User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Children and COVID: Weekly cases may have doubled in early January
Although new COVID-19 cases in children, as measured by the American Academy of Pediatrics and the Children’s Hospital Association, have remained fairly steady in recent months, data from the Centers for Diseases Control and Prevention suggest that weekly cases took a big jump in early January.
For the most recent week covered . New cases for the first 2 weeks of the year – 31,000 for the week of Dec. 30 to Jan. 5 and 26,000 during Jan. 6-12 – were consistent with the AAP/CHA assertion that “weekly reported child cases have plateaued at an average of about 32,000 cases ... over the past 4 months.”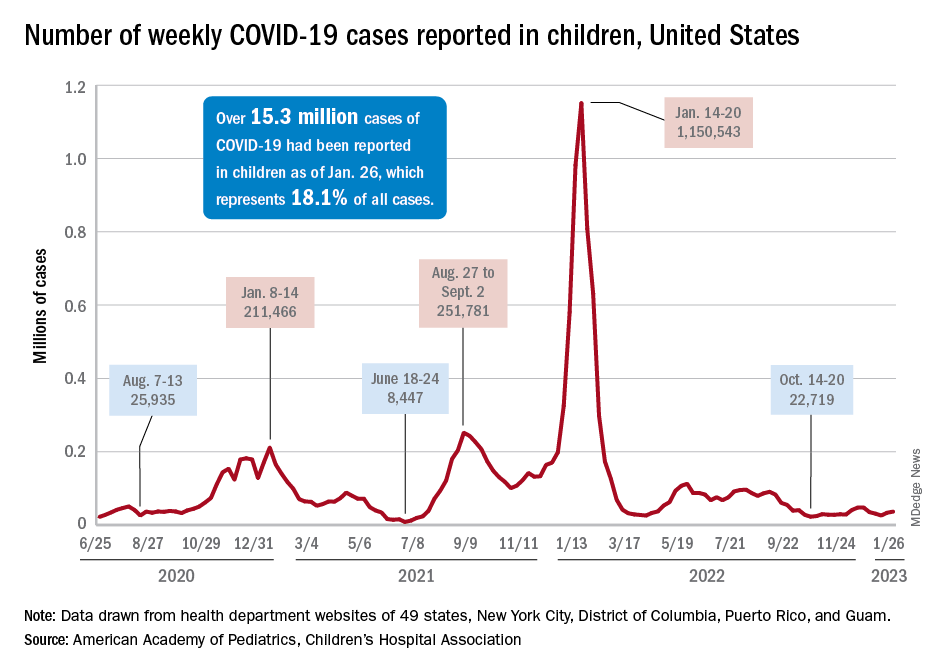
The CDC data, however, show that new cases doubled during the week of Jan. 1-7 to over 65,000, compared with the end of December, and stayed at that level for Jan. 8-14, and since CDC figures are subject to a 6-week reporting delay, the final numbers are likely to be even higher. The composition by age changed somewhat between the 2 weeks, though, as those aged 0-4 years went from almost half of all cases in the first week down to 40% in the second, while cases rose for children aged 5-11 and 12-15, based on data from the COVID-19 response team.
Emergency department visits for January do not show a corresponding increase. ED visits among children aged 0-11 years with COVID-19, measured as a percentage of all ED visits, declined over the course of the month, as did visits for 16- and 17-year-olds, while those aged 12-15 started the month at 1.4% and were at 1.4% on Jan. 27, with a slight dip down to 1.2% in between, the CDC said on its COVID Data Tracker. Daily hospitalizations for children aged 0-17 also declined through mid-January and did not reflect the jump in new cases.
Meanwhile, vaccinated children are still in the minority: 57% of those under age 18 have received no COVID vaccine yet, the AAP said in a separate report. Just 7.4% of children under age 2 years had received at least one dose as of Jan. 25, as had 10.1% of those aged 2-4 years, 39.6% of 5- to 11-year-olds and 71.8% of those 12-17 years old, according to the CDC, with corresponding figures for completion of the primary series at 3.5%, 5.3%, 32.5%, and 61.5%.
Although new COVID-19 cases in children, as measured by the American Academy of Pediatrics and the Children’s Hospital Association, have remained fairly steady in recent months, data from the Centers for Diseases Control and Prevention suggest that weekly cases took a big jump in early January.
For the most recent week covered . New cases for the first 2 weeks of the year – 31,000 for the week of Dec. 30 to Jan. 5 and 26,000 during Jan. 6-12 – were consistent with the AAP/CHA assertion that “weekly reported child cases have plateaued at an average of about 32,000 cases ... over the past 4 months.”
The CDC data, however, show that new cases doubled during the week of Jan. 1-7 to over 65,000, compared with the end of December, and stayed at that level for Jan. 8-14, and since CDC figures are subject to a 6-week reporting delay, the final numbers are likely to be even higher. The composition by age changed somewhat between the 2 weeks, though, as those aged 0-4 years went from almost half of all cases in the first week down to 40% in the second, while cases rose for children aged 5-11 and 12-15, based on data from the COVID-19 response team.
Emergency department visits for January do not show a corresponding increase. ED visits among children aged 0-11 years with COVID-19, measured as a percentage of all ED visits, declined over the course of the month, as did visits for 16- and 17-year-olds, while those aged 12-15 started the month at 1.4% and were at 1.4% on Jan. 27, with a slight dip down to 1.2% in between, the CDC said on its COVID Data Tracker. Daily hospitalizations for children aged 0-17 also declined through mid-January and did not reflect the jump in new cases.
Meanwhile, vaccinated children are still in the minority: 57% of those under age 18 have received no COVID vaccine yet, the AAP said in a separate report. Just 7.4% of children under age 2 years had received at least one dose as of Jan. 25, as had 10.1% of those aged 2-4 years, 39.6% of 5- to 11-year-olds and 71.8% of those 12-17 years old, according to the CDC, with corresponding figures for completion of the primary series at 3.5%, 5.3%, 32.5%, and 61.5%.
Although new COVID-19 cases in children, as measured by the American Academy of Pediatrics and the Children’s Hospital Association, have remained fairly steady in recent months, data from the Centers for Diseases Control and Prevention suggest that weekly cases took a big jump in early January.
For the most recent week covered . New cases for the first 2 weeks of the year – 31,000 for the week of Dec. 30 to Jan. 5 and 26,000 during Jan. 6-12 – were consistent with the AAP/CHA assertion that “weekly reported child cases have plateaued at an average of about 32,000 cases ... over the past 4 months.”
The CDC data, however, show that new cases doubled during the week of Jan. 1-7 to over 65,000, compared with the end of December, and stayed at that level for Jan. 8-14, and since CDC figures are subject to a 6-week reporting delay, the final numbers are likely to be even higher. The composition by age changed somewhat between the 2 weeks, though, as those aged 0-4 years went from almost half of all cases in the first week down to 40% in the second, while cases rose for children aged 5-11 and 12-15, based on data from the COVID-19 response team.
Emergency department visits for January do not show a corresponding increase. ED visits among children aged 0-11 years with COVID-19, measured as a percentage of all ED visits, declined over the course of the month, as did visits for 16- and 17-year-olds, while those aged 12-15 started the month at 1.4% and were at 1.4% on Jan. 27, with a slight dip down to 1.2% in between, the CDC said on its COVID Data Tracker. Daily hospitalizations for children aged 0-17 also declined through mid-January and did not reflect the jump in new cases.
Meanwhile, vaccinated children are still in the minority: 57% of those under age 18 have received no COVID vaccine yet, the AAP said in a separate report. Just 7.4% of children under age 2 years had received at least one dose as of Jan. 25, as had 10.1% of those aged 2-4 years, 39.6% of 5- to 11-year-olds and 71.8% of those 12-17 years old, according to the CDC, with corresponding figures for completion of the primary series at 3.5%, 5.3%, 32.5%, and 61.5%.
Citing workplace violence, one-fourth of critical care workers are ready to quit
A surgeon in Tulsa shot by a disgruntled patient. A doctor in India beaten by a group of bereaved family members. A general practitioner in the United Kingdom threatened with stabbing. A new study identifies this trend and finds that 25% of health care workers polled were willing to quit because of such violence.
“That was pretty appalling,” Rahul Kashyap, MD, MBA, MBBS, recalls. Dr. Kashyap is one of the leaders of the Violence Study of Healthcare Workers and Systems (ViSHWaS), which polled an international sample of physicians, nurses, and hospital staff. This study has worrying implications, Dr. Kashyap says. In a time when hospital staff are reporting burnout in record numbers, further deterrents may be the last thing our health care system needs. But Dr. Kashyap hopes that bringing awareness to these trends may allow physicians, policymakers, and the public to mobilize and intervene before it’s too late.
Previous studies have revealed similar trends. The rate of workplace violence directed at U.S. health care workers is five times that of workers in any other industry, according to the Bureau of Labor Statistics. The same study found that attacks had increased 63% from 2011 to 2018. Other polls that focus on the pandemic show that nearly half of U.S. nurses believe that violence increased since the world shut down. Well before the pandemic, however, a study from the Indian Medical Association found that 75% of doctors experienced workplace violence.
With this history in mind, perhaps it’s not surprising that the idea for the study came from the authors’ personal experiences. They had seen coworkers go through attacks, or they had endured attacks themselves, Dr. Kashyap says. But they couldn’t find any global data to back up these experiences. So Dr. Kashyap and his colleagues formed a web of volunteers dedicated to creating a cross-sectional study.
They got in touch with researchers from countries across Asia, the Middle East, South America, North America, and Africa. The initial group agreed to reach out to their contacts, casting a wide net. Researchers used WhatsApp, LinkedIn, and text messages to distribute the survey. Health care workers in each country completed the brief questionnaire, recalling their prepandemic world and evaluating their current one.
Within 2 months, they had reached health care workers in more than 100 countries. They concluded the study when they received about 5,000 results, according to Dr. Kashyap, and then began the process of stratifying the data. For this report, they focused on critical care, emergency medicine, and anesthesiology, which resulted in 598 responses from 69 countries. Of these, India and the United States had the highest number of participants.
In all, 73% of participants reported facing physical or verbal violence while in the hospital; 48% said they felt less motivated to work because of that violence; 39% of respondents believed that the amount of violence they experienced was the same as before the COVID-19 pandemic; and 36% of respondents believed that violence had increased. Even though they were trained on guidelines from the Occupational Safety and Health Administration, 20% of participants felt unprepared to face violence.
Although the study didn’t analyze the reasons workers felt this way, Dr. Kashyap speculates that it could be related to the medical distrust that grew during the pandemic or the stress patients and health care professionals experienced during its peak.
Regardless, the researchers say their study is a starting point. Now that the trend has been highlighted, it may be acted on.
Moving forward, Dr. Kashyap believes that controlling for different variables could determine whether factors like gender or shift time put a worker at higher risk for violence. He hopes it’s possible to interrupt these patterns and reestablish trust in the hospital environment. “It’s aspirational, but you’re hoping that through studies like ViSHWaS, which means trust in Hindi ... [we could restore] the trust and confidence among health care providers for the patients and family members.”
A version of this article first appeared on Medscape.com.
A surgeon in Tulsa shot by a disgruntled patient. A doctor in India beaten by a group of bereaved family members. A general practitioner in the United Kingdom threatened with stabbing. A new study identifies this trend and finds that 25% of health care workers polled were willing to quit because of such violence.
“That was pretty appalling,” Rahul Kashyap, MD, MBA, MBBS, recalls. Dr. Kashyap is one of the leaders of the Violence Study of Healthcare Workers and Systems (ViSHWaS), which polled an international sample of physicians, nurses, and hospital staff. This study has worrying implications, Dr. Kashyap says. In a time when hospital staff are reporting burnout in record numbers, further deterrents may be the last thing our health care system needs. But Dr. Kashyap hopes that bringing awareness to these trends may allow physicians, policymakers, and the public to mobilize and intervene before it’s too late.
Previous studies have revealed similar trends. The rate of workplace violence directed at U.S. health care workers is five times that of workers in any other industry, according to the Bureau of Labor Statistics. The same study found that attacks had increased 63% from 2011 to 2018. Other polls that focus on the pandemic show that nearly half of U.S. nurses believe that violence increased since the world shut down. Well before the pandemic, however, a study from the Indian Medical Association found that 75% of doctors experienced workplace violence.
With this history in mind, perhaps it’s not surprising that the idea for the study came from the authors’ personal experiences. They had seen coworkers go through attacks, or they had endured attacks themselves, Dr. Kashyap says. But they couldn’t find any global data to back up these experiences. So Dr. Kashyap and his colleagues formed a web of volunteers dedicated to creating a cross-sectional study.
They got in touch with researchers from countries across Asia, the Middle East, South America, North America, and Africa. The initial group agreed to reach out to their contacts, casting a wide net. Researchers used WhatsApp, LinkedIn, and text messages to distribute the survey. Health care workers in each country completed the brief questionnaire, recalling their prepandemic world and evaluating their current one.
Within 2 months, they had reached health care workers in more than 100 countries. They concluded the study when they received about 5,000 results, according to Dr. Kashyap, and then began the process of stratifying the data. For this report, they focused on critical care, emergency medicine, and anesthesiology, which resulted in 598 responses from 69 countries. Of these, India and the United States had the highest number of participants.
In all, 73% of participants reported facing physical or verbal violence while in the hospital; 48% said they felt less motivated to work because of that violence; 39% of respondents believed that the amount of violence they experienced was the same as before the COVID-19 pandemic; and 36% of respondents believed that violence had increased. Even though they were trained on guidelines from the Occupational Safety and Health Administration, 20% of participants felt unprepared to face violence.
Although the study didn’t analyze the reasons workers felt this way, Dr. Kashyap speculates that it could be related to the medical distrust that grew during the pandemic or the stress patients and health care professionals experienced during its peak.
Regardless, the researchers say their study is a starting point. Now that the trend has been highlighted, it may be acted on.
Moving forward, Dr. Kashyap believes that controlling for different variables could determine whether factors like gender or shift time put a worker at higher risk for violence. He hopes it’s possible to interrupt these patterns and reestablish trust in the hospital environment. “It’s aspirational, but you’re hoping that through studies like ViSHWaS, which means trust in Hindi ... [we could restore] the trust and confidence among health care providers for the patients and family members.”
A version of this article first appeared on Medscape.com.
A surgeon in Tulsa shot by a disgruntled patient. A doctor in India beaten by a group of bereaved family members. A general practitioner in the United Kingdom threatened with stabbing. A new study identifies this trend and finds that 25% of health care workers polled were willing to quit because of such violence.
“That was pretty appalling,” Rahul Kashyap, MD, MBA, MBBS, recalls. Dr. Kashyap is one of the leaders of the Violence Study of Healthcare Workers and Systems (ViSHWaS), which polled an international sample of physicians, nurses, and hospital staff. This study has worrying implications, Dr. Kashyap says. In a time when hospital staff are reporting burnout in record numbers, further deterrents may be the last thing our health care system needs. But Dr. Kashyap hopes that bringing awareness to these trends may allow physicians, policymakers, and the public to mobilize and intervene before it’s too late.
Previous studies have revealed similar trends. The rate of workplace violence directed at U.S. health care workers is five times that of workers in any other industry, according to the Bureau of Labor Statistics. The same study found that attacks had increased 63% from 2011 to 2018. Other polls that focus on the pandemic show that nearly half of U.S. nurses believe that violence increased since the world shut down. Well before the pandemic, however, a study from the Indian Medical Association found that 75% of doctors experienced workplace violence.
With this history in mind, perhaps it’s not surprising that the idea for the study came from the authors’ personal experiences. They had seen coworkers go through attacks, or they had endured attacks themselves, Dr. Kashyap says. But they couldn’t find any global data to back up these experiences. So Dr. Kashyap and his colleagues formed a web of volunteers dedicated to creating a cross-sectional study.
They got in touch with researchers from countries across Asia, the Middle East, South America, North America, and Africa. The initial group agreed to reach out to their contacts, casting a wide net. Researchers used WhatsApp, LinkedIn, and text messages to distribute the survey. Health care workers in each country completed the brief questionnaire, recalling their prepandemic world and evaluating their current one.
Within 2 months, they had reached health care workers in more than 100 countries. They concluded the study when they received about 5,000 results, according to Dr. Kashyap, and then began the process of stratifying the data. For this report, they focused on critical care, emergency medicine, and anesthesiology, which resulted in 598 responses from 69 countries. Of these, India and the United States had the highest number of participants.
In all, 73% of participants reported facing physical or verbal violence while in the hospital; 48% said they felt less motivated to work because of that violence; 39% of respondents believed that the amount of violence they experienced was the same as before the COVID-19 pandemic; and 36% of respondents believed that violence had increased. Even though they were trained on guidelines from the Occupational Safety and Health Administration, 20% of participants felt unprepared to face violence.
Although the study didn’t analyze the reasons workers felt this way, Dr. Kashyap speculates that it could be related to the medical distrust that grew during the pandemic or the stress patients and health care professionals experienced during its peak.
Regardless, the researchers say their study is a starting point. Now that the trend has been highlighted, it may be acted on.
Moving forward, Dr. Kashyap believes that controlling for different variables could determine whether factors like gender or shift time put a worker at higher risk for violence. He hopes it’s possible to interrupt these patterns and reestablish trust in the hospital environment. “It’s aspirational, but you’re hoping that through studies like ViSHWaS, which means trust in Hindi ... [we could restore] the trust and confidence among health care providers for the patients and family members.”
A version of this article first appeared on Medscape.com.
Feds charge 25 nursing school execs, staff in fake diploma scheme
The U.S. Department of Justice recently announced charges against 25 owners, operators, and employees of three Florida nursing schools in a fraud scheme in which they sold as many as 7,600 fake nursing degrees.
The purchasers in the diploma scheme paid $10,000 to $15,000 for degrees and transcripts and some 2,800 of the buyers passed the national nursing licensing exam to become registered nurses (RNs) and licensed practice nurses/vocational nurses (LPN/VNs) around the country, according to The New York Times.
Many of the degree recipients went on to work at hospitals, nursing homes, and Veterans Affairs medical centers, according to the U.S. Attorney’s Office for the Southern District of Florida.
Several national nursing organizations cooperated with the investigation, and the Delaware Division of Professional Regulation already annulled 26 licenses, according to the Delaware Nurses Association. Fake licenses were issued in five states, according to federal reports.
“We are deeply unsettled by this egregious act,” DNA President Stephanie McClellan, MSN, RN, CMSRN, said in the group’s press statement. “We want all Delaware nurses to be aware of this active issue and to speak up if there is a concern regarding capacity to practice safely by a colleague/peer,” she said.
The Oregon State Board of Nursing is also investigating at least a dozen nurses who may have paid for their degrees, according to a Portland CBS affiliate.
The National Council of State Boards of Nursing said in a statement that it had helped authorities identify and monitor the individuals who allegedly provided the false degrees.
Nursing community reacts
News of the fraud scheme spread through the nursing community, including social media. “The recent report on falsified nursing school degrees is both heartbreaking and serves as an eye-opener,” tweeted Usha Menon, PhD, RN, FAAN, dean and health professor of the University of South Florida Health College of Nursing. “There was enough of a need that prompted these bad actors to develop a scheme that could’ve endangered dozens of lives.”
Jennifer Mensik Kennedy, PhD, MBA, RN, the new president of the American Nurses Association, also weighed in. “The accusation that personnel at once-accredited nursing schools allegedly participated in this scheme is simply deplorable. These unlawful and unethical acts disparage the reputation of actual nurses everywhere who have rightfully earned [their titles] through their education, hard work, dedication, and time.”
The false degrees and transcripts were issued by three once-accredited and now-shuttered nursing schools in South Florida: Palm Beach School of Nursing, Sacred Heart International Institute, and Sienna College.
The alleged co-conspirators reportedly made $114 million from the scheme, which dates back to 2016, according to several news reports. Each defendant faces up to 20 years in prison.
Most LPN programs charge $10,000 to $15,000 to complete a program, Robert Rosseter, a spokesperson for the American Association of Colleges of Nursing (AACN), told this news organization.
None were AACN members, and none were accredited by the Commission on Collegiate Nursing Education, which is AACN’s autonomous accrediting agency, Mr. Rosseter said. AACN membership is voluntary and is open to schools offering baccalaureate or higher degrees, he explained.
“What is disturbing about this investigation is that there are over 7,600 people around the country with fraudulent nursing credentials who are potentially in critical health care roles treating patients,” Chad Yarbrough, acting special agent in charge for the FBI in Miami, said in the federal justice department release.
‘Operation Nightingale’ based on tip
The federal action, dubbed “Operation Nightingale” after the nursing pioneer Florence Nightingale, began in 2019. It was based on a tip related to a case in Maryland, according to Nurse.org.
That case ensnared Palm Beach School of Nursing owner Johanah Napoleon, who reportedly was selling fake degrees for $6,000 to $18,000 each to two individuals in Maryland and Virginia. Ms. Napoleon was charged in 2021 and eventually pled guilty. The Florida Board of Nursing shut down the Palm Beach school in 2017 owing to its students’ low passing rate on the national licensing exam.
Two participants in the bigger scheme who had also worked with Ms. Napoleon – Geralda Adrien and Woosvelt Predestin – were indicted in 2021. Ms. Adrien owned private education companies for people who at aspired to be nurses, and Mr. Predestin was an employee. They were sentenced to 27 months in prison last year and helped the federal officials build the larger case.
The 25 individuals who were charged Jan. 25 operated in Delaware, New York, New Jersey, Texas, and Florida.
Schemes lured immigrants
In the scheme involving Siena College, some of the individuals acted as recruiters to direct nurses who were looking for employment to the school, where they allegedly would then pay for an RN or LPN/VN degree. The recipients of the false documents then used them to obtain jobs, including at a hospital in Georgia and a Veterans Affairs medical center in Maryland, according to one indictment. The president of Siena and her co-conspirators sold more than 2,000 fake diplomas, according to charging documents.
At the Palm Beach College of Nursing, individuals at various nursing prep and education programs allegedly helped others obtain fake degrees and transcripts, which were then used to pass RN and LPN/VN licensing exams in states that included Massachusetts, New Jersey, New York, and Ohio, according to the indictment.
Some individuals then secured employment with a nursing home in Ohio, a home health agency for pediatric patients in Massachusetts, and skilled nursing facilities in New York and New Jersey.
Prosecutors allege that the president of Sacred Heart International Institute and two other co-conspirators sold 588 fake diplomas.
The FBI said that some of the aspiring nurses who were talked into buying the degrees were LPNs who wanted to become RNs and that most of those lured into the scheme were from South Florida’s Haitian American immigrant community, Nurse.org reported.
A version of this article first appeared on Medscape.com.
The U.S. Department of Justice recently announced charges against 25 owners, operators, and employees of three Florida nursing schools in a fraud scheme in which they sold as many as 7,600 fake nursing degrees.
The purchasers in the diploma scheme paid $10,000 to $15,000 for degrees and transcripts and some 2,800 of the buyers passed the national nursing licensing exam to become registered nurses (RNs) and licensed practice nurses/vocational nurses (LPN/VNs) around the country, according to The New York Times.
Many of the degree recipients went on to work at hospitals, nursing homes, and Veterans Affairs medical centers, according to the U.S. Attorney’s Office for the Southern District of Florida.
Several national nursing organizations cooperated with the investigation, and the Delaware Division of Professional Regulation already annulled 26 licenses, according to the Delaware Nurses Association. Fake licenses were issued in five states, according to federal reports.
“We are deeply unsettled by this egregious act,” DNA President Stephanie McClellan, MSN, RN, CMSRN, said in the group’s press statement. “We want all Delaware nurses to be aware of this active issue and to speak up if there is a concern regarding capacity to practice safely by a colleague/peer,” she said.
The Oregon State Board of Nursing is also investigating at least a dozen nurses who may have paid for their degrees, according to a Portland CBS affiliate.
The National Council of State Boards of Nursing said in a statement that it had helped authorities identify and monitor the individuals who allegedly provided the false degrees.
Nursing community reacts
News of the fraud scheme spread through the nursing community, including social media. “The recent report on falsified nursing school degrees is both heartbreaking and serves as an eye-opener,” tweeted Usha Menon, PhD, RN, FAAN, dean and health professor of the University of South Florida Health College of Nursing. “There was enough of a need that prompted these bad actors to develop a scheme that could’ve endangered dozens of lives.”
Jennifer Mensik Kennedy, PhD, MBA, RN, the new president of the American Nurses Association, also weighed in. “The accusation that personnel at once-accredited nursing schools allegedly participated in this scheme is simply deplorable. These unlawful and unethical acts disparage the reputation of actual nurses everywhere who have rightfully earned [their titles] through their education, hard work, dedication, and time.”
The false degrees and transcripts were issued by three once-accredited and now-shuttered nursing schools in South Florida: Palm Beach School of Nursing, Sacred Heart International Institute, and Sienna College.
The alleged co-conspirators reportedly made $114 million from the scheme, which dates back to 2016, according to several news reports. Each defendant faces up to 20 years in prison.
Most LPN programs charge $10,000 to $15,000 to complete a program, Robert Rosseter, a spokesperson for the American Association of Colleges of Nursing (AACN), told this news organization.
None were AACN members, and none were accredited by the Commission on Collegiate Nursing Education, which is AACN’s autonomous accrediting agency, Mr. Rosseter said. AACN membership is voluntary and is open to schools offering baccalaureate or higher degrees, he explained.
“What is disturbing about this investigation is that there are over 7,600 people around the country with fraudulent nursing credentials who are potentially in critical health care roles treating patients,” Chad Yarbrough, acting special agent in charge for the FBI in Miami, said in the federal justice department release.
‘Operation Nightingale’ based on tip
The federal action, dubbed “Operation Nightingale” after the nursing pioneer Florence Nightingale, began in 2019. It was based on a tip related to a case in Maryland, according to Nurse.org.
That case ensnared Palm Beach School of Nursing owner Johanah Napoleon, who reportedly was selling fake degrees for $6,000 to $18,000 each to two individuals in Maryland and Virginia. Ms. Napoleon was charged in 2021 and eventually pled guilty. The Florida Board of Nursing shut down the Palm Beach school in 2017 owing to its students’ low passing rate on the national licensing exam.
Two participants in the bigger scheme who had also worked with Ms. Napoleon – Geralda Adrien and Woosvelt Predestin – were indicted in 2021. Ms. Adrien owned private education companies for people who at aspired to be nurses, and Mr. Predestin was an employee. They were sentenced to 27 months in prison last year and helped the federal officials build the larger case.
The 25 individuals who were charged Jan. 25 operated in Delaware, New York, New Jersey, Texas, and Florida.
Schemes lured immigrants
In the scheme involving Siena College, some of the individuals acted as recruiters to direct nurses who were looking for employment to the school, where they allegedly would then pay for an RN or LPN/VN degree. The recipients of the false documents then used them to obtain jobs, including at a hospital in Georgia and a Veterans Affairs medical center in Maryland, according to one indictment. The president of Siena and her co-conspirators sold more than 2,000 fake diplomas, according to charging documents.
At the Palm Beach College of Nursing, individuals at various nursing prep and education programs allegedly helped others obtain fake degrees and transcripts, which were then used to pass RN and LPN/VN licensing exams in states that included Massachusetts, New Jersey, New York, and Ohio, according to the indictment.
Some individuals then secured employment with a nursing home in Ohio, a home health agency for pediatric patients in Massachusetts, and skilled nursing facilities in New York and New Jersey.
Prosecutors allege that the president of Sacred Heart International Institute and two other co-conspirators sold 588 fake diplomas.
The FBI said that some of the aspiring nurses who were talked into buying the degrees were LPNs who wanted to become RNs and that most of those lured into the scheme were from South Florida’s Haitian American immigrant community, Nurse.org reported.
A version of this article first appeared on Medscape.com.
The U.S. Department of Justice recently announced charges against 25 owners, operators, and employees of three Florida nursing schools in a fraud scheme in which they sold as many as 7,600 fake nursing degrees.
The purchasers in the diploma scheme paid $10,000 to $15,000 for degrees and transcripts and some 2,800 of the buyers passed the national nursing licensing exam to become registered nurses (RNs) and licensed practice nurses/vocational nurses (LPN/VNs) around the country, according to The New York Times.
Many of the degree recipients went on to work at hospitals, nursing homes, and Veterans Affairs medical centers, according to the U.S. Attorney’s Office for the Southern District of Florida.
Several national nursing organizations cooperated with the investigation, and the Delaware Division of Professional Regulation already annulled 26 licenses, according to the Delaware Nurses Association. Fake licenses were issued in five states, according to federal reports.
“We are deeply unsettled by this egregious act,” DNA President Stephanie McClellan, MSN, RN, CMSRN, said in the group’s press statement. “We want all Delaware nurses to be aware of this active issue and to speak up if there is a concern regarding capacity to practice safely by a colleague/peer,” she said.
The Oregon State Board of Nursing is also investigating at least a dozen nurses who may have paid for their degrees, according to a Portland CBS affiliate.
The National Council of State Boards of Nursing said in a statement that it had helped authorities identify and monitor the individuals who allegedly provided the false degrees.
Nursing community reacts
News of the fraud scheme spread through the nursing community, including social media. “The recent report on falsified nursing school degrees is both heartbreaking and serves as an eye-opener,” tweeted Usha Menon, PhD, RN, FAAN, dean and health professor of the University of South Florida Health College of Nursing. “There was enough of a need that prompted these bad actors to develop a scheme that could’ve endangered dozens of lives.”
Jennifer Mensik Kennedy, PhD, MBA, RN, the new president of the American Nurses Association, also weighed in. “The accusation that personnel at once-accredited nursing schools allegedly participated in this scheme is simply deplorable. These unlawful and unethical acts disparage the reputation of actual nurses everywhere who have rightfully earned [their titles] through their education, hard work, dedication, and time.”
The false degrees and transcripts were issued by three once-accredited and now-shuttered nursing schools in South Florida: Palm Beach School of Nursing, Sacred Heart International Institute, and Sienna College.
The alleged co-conspirators reportedly made $114 million from the scheme, which dates back to 2016, according to several news reports. Each defendant faces up to 20 years in prison.
Most LPN programs charge $10,000 to $15,000 to complete a program, Robert Rosseter, a spokesperson for the American Association of Colleges of Nursing (AACN), told this news organization.
None were AACN members, and none were accredited by the Commission on Collegiate Nursing Education, which is AACN’s autonomous accrediting agency, Mr. Rosseter said. AACN membership is voluntary and is open to schools offering baccalaureate or higher degrees, he explained.
“What is disturbing about this investigation is that there are over 7,600 people around the country with fraudulent nursing credentials who are potentially in critical health care roles treating patients,” Chad Yarbrough, acting special agent in charge for the FBI in Miami, said in the federal justice department release.
‘Operation Nightingale’ based on tip
The federal action, dubbed “Operation Nightingale” after the nursing pioneer Florence Nightingale, began in 2019. It was based on a tip related to a case in Maryland, according to Nurse.org.
That case ensnared Palm Beach School of Nursing owner Johanah Napoleon, who reportedly was selling fake degrees for $6,000 to $18,000 each to two individuals in Maryland and Virginia. Ms. Napoleon was charged in 2021 and eventually pled guilty. The Florida Board of Nursing shut down the Palm Beach school in 2017 owing to its students’ low passing rate on the national licensing exam.
Two participants in the bigger scheme who had also worked with Ms. Napoleon – Geralda Adrien and Woosvelt Predestin – were indicted in 2021. Ms. Adrien owned private education companies for people who at aspired to be nurses, and Mr. Predestin was an employee. They were sentenced to 27 months in prison last year and helped the federal officials build the larger case.
The 25 individuals who were charged Jan. 25 operated in Delaware, New York, New Jersey, Texas, and Florida.
Schemes lured immigrants
In the scheme involving Siena College, some of the individuals acted as recruiters to direct nurses who were looking for employment to the school, where they allegedly would then pay for an RN or LPN/VN degree. The recipients of the false documents then used them to obtain jobs, including at a hospital in Georgia and a Veterans Affairs medical center in Maryland, according to one indictment. The president of Siena and her co-conspirators sold more than 2,000 fake diplomas, according to charging documents.
At the Palm Beach College of Nursing, individuals at various nursing prep and education programs allegedly helped others obtain fake degrees and transcripts, which were then used to pass RN and LPN/VN licensing exams in states that included Massachusetts, New Jersey, New York, and Ohio, according to the indictment.
Some individuals then secured employment with a nursing home in Ohio, a home health agency for pediatric patients in Massachusetts, and skilled nursing facilities in New York and New Jersey.
Prosecutors allege that the president of Sacred Heart International Institute and two other co-conspirators sold 588 fake diplomas.
The FBI said that some of the aspiring nurses who were talked into buying the degrees were LPNs who wanted to become RNs and that most of those lured into the scheme were from South Florida’s Haitian American immigrant community, Nurse.org reported.
A version of this article first appeared on Medscape.com.
Biden to end COVID emergencies in May
Doing so will have many effects, including the end of free vaccines and health services to fight the pandemic. The public health emergency has been renewed every 90 days since it was declared by the Trump administration in January 2020.
The declaration allowed major changes throughout the health care system to deal with the pandemic, including the free distribution of vaccines, testing, and treatments. In addition, telehealth services were expanded, and Medicaid and the Children’s Health Insurance Program were extended to millions more Americans.
Biden said the COVID-19 national emergency is set to expire March 1 while the declared public health emergency would currently expire on April 11. The president said both will be extended to end May 11.
There were nearly 300,000 newly reported COVID-19 cases in the United States for the week ending Jan. 25, according to CDC data, as well as more than 3,750 deaths.
A version of this article first appeared on WebMD.com.
Doing so will have many effects, including the end of free vaccines and health services to fight the pandemic. The public health emergency has been renewed every 90 days since it was declared by the Trump administration in January 2020.
The declaration allowed major changes throughout the health care system to deal with the pandemic, including the free distribution of vaccines, testing, and treatments. In addition, telehealth services were expanded, and Medicaid and the Children’s Health Insurance Program were extended to millions more Americans.
Biden said the COVID-19 national emergency is set to expire March 1 while the declared public health emergency would currently expire on April 11. The president said both will be extended to end May 11.
There were nearly 300,000 newly reported COVID-19 cases in the United States for the week ending Jan. 25, according to CDC data, as well as more than 3,750 deaths.
A version of this article first appeared on WebMD.com.
Doing so will have many effects, including the end of free vaccines and health services to fight the pandemic. The public health emergency has been renewed every 90 days since it was declared by the Trump administration in January 2020.
The declaration allowed major changes throughout the health care system to deal with the pandemic, including the free distribution of vaccines, testing, and treatments. In addition, telehealth services were expanded, and Medicaid and the Children’s Health Insurance Program were extended to millions more Americans.
Biden said the COVID-19 national emergency is set to expire March 1 while the declared public health emergency would currently expire on April 11. The president said both will be extended to end May 11.
There were nearly 300,000 newly reported COVID-19 cases in the United States for the week ending Jan. 25, according to CDC data, as well as more than 3,750 deaths.
A version of this article first appeared on WebMD.com.
Angioedema risk jumps when switching HF meds
New renin-angiotensin-system (RAS) inhibitor therapy using sacubitril-valsartan (Entresto) is no more likely to cause angioedema than starting out with an ACE inhibitor or angiotensin receptor blocker (ARB).
But the risk climbs when such patients start on an ACE inhibitor or ARB and then switch to sacubitril-valsartan, compared with those prescribed the newer drug, the only available angiotensin receptor-neprilysin inhibitor (ARNI), in the first place.
Those findings and others from a large database analysis, by researchers at the Food and Drug Administration and Harvard Medical School, may clarify and help alleviate a residual safety concern about the ARNI – that it might promote angioedema – that persists after the drug’s major HF trials.
The angioedema risk increased the most right after the switch to the ARNI from one of the older RAS inhibitors. For example, the overall risk doubled for patients who started with an ARB then switched to sacubitril-valsartan, compared with those who started on the newer drug. But it went up about 2.5 times during the first 14 days after the switch.
A similar pattern emerged for ACE inhibitors, but the increased angioedema risk reached significance only within 2 weeks of the switch from an ACE inhibitor to sacubitril-valsartan compared to starting on the latter.
The analysis, based on data from the FDA’s Sentinel adverse event reporting system, was published in the Journal of the American College of Cardiology.
A rare complication, but ...
Angioedema was rare overall in the study, with an unadjusted rate of about 6.75 per 1,000 person-years for users of ACE inhibitors, less than half that rate for ARB users, and only one-fifth that rate for sacubitril-valsartan recipients.
But even a rare complication can be a worry for drugs as widely used as RAS inhibitors. And it’s not unusual for patients cautiously started on an ACE inhibitor or ARB to be switched to sacubitril-valsartan, which is only recently a core guideline–recommended therapy for HF with reduced ejection fraction.
Such patients transitioning to the ARNI, the current study suggests, should probably be watched closely for signs of angioedema for 2 weeks but especially during the first few days. Indeed, the study’s event curves show most of the extra risk “popping up” right after the switch to sacubitril-valsartan, lead author Efe Eworuke, PhD, told this news organization.
The ARNI’s labeling, which states the drug should follow ACE inhibitors only after 36-hour washout period, “has done justice to this issue,” she said. But “whether clinicians are adhering to that, we can’t tell.”
Potentially, patients who miss the 36-hour washout between ACE inhibitors or ARBs and sacubitril-valsartan may account for the excess angioedema risk seen in the analysis, said Dr. Eworuke, with the FDA’s Center for Drug Evaluation and Research, Silver Spring, Md.
But the analysis doesn’t nail down the window of excess risk to only 36 hours. It suggests that patients switching to the ARNI – even those pausing for 36 hours in between drugs – should probably be monitored “2 weeks or longer,” she said. “They could still have angioedema after the washout period.”
Indeed, the “timing of the switch may be critical,” according to an editorial accompanying the report. “Perhaps a longer initial exposure period of ACE inhibitor or ARB,” beyond 2 weeks, “should be considered before switching to an ARNI,” contended Robert L. Page II, PharmD, MSPH, University of Colorado Skaggs School of Pharmacy and Pharmaceutical Sciences, Aurora.
Moreover, he wrote, the study suggests that “initiation of an ARNI de novo may be safer compared with trialing an ACE inhibitor or ARB then switching to an ARNI,” and “should be a consideration when beginning guideline-directed medical therapy for patients with HF.”
New RAS inhibition with ARNI ‘protective’
Compared with ARNI “new users” who had not received any RAS inhibitor in the prior 6 months, patients in the study who switched from an ACE inhibitor to ARNI (41,548 matched pairs) showed a hazard ratio (HR) for angioedema of 1.62 (95% confidence interval [CI], 0.91-2.89), that is, only a “trend,” the report states.
But that trend became significant when the analysis considered only angioedema cases in the first 14 days after the drug switch: HR, 1.98 (95% CI, 1.11-3.53).
Those switching from an ARB to ARNI, compared with ARNI new users (37,893 matched pairs), showed a significant HR for angioedema of 2.03 (95% CI, 1.16-3.54). The effect was more pronounced when considering only angioedema arising in the first 2 weeks: HR, 2.45 (95% CI, 1.36-4.43).
Compared with new use of ACE inhibitors, new ARNI use (41,998 matched pairs) was “protective,” the report states, with an HR for angioedema of 0.18 (95% CI, 0.11-0.29). So was a switch from ACE inhibitors to the ARNI (69,639 matched pairs), with an HR of 0.31 (95% CI, 0.23-0.43).
But compared with starting with an ARB, ARNI new use (43,755 matched pairs) had a null effect on angioedema risk, HR, 0.59 (95% CI, 0.35-1.01); as did switching from an ARB to ARNI (49,137 matched pairs), HR, 0.85 (95% CI, 0.58-1.26).
The analysis has limitations, Dr. Eworuke acknowledged. The comparator groups probably differed in unknown ways given the limits of propensity matching, for example, and because the FDA’s Sentinel system data can reflect only cases that are reported, the study probably underestimates the true prevalence of angioedema.
For example, a patient may see a clinician for a milder case that resolves without a significant intervention, she noted. But “those types of angioedema would not have been captured by our study.”
Dr. Eworuke disclosed that her comments reflect her views and are not those of the Food and Drug Administration; she and the other authors, as well as editorialist Dr. Page, report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New renin-angiotensin-system (RAS) inhibitor therapy using sacubitril-valsartan (Entresto) is no more likely to cause angioedema than starting out with an ACE inhibitor or angiotensin receptor blocker (ARB).
But the risk climbs when such patients start on an ACE inhibitor or ARB and then switch to sacubitril-valsartan, compared with those prescribed the newer drug, the only available angiotensin receptor-neprilysin inhibitor (ARNI), in the first place.
Those findings and others from a large database analysis, by researchers at the Food and Drug Administration and Harvard Medical School, may clarify and help alleviate a residual safety concern about the ARNI – that it might promote angioedema – that persists after the drug’s major HF trials.
The angioedema risk increased the most right after the switch to the ARNI from one of the older RAS inhibitors. For example, the overall risk doubled for patients who started with an ARB then switched to sacubitril-valsartan, compared with those who started on the newer drug. But it went up about 2.5 times during the first 14 days after the switch.
A similar pattern emerged for ACE inhibitors, but the increased angioedema risk reached significance only within 2 weeks of the switch from an ACE inhibitor to sacubitril-valsartan compared to starting on the latter.
The analysis, based on data from the FDA’s Sentinel adverse event reporting system, was published in the Journal of the American College of Cardiology.
A rare complication, but ...
Angioedema was rare overall in the study, with an unadjusted rate of about 6.75 per 1,000 person-years for users of ACE inhibitors, less than half that rate for ARB users, and only one-fifth that rate for sacubitril-valsartan recipients.
But even a rare complication can be a worry for drugs as widely used as RAS inhibitors. And it’s not unusual for patients cautiously started on an ACE inhibitor or ARB to be switched to sacubitril-valsartan, which is only recently a core guideline–recommended therapy for HF with reduced ejection fraction.
Such patients transitioning to the ARNI, the current study suggests, should probably be watched closely for signs of angioedema for 2 weeks but especially during the first few days. Indeed, the study’s event curves show most of the extra risk “popping up” right after the switch to sacubitril-valsartan, lead author Efe Eworuke, PhD, told this news organization.
The ARNI’s labeling, which states the drug should follow ACE inhibitors only after 36-hour washout period, “has done justice to this issue,” she said. But “whether clinicians are adhering to that, we can’t tell.”
Potentially, patients who miss the 36-hour washout between ACE inhibitors or ARBs and sacubitril-valsartan may account for the excess angioedema risk seen in the analysis, said Dr. Eworuke, with the FDA’s Center for Drug Evaluation and Research, Silver Spring, Md.
But the analysis doesn’t nail down the window of excess risk to only 36 hours. It suggests that patients switching to the ARNI – even those pausing for 36 hours in between drugs – should probably be monitored “2 weeks or longer,” she said. “They could still have angioedema after the washout period.”
Indeed, the “timing of the switch may be critical,” according to an editorial accompanying the report. “Perhaps a longer initial exposure period of ACE inhibitor or ARB,” beyond 2 weeks, “should be considered before switching to an ARNI,” contended Robert L. Page II, PharmD, MSPH, University of Colorado Skaggs School of Pharmacy and Pharmaceutical Sciences, Aurora.
Moreover, he wrote, the study suggests that “initiation of an ARNI de novo may be safer compared with trialing an ACE inhibitor or ARB then switching to an ARNI,” and “should be a consideration when beginning guideline-directed medical therapy for patients with HF.”
New RAS inhibition with ARNI ‘protective’
Compared with ARNI “new users” who had not received any RAS inhibitor in the prior 6 months, patients in the study who switched from an ACE inhibitor to ARNI (41,548 matched pairs) showed a hazard ratio (HR) for angioedema of 1.62 (95% confidence interval [CI], 0.91-2.89), that is, only a “trend,” the report states.
But that trend became significant when the analysis considered only angioedema cases in the first 14 days after the drug switch: HR, 1.98 (95% CI, 1.11-3.53).
Those switching from an ARB to ARNI, compared with ARNI new users (37,893 matched pairs), showed a significant HR for angioedema of 2.03 (95% CI, 1.16-3.54). The effect was more pronounced when considering only angioedema arising in the first 2 weeks: HR, 2.45 (95% CI, 1.36-4.43).
Compared with new use of ACE inhibitors, new ARNI use (41,998 matched pairs) was “protective,” the report states, with an HR for angioedema of 0.18 (95% CI, 0.11-0.29). So was a switch from ACE inhibitors to the ARNI (69,639 matched pairs), with an HR of 0.31 (95% CI, 0.23-0.43).
But compared with starting with an ARB, ARNI new use (43,755 matched pairs) had a null effect on angioedema risk, HR, 0.59 (95% CI, 0.35-1.01); as did switching from an ARB to ARNI (49,137 matched pairs), HR, 0.85 (95% CI, 0.58-1.26).
The analysis has limitations, Dr. Eworuke acknowledged. The comparator groups probably differed in unknown ways given the limits of propensity matching, for example, and because the FDA’s Sentinel system data can reflect only cases that are reported, the study probably underestimates the true prevalence of angioedema.
For example, a patient may see a clinician for a milder case that resolves without a significant intervention, she noted. But “those types of angioedema would not have been captured by our study.”
Dr. Eworuke disclosed that her comments reflect her views and are not those of the Food and Drug Administration; she and the other authors, as well as editorialist Dr. Page, report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New renin-angiotensin-system (RAS) inhibitor therapy using sacubitril-valsartan (Entresto) is no more likely to cause angioedema than starting out with an ACE inhibitor or angiotensin receptor blocker (ARB).
But the risk climbs when such patients start on an ACE inhibitor or ARB and then switch to sacubitril-valsartan, compared with those prescribed the newer drug, the only available angiotensin receptor-neprilysin inhibitor (ARNI), in the first place.
Those findings and others from a large database analysis, by researchers at the Food and Drug Administration and Harvard Medical School, may clarify and help alleviate a residual safety concern about the ARNI – that it might promote angioedema – that persists after the drug’s major HF trials.
The angioedema risk increased the most right after the switch to the ARNI from one of the older RAS inhibitors. For example, the overall risk doubled for patients who started with an ARB then switched to sacubitril-valsartan, compared with those who started on the newer drug. But it went up about 2.5 times during the first 14 days after the switch.
A similar pattern emerged for ACE inhibitors, but the increased angioedema risk reached significance only within 2 weeks of the switch from an ACE inhibitor to sacubitril-valsartan compared to starting on the latter.
The analysis, based on data from the FDA’s Sentinel adverse event reporting system, was published in the Journal of the American College of Cardiology.
A rare complication, but ...
Angioedema was rare overall in the study, with an unadjusted rate of about 6.75 per 1,000 person-years for users of ACE inhibitors, less than half that rate for ARB users, and only one-fifth that rate for sacubitril-valsartan recipients.
But even a rare complication can be a worry for drugs as widely used as RAS inhibitors. And it’s not unusual for patients cautiously started on an ACE inhibitor or ARB to be switched to sacubitril-valsartan, which is only recently a core guideline–recommended therapy for HF with reduced ejection fraction.
Such patients transitioning to the ARNI, the current study suggests, should probably be watched closely for signs of angioedema for 2 weeks but especially during the first few days. Indeed, the study’s event curves show most of the extra risk “popping up” right after the switch to sacubitril-valsartan, lead author Efe Eworuke, PhD, told this news organization.
The ARNI’s labeling, which states the drug should follow ACE inhibitors only after 36-hour washout period, “has done justice to this issue,” she said. But “whether clinicians are adhering to that, we can’t tell.”
Potentially, patients who miss the 36-hour washout between ACE inhibitors or ARBs and sacubitril-valsartan may account for the excess angioedema risk seen in the analysis, said Dr. Eworuke, with the FDA’s Center for Drug Evaluation and Research, Silver Spring, Md.
But the analysis doesn’t nail down the window of excess risk to only 36 hours. It suggests that patients switching to the ARNI – even those pausing for 36 hours in between drugs – should probably be monitored “2 weeks or longer,” she said. “They could still have angioedema after the washout period.”
Indeed, the “timing of the switch may be critical,” according to an editorial accompanying the report. “Perhaps a longer initial exposure period of ACE inhibitor or ARB,” beyond 2 weeks, “should be considered before switching to an ARNI,” contended Robert L. Page II, PharmD, MSPH, University of Colorado Skaggs School of Pharmacy and Pharmaceutical Sciences, Aurora.
Moreover, he wrote, the study suggests that “initiation of an ARNI de novo may be safer compared with trialing an ACE inhibitor or ARB then switching to an ARNI,” and “should be a consideration when beginning guideline-directed medical therapy for patients with HF.”
New RAS inhibition with ARNI ‘protective’
Compared with ARNI “new users” who had not received any RAS inhibitor in the prior 6 months, patients in the study who switched from an ACE inhibitor to ARNI (41,548 matched pairs) showed a hazard ratio (HR) for angioedema of 1.62 (95% confidence interval [CI], 0.91-2.89), that is, only a “trend,” the report states.
But that trend became significant when the analysis considered only angioedema cases in the first 14 days after the drug switch: HR, 1.98 (95% CI, 1.11-3.53).
Those switching from an ARB to ARNI, compared with ARNI new users (37,893 matched pairs), showed a significant HR for angioedema of 2.03 (95% CI, 1.16-3.54). The effect was more pronounced when considering only angioedema arising in the first 2 weeks: HR, 2.45 (95% CI, 1.36-4.43).
Compared with new use of ACE inhibitors, new ARNI use (41,998 matched pairs) was “protective,” the report states, with an HR for angioedema of 0.18 (95% CI, 0.11-0.29). So was a switch from ACE inhibitors to the ARNI (69,639 matched pairs), with an HR of 0.31 (95% CI, 0.23-0.43).
But compared with starting with an ARB, ARNI new use (43,755 matched pairs) had a null effect on angioedema risk, HR, 0.59 (95% CI, 0.35-1.01); as did switching from an ARB to ARNI (49,137 matched pairs), HR, 0.85 (95% CI, 0.58-1.26).
The analysis has limitations, Dr. Eworuke acknowledged. The comparator groups probably differed in unknown ways given the limits of propensity matching, for example, and because the FDA’s Sentinel system data can reflect only cases that are reported, the study probably underestimates the true prevalence of angioedema.
For example, a patient may see a clinician for a milder case that resolves without a significant intervention, she noted. But “those types of angioedema would not have been captured by our study.”
Dr. Eworuke disclosed that her comments reflect her views and are not those of the Food and Drug Administration; she and the other authors, as well as editorialist Dr. Page, report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Even one head injury boosts all-cause mortality risk
An analysis of more than 13,000 adult participants in the Atherosclerosis Risk in Communities (ARIC) study showed a dose-response pattern in which one head injury was linked to a 66% increased risk for all-cause mortality, and two or more head injuries were associated with twice the risk in comparison with no head injuries.
These findings underscore the importance of preventing head injuries and of swift clinical intervention once a head injury occurs, lead author Holly Elser, MD, PhD, department of neurology, Hospital of the University of Pennsylvania, Philadelphia, told this news organization.
“Clinicians should counsel patients who are at risk for falls about head injuries and ensure patients are promptly evaluated in the hospital setting if they do have a fall – especially with loss of consciousness or other symptoms, such as headache or dizziness,” Dr. Elser added.
The findings were published online in JAMA Neurology.
Consistent evidence
There is “pretty consistent evidence” that mortality rates are increased in the short term after head injury, predominantly among hospitalized patients, Dr. Elser noted.
“But there’s less evidence about the long-term mortality implications of head injuries and less evidence from adults living in the community,” she added.
The analysis included 13,037 participants in the ARIC study, an ongoing study involving adults aged 45-65 years who were recruited from four geographically and racially diverse U.S. communities. The mean age at baseline (1987-1989) was 54 years; 57.7% were women; and 27.9% were Black.
Study participants are followed at routine in-person visits and semiannually via telephone.
Data on head injuries came from hospital diagnostic codes and self-reports. These reports included information on the number of injuries and whether the injury required medical care and involved loss of consciousness.
During the 27-year follow-up, 18.4% of the study sample had at least one head injury. Injuries occurred more frequently among women, which may reflect the predominance of women in the study population, said Dr. Elser.
Overall, about 56% of participants died during the study period. The estimated median amount of survival time after head injury was 4.7 years.
The most common causes of death were neoplasm, cardiovascular disease, and neurologic disorders. Regarding specific neurologic causes of death, the researchers found that 62.2% of deaths were due to neurodegenerative disease among individuals with head injury, vs. 51.4% among those without head injury.
This, said Dr. Elser, raises the possibility of reverse causality. “If you have a neurodegenerative disorder like Alzheimer’s disease dementia or Parkinson’s disease that leads to difficulty walking, you may be more likely to fall and have a head injury. The head injury in turn may lead to increased mortality,” she noted.
However, she stressed that the data on cause-specific mortality are exploratory. “Our research motivates future studies that really examine this time-dependent relationship between neurodegenerative disease and head injuries,” Dr. Elser said.
Dose-dependent response
In the unadjusted analysis, the hazard ratio of mortality among individuals with head injury was 2.21 (95% confidence interval, 2.09-2.34) compared with those who did not have head injury.
The association remained significant with adjustment for sociodemographic factors (HR, 1.99; 95% CI, 1.88-2.11) and with additional adjustment for vascular risk factors (HR, 1.92; 95% CI, 1.81-2.03).
The findings also showed a dose-response pattern in the association of head injuries with mortality. Compared with participants who did not have head injury, the HR was 1.66 (95% CI, 1.56-1.77) for those with one head injury and 2.11 (95% CI, 1.89-2.37) for those with two or more head injuries.
“It’s not as though once you’ve had one head injury, you’ve accrued all the damage you possibly can. We see pretty clearly here that recurrent head injury further increased the rate of deaths from all causes,” said Dr. Elser.
Injury severity was determined from hospital diagnostic codes using established algorithms. Results showed that mortality rates were increased with even mild head injury.
Interestingly, the association between head injury and all-cause mortality was weaker among those whose injuries were self-reported. One possibility is that these injuries were less severe, Dr. Elser noted.
“If you have head injury that’s mild enough that you don’t need to go to the hospital, it’s probably going to confer less long-term health risks than one that’s severe enough that you needed to be examined in an acute care setting,” she said.
Results were similar by race and for sex. “Even though there were more women with head injuries, the rate of mortality associated with head injury doesn’t differ from the rate among men,” Dr. Elser reported.
However, the association was stronger among those younger than 54 years at baseline (HR, 2.26) compared with older individuals (HR, 2.0) in the model that adjusted for demographics and lifestyle factors.
This may be explained by the reference group (those without a head injury) – the mortality rate was in general higher for the older participants, said Dr. Elser. It could also be that younger adults are more likely to have severe head injuries from, for example, motor vehicle accidents or violence, she added.
These new findings underscore the importance of public health measures, such as seatbelt laws, to reduce head injuries, the investigators note.
They add that clinicians with patients at risk for head injuries may recommend steps to lessen the risk of falls, such as having access to durable medical equipment, and ensuring driver safety.
Shorter life span
Commenting for this news organization, Frank Conidi, MD, director of the Florida Center for Headache and Sports Neurology in Port St. Lucie and past president of the Florida Society of Neurology, said the large number of participants “adds validity” to the finding that individuals with head injury are likely to have a shorter life span than those who do not suffer head trauma – and that this “was not purely by chance or from other causes.”
However, patients may not have accurately reported head injuries, in which case the rate of injury in the self-report subgroup would not reflect the actual incidence, noted Dr. Conidi, who was not involved with the research.
“In my practice, most patients have little knowledge as to the signs and symptoms of concussion and traumatic brain injury. Most think there needs to be some form of loss of consciousness to have a head injury, which is of course not true,” he said.
Dr. Conidi added that the finding of a higher incidence of death from neurodegenerative disorders supports the generally accepted consensus view that about 30% of patients with traumatic brain injury experience progression of symptoms and are at risk for early dementia.
The ARIC study is supported by the National Heart, Lung, and Blood Institute. Dr. Elser and Dr. Conidi have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
An analysis of more than 13,000 adult participants in the Atherosclerosis Risk in Communities (ARIC) study showed a dose-response pattern in which one head injury was linked to a 66% increased risk for all-cause mortality, and two or more head injuries were associated with twice the risk in comparison with no head injuries.
These findings underscore the importance of preventing head injuries and of swift clinical intervention once a head injury occurs, lead author Holly Elser, MD, PhD, department of neurology, Hospital of the University of Pennsylvania, Philadelphia, told this news organization.
“Clinicians should counsel patients who are at risk for falls about head injuries and ensure patients are promptly evaluated in the hospital setting if they do have a fall – especially with loss of consciousness or other symptoms, such as headache or dizziness,” Dr. Elser added.
The findings were published online in JAMA Neurology.
Consistent evidence
There is “pretty consistent evidence” that mortality rates are increased in the short term after head injury, predominantly among hospitalized patients, Dr. Elser noted.
“But there’s less evidence about the long-term mortality implications of head injuries and less evidence from adults living in the community,” she added.
The analysis included 13,037 participants in the ARIC study, an ongoing study involving adults aged 45-65 years who were recruited from four geographically and racially diverse U.S. communities. The mean age at baseline (1987-1989) was 54 years; 57.7% were women; and 27.9% were Black.
Study participants are followed at routine in-person visits and semiannually via telephone.
Data on head injuries came from hospital diagnostic codes and self-reports. These reports included information on the number of injuries and whether the injury required medical care and involved loss of consciousness.
During the 27-year follow-up, 18.4% of the study sample had at least one head injury. Injuries occurred more frequently among women, which may reflect the predominance of women in the study population, said Dr. Elser.
Overall, about 56% of participants died during the study period. The estimated median amount of survival time after head injury was 4.7 years.
The most common causes of death were neoplasm, cardiovascular disease, and neurologic disorders. Regarding specific neurologic causes of death, the researchers found that 62.2% of deaths were due to neurodegenerative disease among individuals with head injury, vs. 51.4% among those without head injury.
This, said Dr. Elser, raises the possibility of reverse causality. “If you have a neurodegenerative disorder like Alzheimer’s disease dementia or Parkinson’s disease that leads to difficulty walking, you may be more likely to fall and have a head injury. The head injury in turn may lead to increased mortality,” she noted.
However, she stressed that the data on cause-specific mortality are exploratory. “Our research motivates future studies that really examine this time-dependent relationship between neurodegenerative disease and head injuries,” Dr. Elser said.
Dose-dependent response
In the unadjusted analysis, the hazard ratio of mortality among individuals with head injury was 2.21 (95% confidence interval, 2.09-2.34) compared with those who did not have head injury.
The association remained significant with adjustment for sociodemographic factors (HR, 1.99; 95% CI, 1.88-2.11) and with additional adjustment for vascular risk factors (HR, 1.92; 95% CI, 1.81-2.03).
The findings also showed a dose-response pattern in the association of head injuries with mortality. Compared with participants who did not have head injury, the HR was 1.66 (95% CI, 1.56-1.77) for those with one head injury and 2.11 (95% CI, 1.89-2.37) for those with two or more head injuries.
“It’s not as though once you’ve had one head injury, you’ve accrued all the damage you possibly can. We see pretty clearly here that recurrent head injury further increased the rate of deaths from all causes,” said Dr. Elser.
Injury severity was determined from hospital diagnostic codes using established algorithms. Results showed that mortality rates were increased with even mild head injury.
Interestingly, the association between head injury and all-cause mortality was weaker among those whose injuries were self-reported. One possibility is that these injuries were less severe, Dr. Elser noted.
“If you have head injury that’s mild enough that you don’t need to go to the hospital, it’s probably going to confer less long-term health risks than one that’s severe enough that you needed to be examined in an acute care setting,” she said.
Results were similar by race and for sex. “Even though there were more women with head injuries, the rate of mortality associated with head injury doesn’t differ from the rate among men,” Dr. Elser reported.
However, the association was stronger among those younger than 54 years at baseline (HR, 2.26) compared with older individuals (HR, 2.0) in the model that adjusted for demographics and lifestyle factors.
This may be explained by the reference group (those without a head injury) – the mortality rate was in general higher for the older participants, said Dr. Elser. It could also be that younger adults are more likely to have severe head injuries from, for example, motor vehicle accidents or violence, she added.
These new findings underscore the importance of public health measures, such as seatbelt laws, to reduce head injuries, the investigators note.
They add that clinicians with patients at risk for head injuries may recommend steps to lessen the risk of falls, such as having access to durable medical equipment, and ensuring driver safety.
Shorter life span
Commenting for this news organization, Frank Conidi, MD, director of the Florida Center for Headache and Sports Neurology in Port St. Lucie and past president of the Florida Society of Neurology, said the large number of participants “adds validity” to the finding that individuals with head injury are likely to have a shorter life span than those who do not suffer head trauma – and that this “was not purely by chance or from other causes.”
However, patients may not have accurately reported head injuries, in which case the rate of injury in the self-report subgroup would not reflect the actual incidence, noted Dr. Conidi, who was not involved with the research.
“In my practice, most patients have little knowledge as to the signs and symptoms of concussion and traumatic brain injury. Most think there needs to be some form of loss of consciousness to have a head injury, which is of course not true,” he said.
Dr. Conidi added that the finding of a higher incidence of death from neurodegenerative disorders supports the generally accepted consensus view that about 30% of patients with traumatic brain injury experience progression of symptoms and are at risk for early dementia.
The ARIC study is supported by the National Heart, Lung, and Blood Institute. Dr. Elser and Dr. Conidi have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
An analysis of more than 13,000 adult participants in the Atherosclerosis Risk in Communities (ARIC) study showed a dose-response pattern in which one head injury was linked to a 66% increased risk for all-cause mortality, and two or more head injuries were associated with twice the risk in comparison with no head injuries.
These findings underscore the importance of preventing head injuries and of swift clinical intervention once a head injury occurs, lead author Holly Elser, MD, PhD, department of neurology, Hospital of the University of Pennsylvania, Philadelphia, told this news organization.
“Clinicians should counsel patients who are at risk for falls about head injuries and ensure patients are promptly evaluated in the hospital setting if they do have a fall – especially with loss of consciousness or other symptoms, such as headache or dizziness,” Dr. Elser added.
The findings were published online in JAMA Neurology.
Consistent evidence
There is “pretty consistent evidence” that mortality rates are increased in the short term after head injury, predominantly among hospitalized patients, Dr. Elser noted.
“But there’s less evidence about the long-term mortality implications of head injuries and less evidence from adults living in the community,” she added.
The analysis included 13,037 participants in the ARIC study, an ongoing study involving adults aged 45-65 years who were recruited from four geographically and racially diverse U.S. communities. The mean age at baseline (1987-1989) was 54 years; 57.7% were women; and 27.9% were Black.
Study participants are followed at routine in-person visits and semiannually via telephone.
Data on head injuries came from hospital diagnostic codes and self-reports. These reports included information on the number of injuries and whether the injury required medical care and involved loss of consciousness.
During the 27-year follow-up, 18.4% of the study sample had at least one head injury. Injuries occurred more frequently among women, which may reflect the predominance of women in the study population, said Dr. Elser.
Overall, about 56% of participants died during the study period. The estimated median amount of survival time after head injury was 4.7 years.
The most common causes of death were neoplasm, cardiovascular disease, and neurologic disorders. Regarding specific neurologic causes of death, the researchers found that 62.2% of deaths were due to neurodegenerative disease among individuals with head injury, vs. 51.4% among those without head injury.
This, said Dr. Elser, raises the possibility of reverse causality. “If you have a neurodegenerative disorder like Alzheimer’s disease dementia or Parkinson’s disease that leads to difficulty walking, you may be more likely to fall and have a head injury. The head injury in turn may lead to increased mortality,” she noted.
However, she stressed that the data on cause-specific mortality are exploratory. “Our research motivates future studies that really examine this time-dependent relationship between neurodegenerative disease and head injuries,” Dr. Elser said.
Dose-dependent response
In the unadjusted analysis, the hazard ratio of mortality among individuals with head injury was 2.21 (95% confidence interval, 2.09-2.34) compared with those who did not have head injury.
The association remained significant with adjustment for sociodemographic factors (HR, 1.99; 95% CI, 1.88-2.11) and with additional adjustment for vascular risk factors (HR, 1.92; 95% CI, 1.81-2.03).
The findings also showed a dose-response pattern in the association of head injuries with mortality. Compared with participants who did not have head injury, the HR was 1.66 (95% CI, 1.56-1.77) for those with one head injury and 2.11 (95% CI, 1.89-2.37) for those with two or more head injuries.
“It’s not as though once you’ve had one head injury, you’ve accrued all the damage you possibly can. We see pretty clearly here that recurrent head injury further increased the rate of deaths from all causes,” said Dr. Elser.
Injury severity was determined from hospital diagnostic codes using established algorithms. Results showed that mortality rates were increased with even mild head injury.
Interestingly, the association between head injury and all-cause mortality was weaker among those whose injuries were self-reported. One possibility is that these injuries were less severe, Dr. Elser noted.
“If you have head injury that’s mild enough that you don’t need to go to the hospital, it’s probably going to confer less long-term health risks than one that’s severe enough that you needed to be examined in an acute care setting,” she said.
Results were similar by race and for sex. “Even though there were more women with head injuries, the rate of mortality associated with head injury doesn’t differ from the rate among men,” Dr. Elser reported.
However, the association was stronger among those younger than 54 years at baseline (HR, 2.26) compared with older individuals (HR, 2.0) in the model that adjusted for demographics and lifestyle factors.
This may be explained by the reference group (those without a head injury) – the mortality rate was in general higher for the older participants, said Dr. Elser. It could also be that younger adults are more likely to have severe head injuries from, for example, motor vehicle accidents or violence, she added.
These new findings underscore the importance of public health measures, such as seatbelt laws, to reduce head injuries, the investigators note.
They add that clinicians with patients at risk for head injuries may recommend steps to lessen the risk of falls, such as having access to durable medical equipment, and ensuring driver safety.
Shorter life span
Commenting for this news organization, Frank Conidi, MD, director of the Florida Center for Headache and Sports Neurology in Port St. Lucie and past president of the Florida Society of Neurology, said the large number of participants “adds validity” to the finding that individuals with head injury are likely to have a shorter life span than those who do not suffer head trauma – and that this “was not purely by chance or from other causes.”
However, patients may not have accurately reported head injuries, in which case the rate of injury in the self-report subgroup would not reflect the actual incidence, noted Dr. Conidi, who was not involved with the research.
“In my practice, most patients have little knowledge as to the signs and symptoms of concussion and traumatic brain injury. Most think there needs to be some form of loss of consciousness to have a head injury, which is of course not true,” he said.
Dr. Conidi added that the finding of a higher incidence of death from neurodegenerative disorders supports the generally accepted consensus view that about 30% of patients with traumatic brain injury experience progression of symptoms and are at risk for early dementia.
The ARIC study is supported by the National Heart, Lung, and Blood Institute. Dr. Elser and Dr. Conidi have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM JAMA NEUROLOGY
Acute hepatic porphyrias no longer as rare as previously thought
from the American Gastroenterological Association.
For acute attacks, treatment should include intravenous hemin, and for patients with recurrent attacks, a newly-approved therapy called givosiran should be considered, wrote the authors of the update, which was published Jan. 13 in Gastroenterology.
“Diagnoses of AHPs are often missed, with a delay of more than 15 years from initial presentation. The key to early diagnosis is to consider the diagnosis, especially in patients with recurring severe abdominal pain not ascribable to other causes,” wrote the authors, who were led by Bruce Wang, MD, a hepatologist with the University of California, San Francisco.
AHPs are inherited disorders of heme-metabolism, which include acute intermittent porphyria, hereditary coproporphyria, variegate porphyria, and porphyria due to severe deficiency of 5-aminolevulinic acid dehydratase.
Acute intermittent porphyria (AIP) is the most common type, with an estimated prevalence of symptomatic AHP of 1 in 100,000 patients. However, population-level genetic studies show that the prevalence of pathogenic variants for AIP is between 1 in 1,300 and 1 in 1,785.
The major clinical presentation includes attacks of severe abdominal pain, nausea, vomiting, constipation, muscle weakness, neuropathy, tachycardia, and hypertension, yet without peritoneal signs or abnormalities on cross-sectional imaging.
Recent advances in treatment have improved the outlook for patients with AHP. To provide timely guidance, the authors developed 12 clinical practice advice statements on the diagnosis and management of AHPs based on a review of the published literature and expert opinion.
First, AHP screening should be considered in the evaluation of all patients, particularly among women in their childbearing years between ages 15 and 50 with unexplained, recurrent severe abdominal pain that doesn’t have a clear etiology. About 90% of patients with symptomatic AHP are women, and more than 90% of them experience only one or a few acute attacks in their lifetime, which are often precipitated by factors that increase the activity of the enzyme ALAS1 in the liver.
For initial AHP diagnosis, biochemical testing should measure porphobilinogen (PBG) and delta-aminolevulinic acid (ALA) corrected to creatine on a random urine sample. All patients with significantly elevated urinary PBG or ALA should initially be presumed to have AHP, and during acute attacks, both will be elevated at least five-fold of the upper limit of normal. Because ALA and PBG are porphyrin precursors, urine porphyrin testing should not be used alone for AHP screening.
After that, genetic testing should be used to confirm the AHP diagnosis, as well as the specific type of AHP. Sequencing of the four genes ALAD, HMBS, CPOX, and PPOX leads to aminolevulinic acid dehydrase deficiency, acute intermittent porphyria, hereditary coproporphyria, and variegate porphyria, respectively. When whole-gene sequencing is performed, about 95%-99% of cases can be identified. First-degree family members should be screened with genetic testing, and those who are mutation carriers should be counseled.
For acute attacks of AHP that are severe enough to require hospitalization, the currently approved treatment is intravenous hemin infusion, usually given once daily at a dose of 3-4 mg/kg body weight for 3-5 days. Due to potential thrombophlebitis, it’s best to administer hemin in a high-flow central vein via a peripherally inserted central catheter or central port.
In addition, treatment for acute attacks should include analgesics, antiemetics, and management of systemic arterial hypertension, tachycardia, hyponatremia, and hypomagnesemia. The primary goal of treatment during an acute attack is to decrease ALA production. Patients should be counseled to avoid identifiable triggers, such as porphyrinogenic medications, excess alcohol intake, tobacco use, and caloric deprivation.
Although recent advances have improved treatment for acute attacks, management for patients with frequent attacks remains challenging, the study authors wrote. About 3%-5% of patients with symptomatic AHP experience recurrent attacks, which is defined as four or more attacks per year. These attacks aren’t typically associated with identifiable triggers, although some that occur during the luteal phase of a patient’s menstrual cycle are believed to be triggered by progesterone. However, treatment with hormonal suppression therapy, such as GnRH agonists, has had limited success.
Off-label use of prophylactic intravenous heme therapy is common, although the effectiveness in preventing recurrent attacks isn’t well-established. In addition, chronic hemin use is associated with several complications, including infections, iron overload, and the need for indwelling central venous catheters.
Recently, the Food and Drug Administration approved givosiran, a small interfering RNA-based therapy that targets delta-aminolevulinate synthase 1, for treatment in adults with AHP. Monthly subcutaneous therapy appears to significantly lower rates of acute attacks among patients who experience recurrent attacks.
“We suggest prescribing givosiran only for those patients with recurrent acute attacks that are both biochemically and genetically confirmed,” the authors wrote. “Due to limited safety data, givosiran should not be used in women who are pregnant or planning a pregnancy.”
In the most severe cases, liver transplantation should be limited to patients with intractable symptoms and a significantly decreased quality of life who are refractory to pharmacotherapy. If living donor transplantation is considered, genetic testing should be used to screen related living donors since HMBS pathogenic variants in asymptomatic donors could results in poor posttransplantation outcomes.
In the long-term, patients with AHP should be monitored annually for liver disease and chronic kidney disease with serum creatinine and estimated glomerular filtration rate monitored. Patients also face an increased risk of hepatocellular carcinoma and should start screening at age 50, with a liver ultrasound every 6 months.
“Fortunately, most people with genetic defects never experience severe acute attacks or may experience only one or a few attacks throughout their lives,” the authors wrote.
The authors (Bruce Wang, MD, Herbert L. Bonkovsky, MD, AGAF, and Manisha Balwani, MD, MS) reported that they are part of the Porphyrias Consortium. The Porphyrias Consortium is part of the Rare Diseases Clinical Research Network, an initiative of the Division of Rare Diseases Research Innovation at the National Center for Advancing Translational Sciences. The consortium is funded through a collaboration between the center and the National Institute of Diabetes and Digestive and Kidney Diseases. Several authors disclosed funding support and honoraria for advisory board roles with various pharmaceutical companies, including Alnylam, which makes givosiran.
This article was updated 2/3/23.
from the American Gastroenterological Association.
For acute attacks, treatment should include intravenous hemin, and for patients with recurrent attacks, a newly-approved therapy called givosiran should be considered, wrote the authors of the update, which was published Jan. 13 in Gastroenterology.
“Diagnoses of AHPs are often missed, with a delay of more than 15 years from initial presentation. The key to early diagnosis is to consider the diagnosis, especially in patients with recurring severe abdominal pain not ascribable to other causes,” wrote the authors, who were led by Bruce Wang, MD, a hepatologist with the University of California, San Francisco.
AHPs are inherited disorders of heme-metabolism, which include acute intermittent porphyria, hereditary coproporphyria, variegate porphyria, and porphyria due to severe deficiency of 5-aminolevulinic acid dehydratase.
Acute intermittent porphyria (AIP) is the most common type, with an estimated prevalence of symptomatic AHP of 1 in 100,000 patients. However, population-level genetic studies show that the prevalence of pathogenic variants for AIP is between 1 in 1,300 and 1 in 1,785.
The major clinical presentation includes attacks of severe abdominal pain, nausea, vomiting, constipation, muscle weakness, neuropathy, tachycardia, and hypertension, yet without peritoneal signs or abnormalities on cross-sectional imaging.
Recent advances in treatment have improved the outlook for patients with AHP. To provide timely guidance, the authors developed 12 clinical practice advice statements on the diagnosis and management of AHPs based on a review of the published literature and expert opinion.
First, AHP screening should be considered in the evaluation of all patients, particularly among women in their childbearing years between ages 15 and 50 with unexplained, recurrent severe abdominal pain that doesn’t have a clear etiology. About 90% of patients with symptomatic AHP are women, and more than 90% of them experience only one or a few acute attacks in their lifetime, which are often precipitated by factors that increase the activity of the enzyme ALAS1 in the liver.
For initial AHP diagnosis, biochemical testing should measure porphobilinogen (PBG) and delta-aminolevulinic acid (ALA) corrected to creatine on a random urine sample. All patients with significantly elevated urinary PBG or ALA should initially be presumed to have AHP, and during acute attacks, both will be elevated at least five-fold of the upper limit of normal. Because ALA and PBG are porphyrin precursors, urine porphyrin testing should not be used alone for AHP screening.
After that, genetic testing should be used to confirm the AHP diagnosis, as well as the specific type of AHP. Sequencing of the four genes ALAD, HMBS, CPOX, and PPOX leads to aminolevulinic acid dehydrase deficiency, acute intermittent porphyria, hereditary coproporphyria, and variegate porphyria, respectively. When whole-gene sequencing is performed, about 95%-99% of cases can be identified. First-degree family members should be screened with genetic testing, and those who are mutation carriers should be counseled.
For acute attacks of AHP that are severe enough to require hospitalization, the currently approved treatment is intravenous hemin infusion, usually given once daily at a dose of 3-4 mg/kg body weight for 3-5 days. Due to potential thrombophlebitis, it’s best to administer hemin in a high-flow central vein via a peripherally inserted central catheter or central port.
In addition, treatment for acute attacks should include analgesics, antiemetics, and management of systemic arterial hypertension, tachycardia, hyponatremia, and hypomagnesemia. The primary goal of treatment during an acute attack is to decrease ALA production. Patients should be counseled to avoid identifiable triggers, such as porphyrinogenic medications, excess alcohol intake, tobacco use, and caloric deprivation.
Although recent advances have improved treatment for acute attacks, management for patients with frequent attacks remains challenging, the study authors wrote. About 3%-5% of patients with symptomatic AHP experience recurrent attacks, which is defined as four or more attacks per year. These attacks aren’t typically associated with identifiable triggers, although some that occur during the luteal phase of a patient’s menstrual cycle are believed to be triggered by progesterone. However, treatment with hormonal suppression therapy, such as GnRH agonists, has had limited success.
Off-label use of prophylactic intravenous heme therapy is common, although the effectiveness in preventing recurrent attacks isn’t well-established. In addition, chronic hemin use is associated with several complications, including infections, iron overload, and the need for indwelling central venous catheters.
Recently, the Food and Drug Administration approved givosiran, a small interfering RNA-based therapy that targets delta-aminolevulinate synthase 1, for treatment in adults with AHP. Monthly subcutaneous therapy appears to significantly lower rates of acute attacks among patients who experience recurrent attacks.
“We suggest prescribing givosiran only for those patients with recurrent acute attacks that are both biochemically and genetically confirmed,” the authors wrote. “Due to limited safety data, givosiran should not be used in women who are pregnant or planning a pregnancy.”
In the most severe cases, liver transplantation should be limited to patients with intractable symptoms and a significantly decreased quality of life who are refractory to pharmacotherapy. If living donor transplantation is considered, genetic testing should be used to screen related living donors since HMBS pathogenic variants in asymptomatic donors could results in poor posttransplantation outcomes.
In the long-term, patients with AHP should be monitored annually for liver disease and chronic kidney disease with serum creatinine and estimated glomerular filtration rate monitored. Patients also face an increased risk of hepatocellular carcinoma and should start screening at age 50, with a liver ultrasound every 6 months.
“Fortunately, most people with genetic defects never experience severe acute attacks or may experience only one or a few attacks throughout their lives,” the authors wrote.
The authors (Bruce Wang, MD, Herbert L. Bonkovsky, MD, AGAF, and Manisha Balwani, MD, MS) reported that they are part of the Porphyrias Consortium. The Porphyrias Consortium is part of the Rare Diseases Clinical Research Network, an initiative of the Division of Rare Diseases Research Innovation at the National Center for Advancing Translational Sciences. The consortium is funded through a collaboration between the center and the National Institute of Diabetes and Digestive and Kidney Diseases. Several authors disclosed funding support and honoraria for advisory board roles with various pharmaceutical companies, including Alnylam, which makes givosiran.
This article was updated 2/3/23.
from the American Gastroenterological Association.
For acute attacks, treatment should include intravenous hemin, and for patients with recurrent attacks, a newly-approved therapy called givosiran should be considered, wrote the authors of the update, which was published Jan. 13 in Gastroenterology.
“Diagnoses of AHPs are often missed, with a delay of more than 15 years from initial presentation. The key to early diagnosis is to consider the diagnosis, especially in patients with recurring severe abdominal pain not ascribable to other causes,” wrote the authors, who were led by Bruce Wang, MD, a hepatologist with the University of California, San Francisco.
AHPs are inherited disorders of heme-metabolism, which include acute intermittent porphyria, hereditary coproporphyria, variegate porphyria, and porphyria due to severe deficiency of 5-aminolevulinic acid dehydratase.
Acute intermittent porphyria (AIP) is the most common type, with an estimated prevalence of symptomatic AHP of 1 in 100,000 patients. However, population-level genetic studies show that the prevalence of pathogenic variants for AIP is between 1 in 1,300 and 1 in 1,785.
The major clinical presentation includes attacks of severe abdominal pain, nausea, vomiting, constipation, muscle weakness, neuropathy, tachycardia, and hypertension, yet without peritoneal signs or abnormalities on cross-sectional imaging.
Recent advances in treatment have improved the outlook for patients with AHP. To provide timely guidance, the authors developed 12 clinical practice advice statements on the diagnosis and management of AHPs based on a review of the published literature and expert opinion.
First, AHP screening should be considered in the evaluation of all patients, particularly among women in their childbearing years between ages 15 and 50 with unexplained, recurrent severe abdominal pain that doesn’t have a clear etiology. About 90% of patients with symptomatic AHP are women, and more than 90% of them experience only one or a few acute attacks in their lifetime, which are often precipitated by factors that increase the activity of the enzyme ALAS1 in the liver.
For initial AHP diagnosis, biochemical testing should measure porphobilinogen (PBG) and delta-aminolevulinic acid (ALA) corrected to creatine on a random urine sample. All patients with significantly elevated urinary PBG or ALA should initially be presumed to have AHP, and during acute attacks, both will be elevated at least five-fold of the upper limit of normal. Because ALA and PBG are porphyrin precursors, urine porphyrin testing should not be used alone for AHP screening.
After that, genetic testing should be used to confirm the AHP diagnosis, as well as the specific type of AHP. Sequencing of the four genes ALAD, HMBS, CPOX, and PPOX leads to aminolevulinic acid dehydrase deficiency, acute intermittent porphyria, hereditary coproporphyria, and variegate porphyria, respectively. When whole-gene sequencing is performed, about 95%-99% of cases can be identified. First-degree family members should be screened with genetic testing, and those who are mutation carriers should be counseled.
For acute attacks of AHP that are severe enough to require hospitalization, the currently approved treatment is intravenous hemin infusion, usually given once daily at a dose of 3-4 mg/kg body weight for 3-5 days. Due to potential thrombophlebitis, it’s best to administer hemin in a high-flow central vein via a peripherally inserted central catheter or central port.
In addition, treatment for acute attacks should include analgesics, antiemetics, and management of systemic arterial hypertension, tachycardia, hyponatremia, and hypomagnesemia. The primary goal of treatment during an acute attack is to decrease ALA production. Patients should be counseled to avoid identifiable triggers, such as porphyrinogenic medications, excess alcohol intake, tobacco use, and caloric deprivation.
Although recent advances have improved treatment for acute attacks, management for patients with frequent attacks remains challenging, the study authors wrote. About 3%-5% of patients with symptomatic AHP experience recurrent attacks, which is defined as four or more attacks per year. These attacks aren’t typically associated with identifiable triggers, although some that occur during the luteal phase of a patient’s menstrual cycle are believed to be triggered by progesterone. However, treatment with hormonal suppression therapy, such as GnRH agonists, has had limited success.
Off-label use of prophylactic intravenous heme therapy is common, although the effectiveness in preventing recurrent attacks isn’t well-established. In addition, chronic hemin use is associated with several complications, including infections, iron overload, and the need for indwelling central venous catheters.
Recently, the Food and Drug Administration approved givosiran, a small interfering RNA-based therapy that targets delta-aminolevulinate synthase 1, for treatment in adults with AHP. Monthly subcutaneous therapy appears to significantly lower rates of acute attacks among patients who experience recurrent attacks.
“We suggest prescribing givosiran only for those patients with recurrent acute attacks that are both biochemically and genetically confirmed,” the authors wrote. “Due to limited safety data, givosiran should not be used in women who are pregnant or planning a pregnancy.”
In the most severe cases, liver transplantation should be limited to patients with intractable symptoms and a significantly decreased quality of life who are refractory to pharmacotherapy. If living donor transplantation is considered, genetic testing should be used to screen related living donors since HMBS pathogenic variants in asymptomatic donors could results in poor posttransplantation outcomes.
In the long-term, patients with AHP should be monitored annually for liver disease and chronic kidney disease with serum creatinine and estimated glomerular filtration rate monitored. Patients also face an increased risk of hepatocellular carcinoma and should start screening at age 50, with a liver ultrasound every 6 months.
“Fortunately, most people with genetic defects never experience severe acute attacks or may experience only one or a few attacks throughout their lives,” the authors wrote.
The authors (Bruce Wang, MD, Herbert L. Bonkovsky, MD, AGAF, and Manisha Balwani, MD, MS) reported that they are part of the Porphyrias Consortium. The Porphyrias Consortium is part of the Rare Diseases Clinical Research Network, an initiative of the Division of Rare Diseases Research Innovation at the National Center for Advancing Translational Sciences. The consortium is funded through a collaboration between the center and the National Institute of Diabetes and Digestive and Kidney Diseases. Several authors disclosed funding support and honoraria for advisory board roles with various pharmaceutical companies, including Alnylam, which makes givosiran.
This article was updated 2/3/23.
FROM GASTROENTEROLOGY
Novel resuscitation for patients with nonshockable rhythms in cardiac arrest
This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr Robert Glatter, medical adviser for Medscape Emergency Medicine. with a remarkable increase in neurologically intact survival. Welcome, gentlemen.
Dr. Pepe, I’d like to start off by thanking you for taking time to join us to discuss this novel concept of head-up or what you now refer to as a neuroprotective cardiopulmonary resuscitation (CPR) bundle. Can you define what this entails and why it is referred to as a neuroprotective CPR bundle?
Paul E. Pepe, MD, MPH: CPR has been life saving for 60 years the way we’ve performed it, but probably only in a very small percentage of cases. That’s one of the problems. We have almost a thousand people a day who have sudden cardiac arrest out in the community alone and more in the hospital.
We know that early defibrillation and early CPR can contribute, but it’s still a small percentage of those. About 75%-85% of the cases that we go out to see will have nonshockable rhythms and flatlines. Some cases are what we call “pulseless electrical activity,” meaning that it looks like there is some kind of organized complex, but there is no pulse associated with it.
That’s why it’s a problem, because they don’t come back. Part of the reason why we see poor outcomes is not only that these cases tend to be people who, say, were in ventricular fibrillation and then just went on over time and were not witnessed or resuscitated or had a long response time. They basically either go into flatline or autoconvert into these bizarre rhythms.
The other issue is the way we perform CPR. CPR has been lifesaving, but it only generates about 20% and maybe 15% in some cases of normal blood flow, and particularly, cerebral perfusion pressure. We’ve looked at this nicely in the laboratory.
For example, during chest compressions, we’re hoping during the recoil phase to pull blood down and back into the right heart. The problem is that you’re not only setting a pressure rate up here to the arterial side but also, you’re setting back pressure wave on the venous side. Obviously, the arterial side always wins out, but it’s just not as efficient as it could be, at 20% or 30%.
What does this entail? It entails several independent mechanisms in terms of how they work, but they all do the same thing, which is they help to pull blood out of the brain and back into the right heart by basically manipulating intrathoracic pressure and creating more of a vacuum to get blood back there.
It’s so important that people do quality CPR. You have to have a good release and that helps us suck a little bit of blood and sucks the air in. As soon as the air rushes in, it neutralizes the pressure and there’s no more vacuum and nothing else is happening until the next squeeze.
What we have found is that we can cap the airway just for a second with a little pop-up valve. It acts like when you’re sucking a milkshake through a straw and it creates more of a vacuum in the chest. Just a little pop-up valve that pulls a little bit more blood out of the brain and the rest of the body and into the right heart.
We’ve shown in a human study that, for example, the systolic blood pressure almost doubles. It really goes from 40 mm Hg during standard CPR up to 80 mm Hg, and that would be sustained for 14-15 minutes. That was a nice little study that was done in Milwaukee a few years ago.
The other thing that happens is, if you add on something else, it’s like a toilet plunger. I think many people have seen it; it’s called “active compression-decompression.” It not only compresses, but it decompresses. Where it becomes even more effective is that if you had broken bones or stiff bones as you get older or whatever it may be, as you do the CPR, you’re still getting the push down and then you’re getting the pull out. It helps on several levels. More importantly, when you put the two together, they’re very synergistic.
We, have already done the clinical trial that is the proof of concept, and that was published in The Lancet about 10 years ago. In that study, we found that the combination of those two dramatically improved survival rates by 50%, with 1-year survival neurologically intact. That got us on the right track.
The interesting thing is that someone said, “Can we lift the head up a little bit?” We did a large amount of work in the laboratory over 10 years, fine tuning it. When do you first lift the head? How soon is too soon? It’s probably bad if you just go right to it.
We had to get the pump primed a little bit with these other things to get the flow going better, not only pulling blood out of the brain but now, you have a better flow this way. You have to prime at first for a couple of minutes, and we worked out the timing: Is it 3 or 4 minutes? It seems the timing is right at about 2 minutes, then you gradually elevate the head over about 2 minutes. We’re finding that seems to be the optimal way to do it. About 2 minutes of priming with those other two devices, the adjuncts, and then gradually elevate the head over 2 minutes.
When we do that in the laboratory, we’re getting normalized cerebral perfusion pressures. You’re normalizing the flow back again with that. We’re seeing profound differences in outcome as a result, even in these cases of the nonshockables.
Dr. Glatter: What you’re doing basically is resulting in an increase in cardiac output, essentially. That really is important, especially in these nonshockable rhythms, correct?
Dr. Pepe: Absolutely. As you’re doing this compression and you’re getting these intracranial pulse waves that are going up because they’re colliding up there. It could be even damaging in itself, but we’re seeing these intracranial raises. The intracranial pressure starts going up more and more over time. Also, peripherally in most people, you’re not getting good flow out there; then, your vasculature starts to relax. The arterials are starting to not get oxygen, so they don’t go out.
With this technique where we’re returning the pressure, we’re getting to 40% of normal now with the active compression-decompression CPR plus an impedance threshold device (ACD+ITD CPR) approach. Now, you add this, and you’re almost normalizing. In humans, even in these asystole patients, we’re seeing end-title CO2s which are generally in the 15-20 range with standard CPR are now up with ACD+ITD CPR in the 30%-40% range, where we’re getting through 30 or 40 end-tidal CO2s. Now, we’re seeing even the end-tidal CO2s moving up into the 40s and 50s. We know there’s a surrogate marker telling us that we are generating much better flows not only to the rest of the body, but most importantly, to the brain.
Dr. Glatter: Ryan, could you tell us about the approach in terms of on scene, what you’re doing and how you use the device itself? Maybe you could talk about the backpack that you developed with your fire department?
Ryan P. Quinn, BS, EMS: Our approach has always been to get to the patient quickly, like everybody’s approach on a cardiac arrest when you’re responding. We are an advanced life-support paramedic ambulance service through the fire department – we’re all cross-trained firefighter paramedics. Our first vehicle from the fire department is typically the ambulance. It’s smaller and a little quicker than the fire engine. Two paramedics are going to jump out with two backpacks. One has the automated compressive device (we use the Lucas), and the other one is the sequential patient lifting device, the EleGARD.
Our two paramedics are quick to the patient’s side, and once they make contact with the patient to verify pulseless cardiac arrest, they will unpack. One person will go right to compressions if there’s nobody on compressions already. Sometimes we have a first responder police officer with an automated external defibrillator (AED). We go right to the patient’s side, concentrate on compressions, and within 90 seconds to 2 minutes, we have our bags unpacked, we’ve got the devices turned on, patient lifted up, slid under the device, and we have a supraglottic airway that is placed within 15 seconds already premade with the ITD on top. We have a sealed airway that we can continue to compress with Dr. Pepe’s original discussion of building on what’s previously been shown to work.
Dr. Pepe: Let me make a comment about this. This is so important, what Ryan is saying, because it’s something we found during the study. It’s really a true pit-crew approach. You’re not only getting these materials, which you think you need a medical Sherpa for, but you don’t. They set it up and then when they open it up, it’s all laid out just exactly as you need it. It’s not just how fast you get there; it’s how fast you get this done.
When we look at all cases combined against high-performance systems that had some of the highest survival rates around, when we compare it to those, we found that overall, even if you looked at the ones that had over 20-minute responses, the odds ratios were still three to four times higher. It was impressive.
If you looked at it under 15 minutes, which is really reasonable for most systems that get there by the way, the average time that people start CPR in any system in these studies has been about 8 minutes if you actually start this thing, which takes about 2 minutes more for this new bundle of care with this triad, it’s almost 12-14 times higher in terms of the odds ratio. I’ve never seen anything like that where the higher end is over 100 in terms of your confidence intervals.
Ryan’s system did really well and is one of those with even higher levels of outcomes, mostly because they got it on quickly. It’s like the AED for nonshockables but better because you have a wider range of efficacy where it will work.
Dr. Glatter: When the elapsed time was less than 11 minutes, that seemed to be an inflection point in the study, is that correct? You saw that 11-fold higher incidence in terms of neurologically intact survival, is that correct?
Dr. Pepe: We picked that number because that was the median time to get it on board. Half the people were getting it within that time period. The fact that you have a larger window, we’re talking about 13- almost 14-fold improvements in outcome if it was under 15 minutes. It doesn’t matter about the 11 or the 12. It’s the faster you get it on board, the better off you are.
Dr. Glatter: What’s the next step in the process of doing trials and having implementation on a larger scale based on your Annals of Emergency Medicine study? Where do you go from here?
Dr. Pepe: I’ve come to find out there are many confounding variables. What was the quality of CPR? How did people ventilate? Did they give the breath and hold it? Did they give a large enough breath so that blood can go across the transpulmonary system? There are many confounding variables. That’s why I think, in the future, it’s going to be more of looking at things like propensity score matching because we know all the variables that change outcomes. I think that’s going to be a way for me.
The other thing is that we were looking at only 380 cases here. When this doubles up in numbers, as we accrue more cases around the country of people who are implementing this, these numbers I just quoted are going to go up much higher. Unwitnessed asystole is considered futile, and you just don’t get them back. To be able to get these folks back now, even if it’s a small percentage, and the fact that we know that we’re producing this better flow, is pretty striking.
I’m really impressed, and the main thing is to make sure people are educated about it. Number two is that they understand that it has to be done right. It cannot be done wrong or you’re not going to see the differences. Getting it done right is not only following the procedures, the sequence, and how you do it, but it also has to do with getting there quickly, including assigning the right people to put it on and having well-trained people who know what they’re doing.
Dr. Glatter: In general, the lay public obviously should not attempt this in the field lifting someone’s head up in the sense of trying to do chest compressions. I think that message is important that you just said. It’s not ready for prime time yet in any way. It has to be done right.
Dr. Pepe: Bystanders have to learn CPR – they will buy us time and we’ll have better outcomes when they do that. That’s number one. Number two is that as more and more systems adopt this, you’re going to see more people coming back. If you think about what we’re doing now, if we only get back 5% of these nonshockable vs. less than 1%, it’s 5% of 800 people a day because a thousand people a day die. Several dozens of lives can be saved on a daily basis, coming back neurologically intact. That’s the key thing.
Dr. Glatter: Ryan, can you comment about your experience in the field? Is there anything in terms of your current approach that you think would be ideal to change at this point?
Mr. Quinn: We’ve established that this is the approach that we want to take and we’re just fine tuning it to be more efficient. Using the choreography of which person is going to do which role, we have clearly defined roles and clearly defined command of the scene so we’re not missing anything. Training is extremely important.
Dr. Glatter: Paul, I want to ask you about your anecdotal experience of people waking up quickly and talking after elevating their heads and going through this process. Having people talk about it and waking up is really fascinating. Maybe you can comment further on this.
Dr. Pepe: That’s a great point that you bring up because a 40- to 50-year-old guy who got saved with this approach, when he came around, he said he was hearing what people were saying. When he came out of it, he found out he had been getting CPR for about 25 minutes because he had persistent recurring ventricular fibrillation. He said, “How could I have survived that that long?”
When we told him about the new approach, he added, “Well, that’s like neuroprotective.” He’s right, because in the laboratory, we showed it was neuroprotective and we’re also getting better flows back there. It goes along with everything else, and so we’ve adopted the name because it is.
These are really high-powered systems we are comparing against, and we have the same level of return of spontaneous circulation. The major difference was when you started talking about the neurointact survival. We don’t have enough numbers yet, but next go around, we’re going to look at cerebral performance category (CPC) – CPC1 vs. the CPC2 – which were both considered intact, but CPC1 is actually better. We’re seeing many more of those, anecdotally.
I also wanted to mention that people do bring this up and say, “Well, let’s do a trial.” As far as we’re concerned, the trial’s been done in terms of The Lancet study 10 years ago that showed that the active compression-decompression had tremendously better outcomes. We show in the laboratories that you augment that a little bit. These are all [Food and Drug Administration] approved. You can go out and buy it tomorrow and get it done. I have no conflicts of interest, by the way, with any of this.
To have this device that’s going to have the potential of saving so many more lives is really an exciting breakthrough. More importantly, we’re understanding more now about the physiology of CPR and why it works. It could work much better with the approaches that we’ve been developing over the last 20 years or so.
Dr. Glatter: Absolutely. I want to thank both of you gentlemen. It’s been really an incredible experience to learn more about an advance in resuscitation that could truly be lifesaving. Thank you again for taking time to join us.
Dr. Glatter is an attending physician in the department of emergency medicine, Lenox Hill Hospital, New York. Dr. Pepe is professor, department of management, policy, and community health, University of Texas Health Sciences Center, Houston. Mr. Quinn is EMS Chief, Edina (Minn.) Fire Department. No conflicts of interest were reported.
A version of this article first appeared Jan. 26 on Medscape.com.
This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr Robert Glatter, medical adviser for Medscape Emergency Medicine. with a remarkable increase in neurologically intact survival. Welcome, gentlemen.
Dr. Pepe, I’d like to start off by thanking you for taking time to join us to discuss this novel concept of head-up or what you now refer to as a neuroprotective cardiopulmonary resuscitation (CPR) bundle. Can you define what this entails and why it is referred to as a neuroprotective CPR bundle?
Paul E. Pepe, MD, MPH: CPR has been life saving for 60 years the way we’ve performed it, but probably only in a very small percentage of cases. That’s one of the problems. We have almost a thousand people a day who have sudden cardiac arrest out in the community alone and more in the hospital.
We know that early defibrillation and early CPR can contribute, but it’s still a small percentage of those. About 75%-85% of the cases that we go out to see will have nonshockable rhythms and flatlines. Some cases are what we call “pulseless electrical activity,” meaning that it looks like there is some kind of organized complex, but there is no pulse associated with it.
That’s why it’s a problem, because they don’t come back. Part of the reason why we see poor outcomes is not only that these cases tend to be people who, say, were in ventricular fibrillation and then just went on over time and were not witnessed or resuscitated or had a long response time. They basically either go into flatline or autoconvert into these bizarre rhythms.
The other issue is the way we perform CPR. CPR has been lifesaving, but it only generates about 20% and maybe 15% in some cases of normal blood flow, and particularly, cerebral perfusion pressure. We’ve looked at this nicely in the laboratory.
For example, during chest compressions, we’re hoping during the recoil phase to pull blood down and back into the right heart. The problem is that you’re not only setting a pressure rate up here to the arterial side but also, you’re setting back pressure wave on the venous side. Obviously, the arterial side always wins out, but it’s just not as efficient as it could be, at 20% or 30%.
What does this entail? It entails several independent mechanisms in terms of how they work, but they all do the same thing, which is they help to pull blood out of the brain and back into the right heart by basically manipulating intrathoracic pressure and creating more of a vacuum to get blood back there.
It’s so important that people do quality CPR. You have to have a good release and that helps us suck a little bit of blood and sucks the air in. As soon as the air rushes in, it neutralizes the pressure and there’s no more vacuum and nothing else is happening until the next squeeze.
What we have found is that we can cap the airway just for a second with a little pop-up valve. It acts like when you’re sucking a milkshake through a straw and it creates more of a vacuum in the chest. Just a little pop-up valve that pulls a little bit more blood out of the brain and the rest of the body and into the right heart.
We’ve shown in a human study that, for example, the systolic blood pressure almost doubles. It really goes from 40 mm Hg during standard CPR up to 80 mm Hg, and that would be sustained for 14-15 minutes. That was a nice little study that was done in Milwaukee a few years ago.
The other thing that happens is, if you add on something else, it’s like a toilet plunger. I think many people have seen it; it’s called “active compression-decompression.” It not only compresses, but it decompresses. Where it becomes even more effective is that if you had broken bones or stiff bones as you get older or whatever it may be, as you do the CPR, you’re still getting the push down and then you’re getting the pull out. It helps on several levels. More importantly, when you put the two together, they’re very synergistic.
We, have already done the clinical trial that is the proof of concept, and that was published in The Lancet about 10 years ago. In that study, we found that the combination of those two dramatically improved survival rates by 50%, with 1-year survival neurologically intact. That got us on the right track.
The interesting thing is that someone said, “Can we lift the head up a little bit?” We did a large amount of work in the laboratory over 10 years, fine tuning it. When do you first lift the head? How soon is too soon? It’s probably bad if you just go right to it.
We had to get the pump primed a little bit with these other things to get the flow going better, not only pulling blood out of the brain but now, you have a better flow this way. You have to prime at first for a couple of minutes, and we worked out the timing: Is it 3 or 4 minutes? It seems the timing is right at about 2 minutes, then you gradually elevate the head over about 2 minutes. We’re finding that seems to be the optimal way to do it. About 2 minutes of priming with those other two devices, the adjuncts, and then gradually elevate the head over 2 minutes.
When we do that in the laboratory, we’re getting normalized cerebral perfusion pressures. You’re normalizing the flow back again with that. We’re seeing profound differences in outcome as a result, even in these cases of the nonshockables.
Dr. Glatter: What you’re doing basically is resulting in an increase in cardiac output, essentially. That really is important, especially in these nonshockable rhythms, correct?
Dr. Pepe: Absolutely. As you’re doing this compression and you’re getting these intracranial pulse waves that are going up because they’re colliding up there. It could be even damaging in itself, but we’re seeing these intracranial raises. The intracranial pressure starts going up more and more over time. Also, peripherally in most people, you’re not getting good flow out there; then, your vasculature starts to relax. The arterials are starting to not get oxygen, so they don’t go out.
With this technique where we’re returning the pressure, we’re getting to 40% of normal now with the active compression-decompression CPR plus an impedance threshold device (ACD+ITD CPR) approach. Now, you add this, and you’re almost normalizing. In humans, even in these asystole patients, we’re seeing end-title CO2s which are generally in the 15-20 range with standard CPR are now up with ACD+ITD CPR in the 30%-40% range, where we’re getting through 30 or 40 end-tidal CO2s. Now, we’re seeing even the end-tidal CO2s moving up into the 40s and 50s. We know there’s a surrogate marker telling us that we are generating much better flows not only to the rest of the body, but most importantly, to the brain.
Dr. Glatter: Ryan, could you tell us about the approach in terms of on scene, what you’re doing and how you use the device itself? Maybe you could talk about the backpack that you developed with your fire department?
Ryan P. Quinn, BS, EMS: Our approach has always been to get to the patient quickly, like everybody’s approach on a cardiac arrest when you’re responding. We are an advanced life-support paramedic ambulance service through the fire department – we’re all cross-trained firefighter paramedics. Our first vehicle from the fire department is typically the ambulance. It’s smaller and a little quicker than the fire engine. Two paramedics are going to jump out with two backpacks. One has the automated compressive device (we use the Lucas), and the other one is the sequential patient lifting device, the EleGARD.
Our two paramedics are quick to the patient’s side, and once they make contact with the patient to verify pulseless cardiac arrest, they will unpack. One person will go right to compressions if there’s nobody on compressions already. Sometimes we have a first responder police officer with an automated external defibrillator (AED). We go right to the patient’s side, concentrate on compressions, and within 90 seconds to 2 minutes, we have our bags unpacked, we’ve got the devices turned on, patient lifted up, slid under the device, and we have a supraglottic airway that is placed within 15 seconds already premade with the ITD on top. We have a sealed airway that we can continue to compress with Dr. Pepe’s original discussion of building on what’s previously been shown to work.
Dr. Pepe: Let me make a comment about this. This is so important, what Ryan is saying, because it’s something we found during the study. It’s really a true pit-crew approach. You’re not only getting these materials, which you think you need a medical Sherpa for, but you don’t. They set it up and then when they open it up, it’s all laid out just exactly as you need it. It’s not just how fast you get there; it’s how fast you get this done.
When we look at all cases combined against high-performance systems that had some of the highest survival rates around, when we compare it to those, we found that overall, even if you looked at the ones that had over 20-minute responses, the odds ratios were still three to four times higher. It was impressive.
If you looked at it under 15 minutes, which is really reasonable for most systems that get there by the way, the average time that people start CPR in any system in these studies has been about 8 minutes if you actually start this thing, which takes about 2 minutes more for this new bundle of care with this triad, it’s almost 12-14 times higher in terms of the odds ratio. I’ve never seen anything like that where the higher end is over 100 in terms of your confidence intervals.
Ryan’s system did really well and is one of those with even higher levels of outcomes, mostly because they got it on quickly. It’s like the AED for nonshockables but better because you have a wider range of efficacy where it will work.
Dr. Glatter: When the elapsed time was less than 11 minutes, that seemed to be an inflection point in the study, is that correct? You saw that 11-fold higher incidence in terms of neurologically intact survival, is that correct?
Dr. Pepe: We picked that number because that was the median time to get it on board. Half the people were getting it within that time period. The fact that you have a larger window, we’re talking about 13- almost 14-fold improvements in outcome if it was under 15 minutes. It doesn’t matter about the 11 or the 12. It’s the faster you get it on board, the better off you are.
Dr. Glatter: What’s the next step in the process of doing trials and having implementation on a larger scale based on your Annals of Emergency Medicine study? Where do you go from here?
Dr. Pepe: I’ve come to find out there are many confounding variables. What was the quality of CPR? How did people ventilate? Did they give the breath and hold it? Did they give a large enough breath so that blood can go across the transpulmonary system? There are many confounding variables. That’s why I think, in the future, it’s going to be more of looking at things like propensity score matching because we know all the variables that change outcomes. I think that’s going to be a way for me.
The other thing is that we were looking at only 380 cases here. When this doubles up in numbers, as we accrue more cases around the country of people who are implementing this, these numbers I just quoted are going to go up much higher. Unwitnessed asystole is considered futile, and you just don’t get them back. To be able to get these folks back now, even if it’s a small percentage, and the fact that we know that we’re producing this better flow, is pretty striking.
I’m really impressed, and the main thing is to make sure people are educated about it. Number two is that they understand that it has to be done right. It cannot be done wrong or you’re not going to see the differences. Getting it done right is not only following the procedures, the sequence, and how you do it, but it also has to do with getting there quickly, including assigning the right people to put it on and having well-trained people who know what they’re doing.
Dr. Glatter: In general, the lay public obviously should not attempt this in the field lifting someone’s head up in the sense of trying to do chest compressions. I think that message is important that you just said. It’s not ready for prime time yet in any way. It has to be done right.
Dr. Pepe: Bystanders have to learn CPR – they will buy us time and we’ll have better outcomes when they do that. That’s number one. Number two is that as more and more systems adopt this, you’re going to see more people coming back. If you think about what we’re doing now, if we only get back 5% of these nonshockable vs. less than 1%, it’s 5% of 800 people a day because a thousand people a day die. Several dozens of lives can be saved on a daily basis, coming back neurologically intact. That’s the key thing.
Dr. Glatter: Ryan, can you comment about your experience in the field? Is there anything in terms of your current approach that you think would be ideal to change at this point?
Mr. Quinn: We’ve established that this is the approach that we want to take and we’re just fine tuning it to be more efficient. Using the choreography of which person is going to do which role, we have clearly defined roles and clearly defined command of the scene so we’re not missing anything. Training is extremely important.
Dr. Glatter: Paul, I want to ask you about your anecdotal experience of people waking up quickly and talking after elevating their heads and going through this process. Having people talk about it and waking up is really fascinating. Maybe you can comment further on this.
Dr. Pepe: That’s a great point that you bring up because a 40- to 50-year-old guy who got saved with this approach, when he came around, he said he was hearing what people were saying. When he came out of it, he found out he had been getting CPR for about 25 minutes because he had persistent recurring ventricular fibrillation. He said, “How could I have survived that that long?”
When we told him about the new approach, he added, “Well, that’s like neuroprotective.” He’s right, because in the laboratory, we showed it was neuroprotective and we’re also getting better flows back there. It goes along with everything else, and so we’ve adopted the name because it is.
These are really high-powered systems we are comparing against, and we have the same level of return of spontaneous circulation. The major difference was when you started talking about the neurointact survival. We don’t have enough numbers yet, but next go around, we’re going to look at cerebral performance category (CPC) – CPC1 vs. the CPC2 – which were both considered intact, but CPC1 is actually better. We’re seeing many more of those, anecdotally.
I also wanted to mention that people do bring this up and say, “Well, let’s do a trial.” As far as we’re concerned, the trial’s been done in terms of The Lancet study 10 years ago that showed that the active compression-decompression had tremendously better outcomes. We show in the laboratories that you augment that a little bit. These are all [Food and Drug Administration] approved. You can go out and buy it tomorrow and get it done. I have no conflicts of interest, by the way, with any of this.
To have this device that’s going to have the potential of saving so many more lives is really an exciting breakthrough. More importantly, we’re understanding more now about the physiology of CPR and why it works. It could work much better with the approaches that we’ve been developing over the last 20 years or so.
Dr. Glatter: Absolutely. I want to thank both of you gentlemen. It’s been really an incredible experience to learn more about an advance in resuscitation that could truly be lifesaving. Thank you again for taking time to join us.
Dr. Glatter is an attending physician in the department of emergency medicine, Lenox Hill Hospital, New York. Dr. Pepe is professor, department of management, policy, and community health, University of Texas Health Sciences Center, Houston. Mr. Quinn is EMS Chief, Edina (Minn.) Fire Department. No conflicts of interest were reported.
A version of this article first appeared Jan. 26 on Medscape.com.
This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr Robert Glatter, medical adviser for Medscape Emergency Medicine. with a remarkable increase in neurologically intact survival. Welcome, gentlemen.
Dr. Pepe, I’d like to start off by thanking you for taking time to join us to discuss this novel concept of head-up or what you now refer to as a neuroprotective cardiopulmonary resuscitation (CPR) bundle. Can you define what this entails and why it is referred to as a neuroprotective CPR bundle?
Paul E. Pepe, MD, MPH: CPR has been life saving for 60 years the way we’ve performed it, but probably only in a very small percentage of cases. That’s one of the problems. We have almost a thousand people a day who have sudden cardiac arrest out in the community alone and more in the hospital.
We know that early defibrillation and early CPR can contribute, but it’s still a small percentage of those. About 75%-85% of the cases that we go out to see will have nonshockable rhythms and flatlines. Some cases are what we call “pulseless electrical activity,” meaning that it looks like there is some kind of organized complex, but there is no pulse associated with it.
That’s why it’s a problem, because they don’t come back. Part of the reason why we see poor outcomes is not only that these cases tend to be people who, say, were in ventricular fibrillation and then just went on over time and were not witnessed or resuscitated or had a long response time. They basically either go into flatline or autoconvert into these bizarre rhythms.
The other issue is the way we perform CPR. CPR has been lifesaving, but it only generates about 20% and maybe 15% in some cases of normal blood flow, and particularly, cerebral perfusion pressure. We’ve looked at this nicely in the laboratory.
For example, during chest compressions, we’re hoping during the recoil phase to pull blood down and back into the right heart. The problem is that you’re not only setting a pressure rate up here to the arterial side but also, you’re setting back pressure wave on the venous side. Obviously, the arterial side always wins out, but it’s just not as efficient as it could be, at 20% or 30%.
What does this entail? It entails several independent mechanisms in terms of how they work, but they all do the same thing, which is they help to pull blood out of the brain and back into the right heart by basically manipulating intrathoracic pressure and creating more of a vacuum to get blood back there.
It’s so important that people do quality CPR. You have to have a good release and that helps us suck a little bit of blood and sucks the air in. As soon as the air rushes in, it neutralizes the pressure and there’s no more vacuum and nothing else is happening until the next squeeze.
What we have found is that we can cap the airway just for a second with a little pop-up valve. It acts like when you’re sucking a milkshake through a straw and it creates more of a vacuum in the chest. Just a little pop-up valve that pulls a little bit more blood out of the brain and the rest of the body and into the right heart.
We’ve shown in a human study that, for example, the systolic blood pressure almost doubles. It really goes from 40 mm Hg during standard CPR up to 80 mm Hg, and that would be sustained for 14-15 minutes. That was a nice little study that was done in Milwaukee a few years ago.
The other thing that happens is, if you add on something else, it’s like a toilet plunger. I think many people have seen it; it’s called “active compression-decompression.” It not only compresses, but it decompresses. Where it becomes even more effective is that if you had broken bones or stiff bones as you get older or whatever it may be, as you do the CPR, you’re still getting the push down and then you’re getting the pull out. It helps on several levels. More importantly, when you put the two together, they’re very synergistic.
We, have already done the clinical trial that is the proof of concept, and that was published in The Lancet about 10 years ago. In that study, we found that the combination of those two dramatically improved survival rates by 50%, with 1-year survival neurologically intact. That got us on the right track.
The interesting thing is that someone said, “Can we lift the head up a little bit?” We did a large amount of work in the laboratory over 10 years, fine tuning it. When do you first lift the head? How soon is too soon? It’s probably bad if you just go right to it.
We had to get the pump primed a little bit with these other things to get the flow going better, not only pulling blood out of the brain but now, you have a better flow this way. You have to prime at first for a couple of minutes, and we worked out the timing: Is it 3 or 4 minutes? It seems the timing is right at about 2 minutes, then you gradually elevate the head over about 2 minutes. We’re finding that seems to be the optimal way to do it. About 2 minutes of priming with those other two devices, the adjuncts, and then gradually elevate the head over 2 minutes.
When we do that in the laboratory, we’re getting normalized cerebral perfusion pressures. You’re normalizing the flow back again with that. We’re seeing profound differences in outcome as a result, even in these cases of the nonshockables.
Dr. Glatter: What you’re doing basically is resulting in an increase in cardiac output, essentially. That really is important, especially in these nonshockable rhythms, correct?
Dr. Pepe: Absolutely. As you’re doing this compression and you’re getting these intracranial pulse waves that are going up because they’re colliding up there. It could be even damaging in itself, but we’re seeing these intracranial raises. The intracranial pressure starts going up more and more over time. Also, peripherally in most people, you’re not getting good flow out there; then, your vasculature starts to relax. The arterials are starting to not get oxygen, so they don’t go out.
With this technique where we’re returning the pressure, we’re getting to 40% of normal now with the active compression-decompression CPR plus an impedance threshold device (ACD+ITD CPR) approach. Now, you add this, and you’re almost normalizing. In humans, even in these asystole patients, we’re seeing end-title CO2s which are generally in the 15-20 range with standard CPR are now up with ACD+ITD CPR in the 30%-40% range, where we’re getting through 30 or 40 end-tidal CO2s. Now, we’re seeing even the end-tidal CO2s moving up into the 40s and 50s. We know there’s a surrogate marker telling us that we are generating much better flows not only to the rest of the body, but most importantly, to the brain.
Dr. Glatter: Ryan, could you tell us about the approach in terms of on scene, what you’re doing and how you use the device itself? Maybe you could talk about the backpack that you developed with your fire department?
Ryan P. Quinn, BS, EMS: Our approach has always been to get to the patient quickly, like everybody’s approach on a cardiac arrest when you’re responding. We are an advanced life-support paramedic ambulance service through the fire department – we’re all cross-trained firefighter paramedics. Our first vehicle from the fire department is typically the ambulance. It’s smaller and a little quicker than the fire engine. Two paramedics are going to jump out with two backpacks. One has the automated compressive device (we use the Lucas), and the other one is the sequential patient lifting device, the EleGARD.
Our two paramedics are quick to the patient’s side, and once they make contact with the patient to verify pulseless cardiac arrest, they will unpack. One person will go right to compressions if there’s nobody on compressions already. Sometimes we have a first responder police officer with an automated external defibrillator (AED). We go right to the patient’s side, concentrate on compressions, and within 90 seconds to 2 minutes, we have our bags unpacked, we’ve got the devices turned on, patient lifted up, slid under the device, and we have a supraglottic airway that is placed within 15 seconds already premade with the ITD on top. We have a sealed airway that we can continue to compress with Dr. Pepe’s original discussion of building on what’s previously been shown to work.
Dr. Pepe: Let me make a comment about this. This is so important, what Ryan is saying, because it’s something we found during the study. It’s really a true pit-crew approach. You’re not only getting these materials, which you think you need a medical Sherpa for, but you don’t. They set it up and then when they open it up, it’s all laid out just exactly as you need it. It’s not just how fast you get there; it’s how fast you get this done.
When we look at all cases combined against high-performance systems that had some of the highest survival rates around, when we compare it to those, we found that overall, even if you looked at the ones that had over 20-minute responses, the odds ratios were still three to four times higher. It was impressive.
If you looked at it under 15 minutes, which is really reasonable for most systems that get there by the way, the average time that people start CPR in any system in these studies has been about 8 minutes if you actually start this thing, which takes about 2 minutes more for this new bundle of care with this triad, it’s almost 12-14 times higher in terms of the odds ratio. I’ve never seen anything like that where the higher end is over 100 in terms of your confidence intervals.
Ryan’s system did really well and is one of those with even higher levels of outcomes, mostly because they got it on quickly. It’s like the AED for nonshockables but better because you have a wider range of efficacy where it will work.
Dr. Glatter: When the elapsed time was less than 11 minutes, that seemed to be an inflection point in the study, is that correct? You saw that 11-fold higher incidence in terms of neurologically intact survival, is that correct?
Dr. Pepe: We picked that number because that was the median time to get it on board. Half the people were getting it within that time period. The fact that you have a larger window, we’re talking about 13- almost 14-fold improvements in outcome if it was under 15 minutes. It doesn’t matter about the 11 or the 12. It’s the faster you get it on board, the better off you are.
Dr. Glatter: What’s the next step in the process of doing trials and having implementation on a larger scale based on your Annals of Emergency Medicine study? Where do you go from here?
Dr. Pepe: I’ve come to find out there are many confounding variables. What was the quality of CPR? How did people ventilate? Did they give the breath and hold it? Did they give a large enough breath so that blood can go across the transpulmonary system? There are many confounding variables. That’s why I think, in the future, it’s going to be more of looking at things like propensity score matching because we know all the variables that change outcomes. I think that’s going to be a way for me.
The other thing is that we were looking at only 380 cases here. When this doubles up in numbers, as we accrue more cases around the country of people who are implementing this, these numbers I just quoted are going to go up much higher. Unwitnessed asystole is considered futile, and you just don’t get them back. To be able to get these folks back now, even if it’s a small percentage, and the fact that we know that we’re producing this better flow, is pretty striking.
I’m really impressed, and the main thing is to make sure people are educated about it. Number two is that they understand that it has to be done right. It cannot be done wrong or you’re not going to see the differences. Getting it done right is not only following the procedures, the sequence, and how you do it, but it also has to do with getting there quickly, including assigning the right people to put it on and having well-trained people who know what they’re doing.
Dr. Glatter: In general, the lay public obviously should not attempt this in the field lifting someone’s head up in the sense of trying to do chest compressions. I think that message is important that you just said. It’s not ready for prime time yet in any way. It has to be done right.
Dr. Pepe: Bystanders have to learn CPR – they will buy us time and we’ll have better outcomes when they do that. That’s number one. Number two is that as more and more systems adopt this, you’re going to see more people coming back. If you think about what we’re doing now, if we only get back 5% of these nonshockable vs. less than 1%, it’s 5% of 800 people a day because a thousand people a day die. Several dozens of lives can be saved on a daily basis, coming back neurologically intact. That’s the key thing.
Dr. Glatter: Ryan, can you comment about your experience in the field? Is there anything in terms of your current approach that you think would be ideal to change at this point?
Mr. Quinn: We’ve established that this is the approach that we want to take and we’re just fine tuning it to be more efficient. Using the choreography of which person is going to do which role, we have clearly defined roles and clearly defined command of the scene so we’re not missing anything. Training is extremely important.
Dr. Glatter: Paul, I want to ask you about your anecdotal experience of people waking up quickly and talking after elevating their heads and going through this process. Having people talk about it and waking up is really fascinating. Maybe you can comment further on this.
Dr. Pepe: That’s a great point that you bring up because a 40- to 50-year-old guy who got saved with this approach, when he came around, he said he was hearing what people were saying. When he came out of it, he found out he had been getting CPR for about 25 minutes because he had persistent recurring ventricular fibrillation. He said, “How could I have survived that that long?”
When we told him about the new approach, he added, “Well, that’s like neuroprotective.” He’s right, because in the laboratory, we showed it was neuroprotective and we’re also getting better flows back there. It goes along with everything else, and so we’ve adopted the name because it is.
These are really high-powered systems we are comparing against, and we have the same level of return of spontaneous circulation. The major difference was when you started talking about the neurointact survival. We don’t have enough numbers yet, but next go around, we’re going to look at cerebral performance category (CPC) – CPC1 vs. the CPC2 – which were both considered intact, but CPC1 is actually better. We’re seeing many more of those, anecdotally.
I also wanted to mention that people do bring this up and say, “Well, let’s do a trial.” As far as we’re concerned, the trial’s been done in terms of The Lancet study 10 years ago that showed that the active compression-decompression had tremendously better outcomes. We show in the laboratories that you augment that a little bit. These are all [Food and Drug Administration] approved. You can go out and buy it tomorrow and get it done. I have no conflicts of interest, by the way, with any of this.
To have this device that’s going to have the potential of saving so many more lives is really an exciting breakthrough. More importantly, we’re understanding more now about the physiology of CPR and why it works. It could work much better with the approaches that we’ve been developing over the last 20 years or so.
Dr. Glatter: Absolutely. I want to thank both of you gentlemen. It’s been really an incredible experience to learn more about an advance in resuscitation that could truly be lifesaving. Thank you again for taking time to join us.
Dr. Glatter is an attending physician in the department of emergency medicine, Lenox Hill Hospital, New York. Dr. Pepe is professor, department of management, policy, and community health, University of Texas Health Sciences Center, Houston. Mr. Quinn is EMS Chief, Edina (Minn.) Fire Department. No conflicts of interest were reported.
A version of this article first appeared Jan. 26 on Medscape.com.
Don’t cross the friends line with patients
All that moving can make it hard to maintain friendships. Factor in the challenges from the pandemic, and a physician’s life can be lonely. So, when a patient invites you for coffee or a game of pickleball, do you accept? For almost one-third of the physicians who responded to the Medscape Physician Friendships: The Joys and Challenges 2022, the answer might be yes.
About 29% said they develop friendships with patients. However, a lot depends on the circumstances. As one physician in the report said: “I have been a pediatrician for 35 years, and my patients have grown up and become productive adults in our small, rural, isolated area. You can’t help but know almost everyone.”
As the daughter of a cardiologist, Nishi Mehta, MD, a radiologist and founder of the largest physician-only Facebook group in the country, grew up with that small-town-everyone-knows-the-doctor model.
“When I was a kid, I’d go to the mall, and my friends and I would play a game: How long before a patient [of my dad’s] comes up to me?” she said. At the time, Dr. Mehta was embarrassed, but now she marvels that her dad knew his patients so well that they would recognize his daughter in crowded suburban mall.
In other instances, a physician may develop a friendly relationship after a patient leaves their care. For example, Leo Nissola, MD, now a full-time researcher and immunotherapy scientist in San Francisco, has stayed in touch with some of the patients he treated while at the University of Texas MD Anderson Cancer Center, Houston.
Dr. Nissola said it was important to stay connected with the patients he had meaningful relationships with. “It becomes challenging, though, when a former patient asks for medical advice.” At that moment, “you have to be explicitly clear that the relationship has changed.”
A hard line in the sand
The blurring of lines is one reason many doctors refuse to befriend patients, even after they are no longer treating them. The American College of Physicians Ethics Manual advises against treating anyone with whom you have a close relationship, including family and friends.
“Friendships can get in the way of patients being honest with you, which can interfere with medical care,” Dr. Mehta said. “If a patient has a concern related to something they wouldn’t want you to know as friends, it can get awkward. They may elect not to tell you.”
And on the flip side, friendship can provide a view into your private life that you may not welcome in the exam room.
“Let’s say you go out for drinks [with a patient], and you’re up late, but you have surgery the next day,” said Brandi Ring, MD, an ob.gyn. and the associate medical director at the Center for Children and Women in Houston. Now, one of your patients knows you were out until midnight when you had to be in the OR at 5:00 a.m.
Worse still, your relationship could color your decisions about a patient’s care, even unconsciously. It can be hard to maintain objectivity when you have an emotional investment in someone’s well-being.
“We don’t necessarily treat family and friends to the standards of medical care,” said Dr. Ring. “We go above and beyond. We might order more tests and more scans. We don’t always follow the guidelines, especially in critical illness.”
For all these reasons and more, the ACP advises against treating friends.
Put physician before friend
But adhering to those guidelines can lead physicians to make some painful decisions. Cutting yourself off from the possibility of friendship is never easy, and the Medscape report found that physicians tend to have fewer friends than the average American.
“Especially earlier in my practice, when I was a young parent, and I would see a lot of other young parents in the same stage in life, I’d think, ‘In other circumstances, I would be hanging out at the park with this person,’ “ said Kathleen Rowland, MD, a family medicine physician and vice chair of education in the department of family medicine at Rush University, Chicago. “But the hard part is, the doctor-patient relationship always comes first.”
To a certain extent, one’s specialty may determine the feasibility of becoming friends with a patient. While Dr. Mehta has never done so, as a radiologist, she doesn’t usually see patients repeatedly. Likewise, a young gerontologist may have little in common with his octogenarian patients. And an older pediatrician is not in the same life stage as his patients’ sleep-deprived new parents, possibly making them less attractive friends.
However, practicing family medicine is all about long-term physician-patient relationships. Getting to know patients and their families over many years can lead to a certain intimacy. Dr. Rowland said that, while a wonderful part of being a physician is getting that unique trust whereby patients tell you all sorts of things about their lives, she’s never gone down the friendship path.
“There’s the assumption I’ll take care of someone for a long period of time, and their partner and their kids, maybe another generation or two,” Dr. Rowland said. “People really do rely on that relationship to contribute to their health.”
Worse, nowadays, when people may be starved for connection, many patients want to feel emotionally close and cared for by their doctor, so it’d be easy to cross the line. While patients deserve a compassionate, caring doctor, the physician is left to walk the line between those boundaries. Dr. Rowland said, “It’s up to the clinician to say: ‘My role is as a doctor. You deserve caring friends, but I have to order your mammogram and your blood counts. My role is different.’ ”
Friendly but not friends
It can be tricky to navigate the boundary between a cordial, warm relationship with a patient and that patient inviting you to their daughter’s wedding.
“People may mistake being pleasant and friendly for being friends,” said Larry Blosser, MD, chief medical officer at Central Ohio Primary Care, Westerville. In his position, he sometimes hears from patients who have misunderstood their relationship with a doctor in the practice. When that happens, he advises the physician to consider the persona they’re presenting to the patient. If you’re overly friendly, there’s the potential for confusion, but you can’t be aloof and cold, he said.
Maintaining that awareness helps to prevent a patient’s offhand invitation to catch a movie or go on a hike. And verbalizing it to your patients can make your relationship clear from the get-go.
“I tell patients we’re a team. I’m the captain, and they’re my MVP. When the match is over, whatever the results, we’re done,” said Karenne Fru, MD, PhD, a fertility specialist at Oma Fertility Atlanta. Making deep connections is essential to her practice, so Dr. Fru structures her patient interactions carefully. “Infertility is such an isolating experience. While you’re with us, we care about what’s going on in your life, your pets, and your mom’s chemo. We need mutual trust for you to be compliant with the care.”
However, that approach won’t work when you see patients regularly, as with family practice or specialties that see the same patients repeatedly throughout the year. In those circumstances, the match is never over but one in which the onus is on the physician to establish a friendly yet professional rapport without letting your self-interest, loneliness, or lack of friends interfere.
“It’s been a very difficult couple of years for a lot of us. Depending on what kind of clinical work we do, some of us took care of healthy people that got very sick or passed away,” Dr. Rowland said. “Having the chance to reconnect with people and reestablish some of that closeness, both physical and emotional, is going to be good for us.”
Just continue conveying warm, trusting compassion for your patients without blurring the friend lines.
A version of this article first appeared on Medscape.com.
All that moving can make it hard to maintain friendships. Factor in the challenges from the pandemic, and a physician’s life can be lonely. So, when a patient invites you for coffee or a game of pickleball, do you accept? For almost one-third of the physicians who responded to the Medscape Physician Friendships: The Joys and Challenges 2022, the answer might be yes.
About 29% said they develop friendships with patients. However, a lot depends on the circumstances. As one physician in the report said: “I have been a pediatrician for 35 years, and my patients have grown up and become productive adults in our small, rural, isolated area. You can’t help but know almost everyone.”
As the daughter of a cardiologist, Nishi Mehta, MD, a radiologist and founder of the largest physician-only Facebook group in the country, grew up with that small-town-everyone-knows-the-doctor model.
“When I was a kid, I’d go to the mall, and my friends and I would play a game: How long before a patient [of my dad’s] comes up to me?” she said. At the time, Dr. Mehta was embarrassed, but now she marvels that her dad knew his patients so well that they would recognize his daughter in crowded suburban mall.
In other instances, a physician may develop a friendly relationship after a patient leaves their care. For example, Leo Nissola, MD, now a full-time researcher and immunotherapy scientist in San Francisco, has stayed in touch with some of the patients he treated while at the University of Texas MD Anderson Cancer Center, Houston.
Dr. Nissola said it was important to stay connected with the patients he had meaningful relationships with. “It becomes challenging, though, when a former patient asks for medical advice.” At that moment, “you have to be explicitly clear that the relationship has changed.”
A hard line in the sand
The blurring of lines is one reason many doctors refuse to befriend patients, even after they are no longer treating them. The American College of Physicians Ethics Manual advises against treating anyone with whom you have a close relationship, including family and friends.
“Friendships can get in the way of patients being honest with you, which can interfere with medical care,” Dr. Mehta said. “If a patient has a concern related to something they wouldn’t want you to know as friends, it can get awkward. They may elect not to tell you.”
And on the flip side, friendship can provide a view into your private life that you may not welcome in the exam room.
“Let’s say you go out for drinks [with a patient], and you’re up late, but you have surgery the next day,” said Brandi Ring, MD, an ob.gyn. and the associate medical director at the Center for Children and Women in Houston. Now, one of your patients knows you were out until midnight when you had to be in the OR at 5:00 a.m.
Worse still, your relationship could color your decisions about a patient’s care, even unconsciously. It can be hard to maintain objectivity when you have an emotional investment in someone’s well-being.
“We don’t necessarily treat family and friends to the standards of medical care,” said Dr. Ring. “We go above and beyond. We might order more tests and more scans. We don’t always follow the guidelines, especially in critical illness.”
For all these reasons and more, the ACP advises against treating friends.
Put physician before friend
But adhering to those guidelines can lead physicians to make some painful decisions. Cutting yourself off from the possibility of friendship is never easy, and the Medscape report found that physicians tend to have fewer friends than the average American.
“Especially earlier in my practice, when I was a young parent, and I would see a lot of other young parents in the same stage in life, I’d think, ‘In other circumstances, I would be hanging out at the park with this person,’ “ said Kathleen Rowland, MD, a family medicine physician and vice chair of education in the department of family medicine at Rush University, Chicago. “But the hard part is, the doctor-patient relationship always comes first.”
To a certain extent, one’s specialty may determine the feasibility of becoming friends with a patient. While Dr. Mehta has never done so, as a radiologist, she doesn’t usually see patients repeatedly. Likewise, a young gerontologist may have little in common with his octogenarian patients. And an older pediatrician is not in the same life stage as his patients’ sleep-deprived new parents, possibly making them less attractive friends.
However, practicing family medicine is all about long-term physician-patient relationships. Getting to know patients and their families over many years can lead to a certain intimacy. Dr. Rowland said that, while a wonderful part of being a physician is getting that unique trust whereby patients tell you all sorts of things about their lives, she’s never gone down the friendship path.
“There’s the assumption I’ll take care of someone for a long period of time, and their partner and their kids, maybe another generation or two,” Dr. Rowland said. “People really do rely on that relationship to contribute to their health.”
Worse, nowadays, when people may be starved for connection, many patients want to feel emotionally close and cared for by their doctor, so it’d be easy to cross the line. While patients deserve a compassionate, caring doctor, the physician is left to walk the line between those boundaries. Dr. Rowland said, “It’s up to the clinician to say: ‘My role is as a doctor. You deserve caring friends, but I have to order your mammogram and your blood counts. My role is different.’ ”
Friendly but not friends
It can be tricky to navigate the boundary between a cordial, warm relationship with a patient and that patient inviting you to their daughter’s wedding.
“People may mistake being pleasant and friendly for being friends,” said Larry Blosser, MD, chief medical officer at Central Ohio Primary Care, Westerville. In his position, he sometimes hears from patients who have misunderstood their relationship with a doctor in the practice. When that happens, he advises the physician to consider the persona they’re presenting to the patient. If you’re overly friendly, there’s the potential for confusion, but you can’t be aloof and cold, he said.
Maintaining that awareness helps to prevent a patient’s offhand invitation to catch a movie or go on a hike. And verbalizing it to your patients can make your relationship clear from the get-go.
“I tell patients we’re a team. I’m the captain, and they’re my MVP. When the match is over, whatever the results, we’re done,” said Karenne Fru, MD, PhD, a fertility specialist at Oma Fertility Atlanta. Making deep connections is essential to her practice, so Dr. Fru structures her patient interactions carefully. “Infertility is such an isolating experience. While you’re with us, we care about what’s going on in your life, your pets, and your mom’s chemo. We need mutual trust for you to be compliant with the care.”
However, that approach won’t work when you see patients regularly, as with family practice or specialties that see the same patients repeatedly throughout the year. In those circumstances, the match is never over but one in which the onus is on the physician to establish a friendly yet professional rapport without letting your self-interest, loneliness, or lack of friends interfere.
“It’s been a very difficult couple of years for a lot of us. Depending on what kind of clinical work we do, some of us took care of healthy people that got very sick or passed away,” Dr. Rowland said. “Having the chance to reconnect with people and reestablish some of that closeness, both physical and emotional, is going to be good for us.”
Just continue conveying warm, trusting compassion for your patients without blurring the friend lines.
A version of this article first appeared on Medscape.com.
All that moving can make it hard to maintain friendships. Factor in the challenges from the pandemic, and a physician’s life can be lonely. So, when a patient invites you for coffee or a game of pickleball, do you accept? For almost one-third of the physicians who responded to the Medscape Physician Friendships: The Joys and Challenges 2022, the answer might be yes.
About 29% said they develop friendships with patients. However, a lot depends on the circumstances. As one physician in the report said: “I have been a pediatrician for 35 years, and my patients have grown up and become productive adults in our small, rural, isolated area. You can’t help but know almost everyone.”
As the daughter of a cardiologist, Nishi Mehta, MD, a radiologist and founder of the largest physician-only Facebook group in the country, grew up with that small-town-everyone-knows-the-doctor model.
“When I was a kid, I’d go to the mall, and my friends and I would play a game: How long before a patient [of my dad’s] comes up to me?” she said. At the time, Dr. Mehta was embarrassed, but now she marvels that her dad knew his patients so well that they would recognize his daughter in crowded suburban mall.
In other instances, a physician may develop a friendly relationship after a patient leaves their care. For example, Leo Nissola, MD, now a full-time researcher and immunotherapy scientist in San Francisco, has stayed in touch with some of the patients he treated while at the University of Texas MD Anderson Cancer Center, Houston.
Dr. Nissola said it was important to stay connected with the patients he had meaningful relationships with. “It becomes challenging, though, when a former patient asks for medical advice.” At that moment, “you have to be explicitly clear that the relationship has changed.”
A hard line in the sand
The blurring of lines is one reason many doctors refuse to befriend patients, even after they are no longer treating them. The American College of Physicians Ethics Manual advises against treating anyone with whom you have a close relationship, including family and friends.
“Friendships can get in the way of patients being honest with you, which can interfere with medical care,” Dr. Mehta said. “If a patient has a concern related to something they wouldn’t want you to know as friends, it can get awkward. They may elect not to tell you.”
And on the flip side, friendship can provide a view into your private life that you may not welcome in the exam room.
“Let’s say you go out for drinks [with a patient], and you’re up late, but you have surgery the next day,” said Brandi Ring, MD, an ob.gyn. and the associate medical director at the Center for Children and Women in Houston. Now, one of your patients knows you were out until midnight when you had to be in the OR at 5:00 a.m.
Worse still, your relationship could color your decisions about a patient’s care, even unconsciously. It can be hard to maintain objectivity when you have an emotional investment in someone’s well-being.
“We don’t necessarily treat family and friends to the standards of medical care,” said Dr. Ring. “We go above and beyond. We might order more tests and more scans. We don’t always follow the guidelines, especially in critical illness.”
For all these reasons and more, the ACP advises against treating friends.
Put physician before friend
But adhering to those guidelines can lead physicians to make some painful decisions. Cutting yourself off from the possibility of friendship is never easy, and the Medscape report found that physicians tend to have fewer friends than the average American.
“Especially earlier in my practice, when I was a young parent, and I would see a lot of other young parents in the same stage in life, I’d think, ‘In other circumstances, I would be hanging out at the park with this person,’ “ said Kathleen Rowland, MD, a family medicine physician and vice chair of education in the department of family medicine at Rush University, Chicago. “But the hard part is, the doctor-patient relationship always comes first.”
To a certain extent, one’s specialty may determine the feasibility of becoming friends with a patient. While Dr. Mehta has never done so, as a radiologist, she doesn’t usually see patients repeatedly. Likewise, a young gerontologist may have little in common with his octogenarian patients. And an older pediatrician is not in the same life stage as his patients’ sleep-deprived new parents, possibly making them less attractive friends.
However, practicing family medicine is all about long-term physician-patient relationships. Getting to know patients and their families over many years can lead to a certain intimacy. Dr. Rowland said that, while a wonderful part of being a physician is getting that unique trust whereby patients tell you all sorts of things about their lives, she’s never gone down the friendship path.
“There’s the assumption I’ll take care of someone for a long period of time, and their partner and their kids, maybe another generation or two,” Dr. Rowland said. “People really do rely on that relationship to contribute to their health.”
Worse, nowadays, when people may be starved for connection, many patients want to feel emotionally close and cared for by their doctor, so it’d be easy to cross the line. While patients deserve a compassionate, caring doctor, the physician is left to walk the line between those boundaries. Dr. Rowland said, “It’s up to the clinician to say: ‘My role is as a doctor. You deserve caring friends, but I have to order your mammogram and your blood counts. My role is different.’ ”
Friendly but not friends
It can be tricky to navigate the boundary between a cordial, warm relationship with a patient and that patient inviting you to their daughter’s wedding.
“People may mistake being pleasant and friendly for being friends,” said Larry Blosser, MD, chief medical officer at Central Ohio Primary Care, Westerville. In his position, he sometimes hears from patients who have misunderstood their relationship with a doctor in the practice. When that happens, he advises the physician to consider the persona they’re presenting to the patient. If you’re overly friendly, there’s the potential for confusion, but you can’t be aloof and cold, he said.
Maintaining that awareness helps to prevent a patient’s offhand invitation to catch a movie or go on a hike. And verbalizing it to your patients can make your relationship clear from the get-go.
“I tell patients we’re a team. I’m the captain, and they’re my MVP. When the match is over, whatever the results, we’re done,” said Karenne Fru, MD, PhD, a fertility specialist at Oma Fertility Atlanta. Making deep connections is essential to her practice, so Dr. Fru structures her patient interactions carefully. “Infertility is such an isolating experience. While you’re with us, we care about what’s going on in your life, your pets, and your mom’s chemo. We need mutual trust for you to be compliant with the care.”
However, that approach won’t work when you see patients regularly, as with family practice or specialties that see the same patients repeatedly throughout the year. In those circumstances, the match is never over but one in which the onus is on the physician to establish a friendly yet professional rapport without letting your self-interest, loneliness, or lack of friends interfere.
“It’s been a very difficult couple of years for a lot of us. Depending on what kind of clinical work we do, some of us took care of healthy people that got very sick or passed away,” Dr. Rowland said. “Having the chance to reconnect with people and reestablish some of that closeness, both physical and emotional, is going to be good for us.”
Just continue conveying warm, trusting compassion for your patients without blurring the friend lines.
A version of this article first appeared on Medscape.com.
EMR screening in emergency department tags undiagnosed diabetes
A diabetes screening program built into an electronic medical records system identified diabetes or prediabetes in 52% of individuals flagged for abnormal hemoglobin A1c, based on data from more than 2,000 adults.
“Despite the best efforts of clinicians, researchers, and educators, the number of patients living with undiagnosed diabetes is still rising and is currently at approximately 8.5 million, and the number of people unaware of their prediabetes is approximately 77 million,” lead investigator Kristie K. Danielson, PhD, said in an interview. Screening for diabetes is critical to start treatment early, to potentially reverse prediabetes, and to prevent the long-term complications of diabetes and reduced life expectancy.
In a pilot study published in JAMA Network Open, Dr. Danielson and colleagues reviewed data from 8,441 adults who visited a single emergency department in Chicago during February–April 2021.
The EMR at the hospital contained a built-in best practice alert (BPA) that flagged patients as being at risk for type 2 diabetes based the American Diabetes Association recommendations; the identification algorithm included age 45 years and older, or those aged 18-44 years with a body mass index of 25 kg/m2 or higher, no previous history of diabetes, and no A1c measure in the last 3 years, according to the EMR.
A total of 8,441 adult patients visited the ED during the study period; 2,576 triggered BPA tests, and 2,074 had A1c results for review. Among the patients with A1c results, 52% had elevated values of 5.7% or higher. Of these, a total of 758 individuals were identified with prediabetes (A1c, 5.7%-6.4%), 265 with diabetes (A1c, 6.5%-9.9%), and 62 with severe diabetes (A1c, 10% or higher).
After testing, 352 patients with elevated A1c were contacted by the researchers. The mean age of this group was 52.2 years, 54.5% were women, and nearly two-thirds (64.8%) were non-Hispanic Black. The median income of those contacted was in the 44th percentile, and 50% had public insurance.
Most of those contacted (264 patients) were not aware of a previous diagnosis of prediabetes or diabetes; the remaining 88 had a previous diagnosis, but only 51 self-reported receiving treatment, the researchers noted.
Although the screening program successfully identified a significant number of previously undiagnosed individuals with diabetes, prediabetes, or poorly controlled diabetes, its feasibility in routine practice requires further study, the researchers wrote.
The findings were limited by several factors including the identification of patients previously diagnosed with diabetes but who were not being treated, and the potential bias toward individuals of higher socioeconomic status, the researchers noted. However, the results support further exploration of the program as a way to identify undiagnosed diabetes, especially in underserved populations.
Diabetes in underserved groups goes undetected
“We were surprised by the sheer number of people newly diagnosed with diabetes or prediabetes,” which was far greater than expected, commented Dr. Danielson of the University of Illinois at Chicago. “Clearly, we tapped into a new population that has not often been seen by primary care providers or endocrinologists, as is often the case for underserved and vulnerable individuals who visit the emergency department as a first line for health care.”
The screening alert system is straightforward to build into an existing EMR, with technical support, Dr. Danielson said. “In theory, it should be able to be incorporated into other clinical centers and emergency departments. One of the current limitations that we are seeing is that the EMR is still flagging some people already diagnosed with diabetes to be screened for diabetes.” However, “because of this, we also see this as an opportunity to identify and reach out to those with diabetes who are still underserved and not receiving the appropriate diabetes care they need.”
The study results have broader public health implications, Dr. Danielson added. “We have identified a new, large population of people with diabetes who need medical care and diabetes education. This will further add to the burden of health care and costs, and it raises the ethical question of screening and not having full resources readily available to help.
“In my opinion, the study sheds light on a significant issue that will hopefully help drive change at both a health systems and public health level locally and nationally,” she added.
“One of the significant research gaps that has emerged now is how to link these new patients to health care and diabetes education at our institution after they leave the emergency department,” said Dr. Danielson. Diabetes screening in the ED setting is “a very novel area for health system scientists, social workers, and others to now come to the table and collaborate on next steps to help our patients.”
The study was initiated by the investigators, but was supported by a grant from Novo Nordisk to two coauthors. Dr. Danielson also disclosed grant funding from Novo Nordisk during the conduct of the study.
A diabetes screening program built into an electronic medical records system identified diabetes or prediabetes in 52% of individuals flagged for abnormal hemoglobin A1c, based on data from more than 2,000 adults.
“Despite the best efforts of clinicians, researchers, and educators, the number of patients living with undiagnosed diabetes is still rising and is currently at approximately 8.5 million, and the number of people unaware of their prediabetes is approximately 77 million,” lead investigator Kristie K. Danielson, PhD, said in an interview. Screening for diabetes is critical to start treatment early, to potentially reverse prediabetes, and to prevent the long-term complications of diabetes and reduced life expectancy.
In a pilot study published in JAMA Network Open, Dr. Danielson and colleagues reviewed data from 8,441 adults who visited a single emergency department in Chicago during February–April 2021.
The EMR at the hospital contained a built-in best practice alert (BPA) that flagged patients as being at risk for type 2 diabetes based the American Diabetes Association recommendations; the identification algorithm included age 45 years and older, or those aged 18-44 years with a body mass index of 25 kg/m2 or higher, no previous history of diabetes, and no A1c measure in the last 3 years, according to the EMR.
A total of 8,441 adult patients visited the ED during the study period; 2,576 triggered BPA tests, and 2,074 had A1c results for review. Among the patients with A1c results, 52% had elevated values of 5.7% or higher. Of these, a total of 758 individuals were identified with prediabetes (A1c, 5.7%-6.4%), 265 with diabetes (A1c, 6.5%-9.9%), and 62 with severe diabetes (A1c, 10% or higher).
After testing, 352 patients with elevated A1c were contacted by the researchers. The mean age of this group was 52.2 years, 54.5% were women, and nearly two-thirds (64.8%) were non-Hispanic Black. The median income of those contacted was in the 44th percentile, and 50% had public insurance.
Most of those contacted (264 patients) were not aware of a previous diagnosis of prediabetes or diabetes; the remaining 88 had a previous diagnosis, but only 51 self-reported receiving treatment, the researchers noted.
Although the screening program successfully identified a significant number of previously undiagnosed individuals with diabetes, prediabetes, or poorly controlled diabetes, its feasibility in routine practice requires further study, the researchers wrote.
The findings were limited by several factors including the identification of patients previously diagnosed with diabetes but who were not being treated, and the potential bias toward individuals of higher socioeconomic status, the researchers noted. However, the results support further exploration of the program as a way to identify undiagnosed diabetes, especially in underserved populations.
Diabetes in underserved groups goes undetected
“We were surprised by the sheer number of people newly diagnosed with diabetes or prediabetes,” which was far greater than expected, commented Dr. Danielson of the University of Illinois at Chicago. “Clearly, we tapped into a new population that has not often been seen by primary care providers or endocrinologists, as is often the case for underserved and vulnerable individuals who visit the emergency department as a first line for health care.”
The screening alert system is straightforward to build into an existing EMR, with technical support, Dr. Danielson said. “In theory, it should be able to be incorporated into other clinical centers and emergency departments. One of the current limitations that we are seeing is that the EMR is still flagging some people already diagnosed with diabetes to be screened for diabetes.” However, “because of this, we also see this as an opportunity to identify and reach out to those with diabetes who are still underserved and not receiving the appropriate diabetes care they need.”
The study results have broader public health implications, Dr. Danielson added. “We have identified a new, large population of people with diabetes who need medical care and diabetes education. This will further add to the burden of health care and costs, and it raises the ethical question of screening and not having full resources readily available to help.
“In my opinion, the study sheds light on a significant issue that will hopefully help drive change at both a health systems and public health level locally and nationally,” she added.
“One of the significant research gaps that has emerged now is how to link these new patients to health care and diabetes education at our institution after they leave the emergency department,” said Dr. Danielson. Diabetes screening in the ED setting is “a very novel area for health system scientists, social workers, and others to now come to the table and collaborate on next steps to help our patients.”
The study was initiated by the investigators, but was supported by a grant from Novo Nordisk to two coauthors. Dr. Danielson also disclosed grant funding from Novo Nordisk during the conduct of the study.
A diabetes screening program built into an electronic medical records system identified diabetes or prediabetes in 52% of individuals flagged for abnormal hemoglobin A1c, based on data from more than 2,000 adults.
“Despite the best efforts of clinicians, researchers, and educators, the number of patients living with undiagnosed diabetes is still rising and is currently at approximately 8.5 million, and the number of people unaware of their prediabetes is approximately 77 million,” lead investigator Kristie K. Danielson, PhD, said in an interview. Screening for diabetes is critical to start treatment early, to potentially reverse prediabetes, and to prevent the long-term complications of diabetes and reduced life expectancy.
In a pilot study published in JAMA Network Open, Dr. Danielson and colleagues reviewed data from 8,441 adults who visited a single emergency department in Chicago during February–April 2021.
The EMR at the hospital contained a built-in best practice alert (BPA) that flagged patients as being at risk for type 2 diabetes based the American Diabetes Association recommendations; the identification algorithm included age 45 years and older, or those aged 18-44 years with a body mass index of 25 kg/m2 or higher, no previous history of diabetes, and no A1c measure in the last 3 years, according to the EMR.
A total of 8,441 adult patients visited the ED during the study period; 2,576 triggered BPA tests, and 2,074 had A1c results for review. Among the patients with A1c results, 52% had elevated values of 5.7% or higher. Of these, a total of 758 individuals were identified with prediabetes (A1c, 5.7%-6.4%), 265 with diabetes (A1c, 6.5%-9.9%), and 62 with severe diabetes (A1c, 10% or higher).
After testing, 352 patients with elevated A1c were contacted by the researchers. The mean age of this group was 52.2 years, 54.5% were women, and nearly two-thirds (64.8%) were non-Hispanic Black. The median income of those contacted was in the 44th percentile, and 50% had public insurance.
Most of those contacted (264 patients) were not aware of a previous diagnosis of prediabetes or diabetes; the remaining 88 had a previous diagnosis, but only 51 self-reported receiving treatment, the researchers noted.
Although the screening program successfully identified a significant number of previously undiagnosed individuals with diabetes, prediabetes, or poorly controlled diabetes, its feasibility in routine practice requires further study, the researchers wrote.
The findings were limited by several factors including the identification of patients previously diagnosed with diabetes but who were not being treated, and the potential bias toward individuals of higher socioeconomic status, the researchers noted. However, the results support further exploration of the program as a way to identify undiagnosed diabetes, especially in underserved populations.
Diabetes in underserved groups goes undetected
“We were surprised by the sheer number of people newly diagnosed with diabetes or prediabetes,” which was far greater than expected, commented Dr. Danielson of the University of Illinois at Chicago. “Clearly, we tapped into a new population that has not often been seen by primary care providers or endocrinologists, as is often the case for underserved and vulnerable individuals who visit the emergency department as a first line for health care.”
The screening alert system is straightforward to build into an existing EMR, with technical support, Dr. Danielson said. “In theory, it should be able to be incorporated into other clinical centers and emergency departments. One of the current limitations that we are seeing is that the EMR is still flagging some people already diagnosed with diabetes to be screened for diabetes.” However, “because of this, we also see this as an opportunity to identify and reach out to those with diabetes who are still underserved and not receiving the appropriate diabetes care they need.”
The study results have broader public health implications, Dr. Danielson added. “We have identified a new, large population of people with diabetes who need medical care and diabetes education. This will further add to the burden of health care and costs, and it raises the ethical question of screening and not having full resources readily available to help.
“In my opinion, the study sheds light on a significant issue that will hopefully help drive change at both a health systems and public health level locally and nationally,” she added.
“One of the significant research gaps that has emerged now is how to link these new patients to health care and diabetes education at our institution after they leave the emergency department,” said Dr. Danielson. Diabetes screening in the ED setting is “a very novel area for health system scientists, social workers, and others to now come to the table and collaborate on next steps to help our patients.”
The study was initiated by the investigators, but was supported by a grant from Novo Nordisk to two coauthors. Dr. Danielson also disclosed grant funding from Novo Nordisk during the conduct of the study.
FROM JAMA NETWORK OPEN





