User login
Consider the ‘long game’ in tumor management following Mohs surgery
PORTLAND, ORE. – In his nearly 2 decades of dermatology practice, Keith L. Duffy, MD, has seen his share of cases where Mohs surgery was misused or misappropriated.
, Salt Lake City, said at the annual meeting of the Pacific Dermatologic Association. “I want to protect our specialty. I see patients who have dozens of skin cancers. I want to emphasize the long game of management in those patients. You have to think about the tumors in terms of decades.”
In 2012, an ad hoc task force from the American Academy of Dermatology (AAD), the American College of Mohs Surgery, the American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery developed appropriate use criteria (AUC) for 270 scenarios for which Mohs micrographic surgery (MMS) is frequently considered. The task force used a 9-point scale to rate each indication, as follows:
- A score of 7 to 9: The use of MMS is appropriate for the specific indication and is generally considered acceptable.
- A score of 4 to 6: The use of MMS is uncertain for the specific indication, although its use may be appropriate and acceptable.
- A score of 1 to 3: The use of MMS is inappropriate for the specific indication and is generally not considered acceptable.
These ratings were translated into a free Mohs Surgery Appropriate Use Criteria App developed by the AAD.
Subsequently, Dr. Duffy and colleagues retrospectively examined the University of Utah’s adherence to the Mohs AUC over the course of 3 months. Their analysis, published in 2015, included 1,026 nonmelanoma skin cancers in 724 patients. Of the 1,026 cancers, 350 (34.1%) were treated with MMS. Of these, 339 (96.9%) were deemed appropriate based on the AUC guidelines, 4 (1.1%) were deemed uncertain, and 7 (2%) were deemed inappropriate.
There were also 611 skin cancers that were not treated with Mohs but met criteria for treatment with Mohs. “Most of these were AUC 7 tumors,” Dr. Duffy said. “When I see an AUC 7 tumor, I give high consideration for certain anatomic locations, especially the lower leg, scalp, eyelid, genitalia, ear, hands, and feet. I also think about the patient’s age, the number of skin cancers, and histological characteristics. Consider the long game in management and remember that skin cancer patients can make a near infinite amount of skin cancers, so be conservative when excising skin cancers to preserve precious skin.”
In his opinion, full thickness wounds requiring sutures should be avoided on the scalp and lower leg, if possible. “Most carcinomas in these locations are superficial and not aggressive in immunocompetent patients,” said Dr. Duffy, who said he has had one patient in 12 years who was not a transplant patient who had a metastatic squamous cell carcinoma on the lower leg. “Postop complications can be totally avoided. I don’t worry about these patients bleeding or [about] dehiscence. They can go back and play golf the next day, so you save valuable skin where the real estate is precious. This underscores a practice pearl: Incorporate the Mohs AUC and consideration of anatomic location when considering the most appropriate treatment of skin cancers.”
He also advises dermatologists to consider the histopathologic characteristics of the tumor when treating skin cancers to reduce complications and save tissue, so that patients can resume their lifestyle. “When you read the pathology report, really think about what the dermatopathologist saw under the microscope,” said Dr. Duffy, who is an investigator at the University of Utah’s Huntsman Cancer Institute. He said that he is able to review the slides for 90% of his own cases before surgery. “I’m lucky that way, but if you have any questions, your dermatopathologist should be on speed dial.”
Ultimately, he concluded, proper selection of a treatment modality for a specific tumor and patient rules the day. “Tumors should be thought about in the context of the patient and not as a single or isolated cancer,” he said.
Dr. Duffy reported having no relevant disclosures.
PORTLAND, ORE. – In his nearly 2 decades of dermatology practice, Keith L. Duffy, MD, has seen his share of cases where Mohs surgery was misused or misappropriated.
, Salt Lake City, said at the annual meeting of the Pacific Dermatologic Association. “I want to protect our specialty. I see patients who have dozens of skin cancers. I want to emphasize the long game of management in those patients. You have to think about the tumors in terms of decades.”
In 2012, an ad hoc task force from the American Academy of Dermatology (AAD), the American College of Mohs Surgery, the American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery developed appropriate use criteria (AUC) for 270 scenarios for which Mohs micrographic surgery (MMS) is frequently considered. The task force used a 9-point scale to rate each indication, as follows:
- A score of 7 to 9: The use of MMS is appropriate for the specific indication and is generally considered acceptable.
- A score of 4 to 6: The use of MMS is uncertain for the specific indication, although its use may be appropriate and acceptable.
- A score of 1 to 3: The use of MMS is inappropriate for the specific indication and is generally not considered acceptable.
These ratings were translated into a free Mohs Surgery Appropriate Use Criteria App developed by the AAD.
Subsequently, Dr. Duffy and colleagues retrospectively examined the University of Utah’s adherence to the Mohs AUC over the course of 3 months. Their analysis, published in 2015, included 1,026 nonmelanoma skin cancers in 724 patients. Of the 1,026 cancers, 350 (34.1%) were treated with MMS. Of these, 339 (96.9%) were deemed appropriate based on the AUC guidelines, 4 (1.1%) were deemed uncertain, and 7 (2%) were deemed inappropriate.
There were also 611 skin cancers that were not treated with Mohs but met criteria for treatment with Mohs. “Most of these were AUC 7 tumors,” Dr. Duffy said. “When I see an AUC 7 tumor, I give high consideration for certain anatomic locations, especially the lower leg, scalp, eyelid, genitalia, ear, hands, and feet. I also think about the patient’s age, the number of skin cancers, and histological characteristics. Consider the long game in management and remember that skin cancer patients can make a near infinite amount of skin cancers, so be conservative when excising skin cancers to preserve precious skin.”
In his opinion, full thickness wounds requiring sutures should be avoided on the scalp and lower leg, if possible. “Most carcinomas in these locations are superficial and not aggressive in immunocompetent patients,” said Dr. Duffy, who said he has had one patient in 12 years who was not a transplant patient who had a metastatic squamous cell carcinoma on the lower leg. “Postop complications can be totally avoided. I don’t worry about these patients bleeding or [about] dehiscence. They can go back and play golf the next day, so you save valuable skin where the real estate is precious. This underscores a practice pearl: Incorporate the Mohs AUC and consideration of anatomic location when considering the most appropriate treatment of skin cancers.”
He also advises dermatologists to consider the histopathologic characteristics of the tumor when treating skin cancers to reduce complications and save tissue, so that patients can resume their lifestyle. “When you read the pathology report, really think about what the dermatopathologist saw under the microscope,” said Dr. Duffy, who is an investigator at the University of Utah’s Huntsman Cancer Institute. He said that he is able to review the slides for 90% of his own cases before surgery. “I’m lucky that way, but if you have any questions, your dermatopathologist should be on speed dial.”
Ultimately, he concluded, proper selection of a treatment modality for a specific tumor and patient rules the day. “Tumors should be thought about in the context of the patient and not as a single or isolated cancer,” he said.
Dr. Duffy reported having no relevant disclosures.
PORTLAND, ORE. – In his nearly 2 decades of dermatology practice, Keith L. Duffy, MD, has seen his share of cases where Mohs surgery was misused or misappropriated.
, Salt Lake City, said at the annual meeting of the Pacific Dermatologic Association. “I want to protect our specialty. I see patients who have dozens of skin cancers. I want to emphasize the long game of management in those patients. You have to think about the tumors in terms of decades.”
In 2012, an ad hoc task force from the American Academy of Dermatology (AAD), the American College of Mohs Surgery, the American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery developed appropriate use criteria (AUC) for 270 scenarios for which Mohs micrographic surgery (MMS) is frequently considered. The task force used a 9-point scale to rate each indication, as follows:
- A score of 7 to 9: The use of MMS is appropriate for the specific indication and is generally considered acceptable.
- A score of 4 to 6: The use of MMS is uncertain for the specific indication, although its use may be appropriate and acceptable.
- A score of 1 to 3: The use of MMS is inappropriate for the specific indication and is generally not considered acceptable.
These ratings were translated into a free Mohs Surgery Appropriate Use Criteria App developed by the AAD.
Subsequently, Dr. Duffy and colleagues retrospectively examined the University of Utah’s adherence to the Mohs AUC over the course of 3 months. Their analysis, published in 2015, included 1,026 nonmelanoma skin cancers in 724 patients. Of the 1,026 cancers, 350 (34.1%) were treated with MMS. Of these, 339 (96.9%) were deemed appropriate based on the AUC guidelines, 4 (1.1%) were deemed uncertain, and 7 (2%) were deemed inappropriate.
There were also 611 skin cancers that were not treated with Mohs but met criteria for treatment with Mohs. “Most of these were AUC 7 tumors,” Dr. Duffy said. “When I see an AUC 7 tumor, I give high consideration for certain anatomic locations, especially the lower leg, scalp, eyelid, genitalia, ear, hands, and feet. I also think about the patient’s age, the number of skin cancers, and histological characteristics. Consider the long game in management and remember that skin cancer patients can make a near infinite amount of skin cancers, so be conservative when excising skin cancers to preserve precious skin.”
In his opinion, full thickness wounds requiring sutures should be avoided on the scalp and lower leg, if possible. “Most carcinomas in these locations are superficial and not aggressive in immunocompetent patients,” said Dr. Duffy, who said he has had one patient in 12 years who was not a transplant patient who had a metastatic squamous cell carcinoma on the lower leg. “Postop complications can be totally avoided. I don’t worry about these patients bleeding or [about] dehiscence. They can go back and play golf the next day, so you save valuable skin where the real estate is precious. This underscores a practice pearl: Incorporate the Mohs AUC and consideration of anatomic location when considering the most appropriate treatment of skin cancers.”
He also advises dermatologists to consider the histopathologic characteristics of the tumor when treating skin cancers to reduce complications and save tissue, so that patients can resume their lifestyle. “When you read the pathology report, really think about what the dermatopathologist saw under the microscope,” said Dr. Duffy, who is an investigator at the University of Utah’s Huntsman Cancer Institute. He said that he is able to review the slides for 90% of his own cases before surgery. “I’m lucky that way, but if you have any questions, your dermatopathologist should be on speed dial.”
Ultimately, he concluded, proper selection of a treatment modality for a specific tumor and patient rules the day. “Tumors should be thought about in the context of the patient and not as a single or isolated cancer,” he said.
Dr. Duffy reported having no relevant disclosures.
AT PDA 2022
Evolocumab benefits accrue with longer follow-up: FOURIER OLE
Long-term lipid lowering with evolocumab (Repatha) further reduces cardiovascular events, including CV death, without a safety signal, according to results from the FOURIER open-label extension (OLE) study.
In the parent FOURIER trial, treatment with the proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor over a median of 2.2 years reduced the primary efficacy endpoint by 15% but showed no CV mortality signal, compared with placebo, in patients with atherosclerotic disease on background statin therapy.
Now with follow-up out to 8.4 years – the longest to date in any PCSK9 study – cardiovascular mortality was cut by 23% in patients who remained on evolocumab, compared with those originally assigned to placebo (3.32% vs. 4.45%; hazard ratio, 0.77; 95% confidence interval, 0.60-0.99).
The Kaplan-Meier curves during FOURIER were “essentially superimposed and it was not until the open-label extension period had begun with longer-term follow up that the benefit in terms of cardiovascular mortality reduction became apparent,” said principal investigator Michelle O’Donoghue, MD, MPH, of Brigham and Women’s Hospital, Boston.
The results were reported at the annual congress of the European Society of Cardiology and published simultaneously in Circulation.
Pivotal statin trials have median follow-up times of 4-5 years and demonstrated both a lag effect, meaning clinical benefit grew over time, and a legacy effect, where clinical benefit persisted in extended follow-up after the parent study, Dr. O’Donoghue observed.
With shorter follow-up in the parent FOURIER trial, there was evidence of a lag effect with the risk reduction in CV death, MI, and stroke increasing from 16% in the first year to 25% over time with evolocumab.
FOURIER-OLE enrolled 6,635 patients (3355 randomly assigned to evolocumab and 3280 to placebo), who completed the parent study and self-injected evolocumab subcutaneously with the choice of 140 mg every 2 weeks or 420 mg monthly. Study visits were at week 12 and then every 24 weeks. Median follow-up was 5 years.
Their mean age was 62 years, three-fourths were men, a third had diabetes. Three-fourths were on a high-intensity statin at the time of enrollment in FOURIER, and median LDL cholesterol at randomization was 91 mg/dL (2.4 mmol/L).
At week 12, the median LDL cholesterol was 30 mg/dL (0.78 mmol/L), and this was sustained throughout follow-up, Dr. O’Donoghue reported. Most patients achieved very low LDL cholesterol levels, with 63.2% achieving levels less than 40 mg/dL (1.04 mmol/L) and 26.6% less than 20 mg/dL (0.52 mmol/L).
Patients randomly assigned in the parent trial to evolocumab versus placebo had a 15% lower risk of the primary outcome of CV death, MI, stroke, hospitalization for unstable angina, or coronary revascularization (15.4% vs. 17.5%; HR, 0.85; 95% CI, 0.75-0.96).
Their risk of CV death, MI, or stroke was 20% lower (9.7% vs. 11.9%; HR, 0.80; 95% CI, 0.68-0.93), and, as noted previously, 23% lower for CV death.
When major adverse cardiovascular events data were parsed out by year, the largest LDL cholesterol reduction was in years 1 and 2 of the parent study (delta, 62 mg/dL between treatment arms), “highlighting that lag of benefit that continued to accrue with time,” Dr. O’Donoghue said.
“There was then carryover into the extension period, such that there was legacy effect from the LDL [cholesterol] delta that was seen during the parent study,” she said. “This benefit was most apparent early on during open-label extension and then, as one might expect when all patients were being treated with the same therapy, it began to attenuate somewhat with time.”
Although early studies raised concerns that very low LDL cholesterol may be associated with an increased risk of hemorrhagic stroke and neurocognitive effects, the frequency of adverse events did not increase over time with evolocumab exposure.
Annualized incidence rates for patients initially randomized to evolocumab did not exceed those for placebo-treated patients for any of the following events of interest: serious safety events (10% vs. 13%), hemorrhagic stroke (0.04% vs. 0.05%), new-onset diabetes (1.2% vs. 2.3%), muscle-related events (1.2% vs. 1.9%), injection-site reactions (0.4% vs. 0.7%), and drug-related allergic reactions (0.6% vs. 1.1%).
“Long-term use of evolocumab with a median follow-up of more than 7 years appears both safe and well tolerated,” Dr. O’Donoghue said.
Taken together with the continued accrual of cardiovascular benefit, including CV mortality, “these findings argue for early initiation of a marked and sustained LDL cholesterol reduction to maximize benefit,” she concluded.
Translating the benefits
Ulrich Laufs, MD, Leipzig (Germany) University Hospital, Germany, and invited commentator for the session, said the trial addresses two key issues: the long-term safety of low LDL cholesterol lowering and the long-term safety of inhibiting PCSK9, which is highly expressed not only in the liver but also in the brain, small intestine, and kidneys. Indeed, an LDL cholesterol level below 30 mg/dL is lower than the ESC treatment recommendation for very-high-risk patients and is, in fact, lower than most assays are reliable to interpret.
“So it is very important that we have these very clear data showing us that there were no adverse events, also including cataracts and hemorrhagic stroke, and these were on the level of placebo and did not increase over time,” he said.
The question of efficacy is triggered by observations of another PCSK9, the humanized monoclonal antibody bococizumab, which was associated in the SPIRE trial with an increase in LDL cholesterol over time because of neutralizing antibodies. Reassuringly, there was “completely sustained LDL [cholesterol] reduction” with no neutralizing antibodies with the fully human antibody evolocumab in FOURIER-OLE and in recent data from the OSLER-1 study, Dr. Laufs observed.
Acknowledging the potential for selection bias with an OLE program, Dr. Laufs said there are two important open questions: “Can the safety data observed for extracellular PCSK9 inhibition using an antibody be transferred to other mechanisms of PCSK9 inhibition? And obviously, from the perspective of patient care, how can we implement these important data into patient care and improve access to PCSK9 inhibitors?”
With regard to the latter point, he said physicians should be cautious in using the term “plaque regression,” opting instead for prevention and stabilization of atherosclerosis, and when using the term “legacy,” which may be misinterpreted by patients to imply there was cessation of therapy.
“From my perspective, [what] the open-label extension really shows is that earlier treatment is better,” Dr. Laufs said. “This should be our message.”
In a press conference prior to the presentation, ESC commentator Johann Bauersachs, MD, Hannover (Germany) Medical School, said “this is extremely important data because it confirms that it’s safe, and the criticism of the FOURIER study that mortality, cardiovascular mortality, was not reduced is now also reduced.”
Dr. Bauersachs said it would have been unethical to wait 7 years for a placebo-controlled trial and questioned whether data are available and suggestive of a legacy effect among patients who did not participate in the open-label extension.
Dr. O’Donoghue said unfortunately those data aren’t available but that Kaplan-Meier curves for the primary endpoint in the parent trial continued to diverge over time and that there was somewhat of a lag in terms of that divergence. “So, a median follow-up of 2 years may have been insufficient, especially for the emerging cardiovascular mortality that took longer to appear.”
The study was funded by Amgen. Dr. O’Donoghue reported receiving research grants from Amgen, AstraZeneca, Janssen, Intarcia, and Novartis, and consulting fees from Amgen, Novartis, AstraZeneca, and Janssen. Dr. Laufs reported receiving honoraria/reimbursement for lecture, study participation, and scientific cooperation with Saarland or Leipzig University, as well as relationships with multiple pharmaceutical and device makers.
A version of this article first appeared on Medscape.com.
Long-term lipid lowering with evolocumab (Repatha) further reduces cardiovascular events, including CV death, without a safety signal, according to results from the FOURIER open-label extension (OLE) study.
In the parent FOURIER trial, treatment with the proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor over a median of 2.2 years reduced the primary efficacy endpoint by 15% but showed no CV mortality signal, compared with placebo, in patients with atherosclerotic disease on background statin therapy.
Now with follow-up out to 8.4 years – the longest to date in any PCSK9 study – cardiovascular mortality was cut by 23% in patients who remained on evolocumab, compared with those originally assigned to placebo (3.32% vs. 4.45%; hazard ratio, 0.77; 95% confidence interval, 0.60-0.99).
The Kaplan-Meier curves during FOURIER were “essentially superimposed and it was not until the open-label extension period had begun with longer-term follow up that the benefit in terms of cardiovascular mortality reduction became apparent,” said principal investigator Michelle O’Donoghue, MD, MPH, of Brigham and Women’s Hospital, Boston.
The results were reported at the annual congress of the European Society of Cardiology and published simultaneously in Circulation.
Pivotal statin trials have median follow-up times of 4-5 years and demonstrated both a lag effect, meaning clinical benefit grew over time, and a legacy effect, where clinical benefit persisted in extended follow-up after the parent study, Dr. O’Donoghue observed.
With shorter follow-up in the parent FOURIER trial, there was evidence of a lag effect with the risk reduction in CV death, MI, and stroke increasing from 16% in the first year to 25% over time with evolocumab.
FOURIER-OLE enrolled 6,635 patients (3355 randomly assigned to evolocumab and 3280 to placebo), who completed the parent study and self-injected evolocumab subcutaneously with the choice of 140 mg every 2 weeks or 420 mg monthly. Study visits were at week 12 and then every 24 weeks. Median follow-up was 5 years.
Their mean age was 62 years, three-fourths were men, a third had diabetes. Three-fourths were on a high-intensity statin at the time of enrollment in FOURIER, and median LDL cholesterol at randomization was 91 mg/dL (2.4 mmol/L).
At week 12, the median LDL cholesterol was 30 mg/dL (0.78 mmol/L), and this was sustained throughout follow-up, Dr. O’Donoghue reported. Most patients achieved very low LDL cholesterol levels, with 63.2% achieving levels less than 40 mg/dL (1.04 mmol/L) and 26.6% less than 20 mg/dL (0.52 mmol/L).
Patients randomly assigned in the parent trial to evolocumab versus placebo had a 15% lower risk of the primary outcome of CV death, MI, stroke, hospitalization for unstable angina, or coronary revascularization (15.4% vs. 17.5%; HR, 0.85; 95% CI, 0.75-0.96).
Their risk of CV death, MI, or stroke was 20% lower (9.7% vs. 11.9%; HR, 0.80; 95% CI, 0.68-0.93), and, as noted previously, 23% lower for CV death.
When major adverse cardiovascular events data were parsed out by year, the largest LDL cholesterol reduction was in years 1 and 2 of the parent study (delta, 62 mg/dL between treatment arms), “highlighting that lag of benefit that continued to accrue with time,” Dr. O’Donoghue said.
“There was then carryover into the extension period, such that there was legacy effect from the LDL [cholesterol] delta that was seen during the parent study,” she said. “This benefit was most apparent early on during open-label extension and then, as one might expect when all patients were being treated with the same therapy, it began to attenuate somewhat with time.”
Although early studies raised concerns that very low LDL cholesterol may be associated with an increased risk of hemorrhagic stroke and neurocognitive effects, the frequency of adverse events did not increase over time with evolocumab exposure.
Annualized incidence rates for patients initially randomized to evolocumab did not exceed those for placebo-treated patients for any of the following events of interest: serious safety events (10% vs. 13%), hemorrhagic stroke (0.04% vs. 0.05%), new-onset diabetes (1.2% vs. 2.3%), muscle-related events (1.2% vs. 1.9%), injection-site reactions (0.4% vs. 0.7%), and drug-related allergic reactions (0.6% vs. 1.1%).
“Long-term use of evolocumab with a median follow-up of more than 7 years appears both safe and well tolerated,” Dr. O’Donoghue said.
Taken together with the continued accrual of cardiovascular benefit, including CV mortality, “these findings argue for early initiation of a marked and sustained LDL cholesterol reduction to maximize benefit,” she concluded.
Translating the benefits
Ulrich Laufs, MD, Leipzig (Germany) University Hospital, Germany, and invited commentator for the session, said the trial addresses two key issues: the long-term safety of low LDL cholesterol lowering and the long-term safety of inhibiting PCSK9, which is highly expressed not only in the liver but also in the brain, small intestine, and kidneys. Indeed, an LDL cholesterol level below 30 mg/dL is lower than the ESC treatment recommendation for very-high-risk patients and is, in fact, lower than most assays are reliable to interpret.
“So it is very important that we have these very clear data showing us that there were no adverse events, also including cataracts and hemorrhagic stroke, and these were on the level of placebo and did not increase over time,” he said.
The question of efficacy is triggered by observations of another PCSK9, the humanized monoclonal antibody bococizumab, which was associated in the SPIRE trial with an increase in LDL cholesterol over time because of neutralizing antibodies. Reassuringly, there was “completely sustained LDL [cholesterol] reduction” with no neutralizing antibodies with the fully human antibody evolocumab in FOURIER-OLE and in recent data from the OSLER-1 study, Dr. Laufs observed.
Acknowledging the potential for selection bias with an OLE program, Dr. Laufs said there are two important open questions: “Can the safety data observed for extracellular PCSK9 inhibition using an antibody be transferred to other mechanisms of PCSK9 inhibition? And obviously, from the perspective of patient care, how can we implement these important data into patient care and improve access to PCSK9 inhibitors?”
With regard to the latter point, he said physicians should be cautious in using the term “plaque regression,” opting instead for prevention and stabilization of atherosclerosis, and when using the term “legacy,” which may be misinterpreted by patients to imply there was cessation of therapy.
“From my perspective, [what] the open-label extension really shows is that earlier treatment is better,” Dr. Laufs said. “This should be our message.”
In a press conference prior to the presentation, ESC commentator Johann Bauersachs, MD, Hannover (Germany) Medical School, said “this is extremely important data because it confirms that it’s safe, and the criticism of the FOURIER study that mortality, cardiovascular mortality, was not reduced is now also reduced.”
Dr. Bauersachs said it would have been unethical to wait 7 years for a placebo-controlled trial and questioned whether data are available and suggestive of a legacy effect among patients who did not participate in the open-label extension.
Dr. O’Donoghue said unfortunately those data aren’t available but that Kaplan-Meier curves for the primary endpoint in the parent trial continued to diverge over time and that there was somewhat of a lag in terms of that divergence. “So, a median follow-up of 2 years may have been insufficient, especially for the emerging cardiovascular mortality that took longer to appear.”
The study was funded by Amgen. Dr. O’Donoghue reported receiving research grants from Amgen, AstraZeneca, Janssen, Intarcia, and Novartis, and consulting fees from Amgen, Novartis, AstraZeneca, and Janssen. Dr. Laufs reported receiving honoraria/reimbursement for lecture, study participation, and scientific cooperation with Saarland or Leipzig University, as well as relationships with multiple pharmaceutical and device makers.
A version of this article first appeared on Medscape.com.
Long-term lipid lowering with evolocumab (Repatha) further reduces cardiovascular events, including CV death, without a safety signal, according to results from the FOURIER open-label extension (OLE) study.
In the parent FOURIER trial, treatment with the proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor over a median of 2.2 years reduced the primary efficacy endpoint by 15% but showed no CV mortality signal, compared with placebo, in patients with atherosclerotic disease on background statin therapy.
Now with follow-up out to 8.4 years – the longest to date in any PCSK9 study – cardiovascular mortality was cut by 23% in patients who remained on evolocumab, compared with those originally assigned to placebo (3.32% vs. 4.45%; hazard ratio, 0.77; 95% confidence interval, 0.60-0.99).
The Kaplan-Meier curves during FOURIER were “essentially superimposed and it was not until the open-label extension period had begun with longer-term follow up that the benefit in terms of cardiovascular mortality reduction became apparent,” said principal investigator Michelle O’Donoghue, MD, MPH, of Brigham and Women’s Hospital, Boston.
The results were reported at the annual congress of the European Society of Cardiology and published simultaneously in Circulation.
Pivotal statin trials have median follow-up times of 4-5 years and demonstrated both a lag effect, meaning clinical benefit grew over time, and a legacy effect, where clinical benefit persisted in extended follow-up after the parent study, Dr. O’Donoghue observed.
With shorter follow-up in the parent FOURIER trial, there was evidence of a lag effect with the risk reduction in CV death, MI, and stroke increasing from 16% in the first year to 25% over time with evolocumab.
FOURIER-OLE enrolled 6,635 patients (3355 randomly assigned to evolocumab and 3280 to placebo), who completed the parent study and self-injected evolocumab subcutaneously with the choice of 140 mg every 2 weeks or 420 mg monthly. Study visits were at week 12 and then every 24 weeks. Median follow-up was 5 years.
Their mean age was 62 years, three-fourths were men, a third had diabetes. Three-fourths were on a high-intensity statin at the time of enrollment in FOURIER, and median LDL cholesterol at randomization was 91 mg/dL (2.4 mmol/L).
At week 12, the median LDL cholesterol was 30 mg/dL (0.78 mmol/L), and this was sustained throughout follow-up, Dr. O’Donoghue reported. Most patients achieved very low LDL cholesterol levels, with 63.2% achieving levels less than 40 mg/dL (1.04 mmol/L) and 26.6% less than 20 mg/dL (0.52 mmol/L).
Patients randomly assigned in the parent trial to evolocumab versus placebo had a 15% lower risk of the primary outcome of CV death, MI, stroke, hospitalization for unstable angina, or coronary revascularization (15.4% vs. 17.5%; HR, 0.85; 95% CI, 0.75-0.96).
Their risk of CV death, MI, or stroke was 20% lower (9.7% vs. 11.9%; HR, 0.80; 95% CI, 0.68-0.93), and, as noted previously, 23% lower for CV death.
When major adverse cardiovascular events data were parsed out by year, the largest LDL cholesterol reduction was in years 1 and 2 of the parent study (delta, 62 mg/dL between treatment arms), “highlighting that lag of benefit that continued to accrue with time,” Dr. O’Donoghue said.
“There was then carryover into the extension period, such that there was legacy effect from the LDL [cholesterol] delta that was seen during the parent study,” she said. “This benefit was most apparent early on during open-label extension and then, as one might expect when all patients were being treated with the same therapy, it began to attenuate somewhat with time.”
Although early studies raised concerns that very low LDL cholesterol may be associated with an increased risk of hemorrhagic stroke and neurocognitive effects, the frequency of adverse events did not increase over time with evolocumab exposure.
Annualized incidence rates for patients initially randomized to evolocumab did not exceed those for placebo-treated patients for any of the following events of interest: serious safety events (10% vs. 13%), hemorrhagic stroke (0.04% vs. 0.05%), new-onset diabetes (1.2% vs. 2.3%), muscle-related events (1.2% vs. 1.9%), injection-site reactions (0.4% vs. 0.7%), and drug-related allergic reactions (0.6% vs. 1.1%).
“Long-term use of evolocumab with a median follow-up of more than 7 years appears both safe and well tolerated,” Dr. O’Donoghue said.
Taken together with the continued accrual of cardiovascular benefit, including CV mortality, “these findings argue for early initiation of a marked and sustained LDL cholesterol reduction to maximize benefit,” she concluded.
Translating the benefits
Ulrich Laufs, MD, Leipzig (Germany) University Hospital, Germany, and invited commentator for the session, said the trial addresses two key issues: the long-term safety of low LDL cholesterol lowering and the long-term safety of inhibiting PCSK9, which is highly expressed not only in the liver but also in the brain, small intestine, and kidneys. Indeed, an LDL cholesterol level below 30 mg/dL is lower than the ESC treatment recommendation for very-high-risk patients and is, in fact, lower than most assays are reliable to interpret.
“So it is very important that we have these very clear data showing us that there were no adverse events, also including cataracts and hemorrhagic stroke, and these were on the level of placebo and did not increase over time,” he said.
The question of efficacy is triggered by observations of another PCSK9, the humanized monoclonal antibody bococizumab, which was associated in the SPIRE trial with an increase in LDL cholesterol over time because of neutralizing antibodies. Reassuringly, there was “completely sustained LDL [cholesterol] reduction” with no neutralizing antibodies with the fully human antibody evolocumab in FOURIER-OLE and in recent data from the OSLER-1 study, Dr. Laufs observed.
Acknowledging the potential for selection bias with an OLE program, Dr. Laufs said there are two important open questions: “Can the safety data observed for extracellular PCSK9 inhibition using an antibody be transferred to other mechanisms of PCSK9 inhibition? And obviously, from the perspective of patient care, how can we implement these important data into patient care and improve access to PCSK9 inhibitors?”
With regard to the latter point, he said physicians should be cautious in using the term “plaque regression,” opting instead for prevention and stabilization of atherosclerosis, and when using the term “legacy,” which may be misinterpreted by patients to imply there was cessation of therapy.
“From my perspective, [what] the open-label extension really shows is that earlier treatment is better,” Dr. Laufs said. “This should be our message.”
In a press conference prior to the presentation, ESC commentator Johann Bauersachs, MD, Hannover (Germany) Medical School, said “this is extremely important data because it confirms that it’s safe, and the criticism of the FOURIER study that mortality, cardiovascular mortality, was not reduced is now also reduced.”
Dr. Bauersachs said it would have been unethical to wait 7 years for a placebo-controlled trial and questioned whether data are available and suggestive of a legacy effect among patients who did not participate in the open-label extension.
Dr. O’Donoghue said unfortunately those data aren’t available but that Kaplan-Meier curves for the primary endpoint in the parent trial continued to diverge over time and that there was somewhat of a lag in terms of that divergence. “So, a median follow-up of 2 years may have been insufficient, especially for the emerging cardiovascular mortality that took longer to appear.”
The study was funded by Amgen. Dr. O’Donoghue reported receiving research grants from Amgen, AstraZeneca, Janssen, Intarcia, and Novartis, and consulting fees from Amgen, Novartis, AstraZeneca, and Janssen. Dr. Laufs reported receiving honoraria/reimbursement for lecture, study participation, and scientific cooperation with Saarland or Leipzig University, as well as relationships with multiple pharmaceutical and device makers.
A version of this article first appeared on Medscape.com.
FROM ESC CONGRESS 2022
Mother’s fat metabolism in early pregnancy linked to baby’s weight
A baby’s weight and neurodevelopment in the first 2 years of life could be influenced by maternal fat metabolism in the early stages of pregnancy, according to a study.
Patterns of fetal abdominal growth were associated with maternal lipid metabolites that tracked newborn growth, adiposity, and development into childhood and could be identified as early as the 5th month of pregnancy, according to researchers at the University of Oxford, working with colleagues at the University of California.
These fetal growth patterns were also associated with blood flow and nutrient transfer by the placenta, demonstrating a complex interaction between maternal and fetal nutrition early in pregnancy, with implications for postnatal weight and health in later life, they suggested.
Stephen Kennedy, MD, professor of reproductive medicine at the University of Oxford, who co-led the investigation, said it had “provided valuable new insights into the biological origins of childhood obesity, which is one of the most pressing public health issues facing governments around the world.”
International study
The prospective observational study, published in The Lancet Diabetes and Endocrinology, involved 3,598 pregnant women from six countries – Brazil, Kenya, Pakistan, South Africa, Thailand, and the United Kingdom – aged 18 and older and with a BMI of less than 35 kg/m2. The women were monitored using regular fetal ultrasound scans and metabolomic analysis of early pregnancy maternal blood and umbilical cord venous blood at the time of birth.
Their infants, who were singletons, and conceived naturally, were then followed for 2 years to assess their growth and development.
Fetal abdominal circumference growth was found to accelerate or decelerate within “a crucial 20-25 week gestational age window” that followed 4 trajectories of faltering growth, early accelerating growth, late accelerating growth, or median growth. These traits were matched by fetus-placenta blood flow patterns throughout pregnancy and different growth, adiposity, vision, and neurodevelopment outcomes in early childhood, researchers said.
Overall, 709 maternal metabolites had a positive effect for the faltering growth phenotype, and 54 for the early accelerating growth phenotype, whilst 31 had a negative effect for the faltering growth phenotype and 76 for the early accelerating growth phenotype.
The maternal metabolite signatures included 5-hydroxy-eicosatetraenoic acid and 11 phosphatidylcholines linked to oxylipin or saturated fatty acid sidechains. The fungicide, chlorothalonil, was “highly abundant” in the early accelerating growth phenotype group.
‘A unique insight’
Aris Papageorghiou, professor of Fetal Medicine at the University of Oxford, who co-led the research, said: “This study provides evidence of distinct patterns of fetal abdominal growth and placental transfer and how they relate to longer term health. The finding of an association with maternal lipid metabolism early in pregnancy also provides unique insights into how the mother’s health and diet influence her child’s adiposity.”
First author José Villar, MD, professor of perinatal medicine at Oxford, said: “The study complements our previous work that identified fetal head growth trajectories associated with different developmental, behavioral, visual, and growth outcomes at 2 years of age.” Taken together, “the growth of babies’ bodies and brain[s] track separately and early – while still within the womb,” he said.
According to Dr. Kennedy, the latest results “could contribute to earlier identification of infants at risk of obesity” and urged policymakers to “take notice of these findings in their efforts to prevent the oncoming epidemic of obesity, with all its likely adverse social and economic consequences.”
Funding for the study was provided by the Bill and Melinda Gates Foundation.
A version of this article first appeared on Medscape UK.
A baby’s weight and neurodevelopment in the first 2 years of life could be influenced by maternal fat metabolism in the early stages of pregnancy, according to a study.
Patterns of fetal abdominal growth were associated with maternal lipid metabolites that tracked newborn growth, adiposity, and development into childhood and could be identified as early as the 5th month of pregnancy, according to researchers at the University of Oxford, working with colleagues at the University of California.
These fetal growth patterns were also associated with blood flow and nutrient transfer by the placenta, demonstrating a complex interaction between maternal and fetal nutrition early in pregnancy, with implications for postnatal weight and health in later life, they suggested.
Stephen Kennedy, MD, professor of reproductive medicine at the University of Oxford, who co-led the investigation, said it had “provided valuable new insights into the biological origins of childhood obesity, which is one of the most pressing public health issues facing governments around the world.”
International study
The prospective observational study, published in The Lancet Diabetes and Endocrinology, involved 3,598 pregnant women from six countries – Brazil, Kenya, Pakistan, South Africa, Thailand, and the United Kingdom – aged 18 and older and with a BMI of less than 35 kg/m2. The women were monitored using regular fetal ultrasound scans and metabolomic analysis of early pregnancy maternal blood and umbilical cord venous blood at the time of birth.
Their infants, who were singletons, and conceived naturally, were then followed for 2 years to assess their growth and development.
Fetal abdominal circumference growth was found to accelerate or decelerate within “a crucial 20-25 week gestational age window” that followed 4 trajectories of faltering growth, early accelerating growth, late accelerating growth, or median growth. These traits were matched by fetus-placenta blood flow patterns throughout pregnancy and different growth, adiposity, vision, and neurodevelopment outcomes in early childhood, researchers said.
Overall, 709 maternal metabolites had a positive effect for the faltering growth phenotype, and 54 for the early accelerating growth phenotype, whilst 31 had a negative effect for the faltering growth phenotype and 76 for the early accelerating growth phenotype.
The maternal metabolite signatures included 5-hydroxy-eicosatetraenoic acid and 11 phosphatidylcholines linked to oxylipin or saturated fatty acid sidechains. The fungicide, chlorothalonil, was “highly abundant” in the early accelerating growth phenotype group.
‘A unique insight’
Aris Papageorghiou, professor of Fetal Medicine at the University of Oxford, who co-led the research, said: “This study provides evidence of distinct patterns of fetal abdominal growth and placental transfer and how they relate to longer term health. The finding of an association with maternal lipid metabolism early in pregnancy also provides unique insights into how the mother’s health and diet influence her child’s adiposity.”
First author José Villar, MD, professor of perinatal medicine at Oxford, said: “The study complements our previous work that identified fetal head growth trajectories associated with different developmental, behavioral, visual, and growth outcomes at 2 years of age.” Taken together, “the growth of babies’ bodies and brain[s] track separately and early – while still within the womb,” he said.
According to Dr. Kennedy, the latest results “could contribute to earlier identification of infants at risk of obesity” and urged policymakers to “take notice of these findings in their efforts to prevent the oncoming epidemic of obesity, with all its likely adverse social and economic consequences.”
Funding for the study was provided by the Bill and Melinda Gates Foundation.
A version of this article first appeared on Medscape UK.
A baby’s weight and neurodevelopment in the first 2 years of life could be influenced by maternal fat metabolism in the early stages of pregnancy, according to a study.
Patterns of fetal abdominal growth were associated with maternal lipid metabolites that tracked newborn growth, adiposity, and development into childhood and could be identified as early as the 5th month of pregnancy, according to researchers at the University of Oxford, working with colleagues at the University of California.
These fetal growth patterns were also associated with blood flow and nutrient transfer by the placenta, demonstrating a complex interaction between maternal and fetal nutrition early in pregnancy, with implications for postnatal weight and health in later life, they suggested.
Stephen Kennedy, MD, professor of reproductive medicine at the University of Oxford, who co-led the investigation, said it had “provided valuable new insights into the biological origins of childhood obesity, which is one of the most pressing public health issues facing governments around the world.”
International study
The prospective observational study, published in The Lancet Diabetes and Endocrinology, involved 3,598 pregnant women from six countries – Brazil, Kenya, Pakistan, South Africa, Thailand, and the United Kingdom – aged 18 and older and with a BMI of less than 35 kg/m2. The women were monitored using regular fetal ultrasound scans and metabolomic analysis of early pregnancy maternal blood and umbilical cord venous blood at the time of birth.
Their infants, who were singletons, and conceived naturally, were then followed for 2 years to assess their growth and development.
Fetal abdominal circumference growth was found to accelerate or decelerate within “a crucial 20-25 week gestational age window” that followed 4 trajectories of faltering growth, early accelerating growth, late accelerating growth, or median growth. These traits were matched by fetus-placenta blood flow patterns throughout pregnancy and different growth, adiposity, vision, and neurodevelopment outcomes in early childhood, researchers said.
Overall, 709 maternal metabolites had a positive effect for the faltering growth phenotype, and 54 for the early accelerating growth phenotype, whilst 31 had a negative effect for the faltering growth phenotype and 76 for the early accelerating growth phenotype.
The maternal metabolite signatures included 5-hydroxy-eicosatetraenoic acid and 11 phosphatidylcholines linked to oxylipin or saturated fatty acid sidechains. The fungicide, chlorothalonil, was “highly abundant” in the early accelerating growth phenotype group.
‘A unique insight’
Aris Papageorghiou, professor of Fetal Medicine at the University of Oxford, who co-led the research, said: “This study provides evidence of distinct patterns of fetal abdominal growth and placental transfer and how they relate to longer term health. The finding of an association with maternal lipid metabolism early in pregnancy also provides unique insights into how the mother’s health and diet influence her child’s adiposity.”
First author José Villar, MD, professor of perinatal medicine at Oxford, said: “The study complements our previous work that identified fetal head growth trajectories associated with different developmental, behavioral, visual, and growth outcomes at 2 years of age.” Taken together, “the growth of babies’ bodies and brain[s] track separately and early – while still within the womb,” he said.
According to Dr. Kennedy, the latest results “could contribute to earlier identification of infants at risk of obesity” and urged policymakers to “take notice of these findings in their efforts to prevent the oncoming epidemic of obesity, with all its likely adverse social and economic consequences.”
Funding for the study was provided by the Bill and Melinda Gates Foundation.
A version of this article first appeared on Medscape UK.
ARBs, beta-blockers independently inhibit Marfan syndrome progression
Early start might delay surgery
Beta-blockers have long been recommended to prevent aortic dissection associated with Marfan syndrome despite limited evidence, but a new analysis also supports a benefit from angiotensin receptors blockers (ARBs) and further suggests that beta-blockers and ARBs exert independent effects.
For the endpoint of inhibition of growth of the aortic root, “there is no evidence of any interaction between the effects of ARBs with beta-blockers, and so we think that the treatment effects are likely to be additive,” reported Alex Pitcher, BMBCh, DPhil, Oxford (England) University Hospitals, NHS Trust.
Based on these data, Dr. Pitcher recommended considering ARBs and beta-blockers together soon after the diagnosis of Marfan syndrome. This includes young children.
“We think that medical treatments can delay surgery and dissection substantially if given for a number of years,” he added.
In this study, undertaken by the Marfan Treatment Trialists (MTT) collaboration, data were available from 1,442 Marfan syndrome patients participating in seven treatment trials. The primary outcome was aortic root enlargement, a predictor of life-threatening aortic dissection and rupture. Rather than a meta-analysis of the pooled data, the meta-analysis was conducted with individual patient data that involved collaboration with the original trialists.
Four of the studies with 746 patients compared ARBs to placebo or a control medication. A second group of three trials with 766 patients compared ARBs to beta-blockers.
From the two sets of data, a calculation of the effect of beta-blockers was indirectly estimated.
ARBs slow annualized aortic growth rate significantly
In the first set of trials, the analysis showed a significantly slower annualized aortic root growth rate for those treated with ARBs relative to controls (0.07 vs. 0.13), producing a statistically significant absolute difference (0.7%; P = .01) in favor of the ARB.
“In other words, the rate of growth was nearly double in the control arm,” Dr. Pitcher said.
In the three trials comparing ARBs to beta-blockers, the annualized growth rate among those taking an ARB was similar (0.8%) to that seen in the previous set of controlled trials. This rate of annualized growth was not significantly different from the 0.11% annualized rate of growth in patients receiving beta-blockers. When an analysis of the impact of beta-blockers was conducted by indirectly evaluating the change in growth relative to controls, the estimated impact was an annualized growth rate of 0.9% (P = .042).
A second set of data provided the basis for suggesting that the effects of beta ARBs and beta-blockers are independent and potentially additive.
“We were able to look at subgroups of patients in the ARB trials that were broken down by whether they were or were not on beta-blockers at baseline, and so by doing able to estimate independent effects,” Dr. Pitcher said. The lack of any interactions led Dr. Pitcher to conclude that benefits are likely additive.
Of patients genotyped in the ARB studies, more than 80% had the FBN1 pathogenic variant of Marfan syndrome. When the data were analyzed by subgroups, including age or blood pressure, there were no differences in treatment effect except for those with the FBN1 mutation in whom the benefit of ARB therapy was greater relative to those without.
As FBN1 is one of the most common genetic signatures of Marfan syndrome, the “greater effect of ARBs in this group makes it more plausible that the effect is real,” Dr. Pitcher said.
Results could change treatment guidelines
Current guidelines recommend beta-blockers in Marfan syndrome prior to a dilatation size of 4.5 to 5 cm when surgery is indicated, according to Dr. Pitcher, but he said these data might change guidelines. While reinforcing the benefit of beta-blockers, this analysis suggests ARBs should also be considered, possibly in combination with beta-blockers.
“What I hope this meta-analysis does is add substantially to the certainty with which physicians can discuss treatments with patients.”
As for the mechanism, it is reasonable to speculate the antihypertensive effect of both medications is relevant, but each has plausible independent activities that might contribute to modifying aortic growth, according to Roland R.J. van Kimmenade, MD, PhD, a specialist in aortic diseases and heart failure at Raboud University Medical Center, Nijmegan, the Netherlands.
Citing several studies, he suggested that the benefit of beta-blockers could also stem from their ability to reduce heart rate and aortic stiffness while ARBs are likely to inhibit the interaction between the renin-angiotensin system (RAS) and TGF-beta pathway. Each of these might participate in risk of aortic root growth, according to Dr. van Kimmenade, who was invited by ESC to discuss this study.
On the basis of these data as well as past studies, he agreed that the combination of beta-blockers and ARBs might not just be additive but “even a little bit synergistic.”
While Dr. Pitcher suggested that the evidence supports starting both beta-blockers and ARBs soon after the diagnosis, Dr. van Kimmenade said, “I don’t like using beta-blockers in young patients, but ARBs are now shown to be an excellent alternative.”
Ultimately, “the prescription pencil will not replace the surgical knife” in a disease that is likely to eventually require surgery to prevent life-threatening events, according to Dr. van Kimmenade, but he agreed that these data provide more certainty about the value of beta-blockers and ARBs for slowing progression.
Dr. Pitcher reports no potential conflicts of interest. Dr. van Kimmenade has financial relationships with Bayer and Novartis.
Early start might delay surgery
Early start might delay surgery
Beta-blockers have long been recommended to prevent aortic dissection associated with Marfan syndrome despite limited evidence, but a new analysis also supports a benefit from angiotensin receptors blockers (ARBs) and further suggests that beta-blockers and ARBs exert independent effects.
For the endpoint of inhibition of growth of the aortic root, “there is no evidence of any interaction between the effects of ARBs with beta-blockers, and so we think that the treatment effects are likely to be additive,” reported Alex Pitcher, BMBCh, DPhil, Oxford (England) University Hospitals, NHS Trust.
Based on these data, Dr. Pitcher recommended considering ARBs and beta-blockers together soon after the diagnosis of Marfan syndrome. This includes young children.
“We think that medical treatments can delay surgery and dissection substantially if given for a number of years,” he added.
In this study, undertaken by the Marfan Treatment Trialists (MTT) collaboration, data were available from 1,442 Marfan syndrome patients participating in seven treatment trials. The primary outcome was aortic root enlargement, a predictor of life-threatening aortic dissection and rupture. Rather than a meta-analysis of the pooled data, the meta-analysis was conducted with individual patient data that involved collaboration with the original trialists.
Four of the studies with 746 patients compared ARBs to placebo or a control medication. A second group of three trials with 766 patients compared ARBs to beta-blockers.
From the two sets of data, a calculation of the effect of beta-blockers was indirectly estimated.
ARBs slow annualized aortic growth rate significantly
In the first set of trials, the analysis showed a significantly slower annualized aortic root growth rate for those treated with ARBs relative to controls (0.07 vs. 0.13), producing a statistically significant absolute difference (0.7%; P = .01) in favor of the ARB.
“In other words, the rate of growth was nearly double in the control arm,” Dr. Pitcher said.
In the three trials comparing ARBs to beta-blockers, the annualized growth rate among those taking an ARB was similar (0.8%) to that seen in the previous set of controlled trials. This rate of annualized growth was not significantly different from the 0.11% annualized rate of growth in patients receiving beta-blockers. When an analysis of the impact of beta-blockers was conducted by indirectly evaluating the change in growth relative to controls, the estimated impact was an annualized growth rate of 0.9% (P = .042).
A second set of data provided the basis for suggesting that the effects of beta ARBs and beta-blockers are independent and potentially additive.
“We were able to look at subgroups of patients in the ARB trials that were broken down by whether they were or were not on beta-blockers at baseline, and so by doing able to estimate independent effects,” Dr. Pitcher said. The lack of any interactions led Dr. Pitcher to conclude that benefits are likely additive.
Of patients genotyped in the ARB studies, more than 80% had the FBN1 pathogenic variant of Marfan syndrome. When the data were analyzed by subgroups, including age or blood pressure, there were no differences in treatment effect except for those with the FBN1 mutation in whom the benefit of ARB therapy was greater relative to those without.
As FBN1 is one of the most common genetic signatures of Marfan syndrome, the “greater effect of ARBs in this group makes it more plausible that the effect is real,” Dr. Pitcher said.
Results could change treatment guidelines
Current guidelines recommend beta-blockers in Marfan syndrome prior to a dilatation size of 4.5 to 5 cm when surgery is indicated, according to Dr. Pitcher, but he said these data might change guidelines. While reinforcing the benefit of beta-blockers, this analysis suggests ARBs should also be considered, possibly in combination with beta-blockers.
“What I hope this meta-analysis does is add substantially to the certainty with which physicians can discuss treatments with patients.”
As for the mechanism, it is reasonable to speculate the antihypertensive effect of both medications is relevant, but each has plausible independent activities that might contribute to modifying aortic growth, according to Roland R.J. van Kimmenade, MD, PhD, a specialist in aortic diseases and heart failure at Raboud University Medical Center, Nijmegan, the Netherlands.
Citing several studies, he suggested that the benefit of beta-blockers could also stem from their ability to reduce heart rate and aortic stiffness while ARBs are likely to inhibit the interaction between the renin-angiotensin system (RAS) and TGF-beta pathway. Each of these might participate in risk of aortic root growth, according to Dr. van Kimmenade, who was invited by ESC to discuss this study.
On the basis of these data as well as past studies, he agreed that the combination of beta-blockers and ARBs might not just be additive but “even a little bit synergistic.”
While Dr. Pitcher suggested that the evidence supports starting both beta-blockers and ARBs soon after the diagnosis, Dr. van Kimmenade said, “I don’t like using beta-blockers in young patients, but ARBs are now shown to be an excellent alternative.”
Ultimately, “the prescription pencil will not replace the surgical knife” in a disease that is likely to eventually require surgery to prevent life-threatening events, according to Dr. van Kimmenade, but he agreed that these data provide more certainty about the value of beta-blockers and ARBs for slowing progression.
Dr. Pitcher reports no potential conflicts of interest. Dr. van Kimmenade has financial relationships with Bayer and Novartis.
Beta-blockers have long been recommended to prevent aortic dissection associated with Marfan syndrome despite limited evidence, but a new analysis also supports a benefit from angiotensin receptors blockers (ARBs) and further suggests that beta-blockers and ARBs exert independent effects.
For the endpoint of inhibition of growth of the aortic root, “there is no evidence of any interaction between the effects of ARBs with beta-blockers, and so we think that the treatment effects are likely to be additive,” reported Alex Pitcher, BMBCh, DPhil, Oxford (England) University Hospitals, NHS Trust.
Based on these data, Dr. Pitcher recommended considering ARBs and beta-blockers together soon after the diagnosis of Marfan syndrome. This includes young children.
“We think that medical treatments can delay surgery and dissection substantially if given for a number of years,” he added.
In this study, undertaken by the Marfan Treatment Trialists (MTT) collaboration, data were available from 1,442 Marfan syndrome patients participating in seven treatment trials. The primary outcome was aortic root enlargement, a predictor of life-threatening aortic dissection and rupture. Rather than a meta-analysis of the pooled data, the meta-analysis was conducted with individual patient data that involved collaboration with the original trialists.
Four of the studies with 746 patients compared ARBs to placebo or a control medication. A second group of three trials with 766 patients compared ARBs to beta-blockers.
From the two sets of data, a calculation of the effect of beta-blockers was indirectly estimated.
ARBs slow annualized aortic growth rate significantly
In the first set of trials, the analysis showed a significantly slower annualized aortic root growth rate for those treated with ARBs relative to controls (0.07 vs. 0.13), producing a statistically significant absolute difference (0.7%; P = .01) in favor of the ARB.
“In other words, the rate of growth was nearly double in the control arm,” Dr. Pitcher said.
In the three trials comparing ARBs to beta-blockers, the annualized growth rate among those taking an ARB was similar (0.8%) to that seen in the previous set of controlled trials. This rate of annualized growth was not significantly different from the 0.11% annualized rate of growth in patients receiving beta-blockers. When an analysis of the impact of beta-blockers was conducted by indirectly evaluating the change in growth relative to controls, the estimated impact was an annualized growth rate of 0.9% (P = .042).
A second set of data provided the basis for suggesting that the effects of beta ARBs and beta-blockers are independent and potentially additive.
“We were able to look at subgroups of patients in the ARB trials that were broken down by whether they were or were not on beta-blockers at baseline, and so by doing able to estimate independent effects,” Dr. Pitcher said. The lack of any interactions led Dr. Pitcher to conclude that benefits are likely additive.
Of patients genotyped in the ARB studies, more than 80% had the FBN1 pathogenic variant of Marfan syndrome. When the data were analyzed by subgroups, including age or blood pressure, there were no differences in treatment effect except for those with the FBN1 mutation in whom the benefit of ARB therapy was greater relative to those without.
As FBN1 is one of the most common genetic signatures of Marfan syndrome, the “greater effect of ARBs in this group makes it more plausible that the effect is real,” Dr. Pitcher said.
Results could change treatment guidelines
Current guidelines recommend beta-blockers in Marfan syndrome prior to a dilatation size of 4.5 to 5 cm when surgery is indicated, according to Dr. Pitcher, but he said these data might change guidelines. While reinforcing the benefit of beta-blockers, this analysis suggests ARBs should also be considered, possibly in combination with beta-blockers.
“What I hope this meta-analysis does is add substantially to the certainty with which physicians can discuss treatments with patients.”
As for the mechanism, it is reasonable to speculate the antihypertensive effect of both medications is relevant, but each has plausible independent activities that might contribute to modifying aortic growth, according to Roland R.J. van Kimmenade, MD, PhD, a specialist in aortic diseases and heart failure at Raboud University Medical Center, Nijmegan, the Netherlands.
Citing several studies, he suggested that the benefit of beta-blockers could also stem from their ability to reduce heart rate and aortic stiffness while ARBs are likely to inhibit the interaction between the renin-angiotensin system (RAS) and TGF-beta pathway. Each of these might participate in risk of aortic root growth, according to Dr. van Kimmenade, who was invited by ESC to discuss this study.
On the basis of these data as well as past studies, he agreed that the combination of beta-blockers and ARBs might not just be additive but “even a little bit synergistic.”
While Dr. Pitcher suggested that the evidence supports starting both beta-blockers and ARBs soon after the diagnosis, Dr. van Kimmenade said, “I don’t like using beta-blockers in young patients, but ARBs are now shown to be an excellent alternative.”
Ultimately, “the prescription pencil will not replace the surgical knife” in a disease that is likely to eventually require surgery to prevent life-threatening events, according to Dr. van Kimmenade, but he agreed that these data provide more certainty about the value of beta-blockers and ARBs for slowing progression.
Dr. Pitcher reports no potential conflicts of interest. Dr. van Kimmenade has financial relationships with Bayer and Novartis.
FROM ESC CONGRESS 2022
How much do we really know about gender dysphoria?
At the risk of losing a digit or two I am going to dip my toes into the murky waters of gender-affirming care, sometimes referred to as trans care. Recently, Moira Szilagyi, MD, PhD, president of the American Academy of Pediatrics, released two statements, one in the Aug. 22, 2022, Wall Street Journal, the other summarized in the Aug. 25, 2022, AAP Daily Briefing, in which she attempts to clarify the academy’s position on gender-affirming care. They were well-worded and heroic attempts to clear the air. I fear these explanations will do little to encourage informed and courteous discussions between those entrenched on either side of a disagreement that is unfortunately being played out on media outlets and state legislatures instead of the offices of primary care physicians and specialists where it belongs.
The current mess is an example of what can happen when there is a paucity of reliable data, a superabundance of emotion, and a system that feeds on instant news and sound bites with little understanding of how science should work.
Some of the turmoil is a response to the notion that in certain situations gender dysphoria may be a condition that can be learned or mimicked from exposure to other gender-dysphoric individuals. Two papers anchor either side of the debate. The first paper was published in 2018 by a then–Brown University health expert who hypothesized the existence of a condition which she labeled “rapid-onset gender dysphoria [ROGD]”. One can imagine that “social contagion” might be considered as one of the potential contributors to this hypothesized condition. Unfortunately, the publication of the paper ignited a firestorm of criticism from a segment of the population that advocates for the transgender community, prompting the university and the online publisher to backpedal and reevaluate the quality of the research on which the paper was based.
One of the concerns voiced at the time of publication was that the research could be used to support the transphobic agenda by some state legislatures hoping to ban gender-affirming care. How large a role the paper played in the current spate of legislation in is unclear. I suspect it has been small. But, one can’t deny the potential exists.
Leaping forward to 2022, the second paper was published in the August issue of Pediatrics, in which the authors attempted to test the ROGD hypothesis and question the inference of social contagion.
The investigators found that in 2017 and 2019 the birth ratios of transgender-diverse (TGD) individuals did not favor assigned female-sex-at-birth (AFAB) individuals. They also discovered that in their sample overall there was a decrease in the percentage of adolescents who self-identified as TGD. Not surprisingly, “bullying victimization and suicidality were higher among TGD youth when compared with their cisgender peers.” The authors concluded that their findings were “incongruent with an ROGD hypothesis that posits social contagion” nor should it be used to restrict access to gender-affirming care.
There you have it. Are we any closer to understanding gender dysphoria and its origins? I don’t think so. The media is somewhat less confused. The NBC News online presence headline on Aug. 3, 2022, reads “‘Social contagion’ isn’t causing more youths to be transgender, study finds.”
My sense is that the general population perceives an increase in the prevalence of gender dysphoria. It is very likely that this perception is primarily a reflection of a more compassionate and educated attitude in a significant portion of the population making it less challenging for gender-dysphoric youth to surface. However, it should not surprise us that some parents and observers are concerned that a percentage of this increased prevalence is the result of social contagion. Nor should it surprise us that some advocates for the trans population feel threatened by this hypothesis.
Neither of these studies really answers the question of whether some cases of gender dysphoria are the result of social contagion. Both were small samples using methodology that has been called into question. The bottom line is that we need more studies and must remain open to considering their results. That’s how science should work.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
At the risk of losing a digit or two I am going to dip my toes into the murky waters of gender-affirming care, sometimes referred to as trans care. Recently, Moira Szilagyi, MD, PhD, president of the American Academy of Pediatrics, released two statements, one in the Aug. 22, 2022, Wall Street Journal, the other summarized in the Aug. 25, 2022, AAP Daily Briefing, in which she attempts to clarify the academy’s position on gender-affirming care. They were well-worded and heroic attempts to clear the air. I fear these explanations will do little to encourage informed and courteous discussions between those entrenched on either side of a disagreement that is unfortunately being played out on media outlets and state legislatures instead of the offices of primary care physicians and specialists where it belongs.
The current mess is an example of what can happen when there is a paucity of reliable data, a superabundance of emotion, and a system that feeds on instant news and sound bites with little understanding of how science should work.
Some of the turmoil is a response to the notion that in certain situations gender dysphoria may be a condition that can be learned or mimicked from exposure to other gender-dysphoric individuals. Two papers anchor either side of the debate. The first paper was published in 2018 by a then–Brown University health expert who hypothesized the existence of a condition which she labeled “rapid-onset gender dysphoria [ROGD]”. One can imagine that “social contagion” might be considered as one of the potential contributors to this hypothesized condition. Unfortunately, the publication of the paper ignited a firestorm of criticism from a segment of the population that advocates for the transgender community, prompting the university and the online publisher to backpedal and reevaluate the quality of the research on which the paper was based.
One of the concerns voiced at the time of publication was that the research could be used to support the transphobic agenda by some state legislatures hoping to ban gender-affirming care. How large a role the paper played in the current spate of legislation in is unclear. I suspect it has been small. But, one can’t deny the potential exists.
Leaping forward to 2022, the second paper was published in the August issue of Pediatrics, in which the authors attempted to test the ROGD hypothesis and question the inference of social contagion.
The investigators found that in 2017 and 2019 the birth ratios of transgender-diverse (TGD) individuals did not favor assigned female-sex-at-birth (AFAB) individuals. They also discovered that in their sample overall there was a decrease in the percentage of adolescents who self-identified as TGD. Not surprisingly, “bullying victimization and suicidality were higher among TGD youth when compared with their cisgender peers.” The authors concluded that their findings were “incongruent with an ROGD hypothesis that posits social contagion” nor should it be used to restrict access to gender-affirming care.
There you have it. Are we any closer to understanding gender dysphoria and its origins? I don’t think so. The media is somewhat less confused. The NBC News online presence headline on Aug. 3, 2022, reads “‘Social contagion’ isn’t causing more youths to be transgender, study finds.”
My sense is that the general population perceives an increase in the prevalence of gender dysphoria. It is very likely that this perception is primarily a reflection of a more compassionate and educated attitude in a significant portion of the population making it less challenging for gender-dysphoric youth to surface. However, it should not surprise us that some parents and observers are concerned that a percentage of this increased prevalence is the result of social contagion. Nor should it surprise us that some advocates for the trans population feel threatened by this hypothesis.
Neither of these studies really answers the question of whether some cases of gender dysphoria are the result of social contagion. Both were small samples using methodology that has been called into question. The bottom line is that we need more studies and must remain open to considering their results. That’s how science should work.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
At the risk of losing a digit or two I am going to dip my toes into the murky waters of gender-affirming care, sometimes referred to as trans care. Recently, Moira Szilagyi, MD, PhD, president of the American Academy of Pediatrics, released two statements, one in the Aug. 22, 2022, Wall Street Journal, the other summarized in the Aug. 25, 2022, AAP Daily Briefing, in which she attempts to clarify the academy’s position on gender-affirming care. They were well-worded and heroic attempts to clear the air. I fear these explanations will do little to encourage informed and courteous discussions between those entrenched on either side of a disagreement that is unfortunately being played out on media outlets and state legislatures instead of the offices of primary care physicians and specialists where it belongs.
The current mess is an example of what can happen when there is a paucity of reliable data, a superabundance of emotion, and a system that feeds on instant news and sound bites with little understanding of how science should work.
Some of the turmoil is a response to the notion that in certain situations gender dysphoria may be a condition that can be learned or mimicked from exposure to other gender-dysphoric individuals. Two papers anchor either side of the debate. The first paper was published in 2018 by a then–Brown University health expert who hypothesized the existence of a condition which she labeled “rapid-onset gender dysphoria [ROGD]”. One can imagine that “social contagion” might be considered as one of the potential contributors to this hypothesized condition. Unfortunately, the publication of the paper ignited a firestorm of criticism from a segment of the population that advocates for the transgender community, prompting the university and the online publisher to backpedal and reevaluate the quality of the research on which the paper was based.
One of the concerns voiced at the time of publication was that the research could be used to support the transphobic agenda by some state legislatures hoping to ban gender-affirming care. How large a role the paper played in the current spate of legislation in is unclear. I suspect it has been small. But, one can’t deny the potential exists.
Leaping forward to 2022, the second paper was published in the August issue of Pediatrics, in which the authors attempted to test the ROGD hypothesis and question the inference of social contagion.
The investigators found that in 2017 and 2019 the birth ratios of transgender-diverse (TGD) individuals did not favor assigned female-sex-at-birth (AFAB) individuals. They also discovered that in their sample overall there was a decrease in the percentage of adolescents who self-identified as TGD. Not surprisingly, “bullying victimization and suicidality were higher among TGD youth when compared with their cisgender peers.” The authors concluded that their findings were “incongruent with an ROGD hypothesis that posits social contagion” nor should it be used to restrict access to gender-affirming care.
There you have it. Are we any closer to understanding gender dysphoria and its origins? I don’t think so. The media is somewhat less confused. The NBC News online presence headline on Aug. 3, 2022, reads “‘Social contagion’ isn’t causing more youths to be transgender, study finds.”
My sense is that the general population perceives an increase in the prevalence of gender dysphoria. It is very likely that this perception is primarily a reflection of a more compassionate and educated attitude in a significant portion of the population making it less challenging for gender-dysphoric youth to surface. However, it should not surprise us that some parents and observers are concerned that a percentage of this increased prevalence is the result of social contagion. Nor should it surprise us that some advocates for the trans population feel threatened by this hypothesis.
Neither of these studies really answers the question of whether some cases of gender dysphoria are the result of social contagion. Both were small samples using methodology that has been called into question. The bottom line is that we need more studies and must remain open to considering their results. That’s how science should work.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Omega-3 fatty acids and depression: Are they protective?
New research is suggesting that there are “meaningful” associations between higher dietary intake of omega-3 fatty acids and lower risk for depressive episodes.
In addition, consumption of total fatty acids and alpha-linolenic acid was associated with a reduced risk for incident depressive episodes (9% and 29%, respectively).
“Our results showed an important protective effect from the consumption of omega-3,” Maria de Jesus Mendes da Fonseca, University of the State of Rio de Janeiro, and colleagues write.
The findings were published online in Nutrients.
Mixed bag of studies
Epidemiologic evidence suggests that deficient dietary omega-3 intake is a modifiable risk factor for depression and that individuals with low consumption of omega-3 food sources have more depressive symptoms.
However, the results are inconsistent, and few longitudinal studies have addressed this association, the investigators note.
The new analysis included 13,879 adults (aged 39-65 years or older) participating in the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil) from 2008 to 2014.
Data on depressive episodes were obtained with the Clinical Interview Schedule Revised (CIS-R), and food consumption was measured with the Food Frequency Questionnaire (FFQ).
The target dietary components were total polyunsaturated fatty acids (PUFA) and the omega-3 fatty acids: alpha-linolenic acid, eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and docosapentaenoic acid (DPA).
The majority of participants had adequate dietary intake of omega-3 fatty acids, and none was taking omega-3 supplements.
In the fully adjusted model, consumption of fatty acids from the omega-3 family had a protective effect against maintenance of depressive episodes, showing “important associations, although the significance levels are borderline, possibly due to the sample size,” the researchers report.
In regard to onset of depressive episodes, estimates from the fully adjusted model suggest that a higher consumption of omega-3 acids (total and subtypes) is associated with lower risk for depressive episodes – with significant associations for omega-3 and alpha-linolenic acid.
The investigators note that strengths of the study include “its originality, as it is the first to assess associations between maintenance and incidence of depressive episodes and consumption of omega-3, besides the use of data from the ELSA-Brasil Study, with rigorous data collection protocols and reliable and validated instruments, thus guaranteeing the quality of the sample and the data.”
A study limitation, however, was that the ELSA-Brasil sample consists only of public employees, with the potential for a selection bias such as healthy worker phenomenon, the researchers note. Another was the use of the FFQ, which may underestimate daily intake of foods and depends on individual participant recall – all of which could possibly lead to a differential classification bias.
Interpret cautiously
Commenting on the study, David Mischoulon, MD, PhD, professor of psychiatry, Harvard Medical School, and director of the depression clinical and research program at Massachusetts General Hospital, both in Boston, said that data on omega-3s in depression are “very mixed.”
“A lot of the studies don’t necessarily agree with each other. Certainly, in studies that try to seek an association between omega-3 use and depression, it’s always complicated because it can be difficult to control for all variables that could be contributing to the result that you get,” said Dr. Mischoulon, who is also a member of the Anxiety and Depression Association of America and was not involved in the research.
A caveat to the current study was that diet was assessed only at baseline, “so we don’t really know whether there were any substantial dietary changes over time, he noted.
He also cautioned that it is hard to draw any firm conclusions from this type of study.
“In general, in studies with a large sample, which this study has, it’s easier to find statistically significant differences. But you need to ask yourself: Does it really matter? Is it enough to have a clinical impact and make a difference?” Dr. Mischoulon said.
The ELSA-Brasil study was funded by the Brazilian Ministry of Science, Technology, and Innovation and by the Ministry of Health. The investigators have reported no relevant financial relationships. Dr. Mischoulon has received research support from Nordic Naturals and heckel medizintechnik GmbH and honoraria for speaking from the Massachusetts General Hospital Psychiatry Academy. He also works with the MGH Clinical Trials Network and Institute, which has received research funding from multiple pharmaceutical companies and the National Institute of Mental Health.
A version of this article first appeared on Medscape.com.
New research is suggesting that there are “meaningful” associations between higher dietary intake of omega-3 fatty acids and lower risk for depressive episodes.
In addition, consumption of total fatty acids and alpha-linolenic acid was associated with a reduced risk for incident depressive episodes (9% and 29%, respectively).
“Our results showed an important protective effect from the consumption of omega-3,” Maria de Jesus Mendes da Fonseca, University of the State of Rio de Janeiro, and colleagues write.
The findings were published online in Nutrients.
Mixed bag of studies
Epidemiologic evidence suggests that deficient dietary omega-3 intake is a modifiable risk factor for depression and that individuals with low consumption of omega-3 food sources have more depressive symptoms.
However, the results are inconsistent, and few longitudinal studies have addressed this association, the investigators note.
The new analysis included 13,879 adults (aged 39-65 years or older) participating in the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil) from 2008 to 2014.
Data on depressive episodes were obtained with the Clinical Interview Schedule Revised (CIS-R), and food consumption was measured with the Food Frequency Questionnaire (FFQ).
The target dietary components were total polyunsaturated fatty acids (PUFA) and the omega-3 fatty acids: alpha-linolenic acid, eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and docosapentaenoic acid (DPA).
The majority of participants had adequate dietary intake of omega-3 fatty acids, and none was taking omega-3 supplements.
In the fully adjusted model, consumption of fatty acids from the omega-3 family had a protective effect against maintenance of depressive episodes, showing “important associations, although the significance levels are borderline, possibly due to the sample size,” the researchers report.
In regard to onset of depressive episodes, estimates from the fully adjusted model suggest that a higher consumption of omega-3 acids (total and subtypes) is associated with lower risk for depressive episodes – with significant associations for omega-3 and alpha-linolenic acid.
The investigators note that strengths of the study include “its originality, as it is the first to assess associations between maintenance and incidence of depressive episodes and consumption of omega-3, besides the use of data from the ELSA-Brasil Study, with rigorous data collection protocols and reliable and validated instruments, thus guaranteeing the quality of the sample and the data.”
A study limitation, however, was that the ELSA-Brasil sample consists only of public employees, with the potential for a selection bias such as healthy worker phenomenon, the researchers note. Another was the use of the FFQ, which may underestimate daily intake of foods and depends on individual participant recall – all of which could possibly lead to a differential classification bias.
Interpret cautiously
Commenting on the study, David Mischoulon, MD, PhD, professor of psychiatry, Harvard Medical School, and director of the depression clinical and research program at Massachusetts General Hospital, both in Boston, said that data on omega-3s in depression are “very mixed.”
“A lot of the studies don’t necessarily agree with each other. Certainly, in studies that try to seek an association between omega-3 use and depression, it’s always complicated because it can be difficult to control for all variables that could be contributing to the result that you get,” said Dr. Mischoulon, who is also a member of the Anxiety and Depression Association of America and was not involved in the research.
A caveat to the current study was that diet was assessed only at baseline, “so we don’t really know whether there were any substantial dietary changes over time, he noted.
He also cautioned that it is hard to draw any firm conclusions from this type of study.
“In general, in studies with a large sample, which this study has, it’s easier to find statistically significant differences. But you need to ask yourself: Does it really matter? Is it enough to have a clinical impact and make a difference?” Dr. Mischoulon said.
The ELSA-Brasil study was funded by the Brazilian Ministry of Science, Technology, and Innovation and by the Ministry of Health. The investigators have reported no relevant financial relationships. Dr. Mischoulon has received research support from Nordic Naturals and heckel medizintechnik GmbH and honoraria for speaking from the Massachusetts General Hospital Psychiatry Academy. He also works with the MGH Clinical Trials Network and Institute, which has received research funding from multiple pharmaceutical companies and the National Institute of Mental Health.
A version of this article first appeared on Medscape.com.
New research is suggesting that there are “meaningful” associations between higher dietary intake of omega-3 fatty acids and lower risk for depressive episodes.
In addition, consumption of total fatty acids and alpha-linolenic acid was associated with a reduced risk for incident depressive episodes (9% and 29%, respectively).
“Our results showed an important protective effect from the consumption of omega-3,” Maria de Jesus Mendes da Fonseca, University of the State of Rio de Janeiro, and colleagues write.
The findings were published online in Nutrients.
Mixed bag of studies
Epidemiologic evidence suggests that deficient dietary omega-3 intake is a modifiable risk factor for depression and that individuals with low consumption of omega-3 food sources have more depressive symptoms.
However, the results are inconsistent, and few longitudinal studies have addressed this association, the investigators note.
The new analysis included 13,879 adults (aged 39-65 years or older) participating in the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil) from 2008 to 2014.
Data on depressive episodes were obtained with the Clinical Interview Schedule Revised (CIS-R), and food consumption was measured with the Food Frequency Questionnaire (FFQ).
The target dietary components were total polyunsaturated fatty acids (PUFA) and the omega-3 fatty acids: alpha-linolenic acid, eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and docosapentaenoic acid (DPA).
The majority of participants had adequate dietary intake of omega-3 fatty acids, and none was taking omega-3 supplements.
In the fully adjusted model, consumption of fatty acids from the omega-3 family had a protective effect against maintenance of depressive episodes, showing “important associations, although the significance levels are borderline, possibly due to the sample size,” the researchers report.
In regard to onset of depressive episodes, estimates from the fully adjusted model suggest that a higher consumption of omega-3 acids (total and subtypes) is associated with lower risk for depressive episodes – with significant associations for omega-3 and alpha-linolenic acid.
The investigators note that strengths of the study include “its originality, as it is the first to assess associations between maintenance and incidence of depressive episodes and consumption of omega-3, besides the use of data from the ELSA-Brasil Study, with rigorous data collection protocols and reliable and validated instruments, thus guaranteeing the quality of the sample and the data.”
A study limitation, however, was that the ELSA-Brasil sample consists only of public employees, with the potential for a selection bias such as healthy worker phenomenon, the researchers note. Another was the use of the FFQ, which may underestimate daily intake of foods and depends on individual participant recall – all of which could possibly lead to a differential classification bias.
Interpret cautiously
Commenting on the study, David Mischoulon, MD, PhD, professor of psychiatry, Harvard Medical School, and director of the depression clinical and research program at Massachusetts General Hospital, both in Boston, said that data on omega-3s in depression are “very mixed.”
“A lot of the studies don’t necessarily agree with each other. Certainly, in studies that try to seek an association between omega-3 use and depression, it’s always complicated because it can be difficult to control for all variables that could be contributing to the result that you get,” said Dr. Mischoulon, who is also a member of the Anxiety and Depression Association of America and was not involved in the research.
A caveat to the current study was that diet was assessed only at baseline, “so we don’t really know whether there were any substantial dietary changes over time, he noted.
He also cautioned that it is hard to draw any firm conclusions from this type of study.
“In general, in studies with a large sample, which this study has, it’s easier to find statistically significant differences. But you need to ask yourself: Does it really matter? Is it enough to have a clinical impact and make a difference?” Dr. Mischoulon said.
The ELSA-Brasil study was funded by the Brazilian Ministry of Science, Technology, and Innovation and by the Ministry of Health. The investigators have reported no relevant financial relationships. Dr. Mischoulon has received research support from Nordic Naturals and heckel medizintechnik GmbH and honoraria for speaking from the Massachusetts General Hospital Psychiatry Academy. He also works with the MGH Clinical Trials Network and Institute, which has received research funding from multiple pharmaceutical companies and the National Institute of Mental Health.
A version of this article first appeared on Medscape.com.
FROM NUTRIENTS
New ovulatory disorder classifications from FIGO replace 50-year-old system
The first major revision in the systematic description of ovulatory disorders in nearly 50 years has been proposed by a consensus of experts organized by the International Federation of Gynecology and Obstetrics.
“The FIGO HyPO-P system for the classification of ovulatory disorders is submitted for consideration as a worldwide standard,” according to the writing committee, who published their methodology and their proposed applications in the International Journal of Gynecology and Obstetrics.
The classification system was created to replace the much-modified World Health Organization system first described in 1973. Since that time, many modifications have been proposed to accommodate advances in imaging and new information about underlying pathologies, but there has been no subsequent authoritative reference with these modifications or any other newer organizing system.
The new consensus was developed under the aegis of FIGO, but the development group consisted of representatives from national organizations and the major subspecialty societies. Recognized experts in ovulatory disorders and representatives from lay advocacy organizations also participated.
The HyPO-P system is based largely on anatomy. The acronym refers to ovulatory disorders related to the hypothalamus (type I), the pituitary (type II), and the ovary (type III).
Polycystic ovary syndrome (PCOS), one of the most common ovulatory disorders, was given a separate category (type IV) because of its complexity as well as the fact that PCOS is a heterogeneous systemic disorder with manifestations not limited to an impact on ovarian function.
As the first level of classification, three of the four primary categories (I-III) focus attention on the dominant anatomic source of the change in ovulatory function. The original WHO classification system identified as many as seven major groups, but they were based primarily on assays for gonadotropins and estradiol.
The new system “provides a different structure for determining the diagnosis. Blood tests are not a necessary first step,” explained Malcolm G. Munro, MD, clinical professor, department of obstetrics and gynecology, University of California, Los Angeles. Dr. Munro was the first author of the publication.
The classification system “is not as focused on the specific steps for investigation of ovulatory dysfunction as much as it explains how to structure an investigation of the girl or woman with an ovulatory disorder and then how to characterize the underlying cause,” Dr. Munro said in an interview. “It is designed to allow everyone, whether clinicians, researchers, or patients, to speak the same language.”
New system employs four categories
The four primary categories provide just the first level of classification. The next step is encapsulated in the GAIN-FIT-PIE acronym, which frames the presumed or documented categories of etiologies for the primary categories. GAIN stands for genetic, autoimmune, iatrogenic, or neoplasm etiologies. FIT stands for functional, infectious/inflammatory, or trauma and vascular etiologies. PIE stands for physiological, idiopathic, and endocrine etiologies.
By this methodology, a patient with irregular menses, galactorrhea, and elevated prolactin and an MRI showing a pituitary tumor would be identified a type 2-N, signifying pituitary (type 2) involvement with a neoplasm (N).
A third level of classification permits specific diagnostic entities to be named, allowing the patient in the example above to receive a diagnosis of a prolactin-secreting adenoma.
Not all etiologies can be identified with current diagnostic studies, even assuming clinicians have access to the resources, such as advanced imaging, that will increase diagnostic yield. As a result, the authors acknowledged that the classification system will be “aspirational” in at least some patients, but the structure of this system is expected to lead to greater precision in understanding the causes and defining features of ovulatory disorders, which, in turn, might facilitate new research initiatives.
In the published report, diagnostic protocols based on symptoms were described as being “beyond the spectrum” of this initial description. Rather, Dr. Munro explained that the most important contribution of this new classification system are standardization and communication. The system will be amenable for educating trainees and patients, for communicating between clinicians, and as a framework for research where investigators focus on more homogeneous populations of patients.
“There are many causes of ovulatory disorders that are not related to ovarian function. This is one message. Another is that ovulatory disorders are not binary. They occur on a spectrum. These range from transient instances of delayed or failed ovulation to chronic anovulation,” he said.
The new system is “ a welcome update,” according to Mark P. Trolice, MD, director of the IVF Center and professor of obstetrics and gynecology at the University of Central Florida, both in Orlando.
Dr. Trolice pointed to the clinical value of placing PCOS in a separate category. He noted that it affects 8%-13% of women, making it the most common single cause of ovulatory dysfunction.
“Another area that required clarification from prior WHO classifications was hyperprolactinemia, which is now placed in the type II category,” Dr. Trolice said in an interview.
Better terminology can help address a complex set of disorders with multiple causes and variable manifestations.
“In the evaluation of ovulation dysfunction, it is important to remember that regular menstrual intervals do not ensure ovulation,” Dr. Trolice pointed out. Even though a serum progesterone level of higher than 3 ng/mL is one of the simplest laboratory markers for ovulation, this level, he noted, “can vary through the luteal phase and even throughout the day.”
The proposed classification system, while providing a framework for describing ovulatory disorders, is designed to be adaptable, permitting advances in the understanding of the causes of ovulatory dysfunction, in the diagnosis of the causes, and in the treatments to be incorporated.
“No system should be considered permanent,” according to Dr. Munro and his coauthors. “Review and careful modification and revision should be carried out regularly.”
Dr. Munro reports financial relationships with AbbVie, American Regent, Daiichi Sankyo, Hologic, Myovant, and Pharmacosmos. Dr. Trolice reports no potential conflicts of interest.
The first major revision in the systematic description of ovulatory disorders in nearly 50 years has been proposed by a consensus of experts organized by the International Federation of Gynecology and Obstetrics.
“The FIGO HyPO-P system for the classification of ovulatory disorders is submitted for consideration as a worldwide standard,” according to the writing committee, who published their methodology and their proposed applications in the International Journal of Gynecology and Obstetrics.
The classification system was created to replace the much-modified World Health Organization system first described in 1973. Since that time, many modifications have been proposed to accommodate advances in imaging and new information about underlying pathologies, but there has been no subsequent authoritative reference with these modifications or any other newer organizing system.
The new consensus was developed under the aegis of FIGO, but the development group consisted of representatives from national organizations and the major subspecialty societies. Recognized experts in ovulatory disorders and representatives from lay advocacy organizations also participated.
The HyPO-P system is based largely on anatomy. The acronym refers to ovulatory disorders related to the hypothalamus (type I), the pituitary (type II), and the ovary (type III).
Polycystic ovary syndrome (PCOS), one of the most common ovulatory disorders, was given a separate category (type IV) because of its complexity as well as the fact that PCOS is a heterogeneous systemic disorder with manifestations not limited to an impact on ovarian function.
As the first level of classification, three of the four primary categories (I-III) focus attention on the dominant anatomic source of the change in ovulatory function. The original WHO classification system identified as many as seven major groups, but they were based primarily on assays for gonadotropins and estradiol.
The new system “provides a different structure for determining the diagnosis. Blood tests are not a necessary first step,” explained Malcolm G. Munro, MD, clinical professor, department of obstetrics and gynecology, University of California, Los Angeles. Dr. Munro was the first author of the publication.
The classification system “is not as focused on the specific steps for investigation of ovulatory dysfunction as much as it explains how to structure an investigation of the girl or woman with an ovulatory disorder and then how to characterize the underlying cause,” Dr. Munro said in an interview. “It is designed to allow everyone, whether clinicians, researchers, or patients, to speak the same language.”
New system employs four categories
The four primary categories provide just the first level of classification. The next step is encapsulated in the GAIN-FIT-PIE acronym, which frames the presumed or documented categories of etiologies for the primary categories. GAIN stands for genetic, autoimmune, iatrogenic, or neoplasm etiologies. FIT stands for functional, infectious/inflammatory, or trauma and vascular etiologies. PIE stands for physiological, idiopathic, and endocrine etiologies.
By this methodology, a patient with irregular menses, galactorrhea, and elevated prolactin and an MRI showing a pituitary tumor would be identified a type 2-N, signifying pituitary (type 2) involvement with a neoplasm (N).
A third level of classification permits specific diagnostic entities to be named, allowing the patient in the example above to receive a diagnosis of a prolactin-secreting adenoma.
Not all etiologies can be identified with current diagnostic studies, even assuming clinicians have access to the resources, such as advanced imaging, that will increase diagnostic yield. As a result, the authors acknowledged that the classification system will be “aspirational” in at least some patients, but the structure of this system is expected to lead to greater precision in understanding the causes and defining features of ovulatory disorders, which, in turn, might facilitate new research initiatives.
In the published report, diagnostic protocols based on symptoms were described as being “beyond the spectrum” of this initial description. Rather, Dr. Munro explained that the most important contribution of this new classification system are standardization and communication. The system will be amenable for educating trainees and patients, for communicating between clinicians, and as a framework for research where investigators focus on more homogeneous populations of patients.
“There are many causes of ovulatory disorders that are not related to ovarian function. This is one message. Another is that ovulatory disorders are not binary. They occur on a spectrum. These range from transient instances of delayed or failed ovulation to chronic anovulation,” he said.
The new system is “ a welcome update,” according to Mark P. Trolice, MD, director of the IVF Center and professor of obstetrics and gynecology at the University of Central Florida, both in Orlando.
Dr. Trolice pointed to the clinical value of placing PCOS in a separate category. He noted that it affects 8%-13% of women, making it the most common single cause of ovulatory dysfunction.
“Another area that required clarification from prior WHO classifications was hyperprolactinemia, which is now placed in the type II category,” Dr. Trolice said in an interview.
Better terminology can help address a complex set of disorders with multiple causes and variable manifestations.
“In the evaluation of ovulation dysfunction, it is important to remember that regular menstrual intervals do not ensure ovulation,” Dr. Trolice pointed out. Even though a serum progesterone level of higher than 3 ng/mL is one of the simplest laboratory markers for ovulation, this level, he noted, “can vary through the luteal phase and even throughout the day.”
The proposed classification system, while providing a framework for describing ovulatory disorders, is designed to be adaptable, permitting advances in the understanding of the causes of ovulatory dysfunction, in the diagnosis of the causes, and in the treatments to be incorporated.
“No system should be considered permanent,” according to Dr. Munro and his coauthors. “Review and careful modification and revision should be carried out regularly.”
Dr. Munro reports financial relationships with AbbVie, American Regent, Daiichi Sankyo, Hologic, Myovant, and Pharmacosmos. Dr. Trolice reports no potential conflicts of interest.
The first major revision in the systematic description of ovulatory disorders in nearly 50 years has been proposed by a consensus of experts organized by the International Federation of Gynecology and Obstetrics.
“The FIGO HyPO-P system for the classification of ovulatory disorders is submitted for consideration as a worldwide standard,” according to the writing committee, who published their methodology and their proposed applications in the International Journal of Gynecology and Obstetrics.
The classification system was created to replace the much-modified World Health Organization system first described in 1973. Since that time, many modifications have been proposed to accommodate advances in imaging and new information about underlying pathologies, but there has been no subsequent authoritative reference with these modifications or any other newer organizing system.
The new consensus was developed under the aegis of FIGO, but the development group consisted of representatives from national organizations and the major subspecialty societies. Recognized experts in ovulatory disorders and representatives from lay advocacy organizations also participated.
The HyPO-P system is based largely on anatomy. The acronym refers to ovulatory disorders related to the hypothalamus (type I), the pituitary (type II), and the ovary (type III).
Polycystic ovary syndrome (PCOS), one of the most common ovulatory disorders, was given a separate category (type IV) because of its complexity as well as the fact that PCOS is a heterogeneous systemic disorder with manifestations not limited to an impact on ovarian function.
As the first level of classification, three of the four primary categories (I-III) focus attention on the dominant anatomic source of the change in ovulatory function. The original WHO classification system identified as many as seven major groups, but they were based primarily on assays for gonadotropins and estradiol.
The new system “provides a different structure for determining the diagnosis. Blood tests are not a necessary first step,” explained Malcolm G. Munro, MD, clinical professor, department of obstetrics and gynecology, University of California, Los Angeles. Dr. Munro was the first author of the publication.
The classification system “is not as focused on the specific steps for investigation of ovulatory dysfunction as much as it explains how to structure an investigation of the girl or woman with an ovulatory disorder and then how to characterize the underlying cause,” Dr. Munro said in an interview. “It is designed to allow everyone, whether clinicians, researchers, or patients, to speak the same language.”
New system employs four categories
The four primary categories provide just the first level of classification. The next step is encapsulated in the GAIN-FIT-PIE acronym, which frames the presumed or documented categories of etiologies for the primary categories. GAIN stands for genetic, autoimmune, iatrogenic, or neoplasm etiologies. FIT stands for functional, infectious/inflammatory, or trauma and vascular etiologies. PIE stands for physiological, idiopathic, and endocrine etiologies.
By this methodology, a patient with irregular menses, galactorrhea, and elevated prolactin and an MRI showing a pituitary tumor would be identified a type 2-N, signifying pituitary (type 2) involvement with a neoplasm (N).
A third level of classification permits specific diagnostic entities to be named, allowing the patient in the example above to receive a diagnosis of a prolactin-secreting adenoma.
Not all etiologies can be identified with current diagnostic studies, even assuming clinicians have access to the resources, such as advanced imaging, that will increase diagnostic yield. As a result, the authors acknowledged that the classification system will be “aspirational” in at least some patients, but the structure of this system is expected to lead to greater precision in understanding the causes and defining features of ovulatory disorders, which, in turn, might facilitate new research initiatives.
In the published report, diagnostic protocols based on symptoms were described as being “beyond the spectrum” of this initial description. Rather, Dr. Munro explained that the most important contribution of this new classification system are standardization and communication. The system will be amenable for educating trainees and patients, for communicating between clinicians, and as a framework for research where investigators focus on more homogeneous populations of patients.
“There are many causes of ovulatory disorders that are not related to ovarian function. This is one message. Another is that ovulatory disorders are not binary. They occur on a spectrum. These range from transient instances of delayed or failed ovulation to chronic anovulation,” he said.
The new system is “ a welcome update,” according to Mark P. Trolice, MD, director of the IVF Center and professor of obstetrics and gynecology at the University of Central Florida, both in Orlando.
Dr. Trolice pointed to the clinical value of placing PCOS in a separate category. He noted that it affects 8%-13% of women, making it the most common single cause of ovulatory dysfunction.
“Another area that required clarification from prior WHO classifications was hyperprolactinemia, which is now placed in the type II category,” Dr. Trolice said in an interview.
Better terminology can help address a complex set of disorders with multiple causes and variable manifestations.
“In the evaluation of ovulation dysfunction, it is important to remember that regular menstrual intervals do not ensure ovulation,” Dr. Trolice pointed out. Even though a serum progesterone level of higher than 3 ng/mL is one of the simplest laboratory markers for ovulation, this level, he noted, “can vary through the luteal phase and even throughout the day.”
The proposed classification system, while providing a framework for describing ovulatory disorders, is designed to be adaptable, permitting advances in the understanding of the causes of ovulatory dysfunction, in the diagnosis of the causes, and in the treatments to be incorporated.
“No system should be considered permanent,” according to Dr. Munro and his coauthors. “Review and careful modification and revision should be carried out regularly.”
Dr. Munro reports financial relationships with AbbVie, American Regent, Daiichi Sankyo, Hologic, Myovant, and Pharmacosmos. Dr. Trolice reports no potential conflicts of interest.
FROM INTERNATIONAL JOURNAL OF GYNECOLOGY AND OBSTETRICS
Children and COVID: New cases increase; hospital admissions could follow
New cases of COVID-19 in children were up again after 2 weeks of declines, and preliminary data suggest that hospitalizations may be on the rise as well.
, based on data collected by the American Academy of Pediatrics and the Children’s Hospital Association from state and territorial health departments.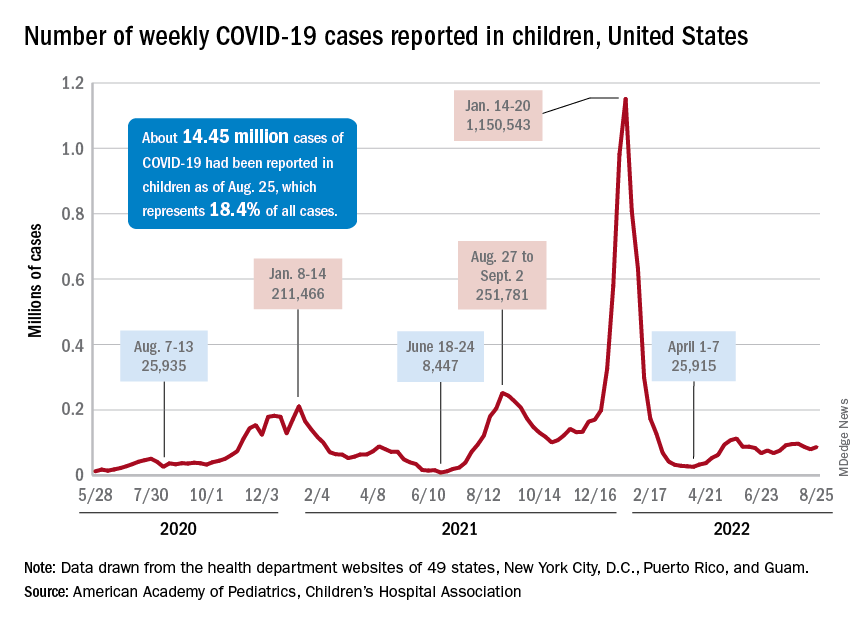
A similar increase seems to be reflected by hospital-level data. The latest 7-day (Aug. 21-27) average is 305 new admissions with diagnosed COVID per day among children aged 0-17 years, compared with 290 per day for the week of Aug. 14-20, the Centers for Disease Control and Prevention reported, while also noting the potential for reporting delays in the most recent 7-day period.
Daily hospital admissions for COVID had been headed downward through the first half of August, falling from 0.46 per 100,000 population at the end of July to 0.40 on Aug. 19, the CDC said on its COVID Data Tracker. Since then, however, admissions have gone the other way, with the preliminary nature of the latest data suggesting that the numbers will be even higher as more hospitals report over the next few days.
Vaccine initiations continue to fall
Initiations among school-age children have fallen for 3 consecutive weeks since Aug. 3, when numbers receiving their first vaccinations reached late-summer highs for those aged 5-11 and 12-17 years. Children under age 5, included in the CDC data for the first time on Aug. 11 as separate groups – under 2 years and 2-4 years – have had vaccine initiations drop by 8.0% and 19.8% over the 2 following weeks, the CDC said.
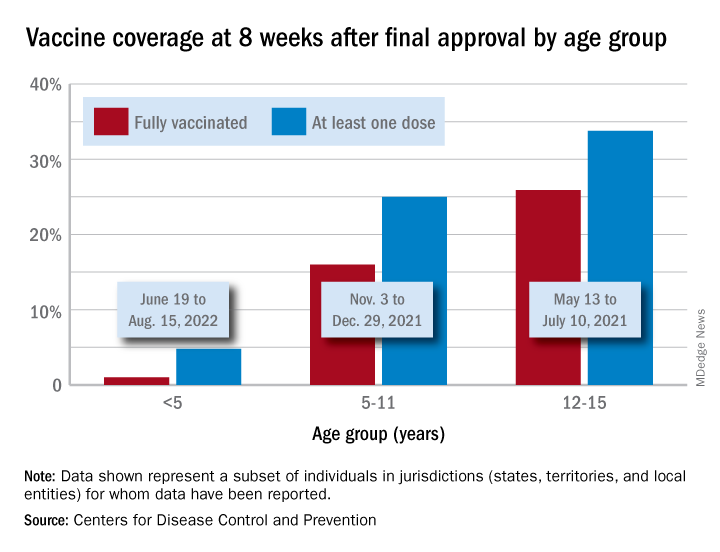
Through their first 8 weeks of vaccine eligibility (June 19 to Aug. 15), 4.8% of children under 5 years of age had received a first vaccination and 1.0% were fully vaccinated. For the two other age groups (5-11 and 12-15) who became eligible after the very first emergency authorization back in 2020, the respective proportions were 25.0% and 16.0% (5-11) and 33.8% and 26.1% (12-15) through the first 8 weeks, according to CDC data.
New cases of COVID-19 in children were up again after 2 weeks of declines, and preliminary data suggest that hospitalizations may be on the rise as well.
, based on data collected by the American Academy of Pediatrics and the Children’s Hospital Association from state and territorial health departments.
A similar increase seems to be reflected by hospital-level data. The latest 7-day (Aug. 21-27) average is 305 new admissions with diagnosed COVID per day among children aged 0-17 years, compared with 290 per day for the week of Aug. 14-20, the Centers for Disease Control and Prevention reported, while also noting the potential for reporting delays in the most recent 7-day period.
Daily hospital admissions for COVID had been headed downward through the first half of August, falling from 0.46 per 100,000 population at the end of July to 0.40 on Aug. 19, the CDC said on its COVID Data Tracker. Since then, however, admissions have gone the other way, with the preliminary nature of the latest data suggesting that the numbers will be even higher as more hospitals report over the next few days.
Vaccine initiations continue to fall
Initiations among school-age children have fallen for 3 consecutive weeks since Aug. 3, when numbers receiving their first vaccinations reached late-summer highs for those aged 5-11 and 12-17 years. Children under age 5, included in the CDC data for the first time on Aug. 11 as separate groups – under 2 years and 2-4 years – have had vaccine initiations drop by 8.0% and 19.8% over the 2 following weeks, the CDC said.

Through their first 8 weeks of vaccine eligibility (June 19 to Aug. 15), 4.8% of children under 5 years of age had received a first vaccination and 1.0% were fully vaccinated. For the two other age groups (5-11 and 12-15) who became eligible after the very first emergency authorization back in 2020, the respective proportions were 25.0% and 16.0% (5-11) and 33.8% and 26.1% (12-15) through the first 8 weeks, according to CDC data.
New cases of COVID-19 in children were up again after 2 weeks of declines, and preliminary data suggest that hospitalizations may be on the rise as well.
, based on data collected by the American Academy of Pediatrics and the Children’s Hospital Association from state and territorial health departments.
A similar increase seems to be reflected by hospital-level data. The latest 7-day (Aug. 21-27) average is 305 new admissions with diagnosed COVID per day among children aged 0-17 years, compared with 290 per day for the week of Aug. 14-20, the Centers for Disease Control and Prevention reported, while also noting the potential for reporting delays in the most recent 7-day period.
Daily hospital admissions for COVID had been headed downward through the first half of August, falling from 0.46 per 100,000 population at the end of July to 0.40 on Aug. 19, the CDC said on its COVID Data Tracker. Since then, however, admissions have gone the other way, with the preliminary nature of the latest data suggesting that the numbers will be even higher as more hospitals report over the next few days.
Vaccine initiations continue to fall
Initiations among school-age children have fallen for 3 consecutive weeks since Aug. 3, when numbers receiving their first vaccinations reached late-summer highs for those aged 5-11 and 12-17 years. Children under age 5, included in the CDC data for the first time on Aug. 11 as separate groups – under 2 years and 2-4 years – have had vaccine initiations drop by 8.0% and 19.8% over the 2 following weeks, the CDC said.

Through their first 8 weeks of vaccine eligibility (June 19 to Aug. 15), 4.8% of children under 5 years of age had received a first vaccination and 1.0% were fully vaccinated. For the two other age groups (5-11 and 12-15) who became eligible after the very first emergency authorization back in 2020, the respective proportions were 25.0% and 16.0% (5-11) and 33.8% and 26.1% (12-15) through the first 8 weeks, according to CDC data.
Ketogenic Diet and Cancer: A Case Report and Feasibility Study at VA Central California Healthcare System
Background
Ketogenic diet (KD) is a high-fat and low carbohydrate diet that has been reported as a treatment option for patients with cancer. KD creates a metabolic state in which blood glucose levels are reduced and ketone bodies are elevated. Cancer cells are unable to use ketone bodies for energy and metabolism due to mitochondrial dysfunction. We published the efficacy of KD in patients with cancer after failure of chemotherapy. 1 This case report is presented to evaluate the feasibility of KD concurrent with chemoimmunotherapy.
Case Report
Patient is a 69-year-old male who presented with iron deficiency anemia in 2018. Colonoscopy and biopsy showed colon adenocarcinoma. He underwent resection which confirmed stage IIIC disease. He received adjuvant treatment with FOLFOX but quickly developed pancreatic and omental metastasis. He was started on FOLFIRI + bevacizumab followed by pancreatic mass resection in 2019. Molecular testing revealed wild type KRAS, positive BRAF V600E, and high MSI. He received encorafenib + cetuximab until disease progression. Treatment was changed to pembrolizumab until PET scan showed progression. His CEA increased to 1031 in January 2021. He was subsequently started on KD concurrent with trifluridine + tipiracil + bevacizumab. He progressed after 10 months. Therapy was changed to ipilimumab + nivolumab with continuation of KD. He was strictly adherent to KD with low Glucose Ketone Index of 8.2 (confirming ketosis) but in 2022 his GKI level started to rise. His CEA, however, significantly decreased to 20 in March 2022 and PET scan showed stable disease. He presently is on maintenance nivolumab + KD while maintaining an excellent quality of life by EORTC QLQ scores.
Conclusions
The use of KD concurrently with chemotherapy and immunotherapy is still under investigation. Our case report shows that KD is tolerable with treatment and can possibly contribute to controlling progression of metastatic cancer. We are starting an investigator initiative KD trial that received a grant from R&D at VACCHCS. We will present the study protocol in poster presentation.
1. Tan-Shalaby JL, Carrick J, Edinger K, et al. Modified Atkins diet in advanced malignancies - final results of a safety and feasibility trial within the Veterans Affairs Pittsburgh Healthcare System]. Nutr Metab (Lond). 2016;13:52. Published 2016 Aug 12. doi:10.1186/s12986-016-0113-y
Background
Ketogenic diet (KD) is a high-fat and low carbohydrate diet that has been reported as a treatment option for patients with cancer. KD creates a metabolic state in which blood glucose levels are reduced and ketone bodies are elevated. Cancer cells are unable to use ketone bodies for energy and metabolism due to mitochondrial dysfunction. We published the efficacy of KD in patients with cancer after failure of chemotherapy. 1 This case report is presented to evaluate the feasibility of KD concurrent with chemoimmunotherapy.
Case Report
Patient is a 69-year-old male who presented with iron deficiency anemia in 2018. Colonoscopy and biopsy showed colon adenocarcinoma. He underwent resection which confirmed stage IIIC disease. He received adjuvant treatment with FOLFOX but quickly developed pancreatic and omental metastasis. He was started on FOLFIRI + bevacizumab followed by pancreatic mass resection in 2019. Molecular testing revealed wild type KRAS, positive BRAF V600E, and high MSI. He received encorafenib + cetuximab until disease progression. Treatment was changed to pembrolizumab until PET scan showed progression. His CEA increased to 1031 in January 2021. He was subsequently started on KD concurrent with trifluridine + tipiracil + bevacizumab. He progressed after 10 months. Therapy was changed to ipilimumab + nivolumab with continuation of KD. He was strictly adherent to KD with low Glucose Ketone Index of 8.2 (confirming ketosis) but in 2022 his GKI level started to rise. His CEA, however, significantly decreased to 20 in March 2022 and PET scan showed stable disease. He presently is on maintenance nivolumab + KD while maintaining an excellent quality of life by EORTC QLQ scores.
Conclusions
The use of KD concurrently with chemotherapy and immunotherapy is still under investigation. Our case report shows that KD is tolerable with treatment and can possibly contribute to controlling progression of metastatic cancer. We are starting an investigator initiative KD trial that received a grant from R&D at VACCHCS. We will present the study protocol in poster presentation.
Background
Ketogenic diet (KD) is a high-fat and low carbohydrate diet that has been reported as a treatment option for patients with cancer. KD creates a metabolic state in which blood glucose levels are reduced and ketone bodies are elevated. Cancer cells are unable to use ketone bodies for energy and metabolism due to mitochondrial dysfunction. We published the efficacy of KD in patients with cancer after failure of chemotherapy. 1 This case report is presented to evaluate the feasibility of KD concurrent with chemoimmunotherapy.
Case Report
Patient is a 69-year-old male who presented with iron deficiency anemia in 2018. Colonoscopy and biopsy showed colon adenocarcinoma. He underwent resection which confirmed stage IIIC disease. He received adjuvant treatment with FOLFOX but quickly developed pancreatic and omental metastasis. He was started on FOLFIRI + bevacizumab followed by pancreatic mass resection in 2019. Molecular testing revealed wild type KRAS, positive BRAF V600E, and high MSI. He received encorafenib + cetuximab until disease progression. Treatment was changed to pembrolizumab until PET scan showed progression. His CEA increased to 1031 in January 2021. He was subsequently started on KD concurrent with trifluridine + tipiracil + bevacizumab. He progressed after 10 months. Therapy was changed to ipilimumab + nivolumab with continuation of KD. He was strictly adherent to KD with low Glucose Ketone Index of 8.2 (confirming ketosis) but in 2022 his GKI level started to rise. His CEA, however, significantly decreased to 20 in March 2022 and PET scan showed stable disease. He presently is on maintenance nivolumab + KD while maintaining an excellent quality of life by EORTC QLQ scores.
Conclusions
The use of KD concurrently with chemotherapy and immunotherapy is still under investigation. Our case report shows that KD is tolerable with treatment and can possibly contribute to controlling progression of metastatic cancer. We are starting an investigator initiative KD trial that received a grant from R&D at VACCHCS. We will present the study protocol in poster presentation.
1. Tan-Shalaby JL, Carrick J, Edinger K, et al. Modified Atkins diet in advanced malignancies - final results of a safety and feasibility trial within the Veterans Affairs Pittsburgh Healthcare System]. Nutr Metab (Lond). 2016;13:52. Published 2016 Aug 12. doi:10.1186/s12986-016-0113-y
1. Tan-Shalaby JL, Carrick J, Edinger K, et al. Modified Atkins diet in advanced malignancies - final results of a safety and feasibility trial within the Veterans Affairs Pittsburgh Healthcare System]. Nutr Metab (Lond). 2016;13:52. Published 2016 Aug 12. doi:10.1186/s12986-016-0113-y
Castration-Resistant Prostate Cancer—Not Only Challenging to Treat, but Difficult to Define
Purpose
Examine the impact of different definitions of castration resistance used to identify patients with castration-resistant prostate cancer (CRPC) using electronic health records (EHR).
Background
CRPC is a form of prostate cancer that is resistant to treatment with androgen deprivation therapy (ADT) and is associated with higher morbidity and mortality. Widely used guidelines like the Prostate Cancer Working Group 3 (PCWG 3), the American Urological Association (AUA), and many others differ in their definitions of castration-resistance. Until now, the feasibility of identifying CRPC using different definitions from EHR data has not been studied.
Methods/Data Analyisis
EHR data from the Veterans Health Administration (01/2006-12/2020) were used to identify veterans with CRPC according to the following criteria: 1) PCWG 3—a PSA increase ?25% from the nadir with a minimum rise of 2 ng/mL, while castrate (testosterone < 50 ng/mL); 2) AUA—2 consecutive PSA rises of ?0.2 ng/mL; 3) CRPC screening—a PSA rise of > 0.0 ng/mL within a window of 7–90 days.
Results
36,101 unique patients were identified using 1 of (or a combination of) the 3 CRPC criteria. Approximately 12,775 (35%) patients met all 3 criteria, while 8,589 (24%) were identified by AUA, 4,785 (13%) by CRPC screening, and 145 (0.4%) by PCWG3. A total of 8,377 (23%) patients met both the AUA and CRPC screening criteria, 1,219 (3%) patients met the AUA and PCWG3 criteria, and 211 (1%) met the PCWG3 and CRPC screening criteria.
Conculsions/Implications
Although several definitions can be used to identify CRPC patients, a combination of these definitions results in the greatest yield of CRPC patients identified using EHR data. Even though the PCWG3 criterion is frequently used in both clinical trials research and retrospective observational research, PCWG3 may miss many patients meeting other criteria and should not be used by itself when studying patients with CRPC identified from EHR data.
Purpose
Examine the impact of different definitions of castration resistance used to identify patients with castration-resistant prostate cancer (CRPC) using electronic health records (EHR).
Background
CRPC is a form of prostate cancer that is resistant to treatment with androgen deprivation therapy (ADT) and is associated with higher morbidity and mortality. Widely used guidelines like the Prostate Cancer Working Group 3 (PCWG 3), the American Urological Association (AUA), and many others differ in their definitions of castration-resistance. Until now, the feasibility of identifying CRPC using different definitions from EHR data has not been studied.
Methods/Data Analyisis
EHR data from the Veterans Health Administration (01/2006-12/2020) were used to identify veterans with CRPC according to the following criteria: 1) PCWG 3—a PSA increase ?25% from the nadir with a minimum rise of 2 ng/mL, while castrate (testosterone < 50 ng/mL); 2) AUA—2 consecutive PSA rises of ?0.2 ng/mL; 3) CRPC screening—a PSA rise of > 0.0 ng/mL within a window of 7–90 days.
Results
36,101 unique patients were identified using 1 of (or a combination of) the 3 CRPC criteria. Approximately 12,775 (35%) patients met all 3 criteria, while 8,589 (24%) were identified by AUA, 4,785 (13%) by CRPC screening, and 145 (0.4%) by PCWG3. A total of 8,377 (23%) patients met both the AUA and CRPC screening criteria, 1,219 (3%) patients met the AUA and PCWG3 criteria, and 211 (1%) met the PCWG3 and CRPC screening criteria.
Conculsions/Implications
Although several definitions can be used to identify CRPC patients, a combination of these definitions results in the greatest yield of CRPC patients identified using EHR data. Even though the PCWG3 criterion is frequently used in both clinical trials research and retrospective observational research, PCWG3 may miss many patients meeting other criteria and should not be used by itself when studying patients with CRPC identified from EHR data.
Purpose
Examine the impact of different definitions of castration resistance used to identify patients with castration-resistant prostate cancer (CRPC) using electronic health records (EHR).
Background
CRPC is a form of prostate cancer that is resistant to treatment with androgen deprivation therapy (ADT) and is associated with higher morbidity and mortality. Widely used guidelines like the Prostate Cancer Working Group 3 (PCWG 3), the American Urological Association (AUA), and many others differ in their definitions of castration-resistance. Until now, the feasibility of identifying CRPC using different definitions from EHR data has not been studied.
Methods/Data Analyisis
EHR data from the Veterans Health Administration (01/2006-12/2020) were used to identify veterans with CRPC according to the following criteria: 1) PCWG 3—a PSA increase ?25% from the nadir with a minimum rise of 2 ng/mL, while castrate (testosterone < 50 ng/mL); 2) AUA—2 consecutive PSA rises of ?0.2 ng/mL; 3) CRPC screening—a PSA rise of > 0.0 ng/mL within a window of 7–90 days.
Results
36,101 unique patients were identified using 1 of (or a combination of) the 3 CRPC criteria. Approximately 12,775 (35%) patients met all 3 criteria, while 8,589 (24%) were identified by AUA, 4,785 (13%) by CRPC screening, and 145 (0.4%) by PCWG3. A total of 8,377 (23%) patients met both the AUA and CRPC screening criteria, 1,219 (3%) patients met the AUA and PCWG3 criteria, and 211 (1%) met the PCWG3 and CRPC screening criteria.
Conculsions/Implications
Although several definitions can be used to identify CRPC patients, a combination of these definitions results in the greatest yield of CRPC patients identified using EHR data. Even though the PCWG3 criterion is frequently used in both clinical trials research and retrospective observational research, PCWG3 may miss many patients meeting other criteria and should not be used by itself when studying patients with CRPC identified from EHR data.