User login
ID Practitioner is an independent news source that provides infectious disease specialists with timely and relevant news and commentary about clinical developments and the impact of health care policy on the infectious disease specialist’s practice. Specialty focus topics include antimicrobial resistance, emerging infections, global ID, hepatitis, HIV, hospital-acquired infections, immunizations and vaccines, influenza, mycoses, pediatric infections, and STIs. Infectious Diseases News is owned by Frontline Medical Communications.
sofosbuvir
ritonavir with dasabuvir
discount
support path
program
ritonavir
greedy
ledipasvir
assistance
viekira pak
vpak
advocacy
needy
protest
abbvie
paritaprevir
ombitasvir
direct-acting antivirals
dasabuvir
gilead
fake-ovir
support
v pak
oasis
harvoni
section[contains(@class, 'footer-nav-section-wrapper')]
div[contains(@class, 'pane-pub-article-idp')]
div[contains(@class, 'pane-medstat-latest-articles-articles-section')]
div[contains(@class, 'pane-pub-home-idp')]
div[contains(@class, 'pane-pub-topic-idp')]
Clean indoor air is vital for infection control
Health workers already know that indoor air quality can be as important to human health as clean water and uncontaminated food. But before the COVID-19 pandemic, its importance in the prevention of respiratory illnesses outside of health circles was only whispered about.
Now, a team of nearly 40 scientists from 14 countries is calling for “a paradigm shift,” so that improvements in indoor air quality are viewed as essential to curb respiratory infections.
Most countries do not have indoor air-quality standards, the scientists point out in their recent report, and those that do often fall short in scope and enforcement.
“We expect everywhere in the world to have clean water flowing from our taps. In most parts of the developed world, it is happening and we take it completely for granted,” said lead investigator Lidia Morawska, PhD, of the International Laboratory for Air Quality and Health at the Queensland University of Technology in Brisbane, Australia.
But bacteria and viruses can circulate freely in the air, and “no one thinks about this, whatsoever, apart from health care facilities,” she said.
A first step is to recognize the risk posed by airborne pathogens, something not yet universally acknowledged. The investigators also want the World Health Organization to extend its guidelines to cover airborne pathogens, and for ventilation standards to include higher airflow and filtration rates.
Germany has been at the forefront of air-quality measures, Dr. Morawska said. Years ago, she observed a monitor showing the carbon dioxide level and relative humidity in the room where she was attending a meeting. The screen was accompanied by red, yellow, and green signals to communicate risk. Such indicators are also commonly displayed in German schools so teachers know when to open the windows or adjust the ventilation.
Monitors show carbon dioxide levels
But this is not yet being done in most other countries, Dr. Morawska said. Levels of carbon dioxide are one measure of indoor air quality, but they serve as a proxy for ventilation, she pointed out. Although the technology is available, sensors that can test a variety of components in a building in real time are not yet affordable.
Dr. Morawska envisions a future where the air quality numbers of the places people frequent are displayed so they know the risk for airborne transmission of respiratory illnesses. And people can begin to expect clean indoor air when they enter a business, office, or entertainment space and request changes when the air quality dips and improvement is needed, she said.
It is a daunting challenge to clean indoor air for several reasons. Air is not containable in the same way water is, which makes it difficult to trace contaminants. And infections transmitted through dirty water and food are usually evident immediately, whereas infections transmitted through airborne pathogens can take days to develop. Plus, the necessary infrastructure changes will be expensive.
However, the initial cost required to change the flow and quality of indoor air might be less than the cost of infections, the scientists pointed out. It is estimated that the global harm caused by COVID-19 alone costs $1 trillion each month.
“In the United States, the yearly cost – direct and indirect – of influenza has been calculated at $11.2 billion. For respiratory infections other than influenza, the yearly cost stood at $40 billion,” the team noted.
“If even half of this was caused by inhalation, we are still talking about massive costs,” said Dr. Morawska.
Bigger is not always better
It is tempting to see the solution as increased ventilation, said Ehsan Mousavi, PhD, assistant professor of construction science and management at Clemson (S.C.) University, who studies indoor air quality and ventilation in hospitals.
“We are ventilating the heck out of hospitals,” he said in an interview. But there is much debate about how much ventilation is the right amount. Too much and “you can blow pathogens into an open wound,” he explained. “Bigger is not always better.”
And there is still debate about the best mix of outside and recirculated air. An increase in the intake of outdoor air can refresh indoor air if it is clean, but that depends on where you live, he pointed out.
The mix used in most standard office buildings is 15% outside air and 85% recirculated air, Dr. Mousavi said. Boosting the percentage of outside air increases costs and energy use.
In fact, it can take five times more energy to ventilate hospital spaces than office spaces, he reported.
Engineers searching for clean-air solutions need to know what particulates are in the air and whether they are harmful to humans, but the sensors currently available can’t identify whether a virus is present in real time.
Samples have to be taken to a lab and, “by the time you know a virus was in the space, the moment is gone,” Dr. Mousavi explained.
More research is needed. “We need a reasonable answer that looks at the problem holistically, not just from the infectious disease perspective,” he said.
Hydrating indoor air
Research is making it clear that health care environments can play a significant role in patient recovery, according to Stephanie Taylor, MD. Dr. Taylor is president of Building4Health, which she founded to help businesses assess the quality of air in their buildings and find solutions. The company uses an algorithm to arrive at a health assessment score.
Air hydration is the most important aspect to target, she said.
Since the 1980s, research has shown that a relative humidity of 40%-60% is healthy for humans, she said. Currently, in an office building in a winter climate, the humidity level is more like 20%.
Canada is the first country to officially recommend the 40%-60% range for senior citizen centers and residential homes.
“Properly hydrated air supports our immune system and prevents skin problems and respiratory problems. It also inactivates many bacteria and viruses,” Dr. Taylor explained. Inhaling dry air compromises the ability of the body to restrict influenza virus infection, researchers showed in a 2019 study.
In the case of COVID-19, as virus particles attach to water molecules, they get bigger and heavier and eventually drop out of the breathing zone and onto surfaces where they can be wiped away, she explained.
But when the particles “are very small – like 5 microns in diameter – and you inhale them, they can lodge deep in the lungs,” she said.
In properly hydrated air, particles will be larger – about 10-20 microns when they attach to the water vapor – so they will get stuck in the nose or the back of the throat, where they can be washed away by mucous and not travel to the lungs.
“Indoor air metrics” can support our health or contribute to disease, “not just over time, but quickly, within minutes or hours,” she said.
No one expects the world’s building stock to suddenly upgrade to the ideal air quality. “But that doesn’t mean we shouldn’t move in that direction,” Dr. Taylor said. Changes can start small and gradually increase.
New research targets indoor air
Humidity is one of the key areas for current research, said Karl Rockne, PhD, director of the environmental engineering program at the National Science Foundation.
“When a virus comes out, it’s not just a naked virus, which is exceptionally small. It’s a virus encapsulated in liquid. And that’s why the humidity is so key. The degree of humidity can determine how fast the water evaporates from the particle,” he said in an interview.
In the wake of COVID-19, his institution is funding more cross-disciplinary research in biology, building science, architecture, and physics, he pointed out.
One such effort involved the development of a sensor that can capture live COVID-19 virus. This so-called “smoking gun,” which proved that the virus can spread through the air, took the combined expertise of professionals in medicine, engineering, and several other disciplines.
Currently, investigators are examining indoor air quality and water supplies in offices that have been left empty during the pandemic, and the effect they will have on human health. And others are looking at the way outside air quality affects indoor air quality, particularly where outdoor air quality is poor, such as in areas experiencing wildfires.
So will COVID-19 be the catalyst that finally drives changes to building design, regulation, and public expectations of air quality in the spaces where we spend close to 90% of our time?
“If not COVID, what else? It affected every country, every sector,” Dr. Morawska said. “There’s enough momentum now to do something about this. And enough realization there is a problem.”
A version of this article first appeared on Medscape.com.
Health workers already know that indoor air quality can be as important to human health as clean water and uncontaminated food. But before the COVID-19 pandemic, its importance in the prevention of respiratory illnesses outside of health circles was only whispered about.
Now, a team of nearly 40 scientists from 14 countries is calling for “a paradigm shift,” so that improvements in indoor air quality are viewed as essential to curb respiratory infections.
Most countries do not have indoor air-quality standards, the scientists point out in their recent report, and those that do often fall short in scope and enforcement.
“We expect everywhere in the world to have clean water flowing from our taps. In most parts of the developed world, it is happening and we take it completely for granted,” said lead investigator Lidia Morawska, PhD, of the International Laboratory for Air Quality and Health at the Queensland University of Technology in Brisbane, Australia.
But bacteria and viruses can circulate freely in the air, and “no one thinks about this, whatsoever, apart from health care facilities,” she said.
A first step is to recognize the risk posed by airborne pathogens, something not yet universally acknowledged. The investigators also want the World Health Organization to extend its guidelines to cover airborne pathogens, and for ventilation standards to include higher airflow and filtration rates.
Germany has been at the forefront of air-quality measures, Dr. Morawska said. Years ago, she observed a monitor showing the carbon dioxide level and relative humidity in the room where she was attending a meeting. The screen was accompanied by red, yellow, and green signals to communicate risk. Such indicators are also commonly displayed in German schools so teachers know when to open the windows or adjust the ventilation.
Monitors show carbon dioxide levels
But this is not yet being done in most other countries, Dr. Morawska said. Levels of carbon dioxide are one measure of indoor air quality, but they serve as a proxy for ventilation, she pointed out. Although the technology is available, sensors that can test a variety of components in a building in real time are not yet affordable.
Dr. Morawska envisions a future where the air quality numbers of the places people frequent are displayed so they know the risk for airborne transmission of respiratory illnesses. And people can begin to expect clean indoor air when they enter a business, office, or entertainment space and request changes when the air quality dips and improvement is needed, she said.
It is a daunting challenge to clean indoor air for several reasons. Air is not containable in the same way water is, which makes it difficult to trace contaminants. And infections transmitted through dirty water and food are usually evident immediately, whereas infections transmitted through airborne pathogens can take days to develop. Plus, the necessary infrastructure changes will be expensive.
However, the initial cost required to change the flow and quality of indoor air might be less than the cost of infections, the scientists pointed out. It is estimated that the global harm caused by COVID-19 alone costs $1 trillion each month.
“In the United States, the yearly cost – direct and indirect – of influenza has been calculated at $11.2 billion. For respiratory infections other than influenza, the yearly cost stood at $40 billion,” the team noted.
“If even half of this was caused by inhalation, we are still talking about massive costs,” said Dr. Morawska.
Bigger is not always better
It is tempting to see the solution as increased ventilation, said Ehsan Mousavi, PhD, assistant professor of construction science and management at Clemson (S.C.) University, who studies indoor air quality and ventilation in hospitals.
“We are ventilating the heck out of hospitals,” he said in an interview. But there is much debate about how much ventilation is the right amount. Too much and “you can blow pathogens into an open wound,” he explained. “Bigger is not always better.”
And there is still debate about the best mix of outside and recirculated air. An increase in the intake of outdoor air can refresh indoor air if it is clean, but that depends on where you live, he pointed out.
The mix used in most standard office buildings is 15% outside air and 85% recirculated air, Dr. Mousavi said. Boosting the percentage of outside air increases costs and energy use.
In fact, it can take five times more energy to ventilate hospital spaces than office spaces, he reported.
Engineers searching for clean-air solutions need to know what particulates are in the air and whether they are harmful to humans, but the sensors currently available can’t identify whether a virus is present in real time.
Samples have to be taken to a lab and, “by the time you know a virus was in the space, the moment is gone,” Dr. Mousavi explained.
More research is needed. “We need a reasonable answer that looks at the problem holistically, not just from the infectious disease perspective,” he said.
Hydrating indoor air
Research is making it clear that health care environments can play a significant role in patient recovery, according to Stephanie Taylor, MD. Dr. Taylor is president of Building4Health, which she founded to help businesses assess the quality of air in their buildings and find solutions. The company uses an algorithm to arrive at a health assessment score.
Air hydration is the most important aspect to target, she said.
Since the 1980s, research has shown that a relative humidity of 40%-60% is healthy for humans, she said. Currently, in an office building in a winter climate, the humidity level is more like 20%.
Canada is the first country to officially recommend the 40%-60% range for senior citizen centers and residential homes.
“Properly hydrated air supports our immune system and prevents skin problems and respiratory problems. It also inactivates many bacteria and viruses,” Dr. Taylor explained. Inhaling dry air compromises the ability of the body to restrict influenza virus infection, researchers showed in a 2019 study.
In the case of COVID-19, as virus particles attach to water molecules, they get bigger and heavier and eventually drop out of the breathing zone and onto surfaces where they can be wiped away, she explained.
But when the particles “are very small – like 5 microns in diameter – and you inhale them, they can lodge deep in the lungs,” she said.
In properly hydrated air, particles will be larger – about 10-20 microns when they attach to the water vapor – so they will get stuck in the nose or the back of the throat, where they can be washed away by mucous and not travel to the lungs.
“Indoor air metrics” can support our health or contribute to disease, “not just over time, but quickly, within minutes or hours,” she said.
No one expects the world’s building stock to suddenly upgrade to the ideal air quality. “But that doesn’t mean we shouldn’t move in that direction,” Dr. Taylor said. Changes can start small and gradually increase.
New research targets indoor air
Humidity is one of the key areas for current research, said Karl Rockne, PhD, director of the environmental engineering program at the National Science Foundation.
“When a virus comes out, it’s not just a naked virus, which is exceptionally small. It’s a virus encapsulated in liquid. And that’s why the humidity is so key. The degree of humidity can determine how fast the water evaporates from the particle,” he said in an interview.
In the wake of COVID-19, his institution is funding more cross-disciplinary research in biology, building science, architecture, and physics, he pointed out.
One such effort involved the development of a sensor that can capture live COVID-19 virus. This so-called “smoking gun,” which proved that the virus can spread through the air, took the combined expertise of professionals in medicine, engineering, and several other disciplines.
Currently, investigators are examining indoor air quality and water supplies in offices that have been left empty during the pandemic, and the effect they will have on human health. And others are looking at the way outside air quality affects indoor air quality, particularly where outdoor air quality is poor, such as in areas experiencing wildfires.
So will COVID-19 be the catalyst that finally drives changes to building design, regulation, and public expectations of air quality in the spaces where we spend close to 90% of our time?
“If not COVID, what else? It affected every country, every sector,” Dr. Morawska said. “There’s enough momentum now to do something about this. And enough realization there is a problem.”
A version of this article first appeared on Medscape.com.
Health workers already know that indoor air quality can be as important to human health as clean water and uncontaminated food. But before the COVID-19 pandemic, its importance in the prevention of respiratory illnesses outside of health circles was only whispered about.
Now, a team of nearly 40 scientists from 14 countries is calling for “a paradigm shift,” so that improvements in indoor air quality are viewed as essential to curb respiratory infections.
Most countries do not have indoor air-quality standards, the scientists point out in their recent report, and those that do often fall short in scope and enforcement.
“We expect everywhere in the world to have clean water flowing from our taps. In most parts of the developed world, it is happening and we take it completely for granted,” said lead investigator Lidia Morawska, PhD, of the International Laboratory for Air Quality and Health at the Queensland University of Technology in Brisbane, Australia.
But bacteria and viruses can circulate freely in the air, and “no one thinks about this, whatsoever, apart from health care facilities,” she said.
A first step is to recognize the risk posed by airborne pathogens, something not yet universally acknowledged. The investigators also want the World Health Organization to extend its guidelines to cover airborne pathogens, and for ventilation standards to include higher airflow and filtration rates.
Germany has been at the forefront of air-quality measures, Dr. Morawska said. Years ago, she observed a monitor showing the carbon dioxide level and relative humidity in the room where she was attending a meeting. The screen was accompanied by red, yellow, and green signals to communicate risk. Such indicators are also commonly displayed in German schools so teachers know when to open the windows or adjust the ventilation.
Monitors show carbon dioxide levels
But this is not yet being done in most other countries, Dr. Morawska said. Levels of carbon dioxide are one measure of indoor air quality, but they serve as a proxy for ventilation, she pointed out. Although the technology is available, sensors that can test a variety of components in a building in real time are not yet affordable.
Dr. Morawska envisions a future where the air quality numbers of the places people frequent are displayed so they know the risk for airborne transmission of respiratory illnesses. And people can begin to expect clean indoor air when they enter a business, office, or entertainment space and request changes when the air quality dips and improvement is needed, she said.
It is a daunting challenge to clean indoor air for several reasons. Air is not containable in the same way water is, which makes it difficult to trace contaminants. And infections transmitted through dirty water and food are usually evident immediately, whereas infections transmitted through airborne pathogens can take days to develop. Plus, the necessary infrastructure changes will be expensive.
However, the initial cost required to change the flow and quality of indoor air might be less than the cost of infections, the scientists pointed out. It is estimated that the global harm caused by COVID-19 alone costs $1 trillion each month.
“In the United States, the yearly cost – direct and indirect – of influenza has been calculated at $11.2 billion. For respiratory infections other than influenza, the yearly cost stood at $40 billion,” the team noted.
“If even half of this was caused by inhalation, we are still talking about massive costs,” said Dr. Morawska.
Bigger is not always better
It is tempting to see the solution as increased ventilation, said Ehsan Mousavi, PhD, assistant professor of construction science and management at Clemson (S.C.) University, who studies indoor air quality and ventilation in hospitals.
“We are ventilating the heck out of hospitals,” he said in an interview. But there is much debate about how much ventilation is the right amount. Too much and “you can blow pathogens into an open wound,” he explained. “Bigger is not always better.”
And there is still debate about the best mix of outside and recirculated air. An increase in the intake of outdoor air can refresh indoor air if it is clean, but that depends on where you live, he pointed out.
The mix used in most standard office buildings is 15% outside air and 85% recirculated air, Dr. Mousavi said. Boosting the percentage of outside air increases costs and energy use.
In fact, it can take five times more energy to ventilate hospital spaces than office spaces, he reported.
Engineers searching for clean-air solutions need to know what particulates are in the air and whether they are harmful to humans, but the sensors currently available can’t identify whether a virus is present in real time.
Samples have to be taken to a lab and, “by the time you know a virus was in the space, the moment is gone,” Dr. Mousavi explained.
More research is needed. “We need a reasonable answer that looks at the problem holistically, not just from the infectious disease perspective,” he said.
Hydrating indoor air
Research is making it clear that health care environments can play a significant role in patient recovery, according to Stephanie Taylor, MD. Dr. Taylor is president of Building4Health, which she founded to help businesses assess the quality of air in their buildings and find solutions. The company uses an algorithm to arrive at a health assessment score.
Air hydration is the most important aspect to target, she said.
Since the 1980s, research has shown that a relative humidity of 40%-60% is healthy for humans, she said. Currently, in an office building in a winter climate, the humidity level is more like 20%.
Canada is the first country to officially recommend the 40%-60% range for senior citizen centers and residential homes.
“Properly hydrated air supports our immune system and prevents skin problems and respiratory problems. It also inactivates many bacteria and viruses,” Dr. Taylor explained. Inhaling dry air compromises the ability of the body to restrict influenza virus infection, researchers showed in a 2019 study.
In the case of COVID-19, as virus particles attach to water molecules, they get bigger and heavier and eventually drop out of the breathing zone and onto surfaces where they can be wiped away, she explained.
But when the particles “are very small – like 5 microns in diameter – and you inhale them, they can lodge deep in the lungs,” she said.
In properly hydrated air, particles will be larger – about 10-20 microns when they attach to the water vapor – so they will get stuck in the nose or the back of the throat, where they can be washed away by mucous and not travel to the lungs.
“Indoor air metrics” can support our health or contribute to disease, “not just over time, but quickly, within minutes or hours,” she said.
No one expects the world’s building stock to suddenly upgrade to the ideal air quality. “But that doesn’t mean we shouldn’t move in that direction,” Dr. Taylor said. Changes can start small and gradually increase.
New research targets indoor air
Humidity is one of the key areas for current research, said Karl Rockne, PhD, director of the environmental engineering program at the National Science Foundation.
“When a virus comes out, it’s not just a naked virus, which is exceptionally small. It’s a virus encapsulated in liquid. And that’s why the humidity is so key. The degree of humidity can determine how fast the water evaporates from the particle,” he said in an interview.
In the wake of COVID-19, his institution is funding more cross-disciplinary research in biology, building science, architecture, and physics, he pointed out.
One such effort involved the development of a sensor that can capture live COVID-19 virus. This so-called “smoking gun,” which proved that the virus can spread through the air, took the combined expertise of professionals in medicine, engineering, and several other disciplines.
Currently, investigators are examining indoor air quality and water supplies in offices that have been left empty during the pandemic, and the effect they will have on human health. And others are looking at the way outside air quality affects indoor air quality, particularly where outdoor air quality is poor, such as in areas experiencing wildfires.
So will COVID-19 be the catalyst that finally drives changes to building design, regulation, and public expectations of air quality in the spaces where we spend close to 90% of our time?
“If not COVID, what else? It affected every country, every sector,” Dr. Morawska said. “There’s enough momentum now to do something about this. And enough realization there is a problem.”
A version of this article first appeared on Medscape.com.
Liver transplant outcomes improving for U.S. patients with HIV/HCV
While liver transplant outcomes were historically poor in people coinfected with HIV and hepatitis C virus (HCV), they have improved significantly in the era of direct-acting antiviral (DAA) therapy, a recent analysis of U.S. organ transplant data showed.
The availability of highly potent DAA therapy should change how transplant specialists view patients coinfected with HIV/HCV who need a liver transplant, according to researcher Jennifer Wang, MD, chief gastroenterology fellow at the University of Chicago, who presented the results of the analysis at the annual Digestive Disease Week® (DDW). Cumulative graft survival rates since the introduction of DAAs are comparable between transplant recipients with HIV/HCV coinfection and recipients who are both HIV and HCV negative, according to the study.
“Having hepatitis C no longer confers worse patient survival in the DAA era, and this is the main takeaway from our study,” Dr. Wang said.
The study also showed that the number of liver transplants among HIV-infected patients has increased over the past 4-5 years. However, the absolute number remains low at 64 cases in 2019, or less than 1% of all liver transplants that year, and only about one-third of those HIV-positive recipients had HCV coinfection, according to Dr. Wang.
Moreover, relatively few centers are performing liver transplants for patients who are HIV/HCV coinfected, and there is significant geographic variation in where the procedures are done, she said in her presentation.
Reassuring data that should prompt referral
Taken together, these results should offer reassurance to transplant centers that patients coinfected with HIV/HCV are no longer at increased risk for poor outcomes after transplantation, said Christine M. Durand, MD, associate professor of medicine at Johns Hopkins University, Baltimore.
“The additional call for action should be beyond the transplantation community to ensure that referrals for liver transplant are where they should be,” Dr. Durand said in an interview.
“With a number of only 64 transplants a year, we’re not doing enough, and there are more patients that could benefit from liver transplants,” added Dr. Durand, who is principal investigator of HOPE in Action, a prospective, multicenter, clinical trial evaluating the safety and survival outcomes of HIV-positive deceased donor liver transplants in HIV-positive recipients.
Impact of the HOPE Act
Liver transplantation for HIV-positive patients has increased since the signing of the HIV Organ Policy Equity (HOPE) Act in 2013, according to Dr. Wang.
The HOPE act expanded the donor pool to include HIV-positive deceased donors, which not only increased the donor supply overall, but specifically helped HIV-positive individuals, who experience a higher rate of waiting-list mortality, according to a review on the topic authored by Dr. Durand and coauthors.
However, some transplant centers may be reluctant to do liver transplants in HIV-positive patients coinfected with HCV. That’s because, in previous studies that were conducted before the DAA era, outcomes after liver transplant in HIV/HCV-coinfected patients were inferior to those in patients with HIV but no HCV infection, Dr. Wang said.
Accordingly, Dr. Wang and colleagues analyzed Organ Procurement and Transplantation Network (OPTN) data on adult patients who underwent liver transplants between 2008 and 2019 to see if the introduction of DAAs had leveled the playing field for those with HCV coinfection.
Progress in a still-underserved population
The practice of liver transplant in the HIV population has been increasing since the HOPE Act, according to Dr. Wang.
Overall, out of 70,125 liver transplant recipients over the 2008-2019 period, 416 (0.6%) were HIV infected, the data show.
In 2014, 28 liver transplants (0.5%) were performed in HIV-infected individuals, which increased to 64 transplants (0.8%) in 2019, data show. Of those 64 HIV-positive liver transplant recipients in 2019, 23 (35.9%) were coinfected with HCV.
Graft survival has greatly improved, from a 3-year survival of only 58% in patients transplanted before the availability of DAAs to 82% in the DAA era, a difference that was statistically significant, Dr. Wang said.
In the DAA era, there was no significant difference in graft failure outcomes when comparing HIV/HCV-coinfected recipients with uninfected recipients, she added.
The largest proportion of liver transplantations in HIV/HCV-coinfected recipients have been done in OPTN Region 9 (New York), both in the pre- and post-DAA eras, according to Dr. Wang. Several regions have very low numbers or have performed no liver transplants in HIV/HCV-coinfected patients in either era.
“The number of transplant centers participating in liver transplant for coinfected patients is still quite low, so this is a very underserved patient population,” Dr. Wang said.
Dr. Wang provided no financial disclosures related to the research. Dr. Durand receives grants to the institution from Abbvie and GlaxoSmithKline and she receives honoraria from Gilead Sciences for serving on a grant review committee.
While liver transplant outcomes were historically poor in people coinfected with HIV and hepatitis C virus (HCV), they have improved significantly in the era of direct-acting antiviral (DAA) therapy, a recent analysis of U.S. organ transplant data showed.
The availability of highly potent DAA therapy should change how transplant specialists view patients coinfected with HIV/HCV who need a liver transplant, according to researcher Jennifer Wang, MD, chief gastroenterology fellow at the University of Chicago, who presented the results of the analysis at the annual Digestive Disease Week® (DDW). Cumulative graft survival rates since the introduction of DAAs are comparable between transplant recipients with HIV/HCV coinfection and recipients who are both HIV and HCV negative, according to the study.
“Having hepatitis C no longer confers worse patient survival in the DAA era, and this is the main takeaway from our study,” Dr. Wang said.
The study also showed that the number of liver transplants among HIV-infected patients has increased over the past 4-5 years. However, the absolute number remains low at 64 cases in 2019, or less than 1% of all liver transplants that year, and only about one-third of those HIV-positive recipients had HCV coinfection, according to Dr. Wang.
Moreover, relatively few centers are performing liver transplants for patients who are HIV/HCV coinfected, and there is significant geographic variation in where the procedures are done, she said in her presentation.
Reassuring data that should prompt referral
Taken together, these results should offer reassurance to transplant centers that patients coinfected with HIV/HCV are no longer at increased risk for poor outcomes after transplantation, said Christine M. Durand, MD, associate professor of medicine at Johns Hopkins University, Baltimore.
“The additional call for action should be beyond the transplantation community to ensure that referrals for liver transplant are where they should be,” Dr. Durand said in an interview.
“With a number of only 64 transplants a year, we’re not doing enough, and there are more patients that could benefit from liver transplants,” added Dr. Durand, who is principal investigator of HOPE in Action, a prospective, multicenter, clinical trial evaluating the safety and survival outcomes of HIV-positive deceased donor liver transplants in HIV-positive recipients.
Impact of the HOPE Act
Liver transplantation for HIV-positive patients has increased since the signing of the HIV Organ Policy Equity (HOPE) Act in 2013, according to Dr. Wang.
The HOPE act expanded the donor pool to include HIV-positive deceased donors, which not only increased the donor supply overall, but specifically helped HIV-positive individuals, who experience a higher rate of waiting-list mortality, according to a review on the topic authored by Dr. Durand and coauthors.
However, some transplant centers may be reluctant to do liver transplants in HIV-positive patients coinfected with HCV. That’s because, in previous studies that were conducted before the DAA era, outcomes after liver transplant in HIV/HCV-coinfected patients were inferior to those in patients with HIV but no HCV infection, Dr. Wang said.
Accordingly, Dr. Wang and colleagues analyzed Organ Procurement and Transplantation Network (OPTN) data on adult patients who underwent liver transplants between 2008 and 2019 to see if the introduction of DAAs had leveled the playing field for those with HCV coinfection.
Progress in a still-underserved population
The practice of liver transplant in the HIV population has been increasing since the HOPE Act, according to Dr. Wang.
Overall, out of 70,125 liver transplant recipients over the 2008-2019 period, 416 (0.6%) were HIV infected, the data show.
In 2014, 28 liver transplants (0.5%) were performed in HIV-infected individuals, which increased to 64 transplants (0.8%) in 2019, data show. Of those 64 HIV-positive liver transplant recipients in 2019, 23 (35.9%) were coinfected with HCV.
Graft survival has greatly improved, from a 3-year survival of only 58% in patients transplanted before the availability of DAAs to 82% in the DAA era, a difference that was statistically significant, Dr. Wang said.
In the DAA era, there was no significant difference in graft failure outcomes when comparing HIV/HCV-coinfected recipients with uninfected recipients, she added.
The largest proportion of liver transplantations in HIV/HCV-coinfected recipients have been done in OPTN Region 9 (New York), both in the pre- and post-DAA eras, according to Dr. Wang. Several regions have very low numbers or have performed no liver transplants in HIV/HCV-coinfected patients in either era.
“The number of transplant centers participating in liver transplant for coinfected patients is still quite low, so this is a very underserved patient population,” Dr. Wang said.
Dr. Wang provided no financial disclosures related to the research. Dr. Durand receives grants to the institution from Abbvie and GlaxoSmithKline and she receives honoraria from Gilead Sciences for serving on a grant review committee.
While liver transplant outcomes were historically poor in people coinfected with HIV and hepatitis C virus (HCV), they have improved significantly in the era of direct-acting antiviral (DAA) therapy, a recent analysis of U.S. organ transplant data showed.
The availability of highly potent DAA therapy should change how transplant specialists view patients coinfected with HIV/HCV who need a liver transplant, according to researcher Jennifer Wang, MD, chief gastroenterology fellow at the University of Chicago, who presented the results of the analysis at the annual Digestive Disease Week® (DDW). Cumulative graft survival rates since the introduction of DAAs are comparable between transplant recipients with HIV/HCV coinfection and recipients who are both HIV and HCV negative, according to the study.
“Having hepatitis C no longer confers worse patient survival in the DAA era, and this is the main takeaway from our study,” Dr. Wang said.
The study also showed that the number of liver transplants among HIV-infected patients has increased over the past 4-5 years. However, the absolute number remains low at 64 cases in 2019, or less than 1% of all liver transplants that year, and only about one-third of those HIV-positive recipients had HCV coinfection, according to Dr. Wang.
Moreover, relatively few centers are performing liver transplants for patients who are HIV/HCV coinfected, and there is significant geographic variation in where the procedures are done, she said in her presentation.
Reassuring data that should prompt referral
Taken together, these results should offer reassurance to transplant centers that patients coinfected with HIV/HCV are no longer at increased risk for poor outcomes after transplantation, said Christine M. Durand, MD, associate professor of medicine at Johns Hopkins University, Baltimore.
“The additional call for action should be beyond the transplantation community to ensure that referrals for liver transplant are where they should be,” Dr. Durand said in an interview.
“With a number of only 64 transplants a year, we’re not doing enough, and there are more patients that could benefit from liver transplants,” added Dr. Durand, who is principal investigator of HOPE in Action, a prospective, multicenter, clinical trial evaluating the safety and survival outcomes of HIV-positive deceased donor liver transplants in HIV-positive recipients.
Impact of the HOPE Act
Liver transplantation for HIV-positive patients has increased since the signing of the HIV Organ Policy Equity (HOPE) Act in 2013, according to Dr. Wang.
The HOPE act expanded the donor pool to include HIV-positive deceased donors, which not only increased the donor supply overall, but specifically helped HIV-positive individuals, who experience a higher rate of waiting-list mortality, according to a review on the topic authored by Dr. Durand and coauthors.
However, some transplant centers may be reluctant to do liver transplants in HIV-positive patients coinfected with HCV. That’s because, in previous studies that were conducted before the DAA era, outcomes after liver transplant in HIV/HCV-coinfected patients were inferior to those in patients with HIV but no HCV infection, Dr. Wang said.
Accordingly, Dr. Wang and colleagues analyzed Organ Procurement and Transplantation Network (OPTN) data on adult patients who underwent liver transplants between 2008 and 2019 to see if the introduction of DAAs had leveled the playing field for those with HCV coinfection.
Progress in a still-underserved population
The practice of liver transplant in the HIV population has been increasing since the HOPE Act, according to Dr. Wang.
Overall, out of 70,125 liver transplant recipients over the 2008-2019 period, 416 (0.6%) were HIV infected, the data show.
In 2014, 28 liver transplants (0.5%) were performed in HIV-infected individuals, which increased to 64 transplants (0.8%) in 2019, data show. Of those 64 HIV-positive liver transplant recipients in 2019, 23 (35.9%) were coinfected with HCV.
Graft survival has greatly improved, from a 3-year survival of only 58% in patients transplanted before the availability of DAAs to 82% in the DAA era, a difference that was statistically significant, Dr. Wang said.
In the DAA era, there was no significant difference in graft failure outcomes when comparing HIV/HCV-coinfected recipients with uninfected recipients, she added.
The largest proportion of liver transplantations in HIV/HCV-coinfected recipients have been done in OPTN Region 9 (New York), both in the pre- and post-DAA eras, according to Dr. Wang. Several regions have very low numbers or have performed no liver transplants in HIV/HCV-coinfected patients in either era.
“The number of transplant centers participating in liver transplant for coinfected patients is still quite low, so this is a very underserved patient population,” Dr. Wang said.
Dr. Wang provided no financial disclosures related to the research. Dr. Durand receives grants to the institution from Abbvie and GlaxoSmithKline and she receives honoraria from Gilead Sciences for serving on a grant review committee.
FROM DDW 2021
Obstructive sleep apnea linked to COVID-19 risk
Greater severity of obstructive sleep apnea (OSA) is associated with a higher risk of contracting COVID-19, and positive airway pressure (PAP) treatment may counter that risk, according to a retrospective analysis from the records of Kaiser Permanente Southern California.
OSA patients often worry that PAP therapy might increase risk of severe COVID-19, said Dennis Hwang, MD, who presented the study at the American Thoracic Society’s virtual international conference (Abstract A1108). But the findings should be reassuring. “If you have obstructive sleep apnea, and you’re supposed to be using PAP, we recommend that you continue using PAP. It’s good for your overall wellness and reducing the risk of cardiovascular disease, but as it relates to COVID-19, it’s possible that it could protect. And there doesn’t appear to be any risk of increased severity of illness (with use of PAP),” Dr. Hwang said in an interview. He is medical director of sleep medicine for Kaiser Permanente San Bernardino County and cochair of sleep medicine for Kaiser Southern California.
He noted that the retrospective nature of the study makes it difficult to pin down whether PAP therapy is truly protective, “but I think there’s enough that we’ve been able conceptually to understand, to suggest that a direct causative relationship is possible,” said Dr. Hwang.
The results may imply that OSA patients should pay special attention to their OSA when there’s concern about exposure to an infectious agent like SARS-CoV-2. “The intermittent hypoxia at night, which can linger over to the day as increased sympathetic activity, increased heart rate. All of these are stresses to the body. So if you’re going to get infected, you want to start at a healthier level. You want to eliminate your sleep apnea to help reduce your risk of morbidity,” said Esra Tasali, MD, who was asked to comment on the study. Dr. Tasali is associate professor of medicine at the University of Chicago, and director of the Sleep Research Center there.
During the Q&A session after the talk, audience members asked about the timing of PAP use during COVID-19 infection, for example how often it was used during the asymptomatic phase of infection and if PAP has a positive effect. The data were not available, but “I think that the way to go is to understand this chronology,” said Dr. Tasali.
The researchers examined records between 2015 and 2020, using sleep study data, remotely collected daily PAP data, and electronic health records, all from Kaiser Permanente Southern California. Included subjects were adults who had enrolled before Feb. 1, 2020, and had sleep diagnostic or PAP data on record by March 1, 2020. The researchers analyzed PAP adherence between March 1, 2020, and the time of COVID-19 diagnosis, or until the study ended on July 31, 2020.
Patients were defined as being untreated (< 2 hours/night PAP), moderately treated (2-3.9 hours/night), or well treated (4 or more hours/night). Apnea hypopnea index (AHI) was used to determine severity. The analysis included 81,932 patients (39.8% were women, mean age was 54.0 years, 9.9% were Black, and 34.5% were Hispanic). A total of 1.7% of subjects without OSA experienced COVID-19 infection, compared to 1.8% with OSA; 0.3% with OSA were hospitalized and 0.07% underwent intensive care or died.
There were some differences between the two groups. The non-USA population was younger (mean age 47.0 vs. 54.5 years), was less likely to be men (44% vs. 60.3%), had a lower mean body mass index (30.4 vs. 34.3), had fewer comorbidities according to the Charleston Comorbidity Index (1.3 vs. 2.0), and were less likely to have hypertension (5.6% vs. 12.4%; P < .0001 for all).
Infection rates were higher in patients with more severe OSA. The rates in untreated mild, moderate, and severe OSA were 2%, 2%, and 2.4% respectively. The rate among all treated patients was 1.4% (P < .0001). Infection rates also dropped among patients with better treatment: untreated, 2.1%; moderately treated, 1.7%; and well treated, 1.3% (P < .0001).
Not having OSA was associated with a lower infection risk than was having OSA (odds ratio [OR], 0.82; 95% confidence interval, 0.70-0.96). Compared to untreated patients, there was lower infection risk in the moderately treated (OR, 0.82; 95% CI, 0.65-1.03) and well treated (OR, 0.68; 95% CI, 0.59-0.79) groups. Higher infection rates were associated with obesity, higher Charlson Comorbidity score (> 2; OR, 1.29; 95% CI, 1.09-1.53), Black (OR, 1.51; 95% CI, 1.24-1.84) and Hispanic ethnicities (OR, 2.23; 95% CI, 1.96-2.54), and Medicaid enrollment. Increasing age was associated with lower risk of infection, with each 5-year increment linked to reduced risk (OR, 0.88; 95% CI, 0.86-0.90). Dr. Hwang suggested that the age association may be because older individuals were more likely to follow social distancing and other precautions.
A multivariate analysis found that OSA was associated with infection risk according to OSA severity, including mild (OR, 1.21; 95% CI, 1.01-1.44), and moderate to severe (OR, 1.27; 95% CI, 1.07-1.51). There was no association between hospitalization rate or ICU admission/death and presence of OSA or PAP adherence in the data presented, but Dr. Hwang said that an updated analysis suggests that OSA may be associated with a risk of greater COVID-19 severity.
The control group was composed of individuals who had undergone sleep testing, but found to not have OSA. Still, they aren’t necessarily representative of the general population, since symptoms likely drove them to testing. A high percentage were also obese, and the average BMI was 30. “It’s certainly not a ‘normal population,’ but the advantage of what we did in terms of using this control group is that they underwent sleep testing, so they were proven to have no obstructive sleep apnea, whereas if we used a general population, we just don’t know,” said Dr. Hwang.
The study received technical and data support from Somnoware, and was funded by Kaiser Permanente. Dr. Tasali has no relevant financial disclosures.
Greater severity of obstructive sleep apnea (OSA) is associated with a higher risk of contracting COVID-19, and positive airway pressure (PAP) treatment may counter that risk, according to a retrospective analysis from the records of Kaiser Permanente Southern California.
OSA patients often worry that PAP therapy might increase risk of severe COVID-19, said Dennis Hwang, MD, who presented the study at the American Thoracic Society’s virtual international conference (Abstract A1108). But the findings should be reassuring. “If you have obstructive sleep apnea, and you’re supposed to be using PAP, we recommend that you continue using PAP. It’s good for your overall wellness and reducing the risk of cardiovascular disease, but as it relates to COVID-19, it’s possible that it could protect. And there doesn’t appear to be any risk of increased severity of illness (with use of PAP),” Dr. Hwang said in an interview. He is medical director of sleep medicine for Kaiser Permanente San Bernardino County and cochair of sleep medicine for Kaiser Southern California.
He noted that the retrospective nature of the study makes it difficult to pin down whether PAP therapy is truly protective, “but I think there’s enough that we’ve been able conceptually to understand, to suggest that a direct causative relationship is possible,” said Dr. Hwang.
The results may imply that OSA patients should pay special attention to their OSA when there’s concern about exposure to an infectious agent like SARS-CoV-2. “The intermittent hypoxia at night, which can linger over to the day as increased sympathetic activity, increased heart rate. All of these are stresses to the body. So if you’re going to get infected, you want to start at a healthier level. You want to eliminate your sleep apnea to help reduce your risk of morbidity,” said Esra Tasali, MD, who was asked to comment on the study. Dr. Tasali is associate professor of medicine at the University of Chicago, and director of the Sleep Research Center there.
During the Q&A session after the talk, audience members asked about the timing of PAP use during COVID-19 infection, for example how often it was used during the asymptomatic phase of infection and if PAP has a positive effect. The data were not available, but “I think that the way to go is to understand this chronology,” said Dr. Tasali.
The researchers examined records between 2015 and 2020, using sleep study data, remotely collected daily PAP data, and electronic health records, all from Kaiser Permanente Southern California. Included subjects were adults who had enrolled before Feb. 1, 2020, and had sleep diagnostic or PAP data on record by March 1, 2020. The researchers analyzed PAP adherence between March 1, 2020, and the time of COVID-19 diagnosis, or until the study ended on July 31, 2020.
Patients were defined as being untreated (< 2 hours/night PAP), moderately treated (2-3.9 hours/night), or well treated (4 or more hours/night). Apnea hypopnea index (AHI) was used to determine severity. The analysis included 81,932 patients (39.8% were women, mean age was 54.0 years, 9.9% were Black, and 34.5% were Hispanic). A total of 1.7% of subjects without OSA experienced COVID-19 infection, compared to 1.8% with OSA; 0.3% with OSA were hospitalized and 0.07% underwent intensive care or died.
There were some differences between the two groups. The non-USA population was younger (mean age 47.0 vs. 54.5 years), was less likely to be men (44% vs. 60.3%), had a lower mean body mass index (30.4 vs. 34.3), had fewer comorbidities according to the Charleston Comorbidity Index (1.3 vs. 2.0), and were less likely to have hypertension (5.6% vs. 12.4%; P < .0001 for all).
Infection rates were higher in patients with more severe OSA. The rates in untreated mild, moderate, and severe OSA were 2%, 2%, and 2.4% respectively. The rate among all treated patients was 1.4% (P < .0001). Infection rates also dropped among patients with better treatment: untreated, 2.1%; moderately treated, 1.7%; and well treated, 1.3% (P < .0001).
Not having OSA was associated with a lower infection risk than was having OSA (odds ratio [OR], 0.82; 95% confidence interval, 0.70-0.96). Compared to untreated patients, there was lower infection risk in the moderately treated (OR, 0.82; 95% CI, 0.65-1.03) and well treated (OR, 0.68; 95% CI, 0.59-0.79) groups. Higher infection rates were associated with obesity, higher Charlson Comorbidity score (> 2; OR, 1.29; 95% CI, 1.09-1.53), Black (OR, 1.51; 95% CI, 1.24-1.84) and Hispanic ethnicities (OR, 2.23; 95% CI, 1.96-2.54), and Medicaid enrollment. Increasing age was associated with lower risk of infection, with each 5-year increment linked to reduced risk (OR, 0.88; 95% CI, 0.86-0.90). Dr. Hwang suggested that the age association may be because older individuals were more likely to follow social distancing and other precautions.
A multivariate analysis found that OSA was associated with infection risk according to OSA severity, including mild (OR, 1.21; 95% CI, 1.01-1.44), and moderate to severe (OR, 1.27; 95% CI, 1.07-1.51). There was no association between hospitalization rate or ICU admission/death and presence of OSA or PAP adherence in the data presented, but Dr. Hwang said that an updated analysis suggests that OSA may be associated with a risk of greater COVID-19 severity.
The control group was composed of individuals who had undergone sleep testing, but found to not have OSA. Still, they aren’t necessarily representative of the general population, since symptoms likely drove them to testing. A high percentage were also obese, and the average BMI was 30. “It’s certainly not a ‘normal population,’ but the advantage of what we did in terms of using this control group is that they underwent sleep testing, so they were proven to have no obstructive sleep apnea, whereas if we used a general population, we just don’t know,” said Dr. Hwang.
The study received technical and data support from Somnoware, and was funded by Kaiser Permanente. Dr. Tasali has no relevant financial disclosures.
Greater severity of obstructive sleep apnea (OSA) is associated with a higher risk of contracting COVID-19, and positive airway pressure (PAP) treatment may counter that risk, according to a retrospective analysis from the records of Kaiser Permanente Southern California.
OSA patients often worry that PAP therapy might increase risk of severe COVID-19, said Dennis Hwang, MD, who presented the study at the American Thoracic Society’s virtual international conference (Abstract A1108). But the findings should be reassuring. “If you have obstructive sleep apnea, and you’re supposed to be using PAP, we recommend that you continue using PAP. It’s good for your overall wellness and reducing the risk of cardiovascular disease, but as it relates to COVID-19, it’s possible that it could protect. And there doesn’t appear to be any risk of increased severity of illness (with use of PAP),” Dr. Hwang said in an interview. He is medical director of sleep medicine for Kaiser Permanente San Bernardino County and cochair of sleep medicine for Kaiser Southern California.
He noted that the retrospective nature of the study makes it difficult to pin down whether PAP therapy is truly protective, “but I think there’s enough that we’ve been able conceptually to understand, to suggest that a direct causative relationship is possible,” said Dr. Hwang.
The results may imply that OSA patients should pay special attention to their OSA when there’s concern about exposure to an infectious agent like SARS-CoV-2. “The intermittent hypoxia at night, which can linger over to the day as increased sympathetic activity, increased heart rate. All of these are stresses to the body. So if you’re going to get infected, you want to start at a healthier level. You want to eliminate your sleep apnea to help reduce your risk of morbidity,” said Esra Tasali, MD, who was asked to comment on the study. Dr. Tasali is associate professor of medicine at the University of Chicago, and director of the Sleep Research Center there.
During the Q&A session after the talk, audience members asked about the timing of PAP use during COVID-19 infection, for example how often it was used during the asymptomatic phase of infection and if PAP has a positive effect. The data were not available, but “I think that the way to go is to understand this chronology,” said Dr. Tasali.
The researchers examined records between 2015 and 2020, using sleep study data, remotely collected daily PAP data, and electronic health records, all from Kaiser Permanente Southern California. Included subjects were adults who had enrolled before Feb. 1, 2020, and had sleep diagnostic or PAP data on record by March 1, 2020. The researchers analyzed PAP adherence between March 1, 2020, and the time of COVID-19 diagnosis, or until the study ended on July 31, 2020.
Patients were defined as being untreated (< 2 hours/night PAP), moderately treated (2-3.9 hours/night), or well treated (4 or more hours/night). Apnea hypopnea index (AHI) was used to determine severity. The analysis included 81,932 patients (39.8% were women, mean age was 54.0 years, 9.9% were Black, and 34.5% were Hispanic). A total of 1.7% of subjects without OSA experienced COVID-19 infection, compared to 1.8% with OSA; 0.3% with OSA were hospitalized and 0.07% underwent intensive care or died.
There were some differences between the two groups. The non-USA population was younger (mean age 47.0 vs. 54.5 years), was less likely to be men (44% vs. 60.3%), had a lower mean body mass index (30.4 vs. 34.3), had fewer comorbidities according to the Charleston Comorbidity Index (1.3 vs. 2.0), and were less likely to have hypertension (5.6% vs. 12.4%; P < .0001 for all).
Infection rates were higher in patients with more severe OSA. The rates in untreated mild, moderate, and severe OSA were 2%, 2%, and 2.4% respectively. The rate among all treated patients was 1.4% (P < .0001). Infection rates also dropped among patients with better treatment: untreated, 2.1%; moderately treated, 1.7%; and well treated, 1.3% (P < .0001).
Not having OSA was associated with a lower infection risk than was having OSA (odds ratio [OR], 0.82; 95% confidence interval, 0.70-0.96). Compared to untreated patients, there was lower infection risk in the moderately treated (OR, 0.82; 95% CI, 0.65-1.03) and well treated (OR, 0.68; 95% CI, 0.59-0.79) groups. Higher infection rates were associated with obesity, higher Charlson Comorbidity score (> 2; OR, 1.29; 95% CI, 1.09-1.53), Black (OR, 1.51; 95% CI, 1.24-1.84) and Hispanic ethnicities (OR, 2.23; 95% CI, 1.96-2.54), and Medicaid enrollment. Increasing age was associated with lower risk of infection, with each 5-year increment linked to reduced risk (OR, 0.88; 95% CI, 0.86-0.90). Dr. Hwang suggested that the age association may be because older individuals were more likely to follow social distancing and other precautions.
A multivariate analysis found that OSA was associated with infection risk according to OSA severity, including mild (OR, 1.21; 95% CI, 1.01-1.44), and moderate to severe (OR, 1.27; 95% CI, 1.07-1.51). There was no association between hospitalization rate or ICU admission/death and presence of OSA or PAP adherence in the data presented, but Dr. Hwang said that an updated analysis suggests that OSA may be associated with a risk of greater COVID-19 severity.
The control group was composed of individuals who had undergone sleep testing, but found to not have OSA. Still, they aren’t necessarily representative of the general population, since symptoms likely drove them to testing. A high percentage were also obese, and the average BMI was 30. “It’s certainly not a ‘normal population,’ but the advantage of what we did in terms of using this control group is that they underwent sleep testing, so they were proven to have no obstructive sleep apnea, whereas if we used a general population, we just don’t know,” said Dr. Hwang.
The study received technical and data support from Somnoware, and was funded by Kaiser Permanente. Dr. Tasali has no relevant financial disclosures.
FROM ATS 2021
Bill seeks to streamline prior authorization in Medicare Advantage plans
A group of bipartisan lawmakers intends to compel insurers to streamline prior authorization processes for Medicare Advantage plans, including a bid to end the use of faxes and develop systems that can allow for real-time decisions.
Rep. Suzan DelBene (D-Wash.); Rep. Mike Kelly (R-Pa.); Rep. Ami Bera, MD (D-Calif.); and Rep. Larry Bucshon, MD, (R-Ind.) on May 13 introduced a bill that would task federal officials with refining standards regarding prior authorization for Medicare Advantage. Titled the Improving Seniors’ Timely Access to Care Act of 2021, the bill would direct the Department of Health & Human Services to create rules intended to make prior authorization more transparent and speedy for the insurer-run Medicare plans. Known as Medicare Advantage, these plans cover about 24.1 million people of the 62 million enrolled in the giant federal health program, according to the nonprofit Kaiser Family Foundation.
These revamped prior authorization systems could not rely on faxes nor could they employ proprietary payer portals that did not meet HHS’ standards, says the text of the bill released by Rep. DelBene. Insurers would also have to report to the Centers for Medicare & Medicaid Services about the extent of their use of prior authorization and the rate of approvals or denials. The bill seeks to encourage plans to adopt prior authorization programs that adhere to evidence-based medical guidelines in consultation with physicians.
There were several reasons for focusing on Medicare Advantage plans, although prior authorization concerns extend more broadly in the U.S. health care system, said Susan Bailey, MD, president of the American Medical Association.
There’s an ample body of research about issues seen in the Medicare Advantage plans. Dr. Bailey also said that, in her experience, Medicare Advantage plans have had some of the most restrictive policies. And, by starting with Medicare Advantage, there’s a potential for a ripple effect in the industry, easing this issue when physicians work with other insurers as well.
“When Medicare adopts a policy whether it be a payment policy or a coverage policy, private insurers typically follow along,” she said.
Strong support among health care groups
There’s strong support for streamlining prior authorization both in the medical community and in Congress.
The bill has the support of about 70 health care organizations, including the AMA and the American Academy of Family Physicians, according to its sponsors. As of May 17, the bill had attracted the backing of 97 members of the House of Representatives, roughly evenly split among Democrats and Republicans.
Rep. DelBene’s previous version of this bill, the Improving Seniors’ Timely Access to Care Act of 2019, attracted 143 Democratic cosponsors and 137 Republican ones, or more than half of the members of the House. This bill was not completed during the previous session of Congress (January 2019–January 2021) because of the more urgent needs of pandemic response, said Rep. Bucshon, who practiced cardiothoracic surgery before joining Congress.
“It wasn’t quite on the radar as much as it might have been if we didn’t have COVID,” Rep. Bucshon said.
Rep. Bucshon added that he expects strong Senate support for a companion measure of the House bill, which could make the difference for efforts to pass it this year.
Insurers have become more aggressive over time in denying payments through prior authorization systems for services that physicians say their patients need, according to Rep. Bucshon. There may be some “bad actors” in medicine who would order unnecessary procedures, Rep. Bucshon allowed, but in most cases, the cumbersome prior authorization processes only put a hurdle for patients seeking needed treatments, he said.
“The premise is that it controls health care costs but actually what it does is it helps insurance company’s bottom line,” Rep. Bucshon said.
In a prepared statement, former Pennsylvania representative Allyson Y. Schwartz, now CEO of the Better Medicare Alliance, said her group had spoken with sponsors of this legislation and appreciates “their receptiveness to feedback in this process.”
“Prior authorization ensures beneficiaries receive clinically appropriate care and reduces exposures to duplicative and unnecessary services,” Ms. Schwartz said. “We share an interest in ensuring prior authorization works as smoothly and effectively as possible for beneficiaries while protecting its essential function of facilitating safe, evidenced-based care.”
The Better Medicare Alliance said its funders include UnitedHealth, Humana, and CVS Health/Aetna, which run Advantage plans. The group also lists as its partners many medical organizations.
“Rationing care by hassling”
Like Rep. Bucshon, Dr. Bailey sees a different motivation in insurers’ persistence in keeping the prior authorization process cumbersome.
Phone calls and faxes remain the key methods for handling prior authorization for medical services, according to the results of a survey done by the AMA in December. Phone calls were always or often required for prior authorization for medical services (59%), with faxes the second-most common approach (46%), followed by health plans’ online portals (39%), electronic health records and practice management systems (29%), and email or U.S. mail (26%), according to the AMA’s report on the survey.
“It seems like every step in the process is designed to make the patient less likely to get the therapy that the doctor thinks that the patient needs,” Dr. Bailey said. “It’s almost like rationing care by hassling the patient and the physician.”
The findings of an investigation by HHS’ internal watchdog unit appear to support Dr. Bailey’s view, showing that insurer-run Medicare plans had a pattern of often walking back their initial rejections.
In 2018, the Office of the Inspector General for HHS reported that Medicare Advantage organizations (MAOs) overturned 75% of their own denials during 2014-16. In addition, independent reviewers within the appeals process overturned additional denials in favor of patients and clinicians, OIG said.
“The high number of overturned denials raises concerns that some Medicare Advantage beneficiaries and providers were initially denied services and payments that should have been provided,” the OIG said in the report. “This is especially concerning because beneficiaries and providers rarely used the appeals process, which is designed to ensure access to care and payment.”
During 2014-2016, patients and clinicians appealed only 1% of denials to the first level of appeal, OIG said. In the report, the watchdog group noted that CMS audits had highlighted “widespread and persistent MAO performance problems related to denials of care and payment.” In 2015, for example, CMS cited 56% of audited contracts for making inappropriate denials.
Dr. Bailey also said in an interview that she routinely encounters problems with prior authorization in her own practice as an allergist and immunologist in Fort Worth, Tex.
In late May, for example, a Medicare Advantage plan made a patient whose chronic asthma had been stable for years change to a new inhaler that resulted in him developing a yeast infection in his mouth, Dr. Bailey said.
“We treated the yeast infection, made some changes in the way he uses his inhaler, so hopefully he would tolerate it better,” Dr. Bailey said. “He had a reaction to the medication to treat the yeast infection and ended up in the hospital. How is that helping anyone? It certainly hasn’t helped my patient.”
Dr. Bailey said insurers have also asked to seek prior authorization to prescribe medications that have been generic for years and have used the process to challenge her on cases of what seem to be common sense in medical practice. This included a bid to have Dr. Bailey prescribe a medication in pill form for a 6-month-old baby who had no teeth.
“Every doctor has got absurd stories like that, but unfortunately, every doctor is going to have tragic stories where prior authorization has resulted in death and harm to the patients,” Dr. Bailey said.
Some physicians leave it to the patient to try to overcome insurers’ decisions on prior authorization, seeing this task as falling outside of their duties, Dr. Bailey said.
“I don’t do that. I fight. I spend a lot of time fighting. I don’t like to lose. I don’t like my patients to lose, so I will go to the mat for them,” Dr. Bailey said. “But I’m blessed to be in a specialty where I’ve got loads more control over my schedule than many other specialties do.”
A version of this article first appeared on Medscape.com.
A group of bipartisan lawmakers intends to compel insurers to streamline prior authorization processes for Medicare Advantage plans, including a bid to end the use of faxes and develop systems that can allow for real-time decisions.
Rep. Suzan DelBene (D-Wash.); Rep. Mike Kelly (R-Pa.); Rep. Ami Bera, MD (D-Calif.); and Rep. Larry Bucshon, MD, (R-Ind.) on May 13 introduced a bill that would task federal officials with refining standards regarding prior authorization for Medicare Advantage. Titled the Improving Seniors’ Timely Access to Care Act of 2021, the bill would direct the Department of Health & Human Services to create rules intended to make prior authorization more transparent and speedy for the insurer-run Medicare plans. Known as Medicare Advantage, these plans cover about 24.1 million people of the 62 million enrolled in the giant federal health program, according to the nonprofit Kaiser Family Foundation.
These revamped prior authorization systems could not rely on faxes nor could they employ proprietary payer portals that did not meet HHS’ standards, says the text of the bill released by Rep. DelBene. Insurers would also have to report to the Centers for Medicare & Medicaid Services about the extent of their use of prior authorization and the rate of approvals or denials. The bill seeks to encourage plans to adopt prior authorization programs that adhere to evidence-based medical guidelines in consultation with physicians.
There were several reasons for focusing on Medicare Advantage plans, although prior authorization concerns extend more broadly in the U.S. health care system, said Susan Bailey, MD, president of the American Medical Association.
There’s an ample body of research about issues seen in the Medicare Advantage plans. Dr. Bailey also said that, in her experience, Medicare Advantage plans have had some of the most restrictive policies. And, by starting with Medicare Advantage, there’s a potential for a ripple effect in the industry, easing this issue when physicians work with other insurers as well.
“When Medicare adopts a policy whether it be a payment policy or a coverage policy, private insurers typically follow along,” she said.
Strong support among health care groups
There’s strong support for streamlining prior authorization both in the medical community and in Congress.
The bill has the support of about 70 health care organizations, including the AMA and the American Academy of Family Physicians, according to its sponsors. As of May 17, the bill had attracted the backing of 97 members of the House of Representatives, roughly evenly split among Democrats and Republicans.
Rep. DelBene’s previous version of this bill, the Improving Seniors’ Timely Access to Care Act of 2019, attracted 143 Democratic cosponsors and 137 Republican ones, or more than half of the members of the House. This bill was not completed during the previous session of Congress (January 2019–January 2021) because of the more urgent needs of pandemic response, said Rep. Bucshon, who practiced cardiothoracic surgery before joining Congress.
“It wasn’t quite on the radar as much as it might have been if we didn’t have COVID,” Rep. Bucshon said.
Rep. Bucshon added that he expects strong Senate support for a companion measure of the House bill, which could make the difference for efforts to pass it this year.
Insurers have become more aggressive over time in denying payments through prior authorization systems for services that physicians say their patients need, according to Rep. Bucshon. There may be some “bad actors” in medicine who would order unnecessary procedures, Rep. Bucshon allowed, but in most cases, the cumbersome prior authorization processes only put a hurdle for patients seeking needed treatments, he said.
“The premise is that it controls health care costs but actually what it does is it helps insurance company’s bottom line,” Rep. Bucshon said.
In a prepared statement, former Pennsylvania representative Allyson Y. Schwartz, now CEO of the Better Medicare Alliance, said her group had spoken with sponsors of this legislation and appreciates “their receptiveness to feedback in this process.”
“Prior authorization ensures beneficiaries receive clinically appropriate care and reduces exposures to duplicative and unnecessary services,” Ms. Schwartz said. “We share an interest in ensuring prior authorization works as smoothly and effectively as possible for beneficiaries while protecting its essential function of facilitating safe, evidenced-based care.”
The Better Medicare Alliance said its funders include UnitedHealth, Humana, and CVS Health/Aetna, which run Advantage plans. The group also lists as its partners many medical organizations.
“Rationing care by hassling”
Like Rep. Bucshon, Dr. Bailey sees a different motivation in insurers’ persistence in keeping the prior authorization process cumbersome.
Phone calls and faxes remain the key methods for handling prior authorization for medical services, according to the results of a survey done by the AMA in December. Phone calls were always or often required for prior authorization for medical services (59%), with faxes the second-most common approach (46%), followed by health plans’ online portals (39%), electronic health records and practice management systems (29%), and email or U.S. mail (26%), according to the AMA’s report on the survey.
“It seems like every step in the process is designed to make the patient less likely to get the therapy that the doctor thinks that the patient needs,” Dr. Bailey said. “It’s almost like rationing care by hassling the patient and the physician.”
The findings of an investigation by HHS’ internal watchdog unit appear to support Dr. Bailey’s view, showing that insurer-run Medicare plans had a pattern of often walking back their initial rejections.
In 2018, the Office of the Inspector General for HHS reported that Medicare Advantage organizations (MAOs) overturned 75% of their own denials during 2014-16. In addition, independent reviewers within the appeals process overturned additional denials in favor of patients and clinicians, OIG said.
“The high number of overturned denials raises concerns that some Medicare Advantage beneficiaries and providers were initially denied services and payments that should have been provided,” the OIG said in the report. “This is especially concerning because beneficiaries and providers rarely used the appeals process, which is designed to ensure access to care and payment.”
During 2014-2016, patients and clinicians appealed only 1% of denials to the first level of appeal, OIG said. In the report, the watchdog group noted that CMS audits had highlighted “widespread and persistent MAO performance problems related to denials of care and payment.” In 2015, for example, CMS cited 56% of audited contracts for making inappropriate denials.
Dr. Bailey also said in an interview that she routinely encounters problems with prior authorization in her own practice as an allergist and immunologist in Fort Worth, Tex.
In late May, for example, a Medicare Advantage plan made a patient whose chronic asthma had been stable for years change to a new inhaler that resulted in him developing a yeast infection in his mouth, Dr. Bailey said.
“We treated the yeast infection, made some changes in the way he uses his inhaler, so hopefully he would tolerate it better,” Dr. Bailey said. “He had a reaction to the medication to treat the yeast infection and ended up in the hospital. How is that helping anyone? It certainly hasn’t helped my patient.”
Dr. Bailey said insurers have also asked to seek prior authorization to prescribe medications that have been generic for years and have used the process to challenge her on cases of what seem to be common sense in medical practice. This included a bid to have Dr. Bailey prescribe a medication in pill form for a 6-month-old baby who had no teeth.
“Every doctor has got absurd stories like that, but unfortunately, every doctor is going to have tragic stories where prior authorization has resulted in death and harm to the patients,” Dr. Bailey said.
Some physicians leave it to the patient to try to overcome insurers’ decisions on prior authorization, seeing this task as falling outside of their duties, Dr. Bailey said.
“I don’t do that. I fight. I spend a lot of time fighting. I don’t like to lose. I don’t like my patients to lose, so I will go to the mat for them,” Dr. Bailey said. “But I’m blessed to be in a specialty where I’ve got loads more control over my schedule than many other specialties do.”
A version of this article first appeared on Medscape.com.
A group of bipartisan lawmakers intends to compel insurers to streamline prior authorization processes for Medicare Advantage plans, including a bid to end the use of faxes and develop systems that can allow for real-time decisions.
Rep. Suzan DelBene (D-Wash.); Rep. Mike Kelly (R-Pa.); Rep. Ami Bera, MD (D-Calif.); and Rep. Larry Bucshon, MD, (R-Ind.) on May 13 introduced a bill that would task federal officials with refining standards regarding prior authorization for Medicare Advantage. Titled the Improving Seniors’ Timely Access to Care Act of 2021, the bill would direct the Department of Health & Human Services to create rules intended to make prior authorization more transparent and speedy for the insurer-run Medicare plans. Known as Medicare Advantage, these plans cover about 24.1 million people of the 62 million enrolled in the giant federal health program, according to the nonprofit Kaiser Family Foundation.
These revamped prior authorization systems could not rely on faxes nor could they employ proprietary payer portals that did not meet HHS’ standards, says the text of the bill released by Rep. DelBene. Insurers would also have to report to the Centers for Medicare & Medicaid Services about the extent of their use of prior authorization and the rate of approvals or denials. The bill seeks to encourage plans to adopt prior authorization programs that adhere to evidence-based medical guidelines in consultation with physicians.
There were several reasons for focusing on Medicare Advantage plans, although prior authorization concerns extend more broadly in the U.S. health care system, said Susan Bailey, MD, president of the American Medical Association.
There’s an ample body of research about issues seen in the Medicare Advantage plans. Dr. Bailey also said that, in her experience, Medicare Advantage plans have had some of the most restrictive policies. And, by starting with Medicare Advantage, there’s a potential for a ripple effect in the industry, easing this issue when physicians work with other insurers as well.
“When Medicare adopts a policy whether it be a payment policy or a coverage policy, private insurers typically follow along,” she said.
Strong support among health care groups
There’s strong support for streamlining prior authorization both in the medical community and in Congress.
The bill has the support of about 70 health care organizations, including the AMA and the American Academy of Family Physicians, according to its sponsors. As of May 17, the bill had attracted the backing of 97 members of the House of Representatives, roughly evenly split among Democrats and Republicans.
Rep. DelBene’s previous version of this bill, the Improving Seniors’ Timely Access to Care Act of 2019, attracted 143 Democratic cosponsors and 137 Republican ones, or more than half of the members of the House. This bill was not completed during the previous session of Congress (January 2019–January 2021) because of the more urgent needs of pandemic response, said Rep. Bucshon, who practiced cardiothoracic surgery before joining Congress.
“It wasn’t quite on the radar as much as it might have been if we didn’t have COVID,” Rep. Bucshon said.
Rep. Bucshon added that he expects strong Senate support for a companion measure of the House bill, which could make the difference for efforts to pass it this year.
Insurers have become more aggressive over time in denying payments through prior authorization systems for services that physicians say their patients need, according to Rep. Bucshon. There may be some “bad actors” in medicine who would order unnecessary procedures, Rep. Bucshon allowed, but in most cases, the cumbersome prior authorization processes only put a hurdle for patients seeking needed treatments, he said.
“The premise is that it controls health care costs but actually what it does is it helps insurance company’s bottom line,” Rep. Bucshon said.
In a prepared statement, former Pennsylvania representative Allyson Y. Schwartz, now CEO of the Better Medicare Alliance, said her group had spoken with sponsors of this legislation and appreciates “their receptiveness to feedback in this process.”
“Prior authorization ensures beneficiaries receive clinically appropriate care and reduces exposures to duplicative and unnecessary services,” Ms. Schwartz said. “We share an interest in ensuring prior authorization works as smoothly and effectively as possible for beneficiaries while protecting its essential function of facilitating safe, evidenced-based care.”
The Better Medicare Alliance said its funders include UnitedHealth, Humana, and CVS Health/Aetna, which run Advantage plans. The group also lists as its partners many medical organizations.
“Rationing care by hassling”
Like Rep. Bucshon, Dr. Bailey sees a different motivation in insurers’ persistence in keeping the prior authorization process cumbersome.
Phone calls and faxes remain the key methods for handling prior authorization for medical services, according to the results of a survey done by the AMA in December. Phone calls were always or often required for prior authorization for medical services (59%), with faxes the second-most common approach (46%), followed by health plans’ online portals (39%), electronic health records and practice management systems (29%), and email or U.S. mail (26%), according to the AMA’s report on the survey.
“It seems like every step in the process is designed to make the patient less likely to get the therapy that the doctor thinks that the patient needs,” Dr. Bailey said. “It’s almost like rationing care by hassling the patient and the physician.”
The findings of an investigation by HHS’ internal watchdog unit appear to support Dr. Bailey’s view, showing that insurer-run Medicare plans had a pattern of often walking back their initial rejections.
In 2018, the Office of the Inspector General for HHS reported that Medicare Advantage organizations (MAOs) overturned 75% of their own denials during 2014-16. In addition, independent reviewers within the appeals process overturned additional denials in favor of patients and clinicians, OIG said.
“The high number of overturned denials raises concerns that some Medicare Advantage beneficiaries and providers were initially denied services and payments that should have been provided,” the OIG said in the report. “This is especially concerning because beneficiaries and providers rarely used the appeals process, which is designed to ensure access to care and payment.”
During 2014-2016, patients and clinicians appealed only 1% of denials to the first level of appeal, OIG said. In the report, the watchdog group noted that CMS audits had highlighted “widespread and persistent MAO performance problems related to denials of care and payment.” In 2015, for example, CMS cited 56% of audited contracts for making inappropriate denials.
Dr. Bailey also said in an interview that she routinely encounters problems with prior authorization in her own practice as an allergist and immunologist in Fort Worth, Tex.
In late May, for example, a Medicare Advantage plan made a patient whose chronic asthma had been stable for years change to a new inhaler that resulted in him developing a yeast infection in his mouth, Dr. Bailey said.
“We treated the yeast infection, made some changes in the way he uses his inhaler, so hopefully he would tolerate it better,” Dr. Bailey said. “He had a reaction to the medication to treat the yeast infection and ended up in the hospital. How is that helping anyone? It certainly hasn’t helped my patient.”
Dr. Bailey said insurers have also asked to seek prior authorization to prescribe medications that have been generic for years and have used the process to challenge her on cases of what seem to be common sense in medical practice. This included a bid to have Dr. Bailey prescribe a medication in pill form for a 6-month-old baby who had no teeth.
“Every doctor has got absurd stories like that, but unfortunately, every doctor is going to have tragic stories where prior authorization has resulted in death and harm to the patients,” Dr. Bailey said.
Some physicians leave it to the patient to try to overcome insurers’ decisions on prior authorization, seeing this task as falling outside of their duties, Dr. Bailey said.
“I don’t do that. I fight. I spend a lot of time fighting. I don’t like to lose. I don’t like my patients to lose, so I will go to the mat for them,” Dr. Bailey said. “But I’m blessed to be in a specialty where I’ve got loads more control over my schedule than many other specialties do.”
A version of this article first appeared on Medscape.com.
What brought me back from the brink of suicide: A physician’s story
William Lynes, MD, had a flourishing medical practice and a fulfilling family life with three children when he first attempted suicide in 1999 at age 45. By 2003, depression and two more suicide attempts led to his early retirement.
In a session at the recent virtual American Psychiatric Association (APA) 2021 annual meeting, Dr. Lynes talked about the challenges of dealing with depression while managing the stresses of a career in medicine. The session in which he spoke was called, “The Suicidal Physician: Narratives From a Physician Who Survived and the Physician Widow of One Who Did Not.”
By writing and speaking about his experiences, he says, he has been able to retain his identity as a physician and avoid obsessive thoughts about suicide. He hopes conversations like these help other physicians feel less alone and enable them to push past stigmas to get the help they need. He suspects they do. More than 600 people joined the APA session, and Dr. Lynes received dozens of thankful messages afterward.
“I love medicine, but intrinsically, the practice of medicine is stressful, and you can’t get away,” said Dr. Lynes, a retired urologist in Temecula, Calif. “As far as feedback, it made me feel like it’s something I should continue to do.”
A way to heal
For Dr. Lynes, his “downward spiral into darkness” began with a series of catastrophic medical events starting in 1998, when he came home from a family vacation in Mexico feeling unwell. He didn’t bother to do anything about it – typical of a physician, he says. Then one night he woke up shaking with chills and fever. Soon he was in the hospital with respiratory failure from septic shock.
Dr. Lynes spent 6 weeks in the intensive care unit, including 4 weeks on a ventilator. He underwent a tracheostomy. He lost 40 pounds and experienced ICU-related delirium. It was a terrifying time, he said. When he tried to return to work 10 months later, he didn’t feel as though he could function normally.
Having once been a driven doctor who worked long hours, he now doubted himself and dreaded giving patients bad news. Spontaneously, he tried to take his own life.
Afterward, he concealed what had happened from everyone except his wife and managed to resume his practice. However, he was unable to regain the enthusiasm he had once had for his work. Although he had experienced depression before, this time it was unrelenting.
He sought help from a psychiatrist, received a diagnosis of bipolar disorder, and began taking medication. Still, he struggled to fulfill his responsibilities. Then in April 2002, he had a snowboarding accident that caused multiple facial fractures and required five operations. When he returned to work this time, he felt like a failure but resisted asking colleagues for help.
A few months later, Dr. Lynes again attempted suicide, which led to another stay in the ICU and more time on a ventilator. Doctors told his family they didn’t think he would survive. When he recovered, he spent time as an inpatient in a psychiatric ward, where he received the first of a series of electroconvulsive therapy sessions. Compounding his anxiety and depression was the inability to come to terms with his life if he were not able to practice medicine.
The next fall, in September 2003, his third suicide attempt took place in his office on a weekend when no one was around. After locking the door, he looked at his reflection in the frame of his medical school diploma. The glass was cracked. “It was dark, it was black, it was cold,” he said. “I can remember seeing my reflection and thinking how disgusted I was.”
For years after that, Dr. Lynes struggled with his sense of self-worth. He hid from the medical system and dreaded doctors’ appointments. Finally in 2016, he found new meaning at a writing conference, where he met a fellow physician whose story was similar to his. She encouraged him to write about his experience. His essay was published in Annals of Internal Medicine that year. “Then I started speaking, and I feel like I’m a physician again,” he said. “That has really healed me quite a bit.”
Why physicians die by suicide
Working in health care can be extremely stressful, even in the best of times, said Michael Myers, MD, a psychiatrist at State University of New York, Brooklyn, and author of the book, “Why Physicians Die By Suicide: Lessons Learned From Their Families and Others Who Cared.”
Years of school and training culminate in a career in which demands are relentless. Societal expectations are high. Many doctors are perfectionists by nature, and physicians tend to feel intense pressure to compete for coveted positions.
Stress starts early in a medical career. A 2016 systematic review and meta-analysis of 183 studies from 43 countries showed that nearly 30% of medical students experienced symptoms of depression and that 11% reported suicidal thoughts, but only 15% sought help.
A 2015 review of 31 studies that involved residents showed that rates of depression remained close to 30% and that about three-quarters of trainees meet criteria for burnout, a type of emotional exhaustion and sense of inadequacy that can result from chronic stress at work.
The stress of medical training appears to be a direct cause of mental health struggles. Rates of depression are higher among those working to become physicians than among their peers of the same age, research shows. In addition, symptoms become more prevalent as people progress through their training.
The COVID-19 pandemic has added stress to an already stressful job. Of more than 2,300 physicians surveyed in August 2020 by the Physicians Foundation, a physicians advocacy organization, 50% indicated that they experienced excessive anger, tearfulness, or anxiety because of the way the pandemic affected their work; 30% felt hopeless or lacking purpose; and 8% had thoughts of self-harm related to the pandemic. Rates of burnout had risen from 40% in 2018 to 58%.
Those problems might be even more acute in places experiencing other types of crises. A 2020 study of 154 emergency department (ED) physicians in Libya, which is in the midst of a civil war, found that 65% were experiencing anxiety, 73% were showing signs of depression, and 68% felt emotionally exhausted.
Every story is different
It is unclear how common suicide is among physicians. One often-repeated estimate is that 300-400 physicians die by suicide each year, but no one is certain how that number was determined, said Dr. Myers, who organized the APA panel.
Studies on suicide are inconsistent, and trends are hard to pinpoint. Anecdotally, he has received just as many calls about physician suicides in the past year as he did before the pandemic started.
Every person is different, and so is every death. Sometimes, career problems have nothing to do with a physician’s suicide, Dr. Myers said. When job stress does play a role, factors are often varied and complex.
After a 35-year career as a double board certified ED physician, Matthew Seaman, MD, retired in January 2017. The same month, a patient filed a complaint against him with the Washington State medical board, which led to an investigation and a lawsuit.
The case was hard on Dr. Seaman, who had continued to work night shifts throughout his career and had won a Hero Award from the American Board of Emergency Medicine, said his wife, Linda Seaman, MD, a family practitioner in Yakima, Wash., who also spoke on the APA panel.
Dr. Seaman said that 2 years after the investigation started, her husband was growing increasingly depressed. In 2019, he testified in a deposition. She said the plaintiff’s attorney “tried every way he could to shame Matt, humiliate Matt, make him believe he was a very bad doctor.” Three days later, he died by suicide at age 62.
Looking back at the year leading up to her husband’s death, Dr. Seaman recognizes multiple obstacles that interfered with her husband’s ability to get help, including frustrating interactions with psychiatrists and the couple’s insurance company.
His identity and experience as a physician also played a role. A couple of months before he died, she tried unsuccessfully to reach his psychiatrist, whose office suggested he go to the ED. However, because he worked as an ED doctor in their small town, he wouldn’t go. Dr. Seaman suspects he was wary of the stigma.
Burnout likely set him up to cave in after decades of work on the front lines, she added. Working in the ED exposes providers to horrific, traumatic cases every day, she said. Physicians learn to suppress their own emotions to deal with what they encounter. Stuffing their feelings can lead to posttraumatic stress. “You just perform,” she said. “You learn to do that.”
A real gift
Whenever Dr. Myers hears stories about doctors who died by suicide or who have written about their mental health struggles to help others, he contacts them. One goal of his own writing and of the conference sessions he organizes is to make it easier for others to share their own stories.
“I tell them, first of all, their courage and honesty is a real gift, and they’re saving lives,” he said. “There are so many suffering doctors out there who think that they’re the only one.”
Public conversations such as those that occurred in the APA session also offer opportunities to share advice, including Dr. Myers’ recommendation that doctors be sure they have a primary care physician of their own.
Many don’t, he says, because they say they are too busy, they can treat their own symptoms, or they can self-refer to specialists when needed. But physicians don’t always recognize symptoms of depression in themselves, and when mental health problems arise, they may not seek help or treat themselves appropriately.
A primary care physician can be the first person to recognize a mental health problem and refer a patient for mental health care, said Dr. Myers, whose latest book, “Becoming a Doctors’ Doctor: A Memoir,” explores his experiences treating doctors with burnout and other mental health problems.
Whether they have a primary care doctor or not, he suggests that physicians talk to anyone they trust – a social worker, a religious leader, or a family member who can then help them find the right sort of care.
In the United States, around-the-clock help is available through the National Suicide Prevention Lifeline at 800-273-8255. A psychiatrist-run hotline specifically for physicians is available at 888-409-0141. “Reach out and get some help,” Dr. Myers said. “Just don’t do it alone.”
Dr. Lynes advocates setting boundaries between life and work. He has also benefited from writing about his experiences. A blog or a diary can help physicians process their feelings, he said. His 2016 essay marked a major turning point in his life, giving his life meaning in helping others.
“Since I wrote that article, I can’t tell you how much better I am,” he said. “Now, I’m not embarrassed to be around physicians. I actually consider myself a physician. I didn’t for many, many years. So, I’m doing pretty well.”
A version of this article first appeared on Medscape.com.
William Lynes, MD, had a flourishing medical practice and a fulfilling family life with three children when he first attempted suicide in 1999 at age 45. By 2003, depression and two more suicide attempts led to his early retirement.
In a session at the recent virtual American Psychiatric Association (APA) 2021 annual meeting, Dr. Lynes talked about the challenges of dealing with depression while managing the stresses of a career in medicine. The session in which he spoke was called, “The Suicidal Physician: Narratives From a Physician Who Survived and the Physician Widow of One Who Did Not.”
By writing and speaking about his experiences, he says, he has been able to retain his identity as a physician and avoid obsessive thoughts about suicide. He hopes conversations like these help other physicians feel less alone and enable them to push past stigmas to get the help they need. He suspects they do. More than 600 people joined the APA session, and Dr. Lynes received dozens of thankful messages afterward.
“I love medicine, but intrinsically, the practice of medicine is stressful, and you can’t get away,” said Dr. Lynes, a retired urologist in Temecula, Calif. “As far as feedback, it made me feel like it’s something I should continue to do.”
A way to heal
For Dr. Lynes, his “downward spiral into darkness” began with a series of catastrophic medical events starting in 1998, when he came home from a family vacation in Mexico feeling unwell. He didn’t bother to do anything about it – typical of a physician, he says. Then one night he woke up shaking with chills and fever. Soon he was in the hospital with respiratory failure from septic shock.
Dr. Lynes spent 6 weeks in the intensive care unit, including 4 weeks on a ventilator. He underwent a tracheostomy. He lost 40 pounds and experienced ICU-related delirium. It was a terrifying time, he said. When he tried to return to work 10 months later, he didn’t feel as though he could function normally.
Having once been a driven doctor who worked long hours, he now doubted himself and dreaded giving patients bad news. Spontaneously, he tried to take his own life.
Afterward, he concealed what had happened from everyone except his wife and managed to resume his practice. However, he was unable to regain the enthusiasm he had once had for his work. Although he had experienced depression before, this time it was unrelenting.
He sought help from a psychiatrist, received a diagnosis of bipolar disorder, and began taking medication. Still, he struggled to fulfill his responsibilities. Then in April 2002, he had a snowboarding accident that caused multiple facial fractures and required five operations. When he returned to work this time, he felt like a failure but resisted asking colleagues for help.
A few months later, Dr. Lynes again attempted suicide, which led to another stay in the ICU and more time on a ventilator. Doctors told his family they didn’t think he would survive. When he recovered, he spent time as an inpatient in a psychiatric ward, where he received the first of a series of electroconvulsive therapy sessions. Compounding his anxiety and depression was the inability to come to terms with his life if he were not able to practice medicine.
The next fall, in September 2003, his third suicide attempt took place in his office on a weekend when no one was around. After locking the door, he looked at his reflection in the frame of his medical school diploma. The glass was cracked. “It was dark, it was black, it was cold,” he said. “I can remember seeing my reflection and thinking how disgusted I was.”
For years after that, Dr. Lynes struggled with his sense of self-worth. He hid from the medical system and dreaded doctors’ appointments. Finally in 2016, he found new meaning at a writing conference, where he met a fellow physician whose story was similar to his. She encouraged him to write about his experience. His essay was published in Annals of Internal Medicine that year. “Then I started speaking, and I feel like I’m a physician again,” he said. “That has really healed me quite a bit.”
Why physicians die by suicide
Working in health care can be extremely stressful, even in the best of times, said Michael Myers, MD, a psychiatrist at State University of New York, Brooklyn, and author of the book, “Why Physicians Die By Suicide: Lessons Learned From Their Families and Others Who Cared.”
Years of school and training culminate in a career in which demands are relentless. Societal expectations are high. Many doctors are perfectionists by nature, and physicians tend to feel intense pressure to compete for coveted positions.
Stress starts early in a medical career. A 2016 systematic review and meta-analysis of 183 studies from 43 countries showed that nearly 30% of medical students experienced symptoms of depression and that 11% reported suicidal thoughts, but only 15% sought help.
A 2015 review of 31 studies that involved residents showed that rates of depression remained close to 30% and that about three-quarters of trainees meet criteria for burnout, a type of emotional exhaustion and sense of inadequacy that can result from chronic stress at work.
The stress of medical training appears to be a direct cause of mental health struggles. Rates of depression are higher among those working to become physicians than among their peers of the same age, research shows. In addition, symptoms become more prevalent as people progress through their training.
The COVID-19 pandemic has added stress to an already stressful job. Of more than 2,300 physicians surveyed in August 2020 by the Physicians Foundation, a physicians advocacy organization, 50% indicated that they experienced excessive anger, tearfulness, or anxiety because of the way the pandemic affected their work; 30% felt hopeless or lacking purpose; and 8% had thoughts of self-harm related to the pandemic. Rates of burnout had risen from 40% in 2018 to 58%.
Those problems might be even more acute in places experiencing other types of crises. A 2020 study of 154 emergency department (ED) physicians in Libya, which is in the midst of a civil war, found that 65% were experiencing anxiety, 73% were showing signs of depression, and 68% felt emotionally exhausted.
Every story is different
It is unclear how common suicide is among physicians. One often-repeated estimate is that 300-400 physicians die by suicide each year, but no one is certain how that number was determined, said Dr. Myers, who organized the APA panel.
Studies on suicide are inconsistent, and trends are hard to pinpoint. Anecdotally, he has received just as many calls about physician suicides in the past year as he did before the pandemic started.
Every person is different, and so is every death. Sometimes, career problems have nothing to do with a physician’s suicide, Dr. Myers said. When job stress does play a role, factors are often varied and complex.
After a 35-year career as a double board certified ED physician, Matthew Seaman, MD, retired in January 2017. The same month, a patient filed a complaint against him with the Washington State medical board, which led to an investigation and a lawsuit.
The case was hard on Dr. Seaman, who had continued to work night shifts throughout his career and had won a Hero Award from the American Board of Emergency Medicine, said his wife, Linda Seaman, MD, a family practitioner in Yakima, Wash., who also spoke on the APA panel.
Dr. Seaman said that 2 years after the investigation started, her husband was growing increasingly depressed. In 2019, he testified in a deposition. She said the plaintiff’s attorney “tried every way he could to shame Matt, humiliate Matt, make him believe he was a very bad doctor.” Three days later, he died by suicide at age 62.
Looking back at the year leading up to her husband’s death, Dr. Seaman recognizes multiple obstacles that interfered with her husband’s ability to get help, including frustrating interactions with psychiatrists and the couple’s insurance company.
His identity and experience as a physician also played a role. A couple of months before he died, she tried unsuccessfully to reach his psychiatrist, whose office suggested he go to the ED. However, because he worked as an ED doctor in their small town, he wouldn’t go. Dr. Seaman suspects he was wary of the stigma.
Burnout likely set him up to cave in after decades of work on the front lines, she added. Working in the ED exposes providers to horrific, traumatic cases every day, she said. Physicians learn to suppress their own emotions to deal with what they encounter. Stuffing their feelings can lead to posttraumatic stress. “You just perform,” she said. “You learn to do that.”
A real gift
Whenever Dr. Myers hears stories about doctors who died by suicide or who have written about their mental health struggles to help others, he contacts them. One goal of his own writing and of the conference sessions he organizes is to make it easier for others to share their own stories.
“I tell them, first of all, their courage and honesty is a real gift, and they’re saving lives,” he said. “There are so many suffering doctors out there who think that they’re the only one.”
Public conversations such as those that occurred in the APA session also offer opportunities to share advice, including Dr. Myers’ recommendation that doctors be sure they have a primary care physician of their own.
Many don’t, he says, because they say they are too busy, they can treat their own symptoms, or they can self-refer to specialists when needed. But physicians don’t always recognize symptoms of depression in themselves, and when mental health problems arise, they may not seek help or treat themselves appropriately.
A primary care physician can be the first person to recognize a mental health problem and refer a patient for mental health care, said Dr. Myers, whose latest book, “Becoming a Doctors’ Doctor: A Memoir,” explores his experiences treating doctors with burnout and other mental health problems.
Whether they have a primary care doctor or not, he suggests that physicians talk to anyone they trust – a social worker, a religious leader, or a family member who can then help them find the right sort of care.
In the United States, around-the-clock help is available through the National Suicide Prevention Lifeline at 800-273-8255. A psychiatrist-run hotline specifically for physicians is available at 888-409-0141. “Reach out and get some help,” Dr. Myers said. “Just don’t do it alone.”
Dr. Lynes advocates setting boundaries between life and work. He has also benefited from writing about his experiences. A blog or a diary can help physicians process their feelings, he said. His 2016 essay marked a major turning point in his life, giving his life meaning in helping others.
“Since I wrote that article, I can’t tell you how much better I am,” he said. “Now, I’m not embarrassed to be around physicians. I actually consider myself a physician. I didn’t for many, many years. So, I’m doing pretty well.”
A version of this article first appeared on Medscape.com.
William Lynes, MD, had a flourishing medical practice and a fulfilling family life with three children when he first attempted suicide in 1999 at age 45. By 2003, depression and two more suicide attempts led to his early retirement.
In a session at the recent virtual American Psychiatric Association (APA) 2021 annual meeting, Dr. Lynes talked about the challenges of dealing with depression while managing the stresses of a career in medicine. The session in which he spoke was called, “The Suicidal Physician: Narratives From a Physician Who Survived and the Physician Widow of One Who Did Not.”
By writing and speaking about his experiences, he says, he has been able to retain his identity as a physician and avoid obsessive thoughts about suicide. He hopes conversations like these help other physicians feel less alone and enable them to push past stigmas to get the help they need. He suspects they do. More than 600 people joined the APA session, and Dr. Lynes received dozens of thankful messages afterward.
“I love medicine, but intrinsically, the practice of medicine is stressful, and you can’t get away,” said Dr. Lynes, a retired urologist in Temecula, Calif. “As far as feedback, it made me feel like it’s something I should continue to do.”
A way to heal
For Dr. Lynes, his “downward spiral into darkness” began with a series of catastrophic medical events starting in 1998, when he came home from a family vacation in Mexico feeling unwell. He didn’t bother to do anything about it – typical of a physician, he says. Then one night he woke up shaking with chills and fever. Soon he was in the hospital with respiratory failure from septic shock.
Dr. Lynes spent 6 weeks in the intensive care unit, including 4 weeks on a ventilator. He underwent a tracheostomy. He lost 40 pounds and experienced ICU-related delirium. It was a terrifying time, he said. When he tried to return to work 10 months later, he didn’t feel as though he could function normally.
Having once been a driven doctor who worked long hours, he now doubted himself and dreaded giving patients bad news. Spontaneously, he tried to take his own life.
Afterward, he concealed what had happened from everyone except his wife and managed to resume his practice. However, he was unable to regain the enthusiasm he had once had for his work. Although he had experienced depression before, this time it was unrelenting.
He sought help from a psychiatrist, received a diagnosis of bipolar disorder, and began taking medication. Still, he struggled to fulfill his responsibilities. Then in April 2002, he had a snowboarding accident that caused multiple facial fractures and required five operations. When he returned to work this time, he felt like a failure but resisted asking colleagues for help.
A few months later, Dr. Lynes again attempted suicide, which led to another stay in the ICU and more time on a ventilator. Doctors told his family they didn’t think he would survive. When he recovered, he spent time as an inpatient in a psychiatric ward, where he received the first of a series of electroconvulsive therapy sessions. Compounding his anxiety and depression was the inability to come to terms with his life if he were not able to practice medicine.
The next fall, in September 2003, his third suicide attempt took place in his office on a weekend when no one was around. After locking the door, he looked at his reflection in the frame of his medical school diploma. The glass was cracked. “It was dark, it was black, it was cold,” he said. “I can remember seeing my reflection and thinking how disgusted I was.”
For years after that, Dr. Lynes struggled with his sense of self-worth. He hid from the medical system and dreaded doctors’ appointments. Finally in 2016, he found new meaning at a writing conference, where he met a fellow physician whose story was similar to his. She encouraged him to write about his experience. His essay was published in Annals of Internal Medicine that year. “Then I started speaking, and I feel like I’m a physician again,” he said. “That has really healed me quite a bit.”
Why physicians die by suicide
Working in health care can be extremely stressful, even in the best of times, said Michael Myers, MD, a psychiatrist at State University of New York, Brooklyn, and author of the book, “Why Physicians Die By Suicide: Lessons Learned From Their Families and Others Who Cared.”
Years of school and training culminate in a career in which demands are relentless. Societal expectations are high. Many doctors are perfectionists by nature, and physicians tend to feel intense pressure to compete for coveted positions.
Stress starts early in a medical career. A 2016 systematic review and meta-analysis of 183 studies from 43 countries showed that nearly 30% of medical students experienced symptoms of depression and that 11% reported suicidal thoughts, but only 15% sought help.
A 2015 review of 31 studies that involved residents showed that rates of depression remained close to 30% and that about three-quarters of trainees meet criteria for burnout, a type of emotional exhaustion and sense of inadequacy that can result from chronic stress at work.
The stress of medical training appears to be a direct cause of mental health struggles. Rates of depression are higher among those working to become physicians than among their peers of the same age, research shows. In addition, symptoms become more prevalent as people progress through their training.
The COVID-19 pandemic has added stress to an already stressful job. Of more than 2,300 physicians surveyed in August 2020 by the Physicians Foundation, a physicians advocacy organization, 50% indicated that they experienced excessive anger, tearfulness, or anxiety because of the way the pandemic affected their work; 30% felt hopeless or lacking purpose; and 8% had thoughts of self-harm related to the pandemic. Rates of burnout had risen from 40% in 2018 to 58%.
Those problems might be even more acute in places experiencing other types of crises. A 2020 study of 154 emergency department (ED) physicians in Libya, which is in the midst of a civil war, found that 65% were experiencing anxiety, 73% were showing signs of depression, and 68% felt emotionally exhausted.
Every story is different
It is unclear how common suicide is among physicians. One often-repeated estimate is that 300-400 physicians die by suicide each year, but no one is certain how that number was determined, said Dr. Myers, who organized the APA panel.
Studies on suicide are inconsistent, and trends are hard to pinpoint. Anecdotally, he has received just as many calls about physician suicides in the past year as he did before the pandemic started.
Every person is different, and so is every death. Sometimes, career problems have nothing to do with a physician’s suicide, Dr. Myers said. When job stress does play a role, factors are often varied and complex.
After a 35-year career as a double board certified ED physician, Matthew Seaman, MD, retired in January 2017. The same month, a patient filed a complaint against him with the Washington State medical board, which led to an investigation and a lawsuit.
The case was hard on Dr. Seaman, who had continued to work night shifts throughout his career and had won a Hero Award from the American Board of Emergency Medicine, said his wife, Linda Seaman, MD, a family practitioner in Yakima, Wash., who also spoke on the APA panel.
Dr. Seaman said that 2 years after the investigation started, her husband was growing increasingly depressed. In 2019, he testified in a deposition. She said the plaintiff’s attorney “tried every way he could to shame Matt, humiliate Matt, make him believe he was a very bad doctor.” Three days later, he died by suicide at age 62.
Looking back at the year leading up to her husband’s death, Dr. Seaman recognizes multiple obstacles that interfered with her husband’s ability to get help, including frustrating interactions with psychiatrists and the couple’s insurance company.
His identity and experience as a physician also played a role. A couple of months before he died, she tried unsuccessfully to reach his psychiatrist, whose office suggested he go to the ED. However, because he worked as an ED doctor in their small town, he wouldn’t go. Dr. Seaman suspects he was wary of the stigma.
Burnout likely set him up to cave in after decades of work on the front lines, she added. Working in the ED exposes providers to horrific, traumatic cases every day, she said. Physicians learn to suppress their own emotions to deal with what they encounter. Stuffing their feelings can lead to posttraumatic stress. “You just perform,” she said. “You learn to do that.”
A real gift
Whenever Dr. Myers hears stories about doctors who died by suicide or who have written about their mental health struggles to help others, he contacts them. One goal of his own writing and of the conference sessions he organizes is to make it easier for others to share their own stories.
“I tell them, first of all, their courage and honesty is a real gift, and they’re saving lives,” he said. “There are so many suffering doctors out there who think that they’re the only one.”
Public conversations such as those that occurred in the APA session also offer opportunities to share advice, including Dr. Myers’ recommendation that doctors be sure they have a primary care physician of their own.
Many don’t, he says, because they say they are too busy, they can treat their own symptoms, or they can self-refer to specialists when needed. But physicians don’t always recognize symptoms of depression in themselves, and when mental health problems arise, they may not seek help or treat themselves appropriately.
A primary care physician can be the first person to recognize a mental health problem and refer a patient for mental health care, said Dr. Myers, whose latest book, “Becoming a Doctors’ Doctor: A Memoir,” explores his experiences treating doctors with burnout and other mental health problems.
Whether they have a primary care doctor or not, he suggests that physicians talk to anyone they trust – a social worker, a religious leader, or a family member who can then help them find the right sort of care.
In the United States, around-the-clock help is available through the National Suicide Prevention Lifeline at 800-273-8255. A psychiatrist-run hotline specifically for physicians is available at 888-409-0141. “Reach out and get some help,” Dr. Myers said. “Just don’t do it alone.”
Dr. Lynes advocates setting boundaries between life and work. He has also benefited from writing about his experiences. A blog or a diary can help physicians process their feelings, he said. His 2016 essay marked a major turning point in his life, giving his life meaning in helping others.
“Since I wrote that article, I can’t tell you how much better I am,” he said. “Now, I’m not embarrassed to be around physicians. I actually consider myself a physician. I didn’t for many, many years. So, I’m doing pretty well.”
A version of this article first appeared on Medscape.com.
Large vessel stroke linked to AstraZeneca COVID vaccine
The three cases (one of which was fatal) occurred in two women and one man in their 30s or 40s and involved blockages of the carotid and middle cerebral artery. Two of the three patients also had venous thrombosis involving the portal and cerebral venous system. All three also had extremely low platelet counts, confirmed antibodies to platelet factor 4, and raised D-dimer levels, all characteristic of the vaccine-induced immune thrombotic thrombocytopenia (VITT) reaction associated with the AstraZeneca vaccine.
They are described in detail in a letter published online on May 25 in the Journal of Neurology, Neurosurgery & Psychiatry
“These are [the] first detailed reports of arterial stroke believed to be caused by VITT after the AstraZeneca COVID vaccine, although stroke has been mentioned previously in the VITT data,” said senior author David Werring, PhD, FRCP.
“VITT has more commonly presented as CVST [Cerebral venous sinus thrombosis] which is stroke caused by a venous thrombosis; these cases are showing that it can also cause stroke caused by an arterial thrombosis,” explained Dr. Werring, professor of clinical neurology at the Stroke Research Centre, University College London.
“In patients who present with ischemic stroke, especially younger patients, and who have had the AstraZeneca vaccine within the past month, clinicians need to consider VITT as a possible cause, as there is a specific treatment needed for this syndrome,” he said.
Young patients presenting with ischemic stroke after receiving the AstraZeneca vaccine should urgently be evaluated for VITT with laboratory tests, including platelet count, D-dimers, fibrinogen, and anti-PF4 antibodies, the authors wrote, and then managed by a multidisciplinary team, including hematology, neurology, stroke, neurosurgery, and neuroradiology, for rapid access to treatments including intravenous immune globulin, methylprednisolone, plasmapheresis, and nonheparin anticoagulants such as fondaparinux, argatroban, or direct oral anticoagulants.
Dr. Werring noted that these reports do not add anything to the overall risk/benefit of the vaccine, as they are only describing three cases. “While VITT is very serious, the benefit of the vaccine still outweighs its risks,” he said. “Around 40% of patients hospitalized with COVID-19 experience some sort of thrombosis and about 1.5% have an ischemic stroke. Whereas latest figures from the U.K. estimate the incidence of VITT with the AstraZeneca vaccine of 1 in 50,000 to 1 in 100,000.
“Our report doesn’t suggest that VITT is more common than these latest figures estimate, but we are just drawing attention to an alternative presentation,” he added.
Three cases
The first patient in the current case series, a woman in her 30s, experienced an intermittent headache on the right side and around her eyes 6 days after the vaccine. Five days later, she awoke feeling drowsy and with weakness to her left face, arm, and leg.
Imaging revealed a blocked right middle cerebral artery with brain infarction and clots in the right portal vein. She underwent brain surgery to reduce the pressure in her skull, plasma removal and replacement, and received the anticoagulant fondaparinux, but she still unfortunately died.
The second patient, a woman in her late 30s, presented with headache, confusion, weakness in her left arm, and loss of vision on the left side 12 days after having received the vaccine. Imaging showed occlusion of both carotid arteries, as well as pulmonary embolism and a left cerebral venous sinus thrombosis.
Her platelet count increased following plasma removal and replacement and intravenous corticosteroids, and her condition improved after fondaparinux treatment.
The third patient, a man in his early 40s, presented 3 weeks after receiving his vaccination with problems speaking. Imaging showed a clot in the left middle cerebral artery, but there was no evidence of clots in the cerebral venous sinuses. He received a platelet and plasma transfusion, and fondaparinux, and remains stable.
High index of suspicion required
In a linked commentary, Hugh Markus, PhD, FRCP, professor of stroke medicine at the University of Cambridge, United Kingdom, wrote: “This report emphasizes that the immune mediated coagulopathy can also cause arterial thrombosis, including ischemic stroke, although venous thrombosis and especially cerebral venous sinus thrombosis appear more frequent.
“During the current period of COVID vaccination, a high index of suspicion is required to identify thrombotic episodes following vaccination,” he added. “However, it is important to remember that these side effects are rare and much less common than both cerebral venous thrombosis and ischemic stroke associated with COVID-19 infection itself.”
Risk/benefit unaltered
Several experts who commented on these reports for the Science Media Centre all agreed with Dr. Werring and Dr. Markus that these reports do not alter the current risk/benefit estimates with the vaccine.
Ian Douglas, PhD, professor of pharmacoepidemiology, London School of Hygiene & Tropical Medicine, who sits on the U.K.’s Medicines and Healthcare Products Regulatory Agency’s Pharmacovigilance Expert Advisory Group, said: “The picture regarding the rare syndrome of blood clots combined with low platelet counts associated with the AstraZeneca vaccine is becoming clearer. Until now, the cases described have tended to involve clots in veins such as cerebral vein thrombosis. In this series of three case reports, we now have some evidence that the types of blood vessels affected include arteries as well as veins.”
“It’s important to stress that such cases remain very rare, and it’s certainly much rarer in people who have had the AstraZeneca vaccine than it is in people affected by COVID-19 itself,” Dr. Douglas emphasized.
“The description of the cases suggests the patients involved presented with the same kind of symptoms as already described in cases involving cerebral vein thrombosis, and they don’t suggest patients need to be on the alert for anything different,” he added.
“However, the emergence of details like this will help guide health professionals who may be faced with similar cases in future; the sooner such cases are recognized, the more chance they will quickly receive the right kind of treatment, hopefully leading to better outcomes.”
Will Lester, MBChB, PhD, consultant hematologist, University Hospitals Birmingham NHS Foundation Trust, said: “VITT remains a rare complication, and patients with a history of thrombosis, including stroke, should not consider themselves to be at any higher risk of this type of rare thrombosis after vaccination, and COVID infection itself is a significant risk for stroke and other types of thrombosis.”
Many countries have paused use of the AstraZeneca vaccine because of its link to the VITT syndrome or restricted its use to older people as the VITT reaction appears to be slightly more common in younger people. In the United Kingdom, the current recommendation is that individuals under 40 years of age should be offered an alternative to the AstraZeneca vaccine where possible.
A version of this article first appeared on Medscape.com.
The three cases (one of which was fatal) occurred in two women and one man in their 30s or 40s and involved blockages of the carotid and middle cerebral artery. Two of the three patients also had venous thrombosis involving the portal and cerebral venous system. All three also had extremely low platelet counts, confirmed antibodies to platelet factor 4, and raised D-dimer levels, all characteristic of the vaccine-induced immune thrombotic thrombocytopenia (VITT) reaction associated with the AstraZeneca vaccine.
They are described in detail in a letter published online on May 25 in the Journal of Neurology, Neurosurgery & Psychiatry
“These are [the] first detailed reports of arterial stroke believed to be caused by VITT after the AstraZeneca COVID vaccine, although stroke has been mentioned previously in the VITT data,” said senior author David Werring, PhD, FRCP.
“VITT has more commonly presented as CVST [Cerebral venous sinus thrombosis] which is stroke caused by a venous thrombosis; these cases are showing that it can also cause stroke caused by an arterial thrombosis,” explained Dr. Werring, professor of clinical neurology at the Stroke Research Centre, University College London.
“In patients who present with ischemic stroke, especially younger patients, and who have had the AstraZeneca vaccine within the past month, clinicians need to consider VITT as a possible cause, as there is a specific treatment needed for this syndrome,” he said.
Young patients presenting with ischemic stroke after receiving the AstraZeneca vaccine should urgently be evaluated for VITT with laboratory tests, including platelet count, D-dimers, fibrinogen, and anti-PF4 antibodies, the authors wrote, and then managed by a multidisciplinary team, including hematology, neurology, stroke, neurosurgery, and neuroradiology, for rapid access to treatments including intravenous immune globulin, methylprednisolone, plasmapheresis, and nonheparin anticoagulants such as fondaparinux, argatroban, or direct oral anticoagulants.
Dr. Werring noted that these reports do not add anything to the overall risk/benefit of the vaccine, as they are only describing three cases. “While VITT is very serious, the benefit of the vaccine still outweighs its risks,” he said. “Around 40% of patients hospitalized with COVID-19 experience some sort of thrombosis and about 1.5% have an ischemic stroke. Whereas latest figures from the U.K. estimate the incidence of VITT with the AstraZeneca vaccine of 1 in 50,000 to 1 in 100,000.
“Our report doesn’t suggest that VITT is more common than these latest figures estimate, but we are just drawing attention to an alternative presentation,” he added.
Three cases
The first patient in the current case series, a woman in her 30s, experienced an intermittent headache on the right side and around her eyes 6 days after the vaccine. Five days later, she awoke feeling drowsy and with weakness to her left face, arm, and leg.
Imaging revealed a blocked right middle cerebral artery with brain infarction and clots in the right portal vein. She underwent brain surgery to reduce the pressure in her skull, plasma removal and replacement, and received the anticoagulant fondaparinux, but she still unfortunately died.
The second patient, a woman in her late 30s, presented with headache, confusion, weakness in her left arm, and loss of vision on the left side 12 days after having received the vaccine. Imaging showed occlusion of both carotid arteries, as well as pulmonary embolism and a left cerebral venous sinus thrombosis.
Her platelet count increased following plasma removal and replacement and intravenous corticosteroids, and her condition improved after fondaparinux treatment.
The third patient, a man in his early 40s, presented 3 weeks after receiving his vaccination with problems speaking. Imaging showed a clot in the left middle cerebral artery, but there was no evidence of clots in the cerebral venous sinuses. He received a platelet and plasma transfusion, and fondaparinux, and remains stable.
High index of suspicion required
In a linked commentary, Hugh Markus, PhD, FRCP, professor of stroke medicine at the University of Cambridge, United Kingdom, wrote: “This report emphasizes that the immune mediated coagulopathy can also cause arterial thrombosis, including ischemic stroke, although venous thrombosis and especially cerebral venous sinus thrombosis appear more frequent.
“During the current period of COVID vaccination, a high index of suspicion is required to identify thrombotic episodes following vaccination,” he added. “However, it is important to remember that these side effects are rare and much less common than both cerebral venous thrombosis and ischemic stroke associated with COVID-19 infection itself.”
Risk/benefit unaltered
Several experts who commented on these reports for the Science Media Centre all agreed with Dr. Werring and Dr. Markus that these reports do not alter the current risk/benefit estimates with the vaccine.
Ian Douglas, PhD, professor of pharmacoepidemiology, London School of Hygiene & Tropical Medicine, who sits on the U.K.’s Medicines and Healthcare Products Regulatory Agency’s Pharmacovigilance Expert Advisory Group, said: “The picture regarding the rare syndrome of blood clots combined with low platelet counts associated with the AstraZeneca vaccine is becoming clearer. Until now, the cases described have tended to involve clots in veins such as cerebral vein thrombosis. In this series of three case reports, we now have some evidence that the types of blood vessels affected include arteries as well as veins.”
“It’s important to stress that such cases remain very rare, and it’s certainly much rarer in people who have had the AstraZeneca vaccine than it is in people affected by COVID-19 itself,” Dr. Douglas emphasized.
“The description of the cases suggests the patients involved presented with the same kind of symptoms as already described in cases involving cerebral vein thrombosis, and they don’t suggest patients need to be on the alert for anything different,” he added.
“However, the emergence of details like this will help guide health professionals who may be faced with similar cases in future; the sooner such cases are recognized, the more chance they will quickly receive the right kind of treatment, hopefully leading to better outcomes.”
Will Lester, MBChB, PhD, consultant hematologist, University Hospitals Birmingham NHS Foundation Trust, said: “VITT remains a rare complication, and patients with a history of thrombosis, including stroke, should not consider themselves to be at any higher risk of this type of rare thrombosis after vaccination, and COVID infection itself is a significant risk for stroke and other types of thrombosis.”
Many countries have paused use of the AstraZeneca vaccine because of its link to the VITT syndrome or restricted its use to older people as the VITT reaction appears to be slightly more common in younger people. In the United Kingdom, the current recommendation is that individuals under 40 years of age should be offered an alternative to the AstraZeneca vaccine where possible.
A version of this article first appeared on Medscape.com.
The three cases (one of which was fatal) occurred in two women and one man in their 30s or 40s and involved blockages of the carotid and middle cerebral artery. Two of the three patients also had venous thrombosis involving the portal and cerebral venous system. All three also had extremely low platelet counts, confirmed antibodies to platelet factor 4, and raised D-dimer levels, all characteristic of the vaccine-induced immune thrombotic thrombocytopenia (VITT) reaction associated with the AstraZeneca vaccine.
They are described in detail in a letter published online on May 25 in the Journal of Neurology, Neurosurgery & Psychiatry
“These are [the] first detailed reports of arterial stroke believed to be caused by VITT after the AstraZeneca COVID vaccine, although stroke has been mentioned previously in the VITT data,” said senior author David Werring, PhD, FRCP.
“VITT has more commonly presented as CVST [Cerebral venous sinus thrombosis] which is stroke caused by a venous thrombosis; these cases are showing that it can also cause stroke caused by an arterial thrombosis,” explained Dr. Werring, professor of clinical neurology at the Stroke Research Centre, University College London.
“In patients who present with ischemic stroke, especially younger patients, and who have had the AstraZeneca vaccine within the past month, clinicians need to consider VITT as a possible cause, as there is a specific treatment needed for this syndrome,” he said.
Young patients presenting with ischemic stroke after receiving the AstraZeneca vaccine should urgently be evaluated for VITT with laboratory tests, including platelet count, D-dimers, fibrinogen, and anti-PF4 antibodies, the authors wrote, and then managed by a multidisciplinary team, including hematology, neurology, stroke, neurosurgery, and neuroradiology, for rapid access to treatments including intravenous immune globulin, methylprednisolone, plasmapheresis, and nonheparin anticoagulants such as fondaparinux, argatroban, or direct oral anticoagulants.
Dr. Werring noted that these reports do not add anything to the overall risk/benefit of the vaccine, as they are only describing three cases. “While VITT is very serious, the benefit of the vaccine still outweighs its risks,” he said. “Around 40% of patients hospitalized with COVID-19 experience some sort of thrombosis and about 1.5% have an ischemic stroke. Whereas latest figures from the U.K. estimate the incidence of VITT with the AstraZeneca vaccine of 1 in 50,000 to 1 in 100,000.
“Our report doesn’t suggest that VITT is more common than these latest figures estimate, but we are just drawing attention to an alternative presentation,” he added.
Three cases
The first patient in the current case series, a woman in her 30s, experienced an intermittent headache on the right side and around her eyes 6 days after the vaccine. Five days later, she awoke feeling drowsy and with weakness to her left face, arm, and leg.
Imaging revealed a blocked right middle cerebral artery with brain infarction and clots in the right portal vein. She underwent brain surgery to reduce the pressure in her skull, plasma removal and replacement, and received the anticoagulant fondaparinux, but she still unfortunately died.
The second patient, a woman in her late 30s, presented with headache, confusion, weakness in her left arm, and loss of vision on the left side 12 days after having received the vaccine. Imaging showed occlusion of both carotid arteries, as well as pulmonary embolism and a left cerebral venous sinus thrombosis.
Her platelet count increased following plasma removal and replacement and intravenous corticosteroids, and her condition improved after fondaparinux treatment.
The third patient, a man in his early 40s, presented 3 weeks after receiving his vaccination with problems speaking. Imaging showed a clot in the left middle cerebral artery, but there was no evidence of clots in the cerebral venous sinuses. He received a platelet and plasma transfusion, and fondaparinux, and remains stable.
High index of suspicion required
In a linked commentary, Hugh Markus, PhD, FRCP, professor of stroke medicine at the University of Cambridge, United Kingdom, wrote: “This report emphasizes that the immune mediated coagulopathy can also cause arterial thrombosis, including ischemic stroke, although venous thrombosis and especially cerebral venous sinus thrombosis appear more frequent.
“During the current period of COVID vaccination, a high index of suspicion is required to identify thrombotic episodes following vaccination,” he added. “However, it is important to remember that these side effects are rare and much less common than both cerebral venous thrombosis and ischemic stroke associated with COVID-19 infection itself.”
Risk/benefit unaltered
Several experts who commented on these reports for the Science Media Centre all agreed with Dr. Werring and Dr. Markus that these reports do not alter the current risk/benefit estimates with the vaccine.
Ian Douglas, PhD, professor of pharmacoepidemiology, London School of Hygiene & Tropical Medicine, who sits on the U.K.’s Medicines and Healthcare Products Regulatory Agency’s Pharmacovigilance Expert Advisory Group, said: “The picture regarding the rare syndrome of blood clots combined with low platelet counts associated with the AstraZeneca vaccine is becoming clearer. Until now, the cases described have tended to involve clots in veins such as cerebral vein thrombosis. In this series of three case reports, we now have some evidence that the types of blood vessels affected include arteries as well as veins.”
“It’s important to stress that such cases remain very rare, and it’s certainly much rarer in people who have had the AstraZeneca vaccine than it is in people affected by COVID-19 itself,” Dr. Douglas emphasized.
“The description of the cases suggests the patients involved presented with the same kind of symptoms as already described in cases involving cerebral vein thrombosis, and they don’t suggest patients need to be on the alert for anything different,” he added.
“However, the emergence of details like this will help guide health professionals who may be faced with similar cases in future; the sooner such cases are recognized, the more chance they will quickly receive the right kind of treatment, hopefully leading to better outcomes.”
Will Lester, MBChB, PhD, consultant hematologist, University Hospitals Birmingham NHS Foundation Trust, said: “VITT remains a rare complication, and patients with a history of thrombosis, including stroke, should not consider themselves to be at any higher risk of this type of rare thrombosis after vaccination, and COVID infection itself is a significant risk for stroke and other types of thrombosis.”
Many countries have paused use of the AstraZeneca vaccine because of its link to the VITT syndrome or restricted its use to older people as the VITT reaction appears to be slightly more common in younger people. In the United Kingdom, the current recommendation is that individuals under 40 years of age should be offered an alternative to the AstraZeneca vaccine where possible.
A version of this article first appeared on Medscape.com.
Lower SARS-CoV-2 vaccine responses seen in patients with immune-mediated inflammatory diseases
Ten percent of patients with immune-mediated inflammatory diseases (IMIDs) fail to respond properly to COVID-19 vaccinations regardless of medication, researchers report, and small new studies suggest those on methotrexate and rituximab may be especially vulnerable to vaccine failure.
Even so, it’s still crucially vital for patients with IMIDs to get vaccinated and for clinicians to follow recommendations to temporarily withhold certain medications around the time of vaccination, rheumatologist Anne R. Bass, MD, of Weill Cornell Medicine and the Hospital for Special Surgery, New York, said in an interview. “We’re not making any significant adjustments,” added Dr. Bass, a coauthor of the American College of Rheumatology’s COVID-19 vaccination guidelines for patients with rheumatic and musculoskeletal diseases.
The findings appear in a trio of studies in Annals of the Rheumatic Diseases. The most recent study, which appeared May 25, 2021, found that more than one-third of patients with IMIDs who took methotrexate didn’t produce adequate antibody levels after vaccination versus 10% of those in other groups. (P < .001) A May 11 study found that 20 of 30 patients with rheumatic diseases on rituximab failed to respond to vaccination. And a May 6 study reported that immune responses against SARS-CoV-2 are “somewhat delayed and reduced” in patients with IMID, with 99.5% of a control group developing neutralizing antibody activity after vaccination versus 90% of those with IMID (P = .0008).
Development of neutralizing antibodies somewhat delayed and reduced
Team members were surprised by the high number of vaccine nonresponders in the May 6 IMID study, coauthor Georg Schett, MD, of Germany’s Friedrich-Alexander University Erlangen-Nuremberg and University Hospital Erlangen, said in an interview.
The researchers compared two groups of patients who had no history of COVID-19 and received COVID-19 vaccinations, mostly two shots of the Pfizer-BioNTech vaccine (96%): 84 with IMID (mean age, 53.1 years; 65.5% females) and 182 healthy controls (mean age, 40.8 years; 57.1% females).
The patients with IMID most commonly had spondyloarthritis (32.1%), RA (29.8%), inflammatory bowel disease (9.5%), and psoriasis (9.5%). Nearly 43% of the patients were treated with biologic and targeted synthetic disease-modifying antirheumatic drugs and 23.9% with conventional synthetic DMARDSs. Another 29% were not treated.
All of the controls developed anti–SARS-CoV-2 IgG, but 6% of the patients with IMID did not (P = .003). The gap in development of neutralizing antibodies was even higher: 99.5% of the controls developed neutralizing antibody activity versus 90% of the IMID group. “Neutralizing antibodies are more relevant because the test shows how much the antibodies interfere with the binding of SARS-CoV-2 proteins to the receptor,” Dr. Schett said.
The study authors concluded that “our study provides evidence that, while vaccination against SARS-CoV-2 is well tolerated and even associated with lower incidence of side effects in patients with IMID, its efficacy is somewhat delayed and reduced. Nonetheless, the data also show that, in principle, patients with IMID respond to SARS-CoV-2 vaccination, supporting an aggressive vaccination strategy.”
Lowered antibody response to vaccination for some methotrexate users
In the newer study, led by Rebecca H. Haberman, MD, of New York University Langone Health, researchers examined COVID-19 vaccine response in cohorts in New York City and Erlangen, Germany.
The New York cohort included 25 patients with IMID who were taking methotrexate by itself or with other immunomodulatory medications (mean age, 63.2 years), 26 with IMID who were on anticytokine therapy and/or other oral immunomodulators (mean age, 49.1 years) and 26 healthy controls (mean age, 49.2 years). Most patients with IMID had psoriasis/psoriatic arthritis or RA.
The German validation cohort included 182 healthy subjects (mean age, 45.0 years), 11 subjects with IMID who received TNF inhibitor monotherapy (mean age, 40.8 years), and 20 subjects with IMID on methotrexate monotherapy (mean age, 54.5 years).
In the New York cohort, 96.1% of healthy controls showed “adequate humoral immune response,” along with 92.3% of patients with IMID who weren’t taking methotrexate. However, those on methotrexate had a lower rate of adequate response (72.0%), and the gap persisted even after researchers removed those who showed signs of previous COVID-19 infection (P = .045).
In the German cohort, 98.3% of healthy cohorts and 90.9% of patients with IMID who didn’t receive methotrexate reached an “adequate” humoral response versus just half (50.0%) of those who were taking methotrexate.
When both cohorts are combined, over 90% of the healthy subjects and the patients with IMID on biologic treatments (mainly TNF blockers, n = 37) showed “robust” antibody response. However, only 62% of patients with IMID who took methotrexate (n = 45) reached an “adequate” level of response. The methotrexate gap remained after researchers accounted for differences in age among the cohorts.
What’s going on? “We think that the underlying chronic immune stimulation in autoimmune patients may cause T-cell exhaustion and thus blunts the immune response,” said Dr. Schett, who’s also a coauthor of this study. “In addition, specific drugs such as methotrexate could additionally impair the immune response.”
Still, the findings “reiterate that vaccinations are safe and effective, which is what the recommendations state,” he said, adding that more testing of vaccination immune response is wise.
Insights into vaccine response while on rituximab
Two more reports, also published in Annals of the Rheumatic Diseases, offer insight into vaccine response in patients with IMID who take rituximab.
In one report, published May 11, U.S. researchers retrospectively tracked 89 rheumatic disease patients (76% female; mean age, 61) at a single clinic who’d received at least one dose of a COVID-19 vaccine. Of those, 21 patients showed no sign of vaccine antibody response, and 20 of them were in the group taking rituximab. (The other patient was taking belimumab.) Another 10 patients taking rituximab did show a response.
“Longer duration from most recent rituximab exposure was associated with a greater likelihood of response,” the report’s authors wrote. “The results suggest that time from last rituximab exposure is an important consideration in maximizing the likelihood of a serological response, but this likely is related to the substantial variation in the period of B-cell depletion following rituximab.”
Finally, an Austrian report published May 6 examined COVID-19 vaccine immune response in five patients who were taking rituximab (four with other drugs such as methotrexate and prednisone). Researchers compared them with eight healthy controls, half who’d been vaccinated.
The researchers found evidence that rituximab “may not have to preclude SARS-CoV-2 vaccination, since a cellular immune response will be mounted even in the absence of circulating B cells. Alternatively, in patients with stable disease, delaying [rituximab] treatment until after the second vaccination may be warranted and, therefore, vaccines with a short interval between first and second vaccination or those showing full protection after a single vaccination may be preferable. Importantly, in the presence of circulating B cells also a humoral immune response may be expected despite prior [rituximab] therapy.”
Dr. Bass said the findings reflect growing awareness that “patients with autoimmune disease, especially when they’re on immunosuppressant medications, don’t quite have as optimal responses to the vaccinations.” However, she said, the vaccines are so potent that they’re likely to still have significant efficacy in these patients even if there’s a reduction in response.
What’s next? Dr. Schett said “testing immune response to vaccination is important for patients with autoimmune disease. Some of them may need a third vaccination.”
The American College of Rheumatology’s COVID-19 vaccination guidelines do not recommend third vaccinations or postvaccination immune testing at this time. However, Dr. Bass, one of the coauthors of the recommendations, said it’s likely that postvaccination immune testing and booster shots will become routine.
Dr. Bass reported no relevant disclosures. Dr. Schett reported receiving consulting fees from AbbVie. The May 6 German vaccine study was funded by Deutsche Forschungsgemeinschaft, Bundesministerium für Bildung und Forschung, the ERC Synergy grant 4D Nanoscope, the IMI funded project RTCure, the Emerging Fields Initiative MIRACLE of the Friedrich-Alexander-Universität Erlangen-Nürnberg, the Schreiber Stiftung, and the Else Kröner-Memorial Scholarship. The study authors reported no disclosures. The May 25 study of German and American cohorts was funded by the National Institute of Arthritis and Musculoskletal and Skin Diseases, National Institute of Allergy and Infectious Diseases, Rheumatology Research Foundation, Bloomberg Philanthropies COVID-19 Initiative, Pfizer COVID-19 Competitive Grant Program, Beatrice Snyder Foundation, Riley Family Foundation, National Psoriasis Foundation, and Deutsche Forschungsgemeinschaft. The authors reported a range of financial relationships with pharmaceutical companies. No specific funding was reported for the other two studies mentioned.
Ten percent of patients with immune-mediated inflammatory diseases (IMIDs) fail to respond properly to COVID-19 vaccinations regardless of medication, researchers report, and small new studies suggest those on methotrexate and rituximab may be especially vulnerable to vaccine failure.
Even so, it’s still crucially vital for patients with IMIDs to get vaccinated and for clinicians to follow recommendations to temporarily withhold certain medications around the time of vaccination, rheumatologist Anne R. Bass, MD, of Weill Cornell Medicine and the Hospital for Special Surgery, New York, said in an interview. “We’re not making any significant adjustments,” added Dr. Bass, a coauthor of the American College of Rheumatology’s COVID-19 vaccination guidelines for patients with rheumatic and musculoskeletal diseases.
The findings appear in a trio of studies in Annals of the Rheumatic Diseases. The most recent study, which appeared May 25, 2021, found that more than one-third of patients with IMIDs who took methotrexate didn’t produce adequate antibody levels after vaccination versus 10% of those in other groups. (P < .001) A May 11 study found that 20 of 30 patients with rheumatic diseases on rituximab failed to respond to vaccination. And a May 6 study reported that immune responses against SARS-CoV-2 are “somewhat delayed and reduced” in patients with IMID, with 99.5% of a control group developing neutralizing antibody activity after vaccination versus 90% of those with IMID (P = .0008).
Development of neutralizing antibodies somewhat delayed and reduced
Team members were surprised by the high number of vaccine nonresponders in the May 6 IMID study, coauthor Georg Schett, MD, of Germany’s Friedrich-Alexander University Erlangen-Nuremberg and University Hospital Erlangen, said in an interview.
The researchers compared two groups of patients who had no history of COVID-19 and received COVID-19 vaccinations, mostly two shots of the Pfizer-BioNTech vaccine (96%): 84 with IMID (mean age, 53.1 years; 65.5% females) and 182 healthy controls (mean age, 40.8 years; 57.1% females).
The patients with IMID most commonly had spondyloarthritis (32.1%), RA (29.8%), inflammatory bowel disease (9.5%), and psoriasis (9.5%). Nearly 43% of the patients were treated with biologic and targeted synthetic disease-modifying antirheumatic drugs and 23.9% with conventional synthetic DMARDSs. Another 29% were not treated.
All of the controls developed anti–SARS-CoV-2 IgG, but 6% of the patients with IMID did not (P = .003). The gap in development of neutralizing antibodies was even higher: 99.5% of the controls developed neutralizing antibody activity versus 90% of the IMID group. “Neutralizing antibodies are more relevant because the test shows how much the antibodies interfere with the binding of SARS-CoV-2 proteins to the receptor,” Dr. Schett said.
The study authors concluded that “our study provides evidence that, while vaccination against SARS-CoV-2 is well tolerated and even associated with lower incidence of side effects in patients with IMID, its efficacy is somewhat delayed and reduced. Nonetheless, the data also show that, in principle, patients with IMID respond to SARS-CoV-2 vaccination, supporting an aggressive vaccination strategy.”
Lowered antibody response to vaccination for some methotrexate users
In the newer study, led by Rebecca H. Haberman, MD, of New York University Langone Health, researchers examined COVID-19 vaccine response in cohorts in New York City and Erlangen, Germany.
The New York cohort included 25 patients with IMID who were taking methotrexate by itself or with other immunomodulatory medications (mean age, 63.2 years), 26 with IMID who were on anticytokine therapy and/or other oral immunomodulators (mean age, 49.1 years) and 26 healthy controls (mean age, 49.2 years). Most patients with IMID had psoriasis/psoriatic arthritis or RA.
The German validation cohort included 182 healthy subjects (mean age, 45.0 years), 11 subjects with IMID who received TNF inhibitor monotherapy (mean age, 40.8 years), and 20 subjects with IMID on methotrexate monotherapy (mean age, 54.5 years).
In the New York cohort, 96.1% of healthy controls showed “adequate humoral immune response,” along with 92.3% of patients with IMID who weren’t taking methotrexate. However, those on methotrexate had a lower rate of adequate response (72.0%), and the gap persisted even after researchers removed those who showed signs of previous COVID-19 infection (P = .045).
In the German cohort, 98.3% of healthy cohorts and 90.9% of patients with IMID who didn’t receive methotrexate reached an “adequate” humoral response versus just half (50.0%) of those who were taking methotrexate.
When both cohorts are combined, over 90% of the healthy subjects and the patients with IMID on biologic treatments (mainly TNF blockers, n = 37) showed “robust” antibody response. However, only 62% of patients with IMID who took methotrexate (n = 45) reached an “adequate” level of response. The methotrexate gap remained after researchers accounted for differences in age among the cohorts.
What’s going on? “We think that the underlying chronic immune stimulation in autoimmune patients may cause T-cell exhaustion and thus blunts the immune response,” said Dr. Schett, who’s also a coauthor of this study. “In addition, specific drugs such as methotrexate could additionally impair the immune response.”
Still, the findings “reiterate that vaccinations are safe and effective, which is what the recommendations state,” he said, adding that more testing of vaccination immune response is wise.
Insights into vaccine response while on rituximab
Two more reports, also published in Annals of the Rheumatic Diseases, offer insight into vaccine response in patients with IMID who take rituximab.
In one report, published May 11, U.S. researchers retrospectively tracked 89 rheumatic disease patients (76% female; mean age, 61) at a single clinic who’d received at least one dose of a COVID-19 vaccine. Of those, 21 patients showed no sign of vaccine antibody response, and 20 of them were in the group taking rituximab. (The other patient was taking belimumab.) Another 10 patients taking rituximab did show a response.
“Longer duration from most recent rituximab exposure was associated with a greater likelihood of response,” the report’s authors wrote. “The results suggest that time from last rituximab exposure is an important consideration in maximizing the likelihood of a serological response, but this likely is related to the substantial variation in the period of B-cell depletion following rituximab.”
Finally, an Austrian report published May 6 examined COVID-19 vaccine immune response in five patients who were taking rituximab (four with other drugs such as methotrexate and prednisone). Researchers compared them with eight healthy controls, half who’d been vaccinated.
The researchers found evidence that rituximab “may not have to preclude SARS-CoV-2 vaccination, since a cellular immune response will be mounted even in the absence of circulating B cells. Alternatively, in patients with stable disease, delaying [rituximab] treatment until after the second vaccination may be warranted and, therefore, vaccines with a short interval between first and second vaccination or those showing full protection after a single vaccination may be preferable. Importantly, in the presence of circulating B cells also a humoral immune response may be expected despite prior [rituximab] therapy.”
Dr. Bass said the findings reflect growing awareness that “patients with autoimmune disease, especially when they’re on immunosuppressant medications, don’t quite have as optimal responses to the vaccinations.” However, she said, the vaccines are so potent that they’re likely to still have significant efficacy in these patients even if there’s a reduction in response.
What’s next? Dr. Schett said “testing immune response to vaccination is important for patients with autoimmune disease. Some of them may need a third vaccination.”
The American College of Rheumatology’s COVID-19 vaccination guidelines do not recommend third vaccinations or postvaccination immune testing at this time. However, Dr. Bass, one of the coauthors of the recommendations, said it’s likely that postvaccination immune testing and booster shots will become routine.
Dr. Bass reported no relevant disclosures. Dr. Schett reported receiving consulting fees from AbbVie. The May 6 German vaccine study was funded by Deutsche Forschungsgemeinschaft, Bundesministerium für Bildung und Forschung, the ERC Synergy grant 4D Nanoscope, the IMI funded project RTCure, the Emerging Fields Initiative MIRACLE of the Friedrich-Alexander-Universität Erlangen-Nürnberg, the Schreiber Stiftung, and the Else Kröner-Memorial Scholarship. The study authors reported no disclosures. The May 25 study of German and American cohorts was funded by the National Institute of Arthritis and Musculoskletal and Skin Diseases, National Institute of Allergy and Infectious Diseases, Rheumatology Research Foundation, Bloomberg Philanthropies COVID-19 Initiative, Pfizer COVID-19 Competitive Grant Program, Beatrice Snyder Foundation, Riley Family Foundation, National Psoriasis Foundation, and Deutsche Forschungsgemeinschaft. The authors reported a range of financial relationships with pharmaceutical companies. No specific funding was reported for the other two studies mentioned.
Ten percent of patients with immune-mediated inflammatory diseases (IMIDs) fail to respond properly to COVID-19 vaccinations regardless of medication, researchers report, and small new studies suggest those on methotrexate and rituximab may be especially vulnerable to vaccine failure.
Even so, it’s still crucially vital for patients with IMIDs to get vaccinated and for clinicians to follow recommendations to temporarily withhold certain medications around the time of vaccination, rheumatologist Anne R. Bass, MD, of Weill Cornell Medicine and the Hospital for Special Surgery, New York, said in an interview. “We’re not making any significant adjustments,” added Dr. Bass, a coauthor of the American College of Rheumatology’s COVID-19 vaccination guidelines for patients with rheumatic and musculoskeletal diseases.
The findings appear in a trio of studies in Annals of the Rheumatic Diseases. The most recent study, which appeared May 25, 2021, found that more than one-third of patients with IMIDs who took methotrexate didn’t produce adequate antibody levels after vaccination versus 10% of those in other groups. (P < .001) A May 11 study found that 20 of 30 patients with rheumatic diseases on rituximab failed to respond to vaccination. And a May 6 study reported that immune responses against SARS-CoV-2 are “somewhat delayed and reduced” in patients with IMID, with 99.5% of a control group developing neutralizing antibody activity after vaccination versus 90% of those with IMID (P = .0008).
Development of neutralizing antibodies somewhat delayed and reduced
Team members were surprised by the high number of vaccine nonresponders in the May 6 IMID study, coauthor Georg Schett, MD, of Germany’s Friedrich-Alexander University Erlangen-Nuremberg and University Hospital Erlangen, said in an interview.
The researchers compared two groups of patients who had no history of COVID-19 and received COVID-19 vaccinations, mostly two shots of the Pfizer-BioNTech vaccine (96%): 84 with IMID (mean age, 53.1 years; 65.5% females) and 182 healthy controls (mean age, 40.8 years; 57.1% females).
The patients with IMID most commonly had spondyloarthritis (32.1%), RA (29.8%), inflammatory bowel disease (9.5%), and psoriasis (9.5%). Nearly 43% of the patients were treated with biologic and targeted synthetic disease-modifying antirheumatic drugs and 23.9% with conventional synthetic DMARDSs. Another 29% were not treated.
All of the controls developed anti–SARS-CoV-2 IgG, but 6% of the patients with IMID did not (P = .003). The gap in development of neutralizing antibodies was even higher: 99.5% of the controls developed neutralizing antibody activity versus 90% of the IMID group. “Neutralizing antibodies are more relevant because the test shows how much the antibodies interfere with the binding of SARS-CoV-2 proteins to the receptor,” Dr. Schett said.
The study authors concluded that “our study provides evidence that, while vaccination against SARS-CoV-2 is well tolerated and even associated with lower incidence of side effects in patients with IMID, its efficacy is somewhat delayed and reduced. Nonetheless, the data also show that, in principle, patients with IMID respond to SARS-CoV-2 vaccination, supporting an aggressive vaccination strategy.”
Lowered antibody response to vaccination for some methotrexate users
In the newer study, led by Rebecca H. Haberman, MD, of New York University Langone Health, researchers examined COVID-19 vaccine response in cohorts in New York City and Erlangen, Germany.
The New York cohort included 25 patients with IMID who were taking methotrexate by itself or with other immunomodulatory medications (mean age, 63.2 years), 26 with IMID who were on anticytokine therapy and/or other oral immunomodulators (mean age, 49.1 years) and 26 healthy controls (mean age, 49.2 years). Most patients with IMID had psoriasis/psoriatic arthritis or RA.
The German validation cohort included 182 healthy subjects (mean age, 45.0 years), 11 subjects with IMID who received TNF inhibitor monotherapy (mean age, 40.8 years), and 20 subjects with IMID on methotrexate monotherapy (mean age, 54.5 years).
In the New York cohort, 96.1% of healthy controls showed “adequate humoral immune response,” along with 92.3% of patients with IMID who weren’t taking methotrexate. However, those on methotrexate had a lower rate of adequate response (72.0%), and the gap persisted even after researchers removed those who showed signs of previous COVID-19 infection (P = .045).
In the German cohort, 98.3% of healthy cohorts and 90.9% of patients with IMID who didn’t receive methotrexate reached an “adequate” humoral response versus just half (50.0%) of those who were taking methotrexate.
When both cohorts are combined, over 90% of the healthy subjects and the patients with IMID on biologic treatments (mainly TNF blockers, n = 37) showed “robust” antibody response. However, only 62% of patients with IMID who took methotrexate (n = 45) reached an “adequate” level of response. The methotrexate gap remained after researchers accounted for differences in age among the cohorts.
What’s going on? “We think that the underlying chronic immune stimulation in autoimmune patients may cause T-cell exhaustion and thus blunts the immune response,” said Dr. Schett, who’s also a coauthor of this study. “In addition, specific drugs such as methotrexate could additionally impair the immune response.”
Still, the findings “reiterate that vaccinations are safe and effective, which is what the recommendations state,” he said, adding that more testing of vaccination immune response is wise.
Insights into vaccine response while on rituximab
Two more reports, also published in Annals of the Rheumatic Diseases, offer insight into vaccine response in patients with IMID who take rituximab.
In one report, published May 11, U.S. researchers retrospectively tracked 89 rheumatic disease patients (76% female; mean age, 61) at a single clinic who’d received at least one dose of a COVID-19 vaccine. Of those, 21 patients showed no sign of vaccine antibody response, and 20 of them were in the group taking rituximab. (The other patient was taking belimumab.) Another 10 patients taking rituximab did show a response.
“Longer duration from most recent rituximab exposure was associated with a greater likelihood of response,” the report’s authors wrote. “The results suggest that time from last rituximab exposure is an important consideration in maximizing the likelihood of a serological response, but this likely is related to the substantial variation in the period of B-cell depletion following rituximab.”
Finally, an Austrian report published May 6 examined COVID-19 vaccine immune response in five patients who were taking rituximab (four with other drugs such as methotrexate and prednisone). Researchers compared them with eight healthy controls, half who’d been vaccinated.
The researchers found evidence that rituximab “may not have to preclude SARS-CoV-2 vaccination, since a cellular immune response will be mounted even in the absence of circulating B cells. Alternatively, in patients with stable disease, delaying [rituximab] treatment until after the second vaccination may be warranted and, therefore, vaccines with a short interval between first and second vaccination or those showing full protection after a single vaccination may be preferable. Importantly, in the presence of circulating B cells also a humoral immune response may be expected despite prior [rituximab] therapy.”
Dr. Bass said the findings reflect growing awareness that “patients with autoimmune disease, especially when they’re on immunosuppressant medications, don’t quite have as optimal responses to the vaccinations.” However, she said, the vaccines are so potent that they’re likely to still have significant efficacy in these patients even if there’s a reduction in response.
What’s next? Dr. Schett said “testing immune response to vaccination is important for patients with autoimmune disease. Some of them may need a third vaccination.”
The American College of Rheumatology’s COVID-19 vaccination guidelines do not recommend third vaccinations or postvaccination immune testing at this time. However, Dr. Bass, one of the coauthors of the recommendations, said it’s likely that postvaccination immune testing and booster shots will become routine.
Dr. Bass reported no relevant disclosures. Dr. Schett reported receiving consulting fees from AbbVie. The May 6 German vaccine study was funded by Deutsche Forschungsgemeinschaft, Bundesministerium für Bildung und Forschung, the ERC Synergy grant 4D Nanoscope, the IMI funded project RTCure, the Emerging Fields Initiative MIRACLE of the Friedrich-Alexander-Universität Erlangen-Nürnberg, the Schreiber Stiftung, and the Else Kröner-Memorial Scholarship. The study authors reported no disclosures. The May 25 study of German and American cohorts was funded by the National Institute of Arthritis and Musculoskletal and Skin Diseases, National Institute of Allergy and Infectious Diseases, Rheumatology Research Foundation, Bloomberg Philanthropies COVID-19 Initiative, Pfizer COVID-19 Competitive Grant Program, Beatrice Snyder Foundation, Riley Family Foundation, National Psoriasis Foundation, and Deutsche Forschungsgemeinschaft. The authors reported a range of financial relationships with pharmaceutical companies. No specific funding was reported for the other two studies mentioned.
FROM ANNALS OF THE RHEUMATIC DISEASES
COVID-19 vaccination rate rising quickly among adolescents
With nearly half of all Americans having received at least one dose of a COVID-19 vaccine, the youngest eligible group is beginning to overcome its late start, according to data from the Centers for Disease Control and Prevention.
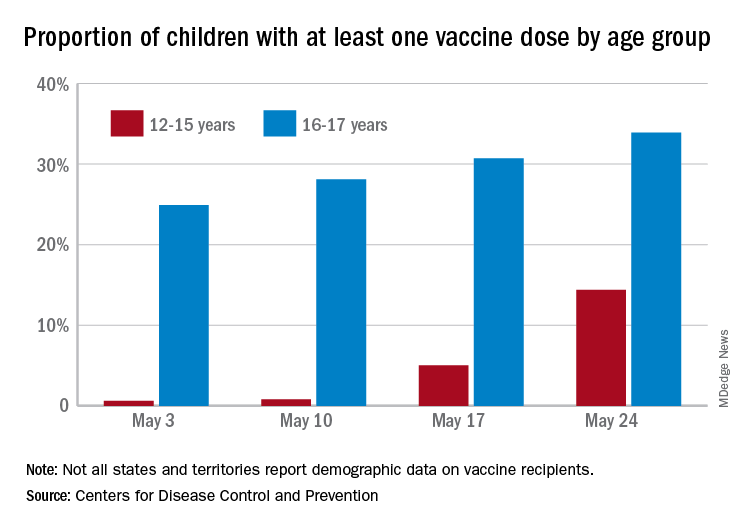
As of May 24, 49.4% of the U.S. population – that’s almost 164 million people – has received at least one dose of vaccine. The corresponding figure for children aged 12-15 years is 14.4%, but that’s up from only 0.6% just 3 weeks before. Among children aged 16-17, who’ve been getting vaccinated since early April in some states, the proportion receiving at least one dose went from 24.9% to 33.9% over those same 3 weeks, the CDC said on its COVID Data Tracker site.
The comparatively rapid increase among the younger group of eligible children can be seen over the last 14 days. To put that into perspective, only those aged 25-39 years were higher at 21.9%, while 18-24 (12.1%), 40-49 (13.4%), 50-64 (18.2%), 65-74 (5.3%), and ≥75 (2.9%) were all lower.
The 12- to 15-year-olds are further behind when it comes to full vaccination status, however, with just 0.6% having received both doses of a two-dose vaccine or one dose of the single-shot variety, compared with 21.6% for those aged 16-17 years. Children aged 12-15 make up 5% of the total U.S. population but just 0.1% of all those who have been fully vaccinated versus 2.5% and 1.4%, respectively, for those aged 16-17, the CDC reported.
With nearly half of all Americans having received at least one dose of a COVID-19 vaccine, the youngest eligible group is beginning to overcome its late start, according to data from the Centers for Disease Control and Prevention.

As of May 24, 49.4% of the U.S. population – that’s almost 164 million people – has received at least one dose of vaccine. The corresponding figure for children aged 12-15 years is 14.4%, but that’s up from only 0.6% just 3 weeks before. Among children aged 16-17, who’ve been getting vaccinated since early April in some states, the proportion receiving at least one dose went from 24.9% to 33.9% over those same 3 weeks, the CDC said on its COVID Data Tracker site.
The comparatively rapid increase among the younger group of eligible children can be seen over the last 14 days. To put that into perspective, only those aged 25-39 years were higher at 21.9%, while 18-24 (12.1%), 40-49 (13.4%), 50-64 (18.2%), 65-74 (5.3%), and ≥75 (2.9%) were all lower.
The 12- to 15-year-olds are further behind when it comes to full vaccination status, however, with just 0.6% having received both doses of a two-dose vaccine or one dose of the single-shot variety, compared with 21.6% for those aged 16-17 years. Children aged 12-15 make up 5% of the total U.S. population but just 0.1% of all those who have been fully vaccinated versus 2.5% and 1.4%, respectively, for those aged 16-17, the CDC reported.
With nearly half of all Americans having received at least one dose of a COVID-19 vaccine, the youngest eligible group is beginning to overcome its late start, according to data from the Centers for Disease Control and Prevention.

As of May 24, 49.4% of the U.S. population – that’s almost 164 million people – has received at least one dose of vaccine. The corresponding figure for children aged 12-15 years is 14.4%, but that’s up from only 0.6% just 3 weeks before. Among children aged 16-17, who’ve been getting vaccinated since early April in some states, the proportion receiving at least one dose went from 24.9% to 33.9% over those same 3 weeks, the CDC said on its COVID Data Tracker site.
The comparatively rapid increase among the younger group of eligible children can be seen over the last 14 days. To put that into perspective, only those aged 25-39 years were higher at 21.9%, while 18-24 (12.1%), 40-49 (13.4%), 50-64 (18.2%), 65-74 (5.3%), and ≥75 (2.9%) were all lower.
The 12- to 15-year-olds are further behind when it comes to full vaccination status, however, with just 0.6% having received both doses of a two-dose vaccine or one dose of the single-shot variety, compared with 21.6% for those aged 16-17 years. Children aged 12-15 make up 5% of the total U.S. population but just 0.1% of all those who have been fully vaccinated versus 2.5% and 1.4%, respectively, for those aged 16-17, the CDC reported.
Dengue may double the risk of symptomatic COVID-19
In a surprising study from the Amazon rain forest, Brazilian scientists found that symptomatic COVID-19 infections were twice as likely to occur in people who had prior dengue.
The study, led by Marcelo Urbano Ferreira, MD, PhD, of the University of São Paulo Biomedical Sciences Institute, was conducted in Mâncio Lima, a town in the Amazon region of Brazil, and published May 6, 2021, in Clinical Infectious Diseases.
In the study, supported by the São Paulo Research Foundation, Ferreira’s team looked at sequential blood samples from 1,285 residents of Mâncio Lima.
An earlier study by Miguel Nicolelis, MD, PhD, and colleagues (published as a preprint) had analyzed data from the first COVID-19 wave in Brazil in 2020. It was an “ecological study” and examined dengue cases in different geographic regions of Brazil. That study concluded that dengue actually seemed to protect people from later developing COVID-19.
Dr. Ferreira anticipated finding a similar effect. Instead, he found the opposite effect. Although dengue did not increase the risk of subsequent COVID-19 infection, symptomatic COVID-19 became twice as likely in people with prior dengue. His study was longitudinal, following a single group of patients in Mâncio Lima over time.
Dr. Ferreira explained that ecological studies are inherently less accurate, as they look at populations in different places. “All the older cases are diagnosed on clinical grounds ... Because most infections are either asymptomatic or symptoms can be easily confused with” other diseases, many cases are missed. So, during the dengue transmission season, “We have some overestimation of the actual number of cases, and outside the transmission season, we have underestimation of the cases.”
On the apparent discrepancy with the earlier study by Dr. Nicolelis, Dr. Ferreira commented, “It’s a wonderful study because it’s something you can do quickly and test a hypothesis [in a] very, very timely [manner], but the problem is if your diagnosis is not very reliable.”
Dr. Ferreira had another advantage: Knowing from sequential blood samples that his patients were exposed to dengue within the past 5 years. He also could tell serologically when they became infected with the SARS-CoV-2 virus, which causes COVID-19.
Dr. Ferreira told this news organization that very few of their patients became seriously ill or required hospitalization. Because their sample size was too small, he could not say if prior dengue made the COVID-19 infection worse.
The type of interaction between two infections like dengue and COVID-19 is called a “syndemic,” which the CDC defines as “synergistically interacting epidemics.” Dr. Ferreira hypothesized about some of the factors that might be at play but does not yet have enough data. For example, he speculated about a biologic basis, such as a link to autoimmunity or vasculitis from prior dengue, but “has no real data to either support or reject these things.”
Dr. Ferreira added that perhaps there are social factors that put certain people at higher risk of infection; for example, maybe some people are “more exposed to high viral loads.”
In Brazil’s first wave of COVID-19, Dr. Ferreira’s team calculated dengue seroconversion as about 10%; many cases of dengue were asymptomatic. Ferreira expects they will “have a very different clinical spectrum during the second wave,” with young people becoming much more ill from the P1 variant of concern.
Scott O’Neill, PhD, founder and director of the World Mosquito Program, told this news organization that, while he found the Brazil results intriguing, at present they are not sufficient to say that there’s a causal relationship between dengue and COVID-19. He expressed concern that the results seem counterintuitive and doubts there’s a biological or mechanistic cause. Instead, Dr. O’Neill wondered if “there could be something about social or economic conditions or living conditions” that might account for the correlation. For example, perhaps poverty increases exposure to both dengue and COVID-19.
Furthermore, Dr. O’Neill said in an interview that he suspects that, with the COVID-19 lockdowns, “You might expect to see more dengue.” This is because “most transmission occurs around the house, and so [with] having more people confined to houses, you might expect to see more dengue.” Such appears to be the case in Singapore.
In an article in the Journal of Infectious Diseases, Jue Tao Lim and colleagues described increased dengue in Singapore during COVID-19. They noted that most employees in Singapore work in air-conditioned settings. With social distancing enforced to try to reduce COVID-19, people stayed at home. The mosquito that transmits dengue, Aedes aegypti, gathers in wet spots in residential areas and bites during the daytime. The authors hypothesized that the spike in dengue was because of this change in habits, which shifted people’s exposure.
The syndemic in Brazil is complicated, with malaria and multiple arboviral diseases (chikungunya, dengue, Zika) overlapping with COVID-19 in areas of high population density, poverty, and poor sanitation, among other social ills. Such overlapping factors make it harder to distinguish correlations from causations. Prospective longitudinal studies might be needed to provide definitive answers.
Dr. Ferreira and Dr. O’Neill have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a surprising study from the Amazon rain forest, Brazilian scientists found that symptomatic COVID-19 infections were twice as likely to occur in people who had prior dengue.
The study, led by Marcelo Urbano Ferreira, MD, PhD, of the University of São Paulo Biomedical Sciences Institute, was conducted in Mâncio Lima, a town in the Amazon region of Brazil, and published May 6, 2021, in Clinical Infectious Diseases.
In the study, supported by the São Paulo Research Foundation, Ferreira’s team looked at sequential blood samples from 1,285 residents of Mâncio Lima.
An earlier study by Miguel Nicolelis, MD, PhD, and colleagues (published as a preprint) had analyzed data from the first COVID-19 wave in Brazil in 2020. It was an “ecological study” and examined dengue cases in different geographic regions of Brazil. That study concluded that dengue actually seemed to protect people from later developing COVID-19.
Dr. Ferreira anticipated finding a similar effect. Instead, he found the opposite effect. Although dengue did not increase the risk of subsequent COVID-19 infection, symptomatic COVID-19 became twice as likely in people with prior dengue. His study was longitudinal, following a single group of patients in Mâncio Lima over time.
Dr. Ferreira explained that ecological studies are inherently less accurate, as they look at populations in different places. “All the older cases are diagnosed on clinical grounds ... Because most infections are either asymptomatic or symptoms can be easily confused with” other diseases, many cases are missed. So, during the dengue transmission season, “We have some overestimation of the actual number of cases, and outside the transmission season, we have underestimation of the cases.”
On the apparent discrepancy with the earlier study by Dr. Nicolelis, Dr. Ferreira commented, “It’s a wonderful study because it’s something you can do quickly and test a hypothesis [in a] very, very timely [manner], but the problem is if your diagnosis is not very reliable.”
Dr. Ferreira had another advantage: Knowing from sequential blood samples that his patients were exposed to dengue within the past 5 years. He also could tell serologically when they became infected with the SARS-CoV-2 virus, which causes COVID-19.
Dr. Ferreira told this news organization that very few of their patients became seriously ill or required hospitalization. Because their sample size was too small, he could not say if prior dengue made the COVID-19 infection worse.
The type of interaction between two infections like dengue and COVID-19 is called a “syndemic,” which the CDC defines as “synergistically interacting epidemics.” Dr. Ferreira hypothesized about some of the factors that might be at play but does not yet have enough data. For example, he speculated about a biologic basis, such as a link to autoimmunity or vasculitis from prior dengue, but “has no real data to either support or reject these things.”
Dr. Ferreira added that perhaps there are social factors that put certain people at higher risk of infection; for example, maybe some people are “more exposed to high viral loads.”
In Brazil’s first wave of COVID-19, Dr. Ferreira’s team calculated dengue seroconversion as about 10%; many cases of dengue were asymptomatic. Ferreira expects they will “have a very different clinical spectrum during the second wave,” with young people becoming much more ill from the P1 variant of concern.
Scott O’Neill, PhD, founder and director of the World Mosquito Program, told this news organization that, while he found the Brazil results intriguing, at present they are not sufficient to say that there’s a causal relationship between dengue and COVID-19. He expressed concern that the results seem counterintuitive and doubts there’s a biological or mechanistic cause. Instead, Dr. O’Neill wondered if “there could be something about social or economic conditions or living conditions” that might account for the correlation. For example, perhaps poverty increases exposure to both dengue and COVID-19.
Furthermore, Dr. O’Neill said in an interview that he suspects that, with the COVID-19 lockdowns, “You might expect to see more dengue.” This is because “most transmission occurs around the house, and so [with] having more people confined to houses, you might expect to see more dengue.” Such appears to be the case in Singapore.
In an article in the Journal of Infectious Diseases, Jue Tao Lim and colleagues described increased dengue in Singapore during COVID-19. They noted that most employees in Singapore work in air-conditioned settings. With social distancing enforced to try to reduce COVID-19, people stayed at home. The mosquito that transmits dengue, Aedes aegypti, gathers in wet spots in residential areas and bites during the daytime. The authors hypothesized that the spike in dengue was because of this change in habits, which shifted people’s exposure.
The syndemic in Brazil is complicated, with malaria and multiple arboviral diseases (chikungunya, dengue, Zika) overlapping with COVID-19 in areas of high population density, poverty, and poor sanitation, among other social ills. Such overlapping factors make it harder to distinguish correlations from causations. Prospective longitudinal studies might be needed to provide definitive answers.
Dr. Ferreira and Dr. O’Neill have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a surprising study from the Amazon rain forest, Brazilian scientists found that symptomatic COVID-19 infections were twice as likely to occur in people who had prior dengue.
The study, led by Marcelo Urbano Ferreira, MD, PhD, of the University of São Paulo Biomedical Sciences Institute, was conducted in Mâncio Lima, a town in the Amazon region of Brazil, and published May 6, 2021, in Clinical Infectious Diseases.
In the study, supported by the São Paulo Research Foundation, Ferreira’s team looked at sequential blood samples from 1,285 residents of Mâncio Lima.
An earlier study by Miguel Nicolelis, MD, PhD, and colleagues (published as a preprint) had analyzed data from the first COVID-19 wave in Brazil in 2020. It was an “ecological study” and examined dengue cases in different geographic regions of Brazil. That study concluded that dengue actually seemed to protect people from later developing COVID-19.
Dr. Ferreira anticipated finding a similar effect. Instead, he found the opposite effect. Although dengue did not increase the risk of subsequent COVID-19 infection, symptomatic COVID-19 became twice as likely in people with prior dengue. His study was longitudinal, following a single group of patients in Mâncio Lima over time.
Dr. Ferreira explained that ecological studies are inherently less accurate, as they look at populations in different places. “All the older cases are diagnosed on clinical grounds ... Because most infections are either asymptomatic or symptoms can be easily confused with” other diseases, many cases are missed. So, during the dengue transmission season, “We have some overestimation of the actual number of cases, and outside the transmission season, we have underestimation of the cases.”
On the apparent discrepancy with the earlier study by Dr. Nicolelis, Dr. Ferreira commented, “It’s a wonderful study because it’s something you can do quickly and test a hypothesis [in a] very, very timely [manner], but the problem is if your diagnosis is not very reliable.”
Dr. Ferreira had another advantage: Knowing from sequential blood samples that his patients were exposed to dengue within the past 5 years. He also could tell serologically when they became infected with the SARS-CoV-2 virus, which causes COVID-19.
Dr. Ferreira told this news organization that very few of their patients became seriously ill or required hospitalization. Because their sample size was too small, he could not say if prior dengue made the COVID-19 infection worse.
The type of interaction between two infections like dengue and COVID-19 is called a “syndemic,” which the CDC defines as “synergistically interacting epidemics.” Dr. Ferreira hypothesized about some of the factors that might be at play but does not yet have enough data. For example, he speculated about a biologic basis, such as a link to autoimmunity or vasculitis from prior dengue, but “has no real data to either support or reject these things.”
Dr. Ferreira added that perhaps there are social factors that put certain people at higher risk of infection; for example, maybe some people are “more exposed to high viral loads.”
In Brazil’s first wave of COVID-19, Dr. Ferreira’s team calculated dengue seroconversion as about 10%; many cases of dengue were asymptomatic. Ferreira expects they will “have a very different clinical spectrum during the second wave,” with young people becoming much more ill from the P1 variant of concern.
Scott O’Neill, PhD, founder and director of the World Mosquito Program, told this news organization that, while he found the Brazil results intriguing, at present they are not sufficient to say that there’s a causal relationship between dengue and COVID-19. He expressed concern that the results seem counterintuitive and doubts there’s a biological or mechanistic cause. Instead, Dr. O’Neill wondered if “there could be something about social or economic conditions or living conditions” that might account for the correlation. For example, perhaps poverty increases exposure to both dengue and COVID-19.
Furthermore, Dr. O’Neill said in an interview that he suspects that, with the COVID-19 lockdowns, “You might expect to see more dengue.” This is because “most transmission occurs around the house, and so [with] having more people confined to houses, you might expect to see more dengue.” Such appears to be the case in Singapore.
In an article in the Journal of Infectious Diseases, Jue Tao Lim and colleagues described increased dengue in Singapore during COVID-19. They noted that most employees in Singapore work in air-conditioned settings. With social distancing enforced to try to reduce COVID-19, people stayed at home. The mosquito that transmits dengue, Aedes aegypti, gathers in wet spots in residential areas and bites during the daytime. The authors hypothesized that the spike in dengue was because of this change in habits, which shifted people’s exposure.
The syndemic in Brazil is complicated, with malaria and multiple arboviral diseases (chikungunya, dengue, Zika) overlapping with COVID-19 in areas of high population density, poverty, and poor sanitation, among other social ills. Such overlapping factors make it harder to distinguish correlations from causations. Prospective longitudinal studies might be needed to provide definitive answers.
Dr. Ferreira and Dr. O’Neill have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Physicians’ trust in health care leadership drops in pandemic
according to a survey conducted by NORC at the University of Chicago on behalf of the American Board of Internal Medicine Foundation.
Survey results, released May 21, indicate that 30% of physicians say their trust in the U.S. health care system and health care leadership has decreased during the pandemic. Only 18% reported an increase in trust.
Physicians, however, have great trust in their fellow clinicians.
In the survey of 600 physicians, 94% said they trust doctors within their practice; 85% trusted doctors outside of their practice; and 89% trusted nurses. That trust increased during the pandemic, with 41% saying their trust in fellow physicians rose and 37% saying their trust in nurses did.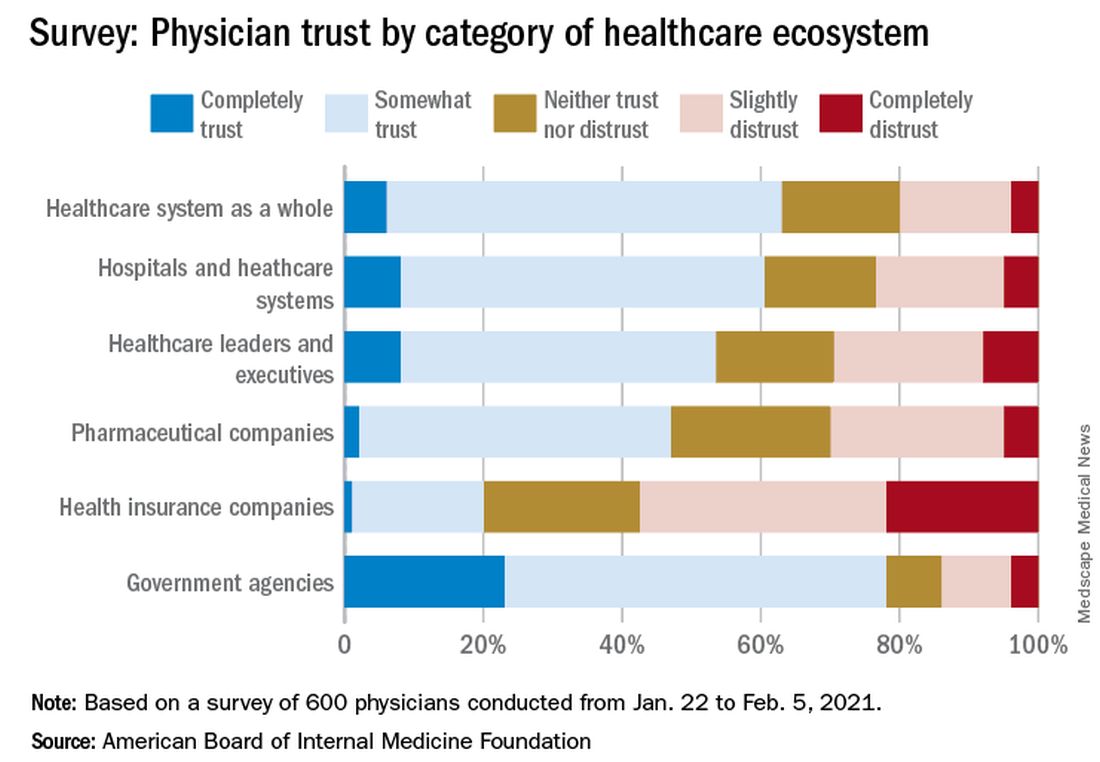
In a separate survey, NORC asked patients about their trust in various aspects of health care. Among 2,069 respondents, a wide majority reported that they trust doctors (84%) and nurses (85%), but only 64% trusted the health care system as a whole. One in three consumers (32%) said their trust in the health care system decreased during the pandemic, compared with 11% who said their trust increased.
The ABIM Foundation released the research findings on May 21 as part of Building Trust, a national campaign that aims to boost trust among patients, clinicians, system leaders, researchers, and others.
Richard J. Baron, MD, president and chief executive officer of the ABIM Foundation, said in an interview, “Clearly there’s lower trust in health care organization leaders and executives, and that’s troubling.
“Science by itself is not enough,” he said. “Becoming trustworthy has to be a core project of everybody in health care.”
Deterioration in physicians’ trust during the pandemic comes in part from failed promises of adequate personal protective equipment and some physicians’ loss of income as a result of the crisis, Dr. Baron said.
He added that the vaccine rollout was very uneven and that policies as to which elective procedures could be performed were handled differently in different parts of the country.
He also noted that, early on, transparency was lacking as to how many COVID patients hospitals were treating, which may have contributed to the decrease in trust in the system.
Fear of being known as ‘the COVID hospital’
Hospitals were afraid of being known as “the COVID hospital” and losing patients who were afraid to come there, Dr. Baron said.
He said the COVID-19 epidemic exacerbated problems regarding trust, but that trust has been declining for some time. The Building Trust campaign will focus on solutions in breaches of trust as physicians move increasingly toward being employees of huge systems, according to Dr. Baron.
However, trust works both ways, Dr. Baron notes. Physicians can be champions for their health care system or “throw the system under the bus,” he said.
For example, if a patient complains about the appointment system, clinicians who trust their institutions may say the system usually works and that they will try to make sure the patient has a better experience next time. Clinicians without trust may say they agree that the health care system doesn’t know what it is doing, and patients may further lose confidence when physicians validate their complaint, and patients may then go elsewhere.
78% of patients trust primary care doctor
When asked whether they trust their primary care physician, 78% of patients said yes. However, trust in doctors was higher among people who were older (90%), White (82%), or had high income (89%). Among people reporting lower trust, 25% said their physician spends too little time with them, and 14% said their doctor does not know or listen to them.
The survey shows that government agencies have work to do to earn trust. Responses indicate that 43% of physicians said they have “complete trust” in government health care agencies, such as the U.S. Food and Drug Administration and the Centers for Disease Control and Prevention, which is substantially higher than other parts of the health care system. However, trust in agencies declined for 43% of physician respondents and increased for 21%.
Dhruv Khullar, MD, MPP, of the department of health policy and economics at Weill Cornell Medical College in New York, told this news organization the survey results match what he sees anecdotally in medicine – that physicians have been losing trust in the system but not in their colleagues.
He said the sample size of 600 is enough to be influential, though he said he would like to know the response rate, which was not calculated for this survey.
He added that, in large part, physicians’ lack of trust in their systems may come from generally being asked to see more patients and to meet more metrics during the same or shorter periods.
Physicians’ lack of trust in the system can have significant consequences, he said. It can lead to burnout, which has been linked with poorer quality of care and physician turnover, he noted.
COVID-19 led some physicians to wonder whether their system had their best interests at heart, insofar as access to adequate medicines and supplies as well as emotional support were inconsistent, Dr. Khullar said.
He said that to regain trust health care systems need to ask themselves questions in three areas. The first is whether their goals are focused on the best interest of the organization or the best interest of the patient.
“Next is competency,” Dr. Khullar said. “Maybe your motives are right, but are you able to deliver? Are you delivering a good product, whether clinical services or something else?”
The third area is transparency, he said. “Are you going to be honest and forthright in what we’re doing and where we’re going?”
Caroline Pearson, senior vice president of health care strategy for NORC, said the emailed survey was conducted between Dec. 29, 2020, and Feb. 5, 2021, with a health care survey partner that maintains a nationwide panel of physicians across specialties.
She said this report is fairly novel insofar as surveys are more typically conducted regarding patients’ trust of their doctors or of the health care system.
Ms. Pearson said because health care is delivered in teams, understanding the level of trust among the entities helps ensure that care will be delivered effectively and seamlessly with high quality.
“We want our patients to trust our doctors, but we really want doctors to trust each other and trust the hospitals and systems in which they’re working,” she said.
Dr. Baron, Ms. Pearson, and Dr. Khullar report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a survey conducted by NORC at the University of Chicago on behalf of the American Board of Internal Medicine Foundation.
Survey results, released May 21, indicate that 30% of physicians say their trust in the U.S. health care system and health care leadership has decreased during the pandemic. Only 18% reported an increase in trust.
Physicians, however, have great trust in their fellow clinicians.
In the survey of 600 physicians, 94% said they trust doctors within their practice; 85% trusted doctors outside of their practice; and 89% trusted nurses. That trust increased during the pandemic, with 41% saying their trust in fellow physicians rose and 37% saying their trust in nurses did.
In a separate survey, NORC asked patients about their trust in various aspects of health care. Among 2,069 respondents, a wide majority reported that they trust doctors (84%) and nurses (85%), but only 64% trusted the health care system as a whole. One in three consumers (32%) said their trust in the health care system decreased during the pandemic, compared with 11% who said their trust increased.
The ABIM Foundation released the research findings on May 21 as part of Building Trust, a national campaign that aims to boost trust among patients, clinicians, system leaders, researchers, and others.
Richard J. Baron, MD, president and chief executive officer of the ABIM Foundation, said in an interview, “Clearly there’s lower trust in health care organization leaders and executives, and that’s troubling.
“Science by itself is not enough,” he said. “Becoming trustworthy has to be a core project of everybody in health care.”
Deterioration in physicians’ trust during the pandemic comes in part from failed promises of adequate personal protective equipment and some physicians’ loss of income as a result of the crisis, Dr. Baron said.
He added that the vaccine rollout was very uneven and that policies as to which elective procedures could be performed were handled differently in different parts of the country.
He also noted that, early on, transparency was lacking as to how many COVID patients hospitals were treating, which may have contributed to the decrease in trust in the system.
Fear of being known as ‘the COVID hospital’
Hospitals were afraid of being known as “the COVID hospital” and losing patients who were afraid to come there, Dr. Baron said.
He said the COVID-19 epidemic exacerbated problems regarding trust, but that trust has been declining for some time. The Building Trust campaign will focus on solutions in breaches of trust as physicians move increasingly toward being employees of huge systems, according to Dr. Baron.
However, trust works both ways, Dr. Baron notes. Physicians can be champions for their health care system or “throw the system under the bus,” he said.
For example, if a patient complains about the appointment system, clinicians who trust their institutions may say the system usually works and that they will try to make sure the patient has a better experience next time. Clinicians without trust may say they agree that the health care system doesn’t know what it is doing, and patients may further lose confidence when physicians validate their complaint, and patients may then go elsewhere.
78% of patients trust primary care doctor
When asked whether they trust their primary care physician, 78% of patients said yes. However, trust in doctors was higher among people who were older (90%), White (82%), or had high income (89%). Among people reporting lower trust, 25% said their physician spends too little time with them, and 14% said their doctor does not know or listen to them.
The survey shows that government agencies have work to do to earn trust. Responses indicate that 43% of physicians said they have “complete trust” in government health care agencies, such as the U.S. Food and Drug Administration and the Centers for Disease Control and Prevention, which is substantially higher than other parts of the health care system. However, trust in agencies declined for 43% of physician respondents and increased for 21%.
Dhruv Khullar, MD, MPP, of the department of health policy and economics at Weill Cornell Medical College in New York, told this news organization the survey results match what he sees anecdotally in medicine – that physicians have been losing trust in the system but not in their colleagues.
He said the sample size of 600 is enough to be influential, though he said he would like to know the response rate, which was not calculated for this survey.
He added that, in large part, physicians’ lack of trust in their systems may come from generally being asked to see more patients and to meet more metrics during the same or shorter periods.
Physicians’ lack of trust in the system can have significant consequences, he said. It can lead to burnout, which has been linked with poorer quality of care and physician turnover, he noted.
COVID-19 led some physicians to wonder whether their system had their best interests at heart, insofar as access to adequate medicines and supplies as well as emotional support were inconsistent, Dr. Khullar said.
He said that to regain trust health care systems need to ask themselves questions in three areas. The first is whether their goals are focused on the best interest of the organization or the best interest of the patient.
“Next is competency,” Dr. Khullar said. “Maybe your motives are right, but are you able to deliver? Are you delivering a good product, whether clinical services or something else?”
The third area is transparency, he said. “Are you going to be honest and forthright in what we’re doing and where we’re going?”
Caroline Pearson, senior vice president of health care strategy for NORC, said the emailed survey was conducted between Dec. 29, 2020, and Feb. 5, 2021, with a health care survey partner that maintains a nationwide panel of physicians across specialties.
She said this report is fairly novel insofar as surveys are more typically conducted regarding patients’ trust of their doctors or of the health care system.
Ms. Pearson said because health care is delivered in teams, understanding the level of trust among the entities helps ensure that care will be delivered effectively and seamlessly with high quality.
“We want our patients to trust our doctors, but we really want doctors to trust each other and trust the hospitals and systems in which they’re working,” she said.
Dr. Baron, Ms. Pearson, and Dr. Khullar report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a survey conducted by NORC at the University of Chicago on behalf of the American Board of Internal Medicine Foundation.
Survey results, released May 21, indicate that 30% of physicians say their trust in the U.S. health care system and health care leadership has decreased during the pandemic. Only 18% reported an increase in trust.
Physicians, however, have great trust in their fellow clinicians.
In the survey of 600 physicians, 94% said they trust doctors within their practice; 85% trusted doctors outside of their practice; and 89% trusted nurses. That trust increased during the pandemic, with 41% saying their trust in fellow physicians rose and 37% saying their trust in nurses did.
In a separate survey, NORC asked patients about their trust in various aspects of health care. Among 2,069 respondents, a wide majority reported that they trust doctors (84%) and nurses (85%), but only 64% trusted the health care system as a whole. One in three consumers (32%) said their trust in the health care system decreased during the pandemic, compared with 11% who said their trust increased.
The ABIM Foundation released the research findings on May 21 as part of Building Trust, a national campaign that aims to boost trust among patients, clinicians, system leaders, researchers, and others.
Richard J. Baron, MD, president and chief executive officer of the ABIM Foundation, said in an interview, “Clearly there’s lower trust in health care organization leaders and executives, and that’s troubling.
“Science by itself is not enough,” he said. “Becoming trustworthy has to be a core project of everybody in health care.”
Deterioration in physicians’ trust during the pandemic comes in part from failed promises of adequate personal protective equipment and some physicians’ loss of income as a result of the crisis, Dr. Baron said.
He added that the vaccine rollout was very uneven and that policies as to which elective procedures could be performed were handled differently in different parts of the country.
He also noted that, early on, transparency was lacking as to how many COVID patients hospitals were treating, which may have contributed to the decrease in trust in the system.
Fear of being known as ‘the COVID hospital’
Hospitals were afraid of being known as “the COVID hospital” and losing patients who were afraid to come there, Dr. Baron said.
He said the COVID-19 epidemic exacerbated problems regarding trust, but that trust has been declining for some time. The Building Trust campaign will focus on solutions in breaches of trust as physicians move increasingly toward being employees of huge systems, according to Dr. Baron.
However, trust works both ways, Dr. Baron notes. Physicians can be champions for their health care system or “throw the system under the bus,” he said.
For example, if a patient complains about the appointment system, clinicians who trust their institutions may say the system usually works and that they will try to make sure the patient has a better experience next time. Clinicians without trust may say they agree that the health care system doesn’t know what it is doing, and patients may further lose confidence when physicians validate their complaint, and patients may then go elsewhere.
78% of patients trust primary care doctor
When asked whether they trust their primary care physician, 78% of patients said yes. However, trust in doctors was higher among people who were older (90%), White (82%), or had high income (89%). Among people reporting lower trust, 25% said their physician spends too little time with them, and 14% said their doctor does not know or listen to them.
The survey shows that government agencies have work to do to earn trust. Responses indicate that 43% of physicians said they have “complete trust” in government health care agencies, such as the U.S. Food and Drug Administration and the Centers for Disease Control and Prevention, which is substantially higher than other parts of the health care system. However, trust in agencies declined for 43% of physician respondents and increased for 21%.
Dhruv Khullar, MD, MPP, of the department of health policy and economics at Weill Cornell Medical College in New York, told this news organization the survey results match what he sees anecdotally in medicine – that physicians have been losing trust in the system but not in their colleagues.
He said the sample size of 600 is enough to be influential, though he said he would like to know the response rate, which was not calculated for this survey.
He added that, in large part, physicians’ lack of trust in their systems may come from generally being asked to see more patients and to meet more metrics during the same or shorter periods.
Physicians’ lack of trust in the system can have significant consequences, he said. It can lead to burnout, which has been linked with poorer quality of care and physician turnover, he noted.
COVID-19 led some physicians to wonder whether their system had their best interests at heart, insofar as access to adequate medicines and supplies as well as emotional support were inconsistent, Dr. Khullar said.
He said that to regain trust health care systems need to ask themselves questions in three areas. The first is whether their goals are focused on the best interest of the organization or the best interest of the patient.
“Next is competency,” Dr. Khullar said. “Maybe your motives are right, but are you able to deliver? Are you delivering a good product, whether clinical services or something else?”
The third area is transparency, he said. “Are you going to be honest and forthright in what we’re doing and where we’re going?”
Caroline Pearson, senior vice president of health care strategy for NORC, said the emailed survey was conducted between Dec. 29, 2020, and Feb. 5, 2021, with a health care survey partner that maintains a nationwide panel of physicians across specialties.
She said this report is fairly novel insofar as surveys are more typically conducted regarding patients’ trust of their doctors or of the health care system.
Ms. Pearson said because health care is delivered in teams, understanding the level of trust among the entities helps ensure that care will be delivered effectively and seamlessly with high quality.
“We want our patients to trust our doctors, but we really want doctors to trust each other and trust the hospitals and systems in which they’re working,” she said.
Dr. Baron, Ms. Pearson, and Dr. Khullar report no relevant financial relationships.
A version of this article first appeared on Medscape.com.