User login
From neuroplasticity to psychoplasticity: Psilocybin may reverse personality disorders and political fanaticism
One of psychiatry’s long-standing dogmas is that personality disorders are enduring, unchangeable, and not amenable to treatment with potent psychotropics or intensive psychotherapy. I propose that this dogma may soon be shattered.
Several other dogmas in psychiatry have been demolished over the past several decades:
- that “insanity” is completely irreversible and requires lifetime institutionalization. The serendipitous discovery of chlorpromazine1 annihilated this centuries-old dogma
- that chronic, severe, refractory depression (with ongoing suicidal urges) that fails to improve with pharmacotherapy or electroconvulsive therapy (ECT) is hopeless and untreatable, until ketamine not only pulverized this dogma, but did it with lightning speed, dazzling us all2
- that dissociative agents such as ketamine are dangerous and condemnable drugs of abuse, until the therapeutic effect of ketamine slayed that dragon3
- that ECT “fries” the brain (as malevolently propagated by antipsychiatry cults), which was completely disproven by neuroimaging studies that show the hippocampus (which shrinks during depression) actually grows by >10% after a few ECT sessions4
- that psychotherapy is not a “real” treatment because talking cannot reverse a psychiatric brain disorder, until studies showed significant neuroplasticity with psychotherapy and decrease in inflammatory biomarkers with cognitive-behavioral therapy (CBT)5
- that persons with refractory hallucinations and delusions are doomed to a life of disability, until clozapine torpedoed that pessimistic dogma6
- that hallucinogens/psychedelics are dangerous and should be banned, until a jarring paradigm shift occurred with the discovery of psilocybin’s transformative effects, and the remarkable therapeutic effects of its mystical trips.7
Psilocybin’s therapeutic effects
Psilocybin has already proved to have a strong and lasting effect on depression and promises to have therapeutic benefits for patients with substance use disorders, posttraumatic stress disorder (PTSD), and anxiety.8 In addition, when the multiple psychological and neurobiological effects of psilocybin (and of other psychedelics) are examined, I see a very promising path to amelioration of severe personality disorders such as psychopathy, antisocial behavior, and narcissism. The mechanism(s) of action of psilocybin on the human brain are drastically different from any man-made psychotropic agent. As a psychiatric neuroscientist, I envision the neurologic impact of psilocybin to be conducive to a complete transformation of a patient’s view of themself, other people, and the meaning of life. It is reminiscent of religious conversion.
The psychological effects of psilocybin in humans have been described as follows:
- emotional breakthrough9
- increased psychological flexibility,10,11 a very cortical effect
- mystical experience,12 which results in sudden and significant changes in behavior and perception and includes the following dimensions: sacredness, noetic quality, deeply felt positive mood, ineffability, paradoxicality, and transcendence of time and space13
- oceanic boundlessness, feeling “one with the universe”14
- universal interconnectedness, insightfulness, blissful state, spiritual experience14
- ego dissolution,15 with loss of one’s personal identity
- increased neuroplasticity16
- changes in cognition and increase in insight.17
The neurobiological effects of psilocybin are mediated by serotonin 5HT2A agonism and include the following18:
- reduction in the activity of the medial prefrontal cortex, which regulates memory, attention, inhibitory control, and habit
- a decrease in the connectivity between the medial prefrontal cortex and the posterior cingulate cortex, which regulates memory and emotions
- reducing the default mode network, which is active during rest, stimulating internal thoughts and reminiscing about previous feelings and events, sometimes including ruminations. Psilocybin reverses those processes to thinking about others, not just the self, and becoming more open-minded about the world and other people. This can be therapeutic for depression, which is often associated with negative ruminations but also with entrenched habits (addictive behaviors), anxiety, PTSD, and obsessive-compulsive disorders
- increased global functional connectivity among various brain networks, leading to stronger functional integration of behavior
- collapse of major cortical oscillatory rhythms such as alpha and others that perpetuate “prior” beliefs
- extensive neuroplasticity and recalibration of thought processes and decomposition of pathological beliefs, referred to as REBUS (relaxed beliefs under psychedelics).
The bottom line is psilocybin and other psychedelics can dramatically alter, reshape, and relax rigid beliefs and personality traits by decreasing “neuroticism” and increasing “extraversion,” insightfulness, openness, and possibly conscientiousness.19 Although no studies of psychedelics in psychopathic, antisocial, or narcissistic personality disorders have been conducted, it is very reasonable to speculate that psilocybin may reverse traits of these disorders such as callousness, lack of empathy, and pathological self-centeredness.
Going further, a preliminary report suggests psilocybin can modify political views by decreasing authoritarianism and increasing libertarianism.20,21 In the current political zeitgeist, could psychedelics such as psilocybin reduce or even eliminate political extremism and visceral hatred on all sides? It would be remarkable research to carry out to heal a politically divided populace.The dogma of untreatable personality disorders or hopelessly entrenched political extremism is on the chopping block, and psychedelics offer hope to splinter those beliefs by concurrently remodeling brain tissue (neuroplasticity) and rectifying the mindset (psychoplasticity).
1. Delay J, Deniker P. Neuroleptic effects of chlorpromazine in therapeutics of neuropsychiatry. J Clin Exp Psychopathol. 1955;16(2):104-112.
2. Walsh Z, Mollaahmetoglu OM, Rootman, J, et al. Ketamine for the treatment of mental health and substance use disorders: comprehensive systematic review. BJPsych Open. 2021;8(1):e19. doi:10.1192/bjo.2021.1061
3. Lener MS, Kadriu B, Zarate CA Jr. Ketamine and beyond: investigations into the potential of glutamatergic agents to treat depression. Drugs. 2017;77(4):381-401.
4. Ayers B, Leaver A, Woods RP, et al. Structural plasticity of the hippocampus and amygdala induced by electroconvulsive therapy in major depression. Biol Psychiatry. 2016;79(4):282-292.
5. Cao B, Li R, Ding L, Xu J, et al. Does cognitive behaviour therapy affect peripheral inflammation of depression? A protocol for the systematic review and meta-analysis. BMJ Open. 2021;11(12):e048162. doi:10.1136/bmjopen-2020-048162
6. Wagner E, Siafis S, Fernando P, et al. Efficacy and safety of clozapine in psychotic disorders—a systematic quantitative meta-review. Transl Psychiatry. 2021;11(1):487.
7. Daws RE, Timmermann C, Giribaldi B, et al. Increased global integration in the brain after psilocybin therapy for depression. Nat Med. 2022;28(4):844-851.
8. Pearson C, Siegel J, Gold JA. Psilocybin-assisted psychotherapy for depression: emerging research on a psychedelic compound with a rich history. J Neurol Sci. 2022;434:120096. doi:10.1016/j.jns.2021.120096
9. Roseman L, Haijen E, Idialu-Ikato K, et al. Emotional breakthrough and psychedelics: validation of the Emotional Breakthrough Inventory. J Psychopharmacol. 2019;33(9):1076-1087.
10. Davis AK, Barrett FS, Griffiths RR. Psychological flexibility mediates the relations between acute psychedelic effects and subjective decreases in depression and anxiety. J Contextual Behav Sci. 2020;15:39-45.
11. Hayes SC, Luoma JB, Bond FW, et al. Acceptance and commitment therapy: model, processes and outcomes. Behav Res Ther. 2006;44(1):1-25.
12. Ross S, Bossis A, Guss J, et al. Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: a randomized controlled trial. J Psychopharmacol. 2016;30(12):1165-1180.
13. Stace WT. Mysticism and Philosophy. Macmillan Pub Ltd; 1960:37.
14. Barrett FS, Griffiths RR. Classic hallucinogens and mystical experiences: phenomenology and neural correlates. Curr Top Behav Neurosci. 2018;36:393-430.
15. Nour MM, Evans L, Nutt D, et al. Ego-dissolution and psychedelics: validation of the Ego-Dissolution Inventory (EDI). Front Hum Neurosci. 2016;10:269. doi:10.3389/fnhum.2016.00269
16. Olson DE. The subjective effects of psychedelics may not be necessary for their enduring therapeutic effects. ACS Pharmacol Transl Sci. 2020;4(2):563-567.
17. Carhart-Harris RL, Bolstridge M, Day CMJ, et al. Psilocybin with psychological support for treatment-resistant depression: six-month follow-up. Psychopharmacology (Berl). 2018;235(2):399-408.
18. Carhart-Harris RL. How do psychedelics work? Curr Opin Psychiatry. 2019;32(1):16-21.
19. Erritzoe D, Roseman L, Nour MM, et al. Effects of psilocybin therapy on personality structure. Acta Psychiatr Scand. 2018;138(5):368-378.
20. Lyons T, Carhart-Harris RL. Increased nature relatedness and decreased authoritarian political views after psilocybin for treatment-resistant depression. J Psychopharmacol. 2018;32(7):811-819.
21. Nour MM, Evans L, Carhart-Harris RL. Psychedelics, personality and political perspectives. J Psychoactive Drugs. 2017;49(3):182-191.
One of psychiatry’s long-standing dogmas is that personality disorders are enduring, unchangeable, and not amenable to treatment with potent psychotropics or intensive psychotherapy. I propose that this dogma may soon be shattered.
Several other dogmas in psychiatry have been demolished over the past several decades:
- that “insanity” is completely irreversible and requires lifetime institutionalization. The serendipitous discovery of chlorpromazine1 annihilated this centuries-old dogma
- that chronic, severe, refractory depression (with ongoing suicidal urges) that fails to improve with pharmacotherapy or electroconvulsive therapy (ECT) is hopeless and untreatable, until ketamine not only pulverized this dogma, but did it with lightning speed, dazzling us all2
- that dissociative agents such as ketamine are dangerous and condemnable drugs of abuse, until the therapeutic effect of ketamine slayed that dragon3
- that ECT “fries” the brain (as malevolently propagated by antipsychiatry cults), which was completely disproven by neuroimaging studies that show the hippocampus (which shrinks during depression) actually grows by >10% after a few ECT sessions4
- that psychotherapy is not a “real” treatment because talking cannot reverse a psychiatric brain disorder, until studies showed significant neuroplasticity with psychotherapy and decrease in inflammatory biomarkers with cognitive-behavioral therapy (CBT)5
- that persons with refractory hallucinations and delusions are doomed to a life of disability, until clozapine torpedoed that pessimistic dogma6
- that hallucinogens/psychedelics are dangerous and should be banned, until a jarring paradigm shift occurred with the discovery of psilocybin’s transformative effects, and the remarkable therapeutic effects of its mystical trips.7
Psilocybin’s therapeutic effects
Psilocybin has already proved to have a strong and lasting effect on depression and promises to have therapeutic benefits for patients with substance use disorders, posttraumatic stress disorder (PTSD), and anxiety.8 In addition, when the multiple psychological and neurobiological effects of psilocybin (and of other psychedelics) are examined, I see a very promising path to amelioration of severe personality disorders such as psychopathy, antisocial behavior, and narcissism. The mechanism(s) of action of psilocybin on the human brain are drastically different from any man-made psychotropic agent. As a psychiatric neuroscientist, I envision the neurologic impact of psilocybin to be conducive to a complete transformation of a patient’s view of themself, other people, and the meaning of life. It is reminiscent of religious conversion.
The psychological effects of psilocybin in humans have been described as follows:
- emotional breakthrough9
- increased psychological flexibility,10,11 a very cortical effect
- mystical experience,12 which results in sudden and significant changes in behavior and perception and includes the following dimensions: sacredness, noetic quality, deeply felt positive mood, ineffability, paradoxicality, and transcendence of time and space13
- oceanic boundlessness, feeling “one with the universe”14
- universal interconnectedness, insightfulness, blissful state, spiritual experience14
- ego dissolution,15 with loss of one’s personal identity
- increased neuroplasticity16
- changes in cognition and increase in insight.17
The neurobiological effects of psilocybin are mediated by serotonin 5HT2A agonism and include the following18:
- reduction in the activity of the medial prefrontal cortex, which regulates memory, attention, inhibitory control, and habit
- a decrease in the connectivity between the medial prefrontal cortex and the posterior cingulate cortex, which regulates memory and emotions
- reducing the default mode network, which is active during rest, stimulating internal thoughts and reminiscing about previous feelings and events, sometimes including ruminations. Psilocybin reverses those processes to thinking about others, not just the self, and becoming more open-minded about the world and other people. This can be therapeutic for depression, which is often associated with negative ruminations but also with entrenched habits (addictive behaviors), anxiety, PTSD, and obsessive-compulsive disorders
- increased global functional connectivity among various brain networks, leading to stronger functional integration of behavior
- collapse of major cortical oscillatory rhythms such as alpha and others that perpetuate “prior” beliefs
- extensive neuroplasticity and recalibration of thought processes and decomposition of pathological beliefs, referred to as REBUS (relaxed beliefs under psychedelics).
The bottom line is psilocybin and other psychedelics can dramatically alter, reshape, and relax rigid beliefs and personality traits by decreasing “neuroticism” and increasing “extraversion,” insightfulness, openness, and possibly conscientiousness.19 Although no studies of psychedelics in psychopathic, antisocial, or narcissistic personality disorders have been conducted, it is very reasonable to speculate that psilocybin may reverse traits of these disorders such as callousness, lack of empathy, and pathological self-centeredness.
Going further, a preliminary report suggests psilocybin can modify political views by decreasing authoritarianism and increasing libertarianism.20,21 In the current political zeitgeist, could psychedelics such as psilocybin reduce or even eliminate political extremism and visceral hatred on all sides? It would be remarkable research to carry out to heal a politically divided populace.The dogma of untreatable personality disorders or hopelessly entrenched political extremism is on the chopping block, and psychedelics offer hope to splinter those beliefs by concurrently remodeling brain tissue (neuroplasticity) and rectifying the mindset (psychoplasticity).
One of psychiatry’s long-standing dogmas is that personality disorders are enduring, unchangeable, and not amenable to treatment with potent psychotropics or intensive psychotherapy. I propose that this dogma may soon be shattered.
Several other dogmas in psychiatry have been demolished over the past several decades:
- that “insanity” is completely irreversible and requires lifetime institutionalization. The serendipitous discovery of chlorpromazine1 annihilated this centuries-old dogma
- that chronic, severe, refractory depression (with ongoing suicidal urges) that fails to improve with pharmacotherapy or electroconvulsive therapy (ECT) is hopeless and untreatable, until ketamine not only pulverized this dogma, but did it with lightning speed, dazzling us all2
- that dissociative agents such as ketamine are dangerous and condemnable drugs of abuse, until the therapeutic effect of ketamine slayed that dragon3
- that ECT “fries” the brain (as malevolently propagated by antipsychiatry cults), which was completely disproven by neuroimaging studies that show the hippocampus (which shrinks during depression) actually grows by >10% after a few ECT sessions4
- that psychotherapy is not a “real” treatment because talking cannot reverse a psychiatric brain disorder, until studies showed significant neuroplasticity with psychotherapy and decrease in inflammatory biomarkers with cognitive-behavioral therapy (CBT)5
- that persons with refractory hallucinations and delusions are doomed to a life of disability, until clozapine torpedoed that pessimistic dogma6
- that hallucinogens/psychedelics are dangerous and should be banned, until a jarring paradigm shift occurred with the discovery of psilocybin’s transformative effects, and the remarkable therapeutic effects of its mystical trips.7
Psilocybin’s therapeutic effects
Psilocybin has already proved to have a strong and lasting effect on depression and promises to have therapeutic benefits for patients with substance use disorders, posttraumatic stress disorder (PTSD), and anxiety.8 In addition, when the multiple psychological and neurobiological effects of psilocybin (and of other psychedelics) are examined, I see a very promising path to amelioration of severe personality disorders such as psychopathy, antisocial behavior, and narcissism. The mechanism(s) of action of psilocybin on the human brain are drastically different from any man-made psychotropic agent. As a psychiatric neuroscientist, I envision the neurologic impact of psilocybin to be conducive to a complete transformation of a patient’s view of themself, other people, and the meaning of life. It is reminiscent of religious conversion.
The psychological effects of psilocybin in humans have been described as follows:
- emotional breakthrough9
- increased psychological flexibility,10,11 a very cortical effect
- mystical experience,12 which results in sudden and significant changes in behavior and perception and includes the following dimensions: sacredness, noetic quality, deeply felt positive mood, ineffability, paradoxicality, and transcendence of time and space13
- oceanic boundlessness, feeling “one with the universe”14
- universal interconnectedness, insightfulness, blissful state, spiritual experience14
- ego dissolution,15 with loss of one’s personal identity
- increased neuroplasticity16
- changes in cognition and increase in insight.17
The neurobiological effects of psilocybin are mediated by serotonin 5HT2A agonism and include the following18:
- reduction in the activity of the medial prefrontal cortex, which regulates memory, attention, inhibitory control, and habit
- a decrease in the connectivity between the medial prefrontal cortex and the posterior cingulate cortex, which regulates memory and emotions
- reducing the default mode network, which is active during rest, stimulating internal thoughts and reminiscing about previous feelings and events, sometimes including ruminations. Psilocybin reverses those processes to thinking about others, not just the self, and becoming more open-minded about the world and other people. This can be therapeutic for depression, which is often associated with negative ruminations but also with entrenched habits (addictive behaviors), anxiety, PTSD, and obsessive-compulsive disorders
- increased global functional connectivity among various brain networks, leading to stronger functional integration of behavior
- collapse of major cortical oscillatory rhythms such as alpha and others that perpetuate “prior” beliefs
- extensive neuroplasticity and recalibration of thought processes and decomposition of pathological beliefs, referred to as REBUS (relaxed beliefs under psychedelics).
The bottom line is psilocybin and other psychedelics can dramatically alter, reshape, and relax rigid beliefs and personality traits by decreasing “neuroticism” and increasing “extraversion,” insightfulness, openness, and possibly conscientiousness.19 Although no studies of psychedelics in psychopathic, antisocial, or narcissistic personality disorders have been conducted, it is very reasonable to speculate that psilocybin may reverse traits of these disorders such as callousness, lack of empathy, and pathological self-centeredness.
Going further, a preliminary report suggests psilocybin can modify political views by decreasing authoritarianism and increasing libertarianism.20,21 In the current political zeitgeist, could psychedelics such as psilocybin reduce or even eliminate political extremism and visceral hatred on all sides? It would be remarkable research to carry out to heal a politically divided populace.The dogma of untreatable personality disorders or hopelessly entrenched political extremism is on the chopping block, and psychedelics offer hope to splinter those beliefs by concurrently remodeling brain tissue (neuroplasticity) and rectifying the mindset (psychoplasticity).
1. Delay J, Deniker P. Neuroleptic effects of chlorpromazine in therapeutics of neuropsychiatry. J Clin Exp Psychopathol. 1955;16(2):104-112.
2. Walsh Z, Mollaahmetoglu OM, Rootman, J, et al. Ketamine for the treatment of mental health and substance use disorders: comprehensive systematic review. BJPsych Open. 2021;8(1):e19. doi:10.1192/bjo.2021.1061
3. Lener MS, Kadriu B, Zarate CA Jr. Ketamine and beyond: investigations into the potential of glutamatergic agents to treat depression. Drugs. 2017;77(4):381-401.
4. Ayers B, Leaver A, Woods RP, et al. Structural plasticity of the hippocampus and amygdala induced by electroconvulsive therapy in major depression. Biol Psychiatry. 2016;79(4):282-292.
5. Cao B, Li R, Ding L, Xu J, et al. Does cognitive behaviour therapy affect peripheral inflammation of depression? A protocol for the systematic review and meta-analysis. BMJ Open. 2021;11(12):e048162. doi:10.1136/bmjopen-2020-048162
6. Wagner E, Siafis S, Fernando P, et al. Efficacy and safety of clozapine in psychotic disorders—a systematic quantitative meta-review. Transl Psychiatry. 2021;11(1):487.
7. Daws RE, Timmermann C, Giribaldi B, et al. Increased global integration in the brain after psilocybin therapy for depression. Nat Med. 2022;28(4):844-851.
8. Pearson C, Siegel J, Gold JA. Psilocybin-assisted psychotherapy for depression: emerging research on a psychedelic compound with a rich history. J Neurol Sci. 2022;434:120096. doi:10.1016/j.jns.2021.120096
9. Roseman L, Haijen E, Idialu-Ikato K, et al. Emotional breakthrough and psychedelics: validation of the Emotional Breakthrough Inventory. J Psychopharmacol. 2019;33(9):1076-1087.
10. Davis AK, Barrett FS, Griffiths RR. Psychological flexibility mediates the relations between acute psychedelic effects and subjective decreases in depression and anxiety. J Contextual Behav Sci. 2020;15:39-45.
11. Hayes SC, Luoma JB, Bond FW, et al. Acceptance and commitment therapy: model, processes and outcomes. Behav Res Ther. 2006;44(1):1-25.
12. Ross S, Bossis A, Guss J, et al. Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: a randomized controlled trial. J Psychopharmacol. 2016;30(12):1165-1180.
13. Stace WT. Mysticism and Philosophy. Macmillan Pub Ltd; 1960:37.
14. Barrett FS, Griffiths RR. Classic hallucinogens and mystical experiences: phenomenology and neural correlates. Curr Top Behav Neurosci. 2018;36:393-430.
15. Nour MM, Evans L, Nutt D, et al. Ego-dissolution and psychedelics: validation of the Ego-Dissolution Inventory (EDI). Front Hum Neurosci. 2016;10:269. doi:10.3389/fnhum.2016.00269
16. Olson DE. The subjective effects of psychedelics may not be necessary for their enduring therapeutic effects. ACS Pharmacol Transl Sci. 2020;4(2):563-567.
17. Carhart-Harris RL, Bolstridge M, Day CMJ, et al. Psilocybin with psychological support for treatment-resistant depression: six-month follow-up. Psychopharmacology (Berl). 2018;235(2):399-408.
18. Carhart-Harris RL. How do psychedelics work? Curr Opin Psychiatry. 2019;32(1):16-21.
19. Erritzoe D, Roseman L, Nour MM, et al. Effects of psilocybin therapy on personality structure. Acta Psychiatr Scand. 2018;138(5):368-378.
20. Lyons T, Carhart-Harris RL. Increased nature relatedness and decreased authoritarian political views after psilocybin for treatment-resistant depression. J Psychopharmacol. 2018;32(7):811-819.
21. Nour MM, Evans L, Carhart-Harris RL. Psychedelics, personality and political perspectives. J Psychoactive Drugs. 2017;49(3):182-191.
1. Delay J, Deniker P. Neuroleptic effects of chlorpromazine in therapeutics of neuropsychiatry. J Clin Exp Psychopathol. 1955;16(2):104-112.
2. Walsh Z, Mollaahmetoglu OM, Rootman, J, et al. Ketamine for the treatment of mental health and substance use disorders: comprehensive systematic review. BJPsych Open. 2021;8(1):e19. doi:10.1192/bjo.2021.1061
3. Lener MS, Kadriu B, Zarate CA Jr. Ketamine and beyond: investigations into the potential of glutamatergic agents to treat depression. Drugs. 2017;77(4):381-401.
4. Ayers B, Leaver A, Woods RP, et al. Structural plasticity of the hippocampus and amygdala induced by electroconvulsive therapy in major depression. Biol Psychiatry. 2016;79(4):282-292.
5. Cao B, Li R, Ding L, Xu J, et al. Does cognitive behaviour therapy affect peripheral inflammation of depression? A protocol for the systematic review and meta-analysis. BMJ Open. 2021;11(12):e048162. doi:10.1136/bmjopen-2020-048162
6. Wagner E, Siafis S, Fernando P, et al. Efficacy and safety of clozapine in psychotic disorders—a systematic quantitative meta-review. Transl Psychiatry. 2021;11(1):487.
7. Daws RE, Timmermann C, Giribaldi B, et al. Increased global integration in the brain after psilocybin therapy for depression. Nat Med. 2022;28(4):844-851.
8. Pearson C, Siegel J, Gold JA. Psilocybin-assisted psychotherapy for depression: emerging research on a psychedelic compound with a rich history. J Neurol Sci. 2022;434:120096. doi:10.1016/j.jns.2021.120096
9. Roseman L, Haijen E, Idialu-Ikato K, et al. Emotional breakthrough and psychedelics: validation of the Emotional Breakthrough Inventory. J Psychopharmacol. 2019;33(9):1076-1087.
10. Davis AK, Barrett FS, Griffiths RR. Psychological flexibility mediates the relations between acute psychedelic effects and subjective decreases in depression and anxiety. J Contextual Behav Sci. 2020;15:39-45.
11. Hayes SC, Luoma JB, Bond FW, et al. Acceptance and commitment therapy: model, processes and outcomes. Behav Res Ther. 2006;44(1):1-25.
12. Ross S, Bossis A, Guss J, et al. Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: a randomized controlled trial. J Psychopharmacol. 2016;30(12):1165-1180.
13. Stace WT. Mysticism and Philosophy. Macmillan Pub Ltd; 1960:37.
14. Barrett FS, Griffiths RR. Classic hallucinogens and mystical experiences: phenomenology and neural correlates. Curr Top Behav Neurosci. 2018;36:393-430.
15. Nour MM, Evans L, Nutt D, et al. Ego-dissolution and psychedelics: validation of the Ego-Dissolution Inventory (EDI). Front Hum Neurosci. 2016;10:269. doi:10.3389/fnhum.2016.00269
16. Olson DE. The subjective effects of psychedelics may not be necessary for their enduring therapeutic effects. ACS Pharmacol Transl Sci. 2020;4(2):563-567.
17. Carhart-Harris RL, Bolstridge M, Day CMJ, et al. Psilocybin with psychological support for treatment-resistant depression: six-month follow-up. Psychopharmacology (Berl). 2018;235(2):399-408.
18. Carhart-Harris RL. How do psychedelics work? Curr Opin Psychiatry. 2019;32(1):16-21.
19. Erritzoe D, Roseman L, Nour MM, et al. Effects of psilocybin therapy on personality structure. Acta Psychiatr Scand. 2018;138(5):368-378.
20. Lyons T, Carhart-Harris RL. Increased nature relatedness and decreased authoritarian political views after psilocybin for treatment-resistant depression. J Psychopharmacol. 2018;32(7):811-819.
21. Nour MM, Evans L, Carhart-Harris RL. Psychedelics, personality and political perspectives. J Psychoactive Drugs. 2017;49(3):182-191.
More on neurotransmitters
The series “Neurotransmitter-based diagnosis and treatment: A hypothesis” (Part 1:
The presentation of abnormal neurotransmission may occur along a continuum. For example, extreme dopamine deficiency can present as catatonia, moderate deficiency may present with inattention, normal activity permits adaptive functioning, and excitatory delirium and sudden death may be at the extreme end of dopaminergic excess.1
The amplitude, rate of change, and location of neurotransmitter dysfunction may determine which specialty takes the primary treatment role. Fatigue, pain, sleep difficulty, and emotional distress require clinicians to understand the whole patient, which is why health care professionals need cross training in psychiatry, and psychiatry recognizes multisystem pathology.
The recognition and treatment of substance use disorders requires an understanding of neurotransmitter symptoms, in terms of both acute drug effects and withdrawal. Fallows2 provides this information in an accessible chart. Discussions of neurotransmitters also have value in managing psychotropic medication withdrawal.3
Acetylcholine is another neurotransmitter of importance; it is essential to normal motor, cognitive, and emotional function. Extreme cholinergic deficiency or anticholinergic crisis has symptoms of pupillary dilation, psychosis, and delirium.4-6 The progressive decline seen in certain dementias is related in part to cholinergic deficit. Dominance of cholinergic activity is associated with depression and biomarkers such as increased rapid eye movement (REM) density, a measure of the frequency of rapid eye movements during REM sleep.7 Cholinergic excess or cholinergic crisis may present with symptoms of salivation, lacrimation, muscle weakness, delirium, or paralysis.8
The articles alluded to the interaction of neurotransmitter systems (eg, “dopamine blockade helps with endorphin suppression”). Isolating the effects of a single neurotransmitter is useful, but covariance of neurotransmitter activity also has diagnostic and treatment implications.9-11 Abnormalities in these interactions may be part of the causal process in fundamental cognitive functions.12 If endorphin suppression is insensitive to dopamine blockade, a relative endorphin excess may create symptoms. If acetylcholine changes are normally balanced by a relative increase in dopamine and norepinephrine, then a weak catecholamine response would fit the catecholamine-cholinergic balance hypothesis of depression. Neurotransmitter interactions are well worked out in the neurology of the basal ganglia but less clear in the frontal and limbic systems.13
Quantification has been applied in some areas of clinical care. Morphine equivalents are used to express opiate potency, and there are algorithms to summarize multiple medication effects on respiratory depression/overdose risk.14,15 Chlorpromazine equivalents were used to translate a range of antipsychotic potencies in the early days of antipsychotic treatment. Adverse effects and some treatment responses partially corresponded to the level of dopamine blockade, but not without noise. There is a wide range of variance as antipsychotic potency is assessed for clinical efficacy.16 We are still working on the array of medication potency and selectivity across neurotransmitter systems.17,18 For example, paroxetine is a potent serotonin reuptake blocker but less selective than citalopram, particularly antagonizing cholinergic muscarinic receptors.
The authors noted their hypothesis needs further elaboration and quantification as psychiatry moves from impressionistic practice to firmer science. Measurement of neurotransmitter activity is an area of intense research. Biomeasures have yet to add much value to the clinical practice of psychiatry, but we hope for progress. Functional neuroimaging with sophisticated algorithms is beginning to detail neocortical activity.19 CSF measurement of dopamine and serotonin metabolites seem to correlate with severe depression and suicidal behavior. Noninvasive, wearable technologies to measure galvanic skin response, oxygenation, and neurotransmitter metabolic products may add to neuro-transmitter-based assessment and treatment.
Neurotransmitters are one aspect of brain function. Other processes, such as hormonal neuromodulation20 and ion channels, may be over- or underactive. Channelopathies are of particular interest in cardiology and neurology but are also notable in pain and emotional disorders.21-26 Voltage-gated sodium channels are thought to be involved in general anesthesia.27 Adverse effects of some psychotropic medications are best understood as ion channel dysfunction.28 Using the strategy of this hypothesis applied to activation or inactivation of sodium, potassium, and calcium channels can guide useful diagnostic and treatment ideas for further study.
Mark C. Chandler, MD
Triangle Neuropsychiatry
Durham, North Carolina
Disclosures
The author reports no financial relationships with any companies whose products are mentioned in his letter, or with manufacturers of competing products.
References
1. Mash DC. Excited delirium and sudden death: a syndromal disorder at the extreme end of the neuropsychiatric continuum. Front Physiol. 2016;7:435.
2. Fallows Z. MIT MedLinks. Accessed August 8, 2022. http://web.mit.edu/zakf/www/drugchart/drugchart11.html
3. Groot PC, van Os J. How user knowledge of psychotropic drug withdrawal resulted in the development of person-specific tapering medication. Ther Adv Psychopharmacol. 2020;10:2045125320932452. doi:10.1177/2045125320932452
4. Picciotto MR, Higley MJ, Mineur YS. Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron. 2012;76(1):116-129.
5. Nair VP, Hunter JM. Anticholinesterases and anticholinergic drugs. Continuing Education in Anaesthesia Critical Care & Pain. 2004;4(5):164-168.
6. Dawson AH, Buckley NA. Pharmacological management of anticholinergic delirium--theory, evidence and practice. Br J Clin Pharmacol. 2016;81(3):516-524.
7. Dulawa SC, Janowsky DS. Cholinergic regulation of mood: from basic and clinical studies to emerging therapeutics. Mol Psychiatry. 2019;24(5):694-709.
8. Adeyinka A, Kondamudi NP. Cholinergic Crisis. StatPearls Publishing; 2022.
9. El Mansari M, Guiard BP, Chernoloz O, et al. Relevance of norepinephrine-dopamine interactions in the treatment of major depressive disorder. CNS Neurosci Ther. 2010;16(3):e1-e17.
10. Esposito E. Serotonin-dopamine interaction as a focus of novel antidepressant drugs. Curr Drug Targets. 2006;7(2):177-185.
11. Kringelbach ML, Cruzat J, Cabral J, et al. Dynamic coupling of whole-brain neuronal and neurotransmitter systems. Proc Natl Acad Sci U S A. 2020;117(17):9566-9576.
12. Thiele A, Bellgrove MA. Neuromodulation of attention. Neuron. 2018;97(4):769-785.
13. Muñoz A, Lopez-Lopez A, Labandeira CM, et al. Interactions between the serotonergic and other neurotransmitter systems in the basal ganglia: role in Parkinson’s disease and adverse effects of L-DOPA. Front Neuroanat. 2020;14:26.
14. Nielsen S, Degenhardt L, Hoban B, et al. A synthesis of oral morphine equivalents (OME) for opioid utilisation studies. Pharmacoepidemiol Drug Saf. 2016;25(6):733-737.
15. Lo-Ciganic WH, Huang JL, Zhang HH, et al. Evaluation of machine-learning algorithms for predicting opioid overdose risk among Medicare beneficiaries with opioid prescriptions. JAMA Netw Open. 2019;2(3):e190968. doi:10.1001/jamanetworkopen.2019.0968
16. Dewan MJ, Koss M. The clinical impact of reported variance in potency of antipsychotic agents. Acta Psychiatr Scand. 1995;91(4):229-232.
17. Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64(6):663-667.
18. Hayasaka Y, Purgato M, Magni LR, et al. Dose equivalents of antidepressants: evidence-based recommendations from randomized controlled trials. J Affect Disord. 2015;180:179-184.
19. Hansen JY, Shafiei G, Markello RD, et al. Mapping neurotransmitter systems to the structural and functional organization of the human neocortex. bioRxiv. 2021. https://doi.org/10.1101/2021.10.28.466336
20. Hwang WJ, Lee TY, Kim NS, et al. The role of estrogen receptors and their signaling across psychiatric disorders. Int J Mol Sci. 2020;22(1):373.
21. Lawrence JH, Tomaselli GF, Marban E. Ion channels: structure and function. Heart Dis Stroke. 1993;2(1):75-80.
22. Fedele F, Severino P, Bruno N, et al. Role of ion channels in coronary microcirculation: a review of the literature. Future Cardiol. 2013;9(6):897-905.
23. Kumar P, Kumar D, Jha SK, et al. Ion channels in neurological disorders. Adv Protein Chem Struct Biol. 2016;103:97-136.
24. Quagliato LA, Nardi AE. The role of convergent ion channel pathways in microglial phenotypes: a systematic review of the implications for neurological and psychiatric disorders. Transl Psychiatry. 2018;8(1):259.
25. Bianchi MT, Botzolakis EJ. Targeting ligand-gated ion channels in neurology and psychiatry: is pharmacological promiscuity an obstacle or an opportunity? BMC Pharmacol. 2010;10:3.
26. Imbrici P, Camerino DC, Tricarico D. Major channels involved in neuropsychiatric disorders and therapeutic perspectives. Front Genet. 2013;4:76.
27. Xiao J, Chen Z, Yu B. A potential mechanism of sodium channel mediating the general anesthesia induced by propofol. Front Cell Neurosci. 2020;14:593050. doi:10.3389/fncel.2020.593050
28. Kamei S, Sato N, Harayama Y, et al. Molecular analysis of potassium ion channel genes in sudden death cases among patients administered psychotropic drug therapy: are polymorphisms in LQT genes a potential risk factor? J Hum Genet. 2014;59(2):95-99.
The authors respond
Thank you for your thoughtful commentary. Our conceptual article was not designed to cover enough ground to be completely thorough. Everything you wrote adds to what we wanted to bring to the reader’s attention. The mechanisms of disease in psychiatry are numerous and still elusive, and the brain’s complexity is staggering. Our main goal was to point out possible correlations between specific symptoms and specific neurotransmitter activity. We had to oversimplify to make the article concise enough for publication. Neurotransmitter effects are based on their synthesis, storage, release, reuptake, and degradation. A receptor’s quantity and quality of function, inhibitors, inducers, and many other factors are involved in neurotransmitter performance. And, of course, there are additional fundamental neurotransmitters beyond the 6 we touched on. Our ability to sort through all of this is still rudimentary. You also reflect on the emerging methods to objectively measure neurotransmitter activity, which will eventually find their way to clinical practice and become invaluable. Still, we treat people, not tests or pictures, so diagnostic thinking based on clinical presentation will forever remain a cornerstone of dealing with individual patients.
We hope scientists and clinicians such as yourself will improve our concept and make it truly practical.
Dmitry M. Arbuck, MD
Clinical Assistant Professor of Psychiatry and Medicine
Indiana University School of Medicine
Indianapolis, Indiana
President and Medical Director
Indiana Polyclinic
Carmel, Indiana
José Miguel Salmerón, MD
Professor
Department of Psychiatry
Universidad del Valle School of Medicine/Hospital
Universitario del Valle
Cali, Colombia
Disclosures
The authors report no financial relationships with any companies whose products are mentioned in their response, or with manufacturers of competing products.
The series “Neurotransmitter-based diagnosis and treatment: A hypothesis” (Part 1:
The presentation of abnormal neurotransmission may occur along a continuum. For example, extreme dopamine deficiency can present as catatonia, moderate deficiency may present with inattention, normal activity permits adaptive functioning, and excitatory delirium and sudden death may be at the extreme end of dopaminergic excess.1
The amplitude, rate of change, and location of neurotransmitter dysfunction may determine which specialty takes the primary treatment role. Fatigue, pain, sleep difficulty, and emotional distress require clinicians to understand the whole patient, which is why health care professionals need cross training in psychiatry, and psychiatry recognizes multisystem pathology.
The recognition and treatment of substance use disorders requires an understanding of neurotransmitter symptoms, in terms of both acute drug effects and withdrawal. Fallows2 provides this information in an accessible chart. Discussions of neurotransmitters also have value in managing psychotropic medication withdrawal.3
Acetylcholine is another neurotransmitter of importance; it is essential to normal motor, cognitive, and emotional function. Extreme cholinergic deficiency or anticholinergic crisis has symptoms of pupillary dilation, psychosis, and delirium.4-6 The progressive decline seen in certain dementias is related in part to cholinergic deficit. Dominance of cholinergic activity is associated with depression and biomarkers such as increased rapid eye movement (REM) density, a measure of the frequency of rapid eye movements during REM sleep.7 Cholinergic excess or cholinergic crisis may present with symptoms of salivation, lacrimation, muscle weakness, delirium, or paralysis.8
The articles alluded to the interaction of neurotransmitter systems (eg, “dopamine blockade helps with endorphin suppression”). Isolating the effects of a single neurotransmitter is useful, but covariance of neurotransmitter activity also has diagnostic and treatment implications.9-11 Abnormalities in these interactions may be part of the causal process in fundamental cognitive functions.12 If endorphin suppression is insensitive to dopamine blockade, a relative endorphin excess may create symptoms. If acetylcholine changes are normally balanced by a relative increase in dopamine and norepinephrine, then a weak catecholamine response would fit the catecholamine-cholinergic balance hypothesis of depression. Neurotransmitter interactions are well worked out in the neurology of the basal ganglia but less clear in the frontal and limbic systems.13
Quantification has been applied in some areas of clinical care. Morphine equivalents are used to express opiate potency, and there are algorithms to summarize multiple medication effects on respiratory depression/overdose risk.14,15 Chlorpromazine equivalents were used to translate a range of antipsychotic potencies in the early days of antipsychotic treatment. Adverse effects and some treatment responses partially corresponded to the level of dopamine blockade, but not without noise. There is a wide range of variance as antipsychotic potency is assessed for clinical efficacy.16 We are still working on the array of medication potency and selectivity across neurotransmitter systems.17,18 For example, paroxetine is a potent serotonin reuptake blocker but less selective than citalopram, particularly antagonizing cholinergic muscarinic receptors.
The authors noted their hypothesis needs further elaboration and quantification as psychiatry moves from impressionistic practice to firmer science. Measurement of neurotransmitter activity is an area of intense research. Biomeasures have yet to add much value to the clinical practice of psychiatry, but we hope for progress. Functional neuroimaging with sophisticated algorithms is beginning to detail neocortical activity.19 CSF measurement of dopamine and serotonin metabolites seem to correlate with severe depression and suicidal behavior. Noninvasive, wearable technologies to measure galvanic skin response, oxygenation, and neurotransmitter metabolic products may add to neuro-transmitter-based assessment and treatment.
Neurotransmitters are one aspect of brain function. Other processes, such as hormonal neuromodulation20 and ion channels, may be over- or underactive. Channelopathies are of particular interest in cardiology and neurology but are also notable in pain and emotional disorders.21-26 Voltage-gated sodium channels are thought to be involved in general anesthesia.27 Adverse effects of some psychotropic medications are best understood as ion channel dysfunction.28 Using the strategy of this hypothesis applied to activation or inactivation of sodium, potassium, and calcium channels can guide useful diagnostic and treatment ideas for further study.
Mark C. Chandler, MD
Triangle Neuropsychiatry
Durham, North Carolina
Disclosures
The author reports no financial relationships with any companies whose products are mentioned in his letter, or with manufacturers of competing products.
References
1. Mash DC. Excited delirium and sudden death: a syndromal disorder at the extreme end of the neuropsychiatric continuum. Front Physiol. 2016;7:435.
2. Fallows Z. MIT MedLinks. Accessed August 8, 2022. http://web.mit.edu/zakf/www/drugchart/drugchart11.html
3. Groot PC, van Os J. How user knowledge of psychotropic drug withdrawal resulted in the development of person-specific tapering medication. Ther Adv Psychopharmacol. 2020;10:2045125320932452. doi:10.1177/2045125320932452
4. Picciotto MR, Higley MJ, Mineur YS. Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron. 2012;76(1):116-129.
5. Nair VP, Hunter JM. Anticholinesterases and anticholinergic drugs. Continuing Education in Anaesthesia Critical Care & Pain. 2004;4(5):164-168.
6. Dawson AH, Buckley NA. Pharmacological management of anticholinergic delirium--theory, evidence and practice. Br J Clin Pharmacol. 2016;81(3):516-524.
7. Dulawa SC, Janowsky DS. Cholinergic regulation of mood: from basic and clinical studies to emerging therapeutics. Mol Psychiatry. 2019;24(5):694-709.
8. Adeyinka A, Kondamudi NP. Cholinergic Crisis. StatPearls Publishing; 2022.
9. El Mansari M, Guiard BP, Chernoloz O, et al. Relevance of norepinephrine-dopamine interactions in the treatment of major depressive disorder. CNS Neurosci Ther. 2010;16(3):e1-e17.
10. Esposito E. Serotonin-dopamine interaction as a focus of novel antidepressant drugs. Curr Drug Targets. 2006;7(2):177-185.
11. Kringelbach ML, Cruzat J, Cabral J, et al. Dynamic coupling of whole-brain neuronal and neurotransmitter systems. Proc Natl Acad Sci U S A. 2020;117(17):9566-9576.
12. Thiele A, Bellgrove MA. Neuromodulation of attention. Neuron. 2018;97(4):769-785.
13. Muñoz A, Lopez-Lopez A, Labandeira CM, et al. Interactions between the serotonergic and other neurotransmitter systems in the basal ganglia: role in Parkinson’s disease and adverse effects of L-DOPA. Front Neuroanat. 2020;14:26.
14. Nielsen S, Degenhardt L, Hoban B, et al. A synthesis of oral morphine equivalents (OME) for opioid utilisation studies. Pharmacoepidemiol Drug Saf. 2016;25(6):733-737.
15. Lo-Ciganic WH, Huang JL, Zhang HH, et al. Evaluation of machine-learning algorithms for predicting opioid overdose risk among Medicare beneficiaries with opioid prescriptions. JAMA Netw Open. 2019;2(3):e190968. doi:10.1001/jamanetworkopen.2019.0968
16. Dewan MJ, Koss M. The clinical impact of reported variance in potency of antipsychotic agents. Acta Psychiatr Scand. 1995;91(4):229-232.
17. Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64(6):663-667.
18. Hayasaka Y, Purgato M, Magni LR, et al. Dose equivalents of antidepressants: evidence-based recommendations from randomized controlled trials. J Affect Disord. 2015;180:179-184.
19. Hansen JY, Shafiei G, Markello RD, et al. Mapping neurotransmitter systems to the structural and functional organization of the human neocortex. bioRxiv. 2021. https://doi.org/10.1101/2021.10.28.466336
20. Hwang WJ, Lee TY, Kim NS, et al. The role of estrogen receptors and their signaling across psychiatric disorders. Int J Mol Sci. 2020;22(1):373.
21. Lawrence JH, Tomaselli GF, Marban E. Ion channels: structure and function. Heart Dis Stroke. 1993;2(1):75-80.
22. Fedele F, Severino P, Bruno N, et al. Role of ion channels in coronary microcirculation: a review of the literature. Future Cardiol. 2013;9(6):897-905.
23. Kumar P, Kumar D, Jha SK, et al. Ion channels in neurological disorders. Adv Protein Chem Struct Biol. 2016;103:97-136.
24. Quagliato LA, Nardi AE. The role of convergent ion channel pathways in microglial phenotypes: a systematic review of the implications for neurological and psychiatric disorders. Transl Psychiatry. 2018;8(1):259.
25. Bianchi MT, Botzolakis EJ. Targeting ligand-gated ion channels in neurology and psychiatry: is pharmacological promiscuity an obstacle or an opportunity? BMC Pharmacol. 2010;10:3.
26. Imbrici P, Camerino DC, Tricarico D. Major channels involved in neuropsychiatric disorders and therapeutic perspectives. Front Genet. 2013;4:76.
27. Xiao J, Chen Z, Yu B. A potential mechanism of sodium channel mediating the general anesthesia induced by propofol. Front Cell Neurosci. 2020;14:593050. doi:10.3389/fncel.2020.593050
28. Kamei S, Sato N, Harayama Y, et al. Molecular analysis of potassium ion channel genes in sudden death cases among patients administered psychotropic drug therapy: are polymorphisms in LQT genes a potential risk factor? J Hum Genet. 2014;59(2):95-99.
The authors respond
Thank you for your thoughtful commentary. Our conceptual article was not designed to cover enough ground to be completely thorough. Everything you wrote adds to what we wanted to bring to the reader’s attention. The mechanisms of disease in psychiatry are numerous and still elusive, and the brain’s complexity is staggering. Our main goal was to point out possible correlations between specific symptoms and specific neurotransmitter activity. We had to oversimplify to make the article concise enough for publication. Neurotransmitter effects are based on their synthesis, storage, release, reuptake, and degradation. A receptor’s quantity and quality of function, inhibitors, inducers, and many other factors are involved in neurotransmitter performance. And, of course, there are additional fundamental neurotransmitters beyond the 6 we touched on. Our ability to sort through all of this is still rudimentary. You also reflect on the emerging methods to objectively measure neurotransmitter activity, which will eventually find their way to clinical practice and become invaluable. Still, we treat people, not tests or pictures, so diagnostic thinking based on clinical presentation will forever remain a cornerstone of dealing with individual patients.
We hope scientists and clinicians such as yourself will improve our concept and make it truly practical.
Dmitry M. Arbuck, MD
Clinical Assistant Professor of Psychiatry and Medicine
Indiana University School of Medicine
Indianapolis, Indiana
President and Medical Director
Indiana Polyclinic
Carmel, Indiana
José Miguel Salmerón, MD
Professor
Department of Psychiatry
Universidad del Valle School of Medicine/Hospital
Universitario del Valle
Cali, Colombia
Disclosures
The authors report no financial relationships with any companies whose products are mentioned in their response, or with manufacturers of competing products.
The series “Neurotransmitter-based diagnosis and treatment: A hypothesis” (Part 1:
The presentation of abnormal neurotransmission may occur along a continuum. For example, extreme dopamine deficiency can present as catatonia, moderate deficiency may present with inattention, normal activity permits adaptive functioning, and excitatory delirium and sudden death may be at the extreme end of dopaminergic excess.1
The amplitude, rate of change, and location of neurotransmitter dysfunction may determine which specialty takes the primary treatment role. Fatigue, pain, sleep difficulty, and emotional distress require clinicians to understand the whole patient, which is why health care professionals need cross training in psychiatry, and psychiatry recognizes multisystem pathology.
The recognition and treatment of substance use disorders requires an understanding of neurotransmitter symptoms, in terms of both acute drug effects and withdrawal. Fallows2 provides this information in an accessible chart. Discussions of neurotransmitters also have value in managing psychotropic medication withdrawal.3
Acetylcholine is another neurotransmitter of importance; it is essential to normal motor, cognitive, and emotional function. Extreme cholinergic deficiency or anticholinergic crisis has symptoms of pupillary dilation, psychosis, and delirium.4-6 The progressive decline seen in certain dementias is related in part to cholinergic deficit. Dominance of cholinergic activity is associated with depression and biomarkers such as increased rapid eye movement (REM) density, a measure of the frequency of rapid eye movements during REM sleep.7 Cholinergic excess or cholinergic crisis may present with symptoms of salivation, lacrimation, muscle weakness, delirium, or paralysis.8
The articles alluded to the interaction of neurotransmitter systems (eg, “dopamine blockade helps with endorphin suppression”). Isolating the effects of a single neurotransmitter is useful, but covariance of neurotransmitter activity also has diagnostic and treatment implications.9-11 Abnormalities in these interactions may be part of the causal process in fundamental cognitive functions.12 If endorphin suppression is insensitive to dopamine blockade, a relative endorphin excess may create symptoms. If acetylcholine changes are normally balanced by a relative increase in dopamine and norepinephrine, then a weak catecholamine response would fit the catecholamine-cholinergic balance hypothesis of depression. Neurotransmitter interactions are well worked out in the neurology of the basal ganglia but less clear in the frontal and limbic systems.13
Quantification has been applied in some areas of clinical care. Morphine equivalents are used to express opiate potency, and there are algorithms to summarize multiple medication effects on respiratory depression/overdose risk.14,15 Chlorpromazine equivalents were used to translate a range of antipsychotic potencies in the early days of antipsychotic treatment. Adverse effects and some treatment responses partially corresponded to the level of dopamine blockade, but not without noise. There is a wide range of variance as antipsychotic potency is assessed for clinical efficacy.16 We are still working on the array of medication potency and selectivity across neurotransmitter systems.17,18 For example, paroxetine is a potent serotonin reuptake blocker but less selective than citalopram, particularly antagonizing cholinergic muscarinic receptors.
The authors noted their hypothesis needs further elaboration and quantification as psychiatry moves from impressionistic practice to firmer science. Measurement of neurotransmitter activity is an area of intense research. Biomeasures have yet to add much value to the clinical practice of psychiatry, but we hope for progress. Functional neuroimaging with sophisticated algorithms is beginning to detail neocortical activity.19 CSF measurement of dopamine and serotonin metabolites seem to correlate with severe depression and suicidal behavior. Noninvasive, wearable technologies to measure galvanic skin response, oxygenation, and neurotransmitter metabolic products may add to neuro-transmitter-based assessment and treatment.
Neurotransmitters are one aspect of brain function. Other processes, such as hormonal neuromodulation20 and ion channels, may be over- or underactive. Channelopathies are of particular interest in cardiology and neurology but are also notable in pain and emotional disorders.21-26 Voltage-gated sodium channels are thought to be involved in general anesthesia.27 Adverse effects of some psychotropic medications are best understood as ion channel dysfunction.28 Using the strategy of this hypothesis applied to activation or inactivation of sodium, potassium, and calcium channels can guide useful diagnostic and treatment ideas for further study.
Mark C. Chandler, MD
Triangle Neuropsychiatry
Durham, North Carolina
Disclosures
The author reports no financial relationships with any companies whose products are mentioned in his letter, or with manufacturers of competing products.
References
1. Mash DC. Excited delirium and sudden death: a syndromal disorder at the extreme end of the neuropsychiatric continuum. Front Physiol. 2016;7:435.
2. Fallows Z. MIT MedLinks. Accessed August 8, 2022. http://web.mit.edu/zakf/www/drugchart/drugchart11.html
3. Groot PC, van Os J. How user knowledge of psychotropic drug withdrawal resulted in the development of person-specific tapering medication. Ther Adv Psychopharmacol. 2020;10:2045125320932452. doi:10.1177/2045125320932452
4. Picciotto MR, Higley MJ, Mineur YS. Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron. 2012;76(1):116-129.
5. Nair VP, Hunter JM. Anticholinesterases and anticholinergic drugs. Continuing Education in Anaesthesia Critical Care & Pain. 2004;4(5):164-168.
6. Dawson AH, Buckley NA. Pharmacological management of anticholinergic delirium--theory, evidence and practice. Br J Clin Pharmacol. 2016;81(3):516-524.
7. Dulawa SC, Janowsky DS. Cholinergic regulation of mood: from basic and clinical studies to emerging therapeutics. Mol Psychiatry. 2019;24(5):694-709.
8. Adeyinka A, Kondamudi NP. Cholinergic Crisis. StatPearls Publishing; 2022.
9. El Mansari M, Guiard BP, Chernoloz O, et al. Relevance of norepinephrine-dopamine interactions in the treatment of major depressive disorder. CNS Neurosci Ther. 2010;16(3):e1-e17.
10. Esposito E. Serotonin-dopamine interaction as a focus of novel antidepressant drugs. Curr Drug Targets. 2006;7(2):177-185.
11. Kringelbach ML, Cruzat J, Cabral J, et al. Dynamic coupling of whole-brain neuronal and neurotransmitter systems. Proc Natl Acad Sci U S A. 2020;117(17):9566-9576.
12. Thiele A, Bellgrove MA. Neuromodulation of attention. Neuron. 2018;97(4):769-785.
13. Muñoz A, Lopez-Lopez A, Labandeira CM, et al. Interactions between the serotonergic and other neurotransmitter systems in the basal ganglia: role in Parkinson’s disease and adverse effects of L-DOPA. Front Neuroanat. 2020;14:26.
14. Nielsen S, Degenhardt L, Hoban B, et al. A synthesis of oral morphine equivalents (OME) for opioid utilisation studies. Pharmacoepidemiol Drug Saf. 2016;25(6):733-737.
15. Lo-Ciganic WH, Huang JL, Zhang HH, et al. Evaluation of machine-learning algorithms for predicting opioid overdose risk among Medicare beneficiaries with opioid prescriptions. JAMA Netw Open. 2019;2(3):e190968. doi:10.1001/jamanetworkopen.2019.0968
16. Dewan MJ, Koss M. The clinical impact of reported variance in potency of antipsychotic agents. Acta Psychiatr Scand. 1995;91(4):229-232.
17. Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64(6):663-667.
18. Hayasaka Y, Purgato M, Magni LR, et al. Dose equivalents of antidepressants: evidence-based recommendations from randomized controlled trials. J Affect Disord. 2015;180:179-184.
19. Hansen JY, Shafiei G, Markello RD, et al. Mapping neurotransmitter systems to the structural and functional organization of the human neocortex. bioRxiv. 2021. https://doi.org/10.1101/2021.10.28.466336
20. Hwang WJ, Lee TY, Kim NS, et al. The role of estrogen receptors and their signaling across psychiatric disorders. Int J Mol Sci. 2020;22(1):373.
21. Lawrence JH, Tomaselli GF, Marban E. Ion channels: structure and function. Heart Dis Stroke. 1993;2(1):75-80.
22. Fedele F, Severino P, Bruno N, et al. Role of ion channels in coronary microcirculation: a review of the literature. Future Cardiol. 2013;9(6):897-905.
23. Kumar P, Kumar D, Jha SK, et al. Ion channels in neurological disorders. Adv Protein Chem Struct Biol. 2016;103:97-136.
24. Quagliato LA, Nardi AE. The role of convergent ion channel pathways in microglial phenotypes: a systematic review of the implications for neurological and psychiatric disorders. Transl Psychiatry. 2018;8(1):259.
25. Bianchi MT, Botzolakis EJ. Targeting ligand-gated ion channels in neurology and psychiatry: is pharmacological promiscuity an obstacle or an opportunity? BMC Pharmacol. 2010;10:3.
26. Imbrici P, Camerino DC, Tricarico D. Major channels involved in neuropsychiatric disorders and therapeutic perspectives. Front Genet. 2013;4:76.
27. Xiao J, Chen Z, Yu B. A potential mechanism of sodium channel mediating the general anesthesia induced by propofol. Front Cell Neurosci. 2020;14:593050. doi:10.3389/fncel.2020.593050
28. Kamei S, Sato N, Harayama Y, et al. Molecular analysis of potassium ion channel genes in sudden death cases among patients administered psychotropic drug therapy: are polymorphisms in LQT genes a potential risk factor? J Hum Genet. 2014;59(2):95-99.
The authors respond
Thank you for your thoughtful commentary. Our conceptual article was not designed to cover enough ground to be completely thorough. Everything you wrote adds to what we wanted to bring to the reader’s attention. The mechanisms of disease in psychiatry are numerous and still elusive, and the brain’s complexity is staggering. Our main goal was to point out possible correlations between specific symptoms and specific neurotransmitter activity. We had to oversimplify to make the article concise enough for publication. Neurotransmitter effects are based on their synthesis, storage, release, reuptake, and degradation. A receptor’s quantity and quality of function, inhibitors, inducers, and many other factors are involved in neurotransmitter performance. And, of course, there are additional fundamental neurotransmitters beyond the 6 we touched on. Our ability to sort through all of this is still rudimentary. You also reflect on the emerging methods to objectively measure neurotransmitter activity, which will eventually find their way to clinical practice and become invaluable. Still, we treat people, not tests or pictures, so diagnostic thinking based on clinical presentation will forever remain a cornerstone of dealing with individual patients.
We hope scientists and clinicians such as yourself will improve our concept and make it truly practical.
Dmitry M. Arbuck, MD
Clinical Assistant Professor of Psychiatry and Medicine
Indiana University School of Medicine
Indianapolis, Indiana
President and Medical Director
Indiana Polyclinic
Carmel, Indiana
José Miguel Salmerón, MD
Professor
Department of Psychiatry
Universidad del Valle School of Medicine/Hospital
Universitario del Valle
Cali, Colombia
Disclosures
The authors report no financial relationships with any companies whose products are mentioned in their response, or with manufacturers of competing products.
Drug-induced progressive multifocal leukoencephalopathy: Rare but serious
Mr. P, age 67, presents to the clinic with vision changes and memory loss following a fall in his home due to limb weakness. Six years ago, his care team diagnosed him with rheumatoid arthritis (RA). Mr. P’s current medication regimen includes methotrexate 20 mg once weekly and etanercept 50 mg once weekly, and he has been stable on this plan for 3 years. Mr. P also was recently diagnosed with major depressive disorder (MDD), but has not yet started treatment. Following a complete workup, an MRI of Mr. P’s brain revealed white matter demyelination. Due to these findings, he is scheduled for a brain biopsy, which confirms a diagnosis of progressive multifocal leukoencephalopathy (PML).
PML is a demyelinating disease of the central nervous system caused by the John Cunningham virus (JCV), or JC polyomavirus, named for the first patient identified to have contracted the virus.1 Asymptomatic infection of JCV often occurs in childhood, and antibodies are found in ≤70% of healthy adults. In most individuals, JCV remains latent in the kidneys and lymphoid organs, but immunosuppression can cause it to reactivate.2
JCV infects oligodendrocytes, astrocytes, and neurons, which results in white matter demyelination. Due to this demyelination, individuals can experience visual field defects, speech disturbances, ataxia, paresthesia, and cognitive impairments.2 Limb weakness presents in 60% of patients with PML, visual disturbances in 20%, and gait disturbances in 65%.3 Progression of these symptoms can lead to a more severe clinical presentation, including focal seizures in ≤10% of patients, and the mortality rate is 30% to 50%.3 Patients with comorbid HIV have a mortality rate ≤90%.2
Currently, there are no biomarkers that can identify PML in its early stages. A PML diagnosis is typically based on the patient’s clinical presentation, radiological imaging, and detection of JCV DNA. A brain biopsy is the gold standard for PML diagnosis.1
Interestingly, data suggest that glial cells harboring JCV in the brain express receptors for serotonin and dopamine.4 Researchers pinpointed 5HT2A receptors as JCV entry points into cells, and theorized that medications competing for binding, such as certain psychotropic agents, might decrease JCV entry. Cells lacking the 5HT2A receptor have shown immunity to JCV infection and the ability of cells to be infected was restored through transfection of 5HT2A receptors.4
Immunosuppressant medications can cause PML
PML was initially seen in individuals with conditions that cause immunosuppression, such as malignancies and HIV. However, “drug-induced PML” refers to cases in which drug-induced immunosuppression creates an environment that allows JCV to reactivate and disseminate back into the CNS.4 It is important to emphasize that drug-induced PML is a very rare effect of certain immunosuppressant medications. Medications that can weaken the immune system include glucocorticoids, monoclonal antibodies, alkylating agents, purine analogues, antimetabolites, and immunosuppressants (Table).1
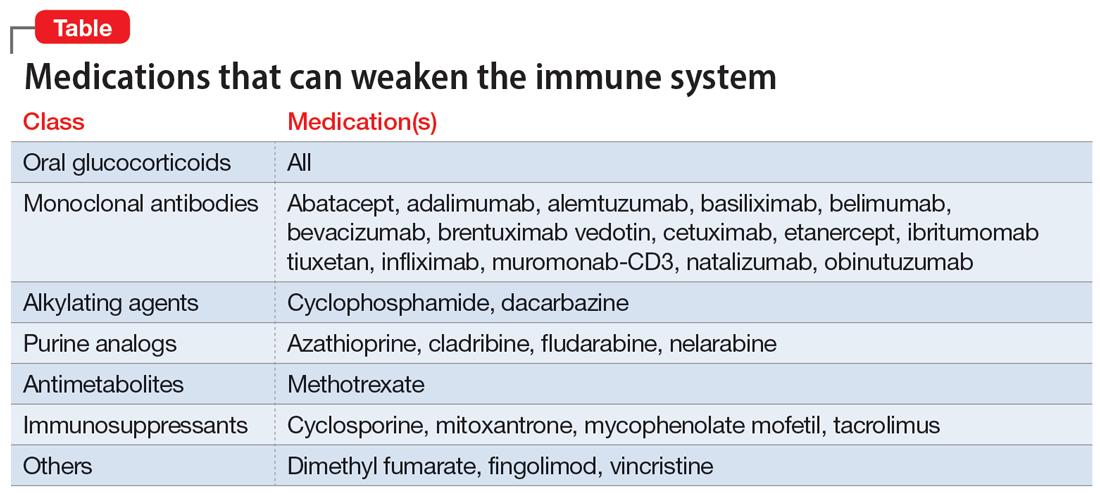
These medications are used to treat conditions such as multiple sclerosis, RA, psoriatic arthritis, and lupus. Although drug-induced PML can result from the use of any of these agents, the highest incidence (1%) is found with natalizumab. Rates of incidence with other agents are either unknown or as low as .002%.1 Evidence suggests that the risk for PML increases with the duration of therapy.5
Continue to: Management
Management: Stop the offending agent, restore immune function
Specific pharmacologic treatments for PML are lacking. Management of drug-induced PML starts with discontinuing the offending agent. Restoring immune function has been found to be the most effective approach to treat PML.3 Restoration is possible through interleukin-2 (IL-2), IL-7, and T-cell infusions. Other treatment options are theoretical and include the development of a JCV vaccine to stimulate host response, plasma exchange to remove the medication from the host, and antiviral therapy targeting JCV replication. Diclofenac, isotretinoin, and mefloquine can inhibit JCV replication.3
Based on the theory that JCV requires 5HT2A receptors for entry into cells, researchers have studied medications that block this receptor as a treatment for PML. The first-generation antipsychotic chlorpromazine did not show benefit when combined with cidofovir, a replication inhibitor.3 Antipsychotics agents such as ziprasidone and olanzapine have shown in vitro inhibition of JCV, while risperidone has mixed results, with 1 trial failing to find a difference on JCV in fetal glial cells.3 Second-generation antipsychotics may be the preferred option due to more potent antagonism of the 5HT2A receptors and fewer adverse effects compared to agents such as chlorpromazine.4 The antidepressant mirtazapine has shown to have promising results, with evidence indicating that earlier initiation is more beneficial.3 Overall, data involving the use of medications that act on the 5HT2A receptor are mixed. Recent data suggest that JCV might enter cells independent of 5HT2A receptors; however, more research in this area is needed.2
The best strategy for treating drug-induced PML has not yet been determined. While combination therapy is thought to be more successful than monotherapy, ultimately, it depends on the patient’s immune response. If a psychotropic medication is chosen as adjunct treatment for drug-induced PML, it is prudent to assess the patient’s entire clinical picture to determine the specific indication for therapy (ie, treating symptomatology or drug-induced PML).
CASE CONTINUED
Following diagnosis, Mr. P is provided supportive therapy, and his care team discontinues methotrexate and etanercept. Although data are mixed on the efficacy of medications that work on 5HT2A receptors, because Mr. P was recently diagnosed with MDD, he is started on mirtazapine 15 mg/d at night in an attempt to manage both MDD and PML. It is possible that his depressive symptoms developed as a result of drug-induced PML rather than major depressive disorder. Discontinuing methotrexate and etanercept stabilizes Mr. P’s PML symptoms but leads to an exacerbation of his RA symptoms. Mr. P is initiated on hyd
Related Resources
- Castle D, Robertson NP. Treatment of progressive multifocal leukoencephalopathy. J Neurol. 2019;266(10):2587-2589. doi:10.1007/s00415-019-09501-y
Drug Brand Names
Abatacept • Orencia
Adalimumab • Humira
Alemtuzumab • Campath
Azathioprine • Azasan, Imuran
Basiliximab • Simulect
Belimumab • Benlysta
Bevacizumab • Avastin
Brentuximab vedotin • Adcetris
Cetuximab • Erbitux
Chlorpromazine • Thorazine, Largactil
Cidofovir • Vistide
Cladribine • Mavenclad
Cyclophosphamide • Cytoxan
Cyclosporine • Gengraf, Neoral
Dacarbazine • DTIC-Dome
Diclofenac • Cambia, Zorvolex
Dimethyl fumarate • Tecfidera
Etanercept • Enbrel
Fingolimod • Gilenya
Fludarabine • Fludara
Hydroxychloroquine • Plaquenil
Ibritumomab tiuxetan • Zevalin
Infliximab • Avsola, Inflectra
Isotretinoin • Absorica, Claravis
Mefloquine • Lariam
Methotrexate • Rheumatrex, Trexall
Mirtazapine • Remeron
Mitoxantrone • Novantrone
Muromonab-CD3 • Orthoclone OKT3
Mycophenolate mofetil • CellCept
Natalizumab • Tysabri
Nelarabine • Arranon
Obinutuzumab • Gazyva
Olanzapine • Zyprexa
Risperidone • Risperdal
Tacrolimus • Prograf
Vincristine • Vincasar PFS
Ziprasidone • Geodon
1. Yukitake M. Drug-induced progressive multifocal leukoencephalopathy in multiple sclerosis: a comprehensive review. Clin Exp Neuroimmunol. 2018;9(1):37-47. doi:10.1111/cen3.12440
2. Alstadhaug KB, Myhr KM, Rinaldo CH. Progressive multifocal leukoencephalopathy. Tidsskr Nor Laegeforen. 2017;137(23-24):10.4045/tidsskr.16.1092. doi:10.4045/tidsskr.16.1092
3. Williamson EML, Berger JR. Diagnosis and treatment of progressive multifocal leukoencephalopathy associated with multiple sclerosis therapies. Neurotherapeutics. 2017;14(4):961-973. doi:10.1007/s13311-017-0570-7
4. Altschuler EL, Kast RE. The atypical antipsychotic agents ziprasidone, risperidone and olanzapine as treatment for and prophylaxis against progressive multifocal leukoencephalopathy. Med Hypotheses. 2005;65(3):585-586.
5. Vinhas de Souza M, Keller-Stanislawski B, Blake K, et al. Drug-induced PML: a global agenda for a global challenge. Clin Pharmacol Ther. 2012;91(4):747-750. doi:10.1038/clpt.2012.4
Mr. P, age 67, presents to the clinic with vision changes and memory loss following a fall in his home due to limb weakness. Six years ago, his care team diagnosed him with rheumatoid arthritis (RA). Mr. P’s current medication regimen includes methotrexate 20 mg once weekly and etanercept 50 mg once weekly, and he has been stable on this plan for 3 years. Mr. P also was recently diagnosed with major depressive disorder (MDD), but has not yet started treatment. Following a complete workup, an MRI of Mr. P’s brain revealed white matter demyelination. Due to these findings, he is scheduled for a brain biopsy, which confirms a diagnosis of progressive multifocal leukoencephalopathy (PML).
PML is a demyelinating disease of the central nervous system caused by the John Cunningham virus (JCV), or JC polyomavirus, named for the first patient identified to have contracted the virus.1 Asymptomatic infection of JCV often occurs in childhood, and antibodies are found in ≤70% of healthy adults. In most individuals, JCV remains latent in the kidneys and lymphoid organs, but immunosuppression can cause it to reactivate.2
JCV infects oligodendrocytes, astrocytes, and neurons, which results in white matter demyelination. Due to this demyelination, individuals can experience visual field defects, speech disturbances, ataxia, paresthesia, and cognitive impairments.2 Limb weakness presents in 60% of patients with PML, visual disturbances in 20%, and gait disturbances in 65%.3 Progression of these symptoms can lead to a more severe clinical presentation, including focal seizures in ≤10% of patients, and the mortality rate is 30% to 50%.3 Patients with comorbid HIV have a mortality rate ≤90%.2
Currently, there are no biomarkers that can identify PML in its early stages. A PML diagnosis is typically based on the patient’s clinical presentation, radiological imaging, and detection of JCV DNA. A brain biopsy is the gold standard for PML diagnosis.1
Interestingly, data suggest that glial cells harboring JCV in the brain express receptors for serotonin and dopamine.4 Researchers pinpointed 5HT2A receptors as JCV entry points into cells, and theorized that medications competing for binding, such as certain psychotropic agents, might decrease JCV entry. Cells lacking the 5HT2A receptor have shown immunity to JCV infection and the ability of cells to be infected was restored through transfection of 5HT2A receptors.4
Immunosuppressant medications can cause PML
PML was initially seen in individuals with conditions that cause immunosuppression, such as malignancies and HIV. However, “drug-induced PML” refers to cases in which drug-induced immunosuppression creates an environment that allows JCV to reactivate and disseminate back into the CNS.4 It is important to emphasize that drug-induced PML is a very rare effect of certain immunosuppressant medications. Medications that can weaken the immune system include glucocorticoids, monoclonal antibodies, alkylating agents, purine analogues, antimetabolites, and immunosuppressants (Table).1

These medications are used to treat conditions such as multiple sclerosis, RA, psoriatic arthritis, and lupus. Although drug-induced PML can result from the use of any of these agents, the highest incidence (1%) is found with natalizumab. Rates of incidence with other agents are either unknown or as low as .002%.1 Evidence suggests that the risk for PML increases with the duration of therapy.5
Continue to: Management
Management: Stop the offending agent, restore immune function
Specific pharmacologic treatments for PML are lacking. Management of drug-induced PML starts with discontinuing the offending agent. Restoring immune function has been found to be the most effective approach to treat PML.3 Restoration is possible through interleukin-2 (IL-2), IL-7, and T-cell infusions. Other treatment options are theoretical and include the development of a JCV vaccine to stimulate host response, plasma exchange to remove the medication from the host, and antiviral therapy targeting JCV replication. Diclofenac, isotretinoin, and mefloquine can inhibit JCV replication.3
Based on the theory that JCV requires 5HT2A receptors for entry into cells, researchers have studied medications that block this receptor as a treatment for PML. The first-generation antipsychotic chlorpromazine did not show benefit when combined with cidofovir, a replication inhibitor.3 Antipsychotics agents such as ziprasidone and olanzapine have shown in vitro inhibition of JCV, while risperidone has mixed results, with 1 trial failing to find a difference on JCV in fetal glial cells.3 Second-generation antipsychotics may be the preferred option due to more potent antagonism of the 5HT2A receptors and fewer adverse effects compared to agents such as chlorpromazine.4 The antidepressant mirtazapine has shown to have promising results, with evidence indicating that earlier initiation is more beneficial.3 Overall, data involving the use of medications that act on the 5HT2A receptor are mixed. Recent data suggest that JCV might enter cells independent of 5HT2A receptors; however, more research in this area is needed.2
The best strategy for treating drug-induced PML has not yet been determined. While combination therapy is thought to be more successful than monotherapy, ultimately, it depends on the patient’s immune response. If a psychotropic medication is chosen as adjunct treatment for drug-induced PML, it is prudent to assess the patient’s entire clinical picture to determine the specific indication for therapy (ie, treating symptomatology or drug-induced PML).
CASE CONTINUED
Following diagnosis, Mr. P is provided supportive therapy, and his care team discontinues methotrexate and etanercept. Although data are mixed on the efficacy of medications that work on 5HT2A receptors, because Mr. P was recently diagnosed with MDD, he is started on mirtazapine 15 mg/d at night in an attempt to manage both MDD and PML. It is possible that his depressive symptoms developed as a result of drug-induced PML rather than major depressive disorder. Discontinuing methotrexate and etanercept stabilizes Mr. P’s PML symptoms but leads to an exacerbation of his RA symptoms. Mr. P is initiated on hyd
Related Resources
- Castle D, Robertson NP. Treatment of progressive multifocal leukoencephalopathy. J Neurol. 2019;266(10):2587-2589. doi:10.1007/s00415-019-09501-y
Drug Brand Names
Abatacept • Orencia
Adalimumab • Humira
Alemtuzumab • Campath
Azathioprine • Azasan, Imuran
Basiliximab • Simulect
Belimumab • Benlysta
Bevacizumab • Avastin
Brentuximab vedotin • Adcetris
Cetuximab • Erbitux
Chlorpromazine • Thorazine, Largactil
Cidofovir • Vistide
Cladribine • Mavenclad
Cyclophosphamide • Cytoxan
Cyclosporine • Gengraf, Neoral
Dacarbazine • DTIC-Dome
Diclofenac • Cambia, Zorvolex
Dimethyl fumarate • Tecfidera
Etanercept • Enbrel
Fingolimod • Gilenya
Fludarabine • Fludara
Hydroxychloroquine • Plaquenil
Ibritumomab tiuxetan • Zevalin
Infliximab • Avsola, Inflectra
Isotretinoin • Absorica, Claravis
Mefloquine • Lariam
Methotrexate • Rheumatrex, Trexall
Mirtazapine • Remeron
Mitoxantrone • Novantrone
Muromonab-CD3 • Orthoclone OKT3
Mycophenolate mofetil • CellCept
Natalizumab • Tysabri
Nelarabine • Arranon
Obinutuzumab • Gazyva
Olanzapine • Zyprexa
Risperidone • Risperdal
Tacrolimus • Prograf
Vincristine • Vincasar PFS
Ziprasidone • Geodon
Mr. P, age 67, presents to the clinic with vision changes and memory loss following a fall in his home due to limb weakness. Six years ago, his care team diagnosed him with rheumatoid arthritis (RA). Mr. P’s current medication regimen includes methotrexate 20 mg once weekly and etanercept 50 mg once weekly, and he has been stable on this plan for 3 years. Mr. P also was recently diagnosed with major depressive disorder (MDD), but has not yet started treatment. Following a complete workup, an MRI of Mr. P’s brain revealed white matter demyelination. Due to these findings, he is scheduled for a brain biopsy, which confirms a diagnosis of progressive multifocal leukoencephalopathy (PML).
PML is a demyelinating disease of the central nervous system caused by the John Cunningham virus (JCV), or JC polyomavirus, named for the first patient identified to have contracted the virus.1 Asymptomatic infection of JCV often occurs in childhood, and antibodies are found in ≤70% of healthy adults. In most individuals, JCV remains latent in the kidneys and lymphoid organs, but immunosuppression can cause it to reactivate.2
JCV infects oligodendrocytes, astrocytes, and neurons, which results in white matter demyelination. Due to this demyelination, individuals can experience visual field defects, speech disturbances, ataxia, paresthesia, and cognitive impairments.2 Limb weakness presents in 60% of patients with PML, visual disturbances in 20%, and gait disturbances in 65%.3 Progression of these symptoms can lead to a more severe clinical presentation, including focal seizures in ≤10% of patients, and the mortality rate is 30% to 50%.3 Patients with comorbid HIV have a mortality rate ≤90%.2
Currently, there are no biomarkers that can identify PML in its early stages. A PML diagnosis is typically based on the patient’s clinical presentation, radiological imaging, and detection of JCV DNA. A brain biopsy is the gold standard for PML diagnosis.1
Interestingly, data suggest that glial cells harboring JCV in the brain express receptors for serotonin and dopamine.4 Researchers pinpointed 5HT2A receptors as JCV entry points into cells, and theorized that medications competing for binding, such as certain psychotropic agents, might decrease JCV entry. Cells lacking the 5HT2A receptor have shown immunity to JCV infection and the ability of cells to be infected was restored through transfection of 5HT2A receptors.4
Immunosuppressant medications can cause PML
PML was initially seen in individuals with conditions that cause immunosuppression, such as malignancies and HIV. However, “drug-induced PML” refers to cases in which drug-induced immunosuppression creates an environment that allows JCV to reactivate and disseminate back into the CNS.4 It is important to emphasize that drug-induced PML is a very rare effect of certain immunosuppressant medications. Medications that can weaken the immune system include glucocorticoids, monoclonal antibodies, alkylating agents, purine analogues, antimetabolites, and immunosuppressants (Table).1

These medications are used to treat conditions such as multiple sclerosis, RA, psoriatic arthritis, and lupus. Although drug-induced PML can result from the use of any of these agents, the highest incidence (1%) is found with natalizumab. Rates of incidence with other agents are either unknown or as low as .002%.1 Evidence suggests that the risk for PML increases with the duration of therapy.5
Continue to: Management
Management: Stop the offending agent, restore immune function
Specific pharmacologic treatments for PML are lacking. Management of drug-induced PML starts with discontinuing the offending agent. Restoring immune function has been found to be the most effective approach to treat PML.3 Restoration is possible through interleukin-2 (IL-2), IL-7, and T-cell infusions. Other treatment options are theoretical and include the development of a JCV vaccine to stimulate host response, plasma exchange to remove the medication from the host, and antiviral therapy targeting JCV replication. Diclofenac, isotretinoin, and mefloquine can inhibit JCV replication.3
Based on the theory that JCV requires 5HT2A receptors for entry into cells, researchers have studied medications that block this receptor as a treatment for PML. The first-generation antipsychotic chlorpromazine did not show benefit when combined with cidofovir, a replication inhibitor.3 Antipsychotics agents such as ziprasidone and olanzapine have shown in vitro inhibition of JCV, while risperidone has mixed results, with 1 trial failing to find a difference on JCV in fetal glial cells.3 Second-generation antipsychotics may be the preferred option due to more potent antagonism of the 5HT2A receptors and fewer adverse effects compared to agents such as chlorpromazine.4 The antidepressant mirtazapine has shown to have promising results, with evidence indicating that earlier initiation is more beneficial.3 Overall, data involving the use of medications that act on the 5HT2A receptor are mixed. Recent data suggest that JCV might enter cells independent of 5HT2A receptors; however, more research in this area is needed.2
The best strategy for treating drug-induced PML has not yet been determined. While combination therapy is thought to be more successful than monotherapy, ultimately, it depends on the patient’s immune response. If a psychotropic medication is chosen as adjunct treatment for drug-induced PML, it is prudent to assess the patient’s entire clinical picture to determine the specific indication for therapy (ie, treating symptomatology or drug-induced PML).
CASE CONTINUED
Following diagnosis, Mr. P is provided supportive therapy, and his care team discontinues methotrexate and etanercept. Although data are mixed on the efficacy of medications that work on 5HT2A receptors, because Mr. P was recently diagnosed with MDD, he is started on mirtazapine 15 mg/d at night in an attempt to manage both MDD and PML. It is possible that his depressive symptoms developed as a result of drug-induced PML rather than major depressive disorder. Discontinuing methotrexate and etanercept stabilizes Mr. P’s PML symptoms but leads to an exacerbation of his RA symptoms. Mr. P is initiated on hyd
Related Resources
- Castle D, Robertson NP. Treatment of progressive multifocal leukoencephalopathy. J Neurol. 2019;266(10):2587-2589. doi:10.1007/s00415-019-09501-y
Drug Brand Names
Abatacept • Orencia
Adalimumab • Humira
Alemtuzumab • Campath
Azathioprine • Azasan, Imuran
Basiliximab • Simulect
Belimumab • Benlysta
Bevacizumab • Avastin
Brentuximab vedotin • Adcetris
Cetuximab • Erbitux
Chlorpromazine • Thorazine, Largactil
Cidofovir • Vistide
Cladribine • Mavenclad
Cyclophosphamide • Cytoxan
Cyclosporine • Gengraf, Neoral
Dacarbazine • DTIC-Dome
Diclofenac • Cambia, Zorvolex
Dimethyl fumarate • Tecfidera
Etanercept • Enbrel
Fingolimod • Gilenya
Fludarabine • Fludara
Hydroxychloroquine • Plaquenil
Ibritumomab tiuxetan • Zevalin
Infliximab • Avsola, Inflectra
Isotretinoin • Absorica, Claravis
Mefloquine • Lariam
Methotrexate • Rheumatrex, Trexall
Mirtazapine • Remeron
Mitoxantrone • Novantrone
Muromonab-CD3 • Orthoclone OKT3
Mycophenolate mofetil • CellCept
Natalizumab • Tysabri
Nelarabine • Arranon
Obinutuzumab • Gazyva
Olanzapine • Zyprexa
Risperidone • Risperdal
Tacrolimus • Prograf
Vincristine • Vincasar PFS
Ziprasidone • Geodon
1. Yukitake M. Drug-induced progressive multifocal leukoencephalopathy in multiple sclerosis: a comprehensive review. Clin Exp Neuroimmunol. 2018;9(1):37-47. doi:10.1111/cen3.12440
2. Alstadhaug KB, Myhr KM, Rinaldo CH. Progressive multifocal leukoencephalopathy. Tidsskr Nor Laegeforen. 2017;137(23-24):10.4045/tidsskr.16.1092. doi:10.4045/tidsskr.16.1092
3. Williamson EML, Berger JR. Diagnosis and treatment of progressive multifocal leukoencephalopathy associated with multiple sclerosis therapies. Neurotherapeutics. 2017;14(4):961-973. doi:10.1007/s13311-017-0570-7
4. Altschuler EL, Kast RE. The atypical antipsychotic agents ziprasidone, risperidone and olanzapine as treatment for and prophylaxis against progressive multifocal leukoencephalopathy. Med Hypotheses. 2005;65(3):585-586.
5. Vinhas de Souza M, Keller-Stanislawski B, Blake K, et al. Drug-induced PML: a global agenda for a global challenge. Clin Pharmacol Ther. 2012;91(4):747-750. doi:10.1038/clpt.2012.4
1. Yukitake M. Drug-induced progressive multifocal leukoencephalopathy in multiple sclerosis: a comprehensive review. Clin Exp Neuroimmunol. 2018;9(1):37-47. doi:10.1111/cen3.12440
2. Alstadhaug KB, Myhr KM, Rinaldo CH. Progressive multifocal leukoencephalopathy. Tidsskr Nor Laegeforen. 2017;137(23-24):10.4045/tidsskr.16.1092. doi:10.4045/tidsskr.16.1092
3. Williamson EML, Berger JR. Diagnosis and treatment of progressive multifocal leukoencephalopathy associated with multiple sclerosis therapies. Neurotherapeutics. 2017;14(4):961-973. doi:10.1007/s13311-017-0570-7
4. Altschuler EL, Kast RE. The atypical antipsychotic agents ziprasidone, risperidone and olanzapine as treatment for and prophylaxis against progressive multifocal leukoencephalopathy. Med Hypotheses. 2005;65(3):585-586.
5. Vinhas de Souza M, Keller-Stanislawski B, Blake K, et al. Drug-induced PML: a global agenda for a global challenge. Clin Pharmacol Ther. 2012;91(4):747-750. doi:10.1038/clpt.2012.4
Hold or not to hold: Navigating involuntary commitment
CASE Depressed and suicidal
Police arrive at the home of Mr. H, age 50, after his wife calls 911. She reports he has depression and she saw him in bed brandishing a firearm as if he wanted to hurt himself. Upon arrival, the officers enter the house and find Mr. H in bed without a firearm. Mr. H says little to the officers about the alleged events, but acknowledges he has depression and is willing to go the hospital for further evaluation. Neither his wife nor the officers locate a firearm in the home.
EVALUATION Emergency detention
In the emergency department (ED), Mr. H’s laboratory results and physical examination findings are normal. He acknowledges feeling depressed over the past 2 weeks. Though he cannot identify any precipitants, he says he has experienced anhedonia, lack of appetite, decreased energy, and changes in his sleep patterns. When asked about the day’s events concerning the firearm, Mr. H becomes guarded and does not give a clear answer regarding having thoughts of suicide.
The evaluating psychiatrist obtains collateral from Mr. H’s wife and reviews his medical records. There are no active prescriptions on file and the psychiatrist notices that last year there was a suicide attempt involving a firearm. Following that episode, Mr. H was hospitalized, treated with sertraline 50 mg/d, and discharged with a diagnosis of major depressive disorder. There was no legal or substance abuse history.
In the ED, the psychiatrist conducts a psychiatric evaluation, including a suicide risk assessment, and determines Mr. H is at imminent risk of ending his life. Mr. H’s psychiatrist explains there are 2 treatment options: to be admitted to the hospital or to be discharged. The psychiatrist recommends hospital admission to Mr. H for his safety and stabilization. Mr. H says he prefers to return home.
Because the psychiatrist believes Mr. H is at imminent risk of ending his life and there is no less restrictive setting for treatment, he implements an emergency detention. In Ohio, this allows Mr. H to be held in the hospital for no more than 3 court days in accordance with state law. Before Mr. H’s emergency detention periods ends, the psychiatrist will need to decide whether Mr. H can be safely discharged. If the psychiatrist determines that Mr. H still needs treatment, the court will be petitioned for a civil commitment hearing.
[polldaddy:11189291]
The author’s observations
In some cases, courts allow information a psychiatrist does not directly obtain from a patient to be admitted as testimony in a civil commitment hearing. However, some jurisdictions consider sources of information not obtained directly from the patient as hearsay and thus inadmissible.1 Though each source listed may provide credible information that could be presented at a hearing, the psychiatrist should discuss with the patient the information obtained from these sources to ensure it is admissable.2 A discussion with Mr. H about the factors that led to his hospital arrival will avoid the psychiatrist’s reliance on what another person has heard or seen when providing testimony. Even when a psychiatrist is not faced with an issue of admissibility, caution must be taken with third-party reports.3
TREATMENT Civil commitment hearing
Before the emergency detention period expires, Mr. H’s psychiatrist determines that he remains at imminent risk of self-harm. To continue hospitalization, the psychiatrist files a petition for civil commitment and testifies at the commitment hearing. He reports that Mr. H suffers from a substantial mood disorder that grossly impairs his judgment and behavior. The psychiatrist also testifies that the least restrictive environment for treatment continues to be inpatient hospitalization, because Mr. H is still at imminent risk of harming himself.
Continue to: Following the psychiatrist's...
Following the psychiatrist’s testimony, the magistrate finds that Mr. H is a mentally ill person subject to hospitalization given his mood disorder that grossly impairs his judgment and behavior. The magistrate orders that Mr. H be civilly committed to the hospital.
[polldaddy:11189293]
The author’s observations
The psychiatrist’s testimony mirrors the language regarding civil commitment in the Ohio Revised Code.4 Other elements considered for mental illness in Ohio are a substantial disorder of memory, thought, orientation, or perception that grossly impairs one’s capacity to recognize reality or meet the demands of life.4 The definition of what constitutes a mental disorder varies by state, but the burden of persuasion—the standard by which the court must be convinced—is generally uniform.5 In the 1979 case Addington v Texas, the United States Supreme Court concluded that in a civil commitment hearing, the minimum standard of proof for involuntary commitment must be clear and convincing evidence.6 Neither medical certainty nor substantial probability are burdens of persuasions.6 Instead, these terms may be presented in a forensic report when an examiner outlines their opinion. Table 1 and the Figure provide more detail on burdens of persuasion.
TREATMENT Civil commitment and patient rights
At a regularly scheduled treatment team meeting, the team informs Mr. H that he has been civilly committed for further treatment. Mr. H becomes upset and tells the team the decision is a complete violation of his rights. After a long rant, Mr. H walks out of the room, saying, “I did not even know when this hearing was.” A member of the treatment team becomes concerned that Mr. H may not have been notified of the hearing.
[polldaddy:11189294]
The author’s observations
It is not clear if Mr. H had been notified of his civil commitment hearing. If Mr. H had not been notified, his rights would have been compromised. Lessard v Schmidt (1972) outlined that individuals involved in a civil commitment hearing should be afforded the same rights as those involved in criminal proceedings.7 Mr. H should have been notified of his hearing and afforded the opportunity to have the assignment of counsel, the right to appear, the right to testify, the right to present witnesses and other evidence, and the right to confront witnesses.
Without notification of the hearing, the only right that would have remained intact for Mr. H would have been the assignment of counsel in his absence and without his knowledge. If Mr. H had been notified of the hearing and did not want to attend, yet still desired legal counsel, he could have waived his presence voluntarily after discussing this option with his attorney.8,9
Continue to: OUTCOME Stabilization and discharge
OUTCOME Stabilization and discharge
During his 10-day stay, Mr. H is treated with sertraline 50 mg/d and engages in individual and group therapy. He shows noticeable improvement in his depressive symptoms and reports having no thoughts of suicide or self-harm. The treatment team determines it is appropriate to discharge him home (the firearm was never found) and involves his wife in safety planning and follow-up care. On the day of his discharge, Mr. H reflects on his treatment and civil commitment. He says, “I did not know a judge could order me to be hospitalized.”
[polldaddy:11189297]
The author’s observations
The physician’s decision to pursue civil commitment is best described by the legal doctrines of police powers and parens patriae. Other relevant ethical principles are described in Table 2.10

Though ethical principles may play a role in civil commitment, parens patriae and police powers is the answer with respect to the State.11Parens patriae is Latin for the “parent of the country” and grants the State the power to protect those residents who are most vulnerable. Police power is the authority of the State to enact and enforce rules that limit the rights of individuals for the greater good of ensuring health, safety, and welfare of all citizens.
Bottom Line
Psychiatrists are entrusted with recognizing when a patient, due to mental illness, is a danger to themselves or others and in need of treatment. After an emergency detention period, if the patient remains a danger to themselves or others and does not want to voluntarily receive treatment, a court hearing is required. As an expert witness, the treating psychiatrist should know the factors of law in their jurisdiction that determine civil commitment.
Related Resources
- Extreme Risk Protection Orders. Johns Hopkins Bloomberg School of Public Health. https://www.jhsph.edu/research/ centers-and-institutes/johns-hopkins-center-for-gun-violenceprevention-and-policy/research/extreme-risk-protectionorders/
- Gutheil TG. The Psychiatrist as Expert Witness. 2nd ed. American Psychiatric Association Publishing; 2009.
- Landmark Cases 2014. American Academy of Psychiatry and the Law. https://www.aapl.org/landmark-cases
Drug Brand Names
Sertraline • Zoloft
1. Pinals DA, Mossman D. Evaluation for Civil Commitment. Oxford University Press; 2012.
2. Thatcher BT, Mossman D. Testifying for civil commitment: help unwilling patients get the treatment they need. Current Psychiatry. 2009;8(11):51-56.
3. Marett CP, Mossman D. What is your liability for involuntary commitment based on faulty information? Current Psychiatry. 2017;16(3):21-25,33.
4. Ohio Rev Code § 5122.01 (2018).
5. The Burden of Proof. University of Minnesota. Accessed January 23, 2022. https://open.lib.umn.edu/criminallaw/chapter/2-4-the-burden-of-proof/
6. Gold LH, Frierson RL, eds. The American Psychiatric Association Publishing Textbook of Forensic Psychiatry. 3rd ed. American Psychiatric Association Publishing; 2018.
7. Gold LH, Frierson RL, eds. The American Psychiatric Association Publishing Textbook of Suicide Assessment and Management. 3rd ed. American Psychiatric Association Publishing; 2020.
8. Cook J. Good lawyering and bad role models: the role of respondent’s counsel in a civil commitment hearing. Georgetown Journal of Legal Ethics. 2000;14(1):179-195.
9. Ferris CE. The search for due process in civil commitment hearings: how procedural realities have altered substantive standards. Vanderbilt Law Rev. 2008;61(3):959-981.
10. Substance Abuse and Mental Health Services Administration. Civil Commitment and the Mental Health Care Continuum: Historical Trends and Principles for Law and Practice. 2019. Accessed January 23, 2022. https://www.samhsa.gov/resource/ebp/civil-commitment-mental-health-care-continuum-historical-trends-principles-law
11. Melton GB, Petrila J, Poythress NG, et al. Psychological Evaluations for the Courts: A Handbook for Mental Health Profession. 4th ed. Guilford Press; 2018.
CASE Depressed and suicidal
Police arrive at the home of Mr. H, age 50, after his wife calls 911. She reports he has depression and she saw him in bed brandishing a firearm as if he wanted to hurt himself. Upon arrival, the officers enter the house and find Mr. H in bed without a firearm. Mr. H says little to the officers about the alleged events, but acknowledges he has depression and is willing to go the hospital for further evaluation. Neither his wife nor the officers locate a firearm in the home.
EVALUATION Emergency detention
In the emergency department (ED), Mr. H’s laboratory results and physical examination findings are normal. He acknowledges feeling depressed over the past 2 weeks. Though he cannot identify any precipitants, he says he has experienced anhedonia, lack of appetite, decreased energy, and changes in his sleep patterns. When asked about the day’s events concerning the firearm, Mr. H becomes guarded and does not give a clear answer regarding having thoughts of suicide.
The evaluating psychiatrist obtains collateral from Mr. H’s wife and reviews his medical records. There are no active prescriptions on file and the psychiatrist notices that last year there was a suicide attempt involving a firearm. Following that episode, Mr. H was hospitalized, treated with sertraline 50 mg/d, and discharged with a diagnosis of major depressive disorder. There was no legal or substance abuse history.
In the ED, the psychiatrist conducts a psychiatric evaluation, including a suicide risk assessment, and determines Mr. H is at imminent risk of ending his life. Mr. H’s psychiatrist explains there are 2 treatment options: to be admitted to the hospital or to be discharged. The psychiatrist recommends hospital admission to Mr. H for his safety and stabilization. Mr. H says he prefers to return home.
Because the psychiatrist believes Mr. H is at imminent risk of ending his life and there is no less restrictive setting for treatment, he implements an emergency detention. In Ohio, this allows Mr. H to be held in the hospital for no more than 3 court days in accordance with state law. Before Mr. H’s emergency detention periods ends, the psychiatrist will need to decide whether Mr. H can be safely discharged. If the psychiatrist determines that Mr. H still needs treatment, the court will be petitioned for a civil commitment hearing.
[polldaddy:11189291]
The author’s observations
In some cases, courts allow information a psychiatrist does not directly obtain from a patient to be admitted as testimony in a civil commitment hearing. However, some jurisdictions consider sources of information not obtained directly from the patient as hearsay and thus inadmissible.1 Though each source listed may provide credible information that could be presented at a hearing, the psychiatrist should discuss with the patient the information obtained from these sources to ensure it is admissable.2 A discussion with Mr. H about the factors that led to his hospital arrival will avoid the psychiatrist’s reliance on what another person has heard or seen when providing testimony. Even when a psychiatrist is not faced with an issue of admissibility, caution must be taken with third-party reports.3
TREATMENT Civil commitment hearing
Before the emergency detention period expires, Mr. H’s psychiatrist determines that he remains at imminent risk of self-harm. To continue hospitalization, the psychiatrist files a petition for civil commitment and testifies at the commitment hearing. He reports that Mr. H suffers from a substantial mood disorder that grossly impairs his judgment and behavior. The psychiatrist also testifies that the least restrictive environment for treatment continues to be inpatient hospitalization, because Mr. H is still at imminent risk of harming himself.
Continue to: Following the psychiatrist's...
Following the psychiatrist’s testimony, the magistrate finds that Mr. H is a mentally ill person subject to hospitalization given his mood disorder that grossly impairs his judgment and behavior. The magistrate orders that Mr. H be civilly committed to the hospital.
[polldaddy:11189293]
The author’s observations
The psychiatrist’s testimony mirrors the language regarding civil commitment in the Ohio Revised Code.4 Other elements considered for mental illness in Ohio are a substantial disorder of memory, thought, orientation, or perception that grossly impairs one’s capacity to recognize reality or meet the demands of life.4 The definition of what constitutes a mental disorder varies by state, but the burden of persuasion—the standard by which the court must be convinced—is generally uniform.5 In the 1979 case Addington v Texas, the United States Supreme Court concluded that in a civil commitment hearing, the minimum standard of proof for involuntary commitment must be clear and convincing evidence.6 Neither medical certainty nor substantial probability are burdens of persuasions.6 Instead, these terms may be presented in a forensic report when an examiner outlines their opinion. Table 1 and the Figure provide more detail on burdens of persuasion.

TREATMENT Civil commitment and patient rights
At a regularly scheduled treatment team meeting, the team informs Mr. H that he has been civilly committed for further treatment. Mr. H becomes upset and tells the team the decision is a complete violation of his rights. After a long rant, Mr. H walks out of the room, saying, “I did not even know when this hearing was.” A member of the treatment team becomes concerned that Mr. H may not have been notified of the hearing.

[polldaddy:11189294]
The author’s observations
It is not clear if Mr. H had been notified of his civil commitment hearing. If Mr. H had not been notified, his rights would have been compromised. Lessard v Schmidt (1972) outlined that individuals involved in a civil commitment hearing should be afforded the same rights as those involved in criminal proceedings.7 Mr. H should have been notified of his hearing and afforded the opportunity to have the assignment of counsel, the right to appear, the right to testify, the right to present witnesses and other evidence, and the right to confront witnesses.
Without notification of the hearing, the only right that would have remained intact for Mr. H would have been the assignment of counsel in his absence and without his knowledge. If Mr. H had been notified of the hearing and did not want to attend, yet still desired legal counsel, he could have waived his presence voluntarily after discussing this option with his attorney.8,9
Continue to: OUTCOME Stabilization and discharge
OUTCOME Stabilization and discharge
During his 10-day stay, Mr. H is treated with sertraline 50 mg/d and engages in individual and group therapy. He shows noticeable improvement in his depressive symptoms and reports having no thoughts of suicide or self-harm. The treatment team determines it is appropriate to discharge him home (the firearm was never found) and involves his wife in safety planning and follow-up care. On the day of his discharge, Mr. H reflects on his treatment and civil commitment. He says, “I did not know a judge could order me to be hospitalized.”
[polldaddy:11189297]
The author’s observations
The physician’s decision to pursue civil commitment is best described by the legal doctrines of police powers and parens patriae. Other relevant ethical principles are described in Table 2.10

Though ethical principles may play a role in civil commitment, parens patriae and police powers is the answer with respect to the State.11Parens patriae is Latin for the “parent of the country” and grants the State the power to protect those residents who are most vulnerable. Police power is the authority of the State to enact and enforce rules that limit the rights of individuals for the greater good of ensuring health, safety, and welfare of all citizens.
Bottom Line
Psychiatrists are entrusted with recognizing when a patient, due to mental illness, is a danger to themselves or others and in need of treatment. After an emergency detention period, if the patient remains a danger to themselves or others and does not want to voluntarily receive treatment, a court hearing is required. As an expert witness, the treating psychiatrist should know the factors of law in their jurisdiction that determine civil commitment.
Related Resources
- Extreme Risk Protection Orders. Johns Hopkins Bloomberg School of Public Health. https://www.jhsph.edu/research/ centers-and-institutes/johns-hopkins-center-for-gun-violenceprevention-and-policy/research/extreme-risk-protectionorders/
- Gutheil TG. The Psychiatrist as Expert Witness. 2nd ed. American Psychiatric Association Publishing; 2009.
- Landmark Cases 2014. American Academy of Psychiatry and the Law. https://www.aapl.org/landmark-cases
Drug Brand Names
Sertraline • Zoloft
CASE Depressed and suicidal
Police arrive at the home of Mr. H, age 50, after his wife calls 911. She reports he has depression and she saw him in bed brandishing a firearm as if he wanted to hurt himself. Upon arrival, the officers enter the house and find Mr. H in bed without a firearm. Mr. H says little to the officers about the alleged events, but acknowledges he has depression and is willing to go the hospital for further evaluation. Neither his wife nor the officers locate a firearm in the home.
EVALUATION Emergency detention
In the emergency department (ED), Mr. H’s laboratory results and physical examination findings are normal. He acknowledges feeling depressed over the past 2 weeks. Though he cannot identify any precipitants, he says he has experienced anhedonia, lack of appetite, decreased energy, and changes in his sleep patterns. When asked about the day’s events concerning the firearm, Mr. H becomes guarded and does not give a clear answer regarding having thoughts of suicide.
The evaluating psychiatrist obtains collateral from Mr. H’s wife and reviews his medical records. There are no active prescriptions on file and the psychiatrist notices that last year there was a suicide attempt involving a firearm. Following that episode, Mr. H was hospitalized, treated with sertraline 50 mg/d, and discharged with a diagnosis of major depressive disorder. There was no legal or substance abuse history.
In the ED, the psychiatrist conducts a psychiatric evaluation, including a suicide risk assessment, and determines Mr. H is at imminent risk of ending his life. Mr. H’s psychiatrist explains there are 2 treatment options: to be admitted to the hospital or to be discharged. The psychiatrist recommends hospital admission to Mr. H for his safety and stabilization. Mr. H says he prefers to return home.
Because the psychiatrist believes Mr. H is at imminent risk of ending his life and there is no less restrictive setting for treatment, he implements an emergency detention. In Ohio, this allows Mr. H to be held in the hospital for no more than 3 court days in accordance with state law. Before Mr. H’s emergency detention periods ends, the psychiatrist will need to decide whether Mr. H can be safely discharged. If the psychiatrist determines that Mr. H still needs treatment, the court will be petitioned for a civil commitment hearing.
[polldaddy:11189291]
The author’s observations
In some cases, courts allow information a psychiatrist does not directly obtain from a patient to be admitted as testimony in a civil commitment hearing. However, some jurisdictions consider sources of information not obtained directly from the patient as hearsay and thus inadmissible.1 Though each source listed may provide credible information that could be presented at a hearing, the psychiatrist should discuss with the patient the information obtained from these sources to ensure it is admissable.2 A discussion with Mr. H about the factors that led to his hospital arrival will avoid the psychiatrist’s reliance on what another person has heard or seen when providing testimony. Even when a psychiatrist is not faced with an issue of admissibility, caution must be taken with third-party reports.3
TREATMENT Civil commitment hearing
Before the emergency detention period expires, Mr. H’s psychiatrist determines that he remains at imminent risk of self-harm. To continue hospitalization, the psychiatrist files a petition for civil commitment and testifies at the commitment hearing. He reports that Mr. H suffers from a substantial mood disorder that grossly impairs his judgment and behavior. The psychiatrist also testifies that the least restrictive environment for treatment continues to be inpatient hospitalization, because Mr. H is still at imminent risk of harming himself.
Continue to: Following the psychiatrist's...
Following the psychiatrist’s testimony, the magistrate finds that Mr. H is a mentally ill person subject to hospitalization given his mood disorder that grossly impairs his judgment and behavior. The magistrate orders that Mr. H be civilly committed to the hospital.
[polldaddy:11189293]
The author’s observations
The psychiatrist’s testimony mirrors the language regarding civil commitment in the Ohio Revised Code.4 Other elements considered for mental illness in Ohio are a substantial disorder of memory, thought, orientation, or perception that grossly impairs one’s capacity to recognize reality or meet the demands of life.4 The definition of what constitutes a mental disorder varies by state, but the burden of persuasion—the standard by which the court must be convinced—is generally uniform.5 In the 1979 case Addington v Texas, the United States Supreme Court concluded that in a civil commitment hearing, the minimum standard of proof for involuntary commitment must be clear and convincing evidence.6 Neither medical certainty nor substantial probability are burdens of persuasions.6 Instead, these terms may be presented in a forensic report when an examiner outlines their opinion. Table 1 and the Figure provide more detail on burdens of persuasion.

TREATMENT Civil commitment and patient rights
At a regularly scheduled treatment team meeting, the team informs Mr. H that he has been civilly committed for further treatment. Mr. H becomes upset and tells the team the decision is a complete violation of his rights. After a long rant, Mr. H walks out of the room, saying, “I did not even know when this hearing was.” A member of the treatment team becomes concerned that Mr. H may not have been notified of the hearing.

[polldaddy:11189294]
The author’s observations
It is not clear if Mr. H had been notified of his civil commitment hearing. If Mr. H had not been notified, his rights would have been compromised. Lessard v Schmidt (1972) outlined that individuals involved in a civil commitment hearing should be afforded the same rights as those involved in criminal proceedings.7 Mr. H should have been notified of his hearing and afforded the opportunity to have the assignment of counsel, the right to appear, the right to testify, the right to present witnesses and other evidence, and the right to confront witnesses.
Without notification of the hearing, the only right that would have remained intact for Mr. H would have been the assignment of counsel in his absence and without his knowledge. If Mr. H had been notified of the hearing and did not want to attend, yet still desired legal counsel, he could have waived his presence voluntarily after discussing this option with his attorney.8,9
Continue to: OUTCOME Stabilization and discharge
OUTCOME Stabilization and discharge
During his 10-day stay, Mr. H is treated with sertraline 50 mg/d and engages in individual and group therapy. He shows noticeable improvement in his depressive symptoms and reports having no thoughts of suicide or self-harm. The treatment team determines it is appropriate to discharge him home (the firearm was never found) and involves his wife in safety planning and follow-up care. On the day of his discharge, Mr. H reflects on his treatment and civil commitment. He says, “I did not know a judge could order me to be hospitalized.”
[polldaddy:11189297]
The author’s observations
The physician’s decision to pursue civil commitment is best described by the legal doctrines of police powers and parens patriae. Other relevant ethical principles are described in Table 2.10

Though ethical principles may play a role in civil commitment, parens patriae and police powers is the answer with respect to the State.11Parens patriae is Latin for the “parent of the country” and grants the State the power to protect those residents who are most vulnerable. Police power is the authority of the State to enact and enforce rules that limit the rights of individuals for the greater good of ensuring health, safety, and welfare of all citizens.
Bottom Line
Psychiatrists are entrusted with recognizing when a patient, due to mental illness, is a danger to themselves or others and in need of treatment. After an emergency detention period, if the patient remains a danger to themselves or others and does not want to voluntarily receive treatment, a court hearing is required. As an expert witness, the treating psychiatrist should know the factors of law in their jurisdiction that determine civil commitment.
Related Resources
- Extreme Risk Protection Orders. Johns Hopkins Bloomberg School of Public Health. https://www.jhsph.edu/research/ centers-and-institutes/johns-hopkins-center-for-gun-violenceprevention-and-policy/research/extreme-risk-protectionorders/
- Gutheil TG. The Psychiatrist as Expert Witness. 2nd ed. American Psychiatric Association Publishing; 2009.
- Landmark Cases 2014. American Academy of Psychiatry and the Law. https://www.aapl.org/landmark-cases
Drug Brand Names
Sertraline • Zoloft
1. Pinals DA, Mossman D. Evaluation for Civil Commitment. Oxford University Press; 2012.
2. Thatcher BT, Mossman D. Testifying for civil commitment: help unwilling patients get the treatment they need. Current Psychiatry. 2009;8(11):51-56.
3. Marett CP, Mossman D. What is your liability for involuntary commitment based on faulty information? Current Psychiatry. 2017;16(3):21-25,33.
4. Ohio Rev Code § 5122.01 (2018).
5. The Burden of Proof. University of Minnesota. Accessed January 23, 2022. https://open.lib.umn.edu/criminallaw/chapter/2-4-the-burden-of-proof/
6. Gold LH, Frierson RL, eds. The American Psychiatric Association Publishing Textbook of Forensic Psychiatry. 3rd ed. American Psychiatric Association Publishing; 2018.
7. Gold LH, Frierson RL, eds. The American Psychiatric Association Publishing Textbook of Suicide Assessment and Management. 3rd ed. American Psychiatric Association Publishing; 2020.
8. Cook J. Good lawyering and bad role models: the role of respondent’s counsel in a civil commitment hearing. Georgetown Journal of Legal Ethics. 2000;14(1):179-195.
9. Ferris CE. The search for due process in civil commitment hearings: how procedural realities have altered substantive standards. Vanderbilt Law Rev. 2008;61(3):959-981.
10. Substance Abuse and Mental Health Services Administration. Civil Commitment and the Mental Health Care Continuum: Historical Trends and Principles for Law and Practice. 2019. Accessed January 23, 2022. https://www.samhsa.gov/resource/ebp/civil-commitment-mental-health-care-continuum-historical-trends-principles-law
11. Melton GB, Petrila J, Poythress NG, et al. Psychological Evaluations for the Courts: A Handbook for Mental Health Profession. 4th ed. Guilford Press; 2018.
1. Pinals DA, Mossman D. Evaluation for Civil Commitment. Oxford University Press; 2012.
2. Thatcher BT, Mossman D. Testifying for civil commitment: help unwilling patients get the treatment they need. Current Psychiatry. 2009;8(11):51-56.
3. Marett CP, Mossman D. What is your liability for involuntary commitment based on faulty information? Current Psychiatry. 2017;16(3):21-25,33.
4. Ohio Rev Code § 5122.01 (2018).
5. The Burden of Proof. University of Minnesota. Accessed January 23, 2022. https://open.lib.umn.edu/criminallaw/chapter/2-4-the-burden-of-proof/
6. Gold LH, Frierson RL, eds. The American Psychiatric Association Publishing Textbook of Forensic Psychiatry. 3rd ed. American Psychiatric Association Publishing; 2018.
7. Gold LH, Frierson RL, eds. The American Psychiatric Association Publishing Textbook of Suicide Assessment and Management. 3rd ed. American Psychiatric Association Publishing; 2020.
8. Cook J. Good lawyering and bad role models: the role of respondent’s counsel in a civil commitment hearing. Georgetown Journal of Legal Ethics. 2000;14(1):179-195.
9. Ferris CE. The search for due process in civil commitment hearings: how procedural realities have altered substantive standards. Vanderbilt Law Rev. 2008;61(3):959-981.
10. Substance Abuse and Mental Health Services Administration. Civil Commitment and the Mental Health Care Continuum: Historical Trends and Principles for Law and Practice. 2019. Accessed January 23, 2022. https://www.samhsa.gov/resource/ebp/civil-commitment-mental-health-care-continuum-historical-trends-principles-law
11. Melton GB, Petrila J, Poythress NG, et al. Psychological Evaluations for the Courts: A Handbook for Mental Health Profession. 4th ed. Guilford Press; 2018.
Preparing patients with serious mental illness for extreme HEAT
Climate change is causing intense heat waves that threaten human health across the globe.
A confluence of factors increases risk
Thermoregulatory dysfunction is thought to be intrinsic to patients with schizophrenia partly due to dysregulated dopaminergic neurotransmission.2 This is compounded by these patients’ higher burden of chronic medical comorbidities such as cardiovascular and respiratory illnesses, which together with psychotropic (ie, antipsychotics, antidepressants, lithium, benzodiazepines) and medical medications (ie, certain antihypertensives, diuretics, treatment for urinary incontinence) further disrupt the body’s cooling strategies and increase vulnerability to heat-related illnesses.1,3 Antipsychotics commonly prescribed to patients with SMI increase hyperthermia risk largely by 2 mechanisms: central and peripheral thermal dysregulation, and anticholinergic properties (ie, olanzapine, clozapine, chlorpromazine).2,3 Other anticholinergic medications prescribed to treat extrapyramidal symptoms (ie, diphenhydramine, benztropine, trihexyphenidyl), anxiety, depression, and insomnia (ie, paroxetine, trazodone, doxepin) further add insult to injury because they impair sweating, which decreases the body’s ability to eliminate heat through evaporation.2,3 Additionally, high temperature exacerbates psychiatric symptoms in patients with SMI, resulting in increased hospitalizations and emergency department visits.
How to keep patients safe
The acronym HEAT provides a framework that psychiatrists can use to highlight the importance of planning for heat waves in their institution and guiding discussions with individual patients about heat-related illnesses (Table 1).

Help the health care system where you work plan and prepare for heat waves. In-service training in mental health settings such as outpatient clinics, shelters, group homes, and residential programs can help staff identify patients at particular risk and reinforce key prevention messages.
Educate patients and their caregivers on strategies for preventing heat-related illness. Informational materials can be distributed in clinics, residential settings, and day programs. A 1-page downloadable pamphlet available at https://smiadviser.org/wp-content/uploads/2022/08/SMI-Heat-Stroke-ver1.0-FINAL.pdf summarizes key prevention messages of staying hydrated, staying cool, and staying safe.
Assess personalized heat-related risks. Inquire about patients’ daily activities, access to air conditioning, and water intake. Minimize the use of anticholinergic medications. Identify who patients can turn to for assistance, especially for those who struggle with cognitive impairment and social isolation.
Teach patients, caregivers, and staff the signs and symptoms of heat exhaustion and heat stroke and how to respond in such situations.
HEAT focuses psychiatric clinicians on preparing and protecting patients with SMI against dangerous heat waves. Clinicians can take a proactive leadership role in disseminating basic principles of heat-related illness prevention and heat-wave toolkits by using resources available from organizations such as the Climate Psychiatry Alliance (Table 2). They can also initiate advocacy efforts to raise awareness about the elevated risks of heat-related illnesses in this vulnerable population.

1. Schmeltz MT, Gamble JL. Risk characterization of hospitalizations for mental illness and/or behavioral disorders with concurrent heat-related illness. PLoS One. 2017;12(10):e0186509. doi:10.1371/journal.pone.0186509
2. Lee CP, Chen PJ, Chang CM. Heat stroke during treatment with olanzapine, trihexyphenidyl, and trazodone in a patient with schizophrenia. Acta Neuropsychiatrica. 2015;27(6):380-385.
3. Bongers KS, Salahudeen MS, Peterson GM. Drug-associated non-pyrogenic hyperthermia: a narrative review. Eur J Clin Pharmacol. 2020;76(1):9-16.
Climate change is causing intense heat waves that threaten human health across the globe.
A confluence of factors increases risk
Thermoregulatory dysfunction is thought to be intrinsic to patients with schizophrenia partly due to dysregulated dopaminergic neurotransmission.2 This is compounded by these patients’ higher burden of chronic medical comorbidities such as cardiovascular and respiratory illnesses, which together with psychotropic (ie, antipsychotics, antidepressants, lithium, benzodiazepines) and medical medications (ie, certain antihypertensives, diuretics, treatment for urinary incontinence) further disrupt the body’s cooling strategies and increase vulnerability to heat-related illnesses.1,3 Antipsychotics commonly prescribed to patients with SMI increase hyperthermia risk largely by 2 mechanisms: central and peripheral thermal dysregulation, and anticholinergic properties (ie, olanzapine, clozapine, chlorpromazine).2,3 Other anticholinergic medications prescribed to treat extrapyramidal symptoms (ie, diphenhydramine, benztropine, trihexyphenidyl), anxiety, depression, and insomnia (ie, paroxetine, trazodone, doxepin) further add insult to injury because they impair sweating, which decreases the body’s ability to eliminate heat through evaporation.2,3 Additionally, high temperature exacerbates psychiatric symptoms in patients with SMI, resulting in increased hospitalizations and emergency department visits.
How to keep patients safe
The acronym HEAT provides a framework that psychiatrists can use to highlight the importance of planning for heat waves in their institution and guiding discussions with individual patients about heat-related illnesses (Table 1).

Help the health care system where you work plan and prepare for heat waves. In-service training in mental health settings such as outpatient clinics, shelters, group homes, and residential programs can help staff identify patients at particular risk and reinforce key prevention messages.
Educate patients and their caregivers on strategies for preventing heat-related illness. Informational materials can be distributed in clinics, residential settings, and day programs. A 1-page downloadable pamphlet available at https://smiadviser.org/wp-content/uploads/2022/08/SMI-Heat-Stroke-ver1.0-FINAL.pdf summarizes key prevention messages of staying hydrated, staying cool, and staying safe.
Assess personalized heat-related risks. Inquire about patients’ daily activities, access to air conditioning, and water intake. Minimize the use of anticholinergic medications. Identify who patients can turn to for assistance, especially for those who struggle with cognitive impairment and social isolation.
Teach patients, caregivers, and staff the signs and symptoms of heat exhaustion and heat stroke and how to respond in such situations.
HEAT focuses psychiatric clinicians on preparing and protecting patients with SMI against dangerous heat waves. Clinicians can take a proactive leadership role in disseminating basic principles of heat-related illness prevention and heat-wave toolkits by using resources available from organizations such as the Climate Psychiatry Alliance (Table 2). They can also initiate advocacy efforts to raise awareness about the elevated risks of heat-related illnesses in this vulnerable population.

Climate change is causing intense heat waves that threaten human health across the globe.
A confluence of factors increases risk
Thermoregulatory dysfunction is thought to be intrinsic to patients with schizophrenia partly due to dysregulated dopaminergic neurotransmission.2 This is compounded by these patients’ higher burden of chronic medical comorbidities such as cardiovascular and respiratory illnesses, which together with psychotropic (ie, antipsychotics, antidepressants, lithium, benzodiazepines) and medical medications (ie, certain antihypertensives, diuretics, treatment for urinary incontinence) further disrupt the body’s cooling strategies and increase vulnerability to heat-related illnesses.1,3 Antipsychotics commonly prescribed to patients with SMI increase hyperthermia risk largely by 2 mechanisms: central and peripheral thermal dysregulation, and anticholinergic properties (ie, olanzapine, clozapine, chlorpromazine).2,3 Other anticholinergic medications prescribed to treat extrapyramidal symptoms (ie, diphenhydramine, benztropine, trihexyphenidyl), anxiety, depression, and insomnia (ie, paroxetine, trazodone, doxepin) further add insult to injury because they impair sweating, which decreases the body’s ability to eliminate heat through evaporation.2,3 Additionally, high temperature exacerbates psychiatric symptoms in patients with SMI, resulting in increased hospitalizations and emergency department visits.
How to keep patients safe
The acronym HEAT provides a framework that psychiatrists can use to highlight the importance of planning for heat waves in their institution and guiding discussions with individual patients about heat-related illnesses (Table 1).

Help the health care system where you work plan and prepare for heat waves. In-service training in mental health settings such as outpatient clinics, shelters, group homes, and residential programs can help staff identify patients at particular risk and reinforce key prevention messages.
Educate patients and their caregivers on strategies for preventing heat-related illness. Informational materials can be distributed in clinics, residential settings, and day programs. A 1-page downloadable pamphlet available at https://smiadviser.org/wp-content/uploads/2022/08/SMI-Heat-Stroke-ver1.0-FINAL.pdf summarizes key prevention messages of staying hydrated, staying cool, and staying safe.
Assess personalized heat-related risks. Inquire about patients’ daily activities, access to air conditioning, and water intake. Minimize the use of anticholinergic medications. Identify who patients can turn to for assistance, especially for those who struggle with cognitive impairment and social isolation.
Teach patients, caregivers, and staff the signs and symptoms of heat exhaustion and heat stroke and how to respond in such situations.
HEAT focuses psychiatric clinicians on preparing and protecting patients with SMI against dangerous heat waves. Clinicians can take a proactive leadership role in disseminating basic principles of heat-related illness prevention and heat-wave toolkits by using resources available from organizations such as the Climate Psychiatry Alliance (Table 2). They can also initiate advocacy efforts to raise awareness about the elevated risks of heat-related illnesses in this vulnerable population.

1. Schmeltz MT, Gamble JL. Risk characterization of hospitalizations for mental illness and/or behavioral disorders with concurrent heat-related illness. PLoS One. 2017;12(10):e0186509. doi:10.1371/journal.pone.0186509
2. Lee CP, Chen PJ, Chang CM. Heat stroke during treatment with olanzapine, trihexyphenidyl, and trazodone in a patient with schizophrenia. Acta Neuropsychiatrica. 2015;27(6):380-385.
3. Bongers KS, Salahudeen MS, Peterson GM. Drug-associated non-pyrogenic hyperthermia: a narrative review. Eur J Clin Pharmacol. 2020;76(1):9-16.
1. Schmeltz MT, Gamble JL. Risk characterization of hospitalizations for mental illness and/or behavioral disorders with concurrent heat-related illness. PLoS One. 2017;12(10):e0186509. doi:10.1371/journal.pone.0186509
2. Lee CP, Chen PJ, Chang CM. Heat stroke during treatment with olanzapine, trihexyphenidyl, and trazodone in a patient with schizophrenia. Acta Neuropsychiatrica. 2015;27(6):380-385.
3. Bongers KS, Salahudeen MS, Peterson GM. Drug-associated non-pyrogenic hyperthermia: a narrative review. Eur J Clin Pharmacol. 2020;76(1):9-16.
Lithium for bipolar disorder: Which patients will respond?
Though Cade discovered it 70 years ago, lithium is still considered the gold standard treatment for preventing manic and depressive phases of bipolar disorder (BD). In addition to its primary indication as a mood stabilizer, lithium has demonstrated efficacy as an augmenting medication for unipolar major depressive disorder.1 While lithium is a first-line agent for BD, it does not improve symptoms in every patient. In a 2004 meta-analysis of 5 randomized controlled trials of patients with BD, Geddes et al2 found lithium was more effective than placebo in preventing the recurrence of mania, with 60% in the lithium group remaining stable compared to 40% in the placebo group. Being able to predict which patients will respond to lithium is crucial to prevent unnecessary exposure to lithium, which can produce significant adverse effects, including somnolence, nausea, diarrhea, and hypothyroidism.2
Several studies have investigated various clinical factors that might predict which patients with BD will respond to lithium. In a review, Kleindienst et al3 highlighted 3 factors that predicted a positive response to lithium:
- fewer hospitalizations prior to treatment
- an episodic course characterized sequentially by mania, depression, and then euthymia
- a later age (>50) at onset of BD.
Recent studies and reviews have isolated additional positive predictors, including having a family history of BD and a shorter duration of illness before receiving lithium, as well as negative predictors, such as rapid cycling, a large number of previous hospitalizations, a depression/mania/euthymia pattern, mood-incongruent psychotic features, and the presence of residual symptoms between mood episodes.3,4
The Table provides a list of probable and possible positive and negative predictors for therapeutic response to lithium in patients with BD.3-6 While relevant, the factors listed as possible predictors may not carry as much influence on lithium responsivity as those categorized as probable predictors.
Because of heterogeneity among studies, clinicians should consider their patient’s presentation as a whole, rather than basing medication choice on independent factors. Ultimately, more studies are required to fully determine the most relevant clinical parameters for lithium response. Overall, however, it appears these clinical factors could be extremely useful to guide psychiatrists in the optimal use of lithium while caring for patients with BD.
1. Crossley NA, Bauer M. Acceleration and augmentation of antidepressants with lithium for depressive disorders: two meta-analyses of randomized, placebo-controlled trials. J Clin Psychiatry. 2007;68(6):935-940.
2. Geddes JR, Burgess S, Hawton K, et al. Long-term lithium therapy for bipolar disorder: systematic review and meta-analysis of randomized controlled trials. Am J Psychiatry. 2004;1m61(2):217-222.
3. Kleindienst N, Engel RR, Greil W. Which clinical factors predict response to prophylactic lithium? A systematic review for bipolar disorders. Bipolar Disord. 2005;7(5):404-417.
4. Kleindienst N, Engel RR, Greil W. Psychosocial and demographic factors associated with response to prophylactic lithium: a systematic review for bipolar disorders. Psychol Med. 2005;35(12):1685-1694.
5. Hui TP, Kandola A, Shen L, et al. A systematic review and meta-analysis of clinical predictors of lithium response in bipolar disorder. Acta Psychiatr Scand. 2019;140(2):94-115.
6. Grillault Laroche D, Etain B, Severus E, et al. Socio-demographic and clinical predictors of outcome to long-term treatment with lithium in bipolar disorders: a systematic review of the contemporary literature and recommendations from the ISBD/IGSLI Task Force on treatment with lithium. Int J Bipolar Disord. 2020;8(1):40.
Though Cade discovered it 70 years ago, lithium is still considered the gold standard treatment for preventing manic and depressive phases of bipolar disorder (BD). In addition to its primary indication as a mood stabilizer, lithium has demonstrated efficacy as an augmenting medication for unipolar major depressive disorder.1 While lithium is a first-line agent for BD, it does not improve symptoms in every patient. In a 2004 meta-analysis of 5 randomized controlled trials of patients with BD, Geddes et al2 found lithium was more effective than placebo in preventing the recurrence of mania, with 60% in the lithium group remaining stable compared to 40% in the placebo group. Being able to predict which patients will respond to lithium is crucial to prevent unnecessary exposure to lithium, which can produce significant adverse effects, including somnolence, nausea, diarrhea, and hypothyroidism.2
Several studies have investigated various clinical factors that might predict which patients with BD will respond to lithium. In a review, Kleindienst et al3 highlighted 3 factors that predicted a positive response to lithium:
- fewer hospitalizations prior to treatment
- an episodic course characterized sequentially by mania, depression, and then euthymia
- a later age (>50) at onset of BD.
Recent studies and reviews have isolated additional positive predictors, including having a family history of BD and a shorter duration of illness before receiving lithium, as well as negative predictors, such as rapid cycling, a large number of previous hospitalizations, a depression/mania/euthymia pattern, mood-incongruent psychotic features, and the presence of residual symptoms between mood episodes.3,4
The Table provides a list of probable and possible positive and negative predictors for therapeutic response to lithium in patients with BD.3-6 While relevant, the factors listed as possible predictors may not carry as much influence on lithium responsivity as those categorized as probable predictors.

Because of heterogeneity among studies, clinicians should consider their patient’s presentation as a whole, rather than basing medication choice on independent factors. Ultimately, more studies are required to fully determine the most relevant clinical parameters for lithium response. Overall, however, it appears these clinical factors could be extremely useful to guide psychiatrists in the optimal use of lithium while caring for patients with BD.
Though Cade discovered it 70 years ago, lithium is still considered the gold standard treatment for preventing manic and depressive phases of bipolar disorder (BD). In addition to its primary indication as a mood stabilizer, lithium has demonstrated efficacy as an augmenting medication for unipolar major depressive disorder.1 While lithium is a first-line agent for BD, it does not improve symptoms in every patient. In a 2004 meta-analysis of 5 randomized controlled trials of patients with BD, Geddes et al2 found lithium was more effective than placebo in preventing the recurrence of mania, with 60% in the lithium group remaining stable compared to 40% in the placebo group. Being able to predict which patients will respond to lithium is crucial to prevent unnecessary exposure to lithium, which can produce significant adverse effects, including somnolence, nausea, diarrhea, and hypothyroidism.2
Several studies have investigated various clinical factors that might predict which patients with BD will respond to lithium. In a review, Kleindienst et al3 highlighted 3 factors that predicted a positive response to lithium:
- fewer hospitalizations prior to treatment
- an episodic course characterized sequentially by mania, depression, and then euthymia
- a later age (>50) at onset of BD.
Recent studies and reviews have isolated additional positive predictors, including having a family history of BD and a shorter duration of illness before receiving lithium, as well as negative predictors, such as rapid cycling, a large number of previous hospitalizations, a depression/mania/euthymia pattern, mood-incongruent psychotic features, and the presence of residual symptoms between mood episodes.3,4
The Table provides a list of probable and possible positive and negative predictors for therapeutic response to lithium in patients with BD.3-6 While relevant, the factors listed as possible predictors may not carry as much influence on lithium responsivity as those categorized as probable predictors.

Because of heterogeneity among studies, clinicians should consider their patient’s presentation as a whole, rather than basing medication choice on independent factors. Ultimately, more studies are required to fully determine the most relevant clinical parameters for lithium response. Overall, however, it appears these clinical factors could be extremely useful to guide psychiatrists in the optimal use of lithium while caring for patients with BD.
1. Crossley NA, Bauer M. Acceleration and augmentation of antidepressants with lithium for depressive disorders: two meta-analyses of randomized, placebo-controlled trials. J Clin Psychiatry. 2007;68(6):935-940.
2. Geddes JR, Burgess S, Hawton K, et al. Long-term lithium therapy for bipolar disorder: systematic review and meta-analysis of randomized controlled trials. Am J Psychiatry. 2004;1m61(2):217-222.
3. Kleindienst N, Engel RR, Greil W. Which clinical factors predict response to prophylactic lithium? A systematic review for bipolar disorders. Bipolar Disord. 2005;7(5):404-417.
4. Kleindienst N, Engel RR, Greil W. Psychosocial and demographic factors associated with response to prophylactic lithium: a systematic review for bipolar disorders. Psychol Med. 2005;35(12):1685-1694.
5. Hui TP, Kandola A, Shen L, et al. A systematic review and meta-analysis of clinical predictors of lithium response in bipolar disorder. Acta Psychiatr Scand. 2019;140(2):94-115.
6. Grillault Laroche D, Etain B, Severus E, et al. Socio-demographic and clinical predictors of outcome to long-term treatment with lithium in bipolar disorders: a systematic review of the contemporary literature and recommendations from the ISBD/IGSLI Task Force on treatment with lithium. Int J Bipolar Disord. 2020;8(1):40.
1. Crossley NA, Bauer M. Acceleration and augmentation of antidepressants with lithium for depressive disorders: two meta-analyses of randomized, placebo-controlled trials. J Clin Psychiatry. 2007;68(6):935-940.
2. Geddes JR, Burgess S, Hawton K, et al. Long-term lithium therapy for bipolar disorder: systematic review and meta-analysis of randomized controlled trials. Am J Psychiatry. 2004;1m61(2):217-222.
3. Kleindienst N, Engel RR, Greil W. Which clinical factors predict response to prophylactic lithium? A systematic review for bipolar disorders. Bipolar Disord. 2005;7(5):404-417.
4. Kleindienst N, Engel RR, Greil W. Psychosocial and demographic factors associated with response to prophylactic lithium: a systematic review for bipolar disorders. Psychol Med. 2005;35(12):1685-1694.
5. Hui TP, Kandola A, Shen L, et al. A systematic review and meta-analysis of clinical predictors of lithium response in bipolar disorder. Acta Psychiatr Scand. 2019;140(2):94-115.
6. Grillault Laroche D, Etain B, Severus E, et al. Socio-demographic and clinical predictors of outcome to long-term treatment with lithium in bipolar disorders: a systematic review of the contemporary literature and recommendations from the ISBD/IGSLI Task Force on treatment with lithium. Int J Bipolar Disord. 2020;8(1):40.
Melatonin as a sleep aid: Are you prescribing it correctly?
Difficulty achieving regular restorative sleep is a common symptom of many psychiatric illnesses and can pose a pharmaceutical challenge, particularly for patients who have contraindications to benzodiazepines or sedative-hypnotics. Melatonin is commonly used to treat insomnia and circadian rhythm disorders in hospitalized patients because it is largely considered safe, nonhabit forming, unlikely to interact with other medications, and possibly protective against delirium.1 We support its short-term use in patients with sleep disruption, even if they do not meet the diagnostic criteria for insomnia or a circadian rhythm sleep-wake disorder. However, this use should be guided by consideration of the known physiological actions of melatonin, and not by an assumption that it acts as a simple sedative-hypnotic.
How melatonin works
Melatonin is an endogenous neurohormone involved in circadian rhythm regulation (sleep/wake regulation), a fundamental process in the functioning of the CNS and in the development of psychiatric disorders.2 Melatonin is commonly described as a sleep-promoting neurotransmitter, but it is more accurately described as a “darkness hormone.”3 With an onset at dusk and offset at sunrise, melatonin is the signal for biological night, not the signal for sleep. Melanopsin-containing retina neurons sensitive to blue light sense the diminishing light of the evening and communicate this cue to the brain’s master clock in the suprachiasmatic nucleus (SCN) of the hypothalamus (via the retinohypothalamic pathway). The SCN then releases its inhibition on the pineal gland, allowing it to release melatonin into the bloodstream and CSF. The timing of this release is known as the dim-light melatonin onset (DLMO).
Selecting the optimal timing and dose
Studies in laboratory and home settings have consistently shown that the DLMO precedes the onset of sleep by approximately 2 to 4 hours.4 Thus, we recommend scheduling melatonin administration for 2 to 4 hours before the intended bedtime.
Lower doses better replicate physiological levels of melatonin. A lower dose is also less likely to lead to a compromise of the entrainment process and the induction of a delayed sleep phase due to the lingering presence of melatonin, or the phase-delaying effects of a strong melatonin signal much later than the ideal DLMO. Giving higher doses at bedtime will induce sleep but may cause a circadian phase delay, effectively “jet lagging” the patient. We recommend prescribing low-dose melatonin (LDM; 0.5 to 1 mg) 2 to 4 hours before the intended bedtime rather than higher doses (≥5 mg) given at bedtime as is common practice and recommended by many melatonin manufacturers. LDM better simulates the natural release and function of melatonin and avoids potential adverse circadian phase delays. T
1. Joseph SG. Melatonin supplementation for the prevention of hospital-associated delirium. Ment Health Clin. 2018;7(4):143-146. doi:10.9740/mhc.2017.07.143
2. Arendt J, Skene DJ. Melatonin as a chronobiotic. Sleep Med Rev. 2005;9(1):25-39. doi:10.1016/j.smrv.2004.05.002
3. Tallavajhula S, Rodgers JJ, Slater JD. Sleep and sleep-wake disorders. In: Arciniengas DB, Yudofsky SC, Hales RE, eds. Textbook of Neuropsychiatry and Clinical Neurosciences. American Psychiatric Association Publishing; 2018:373-393.
4. Sletten TL, Vincenzi S, Redman JR, et al. Timing of sleep and its relationship with the endogenous melatonin rhythm. Front Neurol. 2010;1:137. doi:10.3389/fneur.2010.00137
Difficulty achieving regular restorative sleep is a common symptom of many psychiatric illnesses and can pose a pharmaceutical challenge, particularly for patients who have contraindications to benzodiazepines or sedative-hypnotics. Melatonin is commonly used to treat insomnia and circadian rhythm disorders in hospitalized patients because it is largely considered safe, nonhabit forming, unlikely to interact with other medications, and possibly protective against delirium.1 We support its short-term use in patients with sleep disruption, even if they do not meet the diagnostic criteria for insomnia or a circadian rhythm sleep-wake disorder. However, this use should be guided by consideration of the known physiological actions of melatonin, and not by an assumption that it acts as a simple sedative-hypnotic.
How melatonin works
Melatonin is an endogenous neurohormone involved in circadian rhythm regulation (sleep/wake regulation), a fundamental process in the functioning of the CNS and in the development of psychiatric disorders.2 Melatonin is commonly described as a sleep-promoting neurotransmitter, but it is more accurately described as a “darkness hormone.”3 With an onset at dusk and offset at sunrise, melatonin is the signal for biological night, not the signal for sleep. Melanopsin-containing retina neurons sensitive to blue light sense the diminishing light of the evening and communicate this cue to the brain’s master clock in the suprachiasmatic nucleus (SCN) of the hypothalamus (via the retinohypothalamic pathway). The SCN then releases its inhibition on the pineal gland, allowing it to release melatonin into the bloodstream and CSF. The timing of this release is known as the dim-light melatonin onset (DLMO).
Selecting the optimal timing and dose
Studies in laboratory and home settings have consistently shown that the DLMO precedes the onset of sleep by approximately 2 to 4 hours.4 Thus, we recommend scheduling melatonin administration for 2 to 4 hours before the intended bedtime.
Lower doses better replicate physiological levels of melatonin. A lower dose is also less likely to lead to a compromise of the entrainment process and the induction of a delayed sleep phase due to the lingering presence of melatonin, or the phase-delaying effects of a strong melatonin signal much later than the ideal DLMO. Giving higher doses at bedtime will induce sleep but may cause a circadian phase delay, effectively “jet lagging” the patient. We recommend prescribing low-dose melatonin (LDM; 0.5 to 1 mg) 2 to 4 hours before the intended bedtime rather than higher doses (≥5 mg) given at bedtime as is common practice and recommended by many melatonin manufacturers. LDM better simulates the natural release and function of melatonin and avoids potential adverse circadian phase delays. T
Difficulty achieving regular restorative sleep is a common symptom of many psychiatric illnesses and can pose a pharmaceutical challenge, particularly for patients who have contraindications to benzodiazepines or sedative-hypnotics. Melatonin is commonly used to treat insomnia and circadian rhythm disorders in hospitalized patients because it is largely considered safe, nonhabit forming, unlikely to interact with other medications, and possibly protective against delirium.1 We support its short-term use in patients with sleep disruption, even if they do not meet the diagnostic criteria for insomnia or a circadian rhythm sleep-wake disorder. However, this use should be guided by consideration of the known physiological actions of melatonin, and not by an assumption that it acts as a simple sedative-hypnotic.
How melatonin works
Melatonin is an endogenous neurohormone involved in circadian rhythm regulation (sleep/wake regulation), a fundamental process in the functioning of the CNS and in the development of psychiatric disorders.2 Melatonin is commonly described as a sleep-promoting neurotransmitter, but it is more accurately described as a “darkness hormone.”3 With an onset at dusk and offset at sunrise, melatonin is the signal for biological night, not the signal for sleep. Melanopsin-containing retina neurons sensitive to blue light sense the diminishing light of the evening and communicate this cue to the brain’s master clock in the suprachiasmatic nucleus (SCN) of the hypothalamus (via the retinohypothalamic pathway). The SCN then releases its inhibition on the pineal gland, allowing it to release melatonin into the bloodstream and CSF. The timing of this release is known as the dim-light melatonin onset (DLMO).
Selecting the optimal timing and dose
Studies in laboratory and home settings have consistently shown that the DLMO precedes the onset of sleep by approximately 2 to 4 hours.4 Thus, we recommend scheduling melatonin administration for 2 to 4 hours before the intended bedtime.
Lower doses better replicate physiological levels of melatonin. A lower dose is also less likely to lead to a compromise of the entrainment process and the induction of a delayed sleep phase due to the lingering presence of melatonin, or the phase-delaying effects of a strong melatonin signal much later than the ideal DLMO. Giving higher doses at bedtime will induce sleep but may cause a circadian phase delay, effectively “jet lagging” the patient. We recommend prescribing low-dose melatonin (LDM; 0.5 to 1 mg) 2 to 4 hours before the intended bedtime rather than higher doses (≥5 mg) given at bedtime as is common practice and recommended by many melatonin manufacturers. LDM better simulates the natural release and function of melatonin and avoids potential adverse circadian phase delays. T
1. Joseph SG. Melatonin supplementation for the prevention of hospital-associated delirium. Ment Health Clin. 2018;7(4):143-146. doi:10.9740/mhc.2017.07.143
2. Arendt J, Skene DJ. Melatonin as a chronobiotic. Sleep Med Rev. 2005;9(1):25-39. doi:10.1016/j.smrv.2004.05.002
3. Tallavajhula S, Rodgers JJ, Slater JD. Sleep and sleep-wake disorders. In: Arciniengas DB, Yudofsky SC, Hales RE, eds. Textbook of Neuropsychiatry and Clinical Neurosciences. American Psychiatric Association Publishing; 2018:373-393.
4. Sletten TL, Vincenzi S, Redman JR, et al. Timing of sleep and its relationship with the endogenous melatonin rhythm. Front Neurol. 2010;1:137. doi:10.3389/fneur.2010.00137
1. Joseph SG. Melatonin supplementation for the prevention of hospital-associated delirium. Ment Health Clin. 2018;7(4):143-146. doi:10.9740/mhc.2017.07.143
2. Arendt J, Skene DJ. Melatonin as a chronobiotic. Sleep Med Rev. 2005;9(1):25-39. doi:10.1016/j.smrv.2004.05.002
3. Tallavajhula S, Rodgers JJ, Slater JD. Sleep and sleep-wake disorders. In: Arciniengas DB, Yudofsky SC, Hales RE, eds. Textbook of Neuropsychiatry and Clinical Neurosciences. American Psychiatric Association Publishing; 2018:373-393.
4. Sletten TL, Vincenzi S, Redman JR, et al. Timing of sleep and its relationship with the endogenous melatonin rhythm. Front Neurol. 2010;1:137. doi:10.3389/fneur.2010.00137
Proposal for a new diagnosis: Acute anxiety disorder
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Mr. F, age 42, says he has always been a very anxious person and has chronically found his worrying to negatively affect his life. He says that over the last month his anxiety has been “off the charts” and he is worrying “24/7” due to taking on new responsibilities at his job and his son being diagnosed with lupus. He says his constant worrying is significantly impairing his ability to focus at his job, and he is considering taking a mental health leave from work. His wife reports that she is extremely frustrated because Mr. F has been isolating himself from family and friends; he admits this is true and attributes it to being preoccupied by his worries.
Mr. F endorses chronic insomnia, muscle tension, and irritability associated with anxiety; these have all substantially worsened over the last month. He admits that recently he has occasionally thought it would be easier if he weren’t alive. Mr. F denies having problems with his energy or motivation levels and insists that he generally feels very anxious, but not depressed. He says he drinks 1 alcoholic drink per week and denies any other substance use. Mr. F is overweight and has slightly elevated cholesterol but denies any other health conditions. He takes melatonin to help him sleep but does not take any prescribed medications.
Although this vignette provides limited details, on the surface it appears that Mr. F is experiencing an exacerbation of chronic generalized anxiety disorder (GAD). However, in this article, I propose establishing a new diagnosis: “acute anxiety disorder,” which would encapsulate severe exacerbations of a pre-existing anxiety disorder. Among the patients I have encountered for whom this diagnosis would fit, most have pre-existing GAD or panic disorder.
A look at the differential diagnosis
It is important to determine whether Mr. F is using any substances or has a medical condition that could be contributing to his anxiety. Other psychiatric diagnoses that could be considered include:
Adjustment disorder. This diagnosis would make sense if Mr. F didn’t have an apparent chronic history of symptoms that meet criteria for GAD.
Major depressive disorder with anxious distress. Many patients experiencing a major depressive episode meet the criteria for the specifier “with anxious distress,” even those who do not have a comorbid anxiety disorder.1 However, it is not evident from this vignette that Mr. F is experiencing a major depressive episode.
Continue to: Panic disorder and GAD...
Panic disorder and GAD. It is possible for a patient with GAD to develop panic disorder, which, at times, occurs after experiencing significant life stressors. Panic disorder requires the presence of recurrent panic attacks. Mr. F describes experiencing chronic, intense symptoms of anxiety rather than the discreet episodes of acute symptoms that characterize panic attacks.
Acute stress disorder. This diagnosis involves psychological symptoms that occur in response to exposure to actual or threatened death, serious injury, or sexual violation. Mr. F was not exposed to any of these stressors.
Why this new diagnosis would be helpful
A new diagnosis, acute anxiety disorder, would indicate that a patient is currently experiencing an acute exacerbation of a chronic anxiety disorder that is leading to a significant decrease in their baseline functioning. My proposed criteria for acute anxiety disorder appear in the Table. Here are some reasons this diagnosis would be helpful:

Signifier of severity. Anxiety disorders such as GAD are generally not considered severe conditions and not considered to fall under the rubric of SPMI (severe and persistent mental illness).2 Posttraumatic stress disorder is the anxiety disorder–like condition most often found in the SPMI category. A diagnosis of acute anxiety disorder would indicate a patient is experiencing an episode of anxiety that is distinct from their chronic anxiety condition due to its severe impact on functional capabilities. Acute anxiety disorder would certainly not qualify as a “SPMI diagnosis” that would facilitate someone being considered eligible for supplemental security income, but it might be a legitimate justification for someone to receive short-term disability.
Treatment approach. The pharmacologic treatment of anxiety disorders usually involves a selective serotonin reuptake inhibitor (SSRI) or serotonin-norepinephrine reuptake inhibitor (SNRI). However, these medications can sometimes briefly increase anxiety when they are started. Individuals with acute anxiety are the most vulnerable to the possibility of experiencing increased anxiety when starting an SSRI or SNRI and may benefit from a slower titration of these medications. In light of this and the length of time required for SSRIs or SNRIs to exert a positive effect (typically a few weeks), patients with acute anxiety are best served by treatment with a medication with an immediate onset of action, such as a benzodiazepine or a sleep medication (eg, zolpidem). Benzodiazepines and hypnotics such as zolpidem are best prescribed for as-needed use because they carry a risk of dependence. One might consider prescribing mirtazapine or pregabalin (both of which are used off-label to treat anxiety) because these medications also have a relatively rapid onset of action and can treat both anxiety and insomnia (particularly mirtazapine).
Research considerations. It would be helpful to study which treatments are most effective for the subset of patients who experience acute anxiety disorder as I define it. Perhaps psychotherapy treatment protocols could be adapted or created. Treatment with esketamine or IV ketamine might be further studied as a treatment for acute anxiety because some evidence suggests ketamine is efficacious for this indication.3
1. Otsubo T, Hokama C, Sano N, et al. How significant is the assessment of the DSM-5 ‘anxious distress’ specifier in patients with major depressive disorder without comorbid anxiety disorders in the continuation/maintenance phase? Int J Psychiatry Clin Pract. 2021;25(4):385-392. doi:10.1080/13651501.2021.1907415
2. Butler H, O’Brien AJ. Access to specialist palliative care services by people with severe and persistent mental illness: a retrospective cohort study. Int J Ment Health Nurs. 2018;27(2):737-746. doi:10.1111/inm.12360
3. Glue P, Neehoff SM, Medlicott NJ, et al. Safety and efficacy of maintenance ketamine treatment in patients with treatment-refractory generalised anxiety and social anxiety disorders. J Psychopharmacol. 2018;32(6):663-667. doi:10.1177/0269881118762073
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Mr. F, age 42, says he has always been a very anxious person and has chronically found his worrying to negatively affect his life. He says that over the last month his anxiety has been “off the charts” and he is worrying “24/7” due to taking on new responsibilities at his job and his son being diagnosed with lupus. He says his constant worrying is significantly impairing his ability to focus at his job, and he is considering taking a mental health leave from work. His wife reports that she is extremely frustrated because Mr. F has been isolating himself from family and friends; he admits this is true and attributes it to being preoccupied by his worries.
Mr. F endorses chronic insomnia, muscle tension, and irritability associated with anxiety; these have all substantially worsened over the last month. He admits that recently he has occasionally thought it would be easier if he weren’t alive. Mr. F denies having problems with his energy or motivation levels and insists that he generally feels very anxious, but not depressed. He says he drinks 1 alcoholic drink per week and denies any other substance use. Mr. F is overweight and has slightly elevated cholesterol but denies any other health conditions. He takes melatonin to help him sleep but does not take any prescribed medications.
Although this vignette provides limited details, on the surface it appears that Mr. F is experiencing an exacerbation of chronic generalized anxiety disorder (GAD). However, in this article, I propose establishing a new diagnosis: “acute anxiety disorder,” which would encapsulate severe exacerbations of a pre-existing anxiety disorder. Among the patients I have encountered for whom this diagnosis would fit, most have pre-existing GAD or panic disorder.
A look at the differential diagnosis
It is important to determine whether Mr. F is using any substances or has a medical condition that could be contributing to his anxiety. Other psychiatric diagnoses that could be considered include:
Adjustment disorder. This diagnosis would make sense if Mr. F didn’t have an apparent chronic history of symptoms that meet criteria for GAD.
Major depressive disorder with anxious distress. Many patients experiencing a major depressive episode meet the criteria for the specifier “with anxious distress,” even those who do not have a comorbid anxiety disorder.1 However, it is not evident from this vignette that Mr. F is experiencing a major depressive episode.
Continue to: Panic disorder and GAD...
Panic disorder and GAD. It is possible for a patient with GAD to develop panic disorder, which, at times, occurs after experiencing significant life stressors. Panic disorder requires the presence of recurrent panic attacks. Mr. F describes experiencing chronic, intense symptoms of anxiety rather than the discreet episodes of acute symptoms that characterize panic attacks.
Acute stress disorder. This diagnosis involves psychological symptoms that occur in response to exposure to actual or threatened death, serious injury, or sexual violation. Mr. F was not exposed to any of these stressors.
Why this new diagnosis would be helpful
A new diagnosis, acute anxiety disorder, would indicate that a patient is currently experiencing an acute exacerbation of a chronic anxiety disorder that is leading to a significant decrease in their baseline functioning. My proposed criteria for acute anxiety disorder appear in the Table. Here are some reasons this diagnosis would be helpful:

Signifier of severity. Anxiety disorders such as GAD are generally not considered severe conditions and not considered to fall under the rubric of SPMI (severe and persistent mental illness).2 Posttraumatic stress disorder is the anxiety disorder–like condition most often found in the SPMI category. A diagnosis of acute anxiety disorder would indicate a patient is experiencing an episode of anxiety that is distinct from their chronic anxiety condition due to its severe impact on functional capabilities. Acute anxiety disorder would certainly not qualify as a “SPMI diagnosis” that would facilitate someone being considered eligible for supplemental security income, but it might be a legitimate justification for someone to receive short-term disability.
Treatment approach. The pharmacologic treatment of anxiety disorders usually involves a selective serotonin reuptake inhibitor (SSRI) or serotonin-norepinephrine reuptake inhibitor (SNRI). However, these medications can sometimes briefly increase anxiety when they are started. Individuals with acute anxiety are the most vulnerable to the possibility of experiencing increased anxiety when starting an SSRI or SNRI and may benefit from a slower titration of these medications. In light of this and the length of time required for SSRIs or SNRIs to exert a positive effect (typically a few weeks), patients with acute anxiety are best served by treatment with a medication with an immediate onset of action, such as a benzodiazepine or a sleep medication (eg, zolpidem). Benzodiazepines and hypnotics such as zolpidem are best prescribed for as-needed use because they carry a risk of dependence. One might consider prescribing mirtazapine or pregabalin (both of which are used off-label to treat anxiety) because these medications also have a relatively rapid onset of action and can treat both anxiety and insomnia (particularly mirtazapine).
Research considerations. It would be helpful to study which treatments are most effective for the subset of patients who experience acute anxiety disorder as I define it. Perhaps psychotherapy treatment protocols could be adapted or created. Treatment with esketamine or IV ketamine might be further studied as a treatment for acute anxiety because some evidence suggests ketamine is efficacious for this indication.3
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Mr. F, age 42, says he has always been a very anxious person and has chronically found his worrying to negatively affect his life. He says that over the last month his anxiety has been “off the charts” and he is worrying “24/7” due to taking on new responsibilities at his job and his son being diagnosed with lupus. He says his constant worrying is significantly impairing his ability to focus at his job, and he is considering taking a mental health leave from work. His wife reports that she is extremely frustrated because Mr. F has been isolating himself from family and friends; he admits this is true and attributes it to being preoccupied by his worries.
Mr. F endorses chronic insomnia, muscle tension, and irritability associated with anxiety; these have all substantially worsened over the last month. He admits that recently he has occasionally thought it would be easier if he weren’t alive. Mr. F denies having problems with his energy or motivation levels and insists that he generally feels very anxious, but not depressed. He says he drinks 1 alcoholic drink per week and denies any other substance use. Mr. F is overweight and has slightly elevated cholesterol but denies any other health conditions. He takes melatonin to help him sleep but does not take any prescribed medications.
Although this vignette provides limited details, on the surface it appears that Mr. F is experiencing an exacerbation of chronic generalized anxiety disorder (GAD). However, in this article, I propose establishing a new diagnosis: “acute anxiety disorder,” which would encapsulate severe exacerbations of a pre-existing anxiety disorder. Among the patients I have encountered for whom this diagnosis would fit, most have pre-existing GAD or panic disorder.
A look at the differential diagnosis
It is important to determine whether Mr. F is using any substances or has a medical condition that could be contributing to his anxiety. Other psychiatric diagnoses that could be considered include:
Adjustment disorder. This diagnosis would make sense if Mr. F didn’t have an apparent chronic history of symptoms that meet criteria for GAD.
Major depressive disorder with anxious distress. Many patients experiencing a major depressive episode meet the criteria for the specifier “with anxious distress,” even those who do not have a comorbid anxiety disorder.1 However, it is not evident from this vignette that Mr. F is experiencing a major depressive episode.
Continue to: Panic disorder and GAD...
Panic disorder and GAD. It is possible for a patient with GAD to develop panic disorder, which, at times, occurs after experiencing significant life stressors. Panic disorder requires the presence of recurrent panic attacks. Mr. F describes experiencing chronic, intense symptoms of anxiety rather than the discreet episodes of acute symptoms that characterize panic attacks.
Acute stress disorder. This diagnosis involves psychological symptoms that occur in response to exposure to actual or threatened death, serious injury, or sexual violation. Mr. F was not exposed to any of these stressors.
Why this new diagnosis would be helpful
A new diagnosis, acute anxiety disorder, would indicate that a patient is currently experiencing an acute exacerbation of a chronic anxiety disorder that is leading to a significant decrease in their baseline functioning. My proposed criteria for acute anxiety disorder appear in the Table. Here are some reasons this diagnosis would be helpful:

Signifier of severity. Anxiety disorders such as GAD are generally not considered severe conditions and not considered to fall under the rubric of SPMI (severe and persistent mental illness).2 Posttraumatic stress disorder is the anxiety disorder–like condition most often found in the SPMI category. A diagnosis of acute anxiety disorder would indicate a patient is experiencing an episode of anxiety that is distinct from their chronic anxiety condition due to its severe impact on functional capabilities. Acute anxiety disorder would certainly not qualify as a “SPMI diagnosis” that would facilitate someone being considered eligible for supplemental security income, but it might be a legitimate justification for someone to receive short-term disability.
Treatment approach. The pharmacologic treatment of anxiety disorders usually involves a selective serotonin reuptake inhibitor (SSRI) or serotonin-norepinephrine reuptake inhibitor (SNRI). However, these medications can sometimes briefly increase anxiety when they are started. Individuals with acute anxiety are the most vulnerable to the possibility of experiencing increased anxiety when starting an SSRI or SNRI and may benefit from a slower titration of these medications. In light of this and the length of time required for SSRIs or SNRIs to exert a positive effect (typically a few weeks), patients with acute anxiety are best served by treatment with a medication with an immediate onset of action, such as a benzodiazepine or a sleep medication (eg, zolpidem). Benzodiazepines and hypnotics such as zolpidem are best prescribed for as-needed use because they carry a risk of dependence. One might consider prescribing mirtazapine or pregabalin (both of which are used off-label to treat anxiety) because these medications also have a relatively rapid onset of action and can treat both anxiety and insomnia (particularly mirtazapine).
Research considerations. It would be helpful to study which treatments are most effective for the subset of patients who experience acute anxiety disorder as I define it. Perhaps psychotherapy treatment protocols could be adapted or created. Treatment with esketamine or IV ketamine might be further studied as a treatment for acute anxiety because some evidence suggests ketamine is efficacious for this indication.3
1. Otsubo T, Hokama C, Sano N, et al. How significant is the assessment of the DSM-5 ‘anxious distress’ specifier in patients with major depressive disorder without comorbid anxiety disorders in the continuation/maintenance phase? Int J Psychiatry Clin Pract. 2021;25(4):385-392. doi:10.1080/13651501.2021.1907415
2. Butler H, O’Brien AJ. Access to specialist palliative care services by people with severe and persistent mental illness: a retrospective cohort study. Int J Ment Health Nurs. 2018;27(2):737-746. doi:10.1111/inm.12360
3. Glue P, Neehoff SM, Medlicott NJ, et al. Safety and efficacy of maintenance ketamine treatment in patients with treatment-refractory generalised anxiety and social anxiety disorders. J Psychopharmacol. 2018;32(6):663-667. doi:10.1177/0269881118762073
1. Otsubo T, Hokama C, Sano N, et al. How significant is the assessment of the DSM-5 ‘anxious distress’ specifier in patients with major depressive disorder without comorbid anxiety disorders in the continuation/maintenance phase? Int J Psychiatry Clin Pract. 2021;25(4):385-392. doi:10.1080/13651501.2021.1907415
2. Butler H, O’Brien AJ. Access to specialist palliative care services by people with severe and persistent mental illness: a retrospective cohort study. Int J Ment Health Nurs. 2018;27(2):737-746. doi:10.1111/inm.12360
3. Glue P, Neehoff SM, Medlicott NJ, et al. Safety and efficacy of maintenance ketamine treatment in patients with treatment-refractory generalised anxiety and social anxiety disorders. J Psychopharmacol. 2018;32(6):663-667. doi:10.1177/0269881118762073
Neurosurgical treatment of OCD: Patient selection, safety, and access
Obsessive-compulsive disorder (OCD) is typically a severe, chronic illness in which patients have recurrent, unwanted thoughts, urges, and compulsions.1 It causes significant morbidity and lost potential over time, and is the world’s 10th-most disabling disorder in terms of lost income and decreased quality of life, and the fifth-most disabling mental health condition.2 Patients with OCD (and their clinicians) are often desperate for an efficacious treatment, but we must ensure that those who are not helped by traditional psychotherapeutic and/or pharmacologic treatments are appropriate for safe neurosurgical intervention.
Pros and cons of neurosurgical therapies
Most patients with OCD are effectively treated with cognitive-behavioral therapy and pharmacotherapy in the form of selective serotonin reuptake inhibitors, clomipramine, or second-generation antipsychotics. However, up to 5% of individuals with OCD will have symptoms refractory to these traditional therapies.3 These cases require more aggressive forms of therapy, including radiofrequency ablation surgeries and deep brain stimulation (DBS). The efficacy of both therapies is similar at 40% to 60%.4,5 While these treatments can be life-changing for patients fortunate to receive them, they are not without issue.
Only a limited number of institutions offer these neurosurgical techniques, and for many patients, those locations may be inaccessible. Patients may not experience relief simply due to where they live, difficult logistics, and the high cost requisite to receive care. If fortunate enough to live near a participating institution or have the means to travel to one, the patient and clinician must then choose the best option based on the nuances of the patient’s situation.
Ablation techniques, such as gamma knife or magnetic resonance–guided ultrasound, are simpler and more cost-effective. A drawback of this approach, however, is that it is irreversible. Lesioned structures are irreparable, as are the adverse effects of the surgery, which, while rare, may include a persistent minimally conscious state or necrotic cysts.4 A benefit of this approach is that there is no need for lengthy follow-up as seen with DBS.
DBS is more complicated. In addition to having to undergo an open neurosurgical procedure, these patients require long-term follow-up and monitoring. A positive aspect is the device can be turned off or removed. However, the amount of follow-up and adjustments is significant. These patients need access to clinicians skilled in DBS device management.
Finally, we must consider the chronically ill patient’s perspective after successful treatment. While the patient’s symptoms may improve, their lives and identities likely developed around their symptoms. Bosanac et al6 describe this reality well in a case study in which a patient with OCD was “burdened with normality” after successful DBS treatment. He was finally able to work, build meaningful relationships, and approach previously unattainable social milestones. This was an overwhelming experience for him, and he and his family needed guidance into the world in which most of us find comfort.
As ablation techniques, DBS, and other cutting-edge therapies for OCD come to the forefront of modern care, clinicians must remember to keep patient safety first. Verify follow-up care before committing patients to invasive and irreversible treatments. While general access is currently poor, participating institutions should consider advertising and communicating that there is an accessible network available for these chronically ill individuals.
1. Ruscio AM, Stein DJ, Chiu WT, et al. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry. 2010;15(1):53-63.
2. World Health Organization. The Global Burden of Disease: 2004 Update. World Health Organization; 2008.
3. Jenike MA, Rauch SL. Managing the patient with treatment-resistant obsessive compulsive disorder: current strategies. J Clin Psychiatry. 1994;55 Suppl:11-17.
4. Rasmussen SA, Noren G, Greenberg BD, et al. Gamma ventral capsulotomy in intractable obsessive-compulsive disorder. Biol Psychiatry. 2018;84(5):355-364.
5. Kumar KK, Appelboom, G, Lamsam L, et al. Comparative effectiveness of neuroablation and deep brain stimulation for treatment-resistant obsessive-compulsive disorder: a meta-analytic study. J Neurol Neurosurg Psychiatry. 2019;90(4):469-473.
6. Bosanac P, Hamilton BE, Lucak J, et al. Identity challenges and ‘burden of normality’ after DBS for severe OCD: a narrative case study. BMC Psychiatry. 2018;18(1):186.
Obsessive-compulsive disorder (OCD) is typically a severe, chronic illness in which patients have recurrent, unwanted thoughts, urges, and compulsions.1 It causes significant morbidity and lost potential over time, and is the world’s 10th-most disabling disorder in terms of lost income and decreased quality of life, and the fifth-most disabling mental health condition.2 Patients with OCD (and their clinicians) are often desperate for an efficacious treatment, but we must ensure that those who are not helped by traditional psychotherapeutic and/or pharmacologic treatments are appropriate for safe neurosurgical intervention.
Pros and cons of neurosurgical therapies
Most patients with OCD are effectively treated with cognitive-behavioral therapy and pharmacotherapy in the form of selective serotonin reuptake inhibitors, clomipramine, or second-generation antipsychotics. However, up to 5% of individuals with OCD will have symptoms refractory to these traditional therapies.3 These cases require more aggressive forms of therapy, including radiofrequency ablation surgeries and deep brain stimulation (DBS). The efficacy of both therapies is similar at 40% to 60%.4,5 While these treatments can be life-changing for patients fortunate to receive them, they are not without issue.
Only a limited number of institutions offer these neurosurgical techniques, and for many patients, those locations may be inaccessible. Patients may not experience relief simply due to where they live, difficult logistics, and the high cost requisite to receive care. If fortunate enough to live near a participating institution or have the means to travel to one, the patient and clinician must then choose the best option based on the nuances of the patient’s situation.
Ablation techniques, such as gamma knife or magnetic resonance–guided ultrasound, are simpler and more cost-effective. A drawback of this approach, however, is that it is irreversible. Lesioned structures are irreparable, as are the adverse effects of the surgery, which, while rare, may include a persistent minimally conscious state or necrotic cysts.4 A benefit of this approach is that there is no need for lengthy follow-up as seen with DBS.
DBS is more complicated. In addition to having to undergo an open neurosurgical procedure, these patients require long-term follow-up and monitoring. A positive aspect is the device can be turned off or removed. However, the amount of follow-up and adjustments is significant. These patients need access to clinicians skilled in DBS device management.
Finally, we must consider the chronically ill patient’s perspective after successful treatment. While the patient’s symptoms may improve, their lives and identities likely developed around their symptoms. Bosanac et al6 describe this reality well in a case study in which a patient with OCD was “burdened with normality” after successful DBS treatment. He was finally able to work, build meaningful relationships, and approach previously unattainable social milestones. This was an overwhelming experience for him, and he and his family needed guidance into the world in which most of us find comfort.
As ablation techniques, DBS, and other cutting-edge therapies for OCD come to the forefront of modern care, clinicians must remember to keep patient safety first. Verify follow-up care before committing patients to invasive and irreversible treatments. While general access is currently poor, participating institutions should consider advertising and communicating that there is an accessible network available for these chronically ill individuals.
Obsessive-compulsive disorder (OCD) is typically a severe, chronic illness in which patients have recurrent, unwanted thoughts, urges, and compulsions.1 It causes significant morbidity and lost potential over time, and is the world’s 10th-most disabling disorder in terms of lost income and decreased quality of life, and the fifth-most disabling mental health condition.2 Patients with OCD (and their clinicians) are often desperate for an efficacious treatment, but we must ensure that those who are not helped by traditional psychotherapeutic and/or pharmacologic treatments are appropriate for safe neurosurgical intervention.
Pros and cons of neurosurgical therapies
Most patients with OCD are effectively treated with cognitive-behavioral therapy and pharmacotherapy in the form of selective serotonin reuptake inhibitors, clomipramine, or second-generation antipsychotics. However, up to 5% of individuals with OCD will have symptoms refractory to these traditional therapies.3 These cases require more aggressive forms of therapy, including radiofrequency ablation surgeries and deep brain stimulation (DBS). The efficacy of both therapies is similar at 40% to 60%.4,5 While these treatments can be life-changing for patients fortunate to receive them, they are not without issue.
Only a limited number of institutions offer these neurosurgical techniques, and for many patients, those locations may be inaccessible. Patients may not experience relief simply due to where they live, difficult logistics, and the high cost requisite to receive care. If fortunate enough to live near a participating institution or have the means to travel to one, the patient and clinician must then choose the best option based on the nuances of the patient’s situation.
Ablation techniques, such as gamma knife or magnetic resonance–guided ultrasound, are simpler and more cost-effective. A drawback of this approach, however, is that it is irreversible. Lesioned structures are irreparable, as are the adverse effects of the surgery, which, while rare, may include a persistent minimally conscious state or necrotic cysts.4 A benefit of this approach is that there is no need for lengthy follow-up as seen with DBS.
DBS is more complicated. In addition to having to undergo an open neurosurgical procedure, these patients require long-term follow-up and monitoring. A positive aspect is the device can be turned off or removed. However, the amount of follow-up and adjustments is significant. These patients need access to clinicians skilled in DBS device management.
Finally, we must consider the chronically ill patient’s perspective after successful treatment. While the patient’s symptoms may improve, their lives and identities likely developed around their symptoms. Bosanac et al6 describe this reality well in a case study in which a patient with OCD was “burdened with normality” after successful DBS treatment. He was finally able to work, build meaningful relationships, and approach previously unattainable social milestones. This was an overwhelming experience for him, and he and his family needed guidance into the world in which most of us find comfort.
As ablation techniques, DBS, and other cutting-edge therapies for OCD come to the forefront of modern care, clinicians must remember to keep patient safety first. Verify follow-up care before committing patients to invasive and irreversible treatments. While general access is currently poor, participating institutions should consider advertising and communicating that there is an accessible network available for these chronically ill individuals.
1. Ruscio AM, Stein DJ, Chiu WT, et al. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry. 2010;15(1):53-63.
2. World Health Organization. The Global Burden of Disease: 2004 Update. World Health Organization; 2008.
3. Jenike MA, Rauch SL. Managing the patient with treatment-resistant obsessive compulsive disorder: current strategies. J Clin Psychiatry. 1994;55 Suppl:11-17.
4. Rasmussen SA, Noren G, Greenberg BD, et al. Gamma ventral capsulotomy in intractable obsessive-compulsive disorder. Biol Psychiatry. 2018;84(5):355-364.
5. Kumar KK, Appelboom, G, Lamsam L, et al. Comparative effectiveness of neuroablation and deep brain stimulation for treatment-resistant obsessive-compulsive disorder: a meta-analytic study. J Neurol Neurosurg Psychiatry. 2019;90(4):469-473.
6. Bosanac P, Hamilton BE, Lucak J, et al. Identity challenges and ‘burden of normality’ after DBS for severe OCD: a narrative case study. BMC Psychiatry. 2018;18(1):186.
1. Ruscio AM, Stein DJ, Chiu WT, et al. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry. 2010;15(1):53-63.
2. World Health Organization. The Global Burden of Disease: 2004 Update. World Health Organization; 2008.
3. Jenike MA, Rauch SL. Managing the patient with treatment-resistant obsessive compulsive disorder: current strategies. J Clin Psychiatry. 1994;55 Suppl:11-17.
4. Rasmussen SA, Noren G, Greenberg BD, et al. Gamma ventral capsulotomy in intractable obsessive-compulsive disorder. Biol Psychiatry. 2018;84(5):355-364.
5. Kumar KK, Appelboom, G, Lamsam L, et al. Comparative effectiveness of neuroablation and deep brain stimulation for treatment-resistant obsessive-compulsive disorder: a meta-analytic study. J Neurol Neurosurg Psychiatry. 2019;90(4):469-473.
6. Bosanac P, Hamilton BE, Lucak J, et al. Identity challenges and ‘burden of normality’ after DBS for severe OCD: a narrative case study. BMC Psychiatry. 2018;18(1):186.
What role does social media have in GI?
Dear colleagues,
Most of us engage with social media, whether actively tweeting, following friends on Facebook, or discussing TikTok videos with family. Many gastroenterologists leverage social media to build their professional brand and to reach a wider audience. Others remain wary of committing a social media faux paux or worry about patient confidentiality. In this Perspectives column, Dr. Stephen Chris Pappas and Dr. Mohammad Bilal discuss the risks and benefits of social media for the practicing gastroenterologist. Dr. Pappas has a unique perspective as a gastroenterologist who is also trained as a lawyer, and Dr. Bilal speaks from a wealth of experience leading educational activities on social media. We look forward to hearing your thoughts on Twitter @AGA_GIHN and by email at [email protected].
Gyanprakash A. Ketwaroo, MD, MSc, an associate professor of medicine, Yale University, New Haven, Conn., and chief of endoscopy at West Haven (Conn.) VA Medical Center. He is an associate editor for GI & Hepatology News.
Carefully consider the plentiful risks, concerns
BY STEPHEN CHRIS PAPPAS, MD, JD, FAASLD, FACLM
Social media for gastroenterologists comes with benefits accompanied by pesky risks. The risks are pesky like a mosquito bite: An itching bite is annoying, but getting malaria is serious. Managing your unprofessional tweet to salvage your reputation is going to be annoying. Disclosing a patient's identity on social media is going to be serious; you could find yourself fired, fined, reprimanded, and without hospital privileges, as happened recently to a Rhode Island physician. I divide the risks of social media into legal risks (for example, disclosing patient identity or inadvertently creating a doctor-patient relationship), risks of compromising ethical standards (for example, impairing the doctor-patient relationship), and mixed legal/ethics risks (for example, inappropriate Twitter banter disparaging individuals, promotion of “fake news”). Fortunately, these risks are intuitive and can be mitigated by attention to some simple principles.
Disclosing a patient’s identity on social media is clearly in violation of privacy laws and other regulations. Since privacy compliance is drummed into us ad nauseum via annual compliance training, we could ask “how on earth could an inadvertent disclosure of identity occur?” We must remember that sites that are nominally termed “secure” may not be. As a general suggestion, I would regard social media of all types as open public forums with permanent postings. Even limited descriptions of a patient on social media may allow identification of the actual patient. The risk may be highest in smaller communities; in the past I assisted a small-town practitioner manage the fallout from inadvertently identifying a patient on his professional Facebook page by simply saying “I recently managed a 38-year-old pregnant woman with Crohn’s disease ...” That small amount of information allowed some members of his community to identify the specific patient. My suggestion would be to never talk about individual patients on social media. Phrase comments or questions generically; for example, “Crohn’s disease in pregnancy is managed with attention to ...”.
Another legal risk of social media engagement is to unknowingly create a patient-doctor relationship with a duty to treat, opening the door for exposure to malpractice litigation if something goes awry. A patient may interpret a social media interaction as establishing a patient-doctor relationship. While we think we know what defines a doctor-patient relationship, it’s not always clear and varies between jurisdictions. Indeed, a physician-patient relationship may not even be a necessary element of a claim for professional negligence (an issue shared with “curbside” consults). A recent court case in Minnesota ruled that a duty to care is established if “... it is reasonably foreseeable that the third party will rely on the physician’s acts and be harmed by a breach of the standard of care.” That case involved a telephone call, but you could see the standard easily morphing to apply to social media posts. Gastroenterologists should always talk about disease and treatment on social media in generic terms, preferably with appropriate caveats (for example, “Patients with cholestasis and intense itching may be treated with naloxone in selected cases after detailed assessment by a hepatologist”).
Impairing an established doctor-patient relationship by “friending” a patient on your personal Facebook risks a potential compromise of professional ethics, breaking the boundaries between profession and person for the gastroenterologist. The approach by most professional societies is that a “friend” on social media is equal to a friend in the real world; the same legal and ethical standards apply. Doctor-patient friendships may compromise objectivity, lead to preferential but not optimal therapy, and increase the risk of skirting around informed consent among other issues. Being friends on social media is discouraged, but not prohibited, by most professional societies and licensing bodies. In my opinion, that is sound advice. Over a career of more than 40 years, I have had patients who became friends, but only after I had transferred their care to another hepatologist.
More recently with escalating, aggressive, tones for social media communications, GI/hepatology practitioners must be aware of the serious risk of blurring their personal and professional online lives, particularly where Twitter is involved. The rapidity which people seem to want to reply to a tweet, the public and durable natures of a tweet, and the ability to significantly retweet and repost all spell potential disasters for the physician tweeting an inappropriate communication. Separation of personal and professional social media accounts is strongly encouraged but alone is not enough; you are never totally anonymous online. The reality is that a physician will be judged for an inappropriate communication whether it’s found on their professional or personal site. Either posting could result in reputation damage, reprimands, medical license restrictions or revocations, and litigation. Nationally, medical boards now regularly deal with disciplinary actions for inappropriate social media activity. The best preventive measures include pausing before you post, check the veracity of what you are posting, place your post in context, and assess the tone of your post and the tone of the site that you are posting to. A perfect storm for disaster is that the material is not clearly evidence based and could be construed as “fake,” you are personally emotionally charged, and the site you are posting to is a known cauldron of emotion and fake news.
In summary, social media affords benefits in a health care setting but it comes with some baggage. However, the risks of a social media presence are largely instinctive. An initial starting point is pausing to consider, “Would I say/do this in a public venue where everybody could hear/see me?” If there is any concern, don’t post. Subsequently, conduct yourself on social media with meticulous attention to protecting confidentiality, avoiding any impression of creating a doctor-patient relationship, avoiding doctor-friend relationships, being aware of key legal, institutional, and professional society guidance, separating personal and professional activities, and maintaining professionalism.
Dr. Pappas is in the GI and hepatology section of the department of medicine at Baylor College of Medicine, Houston. He has no relevant conflicts of interest to disclose.
References
Attai DJ et al. Semin Hematol. 2017 Oct; 54(4): 198-204.
Bal BS et al. Clin Orthop Relat Res. 2019 Oct; 477(10): 2204-6.
Ekrem, D et al. 20111 Jun 6. https://www.kevinmd.com/2011/06/7-tips-avoid-hipaa-violations-social-media.html
Hallenbeck J. Doctor and Friend. 2005 Jun. https://journalofethics.ama-assn.org/article/doctor-and-friend/2005-06
Moses RE et al. Am J Gastroenterol. 2014 Aug;109(8):1128-32.
Understand its multifaceted importance
BY MOHAMMAD BILAL, MD, FACP
Merriam-Webster’s dictionary defines social media as “forms of electronic communication (such as websites for social networking and microblogging) through which users create online communities to share information, ideas, personal messages, and other content.” Over the last few years, there has been an increase in use of social media by medical professionals. Whether we like it or not, social media is here to stay. Patients use social media to look up information regarding their doctors, medical practices use it to promote the services they offer, institutions share their programs and initiatives, and doctors use it for education, to engage with like-minded colleagues, collaborate, spread awareness, network, and combat medical misinformation. Social media is now rapidly being used by gastroenterologists and hepatologists, as well as majority of professional GI organizations, and hashtags such as “#MedTwitter”, “#GITwitter,” and “#LiverTwitter” have developed into popular academic forums.1 Therefore, the impact of social media in GI is multifaceted and includes its role in medical education, promoting your practice or division, finding collaborations, building your network and establishing mentors and peer-mentors, disseminating your work, and building your brand.2
What is your goal?
Gastroenterologists could have one or more of the goals mentioned above for using social media. Determining the goals for social media use a priori will allow for determining which social media platform will be appropriate for you. Therefore, it is important to understand the users of various social media platforms. In 2017, Facebook was the highest used social media platform in all age groups, whereas Instagram was most popular amongst ages 18-29 years, while Twitter was used more commonly in ages 30-59 years as compared with Instagram. If your goal is to share scientific knowledge and literature with like-minded physicians and interact with leaders in the field, then Twitter may be ideal. If you want to connect with a younger, more diverse audience, Instagram might be a good option. While many physicians may have a Facebook account, this is often reserved for personal use. Many have separated of personal and professional social media use, although they do not need to exist in silos. Defining your goal with social media use will direct you to the best platform to reach your audience.
Medical education
The use of social media especially Twitter for medical education is continuously increasing. Several leaders in the field use “Tweetorials” as a means to educate others. Tweetorials are a collective set of tweets that systematically cover a specialized topic.3 Other educational forums such as @ScopingSundays, @MondayNightIBD, @IBDClub and @GIJournal provide structured platforms for GI focused discussion.4 @MondayNightIBD is also a source for official continued medical education. Other social media educational platforms include “Liver Fellow Network” which has wide variety of educational materials pertaining to hepatology. In addition, there is continuous opportunity to engage with leaders in the field and authors of published studies and guidelines. Several endoscopy educators have dedicated YouTube channels which have endless supply of educational videos.
Networking
As mentioned above, platforms such as #GITwitter and #LiverTwitter have become popular forums for engaging and connecting with like minded colleagues. Social media provides a space to share ideas and build collaborations with colleagues working on similar projects. The concept “#Twitter2Paper” has been proposed which signifies an idea that generated on Twitter and was eventually converted to a manuscript.5
Institutional, divisional, and practice promotion
Social media is a great tool to showcase the clinical, educational and scholarship services and efforts by programs, practices or divisions. During the COVID-19 pandemic, recruitment efforts at all stages were mainly shifted to virtual platforms, and social media was an instrumental way for programs to highlight their culture and initiatives. Prospective applicants can often refer to social media to get a better understanding of what the program offers. Similarly, if a new clinical service is being provided, targeted efforts can be made to ensure that patients are aware of the available services.
Patient education and combating misinformation
Several gastroenterologists also use social media to spread awareness regarding GI diseases. Instagram, Facebook, and TikTok are effective mediums where one can reach a wider audience. It is important for gastroenterologists to provide accurate information since there is a sea of misinformation available on the internet as well. Posts regarding colonoscopy and colon cancer awareness can help alleviate myths regarding role of colonoscopy. In addition, patient advocates use social media to provide peer support to others who deal with challenges related to chronic illnesses such as inflammatory bowel disease.
Sharing your work
Sharing your work on social media can help your work reach a broader audience. Studies have shown that work shared on social media has higher altmetric scores and can also lead to increased citations.
Diversity, equity, and inclusion
Social media offers a platform where one can promote or showcase their support for causes they believe in. The hashtag “#DiversityinGI” has been instrumental in promoting causes pertaining to diversity and inclusion in GI.
Pitfalls
As gastroenterologists continue to use social media, it is important to be mindful of potential pitfalls. The most critical aspect is to always remember that no post should intentionally or unintentionally violate HIPAA. It is advisable to know your institutional and state social media policies.
Social media is beaming with knowledge, education, science and inspiration. There are endless opportunities for professional and personal growth with effective and responsible use of social media. Its never to late to join the conversation.
Dr. Bilal is an assistant professor of medicine at the University of Minnesota, Minneapolis and an advanced endoscopist in the division of gastroenterology at Minneapolis VA Medical Center. He has no relevant conflicts of interest to disclose.
References
1. Mikolajczyk AE et al. Hepatol Commun. 2020 Jul 5;4(8):1229-33.
2. Bilal M and Oxentenko AS. Am J Gastroenterol. 2020 Oct;115(10):1549-52.
3. Breu AC. N Engl J Med. 2019 Sep 19;381(12):1097-8.
4. Bilal M et al. Nat Rev Gastroenterol Hepatol. 2021 Aug;18(8):519-20.
5. Pawlak KM et al. United European Gastroenterol J. 2021 Feb;9(1):129-32.
Dear colleagues,
Most of us engage with social media, whether actively tweeting, following friends on Facebook, or discussing TikTok videos with family. Many gastroenterologists leverage social media to build their professional brand and to reach a wider audience. Others remain wary of committing a social media faux paux or worry about patient confidentiality. In this Perspectives column, Dr. Stephen Chris Pappas and Dr. Mohammad Bilal discuss the risks and benefits of social media for the practicing gastroenterologist. Dr. Pappas has a unique perspective as a gastroenterologist who is also trained as a lawyer, and Dr. Bilal speaks from a wealth of experience leading educational activities on social media. We look forward to hearing your thoughts on Twitter @AGA_GIHN and by email at [email protected].
Gyanprakash A. Ketwaroo, MD, MSc, an associate professor of medicine, Yale University, New Haven, Conn., and chief of endoscopy at West Haven (Conn.) VA Medical Center. He is an associate editor for GI & Hepatology News.
Carefully consider the plentiful risks, concerns
BY STEPHEN CHRIS PAPPAS, MD, JD, FAASLD, FACLM
Social media for gastroenterologists comes with benefits accompanied by pesky risks. The risks are pesky like a mosquito bite: An itching bite is annoying, but getting malaria is serious. Managing your unprofessional tweet to salvage your reputation is going to be annoying. Disclosing a patient's identity on social media is going to be serious; you could find yourself fired, fined, reprimanded, and without hospital privileges, as happened recently to a Rhode Island physician. I divide the risks of social media into legal risks (for example, disclosing patient identity or inadvertently creating a doctor-patient relationship), risks of compromising ethical standards (for example, impairing the doctor-patient relationship), and mixed legal/ethics risks (for example, inappropriate Twitter banter disparaging individuals, promotion of “fake news”). Fortunately, these risks are intuitive and can be mitigated by attention to some simple principles.
Disclosing a patient’s identity on social media is clearly in violation of privacy laws and other regulations. Since privacy compliance is drummed into us ad nauseum via annual compliance training, we could ask “how on earth could an inadvertent disclosure of identity occur?” We must remember that sites that are nominally termed “secure” may not be. As a general suggestion, I would regard social media of all types as open public forums with permanent postings. Even limited descriptions of a patient on social media may allow identification of the actual patient. The risk may be highest in smaller communities; in the past I assisted a small-town practitioner manage the fallout from inadvertently identifying a patient on his professional Facebook page by simply saying “I recently managed a 38-year-old pregnant woman with Crohn’s disease ...” That small amount of information allowed some members of his community to identify the specific patient. My suggestion would be to never talk about individual patients on social media. Phrase comments or questions generically; for example, “Crohn’s disease in pregnancy is managed with attention to ...”.
Another legal risk of social media engagement is to unknowingly create a patient-doctor relationship with a duty to treat, opening the door for exposure to malpractice litigation if something goes awry. A patient may interpret a social media interaction as establishing a patient-doctor relationship. While we think we know what defines a doctor-patient relationship, it’s not always clear and varies between jurisdictions. Indeed, a physician-patient relationship may not even be a necessary element of a claim for professional negligence (an issue shared with “curbside” consults). A recent court case in Minnesota ruled that a duty to care is established if “... it is reasonably foreseeable that the third party will rely on the physician’s acts and be harmed by a breach of the standard of care.” That case involved a telephone call, but you could see the standard easily morphing to apply to social media posts. Gastroenterologists should always talk about disease and treatment on social media in generic terms, preferably with appropriate caveats (for example, “Patients with cholestasis and intense itching may be treated with naloxone in selected cases after detailed assessment by a hepatologist”).
Impairing an established doctor-patient relationship by “friending” a patient on your personal Facebook risks a potential compromise of professional ethics, breaking the boundaries between profession and person for the gastroenterologist. The approach by most professional societies is that a “friend” on social media is equal to a friend in the real world; the same legal and ethical standards apply. Doctor-patient friendships may compromise objectivity, lead to preferential but not optimal therapy, and increase the risk of skirting around informed consent among other issues. Being friends on social media is discouraged, but not prohibited, by most professional societies and licensing bodies. In my opinion, that is sound advice. Over a career of more than 40 years, I have had patients who became friends, but only after I had transferred their care to another hepatologist.
More recently with escalating, aggressive, tones for social media communications, GI/hepatology practitioners must be aware of the serious risk of blurring their personal and professional online lives, particularly where Twitter is involved. The rapidity which people seem to want to reply to a tweet, the public and durable natures of a tweet, and the ability to significantly retweet and repost all spell potential disasters for the physician tweeting an inappropriate communication. Separation of personal and professional social media accounts is strongly encouraged but alone is not enough; you are never totally anonymous online. The reality is that a physician will be judged for an inappropriate communication whether it’s found on their professional or personal site. Either posting could result in reputation damage, reprimands, medical license restrictions or revocations, and litigation. Nationally, medical boards now regularly deal with disciplinary actions for inappropriate social media activity. The best preventive measures include pausing before you post, check the veracity of what you are posting, place your post in context, and assess the tone of your post and the tone of the site that you are posting to. A perfect storm for disaster is that the material is not clearly evidence based and could be construed as “fake,” you are personally emotionally charged, and the site you are posting to is a known cauldron of emotion and fake news.
In summary, social media affords benefits in a health care setting but it comes with some baggage. However, the risks of a social media presence are largely instinctive. An initial starting point is pausing to consider, “Would I say/do this in a public venue where everybody could hear/see me?” If there is any concern, don’t post. Subsequently, conduct yourself on social media with meticulous attention to protecting confidentiality, avoiding any impression of creating a doctor-patient relationship, avoiding doctor-friend relationships, being aware of key legal, institutional, and professional society guidance, separating personal and professional activities, and maintaining professionalism.
Dr. Pappas is in the GI and hepatology section of the department of medicine at Baylor College of Medicine, Houston. He has no relevant conflicts of interest to disclose.
References
Attai DJ et al. Semin Hematol. 2017 Oct; 54(4): 198-204.
Bal BS et al. Clin Orthop Relat Res. 2019 Oct; 477(10): 2204-6.
Ekrem, D et al. 20111 Jun 6. https://www.kevinmd.com/2011/06/7-tips-avoid-hipaa-violations-social-media.html
Hallenbeck J. Doctor and Friend. 2005 Jun. https://journalofethics.ama-assn.org/article/doctor-and-friend/2005-06
Moses RE et al. Am J Gastroenterol. 2014 Aug;109(8):1128-32.
Understand its multifaceted importance
BY MOHAMMAD BILAL, MD, FACP
Merriam-Webster’s dictionary defines social media as “forms of electronic communication (such as websites for social networking and microblogging) through which users create online communities to share information, ideas, personal messages, and other content.” Over the last few years, there has been an increase in use of social media by medical professionals. Whether we like it or not, social media is here to stay. Patients use social media to look up information regarding their doctors, medical practices use it to promote the services they offer, institutions share their programs and initiatives, and doctors use it for education, to engage with like-minded colleagues, collaborate, spread awareness, network, and combat medical misinformation. Social media is now rapidly being used by gastroenterologists and hepatologists, as well as majority of professional GI organizations, and hashtags such as “#MedTwitter”, “#GITwitter,” and “#LiverTwitter” have developed into popular academic forums.1 Therefore, the impact of social media in GI is multifaceted and includes its role in medical education, promoting your practice or division, finding collaborations, building your network and establishing mentors and peer-mentors, disseminating your work, and building your brand.2
What is your goal?
Gastroenterologists could have one or more of the goals mentioned above for using social media. Determining the goals for social media use a priori will allow for determining which social media platform will be appropriate for you. Therefore, it is important to understand the users of various social media platforms. In 2017, Facebook was the highest used social media platform in all age groups, whereas Instagram was most popular amongst ages 18-29 years, while Twitter was used more commonly in ages 30-59 years as compared with Instagram. If your goal is to share scientific knowledge and literature with like-minded physicians and interact with leaders in the field, then Twitter may be ideal. If you want to connect with a younger, more diverse audience, Instagram might be a good option. While many physicians may have a Facebook account, this is often reserved for personal use. Many have separated of personal and professional social media use, although they do not need to exist in silos. Defining your goal with social media use will direct you to the best platform to reach your audience.
Medical education
The use of social media especially Twitter for medical education is continuously increasing. Several leaders in the field use “Tweetorials” as a means to educate others. Tweetorials are a collective set of tweets that systematically cover a specialized topic.3 Other educational forums such as @ScopingSundays, @MondayNightIBD, @IBDClub and @GIJournal provide structured platforms for GI focused discussion.4 @MondayNightIBD is also a source for official continued medical education. Other social media educational platforms include “Liver Fellow Network” which has wide variety of educational materials pertaining to hepatology. In addition, there is continuous opportunity to engage with leaders in the field and authors of published studies and guidelines. Several endoscopy educators have dedicated YouTube channels which have endless supply of educational videos.
Networking
As mentioned above, platforms such as #GITwitter and #LiverTwitter have become popular forums for engaging and connecting with like minded colleagues. Social media provides a space to share ideas and build collaborations with colleagues working on similar projects. The concept “#Twitter2Paper” has been proposed which signifies an idea that generated on Twitter and was eventually converted to a manuscript.5
Institutional, divisional, and practice promotion
Social media is a great tool to showcase the clinical, educational and scholarship services and efforts by programs, practices or divisions. During the COVID-19 pandemic, recruitment efforts at all stages were mainly shifted to virtual platforms, and social media was an instrumental way for programs to highlight their culture and initiatives. Prospective applicants can often refer to social media to get a better understanding of what the program offers. Similarly, if a new clinical service is being provided, targeted efforts can be made to ensure that patients are aware of the available services.
Patient education and combating misinformation
Several gastroenterologists also use social media to spread awareness regarding GI diseases. Instagram, Facebook, and TikTok are effective mediums where one can reach a wider audience. It is important for gastroenterologists to provide accurate information since there is a sea of misinformation available on the internet as well. Posts regarding colonoscopy and colon cancer awareness can help alleviate myths regarding role of colonoscopy. In addition, patient advocates use social media to provide peer support to others who deal with challenges related to chronic illnesses such as inflammatory bowel disease.
Sharing your work
Sharing your work on social media can help your work reach a broader audience. Studies have shown that work shared on social media has higher altmetric scores and can also lead to increased citations.
Diversity, equity, and inclusion
Social media offers a platform where one can promote or showcase their support for causes they believe in. The hashtag “#DiversityinGI” has been instrumental in promoting causes pertaining to diversity and inclusion in GI.
Pitfalls
As gastroenterologists continue to use social media, it is important to be mindful of potential pitfalls. The most critical aspect is to always remember that no post should intentionally or unintentionally violate HIPAA. It is advisable to know your institutional and state social media policies.
Social media is beaming with knowledge, education, science and inspiration. There are endless opportunities for professional and personal growth with effective and responsible use of social media. Its never to late to join the conversation.
Dr. Bilal is an assistant professor of medicine at the University of Minnesota, Minneapolis and an advanced endoscopist in the division of gastroenterology at Minneapolis VA Medical Center. He has no relevant conflicts of interest to disclose.
References
1. Mikolajczyk AE et al. Hepatol Commun. 2020 Jul 5;4(8):1229-33.
2. Bilal M and Oxentenko AS. Am J Gastroenterol. 2020 Oct;115(10):1549-52.
3. Breu AC. N Engl J Med. 2019 Sep 19;381(12):1097-8.
4. Bilal M et al. Nat Rev Gastroenterol Hepatol. 2021 Aug;18(8):519-20.
5. Pawlak KM et al. United European Gastroenterol J. 2021 Feb;9(1):129-32.
Dear colleagues,
Most of us engage with social media, whether actively tweeting, following friends on Facebook, or discussing TikTok videos with family. Many gastroenterologists leverage social media to build their professional brand and to reach a wider audience. Others remain wary of committing a social media faux paux or worry about patient confidentiality. In this Perspectives column, Dr. Stephen Chris Pappas and Dr. Mohammad Bilal discuss the risks and benefits of social media for the practicing gastroenterologist. Dr. Pappas has a unique perspective as a gastroenterologist who is also trained as a lawyer, and Dr. Bilal speaks from a wealth of experience leading educational activities on social media. We look forward to hearing your thoughts on Twitter @AGA_GIHN and by email at [email protected].
Gyanprakash A. Ketwaroo, MD, MSc, an associate professor of medicine, Yale University, New Haven, Conn., and chief of endoscopy at West Haven (Conn.) VA Medical Center. He is an associate editor for GI & Hepatology News.
Carefully consider the plentiful risks, concerns
BY STEPHEN CHRIS PAPPAS, MD, JD, FAASLD, FACLM
Social media for gastroenterologists comes with benefits accompanied by pesky risks. The risks are pesky like a mosquito bite: An itching bite is annoying, but getting malaria is serious. Managing your unprofessional tweet to salvage your reputation is going to be annoying. Disclosing a patient's identity on social media is going to be serious; you could find yourself fired, fined, reprimanded, and without hospital privileges, as happened recently to a Rhode Island physician. I divide the risks of social media into legal risks (for example, disclosing patient identity or inadvertently creating a doctor-patient relationship), risks of compromising ethical standards (for example, impairing the doctor-patient relationship), and mixed legal/ethics risks (for example, inappropriate Twitter banter disparaging individuals, promotion of “fake news”). Fortunately, these risks are intuitive and can be mitigated by attention to some simple principles.
Disclosing a patient’s identity on social media is clearly in violation of privacy laws and other regulations. Since privacy compliance is drummed into us ad nauseum via annual compliance training, we could ask “how on earth could an inadvertent disclosure of identity occur?” We must remember that sites that are nominally termed “secure” may not be. As a general suggestion, I would regard social media of all types as open public forums with permanent postings. Even limited descriptions of a patient on social media may allow identification of the actual patient. The risk may be highest in smaller communities; in the past I assisted a small-town practitioner manage the fallout from inadvertently identifying a patient on his professional Facebook page by simply saying “I recently managed a 38-year-old pregnant woman with Crohn’s disease ...” That small amount of information allowed some members of his community to identify the specific patient. My suggestion would be to never talk about individual patients on social media. Phrase comments or questions generically; for example, “Crohn’s disease in pregnancy is managed with attention to ...”.
Another legal risk of social media engagement is to unknowingly create a patient-doctor relationship with a duty to treat, opening the door for exposure to malpractice litigation if something goes awry. A patient may interpret a social media interaction as establishing a patient-doctor relationship. While we think we know what defines a doctor-patient relationship, it’s not always clear and varies between jurisdictions. Indeed, a physician-patient relationship may not even be a necessary element of a claim for professional negligence (an issue shared with “curbside” consults). A recent court case in Minnesota ruled that a duty to care is established if “... it is reasonably foreseeable that the third party will rely on the physician’s acts and be harmed by a breach of the standard of care.” That case involved a telephone call, but you could see the standard easily morphing to apply to social media posts. Gastroenterologists should always talk about disease and treatment on social media in generic terms, preferably with appropriate caveats (for example, “Patients with cholestasis and intense itching may be treated with naloxone in selected cases after detailed assessment by a hepatologist”).
Impairing an established doctor-patient relationship by “friending” a patient on your personal Facebook risks a potential compromise of professional ethics, breaking the boundaries between profession and person for the gastroenterologist. The approach by most professional societies is that a “friend” on social media is equal to a friend in the real world; the same legal and ethical standards apply. Doctor-patient friendships may compromise objectivity, lead to preferential but not optimal therapy, and increase the risk of skirting around informed consent among other issues. Being friends on social media is discouraged, but not prohibited, by most professional societies and licensing bodies. In my opinion, that is sound advice. Over a career of more than 40 years, I have had patients who became friends, but only after I had transferred their care to another hepatologist.
More recently with escalating, aggressive, tones for social media communications, GI/hepatology practitioners must be aware of the serious risk of blurring their personal and professional online lives, particularly where Twitter is involved. The rapidity which people seem to want to reply to a tweet, the public and durable natures of a tweet, and the ability to significantly retweet and repost all spell potential disasters for the physician tweeting an inappropriate communication. Separation of personal and professional social media accounts is strongly encouraged but alone is not enough; you are never totally anonymous online. The reality is that a physician will be judged for an inappropriate communication whether it’s found on their professional or personal site. Either posting could result in reputation damage, reprimands, medical license restrictions or revocations, and litigation. Nationally, medical boards now regularly deal with disciplinary actions for inappropriate social media activity. The best preventive measures include pausing before you post, check the veracity of what you are posting, place your post in context, and assess the tone of your post and the tone of the site that you are posting to. A perfect storm for disaster is that the material is not clearly evidence based and could be construed as “fake,” you are personally emotionally charged, and the site you are posting to is a known cauldron of emotion and fake news.
In summary, social media affords benefits in a health care setting but it comes with some baggage. However, the risks of a social media presence are largely instinctive. An initial starting point is pausing to consider, “Would I say/do this in a public venue where everybody could hear/see me?” If there is any concern, don’t post. Subsequently, conduct yourself on social media with meticulous attention to protecting confidentiality, avoiding any impression of creating a doctor-patient relationship, avoiding doctor-friend relationships, being aware of key legal, institutional, and professional society guidance, separating personal and professional activities, and maintaining professionalism.
Dr. Pappas is in the GI and hepatology section of the department of medicine at Baylor College of Medicine, Houston. He has no relevant conflicts of interest to disclose.
References
Attai DJ et al. Semin Hematol. 2017 Oct; 54(4): 198-204.
Bal BS et al. Clin Orthop Relat Res. 2019 Oct; 477(10): 2204-6.
Ekrem, D et al. 20111 Jun 6. https://www.kevinmd.com/2011/06/7-tips-avoid-hipaa-violations-social-media.html
Hallenbeck J. Doctor and Friend. 2005 Jun. https://journalofethics.ama-assn.org/article/doctor-and-friend/2005-06
Moses RE et al. Am J Gastroenterol. 2014 Aug;109(8):1128-32.
Understand its multifaceted importance
BY MOHAMMAD BILAL, MD, FACP
Merriam-Webster’s dictionary defines social media as “forms of electronic communication (such as websites for social networking and microblogging) through which users create online communities to share information, ideas, personal messages, and other content.” Over the last few years, there has been an increase in use of social media by medical professionals. Whether we like it or not, social media is here to stay. Patients use social media to look up information regarding their doctors, medical practices use it to promote the services they offer, institutions share their programs and initiatives, and doctors use it for education, to engage with like-minded colleagues, collaborate, spread awareness, network, and combat medical misinformation. Social media is now rapidly being used by gastroenterologists and hepatologists, as well as majority of professional GI organizations, and hashtags such as “#MedTwitter”, “#GITwitter,” and “#LiverTwitter” have developed into popular academic forums.1 Therefore, the impact of social media in GI is multifaceted and includes its role in medical education, promoting your practice or division, finding collaborations, building your network and establishing mentors and peer-mentors, disseminating your work, and building your brand.2
What is your goal?
Gastroenterologists could have one or more of the goals mentioned above for using social media. Determining the goals for social media use a priori will allow for determining which social media platform will be appropriate for you. Therefore, it is important to understand the users of various social media platforms. In 2017, Facebook was the highest used social media platform in all age groups, whereas Instagram was most popular amongst ages 18-29 years, while Twitter was used more commonly in ages 30-59 years as compared with Instagram. If your goal is to share scientific knowledge and literature with like-minded physicians and interact with leaders in the field, then Twitter may be ideal. If you want to connect with a younger, more diverse audience, Instagram might be a good option. While many physicians may have a Facebook account, this is often reserved for personal use. Many have separated of personal and professional social media use, although they do not need to exist in silos. Defining your goal with social media use will direct you to the best platform to reach your audience.
Medical education
The use of social media especially Twitter for medical education is continuously increasing. Several leaders in the field use “Tweetorials” as a means to educate others. Tweetorials are a collective set of tweets that systematically cover a specialized topic.3 Other educational forums such as @ScopingSundays, @MondayNightIBD, @IBDClub and @GIJournal provide structured platforms for GI focused discussion.4 @MondayNightIBD is also a source for official continued medical education. Other social media educational platforms include “Liver Fellow Network” which has wide variety of educational materials pertaining to hepatology. In addition, there is continuous opportunity to engage with leaders in the field and authors of published studies and guidelines. Several endoscopy educators have dedicated YouTube channels which have endless supply of educational videos.
Networking
As mentioned above, platforms such as #GITwitter and #LiverTwitter have become popular forums for engaging and connecting with like minded colleagues. Social media provides a space to share ideas and build collaborations with colleagues working on similar projects. The concept “#Twitter2Paper” has been proposed which signifies an idea that generated on Twitter and was eventually converted to a manuscript.5
Institutional, divisional, and practice promotion
Social media is a great tool to showcase the clinical, educational and scholarship services and efforts by programs, practices or divisions. During the COVID-19 pandemic, recruitment efforts at all stages were mainly shifted to virtual platforms, and social media was an instrumental way for programs to highlight their culture and initiatives. Prospective applicants can often refer to social media to get a better understanding of what the program offers. Similarly, if a new clinical service is being provided, targeted efforts can be made to ensure that patients are aware of the available services.
Patient education and combating misinformation
Several gastroenterologists also use social media to spread awareness regarding GI diseases. Instagram, Facebook, and TikTok are effective mediums where one can reach a wider audience. It is important for gastroenterologists to provide accurate information since there is a sea of misinformation available on the internet as well. Posts regarding colonoscopy and colon cancer awareness can help alleviate myths regarding role of colonoscopy. In addition, patient advocates use social media to provide peer support to others who deal with challenges related to chronic illnesses such as inflammatory bowel disease.
Sharing your work
Sharing your work on social media can help your work reach a broader audience. Studies have shown that work shared on social media has higher altmetric scores and can also lead to increased citations.
Diversity, equity, and inclusion
Social media offers a platform where one can promote or showcase their support for causes they believe in. The hashtag “#DiversityinGI” has been instrumental in promoting causes pertaining to diversity and inclusion in GI.
Pitfalls
As gastroenterologists continue to use social media, it is important to be mindful of potential pitfalls. The most critical aspect is to always remember that no post should intentionally or unintentionally violate HIPAA. It is advisable to know your institutional and state social media policies.
Social media is beaming with knowledge, education, science and inspiration. There are endless opportunities for professional and personal growth with effective and responsible use of social media. Its never to late to join the conversation.
Dr. Bilal is an assistant professor of medicine at the University of Minnesota, Minneapolis and an advanced endoscopist in the division of gastroenterology at Minneapolis VA Medical Center. He has no relevant conflicts of interest to disclose.
References
1. Mikolajczyk AE et al. Hepatol Commun. 2020 Jul 5;4(8):1229-33.
2. Bilal M and Oxentenko AS. Am J Gastroenterol. 2020 Oct;115(10):1549-52.
3. Breu AC. N Engl J Med. 2019 Sep 19;381(12):1097-8.
4. Bilal M et al. Nat Rev Gastroenterol Hepatol. 2021 Aug;18(8):519-20.
5. Pawlak KM et al. United European Gastroenterol J. 2021 Feb;9(1):129-32.