User login
EMERGENCY MEDICINE is a practical, peer-reviewed monthly publication and Web site that meets the educational needs of emergency clinicians and urgent care clinicians for their practice.
CDC director cites rise in hospitalizations in urging teen vaccinations
“I am deeply concerned by the numbers of hospitalized adolescents and saddened to see the number of adolescents who required treatment in intensive care units or mechanical ventilation,” CDC Director Rochelle Walensky, MD, said in a statement.
While urging teenagers to wear masks and take precautions around others, she asked “parents, relatives, and close friends to join me and talk with teens about the importance of these prevention strategies and to encourage them to get vaccinated.”
Dr. Walensky referred to the CDC’s Morbidity and Mortality Weekly Report that showed adolescent hospitalizations peaked at 2.1 per 100,000 in early January 2021, then dropped to 0.6 per 100,000 in mid-March.
Alarmingly, hospitalizations rose to 1.3 per 100,000 in April, and a number of teens required serious interventions.
“Among hospitalized adolescents, nearly one-third required intensive care unit admission, and 5% required invasive mechanical ventilation,” the report said. No deaths occurred.
The study looked at 376 adolescents aged 12-17 who were hospitalized and tested positive for coronavirus. Of that group, 204 were hospitalized for COVID-19 and the other 172 were hospitalized for reasons not directly related to COVID-19.
Of the 204 hospitalized for COVID-19, 70.6% had an underlying medical condition such as obesity or chronic lung disease.
The study noted that children and teenagers have lower hospitalization rates and generally show less severe symptoms than do older people.
Possible causes for the rise in adolescent COVID-19 hospitalizations include the arrival of variants, the growing number of children returning to in-person education, and the changes in mask-wearing and other safety precautions, the study said.
The American Academy of Pediatrics said that as of May 27, 4 million children have tested positive for COVID-19 since the pandemic began, with about 34,500 new child cases reported for the week ending May 27.
The AAP said children have represented 14.1% of total cases since the pandemic began, but for the week ending May 27, children represented 24.3% of new reported weekly COVID-19 cases.
On May 10, the FDA granted emergency use authorization for the Pfizer coronavirus vaccine to be given to children aged 12-15 years. Previously, the FDA had authorized the Pfizer vaccine for people aged 16 years and up, whereas the Moderna and Johnson & Johnson vaccines are authorized for people aged 18 years and up.
“Vaccination is our way out of this pandemic,” Dr. Walensky said in her statement. “I continue to see promising signs in CDC data that we are nearing the end of this pandemic in this country; however, we all have to do our part and get vaccinated to cross the finish line.”
A version of this article was first published on WebMD.com.
“I am deeply concerned by the numbers of hospitalized adolescents and saddened to see the number of adolescents who required treatment in intensive care units or mechanical ventilation,” CDC Director Rochelle Walensky, MD, said in a statement.
While urging teenagers to wear masks and take precautions around others, she asked “parents, relatives, and close friends to join me and talk with teens about the importance of these prevention strategies and to encourage them to get vaccinated.”
Dr. Walensky referred to the CDC’s Morbidity and Mortality Weekly Report that showed adolescent hospitalizations peaked at 2.1 per 100,000 in early January 2021, then dropped to 0.6 per 100,000 in mid-March.
Alarmingly, hospitalizations rose to 1.3 per 100,000 in April, and a number of teens required serious interventions.
“Among hospitalized adolescents, nearly one-third required intensive care unit admission, and 5% required invasive mechanical ventilation,” the report said. No deaths occurred.
The study looked at 376 adolescents aged 12-17 who were hospitalized and tested positive for coronavirus. Of that group, 204 were hospitalized for COVID-19 and the other 172 were hospitalized for reasons not directly related to COVID-19.
Of the 204 hospitalized for COVID-19, 70.6% had an underlying medical condition such as obesity or chronic lung disease.
The study noted that children and teenagers have lower hospitalization rates and generally show less severe symptoms than do older people.
Possible causes for the rise in adolescent COVID-19 hospitalizations include the arrival of variants, the growing number of children returning to in-person education, and the changes in mask-wearing and other safety precautions, the study said.
The American Academy of Pediatrics said that as of May 27, 4 million children have tested positive for COVID-19 since the pandemic began, with about 34,500 new child cases reported for the week ending May 27.
The AAP said children have represented 14.1% of total cases since the pandemic began, but for the week ending May 27, children represented 24.3% of new reported weekly COVID-19 cases.
On May 10, the FDA granted emergency use authorization for the Pfizer coronavirus vaccine to be given to children aged 12-15 years. Previously, the FDA had authorized the Pfizer vaccine for people aged 16 years and up, whereas the Moderna and Johnson & Johnson vaccines are authorized for people aged 18 years and up.
“Vaccination is our way out of this pandemic,” Dr. Walensky said in her statement. “I continue to see promising signs in CDC data that we are nearing the end of this pandemic in this country; however, we all have to do our part and get vaccinated to cross the finish line.”
A version of this article was first published on WebMD.com.
“I am deeply concerned by the numbers of hospitalized adolescents and saddened to see the number of adolescents who required treatment in intensive care units or mechanical ventilation,” CDC Director Rochelle Walensky, MD, said in a statement.
While urging teenagers to wear masks and take precautions around others, she asked “parents, relatives, and close friends to join me and talk with teens about the importance of these prevention strategies and to encourage them to get vaccinated.”
Dr. Walensky referred to the CDC’s Morbidity and Mortality Weekly Report that showed adolescent hospitalizations peaked at 2.1 per 100,000 in early January 2021, then dropped to 0.6 per 100,000 in mid-March.
Alarmingly, hospitalizations rose to 1.3 per 100,000 in April, and a number of teens required serious interventions.
“Among hospitalized adolescents, nearly one-third required intensive care unit admission, and 5% required invasive mechanical ventilation,” the report said. No deaths occurred.
The study looked at 376 adolescents aged 12-17 who were hospitalized and tested positive for coronavirus. Of that group, 204 were hospitalized for COVID-19 and the other 172 were hospitalized for reasons not directly related to COVID-19.
Of the 204 hospitalized for COVID-19, 70.6% had an underlying medical condition such as obesity or chronic lung disease.
The study noted that children and teenagers have lower hospitalization rates and generally show less severe symptoms than do older people.
Possible causes for the rise in adolescent COVID-19 hospitalizations include the arrival of variants, the growing number of children returning to in-person education, and the changes in mask-wearing and other safety precautions, the study said.
The American Academy of Pediatrics said that as of May 27, 4 million children have tested positive for COVID-19 since the pandemic began, with about 34,500 new child cases reported for the week ending May 27.
The AAP said children have represented 14.1% of total cases since the pandemic began, but for the week ending May 27, children represented 24.3% of new reported weekly COVID-19 cases.
On May 10, the FDA granted emergency use authorization for the Pfizer coronavirus vaccine to be given to children aged 12-15 years. Previously, the FDA had authorized the Pfizer vaccine for people aged 16 years and up, whereas the Moderna and Johnson & Johnson vaccines are authorized for people aged 18 years and up.
“Vaccination is our way out of this pandemic,” Dr. Walensky said in her statement. “I continue to see promising signs in CDC data that we are nearing the end of this pandemic in this country; however, we all have to do our part and get vaccinated to cross the finish line.”
A version of this article was first published on WebMD.com.
DOJ charges 14 with COVID-19–related fraud nearing $150M
The U.S. Department of Justice (DOJ) on May 26 announced charges against 14 defendants across the country who allegedly engaged in health care fraud schemes that exploited the COVID-19 pandemic and resulted in over $143 million in false billings to Medicare.
Among the defendants, a DOJ news release said, were a telemedicine company executive, a physician, marketers, and medical business owners.
In addition, the Centers for Medicare and Medicaid Services separately announced that it had taken “adverse administrative actions” against more than 50 providers for their involvement in fraud schemes related to COVID-19 or the abuse of CMS programs that were designed to encourage access to medical care during the pandemic.
Several of the defendants allegedly offered COVID-19 tests to Medicare beneficiaries in senior living facilities, drive-through COVID-19 testing sites, and medical offices to induce the beneficiaries to provide their personal identifying information and a saliva or a blood sample.
The DOJ charges claim the defendants then misused the information and the samples to submit claims to Medicare for unrelated, medically unnecessary, and far more expensive lab tests, including cancer genetic testing, allergy testing, and respiratory pathogen panel tests.
In some cases, it’s alleged, the lab results were not provided to the individuals in a timely fashion or were not reliable.
Other defendants are charged with exploiting temporary changes in CMS telehealth regulations that were designed to increase access to health care during the pandemic. In these cases, which the DOJ said were the first charges related to the expansion of telehealth under the COVID-19 emergency declaration, the defendants allegedly submitted false and fraudulent claims to Medicare for sham telemedicine encounters that did not occur.
“As part of these cases, medical professionals are alleged to have [been] offered and paid bribes in exchange for the medical professionals’ referral of unnecessary testing,” the DOJ news release said. However, no physicians were identified by the department.
Commenting on this aspect of the law enforcement action, FBI Director Christopher Wray said in the release: “Medical providers have been the unsung heroes for the American public throughout the pandemic. It’s disheartening that some have abused their authorities and committed COVID-19–related fraud against trusting citizens. The FBI, along with our federal law enforcement and private sector partners, are committed to continuing to combat health care fraud and protect the American people.”
The law enforcement action includes the third set of criminal charges related to the misuse of Provider Relief Fund monies, according to the release.
More than 340 individuals were charged in September 2020 with submitting $6 billion in fraudulent claims to federal health care programs and private insurers for telehealth consultations and substance abuse treatment. About $4.5 billion of that was related to telehealth, as reported by this news organization.
The new criminal charges were brought in federal district courts in Arkansas, California, Louisiana, Florida, New Jersey, and New York.
Case summaries
The DOJ provided several case summaries. One defendant, lab owner Billy Joe Taylor of Lavaca, Ark., was charged with participating in a scheme to defraud the government of over $42 million by filing false claims that were billed in combination with COVID-19 testing claims. He also allegedly billed for tests that were not performed.
Petros Hannesyan of Burbank, Calif., the owner of a home health agency, was charged with obtaining over $229,000 from COVID-19 relief programs under false pretenses. His firm allegedly misappropriated funds from the CARES Act Provider Relief Fund and submitted false loan applications and a false loan agreement to the Economic Injury Disaster Loan Program.
Michael Stein and Leonel Palatnik of Palm Beach County, Fla., were charged in a connection with an alleged $73 million conspiracy to defraud the government and to pay and receive health care kickbacks during the pandemic.
Mr. Stein, who owned a “purported” consulting company, and Mr. Palatnik, who owned testing labs in Texas, allegedly exploited Medicare’s waiver of telehealth restrictions “by offering telehealth providers access to Medicare beneficiaries for whom they could bill consultations. In exchange, these providers agreed to refer beneficiaries to [Mr. Palatnik’s] laboratories for expensive and medically unnecessary cancer and cardiovascular genetic testing.”
A version of this article first appeared on Medscape.com.
The U.S. Department of Justice (DOJ) on May 26 announced charges against 14 defendants across the country who allegedly engaged in health care fraud schemes that exploited the COVID-19 pandemic and resulted in over $143 million in false billings to Medicare.
Among the defendants, a DOJ news release said, were a telemedicine company executive, a physician, marketers, and medical business owners.
In addition, the Centers for Medicare and Medicaid Services separately announced that it had taken “adverse administrative actions” against more than 50 providers for their involvement in fraud schemes related to COVID-19 or the abuse of CMS programs that were designed to encourage access to medical care during the pandemic.
Several of the defendants allegedly offered COVID-19 tests to Medicare beneficiaries in senior living facilities, drive-through COVID-19 testing sites, and medical offices to induce the beneficiaries to provide their personal identifying information and a saliva or a blood sample.
The DOJ charges claim the defendants then misused the information and the samples to submit claims to Medicare for unrelated, medically unnecessary, and far more expensive lab tests, including cancer genetic testing, allergy testing, and respiratory pathogen panel tests.
In some cases, it’s alleged, the lab results were not provided to the individuals in a timely fashion or were not reliable.
Other defendants are charged with exploiting temporary changes in CMS telehealth regulations that were designed to increase access to health care during the pandemic. In these cases, which the DOJ said were the first charges related to the expansion of telehealth under the COVID-19 emergency declaration, the defendants allegedly submitted false and fraudulent claims to Medicare for sham telemedicine encounters that did not occur.
“As part of these cases, medical professionals are alleged to have [been] offered and paid bribes in exchange for the medical professionals’ referral of unnecessary testing,” the DOJ news release said. However, no physicians were identified by the department.
Commenting on this aspect of the law enforcement action, FBI Director Christopher Wray said in the release: “Medical providers have been the unsung heroes for the American public throughout the pandemic. It’s disheartening that some have abused their authorities and committed COVID-19–related fraud against trusting citizens. The FBI, along with our federal law enforcement and private sector partners, are committed to continuing to combat health care fraud and protect the American people.”
The law enforcement action includes the third set of criminal charges related to the misuse of Provider Relief Fund monies, according to the release.
More than 340 individuals were charged in September 2020 with submitting $6 billion in fraudulent claims to federal health care programs and private insurers for telehealth consultations and substance abuse treatment. About $4.5 billion of that was related to telehealth, as reported by this news organization.
The new criminal charges were brought in federal district courts in Arkansas, California, Louisiana, Florida, New Jersey, and New York.
Case summaries
The DOJ provided several case summaries. One defendant, lab owner Billy Joe Taylor of Lavaca, Ark., was charged with participating in a scheme to defraud the government of over $42 million by filing false claims that were billed in combination with COVID-19 testing claims. He also allegedly billed for tests that were not performed.
Petros Hannesyan of Burbank, Calif., the owner of a home health agency, was charged with obtaining over $229,000 from COVID-19 relief programs under false pretenses. His firm allegedly misappropriated funds from the CARES Act Provider Relief Fund and submitted false loan applications and a false loan agreement to the Economic Injury Disaster Loan Program.
Michael Stein and Leonel Palatnik of Palm Beach County, Fla., were charged in a connection with an alleged $73 million conspiracy to defraud the government and to pay and receive health care kickbacks during the pandemic.
Mr. Stein, who owned a “purported” consulting company, and Mr. Palatnik, who owned testing labs in Texas, allegedly exploited Medicare’s waiver of telehealth restrictions “by offering telehealth providers access to Medicare beneficiaries for whom they could bill consultations. In exchange, these providers agreed to refer beneficiaries to [Mr. Palatnik’s] laboratories for expensive and medically unnecessary cancer and cardiovascular genetic testing.”
A version of this article first appeared on Medscape.com.
The U.S. Department of Justice (DOJ) on May 26 announced charges against 14 defendants across the country who allegedly engaged in health care fraud schemes that exploited the COVID-19 pandemic and resulted in over $143 million in false billings to Medicare.
Among the defendants, a DOJ news release said, were a telemedicine company executive, a physician, marketers, and medical business owners.
In addition, the Centers for Medicare and Medicaid Services separately announced that it had taken “adverse administrative actions” against more than 50 providers for their involvement in fraud schemes related to COVID-19 or the abuse of CMS programs that were designed to encourage access to medical care during the pandemic.
Several of the defendants allegedly offered COVID-19 tests to Medicare beneficiaries in senior living facilities, drive-through COVID-19 testing sites, and medical offices to induce the beneficiaries to provide their personal identifying information and a saliva or a blood sample.
The DOJ charges claim the defendants then misused the information and the samples to submit claims to Medicare for unrelated, medically unnecessary, and far more expensive lab tests, including cancer genetic testing, allergy testing, and respiratory pathogen panel tests.
In some cases, it’s alleged, the lab results were not provided to the individuals in a timely fashion or were not reliable.
Other defendants are charged with exploiting temporary changes in CMS telehealth regulations that were designed to increase access to health care during the pandemic. In these cases, which the DOJ said were the first charges related to the expansion of telehealth under the COVID-19 emergency declaration, the defendants allegedly submitted false and fraudulent claims to Medicare for sham telemedicine encounters that did not occur.
“As part of these cases, medical professionals are alleged to have [been] offered and paid bribes in exchange for the medical professionals’ referral of unnecessary testing,” the DOJ news release said. However, no physicians were identified by the department.
Commenting on this aspect of the law enforcement action, FBI Director Christopher Wray said in the release: “Medical providers have been the unsung heroes for the American public throughout the pandemic. It’s disheartening that some have abused their authorities and committed COVID-19–related fraud against trusting citizens. The FBI, along with our federal law enforcement and private sector partners, are committed to continuing to combat health care fraud and protect the American people.”
The law enforcement action includes the third set of criminal charges related to the misuse of Provider Relief Fund monies, according to the release.
More than 340 individuals were charged in September 2020 with submitting $6 billion in fraudulent claims to federal health care programs and private insurers for telehealth consultations and substance abuse treatment. About $4.5 billion of that was related to telehealth, as reported by this news organization.
The new criminal charges were brought in federal district courts in Arkansas, California, Louisiana, Florida, New Jersey, and New York.
Case summaries
The DOJ provided several case summaries. One defendant, lab owner Billy Joe Taylor of Lavaca, Ark., was charged with participating in a scheme to defraud the government of over $42 million by filing false claims that were billed in combination with COVID-19 testing claims. He also allegedly billed for tests that were not performed.
Petros Hannesyan of Burbank, Calif., the owner of a home health agency, was charged with obtaining over $229,000 from COVID-19 relief programs under false pretenses. His firm allegedly misappropriated funds from the CARES Act Provider Relief Fund and submitted false loan applications and a false loan agreement to the Economic Injury Disaster Loan Program.
Michael Stein and Leonel Palatnik of Palm Beach County, Fla., were charged in a connection with an alleged $73 million conspiracy to defraud the government and to pay and receive health care kickbacks during the pandemic.
Mr. Stein, who owned a “purported” consulting company, and Mr. Palatnik, who owned testing labs in Texas, allegedly exploited Medicare’s waiver of telehealth restrictions “by offering telehealth providers access to Medicare beneficiaries for whom they could bill consultations. In exchange, these providers agreed to refer beneficiaries to [Mr. Palatnik’s] laboratories for expensive and medically unnecessary cancer and cardiovascular genetic testing.”
A version of this article first appeared on Medscape.com.
What brought me back from the brink of suicide: A physician’s story
William Lynes, MD, had a flourishing medical practice and a fulfilling family life with three children when he first attempted suicide in 1999 at age 45. By 2003, depression and two more suicide attempts led to his early retirement.
In a session at the recent virtual American Psychiatric Association (APA) 2021 annual meeting, Dr. Lynes talked about the challenges of dealing with depression while managing the stresses of a career in medicine. The session in which he spoke was called, “The Suicidal Physician: Narratives From a Physician Who Survived and the Physician Widow of One Who Did Not.”
By writing and speaking about his experiences, he says, he has been able to retain his identity as a physician and avoid obsessive thoughts about suicide. He hopes conversations like these help other physicians feel less alone and enable them to push past stigmas to get the help they need. He suspects they do. More than 600 people joined the APA session, and Dr. Lynes received dozens of thankful messages afterward.
“I love medicine, but intrinsically, the practice of medicine is stressful, and you can’t get away,” said Dr. Lynes, a retired urologist in Temecula, Calif. “As far as feedback, it made me feel like it’s something I should continue to do.”
A way to heal
For Dr. Lynes, his “downward spiral into darkness” began with a series of catastrophic medical events starting in 1998, when he came home from a family vacation in Mexico feeling unwell. He didn’t bother to do anything about it – typical of a physician, he says. Then one night he woke up shaking with chills and fever. Soon he was in the hospital with respiratory failure from septic shock.
Dr. Lynes spent 6 weeks in the intensive care unit, including 4 weeks on a ventilator. He underwent a tracheostomy. He lost 40 pounds and experienced ICU-related delirium. It was a terrifying time, he said. When he tried to return to work 10 months later, he didn’t feel as though he could function normally.
Having once been a driven doctor who worked long hours, he now doubted himself and dreaded giving patients bad news. Spontaneously, he tried to take his own life.
Afterward, he concealed what had happened from everyone except his wife and managed to resume his practice. However, he was unable to regain the enthusiasm he had once had for his work. Although he had experienced depression before, this time it was unrelenting.
He sought help from a psychiatrist, received a diagnosis of bipolar disorder, and began taking medication. Still, he struggled to fulfill his responsibilities. Then in April 2002, he had a snowboarding accident that caused multiple facial fractures and required five operations. When he returned to work this time, he felt like a failure but resisted asking colleagues for help.
A few months later, Dr. Lynes again attempted suicide, which led to another stay in the ICU and more time on a ventilator. Doctors told his family they didn’t think he would survive. When he recovered, he spent time as an inpatient in a psychiatric ward, where he received the first of a series of electroconvulsive therapy sessions. Compounding his anxiety and depression was the inability to come to terms with his life if he were not able to practice medicine.
The next fall, in September 2003, his third suicide attempt took place in his office on a weekend when no one was around. After locking the door, he looked at his reflection in the frame of his medical school diploma. The glass was cracked. “It was dark, it was black, it was cold,” he said. “I can remember seeing my reflection and thinking how disgusted I was.”
For years after that, Dr. Lynes struggled with his sense of self-worth. He hid from the medical system and dreaded doctors’ appointments. Finally in 2016, he found new meaning at a writing conference, where he met a fellow physician whose story was similar to his. She encouraged him to write about his experience. His essay was published in Annals of Internal Medicine that year. “Then I started speaking, and I feel like I’m a physician again,” he said. “That has really healed me quite a bit.”
Why physicians die by suicide
Working in health care can be extremely stressful, even in the best of times, said Michael Myers, MD, a psychiatrist at State University of New York, Brooklyn, and author of the book, “Why Physicians Die By Suicide: Lessons Learned From Their Families and Others Who Cared.”
Years of school and training culminate in a career in which demands are relentless. Societal expectations are high. Many doctors are perfectionists by nature, and physicians tend to feel intense pressure to compete for coveted positions.
Stress starts early in a medical career. A 2016 systematic review and meta-analysis of 183 studies from 43 countries showed that nearly 30% of medical students experienced symptoms of depression and that 11% reported suicidal thoughts, but only 15% sought help.
A 2015 review of 31 studies that involved residents showed that rates of depression remained close to 30% and that about three-quarters of trainees meet criteria for burnout, a type of emotional exhaustion and sense of inadequacy that can result from chronic stress at work.
The stress of medical training appears to be a direct cause of mental health struggles. Rates of depression are higher among those working to become physicians than among their peers of the same age, research shows. In addition, symptoms become more prevalent as people progress through their training.
The COVID-19 pandemic has added stress to an already stressful job. Of more than 2,300 physicians surveyed in August 2020 by the Physicians Foundation, a physicians advocacy organization, 50% indicated that they experienced excessive anger, tearfulness, or anxiety because of the way the pandemic affected their work; 30% felt hopeless or lacking purpose; and 8% had thoughts of self-harm related to the pandemic. Rates of burnout had risen from 40% in 2018 to 58%.
Those problems might be even more acute in places experiencing other types of crises. A 2020 study of 154 emergency department (ED) physicians in Libya, which is in the midst of a civil war, found that 65% were experiencing anxiety, 73% were showing signs of depression, and 68% felt emotionally exhausted.
Every story is different
It is unclear how common suicide is among physicians. One often-repeated estimate is that 300-400 physicians die by suicide each year, but no one is certain how that number was determined, said Dr. Myers, who organized the APA panel.
Studies on suicide are inconsistent, and trends are hard to pinpoint. Anecdotally, he has received just as many calls about physician suicides in the past year as he did before the pandemic started.
Every person is different, and so is every death. Sometimes, career problems have nothing to do with a physician’s suicide, Dr. Myers said. When job stress does play a role, factors are often varied and complex.
After a 35-year career as a double board certified ED physician, Matthew Seaman, MD, retired in January 2017. The same month, a patient filed a complaint against him with the Washington State medical board, which led to an investigation and a lawsuit.
The case was hard on Dr. Seaman, who had continued to work night shifts throughout his career and had won a Hero Award from the American Board of Emergency Medicine, said his wife, Linda Seaman, MD, a family practitioner in Yakima, Wash., who also spoke on the APA panel.
Dr. Seaman said that 2 years after the investigation started, her husband was growing increasingly depressed. In 2019, he testified in a deposition. She said the plaintiff’s attorney “tried every way he could to shame Matt, humiliate Matt, make him believe he was a very bad doctor.” Three days later, he died by suicide at age 62.
Looking back at the year leading up to her husband’s death, Dr. Seaman recognizes multiple obstacles that interfered with her husband’s ability to get help, including frustrating interactions with psychiatrists and the couple’s insurance company.
His identity and experience as a physician also played a role. A couple of months before he died, she tried unsuccessfully to reach his psychiatrist, whose office suggested he go to the ED. However, because he worked as an ED doctor in their small town, he wouldn’t go. Dr. Seaman suspects he was wary of the stigma.
Burnout likely set him up to cave in after decades of work on the front lines, she added. Working in the ED exposes providers to horrific, traumatic cases every day, she said. Physicians learn to suppress their own emotions to deal with what they encounter. Stuffing their feelings can lead to posttraumatic stress. “You just perform,” she said. “You learn to do that.”
A real gift
Whenever Dr. Myers hears stories about doctors who died by suicide or who have written about their mental health struggles to help others, he contacts them. One goal of his own writing and of the conference sessions he organizes is to make it easier for others to share their own stories.
“I tell them, first of all, their courage and honesty is a real gift, and they’re saving lives,” he said. “There are so many suffering doctors out there who think that they’re the only one.”
Public conversations such as those that occurred in the APA session also offer opportunities to share advice, including Dr. Myers’ recommendation that doctors be sure they have a primary care physician of their own.
Many don’t, he says, because they say they are too busy, they can treat their own symptoms, or they can self-refer to specialists when needed. But physicians don’t always recognize symptoms of depression in themselves, and when mental health problems arise, they may not seek help or treat themselves appropriately.
A primary care physician can be the first person to recognize a mental health problem and refer a patient for mental health care, said Dr. Myers, whose latest book, “Becoming a Doctors’ Doctor: A Memoir,” explores his experiences treating doctors with burnout and other mental health problems.
Whether they have a primary care doctor or not, he suggests that physicians talk to anyone they trust – a social worker, a religious leader, or a family member who can then help them find the right sort of care.
In the United States, around-the-clock help is available through the National Suicide Prevention Lifeline at 800-273-8255. A psychiatrist-run hotline specifically for physicians is available at 888-409-0141. “Reach out and get some help,” Dr. Myers said. “Just don’t do it alone.”
Dr. Lynes advocates setting boundaries between life and work. He has also benefited from writing about his experiences. A blog or a diary can help physicians process their feelings, he said. His 2016 essay marked a major turning point in his life, giving his life meaning in helping others.
“Since I wrote that article, I can’t tell you how much better I am,” he said. “Now, I’m not embarrassed to be around physicians. I actually consider myself a physician. I didn’t for many, many years. So, I’m doing pretty well.”
A version of this article first appeared on Medscape.com.
William Lynes, MD, had a flourishing medical practice and a fulfilling family life with three children when he first attempted suicide in 1999 at age 45. By 2003, depression and two more suicide attempts led to his early retirement.
In a session at the recent virtual American Psychiatric Association (APA) 2021 annual meeting, Dr. Lynes talked about the challenges of dealing with depression while managing the stresses of a career in medicine. The session in which he spoke was called, “The Suicidal Physician: Narratives From a Physician Who Survived and the Physician Widow of One Who Did Not.”
By writing and speaking about his experiences, he says, he has been able to retain his identity as a physician and avoid obsessive thoughts about suicide. He hopes conversations like these help other physicians feel less alone and enable them to push past stigmas to get the help they need. He suspects they do. More than 600 people joined the APA session, and Dr. Lynes received dozens of thankful messages afterward.
“I love medicine, but intrinsically, the practice of medicine is stressful, and you can’t get away,” said Dr. Lynes, a retired urologist in Temecula, Calif. “As far as feedback, it made me feel like it’s something I should continue to do.”
A way to heal
For Dr. Lynes, his “downward spiral into darkness” began with a series of catastrophic medical events starting in 1998, when he came home from a family vacation in Mexico feeling unwell. He didn’t bother to do anything about it – typical of a physician, he says. Then one night he woke up shaking with chills and fever. Soon he was in the hospital with respiratory failure from septic shock.
Dr. Lynes spent 6 weeks in the intensive care unit, including 4 weeks on a ventilator. He underwent a tracheostomy. He lost 40 pounds and experienced ICU-related delirium. It was a terrifying time, he said. When he tried to return to work 10 months later, he didn’t feel as though he could function normally.
Having once been a driven doctor who worked long hours, he now doubted himself and dreaded giving patients bad news. Spontaneously, he tried to take his own life.
Afterward, he concealed what had happened from everyone except his wife and managed to resume his practice. However, he was unable to regain the enthusiasm he had once had for his work. Although he had experienced depression before, this time it was unrelenting.
He sought help from a psychiatrist, received a diagnosis of bipolar disorder, and began taking medication. Still, he struggled to fulfill his responsibilities. Then in April 2002, he had a snowboarding accident that caused multiple facial fractures and required five operations. When he returned to work this time, he felt like a failure but resisted asking colleagues for help.
A few months later, Dr. Lynes again attempted suicide, which led to another stay in the ICU and more time on a ventilator. Doctors told his family they didn’t think he would survive. When he recovered, he spent time as an inpatient in a psychiatric ward, where he received the first of a series of electroconvulsive therapy sessions. Compounding his anxiety and depression was the inability to come to terms with his life if he were not able to practice medicine.
The next fall, in September 2003, his third suicide attempt took place in his office on a weekend when no one was around. After locking the door, he looked at his reflection in the frame of his medical school diploma. The glass was cracked. “It was dark, it was black, it was cold,” he said. “I can remember seeing my reflection and thinking how disgusted I was.”
For years after that, Dr. Lynes struggled with his sense of self-worth. He hid from the medical system and dreaded doctors’ appointments. Finally in 2016, he found new meaning at a writing conference, where he met a fellow physician whose story was similar to his. She encouraged him to write about his experience. His essay was published in Annals of Internal Medicine that year. “Then I started speaking, and I feel like I’m a physician again,” he said. “That has really healed me quite a bit.”
Why physicians die by suicide
Working in health care can be extremely stressful, even in the best of times, said Michael Myers, MD, a psychiatrist at State University of New York, Brooklyn, and author of the book, “Why Physicians Die By Suicide: Lessons Learned From Their Families and Others Who Cared.”
Years of school and training culminate in a career in which demands are relentless. Societal expectations are high. Many doctors are perfectionists by nature, and physicians tend to feel intense pressure to compete for coveted positions.
Stress starts early in a medical career. A 2016 systematic review and meta-analysis of 183 studies from 43 countries showed that nearly 30% of medical students experienced symptoms of depression and that 11% reported suicidal thoughts, but only 15% sought help.
A 2015 review of 31 studies that involved residents showed that rates of depression remained close to 30% and that about three-quarters of trainees meet criteria for burnout, a type of emotional exhaustion and sense of inadequacy that can result from chronic stress at work.
The stress of medical training appears to be a direct cause of mental health struggles. Rates of depression are higher among those working to become physicians than among their peers of the same age, research shows. In addition, symptoms become more prevalent as people progress through their training.
The COVID-19 pandemic has added stress to an already stressful job. Of more than 2,300 physicians surveyed in August 2020 by the Physicians Foundation, a physicians advocacy organization, 50% indicated that they experienced excessive anger, tearfulness, or anxiety because of the way the pandemic affected their work; 30% felt hopeless or lacking purpose; and 8% had thoughts of self-harm related to the pandemic. Rates of burnout had risen from 40% in 2018 to 58%.
Those problems might be even more acute in places experiencing other types of crises. A 2020 study of 154 emergency department (ED) physicians in Libya, which is in the midst of a civil war, found that 65% were experiencing anxiety, 73% were showing signs of depression, and 68% felt emotionally exhausted.
Every story is different
It is unclear how common suicide is among physicians. One often-repeated estimate is that 300-400 physicians die by suicide each year, but no one is certain how that number was determined, said Dr. Myers, who organized the APA panel.
Studies on suicide are inconsistent, and trends are hard to pinpoint. Anecdotally, he has received just as many calls about physician suicides in the past year as he did before the pandemic started.
Every person is different, and so is every death. Sometimes, career problems have nothing to do with a physician’s suicide, Dr. Myers said. When job stress does play a role, factors are often varied and complex.
After a 35-year career as a double board certified ED physician, Matthew Seaman, MD, retired in January 2017. The same month, a patient filed a complaint against him with the Washington State medical board, which led to an investigation and a lawsuit.
The case was hard on Dr. Seaman, who had continued to work night shifts throughout his career and had won a Hero Award from the American Board of Emergency Medicine, said his wife, Linda Seaman, MD, a family practitioner in Yakima, Wash., who also spoke on the APA panel.
Dr. Seaman said that 2 years after the investigation started, her husband was growing increasingly depressed. In 2019, he testified in a deposition. She said the plaintiff’s attorney “tried every way he could to shame Matt, humiliate Matt, make him believe he was a very bad doctor.” Three days later, he died by suicide at age 62.
Looking back at the year leading up to her husband’s death, Dr. Seaman recognizes multiple obstacles that interfered with her husband’s ability to get help, including frustrating interactions with psychiatrists and the couple’s insurance company.
His identity and experience as a physician also played a role. A couple of months before he died, she tried unsuccessfully to reach his psychiatrist, whose office suggested he go to the ED. However, because he worked as an ED doctor in their small town, he wouldn’t go. Dr. Seaman suspects he was wary of the stigma.
Burnout likely set him up to cave in after decades of work on the front lines, she added. Working in the ED exposes providers to horrific, traumatic cases every day, she said. Physicians learn to suppress their own emotions to deal with what they encounter. Stuffing their feelings can lead to posttraumatic stress. “You just perform,” she said. “You learn to do that.”
A real gift
Whenever Dr. Myers hears stories about doctors who died by suicide or who have written about their mental health struggles to help others, he contacts them. One goal of his own writing and of the conference sessions he organizes is to make it easier for others to share their own stories.
“I tell them, first of all, their courage and honesty is a real gift, and they’re saving lives,” he said. “There are so many suffering doctors out there who think that they’re the only one.”
Public conversations such as those that occurred in the APA session also offer opportunities to share advice, including Dr. Myers’ recommendation that doctors be sure they have a primary care physician of their own.
Many don’t, he says, because they say they are too busy, they can treat their own symptoms, or they can self-refer to specialists when needed. But physicians don’t always recognize symptoms of depression in themselves, and when mental health problems arise, they may not seek help or treat themselves appropriately.
A primary care physician can be the first person to recognize a mental health problem and refer a patient for mental health care, said Dr. Myers, whose latest book, “Becoming a Doctors’ Doctor: A Memoir,” explores his experiences treating doctors with burnout and other mental health problems.
Whether they have a primary care doctor or not, he suggests that physicians talk to anyone they trust – a social worker, a religious leader, or a family member who can then help them find the right sort of care.
In the United States, around-the-clock help is available through the National Suicide Prevention Lifeline at 800-273-8255. A psychiatrist-run hotline specifically for physicians is available at 888-409-0141. “Reach out and get some help,” Dr. Myers said. “Just don’t do it alone.”
Dr. Lynes advocates setting boundaries between life and work. He has also benefited from writing about his experiences. A blog or a diary can help physicians process their feelings, he said. His 2016 essay marked a major turning point in his life, giving his life meaning in helping others.
“Since I wrote that article, I can’t tell you how much better I am,” he said. “Now, I’m not embarrassed to be around physicians. I actually consider myself a physician. I didn’t for many, many years. So, I’m doing pretty well.”
A version of this article first appeared on Medscape.com.
William Lynes, MD, had a flourishing medical practice and a fulfilling family life with three children when he first attempted suicide in 1999 at age 45. By 2003, depression and two more suicide attempts led to his early retirement.
In a session at the recent virtual American Psychiatric Association (APA) 2021 annual meeting, Dr. Lynes talked about the challenges of dealing with depression while managing the stresses of a career in medicine. The session in which he spoke was called, “The Suicidal Physician: Narratives From a Physician Who Survived and the Physician Widow of One Who Did Not.”
By writing and speaking about his experiences, he says, he has been able to retain his identity as a physician and avoid obsessive thoughts about suicide. He hopes conversations like these help other physicians feel less alone and enable them to push past stigmas to get the help they need. He suspects they do. More than 600 people joined the APA session, and Dr. Lynes received dozens of thankful messages afterward.
“I love medicine, but intrinsically, the practice of medicine is stressful, and you can’t get away,” said Dr. Lynes, a retired urologist in Temecula, Calif. “As far as feedback, it made me feel like it’s something I should continue to do.”
A way to heal
For Dr. Lynes, his “downward spiral into darkness” began with a series of catastrophic medical events starting in 1998, when he came home from a family vacation in Mexico feeling unwell. He didn’t bother to do anything about it – typical of a physician, he says. Then one night he woke up shaking with chills and fever. Soon he was in the hospital with respiratory failure from septic shock.
Dr. Lynes spent 6 weeks in the intensive care unit, including 4 weeks on a ventilator. He underwent a tracheostomy. He lost 40 pounds and experienced ICU-related delirium. It was a terrifying time, he said. When he tried to return to work 10 months later, he didn’t feel as though he could function normally.
Having once been a driven doctor who worked long hours, he now doubted himself and dreaded giving patients bad news. Spontaneously, he tried to take his own life.
Afterward, he concealed what had happened from everyone except his wife and managed to resume his practice. However, he was unable to regain the enthusiasm he had once had for his work. Although he had experienced depression before, this time it was unrelenting.
He sought help from a psychiatrist, received a diagnosis of bipolar disorder, and began taking medication. Still, he struggled to fulfill his responsibilities. Then in April 2002, he had a snowboarding accident that caused multiple facial fractures and required five operations. When he returned to work this time, he felt like a failure but resisted asking colleagues for help.
A few months later, Dr. Lynes again attempted suicide, which led to another stay in the ICU and more time on a ventilator. Doctors told his family they didn’t think he would survive. When he recovered, he spent time as an inpatient in a psychiatric ward, where he received the first of a series of electroconvulsive therapy sessions. Compounding his anxiety and depression was the inability to come to terms with his life if he were not able to practice medicine.
The next fall, in September 2003, his third suicide attempt took place in his office on a weekend when no one was around. After locking the door, he looked at his reflection in the frame of his medical school diploma. The glass was cracked. “It was dark, it was black, it was cold,” he said. “I can remember seeing my reflection and thinking how disgusted I was.”
For years after that, Dr. Lynes struggled with his sense of self-worth. He hid from the medical system and dreaded doctors’ appointments. Finally in 2016, he found new meaning at a writing conference, where he met a fellow physician whose story was similar to his. She encouraged him to write about his experience. His essay was published in Annals of Internal Medicine that year. “Then I started speaking, and I feel like I’m a physician again,” he said. “That has really healed me quite a bit.”
Why physicians die by suicide
Working in health care can be extremely stressful, even in the best of times, said Michael Myers, MD, a psychiatrist at State University of New York, Brooklyn, and author of the book, “Why Physicians Die By Suicide: Lessons Learned From Their Families and Others Who Cared.”
Years of school and training culminate in a career in which demands are relentless. Societal expectations are high. Many doctors are perfectionists by nature, and physicians tend to feel intense pressure to compete for coveted positions.
Stress starts early in a medical career. A 2016 systematic review and meta-analysis of 183 studies from 43 countries showed that nearly 30% of medical students experienced symptoms of depression and that 11% reported suicidal thoughts, but only 15% sought help.
A 2015 review of 31 studies that involved residents showed that rates of depression remained close to 30% and that about three-quarters of trainees meet criteria for burnout, a type of emotional exhaustion and sense of inadequacy that can result from chronic stress at work.
The stress of medical training appears to be a direct cause of mental health struggles. Rates of depression are higher among those working to become physicians than among their peers of the same age, research shows. In addition, symptoms become more prevalent as people progress through their training.
The COVID-19 pandemic has added stress to an already stressful job. Of more than 2,300 physicians surveyed in August 2020 by the Physicians Foundation, a physicians advocacy organization, 50% indicated that they experienced excessive anger, tearfulness, or anxiety because of the way the pandemic affected their work; 30% felt hopeless or lacking purpose; and 8% had thoughts of self-harm related to the pandemic. Rates of burnout had risen from 40% in 2018 to 58%.
Those problems might be even more acute in places experiencing other types of crises. A 2020 study of 154 emergency department (ED) physicians in Libya, which is in the midst of a civil war, found that 65% were experiencing anxiety, 73% were showing signs of depression, and 68% felt emotionally exhausted.
Every story is different
It is unclear how common suicide is among physicians. One often-repeated estimate is that 300-400 physicians die by suicide each year, but no one is certain how that number was determined, said Dr. Myers, who organized the APA panel.
Studies on suicide are inconsistent, and trends are hard to pinpoint. Anecdotally, he has received just as many calls about physician suicides in the past year as he did before the pandemic started.
Every person is different, and so is every death. Sometimes, career problems have nothing to do with a physician’s suicide, Dr. Myers said. When job stress does play a role, factors are often varied and complex.
After a 35-year career as a double board certified ED physician, Matthew Seaman, MD, retired in January 2017. The same month, a patient filed a complaint against him with the Washington State medical board, which led to an investigation and a lawsuit.
The case was hard on Dr. Seaman, who had continued to work night shifts throughout his career and had won a Hero Award from the American Board of Emergency Medicine, said his wife, Linda Seaman, MD, a family practitioner in Yakima, Wash., who also spoke on the APA panel.
Dr. Seaman said that 2 years after the investigation started, her husband was growing increasingly depressed. In 2019, he testified in a deposition. She said the plaintiff’s attorney “tried every way he could to shame Matt, humiliate Matt, make him believe he was a very bad doctor.” Three days later, he died by suicide at age 62.
Looking back at the year leading up to her husband’s death, Dr. Seaman recognizes multiple obstacles that interfered with her husband’s ability to get help, including frustrating interactions with psychiatrists and the couple’s insurance company.
His identity and experience as a physician also played a role. A couple of months before he died, she tried unsuccessfully to reach his psychiatrist, whose office suggested he go to the ED. However, because he worked as an ED doctor in their small town, he wouldn’t go. Dr. Seaman suspects he was wary of the stigma.
Burnout likely set him up to cave in after decades of work on the front lines, she added. Working in the ED exposes providers to horrific, traumatic cases every day, she said. Physicians learn to suppress their own emotions to deal with what they encounter. Stuffing their feelings can lead to posttraumatic stress. “You just perform,” she said. “You learn to do that.”
A real gift
Whenever Dr. Myers hears stories about doctors who died by suicide or who have written about their mental health struggles to help others, he contacts them. One goal of his own writing and of the conference sessions he organizes is to make it easier for others to share their own stories.
“I tell them, first of all, their courage and honesty is a real gift, and they’re saving lives,” he said. “There are so many suffering doctors out there who think that they’re the only one.”
Public conversations such as those that occurred in the APA session also offer opportunities to share advice, including Dr. Myers’ recommendation that doctors be sure they have a primary care physician of their own.
Many don’t, he says, because they say they are too busy, they can treat their own symptoms, or they can self-refer to specialists when needed. But physicians don’t always recognize symptoms of depression in themselves, and when mental health problems arise, they may not seek help or treat themselves appropriately.
A primary care physician can be the first person to recognize a mental health problem and refer a patient for mental health care, said Dr. Myers, whose latest book, “Becoming a Doctors’ Doctor: A Memoir,” explores his experiences treating doctors with burnout and other mental health problems.
Whether they have a primary care doctor or not, he suggests that physicians talk to anyone they trust – a social worker, a religious leader, or a family member who can then help them find the right sort of care.
In the United States, around-the-clock help is available through the National Suicide Prevention Lifeline at 800-273-8255. A psychiatrist-run hotline specifically for physicians is available at 888-409-0141. “Reach out and get some help,” Dr. Myers said. “Just don’t do it alone.”
Dr. Lynes advocates setting boundaries between life and work. He has also benefited from writing about his experiences. A blog or a diary can help physicians process their feelings, he said. His 2016 essay marked a major turning point in his life, giving his life meaning in helping others.
“Since I wrote that article, I can’t tell you how much better I am,” he said. “Now, I’m not embarrassed to be around physicians. I actually consider myself a physician. I didn’t for many, many years. So, I’m doing pretty well.”
A version of this article first appeared on Medscape.com.
Two studies add to knowledge base of biosimilar use in psoriasis, HS
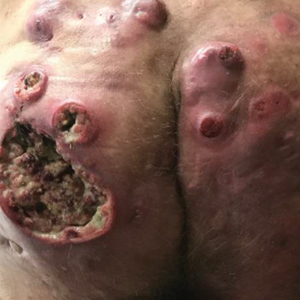
And another study, a small, single-center retrospective study of patients with hidradenitis suppurativa (HS), found that administration of infliximab and biosimilar infliximab were associated with similar and significant improvement in disease.
Both studies were published online in April in JAMA Dermatology and add to mounting evidence that biosimilars may be interchangeable in certain dermatologic conditions.
“Biosimilars are an exciting innovation in the field,” Joseph Zahn, MD, assistant professor of dermatology at George Washington University, Washington, said in an interview. “Their efficacy and price point will allow patients greater access to effective treatment.” To date, biosimilars approved in the United States that could be prescribed by dermatologists include those for rituximab, etanercept, adalimumab, and infliximab.
In the trial from Denmark, Nikolai Loft, MD, of the University of Copenhagen and colleagues evaluated outcomes following a mandatory medical switch from the brand name adalimumab, referred to as adalimumab originator, to adalimumab biosimilars among 726 individuals who were enrolled in a Danish nationwide registry of patients treated with biologics since 2007. The primary outcome was 1-year drug retention in patients switching to adalimumab biosimilars compared with patients treated with adalimumab originator.
The study population consisted of 348 patients with at least 2 years of exposure to adalimumab who had switched from originator to adalimumab biosimilars (a mean age of 52 and 72% male) and 378 patients who served as the adalimumab cohort (a mean age of 51 and 71% male). When the researchers compared the 1-year drug retention rates between the adalimumab biosimilar cohort and the adalimumab originator cohort, the rates were similar (92% vs. 92.1%, respectively).
The hazard ratios for other outcomes were similar as well. Specifically, the crude hazard ratios were 1.02 (P = .94) for all causes of drug discontinuation, 0.82 (P = .60) for insufficient effect, and 1.41 (P = .50) for adverse events (AEs) in the adalimumab biosimilar cohort, compared with the adalimumab originator cohort.
“Overall, results for any AEs were contradicting, but certain AEs were more prevalent in the adalimumab biosimilar cohort,” the authors wrote. Dermatologic AEs and AEs in the “other” category “were more prevalent, which could be attributable to more patients experiencing injection site reactions as a result of larger volumes and differences in excipients and syringes in the adalimumab biosimilars and the adalimumab originator.” Other potential explanations they offered were the nocebo effect and greater awareness of AEs among practitioners and patients.
“This study concludes that, when switched to a biosimilar medication, patients do not have worse control of their psoriasis nor do they switch to other medications,” Dr. Zahn, who was asked to comment about these results, said in the interview. “However, there was a trend toward a higher number of side effects in the biosimilar group. The main takeaway point from this study is that biosimilars of adalimumab seem to be relatively interchangeable in patients with psoriasis without loss of efficacy or significant increase in side effects that lead to a medication change for the patient.”
The researchers acknowledged certain limitations of their study, including the fact that it was limited to Danish patients and that individual AEs could not be examined. “Moreover, the surveillance of AEs is not as vigilant as in clinical trials, and AEs are most likely underreported,” they wrote. “Although no major differences were found when switching from adalimumab originator to adalimumab biosimilar versions, it was not possible to assess the performance of individual adalimumab biosimilar versions in this study.”
In the second study, Christopher Sayed, MD, associate professor of dermatology, University of North Carolina, Chapel Hill, and colleagues retrospectively evaluated the effectiveness of infliximab-abda versus infliximab administration in the treatment of 34 patients with HS who were cared for at the university’s dermatology clinic. Patients were treated with either agent for at least 10 weeks. The infliximab treatment group consisted of 20 patients with a mean age of 42 years who were mostly female (17; 85%), while the infliximab-abda treatment group included 14 patients with a mean age of 36 years who also were mostly female (13; 93%).
Both groups received loading doses of 10 mg/kg at weeks 0, 2, and 6, and treatment was continued with a maintenance dose administered every 4-8 weeks. The patients were followed between February 2016 and June 2020 and the primary outcome measure was Hidradenitis Suppurative Clinical Response (HiSCR), which was defined as at least a 50% decrease in inflammatory nodule count without any increase in the number of abscesses or draining sinuses.
The researchers found that 71% of patients in the infliximab-abda treatment group achieved a HiSCR, compared with 60% of their counterparts in the infliximab treatment group, a difference that did not reach statistical significance (P = .47). Three patients in the infliximab treatment group experienced AEs, compared with none in the infliximab-abda treatment group.
“The data are promising,” Dr. Zahn said. “Although this is a small study with a limited number of patients, it suggests that this particular biosimilar may be a reasonable or possibly even equivalent alternative to infliximab. A larger, prospective trial will be needed before we can be sure the results are equivalent.”
Dr. Sayed and colleagues noted certain limitations of their study, including the retrospective design and the use of concomitant medications by some participants. “There is also a risk of selection bias because copay and medication assistance programs are not available for infliximab-abda for patients with HS,” they wrote.
In an editorial accompanying the two studies, Mark Lebwohl, MD, professor of dermatology, Icahn School of Medicine at Mount Sinai, New York, wrote that the introduction of biosimilars have been justified by “the hope that lower costs” will increase availability of treatments to patients with moderate to severe psoriasis. “Inroads in the U.S. market, however, have been limited,” he added, and there is concern that they “may be used to prevent access to newer interleukin-17 blockers and interleukin-23 blockers for which biosimilars are available and that do not carry the boxed warnings found on tumor necrosis factor blockers.”
Dr. Loft reported receiving personal fees from Eli Lilly and Janssen outside of the submitted work. Many of his coauthors reporting having numerous financial conflicts of interest with the pharmaceutical industry. The HS study was supported by a public health service research award from the National Institutes of Health. Dr. Sayed reported receiving personal fees or personal fees paid to the institution from AbbVie, Novartis, Chemocentryx, GlaxoSmithKline, Incyte, InflaRx, and UCB. No other disclosures were reported. Dr. Lebwohl disclosed receiving research funds from companies including AbbVie, Amgen, Boehringer Ingelheim, Eli Lilly, and Incyte; and receiving personal fees from multiple companies, outside of the submitted work. Dr. Zahn reported having no disclosures.
And another study, a small, single-center retrospective study of patients with hidradenitis suppurativa (HS), found that administration of infliximab and biosimilar infliximab were associated with similar and significant improvement in disease.
Both studies were published online in April in JAMA Dermatology and add to mounting evidence that biosimilars may be interchangeable in certain dermatologic conditions.
“Biosimilars are an exciting innovation in the field,” Joseph Zahn, MD, assistant professor of dermatology at George Washington University, Washington, said in an interview. “Their efficacy and price point will allow patients greater access to effective treatment.” To date, biosimilars approved in the United States that could be prescribed by dermatologists include those for rituximab, etanercept, adalimumab, and infliximab.
In the trial from Denmark, Nikolai Loft, MD, of the University of Copenhagen and colleagues evaluated outcomes following a mandatory medical switch from the brand name adalimumab, referred to as adalimumab originator, to adalimumab biosimilars among 726 individuals who were enrolled in a Danish nationwide registry of patients treated with biologics since 2007. The primary outcome was 1-year drug retention in patients switching to adalimumab biosimilars compared with patients treated with adalimumab originator.
The study population consisted of 348 patients with at least 2 years of exposure to adalimumab who had switched from originator to adalimumab biosimilars (a mean age of 52 and 72% male) and 378 patients who served as the adalimumab cohort (a mean age of 51 and 71% male). When the researchers compared the 1-year drug retention rates between the adalimumab biosimilar cohort and the adalimumab originator cohort, the rates were similar (92% vs. 92.1%, respectively).
The hazard ratios for other outcomes were similar as well. Specifically, the crude hazard ratios were 1.02 (P = .94) for all causes of drug discontinuation, 0.82 (P = .60) for insufficient effect, and 1.41 (P = .50) for adverse events (AEs) in the adalimumab biosimilar cohort, compared with the adalimumab originator cohort.
“Overall, results for any AEs were contradicting, but certain AEs were more prevalent in the adalimumab biosimilar cohort,” the authors wrote. Dermatologic AEs and AEs in the “other” category “were more prevalent, which could be attributable to more patients experiencing injection site reactions as a result of larger volumes and differences in excipients and syringes in the adalimumab biosimilars and the adalimumab originator.” Other potential explanations they offered were the nocebo effect and greater awareness of AEs among practitioners and patients.
“This study concludes that, when switched to a biosimilar medication, patients do not have worse control of their psoriasis nor do they switch to other medications,” Dr. Zahn, who was asked to comment about these results, said in the interview. “However, there was a trend toward a higher number of side effects in the biosimilar group. The main takeaway point from this study is that biosimilars of adalimumab seem to be relatively interchangeable in patients with psoriasis without loss of efficacy or significant increase in side effects that lead to a medication change for the patient.”
The researchers acknowledged certain limitations of their study, including the fact that it was limited to Danish patients and that individual AEs could not be examined. “Moreover, the surveillance of AEs is not as vigilant as in clinical trials, and AEs are most likely underreported,” they wrote. “Although no major differences were found when switching from adalimumab originator to adalimumab biosimilar versions, it was not possible to assess the performance of individual adalimumab biosimilar versions in this study.”
In the second study, Christopher Sayed, MD, associate professor of dermatology, University of North Carolina, Chapel Hill, and colleagues retrospectively evaluated the effectiveness of infliximab-abda versus infliximab administration in the treatment of 34 patients with HS who were cared for at the university’s dermatology clinic. Patients were treated with either agent for at least 10 weeks. The infliximab treatment group consisted of 20 patients with a mean age of 42 years who were mostly female (17; 85%), while the infliximab-abda treatment group included 14 patients with a mean age of 36 years who also were mostly female (13; 93%).
Both groups received loading doses of 10 mg/kg at weeks 0, 2, and 6, and treatment was continued with a maintenance dose administered every 4-8 weeks. The patients were followed between February 2016 and June 2020 and the primary outcome measure was Hidradenitis Suppurative Clinical Response (HiSCR), which was defined as at least a 50% decrease in inflammatory nodule count without any increase in the number of abscesses or draining sinuses.
The researchers found that 71% of patients in the infliximab-abda treatment group achieved a HiSCR, compared with 60% of their counterparts in the infliximab treatment group, a difference that did not reach statistical significance (P = .47). Three patients in the infliximab treatment group experienced AEs, compared with none in the infliximab-abda treatment group.
“The data are promising,” Dr. Zahn said. “Although this is a small study with a limited number of patients, it suggests that this particular biosimilar may be a reasonable or possibly even equivalent alternative to infliximab. A larger, prospective trial will be needed before we can be sure the results are equivalent.”
Dr. Sayed and colleagues noted certain limitations of their study, including the retrospective design and the use of concomitant medications by some participants. “There is also a risk of selection bias because copay and medication assistance programs are not available for infliximab-abda for patients with HS,” they wrote.
In an editorial accompanying the two studies, Mark Lebwohl, MD, professor of dermatology, Icahn School of Medicine at Mount Sinai, New York, wrote that the introduction of biosimilars have been justified by “the hope that lower costs” will increase availability of treatments to patients with moderate to severe psoriasis. “Inroads in the U.S. market, however, have been limited,” he added, and there is concern that they “may be used to prevent access to newer interleukin-17 blockers and interleukin-23 blockers for which biosimilars are available and that do not carry the boxed warnings found on tumor necrosis factor blockers.”
Dr. Loft reported receiving personal fees from Eli Lilly and Janssen outside of the submitted work. Many of his coauthors reporting having numerous financial conflicts of interest with the pharmaceutical industry. The HS study was supported by a public health service research award from the National Institutes of Health. Dr. Sayed reported receiving personal fees or personal fees paid to the institution from AbbVie, Novartis, Chemocentryx, GlaxoSmithKline, Incyte, InflaRx, and UCB. No other disclosures were reported. Dr. Lebwohl disclosed receiving research funds from companies including AbbVie, Amgen, Boehringer Ingelheim, Eli Lilly, and Incyte; and receiving personal fees from multiple companies, outside of the submitted work. Dr. Zahn reported having no disclosures.
And another study, a small, single-center retrospective study of patients with hidradenitis suppurativa (HS), found that administration of infliximab and biosimilar infliximab were associated with similar and significant improvement in disease.
Both studies were published online in April in JAMA Dermatology and add to mounting evidence that biosimilars may be interchangeable in certain dermatologic conditions.
“Biosimilars are an exciting innovation in the field,” Joseph Zahn, MD, assistant professor of dermatology at George Washington University, Washington, said in an interview. “Their efficacy and price point will allow patients greater access to effective treatment.” To date, biosimilars approved in the United States that could be prescribed by dermatologists include those for rituximab, etanercept, adalimumab, and infliximab.
In the trial from Denmark, Nikolai Loft, MD, of the University of Copenhagen and colleagues evaluated outcomes following a mandatory medical switch from the brand name adalimumab, referred to as adalimumab originator, to adalimumab biosimilars among 726 individuals who were enrolled in a Danish nationwide registry of patients treated with biologics since 2007. The primary outcome was 1-year drug retention in patients switching to adalimumab biosimilars compared with patients treated with adalimumab originator.
The study population consisted of 348 patients with at least 2 years of exposure to adalimumab who had switched from originator to adalimumab biosimilars (a mean age of 52 and 72% male) and 378 patients who served as the adalimumab cohort (a mean age of 51 and 71% male). When the researchers compared the 1-year drug retention rates between the adalimumab biosimilar cohort and the adalimumab originator cohort, the rates were similar (92% vs. 92.1%, respectively).
The hazard ratios for other outcomes were similar as well. Specifically, the crude hazard ratios were 1.02 (P = .94) for all causes of drug discontinuation, 0.82 (P = .60) for insufficient effect, and 1.41 (P = .50) for adverse events (AEs) in the adalimumab biosimilar cohort, compared with the adalimumab originator cohort.
“Overall, results for any AEs were contradicting, but certain AEs were more prevalent in the adalimumab biosimilar cohort,” the authors wrote. Dermatologic AEs and AEs in the “other” category “were more prevalent, which could be attributable to more patients experiencing injection site reactions as a result of larger volumes and differences in excipients and syringes in the adalimumab biosimilars and the adalimumab originator.” Other potential explanations they offered were the nocebo effect and greater awareness of AEs among practitioners and patients.
“This study concludes that, when switched to a biosimilar medication, patients do not have worse control of their psoriasis nor do they switch to other medications,” Dr. Zahn, who was asked to comment about these results, said in the interview. “However, there was a trend toward a higher number of side effects in the biosimilar group. The main takeaway point from this study is that biosimilars of adalimumab seem to be relatively interchangeable in patients with psoriasis without loss of efficacy or significant increase in side effects that lead to a medication change for the patient.”
The researchers acknowledged certain limitations of their study, including the fact that it was limited to Danish patients and that individual AEs could not be examined. “Moreover, the surveillance of AEs is not as vigilant as in clinical trials, and AEs are most likely underreported,” they wrote. “Although no major differences were found when switching from adalimumab originator to adalimumab biosimilar versions, it was not possible to assess the performance of individual adalimumab biosimilar versions in this study.”
In the second study, Christopher Sayed, MD, associate professor of dermatology, University of North Carolina, Chapel Hill, and colleagues retrospectively evaluated the effectiveness of infliximab-abda versus infliximab administration in the treatment of 34 patients with HS who were cared for at the university’s dermatology clinic. Patients were treated with either agent for at least 10 weeks. The infliximab treatment group consisted of 20 patients with a mean age of 42 years who were mostly female (17; 85%), while the infliximab-abda treatment group included 14 patients with a mean age of 36 years who also were mostly female (13; 93%).
Both groups received loading doses of 10 mg/kg at weeks 0, 2, and 6, and treatment was continued with a maintenance dose administered every 4-8 weeks. The patients were followed between February 2016 and June 2020 and the primary outcome measure was Hidradenitis Suppurative Clinical Response (HiSCR), which was defined as at least a 50% decrease in inflammatory nodule count without any increase in the number of abscesses or draining sinuses.
The researchers found that 71% of patients in the infliximab-abda treatment group achieved a HiSCR, compared with 60% of their counterparts in the infliximab treatment group, a difference that did not reach statistical significance (P = .47). Three patients in the infliximab treatment group experienced AEs, compared with none in the infliximab-abda treatment group.
“The data are promising,” Dr. Zahn said. “Although this is a small study with a limited number of patients, it suggests that this particular biosimilar may be a reasonable or possibly even equivalent alternative to infliximab. A larger, prospective trial will be needed before we can be sure the results are equivalent.”
Dr. Sayed and colleagues noted certain limitations of their study, including the retrospective design and the use of concomitant medications by some participants. “There is also a risk of selection bias because copay and medication assistance programs are not available for infliximab-abda for patients with HS,” they wrote.
In an editorial accompanying the two studies, Mark Lebwohl, MD, professor of dermatology, Icahn School of Medicine at Mount Sinai, New York, wrote that the introduction of biosimilars have been justified by “the hope that lower costs” will increase availability of treatments to patients with moderate to severe psoriasis. “Inroads in the U.S. market, however, have been limited,” he added, and there is concern that they “may be used to prevent access to newer interleukin-17 blockers and interleukin-23 blockers for which biosimilars are available and that do not carry the boxed warnings found on tumor necrosis factor blockers.”
Dr. Loft reported receiving personal fees from Eli Lilly and Janssen outside of the submitted work. Many of his coauthors reporting having numerous financial conflicts of interest with the pharmaceutical industry. The HS study was supported by a public health service research award from the National Institutes of Health. Dr. Sayed reported receiving personal fees or personal fees paid to the institution from AbbVie, Novartis, Chemocentryx, GlaxoSmithKline, Incyte, InflaRx, and UCB. No other disclosures were reported. Dr. Lebwohl disclosed receiving research funds from companies including AbbVie, Amgen, Boehringer Ingelheim, Eli Lilly, and Incyte; and receiving personal fees from multiple companies, outside of the submitted work. Dr. Zahn reported having no disclosures.
FROM JAMA DERMATOLOGY
Percentage of doctors who are Black barely changed in 120 years
according to a new study.
In 1900, 1.3% of physicians were Black. In 1940, 2.8% of physicians were Black, and by 2018 – when almost 13% of the population was Black – 5.4% of doctors were Black, reports Dan Ly, MD, PhD, MPP, an assistant professor of medicine at the University of California, Los Angeles, in a study published online April 19, 2021, in the Journal of General Internal Medicine.
The proportion of male Black physicians was 2.7% in 1940 and 2.6% in 2018.
Dr. Ly also found a significant wage gap. The median income earned by White doctors was $50,000 more than the median income of Black physicians in 2018. Dr. Ly based his findings on the U.S. Census Decennial Census long form, accessed via IPUMS, a free database funded by the National Institutes of Health and other organizations.
“If we care about the health of the population, particularly the health of Black patients, we should care about how small the proportion of our physicians who are Black is and the extremely slow progress we have made as a medical system in increasing that proportion,” Dr. Ly said in an interview.
Dr. Ly said he took on this research in part because previous studies have shown that Black patients are more likely to seek preventive care from Black doctors. Thus, increasing the numbers of Black physicians could narrow gaps in life expectancy between Whites and Blacks.
He also wanted to see whether progress had been made as a result of various medical organizations and the Association of American Medical Colleges undertaking initiatives to increase workforce diversity. There has been “very, very little” progress, he said.
Norma Poll-Hunter, PhD, the AAMC’s senior director of workforce diversity, said Dr. Ly’s report “was not surprising at all.”
The AAMC reported in 2014 that the number of Black men who apply to and matriculate into medical schools has been declining since 1978. That year, there were 1,410 Black male applicants and 542 Black enrollees. In 2014, there were 1,337 applicants and 515 enrollees.
Since 2014, Black male enrollment has increased slightly, rising from 2.4% in the 2014-2015 school year to 2.9% in the 2019-2020 year, the AAMC reported last year.
In addition, among other historically underrepresented minorities, “we really have seen very small progress” despite the increase in the number of medical schools, Dr. Poll-Hunter said in an interview.
The AAMC and the National Medical Association consider the lack of Black male applicants and matriculants to be a national crisis. The two groups started an alliance in 2020 aimed at finding ways to amplify and support Black men’s interest in medicine and the biomedical sciences and to “develop systems-based solutions to address exclusionary practices that create barriers for Black men and prevent them from having equitable opportunities to successfully enroll in medical school.”
Solutions include requiring medical school admissions committees and application screeners to undergo implicit bias awareness and mitigation training, adopting holistic admissions reviews, and incentivizing institutions of higher learning to partner with Black communities in urban and rural school systems to establish K-12 health sciences academies, said NMA President Leon McDougle, MD, MPH.
“There are the systems factors, and racism is a big one that we have to tackle,” said Dr. Poll-Hunter.
Diversity isn’t just about numbers, said Dr. McDougle, a professor of family medicine and associate dean for diversity and inclusion at Ohio State University, Columbus. “We know that medical school graduates who are African American or Black, Hispanic or Latinx, or American Indian or Alaskan Native are more likely to serve those communities as practicing physicians.
“The COVID-19 pandemic highlighted the urgent need for more African American or Black, Hispanic or Latinx, or American Indian or Alaskan Native physicians,” he said. “Inadequate access to culturally competent care has exacerbated existing health disparities, resulting in death and hospitalization rates up to three to four times the rates of European American or White people.”
Dr. Poll-Hunter also said that studies have shown that diversity in the classroom creates a more enriched learning environment and increases civic mindedness and cognitive complexity, “as well as helps us understand people who are different than ourselves.”
The diversity goal “is not about quotas, it’s about excellence,” she said. “We know that there’s talent that exists, and we want to make sure that everyone has an opportunity to be successful.”
Dr. Ly has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a new study.
In 1900, 1.3% of physicians were Black. In 1940, 2.8% of physicians were Black, and by 2018 – when almost 13% of the population was Black – 5.4% of doctors were Black, reports Dan Ly, MD, PhD, MPP, an assistant professor of medicine at the University of California, Los Angeles, in a study published online April 19, 2021, in the Journal of General Internal Medicine.
The proportion of male Black physicians was 2.7% in 1940 and 2.6% in 2018.
Dr. Ly also found a significant wage gap. The median income earned by White doctors was $50,000 more than the median income of Black physicians in 2018. Dr. Ly based his findings on the U.S. Census Decennial Census long form, accessed via IPUMS, a free database funded by the National Institutes of Health and other organizations.
“If we care about the health of the population, particularly the health of Black patients, we should care about how small the proportion of our physicians who are Black is and the extremely slow progress we have made as a medical system in increasing that proportion,” Dr. Ly said in an interview.
Dr. Ly said he took on this research in part because previous studies have shown that Black patients are more likely to seek preventive care from Black doctors. Thus, increasing the numbers of Black physicians could narrow gaps in life expectancy between Whites and Blacks.
He also wanted to see whether progress had been made as a result of various medical organizations and the Association of American Medical Colleges undertaking initiatives to increase workforce diversity. There has been “very, very little” progress, he said.
Norma Poll-Hunter, PhD, the AAMC’s senior director of workforce diversity, said Dr. Ly’s report “was not surprising at all.”
The AAMC reported in 2014 that the number of Black men who apply to and matriculate into medical schools has been declining since 1978. That year, there were 1,410 Black male applicants and 542 Black enrollees. In 2014, there were 1,337 applicants and 515 enrollees.
Since 2014, Black male enrollment has increased slightly, rising from 2.4% in the 2014-2015 school year to 2.9% in the 2019-2020 year, the AAMC reported last year.
In addition, among other historically underrepresented minorities, “we really have seen very small progress” despite the increase in the number of medical schools, Dr. Poll-Hunter said in an interview.
The AAMC and the National Medical Association consider the lack of Black male applicants and matriculants to be a national crisis. The two groups started an alliance in 2020 aimed at finding ways to amplify and support Black men’s interest in medicine and the biomedical sciences and to “develop systems-based solutions to address exclusionary practices that create barriers for Black men and prevent them from having equitable opportunities to successfully enroll in medical school.”
Solutions include requiring medical school admissions committees and application screeners to undergo implicit bias awareness and mitigation training, adopting holistic admissions reviews, and incentivizing institutions of higher learning to partner with Black communities in urban and rural school systems to establish K-12 health sciences academies, said NMA President Leon McDougle, MD, MPH.
“There are the systems factors, and racism is a big one that we have to tackle,” said Dr. Poll-Hunter.
Diversity isn’t just about numbers, said Dr. McDougle, a professor of family medicine and associate dean for diversity and inclusion at Ohio State University, Columbus. “We know that medical school graduates who are African American or Black, Hispanic or Latinx, or American Indian or Alaskan Native are more likely to serve those communities as practicing physicians.
“The COVID-19 pandemic highlighted the urgent need for more African American or Black, Hispanic or Latinx, or American Indian or Alaskan Native physicians,” he said. “Inadequate access to culturally competent care has exacerbated existing health disparities, resulting in death and hospitalization rates up to three to four times the rates of European American or White people.”
Dr. Poll-Hunter also said that studies have shown that diversity in the classroom creates a more enriched learning environment and increases civic mindedness and cognitive complexity, “as well as helps us understand people who are different than ourselves.”
The diversity goal “is not about quotas, it’s about excellence,” she said. “We know that there’s talent that exists, and we want to make sure that everyone has an opportunity to be successful.”
Dr. Ly has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a new study.
In 1900, 1.3% of physicians were Black. In 1940, 2.8% of physicians were Black, and by 2018 – when almost 13% of the population was Black – 5.4% of doctors were Black, reports Dan Ly, MD, PhD, MPP, an assistant professor of medicine at the University of California, Los Angeles, in a study published online April 19, 2021, in the Journal of General Internal Medicine.
The proportion of male Black physicians was 2.7% in 1940 and 2.6% in 2018.
Dr. Ly also found a significant wage gap. The median income earned by White doctors was $50,000 more than the median income of Black physicians in 2018. Dr. Ly based his findings on the U.S. Census Decennial Census long form, accessed via IPUMS, a free database funded by the National Institutes of Health and other organizations.
“If we care about the health of the population, particularly the health of Black patients, we should care about how small the proportion of our physicians who are Black is and the extremely slow progress we have made as a medical system in increasing that proportion,” Dr. Ly said in an interview.
Dr. Ly said he took on this research in part because previous studies have shown that Black patients are more likely to seek preventive care from Black doctors. Thus, increasing the numbers of Black physicians could narrow gaps in life expectancy between Whites and Blacks.
He also wanted to see whether progress had been made as a result of various medical organizations and the Association of American Medical Colleges undertaking initiatives to increase workforce diversity. There has been “very, very little” progress, he said.
Norma Poll-Hunter, PhD, the AAMC’s senior director of workforce diversity, said Dr. Ly’s report “was not surprising at all.”
The AAMC reported in 2014 that the number of Black men who apply to and matriculate into medical schools has been declining since 1978. That year, there were 1,410 Black male applicants and 542 Black enrollees. In 2014, there were 1,337 applicants and 515 enrollees.
Since 2014, Black male enrollment has increased slightly, rising from 2.4% in the 2014-2015 school year to 2.9% in the 2019-2020 year, the AAMC reported last year.
In addition, among other historically underrepresented minorities, “we really have seen very small progress” despite the increase in the number of medical schools, Dr. Poll-Hunter said in an interview.
The AAMC and the National Medical Association consider the lack of Black male applicants and matriculants to be a national crisis. The two groups started an alliance in 2020 aimed at finding ways to amplify and support Black men’s interest in medicine and the biomedical sciences and to “develop systems-based solutions to address exclusionary practices that create barriers for Black men and prevent them from having equitable opportunities to successfully enroll in medical school.”
Solutions include requiring medical school admissions committees and application screeners to undergo implicit bias awareness and mitigation training, adopting holistic admissions reviews, and incentivizing institutions of higher learning to partner with Black communities in urban and rural school systems to establish K-12 health sciences academies, said NMA President Leon McDougle, MD, MPH.
“There are the systems factors, and racism is a big one that we have to tackle,” said Dr. Poll-Hunter.
Diversity isn’t just about numbers, said Dr. McDougle, a professor of family medicine and associate dean for diversity and inclusion at Ohio State University, Columbus. “We know that medical school graduates who are African American or Black, Hispanic or Latinx, or American Indian or Alaskan Native are more likely to serve those communities as practicing physicians.
“The COVID-19 pandemic highlighted the urgent need for more African American or Black, Hispanic or Latinx, or American Indian or Alaskan Native physicians,” he said. “Inadequate access to culturally competent care has exacerbated existing health disparities, resulting in death and hospitalization rates up to three to four times the rates of European American or White people.”
Dr. Poll-Hunter also said that studies have shown that diversity in the classroom creates a more enriched learning environment and increases civic mindedness and cognitive complexity, “as well as helps us understand people who are different than ourselves.”
The diversity goal “is not about quotas, it’s about excellence,” she said. “We know that there’s talent that exists, and we want to make sure that everyone has an opportunity to be successful.”
Dr. Ly has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Remote cardio visits expand access for underserved during COVID
Remote cardiology clinic visits during COVID-19 were used more often by certain traditionally underserved patient groups, but were also associated with less frequent testing and prescribing, new research shows.
“The COVID-19 pandemic has led to an unprecedented shift in ambulatory cardiovascular care from in-person to remote visits,” lead author Neal Yuan, MD, a cardiology fellow at the Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, said in an interview.
Their findings were published online April 5 in JAMA Network Open.
“We wanted to explore whether the transition to remote visits was associated with disparities in how patients accessed care, and also how this transition affected diagnostic test ordering and medication prescribing,” Dr. Yuan said.
The researchers used electronic health records data for all ambulatory cardiology visits at an urban, multisite health system in Los Angeles County during two periods: April 1 to Dec. 31, 2019, the pre-COVID era; and April 1 to Dec. 31, 2020, the COVID era.
The investigators compared patient characteristics and frequencies of medication ordering and cardiology-specific testing across four visit types: pre-COVID in person, used as reference; COVID-era in person; COVID-era video; and COVID-era telephone.
The study looked at 176,781 ambulatory cardiology visits. Of these visits, 87,182 were conducted in person in the pre-COVID period; 74,498 were conducted in person in the COVID era; 4,720 were COVID-era video visits; and 10,381 were COVID-era telephone visits.
In the study cohort, 79,572 patients (45.0%) were female, 127,080 patients (71.9%) were non-Hispanic White, and the mean age was 68.1 years (standard deviation, 17.0).
Patients accessing COVID-era remote visits were more likely to be Asian, Black, or Hispanic, to have private insurance, and to have cardiovascular comorbidities, such as hypertension and heart failure.
Also, patients whose visits were conducted by video were significantly younger than patients whose visits were conducted in person or by telephone (P < .001).
In addition, the study found that clinicians ordered fewer diagnostic tests, such as electrocardiograms and echocardiograms, and were less likely to order any medication, in the pre-COVID era than during the COVID era.
“If you don’t have a patient in front of you, it’s much more difficult to get a physical exam or obtain reliable vital signs,” said Dr. Yuan. Communication can sometimes be difficult, often because of technical issues, like a bad connection. “You might be more reticent to get testing or to prescribe medications if you don’t feel confident knowing what the patient’s vital signs are.”
In addition, he added, “a lot of medications used in the cardiology setting require monitoring patients’ kidney function and electrolytes, and if you can’t do that reliably, you might be more cautious about prescribing those types of medications.”
An eye-opening study
Cardiologist Nieca Goldberg, MD, medical director of the New York University Langone womens’ heart program and spokesperson for the American Heart Association, recounted her experience with telemedicine at the height of the pandemic in New York, when everything, including medical outpatient offices, had to close.
“We were experienced with telemedicine because we had started a virtual urgent care program well ahead of the pandemic,” she said. “We started using that to screen people with potential COVID symptoms so that they wouldn’t have to come into the hospital, the medical center, or to the offices and expose people. We learned that it was great to have the telemedicine option from the infectious disease standpoint, and I did visits like that for my own patient population.”
An equally if not more important finding from the study is the fact that telemedicine increased access to care among traditionally underserved demographics, she said.
“This is eye-opening, that you can actually improve access to care by doing telemedicine visits. It was really important to see that telemedicine has added benefit to the way we can see people in the health care system.”
Telemedicine visits had a positive impact at a time when people were isolated at home, Dr. Goldberg said.
“It was a way for them to connect with their doctor and in some ways it was more personal,” she added. “I actually got to meet some of my patients’ family members. It was like making a remote house call.”
Stable cardiology patients can take their blood pressure at home, weigh themselves, and take their own pulse to give an excellent set of vital signs that will indicate how they are doing, said Dr. Goldberg.
“During a remote visit, we can talk to the patient and notice whether or not they are short of breath or coughing, but we can’t listen to their heart or do an EKG or any of the traditional cardiac testing. Still, for someone who is not having symptoms and is able to reliably monitor their blood pressure and weight, a remote visit is sufficient to give you a good sense of how that patient is doing,” she said. “We can talk to them about their medications, any potential side effects, and we can use their blood pressure information to adjust their medications.”
Many patients are becoming more savvy about using tech gadgets and devices to monitor their health.
“Some of my patients were using Apple watches and the Kardia app to address their heart rate. Many had purchased inexpensive pulse oximeters to check their oxygen during the pandemic, and that also reads the pulse,” Dr. Goldberg said.
In-person visits were reserved for symptomatic cardiac patients, she explained.
“Initially during the pandemic, we did mostly telemedicine visits and we organized the office so that each cardiologist would come in 1 day a week to take care of symptomatic cardiac patients. In that way, we were able to socially distance – they provided us with [personal protective equipment]; at NYU there was no problem with that – and nobody waited in the waiting room. To this day, office issues are more efficient and people are not waiting in the waiting room,” she added. “Telemedicine improves access to health care in populations where such access is limited.”
Dr. Yuan’s research is supported by a grant from the National Institutes of Health. Dr. Goldberg reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Remote cardiology clinic visits during COVID-19 were used more often by certain traditionally underserved patient groups, but were also associated with less frequent testing and prescribing, new research shows.
“The COVID-19 pandemic has led to an unprecedented shift in ambulatory cardiovascular care from in-person to remote visits,” lead author Neal Yuan, MD, a cardiology fellow at the Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, said in an interview.
Their findings were published online April 5 in JAMA Network Open.
“We wanted to explore whether the transition to remote visits was associated with disparities in how patients accessed care, and also how this transition affected diagnostic test ordering and medication prescribing,” Dr. Yuan said.
The researchers used electronic health records data for all ambulatory cardiology visits at an urban, multisite health system in Los Angeles County during two periods: April 1 to Dec. 31, 2019, the pre-COVID era; and April 1 to Dec. 31, 2020, the COVID era.
The investigators compared patient characteristics and frequencies of medication ordering and cardiology-specific testing across four visit types: pre-COVID in person, used as reference; COVID-era in person; COVID-era video; and COVID-era telephone.
The study looked at 176,781 ambulatory cardiology visits. Of these visits, 87,182 were conducted in person in the pre-COVID period; 74,498 were conducted in person in the COVID era; 4,720 were COVID-era video visits; and 10,381 were COVID-era telephone visits.
In the study cohort, 79,572 patients (45.0%) were female, 127,080 patients (71.9%) were non-Hispanic White, and the mean age was 68.1 years (standard deviation, 17.0).
Patients accessing COVID-era remote visits were more likely to be Asian, Black, or Hispanic, to have private insurance, and to have cardiovascular comorbidities, such as hypertension and heart failure.
Also, patients whose visits were conducted by video were significantly younger than patients whose visits were conducted in person or by telephone (P < .001).
In addition, the study found that clinicians ordered fewer diagnostic tests, such as electrocardiograms and echocardiograms, and were less likely to order any medication, in the pre-COVID era than during the COVID era.
“If you don’t have a patient in front of you, it’s much more difficult to get a physical exam or obtain reliable vital signs,” said Dr. Yuan. Communication can sometimes be difficult, often because of technical issues, like a bad connection. “You might be more reticent to get testing or to prescribe medications if you don’t feel confident knowing what the patient’s vital signs are.”
In addition, he added, “a lot of medications used in the cardiology setting require monitoring patients’ kidney function and electrolytes, and if you can’t do that reliably, you might be more cautious about prescribing those types of medications.”
An eye-opening study
Cardiologist Nieca Goldberg, MD, medical director of the New York University Langone womens’ heart program and spokesperson for the American Heart Association, recounted her experience with telemedicine at the height of the pandemic in New York, when everything, including medical outpatient offices, had to close.
“We were experienced with telemedicine because we had started a virtual urgent care program well ahead of the pandemic,” she said. “We started using that to screen people with potential COVID symptoms so that they wouldn’t have to come into the hospital, the medical center, or to the offices and expose people. We learned that it was great to have the telemedicine option from the infectious disease standpoint, and I did visits like that for my own patient population.”
An equally if not more important finding from the study is the fact that telemedicine increased access to care among traditionally underserved demographics, she said.
“This is eye-opening, that you can actually improve access to care by doing telemedicine visits. It was really important to see that telemedicine has added benefit to the way we can see people in the health care system.”
Telemedicine visits had a positive impact at a time when people were isolated at home, Dr. Goldberg said.
“It was a way for them to connect with their doctor and in some ways it was more personal,” she added. “I actually got to meet some of my patients’ family members. It was like making a remote house call.”
Stable cardiology patients can take their blood pressure at home, weigh themselves, and take their own pulse to give an excellent set of vital signs that will indicate how they are doing, said Dr. Goldberg.
“During a remote visit, we can talk to the patient and notice whether or not they are short of breath or coughing, but we can’t listen to their heart or do an EKG or any of the traditional cardiac testing. Still, for someone who is not having symptoms and is able to reliably monitor their blood pressure and weight, a remote visit is sufficient to give you a good sense of how that patient is doing,” she said. “We can talk to them about their medications, any potential side effects, and we can use their blood pressure information to adjust their medications.”
Many patients are becoming more savvy about using tech gadgets and devices to monitor their health.
“Some of my patients were using Apple watches and the Kardia app to address their heart rate. Many had purchased inexpensive pulse oximeters to check their oxygen during the pandemic, and that also reads the pulse,” Dr. Goldberg said.
In-person visits were reserved for symptomatic cardiac patients, she explained.
“Initially during the pandemic, we did mostly telemedicine visits and we organized the office so that each cardiologist would come in 1 day a week to take care of symptomatic cardiac patients. In that way, we were able to socially distance – they provided us with [personal protective equipment]; at NYU there was no problem with that – and nobody waited in the waiting room. To this day, office issues are more efficient and people are not waiting in the waiting room,” she added. “Telemedicine improves access to health care in populations where such access is limited.”
Dr. Yuan’s research is supported by a grant from the National Institutes of Health. Dr. Goldberg reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Remote cardiology clinic visits during COVID-19 were used more often by certain traditionally underserved patient groups, but were also associated with less frequent testing and prescribing, new research shows.
“The COVID-19 pandemic has led to an unprecedented shift in ambulatory cardiovascular care from in-person to remote visits,” lead author Neal Yuan, MD, a cardiology fellow at the Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, said in an interview.
Their findings were published online April 5 in JAMA Network Open.
“We wanted to explore whether the transition to remote visits was associated with disparities in how patients accessed care, and also how this transition affected diagnostic test ordering and medication prescribing,” Dr. Yuan said.
The researchers used electronic health records data for all ambulatory cardiology visits at an urban, multisite health system in Los Angeles County during two periods: April 1 to Dec. 31, 2019, the pre-COVID era; and April 1 to Dec. 31, 2020, the COVID era.
The investigators compared patient characteristics and frequencies of medication ordering and cardiology-specific testing across four visit types: pre-COVID in person, used as reference; COVID-era in person; COVID-era video; and COVID-era telephone.
The study looked at 176,781 ambulatory cardiology visits. Of these visits, 87,182 were conducted in person in the pre-COVID period; 74,498 were conducted in person in the COVID era; 4,720 were COVID-era video visits; and 10,381 were COVID-era telephone visits.
In the study cohort, 79,572 patients (45.0%) were female, 127,080 patients (71.9%) were non-Hispanic White, and the mean age was 68.1 years (standard deviation, 17.0).
Patients accessing COVID-era remote visits were more likely to be Asian, Black, or Hispanic, to have private insurance, and to have cardiovascular comorbidities, such as hypertension and heart failure.
Also, patients whose visits were conducted by video were significantly younger than patients whose visits were conducted in person or by telephone (P < .001).
In addition, the study found that clinicians ordered fewer diagnostic tests, such as electrocardiograms and echocardiograms, and were less likely to order any medication, in the pre-COVID era than during the COVID era.
“If you don’t have a patient in front of you, it’s much more difficult to get a physical exam or obtain reliable vital signs,” said Dr. Yuan. Communication can sometimes be difficult, often because of technical issues, like a bad connection. “You might be more reticent to get testing or to prescribe medications if you don’t feel confident knowing what the patient’s vital signs are.”
In addition, he added, “a lot of medications used in the cardiology setting require monitoring patients’ kidney function and electrolytes, and if you can’t do that reliably, you might be more cautious about prescribing those types of medications.”
An eye-opening study
Cardiologist Nieca Goldberg, MD, medical director of the New York University Langone womens’ heart program and spokesperson for the American Heart Association, recounted her experience with telemedicine at the height of the pandemic in New York, when everything, including medical outpatient offices, had to close.
“We were experienced with telemedicine because we had started a virtual urgent care program well ahead of the pandemic,” she said. “We started using that to screen people with potential COVID symptoms so that they wouldn’t have to come into the hospital, the medical center, or to the offices and expose people. We learned that it was great to have the telemedicine option from the infectious disease standpoint, and I did visits like that for my own patient population.”
An equally if not more important finding from the study is the fact that telemedicine increased access to care among traditionally underserved demographics, she said.
“This is eye-opening, that you can actually improve access to care by doing telemedicine visits. It was really important to see that telemedicine has added benefit to the way we can see people in the health care system.”
Telemedicine visits had a positive impact at a time when people were isolated at home, Dr. Goldberg said.
“It was a way for them to connect with their doctor and in some ways it was more personal,” she added. “I actually got to meet some of my patients’ family members. It was like making a remote house call.”
Stable cardiology patients can take their blood pressure at home, weigh themselves, and take their own pulse to give an excellent set of vital signs that will indicate how they are doing, said Dr. Goldberg.
“During a remote visit, we can talk to the patient and notice whether or not they are short of breath or coughing, but we can’t listen to their heart or do an EKG or any of the traditional cardiac testing. Still, for someone who is not having symptoms and is able to reliably monitor their blood pressure and weight, a remote visit is sufficient to give you a good sense of how that patient is doing,” she said. “We can talk to them about their medications, any potential side effects, and we can use their blood pressure information to adjust their medications.”
Many patients are becoming more savvy about using tech gadgets and devices to monitor their health.
“Some of my patients were using Apple watches and the Kardia app to address their heart rate. Many had purchased inexpensive pulse oximeters to check their oxygen during the pandemic, and that also reads the pulse,” Dr. Goldberg said.
In-person visits were reserved for symptomatic cardiac patients, she explained.
“Initially during the pandemic, we did mostly telemedicine visits and we organized the office so that each cardiologist would come in 1 day a week to take care of symptomatic cardiac patients. In that way, we were able to socially distance – they provided us with [personal protective equipment]; at NYU there was no problem with that – and nobody waited in the waiting room. To this day, office issues are more efficient and people are not waiting in the waiting room,” she added. “Telemedicine improves access to health care in populations where such access is limited.”
Dr. Yuan’s research is supported by a grant from the National Institutes of Health. Dr. Goldberg reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Squamous Cell Carcinoma in Hidradenitis Suppurativa Lesions Following Tumor Necrosis Factor α Inhibitors
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition with high morbidity rates. Symptoms typically develop between puberty and the third decade of life, affecting twice as many females as males, with an overall disease prevalence of 1% to 4%.1 The pathogenesis is theorized to be related to an immune response to follicular occlusion and rupture in genetically susceptible individuals.
Among the complications associated with HS, the development of cutaneous squamous cell carcinoma (SCC) is 4.6-times more likely within HS lesions than in normal skin and typically is seen in the setting of long-standing disease, particularly in men with HS lesions located on the buttocks and genital region for more than 20 years.2 In 2015, the tumor necrosis factor (TNF) inhibitor adalimumab was approved by the US Food and Drug Administration for the treatment of HS. Tumor necrosis factor α inhibitors have been associated with an increased risk for skin cancer in other clinical settings.3,4 We present a case of locally advanced SCC that developed in a patient with HS who was treated with adalimumab and infliximab (both TNF-α inhibitors), ultimately leading to the patient’s death.
A 59-year-old man who smoked with a 40-year history of severe HS, who previously was lost to follow-up, presented to our dermatology clinic with lesions on the buttocks. Physical examination demonstrated confluent, indurated, boggy plaques; scattered sinus tracts with purulent drainage; scattered cystlike nodules; and tenderness to palpation consistent with Hurley stage III disease (Figure 1A). No involvement of the axillae or groin was noted. He was started on doxycycline and a prednisone taper with minimal improvement and subsequently was switched to adalimumab 3 months later. Adalimumab provided little relief and was discontinued; therapy was transitioned to infliximab 3 months later.
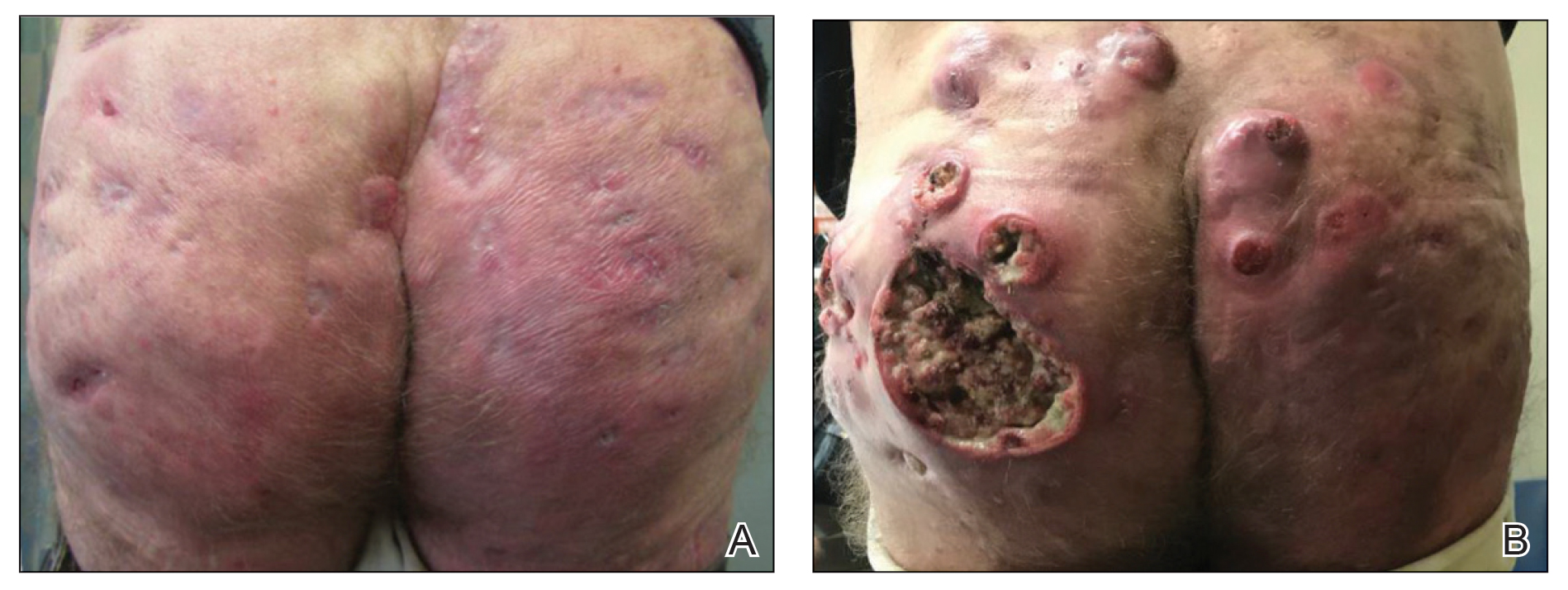
The patient returned to our clinic 3 months later with a severe flare and intractable pain after 4 infusions of infliximab. Physical examination showed a 7×5-cm deep malodorous ulcer with fibrinous exudate on the left buttock, several 2- to 3-cm shallow ulcers draining yellow exudate, and numerous fluctuant subcutaneous nodules on a background of scarring and sinus tracts. He was started again on doxycycline and a prednisone taper. At follow-up 2 weeks later, the largest ulcer had increased to 8 cm, and more indurated and tender subcutaneous nodules and scattered ulcerations developed (Figure 1B). Two punch biopsies of the left buttock revealed an invasive keratinizing carcinoma with no connection to the epidermis, consistent with SCC (Figure 2). Human papillomavirus (HPV) test results with probes for 37 HPV types—13 that were high risk (HPV-16, −18, −31, −33, −35, −39, −45, −51, −52, −56, −58, −59, −68)—were negative. Computerized tomography demonstrated diffuse thickening of the skin on the buttocks, inguinal adenopathy suspicious for nodal metastases, and no evidence of distant metastatic disease. Given the extent of the disease, surgical treatment was not an option, and he began receiving palliative radiotherapy. However, his health declined, and he developed aspiration pneumonia and hypotension requiring pressor support. He was transitioned to hospice care and died 3 months after presentation.
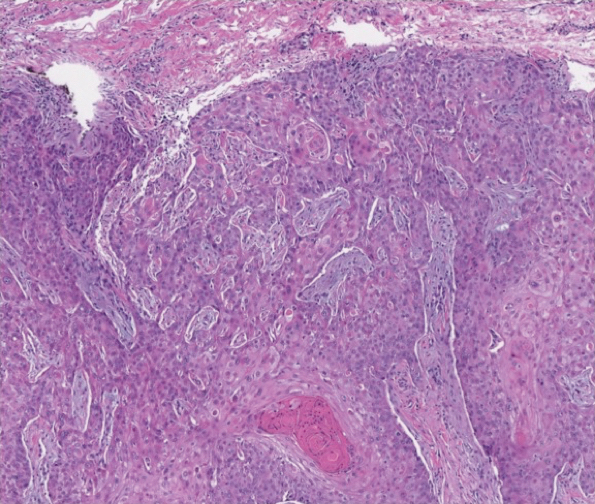
Tumor necrosis factor α antagonist treatment is being increasingly used to control HS but also may increase the risk for SCC development. We performed a search of PubMed articles indexed for MEDLINE as well as Web of Science using the terms hidradenitis suppurativa or acne inversa and one of the following—tumor necrosis factor inhibitor, infliximab, adalimumab, or etanercept—and squamous cell carcinoma or Marjolin ulcer. Seven cases of SCC arising in an HS patient treated with a TNF-α inhibitor have been reported (Table).5-10 Four cases were associated with infliximab use, 2 with adalimumab, and our case occurred after both adalimumab and infliximab treatment. All individuals were men with severe, long-standing disease of the anogenital region. In addition to smoking, HPV-16 positivity also has been reported as a risk factor for developing SCC in the setting of HS.11 In our patient, however, HPV testing did not cover all HPV strains, but several high-risk strains, including HPV-16, were negative.
Hidradenitis suppurativa is caused by an immune response to ruptured follicles and TNF-α antagonists are useful in suppressing this response; however, immunosuppression can lead to an increased susceptibility to malignancy, especially in SCC. It is unclear whether the use of infliximab or adalimumab is causal, additive, or a confounder in the development of SCC in patients with severe HS. It is possible that these agents increase the rapidity of the development of SCC in already-susceptible patients. Although TNF-α antagonists can be an effective therapeutic option for patients with moderate to severe HS, the potential risk for contributing to skin cancer development should raise provider suspicion in high-risk patients. Given the findings in this report, it may be suitable for providers to consider a biopsy prior to initiating TNF-α therapy in men older than 20 years with moderate to severe HS of the groin or buttocks, in addition to more frequent monitoring and a lower threshold to biopsy lesions with rapid growth or ulceration.
- Alikhan A, Lynch PJ, Eisen DB. Hidradenitis suppurativa: a comprehensive review. J Am Acad Dermatol. 2009;60:539-561; quiz 562-533.
- Lapins J, Ye W, Nyren O, et al. Incidence of cancer among patients with hidradenitis suppurativa. Arch Dermatol. 2001;137:730-734.
- Askling J, Fahrbach K, Nordstrom B, et al. Cancer risk with tumor necrosis factor alpha (TNF) inhibitors: meta-analysis of randomized controlled trials of adalimumab, etanercept, and infliximab using patient level data. Pharmacoepidemiol Drug Saf. 2011;20:119-130.
- Mariette X, Matucci-Cerinic M, Pavelka K, et al. Malignancies associated with tumour necrosis factor inhibitors in registries and prospective observational studies: a systematic review and meta-analysis. Ann Rheum Dis. 2011;70:1895-1904.
- Maalouf E, Faye O, Poli F, et al. Fatal epidermoid carcinoma in hidradenitis suppurativa following treatment with infliximab. Ann Dermatol Venereol. 2006;133(5 pt 1):473-474.
- Kurokawa I, Nishimura K, Yamanaka K, et al. Cytokeratin expression in squamous cell carcinoma arising from hidradenitis suppurativa (acne inversa). J Cutan Pathol. 2007;34:675-678.
- Scheinfeld N. A case of a patient with stage III familial hidradenitis suppurativa treated with 3 courses of infliximab and died of metastatic squamous cell carcinoma. Dermatol Online J. 2014;20(3).
- Verdelli A, Antiga E, Bonciani D, et al. A fatal case of hidradenitis suppurativa associated with sepsis and squamous cell carcinoma. Int J Dermatol. 2016;55:E52-E53.
- Giesey R, Delost GR, Honaker J, et al. Metastatic squamous cell carcinoma in a patient treated with adalimumab for hidradenitis suppurativa. JAAD Case Rep. 2017;3:489-491.
- Roy C, Roy S, Ghazawi F, et al. Cutaneous squamous cell carcinoma arising in hidradenitis suppurativa: a case report. SAGE Open Med Case Rep. 2019;7:2050313X19847359.
- Lavogiez C, Delaporte E, Darras-Vercambre S, et al. Clinicopathological study of 13 cases of squamous cell carcinoma complicating hidradenitis suppurativa. Dermatology. 2010;220:147-153.
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition with high morbidity rates. Symptoms typically develop between puberty and the third decade of life, affecting twice as many females as males, with an overall disease prevalence of 1% to 4%.1 The pathogenesis is theorized to be related to an immune response to follicular occlusion and rupture in genetically susceptible individuals.
Among the complications associated with HS, the development of cutaneous squamous cell carcinoma (SCC) is 4.6-times more likely within HS lesions than in normal skin and typically is seen in the setting of long-standing disease, particularly in men with HS lesions located on the buttocks and genital region for more than 20 years.2 In 2015, the tumor necrosis factor (TNF) inhibitor adalimumab was approved by the US Food and Drug Administration for the treatment of HS. Tumor necrosis factor α inhibitors have been associated with an increased risk for skin cancer in other clinical settings.3,4 We present a case of locally advanced SCC that developed in a patient with HS who was treated with adalimumab and infliximab (both TNF-α inhibitors), ultimately leading to the patient’s death.
A 59-year-old man who smoked with a 40-year history of severe HS, who previously was lost to follow-up, presented to our dermatology clinic with lesions on the buttocks. Physical examination demonstrated confluent, indurated, boggy plaques; scattered sinus tracts with purulent drainage; scattered cystlike nodules; and tenderness to palpation consistent with Hurley stage III disease (Figure 1A). No involvement of the axillae or groin was noted. He was started on doxycycline and a prednisone taper with minimal improvement and subsequently was switched to adalimumab 3 months later. Adalimumab provided little relief and was discontinued; therapy was transitioned to infliximab 3 months later.

The patient returned to our clinic 3 months later with a severe flare and intractable pain after 4 infusions of infliximab. Physical examination showed a 7×5-cm deep malodorous ulcer with fibrinous exudate on the left buttock, several 2- to 3-cm shallow ulcers draining yellow exudate, and numerous fluctuant subcutaneous nodules on a background of scarring and sinus tracts. He was started again on doxycycline and a prednisone taper. At follow-up 2 weeks later, the largest ulcer had increased to 8 cm, and more indurated and tender subcutaneous nodules and scattered ulcerations developed (Figure 1B). Two punch biopsies of the left buttock revealed an invasive keratinizing carcinoma with no connection to the epidermis, consistent with SCC (Figure 2). Human papillomavirus (HPV) test results with probes for 37 HPV types—13 that were high risk (HPV-16, −18, −31, −33, −35, −39, −45, −51, −52, −56, −58, −59, −68)—were negative. Computerized tomography demonstrated diffuse thickening of the skin on the buttocks, inguinal adenopathy suspicious for nodal metastases, and no evidence of distant metastatic disease. Given the extent of the disease, surgical treatment was not an option, and he began receiving palliative radiotherapy. However, his health declined, and he developed aspiration pneumonia and hypotension requiring pressor support. He was transitioned to hospice care and died 3 months after presentation.

Tumor necrosis factor α antagonist treatment is being increasingly used to control HS but also may increase the risk for SCC development. We performed a search of PubMed articles indexed for MEDLINE as well as Web of Science using the terms hidradenitis suppurativa or acne inversa and one of the following—tumor necrosis factor inhibitor, infliximab, adalimumab, or etanercept—and squamous cell carcinoma or Marjolin ulcer. Seven cases of SCC arising in an HS patient treated with a TNF-α inhibitor have been reported (Table).5-10 Four cases were associated with infliximab use, 2 with adalimumab, and our case occurred after both adalimumab and infliximab treatment. All individuals were men with severe, long-standing disease of the anogenital region. In addition to smoking, HPV-16 positivity also has been reported as a risk factor for developing SCC in the setting of HS.11 In our patient, however, HPV testing did not cover all HPV strains, but several high-risk strains, including HPV-16, were negative.

Hidradenitis suppurativa is caused by an immune response to ruptured follicles and TNF-α antagonists are useful in suppressing this response; however, immunosuppression can lead to an increased susceptibility to malignancy, especially in SCC. It is unclear whether the use of infliximab or adalimumab is causal, additive, or a confounder in the development of SCC in patients with severe HS. It is possible that these agents increase the rapidity of the development of SCC in already-susceptible patients. Although TNF-α antagonists can be an effective therapeutic option for patients with moderate to severe HS, the potential risk for contributing to skin cancer development should raise provider suspicion in high-risk patients. Given the findings in this report, it may be suitable for providers to consider a biopsy prior to initiating TNF-α therapy in men older than 20 years with moderate to severe HS of the groin or buttocks, in addition to more frequent monitoring and a lower threshold to biopsy lesions with rapid growth or ulceration.
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition with high morbidity rates. Symptoms typically develop between puberty and the third decade of life, affecting twice as many females as males, with an overall disease prevalence of 1% to 4%.1 The pathogenesis is theorized to be related to an immune response to follicular occlusion and rupture in genetically susceptible individuals.
Among the complications associated with HS, the development of cutaneous squamous cell carcinoma (SCC) is 4.6-times more likely within HS lesions than in normal skin and typically is seen in the setting of long-standing disease, particularly in men with HS lesions located on the buttocks and genital region for more than 20 years.2 In 2015, the tumor necrosis factor (TNF) inhibitor adalimumab was approved by the US Food and Drug Administration for the treatment of HS. Tumor necrosis factor α inhibitors have been associated with an increased risk for skin cancer in other clinical settings.3,4 We present a case of locally advanced SCC that developed in a patient with HS who was treated with adalimumab and infliximab (both TNF-α inhibitors), ultimately leading to the patient’s death.
A 59-year-old man who smoked with a 40-year history of severe HS, who previously was lost to follow-up, presented to our dermatology clinic with lesions on the buttocks. Physical examination demonstrated confluent, indurated, boggy plaques; scattered sinus tracts with purulent drainage; scattered cystlike nodules; and tenderness to palpation consistent with Hurley stage III disease (Figure 1A). No involvement of the axillae or groin was noted. He was started on doxycycline and a prednisone taper with minimal improvement and subsequently was switched to adalimumab 3 months later. Adalimumab provided little relief and was discontinued; therapy was transitioned to infliximab 3 months later.

The patient returned to our clinic 3 months later with a severe flare and intractable pain after 4 infusions of infliximab. Physical examination showed a 7×5-cm deep malodorous ulcer with fibrinous exudate on the left buttock, several 2- to 3-cm shallow ulcers draining yellow exudate, and numerous fluctuant subcutaneous nodules on a background of scarring and sinus tracts. He was started again on doxycycline and a prednisone taper. At follow-up 2 weeks later, the largest ulcer had increased to 8 cm, and more indurated and tender subcutaneous nodules and scattered ulcerations developed (Figure 1B). Two punch biopsies of the left buttock revealed an invasive keratinizing carcinoma with no connection to the epidermis, consistent with SCC (Figure 2). Human papillomavirus (HPV) test results with probes for 37 HPV types—13 that were high risk (HPV-16, −18, −31, −33, −35, −39, −45, −51, −52, −56, −58, −59, −68)—were negative. Computerized tomography demonstrated diffuse thickening of the skin on the buttocks, inguinal adenopathy suspicious for nodal metastases, and no evidence of distant metastatic disease. Given the extent of the disease, surgical treatment was not an option, and he began receiving palliative radiotherapy. However, his health declined, and he developed aspiration pneumonia and hypotension requiring pressor support. He was transitioned to hospice care and died 3 months after presentation.

Tumor necrosis factor α antagonist treatment is being increasingly used to control HS but also may increase the risk for SCC development. We performed a search of PubMed articles indexed for MEDLINE as well as Web of Science using the terms hidradenitis suppurativa or acne inversa and one of the following—tumor necrosis factor inhibitor, infliximab, adalimumab, or etanercept—and squamous cell carcinoma or Marjolin ulcer. Seven cases of SCC arising in an HS patient treated with a TNF-α inhibitor have been reported (Table).5-10 Four cases were associated with infliximab use, 2 with adalimumab, and our case occurred after both adalimumab and infliximab treatment. All individuals were men with severe, long-standing disease of the anogenital region. In addition to smoking, HPV-16 positivity also has been reported as a risk factor for developing SCC in the setting of HS.11 In our patient, however, HPV testing did not cover all HPV strains, but several high-risk strains, including HPV-16, were negative.

Hidradenitis suppurativa is caused by an immune response to ruptured follicles and TNF-α antagonists are useful in suppressing this response; however, immunosuppression can lead to an increased susceptibility to malignancy, especially in SCC. It is unclear whether the use of infliximab or adalimumab is causal, additive, or a confounder in the development of SCC in patients with severe HS. It is possible that these agents increase the rapidity of the development of SCC in already-susceptible patients. Although TNF-α antagonists can be an effective therapeutic option for patients with moderate to severe HS, the potential risk for contributing to skin cancer development should raise provider suspicion in high-risk patients. Given the findings in this report, it may be suitable for providers to consider a biopsy prior to initiating TNF-α therapy in men older than 20 years with moderate to severe HS of the groin or buttocks, in addition to more frequent monitoring and a lower threshold to biopsy lesions with rapid growth or ulceration.
- Alikhan A, Lynch PJ, Eisen DB. Hidradenitis suppurativa: a comprehensive review. J Am Acad Dermatol. 2009;60:539-561; quiz 562-533.
- Lapins J, Ye W, Nyren O, et al. Incidence of cancer among patients with hidradenitis suppurativa. Arch Dermatol. 2001;137:730-734.
- Askling J, Fahrbach K, Nordstrom B, et al. Cancer risk with tumor necrosis factor alpha (TNF) inhibitors: meta-analysis of randomized controlled trials of adalimumab, etanercept, and infliximab using patient level data. Pharmacoepidemiol Drug Saf. 2011;20:119-130.
- Mariette X, Matucci-Cerinic M, Pavelka K, et al. Malignancies associated with tumour necrosis factor inhibitors in registries and prospective observational studies: a systematic review and meta-analysis. Ann Rheum Dis. 2011;70:1895-1904.
- Maalouf E, Faye O, Poli F, et al. Fatal epidermoid carcinoma in hidradenitis suppurativa following treatment with infliximab. Ann Dermatol Venereol. 2006;133(5 pt 1):473-474.
- Kurokawa I, Nishimura K, Yamanaka K, et al. Cytokeratin expression in squamous cell carcinoma arising from hidradenitis suppurativa (acne inversa). J Cutan Pathol. 2007;34:675-678.
- Scheinfeld N. A case of a patient with stage III familial hidradenitis suppurativa treated with 3 courses of infliximab and died of metastatic squamous cell carcinoma. Dermatol Online J. 2014;20(3).
- Verdelli A, Antiga E, Bonciani D, et al. A fatal case of hidradenitis suppurativa associated with sepsis and squamous cell carcinoma. Int J Dermatol. 2016;55:E52-E53.
- Giesey R, Delost GR, Honaker J, et al. Metastatic squamous cell carcinoma in a patient treated with adalimumab for hidradenitis suppurativa. JAAD Case Rep. 2017;3:489-491.
- Roy C, Roy S, Ghazawi F, et al. Cutaneous squamous cell carcinoma arising in hidradenitis suppurativa: a case report. SAGE Open Med Case Rep. 2019;7:2050313X19847359.
- Lavogiez C, Delaporte E, Darras-Vercambre S, et al. Clinicopathological study of 13 cases of squamous cell carcinoma complicating hidradenitis suppurativa. Dermatology. 2010;220:147-153.
- Alikhan A, Lynch PJ, Eisen DB. Hidradenitis suppurativa: a comprehensive review. J Am Acad Dermatol. 2009;60:539-561; quiz 562-533.
- Lapins J, Ye W, Nyren O, et al. Incidence of cancer among patients with hidradenitis suppurativa. Arch Dermatol. 2001;137:730-734.
- Askling J, Fahrbach K, Nordstrom B, et al. Cancer risk with tumor necrosis factor alpha (TNF) inhibitors: meta-analysis of randomized controlled trials of adalimumab, etanercept, and infliximab using patient level data. Pharmacoepidemiol Drug Saf. 2011;20:119-130.
- Mariette X, Matucci-Cerinic M, Pavelka K, et al. Malignancies associated with tumour necrosis factor inhibitors in registries and prospective observational studies: a systematic review and meta-analysis. Ann Rheum Dis. 2011;70:1895-1904.
- Maalouf E, Faye O, Poli F, et al. Fatal epidermoid carcinoma in hidradenitis suppurativa following treatment with infliximab. Ann Dermatol Venereol. 2006;133(5 pt 1):473-474.
- Kurokawa I, Nishimura K, Yamanaka K, et al. Cytokeratin expression in squamous cell carcinoma arising from hidradenitis suppurativa (acne inversa). J Cutan Pathol. 2007;34:675-678.
- Scheinfeld N. A case of a patient with stage III familial hidradenitis suppurativa treated with 3 courses of infliximab and died of metastatic squamous cell carcinoma. Dermatol Online J. 2014;20(3).
- Verdelli A, Antiga E, Bonciani D, et al. A fatal case of hidradenitis suppurativa associated with sepsis and squamous cell carcinoma. Int J Dermatol. 2016;55:E52-E53.
- Giesey R, Delost GR, Honaker J, et al. Metastatic squamous cell carcinoma in a patient treated with adalimumab for hidradenitis suppurativa. JAAD Case Rep. 2017;3:489-491.
- Roy C, Roy S, Ghazawi F, et al. Cutaneous squamous cell carcinoma arising in hidradenitis suppurativa: a case report. SAGE Open Med Case Rep. 2019;7:2050313X19847359.
- Lavogiez C, Delaporte E, Darras-Vercambre S, et al. Clinicopathological study of 13 cases of squamous cell carcinoma complicating hidradenitis suppurativa. Dermatology. 2010;220:147-153.
Practice Points
- Consider biopsy of representative lesions in men older than 20 years with moderate to severe disease of the groin and/or buttocks prior to initiation of tumor necrosis factor inhibitors.
- Consider more frequent clinical monitoring with a decrease in threshold to perform biopsy of any new or ulcerating lesions.
Starting April 5, patients can read your notes: 5 things to consider
Change in writing style is not mandated
The mandate, called “open notes” by many, is part of the 21st Century Cures Act, a wide-ranging piece of federal health care legislation. The previous deadline of Nov. 2, 2020, for enacting open notes was extended last year because of the exigencies of the COVID-19 pandemic.
Organizations must provide access via patient portals to the following types of notes: consultations, discharge summaries, histories, physical examination findings, imaging narratives, laboratory and pathology report narratives, and procedure and progress notes. Noncompliant organizations will eventually be subject to fines from the Department of Health & Human Services for “information blocking.”
This news organization reported on the mandate in 2020, and some readers said it was an unwelcome intrusion into practice. Since then, this news organization has run additional open notes stories about physician concerns, a perspective essay addressing those fears, and a reader poll about the phenomenon.
Now, as the legislation turns into a practical clinical matter, there are five key points clinicians should consider.
Clinicians don’t have to change writing style.
The new law mandates timely patient access to notes and test results, but it doesn’t require that clinicians alter their writing, said Scott MacDonald, MD, an internist and electronic health record medical director at University of California Davis Health in Sacramento.
“You don’t have to change your notes,” he said. However, patients are now part of the note audience and some health care systems are directing clinicians to make patient-friendly style changes.
Everyday experience should guide clinicians when writing notes, said one expert.
“When you’re not sure [of how to write a note], just mirror the way you would speak in the office – that’s going to get you right, including for mental health issues,” advised Leonor Fernandez, MD, an internist at Beth Deaconess Israel Medical Center, Boston, in her “take-away” comments in the online video, How to Write an Open Note.
According to a 2020 Medscape poll of 1,050 physicians, a majority (56%) anticipate that they will write notes differently, knowing that patients can read them via open notes. Nearly two-thirds (64%) believe that this new wrinkle in medical records will increase their workload. However, actual practice suggests that this is true for a minority of practitioners, according to the results from a recent study of more than 1,000 physicians in Boston, Seattle, and rural Pennsylvania, who already work in open notes settings. Only about one-third (37%) reported “spending more time on documentation.”
Note writing is going to change because of the addition of the patient reader, and something will be lost, argued Steven Reidbord, MD, a psychiatrist in private practice in San Francisco. By watering down the language for patients, “you are trading away the technical precision and other advantages of having a professional language,” commented Dr. Reidbord, who blogs for Psychology Today and has criticized the open notes movement in the past.
However, years of investigation from OpenNotes, the Boston-based advocacy and research organization, indicates that there are many gains with patient-accessible notes, including improved medical record accuracy, greater medication adherence, and potentially improved health care disparities among a range of patient types. In a 2019 study, researchers said that worry and confusion among note-reading patients are uncommon (5% and 3%, respectively), which addresses two criticisms voiced by multiple people last year.
Some clinical notes can be withheld.
The new rules from the federal government permit information blocking if there is clear evidence that doing so “will substantially reduce the risk of harm” to patients or to other third parties, Tom Delbanco, MD, and Charlotte Blease, PhD, of OpenNotes in Boston wrote in a commentary in February 2021.
There are also state-level laws that can supersede the new U.S. law and block access to notes, points out MacDonald. For example, California law dictates that providers cannot post cancer test results without talking with the patient first.
The OpenNotes organization also points out that, with regard to sensitive psychotherapy notes that are separated from the rest of a medical record, those notes “can be kept from patients without their permission, and such rules vary state by state.”
Some patients are more likely readers.
Some patients are more likely to peer into their files than others, said Liz Salmi, senior strategist at OpenNotes, who is also a brain cancer patient.
“Those patients who have more serious or chronic conditions ... are more likely to read their notes,” she said in an interview.
A new study of nearly 6,000 medical oncology patients at the University of Wisconsin confirmed that opinion. Patients with incurable metastatic disease were much more likely than those with early-stage, curable disease to read notes. Notably, younger patients were more likely than older ones to access notes, likely the result of generational tech savvy.
Despite the unpredictability of serious disease such as cancer, oncology patients find satisfaction in reading their notes, say experts. “We’ve overwhelmingly heard that patients like it,” Thomas LeBlanc, MD, medical oncologist at Duke University, Durham, N.C., where all patients already have access to clinicians’ notes, told this news organization in 2018.
You are part of the avant garde.
The United States and Scandinavian countries are the world leaders in implementing open notes in clinical practice, Dr. Blease said in an interview.
“It’s a phenomenal achievement” to have enacted open notes nationally, she said. For example, there are no open notes in Northern Ireland, Dr. Blease’s home country, or most of Europe.
In the United States, there are more than 200 medical organizations, including at least one in every state, that were voluntarily providing open notes before April 5, including interstate giants such as Banner Health and big-name medical centers such as Cleveland Clinic.
It may be hard for the United States to top Sweden’s embrace of the practice. The national open notes program now has 7.2 million patient accounts in a country of 10 million people, noted Maria Häggland, PhD, of Uppsala (Sweden) MedTech Science Innovation Center during a webinar last year.
The start day will come, and you may not notice.
“When April 5 happens, something brand new is going to happen symbolically,” Ms. Salmi said. Its importance is hard to measure.
“Patients say they trust their doctor more because they understand their thinking with open notes. How do you value that? We don’t have metrics for that,” she said.
Dr. MacDonald suggested that open notes are both new and not new. In the fall of 2020, he predicted that the launch day would come, and few clinicians would notice, in part because many patients already access truncated information via patient portals.
However, there are “sensitive issues,” such as with adolescents and reproductive health, where “we know that some parents have sign-in information for their teen’s portal,” he commented. With clinical notes now on full display, potential problems “may be out of our control.”
Still, the Sacramento-based physician and IT officer acknowledged that concerns about open notes may be a bit inflated. “I’ve been more worried about reassuring physicians that everything will be okay than what’s actually going to happen [as the law takes effect],” Dr. MacDonald said.
The OpenNotes organization is grant funded, and staff disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Change in writing style is not mandated
Change in writing style is not mandated
The mandate, called “open notes” by many, is part of the 21st Century Cures Act, a wide-ranging piece of federal health care legislation. The previous deadline of Nov. 2, 2020, for enacting open notes was extended last year because of the exigencies of the COVID-19 pandemic.
Organizations must provide access via patient portals to the following types of notes: consultations, discharge summaries, histories, physical examination findings, imaging narratives, laboratory and pathology report narratives, and procedure and progress notes. Noncompliant organizations will eventually be subject to fines from the Department of Health & Human Services for “information blocking.”
This news organization reported on the mandate in 2020, and some readers said it was an unwelcome intrusion into practice. Since then, this news organization has run additional open notes stories about physician concerns, a perspective essay addressing those fears, and a reader poll about the phenomenon.
Now, as the legislation turns into a practical clinical matter, there are five key points clinicians should consider.
Clinicians don’t have to change writing style.
The new law mandates timely patient access to notes and test results, but it doesn’t require that clinicians alter their writing, said Scott MacDonald, MD, an internist and electronic health record medical director at University of California Davis Health in Sacramento.
“You don’t have to change your notes,” he said. However, patients are now part of the note audience and some health care systems are directing clinicians to make patient-friendly style changes.
Everyday experience should guide clinicians when writing notes, said one expert.
“When you’re not sure [of how to write a note], just mirror the way you would speak in the office – that’s going to get you right, including for mental health issues,” advised Leonor Fernandez, MD, an internist at Beth Deaconess Israel Medical Center, Boston, in her “take-away” comments in the online video, How to Write an Open Note.
According to a 2020 Medscape poll of 1,050 physicians, a majority (56%) anticipate that they will write notes differently, knowing that patients can read them via open notes. Nearly two-thirds (64%) believe that this new wrinkle in medical records will increase their workload. However, actual practice suggests that this is true for a minority of practitioners, according to the results from a recent study of more than 1,000 physicians in Boston, Seattle, and rural Pennsylvania, who already work in open notes settings. Only about one-third (37%) reported “spending more time on documentation.”
Note writing is going to change because of the addition of the patient reader, and something will be lost, argued Steven Reidbord, MD, a psychiatrist in private practice in San Francisco. By watering down the language for patients, “you are trading away the technical precision and other advantages of having a professional language,” commented Dr. Reidbord, who blogs for Psychology Today and has criticized the open notes movement in the past.
However, years of investigation from OpenNotes, the Boston-based advocacy and research organization, indicates that there are many gains with patient-accessible notes, including improved medical record accuracy, greater medication adherence, and potentially improved health care disparities among a range of patient types. In a 2019 study, researchers said that worry and confusion among note-reading patients are uncommon (5% and 3%, respectively), which addresses two criticisms voiced by multiple people last year.
Some clinical notes can be withheld.
The new rules from the federal government permit information blocking if there is clear evidence that doing so “will substantially reduce the risk of harm” to patients or to other third parties, Tom Delbanco, MD, and Charlotte Blease, PhD, of OpenNotes in Boston wrote in a commentary in February 2021.
There are also state-level laws that can supersede the new U.S. law and block access to notes, points out MacDonald. For example, California law dictates that providers cannot post cancer test results without talking with the patient first.
The OpenNotes organization also points out that, with regard to sensitive psychotherapy notes that are separated from the rest of a medical record, those notes “can be kept from patients without their permission, and such rules vary state by state.”
Some patients are more likely readers.
Some patients are more likely to peer into their files than others, said Liz Salmi, senior strategist at OpenNotes, who is also a brain cancer patient.
“Those patients who have more serious or chronic conditions ... are more likely to read their notes,” she said in an interview.
A new study of nearly 6,000 medical oncology patients at the University of Wisconsin confirmed that opinion. Patients with incurable metastatic disease were much more likely than those with early-stage, curable disease to read notes. Notably, younger patients were more likely than older ones to access notes, likely the result of generational tech savvy.
Despite the unpredictability of serious disease such as cancer, oncology patients find satisfaction in reading their notes, say experts. “We’ve overwhelmingly heard that patients like it,” Thomas LeBlanc, MD, medical oncologist at Duke University, Durham, N.C., where all patients already have access to clinicians’ notes, told this news organization in 2018.
You are part of the avant garde.
The United States and Scandinavian countries are the world leaders in implementing open notes in clinical practice, Dr. Blease said in an interview.
“It’s a phenomenal achievement” to have enacted open notes nationally, she said. For example, there are no open notes in Northern Ireland, Dr. Blease’s home country, or most of Europe.
In the United States, there are more than 200 medical organizations, including at least one in every state, that were voluntarily providing open notes before April 5, including interstate giants such as Banner Health and big-name medical centers such as Cleveland Clinic.
It may be hard for the United States to top Sweden’s embrace of the practice. The national open notes program now has 7.2 million patient accounts in a country of 10 million people, noted Maria Häggland, PhD, of Uppsala (Sweden) MedTech Science Innovation Center during a webinar last year.
The start day will come, and you may not notice.
“When April 5 happens, something brand new is going to happen symbolically,” Ms. Salmi said. Its importance is hard to measure.
“Patients say they trust their doctor more because they understand their thinking with open notes. How do you value that? We don’t have metrics for that,” she said.
Dr. MacDonald suggested that open notes are both new and not new. In the fall of 2020, he predicted that the launch day would come, and few clinicians would notice, in part because many patients already access truncated information via patient portals.
However, there are “sensitive issues,” such as with adolescents and reproductive health, where “we know that some parents have sign-in information for their teen’s portal,” he commented. With clinical notes now on full display, potential problems “may be out of our control.”
Still, the Sacramento-based physician and IT officer acknowledged that concerns about open notes may be a bit inflated. “I’ve been more worried about reassuring physicians that everything will be okay than what’s actually going to happen [as the law takes effect],” Dr. MacDonald said.
The OpenNotes organization is grant funded, and staff disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The mandate, called “open notes” by many, is part of the 21st Century Cures Act, a wide-ranging piece of federal health care legislation. The previous deadline of Nov. 2, 2020, for enacting open notes was extended last year because of the exigencies of the COVID-19 pandemic.
Organizations must provide access via patient portals to the following types of notes: consultations, discharge summaries, histories, physical examination findings, imaging narratives, laboratory and pathology report narratives, and procedure and progress notes. Noncompliant organizations will eventually be subject to fines from the Department of Health & Human Services for “information blocking.”
This news organization reported on the mandate in 2020, and some readers said it was an unwelcome intrusion into practice. Since then, this news organization has run additional open notes stories about physician concerns, a perspective essay addressing those fears, and a reader poll about the phenomenon.
Now, as the legislation turns into a practical clinical matter, there are five key points clinicians should consider.
Clinicians don’t have to change writing style.
The new law mandates timely patient access to notes and test results, but it doesn’t require that clinicians alter their writing, said Scott MacDonald, MD, an internist and electronic health record medical director at University of California Davis Health in Sacramento.
“You don’t have to change your notes,” he said. However, patients are now part of the note audience and some health care systems are directing clinicians to make patient-friendly style changes.
Everyday experience should guide clinicians when writing notes, said one expert.
“When you’re not sure [of how to write a note], just mirror the way you would speak in the office – that’s going to get you right, including for mental health issues,” advised Leonor Fernandez, MD, an internist at Beth Deaconess Israel Medical Center, Boston, in her “take-away” comments in the online video, How to Write an Open Note.
According to a 2020 Medscape poll of 1,050 physicians, a majority (56%) anticipate that they will write notes differently, knowing that patients can read them via open notes. Nearly two-thirds (64%) believe that this new wrinkle in medical records will increase their workload. However, actual practice suggests that this is true for a minority of practitioners, according to the results from a recent study of more than 1,000 physicians in Boston, Seattle, and rural Pennsylvania, who already work in open notes settings. Only about one-third (37%) reported “spending more time on documentation.”
Note writing is going to change because of the addition of the patient reader, and something will be lost, argued Steven Reidbord, MD, a psychiatrist in private practice in San Francisco. By watering down the language for patients, “you are trading away the technical precision and other advantages of having a professional language,” commented Dr. Reidbord, who blogs for Psychology Today and has criticized the open notes movement in the past.
However, years of investigation from OpenNotes, the Boston-based advocacy and research organization, indicates that there are many gains with patient-accessible notes, including improved medical record accuracy, greater medication adherence, and potentially improved health care disparities among a range of patient types. In a 2019 study, researchers said that worry and confusion among note-reading patients are uncommon (5% and 3%, respectively), which addresses two criticisms voiced by multiple people last year.
Some clinical notes can be withheld.
The new rules from the federal government permit information blocking if there is clear evidence that doing so “will substantially reduce the risk of harm” to patients or to other third parties, Tom Delbanco, MD, and Charlotte Blease, PhD, of OpenNotes in Boston wrote in a commentary in February 2021.
There are also state-level laws that can supersede the new U.S. law and block access to notes, points out MacDonald. For example, California law dictates that providers cannot post cancer test results without talking with the patient first.
The OpenNotes organization also points out that, with regard to sensitive psychotherapy notes that are separated from the rest of a medical record, those notes “can be kept from patients without their permission, and such rules vary state by state.”
Some patients are more likely readers.
Some patients are more likely to peer into their files than others, said Liz Salmi, senior strategist at OpenNotes, who is also a brain cancer patient.
“Those patients who have more serious or chronic conditions ... are more likely to read their notes,” she said in an interview.
A new study of nearly 6,000 medical oncology patients at the University of Wisconsin confirmed that opinion. Patients with incurable metastatic disease were much more likely than those with early-stage, curable disease to read notes. Notably, younger patients were more likely than older ones to access notes, likely the result of generational tech savvy.
Despite the unpredictability of serious disease such as cancer, oncology patients find satisfaction in reading their notes, say experts. “We’ve overwhelmingly heard that patients like it,” Thomas LeBlanc, MD, medical oncologist at Duke University, Durham, N.C., where all patients already have access to clinicians’ notes, told this news organization in 2018.
You are part of the avant garde.
The United States and Scandinavian countries are the world leaders in implementing open notes in clinical practice, Dr. Blease said in an interview.
“It’s a phenomenal achievement” to have enacted open notes nationally, she said. For example, there are no open notes in Northern Ireland, Dr. Blease’s home country, or most of Europe.
In the United States, there are more than 200 medical organizations, including at least one in every state, that were voluntarily providing open notes before April 5, including interstate giants such as Banner Health and big-name medical centers such as Cleveland Clinic.
It may be hard for the United States to top Sweden’s embrace of the practice. The national open notes program now has 7.2 million patient accounts in a country of 10 million people, noted Maria Häggland, PhD, of Uppsala (Sweden) MedTech Science Innovation Center during a webinar last year.
The start day will come, and you may not notice.
“When April 5 happens, something brand new is going to happen symbolically,” Ms. Salmi said. Its importance is hard to measure.
“Patients say they trust their doctor more because they understand their thinking with open notes. How do you value that? We don’t have metrics for that,” she said.
Dr. MacDonald suggested that open notes are both new and not new. In the fall of 2020, he predicted that the launch day would come, and few clinicians would notice, in part because many patients already access truncated information via patient portals.
However, there are “sensitive issues,” such as with adolescents and reproductive health, where “we know that some parents have sign-in information for their teen’s portal,” he commented. With clinical notes now on full display, potential problems “may be out of our control.”
Still, the Sacramento-based physician and IT officer acknowledged that concerns about open notes may be a bit inflated. “I’ve been more worried about reassuring physicians that everything will be okay than what’s actually going to happen [as the law takes effect],” Dr. MacDonald said.
The OpenNotes organization is grant funded, and staff disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Top JAMA editor on leave amid podcast investigation
The American Medical Association’s Joint Oversight Committee announced that Howard Bauchner, MD, is on leave beginning at the end of the day on March 25. Dr. Bauchner is the top editor at JAMA, the journal of the AMA.
“The decision to place the editor-in-chief on administrative leave neither implicates nor exonerates individuals and is standard operating procedure for such investigations,” the committee said in a statement.
More than 2,000 people signed a petition on Change.org calling for an investigation at JAMA over the February podcast episode, called “Structural Racism for Doctors: What Is It?”
Already, Edward H. Livingston, MD, the host of the podcast, has resigned as deputy editor of the journal.
During the podcast, Dr. Livingston, who is White, said, “Structural racism is an unfortunate term. Personally, I think taking racism out of the conversation will help. Many of us are offended by the concept that we are racist.”
The audio of the podcast has been deleted from JAMA’s website. In its place is audio of a statement from Dr. Bauchner. In his statement, which he released in the week prior to his being on leave, he said the comments in the podcast, which also featured Mitch Katz, MD, were “inaccurate, offensive, hurtful, and inconsistent with the standards of JAMA.”
Also deleted was a JAMA tweet promoting the podcast episode. The tweet said: “No physician is racist, so how can there be structural racism in health care? An explanation of the idea by doctors for doctors in this user-friendly podcast.”
This story will be updated.
A version of this article first appeared on WedMD.com.
The American Medical Association’s Joint Oversight Committee announced that Howard Bauchner, MD, is on leave beginning at the end of the day on March 25. Dr. Bauchner is the top editor at JAMA, the journal of the AMA.
“The decision to place the editor-in-chief on administrative leave neither implicates nor exonerates individuals and is standard operating procedure for such investigations,” the committee said in a statement.
More than 2,000 people signed a petition on Change.org calling for an investigation at JAMA over the February podcast episode, called “Structural Racism for Doctors: What Is It?”
Already, Edward H. Livingston, MD, the host of the podcast, has resigned as deputy editor of the journal.
During the podcast, Dr. Livingston, who is White, said, “Structural racism is an unfortunate term. Personally, I think taking racism out of the conversation will help. Many of us are offended by the concept that we are racist.”
The audio of the podcast has been deleted from JAMA’s website. In its place is audio of a statement from Dr. Bauchner. In his statement, which he released in the week prior to his being on leave, he said the comments in the podcast, which also featured Mitch Katz, MD, were “inaccurate, offensive, hurtful, and inconsistent with the standards of JAMA.”
Also deleted was a JAMA tweet promoting the podcast episode. The tweet said: “No physician is racist, so how can there be structural racism in health care? An explanation of the idea by doctors for doctors in this user-friendly podcast.”
This story will be updated.
A version of this article first appeared on WedMD.com.
The American Medical Association’s Joint Oversight Committee announced that Howard Bauchner, MD, is on leave beginning at the end of the day on March 25. Dr. Bauchner is the top editor at JAMA, the journal of the AMA.
“The decision to place the editor-in-chief on administrative leave neither implicates nor exonerates individuals and is standard operating procedure for such investigations,” the committee said in a statement.
More than 2,000 people signed a petition on Change.org calling for an investigation at JAMA over the February podcast episode, called “Structural Racism for Doctors: What Is It?”
Already, Edward H. Livingston, MD, the host of the podcast, has resigned as deputy editor of the journal.
During the podcast, Dr. Livingston, who is White, said, “Structural racism is an unfortunate term. Personally, I think taking racism out of the conversation will help. Many of us are offended by the concept that we are racist.”
The audio of the podcast has been deleted from JAMA’s website. In its place is audio of a statement from Dr. Bauchner. In his statement, which he released in the week prior to his being on leave, he said the comments in the podcast, which also featured Mitch Katz, MD, were “inaccurate, offensive, hurtful, and inconsistent with the standards of JAMA.”
Also deleted was a JAMA tweet promoting the podcast episode. The tweet said: “No physician is racist, so how can there be structural racism in health care? An explanation of the idea by doctors for doctors in this user-friendly podcast.”
This story will be updated.
A version of this article first appeared on WedMD.com.
New analysis eyes the surgical landscape for hidradenitis suppurativa
yet these options should be balanced against potentially higher morbidity of extensive procedures.
Those are among the key findings of a systematic review and meta-analysis published online in Dermatologic Surgery.
“There is a major need to better understand the best surgical approaches to HS,” one of the study authors, Christopher Sayed, MD, associate professor of dermatology at the University of North Carolina at Chapel Hill, said in an interview. Previous studies have mostly reviewed outcomes for procedure types in individual cohorts, “but no recent reports have combined and analyzed data from recent studies.”
When Dr. Sayed and colleagues set out to summarize the literature on HS surgery regarding patient characteristics, surgical approaches, and study quality, as well as compare postsurgical recurrence rates, the most recent meta-analysis on postoperative recurrence rates of HS included studies published between 1990 and 2015. “In the past few years, surgical management of HS has become an increasingly popular area of study,” corresponding author Ashley Riddle, MD, MPH, who is currently an internal medicine resident at the Carolinas Medical Center, Charlotte, said in an interview. “We sought to provide an updated picture of the HS surgical landscape by analyzing studies published between 2004 to 2019. We also limited our analysis to studies with follow-up periods of greater than 1 year and included information on disease severity, adverse events, and patient satisfaction when available.”
Of 715 relevant studies identified in the medical literature, the researchers included 59 in the review and 33 in the meta-analysis. Of these 59 studies, 56 were case series, 2 were randomized, controlled trials, and one was a retrospective cohort study.
Of the 50 studies reporting gender and age at time of surgery, 61% of patients were female and their average age was 37 years. Of the 25 studies that reported Hurley scores, 73% had Hurley stage 3 HS. Of the 38 studies reporting the number of procedures per anatomic region, the most commonly operated on regions were the axilla (59%) and the inguinal region (20%).
The researchers found that 22 studies of wide excision had the lowest pooled recurrence rate at 8%, while local excision had the highest pooled recurrence rate at 34%. Meanwhile, among studies of wide/radical excision, flap repair had a pooled recurrence rate of 0%, while delayed primary closure had the highest pooled recurrence rate at 38%.
“Extensive excisions of HS seem to portend a lower risk of postoperative recurrence, but there are many approaches available that may be more appropriate for certain patients,” Dr. Riddle said. “The influence of patient factors such as comorbidities and disease severity on surgical outcomes is unclear and is a potential area of future study.”
Dr. Sayed, an author of the 2019 North American guidelines for the clinical management of HS, pointed out that most studies in the review and meta-analysis included patients who had diabetes, were on biologics or other therapy, were actively smoking, or had other comorbidities that sometimes influence surgeons to delay surgical treatment because they consider it elective. “Most studies indicated minimal or no risk of significant complications relating to these factors, so they should ideally not become obstacles for patients interested in surgical care,” he said.
Dr. Riddle said that she was surprised by how relatively few studies had been published on more conservative surgical approaches such as skin tissue–sparing excision with electrosurgical peeling, deroofing, local excision, and CO2 laser–based evaporation.
The researchers acknowledged certain limitations of their work, including the high risk of bias for most included studies. “Almost all studies were retrospective with substantial methodological limitations, and there were no head-to-head comparisons of different surgical approaches,” Dr. Riddle said. “Patient comorbidities and postoperative complications were variably reported.”
Dr. Sayed disclosed that he is a speaker for AbbVie and Novartis; an investigator for AbbVie, Novartis, InflaRx, and UCB; and on the advisory board of AbbVie and InflaRx. The remaining authors reported having no financial disclosures.
yet these options should be balanced against potentially higher morbidity of extensive procedures.
Those are among the key findings of a systematic review and meta-analysis published online in Dermatologic Surgery.
“There is a major need to better understand the best surgical approaches to HS,” one of the study authors, Christopher Sayed, MD, associate professor of dermatology at the University of North Carolina at Chapel Hill, said in an interview. Previous studies have mostly reviewed outcomes for procedure types in individual cohorts, “but no recent reports have combined and analyzed data from recent studies.”
When Dr. Sayed and colleagues set out to summarize the literature on HS surgery regarding patient characteristics, surgical approaches, and study quality, as well as compare postsurgical recurrence rates, the most recent meta-analysis on postoperative recurrence rates of HS included studies published between 1990 and 2015. “In the past few years, surgical management of HS has become an increasingly popular area of study,” corresponding author Ashley Riddle, MD, MPH, who is currently an internal medicine resident at the Carolinas Medical Center, Charlotte, said in an interview. “We sought to provide an updated picture of the HS surgical landscape by analyzing studies published between 2004 to 2019. We also limited our analysis to studies with follow-up periods of greater than 1 year and included information on disease severity, adverse events, and patient satisfaction when available.”
Of 715 relevant studies identified in the medical literature, the researchers included 59 in the review and 33 in the meta-analysis. Of these 59 studies, 56 were case series, 2 were randomized, controlled trials, and one was a retrospective cohort study.
Of the 50 studies reporting gender and age at time of surgery, 61% of patients were female and their average age was 37 years. Of the 25 studies that reported Hurley scores, 73% had Hurley stage 3 HS. Of the 38 studies reporting the number of procedures per anatomic region, the most commonly operated on regions were the axilla (59%) and the inguinal region (20%).
The researchers found that 22 studies of wide excision had the lowest pooled recurrence rate at 8%, while local excision had the highest pooled recurrence rate at 34%. Meanwhile, among studies of wide/radical excision, flap repair had a pooled recurrence rate of 0%, while delayed primary closure had the highest pooled recurrence rate at 38%.
“Extensive excisions of HS seem to portend a lower risk of postoperative recurrence, but there are many approaches available that may be more appropriate for certain patients,” Dr. Riddle said. “The influence of patient factors such as comorbidities and disease severity on surgical outcomes is unclear and is a potential area of future study.”
Dr. Sayed, an author of the 2019 North American guidelines for the clinical management of HS, pointed out that most studies in the review and meta-analysis included patients who had diabetes, were on biologics or other therapy, were actively smoking, or had other comorbidities that sometimes influence surgeons to delay surgical treatment because they consider it elective. “Most studies indicated minimal or no risk of significant complications relating to these factors, so they should ideally not become obstacles for patients interested in surgical care,” he said.
Dr. Riddle said that she was surprised by how relatively few studies had been published on more conservative surgical approaches such as skin tissue–sparing excision with electrosurgical peeling, deroofing, local excision, and CO2 laser–based evaporation.
The researchers acknowledged certain limitations of their work, including the high risk of bias for most included studies. “Almost all studies were retrospective with substantial methodological limitations, and there were no head-to-head comparisons of different surgical approaches,” Dr. Riddle said. “Patient comorbidities and postoperative complications were variably reported.”
Dr. Sayed disclosed that he is a speaker for AbbVie and Novartis; an investigator for AbbVie, Novartis, InflaRx, and UCB; and on the advisory board of AbbVie and InflaRx. The remaining authors reported having no financial disclosures.
yet these options should be balanced against potentially higher morbidity of extensive procedures.
Those are among the key findings of a systematic review and meta-analysis published online in Dermatologic Surgery.
“There is a major need to better understand the best surgical approaches to HS,” one of the study authors, Christopher Sayed, MD, associate professor of dermatology at the University of North Carolina at Chapel Hill, said in an interview. Previous studies have mostly reviewed outcomes for procedure types in individual cohorts, “but no recent reports have combined and analyzed data from recent studies.”
When Dr. Sayed and colleagues set out to summarize the literature on HS surgery regarding patient characteristics, surgical approaches, and study quality, as well as compare postsurgical recurrence rates, the most recent meta-analysis on postoperative recurrence rates of HS included studies published between 1990 and 2015. “In the past few years, surgical management of HS has become an increasingly popular area of study,” corresponding author Ashley Riddle, MD, MPH, who is currently an internal medicine resident at the Carolinas Medical Center, Charlotte, said in an interview. “We sought to provide an updated picture of the HS surgical landscape by analyzing studies published between 2004 to 2019. We also limited our analysis to studies with follow-up periods of greater than 1 year and included information on disease severity, adverse events, and patient satisfaction when available.”
Of 715 relevant studies identified in the medical literature, the researchers included 59 in the review and 33 in the meta-analysis. Of these 59 studies, 56 were case series, 2 were randomized, controlled trials, and one was a retrospective cohort study.
Of the 50 studies reporting gender and age at time of surgery, 61% of patients were female and their average age was 37 years. Of the 25 studies that reported Hurley scores, 73% had Hurley stage 3 HS. Of the 38 studies reporting the number of procedures per anatomic region, the most commonly operated on regions were the axilla (59%) and the inguinal region (20%).
The researchers found that 22 studies of wide excision had the lowest pooled recurrence rate at 8%, while local excision had the highest pooled recurrence rate at 34%. Meanwhile, among studies of wide/radical excision, flap repair had a pooled recurrence rate of 0%, while delayed primary closure had the highest pooled recurrence rate at 38%.
“Extensive excisions of HS seem to portend a lower risk of postoperative recurrence, but there are many approaches available that may be more appropriate for certain patients,” Dr. Riddle said. “The influence of patient factors such as comorbidities and disease severity on surgical outcomes is unclear and is a potential area of future study.”
Dr. Sayed, an author of the 2019 North American guidelines for the clinical management of HS, pointed out that most studies in the review and meta-analysis included patients who had diabetes, were on biologics or other therapy, were actively smoking, or had other comorbidities that sometimes influence surgeons to delay surgical treatment because they consider it elective. “Most studies indicated minimal or no risk of significant complications relating to these factors, so they should ideally not become obstacles for patients interested in surgical care,” he said.
Dr. Riddle said that she was surprised by how relatively few studies had been published on more conservative surgical approaches such as skin tissue–sparing excision with electrosurgical peeling, deroofing, local excision, and CO2 laser–based evaporation.
The researchers acknowledged certain limitations of their work, including the high risk of bias for most included studies. “Almost all studies were retrospective with substantial methodological limitations, and there were no head-to-head comparisons of different surgical approaches,” Dr. Riddle said. “Patient comorbidities and postoperative complications were variably reported.”
Dr. Sayed disclosed that he is a speaker for AbbVie and Novartis; an investigator for AbbVie, Novartis, InflaRx, and UCB; and on the advisory board of AbbVie and InflaRx. The remaining authors reported having no financial disclosures.
FROM DERMATOLOGIC SURGERY