User login
In Case You Missed It: COVID
Some with long COVID see relief after vaccination
Several weeks after getting his second dose of an mRNA vaccine, Aaron Goyang thinks his long bout with COVID-19 has finally come to an end.
Mr. Goyang, who is 33 and is a radiology technician in Austin, Tex., thinks he got COVID-19 from some of the coughing, gasping patients he treated last spring.
At the time, testing was scarce, and by the time he was tested – several weeks into his illness – it came back negative. He fought off the initial symptoms but experienced relapse a week later.
Mr. Goyang says that, for the next 8 or 9 months, he was on a roller coaster with extreme shortness of breath and chest tightness that could be so severe it would send him to the emergency department. He had to use an inhaler to get through his workdays.
“Even if I was just sitting around, it would come and take me,” he says. “It almost felt like someone was bear-hugging me constantly, and I just couldn’t get in a good enough breath.”
On his best days, he would walk around his neighborhood, being careful not to overdo it. He tried running once, and it nearly sent him to the hospital.
“Very honestly, I didn’t know if I would ever be able to do it again,” he says.
But Mr. Goyang says that, several weeks after getting the Pfizer vaccine, he was able to run a mile again with no problems. “I was very thankful for that,” he says.
Mr. Goyang is not alone. Some social media groups are dedicated to patients who are living with a condition that’s been known as long COVID and that was recently termed postacute sequelae of SARS-CoV-2 infection (PASC). These patients are sometimes referred to as long haulers.
On social media, patients with PASC are eagerly and anxiously quizzing each other about the vaccines and their effects.
Survivor Corps, which has a public Facebook group with 159,000 members, recently took a poll to see whether there was any substance to rumors that those with long COVID were feeling better after being vaccinated.
“Out of 400 people, 36% showed an improvement in symptoms, anywhere between a mild improvement to complete resolution of symptoms,” said Diana Berrent, a long-COVID patient who founded the group. Survivor Corps has become active in patient advocacy and is a resource for researchers studying the new condition.
Ms. Berrent has become such a trusted voice during the pandemic. She interviewed Anthony Fauci, MD, head of the National Institutes of Allergy and Infectious Diseases, last October.
“The implications are huge,” she says.
“Some of this damage is permanent damage. It’s not going to cure the scarring of your heart tissue, it’s not going to cure the irreparable damage to your lungs, but if it’s making people feel better, then that’s an indication there’s viral persistence going on,” says Ms. Berrent.
“I’ve been saying for months and months, we shouldn’t be calling this postacute anything,” she adds.
Patients report improvement
Daniel Griffin, MD, PhD, is equally excited. He’s an infectious disease specialist at Columbia University, New York. He says about one in five patients he treated for COVID-19 last year never got better. Many of them, such as Mr. Goyang, were health care workers.
“I don’t know if people actually catch this, but a lot of our coworkers are either permanently disabled or died,” Dr. Griffin says.
Health care workers were also among the first to be vaccinated. Dr. Griffin says many of his patients began reaching out to him about a week or two after being vaccinated “and saying, ‘You know, I actually feel better.’ And some of them were saying, ‘I feel all better,’ after being sick – a lot of them – for a year.”
Then he was getting calls and texts from other doctors, asking, “Hey, are you seeing this?”
The benefits of vaccination for some long-haulers came as a surprise. Dr. Griffin says that, before the vaccines came out, many of his patients were worried that getting vaccinated might overstimulate their immune systems and cause symptoms to get worse.
Indeed, a small percentage of people – about 3%-5%, based on informal polls on social media – report that they do experience worsening of symptoms after getting the shot. It’s not clear why.
Dr. Griffin estimates that between 30% and 50% of patients’ symptoms improve after they receive the mRNA vaccines. “I’m seeing this chunk of people – they tell me their brain fog has improved, their fatigue is gone, the fevers that wouldn’t resolve have now gone,” he says. “I’m seeing that personally, and I’m hearing it from my colleagues.”
Dr. Griffin says the observation has launched several studies and that there are several theories about how the vaccines might be affecting long COVID.
An immune system boost?
One possibility is that the virus continues to stimulate the immune system, which continues to fight the virus for months. If that is the case, Dr. Griffin says, the vaccine may be giving the immune system the boost it needs to finally clear the virus away.
Donna Farber, PhD, a professor of microbiology and immunology at Columbia University, has heard the stories, too.
“It is possible that the persisting virus in long COVID-19 may be at a low level – not enough to stimulate a potent immune response to clear the virus, but enough to cause symptoms. Activating the immune response therefore is therapeutic in directing viral clearance,” she says.
Dr. Farber explains that long COVID may be a bit like Lyme disease. Some patients with Lyme disease must take antibiotics for months before their symptoms disappear.
Dr. Griffin says there’s another possibility. Several studies have now shown that people with lingering COVID-19 symptoms develop autoantibodies. There’s a theory that SARS-CoV-2 may create an autoimmune condition that leads to long-term symptoms.
If that is the case, Dr. Griffin says, the vaccine may be helping the body to reset its tolerance to itself, “so maybe now you’re getting a healthy immune response.”
More studies are needed to know for sure.
Either way, the vaccines are a much-needed bit of hope for the long-COVID community, and Dr. Griffin tells his patients who are still worried that, at the very least, they’ll be protected from another SARS-CoV-2 infection.
A version of this article first appeared on Medscape.com.
Several weeks after getting his second dose of an mRNA vaccine, Aaron Goyang thinks his long bout with COVID-19 has finally come to an end.
Mr. Goyang, who is 33 and is a radiology technician in Austin, Tex., thinks he got COVID-19 from some of the coughing, gasping patients he treated last spring.
At the time, testing was scarce, and by the time he was tested – several weeks into his illness – it came back negative. He fought off the initial symptoms but experienced relapse a week later.
Mr. Goyang says that, for the next 8 or 9 months, he was on a roller coaster with extreme shortness of breath and chest tightness that could be so severe it would send him to the emergency department. He had to use an inhaler to get through his workdays.
“Even if I was just sitting around, it would come and take me,” he says. “It almost felt like someone was bear-hugging me constantly, and I just couldn’t get in a good enough breath.”
On his best days, he would walk around his neighborhood, being careful not to overdo it. He tried running once, and it nearly sent him to the hospital.
“Very honestly, I didn’t know if I would ever be able to do it again,” he says.
But Mr. Goyang says that, several weeks after getting the Pfizer vaccine, he was able to run a mile again with no problems. “I was very thankful for that,” he says.
Mr. Goyang is not alone. Some social media groups are dedicated to patients who are living with a condition that’s been known as long COVID and that was recently termed postacute sequelae of SARS-CoV-2 infection (PASC). These patients are sometimes referred to as long haulers.
On social media, patients with PASC are eagerly and anxiously quizzing each other about the vaccines and their effects.
Survivor Corps, which has a public Facebook group with 159,000 members, recently took a poll to see whether there was any substance to rumors that those with long COVID were feeling better after being vaccinated.
“Out of 400 people, 36% showed an improvement in symptoms, anywhere between a mild improvement to complete resolution of symptoms,” said Diana Berrent, a long-COVID patient who founded the group. Survivor Corps has become active in patient advocacy and is a resource for researchers studying the new condition.
Ms. Berrent has become such a trusted voice during the pandemic. She interviewed Anthony Fauci, MD, head of the National Institutes of Allergy and Infectious Diseases, last October.
“The implications are huge,” she says.
“Some of this damage is permanent damage. It’s not going to cure the scarring of your heart tissue, it’s not going to cure the irreparable damage to your lungs, but if it’s making people feel better, then that’s an indication there’s viral persistence going on,” says Ms. Berrent.
“I’ve been saying for months and months, we shouldn’t be calling this postacute anything,” she adds.
Patients report improvement
Daniel Griffin, MD, PhD, is equally excited. He’s an infectious disease specialist at Columbia University, New York. He says about one in five patients he treated for COVID-19 last year never got better. Many of them, such as Mr. Goyang, were health care workers.
“I don’t know if people actually catch this, but a lot of our coworkers are either permanently disabled or died,” Dr. Griffin says.
Health care workers were also among the first to be vaccinated. Dr. Griffin says many of his patients began reaching out to him about a week or two after being vaccinated “and saying, ‘You know, I actually feel better.’ And some of them were saying, ‘I feel all better,’ after being sick – a lot of them – for a year.”
Then he was getting calls and texts from other doctors, asking, “Hey, are you seeing this?”
The benefits of vaccination for some long-haulers came as a surprise. Dr. Griffin says that, before the vaccines came out, many of his patients were worried that getting vaccinated might overstimulate their immune systems and cause symptoms to get worse.
Indeed, a small percentage of people – about 3%-5%, based on informal polls on social media – report that they do experience worsening of symptoms after getting the shot. It’s not clear why.
Dr. Griffin estimates that between 30% and 50% of patients’ symptoms improve after they receive the mRNA vaccines. “I’m seeing this chunk of people – they tell me their brain fog has improved, their fatigue is gone, the fevers that wouldn’t resolve have now gone,” he says. “I’m seeing that personally, and I’m hearing it from my colleagues.”
Dr. Griffin says the observation has launched several studies and that there are several theories about how the vaccines might be affecting long COVID.
An immune system boost?
One possibility is that the virus continues to stimulate the immune system, which continues to fight the virus for months. If that is the case, Dr. Griffin says, the vaccine may be giving the immune system the boost it needs to finally clear the virus away.
Donna Farber, PhD, a professor of microbiology and immunology at Columbia University, has heard the stories, too.
“It is possible that the persisting virus in long COVID-19 may be at a low level – not enough to stimulate a potent immune response to clear the virus, but enough to cause symptoms. Activating the immune response therefore is therapeutic in directing viral clearance,” she says.
Dr. Farber explains that long COVID may be a bit like Lyme disease. Some patients with Lyme disease must take antibiotics for months before their symptoms disappear.
Dr. Griffin says there’s another possibility. Several studies have now shown that people with lingering COVID-19 symptoms develop autoantibodies. There’s a theory that SARS-CoV-2 may create an autoimmune condition that leads to long-term symptoms.
If that is the case, Dr. Griffin says, the vaccine may be helping the body to reset its tolerance to itself, “so maybe now you’re getting a healthy immune response.”
More studies are needed to know for sure.
Either way, the vaccines are a much-needed bit of hope for the long-COVID community, and Dr. Griffin tells his patients who are still worried that, at the very least, they’ll be protected from another SARS-CoV-2 infection.
A version of this article first appeared on Medscape.com.
Several weeks after getting his second dose of an mRNA vaccine, Aaron Goyang thinks his long bout with COVID-19 has finally come to an end.
Mr. Goyang, who is 33 and is a radiology technician in Austin, Tex., thinks he got COVID-19 from some of the coughing, gasping patients he treated last spring.
At the time, testing was scarce, and by the time he was tested – several weeks into his illness – it came back negative. He fought off the initial symptoms but experienced relapse a week later.
Mr. Goyang says that, for the next 8 or 9 months, he was on a roller coaster with extreme shortness of breath and chest tightness that could be so severe it would send him to the emergency department. He had to use an inhaler to get through his workdays.
“Even if I was just sitting around, it would come and take me,” he says. “It almost felt like someone was bear-hugging me constantly, and I just couldn’t get in a good enough breath.”
On his best days, he would walk around his neighborhood, being careful not to overdo it. He tried running once, and it nearly sent him to the hospital.
“Very honestly, I didn’t know if I would ever be able to do it again,” he says.
But Mr. Goyang says that, several weeks after getting the Pfizer vaccine, he was able to run a mile again with no problems. “I was very thankful for that,” he says.
Mr. Goyang is not alone. Some social media groups are dedicated to patients who are living with a condition that’s been known as long COVID and that was recently termed postacute sequelae of SARS-CoV-2 infection (PASC). These patients are sometimes referred to as long haulers.
On social media, patients with PASC are eagerly and anxiously quizzing each other about the vaccines and their effects.
Survivor Corps, which has a public Facebook group with 159,000 members, recently took a poll to see whether there was any substance to rumors that those with long COVID were feeling better after being vaccinated.
“Out of 400 people, 36% showed an improvement in symptoms, anywhere between a mild improvement to complete resolution of symptoms,” said Diana Berrent, a long-COVID patient who founded the group. Survivor Corps has become active in patient advocacy and is a resource for researchers studying the new condition.
Ms. Berrent has become such a trusted voice during the pandemic. She interviewed Anthony Fauci, MD, head of the National Institutes of Allergy and Infectious Diseases, last October.
“The implications are huge,” she says.
“Some of this damage is permanent damage. It’s not going to cure the scarring of your heart tissue, it’s not going to cure the irreparable damage to your lungs, but if it’s making people feel better, then that’s an indication there’s viral persistence going on,” says Ms. Berrent.
“I’ve been saying for months and months, we shouldn’t be calling this postacute anything,” she adds.
Patients report improvement
Daniel Griffin, MD, PhD, is equally excited. He’s an infectious disease specialist at Columbia University, New York. He says about one in five patients he treated for COVID-19 last year never got better. Many of them, such as Mr. Goyang, were health care workers.
“I don’t know if people actually catch this, but a lot of our coworkers are either permanently disabled or died,” Dr. Griffin says.
Health care workers were also among the first to be vaccinated. Dr. Griffin says many of his patients began reaching out to him about a week or two after being vaccinated “and saying, ‘You know, I actually feel better.’ And some of them were saying, ‘I feel all better,’ after being sick – a lot of them – for a year.”
Then he was getting calls and texts from other doctors, asking, “Hey, are you seeing this?”
The benefits of vaccination for some long-haulers came as a surprise. Dr. Griffin says that, before the vaccines came out, many of his patients were worried that getting vaccinated might overstimulate their immune systems and cause symptoms to get worse.
Indeed, a small percentage of people – about 3%-5%, based on informal polls on social media – report that they do experience worsening of symptoms after getting the shot. It’s not clear why.
Dr. Griffin estimates that between 30% and 50% of patients’ symptoms improve after they receive the mRNA vaccines. “I’m seeing this chunk of people – they tell me their brain fog has improved, their fatigue is gone, the fevers that wouldn’t resolve have now gone,” he says. “I’m seeing that personally, and I’m hearing it from my colleagues.”
Dr. Griffin says the observation has launched several studies and that there are several theories about how the vaccines might be affecting long COVID.
An immune system boost?
One possibility is that the virus continues to stimulate the immune system, which continues to fight the virus for months. If that is the case, Dr. Griffin says, the vaccine may be giving the immune system the boost it needs to finally clear the virus away.
Donna Farber, PhD, a professor of microbiology and immunology at Columbia University, has heard the stories, too.
“It is possible that the persisting virus in long COVID-19 may be at a low level – not enough to stimulate a potent immune response to clear the virus, but enough to cause symptoms. Activating the immune response therefore is therapeutic in directing viral clearance,” she says.
Dr. Farber explains that long COVID may be a bit like Lyme disease. Some patients with Lyme disease must take antibiotics for months before their symptoms disappear.
Dr. Griffin says there’s another possibility. Several studies have now shown that people with lingering COVID-19 symptoms develop autoantibodies. There’s a theory that SARS-CoV-2 may create an autoimmune condition that leads to long-term symptoms.
If that is the case, Dr. Griffin says, the vaccine may be helping the body to reset its tolerance to itself, “so maybe now you’re getting a healthy immune response.”
More studies are needed to know for sure.
Either way, the vaccines are a much-needed bit of hope for the long-COVID community, and Dr. Griffin tells his patients who are still worried that, at the very least, they’ll be protected from another SARS-CoV-2 infection.
A version of this article first appeared on Medscape.com.
We’re all vaccinated: Can we go back to the office (unmasked) now?
Congratulations, you’ve been vaccinated!
It’s been a year like no other, and outpatient psychiatrists turned to Zoom and other telemental health platforms to provide treatment for our patients. Offices sit empty as the dust lands and the plants wilt. Perhaps a few patients are seen in person, masked and carefully distanced, after health screening and temperature checks, with surfaces sanitized between visits, all in accordance with health department regulations. But now the vaccine offers both safety and the promise of a return to a new normal, one that is certain to look different from the normal that was left behind.
I have been vaccinated and many of my patients have also been vaccinated. I began to wonder if it was safe to start seeing patients in person; could I see fully vaccinated patients, unmasked and without temperature checks and sanitizing? I started asking this question in February, and the response I got then was that it was too soon to tell; we did not have any data on whether vaccinated people could transmit the novel coronavirus. Two vaccinated people might be at risk of transmitting the virus and then infecting others, and the question of whether the vaccines would protect against illness caused by variants remained. Preliminary data out of Israel indicated that the vaccine did reduce transmission, but no one was saying that it was fine to see patients without masks, and video-conferencing remained the safest option.
On Monday, March 8, 2021, the Centers for Disease Control and Prevention released long-awaited interim public health guidelines for fully vaccinated people. The guidelines allowed for two vaccinated people to be in a room together unmasked, and for a fully-vaccinated person to be in a room unmasked with an unvaccinated person who did not have risk factors for becoming severely ill with COVID. Was this the green light that psychiatrists were waiting for? Was there new data about transmission, or was this part of the CDC’s effort to make vaccines more desirable?
Michael Chang, MD, is a pediatric infectious disease specialist at the University of Texas Health Science Center at Houston. We spoke 2 days after the CDC interim guidelines were released. Dr. Chang was optimistic.
“, including data about variants and about transmission. At some point, however, the risk is low enough, and we should probably start thinking about going back to in-person visits,” Dr. Chang said. He said he personally would feel safe meeting unmasked with a vaccinated patient, but noted that his institution still requires doctors to wear masks. “Most vaccinations reduce transmission of illness,” Dr. Chang said, “but SARS-CoV-2 continues to surprise us in many ways.”
Katelyn Jetelina, PhD, MPH, an epidemiologist at the University of Texas School of Public Health in Dallas, distributes a newsletter, “Your Local Epidemiologist,” where she discusses data pertaining to the pandemic. In her newsletter dated March 14, 2021, Dr. Jetelina wrote, “There are now 7 sub-studies/press releases that confirm a 50-95% reduced transmission after vaccination. This is a big range, which is typical for such drastically different scientific studies. Variability is likely due to different sample sizes, locations, vaccines, genetics, cultures, etc. It will be a while until we know the ‘true’ percentage for each vaccine.”
Leslie Walker, MD, is a fully vaccinated psychiatrist in private practice in Shaker Heights, Ohio. She has recently started seeing fully vaccinated patients in person.
“So far it’s only 1 or 2 patients a day. I’m leaving it up to the patient. If they prefer masks, we stay masked. I may reverse course, depending on what information comes out.” She went on to note, “There are benefits to being able to see someone’s full facial expressions and whether they match someone’s words and body language, so the benefit of “unmasking” extends beyond comfort and convenience and must be balanced against the theoretical risk of COVID exposure in the room.”
While the CDC has now said it is safe to meet, the state health departments also have guidelines for medical practices, and everyone is still worried about vulnerable people in their households and potential spread to the community at large.
In Maryland, where I work, Aliya Jones, MD, MBA, is the head of the Behavioral Health Administration (BHA) for the Maryland Department of Health. “It remains risky to not wear masks, however, the risk is low when both individuals are vaccinated,” Dr. Jones wrote. “BHA is not recommending that providers see clients without both parties wearing a mask. All of our general practice recommendations for infection control are unchanged. People should be screened before entering clinical practices and persons who are symptomatic, whether vaccinated or not, should not be seen face-to-face, except in cases of an emergency, in which case additional precautions should be taken.”
So is it safe for a fully-vaccinated psychiatrist to have a session with a fully-vaccinated patient sitting 8 feet apart without masks? I’m left with the idea that it is for those two people, but when it comes to unvaccinated people in their households, we want more certainty than we currently have. The messaging remains unclear. The CDC’s interim guidelines offer hope for a future, but the science is still catching up, and to feel safe enough, we may want to wait a little longer for more definitive data – or herd immunity – before we reveal our smiles.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore.
Congratulations, you’ve been vaccinated!
It’s been a year like no other, and outpatient psychiatrists turned to Zoom and other telemental health platforms to provide treatment for our patients. Offices sit empty as the dust lands and the plants wilt. Perhaps a few patients are seen in person, masked and carefully distanced, after health screening and temperature checks, with surfaces sanitized between visits, all in accordance with health department regulations. But now the vaccine offers both safety and the promise of a return to a new normal, one that is certain to look different from the normal that was left behind.
I have been vaccinated and many of my patients have also been vaccinated. I began to wonder if it was safe to start seeing patients in person; could I see fully vaccinated patients, unmasked and without temperature checks and sanitizing? I started asking this question in February, and the response I got then was that it was too soon to tell; we did not have any data on whether vaccinated people could transmit the novel coronavirus. Two vaccinated people might be at risk of transmitting the virus and then infecting others, and the question of whether the vaccines would protect against illness caused by variants remained. Preliminary data out of Israel indicated that the vaccine did reduce transmission, but no one was saying that it was fine to see patients without masks, and video-conferencing remained the safest option.
On Monday, March 8, 2021, the Centers for Disease Control and Prevention released long-awaited interim public health guidelines for fully vaccinated people. The guidelines allowed for two vaccinated people to be in a room together unmasked, and for a fully-vaccinated person to be in a room unmasked with an unvaccinated person who did not have risk factors for becoming severely ill with COVID. Was this the green light that psychiatrists were waiting for? Was there new data about transmission, or was this part of the CDC’s effort to make vaccines more desirable?
Michael Chang, MD, is a pediatric infectious disease specialist at the University of Texas Health Science Center at Houston. We spoke 2 days after the CDC interim guidelines were released. Dr. Chang was optimistic.
“, including data about variants and about transmission. At some point, however, the risk is low enough, and we should probably start thinking about going back to in-person visits,” Dr. Chang said. He said he personally would feel safe meeting unmasked with a vaccinated patient, but noted that his institution still requires doctors to wear masks. “Most vaccinations reduce transmission of illness,” Dr. Chang said, “but SARS-CoV-2 continues to surprise us in many ways.”
Katelyn Jetelina, PhD, MPH, an epidemiologist at the University of Texas School of Public Health in Dallas, distributes a newsletter, “Your Local Epidemiologist,” where she discusses data pertaining to the pandemic. In her newsletter dated March 14, 2021, Dr. Jetelina wrote, “There are now 7 sub-studies/press releases that confirm a 50-95% reduced transmission after vaccination. This is a big range, which is typical for such drastically different scientific studies. Variability is likely due to different sample sizes, locations, vaccines, genetics, cultures, etc. It will be a while until we know the ‘true’ percentage for each vaccine.”
Leslie Walker, MD, is a fully vaccinated psychiatrist in private practice in Shaker Heights, Ohio. She has recently started seeing fully vaccinated patients in person.
“So far it’s only 1 or 2 patients a day. I’m leaving it up to the patient. If they prefer masks, we stay masked. I may reverse course, depending on what information comes out.” She went on to note, “There are benefits to being able to see someone’s full facial expressions and whether they match someone’s words and body language, so the benefit of “unmasking” extends beyond comfort and convenience and must be balanced against the theoretical risk of COVID exposure in the room.”
While the CDC has now said it is safe to meet, the state health departments also have guidelines for medical practices, and everyone is still worried about vulnerable people in their households and potential spread to the community at large.
In Maryland, where I work, Aliya Jones, MD, MBA, is the head of the Behavioral Health Administration (BHA) for the Maryland Department of Health. “It remains risky to not wear masks, however, the risk is low when both individuals are vaccinated,” Dr. Jones wrote. “BHA is not recommending that providers see clients without both parties wearing a mask. All of our general practice recommendations for infection control are unchanged. People should be screened before entering clinical practices and persons who are symptomatic, whether vaccinated or not, should not be seen face-to-face, except in cases of an emergency, in which case additional precautions should be taken.”
So is it safe for a fully-vaccinated psychiatrist to have a session with a fully-vaccinated patient sitting 8 feet apart without masks? I’m left with the idea that it is for those two people, but when it comes to unvaccinated people in their households, we want more certainty than we currently have. The messaging remains unclear. The CDC’s interim guidelines offer hope for a future, but the science is still catching up, and to feel safe enough, we may want to wait a little longer for more definitive data – or herd immunity – before we reveal our smiles.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore.
Congratulations, you’ve been vaccinated!
It’s been a year like no other, and outpatient psychiatrists turned to Zoom and other telemental health platforms to provide treatment for our patients. Offices sit empty as the dust lands and the plants wilt. Perhaps a few patients are seen in person, masked and carefully distanced, after health screening and temperature checks, with surfaces sanitized between visits, all in accordance with health department regulations. But now the vaccine offers both safety and the promise of a return to a new normal, one that is certain to look different from the normal that was left behind.
I have been vaccinated and many of my patients have also been vaccinated. I began to wonder if it was safe to start seeing patients in person; could I see fully vaccinated patients, unmasked and without temperature checks and sanitizing? I started asking this question in February, and the response I got then was that it was too soon to tell; we did not have any data on whether vaccinated people could transmit the novel coronavirus. Two vaccinated people might be at risk of transmitting the virus and then infecting others, and the question of whether the vaccines would protect against illness caused by variants remained. Preliminary data out of Israel indicated that the vaccine did reduce transmission, but no one was saying that it was fine to see patients without masks, and video-conferencing remained the safest option.
On Monday, March 8, 2021, the Centers for Disease Control and Prevention released long-awaited interim public health guidelines for fully vaccinated people. The guidelines allowed for two vaccinated people to be in a room together unmasked, and for a fully-vaccinated person to be in a room unmasked with an unvaccinated person who did not have risk factors for becoming severely ill with COVID. Was this the green light that psychiatrists were waiting for? Was there new data about transmission, or was this part of the CDC’s effort to make vaccines more desirable?
Michael Chang, MD, is a pediatric infectious disease specialist at the University of Texas Health Science Center at Houston. We spoke 2 days after the CDC interim guidelines were released. Dr. Chang was optimistic.
“, including data about variants and about transmission. At some point, however, the risk is low enough, and we should probably start thinking about going back to in-person visits,” Dr. Chang said. He said he personally would feel safe meeting unmasked with a vaccinated patient, but noted that his institution still requires doctors to wear masks. “Most vaccinations reduce transmission of illness,” Dr. Chang said, “but SARS-CoV-2 continues to surprise us in many ways.”
Katelyn Jetelina, PhD, MPH, an epidemiologist at the University of Texas School of Public Health in Dallas, distributes a newsletter, “Your Local Epidemiologist,” where she discusses data pertaining to the pandemic. In her newsletter dated March 14, 2021, Dr. Jetelina wrote, “There are now 7 sub-studies/press releases that confirm a 50-95% reduced transmission after vaccination. This is a big range, which is typical for such drastically different scientific studies. Variability is likely due to different sample sizes, locations, vaccines, genetics, cultures, etc. It will be a while until we know the ‘true’ percentage for each vaccine.”
Leslie Walker, MD, is a fully vaccinated psychiatrist in private practice in Shaker Heights, Ohio. She has recently started seeing fully vaccinated patients in person.
“So far it’s only 1 or 2 patients a day. I’m leaving it up to the patient. If they prefer masks, we stay masked. I may reverse course, depending on what information comes out.” She went on to note, “There are benefits to being able to see someone’s full facial expressions and whether they match someone’s words and body language, so the benefit of “unmasking” extends beyond comfort and convenience and must be balanced against the theoretical risk of COVID exposure in the room.”
While the CDC has now said it is safe to meet, the state health departments also have guidelines for medical practices, and everyone is still worried about vulnerable people in their households and potential spread to the community at large.
In Maryland, where I work, Aliya Jones, MD, MBA, is the head of the Behavioral Health Administration (BHA) for the Maryland Department of Health. “It remains risky to not wear masks, however, the risk is low when both individuals are vaccinated,” Dr. Jones wrote. “BHA is not recommending that providers see clients without both parties wearing a mask. All of our general practice recommendations for infection control are unchanged. People should be screened before entering clinical practices and persons who are symptomatic, whether vaccinated or not, should not be seen face-to-face, except in cases of an emergency, in which case additional precautions should be taken.”
So is it safe for a fully-vaccinated psychiatrist to have a session with a fully-vaccinated patient sitting 8 feet apart without masks? I’m left with the idea that it is for those two people, but when it comes to unvaccinated people in their households, we want more certainty than we currently have. The messaging remains unclear. The CDC’s interim guidelines offer hope for a future, but the science is still catching up, and to feel safe enough, we may want to wait a little longer for more definitive data – or herd immunity – before we reveal our smiles.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore.
Could pollen be driving COVID-19 infections?
Some scientists say they’ve noticed a pattern to the recurring waves of SARS-CoV-2 infections around the globe: As pollen levels increased in outdoor air in 31 countries, COVID-19 cases accelerated.
Yet other recent studies point in the opposite direction, suggesting that peaks in pollen seasons coincide with a fall-off in the spread of some respiratory viruses, like COVID-19 and influenza. There’s even some evidence that pollen may compete with the virus that causes COVID-19 and may even help prevent infection.
So which is it? The answer may still be up in the air.
Doctors don’t fully understand what makes some viruses – like the ones that cause the flu – circulate in seasonal patterns.
There are, of course, many theories. These revolve around things like temperature and humidity – viruses tend to prefer colder, drier air – something that’s thought to help them spread more easily in the winter months. People are exposed to less sunlight during the winter, as they spend more time indoors, and the earth points away from the sun, providing some natural shielding. That may play a role because ultraviolet light from the sun acts like a natural disinfectant and may help keep circulating viral levels down.
In addition, exposure to sunlight helps the body make vitamin D, which may help keep our immune responses strong. Extreme temperatures – both cold and hot – also change our behavior, so that we spend more time cloistered indoors, where we can more easily cough and sneeze on each other and generally swap more germs.
Spike in pollen, jump in infections
The new study, published in the Proceedings of the National Academy of Sciences, adds a new variable to this mix – pollen. It relies on data from 248 airborne pollen–monitoring sites in 31 countries. The study also took into account other effects, such as population density, temperature, humidity, and lockdown orders. The study authors found that, when pollen in an area spiked, so did infections, after an average lag of about 4 days. The study authors say pollen seemed to account for, on average, 44% of the infection rate variability between countries.
The study authors say pollen could be a culprit in respiratory infections, not because the viruses hitch a ride on pollen grains and travel into our mouth, eyes, and nose, but because pollen seems to perturb our immune defenses, even if a person isn’t allergic to it.
“When we inhale pollen, they end up on our nasal mucosa, and here they diminish the expression of genes that are important for the defense against airborne viruses,” study author Stefanie Gilles, PhD, chair of environmental medicine at the Technical University of Munich, said in a press conference.
In a study published last year, Dr. Gilles found that mice exposed to pollen made less interferon and other protective chemical signals to the immune system. Those then infected with respiratory syncytial virus had more virus in their bodies, compared with mice not exposed to pollen. She seemed to see the same effect in human volunteers.
The study authors think pollen may cause the body to drop its defenses against the airborne virus that causes COVID-19, too.
“If you’re in a crowded room, and other people are there that are asymptomatic, and you’ve just been breathing in pollen all day long, chances are that you’re going to be more susceptible to the virus,” says Lewis Ziska, PhD, a plant physiologist who studies pollen, climate change, and health at Columbia University’s Mailman School of Public Health in New York. “Having a mask is obviously really critical in that regard.”
Masks do a great job of blocking pollen, so wearing one is even more important when pollen and viruses are floating around, he says.
Other researchers, however, say that, while the study raises some interesting questions, it can’t prove that pollen is increasing COVID-19 infections.
“Just because two things happen at the same time doesn’t mean that one causes the other,” says Martijn Hoogeveen, PhD, a professor of technical sciences and environment at the Open University in the Netherlands.
Dr. Hoogeveen’s recent study, published in Science of the Total Environment, found that the arrival of pollen season in the Netherlands coincides with the end of flu season, and that COVID-19 infection peaks tend to follow a similar pattern – exactly the opposite of the PNAS study.
Another preprint study, which focused on the Chicago area, found the same thing – as pollen climbs, flu cases drop. The researchers behind that study think pollen may actually compete with viruses in our airways, helping to block them from infecting our cells.
Patterns may be hard to nail down
Why did these studies reach such different conclusions?
Dr. Hoogeveen’s paper focused on a single country and looked at the incidence of flu infections over four seasons, from 2016 to 2020, while the PNAS study collected data on pollen from January through the first week of April 2020.
He thinks that a single season, or really part of a season, may not be long enough to see meaningful patterns, especially considering that this new-to-humans virus was spreading quickly at nearly the same time. He says it will be interesting to follow what happens with COVID-19 infections and pollen in the coming months and years.
Dr. Hoogeveen says that in a large study spanning so many countries it would have been nearly impossible to account for differences in pandemic control strategies. Some countries embraced the use of masks, stay-at-home orders, and social distancing, for example, while others took less stringent measures in order to let the virus run its course in pursuit of herd immunity.
Limiting the study area to a single country or city, he says, helps researchers better understand all the variables that might have been in play along with pollen.
“There is no scientific consensus yet, about what it is driving, and that’s what makes it such an interesting field,” he says.
A version of this article first appeared on Medscape.com.
Some scientists say they’ve noticed a pattern to the recurring waves of SARS-CoV-2 infections around the globe: As pollen levels increased in outdoor air in 31 countries, COVID-19 cases accelerated.
Yet other recent studies point in the opposite direction, suggesting that peaks in pollen seasons coincide with a fall-off in the spread of some respiratory viruses, like COVID-19 and influenza. There’s even some evidence that pollen may compete with the virus that causes COVID-19 and may even help prevent infection.
So which is it? The answer may still be up in the air.
Doctors don’t fully understand what makes some viruses – like the ones that cause the flu – circulate in seasonal patterns.
There are, of course, many theories. These revolve around things like temperature and humidity – viruses tend to prefer colder, drier air – something that’s thought to help them spread more easily in the winter months. People are exposed to less sunlight during the winter, as they spend more time indoors, and the earth points away from the sun, providing some natural shielding. That may play a role because ultraviolet light from the sun acts like a natural disinfectant and may help keep circulating viral levels down.
In addition, exposure to sunlight helps the body make vitamin D, which may help keep our immune responses strong. Extreme temperatures – both cold and hot – also change our behavior, so that we spend more time cloistered indoors, where we can more easily cough and sneeze on each other and generally swap more germs.
Spike in pollen, jump in infections
The new study, published in the Proceedings of the National Academy of Sciences, adds a new variable to this mix – pollen. It relies on data from 248 airborne pollen–monitoring sites in 31 countries. The study also took into account other effects, such as population density, temperature, humidity, and lockdown orders. The study authors found that, when pollen in an area spiked, so did infections, after an average lag of about 4 days. The study authors say pollen seemed to account for, on average, 44% of the infection rate variability between countries.
The study authors say pollen could be a culprit in respiratory infections, not because the viruses hitch a ride on pollen grains and travel into our mouth, eyes, and nose, but because pollen seems to perturb our immune defenses, even if a person isn’t allergic to it.
“When we inhale pollen, they end up on our nasal mucosa, and here they diminish the expression of genes that are important for the defense against airborne viruses,” study author Stefanie Gilles, PhD, chair of environmental medicine at the Technical University of Munich, said in a press conference.
In a study published last year, Dr. Gilles found that mice exposed to pollen made less interferon and other protective chemical signals to the immune system. Those then infected with respiratory syncytial virus had more virus in their bodies, compared with mice not exposed to pollen. She seemed to see the same effect in human volunteers.
The study authors think pollen may cause the body to drop its defenses against the airborne virus that causes COVID-19, too.
“If you’re in a crowded room, and other people are there that are asymptomatic, and you’ve just been breathing in pollen all day long, chances are that you’re going to be more susceptible to the virus,” says Lewis Ziska, PhD, a plant physiologist who studies pollen, climate change, and health at Columbia University’s Mailman School of Public Health in New York. “Having a mask is obviously really critical in that regard.”
Masks do a great job of blocking pollen, so wearing one is even more important when pollen and viruses are floating around, he says.
Other researchers, however, say that, while the study raises some interesting questions, it can’t prove that pollen is increasing COVID-19 infections.
“Just because two things happen at the same time doesn’t mean that one causes the other,” says Martijn Hoogeveen, PhD, a professor of technical sciences and environment at the Open University in the Netherlands.
Dr. Hoogeveen’s recent study, published in Science of the Total Environment, found that the arrival of pollen season in the Netherlands coincides with the end of flu season, and that COVID-19 infection peaks tend to follow a similar pattern – exactly the opposite of the PNAS study.
Another preprint study, which focused on the Chicago area, found the same thing – as pollen climbs, flu cases drop. The researchers behind that study think pollen may actually compete with viruses in our airways, helping to block them from infecting our cells.
Patterns may be hard to nail down
Why did these studies reach such different conclusions?
Dr. Hoogeveen’s paper focused on a single country and looked at the incidence of flu infections over four seasons, from 2016 to 2020, while the PNAS study collected data on pollen from January through the first week of April 2020.
He thinks that a single season, or really part of a season, may not be long enough to see meaningful patterns, especially considering that this new-to-humans virus was spreading quickly at nearly the same time. He says it will be interesting to follow what happens with COVID-19 infections and pollen in the coming months and years.
Dr. Hoogeveen says that in a large study spanning so many countries it would have been nearly impossible to account for differences in pandemic control strategies. Some countries embraced the use of masks, stay-at-home orders, and social distancing, for example, while others took less stringent measures in order to let the virus run its course in pursuit of herd immunity.
Limiting the study area to a single country or city, he says, helps researchers better understand all the variables that might have been in play along with pollen.
“There is no scientific consensus yet, about what it is driving, and that’s what makes it such an interesting field,” he says.
A version of this article first appeared on Medscape.com.
Some scientists say they’ve noticed a pattern to the recurring waves of SARS-CoV-2 infections around the globe: As pollen levels increased in outdoor air in 31 countries, COVID-19 cases accelerated.
Yet other recent studies point in the opposite direction, suggesting that peaks in pollen seasons coincide with a fall-off in the spread of some respiratory viruses, like COVID-19 and influenza. There’s even some evidence that pollen may compete with the virus that causes COVID-19 and may even help prevent infection.
So which is it? The answer may still be up in the air.
Doctors don’t fully understand what makes some viruses – like the ones that cause the flu – circulate in seasonal patterns.
There are, of course, many theories. These revolve around things like temperature and humidity – viruses tend to prefer colder, drier air – something that’s thought to help them spread more easily in the winter months. People are exposed to less sunlight during the winter, as they spend more time indoors, and the earth points away from the sun, providing some natural shielding. That may play a role because ultraviolet light from the sun acts like a natural disinfectant and may help keep circulating viral levels down.
In addition, exposure to sunlight helps the body make vitamin D, which may help keep our immune responses strong. Extreme temperatures – both cold and hot – also change our behavior, so that we spend more time cloistered indoors, where we can more easily cough and sneeze on each other and generally swap more germs.
Spike in pollen, jump in infections
The new study, published in the Proceedings of the National Academy of Sciences, adds a new variable to this mix – pollen. It relies on data from 248 airborne pollen–monitoring sites in 31 countries. The study also took into account other effects, such as population density, temperature, humidity, and lockdown orders. The study authors found that, when pollen in an area spiked, so did infections, after an average lag of about 4 days. The study authors say pollen seemed to account for, on average, 44% of the infection rate variability between countries.
The study authors say pollen could be a culprit in respiratory infections, not because the viruses hitch a ride on pollen grains and travel into our mouth, eyes, and nose, but because pollen seems to perturb our immune defenses, even if a person isn’t allergic to it.
“When we inhale pollen, they end up on our nasal mucosa, and here they diminish the expression of genes that are important for the defense against airborne viruses,” study author Stefanie Gilles, PhD, chair of environmental medicine at the Technical University of Munich, said in a press conference.
In a study published last year, Dr. Gilles found that mice exposed to pollen made less interferon and other protective chemical signals to the immune system. Those then infected with respiratory syncytial virus had more virus in their bodies, compared with mice not exposed to pollen. She seemed to see the same effect in human volunteers.
The study authors think pollen may cause the body to drop its defenses against the airborne virus that causes COVID-19, too.
“If you’re in a crowded room, and other people are there that are asymptomatic, and you’ve just been breathing in pollen all day long, chances are that you’re going to be more susceptible to the virus,” says Lewis Ziska, PhD, a plant physiologist who studies pollen, climate change, and health at Columbia University’s Mailman School of Public Health in New York. “Having a mask is obviously really critical in that regard.”
Masks do a great job of blocking pollen, so wearing one is even more important when pollen and viruses are floating around, he says.
Other researchers, however, say that, while the study raises some interesting questions, it can’t prove that pollen is increasing COVID-19 infections.
“Just because two things happen at the same time doesn’t mean that one causes the other,” says Martijn Hoogeveen, PhD, a professor of technical sciences and environment at the Open University in the Netherlands.
Dr. Hoogeveen’s recent study, published in Science of the Total Environment, found that the arrival of pollen season in the Netherlands coincides with the end of flu season, and that COVID-19 infection peaks tend to follow a similar pattern – exactly the opposite of the PNAS study.
Another preprint study, which focused on the Chicago area, found the same thing – as pollen climbs, flu cases drop. The researchers behind that study think pollen may actually compete with viruses in our airways, helping to block them from infecting our cells.
Patterns may be hard to nail down
Why did these studies reach such different conclusions?
Dr. Hoogeveen’s paper focused on a single country and looked at the incidence of flu infections over four seasons, from 2016 to 2020, while the PNAS study collected data on pollen from January through the first week of April 2020.
He thinks that a single season, or really part of a season, may not be long enough to see meaningful patterns, especially considering that this new-to-humans virus was spreading quickly at nearly the same time. He says it will be interesting to follow what happens with COVID-19 infections and pollen in the coming months and years.
Dr. Hoogeveen says that in a large study spanning so many countries it would have been nearly impossible to account for differences in pandemic control strategies. Some countries embraced the use of masks, stay-at-home orders, and social distancing, for example, while others took less stringent measures in order to let the virus run its course in pursuit of herd immunity.
Limiting the study area to a single country or city, he says, helps researchers better understand all the variables that might have been in play along with pollen.
“There is no scientific consensus yet, about what it is driving, and that’s what makes it such an interesting field,” he says.
A version of this article first appeared on Medscape.com.
Virtual is the new real
Why did we fall short on maximizing telehealth’s value in the COVID-19 pandemic?
The COVID-19 pandemic catalyzed the transformation of Internet-based, remotely accessible innovative technologies. Internet-based customer service delivery technology was rapidly adopted and utilized by several services industries, but health care systems in most of the countries across the world faced unique challenges in adopting the technology for the delivery of health care services. The health care ecosystem of the United States was not immune to such challenges, and several significant barriers surfaced while the pandemic was underway.
Complexly structured, fragmented, unprepared, and overly burnt-out health systems in the United States arguably have fallen short of maximizing the value of telehealth in delivering safe, easily accessible, comprehensive, and cost-effective health care services. In this essay, we examine the reasons for such a suboptimal performance and discuss a few important strategies that may be useful in maximizing the value of telehealth value in several, appropriate health care services.
Hospitals and telehealth
Are hospitalists preparing ourselves “not to see” patients in a hospital-based health care delivery setting? If you have not yet started yet, now may be the right time! Yes, a certain percentage of doctor-patient encounters in hospital settings will remain virtual forever.
A well-established telehealth infrastructure is rarely found in most U.S. hospitals, although the COVID-19 pandemic has unexpectedly boosted the rapid growth of telehealth in the country.1 Public health emergency declarations in the United States in the face of the COVID-19 crisis have facilitated two important initiatives to restore health care delivery amidst formal and informal lockdowns that brought states to a grinding halt. These extend from expansion of virtual services, including telehealth, virtual check-ins, and e-visits, to the decision by the Department of Health & Human Services Office of Civil Rights to exercise enforcement discretion and waive penalties for the use of relatively inexpensive, non–public-facing mobile and other audiovisual technology tools.2
Hospital-based care in the United States taps nearly 33% of national health expenditure. An additional 30% of national health expenditure that is related to physicians, prescriptions, and other facilities is indirectly influenced by care delivered at health care facilities.3 Studies show that about 20% of ED visits could potentially be avoided via virtual urgent care offerings.4 A rapidly changing health care ecosystem is proving formidable for most hospital systems, and a test for their resilience and agility. Not just the implementation of telehealth is challenging, but getting it right is the key success factor.
Hospital-based telehealth
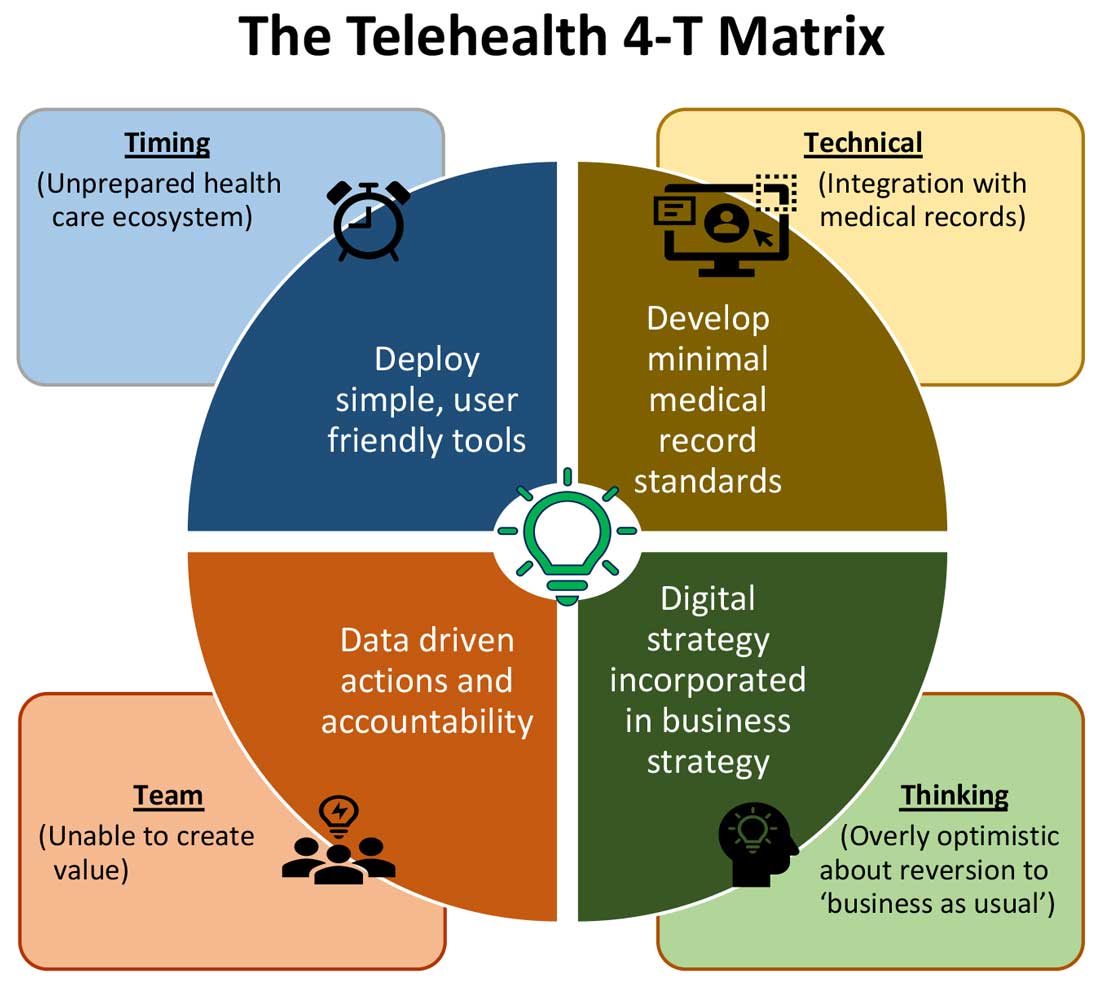
Expansion of telehealth coverage by the Centers for Medicare & Medicaid Services and most commercial payers did not quite ride the pandemic-induced momentum across the care continuum. Hospitals are lagging far behind ambulatory care in implementing telehealth. As illustrated in the “4-T Matrix” (see graphic) we would like to examine four key reasons for such a sluggish initial uptake and try to propose four important strategies that may help us to maximize the value created by telehealth technologies.
1. Timing
The health care system has always lagged far behind other service industries in terms of technology adaptation. Because of the unique nature of health care services, face-to-face interaction supersedes all other forms of communication. A rapidly evolving pandemic was not matched by simultaneous technology education for patients and providers. The enormous choice of hard-to-navigate telehealth tools; time and labor-intensive implementation; and uncertainty around payer, policy, and regulatory expectations might have precluded providers from the rapid adoption of telehealth in the hospital setting. Patients’ specific characteristics, such as the absence of technology-centered education, information, age, comorbidities, lack of technical literacy, and dependency on caregivers contributed to the suboptimal response from patients and families.
Deploying simple, ubiquitous, user-friendly, and technologically less challenging telehealth solutions may be a better approach to increase the adoption of such solutions by providers and patients. Hospitals need to develop and distribute telehealth user guides in all possible modes of communication. Provider-centric in-service sessions, workshops, and live support by “superuser teams” often work well in reducing end-user resistance.
2. Technical
Current electronic medical records vary widely in their features and offerings, and their ability to interact with third-party software and platforms. Dissatisfaction of end users with EMRs is well known, as is their likely relationship to burnout. Recent research continues to show a strong relationship between EMR usability and the odds of burnout among physicians.5 In the current climate, administrators and health informaticists have the responsibility to avoid adding increased burdens to end users.
Another issue is the limited connectivity in many remote/rural areas that would impact implementation of telehealth platforms. Studies indicate that 33% of rural Americans lack access to high-speed broadband Internet to support video visits.6 The recent successful implementation of telehealth across 530 providers in 75 ambulatory practices operated by Munson Healthcare, a rural health system in northern Michigan, sheds light on the technology’s enormous potential in providing safe access to rural populations.6,7
Privacy and safety of patient data is of paramount importance. According to a national poll on healthy aging by the University of Michigan in May 2019, targeting older adults, 47% of survey responders expressed difficulty using technology and 49% of survey responders were concerned about privacy.8 Use of certification and other tools offered by the Office of the National Coordinator for Health Information Technology would help reassure users, and the ability to capture and share images between providers would be of immense benefit in facilitating e-consults.
The need of the hour is redesigned work flow, to help providers adopt and use virtual care/telehealth efficiently. Work flow redesign must be coupled with technological advances to allow seamless integration of third-party telehealth platforms into existing EMR systems or built directly into EMRs. Use of quality metrics and analytical tools specific to telehealth would help measure the technology’s impact on patient care, outcomes, and end-user/provider experience.
3. Teams and training
Outcomes of health care interventions are often determined by the effectiveness of teams. Irrespective of how robust health care systems may have been initially, rapidly spreading infectious diseases like COVID-19 can quickly derail the system, bringing the workforce and patients to a breaking point.5 Decentralized, uncoordinated, and siloed efforts by individual teams across the care continuum were contributing factors for the partial success of telehealth care delivery pathways. The hospital systems with telehealth-ready teams at the start of the COVID-19 pandemic were so rare that the knowledge and technical training opportunities for innovators grew severalfold during the pandemic.
As per the American Medical Association, telehealth success is massively dependent on building the right team. Core, leadership, advisory, and implementation teams comprised of clinical representatives, end users, administrative personnel, executive members of the organization, technical experts, and payment/policy experts should be put together before implementing a telehealth strategy.9 Seamless integration of hospital-based care with ambulatory care via a telehealth platform is only complete when care managers are trained and deployed to fulfill the needs of a diverse group of patients. Deriving overall value from telehealth is only possible when there is a skill development, training and mentoring team put in place.
4. Thinking
In most U.S. hospitals, inpatient health care is equally distributed between nonprocedure and procedure-based services. Hospitals resorted to suspension of nonemergent procedures to mitigate the risk of spreading COVID-19. This was further compounded by many patients’ self-selection to defer care, an abrupt reduction in the influx of patients from the referral base because of suboptimally operating ambulatory care services, leading to low hospital occupancy.
Hospitals across the nation have gone through a massive short-term financial crunch and unfavorable cash-flow forecast, which prompted a paradoxical work-force reduction. While some argue that it may be akin to strategic myopia, the authors believed that such a response is strategically imperative to keep the hospital afloat. It is reasonable to attribute the paucity of innovation to constrained resources, and health systems are simply staying overly optimistic about “weathering the storm” and reverting soon to “business as usual.” The technological framework necessary for deploying a telehealth solution often comes with a price. Financially challenged hospital systems rarely exercise any capital-intensive activities. By contrast, telehealth adoption by ambulatory care can result in quicker resumption of patient care in community settings. A lack of operational and infrastructure synchrony between ambulatory and in-hospital systems has failed to capture telehealth-driven inpatient volume. For example, direct admissions from ambulatory telehealth referrals was a missed opportunity in several places. Referrals for labs, diagnostic tests, and other allied services could have helped hospitals offset their fixed costs. Similarly, work flows related to discharge and postdischarge follow up rarely embrace telehealth tools or telehealth care pathways. A brisk change in the health care ecosystem is partly responsible for this.
Digital strategy needs to be incorporated into business strategy. For the reasons already discussed, telehealth technology is not a “nice to have” anymore, but a “must have.” At present, providers are of the opinion that about 20% of their patient services can be delivered via a telehealth platform. Similar trends are observed among patients, as a new modality of access to care is increasingly beneficial to them. Telehealth must be incorporated in standardized hospital work flows. Use of telehealth for preoperative clearance will greatly minimize same-day surgery cancellations. Given the potential shortage in resources, telehealth adoption for inpatient consultations will help systems conserve personal protective equipment, minimize the risk of staff exposure to COVID-19, and improve efficiency.
Digital strategy also prompts the reengineering of care delivery.10 Excessive and unused physical capacity can be converted into digital care hubs. Health maintenance, prevention, health promotion, health education, and chronic disease management not only can serve a variety of patient groups but can also help address the “last-mile problem” in health care. A successful digital strategy usually has three important components – Commitment: Hospital leadership is committed to include digital transformation as a strategic objective; Cost: Digital strategy is added as a line item in the budget; and Control: Measurable metrics are put in place to monitor the performance, impact, and influence of the digital strategy.
Conclusion
For decades, most U.S. health systems occupied the periphery of technological transformation when compared to the rest of the service industry. While most health systems took a heroic approach to the adoption of telehealth during COVID-19, despite being unprepared, the need for a systematic telehealth deployment is far from being adequately fulfilled. The COVID-19 pandemic brought permanent changes to several business disciplines globally. Given the impact of the pandemic on the health and overall wellbeing of American society, the U.S. health care industry must leave no stone unturned in its quest for transformation.
Dr. Lingisetty is a hospitalist and physician executive at Baptist Health System, Little Rock, Ark, and is cofounder/president of SHM’s Arkansas chapter. Dr. Prasad is medical director of care management and a hospitalist at Advocate Aurora Health in Milwaukee. He is cochair of SHM’s IT Special Interest Group, sits on the HQPS committee, and is president of SHM’s Wisconsin chapter. Dr. Palabindala is the medical director, utilization management, and physician advisory services at the University of Mississippi Medical Center and an associate professor of medicine and academic hospitalist at the University of Mississippi, both in Jackson.
References
1. Finnegan M. “Telehealth booms amid COVID-19 crisis.” Computerworld. 2020 Apr 27. www.computerworld.com/article/3540315/telehealth-booms-amid-covid-19-crisis-virtual-care-is-here-to-stay.html. Accessed 2020 Sep 12.
2. Department of Health & Human Services. “OCR Announces Notification of Enforcement Discretion for Telehealth Remote Communications During the COVID-19 Nationwide Public Health Emergency.” 2020 Mar 17. www.hhs.gov/about/news/2020/03/17/ocr-announces-notification-of-enforcement-discretion-for-telehealth-remote-communications-during-the-covid-19.html. Accessed 2020 Sep 12.
3. National Center for Health Statistics. “Health Expenditures.” www.cdc.gov/nchs/fastats/health-expenditures.htm. Accessed 2020 Sep 12.
4. Bestsennyy O et al. “Telehealth: A post–COVID-19 reality?” McKinsey & Company. 2020 May 29. www.mckinsey.com/industries/healthcare-systems-and-services/our-insights/telehealth-a-quarter-trillion-dollar-post-covid-19-reality. Accessed 2020 Sep 12.
5. Melnick ER et al. The Association Between Perceived Electronic Health Record Usability and Professional Burnout Among U.S. Physicians. Mayo Clin Proc. 2020 March;95(3):476-87.
6. Hirko KA et al. Telehealth in response to the COVID-19 pandemic: Implications for rural health disparities. J Am Med Inform Assoc. 2020 Nov;27(11):1816-8. .
7. American Academy of Family Physicians. “Study Examines Telehealth, Rural Disparities in Pandemic.” 2020 July 30. www.aafp.org/news/practice-professional-issues/20200730ruraltelehealth.html. Accessed 2020 Dec 15.
8. Kurlander J et al. “Virtual Visits: Telehealth and Older Adults.” National Poll on Healthy Aging. 2019 Oct. hdl.handle.net/2027.42/151376.
9. American Medical Association. Telehealth Implementation Playbook. 2019. www.ama-assn.org/system/files/2020-04/ama-telehealth-implementation-playbook.pdf.
10. Smith AC et al. Telehealth for global emergencies: Implications for coronavirus disease 2019 (COVID-19). J Telemed Telecare. 2020 Jun;26(5):309-13.
Why did we fall short on maximizing telehealth’s value in the COVID-19 pandemic?
Why did we fall short on maximizing telehealth’s value in the COVID-19 pandemic?
The COVID-19 pandemic catalyzed the transformation of Internet-based, remotely accessible innovative technologies. Internet-based customer service delivery technology was rapidly adopted and utilized by several services industries, but health care systems in most of the countries across the world faced unique challenges in adopting the technology for the delivery of health care services. The health care ecosystem of the United States was not immune to such challenges, and several significant barriers surfaced while the pandemic was underway.
Complexly structured, fragmented, unprepared, and overly burnt-out health systems in the United States arguably have fallen short of maximizing the value of telehealth in delivering safe, easily accessible, comprehensive, and cost-effective health care services. In this essay, we examine the reasons for such a suboptimal performance and discuss a few important strategies that may be useful in maximizing the value of telehealth value in several, appropriate health care services.
Hospitals and telehealth
Are hospitalists preparing ourselves “not to see” patients in a hospital-based health care delivery setting? If you have not yet started yet, now may be the right time! Yes, a certain percentage of doctor-patient encounters in hospital settings will remain virtual forever.
A well-established telehealth infrastructure is rarely found in most U.S. hospitals, although the COVID-19 pandemic has unexpectedly boosted the rapid growth of telehealth in the country.1 Public health emergency declarations in the United States in the face of the COVID-19 crisis have facilitated two important initiatives to restore health care delivery amidst formal and informal lockdowns that brought states to a grinding halt. These extend from expansion of virtual services, including telehealth, virtual check-ins, and e-visits, to the decision by the Department of Health & Human Services Office of Civil Rights to exercise enforcement discretion and waive penalties for the use of relatively inexpensive, non–public-facing mobile and other audiovisual technology tools.2
Hospital-based care in the United States taps nearly 33% of national health expenditure. An additional 30% of national health expenditure that is related to physicians, prescriptions, and other facilities is indirectly influenced by care delivered at health care facilities.3 Studies show that about 20% of ED visits could potentially be avoided via virtual urgent care offerings.4 A rapidly changing health care ecosystem is proving formidable for most hospital systems, and a test for their resilience and agility. Not just the implementation of telehealth is challenging, but getting it right is the key success factor.
Hospital-based telehealth

Expansion of telehealth coverage by the Centers for Medicare & Medicaid Services and most commercial payers did not quite ride the pandemic-induced momentum across the care continuum. Hospitals are lagging far behind ambulatory care in implementing telehealth. As illustrated in the “4-T Matrix” (see graphic) we would like to examine four key reasons for such a sluggish initial uptake and try to propose four important strategies that may help us to maximize the value created by telehealth technologies.
1. Timing
The health care system has always lagged far behind other service industries in terms of technology adaptation. Because of the unique nature of health care services, face-to-face interaction supersedes all other forms of communication. A rapidly evolving pandemic was not matched by simultaneous technology education for patients and providers. The enormous choice of hard-to-navigate telehealth tools; time and labor-intensive implementation; and uncertainty around payer, policy, and regulatory expectations might have precluded providers from the rapid adoption of telehealth in the hospital setting. Patients’ specific characteristics, such as the absence of technology-centered education, information, age, comorbidities, lack of technical literacy, and dependency on caregivers contributed to the suboptimal response from patients and families.
Deploying simple, ubiquitous, user-friendly, and technologically less challenging telehealth solutions may be a better approach to increase the adoption of such solutions by providers and patients. Hospitals need to develop and distribute telehealth user guides in all possible modes of communication. Provider-centric in-service sessions, workshops, and live support by “superuser teams” often work well in reducing end-user resistance.
2. Technical
Current electronic medical records vary widely in their features and offerings, and their ability to interact with third-party software and platforms. Dissatisfaction of end users with EMRs is well known, as is their likely relationship to burnout. Recent research continues to show a strong relationship between EMR usability and the odds of burnout among physicians.5 In the current climate, administrators and health informaticists have the responsibility to avoid adding increased burdens to end users.
Another issue is the limited connectivity in many remote/rural areas that would impact implementation of telehealth platforms. Studies indicate that 33% of rural Americans lack access to high-speed broadband Internet to support video visits.6 The recent successful implementation of telehealth across 530 providers in 75 ambulatory practices operated by Munson Healthcare, a rural health system in northern Michigan, sheds light on the technology’s enormous potential in providing safe access to rural populations.6,7
Privacy and safety of patient data is of paramount importance. According to a national poll on healthy aging by the University of Michigan in May 2019, targeting older adults, 47% of survey responders expressed difficulty using technology and 49% of survey responders were concerned about privacy.8 Use of certification and other tools offered by the Office of the National Coordinator for Health Information Technology would help reassure users, and the ability to capture and share images between providers would be of immense benefit in facilitating e-consults.
The need of the hour is redesigned work flow, to help providers adopt and use virtual care/telehealth efficiently. Work flow redesign must be coupled with technological advances to allow seamless integration of third-party telehealth platforms into existing EMR systems or built directly into EMRs. Use of quality metrics and analytical tools specific to telehealth would help measure the technology’s impact on patient care, outcomes, and end-user/provider experience.
3. Teams and training
Outcomes of health care interventions are often determined by the effectiveness of teams. Irrespective of how robust health care systems may have been initially, rapidly spreading infectious diseases like COVID-19 can quickly derail the system, bringing the workforce and patients to a breaking point.5 Decentralized, uncoordinated, and siloed efforts by individual teams across the care continuum were contributing factors for the partial success of telehealth care delivery pathways. The hospital systems with telehealth-ready teams at the start of the COVID-19 pandemic were so rare that the knowledge and technical training opportunities for innovators grew severalfold during the pandemic.
As per the American Medical Association, telehealth success is massively dependent on building the right team. Core, leadership, advisory, and implementation teams comprised of clinical representatives, end users, administrative personnel, executive members of the organization, technical experts, and payment/policy experts should be put together before implementing a telehealth strategy.9 Seamless integration of hospital-based care with ambulatory care via a telehealth platform is only complete when care managers are trained and deployed to fulfill the needs of a diverse group of patients. Deriving overall value from telehealth is only possible when there is a skill development, training and mentoring team put in place.
4. Thinking
In most U.S. hospitals, inpatient health care is equally distributed between nonprocedure and procedure-based services. Hospitals resorted to suspension of nonemergent procedures to mitigate the risk of spreading COVID-19. This was further compounded by many patients’ self-selection to defer care, an abrupt reduction in the influx of patients from the referral base because of suboptimally operating ambulatory care services, leading to low hospital occupancy.
Hospitals across the nation have gone through a massive short-term financial crunch and unfavorable cash-flow forecast, which prompted a paradoxical work-force reduction. While some argue that it may be akin to strategic myopia, the authors believed that such a response is strategically imperative to keep the hospital afloat. It is reasonable to attribute the paucity of innovation to constrained resources, and health systems are simply staying overly optimistic about “weathering the storm” and reverting soon to “business as usual.” The technological framework necessary for deploying a telehealth solution often comes with a price. Financially challenged hospital systems rarely exercise any capital-intensive activities. By contrast, telehealth adoption by ambulatory care can result in quicker resumption of patient care in community settings. A lack of operational and infrastructure synchrony between ambulatory and in-hospital systems has failed to capture telehealth-driven inpatient volume. For example, direct admissions from ambulatory telehealth referrals was a missed opportunity in several places. Referrals for labs, diagnostic tests, and other allied services could have helped hospitals offset their fixed costs. Similarly, work flows related to discharge and postdischarge follow up rarely embrace telehealth tools or telehealth care pathways. A brisk change in the health care ecosystem is partly responsible for this.
Digital strategy needs to be incorporated into business strategy. For the reasons already discussed, telehealth technology is not a “nice to have” anymore, but a “must have.” At present, providers are of the opinion that about 20% of their patient services can be delivered via a telehealth platform. Similar trends are observed among patients, as a new modality of access to care is increasingly beneficial to them. Telehealth must be incorporated in standardized hospital work flows. Use of telehealth for preoperative clearance will greatly minimize same-day surgery cancellations. Given the potential shortage in resources, telehealth adoption for inpatient consultations will help systems conserve personal protective equipment, minimize the risk of staff exposure to COVID-19, and improve efficiency.
Digital strategy also prompts the reengineering of care delivery.10 Excessive and unused physical capacity can be converted into digital care hubs. Health maintenance, prevention, health promotion, health education, and chronic disease management not only can serve a variety of patient groups but can also help address the “last-mile problem” in health care. A successful digital strategy usually has three important components – Commitment: Hospital leadership is committed to include digital transformation as a strategic objective; Cost: Digital strategy is added as a line item in the budget; and Control: Measurable metrics are put in place to monitor the performance, impact, and influence of the digital strategy.
Conclusion
For decades, most U.S. health systems occupied the periphery of technological transformation when compared to the rest of the service industry. While most health systems took a heroic approach to the adoption of telehealth during COVID-19, despite being unprepared, the need for a systematic telehealth deployment is far from being adequately fulfilled. The COVID-19 pandemic brought permanent changes to several business disciplines globally. Given the impact of the pandemic on the health and overall wellbeing of American society, the U.S. health care industry must leave no stone unturned in its quest for transformation.
Dr. Lingisetty is a hospitalist and physician executive at Baptist Health System, Little Rock, Ark, and is cofounder/president of SHM’s Arkansas chapter. Dr. Prasad is medical director of care management and a hospitalist at Advocate Aurora Health in Milwaukee. He is cochair of SHM’s IT Special Interest Group, sits on the HQPS committee, and is president of SHM’s Wisconsin chapter. Dr. Palabindala is the medical director, utilization management, and physician advisory services at the University of Mississippi Medical Center and an associate professor of medicine and academic hospitalist at the University of Mississippi, both in Jackson.
References
1. Finnegan M. “Telehealth booms amid COVID-19 crisis.” Computerworld. 2020 Apr 27. www.computerworld.com/article/3540315/telehealth-booms-amid-covid-19-crisis-virtual-care-is-here-to-stay.html. Accessed 2020 Sep 12.
2. Department of Health & Human Services. “OCR Announces Notification of Enforcement Discretion for Telehealth Remote Communications During the COVID-19 Nationwide Public Health Emergency.” 2020 Mar 17. www.hhs.gov/about/news/2020/03/17/ocr-announces-notification-of-enforcement-discretion-for-telehealth-remote-communications-during-the-covid-19.html. Accessed 2020 Sep 12.
3. National Center for Health Statistics. “Health Expenditures.” www.cdc.gov/nchs/fastats/health-expenditures.htm. Accessed 2020 Sep 12.
4. Bestsennyy O et al. “Telehealth: A post–COVID-19 reality?” McKinsey & Company. 2020 May 29. www.mckinsey.com/industries/healthcare-systems-and-services/our-insights/telehealth-a-quarter-trillion-dollar-post-covid-19-reality. Accessed 2020 Sep 12.
5. Melnick ER et al. The Association Between Perceived Electronic Health Record Usability and Professional Burnout Among U.S. Physicians. Mayo Clin Proc. 2020 March;95(3):476-87.
6. Hirko KA et al. Telehealth in response to the COVID-19 pandemic: Implications for rural health disparities. J Am Med Inform Assoc. 2020 Nov;27(11):1816-8. .
7. American Academy of Family Physicians. “Study Examines Telehealth, Rural Disparities in Pandemic.” 2020 July 30. www.aafp.org/news/practice-professional-issues/20200730ruraltelehealth.html. Accessed 2020 Dec 15.
8. Kurlander J et al. “Virtual Visits: Telehealth and Older Adults.” National Poll on Healthy Aging. 2019 Oct. hdl.handle.net/2027.42/151376.
9. American Medical Association. Telehealth Implementation Playbook. 2019. www.ama-assn.org/system/files/2020-04/ama-telehealth-implementation-playbook.pdf.
10. Smith AC et al. Telehealth for global emergencies: Implications for coronavirus disease 2019 (COVID-19). J Telemed Telecare. 2020 Jun;26(5):309-13.
The COVID-19 pandemic catalyzed the transformation of Internet-based, remotely accessible innovative technologies. Internet-based customer service delivery technology was rapidly adopted and utilized by several services industries, but health care systems in most of the countries across the world faced unique challenges in adopting the technology for the delivery of health care services. The health care ecosystem of the United States was not immune to such challenges, and several significant barriers surfaced while the pandemic was underway.
Complexly structured, fragmented, unprepared, and overly burnt-out health systems in the United States arguably have fallen short of maximizing the value of telehealth in delivering safe, easily accessible, comprehensive, and cost-effective health care services. In this essay, we examine the reasons for such a suboptimal performance and discuss a few important strategies that may be useful in maximizing the value of telehealth value in several, appropriate health care services.
Hospitals and telehealth
Are hospitalists preparing ourselves “not to see” patients in a hospital-based health care delivery setting? If you have not yet started yet, now may be the right time! Yes, a certain percentage of doctor-patient encounters in hospital settings will remain virtual forever.
A well-established telehealth infrastructure is rarely found in most U.S. hospitals, although the COVID-19 pandemic has unexpectedly boosted the rapid growth of telehealth in the country.1 Public health emergency declarations in the United States in the face of the COVID-19 crisis have facilitated two important initiatives to restore health care delivery amidst formal and informal lockdowns that brought states to a grinding halt. These extend from expansion of virtual services, including telehealth, virtual check-ins, and e-visits, to the decision by the Department of Health & Human Services Office of Civil Rights to exercise enforcement discretion and waive penalties for the use of relatively inexpensive, non–public-facing mobile and other audiovisual technology tools.2
Hospital-based care in the United States taps nearly 33% of national health expenditure. An additional 30% of national health expenditure that is related to physicians, prescriptions, and other facilities is indirectly influenced by care delivered at health care facilities.3 Studies show that about 20% of ED visits could potentially be avoided via virtual urgent care offerings.4 A rapidly changing health care ecosystem is proving formidable for most hospital systems, and a test for their resilience and agility. Not just the implementation of telehealth is challenging, but getting it right is the key success factor.
Hospital-based telehealth

Expansion of telehealth coverage by the Centers for Medicare & Medicaid Services and most commercial payers did not quite ride the pandemic-induced momentum across the care continuum. Hospitals are lagging far behind ambulatory care in implementing telehealth. As illustrated in the “4-T Matrix” (see graphic) we would like to examine four key reasons for such a sluggish initial uptake and try to propose four important strategies that may help us to maximize the value created by telehealth technologies.
1. Timing
The health care system has always lagged far behind other service industries in terms of technology adaptation. Because of the unique nature of health care services, face-to-face interaction supersedes all other forms of communication. A rapidly evolving pandemic was not matched by simultaneous technology education for patients and providers. The enormous choice of hard-to-navigate telehealth tools; time and labor-intensive implementation; and uncertainty around payer, policy, and regulatory expectations might have precluded providers from the rapid adoption of telehealth in the hospital setting. Patients’ specific characteristics, such as the absence of technology-centered education, information, age, comorbidities, lack of technical literacy, and dependency on caregivers contributed to the suboptimal response from patients and families.
Deploying simple, ubiquitous, user-friendly, and technologically less challenging telehealth solutions may be a better approach to increase the adoption of such solutions by providers and patients. Hospitals need to develop and distribute telehealth user guides in all possible modes of communication. Provider-centric in-service sessions, workshops, and live support by “superuser teams” often work well in reducing end-user resistance.
2. Technical
Current electronic medical records vary widely in their features and offerings, and their ability to interact with third-party software and platforms. Dissatisfaction of end users with EMRs is well known, as is their likely relationship to burnout. Recent research continues to show a strong relationship between EMR usability and the odds of burnout among physicians.5 In the current climate, administrators and health informaticists have the responsibility to avoid adding increased burdens to end users.
Another issue is the limited connectivity in many remote/rural areas that would impact implementation of telehealth platforms. Studies indicate that 33% of rural Americans lack access to high-speed broadband Internet to support video visits.6 The recent successful implementation of telehealth across 530 providers in 75 ambulatory practices operated by Munson Healthcare, a rural health system in northern Michigan, sheds light on the technology’s enormous potential in providing safe access to rural populations.6,7
Privacy and safety of patient data is of paramount importance. According to a national poll on healthy aging by the University of Michigan in May 2019, targeting older adults, 47% of survey responders expressed difficulty using technology and 49% of survey responders were concerned about privacy.8 Use of certification and other tools offered by the Office of the National Coordinator for Health Information Technology would help reassure users, and the ability to capture and share images between providers would be of immense benefit in facilitating e-consults.
The need of the hour is redesigned work flow, to help providers adopt and use virtual care/telehealth efficiently. Work flow redesign must be coupled with technological advances to allow seamless integration of third-party telehealth platforms into existing EMR systems or built directly into EMRs. Use of quality metrics and analytical tools specific to telehealth would help measure the technology’s impact on patient care, outcomes, and end-user/provider experience.
3. Teams and training
Outcomes of health care interventions are often determined by the effectiveness of teams. Irrespective of how robust health care systems may have been initially, rapidly spreading infectious diseases like COVID-19 can quickly derail the system, bringing the workforce and patients to a breaking point.5 Decentralized, uncoordinated, and siloed efforts by individual teams across the care continuum were contributing factors for the partial success of telehealth care delivery pathways. The hospital systems with telehealth-ready teams at the start of the COVID-19 pandemic were so rare that the knowledge and technical training opportunities for innovators grew severalfold during the pandemic.
As per the American Medical Association, telehealth success is massively dependent on building the right team. Core, leadership, advisory, and implementation teams comprised of clinical representatives, end users, administrative personnel, executive members of the organization, technical experts, and payment/policy experts should be put together before implementing a telehealth strategy.9 Seamless integration of hospital-based care with ambulatory care via a telehealth platform is only complete when care managers are trained and deployed to fulfill the needs of a diverse group of patients. Deriving overall value from telehealth is only possible when there is a skill development, training and mentoring team put in place.
4. Thinking
In most U.S. hospitals, inpatient health care is equally distributed between nonprocedure and procedure-based services. Hospitals resorted to suspension of nonemergent procedures to mitigate the risk of spreading COVID-19. This was further compounded by many patients’ self-selection to defer care, an abrupt reduction in the influx of patients from the referral base because of suboptimally operating ambulatory care services, leading to low hospital occupancy.
Hospitals across the nation have gone through a massive short-term financial crunch and unfavorable cash-flow forecast, which prompted a paradoxical work-force reduction. While some argue that it may be akin to strategic myopia, the authors believed that such a response is strategically imperative to keep the hospital afloat. It is reasonable to attribute the paucity of innovation to constrained resources, and health systems are simply staying overly optimistic about “weathering the storm” and reverting soon to “business as usual.” The technological framework necessary for deploying a telehealth solution often comes with a price. Financially challenged hospital systems rarely exercise any capital-intensive activities. By contrast, telehealth adoption by ambulatory care can result in quicker resumption of patient care in community settings. A lack of operational and infrastructure synchrony between ambulatory and in-hospital systems has failed to capture telehealth-driven inpatient volume. For example, direct admissions from ambulatory telehealth referrals was a missed opportunity in several places. Referrals for labs, diagnostic tests, and other allied services could have helped hospitals offset their fixed costs. Similarly, work flows related to discharge and postdischarge follow up rarely embrace telehealth tools or telehealth care pathways. A brisk change in the health care ecosystem is partly responsible for this.
Digital strategy needs to be incorporated into business strategy. For the reasons already discussed, telehealth technology is not a “nice to have” anymore, but a “must have.” At present, providers are of the opinion that about 20% of their patient services can be delivered via a telehealth platform. Similar trends are observed among patients, as a new modality of access to care is increasingly beneficial to them. Telehealth must be incorporated in standardized hospital work flows. Use of telehealth for preoperative clearance will greatly minimize same-day surgery cancellations. Given the potential shortage in resources, telehealth adoption for inpatient consultations will help systems conserve personal protective equipment, minimize the risk of staff exposure to COVID-19, and improve efficiency.
Digital strategy also prompts the reengineering of care delivery.10 Excessive and unused physical capacity can be converted into digital care hubs. Health maintenance, prevention, health promotion, health education, and chronic disease management not only can serve a variety of patient groups but can also help address the “last-mile problem” in health care. A successful digital strategy usually has three important components – Commitment: Hospital leadership is committed to include digital transformation as a strategic objective; Cost: Digital strategy is added as a line item in the budget; and Control: Measurable metrics are put in place to monitor the performance, impact, and influence of the digital strategy.
Conclusion
For decades, most U.S. health systems occupied the periphery of technological transformation when compared to the rest of the service industry. While most health systems took a heroic approach to the adoption of telehealth during COVID-19, despite being unprepared, the need for a systematic telehealth deployment is far from being adequately fulfilled. The COVID-19 pandemic brought permanent changes to several business disciplines globally. Given the impact of the pandemic on the health and overall wellbeing of American society, the U.S. health care industry must leave no stone unturned in its quest for transformation.
Dr. Lingisetty is a hospitalist and physician executive at Baptist Health System, Little Rock, Ark, and is cofounder/president of SHM’s Arkansas chapter. Dr. Prasad is medical director of care management and a hospitalist at Advocate Aurora Health in Milwaukee. He is cochair of SHM’s IT Special Interest Group, sits on the HQPS committee, and is president of SHM’s Wisconsin chapter. Dr. Palabindala is the medical director, utilization management, and physician advisory services at the University of Mississippi Medical Center and an associate professor of medicine and academic hospitalist at the University of Mississippi, both in Jackson.
References
1. Finnegan M. “Telehealth booms amid COVID-19 crisis.” Computerworld. 2020 Apr 27. www.computerworld.com/article/3540315/telehealth-booms-amid-covid-19-crisis-virtual-care-is-here-to-stay.html. Accessed 2020 Sep 12.
2. Department of Health & Human Services. “OCR Announces Notification of Enforcement Discretion for Telehealth Remote Communications During the COVID-19 Nationwide Public Health Emergency.” 2020 Mar 17. www.hhs.gov/about/news/2020/03/17/ocr-announces-notification-of-enforcement-discretion-for-telehealth-remote-communications-during-the-covid-19.html. Accessed 2020 Sep 12.
3. National Center for Health Statistics. “Health Expenditures.” www.cdc.gov/nchs/fastats/health-expenditures.htm. Accessed 2020 Sep 12.
4. Bestsennyy O et al. “Telehealth: A post–COVID-19 reality?” McKinsey & Company. 2020 May 29. www.mckinsey.com/industries/healthcare-systems-and-services/our-insights/telehealth-a-quarter-trillion-dollar-post-covid-19-reality. Accessed 2020 Sep 12.
5. Melnick ER et al. The Association Between Perceived Electronic Health Record Usability and Professional Burnout Among U.S. Physicians. Mayo Clin Proc. 2020 March;95(3):476-87.
6. Hirko KA et al. Telehealth in response to the COVID-19 pandemic: Implications for rural health disparities. J Am Med Inform Assoc. 2020 Nov;27(11):1816-8. .
7. American Academy of Family Physicians. “Study Examines Telehealth, Rural Disparities in Pandemic.” 2020 July 30. www.aafp.org/news/practice-professional-issues/20200730ruraltelehealth.html. Accessed 2020 Dec 15.
8. Kurlander J et al. “Virtual Visits: Telehealth and Older Adults.” National Poll on Healthy Aging. 2019 Oct. hdl.handle.net/2027.42/151376.
9. American Medical Association. Telehealth Implementation Playbook. 2019. www.ama-assn.org/system/files/2020-04/ama-telehealth-implementation-playbook.pdf.
10. Smith AC et al. Telehealth for global emergencies: Implications for coronavirus disease 2019 (COVID-19). J Telemed Telecare. 2020 Jun;26(5):309-13.
Fauci worries about possible post–COVID-19 ‘mental health pandemic’
Anthony Fauci, MD, says he’s concerned about how Americans will react once the coronavirus pandemic is brought under control, CBS News reports.
Noting that an American Psychological Association survey showed people reporting high stress levels because of the pandemic, CBS’s Norah O’Donnell asked if Dr. Fauci was concerned about a possible “mental health pandemic.”
“Very much so,” Dr. Fauci, director of the National Institute of Allergy and Infectious Diseases and a top White House coronavirus adviser, replied.
“That’s the reason why I want to get the virological aspect of this pandemic behind us as quickly as we possibly can because the long-term ravages of this are so multifaceted,” Dr. Fauci said.
, he said.
“And then the other things: Not only the mental health effects, but many people have put off routine types of medical examinations that they normally would have done,” Dr. Fauci said.
“I hope we don’t see an increase in some preventable situations that would not have happened if people had the normal access to medical care, which clearly was interrupted by the shutdown associated with COVID-19,” he added.
The American Psychological Association released the survey results March 11 in what many people consider the 1-year anniversary of the start of the coronavirus pandemic.
“The prolonged stress experienced by adults, especially the high levels of stress reported by Americans directly linked to the pandemic, is seriously affecting mental and physical health, including changes to weight, sleep and alcohol use,” the APA said in a news release.
Some of the key findings of the survey include:
- 61% of respondents reported experiencing undesired weight changes since the start of the pandemic.
- 67% said their sleep habits changed, with 35% saying they slept more and 31% less.
- 23% reported drinking more alcohol to cope with stress.
- 47% said they delayed or canceled health care services because of the pandemic.
- 48% said their stress levels had increased.
A version of this article first appeared on Medscape.com.
Anthony Fauci, MD, says he’s concerned about how Americans will react once the coronavirus pandemic is brought under control, CBS News reports.
Noting that an American Psychological Association survey showed people reporting high stress levels because of the pandemic, CBS’s Norah O’Donnell asked if Dr. Fauci was concerned about a possible “mental health pandemic.”
“Very much so,” Dr. Fauci, director of the National Institute of Allergy and Infectious Diseases and a top White House coronavirus adviser, replied.
“That’s the reason why I want to get the virological aspect of this pandemic behind us as quickly as we possibly can because the long-term ravages of this are so multifaceted,” Dr. Fauci said.
, he said.
“And then the other things: Not only the mental health effects, but many people have put off routine types of medical examinations that they normally would have done,” Dr. Fauci said.
“I hope we don’t see an increase in some preventable situations that would not have happened if people had the normal access to medical care, which clearly was interrupted by the shutdown associated with COVID-19,” he added.
The American Psychological Association released the survey results March 11 in what many people consider the 1-year anniversary of the start of the coronavirus pandemic.
“The prolonged stress experienced by adults, especially the high levels of stress reported by Americans directly linked to the pandemic, is seriously affecting mental and physical health, including changes to weight, sleep and alcohol use,” the APA said in a news release.
Some of the key findings of the survey include:
- 61% of respondents reported experiencing undesired weight changes since the start of the pandemic.
- 67% said their sleep habits changed, with 35% saying they slept more and 31% less.
- 23% reported drinking more alcohol to cope with stress.
- 47% said they delayed or canceled health care services because of the pandemic.
- 48% said their stress levels had increased.
A version of this article first appeared on Medscape.com.
Anthony Fauci, MD, says he’s concerned about how Americans will react once the coronavirus pandemic is brought under control, CBS News reports.
Noting that an American Psychological Association survey showed people reporting high stress levels because of the pandemic, CBS’s Norah O’Donnell asked if Dr. Fauci was concerned about a possible “mental health pandemic.”
“Very much so,” Dr. Fauci, director of the National Institute of Allergy and Infectious Diseases and a top White House coronavirus adviser, replied.
“That’s the reason why I want to get the virological aspect of this pandemic behind us as quickly as we possibly can because the long-term ravages of this are so multifaceted,” Dr. Fauci said.
, he said.
“And then the other things: Not only the mental health effects, but many people have put off routine types of medical examinations that they normally would have done,” Dr. Fauci said.
“I hope we don’t see an increase in some preventable situations that would not have happened if people had the normal access to medical care, which clearly was interrupted by the shutdown associated with COVID-19,” he added.
The American Psychological Association released the survey results March 11 in what many people consider the 1-year anniversary of the start of the coronavirus pandemic.
“The prolonged stress experienced by adults, especially the high levels of stress reported by Americans directly linked to the pandemic, is seriously affecting mental and physical health, including changes to weight, sleep and alcohol use,” the APA said in a news release.
Some of the key findings of the survey include:
- 61% of respondents reported experiencing undesired weight changes since the start of the pandemic.
- 67% said their sleep habits changed, with 35% saying they slept more and 31% less.
- 23% reported drinking more alcohol to cope with stress.
- 47% said they delayed or canceled health care services because of the pandemic.
- 48% said their stress levels had increased.
A version of this article first appeared on Medscape.com.
Decline in child COVID-19 cases picks up after 2-week slowdown
, according to data gathered by the American Academy of Pediatrics and the Children’s Hospital Association.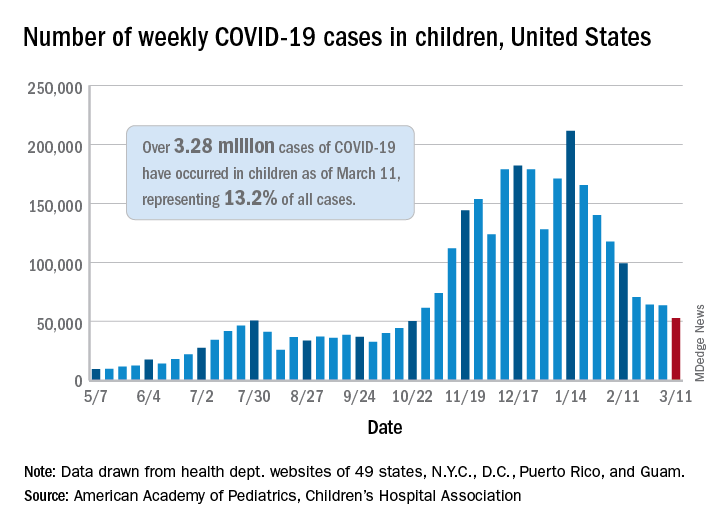
From Feb. 19 to March 4, the drop in new cases averaged just 5% each week, compared with 13.3% per week over the 5-week period from Jan. 15 to Feb. 18. For the week of March 5-11, a total of 52,695 COVID-19 cases were reported in children, down from 63,562 the previous week and the lowest number since late October, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
In those jurisdictions, 3.28 million children have been infected with SARS-CoV-2, representing 13.2% of all cases since the beginning of the pandemic. The cumulative rate of COVID-19 has now risen to 4,364 cases per 100,000 children nationally, with state rates ranging from 1,062 per 100,000 in Hawaii to 8,692 per 100,000 in North Dakota, the AAP and CHA said in their weekly COVID-19 report.
Hospitalization data are more limited – 24 states and New York City – but continue to show that serious illness is much less common in younger individuals: Children represent just 1.9% of all hospitalizations, and only 0.8% of the children who have been infected were hospitalized. Neither rate has changed since early February, the AAP and CHA said.
The number of deaths in children, however, rose from 253 to 266, the largest 1-week increase since early February in the 43 states (along with New York City, Puerto Rico, and Guam) that are tracking mortality data by age, the AAP and CHA reported.
Among those 46 jurisdictions, there are 10 (9 states and the District of Columbia) that have not yet reported a COVID-19–related child death, while Texas has almost twice as many deaths, 47, as the next state, Arizona, which has 24. Meanwhile, California’s total of 452,000 cases is almost 2½ times higher than the 183,000 recorded by Illinois, according to the report.
, according to data gathered by the American Academy of Pediatrics and the Children’s Hospital Association.
From Feb. 19 to March 4, the drop in new cases averaged just 5% each week, compared with 13.3% per week over the 5-week period from Jan. 15 to Feb. 18. For the week of March 5-11, a total of 52,695 COVID-19 cases were reported in children, down from 63,562 the previous week and the lowest number since late October, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
In those jurisdictions, 3.28 million children have been infected with SARS-CoV-2, representing 13.2% of all cases since the beginning of the pandemic. The cumulative rate of COVID-19 has now risen to 4,364 cases per 100,000 children nationally, with state rates ranging from 1,062 per 100,000 in Hawaii to 8,692 per 100,000 in North Dakota, the AAP and CHA said in their weekly COVID-19 report.
Hospitalization data are more limited – 24 states and New York City – but continue to show that serious illness is much less common in younger individuals: Children represent just 1.9% of all hospitalizations, and only 0.8% of the children who have been infected were hospitalized. Neither rate has changed since early February, the AAP and CHA said.
The number of deaths in children, however, rose from 253 to 266, the largest 1-week increase since early February in the 43 states (along with New York City, Puerto Rico, and Guam) that are tracking mortality data by age, the AAP and CHA reported.
Among those 46 jurisdictions, there are 10 (9 states and the District of Columbia) that have not yet reported a COVID-19–related child death, while Texas has almost twice as many deaths, 47, as the next state, Arizona, which has 24. Meanwhile, California’s total of 452,000 cases is almost 2½ times higher than the 183,000 recorded by Illinois, according to the report.
, according to data gathered by the American Academy of Pediatrics and the Children’s Hospital Association.
From Feb. 19 to March 4, the drop in new cases averaged just 5% each week, compared with 13.3% per week over the 5-week period from Jan. 15 to Feb. 18. For the week of March 5-11, a total of 52,695 COVID-19 cases were reported in children, down from 63,562 the previous week and the lowest number since late October, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
In those jurisdictions, 3.28 million children have been infected with SARS-CoV-2, representing 13.2% of all cases since the beginning of the pandemic. The cumulative rate of COVID-19 has now risen to 4,364 cases per 100,000 children nationally, with state rates ranging from 1,062 per 100,000 in Hawaii to 8,692 per 100,000 in North Dakota, the AAP and CHA said in their weekly COVID-19 report.
Hospitalization data are more limited – 24 states and New York City – but continue to show that serious illness is much less common in younger individuals: Children represent just 1.9% of all hospitalizations, and only 0.8% of the children who have been infected were hospitalized. Neither rate has changed since early February, the AAP and CHA said.
The number of deaths in children, however, rose from 253 to 266, the largest 1-week increase since early February in the 43 states (along with New York City, Puerto Rico, and Guam) that are tracking mortality data by age, the AAP and CHA reported.
Among those 46 jurisdictions, there are 10 (9 states and the District of Columbia) that have not yet reported a COVID-19–related child death, while Texas has almost twice as many deaths, 47, as the next state, Arizona, which has 24. Meanwhile, California’s total of 452,000 cases is almost 2½ times higher than the 183,000 recorded by Illinois, according to the report.
Blood cancer patients, survivors hesitate over COVID-19 vaccine
Nearly one in three patients with blood cancer, and survivors, say they are unlikely to get a COVID-19 vaccine or unsure about getting it if one were available. The findings come from a nationwide survey by The Leukemia & Lymphoma Society, which collected 6,517 responses.
“These findings are worrisome, to say the least,” Gwen Nichols, MD, chief medical officer of the society, said in a statement.
“We know cancer patients – and blood cancer patients in particular – are susceptible to the worst effects of the virus [and] all of us in the medical community need to help cancer patients understand the importance of getting vaccinated,” she added.
The survey – the largest ever done in which cancer patients and survivors were asked about their attitudes toward COVID-19 vaccines – was published online March 8 by The Leukemia & Lymphoma Society.
Survey sample
The survey asked patients with blood cancer, and survivors, about their attitudes regarding COVID-19 and COVID-19 vaccines.
“The main outcome [was] vaccine attitudes,” noted the authors, headed by Rena Conti, PhD, dean’s research scholar, Boston University.
Respondents were asked: “How likely are you to choose to get the vaccine?” Participants could indicate they were very unlikely, unlikely, neither likely nor unlikely, likely, or very likely to get vaccinated.
“We found that 17% of respondents indicate[d] that they [were] unlikely or very unlikely to take a vaccine,” Dr. Conti and colleagues observed.
Among the 17% – deemed to be “vaccine hesitant” – slightly over half (54%) stated they had concerns about the side effects associated with COVID-19 vaccination and believed neither of the two newly approved vaccines had been or would ever be tested properly.
The survey authors noted that there is no reason to believe COVID-19 vaccines are any less safe in patients with blood cancers, but concerns have been expressed that patients with some forms of blood cancer or those undergoing certain treatments may not achieve the same immune response to the vaccine as would noncancer controls.
Importantly, the survey was conducted Dec. 1-21, 2020, and responses differed depending on whether respondents answered the survey before or after the Pfizer-BioNTech and Moderna vaccines had been given emergency use authorization by the Food and Drug Administration starting Dec. 10, 2020.
There was a slight increase in positive responses after the vaccines were granted regulatory approval. (One-third of those who responded to the survey after the approval were 3.7% more likely to indicate they would get vaccinated). “This suggests that hesitancy may be influenced by emerging information dissemination, government action, and vaccine availability, transforming the hypothetical opportunity of vaccination to a real one,” the survey authors speculated.
Survey respondents who were vaccine hesitant were also over 14% more likely to indicate that they didn’t think they would require hospitalization should they contract COVID-19. But clinical data have suggested that approximately half of patients with a hematological malignancy who required hospitalization for COVID-19 die from the infection, the authors noted.
“Vaccine hesitant respondents [were] also significantly less likely to engage in protective health behaviors,” the survey authors pointed out. For example, they were almost 4% less likely to have worn a face mask and 1.6% less likely to have taken other protective measures to guard against COVID-19 infection.
Need for clear messaging
To counter vaccine hesitancy, the authors suggest there is a need for clear, consistent messaging targeting patients with cancer that emphasize the risks of COVID-19 and underscore vaccine benefits.
Dr. Conti pointed out that patients with blood cancer are, in fact, being given preferential access to vaccines in many communities, although this clearly doesn’t mean patients are willing to get vaccinated, as she also noted.
“We need both adequate supply and strong demand to keep this vulnerable population safe,” Dr. Conti emphasized.
The Leukemia & Lymphoma Society plans to repeat the survey in the near future to assess patients’ and survivors’ access to vaccines as well as their willingness to get vaccinated.
The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Nearly one in three patients with blood cancer, and survivors, say they are unlikely to get a COVID-19 vaccine or unsure about getting it if one were available. The findings come from a nationwide survey by The Leukemia & Lymphoma Society, which collected 6,517 responses.
“These findings are worrisome, to say the least,” Gwen Nichols, MD, chief medical officer of the society, said in a statement.
“We know cancer patients – and blood cancer patients in particular – are susceptible to the worst effects of the virus [and] all of us in the medical community need to help cancer patients understand the importance of getting vaccinated,” she added.
The survey – the largest ever done in which cancer patients and survivors were asked about their attitudes toward COVID-19 vaccines – was published online March 8 by The Leukemia & Lymphoma Society.
Survey sample
The survey asked patients with blood cancer, and survivors, about their attitudes regarding COVID-19 and COVID-19 vaccines.
“The main outcome [was] vaccine attitudes,” noted the authors, headed by Rena Conti, PhD, dean’s research scholar, Boston University.
Respondents were asked: “How likely are you to choose to get the vaccine?” Participants could indicate they were very unlikely, unlikely, neither likely nor unlikely, likely, or very likely to get vaccinated.
“We found that 17% of respondents indicate[d] that they [were] unlikely or very unlikely to take a vaccine,” Dr. Conti and colleagues observed.
Among the 17% – deemed to be “vaccine hesitant” – slightly over half (54%) stated they had concerns about the side effects associated with COVID-19 vaccination and believed neither of the two newly approved vaccines had been or would ever be tested properly.
The survey authors noted that there is no reason to believe COVID-19 vaccines are any less safe in patients with blood cancers, but concerns have been expressed that patients with some forms of blood cancer or those undergoing certain treatments may not achieve the same immune response to the vaccine as would noncancer controls.
Importantly, the survey was conducted Dec. 1-21, 2020, and responses differed depending on whether respondents answered the survey before or after the Pfizer-BioNTech and Moderna vaccines had been given emergency use authorization by the Food and Drug Administration starting Dec. 10, 2020.
There was a slight increase in positive responses after the vaccines were granted regulatory approval. (One-third of those who responded to the survey after the approval were 3.7% more likely to indicate they would get vaccinated). “This suggests that hesitancy may be influenced by emerging information dissemination, government action, and vaccine availability, transforming the hypothetical opportunity of vaccination to a real one,” the survey authors speculated.
Survey respondents who were vaccine hesitant were also over 14% more likely to indicate that they didn’t think they would require hospitalization should they contract COVID-19. But clinical data have suggested that approximately half of patients with a hematological malignancy who required hospitalization for COVID-19 die from the infection, the authors noted.
“Vaccine hesitant respondents [were] also significantly less likely to engage in protective health behaviors,” the survey authors pointed out. For example, they were almost 4% less likely to have worn a face mask and 1.6% less likely to have taken other protective measures to guard against COVID-19 infection.
Need for clear messaging
To counter vaccine hesitancy, the authors suggest there is a need for clear, consistent messaging targeting patients with cancer that emphasize the risks of COVID-19 and underscore vaccine benefits.
Dr. Conti pointed out that patients with blood cancer are, in fact, being given preferential access to vaccines in many communities, although this clearly doesn’t mean patients are willing to get vaccinated, as she also noted.
“We need both adequate supply and strong demand to keep this vulnerable population safe,” Dr. Conti emphasized.
The Leukemia & Lymphoma Society plans to repeat the survey in the near future to assess patients’ and survivors’ access to vaccines as well as their willingness to get vaccinated.
The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Nearly one in three patients with blood cancer, and survivors, say they are unlikely to get a COVID-19 vaccine or unsure about getting it if one were available. The findings come from a nationwide survey by The Leukemia & Lymphoma Society, which collected 6,517 responses.
“These findings are worrisome, to say the least,” Gwen Nichols, MD, chief medical officer of the society, said in a statement.
“We know cancer patients – and blood cancer patients in particular – are susceptible to the worst effects of the virus [and] all of us in the medical community need to help cancer patients understand the importance of getting vaccinated,” she added.
The survey – the largest ever done in which cancer patients and survivors were asked about their attitudes toward COVID-19 vaccines – was published online March 8 by The Leukemia & Lymphoma Society.
Survey sample
The survey asked patients with blood cancer, and survivors, about their attitudes regarding COVID-19 and COVID-19 vaccines.
“The main outcome [was] vaccine attitudes,” noted the authors, headed by Rena Conti, PhD, dean’s research scholar, Boston University.
Respondents were asked: “How likely are you to choose to get the vaccine?” Participants could indicate they were very unlikely, unlikely, neither likely nor unlikely, likely, or very likely to get vaccinated.
“We found that 17% of respondents indicate[d] that they [were] unlikely or very unlikely to take a vaccine,” Dr. Conti and colleagues observed.
Among the 17% – deemed to be “vaccine hesitant” – slightly over half (54%) stated they had concerns about the side effects associated with COVID-19 vaccination and believed neither of the two newly approved vaccines had been or would ever be tested properly.
The survey authors noted that there is no reason to believe COVID-19 vaccines are any less safe in patients with blood cancers, but concerns have been expressed that patients with some forms of blood cancer or those undergoing certain treatments may not achieve the same immune response to the vaccine as would noncancer controls.
Importantly, the survey was conducted Dec. 1-21, 2020, and responses differed depending on whether respondents answered the survey before or after the Pfizer-BioNTech and Moderna vaccines had been given emergency use authorization by the Food and Drug Administration starting Dec. 10, 2020.
There was a slight increase in positive responses after the vaccines were granted regulatory approval. (One-third of those who responded to the survey after the approval were 3.7% more likely to indicate they would get vaccinated). “This suggests that hesitancy may be influenced by emerging information dissemination, government action, and vaccine availability, transforming the hypothetical opportunity of vaccination to a real one,” the survey authors speculated.
Survey respondents who were vaccine hesitant were also over 14% more likely to indicate that they didn’t think they would require hospitalization should they contract COVID-19. But clinical data have suggested that approximately half of patients with a hematological malignancy who required hospitalization for COVID-19 die from the infection, the authors noted.
“Vaccine hesitant respondents [were] also significantly less likely to engage in protective health behaviors,” the survey authors pointed out. For example, they were almost 4% less likely to have worn a face mask and 1.6% less likely to have taken other protective measures to guard against COVID-19 infection.
Need for clear messaging
To counter vaccine hesitancy, the authors suggest there is a need for clear, consistent messaging targeting patients with cancer that emphasize the risks of COVID-19 and underscore vaccine benefits.
Dr. Conti pointed out that patients with blood cancer are, in fact, being given preferential access to vaccines in many communities, although this clearly doesn’t mean patients are willing to get vaccinated, as she also noted.
“We need both adequate supply and strong demand to keep this vulnerable population safe,” Dr. Conti emphasized.
The Leukemia & Lymphoma Society plans to repeat the survey in the near future to assess patients’ and survivors’ access to vaccines as well as their willingness to get vaccinated.
The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Don’t delay: Cancer patients need both doses of COVID vaccine
The new findings, which are soon to be published as a preprint, cast doubt on the current U.K. policy of delaying the second dose of the vaccine.
Delaying the second dose can leave most patients with cancer wholly or partially unprotected, according to the researchers. Moreover, such a delay has implications for transmission of SARS-CoV-2 in the cancer patient’s environs as well as for the evolution of virus variants that could be of concern, the researchers concluded.
The data come from a British study that included 151 patients with cancer and 54 healthy control persons. All participants received the COVID-19 mRNA BNT162b2 vaccine (Pfizer-BioNTech).
This vaccine requires two doses. The first few participants in this study were given the second dose 21 days after they had received the first dose, but then national guidelines changed, and the remaining participants had to wait 12 weeks to receive their second dose.
The researchers reported that, among health controls, the immune efficacy of the first dose was very high (97% efficacious). By contrast, among patients with solid tumors, the immune efficacy of a single dose was strikingly low (39%), and it was even lower in patients with hematologic malignancies (13%).
The second dose of vaccine greatly and rapidly increased the immune efficacy in patients with solid tumors (95% within 2 weeks of receiving the second dose), the researchers added.
Too few patients with hematologic cancers had received the second dose before the study ended for clear conclusions to be drawn. Nevertheless, the available data suggest that 50% of patients with hematologic cancers who had received the booster at day 21 were seropositive at 5 weeks vs. only 8% of those who had not received the booster.
“Our data provide the first real-world evidence of immune efficacy following one dose of the Pfizer vaccine in immunocompromised patient populations [and] clearly show that the poor one-dose efficacy in cancer patients can be rescued with an early booster at day 21,” commented senior author Sheeba Irshad, MD, senior clinical lecturer, King’s College London.
“Based on our findings, we would recommend an urgent review of the vaccine strategy for clinically extremely vulnerable groups. Until then, it is important that cancer patients continue to observe all public health measures in place, such as social distancing and shielding when attending hospitals, even after vaccination,” Dr. Irshad added.
The paper, with first author Leticia Monin-Aldama, PhD, is scheduled to appear on the preprint server medRxiv. It has not undergone peer review. The paper was distributed to journalists, with comments from experts not involved in the study, by the UK Science Media Centre.
These data are “of immediate importance” to patients with cancer, commented Shoba Amarnath, PhD, Newcastle University research fellow, Laboratory of T-cell Regulation, Newcastle University Center for Cancer, Newcastle upon Tyne, England.
“These findings are consistent with our understanding. … We know that the immune system within cancer patients is compromised as compared to healthy controls,” Dr. Amarnath said. “The data in the study support the notion that, in solid cancer patients, a considerable delay in second dose will extend the period when cancer patients are at risk of SARS-CoV-2 infection.”
Although more data are required, “this study does raise the issue of whether patients with cancer, other diseases, or those undergoing therapies that affect the body’s immune response should be fast-tracked for their second vaccine dose,” commented Lawrence Young, PhD, professor of molecular oncology and director of the Warwick Cancer Research Center, University of Warwick, Coventry, England.
Stephen Evans, MSc, professor of pharmacoepidemiology, London School of Hygiene and Tropical Medicine, underlined that the study is “essentially” observational and “inevitable limitations must be taken into account.
“Nevertheless, these results do suggest that the vaccines may well not protect those patients with cancer as well as those without cancer,” Mr. Evans said. He added that it is “important that this population continues to observe all COVID-19–associated measures, such as social distancing and shielding when attending hospitals, even after vaccination.”
Study details
Previous studies have shown that some patients with cancer have prolonged responses to SARS-CoV-2 infection, with ongoing immune dysregulation, inefficient seroconversion, and prolonged viral shedding.
There are few data, however, on how these patients respond to COVID-19 vaccination. The authors point out that, among the 18,860 individuals who received the Pfizer vaccine during its development trials, “none with an active oncological diagnosis was included.”
To investigate this issue, they launched the SARS-CoV-2 for Cancer Patients (SOAP-02) study.
The 151 patients with cancer who participated in this study were mostly elderly, the authors noted (75% were older than 65 years; the median age was 73 years). The majority (63%) had solid-tumor malignancies. Of those, 8% had late-stage disease and had been living with their cancer for more than 24 months.
The healthy control persons were vaccine-eligible primary health care workers who were not age matched to the cancer patients.
All participants received the first dose of vaccine; 31 (of 151) patients with cancer and 16 (of 54) healthy control persons received the second dose on day 21.
The remaining participants were scheduled to receive their second dose 12 weeks later (after the study ended), in line with the changes in the national guidelines.
The team reported that, approximately 21 days after receiving the first vaccine dose, the immune efficacy of the vaccine was estimated to be 97% among healthy control persons vs. 39% for patients with solid tumors and only 13% for those with hematologic malignancies (P < .0001 for both).
T-cell responses, as assessed via interferon-gamma and/or interleukin-2 production, were observed in 82% of healthy control persons, 71% of patients with solid tumors, and 50% of those with hematologic cancers.
Vaccine boosting at day 21 resulted in immune efficacy of 100% for healthy control persons and 95% for patients with solid tumors. In contrast, only 43% of those who did not receive the second dose were seropositive 2 weeks later.
Further analysis suggested that participants who did not have a serologic response were “spread evenly” across different cancer types, but the reduced responses were more frequent among patients who had received the vaccine within 15 days of cancer treatment, especially chemotherapy, and had undergone intensive treatments.
The SOAP study is sponsored by King’s College London and Guy’s and St. Thomas Trust Foundation NHS Trust. It is funded from grants from the KCL Charity, Cancer Research UK, and program grants from Breast Cancer Now. The investigators have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The new findings, which are soon to be published as a preprint, cast doubt on the current U.K. policy of delaying the second dose of the vaccine.
Delaying the second dose can leave most patients with cancer wholly or partially unprotected, according to the researchers. Moreover, such a delay has implications for transmission of SARS-CoV-2 in the cancer patient’s environs as well as for the evolution of virus variants that could be of concern, the researchers concluded.
The data come from a British study that included 151 patients with cancer and 54 healthy control persons. All participants received the COVID-19 mRNA BNT162b2 vaccine (Pfizer-BioNTech).
This vaccine requires two doses. The first few participants in this study were given the second dose 21 days after they had received the first dose, but then national guidelines changed, and the remaining participants had to wait 12 weeks to receive their second dose.
The researchers reported that, among health controls, the immune efficacy of the first dose was very high (97% efficacious). By contrast, among patients with solid tumors, the immune efficacy of a single dose was strikingly low (39%), and it was even lower in patients with hematologic malignancies (13%).
The second dose of vaccine greatly and rapidly increased the immune efficacy in patients with solid tumors (95% within 2 weeks of receiving the second dose), the researchers added.
Too few patients with hematologic cancers had received the second dose before the study ended for clear conclusions to be drawn. Nevertheless, the available data suggest that 50% of patients with hematologic cancers who had received the booster at day 21 were seropositive at 5 weeks vs. only 8% of those who had not received the booster.
“Our data provide the first real-world evidence of immune efficacy following one dose of the Pfizer vaccine in immunocompromised patient populations [and] clearly show that the poor one-dose efficacy in cancer patients can be rescued with an early booster at day 21,” commented senior author Sheeba Irshad, MD, senior clinical lecturer, King’s College London.
“Based on our findings, we would recommend an urgent review of the vaccine strategy for clinically extremely vulnerable groups. Until then, it is important that cancer patients continue to observe all public health measures in place, such as social distancing and shielding when attending hospitals, even after vaccination,” Dr. Irshad added.
The paper, with first author Leticia Monin-Aldama, PhD, is scheduled to appear on the preprint server medRxiv. It has not undergone peer review. The paper was distributed to journalists, with comments from experts not involved in the study, by the UK Science Media Centre.
These data are “of immediate importance” to patients with cancer, commented Shoba Amarnath, PhD, Newcastle University research fellow, Laboratory of T-cell Regulation, Newcastle University Center for Cancer, Newcastle upon Tyne, England.
“These findings are consistent with our understanding. … We know that the immune system within cancer patients is compromised as compared to healthy controls,” Dr. Amarnath said. “The data in the study support the notion that, in solid cancer patients, a considerable delay in second dose will extend the period when cancer patients are at risk of SARS-CoV-2 infection.”
Although more data are required, “this study does raise the issue of whether patients with cancer, other diseases, or those undergoing therapies that affect the body’s immune response should be fast-tracked for their second vaccine dose,” commented Lawrence Young, PhD, professor of molecular oncology and director of the Warwick Cancer Research Center, University of Warwick, Coventry, England.
Stephen Evans, MSc, professor of pharmacoepidemiology, London School of Hygiene and Tropical Medicine, underlined that the study is “essentially” observational and “inevitable limitations must be taken into account.
“Nevertheless, these results do suggest that the vaccines may well not protect those patients with cancer as well as those without cancer,” Mr. Evans said. He added that it is “important that this population continues to observe all COVID-19–associated measures, such as social distancing and shielding when attending hospitals, even after vaccination.”
Study details
Previous studies have shown that some patients with cancer have prolonged responses to SARS-CoV-2 infection, with ongoing immune dysregulation, inefficient seroconversion, and prolonged viral shedding.
There are few data, however, on how these patients respond to COVID-19 vaccination. The authors point out that, among the 18,860 individuals who received the Pfizer vaccine during its development trials, “none with an active oncological diagnosis was included.”
To investigate this issue, they launched the SARS-CoV-2 for Cancer Patients (SOAP-02) study.
The 151 patients with cancer who participated in this study were mostly elderly, the authors noted (75% were older than 65 years; the median age was 73 years). The majority (63%) had solid-tumor malignancies. Of those, 8% had late-stage disease and had been living with their cancer for more than 24 months.
The healthy control persons were vaccine-eligible primary health care workers who were not age matched to the cancer patients.
All participants received the first dose of vaccine; 31 (of 151) patients with cancer and 16 (of 54) healthy control persons received the second dose on day 21.
The remaining participants were scheduled to receive their second dose 12 weeks later (after the study ended), in line with the changes in the national guidelines.
The team reported that, approximately 21 days after receiving the first vaccine dose, the immune efficacy of the vaccine was estimated to be 97% among healthy control persons vs. 39% for patients with solid tumors and only 13% for those with hematologic malignancies (P < .0001 for both).
T-cell responses, as assessed via interferon-gamma and/or interleukin-2 production, were observed in 82% of healthy control persons, 71% of patients with solid tumors, and 50% of those with hematologic cancers.
Vaccine boosting at day 21 resulted in immune efficacy of 100% for healthy control persons and 95% for patients with solid tumors. In contrast, only 43% of those who did not receive the second dose were seropositive 2 weeks later.
Further analysis suggested that participants who did not have a serologic response were “spread evenly” across different cancer types, but the reduced responses were more frequent among patients who had received the vaccine within 15 days of cancer treatment, especially chemotherapy, and had undergone intensive treatments.
The SOAP study is sponsored by King’s College London and Guy’s and St. Thomas Trust Foundation NHS Trust. It is funded from grants from the KCL Charity, Cancer Research UK, and program grants from Breast Cancer Now. The investigators have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The new findings, which are soon to be published as a preprint, cast doubt on the current U.K. policy of delaying the second dose of the vaccine.
Delaying the second dose can leave most patients with cancer wholly or partially unprotected, according to the researchers. Moreover, such a delay has implications for transmission of SARS-CoV-2 in the cancer patient’s environs as well as for the evolution of virus variants that could be of concern, the researchers concluded.
The data come from a British study that included 151 patients with cancer and 54 healthy control persons. All participants received the COVID-19 mRNA BNT162b2 vaccine (Pfizer-BioNTech).
This vaccine requires two doses. The first few participants in this study were given the second dose 21 days after they had received the first dose, but then national guidelines changed, and the remaining participants had to wait 12 weeks to receive their second dose.
The researchers reported that, among health controls, the immune efficacy of the first dose was very high (97% efficacious). By contrast, among patients with solid tumors, the immune efficacy of a single dose was strikingly low (39%), and it was even lower in patients with hematologic malignancies (13%).
The second dose of vaccine greatly and rapidly increased the immune efficacy in patients with solid tumors (95% within 2 weeks of receiving the second dose), the researchers added.
Too few patients with hematologic cancers had received the second dose before the study ended for clear conclusions to be drawn. Nevertheless, the available data suggest that 50% of patients with hematologic cancers who had received the booster at day 21 were seropositive at 5 weeks vs. only 8% of those who had not received the booster.
“Our data provide the first real-world evidence of immune efficacy following one dose of the Pfizer vaccine in immunocompromised patient populations [and] clearly show that the poor one-dose efficacy in cancer patients can be rescued with an early booster at day 21,” commented senior author Sheeba Irshad, MD, senior clinical lecturer, King’s College London.
“Based on our findings, we would recommend an urgent review of the vaccine strategy for clinically extremely vulnerable groups. Until then, it is important that cancer patients continue to observe all public health measures in place, such as social distancing and shielding when attending hospitals, even after vaccination,” Dr. Irshad added.
The paper, with first author Leticia Monin-Aldama, PhD, is scheduled to appear on the preprint server medRxiv. It has not undergone peer review. The paper was distributed to journalists, with comments from experts not involved in the study, by the UK Science Media Centre.
These data are “of immediate importance” to patients with cancer, commented Shoba Amarnath, PhD, Newcastle University research fellow, Laboratory of T-cell Regulation, Newcastle University Center for Cancer, Newcastle upon Tyne, England.
“These findings are consistent with our understanding. … We know that the immune system within cancer patients is compromised as compared to healthy controls,” Dr. Amarnath said. “The data in the study support the notion that, in solid cancer patients, a considerable delay in second dose will extend the period when cancer patients are at risk of SARS-CoV-2 infection.”
Although more data are required, “this study does raise the issue of whether patients with cancer, other diseases, or those undergoing therapies that affect the body’s immune response should be fast-tracked for their second vaccine dose,” commented Lawrence Young, PhD, professor of molecular oncology and director of the Warwick Cancer Research Center, University of Warwick, Coventry, England.
Stephen Evans, MSc, professor of pharmacoepidemiology, London School of Hygiene and Tropical Medicine, underlined that the study is “essentially” observational and “inevitable limitations must be taken into account.
“Nevertheless, these results do suggest that the vaccines may well not protect those patients with cancer as well as those without cancer,” Mr. Evans said. He added that it is “important that this population continues to observe all COVID-19–associated measures, such as social distancing and shielding when attending hospitals, even after vaccination.”
Study details
Previous studies have shown that some patients with cancer have prolonged responses to SARS-CoV-2 infection, with ongoing immune dysregulation, inefficient seroconversion, and prolonged viral shedding.
There are few data, however, on how these patients respond to COVID-19 vaccination. The authors point out that, among the 18,860 individuals who received the Pfizer vaccine during its development trials, “none with an active oncological diagnosis was included.”
To investigate this issue, they launched the SARS-CoV-2 for Cancer Patients (SOAP-02) study.
The 151 patients with cancer who participated in this study were mostly elderly, the authors noted (75% were older than 65 years; the median age was 73 years). The majority (63%) had solid-tumor malignancies. Of those, 8% had late-stage disease and had been living with their cancer for more than 24 months.
The healthy control persons were vaccine-eligible primary health care workers who were not age matched to the cancer patients.
All participants received the first dose of vaccine; 31 (of 151) patients with cancer and 16 (of 54) healthy control persons received the second dose on day 21.
The remaining participants were scheduled to receive their second dose 12 weeks later (after the study ended), in line with the changes in the national guidelines.
The team reported that, approximately 21 days after receiving the first vaccine dose, the immune efficacy of the vaccine was estimated to be 97% among healthy control persons vs. 39% for patients with solid tumors and only 13% for those with hematologic malignancies (P < .0001 for both).
T-cell responses, as assessed via interferon-gamma and/or interleukin-2 production, were observed in 82% of healthy control persons, 71% of patients with solid tumors, and 50% of those with hematologic cancers.
Vaccine boosting at day 21 resulted in immune efficacy of 100% for healthy control persons and 95% for patients with solid tumors. In contrast, only 43% of those who did not receive the second dose were seropositive 2 weeks later.
Further analysis suggested that participants who did not have a serologic response were “spread evenly” across different cancer types, but the reduced responses were more frequent among patients who had received the vaccine within 15 days of cancer treatment, especially chemotherapy, and had undergone intensive treatments.
The SOAP study is sponsored by King’s College London and Guy’s and St. Thomas Trust Foundation NHS Trust. It is funded from grants from the KCL Charity, Cancer Research UK, and program grants from Breast Cancer Now. The investigators have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
First pill for COVID-19 could be ready by year’s end
New pills to treat patients with COVID-19 are currently in midstage clinical trials and, if successful, could be ready by the end of the year.
Only one treatment – remdesivir (Veklury) – has been fully approved by the U.S. Food and Drug Administration for patients in the hospital and it must be administered intravenously.
Hopes for a day when patients with COVID-19 can take a pill to rid their bodies of the virus got a boost when early trial results were presented at a medical conference.
Interim phase 2 results for the oral experimental COVID-19 drug molnupiravir, designed to do for patients with COVID-19 what oseltamivir (Tamiflu) can do for patients with the flu, were presented at the Conference on Retroviruses and Opportunistic Infections 2021 Annual Meeting, as reported by this news organization.
In the small study, the pill significantly reduced infectious virus in patients who were symptomatic and had tested positive for COVID-19 during the previous 4 days but were not hospitalized.
After 5 days of treatment, no participants who received molnupiravir had detectable virus, whereas 24% who received placebo did.
Two other oral agents are being developed by RedHill Biopharma: one for severe COVID-19 infection for hospitalized patients and one for patients at home with mild infection.
The first, opaganib (Yeliva), proceeded to a phase 2/3 global trial for hospitalized patients after the company announced top-line safety and efficacy data in December. In phase 2, the drug was shown to be safe in patients requiring oxygen and effectively reduced the need for oxygen by the end of the treatment period.
A key feature is that it is both an antiviral and an anti-inflammatory, Gilead Raday, RedHill’s chief operating officer, said in an interview. Data are expected midyear on its performance in 464 patients. The drug is being tested on top of remdesivir or in addition to dexamethasone.
The second, upamostat (RHB-107), is currently undergoing a phase 2/3 trial in the United States and is being investigated for use in nonhospitalized COVID-19 patients.
“I would expect data to be available in the second half of this year,” Mr. Raday said.
Upamostat is a novel serine protease inhibitor expected to be effective against emerging variants because it targets human cell factors involved in viral entry, according to the company.
Other drugs are being investigated in trials that are in earlier stages.
Urgent need for oral agents
Infectious disease specialists are watching the move toward a COVID-19 pill enthusiastically.
“We badly need an oral treatment option for COVID,” said Sarah Doernberg, MD, an infectious disease specialist from the University of California, San Francisco.
“It’s a real gap in our armamentarium for COVID in outpatient treatment, which is where most who contract COVID-19 will seek care,” she said in an interview.
Although some studies have shown the benefit of monoclonal antibodies for prevention and early treatment, there are major logistical issues because all the current options require IV administration, she explained.
“If we had a pill to treat early COVID, especially in high-risk patients, it would fill a gap,” she said, noting that a pill could help people get better faster and prevent hospital stays.
Studies of molnupiravir suggest that it decreases viral shedding in the first few days after COVID infection, Dr. Doernberg reported.
There is excitement around the drug, but it will be important to see whether the results translate into fewer people requiring hospital admission and whether people feel better faster.
“I want to see the clinical data,” Dr. Doernberg said.
She will also be watching for the upamostat and opaganib results in the coming weeks.
“If these drugs are successful, I think it’s possible we could use them – maybe under an emergency use authorization – this year,” she said.
Once antiviral pills are a viable option for COVID-19 treatment, questions will arise about their use, she said.
One question is whether patients who are getting remdesivir in the hospital and are ready to leave after 5 days should continue treatment with antiviral pills at home.
Another is whether the pills – if they are shown to be effective – will be helpful for COVID post exposure. That use would be important for people who do not have COVID-19 but who are in close contact with someone who does, such as a member of their household.
“We have that model,” Dr. Doernberg said. “We know that oseltamivir can be used for postexposure prophylaxis and can help to prevent development of clinical disease.”
But she cautioned that a challenge with COVID is that people are contagious very early. A pill would need to come with the ability to test for COVID-19 early and get patients linked to care immediately.
“Those are not small challenges,” she said.
Vaccines alone won’t end the COVID threat
Treatments are part of the “belt-and-suspenders” approach, along with vaccines to combat COVID-19, Dr. Doernberg said.
“We’re not going to eradicate COVID,” she said. “We’re still going to need treatments for people who either don’t respond to the vaccine or haven’t gotten the vaccine or developed disease despite the vaccine.”
Oral formulations are desperately needed, agreed Kenneth Johnson, PhD, professor of molecular biosciences at the University of Texas at Austin.
Right now, remdesivir treatments involve patients being hooked up to an IV for 30-120 minutes each day for 5 days. And the cost of a 5-day course of remdesivir ranges from $2340 to $3120 in the United States.
“We’re hoping we can come up with something that is a little bit easier to administer, and without as many concerns for toxic side effects,” he said.
Dr. Johnson’s team at UT-Austin recently made a key discovery about the way remdesivir stops the replication of viral RNA.
The understanding of where the virus starts to replicate in the infection chain of events and how and where it reacts with remdesivir might lead to the development of better, more concentrated pill forms of antivirals in the future, with fewer toxicities, he said.
The team used a lab dish to recreate the step-by-step process that occurs when a patient who is infected with SARS-CoV-2 receives remdesivir.
The discovery was published online in Molecular Cell in January and will be printed in the April issue of the journal.
The discovery won’t lead to an effective COVID-19 pill for our current crisis, but will be important for the next generation of drugs needed to deal with future coronaviruses, Dr. Johnson explained.
And there will be other coronaviruses, he said, noting that this one is the third in 20 years to jump from animals to humans. “It’s just a matter of time,” he said.
A version of this article first appeared on Medscape.com.
New pills to treat patients with COVID-19 are currently in midstage clinical trials and, if successful, could be ready by the end of the year.
Only one treatment – remdesivir (Veklury) – has been fully approved by the U.S. Food and Drug Administration for patients in the hospital and it must be administered intravenously.
Hopes for a day when patients with COVID-19 can take a pill to rid their bodies of the virus got a boost when early trial results were presented at a medical conference.
Interim phase 2 results for the oral experimental COVID-19 drug molnupiravir, designed to do for patients with COVID-19 what oseltamivir (Tamiflu) can do for patients with the flu, were presented at the Conference on Retroviruses and Opportunistic Infections 2021 Annual Meeting, as reported by this news organization.
In the small study, the pill significantly reduced infectious virus in patients who were symptomatic and had tested positive for COVID-19 during the previous 4 days but were not hospitalized.
After 5 days of treatment, no participants who received molnupiravir had detectable virus, whereas 24% who received placebo did.
Two other oral agents are being developed by RedHill Biopharma: one for severe COVID-19 infection for hospitalized patients and one for patients at home with mild infection.
The first, opaganib (Yeliva), proceeded to a phase 2/3 global trial for hospitalized patients after the company announced top-line safety and efficacy data in December. In phase 2, the drug was shown to be safe in patients requiring oxygen and effectively reduced the need for oxygen by the end of the treatment period.
A key feature is that it is both an antiviral and an anti-inflammatory, Gilead Raday, RedHill’s chief operating officer, said in an interview. Data are expected midyear on its performance in 464 patients. The drug is being tested on top of remdesivir or in addition to dexamethasone.
The second, upamostat (RHB-107), is currently undergoing a phase 2/3 trial in the United States and is being investigated for use in nonhospitalized COVID-19 patients.
“I would expect data to be available in the second half of this year,” Mr. Raday said.
Upamostat is a novel serine protease inhibitor expected to be effective against emerging variants because it targets human cell factors involved in viral entry, according to the company.
Other drugs are being investigated in trials that are in earlier stages.
Urgent need for oral agents
Infectious disease specialists are watching the move toward a COVID-19 pill enthusiastically.
“We badly need an oral treatment option for COVID,” said Sarah Doernberg, MD, an infectious disease specialist from the University of California, San Francisco.
“It’s a real gap in our armamentarium for COVID in outpatient treatment, which is where most who contract COVID-19 will seek care,” she said in an interview.
Although some studies have shown the benefit of monoclonal antibodies for prevention and early treatment, there are major logistical issues because all the current options require IV administration, she explained.
“If we had a pill to treat early COVID, especially in high-risk patients, it would fill a gap,” she said, noting that a pill could help people get better faster and prevent hospital stays.
Studies of molnupiravir suggest that it decreases viral shedding in the first few days after COVID infection, Dr. Doernberg reported.
There is excitement around the drug, but it will be important to see whether the results translate into fewer people requiring hospital admission and whether people feel better faster.
“I want to see the clinical data,” Dr. Doernberg said.
She will also be watching for the upamostat and opaganib results in the coming weeks.
“If these drugs are successful, I think it’s possible we could use them – maybe under an emergency use authorization – this year,” she said.
Once antiviral pills are a viable option for COVID-19 treatment, questions will arise about their use, she said.
One question is whether patients who are getting remdesivir in the hospital and are ready to leave after 5 days should continue treatment with antiviral pills at home.
Another is whether the pills – if they are shown to be effective – will be helpful for COVID post exposure. That use would be important for people who do not have COVID-19 but who are in close contact with someone who does, such as a member of their household.
“We have that model,” Dr. Doernberg said. “We know that oseltamivir can be used for postexposure prophylaxis and can help to prevent development of clinical disease.”
But she cautioned that a challenge with COVID is that people are contagious very early. A pill would need to come with the ability to test for COVID-19 early and get patients linked to care immediately.
“Those are not small challenges,” she said.
Vaccines alone won’t end the COVID threat
Treatments are part of the “belt-and-suspenders” approach, along with vaccines to combat COVID-19, Dr. Doernberg said.
“We’re not going to eradicate COVID,” she said. “We’re still going to need treatments for people who either don’t respond to the vaccine or haven’t gotten the vaccine or developed disease despite the vaccine.”
Oral formulations are desperately needed, agreed Kenneth Johnson, PhD, professor of molecular biosciences at the University of Texas at Austin.
Right now, remdesivir treatments involve patients being hooked up to an IV for 30-120 minutes each day for 5 days. And the cost of a 5-day course of remdesivir ranges from $2340 to $3120 in the United States.
“We’re hoping we can come up with something that is a little bit easier to administer, and without as many concerns for toxic side effects,” he said.
Dr. Johnson’s team at UT-Austin recently made a key discovery about the way remdesivir stops the replication of viral RNA.
The understanding of where the virus starts to replicate in the infection chain of events and how and where it reacts with remdesivir might lead to the development of better, more concentrated pill forms of antivirals in the future, with fewer toxicities, he said.
The team used a lab dish to recreate the step-by-step process that occurs when a patient who is infected with SARS-CoV-2 receives remdesivir.
The discovery was published online in Molecular Cell in January and will be printed in the April issue of the journal.
The discovery won’t lead to an effective COVID-19 pill for our current crisis, but will be important for the next generation of drugs needed to deal with future coronaviruses, Dr. Johnson explained.
And there will be other coronaviruses, he said, noting that this one is the third in 20 years to jump from animals to humans. “It’s just a matter of time,” he said.
A version of this article first appeared on Medscape.com.
New pills to treat patients with COVID-19 are currently in midstage clinical trials and, if successful, could be ready by the end of the year.
Only one treatment – remdesivir (Veklury) – has been fully approved by the U.S. Food and Drug Administration for patients in the hospital and it must be administered intravenously.
Hopes for a day when patients with COVID-19 can take a pill to rid their bodies of the virus got a boost when early trial results were presented at a medical conference.
Interim phase 2 results for the oral experimental COVID-19 drug molnupiravir, designed to do for patients with COVID-19 what oseltamivir (Tamiflu) can do for patients with the flu, were presented at the Conference on Retroviruses and Opportunistic Infections 2021 Annual Meeting, as reported by this news organization.
In the small study, the pill significantly reduced infectious virus in patients who were symptomatic and had tested positive for COVID-19 during the previous 4 days but were not hospitalized.
After 5 days of treatment, no participants who received molnupiravir had detectable virus, whereas 24% who received placebo did.
Two other oral agents are being developed by RedHill Biopharma: one for severe COVID-19 infection for hospitalized patients and one for patients at home with mild infection.
The first, opaganib (Yeliva), proceeded to a phase 2/3 global trial for hospitalized patients after the company announced top-line safety and efficacy data in December. In phase 2, the drug was shown to be safe in patients requiring oxygen and effectively reduced the need for oxygen by the end of the treatment period.
A key feature is that it is both an antiviral and an anti-inflammatory, Gilead Raday, RedHill’s chief operating officer, said in an interview. Data are expected midyear on its performance in 464 patients. The drug is being tested on top of remdesivir or in addition to dexamethasone.
The second, upamostat (RHB-107), is currently undergoing a phase 2/3 trial in the United States and is being investigated for use in nonhospitalized COVID-19 patients.
“I would expect data to be available in the second half of this year,” Mr. Raday said.
Upamostat is a novel serine protease inhibitor expected to be effective against emerging variants because it targets human cell factors involved in viral entry, according to the company.
Other drugs are being investigated in trials that are in earlier stages.
Urgent need for oral agents
Infectious disease specialists are watching the move toward a COVID-19 pill enthusiastically.
“We badly need an oral treatment option for COVID,” said Sarah Doernberg, MD, an infectious disease specialist from the University of California, San Francisco.
“It’s a real gap in our armamentarium for COVID in outpatient treatment, which is where most who contract COVID-19 will seek care,” she said in an interview.
Although some studies have shown the benefit of monoclonal antibodies for prevention and early treatment, there are major logistical issues because all the current options require IV administration, she explained.
“If we had a pill to treat early COVID, especially in high-risk patients, it would fill a gap,” she said, noting that a pill could help people get better faster and prevent hospital stays.
Studies of molnupiravir suggest that it decreases viral shedding in the first few days after COVID infection, Dr. Doernberg reported.
There is excitement around the drug, but it will be important to see whether the results translate into fewer people requiring hospital admission and whether people feel better faster.
“I want to see the clinical data,” Dr. Doernberg said.
She will also be watching for the upamostat and opaganib results in the coming weeks.
“If these drugs are successful, I think it’s possible we could use them – maybe under an emergency use authorization – this year,” she said.
Once antiviral pills are a viable option for COVID-19 treatment, questions will arise about their use, she said.
One question is whether patients who are getting remdesivir in the hospital and are ready to leave after 5 days should continue treatment with antiviral pills at home.
Another is whether the pills – if they are shown to be effective – will be helpful for COVID post exposure. That use would be important for people who do not have COVID-19 but who are in close contact with someone who does, such as a member of their household.
“We have that model,” Dr. Doernberg said. “We know that oseltamivir can be used for postexposure prophylaxis and can help to prevent development of clinical disease.”
But she cautioned that a challenge with COVID is that people are contagious very early. A pill would need to come with the ability to test for COVID-19 early and get patients linked to care immediately.
“Those are not small challenges,” she said.
Vaccines alone won’t end the COVID threat
Treatments are part of the “belt-and-suspenders” approach, along with vaccines to combat COVID-19, Dr. Doernberg said.
“We’re not going to eradicate COVID,” she said. “We’re still going to need treatments for people who either don’t respond to the vaccine or haven’t gotten the vaccine or developed disease despite the vaccine.”
Oral formulations are desperately needed, agreed Kenneth Johnson, PhD, professor of molecular biosciences at the University of Texas at Austin.
Right now, remdesivir treatments involve patients being hooked up to an IV for 30-120 minutes each day for 5 days. And the cost of a 5-day course of remdesivir ranges from $2340 to $3120 in the United States.
“We’re hoping we can come up with something that is a little bit easier to administer, and without as many concerns for toxic side effects,” he said.
Dr. Johnson’s team at UT-Austin recently made a key discovery about the way remdesivir stops the replication of viral RNA.
The understanding of where the virus starts to replicate in the infection chain of events and how and where it reacts with remdesivir might lead to the development of better, more concentrated pill forms of antivirals in the future, with fewer toxicities, he said.
The team used a lab dish to recreate the step-by-step process that occurs when a patient who is infected with SARS-CoV-2 receives remdesivir.
The discovery was published online in Molecular Cell in January and will be printed in the April issue of the journal.
The discovery won’t lead to an effective COVID-19 pill for our current crisis, but will be important for the next generation of drugs needed to deal with future coronaviruses, Dr. Johnson explained.
And there will be other coronaviruses, he said, noting that this one is the third in 20 years to jump from animals to humans. “It’s just a matter of time,” he said.
A version of this article first appeared on Medscape.com.
Pregnant patients with severe COVID-19 disease at increased risk of complications
Pregnant patients with COVID-19 infections were more likely to experience severe disease if they had preexisting comorbidities, such as chronic hypertension, asthma, or pregestational diabetes, according to findings from a new study presented at the meeting sponsored by the Society for Maternal-Fetal Medicine.
The study included outcomes for the largest multistate cohort of pregnant patients with COVID-19 outside of what the Centers for Disease Control and Prevention is tracking. Its findings also mirrored those of a multicenter, retrospective study in Washington state, published in the American Journal of Obstetrics & Gynecology. That study also found that pregnant patients hospitalized for COVID-19 were more likely to have comorbidities, and both studies found an increased likelihood of preterm birth among pregnant patients with severe or critical disease.
Disease severity linked to risk of perinatal complications
In the abstract presented at the SMFM meeting, more severe disease was associated with older age and a higher median body mass index, as seen in the general population, but the researchers found no differences in disease severity occurred by race or ethnicity, Torri D. Metz, MD, of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network, told attendees of the conference. The researchers also found that perinatal complications were more prevalent in those with severe or critical COVID-19 disease but not in those with mild or moderate disease. Vertical COVID-19 transmission from mother to child was rare.
The observational study included all patients who had a singleton pregnancy, had a positive SARS-CoV-2 test, and delivered between March 1 and July 31, 2020, at one of the 33 U.S. hospitals in the NICHD Maternal-Fetal Medicine Units Network, spread across 14 states. The researchers used electronic medical records to determine incidence of cesarean delivery, postpartum hemorrhage, hypertensive disorders of pregnancy, preterm birth (less than 37 weeks), maternal death, infant death, and positive infant COVID-19 test. They tracked mothers through 6 weeks post partum and newborns through delivery hospitalization.
Of 1,291 patients in the cohort, 1,219 received their first positive COVID-19 test during pregnancy. The others tested positive while in the hospital for delivery or within a month and a half after discharge. Limiting their analysis to those who developed COVID-19 while pregnant prior to delivery, nearly half (47%) were asymptomatic.
The disease was mild in 27%, moderate in 14%, severe in 8%, and critical in 4%. The researchers used the National Institutes of Health classifications for severity and included deaths in the critical group. The most common symptom was a cough, reported by a third of the patients (34%). Four of six maternal deaths that occurred were caused by COVID-19.
Compared with an average age of 28 in those without symptoms, the mean age was 29 in those with mild/moderate disease and 30 in those with severe/critical disease (P = .006). Similarly, the mean BMI was 28.3 in asymptomatic patients, 29 in those with mild/moderate disease, and 32.3 in those with severe/critical disease (P < .001). Despite a diverse cohort – 53% Hispanic, 23% Black, and 15% White – the researches found no racial/ethnic trends in disease severity.
Patients who had asthma, chronic obstructive pulmonary disorder, pregestational diabetes, chronic hypertension, chronic liver disease, or a seizure disorder were all significantly more likely to have critical/severe disease than mild/moderate disease, and more likely to have mild/moderate disease than asymptomatic (P values ranged from < .001 to .02).
The mothers with critical or severe illness were 1.6 times more likely to have cesarean births and to have hypertensive disorders of pregnancy, and they were twice as likely to have postpartum hemorrhage (P < .001; P = .007). Those with mild or moderate disease, however, had no increased risks for perinatal complications over asymptomatic patients.
Critical or severe illness was also associated with more than triple the risk of preterm birth (adjusted risk ratio, 3.6; P < .001). Newborns of mothers with critical or severe illness also had three times greater risk of neonatal ICU admission (ARR, 3.1; P <. 001) and weighed an average 385 g less than newborns of asymptomatic mothers. COVID-19 rate among infants was only 1% during delivery hospitalization.
Since the study cutoff was July 30 and COVID infections only became prevalent in March, the researchers were unable to evaluate women for outcomes resulting from COVID infections in early pregnancy, such as congenital anomalies or early miscarriage, Dr. Metz said. In addition, since many of the sites are urban centers, the data may not be generalizable to rural areas.
Peter S. Bernstein, MD, MPH, of Montefiore Medical Center, New York, asked whether the increased cesarean deliveries and preterm births in the group of women with severe disease were caused by usual obstetric causes or the treatment of COVID-19 infection. Dr. Metz said the vast majority of preterm deliveries were indicated, but only a small proportion were induced for COVID-19 alone. “A lot had hypertensive disorders of pregnancies or PPROM, so it’s partly driven by the infection itself but also partly driven by some of those perinatal complications,” she said.
Similar findings in Washington
In the Washington study, among 240 pregnant patients with confirmed COVID-19 infection between March 1 and July 30, 2020, 1 in 11 developed severe or critical disease, and 1 in 10 were hospitalized. The pregnant patients had more than triple the risk of hospitalization compared with adults of similar ages in the general population (10% vs. 2.8%; rate ratio, 3.5). Similar to the multistate NICHD study, women were more likely to be hospitalized if they had asthma, hypertension, type 2 diabetes, autoimmune disease, or class III obesity.
Three mothers died of COVID-19, resulting in a case fatality rate 13.6 times greater than nonpregnant patients with COVID-19 in the general population. The absolute difference in the rate was 1.2%. As seen in the NICHD study, preterm birth was more common in mothers with severe or critical COVID-19. Nearly half (45.4%) of mothers with severe or critical COVID-19 delivered preterm compared to 5.2% in those with mild COVID-19 (P < .001).
“Our finding that deaths in pregnant patients contributed disproportionately to deaths from COVID-19 among 20- to 39-year-olds in Washington state is similar to what was observed during the influenza A virus H1N1 2009 pandemic,” Erica M. Lokken, PhD, MS, of the departments of global health and ob.gyn. at the University of Washington, Seattle, and colleagues wrote in the Washington study. But they noted that it took 8 months into the pandemic before pregnant patients were identified as a high-risk group for COVID-19.
“Given the similarity in clinical course between COVID-19 and IAV H1N1 2009 with an increased risk for mortality during pregnancy and the postpartum period, we strongly recommend that pregnant patients should be considered a high-risk population to novel highly pathogenic respiratory viruses until proven otherwise by population-based studies with good ascertainment of pregnancy status,” they wrote.
Judette Louis, MD, MPH, associate professor of ob.gyn. and department chair at the University of South Florida, Tampa, said in an interview that the findings in these studies were fairly expected, but it’s important to have data from such a large cohort as the one presented at SMFM.
“It confirmed that those who had severe disease were more likely to have chronic medical conditions, mirroring what we saw in the general population who isn’t pregnant,” Dr. Louis said. “I thought this was very crucial because as pregnant women are trying to decide whether they should get the COVID vaccine, this provides support to say that if you’re pregnant, you’re more likely to have severe disease [if you have] other chronic medical conditions.”
The findings also confirm the importance of pregnant people taking precautions to avoid infection.
“Even though these individuals are, as a group, in an age cohort that mostly has asymptomatic disease, for some of them, it results in severe disease and even maternal death,” she said. “They should still take it seriously if they’re pregnant.”
The SMFM abstract study was funded by the NICHD. The Washington study was funded by the University of Washington Population Health Initiative, the National Institutes of Health, and philanthropic gift funds. One coauthor of the Washington study is on a Pfizer and GlaxoSmithKline advisory board for immunizations. No other authors or individuals interviewed reported any disclosures.
Pregnant patients with COVID-19 infections were more likely to experience severe disease if they had preexisting comorbidities, such as chronic hypertension, asthma, or pregestational diabetes, according to findings from a new study presented at the meeting sponsored by the Society for Maternal-Fetal Medicine.
The study included outcomes for the largest multistate cohort of pregnant patients with COVID-19 outside of what the Centers for Disease Control and Prevention is tracking. Its findings also mirrored those of a multicenter, retrospective study in Washington state, published in the American Journal of Obstetrics & Gynecology. That study also found that pregnant patients hospitalized for COVID-19 were more likely to have comorbidities, and both studies found an increased likelihood of preterm birth among pregnant patients with severe or critical disease.
Disease severity linked to risk of perinatal complications
In the abstract presented at the SMFM meeting, more severe disease was associated with older age and a higher median body mass index, as seen in the general population, but the researchers found no differences in disease severity occurred by race or ethnicity, Torri D. Metz, MD, of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network, told attendees of the conference. The researchers also found that perinatal complications were more prevalent in those with severe or critical COVID-19 disease but not in those with mild or moderate disease. Vertical COVID-19 transmission from mother to child was rare.
The observational study included all patients who had a singleton pregnancy, had a positive SARS-CoV-2 test, and delivered between March 1 and July 31, 2020, at one of the 33 U.S. hospitals in the NICHD Maternal-Fetal Medicine Units Network, spread across 14 states. The researchers used electronic medical records to determine incidence of cesarean delivery, postpartum hemorrhage, hypertensive disorders of pregnancy, preterm birth (less than 37 weeks), maternal death, infant death, and positive infant COVID-19 test. They tracked mothers through 6 weeks post partum and newborns through delivery hospitalization.
Of 1,291 patients in the cohort, 1,219 received their first positive COVID-19 test during pregnancy. The others tested positive while in the hospital for delivery or within a month and a half after discharge. Limiting their analysis to those who developed COVID-19 while pregnant prior to delivery, nearly half (47%) were asymptomatic.
The disease was mild in 27%, moderate in 14%, severe in 8%, and critical in 4%. The researchers used the National Institutes of Health classifications for severity and included deaths in the critical group. The most common symptom was a cough, reported by a third of the patients (34%). Four of six maternal deaths that occurred were caused by COVID-19.
Compared with an average age of 28 in those without symptoms, the mean age was 29 in those with mild/moderate disease and 30 in those with severe/critical disease (P = .006). Similarly, the mean BMI was 28.3 in asymptomatic patients, 29 in those with mild/moderate disease, and 32.3 in those with severe/critical disease (P < .001). Despite a diverse cohort – 53% Hispanic, 23% Black, and 15% White – the researches found no racial/ethnic trends in disease severity.
Patients who had asthma, chronic obstructive pulmonary disorder, pregestational diabetes, chronic hypertension, chronic liver disease, or a seizure disorder were all significantly more likely to have critical/severe disease than mild/moderate disease, and more likely to have mild/moderate disease than asymptomatic (P values ranged from < .001 to .02).
The mothers with critical or severe illness were 1.6 times more likely to have cesarean births and to have hypertensive disorders of pregnancy, and they were twice as likely to have postpartum hemorrhage (P < .001; P = .007). Those with mild or moderate disease, however, had no increased risks for perinatal complications over asymptomatic patients.
Critical or severe illness was also associated with more than triple the risk of preterm birth (adjusted risk ratio, 3.6; P < .001). Newborns of mothers with critical or severe illness also had three times greater risk of neonatal ICU admission (ARR, 3.1; P <. 001) and weighed an average 385 g less than newborns of asymptomatic mothers. COVID-19 rate among infants was only 1% during delivery hospitalization.
Since the study cutoff was July 30 and COVID infections only became prevalent in March, the researchers were unable to evaluate women for outcomes resulting from COVID infections in early pregnancy, such as congenital anomalies or early miscarriage, Dr. Metz said. In addition, since many of the sites are urban centers, the data may not be generalizable to rural areas.
Peter S. Bernstein, MD, MPH, of Montefiore Medical Center, New York, asked whether the increased cesarean deliveries and preterm births in the group of women with severe disease were caused by usual obstetric causes or the treatment of COVID-19 infection. Dr. Metz said the vast majority of preterm deliveries were indicated, but only a small proportion were induced for COVID-19 alone. “A lot had hypertensive disorders of pregnancies or PPROM, so it’s partly driven by the infection itself but also partly driven by some of those perinatal complications,” she said.
Similar findings in Washington
In the Washington study, among 240 pregnant patients with confirmed COVID-19 infection between March 1 and July 30, 2020, 1 in 11 developed severe or critical disease, and 1 in 10 were hospitalized. The pregnant patients had more than triple the risk of hospitalization compared with adults of similar ages in the general population (10% vs. 2.8%; rate ratio, 3.5). Similar to the multistate NICHD study, women were more likely to be hospitalized if they had asthma, hypertension, type 2 diabetes, autoimmune disease, or class III obesity.
Three mothers died of COVID-19, resulting in a case fatality rate 13.6 times greater than nonpregnant patients with COVID-19 in the general population. The absolute difference in the rate was 1.2%. As seen in the NICHD study, preterm birth was more common in mothers with severe or critical COVID-19. Nearly half (45.4%) of mothers with severe or critical COVID-19 delivered preterm compared to 5.2% in those with mild COVID-19 (P < .001).
“Our finding that deaths in pregnant patients contributed disproportionately to deaths from COVID-19 among 20- to 39-year-olds in Washington state is similar to what was observed during the influenza A virus H1N1 2009 pandemic,” Erica M. Lokken, PhD, MS, of the departments of global health and ob.gyn. at the University of Washington, Seattle, and colleagues wrote in the Washington study. But they noted that it took 8 months into the pandemic before pregnant patients were identified as a high-risk group for COVID-19.
“Given the similarity in clinical course between COVID-19 and IAV H1N1 2009 with an increased risk for mortality during pregnancy and the postpartum period, we strongly recommend that pregnant patients should be considered a high-risk population to novel highly pathogenic respiratory viruses until proven otherwise by population-based studies with good ascertainment of pregnancy status,” they wrote.
Judette Louis, MD, MPH, associate professor of ob.gyn. and department chair at the University of South Florida, Tampa, said in an interview that the findings in these studies were fairly expected, but it’s important to have data from such a large cohort as the one presented at SMFM.
“It confirmed that those who had severe disease were more likely to have chronic medical conditions, mirroring what we saw in the general population who isn’t pregnant,” Dr. Louis said. “I thought this was very crucial because as pregnant women are trying to decide whether they should get the COVID vaccine, this provides support to say that if you’re pregnant, you’re more likely to have severe disease [if you have] other chronic medical conditions.”
The findings also confirm the importance of pregnant people taking precautions to avoid infection.
“Even though these individuals are, as a group, in an age cohort that mostly has asymptomatic disease, for some of them, it results in severe disease and even maternal death,” she said. “They should still take it seriously if they’re pregnant.”
The SMFM abstract study was funded by the NICHD. The Washington study was funded by the University of Washington Population Health Initiative, the National Institutes of Health, and philanthropic gift funds. One coauthor of the Washington study is on a Pfizer and GlaxoSmithKline advisory board for immunizations. No other authors or individuals interviewed reported any disclosures.
Pregnant patients with COVID-19 infections were more likely to experience severe disease if they had preexisting comorbidities, such as chronic hypertension, asthma, or pregestational diabetes, according to findings from a new study presented at the meeting sponsored by the Society for Maternal-Fetal Medicine.
The study included outcomes for the largest multistate cohort of pregnant patients with COVID-19 outside of what the Centers for Disease Control and Prevention is tracking. Its findings also mirrored those of a multicenter, retrospective study in Washington state, published in the American Journal of Obstetrics & Gynecology. That study also found that pregnant patients hospitalized for COVID-19 were more likely to have comorbidities, and both studies found an increased likelihood of preterm birth among pregnant patients with severe or critical disease.
Disease severity linked to risk of perinatal complications
In the abstract presented at the SMFM meeting, more severe disease was associated with older age and a higher median body mass index, as seen in the general population, but the researchers found no differences in disease severity occurred by race or ethnicity, Torri D. Metz, MD, of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network, told attendees of the conference. The researchers also found that perinatal complications were more prevalent in those with severe or critical COVID-19 disease but not in those with mild or moderate disease. Vertical COVID-19 transmission from mother to child was rare.
The observational study included all patients who had a singleton pregnancy, had a positive SARS-CoV-2 test, and delivered between March 1 and July 31, 2020, at one of the 33 U.S. hospitals in the NICHD Maternal-Fetal Medicine Units Network, spread across 14 states. The researchers used electronic medical records to determine incidence of cesarean delivery, postpartum hemorrhage, hypertensive disorders of pregnancy, preterm birth (less than 37 weeks), maternal death, infant death, and positive infant COVID-19 test. They tracked mothers through 6 weeks post partum and newborns through delivery hospitalization.
Of 1,291 patients in the cohort, 1,219 received their first positive COVID-19 test during pregnancy. The others tested positive while in the hospital for delivery or within a month and a half after discharge. Limiting their analysis to those who developed COVID-19 while pregnant prior to delivery, nearly half (47%) were asymptomatic.
The disease was mild in 27%, moderate in 14%, severe in 8%, and critical in 4%. The researchers used the National Institutes of Health classifications for severity and included deaths in the critical group. The most common symptom was a cough, reported by a third of the patients (34%). Four of six maternal deaths that occurred were caused by COVID-19.
Compared with an average age of 28 in those without symptoms, the mean age was 29 in those with mild/moderate disease and 30 in those with severe/critical disease (P = .006). Similarly, the mean BMI was 28.3 in asymptomatic patients, 29 in those with mild/moderate disease, and 32.3 in those with severe/critical disease (P < .001). Despite a diverse cohort – 53% Hispanic, 23% Black, and 15% White – the researches found no racial/ethnic trends in disease severity.
Patients who had asthma, chronic obstructive pulmonary disorder, pregestational diabetes, chronic hypertension, chronic liver disease, or a seizure disorder were all significantly more likely to have critical/severe disease than mild/moderate disease, and more likely to have mild/moderate disease than asymptomatic (P values ranged from < .001 to .02).
The mothers with critical or severe illness were 1.6 times more likely to have cesarean births and to have hypertensive disorders of pregnancy, and they were twice as likely to have postpartum hemorrhage (P < .001; P = .007). Those with mild or moderate disease, however, had no increased risks for perinatal complications over asymptomatic patients.
Critical or severe illness was also associated with more than triple the risk of preterm birth (adjusted risk ratio, 3.6; P < .001). Newborns of mothers with critical or severe illness also had three times greater risk of neonatal ICU admission (ARR, 3.1; P <. 001) and weighed an average 385 g less than newborns of asymptomatic mothers. COVID-19 rate among infants was only 1% during delivery hospitalization.
Since the study cutoff was July 30 and COVID infections only became prevalent in March, the researchers were unable to evaluate women for outcomes resulting from COVID infections in early pregnancy, such as congenital anomalies or early miscarriage, Dr. Metz said. In addition, since many of the sites are urban centers, the data may not be generalizable to rural areas.
Peter S. Bernstein, MD, MPH, of Montefiore Medical Center, New York, asked whether the increased cesarean deliveries and preterm births in the group of women with severe disease were caused by usual obstetric causes or the treatment of COVID-19 infection. Dr. Metz said the vast majority of preterm deliveries were indicated, but only a small proportion were induced for COVID-19 alone. “A lot had hypertensive disorders of pregnancies or PPROM, so it’s partly driven by the infection itself but also partly driven by some of those perinatal complications,” she said.
Similar findings in Washington
In the Washington study, among 240 pregnant patients with confirmed COVID-19 infection between March 1 and July 30, 2020, 1 in 11 developed severe or critical disease, and 1 in 10 were hospitalized. The pregnant patients had more than triple the risk of hospitalization compared with adults of similar ages in the general population (10% vs. 2.8%; rate ratio, 3.5). Similar to the multistate NICHD study, women were more likely to be hospitalized if they had asthma, hypertension, type 2 diabetes, autoimmune disease, or class III obesity.
Three mothers died of COVID-19, resulting in a case fatality rate 13.6 times greater than nonpregnant patients with COVID-19 in the general population. The absolute difference in the rate was 1.2%. As seen in the NICHD study, preterm birth was more common in mothers with severe or critical COVID-19. Nearly half (45.4%) of mothers with severe or critical COVID-19 delivered preterm compared to 5.2% in those with mild COVID-19 (P < .001).
“Our finding that deaths in pregnant patients contributed disproportionately to deaths from COVID-19 among 20- to 39-year-olds in Washington state is similar to what was observed during the influenza A virus H1N1 2009 pandemic,” Erica M. Lokken, PhD, MS, of the departments of global health and ob.gyn. at the University of Washington, Seattle, and colleagues wrote in the Washington study. But they noted that it took 8 months into the pandemic before pregnant patients were identified as a high-risk group for COVID-19.
“Given the similarity in clinical course between COVID-19 and IAV H1N1 2009 with an increased risk for mortality during pregnancy and the postpartum period, we strongly recommend that pregnant patients should be considered a high-risk population to novel highly pathogenic respiratory viruses until proven otherwise by population-based studies with good ascertainment of pregnancy status,” they wrote.
Judette Louis, MD, MPH, associate professor of ob.gyn. and department chair at the University of South Florida, Tampa, said in an interview that the findings in these studies were fairly expected, but it’s important to have data from such a large cohort as the one presented at SMFM.
“It confirmed that those who had severe disease were more likely to have chronic medical conditions, mirroring what we saw in the general population who isn’t pregnant,” Dr. Louis said. “I thought this was very crucial because as pregnant women are trying to decide whether they should get the COVID vaccine, this provides support to say that if you’re pregnant, you’re more likely to have severe disease [if you have] other chronic medical conditions.”
The findings also confirm the importance of pregnant people taking precautions to avoid infection.
“Even though these individuals are, as a group, in an age cohort that mostly has asymptomatic disease, for some of them, it results in severe disease and even maternal death,” she said. “They should still take it seriously if they’re pregnant.”
The SMFM abstract study was funded by the NICHD. The Washington study was funded by the University of Washington Population Health Initiative, the National Institutes of Health, and philanthropic gift funds. One coauthor of the Washington study is on a Pfizer and GlaxoSmithKline advisory board for immunizations. No other authors or individuals interviewed reported any disclosures.
FROM THE PREGNANCY MEETING