User login
‘Superior’ CLL regimen cuts chemo in half
“Overall, our data suggests that [the chemoimmunotherapy] regimen is very effective and appears superior to published six cycles of chemotherapy regimen for the same favorable risk features,” first author Dr. Nitin Jain, an associate professor in the department of leukemia at the University of Texas MD Anderson Cancer Center, Houston, told MDedge.
Chemoimmunotherapy with fludarabine, cyclophosphamide and rituximab (FCR) has been a standard frontline treatment for young, fit patients with CLL, resulting in 10-year PFS rates above 55% in patients with mutated IGHV status, said coauthor Dr. Alessandra Ferrajoli, also of the MD Anderson Cancer Center, in presenting the findings at the European Hematology Association annual congress.
The authors sought to investigate the efficacy of a targeted therapy combination of ibrutinib and obinutuzumab with fludarabine and cyclophosphamide (iFCG). They also sought to determine whether a three-cycle regimen of the chemotherapy, as compared to six cycles, could reduce the risk of myelodysplastic syndrome (MDS), which increases with chemotherapy in CLL patients who have mutated IGHV status.
For the phase 2 study, 45 previously untreated patients with CLL, who had mutated IGHV and an absence of del(17p)/TP53 mutation (both of which are associated with more favorable outcomes in CLL) were enrolled between March 2016 and August 2018. The patients were deemed fit for chemotherapy and had a median age of 60.
All patients were initially treated with three cycles of the iFCG regimen, and among them, 39 (87%) achieved undetectable measurable residual disease (MRD) in their bone marrow.
After the three cycles, an MRD-driven strategy was then used to determine subsequent treatment: All patients received nine courses of ibrutinib, and for those achieving complete remission (CR) or CR with incomplete count recovery (CRi) and undetectable MRD, three cycles of obinutuzumab were administered, while all others received nine additional cycles of obinutuzumab.
At completion of the 12 courses, those who still had MRD positivity continued on ibrutinib, while those with undetectable MRD discontinued ibrutinib.
By cycle six of iFCG, 40 (89%) of the patients achieved undetectable MRD. Overall, 44 of the 45 patients (98%) achieved undetectable MRD as their best response at any time during the study, with 69% of patients achieving CR/CRi. Four patients came off the study prior to cycle 12, including one death, one infection, and one patient who opted to pursue treatment locally. With a median follow-up of 59.6 months, there were no cases of CLL progression or Richter transformation and the lone death was from heart failure.
One patient developed treatment-related myelodysplastic syndrome (MDS), and that patient has maintained normal blood counts over 38 months of monitoring and has not required MDS therapy, Dr. Ferrajoli reported.
Over the follow-up, the six patients who were MRD positive after the completion of three cycles experienced a recurrence of MRD, defined as two consecutive values of 0.01% or higher in peripheral blood by flow cytometry, at a median of 27.2 months after stopping all therapy.
“Not unexpectedly, MRD recurrence during follow-up correlated with MRD positivity during therapy,” Dr. Ferrajoli said.
She noted that all six of the patients were being monitored, with no clinical progression or active therapy. However, with a median follow-up of 5 years, the progression-free survival (PFS) rate among the 45 patients was 97.7%, and the overall survival (OS) rate was 97.8%. Dr. Ferrajoli noted that, while the study population was clearly different, the results compare favorably with CLL clinical trial results that have previously shown a 5-year PFS of approximately 65% with FCR alone; approximately 70% with ibrutinib; and 81% with ibrutinib among patients with mutated IGHV status.
Furthermore, the rate of undetectable MRD status in mutated IGHV patients being 95% in evaluable patients in the current study is notably higher than rates of 51% through 67% reported in five other trials of CLL treatment with six cycles of FCR and with a rate of 79% in the DFCI trial of six-cycle chemotherapy plus ibrutinib.
And the current study’s undetectable MRD rate of 89% in the intention-to-treat population compares with just 13% though 40% in the five other chemotherapy trials and 79% in the DFCI trial, the authors note.
The current trial was the only one of any of their comparisons to utilize the three-cycle regimen.
Asked at the meeting about concerns of toxicities reported with obinutuzumab and chemotherapy, Dr. Ferrajoli said “the treatment was very well tolerated.”
“Myelosuppression is a concern with this combination, but we did make the use of prophylactic growth-factor mandatory in the study, so we were able to control that,” she said.
Dr. Jain noted that, while treatment trends have moved largely to chemo-free regimens, particularly in the United States because of concerns about the MDS, the current study’s results importantly shed light on a potentially beneficial approach of just three cycles of chemotherapy.
“In Europe and the rest of the world where chemo use is still common, this regimen could be considered,” he told MDedge. “The findings show that if you still use chemo in your practice, this regimen uses 50% less chemotherapy, yet seems to give higher response rates.”
“While MDS and acute myeloid leukemia (AML) remain a concern with any chemotherapy regimen, it is possible that 50% less chemo will lead to less risk of MDS AML, but longer-term follow-up [is needed],” he said.
Dr. Ferrajoli reported that she has received research support from Astra-Zeneca and Beigene. Dr. Jain has received research funding and honoraria from Genentech and Pharmacyclics.
“Overall, our data suggests that [the chemoimmunotherapy] regimen is very effective and appears superior to published six cycles of chemotherapy regimen for the same favorable risk features,” first author Dr. Nitin Jain, an associate professor in the department of leukemia at the University of Texas MD Anderson Cancer Center, Houston, told MDedge.
Chemoimmunotherapy with fludarabine, cyclophosphamide and rituximab (FCR) has been a standard frontline treatment for young, fit patients with CLL, resulting in 10-year PFS rates above 55% in patients with mutated IGHV status, said coauthor Dr. Alessandra Ferrajoli, also of the MD Anderson Cancer Center, in presenting the findings at the European Hematology Association annual congress.
The authors sought to investigate the efficacy of a targeted therapy combination of ibrutinib and obinutuzumab with fludarabine and cyclophosphamide (iFCG). They also sought to determine whether a three-cycle regimen of the chemotherapy, as compared to six cycles, could reduce the risk of myelodysplastic syndrome (MDS), which increases with chemotherapy in CLL patients who have mutated IGHV status.
For the phase 2 study, 45 previously untreated patients with CLL, who had mutated IGHV and an absence of del(17p)/TP53 mutation (both of which are associated with more favorable outcomes in CLL) were enrolled between March 2016 and August 2018. The patients were deemed fit for chemotherapy and had a median age of 60.
All patients were initially treated with three cycles of the iFCG regimen, and among them, 39 (87%) achieved undetectable measurable residual disease (MRD) in their bone marrow.
After the three cycles, an MRD-driven strategy was then used to determine subsequent treatment: All patients received nine courses of ibrutinib, and for those achieving complete remission (CR) or CR with incomplete count recovery (CRi) and undetectable MRD, three cycles of obinutuzumab were administered, while all others received nine additional cycles of obinutuzumab.
At completion of the 12 courses, those who still had MRD positivity continued on ibrutinib, while those with undetectable MRD discontinued ibrutinib.
By cycle six of iFCG, 40 (89%) of the patients achieved undetectable MRD. Overall, 44 of the 45 patients (98%) achieved undetectable MRD as their best response at any time during the study, with 69% of patients achieving CR/CRi. Four patients came off the study prior to cycle 12, including one death, one infection, and one patient who opted to pursue treatment locally. With a median follow-up of 59.6 months, there were no cases of CLL progression or Richter transformation and the lone death was from heart failure.
One patient developed treatment-related myelodysplastic syndrome (MDS), and that patient has maintained normal blood counts over 38 months of monitoring and has not required MDS therapy, Dr. Ferrajoli reported.
Over the follow-up, the six patients who were MRD positive after the completion of three cycles experienced a recurrence of MRD, defined as two consecutive values of 0.01% or higher in peripheral blood by flow cytometry, at a median of 27.2 months after stopping all therapy.
“Not unexpectedly, MRD recurrence during follow-up correlated with MRD positivity during therapy,” Dr. Ferrajoli said.
She noted that all six of the patients were being monitored, with no clinical progression or active therapy. However, with a median follow-up of 5 years, the progression-free survival (PFS) rate among the 45 patients was 97.7%, and the overall survival (OS) rate was 97.8%. Dr. Ferrajoli noted that, while the study population was clearly different, the results compare favorably with CLL clinical trial results that have previously shown a 5-year PFS of approximately 65% with FCR alone; approximately 70% with ibrutinib; and 81% with ibrutinib among patients with mutated IGHV status.
Furthermore, the rate of undetectable MRD status in mutated IGHV patients being 95% in evaluable patients in the current study is notably higher than rates of 51% through 67% reported in five other trials of CLL treatment with six cycles of FCR and with a rate of 79% in the DFCI trial of six-cycle chemotherapy plus ibrutinib.
And the current study’s undetectable MRD rate of 89% in the intention-to-treat population compares with just 13% though 40% in the five other chemotherapy trials and 79% in the DFCI trial, the authors note.
The current trial was the only one of any of their comparisons to utilize the three-cycle regimen.
Asked at the meeting about concerns of toxicities reported with obinutuzumab and chemotherapy, Dr. Ferrajoli said “the treatment was very well tolerated.”
“Myelosuppression is a concern with this combination, but we did make the use of prophylactic growth-factor mandatory in the study, so we were able to control that,” she said.
Dr. Jain noted that, while treatment trends have moved largely to chemo-free regimens, particularly in the United States because of concerns about the MDS, the current study’s results importantly shed light on a potentially beneficial approach of just three cycles of chemotherapy.
“In Europe and the rest of the world where chemo use is still common, this regimen could be considered,” he told MDedge. “The findings show that if you still use chemo in your practice, this regimen uses 50% less chemotherapy, yet seems to give higher response rates.”
“While MDS and acute myeloid leukemia (AML) remain a concern with any chemotherapy regimen, it is possible that 50% less chemo will lead to less risk of MDS AML, but longer-term follow-up [is needed],” he said.
Dr. Ferrajoli reported that she has received research support from Astra-Zeneca and Beigene. Dr. Jain has received research funding and honoraria from Genentech and Pharmacyclics.
“Overall, our data suggests that [the chemoimmunotherapy] regimen is very effective and appears superior to published six cycles of chemotherapy regimen for the same favorable risk features,” first author Dr. Nitin Jain, an associate professor in the department of leukemia at the University of Texas MD Anderson Cancer Center, Houston, told MDedge.
Chemoimmunotherapy with fludarabine, cyclophosphamide and rituximab (FCR) has been a standard frontline treatment for young, fit patients with CLL, resulting in 10-year PFS rates above 55% in patients with mutated IGHV status, said coauthor Dr. Alessandra Ferrajoli, also of the MD Anderson Cancer Center, in presenting the findings at the European Hematology Association annual congress.
The authors sought to investigate the efficacy of a targeted therapy combination of ibrutinib and obinutuzumab with fludarabine and cyclophosphamide (iFCG). They also sought to determine whether a three-cycle regimen of the chemotherapy, as compared to six cycles, could reduce the risk of myelodysplastic syndrome (MDS), which increases with chemotherapy in CLL patients who have mutated IGHV status.
For the phase 2 study, 45 previously untreated patients with CLL, who had mutated IGHV and an absence of del(17p)/TP53 mutation (both of which are associated with more favorable outcomes in CLL) were enrolled between March 2016 and August 2018. The patients were deemed fit for chemotherapy and had a median age of 60.
All patients were initially treated with three cycles of the iFCG regimen, and among them, 39 (87%) achieved undetectable measurable residual disease (MRD) in their bone marrow.
After the three cycles, an MRD-driven strategy was then used to determine subsequent treatment: All patients received nine courses of ibrutinib, and for those achieving complete remission (CR) or CR with incomplete count recovery (CRi) and undetectable MRD, three cycles of obinutuzumab were administered, while all others received nine additional cycles of obinutuzumab.
At completion of the 12 courses, those who still had MRD positivity continued on ibrutinib, while those with undetectable MRD discontinued ibrutinib.
By cycle six of iFCG, 40 (89%) of the patients achieved undetectable MRD. Overall, 44 of the 45 patients (98%) achieved undetectable MRD as their best response at any time during the study, with 69% of patients achieving CR/CRi. Four patients came off the study prior to cycle 12, including one death, one infection, and one patient who opted to pursue treatment locally. With a median follow-up of 59.6 months, there were no cases of CLL progression or Richter transformation and the lone death was from heart failure.
One patient developed treatment-related myelodysplastic syndrome (MDS), and that patient has maintained normal blood counts over 38 months of monitoring and has not required MDS therapy, Dr. Ferrajoli reported.
Over the follow-up, the six patients who were MRD positive after the completion of three cycles experienced a recurrence of MRD, defined as two consecutive values of 0.01% or higher in peripheral blood by flow cytometry, at a median of 27.2 months after stopping all therapy.
“Not unexpectedly, MRD recurrence during follow-up correlated with MRD positivity during therapy,” Dr. Ferrajoli said.
She noted that all six of the patients were being monitored, with no clinical progression or active therapy. However, with a median follow-up of 5 years, the progression-free survival (PFS) rate among the 45 patients was 97.7%, and the overall survival (OS) rate was 97.8%. Dr. Ferrajoli noted that, while the study population was clearly different, the results compare favorably with CLL clinical trial results that have previously shown a 5-year PFS of approximately 65% with FCR alone; approximately 70% with ibrutinib; and 81% with ibrutinib among patients with mutated IGHV status.
Furthermore, the rate of undetectable MRD status in mutated IGHV patients being 95% in evaluable patients in the current study is notably higher than rates of 51% through 67% reported in five other trials of CLL treatment with six cycles of FCR and with a rate of 79% in the DFCI trial of six-cycle chemotherapy plus ibrutinib.
And the current study’s undetectable MRD rate of 89% in the intention-to-treat population compares with just 13% though 40% in the five other chemotherapy trials and 79% in the DFCI trial, the authors note.
The current trial was the only one of any of their comparisons to utilize the three-cycle regimen.
Asked at the meeting about concerns of toxicities reported with obinutuzumab and chemotherapy, Dr. Ferrajoli said “the treatment was very well tolerated.”
“Myelosuppression is a concern with this combination, but we did make the use of prophylactic growth-factor mandatory in the study, so we were able to control that,” she said.
Dr. Jain noted that, while treatment trends have moved largely to chemo-free regimens, particularly in the United States because of concerns about the MDS, the current study’s results importantly shed light on a potentially beneficial approach of just three cycles of chemotherapy.
“In Europe and the rest of the world where chemo use is still common, this regimen could be considered,” he told MDedge. “The findings show that if you still use chemo in your practice, this regimen uses 50% less chemotherapy, yet seems to give higher response rates.”
“While MDS and acute myeloid leukemia (AML) remain a concern with any chemotherapy regimen, it is possible that 50% less chemo will lead to less risk of MDS AML, but longer-term follow-up [is needed],” he said.
Dr. Ferrajoli reported that she has received research support from Astra-Zeneca and Beigene. Dr. Jain has received research funding and honoraria from Genentech and Pharmacyclics.
FROM EHA 2022
Upadacitinib recommended for nonradiographic axSpA in Europe
Upadacitinib may soon be used for the treatment of nonradiographic axial spondyloarthritis (nr-axSpA) after the European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) gave it its stamp of approval.
AbbVie, the drug’s manufacturer, announced on June 27 that the committee approved the use on June 23. The recommendation to approve market authorization for upadacitinib for nr-axSpA now goes to the European Commission, which is expected to make a decision by the third quarter of 2022.
“The CHMP’s recommendation to approve upadacitinib for patients with nr-axSpA is an important milestone in providing a new treatment option to patients in need,” said Neil Gallagher, MD, vice president of development and chief medical officer of AbbVie. He noted that currently, there are few options to treat symptoms such as inflammation, back pain, and stiffness for these patients.
Officially, the new indication for upadacitinib (Rinvoq) 15 mg once daily is for the treatment of active nr-axSpA in adult patients with objective signs of inflammation, as indicated by elevated C-reactive protein and/or MRI, whose condition has responded inadequately to NSAIDs.
Upadacitinib is a selective Janus kinase (JAK) inhibitor that in human cellular assays preferentially inhibits signaling by JAK1 or JAK1/3.
In the European Union, upadacitinib is currently approved for use in patients with moderate to severe active rheumatoid arthritis, active psoriatic arthritis, active ankylosing spondylitis, and moderate to severe atopic dermatitis. In addition to these indications, it is approved in the United States for ulcerative colitis but not for nr-axSpA.
The committee based its decision on the results of the nr-axSpA study within the SELECT-AXIS-2 trial, recently reported at the European Alliance of Associations for Rheumatology (EULAR) 2022 annual meeting.
The nr-axSpA study met the primary endpoint of a 40% improvement in Assessment of SpondyloArthritis International Society response criteria (ASAS40) and the first 12 of 14 ranked secondary endpoints, according to AbbVie.
The most commonly reported adverse reactions with upadacitinib 15 mg were upper respiratory tract infections, elevated blood creatine phosphokinase levels, elevated alanine transaminase levels, bronchitis, nausea, cough, elevated aspartate transaminase levels, and hypercholesterolemia. These occurred in 2% or more of patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis in clinical trials.
The safety profile of upadacitinib with long-term treatment was generally similar to the safety profile during the placebo-controlled period across indications, AbbVie said.
A version of this article first appeared on Medscape.com.
Upadacitinib may soon be used for the treatment of nonradiographic axial spondyloarthritis (nr-axSpA) after the European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) gave it its stamp of approval.
AbbVie, the drug’s manufacturer, announced on June 27 that the committee approved the use on June 23. The recommendation to approve market authorization for upadacitinib for nr-axSpA now goes to the European Commission, which is expected to make a decision by the third quarter of 2022.
“The CHMP’s recommendation to approve upadacitinib for patients with nr-axSpA is an important milestone in providing a new treatment option to patients in need,” said Neil Gallagher, MD, vice president of development and chief medical officer of AbbVie. He noted that currently, there are few options to treat symptoms such as inflammation, back pain, and stiffness for these patients.
Officially, the new indication for upadacitinib (Rinvoq) 15 mg once daily is for the treatment of active nr-axSpA in adult patients with objective signs of inflammation, as indicated by elevated C-reactive protein and/or MRI, whose condition has responded inadequately to NSAIDs.
Upadacitinib is a selective Janus kinase (JAK) inhibitor that in human cellular assays preferentially inhibits signaling by JAK1 or JAK1/3.
In the European Union, upadacitinib is currently approved for use in patients with moderate to severe active rheumatoid arthritis, active psoriatic arthritis, active ankylosing spondylitis, and moderate to severe atopic dermatitis. In addition to these indications, it is approved in the United States for ulcerative colitis but not for nr-axSpA.
The committee based its decision on the results of the nr-axSpA study within the SELECT-AXIS-2 trial, recently reported at the European Alliance of Associations for Rheumatology (EULAR) 2022 annual meeting.
The nr-axSpA study met the primary endpoint of a 40% improvement in Assessment of SpondyloArthritis International Society response criteria (ASAS40) and the first 12 of 14 ranked secondary endpoints, according to AbbVie.
The most commonly reported adverse reactions with upadacitinib 15 mg were upper respiratory tract infections, elevated blood creatine phosphokinase levels, elevated alanine transaminase levels, bronchitis, nausea, cough, elevated aspartate transaminase levels, and hypercholesterolemia. These occurred in 2% or more of patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis in clinical trials.
The safety profile of upadacitinib with long-term treatment was generally similar to the safety profile during the placebo-controlled period across indications, AbbVie said.
A version of this article first appeared on Medscape.com.
Upadacitinib may soon be used for the treatment of nonradiographic axial spondyloarthritis (nr-axSpA) after the European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) gave it its stamp of approval.
AbbVie, the drug’s manufacturer, announced on June 27 that the committee approved the use on June 23. The recommendation to approve market authorization for upadacitinib for nr-axSpA now goes to the European Commission, which is expected to make a decision by the third quarter of 2022.
“The CHMP’s recommendation to approve upadacitinib for patients with nr-axSpA is an important milestone in providing a new treatment option to patients in need,” said Neil Gallagher, MD, vice president of development and chief medical officer of AbbVie. He noted that currently, there are few options to treat symptoms such as inflammation, back pain, and stiffness for these patients.
Officially, the new indication for upadacitinib (Rinvoq) 15 mg once daily is for the treatment of active nr-axSpA in adult patients with objective signs of inflammation, as indicated by elevated C-reactive protein and/or MRI, whose condition has responded inadequately to NSAIDs.
Upadacitinib is a selective Janus kinase (JAK) inhibitor that in human cellular assays preferentially inhibits signaling by JAK1 or JAK1/3.
In the European Union, upadacitinib is currently approved for use in patients with moderate to severe active rheumatoid arthritis, active psoriatic arthritis, active ankylosing spondylitis, and moderate to severe atopic dermatitis. In addition to these indications, it is approved in the United States for ulcerative colitis but not for nr-axSpA.
The committee based its decision on the results of the nr-axSpA study within the SELECT-AXIS-2 trial, recently reported at the European Alliance of Associations for Rheumatology (EULAR) 2022 annual meeting.
The nr-axSpA study met the primary endpoint of a 40% improvement in Assessment of SpondyloArthritis International Society response criteria (ASAS40) and the first 12 of 14 ranked secondary endpoints, according to AbbVie.
The most commonly reported adverse reactions with upadacitinib 15 mg were upper respiratory tract infections, elevated blood creatine phosphokinase levels, elevated alanine transaminase levels, bronchitis, nausea, cough, elevated aspartate transaminase levels, and hypercholesterolemia. These occurred in 2% or more of patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis in clinical trials.
The safety profile of upadacitinib with long-term treatment was generally similar to the safety profile during the placebo-controlled period across indications, AbbVie said.
A version of this article first appeared on Medscape.com.
Children and COVID: Vaccination off to slow start for the newly eligible
New cases of COVID-19 continue to drop among children, but the vaccination effort in those under age 5 years began with something less than a bang.
In the first 2 days after their respective approvals, almost 99,000 children aged 5-11 years and over 675,000 children aged 12-15 were vaccinated, according to data from the Centers for Disease Control and Prevention. Children aged 0-4 years represent almost 6% of the overall population, compared with 8.7% for the 5- to 11-year-olds and 5.1% for those aged 12-15.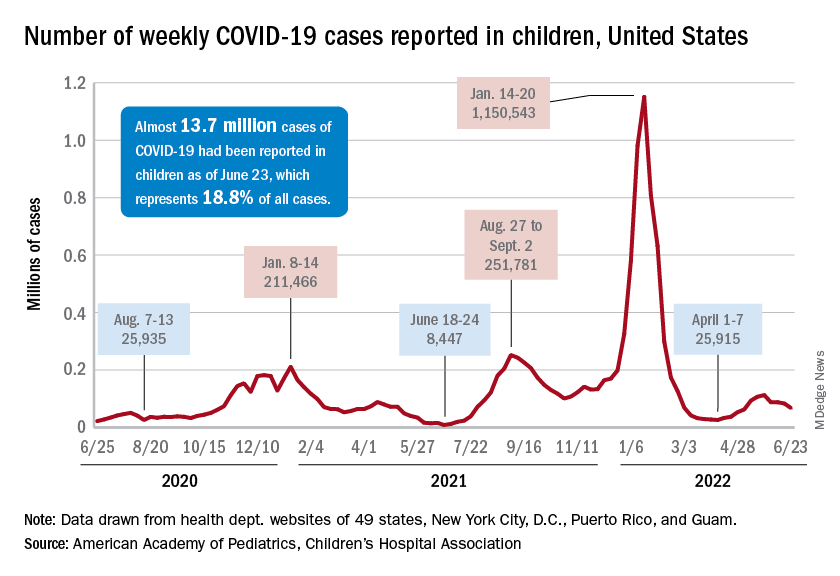
The recent decline in new cases over the past 4 weeks and the substantial decline since the Omicron surge could be a factor in the lack of response, but it is worth noting that the almost 68,000 new child cases reported in the past week, June 17-23, are “far higher than 1 year ago, June 24, 2021, when 8,400 child cases were reported,” the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID report.
That total for June 17-23 was 19% lower than the previous week and down by 40% since new cases hit a spring peak of 112,000 in late May. Regionally, new cases were down in the Midwest, the South, and the West, the AAP/CHA report showed, but the Northeast saw a small increase, which could be a signal of things to come for the summer.
The decline in new cases, however, has not been accompanied by decreases in hospitalizations or emergency department visits. New admissions of children aged 0-17 with confirmed COVID were at 0.31 per 100,000 population on June 24 after reaching that level on June 15, so no drop-off has occurred yet but there are signs of leveling off, based on CDC data.
The ED visit rates have been fairly steady through June, although COVID-related visits were up to 3.4% of all ED visits on June 22 for children aged 0-11 years, after being below 3% for the first 2 weeks of the month. The rate for children aged 12-15 has been between 1.6% and 1.9% for the past 3 weeks and the rate for 16- and 17-year-olds has been hovering between 1.7% and 2.2% for most of June, after going as high as 2.7% in late May, the CDC said on its COVID Data Tracker.
New cases of COVID-19 continue to drop among children, but the vaccination effort in those under age 5 years began with something less than a bang.
In the first 2 days after their respective approvals, almost 99,000 children aged 5-11 years and over 675,000 children aged 12-15 were vaccinated, according to data from the Centers for Disease Control and Prevention. Children aged 0-4 years represent almost 6% of the overall population, compared with 8.7% for the 5- to 11-year-olds and 5.1% for those aged 12-15.
The recent decline in new cases over the past 4 weeks and the substantial decline since the Omicron surge could be a factor in the lack of response, but it is worth noting that the almost 68,000 new child cases reported in the past week, June 17-23, are “far higher than 1 year ago, June 24, 2021, when 8,400 child cases were reported,” the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID report.
That total for June 17-23 was 19% lower than the previous week and down by 40% since new cases hit a spring peak of 112,000 in late May. Regionally, new cases were down in the Midwest, the South, and the West, the AAP/CHA report showed, but the Northeast saw a small increase, which could be a signal of things to come for the summer.
The decline in new cases, however, has not been accompanied by decreases in hospitalizations or emergency department visits. New admissions of children aged 0-17 with confirmed COVID were at 0.31 per 100,000 population on June 24 after reaching that level on June 15, so no drop-off has occurred yet but there are signs of leveling off, based on CDC data.
The ED visit rates have been fairly steady through June, although COVID-related visits were up to 3.4% of all ED visits on June 22 for children aged 0-11 years, after being below 3% for the first 2 weeks of the month. The rate for children aged 12-15 has been between 1.6% and 1.9% for the past 3 weeks and the rate for 16- and 17-year-olds has been hovering between 1.7% and 2.2% for most of June, after going as high as 2.7% in late May, the CDC said on its COVID Data Tracker.
New cases of COVID-19 continue to drop among children, but the vaccination effort in those under age 5 years began with something less than a bang.
In the first 2 days after their respective approvals, almost 99,000 children aged 5-11 years and over 675,000 children aged 12-15 were vaccinated, according to data from the Centers for Disease Control and Prevention. Children aged 0-4 years represent almost 6% of the overall population, compared with 8.7% for the 5- to 11-year-olds and 5.1% for those aged 12-15.
The recent decline in new cases over the past 4 weeks and the substantial decline since the Omicron surge could be a factor in the lack of response, but it is worth noting that the almost 68,000 new child cases reported in the past week, June 17-23, are “far higher than 1 year ago, June 24, 2021, when 8,400 child cases were reported,” the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID report.
That total for June 17-23 was 19% lower than the previous week and down by 40% since new cases hit a spring peak of 112,000 in late May. Regionally, new cases were down in the Midwest, the South, and the West, the AAP/CHA report showed, but the Northeast saw a small increase, which could be a signal of things to come for the summer.
The decline in new cases, however, has not been accompanied by decreases in hospitalizations or emergency department visits. New admissions of children aged 0-17 with confirmed COVID were at 0.31 per 100,000 population on June 24 after reaching that level on June 15, so no drop-off has occurred yet but there are signs of leveling off, based on CDC data.
The ED visit rates have been fairly steady through June, although COVID-related visits were up to 3.4% of all ED visits on June 22 for children aged 0-11 years, after being below 3% for the first 2 weeks of the month. The rate for children aged 12-15 has been between 1.6% and 1.9% for the past 3 weeks and the rate for 16- and 17-year-olds has been hovering between 1.7% and 2.2% for most of June, after going as high as 2.7% in late May, the CDC said on its COVID Data Tracker.
FDA approves Qsymia for treating teens with obesity
The indication is for use as additional therapy along with a reduced-calorie diet and increased physical activity in youth with obesity, defined as a body mass index of the 95th percentile or greater when standardized for age and sex.
Qsymia was first approved in July 2012 for chronic weight management in adults with an initial BMI of 30 kg/m2 or greater (obese) or 27 kg/m2 or greater (overweight) with one or more weight-related comorbidities, as an adjunct to lifestyle modification.
About 1 in 5 adolescents in the United States has obesity, according to the FDA.
The drug is the fourth to be approved for treating obesity in youth, along with liraglutide (Saxenda) and orlistat (Alli, Xenical), both approved down to age 12, and phentermine for those aged 16 and older.
The Qsymia approval was based on data from a phase 4 double-blind, placebo-controlled trial of 223 youth aged 12-16 with obesity who had not lost weight with lifestyle modifications. They were randomly assigned to Qsymia in doses of 7.5 mg phentermine/46 mg topiramate, 15 mg phentermine/92 mg topiramate, or placebo once daily, along with lifestyle counseling for all.
At 56 weeks, those taking the lower Qsymia dose lost an average of 4.8% of their BMI, and those on the higher dose lost 7.1%. In contrast, the placebo group gained about 3.3% of their BMI.
Because Qsymia increases the risk for oral clefts (lip and palate) in a fetus if taken during pregnancy, female patients should obtain negative pregnancy tests before starting the drug, take monthly pregnancy tests while on the drug, and use effective contraception throughout. Also because of the oral cleft risk, Qsymia is available only through an FDA program called a Risk Evaluation and Mitigation Strategy.
Additional potential adverse effects with Qsymia include increased heart rate and suicidal behavior/ideation. Patients should be advised to monitor for mood changes and discontinue the drug if depression or suicidal thoughts develop. The drug has also been linked to slowing of linear growth, so growth should be monitored in adolescents taking the drug, according to the FDA.
Qsymia is also associated with acute myopia, secondary angle closure glaucoma, visual problems, sleep disorders, cognitive impairment, metabolic acidosis, and decreased renal function.
The most common adverse reactions reported in the pediatric clinical trial included depression, dizziness, joint pain, fever, flu, and ankle sprain.
A version of this article first appeared on Medscape.com.
The indication is for use as additional therapy along with a reduced-calorie diet and increased physical activity in youth with obesity, defined as a body mass index of the 95th percentile or greater when standardized for age and sex.
Qsymia was first approved in July 2012 for chronic weight management in adults with an initial BMI of 30 kg/m2 or greater (obese) or 27 kg/m2 or greater (overweight) with one or more weight-related comorbidities, as an adjunct to lifestyle modification.
About 1 in 5 adolescents in the United States has obesity, according to the FDA.
The drug is the fourth to be approved for treating obesity in youth, along with liraglutide (Saxenda) and orlistat (Alli, Xenical), both approved down to age 12, and phentermine for those aged 16 and older.
The Qsymia approval was based on data from a phase 4 double-blind, placebo-controlled trial of 223 youth aged 12-16 with obesity who had not lost weight with lifestyle modifications. They were randomly assigned to Qsymia in doses of 7.5 mg phentermine/46 mg topiramate, 15 mg phentermine/92 mg topiramate, or placebo once daily, along with lifestyle counseling for all.
At 56 weeks, those taking the lower Qsymia dose lost an average of 4.8% of their BMI, and those on the higher dose lost 7.1%. In contrast, the placebo group gained about 3.3% of their BMI.
Because Qsymia increases the risk for oral clefts (lip and palate) in a fetus if taken during pregnancy, female patients should obtain negative pregnancy tests before starting the drug, take monthly pregnancy tests while on the drug, and use effective contraception throughout. Also because of the oral cleft risk, Qsymia is available only through an FDA program called a Risk Evaluation and Mitigation Strategy.
Additional potential adverse effects with Qsymia include increased heart rate and suicidal behavior/ideation. Patients should be advised to monitor for mood changes and discontinue the drug if depression or suicidal thoughts develop. The drug has also been linked to slowing of linear growth, so growth should be monitored in adolescents taking the drug, according to the FDA.
Qsymia is also associated with acute myopia, secondary angle closure glaucoma, visual problems, sleep disorders, cognitive impairment, metabolic acidosis, and decreased renal function.
The most common adverse reactions reported in the pediatric clinical trial included depression, dizziness, joint pain, fever, flu, and ankle sprain.
A version of this article first appeared on Medscape.com.
The indication is for use as additional therapy along with a reduced-calorie diet and increased physical activity in youth with obesity, defined as a body mass index of the 95th percentile or greater when standardized for age and sex.
Qsymia was first approved in July 2012 for chronic weight management in adults with an initial BMI of 30 kg/m2 or greater (obese) or 27 kg/m2 or greater (overweight) with one or more weight-related comorbidities, as an adjunct to lifestyle modification.
About 1 in 5 adolescents in the United States has obesity, according to the FDA.
The drug is the fourth to be approved for treating obesity in youth, along with liraglutide (Saxenda) and orlistat (Alli, Xenical), both approved down to age 12, and phentermine for those aged 16 and older.
The Qsymia approval was based on data from a phase 4 double-blind, placebo-controlled trial of 223 youth aged 12-16 with obesity who had not lost weight with lifestyle modifications. They were randomly assigned to Qsymia in doses of 7.5 mg phentermine/46 mg topiramate, 15 mg phentermine/92 mg topiramate, or placebo once daily, along with lifestyle counseling for all.
At 56 weeks, those taking the lower Qsymia dose lost an average of 4.8% of their BMI, and those on the higher dose lost 7.1%. In contrast, the placebo group gained about 3.3% of their BMI.
Because Qsymia increases the risk for oral clefts (lip and palate) in a fetus if taken during pregnancy, female patients should obtain negative pregnancy tests before starting the drug, take monthly pregnancy tests while on the drug, and use effective contraception throughout. Also because of the oral cleft risk, Qsymia is available only through an FDA program called a Risk Evaluation and Mitigation Strategy.
Additional potential adverse effects with Qsymia include increased heart rate and suicidal behavior/ideation. Patients should be advised to monitor for mood changes and discontinue the drug if depression or suicidal thoughts develop. The drug has also been linked to slowing of linear growth, so growth should be monitored in adolescents taking the drug, according to the FDA.
Qsymia is also associated with acute myopia, secondary angle closure glaucoma, visual problems, sleep disorders, cognitive impairment, metabolic acidosis, and decreased renal function.
The most common adverse reactions reported in the pediatric clinical trial included depression, dizziness, joint pain, fever, flu, and ankle sprain.
A version of this article first appeared on Medscape.com.
Simultaneous Cases of Carfilzomib-Induced Thrombotic Microangiopathy in 2 Patients With Multiple Myeloma
As a class of drugs, proteasome inhibitors are known to rarely cause drug-induced thrombotic microangiopathy (DITMA). In particular, carfilzomib is a second-generation, irreversible proteasome inhibitor approved for the treatment of relapsed, refractory multiple myeloma (MM) in combination with other therapeutic agents.1 Although generally well tolerated, carfilzomib has been associated with serious adverse events such as cardiovascular toxicity and DITMA.2-4 Thrombotic microangiopathy (TMA) is a life-threatening disorder characterized by thrombocytopenia, microangiopathic hemolytic anemia, and end-organ damage.5 Its occurrence secondary to carfilzomib has been reported only rarely in clinical trials of MM, and the most effective management of the disorder as well as the concurrent risk factors that contribute to its development remain incompletely understood.6,7 As a result, given both the expanding use of carfilzomib in practice and the morbidity of TMA, descriptions of carfilzomib-induced TMA from the real-world setting continue to provide important contributions to our understanding of the disorder.
At our US Department of Veterans Affairs (VA) medical center, 2 patients developed severe carfilzomib-induced TMA within days of one another. The presentation of simultaneous cases was highly unexpected and offered the unique opportunity to compare clinical features in real time. Here, we describe our 2 cases in detail, review their presentations and management in the context of the prior literature, and discuss potential insights gained into the disease.
Case Presentation
Case 1
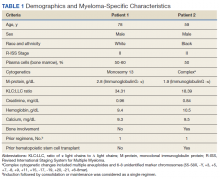
A 78-year-old male patient was diagnosed with monoclonal gammopathy of undetermined significance in 2012 that progressed to Revised International Staging System stage II IgG-κ MM in 2016 due to worsening anemia with a hemoglobin level < 10 g/dL (Table 1). He was treated initially with 8 cycles of first-line bortezomib, lenalidomide, and dexamethasone, to which he achieved a partial response with > 50% reduction in serum M-protein. He then received 3 cycles of maintenance bortezomib until relapse, at which time he was switched to second-line therapy consisting of carfilzomib 20 mg/m2 on days 1 and 2 and 56 mg/m2 on days 8, 9, 15, and 16 for cycle 1, followed by 56 mg/m2 on days 1, 2, 8, 9, 15, and 16 for subsequent cycles plus dexamethasone 20 mg twice weekly every 28 days.
After the patient received cycle 3, day 1 of carfilzomib, he developed subjective fevers, chills, and diarrhea. He missed his day 2 infusion and instead presented to the VA emergency department, where his vital signs were stable and laboratory tests were notable for the following levels: leukocytosis of20.3 K/µL (91.7% neutrophils), hemoglobin 12.4 g/dL (prior 13.5 g/dL), platelet count 171 K/µL, and creatinine 1.39 mg/dL (prior 1.13 g/dL). A chest X-ray demonstrated diffuse bilateral opacities concerning for edema vs infection, and he was started empirically on vancomycin, piperacillin-tazobactam, and azithromycin. His outpatient medications, which included acyclovir, aspirin, finasteride, oxybutynin, ranitidine, omega-3 fatty acids, fish oil, vitamin D, and senna, were continued as indicated.
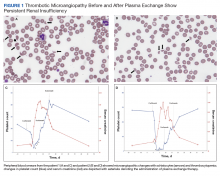
On hospital day 2, the patient’s platelet count dropped to 81 K/µL and creatinine level rose to 1.78 mg/dL. He developed dark urine (urinalysis [UA] 3+ blood, 6-11 red blood cells per high power field [RBC/HPF]) and had laboratory tests suggestive of hemolysis, including lactic dehydrogenase (LDH) > 1,200 IU/L (reference range, 60-250 IU/L), haptoglobin < 30 mg/dL (reference range, 44-215 mg/dL), total bilirubin 3.2 mg/dL (reference range, 0.2-1.3 mg/dL; indirect bilirubin, 2.6 mg/dL), and a peripheral blood smear demonstrating moderate microangiopathy (Figure 1).
Workup for alternative causes of thrombocytopenia included a negative heparin-induced thrombocytopenia panel and a disseminated intravascular coagulation (DIC) panel showing elevated fibrinogen (515 mg/dL; reference range, 200-400 mg/dL) and mildly elevated international normalized ratio (INR) (1.3). Blood cultures were negative, and a 22-pathogen gastrointestinal polymerase chain reaction (PCR) panel failed to identify viral or bacterial pathogens, including Escherichia coli O157:H7. C3 (81 mg/dL; reference range, 90-180 mg/dL) and C4 (16 mg/dL; reference range, 16-47 mg/dL) complement levels were borderline to mildly reduced.
Based on this constellation of findings, a diagnosis of TMA was made, and the patient was started empirically on plasma exchange and pulse-dosed steroids. After 4 cycles of plasma exchange, the platelet count had normalized from its nadir of 29 K/µL. ADAMTS13 activity (98% enzyme activity) ruled out thrombotic thrombocytopenic purpura (TTP), and the patient continued to have anuric renal failure (creatinine, 8.62 mg/dL) necessitating the initiation of hemodialysis. Given persistent renal insufficiency, a diagnosis of atypical hemolytic uremic syndrome (HUS) was considered, and eculizumab 900 mg was administered on days 8 and 15 with stabilization of renal function. By the time of discharge on day 18, the patient’s creatinine level had decreased to 3.89 mg/dL, and platelet count was 403 K/µL. Creatinine normalized to 1.07 mg/dL by day 46.
Outpatient genetic testing through the BloodCenter of Wisconsin Diagnostic Laboratories was negative for mutations in the following genes associated with atypical HUS: CFH, CFI, MCP (CD46), THBD, CFB, C3, DGKE, ADAMTS13, C4BPA, C4BPB, LMNA, CFHR1, CFHR3, CFHR4, and CFHR5. The patient subsequently remained off all antimyeloma therapy for > 1 year until eventually starting third-line pomalidomide plus dexamethasone without reinitiation of proteasome inhibitor therapy.
Case 2
A 59-year-old male patient, diagnosed in 2013 with ISS stage I IgG-κ MM after presenting with compression fractures, completed 8 cycles of cyclophosphamide, bortezomib, and dexamethasone before undergoing autologous hematopoietic stem cell transplantation with complete response (Table 1). He subsequently received single-agent maintenance bortezomib until relapse nearly 2 years later, at which time he started second-line carfilzomib 20 mg/m2 on days 1 and 2 and 27 mg/m2 on days 8, 9, 15, and 16 for cycle 1, followed by 27 mg/m2 on days 8, 9, 15, and 16 for cycles 2 to 8, lenalidomide 25 mg on days 1 to 21, and dexamethasone 40 mg weekly every 28 days. Serum free light chain levels normalized after 9 cycles, and he subsequently began maintenance carfilzomib 70 mg/m2 on days 1 and 15 plus lenalidomide 10 mg on days 1 to 21 every 28 days.
On the morning before admission, the patient received C6D17 of maintenance carfilzomib, which had been delayed from day 15 because of the holiday. Later that evening, he developed nausea, vomiting, and fever of 101.3 °F. He presented to the VA emergency department and was tachycardic (108 beats per minute) and hypotensive (86/55 mm Hg). Laboratory tests were notable for hemoglobin level 9.9 g/dL (prior 11.6 g/dL), platelet count 270 K/µL, and creatinine level 1.86 mg/dL (prior 1.12 mg/dL). A respiratory viral panel was positive for influenza A, and antimicrobial agents were eventually broadened to piperacillin-tazobactam, azithromycin, and oseltamivir. His outpatient medications, which included acyclovir, zoledronic acid, sulfamethoxazole/trimethoprim, aspirin, amlodipine, atorvastatin, omeprazole, zolpidem, calcium, vitamin D, loratadine, ascorbic acid, and prochlorperazine, were continued as indicated.
On hospital day 2, the patient’s platelet count declined from 211 to 57 K/µL. He developed tea-colored urine (UA 2+ blood, 0-2 RBC/HPF) and had laboratory tests suggestive of hemolysis, including LDH 910 IU/L (reference range, 60-250 IU/L), total bilirubin 3.3 mg/dL (reference range, 0.2-1.3 mg/dL; no direct or indirect available), and a peripheral blood smear demonstrating moderate microangiopathy. Although haptoglobin level was normal at this time (206 mg/dL; reference range, 44-215 mg/dL), it decreased to 42 mg/dL by the following day. Additional workup included a negative direct Coombs and a DIC panel showing elevated fibrinogen (596 mg/dL; reference range, 200-400 mg/dL) and mildly elevated INR (1.16). Blood cultures remained negative, and a 22-pathogen GI PCR panel identified no viral or bacterial pathogens, including E coli O157:H7. C3 (114 mg/dL; reference range, 90-180 mg/dL) and C4 (40 mg/dL; reference range, 16-47 mg/dL) complement levels were both normal.
Based on these findings, empiric treatment was started with plasma exchange and pulse-dosed steroids. The patient received 3 cycles of plasma exchange until the results of the ADAMTS13 activity ruled out TTP (63% enzyme activity). Over the next 6 days, his platelet count reached a nadir of 6 K/µL and creatinine level peaked at 10.36 mg/dL, necessitating the initiation of hemodialysis. Given severe renal insufficiency, a diagnosis of atypical HUS was again considered, and eculizumab 900 mg was administered on days 9 and 16 with stabilization of renal function. By the time of discharge on day 17, the patient’s creatinine level had decreased to 4.17 mg/dL and platelet count was 164 K/µL. Creatinine level normalized to 1.02 mg/dL by day 72.
Outpatient genetic testing through the BloodCenter of Wisconsin Diagnostic Laboratories was negative for gene mutations associated with atypical HUS. Approximately 1 month after discharge, the patient resumed maintenance lenalidomide alone without reinitiation of proteasome inhibitor therapy.
Discussion
In this case series, we describe the uncommon drug-related adverse event of TMA occurring in 2 patients with MM after receiving carfilzomib. Although the incidence of TMA disorders is low, reaching up to 2.8% in patients receiving carfilzomib plus cyclophosphamide and dexamethasone in the phase 2 CARDAMON trial, our experience suggests that a high index of suspicion for carfilzomib-induced TMA is warranted in the real-world setting.8 TMA syndromes, including TTP, HUS, and DITMA, are characterized by microvascular endothelial injury and thrombosis leading to thrombocytopenia and microangiopathic hemolytic anemia.5,9 Several drug culprits of DITMA are recognized, including quinine, gemcitabine, tacrolimus, and proteasome inhibitors (bortezomib, carfilzomib, ixazomib).10-12 In a real-world series of patients receiving proteasome inhibitor therapy, either carfilzomib (n=8) or bortezomib (n=3), common clinical features of DITMA included thrombocytopenia, microangiopathic hemolytic anemia, gastrointestinal symptoms, and renal insufficiency with or without a need for hemodialysis.2 Although DITMA has been described primarily as an early event, its occurrence after 12 months of proteasome inhibitor therapy has also been reported, both in this series and elsewhere, thereby suggesting an ongoing risk for DITMA throughout the duration of carfilzomib treatment.2,13
The diagnosis of DITMA can be challenging given its nonspecific symptoms that overlap with other TMA syndromes. Previous studies have proposed that for a drug to be associated with DITMA, there should be: (1) evidence of clinical and/or pathologic findings of TMA; (2) exclusion of alternative causes of TMA; (3) no other new drug exposures other than the suspected culprit medication; and (4) a lack of recurrence of TMA in absence of the drug.10 In the case of patients with MM, other causes of TMA have also been described, including the underlying plasma cell disorder itself and stem cell transplantation.14 In the 2 cases we have described, these alternative causes were considered unlikely given that only 1 patient underwent transplantation remotely and neither had a previous history of TMA secondary to their disease. With respect to other TMA syndromes, ADAMTS13 levels > 10% and negative stool studies for E coli O157:H7 suggested against TTP or typical HUS, respectively. No other drug culprits were identified, and the close timing between the receipt of carfilzomib and symptom onset supported a causal relationship.
Because specific therapies are lacking, management of DITMA has traditionally included drug discontinuation and supportive care for end-organ injury.5 The terminal complement inhibitor, eculizumab, improves hematologic abnormalities and renal function in patients with atypical HUS but its use for treating patients with DITMA is not standard.15 Therefore, the decision to administer eculizumab to our 2 patients was driven by their severe renal insufficiency without improvement after plasma exchange, which suggested a phenotype similar to atypical HUS. After administration of eculizumab, renal function stabilized and then gradually improved over weeks to months, a time course similar to that described in cases of patients with DITMA secondary to other anticancer therapies treated with eculizumab.16 Although these results suggest a potential role for eculizumab in proteasome inhibitor–induced TMA, distinguishing the benefit of eculizumab over drug discontinuation alone remains challenging, and well-designed prospective investigations are needed.
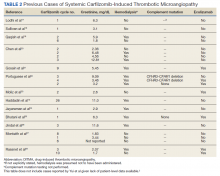
The clustered occurrence of our 2 cases is unique from previous reports that describe carfilzomib-induced TMA as a sporadic event (Table 2).13,17-28 Both immune-mediated and direct toxic effects have been proposed as mechanisms of DITMA, and while our cases do not differentiate between these mechanisms, we considered whether a combined model of initiation, whereby patient or environmental risk factors modulate occurrence of the disease in conjunction with the inciting drug, could explain the clustered occurrence of cases. In this series, drug manufacturing was not a shared risk factor as each patient received carfilzomib from different lot numbers. Furthermore, other patients at our center received carfilzomib from the same batches without developing DITMA. We also considered the role of infection given that 1 patient was diagnosed with influenza A and both presented with nonspecific, viral-like symptoms during the winter season. Interestingly, concurrent viral infections have been reported in other cases of carfilzomib-induced DITMA as well and have also been discussed as a trigger of atypical HUS.20,29 Finally, genetic testing was negative for complement pathway mutations that might predispose to complement dysregulation.
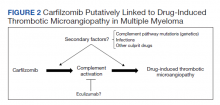
The absence of complement mutations in our 2 patients differs from a recent series describing heterozygous CFHR3-CHFR1 deletions in association with carfilzomib-induced TMA.22 In that report, the authors hypothesized that carfilzomib decreases expression of complement factor H (CFH), a negative regulator of complement activation, thereby leading to complement dysregulation in patients who are genetically predisposed. In a second series, plasma from patients with DITMA secondary to carfilzomib induced the deposition of the complement complex, C5b-9, on endothelial cells in culture, suggesting activation of the complement pathway.30 The effective use of eculizumab would also point to a role for complement activation, and ongoing investigations should aim to identify the triggers and mechanisms of complement dysregulation in this setting, especially for patients like ours in whom genetic testing for complement pathway mutations is negative (Figure 2).
Conclusions
DITMA is a known risk of proteasome inhibitors and is listed as a safety warning in the prescribing information for bortezomib, carfilzomib, and ixazomib.12 Given the overall rarity of this adverse event, the simultaneous presentation of our 2 cases was unexpected and underscores the need for heightened awareness in clinical practice. In addition, while no underlying complement mutations were identified, eculizumab was used in both cases to successfully stabilize renal function. Further research investigating the efficacy of eculizumab and the role of complement activation in proteasome inhibitor–induced TMA will be valuable.
Acknowledgments
The authors would like to thank the patients whose histories are reported in this manuscript as well as the physicians and staff who provided care during the hospitalizations and beyond. We also thank Oscar Silva, MD, PhD, for his assistance in reviewing and formatting the peripheral blood smear images.
1. McBride A, Klaus JO, Stockeri-Goldstein K. Carfilzomib: a second-generation proteasome inhibitor for the treatment of multiple myeloma. Am J Health Syst Pharm. 2015;72(5):353-360. doi:10.2146/ajhp130281
2. Yui JC, Van Keer J, Weiss BM, et al. Proteasome inhibitor associated thrombotic microangiopathy. Am J Hematol. 2016;91(9):E348-E352. doi:10.1002/ajh.24447
3. Dimopoulos MA, Roussou M, Gavriatopoulou M, et al. Cardiac and renal complications of carfilzomib in patients with multiple myeloma. Blood Adv. 2017;1(7):449-454. doi:10.1182/bloodadvances.2016003269
4. Chari A, Stewart AK, Russell SD, et al. Analysis of carfilzomib cardiovascular safety profile across relapsed and/or refractory multiple myeloma clinical trials. Blood Adv. 2018;2(13):1633-1644. doi:10.1182/bloodadvances.2017015545
5. George JN, Nester CM. Syndromes of thrombotic microangiopathy. N Engl J Med. 2014;371(7):654-666. doi:10.1056/NEJMra1312353
6. Dimopoulos MA, Moreau P, Palumbo A, et al. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study. Lancet Oncol. 2016;17(1):27-38. doi:10.1016/S1470-2045(15)00464-7
7. Dimopoulos M, Quach H, Mateos MV, et al. Carfilzomib, dexamethasone, and daratumumab versus carfilzomib and dexamethasone for patients with relapsed or refractory multiple myeloma (CANDOR): results from a randomised, multicentre, open-label, phase 3 study. Lancet. 2020;396(10245):186-197. doi:10.1016/S0140-6736(20)30734-0
8. Camilleri M, Cuadrado M, Phillips E, et al. Thrombotic microangiopathy in untreated myeloma patients receiving carfilzomib, cyclophosphamide and dexamethasone on the CARDAMON study. Br J Haematol. 2021;193(4):750-760. doi:10.1111/bjh.17377
9. Masias C, Vasu S, Cataland SR. None of the above: thrombotic microangiopathy beyond TTP and HUS. Blood. 2017;129(21):2857-2863. doi:10.1182/blood-2016-11-743104
10. Al-Nouri ZL, Reese JA, Terrell DR, Vesely SK, George JN. Drug-induced thrombotic microangiopathy: a systemic review of published reports. Blood. 2015;125(4):616-618. doi:10.1182/blood-2014-11-611335
11. Saleem R, Reese JA, George JN. Drug-induced thrombotic-microangiopathy: an updated systematic review, 2014-2018. Am J Hematol. 2018;93(9):E241-E243. doi:10.1002/ajh.25208
12 Nguyen MN, Nayernama A, Jones SC, Kanapuru B, Gormley N, Waldron PE. Proteasome inhibitor-associated thrombotic microangiopathy: a review of cases reported to the FDA adverse event reporting system and published in the literature. Am J Hematol. 2020;95(9):E218-E222. doi:10.1002/ajh.25832
13. Haddadin M, Al-Sadawi M, Madanat S, et al. Late presentation of carfilzomib associated thrombotic microangiopathy. Am J Med Case Rep. 2019;7(10):240-243. doi:10.12691/ajmcr-7-10-5
14 Portuguese AJ, Gleber C, Passero Jr FC, Lipe B. A review of thrombotic microangiopathies in multiple myeloma. Leuk Res. 2019;85:106195. doi:10.1016/j.leukres.2019.106195
15. Legendre CM, Licht C, Muus P, et al. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med. 2013;368(23):2169-2181. doi:10.1056/NEJMoa1208981
16. Olson SR, Lu E, Sulpizio E, Shatzel JJ, Rueda JF, DeLoughery TG. When to stop eculizumab in complement-mediated thrombotic microangiopathies. Am J Nephrol. 2018;48(2):96-107. doi:10.1159/000492033
17. Lodhi A, Kumar A, Saqlain MU, Suneja M. Thrombotic microangiopathy associated with proteasome inhibitors. Clin Kidney J. 2015;8(5):632-636. doi:10.1093/ckj/sfv059
18. Sullivan MR, Danilov AV, Lansigan F, Dunbar NM. Carfilzomib associated thrombotic microangiopathy initially treated with therapeutic plasma exchange. J Clin Apher., 2015;30(5):308-310. doi:10.1002/jca.21371
19. Qaqish I, Schlam IM, Chakkera HA, Fonseca R, Adamski J. Carfilzomib: a cause of drug associated thrombotic microangiopathy. Transfus Apher Sci. 2016;54(3):401-404. doi:10.1016/j.transci.2016.03.002
20. Chen Y, Ooi M, Lim SF, et al. Thrombotic microangiopathy during carfilzomib use: case series in Singapore. Blood Cancer J. 2016;6(7):e450. doi:10.1038/bcj.2016.62
21. Gosain R, Gill A, Fuqua J, et al. Gemcitabine and carfilzomib induced thrombotic microangiopathy: eculizumab as a life-saving treatment. Clin Case Rep. 2017;5(12):1926-1930. doi:10.1002/ccr3.1214
22. Portuguese AJ, Lipe B. Carfilzomib-induced aHUS responds to early eculizumab and may be associated with heterozygrous CFHR3-CFHR1 deletion. Blood Adv. 2018;2(23):3443-3446. doi:10.1182/bloodadvances.2018027532
23. Moliz C, Gutiérrez E, Cavero T, Redondo B, Praga M. Eculizumab as a treatment for atypical hemolytic syndrome secondary to carfilzomib. Nefrologia (Engl Ed). 2019;39(1):86-88. doi:10.1016/j.nefro.2018.02.005
24. Jeyaraman P, Borah P, Singh A, et al., Thrombotic microangiopathy after carfilzomib in a very young myeloma patient. Blood Cells Mol Dis. 2020;81:102400. doi:10.1016/j.bcmd.2019.102400
25. Bhutani D, Assal A, Mapara MY, Prinzing S, Lentzsch S. Case report: carfilzomib-induced thrombotic microangiopathy with complement activation treated successfully with eculizumab. Clin Lymphoma Myeloma Leuk. 2020;20(4):e155-e157. doi:10.1016/j.clml.2020.01.016
26. Jindal N, Jandial A, Jain A, et al. Carfilzomib-induced thrombotic microangiopathy: a case based review. Hematol Oncol Stem Cell Ther. 2020;S1658-3876(20)30118-7. doi:10.1016/j.hemonc.2020.07.001
27. Monteith BE, Venner CP, Reece DE, et al. Drug-induced thrombotic microangiopathy with concurrent proteasome inhibitor use in the treatment of multiple myeloma: a case series and review of the literature. Clin Lymphoma Myeloma Leuk. 2020;20(11):e791-e780. doi:10.1016/j.clml.2020.04.014
28. Rassner M, Baur R, Wäsch R, et al. Two cases of carfilzomib-induced thrombotic microangiopathy successfully treated with eculizumab in multiple myeloma. BMC Nephrol. 2021;22(1):32. doi:10.1186/s12882-020-02226-5
29. Kavanagh D, Goodship THJ. Atypical hemolytic uremic syndrome, genetic basis, and clinical manifestations. Hematology Am Soc Hematol Educ Program. 2011;2011:15-20. doi:10.1182/asheducation-2011.1.15
30. Blasco M, Martínez-Roca A, Rodríguez-Lobato LG, et al. Complement as the enabler of carfilzomib-induced thrombotic microangiopathy. Br J Haematol. 2021;193(1):181-187. doi:10.1111/bjh.16796
As a class of drugs, proteasome inhibitors are known to rarely cause drug-induced thrombotic microangiopathy (DITMA). In particular, carfilzomib is a second-generation, irreversible proteasome inhibitor approved for the treatment of relapsed, refractory multiple myeloma (MM) in combination with other therapeutic agents.1 Although generally well tolerated, carfilzomib has been associated with serious adverse events such as cardiovascular toxicity and DITMA.2-4 Thrombotic microangiopathy (TMA) is a life-threatening disorder characterized by thrombocytopenia, microangiopathic hemolytic anemia, and end-organ damage.5 Its occurrence secondary to carfilzomib has been reported only rarely in clinical trials of MM, and the most effective management of the disorder as well as the concurrent risk factors that contribute to its development remain incompletely understood.6,7 As a result, given both the expanding use of carfilzomib in practice and the morbidity of TMA, descriptions of carfilzomib-induced TMA from the real-world setting continue to provide important contributions to our understanding of the disorder.
At our US Department of Veterans Affairs (VA) medical center, 2 patients developed severe carfilzomib-induced TMA within days of one another. The presentation of simultaneous cases was highly unexpected and offered the unique opportunity to compare clinical features in real time. Here, we describe our 2 cases in detail, review their presentations and management in the context of the prior literature, and discuss potential insights gained into the disease.
Case Presentation
Case 1
A 78-year-old male patient was diagnosed with monoclonal gammopathy of undetermined significance in 2012 that progressed to Revised International Staging System stage II IgG-κ MM in 2016 due to worsening anemia with a hemoglobin level < 10 g/dL (Table 1). He was treated initially with 8 cycles of first-line bortezomib, lenalidomide, and dexamethasone, to which he achieved a partial response with > 50% reduction in serum M-protein. He then received 3 cycles of maintenance bortezomib until relapse, at which time he was switched to second-line therapy consisting of carfilzomib 20 mg/m2 on days 1 and 2 and 56 mg/m2 on days 8, 9, 15, and 16 for cycle 1, followed by 56 mg/m2 on days 1, 2, 8, 9, 15, and 16 for subsequent cycles plus dexamethasone 20 mg twice weekly every 28 days.
After the patient received cycle 3, day 1 of carfilzomib, he developed subjective fevers, chills, and diarrhea. He missed his day 2 infusion and instead presented to the VA emergency department, where his vital signs were stable and laboratory tests were notable for the following levels: leukocytosis of20.3 K/µL (91.7% neutrophils), hemoglobin 12.4 g/dL (prior 13.5 g/dL), platelet count 171 K/µL, and creatinine 1.39 mg/dL (prior 1.13 g/dL). A chest X-ray demonstrated diffuse bilateral opacities concerning for edema vs infection, and he was started empirically on vancomycin, piperacillin-tazobactam, and azithromycin. His outpatient medications, which included acyclovir, aspirin, finasteride, oxybutynin, ranitidine, omega-3 fatty acids, fish oil, vitamin D, and senna, were continued as indicated.
On hospital day 2, the patient’s platelet count dropped to 81 K/µL and creatinine level rose to 1.78 mg/dL. He developed dark urine (urinalysis [UA] 3+ blood, 6-11 red blood cells per high power field [RBC/HPF]) and had laboratory tests suggestive of hemolysis, including lactic dehydrogenase (LDH) > 1,200 IU/L (reference range, 60-250 IU/L), haptoglobin < 30 mg/dL (reference range, 44-215 mg/dL), total bilirubin 3.2 mg/dL (reference range, 0.2-1.3 mg/dL; indirect bilirubin, 2.6 mg/dL), and a peripheral blood smear demonstrating moderate microangiopathy (Figure 1).
Workup for alternative causes of thrombocytopenia included a negative heparin-induced thrombocytopenia panel and a disseminated intravascular coagulation (DIC) panel showing elevated fibrinogen (515 mg/dL; reference range, 200-400 mg/dL) and mildly elevated international normalized ratio (INR) (1.3). Blood cultures were negative, and a 22-pathogen gastrointestinal polymerase chain reaction (PCR) panel failed to identify viral or bacterial pathogens, including Escherichia coli O157:H7. C3 (81 mg/dL; reference range, 90-180 mg/dL) and C4 (16 mg/dL; reference range, 16-47 mg/dL) complement levels were borderline to mildly reduced.
Based on this constellation of findings, a diagnosis of TMA was made, and the patient was started empirically on plasma exchange and pulse-dosed steroids. After 4 cycles of plasma exchange, the platelet count had normalized from its nadir of 29 K/µL. ADAMTS13 activity (98% enzyme activity) ruled out thrombotic thrombocytopenic purpura (TTP), and the patient continued to have anuric renal failure (creatinine, 8.62 mg/dL) necessitating the initiation of hemodialysis. Given persistent renal insufficiency, a diagnosis of atypical hemolytic uremic syndrome (HUS) was considered, and eculizumab 900 mg was administered on days 8 and 15 with stabilization of renal function. By the time of discharge on day 18, the patient’s creatinine level had decreased to 3.89 mg/dL, and platelet count was 403 K/µL. Creatinine normalized to 1.07 mg/dL by day 46.
Outpatient genetic testing through the BloodCenter of Wisconsin Diagnostic Laboratories was negative for mutations in the following genes associated with atypical HUS: CFH, CFI, MCP (CD46), THBD, CFB, C3, DGKE, ADAMTS13, C4BPA, C4BPB, LMNA, CFHR1, CFHR3, CFHR4, and CFHR5. The patient subsequently remained off all antimyeloma therapy for > 1 year until eventually starting third-line pomalidomide plus dexamethasone without reinitiation of proteasome inhibitor therapy.
Case 2
A 59-year-old male patient, diagnosed in 2013 with ISS stage I IgG-κ MM after presenting with compression fractures, completed 8 cycles of cyclophosphamide, bortezomib, and dexamethasone before undergoing autologous hematopoietic stem cell transplantation with complete response (Table 1). He subsequently received single-agent maintenance bortezomib until relapse nearly 2 years later, at which time he started second-line carfilzomib 20 mg/m2 on days 1 and 2 and 27 mg/m2 on days 8, 9, 15, and 16 for cycle 1, followed by 27 mg/m2 on days 8, 9, 15, and 16 for cycles 2 to 8, lenalidomide 25 mg on days 1 to 21, and dexamethasone 40 mg weekly every 28 days. Serum free light chain levels normalized after 9 cycles, and he subsequently began maintenance carfilzomib 70 mg/m2 on days 1 and 15 plus lenalidomide 10 mg on days 1 to 21 every 28 days.
On the morning before admission, the patient received C6D17 of maintenance carfilzomib, which had been delayed from day 15 because of the holiday. Later that evening, he developed nausea, vomiting, and fever of 101.3 °F. He presented to the VA emergency department and was tachycardic (108 beats per minute) and hypotensive (86/55 mm Hg). Laboratory tests were notable for hemoglobin level 9.9 g/dL (prior 11.6 g/dL), platelet count 270 K/µL, and creatinine level 1.86 mg/dL (prior 1.12 mg/dL). A respiratory viral panel was positive for influenza A, and antimicrobial agents were eventually broadened to piperacillin-tazobactam, azithromycin, and oseltamivir. His outpatient medications, which included acyclovir, zoledronic acid, sulfamethoxazole/trimethoprim, aspirin, amlodipine, atorvastatin, omeprazole, zolpidem, calcium, vitamin D, loratadine, ascorbic acid, and prochlorperazine, were continued as indicated.
On hospital day 2, the patient’s platelet count declined from 211 to 57 K/µL. He developed tea-colored urine (UA 2+ blood, 0-2 RBC/HPF) and had laboratory tests suggestive of hemolysis, including LDH 910 IU/L (reference range, 60-250 IU/L), total bilirubin 3.3 mg/dL (reference range, 0.2-1.3 mg/dL; no direct or indirect available), and a peripheral blood smear demonstrating moderate microangiopathy. Although haptoglobin level was normal at this time (206 mg/dL; reference range, 44-215 mg/dL), it decreased to 42 mg/dL by the following day. Additional workup included a negative direct Coombs and a DIC panel showing elevated fibrinogen (596 mg/dL; reference range, 200-400 mg/dL) and mildly elevated INR (1.16). Blood cultures remained negative, and a 22-pathogen GI PCR panel identified no viral or bacterial pathogens, including E coli O157:H7. C3 (114 mg/dL; reference range, 90-180 mg/dL) and C4 (40 mg/dL; reference range, 16-47 mg/dL) complement levels were both normal.
Based on these findings, empiric treatment was started with plasma exchange and pulse-dosed steroids. The patient received 3 cycles of plasma exchange until the results of the ADAMTS13 activity ruled out TTP (63% enzyme activity). Over the next 6 days, his platelet count reached a nadir of 6 K/µL and creatinine level peaked at 10.36 mg/dL, necessitating the initiation of hemodialysis. Given severe renal insufficiency, a diagnosis of atypical HUS was again considered, and eculizumab 900 mg was administered on days 9 and 16 with stabilization of renal function. By the time of discharge on day 17, the patient’s creatinine level had decreased to 4.17 mg/dL and platelet count was 164 K/µL. Creatinine level normalized to 1.02 mg/dL by day 72.
Outpatient genetic testing through the BloodCenter of Wisconsin Diagnostic Laboratories was negative for gene mutations associated with atypical HUS. Approximately 1 month after discharge, the patient resumed maintenance lenalidomide alone without reinitiation of proteasome inhibitor therapy.
Discussion
In this case series, we describe the uncommon drug-related adverse event of TMA occurring in 2 patients with MM after receiving carfilzomib. Although the incidence of TMA disorders is low, reaching up to 2.8% in patients receiving carfilzomib plus cyclophosphamide and dexamethasone in the phase 2 CARDAMON trial, our experience suggests that a high index of suspicion for carfilzomib-induced TMA is warranted in the real-world setting.8 TMA syndromes, including TTP, HUS, and DITMA, are characterized by microvascular endothelial injury and thrombosis leading to thrombocytopenia and microangiopathic hemolytic anemia.5,9 Several drug culprits of DITMA are recognized, including quinine, gemcitabine, tacrolimus, and proteasome inhibitors (bortezomib, carfilzomib, ixazomib).10-12 In a real-world series of patients receiving proteasome inhibitor therapy, either carfilzomib (n=8) or bortezomib (n=3), common clinical features of DITMA included thrombocytopenia, microangiopathic hemolytic anemia, gastrointestinal symptoms, and renal insufficiency with or without a need for hemodialysis.2 Although DITMA has been described primarily as an early event, its occurrence after 12 months of proteasome inhibitor therapy has also been reported, both in this series and elsewhere, thereby suggesting an ongoing risk for DITMA throughout the duration of carfilzomib treatment.2,13
The diagnosis of DITMA can be challenging given its nonspecific symptoms that overlap with other TMA syndromes. Previous studies have proposed that for a drug to be associated with DITMA, there should be: (1) evidence of clinical and/or pathologic findings of TMA; (2) exclusion of alternative causes of TMA; (3) no other new drug exposures other than the suspected culprit medication; and (4) a lack of recurrence of TMA in absence of the drug.10 In the case of patients with MM, other causes of TMA have also been described, including the underlying plasma cell disorder itself and stem cell transplantation.14 In the 2 cases we have described, these alternative causes were considered unlikely given that only 1 patient underwent transplantation remotely and neither had a previous history of TMA secondary to their disease. With respect to other TMA syndromes, ADAMTS13 levels > 10% and negative stool studies for E coli O157:H7 suggested against TTP or typical HUS, respectively. No other drug culprits were identified, and the close timing between the receipt of carfilzomib and symptom onset supported a causal relationship.
Because specific therapies are lacking, management of DITMA has traditionally included drug discontinuation and supportive care for end-organ injury.5 The terminal complement inhibitor, eculizumab, improves hematologic abnormalities and renal function in patients with atypical HUS but its use for treating patients with DITMA is not standard.15 Therefore, the decision to administer eculizumab to our 2 patients was driven by their severe renal insufficiency without improvement after plasma exchange, which suggested a phenotype similar to atypical HUS. After administration of eculizumab, renal function stabilized and then gradually improved over weeks to months, a time course similar to that described in cases of patients with DITMA secondary to other anticancer therapies treated with eculizumab.16 Although these results suggest a potential role for eculizumab in proteasome inhibitor–induced TMA, distinguishing the benefit of eculizumab over drug discontinuation alone remains challenging, and well-designed prospective investigations are needed.
The clustered occurrence of our 2 cases is unique from previous reports that describe carfilzomib-induced TMA as a sporadic event (Table 2).13,17-28 Both immune-mediated and direct toxic effects have been proposed as mechanisms of DITMA, and while our cases do not differentiate between these mechanisms, we considered whether a combined model of initiation, whereby patient or environmental risk factors modulate occurrence of the disease in conjunction with the inciting drug, could explain the clustered occurrence of cases. In this series, drug manufacturing was not a shared risk factor as each patient received carfilzomib from different lot numbers. Furthermore, other patients at our center received carfilzomib from the same batches without developing DITMA. We also considered the role of infection given that 1 patient was diagnosed with influenza A and both presented with nonspecific, viral-like symptoms during the winter season. Interestingly, concurrent viral infections have been reported in other cases of carfilzomib-induced DITMA as well and have also been discussed as a trigger of atypical HUS.20,29 Finally, genetic testing was negative for complement pathway mutations that might predispose to complement dysregulation.
The absence of complement mutations in our 2 patients differs from a recent series describing heterozygous CFHR3-CHFR1 deletions in association with carfilzomib-induced TMA.22 In that report, the authors hypothesized that carfilzomib decreases expression of complement factor H (CFH), a negative regulator of complement activation, thereby leading to complement dysregulation in patients who are genetically predisposed. In a second series, plasma from patients with DITMA secondary to carfilzomib induced the deposition of the complement complex, C5b-9, on endothelial cells in culture, suggesting activation of the complement pathway.30 The effective use of eculizumab would also point to a role for complement activation, and ongoing investigations should aim to identify the triggers and mechanisms of complement dysregulation in this setting, especially for patients like ours in whom genetic testing for complement pathway mutations is negative (Figure 2).
Conclusions
DITMA is a known risk of proteasome inhibitors and is listed as a safety warning in the prescribing information for bortezomib, carfilzomib, and ixazomib.12 Given the overall rarity of this adverse event, the simultaneous presentation of our 2 cases was unexpected and underscores the need for heightened awareness in clinical practice. In addition, while no underlying complement mutations were identified, eculizumab was used in both cases to successfully stabilize renal function. Further research investigating the efficacy of eculizumab and the role of complement activation in proteasome inhibitor–induced TMA will be valuable.
Acknowledgments
The authors would like to thank the patients whose histories are reported in this manuscript as well as the physicians and staff who provided care during the hospitalizations and beyond. We also thank Oscar Silva, MD, PhD, for his assistance in reviewing and formatting the peripheral blood smear images.
As a class of drugs, proteasome inhibitors are known to rarely cause drug-induced thrombotic microangiopathy (DITMA). In particular, carfilzomib is a second-generation, irreversible proteasome inhibitor approved for the treatment of relapsed, refractory multiple myeloma (MM) in combination with other therapeutic agents.1 Although generally well tolerated, carfilzomib has been associated with serious adverse events such as cardiovascular toxicity and DITMA.2-4 Thrombotic microangiopathy (TMA) is a life-threatening disorder characterized by thrombocytopenia, microangiopathic hemolytic anemia, and end-organ damage.5 Its occurrence secondary to carfilzomib has been reported only rarely in clinical trials of MM, and the most effective management of the disorder as well as the concurrent risk factors that contribute to its development remain incompletely understood.6,7 As a result, given both the expanding use of carfilzomib in practice and the morbidity of TMA, descriptions of carfilzomib-induced TMA from the real-world setting continue to provide important contributions to our understanding of the disorder.
At our US Department of Veterans Affairs (VA) medical center, 2 patients developed severe carfilzomib-induced TMA within days of one another. The presentation of simultaneous cases was highly unexpected and offered the unique opportunity to compare clinical features in real time. Here, we describe our 2 cases in detail, review their presentations and management in the context of the prior literature, and discuss potential insights gained into the disease.
Case Presentation
Case 1
A 78-year-old male patient was diagnosed with monoclonal gammopathy of undetermined significance in 2012 that progressed to Revised International Staging System stage II IgG-κ MM in 2016 due to worsening anemia with a hemoglobin level < 10 g/dL (Table 1). He was treated initially with 8 cycles of first-line bortezomib, lenalidomide, and dexamethasone, to which he achieved a partial response with > 50% reduction in serum M-protein. He then received 3 cycles of maintenance bortezomib until relapse, at which time he was switched to second-line therapy consisting of carfilzomib 20 mg/m2 on days 1 and 2 and 56 mg/m2 on days 8, 9, 15, and 16 for cycle 1, followed by 56 mg/m2 on days 1, 2, 8, 9, 15, and 16 for subsequent cycles plus dexamethasone 20 mg twice weekly every 28 days.
After the patient received cycle 3, day 1 of carfilzomib, he developed subjective fevers, chills, and diarrhea. He missed his day 2 infusion and instead presented to the VA emergency department, where his vital signs were stable and laboratory tests were notable for the following levels: leukocytosis of20.3 K/µL (91.7% neutrophils), hemoglobin 12.4 g/dL (prior 13.5 g/dL), platelet count 171 K/µL, and creatinine 1.39 mg/dL (prior 1.13 g/dL). A chest X-ray demonstrated diffuse bilateral opacities concerning for edema vs infection, and he was started empirically on vancomycin, piperacillin-tazobactam, and azithromycin. His outpatient medications, which included acyclovir, aspirin, finasteride, oxybutynin, ranitidine, omega-3 fatty acids, fish oil, vitamin D, and senna, were continued as indicated.
On hospital day 2, the patient’s platelet count dropped to 81 K/µL and creatinine level rose to 1.78 mg/dL. He developed dark urine (urinalysis [UA] 3+ blood, 6-11 red blood cells per high power field [RBC/HPF]) and had laboratory tests suggestive of hemolysis, including lactic dehydrogenase (LDH) > 1,200 IU/L (reference range, 60-250 IU/L), haptoglobin < 30 mg/dL (reference range, 44-215 mg/dL), total bilirubin 3.2 mg/dL (reference range, 0.2-1.3 mg/dL; indirect bilirubin, 2.6 mg/dL), and a peripheral blood smear demonstrating moderate microangiopathy (Figure 1).
Workup for alternative causes of thrombocytopenia included a negative heparin-induced thrombocytopenia panel and a disseminated intravascular coagulation (DIC) panel showing elevated fibrinogen (515 mg/dL; reference range, 200-400 mg/dL) and mildly elevated international normalized ratio (INR) (1.3). Blood cultures were negative, and a 22-pathogen gastrointestinal polymerase chain reaction (PCR) panel failed to identify viral or bacterial pathogens, including Escherichia coli O157:H7. C3 (81 mg/dL; reference range, 90-180 mg/dL) and C4 (16 mg/dL; reference range, 16-47 mg/dL) complement levels were borderline to mildly reduced.
Based on this constellation of findings, a diagnosis of TMA was made, and the patient was started empirically on plasma exchange and pulse-dosed steroids. After 4 cycles of plasma exchange, the platelet count had normalized from its nadir of 29 K/µL. ADAMTS13 activity (98% enzyme activity) ruled out thrombotic thrombocytopenic purpura (TTP), and the patient continued to have anuric renal failure (creatinine, 8.62 mg/dL) necessitating the initiation of hemodialysis. Given persistent renal insufficiency, a diagnosis of atypical hemolytic uremic syndrome (HUS) was considered, and eculizumab 900 mg was administered on days 8 and 15 with stabilization of renal function. By the time of discharge on day 18, the patient’s creatinine level had decreased to 3.89 mg/dL, and platelet count was 403 K/µL. Creatinine normalized to 1.07 mg/dL by day 46.
Outpatient genetic testing through the BloodCenter of Wisconsin Diagnostic Laboratories was negative for mutations in the following genes associated with atypical HUS: CFH, CFI, MCP (CD46), THBD, CFB, C3, DGKE, ADAMTS13, C4BPA, C4BPB, LMNA, CFHR1, CFHR3, CFHR4, and CFHR5. The patient subsequently remained off all antimyeloma therapy for > 1 year until eventually starting third-line pomalidomide plus dexamethasone without reinitiation of proteasome inhibitor therapy.
Case 2
A 59-year-old male patient, diagnosed in 2013 with ISS stage I IgG-κ MM after presenting with compression fractures, completed 8 cycles of cyclophosphamide, bortezomib, and dexamethasone before undergoing autologous hematopoietic stem cell transplantation with complete response (Table 1). He subsequently received single-agent maintenance bortezomib until relapse nearly 2 years later, at which time he started second-line carfilzomib 20 mg/m2 on days 1 and 2 and 27 mg/m2 on days 8, 9, 15, and 16 for cycle 1, followed by 27 mg/m2 on days 8, 9, 15, and 16 for cycles 2 to 8, lenalidomide 25 mg on days 1 to 21, and dexamethasone 40 mg weekly every 28 days. Serum free light chain levels normalized after 9 cycles, and he subsequently began maintenance carfilzomib 70 mg/m2 on days 1 and 15 plus lenalidomide 10 mg on days 1 to 21 every 28 days.
On the morning before admission, the patient received C6D17 of maintenance carfilzomib, which had been delayed from day 15 because of the holiday. Later that evening, he developed nausea, vomiting, and fever of 101.3 °F. He presented to the VA emergency department and was tachycardic (108 beats per minute) and hypotensive (86/55 mm Hg). Laboratory tests were notable for hemoglobin level 9.9 g/dL (prior 11.6 g/dL), platelet count 270 K/µL, and creatinine level 1.86 mg/dL (prior 1.12 mg/dL). A respiratory viral panel was positive for influenza A, and antimicrobial agents were eventually broadened to piperacillin-tazobactam, azithromycin, and oseltamivir. His outpatient medications, which included acyclovir, zoledronic acid, sulfamethoxazole/trimethoprim, aspirin, amlodipine, atorvastatin, omeprazole, zolpidem, calcium, vitamin D, loratadine, ascorbic acid, and prochlorperazine, were continued as indicated.
On hospital day 2, the patient’s platelet count declined from 211 to 57 K/µL. He developed tea-colored urine (UA 2+ blood, 0-2 RBC/HPF) and had laboratory tests suggestive of hemolysis, including LDH 910 IU/L (reference range, 60-250 IU/L), total bilirubin 3.3 mg/dL (reference range, 0.2-1.3 mg/dL; no direct or indirect available), and a peripheral blood smear demonstrating moderate microangiopathy. Although haptoglobin level was normal at this time (206 mg/dL; reference range, 44-215 mg/dL), it decreased to 42 mg/dL by the following day. Additional workup included a negative direct Coombs and a DIC panel showing elevated fibrinogen (596 mg/dL; reference range, 200-400 mg/dL) and mildly elevated INR (1.16). Blood cultures remained negative, and a 22-pathogen GI PCR panel identified no viral or bacterial pathogens, including E coli O157:H7. C3 (114 mg/dL; reference range, 90-180 mg/dL) and C4 (40 mg/dL; reference range, 16-47 mg/dL) complement levels were both normal.
Based on these findings, empiric treatment was started with plasma exchange and pulse-dosed steroids. The patient received 3 cycles of plasma exchange until the results of the ADAMTS13 activity ruled out TTP (63% enzyme activity). Over the next 6 days, his platelet count reached a nadir of 6 K/µL and creatinine level peaked at 10.36 mg/dL, necessitating the initiation of hemodialysis. Given severe renal insufficiency, a diagnosis of atypical HUS was again considered, and eculizumab 900 mg was administered on days 9 and 16 with stabilization of renal function. By the time of discharge on day 17, the patient’s creatinine level had decreased to 4.17 mg/dL and platelet count was 164 K/µL. Creatinine level normalized to 1.02 mg/dL by day 72.
Outpatient genetic testing through the BloodCenter of Wisconsin Diagnostic Laboratories was negative for gene mutations associated with atypical HUS. Approximately 1 month after discharge, the patient resumed maintenance lenalidomide alone without reinitiation of proteasome inhibitor therapy.
Discussion
In this case series, we describe the uncommon drug-related adverse event of TMA occurring in 2 patients with MM after receiving carfilzomib. Although the incidence of TMA disorders is low, reaching up to 2.8% in patients receiving carfilzomib plus cyclophosphamide and dexamethasone in the phase 2 CARDAMON trial, our experience suggests that a high index of suspicion for carfilzomib-induced TMA is warranted in the real-world setting.8 TMA syndromes, including TTP, HUS, and DITMA, are characterized by microvascular endothelial injury and thrombosis leading to thrombocytopenia and microangiopathic hemolytic anemia.5,9 Several drug culprits of DITMA are recognized, including quinine, gemcitabine, tacrolimus, and proteasome inhibitors (bortezomib, carfilzomib, ixazomib).10-12 In a real-world series of patients receiving proteasome inhibitor therapy, either carfilzomib (n=8) or bortezomib (n=3), common clinical features of DITMA included thrombocytopenia, microangiopathic hemolytic anemia, gastrointestinal symptoms, and renal insufficiency with or without a need for hemodialysis.2 Although DITMA has been described primarily as an early event, its occurrence after 12 months of proteasome inhibitor therapy has also been reported, both in this series and elsewhere, thereby suggesting an ongoing risk for DITMA throughout the duration of carfilzomib treatment.2,13
The diagnosis of DITMA can be challenging given its nonspecific symptoms that overlap with other TMA syndromes. Previous studies have proposed that for a drug to be associated with DITMA, there should be: (1) evidence of clinical and/or pathologic findings of TMA; (2) exclusion of alternative causes of TMA; (3) no other new drug exposures other than the suspected culprit medication; and (4) a lack of recurrence of TMA in absence of the drug.10 In the case of patients with MM, other causes of TMA have also been described, including the underlying plasma cell disorder itself and stem cell transplantation.14 In the 2 cases we have described, these alternative causes were considered unlikely given that only 1 patient underwent transplantation remotely and neither had a previous history of TMA secondary to their disease. With respect to other TMA syndromes, ADAMTS13 levels > 10% and negative stool studies for E coli O157:H7 suggested against TTP or typical HUS, respectively. No other drug culprits were identified, and the close timing between the receipt of carfilzomib and symptom onset supported a causal relationship.
Because specific therapies are lacking, management of DITMA has traditionally included drug discontinuation and supportive care for end-organ injury.5 The terminal complement inhibitor, eculizumab, improves hematologic abnormalities and renal function in patients with atypical HUS but its use for treating patients with DITMA is not standard.15 Therefore, the decision to administer eculizumab to our 2 patients was driven by their severe renal insufficiency without improvement after plasma exchange, which suggested a phenotype similar to atypical HUS. After administration of eculizumab, renal function stabilized and then gradually improved over weeks to months, a time course similar to that described in cases of patients with DITMA secondary to other anticancer therapies treated with eculizumab.16 Although these results suggest a potential role for eculizumab in proteasome inhibitor–induced TMA, distinguishing the benefit of eculizumab over drug discontinuation alone remains challenging, and well-designed prospective investigations are needed.
The clustered occurrence of our 2 cases is unique from previous reports that describe carfilzomib-induced TMA as a sporadic event (Table 2).13,17-28 Both immune-mediated and direct toxic effects have been proposed as mechanisms of DITMA, and while our cases do not differentiate between these mechanisms, we considered whether a combined model of initiation, whereby patient or environmental risk factors modulate occurrence of the disease in conjunction with the inciting drug, could explain the clustered occurrence of cases. In this series, drug manufacturing was not a shared risk factor as each patient received carfilzomib from different lot numbers. Furthermore, other patients at our center received carfilzomib from the same batches without developing DITMA. We also considered the role of infection given that 1 patient was diagnosed with influenza A and both presented with nonspecific, viral-like symptoms during the winter season. Interestingly, concurrent viral infections have been reported in other cases of carfilzomib-induced DITMA as well and have also been discussed as a trigger of atypical HUS.20,29 Finally, genetic testing was negative for complement pathway mutations that might predispose to complement dysregulation.
The absence of complement mutations in our 2 patients differs from a recent series describing heterozygous CFHR3-CHFR1 deletions in association with carfilzomib-induced TMA.22 In that report, the authors hypothesized that carfilzomib decreases expression of complement factor H (CFH), a negative regulator of complement activation, thereby leading to complement dysregulation in patients who are genetically predisposed. In a second series, plasma from patients with DITMA secondary to carfilzomib induced the deposition of the complement complex, C5b-9, on endothelial cells in culture, suggesting activation of the complement pathway.30 The effective use of eculizumab would also point to a role for complement activation, and ongoing investigations should aim to identify the triggers and mechanisms of complement dysregulation in this setting, especially for patients like ours in whom genetic testing for complement pathway mutations is negative (Figure 2).
Conclusions
DITMA is a known risk of proteasome inhibitors and is listed as a safety warning in the prescribing information for bortezomib, carfilzomib, and ixazomib.12 Given the overall rarity of this adverse event, the simultaneous presentation of our 2 cases was unexpected and underscores the need for heightened awareness in clinical practice. In addition, while no underlying complement mutations were identified, eculizumab was used in both cases to successfully stabilize renal function. Further research investigating the efficacy of eculizumab and the role of complement activation in proteasome inhibitor–induced TMA will be valuable.
Acknowledgments
The authors would like to thank the patients whose histories are reported in this manuscript as well as the physicians and staff who provided care during the hospitalizations and beyond. We also thank Oscar Silva, MD, PhD, for his assistance in reviewing and formatting the peripheral blood smear images.
1. McBride A, Klaus JO, Stockeri-Goldstein K. Carfilzomib: a second-generation proteasome inhibitor for the treatment of multiple myeloma. Am J Health Syst Pharm. 2015;72(5):353-360. doi:10.2146/ajhp130281
2. Yui JC, Van Keer J, Weiss BM, et al. Proteasome inhibitor associated thrombotic microangiopathy. Am J Hematol. 2016;91(9):E348-E352. doi:10.1002/ajh.24447
3. Dimopoulos MA, Roussou M, Gavriatopoulou M, et al. Cardiac and renal complications of carfilzomib in patients with multiple myeloma. Blood Adv. 2017;1(7):449-454. doi:10.1182/bloodadvances.2016003269
4. Chari A, Stewart AK, Russell SD, et al. Analysis of carfilzomib cardiovascular safety profile across relapsed and/or refractory multiple myeloma clinical trials. Blood Adv. 2018;2(13):1633-1644. doi:10.1182/bloodadvances.2017015545
5. George JN, Nester CM. Syndromes of thrombotic microangiopathy. N Engl J Med. 2014;371(7):654-666. doi:10.1056/NEJMra1312353
6. Dimopoulos MA, Moreau P, Palumbo A, et al. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study. Lancet Oncol. 2016;17(1):27-38. doi:10.1016/S1470-2045(15)00464-7
7. Dimopoulos M, Quach H, Mateos MV, et al. Carfilzomib, dexamethasone, and daratumumab versus carfilzomib and dexamethasone for patients with relapsed or refractory multiple myeloma (CANDOR): results from a randomised, multicentre, open-label, phase 3 study. Lancet. 2020;396(10245):186-197. doi:10.1016/S0140-6736(20)30734-0
8. Camilleri M, Cuadrado M, Phillips E, et al. Thrombotic microangiopathy in untreated myeloma patients receiving carfilzomib, cyclophosphamide and dexamethasone on the CARDAMON study. Br J Haematol. 2021;193(4):750-760. doi:10.1111/bjh.17377
9. Masias C, Vasu S, Cataland SR. None of the above: thrombotic microangiopathy beyond TTP and HUS. Blood. 2017;129(21):2857-2863. doi:10.1182/blood-2016-11-743104
10. Al-Nouri ZL, Reese JA, Terrell DR, Vesely SK, George JN. Drug-induced thrombotic microangiopathy: a systemic review of published reports. Blood. 2015;125(4):616-618. doi:10.1182/blood-2014-11-611335
11. Saleem R, Reese JA, George JN. Drug-induced thrombotic-microangiopathy: an updated systematic review, 2014-2018. Am J Hematol. 2018;93(9):E241-E243. doi:10.1002/ajh.25208
12 Nguyen MN, Nayernama A, Jones SC, Kanapuru B, Gormley N, Waldron PE. Proteasome inhibitor-associated thrombotic microangiopathy: a review of cases reported to the FDA adverse event reporting system and published in the literature. Am J Hematol. 2020;95(9):E218-E222. doi:10.1002/ajh.25832
13. Haddadin M, Al-Sadawi M, Madanat S, et al. Late presentation of carfilzomib associated thrombotic microangiopathy. Am J Med Case Rep. 2019;7(10):240-243. doi:10.12691/ajmcr-7-10-5
14 Portuguese AJ, Gleber C, Passero Jr FC, Lipe B. A review of thrombotic microangiopathies in multiple myeloma. Leuk Res. 2019;85:106195. doi:10.1016/j.leukres.2019.106195
15. Legendre CM, Licht C, Muus P, et al. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med. 2013;368(23):2169-2181. doi:10.1056/NEJMoa1208981
16. Olson SR, Lu E, Sulpizio E, Shatzel JJ, Rueda JF, DeLoughery TG. When to stop eculizumab in complement-mediated thrombotic microangiopathies. Am J Nephrol. 2018;48(2):96-107. doi:10.1159/000492033
17. Lodhi A, Kumar A, Saqlain MU, Suneja M. Thrombotic microangiopathy associated with proteasome inhibitors. Clin Kidney J. 2015;8(5):632-636. doi:10.1093/ckj/sfv059
18. Sullivan MR, Danilov AV, Lansigan F, Dunbar NM. Carfilzomib associated thrombotic microangiopathy initially treated with therapeutic plasma exchange. J Clin Apher., 2015;30(5):308-310. doi:10.1002/jca.21371
19. Qaqish I, Schlam IM, Chakkera HA, Fonseca R, Adamski J. Carfilzomib: a cause of drug associated thrombotic microangiopathy. Transfus Apher Sci. 2016;54(3):401-404. doi:10.1016/j.transci.2016.03.002
20. Chen Y, Ooi M, Lim SF, et al. Thrombotic microangiopathy during carfilzomib use: case series in Singapore. Blood Cancer J. 2016;6(7):e450. doi:10.1038/bcj.2016.62
21. Gosain R, Gill A, Fuqua J, et al. Gemcitabine and carfilzomib induced thrombotic microangiopathy: eculizumab as a life-saving treatment. Clin Case Rep. 2017;5(12):1926-1930. doi:10.1002/ccr3.1214
22. Portuguese AJ, Lipe B. Carfilzomib-induced aHUS responds to early eculizumab and may be associated with heterozygrous CFHR3-CFHR1 deletion. Blood Adv. 2018;2(23):3443-3446. doi:10.1182/bloodadvances.2018027532
23. Moliz C, Gutiérrez E, Cavero T, Redondo B, Praga M. Eculizumab as a treatment for atypical hemolytic syndrome secondary to carfilzomib. Nefrologia (Engl Ed). 2019;39(1):86-88. doi:10.1016/j.nefro.2018.02.005
24. Jeyaraman P, Borah P, Singh A, et al., Thrombotic microangiopathy after carfilzomib in a very young myeloma patient. Blood Cells Mol Dis. 2020;81:102400. doi:10.1016/j.bcmd.2019.102400
25. Bhutani D, Assal A, Mapara MY, Prinzing S, Lentzsch S. Case report: carfilzomib-induced thrombotic microangiopathy with complement activation treated successfully with eculizumab. Clin Lymphoma Myeloma Leuk. 2020;20(4):e155-e157. doi:10.1016/j.clml.2020.01.016
26. Jindal N, Jandial A, Jain A, et al. Carfilzomib-induced thrombotic microangiopathy: a case based review. Hematol Oncol Stem Cell Ther. 2020;S1658-3876(20)30118-7. doi:10.1016/j.hemonc.2020.07.001
27. Monteith BE, Venner CP, Reece DE, et al. Drug-induced thrombotic microangiopathy with concurrent proteasome inhibitor use in the treatment of multiple myeloma: a case series and review of the literature. Clin Lymphoma Myeloma Leuk. 2020;20(11):e791-e780. doi:10.1016/j.clml.2020.04.014
28. Rassner M, Baur R, Wäsch R, et al. Two cases of carfilzomib-induced thrombotic microangiopathy successfully treated with eculizumab in multiple myeloma. BMC Nephrol. 2021;22(1):32. doi:10.1186/s12882-020-02226-5
29. Kavanagh D, Goodship THJ. Atypical hemolytic uremic syndrome, genetic basis, and clinical manifestations. Hematology Am Soc Hematol Educ Program. 2011;2011:15-20. doi:10.1182/asheducation-2011.1.15
30. Blasco M, Martínez-Roca A, Rodríguez-Lobato LG, et al. Complement as the enabler of carfilzomib-induced thrombotic microangiopathy. Br J Haematol. 2021;193(1):181-187. doi:10.1111/bjh.16796
1. McBride A, Klaus JO, Stockeri-Goldstein K. Carfilzomib: a second-generation proteasome inhibitor for the treatment of multiple myeloma. Am J Health Syst Pharm. 2015;72(5):353-360. doi:10.2146/ajhp130281
2. Yui JC, Van Keer J, Weiss BM, et al. Proteasome inhibitor associated thrombotic microangiopathy. Am J Hematol. 2016;91(9):E348-E352. doi:10.1002/ajh.24447
3. Dimopoulos MA, Roussou M, Gavriatopoulou M, et al. Cardiac and renal complications of carfilzomib in patients with multiple myeloma. Blood Adv. 2017;1(7):449-454. doi:10.1182/bloodadvances.2016003269
4. Chari A, Stewart AK, Russell SD, et al. Analysis of carfilzomib cardiovascular safety profile across relapsed and/or refractory multiple myeloma clinical trials. Blood Adv. 2018;2(13):1633-1644. doi:10.1182/bloodadvances.2017015545
5. George JN, Nester CM. Syndromes of thrombotic microangiopathy. N Engl J Med. 2014;371(7):654-666. doi:10.1056/NEJMra1312353
6. Dimopoulos MA, Moreau P, Palumbo A, et al. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study. Lancet Oncol. 2016;17(1):27-38. doi:10.1016/S1470-2045(15)00464-7
7. Dimopoulos M, Quach H, Mateos MV, et al. Carfilzomib, dexamethasone, and daratumumab versus carfilzomib and dexamethasone for patients with relapsed or refractory multiple myeloma (CANDOR): results from a randomised, multicentre, open-label, phase 3 study. Lancet. 2020;396(10245):186-197. doi:10.1016/S0140-6736(20)30734-0
8. Camilleri M, Cuadrado M, Phillips E, et al. Thrombotic microangiopathy in untreated myeloma patients receiving carfilzomib, cyclophosphamide and dexamethasone on the CARDAMON study. Br J Haematol. 2021;193(4):750-760. doi:10.1111/bjh.17377
9. Masias C, Vasu S, Cataland SR. None of the above: thrombotic microangiopathy beyond TTP and HUS. Blood. 2017;129(21):2857-2863. doi:10.1182/blood-2016-11-743104
10. Al-Nouri ZL, Reese JA, Terrell DR, Vesely SK, George JN. Drug-induced thrombotic microangiopathy: a systemic review of published reports. Blood. 2015;125(4):616-618. doi:10.1182/blood-2014-11-611335
11. Saleem R, Reese JA, George JN. Drug-induced thrombotic-microangiopathy: an updated systematic review, 2014-2018. Am J Hematol. 2018;93(9):E241-E243. doi:10.1002/ajh.25208
12 Nguyen MN, Nayernama A, Jones SC, Kanapuru B, Gormley N, Waldron PE. Proteasome inhibitor-associated thrombotic microangiopathy: a review of cases reported to the FDA adverse event reporting system and published in the literature. Am J Hematol. 2020;95(9):E218-E222. doi:10.1002/ajh.25832
13. Haddadin M, Al-Sadawi M, Madanat S, et al. Late presentation of carfilzomib associated thrombotic microangiopathy. Am J Med Case Rep. 2019;7(10):240-243. doi:10.12691/ajmcr-7-10-5
14 Portuguese AJ, Gleber C, Passero Jr FC, Lipe B. A review of thrombotic microangiopathies in multiple myeloma. Leuk Res. 2019;85:106195. doi:10.1016/j.leukres.2019.106195
15. Legendre CM, Licht C, Muus P, et al. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med. 2013;368(23):2169-2181. doi:10.1056/NEJMoa1208981
16. Olson SR, Lu E, Sulpizio E, Shatzel JJ, Rueda JF, DeLoughery TG. When to stop eculizumab in complement-mediated thrombotic microangiopathies. Am J Nephrol. 2018;48(2):96-107. doi:10.1159/000492033
17. Lodhi A, Kumar A, Saqlain MU, Suneja M. Thrombotic microangiopathy associated with proteasome inhibitors. Clin Kidney J. 2015;8(5):632-636. doi:10.1093/ckj/sfv059
18. Sullivan MR, Danilov AV, Lansigan F, Dunbar NM. Carfilzomib associated thrombotic microangiopathy initially treated with therapeutic plasma exchange. J Clin Apher., 2015;30(5):308-310. doi:10.1002/jca.21371
19. Qaqish I, Schlam IM, Chakkera HA, Fonseca R, Adamski J. Carfilzomib: a cause of drug associated thrombotic microangiopathy. Transfus Apher Sci. 2016;54(3):401-404. doi:10.1016/j.transci.2016.03.002
20. Chen Y, Ooi M, Lim SF, et al. Thrombotic microangiopathy during carfilzomib use: case series in Singapore. Blood Cancer J. 2016;6(7):e450. doi:10.1038/bcj.2016.62
21. Gosain R, Gill A, Fuqua J, et al. Gemcitabine and carfilzomib induced thrombotic microangiopathy: eculizumab as a life-saving treatment. Clin Case Rep. 2017;5(12):1926-1930. doi:10.1002/ccr3.1214
22. Portuguese AJ, Lipe B. Carfilzomib-induced aHUS responds to early eculizumab and may be associated with heterozygrous CFHR3-CFHR1 deletion. Blood Adv. 2018;2(23):3443-3446. doi:10.1182/bloodadvances.2018027532
23. Moliz C, Gutiérrez E, Cavero T, Redondo B, Praga M. Eculizumab as a treatment for atypical hemolytic syndrome secondary to carfilzomib. Nefrologia (Engl Ed). 2019;39(1):86-88. doi:10.1016/j.nefro.2018.02.005
24. Jeyaraman P, Borah P, Singh A, et al., Thrombotic microangiopathy after carfilzomib in a very young myeloma patient. Blood Cells Mol Dis. 2020;81:102400. doi:10.1016/j.bcmd.2019.102400
25. Bhutani D, Assal A, Mapara MY, Prinzing S, Lentzsch S. Case report: carfilzomib-induced thrombotic microangiopathy with complement activation treated successfully with eculizumab. Clin Lymphoma Myeloma Leuk. 2020;20(4):e155-e157. doi:10.1016/j.clml.2020.01.016
26. Jindal N, Jandial A, Jain A, et al. Carfilzomib-induced thrombotic microangiopathy: a case based review. Hematol Oncol Stem Cell Ther. 2020;S1658-3876(20)30118-7. doi:10.1016/j.hemonc.2020.07.001
27. Monteith BE, Venner CP, Reece DE, et al. Drug-induced thrombotic microangiopathy with concurrent proteasome inhibitor use in the treatment of multiple myeloma: a case series and review of the literature. Clin Lymphoma Myeloma Leuk. 2020;20(11):e791-e780. doi:10.1016/j.clml.2020.04.014
28. Rassner M, Baur R, Wäsch R, et al. Two cases of carfilzomib-induced thrombotic microangiopathy successfully treated with eculizumab in multiple myeloma. BMC Nephrol. 2021;22(1):32. doi:10.1186/s12882-020-02226-5
29. Kavanagh D, Goodship THJ. Atypical hemolytic uremic syndrome, genetic basis, and clinical manifestations. Hematology Am Soc Hematol Educ Program. 2011;2011:15-20. doi:10.1182/asheducation-2011.1.15
30. Blasco M, Martínez-Roca A, Rodríguez-Lobato LG, et al. Complement as the enabler of carfilzomib-induced thrombotic microangiopathy. Br J Haematol. 2021;193(1):181-187. doi:10.1111/bjh.16796
‘Unexpected’: Breast cancer spreads most during sleep
a discovery the investigators called “striking and unexpected.”
“This has not been shown before [and] we were surprised, indeed,” Nicola Aceto, PhD, professor of molecular oncology, Swiss Federal Institute of Technology Zürich, said in an interview.
The findings carry potential implications for the timing of biopsy and treatment of metastasis-prone cancers, the authors said.
The study was published online in Nature.
Circulating tumor cells (CTCs) are generally believed to shed constantly or following particular events such as surgery or physical activity; however, the processes that regulate tumor cell metastasis and how circadian rhythms may play into tumorigenesis remain unclear.
To better understand these processes, Dr. Aceto and colleagues collected blood samples from 30 women with breast cancer at 4:00 a.m. and 10:00 a.m. – times representing the body’s resting and active phases, respectively.
The researchers observed that more than 78% of all CTCs obtained were from samples taken during the resting phase.
This finding is astounding, Harrison Ball, a PhD candidate, and Sunitha Nagrath, PhD, with the University of Michigan, Ann Arbor, wrote in Nature News & Views .
Dr. Aceto and colleagues also found that CTCs generated at night divide more quickly and therefore have a higher potential to metastasize, compared with those generated during the day, which “are devoid of metastatic ability,” according to the authors, who obtained similar results in a series of mouse models.
The team also observed that key circadian rhythm hormones (such as melatonin, testosterone, and glucocorticoids) regulate CTC generation, and insulin promotes tumor cell proliferation in a time-dependent manner, suggesting a “need for time-controlled approaches for the characterization and treatment of breast cancer,” the authors wrote.
Practice changing?
Dr. Ball and Dr. Nagrath said the time-dependent nature of CTC dynamics could very well transform how cancer patients are assessed and treated.
“The data pointing to CTC proliferation and release during the rest phase suggest that doctors might need to become more conscious of when to administer specific treatments,” they wrote.
Both cautioned, however, that large clinical trials would be needed before any consideration of circadian rhythms is incorporated into standard practice. It’s also unclear whether these results in breast cancer hold true for other tumor types.
Mariana G. Figueiro, PhD, who was not involved in the research, agreed that, if studies confirm more metastatic spread at night, “there is an opportunity to treat patients at strategic times.”
Dr. Figueiro, of the Icahn School of Medicine at Mount Sinai, New York, also saw a potential impact on the timing of blood draws. “I think tightening up on how people do biopsies and bloodwork based on circadian time is important.”
Marleen Meyers, MD, agreed that these findings could have many clinical implications.
“The most obvious is that the time of day [that] treatment is administered may influence efficacy,” said Dr. Meyers, clinical professor of medicine at New York University Langone’s Perlmutter Cancer Center.
But, Dr. Meyers noted, the benefits of treating someone at night would need to be weighed against the downsides of interrupting a person’s normal sleep-wake cycle. “If this finding is clinically important it will be a challenge incorporating this into clinical care,” she said.
The study had no funding reported. Dr. Aceto is a cofounder and member of the board of PAGE Therapeutics, listed as an inventor in patent applications related to circulating tumor cells, a paid consultant for several companies, and a Novartis shareholder. One coauthor is a cofounder of PAGE Therapeutics. All other authors declare no competing interests. Dr. Meyers and Dr. Figueiro reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a discovery the investigators called “striking and unexpected.”
“This has not been shown before [and] we were surprised, indeed,” Nicola Aceto, PhD, professor of molecular oncology, Swiss Federal Institute of Technology Zürich, said in an interview.
The findings carry potential implications for the timing of biopsy and treatment of metastasis-prone cancers, the authors said.
The study was published online in Nature.
Circulating tumor cells (CTCs) are generally believed to shed constantly or following particular events such as surgery or physical activity; however, the processes that regulate tumor cell metastasis and how circadian rhythms may play into tumorigenesis remain unclear.
To better understand these processes, Dr. Aceto and colleagues collected blood samples from 30 women with breast cancer at 4:00 a.m. and 10:00 a.m. – times representing the body’s resting and active phases, respectively.
The researchers observed that more than 78% of all CTCs obtained were from samples taken during the resting phase.
This finding is astounding, Harrison Ball, a PhD candidate, and Sunitha Nagrath, PhD, with the University of Michigan, Ann Arbor, wrote in Nature News & Views .
Dr. Aceto and colleagues also found that CTCs generated at night divide more quickly and therefore have a higher potential to metastasize, compared with those generated during the day, which “are devoid of metastatic ability,” according to the authors, who obtained similar results in a series of mouse models.
The team also observed that key circadian rhythm hormones (such as melatonin, testosterone, and glucocorticoids) regulate CTC generation, and insulin promotes tumor cell proliferation in a time-dependent manner, suggesting a “need for time-controlled approaches for the characterization and treatment of breast cancer,” the authors wrote.
Practice changing?
Dr. Ball and Dr. Nagrath said the time-dependent nature of CTC dynamics could very well transform how cancer patients are assessed and treated.
“The data pointing to CTC proliferation and release during the rest phase suggest that doctors might need to become more conscious of when to administer specific treatments,” they wrote.
Both cautioned, however, that large clinical trials would be needed before any consideration of circadian rhythms is incorporated into standard practice. It’s also unclear whether these results in breast cancer hold true for other tumor types.
Mariana G. Figueiro, PhD, who was not involved in the research, agreed that, if studies confirm more metastatic spread at night, “there is an opportunity to treat patients at strategic times.”
Dr. Figueiro, of the Icahn School of Medicine at Mount Sinai, New York, also saw a potential impact on the timing of blood draws. “I think tightening up on how people do biopsies and bloodwork based on circadian time is important.”
Marleen Meyers, MD, agreed that these findings could have many clinical implications.
“The most obvious is that the time of day [that] treatment is administered may influence efficacy,” said Dr. Meyers, clinical professor of medicine at New York University Langone’s Perlmutter Cancer Center.
But, Dr. Meyers noted, the benefits of treating someone at night would need to be weighed against the downsides of interrupting a person’s normal sleep-wake cycle. “If this finding is clinically important it will be a challenge incorporating this into clinical care,” she said.
The study had no funding reported. Dr. Aceto is a cofounder and member of the board of PAGE Therapeutics, listed as an inventor in patent applications related to circulating tumor cells, a paid consultant for several companies, and a Novartis shareholder. One coauthor is a cofounder of PAGE Therapeutics. All other authors declare no competing interests. Dr. Meyers and Dr. Figueiro reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a discovery the investigators called “striking and unexpected.”
“This has not been shown before [and] we were surprised, indeed,” Nicola Aceto, PhD, professor of molecular oncology, Swiss Federal Institute of Technology Zürich, said in an interview.
The findings carry potential implications for the timing of biopsy and treatment of metastasis-prone cancers, the authors said.
The study was published online in Nature.
Circulating tumor cells (CTCs) are generally believed to shed constantly or following particular events such as surgery or physical activity; however, the processes that regulate tumor cell metastasis and how circadian rhythms may play into tumorigenesis remain unclear.
To better understand these processes, Dr. Aceto and colleagues collected blood samples from 30 women with breast cancer at 4:00 a.m. and 10:00 a.m. – times representing the body’s resting and active phases, respectively.
The researchers observed that more than 78% of all CTCs obtained were from samples taken during the resting phase.
This finding is astounding, Harrison Ball, a PhD candidate, and Sunitha Nagrath, PhD, with the University of Michigan, Ann Arbor, wrote in Nature News & Views .
Dr. Aceto and colleagues also found that CTCs generated at night divide more quickly and therefore have a higher potential to metastasize, compared with those generated during the day, which “are devoid of metastatic ability,” according to the authors, who obtained similar results in a series of mouse models.
The team also observed that key circadian rhythm hormones (such as melatonin, testosterone, and glucocorticoids) regulate CTC generation, and insulin promotes tumor cell proliferation in a time-dependent manner, suggesting a “need for time-controlled approaches for the characterization and treatment of breast cancer,” the authors wrote.
Practice changing?
Dr. Ball and Dr. Nagrath said the time-dependent nature of CTC dynamics could very well transform how cancer patients are assessed and treated.
“The data pointing to CTC proliferation and release during the rest phase suggest that doctors might need to become more conscious of when to administer specific treatments,” they wrote.
Both cautioned, however, that large clinical trials would be needed before any consideration of circadian rhythms is incorporated into standard practice. It’s also unclear whether these results in breast cancer hold true for other tumor types.
Mariana G. Figueiro, PhD, who was not involved in the research, agreed that, if studies confirm more metastatic spread at night, “there is an opportunity to treat patients at strategic times.”
Dr. Figueiro, of the Icahn School of Medicine at Mount Sinai, New York, also saw a potential impact on the timing of blood draws. “I think tightening up on how people do biopsies and bloodwork based on circadian time is important.”
Marleen Meyers, MD, agreed that these findings could have many clinical implications.
“The most obvious is that the time of day [that] treatment is administered may influence efficacy,” said Dr. Meyers, clinical professor of medicine at New York University Langone’s Perlmutter Cancer Center.
But, Dr. Meyers noted, the benefits of treating someone at night would need to be weighed against the downsides of interrupting a person’s normal sleep-wake cycle. “If this finding is clinically important it will be a challenge incorporating this into clinical care,” she said.
The study had no funding reported. Dr. Aceto is a cofounder and member of the board of PAGE Therapeutics, listed as an inventor in patent applications related to circulating tumor cells, a paid consultant for several companies, and a Novartis shareholder. One coauthor is a cofounder of PAGE Therapeutics. All other authors declare no competing interests. Dr. Meyers and Dr. Figueiro reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NATURE
Hydroxychloroquine risk found in some older patients with RA
Hydroxychloroquine should be initiated with caution in older patients with rheumatoid arthritis who also have heart failure or are at risk for it, say the authors of a study suggesting that the drug could increase their risk for major adverse cardiovascular events (MACE), compared with methotrexate.
A cohort study published online in the Journal of the American College of Cardiology looked at outcomes in 54,462 patients with RA aged 65 years or older and not previously treated with disease-modifying antirheumatic drugs. Half were initiated on methotrexate and half on hydroxychloroquine, making 27,231 propensity-matched pairs.
Across the entire cohort, hydroxychloroquine was not associated with a higher risk for sudden cardiac arrest, ventricular arrhythmia, or MACE, compared with methotrexate. When broken down into individual cardiovascular events, the data suggested a statistically significant 17% increase in the risk for cardiovascular mortality and 10% increase in all-cause mortality with hydroxychloroquine, although there were no differences in the risks for myocardial infarction or stroke.
However, a subgroup analysis revealed a significant 30% increase in the risk for MACE among patients starting hydroxychloroquine who also had a history of heart failure, compared with patients taking methotrexate. The researchers found no difference between the two drugs in patients without a history of heart failure. The study also suggested an overall 41% increase in the risk for hospitalization with heart failure with hydroxychloroquine, regardless of heart failure history.
Hydroxychloroquine was also associated with a 34% increase in the risk for cardiovascular mortality, a 22% increase in the risk for all-cause mortality, and a 74% increase in the risk for MI.
The lead author of the study, Elvira D’Andrea, MD, PhD, of Brigham and Women’s Hospital and Harvard Medical School in Boston, said that hydroxychloroquine is used as a first-line treatment for RA, but there was limited evidence on its cardiovascular risks. The pandemic in particular shined a spotlight on these concerns and prompted the researchers to extend their original prepandemic study to encompass additional cardiovascular outcomes.
“The emerging concerns on its cardiovascular safety in early 2020 has led the rheumatological community, and patients regularly taking hydroxychloroquine for rheumatoid arthritis, to confusion,” Dr. D’Andrea said in an interview.
She advised that clinicians be cautious when initiating hydroxychloroquine in older patients with existing heart failure or who have risk factors for it. “Although heart failure is a known concern for hydroxychloroquine use, these findings helped to clarify the relationship between the use of hydroxychloroquine or methotrexate and heart failure. Clinicians should pay careful attention to clinical manifestations of cardiomyopathy or heart failure in older patients with rheumatoid arthritis treated with hydroxychloroquine.”
Hydroxychloroquine is associated with cardiotoxicity, particularly cardiomyopathy, which may help precipitate MACE or heart failure exacerbations in patients who already have deterioration of their cardiac tissue, the authors suggested.
Short follow-up period leaves risk attribution under question
In an accompanying editorial, Elizabeth Blair Solow, MD, and Bonnie L. Bermas, MD, of the University of Texas Southwestern Medical Center, Dallas, commented that the lack of an increased risk for arrhythmic events or MACE in the overall cohort taking hydroxychloroquine was reassuring. They also suggested the subgroup analysis findings among patients with preexisting heart failure were still “exploratory and hypothesis-generating” and should be interpreted with caution.
They noted that the follow-up time of the study was relatively short – a median of 209 days – given that hydroxychloroquine does not reach a steady-state level for 6 months.
“Evidence to date suggests cardiomyopathy from HCQ [hydroxychloroquine] takes years to develop, many months beyond the exposures described here, bringing into question as to whether HCQ itself increased HF hospitalizations,” the editorialists wrote.
The editorial also raised the question of whether the association observed in the study was related to a possible cardioprotective effect of methotrexate, given that previous studies have suggested this effect in older patients with RA.
The study authors did an exploratory analysis comparing hydroxychloroquine with sulfasalazine, which appeared to support their main findings of a possible cardiovascular effect of hydroxychloroquine. However, they qualified this by pointing out that the analysis involved small numbers of patients.
Senior investigator Seoyoung C. Kim, MD, ScD, of Brigham and Women’s Hospital and Harvard Medical School, both in Boston, also noted that the study only looked at outcomes in patients aged 65 years and older.
“It would be clinically important to further examine the cardiovascular safety of hydroxychloroquine versus methotrexate in a younger population with rheumatic conditions,” she said.
The study was supported by the National Institutes of Health, Brigham and Women’s Hospital, and Harvard Medical School. Four authors declared unrelated research grants from the pharmaceutical sector, with one also declaring stock options and consulting work with the pharmaceutical sector. No other conflicts of interest were declared.
A version of this article first appeared on Medscape.com.
Hydroxychloroquine should be initiated with caution in older patients with rheumatoid arthritis who also have heart failure or are at risk for it, say the authors of a study suggesting that the drug could increase their risk for major adverse cardiovascular events (MACE), compared with methotrexate.
A cohort study published online in the Journal of the American College of Cardiology looked at outcomes in 54,462 patients with RA aged 65 years or older and not previously treated with disease-modifying antirheumatic drugs. Half were initiated on methotrexate and half on hydroxychloroquine, making 27,231 propensity-matched pairs.
Across the entire cohort, hydroxychloroquine was not associated with a higher risk for sudden cardiac arrest, ventricular arrhythmia, or MACE, compared with methotrexate. When broken down into individual cardiovascular events, the data suggested a statistically significant 17% increase in the risk for cardiovascular mortality and 10% increase in all-cause mortality with hydroxychloroquine, although there were no differences in the risks for myocardial infarction or stroke.
However, a subgroup analysis revealed a significant 30% increase in the risk for MACE among patients starting hydroxychloroquine who also had a history of heart failure, compared with patients taking methotrexate. The researchers found no difference between the two drugs in patients without a history of heart failure. The study also suggested an overall 41% increase in the risk for hospitalization with heart failure with hydroxychloroquine, regardless of heart failure history.
Hydroxychloroquine was also associated with a 34% increase in the risk for cardiovascular mortality, a 22% increase in the risk for all-cause mortality, and a 74% increase in the risk for MI.
The lead author of the study, Elvira D’Andrea, MD, PhD, of Brigham and Women’s Hospital and Harvard Medical School in Boston, said that hydroxychloroquine is used as a first-line treatment for RA, but there was limited evidence on its cardiovascular risks. The pandemic in particular shined a spotlight on these concerns and prompted the researchers to extend their original prepandemic study to encompass additional cardiovascular outcomes.
“The emerging concerns on its cardiovascular safety in early 2020 has led the rheumatological community, and patients regularly taking hydroxychloroquine for rheumatoid arthritis, to confusion,” Dr. D’Andrea said in an interview.
She advised that clinicians be cautious when initiating hydroxychloroquine in older patients with existing heart failure or who have risk factors for it. “Although heart failure is a known concern for hydroxychloroquine use, these findings helped to clarify the relationship between the use of hydroxychloroquine or methotrexate and heart failure. Clinicians should pay careful attention to clinical manifestations of cardiomyopathy or heart failure in older patients with rheumatoid arthritis treated with hydroxychloroquine.”
Hydroxychloroquine is associated with cardiotoxicity, particularly cardiomyopathy, which may help precipitate MACE or heart failure exacerbations in patients who already have deterioration of their cardiac tissue, the authors suggested.
Short follow-up period leaves risk attribution under question
In an accompanying editorial, Elizabeth Blair Solow, MD, and Bonnie L. Bermas, MD, of the University of Texas Southwestern Medical Center, Dallas, commented that the lack of an increased risk for arrhythmic events or MACE in the overall cohort taking hydroxychloroquine was reassuring. They also suggested the subgroup analysis findings among patients with preexisting heart failure were still “exploratory and hypothesis-generating” and should be interpreted with caution.
They noted that the follow-up time of the study was relatively short – a median of 209 days – given that hydroxychloroquine does not reach a steady-state level for 6 months.
“Evidence to date suggests cardiomyopathy from HCQ [hydroxychloroquine] takes years to develop, many months beyond the exposures described here, bringing into question as to whether HCQ itself increased HF hospitalizations,” the editorialists wrote.
The editorial also raised the question of whether the association observed in the study was related to a possible cardioprotective effect of methotrexate, given that previous studies have suggested this effect in older patients with RA.
The study authors did an exploratory analysis comparing hydroxychloroquine with sulfasalazine, which appeared to support their main findings of a possible cardiovascular effect of hydroxychloroquine. However, they qualified this by pointing out that the analysis involved small numbers of patients.
Senior investigator Seoyoung C. Kim, MD, ScD, of Brigham and Women’s Hospital and Harvard Medical School, both in Boston, also noted that the study only looked at outcomes in patients aged 65 years and older.
“It would be clinically important to further examine the cardiovascular safety of hydroxychloroquine versus methotrexate in a younger population with rheumatic conditions,” she said.
The study was supported by the National Institutes of Health, Brigham and Women’s Hospital, and Harvard Medical School. Four authors declared unrelated research grants from the pharmaceutical sector, with one also declaring stock options and consulting work with the pharmaceutical sector. No other conflicts of interest were declared.
A version of this article first appeared on Medscape.com.
Hydroxychloroquine should be initiated with caution in older patients with rheumatoid arthritis who also have heart failure or are at risk for it, say the authors of a study suggesting that the drug could increase their risk for major adverse cardiovascular events (MACE), compared with methotrexate.
A cohort study published online in the Journal of the American College of Cardiology looked at outcomes in 54,462 patients with RA aged 65 years or older and not previously treated with disease-modifying antirheumatic drugs. Half were initiated on methotrexate and half on hydroxychloroquine, making 27,231 propensity-matched pairs.
Across the entire cohort, hydroxychloroquine was not associated with a higher risk for sudden cardiac arrest, ventricular arrhythmia, or MACE, compared with methotrexate. When broken down into individual cardiovascular events, the data suggested a statistically significant 17% increase in the risk for cardiovascular mortality and 10% increase in all-cause mortality with hydroxychloroquine, although there were no differences in the risks for myocardial infarction or stroke.
However, a subgroup analysis revealed a significant 30% increase in the risk for MACE among patients starting hydroxychloroquine who also had a history of heart failure, compared with patients taking methotrexate. The researchers found no difference between the two drugs in patients without a history of heart failure. The study also suggested an overall 41% increase in the risk for hospitalization with heart failure with hydroxychloroquine, regardless of heart failure history.
Hydroxychloroquine was also associated with a 34% increase in the risk for cardiovascular mortality, a 22% increase in the risk for all-cause mortality, and a 74% increase in the risk for MI.
The lead author of the study, Elvira D’Andrea, MD, PhD, of Brigham and Women’s Hospital and Harvard Medical School in Boston, said that hydroxychloroquine is used as a first-line treatment for RA, but there was limited evidence on its cardiovascular risks. The pandemic in particular shined a spotlight on these concerns and prompted the researchers to extend their original prepandemic study to encompass additional cardiovascular outcomes.
“The emerging concerns on its cardiovascular safety in early 2020 has led the rheumatological community, and patients regularly taking hydroxychloroquine for rheumatoid arthritis, to confusion,” Dr. D’Andrea said in an interview.
She advised that clinicians be cautious when initiating hydroxychloroquine in older patients with existing heart failure or who have risk factors for it. “Although heart failure is a known concern for hydroxychloroquine use, these findings helped to clarify the relationship between the use of hydroxychloroquine or methotrexate and heart failure. Clinicians should pay careful attention to clinical manifestations of cardiomyopathy or heart failure in older patients with rheumatoid arthritis treated with hydroxychloroquine.”
Hydroxychloroquine is associated with cardiotoxicity, particularly cardiomyopathy, which may help precipitate MACE or heart failure exacerbations in patients who already have deterioration of their cardiac tissue, the authors suggested.
Short follow-up period leaves risk attribution under question
In an accompanying editorial, Elizabeth Blair Solow, MD, and Bonnie L. Bermas, MD, of the University of Texas Southwestern Medical Center, Dallas, commented that the lack of an increased risk for arrhythmic events or MACE in the overall cohort taking hydroxychloroquine was reassuring. They also suggested the subgroup analysis findings among patients with preexisting heart failure were still “exploratory and hypothesis-generating” and should be interpreted with caution.
They noted that the follow-up time of the study was relatively short – a median of 209 days – given that hydroxychloroquine does not reach a steady-state level for 6 months.
“Evidence to date suggests cardiomyopathy from HCQ [hydroxychloroquine] takes years to develop, many months beyond the exposures described here, bringing into question as to whether HCQ itself increased HF hospitalizations,” the editorialists wrote.
The editorial also raised the question of whether the association observed in the study was related to a possible cardioprotective effect of methotrexate, given that previous studies have suggested this effect in older patients with RA.
The study authors did an exploratory analysis comparing hydroxychloroquine with sulfasalazine, which appeared to support their main findings of a possible cardiovascular effect of hydroxychloroquine. However, they qualified this by pointing out that the analysis involved small numbers of patients.
Senior investigator Seoyoung C. Kim, MD, ScD, of Brigham and Women’s Hospital and Harvard Medical School, both in Boston, also noted that the study only looked at outcomes in patients aged 65 years and older.
“It would be clinically important to further examine the cardiovascular safety of hydroxychloroquine versus methotrexate in a younger population with rheumatic conditions,” she said.
The study was supported by the National Institutes of Health, Brigham and Women’s Hospital, and Harvard Medical School. Four authors declared unrelated research grants from the pharmaceutical sector, with one also declaring stock options and consulting work with the pharmaceutical sector. No other conflicts of interest were declared.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Ob.gyns. on the day that Roe v. Wade was overturned
“I’m happy to contribute, but can you keep it anonymous? It’s a safety concern for me.”
On the day that the Supreme Court of the United States voted to strike down Roe v. Wade, I reached out to ob.gyn.s across the country, wanting to hear their reactions. My own response, like that of many doctors and women, was a visceral mix of anger, fear, and grief. I could only begin to imagine what the real experts on reproductive health care were going through.
When the first ob.gyn. responded to my request by expressing concerns around anonymity and personal safety, I was shocked – but I shouldn’t have been. For starters, there is already a storied history in this country of deadly attacks on abortion providers. David Gunn, MD; Barnett Slepian, MD; and George Tiller, MD, were all tragically murdered by antiabortion extremists. Then, there’s the existence of websites that keep logs of abortion providers and sometimes include photos, office contact information, or even home addresses.
The idea that any reproductive health care provider should have to think twice before offering their uniquely qualified opinion is profoundly disturbing, nearly as disturbing as the Supreme Court’s decision itself. But it’s more critical than ever for ob.gyn. voices to be amplified. This is the time for the healthcare community to rally around women’s health providers, to learn from them, to support them.
I asked ob.gyns. around the country to tell me what they were thinking and feeling on the day that Roe v. Wade was overturned. We agreed to keep the responses anonymous, given that several people expressed very understandable safety concerns.
Here’s what they had to say.
Tennessee ob.gyn.
“Today is an emotionally charged day for many people in this country, yet as I type this, with my ob.gyn. practice continuing around me, with my own almost 10-week pregnancy growing inside me, I feel quite blunted. I feel powerless to answer questions that are variations on ‘what next?’ or ‘how do we fight back?’ All I can think of is, I am so glad I do not have anyone on my schedule right now who does not want to be pregnant. But what will happen when that eventually changes? What about my colleagues who do have these patients on their schedules today? On a personal level, what if my prenatal genetic testing comes back abnormal? How can we so blatantly disregard a separation of church and state in this country? What ways will our government interfere with my practice next? My head is spinning, but I have to go see my next patient. She is a 25-year-old who is here to have an IUD placed, and that seems like the most important thing I can do today.”
South Carolina ob.gyn.
“I’m really scared. For my patients and for myself. I don’t know how to be a good ob.gyn. if my ability to offer safe and accessible abortion care is being threatened.”
Massachusetts ob.gyn.
“Livid and devastated and sad and terrified.”
California family planning specialist
“The fact is that about one in four people with uteruses have had an abortion. I can’t tell you how many abortions I’ve provided for people who say that they don’t ‘believe’ in them or that they thought they’d never be in this situation. ... The fact is that pregnancy is a life-threatening condition in and of itself. I am an ob.gyn., a medical doctor, and an abortion provider. I will not stop providing abortions or helping people access them. I will dedicate my life to ensuring this right to bodily autonomy. Today I am devastated by the Supreme Court’s decision to force parenthood that will result in increased maternal mortality. I am broken, but I have never been more proud to be an abortion provider.”
New York ob.gyn.
“Grateful to live in a state and work for a hospital where I can provide abortions but feel terrible for so many people less fortunate and underserved.”
Illinois maternal-fetal medicine specialist
“As a maternal-fetal medicine specialist, I fear for my patients who are at the highest risk of pregnancy complications having their freedom taken away. For the tragic ultrasound findings that make a pregnant person carry a baby who will never live. For the patients who cannot use most forms of contraception because of their medical comorbidities. For the patients who are victims of intimate partner violence or under the influence of their culture, to continue having children regardless of their desires or their health. ... The freedom to prevent or end a pregnancy has enabled women to become independent and productive members of society on their own terms, with or without children. My heart breaks for the children and adolescents and adults who are being told they are second-class citizens, not worthy of making their own decisions. Politicians and Supreme Court justices are not in the clinic room, ultrasound suite, operating room, or delivery room when we have these intense conversations and pregnancy outcomes. They have no idea that of which they speak, and it’s unconscionable that they can determine what healthcare decisions my patients can make for their own lives. Nobody knows a body better than the patient themselves.”
Texas ob.gyn.
“In the area where I live and practice, it feels like guns and the people who use them have more legal rights than people with uteruses in desperate or life-threatening situations. I’m afraid for my personal safety as a women’s health practitioner in this political climate. I feel helpless, but I’m supposed to be able to help my patients.”
Missouri family planning specialist
“Abortion is an essential part of healthcare, and the only people that should get a say in it are the patient and their doctor. Period. The fact that some far-off court without any medical expertise can insert itself into individual medical decisions is oppressive and unethical.”
Georgia ob.gyn.
“I can’t even think straight right now. I feel sick. Honestly, I’ve been thinking about moving for a long time now. Somewhere where I would actually be able to offer good, comprehensive care.”
New York ob.gyn.
“I graduated from my ob.gyn. residency hours after the Roe v. Wade news broke. It was so emotional for me. I’ve dedicated my life to caring for people with uteruses and I will not let this heartbreaking news change that. I feel more committed than ever to women’s health. I fully plan to continue delivering babies, providing contraception, and performing abortions. I will be there to help women with desired pregnancies who received unspeakably bad news about fetal anomalies. I will be there to help women with life-threatening pregnancy complications before fetal viability. I will be there to help women with ectopic pregnancies. I will be there to help women who were raped or otherwise forced into pregnancy. I will always be there to help women.”
Dr. Croll is a neurovascular fellow at New York University Langone Health. She disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
“I’m happy to contribute, but can you keep it anonymous? It’s a safety concern for me.”
On the day that the Supreme Court of the United States voted to strike down Roe v. Wade, I reached out to ob.gyn.s across the country, wanting to hear their reactions. My own response, like that of many doctors and women, was a visceral mix of anger, fear, and grief. I could only begin to imagine what the real experts on reproductive health care were going through.
When the first ob.gyn. responded to my request by expressing concerns around anonymity and personal safety, I was shocked – but I shouldn’t have been. For starters, there is already a storied history in this country of deadly attacks on abortion providers. David Gunn, MD; Barnett Slepian, MD; and George Tiller, MD, were all tragically murdered by antiabortion extremists. Then, there’s the existence of websites that keep logs of abortion providers and sometimes include photos, office contact information, or even home addresses.
The idea that any reproductive health care provider should have to think twice before offering their uniquely qualified opinion is profoundly disturbing, nearly as disturbing as the Supreme Court’s decision itself. But it’s more critical than ever for ob.gyn. voices to be amplified. This is the time for the healthcare community to rally around women’s health providers, to learn from them, to support them.
I asked ob.gyns. around the country to tell me what they were thinking and feeling on the day that Roe v. Wade was overturned. We agreed to keep the responses anonymous, given that several people expressed very understandable safety concerns.
Here’s what they had to say.
Tennessee ob.gyn.
“Today is an emotionally charged day for many people in this country, yet as I type this, with my ob.gyn. practice continuing around me, with my own almost 10-week pregnancy growing inside me, I feel quite blunted. I feel powerless to answer questions that are variations on ‘what next?’ or ‘how do we fight back?’ All I can think of is, I am so glad I do not have anyone on my schedule right now who does not want to be pregnant. But what will happen when that eventually changes? What about my colleagues who do have these patients on their schedules today? On a personal level, what if my prenatal genetic testing comes back abnormal? How can we so blatantly disregard a separation of church and state in this country? What ways will our government interfere with my practice next? My head is spinning, but I have to go see my next patient. She is a 25-year-old who is here to have an IUD placed, and that seems like the most important thing I can do today.”
South Carolina ob.gyn.
“I’m really scared. For my patients and for myself. I don’t know how to be a good ob.gyn. if my ability to offer safe and accessible abortion care is being threatened.”
Massachusetts ob.gyn.
“Livid and devastated and sad and terrified.”
California family planning specialist
“The fact is that about one in four people with uteruses have had an abortion. I can’t tell you how many abortions I’ve provided for people who say that they don’t ‘believe’ in them or that they thought they’d never be in this situation. ... The fact is that pregnancy is a life-threatening condition in and of itself. I am an ob.gyn., a medical doctor, and an abortion provider. I will not stop providing abortions or helping people access them. I will dedicate my life to ensuring this right to bodily autonomy. Today I am devastated by the Supreme Court’s decision to force parenthood that will result in increased maternal mortality. I am broken, but I have never been more proud to be an abortion provider.”
New York ob.gyn.
“Grateful to live in a state and work for a hospital where I can provide abortions but feel terrible for so many people less fortunate and underserved.”
Illinois maternal-fetal medicine specialist
“As a maternal-fetal medicine specialist, I fear for my patients who are at the highest risk of pregnancy complications having their freedom taken away. For the tragic ultrasound findings that make a pregnant person carry a baby who will never live. For the patients who cannot use most forms of contraception because of their medical comorbidities. For the patients who are victims of intimate partner violence or under the influence of their culture, to continue having children regardless of their desires or their health. ... The freedom to prevent or end a pregnancy has enabled women to become independent and productive members of society on their own terms, with or without children. My heart breaks for the children and adolescents and adults who are being told they are second-class citizens, not worthy of making their own decisions. Politicians and Supreme Court justices are not in the clinic room, ultrasound suite, operating room, or delivery room when we have these intense conversations and pregnancy outcomes. They have no idea that of which they speak, and it’s unconscionable that they can determine what healthcare decisions my patients can make for their own lives. Nobody knows a body better than the patient themselves.”
Texas ob.gyn.
“In the area where I live and practice, it feels like guns and the people who use them have more legal rights than people with uteruses in desperate or life-threatening situations. I’m afraid for my personal safety as a women’s health practitioner in this political climate. I feel helpless, but I’m supposed to be able to help my patients.”
Missouri family planning specialist
“Abortion is an essential part of healthcare, and the only people that should get a say in it are the patient and their doctor. Period. The fact that some far-off court without any medical expertise can insert itself into individual medical decisions is oppressive and unethical.”
Georgia ob.gyn.
“I can’t even think straight right now. I feel sick. Honestly, I’ve been thinking about moving for a long time now. Somewhere where I would actually be able to offer good, comprehensive care.”
New York ob.gyn.
“I graduated from my ob.gyn. residency hours after the Roe v. Wade news broke. It was so emotional for me. I’ve dedicated my life to caring for people with uteruses and I will not let this heartbreaking news change that. I feel more committed than ever to women’s health. I fully plan to continue delivering babies, providing contraception, and performing abortions. I will be there to help women with desired pregnancies who received unspeakably bad news about fetal anomalies. I will be there to help women with life-threatening pregnancy complications before fetal viability. I will be there to help women with ectopic pregnancies. I will be there to help women who were raped or otherwise forced into pregnancy. I will always be there to help women.”
Dr. Croll is a neurovascular fellow at New York University Langone Health. She disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
“I’m happy to contribute, but can you keep it anonymous? It’s a safety concern for me.”
On the day that the Supreme Court of the United States voted to strike down Roe v. Wade, I reached out to ob.gyn.s across the country, wanting to hear their reactions. My own response, like that of many doctors and women, was a visceral mix of anger, fear, and grief. I could only begin to imagine what the real experts on reproductive health care were going through.
When the first ob.gyn. responded to my request by expressing concerns around anonymity and personal safety, I was shocked – but I shouldn’t have been. For starters, there is already a storied history in this country of deadly attacks on abortion providers. David Gunn, MD; Barnett Slepian, MD; and George Tiller, MD, were all tragically murdered by antiabortion extremists. Then, there’s the existence of websites that keep logs of abortion providers and sometimes include photos, office contact information, or even home addresses.
The idea that any reproductive health care provider should have to think twice before offering their uniquely qualified opinion is profoundly disturbing, nearly as disturbing as the Supreme Court’s decision itself. But it’s more critical than ever for ob.gyn. voices to be amplified. This is the time for the healthcare community to rally around women’s health providers, to learn from them, to support them.
I asked ob.gyns. around the country to tell me what they were thinking and feeling on the day that Roe v. Wade was overturned. We agreed to keep the responses anonymous, given that several people expressed very understandable safety concerns.
Here’s what they had to say.
Tennessee ob.gyn.
“Today is an emotionally charged day for many people in this country, yet as I type this, with my ob.gyn. practice continuing around me, with my own almost 10-week pregnancy growing inside me, I feel quite blunted. I feel powerless to answer questions that are variations on ‘what next?’ or ‘how do we fight back?’ All I can think of is, I am so glad I do not have anyone on my schedule right now who does not want to be pregnant. But what will happen when that eventually changes? What about my colleagues who do have these patients on their schedules today? On a personal level, what if my prenatal genetic testing comes back abnormal? How can we so blatantly disregard a separation of church and state in this country? What ways will our government interfere with my practice next? My head is spinning, but I have to go see my next patient. She is a 25-year-old who is here to have an IUD placed, and that seems like the most important thing I can do today.”
South Carolina ob.gyn.
“I’m really scared. For my patients and for myself. I don’t know how to be a good ob.gyn. if my ability to offer safe and accessible abortion care is being threatened.”
Massachusetts ob.gyn.
“Livid and devastated and sad and terrified.”
California family planning specialist
“The fact is that about one in four people with uteruses have had an abortion. I can’t tell you how many abortions I’ve provided for people who say that they don’t ‘believe’ in them or that they thought they’d never be in this situation. ... The fact is that pregnancy is a life-threatening condition in and of itself. I am an ob.gyn., a medical doctor, and an abortion provider. I will not stop providing abortions or helping people access them. I will dedicate my life to ensuring this right to bodily autonomy. Today I am devastated by the Supreme Court’s decision to force parenthood that will result in increased maternal mortality. I am broken, but I have never been more proud to be an abortion provider.”
New York ob.gyn.
“Grateful to live in a state and work for a hospital where I can provide abortions but feel terrible for so many people less fortunate and underserved.”
Illinois maternal-fetal medicine specialist
“As a maternal-fetal medicine specialist, I fear for my patients who are at the highest risk of pregnancy complications having their freedom taken away. For the tragic ultrasound findings that make a pregnant person carry a baby who will never live. For the patients who cannot use most forms of contraception because of their medical comorbidities. For the patients who are victims of intimate partner violence or under the influence of their culture, to continue having children regardless of their desires or their health. ... The freedom to prevent or end a pregnancy has enabled women to become independent and productive members of society on their own terms, with or without children. My heart breaks for the children and adolescents and adults who are being told they are second-class citizens, not worthy of making their own decisions. Politicians and Supreme Court justices are not in the clinic room, ultrasound suite, operating room, or delivery room when we have these intense conversations and pregnancy outcomes. They have no idea that of which they speak, and it’s unconscionable that they can determine what healthcare decisions my patients can make for their own lives. Nobody knows a body better than the patient themselves.”
Texas ob.gyn.
“In the area where I live and practice, it feels like guns and the people who use them have more legal rights than people with uteruses in desperate or life-threatening situations. I’m afraid for my personal safety as a women’s health practitioner in this political climate. I feel helpless, but I’m supposed to be able to help my patients.”
Missouri family planning specialist
“Abortion is an essential part of healthcare, and the only people that should get a say in it are the patient and their doctor. Period. The fact that some far-off court without any medical expertise can insert itself into individual medical decisions is oppressive and unethical.”
Georgia ob.gyn.
“I can’t even think straight right now. I feel sick. Honestly, I’ve been thinking about moving for a long time now. Somewhere where I would actually be able to offer good, comprehensive care.”
New York ob.gyn.
“I graduated from my ob.gyn. residency hours after the Roe v. Wade news broke. It was so emotional for me. I’ve dedicated my life to caring for people with uteruses and I will not let this heartbreaking news change that. I feel more committed than ever to women’s health. I fully plan to continue delivering babies, providing contraception, and performing abortions. I will be there to help women with desired pregnancies who received unspeakably bad news about fetal anomalies. I will be there to help women with life-threatening pregnancy complications before fetal viability. I will be there to help women with ectopic pregnancies. I will be there to help women who were raped or otherwise forced into pregnancy. I will always be there to help women.”
Dr. Croll is a neurovascular fellow at New York University Langone Health. She disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
Suicide risk rises for cyberbullying victims
Experiencing cyberbullying as a victim was a significant risk factor for suicidality in early adolescents aged 10-13 years, based on data from more than 10,000 individuals.
Adolescent suicidality, defined as suicidal ideation or suicide attempts, remains a major public health issue, Shay Arnon, MA, of Reichman University, Herzliya, Israel, and colleagues wrote.
Although cyberbullying experiences and perpetration have been associated with mental health issues, their roles as specific suicidality risk factors have not been explored, they said.
In a study published in JAMA Network Open, the researchers analyzed data on cyberbullying experiences collected between July 2018 and January 2021 as part of the Adolescent Brain Cognitive Development (ABCD) study, with a diverse population of young adolescents aged 10-13 years.
The study population included 10,414 participants; the mean age was 12 years, 47.6% were female.
Overall, 7.6% of the participants had reported suicidality during the study period. A total of 930 (8.9%) reported experiencing cyberbullying as victims, and 96 (0.9%) reported perpetrating cyberbullying; 66 (69%) of the perpetrators also experienced cyberbullying.
Experiencing cyberbullying was associated with a fourfold increased risk of suicidality (odds ratio, 4.2), that remained significant after controlling for factors including demographics and multiple environmental risk and protective factors, including negative life events, family conflict, parental monitoring, school environment, and racial/ethnic discrimination (OR, 2.5), and after controlling for internalizing and externalizing psychopathology (OR, 1.8).
Adolescents who were both target and perpetrator of offline peer aggression had an increased risk of suicidality (OR, 1.5 for both), and cyberbullying experiences also remained associated with suicidality when included with offline bullying as target and perpetrator (OR, 1.7).
The results contradict previous studies showing an increased risk of suicidality in cyberbullying perpetrators as well as victims, the researchers noted. Some possible reasons for this difference are the anonymity of many cyberbullying perpetrators, and the tendency of many adolescents on social media to make quick-turn comments without thinking of their actions as offensive to others.
The study findings were limited by several factors including the cross-sectional design, which prevented conclusions about causality, a low-resolution screening for cyberbullying experiences, and the effect of unmeasured confounding variables, the researchers noted. Other limitations include the collection of data before the COVID-19 pandemic, so the effects of the pandemic on peer online communication and cyberbullying could not be determined.
However, the results suggest that experiencing cyberbullying is significantly associated with suicidality in young adolescents independent of other peer aggression experiences. “Assessment of cyberbullying experiences among children and adolescents should be a component of the comprehensive suicide risk assessment,” they concluded.
Pandemic pushed existing cyberbullying problems
“Electronic media use has increased significantly in the early adolescent demographic, particularly during the COVID-19 pandemic,” Peter L. Loper Jr., MD, of the University of South Carolina, Columbia, said in an interview.
“In many cases, the majority of an adolescent’s peer-peer interactions are now occurring on electronic devices. This has dramatically increased the incidence and prevalence of cyberbullying, making this study very timely and relevant,” said Dr. Loper, who was not involved in the study.
“From an experiential, ethnographic standpoint working on a psychiatric acute crisis stabilization unit, we have consistently recognized cyberbullying as a common and frequent etiology of suicidal ideation or attempt in the adolescents admitted to our unit,” said Dr. Loper.
“Unfortunately, much of the peer-peer interactions vital to supporting healthy adolescent development are now occurring on electronic devices instead of real-time and in person,” said Dr. Loper. “This comes with great risk to our adolescents and makes them susceptible to multiple potential dangers, not the least of which is cyberbullying.
“The biggest challenge in mitigating the impact of cyberbullying is that most adolescences want to have access to electronic media,” he said. “Limiting adolescents’ access to electronic media, and monitoring adolescents’ electronic media use are vital steps to preventing cyberbullying. Apps such as ‘Bark’ can used by parents to monitor their adolescents’ electronic media activity to ensure their safety and well-being.”
Additional research is needed to focus on other areas in which electronic media use may be affecting adolescents’ social, emotional, and psychological well-being and development, “which will become more and more important as electronic media use in this demographic continues to increase,” Dr. Loper said.
The study was supported by the National Institute of Mental Health and the Lifespan Brain Institute of Children’s Hospital of Philadelphia and Penn Medicine, University of Pennsylvania. The researchers had no financial conflicts to disclose. Dr. Loper had no financial conflicts to disclose.
Experiencing cyberbullying as a victim was a significant risk factor for suicidality in early adolescents aged 10-13 years, based on data from more than 10,000 individuals.
Adolescent suicidality, defined as suicidal ideation or suicide attempts, remains a major public health issue, Shay Arnon, MA, of Reichman University, Herzliya, Israel, and colleagues wrote.
Although cyberbullying experiences and perpetration have been associated with mental health issues, their roles as specific suicidality risk factors have not been explored, they said.
In a study published in JAMA Network Open, the researchers analyzed data on cyberbullying experiences collected between July 2018 and January 2021 as part of the Adolescent Brain Cognitive Development (ABCD) study, with a diverse population of young adolescents aged 10-13 years.
The study population included 10,414 participants; the mean age was 12 years, 47.6% were female.
Overall, 7.6% of the participants had reported suicidality during the study period. A total of 930 (8.9%) reported experiencing cyberbullying as victims, and 96 (0.9%) reported perpetrating cyberbullying; 66 (69%) of the perpetrators also experienced cyberbullying.
Experiencing cyberbullying was associated with a fourfold increased risk of suicidality (odds ratio, 4.2), that remained significant after controlling for factors including demographics and multiple environmental risk and protective factors, including negative life events, family conflict, parental monitoring, school environment, and racial/ethnic discrimination (OR, 2.5), and after controlling for internalizing and externalizing psychopathology (OR, 1.8).
Adolescents who were both target and perpetrator of offline peer aggression had an increased risk of suicidality (OR, 1.5 for both), and cyberbullying experiences also remained associated with suicidality when included with offline bullying as target and perpetrator (OR, 1.7).
The results contradict previous studies showing an increased risk of suicidality in cyberbullying perpetrators as well as victims, the researchers noted. Some possible reasons for this difference are the anonymity of many cyberbullying perpetrators, and the tendency of many adolescents on social media to make quick-turn comments without thinking of their actions as offensive to others.
The study findings were limited by several factors including the cross-sectional design, which prevented conclusions about causality, a low-resolution screening for cyberbullying experiences, and the effect of unmeasured confounding variables, the researchers noted. Other limitations include the collection of data before the COVID-19 pandemic, so the effects of the pandemic on peer online communication and cyberbullying could not be determined.
However, the results suggest that experiencing cyberbullying is significantly associated with suicidality in young adolescents independent of other peer aggression experiences. “Assessment of cyberbullying experiences among children and adolescents should be a component of the comprehensive suicide risk assessment,” they concluded.
Pandemic pushed existing cyberbullying problems
“Electronic media use has increased significantly in the early adolescent demographic, particularly during the COVID-19 pandemic,” Peter L. Loper Jr., MD, of the University of South Carolina, Columbia, said in an interview.
“In many cases, the majority of an adolescent’s peer-peer interactions are now occurring on electronic devices. This has dramatically increased the incidence and prevalence of cyberbullying, making this study very timely and relevant,” said Dr. Loper, who was not involved in the study.
“From an experiential, ethnographic standpoint working on a psychiatric acute crisis stabilization unit, we have consistently recognized cyberbullying as a common and frequent etiology of suicidal ideation or attempt in the adolescents admitted to our unit,” said Dr. Loper.
“Unfortunately, much of the peer-peer interactions vital to supporting healthy adolescent development are now occurring on electronic devices instead of real-time and in person,” said Dr. Loper. “This comes with great risk to our adolescents and makes them susceptible to multiple potential dangers, not the least of which is cyberbullying.
“The biggest challenge in mitigating the impact of cyberbullying is that most adolescences want to have access to electronic media,” he said. “Limiting adolescents’ access to electronic media, and monitoring adolescents’ electronic media use are vital steps to preventing cyberbullying. Apps such as ‘Bark’ can used by parents to monitor their adolescents’ electronic media activity to ensure their safety and well-being.”
Additional research is needed to focus on other areas in which electronic media use may be affecting adolescents’ social, emotional, and psychological well-being and development, “which will become more and more important as electronic media use in this demographic continues to increase,” Dr. Loper said.
The study was supported by the National Institute of Mental Health and the Lifespan Brain Institute of Children’s Hospital of Philadelphia and Penn Medicine, University of Pennsylvania. The researchers had no financial conflicts to disclose. Dr. Loper had no financial conflicts to disclose.
Experiencing cyberbullying as a victim was a significant risk factor for suicidality in early adolescents aged 10-13 years, based on data from more than 10,000 individuals.
Adolescent suicidality, defined as suicidal ideation or suicide attempts, remains a major public health issue, Shay Arnon, MA, of Reichman University, Herzliya, Israel, and colleagues wrote.
Although cyberbullying experiences and perpetration have been associated with mental health issues, their roles as specific suicidality risk factors have not been explored, they said.
In a study published in JAMA Network Open, the researchers analyzed data on cyberbullying experiences collected between July 2018 and January 2021 as part of the Adolescent Brain Cognitive Development (ABCD) study, with a diverse population of young adolescents aged 10-13 years.
The study population included 10,414 participants; the mean age was 12 years, 47.6% were female.
Overall, 7.6% of the participants had reported suicidality during the study period. A total of 930 (8.9%) reported experiencing cyberbullying as victims, and 96 (0.9%) reported perpetrating cyberbullying; 66 (69%) of the perpetrators also experienced cyberbullying.
Experiencing cyberbullying was associated with a fourfold increased risk of suicidality (odds ratio, 4.2), that remained significant after controlling for factors including demographics and multiple environmental risk and protective factors, including negative life events, family conflict, parental monitoring, school environment, and racial/ethnic discrimination (OR, 2.5), and after controlling for internalizing and externalizing psychopathology (OR, 1.8).
Adolescents who were both target and perpetrator of offline peer aggression had an increased risk of suicidality (OR, 1.5 for both), and cyberbullying experiences also remained associated with suicidality when included with offline bullying as target and perpetrator (OR, 1.7).
The results contradict previous studies showing an increased risk of suicidality in cyberbullying perpetrators as well as victims, the researchers noted. Some possible reasons for this difference are the anonymity of many cyberbullying perpetrators, and the tendency of many adolescents on social media to make quick-turn comments without thinking of their actions as offensive to others.
The study findings were limited by several factors including the cross-sectional design, which prevented conclusions about causality, a low-resolution screening for cyberbullying experiences, and the effect of unmeasured confounding variables, the researchers noted. Other limitations include the collection of data before the COVID-19 pandemic, so the effects of the pandemic on peer online communication and cyberbullying could not be determined.
However, the results suggest that experiencing cyberbullying is significantly associated with suicidality in young adolescents independent of other peer aggression experiences. “Assessment of cyberbullying experiences among children and adolescents should be a component of the comprehensive suicide risk assessment,” they concluded.
Pandemic pushed existing cyberbullying problems
“Electronic media use has increased significantly in the early adolescent demographic, particularly during the COVID-19 pandemic,” Peter L. Loper Jr., MD, of the University of South Carolina, Columbia, said in an interview.
“In many cases, the majority of an adolescent’s peer-peer interactions are now occurring on electronic devices. This has dramatically increased the incidence and prevalence of cyberbullying, making this study very timely and relevant,” said Dr. Loper, who was not involved in the study.
“From an experiential, ethnographic standpoint working on a psychiatric acute crisis stabilization unit, we have consistently recognized cyberbullying as a common and frequent etiology of suicidal ideation or attempt in the adolescents admitted to our unit,” said Dr. Loper.
“Unfortunately, much of the peer-peer interactions vital to supporting healthy adolescent development are now occurring on electronic devices instead of real-time and in person,” said Dr. Loper. “This comes with great risk to our adolescents and makes them susceptible to multiple potential dangers, not the least of which is cyberbullying.
“The biggest challenge in mitigating the impact of cyberbullying is that most adolescences want to have access to electronic media,” he said. “Limiting adolescents’ access to electronic media, and monitoring adolescents’ electronic media use are vital steps to preventing cyberbullying. Apps such as ‘Bark’ can used by parents to monitor their adolescents’ electronic media activity to ensure their safety and well-being.”
Additional research is needed to focus on other areas in which electronic media use may be affecting adolescents’ social, emotional, and psychological well-being and development, “which will become more and more important as electronic media use in this demographic continues to increase,” Dr. Loper said.
The study was supported by the National Institute of Mental Health and the Lifespan Brain Institute of Children’s Hospital of Philadelphia and Penn Medicine, University of Pennsylvania. The researchers had no financial conflicts to disclose. Dr. Loper had no financial conflicts to disclose.
FROM JAMA NETWORK OPEN
Good chemo vs. bad chemo: When too much is a bad thing
A new study finds that mortality is significantly higher among patients with advanced solid tumors who are admitted to the hospital for chemotherapy treatment.
The findings – released in a poster session at the annual meeting of the American Society of Clinical Oncology – found that patients with solid tumors were more likely to be treated for nonurgent indications, not be referred to palliative care, and die within 60 days, compared with patients with hematologic malignancies.
Decisions about inpatient chemotherapy should not be uniform and instead should be based on a case-by-case basis, said Natalie Berger, MD, a hematologist-oncologist at Mount Sinai Hospital,, New York, and the study’s lead author.
Inpatient chemotherapy can be appropriate in certain situations, such as when chemotherapy must be given in the hospital and when it must be administered quickly after a patient presents with cancer symptoms and needs relief, she said.
However, “sometimes patients are admitted due to infection, side effects of chemotherapy or cancer, or for reasons unrelated to their cancer, and chemotherapy may be administered when it is not appropriate. It is also overutilized at the end of life which can lead to more aggressive end-of-life care rather than focusing on quality of life and supportive care,” Dr. Berger said.
The study is based on a retrospective chart review of 880 patients admitted to Mount Sinai Hospital between January 2016 and December 2017 to receive chemotherapy.
They found that the type of tumor was used to determine the urgency of an in-hospital stay for chemotherapy (odds ratio, 0.42; 95% CI, 0.25-0.72; P = .001). Patients with solid tumors or older patients or patients with a functional impairment score (Karnofsky Performance Scale) of 50% were less likely to respond to chemotherapy. There was also a decrease in quality of life among these patients, but only 46% of patients with solid tumors and 15% of patients with hematologic malignancies met with a palliative care professional.
One-third (34%) of patients with solid tumors didn’t have urgent indications, 43% of patients had no response to inpatient chemotherapy, and 20% died within 60 days, compared with patients with hematologic malignancies (19%, 19%, and 9%, respectively).
“There are many reasons why this [high mortality rate in patients with solid tumors] may be happening. Solid tumor patients are more often admitted at a later stage of their cancer when they are sicker, and they were also less likely to have a response to inpatient chemotherapy. Older patients and patients with a poor performance status were also less likely to respond to chemotherapy. This indicates that these patients were sicker, and chemotherapy use may not have been appropriate and palliative care may be underutilized,” she said.
Dr. Berger and colleagues have created a standardized protocol to assess “the appropriateness” of inpatient chemotherapy, improve quality of life, and reduce chemotherapy and health care utilization at the end of life. The protocol has been implemented as a pilot program at Mount Sinai Hospital, Dr. Berger said.
“Any inpatient chemotherapy case that meets standard accepted criteria for required inpatient administration are auto-approved through the electronic survey. For cases outside of standard criteria, further information must be inputted to determine appropriateness of inpatient treatment and are then scored electronically and reviewed by committee physicians and pharmacists,” she said.
Gabriel A. Brooks, MD, MPH, an oncologist with Dartmouth Hitchcock Medical Center, Lebanon, N.H., who was not affiliated with the study, said that inpatient chemotherapy treatment is under scrutiny elsewhere as well.
“There has been recognition that patients who are otherwise sick enough to require hospital admission are often too sick to benefit from chemotherapy,” although there are exceptions. “There is certainly a movement to limit inpatient chemotherapy to situations where it is most likely to be beneficial. Some of this is driven by cost pressures. For instance, Medicare pays for inpatient hospitalizations using the DRG [diagnosis-related group] system. Hospitals cannot charge a la carte for treatments given in the hospital. Instead, they are reimbursed at a fixed rate based on the hospital diagnoses. This will often lead to poor reimbursement of high-cost cancer treatments.”
Dr. Brooks said the study offers insight into who’s getting inpatient chemotherapy. However, “what I can’t tell from this poster is how often the solid tumor patients are getting first-line chemotherapy [as] these patients may be presenting late or may have a potentially treatable cancer with a narrow closing window for treatment versus later-line chemotherapy.”
He also noted that patient and family wishes are missing from the research. “This is critical. Patients and families should be informed that inpatient chemotherapy may not provide the benefit they are hoping for, especially for patients with solid tumors starting later lines of therapy. Patients should be informed that there are alternatives to inpatient chemotherapy, such as hospice referral or waiting for possible outpatient treatment – if their condition improves. But when a patient wants to try inpatient chemotherapy and their doctor wants to offer it, then it is likely a reasonable thing to try.”
Going forward, he said, “qualitative study is needed to better understand when and why inpatient chemotherapy is used. There are likely some clear good uses and some clear bad uses of inpatient chemotherapy. Can outpatient regimens be substituted for the regimens where patients are directly admitted? Or, can outpatient protocols be devised for these regimens? Are there specific situations where inpatient chemotherapy is the right thing (leukemia, esophageal cancer with worsening dysphagia, etc.)?”
No study funding was received.
A new study finds that mortality is significantly higher among patients with advanced solid tumors who are admitted to the hospital for chemotherapy treatment.
The findings – released in a poster session at the annual meeting of the American Society of Clinical Oncology – found that patients with solid tumors were more likely to be treated for nonurgent indications, not be referred to palliative care, and die within 60 days, compared with patients with hematologic malignancies.
Decisions about inpatient chemotherapy should not be uniform and instead should be based on a case-by-case basis, said Natalie Berger, MD, a hematologist-oncologist at Mount Sinai Hospital,, New York, and the study’s lead author.
Inpatient chemotherapy can be appropriate in certain situations, such as when chemotherapy must be given in the hospital and when it must be administered quickly after a patient presents with cancer symptoms and needs relief, she said.
However, “sometimes patients are admitted due to infection, side effects of chemotherapy or cancer, or for reasons unrelated to their cancer, and chemotherapy may be administered when it is not appropriate. It is also overutilized at the end of life which can lead to more aggressive end-of-life care rather than focusing on quality of life and supportive care,” Dr. Berger said.
The study is based on a retrospective chart review of 880 patients admitted to Mount Sinai Hospital between January 2016 and December 2017 to receive chemotherapy.
They found that the type of tumor was used to determine the urgency of an in-hospital stay for chemotherapy (odds ratio, 0.42; 95% CI, 0.25-0.72; P = .001). Patients with solid tumors or older patients or patients with a functional impairment score (Karnofsky Performance Scale) of 50% were less likely to respond to chemotherapy. There was also a decrease in quality of life among these patients, but only 46% of patients with solid tumors and 15% of patients with hematologic malignancies met with a palliative care professional.
One-third (34%) of patients with solid tumors didn’t have urgent indications, 43% of patients had no response to inpatient chemotherapy, and 20% died within 60 days, compared with patients with hematologic malignancies (19%, 19%, and 9%, respectively).
“There are many reasons why this [high mortality rate in patients with solid tumors] may be happening. Solid tumor patients are more often admitted at a later stage of their cancer when they are sicker, and they were also less likely to have a response to inpatient chemotherapy. Older patients and patients with a poor performance status were also less likely to respond to chemotherapy. This indicates that these patients were sicker, and chemotherapy use may not have been appropriate and palliative care may be underutilized,” she said.
Dr. Berger and colleagues have created a standardized protocol to assess “the appropriateness” of inpatient chemotherapy, improve quality of life, and reduce chemotherapy and health care utilization at the end of life. The protocol has been implemented as a pilot program at Mount Sinai Hospital, Dr. Berger said.
“Any inpatient chemotherapy case that meets standard accepted criteria for required inpatient administration are auto-approved through the electronic survey. For cases outside of standard criteria, further information must be inputted to determine appropriateness of inpatient treatment and are then scored electronically and reviewed by committee physicians and pharmacists,” she said.
Gabriel A. Brooks, MD, MPH, an oncologist with Dartmouth Hitchcock Medical Center, Lebanon, N.H., who was not affiliated with the study, said that inpatient chemotherapy treatment is under scrutiny elsewhere as well.
“There has been recognition that patients who are otherwise sick enough to require hospital admission are often too sick to benefit from chemotherapy,” although there are exceptions. “There is certainly a movement to limit inpatient chemotherapy to situations where it is most likely to be beneficial. Some of this is driven by cost pressures. For instance, Medicare pays for inpatient hospitalizations using the DRG [diagnosis-related group] system. Hospitals cannot charge a la carte for treatments given in the hospital. Instead, they are reimbursed at a fixed rate based on the hospital diagnoses. This will often lead to poor reimbursement of high-cost cancer treatments.”
Dr. Brooks said the study offers insight into who’s getting inpatient chemotherapy. However, “what I can’t tell from this poster is how often the solid tumor patients are getting first-line chemotherapy [as] these patients may be presenting late or may have a potentially treatable cancer with a narrow closing window for treatment versus later-line chemotherapy.”
He also noted that patient and family wishes are missing from the research. “This is critical. Patients and families should be informed that inpatient chemotherapy may not provide the benefit they are hoping for, especially for patients with solid tumors starting later lines of therapy. Patients should be informed that there are alternatives to inpatient chemotherapy, such as hospice referral or waiting for possible outpatient treatment – if their condition improves. But when a patient wants to try inpatient chemotherapy and their doctor wants to offer it, then it is likely a reasonable thing to try.”
Going forward, he said, “qualitative study is needed to better understand when and why inpatient chemotherapy is used. There are likely some clear good uses and some clear bad uses of inpatient chemotherapy. Can outpatient regimens be substituted for the regimens where patients are directly admitted? Or, can outpatient protocols be devised for these regimens? Are there specific situations where inpatient chemotherapy is the right thing (leukemia, esophageal cancer with worsening dysphagia, etc.)?”
No study funding was received.
A new study finds that mortality is significantly higher among patients with advanced solid tumors who are admitted to the hospital for chemotherapy treatment.
The findings – released in a poster session at the annual meeting of the American Society of Clinical Oncology – found that patients with solid tumors were more likely to be treated for nonurgent indications, not be referred to palliative care, and die within 60 days, compared with patients with hematologic malignancies.
Decisions about inpatient chemotherapy should not be uniform and instead should be based on a case-by-case basis, said Natalie Berger, MD, a hematologist-oncologist at Mount Sinai Hospital,, New York, and the study’s lead author.
Inpatient chemotherapy can be appropriate in certain situations, such as when chemotherapy must be given in the hospital and when it must be administered quickly after a patient presents with cancer symptoms and needs relief, she said.
However, “sometimes patients are admitted due to infection, side effects of chemotherapy or cancer, or for reasons unrelated to their cancer, and chemotherapy may be administered when it is not appropriate. It is also overutilized at the end of life which can lead to more aggressive end-of-life care rather than focusing on quality of life and supportive care,” Dr. Berger said.
The study is based on a retrospective chart review of 880 patients admitted to Mount Sinai Hospital between January 2016 and December 2017 to receive chemotherapy.
They found that the type of tumor was used to determine the urgency of an in-hospital stay for chemotherapy (odds ratio, 0.42; 95% CI, 0.25-0.72; P = .001). Patients with solid tumors or older patients or patients with a functional impairment score (Karnofsky Performance Scale) of 50% were less likely to respond to chemotherapy. There was also a decrease in quality of life among these patients, but only 46% of patients with solid tumors and 15% of patients with hematologic malignancies met with a palliative care professional.
One-third (34%) of patients with solid tumors didn’t have urgent indications, 43% of patients had no response to inpatient chemotherapy, and 20% died within 60 days, compared with patients with hematologic malignancies (19%, 19%, and 9%, respectively).
“There are many reasons why this [high mortality rate in patients with solid tumors] may be happening. Solid tumor patients are more often admitted at a later stage of their cancer when they are sicker, and they were also less likely to have a response to inpatient chemotherapy. Older patients and patients with a poor performance status were also less likely to respond to chemotherapy. This indicates that these patients were sicker, and chemotherapy use may not have been appropriate and palliative care may be underutilized,” she said.
Dr. Berger and colleagues have created a standardized protocol to assess “the appropriateness” of inpatient chemotherapy, improve quality of life, and reduce chemotherapy and health care utilization at the end of life. The protocol has been implemented as a pilot program at Mount Sinai Hospital, Dr. Berger said.
“Any inpatient chemotherapy case that meets standard accepted criteria for required inpatient administration are auto-approved through the electronic survey. For cases outside of standard criteria, further information must be inputted to determine appropriateness of inpatient treatment and are then scored electronically and reviewed by committee physicians and pharmacists,” she said.
Gabriel A. Brooks, MD, MPH, an oncologist with Dartmouth Hitchcock Medical Center, Lebanon, N.H., who was not affiliated with the study, said that inpatient chemotherapy treatment is under scrutiny elsewhere as well.
“There has been recognition that patients who are otherwise sick enough to require hospital admission are often too sick to benefit from chemotherapy,” although there are exceptions. “There is certainly a movement to limit inpatient chemotherapy to situations where it is most likely to be beneficial. Some of this is driven by cost pressures. For instance, Medicare pays for inpatient hospitalizations using the DRG [diagnosis-related group] system. Hospitals cannot charge a la carte for treatments given in the hospital. Instead, they are reimbursed at a fixed rate based on the hospital diagnoses. This will often lead to poor reimbursement of high-cost cancer treatments.”
Dr. Brooks said the study offers insight into who’s getting inpatient chemotherapy. However, “what I can’t tell from this poster is how often the solid tumor patients are getting first-line chemotherapy [as] these patients may be presenting late or may have a potentially treatable cancer with a narrow closing window for treatment versus later-line chemotherapy.”
He also noted that patient and family wishes are missing from the research. “This is critical. Patients and families should be informed that inpatient chemotherapy may not provide the benefit they are hoping for, especially for patients with solid tumors starting later lines of therapy. Patients should be informed that there are alternatives to inpatient chemotherapy, such as hospice referral or waiting for possible outpatient treatment – if their condition improves. But when a patient wants to try inpatient chemotherapy and their doctor wants to offer it, then it is likely a reasonable thing to try.”
Going forward, he said, “qualitative study is needed to better understand when and why inpatient chemotherapy is used. There are likely some clear good uses and some clear bad uses of inpatient chemotherapy. Can outpatient regimens be substituted for the regimens where patients are directly admitted? Or, can outpatient protocols be devised for these regimens? Are there specific situations where inpatient chemotherapy is the right thing (leukemia, esophageal cancer with worsening dysphagia, etc.)?”
No study funding was received.
FROM ASCO 2022