User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
The longevity gene: Healthy mutant reverses heart aging
Everybody wants a younger heart
As more people live well past 90, scientists have been taking a closer look at how they’ve been doing it. Mostly it boiled down to genetics. You either had it or you didn’t. Well, a recent study suggests that doesn’t have to be true anymore, at least for the heart.
Scientists from the United Kingdom and Italy found an antiaging gene in some centenarians that has shown possible antiaging effects in mice and in human heart cells. A single administration of the mutant antiaging gene, they found, stopped heart function decay in middle-aged mice and even reversed the biological clock by the human equivalent of 10 years in elderly mice.
When the researchers applied the antiaging gene to samples of human heart cells from elderly people with heart problems, the cells “resumed functioning properly, proving to be more efficient in building new blood vessels,” they said in a written statement. It all kind of sounds like something out of Dr. Frankenstein’s lab.
I want to believe … in better sleep
The “X-Files” theme song plays. Mulder and Scully are sitting in a diner, breakfast laid out around them. The diner is quiet, with only a few people inside.
Mulder: I’m telling you, Scully, there’s something spooky going on here.
Scully: You mean other than the fact that this town in Georgia looks suspiciously like Vancouver?
Mulder: Not one person we spoke to yesterday has gotten a full night’s sleep since the UFO sighting last month. I’m telling you, they’re here, they’re experimenting.
Scully: Do you really want me to do this to you again?
Mulder: Do what again?
Scully: There’s nothing going on here that can’t be explained by the current research. Why, in January 2023 a study was published revealing a link between poor sleep and belief in paranormal phenomena like UFOS, demons, or ghosts. Which probably explains why you’re on your third cup of coffee for the morning.
Mulder: Scully, you’ve literally been abducted by aliens. Do we have to play this game every time?
Scully: Look, it’s simple. In a sample of nearly 9,000 people, nearly two-thirds of those who reported experiencing sleep paralysis or exploding head syndrome reported believing in UFOs and aliens walking amongst humanity, despite making up just 3% of the overall sample.
Furthermore, about 60% of those reporting sleep paralysis also reported believing near-death experiences prove the soul lingers on after death, and those with stronger insomnia symptoms were more likely to believe in the devil.
Mulder: Aha!
Scully: Aha what?
Mulder: You’re a devout Christian. You believe in the devil and the soul.
Scully: Yes, but I don’t let it interfere with a good night’s sleep, Mulder. These people saw something strange, convinced themselves it was a UFO, and now they can’t sleep. It’s a vicious cycle. The study authors even said that people experiencing strange nighttime phenomena could interpret this as evidence of aliens or other paranormal beings, thus making them even more susceptible to further sleep disruption and deepening beliefs. Look who I’m talking to.
Mulder: Always with the facts, eh?
Scully: I am a doctor, after all. And if you want more research into how paranormal belief and poor sleep quality are linked, I’d be happy to dig out the literature, because the truth is out there, Mulder.
Mulder: I hate you sometimes.
It’s ChatGPT’s world. We’re just living in it
Have you heard about ChatGPT? The artificial intelligence chatbot was just launched in November and it’s already more important to the Internet than either Vladimir Putin or “Rick and Morty.”
What’s that? You’re wondering why you should care? Well, excuuuuuse us, but we thought you might want to know that ChatGPT is in the process of taking over the world. Let’s take a quick look at what it’s been up to.
“ChatGPT bot passes law school exam”
“ChatGPT passes MBA exam given by a Wharton professor”
“A freelance writer says ChatGPT wrote a $600 article in just 30 seconds”
And here’s one that might be of interest to those of the health care persuasion: “ChatGPT can pass part of the U.S. Medical Licensing Exam.” See? It’s coming for you, too.
The artificial intelligence known as ChatGPT “performed at >50% accuracy across [the three USMLE] examinations, exceeding 60% in most analyses,” a group of researchers wrote on the preprint server medRxiv, noting that 60% is usually the pass threshold for humans taking the exam in any given year.
ChatGPT was not given any special medical training before the exam, but the investigators pointed out that another AI, PubMedGPT, which is trained exclusively on biomedical domain literature, was only 50.8% accurate on the USMLE. Its reliance on “ongoing academic discourse that tends to be inconclusive, contradictory, or highly conservative or noncommittal in its language” was its undoing, the team suggested.
To top it off, ChatGPT is listed as one of the authors at the top of the medRxiv report, with an acknowledgment at the end saying that “ChatGPT contributed to the writing of several sections of this manuscript.”
We’ve said it before, and no doubt we’ll say it again: We’re doomed.
Everybody wants a younger heart
As more people live well past 90, scientists have been taking a closer look at how they’ve been doing it. Mostly it boiled down to genetics. You either had it or you didn’t. Well, a recent study suggests that doesn’t have to be true anymore, at least for the heart.
Scientists from the United Kingdom and Italy found an antiaging gene in some centenarians that has shown possible antiaging effects in mice and in human heart cells. A single administration of the mutant antiaging gene, they found, stopped heart function decay in middle-aged mice and even reversed the biological clock by the human equivalent of 10 years in elderly mice.
When the researchers applied the antiaging gene to samples of human heart cells from elderly people with heart problems, the cells “resumed functioning properly, proving to be more efficient in building new blood vessels,” they said in a written statement. It all kind of sounds like something out of Dr. Frankenstein’s lab.
I want to believe … in better sleep
The “X-Files” theme song plays. Mulder and Scully are sitting in a diner, breakfast laid out around them. The diner is quiet, with only a few people inside.
Mulder: I’m telling you, Scully, there’s something spooky going on here.
Scully: You mean other than the fact that this town in Georgia looks suspiciously like Vancouver?
Mulder: Not one person we spoke to yesterday has gotten a full night’s sleep since the UFO sighting last month. I’m telling you, they’re here, they’re experimenting.
Scully: Do you really want me to do this to you again?
Mulder: Do what again?
Scully: There’s nothing going on here that can’t be explained by the current research. Why, in January 2023 a study was published revealing a link between poor sleep and belief in paranormal phenomena like UFOS, demons, or ghosts. Which probably explains why you’re on your third cup of coffee for the morning.
Mulder: Scully, you’ve literally been abducted by aliens. Do we have to play this game every time?
Scully: Look, it’s simple. In a sample of nearly 9,000 people, nearly two-thirds of those who reported experiencing sleep paralysis or exploding head syndrome reported believing in UFOs and aliens walking amongst humanity, despite making up just 3% of the overall sample.
Furthermore, about 60% of those reporting sleep paralysis also reported believing near-death experiences prove the soul lingers on after death, and those with stronger insomnia symptoms were more likely to believe in the devil.
Mulder: Aha!
Scully: Aha what?
Mulder: You’re a devout Christian. You believe in the devil and the soul.
Scully: Yes, but I don’t let it interfere with a good night’s sleep, Mulder. These people saw something strange, convinced themselves it was a UFO, and now they can’t sleep. It’s a vicious cycle. The study authors even said that people experiencing strange nighttime phenomena could interpret this as evidence of aliens or other paranormal beings, thus making them even more susceptible to further sleep disruption and deepening beliefs. Look who I’m talking to.
Mulder: Always with the facts, eh?
Scully: I am a doctor, after all. And if you want more research into how paranormal belief and poor sleep quality are linked, I’d be happy to dig out the literature, because the truth is out there, Mulder.
Mulder: I hate you sometimes.
It’s ChatGPT’s world. We’re just living in it
Have you heard about ChatGPT? The artificial intelligence chatbot was just launched in November and it’s already more important to the Internet than either Vladimir Putin or “Rick and Morty.”
What’s that? You’re wondering why you should care? Well, excuuuuuse us, but we thought you might want to know that ChatGPT is in the process of taking over the world. Let’s take a quick look at what it’s been up to.
“ChatGPT bot passes law school exam”
“ChatGPT passes MBA exam given by a Wharton professor”
“A freelance writer says ChatGPT wrote a $600 article in just 30 seconds”
And here’s one that might be of interest to those of the health care persuasion: “ChatGPT can pass part of the U.S. Medical Licensing Exam.” See? It’s coming for you, too.
The artificial intelligence known as ChatGPT “performed at >50% accuracy across [the three USMLE] examinations, exceeding 60% in most analyses,” a group of researchers wrote on the preprint server medRxiv, noting that 60% is usually the pass threshold for humans taking the exam in any given year.
ChatGPT was not given any special medical training before the exam, but the investigators pointed out that another AI, PubMedGPT, which is trained exclusively on biomedical domain literature, was only 50.8% accurate on the USMLE. Its reliance on “ongoing academic discourse that tends to be inconclusive, contradictory, or highly conservative or noncommittal in its language” was its undoing, the team suggested.
To top it off, ChatGPT is listed as one of the authors at the top of the medRxiv report, with an acknowledgment at the end saying that “ChatGPT contributed to the writing of several sections of this manuscript.”
We’ve said it before, and no doubt we’ll say it again: We’re doomed.
Everybody wants a younger heart
As more people live well past 90, scientists have been taking a closer look at how they’ve been doing it. Mostly it boiled down to genetics. You either had it or you didn’t. Well, a recent study suggests that doesn’t have to be true anymore, at least for the heart.
Scientists from the United Kingdom and Italy found an antiaging gene in some centenarians that has shown possible antiaging effects in mice and in human heart cells. A single administration of the mutant antiaging gene, they found, stopped heart function decay in middle-aged mice and even reversed the biological clock by the human equivalent of 10 years in elderly mice.
When the researchers applied the antiaging gene to samples of human heart cells from elderly people with heart problems, the cells “resumed functioning properly, proving to be more efficient in building new blood vessels,” they said in a written statement. It all kind of sounds like something out of Dr. Frankenstein’s lab.
I want to believe … in better sleep
The “X-Files” theme song plays. Mulder and Scully are sitting in a diner, breakfast laid out around them. The diner is quiet, with only a few people inside.
Mulder: I’m telling you, Scully, there’s something spooky going on here.
Scully: You mean other than the fact that this town in Georgia looks suspiciously like Vancouver?
Mulder: Not one person we spoke to yesterday has gotten a full night’s sleep since the UFO sighting last month. I’m telling you, they’re here, they’re experimenting.
Scully: Do you really want me to do this to you again?
Mulder: Do what again?
Scully: There’s nothing going on here that can’t be explained by the current research. Why, in January 2023 a study was published revealing a link between poor sleep and belief in paranormal phenomena like UFOS, demons, or ghosts. Which probably explains why you’re on your third cup of coffee for the morning.
Mulder: Scully, you’ve literally been abducted by aliens. Do we have to play this game every time?
Scully: Look, it’s simple. In a sample of nearly 9,000 people, nearly two-thirds of those who reported experiencing sleep paralysis or exploding head syndrome reported believing in UFOs and aliens walking amongst humanity, despite making up just 3% of the overall sample.
Furthermore, about 60% of those reporting sleep paralysis also reported believing near-death experiences prove the soul lingers on after death, and those with stronger insomnia symptoms were more likely to believe in the devil.
Mulder: Aha!
Scully: Aha what?
Mulder: You’re a devout Christian. You believe in the devil and the soul.
Scully: Yes, but I don’t let it interfere with a good night’s sleep, Mulder. These people saw something strange, convinced themselves it was a UFO, and now they can’t sleep. It’s a vicious cycle. The study authors even said that people experiencing strange nighttime phenomena could interpret this as evidence of aliens or other paranormal beings, thus making them even more susceptible to further sleep disruption and deepening beliefs. Look who I’m talking to.
Mulder: Always with the facts, eh?
Scully: I am a doctor, after all. And if you want more research into how paranormal belief and poor sleep quality are linked, I’d be happy to dig out the literature, because the truth is out there, Mulder.
Mulder: I hate you sometimes.
It’s ChatGPT’s world. We’re just living in it
Have you heard about ChatGPT? The artificial intelligence chatbot was just launched in November and it’s already more important to the Internet than either Vladimir Putin or “Rick and Morty.”
What’s that? You’re wondering why you should care? Well, excuuuuuse us, but we thought you might want to know that ChatGPT is in the process of taking over the world. Let’s take a quick look at what it’s been up to.
“ChatGPT bot passes law school exam”
“ChatGPT passes MBA exam given by a Wharton professor”
“A freelance writer says ChatGPT wrote a $600 article in just 30 seconds”
And here’s one that might be of interest to those of the health care persuasion: “ChatGPT can pass part of the U.S. Medical Licensing Exam.” See? It’s coming for you, too.
The artificial intelligence known as ChatGPT “performed at >50% accuracy across [the three USMLE] examinations, exceeding 60% in most analyses,” a group of researchers wrote on the preprint server medRxiv, noting that 60% is usually the pass threshold for humans taking the exam in any given year.
ChatGPT was not given any special medical training before the exam, but the investigators pointed out that another AI, PubMedGPT, which is trained exclusively on biomedical domain literature, was only 50.8% accurate on the USMLE. Its reliance on “ongoing academic discourse that tends to be inconclusive, contradictory, or highly conservative or noncommittal in its language” was its undoing, the team suggested.
To top it off, ChatGPT is listed as one of the authors at the top of the medRxiv report, with an acknowledgment at the end saying that “ChatGPT contributed to the writing of several sections of this manuscript.”
We’ve said it before, and no doubt we’ll say it again: We’re doomed.
Over half of ED visits from cancer patients could be prevented
Overall, researchers found that 18.3 million (52%) ED visits among patients with cancer between 2012 and 2019 were potentially avoidable. Pain was the most common reason for such a visit. Notably, the number of potentially preventable ED visits documented each year increased over the study period.
“These findings highlight the need for cancer care programs to implement evidence-based interventions to better manage cancer treatment complications, such as uncontrolled pain, in outpatient and ambulatory settings,” said the authors, led by Amir Alishahi Tabriz, MD, PhD, MPH, department of health outcomes and behavior, Moffitt Cancer Center, Tampa.
Authors of an accompanying editorial agree, noting that “patients at risk for having uncontrolled pain could potentially be identified earlier, and steps could be taken that would address their pain and help prevent acute care visits.”
The study and the editorial were published online Jan. 19, 2022, in JAMA Network Open.
Patients with cancer experience a range of side effects from their cancer and treatment. Many such problems can be managed in the ambulatory setting but are often managed in the ED, which is far from optimal for patients with cancer from both a complications and cost perspective. Still, little is known about whether ED visits among patients with cancer are avoidable.
To better understand unnecessary emergency care use by these patients, Dr. Tabriz and colleagues evaluated trends and characteristics of potentially preventable ED visits among adults with cancer who had an ED visit between 2012 and 2019. The authors used the Centers for Medicare & Medicaid Services definition for a potentially preventable ED visit among patients receiving chemotherapy.
Among the 35.5 million ED visits made by patients with cancer during the study period, 18.3 million (52%) were identified as potentially preventable. Nearly 5.8 million of these visits (21%) were classified as being of “high acuity,” and almost 30% resulted in unplanned hospitalizations.
Pain was the most common reason for potentially preventable ED visits, accounting for 37% of these visits.
The absolute number of potentially preventable ED visits among cancer patients increased from about 1.8 million in 2012 to 3.2 million in 2019. The number of patients who visited the ED because of pain more than doubled, from roughly 1.2 million in 2012 to 2.4 million in 2019.
“The disproportionate increase in the number of ED visits by patients with cancer has put a substantial burden on EDs that are already operating at peak capacity” and “reinforces the need for cancer care programs to devise innovative ways to manage complications associated with cancer treatment in the outpatient and ambulatory settings,” Dr. Tabriz and coauthors wrote.
The increase could be an “unintended” consequence of efforts to decrease overall opioid administration in response to the opioid epidemic, Dr. Tabriz and colleagues noted. For example, the authors point to a recent study that found that about half of patients with cancer who had severe pain did not receive outpatient opioids in the week before visiting the ED.
“Even access to outpatient care does not mean patients can get the care they need outside an ED,” wrote editorialists Erek Majka, MD, with Summerlin Hospital, Las Vegas, and N. Seth Trueger, MD, MPH, with Northwestern University, Chicago. Thus, “it is no surprise that patients are sent to the ED if the alternatives do not have the staff or diagnostic and therapeutic capabilities the patients need.”
Overall, however, the “goal is not to eliminate ED visits for their own sake; rather, the goal is better care of patients with cancer, and secondarily, in a manner that is cost-effective,” Dr. Majka and Dr. Trueger explained.
No specific funding for the study was reported. The authors disclosed no relevant financial relationships. Dr. Trueger is digital media editor of JAMA Network Open, but he was not involved in decisions regarding review of the manuscript or its acceptance.
A version of this article first appeared on Medscape.com.
Overall, researchers found that 18.3 million (52%) ED visits among patients with cancer between 2012 and 2019 were potentially avoidable. Pain was the most common reason for such a visit. Notably, the number of potentially preventable ED visits documented each year increased over the study period.
“These findings highlight the need for cancer care programs to implement evidence-based interventions to better manage cancer treatment complications, such as uncontrolled pain, in outpatient and ambulatory settings,” said the authors, led by Amir Alishahi Tabriz, MD, PhD, MPH, department of health outcomes and behavior, Moffitt Cancer Center, Tampa.
Authors of an accompanying editorial agree, noting that “patients at risk for having uncontrolled pain could potentially be identified earlier, and steps could be taken that would address their pain and help prevent acute care visits.”
The study and the editorial were published online Jan. 19, 2022, in JAMA Network Open.
Patients with cancer experience a range of side effects from their cancer and treatment. Many such problems can be managed in the ambulatory setting but are often managed in the ED, which is far from optimal for patients with cancer from both a complications and cost perspective. Still, little is known about whether ED visits among patients with cancer are avoidable.
To better understand unnecessary emergency care use by these patients, Dr. Tabriz and colleagues evaluated trends and characteristics of potentially preventable ED visits among adults with cancer who had an ED visit between 2012 and 2019. The authors used the Centers for Medicare & Medicaid Services definition for a potentially preventable ED visit among patients receiving chemotherapy.
Among the 35.5 million ED visits made by patients with cancer during the study period, 18.3 million (52%) were identified as potentially preventable. Nearly 5.8 million of these visits (21%) were classified as being of “high acuity,” and almost 30% resulted in unplanned hospitalizations.
Pain was the most common reason for potentially preventable ED visits, accounting for 37% of these visits.
The absolute number of potentially preventable ED visits among cancer patients increased from about 1.8 million in 2012 to 3.2 million in 2019. The number of patients who visited the ED because of pain more than doubled, from roughly 1.2 million in 2012 to 2.4 million in 2019.
“The disproportionate increase in the number of ED visits by patients with cancer has put a substantial burden on EDs that are already operating at peak capacity” and “reinforces the need for cancer care programs to devise innovative ways to manage complications associated with cancer treatment in the outpatient and ambulatory settings,” Dr. Tabriz and coauthors wrote.
The increase could be an “unintended” consequence of efforts to decrease overall opioid administration in response to the opioid epidemic, Dr. Tabriz and colleagues noted. For example, the authors point to a recent study that found that about half of patients with cancer who had severe pain did not receive outpatient opioids in the week before visiting the ED.
“Even access to outpatient care does not mean patients can get the care they need outside an ED,” wrote editorialists Erek Majka, MD, with Summerlin Hospital, Las Vegas, and N. Seth Trueger, MD, MPH, with Northwestern University, Chicago. Thus, “it is no surprise that patients are sent to the ED if the alternatives do not have the staff or diagnostic and therapeutic capabilities the patients need.”
Overall, however, the “goal is not to eliminate ED visits for their own sake; rather, the goal is better care of patients with cancer, and secondarily, in a manner that is cost-effective,” Dr. Majka and Dr. Trueger explained.
No specific funding for the study was reported. The authors disclosed no relevant financial relationships. Dr. Trueger is digital media editor of JAMA Network Open, but he was not involved in decisions regarding review of the manuscript or its acceptance.
A version of this article first appeared on Medscape.com.
Overall, researchers found that 18.3 million (52%) ED visits among patients with cancer between 2012 and 2019 were potentially avoidable. Pain was the most common reason for such a visit. Notably, the number of potentially preventable ED visits documented each year increased over the study period.
“These findings highlight the need for cancer care programs to implement evidence-based interventions to better manage cancer treatment complications, such as uncontrolled pain, in outpatient and ambulatory settings,” said the authors, led by Amir Alishahi Tabriz, MD, PhD, MPH, department of health outcomes and behavior, Moffitt Cancer Center, Tampa.
Authors of an accompanying editorial agree, noting that “patients at risk for having uncontrolled pain could potentially be identified earlier, and steps could be taken that would address their pain and help prevent acute care visits.”
The study and the editorial were published online Jan. 19, 2022, in JAMA Network Open.
Patients with cancer experience a range of side effects from their cancer and treatment. Many such problems can be managed in the ambulatory setting but are often managed in the ED, which is far from optimal for patients with cancer from both a complications and cost perspective. Still, little is known about whether ED visits among patients with cancer are avoidable.
To better understand unnecessary emergency care use by these patients, Dr. Tabriz and colleagues evaluated trends and characteristics of potentially preventable ED visits among adults with cancer who had an ED visit between 2012 and 2019. The authors used the Centers for Medicare & Medicaid Services definition for a potentially preventable ED visit among patients receiving chemotherapy.
Among the 35.5 million ED visits made by patients with cancer during the study period, 18.3 million (52%) were identified as potentially preventable. Nearly 5.8 million of these visits (21%) were classified as being of “high acuity,” and almost 30% resulted in unplanned hospitalizations.
Pain was the most common reason for potentially preventable ED visits, accounting for 37% of these visits.
The absolute number of potentially preventable ED visits among cancer patients increased from about 1.8 million in 2012 to 3.2 million in 2019. The number of patients who visited the ED because of pain more than doubled, from roughly 1.2 million in 2012 to 2.4 million in 2019.
“The disproportionate increase in the number of ED visits by patients with cancer has put a substantial burden on EDs that are already operating at peak capacity” and “reinforces the need for cancer care programs to devise innovative ways to manage complications associated with cancer treatment in the outpatient and ambulatory settings,” Dr. Tabriz and coauthors wrote.
The increase could be an “unintended” consequence of efforts to decrease overall opioid administration in response to the opioid epidemic, Dr. Tabriz and colleagues noted. For example, the authors point to a recent study that found that about half of patients with cancer who had severe pain did not receive outpatient opioids in the week before visiting the ED.
“Even access to outpatient care does not mean patients can get the care they need outside an ED,” wrote editorialists Erek Majka, MD, with Summerlin Hospital, Las Vegas, and N. Seth Trueger, MD, MPH, with Northwestern University, Chicago. Thus, “it is no surprise that patients are sent to the ED if the alternatives do not have the staff or diagnostic and therapeutic capabilities the patients need.”
Overall, however, the “goal is not to eliminate ED visits for their own sake; rather, the goal is better care of patients with cancer, and secondarily, in a manner that is cost-effective,” Dr. Majka and Dr. Trueger explained.
No specific funding for the study was reported. The authors disclosed no relevant financial relationships. Dr. Trueger is digital media editor of JAMA Network Open, but he was not involved in decisions regarding review of the manuscript or its acceptance.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
VEXAS syndrome: More common, variable, and severe than expected
A recently discovered inflammatory disease known as VEXAS syndrome is more common, variable, and dangerous than previously understood, according to results of a retrospective observational study of a large health care system database. The findings, published in JAMA, found that it struck 1 in 4,269 men over the age of 50 in a largely White population and caused a wide variety of symptoms.
“The disease is quite severe,” study lead author David Beck, MD, PhD, of the department of medicine at NYU Langone Health, said in an interview. Patients with the condition “have a variety of clinical symptoms affecting different parts of the body and are being managed by different medical specialties.”
Dr. Beck and colleagues first described VEXAS (vacuoles, E1-ubiquitin-activating enzyme, X-linked, autoinflammatory, somatic) syndrome in 2020. They linked it to mutations in the UBA1 (ubiquitin-like modifier activating enzyme 1) gene. The enzyme initiates a process that identifies misfolded proteins as targets for degradation.
“VEXAS syndrome is characterized by anemia and inflammation in the skin, lungs, cartilage, and joints,” Dr. Beck said. “These symptoms are frequently mistaken for other rheumatic or hematologic diseases. However, this syndrome has a different cause, is treated differently, requires additional monitoring, and can be far more severe.”
According to him, hundreds of people have been diagnosed with the disease in the short time since it was defined. The disease is believed to be fatal in some cases. A previous report found that the median survival was 9 years among patients with a certain variant; that was significantly less than patients with two other variants.
For the new study, researchers searched for UBA1 variants in genetic data from 163,096 subjects (mean age, 52.8 years; 94% White, 61% women) who took part in the Geisinger MyCode Community Health Initiative. The 1996-2022 data comes from patients at 10 Pennsylvania hospitals.
Eleven people (9 males, 2 females) had likely UBA1 variants, and all had anemia. The cases accounted for 1 in 13,591 unrelated people (95% confidence interval, 1:7,775-1:23,758), 1 in 4,269 men older than 50 years (95% CI, 1:2,319-1:7,859), and 1 in 26,238 women older than 50 years (95% CI, 1:7,196-1:147,669).
Other common findings included macrocytosis (91%), skin problems (73%), and pulmonary disease (91%). Ten patients (91%) required transfusions.
Five of the 11 subjects didn’t meet the previously defined criteria for VEXAS syndrome. None had been diagnosed with the condition, which is not surprising considering that it hadn’t been discovered and described until recently.
Just over half of the patients – 55% – had a clinical diagnosis that was previously linked to VEXAS syndrome. “This means that slightly less than half of the patients with VEXAS syndrome had no clear associated clinical diagnosis,” Dr. Beck said. “The lack of associated clinical diagnoses may be due to the variety of nonspecific clinical characteristics that span different subspecialities in VEXAS syndrome. VEXAS syndrome represents an example of a multisystem disease where patients and their symptoms may get lost in the shuffle.”
In the future, “professionals should look out for patients with unexplained inflammation – and some combination of hematologic, rheumatologic, pulmonary, and dermatologic clinical manifestations – that either don’t carry a clinical diagnosis or don’t respond to first-line therapies,” Dr. Beck said. “These patients will also frequently be anemic, have low platelet counts, elevated markers of inflammation in the blood, and be dependent on corticosteroids.”
Diagnosis can be made via genetic testing, but the study authors note that it “is not routinely offered on standard workup for myeloid neoplasms or immune dysregulation diagnostic panels.”
As for treatment, Dr. Beck said the disease “can be partially controlled by multiple different anticytokine therapies or biologics. However, in most cases, patients still need additional steroids and/or disease-modifying antirheumatic agents [DMARDs]. In addition, bone marrow transplantation has shown signs of being a highly effective therapy.”
The study authors say more research is needed to understand the disease’s prevalence in more diverse populations.
In an interview, Matthew J. Koster, MD, a rheumatologist at Mayo Clinic in Rochester, Minn., who’s studied the disease but didn’t take part in this research project, said the findings are valid and “highly important.
“The findings of this study highlight what many academic and quaternary referral centers were wondering: Is VEXAS really more common than we think, with patients hiding in plain sight? The answer is yes,” he said. “Currently, there are less than 400 cases reported in the literature of VEXAS, but large centers are diagnosing this condition with some frequency. For example, at Mayo Clinic in Rochester, we diagnose on average one new patient with VEXAS every 7-14 days and have diagnosed 60 in the past 18 months. A national collaborative group in France has diagnosed approximately 250 patients over that same time frame when pooling patients nationwide.”
The prevalence is high enough, he said, that “clinicians should consider that some of the patients with diseases that are not responding to treatment may in fact have VEXAS rather than ‘refractory’ relapsing polychondritis or ‘recalcitrant’ rheumatoid arthritis, etc.”
The National Institute of Health funded the study. Dr. Beck, the other authors, and Dr. Koster report no disclosures.
A recently discovered inflammatory disease known as VEXAS syndrome is more common, variable, and dangerous than previously understood, according to results of a retrospective observational study of a large health care system database. The findings, published in JAMA, found that it struck 1 in 4,269 men over the age of 50 in a largely White population and caused a wide variety of symptoms.
“The disease is quite severe,” study lead author David Beck, MD, PhD, of the department of medicine at NYU Langone Health, said in an interview. Patients with the condition “have a variety of clinical symptoms affecting different parts of the body and are being managed by different medical specialties.”
Dr. Beck and colleagues first described VEXAS (vacuoles, E1-ubiquitin-activating enzyme, X-linked, autoinflammatory, somatic) syndrome in 2020. They linked it to mutations in the UBA1 (ubiquitin-like modifier activating enzyme 1) gene. The enzyme initiates a process that identifies misfolded proteins as targets for degradation.
“VEXAS syndrome is characterized by anemia and inflammation in the skin, lungs, cartilage, and joints,” Dr. Beck said. “These symptoms are frequently mistaken for other rheumatic or hematologic diseases. However, this syndrome has a different cause, is treated differently, requires additional monitoring, and can be far more severe.”
According to him, hundreds of people have been diagnosed with the disease in the short time since it was defined. The disease is believed to be fatal in some cases. A previous report found that the median survival was 9 years among patients with a certain variant; that was significantly less than patients with two other variants.
For the new study, researchers searched for UBA1 variants in genetic data from 163,096 subjects (mean age, 52.8 years; 94% White, 61% women) who took part in the Geisinger MyCode Community Health Initiative. The 1996-2022 data comes from patients at 10 Pennsylvania hospitals.
Eleven people (9 males, 2 females) had likely UBA1 variants, and all had anemia. The cases accounted for 1 in 13,591 unrelated people (95% confidence interval, 1:7,775-1:23,758), 1 in 4,269 men older than 50 years (95% CI, 1:2,319-1:7,859), and 1 in 26,238 women older than 50 years (95% CI, 1:7,196-1:147,669).
Other common findings included macrocytosis (91%), skin problems (73%), and pulmonary disease (91%). Ten patients (91%) required transfusions.
Five of the 11 subjects didn’t meet the previously defined criteria for VEXAS syndrome. None had been diagnosed with the condition, which is not surprising considering that it hadn’t been discovered and described until recently.
Just over half of the patients – 55% – had a clinical diagnosis that was previously linked to VEXAS syndrome. “This means that slightly less than half of the patients with VEXAS syndrome had no clear associated clinical diagnosis,” Dr. Beck said. “The lack of associated clinical diagnoses may be due to the variety of nonspecific clinical characteristics that span different subspecialities in VEXAS syndrome. VEXAS syndrome represents an example of a multisystem disease where patients and their symptoms may get lost in the shuffle.”
In the future, “professionals should look out for patients with unexplained inflammation – and some combination of hematologic, rheumatologic, pulmonary, and dermatologic clinical manifestations – that either don’t carry a clinical diagnosis or don’t respond to first-line therapies,” Dr. Beck said. “These patients will also frequently be anemic, have low platelet counts, elevated markers of inflammation in the blood, and be dependent on corticosteroids.”
Diagnosis can be made via genetic testing, but the study authors note that it “is not routinely offered on standard workup for myeloid neoplasms or immune dysregulation diagnostic panels.”
As for treatment, Dr. Beck said the disease “can be partially controlled by multiple different anticytokine therapies or biologics. However, in most cases, patients still need additional steroids and/or disease-modifying antirheumatic agents [DMARDs]. In addition, bone marrow transplantation has shown signs of being a highly effective therapy.”
The study authors say more research is needed to understand the disease’s prevalence in more diverse populations.
In an interview, Matthew J. Koster, MD, a rheumatologist at Mayo Clinic in Rochester, Minn., who’s studied the disease but didn’t take part in this research project, said the findings are valid and “highly important.
“The findings of this study highlight what many academic and quaternary referral centers were wondering: Is VEXAS really more common than we think, with patients hiding in plain sight? The answer is yes,” he said. “Currently, there are less than 400 cases reported in the literature of VEXAS, but large centers are diagnosing this condition with some frequency. For example, at Mayo Clinic in Rochester, we diagnose on average one new patient with VEXAS every 7-14 days and have diagnosed 60 in the past 18 months. A national collaborative group in France has diagnosed approximately 250 patients over that same time frame when pooling patients nationwide.”
The prevalence is high enough, he said, that “clinicians should consider that some of the patients with diseases that are not responding to treatment may in fact have VEXAS rather than ‘refractory’ relapsing polychondritis or ‘recalcitrant’ rheumatoid arthritis, etc.”
The National Institute of Health funded the study. Dr. Beck, the other authors, and Dr. Koster report no disclosures.
A recently discovered inflammatory disease known as VEXAS syndrome is more common, variable, and dangerous than previously understood, according to results of a retrospective observational study of a large health care system database. The findings, published in JAMA, found that it struck 1 in 4,269 men over the age of 50 in a largely White population and caused a wide variety of symptoms.
“The disease is quite severe,” study lead author David Beck, MD, PhD, of the department of medicine at NYU Langone Health, said in an interview. Patients with the condition “have a variety of clinical symptoms affecting different parts of the body and are being managed by different medical specialties.”
Dr. Beck and colleagues first described VEXAS (vacuoles, E1-ubiquitin-activating enzyme, X-linked, autoinflammatory, somatic) syndrome in 2020. They linked it to mutations in the UBA1 (ubiquitin-like modifier activating enzyme 1) gene. The enzyme initiates a process that identifies misfolded proteins as targets for degradation.
“VEXAS syndrome is characterized by anemia and inflammation in the skin, lungs, cartilage, and joints,” Dr. Beck said. “These symptoms are frequently mistaken for other rheumatic or hematologic diseases. However, this syndrome has a different cause, is treated differently, requires additional monitoring, and can be far more severe.”
According to him, hundreds of people have been diagnosed with the disease in the short time since it was defined. The disease is believed to be fatal in some cases. A previous report found that the median survival was 9 years among patients with a certain variant; that was significantly less than patients with two other variants.
For the new study, researchers searched for UBA1 variants in genetic data from 163,096 subjects (mean age, 52.8 years; 94% White, 61% women) who took part in the Geisinger MyCode Community Health Initiative. The 1996-2022 data comes from patients at 10 Pennsylvania hospitals.
Eleven people (9 males, 2 females) had likely UBA1 variants, and all had anemia. The cases accounted for 1 in 13,591 unrelated people (95% confidence interval, 1:7,775-1:23,758), 1 in 4,269 men older than 50 years (95% CI, 1:2,319-1:7,859), and 1 in 26,238 women older than 50 years (95% CI, 1:7,196-1:147,669).
Other common findings included macrocytosis (91%), skin problems (73%), and pulmonary disease (91%). Ten patients (91%) required transfusions.
Five of the 11 subjects didn’t meet the previously defined criteria for VEXAS syndrome. None had been diagnosed with the condition, which is not surprising considering that it hadn’t been discovered and described until recently.
Just over half of the patients – 55% – had a clinical diagnosis that was previously linked to VEXAS syndrome. “This means that slightly less than half of the patients with VEXAS syndrome had no clear associated clinical diagnosis,” Dr. Beck said. “The lack of associated clinical diagnoses may be due to the variety of nonspecific clinical characteristics that span different subspecialities in VEXAS syndrome. VEXAS syndrome represents an example of a multisystem disease where patients and their symptoms may get lost in the shuffle.”
In the future, “professionals should look out for patients with unexplained inflammation – and some combination of hematologic, rheumatologic, pulmonary, and dermatologic clinical manifestations – that either don’t carry a clinical diagnosis or don’t respond to first-line therapies,” Dr. Beck said. “These patients will also frequently be anemic, have low platelet counts, elevated markers of inflammation in the blood, and be dependent on corticosteroids.”
Diagnosis can be made via genetic testing, but the study authors note that it “is not routinely offered on standard workup for myeloid neoplasms or immune dysregulation diagnostic panels.”
As for treatment, Dr. Beck said the disease “can be partially controlled by multiple different anticytokine therapies or biologics. However, in most cases, patients still need additional steroids and/or disease-modifying antirheumatic agents [DMARDs]. In addition, bone marrow transplantation has shown signs of being a highly effective therapy.”
The study authors say more research is needed to understand the disease’s prevalence in more diverse populations.
In an interview, Matthew J. Koster, MD, a rheumatologist at Mayo Clinic in Rochester, Minn., who’s studied the disease but didn’t take part in this research project, said the findings are valid and “highly important.
“The findings of this study highlight what many academic and quaternary referral centers were wondering: Is VEXAS really more common than we think, with patients hiding in plain sight? The answer is yes,” he said. “Currently, there are less than 400 cases reported in the literature of VEXAS, but large centers are diagnosing this condition with some frequency. For example, at Mayo Clinic in Rochester, we diagnose on average one new patient with VEXAS every 7-14 days and have diagnosed 60 in the past 18 months. A national collaborative group in France has diagnosed approximately 250 patients over that same time frame when pooling patients nationwide.”
The prevalence is high enough, he said, that “clinicians should consider that some of the patients with diseases that are not responding to treatment may in fact have VEXAS rather than ‘refractory’ relapsing polychondritis or ‘recalcitrant’ rheumatoid arthritis, etc.”
The National Institute of Health funded the study. Dr. Beck, the other authors, and Dr. Koster report no disclosures.
FROM JAMA
Evaluation after a suicide attempt: What to ask
In 2021, suicide was the 11th leading cause of death in the United States.1 Suicide resulted in 49,000 US deaths during 2021; it was the second most common cause of death in individuals age 10 to 34, and the fifth leading cause among children.1,2 Women are 3 to 4 times more likely than men to attempt suicide, but men are 4 times more likely to die by suicide.2
The evaluation of patients with suicidal ideation who have not made an attempt generally involves assessing 4 factors: the specific plan, access to lethal means, any recent social stressors, and the presence of a psychiatric disorder.3 The clinician should also assess which potential deterrents, such as religious beliefs or dependent children, might be present.
Mental health clinicians are often called upon to evaluate a patient after a suicide attempt to assess intent for continued self-harm and to determine appropriate disposition. Such an evaluation must consider multiple factors, including the method used, premeditation, consequences of the attempt, the presence of severe depression and/or psychosis, and the role of substance use. Assessment after a suicide attempt differs from the examination of individuals who harbor suicidal thoughts but have not made an attempt; the latter group may be more likely to respond to interventions such as intensive outpatient care, mobilization of family support, and religious proscriptions against suicide. However, for patients who make an attempt to end their life, whatever potential safeguards or deterrents to suicide that were in place obviously did not prevent the self-harm act. The consequences of the attempt, such as disabling injuries or medical complications, and possible involuntary commitment, need to be considered. Assessment of the patient’s feelings about having survived the attempt is important because the psychological impact of the attempt on family members may serve to intensify the patient’s depression and make a subsequent attempt more likely.
Many individuals who think of suicide have communicated self-harm thoughts or intentions, but such comments are often minimized or ignored. There is a common but erroneous belief that if patients are encouraged to discuss thoughts of self-harm, they will be more likely to act upon them. Because the opposite is true,4 clinicians should ask vulnerable patients about suicidal ideation or intent. Importantly, noncompliance with life-saving medical care, risk-taking behaviors, and substance use may also signal a desire for self-harm. Passive thoughts of death, typified by comments such as “I don’t care whether I wake up or not,” should also be elicited. Many patients who think of suicide speak of being in a “bad place” where reason and logic give way to an intense desire to end their misery.

The evaluation of a patient who has attempted suicide is an important component of providing psychiatric care. This article reflects our 45 years of evaluating such patients. As such, it reflects our clinical experience and is not evidence-based. We offer a checklist of 14 questions that we have found helpful when determining if it would be best for a patient to receive inpatient psychiatric hospitalization or a discharge referral for outpatient care (Table). Questions 1 through 6 are specific for patients who have made a suicide attempt, while questions 7 through 14 are helpful for assessing global risk factors for suicide.
1. Was the attempt premeditated?
Determining premeditation vs impulsivity is an essential element of the assessment following a suicide attempt. Many such acts may occur without forethought in response to an unexpected stressor, such as an altercation between partners or family conflicts. Impulsive attempts can occur when an individual is involved in a distressing event and/or while intoxicated. Conversely, premeditation involves forethought and planning, which may increase the risk of suicide in the near future.
Examples of premeditated behavior include:
- Contemplating the attempt days or weeks beforehand
- Researching the effects of a medication or combination of medications in terms of potential lethality
- Engaging in behavior that would decrease the likelihood of their body being discovered after the attempt
- Obtaining weapons and/or stockpiling pills
- Canvassing potential sites such as bridges or tall buildings
- Engaging in a suicide attempt “practice run”
- Leaving a suicide note or message on social media
- Making funeral arrangements, such as choosing burial clothing
- Writing a will and arranging for the custody of dependent children
- Purchasing life insurance that does not deny payment of benefits in cases of death by suicide.
Continue to: Patients with a premeditated...
Patients with a premeditated suicide attempt generally do not expect to survive and are often surprised or upset that the act was not fatal. The presence of indicators that the attempt was premeditated should direct the disposition more toward hospitalization than discharge. In assessing the impact of premeditation, it is important to gauge not just the examples listed above, but also the patient’s perception of these issues (such as potential loss of child custody). Consider how much the patient is emotionally affected by such thinking.
2. What were the consequences of the attempt?
Assessing the reason for the attempt (if any) and determining whether the inciting circumstance has changed due to the suicide attempt are an important part of the evaluation. A suicide attempt may result in reconciliation with and/or renewed support from family members or partners, who might not have been aware of the patient’s emotional distress. Such unexpected support often results in the patient exhibiting improved mood and affect, and possibly temporary resolution of suicidal thoughts. This “flight into health” may be short-lived, but it also may be enough to engage the patient in a therapeutic alliance. That may permit a discharge with safe disposition to the outpatient clinic while in the custody of a family member, partner, or close friend.
Alternatively, some people experience a troubling worsening of precipitants following a suicide attempt. Preexisting medical conditions and financial, occupational, and/or social woes may be exacerbated. Child custody determinations may be affected, assuming the patient understands the possibility of this adverse consequence. Violent methods may result in disfigurement and body image issues. Individuals from small, close-knit communities may experience stigmatization and unwanted notoriety because of their suicide attempt. Such negative consequences may render some patients more likely to make another attempt to die by suicide. It is crucial to consider how a suicide attempt may have changed the original stress that led to the attempt.
3. Which method was used?
Most fatal suicides in the US are by firearms, and many individuals who survive such attempts do so because of unfamiliarity with the weapon, gun malfunction, faulty aim, or alcohol use.5-7 Some survivors report intending to shoot themselves in the heart, but instead suffered shoulder injuries. Unfortunately, for a patient who survives self-inflicted gunshot wounds, the sequelae of chronic pain, multiple surgical procedures, disability, and disfigurement may serve as constant negative reminders of the event. Some individuals with suicidal intent eschew the idea of using firearms because they hope to avoid having a family member be the first to discover them. Witnessing the aftermath of a fatal suicide by gunshot can induce symptoms of posttraumatic stress disorder in family members and/or partners.8
For a patient with self-inflicted gunshot wounds, always determine whether the weapon has been secured or if the patient still has access to it. Asking about weapon availability is essential during the evaluation of any patient with depression, major life crises, or other factors that may yield a desire to die; this is especially true for individuals with substance use disorders (SUDs). Whenever readily available to such individuals, weapons need to be safely removed.
Continue to: Other self-harm methods...
Other self-harm methods with a high degree of lethality include jumping from bridges or buildings, poisonings, self-immolation, cutting, and hangings. Individuals who choose these approaches generally do not intend to survive. Many of these methods also entail premeditation, as in the case of individuals who canvass bridges and note time when traffic is light so they are less likely to be interrupted. Between 1937 and 2012, there were >1,600 deaths by suicide from San Francisco’s Golden Gate Bridge.9 Patients who choose highly lethal methods are often irritated during the postattempt evaluation because their plans were not fatal. Usually, patients who choose such potentially lethal methods are hospitalized initially on medical and surgical floors, and receive most of their psychiatric care from consultation psychiatrists. Following discharge, these patients may be at high risk for subsequent suicide attempts.
In the US, the most common method of attempting suicide is by overdose.4 Lethality is determined by the agent or combination of substances ingested, the amount taken, the person’s health status, and the length of time before they are discovered. Many patients mistakenly assume that readily available agents such as acetaminophen and aspirin are less likely to be fatal than prescription medications. Evaluators may want to assess for suicidality in individuals with erratic, risk-taking behaviors, who are at especially high risk for death. Learning about the method the patient used can help the clinician determine the imminent risk of another suicide attempt. The more potentially fatal the patient’s method, the more serious their suicide intent, and the higher the risk they will make another suicide attempt, possibly using an even more lethal method.
4. What was the intent?
“What did you want to happen when you made this attempt?” Many patients will respond that they wanted to die, sleep, not wake up, or did not care what happened. Others say it was a gesture to evoke a certain response from another person. If this is the case, it is important to know whether the desired outcome was achieved. These so-called gestures often involve making sure the intended person is aware of the attempt, often by writing a letter, sending a text, or posting on social media. Such behaviors may be exhibited by patients with personality disorders. While such attempts often are impulsive, if the attempt fails to generate the anticipated effect, the patient may try to gain more attention by escalating their suicide actions.
Conversely, if a spouse or partner reconciles with the patient solely because of a suicide attempt, this may set a pattern for future self-harm events in which the patient hopes to achieve the same outcome. Nevertheless, it is better to err for safety because some of these patients will make another attempt, just to prove that they should have been taken more seriously. An exploration of such intent can help the evaluation because even supposed “gestures” can have dangerous consequences. Acts that do not result in the desired outcome should precipitate hospitalization rather than discharge.
5. What facilitated the patient’s rescue?
“Why is this patient still alive?” Determine if the patient did anything to save themself, such as calling an ambulance, inducing emesis, telling someone what they did, or coming to the hospital on their own. If yes, asking them what changed their mind may provide information about what exists in their lives to potentially prevent future attempts, or about wishes to stay alive. These issues can be used to guide outpatient therapy.
Continue to: How does the patient feel about having survived?
6. How does the patient feel about having survived?
When a patient is asked how they feel about having survived a suicide attempt, some will label their act “stupid” and profess embarrassment. Others exhibit future-oriented thought, which is a very good prognostic sign. More ominous is subsequent dysphoria or lamenting that “I could not even do this right.” Patients often express anger toward anyone who rescued them, especially those whose attempts were carefully planned or were discovered by accident. Some patients might also express ambivalence about having survived.
The patient’s response to this question may be shaped by their desire to avoid hospitalization, so beyond their verbal answers, be attentive to clinical cues that may suggest the patient is not being fully transparent. Anger or ambivalence about having survived, a lack of future-oriented thought, and a restricted affect despite verbalizing joy about still being alive are features that suggest psychiatric hospitalization may be warranted.
7. Has the patient made previous suicide attempts?
Compared to individuals with no previous suicide attempts, patients with a history of suicide attempts are 30 to 40 times more likely to die by suicide.2 Many patients who present after a suicide attempt have tried to kill themselves multiple times. Exploring the number of past attempts, how recent the attempts were, and what dispositions were made can be of benefit. Reviewing the potential lethality of past attempts (eg, was hospitalization required, was the patient placed in an intensive care unit, and/or was intubation needed) is recommended. If outpatient care was suggested or medication prescribed, was the patient adherent? Consider asking about passive suicidal behavior, such as not seeking care for medical issues, discontinuing life-saving medication, or engaging in reckless behavior. While such behaviors may not have been classified as a suicide attempt, it might indicate a feeling of indifference toward staying alive. A patient with a past attempt, especially if recent, merits consideration for inpatient care. Once again, referring previously nonadherent patients to outpatient treatment is less likely to be effective.
8. Does the patient have a support network?
Before discharging a patient who has made a suicide attempt, consider the quality of their support network. Gauging the response of the family and friends to the patient’s attempt can be beneficial. Indifference or resentment on the part of loved ones is a bad sign. Some patients have access to support networks they either did not know were available or chose not to utilize. In other instances, after realizing how depressed the patient has been, the family might provide a new safety net. Strong religious affiliations can also be valuable because devout spirituality can be a deterrent to suicide behaviors.10 For an individual whose attempt was motivated by loneliness or feeling unloved or underappreciated, a newly realized support network can be an additional protective deterrent.
9. Does the patient have a family history of suicide?
There may be a familial component to suicide. Knowing about any suicide history in the family contributes to future therapeutic planning. The clinician may want to explore the patient’s family suicide history in detail because such information can have substantial impact on the patient’s motivation for attempting suicide. The evaluator may want to determine if the anniversary of a family suicide is coming. Triggers for a suicide attempt could include the anniversary of a death, birthdays, family-oriented holidays, and similar events. It is productive to understand how the patient feels about family members who have died by suicide. Some will empathize with the deceased, commenting that they did the “right thing.” Others, upon realizing how their own attempt affected others, will be remorseful and determined not to inflict more pain on their family. Such patients may need to be reminded of the misery associated with their family being left without them. These understandings are helpful at setting a safe disposition. However, a history of death by suicide in the family should always be thoroughly evaluated, regardless of the patient’s attitude about that death.
Continue to: Was the attempt the result of depression?
10. Was the attempt the result of depression?
For a patient experiencing depressive symptoms, the prognosis is less positive; they are more likely to harbor serious intent, premeditation, hopelessness, and social isolation, and less likely to express future-oriented thought. They often exhibit a temporary “flight into health.” Such progress is often transitory and may not represent recovery. Because mood disorders may still be present despite a temporary improvement, inpatient and pharmacologic treatment may be needed. If a patient’s suicide attempt occurred as a result of severe depression, it is possible they will make another suicide attempt unless their depression is addressed in a safe and secure setting, such as inpatient hospitalization, or through close family observation while the patient is receiving intensive outpatient treatment.
11. Does the patient have a psychotic disorder?
Many patients with a psychotic illness die following their first attempt without ever having contact with a mental health professional.11 Features of psychosis might include malevolent auditory hallucinations that suggest self-destruction.11 Such “voices” can be intense and self-deprecating; many patients with this type of hallucination report having made a suicide attempt “just to make the voices stop.”
Symptoms of paranoia can make it less likely for individuals with psychosis to confide in family members, friends, or medical personnel. Religious elements are often of a delusional nature and can be dangerous. Psychosis is more difficult to hide than depression and the presence of psychoses concurrent with major depressive disorder (MDD) increases the probability of suicidality.11 Psychosis secondary to substance use may diminish inhibitions and heighten impulsivity, thereby exacerbating the likelihood of self-harm. Usually, the presence of psychotic features precipitating or following a suicide attempt leads to psychiatric hospitalization.
12. Is the patient in a high-risk demographic group?
When evaluating a patient who has attempted suicide, it helps to consider not just what they did, but who they are. Specifically, does the individual belong to a demographic group that traditionally has a high rate of suicide? For example, patients who are Native American or Alaska Natives warrant extra caution.2 Older White males, especially those who are divorced, widowed, retired, and/or have chronic health problems, are also at greater risk. Compared to the general population, individuals age >80 have a massively elevated chance for self-induced death.12 Some of the reasons include:
- medical comorbidities make surviving an attempt less likely
- access to large amounts of medications
- more irreversible issues, such as chronic pain, disability, or widowhood
- living alone, which may delay discovery.
Patients who are members of any of these demographic groups may deserve serious consideration for inpatient psychiatric admission, regardless of other factors.
Continue to: Were drugs or alcohol involved?
13. Were drugs or alcohol involved?
This factor is unique in that it is both a chronic risk factor (SUDs) and a warning sign for imminent suicide, as in the case of an individual who gets intoxicated to disinhibit their fear of death so they can attempt suicide. Alcohol use disorders are associated with depression and suicide. Overdoses by fentanyl and other opiates have become more frequent.13 In many cases, fatalities are unintentional because users overestimate their tolerance or ingest contaminated substances.14 Disinhibition by alcohol and/or other drugs is a risk factor for attempting suicide and can intensify the depth of MDD. Some patients will ingest substances before an attempt just to give them the courage to act; many think of suicide only when intoxicated. Toxicology screens are indicated as part of the evaluation after a suicide attempt.
Depressive and suicidal thoughts often occur in people “coming down” from cocaine or other stimulants. These circumstances require determining whether to refer the patient for treatment for an SUD or psychiatric hospitalization.
In summary, getting intoxicated solely to diminish anxiety about suicide is a dangerous feature, whereas attempting suicide due to intoxication is less concerning. The latter patient may not consider suicide unless they become intoxicated again. When available, dual diagnosis treatment facilities can be an appropriate referral for such patients. Emergency department holding beds can allow these individuals to detoxify prior to the evaluation.
14. Does the patient have future-oriented thoughts?
When evaluating a patient who has attempted suicide, the presence of future planning and anticipation can be reassuring, but these features should be carefully assessed.14-16
After-the-fact comments may be more reliable when a patient offers them spontaneously, as opposed to in response to direct questioning.
- the specificity of the future plans
- corroboration from the family and others about the patient’s previous investment in the upcoming event
- whether the patient mentions such plans spontaneously or only in response to direct questioning
- the patient’s emotional expression or affect when discussing their future
- whether such plans are reasonable, grandiose, and/or unrealistic.
Bottom Line
When assessing a patient after a suicide attempt, both the patient’s presentation and history and the clinician’s instincts are important. Careful consideration of the method, stated intent, premeditation vs impulsivity, feelings about having survived, presence of psychiatric illness, high-risk demographic, postattempt demeanor and affect, quality of support, presence of self-rescue behaviors, future-oriented thoughts, and other factors can help in making the appropriate disposition.
Related Resources
- Kim H, Kim Y, Shin MH, et al. Early psychiatric referral after attempted suicide helps prevent suicide reattempts: a longitudinal national cohort study in South Korea. Front Psychiatry. 2022;13:607892. doi:10.3389/fpsyt.2022.607892
- Michaud L, Berva S, Ostertag L, et al. When to discharge and when to voluntary or compulsory hospitalize? Factors associated with treatment decision after self-harm. Psychiatry Res. 2022;317:114810. doi:10.1016/j.psychres.2022.114810
1. Ten Leading Causes of Death, United States 2020. Centers for Disease Control and Prevention WISQARS. Accessed March 4, 2022. https://wisqars.cdc.gov/data/lcd/home
2. Norris D, Clark MS. Evaluation and treatment of suicidal patients. Am Fam Physician. 2012;15;85(6):602-605.
3. Gliatto MF, Rai AK. Evaluation and treatment patients with suicidal ideation. Am Fam Phys. 1999;59(6):1500-1506.
4. Dazzi T, Gribble R, Wessely S, et al. Does asking about suicide and related behaviors induce suicidal ideation? What is the evidence? Psychol Med. 2014;44(16):3361-3363.
5. Lewiecki EM, Miller SA. Suicide, guns and public policy. Am J Public Health. 2013;103(1):27-31.
6. Frierson RL. Women who shoot themselves. Hosp Community Psychiatry. 1989;40(8):841-843.
7. Frierson RL, Lippmann SB. Psychiatric consultation for patients with self-inflicted gunshot wounds. Psychosomatics. 1990;31(1):67-74.
8. Mitchell AM, Terhorst L. PTSD symptoms in survivors bereaved by the suicide of a significant other. J Am Psychiatr Nurses Assoc. 2017;23(1):61-65.
9. Bateson J. The Golden Gate Bridge’s fatal flaw. Los Angeles Times. May 25, 2012. Accessed March 2, 2022. https://www.latimes.com/opinion/la-xpm-2012-may-25-la-oe-adv-bateson-golden-gate-20120525-story.html
10. Dervic K, Oquendoma MA, Grunebaum MF, et al. Religious affiliation and suicide attempt. Am J Psychiatry. 2004;161(12):2303-2308.
11. Nordentoft H, Madsen T, Fedyszyn IF. Suicidal behavior and mortality in first episode psychosis. J Nerv Ment Dis. 2015;203(5):387-392.
12. Frierson R, Lippmann S. Suicide attempts by the old and the very old. Arch Intern Med. 1991;151(1):141-144.
13. Braden JB, Edlund MJ, Sullivan MD. Suicide deaths with opiate poisonings in the United States: 1999-2014. Am J Public Health. 2017;107(3):421-426.
14. Morin KA, Acharya S, Eibl JK, et al: Evidence of increased fentanyl use during the COVID-19 pandemic among opioid agonist treated patients in Ontario, Canada. Int J Drug Policy. 2021;90:103088.
15. Shobassy A, Abu-Mohammad AS. Assessing imminent suicide risk: what about future planning? Current Psychiatry. 2022;21(2):12-17.
16. MacLeod AK, Pankhania B, Lee M, et al. Parasuicide, depression and the anticipation of positive and negative future experiences. Psychol Med. 1997;27(4):973-977.
17. Macleod AK, Tata P, Tyrer P, et al. Hopelessness and positive and negative future thinking in parasuicide. Br J Clin Psychol. 2010;44(Pt 4):495-504.
In 2021, suicide was the 11th leading cause of death in the United States.1 Suicide resulted in 49,000 US deaths during 2021; it was the second most common cause of death in individuals age 10 to 34, and the fifth leading cause among children.1,2 Women are 3 to 4 times more likely than men to attempt suicide, but men are 4 times more likely to die by suicide.2
The evaluation of patients with suicidal ideation who have not made an attempt generally involves assessing 4 factors: the specific plan, access to lethal means, any recent social stressors, and the presence of a psychiatric disorder.3 The clinician should also assess which potential deterrents, such as religious beliefs or dependent children, might be present.
Mental health clinicians are often called upon to evaluate a patient after a suicide attempt to assess intent for continued self-harm and to determine appropriate disposition. Such an evaluation must consider multiple factors, including the method used, premeditation, consequences of the attempt, the presence of severe depression and/or psychosis, and the role of substance use. Assessment after a suicide attempt differs from the examination of individuals who harbor suicidal thoughts but have not made an attempt; the latter group may be more likely to respond to interventions such as intensive outpatient care, mobilization of family support, and religious proscriptions against suicide. However, for patients who make an attempt to end their life, whatever potential safeguards or deterrents to suicide that were in place obviously did not prevent the self-harm act. The consequences of the attempt, such as disabling injuries or medical complications, and possible involuntary commitment, need to be considered. Assessment of the patient’s feelings about having survived the attempt is important because the psychological impact of the attempt on family members may serve to intensify the patient’s depression and make a subsequent attempt more likely.
Many individuals who think of suicide have communicated self-harm thoughts or intentions, but such comments are often minimized or ignored. There is a common but erroneous belief that if patients are encouraged to discuss thoughts of self-harm, they will be more likely to act upon them. Because the opposite is true,4 clinicians should ask vulnerable patients about suicidal ideation or intent. Importantly, noncompliance with life-saving medical care, risk-taking behaviors, and substance use may also signal a desire for self-harm. Passive thoughts of death, typified by comments such as “I don’t care whether I wake up or not,” should also be elicited. Many patients who think of suicide speak of being in a “bad place” where reason and logic give way to an intense desire to end their misery.

The evaluation of a patient who has attempted suicide is an important component of providing psychiatric care. This article reflects our 45 years of evaluating such patients. As such, it reflects our clinical experience and is not evidence-based. We offer a checklist of 14 questions that we have found helpful when determining if it would be best for a patient to receive inpatient psychiatric hospitalization or a discharge referral for outpatient care (Table). Questions 1 through 6 are specific for patients who have made a suicide attempt, while questions 7 through 14 are helpful for assessing global risk factors for suicide.
1. Was the attempt premeditated?
Determining premeditation vs impulsivity is an essential element of the assessment following a suicide attempt. Many such acts may occur without forethought in response to an unexpected stressor, such as an altercation between partners or family conflicts. Impulsive attempts can occur when an individual is involved in a distressing event and/or while intoxicated. Conversely, premeditation involves forethought and planning, which may increase the risk of suicide in the near future.
Examples of premeditated behavior include:
- Contemplating the attempt days or weeks beforehand
- Researching the effects of a medication or combination of medications in terms of potential lethality
- Engaging in behavior that would decrease the likelihood of their body being discovered after the attempt
- Obtaining weapons and/or stockpiling pills
- Canvassing potential sites such as bridges or tall buildings
- Engaging in a suicide attempt “practice run”
- Leaving a suicide note or message on social media
- Making funeral arrangements, such as choosing burial clothing
- Writing a will and arranging for the custody of dependent children
- Purchasing life insurance that does not deny payment of benefits in cases of death by suicide.
Continue to: Patients with a premeditated...
Patients with a premeditated suicide attempt generally do not expect to survive and are often surprised or upset that the act was not fatal. The presence of indicators that the attempt was premeditated should direct the disposition more toward hospitalization than discharge. In assessing the impact of premeditation, it is important to gauge not just the examples listed above, but also the patient’s perception of these issues (such as potential loss of child custody). Consider how much the patient is emotionally affected by such thinking.
2. What were the consequences of the attempt?
Assessing the reason for the attempt (if any) and determining whether the inciting circumstance has changed due to the suicide attempt are an important part of the evaluation. A suicide attempt may result in reconciliation with and/or renewed support from family members or partners, who might not have been aware of the patient’s emotional distress. Such unexpected support often results in the patient exhibiting improved mood and affect, and possibly temporary resolution of suicidal thoughts. This “flight into health” may be short-lived, but it also may be enough to engage the patient in a therapeutic alliance. That may permit a discharge with safe disposition to the outpatient clinic while in the custody of a family member, partner, or close friend.
Alternatively, some people experience a troubling worsening of precipitants following a suicide attempt. Preexisting medical conditions and financial, occupational, and/or social woes may be exacerbated. Child custody determinations may be affected, assuming the patient understands the possibility of this adverse consequence. Violent methods may result in disfigurement and body image issues. Individuals from small, close-knit communities may experience stigmatization and unwanted notoriety because of their suicide attempt. Such negative consequences may render some patients more likely to make another attempt to die by suicide. It is crucial to consider how a suicide attempt may have changed the original stress that led to the attempt.
3. Which method was used?
Most fatal suicides in the US are by firearms, and many individuals who survive such attempts do so because of unfamiliarity with the weapon, gun malfunction, faulty aim, or alcohol use.5-7 Some survivors report intending to shoot themselves in the heart, but instead suffered shoulder injuries. Unfortunately, for a patient who survives self-inflicted gunshot wounds, the sequelae of chronic pain, multiple surgical procedures, disability, and disfigurement may serve as constant negative reminders of the event. Some individuals with suicidal intent eschew the idea of using firearms because they hope to avoid having a family member be the first to discover them. Witnessing the aftermath of a fatal suicide by gunshot can induce symptoms of posttraumatic stress disorder in family members and/or partners.8
For a patient with self-inflicted gunshot wounds, always determine whether the weapon has been secured or if the patient still has access to it. Asking about weapon availability is essential during the evaluation of any patient with depression, major life crises, or other factors that may yield a desire to die; this is especially true for individuals with substance use disorders (SUDs). Whenever readily available to such individuals, weapons need to be safely removed.
Continue to: Other self-harm methods...
Other self-harm methods with a high degree of lethality include jumping from bridges or buildings, poisonings, self-immolation, cutting, and hangings. Individuals who choose these approaches generally do not intend to survive. Many of these methods also entail premeditation, as in the case of individuals who canvass bridges and note time when traffic is light so they are less likely to be interrupted. Between 1937 and 2012, there were >1,600 deaths by suicide from San Francisco’s Golden Gate Bridge.9 Patients who choose highly lethal methods are often irritated during the postattempt evaluation because their plans were not fatal. Usually, patients who choose such potentially lethal methods are hospitalized initially on medical and surgical floors, and receive most of their psychiatric care from consultation psychiatrists. Following discharge, these patients may be at high risk for subsequent suicide attempts.
In the US, the most common method of attempting suicide is by overdose.4 Lethality is determined by the agent or combination of substances ingested, the amount taken, the person’s health status, and the length of time before they are discovered. Many patients mistakenly assume that readily available agents such as acetaminophen and aspirin are less likely to be fatal than prescription medications. Evaluators may want to assess for suicidality in individuals with erratic, risk-taking behaviors, who are at especially high risk for death. Learning about the method the patient used can help the clinician determine the imminent risk of another suicide attempt. The more potentially fatal the patient’s method, the more serious their suicide intent, and the higher the risk they will make another suicide attempt, possibly using an even more lethal method.
4. What was the intent?
“What did you want to happen when you made this attempt?” Many patients will respond that they wanted to die, sleep, not wake up, or did not care what happened. Others say it was a gesture to evoke a certain response from another person. If this is the case, it is important to know whether the desired outcome was achieved. These so-called gestures often involve making sure the intended person is aware of the attempt, often by writing a letter, sending a text, or posting on social media. Such behaviors may be exhibited by patients with personality disorders. While such attempts often are impulsive, if the attempt fails to generate the anticipated effect, the patient may try to gain more attention by escalating their suicide actions.
Conversely, if a spouse or partner reconciles with the patient solely because of a suicide attempt, this may set a pattern for future self-harm events in which the patient hopes to achieve the same outcome. Nevertheless, it is better to err for safety because some of these patients will make another attempt, just to prove that they should have been taken more seriously. An exploration of such intent can help the evaluation because even supposed “gestures” can have dangerous consequences. Acts that do not result in the desired outcome should precipitate hospitalization rather than discharge.
5. What facilitated the patient’s rescue?
“Why is this patient still alive?” Determine if the patient did anything to save themself, such as calling an ambulance, inducing emesis, telling someone what they did, or coming to the hospital on their own. If yes, asking them what changed their mind may provide information about what exists in their lives to potentially prevent future attempts, or about wishes to stay alive. These issues can be used to guide outpatient therapy.
Continue to: How does the patient feel about having survived?
6. How does the patient feel about having survived?
When a patient is asked how they feel about having survived a suicide attempt, some will label their act “stupid” and profess embarrassment. Others exhibit future-oriented thought, which is a very good prognostic sign. More ominous is subsequent dysphoria or lamenting that “I could not even do this right.” Patients often express anger toward anyone who rescued them, especially those whose attempts were carefully planned or were discovered by accident. Some patients might also express ambivalence about having survived.
The patient’s response to this question may be shaped by their desire to avoid hospitalization, so beyond their verbal answers, be attentive to clinical cues that may suggest the patient is not being fully transparent. Anger or ambivalence about having survived, a lack of future-oriented thought, and a restricted affect despite verbalizing joy about still being alive are features that suggest psychiatric hospitalization may be warranted.
7. Has the patient made previous suicide attempts?
Compared to individuals with no previous suicide attempts, patients with a history of suicide attempts are 30 to 40 times more likely to die by suicide.2 Many patients who present after a suicide attempt have tried to kill themselves multiple times. Exploring the number of past attempts, how recent the attempts were, and what dispositions were made can be of benefit. Reviewing the potential lethality of past attempts (eg, was hospitalization required, was the patient placed in an intensive care unit, and/or was intubation needed) is recommended. If outpatient care was suggested or medication prescribed, was the patient adherent? Consider asking about passive suicidal behavior, such as not seeking care for medical issues, discontinuing life-saving medication, or engaging in reckless behavior. While such behaviors may not have been classified as a suicide attempt, it might indicate a feeling of indifference toward staying alive. A patient with a past attempt, especially if recent, merits consideration for inpatient care. Once again, referring previously nonadherent patients to outpatient treatment is less likely to be effective.
8. Does the patient have a support network?
Before discharging a patient who has made a suicide attempt, consider the quality of their support network. Gauging the response of the family and friends to the patient’s attempt can be beneficial. Indifference or resentment on the part of loved ones is a bad sign. Some patients have access to support networks they either did not know were available or chose not to utilize. In other instances, after realizing how depressed the patient has been, the family might provide a new safety net. Strong religious affiliations can also be valuable because devout spirituality can be a deterrent to suicide behaviors.10 For an individual whose attempt was motivated by loneliness or feeling unloved or underappreciated, a newly realized support network can be an additional protective deterrent.
9. Does the patient have a family history of suicide?
There may be a familial component to suicide. Knowing about any suicide history in the family contributes to future therapeutic planning. The clinician may want to explore the patient’s family suicide history in detail because such information can have substantial impact on the patient’s motivation for attempting suicide. The evaluator may want to determine if the anniversary of a family suicide is coming. Triggers for a suicide attempt could include the anniversary of a death, birthdays, family-oriented holidays, and similar events. It is productive to understand how the patient feels about family members who have died by suicide. Some will empathize with the deceased, commenting that they did the “right thing.” Others, upon realizing how their own attempt affected others, will be remorseful and determined not to inflict more pain on their family. Such patients may need to be reminded of the misery associated with their family being left without them. These understandings are helpful at setting a safe disposition. However, a history of death by suicide in the family should always be thoroughly evaluated, regardless of the patient’s attitude about that death.
Continue to: Was the attempt the result of depression?
10. Was the attempt the result of depression?
For a patient experiencing depressive symptoms, the prognosis is less positive; they are more likely to harbor serious intent, premeditation, hopelessness, and social isolation, and less likely to express future-oriented thought. They often exhibit a temporary “flight into health.” Such progress is often transitory and may not represent recovery. Because mood disorders may still be present despite a temporary improvement, inpatient and pharmacologic treatment may be needed. If a patient’s suicide attempt occurred as a result of severe depression, it is possible they will make another suicide attempt unless their depression is addressed in a safe and secure setting, such as inpatient hospitalization, or through close family observation while the patient is receiving intensive outpatient treatment.
11. Does the patient have a psychotic disorder?
Many patients with a psychotic illness die following their first attempt without ever having contact with a mental health professional.11 Features of psychosis might include malevolent auditory hallucinations that suggest self-destruction.11 Such “voices” can be intense and self-deprecating; many patients with this type of hallucination report having made a suicide attempt “just to make the voices stop.”
Symptoms of paranoia can make it less likely for individuals with psychosis to confide in family members, friends, or medical personnel. Religious elements are often of a delusional nature and can be dangerous. Psychosis is more difficult to hide than depression and the presence of psychoses concurrent with major depressive disorder (MDD) increases the probability of suicidality.11 Psychosis secondary to substance use may diminish inhibitions and heighten impulsivity, thereby exacerbating the likelihood of self-harm. Usually, the presence of psychotic features precipitating or following a suicide attempt leads to psychiatric hospitalization.
12. Is the patient in a high-risk demographic group?
When evaluating a patient who has attempted suicide, it helps to consider not just what they did, but who they are. Specifically, does the individual belong to a demographic group that traditionally has a high rate of suicide? For example, patients who are Native American or Alaska Natives warrant extra caution.2 Older White males, especially those who are divorced, widowed, retired, and/or have chronic health problems, are also at greater risk. Compared to the general population, individuals age >80 have a massively elevated chance for self-induced death.12 Some of the reasons include:
- medical comorbidities make surviving an attempt less likely
- access to large amounts of medications
- more irreversible issues, such as chronic pain, disability, or widowhood
- living alone, which may delay discovery.
Patients who are members of any of these demographic groups may deserve serious consideration for inpatient psychiatric admission, regardless of other factors.
Continue to: Were drugs or alcohol involved?
13. Were drugs or alcohol involved?
This factor is unique in that it is both a chronic risk factor (SUDs) and a warning sign for imminent suicide, as in the case of an individual who gets intoxicated to disinhibit their fear of death so they can attempt suicide. Alcohol use disorders are associated with depression and suicide. Overdoses by fentanyl and other opiates have become more frequent.13 In many cases, fatalities are unintentional because users overestimate their tolerance or ingest contaminated substances.14 Disinhibition by alcohol and/or other drugs is a risk factor for attempting suicide and can intensify the depth of MDD. Some patients will ingest substances before an attempt just to give them the courage to act; many think of suicide only when intoxicated. Toxicology screens are indicated as part of the evaluation after a suicide attempt.
Depressive and suicidal thoughts often occur in people “coming down” from cocaine or other stimulants. These circumstances require determining whether to refer the patient for treatment for an SUD or psychiatric hospitalization.
In summary, getting intoxicated solely to diminish anxiety about suicide is a dangerous feature, whereas attempting suicide due to intoxication is less concerning. The latter patient may not consider suicide unless they become intoxicated again. When available, dual diagnosis treatment facilities can be an appropriate referral for such patients. Emergency department holding beds can allow these individuals to detoxify prior to the evaluation.
14. Does the patient have future-oriented thoughts?
When evaluating a patient who has attempted suicide, the presence of future planning and anticipation can be reassuring, but these features should be carefully assessed.14-16
After-the-fact comments may be more reliable when a patient offers them spontaneously, as opposed to in response to direct questioning.
- the specificity of the future plans
- corroboration from the family and others about the patient’s previous investment in the upcoming event
- whether the patient mentions such plans spontaneously or only in response to direct questioning
- the patient’s emotional expression or affect when discussing their future
- whether such plans are reasonable, grandiose, and/or unrealistic.
Bottom Line
When assessing a patient after a suicide attempt, both the patient’s presentation and history and the clinician’s instincts are important. Careful consideration of the method, stated intent, premeditation vs impulsivity, feelings about having survived, presence of psychiatric illness, high-risk demographic, postattempt demeanor and affect, quality of support, presence of self-rescue behaviors, future-oriented thoughts, and other factors can help in making the appropriate disposition.
Related Resources
- Kim H, Kim Y, Shin MH, et al. Early psychiatric referral after attempted suicide helps prevent suicide reattempts: a longitudinal national cohort study in South Korea. Front Psychiatry. 2022;13:607892. doi:10.3389/fpsyt.2022.607892
- Michaud L, Berva S, Ostertag L, et al. When to discharge and when to voluntary or compulsory hospitalize? Factors associated with treatment decision after self-harm. Psychiatry Res. 2022;317:114810. doi:10.1016/j.psychres.2022.114810
In 2021, suicide was the 11th leading cause of death in the United States.1 Suicide resulted in 49,000 US deaths during 2021; it was the second most common cause of death in individuals age 10 to 34, and the fifth leading cause among children.1,2 Women are 3 to 4 times more likely than men to attempt suicide, but men are 4 times more likely to die by suicide.2
The evaluation of patients with suicidal ideation who have not made an attempt generally involves assessing 4 factors: the specific plan, access to lethal means, any recent social stressors, and the presence of a psychiatric disorder.3 The clinician should also assess which potential deterrents, such as religious beliefs or dependent children, might be present.
Mental health clinicians are often called upon to evaluate a patient after a suicide attempt to assess intent for continued self-harm and to determine appropriate disposition. Such an evaluation must consider multiple factors, including the method used, premeditation, consequences of the attempt, the presence of severe depression and/or psychosis, and the role of substance use. Assessment after a suicide attempt differs from the examination of individuals who harbor suicidal thoughts but have not made an attempt; the latter group may be more likely to respond to interventions such as intensive outpatient care, mobilization of family support, and religious proscriptions against suicide. However, for patients who make an attempt to end their life, whatever potential safeguards or deterrents to suicide that were in place obviously did not prevent the self-harm act. The consequences of the attempt, such as disabling injuries or medical complications, and possible involuntary commitment, need to be considered. Assessment of the patient’s feelings about having survived the attempt is important because the psychological impact of the attempt on family members may serve to intensify the patient’s depression and make a subsequent attempt more likely.
Many individuals who think of suicide have communicated self-harm thoughts or intentions, but such comments are often minimized or ignored. There is a common but erroneous belief that if patients are encouraged to discuss thoughts of self-harm, they will be more likely to act upon them. Because the opposite is true,4 clinicians should ask vulnerable patients about suicidal ideation or intent. Importantly, noncompliance with life-saving medical care, risk-taking behaviors, and substance use may also signal a desire for self-harm. Passive thoughts of death, typified by comments such as “I don’t care whether I wake up or not,” should also be elicited. Many patients who think of suicide speak of being in a “bad place” where reason and logic give way to an intense desire to end their misery.

The evaluation of a patient who has attempted suicide is an important component of providing psychiatric care. This article reflects our 45 years of evaluating such patients. As such, it reflects our clinical experience and is not evidence-based. We offer a checklist of 14 questions that we have found helpful when determining if it would be best for a patient to receive inpatient psychiatric hospitalization or a discharge referral for outpatient care (Table). Questions 1 through 6 are specific for patients who have made a suicide attempt, while questions 7 through 14 are helpful for assessing global risk factors for suicide.
1. Was the attempt premeditated?
Determining premeditation vs impulsivity is an essential element of the assessment following a suicide attempt. Many such acts may occur without forethought in response to an unexpected stressor, such as an altercation between partners or family conflicts. Impulsive attempts can occur when an individual is involved in a distressing event and/or while intoxicated. Conversely, premeditation involves forethought and planning, which may increase the risk of suicide in the near future.
Examples of premeditated behavior include:
- Contemplating the attempt days or weeks beforehand
- Researching the effects of a medication or combination of medications in terms of potential lethality
- Engaging in behavior that would decrease the likelihood of their body being discovered after the attempt
- Obtaining weapons and/or stockpiling pills
- Canvassing potential sites such as bridges or tall buildings
- Engaging in a suicide attempt “practice run”
- Leaving a suicide note or message on social media
- Making funeral arrangements, such as choosing burial clothing
- Writing a will and arranging for the custody of dependent children
- Purchasing life insurance that does not deny payment of benefits in cases of death by suicide.
Continue to: Patients with a premeditated...
Patients with a premeditated suicide attempt generally do not expect to survive and are often surprised or upset that the act was not fatal. The presence of indicators that the attempt was premeditated should direct the disposition more toward hospitalization than discharge. In assessing the impact of premeditation, it is important to gauge not just the examples listed above, but also the patient’s perception of these issues (such as potential loss of child custody). Consider how much the patient is emotionally affected by such thinking.
2. What were the consequences of the attempt?
Assessing the reason for the attempt (if any) and determining whether the inciting circumstance has changed due to the suicide attempt are an important part of the evaluation. A suicide attempt may result in reconciliation with and/or renewed support from family members or partners, who might not have been aware of the patient’s emotional distress. Such unexpected support often results in the patient exhibiting improved mood and affect, and possibly temporary resolution of suicidal thoughts. This “flight into health” may be short-lived, but it also may be enough to engage the patient in a therapeutic alliance. That may permit a discharge with safe disposition to the outpatient clinic while in the custody of a family member, partner, or close friend.
Alternatively, some people experience a troubling worsening of precipitants following a suicide attempt. Preexisting medical conditions and financial, occupational, and/or social woes may be exacerbated. Child custody determinations may be affected, assuming the patient understands the possibility of this adverse consequence. Violent methods may result in disfigurement and body image issues. Individuals from small, close-knit communities may experience stigmatization and unwanted notoriety because of their suicide attempt. Such negative consequences may render some patients more likely to make another attempt to die by suicide. It is crucial to consider how a suicide attempt may have changed the original stress that led to the attempt.
3. Which method was used?
Most fatal suicides in the US are by firearms, and many individuals who survive such attempts do so because of unfamiliarity with the weapon, gun malfunction, faulty aim, or alcohol use.5-7 Some survivors report intending to shoot themselves in the heart, but instead suffered shoulder injuries. Unfortunately, for a patient who survives self-inflicted gunshot wounds, the sequelae of chronic pain, multiple surgical procedures, disability, and disfigurement may serve as constant negative reminders of the event. Some individuals with suicidal intent eschew the idea of using firearms because they hope to avoid having a family member be the first to discover them. Witnessing the aftermath of a fatal suicide by gunshot can induce symptoms of posttraumatic stress disorder in family members and/or partners.8
For a patient with self-inflicted gunshot wounds, always determine whether the weapon has been secured or if the patient still has access to it. Asking about weapon availability is essential during the evaluation of any patient with depression, major life crises, or other factors that may yield a desire to die; this is especially true for individuals with substance use disorders (SUDs). Whenever readily available to such individuals, weapons need to be safely removed.
Continue to: Other self-harm methods...
Other self-harm methods with a high degree of lethality include jumping from bridges or buildings, poisonings, self-immolation, cutting, and hangings. Individuals who choose these approaches generally do not intend to survive. Many of these methods also entail premeditation, as in the case of individuals who canvass bridges and note time when traffic is light so they are less likely to be interrupted. Between 1937 and 2012, there were >1,600 deaths by suicide from San Francisco’s Golden Gate Bridge.9 Patients who choose highly lethal methods are often irritated during the postattempt evaluation because their plans were not fatal. Usually, patients who choose such potentially lethal methods are hospitalized initially on medical and surgical floors, and receive most of their psychiatric care from consultation psychiatrists. Following discharge, these patients may be at high risk for subsequent suicide attempts.
In the US, the most common method of attempting suicide is by overdose.4 Lethality is determined by the agent or combination of substances ingested, the amount taken, the person’s health status, and the length of time before they are discovered. Many patients mistakenly assume that readily available agents such as acetaminophen and aspirin are less likely to be fatal than prescription medications. Evaluators may want to assess for suicidality in individuals with erratic, risk-taking behaviors, who are at especially high risk for death. Learning about the method the patient used can help the clinician determine the imminent risk of another suicide attempt. The more potentially fatal the patient’s method, the more serious their suicide intent, and the higher the risk they will make another suicide attempt, possibly using an even more lethal method.
4. What was the intent?
“What did you want to happen when you made this attempt?” Many patients will respond that they wanted to die, sleep, not wake up, or did not care what happened. Others say it was a gesture to evoke a certain response from another person. If this is the case, it is important to know whether the desired outcome was achieved. These so-called gestures often involve making sure the intended person is aware of the attempt, often by writing a letter, sending a text, or posting on social media. Such behaviors may be exhibited by patients with personality disorders. While such attempts often are impulsive, if the attempt fails to generate the anticipated effect, the patient may try to gain more attention by escalating their suicide actions.
Conversely, if a spouse or partner reconciles with the patient solely because of a suicide attempt, this may set a pattern for future self-harm events in which the patient hopes to achieve the same outcome. Nevertheless, it is better to err for safety because some of these patients will make another attempt, just to prove that they should have been taken more seriously. An exploration of such intent can help the evaluation because even supposed “gestures” can have dangerous consequences. Acts that do not result in the desired outcome should precipitate hospitalization rather than discharge.
5. What facilitated the patient’s rescue?
“Why is this patient still alive?” Determine if the patient did anything to save themself, such as calling an ambulance, inducing emesis, telling someone what they did, or coming to the hospital on their own. If yes, asking them what changed their mind may provide information about what exists in their lives to potentially prevent future attempts, or about wishes to stay alive. These issues can be used to guide outpatient therapy.
Continue to: How does the patient feel about having survived?
6. How does the patient feel about having survived?
When a patient is asked how they feel about having survived a suicide attempt, some will label their act “stupid” and profess embarrassment. Others exhibit future-oriented thought, which is a very good prognostic sign. More ominous is subsequent dysphoria or lamenting that “I could not even do this right.” Patients often express anger toward anyone who rescued them, especially those whose attempts were carefully planned or were discovered by accident. Some patients might also express ambivalence about having survived.
The patient’s response to this question may be shaped by their desire to avoid hospitalization, so beyond their verbal answers, be attentive to clinical cues that may suggest the patient is not being fully transparent. Anger or ambivalence about having survived, a lack of future-oriented thought, and a restricted affect despite verbalizing joy about still being alive are features that suggest psychiatric hospitalization may be warranted.
7. Has the patient made previous suicide attempts?
Compared to individuals with no previous suicide attempts, patients with a history of suicide attempts are 30 to 40 times more likely to die by suicide.2 Many patients who present after a suicide attempt have tried to kill themselves multiple times. Exploring the number of past attempts, how recent the attempts were, and what dispositions were made can be of benefit. Reviewing the potential lethality of past attempts (eg, was hospitalization required, was the patient placed in an intensive care unit, and/or was intubation needed) is recommended. If outpatient care was suggested or medication prescribed, was the patient adherent? Consider asking about passive suicidal behavior, such as not seeking care for medical issues, discontinuing life-saving medication, or engaging in reckless behavior. While such behaviors may not have been classified as a suicide attempt, it might indicate a feeling of indifference toward staying alive. A patient with a past attempt, especially if recent, merits consideration for inpatient care. Once again, referring previously nonadherent patients to outpatient treatment is less likely to be effective.
8. Does the patient have a support network?
Before discharging a patient who has made a suicide attempt, consider the quality of their support network. Gauging the response of the family and friends to the patient’s attempt can be beneficial. Indifference or resentment on the part of loved ones is a bad sign. Some patients have access to support networks they either did not know were available or chose not to utilize. In other instances, after realizing how depressed the patient has been, the family might provide a new safety net. Strong religious affiliations can also be valuable because devout spirituality can be a deterrent to suicide behaviors.10 For an individual whose attempt was motivated by loneliness or feeling unloved or underappreciated, a newly realized support network can be an additional protective deterrent.
9. Does the patient have a family history of suicide?
There may be a familial component to suicide. Knowing about any suicide history in the family contributes to future therapeutic planning. The clinician may want to explore the patient’s family suicide history in detail because such information can have substantial impact on the patient’s motivation for attempting suicide. The evaluator may want to determine if the anniversary of a family suicide is coming. Triggers for a suicide attempt could include the anniversary of a death, birthdays, family-oriented holidays, and similar events. It is productive to understand how the patient feels about family members who have died by suicide. Some will empathize with the deceased, commenting that they did the “right thing.” Others, upon realizing how their own attempt affected others, will be remorseful and determined not to inflict more pain on their family. Such patients may need to be reminded of the misery associated with their family being left without them. These understandings are helpful at setting a safe disposition. However, a history of death by suicide in the family should always be thoroughly evaluated, regardless of the patient’s attitude about that death.
Continue to: Was the attempt the result of depression?
10. Was the attempt the result of depression?
For a patient experiencing depressive symptoms, the prognosis is less positive; they are more likely to harbor serious intent, premeditation, hopelessness, and social isolation, and less likely to express future-oriented thought. They often exhibit a temporary “flight into health.” Such progress is often transitory and may not represent recovery. Because mood disorders may still be present despite a temporary improvement, inpatient and pharmacologic treatment may be needed. If a patient’s suicide attempt occurred as a result of severe depression, it is possible they will make another suicide attempt unless their depression is addressed in a safe and secure setting, such as inpatient hospitalization, or through close family observation while the patient is receiving intensive outpatient treatment.
11. Does the patient have a psychotic disorder?
Many patients with a psychotic illness die following their first attempt without ever having contact with a mental health professional.11 Features of psychosis might include malevolent auditory hallucinations that suggest self-destruction.11 Such “voices” can be intense and self-deprecating; many patients with this type of hallucination report having made a suicide attempt “just to make the voices stop.”
Symptoms of paranoia can make it less likely for individuals with psychosis to confide in family members, friends, or medical personnel. Religious elements are often of a delusional nature and can be dangerous. Psychosis is more difficult to hide than depression and the presence of psychoses concurrent with major depressive disorder (MDD) increases the probability of suicidality.11 Psychosis secondary to substance use may diminish inhibitions and heighten impulsivity, thereby exacerbating the likelihood of self-harm. Usually, the presence of psychotic features precipitating or following a suicide attempt leads to psychiatric hospitalization.
12. Is the patient in a high-risk demographic group?
When evaluating a patient who has attempted suicide, it helps to consider not just what they did, but who they are. Specifically, does the individual belong to a demographic group that traditionally has a high rate of suicide? For example, patients who are Native American or Alaska Natives warrant extra caution.2 Older White males, especially those who are divorced, widowed, retired, and/or have chronic health problems, are also at greater risk. Compared to the general population, individuals age >80 have a massively elevated chance for self-induced death.12 Some of the reasons include:
- medical comorbidities make surviving an attempt less likely
- access to large amounts of medications
- more irreversible issues, such as chronic pain, disability, or widowhood
- living alone, which may delay discovery.
Patients who are members of any of these demographic groups may deserve serious consideration for inpatient psychiatric admission, regardless of other factors.
Continue to: Were drugs or alcohol involved?
13. Were drugs or alcohol involved?
This factor is unique in that it is both a chronic risk factor (SUDs) and a warning sign for imminent suicide, as in the case of an individual who gets intoxicated to disinhibit their fear of death so they can attempt suicide. Alcohol use disorders are associated with depression and suicide. Overdoses by fentanyl and other opiates have become more frequent.13 In many cases, fatalities are unintentional because users overestimate their tolerance or ingest contaminated substances.14 Disinhibition by alcohol and/or other drugs is a risk factor for attempting suicide and can intensify the depth of MDD. Some patients will ingest substances before an attempt just to give them the courage to act; many think of suicide only when intoxicated. Toxicology screens are indicated as part of the evaluation after a suicide attempt.
Depressive and suicidal thoughts often occur in people “coming down” from cocaine or other stimulants. These circumstances require determining whether to refer the patient for treatment for an SUD or psychiatric hospitalization.
In summary, getting intoxicated solely to diminish anxiety about suicide is a dangerous feature, whereas attempting suicide due to intoxication is less concerning. The latter patient may not consider suicide unless they become intoxicated again. When available, dual diagnosis treatment facilities can be an appropriate referral for such patients. Emergency department holding beds can allow these individuals to detoxify prior to the evaluation.
14. Does the patient have future-oriented thoughts?
When evaluating a patient who has attempted suicide, the presence of future planning and anticipation can be reassuring, but these features should be carefully assessed.14-16
After-the-fact comments may be more reliable when a patient offers them spontaneously, as opposed to in response to direct questioning.
- the specificity of the future plans
- corroboration from the family and others about the patient’s previous investment in the upcoming event
- whether the patient mentions such plans spontaneously or only in response to direct questioning
- the patient’s emotional expression or affect when discussing their future
- whether such plans are reasonable, grandiose, and/or unrealistic.
Bottom Line
When assessing a patient after a suicide attempt, both the patient’s presentation and history and the clinician’s instincts are important. Careful consideration of the method, stated intent, premeditation vs impulsivity, feelings about having survived, presence of psychiatric illness, high-risk demographic, postattempt demeanor and affect, quality of support, presence of self-rescue behaviors, future-oriented thoughts, and other factors can help in making the appropriate disposition.
Related Resources
- Kim H, Kim Y, Shin MH, et al. Early psychiatric referral after attempted suicide helps prevent suicide reattempts: a longitudinal national cohort study in South Korea. Front Psychiatry. 2022;13:607892. doi:10.3389/fpsyt.2022.607892
- Michaud L, Berva S, Ostertag L, et al. When to discharge and when to voluntary or compulsory hospitalize? Factors associated with treatment decision after self-harm. Psychiatry Res. 2022;317:114810. doi:10.1016/j.psychres.2022.114810
1. Ten Leading Causes of Death, United States 2020. Centers for Disease Control and Prevention WISQARS. Accessed March 4, 2022. https://wisqars.cdc.gov/data/lcd/home
2. Norris D, Clark MS. Evaluation and treatment of suicidal patients. Am Fam Physician. 2012;15;85(6):602-605.
3. Gliatto MF, Rai AK. Evaluation and treatment patients with suicidal ideation. Am Fam Phys. 1999;59(6):1500-1506.
4. Dazzi T, Gribble R, Wessely S, et al. Does asking about suicide and related behaviors induce suicidal ideation? What is the evidence? Psychol Med. 2014;44(16):3361-3363.
5. Lewiecki EM, Miller SA. Suicide, guns and public policy. Am J Public Health. 2013;103(1):27-31.
6. Frierson RL. Women who shoot themselves. Hosp Community Psychiatry. 1989;40(8):841-843.
7. Frierson RL, Lippmann SB. Psychiatric consultation for patients with self-inflicted gunshot wounds. Psychosomatics. 1990;31(1):67-74.
8. Mitchell AM, Terhorst L. PTSD symptoms in survivors bereaved by the suicide of a significant other. J Am Psychiatr Nurses Assoc. 2017;23(1):61-65.
9. Bateson J. The Golden Gate Bridge’s fatal flaw. Los Angeles Times. May 25, 2012. Accessed March 2, 2022. https://www.latimes.com/opinion/la-xpm-2012-may-25-la-oe-adv-bateson-golden-gate-20120525-story.html
10. Dervic K, Oquendoma MA, Grunebaum MF, et al. Religious affiliation and suicide attempt. Am J Psychiatry. 2004;161(12):2303-2308.
11. Nordentoft H, Madsen T, Fedyszyn IF. Suicidal behavior and mortality in first episode psychosis. J Nerv Ment Dis. 2015;203(5):387-392.
12. Frierson R, Lippmann S. Suicide attempts by the old and the very old. Arch Intern Med. 1991;151(1):141-144.
13. Braden JB, Edlund MJ, Sullivan MD. Suicide deaths with opiate poisonings in the United States: 1999-2014. Am J Public Health. 2017;107(3):421-426.
14. Morin KA, Acharya S, Eibl JK, et al: Evidence of increased fentanyl use during the COVID-19 pandemic among opioid agonist treated patients in Ontario, Canada. Int J Drug Policy. 2021;90:103088.
15. Shobassy A, Abu-Mohammad AS. Assessing imminent suicide risk: what about future planning? Current Psychiatry. 2022;21(2):12-17.
16. MacLeod AK, Pankhania B, Lee M, et al. Parasuicide, depression and the anticipation of positive and negative future experiences. Psychol Med. 1997;27(4):973-977.
17. Macleod AK, Tata P, Tyrer P, et al. Hopelessness and positive and negative future thinking in parasuicide. Br J Clin Psychol. 2010;44(Pt 4):495-504.
1. Ten Leading Causes of Death, United States 2020. Centers for Disease Control and Prevention WISQARS. Accessed March 4, 2022. https://wisqars.cdc.gov/data/lcd/home
2. Norris D, Clark MS. Evaluation and treatment of suicidal patients. Am Fam Physician. 2012;15;85(6):602-605.
3. Gliatto MF, Rai AK. Evaluation and treatment patients with suicidal ideation. Am Fam Phys. 1999;59(6):1500-1506.
4. Dazzi T, Gribble R, Wessely S, et al. Does asking about suicide and related behaviors induce suicidal ideation? What is the evidence? Psychol Med. 2014;44(16):3361-3363.
5. Lewiecki EM, Miller SA. Suicide, guns and public policy. Am J Public Health. 2013;103(1):27-31.
6. Frierson RL. Women who shoot themselves. Hosp Community Psychiatry. 1989;40(8):841-843.
7. Frierson RL, Lippmann SB. Psychiatric consultation for patients with self-inflicted gunshot wounds. Psychosomatics. 1990;31(1):67-74.
8. Mitchell AM, Terhorst L. PTSD symptoms in survivors bereaved by the suicide of a significant other. J Am Psychiatr Nurses Assoc. 2017;23(1):61-65.
9. Bateson J. The Golden Gate Bridge’s fatal flaw. Los Angeles Times. May 25, 2012. Accessed March 2, 2022. https://www.latimes.com/opinion/la-xpm-2012-may-25-la-oe-adv-bateson-golden-gate-20120525-story.html
10. Dervic K, Oquendoma MA, Grunebaum MF, et al. Religious affiliation and suicide attempt. Am J Psychiatry. 2004;161(12):2303-2308.
11. Nordentoft H, Madsen T, Fedyszyn IF. Suicidal behavior and mortality in first episode psychosis. J Nerv Ment Dis. 2015;203(5):387-392.
12. Frierson R, Lippmann S. Suicide attempts by the old and the very old. Arch Intern Med. 1991;151(1):141-144.
13. Braden JB, Edlund MJ, Sullivan MD. Suicide deaths with opiate poisonings in the United States: 1999-2014. Am J Public Health. 2017;107(3):421-426.
14. Morin KA, Acharya S, Eibl JK, et al: Evidence of increased fentanyl use during the COVID-19 pandemic among opioid agonist treated patients in Ontario, Canada. Int J Drug Policy. 2021;90:103088.
15. Shobassy A, Abu-Mohammad AS. Assessing imminent suicide risk: what about future planning? Current Psychiatry. 2022;21(2):12-17.
16. MacLeod AK, Pankhania B, Lee M, et al. Parasuicide, depression and the anticipation of positive and negative future experiences. Psychol Med. 1997;27(4):973-977.
17. Macleod AK, Tata P, Tyrer P, et al. Hopelessness and positive and negative future thinking in parasuicide. Br J Clin Psychol. 2010;44(Pt 4):495-504.
PCSK9 inhibitors for severe COVID? Pilot trial signals of benefit
PCSK9 inhibitors may best be known for their powerful LDL-lowering effects but are less appreciated as anti-inflammatory agents with potential beyond cardiovascular health.
In a small pilot trial, for example, patients hospitalized with severe COVID-19 who received a single injection of PCSK9 inhibitor became less sick and more likely to survive than those given a placebo. Their 30-day risk of death or intubation fell significantly, as did their levels of the inflammatory cytokine interleukin 6 (IL-6).
Indeed, survival gains in the PCSK9-inhibitor group were greatest among patients with higher baseline concentrations of IL-6. Although the trial wasn’t powered for clinical outcomes, it suggests the drugs’ efficacy in COVID-19 tracks with intensity of inflammation, proposes a report published in the Journal of the American College of Cardiology.
Therefore, “PCSK9 inhibition may represent a novel therapeutic pathway in addition to currently recommended therapeutic approaches for severe COVID-19,” conclude the authors, led by Eliano P. Navarese, MD, PhD, Nicolaus Copernicus University, Bydgoszcz, Poland.
PCSK9 inhibitors as anti-inflammatories
Although the study was small and only hypothesis-generating, the fact that outcomes for actively treated patients were proportional to baseline IL-6 levels “strongly suggests that PCSK9 inhibition can directly modulate inflammation in COVID-19,” argues an editorial accompanying the report.
and likely sheds light on “mechanisms through which PCSK9 inhibition dually modulates lipoprotein metabolism and inflammation,” write Sascha N. Goonewardena, MD, University of Michigan, Ann Arbor, and Robert S. Rosenson, MD, Icahn School of Medicine at Mount Sinai, New York.
The results are consistent with prior evidence that the drugs are anti-inflammatory at least partly because of their interference with inflammatory pathways triggered by PCSK9 and mediated by IL-6, as described by Dr. Navarese and colleagues.
Indeed, they write, PCSK9 inhibitors may improve COVID outcomes mostly through mechanisms unrelated to LDL-receptor expression, “including direct inhibition of PCSK9-triggered inflammation.”
If true, the authors observe, it might explain “why the positive findings of the present study have not been consistently observed in trials involving other lipid-lowering agents, such as statins.” Those drugs are well-known to decrease levels of the inflammatory biomarker C-reactive protein.
In patients with stable coronary disease, in whom inflammation is typically tracked by measuring CRP, “the PCSK9 inhibitors have not been shown to have an anti-inflammatory effect,” Dr. Rosenson further explained.
But the current study’s patients with acute, severe COVID-19, a “profound inflammatory insult” with upregulation of IL-6, were “a good population” for evaluating the drugs’ potential anti-inflammatory effects, Dr. Rosenson said in an interview. The results “are quite enticing but require corroboration in a larger trial.”
A single injection
The IMPACT-SIRIO 5 trial entered 60 adults hospitalized with severe COVID-19 and elevated IL-6 at four centers in Poland. Patients with other known active infections were excluded.
They were randomly assigned double-blind to receive a 140 mg injection of evolocumab (Repatha) or placebo. The 2 groups were similar with respect to demographics, body-mass index, time since symptom onset, and treatments for managing COVID-19 and its complications.
Rates of death or need for intubation at 30 days, the primary endpoint, were 23.3% in the PCSK9-inhibitor group and 53.3% for controls, a risk difference of 30% (95% confidence interval –53.4% to –6.6%). The median durations of oxygen therapy were significantly different at 13 days and 20 days, respectively, the report states.
Serum IL-6 levels fell further over 30 days in the PCSK9-inhibitor group (–56% vs. –21% among controls). A drop by more than 90% was seen in 60% of patients in the PCSK9-inhibitor group and in 27% of controls.
The average hospital stay was shorter for those getting the PCSK9 inhibitor, compared with placebo, 16 days versus 22 days, and their 30-day mortality was numerically lower, 16% versus 33.3%.
Patients’ baseline IL-6 levels above the median, the report states, had a lower mortality on the PCSK9 inhibitor versus placebo (risk difference –37.5%; 95% CI –68.2% to –6.70%).
A larger trial to corroborate these results would potentially enter similar patients hospitalized with COVID-19 with reproducible evidence of an ongoing cytokine storm, such as elevated levels of IL-6, who would be assigned to either a PCSK9 inhibitor or placebo, Dr. Rosenson proposed.
Although the current primary endpoint that combines mortality and intubation was “reasonable” for a small pilot trial, he said, if the researchers embark on a larger study, “they’ll want to look at those events separately.”
Dr. Navarese discloses receiving speaker and consultancy fees from Amgen, Sanofi-Regeneron, Bayer; and grants from Abbott. Disclosures for the other authors are in the report. Rosenson discloses receiving research funding to his institution from Amgen, Arrowhead, Eli Lilly, Novartis, and Regeneron; consulting fees from Amgen, Arrowhead, CRISPR Therapeutics, Eli Lilly, Lipigon, Novartis, Precision Biosciences, Regeneron, Ultragenyx, and Verve; speaking fees from Amgen, Kowa, and Regeneron; and royalties from Wolters Kluwer; and owning stock in MediMergent. Dr. Goonewardena reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
PCSK9 inhibitors may best be known for their powerful LDL-lowering effects but are less appreciated as anti-inflammatory agents with potential beyond cardiovascular health.
In a small pilot trial, for example, patients hospitalized with severe COVID-19 who received a single injection of PCSK9 inhibitor became less sick and more likely to survive than those given a placebo. Their 30-day risk of death or intubation fell significantly, as did their levels of the inflammatory cytokine interleukin 6 (IL-6).
Indeed, survival gains in the PCSK9-inhibitor group were greatest among patients with higher baseline concentrations of IL-6. Although the trial wasn’t powered for clinical outcomes, it suggests the drugs’ efficacy in COVID-19 tracks with intensity of inflammation, proposes a report published in the Journal of the American College of Cardiology.
Therefore, “PCSK9 inhibition may represent a novel therapeutic pathway in addition to currently recommended therapeutic approaches for severe COVID-19,” conclude the authors, led by Eliano P. Navarese, MD, PhD, Nicolaus Copernicus University, Bydgoszcz, Poland.
PCSK9 inhibitors as anti-inflammatories
Although the study was small and only hypothesis-generating, the fact that outcomes for actively treated patients were proportional to baseline IL-6 levels “strongly suggests that PCSK9 inhibition can directly modulate inflammation in COVID-19,” argues an editorial accompanying the report.
and likely sheds light on “mechanisms through which PCSK9 inhibition dually modulates lipoprotein metabolism and inflammation,” write Sascha N. Goonewardena, MD, University of Michigan, Ann Arbor, and Robert S. Rosenson, MD, Icahn School of Medicine at Mount Sinai, New York.
The results are consistent with prior evidence that the drugs are anti-inflammatory at least partly because of their interference with inflammatory pathways triggered by PCSK9 and mediated by IL-6, as described by Dr. Navarese and colleagues.
Indeed, they write, PCSK9 inhibitors may improve COVID outcomes mostly through mechanisms unrelated to LDL-receptor expression, “including direct inhibition of PCSK9-triggered inflammation.”
If true, the authors observe, it might explain “why the positive findings of the present study have not been consistently observed in trials involving other lipid-lowering agents, such as statins.” Those drugs are well-known to decrease levels of the inflammatory biomarker C-reactive protein.
In patients with stable coronary disease, in whom inflammation is typically tracked by measuring CRP, “the PCSK9 inhibitors have not been shown to have an anti-inflammatory effect,” Dr. Rosenson further explained.
But the current study’s patients with acute, severe COVID-19, a “profound inflammatory insult” with upregulation of IL-6, were “a good population” for evaluating the drugs’ potential anti-inflammatory effects, Dr. Rosenson said in an interview. The results “are quite enticing but require corroboration in a larger trial.”
A single injection
The IMPACT-SIRIO 5 trial entered 60 adults hospitalized with severe COVID-19 and elevated IL-6 at four centers in Poland. Patients with other known active infections were excluded.
They were randomly assigned double-blind to receive a 140 mg injection of evolocumab (Repatha) or placebo. The 2 groups were similar with respect to demographics, body-mass index, time since symptom onset, and treatments for managing COVID-19 and its complications.
Rates of death or need for intubation at 30 days, the primary endpoint, were 23.3% in the PCSK9-inhibitor group and 53.3% for controls, a risk difference of 30% (95% confidence interval –53.4% to –6.6%). The median durations of oxygen therapy were significantly different at 13 days and 20 days, respectively, the report states.
Serum IL-6 levels fell further over 30 days in the PCSK9-inhibitor group (–56% vs. –21% among controls). A drop by more than 90% was seen in 60% of patients in the PCSK9-inhibitor group and in 27% of controls.
The average hospital stay was shorter for those getting the PCSK9 inhibitor, compared with placebo, 16 days versus 22 days, and their 30-day mortality was numerically lower, 16% versus 33.3%.
Patients’ baseline IL-6 levels above the median, the report states, had a lower mortality on the PCSK9 inhibitor versus placebo (risk difference –37.5%; 95% CI –68.2% to –6.70%).
A larger trial to corroborate these results would potentially enter similar patients hospitalized with COVID-19 with reproducible evidence of an ongoing cytokine storm, such as elevated levels of IL-6, who would be assigned to either a PCSK9 inhibitor or placebo, Dr. Rosenson proposed.
Although the current primary endpoint that combines mortality and intubation was “reasonable” for a small pilot trial, he said, if the researchers embark on a larger study, “they’ll want to look at those events separately.”
Dr. Navarese discloses receiving speaker and consultancy fees from Amgen, Sanofi-Regeneron, Bayer; and grants from Abbott. Disclosures for the other authors are in the report. Rosenson discloses receiving research funding to his institution from Amgen, Arrowhead, Eli Lilly, Novartis, and Regeneron; consulting fees from Amgen, Arrowhead, CRISPR Therapeutics, Eli Lilly, Lipigon, Novartis, Precision Biosciences, Regeneron, Ultragenyx, and Verve; speaking fees from Amgen, Kowa, and Regeneron; and royalties from Wolters Kluwer; and owning stock in MediMergent. Dr. Goonewardena reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
PCSK9 inhibitors may best be known for their powerful LDL-lowering effects but are less appreciated as anti-inflammatory agents with potential beyond cardiovascular health.
In a small pilot trial, for example, patients hospitalized with severe COVID-19 who received a single injection of PCSK9 inhibitor became less sick and more likely to survive than those given a placebo. Their 30-day risk of death or intubation fell significantly, as did their levels of the inflammatory cytokine interleukin 6 (IL-6).
Indeed, survival gains in the PCSK9-inhibitor group were greatest among patients with higher baseline concentrations of IL-6. Although the trial wasn’t powered for clinical outcomes, it suggests the drugs’ efficacy in COVID-19 tracks with intensity of inflammation, proposes a report published in the Journal of the American College of Cardiology.
Therefore, “PCSK9 inhibition may represent a novel therapeutic pathway in addition to currently recommended therapeutic approaches for severe COVID-19,” conclude the authors, led by Eliano P. Navarese, MD, PhD, Nicolaus Copernicus University, Bydgoszcz, Poland.
PCSK9 inhibitors as anti-inflammatories
Although the study was small and only hypothesis-generating, the fact that outcomes for actively treated patients were proportional to baseline IL-6 levels “strongly suggests that PCSK9 inhibition can directly modulate inflammation in COVID-19,” argues an editorial accompanying the report.
and likely sheds light on “mechanisms through which PCSK9 inhibition dually modulates lipoprotein metabolism and inflammation,” write Sascha N. Goonewardena, MD, University of Michigan, Ann Arbor, and Robert S. Rosenson, MD, Icahn School of Medicine at Mount Sinai, New York.
The results are consistent with prior evidence that the drugs are anti-inflammatory at least partly because of their interference with inflammatory pathways triggered by PCSK9 and mediated by IL-6, as described by Dr. Navarese and colleagues.
Indeed, they write, PCSK9 inhibitors may improve COVID outcomes mostly through mechanisms unrelated to LDL-receptor expression, “including direct inhibition of PCSK9-triggered inflammation.”
If true, the authors observe, it might explain “why the positive findings of the present study have not been consistently observed in trials involving other lipid-lowering agents, such as statins.” Those drugs are well-known to decrease levels of the inflammatory biomarker C-reactive protein.
In patients with stable coronary disease, in whom inflammation is typically tracked by measuring CRP, “the PCSK9 inhibitors have not been shown to have an anti-inflammatory effect,” Dr. Rosenson further explained.
But the current study’s patients with acute, severe COVID-19, a “profound inflammatory insult” with upregulation of IL-6, were “a good population” for evaluating the drugs’ potential anti-inflammatory effects, Dr. Rosenson said in an interview. The results “are quite enticing but require corroboration in a larger trial.”
A single injection
The IMPACT-SIRIO 5 trial entered 60 adults hospitalized with severe COVID-19 and elevated IL-6 at four centers in Poland. Patients with other known active infections were excluded.
They were randomly assigned double-blind to receive a 140 mg injection of evolocumab (Repatha) or placebo. The 2 groups were similar with respect to demographics, body-mass index, time since symptom onset, and treatments for managing COVID-19 and its complications.
Rates of death or need for intubation at 30 days, the primary endpoint, were 23.3% in the PCSK9-inhibitor group and 53.3% for controls, a risk difference of 30% (95% confidence interval –53.4% to –6.6%). The median durations of oxygen therapy were significantly different at 13 days and 20 days, respectively, the report states.
Serum IL-6 levels fell further over 30 days in the PCSK9-inhibitor group (–56% vs. –21% among controls). A drop by more than 90% was seen in 60% of patients in the PCSK9-inhibitor group and in 27% of controls.
The average hospital stay was shorter for those getting the PCSK9 inhibitor, compared with placebo, 16 days versus 22 days, and their 30-day mortality was numerically lower, 16% versus 33.3%.
Patients’ baseline IL-6 levels above the median, the report states, had a lower mortality on the PCSK9 inhibitor versus placebo (risk difference –37.5%; 95% CI –68.2% to –6.70%).
A larger trial to corroborate these results would potentially enter similar patients hospitalized with COVID-19 with reproducible evidence of an ongoing cytokine storm, such as elevated levels of IL-6, who would be assigned to either a PCSK9 inhibitor or placebo, Dr. Rosenson proposed.
Although the current primary endpoint that combines mortality and intubation was “reasonable” for a small pilot trial, he said, if the researchers embark on a larger study, “they’ll want to look at those events separately.”
Dr. Navarese discloses receiving speaker and consultancy fees from Amgen, Sanofi-Regeneron, Bayer; and grants from Abbott. Disclosures for the other authors are in the report. Rosenson discloses receiving research funding to his institution from Amgen, Arrowhead, Eli Lilly, Novartis, and Regeneron; consulting fees from Amgen, Arrowhead, CRISPR Therapeutics, Eli Lilly, Lipigon, Novartis, Precision Biosciences, Regeneron, Ultragenyx, and Verve; speaking fees from Amgen, Kowa, and Regeneron; and royalties from Wolters Kluwer; and owning stock in MediMergent. Dr. Goonewardena reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Guidelines recommend CBT alone for mild acute depression, more options for more severe cases
The guidelines also state that patients with mild depression should start with CBT alone, and if a patient with moderate to severe depression prefers, they can use a combination of both CBT and an SGA.
These nuanced recommendations contrast sharply with the 2016 ACP guidelines for depression, which lumped all stages and severity levels together, and came with just one recommendation: Clinicians should choose between CBT and an SGA.
More data have come to light over the years, requiring the present update, reported lead author Amir Qaseem, MD, PhD, vice president of Clinical Policy and the Center for Evidence Reviews at the ACP, and adjunct faculty at Thomas Jefferson University, Philadelphia, and colleagues.
In addition to the focus on acute depression, Dr. Qaseem and colleagues highlighted the new guidelines' “consideration of patient values and preferences, and costs,” as well as responses to therapy.
Recommendations were derived from a network meta-analysis that included studies evaluating nonpharmacologic and pharmacologic therapies, the authors wrote in Annals of Internal Medicine. They compared effectiveness across a range of SGAs, “including selective serotonin reuptake inhibitors; serotonin-norepinephrine reuptake inhibitors; and others such as bupropion, mirtazapine, nefazodone, trazodone, vilazodone, and vortioxetine.”
This analysis yielded three pieces of clinical advice.
First, patients in the acute phase of mild depression should receive CBT alone as their initial treatment.
Dr. Qaseem and colleagues noted that many depression studies for pharmacologic therapies excluded these patients in favor of those with moderate to severe depression, leaving an evidence gap.
“Furthermore, the Clinical Guidelines Committee had concerns about adverse effects of SGAs in these patients and suggests that the use of SGAs as initial treatment of these patients should be based on additional considerations, such as limited access to or cost of CBT, history of moderate or severe major depressive disorder, or patient preferences,” they added.
The committee’s next recommendation, based on moderate-certainty evidence, suggested that CBT alone or an SGA alone should be considered for patients in the acute phase of moderate to severe depression. This call for monotherapy is balanced by a conditional recommendation based on low-certainty evidence that the same group may benefit from initial combination therapy with both CBT and an SGA.
“The informed decision on the options of monotherapy with CBT versus SGAs, or combination therapy, should be personalized and based on discussion of potential treatment benefits, harms, adverse effect profiles, cost, feasibility, patients’ specific symptoms (such as insomnia, hypersomnia, or fluctuation in appetite), comorbidities, concomitant medication use, and patient preferences,” the guidelines state.
The third and final recommendation offers an algorithm for patients who do not respond to initial therapy with an SGA. Multiple pathways are provided: Switch to CBT or augment with CBT; or switch to a different SGA or augment with a second pharmacologic therapy, such as mirtazapine, bupropion, or buspirone.
“These second-line treatment strategies show similar efficacy when compared with each other,” the guidelines committee noted.
Again, the guidelines suggest that second-line choices should be personalized based on the various factors previously discussed.
A timely update
“The new guideline is very different from the last guideline,” said Ryan Mire, MD, president of the ACP and practicing internal medicine physician in Nashville, Tenn. in a written comment. “ACP decided to update the depression guidelines with a focus on acute depression because approximately 70% of patients with major depressive disorder do not achieve remission and remain in the acute phase after the initial pharmacologic treatment attempt. In addition, there is new evidence on second-line treatments since the 2016 ACP guideline was published.”
Neil S. Skolnik, MD, of Thomas Jefferson University, Philadelphia, agreed that the guidelines offer a necessary and fresh perspective on caring for patients with depression.
“These guidelines are a helpful update, assuring us that we are using the latest, evidence-based therapies, and [they] are written in a practical, easy-to-implement manner,” Dr. Skolnik said in a written comment.
“First, the guidelines reaffirm that CBT is an effective first-line option, with or without the concurrent use of an SGA,” Dr. Skolnik said, noting that CBT alone may reduce likelihood of recurrence, compared with an SGA alone. “Many patients do not like the idea of medication, or the potential side effects of medications, and CBT is an evidenced-based approach that can be very helpful for patients.”
Dr. Skolnik also applauded the guidelines authors for offering a clear path forward for patients who do not have full remission after treatment – a common clinical scenario.
He went on to offer some more detailed steps forward.
“If someone chooses to be treated with an SGA alone and has not had much response at all to an initial SGA, usually a selective serotonin reuptake inhibitor, I’ll usually switch to a different SSRI or serotonin and norepinephrine reuptake inhibitor (SNRI) and/or add CBT,” Dr. Skolnik said. “If they have had a partial response, I’ll often encourage CBT and consider the addition of augmentation with an additional medication as discussed in the guidelines.”
Valuable despite the gaps
Other experts expressed mixed impressions of the update, noting both highs and lows.
“Although [this guideline] has some gaps, it is more valuable in several ways than other widely consulted practice guidelines for depression,” wrote Miriam Shuchman, MD and Elia Abi-Jaoude, MSc, MD, PhD, of the University of Toronto, in an accompanying editorial.
Specifically, they praised the publication’s focus on shared decision-making in the treatment planning process.
“This effort to respond to patient preferences is crucial and may even increase the chance that patients will improve with treatment,” they wrote.
They also applauded the ACP’s efforts to recuse any committee members who may have had conflicts of interest “that could affect their judgment about treatments for depression.”
After highlighting these attributes, Dr. Shuchman and Dr. Abi-Jaoude noted that the guidelines still contain “significant gaps.”
Foremost, they pointed out the guidelines' emphasis on CBT to the exclusion of other nonpharmacologic options.
“The guideline does patients a disservice by leaving out several nonmedication treatment options that clinicians can offer as first- or second-line therapies,” they wrote.
This oversight may increase risk that patients simply hop from one SGA to another, which is a common, and often ineffective, strategy, according to Dr. Shuchman and Dr. Abi-Jaoude.
“Patients often go from one drug to the next in the hopes of landing on one that ‘works,’ ” the editorialists wrote. “This narrow clinical approach of pursuing medication-based treatments ignores the ways difficulties in a person’s work or relationships may contribute to their struggles with depression. At a time when the COVID-19 pandemic has underscored the importance of the social context of mental health, clinicians may need to consider other forms of support and tailor prescribing to what is most relevant and accessible for a particular patient.”
Dr. Shuchman and Dr. Abi-Jaoude went on to suggest several nonpharmacologic options beyond CBT, including interpersonal therapy, psychodynamic therapy, problem solving, behavioral activation, and guided self-help.
The other key gap they pointed out relates to withdrawal.
Although the guideline does advise physicians to taper antidepressants to reduce risk of withdrawal, the editorialists suggested that this recommendation lacked sufficient emphasis, as it can be a particularly difficult period in the treatment process.
“Tapering of an antidepressant may need to be done over months or years, not weeks, and a patient may need to visit a compounding pharmacy to obtain doses of a second-generation antidepressant not marketed by drug manufacturers so that prescriptions can be tapered even more slowly,” they suggested.
Financial costs remain unclear
Beyond the above medical considerations, one other piece of the depression puzzle remains unsolved: cost.
In a simultaneously published rapid review, Andreea Dobrescu, MD, PhD, of Cochrane Austria, and colleagues evaluated the relative cost-effectiveness of first- and second-step treatment strategies.
For most comparisons, evidence was insufficient to reach a conclusion, although they suggested that CBT may be more cost effective at the 5-year mark.
“For most pharmacologic and nonpharmacologic interventions for major depressive disorder, evidence was missing or was insufficient to draw conclusions about the cost-effectiveness of first- or second-step treatments for MDD,” Dr. Dobrescu and colleagues wrote. “The strongest evidence (albeit still low certainty of evidence) was for the cost-effectiveness of CBT compared with SGA as a first-step treatment over a 5-year time horizon from the societal and health care sector perspectives. However, this evidence should also be interpreted cautiously considering it is based on a single study.”
When asked about the financial findings, Dr. Mire agreed that more data are needed, especially because CBT and SGA costs range widely. He suggested that cost, for each patient, should be considered in the personalized approach now highlighted by the new guidelines.
The guidelines and the Cochrane cost-effectiveness study were supported by the ACP. The guidelines' authors and other individuals quoted in this article reported no conflicts of interest.
The guidelines also state that patients with mild depression should start with CBT alone, and if a patient with moderate to severe depression prefers, they can use a combination of both CBT and an SGA.
These nuanced recommendations contrast sharply with the 2016 ACP guidelines for depression, which lumped all stages and severity levels together, and came with just one recommendation: Clinicians should choose between CBT and an SGA.
More data have come to light over the years, requiring the present update, reported lead author Amir Qaseem, MD, PhD, vice president of Clinical Policy and the Center for Evidence Reviews at the ACP, and adjunct faculty at Thomas Jefferson University, Philadelphia, and colleagues.
In addition to the focus on acute depression, Dr. Qaseem and colleagues highlighted the new guidelines' “consideration of patient values and preferences, and costs,” as well as responses to therapy.
Recommendations were derived from a network meta-analysis that included studies evaluating nonpharmacologic and pharmacologic therapies, the authors wrote in Annals of Internal Medicine. They compared effectiveness across a range of SGAs, “including selective serotonin reuptake inhibitors; serotonin-norepinephrine reuptake inhibitors; and others such as bupropion, mirtazapine, nefazodone, trazodone, vilazodone, and vortioxetine.”
This analysis yielded three pieces of clinical advice.
First, patients in the acute phase of mild depression should receive CBT alone as their initial treatment.
Dr. Qaseem and colleagues noted that many depression studies for pharmacologic therapies excluded these patients in favor of those with moderate to severe depression, leaving an evidence gap.
“Furthermore, the Clinical Guidelines Committee had concerns about adverse effects of SGAs in these patients and suggests that the use of SGAs as initial treatment of these patients should be based on additional considerations, such as limited access to or cost of CBT, history of moderate or severe major depressive disorder, or patient preferences,” they added.
The committee’s next recommendation, based on moderate-certainty evidence, suggested that CBT alone or an SGA alone should be considered for patients in the acute phase of moderate to severe depression. This call for monotherapy is balanced by a conditional recommendation based on low-certainty evidence that the same group may benefit from initial combination therapy with both CBT and an SGA.
“The informed decision on the options of monotherapy with CBT versus SGAs, or combination therapy, should be personalized and based on discussion of potential treatment benefits, harms, adverse effect profiles, cost, feasibility, patients’ specific symptoms (such as insomnia, hypersomnia, or fluctuation in appetite), comorbidities, concomitant medication use, and patient preferences,” the guidelines state.
The third and final recommendation offers an algorithm for patients who do not respond to initial therapy with an SGA. Multiple pathways are provided: Switch to CBT or augment with CBT; or switch to a different SGA or augment with a second pharmacologic therapy, such as mirtazapine, bupropion, or buspirone.
“These second-line treatment strategies show similar efficacy when compared with each other,” the guidelines committee noted.
Again, the guidelines suggest that second-line choices should be personalized based on the various factors previously discussed.
A timely update
“The new guideline is very different from the last guideline,” said Ryan Mire, MD, president of the ACP and practicing internal medicine physician in Nashville, Tenn. in a written comment. “ACP decided to update the depression guidelines with a focus on acute depression because approximately 70% of patients with major depressive disorder do not achieve remission and remain in the acute phase after the initial pharmacologic treatment attempt. In addition, there is new evidence on second-line treatments since the 2016 ACP guideline was published.”
Neil S. Skolnik, MD, of Thomas Jefferson University, Philadelphia, agreed that the guidelines offer a necessary and fresh perspective on caring for patients with depression.
“These guidelines are a helpful update, assuring us that we are using the latest, evidence-based therapies, and [they] are written in a practical, easy-to-implement manner,” Dr. Skolnik said in a written comment.
“First, the guidelines reaffirm that CBT is an effective first-line option, with or without the concurrent use of an SGA,” Dr. Skolnik said, noting that CBT alone may reduce likelihood of recurrence, compared with an SGA alone. “Many patients do not like the idea of medication, or the potential side effects of medications, and CBT is an evidenced-based approach that can be very helpful for patients.”
Dr. Skolnik also applauded the guidelines authors for offering a clear path forward for patients who do not have full remission after treatment – a common clinical scenario.
He went on to offer some more detailed steps forward.
“If someone chooses to be treated with an SGA alone and has not had much response at all to an initial SGA, usually a selective serotonin reuptake inhibitor, I’ll usually switch to a different SSRI or serotonin and norepinephrine reuptake inhibitor (SNRI) and/or add CBT,” Dr. Skolnik said. “If they have had a partial response, I’ll often encourage CBT and consider the addition of augmentation with an additional medication as discussed in the guidelines.”
Valuable despite the gaps
Other experts expressed mixed impressions of the update, noting both highs and lows.
“Although [this guideline] has some gaps, it is more valuable in several ways than other widely consulted practice guidelines for depression,” wrote Miriam Shuchman, MD and Elia Abi-Jaoude, MSc, MD, PhD, of the University of Toronto, in an accompanying editorial.
Specifically, they praised the publication’s focus on shared decision-making in the treatment planning process.
“This effort to respond to patient preferences is crucial and may even increase the chance that patients will improve with treatment,” they wrote.
They also applauded the ACP’s efforts to recuse any committee members who may have had conflicts of interest “that could affect their judgment about treatments for depression.”
After highlighting these attributes, Dr. Shuchman and Dr. Abi-Jaoude noted that the guidelines still contain “significant gaps.”
Foremost, they pointed out the guidelines' emphasis on CBT to the exclusion of other nonpharmacologic options.
“The guideline does patients a disservice by leaving out several nonmedication treatment options that clinicians can offer as first- or second-line therapies,” they wrote.
This oversight may increase risk that patients simply hop from one SGA to another, which is a common, and often ineffective, strategy, according to Dr. Shuchman and Dr. Abi-Jaoude.
“Patients often go from one drug to the next in the hopes of landing on one that ‘works,’ ” the editorialists wrote. “This narrow clinical approach of pursuing medication-based treatments ignores the ways difficulties in a person’s work or relationships may contribute to their struggles with depression. At a time when the COVID-19 pandemic has underscored the importance of the social context of mental health, clinicians may need to consider other forms of support and tailor prescribing to what is most relevant and accessible for a particular patient.”
Dr. Shuchman and Dr. Abi-Jaoude went on to suggest several nonpharmacologic options beyond CBT, including interpersonal therapy, psychodynamic therapy, problem solving, behavioral activation, and guided self-help.
The other key gap they pointed out relates to withdrawal.
Although the guideline does advise physicians to taper antidepressants to reduce risk of withdrawal, the editorialists suggested that this recommendation lacked sufficient emphasis, as it can be a particularly difficult period in the treatment process.
“Tapering of an antidepressant may need to be done over months or years, not weeks, and a patient may need to visit a compounding pharmacy to obtain doses of a second-generation antidepressant not marketed by drug manufacturers so that prescriptions can be tapered even more slowly,” they suggested.
Financial costs remain unclear
Beyond the above medical considerations, one other piece of the depression puzzle remains unsolved: cost.
In a simultaneously published rapid review, Andreea Dobrescu, MD, PhD, of Cochrane Austria, and colleagues evaluated the relative cost-effectiveness of first- and second-step treatment strategies.
For most comparisons, evidence was insufficient to reach a conclusion, although they suggested that CBT may be more cost effective at the 5-year mark.
“For most pharmacologic and nonpharmacologic interventions for major depressive disorder, evidence was missing or was insufficient to draw conclusions about the cost-effectiveness of first- or second-step treatments for MDD,” Dr. Dobrescu and colleagues wrote. “The strongest evidence (albeit still low certainty of evidence) was for the cost-effectiveness of CBT compared with SGA as a first-step treatment over a 5-year time horizon from the societal and health care sector perspectives. However, this evidence should also be interpreted cautiously considering it is based on a single study.”
When asked about the financial findings, Dr. Mire agreed that more data are needed, especially because CBT and SGA costs range widely. He suggested that cost, for each patient, should be considered in the personalized approach now highlighted by the new guidelines.
The guidelines and the Cochrane cost-effectiveness study were supported by the ACP. The guidelines' authors and other individuals quoted in this article reported no conflicts of interest.
The guidelines also state that patients with mild depression should start with CBT alone, and if a patient with moderate to severe depression prefers, they can use a combination of both CBT and an SGA.
These nuanced recommendations contrast sharply with the 2016 ACP guidelines for depression, which lumped all stages and severity levels together, and came with just one recommendation: Clinicians should choose between CBT and an SGA.
More data have come to light over the years, requiring the present update, reported lead author Amir Qaseem, MD, PhD, vice president of Clinical Policy and the Center for Evidence Reviews at the ACP, and adjunct faculty at Thomas Jefferson University, Philadelphia, and colleagues.
In addition to the focus on acute depression, Dr. Qaseem and colleagues highlighted the new guidelines' “consideration of patient values and preferences, and costs,” as well as responses to therapy.
Recommendations were derived from a network meta-analysis that included studies evaluating nonpharmacologic and pharmacologic therapies, the authors wrote in Annals of Internal Medicine. They compared effectiveness across a range of SGAs, “including selective serotonin reuptake inhibitors; serotonin-norepinephrine reuptake inhibitors; and others such as bupropion, mirtazapine, nefazodone, trazodone, vilazodone, and vortioxetine.”
This analysis yielded three pieces of clinical advice.
First, patients in the acute phase of mild depression should receive CBT alone as their initial treatment.
Dr. Qaseem and colleagues noted that many depression studies for pharmacologic therapies excluded these patients in favor of those with moderate to severe depression, leaving an evidence gap.
“Furthermore, the Clinical Guidelines Committee had concerns about adverse effects of SGAs in these patients and suggests that the use of SGAs as initial treatment of these patients should be based on additional considerations, such as limited access to or cost of CBT, history of moderate or severe major depressive disorder, or patient preferences,” they added.
The committee’s next recommendation, based on moderate-certainty evidence, suggested that CBT alone or an SGA alone should be considered for patients in the acute phase of moderate to severe depression. This call for monotherapy is balanced by a conditional recommendation based on low-certainty evidence that the same group may benefit from initial combination therapy with both CBT and an SGA.
“The informed decision on the options of monotherapy with CBT versus SGAs, or combination therapy, should be personalized and based on discussion of potential treatment benefits, harms, adverse effect profiles, cost, feasibility, patients’ specific symptoms (such as insomnia, hypersomnia, or fluctuation in appetite), comorbidities, concomitant medication use, and patient preferences,” the guidelines state.
The third and final recommendation offers an algorithm for patients who do not respond to initial therapy with an SGA. Multiple pathways are provided: Switch to CBT or augment with CBT; or switch to a different SGA or augment with a second pharmacologic therapy, such as mirtazapine, bupropion, or buspirone.
“These second-line treatment strategies show similar efficacy when compared with each other,” the guidelines committee noted.
Again, the guidelines suggest that second-line choices should be personalized based on the various factors previously discussed.
A timely update
“The new guideline is very different from the last guideline,” said Ryan Mire, MD, president of the ACP and practicing internal medicine physician in Nashville, Tenn. in a written comment. “ACP decided to update the depression guidelines with a focus on acute depression because approximately 70% of patients with major depressive disorder do not achieve remission and remain in the acute phase after the initial pharmacologic treatment attempt. In addition, there is new evidence on second-line treatments since the 2016 ACP guideline was published.”
Neil S. Skolnik, MD, of Thomas Jefferson University, Philadelphia, agreed that the guidelines offer a necessary and fresh perspective on caring for patients with depression.
“These guidelines are a helpful update, assuring us that we are using the latest, evidence-based therapies, and [they] are written in a practical, easy-to-implement manner,” Dr. Skolnik said in a written comment.
“First, the guidelines reaffirm that CBT is an effective first-line option, with or without the concurrent use of an SGA,” Dr. Skolnik said, noting that CBT alone may reduce likelihood of recurrence, compared with an SGA alone. “Many patients do not like the idea of medication, or the potential side effects of medications, and CBT is an evidenced-based approach that can be very helpful for patients.”
Dr. Skolnik also applauded the guidelines authors for offering a clear path forward for patients who do not have full remission after treatment – a common clinical scenario.
He went on to offer some more detailed steps forward.
“If someone chooses to be treated with an SGA alone and has not had much response at all to an initial SGA, usually a selective serotonin reuptake inhibitor, I’ll usually switch to a different SSRI or serotonin and norepinephrine reuptake inhibitor (SNRI) and/or add CBT,” Dr. Skolnik said. “If they have had a partial response, I’ll often encourage CBT and consider the addition of augmentation with an additional medication as discussed in the guidelines.”
Valuable despite the gaps
Other experts expressed mixed impressions of the update, noting both highs and lows.
“Although [this guideline] has some gaps, it is more valuable in several ways than other widely consulted practice guidelines for depression,” wrote Miriam Shuchman, MD and Elia Abi-Jaoude, MSc, MD, PhD, of the University of Toronto, in an accompanying editorial.
Specifically, they praised the publication’s focus on shared decision-making in the treatment planning process.
“This effort to respond to patient preferences is crucial and may even increase the chance that patients will improve with treatment,” they wrote.
They also applauded the ACP’s efforts to recuse any committee members who may have had conflicts of interest “that could affect their judgment about treatments for depression.”
After highlighting these attributes, Dr. Shuchman and Dr. Abi-Jaoude noted that the guidelines still contain “significant gaps.”
Foremost, they pointed out the guidelines' emphasis on CBT to the exclusion of other nonpharmacologic options.
“The guideline does patients a disservice by leaving out several nonmedication treatment options that clinicians can offer as first- or second-line therapies,” they wrote.
This oversight may increase risk that patients simply hop from one SGA to another, which is a common, and often ineffective, strategy, according to Dr. Shuchman and Dr. Abi-Jaoude.
“Patients often go from one drug to the next in the hopes of landing on one that ‘works,’ ” the editorialists wrote. “This narrow clinical approach of pursuing medication-based treatments ignores the ways difficulties in a person’s work or relationships may contribute to their struggles with depression. At a time when the COVID-19 pandemic has underscored the importance of the social context of mental health, clinicians may need to consider other forms of support and tailor prescribing to what is most relevant and accessible for a particular patient.”
Dr. Shuchman and Dr. Abi-Jaoude went on to suggest several nonpharmacologic options beyond CBT, including interpersonal therapy, psychodynamic therapy, problem solving, behavioral activation, and guided self-help.
The other key gap they pointed out relates to withdrawal.
Although the guideline does advise physicians to taper antidepressants to reduce risk of withdrawal, the editorialists suggested that this recommendation lacked sufficient emphasis, as it can be a particularly difficult period in the treatment process.
“Tapering of an antidepressant may need to be done over months or years, not weeks, and a patient may need to visit a compounding pharmacy to obtain doses of a second-generation antidepressant not marketed by drug manufacturers so that prescriptions can be tapered even more slowly,” they suggested.
Financial costs remain unclear
Beyond the above medical considerations, one other piece of the depression puzzle remains unsolved: cost.
In a simultaneously published rapid review, Andreea Dobrescu, MD, PhD, of Cochrane Austria, and colleagues evaluated the relative cost-effectiveness of first- and second-step treatment strategies.
For most comparisons, evidence was insufficient to reach a conclusion, although they suggested that CBT may be more cost effective at the 5-year mark.
“For most pharmacologic and nonpharmacologic interventions for major depressive disorder, evidence was missing or was insufficient to draw conclusions about the cost-effectiveness of first- or second-step treatments for MDD,” Dr. Dobrescu and colleagues wrote. “The strongest evidence (albeit still low certainty of evidence) was for the cost-effectiveness of CBT compared with SGA as a first-step treatment over a 5-year time horizon from the societal and health care sector perspectives. However, this evidence should also be interpreted cautiously considering it is based on a single study.”
When asked about the financial findings, Dr. Mire agreed that more data are needed, especially because CBT and SGA costs range widely. He suggested that cost, for each patient, should be considered in the personalized approach now highlighted by the new guidelines.
The guidelines and the Cochrane cost-effectiveness study were supported by the ACP. The guidelines' authors and other individuals quoted in this article reported no conflicts of interest.
FROM ANNALS OF INTERNAL MEDICINE
AHA scientific statement on rapid evaluation for suspected TIA
TIAs are “warning shots” of a future stroke and require emergency evaluation, Hardik Amin, MD, chair of the writing committee and medical stroke director, Yale New Haven (Conn.) Hospital, said in an AHA podcast.
A key aim of the scientific statement is to help clinicians properly risk-stratify patients with suspected TIA and determine which patients need to be admitted to the hospital and which patients might be safely discharged as long as proper and prompt follow-up has been arranged, Dr. Amin explained.
The statement, published online in the journal Stroke, addresses “how we can identify and be confident in diagnosing a TIA patient and what might suggest an alternative diagnosis,” he added.
Diagnostic challenge
It’s estimated that nearly one in five people who suffer a TIA will have a full-blown stroke within 3 months; close to half of these strokes will happen within 2 days.
The challenge with TIAs is that they can be tough to diagnose because many patients no longer have symptoms when they arrive at the emergency department. There is also no confirmatory test. Limited resources and access to stroke specialists in rural centers may exacerbate these challenges, the authors noted.
The statement pointed out that the F.A.S.T. acronym for stroke symptoms (Face drooping, Arm weakness, Speech difficulty, Time to call 911) can also be used to identify a TIA – even if the symptoms resolve.
The statement also provided guidance on how to tell the difference between a TIA and a TIA mimic.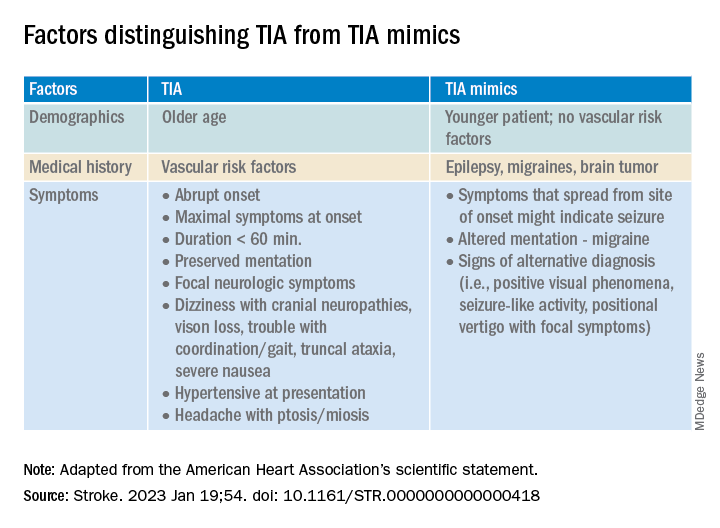
If available, a noncontrast head CT (NCCT) scan should be done initially in the emergency department to evaluate for subacute ischemia, hemorrhage, or mass lesion. Although the sensitivity of NCCT to detect an acute infarct is low, NCCT is useful for ruling out TIA mimics, the writing group said.
Multimodal brain MRI is the “preferred” method to evaluate for acute ischemic infarct and ideally should be obtained within 24 hours of symptom onset, and in most centers will follow an NCCT.
“When MRI cannot be obtained acutely to definitively distinguish TIA from stroke, it remains reasonable to make a clinical diagnosis of TIA in the ED on the basis of a negative NCCT and symptom resolution within 24 hours,” the authors said.
“A potential next step would be hospital admission for MRI, comprehensive workup, and neurology consultation. Other options might include transferring patients to a facility with advanced imaging and vascular neurology expertise or arranging a timely (ideally < 24 hours) outpatient MRI,” they advised.
The statement also provides guidance on the advantages, limitations, and considerations of Doppler ultrasonography, CT angiography, and magnetic resonance angiography for TIA assessment.
Once TIA is diagnosed, a cardiac work-up is advised because of the potential for heart-related factors to cause a TIA.
An individual’s risk of future stroke after TIA can be rapidly assessed using the ABCD2 score, which stratifies patients into low, medium, and high risk based on age, blood pressure, clinical features, duration of symptoms, and diabetes.
“It is up to each center to use the resources available and create a pathway to ensure successful management and disposition of patients with TIA, with the ultimate goal of reducing the risk of future stroke,” the authors concluded.
This scientific statement was prepared by the volunteer writing group on behalf of the American Heart Association’s Emergency Neurovascular Care Committee of the Stroke Council and the Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists, and it is endorsed by the American Association of Neurological Surgeons/Congress of Neurological Surgeons.
A version of this article first appeared on Medscape.com.
TIAs are “warning shots” of a future stroke and require emergency evaluation, Hardik Amin, MD, chair of the writing committee and medical stroke director, Yale New Haven (Conn.) Hospital, said in an AHA podcast.
A key aim of the scientific statement is to help clinicians properly risk-stratify patients with suspected TIA and determine which patients need to be admitted to the hospital and which patients might be safely discharged as long as proper and prompt follow-up has been arranged, Dr. Amin explained.
The statement, published online in the journal Stroke, addresses “how we can identify and be confident in diagnosing a TIA patient and what might suggest an alternative diagnosis,” he added.
Diagnostic challenge
It’s estimated that nearly one in five people who suffer a TIA will have a full-blown stroke within 3 months; close to half of these strokes will happen within 2 days.
The challenge with TIAs is that they can be tough to diagnose because many patients no longer have symptoms when they arrive at the emergency department. There is also no confirmatory test. Limited resources and access to stroke specialists in rural centers may exacerbate these challenges, the authors noted.
The statement pointed out that the F.A.S.T. acronym for stroke symptoms (Face drooping, Arm weakness, Speech difficulty, Time to call 911) can also be used to identify a TIA – even if the symptoms resolve.
The statement also provided guidance on how to tell the difference between a TIA and a TIA mimic.
If available, a noncontrast head CT (NCCT) scan should be done initially in the emergency department to evaluate for subacute ischemia, hemorrhage, or mass lesion. Although the sensitivity of NCCT to detect an acute infarct is low, NCCT is useful for ruling out TIA mimics, the writing group said.
Multimodal brain MRI is the “preferred” method to evaluate for acute ischemic infarct and ideally should be obtained within 24 hours of symptom onset, and in most centers will follow an NCCT.
“When MRI cannot be obtained acutely to definitively distinguish TIA from stroke, it remains reasonable to make a clinical diagnosis of TIA in the ED on the basis of a negative NCCT and symptom resolution within 24 hours,” the authors said.
“A potential next step would be hospital admission for MRI, comprehensive workup, and neurology consultation. Other options might include transferring patients to a facility with advanced imaging and vascular neurology expertise or arranging a timely (ideally < 24 hours) outpatient MRI,” they advised.
The statement also provides guidance on the advantages, limitations, and considerations of Doppler ultrasonography, CT angiography, and magnetic resonance angiography for TIA assessment.
Once TIA is diagnosed, a cardiac work-up is advised because of the potential for heart-related factors to cause a TIA.
An individual’s risk of future stroke after TIA can be rapidly assessed using the ABCD2 score, which stratifies patients into low, medium, and high risk based on age, blood pressure, clinical features, duration of symptoms, and diabetes.
“It is up to each center to use the resources available and create a pathway to ensure successful management and disposition of patients with TIA, with the ultimate goal of reducing the risk of future stroke,” the authors concluded.
This scientific statement was prepared by the volunteer writing group on behalf of the American Heart Association’s Emergency Neurovascular Care Committee of the Stroke Council and the Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists, and it is endorsed by the American Association of Neurological Surgeons/Congress of Neurological Surgeons.
A version of this article first appeared on Medscape.com.
TIAs are “warning shots” of a future stroke and require emergency evaluation, Hardik Amin, MD, chair of the writing committee and medical stroke director, Yale New Haven (Conn.) Hospital, said in an AHA podcast.
A key aim of the scientific statement is to help clinicians properly risk-stratify patients with suspected TIA and determine which patients need to be admitted to the hospital and which patients might be safely discharged as long as proper and prompt follow-up has been arranged, Dr. Amin explained.
The statement, published online in the journal Stroke, addresses “how we can identify and be confident in diagnosing a TIA patient and what might suggest an alternative diagnosis,” he added.
Diagnostic challenge
It’s estimated that nearly one in five people who suffer a TIA will have a full-blown stroke within 3 months; close to half of these strokes will happen within 2 days.
The challenge with TIAs is that they can be tough to diagnose because many patients no longer have symptoms when they arrive at the emergency department. There is also no confirmatory test. Limited resources and access to stroke specialists in rural centers may exacerbate these challenges, the authors noted.
The statement pointed out that the F.A.S.T. acronym for stroke symptoms (Face drooping, Arm weakness, Speech difficulty, Time to call 911) can also be used to identify a TIA – even if the symptoms resolve.
The statement also provided guidance on how to tell the difference between a TIA and a TIA mimic.
If available, a noncontrast head CT (NCCT) scan should be done initially in the emergency department to evaluate for subacute ischemia, hemorrhage, or mass lesion. Although the sensitivity of NCCT to detect an acute infarct is low, NCCT is useful for ruling out TIA mimics, the writing group said.
Multimodal brain MRI is the “preferred” method to evaluate for acute ischemic infarct and ideally should be obtained within 24 hours of symptom onset, and in most centers will follow an NCCT.
“When MRI cannot be obtained acutely to definitively distinguish TIA from stroke, it remains reasonable to make a clinical diagnosis of TIA in the ED on the basis of a negative NCCT and symptom resolution within 24 hours,” the authors said.
“A potential next step would be hospital admission for MRI, comprehensive workup, and neurology consultation. Other options might include transferring patients to a facility with advanced imaging and vascular neurology expertise or arranging a timely (ideally < 24 hours) outpatient MRI,” they advised.
The statement also provides guidance on the advantages, limitations, and considerations of Doppler ultrasonography, CT angiography, and magnetic resonance angiography for TIA assessment.
Once TIA is diagnosed, a cardiac work-up is advised because of the potential for heart-related factors to cause a TIA.
An individual’s risk of future stroke after TIA can be rapidly assessed using the ABCD2 score, which stratifies patients into low, medium, and high risk based on age, blood pressure, clinical features, duration of symptoms, and diabetes.
“It is up to each center to use the resources available and create a pathway to ensure successful management and disposition of patients with TIA, with the ultimate goal of reducing the risk of future stroke,” the authors concluded.
This scientific statement was prepared by the volunteer writing group on behalf of the American Heart Association’s Emergency Neurovascular Care Committee of the Stroke Council and the Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists, and it is endorsed by the American Association of Neurological Surgeons/Congress of Neurological Surgeons.
A version of this article first appeared on Medscape.com.
FROM STROKE
Doctors’ happiness has not rebounded as pandemic drags on
Physicians reported similar levels of unhappiness in 2022 too.
Fewer than half of physicians said they were currently somewhat or very happy at work, compared with 75% of physicians who said they were somewhat or very happy at work in a previous survey conducted before the pandemic, the new Medscape Physician Lifestyle & Happiness Report 2023 shows.*
“I am not surprised that we’re less happy now,” said Amaryllis Sánchez, MD, a board-certified family medicine physician and a certified physician coach.
“I speak to physicians around the country and I hear that their workplaces are understaffed, they’re overworked and they don’t feel safe. Although we’re in a different phase of the pandemic, doctors feel that the ground beneath them is still shaky,” said Dr. Sánchez, the author of “Recapturing Joy in Medicine.”
Most doctors are seeing more patients than they can handle and are expected to do that consistently. “When you no longer have the capacity to give of yourself, that becomes a nearly impossible task,” said Dr. Sánchez.
Also, physicians in understaffed workplaces often must take on additional work such as administrative or nursing duties, said Katie Cole, DO, a board-certified psychiatrist and a physician coach.
While health systems are aware that physicians need time to rest and recharge, staffing shortages prevent doctors from taking time off because they can’t find coverage, said Dr. Cole.
“While we know that it’s important for physicians to take vacations, more than one-third of doctors still take 2 weeks or less of vacation annually,” said Dr. Cole.
Physicians also tend to have less compassion for themselves and sacrifice self-care compared to other health care workers. “When a patient dies, nurses get together, debrief, and hug each other, whereas doctors have another patient to see. The culture of medicine doesn’t support self-compassion for physicians,” said Dr. Cole.
Physicians also felt less safe at work during the pandemic because of to shortages of personal protective equipment, said Dr. Sánchez. They have also witnessed or experienced an increase in abusive behavior, violence and threats of violence.
Physicians’ personal life suffers
Doctors maintain their mental health primarily by spending time with family members and friends, according to 2022’s Medscape Physician Lifestyle & Happiness Report. Yet half of doctors reported in a survey by the Physicians Foundation that they withdrew from family, friends or coworkers in 2022, said Dr. Sánchez.
“When you exceed your mental, emotional, and physical capacity at work, you have no reserve left for your personal life,” said Dr. Cole.
That may explain why only 58% of doctors reported feeling somewhat or very happy outside of work, compared with 84% who felt that way before the pandemic.
More women doctors said they deal with stronger feelings of conflict in trying to balance parenting responsibilities with a highly demanding job. Nearly one in two women physician-parents reported feeling very conflicted at work, compared with about one in four male physician-parents.
When physicians go home, they may be emotionally drained and tired mentally from making a lot of decisions at work, said Dr. Cole.
“As a woman, if you have children and a husband and you’re responsible for dinner, picking up the kids at daycare or helping them with homework, and making all these decisions when you get home, it’s overwhelming,” said Dr. Cole.
Prioritize your well-being
Doctors need to prioritize their own well-being, said Dr. Sánchez. “That’s not being selfish, that’s doing what’s necessary to stay well and be able to take care of patients. If doctors don’t take care of themselves, no one else will.”
Dr. Sánchez recommended that doctors regularly interact with relatives, friends, trusted colleagues, or clergy to help maintain their well-being, rather than waiting until a crisis to reach out.
A good coach, mentor, or counselor can help physicians gain enough self-awareness to handle their emotions and gain more clarity about what changes need to be made, she said.
Dr. Cole suggested that doctors figure out what makes them happy and fulfilled at work and try to spend more time on that activity. “Knowing what makes you happy and your strengths are foundational for creating a life you love.”
She urged doctors to “start thinking now about what you love about medicine and what is going right at home, and what areas you want to change. Then, start advocating for your needs.”
A version of this article originally appeared on Medscape.com.
Correction, 1/26/23: An earlier version of this article misstated the findings of the survey.
Physicians reported similar levels of unhappiness in 2022 too.
Fewer than half of physicians said they were currently somewhat or very happy at work, compared with 75% of physicians who said they were somewhat or very happy at work in a previous survey conducted before the pandemic, the new Medscape Physician Lifestyle & Happiness Report 2023 shows.*
“I am not surprised that we’re less happy now,” said Amaryllis Sánchez, MD, a board-certified family medicine physician and a certified physician coach.
“I speak to physicians around the country and I hear that their workplaces are understaffed, they’re overworked and they don’t feel safe. Although we’re in a different phase of the pandemic, doctors feel that the ground beneath them is still shaky,” said Dr. Sánchez, the author of “Recapturing Joy in Medicine.”
Most doctors are seeing more patients than they can handle and are expected to do that consistently. “When you no longer have the capacity to give of yourself, that becomes a nearly impossible task,” said Dr. Sánchez.
Also, physicians in understaffed workplaces often must take on additional work such as administrative or nursing duties, said Katie Cole, DO, a board-certified psychiatrist and a physician coach.
While health systems are aware that physicians need time to rest and recharge, staffing shortages prevent doctors from taking time off because they can’t find coverage, said Dr. Cole.
“While we know that it’s important for physicians to take vacations, more than one-third of doctors still take 2 weeks or less of vacation annually,” said Dr. Cole.
Physicians also tend to have less compassion for themselves and sacrifice self-care compared to other health care workers. “When a patient dies, nurses get together, debrief, and hug each other, whereas doctors have another patient to see. The culture of medicine doesn’t support self-compassion for physicians,” said Dr. Cole.
Physicians also felt less safe at work during the pandemic because of to shortages of personal protective equipment, said Dr. Sánchez. They have also witnessed or experienced an increase in abusive behavior, violence and threats of violence.
Physicians’ personal life suffers
Doctors maintain their mental health primarily by spending time with family members and friends, according to 2022’s Medscape Physician Lifestyle & Happiness Report. Yet half of doctors reported in a survey by the Physicians Foundation that they withdrew from family, friends or coworkers in 2022, said Dr. Sánchez.
“When you exceed your mental, emotional, and physical capacity at work, you have no reserve left for your personal life,” said Dr. Cole.
That may explain why only 58% of doctors reported feeling somewhat or very happy outside of work, compared with 84% who felt that way before the pandemic.
More women doctors said they deal with stronger feelings of conflict in trying to balance parenting responsibilities with a highly demanding job. Nearly one in two women physician-parents reported feeling very conflicted at work, compared with about one in four male physician-parents.
When physicians go home, they may be emotionally drained and tired mentally from making a lot of decisions at work, said Dr. Cole.
“As a woman, if you have children and a husband and you’re responsible for dinner, picking up the kids at daycare or helping them with homework, and making all these decisions when you get home, it’s overwhelming,” said Dr. Cole.
Prioritize your well-being
Doctors need to prioritize their own well-being, said Dr. Sánchez. “That’s not being selfish, that’s doing what’s necessary to stay well and be able to take care of patients. If doctors don’t take care of themselves, no one else will.”
Dr. Sánchez recommended that doctors regularly interact with relatives, friends, trusted colleagues, or clergy to help maintain their well-being, rather than waiting until a crisis to reach out.
A good coach, mentor, or counselor can help physicians gain enough self-awareness to handle their emotions and gain more clarity about what changes need to be made, she said.
Dr. Cole suggested that doctors figure out what makes them happy and fulfilled at work and try to spend more time on that activity. “Knowing what makes you happy and your strengths are foundational for creating a life you love.”
She urged doctors to “start thinking now about what you love about medicine and what is going right at home, and what areas you want to change. Then, start advocating for your needs.”
A version of this article originally appeared on Medscape.com.
Correction, 1/26/23: An earlier version of this article misstated the findings of the survey.
Physicians reported similar levels of unhappiness in 2022 too.
Fewer than half of physicians said they were currently somewhat or very happy at work, compared with 75% of physicians who said they were somewhat or very happy at work in a previous survey conducted before the pandemic, the new Medscape Physician Lifestyle & Happiness Report 2023 shows.*
“I am not surprised that we’re less happy now,” said Amaryllis Sánchez, MD, a board-certified family medicine physician and a certified physician coach.
“I speak to physicians around the country and I hear that their workplaces are understaffed, they’re overworked and they don’t feel safe. Although we’re in a different phase of the pandemic, doctors feel that the ground beneath them is still shaky,” said Dr. Sánchez, the author of “Recapturing Joy in Medicine.”
Most doctors are seeing more patients than they can handle and are expected to do that consistently. “When you no longer have the capacity to give of yourself, that becomes a nearly impossible task,” said Dr. Sánchez.
Also, physicians in understaffed workplaces often must take on additional work such as administrative or nursing duties, said Katie Cole, DO, a board-certified psychiatrist and a physician coach.
While health systems are aware that physicians need time to rest and recharge, staffing shortages prevent doctors from taking time off because they can’t find coverage, said Dr. Cole.
“While we know that it’s important for physicians to take vacations, more than one-third of doctors still take 2 weeks or less of vacation annually,” said Dr. Cole.
Physicians also tend to have less compassion for themselves and sacrifice self-care compared to other health care workers. “When a patient dies, nurses get together, debrief, and hug each other, whereas doctors have another patient to see. The culture of medicine doesn’t support self-compassion for physicians,” said Dr. Cole.
Physicians also felt less safe at work during the pandemic because of to shortages of personal protective equipment, said Dr. Sánchez. They have also witnessed or experienced an increase in abusive behavior, violence and threats of violence.
Physicians’ personal life suffers
Doctors maintain their mental health primarily by spending time with family members and friends, according to 2022’s Medscape Physician Lifestyle & Happiness Report. Yet half of doctors reported in a survey by the Physicians Foundation that they withdrew from family, friends or coworkers in 2022, said Dr. Sánchez.
“When you exceed your mental, emotional, and physical capacity at work, you have no reserve left for your personal life,” said Dr. Cole.
That may explain why only 58% of doctors reported feeling somewhat or very happy outside of work, compared with 84% who felt that way before the pandemic.
More women doctors said they deal with stronger feelings of conflict in trying to balance parenting responsibilities with a highly demanding job. Nearly one in two women physician-parents reported feeling very conflicted at work, compared with about one in four male physician-parents.
When physicians go home, they may be emotionally drained and tired mentally from making a lot of decisions at work, said Dr. Cole.
“As a woman, if you have children and a husband and you’re responsible for dinner, picking up the kids at daycare or helping them with homework, and making all these decisions when you get home, it’s overwhelming,” said Dr. Cole.
Prioritize your well-being
Doctors need to prioritize their own well-being, said Dr. Sánchez. “That’s not being selfish, that’s doing what’s necessary to stay well and be able to take care of patients. If doctors don’t take care of themselves, no one else will.”
Dr. Sánchez recommended that doctors regularly interact with relatives, friends, trusted colleagues, or clergy to help maintain their well-being, rather than waiting until a crisis to reach out.
A good coach, mentor, or counselor can help physicians gain enough self-awareness to handle their emotions and gain more clarity about what changes need to be made, she said.
Dr. Cole suggested that doctors figure out what makes them happy and fulfilled at work and try to spend more time on that activity. “Knowing what makes you happy and your strengths are foundational for creating a life you love.”
She urged doctors to “start thinking now about what you love about medicine and what is going right at home, and what areas you want to change. Then, start advocating for your needs.”
A version of this article originally appeared on Medscape.com.
Correction, 1/26/23: An earlier version of this article misstated the findings of the survey.
A patient named ‘Settle’ decides to sue instead
On Nov. 1, 2020, Dallas Settle went to Plateau Medical Center, Oak Hill, W.Va., complaining of pain that was later described in court documents as being “in his right mid-abdomen migrating to his right lower abdomen.” Following a CT scan, Mr. Settle was diagnosed with diverticulitis resulting in pneumoperitoneum, which is the presence of air or other gas in the abdominal cavity. The patient, it was decided, required surgery to correct the problem, but Plateau Medical Center didn’t have the staff to perform the procedure.
Mr. Settle was then transferred to another West Virginia hospital, Charleston Area Medical Center (CAMC). Here, he was evaluated by doctors in the facility’s General Division, who initiated treatment with IV fluids and opiate analgesics. He was then placed under the care of a trauma surgeon, who initially decided to treat the patient nonoperatively. If that approach failed, the surgeon believed, Mr. Settle would probably require a laparotomy, bowel resection, and ostomy.
Another surgical team performed an exploratory laparotomy the following day. The team determined that Mr. Settle was suffering from a ruptured appendicitis and allegedly performed an appendectomy. But Mr. Settle’s condition continued to deteriorate the following day.
Another CT scan followed. It revealed various problems – multiple fluid collections, an ileus, distended loops of the patient’s small bowel, a left renal cyst, subcentimeter mesenteric, and retroperitoneal adenopathy. Additional CT scans conducted 4 days later indicated other problems, including fluid collections in the patient’s right- and left-lower quadrants.
Over the next few days, doctors performed further exploratory laparotomies. Finally, on Nov. 22, Mr. Settle was transferred out of the intensive care unit in preparation for his discharge the following day.
His pain continued to worsen, however, and he was readmitted to CAMC a day later. At this point, an examination revealed that his surgical incisions had become infected.
Worse news was on the horizon. On Nov. 28, the trauma surgeon who had first agreed to treat Mr. Settle informed him that, despite claims to the contrary, his appendix hadn’t been removed.
Eventually, Mr. Settle was referred to the Cleveland Clinic, where at press time he was still being treated.
Mr. Settle has hired the firm Calwell Luce diTrapano to sue CAMC, accusing it of medical malpractice, medical negligence, and other lapses in the standard of care. In his complaint, he accused the hospital and its staff of breaching their duty of care “by negligently and improperly treating him” and by failing “to exercise the degree of care, skill, and learning required and expected of reasonable health care providers.”
His suit seeks not only compensatory damages and other relief but also punitive damages.
The content contained in this article is for informational purposes only and does not constitute legal advice. Reliance on any information provided in this article is solely at your own risk.
A version of this article originally appeared on Medscape.com.
On Nov. 1, 2020, Dallas Settle went to Plateau Medical Center, Oak Hill, W.Va., complaining of pain that was later described in court documents as being “in his right mid-abdomen migrating to his right lower abdomen.” Following a CT scan, Mr. Settle was diagnosed with diverticulitis resulting in pneumoperitoneum, which is the presence of air or other gas in the abdominal cavity. The patient, it was decided, required surgery to correct the problem, but Plateau Medical Center didn’t have the staff to perform the procedure.
Mr. Settle was then transferred to another West Virginia hospital, Charleston Area Medical Center (CAMC). Here, he was evaluated by doctors in the facility’s General Division, who initiated treatment with IV fluids and opiate analgesics. He was then placed under the care of a trauma surgeon, who initially decided to treat the patient nonoperatively. If that approach failed, the surgeon believed, Mr. Settle would probably require a laparotomy, bowel resection, and ostomy.
Another surgical team performed an exploratory laparotomy the following day. The team determined that Mr. Settle was suffering from a ruptured appendicitis and allegedly performed an appendectomy. But Mr. Settle’s condition continued to deteriorate the following day.
Another CT scan followed. It revealed various problems – multiple fluid collections, an ileus, distended loops of the patient’s small bowel, a left renal cyst, subcentimeter mesenteric, and retroperitoneal adenopathy. Additional CT scans conducted 4 days later indicated other problems, including fluid collections in the patient’s right- and left-lower quadrants.
Over the next few days, doctors performed further exploratory laparotomies. Finally, on Nov. 22, Mr. Settle was transferred out of the intensive care unit in preparation for his discharge the following day.
His pain continued to worsen, however, and he was readmitted to CAMC a day later. At this point, an examination revealed that his surgical incisions had become infected.
Worse news was on the horizon. On Nov. 28, the trauma surgeon who had first agreed to treat Mr. Settle informed him that, despite claims to the contrary, his appendix hadn’t been removed.
Eventually, Mr. Settle was referred to the Cleveland Clinic, where at press time he was still being treated.
Mr. Settle has hired the firm Calwell Luce diTrapano to sue CAMC, accusing it of medical malpractice, medical negligence, and other lapses in the standard of care. In his complaint, he accused the hospital and its staff of breaching their duty of care “by negligently and improperly treating him” and by failing “to exercise the degree of care, skill, and learning required and expected of reasonable health care providers.”
His suit seeks not only compensatory damages and other relief but also punitive damages.
The content contained in this article is for informational purposes only and does not constitute legal advice. Reliance on any information provided in this article is solely at your own risk.
A version of this article originally appeared on Medscape.com.
On Nov. 1, 2020, Dallas Settle went to Plateau Medical Center, Oak Hill, W.Va., complaining of pain that was later described in court documents as being “in his right mid-abdomen migrating to his right lower abdomen.” Following a CT scan, Mr. Settle was diagnosed with diverticulitis resulting in pneumoperitoneum, which is the presence of air or other gas in the abdominal cavity. The patient, it was decided, required surgery to correct the problem, but Plateau Medical Center didn’t have the staff to perform the procedure.
Mr. Settle was then transferred to another West Virginia hospital, Charleston Area Medical Center (CAMC). Here, he was evaluated by doctors in the facility’s General Division, who initiated treatment with IV fluids and opiate analgesics. He was then placed under the care of a trauma surgeon, who initially decided to treat the patient nonoperatively. If that approach failed, the surgeon believed, Mr. Settle would probably require a laparotomy, bowel resection, and ostomy.
Another surgical team performed an exploratory laparotomy the following day. The team determined that Mr. Settle was suffering from a ruptured appendicitis and allegedly performed an appendectomy. But Mr. Settle’s condition continued to deteriorate the following day.
Another CT scan followed. It revealed various problems – multiple fluid collections, an ileus, distended loops of the patient’s small bowel, a left renal cyst, subcentimeter mesenteric, and retroperitoneal adenopathy. Additional CT scans conducted 4 days later indicated other problems, including fluid collections in the patient’s right- and left-lower quadrants.
Over the next few days, doctors performed further exploratory laparotomies. Finally, on Nov. 22, Mr. Settle was transferred out of the intensive care unit in preparation for his discharge the following day.
His pain continued to worsen, however, and he was readmitted to CAMC a day later. At this point, an examination revealed that his surgical incisions had become infected.
Worse news was on the horizon. On Nov. 28, the trauma surgeon who had first agreed to treat Mr. Settle informed him that, despite claims to the contrary, his appendix hadn’t been removed.
Eventually, Mr. Settle was referred to the Cleveland Clinic, where at press time he was still being treated.
Mr. Settle has hired the firm Calwell Luce diTrapano to sue CAMC, accusing it of medical malpractice, medical negligence, and other lapses in the standard of care. In his complaint, he accused the hospital and its staff of breaching their duty of care “by negligently and improperly treating him” and by failing “to exercise the degree of care, skill, and learning required and expected of reasonable health care providers.”
His suit seeks not only compensatory damages and other relief but also punitive damages.
The content contained in this article is for informational purposes only and does not constitute legal advice. Reliance on any information provided in this article is solely at your own risk.
A version of this article originally appeared on Medscape.com.
Damar Hamlin’s cardiac arrest: Key lessons
This discussion was recorded on Jan. 9, 2023. This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr. Robert D. Glatter, medical adviser for Medscape Emergency Medicine. Today, we have Dr. Paul E. Pepe, an emergency medicine physician based in Florida and a highly recognized expert in emergency medical services (EMS), critical care, sports and event medicine, and resuscitation. Also joining us is Dr. Michael S. (“Mick”) Malloy, an emergency medicine physician based in Ireland, also an expert in prehospital care, resuscitation, and sports and event medicine. Welcome, gentlemen.
Dr. Pepe: Thanks for having us here.
Dr. Glatter: the Buffalo Bills safety who went down suffering a cardiac arrest in front of millions of people. Although we don’t know the exact cause of the events that transpired, the goal of our discussion is to guide our audience through a systematic approach to evaluation and management of an athlete suffering blunt force chest and neck trauma, and then suffering a cardiac arrest. We do know, obviously, that Damar was successfully resuscitated, thanks to the medical staff and trainers.
Almost 50 years ago, Chuck Hughes, a Detroit Lions receiver, went down and died with just a minute to go in the game and, unfortunately, didn’t survive.
Paul, can you tell me your impressions after viewing the replay of the events that evening? What were the most likely causes of this syncopal event and the subsequent cardiac arrest?
Dr. Pepe: We don’t know anything specifically. It’s being kept private about what the events were. It’s a little bit complicated in a sense that he basically had an extended resuscitation in the hospital. My experience has been that most people that have ventricular fibrillation, from whatever cause, will most likely be waking up on the field if you get to them. I’ve had personal experience with that.
More importantly than when it starts, when someone goes down on the field, both Dr. Malloy and I take a broader view. We don’t get tunnel vision and think, “Oh, it was a traumatic event,” or “It was cardiac event,” and we just have our minds open. There are many things that could make you stop breathing on the field. It could be a neck or a severe head injury, and then any kind of other internal injury that occurs.
When I saw in the video that Damar Hamlin stood up, that made it a less likely to be a spinal injury. He seemed to be physically functioning, and then he suddenly collapsed. That went along with something that looks like a ventricular fibrillation or ventricular tachycardia type of event and made me think right away that it was commotio cordis. I’m not a Latin scholar, but commotio is like commotion. A literal translation might be an agitation of the heart. I was thinking that he probably got hit somewhere in the middle of the chest at the right moment where the heart is resetting in that repolarization phase, like an R-on-T phenomenon, and then caused this sudden ventricular dysrhythmia.
Most people associate it to that because we have a couple of dozen cases a year of people getting hockey pucks or a baseball hitting their chest, which is very common with adolescents. On the other hand, you can’t get it from a blunt injury like this, and it was too early for it to be, say, a direct cardiac contusion, unless there was a direct injury there. It just happened so quickly.
In Europe, they’ve had a large amount of experience with this same kind of problem before, even just from a direct shoulder hit, for example. Mick Malloy is the dean of the faculty of sports and exercise medicine at the Royal College of Surgeons in Ireland and has vast experience, and now he is the person overseeing the procedures for this. Mick, have you had those kinds of experiences as well?
Dr. Molloy: Yes. It’s something that has occurred over recent decades and has been more recognized. I note that in professional sports, it’s a very different thing because you’ve got such huge teams and teams trained to respond very quickly. And that’s the most important thing in this scenario – having a team that is well functioning as a high-class emergency response team ready to get out on to that field very quickly after the person collapses, getting the automated external defibrillator (AED) on, and then recognizing whether there needs to be a shock given or not. The machine will tell you all that.
In our scenario, we run courses called CARES (Care of the Athlete Resuscitation and Emergencies in Sport) to make sure that our team physicians and team physiotherapists and trainers are all speaking as one when an emergency arises.
I don’t worry so much about the professional sport. It’s more with the amateur sports and the kids sports that I get a bit more concerned because there isn’t the same level of medical care there. Having everybody trained in basic life support would be very important to reduce unnecessary deaths from these types of conditions.
As Paul mentioned, there is a very specific cardiac cause in some of these circumstances, where you get hit just at the wrong time and that hit occurs at a particular electrical point in time. It causes this ventricular fibrillation, and the only real treatment there is the defibrillator as quickly as possible.
Dr. Glatter: What you’re saying ultimately is an important part about rapid defibrillation, and at first, cardiopulmonary resuscitation (CPR). People are concerned about whether they should begin CPR. We’re talking about out-of-hospital cardiac arrest that is outside of a football stadium, for example. Some people are obsessed with taking a person’s pulse, and that’s been a point of contention. If someone is unconscious and not breathing, we should start CPR. Wouldn›t you agree? They will wake up quickly if you begin chest compressions if they’re not necessary.
Dr. Pepe: I tell people, just do it. You’re right, people will wake up and feel it if they don’t need it.
Getting back to Mick’s point of having things ready to go, for example, 8 years ago, we had a professional player on the bench who suddenly collapsed right there in front of the entire audience. We immediately did CPR, and we got the AED on. We shocked him and he was ready, willing, and able to get back on the bench again. It turns out he had underlying coronary artery disease, but we got him back right away.
I did an initial study where we placed an AED in a public place at the Chicago O’Hare Airport to see if the public would use these. Most cardiac arrests occur at home, of course, but in public places, that was a good place to try it. We had almost 10 cases the first year. What was fascinating was that we had almost no survivors over the previous decade, even though there were paramedics at the airport. When we put these out there, we had nine people go down that first year, and six people who had never operated an AED or seen one before knew to get one and use it. Every one of those people survived neurologically intact, and almost every person was waking up before traditional responders got there. That’s how effective this is, but you need to know where the AED is.
Dr. Glatter: How to turn it on, where it is, and how to operate it.
Dr. Pepe: That was the point: These rescuers saved lives in the first year, and it was tremendous. Two points I make about it are that one, you need to know where it is, and two, just go turn it on. It gives you the instructions to follow through; just be in the Nike mode, because it basically won’t hurt a person. It’s rare that there’s ever been any complication of that. The machine algorithms are so good.
Dr. Glatter: Mick, I want to turn to you about the European experience. Specifically in Denmark, we know that there’s a large public health initiative to have AEDs accessible. There have been studies showing that when the public is engaged, especially with studies looking at an app when access is available, survivability doubled in the past 10 years from having access to AEDs. What’s your experience in Ireland in terms of public access to defibrillators?
Dr. Molloy: We’ve got two different streams here. There was a big push to have more AEDs at all sports venues. That was great, but some of the sporting clubs put them inside the locked door. I said that there’s no point to that because nobody can access it. You need to have an external building and you need to leave it open. If somebody needs to use it, they need to know how to get it, open it, and get away, and not get in through a locked door to get access to a defibrillator. We have AEDs now in most stadiums and even in small rural areas, where you might have only 200 people turn up for a game.
From another public access side, if you dial in – in our scenario, it’s 112, not 911 –we have Community First Responder groups. In the rural areas, you have local people who’ve been trained in basic life support and community first response who have AEDs. They’ll have periods of the day where they come home from work as a teacher, a nurse, a policeman, or a fireman, and they turn on an app on their phone and say, “I’m available for the next 5 hours.” If there’s a cardiac arrest rung in within 5 miles of their community, they will drive directly there with the AED that they have. We’ve had numerous saves from that in the country because it could take 40 minutes to get an EMS vehicle there, and obviously, time is crucial in these scenarios. Our dispatchers will talk people through CPR, and then the community responders arrive with the AED. It has been a fantastic initiative.
Dr. Pepe: In many places, people have apps on their phones where they’re locked into the system, and it will go off and tell them there is something nearby and even GPS them into it, and it’s been fantastic.
The two points I want to make to responding to what we just heard Dean Malloy say is one, we always have a designated spot to have these in various places. If I’m at City Hall, we always have them near the red elevators on every floor and down at security. In all the public high schools, we always have one right below the clock where everybody can see it. We set it up in a very standardized form that anybody and everybody will know where it is at the time an event happens.
The other point he made about having the response teams is fantastic. I live in a large high rise and there are two complexes with many people here, and many are older, so there’s going to be a higher risk for having an event. In fact, we’ve just had one recently. The concept we developed here was a community emergency response team, where we sometimes have doctors, nurses, and paramedics who live here be on call and be responsible, or you could try to find an AED. More importantly, we made sure everybody here knew where they were and where to get them. We’ve got most of the people trained, and we’re doing more training in what actions to take during these periods of time when such events happen.
Dr. Glatter: Yes, it’s critical. I wanted to point out that we’ve looked at the use of drones, especially here in the United States. There have been some pilot studies looking at their utility in the setting of out-of-hospital cardiac arrest. I want to get both of your thoughts on this and the feasibility of this.
Dr. Molloy: In a rural area, it’s a fantastic idea. You’re going to get something there as the crow flies very quickly. You probably have to look at exactly in, say, a rural area like Ireland of 32,000 square kilometers, how many you›ll have to put, what kind of distances they can realistically cover, and make sure the batteries are charged. Certainly, that’s a very good initiative because with the AEDs, you can’t do anything wrong. You can’t give a shock unless a shock needs to be given. The machine directs you what to do, so somebody who has had no training can pick one of these out of the box and start to work with it quickly and confidently that they can’t do anything wrong.
It’s a great idea. It would be a little expensive potentially at the moment in getting the drones and having that volume of drones around. In the U.S., you have completely different air traffic than we have, and in cities, you have more helicopters flying around. We certainly wouldn’t have that in our cities because that could cause a challenge if you’ve got drones flying around as well. It’s about making it safe that nothing else can go wrong from a drone in somebody else’s flight path.
Dr. Pepe: In my experience, the earlier the intervention, the better the results. There is a limit here in terms of the drones if they just can’t get there soon enough. Having said that, we are so fortunate in the city of Seattle to have most citizens knowing CPR, and we’d get that person resuscitated because they were doing such a good job with the CPR up front.
That’s why you’re going to see the Buffalo Bills player survive neurologically intact – because he did get immediate treatment right then and there. In the future, we may even have some better devices that will actually even restore normal blood flow right then and there while you’re still in cardiac arrest. There are limitations in every case. But on the other hand, it’s exciting and it paid off in this case recently.
Dr. Molloy: Just a point of interest coming from this small little country over here. The first portable defibrillator was developed in Belfast, Ireland, in the back of a cardiac response car. Despite us being a tiny little country, we do have some advances ahead of the United States.
Dr. Pepe: That was a breakthrough. Dr. Frank Pantridge and John Geddes did this great work and that caught the imagination of everybody here. At first, they were just going out to give people oxygen and sedate them for their chest pain. It turned out that their defibrillators are what made the difference as they went out there. Absolutely, I have to acknowledge the folks in Ireland for giving us this. Many of the EMS systems got started because of the article they published in The Lancet back in 1967.
Dr. Glatter: I wanted to briefly talk about screening of the athletes at the high school/college level, but also at the professional level. Obviously, there are issues, including the risk for false-positives in terms of low incidence, but there are also false negatives, as the case with Christian Eriksen, who had a cardiac arrest in 2021 and who has been through extensive testing. We can debate the validity of such testing, but I wanted to get both of your takes on the utility of screening in such a population.
Dr. Molloy: That’s a very emotive subject. False-positives are difficult because you’re now saying to somebody that they can’t compete in your sport at a decent level. The difficult part is telling somebody that this is the end of their career.
The false-negative is a little bit more difficult. I don’t know Christian Eriksen and I’m not involved in his team in any way, but that is a one-point examination, and you’re dependent on the scale of the process interpreting the ECG, which is again only a couple of seconds and that particular arrhythmia may not have shown up on that.
Also, athletes, by nature of what they’re doing, are operating at 99% of efficiency on a frequent basis. They are at the peak of their physiologic fitness, and it does make them a little bit more prone to picking up viral illnesses from time to time. They may get a small viral myopericarditis, which causes a new arrhythmia that nobody knew about. They had the screening 2 or 3 years ago, and they now developed a new problem because of what they do, which just may not show up.
I was actually surprised that the gentleman came through it very well, which is fantastic. He wasn’t allowed to play football in the country where he was employed, and he has now moved to another country and is playing football with a defibrillator inserted. I don’t know what the rules are in American football where you can play with implantable defibrillators. I’m not so sure it’s a great idea to do that.
Dr. Pepe: One thing that we should bring up is that there are athletes with underlying cardiomyopathies or hypertrophies and things like that, but that was unlikely in this case. It’s possible, but it’s unlikely, because it would have manifested itself before. In terms of screening, I’ve met some very smart medical doctors who have run those tests, and they have been very encouraged even at the high school levels to have screenings done, whether it’s electrocardiography, echocardiography, and so on. I have to reiterate what Dr Malloy just said in that it may have its downsides as well. If you can pick up real obvious cases, I think that may be of value.
Dr. Glatter: I want to conclude and get some pearls and takeaways from each of you regarding the events that transpired and what our audience can really hold onto.
Dr. Molloy: Look at Formula One in the past 50 years. In Formula One, in the beginning it was a 2-minute job to change a tire. Now, they have this down where they’re measuring in fractions of a second and criticizing each other if one guy is 2.6 seconds and the other guy is 2.9 seconds. For me, that’s phenomenal. It takes me 25 minutes to change a tire.
We’ve looked at that from a resuscitation perspective, and we now do pit crew resuscitation before our events. We’ve planned our team and know who’s going to be occupying what role. After the events at the UEFA championships, we had a new rule brought in by UEFA where they handed me a new document saying, “This is what we would like you to do for resuscitation.” It was a three-man triangle, and I said, “No, we’re not going to do that here.” And they said, “Why, you have to; it’s our rule.”
I said, “No, our rule in Ireland is we have a six-person triangle. We’re not downing our standards because of what you have internationally. You’re covering games in some very low-resource environments, I know that. We have a particular standard here that we’re sticking to. We have a six-person group. We know what we’re all doing; we come very quickly to those downed players and get involved and we’ve had good outcomes, so we’re not going to change the standards.”
That’s the thing: You need to practice these things. The players don’t go out on the weekend and do a move for the very first time without practicing it hundreds of times. We need to look at it the same way as the medical team who are looking after that group of players and the crowd because we also look after the crowd.
A particular challenge in some of our stadiums is that the upper decks are so steep, and it’s very hard to get a patient onto a trolley and do CPR as you’re bringing them down to a zone to get them flat. We’ve had to come up with some innovative techniques to try and do that and accommodate that using some of the mechanical CPR devices. That’s the result you’ll only get from having practiced these events and trying to extricate patients. We want to check response times, so you have to practice your response team activity very frequently.
Dr. Pepe: There are two points made by Mick that I want to react to. One, the pit crew approach is critical in so many ways. We do the same thing in what we call the medical first attack, where we knew who the A, B, and C person would be. When we took it out to the NBA trainers, I recommended for them to have a similar approach so that if an event does happen right in the middle of prime time, they are coordinated.
The second point is that we do mass-gathering medicine. It’s not just the sportspeople on the field or the entertainers that we’re looking after; it is the people in the stands. We will see a cardiac arrest once a month. If you think about it, you might see a cardiac arrest occur in any community on a regular basis. Now you’ve got 100,000 people in one stadium, and something is bound to go wrong over those 3 or 4 hours where they are there and may have a critical emergency. Preparation for all of that is really important as well.
The final point is that on a day-to-day basis, most cardiac arrests do occur in the home. Granted, 80% of them are nonshockable cases, but the people who are more apt to survive are going to be the ones who have an electrical event. In fact, when we looked at our data years ago, we found that, of the cases of people with ventricular fibrillation that we resuscitated, half didn’t even have heart damage. Their enzymes were normal. It was a pure electrical event, and they were more resuscitable. They may have an underlying problem, but we can fix that once we get them back.
Everybody needs to know how to do bystander CPR, and second, we must make sure we have AEDs strategically placed, as I alluded to before. We also go out to other parts of the community and give them advice. All those things must be put in place, but more importantly, just get the training and make the training simple. It’s really a “just do it” philosophy, but make it simple.
For example, when I teach a course, I can do it in 15 minutes, and people retain it because I keep reiterating things like, “Okay, there’s one thing you need to know about choking: Pop the cork.” You give them a physiologic image of what’s happening. Everybody says, “I remember you saying to just do it, pop the cork.”
With AEDs, know where it is – that’s why we should have it in standardized places. Go get it, turn it on, and then follow the instructions. Also, the most important thing is making sure you’re doing quality compressions; and there are videos that can help you with that, as well as classes that you can take that will get you through it.
Dr. Glatter: Absolutely. The public still has the misconception that you need to do mouth-to-mouth resuscitation. The message has not permeated through society that you don’t need to do mouth-to-mouth. Hands-only CPR is the gold standard now.
Dr. Pepe: If people have a reversible cause like ventricular fibrillation, often they’re already gasping, which is better than a delivered breath, by the way. Most important, then, are the compressions to make sure you have oxygen going up to the brain, because you’re still theoretically loaded with oxygen in your bloodstream if you had a sudden cardiac arrest from a ventricular fibrillation.
Your points are well taken, and we found that we had better outcomes when we just gave instructions to do compressions only, and that became the standard. Mick, you’ve had some experiences with that as well.
Dr. Molloy: If we’re going to have a long-term benefit from all this, we have to start doing this in elementary school and teaching kids basic life support and some basic health messaging.
I remember trying to get this across to a teacher one day and the teacher saying, “But why would we teach young kids to resuscitate each other?” I said, “I think you forget that the only 60-year-old person in the room is you. You train them, and we train them. They’re the ones who are going to respond and keep you alive. That’s the way you should be looking at this.” That completely changed the mindset of whether we should be doing this for the kids or not.
Dr. Pepe: In fact, what we find is that that’s exactly who gets saved. I had case after case where the kids at the school had learned CPR and saved the teachers or the administrator at the high school or elementary school. It’s a fantastic point that you bring up, Dr. Malloy.
Dr. Glatter: One other brief thing we can interject here is that the team was excellent on field in that they evaluated Damar Hamlin in a primary survey sense of ABCs (i.e., airway, breathing, and circulation) for things like a tension pneumothorax. In the sense in which he was hit, there are reversible causes. Making sure he didn’t have a tension pneumothorax that caused the arrest, in my mind, was critical.
Dr. Pepe: We do the same thing on a day-to-day basis with a car wreck, because it could be that the person had ventricular fibrillation and then had the wreck. It’s not always trauma. That’s a fantastic point that you’re making. That’s exactly what I think happened, and that’s what we do.
Dr. Glatter: Well, thank you, gentlemen. This was an informative and helpful discussion for our audience. I appreciate your time and expertise.
Dr. Glatter, is an attending physician at Lenox Hill Hospital in New York City and assistant professor of emergency medicine at Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. He is an editorial adviser and hosts the Hot Topics in EM series on Medscape. He is also a medical contributor for Forbes.
Dr. Pepe is a professor of internal medicine, surgery, pediatrics, public health, and emergency medicine at University of Texas Health Science Center in Houston. He’s also a global coordinator of the U.S. Metropolitan Municipalities EMS Medical Directors (“Eagles”) Coalition.
Dr. Molloy works clinically as a consultant in emergency medicine in Wexford General Hospital, part of the Ireland East Hospital Group (IEHG). Internationally, he is a member of the Disaster Medicine Section of the European Society of Emergency Medicine (EUSEM) and has been appointed by the Irish Medical Organization (IMO) as one of two Irish delegates to serve on the European Board and Section of Emergency Medicine of the European Union of Medical Specialists (UEMS), having served for a number of years on its predecessor, the Multidisciplinary Joint Committee on Emergency Medicine.
A version of this article first appeared on Medscape.com.
This discussion was recorded on Jan. 9, 2023. This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr. Robert D. Glatter, medical adviser for Medscape Emergency Medicine. Today, we have Dr. Paul E. Pepe, an emergency medicine physician based in Florida and a highly recognized expert in emergency medical services (EMS), critical care, sports and event medicine, and resuscitation. Also joining us is Dr. Michael S. (“Mick”) Malloy, an emergency medicine physician based in Ireland, also an expert in prehospital care, resuscitation, and sports and event medicine. Welcome, gentlemen.
Dr. Pepe: Thanks for having us here.
Dr. Glatter: the Buffalo Bills safety who went down suffering a cardiac arrest in front of millions of people. Although we don’t know the exact cause of the events that transpired, the goal of our discussion is to guide our audience through a systematic approach to evaluation and management of an athlete suffering blunt force chest and neck trauma, and then suffering a cardiac arrest. We do know, obviously, that Damar was successfully resuscitated, thanks to the medical staff and trainers.
Almost 50 years ago, Chuck Hughes, a Detroit Lions receiver, went down and died with just a minute to go in the game and, unfortunately, didn’t survive.
Paul, can you tell me your impressions after viewing the replay of the events that evening? What were the most likely causes of this syncopal event and the subsequent cardiac arrest?
Dr. Pepe: We don’t know anything specifically. It’s being kept private about what the events were. It’s a little bit complicated in a sense that he basically had an extended resuscitation in the hospital. My experience has been that most people that have ventricular fibrillation, from whatever cause, will most likely be waking up on the field if you get to them. I’ve had personal experience with that.
More importantly than when it starts, when someone goes down on the field, both Dr. Malloy and I take a broader view. We don’t get tunnel vision and think, “Oh, it was a traumatic event,” or “It was cardiac event,” and we just have our minds open. There are many things that could make you stop breathing on the field. It could be a neck or a severe head injury, and then any kind of other internal injury that occurs.
When I saw in the video that Damar Hamlin stood up, that made it a less likely to be a spinal injury. He seemed to be physically functioning, and then he suddenly collapsed. That went along with something that looks like a ventricular fibrillation or ventricular tachycardia type of event and made me think right away that it was commotio cordis. I’m not a Latin scholar, but commotio is like commotion. A literal translation might be an agitation of the heart. I was thinking that he probably got hit somewhere in the middle of the chest at the right moment where the heart is resetting in that repolarization phase, like an R-on-T phenomenon, and then caused this sudden ventricular dysrhythmia.
Most people associate it to that because we have a couple of dozen cases a year of people getting hockey pucks or a baseball hitting their chest, which is very common with adolescents. On the other hand, you can’t get it from a blunt injury like this, and it was too early for it to be, say, a direct cardiac contusion, unless there was a direct injury there. It just happened so quickly.
In Europe, they’ve had a large amount of experience with this same kind of problem before, even just from a direct shoulder hit, for example. Mick Malloy is the dean of the faculty of sports and exercise medicine at the Royal College of Surgeons in Ireland and has vast experience, and now he is the person overseeing the procedures for this. Mick, have you had those kinds of experiences as well?
Dr. Molloy: Yes. It’s something that has occurred over recent decades and has been more recognized. I note that in professional sports, it’s a very different thing because you’ve got such huge teams and teams trained to respond very quickly. And that’s the most important thing in this scenario – having a team that is well functioning as a high-class emergency response team ready to get out on to that field very quickly after the person collapses, getting the automated external defibrillator (AED) on, and then recognizing whether there needs to be a shock given or not. The machine will tell you all that.
In our scenario, we run courses called CARES (Care of the Athlete Resuscitation and Emergencies in Sport) to make sure that our team physicians and team physiotherapists and trainers are all speaking as one when an emergency arises.
I don’t worry so much about the professional sport. It’s more with the amateur sports and the kids sports that I get a bit more concerned because there isn’t the same level of medical care there. Having everybody trained in basic life support would be very important to reduce unnecessary deaths from these types of conditions.
As Paul mentioned, there is a very specific cardiac cause in some of these circumstances, where you get hit just at the wrong time and that hit occurs at a particular electrical point in time. It causes this ventricular fibrillation, and the only real treatment there is the defibrillator as quickly as possible.
Dr. Glatter: What you’re saying ultimately is an important part about rapid defibrillation, and at first, cardiopulmonary resuscitation (CPR). People are concerned about whether they should begin CPR. We’re talking about out-of-hospital cardiac arrest that is outside of a football stadium, for example. Some people are obsessed with taking a person’s pulse, and that’s been a point of contention. If someone is unconscious and not breathing, we should start CPR. Wouldn›t you agree? They will wake up quickly if you begin chest compressions if they’re not necessary.
Dr. Pepe: I tell people, just do it. You’re right, people will wake up and feel it if they don’t need it.
Getting back to Mick’s point of having things ready to go, for example, 8 years ago, we had a professional player on the bench who suddenly collapsed right there in front of the entire audience. We immediately did CPR, and we got the AED on. We shocked him and he was ready, willing, and able to get back on the bench again. It turns out he had underlying coronary artery disease, but we got him back right away.
I did an initial study where we placed an AED in a public place at the Chicago O’Hare Airport to see if the public would use these. Most cardiac arrests occur at home, of course, but in public places, that was a good place to try it. We had almost 10 cases the first year. What was fascinating was that we had almost no survivors over the previous decade, even though there were paramedics at the airport. When we put these out there, we had nine people go down that first year, and six people who had never operated an AED or seen one before knew to get one and use it. Every one of those people survived neurologically intact, and almost every person was waking up before traditional responders got there. That’s how effective this is, but you need to know where the AED is.
Dr. Glatter: How to turn it on, where it is, and how to operate it.
Dr. Pepe: That was the point: These rescuers saved lives in the first year, and it was tremendous. Two points I make about it are that one, you need to know where it is, and two, just go turn it on. It gives you the instructions to follow through; just be in the Nike mode, because it basically won’t hurt a person. It’s rare that there’s ever been any complication of that. The machine algorithms are so good.
Dr. Glatter: Mick, I want to turn to you about the European experience. Specifically in Denmark, we know that there’s a large public health initiative to have AEDs accessible. There have been studies showing that when the public is engaged, especially with studies looking at an app when access is available, survivability doubled in the past 10 years from having access to AEDs. What’s your experience in Ireland in terms of public access to defibrillators?
Dr. Molloy: We’ve got two different streams here. There was a big push to have more AEDs at all sports venues. That was great, but some of the sporting clubs put them inside the locked door. I said that there’s no point to that because nobody can access it. You need to have an external building and you need to leave it open. If somebody needs to use it, they need to know how to get it, open it, and get away, and not get in through a locked door to get access to a defibrillator. We have AEDs now in most stadiums and even in small rural areas, where you might have only 200 people turn up for a game.
From another public access side, if you dial in – in our scenario, it’s 112, not 911 –we have Community First Responder groups. In the rural areas, you have local people who’ve been trained in basic life support and community first response who have AEDs. They’ll have periods of the day where they come home from work as a teacher, a nurse, a policeman, or a fireman, and they turn on an app on their phone and say, “I’m available for the next 5 hours.” If there’s a cardiac arrest rung in within 5 miles of their community, they will drive directly there with the AED that they have. We’ve had numerous saves from that in the country because it could take 40 minutes to get an EMS vehicle there, and obviously, time is crucial in these scenarios. Our dispatchers will talk people through CPR, and then the community responders arrive with the AED. It has been a fantastic initiative.
Dr. Pepe: In many places, people have apps on their phones where they’re locked into the system, and it will go off and tell them there is something nearby and even GPS them into it, and it’s been fantastic.
The two points I want to make to responding to what we just heard Dean Malloy say is one, we always have a designated spot to have these in various places. If I’m at City Hall, we always have them near the red elevators on every floor and down at security. In all the public high schools, we always have one right below the clock where everybody can see it. We set it up in a very standardized form that anybody and everybody will know where it is at the time an event happens.
The other point he made about having the response teams is fantastic. I live in a large high rise and there are two complexes with many people here, and many are older, so there’s going to be a higher risk for having an event. In fact, we’ve just had one recently. The concept we developed here was a community emergency response team, where we sometimes have doctors, nurses, and paramedics who live here be on call and be responsible, or you could try to find an AED. More importantly, we made sure everybody here knew where they were and where to get them. We’ve got most of the people trained, and we’re doing more training in what actions to take during these periods of time when such events happen.
Dr. Glatter: Yes, it’s critical. I wanted to point out that we’ve looked at the use of drones, especially here in the United States. There have been some pilot studies looking at their utility in the setting of out-of-hospital cardiac arrest. I want to get both of your thoughts on this and the feasibility of this.
Dr. Molloy: In a rural area, it’s a fantastic idea. You’re going to get something there as the crow flies very quickly. You probably have to look at exactly in, say, a rural area like Ireland of 32,000 square kilometers, how many you›ll have to put, what kind of distances they can realistically cover, and make sure the batteries are charged. Certainly, that’s a very good initiative because with the AEDs, you can’t do anything wrong. You can’t give a shock unless a shock needs to be given. The machine directs you what to do, so somebody who has had no training can pick one of these out of the box and start to work with it quickly and confidently that they can’t do anything wrong.
It’s a great idea. It would be a little expensive potentially at the moment in getting the drones and having that volume of drones around. In the U.S., you have completely different air traffic than we have, and in cities, you have more helicopters flying around. We certainly wouldn’t have that in our cities because that could cause a challenge if you’ve got drones flying around as well. It’s about making it safe that nothing else can go wrong from a drone in somebody else’s flight path.
Dr. Pepe: In my experience, the earlier the intervention, the better the results. There is a limit here in terms of the drones if they just can’t get there soon enough. Having said that, we are so fortunate in the city of Seattle to have most citizens knowing CPR, and we’d get that person resuscitated because they were doing such a good job with the CPR up front.
That’s why you’re going to see the Buffalo Bills player survive neurologically intact – because he did get immediate treatment right then and there. In the future, we may even have some better devices that will actually even restore normal blood flow right then and there while you’re still in cardiac arrest. There are limitations in every case. But on the other hand, it’s exciting and it paid off in this case recently.
Dr. Molloy: Just a point of interest coming from this small little country over here. The first portable defibrillator was developed in Belfast, Ireland, in the back of a cardiac response car. Despite us being a tiny little country, we do have some advances ahead of the United States.
Dr. Pepe: That was a breakthrough. Dr. Frank Pantridge and John Geddes did this great work and that caught the imagination of everybody here. At first, they were just going out to give people oxygen and sedate them for their chest pain. It turned out that their defibrillators are what made the difference as they went out there. Absolutely, I have to acknowledge the folks in Ireland for giving us this. Many of the EMS systems got started because of the article they published in The Lancet back in 1967.
Dr. Glatter: I wanted to briefly talk about screening of the athletes at the high school/college level, but also at the professional level. Obviously, there are issues, including the risk for false-positives in terms of low incidence, but there are also false negatives, as the case with Christian Eriksen, who had a cardiac arrest in 2021 and who has been through extensive testing. We can debate the validity of such testing, but I wanted to get both of your takes on the utility of screening in such a population.
Dr. Molloy: That’s a very emotive subject. False-positives are difficult because you’re now saying to somebody that they can’t compete in your sport at a decent level. The difficult part is telling somebody that this is the end of their career.
The false-negative is a little bit more difficult. I don’t know Christian Eriksen and I’m not involved in his team in any way, but that is a one-point examination, and you’re dependent on the scale of the process interpreting the ECG, which is again only a couple of seconds and that particular arrhythmia may not have shown up on that.
Also, athletes, by nature of what they’re doing, are operating at 99% of efficiency on a frequent basis. They are at the peak of their physiologic fitness, and it does make them a little bit more prone to picking up viral illnesses from time to time. They may get a small viral myopericarditis, which causes a new arrhythmia that nobody knew about. They had the screening 2 or 3 years ago, and they now developed a new problem because of what they do, which just may not show up.
I was actually surprised that the gentleman came through it very well, which is fantastic. He wasn’t allowed to play football in the country where he was employed, and he has now moved to another country and is playing football with a defibrillator inserted. I don’t know what the rules are in American football where you can play with implantable defibrillators. I’m not so sure it’s a great idea to do that.
Dr. Pepe: One thing that we should bring up is that there are athletes with underlying cardiomyopathies or hypertrophies and things like that, but that was unlikely in this case. It’s possible, but it’s unlikely, because it would have manifested itself before. In terms of screening, I’ve met some very smart medical doctors who have run those tests, and they have been very encouraged even at the high school levels to have screenings done, whether it’s electrocardiography, echocardiography, and so on. I have to reiterate what Dr Malloy just said in that it may have its downsides as well. If you can pick up real obvious cases, I think that may be of value.
Dr. Glatter: I want to conclude and get some pearls and takeaways from each of you regarding the events that transpired and what our audience can really hold onto.
Dr. Molloy: Look at Formula One in the past 50 years. In Formula One, in the beginning it was a 2-minute job to change a tire. Now, they have this down where they’re measuring in fractions of a second and criticizing each other if one guy is 2.6 seconds and the other guy is 2.9 seconds. For me, that’s phenomenal. It takes me 25 minutes to change a tire.
We’ve looked at that from a resuscitation perspective, and we now do pit crew resuscitation before our events. We’ve planned our team and know who’s going to be occupying what role. After the events at the UEFA championships, we had a new rule brought in by UEFA where they handed me a new document saying, “This is what we would like you to do for resuscitation.” It was a three-man triangle, and I said, “No, we’re not going to do that here.” And they said, “Why, you have to; it’s our rule.”
I said, “No, our rule in Ireland is we have a six-person triangle. We’re not downing our standards because of what you have internationally. You’re covering games in some very low-resource environments, I know that. We have a particular standard here that we’re sticking to. We have a six-person group. We know what we’re all doing; we come very quickly to those downed players and get involved and we’ve had good outcomes, so we’re not going to change the standards.”
That’s the thing: You need to practice these things. The players don’t go out on the weekend and do a move for the very first time without practicing it hundreds of times. We need to look at it the same way as the medical team who are looking after that group of players and the crowd because we also look after the crowd.
A particular challenge in some of our stadiums is that the upper decks are so steep, and it’s very hard to get a patient onto a trolley and do CPR as you’re bringing them down to a zone to get them flat. We’ve had to come up with some innovative techniques to try and do that and accommodate that using some of the mechanical CPR devices. That’s the result you’ll only get from having practiced these events and trying to extricate patients. We want to check response times, so you have to practice your response team activity very frequently.
Dr. Pepe: There are two points made by Mick that I want to react to. One, the pit crew approach is critical in so many ways. We do the same thing in what we call the medical first attack, where we knew who the A, B, and C person would be. When we took it out to the NBA trainers, I recommended for them to have a similar approach so that if an event does happen right in the middle of prime time, they are coordinated.
The second point is that we do mass-gathering medicine. It’s not just the sportspeople on the field or the entertainers that we’re looking after; it is the people in the stands. We will see a cardiac arrest once a month. If you think about it, you might see a cardiac arrest occur in any community on a regular basis. Now you’ve got 100,000 people in one stadium, and something is bound to go wrong over those 3 or 4 hours where they are there and may have a critical emergency. Preparation for all of that is really important as well.
The final point is that on a day-to-day basis, most cardiac arrests do occur in the home. Granted, 80% of them are nonshockable cases, but the people who are more apt to survive are going to be the ones who have an electrical event. In fact, when we looked at our data years ago, we found that, of the cases of people with ventricular fibrillation that we resuscitated, half didn’t even have heart damage. Their enzymes were normal. It was a pure electrical event, and they were more resuscitable. They may have an underlying problem, but we can fix that once we get them back.
Everybody needs to know how to do bystander CPR, and second, we must make sure we have AEDs strategically placed, as I alluded to before. We also go out to other parts of the community and give them advice. All those things must be put in place, but more importantly, just get the training and make the training simple. It’s really a “just do it” philosophy, but make it simple.
For example, when I teach a course, I can do it in 15 minutes, and people retain it because I keep reiterating things like, “Okay, there’s one thing you need to know about choking: Pop the cork.” You give them a physiologic image of what’s happening. Everybody says, “I remember you saying to just do it, pop the cork.”
With AEDs, know where it is – that’s why we should have it in standardized places. Go get it, turn it on, and then follow the instructions. Also, the most important thing is making sure you’re doing quality compressions; and there are videos that can help you with that, as well as classes that you can take that will get you through it.
Dr. Glatter: Absolutely. The public still has the misconception that you need to do mouth-to-mouth resuscitation. The message has not permeated through society that you don’t need to do mouth-to-mouth. Hands-only CPR is the gold standard now.
Dr. Pepe: If people have a reversible cause like ventricular fibrillation, often they’re already gasping, which is better than a delivered breath, by the way. Most important, then, are the compressions to make sure you have oxygen going up to the brain, because you’re still theoretically loaded with oxygen in your bloodstream if you had a sudden cardiac arrest from a ventricular fibrillation.
Your points are well taken, and we found that we had better outcomes when we just gave instructions to do compressions only, and that became the standard. Mick, you’ve had some experiences with that as well.
Dr. Molloy: If we’re going to have a long-term benefit from all this, we have to start doing this in elementary school and teaching kids basic life support and some basic health messaging.
I remember trying to get this across to a teacher one day and the teacher saying, “But why would we teach young kids to resuscitate each other?” I said, “I think you forget that the only 60-year-old person in the room is you. You train them, and we train them. They’re the ones who are going to respond and keep you alive. That’s the way you should be looking at this.” That completely changed the mindset of whether we should be doing this for the kids or not.
Dr. Pepe: In fact, what we find is that that’s exactly who gets saved. I had case after case where the kids at the school had learned CPR and saved the teachers or the administrator at the high school or elementary school. It’s a fantastic point that you bring up, Dr. Malloy.
Dr. Glatter: One other brief thing we can interject here is that the team was excellent on field in that they evaluated Damar Hamlin in a primary survey sense of ABCs (i.e., airway, breathing, and circulation) for things like a tension pneumothorax. In the sense in which he was hit, there are reversible causes. Making sure he didn’t have a tension pneumothorax that caused the arrest, in my mind, was critical.
Dr. Pepe: We do the same thing on a day-to-day basis with a car wreck, because it could be that the person had ventricular fibrillation and then had the wreck. It’s not always trauma. That’s a fantastic point that you’re making. That’s exactly what I think happened, and that’s what we do.
Dr. Glatter: Well, thank you, gentlemen. This was an informative and helpful discussion for our audience. I appreciate your time and expertise.
Dr. Glatter, is an attending physician at Lenox Hill Hospital in New York City and assistant professor of emergency medicine at Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. He is an editorial adviser and hosts the Hot Topics in EM series on Medscape. He is also a medical contributor for Forbes.
Dr. Pepe is a professor of internal medicine, surgery, pediatrics, public health, and emergency medicine at University of Texas Health Science Center in Houston. He’s also a global coordinator of the U.S. Metropolitan Municipalities EMS Medical Directors (“Eagles”) Coalition.
Dr. Molloy works clinically as a consultant in emergency medicine in Wexford General Hospital, part of the Ireland East Hospital Group (IEHG). Internationally, he is a member of the Disaster Medicine Section of the European Society of Emergency Medicine (EUSEM) and has been appointed by the Irish Medical Organization (IMO) as one of two Irish delegates to serve on the European Board and Section of Emergency Medicine of the European Union of Medical Specialists (UEMS), having served for a number of years on its predecessor, the Multidisciplinary Joint Committee on Emergency Medicine.
A version of this article first appeared on Medscape.com.
This discussion was recorded on Jan. 9, 2023. This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr. Robert D. Glatter, medical adviser for Medscape Emergency Medicine. Today, we have Dr. Paul E. Pepe, an emergency medicine physician based in Florida and a highly recognized expert in emergency medical services (EMS), critical care, sports and event medicine, and resuscitation. Also joining us is Dr. Michael S. (“Mick”) Malloy, an emergency medicine physician based in Ireland, also an expert in prehospital care, resuscitation, and sports and event medicine. Welcome, gentlemen.
Dr. Pepe: Thanks for having us here.
Dr. Glatter: the Buffalo Bills safety who went down suffering a cardiac arrest in front of millions of people. Although we don’t know the exact cause of the events that transpired, the goal of our discussion is to guide our audience through a systematic approach to evaluation and management of an athlete suffering blunt force chest and neck trauma, and then suffering a cardiac arrest. We do know, obviously, that Damar was successfully resuscitated, thanks to the medical staff and trainers.
Almost 50 years ago, Chuck Hughes, a Detroit Lions receiver, went down and died with just a minute to go in the game and, unfortunately, didn’t survive.
Paul, can you tell me your impressions after viewing the replay of the events that evening? What were the most likely causes of this syncopal event and the subsequent cardiac arrest?
Dr. Pepe: We don’t know anything specifically. It’s being kept private about what the events were. It’s a little bit complicated in a sense that he basically had an extended resuscitation in the hospital. My experience has been that most people that have ventricular fibrillation, from whatever cause, will most likely be waking up on the field if you get to them. I’ve had personal experience with that.
More importantly than when it starts, when someone goes down on the field, both Dr. Malloy and I take a broader view. We don’t get tunnel vision and think, “Oh, it was a traumatic event,” or “It was cardiac event,” and we just have our minds open. There are many things that could make you stop breathing on the field. It could be a neck or a severe head injury, and then any kind of other internal injury that occurs.
When I saw in the video that Damar Hamlin stood up, that made it a less likely to be a spinal injury. He seemed to be physically functioning, and then he suddenly collapsed. That went along with something that looks like a ventricular fibrillation or ventricular tachycardia type of event and made me think right away that it was commotio cordis. I’m not a Latin scholar, but commotio is like commotion. A literal translation might be an agitation of the heart. I was thinking that he probably got hit somewhere in the middle of the chest at the right moment where the heart is resetting in that repolarization phase, like an R-on-T phenomenon, and then caused this sudden ventricular dysrhythmia.
Most people associate it to that because we have a couple of dozen cases a year of people getting hockey pucks or a baseball hitting their chest, which is very common with adolescents. On the other hand, you can’t get it from a blunt injury like this, and it was too early for it to be, say, a direct cardiac contusion, unless there was a direct injury there. It just happened so quickly.
In Europe, they’ve had a large amount of experience with this same kind of problem before, even just from a direct shoulder hit, for example. Mick Malloy is the dean of the faculty of sports and exercise medicine at the Royal College of Surgeons in Ireland and has vast experience, and now he is the person overseeing the procedures for this. Mick, have you had those kinds of experiences as well?
Dr. Molloy: Yes. It’s something that has occurred over recent decades and has been more recognized. I note that in professional sports, it’s a very different thing because you’ve got such huge teams and teams trained to respond very quickly. And that’s the most important thing in this scenario – having a team that is well functioning as a high-class emergency response team ready to get out on to that field very quickly after the person collapses, getting the automated external defibrillator (AED) on, and then recognizing whether there needs to be a shock given or not. The machine will tell you all that.
In our scenario, we run courses called CARES (Care of the Athlete Resuscitation and Emergencies in Sport) to make sure that our team physicians and team physiotherapists and trainers are all speaking as one when an emergency arises.
I don’t worry so much about the professional sport. It’s more with the amateur sports and the kids sports that I get a bit more concerned because there isn’t the same level of medical care there. Having everybody trained in basic life support would be very important to reduce unnecessary deaths from these types of conditions.
As Paul mentioned, there is a very specific cardiac cause in some of these circumstances, where you get hit just at the wrong time and that hit occurs at a particular electrical point in time. It causes this ventricular fibrillation, and the only real treatment there is the defibrillator as quickly as possible.
Dr. Glatter: What you’re saying ultimately is an important part about rapid defibrillation, and at first, cardiopulmonary resuscitation (CPR). People are concerned about whether they should begin CPR. We’re talking about out-of-hospital cardiac arrest that is outside of a football stadium, for example. Some people are obsessed with taking a person’s pulse, and that’s been a point of contention. If someone is unconscious and not breathing, we should start CPR. Wouldn›t you agree? They will wake up quickly if you begin chest compressions if they’re not necessary.
Dr. Pepe: I tell people, just do it. You’re right, people will wake up and feel it if they don’t need it.
Getting back to Mick’s point of having things ready to go, for example, 8 years ago, we had a professional player on the bench who suddenly collapsed right there in front of the entire audience. We immediately did CPR, and we got the AED on. We shocked him and he was ready, willing, and able to get back on the bench again. It turns out he had underlying coronary artery disease, but we got him back right away.
I did an initial study where we placed an AED in a public place at the Chicago O’Hare Airport to see if the public would use these. Most cardiac arrests occur at home, of course, but in public places, that was a good place to try it. We had almost 10 cases the first year. What was fascinating was that we had almost no survivors over the previous decade, even though there were paramedics at the airport. When we put these out there, we had nine people go down that first year, and six people who had never operated an AED or seen one before knew to get one and use it. Every one of those people survived neurologically intact, and almost every person was waking up before traditional responders got there. That’s how effective this is, but you need to know where the AED is.
Dr. Glatter: How to turn it on, where it is, and how to operate it.
Dr. Pepe: That was the point: These rescuers saved lives in the first year, and it was tremendous. Two points I make about it are that one, you need to know where it is, and two, just go turn it on. It gives you the instructions to follow through; just be in the Nike mode, because it basically won’t hurt a person. It’s rare that there’s ever been any complication of that. The machine algorithms are so good.
Dr. Glatter: Mick, I want to turn to you about the European experience. Specifically in Denmark, we know that there’s a large public health initiative to have AEDs accessible. There have been studies showing that when the public is engaged, especially with studies looking at an app when access is available, survivability doubled in the past 10 years from having access to AEDs. What’s your experience in Ireland in terms of public access to defibrillators?
Dr. Molloy: We’ve got two different streams here. There was a big push to have more AEDs at all sports venues. That was great, but some of the sporting clubs put them inside the locked door. I said that there’s no point to that because nobody can access it. You need to have an external building and you need to leave it open. If somebody needs to use it, they need to know how to get it, open it, and get away, and not get in through a locked door to get access to a defibrillator. We have AEDs now in most stadiums and even in small rural areas, where you might have only 200 people turn up for a game.
From another public access side, if you dial in – in our scenario, it’s 112, not 911 –we have Community First Responder groups. In the rural areas, you have local people who’ve been trained in basic life support and community first response who have AEDs. They’ll have periods of the day where they come home from work as a teacher, a nurse, a policeman, or a fireman, and they turn on an app on their phone and say, “I’m available for the next 5 hours.” If there’s a cardiac arrest rung in within 5 miles of their community, they will drive directly there with the AED that they have. We’ve had numerous saves from that in the country because it could take 40 minutes to get an EMS vehicle there, and obviously, time is crucial in these scenarios. Our dispatchers will talk people through CPR, and then the community responders arrive with the AED. It has been a fantastic initiative.
Dr. Pepe: In many places, people have apps on their phones where they’re locked into the system, and it will go off and tell them there is something nearby and even GPS them into it, and it’s been fantastic.
The two points I want to make to responding to what we just heard Dean Malloy say is one, we always have a designated spot to have these in various places. If I’m at City Hall, we always have them near the red elevators on every floor and down at security. In all the public high schools, we always have one right below the clock where everybody can see it. We set it up in a very standardized form that anybody and everybody will know where it is at the time an event happens.
The other point he made about having the response teams is fantastic. I live in a large high rise and there are two complexes with many people here, and many are older, so there’s going to be a higher risk for having an event. In fact, we’ve just had one recently. The concept we developed here was a community emergency response team, where we sometimes have doctors, nurses, and paramedics who live here be on call and be responsible, or you could try to find an AED. More importantly, we made sure everybody here knew where they were and where to get them. We’ve got most of the people trained, and we’re doing more training in what actions to take during these periods of time when such events happen.
Dr. Glatter: Yes, it’s critical. I wanted to point out that we’ve looked at the use of drones, especially here in the United States. There have been some pilot studies looking at their utility in the setting of out-of-hospital cardiac arrest. I want to get both of your thoughts on this and the feasibility of this.
Dr. Molloy: In a rural area, it’s a fantastic idea. You’re going to get something there as the crow flies very quickly. You probably have to look at exactly in, say, a rural area like Ireland of 32,000 square kilometers, how many you›ll have to put, what kind of distances they can realistically cover, and make sure the batteries are charged. Certainly, that’s a very good initiative because with the AEDs, you can’t do anything wrong. You can’t give a shock unless a shock needs to be given. The machine directs you what to do, so somebody who has had no training can pick one of these out of the box and start to work with it quickly and confidently that they can’t do anything wrong.
It’s a great idea. It would be a little expensive potentially at the moment in getting the drones and having that volume of drones around. In the U.S., you have completely different air traffic than we have, and in cities, you have more helicopters flying around. We certainly wouldn’t have that in our cities because that could cause a challenge if you’ve got drones flying around as well. It’s about making it safe that nothing else can go wrong from a drone in somebody else’s flight path.
Dr. Pepe: In my experience, the earlier the intervention, the better the results. There is a limit here in terms of the drones if they just can’t get there soon enough. Having said that, we are so fortunate in the city of Seattle to have most citizens knowing CPR, and we’d get that person resuscitated because they were doing such a good job with the CPR up front.
That’s why you’re going to see the Buffalo Bills player survive neurologically intact – because he did get immediate treatment right then and there. In the future, we may even have some better devices that will actually even restore normal blood flow right then and there while you’re still in cardiac arrest. There are limitations in every case. But on the other hand, it’s exciting and it paid off in this case recently.
Dr. Molloy: Just a point of interest coming from this small little country over here. The first portable defibrillator was developed in Belfast, Ireland, in the back of a cardiac response car. Despite us being a tiny little country, we do have some advances ahead of the United States.
Dr. Pepe: That was a breakthrough. Dr. Frank Pantridge and John Geddes did this great work and that caught the imagination of everybody here. At first, they were just going out to give people oxygen and sedate them for their chest pain. It turned out that their defibrillators are what made the difference as they went out there. Absolutely, I have to acknowledge the folks in Ireland for giving us this. Many of the EMS systems got started because of the article they published in The Lancet back in 1967.
Dr. Glatter: I wanted to briefly talk about screening of the athletes at the high school/college level, but also at the professional level. Obviously, there are issues, including the risk for false-positives in terms of low incidence, but there are also false negatives, as the case with Christian Eriksen, who had a cardiac arrest in 2021 and who has been through extensive testing. We can debate the validity of such testing, but I wanted to get both of your takes on the utility of screening in such a population.
Dr. Molloy: That’s a very emotive subject. False-positives are difficult because you’re now saying to somebody that they can’t compete in your sport at a decent level. The difficult part is telling somebody that this is the end of their career.
The false-negative is a little bit more difficult. I don’t know Christian Eriksen and I’m not involved in his team in any way, but that is a one-point examination, and you’re dependent on the scale of the process interpreting the ECG, which is again only a couple of seconds and that particular arrhythmia may not have shown up on that.
Also, athletes, by nature of what they’re doing, are operating at 99% of efficiency on a frequent basis. They are at the peak of their physiologic fitness, and it does make them a little bit more prone to picking up viral illnesses from time to time. They may get a small viral myopericarditis, which causes a new arrhythmia that nobody knew about. They had the screening 2 or 3 years ago, and they now developed a new problem because of what they do, which just may not show up.
I was actually surprised that the gentleman came through it very well, which is fantastic. He wasn’t allowed to play football in the country where he was employed, and he has now moved to another country and is playing football with a defibrillator inserted. I don’t know what the rules are in American football where you can play with implantable defibrillators. I’m not so sure it’s a great idea to do that.
Dr. Pepe: One thing that we should bring up is that there are athletes with underlying cardiomyopathies or hypertrophies and things like that, but that was unlikely in this case. It’s possible, but it’s unlikely, because it would have manifested itself before. In terms of screening, I’ve met some very smart medical doctors who have run those tests, and they have been very encouraged even at the high school levels to have screenings done, whether it’s electrocardiography, echocardiography, and so on. I have to reiterate what Dr Malloy just said in that it may have its downsides as well. If you can pick up real obvious cases, I think that may be of value.
Dr. Glatter: I want to conclude and get some pearls and takeaways from each of you regarding the events that transpired and what our audience can really hold onto.
Dr. Molloy: Look at Formula One in the past 50 years. In Formula One, in the beginning it was a 2-minute job to change a tire. Now, they have this down where they’re measuring in fractions of a second and criticizing each other if one guy is 2.6 seconds and the other guy is 2.9 seconds. For me, that’s phenomenal. It takes me 25 minutes to change a tire.
We’ve looked at that from a resuscitation perspective, and we now do pit crew resuscitation before our events. We’ve planned our team and know who’s going to be occupying what role. After the events at the UEFA championships, we had a new rule brought in by UEFA where they handed me a new document saying, “This is what we would like you to do for resuscitation.” It was a three-man triangle, and I said, “No, we’re not going to do that here.” And they said, “Why, you have to; it’s our rule.”
I said, “No, our rule in Ireland is we have a six-person triangle. We’re not downing our standards because of what you have internationally. You’re covering games in some very low-resource environments, I know that. We have a particular standard here that we’re sticking to. We have a six-person group. We know what we’re all doing; we come very quickly to those downed players and get involved and we’ve had good outcomes, so we’re not going to change the standards.”
That’s the thing: You need to practice these things. The players don’t go out on the weekend and do a move for the very first time without practicing it hundreds of times. We need to look at it the same way as the medical team who are looking after that group of players and the crowd because we also look after the crowd.
A particular challenge in some of our stadiums is that the upper decks are so steep, and it’s very hard to get a patient onto a trolley and do CPR as you’re bringing them down to a zone to get them flat. We’ve had to come up with some innovative techniques to try and do that and accommodate that using some of the mechanical CPR devices. That’s the result you’ll only get from having practiced these events and trying to extricate patients. We want to check response times, so you have to practice your response team activity very frequently.
Dr. Pepe: There are two points made by Mick that I want to react to. One, the pit crew approach is critical in so many ways. We do the same thing in what we call the medical first attack, where we knew who the A, B, and C person would be. When we took it out to the NBA trainers, I recommended for them to have a similar approach so that if an event does happen right in the middle of prime time, they are coordinated.
The second point is that we do mass-gathering medicine. It’s not just the sportspeople on the field or the entertainers that we’re looking after; it is the people in the stands. We will see a cardiac arrest once a month. If you think about it, you might see a cardiac arrest occur in any community on a regular basis. Now you’ve got 100,000 people in one stadium, and something is bound to go wrong over those 3 or 4 hours where they are there and may have a critical emergency. Preparation for all of that is really important as well.
The final point is that on a day-to-day basis, most cardiac arrests do occur in the home. Granted, 80% of them are nonshockable cases, but the people who are more apt to survive are going to be the ones who have an electrical event. In fact, when we looked at our data years ago, we found that, of the cases of people with ventricular fibrillation that we resuscitated, half didn’t even have heart damage. Their enzymes were normal. It was a pure electrical event, and they were more resuscitable. They may have an underlying problem, but we can fix that once we get them back.
Everybody needs to know how to do bystander CPR, and second, we must make sure we have AEDs strategically placed, as I alluded to before. We also go out to other parts of the community and give them advice. All those things must be put in place, but more importantly, just get the training and make the training simple. It’s really a “just do it” philosophy, but make it simple.
For example, when I teach a course, I can do it in 15 minutes, and people retain it because I keep reiterating things like, “Okay, there’s one thing you need to know about choking: Pop the cork.” You give them a physiologic image of what’s happening. Everybody says, “I remember you saying to just do it, pop the cork.”
With AEDs, know where it is – that’s why we should have it in standardized places. Go get it, turn it on, and then follow the instructions. Also, the most important thing is making sure you’re doing quality compressions; and there are videos that can help you with that, as well as classes that you can take that will get you through it.
Dr. Glatter: Absolutely. The public still has the misconception that you need to do mouth-to-mouth resuscitation. The message has not permeated through society that you don’t need to do mouth-to-mouth. Hands-only CPR is the gold standard now.
Dr. Pepe: If people have a reversible cause like ventricular fibrillation, often they’re already gasping, which is better than a delivered breath, by the way. Most important, then, are the compressions to make sure you have oxygen going up to the brain, because you’re still theoretically loaded with oxygen in your bloodstream if you had a sudden cardiac arrest from a ventricular fibrillation.
Your points are well taken, and we found that we had better outcomes when we just gave instructions to do compressions only, and that became the standard. Mick, you’ve had some experiences with that as well.
Dr. Molloy: If we’re going to have a long-term benefit from all this, we have to start doing this in elementary school and teaching kids basic life support and some basic health messaging.
I remember trying to get this across to a teacher one day and the teacher saying, “But why would we teach young kids to resuscitate each other?” I said, “I think you forget that the only 60-year-old person in the room is you. You train them, and we train them. They’re the ones who are going to respond and keep you alive. That’s the way you should be looking at this.” That completely changed the mindset of whether we should be doing this for the kids or not.
Dr. Pepe: In fact, what we find is that that’s exactly who gets saved. I had case after case where the kids at the school had learned CPR and saved the teachers or the administrator at the high school or elementary school. It’s a fantastic point that you bring up, Dr. Malloy.
Dr. Glatter: One other brief thing we can interject here is that the team was excellent on field in that they evaluated Damar Hamlin in a primary survey sense of ABCs (i.e., airway, breathing, and circulation) for things like a tension pneumothorax. In the sense in which he was hit, there are reversible causes. Making sure he didn’t have a tension pneumothorax that caused the arrest, in my mind, was critical.
Dr. Pepe: We do the same thing on a day-to-day basis with a car wreck, because it could be that the person had ventricular fibrillation and then had the wreck. It’s not always trauma. That’s a fantastic point that you’re making. That’s exactly what I think happened, and that’s what we do.
Dr. Glatter: Well, thank you, gentlemen. This was an informative and helpful discussion for our audience. I appreciate your time and expertise.
Dr. Glatter, is an attending physician at Lenox Hill Hospital in New York City and assistant professor of emergency medicine at Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. He is an editorial adviser and hosts the Hot Topics in EM series on Medscape. He is also a medical contributor for Forbes.
Dr. Pepe is a professor of internal medicine, surgery, pediatrics, public health, and emergency medicine at University of Texas Health Science Center in Houston. He’s also a global coordinator of the U.S. Metropolitan Municipalities EMS Medical Directors (“Eagles”) Coalition.
Dr. Molloy works clinically as a consultant in emergency medicine in Wexford General Hospital, part of the Ireland East Hospital Group (IEHG). Internationally, he is a member of the Disaster Medicine Section of the European Society of Emergency Medicine (EUSEM) and has been appointed by the Irish Medical Organization (IMO) as one of two Irish delegates to serve on the European Board and Section of Emergency Medicine of the European Union of Medical Specialists (UEMS), having served for a number of years on its predecessor, the Multidisciplinary Joint Committee on Emergency Medicine.
A version of this article first appeared on Medscape.com.







