User login
News and Views that Matter to Physicians
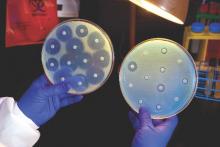
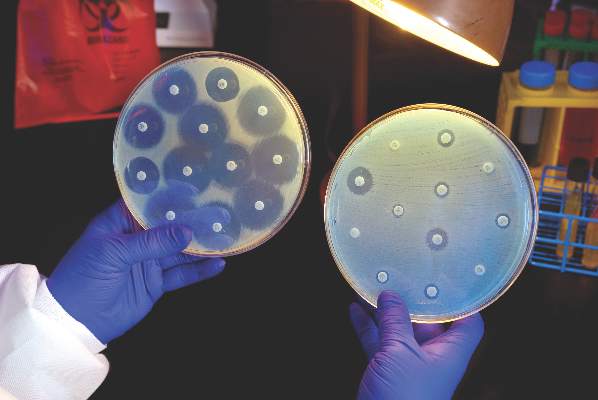
VIDEO: Coronary DES outperform BMS mostly on restenosis
ROME – The difference between contemporary drug-eluting coronary stents and bare-metal stents is not very great, a large Norwegian coronary stent trial showed.
Today’s drug-eluting stents (DES), often called second-generation DES, largely do only what they were designed to do, compared with bare-metal stents (BMS): reduce the rate of stent restenosis and the need for target-lesion revascularization.
“The long-term benefit of contemporary DES over BMS was less that expected,” Kaare H. Bønaa, MD, reported at the annual congress of the European Society of Cardiology.
Results from the Norwegian Coronary Stent Trial (NORSTENT), run with 9,013 patients, showed that patients who received one or more drug-eluting stents had, during nearly 5 years of follow-up, a 5% absolute drop in target-lesion revascularizations (a 53% relative risk reduction), and a 3.3% reduction in all revascularizations (a 24% relative risk reduction), compared with patients who received bare-metal stents, said Dr. Bønaa.
The results also showed that patients who received DES had a 0.4% reduced rate of stent thrombosis (a 36% relative risk reduction), compared with patients treated with BMS during nearly 5 years of follow-up. All three differences were statistically significant.
But the NORSTENT findings also documented that the patients who received either DES or BMS had virtually identical rates of all-cause deaths and nonfatal myocardial infarctions. And, on average, the two different types of coronary stents produced identical improvements in patients’ quality of life, reported Dr. Bønaa, a professor and researcher in the Clinic for Heart Disease at St. Olav’s University Hospital in Trondheim, Norway.
The study’s primary endpoint was the combined rate of death or nonfatal MI, and so the nonsignificant difference in that outcome between the two study arms meant that, formally, the NORSTENT trial produced a neutral result. Concurrently with his report, the results appeared in an article online (New Engl J Med. 2016 Aug 30. doi: 10.1056/NEJMoa1607991).
“The difference between the two stent types is not as great as we thought. Patients who get DES do not live longer or better” than those who receive BMS, Dr. Bønaa said. “We suggest that both contemporary DES and BMS can be recommended for contemporary revascularization. The results open up use of BMS for certain patients,” such as those scheduled for surgery or patients who cannot tolerate or afford the drugs used for dual antiplatelet therapy following coronary stent placement.
But the designated discussant for the study, Stefan James, MD, insisted that recent-generation DES “should remain recommended over BMS,” particularly the specific DES that underwent testing in randomized trials that used hard clinical endpoints. The 2014 revascularization guidelines of the European Society of Cardiology recommend new-generation DES over BMS, he noted.
In addition, “BMS should not be specifically recommended for patients at high risk of stent thrombosis or for patients who do not tolerate dual-antiplatelet therapy,” said Dr. James, professor of cardiology at Uppsala University in Sweden.
NORSTENT ran at eight centers in Norway during 2008-2011, and enrolled patients either had acute coronary syndrome (71% of those in the study) or stable coronary disease. Patients averaged 63 years old. The trial excluded patients with prior stents or bifurcated coronary lesions. Enrolled patients received, on average, 1.7 stents. The specific stent in each class that patients received was left to the discretion of each operator, and 95% of patients in the DES arm received a second-generation device. All patients in both arms of the study received dual-antiplatelet therapy for 9 months.
The finding that DES cut the rate of revascularization procedures by 3.3%, compared with patients treated with BMS, means that, on average, clinicians would need to treat 30 patients with DES to avoid the need for one additional repeat revascularization procedure that would occur if BMS were used instead.
That number needed to treat of 30 to avoid one repeat revascularization may seem high, but the money saved that way would still counterbalance the incremental cost of a DES over a BMS, which today in Europe would be about 50-100 euros, noted one cardiologist.
If you multiply 30 procedures by 100 extra euros per stent and by an average of 1.7 stents per patient, you may spend 5,100 euros, less than the cost of a repeat revascularization procedure, commented Carlo Di Mario, MD, a professor of cardiology and an interventional cardiologist at Royal Brompton & Harefield Hospitals in London.
In a video interview, Steen D. Kristensen, MD, of Aarhus University, Denmark, discussed the NORSTENT findings and their implications.
NORSTENT received no commercial support. Dr. Bønaa and Dr. Di Mario had no disclosures. Dr. James has been a consultant to Boston Scientific and has received research support from Boston Scientific and Abbott Vascular.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
NORSTENT was a very well-performed trial. It produced a neutral result for its primary endpoint, but for the secondary endpoint of repeat revascularization, there were significantly more events using bare-metal stents. This is a major finding, and NORSTENT’s design make the results very generalizable.
It may be slightly surprising that the newer drug-eluting stents did not perform better for the primary endpoint of reducing deaths and MIs during 5 years of follow-up, but seeing a difference in the revascularization rate is not surprising; that is what we would expect. We use DES to reduce the problem of restenosis. Results from several earlier studies that had compared DES with BMS had suggested other benefits from DES, and that is also what the European Society of Cardiology guidelines say.
I will not go home now and start using BMS in my own practice. I will continue to use DES, because they have an advantage. I use BMS in patients who cannot tolerate long-term treatment with dual antiplatelet therapy. The results are encouraging for centers where there is a large price difference between DES and BMS, but that is not the case where I practice in Denmark.
Steen D. Kristensen, MD, is a professor of interventional cardiologist at Aarhus University, Denmark. He made these comments in an interview. He had no relevant disclosures.
NORSTENT was a very well-performed trial. It produced a neutral result for its primary endpoint, but for the secondary endpoint of repeat revascularization, there were significantly more events using bare-metal stents. This is a major finding, and NORSTENT’s design make the results very generalizable.
It may be slightly surprising that the newer drug-eluting stents did not perform better for the primary endpoint of reducing deaths and MIs during 5 years of follow-up, but seeing a difference in the revascularization rate is not surprising; that is what we would expect. We use DES to reduce the problem of restenosis. Results from several earlier studies that had compared DES with BMS had suggested other benefits from DES, and that is also what the European Society of Cardiology guidelines say.
I will not go home now and start using BMS in my own practice. I will continue to use DES, because they have an advantage. I use BMS in patients who cannot tolerate long-term treatment with dual antiplatelet therapy. The results are encouraging for centers where there is a large price difference between DES and BMS, but that is not the case where I practice in Denmark.
Steen D. Kristensen, MD, is a professor of interventional cardiologist at Aarhus University, Denmark. He made these comments in an interview. He had no relevant disclosures.
NORSTENT was a very well-performed trial. It produced a neutral result for its primary endpoint, but for the secondary endpoint of repeat revascularization, there were significantly more events using bare-metal stents. This is a major finding, and NORSTENT’s design make the results very generalizable.
It may be slightly surprising that the newer drug-eluting stents did not perform better for the primary endpoint of reducing deaths and MIs during 5 years of follow-up, but seeing a difference in the revascularization rate is not surprising; that is what we would expect. We use DES to reduce the problem of restenosis. Results from several earlier studies that had compared DES with BMS had suggested other benefits from DES, and that is also what the European Society of Cardiology guidelines say.
I will not go home now and start using BMS in my own practice. I will continue to use DES, because they have an advantage. I use BMS in patients who cannot tolerate long-term treatment with dual antiplatelet therapy. The results are encouraging for centers where there is a large price difference between DES and BMS, but that is not the case where I practice in Denmark.
Steen D. Kristensen, MD, is a professor of interventional cardiologist at Aarhus University, Denmark. He made these comments in an interview. He had no relevant disclosures.
ROME – The difference between contemporary drug-eluting coronary stents and bare-metal stents is not very great, a large Norwegian coronary stent trial showed.
Today’s drug-eluting stents (DES), often called second-generation DES, largely do only what they were designed to do, compared with bare-metal stents (BMS): reduce the rate of stent restenosis and the need for target-lesion revascularization.
“The long-term benefit of contemporary DES over BMS was less that expected,” Kaare H. Bønaa, MD, reported at the annual congress of the European Society of Cardiology.
Results from the Norwegian Coronary Stent Trial (NORSTENT), run with 9,013 patients, showed that patients who received one or more drug-eluting stents had, during nearly 5 years of follow-up, a 5% absolute drop in target-lesion revascularizations (a 53% relative risk reduction), and a 3.3% reduction in all revascularizations (a 24% relative risk reduction), compared with patients who received bare-metal stents, said Dr. Bønaa.
The results also showed that patients who received DES had a 0.4% reduced rate of stent thrombosis (a 36% relative risk reduction), compared with patients treated with BMS during nearly 5 years of follow-up. All three differences were statistically significant.
But the NORSTENT findings also documented that the patients who received either DES or BMS had virtually identical rates of all-cause deaths and nonfatal myocardial infarctions. And, on average, the two different types of coronary stents produced identical improvements in patients’ quality of life, reported Dr. Bønaa, a professor and researcher in the Clinic for Heart Disease at St. Olav’s University Hospital in Trondheim, Norway.
The study’s primary endpoint was the combined rate of death or nonfatal MI, and so the nonsignificant difference in that outcome between the two study arms meant that, formally, the NORSTENT trial produced a neutral result. Concurrently with his report, the results appeared in an article online (New Engl J Med. 2016 Aug 30. doi: 10.1056/NEJMoa1607991).
“The difference between the two stent types is not as great as we thought. Patients who get DES do not live longer or better” than those who receive BMS, Dr. Bønaa said. “We suggest that both contemporary DES and BMS can be recommended for contemporary revascularization. The results open up use of BMS for certain patients,” such as those scheduled for surgery or patients who cannot tolerate or afford the drugs used for dual antiplatelet therapy following coronary stent placement.
But the designated discussant for the study, Stefan James, MD, insisted that recent-generation DES “should remain recommended over BMS,” particularly the specific DES that underwent testing in randomized trials that used hard clinical endpoints. The 2014 revascularization guidelines of the European Society of Cardiology recommend new-generation DES over BMS, he noted.
In addition, “BMS should not be specifically recommended for patients at high risk of stent thrombosis or for patients who do not tolerate dual-antiplatelet therapy,” said Dr. James, professor of cardiology at Uppsala University in Sweden.
NORSTENT ran at eight centers in Norway during 2008-2011, and enrolled patients either had acute coronary syndrome (71% of those in the study) or stable coronary disease. Patients averaged 63 years old. The trial excluded patients with prior stents or bifurcated coronary lesions. Enrolled patients received, on average, 1.7 stents. The specific stent in each class that patients received was left to the discretion of each operator, and 95% of patients in the DES arm received a second-generation device. All patients in both arms of the study received dual-antiplatelet therapy for 9 months.
The finding that DES cut the rate of revascularization procedures by 3.3%, compared with patients treated with BMS, means that, on average, clinicians would need to treat 30 patients with DES to avoid the need for one additional repeat revascularization procedure that would occur if BMS were used instead.
That number needed to treat of 30 to avoid one repeat revascularization may seem high, but the money saved that way would still counterbalance the incremental cost of a DES over a BMS, which today in Europe would be about 50-100 euros, noted one cardiologist.
If you multiply 30 procedures by 100 extra euros per stent and by an average of 1.7 stents per patient, you may spend 5,100 euros, less than the cost of a repeat revascularization procedure, commented Carlo Di Mario, MD, a professor of cardiology and an interventional cardiologist at Royal Brompton & Harefield Hospitals in London.
In a video interview, Steen D. Kristensen, MD, of Aarhus University, Denmark, discussed the NORSTENT findings and their implications.
NORSTENT received no commercial support. Dr. Bønaa and Dr. Di Mario had no disclosures. Dr. James has been a consultant to Boston Scientific and has received research support from Boston Scientific and Abbott Vascular.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
ROME – The difference between contemporary drug-eluting coronary stents and bare-metal stents is not very great, a large Norwegian coronary stent trial showed.
Today’s drug-eluting stents (DES), often called second-generation DES, largely do only what they were designed to do, compared with bare-metal stents (BMS): reduce the rate of stent restenosis and the need for target-lesion revascularization.
“The long-term benefit of contemporary DES over BMS was less that expected,” Kaare H. Bønaa, MD, reported at the annual congress of the European Society of Cardiology.
Results from the Norwegian Coronary Stent Trial (NORSTENT), run with 9,013 patients, showed that patients who received one or more drug-eluting stents had, during nearly 5 years of follow-up, a 5% absolute drop in target-lesion revascularizations (a 53% relative risk reduction), and a 3.3% reduction in all revascularizations (a 24% relative risk reduction), compared with patients who received bare-metal stents, said Dr. Bønaa.
The results also showed that patients who received DES had a 0.4% reduced rate of stent thrombosis (a 36% relative risk reduction), compared with patients treated with BMS during nearly 5 years of follow-up. All three differences were statistically significant.
But the NORSTENT findings also documented that the patients who received either DES or BMS had virtually identical rates of all-cause deaths and nonfatal myocardial infarctions. And, on average, the two different types of coronary stents produced identical improvements in patients’ quality of life, reported Dr. Bønaa, a professor and researcher in the Clinic for Heart Disease at St. Olav’s University Hospital in Trondheim, Norway.
The study’s primary endpoint was the combined rate of death or nonfatal MI, and so the nonsignificant difference in that outcome between the two study arms meant that, formally, the NORSTENT trial produced a neutral result. Concurrently with his report, the results appeared in an article online (New Engl J Med. 2016 Aug 30. doi: 10.1056/NEJMoa1607991).
“The difference between the two stent types is not as great as we thought. Patients who get DES do not live longer or better” than those who receive BMS, Dr. Bønaa said. “We suggest that both contemporary DES and BMS can be recommended for contemporary revascularization. The results open up use of BMS for certain patients,” such as those scheduled for surgery or patients who cannot tolerate or afford the drugs used for dual antiplatelet therapy following coronary stent placement.
But the designated discussant for the study, Stefan James, MD, insisted that recent-generation DES “should remain recommended over BMS,” particularly the specific DES that underwent testing in randomized trials that used hard clinical endpoints. The 2014 revascularization guidelines of the European Society of Cardiology recommend new-generation DES over BMS, he noted.
In addition, “BMS should not be specifically recommended for patients at high risk of stent thrombosis or for patients who do not tolerate dual-antiplatelet therapy,” said Dr. James, professor of cardiology at Uppsala University in Sweden.
NORSTENT ran at eight centers in Norway during 2008-2011, and enrolled patients either had acute coronary syndrome (71% of those in the study) or stable coronary disease. Patients averaged 63 years old. The trial excluded patients with prior stents or bifurcated coronary lesions. Enrolled patients received, on average, 1.7 stents. The specific stent in each class that patients received was left to the discretion of each operator, and 95% of patients in the DES arm received a second-generation device. All patients in both arms of the study received dual-antiplatelet therapy for 9 months.
The finding that DES cut the rate of revascularization procedures by 3.3%, compared with patients treated with BMS, means that, on average, clinicians would need to treat 30 patients with DES to avoid the need for one additional repeat revascularization procedure that would occur if BMS were used instead.
That number needed to treat of 30 to avoid one repeat revascularization may seem high, but the money saved that way would still counterbalance the incremental cost of a DES over a BMS, which today in Europe would be about 50-100 euros, noted one cardiologist.
If you multiply 30 procedures by 100 extra euros per stent and by an average of 1.7 stents per patient, you may spend 5,100 euros, less than the cost of a repeat revascularization procedure, commented Carlo Di Mario, MD, a professor of cardiology and an interventional cardiologist at Royal Brompton & Harefield Hospitals in London.
In a video interview, Steen D. Kristensen, MD, of Aarhus University, Denmark, discussed the NORSTENT findings and their implications.
NORSTENT received no commercial support. Dr. Bønaa and Dr. Di Mario had no disclosures. Dr. James has been a consultant to Boston Scientific and has received research support from Boston Scientific and Abbott Vascular.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
AT THE ESC CONGRESS 2016
Key clinical point: The benefit from coronary revascularization with drug-eluting stents, compared with bare-metal stents, was mostly in a reduced need for repeat revascularization, with no difference in mortality or MIs during 5 years of follow-up.
Major finding: Thirty patients need to be treated with drug-eluting stents to prevent one repeat revascularization, compared with bare-metal stents.
Data source: NORSTENT, a randomized, multicenter trial with 9,013 patients.
Disclosures: NORSTENT received no commercial support. Dr. Bønaa and Dr. Di Mario had no disclosures. Dr. James has been a consultant to Boston Scientific and has received research support from Boston Scientific and Abbott Vascular.
VIDEO: Withdrawing antipsychotics is safe and feasible in long-term care
TORONTO – Antipsychotics can be safely withdrawn from many dementia patients in long-term care facilities, two new studies from Australia and Canada have determined.
When the drugs were withdrawn and supplanted with behavior-centered care in the Australian study, 80% of patients experienced no relapse of symptoms, Henry Brodaty, MD, DSc, said at the Alzheimer’s Association International Conference 2016.
“We saw no significant changes at all in agitation, aggression, delusions, or hallucinations,” Dr. Brodaty, the Scientia Professor of Ageing and Mental Health, University of New South Wales, Australia, said in an interview. “Were we surprised at this? No. Because for the majority of these patients, the medications were inappropriately prescribed.”
The 12-month Australian study is still in the process of tracking outcomes after antipsychotic withdrawal. But the Canadian study found great benefits, said Selma Didic, an improvement analyst with the Canadian Foundation for Healthcare Improvement in Ottawa. “We saw falls decrease by 20%. The incidence of verbal abuse and socially disruptive behavior actually decreased as well.”
In fact, she said, patients who discontinued the medications actually started behaving better than the comparator group that stayed on them.
The Australian experience
Dr. Brodaty discussed the HALT (Halting Antipsychotic Use in Long-Term Care) study. HALT is a single-arm, 12-month longitudinal study carried out in 23 nursing homes in New South Wales.
The study team worked with nursing leadership in each facility to identify patients who might be eligible for the program. In order to enroll, each patient’s family and general physician had to agree to a trial of deprescribing. Physicians were instructed to wean patients off the medication by decreasing the dose by half once a week. Most patients were able to stop within a couple of weeks, Dr. Brodaty said.
Getting buy-in wasn’t always easy, he noted. “Some families didn’t want to rock the boat, and some physicians were resistant,” to the idea. Overall, “Families and nurses were very, very worried” about the prospect of dropping drugs that were seen as helpful in everyday patient management.
But getting rid of the medications was just half the picture. Training nurses and care staff to intervene in problematic behaviors without resorting to drugs was just as important. A nurse-leader at each facility received training in person-centered care, and then trained the rest of the staff. This wasn’t always an easy idea to embrace, either, Dr. Brodaty said, especially since nursing staff often leads the discussion about the need for drugs to manage behavioral problems.
“Nursing staff are very task oriented, focused on dressing, bathing, eating, and toileting. They work very hard, and they don’t always have time to sit down and talk to resistant patients. It takes a much different attitude to show that you can actually save time by spending time and engaging the patient.”
He related one of his favorite illustrative stories – the milkman who caused a ruckus at bath time. “He got upset and aggressive every night when being put to bed and every morning when being given a shower. The staff spoke to his wife about it. She said that for 40 years, he was accustomed to getting up at 4 a.m. to deliver the milk. He would take a bath at night and get on his track suit and go to bed. Then at 4 a.m., he would get up and be ready to jump in the truck and go.”
When the staff started letting him shower at night and go to bed in his track suit, the milkman’s behavior improved without the need for antipsychotic medications.
“This is what we mean by ‘person-centered care,’ ” Dr. Brodaty said. “We use the ABC paradigm: Addressing the antecedent to the behavior, then the behavior, and then the consequences of the behavior.”
The intervention cohort comprised 139 patients with a mean age of 85 years; most were women. The vast majority (93%) had a diagnosis of dementia. About one-third had Alzheimer’s and one-third vascular dementia. The remainder had other diagnoses, including frontotemporal dementia, Lewy body dementia, and Parkinson’s disease. Common comorbid conditions included depression (56%) and previous stroke (36%). None of the patients had a diagnosis of psychosis.
Risperidone was the most common antipsychotic medication (85%). Other medications were olanzapine, quetiapine, and haloperidol. About 30% had come to the facility on the medication; the others had received it since admission.
Despite the national recommendation to review antipsychotic use every 12 weeks, patients had been on their current antipsychotic for an average of 2 years, and on their current dose for 1 year. In reviewing medications, Dr. Brodaty also found a “concerning” lack of informed consent. In Australia, informed consent for antipsychotic drugs can be given by a family member, but 84% of patients had no documented consent at all.
Of the original group, 125 entered the deprescribing protocol. Of these, 26 (21%) have since resumed their medications, but 79% have done well and are without a relapse of their symptoms or problematic behaviors. An ongoing medication review suggests there has been no concomitant upswing in other psychotropic medications, including benzodiazepines.
Neuropsychiatric symptoms remained stable from baseline. The mean total group score on the Neuropsychiatric Index (NPI) has not changed from its baseline of 30. The mean agitation/aggression NPI subscale has remained about 6, and the mean group score on the Cohen-Mansfield Agitation Inventory about 56. The NPI delusion subscale increased, but the change was nonsignificant, Dr. Brodaty said. The NPI hallucinations subscale decreased slightly, but again the change was nonsignificant.
“Look, we all know antipsychotics are bad for old people, and we all know they are overprescribed,” he said. “Inappropriate use of these medications is an old story, yet we’re still talking about it. Why is this? We have the knowledge now, and we have to build on this knowledge so that we can change practice.”
The Canadian experience
Ms. Didic shared a year-long quality improvement process at 24 long-term care facilities that wanted to improve antipsychotic prescribing for their dementia patients.
The program, which was sponsored by the Canadian Foundation for Healthcare Improvement, used a “train-the-trainer” approach to spread support for antipsychotic deprescribing.
The foundation deployed 15 interdisciplinary teams, which comprised 180 members, including physicians, nurses, pharmacists, recreational therapists, and “clinical champions” who took the methodology directly into participating facilities. Interactive webinars on patient-centered care and deprescribing protocols were part of the process, Ms. Didic said.
In all, 416 patients were included in the outcomes report. Within 12 months, antipsychotics were eliminated in 74 patients (18%) and in 148 (36%), the dosage was reduced.
The benefits of these changes were striking, Ms. Didic said. There were fewer falls and reductions in verbal abuse, care resistance, and socially inappropriate behaviors. These issues either remained the same or got worse in patients who did not decrease antipsychotics. Again, there was no concomitant increase in other psychotropic medications.
The results show that changing the focus from medication-first to behavior-first care is institutionally feasible, Ms. Didic said.
Staff members’ assessments of the program and its personal and institutional impact were positive:
• 91% said they instituted regular medication reviews for every resident.
• 92% said old ways of doing things were adjusted to accommodate the new type of care.
• 94% said the new person-centered care was now a standard way of working.
• 84% said the project improved their ability to lead.
• 80% said it improved their ability to communicate.
“Currently, our teams are now spreading and sharing these resources and tools, serving as advisers, and organizing clinical training and workshops,” for other Canadian nursing homes that want to adopt the strategy.
Dr. Richard Caselli, professor of neurology at the Mayo Clinic, Scottsdale, Ariz., commented on the issues surrounding antipsychotic prescribing in long-term care facilities in a video interview.
Neither Ms. Didic nor Dr. Brodaty had any financial declarations.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @alz_gal
TORONTO – Antipsychotics can be safely withdrawn from many dementia patients in long-term care facilities, two new studies from Australia and Canada have determined.
When the drugs were withdrawn and supplanted with behavior-centered care in the Australian study, 80% of patients experienced no relapse of symptoms, Henry Brodaty, MD, DSc, said at the Alzheimer’s Association International Conference 2016.
“We saw no significant changes at all in agitation, aggression, delusions, or hallucinations,” Dr. Brodaty, the Scientia Professor of Ageing and Mental Health, University of New South Wales, Australia, said in an interview. “Were we surprised at this? No. Because for the majority of these patients, the medications were inappropriately prescribed.”
The 12-month Australian study is still in the process of tracking outcomes after antipsychotic withdrawal. But the Canadian study found great benefits, said Selma Didic, an improvement analyst with the Canadian Foundation for Healthcare Improvement in Ottawa. “We saw falls decrease by 20%. The incidence of verbal abuse and socially disruptive behavior actually decreased as well.”
In fact, she said, patients who discontinued the medications actually started behaving better than the comparator group that stayed on them.
The Australian experience
Dr. Brodaty discussed the HALT (Halting Antipsychotic Use in Long-Term Care) study. HALT is a single-arm, 12-month longitudinal study carried out in 23 nursing homes in New South Wales.
The study team worked with nursing leadership in each facility to identify patients who might be eligible for the program. In order to enroll, each patient’s family and general physician had to agree to a trial of deprescribing. Physicians were instructed to wean patients off the medication by decreasing the dose by half once a week. Most patients were able to stop within a couple of weeks, Dr. Brodaty said.
Getting buy-in wasn’t always easy, he noted. “Some families didn’t want to rock the boat, and some physicians were resistant,” to the idea. Overall, “Families and nurses were very, very worried” about the prospect of dropping drugs that were seen as helpful in everyday patient management.
But getting rid of the medications was just half the picture. Training nurses and care staff to intervene in problematic behaviors without resorting to drugs was just as important. A nurse-leader at each facility received training in person-centered care, and then trained the rest of the staff. This wasn’t always an easy idea to embrace, either, Dr. Brodaty said, especially since nursing staff often leads the discussion about the need for drugs to manage behavioral problems.
“Nursing staff are very task oriented, focused on dressing, bathing, eating, and toileting. They work very hard, and they don’t always have time to sit down and talk to resistant patients. It takes a much different attitude to show that you can actually save time by spending time and engaging the patient.”
He related one of his favorite illustrative stories – the milkman who caused a ruckus at bath time. “He got upset and aggressive every night when being put to bed and every morning when being given a shower. The staff spoke to his wife about it. She said that for 40 years, he was accustomed to getting up at 4 a.m. to deliver the milk. He would take a bath at night and get on his track suit and go to bed. Then at 4 a.m., he would get up and be ready to jump in the truck and go.”
When the staff started letting him shower at night and go to bed in his track suit, the milkman’s behavior improved without the need for antipsychotic medications.
“This is what we mean by ‘person-centered care,’ ” Dr. Brodaty said. “We use the ABC paradigm: Addressing the antecedent to the behavior, then the behavior, and then the consequences of the behavior.”
The intervention cohort comprised 139 patients with a mean age of 85 years; most were women. The vast majority (93%) had a diagnosis of dementia. About one-third had Alzheimer’s and one-third vascular dementia. The remainder had other diagnoses, including frontotemporal dementia, Lewy body dementia, and Parkinson’s disease. Common comorbid conditions included depression (56%) and previous stroke (36%). None of the patients had a diagnosis of psychosis.
Risperidone was the most common antipsychotic medication (85%). Other medications were olanzapine, quetiapine, and haloperidol. About 30% had come to the facility on the medication; the others had received it since admission.
Despite the national recommendation to review antipsychotic use every 12 weeks, patients had been on their current antipsychotic for an average of 2 years, and on their current dose for 1 year. In reviewing medications, Dr. Brodaty also found a “concerning” lack of informed consent. In Australia, informed consent for antipsychotic drugs can be given by a family member, but 84% of patients had no documented consent at all.
Of the original group, 125 entered the deprescribing protocol. Of these, 26 (21%) have since resumed their medications, but 79% have done well and are without a relapse of their symptoms or problematic behaviors. An ongoing medication review suggests there has been no concomitant upswing in other psychotropic medications, including benzodiazepines.
Neuropsychiatric symptoms remained stable from baseline. The mean total group score on the Neuropsychiatric Index (NPI) has not changed from its baseline of 30. The mean agitation/aggression NPI subscale has remained about 6, and the mean group score on the Cohen-Mansfield Agitation Inventory about 56. The NPI delusion subscale increased, but the change was nonsignificant, Dr. Brodaty said. The NPI hallucinations subscale decreased slightly, but again the change was nonsignificant.
“Look, we all know antipsychotics are bad for old people, and we all know they are overprescribed,” he said. “Inappropriate use of these medications is an old story, yet we’re still talking about it. Why is this? We have the knowledge now, and we have to build on this knowledge so that we can change practice.”
The Canadian experience
Ms. Didic shared a year-long quality improvement process at 24 long-term care facilities that wanted to improve antipsychotic prescribing for their dementia patients.
The program, which was sponsored by the Canadian Foundation for Healthcare Improvement, used a “train-the-trainer” approach to spread support for antipsychotic deprescribing.
The foundation deployed 15 interdisciplinary teams, which comprised 180 members, including physicians, nurses, pharmacists, recreational therapists, and “clinical champions” who took the methodology directly into participating facilities. Interactive webinars on patient-centered care and deprescribing protocols were part of the process, Ms. Didic said.
In all, 416 patients were included in the outcomes report. Within 12 months, antipsychotics were eliminated in 74 patients (18%) and in 148 (36%), the dosage was reduced.
The benefits of these changes were striking, Ms. Didic said. There were fewer falls and reductions in verbal abuse, care resistance, and socially inappropriate behaviors. These issues either remained the same or got worse in patients who did not decrease antipsychotics. Again, there was no concomitant increase in other psychotropic medications.
The results show that changing the focus from medication-first to behavior-first care is institutionally feasible, Ms. Didic said.
Staff members’ assessments of the program and its personal and institutional impact were positive:
• 91% said they instituted regular medication reviews for every resident.
• 92% said old ways of doing things were adjusted to accommodate the new type of care.
• 94% said the new person-centered care was now a standard way of working.
• 84% said the project improved their ability to lead.
• 80% said it improved their ability to communicate.
“Currently, our teams are now spreading and sharing these resources and tools, serving as advisers, and organizing clinical training and workshops,” for other Canadian nursing homes that want to adopt the strategy.
Dr. Richard Caselli, professor of neurology at the Mayo Clinic, Scottsdale, Ariz., commented on the issues surrounding antipsychotic prescribing in long-term care facilities in a video interview.
Neither Ms. Didic nor Dr. Brodaty had any financial declarations.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @alz_gal
TORONTO – Antipsychotics can be safely withdrawn from many dementia patients in long-term care facilities, two new studies from Australia and Canada have determined.
When the drugs were withdrawn and supplanted with behavior-centered care in the Australian study, 80% of patients experienced no relapse of symptoms, Henry Brodaty, MD, DSc, said at the Alzheimer’s Association International Conference 2016.
“We saw no significant changes at all in agitation, aggression, delusions, or hallucinations,” Dr. Brodaty, the Scientia Professor of Ageing and Mental Health, University of New South Wales, Australia, said in an interview. “Were we surprised at this? No. Because for the majority of these patients, the medications were inappropriately prescribed.”
The 12-month Australian study is still in the process of tracking outcomes after antipsychotic withdrawal. But the Canadian study found great benefits, said Selma Didic, an improvement analyst with the Canadian Foundation for Healthcare Improvement in Ottawa. “We saw falls decrease by 20%. The incidence of verbal abuse and socially disruptive behavior actually decreased as well.”
In fact, she said, patients who discontinued the medications actually started behaving better than the comparator group that stayed on them.
The Australian experience
Dr. Brodaty discussed the HALT (Halting Antipsychotic Use in Long-Term Care) study. HALT is a single-arm, 12-month longitudinal study carried out in 23 nursing homes in New South Wales.
The study team worked with nursing leadership in each facility to identify patients who might be eligible for the program. In order to enroll, each patient’s family and general physician had to agree to a trial of deprescribing. Physicians were instructed to wean patients off the medication by decreasing the dose by half once a week. Most patients were able to stop within a couple of weeks, Dr. Brodaty said.
Getting buy-in wasn’t always easy, he noted. “Some families didn’t want to rock the boat, and some physicians were resistant,” to the idea. Overall, “Families and nurses were very, very worried” about the prospect of dropping drugs that were seen as helpful in everyday patient management.
But getting rid of the medications was just half the picture. Training nurses and care staff to intervene in problematic behaviors without resorting to drugs was just as important. A nurse-leader at each facility received training in person-centered care, and then trained the rest of the staff. This wasn’t always an easy idea to embrace, either, Dr. Brodaty said, especially since nursing staff often leads the discussion about the need for drugs to manage behavioral problems.
“Nursing staff are very task oriented, focused on dressing, bathing, eating, and toileting. They work very hard, and they don’t always have time to sit down and talk to resistant patients. It takes a much different attitude to show that you can actually save time by spending time and engaging the patient.”
He related one of his favorite illustrative stories – the milkman who caused a ruckus at bath time. “He got upset and aggressive every night when being put to bed and every morning when being given a shower. The staff spoke to his wife about it. She said that for 40 years, he was accustomed to getting up at 4 a.m. to deliver the milk. He would take a bath at night and get on his track suit and go to bed. Then at 4 a.m., he would get up and be ready to jump in the truck and go.”
When the staff started letting him shower at night and go to bed in his track suit, the milkman’s behavior improved without the need for antipsychotic medications.
“This is what we mean by ‘person-centered care,’ ” Dr. Brodaty said. “We use the ABC paradigm: Addressing the antecedent to the behavior, then the behavior, and then the consequences of the behavior.”
The intervention cohort comprised 139 patients with a mean age of 85 years; most were women. The vast majority (93%) had a diagnosis of dementia. About one-third had Alzheimer’s and one-third vascular dementia. The remainder had other diagnoses, including frontotemporal dementia, Lewy body dementia, and Parkinson’s disease. Common comorbid conditions included depression (56%) and previous stroke (36%). None of the patients had a diagnosis of psychosis.
Risperidone was the most common antipsychotic medication (85%). Other medications were olanzapine, quetiapine, and haloperidol. About 30% had come to the facility on the medication; the others had received it since admission.
Despite the national recommendation to review antipsychotic use every 12 weeks, patients had been on their current antipsychotic for an average of 2 years, and on their current dose for 1 year. In reviewing medications, Dr. Brodaty also found a “concerning” lack of informed consent. In Australia, informed consent for antipsychotic drugs can be given by a family member, but 84% of patients had no documented consent at all.
Of the original group, 125 entered the deprescribing protocol. Of these, 26 (21%) have since resumed their medications, but 79% have done well and are without a relapse of their symptoms or problematic behaviors. An ongoing medication review suggests there has been no concomitant upswing in other psychotropic medications, including benzodiazepines.
Neuropsychiatric symptoms remained stable from baseline. The mean total group score on the Neuropsychiatric Index (NPI) has not changed from its baseline of 30. The mean agitation/aggression NPI subscale has remained about 6, and the mean group score on the Cohen-Mansfield Agitation Inventory about 56. The NPI delusion subscale increased, but the change was nonsignificant, Dr. Brodaty said. The NPI hallucinations subscale decreased slightly, but again the change was nonsignificant.
“Look, we all know antipsychotics are bad for old people, and we all know they are overprescribed,” he said. “Inappropriate use of these medications is an old story, yet we’re still talking about it. Why is this? We have the knowledge now, and we have to build on this knowledge so that we can change practice.”
The Canadian experience
Ms. Didic shared a year-long quality improvement process at 24 long-term care facilities that wanted to improve antipsychotic prescribing for their dementia patients.
The program, which was sponsored by the Canadian Foundation for Healthcare Improvement, used a “train-the-trainer” approach to spread support for antipsychotic deprescribing.
The foundation deployed 15 interdisciplinary teams, which comprised 180 members, including physicians, nurses, pharmacists, recreational therapists, and “clinical champions” who took the methodology directly into participating facilities. Interactive webinars on patient-centered care and deprescribing protocols were part of the process, Ms. Didic said.
In all, 416 patients were included in the outcomes report. Within 12 months, antipsychotics were eliminated in 74 patients (18%) and in 148 (36%), the dosage was reduced.
The benefits of these changes were striking, Ms. Didic said. There were fewer falls and reductions in verbal abuse, care resistance, and socially inappropriate behaviors. These issues either remained the same or got worse in patients who did not decrease antipsychotics. Again, there was no concomitant increase in other psychotropic medications.
The results show that changing the focus from medication-first to behavior-first care is institutionally feasible, Ms. Didic said.
Staff members’ assessments of the program and its personal and institutional impact were positive:
• 91% said they instituted regular medication reviews for every resident.
• 92% said old ways of doing things were adjusted to accommodate the new type of care.
• 94% said the new person-centered care was now a standard way of working.
• 84% said the project improved their ability to lead.
• 80% said it improved their ability to communicate.
“Currently, our teams are now spreading and sharing these resources and tools, serving as advisers, and organizing clinical training and workshops,” for other Canadian nursing homes that want to adopt the strategy.
Dr. Richard Caselli, professor of neurology at the Mayo Clinic, Scottsdale, Ariz., commented on the issues surrounding antipsychotic prescribing in long-term care facilities in a video interview.
Neither Ms. Didic nor Dr. Brodaty had any financial declarations.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @alz_gal
EXPERT ANALYSIS FROM AAIC 2016
Guillain-Barré incidence rose with Zika across Americas
Increased incidence of Guillain-Barré syndrome corresponded closely with patterns of Zika virus disease incidence in Central and South America from April 2015 through March 2016, according to results from a new temporal and graphic analysis.
The findings show Guillain-Barré syndrome (GBS) cases increasing from 100% to nearly 900% above previously recorded baseline rates during periods of Zika virus transmission in El Salvador, the Dominican Republic, Colombia, Honduras, Suriname, Venezuela, and the Brazilian state of Bahia (N Engl J Med. 2016 Aug 31. doi: 10.1056/NEJMc1609015).
The analysis of the yearlong period also revealed that declines in GBS incidence accompanied declines in Zika virus disease when and where transmission began to wane. The researchers, led by Marcos A. Espinal, MD, DrPH, of the Pan American Health Organization in Washington, did not find significant associations between co-circulation of dengue virus and GBS incidence. The study, which looked at 164,237 confirmed and suspected cases of Zika virus disease and 1,474 cases of GBS, found a 75% higher Zika virus disease incidence rate in women, which Dr. Espinal and colleagues said might be attributable to differences in health care–seeking behavior. GBS incidence, meanwhile, was 28% higher among males. The higher rate of GBS in men, the authors said, was consistent with findings from previous epidemiological studies of GBS.
While the new results did not show that Zika virus causes GBS, Dr. Espinal and colleagues wrote, they argued that they were indicative of a strong association, adding that GBS “could serve as a sentinel for Zika virus disease and other neurological disorders linked to Zika virus,” including microcephaly.
Most of the study authors worked for the Pan American Health Organization or for national health agencies in the data-contributing countries. None declared conflicts of interest.
Increased incidence of Guillain-Barré syndrome corresponded closely with patterns of Zika virus disease incidence in Central and South America from April 2015 through March 2016, according to results from a new temporal and graphic analysis.
The findings show Guillain-Barré syndrome (GBS) cases increasing from 100% to nearly 900% above previously recorded baseline rates during periods of Zika virus transmission in El Salvador, the Dominican Republic, Colombia, Honduras, Suriname, Venezuela, and the Brazilian state of Bahia (N Engl J Med. 2016 Aug 31. doi: 10.1056/NEJMc1609015).
The analysis of the yearlong period also revealed that declines in GBS incidence accompanied declines in Zika virus disease when and where transmission began to wane. The researchers, led by Marcos A. Espinal, MD, DrPH, of the Pan American Health Organization in Washington, did not find significant associations between co-circulation of dengue virus and GBS incidence. The study, which looked at 164,237 confirmed and suspected cases of Zika virus disease and 1,474 cases of GBS, found a 75% higher Zika virus disease incidence rate in women, which Dr. Espinal and colleagues said might be attributable to differences in health care–seeking behavior. GBS incidence, meanwhile, was 28% higher among males. The higher rate of GBS in men, the authors said, was consistent with findings from previous epidemiological studies of GBS.
While the new results did not show that Zika virus causes GBS, Dr. Espinal and colleagues wrote, they argued that they were indicative of a strong association, adding that GBS “could serve as a sentinel for Zika virus disease and other neurological disorders linked to Zika virus,” including microcephaly.
Most of the study authors worked for the Pan American Health Organization or for national health agencies in the data-contributing countries. None declared conflicts of interest.
Increased incidence of Guillain-Barré syndrome corresponded closely with patterns of Zika virus disease incidence in Central and South America from April 2015 through March 2016, according to results from a new temporal and graphic analysis.
The findings show Guillain-Barré syndrome (GBS) cases increasing from 100% to nearly 900% above previously recorded baseline rates during periods of Zika virus transmission in El Salvador, the Dominican Republic, Colombia, Honduras, Suriname, Venezuela, and the Brazilian state of Bahia (N Engl J Med. 2016 Aug 31. doi: 10.1056/NEJMc1609015).
The analysis of the yearlong period also revealed that declines in GBS incidence accompanied declines in Zika virus disease when and where transmission began to wane. The researchers, led by Marcos A. Espinal, MD, DrPH, of the Pan American Health Organization in Washington, did not find significant associations between co-circulation of dengue virus and GBS incidence. The study, which looked at 164,237 confirmed and suspected cases of Zika virus disease and 1,474 cases of GBS, found a 75% higher Zika virus disease incidence rate in women, which Dr. Espinal and colleagues said might be attributable to differences in health care–seeking behavior. GBS incidence, meanwhile, was 28% higher among males. The higher rate of GBS in men, the authors said, was consistent with findings from previous epidemiological studies of GBS.
While the new results did not show that Zika virus causes GBS, Dr. Espinal and colleagues wrote, they argued that they were indicative of a strong association, adding that GBS “could serve as a sentinel for Zika virus disease and other neurological disorders linked to Zika virus,” including microcephaly.
Most of the study authors worked for the Pan American Health Organization or for national health agencies in the data-contributing countries. None declared conflicts of interest.
FROM NEW ENGLAND JOURNAL OF MEDICINE
FDA: New labeling warns against combining opioids, benzodiazepines
Labeling for prescription opioid pain or cough medicines and benzodiazepines will now carry the strongest available warning regarding serious side effects and death associated with their combined use, according to the Food and Drug Administration.
The new boxed warnings urge health care professionals to limit prescribing opioid pain medicines with benzodiazepines or other central nervous system depressants only to patients for whom alternative treatment options are inadequate, and to limit dosages and treatment duration to the minimum possible while achieving the desired clinical effect.
“First, the FDA is requiring companies to update their product labeling for ... benzodiazepines and opioids to include possible harms when they are used together. Second, we are requiring new or updated medication guides for these drugs reflecting those same warnings,” said Doug Throckmorton, MD, deputy director of the FDA’s Center for Drug Evaluation and Research, during a telebriefing.
Opioids will include a warning regarding prescribing with benzodiazepines and other central nervous system depressants, including alcohol. Benzodiazepines will include a warning regarding prescribing with opioids.
In addition, the FDA has issued a safety communication to “warn the public about the serious risk of taking these products together to help make doctors more cautious and patients better informed,” Dr. Throckmorton said.
The action comes amid ongoing efforts to address an epidemic of opioid addiction across the United States, and in response to a first-of-its-kind “citizen petition” calling for the boxed warnings.
A coalition of health officials from multiple cities, states, and U.S. territories initiated that petition in February, and thousands of concerned community members started an additional online petition. Those petitions were in response to both the increasing combined use of opioids and benzodiazepines and a concomitant increase in the risk of serious side effects and deaths associated with their combined use, according to Baltimore City Health Commissioner Leana Wen, MD.
As an emergency physician, Dr. Wen said that she has seen firsthand the alarming trends; one in three unintentional overdose deaths from prescribed opioids also involve benzodiazepines, she noted.
“In my state of Maryland in 2014, benzodiazepines were associated with 19% of prescription opioid deaths, and 59% of benzodiazepine-associated deaths involved prescription opioids. We also noted the growing biological evidence that combining these medications caused sleepiness and slowed breathing, increasing the likelihood of a fatal overdose,” she said.
Dr. Throckmorton further noted that emergency department visits and deaths involving patients prescribed both opioids and benzodiazepines have increased significantly over time. From 2004 to 2011, the rate of nonmedical use–related emergency department visits increased significantly each year, and overdose deaths involving both drug classes during that period nearly tripled on an annual basis.
“Communities have been seeing this trend for some time, but ultimately we needed data in order to act today,” FDA Commissioner Robert Califf, MD, said during the telebriefing.
The current action is just “one part of a larger effort to address this epidemic.
“We remain focused and deeply committed to contributing to the comprehensive effort to address the opioid epidemic,” Dr. Califf said. The FDA “will continue to monitor these products carefully and take additional actions as needed, and will share updates with the public as necessary as we work to address this public health crisis.”
Dr. Califf noted that the current action is part of the FDA’s Opioids Action Plan, which is “importantly not meant just to cover illicit or abusive use of opioids.”
“So, you’ll be hearing a lot more from us, because this is a national crisis that is not going away. We’re making progress on the prescribing, and we’re seeing a reduction in the use of opioids now,” he noted. “But we’re still seeing many overdoses.
“This is a continuum, and we’ll continue to try to do everything we can to address the epidemic,” Dr. Califf concluded.
Labeling for prescription opioid pain or cough medicines and benzodiazepines will now carry the strongest available warning regarding serious side effects and death associated with their combined use, according to the Food and Drug Administration.
The new boxed warnings urge health care professionals to limit prescribing opioid pain medicines with benzodiazepines or other central nervous system depressants only to patients for whom alternative treatment options are inadequate, and to limit dosages and treatment duration to the minimum possible while achieving the desired clinical effect.
“First, the FDA is requiring companies to update their product labeling for ... benzodiazepines and opioids to include possible harms when they are used together. Second, we are requiring new or updated medication guides for these drugs reflecting those same warnings,” said Doug Throckmorton, MD, deputy director of the FDA’s Center for Drug Evaluation and Research, during a telebriefing.
Opioids will include a warning regarding prescribing with benzodiazepines and other central nervous system depressants, including alcohol. Benzodiazepines will include a warning regarding prescribing with opioids.
In addition, the FDA has issued a safety communication to “warn the public about the serious risk of taking these products together to help make doctors more cautious and patients better informed,” Dr. Throckmorton said.
The action comes amid ongoing efforts to address an epidemic of opioid addiction across the United States, and in response to a first-of-its-kind “citizen petition” calling for the boxed warnings.
A coalition of health officials from multiple cities, states, and U.S. territories initiated that petition in February, and thousands of concerned community members started an additional online petition. Those petitions were in response to both the increasing combined use of opioids and benzodiazepines and a concomitant increase in the risk of serious side effects and deaths associated with their combined use, according to Baltimore City Health Commissioner Leana Wen, MD.
As an emergency physician, Dr. Wen said that she has seen firsthand the alarming trends; one in three unintentional overdose deaths from prescribed opioids also involve benzodiazepines, she noted.
“In my state of Maryland in 2014, benzodiazepines were associated with 19% of prescription opioid deaths, and 59% of benzodiazepine-associated deaths involved prescription opioids. We also noted the growing biological evidence that combining these medications caused sleepiness and slowed breathing, increasing the likelihood of a fatal overdose,” she said.
Dr. Throckmorton further noted that emergency department visits and deaths involving patients prescribed both opioids and benzodiazepines have increased significantly over time. From 2004 to 2011, the rate of nonmedical use–related emergency department visits increased significantly each year, and overdose deaths involving both drug classes during that period nearly tripled on an annual basis.
“Communities have been seeing this trend for some time, but ultimately we needed data in order to act today,” FDA Commissioner Robert Califf, MD, said during the telebriefing.
The current action is just “one part of a larger effort to address this epidemic.
“We remain focused and deeply committed to contributing to the comprehensive effort to address the opioid epidemic,” Dr. Califf said. The FDA “will continue to monitor these products carefully and take additional actions as needed, and will share updates with the public as necessary as we work to address this public health crisis.”
Dr. Califf noted that the current action is part of the FDA’s Opioids Action Plan, which is “importantly not meant just to cover illicit or abusive use of opioids.”
“So, you’ll be hearing a lot more from us, because this is a national crisis that is not going away. We’re making progress on the prescribing, and we’re seeing a reduction in the use of opioids now,” he noted. “But we’re still seeing many overdoses.
“This is a continuum, and we’ll continue to try to do everything we can to address the epidemic,” Dr. Califf concluded.
Labeling for prescription opioid pain or cough medicines and benzodiazepines will now carry the strongest available warning regarding serious side effects and death associated with their combined use, according to the Food and Drug Administration.
The new boxed warnings urge health care professionals to limit prescribing opioid pain medicines with benzodiazepines or other central nervous system depressants only to patients for whom alternative treatment options are inadequate, and to limit dosages and treatment duration to the minimum possible while achieving the desired clinical effect.
“First, the FDA is requiring companies to update their product labeling for ... benzodiazepines and opioids to include possible harms when they are used together. Second, we are requiring new or updated medication guides for these drugs reflecting those same warnings,” said Doug Throckmorton, MD, deputy director of the FDA’s Center for Drug Evaluation and Research, during a telebriefing.
Opioids will include a warning regarding prescribing with benzodiazepines and other central nervous system depressants, including alcohol. Benzodiazepines will include a warning regarding prescribing with opioids.
In addition, the FDA has issued a safety communication to “warn the public about the serious risk of taking these products together to help make doctors more cautious and patients better informed,” Dr. Throckmorton said.
The action comes amid ongoing efforts to address an epidemic of opioid addiction across the United States, and in response to a first-of-its-kind “citizen petition” calling for the boxed warnings.
A coalition of health officials from multiple cities, states, and U.S. territories initiated that petition in February, and thousands of concerned community members started an additional online petition. Those petitions were in response to both the increasing combined use of opioids and benzodiazepines and a concomitant increase in the risk of serious side effects and deaths associated with their combined use, according to Baltimore City Health Commissioner Leana Wen, MD.
As an emergency physician, Dr. Wen said that she has seen firsthand the alarming trends; one in three unintentional overdose deaths from prescribed opioids also involve benzodiazepines, she noted.
“In my state of Maryland in 2014, benzodiazepines were associated with 19% of prescription opioid deaths, and 59% of benzodiazepine-associated deaths involved prescription opioids. We also noted the growing biological evidence that combining these medications caused sleepiness and slowed breathing, increasing the likelihood of a fatal overdose,” she said.
Dr. Throckmorton further noted that emergency department visits and deaths involving patients prescribed both opioids and benzodiazepines have increased significantly over time. From 2004 to 2011, the rate of nonmedical use–related emergency department visits increased significantly each year, and overdose deaths involving both drug classes during that period nearly tripled on an annual basis.
“Communities have been seeing this trend for some time, but ultimately we needed data in order to act today,” FDA Commissioner Robert Califf, MD, said during the telebriefing.
The current action is just “one part of a larger effort to address this epidemic.
“We remain focused and deeply committed to contributing to the comprehensive effort to address the opioid epidemic,” Dr. Califf said. The FDA “will continue to monitor these products carefully and take additional actions as needed, and will share updates with the public as necessary as we work to address this public health crisis.”
Dr. Califf noted that the current action is part of the FDA’s Opioids Action Plan, which is “importantly not meant just to cover illicit or abusive use of opioids.”
“So, you’ll be hearing a lot more from us, because this is a national crisis that is not going away. We’re making progress on the prescribing, and we’re seeing a reduction in the use of opioids now,” he noted. “But we’re still seeing many overdoses.
“This is a continuum, and we’ll continue to try to do everything we can to address the epidemic,” Dr. Califf concluded.
Adding salmeterol to steroids didn’t boost kids’ serious asthma events
Adding the long-acting beta-agonist salmeterol to fluticasone in a fixed-dose combination didn’t increase serious asthma-related events among children aged 4-11 years, according to a report published online Sept. 1 in the New England Journal of Medicine.
After long-acting beta-agonists were introduced as add-on therapy for uncontrolled asthma, two large studies involving adults linked the treatment to an increase in asthma-related death. Other studies found no such association.
The FDA mandated that all four manufacturers of those agents in the United States perform large postmarketing safety trials to establish the noninferiority of the approach. In response, GlaxoSmithKline, the only maker of a long-acting beta-agonist with a pediatric indication (salmeterol), performed this international randomized, double-blind, controlled trial at 567 medical centers in 32 countries, said David A. Stempel, MD, of Respiratory Clinical Development, GSK, Research Triangle Park, N.C., and his associates.
The trial involved 6,208 children aged 4-11 years who had controlled or uncontrolled asthma with a history of exacerbations during the preceding year. The participants were randomly assigned to receive 26 weeks of a lower fixed-dose combination of salmeterol plus fluticasone, a higher fixed-dose combination, a lower dose of fluticasone alone, or a higher dose of fluticasone alone, delivered twice daily via a disk device.
The primary safety endpoint was a composite of death, endotracheal intubation, and hospitalization. No deaths or intubations occurred.
A total of 27 patients taking combined therapy and 21 taking fluticasone alone required hospitalization for asthma (hazard ratio, 1.28). The number of severe asthma exacerbations was 14% lower when salmeterol was added to fluticasone, a nonsignificant difference.
The results demonstrate the noninferiority of the combined therapy, Dr. Stempel and his associates said (N Engl J Med. 2016 Sep 1;375[9]:840-9).
The percentage of children who withdrew from the study because of asthma exacerbations was identical in the two groups (1.1% of each), and the percentage who had a serious adverse event was nearly identical (1.8% vs 1.7%, respectively). The mean percentage of rescue therapy–free days also was similar (83.0% vs 81.9%), as was the mean percentage of days in which asthma was controlled (74.8% vs. 73.4%).
At the conclusion of the study, 88.1% of the fluticasone-plus-salmeterol group had controlled asthma, as did 88.5% of the fluticasone-only group. Meaningful differences between the two treatments could not be identified among various subgroups of patients – defined by age, sex, and race – because the overall number of adverse events was so low, the investigators added.
They cautioned that the trial excluded children who had a history of multiple asthma-related hospitalizations and intubations. Therefore, the findings may not be applicable to patients with very severe asthma, the researchers cautioned.
GlaxoSmithKline sponsored the trial in response to a Food and Drug Administration mandate for large postmarketing safety studies from the marketers of long-acting beta agonist–containing products sold in the United States. Dr. Stempel is an employee of GSK; his associates reported ties to numerous industry sources.
These study findings provide reassuring evidence that combination inhalers are safe for the unusual child with asthma who needs more than inhaled glucocorticoids to control the disease or who has persistent, objectively documented variable airflow obstruction.
 |
Dr. Andrew Bush |
But it’s important to emphasize that a combined inhaler is never indicated as first-line preventive therapy in children, because such use is increasingly creeping into practice. And monotherapy with a long-acting beta-agonist in a child should be considered medical negligence.
Andrew Bush, MD, is in the department of respiratory medicine at Royal Brompton Hospital, London. Urs Frey, MD, PhD, is in the department of pediatrics at the University of Basel (Switzerland) Children’s Hospital. They reported having no relevant financial disclosures. Dr. Bush and Dr. Frey made these remarks in an editorial accompanying Dr. Stempel’s report (N Engl J Med. 2016 Sep 1;375[9]:889-91).
These study findings provide reassuring evidence that combination inhalers are safe for the unusual child with asthma who needs more than inhaled glucocorticoids to control the disease or who has persistent, objectively documented variable airflow obstruction.
 |
Dr. Andrew Bush |
But it’s important to emphasize that a combined inhaler is never indicated as first-line preventive therapy in children, because such use is increasingly creeping into practice. And monotherapy with a long-acting beta-agonist in a child should be considered medical negligence.
Andrew Bush, MD, is in the department of respiratory medicine at Royal Brompton Hospital, London. Urs Frey, MD, PhD, is in the department of pediatrics at the University of Basel (Switzerland) Children’s Hospital. They reported having no relevant financial disclosures. Dr. Bush and Dr. Frey made these remarks in an editorial accompanying Dr. Stempel’s report (N Engl J Med. 2016 Sep 1;375[9]:889-91).
These study findings provide reassuring evidence that combination inhalers are safe for the unusual child with asthma who needs more than inhaled glucocorticoids to control the disease or who has persistent, objectively documented variable airflow obstruction.
 |
Dr. Andrew Bush |
But it’s important to emphasize that a combined inhaler is never indicated as first-line preventive therapy in children, because such use is increasingly creeping into practice. And monotherapy with a long-acting beta-agonist in a child should be considered medical negligence.
Andrew Bush, MD, is in the department of respiratory medicine at Royal Brompton Hospital, London. Urs Frey, MD, PhD, is in the department of pediatrics at the University of Basel (Switzerland) Children’s Hospital. They reported having no relevant financial disclosures. Dr. Bush and Dr. Frey made these remarks in an editorial accompanying Dr. Stempel’s report (N Engl J Med. 2016 Sep 1;375[9]:889-91).
Adding the long-acting beta-agonist salmeterol to fluticasone in a fixed-dose combination didn’t increase serious asthma-related events among children aged 4-11 years, according to a report published online Sept. 1 in the New England Journal of Medicine.
After long-acting beta-agonists were introduced as add-on therapy for uncontrolled asthma, two large studies involving adults linked the treatment to an increase in asthma-related death. Other studies found no such association.
The FDA mandated that all four manufacturers of those agents in the United States perform large postmarketing safety trials to establish the noninferiority of the approach. In response, GlaxoSmithKline, the only maker of a long-acting beta-agonist with a pediatric indication (salmeterol), performed this international randomized, double-blind, controlled trial at 567 medical centers in 32 countries, said David A. Stempel, MD, of Respiratory Clinical Development, GSK, Research Triangle Park, N.C., and his associates.
The trial involved 6,208 children aged 4-11 years who had controlled or uncontrolled asthma with a history of exacerbations during the preceding year. The participants were randomly assigned to receive 26 weeks of a lower fixed-dose combination of salmeterol plus fluticasone, a higher fixed-dose combination, a lower dose of fluticasone alone, or a higher dose of fluticasone alone, delivered twice daily via a disk device.
The primary safety endpoint was a composite of death, endotracheal intubation, and hospitalization. No deaths or intubations occurred.
A total of 27 patients taking combined therapy and 21 taking fluticasone alone required hospitalization for asthma (hazard ratio, 1.28). The number of severe asthma exacerbations was 14% lower when salmeterol was added to fluticasone, a nonsignificant difference.
The results demonstrate the noninferiority of the combined therapy, Dr. Stempel and his associates said (N Engl J Med. 2016 Sep 1;375[9]:840-9).
The percentage of children who withdrew from the study because of asthma exacerbations was identical in the two groups (1.1% of each), and the percentage who had a serious adverse event was nearly identical (1.8% vs 1.7%, respectively). The mean percentage of rescue therapy–free days also was similar (83.0% vs 81.9%), as was the mean percentage of days in which asthma was controlled (74.8% vs. 73.4%).
At the conclusion of the study, 88.1% of the fluticasone-plus-salmeterol group had controlled asthma, as did 88.5% of the fluticasone-only group. Meaningful differences between the two treatments could not be identified among various subgroups of patients – defined by age, sex, and race – because the overall number of adverse events was so low, the investigators added.
They cautioned that the trial excluded children who had a history of multiple asthma-related hospitalizations and intubations. Therefore, the findings may not be applicable to patients with very severe asthma, the researchers cautioned.
GlaxoSmithKline sponsored the trial in response to a Food and Drug Administration mandate for large postmarketing safety studies from the marketers of long-acting beta agonist–containing products sold in the United States. Dr. Stempel is an employee of GSK; his associates reported ties to numerous industry sources.
Adding the long-acting beta-agonist salmeterol to fluticasone in a fixed-dose combination didn’t increase serious asthma-related events among children aged 4-11 years, according to a report published online Sept. 1 in the New England Journal of Medicine.
After long-acting beta-agonists were introduced as add-on therapy for uncontrolled asthma, two large studies involving adults linked the treatment to an increase in asthma-related death. Other studies found no such association.
The FDA mandated that all four manufacturers of those agents in the United States perform large postmarketing safety trials to establish the noninferiority of the approach. In response, GlaxoSmithKline, the only maker of a long-acting beta-agonist with a pediatric indication (salmeterol), performed this international randomized, double-blind, controlled trial at 567 medical centers in 32 countries, said David A. Stempel, MD, of Respiratory Clinical Development, GSK, Research Triangle Park, N.C., and his associates.
The trial involved 6,208 children aged 4-11 years who had controlled or uncontrolled asthma with a history of exacerbations during the preceding year. The participants were randomly assigned to receive 26 weeks of a lower fixed-dose combination of salmeterol plus fluticasone, a higher fixed-dose combination, a lower dose of fluticasone alone, or a higher dose of fluticasone alone, delivered twice daily via a disk device.
The primary safety endpoint was a composite of death, endotracheal intubation, and hospitalization. No deaths or intubations occurred.
A total of 27 patients taking combined therapy and 21 taking fluticasone alone required hospitalization for asthma (hazard ratio, 1.28). The number of severe asthma exacerbations was 14% lower when salmeterol was added to fluticasone, a nonsignificant difference.
The results demonstrate the noninferiority of the combined therapy, Dr. Stempel and his associates said (N Engl J Med. 2016 Sep 1;375[9]:840-9).
The percentage of children who withdrew from the study because of asthma exacerbations was identical in the two groups (1.1% of each), and the percentage who had a serious adverse event was nearly identical (1.8% vs 1.7%, respectively). The mean percentage of rescue therapy–free days also was similar (83.0% vs 81.9%), as was the mean percentage of days in which asthma was controlled (74.8% vs. 73.4%).
At the conclusion of the study, 88.1% of the fluticasone-plus-salmeterol group had controlled asthma, as did 88.5% of the fluticasone-only group. Meaningful differences between the two treatments could not be identified among various subgroups of patients – defined by age, sex, and race – because the overall number of adverse events was so low, the investigators added.
They cautioned that the trial excluded children who had a history of multiple asthma-related hospitalizations and intubations. Therefore, the findings may not be applicable to patients with very severe asthma, the researchers cautioned.
GlaxoSmithKline sponsored the trial in response to a Food and Drug Administration mandate for large postmarketing safety studies from the marketers of long-acting beta agonist–containing products sold in the United States. Dr. Stempel is an employee of GSK; his associates reported ties to numerous industry sources.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Adding salmeterol to fluticasone therapy didn’t increase serious asthma-related events among children.
Major finding: 27 patients taking combined therapy and 21 taking fluticasone alone required hospitalization for asthma (HR, 1.28).
Data source: A 26-week international randomized, double-blind trial involving 6,208 patients aged 4-11 years.
Disclosures: GlaxoSmithKline sponsored the trial in response to a Food and Drug Administration mandate for large postmarketing safety studies from the marketers of long-acting beta agonist–containing products sold in the United States. Dr. Stempel is an employee of GSK; his associates reported ties to numerous industry sources.
Hospitals increase CRE risk when they share patients
The more hospitals share patients, the more likely they are to have a problem with carbapenem-resistant Enterobacteriaceae (CRE), especially if long-term acute care hospitals (LTACHs) are in the mix, according to a state-wide investigation from Illinois.
Greater hospital centrality was independently associated with higher rates overall, and sharing four or more patients with a long-term acute care hospital (LTACH) in the 3-month study window doubled the rate of CRE cases.
Although it’s possible that was because of chance (P = 0.11), the link between LTACHs and CRE “is consistent with prior analyses that have shown the central role LTACHs have in” spreading the organism, said the researchers, led by Michael Ray of the Illinois Department of Public Health (Clin Infect Dis. 2016 Aug 2. pii: ciw461).
Patients often spend weeks in LTACH facilities for ongoing, serious health problems. The severity of illness, long stay, and sometimes chronic antibiotic use increase the risk of CRE exposure, and the team found that many LTACH patients are colonized.
“These findings have immediate public health implications. … Early interventions should be focused on the most connected facilities, as well as those with strong connections to LTACHs.” When one hospital has an outbreak, facilities that share its patients need to swing into action screening new admissions and taking other steps to prevent regional spread, the team said.
Meanwhile, “state-wide patient-sharing data, which are now increasingly available through sources like the Healthcare Cost and Utilization Project, provide an important way to assess hospital risk of CRE exposure based on its position in regional patient-sharing networks,” they noted. “Public health can play a critical role in identifying tightly connected hospitals and educating personnel at such facilities about their risk and need for enhanced infection control interventions.”
The team came to their conclusions after linking Illinois’ drug-resistant organisms registry with admissions data for 185 hospitals. About half reported at least one CRE case over 3 months, with a mean of 3.5 cases per hospital.
There was an average of 64 patient-sharing connections per facility, with a minimum of one connection and a maximum of 145 connections. Each additional patient two hospitals shared corresponded to a 3% increase in the CRE rate in urban facilities and a 6% increase in rural ones. The investigators didn’t explain the discrepancy, except to note that rural areas don’t have LTACHs.
Almost two-thirds of hospitals reporting CRE were in Chicago-area counties; almost half had shared at least one patient with an LTACH, and 21% had shared four or more.
CRE cases were an average of 64 years old, and equally distributed between men and women and black and white patients.
The Centers for Disease Control and Prevention funded the work. The authors had no disclosures.
The more hospitals share patients, the more likely they are to have a problem with carbapenem-resistant Enterobacteriaceae (CRE), especially if long-term acute care hospitals (LTACHs) are in the mix, according to a state-wide investigation from Illinois.
Greater hospital centrality was independently associated with higher rates overall, and sharing four or more patients with a long-term acute care hospital (LTACH) in the 3-month study window doubled the rate of CRE cases.
Although it’s possible that was because of chance (P = 0.11), the link between LTACHs and CRE “is consistent with prior analyses that have shown the central role LTACHs have in” spreading the organism, said the researchers, led by Michael Ray of the Illinois Department of Public Health (Clin Infect Dis. 2016 Aug 2. pii: ciw461).
Patients often spend weeks in LTACH facilities for ongoing, serious health problems. The severity of illness, long stay, and sometimes chronic antibiotic use increase the risk of CRE exposure, and the team found that many LTACH patients are colonized.
“These findings have immediate public health implications. … Early interventions should be focused on the most connected facilities, as well as those with strong connections to LTACHs.” When one hospital has an outbreak, facilities that share its patients need to swing into action screening new admissions and taking other steps to prevent regional spread, the team said.
Meanwhile, “state-wide patient-sharing data, which are now increasingly available through sources like the Healthcare Cost and Utilization Project, provide an important way to assess hospital risk of CRE exposure based on its position in regional patient-sharing networks,” they noted. “Public health can play a critical role in identifying tightly connected hospitals and educating personnel at such facilities about their risk and need for enhanced infection control interventions.”
The team came to their conclusions after linking Illinois’ drug-resistant organisms registry with admissions data for 185 hospitals. About half reported at least one CRE case over 3 months, with a mean of 3.5 cases per hospital.
There was an average of 64 patient-sharing connections per facility, with a minimum of one connection and a maximum of 145 connections. Each additional patient two hospitals shared corresponded to a 3% increase in the CRE rate in urban facilities and a 6% increase in rural ones. The investigators didn’t explain the discrepancy, except to note that rural areas don’t have LTACHs.
Almost two-thirds of hospitals reporting CRE were in Chicago-area counties; almost half had shared at least one patient with an LTACH, and 21% had shared four or more.
CRE cases were an average of 64 years old, and equally distributed between men and women and black and white patients.
The Centers for Disease Control and Prevention funded the work. The authors had no disclosures.
The more hospitals share patients, the more likely they are to have a problem with carbapenem-resistant Enterobacteriaceae (CRE), especially if long-term acute care hospitals (LTACHs) are in the mix, according to a state-wide investigation from Illinois.
Greater hospital centrality was independently associated with higher rates overall, and sharing four or more patients with a long-term acute care hospital (LTACH) in the 3-month study window doubled the rate of CRE cases.
Although it’s possible that was because of chance (P = 0.11), the link between LTACHs and CRE “is consistent with prior analyses that have shown the central role LTACHs have in” spreading the organism, said the researchers, led by Michael Ray of the Illinois Department of Public Health (Clin Infect Dis. 2016 Aug 2. pii: ciw461).
Patients often spend weeks in LTACH facilities for ongoing, serious health problems. The severity of illness, long stay, and sometimes chronic antibiotic use increase the risk of CRE exposure, and the team found that many LTACH patients are colonized.
“These findings have immediate public health implications. … Early interventions should be focused on the most connected facilities, as well as those with strong connections to LTACHs.” When one hospital has an outbreak, facilities that share its patients need to swing into action screening new admissions and taking other steps to prevent regional spread, the team said.
Meanwhile, “state-wide patient-sharing data, which are now increasingly available through sources like the Healthcare Cost and Utilization Project, provide an important way to assess hospital risk of CRE exposure based on its position in regional patient-sharing networks,” they noted. “Public health can play a critical role in identifying tightly connected hospitals and educating personnel at such facilities about their risk and need for enhanced infection control interventions.”
The team came to their conclusions after linking Illinois’ drug-resistant organisms registry with admissions data for 185 hospitals. About half reported at least one CRE case over 3 months, with a mean of 3.5 cases per hospital.
There was an average of 64 patient-sharing connections per facility, with a minimum of one connection and a maximum of 145 connections. Each additional patient two hospitals shared corresponded to a 3% increase in the CRE rate in urban facilities and a 6% increase in rural ones. The investigators didn’t explain the discrepancy, except to note that rural areas don’t have LTACHs.
Almost two-thirds of hospitals reporting CRE were in Chicago-area counties; almost half had shared at least one patient with an LTACH, and 21% had shared four or more.
CRE cases were an average of 64 years old, and equally distributed between men and women and black and white patients.
The Centers for Disease Control and Prevention funded the work. The authors had no disclosures.
FROM CLINICAL INFECTIOUS DISEASES
Key clinical point: The more hospitals share patients, the more likely they are to have a problem with CRE, especially if long-term acute care hospitals are in the mix.
Major finding: Sharing four or more patients with a long-term acute care hospital in the 3-month study window doubled the rate of CRE cases (P = 0.11).
Data source: 185 Illinois hospitals.
Disclosures: The Centers for Disease Control and Prevention funded the work. The authors had no disclosures.
ENSURE-AF trial supports edoxaban for electrical cardioversion
ROME – Results of the largest-ever randomized clinical trial of anticoagulation for electrical cardioversion of patients with nonvalvular atrial fibrillation demonstrate that edoxaban is a safe, effective, and convenient alternative to the standard strategy of enoxaparin as a bridge to warfarin.
The ENSURE-AF trial was a phase IIIb study involving 2,199 patients with atrial fibrillation who underwent electrical cardioversion at 239 sites in the United States and 19 European countries. The key finding: The edoxaban-treated group had rates of thromboembolism and major bleeding at 28-30 days follow-up similar to those of the enoxaparin/warfarin-treated controls.
And edoxaban offered a major practical advantage: Because “edoxaban kicks in within 2 hours, you can do the procedure just 2 hours after initiation of therapy in a patient with a reassuring transesophageal echocardiographic exam, which is definitely not possible with warfarin,” Andreas Goette, MD, observed at the annual congress of the European Society of Cardiology.
Roughly half of participants were treated at centers that don’t routinely use a transesophageal echo-guided management strategy and therefore delayed cardioversion until patients were anticoagulated for at least 3 weeks. The safety and efficacy outcomes were similar regardless of whether or not transesophageal echocardiography (TEE) guidance was used, according to Dr. Goette of St. Vincenz Hospital in Paderborn, Germany.
Edoxaban (Savaysa) was prescribed at 60 mg once daily except in patients weighing 60 kg or less or having a creatinine clearance rate of 15-50 mL/min, in which case they received 30 mg once daily. In the control arm, enoxaparin (Lovenox) was used until warfarin achieved an International Normalized Ratio of 2.0-3.0. Patients in the enoxaparin/warfarin arm spent a mean of 71% of their treatment time within the target INR range.
The primary efficacy outcome was the 28-day composite of stroke or other systemic embolic events, MI, or cardiovascular mortality. The rate was 0.5% in the edoxaban arm and 1.0% in the enoxaparin/warfarin group. In patients whose management strategy was TEE-guided, the rate was 0.3% in the edoxaban group and 0.8% with enoxaparin/warfarin. In non-TEE-guided patients, the rates were 0.6% and 1.2% with edoxaban and warfarin, respectively.
Although rates were consistently numerically lower in the edoxaban group, the differences did not reach statistical significance, Dr. Goette explained.
The combined rate of major or clinically relevant nonmajor bleeding through 30 days was 1.5% with edoxaban and similar at 1.0% with enoxaparin plus warfarin. Three patients in the edoxaban group experienced a major bleeding event, as did five in the comparator arm.
Because anticoagulation with edoxaban is so convenient and allows cardioversion to safely be performed in short order, the ENSURE-AF investigators are in the process of calculating the potential savings in health care costs obtainable through this strategy, the cardiologist said.
ENSURE-AF provides the first prospective randomized data on the use of edoxaban as an alternative to warfarin for pericardioversion anticoagulation. There has been one other randomized trial of a novel oral anticoagulant (NOAC) in this setting, the 1,504-patient X-VeRT trial (Eur Heart J. 2014 Dec 14;35[47]:3346-55), involving rivaroxaban (Xarelto).
Riccardo Cappato, MD, first author of the X-VeRT publication, served as the designated discussant for ENSURE-AF. He noted that the results of the two trials are “completely superimposable.” Rates of the composite efficacy endpoint were identical at 0.5% for both NOACs versus 1.0% for the vitamin K antagonist arms of X-VeRT and ENSURE-AF. The major bleeding rates also were identical for edoxaban and rivaroxaban in the two studies. Moreover, the major bleeding rates associated with warfarin or other vitamin K antagonists were spot-on the same in the two trials.
“It’s a rather unusual situation for such large numbers of patients,” observed Dr. Cappato of Humanitas Research Institute in Milan.
“These data go very clearly in the same direction. I think a good take-home message here for us today is that both of these novel oral anticoagulants can be safely and efficaciously applied to patients undergoing elective cardioversion of nonvalvular atrial fibrillation,” he added.
In an interview, Mark A. Creager, MD, immediate past president of the American Heart Association, said that many U.S. physicians are switching to NOACs for this purpose.
“We are already using the novel oral anticoagulants to facilitate anticoagulation for patients undergoing cardioversion, so ENSURE-AF provides objective evidence that edoxaban is a reasonable drug,” said Dr. Creager, director of the Dartmouth-Hitchcock Heart and Vascular Center in New Hampshire.
The ENSURE-AF trial was funded by Daiichi Sankyo. Dr. Goette and Dr. Cappato reported receiving research grants from and serving as consultants to that company and other pharmaceutical and medical device manufacturers.
Simultaneously with Dr. Goette’s presentation in Rome, the ENSURE-AF results were published online Aug. 30 in The Lancet.
ROME – Results of the largest-ever randomized clinical trial of anticoagulation for electrical cardioversion of patients with nonvalvular atrial fibrillation demonstrate that edoxaban is a safe, effective, and convenient alternative to the standard strategy of enoxaparin as a bridge to warfarin.
The ENSURE-AF trial was a phase IIIb study involving 2,199 patients with atrial fibrillation who underwent electrical cardioversion at 239 sites in the United States and 19 European countries. The key finding: The edoxaban-treated group had rates of thromboembolism and major bleeding at 28-30 days follow-up similar to those of the enoxaparin/warfarin-treated controls.
And edoxaban offered a major practical advantage: Because “edoxaban kicks in within 2 hours, you can do the procedure just 2 hours after initiation of therapy in a patient with a reassuring transesophageal echocardiographic exam, which is definitely not possible with warfarin,” Andreas Goette, MD, observed at the annual congress of the European Society of Cardiology.
Roughly half of participants were treated at centers that don’t routinely use a transesophageal echo-guided management strategy and therefore delayed cardioversion until patients were anticoagulated for at least 3 weeks. The safety and efficacy outcomes were similar regardless of whether or not transesophageal echocardiography (TEE) guidance was used, according to Dr. Goette of St. Vincenz Hospital in Paderborn, Germany.
Edoxaban (Savaysa) was prescribed at 60 mg once daily except in patients weighing 60 kg or less or having a creatinine clearance rate of 15-50 mL/min, in which case they received 30 mg once daily. In the control arm, enoxaparin (Lovenox) was used until warfarin achieved an International Normalized Ratio of 2.0-3.0. Patients in the enoxaparin/warfarin arm spent a mean of 71% of their treatment time within the target INR range.
The primary efficacy outcome was the 28-day composite of stroke or other systemic embolic events, MI, or cardiovascular mortality. The rate was 0.5% in the edoxaban arm and 1.0% in the enoxaparin/warfarin group. In patients whose management strategy was TEE-guided, the rate was 0.3% in the edoxaban group and 0.8% with enoxaparin/warfarin. In non-TEE-guided patients, the rates were 0.6% and 1.2% with edoxaban and warfarin, respectively.
Although rates were consistently numerically lower in the edoxaban group, the differences did not reach statistical significance, Dr. Goette explained.
The combined rate of major or clinically relevant nonmajor bleeding through 30 days was 1.5% with edoxaban and similar at 1.0% with enoxaparin plus warfarin. Three patients in the edoxaban group experienced a major bleeding event, as did five in the comparator arm.
Because anticoagulation with edoxaban is so convenient and allows cardioversion to safely be performed in short order, the ENSURE-AF investigators are in the process of calculating the potential savings in health care costs obtainable through this strategy, the cardiologist said.
ENSURE-AF provides the first prospective randomized data on the use of edoxaban as an alternative to warfarin for pericardioversion anticoagulation. There has been one other randomized trial of a novel oral anticoagulant (NOAC) in this setting, the 1,504-patient X-VeRT trial (Eur Heart J. 2014 Dec 14;35[47]:3346-55), involving rivaroxaban (Xarelto).
Riccardo Cappato, MD, first author of the X-VeRT publication, served as the designated discussant for ENSURE-AF. He noted that the results of the two trials are “completely superimposable.” Rates of the composite efficacy endpoint were identical at 0.5% for both NOACs versus 1.0% for the vitamin K antagonist arms of X-VeRT and ENSURE-AF. The major bleeding rates also were identical for edoxaban and rivaroxaban in the two studies. Moreover, the major bleeding rates associated with warfarin or other vitamin K antagonists were spot-on the same in the two trials.
“It’s a rather unusual situation for such large numbers of patients,” observed Dr. Cappato of Humanitas Research Institute in Milan.
“These data go very clearly in the same direction. I think a good take-home message here for us today is that both of these novel oral anticoagulants can be safely and efficaciously applied to patients undergoing elective cardioversion of nonvalvular atrial fibrillation,” he added.
In an interview, Mark A. Creager, MD, immediate past president of the American Heart Association, said that many U.S. physicians are switching to NOACs for this purpose.
“We are already using the novel oral anticoagulants to facilitate anticoagulation for patients undergoing cardioversion, so ENSURE-AF provides objective evidence that edoxaban is a reasonable drug,” said Dr. Creager, director of the Dartmouth-Hitchcock Heart and Vascular Center in New Hampshire.
The ENSURE-AF trial was funded by Daiichi Sankyo. Dr. Goette and Dr. Cappato reported receiving research grants from and serving as consultants to that company and other pharmaceutical and medical device manufacturers.
Simultaneously with Dr. Goette’s presentation in Rome, the ENSURE-AF results were published online Aug. 30 in The Lancet.
ROME – Results of the largest-ever randomized clinical trial of anticoagulation for electrical cardioversion of patients with nonvalvular atrial fibrillation demonstrate that edoxaban is a safe, effective, and convenient alternative to the standard strategy of enoxaparin as a bridge to warfarin.
The ENSURE-AF trial was a phase IIIb study involving 2,199 patients with atrial fibrillation who underwent electrical cardioversion at 239 sites in the United States and 19 European countries. The key finding: The edoxaban-treated group had rates of thromboembolism and major bleeding at 28-30 days follow-up similar to those of the enoxaparin/warfarin-treated controls.
And edoxaban offered a major practical advantage: Because “edoxaban kicks in within 2 hours, you can do the procedure just 2 hours after initiation of therapy in a patient with a reassuring transesophageal echocardiographic exam, which is definitely not possible with warfarin,” Andreas Goette, MD, observed at the annual congress of the European Society of Cardiology.
Roughly half of participants were treated at centers that don’t routinely use a transesophageal echo-guided management strategy and therefore delayed cardioversion until patients were anticoagulated for at least 3 weeks. The safety and efficacy outcomes were similar regardless of whether or not transesophageal echocardiography (TEE) guidance was used, according to Dr. Goette of St. Vincenz Hospital in Paderborn, Germany.
Edoxaban (Savaysa) was prescribed at 60 mg once daily except in patients weighing 60 kg or less or having a creatinine clearance rate of 15-50 mL/min, in which case they received 30 mg once daily. In the control arm, enoxaparin (Lovenox) was used until warfarin achieved an International Normalized Ratio of 2.0-3.0. Patients in the enoxaparin/warfarin arm spent a mean of 71% of their treatment time within the target INR range.
The primary efficacy outcome was the 28-day composite of stroke or other systemic embolic events, MI, or cardiovascular mortality. The rate was 0.5% in the edoxaban arm and 1.0% in the enoxaparin/warfarin group. In patients whose management strategy was TEE-guided, the rate was 0.3% in the edoxaban group and 0.8% with enoxaparin/warfarin. In non-TEE-guided patients, the rates were 0.6% and 1.2% with edoxaban and warfarin, respectively.
Although rates were consistently numerically lower in the edoxaban group, the differences did not reach statistical significance, Dr. Goette explained.
The combined rate of major or clinically relevant nonmajor bleeding through 30 days was 1.5% with edoxaban and similar at 1.0% with enoxaparin plus warfarin. Three patients in the edoxaban group experienced a major bleeding event, as did five in the comparator arm.
Because anticoagulation with edoxaban is so convenient and allows cardioversion to safely be performed in short order, the ENSURE-AF investigators are in the process of calculating the potential savings in health care costs obtainable through this strategy, the cardiologist said.
ENSURE-AF provides the first prospective randomized data on the use of edoxaban as an alternative to warfarin for pericardioversion anticoagulation. There has been one other randomized trial of a novel oral anticoagulant (NOAC) in this setting, the 1,504-patient X-VeRT trial (Eur Heart J. 2014 Dec 14;35[47]:3346-55), involving rivaroxaban (Xarelto).
Riccardo Cappato, MD, first author of the X-VeRT publication, served as the designated discussant for ENSURE-AF. He noted that the results of the two trials are “completely superimposable.” Rates of the composite efficacy endpoint were identical at 0.5% for both NOACs versus 1.0% for the vitamin K antagonist arms of X-VeRT and ENSURE-AF. The major bleeding rates also were identical for edoxaban and rivaroxaban in the two studies. Moreover, the major bleeding rates associated with warfarin or other vitamin K antagonists were spot-on the same in the two trials.
“It’s a rather unusual situation for such large numbers of patients,” observed Dr. Cappato of Humanitas Research Institute in Milan.
“These data go very clearly in the same direction. I think a good take-home message here for us today is that both of these novel oral anticoagulants can be safely and efficaciously applied to patients undergoing elective cardioversion of nonvalvular atrial fibrillation,” he added.
In an interview, Mark A. Creager, MD, immediate past president of the American Heart Association, said that many U.S. physicians are switching to NOACs for this purpose.
“We are already using the novel oral anticoagulants to facilitate anticoagulation for patients undergoing cardioversion, so ENSURE-AF provides objective evidence that edoxaban is a reasonable drug,” said Dr. Creager, director of the Dartmouth-Hitchcock Heart and Vascular Center in New Hampshire.
The ENSURE-AF trial was funded by Daiichi Sankyo. Dr. Goette and Dr. Cappato reported receiving research grants from and serving as consultants to that company and other pharmaceutical and medical device manufacturers.
Simultaneously with Dr. Goette’s presentation in Rome, the ENSURE-AF results were published online Aug. 30 in The Lancet.
AT THE ESC CONGRESS 2016
Key clinical point: Edoxaban is a safe, effective, and convenient alternative to warfarin for anticoagulation in patients undergoing electrical cardioversion of atrial fibrillation.
Major finding: The composite endpoint of stroke, other systemic embolic events, MI, or cardiovascular death occurred in 0.5% of patients with atrial fibrillation assigned to edoxaban for pericardioversion anticoagulation and in 1.0% on enoxaparin bridging to warfarin.
Data source: A randomized prospective multinational trial of 2,199 patients scheduled for electrical cardioversion of their nonvalvular atrial fibrillation.
Disclosures: The ENSURE-AF trial was funded by Daiichi Sankyo. The presenter reported receiving research grants from and serving as a consultant to that company as well as other pharmaceutical and medical device manufacturers.
Rotavirus vaccination herd effect benefits newborns and infants
Unvaccinated newborns and infants 42 days old or younger had significantly fewer rotavirus infections after the introduction of a universal mass vaccination (UMV) program, based on a decade of surveillance data from 11 pediatric care facilities in Austria.
“The present study aimed to investigate the long-term effect of UMV on rotavirus (RV)–associated hospitalizations, with particular focus on neonates and infants less than 6 weeks of age, comparing surveillance data between the prevaccination and postvaccination periods,” wrote Martina Prelog, MD, of University Hospital Wuerzburg (Germany), and her colleagues.
The data included 10,960 laboratory-confirmed cases of RV covering the periods before and after the initiation of the mass vaccination program.
Overall, hospitalizations for community-acquired RV infections dropped by almost 90% across all age groups. Among young infants, nosocomial RV infection rates were 28% prior to the vaccination program and 19% afterwards. However, overall nosocomial RV infection rates increased from 6% before the vaccination program to 13% after the program, and 6% of the cases were breakthrough infections, generally after incomplete RV vaccination.
“High numbers of documented cases and similar trends in all centers bolster the conclusion that UMV with RV vaccination may be associated with lower rates of RV hospitalization in unvaccinated neonates and young infants, supporting the beneficial role of UMV,” Dr. Prelog and her associates wrote.
Find the full study here in the Journal of Infectious Diseases (2016. doi: 10.1093/infdis/jiw186).
Unvaccinated newborns and infants 42 days old or younger had significantly fewer rotavirus infections after the introduction of a universal mass vaccination (UMV) program, based on a decade of surveillance data from 11 pediatric care facilities in Austria.
“The present study aimed to investigate the long-term effect of UMV on rotavirus (RV)–associated hospitalizations, with particular focus on neonates and infants less than 6 weeks of age, comparing surveillance data between the prevaccination and postvaccination periods,” wrote Martina Prelog, MD, of University Hospital Wuerzburg (Germany), and her colleagues.
The data included 10,960 laboratory-confirmed cases of RV covering the periods before and after the initiation of the mass vaccination program.
Overall, hospitalizations for community-acquired RV infections dropped by almost 90% across all age groups. Among young infants, nosocomial RV infection rates were 28% prior to the vaccination program and 19% afterwards. However, overall nosocomial RV infection rates increased from 6% before the vaccination program to 13% after the program, and 6% of the cases were breakthrough infections, generally after incomplete RV vaccination.
“High numbers of documented cases and similar trends in all centers bolster the conclusion that UMV with RV vaccination may be associated with lower rates of RV hospitalization in unvaccinated neonates and young infants, supporting the beneficial role of UMV,” Dr. Prelog and her associates wrote.
Find the full study here in the Journal of Infectious Diseases (2016. doi: 10.1093/infdis/jiw186).
Unvaccinated newborns and infants 42 days old or younger had significantly fewer rotavirus infections after the introduction of a universal mass vaccination (UMV) program, based on a decade of surveillance data from 11 pediatric care facilities in Austria.
“The present study aimed to investigate the long-term effect of UMV on rotavirus (RV)–associated hospitalizations, with particular focus on neonates and infants less than 6 weeks of age, comparing surveillance data between the prevaccination and postvaccination periods,” wrote Martina Prelog, MD, of University Hospital Wuerzburg (Germany), and her colleagues.
The data included 10,960 laboratory-confirmed cases of RV covering the periods before and after the initiation of the mass vaccination program.
Overall, hospitalizations for community-acquired RV infections dropped by almost 90% across all age groups. Among young infants, nosocomial RV infection rates were 28% prior to the vaccination program and 19% afterwards. However, overall nosocomial RV infection rates increased from 6% before the vaccination program to 13% after the program, and 6% of the cases were breakthrough infections, generally after incomplete RV vaccination.
“High numbers of documented cases and similar trends in all centers bolster the conclusion that UMV with RV vaccination may be associated with lower rates of RV hospitalization in unvaccinated neonates and young infants, supporting the beneficial role of UMV,” Dr. Prelog and her associates wrote.
Find the full study here in the Journal of Infectious Diseases (2016. doi: 10.1093/infdis/jiw186).
FROM THE JOURNAL OF INFECTIOUS DISEASES
VIDEO: Functional noninvasive imaging cuts unnecessary angiography
ROME – Functional, noninvasive cardiac imaging using cardiovascular MR or myocardial perfusion scintigraphy was significantly better than was a current and well regarded guideline-based approach to identifying patients with chest pain and suspected coronary artery disease who could safely avoid angiography, thereby cutting the rate of unnecessary angiography by about 75%.
Following the guideline formula adopted by the British National Institute for Health and Care Excellence (NICE) resulted in a 29% rate of unnecessary angiography compared with rates of 7.5% using cardiovascular MR (CMR) and 7.1% using myocardial perfusion scintigraphy (MPS) in a multicenter randomized trial with 1,202 patients, John P. Greenwood, MBChB, said at the annual congress of the European Society of Cardiology.
This universal use of a functional, noninvasive imaging strategy to guide angiography resulted in no significant penalty of missed coronary disease or subsequent coronary events. The rate of positive angiography findings was 12% among the 240 patients managed according to the NICE guidelines, 10% among 481 patients screened by CMR, and 9% among the 481 patients screened using MPS, reported Dr. Greenwood, professor of cardiology at the University of Leeds (England). The rate of major adverse coronary events after 12 months of follow-up were 3% following the NICE protocol and 4% when screening by CMR or with MPS.
Concurrently with Dr. Greenwood’s report, the findings from the Clinical Evaluation of Magnetic Resonance Imaging in Coronary Heart Disease 2 (CE-MARC2) study appeared in an article online (JAMA. 2016 Aug 29. doi: 10.1001/jama.2016.12680).
“We showed that a functional test with CMR or MPS can reduce the rate of unnecessary coronary angiography. Cutting unnecessary angiography is really important to patients, and it may also cost effective,” he said, but cautioned that a formal cost analysis of the options tested in this study is still being run.
The NICE guidelines manage patients with chest pain that could be angina by their pretest probability of having coronary artery disease (CAD), and at the time the study was designed the NICE guidelines, issued in 2010, provided the most up-to-date expert guidance on how to triage these patients. The study enrolled patients with a pretest probability for CAD of 10%-90%; collectively their average probability was 50%. The patients participated in the study at one of six U.K. centers during November 2012 to March 2015. The average age was 56 years.
MPS is “probably the noninvasive imaging approach most commonly used worldwide to detect coronary ischemia,” Dr. Greenwood said. But he led an earlier study that showed that CMR, using a gadolinium-based tracing agent, works even better than MPS (in this study single photon emission CT) to predict a patient’s risk for major cardiac events. He said this superiority is probably because of the greater spatial resolution with CMR.
“The higher spatial resolution of CMR, about 5- to 10-fold greater that MPS, is less likely to produce false negative results,” he said in an interview. “We showed that CMR has higher diagnostic accuracy, is a better prognosticator, and is more cost effective” than MPS. Dr. Greenwood attributed the similar performance of CMR and MPS in CE-MARC2 to the study’s design, which led to fewer patients undergoing each of the two imaging methods and made CE-MARC2 underpowered to discern a difference in specificity. In his earlier study, which included 752 patients who underwent examination with both CMR and MPS, the negative predictive value of CMR was 91% compared with 79% with MPS.
CMR uses conventional MR machines, is now widely available, and is being widely used today as a first-line test in the United Kingdom and Europe, he added.
Dr. Greenwood believes that in his new study functional imaging outperformed the NICE guidelines because the pretest models used in the guidelines “tend to overestimate risk,” the factor that produces angiography overuse.
His report included two additional analyses that assessed the impact of CMR and MPS in the subgroup of patients with a high pretest probability for CAD, 61%-90%, and in the subgroup with a low pretest probability, 10%-29%. Among the patients with a high likelihood for CAD the two functional imaging methods cut the rate of unnecessary angiography by 95%, a statistically significant difference. Among those with a low likelihood functional imaging cut the rate 56%, a difference that did not reach statistical significance.
[email protected]
On Twitter @mitchelzoler
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The results from CE-MARC2 very nicely showed that imaging-guided angiography is as safe as compulsory angiography in the highest-risk subgroup of the enrolled patients, those with a pretest probability of 61%-90% for having coronary artery disease. Findings from the economic analysis of this study that remains pending will be crucial for eventually recommending one strategy over the other in this setting.
 |
| Mitchel L. Zoler/Frontline Medical News Dr. Udo Sechtem |
The 12-month rate of the hardest clinical endpoints measured in this study, cardiovascular deaths and MIs, was very low in this study: 1.3% in the patients managed with NICE guidance, 1% in those who first underwent cardiovascular MR, and 0.8% in the patients who first underwent myocardial perfusion scintigraphy. Despite this low risk, the patients in each of the three arms of the study underwent roughly 500 test procedures.
We should therefore consider a totally different approach. Instead of immediately performing a noninvasive test or the tests called for by the NICE guidelines, what about no testing at all. Instead, patients would first undergo optimal preventive and symptomatic medical treatments. If patients failed this strategy they then could be considered for revascularization. I propose a study that would compare imaging-guided conditional angiography, as tested in CE-MARC2, with symptom-guided conditional angiography. Functional, noninvasive testing for all needs to be compared against optimal management and symptom driven interventions.
Udo Sechtem, Dr Med, is head of cardiology at the Robert-Bosch-Hospital in Stuttgart, Germany. He made these comments as the designated discussant for the study. He had no disclosures.
The results from CE-MARC2 very nicely showed that imaging-guided angiography is as safe as compulsory angiography in the highest-risk subgroup of the enrolled patients, those with a pretest probability of 61%-90% for having coronary artery disease. Findings from the economic analysis of this study that remains pending will be crucial for eventually recommending one strategy over the other in this setting.
 |
| Mitchel L. Zoler/Frontline Medical News Dr. Udo Sechtem |
The 12-month rate of the hardest clinical endpoints measured in this study, cardiovascular deaths and MIs, was very low in this study: 1.3% in the patients managed with NICE guidance, 1% in those who first underwent cardiovascular MR, and 0.8% in the patients who first underwent myocardial perfusion scintigraphy. Despite this low risk, the patients in each of the three arms of the study underwent roughly 500 test procedures.
We should therefore consider a totally different approach. Instead of immediately performing a noninvasive test or the tests called for by the NICE guidelines, what about no testing at all. Instead, patients would first undergo optimal preventive and symptomatic medical treatments. If patients failed this strategy they then could be considered for revascularization. I propose a study that would compare imaging-guided conditional angiography, as tested in CE-MARC2, with symptom-guided conditional angiography. Functional, noninvasive testing for all needs to be compared against optimal management and symptom driven interventions.
Udo Sechtem, Dr Med, is head of cardiology at the Robert-Bosch-Hospital in Stuttgart, Germany. He made these comments as the designated discussant for the study. He had no disclosures.
The results from CE-MARC2 very nicely showed that imaging-guided angiography is as safe as compulsory angiography in the highest-risk subgroup of the enrolled patients, those with a pretest probability of 61%-90% for having coronary artery disease. Findings from the economic analysis of this study that remains pending will be crucial for eventually recommending one strategy over the other in this setting.
 |
| Mitchel L. Zoler/Frontline Medical News Dr. Udo Sechtem |
The 12-month rate of the hardest clinical endpoints measured in this study, cardiovascular deaths and MIs, was very low in this study: 1.3% in the patients managed with NICE guidance, 1% in those who first underwent cardiovascular MR, and 0.8% in the patients who first underwent myocardial perfusion scintigraphy. Despite this low risk, the patients in each of the three arms of the study underwent roughly 500 test procedures.
We should therefore consider a totally different approach. Instead of immediately performing a noninvasive test or the tests called for by the NICE guidelines, what about no testing at all. Instead, patients would first undergo optimal preventive and symptomatic medical treatments. If patients failed this strategy they then could be considered for revascularization. I propose a study that would compare imaging-guided conditional angiography, as tested in CE-MARC2, with symptom-guided conditional angiography. Functional, noninvasive testing for all needs to be compared against optimal management and symptom driven interventions.
Udo Sechtem, Dr Med, is head of cardiology at the Robert-Bosch-Hospital in Stuttgart, Germany. He made these comments as the designated discussant for the study. He had no disclosures.
ROME – Functional, noninvasive cardiac imaging using cardiovascular MR or myocardial perfusion scintigraphy was significantly better than was a current and well regarded guideline-based approach to identifying patients with chest pain and suspected coronary artery disease who could safely avoid angiography, thereby cutting the rate of unnecessary angiography by about 75%.
Following the guideline formula adopted by the British National Institute for Health and Care Excellence (NICE) resulted in a 29% rate of unnecessary angiography compared with rates of 7.5% using cardiovascular MR (CMR) and 7.1% using myocardial perfusion scintigraphy (MPS) in a multicenter randomized trial with 1,202 patients, John P. Greenwood, MBChB, said at the annual congress of the European Society of Cardiology.
This universal use of a functional, noninvasive imaging strategy to guide angiography resulted in no significant penalty of missed coronary disease or subsequent coronary events. The rate of positive angiography findings was 12% among the 240 patients managed according to the NICE guidelines, 10% among 481 patients screened by CMR, and 9% among the 481 patients screened using MPS, reported Dr. Greenwood, professor of cardiology at the University of Leeds (England). The rate of major adverse coronary events after 12 months of follow-up were 3% following the NICE protocol and 4% when screening by CMR or with MPS.
Concurrently with Dr. Greenwood’s report, the findings from the Clinical Evaluation of Magnetic Resonance Imaging in Coronary Heart Disease 2 (CE-MARC2) study appeared in an article online (JAMA. 2016 Aug 29. doi: 10.1001/jama.2016.12680).
“We showed that a functional test with CMR or MPS can reduce the rate of unnecessary coronary angiography. Cutting unnecessary angiography is really important to patients, and it may also cost effective,” he said, but cautioned that a formal cost analysis of the options tested in this study is still being run.
The NICE guidelines manage patients with chest pain that could be angina by their pretest probability of having coronary artery disease (CAD), and at the time the study was designed the NICE guidelines, issued in 2010, provided the most up-to-date expert guidance on how to triage these patients. The study enrolled patients with a pretest probability for CAD of 10%-90%; collectively their average probability was 50%. The patients participated in the study at one of six U.K. centers during November 2012 to March 2015. The average age was 56 years.
MPS is “probably the noninvasive imaging approach most commonly used worldwide to detect coronary ischemia,” Dr. Greenwood said. But he led an earlier study that showed that CMR, using a gadolinium-based tracing agent, works even better than MPS (in this study single photon emission CT) to predict a patient’s risk for major cardiac events. He said this superiority is probably because of the greater spatial resolution with CMR.
“The higher spatial resolution of CMR, about 5- to 10-fold greater that MPS, is less likely to produce false negative results,” he said in an interview. “We showed that CMR has higher diagnostic accuracy, is a better prognosticator, and is more cost effective” than MPS. Dr. Greenwood attributed the similar performance of CMR and MPS in CE-MARC2 to the study’s design, which led to fewer patients undergoing each of the two imaging methods and made CE-MARC2 underpowered to discern a difference in specificity. In his earlier study, which included 752 patients who underwent examination with both CMR and MPS, the negative predictive value of CMR was 91% compared with 79% with MPS.
CMR uses conventional MR machines, is now widely available, and is being widely used today as a first-line test in the United Kingdom and Europe, he added.
Dr. Greenwood believes that in his new study functional imaging outperformed the NICE guidelines because the pretest models used in the guidelines “tend to overestimate risk,” the factor that produces angiography overuse.
His report included two additional analyses that assessed the impact of CMR and MPS in the subgroup of patients with a high pretest probability for CAD, 61%-90%, and in the subgroup with a low pretest probability, 10%-29%. Among the patients with a high likelihood for CAD the two functional imaging methods cut the rate of unnecessary angiography by 95%, a statistically significant difference. Among those with a low likelihood functional imaging cut the rate 56%, a difference that did not reach statistical significance.
[email protected]
On Twitter @mitchelzoler
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ROME – Functional, noninvasive cardiac imaging using cardiovascular MR or myocardial perfusion scintigraphy was significantly better than was a current and well regarded guideline-based approach to identifying patients with chest pain and suspected coronary artery disease who could safely avoid angiography, thereby cutting the rate of unnecessary angiography by about 75%.
Following the guideline formula adopted by the British National Institute for Health and Care Excellence (NICE) resulted in a 29% rate of unnecessary angiography compared with rates of 7.5% using cardiovascular MR (CMR) and 7.1% using myocardial perfusion scintigraphy (MPS) in a multicenter randomized trial with 1,202 patients, John P. Greenwood, MBChB, said at the annual congress of the European Society of Cardiology.
This universal use of a functional, noninvasive imaging strategy to guide angiography resulted in no significant penalty of missed coronary disease or subsequent coronary events. The rate of positive angiography findings was 12% among the 240 patients managed according to the NICE guidelines, 10% among 481 patients screened by CMR, and 9% among the 481 patients screened using MPS, reported Dr. Greenwood, professor of cardiology at the University of Leeds (England). The rate of major adverse coronary events after 12 months of follow-up were 3% following the NICE protocol and 4% when screening by CMR or with MPS.
Concurrently with Dr. Greenwood’s report, the findings from the Clinical Evaluation of Magnetic Resonance Imaging in Coronary Heart Disease 2 (CE-MARC2) study appeared in an article online (JAMA. 2016 Aug 29. doi: 10.1001/jama.2016.12680).
“We showed that a functional test with CMR or MPS can reduce the rate of unnecessary coronary angiography. Cutting unnecessary angiography is really important to patients, and it may also cost effective,” he said, but cautioned that a formal cost analysis of the options tested in this study is still being run.
The NICE guidelines manage patients with chest pain that could be angina by their pretest probability of having coronary artery disease (CAD), and at the time the study was designed the NICE guidelines, issued in 2010, provided the most up-to-date expert guidance on how to triage these patients. The study enrolled patients with a pretest probability for CAD of 10%-90%; collectively their average probability was 50%. The patients participated in the study at one of six U.K. centers during November 2012 to March 2015. The average age was 56 years.
MPS is “probably the noninvasive imaging approach most commonly used worldwide to detect coronary ischemia,” Dr. Greenwood said. But he led an earlier study that showed that CMR, using a gadolinium-based tracing agent, works even better than MPS (in this study single photon emission CT) to predict a patient’s risk for major cardiac events. He said this superiority is probably because of the greater spatial resolution with CMR.
“The higher spatial resolution of CMR, about 5- to 10-fold greater that MPS, is less likely to produce false negative results,” he said in an interview. “We showed that CMR has higher diagnostic accuracy, is a better prognosticator, and is more cost effective” than MPS. Dr. Greenwood attributed the similar performance of CMR and MPS in CE-MARC2 to the study’s design, which led to fewer patients undergoing each of the two imaging methods and made CE-MARC2 underpowered to discern a difference in specificity. In his earlier study, which included 752 patients who underwent examination with both CMR and MPS, the negative predictive value of CMR was 91% compared with 79% with MPS.
CMR uses conventional MR machines, is now widely available, and is being widely used today as a first-line test in the United Kingdom and Europe, he added.
Dr. Greenwood believes that in his new study functional imaging outperformed the NICE guidelines because the pretest models used in the guidelines “tend to overestimate risk,” the factor that produces angiography overuse.
His report included two additional analyses that assessed the impact of CMR and MPS in the subgroup of patients with a high pretest probability for CAD, 61%-90%, and in the subgroup with a low pretest probability, 10%-29%. Among the patients with a high likelihood for CAD the two functional imaging methods cut the rate of unnecessary angiography by 95%, a statistically significant difference. Among those with a low likelihood functional imaging cut the rate 56%, a difference that did not reach statistical significance.
[email protected]
On Twitter @mitchelzoler
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE ESC CONGRESS 2016
Key clinical point: Screening patients with suspected angina via cardiovascular MR or myocardial perfusion imaging substantially reduced the rate of unnecessary angiography compared with the screening algorithm currently endorsed by British national guidelines.
Major finding: The unnecessary angiography rate was 29% with the guideline algorithm, 7.5% with cardiovascular MR, and 7.1% with myocardial perfusion scintigraphy.
Data source: CE MARC2, a multicenter, randomized trial with 1,202 patients.
Disclosures: Dr. Greenwood had no disclosures.
Andexanet controlled factor Xa inhibitor–related bleeding
The factor Xa antidote andexanet achieved effective hemostasis 12 hours after infusion in 79% of patients who had developed serious acute bleeding on factor Xa inhibitor therapy, according to a preliminary analysis of an ongoing study of how reducing anti–factor Xa activity affects clinical hemostatic outcomes.
“The site of bleeding was most often gastrointestinal or intracranial; anti–factor Xa activity was considerably elevated in most patients and, as such, was likely to be a major impediment to clinical hemostasis. The administration of an andexanet bolus and infusion resulted in rapid and substantial reversal of anti–factor Xa activity,” Stuart Connolly, MD, of McMaster University, Hamilton, Ont., and his associates reported at the ESC Congress and in a simultaneously published study (N Engl J Med. 2016 Aug 30. doi: 10.1056/NEJMoa1607887).
Andexanet alfa is a recombinant modified human factor Xa decoy protein that “sharply” reduced plasma levels of unbound factor Xa inhibitors as well as anti-factor Xa activity in healthy older volunteers receiving apixaban or rivaroxaban, the researchers noted. Based on those findings, they designed a prospective, multicenter, single-group, open-label study (Andexanet Alfa, a Novel Antidote to the Anticoagulation Effects of FXA Inhibitors; ANNEXA-4) of andexanet in patients with potentially life-threatening acute major bleeding related to anticoagulation with a factor Xa inhibitor.
This interim report from the ongoing study included 67 patients with data available by June 17, 2016. Participants averaged 77 years of age and were receiving a factor Xa inhibtor because of atrial fibrillation, venous thromboembolism, or both. All patients received a bolus of andexanet for 15 to 30 minutes followed by a 2-hour infusion. Based on previous studies, the researchers used a 400-mg bolus of andexanet followed by a 480-mg infusion when patients had last taken their factor Xa inhibitor more than 7 hours before, and a higher 800-mg bolus followed by a 960-mg infusion when patients had taken their anticoagulant more recently. Bleeding and hemostasis were evaluated based on serial CT or MRI scans of patients with intracranial hemorrhage and corrected hemoglobin and hematocrit levels at 12 hours for patients with gastrointestinal and other nonvisible bleeding.
Among 47 patients in the primary efficacy analysis, 37 (79%) achieved excellent or good hemostasis (95% confidence interval, 64% to 89%), including 81% of patients on rivaroxaban and 75% of patients on enoxaparin, the researchers reported. “The rates of excellent or good efficacy were 84% for gastrointestinal bleeding and 80% for intracranial bleeding, rates that were consistent for other subgroups that were examined,” they said. Among the five patients (10%) with the most residual anti–factor Xa activity, all had received the lower andexanet dose. Four had received rivaroxaban while one had received apixaban.
The safety population included all 67 patients, none of whom developed infusion reactions or antibodies to factors X, Xa, or andexanet. After 30 days of follow-up, 12 patients (18%) had experienced one or more thrombotic events, including deep vein thrombosis (seven patients), stroke (five patients), myocardial infarction (one patient), and pulmonary embolism (one patient). One-third of these events occurred within 3 days of receiving andexanet, while the rest occurred by day 30. A total of 10 patients (15%) died, and six deaths were from cardiovascular causes. “A controlled study would be required to assess whether the frequency of these events exceeded that expected in patients at increased risk for thrombotic events,” the researchers commented.
Portola Pharmaceuticals makes andexanet alfa and funded the study. Dr. Connolly disclosed ties to Bayer, Bristol-Myers Squibb/Pfizer, Daiichi-Sankyo, CSL Behring, Octapharma, and Boehringer Ingelheim outside the submitted work.
The factor Xa antidote andexanet achieved effective hemostasis 12 hours after infusion in 79% of patients who had developed serious acute bleeding on factor Xa inhibitor therapy, according to a preliminary analysis of an ongoing study of how reducing anti–factor Xa activity affects clinical hemostatic outcomes.
“The site of bleeding was most often gastrointestinal or intracranial; anti–factor Xa activity was considerably elevated in most patients and, as such, was likely to be a major impediment to clinical hemostasis. The administration of an andexanet bolus and infusion resulted in rapid and substantial reversal of anti–factor Xa activity,” Stuart Connolly, MD, of McMaster University, Hamilton, Ont., and his associates reported at the ESC Congress and in a simultaneously published study (N Engl J Med. 2016 Aug 30. doi: 10.1056/NEJMoa1607887).
Andexanet alfa is a recombinant modified human factor Xa decoy protein that “sharply” reduced plasma levels of unbound factor Xa inhibitors as well as anti-factor Xa activity in healthy older volunteers receiving apixaban or rivaroxaban, the researchers noted. Based on those findings, they designed a prospective, multicenter, single-group, open-label study (Andexanet Alfa, a Novel Antidote to the Anticoagulation Effects of FXA Inhibitors; ANNEXA-4) of andexanet in patients with potentially life-threatening acute major bleeding related to anticoagulation with a factor Xa inhibitor.
This interim report from the ongoing study included 67 patients with data available by June 17, 2016. Participants averaged 77 years of age and were receiving a factor Xa inhibtor because of atrial fibrillation, venous thromboembolism, or both. All patients received a bolus of andexanet for 15 to 30 minutes followed by a 2-hour infusion. Based on previous studies, the researchers used a 400-mg bolus of andexanet followed by a 480-mg infusion when patients had last taken their factor Xa inhibitor more than 7 hours before, and a higher 800-mg bolus followed by a 960-mg infusion when patients had taken their anticoagulant more recently. Bleeding and hemostasis were evaluated based on serial CT or MRI scans of patients with intracranial hemorrhage and corrected hemoglobin and hematocrit levels at 12 hours for patients with gastrointestinal and other nonvisible bleeding.
Among 47 patients in the primary efficacy analysis, 37 (79%) achieved excellent or good hemostasis (95% confidence interval, 64% to 89%), including 81% of patients on rivaroxaban and 75% of patients on enoxaparin, the researchers reported. “The rates of excellent or good efficacy were 84% for gastrointestinal bleeding and 80% for intracranial bleeding, rates that were consistent for other subgroups that were examined,” they said. Among the five patients (10%) with the most residual anti–factor Xa activity, all had received the lower andexanet dose. Four had received rivaroxaban while one had received apixaban.
The safety population included all 67 patients, none of whom developed infusion reactions or antibodies to factors X, Xa, or andexanet. After 30 days of follow-up, 12 patients (18%) had experienced one or more thrombotic events, including deep vein thrombosis (seven patients), stroke (five patients), myocardial infarction (one patient), and pulmonary embolism (one patient). One-third of these events occurred within 3 days of receiving andexanet, while the rest occurred by day 30. A total of 10 patients (15%) died, and six deaths were from cardiovascular causes. “A controlled study would be required to assess whether the frequency of these events exceeded that expected in patients at increased risk for thrombotic events,” the researchers commented.
Portola Pharmaceuticals makes andexanet alfa and funded the study. Dr. Connolly disclosed ties to Bayer, Bristol-Myers Squibb/Pfizer, Daiichi-Sankyo, CSL Behring, Octapharma, and Boehringer Ingelheim outside the submitted work.
The factor Xa antidote andexanet achieved effective hemostasis 12 hours after infusion in 79% of patients who had developed serious acute bleeding on factor Xa inhibitor therapy, according to a preliminary analysis of an ongoing study of how reducing anti–factor Xa activity affects clinical hemostatic outcomes.
“The site of bleeding was most often gastrointestinal or intracranial; anti–factor Xa activity was considerably elevated in most patients and, as such, was likely to be a major impediment to clinical hemostasis. The administration of an andexanet bolus and infusion resulted in rapid and substantial reversal of anti–factor Xa activity,” Stuart Connolly, MD, of McMaster University, Hamilton, Ont., and his associates reported at the ESC Congress and in a simultaneously published study (N Engl J Med. 2016 Aug 30. doi: 10.1056/NEJMoa1607887).
Andexanet alfa is a recombinant modified human factor Xa decoy protein that “sharply” reduced plasma levels of unbound factor Xa inhibitors as well as anti-factor Xa activity in healthy older volunteers receiving apixaban or rivaroxaban, the researchers noted. Based on those findings, they designed a prospective, multicenter, single-group, open-label study (Andexanet Alfa, a Novel Antidote to the Anticoagulation Effects of FXA Inhibitors; ANNEXA-4) of andexanet in patients with potentially life-threatening acute major bleeding related to anticoagulation with a factor Xa inhibitor.
This interim report from the ongoing study included 67 patients with data available by June 17, 2016. Participants averaged 77 years of age and were receiving a factor Xa inhibtor because of atrial fibrillation, venous thromboembolism, or both. All patients received a bolus of andexanet for 15 to 30 minutes followed by a 2-hour infusion. Based on previous studies, the researchers used a 400-mg bolus of andexanet followed by a 480-mg infusion when patients had last taken their factor Xa inhibitor more than 7 hours before, and a higher 800-mg bolus followed by a 960-mg infusion when patients had taken their anticoagulant more recently. Bleeding and hemostasis were evaluated based on serial CT or MRI scans of patients with intracranial hemorrhage and corrected hemoglobin and hematocrit levels at 12 hours for patients with gastrointestinal and other nonvisible bleeding.
Among 47 patients in the primary efficacy analysis, 37 (79%) achieved excellent or good hemostasis (95% confidence interval, 64% to 89%), including 81% of patients on rivaroxaban and 75% of patients on enoxaparin, the researchers reported. “The rates of excellent or good efficacy were 84% for gastrointestinal bleeding and 80% for intracranial bleeding, rates that were consistent for other subgroups that were examined,” they said. Among the five patients (10%) with the most residual anti–factor Xa activity, all had received the lower andexanet dose. Four had received rivaroxaban while one had received apixaban.
The safety population included all 67 patients, none of whom developed infusion reactions or antibodies to factors X, Xa, or andexanet. After 30 days of follow-up, 12 patients (18%) had experienced one or more thrombotic events, including deep vein thrombosis (seven patients), stroke (five patients), myocardial infarction (one patient), and pulmonary embolism (one patient). One-third of these events occurred within 3 days of receiving andexanet, while the rest occurred by day 30. A total of 10 patients (15%) died, and six deaths were from cardiovascular causes. “A controlled study would be required to assess whether the frequency of these events exceeded that expected in patients at increased risk for thrombotic events,” the researchers commented.
Portola Pharmaceuticals makes andexanet alfa and funded the study. Dr. Connolly disclosed ties to Bayer, Bristol-Myers Squibb/Pfizer, Daiichi-Sankyo, CSL Behring, Octapharma, and Boehringer Ingelheim outside the submitted work.
FROM ESC CONGRESS 2016
Key clinical point: Andexanet alfa achieved effective hemostasis in most patients with acute major bleeding on factor Xa inhibitors.
Major finding: At 12 hours after infusion, 37 of 47 patients in the efficacy analysis achieved excellent or good hemostasis (79%; 95% confidence interval, 64% to 89%).
Data source: A prospective, multicenter, single-group, open-label study of 67 adults, most of whom had substantial cardiovascular disease.
Disclosures: Portola Pharmaceuticals makes andexanet alfa and funded the study. Dr. Connolly disclosed ties to Bayer, Bristol-Myers Squibb/Pfizer, Daiichi-Sankyo, CSL Behring, Octapharma, and Boehringer Ingelheim outside the submitted work.