User login
Official news magazine of the Society of Hospital Medicine
Copyright by Society of Hospital Medicine or related companies. All rights reserved. ISSN 1553-085X
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'pane-pub-article-hospitalist')]


Physician income drops, burnout spikes globally in pandemic
according to the results of a Medscape survey.
More than 7,500 physicians – nearly 5,000 in the United States, and others in Brazil, France, Germany, Mexico, Portugal, Spain, and the United Kingdom – responded to questions about their struggles to save patients and how the pandemic has changed their income and their lives at home and at work.
The pain was evident in this response from an emergency medicine physician in Spain: “It has been the worst time in my life ever, in both my personal and professional life.”
Conversely, some reported positive effects.
An internist in Brazil wrote: “I feel more proud of my career than ever before.”
One quarter of U.S. physicians considering earlier retirement
Physicians in the United States were asked what career changes, if any, they were considering in light of their experience with COVID-19. Although a little more than half (51%) said they were not planning any changes, 25% answered, “retiring earlier than previously planned,” and 12% answered, “a career change away from medicine.”
The number of physicians reporting an income drop was highest in Brazil (63% reported a drop), followed by the United States (62%), Mexico (56%), Portugal (49%), Germany (42%), France (41%), and Spain (31%). The question was not asked in the United Kingdom survey.
In the United States, the size of the drop has been substantial: 9% lost 76%-100% of their income; 14% lost 51%-75%; 28% lost 26%-50%; 33% lost 11%-25%; and 15% lost 1%-10%.
The U.S. specialists with the largest drop in income were ophthalmologists, who lost 51%, followed by allergists (46%), plastic surgeons (46%), and otolaryngologists (45%).
“I’m looking for a new profession due to economic impact,” an otolaryngologist in the United States said. “We are at risk while essentially using our private savings to keep our practice solvent.”
More than half of U.S. physicians (54%) have personally treated patients with COVID-19. Percentages were higher in France, Spain, and the United Kingdom (percentages ranged from 60%-68%).
The United States led all eight countries in treating patients with COVID-19 via telemedicine, at 26%. Germany had the lowest telemedicine percentage, at 10%.
Burnout intensifies
About two thirds of US physicians (64%) said that burnout had intensified during the crisis (70% of female physicians and 61% of male physicians said it had).
Many factors are feeding the burnout.
A critical care physician in the United States responded, “It is terrible to see people arriving at their rooms and assuming they were going to die soon; to see people saying goodbye to their families before dying or before being intubated.”
In all eight countries, a substantial percentage of physicians reported they “sometimes, often or always” treated patients with COVID-19 without the proper personal protective equipment. Spain had by far the largest percentage who answered that way (67%), followed by France (45%), Mexico (40%), the United Kingdom (34%), Brazil and Germany (28% each); and the United States and Portugal (23% each).
A U.S. rheumatologist wrote: “The fact that we were sent to take care of infectious patients without proper protection equipment made me feel we were betrayed in this fight.”
Sense of duty to volunteer to treat COVID-19 patients varied substantially among countries, from 69% who felt that way in Spain to 40% in Brazil. Half (50%) in the United States felt that way.
“Altruism must take second place where a real and present threat exists to my own personal existence,” one U.S. internist wrote.
Numbers personally infected
One fifth of physicians in Spain and the United Kingdom had personally been infected with the virus. Brazil, France, and Mexico had the next highest numbers, with 13%-15% of physicians infected; 5%-6% in the United States, Germany, and Portugal said they had been infected.
The percentage of physicians who reported that immediate family members had been infected ranged from 25% in Spain to 6% in Portugal. Among US physicians, 9% reported that family members had been diagnosed with COVID-19.
In the United States, 44% of respondents who had family living with them at home during the pandemic reported that relationships at home were more stressed because of stay-at-home guidelines and social distancing. Almost half (47%) said there had been no change, and 9% said relationships were less stressed.
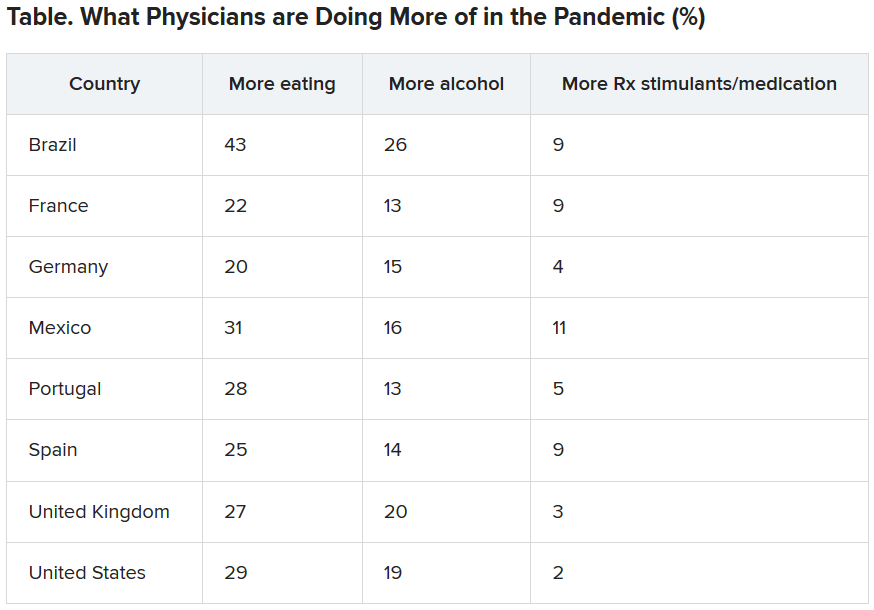
Eating is coping mechanism of choice
Physicians were asked what they were doing more of during the pandemic, and food seemed to be the top source of comfort in all eight countries.
Loneliness reports differ across globe
Portugal had the highest percentage (51%) of physicians reporting increased loneliness. Next were Brazil (48%), the United States (46%), the United Kingdom (42%), France (41%), Spain and Mexico (40% each), and Germany (32%).
All eight countries lacked workplace activities to help physicians with grief. More than half (55%) of U.K. physicians reported having such activities available at their workplace, whereas only 25% of physicians in Germany did; 12%-24% of respondents across the countries were unsure about the offerings.
This article first appeared on Medscape.com.
according to the results of a Medscape survey.
More than 7,500 physicians – nearly 5,000 in the United States, and others in Brazil, France, Germany, Mexico, Portugal, Spain, and the United Kingdom – responded to questions about their struggles to save patients and how the pandemic has changed their income and their lives at home and at work.
The pain was evident in this response from an emergency medicine physician in Spain: “It has been the worst time in my life ever, in both my personal and professional life.”
Conversely, some reported positive effects.
An internist in Brazil wrote: “I feel more proud of my career than ever before.”
One quarter of U.S. physicians considering earlier retirement
Physicians in the United States were asked what career changes, if any, they were considering in light of their experience with COVID-19. Although a little more than half (51%) said they were not planning any changes, 25% answered, “retiring earlier than previously planned,” and 12% answered, “a career change away from medicine.”
The number of physicians reporting an income drop was highest in Brazil (63% reported a drop), followed by the United States (62%), Mexico (56%), Portugal (49%), Germany (42%), France (41%), and Spain (31%). The question was not asked in the United Kingdom survey.
In the United States, the size of the drop has been substantial: 9% lost 76%-100% of their income; 14% lost 51%-75%; 28% lost 26%-50%; 33% lost 11%-25%; and 15% lost 1%-10%.
The U.S. specialists with the largest drop in income were ophthalmologists, who lost 51%, followed by allergists (46%), plastic surgeons (46%), and otolaryngologists (45%).
“I’m looking for a new profession due to economic impact,” an otolaryngologist in the United States said. “We are at risk while essentially using our private savings to keep our practice solvent.”
More than half of U.S. physicians (54%) have personally treated patients with COVID-19. Percentages were higher in France, Spain, and the United Kingdom (percentages ranged from 60%-68%).
The United States led all eight countries in treating patients with COVID-19 via telemedicine, at 26%. Germany had the lowest telemedicine percentage, at 10%.
Burnout intensifies
About two thirds of US physicians (64%) said that burnout had intensified during the crisis (70% of female physicians and 61% of male physicians said it had).
Many factors are feeding the burnout.
A critical care physician in the United States responded, “It is terrible to see people arriving at their rooms and assuming they were going to die soon; to see people saying goodbye to their families before dying or before being intubated.”
In all eight countries, a substantial percentage of physicians reported they “sometimes, often or always” treated patients with COVID-19 without the proper personal protective equipment. Spain had by far the largest percentage who answered that way (67%), followed by France (45%), Mexico (40%), the United Kingdom (34%), Brazil and Germany (28% each); and the United States and Portugal (23% each).
A U.S. rheumatologist wrote: “The fact that we were sent to take care of infectious patients without proper protection equipment made me feel we were betrayed in this fight.”
Sense of duty to volunteer to treat COVID-19 patients varied substantially among countries, from 69% who felt that way in Spain to 40% in Brazil. Half (50%) in the United States felt that way.
“Altruism must take second place where a real and present threat exists to my own personal existence,” one U.S. internist wrote.
Numbers personally infected
One fifth of physicians in Spain and the United Kingdom had personally been infected with the virus. Brazil, France, and Mexico had the next highest numbers, with 13%-15% of physicians infected; 5%-6% in the United States, Germany, and Portugal said they had been infected.
The percentage of physicians who reported that immediate family members had been infected ranged from 25% in Spain to 6% in Portugal. Among US physicians, 9% reported that family members had been diagnosed with COVID-19.
In the United States, 44% of respondents who had family living with them at home during the pandemic reported that relationships at home were more stressed because of stay-at-home guidelines and social distancing. Almost half (47%) said there had been no change, and 9% said relationships were less stressed.
Eating is coping mechanism of choice
Physicians were asked what they were doing more of during the pandemic, and food seemed to be the top source of comfort in all eight countries.
Loneliness reports differ across globe
Portugal had the highest percentage (51%) of physicians reporting increased loneliness. Next were Brazil (48%), the United States (46%), the United Kingdom (42%), France (41%), Spain and Mexico (40% each), and Germany (32%).
All eight countries lacked workplace activities to help physicians with grief. More than half (55%) of U.K. physicians reported having such activities available at their workplace, whereas only 25% of physicians in Germany did; 12%-24% of respondents across the countries were unsure about the offerings.
This article first appeared on Medscape.com.
according to the results of a Medscape survey.
More than 7,500 physicians – nearly 5,000 in the United States, and others in Brazil, France, Germany, Mexico, Portugal, Spain, and the United Kingdom – responded to questions about their struggles to save patients and how the pandemic has changed their income and their lives at home and at work.
The pain was evident in this response from an emergency medicine physician in Spain: “It has been the worst time in my life ever, in both my personal and professional life.”
Conversely, some reported positive effects.
An internist in Brazil wrote: “I feel more proud of my career than ever before.”
One quarter of U.S. physicians considering earlier retirement
Physicians in the United States were asked what career changes, if any, they were considering in light of their experience with COVID-19. Although a little more than half (51%) said they were not planning any changes, 25% answered, “retiring earlier than previously planned,” and 12% answered, “a career change away from medicine.”
The number of physicians reporting an income drop was highest in Brazil (63% reported a drop), followed by the United States (62%), Mexico (56%), Portugal (49%), Germany (42%), France (41%), and Spain (31%). The question was not asked in the United Kingdom survey.
In the United States, the size of the drop has been substantial: 9% lost 76%-100% of their income; 14% lost 51%-75%; 28% lost 26%-50%; 33% lost 11%-25%; and 15% lost 1%-10%.
The U.S. specialists with the largest drop in income were ophthalmologists, who lost 51%, followed by allergists (46%), plastic surgeons (46%), and otolaryngologists (45%).
“I’m looking for a new profession due to economic impact,” an otolaryngologist in the United States said. “We are at risk while essentially using our private savings to keep our practice solvent.”
More than half of U.S. physicians (54%) have personally treated patients with COVID-19. Percentages were higher in France, Spain, and the United Kingdom (percentages ranged from 60%-68%).
The United States led all eight countries in treating patients with COVID-19 via telemedicine, at 26%. Germany had the lowest telemedicine percentage, at 10%.
Burnout intensifies
About two thirds of US physicians (64%) said that burnout had intensified during the crisis (70% of female physicians and 61% of male physicians said it had).
Many factors are feeding the burnout.
A critical care physician in the United States responded, “It is terrible to see people arriving at their rooms and assuming they were going to die soon; to see people saying goodbye to their families before dying or before being intubated.”
In all eight countries, a substantial percentage of physicians reported they “sometimes, often or always” treated patients with COVID-19 without the proper personal protective equipment. Spain had by far the largest percentage who answered that way (67%), followed by France (45%), Mexico (40%), the United Kingdom (34%), Brazil and Germany (28% each); and the United States and Portugal (23% each).
A U.S. rheumatologist wrote: “The fact that we were sent to take care of infectious patients without proper protection equipment made me feel we were betrayed in this fight.”
Sense of duty to volunteer to treat COVID-19 patients varied substantially among countries, from 69% who felt that way in Spain to 40% in Brazil. Half (50%) in the United States felt that way.
“Altruism must take second place where a real and present threat exists to my own personal existence,” one U.S. internist wrote.
Numbers personally infected
One fifth of physicians in Spain and the United Kingdom had personally been infected with the virus. Brazil, France, and Mexico had the next highest numbers, with 13%-15% of physicians infected; 5%-6% in the United States, Germany, and Portugal said they had been infected.
The percentage of physicians who reported that immediate family members had been infected ranged from 25% in Spain to 6% in Portugal. Among US physicians, 9% reported that family members had been diagnosed with COVID-19.
In the United States, 44% of respondents who had family living with them at home during the pandemic reported that relationships at home were more stressed because of stay-at-home guidelines and social distancing. Almost half (47%) said there had been no change, and 9% said relationships were less stressed.
Eating is coping mechanism of choice
Physicians were asked what they were doing more of during the pandemic, and food seemed to be the top source of comfort in all eight countries.
Loneliness reports differ across globe
Portugal had the highest percentage (51%) of physicians reporting increased loneliness. Next were Brazil (48%), the United States (46%), the United Kingdom (42%), France (41%), Spain and Mexico (40% each), and Germany (32%).
All eight countries lacked workplace activities to help physicians with grief. More than half (55%) of U.K. physicians reported having such activities available at their workplace, whereas only 25% of physicians in Germany did; 12%-24% of respondents across the countries were unsure about the offerings.
This article first appeared on Medscape.com.
Infectious COVID-19 can persist in gut for weeks
Stool tests were positive among people with no GI symptoms, and in some cases up to 6 days after nasopharyngeal swabs yielded negative results.
The small pilot study suggests a quiescent but active infection in the gut. Stool testing revealed genomic evidence of active infection in 7 of the 15 participants tested in one of two hospitals in Hong Kong.
“We found active and prolonged SARS-CoV-2 infection in the stool of patients with COVID-19, even after recovery, suggesting that coronavirus could remain in the gut of asymptomatic carriers,” senior author Siew C. Ng, MBBS, PhD, told Medscape Medical News.
“Due to the potential threat of fecal-oral transmission, it is important to maintain long-term coronavirus and health surveillance,” said Ng, Associate Director of the Centre for Gut Microbiota Research at the Chinese University of Hong Kong (CUHK).
“Discharged patients and their caretakers should remain vigilant and observe strict personal and toileting hygiene,” she added.
The prospective, observational study was published online July 20 in Gut.
Ramping up COVID-19 testing
As a follow-up to these and other findings – including the testing of more than 2,000 stool samples in children and the needy arriving at Hong Kong airports starting March 29 – the same investigators are establishing a CUHK Coronavirus Testing Center.
As of Aug. 31, the detection rate in tested children was 0.28%. The Center plans to offer as many as 2,000 COVID-19 tests daily going forward to help identify asymptomatic carriers, the investigators announced in a Sept. 7 news release.
In contrast to nasopharyngeal sampling, stool specimens are “more convenient, safe and non-invasive to collect in the pediatric population,” professor Paul Chan, chairman of the Department of Microbiology, CU Medicine, said in the release. “This makes the stool test a better option for COVID-19 screening in babies, young children and those whose respiratory samples are difficult to collect.”
Even though previous researchers identified SARS-CoV-2 in the stool, the activity and infectivity of the virus in the gastrointestinal tract during and after COVID-19 respiratory positivity remained largely unknown.
Active infection detected in stool
This prospective study involved 15 people hospitalized with COVID-19 in March and April. Participants were a median 55 years old (range, 22-71 years) and all presented with respiratory symptoms. Only one patient had concurrent GI symptoms at admission. Median length of stay was 21 days.
Investigators collected fecal samples serially until discharge. They extracted viral DNA to test for transcriptional genetic evidence of active infection, which they detected in 7 of 15 patients. The patient with GI symptoms was not in this positive group.
The findings suggest a “quiescent but active GI infection,” the researchers note.
Three of the seven patients continued to test positive for active infection in their stool up to 6 days after respiratory clearance of SARS-CoV-2.
Microbiome matters
The investigators also extracted, amplified, and sequenced DNA from the stool samples. Their “metagenomic” profile revealed the type and amounts of bacterial strains in each patient’s gut microbiome.
Interestingly, bacterial strains differed between people with high SARS-CoV-2 infectivity versus participants with low to no evidence of active infection.
“Stool with high viral activity had higher abundance of pathogenic bacteria,” Ng said. In contrast, people with low or no infectivity had more beneficial bacterial strains, including bacteria that play critical roles in boosting host immunity.
Each patient’s microbiome composition changed during the course of the study. Whether the microbiome alters the course of COVID-19 or COVID-19 alters the composition of the microbiome requires further study, the authors note.
The U.S. Food and Drug Administration and officials in other countries have contacted the Hong Kong investigators for more details on their stool testing strategy, professor Francis K.L. Chan, dean of the faculty of medicine and director of the Centre for Gut Microbiota Research at CUHK, stated in the news release.
Further research into revealing the infectivity and pathogenesis of SARS-CoV-2 in the GI tract is warranted. The value of modulating the human gut microbiome in this patient population could be worthwhile to investigate as well, the researchers said.
Novel finding
“Some of it is not-so-new news and some is new,” David A. Johnson, MD, told Medscape Medical News when asked to comment on the study.
For example, previous researchers have detected SARS-CoV-2 virus in the stool. However, this study takes it a step further and shows that the virus present in stool can remain infectious on the basis of metagenomic signatures.
Furthermore, the virus can remain infectious in the gut even after a patient tests negative for COVID-19 through nasopharyngeal sampling – in this report up to 6 days later, said Johnson, professor of medicine, chief of gastroenterology, Eastern Virginia Medical School in Norfolk, Va.
The study carries important implications for people who currently test negative following active COVID-19 infection, he added. Centers for Disease Control and Prevention criteria clear a person as negative after two nasopharyngeal swabs at least 24 hours apart.
People in this category could believe they are no longer infectious and might return to a setting where they could infect others, Johnson said.
One potential means for spreading SARS-CoV-2 from the gut is from a toilet plume, as Johnson previously highlighted in a video report for Medscape Medical News.
The study authors disclosed no relevant financial relationships. Johnson serves as an adviser to WebMD/Medscape.
This article first appeared on Medscape.com.
Stool tests were positive among people with no GI symptoms, and in some cases up to 6 days after nasopharyngeal swabs yielded negative results.
The small pilot study suggests a quiescent but active infection in the gut. Stool testing revealed genomic evidence of active infection in 7 of the 15 participants tested in one of two hospitals in Hong Kong.
“We found active and prolonged SARS-CoV-2 infection in the stool of patients with COVID-19, even after recovery, suggesting that coronavirus could remain in the gut of asymptomatic carriers,” senior author Siew C. Ng, MBBS, PhD, told Medscape Medical News.
“Due to the potential threat of fecal-oral transmission, it is important to maintain long-term coronavirus and health surveillance,” said Ng, Associate Director of the Centre for Gut Microbiota Research at the Chinese University of Hong Kong (CUHK).
“Discharged patients and their caretakers should remain vigilant and observe strict personal and toileting hygiene,” she added.
The prospective, observational study was published online July 20 in Gut.
Ramping up COVID-19 testing
As a follow-up to these and other findings – including the testing of more than 2,000 stool samples in children and the needy arriving at Hong Kong airports starting March 29 – the same investigators are establishing a CUHK Coronavirus Testing Center.
As of Aug. 31, the detection rate in tested children was 0.28%. The Center plans to offer as many as 2,000 COVID-19 tests daily going forward to help identify asymptomatic carriers, the investigators announced in a Sept. 7 news release.
In contrast to nasopharyngeal sampling, stool specimens are “more convenient, safe and non-invasive to collect in the pediatric population,” professor Paul Chan, chairman of the Department of Microbiology, CU Medicine, said in the release. “This makes the stool test a better option for COVID-19 screening in babies, young children and those whose respiratory samples are difficult to collect.”
Even though previous researchers identified SARS-CoV-2 in the stool, the activity and infectivity of the virus in the gastrointestinal tract during and after COVID-19 respiratory positivity remained largely unknown.
Active infection detected in stool
This prospective study involved 15 people hospitalized with COVID-19 in March and April. Participants were a median 55 years old (range, 22-71 years) and all presented with respiratory symptoms. Only one patient had concurrent GI symptoms at admission. Median length of stay was 21 days.
Investigators collected fecal samples serially until discharge. They extracted viral DNA to test for transcriptional genetic evidence of active infection, which they detected in 7 of 15 patients. The patient with GI symptoms was not in this positive group.
The findings suggest a “quiescent but active GI infection,” the researchers note.
Three of the seven patients continued to test positive for active infection in their stool up to 6 days after respiratory clearance of SARS-CoV-2.
Microbiome matters
The investigators also extracted, amplified, and sequenced DNA from the stool samples. Their “metagenomic” profile revealed the type and amounts of bacterial strains in each patient’s gut microbiome.
Interestingly, bacterial strains differed between people with high SARS-CoV-2 infectivity versus participants with low to no evidence of active infection.
“Stool with high viral activity had higher abundance of pathogenic bacteria,” Ng said. In contrast, people with low or no infectivity had more beneficial bacterial strains, including bacteria that play critical roles in boosting host immunity.
Each patient’s microbiome composition changed during the course of the study. Whether the microbiome alters the course of COVID-19 or COVID-19 alters the composition of the microbiome requires further study, the authors note.
The U.S. Food and Drug Administration and officials in other countries have contacted the Hong Kong investigators for more details on their stool testing strategy, professor Francis K.L. Chan, dean of the faculty of medicine and director of the Centre for Gut Microbiota Research at CUHK, stated in the news release.
Further research into revealing the infectivity and pathogenesis of SARS-CoV-2 in the GI tract is warranted. The value of modulating the human gut microbiome in this patient population could be worthwhile to investigate as well, the researchers said.
Novel finding
“Some of it is not-so-new news and some is new,” David A. Johnson, MD, told Medscape Medical News when asked to comment on the study.
For example, previous researchers have detected SARS-CoV-2 virus in the stool. However, this study takes it a step further and shows that the virus present in stool can remain infectious on the basis of metagenomic signatures.
Furthermore, the virus can remain infectious in the gut even after a patient tests negative for COVID-19 through nasopharyngeal sampling – in this report up to 6 days later, said Johnson, professor of medicine, chief of gastroenterology, Eastern Virginia Medical School in Norfolk, Va.
The study carries important implications for people who currently test negative following active COVID-19 infection, he added. Centers for Disease Control and Prevention criteria clear a person as negative after two nasopharyngeal swabs at least 24 hours apart.
People in this category could believe they are no longer infectious and might return to a setting where they could infect others, Johnson said.
One potential means for spreading SARS-CoV-2 from the gut is from a toilet plume, as Johnson previously highlighted in a video report for Medscape Medical News.
The study authors disclosed no relevant financial relationships. Johnson serves as an adviser to WebMD/Medscape.
This article first appeared on Medscape.com.
Stool tests were positive among people with no GI symptoms, and in some cases up to 6 days after nasopharyngeal swabs yielded negative results.
The small pilot study suggests a quiescent but active infection in the gut. Stool testing revealed genomic evidence of active infection in 7 of the 15 participants tested in one of two hospitals in Hong Kong.
“We found active and prolonged SARS-CoV-2 infection in the stool of patients with COVID-19, even after recovery, suggesting that coronavirus could remain in the gut of asymptomatic carriers,” senior author Siew C. Ng, MBBS, PhD, told Medscape Medical News.
“Due to the potential threat of fecal-oral transmission, it is important to maintain long-term coronavirus and health surveillance,” said Ng, Associate Director of the Centre for Gut Microbiota Research at the Chinese University of Hong Kong (CUHK).
“Discharged patients and their caretakers should remain vigilant and observe strict personal and toileting hygiene,” she added.
The prospective, observational study was published online July 20 in Gut.
Ramping up COVID-19 testing
As a follow-up to these and other findings – including the testing of more than 2,000 stool samples in children and the needy arriving at Hong Kong airports starting March 29 – the same investigators are establishing a CUHK Coronavirus Testing Center.
As of Aug. 31, the detection rate in tested children was 0.28%. The Center plans to offer as many as 2,000 COVID-19 tests daily going forward to help identify asymptomatic carriers, the investigators announced in a Sept. 7 news release.
In contrast to nasopharyngeal sampling, stool specimens are “more convenient, safe and non-invasive to collect in the pediatric population,” professor Paul Chan, chairman of the Department of Microbiology, CU Medicine, said in the release. “This makes the stool test a better option for COVID-19 screening in babies, young children and those whose respiratory samples are difficult to collect.”
Even though previous researchers identified SARS-CoV-2 in the stool, the activity and infectivity of the virus in the gastrointestinal tract during and after COVID-19 respiratory positivity remained largely unknown.
Active infection detected in stool
This prospective study involved 15 people hospitalized with COVID-19 in March and April. Participants were a median 55 years old (range, 22-71 years) and all presented with respiratory symptoms. Only one patient had concurrent GI symptoms at admission. Median length of stay was 21 days.
Investigators collected fecal samples serially until discharge. They extracted viral DNA to test for transcriptional genetic evidence of active infection, which they detected in 7 of 15 patients. The patient with GI symptoms was not in this positive group.
The findings suggest a “quiescent but active GI infection,” the researchers note.
Three of the seven patients continued to test positive for active infection in their stool up to 6 days after respiratory clearance of SARS-CoV-2.
Microbiome matters
The investigators also extracted, amplified, and sequenced DNA from the stool samples. Their “metagenomic” profile revealed the type and amounts of bacterial strains in each patient’s gut microbiome.
Interestingly, bacterial strains differed between people with high SARS-CoV-2 infectivity versus participants with low to no evidence of active infection.
“Stool with high viral activity had higher abundance of pathogenic bacteria,” Ng said. In contrast, people with low or no infectivity had more beneficial bacterial strains, including bacteria that play critical roles in boosting host immunity.
Each patient’s microbiome composition changed during the course of the study. Whether the microbiome alters the course of COVID-19 or COVID-19 alters the composition of the microbiome requires further study, the authors note.
The U.S. Food and Drug Administration and officials in other countries have contacted the Hong Kong investigators for more details on their stool testing strategy, professor Francis K.L. Chan, dean of the faculty of medicine and director of the Centre for Gut Microbiota Research at CUHK, stated in the news release.
Further research into revealing the infectivity and pathogenesis of SARS-CoV-2 in the GI tract is warranted. The value of modulating the human gut microbiome in this patient population could be worthwhile to investigate as well, the researchers said.
Novel finding
“Some of it is not-so-new news and some is new,” David A. Johnson, MD, told Medscape Medical News when asked to comment on the study.
For example, previous researchers have detected SARS-CoV-2 virus in the stool. However, this study takes it a step further and shows that the virus present in stool can remain infectious on the basis of metagenomic signatures.
Furthermore, the virus can remain infectious in the gut even after a patient tests negative for COVID-19 through nasopharyngeal sampling – in this report up to 6 days later, said Johnson, professor of medicine, chief of gastroenterology, Eastern Virginia Medical School in Norfolk, Va.
The study carries important implications for people who currently test negative following active COVID-19 infection, he added. Centers for Disease Control and Prevention criteria clear a person as negative after two nasopharyngeal swabs at least 24 hours apart.
People in this category could believe they are no longer infectious and might return to a setting where they could infect others, Johnson said.
One potential means for spreading SARS-CoV-2 from the gut is from a toilet plume, as Johnson previously highlighted in a video report for Medscape Medical News.
The study authors disclosed no relevant financial relationships. Johnson serves as an adviser to WebMD/Medscape.
This article first appeared on Medscape.com.
In a time of two pandemics, a recommitment to work together
Overwhelmed. As if we weren’t already overwhelmed. For decades, hospitalists have been on the forefront of improving acute care amidst a rapidly changing environment. These last few decades have seen tremendous advances in medicine, technology, safety culture, innovations in payment models, transformation in business models, and a rising tide of health care policy. There was never a year we didn’t face major change … and adapt to it. Then 2020 came upon us.
This year, we adapt to more than a score and 4 years’ worth of change.
The two pandemics that have come upon us are like tsunamis. And many of us are drowning. We know of threats of pandemics: influenza, Ebola, and the like. But SARS-CoV-2 is new and like no other. We live in fear and isolation, each and every day learning new information and debunking others. We also know of racial injustice and racism, implicit or explicit in our nation, whether we live it or just read of it. George Floyd’s death in my hometown marked another tsunami, a great realization in our nation, and a great unmasking of our denial.
Yet our country is not united.
Hospital medicine is not immune to this disunity. At a time that we are all treading water, staying afloat in our own hospitals and communities, confronting these issues beyond our immediate spheres of influence is overwhelming. We are impacted by these pandemics, personally and professionally. And admittedly, we can be both victim and perpetrator.
In the face of a novel infectious agent, medicine responded quickly and pushed us beyond our limits. We have developed new infection prevention guidelines. We worked creatively to solve PPE shortages. We fashioned new work flows and new care models. We accelerated telehealth applications. We expanded the boundaries on home-based programs and reached out to vulnerable elderly in congregate living – an isolation no older person should have to endure. We cared for our colleagues, neighbors, and family members who fell ill, some who recovered, and sadly, some who fell. We developed best-practice guidelines, research protocols, created new order sets, note templates, and documentation standards. We flexed into EDs, ICUs, and field hospitals. Amidst the turmoil, we took pay cuts and saw colleagues go on furlough. And still, we mentored leaders in our schools, churches, synagogues, mosques, and civic communities.
And just when we thought we could endure no more, on May 25, we witnessed a black man in Minneapolis killed by a policeman’s knee. The same knee that divided Americans when black American athletes knelt to protest the injustice their people have endured for centuries. A knee that has been confused for insolence, when it was meant for justice ... yes, justice, for all. So, in early June, around the nation in support of black lives we also knelt, for almost 9 minutes.
This was the third time I cried during the pandemics.
For many of us, structural racism in America had finally been unmasked. The nation protested and rioted for weeks, and some communities have continued. Indeed, these two pandemics are still surging.
Side by side COVID-19 case conferences we lay transparent data demonstrating health disparities that we have tolerated for so long. We have vowed to resource equity work, and we opened dialogue, not only with patients and communities of color, but also with colleagues of color – some ready and some not yet ready to share and relive the traumas of their past and their present.
And still, we are not united.
While we physically mask to prevent the spread of COVID-19, we must make efforts to unmask the truths of SARS-CoV-2, the failings of our health system, the richness of our communities of color, and the injustice in the fabric of our society. More importantly, we must work together to create solutions. While we have diverse interests and priorities, at SHM, we can find common ground with kindred spirits, enhance the role of our specialty, and advance the health of our patients.
Let’s not be mistaken. These pandemics add to a growing list of interwoven issues in our society. In 2018, I wrote a piece on the role of hospitalists in addressing rural health disparities.1 According to the Sheps Center for Health Services Research, 129 rural hospitals have closed since 2010, closures that have accelerated with the COVID-19 pandemic.2 More than ever, we must stand above our inner and outer conflicts and be united to promote the health of our nation during these pandemics, because “all policy is health policy.”3
Most SHM presidents and president-elects come in with a platform, a priority for the specialty and for the society. This year, the platform has chosen us. For 20 years, I have witnessed SHM be a workshop for our members to address the pressing needs of our specialty and our patients. In 2020, we’ve continued to see SHM as a workshop for our members and a tour de force addressing these pandemics, from just in time publications of research and perspectives in the Journal of Hospital Medicine, to webinars and open access education in the Learning Portal, to advocacy on Capitol Hill. All of that work has been informed by you and for you. While there is still so much to do, we need not be overwhelmed when we do it together.
A score and 4 years ago, Robert Wachter, MD, and Lee Goldman, MD, dubbed us “hospitalists.” A year later, our shared workshop was born. Through one name change and now our first CEO transition from Larry Wellikson, MD, to Eric Howell, MD, SHM will continue to be where hospitalists both adapt and shape our nation through solutions that put an end to these pandemics. Let’s recommit to this work together.
Dr. Siy is division medical director, hospital specialties, in the departments of hospital medicine and community senior and palliative care, at HealthPartners in Bloomington, Minn. He is president-elect of SHM.
Sources
1. Hardeman RR et al. Stolen Breaths. N Engl J Med. 2020 Jul 16;383:197-9.
2. Siy JC. Reviving Rural Health Care. The Hospitalist. 2018 Sep 24.
3. The Cecil G. Sheps Center For Health Services Research. Rural Hospital Closures. 2014. https://www.shepscenter.unc.edu/programs-projects/rural-health/rural-hospital-closures/
Overwhelmed. As if we weren’t already overwhelmed. For decades, hospitalists have been on the forefront of improving acute care amidst a rapidly changing environment. These last few decades have seen tremendous advances in medicine, technology, safety culture, innovations in payment models, transformation in business models, and a rising tide of health care policy. There was never a year we didn’t face major change … and adapt to it. Then 2020 came upon us.
This year, we adapt to more than a score and 4 years’ worth of change.
The two pandemics that have come upon us are like tsunamis. And many of us are drowning. We know of threats of pandemics: influenza, Ebola, and the like. But SARS-CoV-2 is new and like no other. We live in fear and isolation, each and every day learning new information and debunking others. We also know of racial injustice and racism, implicit or explicit in our nation, whether we live it or just read of it. George Floyd’s death in my hometown marked another tsunami, a great realization in our nation, and a great unmasking of our denial.
Yet our country is not united.
Hospital medicine is not immune to this disunity. At a time that we are all treading water, staying afloat in our own hospitals and communities, confronting these issues beyond our immediate spheres of influence is overwhelming. We are impacted by these pandemics, personally and professionally. And admittedly, we can be both victim and perpetrator.
In the face of a novel infectious agent, medicine responded quickly and pushed us beyond our limits. We have developed new infection prevention guidelines. We worked creatively to solve PPE shortages. We fashioned new work flows and new care models. We accelerated telehealth applications. We expanded the boundaries on home-based programs and reached out to vulnerable elderly in congregate living – an isolation no older person should have to endure. We cared for our colleagues, neighbors, and family members who fell ill, some who recovered, and sadly, some who fell. We developed best-practice guidelines, research protocols, created new order sets, note templates, and documentation standards. We flexed into EDs, ICUs, and field hospitals. Amidst the turmoil, we took pay cuts and saw colleagues go on furlough. And still, we mentored leaders in our schools, churches, synagogues, mosques, and civic communities.
And just when we thought we could endure no more, on May 25, we witnessed a black man in Minneapolis killed by a policeman’s knee. The same knee that divided Americans when black American athletes knelt to protest the injustice their people have endured for centuries. A knee that has been confused for insolence, when it was meant for justice ... yes, justice, for all. So, in early June, around the nation in support of black lives we also knelt, for almost 9 minutes.
This was the third time I cried during the pandemics.
For many of us, structural racism in America had finally been unmasked. The nation protested and rioted for weeks, and some communities have continued. Indeed, these two pandemics are still surging.
Side by side COVID-19 case conferences we lay transparent data demonstrating health disparities that we have tolerated for so long. We have vowed to resource equity work, and we opened dialogue, not only with patients and communities of color, but also with colleagues of color – some ready and some not yet ready to share and relive the traumas of their past and their present.
And still, we are not united.
While we physically mask to prevent the spread of COVID-19, we must make efforts to unmask the truths of SARS-CoV-2, the failings of our health system, the richness of our communities of color, and the injustice in the fabric of our society. More importantly, we must work together to create solutions. While we have diverse interests and priorities, at SHM, we can find common ground with kindred spirits, enhance the role of our specialty, and advance the health of our patients.
Let’s not be mistaken. These pandemics add to a growing list of interwoven issues in our society. In 2018, I wrote a piece on the role of hospitalists in addressing rural health disparities.1 According to the Sheps Center for Health Services Research, 129 rural hospitals have closed since 2010, closures that have accelerated with the COVID-19 pandemic.2 More than ever, we must stand above our inner and outer conflicts and be united to promote the health of our nation during these pandemics, because “all policy is health policy.”3
Most SHM presidents and president-elects come in with a platform, a priority for the specialty and for the society. This year, the platform has chosen us. For 20 years, I have witnessed SHM be a workshop for our members to address the pressing needs of our specialty and our patients. In 2020, we’ve continued to see SHM as a workshop for our members and a tour de force addressing these pandemics, from just in time publications of research and perspectives in the Journal of Hospital Medicine, to webinars and open access education in the Learning Portal, to advocacy on Capitol Hill. All of that work has been informed by you and for you. While there is still so much to do, we need not be overwhelmed when we do it together.
A score and 4 years ago, Robert Wachter, MD, and Lee Goldman, MD, dubbed us “hospitalists.” A year later, our shared workshop was born. Through one name change and now our first CEO transition from Larry Wellikson, MD, to Eric Howell, MD, SHM will continue to be where hospitalists both adapt and shape our nation through solutions that put an end to these pandemics. Let’s recommit to this work together.
Dr. Siy is division medical director, hospital specialties, in the departments of hospital medicine and community senior and palliative care, at HealthPartners in Bloomington, Minn. He is president-elect of SHM.
Sources
1. Hardeman RR et al. Stolen Breaths. N Engl J Med. 2020 Jul 16;383:197-9.
2. Siy JC. Reviving Rural Health Care. The Hospitalist. 2018 Sep 24.
3. The Cecil G. Sheps Center For Health Services Research. Rural Hospital Closures. 2014. https://www.shepscenter.unc.edu/programs-projects/rural-health/rural-hospital-closures/
Overwhelmed. As if we weren’t already overwhelmed. For decades, hospitalists have been on the forefront of improving acute care amidst a rapidly changing environment. These last few decades have seen tremendous advances in medicine, technology, safety culture, innovations in payment models, transformation in business models, and a rising tide of health care policy. There was never a year we didn’t face major change … and adapt to it. Then 2020 came upon us.
This year, we adapt to more than a score and 4 years’ worth of change.
The two pandemics that have come upon us are like tsunamis. And many of us are drowning. We know of threats of pandemics: influenza, Ebola, and the like. But SARS-CoV-2 is new and like no other. We live in fear and isolation, each and every day learning new information and debunking others. We also know of racial injustice and racism, implicit or explicit in our nation, whether we live it or just read of it. George Floyd’s death in my hometown marked another tsunami, a great realization in our nation, and a great unmasking of our denial.
Yet our country is not united.
Hospital medicine is not immune to this disunity. At a time that we are all treading water, staying afloat in our own hospitals and communities, confronting these issues beyond our immediate spheres of influence is overwhelming. We are impacted by these pandemics, personally and professionally. And admittedly, we can be both victim and perpetrator.
In the face of a novel infectious agent, medicine responded quickly and pushed us beyond our limits. We have developed new infection prevention guidelines. We worked creatively to solve PPE shortages. We fashioned new work flows and new care models. We accelerated telehealth applications. We expanded the boundaries on home-based programs and reached out to vulnerable elderly in congregate living – an isolation no older person should have to endure. We cared for our colleagues, neighbors, and family members who fell ill, some who recovered, and sadly, some who fell. We developed best-practice guidelines, research protocols, created new order sets, note templates, and documentation standards. We flexed into EDs, ICUs, and field hospitals. Amidst the turmoil, we took pay cuts and saw colleagues go on furlough. And still, we mentored leaders in our schools, churches, synagogues, mosques, and civic communities.
And just when we thought we could endure no more, on May 25, we witnessed a black man in Minneapolis killed by a policeman’s knee. The same knee that divided Americans when black American athletes knelt to protest the injustice their people have endured for centuries. A knee that has been confused for insolence, when it was meant for justice ... yes, justice, for all. So, in early June, around the nation in support of black lives we also knelt, for almost 9 minutes.
This was the third time I cried during the pandemics.
For many of us, structural racism in America had finally been unmasked. The nation protested and rioted for weeks, and some communities have continued. Indeed, these two pandemics are still surging.
Side by side COVID-19 case conferences we lay transparent data demonstrating health disparities that we have tolerated for so long. We have vowed to resource equity work, and we opened dialogue, not only with patients and communities of color, but also with colleagues of color – some ready and some not yet ready to share and relive the traumas of their past and their present.
And still, we are not united.
While we physically mask to prevent the spread of COVID-19, we must make efforts to unmask the truths of SARS-CoV-2, the failings of our health system, the richness of our communities of color, and the injustice in the fabric of our society. More importantly, we must work together to create solutions. While we have diverse interests and priorities, at SHM, we can find common ground with kindred spirits, enhance the role of our specialty, and advance the health of our patients.
Let’s not be mistaken. These pandemics add to a growing list of interwoven issues in our society. In 2018, I wrote a piece on the role of hospitalists in addressing rural health disparities.1 According to the Sheps Center for Health Services Research, 129 rural hospitals have closed since 2010, closures that have accelerated with the COVID-19 pandemic.2 More than ever, we must stand above our inner and outer conflicts and be united to promote the health of our nation during these pandemics, because “all policy is health policy.”3
Most SHM presidents and president-elects come in with a platform, a priority for the specialty and for the society. This year, the platform has chosen us. For 20 years, I have witnessed SHM be a workshop for our members to address the pressing needs of our specialty and our patients. In 2020, we’ve continued to see SHM as a workshop for our members and a tour de force addressing these pandemics, from just in time publications of research and perspectives in the Journal of Hospital Medicine, to webinars and open access education in the Learning Portal, to advocacy on Capitol Hill. All of that work has been informed by you and for you. While there is still so much to do, we need not be overwhelmed when we do it together.
A score and 4 years ago, Robert Wachter, MD, and Lee Goldman, MD, dubbed us “hospitalists.” A year later, our shared workshop was born. Through one name change and now our first CEO transition from Larry Wellikson, MD, to Eric Howell, MD, SHM will continue to be where hospitalists both adapt and shape our nation through solutions that put an end to these pandemics. Let’s recommit to this work together.
Dr. Siy is division medical director, hospital specialties, in the departments of hospital medicine and community senior and palliative care, at HealthPartners in Bloomington, Minn. He is president-elect of SHM.
Sources
1. Hardeman RR et al. Stolen Breaths. N Engl J Med. 2020 Jul 16;383:197-9.
2. Siy JC. Reviving Rural Health Care. The Hospitalist. 2018 Sep 24.
3. The Cecil G. Sheps Center For Health Services Research. Rural Hospital Closures. 2014. https://www.shepscenter.unc.edu/programs-projects/rural-health/rural-hospital-closures/
Worry over family, friends the main driver of COVID-19 stress
Individuals are more worried about family members becoming ill with COVID-19 or about unknowingly transmitting the disease to family members than they are about contracting it themselves, results of a new survey show.
Investigators surveyed over 3,000 adults, using an online questionnaire. Of the respondents, about 20% were health care workers, and most were living in locations with active stay-at-home orders at the time of the survey.
Close to half of participants were worried about family members contracting the virus, one third were worried about unknowingly infecting others, and 20% were worried about contracting the virus themselves.
“We were a little surprised to see that people were more concerned about others than about themselves, specifically worrying about whether a family member would contract COVID-19 and whether they might unintentionally infect others,” lead author Ran Barzilay, MD, PhD, child and adolescent psychiatrist at the Children’s Hospital of Philadelphia (CHOP), told Medscape Medical News.
The study was published online August 20 in Translational Psychiatry.
Interactive platform
“The pandemic has provided a unique opportunity to study resilience in healthcare professionals and others,” said Barzilay, assistant professor at the Lifespan Brain Institute, a collaboration between CHOP and the University of Pennsylvania, under the directorship of Raquel Gur, MD, PhD.
“After the pandemic broke out in March, we launched a website in early April where we surveyed people for levels of resilience, mental health, and well-being during the outbreak,” he added.
Survey participants then shared it with their contacts.
“To date, over 7000 people have completed it – mostly from the US but also from Israel,” Barzilay said.
The survey was anonymous, but participants could choose to have follow-up contact. The survey included an interactive 21-item resilience questionnaire and an assessment of COVID-19-related items related to worries concerning the following: contracting, dying from, or currently having the illness; having a family member contract the illness; unknowingly infecting others; and experiencing significant financial burden.
A total of 1350 participants took a second survey on anxiety and depression that utilized the Generalized Anxiety Disorder–7 and the Patient Health Questionnaire–2.
“What makes the survey unique is that it’s not just a means of collecting data but also an interactive platform that gives participants immediate personalized feedback, based on their responses to the resilience and well-being surveys, with practical tips and recommendations for stress management and ways of boosting resilience,” Barzilay said.
Tend and befriend
Ten days into the survey, data were available on 3,042 participants (64% women, 54% with advanced education, 20.5% health care providers), who ranged in age from 18 to 70 years (mean [SD], 38.9 [11.9] years).
After accounting for covariates, the researchers found that participants reported more distress about family members contracting COVID-19 and about unknowingly infecting others than about getting COVID-19 themselves (48.5% and 36% vs. 19.9%, respectively; P < .0005).
Increased COVID-19-related worries were associated with 22% higher anxiety and 16.1% higher depression scores; women had higher scores than men on both.
Each 1-SD increase in the composite score of COVID-19 worries was associated with over twice the increased probability of generalized anxiety and depression (odds ratio, 2.23; 95% confidence interval, 1.88-2.65; and OR, 1.67; 95% CI, 1.41-1.98, respectively; for both, P < .001).
On the other hand, for every 1-SD increase in the resilience score, there was a 64.9% decrease in the possibility of screening positive for generalized anxiety disorder and a 69.3% decrease in the possibility of screening positive for depression (for both, P < .0001).
Compared to participants from Israel, US participants were “more stressed” about contracting, dying from, and currently having COVID-19 themselves. Overall, Israeli participants scored higher than US participants on the resilience scale.
Rates of anxiety and depression did not differ significantly between healthcare providers and others. Health care providers worried more about contracting COVID-19 themselves and worried less about finances after COVID-19.
The authors propose that survey participants were more worried about others than about themselves because of “prosocial behavior under stress” and “tend-and-befriend,” whereby, “in response to threat, humans tend to protect their close ones (tending) and seek out their social group for mutual defense (befriending).”
This type of altruistic behavior has been “described in acute situations throughout history” and has been “linked to mechanisms of resilience for overcoming adversity,” the authors indicate.
Demographic biases
Commenting on the findings for Medscape Medical News, Golnaz Tabibnia, PhD, a neuroscientist at the University of California, Irvine, who was not involved in the research, suggested that although higher resilience scores were associated with lower COVID-related worries, it is possible, “as the authors suggest, that having more resilience resources makes you less worried, but the causality could go the other direction as well, and less worry/rumination may lead to more resilience.”
Also commenting on the study for Medscape Medical News, Christiaan Vinkers, MD, PhD, a psychiatrist at the Amsterdam University Medical Center, Amsterdam, the Netherlands, said it was noteworthy that healthcare providers reported similar levels of mood and anxiety symptoms, compared to others.
“This is encouraging, as it suggests adequate resilience levels in professionals who work in the front lines of the COVID-19 pandemic,” he said.
Resilience occurs not only at the individual level but also at the community level, which may help explain the striking differences in COVID-19-related worries and anxiety between participants from the United States and Israel, Vinkers added.
E. Alison Holman, PhD, professor, Sue and Bill Gross School of Nursing, University of California, Irvine, noted that respondents were predominantly white, female, and had relatively high incomes, “suggesting strong demographic biases in those who chose to participate.”
Holman, who was not involved with the study, told Medscape Medical News that the “findings do not address the real impact of COVID-19 on the hardest-hit communities in America – poor, Black, and Latinx communities, where a large proportion of essential workers live.”
Barzilay acknowledged that, “unfortunately, because of the way the study was circulated, it did not reach minorities, which is one of the things we want to improve.”
The study is ongoing and has been translated into Spanish, French, and Hebrew. The team plans to collect data on diverse populations.
The study was supported by grants from the National Institute of Mental Health, the Lifespan Brain Institute of Children’s Hospital of Philadelphia, Penn Medicine, the University of Pennsylvania, and in part by the Zuckerman STEM Leadership Program. Barzilay serves on the scientific board and reports stock ownership in Taliaz Health. The other authors, Golnaz, Vinkers, and Holman have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Individuals are more worried about family members becoming ill with COVID-19 or about unknowingly transmitting the disease to family members than they are about contracting it themselves, results of a new survey show.
Investigators surveyed over 3,000 adults, using an online questionnaire. Of the respondents, about 20% were health care workers, and most were living in locations with active stay-at-home orders at the time of the survey.
Close to half of participants were worried about family members contracting the virus, one third were worried about unknowingly infecting others, and 20% were worried about contracting the virus themselves.
“We were a little surprised to see that people were more concerned about others than about themselves, specifically worrying about whether a family member would contract COVID-19 and whether they might unintentionally infect others,” lead author Ran Barzilay, MD, PhD, child and adolescent psychiatrist at the Children’s Hospital of Philadelphia (CHOP), told Medscape Medical News.
The study was published online August 20 in Translational Psychiatry.
Interactive platform
“The pandemic has provided a unique opportunity to study resilience in healthcare professionals and others,” said Barzilay, assistant professor at the Lifespan Brain Institute, a collaboration between CHOP and the University of Pennsylvania, under the directorship of Raquel Gur, MD, PhD.
“After the pandemic broke out in March, we launched a website in early April where we surveyed people for levels of resilience, mental health, and well-being during the outbreak,” he added.
Survey participants then shared it with their contacts.
“To date, over 7000 people have completed it – mostly from the US but also from Israel,” Barzilay said.
The survey was anonymous, but participants could choose to have follow-up contact. The survey included an interactive 21-item resilience questionnaire and an assessment of COVID-19-related items related to worries concerning the following: contracting, dying from, or currently having the illness; having a family member contract the illness; unknowingly infecting others; and experiencing significant financial burden.
A total of 1350 participants took a second survey on anxiety and depression that utilized the Generalized Anxiety Disorder–7 and the Patient Health Questionnaire–2.
“What makes the survey unique is that it’s not just a means of collecting data but also an interactive platform that gives participants immediate personalized feedback, based on their responses to the resilience and well-being surveys, with practical tips and recommendations for stress management and ways of boosting resilience,” Barzilay said.
Tend and befriend
Ten days into the survey, data were available on 3,042 participants (64% women, 54% with advanced education, 20.5% health care providers), who ranged in age from 18 to 70 years (mean [SD], 38.9 [11.9] years).
After accounting for covariates, the researchers found that participants reported more distress about family members contracting COVID-19 and about unknowingly infecting others than about getting COVID-19 themselves (48.5% and 36% vs. 19.9%, respectively; P < .0005).
Increased COVID-19-related worries were associated with 22% higher anxiety and 16.1% higher depression scores; women had higher scores than men on both.
Each 1-SD increase in the composite score of COVID-19 worries was associated with over twice the increased probability of generalized anxiety and depression (odds ratio, 2.23; 95% confidence interval, 1.88-2.65; and OR, 1.67; 95% CI, 1.41-1.98, respectively; for both, P < .001).
On the other hand, for every 1-SD increase in the resilience score, there was a 64.9% decrease in the possibility of screening positive for generalized anxiety disorder and a 69.3% decrease in the possibility of screening positive for depression (for both, P < .0001).
Compared to participants from Israel, US participants were “more stressed” about contracting, dying from, and currently having COVID-19 themselves. Overall, Israeli participants scored higher than US participants on the resilience scale.
Rates of anxiety and depression did not differ significantly between healthcare providers and others. Health care providers worried more about contracting COVID-19 themselves and worried less about finances after COVID-19.
The authors propose that survey participants were more worried about others than about themselves because of “prosocial behavior under stress” and “tend-and-befriend,” whereby, “in response to threat, humans tend to protect their close ones (tending) and seek out their social group for mutual defense (befriending).”
This type of altruistic behavior has been “described in acute situations throughout history” and has been “linked to mechanisms of resilience for overcoming adversity,” the authors indicate.
Demographic biases
Commenting on the findings for Medscape Medical News, Golnaz Tabibnia, PhD, a neuroscientist at the University of California, Irvine, who was not involved in the research, suggested that although higher resilience scores were associated with lower COVID-related worries, it is possible, “as the authors suggest, that having more resilience resources makes you less worried, but the causality could go the other direction as well, and less worry/rumination may lead to more resilience.”
Also commenting on the study for Medscape Medical News, Christiaan Vinkers, MD, PhD, a psychiatrist at the Amsterdam University Medical Center, Amsterdam, the Netherlands, said it was noteworthy that healthcare providers reported similar levels of mood and anxiety symptoms, compared to others.
“This is encouraging, as it suggests adequate resilience levels in professionals who work in the front lines of the COVID-19 pandemic,” he said.
Resilience occurs not only at the individual level but also at the community level, which may help explain the striking differences in COVID-19-related worries and anxiety between participants from the United States and Israel, Vinkers added.
E. Alison Holman, PhD, professor, Sue and Bill Gross School of Nursing, University of California, Irvine, noted that respondents were predominantly white, female, and had relatively high incomes, “suggesting strong demographic biases in those who chose to participate.”
Holman, who was not involved with the study, told Medscape Medical News that the “findings do not address the real impact of COVID-19 on the hardest-hit communities in America – poor, Black, and Latinx communities, where a large proportion of essential workers live.”
Barzilay acknowledged that, “unfortunately, because of the way the study was circulated, it did not reach minorities, which is one of the things we want to improve.”
The study is ongoing and has been translated into Spanish, French, and Hebrew. The team plans to collect data on diverse populations.
The study was supported by grants from the National Institute of Mental Health, the Lifespan Brain Institute of Children’s Hospital of Philadelphia, Penn Medicine, the University of Pennsylvania, and in part by the Zuckerman STEM Leadership Program. Barzilay serves on the scientific board and reports stock ownership in Taliaz Health. The other authors, Golnaz, Vinkers, and Holman have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Individuals are more worried about family members becoming ill with COVID-19 or about unknowingly transmitting the disease to family members than they are about contracting it themselves, results of a new survey show.
Investigators surveyed over 3,000 adults, using an online questionnaire. Of the respondents, about 20% were health care workers, and most were living in locations with active stay-at-home orders at the time of the survey.
Close to half of participants were worried about family members contracting the virus, one third were worried about unknowingly infecting others, and 20% were worried about contracting the virus themselves.
“We were a little surprised to see that people were more concerned about others than about themselves, specifically worrying about whether a family member would contract COVID-19 and whether they might unintentionally infect others,” lead author Ran Barzilay, MD, PhD, child and adolescent psychiatrist at the Children’s Hospital of Philadelphia (CHOP), told Medscape Medical News.
The study was published online August 20 in Translational Psychiatry.
Interactive platform
“The pandemic has provided a unique opportunity to study resilience in healthcare professionals and others,” said Barzilay, assistant professor at the Lifespan Brain Institute, a collaboration between CHOP and the University of Pennsylvania, under the directorship of Raquel Gur, MD, PhD.
“After the pandemic broke out in March, we launched a website in early April where we surveyed people for levels of resilience, mental health, and well-being during the outbreak,” he added.
Survey participants then shared it with their contacts.
“To date, over 7000 people have completed it – mostly from the US but also from Israel,” Barzilay said.
The survey was anonymous, but participants could choose to have follow-up contact. The survey included an interactive 21-item resilience questionnaire and an assessment of COVID-19-related items related to worries concerning the following: contracting, dying from, or currently having the illness; having a family member contract the illness; unknowingly infecting others; and experiencing significant financial burden.
A total of 1350 participants took a second survey on anxiety and depression that utilized the Generalized Anxiety Disorder–7 and the Patient Health Questionnaire–2.
“What makes the survey unique is that it’s not just a means of collecting data but also an interactive platform that gives participants immediate personalized feedback, based on their responses to the resilience and well-being surveys, with practical tips and recommendations for stress management and ways of boosting resilience,” Barzilay said.
Tend and befriend
Ten days into the survey, data were available on 3,042 participants (64% women, 54% with advanced education, 20.5% health care providers), who ranged in age from 18 to 70 years (mean [SD], 38.9 [11.9] years).
After accounting for covariates, the researchers found that participants reported more distress about family members contracting COVID-19 and about unknowingly infecting others than about getting COVID-19 themselves (48.5% and 36% vs. 19.9%, respectively; P < .0005).
Increased COVID-19-related worries were associated with 22% higher anxiety and 16.1% higher depression scores; women had higher scores than men on both.
Each 1-SD increase in the composite score of COVID-19 worries was associated with over twice the increased probability of generalized anxiety and depression (odds ratio, 2.23; 95% confidence interval, 1.88-2.65; and OR, 1.67; 95% CI, 1.41-1.98, respectively; for both, P < .001).
On the other hand, for every 1-SD increase in the resilience score, there was a 64.9% decrease in the possibility of screening positive for generalized anxiety disorder and a 69.3% decrease in the possibility of screening positive for depression (for both, P < .0001).
Compared to participants from Israel, US participants were “more stressed” about contracting, dying from, and currently having COVID-19 themselves. Overall, Israeli participants scored higher than US participants on the resilience scale.
Rates of anxiety and depression did not differ significantly between healthcare providers and others. Health care providers worried more about contracting COVID-19 themselves and worried less about finances after COVID-19.
The authors propose that survey participants were more worried about others than about themselves because of “prosocial behavior under stress” and “tend-and-befriend,” whereby, “in response to threat, humans tend to protect their close ones (tending) and seek out their social group for mutual defense (befriending).”
This type of altruistic behavior has been “described in acute situations throughout history” and has been “linked to mechanisms of resilience for overcoming adversity,” the authors indicate.
Demographic biases
Commenting on the findings for Medscape Medical News, Golnaz Tabibnia, PhD, a neuroscientist at the University of California, Irvine, who was not involved in the research, suggested that although higher resilience scores were associated with lower COVID-related worries, it is possible, “as the authors suggest, that having more resilience resources makes you less worried, but the causality could go the other direction as well, and less worry/rumination may lead to more resilience.”
Also commenting on the study for Medscape Medical News, Christiaan Vinkers, MD, PhD, a psychiatrist at the Amsterdam University Medical Center, Amsterdam, the Netherlands, said it was noteworthy that healthcare providers reported similar levels of mood and anxiety symptoms, compared to others.
“This is encouraging, as it suggests adequate resilience levels in professionals who work in the front lines of the COVID-19 pandemic,” he said.
Resilience occurs not only at the individual level but also at the community level, which may help explain the striking differences in COVID-19-related worries and anxiety between participants from the United States and Israel, Vinkers added.
E. Alison Holman, PhD, professor, Sue and Bill Gross School of Nursing, University of California, Irvine, noted that respondents were predominantly white, female, and had relatively high incomes, “suggesting strong demographic biases in those who chose to participate.”
Holman, who was not involved with the study, told Medscape Medical News that the “findings do not address the real impact of COVID-19 on the hardest-hit communities in America – poor, Black, and Latinx communities, where a large proportion of essential workers live.”
Barzilay acknowledged that, “unfortunately, because of the way the study was circulated, it did not reach minorities, which is one of the things we want to improve.”
The study is ongoing and has been translated into Spanish, French, and Hebrew. The team plans to collect data on diverse populations.
The study was supported by grants from the National Institute of Mental Health, the Lifespan Brain Institute of Children’s Hospital of Philadelphia, Penn Medicine, the University of Pennsylvania, and in part by the Zuckerman STEM Leadership Program. Barzilay serves on the scientific board and reports stock ownership in Taliaz Health. The other authors, Golnaz, Vinkers, and Holman have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The earlier the better for colchicine post-MI: COLCOT
The earlier the anti-inflammatory drug colchicine is initiated after a myocardial infarction (MI) the greater the benefit, a new COLCOT analysis suggests.
The parent trial was conducted in patients with a recent MI because of the intense inflammation present at that time, and added colchicine 0.5 mg daily to standard care within 30 days following MI.
As previously reported, colchicine significantly reduced the risk of the primary end point – a composite of cardiovascular (CV) death, resuscitated cardiac arrest, MI, stroke, or urgent hospitalization for angina requiring revascularization – by 23% compared with placebo.
This new analysis shows the risk was reduced by 48% in patients receiving colchicine within 3 days of an MI (4.3% vs. 8.3%; adjusted hazard ratio, 0.52; 95% confidence interval, 0.32-0.84, P = .007).
Risk of a secondary efficacy end point – CV death, resuscitated cardiac arrest, MI, or stroke – was reduced by 45% over an average follow up of 22.7 months (3.3% vs 6.1%; adjusted HR, 0.55; 95% CI, 0.32-0.95, P = .031).
“We believe that our results support an early, in-hospital initiation of adjunctive colchicine for post-MI prevention,” Nadia Bouabdallaoui, MD, Montreal Heart Institute, Quebec, Canada, said during an online session devoted to colchicine at the European Society of Cardiology Congress 2020.
Session moderator Massimo Imazio, MD, professor of cardiology at the University of Turin, Italy, said the improved outcomes suggest that earlier treatment is better – a finding that parallels his own experience using colchicine in patients with pericarditis.
“This substudy is very important because this is probably also the year in cardiovascular applications [that] early use of the drug could improve outcomes,” he said.
Positive data have been accumulating for colchicine from COLCOT, LoDoCo, and, most recently, the LoDoCo2 trial, even as another anti-inflammatory drug, methotrexate, flamed out as secondary prevention in the CIRT trial.
The new COLCOT substudy included 4,661 of the 4,745 original patients and examined treatment initiation using three strata: within 0-3 days (n = 1,193), 4-7 days (n = 720), and 8-30 days (n = 2,748). Patients who received treatment within 3 days were slightly younger, more likely to be smokers, and to have a shorter time from MI to randomization (2.1 days vs 5.1 days vs. 20.8 days, respectively).
In the subset receiving treatment within 3 days, those assigned to colchicine had the same number of cardiac deaths as those given placebo (2 vs. 2) but fewer resuscitated cardiac arrests (1 vs. 3), MIs (17 vs. 29), strokes (1 vs. 5), and urgent hospitalizations for angina requiring revascularization (6 vs. 17).
“A larger trial might have allowed for a better assessment of individual endpoints and subgroups,” observed Bouabdallaoui.
Although there is growing support for colchicine, experts caution that the drug many not be for everyone. In COLCOT, 1 in 10 patients were unable to tolerate the drug, largely because of gastrointestinal (GI) issues.
Pharmacogenomics substudy
A second COLCOT substudy aimed to identify genetic markers predictive of colchicine response and to gain insights into the mechanisms behind this response. It included 767 patients treated with colchicine and another 755 treated with placebo – or about one-third the patients in the original trial.
A genome-wide association study did not find a significant association for the primary CV endpoint, although a prespecified subgroup analysis in men identified an interesting region on chromosome 9 (variant: rs10811106), which just missed reaching genomewide significance, said Marie-Pierre Dubé, PhD, director of the Université de Montréal Beaulieu-Saucier Pharmacogenomics Centre at the Montreal Heart Institute.
In addition, the genomewide analysis found two significant regions for GI events: one on chromosome 6 (variant: rs6916345) and one on chromosome 10 (variant: rs74795203).
For each of the identified regions, the researchers then tested the effect of the allele in the placebo group and the interaction between the genetic variant and treatment with colchicine. For the chromosome 9 region in males, there was no effect in the placebo group and a significant interaction in the colchicine group.
For the significant GI event findings, there was a small effect for the chromosome 6 region in the placebo group and a very significant interaction with colchicine, Dubé said. Similarly, there was no effect for the chromosome 10 region in the placebo group and a significant interaction with colchicine.
Additional analyses in stratified patient populations showed that males with the protective allele (CC) for the chromosome 9 region represented 83% of the population. The primary CV endpoint occurred in 3.2% of these men treated with colchicine and 6.3% treated with placebo (HR, 0.46; 95% CI, 0.24 - 0.86).
For the gastrointestinal events, 25% of patients carried the risk allele (AA) for the chromosome 6 region and 36.9% of these had GI events when treated with colchicine versus 18.6% when treated with placebo (HR, 2.42; 95% CI, 1.57-3.72).
Similarly, 13% of individuals carried one or two copies of the risk allele (AG+GG) for the chromosome 10 region and the risk of GI events in these was nearly four times higher with colchicine (47.1% vs. 18.9%; HR, 3.98; 95% CI 2.24-7.07).
Functional genomic analyses of the identified regions were also performed and showed that the chromosome 9 locus overlaps with the SAXO1 gene, a stabilizer of axonemal microtubules 1.
“The leading variant at this locus (rs10811106 C allele) correlated with the expression of the HAUS6 gene, which is involved in microtubule generation from existing microtubules, and may interact with the effect of colchicine, which is known to inhibit microtubule formation,” observed Dubé.
Also, the chromosome 6 locus associated with gastrointestinal events was colocalizing with the Crohn’s disease locus, adding further support for this region.
“The results support potential personalized approaches to inflammation reduction for cardiovascular prevention,” Dubé said.
This is a post hoc subgroup analysis, however, and replication is necessary, ideally in prospective randomized trials, she noted.
The substudy is important because it provides further insights into the link between colchicine and microtubule polymerization, affecting the activation of the inflammasome, session moderator Imazio said.
“Second, it is important because pharmacogenomics can help us to better understand the optimal responder to colchicine and colchicine resistance,” he said. “So it can be useful for personalized medicine, leading to the proper use of the drug for the proper patient.”
COLCOT was supported by the government of Quebec, the Canadian Institutes of Health Research, and philanthropic foundations. Bouabdallaoui has disclosed no relevant financial relationships. Dubé reported grants from the government of Quebec; personal fees from DalCor and GlaxoSmithKline; research support from AstraZeneca, Pfizer, Servier, Sanofi; and minor equity interest in DalCor. Dubé is also coauthor of patents on pharmacogenomics-guided CETP inhibition, and pharmacogenomics markers of response to colchicine.
This article first appeared on Medscape.com.
The earlier the anti-inflammatory drug colchicine is initiated after a myocardial infarction (MI) the greater the benefit, a new COLCOT analysis suggests.
The parent trial was conducted in patients with a recent MI because of the intense inflammation present at that time, and added colchicine 0.5 mg daily to standard care within 30 days following MI.
As previously reported, colchicine significantly reduced the risk of the primary end point – a composite of cardiovascular (CV) death, resuscitated cardiac arrest, MI, stroke, or urgent hospitalization for angina requiring revascularization – by 23% compared with placebo.
This new analysis shows the risk was reduced by 48% in patients receiving colchicine within 3 days of an MI (4.3% vs. 8.3%; adjusted hazard ratio, 0.52; 95% confidence interval, 0.32-0.84, P = .007).
Risk of a secondary efficacy end point – CV death, resuscitated cardiac arrest, MI, or stroke – was reduced by 45% over an average follow up of 22.7 months (3.3% vs 6.1%; adjusted HR, 0.55; 95% CI, 0.32-0.95, P = .031).
“We believe that our results support an early, in-hospital initiation of adjunctive colchicine for post-MI prevention,” Nadia Bouabdallaoui, MD, Montreal Heart Institute, Quebec, Canada, said during an online session devoted to colchicine at the European Society of Cardiology Congress 2020.
Session moderator Massimo Imazio, MD, professor of cardiology at the University of Turin, Italy, said the improved outcomes suggest that earlier treatment is better – a finding that parallels his own experience using colchicine in patients with pericarditis.
“This substudy is very important because this is probably also the year in cardiovascular applications [that] early use of the drug could improve outcomes,” he said.
Positive data have been accumulating for colchicine from COLCOT, LoDoCo, and, most recently, the LoDoCo2 trial, even as another anti-inflammatory drug, methotrexate, flamed out as secondary prevention in the CIRT trial.
The new COLCOT substudy included 4,661 of the 4,745 original patients and examined treatment initiation using three strata: within 0-3 days (n = 1,193), 4-7 days (n = 720), and 8-30 days (n = 2,748). Patients who received treatment within 3 days were slightly younger, more likely to be smokers, and to have a shorter time from MI to randomization (2.1 days vs 5.1 days vs. 20.8 days, respectively).
In the subset receiving treatment within 3 days, those assigned to colchicine had the same number of cardiac deaths as those given placebo (2 vs. 2) but fewer resuscitated cardiac arrests (1 vs. 3), MIs (17 vs. 29), strokes (1 vs. 5), and urgent hospitalizations for angina requiring revascularization (6 vs. 17).
“A larger trial might have allowed for a better assessment of individual endpoints and subgroups,” observed Bouabdallaoui.
Although there is growing support for colchicine, experts caution that the drug many not be for everyone. In COLCOT, 1 in 10 patients were unable to tolerate the drug, largely because of gastrointestinal (GI) issues.
Pharmacogenomics substudy
A second COLCOT substudy aimed to identify genetic markers predictive of colchicine response and to gain insights into the mechanisms behind this response. It included 767 patients treated with colchicine and another 755 treated with placebo – or about one-third the patients in the original trial.
A genome-wide association study did not find a significant association for the primary CV endpoint, although a prespecified subgroup analysis in men identified an interesting region on chromosome 9 (variant: rs10811106), which just missed reaching genomewide significance, said Marie-Pierre Dubé, PhD, director of the Université de Montréal Beaulieu-Saucier Pharmacogenomics Centre at the Montreal Heart Institute.
In addition, the genomewide analysis found two significant regions for GI events: one on chromosome 6 (variant: rs6916345) and one on chromosome 10 (variant: rs74795203).
For each of the identified regions, the researchers then tested the effect of the allele in the placebo group and the interaction between the genetic variant and treatment with colchicine. For the chromosome 9 region in males, there was no effect in the placebo group and a significant interaction in the colchicine group.
For the significant GI event findings, there was a small effect for the chromosome 6 region in the placebo group and a very significant interaction with colchicine, Dubé said. Similarly, there was no effect for the chromosome 10 region in the placebo group and a significant interaction with colchicine.
Additional analyses in stratified patient populations showed that males with the protective allele (CC) for the chromosome 9 region represented 83% of the population. The primary CV endpoint occurred in 3.2% of these men treated with colchicine and 6.3% treated with placebo (HR, 0.46; 95% CI, 0.24 - 0.86).
For the gastrointestinal events, 25% of patients carried the risk allele (AA) for the chromosome 6 region and 36.9% of these had GI events when treated with colchicine versus 18.6% when treated with placebo (HR, 2.42; 95% CI, 1.57-3.72).
Similarly, 13% of individuals carried one or two copies of the risk allele (AG+GG) for the chromosome 10 region and the risk of GI events in these was nearly four times higher with colchicine (47.1% vs. 18.9%; HR, 3.98; 95% CI 2.24-7.07).
Functional genomic analyses of the identified regions were also performed and showed that the chromosome 9 locus overlaps with the SAXO1 gene, a stabilizer of axonemal microtubules 1.
“The leading variant at this locus (rs10811106 C allele) correlated with the expression of the HAUS6 gene, which is involved in microtubule generation from existing microtubules, and may interact with the effect of colchicine, which is known to inhibit microtubule formation,” observed Dubé.
Also, the chromosome 6 locus associated with gastrointestinal events was colocalizing with the Crohn’s disease locus, adding further support for this region.
“The results support potential personalized approaches to inflammation reduction for cardiovascular prevention,” Dubé said.
This is a post hoc subgroup analysis, however, and replication is necessary, ideally in prospective randomized trials, she noted.
The substudy is important because it provides further insights into the link between colchicine and microtubule polymerization, affecting the activation of the inflammasome, session moderator Imazio said.
“Second, it is important because pharmacogenomics can help us to better understand the optimal responder to colchicine and colchicine resistance,” he said. “So it can be useful for personalized medicine, leading to the proper use of the drug for the proper patient.”
COLCOT was supported by the government of Quebec, the Canadian Institutes of Health Research, and philanthropic foundations. Bouabdallaoui has disclosed no relevant financial relationships. Dubé reported grants from the government of Quebec; personal fees from DalCor and GlaxoSmithKline; research support from AstraZeneca, Pfizer, Servier, Sanofi; and minor equity interest in DalCor. Dubé is also coauthor of patents on pharmacogenomics-guided CETP inhibition, and pharmacogenomics markers of response to colchicine.
This article first appeared on Medscape.com.
The earlier the anti-inflammatory drug colchicine is initiated after a myocardial infarction (MI) the greater the benefit, a new COLCOT analysis suggests.
The parent trial was conducted in patients with a recent MI because of the intense inflammation present at that time, and added colchicine 0.5 mg daily to standard care within 30 days following MI.
As previously reported, colchicine significantly reduced the risk of the primary end point – a composite of cardiovascular (CV) death, resuscitated cardiac arrest, MI, stroke, or urgent hospitalization for angina requiring revascularization – by 23% compared with placebo.
This new analysis shows the risk was reduced by 48% in patients receiving colchicine within 3 days of an MI (4.3% vs. 8.3%; adjusted hazard ratio, 0.52; 95% confidence interval, 0.32-0.84, P = .007).
Risk of a secondary efficacy end point – CV death, resuscitated cardiac arrest, MI, or stroke – was reduced by 45% over an average follow up of 22.7 months (3.3% vs 6.1%; adjusted HR, 0.55; 95% CI, 0.32-0.95, P = .031).
“We believe that our results support an early, in-hospital initiation of adjunctive colchicine for post-MI prevention,” Nadia Bouabdallaoui, MD, Montreal Heart Institute, Quebec, Canada, said during an online session devoted to colchicine at the European Society of Cardiology Congress 2020.
Session moderator Massimo Imazio, MD, professor of cardiology at the University of Turin, Italy, said the improved outcomes suggest that earlier treatment is better – a finding that parallels his own experience using colchicine in patients with pericarditis.
“This substudy is very important because this is probably also the year in cardiovascular applications [that] early use of the drug could improve outcomes,” he said.
Positive data have been accumulating for colchicine from COLCOT, LoDoCo, and, most recently, the LoDoCo2 trial, even as another anti-inflammatory drug, methotrexate, flamed out as secondary prevention in the CIRT trial.
The new COLCOT substudy included 4,661 of the 4,745 original patients and examined treatment initiation using three strata: within 0-3 days (n = 1,193), 4-7 days (n = 720), and 8-30 days (n = 2,748). Patients who received treatment within 3 days were slightly younger, more likely to be smokers, and to have a shorter time from MI to randomization (2.1 days vs 5.1 days vs. 20.8 days, respectively).
In the subset receiving treatment within 3 days, those assigned to colchicine had the same number of cardiac deaths as those given placebo (2 vs. 2) but fewer resuscitated cardiac arrests (1 vs. 3), MIs (17 vs. 29), strokes (1 vs. 5), and urgent hospitalizations for angina requiring revascularization (6 vs. 17).
“A larger trial might have allowed for a better assessment of individual endpoints and subgroups,” observed Bouabdallaoui.
Although there is growing support for colchicine, experts caution that the drug many not be for everyone. In COLCOT, 1 in 10 patients were unable to tolerate the drug, largely because of gastrointestinal (GI) issues.
Pharmacogenomics substudy
A second COLCOT substudy aimed to identify genetic markers predictive of colchicine response and to gain insights into the mechanisms behind this response. It included 767 patients treated with colchicine and another 755 treated with placebo – or about one-third the patients in the original trial.
A genome-wide association study did not find a significant association for the primary CV endpoint, although a prespecified subgroup analysis in men identified an interesting region on chromosome 9 (variant: rs10811106), which just missed reaching genomewide significance, said Marie-Pierre Dubé, PhD, director of the Université de Montréal Beaulieu-Saucier Pharmacogenomics Centre at the Montreal Heart Institute.
In addition, the genomewide analysis found two significant regions for GI events: one on chromosome 6 (variant: rs6916345) and one on chromosome 10 (variant: rs74795203).
For each of the identified regions, the researchers then tested the effect of the allele in the placebo group and the interaction between the genetic variant and treatment with colchicine. For the chromosome 9 region in males, there was no effect in the placebo group and a significant interaction in the colchicine group.
For the significant GI event findings, there was a small effect for the chromosome 6 region in the placebo group and a very significant interaction with colchicine, Dubé said. Similarly, there was no effect for the chromosome 10 region in the placebo group and a significant interaction with colchicine.
Additional analyses in stratified patient populations showed that males with the protective allele (CC) for the chromosome 9 region represented 83% of the population. The primary CV endpoint occurred in 3.2% of these men treated with colchicine and 6.3% treated with placebo (HR, 0.46; 95% CI, 0.24 - 0.86).
For the gastrointestinal events, 25% of patients carried the risk allele (AA) for the chromosome 6 region and 36.9% of these had GI events when treated with colchicine versus 18.6% when treated with placebo (HR, 2.42; 95% CI, 1.57-3.72).
Similarly, 13% of individuals carried one or two copies of the risk allele (AG+GG) for the chromosome 10 region and the risk of GI events in these was nearly four times higher with colchicine (47.1% vs. 18.9%; HR, 3.98; 95% CI 2.24-7.07).
Functional genomic analyses of the identified regions were also performed and showed that the chromosome 9 locus overlaps with the SAXO1 gene, a stabilizer of axonemal microtubules 1.
“The leading variant at this locus (rs10811106 C allele) correlated with the expression of the HAUS6 gene, which is involved in microtubule generation from existing microtubules, and may interact with the effect of colchicine, which is known to inhibit microtubule formation,” observed Dubé.
Also, the chromosome 6 locus associated with gastrointestinal events was colocalizing with the Crohn’s disease locus, adding further support for this region.
“The results support potential personalized approaches to inflammation reduction for cardiovascular prevention,” Dubé said.
This is a post hoc subgroup analysis, however, and replication is necessary, ideally in prospective randomized trials, she noted.
The substudy is important because it provides further insights into the link between colchicine and microtubule polymerization, affecting the activation of the inflammasome, session moderator Imazio said.
“Second, it is important because pharmacogenomics can help us to better understand the optimal responder to colchicine and colchicine resistance,” he said. “So it can be useful for personalized medicine, leading to the proper use of the drug for the proper patient.”
COLCOT was supported by the government of Quebec, the Canadian Institutes of Health Research, and philanthropic foundations. Bouabdallaoui has disclosed no relevant financial relationships. Dubé reported grants from the government of Quebec; personal fees from DalCor and GlaxoSmithKline; research support from AstraZeneca, Pfizer, Servier, Sanofi; and minor equity interest in DalCor. Dubé is also coauthor of patents on pharmacogenomics-guided CETP inhibition, and pharmacogenomics markers of response to colchicine.
This article first appeared on Medscape.com.
Lessons for patients with MS and COVID-19
Two important lessons about managing patients with multiple sclerosis (MS) and COVID-19 have emerged from a hospital clinic in Madrid that managed COVID-infected patients with MS through the peak of the pandemic: Combined polymeric chain reaction and serology testing helped avoid disease reactivation in asymptomatic carriers during the pandemic peak, although after the peak PCR alone proved just as effective; and
Virginia Meca-Lallana, MD, a neurologist and coordinator of the demyelinating diseases unit at the Hospital of the University of the Princess in Madrid, and colleagues presented their findings in two posters at the Joint European Committee for Treatment and Research in Multiple Sclerosis-Americas Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS-ACTRIMS) 2020, this year known as MSVirtual2020.
“MS treatments don’t seem to make the prognosis of COVID-19 worse, but it is very important to evaluate other risk factors,” Dr. Meca-Lallana said in an interview. “MS treatments prevent the patients’ disability, and it is very important not to stop them if it isn’t necessary.”
The results arose from a multidisciplinary safety protocol involving neurology, microbiology, and preventive medicine that the University of Princess physicians developed to keep MS stable in patients diagnosed with SARS-CoV-2.
The researchers obtained 152 PCR nasopharyngeal swabs and 140 serology tests in 90 patients with MS over 3 months before starting a variety of MS treatments: Natalizumab (96 tests), ocrelizumab (36), rituximab (3), methylprednisolone (7), cladribine (4), and dimethyl fumarate (3). The protocol identified 7 asymptomatic carriers—7.8% of the total population—5 of whom had positive immunoglobulin M and G serology. The study also confirmed 5 patients with positive IgM+IgG serology post-infection, but no COVID-19 reactivations were detected after implementation of the protocol.
“The safety protocol reached its objective of avoiding disease reactivation and clinical activation in asymptomatic carriers,” Dr. Meca-Lallana said.
The second poster she presented reported on the real-world experience with SARS-CoV-2 in the MS unit at her hospital. The observational, prospective study included 41 cases, 38 of which were relapsing-remitting MS and the remainder progressive MS. The patients had MS for an average of 9 years.
“We need more patients to draw more robust conclusions, but in our patients, MS treatments seem safe in this situation,” Dr. Meca-Lallana said. “We did not discontinue treatments, and after our first results, we only delayed treatments in patients with any additional comorbidity or when coming to the hospital was not safe.”
A total of 39 patients were taking disease-modifying therapies (DMTs): 46.3% with oral agents, 39% with monoclonal antibodies, and 10% with injectable agents; 27 patients were previously treated with other DMTs. The median Expanded Disability Status Scale (EDSS) was 2.5, and 11 patients had clinical activity the previous year. Eighteen cases were confirmed by PCR or serology, or both, and 23 were diagnosed clinically.
Among the patients with MS and COVID-19, 17% were admitted to the hospital. Six patients had pneumonia, but none required admission to the intensive care unit, and no deaths occurred. Three patients had other comorbidities. Admitted patients tended to be older and had higher EDSS scores, although the difference was not statistically significant. MS worsened in 7 patients, and 10 patients stopped or paused DMTs because of the infection.
“Multiple sclerosis is a weakening illness,” Dr. Meca-Lallana said. “MS treatments do not seem to make the prognosis of COVID-19 worse, but it is very important to evaluate other risk factors.”
The SARS-CoV-2 infection does not seem to result in a more aggressive form of the disease in MS patients, and selective immunosuppression may improve their outcomes, she noted.
“MS treatments avoid the patient’s disability,” the investigator added, “and it is very important not to stop them if it isn’t necessary.”
Dr. Meca-Lallana had no relevant financial disclosures.
Two important lessons about managing patients with multiple sclerosis (MS) and COVID-19 have emerged from a hospital clinic in Madrid that managed COVID-infected patients with MS through the peak of the pandemic: Combined polymeric chain reaction and serology testing helped avoid disease reactivation in asymptomatic carriers during the pandemic peak, although after the peak PCR alone proved just as effective; and
Virginia Meca-Lallana, MD, a neurologist and coordinator of the demyelinating diseases unit at the Hospital of the University of the Princess in Madrid, and colleagues presented their findings in two posters at the Joint European Committee for Treatment and Research in Multiple Sclerosis-Americas Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS-ACTRIMS) 2020, this year known as MSVirtual2020.
“MS treatments don’t seem to make the prognosis of COVID-19 worse, but it is very important to evaluate other risk factors,” Dr. Meca-Lallana said in an interview. “MS treatments prevent the patients’ disability, and it is very important not to stop them if it isn’t necessary.”
The results arose from a multidisciplinary safety protocol involving neurology, microbiology, and preventive medicine that the University of Princess physicians developed to keep MS stable in patients diagnosed with SARS-CoV-2.
The researchers obtained 152 PCR nasopharyngeal swabs and 140 serology tests in 90 patients with MS over 3 months before starting a variety of MS treatments: Natalizumab (96 tests), ocrelizumab (36), rituximab (3), methylprednisolone (7), cladribine (4), and dimethyl fumarate (3). The protocol identified 7 asymptomatic carriers—7.8% of the total population—5 of whom had positive immunoglobulin M and G serology. The study also confirmed 5 patients with positive IgM+IgG serology post-infection, but no COVID-19 reactivations were detected after implementation of the protocol.
“The safety protocol reached its objective of avoiding disease reactivation and clinical activation in asymptomatic carriers,” Dr. Meca-Lallana said.
The second poster she presented reported on the real-world experience with SARS-CoV-2 in the MS unit at her hospital. The observational, prospective study included 41 cases, 38 of which were relapsing-remitting MS and the remainder progressive MS. The patients had MS for an average of 9 years.
“We need more patients to draw more robust conclusions, but in our patients, MS treatments seem safe in this situation,” Dr. Meca-Lallana said. “We did not discontinue treatments, and after our first results, we only delayed treatments in patients with any additional comorbidity or when coming to the hospital was not safe.”
A total of 39 patients were taking disease-modifying therapies (DMTs): 46.3% with oral agents, 39% with monoclonal antibodies, and 10% with injectable agents; 27 patients were previously treated with other DMTs. The median Expanded Disability Status Scale (EDSS) was 2.5, and 11 patients had clinical activity the previous year. Eighteen cases were confirmed by PCR or serology, or both, and 23 were diagnosed clinically.
Among the patients with MS and COVID-19, 17% were admitted to the hospital. Six patients had pneumonia, but none required admission to the intensive care unit, and no deaths occurred. Three patients had other comorbidities. Admitted patients tended to be older and had higher EDSS scores, although the difference was not statistically significant. MS worsened in 7 patients, and 10 patients stopped or paused DMTs because of the infection.
“Multiple sclerosis is a weakening illness,” Dr. Meca-Lallana said. “MS treatments do not seem to make the prognosis of COVID-19 worse, but it is very important to evaluate other risk factors.”
The SARS-CoV-2 infection does not seem to result in a more aggressive form of the disease in MS patients, and selective immunosuppression may improve their outcomes, she noted.
“MS treatments avoid the patient’s disability,” the investigator added, “and it is very important not to stop them if it isn’t necessary.”
Dr. Meca-Lallana had no relevant financial disclosures.
Two important lessons about managing patients with multiple sclerosis (MS) and COVID-19 have emerged from a hospital clinic in Madrid that managed COVID-infected patients with MS through the peak of the pandemic: Combined polymeric chain reaction and serology testing helped avoid disease reactivation in asymptomatic carriers during the pandemic peak, although after the peak PCR alone proved just as effective; and
Virginia Meca-Lallana, MD, a neurologist and coordinator of the demyelinating diseases unit at the Hospital of the University of the Princess in Madrid, and colleagues presented their findings in two posters at the Joint European Committee for Treatment and Research in Multiple Sclerosis-Americas Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS-ACTRIMS) 2020, this year known as MSVirtual2020.
“MS treatments don’t seem to make the prognosis of COVID-19 worse, but it is very important to evaluate other risk factors,” Dr. Meca-Lallana said in an interview. “MS treatments prevent the patients’ disability, and it is very important not to stop them if it isn’t necessary.”
The results arose from a multidisciplinary safety protocol involving neurology, microbiology, and preventive medicine that the University of Princess physicians developed to keep MS stable in patients diagnosed with SARS-CoV-2.
The researchers obtained 152 PCR nasopharyngeal swabs and 140 serology tests in 90 patients with MS over 3 months before starting a variety of MS treatments: Natalizumab (96 tests), ocrelizumab (36), rituximab (3), methylprednisolone (7), cladribine (4), and dimethyl fumarate (3). The protocol identified 7 asymptomatic carriers—7.8% of the total population—5 of whom had positive immunoglobulin M and G serology. The study also confirmed 5 patients with positive IgM+IgG serology post-infection, but no COVID-19 reactivations were detected after implementation of the protocol.
“The safety protocol reached its objective of avoiding disease reactivation and clinical activation in asymptomatic carriers,” Dr. Meca-Lallana said.
The second poster she presented reported on the real-world experience with SARS-CoV-2 in the MS unit at her hospital. The observational, prospective study included 41 cases, 38 of which were relapsing-remitting MS and the remainder progressive MS. The patients had MS for an average of 9 years.
“We need more patients to draw more robust conclusions, but in our patients, MS treatments seem safe in this situation,” Dr. Meca-Lallana said. “We did not discontinue treatments, and after our first results, we only delayed treatments in patients with any additional comorbidity or when coming to the hospital was not safe.”
A total of 39 patients were taking disease-modifying therapies (DMTs): 46.3% with oral agents, 39% with monoclonal antibodies, and 10% with injectable agents; 27 patients were previously treated with other DMTs. The median Expanded Disability Status Scale (EDSS) was 2.5, and 11 patients had clinical activity the previous year. Eighteen cases were confirmed by PCR or serology, or both, and 23 were diagnosed clinically.
Among the patients with MS and COVID-19, 17% were admitted to the hospital. Six patients had pneumonia, but none required admission to the intensive care unit, and no deaths occurred. Three patients had other comorbidities. Admitted patients tended to be older and had higher EDSS scores, although the difference was not statistically significant. MS worsened in 7 patients, and 10 patients stopped or paused DMTs because of the infection.
“Multiple sclerosis is a weakening illness,” Dr. Meca-Lallana said. “MS treatments do not seem to make the prognosis of COVID-19 worse, but it is very important to evaluate other risk factors.”
The SARS-CoV-2 infection does not seem to result in a more aggressive form of the disease in MS patients, and selective immunosuppression may improve their outcomes, she noted.
“MS treatments avoid the patient’s disability,” the investigator added, “and it is very important not to stop them if it isn’t necessary.”
Dr. Meca-Lallana had no relevant financial disclosures.
FROM MSVirtual2020
Hospitalist movers and shakers – September 2020
The American Board of Internal Medicine has named David Pizzimenti, DO, to its board of trustees. The appointment comes with a 3-year term.
Dr. Pizzimenti has been a practicing internist in Mississippi since 2005. He currently serves as associate medical officer of acute care at North Mississippi Medical Center, Tupelo, where he also directs the hospitalist program and the internal medicine residency program. Prior to joining NMMC, he managed the same role at Magnolia Regional Health Center (Corinth, Miss.).
Dr. Pizzimenti is an inducted member of the American College of Osteopathic Internist College of Fellows, as well as a certified wound care specialist.
Tommy Ibrahim, MD, FHM, recently was named the new president and CEO for Bassett Healthcare Network, replacing William Streck, who had served in the role from 1984 to 2014, and then on an interim basis since 2018.
Dr. Ibrahim comes to Bassett from Integris Health, the largest nonprofit health care system in Oklahoma, where he was executive vice president and chief physician executive. He started his career as a hospitalist before moving into administration, and is a fellow in hospital medicine as well as a fellow of the American College of Healthcare Executives.
Bassett Healthcare Network is based at Bassett Medical Center in Cooperstown, N.Y., and includes four hospitals and more than two dozen primary care centers in eight New York counties.
Russell Kerbel, MD, MBA, has been named medical director for sepsis prevention at the University of California, Los Angeles. Since his arrival at UCLA in 2014, Dr. Kerbel – a hospitalist by training – has worked to increase awareness and standardize sepsis treatment through his advocacy, interdepartmental collaboration, and informatics knowledge.
Joshua Lenchus, DO, RPh, SFHM, was installed as vice president of the Florida Medical Association during the all-virtual 2020 FMA annual meeting in August. Dr. Lenchus is a hospitalist and chief medical officer at the Broward Health Medical Center in Fort Lauderdale, Fla.
Christopher Carpenter, MD, has been elevated to chief of staff at Natividad, a 172-bed, county-owned hospital in Salinas, Calif. Dr. Carpenter has served Natividad for the past 4 years, holding the positions of chief hospitalist, chief of service for pediatrics, vice chief of staff, and most recently director of pediatric services.
Dr. Carpenter’s term as chief of staff is limited to 2 years, during which he said his goals include promoting diversity within the facility’s leadership.
Prior to arriving at Natividad, Dr. Carpenter was instructor of pediatrics at Harvard Medical School, Boston, as well as associate director of the Boston Children’s Hospital Pediatric Global Health Fellowship.
David Fagan, MD, recently was promoted to medical director at Mid-State Health Center (Plymouth, N.H.), where he has served for the past 10 years. The 30-year medical veteran began working in his new role in May 2020.
Previously, Dr. Fagan has served the facility as an internist and hospitalist, and he has been among the leaders at Mid-State in ensuring safety for patients and staff during the COVID-19 response.
The Carroll County Memorial Hospital (Carrolton, Mo.) recently announced its new hospitalist program, which officially began on June 1, 2020. CCMH officials said the focus of the hospitalists will be to maintain communication with primary care physicians once patients leave the hospital facility.
CCMH added three physicians to its staff to work in the hospitalist program: Reuben I. Thaker, MD; Samuel C. Evans, MD; and Charles C. Glendenning, DO.
NorthShore University HealthSystem (Evanston, Ill.) has agreed to purchase Northwest Community Healthcare, a single-hospital health system located in Arlington Heights, Ill. NCH will become a hospital hub for NorthShore in the northwest Chicago suburbs.
When the agreement is finalized, NorthShore’s stable of hospitals will rise to six in and around Chicago. The system also provides outpatient care, labwork, and pharmacy services.
The American Board of Internal Medicine has named David Pizzimenti, DO, to its board of trustees. The appointment comes with a 3-year term.
Dr. Pizzimenti has been a practicing internist in Mississippi since 2005. He currently serves as associate medical officer of acute care at North Mississippi Medical Center, Tupelo, where he also directs the hospitalist program and the internal medicine residency program. Prior to joining NMMC, he managed the same role at Magnolia Regional Health Center (Corinth, Miss.).
Dr. Pizzimenti is an inducted member of the American College of Osteopathic Internist College of Fellows, as well as a certified wound care specialist.
Tommy Ibrahim, MD, FHM, recently was named the new president and CEO for Bassett Healthcare Network, replacing William Streck, who had served in the role from 1984 to 2014, and then on an interim basis since 2018.
Dr. Ibrahim comes to Bassett from Integris Health, the largest nonprofit health care system in Oklahoma, where he was executive vice president and chief physician executive. He started his career as a hospitalist before moving into administration, and is a fellow in hospital medicine as well as a fellow of the American College of Healthcare Executives.
Bassett Healthcare Network is based at Bassett Medical Center in Cooperstown, N.Y., and includes four hospitals and more than two dozen primary care centers in eight New York counties.
Russell Kerbel, MD, MBA, has been named medical director for sepsis prevention at the University of California, Los Angeles. Since his arrival at UCLA in 2014, Dr. Kerbel – a hospitalist by training – has worked to increase awareness and standardize sepsis treatment through his advocacy, interdepartmental collaboration, and informatics knowledge.
Joshua Lenchus, DO, RPh, SFHM, was installed as vice president of the Florida Medical Association during the all-virtual 2020 FMA annual meeting in August. Dr. Lenchus is a hospitalist and chief medical officer at the Broward Health Medical Center in Fort Lauderdale, Fla.
Christopher Carpenter, MD, has been elevated to chief of staff at Natividad, a 172-bed, county-owned hospital in Salinas, Calif. Dr. Carpenter has served Natividad for the past 4 years, holding the positions of chief hospitalist, chief of service for pediatrics, vice chief of staff, and most recently director of pediatric services.
Dr. Carpenter’s term as chief of staff is limited to 2 years, during which he said his goals include promoting diversity within the facility’s leadership.
Prior to arriving at Natividad, Dr. Carpenter was instructor of pediatrics at Harvard Medical School, Boston, as well as associate director of the Boston Children’s Hospital Pediatric Global Health Fellowship.
David Fagan, MD, recently was promoted to medical director at Mid-State Health Center (Plymouth, N.H.), where he has served for the past 10 years. The 30-year medical veteran began working in his new role in May 2020.
Previously, Dr. Fagan has served the facility as an internist and hospitalist, and he has been among the leaders at Mid-State in ensuring safety for patients and staff during the COVID-19 response.
The Carroll County Memorial Hospital (Carrolton, Mo.) recently announced its new hospitalist program, which officially began on June 1, 2020. CCMH officials said the focus of the hospitalists will be to maintain communication with primary care physicians once patients leave the hospital facility.
CCMH added three physicians to its staff to work in the hospitalist program: Reuben I. Thaker, MD; Samuel C. Evans, MD; and Charles C. Glendenning, DO.
NorthShore University HealthSystem (Evanston, Ill.) has agreed to purchase Northwest Community Healthcare, a single-hospital health system located in Arlington Heights, Ill. NCH will become a hospital hub for NorthShore in the northwest Chicago suburbs.
When the agreement is finalized, NorthShore’s stable of hospitals will rise to six in and around Chicago. The system also provides outpatient care, labwork, and pharmacy services.
The American Board of Internal Medicine has named David Pizzimenti, DO, to its board of trustees. The appointment comes with a 3-year term.
Dr. Pizzimenti has been a practicing internist in Mississippi since 2005. He currently serves as associate medical officer of acute care at North Mississippi Medical Center, Tupelo, where he also directs the hospitalist program and the internal medicine residency program. Prior to joining NMMC, he managed the same role at Magnolia Regional Health Center (Corinth, Miss.).
Dr. Pizzimenti is an inducted member of the American College of Osteopathic Internist College of Fellows, as well as a certified wound care specialist.
Tommy Ibrahim, MD, FHM, recently was named the new president and CEO for Bassett Healthcare Network, replacing William Streck, who had served in the role from 1984 to 2014, and then on an interim basis since 2018.
Dr. Ibrahim comes to Bassett from Integris Health, the largest nonprofit health care system in Oklahoma, where he was executive vice president and chief physician executive. He started his career as a hospitalist before moving into administration, and is a fellow in hospital medicine as well as a fellow of the American College of Healthcare Executives.
Bassett Healthcare Network is based at Bassett Medical Center in Cooperstown, N.Y., and includes four hospitals and more than two dozen primary care centers in eight New York counties.
Russell Kerbel, MD, MBA, has been named medical director for sepsis prevention at the University of California, Los Angeles. Since his arrival at UCLA in 2014, Dr. Kerbel – a hospitalist by training – has worked to increase awareness and standardize sepsis treatment through his advocacy, interdepartmental collaboration, and informatics knowledge.
Joshua Lenchus, DO, RPh, SFHM, was installed as vice president of the Florida Medical Association during the all-virtual 2020 FMA annual meeting in August. Dr. Lenchus is a hospitalist and chief medical officer at the Broward Health Medical Center in Fort Lauderdale, Fla.
Christopher Carpenter, MD, has been elevated to chief of staff at Natividad, a 172-bed, county-owned hospital in Salinas, Calif. Dr. Carpenter has served Natividad for the past 4 years, holding the positions of chief hospitalist, chief of service for pediatrics, vice chief of staff, and most recently director of pediatric services.
Dr. Carpenter’s term as chief of staff is limited to 2 years, during which he said his goals include promoting diversity within the facility’s leadership.
Prior to arriving at Natividad, Dr. Carpenter was instructor of pediatrics at Harvard Medical School, Boston, as well as associate director of the Boston Children’s Hospital Pediatric Global Health Fellowship.
David Fagan, MD, recently was promoted to medical director at Mid-State Health Center (Plymouth, N.H.), where he has served for the past 10 years. The 30-year medical veteran began working in his new role in May 2020.
Previously, Dr. Fagan has served the facility as an internist and hospitalist, and he has been among the leaders at Mid-State in ensuring safety for patients and staff during the COVID-19 response.
The Carroll County Memorial Hospital (Carrolton, Mo.) recently announced its new hospitalist program, which officially began on June 1, 2020. CCMH officials said the focus of the hospitalists will be to maintain communication with primary care physicians once patients leave the hospital facility.
CCMH added three physicians to its staff to work in the hospitalist program: Reuben I. Thaker, MD; Samuel C. Evans, MD; and Charles C. Glendenning, DO.
NorthShore University HealthSystem (Evanston, Ill.) has agreed to purchase Northwest Community Healthcare, a single-hospital health system located in Arlington Heights, Ill. NCH will become a hospital hub for NorthShore in the northwest Chicago suburbs.
When the agreement is finalized, NorthShore’s stable of hospitals will rise to six in and around Chicago. The system also provides outpatient care, labwork, and pharmacy services.
Distinguishing COVID-19 from flu in kids remains challenging
For children with COVID-19, rates of hospitalization, ICU admission, and ventilator use were similar to those of children with influenza, but rates differed in other respects, according to results of a study published online Sept. 11 in JAMA Network Open.
As winter approaches, distinguishing patients with COVID-19 from those with influenza will become a problem. To assist with that, Xiaoyan Song, PhD, director of the office of infection control and epidemiology at Children’s National Hospital in Washington, D.C., and colleagues investigated commonalities and differences between the clinical symptoms of COVID-19 and influenza in children.
“Distinguishing COVID-19 from flu and other respiratory viral infections remains a challenge to clinicians. Although our study showed that patients with COVID-19 were more likely than patients with flu to report fever, gastrointestinal, and other clinical symptoms at the time of diagnosis, the two groups do have many overlapping clinical symptoms,” Dr. Song said. “Until future data show us otherwise, clinicians need to prepare for managing coinfections of COVID-19 with flu and/or other respiratory viral infections in the upcoming flu season.”
The retrospective cohort study included 315 children diagnosed with laboratory-confirmed COVID-19 between March 25 and May 15, 2020, and 1,402 children diagnosed with laboratory-confirmed seasonal influenza A or influenza B between Oct. 1, 2019, and June 6, 2020, at Children’s National Hospital. The investigation excluded asymptomatic patients who tested positive for COVID-19.
Patients with COVID-19 and patients with influenza were similar with respect to rates of hospitalization (17% vs. 21%; odds ratio, 0.8; 95% confidence interval, 0.6-1.1; P = .15), admission to the ICU (6% vs. 7%; OR, 0.8; 95% CI, 0.5-1.3; P = .42), and use of mechanical ventilation (3% vs. 2%; OR, 1.5; 95% CI, 0.9-2.6; P =.17).
The difference in the duration of ventilation for the two groups was not statistically significant. None of the patients who had COVID-19 or influenza B died, but two patients with influenza A did.
No patients had coinfections, which the researchers attribute to the mid-March shutdown of many schools, which they believe limited the spread of seasonal influenza.
Patients who were hospitalized with COVID-19 were older (median age, 9.7 years; range, 0.06-23.2 years) than those hospitalized with either type of influenza (median age, 4.2 years; range, 0.04-23.1). Patients older than 15 years made up 37% of patients with COVID-19 but only 6% of those with influenza.
Among patients hospitalized with COVID-19, 65% had at least one underlying medical condition, compared with 42% of those hospitalized for either type of influenza (OR, 2.6; 95% CI, 1.4-4.7; P = .002).
The most common underlying condition was neurologic problems from global developmental delay or seizures, identified in 11 patients (20%) hospitalized with COVID-19 and in 24 patients (8%) hospitalized with influenza (OR, 2.8; 95% CI, 1.3-6.2; P = .002). There was no significant difference between the two groups with respect to a history of asthma, cardiac disease, hematologic disease, and cancer.
For both groups, fever and cough were the most frequently reported symptoms at the time of diagnosis. However, more patients hospitalized with COVID-19 reported fever (76% vs. 55%; OR, 2.6; 95% CI, 1.4-5.1; P = 01), diarrhea or vomiting (26% vs. 12%; OR, 2.5; 95% CI, 1.2-5.0; P = .01), headache (11% vs. 3%; OR, 3.9; 95% CI, 1.3-11.5; P = .01), myalgia (22% vs. 7%; OR, 3.9; 95% CI, 1.8-8.5; P = .001), or chest pain (11% vs. 3%; OR, 3.9; 95% CI, 1.3-11.5; P = .01).
The researchers found no statistically significant differences between the two groups in rates of cough, congestion, sore throat, or shortness of breath.
Comparison of the symptom spectrum between COVID-19 and flu differed with respect to influenza type. More patients with COVID-19 reported fever, cough, diarrhea and vomiting, and myalgia than patients hospitalized with influenza A. But rates of fever, cough, diarrhea or vomiting, headache, or chest pain didn’t differ significantly in patients with COVID-19 and those with influenza B.
Larry K. Kociolek, MD, medical director of infection prevention and control at Ann and Robert H. Lurie Children’s Hospital of Chicago, noted the lower age of patients with flu. “Differentiating the two infections, which is difficult if not impossible based on symptoms alone, may have prognostic implications, depending on the age of the child. Because this study was performed outside peak influenza season, when coinfections would be less likely to occur, we must be vigilant about the potential clinical implications of influenza and SARS-CoV-2 coinfection this fall and winter.”
Clinicians will still have to use a combination of symptoms, examinations, and testing to distinguish the two diseases, said Aimee Sznewajs, MD, medical director of the pediatric hospital medicine department at Children’s Minnesota, Minneapolis. “We will continue to test for influenza and COVID-19 prior to hospitalizations and make decisions about whether to hospitalize based on other clinical factors, such as dehydration, oxygen requirement, and vital sign changes.”
Dr. Sznewajs stressed the importance of maintaining public health strategies, including “ensuring all children get the flu vaccine, encouraging mask wearing and hand hygiene, adequate testing to determine which virus is present, and other mitigation measures if the prevalence of COVID-19 is increasing in the community.”
Dr. Song reiterated those points, noting that clinicians need to make the most of the options they have. “Clinicians already have many great tools on hand. It is extremely important to get the flu vaccine now, especially for kids with underlying medical conditions. Diagnostic tests are available for both COVID-19 and flu. Antiviral treatment for flu is available. Judicious use of these tools will protect the health of providers, kids, and well-being at large.”
The authors noted several limitations for the study, including its retrospective design, that the data came from a single center, and that different platforms were used to detect the viruses.
A version of this article originally appeared on Medscape.com.
For children with COVID-19, rates of hospitalization, ICU admission, and ventilator use were similar to those of children with influenza, but rates differed in other respects, according to results of a study published online Sept. 11 in JAMA Network Open.
As winter approaches, distinguishing patients with COVID-19 from those with influenza will become a problem. To assist with that, Xiaoyan Song, PhD, director of the office of infection control and epidemiology at Children’s National Hospital in Washington, D.C., and colleagues investigated commonalities and differences between the clinical symptoms of COVID-19 and influenza in children.
“Distinguishing COVID-19 from flu and other respiratory viral infections remains a challenge to clinicians. Although our study showed that patients with COVID-19 were more likely than patients with flu to report fever, gastrointestinal, and other clinical symptoms at the time of diagnosis, the two groups do have many overlapping clinical symptoms,” Dr. Song said. “Until future data show us otherwise, clinicians need to prepare for managing coinfections of COVID-19 with flu and/or other respiratory viral infections in the upcoming flu season.”
The retrospective cohort study included 315 children diagnosed with laboratory-confirmed COVID-19 between March 25 and May 15, 2020, and 1,402 children diagnosed with laboratory-confirmed seasonal influenza A or influenza B between Oct. 1, 2019, and June 6, 2020, at Children’s National Hospital. The investigation excluded asymptomatic patients who tested positive for COVID-19.
Patients with COVID-19 and patients with influenza were similar with respect to rates of hospitalization (17% vs. 21%; odds ratio, 0.8; 95% confidence interval, 0.6-1.1; P = .15), admission to the ICU (6% vs. 7%; OR, 0.8; 95% CI, 0.5-1.3; P = .42), and use of mechanical ventilation (3% vs. 2%; OR, 1.5; 95% CI, 0.9-2.6; P =.17).
The difference in the duration of ventilation for the two groups was not statistically significant. None of the patients who had COVID-19 or influenza B died, but two patients with influenza A did.
No patients had coinfections, which the researchers attribute to the mid-March shutdown of many schools, which they believe limited the spread of seasonal influenza.
Patients who were hospitalized with COVID-19 were older (median age, 9.7 years; range, 0.06-23.2 years) than those hospitalized with either type of influenza (median age, 4.2 years; range, 0.04-23.1). Patients older than 15 years made up 37% of patients with COVID-19 but only 6% of those with influenza.
Among patients hospitalized with COVID-19, 65% had at least one underlying medical condition, compared with 42% of those hospitalized for either type of influenza (OR, 2.6; 95% CI, 1.4-4.7; P = .002).
The most common underlying condition was neurologic problems from global developmental delay or seizures, identified in 11 patients (20%) hospitalized with COVID-19 and in 24 patients (8%) hospitalized with influenza (OR, 2.8; 95% CI, 1.3-6.2; P = .002). There was no significant difference between the two groups with respect to a history of asthma, cardiac disease, hematologic disease, and cancer.
For both groups, fever and cough were the most frequently reported symptoms at the time of diagnosis. However, more patients hospitalized with COVID-19 reported fever (76% vs. 55%; OR, 2.6; 95% CI, 1.4-5.1; P = 01), diarrhea or vomiting (26% vs. 12%; OR, 2.5; 95% CI, 1.2-5.0; P = .01), headache (11% vs. 3%; OR, 3.9; 95% CI, 1.3-11.5; P = .01), myalgia (22% vs. 7%; OR, 3.9; 95% CI, 1.8-8.5; P = .001), or chest pain (11% vs. 3%; OR, 3.9; 95% CI, 1.3-11.5; P = .01).
The researchers found no statistically significant differences between the two groups in rates of cough, congestion, sore throat, or shortness of breath.
Comparison of the symptom spectrum between COVID-19 and flu differed with respect to influenza type. More patients with COVID-19 reported fever, cough, diarrhea and vomiting, and myalgia than patients hospitalized with influenza A. But rates of fever, cough, diarrhea or vomiting, headache, or chest pain didn’t differ significantly in patients with COVID-19 and those with influenza B.
Larry K. Kociolek, MD, medical director of infection prevention and control at Ann and Robert H. Lurie Children’s Hospital of Chicago, noted the lower age of patients with flu. “Differentiating the two infections, which is difficult if not impossible based on symptoms alone, may have prognostic implications, depending on the age of the child. Because this study was performed outside peak influenza season, when coinfections would be less likely to occur, we must be vigilant about the potential clinical implications of influenza and SARS-CoV-2 coinfection this fall and winter.”
Clinicians will still have to use a combination of symptoms, examinations, and testing to distinguish the two diseases, said Aimee Sznewajs, MD, medical director of the pediatric hospital medicine department at Children’s Minnesota, Minneapolis. “We will continue to test for influenza and COVID-19 prior to hospitalizations and make decisions about whether to hospitalize based on other clinical factors, such as dehydration, oxygen requirement, and vital sign changes.”
Dr. Sznewajs stressed the importance of maintaining public health strategies, including “ensuring all children get the flu vaccine, encouraging mask wearing and hand hygiene, adequate testing to determine which virus is present, and other mitigation measures if the prevalence of COVID-19 is increasing in the community.”
Dr. Song reiterated those points, noting that clinicians need to make the most of the options they have. “Clinicians already have many great tools on hand. It is extremely important to get the flu vaccine now, especially for kids with underlying medical conditions. Diagnostic tests are available for both COVID-19 and flu. Antiviral treatment for flu is available. Judicious use of these tools will protect the health of providers, kids, and well-being at large.”
The authors noted several limitations for the study, including its retrospective design, that the data came from a single center, and that different platforms were used to detect the viruses.
A version of this article originally appeared on Medscape.com.
For children with COVID-19, rates of hospitalization, ICU admission, and ventilator use were similar to those of children with influenza, but rates differed in other respects, according to results of a study published online Sept. 11 in JAMA Network Open.
As winter approaches, distinguishing patients with COVID-19 from those with influenza will become a problem. To assist with that, Xiaoyan Song, PhD, director of the office of infection control and epidemiology at Children’s National Hospital in Washington, D.C., and colleagues investigated commonalities and differences between the clinical symptoms of COVID-19 and influenza in children.
“Distinguishing COVID-19 from flu and other respiratory viral infections remains a challenge to clinicians. Although our study showed that patients with COVID-19 were more likely than patients with flu to report fever, gastrointestinal, and other clinical symptoms at the time of diagnosis, the two groups do have many overlapping clinical symptoms,” Dr. Song said. “Until future data show us otherwise, clinicians need to prepare for managing coinfections of COVID-19 with flu and/or other respiratory viral infections in the upcoming flu season.”
The retrospective cohort study included 315 children diagnosed with laboratory-confirmed COVID-19 between March 25 and May 15, 2020, and 1,402 children diagnosed with laboratory-confirmed seasonal influenza A or influenza B between Oct. 1, 2019, and June 6, 2020, at Children’s National Hospital. The investigation excluded asymptomatic patients who tested positive for COVID-19.
Patients with COVID-19 and patients with influenza were similar with respect to rates of hospitalization (17% vs. 21%; odds ratio, 0.8; 95% confidence interval, 0.6-1.1; P = .15), admission to the ICU (6% vs. 7%; OR, 0.8; 95% CI, 0.5-1.3; P = .42), and use of mechanical ventilation (3% vs. 2%; OR, 1.5; 95% CI, 0.9-2.6; P =.17).
The difference in the duration of ventilation for the two groups was not statistically significant. None of the patients who had COVID-19 or influenza B died, but two patients with influenza A did.
No patients had coinfections, which the researchers attribute to the mid-March shutdown of many schools, which they believe limited the spread of seasonal influenza.
Patients who were hospitalized with COVID-19 were older (median age, 9.7 years; range, 0.06-23.2 years) than those hospitalized with either type of influenza (median age, 4.2 years; range, 0.04-23.1). Patients older than 15 years made up 37% of patients with COVID-19 but only 6% of those with influenza.
Among patients hospitalized with COVID-19, 65% had at least one underlying medical condition, compared with 42% of those hospitalized for either type of influenza (OR, 2.6; 95% CI, 1.4-4.7; P = .002).
The most common underlying condition was neurologic problems from global developmental delay or seizures, identified in 11 patients (20%) hospitalized with COVID-19 and in 24 patients (8%) hospitalized with influenza (OR, 2.8; 95% CI, 1.3-6.2; P = .002). There was no significant difference between the two groups with respect to a history of asthma, cardiac disease, hematologic disease, and cancer.
For both groups, fever and cough were the most frequently reported symptoms at the time of diagnosis. However, more patients hospitalized with COVID-19 reported fever (76% vs. 55%; OR, 2.6; 95% CI, 1.4-5.1; P = 01), diarrhea or vomiting (26% vs. 12%; OR, 2.5; 95% CI, 1.2-5.0; P = .01), headache (11% vs. 3%; OR, 3.9; 95% CI, 1.3-11.5; P = .01), myalgia (22% vs. 7%; OR, 3.9; 95% CI, 1.8-8.5; P = .001), or chest pain (11% vs. 3%; OR, 3.9; 95% CI, 1.3-11.5; P = .01).
The researchers found no statistically significant differences between the two groups in rates of cough, congestion, sore throat, or shortness of breath.
Comparison of the symptom spectrum between COVID-19 and flu differed with respect to influenza type. More patients with COVID-19 reported fever, cough, diarrhea and vomiting, and myalgia than patients hospitalized with influenza A. But rates of fever, cough, diarrhea or vomiting, headache, or chest pain didn’t differ significantly in patients with COVID-19 and those with influenza B.
Larry K. Kociolek, MD, medical director of infection prevention and control at Ann and Robert H. Lurie Children’s Hospital of Chicago, noted the lower age of patients with flu. “Differentiating the two infections, which is difficult if not impossible based on symptoms alone, may have prognostic implications, depending on the age of the child. Because this study was performed outside peak influenza season, when coinfections would be less likely to occur, we must be vigilant about the potential clinical implications of influenza and SARS-CoV-2 coinfection this fall and winter.”
Clinicians will still have to use a combination of symptoms, examinations, and testing to distinguish the two diseases, said Aimee Sznewajs, MD, medical director of the pediatric hospital medicine department at Children’s Minnesota, Minneapolis. “We will continue to test for influenza and COVID-19 prior to hospitalizations and make decisions about whether to hospitalize based on other clinical factors, such as dehydration, oxygen requirement, and vital sign changes.”
Dr. Sznewajs stressed the importance of maintaining public health strategies, including “ensuring all children get the flu vaccine, encouraging mask wearing and hand hygiene, adequate testing to determine which virus is present, and other mitigation measures if the prevalence of COVID-19 is increasing in the community.”
Dr. Song reiterated those points, noting that clinicians need to make the most of the options they have. “Clinicians already have many great tools on hand. It is extremely important to get the flu vaccine now, especially for kids with underlying medical conditions. Diagnostic tests are available for both COVID-19 and flu. Antiviral treatment for flu is available. Judicious use of these tools will protect the health of providers, kids, and well-being at large.”
The authors noted several limitations for the study, including its retrospective design, that the data came from a single center, and that different platforms were used to detect the viruses.
A version of this article originally appeared on Medscape.com.
AI can pinpoint COVID-19 from chest x-rays
Conventional chest x-rays combined with artificial intelligence (AI) can identify lung damage from COVID-19 and differentiate coronavirus patients from other patients, improving triage efforts, new research suggests.
The AI tool – developed by Jason Fleischer, PhD, and graduate student Mohammad Tariqul Islam, both from Princeton (N.J.) University – can distinguish COVID-19 patients from those with pneumonia or normal lung tissue with an accuracy of more than 95%.
“We were able to separate the COVID-19 patients with very high fidelity,” Dr. Fleischer said in an interview. “If you give me an x-ray now, I can say with very high confidence whether a patient has COVID-19.”
The diagnostic tool pinpoints patterns on x-ray images that are too subtle for even trained experts to notice. The precision of CT scanning is similar to that of the AI tool, but CT costs much more and has other disadvantages, said Dr. Fleischer, who presented his findings at the virtual European Respiratory Society International Congress 2020.
“CT is more expensive and uses higher doses of radiation,” he said. “Another big thing is that not everyone has tomography facilities – including a lot of rural places and developing countries – so you need something that’s on the spot.”
With machine learning, Dr. Fleischer analyzed 2,300 x-ray images: 1,018 “normal” images from patients who had neither pneumonia nor COVID-19, 1,011 from patients with pneumonia, and 271 from patients with COVID-19.
The AI tool uses a neural network to refine the number and type of lung features being tracked. A UMAP (Uniform Manifold Approximation and Projection) clustering algorithm then looks for similarities and differences in those images, he explained.
“We, as users, knew which type each x-ray was – normal, pneumonia positive, or COVID-19 positive – but the network did not,” he added.
Clinicians have observed two basic types of lung problems in COVID-19 patients: pneumonia that fills lung air sacs with fluid and dangerously low blood-oxygen levels despite nearly normal breathing patterns. Because treatment can vary according to type, it would be beneficial to quickly distinguish between them, Dr. Fleischer said.
The AI tool showed that there is a distinct difference in chest x-rays from pneumonia-positive patients and healthy people, he said. It also demonstrated two distinct clusters of COVID-19–positive chest x-rays: those that looked like pneumonia and those with a more normal presentation.
The fact that “the AI system recognizes something unique in chest x-rays from COVID-19–positive patients” indicates that the computer is able to identify visual markers for coronavirus, he explained. “We currently do not know what these markers are.”
Dr. Fleischer said his goal is not to replace physician decision-making, but to supplement it.
“I’m uncomfortable with having computers make the final decision,” he said. “They often have a narrow focus, whereas doctors have the big picture in mind.”
This AI tool is “very interesting,” especially in the context of expanding AI applications in various specialties, said Thierry Fumeaux, MD, from Nyon (Switzerland) Hospital. Some physicians currently disagree on whether a chest x-ray or CT scan is the better tool to help diagnose COVID-19.
“It seems better than the human eye and brain” to pinpoint COVID-19 lung damage, “so it’s very attractive as a technology,” Dr. Fumeaux said in an interview.
And AI can be used to supplement the efforts of busy and fatigued clinicians who might be stretched thin by large caseloads. “I cannot read 200 chest x-rays in a day, but a computer can do that in 2 minutes,” he said.
But Dr. Fumeaux offered a caveat: “Pattern recognition is promising, but at the moment I’m not aware of papers showing that, by using AI, you’re changing anything in the outcome of a patient.”
Ideally, Dr. Fleischer said he hopes that AI will soon be able to accurately indicate which treatments are most effective for individual COVID-19 patients. And the technology might eventually be used to help with treatment decisions for patients with asthma or chronic obstructive pulmonary disease, he noted.
But he needs more data before results indicate whether a COVID-19 patient would benefit from ventilator support, for example, and the tool can be used more widely. To contribute data or collaborate with Dr. Fleischer’s efforts, contact him.
“Machine learning is all about data, so you can find these correlations,” he said. “It would be nice to be able to use it to reassure a worried patient that their prognosis is good; to say that most of the people with symptoms like yours will be just fine.”
Dr. Fleischer and Dr. Fumeaux have declared no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Conventional chest x-rays combined with artificial intelligence (AI) can identify lung damage from COVID-19 and differentiate coronavirus patients from other patients, improving triage efforts, new research suggests.
The AI tool – developed by Jason Fleischer, PhD, and graduate student Mohammad Tariqul Islam, both from Princeton (N.J.) University – can distinguish COVID-19 patients from those with pneumonia or normal lung tissue with an accuracy of more than 95%.
“We were able to separate the COVID-19 patients with very high fidelity,” Dr. Fleischer said in an interview. “If you give me an x-ray now, I can say with very high confidence whether a patient has COVID-19.”
The diagnostic tool pinpoints patterns on x-ray images that are too subtle for even trained experts to notice. The precision of CT scanning is similar to that of the AI tool, but CT costs much more and has other disadvantages, said Dr. Fleischer, who presented his findings at the virtual European Respiratory Society International Congress 2020.
“CT is more expensive and uses higher doses of radiation,” he said. “Another big thing is that not everyone has tomography facilities – including a lot of rural places and developing countries – so you need something that’s on the spot.”
With machine learning, Dr. Fleischer analyzed 2,300 x-ray images: 1,018 “normal” images from patients who had neither pneumonia nor COVID-19, 1,011 from patients with pneumonia, and 271 from patients with COVID-19.
The AI tool uses a neural network to refine the number and type of lung features being tracked. A UMAP (Uniform Manifold Approximation and Projection) clustering algorithm then looks for similarities and differences in those images, he explained.
“We, as users, knew which type each x-ray was – normal, pneumonia positive, or COVID-19 positive – but the network did not,” he added.
Clinicians have observed two basic types of lung problems in COVID-19 patients: pneumonia that fills lung air sacs with fluid and dangerously low blood-oxygen levels despite nearly normal breathing patterns. Because treatment can vary according to type, it would be beneficial to quickly distinguish between them, Dr. Fleischer said.
The AI tool showed that there is a distinct difference in chest x-rays from pneumonia-positive patients and healthy people, he said. It also demonstrated two distinct clusters of COVID-19–positive chest x-rays: those that looked like pneumonia and those with a more normal presentation.
The fact that “the AI system recognizes something unique in chest x-rays from COVID-19–positive patients” indicates that the computer is able to identify visual markers for coronavirus, he explained. “We currently do not know what these markers are.”
Dr. Fleischer said his goal is not to replace physician decision-making, but to supplement it.
“I’m uncomfortable with having computers make the final decision,” he said. “They often have a narrow focus, whereas doctors have the big picture in mind.”
This AI tool is “very interesting,” especially in the context of expanding AI applications in various specialties, said Thierry Fumeaux, MD, from Nyon (Switzerland) Hospital. Some physicians currently disagree on whether a chest x-ray or CT scan is the better tool to help diagnose COVID-19.
“It seems better than the human eye and brain” to pinpoint COVID-19 lung damage, “so it’s very attractive as a technology,” Dr. Fumeaux said in an interview.
And AI can be used to supplement the efforts of busy and fatigued clinicians who might be stretched thin by large caseloads. “I cannot read 200 chest x-rays in a day, but a computer can do that in 2 minutes,” he said.
But Dr. Fumeaux offered a caveat: “Pattern recognition is promising, but at the moment I’m not aware of papers showing that, by using AI, you’re changing anything in the outcome of a patient.”
Ideally, Dr. Fleischer said he hopes that AI will soon be able to accurately indicate which treatments are most effective for individual COVID-19 patients. And the technology might eventually be used to help with treatment decisions for patients with asthma or chronic obstructive pulmonary disease, he noted.
But he needs more data before results indicate whether a COVID-19 patient would benefit from ventilator support, for example, and the tool can be used more widely. To contribute data or collaborate with Dr. Fleischer’s efforts, contact him.
“Machine learning is all about data, so you can find these correlations,” he said. “It would be nice to be able to use it to reassure a worried patient that their prognosis is good; to say that most of the people with symptoms like yours will be just fine.”
Dr. Fleischer and Dr. Fumeaux have declared no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Conventional chest x-rays combined with artificial intelligence (AI) can identify lung damage from COVID-19 and differentiate coronavirus patients from other patients, improving triage efforts, new research suggests.
The AI tool – developed by Jason Fleischer, PhD, and graduate student Mohammad Tariqul Islam, both from Princeton (N.J.) University – can distinguish COVID-19 patients from those with pneumonia or normal lung tissue with an accuracy of more than 95%.
“We were able to separate the COVID-19 patients with very high fidelity,” Dr. Fleischer said in an interview. “If you give me an x-ray now, I can say with very high confidence whether a patient has COVID-19.”
The diagnostic tool pinpoints patterns on x-ray images that are too subtle for even trained experts to notice. The precision of CT scanning is similar to that of the AI tool, but CT costs much more and has other disadvantages, said Dr. Fleischer, who presented his findings at the virtual European Respiratory Society International Congress 2020.
“CT is more expensive and uses higher doses of radiation,” he said. “Another big thing is that not everyone has tomography facilities – including a lot of rural places and developing countries – so you need something that’s on the spot.”
With machine learning, Dr. Fleischer analyzed 2,300 x-ray images: 1,018 “normal” images from patients who had neither pneumonia nor COVID-19, 1,011 from patients with pneumonia, and 271 from patients with COVID-19.
The AI tool uses a neural network to refine the number and type of lung features being tracked. A UMAP (Uniform Manifold Approximation and Projection) clustering algorithm then looks for similarities and differences in those images, he explained.
“We, as users, knew which type each x-ray was – normal, pneumonia positive, or COVID-19 positive – but the network did not,” he added.
Clinicians have observed two basic types of lung problems in COVID-19 patients: pneumonia that fills lung air sacs with fluid and dangerously low blood-oxygen levels despite nearly normal breathing patterns. Because treatment can vary according to type, it would be beneficial to quickly distinguish between them, Dr. Fleischer said.
The AI tool showed that there is a distinct difference in chest x-rays from pneumonia-positive patients and healthy people, he said. It also demonstrated two distinct clusters of COVID-19–positive chest x-rays: those that looked like pneumonia and those with a more normal presentation.
The fact that “the AI system recognizes something unique in chest x-rays from COVID-19–positive patients” indicates that the computer is able to identify visual markers for coronavirus, he explained. “We currently do not know what these markers are.”
Dr. Fleischer said his goal is not to replace physician decision-making, but to supplement it.
“I’m uncomfortable with having computers make the final decision,” he said. “They often have a narrow focus, whereas doctors have the big picture in mind.”
This AI tool is “very interesting,” especially in the context of expanding AI applications in various specialties, said Thierry Fumeaux, MD, from Nyon (Switzerland) Hospital. Some physicians currently disagree on whether a chest x-ray or CT scan is the better tool to help diagnose COVID-19.
“It seems better than the human eye and brain” to pinpoint COVID-19 lung damage, “so it’s very attractive as a technology,” Dr. Fumeaux said in an interview.
And AI can be used to supplement the efforts of busy and fatigued clinicians who might be stretched thin by large caseloads. “I cannot read 200 chest x-rays in a day, but a computer can do that in 2 minutes,” he said.
But Dr. Fumeaux offered a caveat: “Pattern recognition is promising, but at the moment I’m not aware of papers showing that, by using AI, you’re changing anything in the outcome of a patient.”
Ideally, Dr. Fleischer said he hopes that AI will soon be able to accurately indicate which treatments are most effective for individual COVID-19 patients. And the technology might eventually be used to help with treatment decisions for patients with asthma or chronic obstructive pulmonary disease, he noted.
But he needs more data before results indicate whether a COVID-19 patient would benefit from ventilator support, for example, and the tool can be used more widely. To contribute data or collaborate with Dr. Fleischer’s efforts, contact him.
“Machine learning is all about data, so you can find these correlations,” he said. “It would be nice to be able to use it to reassure a worried patient that their prognosis is good; to say that most of the people with symptoms like yours will be just fine.”
Dr. Fleischer and Dr. Fumeaux have declared no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Social distancing impacts other infectious diseases
Diagnoses of 12 common pediatric infectious diseases in a large pediatric primary care network declined significantly in the weeks after COVID-19 social distancing (SD) was enacted in Massachusetts, compared with the same time period in 2019, an analysis of EHR data has shown.
While declines in infectious disease transmission with SD are not surprising, “these data demonstrate the extent to which transmission of common pediatric infections can be altered when close contact with other children is eliminated,” Jonathan Hatoun, MD, MPH of the Pediatric Physicians’ Organization at Children’s in Brookline, Mass., and coauthors wrote in Pediatrics . “Notably, three of the studied diseases, namely, influenza, croup, and bronchiolitis, essentially disappeared with [social distancing].”
The researchers analyzed the weekly incidence of each diagnosis for similar calendar periods in 2019 and 2020. A pre-SD period was defined as week 1-9, starting on Jan. 1, and a post-SD period was defined as week 13-18. (The several-week gap represented an implementation period as social distancing was enacted in the state earlier in 2020, from a declared statewide state of emergency through school closures and stay-at-home advisories.)
To isolate the effect of widespread SD, they performed a “difference-in-differences regression analysis, with diagnosis count as a function of calendar year, time period (pre-SD versus post-SD) and the interaction between the two.” The Massachusetts pediatric network provides care for approximately 375,000 children in 100 locations around the state.
In their research brief, Dr. Hatoun and coauthors presented weekly rates expressed as diagnoses per 100,000 patients per day. The rate of bronchiolitis, for instance, was 18 and 8 in the pre- and post-SD–equivalent weeks of 2019, respectively, and 20 and 0.6 in the pre- and post-SD weeks of 2020. Their analysis showed the rate in the 2020 post-SD period to be 10 diagnoses per 100,000 patients per day lower than they would have expected based on the 2019 trend.
Rates of pneumonia, acute otitis media, and streptococcal pharyngitis were similarly 14, 85, and 31 diagnoses per 100,000 patients per day lower, respectively. The prevalence of each of the other conditions analyzed – the common cold, croup, gastroenteritis, nonstreptococcal pharyngitis, sinusitis, skin and soft tissue infections, and urinary tract infection (UTI) – also was significantly lower in the 2020 post-SD period than would be expected based on 2019 data (P < .001 for all diagnoses).
Putting things in perspective
“This study puts numbers to the sense that we have all had in pediatrics – that social distancing appears to have had a dramatic impact on the transmission of common childhood infectious diseases, especially other respiratory viral pathogens,” Audrey R. John, MD, PhD, chief of the division of pediatric infectious disease at Children’s Hospital of Philadelphia, said in an interview.
The authors acknowledged the possible role of families not seeking care, but said that a smaller decrease in diagnoses of UTI – generally not a contagious disease – “suggests that changes in care-seeking behavior had a relatively modest effect on the other observed declines.” (The rate of UTI for the pre- and post-SD periods was 3.3 and 3.7 per 100,000 patients per day in 2019, and 3.4 and 2.4 in 2020, for a difference in differences of –1.5).
In an accompanying editorial, David W. Kimberlin, MD and Erica C. Bjornstad, MD, PhD, MPH, of the University of Alabama at Birmingham, called the report “provocative” and wrote that similar observations of infections dropping during periods of isolation – namely, dramatic declines in influenza and other respiratory viruses in Seattle after a record snowstorm in 2019 – combined with findings from other modeling studies “suggest that the decline [reported in Boston] is indeed real” (Pediatrics 2020. doi: 10.1542/peds.2020-019232).
However, “we also now know that immunization rates for American children have plummeted since the onset of the SARS-CoV-2 pandemic [because of a] ... dramatic decrease in the use of health care during the first months of the pandemic,” they wrote. “Viewed through this lens,” the declines reported in Boston may reflect inflections going “undiagnosed and untreated.”
Ultimately, Dr. Kimberlin and Dr. Bjornstad said, “the verdict remains out.”
Dr. John said that she and others are “concerned about children not seeking care in a timely manner, and [concerned] that reductions in reported infections might be due to a lack of recognition rather than a lack of transmission.”
In Philadelphia, however, declines in admissions for asthma exacerbations, “which are often caused by respiratory viral infections, suggests that this may not be the case,” said Dr. John, who was asked to comment on the study.
In addition, she said, the Massachusetts data showing that UTI diagnoses “are nearly as common this year as in 2019” are “reassuring.”
Are there lessons for the future?
Coauthor Louis Vernacchio, MD, MSc, chief medical officer of the Pediatric Physicians’ Organization at Children’s network, said in an interview that beyond the pandemic, it’s likely that “more careful attention to proven infection control practices in daycares and schools could reduce the burden of common infectious diseases in children.”
Dr. John similarly sees a long-term value of quantifying the impact of social distancing. “We’ve always known [for instance] that bronchiolitis is the result of viral infection.” Findings like the Massachusetts data “will help us advise families who might be trying to protect their premature infants (at risk for severe bronchiolitis) through social distancing.”
The analysis covered both in-person and telemedicine encounters occurring on weekdays.
The authors of the research brief indicated they have no relevant financial disclosures and there was no external funding. The authors of the commentary also reported they have no relevant financial disclosures, and Dr. John said she had no relevant financial disclosures.
SOURCE: Hatoun J et al. Pediatrics. 2020. doi: 10.1542/peds.2020-006460.
Diagnoses of 12 common pediatric infectious diseases in a large pediatric primary care network declined significantly in the weeks after COVID-19 social distancing (SD) was enacted in Massachusetts, compared with the same time period in 2019, an analysis of EHR data has shown.
While declines in infectious disease transmission with SD are not surprising, “these data demonstrate the extent to which transmission of common pediatric infections can be altered when close contact with other children is eliminated,” Jonathan Hatoun, MD, MPH of the Pediatric Physicians’ Organization at Children’s in Brookline, Mass., and coauthors wrote in Pediatrics . “Notably, three of the studied diseases, namely, influenza, croup, and bronchiolitis, essentially disappeared with [social distancing].”
The researchers analyzed the weekly incidence of each diagnosis for similar calendar periods in 2019 and 2020. A pre-SD period was defined as week 1-9, starting on Jan. 1, and a post-SD period was defined as week 13-18. (The several-week gap represented an implementation period as social distancing was enacted in the state earlier in 2020, from a declared statewide state of emergency through school closures and stay-at-home advisories.)
To isolate the effect of widespread SD, they performed a “difference-in-differences regression analysis, with diagnosis count as a function of calendar year, time period (pre-SD versus post-SD) and the interaction between the two.” The Massachusetts pediatric network provides care for approximately 375,000 children in 100 locations around the state.
In their research brief, Dr. Hatoun and coauthors presented weekly rates expressed as diagnoses per 100,000 patients per day. The rate of bronchiolitis, for instance, was 18 and 8 in the pre- and post-SD–equivalent weeks of 2019, respectively, and 20 and 0.6 in the pre- and post-SD weeks of 2020. Their analysis showed the rate in the 2020 post-SD period to be 10 diagnoses per 100,000 patients per day lower than they would have expected based on the 2019 trend.
Rates of pneumonia, acute otitis media, and streptococcal pharyngitis were similarly 14, 85, and 31 diagnoses per 100,000 patients per day lower, respectively. The prevalence of each of the other conditions analyzed – the common cold, croup, gastroenteritis, nonstreptococcal pharyngitis, sinusitis, skin and soft tissue infections, and urinary tract infection (UTI) – also was significantly lower in the 2020 post-SD period than would be expected based on 2019 data (P < .001 for all diagnoses).
Putting things in perspective
“This study puts numbers to the sense that we have all had in pediatrics – that social distancing appears to have had a dramatic impact on the transmission of common childhood infectious diseases, especially other respiratory viral pathogens,” Audrey R. John, MD, PhD, chief of the division of pediatric infectious disease at Children’s Hospital of Philadelphia, said in an interview.
The authors acknowledged the possible role of families not seeking care, but said that a smaller decrease in diagnoses of UTI – generally not a contagious disease – “suggests that changes in care-seeking behavior had a relatively modest effect on the other observed declines.” (The rate of UTI for the pre- and post-SD periods was 3.3 and 3.7 per 100,000 patients per day in 2019, and 3.4 and 2.4 in 2020, for a difference in differences of –1.5).
In an accompanying editorial, David W. Kimberlin, MD and Erica C. Bjornstad, MD, PhD, MPH, of the University of Alabama at Birmingham, called the report “provocative” and wrote that similar observations of infections dropping during periods of isolation – namely, dramatic declines in influenza and other respiratory viruses in Seattle after a record snowstorm in 2019 – combined with findings from other modeling studies “suggest that the decline [reported in Boston] is indeed real” (Pediatrics 2020. doi: 10.1542/peds.2020-019232).
However, “we also now know that immunization rates for American children have plummeted since the onset of the SARS-CoV-2 pandemic [because of a] ... dramatic decrease in the use of health care during the first months of the pandemic,” they wrote. “Viewed through this lens,” the declines reported in Boston may reflect inflections going “undiagnosed and untreated.”
Ultimately, Dr. Kimberlin and Dr. Bjornstad said, “the verdict remains out.”
Dr. John said that she and others are “concerned about children not seeking care in a timely manner, and [concerned] that reductions in reported infections might be due to a lack of recognition rather than a lack of transmission.”
In Philadelphia, however, declines in admissions for asthma exacerbations, “which are often caused by respiratory viral infections, suggests that this may not be the case,” said Dr. John, who was asked to comment on the study.
In addition, she said, the Massachusetts data showing that UTI diagnoses “are nearly as common this year as in 2019” are “reassuring.”
Are there lessons for the future?
Coauthor Louis Vernacchio, MD, MSc, chief medical officer of the Pediatric Physicians’ Organization at Children’s network, said in an interview that beyond the pandemic, it’s likely that “more careful attention to proven infection control practices in daycares and schools could reduce the burden of common infectious diseases in children.”
Dr. John similarly sees a long-term value of quantifying the impact of social distancing. “We’ve always known [for instance] that bronchiolitis is the result of viral infection.” Findings like the Massachusetts data “will help us advise families who might be trying to protect their premature infants (at risk for severe bronchiolitis) through social distancing.”
The analysis covered both in-person and telemedicine encounters occurring on weekdays.
The authors of the research brief indicated they have no relevant financial disclosures and there was no external funding. The authors of the commentary also reported they have no relevant financial disclosures, and Dr. John said she had no relevant financial disclosures.
SOURCE: Hatoun J et al. Pediatrics. 2020. doi: 10.1542/peds.2020-006460.
Diagnoses of 12 common pediatric infectious diseases in a large pediatric primary care network declined significantly in the weeks after COVID-19 social distancing (SD) was enacted in Massachusetts, compared with the same time period in 2019, an analysis of EHR data has shown.
While declines in infectious disease transmission with SD are not surprising, “these data demonstrate the extent to which transmission of common pediatric infections can be altered when close contact with other children is eliminated,” Jonathan Hatoun, MD, MPH of the Pediatric Physicians’ Organization at Children’s in Brookline, Mass., and coauthors wrote in Pediatrics . “Notably, three of the studied diseases, namely, influenza, croup, and bronchiolitis, essentially disappeared with [social distancing].”
The researchers analyzed the weekly incidence of each diagnosis for similar calendar periods in 2019 and 2020. A pre-SD period was defined as week 1-9, starting on Jan. 1, and a post-SD period was defined as week 13-18. (The several-week gap represented an implementation period as social distancing was enacted in the state earlier in 2020, from a declared statewide state of emergency through school closures and stay-at-home advisories.)
To isolate the effect of widespread SD, they performed a “difference-in-differences regression analysis, with diagnosis count as a function of calendar year, time period (pre-SD versus post-SD) and the interaction between the two.” The Massachusetts pediatric network provides care for approximately 375,000 children in 100 locations around the state.
In their research brief, Dr. Hatoun and coauthors presented weekly rates expressed as diagnoses per 100,000 patients per day. The rate of bronchiolitis, for instance, was 18 and 8 in the pre- and post-SD–equivalent weeks of 2019, respectively, and 20 and 0.6 in the pre- and post-SD weeks of 2020. Their analysis showed the rate in the 2020 post-SD period to be 10 diagnoses per 100,000 patients per day lower than they would have expected based on the 2019 trend.
Rates of pneumonia, acute otitis media, and streptococcal pharyngitis were similarly 14, 85, and 31 diagnoses per 100,000 patients per day lower, respectively. The prevalence of each of the other conditions analyzed – the common cold, croup, gastroenteritis, nonstreptococcal pharyngitis, sinusitis, skin and soft tissue infections, and urinary tract infection (UTI) – also was significantly lower in the 2020 post-SD period than would be expected based on 2019 data (P < .001 for all diagnoses).
Putting things in perspective
“This study puts numbers to the sense that we have all had in pediatrics – that social distancing appears to have had a dramatic impact on the transmission of common childhood infectious diseases, especially other respiratory viral pathogens,” Audrey R. John, MD, PhD, chief of the division of pediatric infectious disease at Children’s Hospital of Philadelphia, said in an interview.
The authors acknowledged the possible role of families not seeking care, but said that a smaller decrease in diagnoses of UTI – generally not a contagious disease – “suggests that changes in care-seeking behavior had a relatively modest effect on the other observed declines.” (The rate of UTI for the pre- and post-SD periods was 3.3 and 3.7 per 100,000 patients per day in 2019, and 3.4 and 2.4 in 2020, for a difference in differences of –1.5).
In an accompanying editorial, David W. Kimberlin, MD and Erica C. Bjornstad, MD, PhD, MPH, of the University of Alabama at Birmingham, called the report “provocative” and wrote that similar observations of infections dropping during periods of isolation – namely, dramatic declines in influenza and other respiratory viruses in Seattle after a record snowstorm in 2019 – combined with findings from other modeling studies “suggest that the decline [reported in Boston] is indeed real” (Pediatrics 2020. doi: 10.1542/peds.2020-019232).
However, “we also now know that immunization rates for American children have plummeted since the onset of the SARS-CoV-2 pandemic [because of a] ... dramatic decrease in the use of health care during the first months of the pandemic,” they wrote. “Viewed through this lens,” the declines reported in Boston may reflect inflections going “undiagnosed and untreated.”
Ultimately, Dr. Kimberlin and Dr. Bjornstad said, “the verdict remains out.”
Dr. John said that she and others are “concerned about children not seeking care in a timely manner, and [concerned] that reductions in reported infections might be due to a lack of recognition rather than a lack of transmission.”
In Philadelphia, however, declines in admissions for asthma exacerbations, “which are often caused by respiratory viral infections, suggests that this may not be the case,” said Dr. John, who was asked to comment on the study.
In addition, she said, the Massachusetts data showing that UTI diagnoses “are nearly as common this year as in 2019” are “reassuring.”
Are there lessons for the future?
Coauthor Louis Vernacchio, MD, MSc, chief medical officer of the Pediatric Physicians’ Organization at Children’s network, said in an interview that beyond the pandemic, it’s likely that “more careful attention to proven infection control practices in daycares and schools could reduce the burden of common infectious diseases in children.”
Dr. John similarly sees a long-term value of quantifying the impact of social distancing. “We’ve always known [for instance] that bronchiolitis is the result of viral infection.” Findings like the Massachusetts data “will help us advise families who might be trying to protect their premature infants (at risk for severe bronchiolitis) through social distancing.”
The analysis covered both in-person and telemedicine encounters occurring on weekdays.
The authors of the research brief indicated they have no relevant financial disclosures and there was no external funding. The authors of the commentary also reported they have no relevant financial disclosures, and Dr. John said she had no relevant financial disclosures.
SOURCE: Hatoun J et al. Pediatrics. 2020. doi: 10.1542/peds.2020-006460.
FROM PEDIATRICS