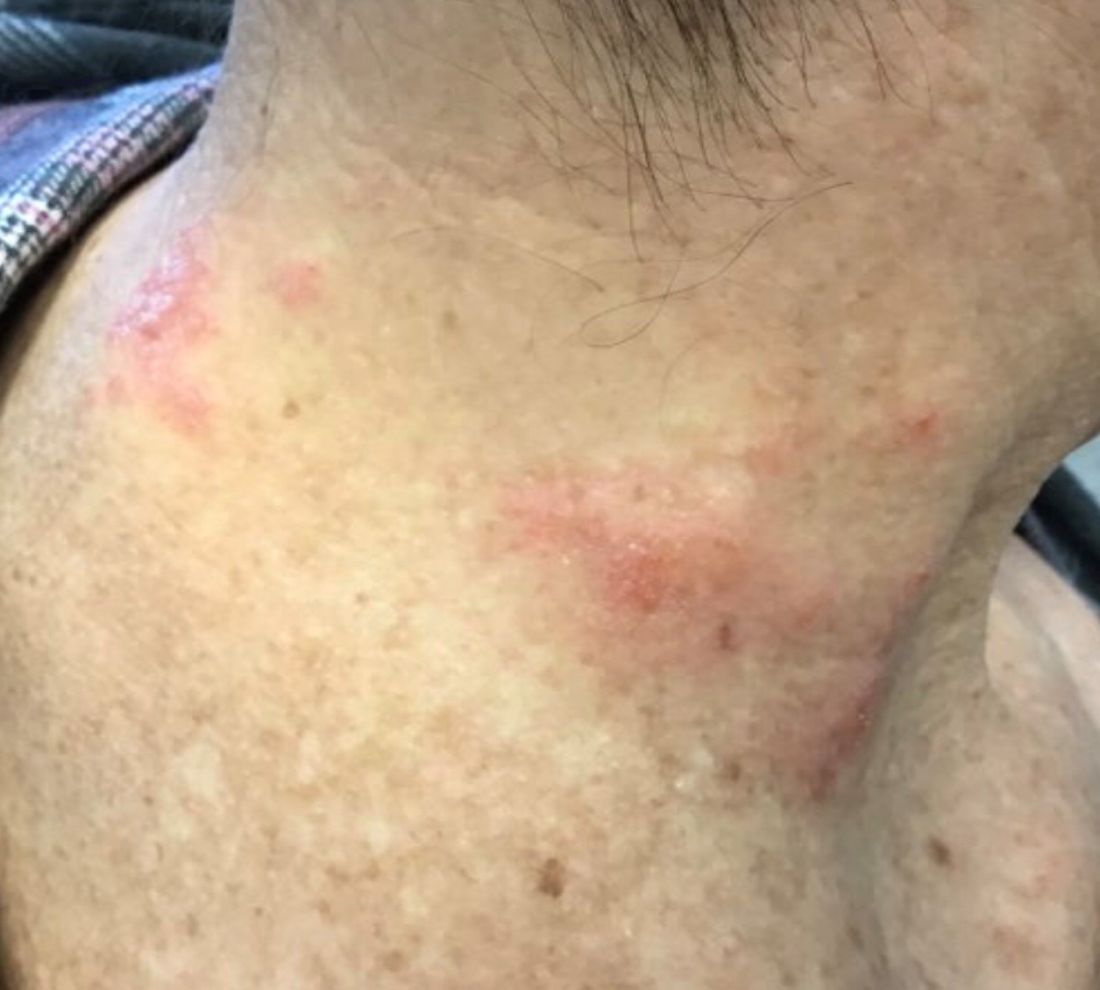
The patient’s biopsy showed sparse and grouped and slightly enlarged atypical stained mononuclear cells in mostly perifollicular areas with focal epidermotropism. CD30 staining was positive. She responded to potent topical steroids.
The etiology of LyP is unknown. It is unclear whether the proliferation of T-cells is a benign and chronic disorder, or an indolent T-cell malignancy.
In addition, 10% of LyP cases are associated with anaplastic large-cell lymphoma, cutaneous T-cell lymphoma (mycosis fungoides), or Hodgkin lymphoma. Borderline cases are those that overlap LyP and lymphoma.
Patients typically present with crops of asymptomatic erythematous to brown papules that may become pustular, vesicular, or necrotic. Lesions tend to resolve within 2-8 weeks with or without scarring. The trunk and extremities are commonly affected. The condition tends to be chronic over months to years. The waxing and waning course is characteristic of LyP. Constitutional symptoms are generally absent in cases not associated with systemic disease.
Histopathologic examination reveals a dense wedge-shaped dermal infiltrate of atypical lymphocytes along with numerous eosinophils and neutrophils. Epidermotropism may be present and lymphocytes stain positive for CD30+. Vessels in the dermis may exhibit fibrin deposition and red blood cell extravasation. Histologically, LyP can be classified as Type A to E. These subtypes are determined by the size and type of atypical cells, location and amount of infiltrate, and staining of CD30 and CD8.
The differential diagnosis of LyP includes pityriasis lichenoides, anaplastic large cell lymphoma, cutaneous T-cell lymphoma, folliculitis, arthropod assault, Langerhans cell histiocytosis, and leukemia cutis. Treatment is symptomatic. Mild forms of LyP can many times be managed with superpotent topical corticosteroids. Bexarotene gel has been used for early lesions. For more widespread or persistent disease, intralesional corticosteroids, phototherapy (UVB or PUVA), tetracycline antibiotics, and methotrexate have been reported to be effective. Refractory cases may respond to interferon alpha or oral bexarotene. Routine evaluations are recommended as patients may be at increased risk for the development of lymphoma.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
The patient’s biopsy showed sparse and grouped and slightly enlarged atypical stained mononuclear cells in mostly perifollicular areas with focal epidermotropism. CD30 staining was positive. She responded to potent topical steroids.
The etiology of LyP is unknown. It is unclear whether the proliferation of T-cells is a benign and chronic disorder, or an indolent T-cell malignancy.
In addition, 10% of LyP cases are associated with anaplastic large-cell lymphoma, cutaneous T-cell lymphoma (mycosis fungoides), or Hodgkin lymphoma. Borderline cases are those that overlap LyP and lymphoma.
Patients typically present with crops of asymptomatic erythematous to brown papules that may become pustular, vesicular, or necrotic. Lesions tend to resolve within 2-8 weeks with or without scarring. The trunk and extremities are commonly affected. The condition tends to be chronic over months to years. The waxing and waning course is characteristic of LyP. Constitutional symptoms are generally absent in cases not associated with systemic disease.
Histopathologic examination reveals a dense wedge-shaped dermal infiltrate of atypical lymphocytes along with numerous eosinophils and neutrophils. Epidermotropism may be present and lymphocytes stain positive for CD30+. Vessels in the dermis may exhibit fibrin deposition and red blood cell extravasation. Histologically, LyP can be classified as Type A to E. These subtypes are determined by the size and type of atypical cells, location and amount of infiltrate, and staining of CD30 and CD8.
The differential diagnosis of LyP includes pityriasis lichenoides, anaplastic large cell lymphoma, cutaneous T-cell lymphoma, folliculitis, arthropod assault, Langerhans cell histiocytosis, and leukemia cutis. Treatment is symptomatic. Mild forms of LyP can many times be managed with superpotent topical corticosteroids. Bexarotene gel has been used for early lesions. For more widespread or persistent disease, intralesional corticosteroids, phototherapy (UVB or PUVA), tetracycline antibiotics, and methotrexate have been reported to be effective. Refractory cases may respond to interferon alpha or oral bexarotene. Routine evaluations are recommended as patients may be at increased risk for the development of lymphoma.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
The patient’s biopsy showed sparse and grouped and slightly enlarged atypical stained mononuclear cells in mostly perifollicular areas with focal epidermotropism. CD30 staining was positive. She responded to potent topical steroids.
The etiology of LyP is unknown. It is unclear whether the proliferation of T-cells is a benign and chronic disorder, or an indolent T-cell malignancy.
In addition, 10% of LyP cases are associated with anaplastic large-cell lymphoma, cutaneous T-cell lymphoma (mycosis fungoides), or Hodgkin lymphoma. Borderline cases are those that overlap LyP and lymphoma.
Patients typically present with crops of asymptomatic erythematous to brown papules that may become pustular, vesicular, or necrotic. Lesions tend to resolve within 2-8 weeks with or without scarring. The trunk and extremities are commonly affected. The condition tends to be chronic over months to years. The waxing and waning course is characteristic of LyP. Constitutional symptoms are generally absent in cases not associated with systemic disease.
Histopathologic examination reveals a dense wedge-shaped dermal infiltrate of atypical lymphocytes along with numerous eosinophils and neutrophils. Epidermotropism may be present and lymphocytes stain positive for CD30+. Vessels in the dermis may exhibit fibrin deposition and red blood cell extravasation. Histologically, LyP can be classified as Type A to E. These subtypes are determined by the size and type of atypical cells, location and amount of infiltrate, and staining of CD30 and CD8.
The differential diagnosis of LyP includes pityriasis lichenoides, anaplastic large cell lymphoma, cutaneous T-cell lymphoma, folliculitis, arthropod assault, Langerhans cell histiocytosis, and leukemia cutis. Treatment is symptomatic. Mild forms of LyP can many times be managed with superpotent topical corticosteroids. Bexarotene gel has been used for early lesions. For more widespread or persistent disease, intralesional corticosteroids, phototherapy (UVB or PUVA), tetracycline antibiotics, and methotrexate have been reported to be effective. Refractory cases may respond to interferon alpha or oral bexarotene. Routine evaluations are recommended as patients may be at increased risk for the development of lymphoma.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
Cosmeceutical ingredients to use before and after antiaging procedures
Outcomes are improved when proper skincare is practiced before and after any type of dermatologic procedure. This column reviews These are ingredients commonly used before, during, and after procedures.
I will use the first person when I am expressing my personal opinion or experience versus data reported in published studies that I reference.
Ascorbic acid
Ascorbic acid (vitamin C) is an essential cofactor necessary for lysyl hydroxylase and prolyl hydroxylase to produce collagen. Many studies have demonstrated that the use of oral and topical ascorbic acid increases collagen production by fibroblasts.1-3 Several different ascorbic acid products, varying greatly in quality, are available on the market.
Ascorbic acid is very sensitive to light and air exposure and does not penetrate well if not at a pH of 2 or 2.5. There are aqueous and lipophilic formulations. Some are produced from L-ascorbic acid, while others are made from ascorbyl palmitate, or salts such as calcium ascorbate, magnesium ascorbate, magnesium ascorbyl phosphate, sodium ascorbate, and sodium ascorbyl phosphate. Consequently, one must closely evaluate any chosen ascorbic acid preparation and pay close attention to the form used in any studies. I am discussing ascorbic acid in general, but my statements only apply to properly formulated products. Most of the studies I quote used L-ascorbic acid, which is the form studied by the late Sheldon Pinnell, MD, who was an expert on ascorbic acid.
Properly formulated L-ascorbic acid products have a low pH. Unless formulated specifically to deter stinging, these low-pH preparations will sting wounded skin. For this reason, most ascorbic acid preparations should be avoided until the skin has completely re-epithelialized. I prefer using it preprocedure and after the procedure once the skin has re-epithelialized. Alster and West showed that use of ascorbic acid – in an aqueous solution formulated not to sting – after laser resurfacing resulted in a significant decrease in post‐CO2 laser resurfacing erythema by the eighth postoperative week when compared with laser‐irradiated skin that had not received topical vitamin C.4
I prefer using ascorbic acid in patients before and after procedures involving fillers, toxins, skin tightening, and nonablative lasers. In my experience, this improves collagen production. Also, I use ascorbic acid before microneedling, but not during or after. Several case reports have cited allergic granulomatous reactions when ascorbic acid is used during microneedling procedures,5 although these reports did not involve aqueous formulations.
Defensin
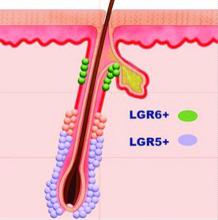
Defensins are peptides that play an important role in wound repair. Defensin has exhibited the capacity to activate the leucine-rich repeat-containing G-protein–coupled receptors 5 and 6 (also known as LGR5+ and LGR6+) stem cells.6 This accelerates wound healing by stimulating LGR stem cells to form new keratinocytes that populate the epidermis.7 Using defensins prior to procedures would theoretically speed wound healing, but no studies have been published in this area. Anecdotally, it has been used after microneedling without complication. I have not used defensin in this situation, but when I have asked the audience during lectures, many practitioners have reported using it and found that it accelerates healing.
Growth factors
Growth factors are essential in the skin because they are responsible for immunomodulation, regulation of cell division, wound healing, and tissue generation.1 There are several important growth factor families, including: transforming growth factor-beta (TGF-beta), epidermal growth factor (EGF), insulin-like growth factor (IGF), platelet-derived growth factor (PDGF), and fibroblast growth factor (FGF).2 Because of the numerous different variables that play a role with growth factor function, it is difficult to know exactly which combinations are the most helpful to improve outcomes after procedures. There is some evidence to support the use of FGF, TGF-beta, and EGF, IGF, and PDGF to hasten skin healing.8,9 It is certain that growth factors play an important role in pre- and postprocedure skincare, but we do not yet know which growth factor combinations are the most effective.
Heparan sulfate
Heparan sulfate is a glycosaminoglycan found in the skin. Older cells are less responsive to growth factors than are younger cells; therefore, it is desirable to amplify the growth factor signal in older patients. Heparan sulfate has been shown to contribute to growth factors reaching the receptors on the cell surface and enhancing the cell’s ability to “hear” growth factor signals. Combining growth factors with enhancers such as heparan sulfate, defensins, ascorbic acid, and matrikines can improve outcomes of cosmetic procedures. There are not enough studies yet to substantiate which combinations are the most effective. However, I believe that if you are using a growth factor–containing product after a procedure, you should combine it with heparan sulfate to improve efficacy.
Heparan sulfate is not the same as the blood thinner heparin; however, it may affect clotting factors. It is prudent to stop heparan sulfate the day before a dermal filler procedure because of this theoretical risk. (I have not seen an increase in bruising in patients who use heparan sulfate prior to getting fillers.) I suggest using heparan sulfate–containing products with growth factors 24 hours after injecting fillers to try and enhance collagen synthesis that occurs after hyaluronic acid (HA) filler injections.10
Hyaluronic acid
Hyaluronic acid (HA) is known to increase penetration of drugs, as well as cosmeceutical ingredients.11 For this reason, it is often used before a procedure to increase efficacy of growth factors. Many practitioners report using it during microneedling to help the device glide across the skin. I have not observed or heard of any reports of adverse events from using it during microneedling.
HA has been shown to accelerate wound healing in rats12 and dental procedures.13 For this reason, it is often used after laser resurfacing and microneedling procedures and on sutured and open wounds. HA can vary in chain link and molecular weight and whether or not it is cross linked. These differences affect efficacy and should be taken into consideration when choosing an HA product. Some formulations combine various forms of HA. Because HA may increase bruising because of its effects on fibrin formation,14 I prefer not to use it 2 days prior to or the day of filler injections.15
Hydroxy acids
Pretreating skin with hydroxy acids increases dermal matrix formation,16 promotes collagen synthesis,17 and hastens stratum corneum turnover.18 Although postprocedure healing times after pretreatment with hydroxy acids has not been studied, it is very likely that pretreatment with hydroxy acids speeds healing time by increasing collagen production and cell turnover. West and Alster showed that pretreating skin with hydroxy acids prior to CO2 resurfacing did not affect the incidence of postprocedure hyperpigmentation.19
Matrikines
Matrikines are peptides that occur when extracellular matrix (ECM) macromolecules are partially degraded. These peptides interact with cell surface receptors and activate intracellular signalling pathways to modulate ECM remodeling.20 Matrikines, such as tripeptides and hexapeptides, have been shown to remove damaged collagen and elastin from the ECM.21 It is thought that these matrikines help to prepare the skin for procedures by freeing up space to allow room for newly formed collagen. Using matrikines at least 2 weeks before procedures may precondition the skin to heal faster.22
The tripeptide glycyl-histidyl-lysine (GHK) is a good example of a matrikine. When it forms a complex with copper (II) ions (GHK–Cu) it can stimulate collagen and glycosaminoglycan synthesis23 and increase tissue inhibitors of metalloproteinases, TIMP-1 and TIMP-2, which play a role in wound remodeling.24
A serum that contains tripeptide-1, hexapeptide-12, lactoferrin, and phosphatidyl serine has been shown to speed resolution of bruises and inflammation when applied after procedures. It is believed that these ingredients activate macrophages to clear hemosiderin from the skin.
Retinoids
Derived from vitamin A, the retinoid family includes compounds such as adapalene, retinol, tazarotene, trifarotene, and tretinoin. Retinoids should be used for at least 2-4 weeks prior to procedures to improve outcomes. Multiple studies have cogently revealed that pretreatment with tretinoin accelerates wound healing.25-27 Kligman assessed healing after punch biopsy in the mid-1990s and found that the wounds on arms pretreated with tretinoin cream 0.05%-0.1% were significantly diminished by 35%-37% on days 1 and 4 and 47%-50% reduced on days 6, 8, and 11 as compared with the wounds on untreated arms.28 A tretinoin pretreatment regimen of 2-4 weeks is supported by the preponderance of studies29 because peak epidermal hypertrophy emerges after 7 days of tretinoin application and normalizes after 14 days of continued treatment.30 Such an approach gives the skin time to recover from any retinoid dermatitis before the procedure is performed. Pretreatment with adapalene requires an earlier initiation period and should be introduced 5-6 weeks before procedures because it exhibits a longer half-life.31
Topical retinoids should not be used after a procedure until re-epithelialization is complete. Hung et al. applied 0.05% tretinoin cream daily for 10 days prior to partial-thickness skin wounding in a porcine model, with results revealing that re-epithelialization was accelerated with preprocedure treatment while use after the procedure slowed wound healing.32
Skin care regimen design by procedure type
Procedures can be divided into six main types: nonablative, such as peels, intense pulsed light (IPL), and vascular or pigmented lasers; microneedling or other procedures that cause open channels into the dermis; injectables such as toxins and fillers; ablative, such as CO2, erbium, and fractionated lasers; sutured wounds; and unsutured wounds. Skincare regimens that are prescribed before and after each of these procedures should take into account the Baumann Skin Type, the procedure type, whether it is pre- or postprocedure, and lifestyle issues such as sun exposure. Once the pre- and postprocedure regimen has been designed, patients should be given specific instructions as to which brands, the exact products, and the order in which to apply them.
Conclusion
To ensure the best outcomes from surgical treatments, patient education is a key step. The more that patients know and understand about the ways in which they can prepare for their procedure and treat their skin after the procedure, the better the results. Providers should give this type of information in an easy-to-follow printed instruction sheet because studies show that patients cannot remember most of the oral instructions offered by practitioners. Patients should be encouraged to ask questions during their consultation and procedure and to express any concerns with the practitioner’s office should any arise after they have returned home. These steps help improve patient compliance, satisfaction, and outcomes. Please discuss your opinions and experience with me on LinkedIn. You can also see a lecture on this topic on my website, SkinGuru.com.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), as well as a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at [email protected].
References
1. Murad S et al. Proc Natl Acad Sci U S A. 1981 May;78(5):2879-82.
2. Tajima S, Pinnell SR. J Dermatol Sci. 1996 Mar;11(3):250-3.
3. Geesin JC et al. J Invest Dermatol. 1988 Apr;90(4):420-4.
4. Alster TS, West TB. Dermatol Surg. 1998 Mar;24(3):331-4.
5. Soltani-Arabshahi R et al. JAMA Dermatol. 2014 Jan;150(1):68-72.
6. Lough D et al. Plast Reconstr Surg. 2013 Nov;132(5):1159-71.
7. Hirsch T et al. J Gene Med. 2009 Mar;11(3):220-8.
8. Van Brunt J, Klausner A. Nat Biotechnol. 1988 Jan 1;6:25-30.
9. Lynch SE et al. J Clin Invest. 1989 Aug;84(2):640-6.
10. Wang F et al. Arch Dermatol. 2007 Feb;143(2):155-63.
11. Huang G, Huang H. Drug Deliv. 2018 Nov;25(1):766-72.
12. Celani LM. J Surg Clin Res. 2019 Oct. doi: 10.20398/jscr.v10i2.18825.
13. Yildirim S et al. J Periodontol. 2018 Jan;89(1):36-45.
14. Weigel PH et al. Ciba Found Symp. 1989;143:248-61; discussion 261-4, 281-5.
15. Basora JF et al. Am J Case Rep. 2014 May 9;15:199-202.
16. Okano Yet al. Exp Dermatol. 2003;12 Suppl 2:57-63.
17. Bernstein EF et al. Dermatol Surg. 2001 May;27(5):429-33.
18. Hood HL et al. Food Chem Toxicol. 1999 Nov;37(11):1105-11.
19. West TB, Alster TS. Dermatol Surg. 1999 Jan;25(1):15-7.
20. Maquart FX et al. M. Biochimie. 2005 Mar-Apr;87(3-4):353-60.
21. Pickart L et al. Biomed Res Int. 2015;2015:648108.
22. Widgerow AD et al. Aesthet Surg J. 2019 Apr 8;39 (Supplement 3):S103-11.
23. Maquart FX et al. FEBS Lett. 1988 Oct 10;238(2):343-6.
24. Siméon A et al. J Invest Dermatol. 1999 Jun;112(6):957-64.
25. Vagotis FL, Brundage SR. Aesthetic Plast Surg. 1995 May-Jun;19(3):243-6.
26. Stuzin JM. Plast Reconstr Surg. 2011 Mar;127(3):1343-5.
27. Elson ML. J Am Acad Dermatol. 1998 Aug;39:S79-81.
28. Popp C et al. Br J Dermatol. 1995 Jan;132(1):46-53.
29. Orringer JS et al. J Am Acad Dermatol. 2004 Dec;51(6):940-6.
30. Kim IH et al. J Korean Med Sci. 1996 Aug;11(4):335-41.
31. Basak PY et al. Eur J Dermatol. 2002 Mar-Apr;12(2):145-8.
32. Hung VC et al. Arch Dermatol. 1989 Jan;125(1):65-9.
Outcomes are improved when proper skincare is practiced before and after any type of dermatologic procedure. This column reviews These are ingredients commonly used before, during, and after procedures.
I will use the first person when I am expressing my personal opinion or experience versus data reported in published studies that I reference.
Ascorbic acid
Ascorbic acid (vitamin C) is an essential cofactor necessary for lysyl hydroxylase and prolyl hydroxylase to produce collagen. Many studies have demonstrated that the use of oral and topical ascorbic acid increases collagen production by fibroblasts.1-3 Several different ascorbic acid products, varying greatly in quality, are available on the market.
Ascorbic acid is very sensitive to light and air exposure and does not penetrate well if not at a pH of 2 or 2.5. There are aqueous and lipophilic formulations. Some are produced from L-ascorbic acid, while others are made from ascorbyl palmitate, or salts such as calcium ascorbate, magnesium ascorbate, magnesium ascorbyl phosphate, sodium ascorbate, and sodium ascorbyl phosphate. Consequently, one must closely evaluate any chosen ascorbic acid preparation and pay close attention to the form used in any studies. I am discussing ascorbic acid in general, but my statements only apply to properly formulated products. Most of the studies I quote used L-ascorbic acid, which is the form studied by the late Sheldon Pinnell, MD, who was an expert on ascorbic acid.
Properly formulated L-ascorbic acid products have a low pH. Unless formulated specifically to deter stinging, these low-pH preparations will sting wounded skin. For this reason, most ascorbic acid preparations should be avoided until the skin has completely re-epithelialized. I prefer using it preprocedure and after the procedure once the skin has re-epithelialized. Alster and West showed that use of ascorbic acid – in an aqueous solution formulated not to sting – after laser resurfacing resulted in a significant decrease in post‐CO2 laser resurfacing erythema by the eighth postoperative week when compared with laser‐irradiated skin that had not received topical vitamin C.4
I prefer using ascorbic acid in patients before and after procedures involving fillers, toxins, skin tightening, and nonablative lasers. In my experience, this improves collagen production. Also, I use ascorbic acid before microneedling, but not during or after. Several case reports have cited allergic granulomatous reactions when ascorbic acid is used during microneedling procedures,5 although these reports did not involve aqueous formulations.
Defensin
Defensins are peptides that play an important role in wound repair. Defensin has exhibited the capacity to activate the leucine-rich repeat-containing G-protein–coupled receptors 5 and 6 (also known as LGR5+ and LGR6+) stem cells.6 This accelerates wound healing by stimulating LGR stem cells to form new keratinocytes that populate the epidermis.7 Using defensins prior to procedures would theoretically speed wound healing, but no studies have been published in this area. Anecdotally, it has been used after microneedling without complication. I have not used defensin in this situation, but when I have asked the audience during lectures, many practitioners have reported using it and found that it accelerates healing.
Growth factors
Growth factors are essential in the skin because they are responsible for immunomodulation, regulation of cell division, wound healing, and tissue generation.1 There are several important growth factor families, including: transforming growth factor-beta (TGF-beta), epidermal growth factor (EGF), insulin-like growth factor (IGF), platelet-derived growth factor (PDGF), and fibroblast growth factor (FGF).2 Because of the numerous different variables that play a role with growth factor function, it is difficult to know exactly which combinations are the most helpful to improve outcomes after procedures. There is some evidence to support the use of FGF, TGF-beta, and EGF, IGF, and PDGF to hasten skin healing.8,9 It is certain that growth factors play an important role in pre- and postprocedure skincare, but we do not yet know which growth factor combinations are the most effective.
Heparan sulfate
Heparan sulfate is a glycosaminoglycan found in the skin. Older cells are less responsive to growth factors than are younger cells; therefore, it is desirable to amplify the growth factor signal in older patients. Heparan sulfate has been shown to contribute to growth factors reaching the receptors on the cell surface and enhancing the cell’s ability to “hear” growth factor signals. Combining growth factors with enhancers such as heparan sulfate, defensins, ascorbic acid, and matrikines can improve outcomes of cosmetic procedures. There are not enough studies yet to substantiate which combinations are the most effective. However, I believe that if you are using a growth factor–containing product after a procedure, you should combine it with heparan sulfate to improve efficacy.
Heparan sulfate is not the same as the blood thinner heparin; however, it may affect clotting factors. It is prudent to stop heparan sulfate the day before a dermal filler procedure because of this theoretical risk. (I have not seen an increase in bruising in patients who use heparan sulfate prior to getting fillers.) I suggest using heparan sulfate–containing products with growth factors 24 hours after injecting fillers to try and enhance collagen synthesis that occurs after hyaluronic acid (HA) filler injections.10
Hyaluronic acid
Hyaluronic acid (HA) is known to increase penetration of drugs, as well as cosmeceutical ingredients.11 For this reason, it is often used before a procedure to increase efficacy of growth factors. Many practitioners report using it during microneedling to help the device glide across the skin. I have not observed or heard of any reports of adverse events from using it during microneedling.
HA has been shown to accelerate wound healing in rats12 and dental procedures.13 For this reason, it is often used after laser resurfacing and microneedling procedures and on sutured and open wounds. HA can vary in chain link and molecular weight and whether or not it is cross linked. These differences affect efficacy and should be taken into consideration when choosing an HA product. Some formulations combine various forms of HA. Because HA may increase bruising because of its effects on fibrin formation,14 I prefer not to use it 2 days prior to or the day of filler injections.15
Hydroxy acids
Pretreating skin with hydroxy acids increases dermal matrix formation,16 promotes collagen synthesis,17 and hastens stratum corneum turnover.18 Although postprocedure healing times after pretreatment with hydroxy acids has not been studied, it is very likely that pretreatment with hydroxy acids speeds healing time by increasing collagen production and cell turnover. West and Alster showed that pretreating skin with hydroxy acids prior to CO2 resurfacing did not affect the incidence of postprocedure hyperpigmentation.19
Matrikines
Matrikines are peptides that occur when extracellular matrix (ECM) macromolecules are partially degraded. These peptides interact with cell surface receptors and activate intracellular signalling pathways to modulate ECM remodeling.20 Matrikines, such as tripeptides and hexapeptides, have been shown to remove damaged collagen and elastin from the ECM.21 It is thought that these matrikines help to prepare the skin for procedures by freeing up space to allow room for newly formed collagen. Using matrikines at least 2 weeks before procedures may precondition the skin to heal faster.22
The tripeptide glycyl-histidyl-lysine (GHK) is a good example of a matrikine. When it forms a complex with copper (II) ions (GHK–Cu) it can stimulate collagen and glycosaminoglycan synthesis23 and increase tissue inhibitors of metalloproteinases, TIMP-1 and TIMP-2, which play a role in wound remodeling.24
A serum that contains tripeptide-1, hexapeptide-12, lactoferrin, and phosphatidyl serine has been shown to speed resolution of bruises and inflammation when applied after procedures. It is believed that these ingredients activate macrophages to clear hemosiderin from the skin.
Retinoids
Derived from vitamin A, the retinoid family includes compounds such as adapalene, retinol, tazarotene, trifarotene, and tretinoin. Retinoids should be used for at least 2-4 weeks prior to procedures to improve outcomes. Multiple studies have cogently revealed that pretreatment with tretinoin accelerates wound healing.25-27 Kligman assessed healing after punch biopsy in the mid-1990s and found that the wounds on arms pretreated with tretinoin cream 0.05%-0.1% were significantly diminished by 35%-37% on days 1 and 4 and 47%-50% reduced on days 6, 8, and 11 as compared with the wounds on untreated arms.28 A tretinoin pretreatment regimen of 2-4 weeks is supported by the preponderance of studies29 because peak epidermal hypertrophy emerges after 7 days of tretinoin application and normalizes after 14 days of continued treatment.30 Such an approach gives the skin time to recover from any retinoid dermatitis before the procedure is performed. Pretreatment with adapalene requires an earlier initiation period and should be introduced 5-6 weeks before procedures because it exhibits a longer half-life.31
Topical retinoids should not be used after a procedure until re-epithelialization is complete. Hung et al. applied 0.05% tretinoin cream daily for 10 days prior to partial-thickness skin wounding in a porcine model, with results revealing that re-epithelialization was accelerated with preprocedure treatment while use after the procedure slowed wound healing.32
Skin care regimen design by procedure type
Procedures can be divided into six main types: nonablative, such as peels, intense pulsed light (IPL), and vascular or pigmented lasers; microneedling or other procedures that cause open channels into the dermis; injectables such as toxins and fillers; ablative, such as CO2, erbium, and fractionated lasers; sutured wounds; and unsutured wounds. Skincare regimens that are prescribed before and after each of these procedures should take into account the Baumann Skin Type, the procedure type, whether it is pre- or postprocedure, and lifestyle issues such as sun exposure. Once the pre- and postprocedure regimen has been designed, patients should be given specific instructions as to which brands, the exact products, and the order in which to apply them.
Conclusion
To ensure the best outcomes from surgical treatments, patient education is a key step. The more that patients know and understand about the ways in which they can prepare for their procedure and treat their skin after the procedure, the better the results. Providers should give this type of information in an easy-to-follow printed instruction sheet because studies show that patients cannot remember most of the oral instructions offered by practitioners. Patients should be encouraged to ask questions during their consultation and procedure and to express any concerns with the practitioner’s office should any arise after they have returned home. These steps help improve patient compliance, satisfaction, and outcomes. Please discuss your opinions and experience with me on LinkedIn. You can also see a lecture on this topic on my website, SkinGuru.com.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), as well as a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at [email protected].
References
1. Murad S et al. Proc Natl Acad Sci U S A. 1981 May;78(5):2879-82.
2. Tajima S, Pinnell SR. J Dermatol Sci. 1996 Mar;11(3):250-3.
3. Geesin JC et al. J Invest Dermatol. 1988 Apr;90(4):420-4.
4. Alster TS, West TB. Dermatol Surg. 1998 Mar;24(3):331-4.
5. Soltani-Arabshahi R et al. JAMA Dermatol. 2014 Jan;150(1):68-72.
6. Lough D et al. Plast Reconstr Surg. 2013 Nov;132(5):1159-71.
7. Hirsch T et al. J Gene Med. 2009 Mar;11(3):220-8.
8. Van Brunt J, Klausner A. Nat Biotechnol. 1988 Jan 1;6:25-30.
9. Lynch SE et al. J Clin Invest. 1989 Aug;84(2):640-6.
10. Wang F et al. Arch Dermatol. 2007 Feb;143(2):155-63.
11. Huang G, Huang H. Drug Deliv. 2018 Nov;25(1):766-72.
12. Celani LM. J Surg Clin Res. 2019 Oct. doi: 10.20398/jscr.v10i2.18825.
13. Yildirim S et al. J Periodontol. 2018 Jan;89(1):36-45.
14. Weigel PH et al. Ciba Found Symp. 1989;143:248-61; discussion 261-4, 281-5.
15. Basora JF et al. Am J Case Rep. 2014 May 9;15:199-202.
16. Okano Yet al. Exp Dermatol. 2003;12 Suppl 2:57-63.
17. Bernstein EF et al. Dermatol Surg. 2001 May;27(5):429-33.
18. Hood HL et al. Food Chem Toxicol. 1999 Nov;37(11):1105-11.
19. West TB, Alster TS. Dermatol Surg. 1999 Jan;25(1):15-7.
20. Maquart FX et al. M. Biochimie. 2005 Mar-Apr;87(3-4):353-60.
21. Pickart L et al. Biomed Res Int. 2015;2015:648108.
22. Widgerow AD et al. Aesthet Surg J. 2019 Apr 8;39 (Supplement 3):S103-11.
23. Maquart FX et al. FEBS Lett. 1988 Oct 10;238(2):343-6.
24. Siméon A et al. J Invest Dermatol. 1999 Jun;112(6):957-64.
25. Vagotis FL, Brundage SR. Aesthetic Plast Surg. 1995 May-Jun;19(3):243-6.
26. Stuzin JM. Plast Reconstr Surg. 2011 Mar;127(3):1343-5.
27. Elson ML. J Am Acad Dermatol. 1998 Aug;39:S79-81.
28. Popp C et al. Br J Dermatol. 1995 Jan;132(1):46-53.
29. Orringer JS et al. J Am Acad Dermatol. 2004 Dec;51(6):940-6.
30. Kim IH et al. J Korean Med Sci. 1996 Aug;11(4):335-41.
31. Basak PY et al. Eur J Dermatol. 2002 Mar-Apr;12(2):145-8.
32. Hung VC et al. Arch Dermatol. 1989 Jan;125(1):65-9.
Outcomes are improved when proper skincare is practiced before and after any type of dermatologic procedure. This column reviews These are ingredients commonly used before, during, and after procedures.
I will use the first person when I am expressing my personal opinion or experience versus data reported in published studies that I reference.
Ascorbic acid
Ascorbic acid (vitamin C) is an essential cofactor necessary for lysyl hydroxylase and prolyl hydroxylase to produce collagen. Many studies have demonstrated that the use of oral and topical ascorbic acid increases collagen production by fibroblasts.1-3 Several different ascorbic acid products, varying greatly in quality, are available on the market.
Ascorbic acid is very sensitive to light and air exposure and does not penetrate well if not at a pH of 2 or 2.5. There are aqueous and lipophilic formulations. Some are produced from L-ascorbic acid, while others are made from ascorbyl palmitate, or salts such as calcium ascorbate, magnesium ascorbate, magnesium ascorbyl phosphate, sodium ascorbate, and sodium ascorbyl phosphate. Consequently, one must closely evaluate any chosen ascorbic acid preparation and pay close attention to the form used in any studies. I am discussing ascorbic acid in general, but my statements only apply to properly formulated products. Most of the studies I quote used L-ascorbic acid, which is the form studied by the late Sheldon Pinnell, MD, who was an expert on ascorbic acid.
Properly formulated L-ascorbic acid products have a low pH. Unless formulated specifically to deter stinging, these low-pH preparations will sting wounded skin. For this reason, most ascorbic acid preparations should be avoided until the skin has completely re-epithelialized. I prefer using it preprocedure and after the procedure once the skin has re-epithelialized. Alster and West showed that use of ascorbic acid – in an aqueous solution formulated not to sting – after laser resurfacing resulted in a significant decrease in post‐CO2 laser resurfacing erythema by the eighth postoperative week when compared with laser‐irradiated skin that had not received topical vitamin C.4
I prefer using ascorbic acid in patients before and after procedures involving fillers, toxins, skin tightening, and nonablative lasers. In my experience, this improves collagen production. Also, I use ascorbic acid before microneedling, but not during or after. Several case reports have cited allergic granulomatous reactions when ascorbic acid is used during microneedling procedures,5 although these reports did not involve aqueous formulations.
Defensin
Defensins are peptides that play an important role in wound repair. Defensin has exhibited the capacity to activate the leucine-rich repeat-containing G-protein–coupled receptors 5 and 6 (also known as LGR5+ and LGR6+) stem cells.6 This accelerates wound healing by stimulating LGR stem cells to form new keratinocytes that populate the epidermis.7 Using defensins prior to procedures would theoretically speed wound healing, but no studies have been published in this area. Anecdotally, it has been used after microneedling without complication. I have not used defensin in this situation, but when I have asked the audience during lectures, many practitioners have reported using it and found that it accelerates healing.
Growth factors
Growth factors are essential in the skin because they are responsible for immunomodulation, regulation of cell division, wound healing, and tissue generation.1 There are several important growth factor families, including: transforming growth factor-beta (TGF-beta), epidermal growth factor (EGF), insulin-like growth factor (IGF), platelet-derived growth factor (PDGF), and fibroblast growth factor (FGF).2 Because of the numerous different variables that play a role with growth factor function, it is difficult to know exactly which combinations are the most helpful to improve outcomes after procedures. There is some evidence to support the use of FGF, TGF-beta, and EGF, IGF, and PDGF to hasten skin healing.8,9 It is certain that growth factors play an important role in pre- and postprocedure skincare, but we do not yet know which growth factor combinations are the most effective.
Heparan sulfate
Heparan sulfate is a glycosaminoglycan found in the skin. Older cells are less responsive to growth factors than are younger cells; therefore, it is desirable to amplify the growth factor signal in older patients. Heparan sulfate has been shown to contribute to growth factors reaching the receptors on the cell surface and enhancing the cell’s ability to “hear” growth factor signals. Combining growth factors with enhancers such as heparan sulfate, defensins, ascorbic acid, and matrikines can improve outcomes of cosmetic procedures. There are not enough studies yet to substantiate which combinations are the most effective. However, I believe that if you are using a growth factor–containing product after a procedure, you should combine it with heparan sulfate to improve efficacy.
Heparan sulfate is not the same as the blood thinner heparin; however, it may affect clotting factors. It is prudent to stop heparan sulfate the day before a dermal filler procedure because of this theoretical risk. (I have not seen an increase in bruising in patients who use heparan sulfate prior to getting fillers.) I suggest using heparan sulfate–containing products with growth factors 24 hours after injecting fillers to try and enhance collagen synthesis that occurs after hyaluronic acid (HA) filler injections.10
Hyaluronic acid
Hyaluronic acid (HA) is known to increase penetration of drugs, as well as cosmeceutical ingredients.11 For this reason, it is often used before a procedure to increase efficacy of growth factors. Many practitioners report using it during microneedling to help the device glide across the skin. I have not observed or heard of any reports of adverse events from using it during microneedling.
HA has been shown to accelerate wound healing in rats12 and dental procedures.13 For this reason, it is often used after laser resurfacing and microneedling procedures and on sutured and open wounds. HA can vary in chain link and molecular weight and whether or not it is cross linked. These differences affect efficacy and should be taken into consideration when choosing an HA product. Some formulations combine various forms of HA. Because HA may increase bruising because of its effects on fibrin formation,14 I prefer not to use it 2 days prior to or the day of filler injections.15
Hydroxy acids
Pretreating skin with hydroxy acids increases dermal matrix formation,16 promotes collagen synthesis,17 and hastens stratum corneum turnover.18 Although postprocedure healing times after pretreatment with hydroxy acids has not been studied, it is very likely that pretreatment with hydroxy acids speeds healing time by increasing collagen production and cell turnover. West and Alster showed that pretreating skin with hydroxy acids prior to CO2 resurfacing did not affect the incidence of postprocedure hyperpigmentation.19
Matrikines
Matrikines are peptides that occur when extracellular matrix (ECM) macromolecules are partially degraded. These peptides interact with cell surface receptors and activate intracellular signalling pathways to modulate ECM remodeling.20 Matrikines, such as tripeptides and hexapeptides, have been shown to remove damaged collagen and elastin from the ECM.21 It is thought that these matrikines help to prepare the skin for procedures by freeing up space to allow room for newly formed collagen. Using matrikines at least 2 weeks before procedures may precondition the skin to heal faster.22
The tripeptide glycyl-histidyl-lysine (GHK) is a good example of a matrikine. When it forms a complex with copper (II) ions (GHK–Cu) it can stimulate collagen and glycosaminoglycan synthesis23 and increase tissue inhibitors of metalloproteinases, TIMP-1 and TIMP-2, which play a role in wound remodeling.24
A serum that contains tripeptide-1, hexapeptide-12, lactoferrin, and phosphatidyl serine has been shown to speed resolution of bruises and inflammation when applied after procedures. It is believed that these ingredients activate macrophages to clear hemosiderin from the skin.
Retinoids
Derived from vitamin A, the retinoid family includes compounds such as adapalene, retinol, tazarotene, trifarotene, and tretinoin. Retinoids should be used for at least 2-4 weeks prior to procedures to improve outcomes. Multiple studies have cogently revealed that pretreatment with tretinoin accelerates wound healing.25-27 Kligman assessed healing after punch biopsy in the mid-1990s and found that the wounds on arms pretreated with tretinoin cream 0.05%-0.1% were significantly diminished by 35%-37% on days 1 and 4 and 47%-50% reduced on days 6, 8, and 11 as compared with the wounds on untreated arms.28 A tretinoin pretreatment regimen of 2-4 weeks is supported by the preponderance of studies29 because peak epidermal hypertrophy emerges after 7 days of tretinoin application and normalizes after 14 days of continued treatment.30 Such an approach gives the skin time to recover from any retinoid dermatitis before the procedure is performed. Pretreatment with adapalene requires an earlier initiation period and should be introduced 5-6 weeks before procedures because it exhibits a longer half-life.31
Topical retinoids should not be used after a procedure until re-epithelialization is complete. Hung et al. applied 0.05% tretinoin cream daily for 10 days prior to partial-thickness skin wounding in a porcine model, with results revealing that re-epithelialization was accelerated with preprocedure treatment while use after the procedure slowed wound healing.32
Skin care regimen design by procedure type
Procedures can be divided into six main types: nonablative, such as peels, intense pulsed light (IPL), and vascular or pigmented lasers; microneedling or other procedures that cause open channels into the dermis; injectables such as toxins and fillers; ablative, such as CO2, erbium, and fractionated lasers; sutured wounds; and unsutured wounds. Skincare regimens that are prescribed before and after each of these procedures should take into account the Baumann Skin Type, the procedure type, whether it is pre- or postprocedure, and lifestyle issues such as sun exposure. Once the pre- and postprocedure regimen has been designed, patients should be given specific instructions as to which brands, the exact products, and the order in which to apply them.
Conclusion
To ensure the best outcomes from surgical treatments, patient education is a key step. The more that patients know and understand about the ways in which they can prepare for their procedure and treat their skin after the procedure, the better the results. Providers should give this type of information in an easy-to-follow printed instruction sheet because studies show that patients cannot remember most of the oral instructions offered by practitioners. Patients should be encouraged to ask questions during their consultation and procedure and to express any concerns with the practitioner’s office should any arise after they have returned home. These steps help improve patient compliance, satisfaction, and outcomes. Please discuss your opinions and experience with me on LinkedIn. You can also see a lecture on this topic on my website, SkinGuru.com.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), as well as a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at [email protected].
References
1. Murad S et al. Proc Natl Acad Sci U S A. 1981 May;78(5):2879-82.
2. Tajima S, Pinnell SR. J Dermatol Sci. 1996 Mar;11(3):250-3.
3. Geesin JC et al. J Invest Dermatol. 1988 Apr;90(4):420-4.
4. Alster TS, West TB. Dermatol Surg. 1998 Mar;24(3):331-4.
5. Soltani-Arabshahi R et al. JAMA Dermatol. 2014 Jan;150(1):68-72.
6. Lough D et al. Plast Reconstr Surg. 2013 Nov;132(5):1159-71.
7. Hirsch T et al. J Gene Med. 2009 Mar;11(3):220-8.
8. Van Brunt J, Klausner A. Nat Biotechnol. 1988 Jan 1;6:25-30.
9. Lynch SE et al. J Clin Invest. 1989 Aug;84(2):640-6.
10. Wang F et al. Arch Dermatol. 2007 Feb;143(2):155-63.
11. Huang G, Huang H. Drug Deliv. 2018 Nov;25(1):766-72.
12. Celani LM. J Surg Clin Res. 2019 Oct. doi: 10.20398/jscr.v10i2.18825.
13. Yildirim S et al. J Periodontol. 2018 Jan;89(1):36-45.
14. Weigel PH et al. Ciba Found Symp. 1989;143:248-61; discussion 261-4, 281-5.
15. Basora JF et al. Am J Case Rep. 2014 May 9;15:199-202.
16. Okano Yet al. Exp Dermatol. 2003;12 Suppl 2:57-63.
17. Bernstein EF et al. Dermatol Surg. 2001 May;27(5):429-33.
18. Hood HL et al. Food Chem Toxicol. 1999 Nov;37(11):1105-11.
19. West TB, Alster TS. Dermatol Surg. 1999 Jan;25(1):15-7.
20. Maquart FX et al. M. Biochimie. 2005 Mar-Apr;87(3-4):353-60.
21. Pickart L et al. Biomed Res Int. 2015;2015:648108.
22. Widgerow AD et al. Aesthet Surg J. 2019 Apr 8;39 (Supplement 3):S103-11.
23. Maquart FX et al. FEBS Lett. 1988 Oct 10;238(2):343-6.
24. Siméon A et al. J Invest Dermatol. 1999 Jun;112(6):957-64.
25. Vagotis FL, Brundage SR. Aesthetic Plast Surg. 1995 May-Jun;19(3):243-6.
26. Stuzin JM. Plast Reconstr Surg. 2011 Mar;127(3):1343-5.
27. Elson ML. J Am Acad Dermatol. 1998 Aug;39:S79-81.
28. Popp C et al. Br J Dermatol. 1995 Jan;132(1):46-53.
29. Orringer JS et al. J Am Acad Dermatol. 2004 Dec;51(6):940-6.
30. Kim IH et al. J Korean Med Sci. 1996 Aug;11(4):335-41.
31. Basak PY et al. Eur J Dermatol. 2002 Mar-Apr;12(2):145-8.
32. Hung VC et al. Arch Dermatol. 1989 Jan;125(1):65-9.
Trump seeks to cut NIH, CDC budgets, some Medicare spending
The Trump administration on Feb. 10 argued for cutting spending for a federal agency at the forefront of the efforts to combat the coronavirus, while also seeking to slow spending in certain parts of the Medicare and Medicaid programs.
President Donald Trump presented his fiscal 2021 request to Congress for refilling the coffers of federal agencies. In any administration, an annual budget serves only as a political blueprint, as the White House document itself makes no changes in federal spending.
In Mr. Trump’s case, several of his requests for agencies within the Department of Health & Human Services run counter to recent budget trends. Republicans and Democrats in Congress have worked together in recent years to increase budgets for major federal health agencies.
But Mr. Trump asked Congress to cut annual budget authority for the National Institute of Allergy and Infectious Diseases by $430 million to $5.446 billion for fiscal 2021. In contrast, Congress has raised the annual budget for NIAID, a key agency in combating the coronavirus, from $5.545 billion in fiscal 2019 to $5.876 billion in fiscal 2020, which began in October, according to an HHS summary of Mr. Trump’s request.
For the Centers for Disease Control and Prevention, which is central to the battle against the coronavirus, Mr. Trump proposed a drop in discretionary funding to $5.627 billion. In contrast, Congress raised the CDC budget from $6.544 billion in fiscal 2019 to $6.917 in fiscal 2020.
Mr. Trump also wants to cut $559 million from the budget of the National Cancer Institute, dropping it to $5.881 billion in fiscal 2021. In contrast, Congress raised NCI’s budget from $6.121 billion in fiscal 2019 to $6.440 billion in fiscal 2020.
Mr. Trump requested a $2.6 billion reduction in the National Institutes of Health’s total discretionary budget, seeking to drop it to $37.70 billion. In contrast, Congress raised NIH’s budget from $37.887 in fiscal 2019 to $40.304 billion in fiscal 2020.
Mr. Trump’s budget proposal also includes an estimate of $152 billion in savings over a decade for Medicaid through the implementation of what the administration calls “community engagement” requirements.
The Trump administration has been at odds with Democrats for years about whether work requirements should be attached to Medicaid. “Well-designed community engagement incentives have great potential to improve health and well-being while empowering beneficiaries to rise out of poverty,” HHS said in a budget document.
Yet researchers last year reported that Arkansas’ attempt to attach work requirements to Medicaid caused almost 17,000 adults to lose this health care coverage within the first 6 months, and there was no significant difference in employment.
The researchers say this loss of coverage was partly a result of bureaucratic obstacles and confusion about the new rules. In June 2018, Arkansas became the first state to implement work requirements for Medicaid, Benjamin D. Sommers, MD, PhD, of the Harvard T.H. Chan School of Public Health, Boston, and colleagues wrote in the New England Journal of Medicine (2019 Sep 12;381[11]:1073-82).
Budget ‘would thwart’ progress
A few medical groups on Monday quickly criticized Mr. Trump’s proposals.
“In a time where our nation continues to face significant public health challenges — including 2019 novel coronavirus, climate change, gun violence, and costly chronic diseases such as heart disease and cancer – the administration should be investing more resources in better health, not cutting federal health budgets,” said Georges C. Benjamin, MD, executive director of the American Public Health Association, in a statement.
David J. Skorton, MD, chief executive and president of the Association of American Medical Colleges (AAMC) also urged increased investment in fighting disease.
“We must continue the bipartisan budget trajectory set forth by Congress over the last several years, not reverse course,” Dr. Skorton said in a statement.
Mr. Trump’s proposed cuts in medical research “would thwart scientific progress on strategies to prevent, diagnose, treat, and cure medical conditions that affect countless patients nationwide,” he said.
In total, the new 2021 appropriations for HHS would fall by $9.46 billion to $85.667 billion under Mr. Trump’s proposal. Appropriations, also called discretionary budget authority, represents the operating budgets for federal agencies. These are decided through annual spending bills.
Congress has separate sets of laws for handling payments the federal government makes through Medicare and Medicaid. These are known as mandatory spending.
‘Untenable cuts’
AAMC’s Dr. Skorton also objected to what he termed Mr. Trump’s bid “to reduce and consolidate Medicare, Medicaid, and children’s hospital graduate medical education into a single grant program.”
This would force teaching hospitals to absorb $52 billion in “untenable cuts,” he said.
“The proposal ignores the intent of the Medicare GME program, which is to ensure an adequate physician workforce to care for Medicare beneficiaries and support the critical patient care missions of America’s teaching hospitals,” Dr. Skorton said.
The budget also seeks cuts to Medicaid, which come in addition to the administration’s “recent proposals to scale back Medicaid coverage,” Dr. Skorton said.
“More than 26% of all Medicaid hospitalizations occur at AAMC-member teaching hospitals, even though these institutions represent only 5% of all hospitals,” Dr. Skorton said. “Each of the administration’s proposals on their own would be devastating for patients – and combined, they would be disastrous.”
Rick Pollack, the chief executive and president of the American Hospital Association, described Mr. Trump’s fiscal 2021 proposal as another bid to undermine medical care in the United States.
“Every year, we adapt to a constantly changing environment, but every year, the administration aims to gut our nation’s health care infrastructure,” Mr. Pollack said in a statement.
In it, he noted that about one in five people in America depend on Medicaid, with children accounting for a large proportion of those covered by the state-federal program.
“The budget’s proposal on Medicaid financing and service delivery would cut hundreds of billions of dollars from the Medicaid program annually,” Mr. Pollack said.
He also objected to “hundreds of billions of proposed reductions to Medicare” endorsed by Mr. Trump.
Medical malpractice overhaul
The Trump administration also offered many suggestions for changing federal laws to reduce health care spending. Among these was a proposed overhaul of the approach to medical malpractice cases.
The president’s budget proposal estimates $40 billion in savings over a decade from steps to limit medical liability, according to a report from the Office of Management and Budget (OMB).
“The current medical liability system does not work for patients or providers, nor does it promote high-quality, evidence-based care,” OMB said. “Providers practice with a threat of potentially frivolous lawsuits, and injured patients often do not receive just compensation for their injuries.”
Mr. Trump’s fiscal 2021 budget calls for a cap on noneconomic damage awards of $250,000, which would increase with inflation over time, and a 3-year statute of limitations. Under this plan, courts could also modify attorney’s fee arrangements. HHS could provide guidance to states on how to create expert panels and administrative health care tribunals to review medical liability.
These steps would lead to lower health care spending, with clinicians dropping “defensive medicine practices,” OMB said. That would benefit the Medicare and Medicaid programs as well as lowering costs of health insurance in general.
Mr. Trump’s fiscal 2021 budget also includes a series of proposals for Medicare that it estimates would, in aggregate, save $755.5 billion over a decade.
Site-neutral policy
A large chunk of the estimated Medicare savings in Mr. Trump’s fiscal 2021 health budget would come from lowering payments to hospitals for services provided in their outpatient and physician offices.
In the fiscal 2021 proposal, HHS noted that “Medicare generally pays on-campus hospital outpatient departments substantially more than physician offices for the same services.”
Mr. Trump’s budget proposal seeks a more expansive shift to what’s called a “site-neutral” payment for services delivered in hospital outpatient programs or physician offices. This would bring these payments more in line with those made to independent physician practices.
“This proposal would eliminate the often significant disparity between what Medicare pays in these different settings for the same services,” HHS said in the budget summary.
HHS estimated this change in policy would generate $117.2 billion in savings over a decade. Combined with saving from medical malpractice reforms, the Trump administration estimates these two moves combined could save about $164 billion over a decade.
The site-neutral policy has been a legal battleground, with hospital and physician groups winning a round last year.
Another Medicare proposal included in Mr. Trump’s fiscal 2021 budget homes in on this issue for cases where a hospital owns a physician office. Medicare now pays most off-campus hospital outpatient departments higher rates than the program’s physician fee schedule dictates for the same services.
Switching to a site-neutral policy for these hospital-owned physician offices would result in $47.2 billion in savings over a decade, HHS said in the budget document.
This article first appeared on Medscape.com.
The Trump administration on Feb. 10 argued for cutting spending for a federal agency at the forefront of the efforts to combat the coronavirus, while also seeking to slow spending in certain parts of the Medicare and Medicaid programs.
President Donald Trump presented his fiscal 2021 request to Congress for refilling the coffers of federal agencies. In any administration, an annual budget serves only as a political blueprint, as the White House document itself makes no changes in federal spending.
In Mr. Trump’s case, several of his requests for agencies within the Department of Health & Human Services run counter to recent budget trends. Republicans and Democrats in Congress have worked together in recent years to increase budgets for major federal health agencies.
But Mr. Trump asked Congress to cut annual budget authority for the National Institute of Allergy and Infectious Diseases by $430 million to $5.446 billion for fiscal 2021. In contrast, Congress has raised the annual budget for NIAID, a key agency in combating the coronavirus, from $5.545 billion in fiscal 2019 to $5.876 billion in fiscal 2020, which began in October, according to an HHS summary of Mr. Trump’s request.
For the Centers for Disease Control and Prevention, which is central to the battle against the coronavirus, Mr. Trump proposed a drop in discretionary funding to $5.627 billion. In contrast, Congress raised the CDC budget from $6.544 billion in fiscal 2019 to $6.917 in fiscal 2020.
Mr. Trump also wants to cut $559 million from the budget of the National Cancer Institute, dropping it to $5.881 billion in fiscal 2021. In contrast, Congress raised NCI’s budget from $6.121 billion in fiscal 2019 to $6.440 billion in fiscal 2020.
Mr. Trump requested a $2.6 billion reduction in the National Institutes of Health’s total discretionary budget, seeking to drop it to $37.70 billion. In contrast, Congress raised NIH’s budget from $37.887 in fiscal 2019 to $40.304 billion in fiscal 2020.
Mr. Trump’s budget proposal also includes an estimate of $152 billion in savings over a decade for Medicaid through the implementation of what the administration calls “community engagement” requirements.
The Trump administration has been at odds with Democrats for years about whether work requirements should be attached to Medicaid. “Well-designed community engagement incentives have great potential to improve health and well-being while empowering beneficiaries to rise out of poverty,” HHS said in a budget document.
Yet researchers last year reported that Arkansas’ attempt to attach work requirements to Medicaid caused almost 17,000 adults to lose this health care coverage within the first 6 months, and there was no significant difference in employment.
The researchers say this loss of coverage was partly a result of bureaucratic obstacles and confusion about the new rules. In June 2018, Arkansas became the first state to implement work requirements for Medicaid, Benjamin D. Sommers, MD, PhD, of the Harvard T.H. Chan School of Public Health, Boston, and colleagues wrote in the New England Journal of Medicine (2019 Sep 12;381[11]:1073-82).
Budget ‘would thwart’ progress
A few medical groups on Monday quickly criticized Mr. Trump’s proposals.
“In a time where our nation continues to face significant public health challenges — including 2019 novel coronavirus, climate change, gun violence, and costly chronic diseases such as heart disease and cancer – the administration should be investing more resources in better health, not cutting federal health budgets,” said Georges C. Benjamin, MD, executive director of the American Public Health Association, in a statement.
David J. Skorton, MD, chief executive and president of the Association of American Medical Colleges (AAMC) also urged increased investment in fighting disease.
“We must continue the bipartisan budget trajectory set forth by Congress over the last several years, not reverse course,” Dr. Skorton said in a statement.
Mr. Trump’s proposed cuts in medical research “would thwart scientific progress on strategies to prevent, diagnose, treat, and cure medical conditions that affect countless patients nationwide,” he said.
In total, the new 2021 appropriations for HHS would fall by $9.46 billion to $85.667 billion under Mr. Trump’s proposal. Appropriations, also called discretionary budget authority, represents the operating budgets for federal agencies. These are decided through annual spending bills.
Congress has separate sets of laws for handling payments the federal government makes through Medicare and Medicaid. These are known as mandatory spending.
‘Untenable cuts’
AAMC’s Dr. Skorton also objected to what he termed Mr. Trump’s bid “to reduce and consolidate Medicare, Medicaid, and children’s hospital graduate medical education into a single grant program.”
This would force teaching hospitals to absorb $52 billion in “untenable cuts,” he said.
“The proposal ignores the intent of the Medicare GME program, which is to ensure an adequate physician workforce to care for Medicare beneficiaries and support the critical patient care missions of America’s teaching hospitals,” Dr. Skorton said.
The budget also seeks cuts to Medicaid, which come in addition to the administration’s “recent proposals to scale back Medicaid coverage,” Dr. Skorton said.
“More than 26% of all Medicaid hospitalizations occur at AAMC-member teaching hospitals, even though these institutions represent only 5% of all hospitals,” Dr. Skorton said. “Each of the administration’s proposals on their own would be devastating for patients – and combined, they would be disastrous.”
Rick Pollack, the chief executive and president of the American Hospital Association, described Mr. Trump’s fiscal 2021 proposal as another bid to undermine medical care in the United States.
“Every year, we adapt to a constantly changing environment, but every year, the administration aims to gut our nation’s health care infrastructure,” Mr. Pollack said in a statement.
In it, he noted that about one in five people in America depend on Medicaid, with children accounting for a large proportion of those covered by the state-federal program.
“The budget’s proposal on Medicaid financing and service delivery would cut hundreds of billions of dollars from the Medicaid program annually,” Mr. Pollack said.
He also objected to “hundreds of billions of proposed reductions to Medicare” endorsed by Mr. Trump.
Medical malpractice overhaul
The Trump administration also offered many suggestions for changing federal laws to reduce health care spending. Among these was a proposed overhaul of the approach to medical malpractice cases.
The president’s budget proposal estimates $40 billion in savings over a decade from steps to limit medical liability, according to a report from the Office of Management and Budget (OMB).
“The current medical liability system does not work for patients or providers, nor does it promote high-quality, evidence-based care,” OMB said. “Providers practice with a threat of potentially frivolous lawsuits, and injured patients often do not receive just compensation for their injuries.”
Mr. Trump’s fiscal 2021 budget calls for a cap on noneconomic damage awards of $250,000, which would increase with inflation over time, and a 3-year statute of limitations. Under this plan, courts could also modify attorney’s fee arrangements. HHS could provide guidance to states on how to create expert panels and administrative health care tribunals to review medical liability.
These steps would lead to lower health care spending, with clinicians dropping “defensive medicine practices,” OMB said. That would benefit the Medicare and Medicaid programs as well as lowering costs of health insurance in general.
Mr. Trump’s fiscal 2021 budget also includes a series of proposals for Medicare that it estimates would, in aggregate, save $755.5 billion over a decade.
Site-neutral policy
A large chunk of the estimated Medicare savings in Mr. Trump’s fiscal 2021 health budget would come from lowering payments to hospitals for services provided in their outpatient and physician offices.
In the fiscal 2021 proposal, HHS noted that “Medicare generally pays on-campus hospital outpatient departments substantially more than physician offices for the same services.”
Mr. Trump’s budget proposal seeks a more expansive shift to what’s called a “site-neutral” payment for services delivered in hospital outpatient programs or physician offices. This would bring these payments more in line with those made to independent physician practices.
“This proposal would eliminate the often significant disparity between what Medicare pays in these different settings for the same services,” HHS said in the budget summary.
HHS estimated this change in policy would generate $117.2 billion in savings over a decade. Combined with saving from medical malpractice reforms, the Trump administration estimates these two moves combined could save about $164 billion over a decade.
The site-neutral policy has been a legal battleground, with hospital and physician groups winning a round last year.
Another Medicare proposal included in Mr. Trump’s fiscal 2021 budget homes in on this issue for cases where a hospital owns a physician office. Medicare now pays most off-campus hospital outpatient departments higher rates than the program’s physician fee schedule dictates for the same services.
Switching to a site-neutral policy for these hospital-owned physician offices would result in $47.2 billion in savings over a decade, HHS said in the budget document.
This article first appeared on Medscape.com.
The Trump administration on Feb. 10 argued for cutting spending for a federal agency at the forefront of the efforts to combat the coronavirus, while also seeking to slow spending in certain parts of the Medicare and Medicaid programs.
President Donald Trump presented his fiscal 2021 request to Congress for refilling the coffers of federal agencies. In any administration, an annual budget serves only as a political blueprint, as the White House document itself makes no changes in federal spending.
In Mr. Trump’s case, several of his requests for agencies within the Department of Health & Human Services run counter to recent budget trends. Republicans and Democrats in Congress have worked together in recent years to increase budgets for major federal health agencies.
But Mr. Trump asked Congress to cut annual budget authority for the National Institute of Allergy and Infectious Diseases by $430 million to $5.446 billion for fiscal 2021. In contrast, Congress has raised the annual budget for NIAID, a key agency in combating the coronavirus, from $5.545 billion in fiscal 2019 to $5.876 billion in fiscal 2020, which began in October, according to an HHS summary of Mr. Trump’s request.
For the Centers for Disease Control and Prevention, which is central to the battle against the coronavirus, Mr. Trump proposed a drop in discretionary funding to $5.627 billion. In contrast, Congress raised the CDC budget from $6.544 billion in fiscal 2019 to $6.917 in fiscal 2020.
Mr. Trump also wants to cut $559 million from the budget of the National Cancer Institute, dropping it to $5.881 billion in fiscal 2021. In contrast, Congress raised NCI’s budget from $6.121 billion in fiscal 2019 to $6.440 billion in fiscal 2020.
Mr. Trump requested a $2.6 billion reduction in the National Institutes of Health’s total discretionary budget, seeking to drop it to $37.70 billion. In contrast, Congress raised NIH’s budget from $37.887 in fiscal 2019 to $40.304 billion in fiscal 2020.
Mr. Trump’s budget proposal also includes an estimate of $152 billion in savings over a decade for Medicaid through the implementation of what the administration calls “community engagement” requirements.
The Trump administration has been at odds with Democrats for years about whether work requirements should be attached to Medicaid. “Well-designed community engagement incentives have great potential to improve health and well-being while empowering beneficiaries to rise out of poverty,” HHS said in a budget document.
Yet researchers last year reported that Arkansas’ attempt to attach work requirements to Medicaid caused almost 17,000 adults to lose this health care coverage within the first 6 months, and there was no significant difference in employment.
The researchers say this loss of coverage was partly a result of bureaucratic obstacles and confusion about the new rules. In June 2018, Arkansas became the first state to implement work requirements for Medicaid, Benjamin D. Sommers, MD, PhD, of the Harvard T.H. Chan School of Public Health, Boston, and colleagues wrote in the New England Journal of Medicine (2019 Sep 12;381[11]:1073-82).
Budget ‘would thwart’ progress
A few medical groups on Monday quickly criticized Mr. Trump’s proposals.
“In a time where our nation continues to face significant public health challenges — including 2019 novel coronavirus, climate change, gun violence, and costly chronic diseases such as heart disease and cancer – the administration should be investing more resources in better health, not cutting federal health budgets,” said Georges C. Benjamin, MD, executive director of the American Public Health Association, in a statement.
David J. Skorton, MD, chief executive and president of the Association of American Medical Colleges (AAMC) also urged increased investment in fighting disease.
“We must continue the bipartisan budget trajectory set forth by Congress over the last several years, not reverse course,” Dr. Skorton said in a statement.
Mr. Trump’s proposed cuts in medical research “would thwart scientific progress on strategies to prevent, diagnose, treat, and cure medical conditions that affect countless patients nationwide,” he said.
In total, the new 2021 appropriations for HHS would fall by $9.46 billion to $85.667 billion under Mr. Trump’s proposal. Appropriations, also called discretionary budget authority, represents the operating budgets for federal agencies. These are decided through annual spending bills.
Congress has separate sets of laws for handling payments the federal government makes through Medicare and Medicaid. These are known as mandatory spending.
‘Untenable cuts’
AAMC’s Dr. Skorton also objected to what he termed Mr. Trump’s bid “to reduce and consolidate Medicare, Medicaid, and children’s hospital graduate medical education into a single grant program.”
This would force teaching hospitals to absorb $52 billion in “untenable cuts,” he said.
“The proposal ignores the intent of the Medicare GME program, which is to ensure an adequate physician workforce to care for Medicare beneficiaries and support the critical patient care missions of America’s teaching hospitals,” Dr. Skorton said.
The budget also seeks cuts to Medicaid, which come in addition to the administration’s “recent proposals to scale back Medicaid coverage,” Dr. Skorton said.
“More than 26% of all Medicaid hospitalizations occur at AAMC-member teaching hospitals, even though these institutions represent only 5% of all hospitals,” Dr. Skorton said. “Each of the administration’s proposals on their own would be devastating for patients – and combined, they would be disastrous.”
Rick Pollack, the chief executive and president of the American Hospital Association, described Mr. Trump’s fiscal 2021 proposal as another bid to undermine medical care in the United States.
“Every year, we adapt to a constantly changing environment, but every year, the administration aims to gut our nation’s health care infrastructure,” Mr. Pollack said in a statement.
In it, he noted that about one in five people in America depend on Medicaid, with children accounting for a large proportion of those covered by the state-federal program.
“The budget’s proposal on Medicaid financing and service delivery would cut hundreds of billions of dollars from the Medicaid program annually,” Mr. Pollack said.
He also objected to “hundreds of billions of proposed reductions to Medicare” endorsed by Mr. Trump.
Medical malpractice overhaul
The Trump administration also offered many suggestions for changing federal laws to reduce health care spending. Among these was a proposed overhaul of the approach to medical malpractice cases.
The president’s budget proposal estimates $40 billion in savings over a decade from steps to limit medical liability, according to a report from the Office of Management and Budget (OMB).
“The current medical liability system does not work for patients or providers, nor does it promote high-quality, evidence-based care,” OMB said. “Providers practice with a threat of potentially frivolous lawsuits, and injured patients often do not receive just compensation for their injuries.”
Mr. Trump’s fiscal 2021 budget calls for a cap on noneconomic damage awards of $250,000, which would increase with inflation over time, and a 3-year statute of limitations. Under this plan, courts could also modify attorney’s fee arrangements. HHS could provide guidance to states on how to create expert panels and administrative health care tribunals to review medical liability.
These steps would lead to lower health care spending, with clinicians dropping “defensive medicine practices,” OMB said. That would benefit the Medicare and Medicaid programs as well as lowering costs of health insurance in general.
Mr. Trump’s fiscal 2021 budget also includes a series of proposals for Medicare that it estimates would, in aggregate, save $755.5 billion over a decade.
Site-neutral policy
A large chunk of the estimated Medicare savings in Mr. Trump’s fiscal 2021 health budget would come from lowering payments to hospitals for services provided in their outpatient and physician offices.
In the fiscal 2021 proposal, HHS noted that “Medicare generally pays on-campus hospital outpatient departments substantially more than physician offices for the same services.”
Mr. Trump’s budget proposal seeks a more expansive shift to what’s called a “site-neutral” payment for services delivered in hospital outpatient programs or physician offices. This would bring these payments more in line with those made to independent physician practices.
“This proposal would eliminate the often significant disparity between what Medicare pays in these different settings for the same services,” HHS said in the budget summary.
HHS estimated this change in policy would generate $117.2 billion in savings over a decade. Combined with saving from medical malpractice reforms, the Trump administration estimates these two moves combined could save about $164 billion over a decade.
The site-neutral policy has been a legal battleground, with hospital and physician groups winning a round last year.
Another Medicare proposal included in Mr. Trump’s fiscal 2021 budget homes in on this issue for cases where a hospital owns a physician office. Medicare now pays most off-campus hospital outpatient departments higher rates than the program’s physician fee schedule dictates for the same services.
Switching to a site-neutral policy for these hospital-owned physician offices would result in $47.2 billion in savings over a decade, HHS said in the budget document.
This article first appeared on Medscape.com.
Epidermolysis bullosa classification criteria refined and ready
LONDON – have come in understanding this debilitating group of genetic skin diseases, but also how far there is still to go towards improving the management of those affected.
Previous criteria issued in 2014 represented “important progress” and “built on the achievements of several generations of physicians and researchers who described the phenotypes, the level of skin cleavage, developed and characterized antibodies, and discovered EB-associated genes,” Cristina Has, MD, said at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA).
Dr. Has, a senior dermatologist and professor of experimental dermatology at the University of Freiburg (Germany), observed that prior criteria had “introduced genetic and molecular data in a so-called onion-skin classification of EB, and removed most of the eponyms,” which had been maintained in the latest update.
“What is new, and probably the most important change, is making the distinction between classical EB and other disorders with skin fragility,” she said, noting that the revised classification criteria for EB included minor changes to the nomenclature of EB. Six new EB subtypes and genes have also been added, and there are new sections on genotype/phenotype correlations, disease modifying factors, and the natural history of EB. Furthermore, supporting information included a concise description of clinical and genetic features of all EB types and subtypes.
The updated criteria are the result of an expert meeting held in April 2019 and have been accepted for publication. The expert panel that developed the criteria think that the revised classification criteria will be “useful and, we hope, inspiring and motivating for the young generation of dermatologists, pediatricians, and for the researchers who work in this field,” Dr. Has said.
“The term EB has been used in the last years for many new disorders, and this is the reason why we thought we have to somehow control this, and to make the distinction between classical epidermolysis bullosa due to defects at the dermal junction and other disorders with skin fragility where the anomalies occur within other layers of the epidermis or in the dermis,” Dr. Has explained.
There are still 4 main types of classical EB: EB simplex (EBS), dystrophic EB (DEB), junctional EB, and Kindler EB, but there are now 34 subtypes, slightly fewer than before. The updated criteria distinguish between the types and subtypes according to the level of skin cleavage, the inheritance pattern, the mutated gene, and the targeted protein, Dr. Has said.
As for peeling disorders, these have been classified as being erosive or hyperkeratotic, or as affecting the connective tissue with skin blistering. Similar to classical EB, these disorders are associated with fragility of the skin and mucosa and share some pathogenetic mechanisms. Moreover, as “the suffering of the patient is similar,” Dr. Has said, “we’d like to consider them under the umbrella of EB.” Most of the disorders she listed were inherited via an autosomal recessive mechanism, with intraepidermal disorders inherited via an autosomal dominant mechanism. New genes are being identified the time, she added, so these groupings will no doubt be subject to future revisions.
Minor changes to nomenclature were made to avoid confusion among clinicians and those living with the condition. As such, Kindler EB replaces Kindler syndrome, names of some subtypes were simplified, and a new “self-improving” type of DEB was introduced to replace the term “transient dermolysis of the newborn.” Altogether, there are now 11 subtypes of DEB. A distinction was also made between syndromic and nonsyndromic EB. “We all know that EB can be a systemic disorder with secondary manifestations within different organs,” Dr. Has told conference attendees. Anemia and failure to thrive can be associated, but it still remains a nonsyndromic disorder, she said. By contrast, “syndromic EB is due to genetic defects, which are also expressed in other organs than the skin or mucosal membranes, and lead to primary extracutaneous manifestations, such as cardiomyopathy, nephropathy, and so on.”
There are fewer subtypes of EBS and “we think they are better defined,” Dr. Has stated. “EB simplex is the most heterogenous EB type, clinically and genetically, and includes several syndromic disorders,” and the new classification criteria should be useful in helping categorize individuals with EBS and thus help target their management.
One of the six new subtypes of EB included in the revised classification criteria is “syndromic EBS with cardiomyopathy” caused by the KLH24 mutation. This gene was discovered in 2016 and more than 40 cases have so far been identified, 50% of which have been sporadic de novo mutations.
Other new EB subtypes are:
- “EBS with localized nephropathy” caused by a mutation in the CD151 gene.
- An autosomal recessive EBS linked to the KRT5 gene.
- A new phenotype that manifests with oral mucosal blisters linked to the DSG3 gene. (Although only a single case has been reported to date, it was felt worthy of inclusion.)
- Another linked to DSG3 that leads to skin fragility and hypertrichosis.
- A new dystrophic EB subtype linked to mutations in the PLOD3 gene.
In an interview, Dr. Has reiterated the importance of keeping classification criteria updated in line with current research findings. She emphasized that there were many types of EB and how important it was to refine how these were classified based on the underlying genetics.
“We brought much more genetic data into the paper, because we are in the era of personalized medicine,” she said. “There are specific therapies for mutations and for different subtypes and that’s why we think that, step by step, we have to bring in more and more data into the classification.”
There are many people with EBS, she observed, and while these individuals may not have such a dramatic clinical presentation as those with recessive DEB, for example, the effect of the condition on their daily lives is no less. “These people are active, they have jobs, they have to work, and they have pain, they have blister,” Dr. Has said.
While the criteria are intended only for classification of EB, they might help in practice. Dr. Has gave an anecdotal example of a woman that has been misdiagnosed as having a type of DEB with a high risk of squamous cell carcinoma but in fact had a different form of EB with no risk of developing SCC. “That’s why criteria are important,” she said.
Dr. Has had no conflicts of interest to disclose.
LONDON – have come in understanding this debilitating group of genetic skin diseases, but also how far there is still to go towards improving the management of those affected.
Previous criteria issued in 2014 represented “important progress” and “built on the achievements of several generations of physicians and researchers who described the phenotypes, the level of skin cleavage, developed and characterized antibodies, and discovered EB-associated genes,” Cristina Has, MD, said at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA).
Dr. Has, a senior dermatologist and professor of experimental dermatology at the University of Freiburg (Germany), observed that prior criteria had “introduced genetic and molecular data in a so-called onion-skin classification of EB, and removed most of the eponyms,” which had been maintained in the latest update.
“What is new, and probably the most important change, is making the distinction between classical EB and other disorders with skin fragility,” she said, noting that the revised classification criteria for EB included minor changes to the nomenclature of EB. Six new EB subtypes and genes have also been added, and there are new sections on genotype/phenotype correlations, disease modifying factors, and the natural history of EB. Furthermore, supporting information included a concise description of clinical and genetic features of all EB types and subtypes.
The updated criteria are the result of an expert meeting held in April 2019 and have been accepted for publication. The expert panel that developed the criteria think that the revised classification criteria will be “useful and, we hope, inspiring and motivating for the young generation of dermatologists, pediatricians, and for the researchers who work in this field,” Dr. Has said.
“The term EB has been used in the last years for many new disorders, and this is the reason why we thought we have to somehow control this, and to make the distinction between classical epidermolysis bullosa due to defects at the dermal junction and other disorders with skin fragility where the anomalies occur within other layers of the epidermis or in the dermis,” Dr. Has explained.
There are still 4 main types of classical EB: EB simplex (EBS), dystrophic EB (DEB), junctional EB, and Kindler EB, but there are now 34 subtypes, slightly fewer than before. The updated criteria distinguish between the types and subtypes according to the level of skin cleavage, the inheritance pattern, the mutated gene, and the targeted protein, Dr. Has said.
As for peeling disorders, these have been classified as being erosive or hyperkeratotic, or as affecting the connective tissue with skin blistering. Similar to classical EB, these disorders are associated with fragility of the skin and mucosa and share some pathogenetic mechanisms. Moreover, as “the suffering of the patient is similar,” Dr. Has said, “we’d like to consider them under the umbrella of EB.” Most of the disorders she listed were inherited via an autosomal recessive mechanism, with intraepidermal disorders inherited via an autosomal dominant mechanism. New genes are being identified the time, she added, so these groupings will no doubt be subject to future revisions.
Minor changes to nomenclature were made to avoid confusion among clinicians and those living with the condition. As such, Kindler EB replaces Kindler syndrome, names of some subtypes were simplified, and a new “self-improving” type of DEB was introduced to replace the term “transient dermolysis of the newborn.” Altogether, there are now 11 subtypes of DEB. A distinction was also made between syndromic and nonsyndromic EB. “We all know that EB can be a systemic disorder with secondary manifestations within different organs,” Dr. Has told conference attendees. Anemia and failure to thrive can be associated, but it still remains a nonsyndromic disorder, she said. By contrast, “syndromic EB is due to genetic defects, which are also expressed in other organs than the skin or mucosal membranes, and lead to primary extracutaneous manifestations, such as cardiomyopathy, nephropathy, and so on.”
There are fewer subtypes of EBS and “we think they are better defined,” Dr. Has stated. “EB simplex is the most heterogenous EB type, clinically and genetically, and includes several syndromic disorders,” and the new classification criteria should be useful in helping categorize individuals with EBS and thus help target their management.
One of the six new subtypes of EB included in the revised classification criteria is “syndromic EBS with cardiomyopathy” caused by the KLH24 mutation. This gene was discovered in 2016 and more than 40 cases have so far been identified, 50% of which have been sporadic de novo mutations.
Other new EB subtypes are:
- “EBS with localized nephropathy” caused by a mutation in the CD151 gene.
- An autosomal recessive EBS linked to the KRT5 gene.
- A new phenotype that manifests with oral mucosal blisters linked to the DSG3 gene. (Although only a single case has been reported to date, it was felt worthy of inclusion.)
- Another linked to DSG3 that leads to skin fragility and hypertrichosis.
- A new dystrophic EB subtype linked to mutations in the PLOD3 gene.
In an interview, Dr. Has reiterated the importance of keeping classification criteria updated in line with current research findings. She emphasized that there were many types of EB and how important it was to refine how these were classified based on the underlying genetics.
“We brought much more genetic data into the paper, because we are in the era of personalized medicine,” she said. “There are specific therapies for mutations and for different subtypes and that’s why we think that, step by step, we have to bring in more and more data into the classification.”
There are many people with EBS, she observed, and while these individuals may not have such a dramatic clinical presentation as those with recessive DEB, for example, the effect of the condition on their daily lives is no less. “These people are active, they have jobs, they have to work, and they have pain, they have blister,” Dr. Has said.
While the criteria are intended only for classification of EB, they might help in practice. Dr. Has gave an anecdotal example of a woman that has been misdiagnosed as having a type of DEB with a high risk of squamous cell carcinoma but in fact had a different form of EB with no risk of developing SCC. “That’s why criteria are important,” she said.
Dr. Has had no conflicts of interest to disclose.
LONDON – have come in understanding this debilitating group of genetic skin diseases, but also how far there is still to go towards improving the management of those affected.
Previous criteria issued in 2014 represented “important progress” and “built on the achievements of several generations of physicians and researchers who described the phenotypes, the level of skin cleavage, developed and characterized antibodies, and discovered EB-associated genes,” Cristina Has, MD, said at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA).
Dr. Has, a senior dermatologist and professor of experimental dermatology at the University of Freiburg (Germany), observed that prior criteria had “introduced genetic and molecular data in a so-called onion-skin classification of EB, and removed most of the eponyms,” which had been maintained in the latest update.
“What is new, and probably the most important change, is making the distinction between classical EB and other disorders with skin fragility,” she said, noting that the revised classification criteria for EB included minor changes to the nomenclature of EB. Six new EB subtypes and genes have also been added, and there are new sections on genotype/phenotype correlations, disease modifying factors, and the natural history of EB. Furthermore, supporting information included a concise description of clinical and genetic features of all EB types and subtypes.
The updated criteria are the result of an expert meeting held in April 2019 and have been accepted for publication. The expert panel that developed the criteria think that the revised classification criteria will be “useful and, we hope, inspiring and motivating for the young generation of dermatologists, pediatricians, and for the researchers who work in this field,” Dr. Has said.
“The term EB has been used in the last years for many new disorders, and this is the reason why we thought we have to somehow control this, and to make the distinction between classical epidermolysis bullosa due to defects at the dermal junction and other disorders with skin fragility where the anomalies occur within other layers of the epidermis or in the dermis,” Dr. Has explained.
There are still 4 main types of classical EB: EB simplex (EBS), dystrophic EB (DEB), junctional EB, and Kindler EB, but there are now 34 subtypes, slightly fewer than before. The updated criteria distinguish between the types and subtypes according to the level of skin cleavage, the inheritance pattern, the mutated gene, and the targeted protein, Dr. Has said.
As for peeling disorders, these have been classified as being erosive or hyperkeratotic, or as affecting the connective tissue with skin blistering. Similar to classical EB, these disorders are associated with fragility of the skin and mucosa and share some pathogenetic mechanisms. Moreover, as “the suffering of the patient is similar,” Dr. Has said, “we’d like to consider them under the umbrella of EB.” Most of the disorders she listed were inherited via an autosomal recessive mechanism, with intraepidermal disorders inherited via an autosomal dominant mechanism. New genes are being identified the time, she added, so these groupings will no doubt be subject to future revisions.
Minor changes to nomenclature were made to avoid confusion among clinicians and those living with the condition. As such, Kindler EB replaces Kindler syndrome, names of some subtypes were simplified, and a new “self-improving” type of DEB was introduced to replace the term “transient dermolysis of the newborn.” Altogether, there are now 11 subtypes of DEB. A distinction was also made between syndromic and nonsyndromic EB. “We all know that EB can be a systemic disorder with secondary manifestations within different organs,” Dr. Has told conference attendees. Anemia and failure to thrive can be associated, but it still remains a nonsyndromic disorder, she said. By contrast, “syndromic EB is due to genetic defects, which are also expressed in other organs than the skin or mucosal membranes, and lead to primary extracutaneous manifestations, such as cardiomyopathy, nephropathy, and so on.”
There are fewer subtypes of EBS and “we think they are better defined,” Dr. Has stated. “EB simplex is the most heterogenous EB type, clinically and genetically, and includes several syndromic disorders,” and the new classification criteria should be useful in helping categorize individuals with EBS and thus help target their management.
One of the six new subtypes of EB included in the revised classification criteria is “syndromic EBS with cardiomyopathy” caused by the KLH24 mutation. This gene was discovered in 2016 and more than 40 cases have so far been identified, 50% of which have been sporadic de novo mutations.
Other new EB subtypes are:
- “EBS with localized nephropathy” caused by a mutation in the CD151 gene.
- An autosomal recessive EBS linked to the KRT5 gene.
- A new phenotype that manifests with oral mucosal blisters linked to the DSG3 gene. (Although only a single case has been reported to date, it was felt worthy of inclusion.)
- Another linked to DSG3 that leads to skin fragility and hypertrichosis.
- A new dystrophic EB subtype linked to mutations in the PLOD3 gene.
In an interview, Dr. Has reiterated the importance of keeping classification criteria updated in line with current research findings. She emphasized that there were many types of EB and how important it was to refine how these were classified based on the underlying genetics.
“We brought much more genetic data into the paper, because we are in the era of personalized medicine,” she said. “There are specific therapies for mutations and for different subtypes and that’s why we think that, step by step, we have to bring in more and more data into the classification.”
There are many people with EBS, she observed, and while these individuals may not have such a dramatic clinical presentation as those with recessive DEB, for example, the effect of the condition on their daily lives is no less. “These people are active, they have jobs, they have to work, and they have pain, they have blister,” Dr. Has said.
While the criteria are intended only for classification of EB, they might help in practice. Dr. Has gave an anecdotal example of a woman that has been misdiagnosed as having a type of DEB with a high risk of squamous cell carcinoma but in fact had a different form of EB with no risk of developing SCC. “That’s why criteria are important,” she said.
Dr. Has had no conflicts of interest to disclose.
REPORTING FROM EB 2020
Acne treatment may vary based on race, gender, insurance
based on findings from a retrospective, cohort study of 29,928 individuals with acne.
“Our findings suggest the presence of racial/ethnic, sex, and insurance-based disparities in health care use and treatment for acne and raise particular concern for undertreatment among racial/ethnic minority and female patients,” John S. Barbieri, MD, a dermatology research fellow at the University of Pennsylvania, Philadelphia, and colleagues wrote in a study published in JAMA Dermatology.
Data from previous studies have suggested racial disparities in the management of several dermatologic conditions, including atopic dermatitis and psoriasis, but associations between social demographics and prescribing patterns have not been well studied for acne treatment, the authors noted.
For the current study, the researchers used deidentified data from the Optum electronic health record from Jan. 1, 2007 to June 30, 2017. In all, 29,928 patients aged 15-35 years and who were being treated for acne were included in the study. Of that total, 64% were women, 8% were non-Hispanic black and 68% were white, with the remaining patients grouped as non-Hispanic Asian, Hispanic, or other.
Non-Hispanic black patients were significantly more likely to be seen by a dermatologist, compared with non-Hispanic white patients, who were designated as the reference (odds ratio, 1.20). However, the black patients were less likely to receive prescriptions for any acne medication (incidence rate ratio, 0.89).
Non-Hispanic black patients were more likely than non-Hispanic white patients to be prescribed topical retinoids or topical antibiotics (OR, 1.25 and 1.35, respectively). They were also were less likely than their white counterparts to be prescribed oral antibiotics, spironolactone, and isotretinoin (OR, 0.80, 0.68, and 0.39, respectively).
Overall, men were more than twice as likely as women to receive prescriptions for isotretinoin (OR, 2.44). They were also more likely to receive prescriptions for the other treatments, but the differences were not as high as those for isotretinoin.
In addition, patients with Medicaid insurance were significantly less likely than those with commercial insurance (the reference) to see a dermatologist (OR, 0.46). Medicaid patients also were less likely to be prescribed topical retinoids, oral antibiotics, spironolactone, or isotretinoin (OR, 0.82, 0.87, 0.50, and 0.43, respectively).
The study findings were limited by several factors, among them, the use of automated pharmacy data without confirmation that patients had picked up the medications they had been prescribed, the researchers said. The study also lacked data on acne severity, clinical outcomes, and the use of over-the-counter acne treatments.
“Further study is needed to confirm our findings, provide understanding of the reasons for these potential disparities, and develop strategies to ensure equitable care for patients with acne,” the researchers concluded.
The study was supported in part by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health, and by a Pfizer Fellowship in Dermatology Patient Oriented Research grant to the Trustees of the University of Pennsylvania. Dr. Barbieri had no financial conflicts to disclose. One of the study coauthors disclosed relationships with Pfizer, Eli Lilly, and Novartis.
SOURCE: Barbieri JS et al. JAMA Dermatol. 2020 Feb 5. doi: 10.1001/jamadermatol.2019.4818.
based on findings from a retrospective, cohort study of 29,928 individuals with acne.
“Our findings suggest the presence of racial/ethnic, sex, and insurance-based disparities in health care use and treatment for acne and raise particular concern for undertreatment among racial/ethnic minority and female patients,” John S. Barbieri, MD, a dermatology research fellow at the University of Pennsylvania, Philadelphia, and colleagues wrote in a study published in JAMA Dermatology.
Data from previous studies have suggested racial disparities in the management of several dermatologic conditions, including atopic dermatitis and psoriasis, but associations between social demographics and prescribing patterns have not been well studied for acne treatment, the authors noted.
For the current study, the researchers used deidentified data from the Optum electronic health record from Jan. 1, 2007 to June 30, 2017. In all, 29,928 patients aged 15-35 years and who were being treated for acne were included in the study. Of that total, 64% were women, 8% were non-Hispanic black and 68% were white, with the remaining patients grouped as non-Hispanic Asian, Hispanic, or other.
Non-Hispanic black patients were significantly more likely to be seen by a dermatologist, compared with non-Hispanic white patients, who were designated as the reference (odds ratio, 1.20). However, the black patients were less likely to receive prescriptions for any acne medication (incidence rate ratio, 0.89).
Non-Hispanic black patients were more likely than non-Hispanic white patients to be prescribed topical retinoids or topical antibiotics (OR, 1.25 and 1.35, respectively). They were also were less likely than their white counterparts to be prescribed oral antibiotics, spironolactone, and isotretinoin (OR, 0.80, 0.68, and 0.39, respectively).
Overall, men were more than twice as likely as women to receive prescriptions for isotretinoin (OR, 2.44). They were also more likely to receive prescriptions for the other treatments, but the differences were not as high as those for isotretinoin.
In addition, patients with Medicaid insurance were significantly less likely than those with commercial insurance (the reference) to see a dermatologist (OR, 0.46). Medicaid patients also were less likely to be prescribed topical retinoids, oral antibiotics, spironolactone, or isotretinoin (OR, 0.82, 0.87, 0.50, and 0.43, respectively).
The study findings were limited by several factors, among them, the use of automated pharmacy data without confirmation that patients had picked up the medications they had been prescribed, the researchers said. The study also lacked data on acne severity, clinical outcomes, and the use of over-the-counter acne treatments.
“Further study is needed to confirm our findings, provide understanding of the reasons for these potential disparities, and develop strategies to ensure equitable care for patients with acne,” the researchers concluded.
The study was supported in part by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health, and by a Pfizer Fellowship in Dermatology Patient Oriented Research grant to the Trustees of the University of Pennsylvania. Dr. Barbieri had no financial conflicts to disclose. One of the study coauthors disclosed relationships with Pfizer, Eli Lilly, and Novartis.
SOURCE: Barbieri JS et al. JAMA Dermatol. 2020 Feb 5. doi: 10.1001/jamadermatol.2019.4818.
based on findings from a retrospective, cohort study of 29,928 individuals with acne.
“Our findings suggest the presence of racial/ethnic, sex, and insurance-based disparities in health care use and treatment for acne and raise particular concern for undertreatment among racial/ethnic minority and female patients,” John S. Barbieri, MD, a dermatology research fellow at the University of Pennsylvania, Philadelphia, and colleagues wrote in a study published in JAMA Dermatology.
Data from previous studies have suggested racial disparities in the management of several dermatologic conditions, including atopic dermatitis and psoriasis, but associations between social demographics and prescribing patterns have not been well studied for acne treatment, the authors noted.
For the current study, the researchers used deidentified data from the Optum electronic health record from Jan. 1, 2007 to June 30, 2017. In all, 29,928 patients aged 15-35 years and who were being treated for acne were included in the study. Of that total, 64% were women, 8% were non-Hispanic black and 68% were white, with the remaining patients grouped as non-Hispanic Asian, Hispanic, or other.
Non-Hispanic black patients were significantly more likely to be seen by a dermatologist, compared with non-Hispanic white patients, who were designated as the reference (odds ratio, 1.20). However, the black patients were less likely to receive prescriptions for any acne medication (incidence rate ratio, 0.89).
Non-Hispanic black patients were more likely than non-Hispanic white patients to be prescribed topical retinoids or topical antibiotics (OR, 1.25 and 1.35, respectively). They were also were less likely than their white counterparts to be prescribed oral antibiotics, spironolactone, and isotretinoin (OR, 0.80, 0.68, and 0.39, respectively).
Overall, men were more than twice as likely as women to receive prescriptions for isotretinoin (OR, 2.44). They were also more likely to receive prescriptions for the other treatments, but the differences were not as high as those for isotretinoin.
In addition, patients with Medicaid insurance were significantly less likely than those with commercial insurance (the reference) to see a dermatologist (OR, 0.46). Medicaid patients also were less likely to be prescribed topical retinoids, oral antibiotics, spironolactone, or isotretinoin (OR, 0.82, 0.87, 0.50, and 0.43, respectively).
The study findings were limited by several factors, among them, the use of automated pharmacy data without confirmation that patients had picked up the medications they had been prescribed, the researchers said. The study also lacked data on acne severity, clinical outcomes, and the use of over-the-counter acne treatments.
“Further study is needed to confirm our findings, provide understanding of the reasons for these potential disparities, and develop strategies to ensure equitable care for patients with acne,” the researchers concluded.
The study was supported in part by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health, and by a Pfizer Fellowship in Dermatology Patient Oriented Research grant to the Trustees of the University of Pennsylvania. Dr. Barbieri had no financial conflicts to disclose. One of the study coauthors disclosed relationships with Pfizer, Eli Lilly, and Novartis.
SOURCE: Barbieri JS et al. JAMA Dermatol. 2020 Feb 5. doi: 10.1001/jamadermatol.2019.4818.
FROM JAMA DERMATOLOGY
Be alert for embezzlement
With myriad complex, high-tech problems facing private practice in this modern era, I am periodically reminded by long-time readers to revisit some of the low-tech issues that will always require our attention.
Few are lower tech (in most cases) and more easily overlooked than theft from within. Embezzlement remains far more common in medical offices than generally assumed – and it often occurs in full view of physicians who think everything is fine. Most embezzlers are not skillful or discreet; their transgressions may go undetected for years, simply because no one suspects it is happening.
Detecting fraud is an inexact science. There is no textbook approach that one can follow, but a few simple measures will prevent or expose the most common forms:
- Make it more difficult. Theft and embezzlement are usually products of opportunity, so minimize those opportunities. No one person should be in charge of the entire bookkeeping process: The person who enters charges should be different from the one who enters payments. The one who writes checks or makes electronic fund transfers should not balance the books, and so on. Internal audits should be done on a regular basis, and all employees should know that. Your accountant can help.
- Reconcile cash receipts daily. Embezzlement does not require sophisticated technology; the most common form is simply taking cash out of the till. In a typical scenario, a patient pays a copay of $15 in cash; the receptionist records the payment as $5, and pockets the rest. Make sure a receipt is generated for every cash transaction, and that someone other than the person accepting cash reconciles the charges, receipts, and cash totals daily.
- Inventory your stock. Cash isn’t the only susceptible commodity. If you sell cosmetics or other products, inventory your stock frequently. And office personnel are not the only potential thieves: Last year, a locum tenens physician down the street conspired with a receptionist to take cash transactions for cosmetic neurotoxins and fillers “off the books” and split the spoils. That office was being ripped off twice; first for the neurotoxin and filler materials themselves, and then for the cash proceeds.
- Separate all accounting duties. Another popular ploy is false invoicing for imaginary supplies. A friend’s experience provides a good example (retold with his permission): His bookkeeper wrote sizable checks to herself, disguising them in the ledger as payments to vendors commonly used by his practice. Since the same employee also balanced the checkbook, she got away with it for years. “It wasn’t at all clever,” he told me, “and I’m embarrassed to admit that it happened to me.” Once again, separation of duties is the key to prevention. One employee should enter invoices into the data system, another should issue the check or make the electronic transfer, and a third should match invoices to goods and services received.
- Verify expense reports. False expense reporting is a subset of the fake invoice scam. When an employee asks for reimbursement of expenses, make sure those expenses are real.
- Consider computer safeguards. Computers facilitate a lot of financial chores, but they also consolidate financial data in one place, where it is potentially accessible to anybody, anywhere. Your computer vendor should be aware of this, and there should be safeguards built into your system. Ask about them. If they aren’t there, ask why.
- Hire honest employees. All applicants look great on paper, so check their references; and with their permission, you can run background checks for a few dollars on any of several public information web sites. My columns on hiring are available on the MDedge Dermatology website.
- Look for “red flags.” Examples include employees who refuse to take vacations, because someone else will have do their work or who insist on posting expenses that are a coworker’s responsibility, “just to be nice.” Anyone obviously living beyond his or her means merits suspicion as well.
- Consider bonding your employees. Dishonesty bonds are relatively inexpensive, and provide assurance of some measure of recovery if your safeguards fail. Also, just knowing that your staff is bonded will scare off most dishonest applicants. One effective screen is a question on your employment application: “Would you object to being bonded?”
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
With myriad complex, high-tech problems facing private practice in this modern era, I am periodically reminded by long-time readers to revisit some of the low-tech issues that will always require our attention.
Few are lower tech (in most cases) and more easily overlooked than theft from within. Embezzlement remains far more common in medical offices than generally assumed – and it often occurs in full view of physicians who think everything is fine. Most embezzlers are not skillful or discreet; their transgressions may go undetected for years, simply because no one suspects it is happening.
Detecting fraud is an inexact science. There is no textbook approach that one can follow, but a few simple measures will prevent or expose the most common forms:
- Make it more difficult. Theft and embezzlement are usually products of opportunity, so minimize those opportunities. No one person should be in charge of the entire bookkeeping process: The person who enters charges should be different from the one who enters payments. The one who writes checks or makes electronic fund transfers should not balance the books, and so on. Internal audits should be done on a regular basis, and all employees should know that. Your accountant can help.
- Reconcile cash receipts daily. Embezzlement does not require sophisticated technology; the most common form is simply taking cash out of the till. In a typical scenario, a patient pays a copay of $15 in cash; the receptionist records the payment as $5, and pockets the rest. Make sure a receipt is generated for every cash transaction, and that someone other than the person accepting cash reconciles the charges, receipts, and cash totals daily.
- Inventory your stock. Cash isn’t the only susceptible commodity. If you sell cosmetics or other products, inventory your stock frequently. And office personnel are not the only potential thieves: Last year, a locum tenens physician down the street conspired with a receptionist to take cash transactions for cosmetic neurotoxins and fillers “off the books” and split the spoils. That office was being ripped off twice; first for the neurotoxin and filler materials themselves, and then for the cash proceeds.
- Separate all accounting duties. Another popular ploy is false invoicing for imaginary supplies. A friend’s experience provides a good example (retold with his permission): His bookkeeper wrote sizable checks to herself, disguising them in the ledger as payments to vendors commonly used by his practice. Since the same employee also balanced the checkbook, she got away with it for years. “It wasn’t at all clever,” he told me, “and I’m embarrassed to admit that it happened to me.” Once again, separation of duties is the key to prevention. One employee should enter invoices into the data system, another should issue the check or make the electronic transfer, and a third should match invoices to goods and services received.
- Verify expense reports. False expense reporting is a subset of the fake invoice scam. When an employee asks for reimbursement of expenses, make sure those expenses are real.
- Consider computer safeguards. Computers facilitate a lot of financial chores, but they also consolidate financial data in one place, where it is potentially accessible to anybody, anywhere. Your computer vendor should be aware of this, and there should be safeguards built into your system. Ask about them. If they aren’t there, ask why.
- Hire honest employees. All applicants look great on paper, so check their references; and with their permission, you can run background checks for a few dollars on any of several public information web sites. My columns on hiring are available on the MDedge Dermatology website.
- Look for “red flags.” Examples include employees who refuse to take vacations, because someone else will have do their work or who insist on posting expenses that are a coworker’s responsibility, “just to be nice.” Anyone obviously living beyond his or her means merits suspicion as well.
- Consider bonding your employees. Dishonesty bonds are relatively inexpensive, and provide assurance of some measure of recovery if your safeguards fail. Also, just knowing that your staff is bonded will scare off most dishonest applicants. One effective screen is a question on your employment application: “Would you object to being bonded?”
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
With myriad complex, high-tech problems facing private practice in this modern era, I am periodically reminded by long-time readers to revisit some of the low-tech issues that will always require our attention.
Few are lower tech (in most cases) and more easily overlooked than theft from within. Embezzlement remains far more common in medical offices than generally assumed – and it often occurs in full view of physicians who think everything is fine. Most embezzlers are not skillful or discreet; their transgressions may go undetected for years, simply because no one suspects it is happening.
Detecting fraud is an inexact science. There is no textbook approach that one can follow, but a few simple measures will prevent or expose the most common forms:
- Make it more difficult. Theft and embezzlement are usually products of opportunity, so minimize those opportunities. No one person should be in charge of the entire bookkeeping process: The person who enters charges should be different from the one who enters payments. The one who writes checks or makes electronic fund transfers should not balance the books, and so on. Internal audits should be done on a regular basis, and all employees should know that. Your accountant can help.
- Reconcile cash receipts daily. Embezzlement does not require sophisticated technology; the most common form is simply taking cash out of the till. In a typical scenario, a patient pays a copay of $15 in cash; the receptionist records the payment as $5, and pockets the rest. Make sure a receipt is generated for every cash transaction, and that someone other than the person accepting cash reconciles the charges, receipts, and cash totals daily.
- Inventory your stock. Cash isn’t the only susceptible commodity. If you sell cosmetics or other products, inventory your stock frequently. And office personnel are not the only potential thieves: Last year, a locum tenens physician down the street conspired with a receptionist to take cash transactions for cosmetic neurotoxins and fillers “off the books” and split the spoils. That office was being ripped off twice; first for the neurotoxin and filler materials themselves, and then for the cash proceeds.
- Separate all accounting duties. Another popular ploy is false invoicing for imaginary supplies. A friend’s experience provides a good example (retold with his permission): His bookkeeper wrote sizable checks to herself, disguising them in the ledger as payments to vendors commonly used by his practice. Since the same employee also balanced the checkbook, she got away with it for years. “It wasn’t at all clever,” he told me, “and I’m embarrassed to admit that it happened to me.” Once again, separation of duties is the key to prevention. One employee should enter invoices into the data system, another should issue the check or make the electronic transfer, and a third should match invoices to goods and services received.
- Verify expense reports. False expense reporting is a subset of the fake invoice scam. When an employee asks for reimbursement of expenses, make sure those expenses are real.
- Consider computer safeguards. Computers facilitate a lot of financial chores, but they also consolidate financial data in one place, where it is potentially accessible to anybody, anywhere. Your computer vendor should be aware of this, and there should be safeguards built into your system. Ask about them. If they aren’t there, ask why.
- Hire honest employees. All applicants look great on paper, so check their references; and with their permission, you can run background checks for a few dollars on any of several public information web sites. My columns on hiring are available on the MDedge Dermatology website.
- Look for “red flags.” Examples include employees who refuse to take vacations, because someone else will have do their work or who insist on posting expenses that are a coworker’s responsibility, “just to be nice.” Anyone obviously living beyond his or her means merits suspicion as well.
- Consider bonding your employees. Dishonesty bonds are relatively inexpensive, and provide assurance of some measure of recovery if your safeguards fail. Also, just knowing that your staff is bonded will scare off most dishonest applicants. One effective screen is a question on your employment application: “Would you object to being bonded?”
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Smartphone apps for suspicious skin lesions unreliable
Smartphone applications ( said U.K. researchers reporting a systematic review.
These apps are providing information that could lead to “potentially life-or-death decisions,” commented co-lead author Hywel C. Williams, MD, from the Centre of Evidence Based Dermatology, University of Nottingham (England).
“The one thing you mustn’t do in a situation where early diagnosis can make a difference between life and death is you mustn’t miss the melanoma,” he said in an interview.
“These apps were missing melanomas, and that’s very worrisome,” he commented.
The review included nine studies of skin cancer smartphone apps, including two apps, SkinScan and SkinVision, that have been given Conformit Europenne (CE) marks, allowing them to be marketed across Europe. These apps are also available in Australia and New Zealand, but not in the United States.
The review found that SkinScan was not able to identify any melanomas in the one study that assessed this app, while SkinVision had a relatively low sensitivity and specificity, with 12% of cancerous or precancerous lesions missed and 21% of benign lesions wrongly identified as cancerous.
This means that among 1,000 people with a melanoma prevalence of 3%, 4 of 30 melanomas would be missed, and 200 people would be incorrectly told that a mole was of high concern, the authors estimated.
The research was published by The BMJ on Feb. 10.
“Although I was broad minded on the potential benefit of apps for diagnosing skin cancer, I am now worried given the results of our study and the overall poor quality of studies used to test these apps,” Dr. Williams commented in a statement.
Coauthor Jac Dinnes, PhD, from the Institute of Applied Health Research at the University of Birmingham (England), added it is “really disappointing that there is not better quality evidence available to judge the efficacy of these apps.”
“It is vital that health care professionals are aware of the current limitations both in the technologies and in their evaluations,” she added.
The results also highlight the limitations of the regulatory system governing smartphone apps in that they are currently not subject to assessment by bodies such as the U.K.’s Medicines and Healthcare Products Regulatory Agency (MHRA), the authors commented.
“Regulators need to become alert to the potential harm that poorly performing algorithm-based diagnostic or risk monitoring apps create,” said co-lead author Jonathan J. Deeks, PhD, also at the Institute of Applied Health Research.
“We rely on the CE mark as a sign of quality, but the current CE mark assessment processes are not fit for protecting the public against the risks that these apps present.”
Speaking in an interview, Williams lamented the poor quality of the research that had been conducted. “These studies were not good enough,” he said, adding that “there’s no excuse for really poor study design and poor reporting.”
He would like to see the regulations tightened around AI apps purporting to inform decision making for the general public and suggests that these devices should be assessed by the MHRA. “I really do think a CE mark is not enough,” he said.
The team noted that the skin cancer apps “all include disclaimers that the results should only be used as a guide and cannot replace health care advice,” through which the manufacturers “attempt to evade any responsibility for negative outcomes experienced by users.”
Nevertheless, the “poor and variable performance” of the apps revealed by their review indicates that they “have not yet shown sufficient promise to recommend their use,” they concluded.
The “official approval” implied by a CE mark “will give consumers the impression that the apps have been assessed as effective and safe,” wrote Ben Goldacre, DataLab director, Nuffield Department of Primary Care, University of Oxford (England), and colleagues in an accompanying editorial.
“The implicit assumption is that apps are similarly low-risk technology” to devices such as sticking plasters and reading glasses, they comment.
“But shortcomings in diagnostic apps can have serious implications,” they warn. The “risks include psychological harm from health anxiety or ‘cyberchondria,’ and physical harm from misdiagnosis or overdiagnosis; for clinicians there is a risk of increased workload, and changes to ethical or legal responsibilities around triage, referral, diagnosis, and treatment.” There is also potential for “inappropriate resource use, and even loss of credibility for digital technology in general.”
Details of the review
For their review, the authors searched the Cochrane Central Register on Controlled Trials, the MEDLNE, Embase, Cumulative Index to Nursing and Allied Health Literature, Conference Proceedings Citation index, Zetoc, and Science Citation Index databases, and online trial registers for studies published between August 2016 and April 2019.
From 80 studies identified, 9 met the eligibility criteria.
Of those, six studies, evaluating a total of 725 skin lesions, determined the accuracy of smartphone apps in risk stratifying suspicious skin lesions by comparing them against a histopathological reference standard diagnosis or expert follow-up.
Five of these studies aimed to detect only melanoma, while one sought to differentiate between malignant or premalignant lesions (including melanoma, basal cell carcinoma, and squamous cell carcinoma) and benign lesions.
The three remaining studies, which evaluated 407 lesions in all, compared smartphone app recommendations against a reference standard of expert recommendations for further investigation or intervention.
The researchers found the studies had a string of potential biases and limitations.
For example, only four studies recruited a consecutive sample of study participants and lesions, and only two included lesions selected by study participants, whereas five studies used lesions that had been selected by a clinician.
Three studies reported that it took 5-10 attempts to obtain an adequate image. In seven studies, it was the researchers and not the patients who used the app to photograph the lesions, and two studies used images obtained from dermatology databases.
This “raised concerns that the results of the studies were unlikely to be representative of real life use,” the authors comment.
In addition, the exclusion of unevaluable images “might have systematically inflated the diagnostic performance of the tested apps,” they add.
The independent research was supported by the National Institute for Health Research (NIHR) Birmingham Biomedical Research Centre at the University Hospitals Birmingham NHS Foundation Trust and the University of Birmingham and is an update of one of a collection of reviews funded by the NIHR through its Cochrane Systematic Review Programme Grant.
This article first appeared on Medscape.com.
Smartphone applications ( said U.K. researchers reporting a systematic review.
These apps are providing information that could lead to “potentially life-or-death decisions,” commented co-lead author Hywel C. Williams, MD, from the Centre of Evidence Based Dermatology, University of Nottingham (England).
“The one thing you mustn’t do in a situation where early diagnosis can make a difference between life and death is you mustn’t miss the melanoma,” he said in an interview.
“These apps were missing melanomas, and that’s very worrisome,” he commented.
The review included nine studies of skin cancer smartphone apps, including two apps, SkinScan and SkinVision, that have been given Conformit Europenne (CE) marks, allowing them to be marketed across Europe. These apps are also available in Australia and New Zealand, but not in the United States.
The review found that SkinScan was not able to identify any melanomas in the one study that assessed this app, while SkinVision had a relatively low sensitivity and specificity, with 12% of cancerous or precancerous lesions missed and 21% of benign lesions wrongly identified as cancerous.
This means that among 1,000 people with a melanoma prevalence of 3%, 4 of 30 melanomas would be missed, and 200 people would be incorrectly told that a mole was of high concern, the authors estimated.
The research was published by The BMJ on Feb. 10.
“Although I was broad minded on the potential benefit of apps for diagnosing skin cancer, I am now worried given the results of our study and the overall poor quality of studies used to test these apps,” Dr. Williams commented in a statement.
Coauthor Jac Dinnes, PhD, from the Institute of Applied Health Research at the University of Birmingham (England), added it is “really disappointing that there is not better quality evidence available to judge the efficacy of these apps.”
“It is vital that health care professionals are aware of the current limitations both in the technologies and in their evaluations,” she added.
The results also highlight the limitations of the regulatory system governing smartphone apps in that they are currently not subject to assessment by bodies such as the U.K.’s Medicines and Healthcare Products Regulatory Agency (MHRA), the authors commented.
“Regulators need to become alert to the potential harm that poorly performing algorithm-based diagnostic or risk monitoring apps create,” said co-lead author Jonathan J. Deeks, PhD, also at the Institute of Applied Health Research.
“We rely on the CE mark as a sign of quality, but the current CE mark assessment processes are not fit for protecting the public against the risks that these apps present.”
Speaking in an interview, Williams lamented the poor quality of the research that had been conducted. “These studies were not good enough,” he said, adding that “there’s no excuse for really poor study design and poor reporting.”
He would like to see the regulations tightened around AI apps purporting to inform decision making for the general public and suggests that these devices should be assessed by the MHRA. “I really do think a CE mark is not enough,” he said.
The team noted that the skin cancer apps “all include disclaimers that the results should only be used as a guide and cannot replace health care advice,” through which the manufacturers “attempt to evade any responsibility for negative outcomes experienced by users.”
Nevertheless, the “poor and variable performance” of the apps revealed by their review indicates that they “have not yet shown sufficient promise to recommend their use,” they concluded.
The “official approval” implied by a CE mark “will give consumers the impression that the apps have been assessed as effective and safe,” wrote Ben Goldacre, DataLab director, Nuffield Department of Primary Care, University of Oxford (England), and colleagues in an accompanying editorial.
“The implicit assumption is that apps are similarly low-risk technology” to devices such as sticking plasters and reading glasses, they comment.
“But shortcomings in diagnostic apps can have serious implications,” they warn. The “risks include psychological harm from health anxiety or ‘cyberchondria,’ and physical harm from misdiagnosis or overdiagnosis; for clinicians there is a risk of increased workload, and changes to ethical or legal responsibilities around triage, referral, diagnosis, and treatment.” There is also potential for “inappropriate resource use, and even loss of credibility for digital technology in general.”
Details of the review
For their review, the authors searched the Cochrane Central Register on Controlled Trials, the MEDLNE, Embase, Cumulative Index to Nursing and Allied Health Literature, Conference Proceedings Citation index, Zetoc, and Science Citation Index databases, and online trial registers for studies published between August 2016 and April 2019.
From 80 studies identified, 9 met the eligibility criteria.
Of those, six studies, evaluating a total of 725 skin lesions, determined the accuracy of smartphone apps in risk stratifying suspicious skin lesions by comparing them against a histopathological reference standard diagnosis or expert follow-up.
Five of these studies aimed to detect only melanoma, while one sought to differentiate between malignant or premalignant lesions (including melanoma, basal cell carcinoma, and squamous cell carcinoma) and benign lesions.
The three remaining studies, which evaluated 407 lesions in all, compared smartphone app recommendations against a reference standard of expert recommendations for further investigation or intervention.
The researchers found the studies had a string of potential biases and limitations.
For example, only four studies recruited a consecutive sample of study participants and lesions, and only two included lesions selected by study participants, whereas five studies used lesions that had been selected by a clinician.
Three studies reported that it took 5-10 attempts to obtain an adequate image. In seven studies, it was the researchers and not the patients who used the app to photograph the lesions, and two studies used images obtained from dermatology databases.
This “raised concerns that the results of the studies were unlikely to be representative of real life use,” the authors comment.
In addition, the exclusion of unevaluable images “might have systematically inflated the diagnostic performance of the tested apps,” they add.
The independent research was supported by the National Institute for Health Research (NIHR) Birmingham Biomedical Research Centre at the University Hospitals Birmingham NHS Foundation Trust and the University of Birmingham and is an update of one of a collection of reviews funded by the NIHR through its Cochrane Systematic Review Programme Grant.
This article first appeared on Medscape.com.
Smartphone applications ( said U.K. researchers reporting a systematic review.
These apps are providing information that could lead to “potentially life-or-death decisions,” commented co-lead author Hywel C. Williams, MD, from the Centre of Evidence Based Dermatology, University of Nottingham (England).
“The one thing you mustn’t do in a situation where early diagnosis can make a difference between life and death is you mustn’t miss the melanoma,” he said in an interview.
“These apps were missing melanomas, and that’s very worrisome,” he commented.
The review included nine studies of skin cancer smartphone apps, including two apps, SkinScan and SkinVision, that have been given Conformit Europenne (CE) marks, allowing them to be marketed across Europe. These apps are also available in Australia and New Zealand, but not in the United States.
The review found that SkinScan was not able to identify any melanomas in the one study that assessed this app, while SkinVision had a relatively low sensitivity and specificity, with 12% of cancerous or precancerous lesions missed and 21% of benign lesions wrongly identified as cancerous.
This means that among 1,000 people with a melanoma prevalence of 3%, 4 of 30 melanomas would be missed, and 200 people would be incorrectly told that a mole was of high concern, the authors estimated.
The research was published by The BMJ on Feb. 10.
“Although I was broad minded on the potential benefit of apps for diagnosing skin cancer, I am now worried given the results of our study and the overall poor quality of studies used to test these apps,” Dr. Williams commented in a statement.
Coauthor Jac Dinnes, PhD, from the Institute of Applied Health Research at the University of Birmingham (England), added it is “really disappointing that there is not better quality evidence available to judge the efficacy of these apps.”
“It is vital that health care professionals are aware of the current limitations both in the technologies and in their evaluations,” she added.
The results also highlight the limitations of the regulatory system governing smartphone apps in that they are currently not subject to assessment by bodies such as the U.K.’s Medicines and Healthcare Products Regulatory Agency (MHRA), the authors commented.
“Regulators need to become alert to the potential harm that poorly performing algorithm-based diagnostic or risk monitoring apps create,” said co-lead author Jonathan J. Deeks, PhD, also at the Institute of Applied Health Research.
“We rely on the CE mark as a sign of quality, but the current CE mark assessment processes are not fit for protecting the public against the risks that these apps present.”
Speaking in an interview, Williams lamented the poor quality of the research that had been conducted. “These studies were not good enough,” he said, adding that “there’s no excuse for really poor study design and poor reporting.”
He would like to see the regulations tightened around AI apps purporting to inform decision making for the general public and suggests that these devices should be assessed by the MHRA. “I really do think a CE mark is not enough,” he said.
The team noted that the skin cancer apps “all include disclaimers that the results should only be used as a guide and cannot replace health care advice,” through which the manufacturers “attempt to evade any responsibility for negative outcomes experienced by users.”
Nevertheless, the “poor and variable performance” of the apps revealed by their review indicates that they “have not yet shown sufficient promise to recommend their use,” they concluded.
The “official approval” implied by a CE mark “will give consumers the impression that the apps have been assessed as effective and safe,” wrote Ben Goldacre, DataLab director, Nuffield Department of Primary Care, University of Oxford (England), and colleagues in an accompanying editorial.
“The implicit assumption is that apps are similarly low-risk technology” to devices such as sticking plasters and reading glasses, they comment.
“But shortcomings in diagnostic apps can have serious implications,” they warn. The “risks include psychological harm from health anxiety or ‘cyberchondria,’ and physical harm from misdiagnosis or overdiagnosis; for clinicians there is a risk of increased workload, and changes to ethical or legal responsibilities around triage, referral, diagnosis, and treatment.” There is also potential for “inappropriate resource use, and even loss of credibility for digital technology in general.”
Details of the review
For their review, the authors searched the Cochrane Central Register on Controlled Trials, the MEDLNE, Embase, Cumulative Index to Nursing and Allied Health Literature, Conference Proceedings Citation index, Zetoc, and Science Citation Index databases, and online trial registers for studies published between August 2016 and April 2019.
From 80 studies identified, 9 met the eligibility criteria.
Of those, six studies, evaluating a total of 725 skin lesions, determined the accuracy of smartphone apps in risk stratifying suspicious skin lesions by comparing them against a histopathological reference standard diagnosis or expert follow-up.
Five of these studies aimed to detect only melanoma, while one sought to differentiate between malignant or premalignant lesions (including melanoma, basal cell carcinoma, and squamous cell carcinoma) and benign lesions.
The three remaining studies, which evaluated 407 lesions in all, compared smartphone app recommendations against a reference standard of expert recommendations for further investigation or intervention.
The researchers found the studies had a string of potential biases and limitations.
For example, only four studies recruited a consecutive sample of study participants and lesions, and only two included lesions selected by study participants, whereas five studies used lesions that had been selected by a clinician.
Three studies reported that it took 5-10 attempts to obtain an adequate image. In seven studies, it was the researchers and not the patients who used the app to photograph the lesions, and two studies used images obtained from dermatology databases.
This “raised concerns that the results of the studies were unlikely to be representative of real life use,” the authors comment.
In addition, the exclusion of unevaluable images “might have systematically inflated the diagnostic performance of the tested apps,” they add.
The independent research was supported by the National Institute for Health Research (NIHR) Birmingham Biomedical Research Centre at the University Hospitals Birmingham NHS Foundation Trust and the University of Birmingham and is an update of one of a collection of reviews funded by the NIHR through its Cochrane Systematic Review Programme Grant.
This article first appeared on Medscape.com.
CDC confirms 13th case of coronavirus in U.S.
The Centers for Disease Control and Prevention announced the number of confirmed cases of the 2019 Novel Coronavirus (2019-nCoV) in the United States has reached 13.
The latest case, announced Feb. 11, 2020, by the CDC, was in a person in California who was previously under federal quarantine because the patient had traveled to Wuhan, China.
The CDC is currently looking into who the patient may have come in contact with to understand the potential for further spread of the coronavirus.
“The contact investigation is ongoing,” CDC principal deputy director Anne Schuchat, MD, said during a Feb. 11 press conference to provide an update on coronavirus containment activities being taken by the CDC.
Dr. Schuchat also addressed issues related to the laboratory test, as the patient in California was initially thought to be negative for the coronavirus.
“With other cases around the country that we are evaluating, we have been doing serial tests to understand whether they are still infectious” and to gather other information about how results change over time, Dr. Schuchat said.
She noted that the CDC does not “have as much information as we would like on the severity of the virus,” noting that there are many cases in China with severe reactions, while the 13 cases in the United States represent a much more mild reaction to the virus so far.
With the latest case in California, she noted that there was “probably a mix-up and the original test wasn’t negative,” although she did not elaborate on what the nature of the mix-up was, stating that was all the information that she had.
In general, Dr. Schuchat touted the actions taken by the CDC and the federal government focused primarily on containing the spread of the virus in the United States, including the implementation of travel advisories, quarantining passengers returning from China, as well as the new test kits that are being distributed by the agency across the nation and around the world. She also mentioned CDC staff are being deployed around the world to monitor the spreading of the disease and highlighted the outreach efforts to keep the public informed.
Dr. Schuchat highlighted the fact that, of the 13 cases in the United States, 11 were with patients that were in Wuhan, and only 2 were because of close contact with a patient, something that she attributed to the actions being taken.
She also noted that cases in the United States have not been as severe as they have been in China, where deaths have been attributed to the coronavirus outbreak. She added that there have been only two deaths outside of mainland China attributed to the coronavirus.
“Some of the steps the CDC has taken have really put us in better shape should widespread transmission occur in the United States,” she said.
Dr. Schuchat also highlighted that the first charter flight of people quarantined after returning from Wuhan have reached the 14-day milestone and should be on their way home beginning today.
The Centers for Disease Control and Prevention announced the number of confirmed cases of the 2019 Novel Coronavirus (2019-nCoV) in the United States has reached 13.
The latest case, announced Feb. 11, 2020, by the CDC, was in a person in California who was previously under federal quarantine because the patient had traveled to Wuhan, China.
The CDC is currently looking into who the patient may have come in contact with to understand the potential for further spread of the coronavirus.
“The contact investigation is ongoing,” CDC principal deputy director Anne Schuchat, MD, said during a Feb. 11 press conference to provide an update on coronavirus containment activities being taken by the CDC.
Dr. Schuchat also addressed issues related to the laboratory test, as the patient in California was initially thought to be negative for the coronavirus.
“With other cases around the country that we are evaluating, we have been doing serial tests to understand whether they are still infectious” and to gather other information about how results change over time, Dr. Schuchat said.
She noted that the CDC does not “have as much information as we would like on the severity of the virus,” noting that there are many cases in China with severe reactions, while the 13 cases in the United States represent a much more mild reaction to the virus so far.
With the latest case in California, she noted that there was “probably a mix-up and the original test wasn’t negative,” although she did not elaborate on what the nature of the mix-up was, stating that was all the information that she had.
In general, Dr. Schuchat touted the actions taken by the CDC and the federal government focused primarily on containing the spread of the virus in the United States, including the implementation of travel advisories, quarantining passengers returning from China, as well as the new test kits that are being distributed by the agency across the nation and around the world. She also mentioned CDC staff are being deployed around the world to monitor the spreading of the disease and highlighted the outreach efforts to keep the public informed.
Dr. Schuchat highlighted the fact that, of the 13 cases in the United States, 11 were with patients that were in Wuhan, and only 2 were because of close contact with a patient, something that she attributed to the actions being taken.
She also noted that cases in the United States have not been as severe as they have been in China, where deaths have been attributed to the coronavirus outbreak. She added that there have been only two deaths outside of mainland China attributed to the coronavirus.
“Some of the steps the CDC has taken have really put us in better shape should widespread transmission occur in the United States,” she said.
Dr. Schuchat also highlighted that the first charter flight of people quarantined after returning from Wuhan have reached the 14-day milestone and should be on their way home beginning today.
The Centers for Disease Control and Prevention announced the number of confirmed cases of the 2019 Novel Coronavirus (2019-nCoV) in the United States has reached 13.
The latest case, announced Feb. 11, 2020, by the CDC, was in a person in California who was previously under federal quarantine because the patient had traveled to Wuhan, China.
The CDC is currently looking into who the patient may have come in contact with to understand the potential for further spread of the coronavirus.
“The contact investigation is ongoing,” CDC principal deputy director Anne Schuchat, MD, said during a Feb. 11 press conference to provide an update on coronavirus containment activities being taken by the CDC.
Dr. Schuchat also addressed issues related to the laboratory test, as the patient in California was initially thought to be negative for the coronavirus.
“With other cases around the country that we are evaluating, we have been doing serial tests to understand whether they are still infectious” and to gather other information about how results change over time, Dr. Schuchat said.
She noted that the CDC does not “have as much information as we would like on the severity of the virus,” noting that there are many cases in China with severe reactions, while the 13 cases in the United States represent a much more mild reaction to the virus so far.
With the latest case in California, she noted that there was “probably a mix-up and the original test wasn’t negative,” although she did not elaborate on what the nature of the mix-up was, stating that was all the information that she had.
In general, Dr. Schuchat touted the actions taken by the CDC and the federal government focused primarily on containing the spread of the virus in the United States, including the implementation of travel advisories, quarantining passengers returning from China, as well as the new test kits that are being distributed by the agency across the nation and around the world. She also mentioned CDC staff are being deployed around the world to monitor the spreading of the disease and highlighted the outreach efforts to keep the public informed.
Dr. Schuchat highlighted the fact that, of the 13 cases in the United States, 11 were with patients that were in Wuhan, and only 2 were because of close contact with a patient, something that she attributed to the actions being taken.
She also noted that cases in the United States have not been as severe as they have been in China, where deaths have been attributed to the coronavirus outbreak. She added that there have been only two deaths outside of mainland China attributed to the coronavirus.
“Some of the steps the CDC has taken have really put us in better shape should widespread transmission occur in the United States,” she said.
Dr. Schuchat also highlighted that the first charter flight of people quarantined after returning from Wuhan have reached the 14-day milestone and should be on their way home beginning today.
Psoriasis ointment helped with itch, healing in phase 2 EB study
LONDON – , in a small, placebo-controlled, phase 2 study.
More importantly, use of the ointment promoted wound healing in those with the severe skin-blistering condition. Indeed, compared with placebo, a greater reduction in wound size was observed after 2 weeks when the ointment was applied (a mean reduction of 65.5% vs. 88.4%; P less than .006). However, at 1 month, no significant differences were seen in the size of the wounds between the two treatment arms.
“Calcipotriol is a vitamin D analog and it is well known that vitamin D is a very critical factor for skin homeostasis and proper wound healing,” Christina Guttmann-Gruber, PhD, said at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA). Dr. Guttmann-Gruber, a group lead researcher for EB House Austria, which is based at the Salzburg (Austria) University Clinic for Dermatology, noted that vitamin D also helps with tissue repair and immune modulation, and enhances local antimicrobial activity.
During an oral poster presentation at the meeting, Dr. Guttmann-Gruber explained that in previous in vitro studies, it was found that low concentrations (100 nmol) of calcipotriol inhibited proliferation of RDEB tumor cells (Sci Rep. 2018 Sep 7;8:13430). Calcipotriol (also known as calcipotriene) also was found to improve the expression of antimicrobial peptides and promote wound closure. “Therefore, we thought that applying calcipotriol at the site of injury, on chronic wounds prone to superinfection where it is needed, might be beneficial for our patients.”
She and her associates designed a two-arm, randomized, double-blind crossover study to assess the effects of an existing calcipotriol-containing ointment on wound healing in patients with RDEB. The ointment used in the study is approved for treating psoriasis but was adapted by the in-house pharmacy team to reduce the concentration of calcipotriol to about 0.05 mcg/g, or around 121 nmol. The reason for the reduction was that, at higher doses, keratinocyte proliferation was reduced, which would be detrimental in RDEB patients.
Nine patients were included in the study and were randomized to either apply 1 g of the active or placebo ointment to each of two designated wounds, of at least 6 cm2 in size, every day for 4 weeks. A 2-month washout period then followed before the groups switched to use the other ointment for 1 month. Six out of the nine patients completed both treatment phases. The reasons for the patients not completing both intervention phases were not related to the drug.
Calcipotriol treatment resulted in a significant and steady reduction in itch over the entire course of treatment, which was not seen among those on placebo, Dr. Guttmann-Gruber observed. The reduction in itch was “striking,” but only while the treatment was being used, she said. Results for pain were less clear, with a significant reduction in pain after 2 weeks seen only in the placebo group, while both treatments reduced pain to the same degree by 1 month.
No serious adverse events were observed at any time point and topical use of the low-dose calcipotriol did not significantly change serum levels of calcium or vitamin D in the two patients in which this was studied, Dr. Guttmann-Gruber said.
“This is an approved drug; it’s used in psoriasis, but at a very high concentration. We were able to use it off label and make a diluted version,” she observed. “Any pharmacy can do it.” Although it was applied topically, it could be done by applying it to the dressing rather directly onto the wounded skin, she said.
Data on the skin microbiome response to treatment were also collected but were not available to analyze in time for presentation, but it appeared that there was improvement with the low-dose calcipotriol treatment, Dr. Guttmann-Gruber said. “When the wounds are healing, the microbial flora is improving.”
The next step will probably be to plan a multicenter trial of this treatment, Dr. Guttmann-Gruber said in an interview. The questions is whether such a trial would get the financial backing it needed, but if an orphan drug designation could be obtained for calcipotriol for EB, then it would be possible to conduct such a trial.
The study was funded by DEBRA Austria. The presenting author, Dr. Guttmann-Gruber, had no conflicts of interest to disclose.
SOURCE: Guttmann-Gruber C et al. EB World Congress 2020. Poster 34.
LONDON – , in a small, placebo-controlled, phase 2 study.
More importantly, use of the ointment promoted wound healing in those with the severe skin-blistering condition. Indeed, compared with placebo, a greater reduction in wound size was observed after 2 weeks when the ointment was applied (a mean reduction of 65.5% vs. 88.4%; P less than .006). However, at 1 month, no significant differences were seen in the size of the wounds between the two treatment arms.
“Calcipotriol is a vitamin D analog and it is well known that vitamin D is a very critical factor for skin homeostasis and proper wound healing,” Christina Guttmann-Gruber, PhD, said at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA). Dr. Guttmann-Gruber, a group lead researcher for EB House Austria, which is based at the Salzburg (Austria) University Clinic for Dermatology, noted that vitamin D also helps with tissue repair and immune modulation, and enhances local antimicrobial activity.
During an oral poster presentation at the meeting, Dr. Guttmann-Gruber explained that in previous in vitro studies, it was found that low concentrations (100 nmol) of calcipotriol inhibited proliferation of RDEB tumor cells (Sci Rep. 2018 Sep 7;8:13430). Calcipotriol (also known as calcipotriene) also was found to improve the expression of antimicrobial peptides and promote wound closure. “Therefore, we thought that applying calcipotriol at the site of injury, on chronic wounds prone to superinfection where it is needed, might be beneficial for our patients.”
She and her associates designed a two-arm, randomized, double-blind crossover study to assess the effects of an existing calcipotriol-containing ointment on wound healing in patients with RDEB. The ointment used in the study is approved for treating psoriasis but was adapted by the in-house pharmacy team to reduce the concentration of calcipotriol to about 0.05 mcg/g, or around 121 nmol. The reason for the reduction was that, at higher doses, keratinocyte proliferation was reduced, which would be detrimental in RDEB patients.
Nine patients were included in the study and were randomized to either apply 1 g of the active or placebo ointment to each of two designated wounds, of at least 6 cm2 in size, every day for 4 weeks. A 2-month washout period then followed before the groups switched to use the other ointment for 1 month. Six out of the nine patients completed both treatment phases. The reasons for the patients not completing both intervention phases were not related to the drug.
Calcipotriol treatment resulted in a significant and steady reduction in itch over the entire course of treatment, which was not seen among those on placebo, Dr. Guttmann-Gruber observed. The reduction in itch was “striking,” but only while the treatment was being used, she said. Results for pain were less clear, with a significant reduction in pain after 2 weeks seen only in the placebo group, while both treatments reduced pain to the same degree by 1 month.
No serious adverse events were observed at any time point and topical use of the low-dose calcipotriol did not significantly change serum levels of calcium or vitamin D in the two patients in which this was studied, Dr. Guttmann-Gruber said.
“This is an approved drug; it’s used in psoriasis, but at a very high concentration. We were able to use it off label and make a diluted version,” she observed. “Any pharmacy can do it.” Although it was applied topically, it could be done by applying it to the dressing rather directly onto the wounded skin, she said.
Data on the skin microbiome response to treatment were also collected but were not available to analyze in time for presentation, but it appeared that there was improvement with the low-dose calcipotriol treatment, Dr. Guttmann-Gruber said. “When the wounds are healing, the microbial flora is improving.”
The next step will probably be to plan a multicenter trial of this treatment, Dr. Guttmann-Gruber said in an interview. The questions is whether such a trial would get the financial backing it needed, but if an orphan drug designation could be obtained for calcipotriol for EB, then it would be possible to conduct such a trial.
The study was funded by DEBRA Austria. The presenting author, Dr. Guttmann-Gruber, had no conflicts of interest to disclose.
SOURCE: Guttmann-Gruber C et al. EB World Congress 2020. Poster 34.
LONDON – , in a small, placebo-controlled, phase 2 study.
More importantly, use of the ointment promoted wound healing in those with the severe skin-blistering condition. Indeed, compared with placebo, a greater reduction in wound size was observed after 2 weeks when the ointment was applied (a mean reduction of 65.5% vs. 88.4%; P less than .006). However, at 1 month, no significant differences were seen in the size of the wounds between the two treatment arms.
“Calcipotriol is a vitamin D analog and it is well known that vitamin D is a very critical factor for skin homeostasis and proper wound healing,” Christina Guttmann-Gruber, PhD, said at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA). Dr. Guttmann-Gruber, a group lead researcher for EB House Austria, which is based at the Salzburg (Austria) University Clinic for Dermatology, noted that vitamin D also helps with tissue repair and immune modulation, and enhances local antimicrobial activity.
During an oral poster presentation at the meeting, Dr. Guttmann-Gruber explained that in previous in vitro studies, it was found that low concentrations (100 nmol) of calcipotriol inhibited proliferation of RDEB tumor cells (Sci Rep. 2018 Sep 7;8:13430). Calcipotriol (also known as calcipotriene) also was found to improve the expression of antimicrobial peptides and promote wound closure. “Therefore, we thought that applying calcipotriol at the site of injury, on chronic wounds prone to superinfection where it is needed, might be beneficial for our patients.”
She and her associates designed a two-arm, randomized, double-blind crossover study to assess the effects of an existing calcipotriol-containing ointment on wound healing in patients with RDEB. The ointment used in the study is approved for treating psoriasis but was adapted by the in-house pharmacy team to reduce the concentration of calcipotriol to about 0.05 mcg/g, or around 121 nmol. The reason for the reduction was that, at higher doses, keratinocyte proliferation was reduced, which would be detrimental in RDEB patients.
Nine patients were included in the study and were randomized to either apply 1 g of the active or placebo ointment to each of two designated wounds, of at least 6 cm2 in size, every day for 4 weeks. A 2-month washout period then followed before the groups switched to use the other ointment for 1 month. Six out of the nine patients completed both treatment phases. The reasons for the patients not completing both intervention phases were not related to the drug.
Calcipotriol treatment resulted in a significant and steady reduction in itch over the entire course of treatment, which was not seen among those on placebo, Dr. Guttmann-Gruber observed. The reduction in itch was “striking,” but only while the treatment was being used, she said. Results for pain were less clear, with a significant reduction in pain after 2 weeks seen only in the placebo group, while both treatments reduced pain to the same degree by 1 month.
No serious adverse events were observed at any time point and topical use of the low-dose calcipotriol did not significantly change serum levels of calcium or vitamin D in the two patients in which this was studied, Dr. Guttmann-Gruber said.
“This is an approved drug; it’s used in psoriasis, but at a very high concentration. We were able to use it off label and make a diluted version,” she observed. “Any pharmacy can do it.” Although it was applied topically, it could be done by applying it to the dressing rather directly onto the wounded skin, she said.
Data on the skin microbiome response to treatment were also collected but were not available to analyze in time for presentation, but it appeared that there was improvement with the low-dose calcipotriol treatment, Dr. Guttmann-Gruber said. “When the wounds are healing, the microbial flora is improving.”
The next step will probably be to plan a multicenter trial of this treatment, Dr. Guttmann-Gruber said in an interview. The questions is whether such a trial would get the financial backing it needed, but if an orphan drug designation could be obtained for calcipotriol for EB, then it would be possible to conduct such a trial.
The study was funded by DEBRA Austria. The presenting author, Dr. Guttmann-Gruber, had no conflicts of interest to disclose.
SOURCE: Guttmann-Gruber C et al. EB World Congress 2020. Poster 34.
REPORTING FROM EB 2020
What you absolutely need to know about tail coverage
A 28-year-old pediatrician working in a large group practice in California found a new job in Pennsylvania. The job would allow her to live with her husband, who was a nonphysician.
On her last day of work at the California job, the practice’s office manager asked her, “Do you know about the tail coverage?”
He explained that it is malpractice insurance for any cases filed against her after leaving the job. Without it, he said, she would not be covered for those claims.
The physician (who asked not to be identified) had very little savings and suddenly had to pay a five-figure bill for tail coverage. To provide the extra malpractice coverage, she and her husband had to use savings they’d set aside to buy a house.
Getting tail coverage, known formally as an extended reporting endorsement, often comes as a complete and costly surprise for new doctors, says Dennis Hursh, Esq, a health care attorney based in Middletown, Penn., who deals with physicians’ employment contracts.
“Having to pay for a tail can disrupt lives,” Hursh said. “A tail can cost about one third of a young doctor’s salary. If you don’t feel you can afford to pay that, you may be forced to stay with a job you don’t like.”
Most medical residents don’t think about tail coverage until they apply for their first job, but last year, residents at Hahnemann University Hospital in Philadelphia got a painful early lesson.
In the summer, the hospital went out of business because of financial problems. Hundreds of medical residents and fellows not only were forced to find new programs but also had to prepare to buy tail coverage for their training years at Hahnemann.
“All the guarantees have been yanked out from under us,” said Tom Sibert, MD, a former internal medicine resident at the hospital, who is now finishing his training in California. “Residents don’t have that kind of money.”
Hahnemann trainees have asked the judge in the bankruptcy proceedings to put them ahead of other creditors and to ensure their tail coverage is paid. As of early February, the issue had not been resolved.
Meanwhile, Sibert and many other former trainees were trying to get quotes for purchasing tail coverage. They have been shocked by the amounts they would have to pay.
How tail coverage works
Medical malpractice tail coverage protects from incidents that took place when doctors were at their previous jobs but that later resulted in malpractice claims after they had left that employer.
One type of malpractice insurance, an occurrence policy, does not need tail coverage. Occurrence policies cover any incident that occurred when the policy was in force, no matter when a claim was filed – even if it is filed many years after the claims-filing period of the policy ends.
However, most malpractice policies – as many as 85%, according to one estimate – are claims-made policies. Claims-made policies are more much common because they’re significantly less expensive than occurrence policies.
Under a claims-made policy, coverage for malpractice claims completely stops when the policy ends. It does not cover incidents that occurred when the policy was in force but for which the patients later filed claims, as the occurrence policy does. So a tail is needed to cover these claims.
Physicians in all stages of their career may need tail coverage when they leave a job, change malpractice carriers, or retire.
But young physicians often have greater problems with tail coverage, for several reasons. They tend to be employed, and as such, they cannot choose the coverage they want. As a result, they most likely get claims-made coverage. In addition, the job turnover tends to be higher for these doctors. When leaving a job, the tail comes into play. More than half of new physicians leave their first job within 5 years, and of those, more than half leave after only 1 or 2 years.
Young physicians have no experience with tails and may not even know what they are. “In training, malpractice coverage is not a problem because the program handles it,” Mr. Hursh said. Accreditation standards require that teaching hospitals buy coverage, including a tail when residents leave.
So when young physicians are offered their first job and are handed an employment contract to sign, they may not even look for tail coverage, says Mr. Hursh, who wrote The Final Hurdle, a Physician’s Guide to Negotiating a Fair Employment Agreement. Instead, “young physicians tend to focus on issues like salary, benefits, and signing bonuses,” he said.
Mr. Hursh says the tail is usually the most expensive potential cost in the contract.
There’s no easy way to get out of paying the tail coverage once it is enshrined in the contract. The full tail can cost five or even six figures, depending on the physicians’ specialty, the local malpractice premium, and the physician’s own claims history.
Can you negotiate your tail coverage?
Negotiating tail coverage in the employment contract involves some familiarity with medical malpractice insurance and a close reading of the contract. First, you have to determine that the employer is providing claims-made coverage, which would require a tail if you leave. Then you have to determine whether the employer will pay for the tail coverage.
Often, the contract does not even mention tail coverage. “It could merely state that the practice will be responsible for malpractice coverage while you are working there,” Mr. Hursh said. Although it never specifies the tail, this language indicates that you will be paying for it, he says.
Therefore, it’s wise to have a conversation with your prospective employer about the tail. “Some new doctors never ask the question ‘What happens if I leave? Do I get tail coverage?’ ” said Israel Teitelbaum, an attorney who is chairman of Contemporary Insurance Services, an insurance broker in Silver Spring, Md.
Talking about the tail, however, can be a touchy subject for many young doctors applying for their first job. The tail matters only if you leave the job, and you may not want to imply that you would ever want to leave. Too much money, however, is on the line for you not to ask, Mr. Teitelbaum said.
Even if the employer verbally agrees to pay for the tail coverage, experts advise that you try to get the employer’s commitment in writing and have it put it into the contract.
Getting the employer to cover the tail in the initial contract is crucial because once you have agreed to work there, “it’s much more difficult to get it changed,” Mr. Teitelbaum said. However, even if tail coverage is not in the first contract, you shouldn’t give up, he says. You should try again in the next contract a few years later.
“It’s never too late to bring it up,” Mr. Teitelbaum said. After a few years of employment, you have a track record at the job. “A doctor who is very desirable to the employer may be able to get tail coverage on contract renewal.”
Coverage: Large employers vs. small employers
Willingness to pay for an employee’s tail coverage varies depending on the size of the employer. Large employers – systems, hospitals, and large practices – are much more likely to cover the tail than small and medium-sized practices.
Large employers tend to pay for at least part of the tail because they realize that it is in their interest to do so. Since they have the deepest pockets, they’re often the first to be named in a lawsuit. They might have to pay the whole claim if the physician did not have tail coverage.
However, many large employers want to use tail coverage as a bargaining chip to make sure doctors stay for a while at least. One typical arrangement, Mr. Hursh says, is to pay only one-fifth of the tail if the physician leaves in the first year of employment and then to pay one fifth more in each succeeding year until year five, when the employer assumes the entire cost of the tail.
Smaller practices, on the other hand, are usually close-fisted about tail coverage. “They tend to view the tail as an unnecessary expense,” Mr. Hursh said. “They don’t want to pay for a doctor who is not generating revenue for them any more.”
Traditionally, when physicians become partners, practices are more generous and agree to pay their tails if they leave, Mr. Hursh says. But he thinks this is changing, too – recent partnership contracts he has reviewed did not provide for tail coverage.
Times you don’t need to pay for tail coverage
Even if you’re responsible for the tail coverage, your insurance arrangement may be such that you don’t have to pay for it, says Michelle Perron, a malpractice insurance broker in North Hampton, N.H.
For example, if the carrier at your new job is the same as the one at your old job, your coverage would continue with no break, and you would not need a tail, she says. Even if you move to another state, your old carrier might also sell policies there, and you would then likely have seamless coverage, Ms. Perron says. This would be handy if you could choose your new carrier.
Even when you change carriers, Ms. Perron says, the new one might agree to pick up the old carrier’s coverage in return for getting your business, assuming you are an independent physician buying your own coverage. The new carrier would issue prior acts coverage, also known as nose coverage.
Older doctors going into retirement also have a potential tail coverage problem, but their tail coverage premium is often waived, Ms. Perron says. The need for a tail has to do with claims arising post retirement, after your coverage has ended. Typically, if you have been with the carrier for at least 5 years and you are age 55 years or older, your carrier will waive the tail coverage premium, she says.
However, if the retired doctor starts practicing again, even part time, the carrier may want to take back the free tail, she says. Some retired doctors get around this by buying a lower-priced tail from another company, but the former carrier may still want its money back, Ms. Perron says.
Can you just go without tail coverage?
What happens if physicians with a tail commitment choose to wing it and not pay for the tail? If a claim was never made against them, they may believe that the expense is unnecessary. The situation, however, is not so simple.
Some states require having tail coverage. Malpractice coverage is required in seven states, and at least some of those states explicitly extend this requirement to tails. They are Colorado, Connecticut, Kansas, Massachusetts, New Jersey, Rhode Island, and Wisconsin. Eleven more states tie malpractice coverage, perhaps including tails, to some benefit for the doctor, such as tort reform. These states include Indiana, Nebraska, New Mexico, New York, and Pennsylvania.
Many hospitals require tail coverage for privileges, and some insurers do as well. In addition, Ms. Perron says a missing tail reduces your prospects when looking for a job. “For the employer, having to pay coverage for a new hire will cost more than starting fresh with someone else,” she said.
Still, it’s important to remember the risk of being sued. “If you don’t buy the tail coverage, you are at risk for a lawsuit for many years to come,” Mr. Teitelbaum said.
Doctors should consider their potential lifetime risk, not just their current risk. Although only 8% of doctors younger than age 40 have been sued for malpractice, that figure climbs to almost half by the time doctors reach age 55.
The risks are higher in some specialties. About 63% of general surgeons and ob.gyns. have been sued.
Many of these claims are without merit, and doctors pay only the legal expenses of defending the case. Some doctors may think they could risk frivolous suits and cover legal expenses out of pocket. An American Medical Association survey showed that 68% of closed claims against doctors were dropped, dismissed, or withdrawn. It said these claims cost an average of more than $30,000 to defend.
However, Mr. Teitelbaum puts the defense costs for so-called frivolous suits much higher than the AMA, at $250,000 or more. “Even if you’re sure you won’t have to pay a claim, you still have to defend yourself against frivolous suits,” he said. “You won’t recover those expenses.”
How to lower your tail coverage cost
Physicians typically have 60 days to buy tail coverage after their regular coverage has ended. Specialized brokers such as Mr. Teitelbaum and Ms. Perron help physicians look for the best tails to buy.
The cost of the tail depends on how long you’ve been at your job when you leave it, Ms. Perron says. If you leave in the first 1 or 2 years of the policy, she says, the tail price will be lower because the coverage period is shorter.
Usually the most expensive tail available is from the carrier that issued the original policy. Why is this? “Carriers rarely sell a tail that undercuts their retail price,” Mr. Teitelbaum said. “They don’t want to compete with themselves, and in fact doing so could pose regulatory problems for them.”
Instead of buying from their own carrier, doctors can purchase stand-alone tails from competitors, which Mr. Teitelbaum says are 10%-30% less expensive than the policy the original carrier issues. However, stand-alone tails are not always easy to find, especially for high-cost specialties such as neurosurgery and ob.gyn., he says.
Some physicians try to bring down the cost of the tail by limiting the duration of the tail. You can buy tails that only cover claims filed 1-5 years after the incident took place, rather than indefinitely. These limits mirror the typical statute of limitations – the time limit to file a claim in each state. This limit is as little as 2 years in some states, though it can be as long as 6 years in others.
However, some states make exceptions to the statute of limitations. The 2- to 6-year clock doesn’t start ticking until the mistake is discovered or, in the case of children, when they reach adulthood. “This means that with a limited tail, you always have risk,” Perron said.
And yet some doctors insist on these time-limited tails. “If a doctor opts for 3 years’ coverage, that’s better than no years,” Mr. Teitelbaum said. “But I would advise them to take at least 5 years because that gives you coverage for the basic statute of limitations in most states. Three-year tails do yield savings, but often they’re not enough to warrant the risk.”
Another way to reduce costs is to lower the coverage limits of the tail. The standard coverage limit is $1 million per case and $3 million per year, so doctors might be able to save money on the premium by buying limits of $200,000/$600,000. But Mr. Teitelbaum says most companies would refuse to sell a policy with a limit lower than that of the expiring policy.
Further ways to reduce the cost of the tail include buying tail coverage that doesn’t give the physician the right to approve a settlement or that doesn’t include legal fees in the coverage limits. But these options, too, raise the physician’s risks. Whichever option you choose, the important thing is to protect yourself against costly lawsuits.
This article first appeared on Medscape.com.
A 28-year-old pediatrician working in a large group practice in California found a new job in Pennsylvania. The job would allow her to live with her husband, who was a nonphysician.
On her last day of work at the California job, the practice’s office manager asked her, “Do you know about the tail coverage?”
He explained that it is malpractice insurance for any cases filed against her after leaving the job. Without it, he said, she would not be covered for those claims.
The physician (who asked not to be identified) had very little savings and suddenly had to pay a five-figure bill for tail coverage. To provide the extra malpractice coverage, she and her husband had to use savings they’d set aside to buy a house.
Getting tail coverage, known formally as an extended reporting endorsement, often comes as a complete and costly surprise for new doctors, says Dennis Hursh, Esq, a health care attorney based in Middletown, Penn., who deals with physicians’ employment contracts.
“Having to pay for a tail can disrupt lives,” Hursh said. “A tail can cost about one third of a young doctor’s salary. If you don’t feel you can afford to pay that, you may be forced to stay with a job you don’t like.”
Most medical residents don’t think about tail coverage until they apply for their first job, but last year, residents at Hahnemann University Hospital in Philadelphia got a painful early lesson.
In the summer, the hospital went out of business because of financial problems. Hundreds of medical residents and fellows not only were forced to find new programs but also had to prepare to buy tail coverage for their training years at Hahnemann.
“All the guarantees have been yanked out from under us,” said Tom Sibert, MD, a former internal medicine resident at the hospital, who is now finishing his training in California. “Residents don’t have that kind of money.”
Hahnemann trainees have asked the judge in the bankruptcy proceedings to put them ahead of other creditors and to ensure their tail coverage is paid. As of early February, the issue had not been resolved.
Meanwhile, Sibert and many other former trainees were trying to get quotes for purchasing tail coverage. They have been shocked by the amounts they would have to pay.
How tail coverage works
Medical malpractice tail coverage protects from incidents that took place when doctors were at their previous jobs but that later resulted in malpractice claims after they had left that employer.
One type of malpractice insurance, an occurrence policy, does not need tail coverage. Occurrence policies cover any incident that occurred when the policy was in force, no matter when a claim was filed – even if it is filed many years after the claims-filing period of the policy ends.
However, most malpractice policies – as many as 85%, according to one estimate – are claims-made policies. Claims-made policies are more much common because they’re significantly less expensive than occurrence policies.
Under a claims-made policy, coverage for malpractice claims completely stops when the policy ends. It does not cover incidents that occurred when the policy was in force but for which the patients later filed claims, as the occurrence policy does. So a tail is needed to cover these claims.
Physicians in all stages of their career may need tail coverage when they leave a job, change malpractice carriers, or retire.
But young physicians often have greater problems with tail coverage, for several reasons. They tend to be employed, and as such, they cannot choose the coverage they want. As a result, they most likely get claims-made coverage. In addition, the job turnover tends to be higher for these doctors. When leaving a job, the tail comes into play. More than half of new physicians leave their first job within 5 years, and of those, more than half leave after only 1 or 2 years.
Young physicians have no experience with tails and may not even know what they are. “In training, malpractice coverage is not a problem because the program handles it,” Mr. Hursh said. Accreditation standards require that teaching hospitals buy coverage, including a tail when residents leave.
So when young physicians are offered their first job and are handed an employment contract to sign, they may not even look for tail coverage, says Mr. Hursh, who wrote The Final Hurdle, a Physician’s Guide to Negotiating a Fair Employment Agreement. Instead, “young physicians tend to focus on issues like salary, benefits, and signing bonuses,” he said.
Mr. Hursh says the tail is usually the most expensive potential cost in the contract.
There’s no easy way to get out of paying the tail coverage once it is enshrined in the contract. The full tail can cost five or even six figures, depending on the physicians’ specialty, the local malpractice premium, and the physician’s own claims history.
Can you negotiate your tail coverage?
Negotiating tail coverage in the employment contract involves some familiarity with medical malpractice insurance and a close reading of the contract. First, you have to determine that the employer is providing claims-made coverage, which would require a tail if you leave. Then you have to determine whether the employer will pay for the tail coverage.
Often, the contract does not even mention tail coverage. “It could merely state that the practice will be responsible for malpractice coverage while you are working there,” Mr. Hursh said. Although it never specifies the tail, this language indicates that you will be paying for it, he says.
Therefore, it’s wise to have a conversation with your prospective employer about the tail. “Some new doctors never ask the question ‘What happens if I leave? Do I get tail coverage?’ ” said Israel Teitelbaum, an attorney who is chairman of Contemporary Insurance Services, an insurance broker in Silver Spring, Md.
Talking about the tail, however, can be a touchy subject for many young doctors applying for their first job. The tail matters only if you leave the job, and you may not want to imply that you would ever want to leave. Too much money, however, is on the line for you not to ask, Mr. Teitelbaum said.
Even if the employer verbally agrees to pay for the tail coverage, experts advise that you try to get the employer’s commitment in writing and have it put it into the contract.
Getting the employer to cover the tail in the initial contract is crucial because once you have agreed to work there, “it’s much more difficult to get it changed,” Mr. Teitelbaum said. However, even if tail coverage is not in the first contract, you shouldn’t give up, he says. You should try again in the next contract a few years later.
“It’s never too late to bring it up,” Mr. Teitelbaum said. After a few years of employment, you have a track record at the job. “A doctor who is very desirable to the employer may be able to get tail coverage on contract renewal.”
Coverage: Large employers vs. small employers
Willingness to pay for an employee’s tail coverage varies depending on the size of the employer. Large employers – systems, hospitals, and large practices – are much more likely to cover the tail than small and medium-sized practices.
Large employers tend to pay for at least part of the tail because they realize that it is in their interest to do so. Since they have the deepest pockets, they’re often the first to be named in a lawsuit. They might have to pay the whole claim if the physician did not have tail coverage.
However, many large employers want to use tail coverage as a bargaining chip to make sure doctors stay for a while at least. One typical arrangement, Mr. Hursh says, is to pay only one-fifth of the tail if the physician leaves in the first year of employment and then to pay one fifth more in each succeeding year until year five, when the employer assumes the entire cost of the tail.
Smaller practices, on the other hand, are usually close-fisted about tail coverage. “They tend to view the tail as an unnecessary expense,” Mr. Hursh said. “They don’t want to pay for a doctor who is not generating revenue for them any more.”
Traditionally, when physicians become partners, practices are more generous and agree to pay their tails if they leave, Mr. Hursh says. But he thinks this is changing, too – recent partnership contracts he has reviewed did not provide for tail coverage.
Times you don’t need to pay for tail coverage
Even if you’re responsible for the tail coverage, your insurance arrangement may be such that you don’t have to pay for it, says Michelle Perron, a malpractice insurance broker in North Hampton, N.H.
For example, if the carrier at your new job is the same as the one at your old job, your coverage would continue with no break, and you would not need a tail, she says. Even if you move to another state, your old carrier might also sell policies there, and you would then likely have seamless coverage, Ms. Perron says. This would be handy if you could choose your new carrier.
Even when you change carriers, Ms. Perron says, the new one might agree to pick up the old carrier’s coverage in return for getting your business, assuming you are an independent physician buying your own coverage. The new carrier would issue prior acts coverage, also known as nose coverage.
Older doctors going into retirement also have a potential tail coverage problem, but their tail coverage premium is often waived, Ms. Perron says. The need for a tail has to do with claims arising post retirement, after your coverage has ended. Typically, if you have been with the carrier for at least 5 years and you are age 55 years or older, your carrier will waive the tail coverage premium, she says.
However, if the retired doctor starts practicing again, even part time, the carrier may want to take back the free tail, she says. Some retired doctors get around this by buying a lower-priced tail from another company, but the former carrier may still want its money back, Ms. Perron says.
Can you just go without tail coverage?
What happens if physicians with a tail commitment choose to wing it and not pay for the tail? If a claim was never made against them, they may believe that the expense is unnecessary. The situation, however, is not so simple.
Some states require having tail coverage. Malpractice coverage is required in seven states, and at least some of those states explicitly extend this requirement to tails. They are Colorado, Connecticut, Kansas, Massachusetts, New Jersey, Rhode Island, and Wisconsin. Eleven more states tie malpractice coverage, perhaps including tails, to some benefit for the doctor, such as tort reform. These states include Indiana, Nebraska, New Mexico, New York, and Pennsylvania.
Many hospitals require tail coverage for privileges, and some insurers do as well. In addition, Ms. Perron says a missing tail reduces your prospects when looking for a job. “For the employer, having to pay coverage for a new hire will cost more than starting fresh with someone else,” she said.
Still, it’s important to remember the risk of being sued. “If you don’t buy the tail coverage, you are at risk for a lawsuit for many years to come,” Mr. Teitelbaum said.
Doctors should consider their potential lifetime risk, not just their current risk. Although only 8% of doctors younger than age 40 have been sued for malpractice, that figure climbs to almost half by the time doctors reach age 55.
The risks are higher in some specialties. About 63% of general surgeons and ob.gyns. have been sued.
Many of these claims are without merit, and doctors pay only the legal expenses of defending the case. Some doctors may think they could risk frivolous suits and cover legal expenses out of pocket. An American Medical Association survey showed that 68% of closed claims against doctors were dropped, dismissed, or withdrawn. It said these claims cost an average of more than $30,000 to defend.
However, Mr. Teitelbaum puts the defense costs for so-called frivolous suits much higher than the AMA, at $250,000 or more. “Even if you’re sure you won’t have to pay a claim, you still have to defend yourself against frivolous suits,” he said. “You won’t recover those expenses.”
How to lower your tail coverage cost
Physicians typically have 60 days to buy tail coverage after their regular coverage has ended. Specialized brokers such as Mr. Teitelbaum and Ms. Perron help physicians look for the best tails to buy.
The cost of the tail depends on how long you’ve been at your job when you leave it, Ms. Perron says. If you leave in the first 1 or 2 years of the policy, she says, the tail price will be lower because the coverage period is shorter.
Usually the most expensive tail available is from the carrier that issued the original policy. Why is this? “Carriers rarely sell a tail that undercuts their retail price,” Mr. Teitelbaum said. “They don’t want to compete with themselves, and in fact doing so could pose regulatory problems for them.”
Instead of buying from their own carrier, doctors can purchase stand-alone tails from competitors, which Mr. Teitelbaum says are 10%-30% less expensive than the policy the original carrier issues. However, stand-alone tails are not always easy to find, especially for high-cost specialties such as neurosurgery and ob.gyn., he says.
Some physicians try to bring down the cost of the tail by limiting the duration of the tail. You can buy tails that only cover claims filed 1-5 years after the incident took place, rather than indefinitely. These limits mirror the typical statute of limitations – the time limit to file a claim in each state. This limit is as little as 2 years in some states, though it can be as long as 6 years in others.
However, some states make exceptions to the statute of limitations. The 2- to 6-year clock doesn’t start ticking until the mistake is discovered or, in the case of children, when they reach adulthood. “This means that with a limited tail, you always have risk,” Perron said.
And yet some doctors insist on these time-limited tails. “If a doctor opts for 3 years’ coverage, that’s better than no years,” Mr. Teitelbaum said. “But I would advise them to take at least 5 years because that gives you coverage for the basic statute of limitations in most states. Three-year tails do yield savings, but often they’re not enough to warrant the risk.”
Another way to reduce costs is to lower the coverage limits of the tail. The standard coverage limit is $1 million per case and $3 million per year, so doctors might be able to save money on the premium by buying limits of $200,000/$600,000. But Mr. Teitelbaum says most companies would refuse to sell a policy with a limit lower than that of the expiring policy.
Further ways to reduce the cost of the tail include buying tail coverage that doesn’t give the physician the right to approve a settlement or that doesn’t include legal fees in the coverage limits. But these options, too, raise the physician’s risks. Whichever option you choose, the important thing is to protect yourself against costly lawsuits.
This article first appeared on Medscape.com.
A 28-year-old pediatrician working in a large group practice in California found a new job in Pennsylvania. The job would allow her to live with her husband, who was a nonphysician.
On her last day of work at the California job, the practice’s office manager asked her, “Do you know about the tail coverage?”
He explained that it is malpractice insurance for any cases filed against her after leaving the job. Without it, he said, she would not be covered for those claims.
The physician (who asked not to be identified) had very little savings and suddenly had to pay a five-figure bill for tail coverage. To provide the extra malpractice coverage, she and her husband had to use savings they’d set aside to buy a house.
Getting tail coverage, known formally as an extended reporting endorsement, often comes as a complete and costly surprise for new doctors, says Dennis Hursh, Esq, a health care attorney based in Middletown, Penn., who deals with physicians’ employment contracts.
“Having to pay for a tail can disrupt lives,” Hursh said. “A tail can cost about one third of a young doctor’s salary. If you don’t feel you can afford to pay that, you may be forced to stay with a job you don’t like.”
Most medical residents don’t think about tail coverage until they apply for their first job, but last year, residents at Hahnemann University Hospital in Philadelphia got a painful early lesson.
In the summer, the hospital went out of business because of financial problems. Hundreds of medical residents and fellows not only were forced to find new programs but also had to prepare to buy tail coverage for their training years at Hahnemann.
“All the guarantees have been yanked out from under us,” said Tom Sibert, MD, a former internal medicine resident at the hospital, who is now finishing his training in California. “Residents don’t have that kind of money.”
Hahnemann trainees have asked the judge in the bankruptcy proceedings to put them ahead of other creditors and to ensure their tail coverage is paid. As of early February, the issue had not been resolved.
Meanwhile, Sibert and many other former trainees were trying to get quotes for purchasing tail coverage. They have been shocked by the amounts they would have to pay.
How tail coverage works
Medical malpractice tail coverage protects from incidents that took place when doctors were at their previous jobs but that later resulted in malpractice claims after they had left that employer.
One type of malpractice insurance, an occurrence policy, does not need tail coverage. Occurrence policies cover any incident that occurred when the policy was in force, no matter when a claim was filed – even if it is filed many years after the claims-filing period of the policy ends.
However, most malpractice policies – as many as 85%, according to one estimate – are claims-made policies. Claims-made policies are more much common because they’re significantly less expensive than occurrence policies.
Under a claims-made policy, coverage for malpractice claims completely stops when the policy ends. It does not cover incidents that occurred when the policy was in force but for which the patients later filed claims, as the occurrence policy does. So a tail is needed to cover these claims.
Physicians in all stages of their career may need tail coverage when they leave a job, change malpractice carriers, or retire.
But young physicians often have greater problems with tail coverage, for several reasons. They tend to be employed, and as such, they cannot choose the coverage they want. As a result, they most likely get claims-made coverage. In addition, the job turnover tends to be higher for these doctors. When leaving a job, the tail comes into play. More than half of new physicians leave their first job within 5 years, and of those, more than half leave after only 1 or 2 years.
Young physicians have no experience with tails and may not even know what they are. “In training, malpractice coverage is not a problem because the program handles it,” Mr. Hursh said. Accreditation standards require that teaching hospitals buy coverage, including a tail when residents leave.
So when young physicians are offered their first job and are handed an employment contract to sign, they may not even look for tail coverage, says Mr. Hursh, who wrote The Final Hurdle, a Physician’s Guide to Negotiating a Fair Employment Agreement. Instead, “young physicians tend to focus on issues like salary, benefits, and signing bonuses,” he said.
Mr. Hursh says the tail is usually the most expensive potential cost in the contract.
There’s no easy way to get out of paying the tail coverage once it is enshrined in the contract. The full tail can cost five or even six figures, depending on the physicians’ specialty, the local malpractice premium, and the physician’s own claims history.
Can you negotiate your tail coverage?
Negotiating tail coverage in the employment contract involves some familiarity with medical malpractice insurance and a close reading of the contract. First, you have to determine that the employer is providing claims-made coverage, which would require a tail if you leave. Then you have to determine whether the employer will pay for the tail coverage.
Often, the contract does not even mention tail coverage. “It could merely state that the practice will be responsible for malpractice coverage while you are working there,” Mr. Hursh said. Although it never specifies the tail, this language indicates that you will be paying for it, he says.
Therefore, it’s wise to have a conversation with your prospective employer about the tail. “Some new doctors never ask the question ‘What happens if I leave? Do I get tail coverage?’ ” said Israel Teitelbaum, an attorney who is chairman of Contemporary Insurance Services, an insurance broker in Silver Spring, Md.
Talking about the tail, however, can be a touchy subject for many young doctors applying for their first job. The tail matters only if you leave the job, and you may not want to imply that you would ever want to leave. Too much money, however, is on the line for you not to ask, Mr. Teitelbaum said.
Even if the employer verbally agrees to pay for the tail coverage, experts advise that you try to get the employer’s commitment in writing and have it put it into the contract.
Getting the employer to cover the tail in the initial contract is crucial because once you have agreed to work there, “it’s much more difficult to get it changed,” Mr. Teitelbaum said. However, even if tail coverage is not in the first contract, you shouldn’t give up, he says. You should try again in the next contract a few years later.
“It’s never too late to bring it up,” Mr. Teitelbaum said. After a few years of employment, you have a track record at the job. “A doctor who is very desirable to the employer may be able to get tail coverage on contract renewal.”
Coverage: Large employers vs. small employers
Willingness to pay for an employee’s tail coverage varies depending on the size of the employer. Large employers – systems, hospitals, and large practices – are much more likely to cover the tail than small and medium-sized practices.
Large employers tend to pay for at least part of the tail because they realize that it is in their interest to do so. Since they have the deepest pockets, they’re often the first to be named in a lawsuit. They might have to pay the whole claim if the physician did not have tail coverage.
However, many large employers want to use tail coverage as a bargaining chip to make sure doctors stay for a while at least. One typical arrangement, Mr. Hursh says, is to pay only one-fifth of the tail if the physician leaves in the first year of employment and then to pay one fifth more in each succeeding year until year five, when the employer assumes the entire cost of the tail.
Smaller practices, on the other hand, are usually close-fisted about tail coverage. “They tend to view the tail as an unnecessary expense,” Mr. Hursh said. “They don’t want to pay for a doctor who is not generating revenue for them any more.”
Traditionally, when physicians become partners, practices are more generous and agree to pay their tails if they leave, Mr. Hursh says. But he thinks this is changing, too – recent partnership contracts he has reviewed did not provide for tail coverage.
Times you don’t need to pay for tail coverage
Even if you’re responsible for the tail coverage, your insurance arrangement may be such that you don’t have to pay for it, says Michelle Perron, a malpractice insurance broker in North Hampton, N.H.
For example, if the carrier at your new job is the same as the one at your old job, your coverage would continue with no break, and you would not need a tail, she says. Even if you move to another state, your old carrier might also sell policies there, and you would then likely have seamless coverage, Ms. Perron says. This would be handy if you could choose your new carrier.
Even when you change carriers, Ms. Perron says, the new one might agree to pick up the old carrier’s coverage in return for getting your business, assuming you are an independent physician buying your own coverage. The new carrier would issue prior acts coverage, also known as nose coverage.
Older doctors going into retirement also have a potential tail coverage problem, but their tail coverage premium is often waived, Ms. Perron says. The need for a tail has to do with claims arising post retirement, after your coverage has ended. Typically, if you have been with the carrier for at least 5 years and you are age 55 years or older, your carrier will waive the tail coverage premium, she says.
However, if the retired doctor starts practicing again, even part time, the carrier may want to take back the free tail, she says. Some retired doctors get around this by buying a lower-priced tail from another company, but the former carrier may still want its money back, Ms. Perron says.
Can you just go without tail coverage?
What happens if physicians with a tail commitment choose to wing it and not pay for the tail? If a claim was never made against them, they may believe that the expense is unnecessary. The situation, however, is not so simple.
Some states require having tail coverage. Malpractice coverage is required in seven states, and at least some of those states explicitly extend this requirement to tails. They are Colorado, Connecticut, Kansas, Massachusetts, New Jersey, Rhode Island, and Wisconsin. Eleven more states tie malpractice coverage, perhaps including tails, to some benefit for the doctor, such as tort reform. These states include Indiana, Nebraska, New Mexico, New York, and Pennsylvania.
Many hospitals require tail coverage for privileges, and some insurers do as well. In addition, Ms. Perron says a missing tail reduces your prospects when looking for a job. “For the employer, having to pay coverage for a new hire will cost more than starting fresh with someone else,” she said.
Still, it’s important to remember the risk of being sued. “If you don’t buy the tail coverage, you are at risk for a lawsuit for many years to come,” Mr. Teitelbaum said.
Doctors should consider their potential lifetime risk, not just their current risk. Although only 8% of doctors younger than age 40 have been sued for malpractice, that figure climbs to almost half by the time doctors reach age 55.
The risks are higher in some specialties. About 63% of general surgeons and ob.gyns. have been sued.
Many of these claims are without merit, and doctors pay only the legal expenses of defending the case. Some doctors may think they could risk frivolous suits and cover legal expenses out of pocket. An American Medical Association survey showed that 68% of closed claims against doctors were dropped, dismissed, or withdrawn. It said these claims cost an average of more than $30,000 to defend.
However, Mr. Teitelbaum puts the defense costs for so-called frivolous suits much higher than the AMA, at $250,000 or more. “Even if you’re sure you won’t have to pay a claim, you still have to defend yourself against frivolous suits,” he said. “You won’t recover those expenses.”
How to lower your tail coverage cost
Physicians typically have 60 days to buy tail coverage after their regular coverage has ended. Specialized brokers such as Mr. Teitelbaum and Ms. Perron help physicians look for the best tails to buy.
The cost of the tail depends on how long you’ve been at your job when you leave it, Ms. Perron says. If you leave in the first 1 or 2 years of the policy, she says, the tail price will be lower because the coverage period is shorter.
Usually the most expensive tail available is from the carrier that issued the original policy. Why is this? “Carriers rarely sell a tail that undercuts their retail price,” Mr. Teitelbaum said. “They don’t want to compete with themselves, and in fact doing so could pose regulatory problems for them.”
Instead of buying from their own carrier, doctors can purchase stand-alone tails from competitors, which Mr. Teitelbaum says are 10%-30% less expensive than the policy the original carrier issues. However, stand-alone tails are not always easy to find, especially for high-cost specialties such as neurosurgery and ob.gyn., he says.
Some physicians try to bring down the cost of the tail by limiting the duration of the tail. You can buy tails that only cover claims filed 1-5 years after the incident took place, rather than indefinitely. These limits mirror the typical statute of limitations – the time limit to file a claim in each state. This limit is as little as 2 years in some states, though it can be as long as 6 years in others.
However, some states make exceptions to the statute of limitations. The 2- to 6-year clock doesn’t start ticking until the mistake is discovered or, in the case of children, when they reach adulthood. “This means that with a limited tail, you always have risk,” Perron said.
And yet some doctors insist on these time-limited tails. “If a doctor opts for 3 years’ coverage, that’s better than no years,” Mr. Teitelbaum said. “But I would advise them to take at least 5 years because that gives you coverage for the basic statute of limitations in most states. Three-year tails do yield savings, but often they’re not enough to warrant the risk.”
Another way to reduce costs is to lower the coverage limits of the tail. The standard coverage limit is $1 million per case and $3 million per year, so doctors might be able to save money on the premium by buying limits of $200,000/$600,000. But Mr. Teitelbaum says most companies would refuse to sell a policy with a limit lower than that of the expiring policy.
Further ways to reduce the cost of the tail include buying tail coverage that doesn’t give the physician the right to approve a settlement or that doesn’t include legal fees in the coverage limits. But these options, too, raise the physician’s risks. Whichever option you choose, the important thing is to protect yourself against costly lawsuits.
This article first appeared on Medscape.com.