User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Would a national provider directory save docs’ time, help patients?
When a consumer uses a health plan provider directory to look up a physician, there’s a high probability that the entry for that doctor is incomplete or inaccurate. The Centers for Medicare & Medicaid Services would like to change that by creating a National Directory of Healthcare Providers and Services, which the agency believes would be more valuable to consumers.
In asking for public comments on whether and how it should establish the directory, CMS argues that this data repository would help patients locate physicians and could help with care coordination, health information exchange, and public health data reporting.
However, it’s not clear that such a directory would be any better than current insurance company listings or that people would use it. But a national directory could benefit physician practices by reducing their administrative work, according to observers.
In requesting public comment on the proposed national directory, CMS explains that provider organizations face “redundant and burdensome reporting requirements to multiple databases.” The directory could greatly reduce this challenge by requiring health care organizations to report provider information to a single database. Currently, physician practices have to submit these data to an average of 20 payers each, according to CMS.
“Right now, [physicians are] inundated with requests, and it takes a lot of time to update this stuff,” said David Zetter, a practice management consultant in Mechanicsburg, Pa.. “If there were one national repository of this information, that would be a good move.”
CMS envisions the National Directory as a central hub from which payers could obtain the latest provider data, which would be updated through a standardized application programming interface (API). Consequently, the insurers would no longer need to have providers submit this information to them separately.
CMS is soliciting input on what should be included in the directory. It notes that in addition to contact information, insurer directories also include a physicians’ specialties, health plan affiliations, and whether they accept new patients.
CMS’ 60-day public comment period ends Dec. 6. After that, the agency will decide what steps to take if it is decided that CMS has the legal authority to create the directory.
Terrible track record
In its annual reviews of health plan directories, CMS found that, from 2017 to 2022, only 47% of provider entries were complete. Only 73% of the providers could be matched to published directories. And only 28% of the provider names, addresses, and specialties in the directories matched those in the National Provider Identifier (NPI) registry.
Many of the mistakes in provider directories stem from errors made by practice staff, who have many other duties besides updating directory data. Yet an astonishing amount of time and effort is devoted to this task. A 2019 survey found that physician practices spend $2.76 billion annually on directory maintenance, or nearly $1000 per month per practice, on average.
The Council for Affordable Quality Healthcare, which conducted the survey, estimated that placing all directory data collection on a single platform could save the average practice $4,746 per year. For all practices in the United States, that works out to about $1.1 billion annually, CAQH said.
Pros and cons of national directory
For all the money spent on maintaining provider directories, consumers don’t use them very much. According to a 2021 Press Ganey survey, fewer than 5% of consumers seeking a primary care doctor get their information from an insurer or a benefits manager. About half search the internet first, and 24% seek a referral from a physician.
A national provider directory would be useful only if it were done right, Mr. Zetter said. Citing the inaccuracy and incompleteness of health plan directories, he said it was likely that a national directory would have similar problems. Data entered by practice staff would have to be automatically validated, perhaps through use of some kind of AI algorithm.
Effect on coordination of care
Mr. Zetter doubts the directory could improve care coordination, because primary care doctors usually refer patients to specialists they already know.
But Julia Adler-Milstein, PhD, professor of medicine and director of the Center for Clinical Informatics at the University of California, San Francisco, said that a national directory could improve communications among providers when patients select specialists outside of their primary care physician’s referral network.
“Especially if it’s not an established referral relationship, that’s where a national directory would be helpful, not only to locate the physicians but also to understand their preferences in how they’d like to receive information,” she said in an interview.
Dr. Adler-Milstein worries less than Mr. Zetter does about the challenge of ensuring the accuracy of data in the directory. She pointed out that the National Plan and Provider Enumeration System, which includes the NPI registry, has done a good job of validating provider name, address, and specialty information.
Dr. Adler-Milstein is more concerned about whether the proposed directory would address physician preferences as to how they wish to receive information. For example, while some physicians may prefer to be contacted directly, others may prefer or are required to communicate through their practices or health systems.
Efficiency in data exchange
The API used by the proposed directory would be based on the Fast Health Interoperability Resources standard that all electronic health record vendors must now include in their products. That raises the question of whether communications using contact information from the directory would be sent through a secure email system or through integrated EHR systems, Dr. Adler-Milstein said.
“I’m not sure whether the directory could support that [integration],” she said. “If it focuses on the concept of secure email exchange, that’s a relatively inefficient way of doing it,” because providers want clinical messages to pop up in their EHR workflow rather than their inboxes.
Nevertheless, Dr. Milstein-Adler added, the directory “would clearly take a lot of today’s manual work out of the system. I think organizations like UCSF would be very motivated to support the directory, knowing that people were going to a single source to find the updated information, including preferences in how we’d like people to communicate with us. There would be a lot of efficiency reasons for organizations to use this national directory.”
A version of this article first appeared on Medscape.com.
When a consumer uses a health plan provider directory to look up a physician, there’s a high probability that the entry for that doctor is incomplete or inaccurate. The Centers for Medicare & Medicaid Services would like to change that by creating a National Directory of Healthcare Providers and Services, which the agency believes would be more valuable to consumers.
In asking for public comments on whether and how it should establish the directory, CMS argues that this data repository would help patients locate physicians and could help with care coordination, health information exchange, and public health data reporting.
However, it’s not clear that such a directory would be any better than current insurance company listings or that people would use it. But a national directory could benefit physician practices by reducing their administrative work, according to observers.
In requesting public comment on the proposed national directory, CMS explains that provider organizations face “redundant and burdensome reporting requirements to multiple databases.” The directory could greatly reduce this challenge by requiring health care organizations to report provider information to a single database. Currently, physician practices have to submit these data to an average of 20 payers each, according to CMS.
“Right now, [physicians are] inundated with requests, and it takes a lot of time to update this stuff,” said David Zetter, a practice management consultant in Mechanicsburg, Pa.. “If there were one national repository of this information, that would be a good move.”
CMS envisions the National Directory as a central hub from which payers could obtain the latest provider data, which would be updated through a standardized application programming interface (API). Consequently, the insurers would no longer need to have providers submit this information to them separately.
CMS is soliciting input on what should be included in the directory. It notes that in addition to contact information, insurer directories also include a physicians’ specialties, health plan affiliations, and whether they accept new patients.
CMS’ 60-day public comment period ends Dec. 6. After that, the agency will decide what steps to take if it is decided that CMS has the legal authority to create the directory.
Terrible track record
In its annual reviews of health plan directories, CMS found that, from 2017 to 2022, only 47% of provider entries were complete. Only 73% of the providers could be matched to published directories. And only 28% of the provider names, addresses, and specialties in the directories matched those in the National Provider Identifier (NPI) registry.
Many of the mistakes in provider directories stem from errors made by practice staff, who have many other duties besides updating directory data. Yet an astonishing amount of time and effort is devoted to this task. A 2019 survey found that physician practices spend $2.76 billion annually on directory maintenance, or nearly $1000 per month per practice, on average.
The Council for Affordable Quality Healthcare, which conducted the survey, estimated that placing all directory data collection on a single platform could save the average practice $4,746 per year. For all practices in the United States, that works out to about $1.1 billion annually, CAQH said.
Pros and cons of national directory
For all the money spent on maintaining provider directories, consumers don’t use them very much. According to a 2021 Press Ganey survey, fewer than 5% of consumers seeking a primary care doctor get their information from an insurer or a benefits manager. About half search the internet first, and 24% seek a referral from a physician.
A national provider directory would be useful only if it were done right, Mr. Zetter said. Citing the inaccuracy and incompleteness of health plan directories, he said it was likely that a national directory would have similar problems. Data entered by practice staff would have to be automatically validated, perhaps through use of some kind of AI algorithm.
Effect on coordination of care
Mr. Zetter doubts the directory could improve care coordination, because primary care doctors usually refer patients to specialists they already know.
But Julia Adler-Milstein, PhD, professor of medicine and director of the Center for Clinical Informatics at the University of California, San Francisco, said that a national directory could improve communications among providers when patients select specialists outside of their primary care physician’s referral network.
“Especially if it’s not an established referral relationship, that’s where a national directory would be helpful, not only to locate the physicians but also to understand their preferences in how they’d like to receive information,” she said in an interview.
Dr. Adler-Milstein worries less than Mr. Zetter does about the challenge of ensuring the accuracy of data in the directory. She pointed out that the National Plan and Provider Enumeration System, which includes the NPI registry, has done a good job of validating provider name, address, and specialty information.
Dr. Adler-Milstein is more concerned about whether the proposed directory would address physician preferences as to how they wish to receive information. For example, while some physicians may prefer to be contacted directly, others may prefer or are required to communicate through their practices or health systems.
Efficiency in data exchange
The API used by the proposed directory would be based on the Fast Health Interoperability Resources standard that all electronic health record vendors must now include in their products. That raises the question of whether communications using contact information from the directory would be sent through a secure email system or through integrated EHR systems, Dr. Adler-Milstein said.
“I’m not sure whether the directory could support that [integration],” she said. “If it focuses on the concept of secure email exchange, that’s a relatively inefficient way of doing it,” because providers want clinical messages to pop up in their EHR workflow rather than their inboxes.
Nevertheless, Dr. Milstein-Adler added, the directory “would clearly take a lot of today’s manual work out of the system. I think organizations like UCSF would be very motivated to support the directory, knowing that people were going to a single source to find the updated information, including preferences in how we’d like people to communicate with us. There would be a lot of efficiency reasons for organizations to use this national directory.”
A version of this article first appeared on Medscape.com.
When a consumer uses a health plan provider directory to look up a physician, there’s a high probability that the entry for that doctor is incomplete or inaccurate. The Centers for Medicare & Medicaid Services would like to change that by creating a National Directory of Healthcare Providers and Services, which the agency believes would be more valuable to consumers.
In asking for public comments on whether and how it should establish the directory, CMS argues that this data repository would help patients locate physicians and could help with care coordination, health information exchange, and public health data reporting.
However, it’s not clear that such a directory would be any better than current insurance company listings or that people would use it. But a national directory could benefit physician practices by reducing their administrative work, according to observers.
In requesting public comment on the proposed national directory, CMS explains that provider organizations face “redundant and burdensome reporting requirements to multiple databases.” The directory could greatly reduce this challenge by requiring health care organizations to report provider information to a single database. Currently, physician practices have to submit these data to an average of 20 payers each, according to CMS.
“Right now, [physicians are] inundated with requests, and it takes a lot of time to update this stuff,” said David Zetter, a practice management consultant in Mechanicsburg, Pa.. “If there were one national repository of this information, that would be a good move.”
CMS envisions the National Directory as a central hub from which payers could obtain the latest provider data, which would be updated through a standardized application programming interface (API). Consequently, the insurers would no longer need to have providers submit this information to them separately.
CMS is soliciting input on what should be included in the directory. It notes that in addition to contact information, insurer directories also include a physicians’ specialties, health plan affiliations, and whether they accept new patients.
CMS’ 60-day public comment period ends Dec. 6. After that, the agency will decide what steps to take if it is decided that CMS has the legal authority to create the directory.
Terrible track record
In its annual reviews of health plan directories, CMS found that, from 2017 to 2022, only 47% of provider entries were complete. Only 73% of the providers could be matched to published directories. And only 28% of the provider names, addresses, and specialties in the directories matched those in the National Provider Identifier (NPI) registry.
Many of the mistakes in provider directories stem from errors made by practice staff, who have many other duties besides updating directory data. Yet an astonishing amount of time and effort is devoted to this task. A 2019 survey found that physician practices spend $2.76 billion annually on directory maintenance, or nearly $1000 per month per practice, on average.
The Council for Affordable Quality Healthcare, which conducted the survey, estimated that placing all directory data collection on a single platform could save the average practice $4,746 per year. For all practices in the United States, that works out to about $1.1 billion annually, CAQH said.
Pros and cons of national directory
For all the money spent on maintaining provider directories, consumers don’t use them very much. According to a 2021 Press Ganey survey, fewer than 5% of consumers seeking a primary care doctor get their information from an insurer or a benefits manager. About half search the internet first, and 24% seek a referral from a physician.
A national provider directory would be useful only if it were done right, Mr. Zetter said. Citing the inaccuracy and incompleteness of health plan directories, he said it was likely that a national directory would have similar problems. Data entered by practice staff would have to be automatically validated, perhaps through use of some kind of AI algorithm.
Effect on coordination of care
Mr. Zetter doubts the directory could improve care coordination, because primary care doctors usually refer patients to specialists they already know.
But Julia Adler-Milstein, PhD, professor of medicine and director of the Center for Clinical Informatics at the University of California, San Francisco, said that a national directory could improve communications among providers when patients select specialists outside of their primary care physician’s referral network.
“Especially if it’s not an established referral relationship, that’s where a national directory would be helpful, not only to locate the physicians but also to understand their preferences in how they’d like to receive information,” she said in an interview.
Dr. Adler-Milstein worries less than Mr. Zetter does about the challenge of ensuring the accuracy of data in the directory. She pointed out that the National Plan and Provider Enumeration System, which includes the NPI registry, has done a good job of validating provider name, address, and specialty information.
Dr. Adler-Milstein is more concerned about whether the proposed directory would address physician preferences as to how they wish to receive information. For example, while some physicians may prefer to be contacted directly, others may prefer or are required to communicate through their practices or health systems.
Efficiency in data exchange
The API used by the proposed directory would be based on the Fast Health Interoperability Resources standard that all electronic health record vendors must now include in their products. That raises the question of whether communications using contact information from the directory would be sent through a secure email system or through integrated EHR systems, Dr. Adler-Milstein said.
“I’m not sure whether the directory could support that [integration],” she said. “If it focuses on the concept of secure email exchange, that’s a relatively inefficient way of doing it,” because providers want clinical messages to pop up in their EHR workflow rather than their inboxes.
Nevertheless, Dr. Milstein-Adler added, the directory “would clearly take a lot of today’s manual work out of the system. I think organizations like UCSF would be very motivated to support the directory, knowing that people were going to a single source to find the updated information, including preferences in how we’d like people to communicate with us. There would be a lot of efficiency reasons for organizations to use this national directory.”
A version of this article first appeared on Medscape.com.
Is it flu, RSV, or COVID? Experts fear the ‘tripledemic’
Just when we thought this holiday season, finally, would be the back-to-normal one, some infectious disease experts are warning that a so-called “tripledemic” – influenza, COVID-19, and RSV – may be in the forecast.
The warning isn’t without basis.
The flu season has gotten an early start. As of Oct. 21, early increases in seasonal flu activity have been reported in most of the country, the Centers for Disease Control and Prevention said, with the southeast and south-central areas having the highest activity levels.
Children’s hospitals and EDs are seeing a surge in children with RSV.
COVID-19 cases are trending down, according to the CDC, but epidemiologists – scientists who study disease outbreaks – always have their eyes on emerging variants.
said Justin Lessler, PhD, a professor of epidemiology at the University of North Carolina at Chapel Hill. Dr. Lessler is on the coordinating team for the COVID-19 Scenario Modeling Hub, which aims to predict the course COVID-19, and the Flu Scenario Modeling Hub, which does the same for influenza.
For COVID-19, some models are predicting some spikes before Christmas, he said, and others see a new wave in 2023. For the flu, the model is predicting an earlier-than-usual start, as the CDC has reported.
While flu activity is relatively low, the CDC said, the season is off to an early start. For the week ending Oct. 21, 1,674 patients were hospitalized for flu, higher than in the summer months but fewer than the 2,675 hospitalizations for the week of May 15, 2022.
As of Oct. 20, COVID-19 cases have declined 12% over the last 2 weeks, nationwide. But hospitalizations are up 10% in much of the Northeast, The New York Times reports, and the improvement in cases and deaths has been slowing down.
As of Oct. 15, 15% of RSV tests reported nationwide were positive, compared with about 11% at that time in 2021, the CDC said. The surveillance collects information from 75 counties in 12 states.
Experts point out that the viruses – all three are respiratory viruses – are simply playing catchup.
“They spread the same way and along with lots of other viruses, and you tend to see an increase in them during the cold months,” said Timothy Brewer, MD, professor of medicine and epidemiology at UCLA.
The increase in all three viruses “is almost predictable at this point in the pandemic,” said Dean Blumberg, MD, a professor and chief of pediatric infectious diseases at the University of California Davis Health. “All the respiratory viruses are out of whack.”
Last year, RSV cases were up, too, and began to appear very early, he said, in the summer instead of in the cooler months. Flu also appeared early in 2021, as it has in 2022.
That contrasts with the flu season of 2020-2021, when COVID precautions were nearly universal, and cases were down. At UC Davis, “we didn’t have one pediatric admission due to influenza in the 2020-2021 [flu] season,” Dr. Blumberg said.
The number of pediatric flu deaths usually range from 37 to 199 per year, according to CDC records. But in the 2020-2021 season, the CDC recorded one pediatric flu death in the U.S.
Both children and adults have had less contact with others the past two seasons, Dr. Blumberg said, “and they don’t get the immunity they got with those infections [previously]. That’s why we are seeing out-of-season, early season [viruses].”
Eventually, he said, the cases of flu and RSV will return to previous levels. “It could be as soon as next year,” Dr. Blumberg said. And COVID-19, hopefully, will become like influenza, he said.
“RSV has always come around in the fall and winter,” said Elizabeth Murray, DO, a pediatric emergency medicine doctor at the University of Rochester (N.Y.) Medical Center and a spokesperson for the American Academy of Pediatrics. In 2022, children are back in school and for the most part not masking. “It’s a perfect storm for all the germs to spread now. They’ve just been waiting for their opportunity to come back.”
Self-care vs. not
RSV can pose a risk for anyone, but most at risk are children under age 5, especially infants under age 1, and adults over age 65. There is no vaccine for it. Symptoms include a runny nose, decreased appetite, coughing, sneezing, fever, and wheezing. But in young infants, there may only be decreased activity, crankiness, and breathing issues, the CDC said.
Keep an eye on the breathing if RSV is suspected, Dr. Murray tells parents. If your child can’t breathe easily, is unable to lie down comfortably, can’t speak clearly, or is sucking in the chest muscles to breathe, get medical help. Most kids with RSV can stay home and recover, she said, but often will need to be checked by a medical professional.
She advises against getting an oximeter to measure oxygen levels for home use. “They are often not accurate,” she said. If in doubt about how serious your child’s symptoms are, “don’t wait it out,” and don’t hesitate to call 911.
Symptoms of flu, COVID, and RSV can overlap. But each can involve breathing problems, which can be an emergency.
“It’s important to seek medical attention for any concerning symptoms, but especially severe shortness of breath or difficulty breathing, as these could signal the need for supplemental oxygen or other emergency interventions,” said Mandy De Vries, a respiratory therapist and director of education at the American Association for Respiratory Care. Inhalation treatment or mechanical ventilation may be needed for severe respiratory issues.
Precautions
To avoid the tripledemic – or any single infection – Timothy Brewer, MD, a professor of medicine and epidemiology at the University of California, Los Angeles, suggests some familiar measures: “Stay home if you’re feeling sick. Make sure you are up to date on your vaccinations. Wear a mask indoors.”
A version of this article first appeared on Medscape.com.
Just when we thought this holiday season, finally, would be the back-to-normal one, some infectious disease experts are warning that a so-called “tripledemic” – influenza, COVID-19, and RSV – may be in the forecast.
The warning isn’t without basis.
The flu season has gotten an early start. As of Oct. 21, early increases in seasonal flu activity have been reported in most of the country, the Centers for Disease Control and Prevention said, with the southeast and south-central areas having the highest activity levels.
Children’s hospitals and EDs are seeing a surge in children with RSV.
COVID-19 cases are trending down, according to the CDC, but epidemiologists – scientists who study disease outbreaks – always have their eyes on emerging variants.
said Justin Lessler, PhD, a professor of epidemiology at the University of North Carolina at Chapel Hill. Dr. Lessler is on the coordinating team for the COVID-19 Scenario Modeling Hub, which aims to predict the course COVID-19, and the Flu Scenario Modeling Hub, which does the same for influenza.
For COVID-19, some models are predicting some spikes before Christmas, he said, and others see a new wave in 2023. For the flu, the model is predicting an earlier-than-usual start, as the CDC has reported.
While flu activity is relatively low, the CDC said, the season is off to an early start. For the week ending Oct. 21, 1,674 patients were hospitalized for flu, higher than in the summer months but fewer than the 2,675 hospitalizations for the week of May 15, 2022.
As of Oct. 20, COVID-19 cases have declined 12% over the last 2 weeks, nationwide. But hospitalizations are up 10% in much of the Northeast, The New York Times reports, and the improvement in cases and deaths has been slowing down.
As of Oct. 15, 15% of RSV tests reported nationwide were positive, compared with about 11% at that time in 2021, the CDC said. The surveillance collects information from 75 counties in 12 states.
Experts point out that the viruses – all three are respiratory viruses – are simply playing catchup.
“They spread the same way and along with lots of other viruses, and you tend to see an increase in them during the cold months,” said Timothy Brewer, MD, professor of medicine and epidemiology at UCLA.
The increase in all three viruses “is almost predictable at this point in the pandemic,” said Dean Blumberg, MD, a professor and chief of pediatric infectious diseases at the University of California Davis Health. “All the respiratory viruses are out of whack.”
Last year, RSV cases were up, too, and began to appear very early, he said, in the summer instead of in the cooler months. Flu also appeared early in 2021, as it has in 2022.
That contrasts with the flu season of 2020-2021, when COVID precautions were nearly universal, and cases were down. At UC Davis, “we didn’t have one pediatric admission due to influenza in the 2020-2021 [flu] season,” Dr. Blumberg said.
The number of pediatric flu deaths usually range from 37 to 199 per year, according to CDC records. But in the 2020-2021 season, the CDC recorded one pediatric flu death in the U.S.
Both children and adults have had less contact with others the past two seasons, Dr. Blumberg said, “and they don’t get the immunity they got with those infections [previously]. That’s why we are seeing out-of-season, early season [viruses].”
Eventually, he said, the cases of flu and RSV will return to previous levels. “It could be as soon as next year,” Dr. Blumberg said. And COVID-19, hopefully, will become like influenza, he said.
“RSV has always come around in the fall and winter,” said Elizabeth Murray, DO, a pediatric emergency medicine doctor at the University of Rochester (N.Y.) Medical Center and a spokesperson for the American Academy of Pediatrics. In 2022, children are back in school and for the most part not masking. “It’s a perfect storm for all the germs to spread now. They’ve just been waiting for their opportunity to come back.”
Self-care vs. not
RSV can pose a risk for anyone, but most at risk are children under age 5, especially infants under age 1, and adults over age 65. There is no vaccine for it. Symptoms include a runny nose, decreased appetite, coughing, sneezing, fever, and wheezing. But in young infants, there may only be decreased activity, crankiness, and breathing issues, the CDC said.
Keep an eye on the breathing if RSV is suspected, Dr. Murray tells parents. If your child can’t breathe easily, is unable to lie down comfortably, can’t speak clearly, or is sucking in the chest muscles to breathe, get medical help. Most kids with RSV can stay home and recover, she said, but often will need to be checked by a medical professional.
She advises against getting an oximeter to measure oxygen levels for home use. “They are often not accurate,” she said. If in doubt about how serious your child’s symptoms are, “don’t wait it out,” and don’t hesitate to call 911.
Symptoms of flu, COVID, and RSV can overlap. But each can involve breathing problems, which can be an emergency.
“It’s important to seek medical attention for any concerning symptoms, but especially severe shortness of breath or difficulty breathing, as these could signal the need for supplemental oxygen or other emergency interventions,” said Mandy De Vries, a respiratory therapist and director of education at the American Association for Respiratory Care. Inhalation treatment or mechanical ventilation may be needed for severe respiratory issues.
Precautions
To avoid the tripledemic – or any single infection – Timothy Brewer, MD, a professor of medicine and epidemiology at the University of California, Los Angeles, suggests some familiar measures: “Stay home if you’re feeling sick. Make sure you are up to date on your vaccinations. Wear a mask indoors.”
A version of this article first appeared on Medscape.com.
Just when we thought this holiday season, finally, would be the back-to-normal one, some infectious disease experts are warning that a so-called “tripledemic” – influenza, COVID-19, and RSV – may be in the forecast.
The warning isn’t without basis.
The flu season has gotten an early start. As of Oct. 21, early increases in seasonal flu activity have been reported in most of the country, the Centers for Disease Control and Prevention said, with the southeast and south-central areas having the highest activity levels.
Children’s hospitals and EDs are seeing a surge in children with RSV.
COVID-19 cases are trending down, according to the CDC, but epidemiologists – scientists who study disease outbreaks – always have their eyes on emerging variants.
said Justin Lessler, PhD, a professor of epidemiology at the University of North Carolina at Chapel Hill. Dr. Lessler is on the coordinating team for the COVID-19 Scenario Modeling Hub, which aims to predict the course COVID-19, and the Flu Scenario Modeling Hub, which does the same for influenza.
For COVID-19, some models are predicting some spikes before Christmas, he said, and others see a new wave in 2023. For the flu, the model is predicting an earlier-than-usual start, as the CDC has reported.
While flu activity is relatively low, the CDC said, the season is off to an early start. For the week ending Oct. 21, 1,674 patients were hospitalized for flu, higher than in the summer months but fewer than the 2,675 hospitalizations for the week of May 15, 2022.
As of Oct. 20, COVID-19 cases have declined 12% over the last 2 weeks, nationwide. But hospitalizations are up 10% in much of the Northeast, The New York Times reports, and the improvement in cases and deaths has been slowing down.
As of Oct. 15, 15% of RSV tests reported nationwide were positive, compared with about 11% at that time in 2021, the CDC said. The surveillance collects information from 75 counties in 12 states.
Experts point out that the viruses – all three are respiratory viruses – are simply playing catchup.
“They spread the same way and along with lots of other viruses, and you tend to see an increase in them during the cold months,” said Timothy Brewer, MD, professor of medicine and epidemiology at UCLA.
The increase in all three viruses “is almost predictable at this point in the pandemic,” said Dean Blumberg, MD, a professor and chief of pediatric infectious diseases at the University of California Davis Health. “All the respiratory viruses are out of whack.”
Last year, RSV cases were up, too, and began to appear very early, he said, in the summer instead of in the cooler months. Flu also appeared early in 2021, as it has in 2022.
That contrasts with the flu season of 2020-2021, when COVID precautions were nearly universal, and cases were down. At UC Davis, “we didn’t have one pediatric admission due to influenza in the 2020-2021 [flu] season,” Dr. Blumberg said.
The number of pediatric flu deaths usually range from 37 to 199 per year, according to CDC records. But in the 2020-2021 season, the CDC recorded one pediatric flu death in the U.S.
Both children and adults have had less contact with others the past two seasons, Dr. Blumberg said, “and they don’t get the immunity they got with those infections [previously]. That’s why we are seeing out-of-season, early season [viruses].”
Eventually, he said, the cases of flu and RSV will return to previous levels. “It could be as soon as next year,” Dr. Blumberg said. And COVID-19, hopefully, will become like influenza, he said.
“RSV has always come around in the fall and winter,” said Elizabeth Murray, DO, a pediatric emergency medicine doctor at the University of Rochester (N.Y.) Medical Center and a spokesperson for the American Academy of Pediatrics. In 2022, children are back in school and for the most part not masking. “It’s a perfect storm for all the germs to spread now. They’ve just been waiting for their opportunity to come back.”
Self-care vs. not
RSV can pose a risk for anyone, but most at risk are children under age 5, especially infants under age 1, and adults over age 65. There is no vaccine for it. Symptoms include a runny nose, decreased appetite, coughing, sneezing, fever, and wheezing. But in young infants, there may only be decreased activity, crankiness, and breathing issues, the CDC said.
Keep an eye on the breathing if RSV is suspected, Dr. Murray tells parents. If your child can’t breathe easily, is unable to lie down comfortably, can’t speak clearly, or is sucking in the chest muscles to breathe, get medical help. Most kids with RSV can stay home and recover, she said, but often will need to be checked by a medical professional.
She advises against getting an oximeter to measure oxygen levels for home use. “They are often not accurate,” she said. If in doubt about how serious your child’s symptoms are, “don’t wait it out,” and don’t hesitate to call 911.
Symptoms of flu, COVID, and RSV can overlap. But each can involve breathing problems, which can be an emergency.
“It’s important to seek medical attention for any concerning symptoms, but especially severe shortness of breath or difficulty breathing, as these could signal the need for supplemental oxygen or other emergency interventions,” said Mandy De Vries, a respiratory therapist and director of education at the American Association for Respiratory Care. Inhalation treatment or mechanical ventilation may be needed for severe respiratory issues.
Precautions
To avoid the tripledemic – or any single infection – Timothy Brewer, MD, a professor of medicine and epidemiology at the University of California, Los Angeles, suggests some familiar measures: “Stay home if you’re feeling sick. Make sure you are up to date on your vaccinations. Wear a mask indoors.”
A version of this article first appeared on Medscape.com.
Many specialists are on the wrong side of the patient-jargon relationship
Doctor, doctor, gimme the news. I got a bad case of misidentifying you
There are a lot of medical specialties out there. A lot. Everything from allergists to urologists, with something like 150 subspecialties grouped in among the larger specialties. Can you name every one? Do you know what they do?
The point is, telling a patient or anyone in the general public that you’re an ophthalmologist may not be as helpful as you might think, if a recent study is to be believed. In a survey of 204 adults, conducted at the Minnesota State Fair of all places, researchers asked volunteers to define 14 different specialties, as well as five medical seniority titles.
The results were less than stellar. While more than 90% of people correctly defined what cardiologists and dermatologists do, 6 of the other 12 specialists were correctly identified by less than half of those surveyed. Nephrology was at the bottom, correctly identified by just 20% of the fair-attending public, followed by internists (21%), intensivists (29%), hospitalists (31%), pulmonologists (43%), and neonatologists at 48%. The hospitalists are particularly concerning. They’re doctors, but in hospitals. How hard is that? (Yes, it’s obviously more complicated than that, but still.)
The general public didn’t fare much better when it came to correctly lining up the order of progression from medical student to attending. Just 12% managed to place all five in the correct order of med student, intern, senior resident, fellow, then attending, with senior resident proving especially troublesome. More than 40% put senior resident at the end, compared with 27% for attending. Which does make a certain amount of sense, since it has senior in the name.
While the results speak for themselves – maybe elaborate on what the heck your fancy title actually means – it’s too bad the researchers didn’t throw in something really tricky. If two-thirds of the population can’t identify a hospitalist, just imagine how many people would misidentify an otolaryngologist.
Beach-to-table sand could fight obesity
People are always looking for the new weight loss solution. Whether it’s to just look good in a new pair of jeans or reduce the risk of cardiovascular disease, there are millions of diets and exercise routines out here. We’re here to tell you that the next new therapy to reduce fat comes from a very unsuspecting place: Sand.
Like sand from the beach and desert, sand? Well, yes and no.
The research involved engineered porous silica particles made from sand that are designed to have a high surface area. Investigators used a two-step GI model in which gastric digestion was modeled for 30 minutes, followed by a 60-minute intestinal phase, to show that the porous silica particles helped prevent fat and sugar adsorption within the GI tract.
By mimicking the gastrointestinal environment during digestion of a high-fat, high-carb meal, the researchers found that the porous silica created an “anti-obesity effect” by restricting the adsorption of those fats and carbohydrates.
Okay, but how is that on the tummy? Much gentler on the stomach than a drug such as orlistat, said senior researcher Paul Joyce, PhD, of the University of South Australia, Adelaide, who noted the lack of effective therapies without side effects, such as bloating, diarrhea, and abdominal pain, that deter people from treatment.
Obesity affects over 1.9 billion people worldwide, so the researchers think this could be a breakthrough. Reducing obesity may be one of the most preventable ways to reduce the risk of type 2 diabetes, heart disease, and other weight-related chronic conditions. A treatment solution this simple could be the answer to this global health crisis.
Who would have thought the solution would be as simple as sand? But how would the sand get in our stomachs? Do we sprinkle it on our food? Mix it in during cooking? Or will the sand come in pill form? We sure hope it’s that third one.
I am Reliebo. I am here to help you
Halloween is almost here, and the LOTME staff has been trying to make the office look as scary as possible: Headless vampires, ghost clowns, Ted Cruz, gray tombstones, pink hearts, green clovers, red balloons. Wait a second, those last three are Lucky Charms marshmallows, aren’t they? We’ll use those some other time.
What are we not using to decorate? Well, besides marshmallows from cereal, we’re not using Reliebo. That’s what we’re not using. Reliebo is a cute little fuzzy robot, and is not at all scary. Reliebo was designed to be the opposite of scary. Reliebo “may reduce fear as well as alleviate the perception of pain during medical treatments, including vaccinations,” senior author Fumihide Tanaka, PhD, of the University of Tsukuba (Japan) said in a written statement.
The soft, fur-covered robot contains small airbags that can inflate in response to hand movements. When study participants were subjected to a moderate heat stimulus on one arm, those who held the robot with the other arm experienced less pain than those who did not have a Reliebo.
The results also were encouraging when Dr. Tanaka and associates measured the levels of oxytocin and cortisol (biomarkers for stress) from the subjects’ saliva samples and evaluated their fear of injections and their psychological state before and after the experiments.
After looking at that photo of Reliebo for a while, though, we have to admit that we’re having a bit of a rethink about its cuteness. Is it cute, or weird-looking? An office full of fuzzy little inflating robots just could be seriously creepy. Please don’t tell the rest of the staff about this. We want to surprise them on Monday.
Doctor, doctor, gimme the news. I got a bad case of misidentifying you
There are a lot of medical specialties out there. A lot. Everything from allergists to urologists, with something like 150 subspecialties grouped in among the larger specialties. Can you name every one? Do you know what they do?
The point is, telling a patient or anyone in the general public that you’re an ophthalmologist may not be as helpful as you might think, if a recent study is to be believed. In a survey of 204 adults, conducted at the Minnesota State Fair of all places, researchers asked volunteers to define 14 different specialties, as well as five medical seniority titles.
The results were less than stellar. While more than 90% of people correctly defined what cardiologists and dermatologists do, 6 of the other 12 specialists were correctly identified by less than half of those surveyed. Nephrology was at the bottom, correctly identified by just 20% of the fair-attending public, followed by internists (21%), intensivists (29%), hospitalists (31%), pulmonologists (43%), and neonatologists at 48%. The hospitalists are particularly concerning. They’re doctors, but in hospitals. How hard is that? (Yes, it’s obviously more complicated than that, but still.)
The general public didn’t fare much better when it came to correctly lining up the order of progression from medical student to attending. Just 12% managed to place all five in the correct order of med student, intern, senior resident, fellow, then attending, with senior resident proving especially troublesome. More than 40% put senior resident at the end, compared with 27% for attending. Which does make a certain amount of sense, since it has senior in the name.
While the results speak for themselves – maybe elaborate on what the heck your fancy title actually means – it’s too bad the researchers didn’t throw in something really tricky. If two-thirds of the population can’t identify a hospitalist, just imagine how many people would misidentify an otolaryngologist.
Beach-to-table sand could fight obesity
People are always looking for the new weight loss solution. Whether it’s to just look good in a new pair of jeans or reduce the risk of cardiovascular disease, there are millions of diets and exercise routines out here. We’re here to tell you that the next new therapy to reduce fat comes from a very unsuspecting place: Sand.
Like sand from the beach and desert, sand? Well, yes and no.
The research involved engineered porous silica particles made from sand that are designed to have a high surface area. Investigators used a two-step GI model in which gastric digestion was modeled for 30 minutes, followed by a 60-minute intestinal phase, to show that the porous silica particles helped prevent fat and sugar adsorption within the GI tract.
By mimicking the gastrointestinal environment during digestion of a high-fat, high-carb meal, the researchers found that the porous silica created an “anti-obesity effect” by restricting the adsorption of those fats and carbohydrates.
Okay, but how is that on the tummy? Much gentler on the stomach than a drug such as orlistat, said senior researcher Paul Joyce, PhD, of the University of South Australia, Adelaide, who noted the lack of effective therapies without side effects, such as bloating, diarrhea, and abdominal pain, that deter people from treatment.
Obesity affects over 1.9 billion people worldwide, so the researchers think this could be a breakthrough. Reducing obesity may be one of the most preventable ways to reduce the risk of type 2 diabetes, heart disease, and other weight-related chronic conditions. A treatment solution this simple could be the answer to this global health crisis.
Who would have thought the solution would be as simple as sand? But how would the sand get in our stomachs? Do we sprinkle it on our food? Mix it in during cooking? Or will the sand come in pill form? We sure hope it’s that third one.
I am Reliebo. I am here to help you
Halloween is almost here, and the LOTME staff has been trying to make the office look as scary as possible: Headless vampires, ghost clowns, Ted Cruz, gray tombstones, pink hearts, green clovers, red balloons. Wait a second, those last three are Lucky Charms marshmallows, aren’t they? We’ll use those some other time.
What are we not using to decorate? Well, besides marshmallows from cereal, we’re not using Reliebo. That’s what we’re not using. Reliebo is a cute little fuzzy robot, and is not at all scary. Reliebo was designed to be the opposite of scary. Reliebo “may reduce fear as well as alleviate the perception of pain during medical treatments, including vaccinations,” senior author Fumihide Tanaka, PhD, of the University of Tsukuba (Japan) said in a written statement.
The soft, fur-covered robot contains small airbags that can inflate in response to hand movements. When study participants were subjected to a moderate heat stimulus on one arm, those who held the robot with the other arm experienced less pain than those who did not have a Reliebo.
The results also were encouraging when Dr. Tanaka and associates measured the levels of oxytocin and cortisol (biomarkers for stress) from the subjects’ saliva samples and evaluated their fear of injections and their psychological state before and after the experiments.
After looking at that photo of Reliebo for a while, though, we have to admit that we’re having a bit of a rethink about its cuteness. Is it cute, or weird-looking? An office full of fuzzy little inflating robots just could be seriously creepy. Please don’t tell the rest of the staff about this. We want to surprise them on Monday.
Doctor, doctor, gimme the news. I got a bad case of misidentifying you
There are a lot of medical specialties out there. A lot. Everything from allergists to urologists, with something like 150 subspecialties grouped in among the larger specialties. Can you name every one? Do you know what they do?
The point is, telling a patient or anyone in the general public that you’re an ophthalmologist may not be as helpful as you might think, if a recent study is to be believed. In a survey of 204 adults, conducted at the Minnesota State Fair of all places, researchers asked volunteers to define 14 different specialties, as well as five medical seniority titles.
The results were less than stellar. While more than 90% of people correctly defined what cardiologists and dermatologists do, 6 of the other 12 specialists were correctly identified by less than half of those surveyed. Nephrology was at the bottom, correctly identified by just 20% of the fair-attending public, followed by internists (21%), intensivists (29%), hospitalists (31%), pulmonologists (43%), and neonatologists at 48%. The hospitalists are particularly concerning. They’re doctors, but in hospitals. How hard is that? (Yes, it’s obviously more complicated than that, but still.)
The general public didn’t fare much better when it came to correctly lining up the order of progression from medical student to attending. Just 12% managed to place all five in the correct order of med student, intern, senior resident, fellow, then attending, with senior resident proving especially troublesome. More than 40% put senior resident at the end, compared with 27% for attending. Which does make a certain amount of sense, since it has senior in the name.
While the results speak for themselves – maybe elaborate on what the heck your fancy title actually means – it’s too bad the researchers didn’t throw in something really tricky. If two-thirds of the population can’t identify a hospitalist, just imagine how many people would misidentify an otolaryngologist.
Beach-to-table sand could fight obesity
People are always looking for the new weight loss solution. Whether it’s to just look good in a new pair of jeans or reduce the risk of cardiovascular disease, there are millions of diets and exercise routines out here. We’re here to tell you that the next new therapy to reduce fat comes from a very unsuspecting place: Sand.
Like sand from the beach and desert, sand? Well, yes and no.
The research involved engineered porous silica particles made from sand that are designed to have a high surface area. Investigators used a two-step GI model in which gastric digestion was modeled for 30 minutes, followed by a 60-minute intestinal phase, to show that the porous silica particles helped prevent fat and sugar adsorption within the GI tract.
By mimicking the gastrointestinal environment during digestion of a high-fat, high-carb meal, the researchers found that the porous silica created an “anti-obesity effect” by restricting the adsorption of those fats and carbohydrates.
Okay, but how is that on the tummy? Much gentler on the stomach than a drug such as orlistat, said senior researcher Paul Joyce, PhD, of the University of South Australia, Adelaide, who noted the lack of effective therapies without side effects, such as bloating, diarrhea, and abdominal pain, that deter people from treatment.
Obesity affects over 1.9 billion people worldwide, so the researchers think this could be a breakthrough. Reducing obesity may be one of the most preventable ways to reduce the risk of type 2 diabetes, heart disease, and other weight-related chronic conditions. A treatment solution this simple could be the answer to this global health crisis.
Who would have thought the solution would be as simple as sand? But how would the sand get in our stomachs? Do we sprinkle it on our food? Mix it in during cooking? Or will the sand come in pill form? We sure hope it’s that third one.
I am Reliebo. I am here to help you
Halloween is almost here, and the LOTME staff has been trying to make the office look as scary as possible: Headless vampires, ghost clowns, Ted Cruz, gray tombstones, pink hearts, green clovers, red balloons. Wait a second, those last three are Lucky Charms marshmallows, aren’t they? We’ll use those some other time.
What are we not using to decorate? Well, besides marshmallows from cereal, we’re not using Reliebo. That’s what we’re not using. Reliebo is a cute little fuzzy robot, and is not at all scary. Reliebo was designed to be the opposite of scary. Reliebo “may reduce fear as well as alleviate the perception of pain during medical treatments, including vaccinations,” senior author Fumihide Tanaka, PhD, of the University of Tsukuba (Japan) said in a written statement.
The soft, fur-covered robot contains small airbags that can inflate in response to hand movements. When study participants were subjected to a moderate heat stimulus on one arm, those who held the robot with the other arm experienced less pain than those who did not have a Reliebo.
The results also were encouraging when Dr. Tanaka and associates measured the levels of oxytocin and cortisol (biomarkers for stress) from the subjects’ saliva samples and evaluated their fear of injections and their psychological state before and after the experiments.
After looking at that photo of Reliebo for a while, though, we have to admit that we’re having a bit of a rethink about its cuteness. Is it cute, or weird-looking? An office full of fuzzy little inflating robots just could be seriously creepy. Please don’t tell the rest of the staff about this. We want to surprise them on Monday.
Ivermectin for COVID-19: Final nail in the coffin
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
It began in a petri dish.
Ivermectin, a widely available, cheap, and well-tolerated drug on the WHO’s list of essential medicines for its critical role in treating river blindness, was shown to dramatically reduce the proliferation of SARS-CoV-2 virus in cell culture.
You know the rest of the story. Despite the fact that the median inhibitory concentration in cell culture is about 100-fold higher than what one can achieve with oral dosing in humans, anecdotal reports of miraculous cures proliferated.
Cohort studies suggested that people who got ivermectin did very well in terms of COVID outcomes.
A narrative started to develop online – one that is still quite present today – that authorities were suppressing the good news about ivermectin in order to line their own pockets and those of the execs at Big Pharma. The official Twitter account of the Food and Drug Administration clapped back, reminding the populace that we are not horses or cows.
And every time a study came out that seemed like the nail in the coffin for the so-called horse paste, it rose again, vampire-like, feasting on the blood of social media outrage.
The truth is that, while excitement for ivermectin mounted online, it crashed quite quickly in scientific circles. Most randomized trials showed no effect of the drug. A couple of larger trials which seemed to show dramatic effects were subsequently shown to be fraudulent.
Then the TOGETHER trial was published. The 1,400-patient study from Brazil, which treated outpatients with COVID-19, found no significant difference in hospitalization or ER visits – the primary outcome – between those randomized to ivermectin vs. placebo or another therapy.
But still, Brazil. Different population than the United States. Different health systems. And very different rates of Strongyloides infections (this is a parasite that may be incidentally treated by ivermectin, leading to improvement independent of the drug’s effect on COVID). We all wanted a U.S. trial.
And now we have it. ACTIV-6 was published Oct. 21 in JAMA, a study randomizing outpatients with COVID-19 from 93 sites around the United States to ivermectin or placebo.
A total of 1,591 individuals – median age 47, 60% female – with confirmed symptomatic COVID-19 were randomized from June 2021 to February 2022. About half had been vaccinated.
The primary outcome was straightforward: time to clinical recovery. The time to recovery, defined as having three symptom-free days, was 12 days in the ivermectin group and 13 days in the placebo group – that’s within the margin of error.
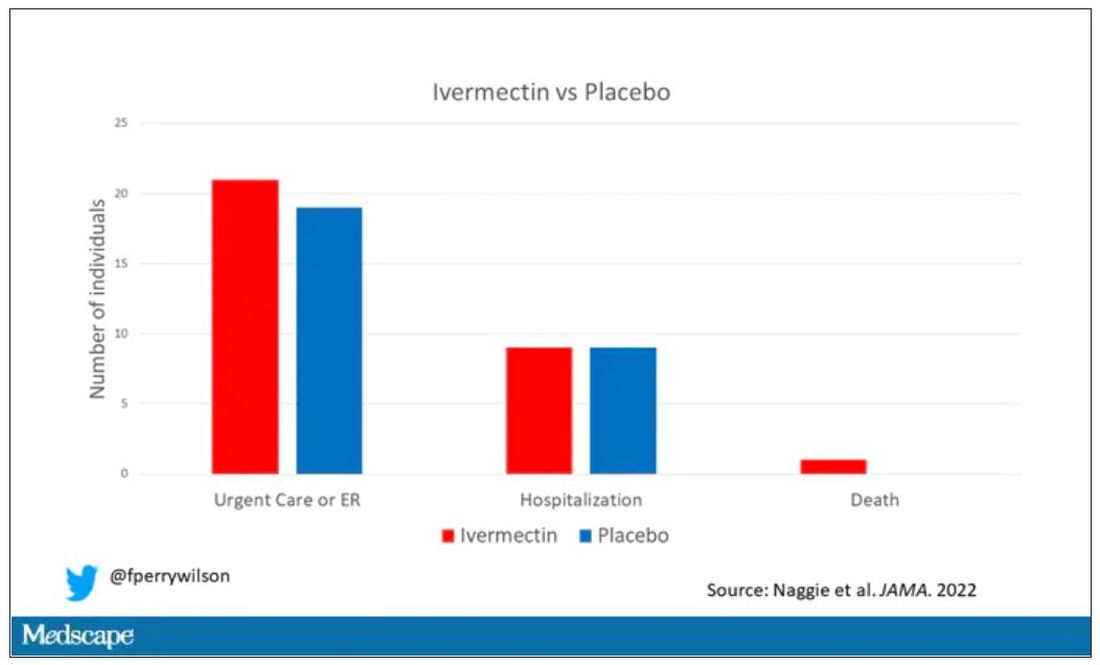
But overall, everyone in the trial did fairly well. Serious outcomes, like death, hospitalization, urgent care, or ER visits, occurred in 32 people in the ivermectin group and 28 in the placebo group. Death itself was rare – just one occurred in the trial, in someone receiving ivermectin.OK, are we done with this drug yet? Is this nice U.S. randomized trial enough to convince people that results from a petri dish don’t always transfer to humans, regardless of the presence or absence of an evil pharmaceutical cabal?
No, of course not. At this point, I can predict the responses. The dose wasn’t high enough. It wasn’t given early enough. The patients weren’t sick enough, or they were too sick. This is motivated reasoning, plain and simple. It’s not to say that there isn’t a chance that this drug has some off-target effects on COVID that we haven’t adequately measured, but studies like ACTIV-6 effectively rule out the idea that it’s a miracle cure. And you know what? That’s OK. Miracle cures are vanishingly rare. Most things that work in medicine work OK; they make us a little better, and we learn why they do that and improve on them, and try again and again. It’s not flashy; it doesn’t have that allure of secret knowledge. But it’s what separates science from magic.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator; his science communication work can be found in the Huffington Post, on NPR, and on Medscape.
A version of this article first appeared on Medscape.com.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
It began in a petri dish.
Ivermectin, a widely available, cheap, and well-tolerated drug on the WHO’s list of essential medicines for its critical role in treating river blindness, was shown to dramatically reduce the proliferation of SARS-CoV-2 virus in cell culture.
You know the rest of the story. Despite the fact that the median inhibitory concentration in cell culture is about 100-fold higher than what one can achieve with oral dosing in humans, anecdotal reports of miraculous cures proliferated.
Cohort studies suggested that people who got ivermectin did very well in terms of COVID outcomes.
A narrative started to develop online – one that is still quite present today – that authorities were suppressing the good news about ivermectin in order to line their own pockets and those of the execs at Big Pharma. The official Twitter account of the Food and Drug Administration clapped back, reminding the populace that we are not horses or cows.
And every time a study came out that seemed like the nail in the coffin for the so-called horse paste, it rose again, vampire-like, feasting on the blood of social media outrage.
The truth is that, while excitement for ivermectin mounted online, it crashed quite quickly in scientific circles. Most randomized trials showed no effect of the drug. A couple of larger trials which seemed to show dramatic effects were subsequently shown to be fraudulent.
Then the TOGETHER trial was published. The 1,400-patient study from Brazil, which treated outpatients with COVID-19, found no significant difference in hospitalization or ER visits – the primary outcome – between those randomized to ivermectin vs. placebo or another therapy.
But still, Brazil. Different population than the United States. Different health systems. And very different rates of Strongyloides infections (this is a parasite that may be incidentally treated by ivermectin, leading to improvement independent of the drug’s effect on COVID). We all wanted a U.S. trial.
And now we have it. ACTIV-6 was published Oct. 21 in JAMA, a study randomizing outpatients with COVID-19 from 93 sites around the United States to ivermectin or placebo.
A total of 1,591 individuals – median age 47, 60% female – with confirmed symptomatic COVID-19 were randomized from June 2021 to February 2022. About half had been vaccinated.
The primary outcome was straightforward: time to clinical recovery. The time to recovery, defined as having three symptom-free days, was 12 days in the ivermectin group and 13 days in the placebo group – that’s within the margin of error.

But overall, everyone in the trial did fairly well. Serious outcomes, like death, hospitalization, urgent care, or ER visits, occurred in 32 people in the ivermectin group and 28 in the placebo group. Death itself was rare – just one occurred in the trial, in someone receiving ivermectin.OK, are we done with this drug yet? Is this nice U.S. randomized trial enough to convince people that results from a petri dish don’t always transfer to humans, regardless of the presence or absence of an evil pharmaceutical cabal?
No, of course not. At this point, I can predict the responses. The dose wasn’t high enough. It wasn’t given early enough. The patients weren’t sick enough, or they were too sick. This is motivated reasoning, plain and simple. It’s not to say that there isn’t a chance that this drug has some off-target effects on COVID that we haven’t adequately measured, but studies like ACTIV-6 effectively rule out the idea that it’s a miracle cure. And you know what? That’s OK. Miracle cures are vanishingly rare. Most things that work in medicine work OK; they make us a little better, and we learn why they do that and improve on them, and try again and again. It’s not flashy; it doesn’t have that allure of secret knowledge. But it’s what separates science from magic.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator; his science communication work can be found in the Huffington Post, on NPR, and on Medscape.
A version of this article first appeared on Medscape.com.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
It began in a petri dish.
Ivermectin, a widely available, cheap, and well-tolerated drug on the WHO’s list of essential medicines for its critical role in treating river blindness, was shown to dramatically reduce the proliferation of SARS-CoV-2 virus in cell culture.
You know the rest of the story. Despite the fact that the median inhibitory concentration in cell culture is about 100-fold higher than what one can achieve with oral dosing in humans, anecdotal reports of miraculous cures proliferated.
Cohort studies suggested that people who got ivermectin did very well in terms of COVID outcomes.
A narrative started to develop online – one that is still quite present today – that authorities were suppressing the good news about ivermectin in order to line their own pockets and those of the execs at Big Pharma. The official Twitter account of the Food and Drug Administration clapped back, reminding the populace that we are not horses or cows.
And every time a study came out that seemed like the nail in the coffin for the so-called horse paste, it rose again, vampire-like, feasting on the blood of social media outrage.
The truth is that, while excitement for ivermectin mounted online, it crashed quite quickly in scientific circles. Most randomized trials showed no effect of the drug. A couple of larger trials which seemed to show dramatic effects were subsequently shown to be fraudulent.
Then the TOGETHER trial was published. The 1,400-patient study from Brazil, which treated outpatients with COVID-19, found no significant difference in hospitalization or ER visits – the primary outcome – between those randomized to ivermectin vs. placebo or another therapy.
But still, Brazil. Different population than the United States. Different health systems. And very different rates of Strongyloides infections (this is a parasite that may be incidentally treated by ivermectin, leading to improvement independent of the drug’s effect on COVID). We all wanted a U.S. trial.
And now we have it. ACTIV-6 was published Oct. 21 in JAMA, a study randomizing outpatients with COVID-19 from 93 sites around the United States to ivermectin or placebo.
A total of 1,591 individuals – median age 47, 60% female – with confirmed symptomatic COVID-19 were randomized from June 2021 to February 2022. About half had been vaccinated.
The primary outcome was straightforward: time to clinical recovery. The time to recovery, defined as having three symptom-free days, was 12 days in the ivermectin group and 13 days in the placebo group – that’s within the margin of error.

But overall, everyone in the trial did fairly well. Serious outcomes, like death, hospitalization, urgent care, or ER visits, occurred in 32 people in the ivermectin group and 28 in the placebo group. Death itself was rare – just one occurred in the trial, in someone receiving ivermectin.OK, are we done with this drug yet? Is this nice U.S. randomized trial enough to convince people that results from a petri dish don’t always transfer to humans, regardless of the presence or absence of an evil pharmaceutical cabal?
No, of course not. At this point, I can predict the responses. The dose wasn’t high enough. It wasn’t given early enough. The patients weren’t sick enough, or they were too sick. This is motivated reasoning, plain and simple. It’s not to say that there isn’t a chance that this drug has some off-target effects on COVID that we haven’t adequately measured, but studies like ACTIV-6 effectively rule out the idea that it’s a miracle cure. And you know what? That’s OK. Miracle cures are vanishingly rare. Most things that work in medicine work OK; they make us a little better, and we learn why they do that and improve on them, and try again and again. It’s not flashy; it doesn’t have that allure of secret knowledge. But it’s what separates science from magic.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator; his science communication work can be found in the Huffington Post, on NPR, and on Medscape.
A version of this article first appeared on Medscape.com.
Vitamin D deficiency linked to death, new study finds
Vitamin D deficiency increases mortality risk and raising levels even slightly could decrease the risk, researchers examining data from the UK Biobank have found.
They used a Mendelian randomization approach, which uses genetic variants as “proxy indicators” for external factors that affect vitamin D levels, such as sun exposure or dietary intake. It allows for analysis of the relationship between deficiency and outcomes including mortality, which can’t be done in randomized clinical trials for ethical reasons.
Using this method, nutritionist Joshua P. Sutherland, PhD, of the Australian Centre for Precision Health, Adelaide, and colleagues found an association between genetically predicted vitamin D levels [25-(OH)D] and mortality from several major causes, with evidence of causality among people with measured concentrations below, but not above, 50 nmol/L. The findings were published online in Annals of Internal Medicine.
“Unlike other types of observational studies, we have overcome some of the methodological obstacles. What is special about this new study is we were able to look at people with very low vitamin D concentrations and what would happen if their concentrations were a little bit higher. Most randomized controlled trials don’t show much of an effect. That’s because most people have sufficient concentrations. Ethically you can’t do a trial of people with very low levels without treating them,” senior author Elina Hypp
The data support the 50 nmol/L cut-off endorsed by the United States National Academy of Medicine and align with previous data suggesting the benefit of vitamin D supplementation is largely seen in people with deficiency.
“Everybody with vitamin D levels less than 50 nmol/L is recommended to increase their levels. Our results suggest there’s no need to go very high. The positive message is that if we are able to raise levels to just the current U.S. recommendations, that’s fine. There’s no need to use large supplement doses,” Dr. Hyppönen explained.
Thus, she advised, “Supplementation will clearly help, especially during wintertime or if a person isn’t getting enough vitamin D from the sun or in places where food isn’t fortified with vitamin D.”
But the data don’t support the approach of using large intermittent doses, she added.
“Sometimes doctors want to fix the deficiency quickly with a large ‘bolus’ dose, then continue with a maintenance dose. Increasing evidence suggests that’s not beneficial and might disturb the body’s metabolism so that it can’t get the amount it needs. It’s safe overall but might not work the way we want it to work.”
Rather, Dr. Hyppönen said, “My sense is that daily modest vitamin D dose supplementation when it’s needed is the best way forward.”
Genetic approach reveals causal relationship
The investigators analyzed data from 307,601 individuals in the UK Biobank, a prospective cohort of people recruited from England, Scotland, and Wales during March 2006 and July 2010. Most were of White European ancestry and were aged 37-73 years at baseline.
Genetically predicted vitamin D levels were estimated using 35 confirmed 25-(OH)D variants. Participants were followed for outcomes up to June 2020.
The average baseline measured 25-(OH)D concentration was 45.2 nmol/L, and 11.7% (n = 36,009) of participants had levels between 10.0 and 24.9 nmol/L. Higher levels were seen in people living in southern areas and nonsmokers as well as those with a higher level of physical activity, less socioeconomic deprivation, and lower body mass index.
During follow-up, 6.1% of participants died (n = 18,700). After adjustment for variables, odds ratios for all causes of mortality were highest among people with 25-(OH)D levels below 25 nmol/L and appeared to plateau between 50 and 75 nmol/L, with no further reduction in mortality at values of 75-125 nmol/L.
Mortality 36% higher in those deficient in vitamin D
The risk for mortality was a significant 36% higher for participants with 25-(OH)D 25 nmol/L compared with 50 nmol/L.
With the Mendelian randomization, there was an L-shaped association between genetically predicted 25-(OH)D level and all-cause mortality (P for nonlinearity < .001) and for mortality because of cancer and cardiovascular disease (P for nonlinearity ≤ .033).
Again, the strongest association with those outcomes and genetically predicted 25-(OH)D was found at levels below 25 nmol/L and a plateau was seen by 50 nmol/L.
Compared with a measured 25-(OH)D concentration of 50 nmol/L, investigators estimated that the genetically predicted odds of all-cause mortality would increase sixfold (odds ratio, 6.00) for participants at 10 nmol/L and by 25% (OR, 1.25) for those at 25 nmol/L.
And, compared with a measured 25-(OH)D concentration of 50 nmol/L, those with 10 nmol/L had genetically predicted odds ratios of 5.98 for cardiovascular mortality, 3.37 for cancer mortality, and 12.44 for respiratory mortality.
Comparing measured 25-(OH)D concentrations of 25 nmol/L versus 50 nmol/L, odds ratios for those outcomes were 1.25, 1.16, and 1.96 (95% confidence interval, 1.88-4.67), respectively. All were statistically significant.
Consistent results supportive of a causal effect of genetically predicted 25-(OH)D on all-cause mortality in those with low measured vitamin D concentrations were also found in a sensitivity analysis of 20,837 people of non-White ethnic origin.
The study was funded by the Australian National Health and Medical Research Council. Dr. Sutherland’s studentship is funded by an Australian Research Training Program Scholarship.
A version of this article first appeared on Medscape.com.
Vitamin D deficiency increases mortality risk and raising levels even slightly could decrease the risk, researchers examining data from the UK Biobank have found.
They used a Mendelian randomization approach, which uses genetic variants as “proxy indicators” for external factors that affect vitamin D levels, such as sun exposure or dietary intake. It allows for analysis of the relationship between deficiency and outcomes including mortality, which can’t be done in randomized clinical trials for ethical reasons.
Using this method, nutritionist Joshua P. Sutherland, PhD, of the Australian Centre for Precision Health, Adelaide, and colleagues found an association between genetically predicted vitamin D levels [25-(OH)D] and mortality from several major causes, with evidence of causality among people with measured concentrations below, but not above, 50 nmol/L. The findings were published online in Annals of Internal Medicine.
“Unlike other types of observational studies, we have overcome some of the methodological obstacles. What is special about this new study is we were able to look at people with very low vitamin D concentrations and what would happen if their concentrations were a little bit higher. Most randomized controlled trials don’t show much of an effect. That’s because most people have sufficient concentrations. Ethically you can’t do a trial of people with very low levels without treating them,” senior author Elina Hypp
The data support the 50 nmol/L cut-off endorsed by the United States National Academy of Medicine and align with previous data suggesting the benefit of vitamin D supplementation is largely seen in people with deficiency.
“Everybody with vitamin D levels less than 50 nmol/L is recommended to increase their levels. Our results suggest there’s no need to go very high. The positive message is that if we are able to raise levels to just the current U.S. recommendations, that’s fine. There’s no need to use large supplement doses,” Dr. Hyppönen explained.
Thus, she advised, “Supplementation will clearly help, especially during wintertime or if a person isn’t getting enough vitamin D from the sun or in places where food isn’t fortified with vitamin D.”
But the data don’t support the approach of using large intermittent doses, she added.
“Sometimes doctors want to fix the deficiency quickly with a large ‘bolus’ dose, then continue with a maintenance dose. Increasing evidence suggests that’s not beneficial and might disturb the body’s metabolism so that it can’t get the amount it needs. It’s safe overall but might not work the way we want it to work.”
Rather, Dr. Hyppönen said, “My sense is that daily modest vitamin D dose supplementation when it’s needed is the best way forward.”
Genetic approach reveals causal relationship
The investigators analyzed data from 307,601 individuals in the UK Biobank, a prospective cohort of people recruited from England, Scotland, and Wales during March 2006 and July 2010. Most were of White European ancestry and were aged 37-73 years at baseline.
Genetically predicted vitamin D levels were estimated using 35 confirmed 25-(OH)D variants. Participants were followed for outcomes up to June 2020.
The average baseline measured 25-(OH)D concentration was 45.2 nmol/L, and 11.7% (n = 36,009) of participants had levels between 10.0 and 24.9 nmol/L. Higher levels were seen in people living in southern areas and nonsmokers as well as those with a higher level of physical activity, less socioeconomic deprivation, and lower body mass index.
During follow-up, 6.1% of participants died (n = 18,700). After adjustment for variables, odds ratios for all causes of mortality were highest among people with 25-(OH)D levels below 25 nmol/L and appeared to plateau between 50 and 75 nmol/L, with no further reduction in mortality at values of 75-125 nmol/L.
Mortality 36% higher in those deficient in vitamin D
The risk for mortality was a significant 36% higher for participants with 25-(OH)D 25 nmol/L compared with 50 nmol/L.
With the Mendelian randomization, there was an L-shaped association between genetically predicted 25-(OH)D level and all-cause mortality (P for nonlinearity < .001) and for mortality because of cancer and cardiovascular disease (P for nonlinearity ≤ .033).
Again, the strongest association with those outcomes and genetically predicted 25-(OH)D was found at levels below 25 nmol/L and a plateau was seen by 50 nmol/L.
Compared with a measured 25-(OH)D concentration of 50 nmol/L, investigators estimated that the genetically predicted odds of all-cause mortality would increase sixfold (odds ratio, 6.00) for participants at 10 nmol/L and by 25% (OR, 1.25) for those at 25 nmol/L.
And, compared with a measured 25-(OH)D concentration of 50 nmol/L, those with 10 nmol/L had genetically predicted odds ratios of 5.98 for cardiovascular mortality, 3.37 for cancer mortality, and 12.44 for respiratory mortality.
Comparing measured 25-(OH)D concentrations of 25 nmol/L versus 50 nmol/L, odds ratios for those outcomes were 1.25, 1.16, and 1.96 (95% confidence interval, 1.88-4.67), respectively. All were statistically significant.
Consistent results supportive of a causal effect of genetically predicted 25-(OH)D on all-cause mortality in those with low measured vitamin D concentrations were also found in a sensitivity analysis of 20,837 people of non-White ethnic origin.
The study was funded by the Australian National Health and Medical Research Council. Dr. Sutherland’s studentship is funded by an Australian Research Training Program Scholarship.
A version of this article first appeared on Medscape.com.
Vitamin D deficiency increases mortality risk and raising levels even slightly could decrease the risk, researchers examining data from the UK Biobank have found.
They used a Mendelian randomization approach, which uses genetic variants as “proxy indicators” for external factors that affect vitamin D levels, such as sun exposure or dietary intake. It allows for analysis of the relationship between deficiency and outcomes including mortality, which can’t be done in randomized clinical trials for ethical reasons.
Using this method, nutritionist Joshua P. Sutherland, PhD, of the Australian Centre for Precision Health, Adelaide, and colleagues found an association between genetically predicted vitamin D levels [25-(OH)D] and mortality from several major causes, with evidence of causality among people with measured concentrations below, but not above, 50 nmol/L. The findings were published online in Annals of Internal Medicine.
“Unlike other types of observational studies, we have overcome some of the methodological obstacles. What is special about this new study is we were able to look at people with very low vitamin D concentrations and what would happen if their concentrations were a little bit higher. Most randomized controlled trials don’t show much of an effect. That’s because most people have sufficient concentrations. Ethically you can’t do a trial of people with very low levels without treating them,” senior author Elina Hypp
The data support the 50 nmol/L cut-off endorsed by the United States National Academy of Medicine and align with previous data suggesting the benefit of vitamin D supplementation is largely seen in people with deficiency.
“Everybody with vitamin D levels less than 50 nmol/L is recommended to increase their levels. Our results suggest there’s no need to go very high. The positive message is that if we are able to raise levels to just the current U.S. recommendations, that’s fine. There’s no need to use large supplement doses,” Dr. Hyppönen explained.
Thus, she advised, “Supplementation will clearly help, especially during wintertime or if a person isn’t getting enough vitamin D from the sun or in places where food isn’t fortified with vitamin D.”
But the data don’t support the approach of using large intermittent doses, she added.
“Sometimes doctors want to fix the deficiency quickly with a large ‘bolus’ dose, then continue with a maintenance dose. Increasing evidence suggests that’s not beneficial and might disturb the body’s metabolism so that it can’t get the amount it needs. It’s safe overall but might not work the way we want it to work.”
Rather, Dr. Hyppönen said, “My sense is that daily modest vitamin D dose supplementation when it’s needed is the best way forward.”
Genetic approach reveals causal relationship
The investigators analyzed data from 307,601 individuals in the UK Biobank, a prospective cohort of people recruited from England, Scotland, and Wales during March 2006 and July 2010. Most were of White European ancestry and were aged 37-73 years at baseline.
Genetically predicted vitamin D levels were estimated using 35 confirmed 25-(OH)D variants. Participants were followed for outcomes up to June 2020.
The average baseline measured 25-(OH)D concentration was 45.2 nmol/L, and 11.7% (n = 36,009) of participants had levels between 10.0 and 24.9 nmol/L. Higher levels were seen in people living in southern areas and nonsmokers as well as those with a higher level of physical activity, less socioeconomic deprivation, and lower body mass index.
During follow-up, 6.1% of participants died (n = 18,700). After adjustment for variables, odds ratios for all causes of mortality were highest among people with 25-(OH)D levels below 25 nmol/L and appeared to plateau between 50 and 75 nmol/L, with no further reduction in mortality at values of 75-125 nmol/L.
Mortality 36% higher in those deficient in vitamin D
The risk for mortality was a significant 36% higher for participants with 25-(OH)D 25 nmol/L compared with 50 nmol/L.
With the Mendelian randomization, there was an L-shaped association between genetically predicted 25-(OH)D level and all-cause mortality (P for nonlinearity < .001) and for mortality because of cancer and cardiovascular disease (P for nonlinearity ≤ .033).
Again, the strongest association with those outcomes and genetically predicted 25-(OH)D was found at levels below 25 nmol/L and a plateau was seen by 50 nmol/L.
Compared with a measured 25-(OH)D concentration of 50 nmol/L, investigators estimated that the genetically predicted odds of all-cause mortality would increase sixfold (odds ratio, 6.00) for participants at 10 nmol/L and by 25% (OR, 1.25) for those at 25 nmol/L.
And, compared with a measured 25-(OH)D concentration of 50 nmol/L, those with 10 nmol/L had genetically predicted odds ratios of 5.98 for cardiovascular mortality, 3.37 for cancer mortality, and 12.44 for respiratory mortality.
Comparing measured 25-(OH)D concentrations of 25 nmol/L versus 50 nmol/L, odds ratios for those outcomes were 1.25, 1.16, and 1.96 (95% confidence interval, 1.88-4.67), respectively. All were statistically significant.
Consistent results supportive of a causal effect of genetically predicted 25-(OH)D on all-cause mortality in those with low measured vitamin D concentrations were also found in a sensitivity analysis of 20,837 people of non-White ethnic origin.
The study was funded by the Australian National Health and Medical Research Council. Dr. Sutherland’s studentship is funded by an Australian Research Training Program Scholarship.
A version of this article first appeared on Medscape.com.
FROM ANNALS OF INTERNAL MEDICINE
Study reveals racial disparities in advanced HF therapies
A new study shows that Black Americans received ventricular assist devices (VADs) and heart transplants about half as often as White Americans, even when receiving care at an advanced heart failure (HF) center.
The analysis, drawn from 377 patients treated at one of 21 VAD centers in the United States as part of the RIVIVAL study, found that 22.3% of White adults received a heart transplant or VAD, compared with 11% of Black adults.
“That’s what is so concerning to us, that we’re seeing this pattern within this select population. I think it would be too reasonable to hypothesize that it very well could be worse in the general population,” study author Thomas Cascino, MD, MSc, University of Michigan, Ann Arbor, commented.
The study was published online in Circulation: Heart Failure, and it builds on previous work by the researchers, showing that patient preference for early VAD therapy is associated with higher New York Heart Association (NYHA) class and lower income level but not race.
In the present analysis, the number of Black and White participants who said they “definitely or probably” wanted VAD therapy was similar (27% vs. 29%), as was the number wanting “any and all life-sustaining therapies” (74% vs. 65%).
Two-thirds of the cohort was NYHA class III, the average EuroQoL visual analog scale (EQ-VAS) score was 64.6 among the 100 participants who identified as Black and 62.1 in the 277 White participants, and the average age was 58 and 61 years, respectively.
Death rates were also similar during the 2-year follow-up: 18% of Black patients and 13% of White patients.
After controlling for multiple clinical and social determinants of health, including age, Interagency Registry for Mechanically Assisted Circulator Support (INTERMACS) patient profile, EQ-VAS score, and level of education, Black participants had a 55% lower rate of VAD or transplant, compared with White participants (hazard ratio, 0.45; 95% confidence interval, 0.23-0.85). Adding VAD preference to the model did not affect the association.
“Our study suggests that we as providers may be making decisions differently,” Dr. Cascino said. “We can’t say for sure what the reasons are but certainly structural racism, discrimination, and provider biases are the things I worry about.”
“There’s an absolute need for us to look inwards, reflect, and acknowledge that we are likely playing a role in this and then start to be part of the change,” he added.
“The lives disabled or lost are simply too many,” coauthor Wendy Taddei-Peters, PhD, a clinical trials project official at the National Heart, Lung, and Blood Institute, said in an NIH statement. “An immediate step could be to require implicit bias training, particularly for transplant and VAD team members.”
Other suggestions are better tracking of underserved patients and the reasons why they do not receive VAD or become listed for transplant; inclusion of psychosocial components into decision-making about advanced therapy candidacy; and having “disparity experts” join in heart team meetings to help identify biases in real time.
Commenting on the study, Khadijah Breathett, MD, HF/transplant cardiologist and tenured associate professor of medicine, Indiana University Bloomington, said, “I’m glad there’s more push for awareness, because there’s still a population of people that don’t believe this is a real problem.”
Dr. Breathett, who is also a racial equity researcher, noted that the findings are similar to those of multiple studies suggesting racial disparities in HF care. In her own 2019 study of 400 providers shown identical clinical vignettes except for race, survey results and think-aloud interviews showed that decisions about advanced HF therapies are hierarchal and not democratic, social history and adherence are the most influential factors, and Black men are seen as not trustworthy and adherent, despite identical social histories, which ultimately led to White men being offered transplantation and Black men VAD implantation. The bias was particularly evident among older providers.
“This problem is real,” Dr. Breathett said. “The process of allocating life-saving therapies is not fair, and there is some level of discrimination that’s taking place towards persons of color, particularly Black patients. It’s time that we consider how we fix these issues.”
To see whether centers can move the needle and put systemic level changes into practice, Dr. Breathett and colleagues are launching the Seeking Objectivity in Allocation of Advanced Heart Failure (SOCIAL HF) Therapies Trial at 14 sites in the United States. It will measure the number of minority and female patients receiving advanced HF therapies at centers randomized to usual care or HF training, including evidence-based bias reduction training, use of objective measures of social support, and changes to facilitate group dynamics. The trial is set to start in January and be completed in September 2026.
“The main takeaway from this study is that it highlights and re-highlights the fact that racial disparities do exist in access to advanced therapy care,” Jaimin Trivedi, MD, MPH, associate professor of cardiothoracic surgery and director of clinical research and bioinformatics, University of Louisville, Ky., said in an interview.
He also called for education and training for all professionals, not just during residency or fellowship, to specifically identify issues with Black patients and encourage Black patients and their family members to get more involved in their HF care.
Dr. Trivedi said that further studies should examine why death rates were similar in the study despite the observed disparities in VAD implantation and transplantation.
He also pointed out that while patients in the study were treated from July 2015 to June 2016, a recent analysis by his team of the United Network for Organ Sharing (UNOS) database showed that 26% of transplants in 2019 were among Black patients, up from just 5% in 1987. “So, there are some encouraging signs as well.”
The study was funded by the National Institutes of Health/National Heart, Lung, and Blood Institute (NHLBI) and the National Center for Advancing Translational Sciences. Dr. Cascino reports having no relevant financial relationships. Four coauthors report financial relationships, including David Lanfear, who serves on the advisory board at Medscape. Dr. Breathett reported funding from multiple NHLBI grants.
A version of this article first appeared on Medscape.com.
A new study shows that Black Americans received ventricular assist devices (VADs) and heart transplants about half as often as White Americans, even when receiving care at an advanced heart failure (HF) center.
The analysis, drawn from 377 patients treated at one of 21 VAD centers in the United States as part of the RIVIVAL study, found that 22.3% of White adults received a heart transplant or VAD, compared with 11% of Black adults.
“That’s what is so concerning to us, that we’re seeing this pattern within this select population. I think it would be too reasonable to hypothesize that it very well could be worse in the general population,” study author Thomas Cascino, MD, MSc, University of Michigan, Ann Arbor, commented.
The study was published online in Circulation: Heart Failure, and it builds on previous work by the researchers, showing that patient preference for early VAD therapy is associated with higher New York Heart Association (NYHA) class and lower income level but not race.
In the present analysis, the number of Black and White participants who said they “definitely or probably” wanted VAD therapy was similar (27% vs. 29%), as was the number wanting “any and all life-sustaining therapies” (74% vs. 65%).
Two-thirds of the cohort was NYHA class III, the average EuroQoL visual analog scale (EQ-VAS) score was 64.6 among the 100 participants who identified as Black and 62.1 in the 277 White participants, and the average age was 58 and 61 years, respectively.
Death rates were also similar during the 2-year follow-up: 18% of Black patients and 13% of White patients.
After controlling for multiple clinical and social determinants of health, including age, Interagency Registry for Mechanically Assisted Circulator Support (INTERMACS) patient profile, EQ-VAS score, and level of education, Black participants had a 55% lower rate of VAD or transplant, compared with White participants (hazard ratio, 0.45; 95% confidence interval, 0.23-0.85). Adding VAD preference to the model did not affect the association.
“Our study suggests that we as providers may be making decisions differently,” Dr. Cascino said. “We can’t say for sure what the reasons are but certainly structural racism, discrimination, and provider biases are the things I worry about.”
“There’s an absolute need for us to look inwards, reflect, and acknowledge that we are likely playing a role in this and then start to be part of the change,” he added.
“The lives disabled or lost are simply too many,” coauthor Wendy Taddei-Peters, PhD, a clinical trials project official at the National Heart, Lung, and Blood Institute, said in an NIH statement. “An immediate step could be to require implicit bias training, particularly for transplant and VAD team members.”
Other suggestions are better tracking of underserved patients and the reasons why they do not receive VAD or become listed for transplant; inclusion of psychosocial components into decision-making about advanced therapy candidacy; and having “disparity experts” join in heart team meetings to help identify biases in real time.
Commenting on the study, Khadijah Breathett, MD, HF/transplant cardiologist and tenured associate professor of medicine, Indiana University Bloomington, said, “I’m glad there’s more push for awareness, because there’s still a population of people that don’t believe this is a real problem.”
Dr. Breathett, who is also a racial equity researcher, noted that the findings are similar to those of multiple studies suggesting racial disparities in HF care. In her own 2019 study of 400 providers shown identical clinical vignettes except for race, survey results and think-aloud interviews showed that decisions about advanced HF therapies are hierarchal and not democratic, social history and adherence are the most influential factors, and Black men are seen as not trustworthy and adherent, despite identical social histories, which ultimately led to White men being offered transplantation and Black men VAD implantation. The bias was particularly evident among older providers.
“This problem is real,” Dr. Breathett said. “The process of allocating life-saving therapies is not fair, and there is some level of discrimination that’s taking place towards persons of color, particularly Black patients. It’s time that we consider how we fix these issues.”
To see whether centers can move the needle and put systemic level changes into practice, Dr. Breathett and colleagues are launching the Seeking Objectivity in Allocation of Advanced Heart Failure (SOCIAL HF) Therapies Trial at 14 sites in the United States. It will measure the number of minority and female patients receiving advanced HF therapies at centers randomized to usual care or HF training, including evidence-based bias reduction training, use of objective measures of social support, and changes to facilitate group dynamics. The trial is set to start in January and be completed in September 2026.
“The main takeaway from this study is that it highlights and re-highlights the fact that racial disparities do exist in access to advanced therapy care,” Jaimin Trivedi, MD, MPH, associate professor of cardiothoracic surgery and director of clinical research and bioinformatics, University of Louisville, Ky., said in an interview.
He also called for education and training for all professionals, not just during residency or fellowship, to specifically identify issues with Black patients and encourage Black patients and their family members to get more involved in their HF care.
Dr. Trivedi said that further studies should examine why death rates were similar in the study despite the observed disparities in VAD implantation and transplantation.
He also pointed out that while patients in the study were treated from July 2015 to June 2016, a recent analysis by his team of the United Network for Organ Sharing (UNOS) database showed that 26% of transplants in 2019 were among Black patients, up from just 5% in 1987. “So, there are some encouraging signs as well.”
The study was funded by the National Institutes of Health/National Heart, Lung, and Blood Institute (NHLBI) and the National Center for Advancing Translational Sciences. Dr. Cascino reports having no relevant financial relationships. Four coauthors report financial relationships, including David Lanfear, who serves on the advisory board at Medscape. Dr. Breathett reported funding from multiple NHLBI grants.
A version of this article first appeared on Medscape.com.
A new study shows that Black Americans received ventricular assist devices (VADs) and heart transplants about half as often as White Americans, even when receiving care at an advanced heart failure (HF) center.
The analysis, drawn from 377 patients treated at one of 21 VAD centers in the United States as part of the RIVIVAL study, found that 22.3% of White adults received a heart transplant or VAD, compared with 11% of Black adults.
“That’s what is so concerning to us, that we’re seeing this pattern within this select population. I think it would be too reasonable to hypothesize that it very well could be worse in the general population,” study author Thomas Cascino, MD, MSc, University of Michigan, Ann Arbor, commented.
The study was published online in Circulation: Heart Failure, and it builds on previous work by the researchers, showing that patient preference for early VAD therapy is associated with higher New York Heart Association (NYHA) class and lower income level but not race.
In the present analysis, the number of Black and White participants who said they “definitely or probably” wanted VAD therapy was similar (27% vs. 29%), as was the number wanting “any and all life-sustaining therapies” (74% vs. 65%).
Two-thirds of the cohort was NYHA class III, the average EuroQoL visual analog scale (EQ-VAS) score was 64.6 among the 100 participants who identified as Black and 62.1 in the 277 White participants, and the average age was 58 and 61 years, respectively.
Death rates were also similar during the 2-year follow-up: 18% of Black patients and 13% of White patients.
After controlling for multiple clinical and social determinants of health, including age, Interagency Registry for Mechanically Assisted Circulator Support (INTERMACS) patient profile, EQ-VAS score, and level of education, Black participants had a 55% lower rate of VAD or transplant, compared with White participants (hazard ratio, 0.45; 95% confidence interval, 0.23-0.85). Adding VAD preference to the model did not affect the association.
“Our study suggests that we as providers may be making decisions differently,” Dr. Cascino said. “We can’t say for sure what the reasons are but certainly structural racism, discrimination, and provider biases are the things I worry about.”
“There’s an absolute need for us to look inwards, reflect, and acknowledge that we are likely playing a role in this and then start to be part of the change,” he added.
“The lives disabled or lost are simply too many,” coauthor Wendy Taddei-Peters, PhD, a clinical trials project official at the National Heart, Lung, and Blood Institute, said in an NIH statement. “An immediate step could be to require implicit bias training, particularly for transplant and VAD team members.”
Other suggestions are better tracking of underserved patients and the reasons why they do not receive VAD or become listed for transplant; inclusion of psychosocial components into decision-making about advanced therapy candidacy; and having “disparity experts” join in heart team meetings to help identify biases in real time.
Commenting on the study, Khadijah Breathett, MD, HF/transplant cardiologist and tenured associate professor of medicine, Indiana University Bloomington, said, “I’m glad there’s more push for awareness, because there’s still a population of people that don’t believe this is a real problem.”
Dr. Breathett, who is also a racial equity researcher, noted that the findings are similar to those of multiple studies suggesting racial disparities in HF care. In her own 2019 study of 400 providers shown identical clinical vignettes except for race, survey results and think-aloud interviews showed that decisions about advanced HF therapies are hierarchal and not democratic, social history and adherence are the most influential factors, and Black men are seen as not trustworthy and adherent, despite identical social histories, which ultimately led to White men being offered transplantation and Black men VAD implantation. The bias was particularly evident among older providers.
“This problem is real,” Dr. Breathett said. “The process of allocating life-saving therapies is not fair, and there is some level of discrimination that’s taking place towards persons of color, particularly Black patients. It’s time that we consider how we fix these issues.”
To see whether centers can move the needle and put systemic level changes into practice, Dr. Breathett and colleagues are launching the Seeking Objectivity in Allocation of Advanced Heart Failure (SOCIAL HF) Therapies Trial at 14 sites in the United States. It will measure the number of minority and female patients receiving advanced HF therapies at centers randomized to usual care or HF training, including evidence-based bias reduction training, use of objective measures of social support, and changes to facilitate group dynamics. The trial is set to start in January and be completed in September 2026.
“The main takeaway from this study is that it highlights and re-highlights the fact that racial disparities do exist in access to advanced therapy care,” Jaimin Trivedi, MD, MPH, associate professor of cardiothoracic surgery and director of clinical research and bioinformatics, University of Louisville, Ky., said in an interview.
He also called for education and training for all professionals, not just during residency or fellowship, to specifically identify issues with Black patients and encourage Black patients and their family members to get more involved in their HF care.
Dr. Trivedi said that further studies should examine why death rates were similar in the study despite the observed disparities in VAD implantation and transplantation.
He also pointed out that while patients in the study were treated from July 2015 to June 2016, a recent analysis by his team of the United Network for Organ Sharing (UNOS) database showed that 26% of transplants in 2019 were among Black patients, up from just 5% in 1987. “So, there are some encouraging signs as well.”
The study was funded by the National Institutes of Health/National Heart, Lung, and Blood Institute (NHLBI) and the National Center for Advancing Translational Sciences. Dr. Cascino reports having no relevant financial relationships. Four coauthors report financial relationships, including David Lanfear, who serves on the advisory board at Medscape. Dr. Breathett reported funding from multiple NHLBI grants.
A version of this article first appeared on Medscape.com.
Four commonly abused drugs linked with atrial fibrillation
Cocaine, methamphetamine, opioids, and cannabis may independently increase risk of atrial fibrillation (AFib), based on data from almost 24 million people.
While more work is needed to uncover causal links, physicians should be aware that these commonly abused substances could be driving new cases of AFib, reported investigators from the University of California, San Francisco.
“Though alcohol and tobacco smoking have each been associated with a heightened risk of [AFib], relationships between other drug use and [AFib] are poorly understood,” they wrote in European Heart Journal.
Some previous studies have ventured into this terrain, but most focused on fatal arrhythmias, or offered anecdotal evidence. This knowledge gap is particularly concerning for cannabis, the researchers noted, as medical and recreational use are on the rise.
The present analysis included data from 23.5 million adults in California who received care through a hospital, emergency department, or outpatient surgery center during 2005-2015. Based on ICD-9 diagnostic codes, 132,834 of these patients used cannabis, 98,271 used methamphetamines, 48,701 used cocaine, and 10,032 used opiates. Inclusion required lack of AFib at baseline.
Reliance on ICD-9 codes makes the data “quite specific,” but lacking sensitivity, according to principal author Gregory M. Marcus, MD, cardiologist and professor of medicine at UCSF.
“If they were designated as using these drugs, that is very likely true,” Dr. Marcus said in an interview. “But certainly, the absence of any mention of use of these drugs does not exclude the possibility that some people were still using them. That would not create spurious false-positive relationships; if anything, it attenuates existing relationships.”
In other words, using ICD-9 codes reduced the power to detect an association between each drug and AFib, meaning any relationship needed to be sufficiently strong enough to generate a significant result.
At the end of the decade-long study period, 998,747 patients (4.2%) had developed incident AFib. After adjusting for potential confounders and mediators, all four drugs showed significant, independent associations with AFib. Methamphetamines presented the greatest risk (hazard ratio, 1.86%), followed by opiates (HR, 1.74), cocaine (HR, 1.61), and cannabis (HR, 1.35).
“Our findings provide the first evidence utilizing a longitudinal cohort to demonstrate that cannabis use predicts the future onset of AFib,” Dr. Marcus and colleagues wrote.
Dose-response relationships were not detected for any of the substances; however, usage levels were also derived from ICD-9 codes, which may have been insufficient for this purpose, according to the investigators.
Causal mechanisms deserve a closer look
Causal links between AFib and each of the drugs remain unclear. Citing prior research, Dr. Marcus and colleagues explained how methamphetamines are capable of “significant cardiac electrical remodeling,” while cocaine may cause sodium channel dysregulation, and opioids can render atrial myocytes more susceptible to oxidative damage. Although cannabis has previously been linked with hospitalization for arrhythmia, a pharmacologic driver of this phenomenon remains largely unexamined.
“We don’t know for sure precisely what the constituents are that are responsible for our findings,” Dr. Marcus said. “It’s possible that there are some effects that are much more generic, such as inhaling a burned substance. There is good evidence that if you inhale pretty much any sort of particulate matter, that increases inflammation in the body. Inflammation is known to be a trigger for atrial fibrillation.”
Alternatively, all four drugs – whether stimulants or depressants – cause “quite dramatic and often rapid effects on the autonomic nervous system,” Dr. Marcus said, noting that these rapid swings are a known trigger for AFib.
Brian Olshansky, MD, emeritus professor of internal medicine-cardiovascular medicine at the University of Iowa, Iowa City, suggested that nonpharmacologic factors are likely also playing a role.
“All these drugs have slightly different mechanisms of action, so there’s not one mechanism that would explain why all of them would cause atrial fibrillation,” Dr. Olshansky said in an interview. “That does suggest that there’s something else going on, besides just the drug itself. It would be potentially concerning if we were to lay the blame totally on these drugs.”
Dr. Olshansky, who recently coauthored a review of stimulant drugs and arrhythmias, suggested that lifestyle, comorbidities, and drug impurities may have added to the risk of AF.
“[The investigators] did try to correct for that kind of stuff, but it’s very hard to correct for a lot of the issues that may be ongoing with individuals who partake in these drugs,” Dr. Olshansky said in an interview. “They may not be a healthy lot, in general.”
Still, considering previous data linking drugs of abuse with arrhythmias, he said the detected risks were “intriguing,” and deserved a closer look.
“It’s a nice groundbreaking study, with regard to the fact that they showed unique relationships that we don’t completely understand,” Dr. Olshansky said. “It opens up a new opportunity for further investigation.”
The investigators disclosed relationships with InCarda, Baylis Medical, Johnson & Johnson, and others. Dr. Olshansky disclosed no relevant competing interests.
Cocaine, methamphetamine, opioids, and cannabis may independently increase risk of atrial fibrillation (AFib), based on data from almost 24 million people.
While more work is needed to uncover causal links, physicians should be aware that these commonly abused substances could be driving new cases of AFib, reported investigators from the University of California, San Francisco.
“Though alcohol and tobacco smoking have each been associated with a heightened risk of [AFib], relationships between other drug use and [AFib] are poorly understood,” they wrote in European Heart Journal.
Some previous studies have ventured into this terrain, but most focused on fatal arrhythmias, or offered anecdotal evidence. This knowledge gap is particularly concerning for cannabis, the researchers noted, as medical and recreational use are on the rise.
The present analysis included data from 23.5 million adults in California who received care through a hospital, emergency department, or outpatient surgery center during 2005-2015. Based on ICD-9 diagnostic codes, 132,834 of these patients used cannabis, 98,271 used methamphetamines, 48,701 used cocaine, and 10,032 used opiates. Inclusion required lack of AFib at baseline.
Reliance on ICD-9 codes makes the data “quite specific,” but lacking sensitivity, according to principal author Gregory M. Marcus, MD, cardiologist and professor of medicine at UCSF.
“If they were designated as using these drugs, that is very likely true,” Dr. Marcus said in an interview. “But certainly, the absence of any mention of use of these drugs does not exclude the possibility that some people were still using them. That would not create spurious false-positive relationships; if anything, it attenuates existing relationships.”
In other words, using ICD-9 codes reduced the power to detect an association between each drug and AFib, meaning any relationship needed to be sufficiently strong enough to generate a significant result.
At the end of the decade-long study period, 998,747 patients (4.2%) had developed incident AFib. After adjusting for potential confounders and mediators, all four drugs showed significant, independent associations with AFib. Methamphetamines presented the greatest risk (hazard ratio, 1.86%), followed by opiates (HR, 1.74), cocaine (HR, 1.61), and cannabis (HR, 1.35).
“Our findings provide the first evidence utilizing a longitudinal cohort to demonstrate that cannabis use predicts the future onset of AFib,” Dr. Marcus and colleagues wrote.
Dose-response relationships were not detected for any of the substances; however, usage levels were also derived from ICD-9 codes, which may have been insufficient for this purpose, according to the investigators.
Causal mechanisms deserve a closer look
Causal links between AFib and each of the drugs remain unclear. Citing prior research, Dr. Marcus and colleagues explained how methamphetamines are capable of “significant cardiac electrical remodeling,” while cocaine may cause sodium channel dysregulation, and opioids can render atrial myocytes more susceptible to oxidative damage. Although cannabis has previously been linked with hospitalization for arrhythmia, a pharmacologic driver of this phenomenon remains largely unexamined.
“We don’t know for sure precisely what the constituents are that are responsible for our findings,” Dr. Marcus said. “It’s possible that there are some effects that are much more generic, such as inhaling a burned substance. There is good evidence that if you inhale pretty much any sort of particulate matter, that increases inflammation in the body. Inflammation is known to be a trigger for atrial fibrillation.”
Alternatively, all four drugs – whether stimulants or depressants – cause “quite dramatic and often rapid effects on the autonomic nervous system,” Dr. Marcus said, noting that these rapid swings are a known trigger for AFib.
Brian Olshansky, MD, emeritus professor of internal medicine-cardiovascular medicine at the University of Iowa, Iowa City, suggested that nonpharmacologic factors are likely also playing a role.
“All these drugs have slightly different mechanisms of action, so there’s not one mechanism that would explain why all of them would cause atrial fibrillation,” Dr. Olshansky said in an interview. “That does suggest that there’s something else going on, besides just the drug itself. It would be potentially concerning if we were to lay the blame totally on these drugs.”
Dr. Olshansky, who recently coauthored a review of stimulant drugs and arrhythmias, suggested that lifestyle, comorbidities, and drug impurities may have added to the risk of AF.
“[The investigators] did try to correct for that kind of stuff, but it’s very hard to correct for a lot of the issues that may be ongoing with individuals who partake in these drugs,” Dr. Olshansky said in an interview. “They may not be a healthy lot, in general.”
Still, considering previous data linking drugs of abuse with arrhythmias, he said the detected risks were “intriguing,” and deserved a closer look.
“It’s a nice groundbreaking study, with regard to the fact that they showed unique relationships that we don’t completely understand,” Dr. Olshansky said. “It opens up a new opportunity for further investigation.”
The investigators disclosed relationships with InCarda, Baylis Medical, Johnson & Johnson, and others. Dr. Olshansky disclosed no relevant competing interests.
Cocaine, methamphetamine, opioids, and cannabis may independently increase risk of atrial fibrillation (AFib), based on data from almost 24 million people.
While more work is needed to uncover causal links, physicians should be aware that these commonly abused substances could be driving new cases of AFib, reported investigators from the University of California, San Francisco.
“Though alcohol and tobacco smoking have each been associated with a heightened risk of [AFib], relationships between other drug use and [AFib] are poorly understood,” they wrote in European Heart Journal.
Some previous studies have ventured into this terrain, but most focused on fatal arrhythmias, or offered anecdotal evidence. This knowledge gap is particularly concerning for cannabis, the researchers noted, as medical and recreational use are on the rise.
The present analysis included data from 23.5 million adults in California who received care through a hospital, emergency department, or outpatient surgery center during 2005-2015. Based on ICD-9 diagnostic codes, 132,834 of these patients used cannabis, 98,271 used methamphetamines, 48,701 used cocaine, and 10,032 used opiates. Inclusion required lack of AFib at baseline.
Reliance on ICD-9 codes makes the data “quite specific,” but lacking sensitivity, according to principal author Gregory M. Marcus, MD, cardiologist and professor of medicine at UCSF.
“If they were designated as using these drugs, that is very likely true,” Dr. Marcus said in an interview. “But certainly, the absence of any mention of use of these drugs does not exclude the possibility that some people were still using them. That would not create spurious false-positive relationships; if anything, it attenuates existing relationships.”
In other words, using ICD-9 codes reduced the power to detect an association between each drug and AFib, meaning any relationship needed to be sufficiently strong enough to generate a significant result.
At the end of the decade-long study period, 998,747 patients (4.2%) had developed incident AFib. After adjusting for potential confounders and mediators, all four drugs showed significant, independent associations with AFib. Methamphetamines presented the greatest risk (hazard ratio, 1.86%), followed by opiates (HR, 1.74), cocaine (HR, 1.61), and cannabis (HR, 1.35).
“Our findings provide the first evidence utilizing a longitudinal cohort to demonstrate that cannabis use predicts the future onset of AFib,” Dr. Marcus and colleagues wrote.
Dose-response relationships were not detected for any of the substances; however, usage levels were also derived from ICD-9 codes, which may have been insufficient for this purpose, according to the investigators.
Causal mechanisms deserve a closer look
Causal links between AFib and each of the drugs remain unclear. Citing prior research, Dr. Marcus and colleagues explained how methamphetamines are capable of “significant cardiac electrical remodeling,” while cocaine may cause sodium channel dysregulation, and opioids can render atrial myocytes more susceptible to oxidative damage. Although cannabis has previously been linked with hospitalization for arrhythmia, a pharmacologic driver of this phenomenon remains largely unexamined.
“We don’t know for sure precisely what the constituents are that are responsible for our findings,” Dr. Marcus said. “It’s possible that there are some effects that are much more generic, such as inhaling a burned substance. There is good evidence that if you inhale pretty much any sort of particulate matter, that increases inflammation in the body. Inflammation is known to be a trigger for atrial fibrillation.”
Alternatively, all four drugs – whether stimulants or depressants – cause “quite dramatic and often rapid effects on the autonomic nervous system,” Dr. Marcus said, noting that these rapid swings are a known trigger for AFib.
Brian Olshansky, MD, emeritus professor of internal medicine-cardiovascular medicine at the University of Iowa, Iowa City, suggested that nonpharmacologic factors are likely also playing a role.
“All these drugs have slightly different mechanisms of action, so there’s not one mechanism that would explain why all of them would cause atrial fibrillation,” Dr. Olshansky said in an interview. “That does suggest that there’s something else going on, besides just the drug itself. It would be potentially concerning if we were to lay the blame totally on these drugs.”
Dr. Olshansky, who recently coauthored a review of stimulant drugs and arrhythmias, suggested that lifestyle, comorbidities, and drug impurities may have added to the risk of AF.
“[The investigators] did try to correct for that kind of stuff, but it’s very hard to correct for a lot of the issues that may be ongoing with individuals who partake in these drugs,” Dr. Olshansky said in an interview. “They may not be a healthy lot, in general.”
Still, considering previous data linking drugs of abuse with arrhythmias, he said the detected risks were “intriguing,” and deserved a closer look.
“It’s a nice groundbreaking study, with regard to the fact that they showed unique relationships that we don’t completely understand,” Dr. Olshansky said. “It opens up a new opportunity for further investigation.”
The investigators disclosed relationships with InCarda, Baylis Medical, Johnson & Johnson, and others. Dr. Olshansky disclosed no relevant competing interests.
FROM EUROPEAN HEART JOURNAL
Children and COVID: Weekly cases fall to lowest level in over a year
With the third autumn of the COVID era now upon us, the discussion has turned again to a possible influenza/COVID twindemic, as well as the new-for-2022 influenza/COVID/respiratory syncytial virus tripledemic. It appears, however, that COVID may have missed the memo.
For the sixth time in the last 7 weeks, the number of new COVID cases in children fell, with just under 23,000 reported during the week of Oct. 14-20, according to the American Academy of Pediatrics and the Children’s Hospital Association. That is the lowest weekly count so far this year, and the lowest since early July of 2021, just as the Delta surge was starting. New pediatric cases had dipped to 8,500, the lowest for any week during the pandemic, a couple of weeks before that, the AAP/CHA data show.
Weekly cases have fallen by almost 75% since over 90,000 were reported for the week of Aug. 26 to Sept. 1, even as children have returned to school and vaccine uptake remains slow in the youngest age groups. Rates of emergency department visits with diagnosed COVID also have continued to drop, as have new admissions, and both are nearing their 2021 lows, according to the Centers for Disease Control and Prevention.
New vaccinations in children under age 5 years were up slightly for the most recent week (Oct. 13-19), but total uptake for that age group is only 7.1% for an initial dose and 2.9% for full vaccination. Among children aged 5-11 years, 38.7% have received at least one dose and 31.6% have completed the primary series, with corresponding figures of 71.2% and 60.9% for those aged 12-17, the CDC said on its COVID Data Tracker.
Despite the low overall numbers, though, the youngest children are, in one respect, punching above their weight when it comes to vaccinations. In the 2 weeks from Oct. 6 to Oct. 19, children under 5 years of age, who represent 5.9% of the U.S. population, received 9.2% of the initial vaccine doses administered. Children aged 5-11 years, who represent 8.7% of the total population, got just 4.2% of all first doses over those same 2 weeks, while 12- to 17-year-olds, who make up 7.6% of the population, got 3.4% of the vaccine doses, the CDC reported.
On the vaccine-approval front, the Food and Drug Administration recently announced that the new bivalent COVID-19 vaccines are now included in the emergency use authorizations for children who have completed primary or booster vaccination. The Moderna vaccine is authorized as a single-dose booster for children as young as 6 years and the Pfizer-BioNTech vaccine can be given as a single booster dose in children as young as 5 years, the FDA said.
“These bivalent COVID-19 vaccines include an mRNA component of the original strain to provide an immune response that is broadly protective against COVID-19 and an mRNA component in common between the omicron variant BA.4 and BA.5 lineages,” the FDA said.
With the third autumn of the COVID era now upon us, the discussion has turned again to a possible influenza/COVID twindemic, as well as the new-for-2022 influenza/COVID/respiratory syncytial virus tripledemic. It appears, however, that COVID may have missed the memo.
For the sixth time in the last 7 weeks, the number of new COVID cases in children fell, with just under 23,000 reported during the week of Oct. 14-20, according to the American Academy of Pediatrics and the Children’s Hospital Association. That is the lowest weekly count so far this year, and the lowest since early July of 2021, just as the Delta surge was starting. New pediatric cases had dipped to 8,500, the lowest for any week during the pandemic, a couple of weeks before that, the AAP/CHA data show.
Weekly cases have fallen by almost 75% since over 90,000 were reported for the week of Aug. 26 to Sept. 1, even as children have returned to school and vaccine uptake remains slow in the youngest age groups. Rates of emergency department visits with diagnosed COVID also have continued to drop, as have new admissions, and both are nearing their 2021 lows, according to the Centers for Disease Control and Prevention.
New vaccinations in children under age 5 years were up slightly for the most recent week (Oct. 13-19), but total uptake for that age group is only 7.1% for an initial dose and 2.9% for full vaccination. Among children aged 5-11 years, 38.7% have received at least one dose and 31.6% have completed the primary series, with corresponding figures of 71.2% and 60.9% for those aged 12-17, the CDC said on its COVID Data Tracker.
Despite the low overall numbers, though, the youngest children are, in one respect, punching above their weight when it comes to vaccinations. In the 2 weeks from Oct. 6 to Oct. 19, children under 5 years of age, who represent 5.9% of the U.S. population, received 9.2% of the initial vaccine doses administered. Children aged 5-11 years, who represent 8.7% of the total population, got just 4.2% of all first doses over those same 2 weeks, while 12- to 17-year-olds, who make up 7.6% of the population, got 3.4% of the vaccine doses, the CDC reported.
On the vaccine-approval front, the Food and Drug Administration recently announced that the new bivalent COVID-19 vaccines are now included in the emergency use authorizations for children who have completed primary or booster vaccination. The Moderna vaccine is authorized as a single-dose booster for children as young as 6 years and the Pfizer-BioNTech vaccine can be given as a single booster dose in children as young as 5 years, the FDA said.
“These bivalent COVID-19 vaccines include an mRNA component of the original strain to provide an immune response that is broadly protective against COVID-19 and an mRNA component in common between the omicron variant BA.4 and BA.5 lineages,” the FDA said.
With the third autumn of the COVID era now upon us, the discussion has turned again to a possible influenza/COVID twindemic, as well as the new-for-2022 influenza/COVID/respiratory syncytial virus tripledemic. It appears, however, that COVID may have missed the memo.
For the sixth time in the last 7 weeks, the number of new COVID cases in children fell, with just under 23,000 reported during the week of Oct. 14-20, according to the American Academy of Pediatrics and the Children’s Hospital Association. That is the lowest weekly count so far this year, and the lowest since early July of 2021, just as the Delta surge was starting. New pediatric cases had dipped to 8,500, the lowest for any week during the pandemic, a couple of weeks before that, the AAP/CHA data show.
Weekly cases have fallen by almost 75% since over 90,000 were reported for the week of Aug. 26 to Sept. 1, even as children have returned to school and vaccine uptake remains slow in the youngest age groups. Rates of emergency department visits with diagnosed COVID also have continued to drop, as have new admissions, and both are nearing their 2021 lows, according to the Centers for Disease Control and Prevention.
New vaccinations in children under age 5 years were up slightly for the most recent week (Oct. 13-19), but total uptake for that age group is only 7.1% for an initial dose and 2.9% for full vaccination. Among children aged 5-11 years, 38.7% have received at least one dose and 31.6% have completed the primary series, with corresponding figures of 71.2% and 60.9% for those aged 12-17, the CDC said on its COVID Data Tracker.
Despite the low overall numbers, though, the youngest children are, in one respect, punching above their weight when it comes to vaccinations. In the 2 weeks from Oct. 6 to Oct. 19, children under 5 years of age, who represent 5.9% of the U.S. population, received 9.2% of the initial vaccine doses administered. Children aged 5-11 years, who represent 8.7% of the total population, got just 4.2% of all first doses over those same 2 weeks, while 12- to 17-year-olds, who make up 7.6% of the population, got 3.4% of the vaccine doses, the CDC reported.
On the vaccine-approval front, the Food and Drug Administration recently announced that the new bivalent COVID-19 vaccines are now included in the emergency use authorizations for children who have completed primary or booster vaccination. The Moderna vaccine is authorized as a single-dose booster for children as young as 6 years and the Pfizer-BioNTech vaccine can be given as a single booster dose in children as young as 5 years, the FDA said.
“These bivalent COVID-19 vaccines include an mRNA component of the original strain to provide an immune response that is broadly protective against COVID-19 and an mRNA component in common between the omicron variant BA.4 and BA.5 lineages,” the FDA said.
Time to ditch clarithromycin for H. pylori?
Rates of resistance to clarithromycin among Helicobacter pylori isolates in the United States and Europe are high enough to warrant discontinuation of empiric use of proton pump inhibitor (PPI)–based triple therapy that includes the antibiotic in these regions, a new study has found.
Overall, 22.2% of participants were resistant to clarithromycin – a rate that is above the currently recommended threshold of 15% or higher for avoidance of PPI-based triple therapy that includes clarithromycin.
, study investigator William Chey, MD, professor and chief, Division of Gastroenterology and Hepatology, Michigan Medicine, Ann Arbor, said in an interview.
Judith Kim, MD, a gastroenterologist at NYU Langone Health and clinical instructor of medicine at NYU Grossman School of Medicine, who wasn’t involved in the study, agrees.
“The use of PPI-based triple therapy is still common practice despite recent recommendations to avoid clarithromycin in areas with high resistance rates,” Dr. Kim told this news organization.
“This study shows that multiple parts of the United States and Europe have high resistance rates,” rendering clarithromycin-based regimens “more likely to ineffectively eradicate H pylori,” Dr. Kim said.
The study was published online in The American Journal of Gastroenterology.
Better options now available
Guidelines advise against the use of PPI-based triple regimens with clarithromycin for H. pylori infection in areas where resistance is 15% or higher or for patients who have previously received macrolides. However, up-to-date information on H. pylori antimicrobial resistance patterns is limited, especially in the United States.
Dr. Chey and colleagues assessed resistance rates to antibiotics commonly used to treat H. pylori in isolates from 907 adults with the infection in the United States and Europe. They included four U.S. subregions and five participating European countries.
In all U.S. subregions and European countries, clarithromycin resistance rates were above 15% except possibly in the United Kingdom, where the sample size was too small to provide a reliable estimate.
Three-quarters of the clarithromycin-resistant isolates were also resistant to metronidazole.
The study also found that, overall, 1.2% of patients had isolates that were resistant to amoxicillin, and 69.2% had isolates resistant to metronidazole. Resistance patterns were similar in the United States and Europe; metronidazole resistance was the most common (50%-79% of isolates), and amoxicillin was the least common (≤ 5%).
“Overall, these data provide robust evidence to support a shift away from the default empiric prescription of triple combinations containing a PPI and clarithromycin for H. pylori infection in the United States and Europe,” the study team writes.
The high prevalence of resistance, including dual resistance, highlights the need for antibiotic stewardship and resistance surveillance, as well as novel treatment strategies for H. pylori infection, they add.
Last spring, as previously reported, the United States Food and Drug Administration approved two vonoprazan-based treatments for H. pylori: Voquezna Triple Pak (vonoprazan, amoxicillin, clarithromycin) and Voquezna Dual Pak (vonoprazan, amoxicillin), both from Phathom Pharmaceuticals.
“Vonoprazan-based treatment may be superior to standard PPI triple therapy for clarithromycin-resistant infections based on prior studies and is a potential good option,” Dr. Kim said.
Still, she added, she “would most likely first recommend regimens that do not have clarithromycin, such as bismuth quadruple therapy.”
Study’s importance
Because the study drew upon the largest dataset to date on U.S. resistance rates, it should be used to more precisely guide first-line therapy decisions, said Richard Peek, Jr., MD, professor of medicine and director of gastroenterology at Vanderbilt University Medical Center, Nashville, Tenn.
“To date, there has been a dearth of information in the United States regarding H. pylori resistance rates, which has often led to the use of ineffective empiric therapies and inappropriate exposure to antibiotics,” Dr. Peek, who wasn’t involved in the study, told this news organization.
“These data are particularly exciting when viewed within the context of new genomic sequencing tests that can determine H. pylori resistance patterns using DNA isolated from the stomach or the stool,” he said.
Dr. Peek agreed that the recent approval of vonoprazan-based therapies “adds another regimen to the therapeutic armamentarium available for eradicating H. pylori, and its value seems to be particularly beneficial for eradication failures.”
The research was funded by Phathom Pharmaceuticals. Dr. Chey is a board member of the American College of Gastroenterology, GI on Demand, the International Foundation of Functional GI Disorders, and the Rome Foundation. He has received compensation as a consultant from AbbVie, Alfasigma, Allakos, Alnylam, Bayer, BioAmerica, Cosmo, Intrinsic Medicine, Ironwood Pharmaceuticals, QOL Medical, Nestle, Phathom Pharmaceuticals, RedHill Biopharma, Salix/Valeant, Takeda, Urovant, and Vibrant; grant/research support from BioAmerica, Commonwealth Diagnostics International, QOL Medical, Salix, and Vibrant; owns stock/stock options in GI on Demand and Modify Health; and owns patents relating to methods and kits for identifying food sensitivities and intolerances, digital manometry, and a rectal expulsion device. Dr. Peek and Dr. Kim report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Rates of resistance to clarithromycin among Helicobacter pylori isolates in the United States and Europe are high enough to warrant discontinuation of empiric use of proton pump inhibitor (PPI)–based triple therapy that includes the antibiotic in these regions, a new study has found.
Overall, 22.2% of participants were resistant to clarithromycin – a rate that is above the currently recommended threshold of 15% or higher for avoidance of PPI-based triple therapy that includes clarithromycin.
, study investigator William Chey, MD, professor and chief, Division of Gastroenterology and Hepatology, Michigan Medicine, Ann Arbor, said in an interview.
Judith Kim, MD, a gastroenterologist at NYU Langone Health and clinical instructor of medicine at NYU Grossman School of Medicine, who wasn’t involved in the study, agrees.
“The use of PPI-based triple therapy is still common practice despite recent recommendations to avoid clarithromycin in areas with high resistance rates,” Dr. Kim told this news organization.
“This study shows that multiple parts of the United States and Europe have high resistance rates,” rendering clarithromycin-based regimens “more likely to ineffectively eradicate H pylori,” Dr. Kim said.
The study was published online in The American Journal of Gastroenterology.
Better options now available
Guidelines advise against the use of PPI-based triple regimens with clarithromycin for H. pylori infection in areas where resistance is 15% or higher or for patients who have previously received macrolides. However, up-to-date information on H. pylori antimicrobial resistance patterns is limited, especially in the United States.
Dr. Chey and colleagues assessed resistance rates to antibiotics commonly used to treat H. pylori in isolates from 907 adults with the infection in the United States and Europe. They included four U.S. subregions and five participating European countries.
In all U.S. subregions and European countries, clarithromycin resistance rates were above 15% except possibly in the United Kingdom, where the sample size was too small to provide a reliable estimate.
Three-quarters of the clarithromycin-resistant isolates were also resistant to metronidazole.
The study also found that, overall, 1.2% of patients had isolates that were resistant to amoxicillin, and 69.2% had isolates resistant to metronidazole. Resistance patterns were similar in the United States and Europe; metronidazole resistance was the most common (50%-79% of isolates), and amoxicillin was the least common (≤ 5%).
“Overall, these data provide robust evidence to support a shift away from the default empiric prescription of triple combinations containing a PPI and clarithromycin for H. pylori infection in the United States and Europe,” the study team writes.
The high prevalence of resistance, including dual resistance, highlights the need for antibiotic stewardship and resistance surveillance, as well as novel treatment strategies for H. pylori infection, they add.
Last spring, as previously reported, the United States Food and Drug Administration approved two vonoprazan-based treatments for H. pylori: Voquezna Triple Pak (vonoprazan, amoxicillin, clarithromycin) and Voquezna Dual Pak (vonoprazan, amoxicillin), both from Phathom Pharmaceuticals.
“Vonoprazan-based treatment may be superior to standard PPI triple therapy for clarithromycin-resistant infections based on prior studies and is a potential good option,” Dr. Kim said.
Still, she added, she “would most likely first recommend regimens that do not have clarithromycin, such as bismuth quadruple therapy.”
Study’s importance
Because the study drew upon the largest dataset to date on U.S. resistance rates, it should be used to more precisely guide first-line therapy decisions, said Richard Peek, Jr., MD, professor of medicine and director of gastroenterology at Vanderbilt University Medical Center, Nashville, Tenn.
“To date, there has been a dearth of information in the United States regarding H. pylori resistance rates, which has often led to the use of ineffective empiric therapies and inappropriate exposure to antibiotics,” Dr. Peek, who wasn’t involved in the study, told this news organization.
“These data are particularly exciting when viewed within the context of new genomic sequencing tests that can determine H. pylori resistance patterns using DNA isolated from the stomach or the stool,” he said.
Dr. Peek agreed that the recent approval of vonoprazan-based therapies “adds another regimen to the therapeutic armamentarium available for eradicating H. pylori, and its value seems to be particularly beneficial for eradication failures.”
The research was funded by Phathom Pharmaceuticals. Dr. Chey is a board member of the American College of Gastroenterology, GI on Demand, the International Foundation of Functional GI Disorders, and the Rome Foundation. He has received compensation as a consultant from AbbVie, Alfasigma, Allakos, Alnylam, Bayer, BioAmerica, Cosmo, Intrinsic Medicine, Ironwood Pharmaceuticals, QOL Medical, Nestle, Phathom Pharmaceuticals, RedHill Biopharma, Salix/Valeant, Takeda, Urovant, and Vibrant; grant/research support from BioAmerica, Commonwealth Diagnostics International, QOL Medical, Salix, and Vibrant; owns stock/stock options in GI on Demand and Modify Health; and owns patents relating to methods and kits for identifying food sensitivities and intolerances, digital manometry, and a rectal expulsion device. Dr. Peek and Dr. Kim report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Rates of resistance to clarithromycin among Helicobacter pylori isolates in the United States and Europe are high enough to warrant discontinuation of empiric use of proton pump inhibitor (PPI)–based triple therapy that includes the antibiotic in these regions, a new study has found.
Overall, 22.2% of participants were resistant to clarithromycin – a rate that is above the currently recommended threshold of 15% or higher for avoidance of PPI-based triple therapy that includes clarithromycin.
, study investigator William Chey, MD, professor and chief, Division of Gastroenterology and Hepatology, Michigan Medicine, Ann Arbor, said in an interview.
Judith Kim, MD, a gastroenterologist at NYU Langone Health and clinical instructor of medicine at NYU Grossman School of Medicine, who wasn’t involved in the study, agrees.
“The use of PPI-based triple therapy is still common practice despite recent recommendations to avoid clarithromycin in areas with high resistance rates,” Dr. Kim told this news organization.
“This study shows that multiple parts of the United States and Europe have high resistance rates,” rendering clarithromycin-based regimens “more likely to ineffectively eradicate H pylori,” Dr. Kim said.
The study was published online in The American Journal of Gastroenterology.
Better options now available
Guidelines advise against the use of PPI-based triple regimens with clarithromycin for H. pylori infection in areas where resistance is 15% or higher or for patients who have previously received macrolides. However, up-to-date information on H. pylori antimicrobial resistance patterns is limited, especially in the United States.
Dr. Chey and colleagues assessed resistance rates to antibiotics commonly used to treat H. pylori in isolates from 907 adults with the infection in the United States and Europe. They included four U.S. subregions and five participating European countries.
In all U.S. subregions and European countries, clarithromycin resistance rates were above 15% except possibly in the United Kingdom, where the sample size was too small to provide a reliable estimate.
Three-quarters of the clarithromycin-resistant isolates were also resistant to metronidazole.
The study also found that, overall, 1.2% of patients had isolates that were resistant to amoxicillin, and 69.2% had isolates resistant to metronidazole. Resistance patterns were similar in the United States and Europe; metronidazole resistance was the most common (50%-79% of isolates), and amoxicillin was the least common (≤ 5%).
“Overall, these data provide robust evidence to support a shift away from the default empiric prescription of triple combinations containing a PPI and clarithromycin for H. pylori infection in the United States and Europe,” the study team writes.
The high prevalence of resistance, including dual resistance, highlights the need for antibiotic stewardship and resistance surveillance, as well as novel treatment strategies for H. pylori infection, they add.
Last spring, as previously reported, the United States Food and Drug Administration approved two vonoprazan-based treatments for H. pylori: Voquezna Triple Pak (vonoprazan, amoxicillin, clarithromycin) and Voquezna Dual Pak (vonoprazan, amoxicillin), both from Phathom Pharmaceuticals.
“Vonoprazan-based treatment may be superior to standard PPI triple therapy for clarithromycin-resistant infections based on prior studies and is a potential good option,” Dr. Kim said.
Still, she added, she “would most likely first recommend regimens that do not have clarithromycin, such as bismuth quadruple therapy.”
Study’s importance
Because the study drew upon the largest dataset to date on U.S. resistance rates, it should be used to more precisely guide first-line therapy decisions, said Richard Peek, Jr., MD, professor of medicine and director of gastroenterology at Vanderbilt University Medical Center, Nashville, Tenn.
“To date, there has been a dearth of information in the United States regarding H. pylori resistance rates, which has often led to the use of ineffective empiric therapies and inappropriate exposure to antibiotics,” Dr. Peek, who wasn’t involved in the study, told this news organization.
“These data are particularly exciting when viewed within the context of new genomic sequencing tests that can determine H. pylori resistance patterns using DNA isolated from the stomach or the stool,” he said.
Dr. Peek agreed that the recent approval of vonoprazan-based therapies “adds another regimen to the therapeutic armamentarium available for eradicating H. pylori, and its value seems to be particularly beneficial for eradication failures.”
The research was funded by Phathom Pharmaceuticals. Dr. Chey is a board member of the American College of Gastroenterology, GI on Demand, the International Foundation of Functional GI Disorders, and the Rome Foundation. He has received compensation as a consultant from AbbVie, Alfasigma, Allakos, Alnylam, Bayer, BioAmerica, Cosmo, Intrinsic Medicine, Ironwood Pharmaceuticals, QOL Medical, Nestle, Phathom Pharmaceuticals, RedHill Biopharma, Salix/Valeant, Takeda, Urovant, and Vibrant; grant/research support from BioAmerica, Commonwealth Diagnostics International, QOL Medical, Salix, and Vibrant; owns stock/stock options in GI on Demand and Modify Health; and owns patents relating to methods and kits for identifying food sensitivities and intolerances, digital manometry, and a rectal expulsion device. Dr. Peek and Dr. Kim report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Doctors favor euphemisms and jargon in discussions of death
Words including death, die, dying, or stillborn were frequently replaced by euphemisms in meetings between clinicians and families of critically ill children, based on data from 33 family meetings that involved discussions of death.
Clear communication is essential in discussing death with patients and families and current consensus guidelines recommend against use of euphemisms; data also suggest that patients and families prefer clear and direct language, wrote Margaret H. Barlet, of Duke University, Durham, N.C., and colleagues.
However, data on the language used in discussions of death in neonatal or pediatric contexts are limited, they said.
In a study published in JAMA Network Open, the researchers reviewed conversations between clinicians and parents of critically ill children. The study participants included 20 parents of 13 infants with neurological conditions who were hospitalized in a pediatric ICU in a single center in the southeastern United States between September 2018 and September 2020. Family meetings were scheduled to discuss prognosis and whether to start, not start, or discontinue life-sustaining treatment. The discussions were recorded, transcribed, and deidentified. The median age of the parents was 28.5 years; 60% identified as Black, 40% as White, and 10% as Asian; with some selecting more than one race.
For all 13 infants, one parent identified as the infant’s mother, and another parent identified as the father for seven of the infants. The median gestational age of the infants was 37 weeks; 54% were female, and the median hospital stay was 86 days.
Twelve infants (92%) required mechanical ventilation, six required chest compressions, and five had a do-not-attempt resuscitation order placed. Two infants died during the hospital admission process.
The primary outcome of the study was language used to reference death during family meetings between doctors and families. In the family conversations, death was referenced 406 times (275 times by clinicians and 131 times by family members).
Families were more likely than were clinicians to use the words die, death, dying, or stillborn; these terms appeared in 19 of 131 references by families and 13 of 275 references by clinicians (15% vs. 5%).
In addition to a category for use of words such as die, death, dying, or stillborn, the researchers identified four types of euphemisms used in place of these terms. They characterized the types of euphemisms as survival framing (for example, not live), colloquialisms (for example, pass away), medical jargon or use of physiologic terms (for example, code event or irrecoverable heart rate drop) and the use of pronouns without an antecedent (for example, it might happen soon).
Overall, 92% of references to death in the conversations were euphemistic. Medical jargon was the most common type of euphemism used by clinicians (118 of 275 references, 43%), while colloquialism was the most common type used by family members (44 of 131 references, 34%).
The results are consistent with limited research on this topic and show the high rates of euphemistic language used in discussions of death, the researchers wrote in their discussion. “Although our work did not directly evaluate the comparative clarity of different ways to reference death, our results raise questions about what language is most clear,” they said. The researchers proposed that their classification of euphemistic language may provide a framework for the use of language in discussions of death and may prompt clinicians to notice the language they use and hear from patients and families. “Empirically evaluating the perceived clarity of euphemism types and their effects on shared decision-making should be a priority for future study and should be used to inform interventions for improving communication in this context,” they said.
The findings were limited by several factors including the use of data from a single institution and the exclusion of non-English speaking families, the researchers noted. In addition, the researchers studied only what was said, therefore “questions about speaker motivation, listener understanding, and the effects of language choice on decision-making remain unanswered,” they added.
However, the results reflect the frequent use of euphemisms by both clinicians and families, and more research is needed to assess the effect of language on understanding, decision-making, and doctor-patient relationships, the researchers concluded.
Euphemisms can create confusion but may increase empathy
“Ms. Barlet and colleagues provide further consideration of types of speech that may obscure a clinician’s intended meaning or distract from their true point in the context of family discussions about critically ill patients,” Michael B. Pitt, MD, of the University of Minnesota, Minneapolis, and colleagues wrote in an accompanying editorial. Using a euphemism such as “pass on” instead of “die” may be an intentional choice by physicians to use less harsh language but it may still cause confusion, they noted.
The study showed how frequently physicians use euphemisms to talk about death but was distinctive in the inclusion of data on language use by families as well, they said.
“This pattern of use identified among the infants’ families may indicate that despite the clinical recommendation that end-of-life discussions avoid the use of euphemisms, it may be worth noting and responding to families’ language preferences accordingly once it is clear they have expressed understanding that the clinician is speaking of death,” they said. For example, if a family is consistently using softer terminology, clinicians should consider responding with similar terms, rather than using medical jargon or the words death or dying, they wrote.
“As the authors note, family preferences for this type of discussion are an important target for future research aimed at optimizing family-centered communication,” the editorialists added.
Families seek clarity in communication
“Clinicians have an important role in helping parents of seriously ill children understand their child’s health condition and make value-driven decisions about care,” Jennifer W. Mack, MD, of Harvard Medical School and the Dana-Farber Cancer Institute, Boston, said in an interview. “The words that clinicians use can have a significant impact on the knowledge parents take away from encounters and the decisions they make. While there is evidence of euphemistic language in the adult setting, there is limited information about this in children,” said Dr. Mack, a pediatric hematologist/oncologist who was not involved in the study.
Dr. Mack said she was not entirely surprised that in the current study, clinician language often includes medical jargon and an avoidance of direct language about death. “This is consistent with what I have seen in clinical practice,” she said. “One striking aspect of the study is that parents used terms like death or die more often than clinicians, and they sometimes used these terms as a way to clarify what the clinician was saying. This suggests to me that parents often want clarity, even if the information is very difficult,” she said.
The key message of the study is that clinicians should pay attention to the words they use to talk about the possibility of death and recognize the tendency of many clinicians to fall back on medical jargon, said Dr. Mack.
“My personal belief is that it is possible to be both clear and compassionate, and clinicians should strive for both in these conversations, to support families and help them make their best decisions for their children,” she said. “We need to remember a single communication strategy or choice of words is not likely to feel supportive to every family; what is helpful for one family may feel painful to another,” she emphasized. “Being willing to listen to the needs they express and their own language choice can help us to be responsive to individual needs,” she added.
An important next step for research is to learn more about what families experience as supportive during conversations with clinicians about death and dying, Dr. Mack said.
The study was supported by the National Institute of Neurological Disorders and Stroke, National Institutes of Health, and the Doris Duke Charitable Foundation. The researchers, editorial authors, and Dr. Mack had no financial conflicts to disclose.
Words including death, die, dying, or stillborn were frequently replaced by euphemisms in meetings between clinicians and families of critically ill children, based on data from 33 family meetings that involved discussions of death.
Clear communication is essential in discussing death with patients and families and current consensus guidelines recommend against use of euphemisms; data also suggest that patients and families prefer clear and direct language, wrote Margaret H. Barlet, of Duke University, Durham, N.C., and colleagues.
However, data on the language used in discussions of death in neonatal or pediatric contexts are limited, they said.
In a study published in JAMA Network Open, the researchers reviewed conversations between clinicians and parents of critically ill children. The study participants included 20 parents of 13 infants with neurological conditions who were hospitalized in a pediatric ICU in a single center in the southeastern United States between September 2018 and September 2020. Family meetings were scheduled to discuss prognosis and whether to start, not start, or discontinue life-sustaining treatment. The discussions were recorded, transcribed, and deidentified. The median age of the parents was 28.5 years; 60% identified as Black, 40% as White, and 10% as Asian; with some selecting more than one race.
For all 13 infants, one parent identified as the infant’s mother, and another parent identified as the father for seven of the infants. The median gestational age of the infants was 37 weeks; 54% were female, and the median hospital stay was 86 days.
Twelve infants (92%) required mechanical ventilation, six required chest compressions, and five had a do-not-attempt resuscitation order placed. Two infants died during the hospital admission process.
The primary outcome of the study was language used to reference death during family meetings between doctors and families. In the family conversations, death was referenced 406 times (275 times by clinicians and 131 times by family members).
Families were more likely than were clinicians to use the words die, death, dying, or stillborn; these terms appeared in 19 of 131 references by families and 13 of 275 references by clinicians (15% vs. 5%).
In addition to a category for use of words such as die, death, dying, or stillborn, the researchers identified four types of euphemisms used in place of these terms. They characterized the types of euphemisms as survival framing (for example, not live), colloquialisms (for example, pass away), medical jargon or use of physiologic terms (for example, code event or irrecoverable heart rate drop) and the use of pronouns without an antecedent (for example, it might happen soon).
Overall, 92% of references to death in the conversations were euphemistic. Medical jargon was the most common type of euphemism used by clinicians (118 of 275 references, 43%), while colloquialism was the most common type used by family members (44 of 131 references, 34%).
The results are consistent with limited research on this topic and show the high rates of euphemistic language used in discussions of death, the researchers wrote in their discussion. “Although our work did not directly evaluate the comparative clarity of different ways to reference death, our results raise questions about what language is most clear,” they said. The researchers proposed that their classification of euphemistic language may provide a framework for the use of language in discussions of death and may prompt clinicians to notice the language they use and hear from patients and families. “Empirically evaluating the perceived clarity of euphemism types and their effects on shared decision-making should be a priority for future study and should be used to inform interventions for improving communication in this context,” they said.
The findings were limited by several factors including the use of data from a single institution and the exclusion of non-English speaking families, the researchers noted. In addition, the researchers studied only what was said, therefore “questions about speaker motivation, listener understanding, and the effects of language choice on decision-making remain unanswered,” they added.
However, the results reflect the frequent use of euphemisms by both clinicians and families, and more research is needed to assess the effect of language on understanding, decision-making, and doctor-patient relationships, the researchers concluded.
Euphemisms can create confusion but may increase empathy
“Ms. Barlet and colleagues provide further consideration of types of speech that may obscure a clinician’s intended meaning or distract from their true point in the context of family discussions about critically ill patients,” Michael B. Pitt, MD, of the University of Minnesota, Minneapolis, and colleagues wrote in an accompanying editorial. Using a euphemism such as “pass on” instead of “die” may be an intentional choice by physicians to use less harsh language but it may still cause confusion, they noted.
The study showed how frequently physicians use euphemisms to talk about death but was distinctive in the inclusion of data on language use by families as well, they said.
“This pattern of use identified among the infants’ families may indicate that despite the clinical recommendation that end-of-life discussions avoid the use of euphemisms, it may be worth noting and responding to families’ language preferences accordingly once it is clear they have expressed understanding that the clinician is speaking of death,” they said. For example, if a family is consistently using softer terminology, clinicians should consider responding with similar terms, rather than using medical jargon or the words death or dying, they wrote.
“As the authors note, family preferences for this type of discussion are an important target for future research aimed at optimizing family-centered communication,” the editorialists added.
Families seek clarity in communication
“Clinicians have an important role in helping parents of seriously ill children understand their child’s health condition and make value-driven decisions about care,” Jennifer W. Mack, MD, of Harvard Medical School and the Dana-Farber Cancer Institute, Boston, said in an interview. “The words that clinicians use can have a significant impact on the knowledge parents take away from encounters and the decisions they make. While there is evidence of euphemistic language in the adult setting, there is limited information about this in children,” said Dr. Mack, a pediatric hematologist/oncologist who was not involved in the study.
Dr. Mack said she was not entirely surprised that in the current study, clinician language often includes medical jargon and an avoidance of direct language about death. “This is consistent with what I have seen in clinical practice,” she said. “One striking aspect of the study is that parents used terms like death or die more often than clinicians, and they sometimes used these terms as a way to clarify what the clinician was saying. This suggests to me that parents often want clarity, even if the information is very difficult,” she said.
The key message of the study is that clinicians should pay attention to the words they use to talk about the possibility of death and recognize the tendency of many clinicians to fall back on medical jargon, said Dr. Mack.
“My personal belief is that it is possible to be both clear and compassionate, and clinicians should strive for both in these conversations, to support families and help them make their best decisions for their children,” she said. “We need to remember a single communication strategy or choice of words is not likely to feel supportive to every family; what is helpful for one family may feel painful to another,” she emphasized. “Being willing to listen to the needs they express and their own language choice can help us to be responsive to individual needs,” she added.
An important next step for research is to learn more about what families experience as supportive during conversations with clinicians about death and dying, Dr. Mack said.
The study was supported by the National Institute of Neurological Disorders and Stroke, National Institutes of Health, and the Doris Duke Charitable Foundation. The researchers, editorial authors, and Dr. Mack had no financial conflicts to disclose.
Words including death, die, dying, or stillborn were frequently replaced by euphemisms in meetings between clinicians and families of critically ill children, based on data from 33 family meetings that involved discussions of death.
Clear communication is essential in discussing death with patients and families and current consensus guidelines recommend against use of euphemisms; data also suggest that patients and families prefer clear and direct language, wrote Margaret H. Barlet, of Duke University, Durham, N.C., and colleagues.
However, data on the language used in discussions of death in neonatal or pediatric contexts are limited, they said.
In a study published in JAMA Network Open, the researchers reviewed conversations between clinicians and parents of critically ill children. The study participants included 20 parents of 13 infants with neurological conditions who were hospitalized in a pediatric ICU in a single center in the southeastern United States between September 2018 and September 2020. Family meetings were scheduled to discuss prognosis and whether to start, not start, or discontinue life-sustaining treatment. The discussions were recorded, transcribed, and deidentified. The median age of the parents was 28.5 years; 60% identified as Black, 40% as White, and 10% as Asian; with some selecting more than one race.
For all 13 infants, one parent identified as the infant’s mother, and another parent identified as the father for seven of the infants. The median gestational age of the infants was 37 weeks; 54% were female, and the median hospital stay was 86 days.
Twelve infants (92%) required mechanical ventilation, six required chest compressions, and five had a do-not-attempt resuscitation order placed. Two infants died during the hospital admission process.
The primary outcome of the study was language used to reference death during family meetings between doctors and families. In the family conversations, death was referenced 406 times (275 times by clinicians and 131 times by family members).
Families were more likely than were clinicians to use the words die, death, dying, or stillborn; these terms appeared in 19 of 131 references by families and 13 of 275 references by clinicians (15% vs. 5%).
In addition to a category for use of words such as die, death, dying, or stillborn, the researchers identified four types of euphemisms used in place of these terms. They characterized the types of euphemisms as survival framing (for example, not live), colloquialisms (for example, pass away), medical jargon or use of physiologic terms (for example, code event or irrecoverable heart rate drop) and the use of pronouns without an antecedent (for example, it might happen soon).
Overall, 92% of references to death in the conversations were euphemistic. Medical jargon was the most common type of euphemism used by clinicians (118 of 275 references, 43%), while colloquialism was the most common type used by family members (44 of 131 references, 34%).
The results are consistent with limited research on this topic and show the high rates of euphemistic language used in discussions of death, the researchers wrote in their discussion. “Although our work did not directly evaluate the comparative clarity of different ways to reference death, our results raise questions about what language is most clear,” they said. The researchers proposed that their classification of euphemistic language may provide a framework for the use of language in discussions of death and may prompt clinicians to notice the language they use and hear from patients and families. “Empirically evaluating the perceived clarity of euphemism types and their effects on shared decision-making should be a priority for future study and should be used to inform interventions for improving communication in this context,” they said.
The findings were limited by several factors including the use of data from a single institution and the exclusion of non-English speaking families, the researchers noted. In addition, the researchers studied only what was said, therefore “questions about speaker motivation, listener understanding, and the effects of language choice on decision-making remain unanswered,” they added.
However, the results reflect the frequent use of euphemisms by both clinicians and families, and more research is needed to assess the effect of language on understanding, decision-making, and doctor-patient relationships, the researchers concluded.
Euphemisms can create confusion but may increase empathy
“Ms. Barlet and colleagues provide further consideration of types of speech that may obscure a clinician’s intended meaning or distract from their true point in the context of family discussions about critically ill patients,” Michael B. Pitt, MD, of the University of Minnesota, Minneapolis, and colleagues wrote in an accompanying editorial. Using a euphemism such as “pass on” instead of “die” may be an intentional choice by physicians to use less harsh language but it may still cause confusion, they noted.
The study showed how frequently physicians use euphemisms to talk about death but was distinctive in the inclusion of data on language use by families as well, they said.
“This pattern of use identified among the infants’ families may indicate that despite the clinical recommendation that end-of-life discussions avoid the use of euphemisms, it may be worth noting and responding to families’ language preferences accordingly once it is clear they have expressed understanding that the clinician is speaking of death,” they said. For example, if a family is consistently using softer terminology, clinicians should consider responding with similar terms, rather than using medical jargon or the words death or dying, they wrote.
“As the authors note, family preferences for this type of discussion are an important target for future research aimed at optimizing family-centered communication,” the editorialists added.
Families seek clarity in communication
“Clinicians have an important role in helping parents of seriously ill children understand their child’s health condition and make value-driven decisions about care,” Jennifer W. Mack, MD, of Harvard Medical School and the Dana-Farber Cancer Institute, Boston, said in an interview. “The words that clinicians use can have a significant impact on the knowledge parents take away from encounters and the decisions they make. While there is evidence of euphemistic language in the adult setting, there is limited information about this in children,” said Dr. Mack, a pediatric hematologist/oncologist who was not involved in the study.
Dr. Mack said she was not entirely surprised that in the current study, clinician language often includes medical jargon and an avoidance of direct language about death. “This is consistent with what I have seen in clinical practice,” she said. “One striking aspect of the study is that parents used terms like death or die more often than clinicians, and they sometimes used these terms as a way to clarify what the clinician was saying. This suggests to me that parents often want clarity, even if the information is very difficult,” she said.
The key message of the study is that clinicians should pay attention to the words they use to talk about the possibility of death and recognize the tendency of many clinicians to fall back on medical jargon, said Dr. Mack.
“My personal belief is that it is possible to be both clear and compassionate, and clinicians should strive for both in these conversations, to support families and help them make their best decisions for their children,” she said. “We need to remember a single communication strategy or choice of words is not likely to feel supportive to every family; what is helpful for one family may feel painful to another,” she emphasized. “Being willing to listen to the needs they express and their own language choice can help us to be responsive to individual needs,” she added.
An important next step for research is to learn more about what families experience as supportive during conversations with clinicians about death and dying, Dr. Mack said.
The study was supported by the National Institute of Neurological Disorders and Stroke, National Institutes of Health, and the Doris Duke Charitable Foundation. The researchers, editorial authors, and Dr. Mack had no financial conflicts to disclose.
FROM JAMA NETWORK OPEN










