User login
Novel liver-targeting drug offers hope for AAT deficiency
LONDON – The novel liver-targeting agent fazirsiran has fared well in a small, but significant, study looking at its ability to improve liver histology in adults with alpha-1 antitrypsin (AAT) deficiency.
Not only were serum and liver levels of the aberrant Z-AAT protein decreased, but also reductions in key liver enzymes were observed.
AAT is “a greatly understudied disease,” said study investigator Pavel Strnad, MD, who presented the results of the phase 2 AROAAT-2002 study at a meeting sponsored by the European Association for the Study of the Liver. The results were published simultaneously in the New England Journal of Medicine.
“We have a great candidate drug, but we will only be able to bring this drug into the clinic when we understand the disease much better, and for this I would like to ask all of you for your support,” he said to delegates at the meeting.
There is currently no pharmacological treatment for AAT, observed Emmanuel Tsochatzis, MD, who chaired the late-breaker session during which the study findings were presented.
“It is a rare disease, but it really does affect the patients who have it. So it would be great to have a therapeutic solution for these patients,” said Dr. Tsochatzis in an interview.
Dr. Tsochatzis, who is professor of hepatology and Consultant Hepatologist at the UCL Institute for Liver and Digestive Health, Royal Free Hospital in London, noted that AAT was associated with liver impairment, fibrosis and cirrhosis, and ultimately end-stage liver disease in which the only option for some patients would be a liver transplant.
“It depends on the stage; many patients are not at the stage that they require a liver transplant,” he said. “For those with significant liver disease, not yet at the cirrhosis stage, you might be able to intervene early and prevent them from progressing to needing a liver transplant.”
Dr. Tsochatzis speculated: “If this becomes an approved treatment, it is a breakthrough for patients.”
New hope for AAT deficiency
There is still a long way to go before fazirsiran is anywhere near ready for clinical use, but the early data presented by Dr. Strnad offer a glimpse that a treatment could be on the horizon.
Naturally occurring AAT is produced in the liver and is thought to play a role in protecting against lung damage via its antiprotease activity. However, mutations in the SERPINA1 gene coding for the AAT protein leads to loss-of-function pulmonary disease and gain-of-function liver disease.
“The PI ZZ [proteinase inhibitor ZZ] genotype occurs in about one in 2,500 to 3,500 Caucasians, so actually is pretty common, and a third of them may actually have a clinically significant liver fibrosis,” said Dr. Strnad.
As levels of Z-AAT accumulate in the liver this “presumably causes the liver toxicity,” he observed. So, the thinking is that stopping the production of this mutant protein might help to reduce liver damage and allow the liver to regenerate and repair itself.
Study details
To test out this theory, a phase 2, open-label study was performed in 16 patients with confirmed AAT deficiency, all of whom had the PI ZZ genotype.
Eight patients were treated with fazirsiran, also known as ARO-AAT, for 24 weeks (cohorts 1 and 1b), while the other eight were treated for 48 weeks (cohort 2); cohort 1 (n = 4) and cohort 2 (n = 8) received 200 mg, while cohort 1b (n = 4), which was added during the trial to evaluate dose response, received 100 mg. In all cases, fazirsiran was given as a subcutaneous injection on day 1, day 4, and then every 12 weeks. All patients had biopsies at baseline, then at either 24 weeks (cohorts 1/1b) or 48 weeks (cohort 2).
“We saw a dramatic reduction in Z-AAT both in serum and in liver biopsies,” Dr. Strnad reported. Indeed, reductions in Z-AAT were around 80%-88%, he said, with a median reduction of 83% at week 24 or 48 according to the published paper.
To illustrate the clear results, Dr. Strand showed a sample biopsy slide where globules of Z-AAT present at the initial assessment were “virtually gone after 48 weeks of treatment.”
“The change in the serum and hepatic Z-AAT levels translated into improvement in liver function tests,” said Dr. Strnad.
Alanine aminotransferase levels were normalized in all patients and there was “marked improvement” in gamma glutamyl transferase (GGT) with the 200-mg dose of fazirsiran. Substantial changes in liver stiffness were recorded with the higher dose, –18% at 24 weeks and –15% at 48 weeks. There were also reductions in serum Pro-C3 of –36% and –17% at 52 weeks.
“Histological changes showed an improvement in liver inflammation and hepatocyte cell death in most of the patients,” Dr. Strnad said. He also noted that there were multiple cases where there was no change and a few where there was a deterioration.
Liver fibrosis was improved in seven of 12 patients given the highest dose of fazirsiran; however, there was no change in three patients and a worsening of 1 point or more in two patients. None of the three patients with evaluable biopsies who received 100 mg showed regression of fibrosis.
Stopping or rolling back fibrosis will be the final goal of proving clinical benefit in liver disease associated with AAT, Dr. Strnad and coinvestigators observe in their published paper, and while the changes seen so far with fazirsiran may not be uniform, they appear to be in the right direction.
Safety findings
”We didn’t see any drug discontinuation, dose interruption, or premature study withdrawals,” said Dr. Strnad.
“We had four different SAE [serious adverse events] which were kind of all over the place and did not seem to be related to treatment.” Those SAEs included viral myocarditis associated with Epstein-Barr virus infection, diverticulitis, dyspnea in a subject with a history of lung disease, and vestibular neuronitis after COVID-19 vaccination.
No significant safety signals were seen regarding lung function. Time will of course tell, he said in response to a question, but “our hope, our hypothesis, is that this will not change lung function dramatically.
“I would not expect any major things over a few years. Of course, whether this can lead to something over 20 years it is difficult to say.”
The current experience with fazirsiran looks good so far, but placebo-controlled, larger and longer studies are needed.
The study was funded by Arrowhead Pharmaceuticals. Dr. Strnad was an investigator in the study and acknowledged receipt of research funding via his institution from the company. Dr. Strnad also acknowledged paid and unpaid relationships with Alnylam Pharmaceuticals, Alpha1 Deutschland, Alpha1 Global, CSL Behring, Grifols Biologicals, Ono Pharmaceuticals, and Takeda California. Dr. Tsochatzis, chaired the late-breaker session at ILC 2022 during which the study findings were presented, was not connected to the study, and reported no relevant conflicts of interest.
LONDON – The novel liver-targeting agent fazirsiran has fared well in a small, but significant, study looking at its ability to improve liver histology in adults with alpha-1 antitrypsin (AAT) deficiency.
Not only were serum and liver levels of the aberrant Z-AAT protein decreased, but also reductions in key liver enzymes were observed.
AAT is “a greatly understudied disease,” said study investigator Pavel Strnad, MD, who presented the results of the phase 2 AROAAT-2002 study at a meeting sponsored by the European Association for the Study of the Liver. The results were published simultaneously in the New England Journal of Medicine.
“We have a great candidate drug, but we will only be able to bring this drug into the clinic when we understand the disease much better, and for this I would like to ask all of you for your support,” he said to delegates at the meeting.
There is currently no pharmacological treatment for AAT, observed Emmanuel Tsochatzis, MD, who chaired the late-breaker session during which the study findings were presented.
“It is a rare disease, but it really does affect the patients who have it. So it would be great to have a therapeutic solution for these patients,” said Dr. Tsochatzis in an interview.
Dr. Tsochatzis, who is professor of hepatology and Consultant Hepatologist at the UCL Institute for Liver and Digestive Health, Royal Free Hospital in London, noted that AAT was associated with liver impairment, fibrosis and cirrhosis, and ultimately end-stage liver disease in which the only option for some patients would be a liver transplant.
“It depends on the stage; many patients are not at the stage that they require a liver transplant,” he said. “For those with significant liver disease, not yet at the cirrhosis stage, you might be able to intervene early and prevent them from progressing to needing a liver transplant.”
Dr. Tsochatzis speculated: “If this becomes an approved treatment, it is a breakthrough for patients.”
New hope for AAT deficiency
There is still a long way to go before fazirsiran is anywhere near ready for clinical use, but the early data presented by Dr. Strnad offer a glimpse that a treatment could be on the horizon.
Naturally occurring AAT is produced in the liver and is thought to play a role in protecting against lung damage via its antiprotease activity. However, mutations in the SERPINA1 gene coding for the AAT protein leads to loss-of-function pulmonary disease and gain-of-function liver disease.
“The PI ZZ [proteinase inhibitor ZZ] genotype occurs in about one in 2,500 to 3,500 Caucasians, so actually is pretty common, and a third of them may actually have a clinically significant liver fibrosis,” said Dr. Strnad.
As levels of Z-AAT accumulate in the liver this “presumably causes the liver toxicity,” he observed. So, the thinking is that stopping the production of this mutant protein might help to reduce liver damage and allow the liver to regenerate and repair itself.
Study details
To test out this theory, a phase 2, open-label study was performed in 16 patients with confirmed AAT deficiency, all of whom had the PI ZZ genotype.
Eight patients were treated with fazirsiran, also known as ARO-AAT, for 24 weeks (cohorts 1 and 1b), while the other eight were treated for 48 weeks (cohort 2); cohort 1 (n = 4) and cohort 2 (n = 8) received 200 mg, while cohort 1b (n = 4), which was added during the trial to evaluate dose response, received 100 mg. In all cases, fazirsiran was given as a subcutaneous injection on day 1, day 4, and then every 12 weeks. All patients had biopsies at baseline, then at either 24 weeks (cohorts 1/1b) or 48 weeks (cohort 2).
“We saw a dramatic reduction in Z-AAT both in serum and in liver biopsies,” Dr. Strnad reported. Indeed, reductions in Z-AAT were around 80%-88%, he said, with a median reduction of 83% at week 24 or 48 according to the published paper.
To illustrate the clear results, Dr. Strand showed a sample biopsy slide where globules of Z-AAT present at the initial assessment were “virtually gone after 48 weeks of treatment.”
“The change in the serum and hepatic Z-AAT levels translated into improvement in liver function tests,” said Dr. Strnad.
Alanine aminotransferase levels were normalized in all patients and there was “marked improvement” in gamma glutamyl transferase (GGT) with the 200-mg dose of fazirsiran. Substantial changes in liver stiffness were recorded with the higher dose, –18% at 24 weeks and –15% at 48 weeks. There were also reductions in serum Pro-C3 of –36% and –17% at 52 weeks.
“Histological changes showed an improvement in liver inflammation and hepatocyte cell death in most of the patients,” Dr. Strnad said. He also noted that there were multiple cases where there was no change and a few where there was a deterioration.
Liver fibrosis was improved in seven of 12 patients given the highest dose of fazirsiran; however, there was no change in three patients and a worsening of 1 point or more in two patients. None of the three patients with evaluable biopsies who received 100 mg showed regression of fibrosis.
Stopping or rolling back fibrosis will be the final goal of proving clinical benefit in liver disease associated with AAT, Dr. Strnad and coinvestigators observe in their published paper, and while the changes seen so far with fazirsiran may not be uniform, they appear to be in the right direction.
Safety findings
”We didn’t see any drug discontinuation, dose interruption, or premature study withdrawals,” said Dr. Strnad.
“We had four different SAE [serious adverse events] which were kind of all over the place and did not seem to be related to treatment.” Those SAEs included viral myocarditis associated with Epstein-Barr virus infection, diverticulitis, dyspnea in a subject with a history of lung disease, and vestibular neuronitis after COVID-19 vaccination.
No significant safety signals were seen regarding lung function. Time will of course tell, he said in response to a question, but “our hope, our hypothesis, is that this will not change lung function dramatically.
“I would not expect any major things over a few years. Of course, whether this can lead to something over 20 years it is difficult to say.”
The current experience with fazirsiran looks good so far, but placebo-controlled, larger and longer studies are needed.
The study was funded by Arrowhead Pharmaceuticals. Dr. Strnad was an investigator in the study and acknowledged receipt of research funding via his institution from the company. Dr. Strnad also acknowledged paid and unpaid relationships with Alnylam Pharmaceuticals, Alpha1 Deutschland, Alpha1 Global, CSL Behring, Grifols Biologicals, Ono Pharmaceuticals, and Takeda California. Dr. Tsochatzis, chaired the late-breaker session at ILC 2022 during which the study findings were presented, was not connected to the study, and reported no relevant conflicts of interest.
LONDON – The novel liver-targeting agent fazirsiran has fared well in a small, but significant, study looking at its ability to improve liver histology in adults with alpha-1 antitrypsin (AAT) deficiency.
Not only were serum and liver levels of the aberrant Z-AAT protein decreased, but also reductions in key liver enzymes were observed.
AAT is “a greatly understudied disease,” said study investigator Pavel Strnad, MD, who presented the results of the phase 2 AROAAT-2002 study at a meeting sponsored by the European Association for the Study of the Liver. The results were published simultaneously in the New England Journal of Medicine.
“We have a great candidate drug, but we will only be able to bring this drug into the clinic when we understand the disease much better, and for this I would like to ask all of you for your support,” he said to delegates at the meeting.
There is currently no pharmacological treatment for AAT, observed Emmanuel Tsochatzis, MD, who chaired the late-breaker session during which the study findings were presented.
“It is a rare disease, but it really does affect the patients who have it. So it would be great to have a therapeutic solution for these patients,” said Dr. Tsochatzis in an interview.
Dr. Tsochatzis, who is professor of hepatology and Consultant Hepatologist at the UCL Institute for Liver and Digestive Health, Royal Free Hospital in London, noted that AAT was associated with liver impairment, fibrosis and cirrhosis, and ultimately end-stage liver disease in which the only option for some patients would be a liver transplant.
“It depends on the stage; many patients are not at the stage that they require a liver transplant,” he said. “For those with significant liver disease, not yet at the cirrhosis stage, you might be able to intervene early and prevent them from progressing to needing a liver transplant.”
Dr. Tsochatzis speculated: “If this becomes an approved treatment, it is a breakthrough for patients.”
New hope for AAT deficiency
There is still a long way to go before fazirsiran is anywhere near ready for clinical use, but the early data presented by Dr. Strnad offer a glimpse that a treatment could be on the horizon.
Naturally occurring AAT is produced in the liver and is thought to play a role in protecting against lung damage via its antiprotease activity. However, mutations in the SERPINA1 gene coding for the AAT protein leads to loss-of-function pulmonary disease and gain-of-function liver disease.
“The PI ZZ [proteinase inhibitor ZZ] genotype occurs in about one in 2,500 to 3,500 Caucasians, so actually is pretty common, and a third of them may actually have a clinically significant liver fibrosis,” said Dr. Strnad.
As levels of Z-AAT accumulate in the liver this “presumably causes the liver toxicity,” he observed. So, the thinking is that stopping the production of this mutant protein might help to reduce liver damage and allow the liver to regenerate and repair itself.
Study details
To test out this theory, a phase 2, open-label study was performed in 16 patients with confirmed AAT deficiency, all of whom had the PI ZZ genotype.
Eight patients were treated with fazirsiran, also known as ARO-AAT, for 24 weeks (cohorts 1 and 1b), while the other eight were treated for 48 weeks (cohort 2); cohort 1 (n = 4) and cohort 2 (n = 8) received 200 mg, while cohort 1b (n = 4), which was added during the trial to evaluate dose response, received 100 mg. In all cases, fazirsiran was given as a subcutaneous injection on day 1, day 4, and then every 12 weeks. All patients had biopsies at baseline, then at either 24 weeks (cohorts 1/1b) or 48 weeks (cohort 2).
“We saw a dramatic reduction in Z-AAT both in serum and in liver biopsies,” Dr. Strnad reported. Indeed, reductions in Z-AAT were around 80%-88%, he said, with a median reduction of 83% at week 24 or 48 according to the published paper.
To illustrate the clear results, Dr. Strand showed a sample biopsy slide where globules of Z-AAT present at the initial assessment were “virtually gone after 48 weeks of treatment.”
“The change in the serum and hepatic Z-AAT levels translated into improvement in liver function tests,” said Dr. Strnad.
Alanine aminotransferase levels were normalized in all patients and there was “marked improvement” in gamma glutamyl transferase (GGT) with the 200-mg dose of fazirsiran. Substantial changes in liver stiffness were recorded with the higher dose, –18% at 24 weeks and –15% at 48 weeks. There were also reductions in serum Pro-C3 of –36% and –17% at 52 weeks.
“Histological changes showed an improvement in liver inflammation and hepatocyte cell death in most of the patients,” Dr. Strnad said. He also noted that there were multiple cases where there was no change and a few where there was a deterioration.
Liver fibrosis was improved in seven of 12 patients given the highest dose of fazirsiran; however, there was no change in three patients and a worsening of 1 point or more in two patients. None of the three patients with evaluable biopsies who received 100 mg showed regression of fibrosis.
Stopping or rolling back fibrosis will be the final goal of proving clinical benefit in liver disease associated with AAT, Dr. Strnad and coinvestigators observe in their published paper, and while the changes seen so far with fazirsiran may not be uniform, they appear to be in the right direction.
Safety findings
”We didn’t see any drug discontinuation, dose interruption, or premature study withdrawals,” said Dr. Strnad.
“We had four different SAE [serious adverse events] which were kind of all over the place and did not seem to be related to treatment.” Those SAEs included viral myocarditis associated with Epstein-Barr virus infection, diverticulitis, dyspnea in a subject with a history of lung disease, and vestibular neuronitis after COVID-19 vaccination.
No significant safety signals were seen regarding lung function. Time will of course tell, he said in response to a question, but “our hope, our hypothesis, is that this will not change lung function dramatically.
“I would not expect any major things over a few years. Of course, whether this can lead to something over 20 years it is difficult to say.”
The current experience with fazirsiran looks good so far, but placebo-controlled, larger and longer studies are needed.
The study was funded by Arrowhead Pharmaceuticals. Dr. Strnad was an investigator in the study and acknowledged receipt of research funding via his institution from the company. Dr. Strnad also acknowledged paid and unpaid relationships with Alnylam Pharmaceuticals, Alpha1 Deutschland, Alpha1 Global, CSL Behring, Grifols Biologicals, Ono Pharmaceuticals, and Takeda California. Dr. Tsochatzis, chaired the late-breaker session at ILC 2022 during which the study findings were presented, was not connected to the study, and reported no relevant conflicts of interest.
AT ILC 2022
Both parents at risk for depression following birth
Physicians have screened new and expectant mothers for perinatal depression for years. But what about fathers?
A new systematic review and meta-analysis suggests it’s time for health care providers to ask both parents about any mental health symptoms before and after their baby is born.
“We are screening most mothers for signs of perinatal depression,” said Kara Smythe, MD, at the department of primary care and population health and Institute of Epidemiology and Health Care at the University College London, who is the lead author of the study. “But we aren’t always asking about the relationship between them and the person helping them care for this newborn. If we don’t consider the experience of new fathers, we’re doing a disservice to everyone.”
Without screening both parents, health care providers can miss important clues to why child and parents experience adverse health outcomes post birth.
The study, published in JAMA Network Open, found that for 3.18% of couples, both parents concurrently experienced depression before and following a birth. The mental illness was more common in the late postnatal period (3-12 months).
According to the Centers for Disease Control and Prevention, about 1 in 8 women experience symptoms of postpartum depression. Other sources indicate the incidence may be much higher. Findings from a mobile app using the Edinburgh Postnatal Depression Scale presented at the American Psychiatric Association’s annual meeting in 2019 indicated more than half of the 164,237 women who used the free app reported symptoms of depression for up to a year following the birth of their baby.
The findings
Dr. Smythe and her team reviewed previously published observational studies on the prevalence of perinatal depression or anxiety in couples from the Ovid and Web of Science between Jan. 1, 1990, and June 8, 2021.
They ultimately included 23 studies with data from 29,286 couples. They broke the data into subgroups of persons with antenatal depression, early postnatal depression (0-12 weeks), late postnatal depression (3-13 months), and perinatal anxiety.
About 1.7% (P < .001) of couples experienced antenatal depression, and about 2.4% (P < .001) experienced early postnatal depression. About 3.2% (P < .001) experienced late postnatal depression. The data on perinatal anxiety were insufficient, they write.
The vast majority of couples included in the samples were White, heterosexual, and highly educated with a middle to high socioeconomic background. The pregnancies were reportedly wanted, if not planned. The majority of the studies – 21 – included in the analysis were from countries other than the United States.
According to the study, evidence suggests that paternal depression can lead to increased symptoms of depression in mothers during pregnancy and the following 6 months. Men reported perinatal depression at similar rates as women, and Dr. Smythe said it’s becoming clear that men experience similar struggles as they transition into fatherhood.
J. J. Parker, MD, a pediatric and internal attending physician at Lurie Children’s Hospital of Chicago and Northwestern Medicine, said the findings solidify what he has observed from his own experience as a new father and resident.
“You’re at higher risk of having depression if your partner has depression, but it’s important to see that in the numbers,” Dr. Parker told this news organization. “I think from a clinician standpoint, this demonstrates that 3% of infants are living in households where both parents are depressed, and that has major implications for the development and health of those children.”
Dr. Smythe and her colleagues found that if even one parent is experiencing a mood disorder such as depression or anxiety, the newborn can experience impaired bonding, behavioral problems, and other harms later in life.
If both parents are experiencing perinatal depression, those negative outcomes could be amplified, although Dr. Smythe said more research is needed to solidify the link.
“I think one quick takeaway for pediatricians, clinicians, and any other health care providers taking care of mothers and infants is to ask about the nonbirthing parent,” Dr. Parker said. “All clinicians can do that right away, even if the mother does not have depression.”
The study was independently supported. Dr. Smythe and her colleagues report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Physicians have screened new and expectant mothers for perinatal depression for years. But what about fathers?
A new systematic review and meta-analysis suggests it’s time for health care providers to ask both parents about any mental health symptoms before and after their baby is born.
“We are screening most mothers for signs of perinatal depression,” said Kara Smythe, MD, at the department of primary care and population health and Institute of Epidemiology and Health Care at the University College London, who is the lead author of the study. “But we aren’t always asking about the relationship between them and the person helping them care for this newborn. If we don’t consider the experience of new fathers, we’re doing a disservice to everyone.”
Without screening both parents, health care providers can miss important clues to why child and parents experience adverse health outcomes post birth.
The study, published in JAMA Network Open, found that for 3.18% of couples, both parents concurrently experienced depression before and following a birth. The mental illness was more common in the late postnatal period (3-12 months).
According to the Centers for Disease Control and Prevention, about 1 in 8 women experience symptoms of postpartum depression. Other sources indicate the incidence may be much higher. Findings from a mobile app using the Edinburgh Postnatal Depression Scale presented at the American Psychiatric Association’s annual meeting in 2019 indicated more than half of the 164,237 women who used the free app reported symptoms of depression for up to a year following the birth of their baby.
The findings
Dr. Smythe and her team reviewed previously published observational studies on the prevalence of perinatal depression or anxiety in couples from the Ovid and Web of Science between Jan. 1, 1990, and June 8, 2021.
They ultimately included 23 studies with data from 29,286 couples. They broke the data into subgroups of persons with antenatal depression, early postnatal depression (0-12 weeks), late postnatal depression (3-13 months), and perinatal anxiety.
About 1.7% (P < .001) of couples experienced antenatal depression, and about 2.4% (P < .001) experienced early postnatal depression. About 3.2% (P < .001) experienced late postnatal depression. The data on perinatal anxiety were insufficient, they write.
The vast majority of couples included in the samples were White, heterosexual, and highly educated with a middle to high socioeconomic background. The pregnancies were reportedly wanted, if not planned. The majority of the studies – 21 – included in the analysis were from countries other than the United States.
According to the study, evidence suggests that paternal depression can lead to increased symptoms of depression in mothers during pregnancy and the following 6 months. Men reported perinatal depression at similar rates as women, and Dr. Smythe said it’s becoming clear that men experience similar struggles as they transition into fatherhood.
J. J. Parker, MD, a pediatric and internal attending physician at Lurie Children’s Hospital of Chicago and Northwestern Medicine, said the findings solidify what he has observed from his own experience as a new father and resident.
“You’re at higher risk of having depression if your partner has depression, but it’s important to see that in the numbers,” Dr. Parker told this news organization. “I think from a clinician standpoint, this demonstrates that 3% of infants are living in households where both parents are depressed, and that has major implications for the development and health of those children.”
Dr. Smythe and her colleagues found that if even one parent is experiencing a mood disorder such as depression or anxiety, the newborn can experience impaired bonding, behavioral problems, and other harms later in life.
If both parents are experiencing perinatal depression, those negative outcomes could be amplified, although Dr. Smythe said more research is needed to solidify the link.
“I think one quick takeaway for pediatricians, clinicians, and any other health care providers taking care of mothers and infants is to ask about the nonbirthing parent,” Dr. Parker said. “All clinicians can do that right away, even if the mother does not have depression.”
The study was independently supported. Dr. Smythe and her colleagues report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Physicians have screened new and expectant mothers for perinatal depression for years. But what about fathers?
A new systematic review and meta-analysis suggests it’s time for health care providers to ask both parents about any mental health symptoms before and after their baby is born.
“We are screening most mothers for signs of perinatal depression,” said Kara Smythe, MD, at the department of primary care and population health and Institute of Epidemiology and Health Care at the University College London, who is the lead author of the study. “But we aren’t always asking about the relationship between them and the person helping them care for this newborn. If we don’t consider the experience of new fathers, we’re doing a disservice to everyone.”
Without screening both parents, health care providers can miss important clues to why child and parents experience adverse health outcomes post birth.
The study, published in JAMA Network Open, found that for 3.18% of couples, both parents concurrently experienced depression before and following a birth. The mental illness was more common in the late postnatal period (3-12 months).
According to the Centers for Disease Control and Prevention, about 1 in 8 women experience symptoms of postpartum depression. Other sources indicate the incidence may be much higher. Findings from a mobile app using the Edinburgh Postnatal Depression Scale presented at the American Psychiatric Association’s annual meeting in 2019 indicated more than half of the 164,237 women who used the free app reported symptoms of depression for up to a year following the birth of their baby.
The findings
Dr. Smythe and her team reviewed previously published observational studies on the prevalence of perinatal depression or anxiety in couples from the Ovid and Web of Science between Jan. 1, 1990, and June 8, 2021.
They ultimately included 23 studies with data from 29,286 couples. They broke the data into subgroups of persons with antenatal depression, early postnatal depression (0-12 weeks), late postnatal depression (3-13 months), and perinatal anxiety.
About 1.7% (P < .001) of couples experienced antenatal depression, and about 2.4% (P < .001) experienced early postnatal depression. About 3.2% (P < .001) experienced late postnatal depression. The data on perinatal anxiety were insufficient, they write.
The vast majority of couples included in the samples were White, heterosexual, and highly educated with a middle to high socioeconomic background. The pregnancies were reportedly wanted, if not planned. The majority of the studies – 21 – included in the analysis were from countries other than the United States.
According to the study, evidence suggests that paternal depression can lead to increased symptoms of depression in mothers during pregnancy and the following 6 months. Men reported perinatal depression at similar rates as women, and Dr. Smythe said it’s becoming clear that men experience similar struggles as they transition into fatherhood.
J. J. Parker, MD, a pediatric and internal attending physician at Lurie Children’s Hospital of Chicago and Northwestern Medicine, said the findings solidify what he has observed from his own experience as a new father and resident.
“You’re at higher risk of having depression if your partner has depression, but it’s important to see that in the numbers,” Dr. Parker told this news organization. “I think from a clinician standpoint, this demonstrates that 3% of infants are living in households where both parents are depressed, and that has major implications for the development and health of those children.”
Dr. Smythe and her colleagues found that if even one parent is experiencing a mood disorder such as depression or anxiety, the newborn can experience impaired bonding, behavioral problems, and other harms later in life.
If both parents are experiencing perinatal depression, those negative outcomes could be amplified, although Dr. Smythe said more research is needed to solidify the link.
“I think one quick takeaway for pediatricians, clinicians, and any other health care providers taking care of mothers and infants is to ask about the nonbirthing parent,” Dr. Parker said. “All clinicians can do that right away, even if the mother does not have depression.”
The study was independently supported. Dr. Smythe and her colleagues report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Women benefit but lag behind in intracoronary imaging in PCI
A real-world analysis reveals that women are consistently less likely to undergo intracoronary imaging as part of percutaneous coronary intervention (PCI), even though it benefits both sexes equally.
Results from nearly all PCIs performed in England and Wales between 2006 and 2019 showed the absolute rate of intracoronary imaging with either intravascular ultrasound (IVUS) or optical coherence tomography (OCT) was 5% lower in the later study years among women at 14.5%, compared with 19.6% in men (P < .001).
After adjustment, female sex was an independent predictor of lower intracoronary imaging use (odds ratio, 0.93; 95% confidence interval, 0.91-0.96), according to the study, published in JACC: Cardiovascular Interventions.
“One of the thoughts I had when we were running this analysis was, well, maybe the indications for that imaging, as recommended by guidelines, are less common in women,” Mamas Mamas, MD, told this news organization. “So what we did was to look at just cases where imaging is recommended by the EAPCI [European Association of Percutaneous Coronary Intervention].”
Again, the use of intracoronary imaging was consistently lower among women than among men for all of the following EAPCI-recommended indications:
- Acute coronary syndrome: 11.6% vs. 12.3% (P < .01).
- Stent thrombosis: 30.9% vs. 34.9% (P < .01).
- Long lesions: 13.1% vs. 16.3% (P < .01).
- Chronic total occlusions: 16.2% vs. 18.3% (P < .01).
- Left main stem PCI: 55.1% vs. 57.5% (P < .01).
- In-stent restenosis: 28.0% vs. 30.7%.
- Calcified lesions: 36.6% vs. 40.1% (P < .01).
- Renal disease: 17.4% vs. 19.5% (P < .01).
As to what might be driving the lower use, Dr. Mamas dismissed the argument that women undergo much simpler PCI, which wouldn’t benefit from imaging. Women do have smaller coronary arteries, however, and there is a belief that it’s easier to eyeball the size of vessels that are smaller rather than larger.
“I’m not convinced that’s entirely true,” he said. “I don’t have a good answer for you, I’m afraid. I don’t really know why we’re seeing it. I just think it’s one of those disparities that is important to highlight.”
Central to this belief is that the benefits of intracoronary imaging were found to be similar in men and women. Intracoronary imaging was associated with lower adjusted odds of in-hospital mortality (OR, 0.56; 95% CI, 0.48-0.64) and major adverse cardiac and cerebrovascular events (OR, 0.83; 95% CI, 0.76-0.91) in women and men (OR, 0.48; 95% CI, 0.44-0.53 and OR, 0.75; 95% CI, 0.71-0.80, respectively), compared with nonimaging groups.
“This really should be a call to arms, particularly given that we show this disparity persists, even in guideline-recommended cases where we should be using it,” said Dr. Mamas, from the Keele (England) Cardiovascular Research Group, Keele University, and Royal Stoke University Hospital, Stoke-on-Trent, England.
“Actually, I would argue that we should be using more imaging in women than men anyway because many of the presentations for acute coronary syndromes in women, like spontaneous coronary artery dissection or MINOCA [MI with nonobstructive coronary arteries], you often need intracoronary imaging to make that kind of diagnosis,” he observed.
Getting worse, not better
Previous studies have shown that women are less likely than men in acute coronary syndromes to receive the transradial approach and P2Y12 inhibitors, but none have specifically looked at intracoronary imaging, Dr. Mamas said.
To fill the gap, the researchers drew on data from 994,478 patients in the British Cardiovascular Intervention Society registry, of whom, 8.4% of 738,616 men and 7.9% of 255,862 women received intracoronary imaging.
Women in the imaging group were older, more likely to be an ethnic minority, and more likely to undergo PCI for non–ST-segment elevation MI than their male counterparts.
One of the more surprising findings was that rates of IVUS and OCT were superimposable between the sexes at the start of the study but quickly diverged starting in around 2012, when the technology took off, Dr. Mamas said. In the most recent data, use was about 3% lower in women overall and rising to 6% in those with stable angina.
“Whilst the disparities between men and women are significant, the bigger question is why are we using so little imaging in guideline-recommended cases where there is a benefit?” he said.
Possible actionable items, he suggested, include providing older physicians who didn’t have access to intracoronary imaging during their training with opportunities in their cath lab or with industry sponsors to increase their skills and confidence. Intracoronary imaging use could also be routinely captured in U.S. and European PCI registries and used as a quality metric.
“In left main, you see a massive difference between centers, and that’s the kind of data that drives discussion,” Dr. Mamas said. “If we start reporting quality metrics, such as radial use, intracoronary imaging, P2Y12 inhibitors by center, then you’ve got something to benchmark centers against.”
Nathaniel Smilowitz, MD, an interventional cardiologist at New York Langone Health, who was not associated with the study, said that it’s troubling to see that the utilization intravascular imaging is so low, despite randomized trials and large meta-analyses showing a mortality benefit associated with its use in PCI.
“Even among men, only 19.6% in the later years were getting intravascular imaging performed to guide their coronary intervention, so one out of five,” he said. “There are opportunities to improve.”
Dr. Smilowitz said he’s also perplexed as to why adoption would be lower in women but that the findings echo those in other domains where women receive less intensive cardiovascular therapy.
“There’s no biological, really plausible, mechanism as to why the need for intravascular imaging would be lower and, particularly, because they showed in stent thrombosis, for example, where intravascular imaging is tremendously important, there were still sex differences,” he said. “So even with clear indications for imaging, women just received the optimal therapy less often than men. It’s disappointing.”
Dr. Smilowitz agreed that there may be a need to incorporate intravascular imaging into metrics, which are reported back to physicians, potentially even for comparisons with peers or regional rates to incentivize physicians to improve uptake.
“As a society, we’ve been quite slow to integrate intravascular imaging to guide PCI and we can do better,” he said.
A version of this article first appeared on Medscape.com.
A real-world analysis reveals that women are consistently less likely to undergo intracoronary imaging as part of percutaneous coronary intervention (PCI), even though it benefits both sexes equally.
Results from nearly all PCIs performed in England and Wales between 2006 and 2019 showed the absolute rate of intracoronary imaging with either intravascular ultrasound (IVUS) or optical coherence tomography (OCT) was 5% lower in the later study years among women at 14.5%, compared with 19.6% in men (P < .001).
After adjustment, female sex was an independent predictor of lower intracoronary imaging use (odds ratio, 0.93; 95% confidence interval, 0.91-0.96), according to the study, published in JACC: Cardiovascular Interventions.
“One of the thoughts I had when we were running this analysis was, well, maybe the indications for that imaging, as recommended by guidelines, are less common in women,” Mamas Mamas, MD, told this news organization. “So what we did was to look at just cases where imaging is recommended by the EAPCI [European Association of Percutaneous Coronary Intervention].”
Again, the use of intracoronary imaging was consistently lower among women than among men for all of the following EAPCI-recommended indications:
- Acute coronary syndrome: 11.6% vs. 12.3% (P < .01).
- Stent thrombosis: 30.9% vs. 34.9% (P < .01).
- Long lesions: 13.1% vs. 16.3% (P < .01).
- Chronic total occlusions: 16.2% vs. 18.3% (P < .01).
- Left main stem PCI: 55.1% vs. 57.5% (P < .01).
- In-stent restenosis: 28.0% vs. 30.7%.
- Calcified lesions: 36.6% vs. 40.1% (P < .01).
- Renal disease: 17.4% vs. 19.5% (P < .01).
As to what might be driving the lower use, Dr. Mamas dismissed the argument that women undergo much simpler PCI, which wouldn’t benefit from imaging. Women do have smaller coronary arteries, however, and there is a belief that it’s easier to eyeball the size of vessels that are smaller rather than larger.
“I’m not convinced that’s entirely true,” he said. “I don’t have a good answer for you, I’m afraid. I don’t really know why we’re seeing it. I just think it’s one of those disparities that is important to highlight.”
Central to this belief is that the benefits of intracoronary imaging were found to be similar in men and women. Intracoronary imaging was associated with lower adjusted odds of in-hospital mortality (OR, 0.56; 95% CI, 0.48-0.64) and major adverse cardiac and cerebrovascular events (OR, 0.83; 95% CI, 0.76-0.91) in women and men (OR, 0.48; 95% CI, 0.44-0.53 and OR, 0.75; 95% CI, 0.71-0.80, respectively), compared with nonimaging groups.
“This really should be a call to arms, particularly given that we show this disparity persists, even in guideline-recommended cases where we should be using it,” said Dr. Mamas, from the Keele (England) Cardiovascular Research Group, Keele University, and Royal Stoke University Hospital, Stoke-on-Trent, England.
“Actually, I would argue that we should be using more imaging in women than men anyway because many of the presentations for acute coronary syndromes in women, like spontaneous coronary artery dissection or MINOCA [MI with nonobstructive coronary arteries], you often need intracoronary imaging to make that kind of diagnosis,” he observed.
Getting worse, not better
Previous studies have shown that women are less likely than men in acute coronary syndromes to receive the transradial approach and P2Y12 inhibitors, but none have specifically looked at intracoronary imaging, Dr. Mamas said.
To fill the gap, the researchers drew on data from 994,478 patients in the British Cardiovascular Intervention Society registry, of whom, 8.4% of 738,616 men and 7.9% of 255,862 women received intracoronary imaging.
Women in the imaging group were older, more likely to be an ethnic minority, and more likely to undergo PCI for non–ST-segment elevation MI than their male counterparts.
One of the more surprising findings was that rates of IVUS and OCT were superimposable between the sexes at the start of the study but quickly diverged starting in around 2012, when the technology took off, Dr. Mamas said. In the most recent data, use was about 3% lower in women overall and rising to 6% in those with stable angina.
“Whilst the disparities between men and women are significant, the bigger question is why are we using so little imaging in guideline-recommended cases where there is a benefit?” he said.
Possible actionable items, he suggested, include providing older physicians who didn’t have access to intracoronary imaging during their training with opportunities in their cath lab or with industry sponsors to increase their skills and confidence. Intracoronary imaging use could also be routinely captured in U.S. and European PCI registries and used as a quality metric.
“In left main, you see a massive difference between centers, and that’s the kind of data that drives discussion,” Dr. Mamas said. “If we start reporting quality metrics, such as radial use, intracoronary imaging, P2Y12 inhibitors by center, then you’ve got something to benchmark centers against.”
Nathaniel Smilowitz, MD, an interventional cardiologist at New York Langone Health, who was not associated with the study, said that it’s troubling to see that the utilization intravascular imaging is so low, despite randomized trials and large meta-analyses showing a mortality benefit associated with its use in PCI.
“Even among men, only 19.6% in the later years were getting intravascular imaging performed to guide their coronary intervention, so one out of five,” he said. “There are opportunities to improve.”
Dr. Smilowitz said he’s also perplexed as to why adoption would be lower in women but that the findings echo those in other domains where women receive less intensive cardiovascular therapy.
“There’s no biological, really plausible, mechanism as to why the need for intravascular imaging would be lower and, particularly, because they showed in stent thrombosis, for example, where intravascular imaging is tremendously important, there were still sex differences,” he said. “So even with clear indications for imaging, women just received the optimal therapy less often than men. It’s disappointing.”
Dr. Smilowitz agreed that there may be a need to incorporate intravascular imaging into metrics, which are reported back to physicians, potentially even for comparisons with peers or regional rates to incentivize physicians to improve uptake.
“As a society, we’ve been quite slow to integrate intravascular imaging to guide PCI and we can do better,” he said.
A version of this article first appeared on Medscape.com.
A real-world analysis reveals that women are consistently less likely to undergo intracoronary imaging as part of percutaneous coronary intervention (PCI), even though it benefits both sexes equally.
Results from nearly all PCIs performed in England and Wales between 2006 and 2019 showed the absolute rate of intracoronary imaging with either intravascular ultrasound (IVUS) or optical coherence tomography (OCT) was 5% lower in the later study years among women at 14.5%, compared with 19.6% in men (P < .001).
After adjustment, female sex was an independent predictor of lower intracoronary imaging use (odds ratio, 0.93; 95% confidence interval, 0.91-0.96), according to the study, published in JACC: Cardiovascular Interventions.
“One of the thoughts I had when we were running this analysis was, well, maybe the indications for that imaging, as recommended by guidelines, are less common in women,” Mamas Mamas, MD, told this news organization. “So what we did was to look at just cases where imaging is recommended by the EAPCI [European Association of Percutaneous Coronary Intervention].”
Again, the use of intracoronary imaging was consistently lower among women than among men for all of the following EAPCI-recommended indications:
- Acute coronary syndrome: 11.6% vs. 12.3% (P < .01).
- Stent thrombosis: 30.9% vs. 34.9% (P < .01).
- Long lesions: 13.1% vs. 16.3% (P < .01).
- Chronic total occlusions: 16.2% vs. 18.3% (P < .01).
- Left main stem PCI: 55.1% vs. 57.5% (P < .01).
- In-stent restenosis: 28.0% vs. 30.7%.
- Calcified lesions: 36.6% vs. 40.1% (P < .01).
- Renal disease: 17.4% vs. 19.5% (P < .01).
As to what might be driving the lower use, Dr. Mamas dismissed the argument that women undergo much simpler PCI, which wouldn’t benefit from imaging. Women do have smaller coronary arteries, however, and there is a belief that it’s easier to eyeball the size of vessels that are smaller rather than larger.
“I’m not convinced that’s entirely true,” he said. “I don’t have a good answer for you, I’m afraid. I don’t really know why we’re seeing it. I just think it’s one of those disparities that is important to highlight.”
Central to this belief is that the benefits of intracoronary imaging were found to be similar in men and women. Intracoronary imaging was associated with lower adjusted odds of in-hospital mortality (OR, 0.56; 95% CI, 0.48-0.64) and major adverse cardiac and cerebrovascular events (OR, 0.83; 95% CI, 0.76-0.91) in women and men (OR, 0.48; 95% CI, 0.44-0.53 and OR, 0.75; 95% CI, 0.71-0.80, respectively), compared with nonimaging groups.
“This really should be a call to arms, particularly given that we show this disparity persists, even in guideline-recommended cases where we should be using it,” said Dr. Mamas, from the Keele (England) Cardiovascular Research Group, Keele University, and Royal Stoke University Hospital, Stoke-on-Trent, England.
“Actually, I would argue that we should be using more imaging in women than men anyway because many of the presentations for acute coronary syndromes in women, like spontaneous coronary artery dissection or MINOCA [MI with nonobstructive coronary arteries], you often need intracoronary imaging to make that kind of diagnosis,” he observed.
Getting worse, not better
Previous studies have shown that women are less likely than men in acute coronary syndromes to receive the transradial approach and P2Y12 inhibitors, but none have specifically looked at intracoronary imaging, Dr. Mamas said.
To fill the gap, the researchers drew on data from 994,478 patients in the British Cardiovascular Intervention Society registry, of whom, 8.4% of 738,616 men and 7.9% of 255,862 women received intracoronary imaging.
Women in the imaging group were older, more likely to be an ethnic minority, and more likely to undergo PCI for non–ST-segment elevation MI than their male counterparts.
One of the more surprising findings was that rates of IVUS and OCT were superimposable between the sexes at the start of the study but quickly diverged starting in around 2012, when the technology took off, Dr. Mamas said. In the most recent data, use was about 3% lower in women overall and rising to 6% in those with stable angina.
“Whilst the disparities between men and women are significant, the bigger question is why are we using so little imaging in guideline-recommended cases where there is a benefit?” he said.
Possible actionable items, he suggested, include providing older physicians who didn’t have access to intracoronary imaging during their training with opportunities in their cath lab or with industry sponsors to increase their skills and confidence. Intracoronary imaging use could also be routinely captured in U.S. and European PCI registries and used as a quality metric.
“In left main, you see a massive difference between centers, and that’s the kind of data that drives discussion,” Dr. Mamas said. “If we start reporting quality metrics, such as radial use, intracoronary imaging, P2Y12 inhibitors by center, then you’ve got something to benchmark centers against.”
Nathaniel Smilowitz, MD, an interventional cardiologist at New York Langone Health, who was not associated with the study, said that it’s troubling to see that the utilization intravascular imaging is so low, despite randomized trials and large meta-analyses showing a mortality benefit associated with its use in PCI.
“Even among men, only 19.6% in the later years were getting intravascular imaging performed to guide their coronary intervention, so one out of five,” he said. “There are opportunities to improve.”
Dr. Smilowitz said he’s also perplexed as to why adoption would be lower in women but that the findings echo those in other domains where women receive less intensive cardiovascular therapy.
“There’s no biological, really plausible, mechanism as to why the need for intravascular imaging would be lower and, particularly, because they showed in stent thrombosis, for example, where intravascular imaging is tremendously important, there were still sex differences,” he said. “So even with clear indications for imaging, women just received the optimal therapy less often than men. It’s disappointing.”
Dr. Smilowitz agreed that there may be a need to incorporate intravascular imaging into metrics, which are reported back to physicians, potentially even for comparisons with peers or regional rates to incentivize physicians to improve uptake.
“As a society, we’ve been quite slow to integrate intravascular imaging to guide PCI and we can do better,” he said.
A version of this article first appeared on Medscape.com.
FROM JACC: CARDIOVASCULAR INTERVENTIONS
Pig-heart transplant case published with new details, insights
It’s a given that the case of David Bennett, Sr, and his transplanted, genetically modified porcine heart will have a lot to teach, and the peer-reviewed publication this week lends welcome authority to some of its earliest lessons.
Mr. Bennett lived for 2 months after receiving the heart in the pioneering surgery, and the new case report compiles the available clinical, anatomic, and histologic evidence and other potential clues to the underlying cause or causes of death.
It also describes a mystery that came to light at autopsy: a grossly enlarged heart attributable to pervasive interstitial edema, and at the cellular level, a peculiar pattern of myocardial damage that included microvascular deterioration and, potentially as a result, cellular necrosis, according to the new report.
The myocardium itself was described as “thickened and stiff,” consistent with the “diastolic heart failure” that characterized Mr. Bennett’s final 10 days and the likely convergence of several underlying processes. Missing, however, was any conventional sign of graft rejection as it is understood clinically or in animal models, the report states.
If a form of tissue rejection was the cause of graft failure, any implicating cellular evidence may simply have been unrecognizable, given the unprecedented nature of the first pig-to-human heart transplantation, the donor animal’s multiple anti-inflammatory gene deletions, and partly investigational immunosuppression regimen, speculated Bartley P. Griffith, MD, University of Maryland, College Park.
“I’m betting against it being a fulminant rejection,” he told this news organization, “because we saw nothing like the [characteristic] platelet deposition or thrombosis of the capillaries.”
Dr. Griffith, who performed the xenotransplant surgery and led Mr. Bennett’s postoperative care, is lead author on the case report published in the New England Journal of Medicine. “Additional studies are underway to characterize the pathophysiologic mechanisms that resulted in this damage,” the report states.
The report builds on recent meeting presentations on the case, which, as previously reported, gave cursory details regarding the organ damage and other clinical developments during and after the surgery, including evidence that the transplanted heart contained porcine cytomegalovirus (PCMV).
Similar details also appeared in a third-person account based in part on personal communication with Dr. Griffith. The cardiac XTx review that focused on this University of Maryland experience was published June 15 in JACC: Basic to Translational Science, with lead author Jacinthe Boulet, MD, CM, Brigham and Women’s Hospital Heart, Boston.
“The question of how to move XTx forward remains uncertain, and appropriate selection of patients for experimental XTx will be one of the most important challenges to be addressed. The first issue we must contend with is whether we are ready to move to the next XTx in a human. We strongly believe this to be the case,” the review states. “Once early experience is gained, with successive iterations of XTx, the bar for success can be raised with maturation of the technology.”
Evidence has so far not implicated several other potential mechanisms underlying the graft failure that had been the focus of early speculations. For example, the transplanted pig heart was infected with PCMV, as previously reported. Mr. Bennett showed traces of PCMV DNA in his circulation, but no actual virus in his native cells. Still, PCMV remains a suspect.
Mr. Bennett also received intravenous immunoglobulin (IVIG) on several occasions to fight rejection, and also severe infections, including a nasty episode of sepsis. A reaction to the IVIG, derived from pooled donor antibodies, could potentially have caused the unusual myocardial damage seen by the University of Maryland team, Dr. Griffith observed. Alternatively, the damage might have been partly related to the patient’s overall severely diminished condition even before the transplant surgery or his rocky postoperative clinical course.
Indeed, Mr. Bennett’s condition worsened dramatically on postoperative day 50, and echocardiography showed a striking degree of myocardial wall thickening and heart enlargement, determined to be from edema. “The heart got amazingly stiff but maintained a systolic function that wasn›t too terrible, even to the very end. But his heart seemed as though it had swollen overnight,” Dr. Griffith said. “We had never seen that type of process, the suddenness of this swelling, in our nonhuman primate studies.”
The damage to the heart muscle appeared irreversible, based on myocardial biopsy results, so the decision was made to withdraw life support 60 days after the transplant surgery, the report notes.
Among the experience’s apparent lessons for future cardiac xenotransplantation, Dr. Griffith said, would be to select patients for the surgery who are in a bit more robust condition than Mr. Bennett was, who are perhaps ambulatory, not sarcopenic, and not recently on prolonged mechanical circulatory support. “We’re going to try to pick a patient who, on the front end, is less critically ill but who is just as likely not to benefit from continued medical therapy” and who isn’t a candidate for conventional heart transplantation, he said.
Because of universal efforts to manage conditions like diabetes, hypertension, and vascular disease in the population, and “because these conditions cause many of the cases of organ failure and fuel demand for transplantation, one might wonder whether the advances reported by Dr. Griffith and colleagues presage a decreasing demand for organ transplantation,” speculates an accompanying editorialfrom Jeffrey L. Platt, MD, and Marilia Cascalho, MD, PhD, University of Michigan, Ann Arbor.
“We think the answer is no. Since aging is associated with progressive decline in the function of the heart, kidneys, and other organs, advances that extend life expectancy will ultimately increase the prevalence of organ failure and potentially the demand for transplantation.”
The donor pig was developed and provided by Revivicor, and the investigational KPL-404 antibody drug used in the experience was provided by Kiniksa. Other disclosures for the case report and editorial from Dr. Platt and Dr. Cascalho are available at NEJM.com. Dr. Boulet reports no relevant relationships; disclosures for the other authors are in their report.
A version of this article first appeared on Medscape.com.
It’s a given that the case of David Bennett, Sr, and his transplanted, genetically modified porcine heart will have a lot to teach, and the peer-reviewed publication this week lends welcome authority to some of its earliest lessons.
Mr. Bennett lived for 2 months after receiving the heart in the pioneering surgery, and the new case report compiles the available clinical, anatomic, and histologic evidence and other potential clues to the underlying cause or causes of death.
It also describes a mystery that came to light at autopsy: a grossly enlarged heart attributable to pervasive interstitial edema, and at the cellular level, a peculiar pattern of myocardial damage that included microvascular deterioration and, potentially as a result, cellular necrosis, according to the new report.
The myocardium itself was described as “thickened and stiff,” consistent with the “diastolic heart failure” that characterized Mr. Bennett’s final 10 days and the likely convergence of several underlying processes. Missing, however, was any conventional sign of graft rejection as it is understood clinically or in animal models, the report states.
If a form of tissue rejection was the cause of graft failure, any implicating cellular evidence may simply have been unrecognizable, given the unprecedented nature of the first pig-to-human heart transplantation, the donor animal’s multiple anti-inflammatory gene deletions, and partly investigational immunosuppression regimen, speculated Bartley P. Griffith, MD, University of Maryland, College Park.
“I’m betting against it being a fulminant rejection,” he told this news organization, “because we saw nothing like the [characteristic] platelet deposition or thrombosis of the capillaries.”
Dr. Griffith, who performed the xenotransplant surgery and led Mr. Bennett’s postoperative care, is lead author on the case report published in the New England Journal of Medicine. “Additional studies are underway to characterize the pathophysiologic mechanisms that resulted in this damage,” the report states.
The report builds on recent meeting presentations on the case, which, as previously reported, gave cursory details regarding the organ damage and other clinical developments during and after the surgery, including evidence that the transplanted heart contained porcine cytomegalovirus (PCMV).
Similar details also appeared in a third-person account based in part on personal communication with Dr. Griffith. The cardiac XTx review that focused on this University of Maryland experience was published June 15 in JACC: Basic to Translational Science, with lead author Jacinthe Boulet, MD, CM, Brigham and Women’s Hospital Heart, Boston.
“The question of how to move XTx forward remains uncertain, and appropriate selection of patients for experimental XTx will be one of the most important challenges to be addressed. The first issue we must contend with is whether we are ready to move to the next XTx in a human. We strongly believe this to be the case,” the review states. “Once early experience is gained, with successive iterations of XTx, the bar for success can be raised with maturation of the technology.”
Evidence has so far not implicated several other potential mechanisms underlying the graft failure that had been the focus of early speculations. For example, the transplanted pig heart was infected with PCMV, as previously reported. Mr. Bennett showed traces of PCMV DNA in his circulation, but no actual virus in his native cells. Still, PCMV remains a suspect.
Mr. Bennett also received intravenous immunoglobulin (IVIG) on several occasions to fight rejection, and also severe infections, including a nasty episode of sepsis. A reaction to the IVIG, derived from pooled donor antibodies, could potentially have caused the unusual myocardial damage seen by the University of Maryland team, Dr. Griffith observed. Alternatively, the damage might have been partly related to the patient’s overall severely diminished condition even before the transplant surgery or his rocky postoperative clinical course.
Indeed, Mr. Bennett’s condition worsened dramatically on postoperative day 50, and echocardiography showed a striking degree of myocardial wall thickening and heart enlargement, determined to be from edema. “The heart got amazingly stiff but maintained a systolic function that wasn›t too terrible, even to the very end. But his heart seemed as though it had swollen overnight,” Dr. Griffith said. “We had never seen that type of process, the suddenness of this swelling, in our nonhuman primate studies.”
The damage to the heart muscle appeared irreversible, based on myocardial biopsy results, so the decision was made to withdraw life support 60 days after the transplant surgery, the report notes.
Among the experience’s apparent lessons for future cardiac xenotransplantation, Dr. Griffith said, would be to select patients for the surgery who are in a bit more robust condition than Mr. Bennett was, who are perhaps ambulatory, not sarcopenic, and not recently on prolonged mechanical circulatory support. “We’re going to try to pick a patient who, on the front end, is less critically ill but who is just as likely not to benefit from continued medical therapy” and who isn’t a candidate for conventional heart transplantation, he said.
Because of universal efforts to manage conditions like diabetes, hypertension, and vascular disease in the population, and “because these conditions cause many of the cases of organ failure and fuel demand for transplantation, one might wonder whether the advances reported by Dr. Griffith and colleagues presage a decreasing demand for organ transplantation,” speculates an accompanying editorialfrom Jeffrey L. Platt, MD, and Marilia Cascalho, MD, PhD, University of Michigan, Ann Arbor.
“We think the answer is no. Since aging is associated with progressive decline in the function of the heart, kidneys, and other organs, advances that extend life expectancy will ultimately increase the prevalence of organ failure and potentially the demand for transplantation.”
The donor pig was developed and provided by Revivicor, and the investigational KPL-404 antibody drug used in the experience was provided by Kiniksa. Other disclosures for the case report and editorial from Dr. Platt and Dr. Cascalho are available at NEJM.com. Dr. Boulet reports no relevant relationships; disclosures for the other authors are in their report.
A version of this article first appeared on Medscape.com.
It’s a given that the case of David Bennett, Sr, and his transplanted, genetically modified porcine heart will have a lot to teach, and the peer-reviewed publication this week lends welcome authority to some of its earliest lessons.
Mr. Bennett lived for 2 months after receiving the heart in the pioneering surgery, and the new case report compiles the available clinical, anatomic, and histologic evidence and other potential clues to the underlying cause or causes of death.
It also describes a mystery that came to light at autopsy: a grossly enlarged heart attributable to pervasive interstitial edema, and at the cellular level, a peculiar pattern of myocardial damage that included microvascular deterioration and, potentially as a result, cellular necrosis, according to the new report.
The myocardium itself was described as “thickened and stiff,” consistent with the “diastolic heart failure” that characterized Mr. Bennett’s final 10 days and the likely convergence of several underlying processes. Missing, however, was any conventional sign of graft rejection as it is understood clinically or in animal models, the report states.
If a form of tissue rejection was the cause of graft failure, any implicating cellular evidence may simply have been unrecognizable, given the unprecedented nature of the first pig-to-human heart transplantation, the donor animal’s multiple anti-inflammatory gene deletions, and partly investigational immunosuppression regimen, speculated Bartley P. Griffith, MD, University of Maryland, College Park.
“I’m betting against it being a fulminant rejection,” he told this news organization, “because we saw nothing like the [characteristic] platelet deposition or thrombosis of the capillaries.”
Dr. Griffith, who performed the xenotransplant surgery and led Mr. Bennett’s postoperative care, is lead author on the case report published in the New England Journal of Medicine. “Additional studies are underway to characterize the pathophysiologic mechanisms that resulted in this damage,” the report states.
The report builds on recent meeting presentations on the case, which, as previously reported, gave cursory details regarding the organ damage and other clinical developments during and after the surgery, including evidence that the transplanted heart contained porcine cytomegalovirus (PCMV).
Similar details also appeared in a third-person account based in part on personal communication with Dr. Griffith. The cardiac XTx review that focused on this University of Maryland experience was published June 15 in JACC: Basic to Translational Science, with lead author Jacinthe Boulet, MD, CM, Brigham and Women’s Hospital Heart, Boston.
“The question of how to move XTx forward remains uncertain, and appropriate selection of patients for experimental XTx will be one of the most important challenges to be addressed. The first issue we must contend with is whether we are ready to move to the next XTx in a human. We strongly believe this to be the case,” the review states. “Once early experience is gained, with successive iterations of XTx, the bar for success can be raised with maturation of the technology.”
Evidence has so far not implicated several other potential mechanisms underlying the graft failure that had been the focus of early speculations. For example, the transplanted pig heart was infected with PCMV, as previously reported. Mr. Bennett showed traces of PCMV DNA in his circulation, but no actual virus in his native cells. Still, PCMV remains a suspect.
Mr. Bennett also received intravenous immunoglobulin (IVIG) on several occasions to fight rejection, and also severe infections, including a nasty episode of sepsis. A reaction to the IVIG, derived from pooled donor antibodies, could potentially have caused the unusual myocardial damage seen by the University of Maryland team, Dr. Griffith observed. Alternatively, the damage might have been partly related to the patient’s overall severely diminished condition even before the transplant surgery or his rocky postoperative clinical course.
Indeed, Mr. Bennett’s condition worsened dramatically on postoperative day 50, and echocardiography showed a striking degree of myocardial wall thickening and heart enlargement, determined to be from edema. “The heart got amazingly stiff but maintained a systolic function that wasn›t too terrible, even to the very end. But his heart seemed as though it had swollen overnight,” Dr. Griffith said. “We had never seen that type of process, the suddenness of this swelling, in our nonhuman primate studies.”
The damage to the heart muscle appeared irreversible, based on myocardial biopsy results, so the decision was made to withdraw life support 60 days after the transplant surgery, the report notes.
Among the experience’s apparent lessons for future cardiac xenotransplantation, Dr. Griffith said, would be to select patients for the surgery who are in a bit more robust condition than Mr. Bennett was, who are perhaps ambulatory, not sarcopenic, and not recently on prolonged mechanical circulatory support. “We’re going to try to pick a patient who, on the front end, is less critically ill but who is just as likely not to benefit from continued medical therapy” and who isn’t a candidate for conventional heart transplantation, he said.
Because of universal efforts to manage conditions like diabetes, hypertension, and vascular disease in the population, and “because these conditions cause many of the cases of organ failure and fuel demand for transplantation, one might wonder whether the advances reported by Dr. Griffith and colleagues presage a decreasing demand for organ transplantation,” speculates an accompanying editorialfrom Jeffrey L. Platt, MD, and Marilia Cascalho, MD, PhD, University of Michigan, Ann Arbor.
“We think the answer is no. Since aging is associated with progressive decline in the function of the heart, kidneys, and other organs, advances that extend life expectancy will ultimately increase the prevalence of organ failure and potentially the demand for transplantation.”
The donor pig was developed and provided by Revivicor, and the investigational KPL-404 antibody drug used in the experience was provided by Kiniksa. Other disclosures for the case report and editorial from Dr. Platt and Dr. Cascalho are available at NEJM.com. Dr. Boulet reports no relevant relationships; disclosures for the other authors are in their report.
A version of this article first appeared on Medscape.com.
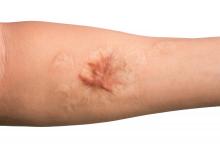
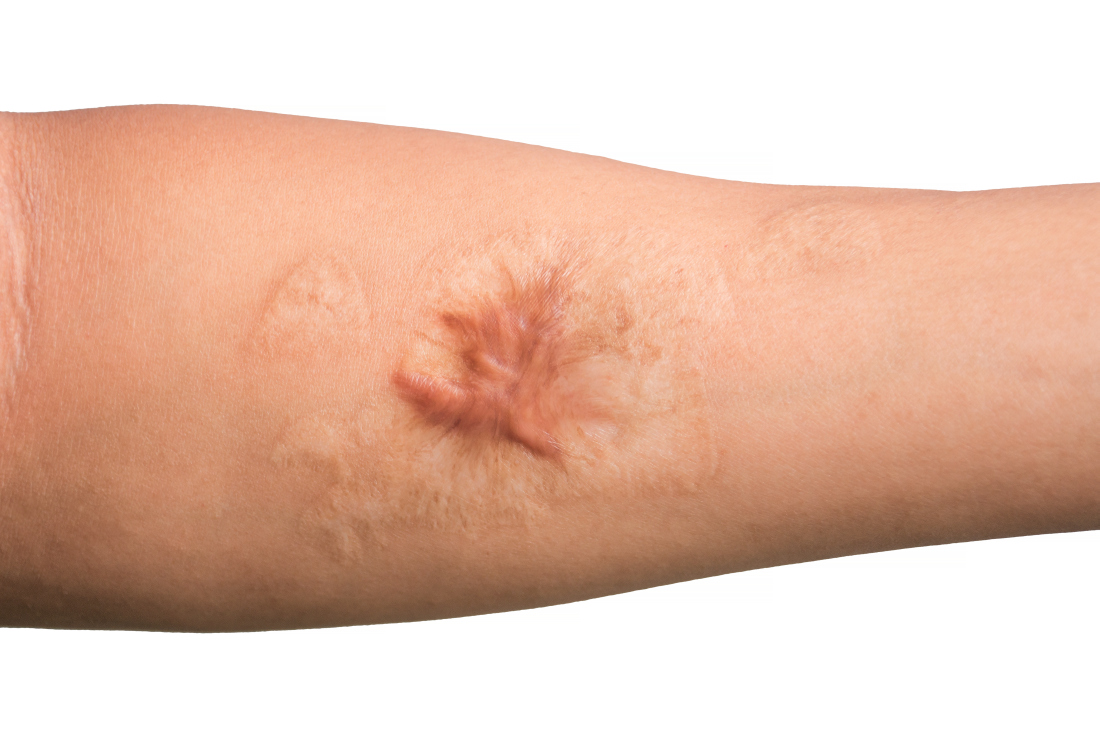
Combo of excision, cryosurgery found to benefit keloid scar outcomes
Treating keloid scars by combining excision and contact cryosurgery is a plausible way to decrease the volume of scars, results from a single-center observational study suggest.
“There is currently no consensus regarding the best treatment of keloid scars,” corresponding author Manon Artz, of the department of plastic, reconstructive, and aesthetic surgery at University Hospital of Brest (France), and colleagues wrote in a research letter published online in JAMA Dermatology.
“Earlier studies report a decreased scar volume and a substantial reduction of recurrence in keloid scars treated by cryosurgery,” they wrote. “In this study, our objective was to assess whether intramarginal excision (shaving) of the keloid scar followed by an immediate single session of contact cryosurgery is associated with decreased scar volume.”
Between March 2014 and May 2020, the researchers evaluated the approach in 31 patients with 40 keloid scars who were treated at University Hospital of Brest. Of these study participants, four were lost to follow-up, leaving 27 patients with 35 keloid scars in the final analysis. Their mean age was 24 years, 60% were female, and there was fairly even distribution of Fitzpatrick skin types II-VI.
Most of the keloid scars were located on the ear (69%) and the chest (23%), while the rest were on the head and neck. The primary outcome was reduction of keloid scar volume after 12 months, which was measured with the Vancouver scar scale. The researchers defined 80%-100% reduction in scar volume as “major,” a 50%-80% reduction as “substantial,” and a 0%-50% reduction or recurrence as “moderate.”
After 12 months, 19 scars (54%) showed a major reduction in volume, while 6 (17%) had a substantial reduction, and seven (20%) experienced no reduction. Across all keloid scars, the median scar volume decreased significantly by 81.9%.
Scar volume reduction differed by anatomical location. Specifically, 84% of ear scars showed major or substantial reduction, while 60% of scars on the chest showed a moderate reduction in scar volume or recurrence. In another key finding, the Vancouver scar scale score was reduced overall in 25 scars by 71.4%, from 7 before treatment to 5 after treatment.
“There remains no silver bullet for the treatment of keloids, but this study adds invaluable evidence that tangential excision followed by contact cryosurgery can be a viable treatment regimen with low recurrence rates,” said Marcus G. Tan, MD, who recently completed his dermatology residency at the University of Ottawa and who was asked to comment on the study. “Clinicians should exercise caution especially when treating individuals with darker skin phototypes due to their increased risk of scarring and dyspigmentation.”
Limitations of this study, he said, include a smaller study population with some patient dropouts and a lack of adverse effects reported.
The researchers and Dr. Tan reported having no financial conflicts.
Treating keloid scars by combining excision and contact cryosurgery is a plausible way to decrease the volume of scars, results from a single-center observational study suggest.
“There is currently no consensus regarding the best treatment of keloid scars,” corresponding author Manon Artz, of the department of plastic, reconstructive, and aesthetic surgery at University Hospital of Brest (France), and colleagues wrote in a research letter published online in JAMA Dermatology.
“Earlier studies report a decreased scar volume and a substantial reduction of recurrence in keloid scars treated by cryosurgery,” they wrote. “In this study, our objective was to assess whether intramarginal excision (shaving) of the keloid scar followed by an immediate single session of contact cryosurgery is associated with decreased scar volume.”
Between March 2014 and May 2020, the researchers evaluated the approach in 31 patients with 40 keloid scars who were treated at University Hospital of Brest. Of these study participants, four were lost to follow-up, leaving 27 patients with 35 keloid scars in the final analysis. Their mean age was 24 years, 60% were female, and there was fairly even distribution of Fitzpatrick skin types II-VI.
Most of the keloid scars were located on the ear (69%) and the chest (23%), while the rest were on the head and neck. The primary outcome was reduction of keloid scar volume after 12 months, which was measured with the Vancouver scar scale. The researchers defined 80%-100% reduction in scar volume as “major,” a 50%-80% reduction as “substantial,” and a 0%-50% reduction or recurrence as “moderate.”
After 12 months, 19 scars (54%) showed a major reduction in volume, while 6 (17%) had a substantial reduction, and seven (20%) experienced no reduction. Across all keloid scars, the median scar volume decreased significantly by 81.9%.
Scar volume reduction differed by anatomical location. Specifically, 84% of ear scars showed major or substantial reduction, while 60% of scars on the chest showed a moderate reduction in scar volume or recurrence. In another key finding, the Vancouver scar scale score was reduced overall in 25 scars by 71.4%, from 7 before treatment to 5 after treatment.
“There remains no silver bullet for the treatment of keloids, but this study adds invaluable evidence that tangential excision followed by contact cryosurgery can be a viable treatment regimen with low recurrence rates,” said Marcus G. Tan, MD, who recently completed his dermatology residency at the University of Ottawa and who was asked to comment on the study. “Clinicians should exercise caution especially when treating individuals with darker skin phototypes due to their increased risk of scarring and dyspigmentation.”
Limitations of this study, he said, include a smaller study population with some patient dropouts and a lack of adverse effects reported.
The researchers and Dr. Tan reported having no financial conflicts.
Treating keloid scars by combining excision and contact cryosurgery is a plausible way to decrease the volume of scars, results from a single-center observational study suggest.
“There is currently no consensus regarding the best treatment of keloid scars,” corresponding author Manon Artz, of the department of plastic, reconstructive, and aesthetic surgery at University Hospital of Brest (France), and colleagues wrote in a research letter published online in JAMA Dermatology.
“Earlier studies report a decreased scar volume and a substantial reduction of recurrence in keloid scars treated by cryosurgery,” they wrote. “In this study, our objective was to assess whether intramarginal excision (shaving) of the keloid scar followed by an immediate single session of contact cryosurgery is associated with decreased scar volume.”
Between March 2014 and May 2020, the researchers evaluated the approach in 31 patients with 40 keloid scars who were treated at University Hospital of Brest. Of these study participants, four were lost to follow-up, leaving 27 patients with 35 keloid scars in the final analysis. Their mean age was 24 years, 60% were female, and there was fairly even distribution of Fitzpatrick skin types II-VI.
Most of the keloid scars were located on the ear (69%) and the chest (23%), while the rest were on the head and neck. The primary outcome was reduction of keloid scar volume after 12 months, which was measured with the Vancouver scar scale. The researchers defined 80%-100% reduction in scar volume as “major,” a 50%-80% reduction as “substantial,” and a 0%-50% reduction or recurrence as “moderate.”
After 12 months, 19 scars (54%) showed a major reduction in volume, while 6 (17%) had a substantial reduction, and seven (20%) experienced no reduction. Across all keloid scars, the median scar volume decreased significantly by 81.9%.
Scar volume reduction differed by anatomical location. Specifically, 84% of ear scars showed major or substantial reduction, while 60% of scars on the chest showed a moderate reduction in scar volume or recurrence. In another key finding, the Vancouver scar scale score was reduced overall in 25 scars by 71.4%, from 7 before treatment to 5 after treatment.
“There remains no silver bullet for the treatment of keloids, but this study adds invaluable evidence that tangential excision followed by contact cryosurgery can be a viable treatment regimen with low recurrence rates,” said Marcus G. Tan, MD, who recently completed his dermatology residency at the University of Ottawa and who was asked to comment on the study. “Clinicians should exercise caution especially when treating individuals with darker skin phototypes due to their increased risk of scarring and dyspigmentation.”
Limitations of this study, he said, include a smaller study population with some patient dropouts and a lack of adverse effects reported.
The researchers and Dr. Tan reported having no financial conflicts.
FROM JAMA DERMATOLOGY
Adjuvant vs. neoadjuvant? What has ASCO 2022 taught us regarding resectable NSCLC?
for non–small cell lung cancer (NSCLC). While there has been some notable progress in this area, we need phase 3 trials that compare the two therapeutic approaches.
Investigators reporting at the 2022 annual meeting of American Society of Clinical Oncology focused primarily on neoadjuvant treatment, which I’ll address here.
In the randomized, phase 2 NADIM II clinical trial reported at the meeting, researchers expanded on the results of NADIM published in 2020 in the Lancet Oncology and in May 2022 in the Journal of Clinical Oncology along with CheckMate 816 results published in the New England Journal of Medicine.
In each of these three studies, researchers compared nivolumab plus chemotherapy versus chemotherapy alone (abstract 8501) as a neoadjuvant treatment for resectable stage IIIA NSCLC. In the study reported at ASCO 2022, patients with resectable clinical stage IIIA-B (per American Joint Committee on Cancer 8th edition) NSCLC and no known EGFR/ALK alterations, were randomized to receive preoperative nivolumab plus chemotherapy (paclitaxel and carboplatin; n = 57) or chemotherapy (n = 29) alone followed by surgery.
The primary endpoint was pathological complete response (pCR); secondary endpoints included major pathological response, safety and tolerability, impact on surgical issues such as delayed or canceled surgeries or length of hospital stay, overall survival and progression free survival. The pCR rate was 36.8% in the neoadjuvant nivolumab plus chemotherapy arm and 6.9% in the chemotherapy alone arm. (P = .0068). 25% of patients on the nivolumab plus chemo arm had grade 3-4 adverse events, compared with 10.3% in the control arm. 93% of patients on the nivolumab plus chemo arm underwent definitive surgery whereas 69.0% of the patients on the chemo alone arm had definitive surgery. (P = .008)
What else did we learn about neoadjuvant treatment at the meeting?
Investigators looking at the optimal number of neoadjuvant cycles (abstract 8500) found that three cycles of sintilimab (an investigational PD-1 inhibitor) produced a numerically higher major pathological response rate, compared with two cycles (when given in concert with platinum-doublet chemotherapy). And, neoadjuvant chemoradiotherapy does not result in significant survival benefits when compared with neoadjuvant chemotherapy alone (abstract 8503).
Of course, when it comes to resectable NSCLC, the goal of treatment is to increase the cure rate and improve survival. No randomized studies have reported yet on overall survival, probably because they are too immature. Instead, disease-free survival (DFS) or event-free survival (EFS) are often used as surrogate endpoints. Since none of the studies reported at ASCO reported on DFS or EFS, we need to look elsewhere. CheckMate 816 was a phase 3 study which randomized patients with stages IB-IIIA NSCLC to receive neoadjuvant nivolumab plus platinum-based chemotherapy or neoadjuvant platinum-based chemotherapy alone, followed by resection. The median EFS was 31.6 months with nivolumab plus chemotherapy and 20.8 months with chemotherapy alone (P = .005). The percentage of patients with a pCR was 24.0% and 2.2%, respectively (P < .001).
We all know one has to be careful when doing cross-trial comparisons as these studies differ by the percentage of patients with various stages of disease, the type of immunotherapy and chemotherapy used, etc. However, I think we can agree that neoadjuvant chemoimmunotherapy results in better outcomes than chemotherapy alone.
Of course, resectable NSCLC is, by definition, resectable. And traditionally, resection is followed by adjuvant chemotherapy to eradicate micrometastases. Unfortunately, the current standard of care for completely resected early-stage NSCLC (stage I [tumor ≥ 4 cm] to IIIA) involves adjuvant platinum-based combination chemotherapy which results in only a modest 4%-5% improvement in survival versus observation.
Given these modest results, as in the neoadjuvant space, investigators have looked at the benefit of adding immunotherapy to adjuvant chemotherapy. One such study has been reported. IMpower 010 randomly assigned patients with completely resected stage IB (tumors ≥ 4 cm) to IIIA NSCLC, whose tumor cells expressed at least 1% PD-L1, to receive adjuvant atezolizumab or best supportive care after adjuvant platinum-based chemotherapy. In the stage II-IIIA population whose tumors expressed PD-L1 on 1% or more of tumor cells, 3-year DFS rates were 60% and 48% in the atezolizumab and best supportive care arms, respectively (hazard ratio, 0·66 P =·.0039). In all patients in the stage II-IIIA population, the 3-year DFS rates were 56% in the atezolizumab group and 49% in the best supportive care group, (HR, 0.79; P = .020).
KEYNOTE-091, reported at the 2021 annual meeting of the European Society for Medical Oncology, randomized early-stage NSCLC patients following complete resection and adjuvant chemotherapy to pembrolizumab or placebo. Median DFS for the overall population was 53.6 months for patients in the pembro arm versus 42 months in the placebo arm (HR, 0.76; P = .0014). Interestingly, the benefit was not seen in patients with PD-L1 with at least 50%, where the 18-month DFS rate was 71.7% in the pembro arm and 70.2% in the placebo arm (HR, 0.82; P = .14). Although the contradictory results of PD-L1 as a biomarker is puzzling, I think we can agree that the addition of immunotherapy following adjuvant chemotherapy improves outcomes compared to adjuvant chemotherapy alone.
What to do when a patient presents with resectable disease?
Cross-trial comparisons are fraught with danger. Until we have a phase 3 study comparing concurrent neoadjuvant chemo/immunotherapy with concurrent adjuvant chemo/immunotherapy, I do not think we can answer the question “which is better?” However, there are some caveats to keep in mind when deciding on which approach to recommend to our patients: First, neoadjuvant treatment requires biomarker testing to ensure the patient does not have EGFR or ALK mutations. This will necessitate a delay in the operation. Will patients be willing to wait? Will the surgeon? Or, would patients prefer to proceed with surgery while the results are pending? Yes, neoadjuvant therapy gives you information regarding the pCR rate, but does that help you in subsequent management of the patient? We do not know.
Secondly, the two adjuvant studies used adjuvant chemotherapy followed by adjuvant immunotherapy, as contrasted to the neoadjuvant study which used concurrent chemo/immunotherapy. Given the longer duration of treatment in postoperative sequential adjuvant studies, there tends to be more drop off because of patients being unwilling or unfit postoperatively to receive long courses of therapy. In IMpower 010, 1,269 patients completed adjuvant chemotherapy; 1,005 were randomized, and of the 507 assigned to the atezolizumab/chemo group, only 323 completed treatment.
Finally, we must beware of using neoadjuvant chemo/immunotherapy to “down-stage” a patient. KEYNOTE-091 included patients with IIIA disease and no benefit to adjuvant chemotherapy followed by immunotherapy was found in this subgroup of patients, which leads me to wonder if these patients were appropriately selected as surgical candidates. In the NADIM II trials, 9 of 29 patients on the neoadjuvant chemotherapy were not resected.
So, many questions remain. In addition to the ones we’ve raised, there is a clear and immediate need for predictive and prognostic biomarkers. In the NADIM II trial, PD-L1 expression was a predictive biomarker of response. The pCR rate for patients with a PD-L1 tumor expression of less than 1%, 1%-49%, and 50% or higher was 15%, 41.7%, and 61.1%, respectively. However, in KEYNOTE-091, the benefit was not seen in patients with PD-L1 of at least than 50%, where the 18-month DFS rate was 71.7% in the pembro arm and 70.2% in the placebo arm.
Another possible biomarker: circulating tumor DNA. In the first NADIM study, three low pretreatment levels of ctDNA were significantly associated with improved progression-free survival and overall survival (HR, 0.20 and HR, 0.07, respectively). Although clinical response did not predict survival outcomes, undetectable ctDNA levels after neoadjuvant treatment were significantly associated with progression-free survival and overall survival (HR, 0.26 and HR0.04, respectively). Similarly, in CheckMate 816, clearance of ctDNA was associated with longer EFS in patients with ctDNA clearance than in those without ctDNA clearance in both the nivolumab/chemotherapy group (HR, 0.60) and the chemotherapy-alone group (HR, 0.63).
Hopefully, ASCO 2023 will provide more answers.
Dr. Schiller is a medical oncologist and founding member of Oncologists United for Climate and Health. She is a former board member of the International Association for the Study of Lung Cancer and a current board member of the Lung Cancer Research Foundation.
for non–small cell lung cancer (NSCLC). While there has been some notable progress in this area, we need phase 3 trials that compare the two therapeutic approaches.
Investigators reporting at the 2022 annual meeting of American Society of Clinical Oncology focused primarily on neoadjuvant treatment, which I’ll address here.
In the randomized, phase 2 NADIM II clinical trial reported at the meeting, researchers expanded on the results of NADIM published in 2020 in the Lancet Oncology and in May 2022 in the Journal of Clinical Oncology along with CheckMate 816 results published in the New England Journal of Medicine.
In each of these three studies, researchers compared nivolumab plus chemotherapy versus chemotherapy alone (abstract 8501) as a neoadjuvant treatment for resectable stage IIIA NSCLC. In the study reported at ASCO 2022, patients with resectable clinical stage IIIA-B (per American Joint Committee on Cancer 8th edition) NSCLC and no known EGFR/ALK alterations, were randomized to receive preoperative nivolumab plus chemotherapy (paclitaxel and carboplatin; n = 57) or chemotherapy (n = 29) alone followed by surgery.
The primary endpoint was pathological complete response (pCR); secondary endpoints included major pathological response, safety and tolerability, impact on surgical issues such as delayed or canceled surgeries or length of hospital stay, overall survival and progression free survival. The pCR rate was 36.8% in the neoadjuvant nivolumab plus chemotherapy arm and 6.9% in the chemotherapy alone arm. (P = .0068). 25% of patients on the nivolumab plus chemo arm had grade 3-4 adverse events, compared with 10.3% in the control arm. 93% of patients on the nivolumab plus chemo arm underwent definitive surgery whereas 69.0% of the patients on the chemo alone arm had definitive surgery. (P = .008)
What else did we learn about neoadjuvant treatment at the meeting?
Investigators looking at the optimal number of neoadjuvant cycles (abstract 8500) found that three cycles of sintilimab (an investigational PD-1 inhibitor) produced a numerically higher major pathological response rate, compared with two cycles (when given in concert with platinum-doublet chemotherapy). And, neoadjuvant chemoradiotherapy does not result in significant survival benefits when compared with neoadjuvant chemotherapy alone (abstract 8503).
Of course, when it comes to resectable NSCLC, the goal of treatment is to increase the cure rate and improve survival. No randomized studies have reported yet on overall survival, probably because they are too immature. Instead, disease-free survival (DFS) or event-free survival (EFS) are often used as surrogate endpoints. Since none of the studies reported at ASCO reported on DFS or EFS, we need to look elsewhere. CheckMate 816 was a phase 3 study which randomized patients with stages IB-IIIA NSCLC to receive neoadjuvant nivolumab plus platinum-based chemotherapy or neoadjuvant platinum-based chemotherapy alone, followed by resection. The median EFS was 31.6 months with nivolumab plus chemotherapy and 20.8 months with chemotherapy alone (P = .005). The percentage of patients with a pCR was 24.0% and 2.2%, respectively (P < .001).
We all know one has to be careful when doing cross-trial comparisons as these studies differ by the percentage of patients with various stages of disease, the type of immunotherapy and chemotherapy used, etc. However, I think we can agree that neoadjuvant chemoimmunotherapy results in better outcomes than chemotherapy alone.
Of course, resectable NSCLC is, by definition, resectable. And traditionally, resection is followed by adjuvant chemotherapy to eradicate micrometastases. Unfortunately, the current standard of care for completely resected early-stage NSCLC (stage I [tumor ≥ 4 cm] to IIIA) involves adjuvant platinum-based combination chemotherapy which results in only a modest 4%-5% improvement in survival versus observation.
Given these modest results, as in the neoadjuvant space, investigators have looked at the benefit of adding immunotherapy to adjuvant chemotherapy. One such study has been reported. IMpower 010 randomly assigned patients with completely resected stage IB (tumors ≥ 4 cm) to IIIA NSCLC, whose tumor cells expressed at least 1% PD-L1, to receive adjuvant atezolizumab or best supportive care after adjuvant platinum-based chemotherapy. In the stage II-IIIA population whose tumors expressed PD-L1 on 1% or more of tumor cells, 3-year DFS rates were 60% and 48% in the atezolizumab and best supportive care arms, respectively (hazard ratio, 0·66 P =·.0039). In all patients in the stage II-IIIA population, the 3-year DFS rates were 56% in the atezolizumab group and 49% in the best supportive care group, (HR, 0.79; P = .020).
KEYNOTE-091, reported at the 2021 annual meeting of the European Society for Medical Oncology, randomized early-stage NSCLC patients following complete resection and adjuvant chemotherapy to pembrolizumab or placebo. Median DFS for the overall population was 53.6 months for patients in the pembro arm versus 42 months in the placebo arm (HR, 0.76; P = .0014). Interestingly, the benefit was not seen in patients with PD-L1 with at least 50%, where the 18-month DFS rate was 71.7% in the pembro arm and 70.2% in the placebo arm (HR, 0.82; P = .14). Although the contradictory results of PD-L1 as a biomarker is puzzling, I think we can agree that the addition of immunotherapy following adjuvant chemotherapy improves outcomes compared to adjuvant chemotherapy alone.
What to do when a patient presents with resectable disease?
Cross-trial comparisons are fraught with danger. Until we have a phase 3 study comparing concurrent neoadjuvant chemo/immunotherapy with concurrent adjuvant chemo/immunotherapy, I do not think we can answer the question “which is better?” However, there are some caveats to keep in mind when deciding on which approach to recommend to our patients: First, neoadjuvant treatment requires biomarker testing to ensure the patient does not have EGFR or ALK mutations. This will necessitate a delay in the operation. Will patients be willing to wait? Will the surgeon? Or, would patients prefer to proceed with surgery while the results are pending? Yes, neoadjuvant therapy gives you information regarding the pCR rate, but does that help you in subsequent management of the patient? We do not know.
Secondly, the two adjuvant studies used adjuvant chemotherapy followed by adjuvant immunotherapy, as contrasted to the neoadjuvant study which used concurrent chemo/immunotherapy. Given the longer duration of treatment in postoperative sequential adjuvant studies, there tends to be more drop off because of patients being unwilling or unfit postoperatively to receive long courses of therapy. In IMpower 010, 1,269 patients completed adjuvant chemotherapy; 1,005 were randomized, and of the 507 assigned to the atezolizumab/chemo group, only 323 completed treatment.
Finally, we must beware of using neoadjuvant chemo/immunotherapy to “down-stage” a patient. KEYNOTE-091 included patients with IIIA disease and no benefit to adjuvant chemotherapy followed by immunotherapy was found in this subgroup of patients, which leads me to wonder if these patients were appropriately selected as surgical candidates. In the NADIM II trials, 9 of 29 patients on the neoadjuvant chemotherapy were not resected.
So, many questions remain. In addition to the ones we’ve raised, there is a clear and immediate need for predictive and prognostic biomarkers. In the NADIM II trial, PD-L1 expression was a predictive biomarker of response. The pCR rate for patients with a PD-L1 tumor expression of less than 1%, 1%-49%, and 50% or higher was 15%, 41.7%, and 61.1%, respectively. However, in KEYNOTE-091, the benefit was not seen in patients with PD-L1 of at least than 50%, where the 18-month DFS rate was 71.7% in the pembro arm and 70.2% in the placebo arm.
Another possible biomarker: circulating tumor DNA. In the first NADIM study, three low pretreatment levels of ctDNA were significantly associated with improved progression-free survival and overall survival (HR, 0.20 and HR, 0.07, respectively). Although clinical response did not predict survival outcomes, undetectable ctDNA levels after neoadjuvant treatment were significantly associated with progression-free survival and overall survival (HR, 0.26 and HR0.04, respectively). Similarly, in CheckMate 816, clearance of ctDNA was associated with longer EFS in patients with ctDNA clearance than in those without ctDNA clearance in both the nivolumab/chemotherapy group (HR, 0.60) and the chemotherapy-alone group (HR, 0.63).
Hopefully, ASCO 2023 will provide more answers.
Dr. Schiller is a medical oncologist and founding member of Oncologists United for Climate and Health. She is a former board member of the International Association for the Study of Lung Cancer and a current board member of the Lung Cancer Research Foundation.
for non–small cell lung cancer (NSCLC). While there has been some notable progress in this area, we need phase 3 trials that compare the two therapeutic approaches.
Investigators reporting at the 2022 annual meeting of American Society of Clinical Oncology focused primarily on neoadjuvant treatment, which I’ll address here.
In the randomized, phase 2 NADIM II clinical trial reported at the meeting, researchers expanded on the results of NADIM published in 2020 in the Lancet Oncology and in May 2022 in the Journal of Clinical Oncology along with CheckMate 816 results published in the New England Journal of Medicine.
In each of these three studies, researchers compared nivolumab plus chemotherapy versus chemotherapy alone (abstract 8501) as a neoadjuvant treatment for resectable stage IIIA NSCLC. In the study reported at ASCO 2022, patients with resectable clinical stage IIIA-B (per American Joint Committee on Cancer 8th edition) NSCLC and no known EGFR/ALK alterations, were randomized to receive preoperative nivolumab plus chemotherapy (paclitaxel and carboplatin; n = 57) or chemotherapy (n = 29) alone followed by surgery.
The primary endpoint was pathological complete response (pCR); secondary endpoints included major pathological response, safety and tolerability, impact on surgical issues such as delayed or canceled surgeries or length of hospital stay, overall survival and progression free survival. The pCR rate was 36.8% in the neoadjuvant nivolumab plus chemotherapy arm and 6.9% in the chemotherapy alone arm. (P = .0068). 25% of patients on the nivolumab plus chemo arm had grade 3-4 adverse events, compared with 10.3% in the control arm. 93% of patients on the nivolumab plus chemo arm underwent definitive surgery whereas 69.0% of the patients on the chemo alone arm had definitive surgery. (P = .008)
What else did we learn about neoadjuvant treatment at the meeting?
Investigators looking at the optimal number of neoadjuvant cycles (abstract 8500) found that three cycles of sintilimab (an investigational PD-1 inhibitor) produced a numerically higher major pathological response rate, compared with two cycles (when given in concert with platinum-doublet chemotherapy). And, neoadjuvant chemoradiotherapy does not result in significant survival benefits when compared with neoadjuvant chemotherapy alone (abstract 8503).
Of course, when it comes to resectable NSCLC, the goal of treatment is to increase the cure rate and improve survival. No randomized studies have reported yet on overall survival, probably because they are too immature. Instead, disease-free survival (DFS) or event-free survival (EFS) are often used as surrogate endpoints. Since none of the studies reported at ASCO reported on DFS or EFS, we need to look elsewhere. CheckMate 816 was a phase 3 study which randomized patients with stages IB-IIIA NSCLC to receive neoadjuvant nivolumab plus platinum-based chemotherapy or neoadjuvant platinum-based chemotherapy alone, followed by resection. The median EFS was 31.6 months with nivolumab plus chemotherapy and 20.8 months with chemotherapy alone (P = .005). The percentage of patients with a pCR was 24.0% and 2.2%, respectively (P < .001).
We all know one has to be careful when doing cross-trial comparisons as these studies differ by the percentage of patients with various stages of disease, the type of immunotherapy and chemotherapy used, etc. However, I think we can agree that neoadjuvant chemoimmunotherapy results in better outcomes than chemotherapy alone.
Of course, resectable NSCLC is, by definition, resectable. And traditionally, resection is followed by adjuvant chemotherapy to eradicate micrometastases. Unfortunately, the current standard of care for completely resected early-stage NSCLC (stage I [tumor ≥ 4 cm] to IIIA) involves adjuvant platinum-based combination chemotherapy which results in only a modest 4%-5% improvement in survival versus observation.
Given these modest results, as in the neoadjuvant space, investigators have looked at the benefit of adding immunotherapy to adjuvant chemotherapy. One such study has been reported. IMpower 010 randomly assigned patients with completely resected stage IB (tumors ≥ 4 cm) to IIIA NSCLC, whose tumor cells expressed at least 1% PD-L1, to receive adjuvant atezolizumab or best supportive care after adjuvant platinum-based chemotherapy. In the stage II-IIIA population whose tumors expressed PD-L1 on 1% or more of tumor cells, 3-year DFS rates were 60% and 48% in the atezolizumab and best supportive care arms, respectively (hazard ratio, 0·66 P =·.0039). In all patients in the stage II-IIIA population, the 3-year DFS rates were 56% in the atezolizumab group and 49% in the best supportive care group, (HR, 0.79; P = .020).
KEYNOTE-091, reported at the 2021 annual meeting of the European Society for Medical Oncology, randomized early-stage NSCLC patients following complete resection and adjuvant chemotherapy to pembrolizumab or placebo. Median DFS for the overall population was 53.6 months for patients in the pembro arm versus 42 months in the placebo arm (HR, 0.76; P = .0014). Interestingly, the benefit was not seen in patients with PD-L1 with at least 50%, where the 18-month DFS rate was 71.7% in the pembro arm and 70.2% in the placebo arm (HR, 0.82; P = .14). Although the contradictory results of PD-L1 as a biomarker is puzzling, I think we can agree that the addition of immunotherapy following adjuvant chemotherapy improves outcomes compared to adjuvant chemotherapy alone.
What to do when a patient presents with resectable disease?
Cross-trial comparisons are fraught with danger. Until we have a phase 3 study comparing concurrent neoadjuvant chemo/immunotherapy with concurrent adjuvant chemo/immunotherapy, I do not think we can answer the question “which is better?” However, there are some caveats to keep in mind when deciding on which approach to recommend to our patients: First, neoadjuvant treatment requires biomarker testing to ensure the patient does not have EGFR or ALK mutations. This will necessitate a delay in the operation. Will patients be willing to wait? Will the surgeon? Or, would patients prefer to proceed with surgery while the results are pending? Yes, neoadjuvant therapy gives you information regarding the pCR rate, but does that help you in subsequent management of the patient? We do not know.
Secondly, the two adjuvant studies used adjuvant chemotherapy followed by adjuvant immunotherapy, as contrasted to the neoadjuvant study which used concurrent chemo/immunotherapy. Given the longer duration of treatment in postoperative sequential adjuvant studies, there tends to be more drop off because of patients being unwilling or unfit postoperatively to receive long courses of therapy. In IMpower 010, 1,269 patients completed adjuvant chemotherapy; 1,005 were randomized, and of the 507 assigned to the atezolizumab/chemo group, only 323 completed treatment.
Finally, we must beware of using neoadjuvant chemo/immunotherapy to “down-stage” a patient. KEYNOTE-091 included patients with IIIA disease and no benefit to adjuvant chemotherapy followed by immunotherapy was found in this subgroup of patients, which leads me to wonder if these patients were appropriately selected as surgical candidates. In the NADIM II trials, 9 of 29 patients on the neoadjuvant chemotherapy were not resected.
So, many questions remain. In addition to the ones we’ve raised, there is a clear and immediate need for predictive and prognostic biomarkers. In the NADIM II trial, PD-L1 expression was a predictive biomarker of response. The pCR rate for patients with a PD-L1 tumor expression of less than 1%, 1%-49%, and 50% or higher was 15%, 41.7%, and 61.1%, respectively. However, in KEYNOTE-091, the benefit was not seen in patients with PD-L1 of at least than 50%, where the 18-month DFS rate was 71.7% in the pembro arm and 70.2% in the placebo arm.
Another possible biomarker: circulating tumor DNA. In the first NADIM study, three low pretreatment levels of ctDNA were significantly associated with improved progression-free survival and overall survival (HR, 0.20 and HR, 0.07, respectively). Although clinical response did not predict survival outcomes, undetectable ctDNA levels after neoadjuvant treatment were significantly associated with progression-free survival and overall survival (HR, 0.26 and HR0.04, respectively). Similarly, in CheckMate 816, clearance of ctDNA was associated with longer EFS in patients with ctDNA clearance than in those without ctDNA clearance in both the nivolumab/chemotherapy group (HR, 0.60) and the chemotherapy-alone group (HR, 0.63).
Hopefully, ASCO 2023 will provide more answers.
Dr. Schiller is a medical oncologist and founding member of Oncologists United for Climate and Health. She is a former board member of the International Association for the Study of Lung Cancer and a current board member of the Lung Cancer Research Foundation.
Roe v. Wade: Medical groups react to Supreme Court decision
The country’s top medical organizations condemned the overturning of Roe v. Wade, saying the removal of federal protections for women to access abortion services marks a “dark day.”
“It is unfathomable. It is unfair. It is wrong,” said the President of the American College of Obstetricians and Gynecologists (ACOG) Iffath Abbasi Hoskins, MD.
“Today is a very dark day in health care. It is a dark day, indeed, for the tens of millions of patients who have suddenly and unfairly lost access to safe legal and evidence-based abortion care,” Dr. Hoskins said at a press conference June 24 sponsored by ACOG.
“It is dark for the thousands of clinicians who now, instead of focusing on providing health care to their patients, have to live with the threats of legal, civil, and even professional penalties,” Dr. Hoskins added.
ACOG has 62,000 members and is the leading group of doctors that provides obstetric and gynecologic care.
Dilemma for some doctors?
“I’d like to take a moment to talk about the future of the medical profession,” said ACOG Chief Executive Officer Maureen G. Phipps, MD, MPH. “[The] decision is, as Dr. Hoskins clearly said, a tragic one for our patients in states across the country, but the harm does not end there.”
Dr. Phipps described overturning Roe v. Wade as “the boldest act of legislative interference that we have seen in this country. It will allow state legislators to tell physicians what care they can and cannot provide to their patients.”
“It will leave physicians looking over our shoulders, wondering if a patient is in enough of a crisis to permit an exception to a law,” Dr. Phipps added. “This is an affront to all that drew my colleagues and me into medicine.”
Although the impact on doctor training remains to be seen, she said 44% of ob.gyn. residents are trained in states now empowered to ban abortions.
The effect of the Supreme Court decision on miscarriage management is another unknown.
“It’s going to be very difficult for us, the clinicians, to manage miscarriage,” Dr. Hoskins said. “Many miscarriages could be what we call ‘incomplete’ in the beginning,” where there is still a heartbeat and the patient is cramping and/or bleeding.
In that instance, Dr. Hoskins said, clinicians may be thinking that they have to wait.
“They may be needing to get additional opinions, whether it’s a legal opinion ... or another medical opinion.”
“It’s going to have a devastating effect on every aspect of a woman’s health care, including if she is spontaneously miscarrying,” Dr. Hoskins predicted.
Physician protect thyself?
To what extent doctors can shield themselves from potential prosecution “is a hard question to answer,” Molly Meegan, JD, ACOG’s chief legal officer and general counsel, said.
Ms. Meegan recommended members speak to the risk managers at their individual institutions for guidance.
“It is a real patchwork [of laws] out there, she said. “And that patchwork itself is a danger to people as they seek essential reproductive health care.”
Also, she added, “If a doctor can’t tell what the law is at the time they’re trying to provide the care, it has a terribly chilling effect on medical care.”
Another potential threat to doctors in states that still allow abortion services is action from a neighboring state.
“We are going to be advocating very strongly that states do not have extra-territorial jurisdiction to reach beyond the edges of their state.”
The worry is if a doctor in New Mexico, where abortion is legal, performs an abortion for a person from Texas, where it will soon be illegal, is then prosecuted by Texas, for example.
Medication abortion
Asked about any potential effects on medication abortions, ACOG’s Jen Villavicencio, MD, said it remains to be seen.
“Certainly many of the laws that we have seen, including trigger ban laws, encompass medication abortion,” she said. Several states have these so-called trigger laws, which put into effect laws passed to ban abortion in case Roe was overturned.
This means, she said, that any abortion option, whether it’s procedural or medication, could be and will be banned in some of these states.
Ms. Meegan added that ACOG will continue to support access to medication abortion and that it should be decided by the U.S. Food and Drug Administration and not individual states.
Maternal mortality may rise
“Maternal mortality in and of itself is a very difficult topic,” Dr. Hoskins said, but [the] decision amplifies the implications. “I think of the patients who will have to manage severe complications and mental health challenges while they are carrying a pregnancy that they are forced to carry.”
“I also think of the patients who need to end their pregnancies in order to save their own lives,” Dr. Hoskins added.
Dr. Hoskins said the United States already has a high maternal mortality rate. This new law, she added, could force women into higher-risk situations if they experience high blood pressure, preeclampsia, or bleeding after the birth of the baby.
Growing inequality possible?
“The grievous inequities that exist in this country will grow and expand unchecked without safe access to legal abortion,” Dr. Phipps said.
She noted that women, based on location, will continue “to have protected access to safe evidence-based abortion. Others will have the means and resources and opportunities to secure the care.”
But the same may not be true for women in underserved or disadvantaged communities, Dr. Phipps added.
American Medical Association
ACOG was not the only group to react. “The American Medical Association is deeply disturbed by the U.S. Supreme Court’s decision to overturn nearly a half century of precedent protecting patients’ right to critical reproductive health care,” President Jack Resneck Jr., MD, said in a statement.
The decision represents “an egregious allowance of government intrusion into the medical examination room, a direct attack on the practice of medicine and the patient-physician relationship, and a brazen violation of patients’ rights to evidence-based reproductive health services.”
American Academy of Family Physicians
“The American Academy of Family Physicians is disappointed and disheartened by the Supreme Court’s decision to strike down longstanding protections afforded by Roe v. Wade and Planned Parenthood v. Casey,” President Sterling N. Ransone Jr., MD, said in a statement.
The organization has 127,600 physician and medical student members.
“This decision negatively impacts our practices and our patients by undermining the patient-physician relationship and potentially criminalizing evidence-based medical care,” added Dr. Ransone.
American College of Physicians
“A patient’s decision about whether to continue a pregnancy should be a private decision made in consultation with a physician or other health care professional, without interference from the government,” President Ryan D. Mire, MD, said in a statement. “We strongly oppose medically unnecessary government restrictions on any health care services,” added Dr. Mire on behalf of the group’s 161,000 members.
American Academy of Pediatrics
“This decision carries grave consequences for our adolescent patients, who already face many more barriers than adults in accessing comprehensive reproductive health care services and abortion care,” President Moira Szilagyi, MD, PhD, said in a statement.
“In the wake of this ruling, the American Academy of Pediatrics will continue to support our chapters as states consider policies affecting access to abortion care, and pediatricians will continue to support our patients,” Dr. Szilagyi added.
American Public Health Association
The court’s decision “is a catastrophic judicial failure that will reverberate differently in each state and portends to jeopardize the health and lives of all Americans,” Executive Director Georges C. Benjamin, MD, said in a statement.
American Urogynecologic Society
“The American Urogynecologic Society opposes any ruling that restricts a person’s access to health care and criminalizes the practice of medicine,” the group said in a statement. “This ruling ultimately poses a serious threat to the patient-provider relationship and subsequent decisionmaking necessary to ensure optimal outcomes for patients. As practitioners, we should be free to provide what is in the best interest of our patients.”
A version of this article first appeared on Medscape.com.
The country’s top medical organizations condemned the overturning of Roe v. Wade, saying the removal of federal protections for women to access abortion services marks a “dark day.”
“It is unfathomable. It is unfair. It is wrong,” said the President of the American College of Obstetricians and Gynecologists (ACOG) Iffath Abbasi Hoskins, MD.
“Today is a very dark day in health care. It is a dark day, indeed, for the tens of millions of patients who have suddenly and unfairly lost access to safe legal and evidence-based abortion care,” Dr. Hoskins said at a press conference June 24 sponsored by ACOG.
“It is dark for the thousands of clinicians who now, instead of focusing on providing health care to their patients, have to live with the threats of legal, civil, and even professional penalties,” Dr. Hoskins added.
ACOG has 62,000 members and is the leading group of doctors that provides obstetric and gynecologic care.
Dilemma for some doctors?
“I’d like to take a moment to talk about the future of the medical profession,” said ACOG Chief Executive Officer Maureen G. Phipps, MD, MPH. “[The] decision is, as Dr. Hoskins clearly said, a tragic one for our patients in states across the country, but the harm does not end there.”
Dr. Phipps described overturning Roe v. Wade as “the boldest act of legislative interference that we have seen in this country. It will allow state legislators to tell physicians what care they can and cannot provide to their patients.”
“It will leave physicians looking over our shoulders, wondering if a patient is in enough of a crisis to permit an exception to a law,” Dr. Phipps added. “This is an affront to all that drew my colleagues and me into medicine.”
Although the impact on doctor training remains to be seen, she said 44% of ob.gyn. residents are trained in states now empowered to ban abortions.
The effect of the Supreme Court decision on miscarriage management is another unknown.
“It’s going to be very difficult for us, the clinicians, to manage miscarriage,” Dr. Hoskins said. “Many miscarriages could be what we call ‘incomplete’ in the beginning,” where there is still a heartbeat and the patient is cramping and/or bleeding.
In that instance, Dr. Hoskins said, clinicians may be thinking that they have to wait.
“They may be needing to get additional opinions, whether it’s a legal opinion ... or another medical opinion.”
“It’s going to have a devastating effect on every aspect of a woman’s health care, including if she is spontaneously miscarrying,” Dr. Hoskins predicted.
Physician protect thyself?
To what extent doctors can shield themselves from potential prosecution “is a hard question to answer,” Molly Meegan, JD, ACOG’s chief legal officer and general counsel, said.
Ms. Meegan recommended members speak to the risk managers at their individual institutions for guidance.
“It is a real patchwork [of laws] out there, she said. “And that patchwork itself is a danger to people as they seek essential reproductive health care.”
Also, she added, “If a doctor can’t tell what the law is at the time they’re trying to provide the care, it has a terribly chilling effect on medical care.”
Another potential threat to doctors in states that still allow abortion services is action from a neighboring state.
“We are going to be advocating very strongly that states do not have extra-territorial jurisdiction to reach beyond the edges of their state.”
The worry is if a doctor in New Mexico, where abortion is legal, performs an abortion for a person from Texas, where it will soon be illegal, is then prosecuted by Texas, for example.
Medication abortion
Asked about any potential effects on medication abortions, ACOG’s Jen Villavicencio, MD, said it remains to be seen.
“Certainly many of the laws that we have seen, including trigger ban laws, encompass medication abortion,” she said. Several states have these so-called trigger laws, which put into effect laws passed to ban abortion in case Roe was overturned.
This means, she said, that any abortion option, whether it’s procedural or medication, could be and will be banned in some of these states.
Ms. Meegan added that ACOG will continue to support access to medication abortion and that it should be decided by the U.S. Food and Drug Administration and not individual states.
Maternal mortality may rise
“Maternal mortality in and of itself is a very difficult topic,” Dr. Hoskins said, but [the] decision amplifies the implications. “I think of the patients who will have to manage severe complications and mental health challenges while they are carrying a pregnancy that they are forced to carry.”
“I also think of the patients who need to end their pregnancies in order to save their own lives,” Dr. Hoskins added.
Dr. Hoskins said the United States already has a high maternal mortality rate. This new law, she added, could force women into higher-risk situations if they experience high blood pressure, preeclampsia, or bleeding after the birth of the baby.
Growing inequality possible?
“The grievous inequities that exist in this country will grow and expand unchecked without safe access to legal abortion,” Dr. Phipps said.
She noted that women, based on location, will continue “to have protected access to safe evidence-based abortion. Others will have the means and resources and opportunities to secure the care.”
But the same may not be true for women in underserved or disadvantaged communities, Dr. Phipps added.
American Medical Association
ACOG was not the only group to react. “The American Medical Association is deeply disturbed by the U.S. Supreme Court’s decision to overturn nearly a half century of precedent protecting patients’ right to critical reproductive health care,” President Jack Resneck Jr., MD, said in a statement.
The decision represents “an egregious allowance of government intrusion into the medical examination room, a direct attack on the practice of medicine and the patient-physician relationship, and a brazen violation of patients’ rights to evidence-based reproductive health services.”
American Academy of Family Physicians
“The American Academy of Family Physicians is disappointed and disheartened by the Supreme Court’s decision to strike down longstanding protections afforded by Roe v. Wade and Planned Parenthood v. Casey,” President Sterling N. Ransone Jr., MD, said in a statement.
The organization has 127,600 physician and medical student members.
“This decision negatively impacts our practices and our patients by undermining the patient-physician relationship and potentially criminalizing evidence-based medical care,” added Dr. Ransone.
American College of Physicians
“A patient’s decision about whether to continue a pregnancy should be a private decision made in consultation with a physician or other health care professional, without interference from the government,” President Ryan D. Mire, MD, said in a statement. “We strongly oppose medically unnecessary government restrictions on any health care services,” added Dr. Mire on behalf of the group’s 161,000 members.
American Academy of Pediatrics
“This decision carries grave consequences for our adolescent patients, who already face many more barriers than adults in accessing comprehensive reproductive health care services and abortion care,” President Moira Szilagyi, MD, PhD, said in a statement.
“In the wake of this ruling, the American Academy of Pediatrics will continue to support our chapters as states consider policies affecting access to abortion care, and pediatricians will continue to support our patients,” Dr. Szilagyi added.
American Public Health Association
The court’s decision “is a catastrophic judicial failure that will reverberate differently in each state and portends to jeopardize the health and lives of all Americans,” Executive Director Georges C. Benjamin, MD, said in a statement.
American Urogynecologic Society
“The American Urogynecologic Society opposes any ruling that restricts a person’s access to health care and criminalizes the practice of medicine,” the group said in a statement. “This ruling ultimately poses a serious threat to the patient-provider relationship and subsequent decisionmaking necessary to ensure optimal outcomes for patients. As practitioners, we should be free to provide what is in the best interest of our patients.”
A version of this article first appeared on Medscape.com.
The country’s top medical organizations condemned the overturning of Roe v. Wade, saying the removal of federal protections for women to access abortion services marks a “dark day.”
“It is unfathomable. It is unfair. It is wrong,” said the President of the American College of Obstetricians and Gynecologists (ACOG) Iffath Abbasi Hoskins, MD.
“Today is a very dark day in health care. It is a dark day, indeed, for the tens of millions of patients who have suddenly and unfairly lost access to safe legal and evidence-based abortion care,” Dr. Hoskins said at a press conference June 24 sponsored by ACOG.
“It is dark for the thousands of clinicians who now, instead of focusing on providing health care to their patients, have to live with the threats of legal, civil, and even professional penalties,” Dr. Hoskins added.
ACOG has 62,000 members and is the leading group of doctors that provides obstetric and gynecologic care.
Dilemma for some doctors?
“I’d like to take a moment to talk about the future of the medical profession,” said ACOG Chief Executive Officer Maureen G. Phipps, MD, MPH. “[The] decision is, as Dr. Hoskins clearly said, a tragic one for our patients in states across the country, but the harm does not end there.”
Dr. Phipps described overturning Roe v. Wade as “the boldest act of legislative interference that we have seen in this country. It will allow state legislators to tell physicians what care they can and cannot provide to their patients.”
“It will leave physicians looking over our shoulders, wondering if a patient is in enough of a crisis to permit an exception to a law,” Dr. Phipps added. “This is an affront to all that drew my colleagues and me into medicine.”
Although the impact on doctor training remains to be seen, she said 44% of ob.gyn. residents are trained in states now empowered to ban abortions.
The effect of the Supreme Court decision on miscarriage management is another unknown.
“It’s going to be very difficult for us, the clinicians, to manage miscarriage,” Dr. Hoskins said. “Many miscarriages could be what we call ‘incomplete’ in the beginning,” where there is still a heartbeat and the patient is cramping and/or bleeding.
In that instance, Dr. Hoskins said, clinicians may be thinking that they have to wait.
“They may be needing to get additional opinions, whether it’s a legal opinion ... or another medical opinion.”
“It’s going to have a devastating effect on every aspect of a woman’s health care, including if she is spontaneously miscarrying,” Dr. Hoskins predicted.
Physician protect thyself?
To what extent doctors can shield themselves from potential prosecution “is a hard question to answer,” Molly Meegan, JD, ACOG’s chief legal officer and general counsel, said.
Ms. Meegan recommended members speak to the risk managers at their individual institutions for guidance.
“It is a real patchwork [of laws] out there, she said. “And that patchwork itself is a danger to people as they seek essential reproductive health care.”
Also, she added, “If a doctor can’t tell what the law is at the time they’re trying to provide the care, it has a terribly chilling effect on medical care.”
Another potential threat to doctors in states that still allow abortion services is action from a neighboring state.
“We are going to be advocating very strongly that states do not have extra-territorial jurisdiction to reach beyond the edges of their state.”
The worry is if a doctor in New Mexico, where abortion is legal, performs an abortion for a person from Texas, where it will soon be illegal, is then prosecuted by Texas, for example.
Medication abortion
Asked about any potential effects on medication abortions, ACOG’s Jen Villavicencio, MD, said it remains to be seen.
“Certainly many of the laws that we have seen, including trigger ban laws, encompass medication abortion,” she said. Several states have these so-called trigger laws, which put into effect laws passed to ban abortion in case Roe was overturned.
This means, she said, that any abortion option, whether it’s procedural or medication, could be and will be banned in some of these states.
Ms. Meegan added that ACOG will continue to support access to medication abortion and that it should be decided by the U.S. Food and Drug Administration and not individual states.
Maternal mortality may rise
“Maternal mortality in and of itself is a very difficult topic,” Dr. Hoskins said, but [the] decision amplifies the implications. “I think of the patients who will have to manage severe complications and mental health challenges while they are carrying a pregnancy that they are forced to carry.”
“I also think of the patients who need to end their pregnancies in order to save their own lives,” Dr. Hoskins added.
Dr. Hoskins said the United States already has a high maternal mortality rate. This new law, she added, could force women into higher-risk situations if they experience high blood pressure, preeclampsia, or bleeding after the birth of the baby.
Growing inequality possible?
“The grievous inequities that exist in this country will grow and expand unchecked without safe access to legal abortion,” Dr. Phipps said.
She noted that women, based on location, will continue “to have protected access to safe evidence-based abortion. Others will have the means and resources and opportunities to secure the care.”
But the same may not be true for women in underserved or disadvantaged communities, Dr. Phipps added.
American Medical Association
ACOG was not the only group to react. “The American Medical Association is deeply disturbed by the U.S. Supreme Court’s decision to overturn nearly a half century of precedent protecting patients’ right to critical reproductive health care,” President Jack Resneck Jr., MD, said in a statement.
The decision represents “an egregious allowance of government intrusion into the medical examination room, a direct attack on the practice of medicine and the patient-physician relationship, and a brazen violation of patients’ rights to evidence-based reproductive health services.”
American Academy of Family Physicians
“The American Academy of Family Physicians is disappointed and disheartened by the Supreme Court’s decision to strike down longstanding protections afforded by Roe v. Wade and Planned Parenthood v. Casey,” President Sterling N. Ransone Jr., MD, said in a statement.
The organization has 127,600 physician and medical student members.
“This decision negatively impacts our practices and our patients by undermining the patient-physician relationship and potentially criminalizing evidence-based medical care,” added Dr. Ransone.
American College of Physicians
“A patient’s decision about whether to continue a pregnancy should be a private decision made in consultation with a physician or other health care professional, without interference from the government,” President Ryan D. Mire, MD, said in a statement. “We strongly oppose medically unnecessary government restrictions on any health care services,” added Dr. Mire on behalf of the group’s 161,000 members.
American Academy of Pediatrics
“This decision carries grave consequences for our adolescent patients, who already face many more barriers than adults in accessing comprehensive reproductive health care services and abortion care,” President Moira Szilagyi, MD, PhD, said in a statement.
“In the wake of this ruling, the American Academy of Pediatrics will continue to support our chapters as states consider policies affecting access to abortion care, and pediatricians will continue to support our patients,” Dr. Szilagyi added.
American Public Health Association
The court’s decision “is a catastrophic judicial failure that will reverberate differently in each state and portends to jeopardize the health and lives of all Americans,” Executive Director Georges C. Benjamin, MD, said in a statement.
American Urogynecologic Society
“The American Urogynecologic Society opposes any ruling that restricts a person’s access to health care and criminalizes the practice of medicine,” the group said in a statement. “This ruling ultimately poses a serious threat to the patient-provider relationship and subsequent decisionmaking necessary to ensure optimal outcomes for patients. As practitioners, we should be free to provide what is in the best interest of our patients.”
A version of this article first appeared on Medscape.com.
Establishing a formalized mentorship program in a community practice
Most GI physicians will tell you that we didn’t get where we are without help along the way. Each of us can point to one – or in most cases, several – specific mentors who provided invaluable guidance when we were in medical school, fellowship, and starting our careers. This is true for gastroenterologists across the spectrum, whether they chose careers in private practice, academic medicine, or within a hospital system.
The leadership at Atlanta Gastroenterology Associates, where I practice, has always recognized the importance of mentorship and its role in developing fulfilling careers for its physicians and a healthy practice culture in which people feel valued and supported. But only recently have we begun to create a formalized program to ensure that everyone has access to mentors.
When I started my career, formalized programs for mentorship did not exist. While I reached out to various doctors in my office and the senior partners throughout the practice, not everyone is comfortable proactively reaching out to ask practice leadership for help and guidance. And as independent GI practices continue to get bigger, there may not be as many opportunities to interact with senior leadership or executives, which means new associates could be left to “cold call” potential mentors by phone or email.
New associates face many challenges
When I was an associate, the path to partnership looked much different than it does in our practice now, and it definitely varies from practice to practice. In our practice, physicians remain associates for 2-3 years before they have the option to become a partner. As they work diligently to provide the best care to patients, new associates face many challenges, like learning how to build a practice and interact with referring physicians, understanding the process to become a partner, and figuring out how to juggle other commitments, such as the balance between work and home life.
Then there are things like buying a home, getting life and disability insurance, and understanding the financial planning aspects of being a business owner. For those whose group model requires buying into the practice to become a partner, medical school doesn’t teach you how to analyze the return on an investment. Providing access to people who have this experience through a mentorship program can help associates be more prepared to become partners, and hopefully be happier and more successful while doing so.
Formalizing a mentorship program
The mentorship program at Atlanta Gastroenterology Associates matches new physicians with a partner-level mentor with whom they are encouraged to meet monthly. We’ve even provided a budget so that mentees and mentors can meet for dinner or coffee and get to know each other better.
The program starts with a meeting of volunteer partners and associates, who then rank their preferred choices for potential mentors. This helps to ensure that the associates are able get to know the mentors a little bit and decide who might be the best fit for their needs. This is also important for new associates who don’t feel comfortable proactively searching for a mentor as they’re able to provide a list of partners they think would be the best match for them.
Each associate is then assigned a partner-level mentor who is responsible for guiding them and providing education around not only clinical care, but also business, marketing, health policy, and all the other critical components of running a successful private practice. Our program specifically pairs mentors and mentees who do not work in the same location. We wanted the mentors to be paired with associates they are not interacting with daily, to ensure our associates get exposed to different perspectives.
As a group, program participants get together every quarter – twice a year in person, and twice virtually. One of the in-person meetings brings together the mentors and mentees with C-suite executives to meet and network with the practice leadership. We organized the program this way because otherwise, most associates wouldn’t get many opportunities to interact with the CEO, chief medical officer, or chief operating officer in any capacity, let alone in a small gathering where they can engage on a personal level.
The second in-person meeting is a dinner with the mentors and mentees, along with their significant others. We understand that all our physicians have responsibilities outside of work, and bringing families together helps provide new associates with a network of support for questions that aren’t work-related. For associates who are not from Atlanta, this can be especially helpful in figuring out housing, schools, and other aspects of work/life balance.
The other two virtual meetings include the C-suite executives and our physician executive board. We develop specific agenda topics related to a career in private practice and provide a forum for the associates to ask practice leadership questions about challenges they may face on their path to becoming a partner.
At the end of each year, we survey the current program participants to see what was successful and what can be improved for the next cohort of incoming associates. So far, the feedback we’ve received from the mentors and mentees has been overwhelmingly positive.
Mentorship benefits the entire practice
As groups continue to grow, more practices may begin to formalize their mentorship programs, particularly those who see the merit they provide in helping recruit and retain valuable associates. To supplement our internal mentorship program, we’ve also started reaching out to local fellowship programs to provide resources to fellows who are considering private practice.
Even though about half of gastroenterology fellows choose independent GI, many aren’t educated about what it means to choose a career in private practice. Our outreach to provide information at the fellowship level is aimed at giving early career physicians an opportunity to know the benefits and challenges associated with private practice. Furthermore, we strive to educate fellows about the resources that are available to guide them through not only joining a group, but also having a successful career.
Leaders of independent GI groups need physicians who want the practice to succeed. Medical school trains physicians to take care of patients but falls short on training physicians to run a business. And building good, strong businesses makes sure that the next generation of leaders are prepared to take over.
Supporting the next generation of practice leaders helps current leadership make changes that will ensure practice sustainability. Often, associates are at the forefront of new technologies, both in terms of patient care, and in terms of practice management, communications, marketing, and advertising. As times change, having associates who are engaged and excited will help any practice be positioned positively for whatever the future holds.
What to look for in joining a practice
Ideally, people should start looking for mentors when they’re looking for a residency program. Joining a practice isn’t much different. If you’re an early career physician who is considering private practice, find an independent GI physician who can tell you about their experiences. And when you’re interviewing with practices, be sure to ask questions about how the group approaches mentorship. If a practice doesn’t have a formal mentorship program, it doesn’t mean it’s an environment where mentorship relationships won’t flourish. In many practices, informal mentorship programs are very successful.
Ask questions about how the practice provides or supports mentorship. Does the practice leadership make themselves available as mentors? Does the practice expect new physicians to find and nurture mentorship relations on their own? Ask about the path to becoming a partner and who is available to discuss challenges, concerns, or any questions that arise.
Independent GI practices are partnerships that seek to provide high-quality care at a lower cost to our community. Strengthening and sustaining that partnership requires us to take the time and continuously invest in the future of new physicians who will go on to serve our community as partner physicians retire. By formalizing a mentorship program, we’re hoping to make it easier for our mentors and mentees to create productive partnerships that will strengthen our group, and ultimately independent gastroenterology overall.
Dr. Sonenshine is a practicing gastroenterologist at Atlanta Gastroenterology Associates, and a partner in United Digestive. He previously served as the chair of communications as a member of the Executive Committee of the Digestive Health Physicians Association. Dr. Sonenshine has no conflicts to declare.
Most GI physicians will tell you that we didn’t get where we are without help along the way. Each of us can point to one – or in most cases, several – specific mentors who provided invaluable guidance when we were in medical school, fellowship, and starting our careers. This is true for gastroenterologists across the spectrum, whether they chose careers in private practice, academic medicine, or within a hospital system.
The leadership at Atlanta Gastroenterology Associates, where I practice, has always recognized the importance of mentorship and its role in developing fulfilling careers for its physicians and a healthy practice culture in which people feel valued and supported. But only recently have we begun to create a formalized program to ensure that everyone has access to mentors.
When I started my career, formalized programs for mentorship did not exist. While I reached out to various doctors in my office and the senior partners throughout the practice, not everyone is comfortable proactively reaching out to ask practice leadership for help and guidance. And as independent GI practices continue to get bigger, there may not be as many opportunities to interact with senior leadership or executives, which means new associates could be left to “cold call” potential mentors by phone or email.
New associates face many challenges
When I was an associate, the path to partnership looked much different than it does in our practice now, and it definitely varies from practice to practice. In our practice, physicians remain associates for 2-3 years before they have the option to become a partner. As they work diligently to provide the best care to patients, new associates face many challenges, like learning how to build a practice and interact with referring physicians, understanding the process to become a partner, and figuring out how to juggle other commitments, such as the balance between work and home life.
Then there are things like buying a home, getting life and disability insurance, and understanding the financial planning aspects of being a business owner. For those whose group model requires buying into the practice to become a partner, medical school doesn’t teach you how to analyze the return on an investment. Providing access to people who have this experience through a mentorship program can help associates be more prepared to become partners, and hopefully be happier and more successful while doing so.
Formalizing a mentorship program
The mentorship program at Atlanta Gastroenterology Associates matches new physicians with a partner-level mentor with whom they are encouraged to meet monthly. We’ve even provided a budget so that mentees and mentors can meet for dinner or coffee and get to know each other better.
The program starts with a meeting of volunteer partners and associates, who then rank their preferred choices for potential mentors. This helps to ensure that the associates are able get to know the mentors a little bit and decide who might be the best fit for their needs. This is also important for new associates who don’t feel comfortable proactively searching for a mentor as they’re able to provide a list of partners they think would be the best match for them.
Each associate is then assigned a partner-level mentor who is responsible for guiding them and providing education around not only clinical care, but also business, marketing, health policy, and all the other critical components of running a successful private practice. Our program specifically pairs mentors and mentees who do not work in the same location. We wanted the mentors to be paired with associates they are not interacting with daily, to ensure our associates get exposed to different perspectives.
As a group, program participants get together every quarter – twice a year in person, and twice virtually. One of the in-person meetings brings together the mentors and mentees with C-suite executives to meet and network with the practice leadership. We organized the program this way because otherwise, most associates wouldn’t get many opportunities to interact with the CEO, chief medical officer, or chief operating officer in any capacity, let alone in a small gathering where they can engage on a personal level.
The second in-person meeting is a dinner with the mentors and mentees, along with their significant others. We understand that all our physicians have responsibilities outside of work, and bringing families together helps provide new associates with a network of support for questions that aren’t work-related. For associates who are not from Atlanta, this can be especially helpful in figuring out housing, schools, and other aspects of work/life balance.
The other two virtual meetings include the C-suite executives and our physician executive board. We develop specific agenda topics related to a career in private practice and provide a forum for the associates to ask practice leadership questions about challenges they may face on their path to becoming a partner.
At the end of each year, we survey the current program participants to see what was successful and what can be improved for the next cohort of incoming associates. So far, the feedback we’ve received from the mentors and mentees has been overwhelmingly positive.
Mentorship benefits the entire practice
As groups continue to grow, more practices may begin to formalize their mentorship programs, particularly those who see the merit they provide in helping recruit and retain valuable associates. To supplement our internal mentorship program, we’ve also started reaching out to local fellowship programs to provide resources to fellows who are considering private practice.
Even though about half of gastroenterology fellows choose independent GI, many aren’t educated about what it means to choose a career in private practice. Our outreach to provide information at the fellowship level is aimed at giving early career physicians an opportunity to know the benefits and challenges associated with private practice. Furthermore, we strive to educate fellows about the resources that are available to guide them through not only joining a group, but also having a successful career.
Leaders of independent GI groups need physicians who want the practice to succeed. Medical school trains physicians to take care of patients but falls short on training physicians to run a business. And building good, strong businesses makes sure that the next generation of leaders are prepared to take over.
Supporting the next generation of practice leaders helps current leadership make changes that will ensure practice sustainability. Often, associates are at the forefront of new technologies, both in terms of patient care, and in terms of practice management, communications, marketing, and advertising. As times change, having associates who are engaged and excited will help any practice be positioned positively for whatever the future holds.
What to look for in joining a practice
Ideally, people should start looking for mentors when they’re looking for a residency program. Joining a practice isn’t much different. If you’re an early career physician who is considering private practice, find an independent GI physician who can tell you about their experiences. And when you’re interviewing with practices, be sure to ask questions about how the group approaches mentorship. If a practice doesn’t have a formal mentorship program, it doesn’t mean it’s an environment where mentorship relationships won’t flourish. In many practices, informal mentorship programs are very successful.
Ask questions about how the practice provides or supports mentorship. Does the practice leadership make themselves available as mentors? Does the practice expect new physicians to find and nurture mentorship relations on their own? Ask about the path to becoming a partner and who is available to discuss challenges, concerns, or any questions that arise.
Independent GI practices are partnerships that seek to provide high-quality care at a lower cost to our community. Strengthening and sustaining that partnership requires us to take the time and continuously invest in the future of new physicians who will go on to serve our community as partner physicians retire. By formalizing a mentorship program, we’re hoping to make it easier for our mentors and mentees to create productive partnerships that will strengthen our group, and ultimately independent gastroenterology overall.
Dr. Sonenshine is a practicing gastroenterologist at Atlanta Gastroenterology Associates, and a partner in United Digestive. He previously served as the chair of communications as a member of the Executive Committee of the Digestive Health Physicians Association. Dr. Sonenshine has no conflicts to declare.
Most GI physicians will tell you that we didn’t get where we are without help along the way. Each of us can point to one – or in most cases, several – specific mentors who provided invaluable guidance when we were in medical school, fellowship, and starting our careers. This is true for gastroenterologists across the spectrum, whether they chose careers in private practice, academic medicine, or within a hospital system.
The leadership at Atlanta Gastroenterology Associates, where I practice, has always recognized the importance of mentorship and its role in developing fulfilling careers for its physicians and a healthy practice culture in which people feel valued and supported. But only recently have we begun to create a formalized program to ensure that everyone has access to mentors.
When I started my career, formalized programs for mentorship did not exist. While I reached out to various doctors in my office and the senior partners throughout the practice, not everyone is comfortable proactively reaching out to ask practice leadership for help and guidance. And as independent GI practices continue to get bigger, there may not be as many opportunities to interact with senior leadership or executives, which means new associates could be left to “cold call” potential mentors by phone or email.
New associates face many challenges
When I was an associate, the path to partnership looked much different than it does in our practice now, and it definitely varies from practice to practice. In our practice, physicians remain associates for 2-3 years before they have the option to become a partner. As they work diligently to provide the best care to patients, new associates face many challenges, like learning how to build a practice and interact with referring physicians, understanding the process to become a partner, and figuring out how to juggle other commitments, such as the balance between work and home life.
Then there are things like buying a home, getting life and disability insurance, and understanding the financial planning aspects of being a business owner. For those whose group model requires buying into the practice to become a partner, medical school doesn’t teach you how to analyze the return on an investment. Providing access to people who have this experience through a mentorship program can help associates be more prepared to become partners, and hopefully be happier and more successful while doing so.
Formalizing a mentorship program
The mentorship program at Atlanta Gastroenterology Associates matches new physicians with a partner-level mentor with whom they are encouraged to meet monthly. We’ve even provided a budget so that mentees and mentors can meet for dinner or coffee and get to know each other better.
The program starts with a meeting of volunteer partners and associates, who then rank their preferred choices for potential mentors. This helps to ensure that the associates are able get to know the mentors a little bit and decide who might be the best fit for their needs. This is also important for new associates who don’t feel comfortable proactively searching for a mentor as they’re able to provide a list of partners they think would be the best match for them.
Each associate is then assigned a partner-level mentor who is responsible for guiding them and providing education around not only clinical care, but also business, marketing, health policy, and all the other critical components of running a successful private practice. Our program specifically pairs mentors and mentees who do not work in the same location. We wanted the mentors to be paired with associates they are not interacting with daily, to ensure our associates get exposed to different perspectives.
As a group, program participants get together every quarter – twice a year in person, and twice virtually. One of the in-person meetings brings together the mentors and mentees with C-suite executives to meet and network with the practice leadership. We organized the program this way because otherwise, most associates wouldn’t get many opportunities to interact with the CEO, chief medical officer, or chief operating officer in any capacity, let alone in a small gathering where they can engage on a personal level.
The second in-person meeting is a dinner with the mentors and mentees, along with their significant others. We understand that all our physicians have responsibilities outside of work, and bringing families together helps provide new associates with a network of support for questions that aren’t work-related. For associates who are not from Atlanta, this can be especially helpful in figuring out housing, schools, and other aspects of work/life balance.
The other two virtual meetings include the C-suite executives and our physician executive board. We develop specific agenda topics related to a career in private practice and provide a forum for the associates to ask practice leadership questions about challenges they may face on their path to becoming a partner.
At the end of each year, we survey the current program participants to see what was successful and what can be improved for the next cohort of incoming associates. So far, the feedback we’ve received from the mentors and mentees has been overwhelmingly positive.
Mentorship benefits the entire practice
As groups continue to grow, more practices may begin to formalize their mentorship programs, particularly those who see the merit they provide in helping recruit and retain valuable associates. To supplement our internal mentorship program, we’ve also started reaching out to local fellowship programs to provide resources to fellows who are considering private practice.
Even though about half of gastroenterology fellows choose independent GI, many aren’t educated about what it means to choose a career in private practice. Our outreach to provide information at the fellowship level is aimed at giving early career physicians an opportunity to know the benefits and challenges associated with private practice. Furthermore, we strive to educate fellows about the resources that are available to guide them through not only joining a group, but also having a successful career.
Leaders of independent GI groups need physicians who want the practice to succeed. Medical school trains physicians to take care of patients but falls short on training physicians to run a business. And building good, strong businesses makes sure that the next generation of leaders are prepared to take over.
Supporting the next generation of practice leaders helps current leadership make changes that will ensure practice sustainability. Often, associates are at the forefront of new technologies, both in terms of patient care, and in terms of practice management, communications, marketing, and advertising. As times change, having associates who are engaged and excited will help any practice be positioned positively for whatever the future holds.
What to look for in joining a practice
Ideally, people should start looking for mentors when they’re looking for a residency program. Joining a practice isn’t much different. If you’re an early career physician who is considering private practice, find an independent GI physician who can tell you about their experiences. And when you’re interviewing with practices, be sure to ask questions about how the group approaches mentorship. If a practice doesn’t have a formal mentorship program, it doesn’t mean it’s an environment where mentorship relationships won’t flourish. In many practices, informal mentorship programs are very successful.
Ask questions about how the practice provides or supports mentorship. Does the practice leadership make themselves available as mentors? Does the practice expect new physicians to find and nurture mentorship relations on their own? Ask about the path to becoming a partner and who is available to discuss challenges, concerns, or any questions that arise.
Independent GI practices are partnerships that seek to provide high-quality care at a lower cost to our community. Strengthening and sustaining that partnership requires us to take the time and continuously invest in the future of new physicians who will go on to serve our community as partner physicians retire. By formalizing a mentorship program, we’re hoping to make it easier for our mentors and mentees to create productive partnerships that will strengthen our group, and ultimately independent gastroenterology overall.
Dr. Sonenshine is a practicing gastroenterologist at Atlanta Gastroenterology Associates, and a partner in United Digestive. He previously served as the chair of communications as a member of the Executive Committee of the Digestive Health Physicians Association. Dr. Sonenshine has no conflicts to declare.
Noninvasive brain stimulation promising for COVID-related smell loss
Noninvasive brain stimulation may help restore a sense of smell in patients with chronic anosmia or hyposmia related to COVID-19, early research suggests.
Results of a small, double-blind, sham-controlled study showed anodal transcranial direct current stimulation (A-tDCS) combined with olfactory training (OT) provided notable and durable improvement in seven patients with persistent COVID-19–related hyposmia or anosmia.
“We are proud and very excited about these results. Although seven patients is a small sample, it is still notable,” lead investigator Fabio Bandini, MD, head of the department of neurology, ASL 3 Genovese, Genoa, Italy, said in an interview.
tDCS is cheap, safe, accessible, and very easy to administer. It has been used in rehabilitative treatment for 15 years, but this is the first time it has been used for this kind of problem, Dr. Bandini added.
The study was published online in the Journal of Neurology, Neurosurgery, and Psychiatry.
First study of its kind
Approximately 1% of patients with COVID will suffer from long-term smell loss, and given the widespread global impact of COVID, this represents a substantial number who have experienced or will potentially experience chronic smell loss because of the disease.
Loss of smell associated with COVID may last anywhere from 15 to 180 days after a SAR-CoV-2 infection, the researchers noted. Research suggests there is central nervous system involvement in COVID anosmia, mostly in the orbitofrontal cortex – the neural substrate for conscious olfactory perception.
“Smell loss has important consequences in everyday life for food, for hazards, for socialization. Usually, you recover from smell loss after 2 or 3 months, but after 6 months, that is considered permanent,” said Dr. Bandini.
Some research has pointed to the activation of the orbital frontal cortex for control of olfactory perception, so Dr. Bandini and colleagues wanted to explore whether stimulating this area could improve smell disturbances in post-COVID patients.
The study included seven consecutive patients with hyposmia or anosmia from COVID-19 lasting at least 6 months and who had a score of less than 12 on the Sniffin’ Sticks identification subtest. Exclusion criteria included severe mood disorder, rhinologic diseases, epilepsy, and sensitive scalp. No medications for alleviating olfactory symptoms were permitted.
Patients’ smell performances were assessed immediately prior to stimulation (t0) and rated on a scale of 0-10, with a score of 0 indicating a complete loss of smell and a score of 10 indicating a full sense of smell as the subjective measure. Sniffin’ Sticks, a validated test that assesses smell threshold, discrimination, and validation, was used as an objective measure.
In the 20-minute OT session, patients had to sniff 10 odors (rose, eucalyptus, lemon, star anise, rosemary, strawberry, coconut, vanilla, pine tree, and bergamot) in a random order for 10 seconds each then were asked to identify the smell and rate its intensity. The training was applied once in each session.
A-tDCS or sham-transcranial direct current stimulation (S-tDCS) was administered at the same time. In the active stimulation the anode was placed over the left prefrontal cortex because the orbitofrontal cortex is not directly accessible by A-tDCS.
The patients participated in olfactory training with S-tDCS for the first 2 weeks. In the second 2 weeks of the study, they received OT with A-tDCS.
The order of sham and A-tDCS stimulation was not counterbalanced to avoid potential carryover effects if A-tDCS had been applied first. The patients and assessors collecting the data were blinded.
The smell assessment was repeated immediately after S-tDCS (t1), A-tDCS (t2) and 3 months from the end of stimulation (t3), using the same odors and the same order of the first assessment.
The Wilcoxon test was used to compare each assessment (t1, t2, and t3) with baseline, indicating a two-sided alpha less than 0.05, which was considered statistically significant.
Both the subjective and objective measures showed a statistically significant improvement at t2 and t3, with average measurements doubled or even tripled, compared with t0 and t1. In addition, all patients demonstrated notable improvement in smell performance.
This study, said Dr. Bandini, is the first to use A-tDCS to treat patients with persistent smell loss due to COVID. Not only did the results show significant improvement in all study participants, compared with baseline but the beneficial effect lasted up to 3 months after treatment, demonstrating a durable effect.
Dr. Bandini noted that the study’s small sample size is a major limitation of the research so he hopes to enlarge it in future research testing A-tDCS for COVID-related smell loss and work toward providing this therapy on an outpatient basis.
Encouraging results offer new hope
Commenting on the research, Cheng-Ying Ho, MD, associate professor of pathology at the Johns Hopkins University, Baltimore, described the study as “interesting and encouraging.
“Even though there is a small percentage of patients that suffer persistent smell loss from COVID, it’s still a large number of people who have smell dysfunction and are unable to recover.”
“So far, there is no treatment for COVID-related or viral infection–related smell loss. The only thing that can be done is olfactory training, but the effect is very limited. There is no drug or other type of therapy for smell loss so far,” said Dr. Ho, whose areas of expertise include neuromuscular pathology, pediatric neuropathology, and neuropathology of infectious diseases.
“Even though it’s a small study with only seven patients, the results are very encouraging. After 2 weeks of stimulation, almost all had smell recovery that lasted several months. The weakness of the study is that they didn’t have a control group. The next step would be to expand the study to include more participants and have an adequate control group that received the sham stimuli to see if their results still stand when they have more participants.
“This very encouraging and relatively noninvasive treatment modality can give patients with smell loss some hope that this therapy can help them recover their sense of smell to some degree. The study seems to suggest that either the tDCS can stimulate nerve regrowth or that it actually can correct the rewiring of the brain,” added Dr. Ho.
The authors have not declared a specific grant for this research from any funding agency in the public, commercial, or not-for-profit sectors. No competing interests were declared.
A version of this article first appeared on Medscape.com.
Noninvasive brain stimulation may help restore a sense of smell in patients with chronic anosmia or hyposmia related to COVID-19, early research suggests.
Results of a small, double-blind, sham-controlled study showed anodal transcranial direct current stimulation (A-tDCS) combined with olfactory training (OT) provided notable and durable improvement in seven patients with persistent COVID-19–related hyposmia or anosmia.
“We are proud and very excited about these results. Although seven patients is a small sample, it is still notable,” lead investigator Fabio Bandini, MD, head of the department of neurology, ASL 3 Genovese, Genoa, Italy, said in an interview.
tDCS is cheap, safe, accessible, and very easy to administer. It has been used in rehabilitative treatment for 15 years, but this is the first time it has been used for this kind of problem, Dr. Bandini added.
The study was published online in the Journal of Neurology, Neurosurgery, and Psychiatry.
First study of its kind
Approximately 1% of patients with COVID will suffer from long-term smell loss, and given the widespread global impact of COVID, this represents a substantial number who have experienced or will potentially experience chronic smell loss because of the disease.
Loss of smell associated with COVID may last anywhere from 15 to 180 days after a SAR-CoV-2 infection, the researchers noted. Research suggests there is central nervous system involvement in COVID anosmia, mostly in the orbitofrontal cortex – the neural substrate for conscious olfactory perception.
“Smell loss has important consequences in everyday life for food, for hazards, for socialization. Usually, you recover from smell loss after 2 or 3 months, but after 6 months, that is considered permanent,” said Dr. Bandini.
Some research has pointed to the activation of the orbital frontal cortex for control of olfactory perception, so Dr. Bandini and colleagues wanted to explore whether stimulating this area could improve smell disturbances in post-COVID patients.
The study included seven consecutive patients with hyposmia or anosmia from COVID-19 lasting at least 6 months and who had a score of less than 12 on the Sniffin’ Sticks identification subtest. Exclusion criteria included severe mood disorder, rhinologic diseases, epilepsy, and sensitive scalp. No medications for alleviating olfactory symptoms were permitted.
Patients’ smell performances were assessed immediately prior to stimulation (t0) and rated on a scale of 0-10, with a score of 0 indicating a complete loss of smell and a score of 10 indicating a full sense of smell as the subjective measure. Sniffin’ Sticks, a validated test that assesses smell threshold, discrimination, and validation, was used as an objective measure.
In the 20-minute OT session, patients had to sniff 10 odors (rose, eucalyptus, lemon, star anise, rosemary, strawberry, coconut, vanilla, pine tree, and bergamot) in a random order for 10 seconds each then were asked to identify the smell and rate its intensity. The training was applied once in each session.
A-tDCS or sham-transcranial direct current stimulation (S-tDCS) was administered at the same time. In the active stimulation the anode was placed over the left prefrontal cortex because the orbitofrontal cortex is not directly accessible by A-tDCS.
The patients participated in olfactory training with S-tDCS for the first 2 weeks. In the second 2 weeks of the study, they received OT with A-tDCS.
The order of sham and A-tDCS stimulation was not counterbalanced to avoid potential carryover effects if A-tDCS had been applied first. The patients and assessors collecting the data were blinded.
The smell assessment was repeated immediately after S-tDCS (t1), A-tDCS (t2) and 3 months from the end of stimulation (t3), using the same odors and the same order of the first assessment.
The Wilcoxon test was used to compare each assessment (t1, t2, and t3) with baseline, indicating a two-sided alpha less than 0.05, which was considered statistically significant.
Both the subjective and objective measures showed a statistically significant improvement at t2 and t3, with average measurements doubled or even tripled, compared with t0 and t1. In addition, all patients demonstrated notable improvement in smell performance.
This study, said Dr. Bandini, is the first to use A-tDCS to treat patients with persistent smell loss due to COVID. Not only did the results show significant improvement in all study participants, compared with baseline but the beneficial effect lasted up to 3 months after treatment, demonstrating a durable effect.
Dr. Bandini noted that the study’s small sample size is a major limitation of the research so he hopes to enlarge it in future research testing A-tDCS for COVID-related smell loss and work toward providing this therapy on an outpatient basis.
Encouraging results offer new hope
Commenting on the research, Cheng-Ying Ho, MD, associate professor of pathology at the Johns Hopkins University, Baltimore, described the study as “interesting and encouraging.
“Even though there is a small percentage of patients that suffer persistent smell loss from COVID, it’s still a large number of people who have smell dysfunction and are unable to recover.”
“So far, there is no treatment for COVID-related or viral infection–related smell loss. The only thing that can be done is olfactory training, but the effect is very limited. There is no drug or other type of therapy for smell loss so far,” said Dr. Ho, whose areas of expertise include neuromuscular pathology, pediatric neuropathology, and neuropathology of infectious diseases.
“Even though it’s a small study with only seven patients, the results are very encouraging. After 2 weeks of stimulation, almost all had smell recovery that lasted several months. The weakness of the study is that they didn’t have a control group. The next step would be to expand the study to include more participants and have an adequate control group that received the sham stimuli to see if their results still stand when they have more participants.
“This very encouraging and relatively noninvasive treatment modality can give patients with smell loss some hope that this therapy can help them recover their sense of smell to some degree. The study seems to suggest that either the tDCS can stimulate nerve regrowth or that it actually can correct the rewiring of the brain,” added Dr. Ho.
The authors have not declared a specific grant for this research from any funding agency in the public, commercial, or not-for-profit sectors. No competing interests were declared.
A version of this article first appeared on Medscape.com.
Noninvasive brain stimulation may help restore a sense of smell in patients with chronic anosmia or hyposmia related to COVID-19, early research suggests.
Results of a small, double-blind, sham-controlled study showed anodal transcranial direct current stimulation (A-tDCS) combined with olfactory training (OT) provided notable and durable improvement in seven patients with persistent COVID-19–related hyposmia or anosmia.
“We are proud and very excited about these results. Although seven patients is a small sample, it is still notable,” lead investigator Fabio Bandini, MD, head of the department of neurology, ASL 3 Genovese, Genoa, Italy, said in an interview.
tDCS is cheap, safe, accessible, and very easy to administer. It has been used in rehabilitative treatment for 15 years, but this is the first time it has been used for this kind of problem, Dr. Bandini added.
The study was published online in the Journal of Neurology, Neurosurgery, and Psychiatry.
First study of its kind
Approximately 1% of patients with COVID will suffer from long-term smell loss, and given the widespread global impact of COVID, this represents a substantial number who have experienced or will potentially experience chronic smell loss because of the disease.
Loss of smell associated with COVID may last anywhere from 15 to 180 days after a SAR-CoV-2 infection, the researchers noted. Research suggests there is central nervous system involvement in COVID anosmia, mostly in the orbitofrontal cortex – the neural substrate for conscious olfactory perception.
“Smell loss has important consequences in everyday life for food, for hazards, for socialization. Usually, you recover from smell loss after 2 or 3 months, but after 6 months, that is considered permanent,” said Dr. Bandini.
Some research has pointed to the activation of the orbital frontal cortex for control of olfactory perception, so Dr. Bandini and colleagues wanted to explore whether stimulating this area could improve smell disturbances in post-COVID patients.
The study included seven consecutive patients with hyposmia or anosmia from COVID-19 lasting at least 6 months and who had a score of less than 12 on the Sniffin’ Sticks identification subtest. Exclusion criteria included severe mood disorder, rhinologic diseases, epilepsy, and sensitive scalp. No medications for alleviating olfactory symptoms were permitted.
Patients’ smell performances were assessed immediately prior to stimulation (t0) and rated on a scale of 0-10, with a score of 0 indicating a complete loss of smell and a score of 10 indicating a full sense of smell as the subjective measure. Sniffin’ Sticks, a validated test that assesses smell threshold, discrimination, and validation, was used as an objective measure.
In the 20-minute OT session, patients had to sniff 10 odors (rose, eucalyptus, lemon, star anise, rosemary, strawberry, coconut, vanilla, pine tree, and bergamot) in a random order for 10 seconds each then were asked to identify the smell and rate its intensity. The training was applied once in each session.
A-tDCS or sham-transcranial direct current stimulation (S-tDCS) was administered at the same time. In the active stimulation the anode was placed over the left prefrontal cortex because the orbitofrontal cortex is not directly accessible by A-tDCS.
The patients participated in olfactory training with S-tDCS for the first 2 weeks. In the second 2 weeks of the study, they received OT with A-tDCS.
The order of sham and A-tDCS stimulation was not counterbalanced to avoid potential carryover effects if A-tDCS had been applied first. The patients and assessors collecting the data were blinded.
The smell assessment was repeated immediately after S-tDCS (t1), A-tDCS (t2) and 3 months from the end of stimulation (t3), using the same odors and the same order of the first assessment.
The Wilcoxon test was used to compare each assessment (t1, t2, and t3) with baseline, indicating a two-sided alpha less than 0.05, which was considered statistically significant.
Both the subjective and objective measures showed a statistically significant improvement at t2 and t3, with average measurements doubled or even tripled, compared with t0 and t1. In addition, all patients demonstrated notable improvement in smell performance.
This study, said Dr. Bandini, is the first to use A-tDCS to treat patients with persistent smell loss due to COVID. Not only did the results show significant improvement in all study participants, compared with baseline but the beneficial effect lasted up to 3 months after treatment, demonstrating a durable effect.
Dr. Bandini noted that the study’s small sample size is a major limitation of the research so he hopes to enlarge it in future research testing A-tDCS for COVID-related smell loss and work toward providing this therapy on an outpatient basis.
Encouraging results offer new hope
Commenting on the research, Cheng-Ying Ho, MD, associate professor of pathology at the Johns Hopkins University, Baltimore, described the study as “interesting and encouraging.
“Even though there is a small percentage of patients that suffer persistent smell loss from COVID, it’s still a large number of people who have smell dysfunction and are unable to recover.”
“So far, there is no treatment for COVID-related or viral infection–related smell loss. The only thing that can be done is olfactory training, but the effect is very limited. There is no drug or other type of therapy for smell loss so far,” said Dr. Ho, whose areas of expertise include neuromuscular pathology, pediatric neuropathology, and neuropathology of infectious diseases.
“Even though it’s a small study with only seven patients, the results are very encouraging. After 2 weeks of stimulation, almost all had smell recovery that lasted several months. The weakness of the study is that they didn’t have a control group. The next step would be to expand the study to include more participants and have an adequate control group that received the sham stimuli to see if their results still stand when they have more participants.
“This very encouraging and relatively noninvasive treatment modality can give patients with smell loss some hope that this therapy can help them recover their sense of smell to some degree. The study seems to suggest that either the tDCS can stimulate nerve regrowth or that it actually can correct the rewiring of the brain,” added Dr. Ho.
The authors have not declared a specific grant for this research from any funding agency in the public, commercial, or not-for-profit sectors. No competing interests were declared.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF NEUROLOGY, NEUROSURGERY, AND PSYCHIATRY
Treat-to-target strategy with tapering proves effective in PsA and axSpA
Aiming for a disease activity target while reducing biologic therapy could be a winning approach for patients with psoriatic arthritis (PsA) and axial spondyloarthritis (axSpA), according to the results of a new study presented at the annual European Congress of Rheumatology.
The findings show that a treat-to-target (T2T) strategy with tapering using a tumor necrosis factor (TNF) inhibitor produces results that are noninferior to a T2T strategy that doesn’t include tapering in these patients.
“Our study has for the first time shown that a treat-to-target tapering strategy is just as good as full-dose continuation, while reducing medication use substantially,” first author Celia Michielsens, MD, a PhD student and researcher at Sint Maartenskliniek in Nijmegen, the Netherlands, said in an interview before her presentation of the study during an oral abstract session at the congress. “Stepwise tapering is also better than fixed-dose reduction or discontinuation, since it is much more individualized.”
The study is now published in Annals of the Rheumatic Diseases.
In the randomized, controlled, open-label, noninferiority study, researchers enrolled patients with PsA or axSpA who were using a TNF inhibitor such as etanercept, adalimumab, or infliximab, and had stable low disease activity for at least 6 months. Patients needed to have a Psoriatic Arthritis Disease Activity Score (PASDAS) of 3.2 or less, or an Ankylosing Spondylitis Disease Activity Score (ASDAS) of at 2.1 or less. In cases of flare, patients were treated with NSAIDs and/or glucorticoids, and if they still had not reached low disease activity after a month, their previous TNF inhibitor dose was reinstated to the last effective interval or dosage, which was maintained throughout the study period. When the patient was already using a full TNF-inhibitor dose or if dose adjustment did not suffice, patients were switched to another biologic or targeted synthetic disease-modifying antirheumatic drug (DMARD).
Participants were randomized, from January 2019 to June 2021, to a tapering or a nontapering T2T strategy in a 2:1 fashion. Then researchers then followed them for 12 months and aimed to determine if the tapering strategy proved noninferior to not tapering within a predefined 20% margin for noninferiority, which Dr. Michielsens said was derived from other studies and what her group determined to be “an acceptable risk.”
Results show strategy is ‘feasible in daily clinical care’
A total of 81 patients – 42 with PsA and 39 with axSpA – were in the group with tapering, and 41 were in the group without tapering: 22 with PsA and 19 with axSpA.
At 12 months, researchers found that 69% of the patients in the group with tapering had low disease activity, measured via the PASDAS and ASDAS, compared with 73% in patients who did not taper. And those in the tapering group saw their medication use dramatically reduced. At the 12-month mark, they were taking just 53% of the defined daily dose for maintenance, compared with 91% of the defined daily dose for the group that didn’t taper.
The researchers were able to successfully taper 72% of the patients in the tapering group, with 28% of them discontinuing their TNF-inhibitor medication entirely. The incidence of flares was 85% in the tapering group and 78% in the nontapering group, a nonsignificant difference (P = .32).
The start of a new medication or an increase in use of an existing medication was more frequent in the tapering group, and significantly so for NSAIDs. An increase in NSAID use was seen in 54% of the tapering group and in just 24% of the nontapering group (P = .002).
Conventional synthetic DMARD use went up in the tapering group, compared with the nontapering group, but this was only among the PsA patients and the change in use was not statistically significant. There were also more frequent increases in glucocorticoid use in the tapering group, compared with the nontapering group, but this was not significant.
Dr. Michielsens said the findings show the value of an individualized approach in treating patients with PsA or axSpA.
“Our study – and those [studies] in rheumatoid arthritis earlier – deliver the highest quality of evidence that disease activity–guided dose personalization can, and in fact should, be used in clinical practice,” she said. “Our pragmatic treat-to-target tapering strategy is feasible in daily clinical care, although treat-to-target using PASDAS and ASDAS needs some implementation. In shared decision-making with patients, a 50% reduction in TNFi use is obtainable, while maintaining low disease activity.”
The increase in the use of NSAIDs is something to be aware of, but it is “not concerning,” Dr. Michielsens added. She pointed out that the NSAID use was typically temporary, used when flares arose, and that the drugs are effective, safe, and inexpensive. She also noted that the use of TNF blockers decreased more than the use of NSAIDs increased.
“This seems a perfectly acceptable trade-off that can be discussed with your patient,” she said.
The 12-month duration of the study is likely long enough to show that the tapering strategy works, Dr. Michielsens said. In rheumatoid arthritis studies, for example, differences in strategies didn’t change after 1 year.
“That said, we are doing an observational extension study to provide more insights in the long-term effects of this treat-to-target strategy,” she said. “At the end of this summer, all patients will have completed their extended follow-up period – a 12-month observational period – so hopefully we can present the results next year at EULAR.”
This study received funding from ReumaNederland. Dr. Michielsens did not have any financial interests to disclose. Two coauthors reported financial relationships with numerous pharmaceutical companies.
Aiming for a disease activity target while reducing biologic therapy could be a winning approach for patients with psoriatic arthritis (PsA) and axial spondyloarthritis (axSpA), according to the results of a new study presented at the annual European Congress of Rheumatology.
The findings show that a treat-to-target (T2T) strategy with tapering using a tumor necrosis factor (TNF) inhibitor produces results that are noninferior to a T2T strategy that doesn’t include tapering in these patients.
“Our study has for the first time shown that a treat-to-target tapering strategy is just as good as full-dose continuation, while reducing medication use substantially,” first author Celia Michielsens, MD, a PhD student and researcher at Sint Maartenskliniek in Nijmegen, the Netherlands, said in an interview before her presentation of the study during an oral abstract session at the congress. “Stepwise tapering is also better than fixed-dose reduction or discontinuation, since it is much more individualized.”
The study is now published in Annals of the Rheumatic Diseases.
In the randomized, controlled, open-label, noninferiority study, researchers enrolled patients with PsA or axSpA who were using a TNF inhibitor such as etanercept, adalimumab, or infliximab, and had stable low disease activity for at least 6 months. Patients needed to have a Psoriatic Arthritis Disease Activity Score (PASDAS) of 3.2 or less, or an Ankylosing Spondylitis Disease Activity Score (ASDAS) of at 2.1 or less. In cases of flare, patients were treated with NSAIDs and/or glucorticoids, and if they still had not reached low disease activity after a month, their previous TNF inhibitor dose was reinstated to the last effective interval or dosage, which was maintained throughout the study period. When the patient was already using a full TNF-inhibitor dose or if dose adjustment did not suffice, patients were switched to another biologic or targeted synthetic disease-modifying antirheumatic drug (DMARD).
Participants were randomized, from January 2019 to June 2021, to a tapering or a nontapering T2T strategy in a 2:1 fashion. Then researchers then followed them for 12 months and aimed to determine if the tapering strategy proved noninferior to not tapering within a predefined 20% margin for noninferiority, which Dr. Michielsens said was derived from other studies and what her group determined to be “an acceptable risk.”
Results show strategy is ‘feasible in daily clinical care’
A total of 81 patients – 42 with PsA and 39 with axSpA – were in the group with tapering, and 41 were in the group without tapering: 22 with PsA and 19 with axSpA.
At 12 months, researchers found that 69% of the patients in the group with tapering had low disease activity, measured via the PASDAS and ASDAS, compared with 73% in patients who did not taper. And those in the tapering group saw their medication use dramatically reduced. At the 12-month mark, they were taking just 53% of the defined daily dose for maintenance, compared with 91% of the defined daily dose for the group that didn’t taper.
The researchers were able to successfully taper 72% of the patients in the tapering group, with 28% of them discontinuing their TNF-inhibitor medication entirely. The incidence of flares was 85% in the tapering group and 78% in the nontapering group, a nonsignificant difference (P = .32).
The start of a new medication or an increase in use of an existing medication was more frequent in the tapering group, and significantly so for NSAIDs. An increase in NSAID use was seen in 54% of the tapering group and in just 24% of the nontapering group (P = .002).
Conventional synthetic DMARD use went up in the tapering group, compared with the nontapering group, but this was only among the PsA patients and the change in use was not statistically significant. There were also more frequent increases in glucocorticoid use in the tapering group, compared with the nontapering group, but this was not significant.
Dr. Michielsens said the findings show the value of an individualized approach in treating patients with PsA or axSpA.
“Our study – and those [studies] in rheumatoid arthritis earlier – deliver the highest quality of evidence that disease activity–guided dose personalization can, and in fact should, be used in clinical practice,” she said. “Our pragmatic treat-to-target tapering strategy is feasible in daily clinical care, although treat-to-target using PASDAS and ASDAS needs some implementation. In shared decision-making with patients, a 50% reduction in TNFi use is obtainable, while maintaining low disease activity.”
The increase in the use of NSAIDs is something to be aware of, but it is “not concerning,” Dr. Michielsens added. She pointed out that the NSAID use was typically temporary, used when flares arose, and that the drugs are effective, safe, and inexpensive. She also noted that the use of TNF blockers decreased more than the use of NSAIDs increased.
“This seems a perfectly acceptable trade-off that can be discussed with your patient,” she said.
The 12-month duration of the study is likely long enough to show that the tapering strategy works, Dr. Michielsens said. In rheumatoid arthritis studies, for example, differences in strategies didn’t change after 1 year.
“That said, we are doing an observational extension study to provide more insights in the long-term effects of this treat-to-target strategy,” she said. “At the end of this summer, all patients will have completed their extended follow-up period – a 12-month observational period – so hopefully we can present the results next year at EULAR.”
This study received funding from ReumaNederland. Dr. Michielsens did not have any financial interests to disclose. Two coauthors reported financial relationships with numerous pharmaceutical companies.
Aiming for a disease activity target while reducing biologic therapy could be a winning approach for patients with psoriatic arthritis (PsA) and axial spondyloarthritis (axSpA), according to the results of a new study presented at the annual European Congress of Rheumatology.
The findings show that a treat-to-target (T2T) strategy with tapering using a tumor necrosis factor (TNF) inhibitor produces results that are noninferior to a T2T strategy that doesn’t include tapering in these patients.
“Our study has for the first time shown that a treat-to-target tapering strategy is just as good as full-dose continuation, while reducing medication use substantially,” first author Celia Michielsens, MD, a PhD student and researcher at Sint Maartenskliniek in Nijmegen, the Netherlands, said in an interview before her presentation of the study during an oral abstract session at the congress. “Stepwise tapering is also better than fixed-dose reduction or discontinuation, since it is much more individualized.”
The study is now published in Annals of the Rheumatic Diseases.
In the randomized, controlled, open-label, noninferiority study, researchers enrolled patients with PsA or axSpA who were using a TNF inhibitor such as etanercept, adalimumab, or infliximab, and had stable low disease activity for at least 6 months. Patients needed to have a Psoriatic Arthritis Disease Activity Score (PASDAS) of 3.2 or less, or an Ankylosing Spondylitis Disease Activity Score (ASDAS) of at 2.1 or less. In cases of flare, patients were treated with NSAIDs and/or glucorticoids, and if they still had not reached low disease activity after a month, their previous TNF inhibitor dose was reinstated to the last effective interval or dosage, which was maintained throughout the study period. When the patient was already using a full TNF-inhibitor dose or if dose adjustment did not suffice, patients were switched to another biologic or targeted synthetic disease-modifying antirheumatic drug (DMARD).
Participants were randomized, from January 2019 to June 2021, to a tapering or a nontapering T2T strategy in a 2:1 fashion. Then researchers then followed them for 12 months and aimed to determine if the tapering strategy proved noninferior to not tapering within a predefined 20% margin for noninferiority, which Dr. Michielsens said was derived from other studies and what her group determined to be “an acceptable risk.”
Results show strategy is ‘feasible in daily clinical care’
A total of 81 patients – 42 with PsA and 39 with axSpA – were in the group with tapering, and 41 were in the group without tapering: 22 with PsA and 19 with axSpA.
At 12 months, researchers found that 69% of the patients in the group with tapering had low disease activity, measured via the PASDAS and ASDAS, compared with 73% in patients who did not taper. And those in the tapering group saw their medication use dramatically reduced. At the 12-month mark, they were taking just 53% of the defined daily dose for maintenance, compared with 91% of the defined daily dose for the group that didn’t taper.
The researchers were able to successfully taper 72% of the patients in the tapering group, with 28% of them discontinuing their TNF-inhibitor medication entirely. The incidence of flares was 85% in the tapering group and 78% in the nontapering group, a nonsignificant difference (P = .32).
The start of a new medication or an increase in use of an existing medication was more frequent in the tapering group, and significantly so for NSAIDs. An increase in NSAID use was seen in 54% of the tapering group and in just 24% of the nontapering group (P = .002).
Conventional synthetic DMARD use went up in the tapering group, compared with the nontapering group, but this was only among the PsA patients and the change in use was not statistically significant. There were also more frequent increases in glucocorticoid use in the tapering group, compared with the nontapering group, but this was not significant.
Dr. Michielsens said the findings show the value of an individualized approach in treating patients with PsA or axSpA.
“Our study – and those [studies] in rheumatoid arthritis earlier – deliver the highest quality of evidence that disease activity–guided dose personalization can, and in fact should, be used in clinical practice,” she said. “Our pragmatic treat-to-target tapering strategy is feasible in daily clinical care, although treat-to-target using PASDAS and ASDAS needs some implementation. In shared decision-making with patients, a 50% reduction in TNFi use is obtainable, while maintaining low disease activity.”
The increase in the use of NSAIDs is something to be aware of, but it is “not concerning,” Dr. Michielsens added. She pointed out that the NSAID use was typically temporary, used when flares arose, and that the drugs are effective, safe, and inexpensive. She also noted that the use of TNF blockers decreased more than the use of NSAIDs increased.
“This seems a perfectly acceptable trade-off that can be discussed with your patient,” she said.
The 12-month duration of the study is likely long enough to show that the tapering strategy works, Dr. Michielsens said. In rheumatoid arthritis studies, for example, differences in strategies didn’t change after 1 year.
“That said, we are doing an observational extension study to provide more insights in the long-term effects of this treat-to-target strategy,” she said. “At the end of this summer, all patients will have completed their extended follow-up period – a 12-month observational period – so hopefully we can present the results next year at EULAR.”
This study received funding from ReumaNederland. Dr. Michielsens did not have any financial interests to disclose. Two coauthors reported financial relationships with numerous pharmaceutical companies.
FROM THE EULAR 2022 CONGRESS