User login
Official news magazine of the Society of Hospital Medicine
Copyright by Society of Hospital Medicine or related companies. All rights reserved. ISSN 1553-085X
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'pane-pub-article-hospitalist')]


Residents curb IV antibiotic overuse in children
ATLANTA – It took less than a year to curb overuse of intravenous antibiotics at the Cincinnati Children’s Hospital, according to a report given at the Pediatric Hospital Medicine meeting.
Overuse of IV antibiotics – continuing IV formulations when oral formulations would work just as well – is a widespread concern in hospital medicine. Patients can often be switched to an oral antibiotic after an initial IV course. It lowers costs, lessens the risk of antimicrobial resistance, and reduces IV complications, but timely transitions don’t always happen.
They certainly weren’t happening at Cincinnati Children’s. “Despite a strong antimicrobial stewardship program, we identified a problem with overuse of IV antibiotics. The majority of pediatric patients admitted to an in-hospital service were started on IV antibiotics regardless of diagnosis or condition. Conversion to enteral antibiotics was often not considered until the day of discharge, even if patients were taking other enteral medications earlier in the admission,” said project leader Sonya Girdwood, MD, a research fellow at the hospital.
To get a handle on the problem, her team focused on two common IV antibiotics, ampicillin and clindamycin, that have oral equivalents with equal bioavailability: amoxicillin in the case of ampicillin, and oral clindamycin. To further define the project, they zeroed in on two common indications: clindamycin for uncomplicated skin and soft-tissue infections, and ampicillin for community-acquired pneumonia, in children over 2 months old.
The team figured that, if patients were able to take other oral medications, they should also be able to take oral antibiotics, so the goal of the project was to increase the rate of antibiotics given orally in children who were taking other enteral medications.
That percentage was 44% at baseline, and increased to 80% by month 8, saving an estimated $30,000 annually. There was no increase in 30-day readmissions. Length of stay held steady overall at about a day and half, but Dr. Girdwood suspected it might have been reduced for cellulitis.
Improvement efforts focused on residents and started in January 2017. Among the first lessons was that IV ampicillin is about 21 times more expensive than amoxicillin and that IV clindamycin is about twice as expensive as its oral formulation.
Residents were tasked with forming a plan at admission to transition children to oral antibiotics as soon as possible and to discuss those plans with attending physicians in preround huddles. Often, “this led to [transition] orders being placed even before rounds started,” Dr. Girdwood said.
A time was set up during evening huddles – 10 p.m. – for residents working overnight to discuss transition timing with attending. Failures – patients still on IV clindamycin or ampicillin when they were taking oral meds – were identified and shared with resident teams.
The gains have been maintained for almost a year with little backsliding; residents are reminded weekly of transition goals.
Children with skin and soft-tissue infections with bone or eye involvement were excluded from the project, along with pneumonia patients with chest tubes or complex or loculated effusions requiring a surgery consult.
There was no external funding, and the investigators had no disclosures.
ATLANTA – It took less than a year to curb overuse of intravenous antibiotics at the Cincinnati Children’s Hospital, according to a report given at the Pediatric Hospital Medicine meeting.
Overuse of IV antibiotics – continuing IV formulations when oral formulations would work just as well – is a widespread concern in hospital medicine. Patients can often be switched to an oral antibiotic after an initial IV course. It lowers costs, lessens the risk of antimicrobial resistance, and reduces IV complications, but timely transitions don’t always happen.
They certainly weren’t happening at Cincinnati Children’s. “Despite a strong antimicrobial stewardship program, we identified a problem with overuse of IV antibiotics. The majority of pediatric patients admitted to an in-hospital service were started on IV antibiotics regardless of diagnosis or condition. Conversion to enteral antibiotics was often not considered until the day of discharge, even if patients were taking other enteral medications earlier in the admission,” said project leader Sonya Girdwood, MD, a research fellow at the hospital.
To get a handle on the problem, her team focused on two common IV antibiotics, ampicillin and clindamycin, that have oral equivalents with equal bioavailability: amoxicillin in the case of ampicillin, and oral clindamycin. To further define the project, they zeroed in on two common indications: clindamycin for uncomplicated skin and soft-tissue infections, and ampicillin for community-acquired pneumonia, in children over 2 months old.
The team figured that, if patients were able to take other oral medications, they should also be able to take oral antibiotics, so the goal of the project was to increase the rate of antibiotics given orally in children who were taking other enteral medications.
That percentage was 44% at baseline, and increased to 80% by month 8, saving an estimated $30,000 annually. There was no increase in 30-day readmissions. Length of stay held steady overall at about a day and half, but Dr. Girdwood suspected it might have been reduced for cellulitis.
Improvement efforts focused on residents and started in January 2017. Among the first lessons was that IV ampicillin is about 21 times more expensive than amoxicillin and that IV clindamycin is about twice as expensive as its oral formulation.
Residents were tasked with forming a plan at admission to transition children to oral antibiotics as soon as possible and to discuss those plans with attending physicians in preround huddles. Often, “this led to [transition] orders being placed even before rounds started,” Dr. Girdwood said.
A time was set up during evening huddles – 10 p.m. – for residents working overnight to discuss transition timing with attending. Failures – patients still on IV clindamycin or ampicillin when they were taking oral meds – were identified and shared with resident teams.
The gains have been maintained for almost a year with little backsliding; residents are reminded weekly of transition goals.
Children with skin and soft-tissue infections with bone or eye involvement were excluded from the project, along with pneumonia patients with chest tubes or complex or loculated effusions requiring a surgery consult.
There was no external funding, and the investigators had no disclosures.
ATLANTA – It took less than a year to curb overuse of intravenous antibiotics at the Cincinnati Children’s Hospital, according to a report given at the Pediatric Hospital Medicine meeting.
Overuse of IV antibiotics – continuing IV formulations when oral formulations would work just as well – is a widespread concern in hospital medicine. Patients can often be switched to an oral antibiotic after an initial IV course. It lowers costs, lessens the risk of antimicrobial resistance, and reduces IV complications, but timely transitions don’t always happen.
They certainly weren’t happening at Cincinnati Children’s. “Despite a strong antimicrobial stewardship program, we identified a problem with overuse of IV antibiotics. The majority of pediatric patients admitted to an in-hospital service were started on IV antibiotics regardless of diagnosis or condition. Conversion to enteral antibiotics was often not considered until the day of discharge, even if patients were taking other enteral medications earlier in the admission,” said project leader Sonya Girdwood, MD, a research fellow at the hospital.
To get a handle on the problem, her team focused on two common IV antibiotics, ampicillin and clindamycin, that have oral equivalents with equal bioavailability: amoxicillin in the case of ampicillin, and oral clindamycin. To further define the project, they zeroed in on two common indications: clindamycin for uncomplicated skin and soft-tissue infections, and ampicillin for community-acquired pneumonia, in children over 2 months old.
The team figured that, if patients were able to take other oral medications, they should also be able to take oral antibiotics, so the goal of the project was to increase the rate of antibiotics given orally in children who were taking other enteral medications.
That percentage was 44% at baseline, and increased to 80% by month 8, saving an estimated $30,000 annually. There was no increase in 30-day readmissions. Length of stay held steady overall at about a day and half, but Dr. Girdwood suspected it might have been reduced for cellulitis.
Improvement efforts focused on residents and started in January 2017. Among the first lessons was that IV ampicillin is about 21 times more expensive than amoxicillin and that IV clindamycin is about twice as expensive as its oral formulation.
Residents were tasked with forming a plan at admission to transition children to oral antibiotics as soon as possible and to discuss those plans with attending physicians in preround huddles. Often, “this led to [transition] orders being placed even before rounds started,” Dr. Girdwood said.
A time was set up during evening huddles – 10 p.m. – for residents working overnight to discuss transition timing with attending. Failures – patients still on IV clindamycin or ampicillin when they were taking oral meds – were identified and shared with resident teams.
The gains have been maintained for almost a year with little backsliding; residents are reminded weekly of transition goals.
Children with skin and soft-tissue infections with bone or eye involvement were excluded from the project, along with pneumonia patients with chest tubes or complex or loculated effusions requiring a surgery consult.
There was no external funding, and the investigators had no disclosures.
REPORTING FROM PHM 2018
Key clinical point:
Major finding: The percentage of antibiotics given orally to children who were taking other enteral medications rose from 44% to 80% over 8 months, saving an estimated $30,000 annually.
Study details: Quality improvement project
Disclosures: There was no external funding, and the investigators didn’t have any disclosures.
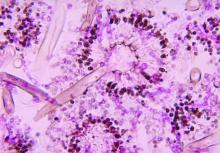
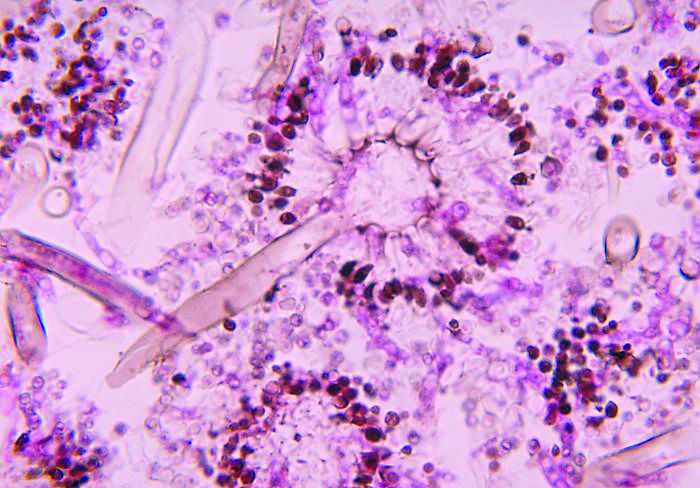
Severe influenza increases risk of invasive pulmonary aspergillosis in the ICU
Severe influenza is an independent risk factor for invasive pulmonary aspergillosis with an accompanying increased mortality in the ICU, according to a multicenter retrospective cohort study at seven tertiary centers in Belgium and the Netherlands.
Data was collected from criteria-meeting adult patients admitted to the ICU for more than 24 hours with acute respiratory failure during the 2009-2016 influenza seasons. The included cohort of 432 patients was composed of 56% men and had a median age of 59 years; all participants were diagnosed as having severe type A or type B influenza infection according to positive airway RT-PCR results.
The full cohort was subcategorized into 117 immunocompromised and 315 as nonimmunocompromised individuals using criteria established by the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study group (EORTC/MSG) . To assess influenza as an independent variable in the development of invasive pulmonary aspergillosis, the 315 nonimmunocompromised influenza positive individuals were compared to an influenza-negative control group of 315 nonimmunocompromised patients admitted to the ICU that presented similar respiratory insufficiency symptoms with community-acquired pneumonia.
Determination of other independent risk factors for incidence of invasive pulmonary aspergillosis was achieved by multivariate analysis of factors such as sex, diabetes status, prednisone use, age, and acute physiology and chronic health evaluation (APACHE) II score. The mean APACHE II score was 22, with the majority of patients requiring intubation for mechanical ventilation for a median duration of 11 days.
Influenza is not considered a host factor for invasive pulmonary aspergillosis and will often miss being diagnosed when using strict interpretation of the current EORTC/MSG or AspICU algorithm criteria, according to the researchers. Consequently for patients with influenza and the noninfluenza control group with community-acquired pneumonia, the definition of invasive pulmonary aspergillosis was modified from the AspICU algorithm. Stringent mycological criteria, including bronchoaveolar lavage (BAL) culture, a positive Aspergillus culture, positive galactomannan test, and/or positive serum galactomannan tests, provided supporting diagnostics for an invasive pulmonary aspergillosis determination.
At a median of 3 days following admission to the ICU, a diagnosis of invasive pulmonary aspergillosis was determined for 19% of the 432 influenza patients. Similar incident percentages of invasive pulmonary aspergillosis occurring for type A and type B, 71/355 (20%) and 12/77 (16%) patients respectively, showed that there was no clear association of the disease development with influenza subtypes that occurred during different annual seasons.
AspICU or EORTC/MSG criteria characterized only 43% and 58% of cases as proven or possible aspergillosis, respectively. On the other hand, stringent mycological tests yielded better invasive pulmonary aspergillosis classification, with 63% of BAL cultures being positive for Aspergillus, 88% of BAL galactomannan tests being positive, and 65% of serum galactomannan tests being positive in the 81/83 patients tested.
The study found that, for influenza patients, being immunocompromised more than doubled the incidence of invasive pulmonary aspergillosis, at 32% versus the 14% of those patients who were nonimmunocompromised. In contrast only 5% in the control group developed invasive pulmonary aspergillosis.
Influenza patients who developed invasive pulmonary aspergillosis in the ICU tended to have their stays significantly lengthened from 9 days (interquartile range, 5-20 days) for those without it to 19 days (IQR, 12-38 days) for those infected (P less than .0001). Likewise, 90-day mortality significantly rose from 28% for those influenza patients without invasive pulmonary aspergillosis to 51% for those with it (P = .0001).
The authors concluded that influenza was “independently associated with invasive pulmonary aspergillosis (adjusted odds ratio, 5.19; P less than.0001) along with a higher APACHE II score, male sex, and use of corticosteroids.”
Furthermore, as influenza appears to be an independent risk factor for invasive pulmonary aspergillosis and its associated high mortality, the authors suggested that “future studies should assess whether a faster diagnosis or antifungal prophylaxis could improve the outcome of influenza-associated aspergillosis.”
The authors reported that they had no conflicts of interest.
SOURCE: Schauwvlieghe AFAD et al. Lancet Respir Med. 2018 Jul 31. doi: 10.1016/S2213-2600(18)30274-1
Severe influenza is an independent risk factor for invasive pulmonary aspergillosis with an accompanying increased mortality in the ICU, according to a multicenter retrospective cohort study at seven tertiary centers in Belgium and the Netherlands.
Data was collected from criteria-meeting adult patients admitted to the ICU for more than 24 hours with acute respiratory failure during the 2009-2016 influenza seasons. The included cohort of 432 patients was composed of 56% men and had a median age of 59 years; all participants were diagnosed as having severe type A or type B influenza infection according to positive airway RT-PCR results.
The full cohort was subcategorized into 117 immunocompromised and 315 as nonimmunocompromised individuals using criteria established by the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study group (EORTC/MSG) . To assess influenza as an independent variable in the development of invasive pulmonary aspergillosis, the 315 nonimmunocompromised influenza positive individuals were compared to an influenza-negative control group of 315 nonimmunocompromised patients admitted to the ICU that presented similar respiratory insufficiency symptoms with community-acquired pneumonia.
Determination of other independent risk factors for incidence of invasive pulmonary aspergillosis was achieved by multivariate analysis of factors such as sex, diabetes status, prednisone use, age, and acute physiology and chronic health evaluation (APACHE) II score. The mean APACHE II score was 22, with the majority of patients requiring intubation for mechanical ventilation for a median duration of 11 days.
Influenza is not considered a host factor for invasive pulmonary aspergillosis and will often miss being diagnosed when using strict interpretation of the current EORTC/MSG or AspICU algorithm criteria, according to the researchers. Consequently for patients with influenza and the noninfluenza control group with community-acquired pneumonia, the definition of invasive pulmonary aspergillosis was modified from the AspICU algorithm. Stringent mycological criteria, including bronchoaveolar lavage (BAL) culture, a positive Aspergillus culture, positive galactomannan test, and/or positive serum galactomannan tests, provided supporting diagnostics for an invasive pulmonary aspergillosis determination.
At a median of 3 days following admission to the ICU, a diagnosis of invasive pulmonary aspergillosis was determined for 19% of the 432 influenza patients. Similar incident percentages of invasive pulmonary aspergillosis occurring for type A and type B, 71/355 (20%) and 12/77 (16%) patients respectively, showed that there was no clear association of the disease development with influenza subtypes that occurred during different annual seasons.
AspICU or EORTC/MSG criteria characterized only 43% and 58% of cases as proven or possible aspergillosis, respectively. On the other hand, stringent mycological tests yielded better invasive pulmonary aspergillosis classification, with 63% of BAL cultures being positive for Aspergillus, 88% of BAL galactomannan tests being positive, and 65% of serum galactomannan tests being positive in the 81/83 patients tested.
The study found that, for influenza patients, being immunocompromised more than doubled the incidence of invasive pulmonary aspergillosis, at 32% versus the 14% of those patients who were nonimmunocompromised. In contrast only 5% in the control group developed invasive pulmonary aspergillosis.
Influenza patients who developed invasive pulmonary aspergillosis in the ICU tended to have their stays significantly lengthened from 9 days (interquartile range, 5-20 days) for those without it to 19 days (IQR, 12-38 days) for those infected (P less than .0001). Likewise, 90-day mortality significantly rose from 28% for those influenza patients without invasive pulmonary aspergillosis to 51% for those with it (P = .0001).
The authors concluded that influenza was “independently associated with invasive pulmonary aspergillosis (adjusted odds ratio, 5.19; P less than.0001) along with a higher APACHE II score, male sex, and use of corticosteroids.”
Furthermore, as influenza appears to be an independent risk factor for invasive pulmonary aspergillosis and its associated high mortality, the authors suggested that “future studies should assess whether a faster diagnosis or antifungal prophylaxis could improve the outcome of influenza-associated aspergillosis.”
The authors reported that they had no conflicts of interest.
SOURCE: Schauwvlieghe AFAD et al. Lancet Respir Med. 2018 Jul 31. doi: 10.1016/S2213-2600(18)30274-1
Severe influenza is an independent risk factor for invasive pulmonary aspergillosis with an accompanying increased mortality in the ICU, according to a multicenter retrospective cohort study at seven tertiary centers in Belgium and the Netherlands.
Data was collected from criteria-meeting adult patients admitted to the ICU for more than 24 hours with acute respiratory failure during the 2009-2016 influenza seasons. The included cohort of 432 patients was composed of 56% men and had a median age of 59 years; all participants were diagnosed as having severe type A or type B influenza infection according to positive airway RT-PCR results.
The full cohort was subcategorized into 117 immunocompromised and 315 as nonimmunocompromised individuals using criteria established by the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study group (EORTC/MSG) . To assess influenza as an independent variable in the development of invasive pulmonary aspergillosis, the 315 nonimmunocompromised influenza positive individuals were compared to an influenza-negative control group of 315 nonimmunocompromised patients admitted to the ICU that presented similar respiratory insufficiency symptoms with community-acquired pneumonia.
Determination of other independent risk factors for incidence of invasive pulmonary aspergillosis was achieved by multivariate analysis of factors such as sex, diabetes status, prednisone use, age, and acute physiology and chronic health evaluation (APACHE) II score. The mean APACHE II score was 22, with the majority of patients requiring intubation for mechanical ventilation for a median duration of 11 days.
Influenza is not considered a host factor for invasive pulmonary aspergillosis and will often miss being diagnosed when using strict interpretation of the current EORTC/MSG or AspICU algorithm criteria, according to the researchers. Consequently for patients with influenza and the noninfluenza control group with community-acquired pneumonia, the definition of invasive pulmonary aspergillosis was modified from the AspICU algorithm. Stringent mycological criteria, including bronchoaveolar lavage (BAL) culture, a positive Aspergillus culture, positive galactomannan test, and/or positive serum galactomannan tests, provided supporting diagnostics for an invasive pulmonary aspergillosis determination.
At a median of 3 days following admission to the ICU, a diagnosis of invasive pulmonary aspergillosis was determined for 19% of the 432 influenza patients. Similar incident percentages of invasive pulmonary aspergillosis occurring for type A and type B, 71/355 (20%) and 12/77 (16%) patients respectively, showed that there was no clear association of the disease development with influenza subtypes that occurred during different annual seasons.
AspICU or EORTC/MSG criteria characterized only 43% and 58% of cases as proven or possible aspergillosis, respectively. On the other hand, stringent mycological tests yielded better invasive pulmonary aspergillosis classification, with 63% of BAL cultures being positive for Aspergillus, 88% of BAL galactomannan tests being positive, and 65% of serum galactomannan tests being positive in the 81/83 patients tested.
The study found that, for influenza patients, being immunocompromised more than doubled the incidence of invasive pulmonary aspergillosis, at 32% versus the 14% of those patients who were nonimmunocompromised. In contrast only 5% in the control group developed invasive pulmonary aspergillosis.
Influenza patients who developed invasive pulmonary aspergillosis in the ICU tended to have their stays significantly lengthened from 9 days (interquartile range, 5-20 days) for those without it to 19 days (IQR, 12-38 days) for those infected (P less than .0001). Likewise, 90-day mortality significantly rose from 28% for those influenza patients without invasive pulmonary aspergillosis to 51% for those with it (P = .0001).
The authors concluded that influenza was “independently associated with invasive pulmonary aspergillosis (adjusted odds ratio, 5.19; P less than.0001) along with a higher APACHE II score, male sex, and use of corticosteroids.”
Furthermore, as influenza appears to be an independent risk factor for invasive pulmonary aspergillosis and its associated high mortality, the authors suggested that “future studies should assess whether a faster diagnosis or antifungal prophylaxis could improve the outcome of influenza-associated aspergillosis.”
The authors reported that they had no conflicts of interest.
SOURCE: Schauwvlieghe AFAD et al. Lancet Respir Med. 2018 Jul 31. doi: 10.1016/S2213-2600(18)30274-1
FROM THE LANCET RESPIRATORY MEDICINE
Key clinical point: ICU admission for severe influenza as significant a risk factor should be included in the existing diagnostic criteria for predicting incidence of invasive pulmonary aspergillosis.
Major finding: Influenza is an independent risk factor associated with invasive pulmonary aspergillosis, with 90-day mortality rising from 28% to 51% when this fungal infection occurs.
Study details: Multicenter retrospective study of 432 adult patients with confirmed severe influenza admitted to the ICU with acute respiratory failure.
Disclosures: The authors reported that they had no conflicts of interest.
Source: Schauwvlieghe AFAD et al. Lancet Respir Med. 2018 Jul 31. doi: 10.1016/S2213-2600(18)30274-1.
Troponin I: Powerful all-cause mortality risk marker in COPD
PARIS – High relative even after researchers adjusted for all major cardiovascular and COPD prognostic indicators, according to a late-breaker presentation at the annual congress of the European Respiratory Society.
Troponin I is detectable in the plasma of most patients with COPD, but relative increases in troponin I correlate with greater relative increases in most cardiovascular and COPD risk factors, according to Benjamin Waschki, MD, Pulmonary Research Institute, LungenClinic, Grosshansdorf, Germany.
The relationship between increased troponin I and increased all-cause mortality was observed in an on-going prospective multicenter cohort of COPD patients followed at 31 centers in Germany. The cohort is called COSYCONET and it began in 2010. The current analysis evaluated 2,020 COPD patients without regard to stage of disease.
There were 136 deaths over the course of follow-up. Without adjustment, the hazard ratio (HR) for death was more than twofold higher in the highest quartile of troponin I (equal to or greater than 6.6 ng/mL), when compared with the lowest (under 2.5 ng/mL) (HR, 2.42; P less than .001). Graphically, the mortality curves for each of the quartiles began to separate at about 12 months, widening in a stepwise manner for greater likelihood of death from the lowest to highest quartiles.
The risk of death from any cause remained elevated for the highest relative to lowest troponin I quartiles after adjusting for cardiovascular risk factors and after adjusting for COPD severity. Again, there was a distinct stepwise separation of the mortality curves for each higher troponin quartile,
Of particular importance, troponin I remained predictive beyond the BODE index, which is a currently employed prognostic mortality predictor in COPD, according to Dr. Waschki. When defining elevated troponin as greater than 6 ng/ML and a high BODE score as greater than 4, mortality was higher for those with a high BODE and low troponin than a high troponin and low BODE, (P less than .001), but a high troponin I was associated with a higher risk of mortality when BODE was low (P less than .001). Moreover, when both troponin I and BODE were elevated, all-cause mortality was more than doubled, relative to those without either risk factor (HR, 2.56; P = .003), Dr. Waschki reported.
After researchers adjusted for major cardiovascular risk factors, such as history of MI and renal impairment, and for major COPD risk factors, such as 6-minute walk test and BODE index, those in the highest quartile had a more than 50% greater risk of death relative to those in the lower quartile over the 3 years of follow-up (HR, 1.69; P = .007), according to Dr. Waschki.
Although troponin I is best known for its diagnostic role in MI, it is now being evaluated as a risk stratifier for many chronic diseases, such as heart failure and chronic kidney disease, explained Dr. Waschki in providing background for this study. He reported that many groups are looking at this as a marker of risk in a variety of chronic diseases.
In fact, a group working independently published a study in COPD just weeks before the ERS Congress that was complementary to those presented by Dr. Waschki. In this study, the goal was to evaluate troponin I as a predictor of cardiovascular events and cardiovascular death (Adamson PD et al. J Am Coll Cardiol 2018;72:1126-37). Performed as a subgroup analysis of 1,599 COPD patients participating in a large treatment trial, there was an almost fourfold increase in the risk of cardiovascular events (HR, 3.7; P = .012) when those in the highest quintile of troponin I (greater than 7.7 ng/ML) were compared with those in the lowest quintile (less than 2.3 ng/mL).
When compared for cardiovascular death, the highest quintile, relative to the lowest quintile, had a more than 20-fold increased risk of cardiovascular death (HR 20.1; P = .005). In the Adamson et al. study, which evaluated inhaled therapies for COPD, treatment response had no impact on troponin I levels or on the risk of cardiovascular events or death.
Based on this study and his own data, Dr. Waschki believes troponin I, which is readily ordered laboratory value, appears to be a useful tool for identifying COPD patients at high risk of death.
“The major message is that after adjusting for all known COPD and cardiovascular risk factors, troponin I remains a significant independent predictor of mortality,” he said.
Dr. Waschki reports no relevant conflicts of interest.
PARIS – High relative even after researchers adjusted for all major cardiovascular and COPD prognostic indicators, according to a late-breaker presentation at the annual congress of the European Respiratory Society.
Troponin I is detectable in the plasma of most patients with COPD, but relative increases in troponin I correlate with greater relative increases in most cardiovascular and COPD risk factors, according to Benjamin Waschki, MD, Pulmonary Research Institute, LungenClinic, Grosshansdorf, Germany.
The relationship between increased troponin I and increased all-cause mortality was observed in an on-going prospective multicenter cohort of COPD patients followed at 31 centers in Germany. The cohort is called COSYCONET and it began in 2010. The current analysis evaluated 2,020 COPD patients without regard to stage of disease.
There were 136 deaths over the course of follow-up. Without adjustment, the hazard ratio (HR) for death was more than twofold higher in the highest quartile of troponin I (equal to or greater than 6.6 ng/mL), when compared with the lowest (under 2.5 ng/mL) (HR, 2.42; P less than .001). Graphically, the mortality curves for each of the quartiles began to separate at about 12 months, widening in a stepwise manner for greater likelihood of death from the lowest to highest quartiles.
The risk of death from any cause remained elevated for the highest relative to lowest troponin I quartiles after adjusting for cardiovascular risk factors and after adjusting for COPD severity. Again, there was a distinct stepwise separation of the mortality curves for each higher troponin quartile,
Of particular importance, troponin I remained predictive beyond the BODE index, which is a currently employed prognostic mortality predictor in COPD, according to Dr. Waschki. When defining elevated troponin as greater than 6 ng/ML and a high BODE score as greater than 4, mortality was higher for those with a high BODE and low troponin than a high troponin and low BODE, (P less than .001), but a high troponin I was associated with a higher risk of mortality when BODE was low (P less than .001). Moreover, when both troponin I and BODE were elevated, all-cause mortality was more than doubled, relative to those without either risk factor (HR, 2.56; P = .003), Dr. Waschki reported.
After researchers adjusted for major cardiovascular risk factors, such as history of MI and renal impairment, and for major COPD risk factors, such as 6-minute walk test and BODE index, those in the highest quartile had a more than 50% greater risk of death relative to those in the lower quartile over the 3 years of follow-up (HR, 1.69; P = .007), according to Dr. Waschki.
Although troponin I is best known for its diagnostic role in MI, it is now being evaluated as a risk stratifier for many chronic diseases, such as heart failure and chronic kidney disease, explained Dr. Waschki in providing background for this study. He reported that many groups are looking at this as a marker of risk in a variety of chronic diseases.
In fact, a group working independently published a study in COPD just weeks before the ERS Congress that was complementary to those presented by Dr. Waschki. In this study, the goal was to evaluate troponin I as a predictor of cardiovascular events and cardiovascular death (Adamson PD et al. J Am Coll Cardiol 2018;72:1126-37). Performed as a subgroup analysis of 1,599 COPD patients participating in a large treatment trial, there was an almost fourfold increase in the risk of cardiovascular events (HR, 3.7; P = .012) when those in the highest quintile of troponin I (greater than 7.7 ng/ML) were compared with those in the lowest quintile (less than 2.3 ng/mL).
When compared for cardiovascular death, the highest quintile, relative to the lowest quintile, had a more than 20-fold increased risk of cardiovascular death (HR 20.1; P = .005). In the Adamson et al. study, which evaluated inhaled therapies for COPD, treatment response had no impact on troponin I levels or on the risk of cardiovascular events or death.
Based on this study and his own data, Dr. Waschki believes troponin I, which is readily ordered laboratory value, appears to be a useful tool for identifying COPD patients at high risk of death.
“The major message is that after adjusting for all known COPD and cardiovascular risk factors, troponin I remains a significant independent predictor of mortality,” he said.
Dr. Waschki reports no relevant conflicts of interest.
PARIS – High relative even after researchers adjusted for all major cardiovascular and COPD prognostic indicators, according to a late-breaker presentation at the annual congress of the European Respiratory Society.
Troponin I is detectable in the plasma of most patients with COPD, but relative increases in troponin I correlate with greater relative increases in most cardiovascular and COPD risk factors, according to Benjamin Waschki, MD, Pulmonary Research Institute, LungenClinic, Grosshansdorf, Germany.
The relationship between increased troponin I and increased all-cause mortality was observed in an on-going prospective multicenter cohort of COPD patients followed at 31 centers in Germany. The cohort is called COSYCONET and it began in 2010. The current analysis evaluated 2,020 COPD patients without regard to stage of disease.
There were 136 deaths over the course of follow-up. Without adjustment, the hazard ratio (HR) for death was more than twofold higher in the highest quartile of troponin I (equal to or greater than 6.6 ng/mL), when compared with the lowest (under 2.5 ng/mL) (HR, 2.42; P less than .001). Graphically, the mortality curves for each of the quartiles began to separate at about 12 months, widening in a stepwise manner for greater likelihood of death from the lowest to highest quartiles.
The risk of death from any cause remained elevated for the highest relative to lowest troponin I quartiles after adjusting for cardiovascular risk factors and after adjusting for COPD severity. Again, there was a distinct stepwise separation of the mortality curves for each higher troponin quartile,
Of particular importance, troponin I remained predictive beyond the BODE index, which is a currently employed prognostic mortality predictor in COPD, according to Dr. Waschki. When defining elevated troponin as greater than 6 ng/ML and a high BODE score as greater than 4, mortality was higher for those with a high BODE and low troponin than a high troponin and low BODE, (P less than .001), but a high troponin I was associated with a higher risk of mortality when BODE was low (P less than .001). Moreover, when both troponin I and BODE were elevated, all-cause mortality was more than doubled, relative to those without either risk factor (HR, 2.56; P = .003), Dr. Waschki reported.
After researchers adjusted for major cardiovascular risk factors, such as history of MI and renal impairment, and for major COPD risk factors, such as 6-minute walk test and BODE index, those in the highest quartile had a more than 50% greater risk of death relative to those in the lower quartile over the 3 years of follow-up (HR, 1.69; P = .007), according to Dr. Waschki.
Although troponin I is best known for its diagnostic role in MI, it is now being evaluated as a risk stratifier for many chronic diseases, such as heart failure and chronic kidney disease, explained Dr. Waschki in providing background for this study. He reported that many groups are looking at this as a marker of risk in a variety of chronic diseases.
In fact, a group working independently published a study in COPD just weeks before the ERS Congress that was complementary to those presented by Dr. Waschki. In this study, the goal was to evaluate troponin I as a predictor of cardiovascular events and cardiovascular death (Adamson PD et al. J Am Coll Cardiol 2018;72:1126-37). Performed as a subgroup analysis of 1,599 COPD patients participating in a large treatment trial, there was an almost fourfold increase in the risk of cardiovascular events (HR, 3.7; P = .012) when those in the highest quintile of troponin I (greater than 7.7 ng/ML) were compared with those in the lowest quintile (less than 2.3 ng/mL).
When compared for cardiovascular death, the highest quintile, relative to the lowest quintile, had a more than 20-fold increased risk of cardiovascular death (HR 20.1; P = .005). In the Adamson et al. study, which evaluated inhaled therapies for COPD, treatment response had no impact on troponin I levels or on the risk of cardiovascular events or death.
Based on this study and his own data, Dr. Waschki believes troponin I, which is readily ordered laboratory value, appears to be a useful tool for identifying COPD patients at high risk of death.
“The major message is that after adjusting for all known COPD and cardiovascular risk factors, troponin I remains a significant independent predictor of mortality,” he said.
Dr. Waschki reports no relevant conflicts of interest.
REPORTING FROM ERS CONGRESS 2018
Key clinical point: Elevated troponin I identifies COPD patients with increased mortality risk independent of all other clinical risk markers.
Major finding: With high troponin I levels, all-cause mortality was increased 69% after researchers adjusted for other risk markers.
Study details: Analysis drawn from on-going multicenter cohort study
Disclosures: Dr. Waschki reports no relevant conflicts of interest.
Trump, not health care, likely focus of midterm elections
Provider community must be “creative and participative”
Come November 2018, Americans will return to the polls to vote for their representatives in Congress, for governors, and for state legislative seats.
Health care has been a topic of debate since the 2016 elections brought a Republican sweep to the executive and legislative branches, but other issues have since moved to the forefront. Will the midterm elections this year prove health care to be a significant issue at the polls?
Unlikely, said Robert Berenson, MD, FACP, Institute Fellow of the Health Policy Center at the Urban Institute. More likely, the election will be a referendum on President Donald Trump, he said. “Things are so partisan right now and it’s all about Trump. I don’t see serious discussion about health policy.”
Ron Greeno, MD, MHM, FCCP, immediate past president of SHM and former chair of the Public Policy Committee, also doesn’t see health care rising to the top of election year issues. But that doesn’t mean health care doesn’t matter to American voters.
“Whether Democrats control the House or Republicans control the House won’t likely make a big difference in terms of impact on the things we care about,” said Dr. Greeno. “The issues they debate in Washington are not going to save the health care system. They are just debating about who is going to pay for what and for whom. To save our health care system, we have to lower the cost of care and only providers can do that.”
What the government can do, he said, is create the right incentives for providers to move away from fee for service and participate in new models that may lower the cost of care. At the same time, “the economy also has to grow at a robust pace, which will make a huge difference. So, recent increases in economic growth rate are welcomed,” said Dr. Greeno.
In 2015, Republicans and Democrats came together to pass bipartisan legislation aimed at moving the health care system away from fee for service: the Medicare and CHIP Reauthorization Act, or MACRA.
However, the law has not been without frustrations, and these concerns will likely not be part of any candidate campaigns in 2018, Dr. Greeno predicted: “There’s not a lot of appetite to reopen the statute (more than) 2 years after it passed.”
MACRA provides clinicians two pathways to reimbursement. The first track, called MIPS (Merit-Based Incentive Payment System), bases a portion of physician reimbursement on scores measured across several categories, including cost and quality. It still operates largely under a fee-for-service framework but is meant to be budget neutral; for every winner there is a loser.
The second track, called the APMs (Alternative Payment Models), requires physicians to take on substantial risk (with potential for reward), if they can achieve specific patient volumes under approved models. However, few providers qualify, especially among hospitalists, though the structure of the program makes it clear that the Centers for Medicare & Medicaid Services intends to have most providers ultimately transition to APMs.
“There’s growing recognition that MACRA, at least the MIPS portion, was a big mistake but Congress can’t go back and say we blew it,” Dr. Berenson said. “CMS has now exempted somewhere between 550,000 and 900,000 clinicians from MACRA,” because they cannot meet the requirements of either pathway without significant hardship.1
CMS wasn’t considering hospitalists specifically when implementing the law, though hospitalists admit half of the Medicare patients in the United States, Dr. Greeno said. There are very few hospitalists currently participating in Advanced APMs and those that are, do not see the volume of patients the pathway requires.
“What hospitalists do is very conducive to alternative payment models, and we can help those alternative payment models drive improved quality and lowered costs,” said Dr. Greeno. “Hospitals use hospitalists to help them manage risk, so it’s frustrating that most hospitalists will not meet the thresholds for the APM track and benefit from the incentives created.”
However, the Society of Hospital Medicine continues to work on behalf of hospitalists. Thanks to its efforts, Dr. Greeno explained, CMS is planning in 2019 to allow hospitalists to choose to be scored under MIPS based on their hospital’s performance across reporting categories. Or, they can choose to report on their own and opt out of this new “facility-based” option.
“We are working with (CMS) to figure out how to make this new option work,” said Dr. Greeno.
At the state level, 36 governorships are up for grabs and those outcomes could influence the direction of Medicaid. In Kentucky, the Trump administration approved a waiver allowing the state to enforce work requirements for Medicaid recipients. However, on June 29, 2018, the D.C. federal district court invalidated the Kentucky HEALTH waiver approval (with the exception of Kentucky’s IMD SUD [institutions for mental disease for substance use disorders] payment waiver authority) and sent it back to HHS to reconsider. Ten other states as of August 2018 had applied for similar waivers.2 However, Dr. Berenson believes that most of what could happen to Medicaid will be a topic after the midterm elections and not before.
He also believes drug prices could become an issue in national elections, though there will not be an easy solution from either side. “Democrats will be reluctant to say they’re going to negotiate drug prices; they’re going to want the government to negotiate for Medicare-like pricing.” Republicans, on the other hand, will be reluctant to consider government regulation.
As a general principle leading into the midterms: “Democrats want to avoid an internal war about whether they are for Medicare for all or single payer or not,” Dr. Berenson said. “What I’m hoping doesn’t happen is that it becomes a litmus test for purity where you have to be for single payer. I think would be huge mistake because it’s not realistic that it would ever get there.”
However, he cites an idea from left-leaning Princeton University’s Paul Starr, a professor of sociology and public affairs, that Democrats could consider: so-called Midlife Medicare, an option that could be made available to Americans beginning at age 50 years.3 It would represent a new Medicare option, funded by general revenues and premiums, available to people age 50 years and older and those younger than 65 years who are without employer-sponsored health insurance.
Regardless, as the United States catapults toward another election that could disrupt the political system or maintain the relative status quo, Dr. Greeno said hospitalists continue to play key roles in improving American health care.
“There are programs in place where we can get the job done if we in the provider community are creative and participative,” he said. “Some of the most important work being done is coming out of the CMS Innovation Center. Hospitalists continue to be a big part of that, but we knew it would take decades of really hard work and I don’t see anything happening in the midterms to derail this or bring about a massive increase in the pace of change.”
References
1. Dickson V. CMS gives more small practices a pass on MACRA. Modern Healthcare. Published June 20, 2017.
2. Medicaid Waiver Tracker: Which States Have Approved and Pending Section 1115 Medicaid Waivers? Kaiser Family Foundation. Published Aug. 8, 2018.
3. Starr P. A new strategy for health care. The American Prospect. Published Jan. 4, 2018. Accessed March 5, 2018.
Provider community must be “creative and participative”
Provider community must be “creative and participative”
Come November 2018, Americans will return to the polls to vote for their representatives in Congress, for governors, and for state legislative seats.
Health care has been a topic of debate since the 2016 elections brought a Republican sweep to the executive and legislative branches, but other issues have since moved to the forefront. Will the midterm elections this year prove health care to be a significant issue at the polls?
Unlikely, said Robert Berenson, MD, FACP, Institute Fellow of the Health Policy Center at the Urban Institute. More likely, the election will be a referendum on President Donald Trump, he said. “Things are so partisan right now and it’s all about Trump. I don’t see serious discussion about health policy.”
Ron Greeno, MD, MHM, FCCP, immediate past president of SHM and former chair of the Public Policy Committee, also doesn’t see health care rising to the top of election year issues. But that doesn’t mean health care doesn’t matter to American voters.
“Whether Democrats control the House or Republicans control the House won’t likely make a big difference in terms of impact on the things we care about,” said Dr. Greeno. “The issues they debate in Washington are not going to save the health care system. They are just debating about who is going to pay for what and for whom. To save our health care system, we have to lower the cost of care and only providers can do that.”
What the government can do, he said, is create the right incentives for providers to move away from fee for service and participate in new models that may lower the cost of care. At the same time, “the economy also has to grow at a robust pace, which will make a huge difference. So, recent increases in economic growth rate are welcomed,” said Dr. Greeno.
In 2015, Republicans and Democrats came together to pass bipartisan legislation aimed at moving the health care system away from fee for service: the Medicare and CHIP Reauthorization Act, or MACRA.
However, the law has not been without frustrations, and these concerns will likely not be part of any candidate campaigns in 2018, Dr. Greeno predicted: “There’s not a lot of appetite to reopen the statute (more than) 2 years after it passed.”
MACRA provides clinicians two pathways to reimbursement. The first track, called MIPS (Merit-Based Incentive Payment System), bases a portion of physician reimbursement on scores measured across several categories, including cost and quality. It still operates largely under a fee-for-service framework but is meant to be budget neutral; for every winner there is a loser.
The second track, called the APMs (Alternative Payment Models), requires physicians to take on substantial risk (with potential for reward), if they can achieve specific patient volumes under approved models. However, few providers qualify, especially among hospitalists, though the structure of the program makes it clear that the Centers for Medicare & Medicaid Services intends to have most providers ultimately transition to APMs.
“There’s growing recognition that MACRA, at least the MIPS portion, was a big mistake but Congress can’t go back and say we blew it,” Dr. Berenson said. “CMS has now exempted somewhere between 550,000 and 900,000 clinicians from MACRA,” because they cannot meet the requirements of either pathway without significant hardship.1
CMS wasn’t considering hospitalists specifically when implementing the law, though hospitalists admit half of the Medicare patients in the United States, Dr. Greeno said. There are very few hospitalists currently participating in Advanced APMs and those that are, do not see the volume of patients the pathway requires.
“What hospitalists do is very conducive to alternative payment models, and we can help those alternative payment models drive improved quality and lowered costs,” said Dr. Greeno. “Hospitals use hospitalists to help them manage risk, so it’s frustrating that most hospitalists will not meet the thresholds for the APM track and benefit from the incentives created.”
However, the Society of Hospital Medicine continues to work on behalf of hospitalists. Thanks to its efforts, Dr. Greeno explained, CMS is planning in 2019 to allow hospitalists to choose to be scored under MIPS based on their hospital’s performance across reporting categories. Or, they can choose to report on their own and opt out of this new “facility-based” option.
“We are working with (CMS) to figure out how to make this new option work,” said Dr. Greeno.
At the state level, 36 governorships are up for grabs and those outcomes could influence the direction of Medicaid. In Kentucky, the Trump administration approved a waiver allowing the state to enforce work requirements for Medicaid recipients. However, on June 29, 2018, the D.C. federal district court invalidated the Kentucky HEALTH waiver approval (with the exception of Kentucky’s IMD SUD [institutions for mental disease for substance use disorders] payment waiver authority) and sent it back to HHS to reconsider. Ten other states as of August 2018 had applied for similar waivers.2 However, Dr. Berenson believes that most of what could happen to Medicaid will be a topic after the midterm elections and not before.
He also believes drug prices could become an issue in national elections, though there will not be an easy solution from either side. “Democrats will be reluctant to say they’re going to negotiate drug prices; they’re going to want the government to negotiate for Medicare-like pricing.” Republicans, on the other hand, will be reluctant to consider government regulation.
As a general principle leading into the midterms: “Democrats want to avoid an internal war about whether they are for Medicare for all or single payer or not,” Dr. Berenson said. “What I’m hoping doesn’t happen is that it becomes a litmus test for purity where you have to be for single payer. I think would be huge mistake because it’s not realistic that it would ever get there.”
However, he cites an idea from left-leaning Princeton University’s Paul Starr, a professor of sociology and public affairs, that Democrats could consider: so-called Midlife Medicare, an option that could be made available to Americans beginning at age 50 years.3 It would represent a new Medicare option, funded by general revenues and premiums, available to people age 50 years and older and those younger than 65 years who are without employer-sponsored health insurance.
Regardless, as the United States catapults toward another election that could disrupt the political system or maintain the relative status quo, Dr. Greeno said hospitalists continue to play key roles in improving American health care.
“There are programs in place where we can get the job done if we in the provider community are creative and participative,” he said. “Some of the most important work being done is coming out of the CMS Innovation Center. Hospitalists continue to be a big part of that, but we knew it would take decades of really hard work and I don’t see anything happening in the midterms to derail this or bring about a massive increase in the pace of change.”
References
1. Dickson V. CMS gives more small practices a pass on MACRA. Modern Healthcare. Published June 20, 2017.
2. Medicaid Waiver Tracker: Which States Have Approved and Pending Section 1115 Medicaid Waivers? Kaiser Family Foundation. Published Aug. 8, 2018.
3. Starr P. A new strategy for health care. The American Prospect. Published Jan. 4, 2018. Accessed March 5, 2018.
Come November 2018, Americans will return to the polls to vote for their representatives in Congress, for governors, and for state legislative seats.
Health care has been a topic of debate since the 2016 elections brought a Republican sweep to the executive and legislative branches, but other issues have since moved to the forefront. Will the midterm elections this year prove health care to be a significant issue at the polls?
Unlikely, said Robert Berenson, MD, FACP, Institute Fellow of the Health Policy Center at the Urban Institute. More likely, the election will be a referendum on President Donald Trump, he said. “Things are so partisan right now and it’s all about Trump. I don’t see serious discussion about health policy.”
Ron Greeno, MD, MHM, FCCP, immediate past president of SHM and former chair of the Public Policy Committee, also doesn’t see health care rising to the top of election year issues. But that doesn’t mean health care doesn’t matter to American voters.
“Whether Democrats control the House or Republicans control the House won’t likely make a big difference in terms of impact on the things we care about,” said Dr. Greeno. “The issues they debate in Washington are not going to save the health care system. They are just debating about who is going to pay for what and for whom. To save our health care system, we have to lower the cost of care and only providers can do that.”
What the government can do, he said, is create the right incentives for providers to move away from fee for service and participate in new models that may lower the cost of care. At the same time, “the economy also has to grow at a robust pace, which will make a huge difference. So, recent increases in economic growth rate are welcomed,” said Dr. Greeno.
In 2015, Republicans and Democrats came together to pass bipartisan legislation aimed at moving the health care system away from fee for service: the Medicare and CHIP Reauthorization Act, or MACRA.
However, the law has not been without frustrations, and these concerns will likely not be part of any candidate campaigns in 2018, Dr. Greeno predicted: “There’s not a lot of appetite to reopen the statute (more than) 2 years after it passed.”
MACRA provides clinicians two pathways to reimbursement. The first track, called MIPS (Merit-Based Incentive Payment System), bases a portion of physician reimbursement on scores measured across several categories, including cost and quality. It still operates largely under a fee-for-service framework but is meant to be budget neutral; for every winner there is a loser.
The second track, called the APMs (Alternative Payment Models), requires physicians to take on substantial risk (with potential for reward), if they can achieve specific patient volumes under approved models. However, few providers qualify, especially among hospitalists, though the structure of the program makes it clear that the Centers for Medicare & Medicaid Services intends to have most providers ultimately transition to APMs.
“There’s growing recognition that MACRA, at least the MIPS portion, was a big mistake but Congress can’t go back and say we blew it,” Dr. Berenson said. “CMS has now exempted somewhere between 550,000 and 900,000 clinicians from MACRA,” because they cannot meet the requirements of either pathway without significant hardship.1
CMS wasn’t considering hospitalists specifically when implementing the law, though hospitalists admit half of the Medicare patients in the United States, Dr. Greeno said. There are very few hospitalists currently participating in Advanced APMs and those that are, do not see the volume of patients the pathway requires.
“What hospitalists do is very conducive to alternative payment models, and we can help those alternative payment models drive improved quality and lowered costs,” said Dr. Greeno. “Hospitals use hospitalists to help them manage risk, so it’s frustrating that most hospitalists will not meet the thresholds for the APM track and benefit from the incentives created.”
However, the Society of Hospital Medicine continues to work on behalf of hospitalists. Thanks to its efforts, Dr. Greeno explained, CMS is planning in 2019 to allow hospitalists to choose to be scored under MIPS based on their hospital’s performance across reporting categories. Or, they can choose to report on their own and opt out of this new “facility-based” option.
“We are working with (CMS) to figure out how to make this new option work,” said Dr. Greeno.
At the state level, 36 governorships are up for grabs and those outcomes could influence the direction of Medicaid. In Kentucky, the Trump administration approved a waiver allowing the state to enforce work requirements for Medicaid recipients. However, on June 29, 2018, the D.C. federal district court invalidated the Kentucky HEALTH waiver approval (with the exception of Kentucky’s IMD SUD [institutions for mental disease for substance use disorders] payment waiver authority) and sent it back to HHS to reconsider. Ten other states as of August 2018 had applied for similar waivers.2 However, Dr. Berenson believes that most of what could happen to Medicaid will be a topic after the midterm elections and not before.
He also believes drug prices could become an issue in national elections, though there will not be an easy solution from either side. “Democrats will be reluctant to say they’re going to negotiate drug prices; they’re going to want the government to negotiate for Medicare-like pricing.” Republicans, on the other hand, will be reluctant to consider government regulation.
As a general principle leading into the midterms: “Democrats want to avoid an internal war about whether they are for Medicare for all or single payer or not,” Dr. Berenson said. “What I’m hoping doesn’t happen is that it becomes a litmus test for purity where you have to be for single payer. I think would be huge mistake because it’s not realistic that it would ever get there.”
However, he cites an idea from left-leaning Princeton University’s Paul Starr, a professor of sociology and public affairs, that Democrats could consider: so-called Midlife Medicare, an option that could be made available to Americans beginning at age 50 years.3 It would represent a new Medicare option, funded by general revenues and premiums, available to people age 50 years and older and those younger than 65 years who are without employer-sponsored health insurance.
Regardless, as the United States catapults toward another election that could disrupt the political system or maintain the relative status quo, Dr. Greeno said hospitalists continue to play key roles in improving American health care.
“There are programs in place where we can get the job done if we in the provider community are creative and participative,” he said. “Some of the most important work being done is coming out of the CMS Innovation Center. Hospitalists continue to be a big part of that, but we knew it would take decades of really hard work and I don’t see anything happening in the midterms to derail this or bring about a massive increase in the pace of change.”
References
1. Dickson V. CMS gives more small practices a pass on MACRA. Modern Healthcare. Published June 20, 2017.
2. Medicaid Waiver Tracker: Which States Have Approved and Pending Section 1115 Medicaid Waivers? Kaiser Family Foundation. Published Aug. 8, 2018.
3. Starr P. A new strategy for health care. The American Prospect. Published Jan. 4, 2018. Accessed March 5, 2018.
Most in-hospital pneumonia deaths may not be preventable
Most in-hospital deaths from community-acquired pneumonia are not preventable with current medical therapy, according to an analysis of deaths at five U.S. hospitals with expertise in pneumonia care.
Adults who are hospitalized with community-acquired pneumonia (CAP) are at high risk for short-term mortality but it is unclear whether an improvement in care could lower this risk, noted the study authors led by Grant W. Waterer, MBBS, PhD, of Northwestern University, Chicago.
“Understanding the circumstances in which CAP patients die could facilitate improvements in the management of CAP by enabling future improvement efforts to focus on common preventable causes of death,” they wrote. Their report was published in CHEST®.
They therefore performed a secondary analysis of the Etiology of Pneumonia in the Community (EPIC) study involving adults hospitalized with CAP between January 2010 and June 2012 across five tertiary-care hospitals in the United States.
The clinical characteristics of patients who died in the hospital were compared with those of patients who survived to hospital discharge. Chronic heart failure, chronic obstructive pulmonary disease, coronary artery disease, chronic liver disease, cerebrovascular disease, cancer (excluding skin cancer), and diabetes were considered as severe chronic comorbidities based on their association with increased mortality and ICU admission in CAP severity scores.
Deaths caused by septic shock, respiratory failure, multisystem organ failure, cardiopulmonary arrest prior to stabilization of CAP, and endocarditis, were considered to be directly related to CAP.
Conversely, causes of death indirectly related to CAP included acute cardiovascular disease, stroke, acute renal failure, and secondary infections developed after hospitalization. Deaths caused by cancer, cirrhosis, and chronic neurologic conditions were considered unrelated to CAP.
Medical notes were assessed to determine whether the patient received management consistent with current recommendations; for example, antibiotics consistent with guidelines from the Infectious Diseases Society of America.
End-of-life limitations in care, such as patient/family decision not to proceed with full medical treatment, also were considered by the research team.
Results showed that among the 2,320 patients with radiographically confirmed CAP, 52 died during initial hospitalization, 33 of whom were aged 65 years or older, and 32 of whom had two or more chronic comorbidities.
Most of the in-hospital deaths occurred early in the hospitalization: 35 within the first 10 days of admission, and 5 after 30 days in hospital.
CAP was judged by an expert physician review panel to be the direct cause of death in 27 of the patients, 10 with CAP having an indirect role with major contribution, 9 with CAP having an indirect role with minor contribution, and 6 with CAP having no role in death.
Do-not-resuscitate orders were present at the time of death for 21 of the patients.
Forty-five of the patients were admitted to an ICU, with 37 dying in the ICU. The eight patients who died on the ward after transfer out of the ICU had end-of-life limitations of care in place.
The researchers noted that the number of patients dying in the ICU was greater in the United States, possibly because in Europe fewer patients are admitted to an ICU.
“This discrepancy likely reflects cultural differences between the U.S. and Europe in the role of intensive care for patients with advanced age and/or advanced comorbid conditions,” they noted.
Overall, the physician review panel identified nine patients who had a lapse in quality of in-hospital CAP care, with four of the deaths potentially linked to this lapse in care.
However, two of the patients had end-of-life limitations of care in place, which according to the authors meant that “only two patients undergoing full medical treatment without end-of-life limitations of care had an identified lapse in quality of in-hospital pneumonia care potentially contributing to in-hospital death, including one with a delay in antibiotics for over an hour in the presence of shock and one with initial antibiotics not consistent with IDSA/ATS guidelines.”
The research team concluded that most in-hospital deaths among adult patients admitted with CAP in their study would not have been preventable with higher quality in-hospital pneumonia care.
“Many of the in-hospital deaths among patients admitted with CAP occurred in older patients with severe comorbidities and end-of-life limitations in care,” they noted.
They said the influence of end-of-life limitations on care short of full palliation was an important finding, with all patients who died outside the ICU having end-of-life limitations in care.
“Current diagnostic related group (DRG) and international classification of diseases (ICD) coding systems do not have the necessary nuances to capture these limitations of care, yet they are clearly important factors in determining whether patients experience in-hospital death,” they added.
Dr. Waterer reported no conflicts. Two coauthors reported potential conflicts of interest in relation to consulting fees from several pharmaceutical companies.
SOURCE: Waterer G. et al. CHEST 2018;154(3):628-35. doi: 10.1016/j.chest.2018.05.021.
Most in-hospital deaths from community-acquired pneumonia are not preventable with current medical therapy, according to an analysis of deaths at five U.S. hospitals with expertise in pneumonia care.
Adults who are hospitalized with community-acquired pneumonia (CAP) are at high risk for short-term mortality but it is unclear whether an improvement in care could lower this risk, noted the study authors led by Grant W. Waterer, MBBS, PhD, of Northwestern University, Chicago.
“Understanding the circumstances in which CAP patients die could facilitate improvements in the management of CAP by enabling future improvement efforts to focus on common preventable causes of death,” they wrote. Their report was published in CHEST®.
They therefore performed a secondary analysis of the Etiology of Pneumonia in the Community (EPIC) study involving adults hospitalized with CAP between January 2010 and June 2012 across five tertiary-care hospitals in the United States.
The clinical characteristics of patients who died in the hospital were compared with those of patients who survived to hospital discharge. Chronic heart failure, chronic obstructive pulmonary disease, coronary artery disease, chronic liver disease, cerebrovascular disease, cancer (excluding skin cancer), and diabetes were considered as severe chronic comorbidities based on their association with increased mortality and ICU admission in CAP severity scores.
Deaths caused by septic shock, respiratory failure, multisystem organ failure, cardiopulmonary arrest prior to stabilization of CAP, and endocarditis, were considered to be directly related to CAP.
Conversely, causes of death indirectly related to CAP included acute cardiovascular disease, stroke, acute renal failure, and secondary infections developed after hospitalization. Deaths caused by cancer, cirrhosis, and chronic neurologic conditions were considered unrelated to CAP.
Medical notes were assessed to determine whether the patient received management consistent with current recommendations; for example, antibiotics consistent with guidelines from the Infectious Diseases Society of America.
End-of-life limitations in care, such as patient/family decision not to proceed with full medical treatment, also were considered by the research team.
Results showed that among the 2,320 patients with radiographically confirmed CAP, 52 died during initial hospitalization, 33 of whom were aged 65 years or older, and 32 of whom had two or more chronic comorbidities.
Most of the in-hospital deaths occurred early in the hospitalization: 35 within the first 10 days of admission, and 5 after 30 days in hospital.
CAP was judged by an expert physician review panel to be the direct cause of death in 27 of the patients, 10 with CAP having an indirect role with major contribution, 9 with CAP having an indirect role with minor contribution, and 6 with CAP having no role in death.
Do-not-resuscitate orders were present at the time of death for 21 of the patients.
Forty-five of the patients were admitted to an ICU, with 37 dying in the ICU. The eight patients who died on the ward after transfer out of the ICU had end-of-life limitations of care in place.
The researchers noted that the number of patients dying in the ICU was greater in the United States, possibly because in Europe fewer patients are admitted to an ICU.
“This discrepancy likely reflects cultural differences between the U.S. and Europe in the role of intensive care for patients with advanced age and/or advanced comorbid conditions,” they noted.
Overall, the physician review panel identified nine patients who had a lapse in quality of in-hospital CAP care, with four of the deaths potentially linked to this lapse in care.
However, two of the patients had end-of-life limitations of care in place, which according to the authors meant that “only two patients undergoing full medical treatment without end-of-life limitations of care had an identified lapse in quality of in-hospital pneumonia care potentially contributing to in-hospital death, including one with a delay in antibiotics for over an hour in the presence of shock and one with initial antibiotics not consistent with IDSA/ATS guidelines.”
The research team concluded that most in-hospital deaths among adult patients admitted with CAP in their study would not have been preventable with higher quality in-hospital pneumonia care.
“Many of the in-hospital deaths among patients admitted with CAP occurred in older patients with severe comorbidities and end-of-life limitations in care,” they noted.
They said the influence of end-of-life limitations on care short of full palliation was an important finding, with all patients who died outside the ICU having end-of-life limitations in care.
“Current diagnostic related group (DRG) and international classification of diseases (ICD) coding systems do not have the necessary nuances to capture these limitations of care, yet they are clearly important factors in determining whether patients experience in-hospital death,” they added.
Dr. Waterer reported no conflicts. Two coauthors reported potential conflicts of interest in relation to consulting fees from several pharmaceutical companies.
SOURCE: Waterer G. et al. CHEST 2018;154(3):628-35. doi: 10.1016/j.chest.2018.05.021.
Most in-hospital deaths from community-acquired pneumonia are not preventable with current medical therapy, according to an analysis of deaths at five U.S. hospitals with expertise in pneumonia care.
Adults who are hospitalized with community-acquired pneumonia (CAP) are at high risk for short-term mortality but it is unclear whether an improvement in care could lower this risk, noted the study authors led by Grant W. Waterer, MBBS, PhD, of Northwestern University, Chicago.
“Understanding the circumstances in which CAP patients die could facilitate improvements in the management of CAP by enabling future improvement efforts to focus on common preventable causes of death,” they wrote. Their report was published in CHEST®.
They therefore performed a secondary analysis of the Etiology of Pneumonia in the Community (EPIC) study involving adults hospitalized with CAP between January 2010 and June 2012 across five tertiary-care hospitals in the United States.
The clinical characteristics of patients who died in the hospital were compared with those of patients who survived to hospital discharge. Chronic heart failure, chronic obstructive pulmonary disease, coronary artery disease, chronic liver disease, cerebrovascular disease, cancer (excluding skin cancer), and diabetes were considered as severe chronic comorbidities based on their association with increased mortality and ICU admission in CAP severity scores.
Deaths caused by septic shock, respiratory failure, multisystem organ failure, cardiopulmonary arrest prior to stabilization of CAP, and endocarditis, were considered to be directly related to CAP.
Conversely, causes of death indirectly related to CAP included acute cardiovascular disease, stroke, acute renal failure, and secondary infections developed after hospitalization. Deaths caused by cancer, cirrhosis, and chronic neurologic conditions were considered unrelated to CAP.
Medical notes were assessed to determine whether the patient received management consistent with current recommendations; for example, antibiotics consistent with guidelines from the Infectious Diseases Society of America.
End-of-life limitations in care, such as patient/family decision not to proceed with full medical treatment, also were considered by the research team.
Results showed that among the 2,320 patients with radiographically confirmed CAP, 52 died during initial hospitalization, 33 of whom were aged 65 years or older, and 32 of whom had two or more chronic comorbidities.
Most of the in-hospital deaths occurred early in the hospitalization: 35 within the first 10 days of admission, and 5 after 30 days in hospital.
CAP was judged by an expert physician review panel to be the direct cause of death in 27 of the patients, 10 with CAP having an indirect role with major contribution, 9 with CAP having an indirect role with minor contribution, and 6 with CAP having no role in death.
Do-not-resuscitate orders were present at the time of death for 21 of the patients.
Forty-five of the patients were admitted to an ICU, with 37 dying in the ICU. The eight patients who died on the ward after transfer out of the ICU had end-of-life limitations of care in place.
The researchers noted that the number of patients dying in the ICU was greater in the United States, possibly because in Europe fewer patients are admitted to an ICU.
“This discrepancy likely reflects cultural differences between the U.S. and Europe in the role of intensive care for patients with advanced age and/or advanced comorbid conditions,” they noted.
Overall, the physician review panel identified nine patients who had a lapse in quality of in-hospital CAP care, with four of the deaths potentially linked to this lapse in care.
However, two of the patients had end-of-life limitations of care in place, which according to the authors meant that “only two patients undergoing full medical treatment without end-of-life limitations of care had an identified lapse in quality of in-hospital pneumonia care potentially contributing to in-hospital death, including one with a delay in antibiotics for over an hour in the presence of shock and one with initial antibiotics not consistent with IDSA/ATS guidelines.”
The research team concluded that most in-hospital deaths among adult patients admitted with CAP in their study would not have been preventable with higher quality in-hospital pneumonia care.
“Many of the in-hospital deaths among patients admitted with CAP occurred in older patients with severe comorbidities and end-of-life limitations in care,” they noted.
They said the influence of end-of-life limitations on care short of full palliation was an important finding, with all patients who died outside the ICU having end-of-life limitations in care.
“Current diagnostic related group (DRG) and international classification of diseases (ICD) coding systems do not have the necessary nuances to capture these limitations of care, yet they are clearly important factors in determining whether patients experience in-hospital death,” they added.
Dr. Waterer reported no conflicts. Two coauthors reported potential conflicts of interest in relation to consulting fees from several pharmaceutical companies.
SOURCE: Waterer G. et al. CHEST 2018;154(3):628-35. doi: 10.1016/j.chest.2018.05.021.
FROM CHEST
Key clinical point: Most in-hospital deaths from community-acquired pneumonia are not preventable with current medical therapy.
Major finding: Two out of 52 patients who died in-hospital from community-acquired pneumonia (CAP) who were undergoing full medical treatment without end-of-life limitations of care had an identified lapse in quality of in-hospital pneumonia care that potentially contributed to their death.
Study details: A secondary analysis of the prospective multicenter Etiology of Pneumonia in the Community (EPIC) study involving 2,320 adults with radiographically confirmed CAP.
Disclosures: Dr. Waterer reported no conflicts. Two coauthors reported potential conflicts of interest in relation to consulting fees from several pharmaceutical companies.
Source: Waterer G. et al. CHEST 2018;154(3):628-35.
Confirmed: Growth in overdose deaths is exponential
Overdose death rates for individual drugs show no particular patterns since the turn of the century, but the exponential growth of overall drug mortality actually started before the opioid epidemic, according to an analysis of almost 600,000 unintentional overdose deaths since 1979.
“The current epidemic of overdose deaths due to prescription opioids, heroin, and fentanyl appears to be the most recent manifestation of a more fundamental, longer-term process,” senior author Donald S. Burke, MD, of the University of Pittsburgh, said in a written statement.
Overdose mortality from all types of drugs rose from 1.13 per 100,000 population in 1979 to 16.96 per 100,000 in 2016, based on data for 599,255 deaths from unintentional drug overdoses in the National Vital Statistics System, they reported in Science.
When the investigators plotted annual drug overdose mortality over that 38-year period, they saw a smooth upward exponential curve with a doubling time of about 9 years. “This remarkably smooth, long-term epidemic growth pattern really caught our attention,” Dr. Burke said. “If we can figure it out, we should be able to bend that curve downward.”
The individual drug types that make up the whole, however, are a different story. “There is no regular or predictable pattern to the overdose rates for any of these drugs. Cocaine overdose death rates curved down and up and down and back up over the past 20 years. Methadone deaths have been on a downturn since the mid-2000s. Prescription opioids have been on a fairly steady, steep climb. Heroin deaths shot up in 2010, followed in 2013 by synthetic opioids, such as fentanyl,” lead author Hawre Jalal, MD, PhD, also of the university, said in the statement.
Geographic and demographic analyses produced the same absence of patterns. the researchers wrote.
The study was supported by grants from the Centers for Disease Control and Prevention and the Robert Wood Johnson Foundation. The investigators said they have no competing interests.
SOURCE: Jalal H et al. Science 2018 Sep 20. doi: 10.1126/science.aau1184.
Overdose death rates for individual drugs show no particular patterns since the turn of the century, but the exponential growth of overall drug mortality actually started before the opioid epidemic, according to an analysis of almost 600,000 unintentional overdose deaths since 1979.
“The current epidemic of overdose deaths due to prescription opioids, heroin, and fentanyl appears to be the most recent manifestation of a more fundamental, longer-term process,” senior author Donald S. Burke, MD, of the University of Pittsburgh, said in a written statement.
Overdose mortality from all types of drugs rose from 1.13 per 100,000 population in 1979 to 16.96 per 100,000 in 2016, based on data for 599,255 deaths from unintentional drug overdoses in the National Vital Statistics System, they reported in Science.
When the investigators plotted annual drug overdose mortality over that 38-year period, they saw a smooth upward exponential curve with a doubling time of about 9 years. “This remarkably smooth, long-term epidemic growth pattern really caught our attention,” Dr. Burke said. “If we can figure it out, we should be able to bend that curve downward.”
The individual drug types that make up the whole, however, are a different story. “There is no regular or predictable pattern to the overdose rates for any of these drugs. Cocaine overdose death rates curved down and up and down and back up over the past 20 years. Methadone deaths have been on a downturn since the mid-2000s. Prescription opioids have been on a fairly steady, steep climb. Heroin deaths shot up in 2010, followed in 2013 by synthetic opioids, such as fentanyl,” lead author Hawre Jalal, MD, PhD, also of the university, said in the statement.
Geographic and demographic analyses produced the same absence of patterns. the researchers wrote.
The study was supported by grants from the Centers for Disease Control and Prevention and the Robert Wood Johnson Foundation. The investigators said they have no competing interests.
SOURCE: Jalal H et al. Science 2018 Sep 20. doi: 10.1126/science.aau1184.
Overdose death rates for individual drugs show no particular patterns since the turn of the century, but the exponential growth of overall drug mortality actually started before the opioid epidemic, according to an analysis of almost 600,000 unintentional overdose deaths since 1979.
“The current epidemic of overdose deaths due to prescription opioids, heroin, and fentanyl appears to be the most recent manifestation of a more fundamental, longer-term process,” senior author Donald S. Burke, MD, of the University of Pittsburgh, said in a written statement.
Overdose mortality from all types of drugs rose from 1.13 per 100,000 population in 1979 to 16.96 per 100,000 in 2016, based on data for 599,255 deaths from unintentional drug overdoses in the National Vital Statistics System, they reported in Science.
When the investigators plotted annual drug overdose mortality over that 38-year period, they saw a smooth upward exponential curve with a doubling time of about 9 years. “This remarkably smooth, long-term epidemic growth pattern really caught our attention,” Dr. Burke said. “If we can figure it out, we should be able to bend that curve downward.”
The individual drug types that make up the whole, however, are a different story. “There is no regular or predictable pattern to the overdose rates for any of these drugs. Cocaine overdose death rates curved down and up and down and back up over the past 20 years. Methadone deaths have been on a downturn since the mid-2000s. Prescription opioids have been on a fairly steady, steep climb. Heroin deaths shot up in 2010, followed in 2013 by synthetic opioids, such as fentanyl,” lead author Hawre Jalal, MD, PhD, also of the university, said in the statement.
Geographic and demographic analyses produced the same absence of patterns. the researchers wrote.
The study was supported by grants from the Centers for Disease Control and Prevention and the Robert Wood Johnson Foundation. The investigators said they have no competing interests.
SOURCE: Jalal H et al. Science 2018 Sep 20. doi: 10.1126/science.aau1184.
FROM SCIENCE
New perspectives keep SHM relevant
Atashi Mandal, MD, finds committee work illuminating and gratifying
Editor’s note: SHM occasionally puts the spotlight on some of our most active members who are making substantial contributions to hospital medicine. Visit www.hospitalmedicine.org for more information on how you can lend your expertise to help improve the care of hospitalized patients.
This month, The Hospitalist spotlights Atashi Mandal, MD , a Med-Peds hospitalist in Huntington Beach, Calif. Dr. Mandal has been a member of SHM since for more than a decade, has served on the Public Policy Committee, and is currently serving on the Patient Experience Committee.
How did you initially hear about SHM, and why did you become a member?
I was a newly minted hospitalist and eagerly searching for a way to use my CME allowance, when I discovered SHM’s annual conference, which happened to be nearby in San Diego that year. I also was intrigued by, and excited to learn more about, an organization that dedicated itself only to hospital medicine. After attending the conference, I was hooked!
As a member of more than a decade, what aspects of your membership have you found to be most valuable?
I’ve always been very impressed by the quality and variety of the educational offerings. As a Med-Peds hospitalist, I can happily attest to greater inclusion of pediatric-specific content and a more robust presence of pediatric hospitalists over the years. Moreover, I am very appreciative of SHM’s progressive attitude as demonstrated by incorporating topics such as gender disparities, LGBTQ health, and the opioid crisis into our curriculum. I also have greatly enjoyed the networking opportunities with fellow hospitalists, some of whom I am happy to say have also become good friends over the years. More recently over the past few years, I’ve participated on committees, which has been an illuminating and gratifying way to help shape SHM’s current and future directives.
Describe your role on the Public Policy Committee. What did the committee accomplish during your term?
I was very honored to serve as a member of this committee for three terms. The staff is truly superhuman and amazing, considering how well they stay abreast of the swiftly changing administrative and legislative currents in health care. Just during my tenure as an SHM member, we’ve witnessed paramount shifts in our practice and culture, from the passage of MACRA, [the Medicare Access and CHIP Reauthorization Act] to the opioid epidemic. The Public Policy Committee identifies issues that affect our practice as hospitalists and advocates on our behalf through various means, from submitting comments and letters as well as personally meeting with our regulatory agencies such as CMS [Centers for Medicare & Medicaid Services], and our federal legislators. Some major victories were the acquisition of our specialty billing code and approval of an advanced care billing code. Additionally, the committee has been tirelessly advocating for reform with observation status. We have submitted comments to legislative committees regarding the opioid crisis and continue to work with MACRA as it affects our membership. While I served, I took a special interest in mental health and pediatric issues, including CHIP [Children’s Health Insurance Program] reauthorization and the 21st Century Cures Act.
What is Hill Day, and what can Hospital Medicine 2019 attendees expect to gain from participating?
Hill Day is a truly educational, exciting – and most important – fun opportunity to hone our advocacy skills and gain some real-world experience interacting with legislators and their staffs. On the last day of the annual conference attendees can travel to D.C., where we will spend about a half-day meeting with our respective state’s legislators or their staff. We typically discuss two or three preselected bills that can directly impact our practice as hospitalists. The legislators and their staffers generally are not aware of how certain legislative items can greatly benefit or adversely affect our patients, and they therefore rely on front-line clinicians like us to provide this narrative, much to their gratitude. I learn a lot and have even more fun each time I go to Capitol Hill, so I strongly encourage everyone to participate in this unique opportunity.
Do you have any advice for early-career hospitalists looking to gain experience and get involved with SHM?
I would encourage you to find your voice and participate! Whether by joining a committee or a Special Interest Group or just chatting on one of the many stimulating forums, we each have something to bring to the table, irrespective of our tenure as hospitalists. The new perspectives mingling with those that are well established is what keeps our organization relevant, so I look forward to new ideas and fresh faces!
Ms. Steele is a marketing communications specialist at the Society of Hospital Medicine.
Atashi Mandal, MD, finds committee work illuminating and gratifying
Atashi Mandal, MD, finds committee work illuminating and gratifying
Editor’s note: SHM occasionally puts the spotlight on some of our most active members who are making substantial contributions to hospital medicine. Visit www.hospitalmedicine.org for more information on how you can lend your expertise to help improve the care of hospitalized patients.
This month, The Hospitalist spotlights Atashi Mandal, MD , a Med-Peds hospitalist in Huntington Beach, Calif. Dr. Mandal has been a member of SHM since for more than a decade, has served on the Public Policy Committee, and is currently serving on the Patient Experience Committee.
How did you initially hear about SHM, and why did you become a member?
I was a newly minted hospitalist and eagerly searching for a way to use my CME allowance, when I discovered SHM’s annual conference, which happened to be nearby in San Diego that year. I also was intrigued by, and excited to learn more about, an organization that dedicated itself only to hospital medicine. After attending the conference, I was hooked!
As a member of more than a decade, what aspects of your membership have you found to be most valuable?
I’ve always been very impressed by the quality and variety of the educational offerings. As a Med-Peds hospitalist, I can happily attest to greater inclusion of pediatric-specific content and a more robust presence of pediatric hospitalists over the years. Moreover, I am very appreciative of SHM’s progressive attitude as demonstrated by incorporating topics such as gender disparities, LGBTQ health, and the opioid crisis into our curriculum. I also have greatly enjoyed the networking opportunities with fellow hospitalists, some of whom I am happy to say have also become good friends over the years. More recently over the past few years, I’ve participated on committees, which has been an illuminating and gratifying way to help shape SHM’s current and future directives.
Describe your role on the Public Policy Committee. What did the committee accomplish during your term?
I was very honored to serve as a member of this committee for three terms. The staff is truly superhuman and amazing, considering how well they stay abreast of the swiftly changing administrative and legislative currents in health care. Just during my tenure as an SHM member, we’ve witnessed paramount shifts in our practice and culture, from the passage of MACRA, [the Medicare Access and CHIP Reauthorization Act] to the opioid epidemic. The Public Policy Committee identifies issues that affect our practice as hospitalists and advocates on our behalf through various means, from submitting comments and letters as well as personally meeting with our regulatory agencies such as CMS [Centers for Medicare & Medicaid Services], and our federal legislators. Some major victories were the acquisition of our specialty billing code and approval of an advanced care billing code. Additionally, the committee has been tirelessly advocating for reform with observation status. We have submitted comments to legislative committees regarding the opioid crisis and continue to work with MACRA as it affects our membership. While I served, I took a special interest in mental health and pediatric issues, including CHIP [Children’s Health Insurance Program] reauthorization and the 21st Century Cures Act.
What is Hill Day, and what can Hospital Medicine 2019 attendees expect to gain from participating?
Hill Day is a truly educational, exciting – and most important – fun opportunity to hone our advocacy skills and gain some real-world experience interacting with legislators and their staffs. On the last day of the annual conference attendees can travel to D.C., where we will spend about a half-day meeting with our respective state’s legislators or their staff. We typically discuss two or three preselected bills that can directly impact our practice as hospitalists. The legislators and their staffers generally are not aware of how certain legislative items can greatly benefit or adversely affect our patients, and they therefore rely on front-line clinicians like us to provide this narrative, much to their gratitude. I learn a lot and have even more fun each time I go to Capitol Hill, so I strongly encourage everyone to participate in this unique opportunity.
Do you have any advice for early-career hospitalists looking to gain experience and get involved with SHM?
I would encourage you to find your voice and participate! Whether by joining a committee or a Special Interest Group or just chatting on one of the many stimulating forums, we each have something to bring to the table, irrespective of our tenure as hospitalists. The new perspectives mingling with those that are well established is what keeps our organization relevant, so I look forward to new ideas and fresh faces!
Ms. Steele is a marketing communications specialist at the Society of Hospital Medicine.
Editor’s note: SHM occasionally puts the spotlight on some of our most active members who are making substantial contributions to hospital medicine. Visit www.hospitalmedicine.org for more information on how you can lend your expertise to help improve the care of hospitalized patients.
This month, The Hospitalist spotlights Atashi Mandal, MD , a Med-Peds hospitalist in Huntington Beach, Calif. Dr. Mandal has been a member of SHM since for more than a decade, has served on the Public Policy Committee, and is currently serving on the Patient Experience Committee.
How did you initially hear about SHM, and why did you become a member?
I was a newly minted hospitalist and eagerly searching for a way to use my CME allowance, when I discovered SHM’s annual conference, which happened to be nearby in San Diego that year. I also was intrigued by, and excited to learn more about, an organization that dedicated itself only to hospital medicine. After attending the conference, I was hooked!
As a member of more than a decade, what aspects of your membership have you found to be most valuable?
I’ve always been very impressed by the quality and variety of the educational offerings. As a Med-Peds hospitalist, I can happily attest to greater inclusion of pediatric-specific content and a more robust presence of pediatric hospitalists over the years. Moreover, I am very appreciative of SHM’s progressive attitude as demonstrated by incorporating topics such as gender disparities, LGBTQ health, and the opioid crisis into our curriculum. I also have greatly enjoyed the networking opportunities with fellow hospitalists, some of whom I am happy to say have also become good friends over the years. More recently over the past few years, I’ve participated on committees, which has been an illuminating and gratifying way to help shape SHM’s current and future directives.
Describe your role on the Public Policy Committee. What did the committee accomplish during your term?
I was very honored to serve as a member of this committee for three terms. The staff is truly superhuman and amazing, considering how well they stay abreast of the swiftly changing administrative and legislative currents in health care. Just during my tenure as an SHM member, we’ve witnessed paramount shifts in our practice and culture, from the passage of MACRA, [the Medicare Access and CHIP Reauthorization Act] to the opioid epidemic. The Public Policy Committee identifies issues that affect our practice as hospitalists and advocates on our behalf through various means, from submitting comments and letters as well as personally meeting with our regulatory agencies such as CMS [Centers for Medicare & Medicaid Services], and our federal legislators. Some major victories were the acquisition of our specialty billing code and approval of an advanced care billing code. Additionally, the committee has been tirelessly advocating for reform with observation status. We have submitted comments to legislative committees regarding the opioid crisis and continue to work with MACRA as it affects our membership. While I served, I took a special interest in mental health and pediatric issues, including CHIP [Children’s Health Insurance Program] reauthorization and the 21st Century Cures Act.
What is Hill Day, and what can Hospital Medicine 2019 attendees expect to gain from participating?
Hill Day is a truly educational, exciting – and most important – fun opportunity to hone our advocacy skills and gain some real-world experience interacting with legislators and their staffs. On the last day of the annual conference attendees can travel to D.C., where we will spend about a half-day meeting with our respective state’s legislators or their staff. We typically discuss two or three preselected bills that can directly impact our practice as hospitalists. The legislators and their staffers generally are not aware of how certain legislative items can greatly benefit or adversely affect our patients, and they therefore rely on front-line clinicians like us to provide this narrative, much to their gratitude. I learn a lot and have even more fun each time I go to Capitol Hill, so I strongly encourage everyone to participate in this unique opportunity.
Do you have any advice for early-career hospitalists looking to gain experience and get involved with SHM?
I would encourage you to find your voice and participate! Whether by joining a committee or a Special Interest Group or just chatting on one of the many stimulating forums, we each have something to bring to the table, irrespective of our tenure as hospitalists. The new perspectives mingling with those that are well established is what keeps our organization relevant, so I look forward to new ideas and fresh faces!
Ms. Steele is a marketing communications specialist at the Society of Hospital Medicine.
Is CMS becoming more open to PTAC recommendations?
Recommendations on potential physician-focused alternative payment models so far have gained little traction with officials at the Centers for Medicare & Medicaid Services, but that could be changing.
At a recent meeting of the Physician-Focused Payment Model Technical Advisory Committee (PTAC), Alex Azar, secretary of the Department of Health & Human Services, and Seema Verma, administrator of the Centers for Medicare & Medicaid Services, attended briefly, according to officials at the American College of Emergency Physicians. ACEP was having its proposal evaluated by PTAC.
Physician organizations have been puzzled as to why federal health officials have yet to approve a physician-developed alternative payment model (APM) under the Quality Payment Program, despite several being recommended by PTAC. In fact, no physician-developed APM has even been sent to the Center for Medicare and Medicaid Innovation for further testing and refinement.
ACEP’s model, the Acute Unscheduled Care Model, “provides incentives to safely discharge Medicare beneficiaries from the ED by facilitating and rewarding postdischarge care coordination,” the organizations notes in the model description submitted to PTAC. “The model ensures that emergency physicians who make the decision to provide safe, efficient outpatient care have the necessary tools to support this transformation and are rewarded for their decision making.”
Going into the meeting, the preliminary evaluation report had the PTAC reviewers agreeing unanimously that the model met 7 of the secretary’s 10 criteria for a physician-focused APM, that the majority agreed on the 8th criterion, and a majority agreed that the model did not meet criteria on the remaining two items.
PTAC reviewers “thought that we met all 10 criteria for models that the secretary put forth for evaluating physician-focused payment models,” Jeffrey Davis, ACEP Director of Regulatory Affairs, said in an interview, adding that the attendance of Mr. Azar and Ms. Verma at the meeting was a positive development.
“I think we were especially inspired by the fact that Secretary Azar, Administrator Verma, and Adam Boehler, the new head of [Center for Medicare and Medicaid Innovation], spoke at the beginning of the PTAC meeting,” he said, adding that while no models have been formally implemented or even designated for testing, they said that ideas are being incorporated into model development going on at the CMS.
Ms. Verma went so far as to tweet that “this group of leading experts volunteer their time to improve our healthcare system, and we greatly value their input.”
“We still think [PTAC] is a great avenue to get our model into the public arena,” Mr. Davis said. “We are optimistic.”
Recommendations on potential physician-focused alternative payment models so far have gained little traction with officials at the Centers for Medicare & Medicaid Services, but that could be changing.
At a recent meeting of the Physician-Focused Payment Model Technical Advisory Committee (PTAC), Alex Azar, secretary of the Department of Health & Human Services, and Seema Verma, administrator of the Centers for Medicare & Medicaid Services, attended briefly, according to officials at the American College of Emergency Physicians. ACEP was having its proposal evaluated by PTAC.
Physician organizations have been puzzled as to why federal health officials have yet to approve a physician-developed alternative payment model (APM) under the Quality Payment Program, despite several being recommended by PTAC. In fact, no physician-developed APM has even been sent to the Center for Medicare and Medicaid Innovation for further testing and refinement.
ACEP’s model, the Acute Unscheduled Care Model, “provides incentives to safely discharge Medicare beneficiaries from the ED by facilitating and rewarding postdischarge care coordination,” the organizations notes in the model description submitted to PTAC. “The model ensures that emergency physicians who make the decision to provide safe, efficient outpatient care have the necessary tools to support this transformation and are rewarded for their decision making.”
Going into the meeting, the preliminary evaluation report had the PTAC reviewers agreeing unanimously that the model met 7 of the secretary’s 10 criteria for a physician-focused APM, that the majority agreed on the 8th criterion, and a majority agreed that the model did not meet criteria on the remaining two items.
PTAC reviewers “thought that we met all 10 criteria for models that the secretary put forth for evaluating physician-focused payment models,” Jeffrey Davis, ACEP Director of Regulatory Affairs, said in an interview, adding that the attendance of Mr. Azar and Ms. Verma at the meeting was a positive development.
“I think we were especially inspired by the fact that Secretary Azar, Administrator Verma, and Adam Boehler, the new head of [Center for Medicare and Medicaid Innovation], spoke at the beginning of the PTAC meeting,” he said, adding that while no models have been formally implemented or even designated for testing, they said that ideas are being incorporated into model development going on at the CMS.
Ms. Verma went so far as to tweet that “this group of leading experts volunteer their time to improve our healthcare system, and we greatly value their input.”
“We still think [PTAC] is a great avenue to get our model into the public arena,” Mr. Davis said. “We are optimistic.”
Recommendations on potential physician-focused alternative payment models so far have gained little traction with officials at the Centers for Medicare & Medicaid Services, but that could be changing.
At a recent meeting of the Physician-Focused Payment Model Technical Advisory Committee (PTAC), Alex Azar, secretary of the Department of Health & Human Services, and Seema Verma, administrator of the Centers for Medicare & Medicaid Services, attended briefly, according to officials at the American College of Emergency Physicians. ACEP was having its proposal evaluated by PTAC.
Physician organizations have been puzzled as to why federal health officials have yet to approve a physician-developed alternative payment model (APM) under the Quality Payment Program, despite several being recommended by PTAC. In fact, no physician-developed APM has even been sent to the Center for Medicare and Medicaid Innovation for further testing and refinement.
ACEP’s model, the Acute Unscheduled Care Model, “provides incentives to safely discharge Medicare beneficiaries from the ED by facilitating and rewarding postdischarge care coordination,” the organizations notes in the model description submitted to PTAC. “The model ensures that emergency physicians who make the decision to provide safe, efficient outpatient care have the necessary tools to support this transformation and are rewarded for their decision making.”
Going into the meeting, the preliminary evaluation report had the PTAC reviewers agreeing unanimously that the model met 7 of the secretary’s 10 criteria for a physician-focused APM, that the majority agreed on the 8th criterion, and a majority agreed that the model did not meet criteria on the remaining two items.
PTAC reviewers “thought that we met all 10 criteria for models that the secretary put forth for evaluating physician-focused payment models,” Jeffrey Davis, ACEP Director of Regulatory Affairs, said in an interview, adding that the attendance of Mr. Azar and Ms. Verma at the meeting was a positive development.
“I think we were especially inspired by the fact that Secretary Azar, Administrator Verma, and Adam Boehler, the new head of [Center for Medicare and Medicaid Innovation], spoke at the beginning of the PTAC meeting,” he said, adding that while no models have been formally implemented or even designated for testing, they said that ideas are being incorporated into model development going on at the CMS.
Ms. Verma went so far as to tweet that “this group of leading experts volunteer their time to improve our healthcare system, and we greatly value their input.”
“We still think [PTAC] is a great avenue to get our model into the public arena,” Mr. Davis said. “We are optimistic.”
Guideline offers comprehensive approach for ICU clinicians
A new (PADIS).
The guideline builds upon the 2013 Clinical Practice Guidelines for the Management of Pain, Agitation, and Delirium (PAD) in Adult Patients in the ICU. Given the comprehensive nature of the PADIS guideline, an accompanying commentary was published simultaneously to help with implementation and interpretation. Both papers are the result of a large-scale, multicenter collaboration and were published in Critical Care Medicine.
A panel of 32 international experts, four methodologists, and four survivors of critical illness used the Grading of Recommendations Assessment, Development and Evaluation approach to develop the PADIS guideline.
“Thousands of hours were invested by these guidelines’ authors, who were in turn were supported by formal and informal collaborators, over the 3.5 years it took to produce this effort,” reported lead author John W. Devlin, PharmD, of the department of pharmacy and health systems sciences, Bouvé College of Health Sciences at Northeastern University, Boston, and his colleagues.
Compared with the 2013 PAD guideline, the PADIS guideline includes new sections regarding rehabilitation/mobility and sleep. “We sought to clarify conceptual definitions within these relatively new critical care research domains,” the panel wrote. “The recommendation rationales, fueled by debate and discussion, circled back to the bedside experience – and the perspective of what was best for patients – held by all panelists and methodology experts.”
The result is extensive and comprehensive, consisting of both broad and specific descriptions of current ICU practices and associated evidence; the guideline includes 37 recommendations, 32 ungraded, nonactionable statements, and two good practice statements. Of note, conditional recommendations far outnumber strong recommendations (34 vs. 3). Reasons for conditional rather than strong recommendations are discussed in rationale sections within the guideline and in the accompanying paper.
“Although our goal was to provide specific recommendations for each question, we suspect some guideline readers may be discouraged by the conditional nature of many recommendations and daunted by the breadth of topics discussed,” wrote Michele C. Balas, PhD, of the Ohio State University College of Nursing in Columbus, and her colleagues. Dr. Balas was on the guideline panel and is the lead author of the accompanying article intended to facilitate implementation and interpretation.
One of the more challenging recommendations surrounds the use of antipsychotics for delirious patients. Although this intervention has become relatively common, the guideline stands against it.
“It should be emphasized that there are few supportive data on ICU antipsychotic use and that the initiation of psychoactive medications during critical illness often results in their inappropriate continuation after ICU discharge,” wrote Dr. Balas and her coauthors.
Along with a hard look at existing practices, the panel actively sought to expand upon the 2013 guideline with new interventions. Discussions ranged from the less conventional, such as aromatherapy, to the more established, such as polypharmacy. Questions, recommendations, and rationale are clearly described for each topic, with clear supporting evidence. Where evidence is missing, the panel recommends future research possibilities.
“One example is the consideration of multiple pharmacologic and nonpharmacologic coanalgesic approaches to the ICU patient,” wrote Dr. Devlin and his coauthors. “When the published evidence was insufficient, limited to a narrow population or specific intervention (e.g., for procedural analgesia), or outright absent to answer the questions we posed, we structured evidence gap descriptors to inform clinicians where the uncertainty lay, and intended to provide sufficient information to apprise and invite researchers to address these gaps.”
The authors disclosed funding from AstraZeneca, Baxter, Covidien, and others.
SOURCE: Devlin JW et al. Crit Care Med. 2018 Sep 1. doi: 10.1097/CCM.0000000000003299; Balas MC et al. Crit Care Med. 2018 Sep 1. doi: 10.1097/CCM.0000000000003307.
A new (PADIS).
The guideline builds upon the 2013 Clinical Practice Guidelines for the Management of Pain, Agitation, and Delirium (PAD) in Adult Patients in the ICU. Given the comprehensive nature of the PADIS guideline, an accompanying commentary was published simultaneously to help with implementation and interpretation. Both papers are the result of a large-scale, multicenter collaboration and were published in Critical Care Medicine.
A panel of 32 international experts, four methodologists, and four survivors of critical illness used the Grading of Recommendations Assessment, Development and Evaluation approach to develop the PADIS guideline.
“Thousands of hours were invested by these guidelines’ authors, who were in turn were supported by formal and informal collaborators, over the 3.5 years it took to produce this effort,” reported lead author John W. Devlin, PharmD, of the department of pharmacy and health systems sciences, Bouvé College of Health Sciences at Northeastern University, Boston, and his colleagues.
Compared with the 2013 PAD guideline, the PADIS guideline includes new sections regarding rehabilitation/mobility and sleep. “We sought to clarify conceptual definitions within these relatively new critical care research domains,” the panel wrote. “The recommendation rationales, fueled by debate and discussion, circled back to the bedside experience – and the perspective of what was best for patients – held by all panelists and methodology experts.”
The result is extensive and comprehensive, consisting of both broad and specific descriptions of current ICU practices and associated evidence; the guideline includes 37 recommendations, 32 ungraded, nonactionable statements, and two good practice statements. Of note, conditional recommendations far outnumber strong recommendations (34 vs. 3). Reasons for conditional rather than strong recommendations are discussed in rationale sections within the guideline and in the accompanying paper.
“Although our goal was to provide specific recommendations for each question, we suspect some guideline readers may be discouraged by the conditional nature of many recommendations and daunted by the breadth of topics discussed,” wrote Michele C. Balas, PhD, of the Ohio State University College of Nursing in Columbus, and her colleagues. Dr. Balas was on the guideline panel and is the lead author of the accompanying article intended to facilitate implementation and interpretation.
One of the more challenging recommendations surrounds the use of antipsychotics for delirious patients. Although this intervention has become relatively common, the guideline stands against it.
“It should be emphasized that there are few supportive data on ICU antipsychotic use and that the initiation of psychoactive medications during critical illness often results in their inappropriate continuation after ICU discharge,” wrote Dr. Balas and her coauthors.
Along with a hard look at existing practices, the panel actively sought to expand upon the 2013 guideline with new interventions. Discussions ranged from the less conventional, such as aromatherapy, to the more established, such as polypharmacy. Questions, recommendations, and rationale are clearly described for each topic, with clear supporting evidence. Where evidence is missing, the panel recommends future research possibilities.
“One example is the consideration of multiple pharmacologic and nonpharmacologic coanalgesic approaches to the ICU patient,” wrote Dr. Devlin and his coauthors. “When the published evidence was insufficient, limited to a narrow population or specific intervention (e.g., for procedural analgesia), or outright absent to answer the questions we posed, we structured evidence gap descriptors to inform clinicians where the uncertainty lay, and intended to provide sufficient information to apprise and invite researchers to address these gaps.”
The authors disclosed funding from AstraZeneca, Baxter, Covidien, and others.
SOURCE: Devlin JW et al. Crit Care Med. 2018 Sep 1. doi: 10.1097/CCM.0000000000003299; Balas MC et al. Crit Care Med. 2018 Sep 1. doi: 10.1097/CCM.0000000000003307.
A new (PADIS).
The guideline builds upon the 2013 Clinical Practice Guidelines for the Management of Pain, Agitation, and Delirium (PAD) in Adult Patients in the ICU. Given the comprehensive nature of the PADIS guideline, an accompanying commentary was published simultaneously to help with implementation and interpretation. Both papers are the result of a large-scale, multicenter collaboration and were published in Critical Care Medicine.
A panel of 32 international experts, four methodologists, and four survivors of critical illness used the Grading of Recommendations Assessment, Development and Evaluation approach to develop the PADIS guideline.
“Thousands of hours were invested by these guidelines’ authors, who were in turn were supported by formal and informal collaborators, over the 3.5 years it took to produce this effort,” reported lead author John W. Devlin, PharmD, of the department of pharmacy and health systems sciences, Bouvé College of Health Sciences at Northeastern University, Boston, and his colleagues.
Compared with the 2013 PAD guideline, the PADIS guideline includes new sections regarding rehabilitation/mobility and sleep. “We sought to clarify conceptual definitions within these relatively new critical care research domains,” the panel wrote. “The recommendation rationales, fueled by debate and discussion, circled back to the bedside experience – and the perspective of what was best for patients – held by all panelists and methodology experts.”
The result is extensive and comprehensive, consisting of both broad and specific descriptions of current ICU practices and associated evidence; the guideline includes 37 recommendations, 32 ungraded, nonactionable statements, and two good practice statements. Of note, conditional recommendations far outnumber strong recommendations (34 vs. 3). Reasons for conditional rather than strong recommendations are discussed in rationale sections within the guideline and in the accompanying paper.
“Although our goal was to provide specific recommendations for each question, we suspect some guideline readers may be discouraged by the conditional nature of many recommendations and daunted by the breadth of topics discussed,” wrote Michele C. Balas, PhD, of the Ohio State University College of Nursing in Columbus, and her colleagues. Dr. Balas was on the guideline panel and is the lead author of the accompanying article intended to facilitate implementation and interpretation.
One of the more challenging recommendations surrounds the use of antipsychotics for delirious patients. Although this intervention has become relatively common, the guideline stands against it.
“It should be emphasized that there are few supportive data on ICU antipsychotic use and that the initiation of psychoactive medications during critical illness often results in their inappropriate continuation after ICU discharge,” wrote Dr. Balas and her coauthors.
Along with a hard look at existing practices, the panel actively sought to expand upon the 2013 guideline with new interventions. Discussions ranged from the less conventional, such as aromatherapy, to the more established, such as polypharmacy. Questions, recommendations, and rationale are clearly described for each topic, with clear supporting evidence. Where evidence is missing, the panel recommends future research possibilities.
“One example is the consideration of multiple pharmacologic and nonpharmacologic coanalgesic approaches to the ICU patient,” wrote Dr. Devlin and his coauthors. “When the published evidence was insufficient, limited to a narrow population or specific intervention (e.g., for procedural analgesia), or outright absent to answer the questions we posed, we structured evidence gap descriptors to inform clinicians where the uncertainty lay, and intended to provide sufficient information to apprise and invite researchers to address these gaps.”
The authors disclosed funding from AstraZeneca, Baxter, Covidien, and others.
SOURCE: Devlin JW et al. Crit Care Med. 2018 Sep 1. doi: 10.1097/CCM.0000000000003299; Balas MC et al. Crit Care Med. 2018 Sep 1. doi: 10.1097/CCM.0000000000003307.
FROM CRITICAL CARE MEDICINE
Key clinical point: The 2018 PADIS guideline recommends intervention strategies for adult ICU patients with pain, agitation/sedation, delirium, immobility, and sleep disruption.
Major finding: The guideline includes 37 recommendations; 32 ungraded, nonactionable statements; and two good practice statements.
Study details: A clinical practice guideline was created by 32 international experts, four methodologists, and four survivors of critical illness.
Disclosures: The authors declared funding from AstraZeneca, Baxter, Covidien, and others.
Sources: Devlin JW et al. Crit Care Med. 2018 Sep 1. doi: 10.1097/CCM.0000000000003299; Balas MC et al. Crit Care Med. 2018 Sep 1. doi: 10.1097/CCM.0000000000003307.
FDA issues new REMS for immediate-release opioids
Opioid prescribers will need to be mindful of a new, expanded Risk Evaluation and Mitigation Strategy issued Sept. 18 by the Food and Drug Administration, covering immediate-release opioid analgesics used in the outpatient setting. The strategy also applies to extended-release and long-acting opioids, which have been subject to REMS since 2012.
The new REMS program requires for the first time that training be made available to health care providers who are involved in pain management. For the purposes of this REMS program, the training is not limited to just the prescriber, but includes nurses and pharmacists.
, including alternatives to opioids for pain management.
The FDA said it is in the process of approving a new label for opioids that will contain information about health care provider education that is now a part of the REMS.
“Our new effort is aimed at arming providers with the most current and comprehensive information on the appropriate management of pain,” FDA Commissioner Scott Gottlieb, MD, said in a statement. “Appropriate prescribing practices and education are important steps that we are prioritizing to help address the human and financial toll of this crisis.”
Dr. Gottlieb added that the goal of the new REMS is to help prescribers with the latest evidence on the appropriate amount of doses that should be prescribed for a given condition and that the “aim is to reduce overall dispensing as a way to further reduce exposure to these drugs. Our goal is to help prevent patients from becoming addicted by decreasing unnecessary or inappropriate exposure to opioids and fostering rational prescribing to enable appropriate access to those patients who have legitimate medical need for these medicines.”
The FDA also approved a new guidance document that includes updated educational content.
Opioid prescribers will need to be mindful of a new, expanded Risk Evaluation and Mitigation Strategy issued Sept. 18 by the Food and Drug Administration, covering immediate-release opioid analgesics used in the outpatient setting. The strategy also applies to extended-release and long-acting opioids, which have been subject to REMS since 2012.
The new REMS program requires for the first time that training be made available to health care providers who are involved in pain management. For the purposes of this REMS program, the training is not limited to just the prescriber, but includes nurses and pharmacists.
, including alternatives to opioids for pain management.
The FDA said it is in the process of approving a new label for opioids that will contain information about health care provider education that is now a part of the REMS.
“Our new effort is aimed at arming providers with the most current and comprehensive information on the appropriate management of pain,” FDA Commissioner Scott Gottlieb, MD, said in a statement. “Appropriate prescribing practices and education are important steps that we are prioritizing to help address the human and financial toll of this crisis.”
Dr. Gottlieb added that the goal of the new REMS is to help prescribers with the latest evidence on the appropriate amount of doses that should be prescribed for a given condition and that the “aim is to reduce overall dispensing as a way to further reduce exposure to these drugs. Our goal is to help prevent patients from becoming addicted by decreasing unnecessary or inappropriate exposure to opioids and fostering rational prescribing to enable appropriate access to those patients who have legitimate medical need for these medicines.”
The FDA also approved a new guidance document that includes updated educational content.
Opioid prescribers will need to be mindful of a new, expanded Risk Evaluation and Mitigation Strategy issued Sept. 18 by the Food and Drug Administration, covering immediate-release opioid analgesics used in the outpatient setting. The strategy also applies to extended-release and long-acting opioids, which have been subject to REMS since 2012.
The new REMS program requires for the first time that training be made available to health care providers who are involved in pain management. For the purposes of this REMS program, the training is not limited to just the prescriber, but includes nurses and pharmacists.
, including alternatives to opioids for pain management.
The FDA said it is in the process of approving a new label for opioids that will contain information about health care provider education that is now a part of the REMS.
“Our new effort is aimed at arming providers with the most current and comprehensive information on the appropriate management of pain,” FDA Commissioner Scott Gottlieb, MD, said in a statement. “Appropriate prescribing practices and education are important steps that we are prioritizing to help address the human and financial toll of this crisis.”
Dr. Gottlieb added that the goal of the new REMS is to help prescribers with the latest evidence on the appropriate amount of doses that should be prescribed for a given condition and that the “aim is to reduce overall dispensing as a way to further reduce exposure to these drugs. Our goal is to help prevent patients from becoming addicted by decreasing unnecessary or inappropriate exposure to opioids and fostering rational prescribing to enable appropriate access to those patients who have legitimate medical need for these medicines.”
The FDA also approved a new guidance document that includes updated educational content.