User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Urticaria Linked to Higher Cancer Risk, Study Finds
TOPLINE:
which decreased to 6% in subsequent years, in a cohort study using Danish healthcare databases.
METHODOLOGY:
- Researchers conducted a retrospective cohort study using data from Danish healthcare registries and compared the incident cancer risk between patients with urticaria and the risk in the general population.
- They identified 87,507 patients (58% women) with a primary or secondary first-time hospital outpatient clinic, emergency room, or inpatient diagnosis of urticaria between 1980 and 2022, who were followed for a median of 10.1 years.
- Incident cancers, including nonmelanoma skin cancer, were identified using the Danish Cancer Registry and classified by the extent of spread at the time of diagnosis.
- This study computed the absolute cancer risk within the first year of an urticaria diagnosis and standardized incidence ratios (SIRs), with 95% CIs standardized to Danish national cancer rates.
TAKEAWAY:
- For the first year of follow-up, the absolute risk for all cancer types was 0.7%, and it was 29.5% for subsequent years. The overall SIR for all types of cancer was 1.09 (95% CI, 1.06-1.11), which was based on 7788 observed cancer cases compared with 7161 cases expected over the entire follow-up period.
- Within the first year of follow-up, 588 patients with urticaria were diagnosed with cancer, for an SIR of 1.49 (95% CI, 1.38-1.62) for all cancer types.
- After the first year, the SIR for all cancer sites decreased and stabilized at 1.06 (95% CI, 1.04-1.09), with 7200 observed cancer cases.
- The risk was highest for hematological cancers in the first year, particularly Hodgkin lymphoma (SIR, 5.35; 95% CI, 2.56-9.85).
IN PRACTICE:
“Our study suggests that urticaria may be a marker of occult cancer and that it is associated with a slightly increased long-term cancer risk,” the authors wrote.
SOURCE:
The study was led by Sissel B.T. Sørensen, departments of dermatology and rheumatology, Aarhus University Hospital, Aarhus, Denmark. It was published online on June 27, 2024, in the British Journal of Dermatology.
LIMITATIONS:
The study is limited by its observational design and reliance on registry data, which may be subject to misclassification or incomplete information. In addition, the study could not assess individual patient factors such as lifestyle or genetic predispositions that may influence cancer risk, and the results may not be generalizable to other populations. Finally, the exact biologic mechanisms linking urticaria and cancer remain unclear, warranting further investigation.
DISCLOSURES:
The study did not receive any funding. The authors reported that they had no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
which decreased to 6% in subsequent years, in a cohort study using Danish healthcare databases.
METHODOLOGY:
- Researchers conducted a retrospective cohort study using data from Danish healthcare registries and compared the incident cancer risk between patients with urticaria and the risk in the general population.
- They identified 87,507 patients (58% women) with a primary or secondary first-time hospital outpatient clinic, emergency room, or inpatient diagnosis of urticaria between 1980 and 2022, who were followed for a median of 10.1 years.
- Incident cancers, including nonmelanoma skin cancer, were identified using the Danish Cancer Registry and classified by the extent of spread at the time of diagnosis.
- This study computed the absolute cancer risk within the first year of an urticaria diagnosis and standardized incidence ratios (SIRs), with 95% CIs standardized to Danish national cancer rates.
TAKEAWAY:
- For the first year of follow-up, the absolute risk for all cancer types was 0.7%, and it was 29.5% for subsequent years. The overall SIR for all types of cancer was 1.09 (95% CI, 1.06-1.11), which was based on 7788 observed cancer cases compared with 7161 cases expected over the entire follow-up period.
- Within the first year of follow-up, 588 patients with urticaria were diagnosed with cancer, for an SIR of 1.49 (95% CI, 1.38-1.62) for all cancer types.
- After the first year, the SIR for all cancer sites decreased and stabilized at 1.06 (95% CI, 1.04-1.09), with 7200 observed cancer cases.
- The risk was highest for hematological cancers in the first year, particularly Hodgkin lymphoma (SIR, 5.35; 95% CI, 2.56-9.85).
IN PRACTICE:
“Our study suggests that urticaria may be a marker of occult cancer and that it is associated with a slightly increased long-term cancer risk,” the authors wrote.
SOURCE:
The study was led by Sissel B.T. Sørensen, departments of dermatology and rheumatology, Aarhus University Hospital, Aarhus, Denmark. It was published online on June 27, 2024, in the British Journal of Dermatology.
LIMITATIONS:
The study is limited by its observational design and reliance on registry data, which may be subject to misclassification or incomplete information. In addition, the study could not assess individual patient factors such as lifestyle or genetic predispositions that may influence cancer risk, and the results may not be generalizable to other populations. Finally, the exact biologic mechanisms linking urticaria and cancer remain unclear, warranting further investigation.
DISCLOSURES:
The study did not receive any funding. The authors reported that they had no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
which decreased to 6% in subsequent years, in a cohort study using Danish healthcare databases.
METHODOLOGY:
- Researchers conducted a retrospective cohort study using data from Danish healthcare registries and compared the incident cancer risk between patients with urticaria and the risk in the general population.
- They identified 87,507 patients (58% women) with a primary or secondary first-time hospital outpatient clinic, emergency room, or inpatient diagnosis of urticaria between 1980 and 2022, who were followed for a median of 10.1 years.
- Incident cancers, including nonmelanoma skin cancer, were identified using the Danish Cancer Registry and classified by the extent of spread at the time of diagnosis.
- This study computed the absolute cancer risk within the first year of an urticaria diagnosis and standardized incidence ratios (SIRs), with 95% CIs standardized to Danish national cancer rates.
TAKEAWAY:
- For the first year of follow-up, the absolute risk for all cancer types was 0.7%, and it was 29.5% for subsequent years. The overall SIR for all types of cancer was 1.09 (95% CI, 1.06-1.11), which was based on 7788 observed cancer cases compared with 7161 cases expected over the entire follow-up period.
- Within the first year of follow-up, 588 patients with urticaria were diagnosed with cancer, for an SIR of 1.49 (95% CI, 1.38-1.62) for all cancer types.
- After the first year, the SIR for all cancer sites decreased and stabilized at 1.06 (95% CI, 1.04-1.09), with 7200 observed cancer cases.
- The risk was highest for hematological cancers in the first year, particularly Hodgkin lymphoma (SIR, 5.35; 95% CI, 2.56-9.85).
IN PRACTICE:
“Our study suggests that urticaria may be a marker of occult cancer and that it is associated with a slightly increased long-term cancer risk,” the authors wrote.
SOURCE:
The study was led by Sissel B.T. Sørensen, departments of dermatology and rheumatology, Aarhus University Hospital, Aarhus, Denmark. It was published online on June 27, 2024, in the British Journal of Dermatology.
LIMITATIONS:
The study is limited by its observational design and reliance on registry data, which may be subject to misclassification or incomplete information. In addition, the study could not assess individual patient factors such as lifestyle or genetic predispositions that may influence cancer risk, and the results may not be generalizable to other populations. Finally, the exact biologic mechanisms linking urticaria and cancer remain unclear, warranting further investigation.
DISCLOSURES:
The study did not receive any funding. The authors reported that they had no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Should South Park: The End of Obesity Be Required Viewing in Medical School?
Yes, there’s still much to find offensive, but South Park: The End of Obesity, in just 51 minutes, does more to explain some of obesity’s realities, its pharmacotherapy, and weight bias than the mainstream media has done perhaps ever.
The mini-movie follows the plight of Eric Cartman, the fictional South Parkian child with severe obesity.
South Park got everything right. The movie starts in a medical center where discussions with Cartman, his mother, and his doctor make it clear that obesity isn’t something that Cartman chose and is perhaps the most distressing aspect of his life. This certainly echoes study findings which report that quality-of-life scores in children with severe obesity are lower than those of children with newly diagnosed on-treatment cancers. As to how obesity erodes a child’s quality of life, no doubt part of its impact stems from obesity being a top source of schoolyard bullying, which is reflected by Cartman as he imagines his life without it.
Cartman’s mother explains that of course they’ve tried diet and exercise, but that intentional behavior change alone hasn’t been sufficient to sustainably move the scale’s needle — a truth for the vast majority of people with obesity. But here, unlike in many actual doctors’ offices, Cartman’s doctor doesn’t spend time doubting or cajoling; instead, he does his job — which is to inform his patient, without judgment, about a pharmaceutical option that has proved to be beneficial. He accurately describes these medications as ushering in “a whole new era of medicine, a miracle really” that can “help people lose vast amounts of weight.”
The kicker, though, comes next. The doctor explains that insurance companies cover the medications only for patients with diabetes, “so if you can’t afford them, you’re just kind of out of luck.” This is changing somewhat now, at least here in Canada, where two of our main private insurers have changed their base coverages to make antiobesity medications something employers need to opt out of rather than opt into, but certainly they’re not covered by US Medicare for weight management, nor by our version of the same here in Canada.
But even for those who have coverage, there are hoops to jump through, which is highlighted by the incredible efforts made by Cartman and his friends to get his insurance plan to cover the medications. Thwarted at every turn, despite the undeniable benefits of these medications to health and quality of life, they are forced to turn to compounding — a phenomenon certainly pervasive here in North America whereby compounding pharmacies claim to be able to provide glucagon-like peptide-1 (GLP-1) analogs with comparable efficacy at a fraction of the price, but without the same rigor of proof of purity or efficacy.
Also covered by South Park is that the GLP-1 analog supply is impacted by use by people who don’t meet approved medical criteria and are using the medications for aesthetic purposes. This speaks to the incredible societal pressure to be thin and to the comfort of some physicians to inappropriately prescribe these medications. This is covered by the subplot of South Park’s weed farmer, Randy, who in turn delivers an important insight into how it feels to use a GLP-1 analog: “I think there’s something wrong with these drugs ... I feel satisfied. With any drugs I want to do more and more, but with these drugs I feel like I want things less. With these drugs you don’t really crave anything.” The sentiment is echoed by Cartman, who exclaims, “I think I’m full. I’ve never known that feeling before in my life, but I’m full.”
It’s remarkable that South Park, a show built on serving up politically incorrect offense, covers obesity and its treatment with more accuracy, nuance, and compassion than does society as a whole. The show notes that obesity is a biological condition (it is), that when it comes to health (in America) “you have to have some f-ing willpower.” But where they explicitly mean having willpower in terms of filing and pursing insurance claims (you do), explains that drug companies are making antiobesity medications more expensive in America than anywhere else in the world (they are), and finally delivers this quote, which, while missing the biological basis of behavior and hunger with respect to obesity, certainly sums up why blame has no place in the discourse:
“We have sugar companies, pharmaceutical companies, and insurance companies all just trying to figure out how to make money off our health. It isn’t fair to put the blame on anyone for their weight.”
No, it’s not.
This movie should be required viewing in medical schools.
Dr. Freedhoff is associate professor, department of family medicine, University of Ottawa, and medical director, Bariatric Medical Institute, Ottawa, Ontario, Canada. He disclosed ties with Bariatric Medical Institute, Constant Health, Novo Nordisk, and Weighty Matters.
A version of this article appeared on Medscape.com.
Yes, there’s still much to find offensive, but South Park: The End of Obesity, in just 51 minutes, does more to explain some of obesity’s realities, its pharmacotherapy, and weight bias than the mainstream media has done perhaps ever.
The mini-movie follows the plight of Eric Cartman, the fictional South Parkian child with severe obesity.
South Park got everything right. The movie starts in a medical center where discussions with Cartman, his mother, and his doctor make it clear that obesity isn’t something that Cartman chose and is perhaps the most distressing aspect of his life. This certainly echoes study findings which report that quality-of-life scores in children with severe obesity are lower than those of children with newly diagnosed on-treatment cancers. As to how obesity erodes a child’s quality of life, no doubt part of its impact stems from obesity being a top source of schoolyard bullying, which is reflected by Cartman as he imagines his life without it.
Cartman’s mother explains that of course they’ve tried diet and exercise, but that intentional behavior change alone hasn’t been sufficient to sustainably move the scale’s needle — a truth for the vast majority of people with obesity. But here, unlike in many actual doctors’ offices, Cartman’s doctor doesn’t spend time doubting or cajoling; instead, he does his job — which is to inform his patient, without judgment, about a pharmaceutical option that has proved to be beneficial. He accurately describes these medications as ushering in “a whole new era of medicine, a miracle really” that can “help people lose vast amounts of weight.”
The kicker, though, comes next. The doctor explains that insurance companies cover the medications only for patients with diabetes, “so if you can’t afford them, you’re just kind of out of luck.” This is changing somewhat now, at least here in Canada, where two of our main private insurers have changed their base coverages to make antiobesity medications something employers need to opt out of rather than opt into, but certainly they’re not covered by US Medicare for weight management, nor by our version of the same here in Canada.
But even for those who have coverage, there are hoops to jump through, which is highlighted by the incredible efforts made by Cartman and his friends to get his insurance plan to cover the medications. Thwarted at every turn, despite the undeniable benefits of these medications to health and quality of life, they are forced to turn to compounding — a phenomenon certainly pervasive here in North America whereby compounding pharmacies claim to be able to provide glucagon-like peptide-1 (GLP-1) analogs with comparable efficacy at a fraction of the price, but without the same rigor of proof of purity or efficacy.
Also covered by South Park is that the GLP-1 analog supply is impacted by use by people who don’t meet approved medical criteria and are using the medications for aesthetic purposes. This speaks to the incredible societal pressure to be thin and to the comfort of some physicians to inappropriately prescribe these medications. This is covered by the subplot of South Park’s weed farmer, Randy, who in turn delivers an important insight into how it feels to use a GLP-1 analog: “I think there’s something wrong with these drugs ... I feel satisfied. With any drugs I want to do more and more, but with these drugs I feel like I want things less. With these drugs you don’t really crave anything.” The sentiment is echoed by Cartman, who exclaims, “I think I’m full. I’ve never known that feeling before in my life, but I’m full.”
It’s remarkable that South Park, a show built on serving up politically incorrect offense, covers obesity and its treatment with more accuracy, nuance, and compassion than does society as a whole. The show notes that obesity is a biological condition (it is), that when it comes to health (in America) “you have to have some f-ing willpower.” But where they explicitly mean having willpower in terms of filing and pursing insurance claims (you do), explains that drug companies are making antiobesity medications more expensive in America than anywhere else in the world (they are), and finally delivers this quote, which, while missing the biological basis of behavior and hunger with respect to obesity, certainly sums up why blame has no place in the discourse:
“We have sugar companies, pharmaceutical companies, and insurance companies all just trying to figure out how to make money off our health. It isn’t fair to put the blame on anyone for their weight.”
No, it’s not.
This movie should be required viewing in medical schools.
Dr. Freedhoff is associate professor, department of family medicine, University of Ottawa, and medical director, Bariatric Medical Institute, Ottawa, Ontario, Canada. He disclosed ties with Bariatric Medical Institute, Constant Health, Novo Nordisk, and Weighty Matters.
A version of this article appeared on Medscape.com.
Yes, there’s still much to find offensive, but South Park: The End of Obesity, in just 51 minutes, does more to explain some of obesity’s realities, its pharmacotherapy, and weight bias than the mainstream media has done perhaps ever.
The mini-movie follows the plight of Eric Cartman, the fictional South Parkian child with severe obesity.
South Park got everything right. The movie starts in a medical center where discussions with Cartman, his mother, and his doctor make it clear that obesity isn’t something that Cartman chose and is perhaps the most distressing aspect of his life. This certainly echoes study findings which report that quality-of-life scores in children with severe obesity are lower than those of children with newly diagnosed on-treatment cancers. As to how obesity erodes a child’s quality of life, no doubt part of its impact stems from obesity being a top source of schoolyard bullying, which is reflected by Cartman as he imagines his life without it.
Cartman’s mother explains that of course they’ve tried diet and exercise, but that intentional behavior change alone hasn’t been sufficient to sustainably move the scale’s needle — a truth for the vast majority of people with obesity. But here, unlike in many actual doctors’ offices, Cartman’s doctor doesn’t spend time doubting or cajoling; instead, he does his job — which is to inform his patient, without judgment, about a pharmaceutical option that has proved to be beneficial. He accurately describes these medications as ushering in “a whole new era of medicine, a miracle really” that can “help people lose vast amounts of weight.”
The kicker, though, comes next. The doctor explains that insurance companies cover the medications only for patients with diabetes, “so if you can’t afford them, you’re just kind of out of luck.” This is changing somewhat now, at least here in Canada, where two of our main private insurers have changed their base coverages to make antiobesity medications something employers need to opt out of rather than opt into, but certainly they’re not covered by US Medicare for weight management, nor by our version of the same here in Canada.
But even for those who have coverage, there are hoops to jump through, which is highlighted by the incredible efforts made by Cartman and his friends to get his insurance plan to cover the medications. Thwarted at every turn, despite the undeniable benefits of these medications to health and quality of life, they are forced to turn to compounding — a phenomenon certainly pervasive here in North America whereby compounding pharmacies claim to be able to provide glucagon-like peptide-1 (GLP-1) analogs with comparable efficacy at a fraction of the price, but without the same rigor of proof of purity or efficacy.
Also covered by South Park is that the GLP-1 analog supply is impacted by use by people who don’t meet approved medical criteria and are using the medications for aesthetic purposes. This speaks to the incredible societal pressure to be thin and to the comfort of some physicians to inappropriately prescribe these medications. This is covered by the subplot of South Park’s weed farmer, Randy, who in turn delivers an important insight into how it feels to use a GLP-1 analog: “I think there’s something wrong with these drugs ... I feel satisfied. With any drugs I want to do more and more, but with these drugs I feel like I want things less. With these drugs you don’t really crave anything.” The sentiment is echoed by Cartman, who exclaims, “I think I’m full. I’ve never known that feeling before in my life, but I’m full.”
It’s remarkable that South Park, a show built on serving up politically incorrect offense, covers obesity and its treatment with more accuracy, nuance, and compassion than does society as a whole. The show notes that obesity is a biological condition (it is), that when it comes to health (in America) “you have to have some f-ing willpower.” But where they explicitly mean having willpower in terms of filing and pursing insurance claims (you do), explains that drug companies are making antiobesity medications more expensive in America than anywhere else in the world (they are), and finally delivers this quote, which, while missing the biological basis of behavior and hunger with respect to obesity, certainly sums up why blame has no place in the discourse:
“We have sugar companies, pharmaceutical companies, and insurance companies all just trying to figure out how to make money off our health. It isn’t fair to put the blame on anyone for their weight.”
No, it’s not.
This movie should be required viewing in medical schools.
Dr. Freedhoff is associate professor, department of family medicine, University of Ottawa, and medical director, Bariatric Medical Institute, Ottawa, Ontario, Canada. He disclosed ties with Bariatric Medical Institute, Constant Health, Novo Nordisk, and Weighty Matters.
A version of this article appeared on Medscape.com.
GLP-1 Thyroid Warning Could Increase Overdiagnosis
ORLANDO, Florida — Clinicians should keep in mind concerns about overdiagnosis of thyroid cancer when prescribing glucagon-like peptide 1 (GLP-1) drugs, as the US boxed warning about this risk for this class of medicines for certain tumors in mice could trigger excess screening, an expert endocrinologist said.
Speaking at the annual American Diabetes Association (ADA) 84th Scientific Sessions, Elizabeth N. Pearce, MD, MSc, a professor of medicine at Boston University, Boston, reviewed the different approaches US and European regulators have taken for the GLP-1 drugs. She also explained the current concerns about the wide use of thyroid screening in general and how these intersect with the rapid uptake of the GLP-1 drugs.
said Dr. Pearce, who is also a former board president of the American Thyroid Association (ATA). “We do not want to contribute to this epidemic of overdiagnosis of thyroid cancer.”
The ATA and the US Preventive Services Task Force (USPSTF) are among the health organizations that have in recent years sought to boost public awareness of the potential risks for excess screening of thyroid nodules. In 2017, the USPSTF, which influences insurance coverage, recommended against routine screening for thyroid cancer in asymptomatic adults. At that time, the incidence of thyroid cancer detection had increased by 4.5% per year over a decade, faster than for any other cancer, but without a corresponding change in the mortality rate, USPSTF said.
“Unequivocally, the thyroid cancer mortality has not kept pace with thyroid cancer detection,” Dr. Pearce said at the ADA meeting. “We’ve been diagnosing a lot of small thyroid cancers that people would otherwise have been destined to die with and not die of.”
Dr. Pearce said clinicians should be careful not to overly restrict access to GLP-1 drugs due to concerns about thyroid cancer — and they should use care in screening nodules.
It’s possible that the weight loss experienced by people taking GLP-1 drugs may make preexisting thyroid nodules more prominent, Dr. Pearce said. It’s also likely that the US boxed warning on thyroid risk on GLP-1 drugs makes clinicians and patients more likely to look for these kinds of growths.
Dr. Pearce urged adherence to guidelines such as the ones the ATA published in 2015 for assessing nodules.
In an interview with this news organization, Dr. Pearce noted the frequency of CT scans in US medical practice in turning up many incidental thyroid nodules, a finding that can cause some panic for patients and their clinicians.
But it helps to put these findings in context, as by the age of 50, about 40% of women will have at least one thyroid nodule, making this a very common finding, she said.
“The vast majority are not malignant,” Dr. Pearce said. “When you explain this to patients, it alleviates anxiety.”
The US, European Union Differences
In the United States, the label for GLP-1 drugs starts with a boxed warning about thyroid C-cell tumors seen in rodents given these medicines in testing.
It’s unknown if the medicines could cause medullary thyroid carcinoma (MTC) in humans, the label adds. The drug is contraindicated in patients with a personal or family history of MTC or multiple endocrine neoplasia syndrome 2, the boxed warning says. This is based largely on data seen in laboratory rats.
“It’s a big black box warning that gets people’s attention,” Dr. Pearce said. “Important to note that if you practice in Europe, you will not be familiar with this labeling because it doesn’t exist there. They’ve never had this warning on the European package.”
The European Medicines Agency (EMA) does include information about the results of rodent studies as part of the discussion of known and potential risks for GLP-1 drugs but has not emphasized it in the same way as the US drug labels do.
For example, the public assessment report posted on the EMA website for semaglutide (Ozempic, Novo Nordisk) notes that nonlethal thyroid C-cell tumors “observed in rodents are a class effect for GLP-1 receptor agonists.” It’s possible that these may be due to a particular sensitivity in rodents, the report said.
“The relevance for humans is considered to be low but cannot be completely excluded,” the EMA report said in the product information section of the report.
There has been ongoing interest in the issue.
The EMA’s Pharmacovigilance Risk Assessment Committee (PRAC) in October concluded that the available evidence does not support a causal association between GLP-1 receptor agonists and thyroid cancer.
The EMA’s PRAC safety committee said it began assessing the evidence about a possible connection following the publication of a study in 2022 in the journal Diabetes Care. That paper reported on an analysis that suggested increased risk for all thyroid cancer and medullary thyroid cancer with the use of GLP-1 drugs, particularly after 1-3 years of treatment.
The EMA’s PRAC said that in making its decision, it also considered other published papers on this topic as well as clinical and postmarketing data on GLP-1 drugs.
In an email interview, Jean-Luc Faillie, MD, PhD, corresponding author of the Diabetes Care paper, called for continued “vigilance and prudence in clinical practice” with GLP-1 drugs.
His paper reported on a case-control analysis on the basis of reports from the French national healthcare insurance system database, looking at people who had taken GLP-1 drugs and similar people who had not.
Due to a lack of a specific diagnostic code for medullary thyroid cancers, the researchers used a composite definition combining thyroid cancer diagnosis with several calcitonin tests, a carcinoembryonic antigen test, or a specific treatment (vandetanib) to identify potential cases of this cancer.
It’s possible that this method could have led to overestimation of MTC among the cases of thyroid cancer, wrote Dr. Faillie, who is a professor at France’s Université de Montpellier, Montpellier, France, and part of its pharmacological vigilance service.
“Nevertheless, it’s crucial to emphasize that any potential overestimation of MTC cases would likely apply equally to both GLP-1 receptor agonist–exposed and unexposed groups,” Dr. Faillie wrote. “Therefore, it should not significantly impact our main findings regarding the suggested increased risk associated with GLP-1 receptor agonist use.”
Dr. Pearce disclosed honoraria for speaking at the Merck China Forum. Dr. Faille and his coauthors reported no conflicts of interest in the publication of their study. Their research was supported by the French Medicines Agency (Agence Nationale de Sécurité du Médicament et des Produits de Santé, grant 2019S015) in the context of a partnership with the Health Product Epidemiology Scientific Interest Group (EPI-PHARE). The study was part of France’s Drugs Systematized Assessment in Real-Life Environment (DRUGS-SAFEr) research program.
A version of this article first appeared on Medscape.com.
ORLANDO, Florida — Clinicians should keep in mind concerns about overdiagnosis of thyroid cancer when prescribing glucagon-like peptide 1 (GLP-1) drugs, as the US boxed warning about this risk for this class of medicines for certain tumors in mice could trigger excess screening, an expert endocrinologist said.
Speaking at the annual American Diabetes Association (ADA) 84th Scientific Sessions, Elizabeth N. Pearce, MD, MSc, a professor of medicine at Boston University, Boston, reviewed the different approaches US and European regulators have taken for the GLP-1 drugs. She also explained the current concerns about the wide use of thyroid screening in general and how these intersect with the rapid uptake of the GLP-1 drugs.
said Dr. Pearce, who is also a former board president of the American Thyroid Association (ATA). “We do not want to contribute to this epidemic of overdiagnosis of thyroid cancer.”
The ATA and the US Preventive Services Task Force (USPSTF) are among the health organizations that have in recent years sought to boost public awareness of the potential risks for excess screening of thyroid nodules. In 2017, the USPSTF, which influences insurance coverage, recommended against routine screening for thyroid cancer in asymptomatic adults. At that time, the incidence of thyroid cancer detection had increased by 4.5% per year over a decade, faster than for any other cancer, but without a corresponding change in the mortality rate, USPSTF said.
“Unequivocally, the thyroid cancer mortality has not kept pace with thyroid cancer detection,” Dr. Pearce said at the ADA meeting. “We’ve been diagnosing a lot of small thyroid cancers that people would otherwise have been destined to die with and not die of.”
Dr. Pearce said clinicians should be careful not to overly restrict access to GLP-1 drugs due to concerns about thyroid cancer — and they should use care in screening nodules.
It’s possible that the weight loss experienced by people taking GLP-1 drugs may make preexisting thyroid nodules more prominent, Dr. Pearce said. It’s also likely that the US boxed warning on thyroid risk on GLP-1 drugs makes clinicians and patients more likely to look for these kinds of growths.
Dr. Pearce urged adherence to guidelines such as the ones the ATA published in 2015 for assessing nodules.
In an interview with this news organization, Dr. Pearce noted the frequency of CT scans in US medical practice in turning up many incidental thyroid nodules, a finding that can cause some panic for patients and their clinicians.
But it helps to put these findings in context, as by the age of 50, about 40% of women will have at least one thyroid nodule, making this a very common finding, she said.
“The vast majority are not malignant,” Dr. Pearce said. “When you explain this to patients, it alleviates anxiety.”
The US, European Union Differences
In the United States, the label for GLP-1 drugs starts with a boxed warning about thyroid C-cell tumors seen in rodents given these medicines in testing.
It’s unknown if the medicines could cause medullary thyroid carcinoma (MTC) in humans, the label adds. The drug is contraindicated in patients with a personal or family history of MTC or multiple endocrine neoplasia syndrome 2, the boxed warning says. This is based largely on data seen in laboratory rats.
“It’s a big black box warning that gets people’s attention,” Dr. Pearce said. “Important to note that if you practice in Europe, you will not be familiar with this labeling because it doesn’t exist there. They’ve never had this warning on the European package.”
The European Medicines Agency (EMA) does include information about the results of rodent studies as part of the discussion of known and potential risks for GLP-1 drugs but has not emphasized it in the same way as the US drug labels do.
For example, the public assessment report posted on the EMA website for semaglutide (Ozempic, Novo Nordisk) notes that nonlethal thyroid C-cell tumors “observed in rodents are a class effect for GLP-1 receptor agonists.” It’s possible that these may be due to a particular sensitivity in rodents, the report said.
“The relevance for humans is considered to be low but cannot be completely excluded,” the EMA report said in the product information section of the report.
There has been ongoing interest in the issue.
The EMA’s Pharmacovigilance Risk Assessment Committee (PRAC) in October concluded that the available evidence does not support a causal association between GLP-1 receptor agonists and thyroid cancer.
The EMA’s PRAC safety committee said it began assessing the evidence about a possible connection following the publication of a study in 2022 in the journal Diabetes Care. That paper reported on an analysis that suggested increased risk for all thyroid cancer and medullary thyroid cancer with the use of GLP-1 drugs, particularly after 1-3 years of treatment.
The EMA’s PRAC said that in making its decision, it also considered other published papers on this topic as well as clinical and postmarketing data on GLP-1 drugs.
In an email interview, Jean-Luc Faillie, MD, PhD, corresponding author of the Diabetes Care paper, called for continued “vigilance and prudence in clinical practice” with GLP-1 drugs.
His paper reported on a case-control analysis on the basis of reports from the French national healthcare insurance system database, looking at people who had taken GLP-1 drugs and similar people who had not.
Due to a lack of a specific diagnostic code for medullary thyroid cancers, the researchers used a composite definition combining thyroid cancer diagnosis with several calcitonin tests, a carcinoembryonic antigen test, or a specific treatment (vandetanib) to identify potential cases of this cancer.
It’s possible that this method could have led to overestimation of MTC among the cases of thyroid cancer, wrote Dr. Faillie, who is a professor at France’s Université de Montpellier, Montpellier, France, and part of its pharmacological vigilance service.
“Nevertheless, it’s crucial to emphasize that any potential overestimation of MTC cases would likely apply equally to both GLP-1 receptor agonist–exposed and unexposed groups,” Dr. Faillie wrote. “Therefore, it should not significantly impact our main findings regarding the suggested increased risk associated with GLP-1 receptor agonist use.”
Dr. Pearce disclosed honoraria for speaking at the Merck China Forum. Dr. Faille and his coauthors reported no conflicts of interest in the publication of their study. Their research was supported by the French Medicines Agency (Agence Nationale de Sécurité du Médicament et des Produits de Santé, grant 2019S015) in the context of a partnership with the Health Product Epidemiology Scientific Interest Group (EPI-PHARE). The study was part of France’s Drugs Systematized Assessment in Real-Life Environment (DRUGS-SAFEr) research program.
A version of this article first appeared on Medscape.com.
ORLANDO, Florida — Clinicians should keep in mind concerns about overdiagnosis of thyroid cancer when prescribing glucagon-like peptide 1 (GLP-1) drugs, as the US boxed warning about this risk for this class of medicines for certain tumors in mice could trigger excess screening, an expert endocrinologist said.
Speaking at the annual American Diabetes Association (ADA) 84th Scientific Sessions, Elizabeth N. Pearce, MD, MSc, a professor of medicine at Boston University, Boston, reviewed the different approaches US and European regulators have taken for the GLP-1 drugs. She also explained the current concerns about the wide use of thyroid screening in general and how these intersect with the rapid uptake of the GLP-1 drugs.
said Dr. Pearce, who is also a former board president of the American Thyroid Association (ATA). “We do not want to contribute to this epidemic of overdiagnosis of thyroid cancer.”
The ATA and the US Preventive Services Task Force (USPSTF) are among the health organizations that have in recent years sought to boost public awareness of the potential risks for excess screening of thyroid nodules. In 2017, the USPSTF, which influences insurance coverage, recommended against routine screening for thyroid cancer in asymptomatic adults. At that time, the incidence of thyroid cancer detection had increased by 4.5% per year over a decade, faster than for any other cancer, but without a corresponding change in the mortality rate, USPSTF said.
“Unequivocally, the thyroid cancer mortality has not kept pace with thyroid cancer detection,” Dr. Pearce said at the ADA meeting. “We’ve been diagnosing a lot of small thyroid cancers that people would otherwise have been destined to die with and not die of.”
Dr. Pearce said clinicians should be careful not to overly restrict access to GLP-1 drugs due to concerns about thyroid cancer — and they should use care in screening nodules.
It’s possible that the weight loss experienced by people taking GLP-1 drugs may make preexisting thyroid nodules more prominent, Dr. Pearce said. It’s also likely that the US boxed warning on thyroid risk on GLP-1 drugs makes clinicians and patients more likely to look for these kinds of growths.
Dr. Pearce urged adherence to guidelines such as the ones the ATA published in 2015 for assessing nodules.
In an interview with this news organization, Dr. Pearce noted the frequency of CT scans in US medical practice in turning up many incidental thyroid nodules, a finding that can cause some panic for patients and their clinicians.
But it helps to put these findings in context, as by the age of 50, about 40% of women will have at least one thyroid nodule, making this a very common finding, she said.
“The vast majority are not malignant,” Dr. Pearce said. “When you explain this to patients, it alleviates anxiety.”
The US, European Union Differences
In the United States, the label for GLP-1 drugs starts with a boxed warning about thyroid C-cell tumors seen in rodents given these medicines in testing.
It’s unknown if the medicines could cause medullary thyroid carcinoma (MTC) in humans, the label adds. The drug is contraindicated in patients with a personal or family history of MTC or multiple endocrine neoplasia syndrome 2, the boxed warning says. This is based largely on data seen in laboratory rats.
“It’s a big black box warning that gets people’s attention,” Dr. Pearce said. “Important to note that if you practice in Europe, you will not be familiar with this labeling because it doesn’t exist there. They’ve never had this warning on the European package.”
The European Medicines Agency (EMA) does include information about the results of rodent studies as part of the discussion of known and potential risks for GLP-1 drugs but has not emphasized it in the same way as the US drug labels do.
For example, the public assessment report posted on the EMA website for semaglutide (Ozempic, Novo Nordisk) notes that nonlethal thyroid C-cell tumors “observed in rodents are a class effect for GLP-1 receptor agonists.” It’s possible that these may be due to a particular sensitivity in rodents, the report said.
“The relevance for humans is considered to be low but cannot be completely excluded,” the EMA report said in the product information section of the report.
There has been ongoing interest in the issue.
The EMA’s Pharmacovigilance Risk Assessment Committee (PRAC) in October concluded that the available evidence does not support a causal association between GLP-1 receptor agonists and thyroid cancer.
The EMA’s PRAC safety committee said it began assessing the evidence about a possible connection following the publication of a study in 2022 in the journal Diabetes Care. That paper reported on an analysis that suggested increased risk for all thyroid cancer and medullary thyroid cancer with the use of GLP-1 drugs, particularly after 1-3 years of treatment.
The EMA’s PRAC said that in making its decision, it also considered other published papers on this topic as well as clinical and postmarketing data on GLP-1 drugs.
In an email interview, Jean-Luc Faillie, MD, PhD, corresponding author of the Diabetes Care paper, called for continued “vigilance and prudence in clinical practice” with GLP-1 drugs.
His paper reported on a case-control analysis on the basis of reports from the French national healthcare insurance system database, looking at people who had taken GLP-1 drugs and similar people who had not.
Due to a lack of a specific diagnostic code for medullary thyroid cancers, the researchers used a composite definition combining thyroid cancer diagnosis with several calcitonin tests, a carcinoembryonic antigen test, or a specific treatment (vandetanib) to identify potential cases of this cancer.
It’s possible that this method could have led to overestimation of MTC among the cases of thyroid cancer, wrote Dr. Faillie, who is a professor at France’s Université de Montpellier, Montpellier, France, and part of its pharmacological vigilance service.
“Nevertheless, it’s crucial to emphasize that any potential overestimation of MTC cases would likely apply equally to both GLP-1 receptor agonist–exposed and unexposed groups,” Dr. Faillie wrote. “Therefore, it should not significantly impact our main findings regarding the suggested increased risk associated with GLP-1 receptor agonist use.”
Dr. Pearce disclosed honoraria for speaking at the Merck China Forum. Dr. Faille and his coauthors reported no conflicts of interest in the publication of their study. Their research was supported by the French Medicines Agency (Agence Nationale de Sécurité du Médicament et des Produits de Santé, grant 2019S015) in the context of a partnership with the Health Product Epidemiology Scientific Interest Group (EPI-PHARE). The study was part of France’s Drugs Systematized Assessment in Real-Life Environment (DRUGS-SAFEr) research program.
A version of this article first appeared on Medscape.com.
FROM ADA 2024
Facial Temperature Can Reveal Age and Disease
This transcript has been edited for clarity.
My oldest daughter is at sleepaway camp for a couple of weeks, and the camp has a photographer who goes around all day taking pictures of the kids, which get uploaded to a private Facebook group. In the past, I would go online every day (or, okay, several times a day) and scroll through all those pictures looking for one that features my kid.
I don’t have to do that anymore. This year, I simply uploaded a picture of my daughter to an app and artificial intelligence (AI) takes care of the rest, recognizing her face amidst the sea of smiling children, and flagging just those photos for me to peruse. It’s amazing, really. And a bit scary.
The fact that facial recognition has penetrated the summer camp market should tell you that the tech is truly ubiquitous. But today we’re going to think a bit more about what AI can do with a picture of your face, because the power of facial recognition is not just skin deep.
What’s got me hot and bothered about facial images is this paper, appearing in Cell Metabolism, which adds a new layer to the standard facial-analysis playbook: facial temperature.
To understand this paper, you need to understand a whole field of research that is developing various different “clocks” for age.
It turns out that age really is just a number. Our cells, our proteins, our biochemistry can be analyzed to give different numbers. These “clocks,” as distinct from the calendar we usually use to measure our age, might have more predictive power than the number itself.
There are numerous molecular clocks, such as telomere length, that not only correlate with calendar age but are superior to calendar age in predicting age-related complications. Testing telomere length typically requires a blood sample — and remains costly. But we can use other sources to estimate age; how about a photo?
I mean, we do this all the time when we meet someone new or, as a physician, when we meet a new patient. I have often written that a patient “appears younger than their stated age,” and we’ve all had the experience of hearing how old someone is and being shocked. I mean, have you seen Sharon Stone recently? She’s 66 years old. Okay — to be fair, there might be some outside help there. But you get the point.
Back to the Cell Metabolism paper. Researchers report on multiple algorithms to obtain an “age” from a picture of an individual’s face.
The first algorithm is pretty straightforward. Researchers collected 2811 images, all of Han Chinese individuals ranging in age from 20 to 90 years, and reconstructed a 3D facial map from those. 
They then trained a convolutional neural network to predict the individuals’ ages from the pictures. It was quite accurate, as you can see here.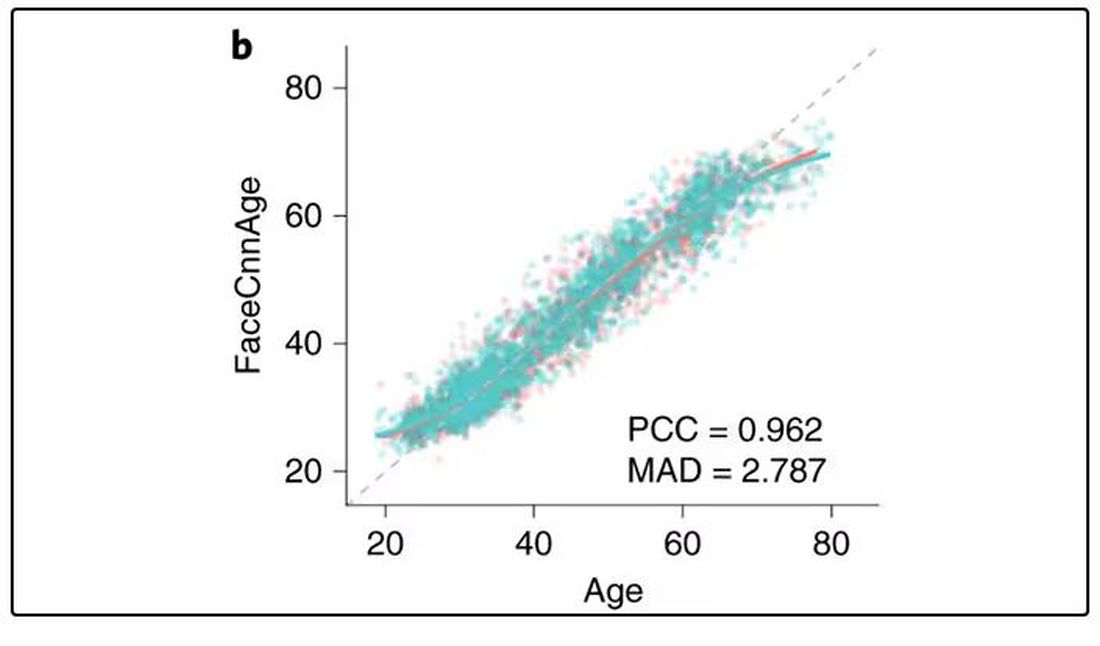
In the AI age, this may not seem that impressive. A brief search online turned up dozens of apps that promised to guess my age from a photo.
I sent this rather unflattering picture of myself to ChatGPT which, after initially demurring and saying it was not designed to guess ages, pegged me at somewhere between 35 and 45, which I am taking as a major victory.
But the Cell Metabolism paper goes deeper. Literally. 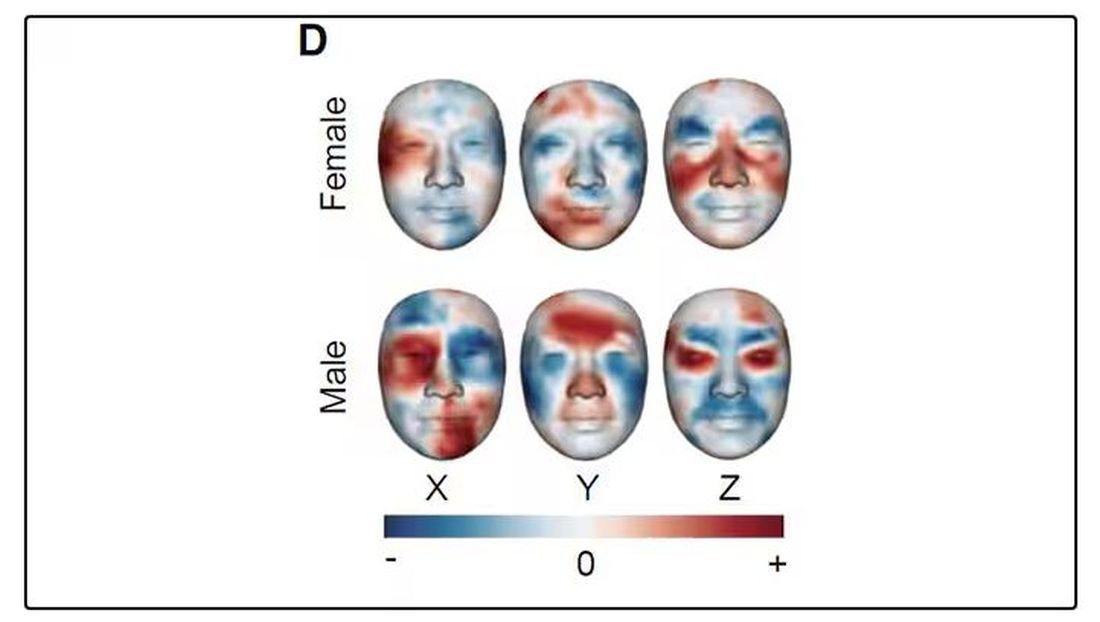
And this is where things start to get interesting. Because sure, the visible part of your face can change depending on makeup, expression, plastic surgery, and the like. But the temperature? That’s harder to fake.
It turns out that the temperature distribution in your face changes as you get older. There is a cooling of the nose and the cheeks, for example.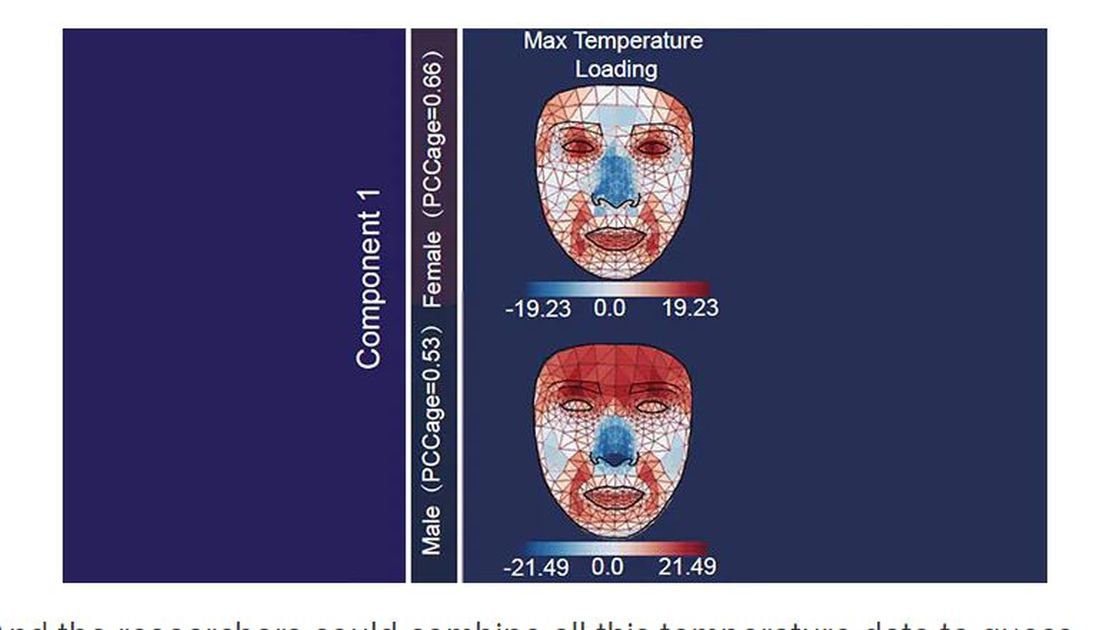
And the researchers could combine all this temperature data to guess someone’s calendar age fairly accurately, though notably not as accurately as the model that just looks at the pictures.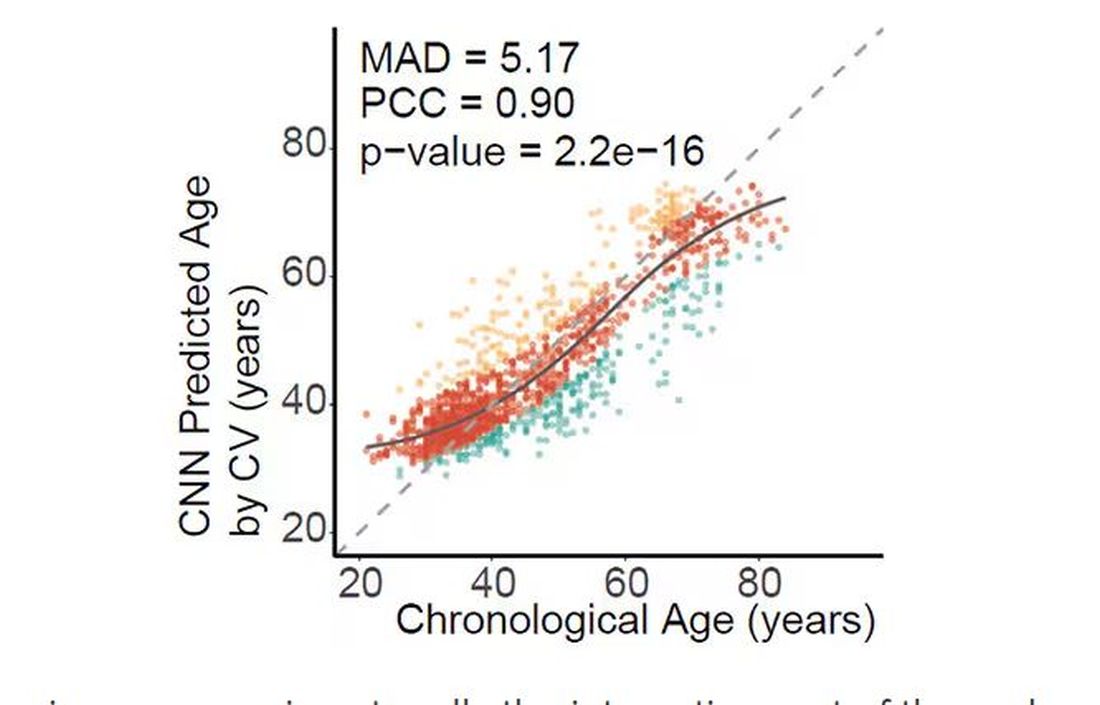
But guessing your age is not really the interesting part of thermal imaging of the face. It’s guessing — or, rather, predicting — the state of your metabolism. All these study participants had extensive metabolic testing performed, as well as detailed analysis of their lifestyle behaviors. And facial images could be used to predict those factors.
For example, the 3D reconstruction of the faces could predict who ate seafood (they tend to look younger than their actual age) compared with who ate poultry and meat (they tend to look older). The thermal imaging could predict who got more sleep (they look younger from a temperature perspective) and who ate more yogurt (also younger-appearing, temperature-wise). Facial temperature patterns could identify those with higher BMI, higher blood pressure, higher fasting glucose.
The researchers used the difference between actual and predicted age as a metric to measure illness as well. You can see here how, on average, individuals with hypertension, diabetes, and even liver cysts are “older,” at least by face temperature.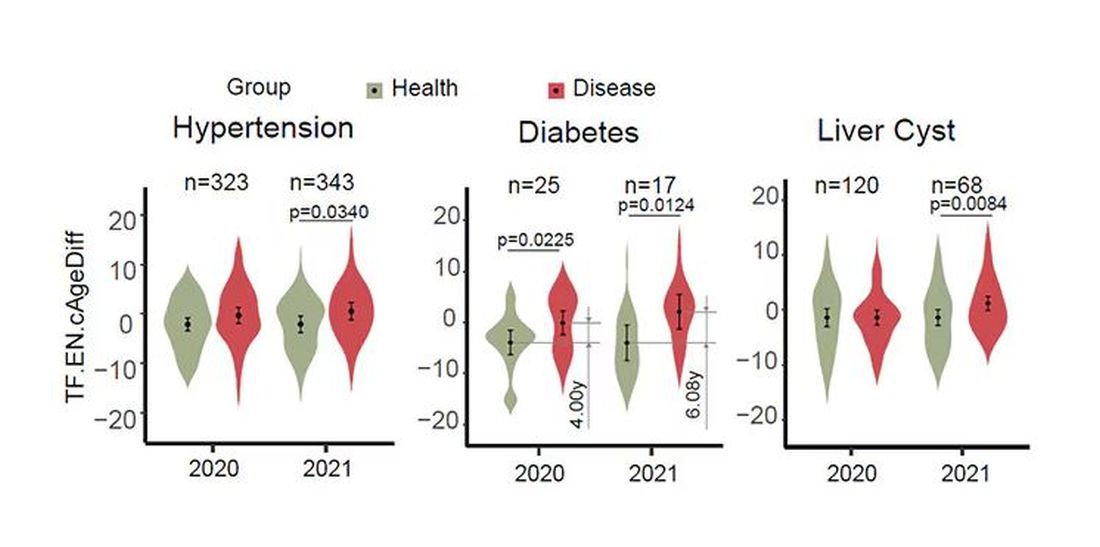
It may even be possible to use facial temperature as biofeedback. In a small study, the researchers measured the difference between facial temperature age and real age before and after 2 weeks of jump-roping. It turns out that 2 weeks of jump-roping can make you look about 5 years younger, at least as judged by a thermal camera. Or like the Predator.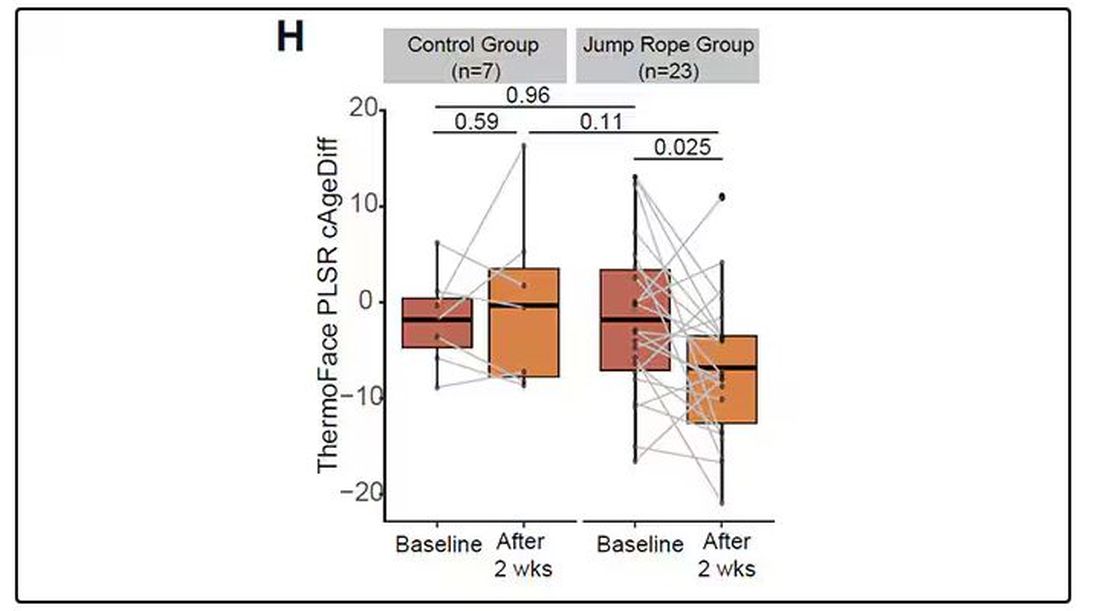
Okay, this is all very cool, but I’m not saying we’ll all be doing facial temperature tests in the near future. No; what this study highlights for me is how much information about ourselves is available to those who know how to decode it. Maybe those data come from the wrinkles in our faces, or the angles of our smiles, or the speed with which we type, or the temperature of our elbows. The data have always been there, actually, but we’ve never had the tools powerful enough to analyze them until now.
When I was a kid, I was obsessed with Star Trek — I know, you’re shocked — and, of course, the famous tricorder, a scanner that could tell everything about someone’s state of health in 5 seconds from 3 feet away. That’s how I thought medicine really would be in the future. Once I got to medical school, I was disabused of that notion. But the age of data, the age of AI, may mean the tricorder age is not actually that far away.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
My oldest daughter is at sleepaway camp for a couple of weeks, and the camp has a photographer who goes around all day taking pictures of the kids, which get uploaded to a private Facebook group. In the past, I would go online every day (or, okay, several times a day) and scroll through all those pictures looking for one that features my kid.
I don’t have to do that anymore. This year, I simply uploaded a picture of my daughter to an app and artificial intelligence (AI) takes care of the rest, recognizing her face amidst the sea of smiling children, and flagging just those photos for me to peruse. It’s amazing, really. And a bit scary.
The fact that facial recognition has penetrated the summer camp market should tell you that the tech is truly ubiquitous. But today we’re going to think a bit more about what AI can do with a picture of your face, because the power of facial recognition is not just skin deep.
What’s got me hot and bothered about facial images is this paper, appearing in Cell Metabolism, which adds a new layer to the standard facial-analysis playbook: facial temperature.
To understand this paper, you need to understand a whole field of research that is developing various different “clocks” for age.
It turns out that age really is just a number. Our cells, our proteins, our biochemistry can be analyzed to give different numbers. These “clocks,” as distinct from the calendar we usually use to measure our age, might have more predictive power than the number itself.
There are numerous molecular clocks, such as telomere length, that not only correlate with calendar age but are superior to calendar age in predicting age-related complications. Testing telomere length typically requires a blood sample — and remains costly. But we can use other sources to estimate age; how about a photo?
I mean, we do this all the time when we meet someone new or, as a physician, when we meet a new patient. I have often written that a patient “appears younger than their stated age,” and we’ve all had the experience of hearing how old someone is and being shocked. I mean, have you seen Sharon Stone recently? She’s 66 years old. Okay — to be fair, there might be some outside help there. But you get the point.
Back to the Cell Metabolism paper. Researchers report on multiple algorithms to obtain an “age” from a picture of an individual’s face.
The first algorithm is pretty straightforward. Researchers collected 2811 images, all of Han Chinese individuals ranging in age from 20 to 90 years, and reconstructed a 3D facial map from those. 
They then trained a convolutional neural network to predict the individuals’ ages from the pictures. It was quite accurate, as you can see here.
In the AI age, this may not seem that impressive. A brief search online turned up dozens of apps that promised to guess my age from a photo.
I sent this rather unflattering picture of myself to ChatGPT which, after initially demurring and saying it was not designed to guess ages, pegged me at somewhere between 35 and 45, which I am taking as a major victory.
But the Cell Metabolism paper goes deeper. Literally. 
And this is where things start to get interesting. Because sure, the visible part of your face can change depending on makeup, expression, plastic surgery, and the like. But the temperature? That’s harder to fake.
It turns out that the temperature distribution in your face changes as you get older. There is a cooling of the nose and the cheeks, for example.
And the researchers could combine all this temperature data to guess someone’s calendar age fairly accurately, though notably not as accurately as the model that just looks at the pictures.
But guessing your age is not really the interesting part of thermal imaging of the face. It’s guessing — or, rather, predicting — the state of your metabolism. All these study participants had extensive metabolic testing performed, as well as detailed analysis of their lifestyle behaviors. And facial images could be used to predict those factors.
For example, the 3D reconstruction of the faces could predict who ate seafood (they tend to look younger than their actual age) compared with who ate poultry and meat (they tend to look older). The thermal imaging could predict who got more sleep (they look younger from a temperature perspective) and who ate more yogurt (also younger-appearing, temperature-wise). Facial temperature patterns could identify those with higher BMI, higher blood pressure, higher fasting glucose.
The researchers used the difference between actual and predicted age as a metric to measure illness as well. You can see here how, on average, individuals with hypertension, diabetes, and even liver cysts are “older,” at least by face temperature.
It may even be possible to use facial temperature as biofeedback. In a small study, the researchers measured the difference between facial temperature age and real age before and after 2 weeks of jump-roping. It turns out that 2 weeks of jump-roping can make you look about 5 years younger, at least as judged by a thermal camera. Or like the Predator.
Okay, this is all very cool, but I’m not saying we’ll all be doing facial temperature tests in the near future. No; what this study highlights for me is how much information about ourselves is available to those who know how to decode it. Maybe those data come from the wrinkles in our faces, or the angles of our smiles, or the speed with which we type, or the temperature of our elbows. The data have always been there, actually, but we’ve never had the tools powerful enough to analyze them until now.
When I was a kid, I was obsessed with Star Trek — I know, you’re shocked — and, of course, the famous tricorder, a scanner that could tell everything about someone’s state of health in 5 seconds from 3 feet away. That’s how I thought medicine really would be in the future. Once I got to medical school, I was disabused of that notion. But the age of data, the age of AI, may mean the tricorder age is not actually that far away.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
My oldest daughter is at sleepaway camp for a couple of weeks, and the camp has a photographer who goes around all day taking pictures of the kids, which get uploaded to a private Facebook group. In the past, I would go online every day (or, okay, several times a day) and scroll through all those pictures looking for one that features my kid.
I don’t have to do that anymore. This year, I simply uploaded a picture of my daughter to an app and artificial intelligence (AI) takes care of the rest, recognizing her face amidst the sea of smiling children, and flagging just those photos for me to peruse. It’s amazing, really. And a bit scary.
The fact that facial recognition has penetrated the summer camp market should tell you that the tech is truly ubiquitous. But today we’re going to think a bit more about what AI can do with a picture of your face, because the power of facial recognition is not just skin deep.
What’s got me hot and bothered about facial images is this paper, appearing in Cell Metabolism, which adds a new layer to the standard facial-analysis playbook: facial temperature.
To understand this paper, you need to understand a whole field of research that is developing various different “clocks” for age.
It turns out that age really is just a number. Our cells, our proteins, our biochemistry can be analyzed to give different numbers. These “clocks,” as distinct from the calendar we usually use to measure our age, might have more predictive power than the number itself.
There are numerous molecular clocks, such as telomere length, that not only correlate with calendar age but are superior to calendar age in predicting age-related complications. Testing telomere length typically requires a blood sample — and remains costly. But we can use other sources to estimate age; how about a photo?
I mean, we do this all the time when we meet someone new or, as a physician, when we meet a new patient. I have often written that a patient “appears younger than their stated age,” and we’ve all had the experience of hearing how old someone is and being shocked. I mean, have you seen Sharon Stone recently? She’s 66 years old. Okay — to be fair, there might be some outside help there. But you get the point.
Back to the Cell Metabolism paper. Researchers report on multiple algorithms to obtain an “age” from a picture of an individual’s face.
The first algorithm is pretty straightforward. Researchers collected 2811 images, all of Han Chinese individuals ranging in age from 20 to 90 years, and reconstructed a 3D facial map from those. 
They then trained a convolutional neural network to predict the individuals’ ages from the pictures. It was quite accurate, as you can see here.
In the AI age, this may not seem that impressive. A brief search online turned up dozens of apps that promised to guess my age from a photo.
I sent this rather unflattering picture of myself to ChatGPT which, after initially demurring and saying it was not designed to guess ages, pegged me at somewhere between 35 and 45, which I am taking as a major victory.
But the Cell Metabolism paper goes deeper. Literally. 
And this is where things start to get interesting. Because sure, the visible part of your face can change depending on makeup, expression, plastic surgery, and the like. But the temperature? That’s harder to fake.
It turns out that the temperature distribution in your face changes as you get older. There is a cooling of the nose and the cheeks, for example.
And the researchers could combine all this temperature data to guess someone’s calendar age fairly accurately, though notably not as accurately as the model that just looks at the pictures.
But guessing your age is not really the interesting part of thermal imaging of the face. It’s guessing — or, rather, predicting — the state of your metabolism. All these study participants had extensive metabolic testing performed, as well as detailed analysis of their lifestyle behaviors. And facial images could be used to predict those factors.
For example, the 3D reconstruction of the faces could predict who ate seafood (they tend to look younger than their actual age) compared with who ate poultry and meat (they tend to look older). The thermal imaging could predict who got more sleep (they look younger from a temperature perspective) and who ate more yogurt (also younger-appearing, temperature-wise). Facial temperature patterns could identify those with higher BMI, higher blood pressure, higher fasting glucose.
The researchers used the difference between actual and predicted age as a metric to measure illness as well. You can see here how, on average, individuals with hypertension, diabetes, and even liver cysts are “older,” at least by face temperature.
It may even be possible to use facial temperature as biofeedback. In a small study, the researchers measured the difference between facial temperature age and real age before and after 2 weeks of jump-roping. It turns out that 2 weeks of jump-roping can make you look about 5 years younger, at least as judged by a thermal camera. Or like the Predator.
Okay, this is all very cool, but I’m not saying we’ll all be doing facial temperature tests in the near future. No; what this study highlights for me is how much information about ourselves is available to those who know how to decode it. Maybe those data come from the wrinkles in our faces, or the angles of our smiles, or the speed with which we type, or the temperature of our elbows. The data have always been there, actually, but we’ve never had the tools powerful enough to analyze them until now.
When I was a kid, I was obsessed with Star Trek — I know, you’re shocked — and, of course, the famous tricorder, a scanner that could tell everything about someone’s state of health in 5 seconds from 3 feet away. That’s how I thought medicine really would be in the future. Once I got to medical school, I was disabused of that notion. But the age of data, the age of AI, may mean the tricorder age is not actually that far away.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
What Should Be Prioritized in Managing Early Diabetes?
ORLANDO, FLORIDA — What to prioritize first in managing early diabetes? That was the question debated on an expert panel at the American Diabetes Association (ADA) 84th Scientific Sessions, with impassioned responses ranging from a plea to “treat obesity first,” to a James Carville–inspired counterpoint of “it’s the glucose, stupid.”
With a focus on preventing complications and inducing remission rounding out the four positions argued,
“In clinical decision-making [for early diabetes], we are faced with weighing each of these variables for the individual patient, and while all are good options, strong arguments can be made for prioritizing each — with the potential of each choice to influence or improve all of the others,” Dr. Retnakaran told this news organization.
Which to Prioritize First?
Making the obesity first argument, Ania M. Jastreboff, MD, PhD, associate professor and director of the Yale Obesity Research Center at Yale School of Medicine, New Haven, Connecticut, noted the striking statistic that nearly 90% of people with type 2 diabetes have overweight or obesity and discussed the ever-expanding data showing the benefits of drugs including glucagon-like peptide 1 (GLP-1) receptor agonists not just in weight loss but also in kidney, cardiovascular, and, as presented at the meeting, sleep apnea improvement.
She contrasted the experiences of two patients with obesity: One treated for the obesity upon type 2 diagnosis — who had a quick normalization of lipids and hypertension soon after the obesity treatment — and the other presenting after 10 years with type 2 diabetes — who was on therapy for hypertension and hyperlipidemia but not for obesity and whose diseases were not as easily treated by that point.
“Why are we treating all the downstream effects and we’re not treating the disease that is potentially the root cause of all these other diseases?” Dr. Jastreboff said.
Complications?
Arguing in favor of focusing on complications, Roopa Mehta, MD, PhD, with the department of endocrinology and metabolism at Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán (INCMNSZ), Mexico City, made the case that stakes don’t get any higher in diabetes than when it comes the looming threat of potentially fatal complications.
Acute myocardial infarction, stroke, amputation, and end-stage renal disease are all on the list of unwanted outcomes and need to be considered even in the earliest stages, as data show early onset type 2 diabetes is linked to life expectancy.
“The main goal of management has always been to prevent complications,” she noted. Citing ADA guidelines, Dr. Mehta underscored the benefits of first- and second-line therapy of metformin, sodium-glucose cotransporter 2 (SGLT2) inhibitors, and GLP-1 receptor agonists for most patients.
Remission?
Discussing the priority of putting patients into disease remission, Roy Taylor, MD, professor of medicine and metabolism at Newcastle University and Newcastle Hospitals NHS in Newcastle upon Tyne, England, and author of the book Life Without Diabetes, focused on an evidence-based alternative to achieving remission — a nonpharmacologic approach that avoids costly and sometimes inaccessible drugs.
In the intervention, described in the DiRECT randomized trial and subsequently in the UK National Health Service Type 2 Diabetes Path to Remission Program, patients with overweight or obesity were placed on a highly restrictive diet of just 800-900 calories a day for 12-20 weeks, followed by maintenance for 12 months, and they not only achieved weight loss but also achieved diabetes remission, in some cases long term.
Acknowledging that “this is not for everyone,” Dr. Taylor asserted that “we have to realize there is a substantial minority of people who want to be healthy but who don’t want to be medicalized,” he said.
“They want their health, and they can do extremely well.”
Glucose?
In taking his self-titled “it’s the glucose, stupid” stand, David M. Nathan, MD, of the Diabetes Center, Massachusetts General Hospital, Harvard Medical School, in Boston, cited extensive evidence showing that early intensive blood glucose control with treatment including sulfonylureas, insulin, or metformin significantly reduced the risk for complications in type 2 diabetes 15 or more years later, including renal failure, blindness, amputation, and myocardial infarctions, in addition to a reduction in diabetes-related death.
“In many of these studies, you saw the benefit even in the setting of weight-gain,” Dr. Nathan underscored.
He further noted the “sobering” findings of the Look AHEAD study, which had to be stopped due to futility when an intensive lifestyle/weight loss intervention showed no significant benefits in terms of cardiovascular disease in people with type 2 diabetes at a median follow-up of 9.6 years.
Ultimately, “diabetes, type 1 and type 2, remains a gluco-centric disease,” Dr. Nathan asserted. “Hyperglycemia is the only universal link between all forms of diabetes and mortality, and the long-term complications of diabetes are intimately associated with hyperglycemia.”
Tackling the Caveats
The ensuing panel discussion did not fail to deliver in delving into key areas of contention, particularly in terms of GLP-1 treatment.
Regarding a lack of data on the potential long-term effects of GLP-1s: “Yes, there are a huge number of studies [on GLP-1 receptor agonists], but they are, in general, over short periods of time and driven by pharma, who get in and get out as quickly as they can and have little in the way of interest to do comparative effectiveness studies,” Dr. Nathan argued.
“Meanwhile, this is like the crack cocaine of medications — patients have to stay on it for a lifetime or they will regain the weight — are you concerned at all about a lifetime of exposure to GLP-1 [drugs]?” he asked the panel.
Dr. Jastreboff responded that the first GLP-1 receptor agonist medications were approved in 2005, nearly 20 years ago, by the US Food and Drug Administration.
“Do I think we need long-term lifetime data? Absolutely,” she said. “We need to do our due diligence, we need to be careful, we need to monitor patients, and when and if there are signals, we need to follow them.”
What about the notorious gastrointestinal side effects of the drugs? “A majority of them are mitigated by slow up-titration,” Dr. Jastreboff noted.
“If patients have nausea, I do not go up [in dose]. I invite patients to tell me if they’re having vomiting because I don’t want anybody to have it, and I can count on one hand how many of my patients do.”
Dr. Mehta added the concern that as the drugs’ popularity soars, “a lot of doctors don’t know when they need to put the brakes on [weight coming off too quickly].”
She underscored that “we are not treating obesity for weight loss or for cosmetic reasons — this is about optimizing health.”
Dr. Jastreboff noted that in her practice, “I down-titrate if they’re losing weight too quickly.”
“If the patient is losing more than 1% per week of their body weight, then I slow down to make sure they’re getting the nutrients that they need, that they have enough energy to exercise, and that they’re prioritizing protein and fruits and vegetables in their diet.
“We just need to go slow, and yes, we need to follow them long term,” she said.
Chiming in from the audience, Julio Rosenstock, MD, a recognized thought leader in type 2 diabetes, offered his own take on the issues, describing Dr. Taylor’s very low–calorie diet suggestion as “not realistic” and Dr. Nathan’s glucose-first argument to be “stuck in the past.”
Based on modern-day evidence, “there is no reason on earth to start [diabetes treatment] with only metformin,” asserted Dr. Rosenstock, director of the Velocity Clinical Research center at Medical City and clinical professor of medicine at the University of Texas Southwestern Medical Center, Dallas.
“We need to start at the very least with metformin and a sodium-glucose cotransporter 2 (SGLT2) inhibitor from day 1, and then, if it’s affordable and there is access, with a GLP-1 receptor agonist,” he said.
“There is nothing better these days than those agents that consistently have shown a reduction of cardiovascular events and slowing of kidney disease progression.”
Overall, however, “I think you are all right,” he added, a sentiment shared by most.
Noting that the discussion as a whole represents a virtual sea change from the evidence-based options that would have been discussed only a decade ago, Dr. Retnakaran summed up his take-home message: “Stay tuned.
“You could easily see things changing in the next decade to come as we get more data and evidence to support what we ultimately should prioritize an early type 2 diabetes, so this is an exciting time.”
Dr. Retnakaran disclosed ties with Novo Nordisk, Boehringer Ingelheim, Novartis, Sanofi, and Eli Lilly. Dr. Jastreboff disclosed ties with Amgen, AstraZeneca, Boehringer Ingelheim, Biohaven, Eli Lilly, Intellihealth, Novo Nordisk, Pfizer, Regeneron, Scholar Rock, Structure Therapeutics, Terms Pharmaceutical, Weight Watchers, and Zealand Pharmaceuticals. Dr. Roopa had relationships with Novo Nordisk, Boehringer Ingelheim, Amgen, AstraZeneca, Eli Lilly, Silanes, and Sanofi. Dr. Taylor received lecture fees from Novartis, Lilly, Abbott, and Nestle Health and research funding from Diabetes UK and is an advisor to Fast800. Dr. Rosenstock reported relationships with Applied Therapeutics, AstraZeneca, Biomea Fusion, Boehringer Ingelheim, Eli Lilly and Company, Hanmi, Merck, Oramed, Structure Therapeutics, Novartis, Novo Nordisk, Pfizer, Ragor, and Sanofi. Dr. Nathan had no disclosures to report.
A version of this article first appeared on Medscape.com.
ORLANDO, FLORIDA — What to prioritize first in managing early diabetes? That was the question debated on an expert panel at the American Diabetes Association (ADA) 84th Scientific Sessions, with impassioned responses ranging from a plea to “treat obesity first,” to a James Carville–inspired counterpoint of “it’s the glucose, stupid.”
With a focus on preventing complications and inducing remission rounding out the four positions argued,
“In clinical decision-making [for early diabetes], we are faced with weighing each of these variables for the individual patient, and while all are good options, strong arguments can be made for prioritizing each — with the potential of each choice to influence or improve all of the others,” Dr. Retnakaran told this news organization.
Which to Prioritize First?
Making the obesity first argument, Ania M. Jastreboff, MD, PhD, associate professor and director of the Yale Obesity Research Center at Yale School of Medicine, New Haven, Connecticut, noted the striking statistic that nearly 90% of people with type 2 diabetes have overweight or obesity and discussed the ever-expanding data showing the benefits of drugs including glucagon-like peptide 1 (GLP-1) receptor agonists not just in weight loss but also in kidney, cardiovascular, and, as presented at the meeting, sleep apnea improvement.
She contrasted the experiences of two patients with obesity: One treated for the obesity upon type 2 diagnosis — who had a quick normalization of lipids and hypertension soon after the obesity treatment — and the other presenting after 10 years with type 2 diabetes — who was on therapy for hypertension and hyperlipidemia but not for obesity and whose diseases were not as easily treated by that point.
“Why are we treating all the downstream effects and we’re not treating the disease that is potentially the root cause of all these other diseases?” Dr. Jastreboff said.
Complications?
Arguing in favor of focusing on complications, Roopa Mehta, MD, PhD, with the department of endocrinology and metabolism at Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán (INCMNSZ), Mexico City, made the case that stakes don’t get any higher in diabetes than when it comes the looming threat of potentially fatal complications.
Acute myocardial infarction, stroke, amputation, and end-stage renal disease are all on the list of unwanted outcomes and need to be considered even in the earliest stages, as data show early onset type 2 diabetes is linked to life expectancy.
“The main goal of management has always been to prevent complications,” she noted. Citing ADA guidelines, Dr. Mehta underscored the benefits of first- and second-line therapy of metformin, sodium-glucose cotransporter 2 (SGLT2) inhibitors, and GLP-1 receptor agonists for most patients.
Remission?
Discussing the priority of putting patients into disease remission, Roy Taylor, MD, professor of medicine and metabolism at Newcastle University and Newcastle Hospitals NHS in Newcastle upon Tyne, England, and author of the book Life Without Diabetes, focused on an evidence-based alternative to achieving remission — a nonpharmacologic approach that avoids costly and sometimes inaccessible drugs.
In the intervention, described in the DiRECT randomized trial and subsequently in the UK National Health Service Type 2 Diabetes Path to Remission Program, patients with overweight or obesity were placed on a highly restrictive diet of just 800-900 calories a day for 12-20 weeks, followed by maintenance for 12 months, and they not only achieved weight loss but also achieved diabetes remission, in some cases long term.
Acknowledging that “this is not for everyone,” Dr. Taylor asserted that “we have to realize there is a substantial minority of people who want to be healthy but who don’t want to be medicalized,” he said.
“They want their health, and they can do extremely well.”
Glucose?
In taking his self-titled “it’s the glucose, stupid” stand, David M. Nathan, MD, of the Diabetes Center, Massachusetts General Hospital, Harvard Medical School, in Boston, cited extensive evidence showing that early intensive blood glucose control with treatment including sulfonylureas, insulin, or metformin significantly reduced the risk for complications in type 2 diabetes 15 or more years later, including renal failure, blindness, amputation, and myocardial infarctions, in addition to a reduction in diabetes-related death.
“In many of these studies, you saw the benefit even in the setting of weight-gain,” Dr. Nathan underscored.
He further noted the “sobering” findings of the Look AHEAD study, which had to be stopped due to futility when an intensive lifestyle/weight loss intervention showed no significant benefits in terms of cardiovascular disease in people with type 2 diabetes at a median follow-up of 9.6 years.
Ultimately, “diabetes, type 1 and type 2, remains a gluco-centric disease,” Dr. Nathan asserted. “Hyperglycemia is the only universal link between all forms of diabetes and mortality, and the long-term complications of diabetes are intimately associated with hyperglycemia.”
Tackling the Caveats
The ensuing panel discussion did not fail to deliver in delving into key areas of contention, particularly in terms of GLP-1 treatment.
Regarding a lack of data on the potential long-term effects of GLP-1s: “Yes, there are a huge number of studies [on GLP-1 receptor agonists], but they are, in general, over short periods of time and driven by pharma, who get in and get out as quickly as they can and have little in the way of interest to do comparative effectiveness studies,” Dr. Nathan argued.
“Meanwhile, this is like the crack cocaine of medications — patients have to stay on it for a lifetime or they will regain the weight — are you concerned at all about a lifetime of exposure to GLP-1 [drugs]?” he asked the panel.
Dr. Jastreboff responded that the first GLP-1 receptor agonist medications were approved in 2005, nearly 20 years ago, by the US Food and Drug Administration.
“Do I think we need long-term lifetime data? Absolutely,” she said. “We need to do our due diligence, we need to be careful, we need to monitor patients, and when and if there are signals, we need to follow them.”
What about the notorious gastrointestinal side effects of the drugs? “A majority of them are mitigated by slow up-titration,” Dr. Jastreboff noted.
“If patients have nausea, I do not go up [in dose]. I invite patients to tell me if they’re having vomiting because I don’t want anybody to have it, and I can count on one hand how many of my patients do.”
Dr. Mehta added the concern that as the drugs’ popularity soars, “a lot of doctors don’t know when they need to put the brakes on [weight coming off too quickly].”
She underscored that “we are not treating obesity for weight loss or for cosmetic reasons — this is about optimizing health.”
Dr. Jastreboff noted that in her practice, “I down-titrate if they’re losing weight too quickly.”
“If the patient is losing more than 1% per week of their body weight, then I slow down to make sure they’re getting the nutrients that they need, that they have enough energy to exercise, and that they’re prioritizing protein and fruits and vegetables in their diet.
“We just need to go slow, and yes, we need to follow them long term,” she said.
Chiming in from the audience, Julio Rosenstock, MD, a recognized thought leader in type 2 diabetes, offered his own take on the issues, describing Dr. Taylor’s very low–calorie diet suggestion as “not realistic” and Dr. Nathan’s glucose-first argument to be “stuck in the past.”
Based on modern-day evidence, “there is no reason on earth to start [diabetes treatment] with only metformin,” asserted Dr. Rosenstock, director of the Velocity Clinical Research center at Medical City and clinical professor of medicine at the University of Texas Southwestern Medical Center, Dallas.
“We need to start at the very least with metformin and a sodium-glucose cotransporter 2 (SGLT2) inhibitor from day 1, and then, if it’s affordable and there is access, with a GLP-1 receptor agonist,” he said.
“There is nothing better these days than those agents that consistently have shown a reduction of cardiovascular events and slowing of kidney disease progression.”
Overall, however, “I think you are all right,” he added, a sentiment shared by most.
Noting that the discussion as a whole represents a virtual sea change from the evidence-based options that would have been discussed only a decade ago, Dr. Retnakaran summed up his take-home message: “Stay tuned.
“You could easily see things changing in the next decade to come as we get more data and evidence to support what we ultimately should prioritize an early type 2 diabetes, so this is an exciting time.”
Dr. Retnakaran disclosed ties with Novo Nordisk, Boehringer Ingelheim, Novartis, Sanofi, and Eli Lilly. Dr. Jastreboff disclosed ties with Amgen, AstraZeneca, Boehringer Ingelheim, Biohaven, Eli Lilly, Intellihealth, Novo Nordisk, Pfizer, Regeneron, Scholar Rock, Structure Therapeutics, Terms Pharmaceutical, Weight Watchers, and Zealand Pharmaceuticals. Dr. Roopa had relationships with Novo Nordisk, Boehringer Ingelheim, Amgen, AstraZeneca, Eli Lilly, Silanes, and Sanofi. Dr. Taylor received lecture fees from Novartis, Lilly, Abbott, and Nestle Health and research funding from Diabetes UK and is an advisor to Fast800. Dr. Rosenstock reported relationships with Applied Therapeutics, AstraZeneca, Biomea Fusion, Boehringer Ingelheim, Eli Lilly and Company, Hanmi, Merck, Oramed, Structure Therapeutics, Novartis, Novo Nordisk, Pfizer, Ragor, and Sanofi. Dr. Nathan had no disclosures to report.
A version of this article first appeared on Medscape.com.
ORLANDO, FLORIDA — What to prioritize first in managing early diabetes? That was the question debated on an expert panel at the American Diabetes Association (ADA) 84th Scientific Sessions, with impassioned responses ranging from a plea to “treat obesity first,” to a James Carville–inspired counterpoint of “it’s the glucose, stupid.”
With a focus on preventing complications and inducing remission rounding out the four positions argued,
“In clinical decision-making [for early diabetes], we are faced with weighing each of these variables for the individual patient, and while all are good options, strong arguments can be made for prioritizing each — with the potential of each choice to influence or improve all of the others,” Dr. Retnakaran told this news organization.
Which to Prioritize First?
Making the obesity first argument, Ania M. Jastreboff, MD, PhD, associate professor and director of the Yale Obesity Research Center at Yale School of Medicine, New Haven, Connecticut, noted the striking statistic that nearly 90% of people with type 2 diabetes have overweight or obesity and discussed the ever-expanding data showing the benefits of drugs including glucagon-like peptide 1 (GLP-1) receptor agonists not just in weight loss but also in kidney, cardiovascular, and, as presented at the meeting, sleep apnea improvement.
She contrasted the experiences of two patients with obesity: One treated for the obesity upon type 2 diagnosis — who had a quick normalization of lipids and hypertension soon after the obesity treatment — and the other presenting after 10 years with type 2 diabetes — who was on therapy for hypertension and hyperlipidemia but not for obesity and whose diseases were not as easily treated by that point.
“Why are we treating all the downstream effects and we’re not treating the disease that is potentially the root cause of all these other diseases?” Dr. Jastreboff said.
Complications?
Arguing in favor of focusing on complications, Roopa Mehta, MD, PhD, with the department of endocrinology and metabolism at Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán (INCMNSZ), Mexico City, made the case that stakes don’t get any higher in diabetes than when it comes the looming threat of potentially fatal complications.
Acute myocardial infarction, stroke, amputation, and end-stage renal disease are all on the list of unwanted outcomes and need to be considered even in the earliest stages, as data show early onset type 2 diabetes is linked to life expectancy.
“The main goal of management has always been to prevent complications,” she noted. Citing ADA guidelines, Dr. Mehta underscored the benefits of first- and second-line therapy of metformin, sodium-glucose cotransporter 2 (SGLT2) inhibitors, and GLP-1 receptor agonists for most patients.
Remission?
Discussing the priority of putting patients into disease remission, Roy Taylor, MD, professor of medicine and metabolism at Newcastle University and Newcastle Hospitals NHS in Newcastle upon Tyne, England, and author of the book Life Without Diabetes, focused on an evidence-based alternative to achieving remission — a nonpharmacologic approach that avoids costly and sometimes inaccessible drugs.
In the intervention, described in the DiRECT randomized trial and subsequently in the UK National Health Service Type 2 Diabetes Path to Remission Program, patients with overweight or obesity were placed on a highly restrictive diet of just 800-900 calories a day for 12-20 weeks, followed by maintenance for 12 months, and they not only achieved weight loss but also achieved diabetes remission, in some cases long term.
Acknowledging that “this is not for everyone,” Dr. Taylor asserted that “we have to realize there is a substantial minority of people who want to be healthy but who don’t want to be medicalized,” he said.
“They want their health, and they can do extremely well.”
Glucose?
In taking his self-titled “it’s the glucose, stupid” stand, David M. Nathan, MD, of the Diabetes Center, Massachusetts General Hospital, Harvard Medical School, in Boston, cited extensive evidence showing that early intensive blood glucose control with treatment including sulfonylureas, insulin, or metformin significantly reduced the risk for complications in type 2 diabetes 15 or more years later, including renal failure, blindness, amputation, and myocardial infarctions, in addition to a reduction in diabetes-related death.
“In many of these studies, you saw the benefit even in the setting of weight-gain,” Dr. Nathan underscored.
He further noted the “sobering” findings of the Look AHEAD study, which had to be stopped due to futility when an intensive lifestyle/weight loss intervention showed no significant benefits in terms of cardiovascular disease in people with type 2 diabetes at a median follow-up of 9.6 years.
Ultimately, “diabetes, type 1 and type 2, remains a gluco-centric disease,” Dr. Nathan asserted. “Hyperglycemia is the only universal link between all forms of diabetes and mortality, and the long-term complications of diabetes are intimately associated with hyperglycemia.”
Tackling the Caveats
The ensuing panel discussion did not fail to deliver in delving into key areas of contention, particularly in terms of GLP-1 treatment.
Regarding a lack of data on the potential long-term effects of GLP-1s: “Yes, there are a huge number of studies [on GLP-1 receptor agonists], but they are, in general, over short periods of time and driven by pharma, who get in and get out as quickly as they can and have little in the way of interest to do comparative effectiveness studies,” Dr. Nathan argued.
“Meanwhile, this is like the crack cocaine of medications — patients have to stay on it for a lifetime or they will regain the weight — are you concerned at all about a lifetime of exposure to GLP-1 [drugs]?” he asked the panel.
Dr. Jastreboff responded that the first GLP-1 receptor agonist medications were approved in 2005, nearly 20 years ago, by the US Food and Drug Administration.
“Do I think we need long-term lifetime data? Absolutely,” she said. “We need to do our due diligence, we need to be careful, we need to monitor patients, and when and if there are signals, we need to follow them.”
What about the notorious gastrointestinal side effects of the drugs? “A majority of them are mitigated by slow up-titration,” Dr. Jastreboff noted.
“If patients have nausea, I do not go up [in dose]. I invite patients to tell me if they’re having vomiting because I don’t want anybody to have it, and I can count on one hand how many of my patients do.”
Dr. Mehta added the concern that as the drugs’ popularity soars, “a lot of doctors don’t know when they need to put the brakes on [weight coming off too quickly].”
She underscored that “we are not treating obesity for weight loss or for cosmetic reasons — this is about optimizing health.”
Dr. Jastreboff noted that in her practice, “I down-titrate if they’re losing weight too quickly.”
“If the patient is losing more than 1% per week of their body weight, then I slow down to make sure they’re getting the nutrients that they need, that they have enough energy to exercise, and that they’re prioritizing protein and fruits and vegetables in their diet.
“We just need to go slow, and yes, we need to follow them long term,” she said.
Chiming in from the audience, Julio Rosenstock, MD, a recognized thought leader in type 2 diabetes, offered his own take on the issues, describing Dr. Taylor’s very low–calorie diet suggestion as “not realistic” and Dr. Nathan’s glucose-first argument to be “stuck in the past.”
Based on modern-day evidence, “there is no reason on earth to start [diabetes treatment] with only metformin,” asserted Dr. Rosenstock, director of the Velocity Clinical Research center at Medical City and clinical professor of medicine at the University of Texas Southwestern Medical Center, Dallas.
“We need to start at the very least with metformin and a sodium-glucose cotransporter 2 (SGLT2) inhibitor from day 1, and then, if it’s affordable and there is access, with a GLP-1 receptor agonist,” he said.
“There is nothing better these days than those agents that consistently have shown a reduction of cardiovascular events and slowing of kidney disease progression.”
Overall, however, “I think you are all right,” he added, a sentiment shared by most.
Noting that the discussion as a whole represents a virtual sea change from the evidence-based options that would have been discussed only a decade ago, Dr. Retnakaran summed up his take-home message: “Stay tuned.
“You could easily see things changing in the next decade to come as we get more data and evidence to support what we ultimately should prioritize an early type 2 diabetes, so this is an exciting time.”
Dr. Retnakaran disclosed ties with Novo Nordisk, Boehringer Ingelheim, Novartis, Sanofi, and Eli Lilly. Dr. Jastreboff disclosed ties with Amgen, AstraZeneca, Boehringer Ingelheim, Biohaven, Eli Lilly, Intellihealth, Novo Nordisk, Pfizer, Regeneron, Scholar Rock, Structure Therapeutics, Terms Pharmaceutical, Weight Watchers, and Zealand Pharmaceuticals. Dr. Roopa had relationships with Novo Nordisk, Boehringer Ingelheim, Amgen, AstraZeneca, Eli Lilly, Silanes, and Sanofi. Dr. Taylor received lecture fees from Novartis, Lilly, Abbott, and Nestle Health and research funding from Diabetes UK and is an advisor to Fast800. Dr. Rosenstock reported relationships with Applied Therapeutics, AstraZeneca, Biomea Fusion, Boehringer Ingelheim, Eli Lilly and Company, Hanmi, Merck, Oramed, Structure Therapeutics, Novartis, Novo Nordisk, Pfizer, Ragor, and Sanofi. Dr. Nathan had no disclosures to report.
A version of this article first appeared on Medscape.com.
Triple Therapy May Be Effective in Drug-Naive T2D
TOPLINE:
A triple combination therapy (TCT) of metformin, dapagliflozin, and saxagliptin is an effective and safe treatment option for drug-naive patients with type 2 diabetes (T2D) compared with stepwise add-on therapy.
METHODOLOGY:
- Current guidelines recommend early combination therapy to extend the time to treatment failure, reduce the risk for diabetic complications, and prevent clinical inertia in patients with T2D.
- This randomized controlled open-label trial conducted at nine sites in South Korea included 105 drug-naive patients with T2D (mean age, 49.5 years; 32.4% women) who either received triple therapy (metformin, dapagliflozin, and saxagliptin) or stepwise add-on therapy (initiated with metformin, followed by glimepiride and sitagliptin for those with baseline hemoglobin A1c levels < 9.0% or with initial dual metformin and glimepiride in those with A1c levels ≥ 9.0% followed by sitagliptin).
- The primary outcome was the proportion of patients who achieved A1c levels < 6.5% without hypoglycemia, weight gain ≥ 5%, or discontinuation of drugs because of adverse events at week 104.
- The secondary outcomes were the proportion of patients whose A1c levels dropped to < 7.0% at weeks 56 and 104 and dropped to < 6.5% at week 56, all without hypoglycemia, weight gain, nor discontinuation due to adverse events.
TAKEAWAY:
- At week 104, a higher proportion of patients in the triple therapy group achieved the primary outcome than those in the stepwise add-on therapy group (39.0% vs 17.1%; P = .027).
- In both groups, a similar proportion of patients (46.3%) achieved A1c levels < 6.5% at week 104, but the proportion of patients without hypoglycemia, weight gain, or discontinuation because of adverse events was higher in the triple therapy group than those in the stepwise add-on therapy group (83.3% vs 38.0%; P < .001).
IN PRACTICE:
The authors wrote: “Although the glycemic efficacy of each drug in the TCT was modest, the combination of these drugs resulted in a 2-year durable glycemic efficacy, with greater than a 2.5% reduction in A1c levels from baseline. The overall results of this study suggest a novel strategy for initial combination therapy in newly diagnosed T2D patients.”
SOURCE:
The study was led by Nam Hoon Kim, MD, of the Department of Internal Medicine, Korea University College of Medicine, Seoul. It was published online in Diabetes, Obesity and Metabolism.
LIMITATIONS:
The study had a relatively small sample size as compared with previous clinical trials. More people in the standard therapy group had A1c levels ≥ 9.0%, which resulted in more than double the number of people receiving dual combination therapy over monotherapy in that group. The trial duration was insufficient to evaluate the cardiovascular outcomes.
DISCLOSURES:
The study was funded by AstraZeneca. Some authors reported financial ties with AstraZeneca and other pharmaceutical and medical device companies as members of advisory boards or recipients of grants, consulting fees, honoraria, or lecture fees.
A version of this article appeared on Medscape.com.
TOPLINE:
A triple combination therapy (TCT) of metformin, dapagliflozin, and saxagliptin is an effective and safe treatment option for drug-naive patients with type 2 diabetes (T2D) compared with stepwise add-on therapy.
METHODOLOGY:
- Current guidelines recommend early combination therapy to extend the time to treatment failure, reduce the risk for diabetic complications, and prevent clinical inertia in patients with T2D.
- This randomized controlled open-label trial conducted at nine sites in South Korea included 105 drug-naive patients with T2D (mean age, 49.5 years; 32.4% women) who either received triple therapy (metformin, dapagliflozin, and saxagliptin) or stepwise add-on therapy (initiated with metformin, followed by glimepiride and sitagliptin for those with baseline hemoglobin A1c levels < 9.0% or with initial dual metformin and glimepiride in those with A1c levels ≥ 9.0% followed by sitagliptin).
- The primary outcome was the proportion of patients who achieved A1c levels < 6.5% without hypoglycemia, weight gain ≥ 5%, or discontinuation of drugs because of adverse events at week 104.
- The secondary outcomes were the proportion of patients whose A1c levels dropped to < 7.0% at weeks 56 and 104 and dropped to < 6.5% at week 56, all without hypoglycemia, weight gain, nor discontinuation due to adverse events.
TAKEAWAY:
- At week 104, a higher proportion of patients in the triple therapy group achieved the primary outcome than those in the stepwise add-on therapy group (39.0% vs 17.1%; P = .027).
- In both groups, a similar proportion of patients (46.3%) achieved A1c levels < 6.5% at week 104, but the proportion of patients without hypoglycemia, weight gain, or discontinuation because of adverse events was higher in the triple therapy group than those in the stepwise add-on therapy group (83.3% vs 38.0%; P < .001).
IN PRACTICE:
The authors wrote: “Although the glycemic efficacy of each drug in the TCT was modest, the combination of these drugs resulted in a 2-year durable glycemic efficacy, with greater than a 2.5% reduction in A1c levels from baseline. The overall results of this study suggest a novel strategy for initial combination therapy in newly diagnosed T2D patients.”
SOURCE:
The study was led by Nam Hoon Kim, MD, of the Department of Internal Medicine, Korea University College of Medicine, Seoul. It was published online in Diabetes, Obesity and Metabolism.
LIMITATIONS:
The study had a relatively small sample size as compared with previous clinical trials. More people in the standard therapy group had A1c levels ≥ 9.0%, which resulted in more than double the number of people receiving dual combination therapy over monotherapy in that group. The trial duration was insufficient to evaluate the cardiovascular outcomes.
DISCLOSURES:
The study was funded by AstraZeneca. Some authors reported financial ties with AstraZeneca and other pharmaceutical and medical device companies as members of advisory boards or recipients of grants, consulting fees, honoraria, or lecture fees.
A version of this article appeared on Medscape.com.
TOPLINE:
A triple combination therapy (TCT) of metformin, dapagliflozin, and saxagliptin is an effective and safe treatment option for drug-naive patients with type 2 diabetes (T2D) compared with stepwise add-on therapy.
METHODOLOGY:
- Current guidelines recommend early combination therapy to extend the time to treatment failure, reduce the risk for diabetic complications, and prevent clinical inertia in patients with T2D.
- This randomized controlled open-label trial conducted at nine sites in South Korea included 105 drug-naive patients with T2D (mean age, 49.5 years; 32.4% women) who either received triple therapy (metformin, dapagliflozin, and saxagliptin) or stepwise add-on therapy (initiated with metformin, followed by glimepiride and sitagliptin for those with baseline hemoglobin A1c levels < 9.0% or with initial dual metformin and glimepiride in those with A1c levels ≥ 9.0% followed by sitagliptin).
- The primary outcome was the proportion of patients who achieved A1c levels < 6.5% without hypoglycemia, weight gain ≥ 5%, or discontinuation of drugs because of adverse events at week 104.
- The secondary outcomes were the proportion of patients whose A1c levels dropped to < 7.0% at weeks 56 and 104 and dropped to < 6.5% at week 56, all without hypoglycemia, weight gain, nor discontinuation due to adverse events.
TAKEAWAY:
- At week 104, a higher proportion of patients in the triple therapy group achieved the primary outcome than those in the stepwise add-on therapy group (39.0% vs 17.1%; P = .027).
- In both groups, a similar proportion of patients (46.3%) achieved A1c levels < 6.5% at week 104, but the proportion of patients without hypoglycemia, weight gain, or discontinuation because of adverse events was higher in the triple therapy group than those in the stepwise add-on therapy group (83.3% vs 38.0%; P < .001).
IN PRACTICE:
The authors wrote: “Although the glycemic efficacy of each drug in the TCT was modest, the combination of these drugs resulted in a 2-year durable glycemic efficacy, with greater than a 2.5% reduction in A1c levels from baseline. The overall results of this study suggest a novel strategy for initial combination therapy in newly diagnosed T2D patients.”
SOURCE:
The study was led by Nam Hoon Kim, MD, of the Department of Internal Medicine, Korea University College of Medicine, Seoul. It was published online in Diabetes, Obesity and Metabolism.
LIMITATIONS:
The study had a relatively small sample size as compared with previous clinical trials. More people in the standard therapy group had A1c levels ≥ 9.0%, which resulted in more than double the number of people receiving dual combination therapy over monotherapy in that group. The trial duration was insufficient to evaluate the cardiovascular outcomes.
DISCLOSURES:
The study was funded by AstraZeneca. Some authors reported financial ties with AstraZeneca and other pharmaceutical and medical device companies as members of advisory boards or recipients of grants, consulting fees, honoraria, or lecture fees.
A version of this article appeared on Medscape.com.
Time Warp: Fax Machines Still Common in Oncology Practice. Why?
One minute, he’s working on sequencing a tumor genome. The next, he’s sifting through pages of disorganized data from a device that has been around for decades: the fax machine.
“If two doctors’ offices aren’t on the same electronic medical record, one of the main ways to transfer records is still by fax,” said Dr. Lewis, director of gastrointestinal oncology at Intermountain Healthcare in Murray, Utah. “I can go from cutting-edge innovation to relying on, at best, 1980s information technology. It just boggles my mind.”
Dr. Lewis, who has posted about his frustration with fax machines, is far from alone. Oncologists are among the many specialists across the country at the mercy of telecopiers.
According to a 2021 report by the Office of the National Coordinator for Health Information Technology, fax and mail continue to be the most common methods for hospitals and health systems to exchange care record summaries. In 2019, nearly 8 in 10 hospitals used mail or fax to send and receive health information, the report found.
Fax machines are still commonplace across the healthcare spectrum, said Robert Havasy, MS, senior director for informatics strategy at the Healthcare Information and Management Systems Society (HIMSS). Inertia, cost, and more pressing priorities for hospitals and medical institutions contribute to the technology sticking around, he explained.
“Post-COVID, my guess is we’re still at over 50% of healthcare practices using fax for some reason, on a daily basis,” Mr. Havasy said in an interview. “A lot of hospitals just don’t have the time, the money, or the staff to fix that problem because there’s always something a little higher up the priority chain they need to focus on.”
If, for instance, “you’re going to do a process redesign to reduce hospital total acquired infections, your fax machine replacement might be 10th or 12th on the list. It just never gets up to 1 or 2 because it’s ‘not that much of a problem,’ ” he added.
Or is it?
Administrators may not view fax machines as a top concern, but clinicians who deal with the machines daily see it differently.
“What worries me is we’re taking records out of an electronic storehouse [and] converting them to a paper medium,” Dr. Lewis said. “And then we are scanning into another electronic storehouse. The more steps, the more can be lost.”
And when information is lost, patient care can be compromised.
Slower Workflows, Care Concerns
Although there are no published data on fax machine use in oncology specifically, this outdated technology does come into play in a variety of ways along the cancer care continuum.
Radiation oncologist David R. Penberthy, MD, said patients often seek his cancer center’s expertise for second opinions, and that requires collecting patient records from many different practices.
“Ideally, it would come electronically, but sometimes it does come by fax,” said Dr. Penberthy, program director of radiation oncology at the University of Virginia School of Medicine in Charlottesville. “The quality of the fax is not always the best. Sometimes it’s literally a fax of a fax. You’re reading something that’s very difficult to read.”
Orders for new tests are also typically sent and received via fax temporarily while IT teams work to integrate them into the electronic health record (EHR), Dr. Penberthy said.
Insurers and third-party laboratories often send test results back by fax as well.
“Even if I haven’t actually sent my patient out of our institution, this crucial result may only be entered back into the record as a scanned document from a fax, which is not great because it can get lost in the other results that are reported electronically,” Dr. Lewis said. The risk here is that an ordering physician won’t see these results, which can lead to delayed or overlooked care for patients, he explained.
“To me, it’s like a blind spot,” Dr. Lewis said. “Every time we use a fax, I see it actually as an opportunity for oversight and missed opportunity to collect data.”
Dr. Penberthy said faxing can slow things down at his practice, particularly if he faxes a document to another office but receives no confirmation and has to track down what happened.
As for cybersecurity, data that are in transit during faxing are generally considered secure and compliant with the Health Insurance Portability and Accountability Act (HIPAA), said Mr. Havasy of HIMSS. However, the Privacy Rule also requires that data remain secure while at rest, which isn’t always possible, he added.
“That’s where faxes fall down, because generally fax machines are in public, if you will, or open areas in a hospital,” he said. “They just sit on a desk. I don’t know that the next nurse who comes up and looks through that stack was the nurse who was treating the patient.”
Important decisions or results can also be missed when sent by fax, creating headaches for physicians and care problems for patients.
Dr. Lewis recently experienced an insurance-related fax mishap over Memorial Day weekend. He believed his patient had access to the antinausea medication he had prescribed. When Dr. Lewis happened to check the fax machine over the weekend, he found a coverage denial for the medication from the insurer but, at that point, had no recourse to appeal because it was a long holiday weekend.
“Had the denial been sent by an electronic means that was quicker and more readily available, it would have been possible to appeal before the holiday weekend,” he said.
Hematologist Aaron Goodman, MD, encountered a similar problem after an insurer denied coverage of an expensive cancer drug for a patient and faxed over its reason for the denial. Dr. Goodman was not directly notified that the information arrived and didn’t learn about the denial for a week, he said.
“There’s no ‘ding’ in my inbox if something is faxed over and scanned,” said Dr. Goodman, associate professor of medicine at UC San Diego Health. “Once I realized it was denied, I was able to rectify it, but it wasted a week of a patient not getting a drug that I felt would be beneficial for them.”
Broader Health Policy Impacts
The use of outdated technology, such as fax machines, also creates ripple effects that burden the health system, health policy experts say.
Duplicate testing and unnecessary care are top impacts, said Julia Adler-Milstein, PhD, professor of medicine and chief of the division of clinical informatics and digital transformation at the University of California, San Francisco.
Studies show that 20%-30% of the $65 billion spent annually on lab tests is used on unnecessary duplicate tests, and another estimated $30 billion is spent each year on unnecessary duplicate medical imaging. These duplicate tests may be mitigated if hospitals adopt certified EHR technology, research shows.
Still, without EHR interoperability between institutions, new providers may be unaware that tests or past labs for patients exist, leading to repeat tests, said Dr. Adler-Milstein, who researches health IT policy with a focus on EHRs. Patients can sometimes fill in the gaps, but not always.
“Fax machines only help close information gaps if the clinician is aware of where to seek out the information and there is someone at the other organization to locate and transmit the information in a timely manner,” Dr. Adler-Milstein said.
Old technology and poor interoperability also greatly affect data collection for disease surveillance and monitoring, said Janet Hamilton, MPH, executive director for the Council of State and Territorial Epidemiologists. This issue was keenly demonstrated during the pandemic, Ms. Hamilton said.
“It was tragic, quite honestly,” she said. “There was such an immense amount of data that needed to be moved quickly, and that’s when computers are at their best.”
But, she said, “we didn’t have the level of systems in place to do it well.”
Specifically, the lack of electronic case reporting in place during the pandemic — where diagnoses are documented in the record and then immediately sent to the public health system — led to reports that were delayed, not made, or had missing or incomplete information, such as patients’ race and ethnicity or other health conditions, Ms. Hamilton said.
Incomplete or missing data hampered the ability of public health officials and researchers to understand how the virus might affect different patients.
“If you had a chronic condition like cancer, you were less likely to have a positive outcome with COVID,” Ms. Hamilton said. “But because electronic case reporting was not in place, we didn’t get some of those additional pieces of information. We didn’t have people’s underlying oncology status to then say, ‘Here are individuals with these types of characteristics, and these are the things that happen if they also have a cancer.’”
Slow, but Steady, Improvements
Efforts at the state and federal levels have targeted improved health information exchange, but progress takes time, Dr. Adler-Milstein said.
Most states have some form of health information exchange, such as statewide exchanges, regional health information organizations, or clinical data registries. Maryland is often held up as a notable example for its health information exchange, Dr. Adler-Milstein noted.
According to Maryland law, all hospitals under the jurisdiction of the Maryland Health Care Commission are required to electronically connect to the state-designated health information exchange. In 2012, Maryland became the first state to connect all its 46 acute care hospitals in the sharing of real-time data.
The Health Information Technology for Economic and Clinical Health (HITECH) Act provided federal-enhanced Medicaid matching funds to states through 2021 to support efforts to advance electronic exchange. Nearly all states used these funds, and most have identified other sources to sustain the efforts, according to a recent US Government Accountability Office (GAO) report. However, GAO found that small and rural providers are less likely to have the financial and technological resources to participate in or maintain electronic exchange capabilities.
Nationally, several recent initiatives have targeted health data interoperability, including for cancer care. The Centers for Disease Control and Prevention’s Data Modernization Initiative is a multiyear, multi–billion-dollar effort to improve data sharing across the federal and state public health landscape.
Meanwhile, in March 2024, the Biden-Harris administration launched United States Core Data for Interoperability Plus Cancer. The program will define a recommended minimum set of cancer-related data to be included in a patient’s EHR to enhance data exchange for research and clinical care.
EHR vendors are also key to improving the landscape, said Dr. Adler-Milstein. Vendors such as Epic have developed strong sharing capabilities for transmitting health information from site to site, but of course, that only helps if providers have Epic, she said.
“That’s where these national frameworks should help, because we don’t want it to break down by what EHR vendor you have,” she said. “It’s a patchwork. You can go to some places and hear success stories because they have Epic or a state health information exchange, but it’s very heterogeneous. In some places, they have nothing and are using a fax machine.”
Mr. Havasy believes fax machines will ultimately go extinct, particularly as a younger, more digitally savvy generation enters the healthcare workforce. He also foresees that the growing use of artificial intelligence will help eradicate the outdated technology.
But, Ms. Hamilton noted, “unless we have consistent, ongoing, sustained funding, it is very hard to move off [an older] technology that can work. That’s one of the biggest barriers.”
“Public health is about protecting the lives of every single person everywhere,” Ms. Hamilton said, “but when we don’t have the data that comes into the system, we can’t achieve our mission.”
A version of this article appeared on Medscape.com.
One minute, he’s working on sequencing a tumor genome. The next, he’s sifting through pages of disorganized data from a device that has been around for decades: the fax machine.
“If two doctors’ offices aren’t on the same electronic medical record, one of the main ways to transfer records is still by fax,” said Dr. Lewis, director of gastrointestinal oncology at Intermountain Healthcare in Murray, Utah. “I can go from cutting-edge innovation to relying on, at best, 1980s information technology. It just boggles my mind.”
Dr. Lewis, who has posted about his frustration with fax machines, is far from alone. Oncologists are among the many specialists across the country at the mercy of telecopiers.
According to a 2021 report by the Office of the National Coordinator for Health Information Technology, fax and mail continue to be the most common methods for hospitals and health systems to exchange care record summaries. In 2019, nearly 8 in 10 hospitals used mail or fax to send and receive health information, the report found.
Fax machines are still commonplace across the healthcare spectrum, said Robert Havasy, MS, senior director for informatics strategy at the Healthcare Information and Management Systems Society (HIMSS). Inertia, cost, and more pressing priorities for hospitals and medical institutions contribute to the technology sticking around, he explained.
“Post-COVID, my guess is we’re still at over 50% of healthcare practices using fax for some reason, on a daily basis,” Mr. Havasy said in an interview. “A lot of hospitals just don’t have the time, the money, or the staff to fix that problem because there’s always something a little higher up the priority chain they need to focus on.”
If, for instance, “you’re going to do a process redesign to reduce hospital total acquired infections, your fax machine replacement might be 10th or 12th on the list. It just never gets up to 1 or 2 because it’s ‘not that much of a problem,’ ” he added.
Or is it?
Administrators may not view fax machines as a top concern, but clinicians who deal with the machines daily see it differently.
“What worries me is we’re taking records out of an electronic storehouse [and] converting them to a paper medium,” Dr. Lewis said. “And then we are scanning into another electronic storehouse. The more steps, the more can be lost.”
And when information is lost, patient care can be compromised.
Slower Workflows, Care Concerns
Although there are no published data on fax machine use in oncology specifically, this outdated technology does come into play in a variety of ways along the cancer care continuum.
Radiation oncologist David R. Penberthy, MD, said patients often seek his cancer center’s expertise for second opinions, and that requires collecting patient records from many different practices.
“Ideally, it would come electronically, but sometimes it does come by fax,” said Dr. Penberthy, program director of radiation oncology at the University of Virginia School of Medicine in Charlottesville. “The quality of the fax is not always the best. Sometimes it’s literally a fax of a fax. You’re reading something that’s very difficult to read.”
Orders for new tests are also typically sent and received via fax temporarily while IT teams work to integrate them into the electronic health record (EHR), Dr. Penberthy said.
Insurers and third-party laboratories often send test results back by fax as well.
“Even if I haven’t actually sent my patient out of our institution, this crucial result may only be entered back into the record as a scanned document from a fax, which is not great because it can get lost in the other results that are reported electronically,” Dr. Lewis said. The risk here is that an ordering physician won’t see these results, which can lead to delayed or overlooked care for patients, he explained.
“To me, it’s like a blind spot,” Dr. Lewis said. “Every time we use a fax, I see it actually as an opportunity for oversight and missed opportunity to collect data.”
Dr. Penberthy said faxing can slow things down at his practice, particularly if he faxes a document to another office but receives no confirmation and has to track down what happened.
As for cybersecurity, data that are in transit during faxing are generally considered secure and compliant with the Health Insurance Portability and Accountability Act (HIPAA), said Mr. Havasy of HIMSS. However, the Privacy Rule also requires that data remain secure while at rest, which isn’t always possible, he added.
“That’s where faxes fall down, because generally fax machines are in public, if you will, or open areas in a hospital,” he said. “They just sit on a desk. I don’t know that the next nurse who comes up and looks through that stack was the nurse who was treating the patient.”
Important decisions or results can also be missed when sent by fax, creating headaches for physicians and care problems for patients.
Dr. Lewis recently experienced an insurance-related fax mishap over Memorial Day weekend. He believed his patient had access to the antinausea medication he had prescribed. When Dr. Lewis happened to check the fax machine over the weekend, he found a coverage denial for the medication from the insurer but, at that point, had no recourse to appeal because it was a long holiday weekend.
“Had the denial been sent by an electronic means that was quicker and more readily available, it would have been possible to appeal before the holiday weekend,” he said.
Hematologist Aaron Goodman, MD, encountered a similar problem after an insurer denied coverage of an expensive cancer drug for a patient and faxed over its reason for the denial. Dr. Goodman was not directly notified that the information arrived and didn’t learn about the denial for a week, he said.
“There’s no ‘ding’ in my inbox if something is faxed over and scanned,” said Dr. Goodman, associate professor of medicine at UC San Diego Health. “Once I realized it was denied, I was able to rectify it, but it wasted a week of a patient not getting a drug that I felt would be beneficial for them.”
Broader Health Policy Impacts
The use of outdated technology, such as fax machines, also creates ripple effects that burden the health system, health policy experts say.
Duplicate testing and unnecessary care are top impacts, said Julia Adler-Milstein, PhD, professor of medicine and chief of the division of clinical informatics and digital transformation at the University of California, San Francisco.
Studies show that 20%-30% of the $65 billion spent annually on lab tests is used on unnecessary duplicate tests, and another estimated $30 billion is spent each year on unnecessary duplicate medical imaging. These duplicate tests may be mitigated if hospitals adopt certified EHR technology, research shows.
Still, without EHR interoperability between institutions, new providers may be unaware that tests or past labs for patients exist, leading to repeat tests, said Dr. Adler-Milstein, who researches health IT policy with a focus on EHRs. Patients can sometimes fill in the gaps, but not always.
“Fax machines only help close information gaps if the clinician is aware of where to seek out the information and there is someone at the other organization to locate and transmit the information in a timely manner,” Dr. Adler-Milstein said.
Old technology and poor interoperability also greatly affect data collection for disease surveillance and monitoring, said Janet Hamilton, MPH, executive director for the Council of State and Territorial Epidemiologists. This issue was keenly demonstrated during the pandemic, Ms. Hamilton said.
“It was tragic, quite honestly,” she said. “There was such an immense amount of data that needed to be moved quickly, and that’s when computers are at their best.”
But, she said, “we didn’t have the level of systems in place to do it well.”
Specifically, the lack of electronic case reporting in place during the pandemic — where diagnoses are documented in the record and then immediately sent to the public health system — led to reports that were delayed, not made, or had missing or incomplete information, such as patients’ race and ethnicity or other health conditions, Ms. Hamilton said.
Incomplete or missing data hampered the ability of public health officials and researchers to understand how the virus might affect different patients.
“If you had a chronic condition like cancer, you were less likely to have a positive outcome with COVID,” Ms. Hamilton said. “But because electronic case reporting was not in place, we didn’t get some of those additional pieces of information. We didn’t have people’s underlying oncology status to then say, ‘Here are individuals with these types of characteristics, and these are the things that happen if they also have a cancer.’”
Slow, but Steady, Improvements
Efforts at the state and federal levels have targeted improved health information exchange, but progress takes time, Dr. Adler-Milstein said.
Most states have some form of health information exchange, such as statewide exchanges, regional health information organizations, or clinical data registries. Maryland is often held up as a notable example for its health information exchange, Dr. Adler-Milstein noted.
According to Maryland law, all hospitals under the jurisdiction of the Maryland Health Care Commission are required to electronically connect to the state-designated health information exchange. In 2012, Maryland became the first state to connect all its 46 acute care hospitals in the sharing of real-time data.
The Health Information Technology for Economic and Clinical Health (HITECH) Act provided federal-enhanced Medicaid matching funds to states through 2021 to support efforts to advance electronic exchange. Nearly all states used these funds, and most have identified other sources to sustain the efforts, according to a recent US Government Accountability Office (GAO) report. However, GAO found that small and rural providers are less likely to have the financial and technological resources to participate in or maintain electronic exchange capabilities.
Nationally, several recent initiatives have targeted health data interoperability, including for cancer care. The Centers for Disease Control and Prevention’s Data Modernization Initiative is a multiyear, multi–billion-dollar effort to improve data sharing across the federal and state public health landscape.
Meanwhile, in March 2024, the Biden-Harris administration launched United States Core Data for Interoperability Plus Cancer. The program will define a recommended minimum set of cancer-related data to be included in a patient’s EHR to enhance data exchange for research and clinical care.
EHR vendors are also key to improving the landscape, said Dr. Adler-Milstein. Vendors such as Epic have developed strong sharing capabilities for transmitting health information from site to site, but of course, that only helps if providers have Epic, she said.
“That’s where these national frameworks should help, because we don’t want it to break down by what EHR vendor you have,” she said. “It’s a patchwork. You can go to some places and hear success stories because they have Epic or a state health information exchange, but it’s very heterogeneous. In some places, they have nothing and are using a fax machine.”
Mr. Havasy believes fax machines will ultimately go extinct, particularly as a younger, more digitally savvy generation enters the healthcare workforce. He also foresees that the growing use of artificial intelligence will help eradicate the outdated technology.
But, Ms. Hamilton noted, “unless we have consistent, ongoing, sustained funding, it is very hard to move off [an older] technology that can work. That’s one of the biggest barriers.”
“Public health is about protecting the lives of every single person everywhere,” Ms. Hamilton said, “but when we don’t have the data that comes into the system, we can’t achieve our mission.”
A version of this article appeared on Medscape.com.
One minute, he’s working on sequencing a tumor genome. The next, he’s sifting through pages of disorganized data from a device that has been around for decades: the fax machine.
“If two doctors’ offices aren’t on the same electronic medical record, one of the main ways to transfer records is still by fax,” said Dr. Lewis, director of gastrointestinal oncology at Intermountain Healthcare in Murray, Utah. “I can go from cutting-edge innovation to relying on, at best, 1980s information technology. It just boggles my mind.”
Dr. Lewis, who has posted about his frustration with fax machines, is far from alone. Oncologists are among the many specialists across the country at the mercy of telecopiers.
According to a 2021 report by the Office of the National Coordinator for Health Information Technology, fax and mail continue to be the most common methods for hospitals and health systems to exchange care record summaries. In 2019, nearly 8 in 10 hospitals used mail or fax to send and receive health information, the report found.
Fax machines are still commonplace across the healthcare spectrum, said Robert Havasy, MS, senior director for informatics strategy at the Healthcare Information and Management Systems Society (HIMSS). Inertia, cost, and more pressing priorities for hospitals and medical institutions contribute to the technology sticking around, he explained.
“Post-COVID, my guess is we’re still at over 50% of healthcare practices using fax for some reason, on a daily basis,” Mr. Havasy said in an interview. “A lot of hospitals just don’t have the time, the money, or the staff to fix that problem because there’s always something a little higher up the priority chain they need to focus on.”
If, for instance, “you’re going to do a process redesign to reduce hospital total acquired infections, your fax machine replacement might be 10th or 12th on the list. It just never gets up to 1 or 2 because it’s ‘not that much of a problem,’ ” he added.
Or is it?
Administrators may not view fax machines as a top concern, but clinicians who deal with the machines daily see it differently.
“What worries me is we’re taking records out of an electronic storehouse [and] converting them to a paper medium,” Dr. Lewis said. “And then we are scanning into another electronic storehouse. The more steps, the more can be lost.”
And when information is lost, patient care can be compromised.
Slower Workflows, Care Concerns
Although there are no published data on fax machine use in oncology specifically, this outdated technology does come into play in a variety of ways along the cancer care continuum.
Radiation oncologist David R. Penberthy, MD, said patients often seek his cancer center’s expertise for second opinions, and that requires collecting patient records from many different practices.
“Ideally, it would come electronically, but sometimes it does come by fax,” said Dr. Penberthy, program director of radiation oncology at the University of Virginia School of Medicine in Charlottesville. “The quality of the fax is not always the best. Sometimes it’s literally a fax of a fax. You’re reading something that’s very difficult to read.”
Orders for new tests are also typically sent and received via fax temporarily while IT teams work to integrate them into the electronic health record (EHR), Dr. Penberthy said.
Insurers and third-party laboratories often send test results back by fax as well.
“Even if I haven’t actually sent my patient out of our institution, this crucial result may only be entered back into the record as a scanned document from a fax, which is not great because it can get lost in the other results that are reported electronically,” Dr. Lewis said. The risk here is that an ordering physician won’t see these results, which can lead to delayed or overlooked care for patients, he explained.
“To me, it’s like a blind spot,” Dr. Lewis said. “Every time we use a fax, I see it actually as an opportunity for oversight and missed opportunity to collect data.”
Dr. Penberthy said faxing can slow things down at his practice, particularly if he faxes a document to another office but receives no confirmation and has to track down what happened.
As for cybersecurity, data that are in transit during faxing are generally considered secure and compliant with the Health Insurance Portability and Accountability Act (HIPAA), said Mr. Havasy of HIMSS. However, the Privacy Rule also requires that data remain secure while at rest, which isn’t always possible, he added.
“That’s where faxes fall down, because generally fax machines are in public, if you will, or open areas in a hospital,” he said. “They just sit on a desk. I don’t know that the next nurse who comes up and looks through that stack was the nurse who was treating the patient.”
Important decisions or results can also be missed when sent by fax, creating headaches for physicians and care problems for patients.
Dr. Lewis recently experienced an insurance-related fax mishap over Memorial Day weekend. He believed his patient had access to the antinausea medication he had prescribed. When Dr. Lewis happened to check the fax machine over the weekend, he found a coverage denial for the medication from the insurer but, at that point, had no recourse to appeal because it was a long holiday weekend.
“Had the denial been sent by an electronic means that was quicker and more readily available, it would have been possible to appeal before the holiday weekend,” he said.
Hematologist Aaron Goodman, MD, encountered a similar problem after an insurer denied coverage of an expensive cancer drug for a patient and faxed over its reason for the denial. Dr. Goodman was not directly notified that the information arrived and didn’t learn about the denial for a week, he said.
“There’s no ‘ding’ in my inbox if something is faxed over and scanned,” said Dr. Goodman, associate professor of medicine at UC San Diego Health. “Once I realized it was denied, I was able to rectify it, but it wasted a week of a patient not getting a drug that I felt would be beneficial for them.”
Broader Health Policy Impacts
The use of outdated technology, such as fax machines, also creates ripple effects that burden the health system, health policy experts say.
Duplicate testing and unnecessary care are top impacts, said Julia Adler-Milstein, PhD, professor of medicine and chief of the division of clinical informatics and digital transformation at the University of California, San Francisco.
Studies show that 20%-30% of the $65 billion spent annually on lab tests is used on unnecessary duplicate tests, and another estimated $30 billion is spent each year on unnecessary duplicate medical imaging. These duplicate tests may be mitigated if hospitals adopt certified EHR technology, research shows.
Still, without EHR interoperability between institutions, new providers may be unaware that tests or past labs for patients exist, leading to repeat tests, said Dr. Adler-Milstein, who researches health IT policy with a focus on EHRs. Patients can sometimes fill in the gaps, but not always.
“Fax machines only help close information gaps if the clinician is aware of where to seek out the information and there is someone at the other organization to locate and transmit the information in a timely manner,” Dr. Adler-Milstein said.
Old technology and poor interoperability also greatly affect data collection for disease surveillance and monitoring, said Janet Hamilton, MPH, executive director for the Council of State and Territorial Epidemiologists. This issue was keenly demonstrated during the pandemic, Ms. Hamilton said.
“It was tragic, quite honestly,” she said. “There was such an immense amount of data that needed to be moved quickly, and that’s when computers are at their best.”
But, she said, “we didn’t have the level of systems in place to do it well.”
Specifically, the lack of electronic case reporting in place during the pandemic — where diagnoses are documented in the record and then immediately sent to the public health system — led to reports that were delayed, not made, or had missing or incomplete information, such as patients’ race and ethnicity or other health conditions, Ms. Hamilton said.
Incomplete or missing data hampered the ability of public health officials and researchers to understand how the virus might affect different patients.
“If you had a chronic condition like cancer, you were less likely to have a positive outcome with COVID,” Ms. Hamilton said. “But because electronic case reporting was not in place, we didn’t get some of those additional pieces of information. We didn’t have people’s underlying oncology status to then say, ‘Here are individuals with these types of characteristics, and these are the things that happen if they also have a cancer.’”
Slow, but Steady, Improvements
Efforts at the state and federal levels have targeted improved health information exchange, but progress takes time, Dr. Adler-Milstein said.
Most states have some form of health information exchange, such as statewide exchanges, regional health information organizations, or clinical data registries. Maryland is often held up as a notable example for its health information exchange, Dr. Adler-Milstein noted.
According to Maryland law, all hospitals under the jurisdiction of the Maryland Health Care Commission are required to electronically connect to the state-designated health information exchange. In 2012, Maryland became the first state to connect all its 46 acute care hospitals in the sharing of real-time data.
The Health Information Technology for Economic and Clinical Health (HITECH) Act provided federal-enhanced Medicaid matching funds to states through 2021 to support efforts to advance electronic exchange. Nearly all states used these funds, and most have identified other sources to sustain the efforts, according to a recent US Government Accountability Office (GAO) report. However, GAO found that small and rural providers are less likely to have the financial and technological resources to participate in or maintain electronic exchange capabilities.
Nationally, several recent initiatives have targeted health data interoperability, including for cancer care. The Centers for Disease Control and Prevention’s Data Modernization Initiative is a multiyear, multi–billion-dollar effort to improve data sharing across the federal and state public health landscape.
Meanwhile, in March 2024, the Biden-Harris administration launched United States Core Data for Interoperability Plus Cancer. The program will define a recommended minimum set of cancer-related data to be included in a patient’s EHR to enhance data exchange for research and clinical care.
EHR vendors are also key to improving the landscape, said Dr. Adler-Milstein. Vendors such as Epic have developed strong sharing capabilities for transmitting health information from site to site, but of course, that only helps if providers have Epic, she said.
“That’s where these national frameworks should help, because we don’t want it to break down by what EHR vendor you have,” she said. “It’s a patchwork. You can go to some places and hear success stories because they have Epic or a state health information exchange, but it’s very heterogeneous. In some places, they have nothing and are using a fax machine.”
Mr. Havasy believes fax machines will ultimately go extinct, particularly as a younger, more digitally savvy generation enters the healthcare workforce. He also foresees that the growing use of artificial intelligence will help eradicate the outdated technology.
But, Ms. Hamilton noted, “unless we have consistent, ongoing, sustained funding, it is very hard to move off [an older] technology that can work. That’s one of the biggest barriers.”
“Public health is about protecting the lives of every single person everywhere,” Ms. Hamilton said, “but when we don’t have the data that comes into the system, we can’t achieve our mission.”
A version of this article appeared on Medscape.com.
Cancer Drug Shortages Continue in the US, Survey Finds
Nearly 90% of the 28 NCCN member centers who responded to the survey, conducted between May 28 and June 11, said they were experiencing a shortage of at least one drug.
“Many drugs that are currently in shortage form the backbones of effective multiagent regimens across both curative and palliative treatment settings,” NCCN’s CEO Crystal S. Denlinger, MD, said in an interview.
The good news is that carboplatin and cisplatin shortages have fallen dramatically since 2023. At the peak of the shortage in 2023, 93% of centers surveyed reported experiencing a shortage of carboplatin and 70% were experiencing a shortage of cisplatin, whereas in 2024, only 11% reported a carboplatin shortage and 7% reported a cisplatin shortage.
“Thankfully, the shortages for carboplatin and cisplatin are mostly resolved at this time,” Dr. Denlinger said.
However, all three NCCN surveys conducted in the past year, including the most recent one, have found shortages of various chemotherapies and supportive care medications, which suggests this is an ongoing issue affecting a significant spectrum of generic drugs.
“The acute crisis associated with the shortage of carboplatin and cisplatin was a singular event that brought the issue into the national spotlight,” but it’s “important to note that the current broad drug shortages found on this survey are not new,” said Dr. Denlinger.
In the latest survey, 89% of NCCN centers continue to report shortages of one or more drugs, and 75% said they are experiencing shortages of two or more drugs.
Overall, 57% of centers are short on vinblastine, 46% are short on etoposide, and 43% are short on topotecan. Other common chemotherapy and supportive care agents in short supply include dacarbazine (18% of centers) as well as 5-fluorouracil (5-FU) and methotrexate (14% of centers).
In 2023, however, shortages of methotrexate and 5-FU were worse, with 67% of centers reporting shortages of methotrexate and 26% of 5-FU.
In the current survey, 75% of NCCN centers also noted they were aware of drug shortages within community practices in their area, and more than one in four centers reported treatment delays requiring additional prior authorization.
Cancer drug shortages impact not only routine treatments but also clinical trials. The recent survey found that 43% of respondents said drug shortages disrupted clinical trials at their center. The biggest issues centers flagged included greater administrative burdens, lower patient enrollment, and fewer open trials.
How are centers dealing with ongoing supply issues?
Top mitigation strategies include reducing waste, limiting use of current stock, and adjusting the timing and dosage within evidence-based ranges.
“The current situation underscores the need for sustainable, long-term solutions that ensure a stable supply of high-quality cancer medications,” Alyssa Schatz, MSW, NCCN senior director of policy and advocacy, said in a news release.
Three-quarters (75%) of survey respondents said they would like to see economic incentives put in place to encourage the high-quality manufacturing of medications, especially generic versions that are often in short supply. Nearly two-thirds (64%) cited a need for a broader buffer stock payment, and the same percentage would like to see more information on user experiences with various generic suppliers to help hospitals contract with those engaging in high-quality practices.
The NCCN also continues to work with federal regulators, agencies, and lawmakers to implement long-term solutions to cancer drug shortages.
“The federal government has a key role to play in addressing this issue,” Ms. Schatz said. “Establishing economic incentives, such as tax breaks or manufacturing grants for generic drugmakers, will help support a robust and resilient supply chain — ultimately safeguarding care for people with cancer across the country.”
A version of this article appeared on Medscape.com.
Nearly 90% of the 28 NCCN member centers who responded to the survey, conducted between May 28 and June 11, said they were experiencing a shortage of at least one drug.
“Many drugs that are currently in shortage form the backbones of effective multiagent regimens across both curative and palliative treatment settings,” NCCN’s CEO Crystal S. Denlinger, MD, said in an interview.
The good news is that carboplatin and cisplatin shortages have fallen dramatically since 2023. At the peak of the shortage in 2023, 93% of centers surveyed reported experiencing a shortage of carboplatin and 70% were experiencing a shortage of cisplatin, whereas in 2024, only 11% reported a carboplatin shortage and 7% reported a cisplatin shortage.
“Thankfully, the shortages for carboplatin and cisplatin are mostly resolved at this time,” Dr. Denlinger said.
However, all three NCCN surveys conducted in the past year, including the most recent one, have found shortages of various chemotherapies and supportive care medications, which suggests this is an ongoing issue affecting a significant spectrum of generic drugs.
“The acute crisis associated with the shortage of carboplatin and cisplatin was a singular event that brought the issue into the national spotlight,” but it’s “important to note that the current broad drug shortages found on this survey are not new,” said Dr. Denlinger.
In the latest survey, 89% of NCCN centers continue to report shortages of one or more drugs, and 75% said they are experiencing shortages of two or more drugs.
Overall, 57% of centers are short on vinblastine, 46% are short on etoposide, and 43% are short on topotecan. Other common chemotherapy and supportive care agents in short supply include dacarbazine (18% of centers) as well as 5-fluorouracil (5-FU) and methotrexate (14% of centers).
In 2023, however, shortages of methotrexate and 5-FU were worse, with 67% of centers reporting shortages of methotrexate and 26% of 5-FU.
In the current survey, 75% of NCCN centers also noted they were aware of drug shortages within community practices in their area, and more than one in four centers reported treatment delays requiring additional prior authorization.
Cancer drug shortages impact not only routine treatments but also clinical trials. The recent survey found that 43% of respondents said drug shortages disrupted clinical trials at their center. The biggest issues centers flagged included greater administrative burdens, lower patient enrollment, and fewer open trials.
How are centers dealing with ongoing supply issues?
Top mitigation strategies include reducing waste, limiting use of current stock, and adjusting the timing and dosage within evidence-based ranges.
“The current situation underscores the need for sustainable, long-term solutions that ensure a stable supply of high-quality cancer medications,” Alyssa Schatz, MSW, NCCN senior director of policy and advocacy, said in a news release.
Three-quarters (75%) of survey respondents said they would like to see economic incentives put in place to encourage the high-quality manufacturing of medications, especially generic versions that are often in short supply. Nearly two-thirds (64%) cited a need for a broader buffer stock payment, and the same percentage would like to see more information on user experiences with various generic suppliers to help hospitals contract with those engaging in high-quality practices.
The NCCN also continues to work with federal regulators, agencies, and lawmakers to implement long-term solutions to cancer drug shortages.
“The federal government has a key role to play in addressing this issue,” Ms. Schatz said. “Establishing economic incentives, such as tax breaks or manufacturing grants for generic drugmakers, will help support a robust and resilient supply chain — ultimately safeguarding care for people with cancer across the country.”
A version of this article appeared on Medscape.com.
Nearly 90% of the 28 NCCN member centers who responded to the survey, conducted between May 28 and June 11, said they were experiencing a shortage of at least one drug.
“Many drugs that are currently in shortage form the backbones of effective multiagent regimens across both curative and palliative treatment settings,” NCCN’s CEO Crystal S. Denlinger, MD, said in an interview.
The good news is that carboplatin and cisplatin shortages have fallen dramatically since 2023. At the peak of the shortage in 2023, 93% of centers surveyed reported experiencing a shortage of carboplatin and 70% were experiencing a shortage of cisplatin, whereas in 2024, only 11% reported a carboplatin shortage and 7% reported a cisplatin shortage.
“Thankfully, the shortages for carboplatin and cisplatin are mostly resolved at this time,” Dr. Denlinger said.
However, all three NCCN surveys conducted in the past year, including the most recent one, have found shortages of various chemotherapies and supportive care medications, which suggests this is an ongoing issue affecting a significant spectrum of generic drugs.
“The acute crisis associated with the shortage of carboplatin and cisplatin was a singular event that brought the issue into the national spotlight,” but it’s “important to note that the current broad drug shortages found on this survey are not new,” said Dr. Denlinger.
In the latest survey, 89% of NCCN centers continue to report shortages of one or more drugs, and 75% said they are experiencing shortages of two or more drugs.
Overall, 57% of centers are short on vinblastine, 46% are short on etoposide, and 43% are short on topotecan. Other common chemotherapy and supportive care agents in short supply include dacarbazine (18% of centers) as well as 5-fluorouracil (5-FU) and methotrexate (14% of centers).
In 2023, however, shortages of methotrexate and 5-FU were worse, with 67% of centers reporting shortages of methotrexate and 26% of 5-FU.
In the current survey, 75% of NCCN centers also noted they were aware of drug shortages within community practices in their area, and more than one in four centers reported treatment delays requiring additional prior authorization.
Cancer drug shortages impact not only routine treatments but also clinical trials. The recent survey found that 43% of respondents said drug shortages disrupted clinical trials at their center. The biggest issues centers flagged included greater administrative burdens, lower patient enrollment, and fewer open trials.
How are centers dealing with ongoing supply issues?
Top mitigation strategies include reducing waste, limiting use of current stock, and adjusting the timing and dosage within evidence-based ranges.
“The current situation underscores the need for sustainable, long-term solutions that ensure a stable supply of high-quality cancer medications,” Alyssa Schatz, MSW, NCCN senior director of policy and advocacy, said in a news release.
Three-quarters (75%) of survey respondents said they would like to see economic incentives put in place to encourage the high-quality manufacturing of medications, especially generic versions that are often in short supply. Nearly two-thirds (64%) cited a need for a broader buffer stock payment, and the same percentage would like to see more information on user experiences with various generic suppliers to help hospitals contract with those engaging in high-quality practices.
The NCCN also continues to work with federal regulators, agencies, and lawmakers to implement long-term solutions to cancer drug shortages.
“The federal government has a key role to play in addressing this issue,” Ms. Schatz said. “Establishing economic incentives, such as tax breaks or manufacturing grants for generic drugmakers, will help support a robust and resilient supply chain — ultimately safeguarding care for people with cancer across the country.”
A version of this article appeared on Medscape.com.
Exercise Plus GLP-1 RAs Upped Weight Loss, Bone Retention
TOPLINE:
People with obesity who exercise while taking glucagon-like peptide 1 receptor agonists (GLP-1 RAs; liraglutide) showed increased weight loss and preserved bone health, according to a study published in JAMA Network Open.
METHODOLOGY:
- Patients were placed on an initial diet that consisted of no more than 800 calories per day for 8 weeks. Those who lost at least 5% of their starting weight were then placed into a 1-year program.
- Participants included 195 adults aged between 18 and 65 years with obesity and no diabetes, 64% of whom were women.
- They were split into four groups of interventions: Exercise only (48 patients), liraglutide only (49 patients), a combination of both (49 participants), and placebo (49 participants), for a 1-year period.
- Patients received liraglutide or volume-matched placebo as daily injections starting at 0.6 mg/d with a weekly increase until 3 mg/d was reached; exercise entailed 30-minute sessions for 4 days a week.
- Researchers studied bone health at each patient’s hip, spine, and forearm after they lost weight, by measuring bone mineral density (BMD).
TAKEAWAY:
- The overall average change in weight loss over the course of 52 weeks was 7.03 kg in the placebo group, 11.19 kg in the exercise group, 13.74 kg in the liraglutide group, and 16.88 kg in the combination group.
- BMD did not change in the combination group in comparison to the placebo group at the hip (mean change, −0.006 g/cm2; 95% CI, −0.017 to 0.004 g/cm2; P = .24) or spine (−0.010 g/cm2; 95% CI, −0.025 to 0.005 g/cm2; P = .20).
- BMD of the spine in the liraglutide group decreased in comparison to the exercise group (mean change, −0.016 g/cm2; 95% CI, −0.032 to −0.001 g/cm2; P = .04) and the placebo group, in addition to decreases in the hip.
IN PRACTICE:
“Our results show that the combination of exercise and GLP-1 RA was the most effective weight loss strategy while preserving bone health,” study authors wrote.
SOURCE:
The study was led by Simon Birk Kjær Jensen, PhD, of the Department of Biomedical Sciences and Faculty of Health and Medical Sciences at the University of Copenhagen in Denmark, and published on June 25 in JAMA Network Open.
LIMITATIONS:
The study only included adults aged between 18 and 65 years without other chronic diseases and may not apply to patients who are older or have diabetes. The study sample was diverse but was conducted in Denmark, with a population of generally similar ancestry.
DISCLOSURES:
One study author reported serving on advisory boards for AstraZeneca, Boehringer Ingelheim, Bayer, and Amgen, among others. Other authors reported various financial interests, including grants, personal fees, and salaries, from Amgen, Novo Nordisk, and Abbott Lab, among others.
A version of this article first appeared on Medscape.com.
TOPLINE:
People with obesity who exercise while taking glucagon-like peptide 1 receptor agonists (GLP-1 RAs; liraglutide) showed increased weight loss and preserved bone health, according to a study published in JAMA Network Open.
METHODOLOGY:
- Patients were placed on an initial diet that consisted of no more than 800 calories per day for 8 weeks. Those who lost at least 5% of their starting weight were then placed into a 1-year program.
- Participants included 195 adults aged between 18 and 65 years with obesity and no diabetes, 64% of whom were women.
- They were split into four groups of interventions: Exercise only (48 patients), liraglutide only (49 patients), a combination of both (49 participants), and placebo (49 participants), for a 1-year period.
- Patients received liraglutide or volume-matched placebo as daily injections starting at 0.6 mg/d with a weekly increase until 3 mg/d was reached; exercise entailed 30-minute sessions for 4 days a week.
- Researchers studied bone health at each patient’s hip, spine, and forearm after they lost weight, by measuring bone mineral density (BMD).
TAKEAWAY:
- The overall average change in weight loss over the course of 52 weeks was 7.03 kg in the placebo group, 11.19 kg in the exercise group, 13.74 kg in the liraglutide group, and 16.88 kg in the combination group.
- BMD did not change in the combination group in comparison to the placebo group at the hip (mean change, −0.006 g/cm2; 95% CI, −0.017 to 0.004 g/cm2; P = .24) or spine (−0.010 g/cm2; 95% CI, −0.025 to 0.005 g/cm2; P = .20).
- BMD of the spine in the liraglutide group decreased in comparison to the exercise group (mean change, −0.016 g/cm2; 95% CI, −0.032 to −0.001 g/cm2; P = .04) and the placebo group, in addition to decreases in the hip.
IN PRACTICE:
“Our results show that the combination of exercise and GLP-1 RA was the most effective weight loss strategy while preserving bone health,” study authors wrote.
SOURCE:
The study was led by Simon Birk Kjær Jensen, PhD, of the Department of Biomedical Sciences and Faculty of Health and Medical Sciences at the University of Copenhagen in Denmark, and published on June 25 in JAMA Network Open.
LIMITATIONS:
The study only included adults aged between 18 and 65 years without other chronic diseases and may not apply to patients who are older or have diabetes. The study sample was diverse but was conducted in Denmark, with a population of generally similar ancestry.
DISCLOSURES:
One study author reported serving on advisory boards for AstraZeneca, Boehringer Ingelheim, Bayer, and Amgen, among others. Other authors reported various financial interests, including grants, personal fees, and salaries, from Amgen, Novo Nordisk, and Abbott Lab, among others.
A version of this article first appeared on Medscape.com.
TOPLINE:
People with obesity who exercise while taking glucagon-like peptide 1 receptor agonists (GLP-1 RAs; liraglutide) showed increased weight loss and preserved bone health, according to a study published in JAMA Network Open.
METHODOLOGY:
- Patients were placed on an initial diet that consisted of no more than 800 calories per day for 8 weeks. Those who lost at least 5% of their starting weight were then placed into a 1-year program.
- Participants included 195 adults aged between 18 and 65 years with obesity and no diabetes, 64% of whom were women.
- They were split into four groups of interventions: Exercise only (48 patients), liraglutide only (49 patients), a combination of both (49 participants), and placebo (49 participants), for a 1-year period.
- Patients received liraglutide or volume-matched placebo as daily injections starting at 0.6 mg/d with a weekly increase until 3 mg/d was reached; exercise entailed 30-minute sessions for 4 days a week.
- Researchers studied bone health at each patient’s hip, spine, and forearm after they lost weight, by measuring bone mineral density (BMD).
TAKEAWAY:
- The overall average change in weight loss over the course of 52 weeks was 7.03 kg in the placebo group, 11.19 kg in the exercise group, 13.74 kg in the liraglutide group, and 16.88 kg in the combination group.
- BMD did not change in the combination group in comparison to the placebo group at the hip (mean change, −0.006 g/cm2; 95% CI, −0.017 to 0.004 g/cm2; P = .24) or spine (−0.010 g/cm2; 95% CI, −0.025 to 0.005 g/cm2; P = .20).
- BMD of the spine in the liraglutide group decreased in comparison to the exercise group (mean change, −0.016 g/cm2; 95% CI, −0.032 to −0.001 g/cm2; P = .04) and the placebo group, in addition to decreases in the hip.
IN PRACTICE:
“Our results show that the combination of exercise and GLP-1 RA was the most effective weight loss strategy while preserving bone health,” study authors wrote.
SOURCE:
The study was led by Simon Birk Kjær Jensen, PhD, of the Department of Biomedical Sciences and Faculty of Health and Medical Sciences at the University of Copenhagen in Denmark, and published on June 25 in JAMA Network Open.
LIMITATIONS:
The study only included adults aged between 18 and 65 years without other chronic diseases and may not apply to patients who are older or have diabetes. The study sample was diverse but was conducted in Denmark, with a population of generally similar ancestry.
DISCLOSURES:
One study author reported serving on advisory boards for AstraZeneca, Boehringer Ingelheim, Bayer, and Amgen, among others. Other authors reported various financial interests, including grants, personal fees, and salaries, from Amgen, Novo Nordisk, and Abbott Lab, among others.
A version of this article first appeared on Medscape.com.
Does Semaglutide Reduce Inflammation?
LYON, FRANCE — The anti-obesity drug semaglutide is associated with significant reductions in the inflammatory marker high-sensitivity C-reactive protein (CRP), even in patients who do not lose substantial amounts of weight with the drug, according to data from the SELECT clinical trial.
The research, presented at the European Atherosclerosis Society 2024, involved over 17,600 patients with overweight or obesity and had established cardiovascular disease but not diabetes.
“Weight loss was associated with greater high-sensitivity CRP reduction in both treatment groups,” said study presenter Jorge Plutzky, MD, director of Preventive Cardiology at Brigham and Women’s Hospital, Boston, but “with increased high-sensitivity CRP reductions in those receiving semaglutide.”
The drug also “significantly reduced high-sensitivity CRP early,” he said, “prior to major weight loss and in those who did not lose significant amounts of weight.” The reductions reached approximately 12% at 4 weeks and around 20% at 8 weeks, when the weight loss “was still quite modest,” at 2% and 3% of body weight, respectively. Even among patients who achieved weight loss of less than 2% body weight, semaglutide was associated with a reduction in high-sensitivity CRP levels.
In the SELECT trial, semaglutide also resulted in a consistent reduction of around 20% vs placebo in major adverse cardiovascular events such as cardiovascular mortality, nonfatal myocardial infarction, or nonfatal stroke.
But Naveed Sattar, MD, PhD, professor of cardiometabolic medicine at the University of Glasgow, Scotland, said in an interview that body weight “is probably the major driver” of CRP levels in the population, accounting for between 20% and 30% of the variation.
Dr. Sattar, who was not involved in the study, said that because drugs like semaglutide lower weight but also have anti-inflammatory effects, the question becomes: “Could the anti-inflammatory effects be part of the mechanisms by which these drugs affect the risk of major adverse cardiovascular events?”
Reducing Cardiovascular Events
The current analysis, however, cannot answer the question, he said. “All it tells us is about associations.”
“What we do know is semaglutide, predominantly by lowering weight, is lowering CRP levels and equally, we know that when you lose weight, you improve blood pressure, you improve lipids, and you reduce the risk of diabetes,” he said.
Dr. Sattar also took issue with the researchers’ conclusion that the high-sensitivity CRP reductions seen in SELECT occurred prior to major weight loss because the “pattern of CRP reduction and weight reduction is almost identical.”
Dr. Sattar also pointed out in a recent editorial that the drug appears to have a direct effect on blood vessels and the heart, which may lead to improvements in systemic inflammation. Consequently, he said, any assertion that semaglutide is genuinely anti-inflammatory is, at this stage, “speculation.”
Dr. Plutzky said that “systemic, chronic inflammation is implicated as a potential mechanism and therapeutic target in atherosclerosis and major adverse cardiovascular events, as well as obesity,” and high-sensitivity CRP levels are an “established biomarker of inflammation and have been shown to predict cardiovascular risk.”
However, the relationship between high-sensitivity CRP, responses to glucagon-like peptide 1 receptor agonists like semaglutide, and cardiovascular outcomes in obesity “remains incompletely understood,” said Dr. Plutzky.
A version of this article appeared on Medscape.com.
LYON, FRANCE — The anti-obesity drug semaglutide is associated with significant reductions in the inflammatory marker high-sensitivity C-reactive protein (CRP), even in patients who do not lose substantial amounts of weight with the drug, according to data from the SELECT clinical trial.
The research, presented at the European Atherosclerosis Society 2024, involved over 17,600 patients with overweight or obesity and had established cardiovascular disease but not diabetes.
“Weight loss was associated with greater high-sensitivity CRP reduction in both treatment groups,” said study presenter Jorge Plutzky, MD, director of Preventive Cardiology at Brigham and Women’s Hospital, Boston, but “with increased high-sensitivity CRP reductions in those receiving semaglutide.”
The drug also “significantly reduced high-sensitivity CRP early,” he said, “prior to major weight loss and in those who did not lose significant amounts of weight.” The reductions reached approximately 12% at 4 weeks and around 20% at 8 weeks, when the weight loss “was still quite modest,” at 2% and 3% of body weight, respectively. Even among patients who achieved weight loss of less than 2% body weight, semaglutide was associated with a reduction in high-sensitivity CRP levels.
In the SELECT trial, semaglutide also resulted in a consistent reduction of around 20% vs placebo in major adverse cardiovascular events such as cardiovascular mortality, nonfatal myocardial infarction, or nonfatal stroke.
But Naveed Sattar, MD, PhD, professor of cardiometabolic medicine at the University of Glasgow, Scotland, said in an interview that body weight “is probably the major driver” of CRP levels in the population, accounting for between 20% and 30% of the variation.
Dr. Sattar, who was not involved in the study, said that because drugs like semaglutide lower weight but also have anti-inflammatory effects, the question becomes: “Could the anti-inflammatory effects be part of the mechanisms by which these drugs affect the risk of major adverse cardiovascular events?”
Reducing Cardiovascular Events
The current analysis, however, cannot answer the question, he said. “All it tells us is about associations.”
“What we do know is semaglutide, predominantly by lowering weight, is lowering CRP levels and equally, we know that when you lose weight, you improve blood pressure, you improve lipids, and you reduce the risk of diabetes,” he said.
Dr. Sattar also took issue with the researchers’ conclusion that the high-sensitivity CRP reductions seen in SELECT occurred prior to major weight loss because the “pattern of CRP reduction and weight reduction is almost identical.”
Dr. Sattar also pointed out in a recent editorial that the drug appears to have a direct effect on blood vessels and the heart, which may lead to improvements in systemic inflammation. Consequently, he said, any assertion that semaglutide is genuinely anti-inflammatory is, at this stage, “speculation.”
Dr. Plutzky said that “systemic, chronic inflammation is implicated as a potential mechanism and therapeutic target in atherosclerosis and major adverse cardiovascular events, as well as obesity,” and high-sensitivity CRP levels are an “established biomarker of inflammation and have been shown to predict cardiovascular risk.”
However, the relationship between high-sensitivity CRP, responses to glucagon-like peptide 1 receptor agonists like semaglutide, and cardiovascular outcomes in obesity “remains incompletely understood,” said Dr. Plutzky.
A version of this article appeared on Medscape.com.
LYON, FRANCE — The anti-obesity drug semaglutide is associated with significant reductions in the inflammatory marker high-sensitivity C-reactive protein (CRP), even in patients who do not lose substantial amounts of weight with the drug, according to data from the SELECT clinical trial.
The research, presented at the European Atherosclerosis Society 2024, involved over 17,600 patients with overweight or obesity and had established cardiovascular disease but not diabetes.
“Weight loss was associated with greater high-sensitivity CRP reduction in both treatment groups,” said study presenter Jorge Plutzky, MD, director of Preventive Cardiology at Brigham and Women’s Hospital, Boston, but “with increased high-sensitivity CRP reductions in those receiving semaglutide.”
The drug also “significantly reduced high-sensitivity CRP early,” he said, “prior to major weight loss and in those who did not lose significant amounts of weight.” The reductions reached approximately 12% at 4 weeks and around 20% at 8 weeks, when the weight loss “was still quite modest,” at 2% and 3% of body weight, respectively. Even among patients who achieved weight loss of less than 2% body weight, semaglutide was associated with a reduction in high-sensitivity CRP levels.
In the SELECT trial, semaglutide also resulted in a consistent reduction of around 20% vs placebo in major adverse cardiovascular events such as cardiovascular mortality, nonfatal myocardial infarction, or nonfatal stroke.
But Naveed Sattar, MD, PhD, professor of cardiometabolic medicine at the University of Glasgow, Scotland, said in an interview that body weight “is probably the major driver” of CRP levels in the population, accounting for between 20% and 30% of the variation.
Dr. Sattar, who was not involved in the study, said that because drugs like semaglutide lower weight but also have anti-inflammatory effects, the question becomes: “Could the anti-inflammatory effects be part of the mechanisms by which these drugs affect the risk of major adverse cardiovascular events?”
Reducing Cardiovascular Events
The current analysis, however, cannot answer the question, he said. “All it tells us is about associations.”
“What we do know is semaglutide, predominantly by lowering weight, is lowering CRP levels and equally, we know that when you lose weight, you improve blood pressure, you improve lipids, and you reduce the risk of diabetes,” he said.
Dr. Sattar also took issue with the researchers’ conclusion that the high-sensitivity CRP reductions seen in SELECT occurred prior to major weight loss because the “pattern of CRP reduction and weight reduction is almost identical.”
Dr. Sattar also pointed out in a recent editorial that the drug appears to have a direct effect on blood vessels and the heart, which may lead to improvements in systemic inflammation. Consequently, he said, any assertion that semaglutide is genuinely anti-inflammatory is, at this stage, “speculation.”
Dr. Plutzky said that “systemic, chronic inflammation is implicated as a potential mechanism and therapeutic target in atherosclerosis and major adverse cardiovascular events, as well as obesity,” and high-sensitivity CRP levels are an “established biomarker of inflammation and have been shown to predict cardiovascular risk.”
However, the relationship between high-sensitivity CRP, responses to glucagon-like peptide 1 receptor agonists like semaglutide, and cardiovascular outcomes in obesity “remains incompletely understood,” said Dr. Plutzky.
A version of this article appeared on Medscape.com.
