User login
FDA-cleared panties could reduce STI risk during oral sex
.
The underwear, sold as Lorals for Protection, are single-use, vanilla-scented, natural latex panties that cover the genitals and anus and block the transfer of bodily fluids during oral sex, according to the company website. They sell in packages of four for $25.
The FDA didn’t run human clinical trials but granted authorization after the company gave it data about the product, The New York Times reported.
“The FDA’s authorization of this product gives people another option to protect against STIs during oral sex,” said Courtney Lias, PhD, director of the FDA office that led the review of the underwear.
Previously, the FDA authorized oral dams to prevent the spread of STIs during oral sex. Oral dams, sometimes called oral sex condoms, are thin latex barriers that go between one partner’s mouth and the other person’s genitals. The dams haven’t been widely used, partly because a person has to hold the dam in place during sex, unlike the panties.
“They’re extremely unpopular,” Jeanne Marrazzo, MD, director of the division of infectious diseases at the University of Alabama at Birmingham, told the Times. “I mean, honestly, could there be anything less sexy than a dental dam?”Melanie Cristol said she came up with the idea for the panties after discovering on her 2014 honeymoon that she had an infection that could be sexually transmitted.
“I wanted to feel sexy and confident and use something that was made with my body and actual sex in mind,” she told the Times.
The panties are made of material about as thin as a condom and form a seal on the thigh to keep fluids inside, she said.
Dr. Marrazzo said the panties are an advancement because there are few options for safe oral sex. She noted that some teenagers have their first sexual experience with oral sex and that the panties could reduce anxiety for people of all ages.
A version of this article first appeared on WebMD.com.
.
The underwear, sold as Lorals for Protection, are single-use, vanilla-scented, natural latex panties that cover the genitals and anus and block the transfer of bodily fluids during oral sex, according to the company website. They sell in packages of four for $25.
The FDA didn’t run human clinical trials but granted authorization after the company gave it data about the product, The New York Times reported.
“The FDA’s authorization of this product gives people another option to protect against STIs during oral sex,” said Courtney Lias, PhD, director of the FDA office that led the review of the underwear.
Previously, the FDA authorized oral dams to prevent the spread of STIs during oral sex. Oral dams, sometimes called oral sex condoms, are thin latex barriers that go between one partner’s mouth and the other person’s genitals. The dams haven’t been widely used, partly because a person has to hold the dam in place during sex, unlike the panties.
“They’re extremely unpopular,” Jeanne Marrazzo, MD, director of the division of infectious diseases at the University of Alabama at Birmingham, told the Times. “I mean, honestly, could there be anything less sexy than a dental dam?”Melanie Cristol said she came up with the idea for the panties after discovering on her 2014 honeymoon that she had an infection that could be sexually transmitted.
“I wanted to feel sexy and confident and use something that was made with my body and actual sex in mind,” she told the Times.
The panties are made of material about as thin as a condom and form a seal on the thigh to keep fluids inside, she said.
Dr. Marrazzo said the panties are an advancement because there are few options for safe oral sex. She noted that some teenagers have their first sexual experience with oral sex and that the panties could reduce anxiety for people of all ages.
A version of this article first appeared on WebMD.com.
.
The underwear, sold as Lorals for Protection, are single-use, vanilla-scented, natural latex panties that cover the genitals and anus and block the transfer of bodily fluids during oral sex, according to the company website. They sell in packages of four for $25.
The FDA didn’t run human clinical trials but granted authorization after the company gave it data about the product, The New York Times reported.
“The FDA’s authorization of this product gives people another option to protect against STIs during oral sex,” said Courtney Lias, PhD, director of the FDA office that led the review of the underwear.
Previously, the FDA authorized oral dams to prevent the spread of STIs during oral sex. Oral dams, sometimes called oral sex condoms, are thin latex barriers that go between one partner’s mouth and the other person’s genitals. The dams haven’t been widely used, partly because a person has to hold the dam in place during sex, unlike the panties.
“They’re extremely unpopular,” Jeanne Marrazzo, MD, director of the division of infectious diseases at the University of Alabama at Birmingham, told the Times. “I mean, honestly, could there be anything less sexy than a dental dam?”Melanie Cristol said she came up with the idea for the panties after discovering on her 2014 honeymoon that she had an infection that could be sexually transmitted.
“I wanted to feel sexy and confident and use something that was made with my body and actual sex in mind,” she told the Times.
The panties are made of material about as thin as a condom and form a seal on the thigh to keep fluids inside, she said.
Dr. Marrazzo said the panties are an advancement because there are few options for safe oral sex. She noted that some teenagers have their first sexual experience with oral sex and that the panties could reduce anxiety for people of all ages.
A version of this article first appeared on WebMD.com.
Psychosocial Barriers and Their Impact on Hepatocellular Carcinoma Care in US Veterans: Tumor Board Model of Care
Hepatocellular carcinoma (HCC) remains a major global health problem and is the third leading cause of cancer-related mortality worldwide.1 Management of HCC is complex; as it largely occurs in the background of chronic liver disease, its management must simultaneously address challenges related to the patient’s tumor burden, as well as their underlying liver dysfunction and performance status. HCC is universally fatal without treatment, with a 5-year survival < 10%.2 However, if detected early HCC is potentially curable, with treatments such as hepatic resection, ablation, and/or liver transplantation, which are associated with 5-year survival rates as high as 70%.2 HCC-specific palliative treatments, including intra-arterial therapies (eg, trans-arterial chemoembolization, radioembolization) and systemic chemotherapy, have also been shown to prolong survival in patients with advanced HCC. Therefore, a key driver of patient survival is receipt of HCC-specific therapy.
There is rising incidence and mortality related to HCC in the US veteran population, largely attributed to acquisition of chronic hepatitis C virus (HCV) infection decades prior.3 There is also a high prevalence of psychosocial barriers in this population, such as low socioeconomic status, homelessness, alcohol and substance use disorders, and psychiatric disorders which can negatively influence receipt of medical treatment, including cancer care.4,5 Given the complexity of managing HCC, as well as the plethora of potential treatment options available, it is widely accepted that a multidisciplinary team approach, such as the multidisciplinary tumor board (MDTB) provides optimal care to patients with HCC.2,6 The aim of the present study was to identify in a population of veterans diagnosed with HCC the prevalence of psychosocial barriers to care and assess their impact and the role of an MDTB on receipt of HCC-specific care.
Methods
In June 2007, a joint institutional MDTB was established for patients with primary liver tumors receiving care at the William S. Middleton Memorial Veterans’ Hospital (WSMMVH) in Madison, Wisconsin. As we have described elsewhere, individual cases with their corresponding imaging studies were reviewed at a weekly conference attended by transplant hepatologists, medical oncologists, hepatobiliary and transplant surgeons, pathologists, diagnostic and interventional radiologists, and nurse coordinators.6 Potential therapies offered included surgical resection, liver transplantation (LT), thermal ablation, intra-arterial therapies (chemo and/or radioembolization), systemic chemotherapy, stereotactic radiation, and best supportive care. Decisions regarding the appropriate treatment modality were made based on patient factors, review of their cross-sectional imaging studies and/or histopathology, and context of their underlying liver dysfunction. The tumor board discussion was summarized in meeting minutes as well as tumor board encounters recorded in each patient’s health record. Although patients with benign tumors were presented at the MDTB, only patients with a diagnosis of HCC were included in this study.
A database analysis was conducted of all veteran patients with HCC managed through the WSMMVH MDTB, since its inception up to December 31, 2016, with follow-up until December 31, 2018. Data for analysis included demographics, laboratory parameters at time of diagnosis and treatment, imaging findings, histopathology and/or surgical pathology, treatment rendered, and follow-up information. The primary outcome measured in this study included receipt of any therapy and secondarily, patient survival.
Discrete variables were analyzed with χ2 statistics or Fisher exact test and continuous variables with the student t test. Multivariable analyses were carried out with logistic regression. Variables with a P < .05 were considered statistically significant. Analyses were carried out using IBM SPSS v24.0.
As a quality-improvement initiative for the care and management of veterans with HCC, this study was determined to be exempt from review by the WSMMVH and University of Wisconsin School of Medicine and Public Health Institutional Review Board.
Results
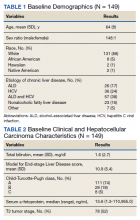
From January 1, 2007, through December 31, 2016, 149 patients with HCC were managed through the MDTB. Baseline demographic data, Model for End-stage Liver Disease (MELD) score and Child-Turcotte-Pugh class, and baseline HCC characteristics of the cohort are shown in Tables 1 and 2.
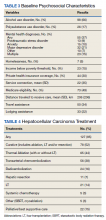
There was a high prevalence of psychosocial barriers in our study cohort, including alcohol or substance use disorder, mental illness diagnosis, and low socioeconomic status (Table 3). The mean distance traveled to WSMMVH for HCC-specific care was 206 km. Fifty patients in the cohort utilized travel assistance and 33 patients accessed lodging assistance.
HCC Treatments
There was a high rate of receipt of treatment in our study cohort with 127 (85%) patients receiving at least one HCC-specific therapy. Care was individualized and coordinated through our institutional MDTB, with both curative and palliative treatment modalities utilized (Table 4).
Curative treatment, which includes LT, ablation, or resection, was offered to 78 (52%) patients who were within T2 stage. Of these 78 patients who were potential candidates for LT as a curative treatment for HCC, 31 were not deemed suitable transplant candidates. Psychosocial barriers precluded consideration for LT in 7 of the 31 patients due to active substance use, homelessness in 1 patient, and severe mental illness in 3 patients. Medical comorbidities, advanced patient age, and patient preference accounted for the remainder.
In a univariate analysis of the cohort of 149 patients, factors that decreased the likelihood of receipt of curative HCC therapy included T2 stage or higher at diagnosis and a diagnosis of depression, whereas provision for lodging was associated with increased likelihood of receiving HCC-specific care (Table 5). Other factors that influenced receipt of any treatment included patient’s MELD score, total bilirubin, and serum α-fetoprotein, a surrogate marker for tumor stage. In the multivariable analysis, predictors of receiving curative therapy included absence of substance use, within T2 stage of tumor, and Child-Turcotte-Pugh class A cirrhosis. The presence of psychosocial barriers apart from substance use did not predict a lower chance of receiving curative HCC therapy (including homelessness, distance traveled to center, mental health disorder, and low income).
Median survival was 727 (95% CI, 488-966) days from diagnosis. Survival from HCC diagnosis in study cohort was 72% at 1 year, 50% at 2 years, 39% at 3 years, and 36% at 5 years. Death occurred in 71 (48%) patients; HCC accounted for death in 52 (73%) patients, complications of end-stage liver disease in 13 (18%) patients, and other causes for the remainder of patients.
Discussion
Increases in prevalence and mortality related to cirrhosis and HCC have been reported among the US veteran population.3 This is in large part attributable to the burden of chronic HCV infection in this population. As mirrored in the US population in general, we may be at a turning point regarding the gradual increase in prevalence in HCC.7 The prevalence of cirrhosis and viral-related HCC related to HCV infection will decline with availability of effective antiviral therapy. Alcoholic liver disease remains a main etiological factor for development of cirrhosis and HCC. Nonalcoholic fatty liver disease is becoming a more prevalent cause for development of cirrhosis, indication for liver transplantation, and development of HCC, and indeed may lead to HCC even in the absence of cirrhosis.8
HCC remains a challenging clinical problem.2 As the vast majority of cases arise in the context of cirrhosis, management of HCC not only must address the cancer stage at diagnosis, but also the patient’s underlying liver dysfunction and performance status. Receipt of HCC-specific therapy is a key driver of patient outcome, with curative therapies available for those diagnosed with early-stage disease. We and others have shown that a multidisciplinary approach to coordinate, individualize, and optimize care for these complex patients can improve the rate of treatment utilization, reduce treatment delays, and improve patient survival.6,9,10
Patient psychosocial barriers, such as low socioeconomic status, homelessness, alcohol and substance use, and psychiatric disorders, are more prevalent among the veteran population and have the potential to negatively influence successful health care delivery. One retrospective study of 100 veterans at a US Department of Veterans Affairs (VA) medical center treated for HCC from 2009 to 2014 showed a majority of the patients lived on a meager income, a high prevalence of homelessness, substance use history in 96% of their cohort, and psychiatric illness in 65% of patients.11 Other studies have documented similar findings in the veteran population, with alcohol, substance use, as well as other uncontrolled comorbidities as barriers to providing care, such as antiviral therapy for chronic HCV infection.12
Herein, we present a cohort of veterans with HCC managed through our MDTB from 2007 to 2016, for whom chronic HCV infection and/or alcoholic liver disease were the main causes of cirrhosis. Our cohort had a high burden of alcohol and substance use disorders while other psychiatric illnesses were also common. Our cohort includes patients who were poor, and even some veterans who lacked a stable home. This profile of poverty and social deprivation among veterans is matched in national data.13-15 Using a tumor board model of nurse navigation and multidisciplinary care, we were able to provide travel and lodging assistance to 50 (34%) and 33 (22%) patients, respectively, in order to facilitate their care.
Our data demonstrate that the impact of psychosocial barriers on our capacity to deliver care varies with the nature of the treatment under consideration: curative vs cancer control. For example, active substance use disorder, homelessness, and severe established mental illness were often considered insurmountable when the treatment in question was LT. Nevertheless, despite the high prevalence in our study group of barriers, such as lack of transport while living far from a VA medical center, or alcohol use disorder, a curative treatment with either LT, tumor ablation, or resection could be offered to over half of our cohort. When noncurative therapies are included, most patients (85%) received HCC-specific care, with good relative survival.
Our reported high receipt of HCC-specific care and patient survival is in contrast to previously reported low rates of HCC-specific care in in a national survey of management of 1296 veteran patients infected with HCV who developed HCC from 1998 to 2006. In this population, HCC-specific treatment was provided to 34%.16 However our data are consistent with our previously published data of patients with HCC managed through an institutional MDTB.6 Indeed, as shown by a univariate analysis in our present study, individualizing care to address modifiable patient barriers, such as providing provisions for lodging if needed, was associated with an increased likelihood of receiving HCC-specific care. On the other hand, advanced tumor stage (> T2) at diagnosis and a diagnosis of depression, which was the most common psychiatric diagnosis in our cohort, were both associated with decreased likelihood of receiving HCC-specific care. Clinical factors such as MELD score, total bilirubin, and serum AFP all affected the likelihood of providing HCC-specific care. In a multivariate analysis, factors that predicted ability to receive curative therapy included absence of substance use, T2 stage of tumor, and Child-Turcotte-Pugh class A cirrhosis. This is expected as patients with HCC within T2 stage (or Milan criteria) with compensated cirrhosis are most likely to receive curative therapies, such as resection, ablation, or LT.2
Conclusions
Our study demonstrates a high burden of psychosocial challenges in veterans with HCC. These accounted for a significant barrier to receive HCC-specific care. Despite the presence of these patient barriers, high rates of HCC-specific treatment are attainable through individualization and coordination of patient care in the context of a MDTB model with nurse navigation. Provision of targeted social support to ameliorate these modifiable factors improves patient outcomes.
1. McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of hepatocellular carcinoma. Hepatology. 2021;73(suppl 1):4-13. doi:10.1002/hep.31288.
2. Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723-750. doi:10.1002/hep.29913
3. Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001-2013. Gastroenterology. 2015;149(6):1471-e18. doi:10.1053/j.gastro.2015.07.056
4. Kazis LE, Miller DR, Clark J, et al. Health-related quality of life in patients served by the Department of Veterans Affairs: results from the Veterans Health Study. Arch Intern Med. 1998;158(6):626-632. doi:10.1001/archinte.158.6.626
5. Slind LM, Keating TM, Fisher AG, Rose TG. A patient navigation model for veterans traveling for cancer care. Fed Pract. 2016;33(suppl 1):40S-45S.
6. Agarwal PD, Phillips P, Hillman L, et al. Multidisciplinary management of hepatocellular carcinoma improves access to therapy and patient survival. J Clin Gastroenterol. 2017;51(9):845-849. doi:10.1097/MCG.0000000000000825
7. White DL, Thrift AP, Kanwal F, Davila J, El-Serag HB. Incidence of hepatocellular carcinoma in all 50 United States, From 2000 Through 2012. Gastroenterology. 2017;152(4):812-820.e5. doi:10.1053/j.gastro.2016.11.020
8. Kanwal F, Kramer JR, Mapakshi S, et al. Risk of hepatocellular cancer in patients with non-alcoholic fatty liver disease. Gastroenterology. 2018;155(6):1828-1837.e2. doi:10.1053/j.gastro.2018.08.024
9. Yopp AC, Mansour JC, Beg MS, et al. Establishment of a multidisciplinary hepatocellular carcinoma clinic is associated with improved clinical outcome. Ann Surg Oncol. 2014;21(4):1287-1295. doi:10.1245/s10434-013-3413-8
10. Chang TT, Sawhney R, Monto A, et al. Implementation of a multidisciplinary treatment team for hepatocellular cancer at a Veterans Affairs Medical Center improves survival. HPB (Oxford). 2008;10(6):405-411. doi:10.1080/13651820802356572
11. Hwa KJ, Dua MM, Wren SM, Visser BC. Missing the obvious: psychosocial obstacles in veterans with hepatocellular carcinoma. HPB (Oxford). 2015;17(12):1124-1129. doi:10.1111/hpb.12508
12. Taylor J, Carr-Lopez S, Robinson A, et al. Determinants of treatment eligibility in veterans with hepatitis C viral infection. Clin Ther. 2017;39(1):130-137. doi:10.1016/j.clinthera.2016.11.019
13. Fargo J, Metraux S, Byrne T, et al. Prevalence and risk of homelessness among US veterans. Prev Chronic Dis. 2012;9:E45.
14. Tsai J, Rosenheck RA. Risk factors for homelessness among US veterans. Epidemiol Rev. 2015;37:177-195. doi:10.1093/epirev/mxu004
15. Tsai J, Link B, Rosenheck RA, Pietrzak RH. Homelessness among a nationally representative sample of US veterans: prevalence, service utilization, and correlates. Soc Psychiatry Psychiatr Epidemiol. 2016;51(6):907-916. doi:10.1007/s00127-016-1210-y
16. Davila JA, Kramer JR, Duan Z, et al. Referral and receipt of treatment for hepatocellular carcinoma in United States veterans: effect of patient and nonpatient factors. Hepatology. 2013;57(5):1858-1868. doi:10.1002/hep.26287
Hepatocellular carcinoma (HCC) remains a major global health problem and is the third leading cause of cancer-related mortality worldwide.1 Management of HCC is complex; as it largely occurs in the background of chronic liver disease, its management must simultaneously address challenges related to the patient’s tumor burden, as well as their underlying liver dysfunction and performance status. HCC is universally fatal without treatment, with a 5-year survival < 10%.2 However, if detected early HCC is potentially curable, with treatments such as hepatic resection, ablation, and/or liver transplantation, which are associated with 5-year survival rates as high as 70%.2 HCC-specific palliative treatments, including intra-arterial therapies (eg, trans-arterial chemoembolization, radioembolization) and systemic chemotherapy, have also been shown to prolong survival in patients with advanced HCC. Therefore, a key driver of patient survival is receipt of HCC-specific therapy.
There is rising incidence and mortality related to HCC in the US veteran population, largely attributed to acquisition of chronic hepatitis C virus (HCV) infection decades prior.3 There is also a high prevalence of psychosocial barriers in this population, such as low socioeconomic status, homelessness, alcohol and substance use disorders, and psychiatric disorders which can negatively influence receipt of medical treatment, including cancer care.4,5 Given the complexity of managing HCC, as well as the plethora of potential treatment options available, it is widely accepted that a multidisciplinary team approach, such as the multidisciplinary tumor board (MDTB) provides optimal care to patients with HCC.2,6 The aim of the present study was to identify in a population of veterans diagnosed with HCC the prevalence of psychosocial barriers to care and assess their impact and the role of an MDTB on receipt of HCC-specific care.
Methods
In June 2007, a joint institutional MDTB was established for patients with primary liver tumors receiving care at the William S. Middleton Memorial Veterans’ Hospital (WSMMVH) in Madison, Wisconsin. As we have described elsewhere, individual cases with their corresponding imaging studies were reviewed at a weekly conference attended by transplant hepatologists, medical oncologists, hepatobiliary and transplant surgeons, pathologists, diagnostic and interventional radiologists, and nurse coordinators.6 Potential therapies offered included surgical resection, liver transplantation (LT), thermal ablation, intra-arterial therapies (chemo and/or radioembolization), systemic chemotherapy, stereotactic radiation, and best supportive care. Decisions regarding the appropriate treatment modality were made based on patient factors, review of their cross-sectional imaging studies and/or histopathology, and context of their underlying liver dysfunction. The tumor board discussion was summarized in meeting minutes as well as tumor board encounters recorded in each patient’s health record. Although patients with benign tumors were presented at the MDTB, only patients with a diagnosis of HCC were included in this study.
A database analysis was conducted of all veteran patients with HCC managed through the WSMMVH MDTB, since its inception up to December 31, 2016, with follow-up until December 31, 2018. Data for analysis included demographics, laboratory parameters at time of diagnosis and treatment, imaging findings, histopathology and/or surgical pathology, treatment rendered, and follow-up information. The primary outcome measured in this study included receipt of any therapy and secondarily, patient survival.
Discrete variables were analyzed with χ2 statistics or Fisher exact test and continuous variables with the student t test. Multivariable analyses were carried out with logistic regression. Variables with a P < .05 were considered statistically significant. Analyses were carried out using IBM SPSS v24.0.
As a quality-improvement initiative for the care and management of veterans with HCC, this study was determined to be exempt from review by the WSMMVH and University of Wisconsin School of Medicine and Public Health Institutional Review Board.
Results
From January 1, 2007, through December 31, 2016, 149 patients with HCC were managed through the MDTB. Baseline demographic data, Model for End-stage Liver Disease (MELD) score and Child-Turcotte-Pugh class, and baseline HCC characteristics of the cohort are shown in Tables 1 and 2.
There was a high prevalence of psychosocial barriers in our study cohort, including alcohol or substance use disorder, mental illness diagnosis, and low socioeconomic status (Table 3). The mean distance traveled to WSMMVH for HCC-specific care was 206 km. Fifty patients in the cohort utilized travel assistance and 33 patients accessed lodging assistance.
HCC Treatments
There was a high rate of receipt of treatment in our study cohort with 127 (85%) patients receiving at least one HCC-specific therapy. Care was individualized and coordinated through our institutional MDTB, with both curative and palliative treatment modalities utilized (Table 4).
Curative treatment, which includes LT, ablation, or resection, was offered to 78 (52%) patients who were within T2 stage. Of these 78 patients who were potential candidates for LT as a curative treatment for HCC, 31 were not deemed suitable transplant candidates. Psychosocial barriers precluded consideration for LT in 7 of the 31 patients due to active substance use, homelessness in 1 patient, and severe mental illness in 3 patients. Medical comorbidities, advanced patient age, and patient preference accounted for the remainder.
In a univariate analysis of the cohort of 149 patients, factors that decreased the likelihood of receipt of curative HCC therapy included T2 stage or higher at diagnosis and a diagnosis of depression, whereas provision for lodging was associated with increased likelihood of receiving HCC-specific care (Table 5). Other factors that influenced receipt of any treatment included patient’s MELD score, total bilirubin, and serum α-fetoprotein, a surrogate marker for tumor stage. In the multivariable analysis, predictors of receiving curative therapy included absence of substance use, within T2 stage of tumor, and Child-Turcotte-Pugh class A cirrhosis. The presence of psychosocial barriers apart from substance use did not predict a lower chance of receiving curative HCC therapy (including homelessness, distance traveled to center, mental health disorder, and low income).
Median survival was 727 (95% CI, 488-966) days from diagnosis. Survival from HCC diagnosis in study cohort was 72% at 1 year, 50% at 2 years, 39% at 3 years, and 36% at 5 years. Death occurred in 71 (48%) patients; HCC accounted for death in 52 (73%) patients, complications of end-stage liver disease in 13 (18%) patients, and other causes for the remainder of patients.
Discussion
Increases in prevalence and mortality related to cirrhosis and HCC have been reported among the US veteran population.3 This is in large part attributable to the burden of chronic HCV infection in this population. As mirrored in the US population in general, we may be at a turning point regarding the gradual increase in prevalence in HCC.7 The prevalence of cirrhosis and viral-related HCC related to HCV infection will decline with availability of effective antiviral therapy. Alcoholic liver disease remains a main etiological factor for development of cirrhosis and HCC. Nonalcoholic fatty liver disease is becoming a more prevalent cause for development of cirrhosis, indication for liver transplantation, and development of HCC, and indeed may lead to HCC even in the absence of cirrhosis.8
HCC remains a challenging clinical problem.2 As the vast majority of cases arise in the context of cirrhosis, management of HCC not only must address the cancer stage at diagnosis, but also the patient’s underlying liver dysfunction and performance status. Receipt of HCC-specific therapy is a key driver of patient outcome, with curative therapies available for those diagnosed with early-stage disease. We and others have shown that a multidisciplinary approach to coordinate, individualize, and optimize care for these complex patients can improve the rate of treatment utilization, reduce treatment delays, and improve patient survival.6,9,10
Patient psychosocial barriers, such as low socioeconomic status, homelessness, alcohol and substance use, and psychiatric disorders, are more prevalent among the veteran population and have the potential to negatively influence successful health care delivery. One retrospective study of 100 veterans at a US Department of Veterans Affairs (VA) medical center treated for HCC from 2009 to 2014 showed a majority of the patients lived on a meager income, a high prevalence of homelessness, substance use history in 96% of their cohort, and psychiatric illness in 65% of patients.11 Other studies have documented similar findings in the veteran population, with alcohol, substance use, as well as other uncontrolled comorbidities as barriers to providing care, such as antiviral therapy for chronic HCV infection.12
Herein, we present a cohort of veterans with HCC managed through our MDTB from 2007 to 2016, for whom chronic HCV infection and/or alcoholic liver disease were the main causes of cirrhosis. Our cohort had a high burden of alcohol and substance use disorders while other psychiatric illnesses were also common. Our cohort includes patients who were poor, and even some veterans who lacked a stable home. This profile of poverty and social deprivation among veterans is matched in national data.13-15 Using a tumor board model of nurse navigation and multidisciplinary care, we were able to provide travel and lodging assistance to 50 (34%) and 33 (22%) patients, respectively, in order to facilitate their care.
Our data demonstrate that the impact of psychosocial barriers on our capacity to deliver care varies with the nature of the treatment under consideration: curative vs cancer control. For example, active substance use disorder, homelessness, and severe established mental illness were often considered insurmountable when the treatment in question was LT. Nevertheless, despite the high prevalence in our study group of barriers, such as lack of transport while living far from a VA medical center, or alcohol use disorder, a curative treatment with either LT, tumor ablation, or resection could be offered to over half of our cohort. When noncurative therapies are included, most patients (85%) received HCC-specific care, with good relative survival.
Our reported high receipt of HCC-specific care and patient survival is in contrast to previously reported low rates of HCC-specific care in in a national survey of management of 1296 veteran patients infected with HCV who developed HCC from 1998 to 2006. In this population, HCC-specific treatment was provided to 34%.16 However our data are consistent with our previously published data of patients with HCC managed through an institutional MDTB.6 Indeed, as shown by a univariate analysis in our present study, individualizing care to address modifiable patient barriers, such as providing provisions for lodging if needed, was associated with an increased likelihood of receiving HCC-specific care. On the other hand, advanced tumor stage (> T2) at diagnosis and a diagnosis of depression, which was the most common psychiatric diagnosis in our cohort, were both associated with decreased likelihood of receiving HCC-specific care. Clinical factors such as MELD score, total bilirubin, and serum AFP all affected the likelihood of providing HCC-specific care. In a multivariate analysis, factors that predicted ability to receive curative therapy included absence of substance use, T2 stage of tumor, and Child-Turcotte-Pugh class A cirrhosis. This is expected as patients with HCC within T2 stage (or Milan criteria) with compensated cirrhosis are most likely to receive curative therapies, such as resection, ablation, or LT.2
Conclusions
Our study demonstrates a high burden of psychosocial challenges in veterans with HCC. These accounted for a significant barrier to receive HCC-specific care. Despite the presence of these patient barriers, high rates of HCC-specific treatment are attainable through individualization and coordination of patient care in the context of a MDTB model with nurse navigation. Provision of targeted social support to ameliorate these modifiable factors improves patient outcomes.
Hepatocellular carcinoma (HCC) remains a major global health problem and is the third leading cause of cancer-related mortality worldwide.1 Management of HCC is complex; as it largely occurs in the background of chronic liver disease, its management must simultaneously address challenges related to the patient’s tumor burden, as well as their underlying liver dysfunction and performance status. HCC is universally fatal without treatment, with a 5-year survival < 10%.2 However, if detected early HCC is potentially curable, with treatments such as hepatic resection, ablation, and/or liver transplantation, which are associated with 5-year survival rates as high as 70%.2 HCC-specific palliative treatments, including intra-arterial therapies (eg, trans-arterial chemoembolization, radioembolization) and systemic chemotherapy, have also been shown to prolong survival in patients with advanced HCC. Therefore, a key driver of patient survival is receipt of HCC-specific therapy.
There is rising incidence and mortality related to HCC in the US veteran population, largely attributed to acquisition of chronic hepatitis C virus (HCV) infection decades prior.3 There is also a high prevalence of psychosocial barriers in this population, such as low socioeconomic status, homelessness, alcohol and substance use disorders, and psychiatric disorders which can negatively influence receipt of medical treatment, including cancer care.4,5 Given the complexity of managing HCC, as well as the plethora of potential treatment options available, it is widely accepted that a multidisciplinary team approach, such as the multidisciplinary tumor board (MDTB) provides optimal care to patients with HCC.2,6 The aim of the present study was to identify in a population of veterans diagnosed with HCC the prevalence of psychosocial barriers to care and assess their impact and the role of an MDTB on receipt of HCC-specific care.
Methods
In June 2007, a joint institutional MDTB was established for patients with primary liver tumors receiving care at the William S. Middleton Memorial Veterans’ Hospital (WSMMVH) in Madison, Wisconsin. As we have described elsewhere, individual cases with their corresponding imaging studies were reviewed at a weekly conference attended by transplant hepatologists, medical oncologists, hepatobiliary and transplant surgeons, pathologists, diagnostic and interventional radiologists, and nurse coordinators.6 Potential therapies offered included surgical resection, liver transplantation (LT), thermal ablation, intra-arterial therapies (chemo and/or radioembolization), systemic chemotherapy, stereotactic radiation, and best supportive care. Decisions regarding the appropriate treatment modality were made based on patient factors, review of their cross-sectional imaging studies and/or histopathology, and context of their underlying liver dysfunction. The tumor board discussion was summarized in meeting minutes as well as tumor board encounters recorded in each patient’s health record. Although patients with benign tumors were presented at the MDTB, only patients with a diagnosis of HCC were included in this study.
A database analysis was conducted of all veteran patients with HCC managed through the WSMMVH MDTB, since its inception up to December 31, 2016, with follow-up until December 31, 2018. Data for analysis included demographics, laboratory parameters at time of diagnosis and treatment, imaging findings, histopathology and/or surgical pathology, treatment rendered, and follow-up information. The primary outcome measured in this study included receipt of any therapy and secondarily, patient survival.
Discrete variables were analyzed with χ2 statistics or Fisher exact test and continuous variables with the student t test. Multivariable analyses were carried out with logistic regression. Variables with a P < .05 were considered statistically significant. Analyses were carried out using IBM SPSS v24.0.
As a quality-improvement initiative for the care and management of veterans with HCC, this study was determined to be exempt from review by the WSMMVH and University of Wisconsin School of Medicine and Public Health Institutional Review Board.
Results
From January 1, 2007, through December 31, 2016, 149 patients with HCC were managed through the MDTB. Baseline demographic data, Model for End-stage Liver Disease (MELD) score and Child-Turcotte-Pugh class, and baseline HCC characteristics of the cohort are shown in Tables 1 and 2.
There was a high prevalence of psychosocial barriers in our study cohort, including alcohol or substance use disorder, mental illness diagnosis, and low socioeconomic status (Table 3). The mean distance traveled to WSMMVH for HCC-specific care was 206 km. Fifty patients in the cohort utilized travel assistance and 33 patients accessed lodging assistance.
HCC Treatments
There was a high rate of receipt of treatment in our study cohort with 127 (85%) patients receiving at least one HCC-specific therapy. Care was individualized and coordinated through our institutional MDTB, with both curative and palliative treatment modalities utilized (Table 4).
Curative treatment, which includes LT, ablation, or resection, was offered to 78 (52%) patients who were within T2 stage. Of these 78 patients who were potential candidates for LT as a curative treatment for HCC, 31 were not deemed suitable transplant candidates. Psychosocial barriers precluded consideration for LT in 7 of the 31 patients due to active substance use, homelessness in 1 patient, and severe mental illness in 3 patients. Medical comorbidities, advanced patient age, and patient preference accounted for the remainder.
In a univariate analysis of the cohort of 149 patients, factors that decreased the likelihood of receipt of curative HCC therapy included T2 stage or higher at diagnosis and a diagnosis of depression, whereas provision for lodging was associated with increased likelihood of receiving HCC-specific care (Table 5). Other factors that influenced receipt of any treatment included patient’s MELD score, total bilirubin, and serum α-fetoprotein, a surrogate marker for tumor stage. In the multivariable analysis, predictors of receiving curative therapy included absence of substance use, within T2 stage of tumor, and Child-Turcotte-Pugh class A cirrhosis. The presence of psychosocial barriers apart from substance use did not predict a lower chance of receiving curative HCC therapy (including homelessness, distance traveled to center, mental health disorder, and low income).
Median survival was 727 (95% CI, 488-966) days from diagnosis. Survival from HCC diagnosis in study cohort was 72% at 1 year, 50% at 2 years, 39% at 3 years, and 36% at 5 years. Death occurred in 71 (48%) patients; HCC accounted for death in 52 (73%) patients, complications of end-stage liver disease in 13 (18%) patients, and other causes for the remainder of patients.
Discussion
Increases in prevalence and mortality related to cirrhosis and HCC have been reported among the US veteran population.3 This is in large part attributable to the burden of chronic HCV infection in this population. As mirrored in the US population in general, we may be at a turning point regarding the gradual increase in prevalence in HCC.7 The prevalence of cirrhosis and viral-related HCC related to HCV infection will decline with availability of effective antiviral therapy. Alcoholic liver disease remains a main etiological factor for development of cirrhosis and HCC. Nonalcoholic fatty liver disease is becoming a more prevalent cause for development of cirrhosis, indication for liver transplantation, and development of HCC, and indeed may lead to HCC even in the absence of cirrhosis.8
HCC remains a challenging clinical problem.2 As the vast majority of cases arise in the context of cirrhosis, management of HCC not only must address the cancer stage at diagnosis, but also the patient’s underlying liver dysfunction and performance status. Receipt of HCC-specific therapy is a key driver of patient outcome, with curative therapies available for those diagnosed with early-stage disease. We and others have shown that a multidisciplinary approach to coordinate, individualize, and optimize care for these complex patients can improve the rate of treatment utilization, reduce treatment delays, and improve patient survival.6,9,10
Patient psychosocial barriers, such as low socioeconomic status, homelessness, alcohol and substance use, and psychiatric disorders, are more prevalent among the veteran population and have the potential to negatively influence successful health care delivery. One retrospective study of 100 veterans at a US Department of Veterans Affairs (VA) medical center treated for HCC from 2009 to 2014 showed a majority of the patients lived on a meager income, a high prevalence of homelessness, substance use history in 96% of their cohort, and psychiatric illness in 65% of patients.11 Other studies have documented similar findings in the veteran population, with alcohol, substance use, as well as other uncontrolled comorbidities as barriers to providing care, such as antiviral therapy for chronic HCV infection.12
Herein, we present a cohort of veterans with HCC managed through our MDTB from 2007 to 2016, for whom chronic HCV infection and/or alcoholic liver disease were the main causes of cirrhosis. Our cohort had a high burden of alcohol and substance use disorders while other psychiatric illnesses were also common. Our cohort includes patients who were poor, and even some veterans who lacked a stable home. This profile of poverty and social deprivation among veterans is matched in national data.13-15 Using a tumor board model of nurse navigation and multidisciplinary care, we were able to provide travel and lodging assistance to 50 (34%) and 33 (22%) patients, respectively, in order to facilitate their care.
Our data demonstrate that the impact of psychosocial barriers on our capacity to deliver care varies with the nature of the treatment under consideration: curative vs cancer control. For example, active substance use disorder, homelessness, and severe established mental illness were often considered insurmountable when the treatment in question was LT. Nevertheless, despite the high prevalence in our study group of barriers, such as lack of transport while living far from a VA medical center, or alcohol use disorder, a curative treatment with either LT, tumor ablation, or resection could be offered to over half of our cohort. When noncurative therapies are included, most patients (85%) received HCC-specific care, with good relative survival.
Our reported high receipt of HCC-specific care and patient survival is in contrast to previously reported low rates of HCC-specific care in in a national survey of management of 1296 veteran patients infected with HCV who developed HCC from 1998 to 2006. In this population, HCC-specific treatment was provided to 34%.16 However our data are consistent with our previously published data of patients with HCC managed through an institutional MDTB.6 Indeed, as shown by a univariate analysis in our present study, individualizing care to address modifiable patient barriers, such as providing provisions for lodging if needed, was associated with an increased likelihood of receiving HCC-specific care. On the other hand, advanced tumor stage (> T2) at diagnosis and a diagnosis of depression, which was the most common psychiatric diagnosis in our cohort, were both associated with decreased likelihood of receiving HCC-specific care. Clinical factors such as MELD score, total bilirubin, and serum AFP all affected the likelihood of providing HCC-specific care. In a multivariate analysis, factors that predicted ability to receive curative therapy included absence of substance use, T2 stage of tumor, and Child-Turcotte-Pugh class A cirrhosis. This is expected as patients with HCC within T2 stage (or Milan criteria) with compensated cirrhosis are most likely to receive curative therapies, such as resection, ablation, or LT.2
Conclusions
Our study demonstrates a high burden of psychosocial challenges in veterans with HCC. These accounted for a significant barrier to receive HCC-specific care. Despite the presence of these patient barriers, high rates of HCC-specific treatment are attainable through individualization and coordination of patient care in the context of a MDTB model with nurse navigation. Provision of targeted social support to ameliorate these modifiable factors improves patient outcomes.
1. McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of hepatocellular carcinoma. Hepatology. 2021;73(suppl 1):4-13. doi:10.1002/hep.31288.
2. Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723-750. doi:10.1002/hep.29913
3. Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001-2013. Gastroenterology. 2015;149(6):1471-e18. doi:10.1053/j.gastro.2015.07.056
4. Kazis LE, Miller DR, Clark J, et al. Health-related quality of life in patients served by the Department of Veterans Affairs: results from the Veterans Health Study. Arch Intern Med. 1998;158(6):626-632. doi:10.1001/archinte.158.6.626
5. Slind LM, Keating TM, Fisher AG, Rose TG. A patient navigation model for veterans traveling for cancer care. Fed Pract. 2016;33(suppl 1):40S-45S.
6. Agarwal PD, Phillips P, Hillman L, et al. Multidisciplinary management of hepatocellular carcinoma improves access to therapy and patient survival. J Clin Gastroenterol. 2017;51(9):845-849. doi:10.1097/MCG.0000000000000825
7. White DL, Thrift AP, Kanwal F, Davila J, El-Serag HB. Incidence of hepatocellular carcinoma in all 50 United States, From 2000 Through 2012. Gastroenterology. 2017;152(4):812-820.e5. doi:10.1053/j.gastro.2016.11.020
8. Kanwal F, Kramer JR, Mapakshi S, et al. Risk of hepatocellular cancer in patients with non-alcoholic fatty liver disease. Gastroenterology. 2018;155(6):1828-1837.e2. doi:10.1053/j.gastro.2018.08.024
9. Yopp AC, Mansour JC, Beg MS, et al. Establishment of a multidisciplinary hepatocellular carcinoma clinic is associated with improved clinical outcome. Ann Surg Oncol. 2014;21(4):1287-1295. doi:10.1245/s10434-013-3413-8
10. Chang TT, Sawhney R, Monto A, et al. Implementation of a multidisciplinary treatment team for hepatocellular cancer at a Veterans Affairs Medical Center improves survival. HPB (Oxford). 2008;10(6):405-411. doi:10.1080/13651820802356572
11. Hwa KJ, Dua MM, Wren SM, Visser BC. Missing the obvious: psychosocial obstacles in veterans with hepatocellular carcinoma. HPB (Oxford). 2015;17(12):1124-1129. doi:10.1111/hpb.12508
12. Taylor J, Carr-Lopez S, Robinson A, et al. Determinants of treatment eligibility in veterans with hepatitis C viral infection. Clin Ther. 2017;39(1):130-137. doi:10.1016/j.clinthera.2016.11.019
13. Fargo J, Metraux S, Byrne T, et al. Prevalence and risk of homelessness among US veterans. Prev Chronic Dis. 2012;9:E45.
14. Tsai J, Rosenheck RA. Risk factors for homelessness among US veterans. Epidemiol Rev. 2015;37:177-195. doi:10.1093/epirev/mxu004
15. Tsai J, Link B, Rosenheck RA, Pietrzak RH. Homelessness among a nationally representative sample of US veterans: prevalence, service utilization, and correlates. Soc Psychiatry Psychiatr Epidemiol. 2016;51(6):907-916. doi:10.1007/s00127-016-1210-y
16. Davila JA, Kramer JR, Duan Z, et al. Referral and receipt of treatment for hepatocellular carcinoma in United States veterans: effect of patient and nonpatient factors. Hepatology. 2013;57(5):1858-1868. doi:10.1002/hep.26287
1. McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of hepatocellular carcinoma. Hepatology. 2021;73(suppl 1):4-13. doi:10.1002/hep.31288.
2. Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723-750. doi:10.1002/hep.29913
3. Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001-2013. Gastroenterology. 2015;149(6):1471-e18. doi:10.1053/j.gastro.2015.07.056
4. Kazis LE, Miller DR, Clark J, et al. Health-related quality of life in patients served by the Department of Veterans Affairs: results from the Veterans Health Study. Arch Intern Med. 1998;158(6):626-632. doi:10.1001/archinte.158.6.626
5. Slind LM, Keating TM, Fisher AG, Rose TG. A patient navigation model for veterans traveling for cancer care. Fed Pract. 2016;33(suppl 1):40S-45S.
6. Agarwal PD, Phillips P, Hillman L, et al. Multidisciplinary management of hepatocellular carcinoma improves access to therapy and patient survival. J Clin Gastroenterol. 2017;51(9):845-849. doi:10.1097/MCG.0000000000000825
7. White DL, Thrift AP, Kanwal F, Davila J, El-Serag HB. Incidence of hepatocellular carcinoma in all 50 United States, From 2000 Through 2012. Gastroenterology. 2017;152(4):812-820.e5. doi:10.1053/j.gastro.2016.11.020
8. Kanwal F, Kramer JR, Mapakshi S, et al. Risk of hepatocellular cancer in patients with non-alcoholic fatty liver disease. Gastroenterology. 2018;155(6):1828-1837.e2. doi:10.1053/j.gastro.2018.08.024
9. Yopp AC, Mansour JC, Beg MS, et al. Establishment of a multidisciplinary hepatocellular carcinoma clinic is associated with improved clinical outcome. Ann Surg Oncol. 2014;21(4):1287-1295. doi:10.1245/s10434-013-3413-8
10. Chang TT, Sawhney R, Monto A, et al. Implementation of a multidisciplinary treatment team for hepatocellular cancer at a Veterans Affairs Medical Center improves survival. HPB (Oxford). 2008;10(6):405-411. doi:10.1080/13651820802356572
11. Hwa KJ, Dua MM, Wren SM, Visser BC. Missing the obvious: psychosocial obstacles in veterans with hepatocellular carcinoma. HPB (Oxford). 2015;17(12):1124-1129. doi:10.1111/hpb.12508
12. Taylor J, Carr-Lopez S, Robinson A, et al. Determinants of treatment eligibility in veterans with hepatitis C viral infection. Clin Ther. 2017;39(1):130-137. doi:10.1016/j.clinthera.2016.11.019
13. Fargo J, Metraux S, Byrne T, et al. Prevalence and risk of homelessness among US veterans. Prev Chronic Dis. 2012;9:E45.
14. Tsai J, Rosenheck RA. Risk factors for homelessness among US veterans. Epidemiol Rev. 2015;37:177-195. doi:10.1093/epirev/mxu004
15. Tsai J, Link B, Rosenheck RA, Pietrzak RH. Homelessness among a nationally representative sample of US veterans: prevalence, service utilization, and correlates. Soc Psychiatry Psychiatr Epidemiol. 2016;51(6):907-916. doi:10.1007/s00127-016-1210-y
16. Davila JA, Kramer JR, Duan Z, et al. Referral and receipt of treatment for hepatocellular carcinoma in United States veterans: effect of patient and nonpatient factors. Hepatology. 2013;57(5):1858-1868. doi:10.1002/hep.26287
Women with lung cancer live longer than men
“In this first Australian prospective study of lung cancer survival comparing men and women, we found that men had a 43% greater risk of dying from their lung cancer than women,” comments lead author Xue Qin Yu, PhD, the Daffodil Centre, the University of Sydney, and colleagues.
“[However], when all prognostic factors were considered together, most of the survival differential disappeared,” they add.
“These results suggest that sex differences in lung cancer survival can be largely explained by known prognostic factors,” Dr. Yu and colleagues emphasize.
The study was published in the May issue of the Journal of Thoracic Oncology.
The ‘45 and up’ study
The findings come from the Sax Institute’s 45 and Up Study, an ongoing trial involving over 267,000 participants aged 45 years and older living in New South Wales, Australia. Patients were recruited to the study between 2006 and 2009. At the time of recruitment, patients were cancer free.
A total of 1,130 participants were diagnosed with having lung cancer during follow-up – 488 women and 642 men. Compared with men, women were, on average, younger at the time of diagnosis, had fewer comorbidities, and were more likely to be never-smokers or to have been exposed to passive smoke.
Women were also more likely to be diagnosed with adenocarcinoma than men and to receive surgery within 6 months of their diagnosis.
“Lung cancer survival was significantly higher for women,” the authors report, at a median of 1.28 years versus 0.77 years for men (P < .0001).
Within each subgroup of major prognostic factors – histologic subtype, cancer stage, cancer treatment, and smoking status – women again survived significantly longer than men.
Interestingly, the authors note that “women with adenocarcinoma had significantly better survival than men with adenocarcinoma independent of smoking status,” (P = .0009). This suggests that sex differences in tumor biology may play a role in explaining the sex survival differential between men and women, they commented. That said, never-smokers had a 16% lower risk for lung cancer death than ever-smokers after adjusting for age, the authors point out.
The authors also note that approximately half of the disparity in survival between the sexes could be explained by differences in the receipt of anticancer therapy within 6 months of the diagnosis. “This could partly be due to a lower proportion of men having surgery within 6 months than women,” investigators speculate, at 17% versus 25%, respectively.
Men were also older than women at the time of diagnosis, were less likely to be never-smokers, and had more comorbidities, all of which might also have prevented them from having surgery. Women with lung cancer may also respond better to chemotherapy than men, although the sex disparity in survival persisted even among patients who did not receive any treatment for their cancer within 6 months of their diagnosis, investigators point out.
Furthermore, “smoking history at baseline was identified as a significant contributing factor to the sex survival disparity, explaining approximately 28% of the overall disparity,” Dr. Yu and colleagues observe.
Only 8% of men diagnosed with lung cancer were never-smokers, compared with 23% of women. The authors note that never-smokers are more likely to receive aggressive or complete treatment and respond well to treatment.
Similarly, tumor-related factors together explained about one-quarter of the overall sex disparity in survival.
Screening guidelines
Commenting on the findings in an accompanying editorial, Claudia Poleri, MD, Hospital María Ferrer, Buenos Aires, says that this Australian study provides “valuable information.”
“The risk of dying from lung cancer was significantly higher for men than for women,” she writes. “Differences in treatment-related factors explained 50% of the sex survival differential, followed by lifestyle and tumor-related factors (28% and 26%, respectively).
“Nevertheless, these differences alone do not explain the higher survival in women,” she comments.
“Does it matter to analyze the differences by sex in lung cancer?” Dr. Poleri asks in the editorial, and then answers herself: “It matters.”
“It is necessary to implement screening programs and build artificial intelligence diagnostic algorithms considering the role of sex and gender equity to ensure that innovative technologies do not induce disparities in clinical care,” she writes.
“It is crucial to conduct education and health public programs that consider these differences, optimizing the use of available resources, [and] it is essential to improve the accuracy of research design and clinical trials,” she adds.
Dr. Yu and Dr. Poleri declared no relevant financial interests.
A version of this article first appeared on Medscape.com.
“In this first Australian prospective study of lung cancer survival comparing men and women, we found that men had a 43% greater risk of dying from their lung cancer than women,” comments lead author Xue Qin Yu, PhD, the Daffodil Centre, the University of Sydney, and colleagues.
“[However], when all prognostic factors were considered together, most of the survival differential disappeared,” they add.
“These results suggest that sex differences in lung cancer survival can be largely explained by known prognostic factors,” Dr. Yu and colleagues emphasize.
The study was published in the May issue of the Journal of Thoracic Oncology.
The ‘45 and up’ study
The findings come from the Sax Institute’s 45 and Up Study, an ongoing trial involving over 267,000 participants aged 45 years and older living in New South Wales, Australia. Patients were recruited to the study between 2006 and 2009. At the time of recruitment, patients were cancer free.
A total of 1,130 participants were diagnosed with having lung cancer during follow-up – 488 women and 642 men. Compared with men, women were, on average, younger at the time of diagnosis, had fewer comorbidities, and were more likely to be never-smokers or to have been exposed to passive smoke.
Women were also more likely to be diagnosed with adenocarcinoma than men and to receive surgery within 6 months of their diagnosis.
“Lung cancer survival was significantly higher for women,” the authors report, at a median of 1.28 years versus 0.77 years for men (P < .0001).
Within each subgroup of major prognostic factors – histologic subtype, cancer stage, cancer treatment, and smoking status – women again survived significantly longer than men.
Interestingly, the authors note that “women with adenocarcinoma had significantly better survival than men with adenocarcinoma independent of smoking status,” (P = .0009). This suggests that sex differences in tumor biology may play a role in explaining the sex survival differential between men and women, they commented. That said, never-smokers had a 16% lower risk for lung cancer death than ever-smokers after adjusting for age, the authors point out.
The authors also note that approximately half of the disparity in survival between the sexes could be explained by differences in the receipt of anticancer therapy within 6 months of the diagnosis. “This could partly be due to a lower proportion of men having surgery within 6 months than women,” investigators speculate, at 17% versus 25%, respectively.
Men were also older than women at the time of diagnosis, were less likely to be never-smokers, and had more comorbidities, all of which might also have prevented them from having surgery. Women with lung cancer may also respond better to chemotherapy than men, although the sex disparity in survival persisted even among patients who did not receive any treatment for their cancer within 6 months of their diagnosis, investigators point out.
Furthermore, “smoking history at baseline was identified as a significant contributing factor to the sex survival disparity, explaining approximately 28% of the overall disparity,” Dr. Yu and colleagues observe.
Only 8% of men diagnosed with lung cancer were never-smokers, compared with 23% of women. The authors note that never-smokers are more likely to receive aggressive or complete treatment and respond well to treatment.
Similarly, tumor-related factors together explained about one-quarter of the overall sex disparity in survival.
Screening guidelines
Commenting on the findings in an accompanying editorial, Claudia Poleri, MD, Hospital María Ferrer, Buenos Aires, says that this Australian study provides “valuable information.”
“The risk of dying from lung cancer was significantly higher for men than for women,” she writes. “Differences in treatment-related factors explained 50% of the sex survival differential, followed by lifestyle and tumor-related factors (28% and 26%, respectively).
“Nevertheless, these differences alone do not explain the higher survival in women,” she comments.
“Does it matter to analyze the differences by sex in lung cancer?” Dr. Poleri asks in the editorial, and then answers herself: “It matters.”
“It is necessary to implement screening programs and build artificial intelligence diagnostic algorithms considering the role of sex and gender equity to ensure that innovative technologies do not induce disparities in clinical care,” she writes.
“It is crucial to conduct education and health public programs that consider these differences, optimizing the use of available resources, [and] it is essential to improve the accuracy of research design and clinical trials,” she adds.
Dr. Yu and Dr. Poleri declared no relevant financial interests.
A version of this article first appeared on Medscape.com.
“In this first Australian prospective study of lung cancer survival comparing men and women, we found that men had a 43% greater risk of dying from their lung cancer than women,” comments lead author Xue Qin Yu, PhD, the Daffodil Centre, the University of Sydney, and colleagues.
“[However], when all prognostic factors were considered together, most of the survival differential disappeared,” they add.
“These results suggest that sex differences in lung cancer survival can be largely explained by known prognostic factors,” Dr. Yu and colleagues emphasize.
The study was published in the May issue of the Journal of Thoracic Oncology.
The ‘45 and up’ study
The findings come from the Sax Institute’s 45 and Up Study, an ongoing trial involving over 267,000 participants aged 45 years and older living in New South Wales, Australia. Patients were recruited to the study between 2006 and 2009. At the time of recruitment, patients were cancer free.
A total of 1,130 participants were diagnosed with having lung cancer during follow-up – 488 women and 642 men. Compared with men, women were, on average, younger at the time of diagnosis, had fewer comorbidities, and were more likely to be never-smokers or to have been exposed to passive smoke.
Women were also more likely to be diagnosed with adenocarcinoma than men and to receive surgery within 6 months of their diagnosis.
“Lung cancer survival was significantly higher for women,” the authors report, at a median of 1.28 years versus 0.77 years for men (P < .0001).
Within each subgroup of major prognostic factors – histologic subtype, cancer stage, cancer treatment, and smoking status – women again survived significantly longer than men.
Interestingly, the authors note that “women with adenocarcinoma had significantly better survival than men with adenocarcinoma independent of smoking status,” (P = .0009). This suggests that sex differences in tumor biology may play a role in explaining the sex survival differential between men and women, they commented. That said, never-smokers had a 16% lower risk for lung cancer death than ever-smokers after adjusting for age, the authors point out.
The authors also note that approximately half of the disparity in survival between the sexes could be explained by differences in the receipt of anticancer therapy within 6 months of the diagnosis. “This could partly be due to a lower proportion of men having surgery within 6 months than women,” investigators speculate, at 17% versus 25%, respectively.
Men were also older than women at the time of diagnosis, were less likely to be never-smokers, and had more comorbidities, all of which might also have prevented them from having surgery. Women with lung cancer may also respond better to chemotherapy than men, although the sex disparity in survival persisted even among patients who did not receive any treatment for their cancer within 6 months of their diagnosis, investigators point out.
Furthermore, “smoking history at baseline was identified as a significant contributing factor to the sex survival disparity, explaining approximately 28% of the overall disparity,” Dr. Yu and colleagues observe.
Only 8% of men diagnosed with lung cancer were never-smokers, compared with 23% of women. The authors note that never-smokers are more likely to receive aggressive or complete treatment and respond well to treatment.
Similarly, tumor-related factors together explained about one-quarter of the overall sex disparity in survival.
Screening guidelines
Commenting on the findings in an accompanying editorial, Claudia Poleri, MD, Hospital María Ferrer, Buenos Aires, says that this Australian study provides “valuable information.”
“The risk of dying from lung cancer was significantly higher for men than for women,” she writes. “Differences in treatment-related factors explained 50% of the sex survival differential, followed by lifestyle and tumor-related factors (28% and 26%, respectively).
“Nevertheless, these differences alone do not explain the higher survival in women,” she comments.
“Does it matter to analyze the differences by sex in lung cancer?” Dr. Poleri asks in the editorial, and then answers herself: “It matters.”
“It is necessary to implement screening programs and build artificial intelligence diagnostic algorithms considering the role of sex and gender equity to ensure that innovative technologies do not induce disparities in clinical care,” she writes.
“It is crucial to conduct education and health public programs that consider these differences, optimizing the use of available resources, [and] it is essential to improve the accuracy of research design and clinical trials,” she adds.
Dr. Yu and Dr. Poleri declared no relevant financial interests.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THORACIC ONCOLOGY
New data support electroconvulsive therapy for severe depression
Advocates and users of electroconvulsive therapy (ECT) have received further scientific backing:
The patient cohort comprised 27,231 men and 40,096 women who had been treated as inpatients. The average age was 45.1 years (range: 18-103 years), and 4,982 patients received ECT. The primary endpoint was death by suicide within 365 days of hospital discharge. The secondary endpoints were death not by suicide and total mortality. The cause-specific hazard ratio (csHR) was calculated for patients with ECT, compared with patients without ECT.
In the propensity score-weighted analysis, ECT was linked to a significantly reduced suicide risk (csHR: 0.53; 95% confidence interval, 0.31-0.92). According to the calculations, ECT was associated with a significantly decreased total mortality risk (hazard ratio, 0.75; 95% CI, 0.58-0.97). However, this was not the case for death from causes other than suicide.
The authors, led by Tyler S. Kaster, PhD, a psychiatrist at Temerty Centre for Therapeutic Brain Intervention, Centre for Addiction and Mental Health, Toronto, concluded that this study underlines the importance of ECT, in particular for people with severe depression.
A well-tested therapy
ECT has been used for decades as a substantial tool for the treatment of patients with severe mental illnesses. Over the past 15 years, new methods for the treatment of severely depressed patients have been tested, such as vagus nerve stimulation, transcranial magnetic stimulation, and intranasal administration of esketamine. However, in a recent review paper in the New England Journal of Medicine, American psychiatrists Randall T. Espinoza, MD, MPH, University of California, Los Angeles, and Charles H. Kellner, MD, University of South Carolina, Charleston, reported that none of these therapies had proven to be an indisputable substitute for ECT for people with severe depression.
Significant clinical benefits
According to these American psychiatrists, the benefit of ECT has been proven many times, and several studies demonstrate the effect on the risk for suicide. Moreover, quality of life is improved, and the rate of new hospital admissions is lowered. ECT can rapidly improve depressive, psychotic, and catatonic symptoms and reduce suicidal urges for certain patient groups.
Studies on ECT involving patients with treatment-refractory depression have shown response rates of 60%-80% and pooled remission rates of 50%-60%. High response rates for ECT have even been reported for patients with psychotic depression or catatonia. In one study that recruited patients with treatment-refractory schizophrenia, the ECT efficacy rates were between 40% and 70%. In some Asian countries, schizophrenia is the main indication for ECT.
Good safety profile
Overall, the psychiatrists consider ECT to be a safe and tolerable therapy. The estimated death rate is around 2.1 deaths per 100,000 treatments. The most common complications are acute cardiopulmonary events, which are estimated to occur in less than 1% of treatments. Rare serious adverse events linked to ECT are arrhythmias, shortness of breath, aspiration, and prolonged seizures. The common but mild side effects are headaches, jaw pain, myalgia, nausea, and vomiting after the procedure, as well as fatigue.
Concerns regarding cognitive impairment still represent an obstacle for the use of ECT. However, in today’s practice, ECT leads to fewer cognitive side effects than previous treatments. The authors stated that it is not possible to predict how an individual patient will be affected, but most patients have only mild or moderate cognitive side effects that generally abate days to weeks after an ECT course has ended.
However, retrograde amnesia linked to ECT can last over a year. In rare cases, acute confusion or delirium can develop that requires interruption or discontinuation of treatment. No indications of structural brain damage after ECT have been detected in neuropathological testing. A Danish cohort study involving 168,015 patients with depression, of whom 3.1% had at least one ECT treatment, did not find a link between ECT with a mean period of almost 5 years and increased onset of dementia.
Bad reputation
Dr. Espinoza and Dr. Kellner criticized the fact that, despite its proven efficacy and safety, ECT is used too little. This judgment is nothing new. Psychiatrists have been complaining for years that this procedure is used too little, including Eric Slade, MD, from the University of Baltimore, in 2017 and German professors Andreas Fallgatter, MD, and Urban Wiesing, MD, PhD, in 2018. Dr. Wiesing and Dr. Fallgatter attribute the low level of use to the fact that ECT is labor-intensive, compared with pharmacotherapy.
Another reason is clearly the bad reputation of this method. However, ECT’s poor image, which has only increased over time, is not a convincing argument to forego today’s ECT as a treatment for patients with severe mental illnesses. According to Dr. Fallgatter and Dr. Wiesing, even the risk of misuse of this method is “not a sufficient argument for categorical refusal, rather for caution at best.” They argued that otherwise, “modern medicine would have to renounce many more therapies.”
This article was translated from Univadis Germany.
Advocates and users of electroconvulsive therapy (ECT) have received further scientific backing:
The patient cohort comprised 27,231 men and 40,096 women who had been treated as inpatients. The average age was 45.1 years (range: 18-103 years), and 4,982 patients received ECT. The primary endpoint was death by suicide within 365 days of hospital discharge. The secondary endpoints were death not by suicide and total mortality. The cause-specific hazard ratio (csHR) was calculated for patients with ECT, compared with patients without ECT.
In the propensity score-weighted analysis, ECT was linked to a significantly reduced suicide risk (csHR: 0.53; 95% confidence interval, 0.31-0.92). According to the calculations, ECT was associated with a significantly decreased total mortality risk (hazard ratio, 0.75; 95% CI, 0.58-0.97). However, this was not the case for death from causes other than suicide.
The authors, led by Tyler S. Kaster, PhD, a psychiatrist at Temerty Centre for Therapeutic Brain Intervention, Centre for Addiction and Mental Health, Toronto, concluded that this study underlines the importance of ECT, in particular for people with severe depression.
A well-tested therapy
ECT has been used for decades as a substantial tool for the treatment of patients with severe mental illnesses. Over the past 15 years, new methods for the treatment of severely depressed patients have been tested, such as vagus nerve stimulation, transcranial magnetic stimulation, and intranasal administration of esketamine. However, in a recent review paper in the New England Journal of Medicine, American psychiatrists Randall T. Espinoza, MD, MPH, University of California, Los Angeles, and Charles H. Kellner, MD, University of South Carolina, Charleston, reported that none of these therapies had proven to be an indisputable substitute for ECT for people with severe depression.
Significant clinical benefits
According to these American psychiatrists, the benefit of ECT has been proven many times, and several studies demonstrate the effect on the risk for suicide. Moreover, quality of life is improved, and the rate of new hospital admissions is lowered. ECT can rapidly improve depressive, psychotic, and catatonic symptoms and reduce suicidal urges for certain patient groups.
Studies on ECT involving patients with treatment-refractory depression have shown response rates of 60%-80% and pooled remission rates of 50%-60%. High response rates for ECT have even been reported for patients with psychotic depression or catatonia. In one study that recruited patients with treatment-refractory schizophrenia, the ECT efficacy rates were between 40% and 70%. In some Asian countries, schizophrenia is the main indication for ECT.
Good safety profile
Overall, the psychiatrists consider ECT to be a safe and tolerable therapy. The estimated death rate is around 2.1 deaths per 100,000 treatments. The most common complications are acute cardiopulmonary events, which are estimated to occur in less than 1% of treatments. Rare serious adverse events linked to ECT are arrhythmias, shortness of breath, aspiration, and prolonged seizures. The common but mild side effects are headaches, jaw pain, myalgia, nausea, and vomiting after the procedure, as well as fatigue.
Concerns regarding cognitive impairment still represent an obstacle for the use of ECT. However, in today’s practice, ECT leads to fewer cognitive side effects than previous treatments. The authors stated that it is not possible to predict how an individual patient will be affected, but most patients have only mild or moderate cognitive side effects that generally abate days to weeks after an ECT course has ended.
However, retrograde amnesia linked to ECT can last over a year. In rare cases, acute confusion or delirium can develop that requires interruption or discontinuation of treatment. No indications of structural brain damage after ECT have been detected in neuropathological testing. A Danish cohort study involving 168,015 patients with depression, of whom 3.1% had at least one ECT treatment, did not find a link between ECT with a mean period of almost 5 years and increased onset of dementia.
Bad reputation
Dr. Espinoza and Dr. Kellner criticized the fact that, despite its proven efficacy and safety, ECT is used too little. This judgment is nothing new. Psychiatrists have been complaining for years that this procedure is used too little, including Eric Slade, MD, from the University of Baltimore, in 2017 and German professors Andreas Fallgatter, MD, and Urban Wiesing, MD, PhD, in 2018. Dr. Wiesing and Dr. Fallgatter attribute the low level of use to the fact that ECT is labor-intensive, compared with pharmacotherapy.
Another reason is clearly the bad reputation of this method. However, ECT’s poor image, which has only increased over time, is not a convincing argument to forego today’s ECT as a treatment for patients with severe mental illnesses. According to Dr. Fallgatter and Dr. Wiesing, even the risk of misuse of this method is “not a sufficient argument for categorical refusal, rather for caution at best.” They argued that otherwise, “modern medicine would have to renounce many more therapies.”
This article was translated from Univadis Germany.
Advocates and users of electroconvulsive therapy (ECT) have received further scientific backing:
The patient cohort comprised 27,231 men and 40,096 women who had been treated as inpatients. The average age was 45.1 years (range: 18-103 years), and 4,982 patients received ECT. The primary endpoint was death by suicide within 365 days of hospital discharge. The secondary endpoints were death not by suicide and total mortality. The cause-specific hazard ratio (csHR) was calculated for patients with ECT, compared with patients without ECT.
In the propensity score-weighted analysis, ECT was linked to a significantly reduced suicide risk (csHR: 0.53; 95% confidence interval, 0.31-0.92). According to the calculations, ECT was associated with a significantly decreased total mortality risk (hazard ratio, 0.75; 95% CI, 0.58-0.97). However, this was not the case for death from causes other than suicide.
The authors, led by Tyler S. Kaster, PhD, a psychiatrist at Temerty Centre for Therapeutic Brain Intervention, Centre for Addiction and Mental Health, Toronto, concluded that this study underlines the importance of ECT, in particular for people with severe depression.
A well-tested therapy
ECT has been used for decades as a substantial tool for the treatment of patients with severe mental illnesses. Over the past 15 years, new methods for the treatment of severely depressed patients have been tested, such as vagus nerve stimulation, transcranial magnetic stimulation, and intranasal administration of esketamine. However, in a recent review paper in the New England Journal of Medicine, American psychiatrists Randall T. Espinoza, MD, MPH, University of California, Los Angeles, and Charles H. Kellner, MD, University of South Carolina, Charleston, reported that none of these therapies had proven to be an indisputable substitute for ECT for people with severe depression.
Significant clinical benefits
According to these American psychiatrists, the benefit of ECT has been proven many times, and several studies demonstrate the effect on the risk for suicide. Moreover, quality of life is improved, and the rate of new hospital admissions is lowered. ECT can rapidly improve depressive, psychotic, and catatonic symptoms and reduce suicidal urges for certain patient groups.
Studies on ECT involving patients with treatment-refractory depression have shown response rates of 60%-80% and pooled remission rates of 50%-60%. High response rates for ECT have even been reported for patients with psychotic depression or catatonia. In one study that recruited patients with treatment-refractory schizophrenia, the ECT efficacy rates were between 40% and 70%. In some Asian countries, schizophrenia is the main indication for ECT.
Good safety profile
Overall, the psychiatrists consider ECT to be a safe and tolerable therapy. The estimated death rate is around 2.1 deaths per 100,000 treatments. The most common complications are acute cardiopulmonary events, which are estimated to occur in less than 1% of treatments. Rare serious adverse events linked to ECT are arrhythmias, shortness of breath, aspiration, and prolonged seizures. The common but mild side effects are headaches, jaw pain, myalgia, nausea, and vomiting after the procedure, as well as fatigue.
Concerns regarding cognitive impairment still represent an obstacle for the use of ECT. However, in today’s practice, ECT leads to fewer cognitive side effects than previous treatments. The authors stated that it is not possible to predict how an individual patient will be affected, but most patients have only mild or moderate cognitive side effects that generally abate days to weeks after an ECT course has ended.
However, retrograde amnesia linked to ECT can last over a year. In rare cases, acute confusion or delirium can develop that requires interruption or discontinuation of treatment. No indications of structural brain damage after ECT have been detected in neuropathological testing. A Danish cohort study involving 168,015 patients with depression, of whom 3.1% had at least one ECT treatment, did not find a link between ECT with a mean period of almost 5 years and increased onset of dementia.
Bad reputation
Dr. Espinoza and Dr. Kellner criticized the fact that, despite its proven efficacy and safety, ECT is used too little. This judgment is nothing new. Psychiatrists have been complaining for years that this procedure is used too little, including Eric Slade, MD, from the University of Baltimore, in 2017 and German professors Andreas Fallgatter, MD, and Urban Wiesing, MD, PhD, in 2018. Dr. Wiesing and Dr. Fallgatter attribute the low level of use to the fact that ECT is labor-intensive, compared with pharmacotherapy.
Another reason is clearly the bad reputation of this method. However, ECT’s poor image, which has only increased over time, is not a convincing argument to forego today’s ECT as a treatment for patients with severe mental illnesses. According to Dr. Fallgatter and Dr. Wiesing, even the risk of misuse of this method is “not a sufficient argument for categorical refusal, rather for caution at best.” They argued that otherwise, “modern medicine would have to renounce many more therapies.”
This article was translated from Univadis Germany.
FROM THE LANCET PSYCHIATRY
Mastectomy may not be necessary for young breast cancer patients
Mastectomies among younger women with nonmetastatic invasive breast cancer may not always be necessary, according to a new study that shows survival outcomes are similar to those of women who had a lumpectomy.
“A lot of times, there’s this assumption that removal of the entire breast is going to prevent cancer from returning in that breast. That makes complete sense, it’s intuitive, so I think a lot of patients are surprised to find that less extensive surgery provides the same overall survival as a really extensive surgery,” said Christine Pestana, MD, a fellow in breast surgical oncology with the Atrium Health Levine Cancer Institute, Charlotte, N.C. Dr. Pestana presented the study at the annual meeting of the American Society of Breast Surgeons earlier this year.
In fact, it has been well-demonstrated among women over age 50 with breast cancer that lumpectomy and mastectomy result in similar outcomes, but efforts to show similar efficacy by analyzing data from randomized trials have been limited by small numbers of women under 40, said the study’s lead author Lejla Hadzikadic-Gusic, MD, who is codirector of the Sandra Levine Young Women’s Breast Cancer Program at Atrium Health. “We’ve done a lot of research since the 1970s to be able to keep a woman’s breasts and just treat her for breast cancer. It’s nice to be able to say the same thing for younger women,” said Dr. Hadzikadic-Gusic, in an interview.
The researchers drew from the Young Women’s Database from the Levine Cancer Institute. The analysis included data from nearly 600 women treated between 2010 and 2018.
The increasing frequency of mastectomies in younger women may be traceable, in part, to high-profile cases of celebrities who have had mastectomies after an early breast cancer diagnosis, with Angelina Jolie being among the most known of examples. But Ms. Jolie had the procedure proactively without a cancer diagnosis because she carried the BRCA1 mutation, which increases breast cancer risk. That information was often lost in press coverage, which can lead to confusion among young women with breast cancer, according to Dr. Hadzikadic-Gusic. “What we’re trying to do is have this data help us educate our patients,” she said.
It’s also important for physicians to help guide patients through these decisions, and family history is a key factor. Dr. Pestana encourages primary care providers to explore family history to help understand cancer risks. “It’s not just breast cancer. It’s also ovarian cancer, colon cancer, prostate cancer. Those all have associations with different genetic mutations. If we start asking those questions, we may be able to identify patients who potentially could have that mutation, refer them to a geneticist, have them tested,” she said.
All of the 591 patients in the study were under age 40, with a median age of 37, and the median follow-up was 67 months. Twelve percent of patients died; 53.3% of patients were HR+/HER2–, 20.8% were HR+/HER2+, 19.3% were triple negative, and 6.6% were HR–/HER2+. There was no association between type of surgery and mortality.
The study was funded by the Levine Family Cancer Institute. Dr. Pestana and Dr. Hadzikadic-Gusic have no relevant financial disclosures.
Mastectomies among younger women with nonmetastatic invasive breast cancer may not always be necessary, according to a new study that shows survival outcomes are similar to those of women who had a lumpectomy.
“A lot of times, there’s this assumption that removal of the entire breast is going to prevent cancer from returning in that breast. That makes complete sense, it’s intuitive, so I think a lot of patients are surprised to find that less extensive surgery provides the same overall survival as a really extensive surgery,” said Christine Pestana, MD, a fellow in breast surgical oncology with the Atrium Health Levine Cancer Institute, Charlotte, N.C. Dr. Pestana presented the study at the annual meeting of the American Society of Breast Surgeons earlier this year.
In fact, it has been well-demonstrated among women over age 50 with breast cancer that lumpectomy and mastectomy result in similar outcomes, but efforts to show similar efficacy by analyzing data from randomized trials have been limited by small numbers of women under 40, said the study’s lead author Lejla Hadzikadic-Gusic, MD, who is codirector of the Sandra Levine Young Women’s Breast Cancer Program at Atrium Health. “We’ve done a lot of research since the 1970s to be able to keep a woman’s breasts and just treat her for breast cancer. It’s nice to be able to say the same thing for younger women,” said Dr. Hadzikadic-Gusic, in an interview.
The researchers drew from the Young Women’s Database from the Levine Cancer Institute. The analysis included data from nearly 600 women treated between 2010 and 2018.
The increasing frequency of mastectomies in younger women may be traceable, in part, to high-profile cases of celebrities who have had mastectomies after an early breast cancer diagnosis, with Angelina Jolie being among the most known of examples. But Ms. Jolie had the procedure proactively without a cancer diagnosis because she carried the BRCA1 mutation, which increases breast cancer risk. That information was often lost in press coverage, which can lead to confusion among young women with breast cancer, according to Dr. Hadzikadic-Gusic. “What we’re trying to do is have this data help us educate our patients,” she said.
It’s also important for physicians to help guide patients through these decisions, and family history is a key factor. Dr. Pestana encourages primary care providers to explore family history to help understand cancer risks. “It’s not just breast cancer. It’s also ovarian cancer, colon cancer, prostate cancer. Those all have associations with different genetic mutations. If we start asking those questions, we may be able to identify patients who potentially could have that mutation, refer them to a geneticist, have them tested,” she said.
All of the 591 patients in the study were under age 40, with a median age of 37, and the median follow-up was 67 months. Twelve percent of patients died; 53.3% of patients were HR+/HER2–, 20.8% were HR+/HER2+, 19.3% were triple negative, and 6.6% were HR–/HER2+. There was no association between type of surgery and mortality.
The study was funded by the Levine Family Cancer Institute. Dr. Pestana and Dr. Hadzikadic-Gusic have no relevant financial disclosures.
Mastectomies among younger women with nonmetastatic invasive breast cancer may not always be necessary, according to a new study that shows survival outcomes are similar to those of women who had a lumpectomy.
“A lot of times, there’s this assumption that removal of the entire breast is going to prevent cancer from returning in that breast. That makes complete sense, it’s intuitive, so I think a lot of patients are surprised to find that less extensive surgery provides the same overall survival as a really extensive surgery,” said Christine Pestana, MD, a fellow in breast surgical oncology with the Atrium Health Levine Cancer Institute, Charlotte, N.C. Dr. Pestana presented the study at the annual meeting of the American Society of Breast Surgeons earlier this year.
In fact, it has been well-demonstrated among women over age 50 with breast cancer that lumpectomy and mastectomy result in similar outcomes, but efforts to show similar efficacy by analyzing data from randomized trials have been limited by small numbers of women under 40, said the study’s lead author Lejla Hadzikadic-Gusic, MD, who is codirector of the Sandra Levine Young Women’s Breast Cancer Program at Atrium Health. “We’ve done a lot of research since the 1970s to be able to keep a woman’s breasts and just treat her for breast cancer. It’s nice to be able to say the same thing for younger women,” said Dr. Hadzikadic-Gusic, in an interview.
The researchers drew from the Young Women’s Database from the Levine Cancer Institute. The analysis included data from nearly 600 women treated between 2010 and 2018.
The increasing frequency of mastectomies in younger women may be traceable, in part, to high-profile cases of celebrities who have had mastectomies after an early breast cancer diagnosis, with Angelina Jolie being among the most known of examples. But Ms. Jolie had the procedure proactively without a cancer diagnosis because she carried the BRCA1 mutation, which increases breast cancer risk. That information was often lost in press coverage, which can lead to confusion among young women with breast cancer, according to Dr. Hadzikadic-Gusic. “What we’re trying to do is have this data help us educate our patients,” she said.
It’s also important for physicians to help guide patients through these decisions, and family history is a key factor. Dr. Pestana encourages primary care providers to explore family history to help understand cancer risks. “It’s not just breast cancer. It’s also ovarian cancer, colon cancer, prostate cancer. Those all have associations with different genetic mutations. If we start asking those questions, we may be able to identify patients who potentially could have that mutation, refer them to a geneticist, have them tested,” she said.
All of the 591 patients in the study were under age 40, with a median age of 37, and the median follow-up was 67 months. Twelve percent of patients died; 53.3% of patients were HR+/HER2–, 20.8% were HR+/HER2+, 19.3% were triple negative, and 6.6% were HR–/HER2+. There was no association between type of surgery and mortality.
The study was funded by the Levine Family Cancer Institute. Dr. Pestana and Dr. Hadzikadic-Gusic have no relevant financial disclosures.
FROM ASBS 2022
Nurses under fire: The stress of medical malpractice
Just because nurses are sued less often than doctors doesn’t mean that their actions aren’t a focus of a large number of medical malpractice lawsuits. A condition known as medical malpractice stress syndrome (MMSS) is increasingly being recognized as affecting medical professionals who are subjected to litigation.
According to a 2019 report by CRICO, the risk management arm of Harvard’s medical facilities, nursing was a “primary service” in 34% of cases with a high-severity injury and in 44% of cases that were closed with a payment. And even though nurses were named as defendants only 14% of the time, likely because many nurses don’t have their own personal malpractice coverage, their hospitals or facilities were sued in most of these cases – making the nurses important witnesses for the defense.
We have every reason to believe that things have gotten worse since the CRICO study was published. Chronic nursing shortages were exacerbated during the COVID pandemic, and we have seen a large number of nurses leave the workforce altogether. In a recent survey of nurses by Hospital IQ, 90% of respondents said they were considering leaving the nursing profession in the next year, with 71% of nurses who have more than 15 years of nursing experience thinking about leaving within the next few months.
Those remaining are faced with increased workloads and extra shifts – often mandated – and working with too little sleep. Their commitment to their mission is heroic, but they are only human; it’s hard to imagine the number of errors, the number of bad outcomes, and the number of lawsuits going anywhere but up.
And of course, the entire profession has been fixated on the recent case of the Tennessee nurse who was prosecuted criminally and convicted in connection with a fatal medication error.
These are all reasons to expect that an increasing number of nurses are going to be trying to cope with symptoms of MMSS. Too many of them will initially be viewed by lawyers or claims professionals as simply defensive, arrogant, or difficult to work with. In fact, it’s impossible to know how many cases are settled just to avoid the risk of such a “difficult client” being deposed.
These caring, hard-working, and committed individuals have had their lives shaken in ways that they never expected. Nurses with MMSS need support, but traditional psychotherapy, with a diffuse focus and long-time horizon, is not the most effective option. What’s necessary is practical support that is short term, goal oriented, and tailored to the specifics of the pending litigation process.
Most important, they need to know that they are not experiencing this alone, that MMSS is a common phenomenon, and that a productive coaching relationship can be highly effective.
When approached and supported effectively, nurses – and indeed all medical professionals – can regain their confidence and focus, continue having productive professional and personal lives, and reduce the likelihood of a downhill spiral. And it makes it more likely that they’ll remain in the profession rather than becoming just another statistic in the ever-worsening shortage of nurses in the United States.
Signs of MMSS in nurses
Mixed with their feelings of anxiety and depression, nurses with MMSS often have thoughts such as:
- Am I going to lose my license?
- Am I going to lose my job?
- Will my reputation be destroyed? Will I ever be able to work as a nurse again?
- What am I going to do for a living?
- If I lose everything, will my spouse divorce me? Will I lose my kids?
- I don’t think I did anything wrong, but what if I’m still found to be at fault?
- Did I miss something? Did I make a mistake? Was there something more that I should have done?
- What’s going to happen next? What else could go wrong?
- Are there more people out there who are going to sue me?
- Everything feels overwhelming and out of control.
- My entire identity is now in question.
- How do I get this case out of my head? I can’t focus on anything else.
- I’m developing medical problems of my own.
- I’m having difficulty focusing at work and relating to patients; how do I know who’s going to sue me next?
- I wish that I could escape it all; I feel like killing myself.
Gail Fiore is president of The Winning Focus, which works with physicians and other professionals involved in litigation who are having difficulty coping with stress, anxiety, and other emotional issues. A version of this article first appeared on Medscape.com.
Just because nurses are sued less often than doctors doesn’t mean that their actions aren’t a focus of a large number of medical malpractice lawsuits. A condition known as medical malpractice stress syndrome (MMSS) is increasingly being recognized as affecting medical professionals who are subjected to litigation.
According to a 2019 report by CRICO, the risk management arm of Harvard’s medical facilities, nursing was a “primary service” in 34% of cases with a high-severity injury and in 44% of cases that were closed with a payment. And even though nurses were named as defendants only 14% of the time, likely because many nurses don’t have their own personal malpractice coverage, their hospitals or facilities were sued in most of these cases – making the nurses important witnesses for the defense.
We have every reason to believe that things have gotten worse since the CRICO study was published. Chronic nursing shortages were exacerbated during the COVID pandemic, and we have seen a large number of nurses leave the workforce altogether. In a recent survey of nurses by Hospital IQ, 90% of respondents said they were considering leaving the nursing profession in the next year, with 71% of nurses who have more than 15 years of nursing experience thinking about leaving within the next few months.
Those remaining are faced with increased workloads and extra shifts – often mandated – and working with too little sleep. Their commitment to their mission is heroic, but they are only human; it’s hard to imagine the number of errors, the number of bad outcomes, and the number of lawsuits going anywhere but up.
And of course, the entire profession has been fixated on the recent case of the Tennessee nurse who was prosecuted criminally and convicted in connection with a fatal medication error.
These are all reasons to expect that an increasing number of nurses are going to be trying to cope with symptoms of MMSS. Too many of them will initially be viewed by lawyers or claims professionals as simply defensive, arrogant, or difficult to work with. In fact, it’s impossible to know how many cases are settled just to avoid the risk of such a “difficult client” being deposed.
These caring, hard-working, and committed individuals have had their lives shaken in ways that they never expected. Nurses with MMSS need support, but traditional psychotherapy, with a diffuse focus and long-time horizon, is not the most effective option. What’s necessary is practical support that is short term, goal oriented, and tailored to the specifics of the pending litigation process.
Most important, they need to know that they are not experiencing this alone, that MMSS is a common phenomenon, and that a productive coaching relationship can be highly effective.
When approached and supported effectively, nurses – and indeed all medical professionals – can regain their confidence and focus, continue having productive professional and personal lives, and reduce the likelihood of a downhill spiral. And it makes it more likely that they’ll remain in the profession rather than becoming just another statistic in the ever-worsening shortage of nurses in the United States.
Signs of MMSS in nurses
Mixed with their feelings of anxiety and depression, nurses with MMSS often have thoughts such as:
- Am I going to lose my license?
- Am I going to lose my job?
- Will my reputation be destroyed? Will I ever be able to work as a nurse again?
- What am I going to do for a living?
- If I lose everything, will my spouse divorce me? Will I lose my kids?
- I don’t think I did anything wrong, but what if I’m still found to be at fault?
- Did I miss something? Did I make a mistake? Was there something more that I should have done?
- What’s going to happen next? What else could go wrong?
- Are there more people out there who are going to sue me?
- Everything feels overwhelming and out of control.
- My entire identity is now in question.
- How do I get this case out of my head? I can’t focus on anything else.
- I’m developing medical problems of my own.
- I’m having difficulty focusing at work and relating to patients; how do I know who’s going to sue me next?
- I wish that I could escape it all; I feel like killing myself.
Gail Fiore is president of The Winning Focus, which works with physicians and other professionals involved in litigation who are having difficulty coping with stress, anxiety, and other emotional issues. A version of this article first appeared on Medscape.com.
Just because nurses are sued less often than doctors doesn’t mean that their actions aren’t a focus of a large number of medical malpractice lawsuits. A condition known as medical malpractice stress syndrome (MMSS) is increasingly being recognized as affecting medical professionals who are subjected to litigation.
According to a 2019 report by CRICO, the risk management arm of Harvard’s medical facilities, nursing was a “primary service” in 34% of cases with a high-severity injury and in 44% of cases that were closed with a payment. And even though nurses were named as defendants only 14% of the time, likely because many nurses don’t have their own personal malpractice coverage, their hospitals or facilities were sued in most of these cases – making the nurses important witnesses for the defense.
We have every reason to believe that things have gotten worse since the CRICO study was published. Chronic nursing shortages were exacerbated during the COVID pandemic, and we have seen a large number of nurses leave the workforce altogether. In a recent survey of nurses by Hospital IQ, 90% of respondents said they were considering leaving the nursing profession in the next year, with 71% of nurses who have more than 15 years of nursing experience thinking about leaving within the next few months.
Those remaining are faced with increased workloads and extra shifts – often mandated – and working with too little sleep. Their commitment to their mission is heroic, but they are only human; it’s hard to imagine the number of errors, the number of bad outcomes, and the number of lawsuits going anywhere but up.
And of course, the entire profession has been fixated on the recent case of the Tennessee nurse who was prosecuted criminally and convicted in connection with a fatal medication error.
These are all reasons to expect that an increasing number of nurses are going to be trying to cope with symptoms of MMSS. Too many of them will initially be viewed by lawyers or claims professionals as simply defensive, arrogant, or difficult to work with. In fact, it’s impossible to know how many cases are settled just to avoid the risk of such a “difficult client” being deposed.
These caring, hard-working, and committed individuals have had their lives shaken in ways that they never expected. Nurses with MMSS need support, but traditional psychotherapy, with a diffuse focus and long-time horizon, is not the most effective option. What’s necessary is practical support that is short term, goal oriented, and tailored to the specifics of the pending litigation process.
Most important, they need to know that they are not experiencing this alone, that MMSS is a common phenomenon, and that a productive coaching relationship can be highly effective.
When approached and supported effectively, nurses – and indeed all medical professionals – can regain their confidence and focus, continue having productive professional and personal lives, and reduce the likelihood of a downhill spiral. And it makes it more likely that they’ll remain in the profession rather than becoming just another statistic in the ever-worsening shortage of nurses in the United States.
Signs of MMSS in nurses
Mixed with their feelings of anxiety and depression, nurses with MMSS often have thoughts such as:
- Am I going to lose my license?
- Am I going to lose my job?
- Will my reputation be destroyed? Will I ever be able to work as a nurse again?
- What am I going to do for a living?
- If I lose everything, will my spouse divorce me? Will I lose my kids?
- I don’t think I did anything wrong, but what if I’m still found to be at fault?
- Did I miss something? Did I make a mistake? Was there something more that I should have done?
- What’s going to happen next? What else could go wrong?
- Are there more people out there who are going to sue me?
- Everything feels overwhelming and out of control.
- My entire identity is now in question.
- How do I get this case out of my head? I can’t focus on anything else.
- I’m developing medical problems of my own.
- I’m having difficulty focusing at work and relating to patients; how do I know who’s going to sue me next?
- I wish that I could escape it all; I feel like killing myself.
Gail Fiore is president of The Winning Focus, which works with physicians and other professionals involved in litigation who are having difficulty coping with stress, anxiety, and other emotional issues. A version of this article first appeared on Medscape.com.
Low butyrylcholinesterase: A possible biomarker of SIDS risk?
Reduced levels of the cholinergic-system enzyme butyrylcholinesterase (BChE) may provide another piece of the puzzle for sudden infant death syndrome (SIDS), preliminary data from Australian researchers suggested.
A small case-control study led by Carmel T. Harrington, PhD,* a sleep medicine expert and honorary research fellow at the Children’s Hospital at Westmead (Australia), found that measurements in 722 dried blood spots taken during neonatal screening 2 or 3 days after birth were lower in babies who subsequently died of SIDS, compared with those of matched surviving controls and other babies who died of non-SIDS causes.
In groups in which cases were reported as SIDS death (n = 26) there was strong evidence that lower BChE-specific activity was associated with death (odds ratio, 0.73 per U/mg; 95% confidence interval, 0.60-0.89, P = .0014). In groups with a non-SIDS death (n = 41), there was no evidence of a linear association between BChE activity and death (OR, 1.001 per U/mg; 95% CI, 0.89-1.13, P = .99). A cohort of 655 age- and sex-matched controls served as a reference group.
Writing online in eBioMedicine, the researchers concluded that a previously unidentified cholinergic deficit, identifiable by abnormal BChE-specific activity, is present at birth in SIDS babies and represents a measurable, specific vulnerability prior to their death. “The finding presents the possibility of identifying infants at future risk for SIDS and it provides a specific avenue for future research into interventions prior to death.”
They hypothesized that the association is evidence of an altered cholinergic homeostasis and claim theirs is the first study to identify a measurable biochemical marker in babies who succumbed to SIDS. The marker “could plausibly produce functional alterations to an infant’s autonomic and arousal responses to an exogenous stressor leaving them vulnerable to sudden death.”
Commenting in a press release, Dr. Harrington said that “babies have a very powerful mechanism to let us know when they are not happy. Usually, if a baby is confronted with a life-threatening situation, such as difficulty breathing during sleep because they are on their tummies, they will arouse and cry out. What this research shows is that some babies don’t have this same robust arousal response.” Despite the sparse data, she believes that BChE is likely involved.
Providing a U.S. perspective on the study but not involved in it, Fern R. Hauck, MD, MS, a professor of family medicine and public health at the University of Virginia, Charlottesville, said that “the media coverage presenting this as the ‘cause of SIDS,’ for which we may find a cure within 5 years, is very disturbing and very misleading. The data are very preliminary and results are based on only 26 SIDS cases.” In addition, the blood samples were more than 2 years old.
This research needs to be repeated in other labs in larger and diverse SIDS populations, she added. “Furthermore, we are not provided any racial-ethnic information about the SIDS cases in this study. In the U.S., the infants who are at greatest risk of dying from SIDS are most commonly African American and Native American/Alaska Native, and thus, these studies would need to be repeated in U.S. populations.”
Dr. Hauck added that, while the differences in blood levels of this enzyme were statistically different, even if this is confirmed by larger studies, there was enough overlap in the blood levels between cases and controls that it could not be used as a blood test at this point with any reasonable predictive value.
As the authors pointed out, she said, the leading theory of SIDS causation is that multiple factors interact. “While everyone would be happy to find one single explanation, it is not so simple. This research does, however, bring into focus the issues of arousal in SIDS and work on biomarkers. The arousal issue is one researchers have been working on for a long time.”
The SIDS research community has long been interested in biomarkers, Dr. Hauck continued. “Dr. Hannah Kinney’s first autoradiography study reported decreased muscarinic cholinergic receptor binding in the arcuate nucleus in SIDS, which the butyrylcholinesterase work further elaborates. More recently, Dr. Kinney reported abnormal cholinergic binding in the mesopontine reticular formation that is related to arousal and REM.”
Moreover, Robin Haynes and colleagues reported in 2017 that differences in serotonin can similarly be found in newborns on a newborn blood test, she said. “Like the butyrylcholinesterase research, there is a lot of work to do before understanding how specifically it can identify risk. The problem with using it prematurely is that it will unnecessarily alarm parents that their baby will die, and, to make it worse, be inaccurate in our warning.”
She also expressed concern that with the focus on a biomarker, parents will forget that SIDS and other sleep-related infant deaths have come down considerably in the United States thanks to greater emphasis on promoting safe infant sleep behaviors.
The research was supported by a crowdfunding campaign and by NSW Health Pathology. The authors disclosed no conflicts of interest. Dr. Hauck disclosed no conflicts of interest.
* This story was corrected on 5/20/2022.
Reduced levels of the cholinergic-system enzyme butyrylcholinesterase (BChE) may provide another piece of the puzzle for sudden infant death syndrome (SIDS), preliminary data from Australian researchers suggested.
A small case-control study led by Carmel T. Harrington, PhD,* a sleep medicine expert and honorary research fellow at the Children’s Hospital at Westmead (Australia), found that measurements in 722 dried blood spots taken during neonatal screening 2 or 3 days after birth were lower in babies who subsequently died of SIDS, compared with those of matched surviving controls and other babies who died of non-SIDS causes.
In groups in which cases were reported as SIDS death (n = 26) there was strong evidence that lower BChE-specific activity was associated with death (odds ratio, 0.73 per U/mg; 95% confidence interval, 0.60-0.89, P = .0014). In groups with a non-SIDS death (n = 41), there was no evidence of a linear association between BChE activity and death (OR, 1.001 per U/mg; 95% CI, 0.89-1.13, P = .99). A cohort of 655 age- and sex-matched controls served as a reference group.
Writing online in eBioMedicine, the researchers concluded that a previously unidentified cholinergic deficit, identifiable by abnormal BChE-specific activity, is present at birth in SIDS babies and represents a measurable, specific vulnerability prior to their death. “The finding presents the possibility of identifying infants at future risk for SIDS and it provides a specific avenue for future research into interventions prior to death.”
They hypothesized that the association is evidence of an altered cholinergic homeostasis and claim theirs is the first study to identify a measurable biochemical marker in babies who succumbed to SIDS. The marker “could plausibly produce functional alterations to an infant’s autonomic and arousal responses to an exogenous stressor leaving them vulnerable to sudden death.”
Commenting in a press release, Dr. Harrington said that “babies have a very powerful mechanism to let us know when they are not happy. Usually, if a baby is confronted with a life-threatening situation, such as difficulty breathing during sleep because they are on their tummies, they will arouse and cry out. What this research shows is that some babies don’t have this same robust arousal response.” Despite the sparse data, she believes that BChE is likely involved.
Providing a U.S. perspective on the study but not involved in it, Fern R. Hauck, MD, MS, a professor of family medicine and public health at the University of Virginia, Charlottesville, said that “the media coverage presenting this as the ‘cause of SIDS,’ for which we may find a cure within 5 years, is very disturbing and very misleading. The data are very preliminary and results are based on only 26 SIDS cases.” In addition, the blood samples were more than 2 years old.
This research needs to be repeated in other labs in larger and diverse SIDS populations, she added. “Furthermore, we are not provided any racial-ethnic information about the SIDS cases in this study. In the U.S., the infants who are at greatest risk of dying from SIDS are most commonly African American and Native American/Alaska Native, and thus, these studies would need to be repeated in U.S. populations.”
Dr. Hauck added that, while the differences in blood levels of this enzyme were statistically different, even if this is confirmed by larger studies, there was enough overlap in the blood levels between cases and controls that it could not be used as a blood test at this point with any reasonable predictive value.
As the authors pointed out, she said, the leading theory of SIDS causation is that multiple factors interact. “While everyone would be happy to find one single explanation, it is not so simple. This research does, however, bring into focus the issues of arousal in SIDS and work on biomarkers. The arousal issue is one researchers have been working on for a long time.”
The SIDS research community has long been interested in biomarkers, Dr. Hauck continued. “Dr. Hannah Kinney’s first autoradiography study reported decreased muscarinic cholinergic receptor binding in the arcuate nucleus in SIDS, which the butyrylcholinesterase work further elaborates. More recently, Dr. Kinney reported abnormal cholinergic binding in the mesopontine reticular formation that is related to arousal and REM.”
Moreover, Robin Haynes and colleagues reported in 2017 that differences in serotonin can similarly be found in newborns on a newborn blood test, she said. “Like the butyrylcholinesterase research, there is a lot of work to do before understanding how specifically it can identify risk. The problem with using it prematurely is that it will unnecessarily alarm parents that their baby will die, and, to make it worse, be inaccurate in our warning.”
She also expressed concern that with the focus on a biomarker, parents will forget that SIDS and other sleep-related infant deaths have come down considerably in the United States thanks to greater emphasis on promoting safe infant sleep behaviors.
The research was supported by a crowdfunding campaign and by NSW Health Pathology. The authors disclosed no conflicts of interest. Dr. Hauck disclosed no conflicts of interest.
* This story was corrected on 5/20/2022.
Reduced levels of the cholinergic-system enzyme butyrylcholinesterase (BChE) may provide another piece of the puzzle for sudden infant death syndrome (SIDS), preliminary data from Australian researchers suggested.
A small case-control study led by Carmel T. Harrington, PhD,* a sleep medicine expert and honorary research fellow at the Children’s Hospital at Westmead (Australia), found that measurements in 722 dried blood spots taken during neonatal screening 2 or 3 days after birth were lower in babies who subsequently died of SIDS, compared with those of matched surviving controls and other babies who died of non-SIDS causes.
In groups in which cases were reported as SIDS death (n = 26) there was strong evidence that lower BChE-specific activity was associated with death (odds ratio, 0.73 per U/mg; 95% confidence interval, 0.60-0.89, P = .0014). In groups with a non-SIDS death (n = 41), there was no evidence of a linear association between BChE activity and death (OR, 1.001 per U/mg; 95% CI, 0.89-1.13, P = .99). A cohort of 655 age- and sex-matched controls served as a reference group.
Writing online in eBioMedicine, the researchers concluded that a previously unidentified cholinergic deficit, identifiable by abnormal BChE-specific activity, is present at birth in SIDS babies and represents a measurable, specific vulnerability prior to their death. “The finding presents the possibility of identifying infants at future risk for SIDS and it provides a specific avenue for future research into interventions prior to death.”
They hypothesized that the association is evidence of an altered cholinergic homeostasis and claim theirs is the first study to identify a measurable biochemical marker in babies who succumbed to SIDS. The marker “could plausibly produce functional alterations to an infant’s autonomic and arousal responses to an exogenous stressor leaving them vulnerable to sudden death.”
Commenting in a press release, Dr. Harrington said that “babies have a very powerful mechanism to let us know when they are not happy. Usually, if a baby is confronted with a life-threatening situation, such as difficulty breathing during sleep because they are on their tummies, they will arouse and cry out. What this research shows is that some babies don’t have this same robust arousal response.” Despite the sparse data, she believes that BChE is likely involved.
Providing a U.S. perspective on the study but not involved in it, Fern R. Hauck, MD, MS, a professor of family medicine and public health at the University of Virginia, Charlottesville, said that “the media coverage presenting this as the ‘cause of SIDS,’ for which we may find a cure within 5 years, is very disturbing and very misleading. The data are very preliminary and results are based on only 26 SIDS cases.” In addition, the blood samples were more than 2 years old.
This research needs to be repeated in other labs in larger and diverse SIDS populations, she added. “Furthermore, we are not provided any racial-ethnic information about the SIDS cases in this study. In the U.S., the infants who are at greatest risk of dying from SIDS are most commonly African American and Native American/Alaska Native, and thus, these studies would need to be repeated in U.S. populations.”
Dr. Hauck added that, while the differences in blood levels of this enzyme were statistically different, even if this is confirmed by larger studies, there was enough overlap in the blood levels between cases and controls that it could not be used as a blood test at this point with any reasonable predictive value.
As the authors pointed out, she said, the leading theory of SIDS causation is that multiple factors interact. “While everyone would be happy to find one single explanation, it is not so simple. This research does, however, bring into focus the issues of arousal in SIDS and work on biomarkers. The arousal issue is one researchers have been working on for a long time.”
The SIDS research community has long been interested in biomarkers, Dr. Hauck continued. “Dr. Hannah Kinney’s first autoradiography study reported decreased muscarinic cholinergic receptor binding in the arcuate nucleus in SIDS, which the butyrylcholinesterase work further elaborates. More recently, Dr. Kinney reported abnormal cholinergic binding in the mesopontine reticular formation that is related to arousal and REM.”
Moreover, Robin Haynes and colleagues reported in 2017 that differences in serotonin can similarly be found in newborns on a newborn blood test, she said. “Like the butyrylcholinesterase research, there is a lot of work to do before understanding how specifically it can identify risk. The problem with using it prematurely is that it will unnecessarily alarm parents that their baby will die, and, to make it worse, be inaccurate in our warning.”
She also expressed concern that with the focus on a biomarker, parents will forget that SIDS and other sleep-related infant deaths have come down considerably in the United States thanks to greater emphasis on promoting safe infant sleep behaviors.
The research was supported by a crowdfunding campaign and by NSW Health Pathology. The authors disclosed no conflicts of interest. Dr. Hauck disclosed no conflicts of interest.
* This story was corrected on 5/20/2022.
FROM EBIOMEDICINE
Low-calorie ketogenic diet improves immune function
According to the latest evidence, This development was revealed during the 8th International Scientific Symposium New Frontiers in Scientific Research, organized by PronoKal Group and held in Barcelona. During this conference, international multidisciplinary experts in the study and management of obesity presented the latest data on the benefits of treatment based on a very-low-calorie ketogenic diet.
“Nutritional ketosis has gained great interest in recent years because it is shown to have beneficial properties for health and promotes healthy aging, increasing longevity,” said Ana Belén Crujeiras, BSc, PhD, principal investigator of the Health Research Institute of Santiago de Compostela-Galician Health Service (IDIS-SERGAS) Group of Epigenomics in Endocrinology and Nutrition and the Biomedical Research Networking Center for Obesity and Nutrition Physiopathology (CIBEROBN). “Furthermore, in the case of obesity, we have more and more evidence that it is an effective treatment, mainly because to achieve this metabolic state (ketosis), routes that require the combustion of fats are activated, and this induces body weight loss.”
The specialist stressed that several strategies are used to induce nutritional ketosis. They are characterized by low carbohydrate consumption (low-carbohydrate and high-fat diet; low-carbohydrate, low-fat diet; and intermittent fasting). But Dr. Crujeiras warned that to use it as a treatment for a disease such as obesity, it must be backed by strong and solid scientific evidence, moving away from the concept of fad diets.
In this sense, since 2010, Dr. Crujeiras’ team has developed several studies focused on analyzing the efficacy and safety of treatment with a very-low-calorie ketogenic diet, the results of which have been published in high-impact journals.
Dr. Crujeiras commented on the main conclusions drawn from these investigations. “Our work has shown that the very-low-calorie ketogenic diet is effective for rapid weight loss and maintenance of lost weight, as well as reducing fat mass, primarily visceral fat mass.
“In this sense, a very interesting result is that despite the strong weight loss it induces, it preserves muscle mass and function and improves resting metabolic rate. These two variables are important, because all therapeutic strategies that exist to lose weight lead to a significant reduction in fat-free mass and also a reduction in energy expenditure at rest. This factor is associated with the risk of regaining lost weight, which is currently the great challenge in the treatment of obesity,” she added.
Specific methylation pattern
Dr. Crujeiras indicated that other notable evidence is the favorable impact on an emotional and psychological level. “To determine whether the caloric restriction of this diet and the strong weight loss that it involves were associated with an increased desire to eat, we also carried out an analysis with psychobiological tests. These results led us to conclude that this guideline is accompanied by a reduction of anxiety about food and an improvement in psychobiological parameters, thus increasing the quality of life of these patients.”
The specialist also mentioned that studies currently in progress show that the beneficial effect of this diet could be mediated by epigenetic mechanisms. “In our group, we have identified a specific DNA methylation pattern in people with obesity and we wondered if the very-low-calorie ketogenic diet would be able to reverse that methylome.
“We conducted a study in which we collected blood samples from patients on the very-low-calorie ketogenic diet (600 to 800 kcal/day) drawn before treatment, at peak ketosis, and at the end of treatment. After determining the global pattern of DNA in all patients with obesity targeted with this strategy and through bioinformatic analysis, we were able to obtain a methylation pattern. The results showed that after weight loss on the very-low-calorie ketogenic diet, the methylome that obese people initially had [was] reversed and matched that of normal-weight people.
“Continuing with this bioinformatic analysis more comprehensively, we wanted to see what kind of genes were differentially methylated, especially by the action of the ketosis itself. We found that most of the genes that exhibited differential methylation (in total, 292 identified) belonged to pathways that were involved in the regulation of metabolism, adipose tissue function, CNS function, and also carcinogenesis,” she continued.
Immunomodulatory effect
Dr. Crujeiras said that her research group also observed the modulatory role of the very-low-calorie ketogenic diet in the functioning of the immune system, “something that was not seen after similar weight loss induced by bariatric surgery. We analyzed this data in the context of the situation created by COVID-19, taking into account the evidence that people with obesity, compared to those with normal weight, have a higher risk of becoming infected and of having a poor evolution of the infection.”
In this regard, Dr. Crujeiras’ team launched an investigation to study the ACE2 gene methylation pattern, comparing obesity with normal weight and the situation after following a very-low-calorie ketogenic diet or undergoing bariatric surgery. “We observed that the methylation pattern of this gene in obese people was increased, compared to normal-weight people,” she explained, “and this increase was observed mainly in visceral adipose tissue. However, we did not see this in subcutaneous adipose tissue, which is in agreement with the hypothesis that visceral adipose tissue is that mostly associated with obesity-related comorbidities.
“Likewise, the very-low-calorie ketogenic diet was associated with decreased ACE2 methylation, along with increased exposure of this gene. However, after bariatric surgery, no significant changes were observed, so we deduce that we are protecting the patient in some way from inflammation and, therefore, from the potential of serious illness if they become infected.
“In light of these results, we wanted to dig deeper into what was happening with the immune system of obese patients and that inflammation after a very-low-calorie ketogenic diet. We conducted a new study, currently under review in the journal Clinical Nutrition, with the same approach, comparing this diet with a standard hypocaloric balanced diet and bariatric surgery, in which we analyzed a wide battery of cytokines (32). We have observed a differential pattern between the very-low-calorie ketogenic diet and bariatric surgery.
“The results confirm our hypothesis that the very-low-calorie ketogenic diet remodels the inflammatory status of obese patients, and we were also able to verify that the increase in ketone bodies has immunomodulatory properties that were previously demonstrated in preclinical and animal models, which is associated with increased immune function in these patients,” added Dr. Crujeiras.
Personalization and weight regain
In regard to the next steps to take in the knowledge and clinical application of the benefits of this dietary strategy, Dr. Crujeiras said that despite the fact that this diet is known to be effective, it is currently prescribed in a standard manner to all patients, “but there is some variability in the response and also a high risk of regaining weight, as is the case with any nutritional intervention strategy, with that ‘regain’ of lost weight being the main challenge in the treatment of obesity. In this sense, the epigenomic and epigenetic markers that we have identified could help us optimize treatment.”
She added that the future lies in establishing an algorithm that encompasses the patient’s exposome data, along with their genetic and epigenetic profile, to properly classify patients and prescribe a personalized precision therapeutic strategy.
Luca Busetto, MD, cochair of the Obesity Management Task Force (OMTF) of the European Association for the Study of Obesity (EASO), also insisted on the challenge posed by the individualized application of the very-low-calorie ketogenic diet, emphasizing that this diet should always be prescribed by a doctor after an appropriate assessment of the patient. “Obesity is not a matter of willpower or motivation, and most people with obesity have struggled their entire lives and failed because their biology tends to cause weight regain. Therefore, we should try to offer them the options that we currently have, including the very-low-calorie ketogenic diet, adapting them as much as possible to the profile of each patient.”
During his speech, Dr. Busetto presented the recent European guidelines for the management of obesity in adults with a very-low-calorie ketogenic diet, endorsed by EASO, and analyzed the main strengths of these recommendations.
Dr. Busetto remarked that three important points clearly justify the use of the very-low-calorie ketogenic diet. The first is the speed with which the initial weight loss occurs. Recent studies have looked at the benefits of a significant loss of excess weight early in a weight-loss diet, and although this is an association rather than a cause, the results strongly suggest that rapid initial weight loss increases the chance of the result being maintained in the long term. This clashes with the traditional recommendation of losing weight little by little as a strategy to achieve long-term results, but it must be taken into account that there are many myths in the treatment of obesity that current evidence is dismantling with new data – and this is one of them.
Secondly, the effect of the very-low-calorie ketogenic diet can be added to other treatments. This has been demonstrated by studies carried out with liraglutide that showed that this dietary strategy optimizes results, compared with patients who had been treated only with this drug. The third point that justifies the use of the very-low-calorie ketogenic diet is the management of obesity comorbidities. Several investigations demonstrate the effectiveness of this diet in this regard, especially in the case of type 2 diabetes. Data suggest that the substantial weight reductions achieved with it also favor the remission of these comorbidities in many patients.
EASO ‘approval’
Dr. Busetto pointed out that, based on this evidence, the OMTF proposed the development of standards to be included in the EASO guidelines, since there had been no specific recommendation on the very-low-calorie ketogenic diet.
“The main objective of these European guidelines was to provide data referenced by scientific evidence and to suggest a common protocol for the use of this dietary strategy,” he added.
For this, a very exhaustive meta-analysis was carried out, researching all the publications that compared the very-low-calorie ketogenic diet with other diets. The results showed the superiority of the former method for the reduction of body mass index and weight and fat mass, with no difference in lean (muscle) mass, despite significant weight loss in these patients.
This evidence also demonstrates a reduction and an improvement in metabolic markers, specifically glucose metabolism and lipid metabolism.
“The final conclusions of the study corroborate that the very-low-calorie ketogenic diet can be recommended as an effective and safe tool for people with obesity, especially those with severe obesity or comorbidities (joint disease, preoperative period to bariatric surgery, metabolic and cardiovascular diseases) who need immediate, substantial weight loss. In addition, it can be prescribed to specific groups of patients with obesity after considering potential contraindications and under medical follow-up,” said Dr. Busetto.
In Dr. Busetto’s opinion, it would be convenient to refer to this approach as a method, instead of as a diet, “because, in reality, the state of ketosis is limited in time, and if the ketogenic phase is stopped without a continuity plan, obviously weight is regained. In addition, the method approach may increase adherence by patients.”
Finally, Dr. Busetto emphasized the importance of integrating this type of treatment into a long-term lifestyle strategy (including habits, exercise, and nutritional advice). “We must start from the basis that obesity is a chronic and relapsing disease, whose management should also be chronic and probably maintained throughout life.”
Dr. Crujeiras and Dr. Busetto have disclosed no relevant financial relationships. PronoKal Group has recently become part of Nestlé Health Science.
A version of this article appeared on Medscape.com. It was translated from Medscape Spanish Edition.
According to the latest evidence, This development was revealed during the 8th International Scientific Symposium New Frontiers in Scientific Research, organized by PronoKal Group and held in Barcelona. During this conference, international multidisciplinary experts in the study and management of obesity presented the latest data on the benefits of treatment based on a very-low-calorie ketogenic diet.
“Nutritional ketosis has gained great interest in recent years because it is shown to have beneficial properties for health and promotes healthy aging, increasing longevity,” said Ana Belén Crujeiras, BSc, PhD, principal investigator of the Health Research Institute of Santiago de Compostela-Galician Health Service (IDIS-SERGAS) Group of Epigenomics in Endocrinology and Nutrition and the Biomedical Research Networking Center for Obesity and Nutrition Physiopathology (CIBEROBN). “Furthermore, in the case of obesity, we have more and more evidence that it is an effective treatment, mainly because to achieve this metabolic state (ketosis), routes that require the combustion of fats are activated, and this induces body weight loss.”
The specialist stressed that several strategies are used to induce nutritional ketosis. They are characterized by low carbohydrate consumption (low-carbohydrate and high-fat diet; low-carbohydrate, low-fat diet; and intermittent fasting). But Dr. Crujeiras warned that to use it as a treatment for a disease such as obesity, it must be backed by strong and solid scientific evidence, moving away from the concept of fad diets.
In this sense, since 2010, Dr. Crujeiras’ team has developed several studies focused on analyzing the efficacy and safety of treatment with a very-low-calorie ketogenic diet, the results of which have been published in high-impact journals.
Dr. Crujeiras commented on the main conclusions drawn from these investigations. “Our work has shown that the very-low-calorie ketogenic diet is effective for rapid weight loss and maintenance of lost weight, as well as reducing fat mass, primarily visceral fat mass.
“In this sense, a very interesting result is that despite the strong weight loss it induces, it preserves muscle mass and function and improves resting metabolic rate. These two variables are important, because all therapeutic strategies that exist to lose weight lead to a significant reduction in fat-free mass and also a reduction in energy expenditure at rest. This factor is associated with the risk of regaining lost weight, which is currently the great challenge in the treatment of obesity,” she added.
Specific methylation pattern
Dr. Crujeiras indicated that other notable evidence is the favorable impact on an emotional and psychological level. “To determine whether the caloric restriction of this diet and the strong weight loss that it involves were associated with an increased desire to eat, we also carried out an analysis with psychobiological tests. These results led us to conclude that this guideline is accompanied by a reduction of anxiety about food and an improvement in psychobiological parameters, thus increasing the quality of life of these patients.”
The specialist also mentioned that studies currently in progress show that the beneficial effect of this diet could be mediated by epigenetic mechanisms. “In our group, we have identified a specific DNA methylation pattern in people with obesity and we wondered if the very-low-calorie ketogenic diet would be able to reverse that methylome.
“We conducted a study in which we collected blood samples from patients on the very-low-calorie ketogenic diet (600 to 800 kcal/day) drawn before treatment, at peak ketosis, and at the end of treatment. After determining the global pattern of DNA in all patients with obesity targeted with this strategy and through bioinformatic analysis, we were able to obtain a methylation pattern. The results showed that after weight loss on the very-low-calorie ketogenic diet, the methylome that obese people initially had [was] reversed and matched that of normal-weight people.
“Continuing with this bioinformatic analysis more comprehensively, we wanted to see what kind of genes were differentially methylated, especially by the action of the ketosis itself. We found that most of the genes that exhibited differential methylation (in total, 292 identified) belonged to pathways that were involved in the regulation of metabolism, adipose tissue function, CNS function, and also carcinogenesis,” she continued.
Immunomodulatory effect
Dr. Crujeiras said that her research group also observed the modulatory role of the very-low-calorie ketogenic diet in the functioning of the immune system, “something that was not seen after similar weight loss induced by bariatric surgery. We analyzed this data in the context of the situation created by COVID-19, taking into account the evidence that people with obesity, compared to those with normal weight, have a higher risk of becoming infected and of having a poor evolution of the infection.”
In this regard, Dr. Crujeiras’ team launched an investigation to study the ACE2 gene methylation pattern, comparing obesity with normal weight and the situation after following a very-low-calorie ketogenic diet or undergoing bariatric surgery. “We observed that the methylation pattern of this gene in obese people was increased, compared to normal-weight people,” she explained, “and this increase was observed mainly in visceral adipose tissue. However, we did not see this in subcutaneous adipose tissue, which is in agreement with the hypothesis that visceral adipose tissue is that mostly associated with obesity-related comorbidities.
“Likewise, the very-low-calorie ketogenic diet was associated with decreased ACE2 methylation, along with increased exposure of this gene. However, after bariatric surgery, no significant changes were observed, so we deduce that we are protecting the patient in some way from inflammation and, therefore, from the potential of serious illness if they become infected.
“In light of these results, we wanted to dig deeper into what was happening with the immune system of obese patients and that inflammation after a very-low-calorie ketogenic diet. We conducted a new study, currently under review in the journal Clinical Nutrition, with the same approach, comparing this diet with a standard hypocaloric balanced diet and bariatric surgery, in which we analyzed a wide battery of cytokines (32). We have observed a differential pattern between the very-low-calorie ketogenic diet and bariatric surgery.
“The results confirm our hypothesis that the very-low-calorie ketogenic diet remodels the inflammatory status of obese patients, and we were also able to verify that the increase in ketone bodies has immunomodulatory properties that were previously demonstrated in preclinical and animal models, which is associated with increased immune function in these patients,” added Dr. Crujeiras.
Personalization and weight regain
In regard to the next steps to take in the knowledge and clinical application of the benefits of this dietary strategy, Dr. Crujeiras said that despite the fact that this diet is known to be effective, it is currently prescribed in a standard manner to all patients, “but there is some variability in the response and also a high risk of regaining weight, as is the case with any nutritional intervention strategy, with that ‘regain’ of lost weight being the main challenge in the treatment of obesity. In this sense, the epigenomic and epigenetic markers that we have identified could help us optimize treatment.”
She added that the future lies in establishing an algorithm that encompasses the patient’s exposome data, along with their genetic and epigenetic profile, to properly classify patients and prescribe a personalized precision therapeutic strategy.
Luca Busetto, MD, cochair of the Obesity Management Task Force (OMTF) of the European Association for the Study of Obesity (EASO), also insisted on the challenge posed by the individualized application of the very-low-calorie ketogenic diet, emphasizing that this diet should always be prescribed by a doctor after an appropriate assessment of the patient. “Obesity is not a matter of willpower or motivation, and most people with obesity have struggled their entire lives and failed because their biology tends to cause weight regain. Therefore, we should try to offer them the options that we currently have, including the very-low-calorie ketogenic diet, adapting them as much as possible to the profile of each patient.”
During his speech, Dr. Busetto presented the recent European guidelines for the management of obesity in adults with a very-low-calorie ketogenic diet, endorsed by EASO, and analyzed the main strengths of these recommendations.
Dr. Busetto remarked that three important points clearly justify the use of the very-low-calorie ketogenic diet. The first is the speed with which the initial weight loss occurs. Recent studies have looked at the benefits of a significant loss of excess weight early in a weight-loss diet, and although this is an association rather than a cause, the results strongly suggest that rapid initial weight loss increases the chance of the result being maintained in the long term. This clashes with the traditional recommendation of losing weight little by little as a strategy to achieve long-term results, but it must be taken into account that there are many myths in the treatment of obesity that current evidence is dismantling with new data – and this is one of them.
Secondly, the effect of the very-low-calorie ketogenic diet can be added to other treatments. This has been demonstrated by studies carried out with liraglutide that showed that this dietary strategy optimizes results, compared with patients who had been treated only with this drug. The third point that justifies the use of the very-low-calorie ketogenic diet is the management of obesity comorbidities. Several investigations demonstrate the effectiveness of this diet in this regard, especially in the case of type 2 diabetes. Data suggest that the substantial weight reductions achieved with it also favor the remission of these comorbidities in many patients.
EASO ‘approval’
Dr. Busetto pointed out that, based on this evidence, the OMTF proposed the development of standards to be included in the EASO guidelines, since there had been no specific recommendation on the very-low-calorie ketogenic diet.
“The main objective of these European guidelines was to provide data referenced by scientific evidence and to suggest a common protocol for the use of this dietary strategy,” he added.
For this, a very exhaustive meta-analysis was carried out, researching all the publications that compared the very-low-calorie ketogenic diet with other diets. The results showed the superiority of the former method for the reduction of body mass index and weight and fat mass, with no difference in lean (muscle) mass, despite significant weight loss in these patients.
This evidence also demonstrates a reduction and an improvement in metabolic markers, specifically glucose metabolism and lipid metabolism.
“The final conclusions of the study corroborate that the very-low-calorie ketogenic diet can be recommended as an effective and safe tool for people with obesity, especially those with severe obesity or comorbidities (joint disease, preoperative period to bariatric surgery, metabolic and cardiovascular diseases) who need immediate, substantial weight loss. In addition, it can be prescribed to specific groups of patients with obesity after considering potential contraindications and under medical follow-up,” said Dr. Busetto.
In Dr. Busetto’s opinion, it would be convenient to refer to this approach as a method, instead of as a diet, “because, in reality, the state of ketosis is limited in time, and if the ketogenic phase is stopped without a continuity plan, obviously weight is regained. In addition, the method approach may increase adherence by patients.”
Finally, Dr. Busetto emphasized the importance of integrating this type of treatment into a long-term lifestyle strategy (including habits, exercise, and nutritional advice). “We must start from the basis that obesity is a chronic and relapsing disease, whose management should also be chronic and probably maintained throughout life.”
Dr. Crujeiras and Dr. Busetto have disclosed no relevant financial relationships. PronoKal Group has recently become part of Nestlé Health Science.
A version of this article appeared on Medscape.com. It was translated from Medscape Spanish Edition.
According to the latest evidence, This development was revealed during the 8th International Scientific Symposium New Frontiers in Scientific Research, organized by PronoKal Group and held in Barcelona. During this conference, international multidisciplinary experts in the study and management of obesity presented the latest data on the benefits of treatment based on a very-low-calorie ketogenic diet.
“Nutritional ketosis has gained great interest in recent years because it is shown to have beneficial properties for health and promotes healthy aging, increasing longevity,” said Ana Belén Crujeiras, BSc, PhD, principal investigator of the Health Research Institute of Santiago de Compostela-Galician Health Service (IDIS-SERGAS) Group of Epigenomics in Endocrinology and Nutrition and the Biomedical Research Networking Center for Obesity and Nutrition Physiopathology (CIBEROBN). “Furthermore, in the case of obesity, we have more and more evidence that it is an effective treatment, mainly because to achieve this metabolic state (ketosis), routes that require the combustion of fats are activated, and this induces body weight loss.”
The specialist stressed that several strategies are used to induce nutritional ketosis. They are characterized by low carbohydrate consumption (low-carbohydrate and high-fat diet; low-carbohydrate, low-fat diet; and intermittent fasting). But Dr. Crujeiras warned that to use it as a treatment for a disease such as obesity, it must be backed by strong and solid scientific evidence, moving away from the concept of fad diets.
In this sense, since 2010, Dr. Crujeiras’ team has developed several studies focused on analyzing the efficacy and safety of treatment with a very-low-calorie ketogenic diet, the results of which have been published in high-impact journals.
Dr. Crujeiras commented on the main conclusions drawn from these investigations. “Our work has shown that the very-low-calorie ketogenic diet is effective for rapid weight loss and maintenance of lost weight, as well as reducing fat mass, primarily visceral fat mass.
“In this sense, a very interesting result is that despite the strong weight loss it induces, it preserves muscle mass and function and improves resting metabolic rate. These two variables are important, because all therapeutic strategies that exist to lose weight lead to a significant reduction in fat-free mass and also a reduction in energy expenditure at rest. This factor is associated with the risk of regaining lost weight, which is currently the great challenge in the treatment of obesity,” she added.
Specific methylation pattern
Dr. Crujeiras indicated that other notable evidence is the favorable impact on an emotional and psychological level. “To determine whether the caloric restriction of this diet and the strong weight loss that it involves were associated with an increased desire to eat, we also carried out an analysis with psychobiological tests. These results led us to conclude that this guideline is accompanied by a reduction of anxiety about food and an improvement in psychobiological parameters, thus increasing the quality of life of these patients.”
The specialist also mentioned that studies currently in progress show that the beneficial effect of this diet could be mediated by epigenetic mechanisms. “In our group, we have identified a specific DNA methylation pattern in people with obesity and we wondered if the very-low-calorie ketogenic diet would be able to reverse that methylome.
“We conducted a study in which we collected blood samples from patients on the very-low-calorie ketogenic diet (600 to 800 kcal/day) drawn before treatment, at peak ketosis, and at the end of treatment. After determining the global pattern of DNA in all patients with obesity targeted with this strategy and through bioinformatic analysis, we were able to obtain a methylation pattern. The results showed that after weight loss on the very-low-calorie ketogenic diet, the methylome that obese people initially had [was] reversed and matched that of normal-weight people.
“Continuing with this bioinformatic analysis more comprehensively, we wanted to see what kind of genes were differentially methylated, especially by the action of the ketosis itself. We found that most of the genes that exhibited differential methylation (in total, 292 identified) belonged to pathways that were involved in the regulation of metabolism, adipose tissue function, CNS function, and also carcinogenesis,” she continued.
Immunomodulatory effect
Dr. Crujeiras said that her research group also observed the modulatory role of the very-low-calorie ketogenic diet in the functioning of the immune system, “something that was not seen after similar weight loss induced by bariatric surgery. We analyzed this data in the context of the situation created by COVID-19, taking into account the evidence that people with obesity, compared to those with normal weight, have a higher risk of becoming infected and of having a poor evolution of the infection.”
In this regard, Dr. Crujeiras’ team launched an investigation to study the ACE2 gene methylation pattern, comparing obesity with normal weight and the situation after following a very-low-calorie ketogenic diet or undergoing bariatric surgery. “We observed that the methylation pattern of this gene in obese people was increased, compared to normal-weight people,” she explained, “and this increase was observed mainly in visceral adipose tissue. However, we did not see this in subcutaneous adipose tissue, which is in agreement with the hypothesis that visceral adipose tissue is that mostly associated with obesity-related comorbidities.
“Likewise, the very-low-calorie ketogenic diet was associated with decreased ACE2 methylation, along with increased exposure of this gene. However, after bariatric surgery, no significant changes were observed, so we deduce that we are protecting the patient in some way from inflammation and, therefore, from the potential of serious illness if they become infected.
“In light of these results, we wanted to dig deeper into what was happening with the immune system of obese patients and that inflammation after a very-low-calorie ketogenic diet. We conducted a new study, currently under review in the journal Clinical Nutrition, with the same approach, comparing this diet with a standard hypocaloric balanced diet and bariatric surgery, in which we analyzed a wide battery of cytokines (32). We have observed a differential pattern between the very-low-calorie ketogenic diet and bariatric surgery.
“The results confirm our hypothesis that the very-low-calorie ketogenic diet remodels the inflammatory status of obese patients, and we were also able to verify that the increase in ketone bodies has immunomodulatory properties that were previously demonstrated in preclinical and animal models, which is associated with increased immune function in these patients,” added Dr. Crujeiras.
Personalization and weight regain
In regard to the next steps to take in the knowledge and clinical application of the benefits of this dietary strategy, Dr. Crujeiras said that despite the fact that this diet is known to be effective, it is currently prescribed in a standard manner to all patients, “but there is some variability in the response and also a high risk of regaining weight, as is the case with any nutritional intervention strategy, with that ‘regain’ of lost weight being the main challenge in the treatment of obesity. In this sense, the epigenomic and epigenetic markers that we have identified could help us optimize treatment.”
She added that the future lies in establishing an algorithm that encompasses the patient’s exposome data, along with their genetic and epigenetic profile, to properly classify patients and prescribe a personalized precision therapeutic strategy.
Luca Busetto, MD, cochair of the Obesity Management Task Force (OMTF) of the European Association for the Study of Obesity (EASO), also insisted on the challenge posed by the individualized application of the very-low-calorie ketogenic diet, emphasizing that this diet should always be prescribed by a doctor after an appropriate assessment of the patient. “Obesity is not a matter of willpower or motivation, and most people with obesity have struggled their entire lives and failed because their biology tends to cause weight regain. Therefore, we should try to offer them the options that we currently have, including the very-low-calorie ketogenic diet, adapting them as much as possible to the profile of each patient.”
During his speech, Dr. Busetto presented the recent European guidelines for the management of obesity in adults with a very-low-calorie ketogenic diet, endorsed by EASO, and analyzed the main strengths of these recommendations.
Dr. Busetto remarked that three important points clearly justify the use of the very-low-calorie ketogenic diet. The first is the speed with which the initial weight loss occurs. Recent studies have looked at the benefits of a significant loss of excess weight early in a weight-loss diet, and although this is an association rather than a cause, the results strongly suggest that rapid initial weight loss increases the chance of the result being maintained in the long term. This clashes with the traditional recommendation of losing weight little by little as a strategy to achieve long-term results, but it must be taken into account that there are many myths in the treatment of obesity that current evidence is dismantling with new data – and this is one of them.
Secondly, the effect of the very-low-calorie ketogenic diet can be added to other treatments. This has been demonstrated by studies carried out with liraglutide that showed that this dietary strategy optimizes results, compared with patients who had been treated only with this drug. The third point that justifies the use of the very-low-calorie ketogenic diet is the management of obesity comorbidities. Several investigations demonstrate the effectiveness of this diet in this regard, especially in the case of type 2 diabetes. Data suggest that the substantial weight reductions achieved with it also favor the remission of these comorbidities in many patients.
EASO ‘approval’
Dr. Busetto pointed out that, based on this evidence, the OMTF proposed the development of standards to be included in the EASO guidelines, since there had been no specific recommendation on the very-low-calorie ketogenic diet.
“The main objective of these European guidelines was to provide data referenced by scientific evidence and to suggest a common protocol for the use of this dietary strategy,” he added.
For this, a very exhaustive meta-analysis was carried out, researching all the publications that compared the very-low-calorie ketogenic diet with other diets. The results showed the superiority of the former method for the reduction of body mass index and weight and fat mass, with no difference in lean (muscle) mass, despite significant weight loss in these patients.
This evidence also demonstrates a reduction and an improvement in metabolic markers, specifically glucose metabolism and lipid metabolism.
“The final conclusions of the study corroborate that the very-low-calorie ketogenic diet can be recommended as an effective and safe tool for people with obesity, especially those with severe obesity or comorbidities (joint disease, preoperative period to bariatric surgery, metabolic and cardiovascular diseases) who need immediate, substantial weight loss. In addition, it can be prescribed to specific groups of patients with obesity after considering potential contraindications and under medical follow-up,” said Dr. Busetto.
In Dr. Busetto’s opinion, it would be convenient to refer to this approach as a method, instead of as a diet, “because, in reality, the state of ketosis is limited in time, and if the ketogenic phase is stopped without a continuity plan, obviously weight is regained. In addition, the method approach may increase adherence by patients.”
Finally, Dr. Busetto emphasized the importance of integrating this type of treatment into a long-term lifestyle strategy (including habits, exercise, and nutritional advice). “We must start from the basis that obesity is a chronic and relapsing disease, whose management should also be chronic and probably maintained throughout life.”
Dr. Crujeiras and Dr. Busetto have disclosed no relevant financial relationships. PronoKal Group has recently become part of Nestlé Health Science.
A version of this article appeared on Medscape.com. It was translated from Medscape Spanish Edition.
Are physician white coats becoming obsolete? How docs dress for work now
Early in the COVID-19 pandemic, Trisha Pasricha, MD, a gastroenterologist and research fellow at Massachusetts General Hospital in Boston, was talking to a patient who had been hospitalized for a peptic ulcer.
Like other physicians in her institution, Dr. Pasricha was wearing scrubs instead of a white coat, out of concern that the white coat might be more prone to accumulating or transmitting COVID-19 pathogens. Her badge identified her as a physician, and she introduced herself clearly as “Dr. Pasricha.”
The patient “required an emergent procedure, which I discussed with him,” Dr. Pasricha told this news organization. “I went over what the procedure entailed, the risks and benefits, and the need for informed consent. The patient nodded and seemed to understand, but at the end of the discussion he said: ‘That all sounds fine, but I need to speak to the doctor first.’ ”
Dr. Pasricha was taken aback. She wondered: “Who did he think I was the whole time that I was reviewing medical concerns, explaining medical concepts, and describing a procedure in a way that a physician would describe it?”
She realized the reason he didn’t correctly identify her was that, clad only in scrubs, she was less easily recognizable as a physician. And to be misidentified as technicians, nurses, physician assistants, or other health care professionals, according to Dr. Pasricha.
Dr. Pasricha said she has been the recipient of this “implicit bias” not only from patients but also from members of the health care team, and added that other female colleagues have told her that they’ve had similar experiences, especially when they’re not wearing a white coat.
Changing times, changing trends
When COVID-19 began to spread, “there was an initial concern that COVID-19 was passed through surfaces, and concerns about whether white coats could carry viral particles,” according to Jordan Steinberg, MD, PhD, surgical director of the craniofacial program at Nicklaus Children’s Pediatric Specialists/Nicklaus Children’s Health System, Miami. “Hospitals didn’t want to launder the white coats as frequently as scrubs, due to cost concerns. There was also a concern raised that a necktie might dangle in patients’ faces, coming in closer contact with pathogens, so more physicians were wearing scrubs.”
Yet even before the pandemic, physician attire in hospital and outpatient settings had started to change. Dr. Steinberg, who is also a clinical associate professor at Florida International University, Miami, told this news organization that, in his previous appointment at Johns Hopkins University, Baltimore, he and his colleagues “had noticed in our institution, as well as other facilities, an increasing trend that moved from white coats worn over professional attire toward more casual dress among medical staff – increased wearing of casual fleece or softshell jackets with the institutional logo.”
This was especially true with trainees and the “younger generation,” who were preferring “what I would almost call ‘warm-up clothes,’ gym clothes, and less shirt-tie-white-coat attire for men or white-coats-and-business attire for women.” Dr. Steinberg thinks that some physicians prefer the fleece with the institutional logo “because it’s like wearing your favorite sports team jersey. It gives a sense of belonging.”
Todd Shaffer, MD, MBA, a family physician at University Physicians Associates, Truman Medical Centers and the Lakewood Medical Pavilion, Kansas City, Mo., has been at his institution for 30 years and has seen a similar trend. “At one point, things were very formal,” he told this news organization. But attire was already becoming less formal before the pandemic, and new changes took place during the pandemic, as physicians began wearing scrubs instead of white coats because of fears of viral contamination.
Now, there is less concern about potential viral contamination with the white coat. Yet many physicians continue to wear scrubs – especially those who interact with patients with COVID – and it has become more acceptable to do so, or to wear personal protective equipment (PPE) over ordinary clothing, but it is less common in routine clinical practice, said Dr. Shaffer, a member of the board of directors of the American Academy of Family Physicians.
“The world has changed since COVID. People feel more comfortable dressing more casually during professional Zoom calls, when they have the convenience of working from home,” said Dr. Shaffer, who is also a professor of family medicine at University of Missouri–Kansas City.
Dr. Shaffer himself hasn’t worn a white coat for years. “I’m more likely to wear medium casual pants. I’ve bought some nicer shirts, so I still look professional and upbeat. I don’t always tuck in my shirt, and I don’t dress as formally.” He wears PPE and a mask and/or face shield when treating patients with COVID-19. And he wears a white coat “when someone wants a photograph taken with the doctors – with the stethoscope draped around my neck.”
Traditional symbol of medicine
Because of the changing mores, Dr. Steinberg and colleagues at Johns Hopkins wondered if there might still be a role for professional attire and white coats and what patients prefer. To investigate the question, they surveyed 487 U.S. adults in the spring of 2020.
Respondents were asked where and how frequently they see health care professionals wearing white coats, scrubs, and fleece or softshell jackets. They were also shown photographs depicting models wearing various types of attire commonly seen in health care settings and were asked to rank the “health care provider’s” level of experience, professionalism, and friendliness.
The majority of participants said they had seen health care practitioners in white coats “most of the time,” in scrubs “sometimes,” and in fleece or softshell jackets “rarely.” Models in white coats were regarded by respondents as more experienced and professional, although those in softshell jackets were perceived as friendlier.
There were age as well as regional differences in the responses, Dr. Steinberg said. Older respondents were significantly more likely than their younger counterparts to perceive a model wearing a white coat over business attire as being more experienced, and – in all regions of the United States except the West coast – respondents gave lower professionalism scores to providers wearing fleece jackets with scrubs underneath.
Respondents tended to prefer surgeons wearing a white coat with scrubs underneath, while a white coat over business attire was the preferred dress code for family physicians and dermatologists.
“People tended to respond as if there was a more professional element in the white coat. The age-old symbol of the white coat still marked something important,” Dr. Steinberg said. “Our data suggest that the white coat isn’t ready to die just yet. People still see an air of authority and a traditional symbol of medicine. Nevertheless, I do think it will become less common than it used to be, especially in certain regions of the country.”
Organic, subtle changes
Christopher Petrilli, MD, assistant professor at New York University, conducted research in 2018 regarding physician attire by surveying over 4,000 patients in 10 U.S. academic hospitals. His team found that most patients continued to prefer physicians to wear formal attire under a white coat, especially older respondents.
Dr. Petrilli and colleagues have been studying the issue of physician attire since 2015. “The big issue when we did our initial study – which might not be accurate anymore – is that few hospitals actually had a uniform dress code,” said Dr. Petrilli, the medical director of clinical documentation improvement and the clinical lead of value-based medicine at NYU Langone Hospitals. “When we looked at ‘honor roll hospitals’ during our study, we cold-called these hospitals and also looked online for their dress code policies. Except for the Mayo Clinic, hospitals that had dress code policies were more generic.”
For example, the American Medical Association guidance merely states that attire should be “clean, unsoiled, and appropriate to the setting of care” and recommends weighing research findings regarding textile transmission of health care–associated infections when individual institutions determine their dress code policies. The AMA’s last policy discussion took place in 2015 and its guidance has not changed since the pandemic.
Regardless of what institutions and patients prefer, some research suggests that many physicians would prefer to stay with wearing scrubs rather than reverting to the white coat. One study of 151 hospitalists, conducted in Ireland, found that three-quarters wanted scrubs to remain standard attire, despite the fact that close to half had experienced changes in patients› perception in the absence of their white coat and “professional attire.”
Jennifer Workman, MD, assistant professor of pediatrics, division of pediatric critical care, University of Utah, Salt Lake City, said in an interview that, as the pandemic has “waxed and waned, some trends have reverted to what they were prepandemic, but other physicians have stayed with wearing scrubs.”
Much depends on practice setting, said Dr. Workman, who is also the medical director of pediatric sepsis at Intermountain Care. In pediatrics, for example, many physicians prefer not to wear white coats when they are interacting with young children or adolescents.
Like Dr. Shaffer, Dr. Workman has seen changes in physicians’ attire during video meetings, where they often dress more casually, perhaps wearing sweatshirts. And in the hospital, more are continuing to wear scrubs. “But I don’t see it as people trying to consciously experiment or push boundaries,” she said. “I see it as a more organic, subtle shift.”
Dr. Petrilli thinks that, at this juncture, it’s “pretty heterogeneous as to who is going to return to formal attire and a white coat and who won’t.” Further research needs to be done into currently evolving trends. “We need a more thorough survey looking at changes. We need to ask [physician respondents]: ‘What is your current attire, and how has it changed?’ ”
Navigating the gender divide
In their study, Dr. Steinberg and colleagues found that respondents perceived a male model wearing business attire underneath any type of outerwear (white coat or fleece) to be significantly more professional than a female model wearing the same attire. Respondents also perceived males wearing scrubs to be more professional than females wearing scrubs.
Male models in white coats over business attire were also more likely to be identified as physicians, compared with female models in the same attire. Females were also more likely to be misidentified as nonphysician health care professionals.
Shikha Jain, MD, assistant professor of medicine at the University of Illinois Cancer Center in Chicago, said that Dr. Steinberg’s study confirmed experiences that she and other female physicians have had. Wearing a white coat makes it more likely that a patient will identify you as a physician, but women are less likely to be identified as physicians, regardless of what they wear.
“I think that individuals of color and especially people with intersectional identities – such as women of color – are even more frequently targeted and stereotyped. Numerous studies have shown that a person of color is less likely to be seen as an authority figure, and studies have shown that physicians of color are less likely to be identified as ‘physicians,’ compared to a Caucasian individual,” she said.
Does that mean that female physicians should revert back to prepandemic white coats rather than scrubs or more casual attire? Not necessarily, according to Dr. Jain.
“The typical dress code guidance is that physicians should dress ‘professionally,’ but what that means is a question that needs to be addressed,” Dr. Jain said. “Medicine has evolved from the days of house calls, in which one’s patient population is a very small, intimate group of people in the physician’s community. Yet now, we’ve given rebirth to the ‘house call’ when we do telemedicine with a patient in his or her home. And in the old days, doctors often had offices their homes and now, with telemedicine, patients often see the interior of their physician’s home.” As the delivery of medicine evolves, concepts of “professionalism” – what is defined as “casual” and what is defined as “formal” – is also evolving.
The more important issue, according to Dr. Jain, is to “continue the conversation” about the discrepancies between how men and women are treated in medicine. Attire is one arena in which this issue plays out, and it’s a “bigger picture” that goes beyond the white coat.
Dr. Jain has been “told by patients that a particular outfit doesn’t make me look like a doctor or that scrubs make me look younger. I don’t think my male colleagues have been subjected to these types of remarks, but my female colleagues have heard them as well.”
Even fellow health care providers have commented on Dr. Jain’s clothing. She was presenting at a major medical conference via video and was wearing a similar outfit to the one she wore for her headshot. “Thirty seconds before beginning my talk, one of the male physicians said: ‘Are you wearing the same outfit you wore for your headshot?’ I can’t imagine a man commenting that another man was wearing the same jacket or tie that he wore in the photograph. I found it odd that this was something that someone felt the need to comment on right before I was about to address a large group of people in a professional capacity.”
Addressing these systemic issues “needs to be done and amplified not only by women but also by men in medicine,” said Dr. Jain, founder and director of Women in Medicine, an organization consisting of women physicians whose goal is to “find and implement solutions to gender inequity.”
Dr. Jain said the organization offers an Inclusive Leadership Development Lab – a course specifically for men in health care leadership positions to learn how to be more equitable, inclusive leaders.
A personal decision
Dr. Pasricha hopes she “handled the patient’s misidentification graciously.” She explained to him that she would be the physician conducting the procedure. The patient was initially “a little embarrassed” that he had misidentified her, but she put him at ease and “we moved forward quickly.”
At this point, although some of her colleagues have continued to wear scrubs or have returned to wearing fleeces with hospital logos, Dr. Pasricha prefers to wear a white coat in both inpatient and outpatient settings because it reduces the likelihood of misidentification.
And white coats can be more convenient – for example, Dr. Jain likes the fact that the white coat has pockets where she can put her stethoscope and other items, while some of her professional clothes don’t always have pockets.
Dr. Jain noted that there are some institutions where everyone seems to wear white coats, not only the physician – “from the chaplain to the phlebotomist to the social worker.” In those settings, the white coat no longer distinguishes physicians from nonphysicians, and so wearing a white coat may not confer additional credibility as a physician.
Nevertheless, “if you want to wear a white coat, if you feel it gives you that added level of authority, if you feel it tells people more clearly that you’re a physician, by all means go ahead and do so,” she said. “There’s no ‘one-size-fits-all’ strategy or solution. What’s more important than your clothing is your professionalism.”
A version of this article first appeared on Medscape.com.
Early in the COVID-19 pandemic, Trisha Pasricha, MD, a gastroenterologist and research fellow at Massachusetts General Hospital in Boston, was talking to a patient who had been hospitalized for a peptic ulcer.
Like other physicians in her institution, Dr. Pasricha was wearing scrubs instead of a white coat, out of concern that the white coat might be more prone to accumulating or transmitting COVID-19 pathogens. Her badge identified her as a physician, and she introduced herself clearly as “Dr. Pasricha.”
The patient “required an emergent procedure, which I discussed with him,” Dr. Pasricha told this news organization. “I went over what the procedure entailed, the risks and benefits, and the need for informed consent. The patient nodded and seemed to understand, but at the end of the discussion he said: ‘That all sounds fine, but I need to speak to the doctor first.’ ”
Dr. Pasricha was taken aback. She wondered: “Who did he think I was the whole time that I was reviewing medical concerns, explaining medical concepts, and describing a procedure in a way that a physician would describe it?”
She realized the reason he didn’t correctly identify her was that, clad only in scrubs, she was less easily recognizable as a physician. And to be misidentified as technicians, nurses, physician assistants, or other health care professionals, according to Dr. Pasricha.
Dr. Pasricha said she has been the recipient of this “implicit bias” not only from patients but also from members of the health care team, and added that other female colleagues have told her that they’ve had similar experiences, especially when they’re not wearing a white coat.
Changing times, changing trends
When COVID-19 began to spread, “there was an initial concern that COVID-19 was passed through surfaces, and concerns about whether white coats could carry viral particles,” according to Jordan Steinberg, MD, PhD, surgical director of the craniofacial program at Nicklaus Children’s Pediatric Specialists/Nicklaus Children’s Health System, Miami. “Hospitals didn’t want to launder the white coats as frequently as scrubs, due to cost concerns. There was also a concern raised that a necktie might dangle in patients’ faces, coming in closer contact with pathogens, so more physicians were wearing scrubs.”
Yet even before the pandemic, physician attire in hospital and outpatient settings had started to change. Dr. Steinberg, who is also a clinical associate professor at Florida International University, Miami, told this news organization that, in his previous appointment at Johns Hopkins University, Baltimore, he and his colleagues “had noticed in our institution, as well as other facilities, an increasing trend that moved from white coats worn over professional attire toward more casual dress among medical staff – increased wearing of casual fleece or softshell jackets with the institutional logo.”
This was especially true with trainees and the “younger generation,” who were preferring “what I would almost call ‘warm-up clothes,’ gym clothes, and less shirt-tie-white-coat attire for men or white-coats-and-business attire for women.” Dr. Steinberg thinks that some physicians prefer the fleece with the institutional logo “because it’s like wearing your favorite sports team jersey. It gives a sense of belonging.”
Todd Shaffer, MD, MBA, a family physician at University Physicians Associates, Truman Medical Centers and the Lakewood Medical Pavilion, Kansas City, Mo., has been at his institution for 30 years and has seen a similar trend. “At one point, things were very formal,” he told this news organization. But attire was already becoming less formal before the pandemic, and new changes took place during the pandemic, as physicians began wearing scrubs instead of white coats because of fears of viral contamination.
Now, there is less concern about potential viral contamination with the white coat. Yet many physicians continue to wear scrubs – especially those who interact with patients with COVID – and it has become more acceptable to do so, or to wear personal protective equipment (PPE) over ordinary clothing, but it is less common in routine clinical practice, said Dr. Shaffer, a member of the board of directors of the American Academy of Family Physicians.
“The world has changed since COVID. People feel more comfortable dressing more casually during professional Zoom calls, when they have the convenience of working from home,” said Dr. Shaffer, who is also a professor of family medicine at University of Missouri–Kansas City.
Dr. Shaffer himself hasn’t worn a white coat for years. “I’m more likely to wear medium casual pants. I’ve bought some nicer shirts, so I still look professional and upbeat. I don’t always tuck in my shirt, and I don’t dress as formally.” He wears PPE and a mask and/or face shield when treating patients with COVID-19. And he wears a white coat “when someone wants a photograph taken with the doctors – with the stethoscope draped around my neck.”
Traditional symbol of medicine
Because of the changing mores, Dr. Steinberg and colleagues at Johns Hopkins wondered if there might still be a role for professional attire and white coats and what patients prefer. To investigate the question, they surveyed 487 U.S. adults in the spring of 2020.
Respondents were asked where and how frequently they see health care professionals wearing white coats, scrubs, and fleece or softshell jackets. They were also shown photographs depicting models wearing various types of attire commonly seen in health care settings and were asked to rank the “health care provider’s” level of experience, professionalism, and friendliness.
The majority of participants said they had seen health care practitioners in white coats “most of the time,” in scrubs “sometimes,” and in fleece or softshell jackets “rarely.” Models in white coats were regarded by respondents as more experienced and professional, although those in softshell jackets were perceived as friendlier.
There were age as well as regional differences in the responses, Dr. Steinberg said. Older respondents were significantly more likely than their younger counterparts to perceive a model wearing a white coat over business attire as being more experienced, and – in all regions of the United States except the West coast – respondents gave lower professionalism scores to providers wearing fleece jackets with scrubs underneath.
Respondents tended to prefer surgeons wearing a white coat with scrubs underneath, while a white coat over business attire was the preferred dress code for family physicians and dermatologists.
“People tended to respond as if there was a more professional element in the white coat. The age-old symbol of the white coat still marked something important,” Dr. Steinberg said. “Our data suggest that the white coat isn’t ready to die just yet. People still see an air of authority and a traditional symbol of medicine. Nevertheless, I do think it will become less common than it used to be, especially in certain regions of the country.”
Organic, subtle changes
Christopher Petrilli, MD, assistant professor at New York University, conducted research in 2018 regarding physician attire by surveying over 4,000 patients in 10 U.S. academic hospitals. His team found that most patients continued to prefer physicians to wear formal attire under a white coat, especially older respondents.
Dr. Petrilli and colleagues have been studying the issue of physician attire since 2015. “The big issue when we did our initial study – which might not be accurate anymore – is that few hospitals actually had a uniform dress code,” said Dr. Petrilli, the medical director of clinical documentation improvement and the clinical lead of value-based medicine at NYU Langone Hospitals. “When we looked at ‘honor roll hospitals’ during our study, we cold-called these hospitals and also looked online for their dress code policies. Except for the Mayo Clinic, hospitals that had dress code policies were more generic.”
For example, the American Medical Association guidance merely states that attire should be “clean, unsoiled, and appropriate to the setting of care” and recommends weighing research findings regarding textile transmission of health care–associated infections when individual institutions determine their dress code policies. The AMA’s last policy discussion took place in 2015 and its guidance has not changed since the pandemic.
Regardless of what institutions and patients prefer, some research suggests that many physicians would prefer to stay with wearing scrubs rather than reverting to the white coat. One study of 151 hospitalists, conducted in Ireland, found that three-quarters wanted scrubs to remain standard attire, despite the fact that close to half had experienced changes in patients› perception in the absence of their white coat and “professional attire.”
Jennifer Workman, MD, assistant professor of pediatrics, division of pediatric critical care, University of Utah, Salt Lake City, said in an interview that, as the pandemic has “waxed and waned, some trends have reverted to what they were prepandemic, but other physicians have stayed with wearing scrubs.”
Much depends on practice setting, said Dr. Workman, who is also the medical director of pediatric sepsis at Intermountain Care. In pediatrics, for example, many physicians prefer not to wear white coats when they are interacting with young children or adolescents.
Like Dr. Shaffer, Dr. Workman has seen changes in physicians’ attire during video meetings, where they often dress more casually, perhaps wearing sweatshirts. And in the hospital, more are continuing to wear scrubs. “But I don’t see it as people trying to consciously experiment or push boundaries,” she said. “I see it as a more organic, subtle shift.”
Dr. Petrilli thinks that, at this juncture, it’s “pretty heterogeneous as to who is going to return to formal attire and a white coat and who won’t.” Further research needs to be done into currently evolving trends. “We need a more thorough survey looking at changes. We need to ask [physician respondents]: ‘What is your current attire, and how has it changed?’ ”
Navigating the gender divide
In their study, Dr. Steinberg and colleagues found that respondents perceived a male model wearing business attire underneath any type of outerwear (white coat or fleece) to be significantly more professional than a female model wearing the same attire. Respondents also perceived males wearing scrubs to be more professional than females wearing scrubs.
Male models in white coats over business attire were also more likely to be identified as physicians, compared with female models in the same attire. Females were also more likely to be misidentified as nonphysician health care professionals.
Shikha Jain, MD, assistant professor of medicine at the University of Illinois Cancer Center in Chicago, said that Dr. Steinberg’s study confirmed experiences that she and other female physicians have had. Wearing a white coat makes it more likely that a patient will identify you as a physician, but women are less likely to be identified as physicians, regardless of what they wear.
“I think that individuals of color and especially people with intersectional identities – such as women of color – are even more frequently targeted and stereotyped. Numerous studies have shown that a person of color is less likely to be seen as an authority figure, and studies have shown that physicians of color are less likely to be identified as ‘physicians,’ compared to a Caucasian individual,” she said.
Does that mean that female physicians should revert back to prepandemic white coats rather than scrubs or more casual attire? Not necessarily, according to Dr. Jain.
“The typical dress code guidance is that physicians should dress ‘professionally,’ but what that means is a question that needs to be addressed,” Dr. Jain said. “Medicine has evolved from the days of house calls, in which one’s patient population is a very small, intimate group of people in the physician’s community. Yet now, we’ve given rebirth to the ‘house call’ when we do telemedicine with a patient in his or her home. And in the old days, doctors often had offices their homes and now, with telemedicine, patients often see the interior of their physician’s home.” As the delivery of medicine evolves, concepts of “professionalism” – what is defined as “casual” and what is defined as “formal” – is also evolving.
The more important issue, according to Dr. Jain, is to “continue the conversation” about the discrepancies between how men and women are treated in medicine. Attire is one arena in which this issue plays out, and it’s a “bigger picture” that goes beyond the white coat.
Dr. Jain has been “told by patients that a particular outfit doesn’t make me look like a doctor or that scrubs make me look younger. I don’t think my male colleagues have been subjected to these types of remarks, but my female colleagues have heard them as well.”
Even fellow health care providers have commented on Dr. Jain’s clothing. She was presenting at a major medical conference via video and was wearing a similar outfit to the one she wore for her headshot. “Thirty seconds before beginning my talk, one of the male physicians said: ‘Are you wearing the same outfit you wore for your headshot?’ I can’t imagine a man commenting that another man was wearing the same jacket or tie that he wore in the photograph. I found it odd that this was something that someone felt the need to comment on right before I was about to address a large group of people in a professional capacity.”
Addressing these systemic issues “needs to be done and amplified not only by women but also by men in medicine,” said Dr. Jain, founder and director of Women in Medicine, an organization consisting of women physicians whose goal is to “find and implement solutions to gender inequity.”
Dr. Jain said the organization offers an Inclusive Leadership Development Lab – a course specifically for men in health care leadership positions to learn how to be more equitable, inclusive leaders.
A personal decision
Dr. Pasricha hopes she “handled the patient’s misidentification graciously.” She explained to him that she would be the physician conducting the procedure. The patient was initially “a little embarrassed” that he had misidentified her, but she put him at ease and “we moved forward quickly.”
At this point, although some of her colleagues have continued to wear scrubs or have returned to wearing fleeces with hospital logos, Dr. Pasricha prefers to wear a white coat in both inpatient and outpatient settings because it reduces the likelihood of misidentification.
And white coats can be more convenient – for example, Dr. Jain likes the fact that the white coat has pockets where she can put her stethoscope and other items, while some of her professional clothes don’t always have pockets.
Dr. Jain noted that there are some institutions where everyone seems to wear white coats, not only the physician – “from the chaplain to the phlebotomist to the social worker.” In those settings, the white coat no longer distinguishes physicians from nonphysicians, and so wearing a white coat may not confer additional credibility as a physician.
Nevertheless, “if you want to wear a white coat, if you feel it gives you that added level of authority, if you feel it tells people more clearly that you’re a physician, by all means go ahead and do so,” she said. “There’s no ‘one-size-fits-all’ strategy or solution. What’s more important than your clothing is your professionalism.”
A version of this article first appeared on Medscape.com.
Early in the COVID-19 pandemic, Trisha Pasricha, MD, a gastroenterologist and research fellow at Massachusetts General Hospital in Boston, was talking to a patient who had been hospitalized for a peptic ulcer.
Like other physicians in her institution, Dr. Pasricha was wearing scrubs instead of a white coat, out of concern that the white coat might be more prone to accumulating or transmitting COVID-19 pathogens. Her badge identified her as a physician, and she introduced herself clearly as “Dr. Pasricha.”
The patient “required an emergent procedure, which I discussed with him,” Dr. Pasricha told this news organization. “I went over what the procedure entailed, the risks and benefits, and the need for informed consent. The patient nodded and seemed to understand, but at the end of the discussion he said: ‘That all sounds fine, but I need to speak to the doctor first.’ ”
Dr. Pasricha was taken aback. She wondered: “Who did he think I was the whole time that I was reviewing medical concerns, explaining medical concepts, and describing a procedure in a way that a physician would describe it?”
She realized the reason he didn’t correctly identify her was that, clad only in scrubs, she was less easily recognizable as a physician. And to be misidentified as technicians, nurses, physician assistants, or other health care professionals, according to Dr. Pasricha.
Dr. Pasricha said she has been the recipient of this “implicit bias” not only from patients but also from members of the health care team, and added that other female colleagues have told her that they’ve had similar experiences, especially when they’re not wearing a white coat.
Changing times, changing trends
When COVID-19 began to spread, “there was an initial concern that COVID-19 was passed through surfaces, and concerns about whether white coats could carry viral particles,” according to Jordan Steinberg, MD, PhD, surgical director of the craniofacial program at Nicklaus Children’s Pediatric Specialists/Nicklaus Children’s Health System, Miami. “Hospitals didn’t want to launder the white coats as frequently as scrubs, due to cost concerns. There was also a concern raised that a necktie might dangle in patients’ faces, coming in closer contact with pathogens, so more physicians were wearing scrubs.”
Yet even before the pandemic, physician attire in hospital and outpatient settings had started to change. Dr. Steinberg, who is also a clinical associate professor at Florida International University, Miami, told this news organization that, in his previous appointment at Johns Hopkins University, Baltimore, he and his colleagues “had noticed in our institution, as well as other facilities, an increasing trend that moved from white coats worn over professional attire toward more casual dress among medical staff – increased wearing of casual fleece or softshell jackets with the institutional logo.”
This was especially true with trainees and the “younger generation,” who were preferring “what I would almost call ‘warm-up clothes,’ gym clothes, and less shirt-tie-white-coat attire for men or white-coats-and-business attire for women.” Dr. Steinberg thinks that some physicians prefer the fleece with the institutional logo “because it’s like wearing your favorite sports team jersey. It gives a sense of belonging.”
Todd Shaffer, MD, MBA, a family physician at University Physicians Associates, Truman Medical Centers and the Lakewood Medical Pavilion, Kansas City, Mo., has been at his institution for 30 years and has seen a similar trend. “At one point, things were very formal,” he told this news organization. But attire was already becoming less formal before the pandemic, and new changes took place during the pandemic, as physicians began wearing scrubs instead of white coats because of fears of viral contamination.
Now, there is less concern about potential viral contamination with the white coat. Yet many physicians continue to wear scrubs – especially those who interact with patients with COVID – and it has become more acceptable to do so, or to wear personal protective equipment (PPE) over ordinary clothing, but it is less common in routine clinical practice, said Dr. Shaffer, a member of the board of directors of the American Academy of Family Physicians.
“The world has changed since COVID. People feel more comfortable dressing more casually during professional Zoom calls, when they have the convenience of working from home,” said Dr. Shaffer, who is also a professor of family medicine at University of Missouri–Kansas City.
Dr. Shaffer himself hasn’t worn a white coat for years. “I’m more likely to wear medium casual pants. I’ve bought some nicer shirts, so I still look professional and upbeat. I don’t always tuck in my shirt, and I don’t dress as formally.” He wears PPE and a mask and/or face shield when treating patients with COVID-19. And he wears a white coat “when someone wants a photograph taken with the doctors – with the stethoscope draped around my neck.”
Traditional symbol of medicine
Because of the changing mores, Dr. Steinberg and colleagues at Johns Hopkins wondered if there might still be a role for professional attire and white coats and what patients prefer. To investigate the question, they surveyed 487 U.S. adults in the spring of 2020.
Respondents were asked where and how frequently they see health care professionals wearing white coats, scrubs, and fleece or softshell jackets. They were also shown photographs depicting models wearing various types of attire commonly seen in health care settings and were asked to rank the “health care provider’s” level of experience, professionalism, and friendliness.
The majority of participants said they had seen health care practitioners in white coats “most of the time,” in scrubs “sometimes,” and in fleece or softshell jackets “rarely.” Models in white coats were regarded by respondents as more experienced and professional, although those in softshell jackets were perceived as friendlier.
There were age as well as regional differences in the responses, Dr. Steinberg said. Older respondents were significantly more likely than their younger counterparts to perceive a model wearing a white coat over business attire as being more experienced, and – in all regions of the United States except the West coast – respondents gave lower professionalism scores to providers wearing fleece jackets with scrubs underneath.
Respondents tended to prefer surgeons wearing a white coat with scrubs underneath, while a white coat over business attire was the preferred dress code for family physicians and dermatologists.
“People tended to respond as if there was a more professional element in the white coat. The age-old symbol of the white coat still marked something important,” Dr. Steinberg said. “Our data suggest that the white coat isn’t ready to die just yet. People still see an air of authority and a traditional symbol of medicine. Nevertheless, I do think it will become less common than it used to be, especially in certain regions of the country.”
Organic, subtle changes
Christopher Petrilli, MD, assistant professor at New York University, conducted research in 2018 regarding physician attire by surveying over 4,000 patients in 10 U.S. academic hospitals. His team found that most patients continued to prefer physicians to wear formal attire under a white coat, especially older respondents.
Dr. Petrilli and colleagues have been studying the issue of physician attire since 2015. “The big issue when we did our initial study – which might not be accurate anymore – is that few hospitals actually had a uniform dress code,” said Dr. Petrilli, the medical director of clinical documentation improvement and the clinical lead of value-based medicine at NYU Langone Hospitals. “When we looked at ‘honor roll hospitals’ during our study, we cold-called these hospitals and also looked online for their dress code policies. Except for the Mayo Clinic, hospitals that had dress code policies were more generic.”
For example, the American Medical Association guidance merely states that attire should be “clean, unsoiled, and appropriate to the setting of care” and recommends weighing research findings regarding textile transmission of health care–associated infections when individual institutions determine their dress code policies. The AMA’s last policy discussion took place in 2015 and its guidance has not changed since the pandemic.
Regardless of what institutions and patients prefer, some research suggests that many physicians would prefer to stay with wearing scrubs rather than reverting to the white coat. One study of 151 hospitalists, conducted in Ireland, found that three-quarters wanted scrubs to remain standard attire, despite the fact that close to half had experienced changes in patients› perception in the absence of their white coat and “professional attire.”
Jennifer Workman, MD, assistant professor of pediatrics, division of pediatric critical care, University of Utah, Salt Lake City, said in an interview that, as the pandemic has “waxed and waned, some trends have reverted to what they were prepandemic, but other physicians have stayed with wearing scrubs.”
Much depends on practice setting, said Dr. Workman, who is also the medical director of pediatric sepsis at Intermountain Care. In pediatrics, for example, many physicians prefer not to wear white coats when they are interacting with young children or adolescents.
Like Dr. Shaffer, Dr. Workman has seen changes in physicians’ attire during video meetings, where they often dress more casually, perhaps wearing sweatshirts. And in the hospital, more are continuing to wear scrubs. “But I don’t see it as people trying to consciously experiment or push boundaries,” she said. “I see it as a more organic, subtle shift.”
Dr. Petrilli thinks that, at this juncture, it’s “pretty heterogeneous as to who is going to return to formal attire and a white coat and who won’t.” Further research needs to be done into currently evolving trends. “We need a more thorough survey looking at changes. We need to ask [physician respondents]: ‘What is your current attire, and how has it changed?’ ”
Navigating the gender divide
In their study, Dr. Steinberg and colleagues found that respondents perceived a male model wearing business attire underneath any type of outerwear (white coat or fleece) to be significantly more professional than a female model wearing the same attire. Respondents also perceived males wearing scrubs to be more professional than females wearing scrubs.
Male models in white coats over business attire were also more likely to be identified as physicians, compared with female models in the same attire. Females were also more likely to be misidentified as nonphysician health care professionals.
Shikha Jain, MD, assistant professor of medicine at the University of Illinois Cancer Center in Chicago, said that Dr. Steinberg’s study confirmed experiences that she and other female physicians have had. Wearing a white coat makes it more likely that a patient will identify you as a physician, but women are less likely to be identified as physicians, regardless of what they wear.
“I think that individuals of color and especially people with intersectional identities – such as women of color – are even more frequently targeted and stereotyped. Numerous studies have shown that a person of color is less likely to be seen as an authority figure, and studies have shown that physicians of color are less likely to be identified as ‘physicians,’ compared to a Caucasian individual,” she said.
Does that mean that female physicians should revert back to prepandemic white coats rather than scrubs or more casual attire? Not necessarily, according to Dr. Jain.
“The typical dress code guidance is that physicians should dress ‘professionally,’ but what that means is a question that needs to be addressed,” Dr. Jain said. “Medicine has evolved from the days of house calls, in which one’s patient population is a very small, intimate group of people in the physician’s community. Yet now, we’ve given rebirth to the ‘house call’ when we do telemedicine with a patient in his or her home. And in the old days, doctors often had offices their homes and now, with telemedicine, patients often see the interior of their physician’s home.” As the delivery of medicine evolves, concepts of “professionalism” – what is defined as “casual” and what is defined as “formal” – is also evolving.
The more important issue, according to Dr. Jain, is to “continue the conversation” about the discrepancies between how men and women are treated in medicine. Attire is one arena in which this issue plays out, and it’s a “bigger picture” that goes beyond the white coat.
Dr. Jain has been “told by patients that a particular outfit doesn’t make me look like a doctor or that scrubs make me look younger. I don’t think my male colleagues have been subjected to these types of remarks, but my female colleagues have heard them as well.”
Even fellow health care providers have commented on Dr. Jain’s clothing. She was presenting at a major medical conference via video and was wearing a similar outfit to the one she wore for her headshot. “Thirty seconds before beginning my talk, one of the male physicians said: ‘Are you wearing the same outfit you wore for your headshot?’ I can’t imagine a man commenting that another man was wearing the same jacket or tie that he wore in the photograph. I found it odd that this was something that someone felt the need to comment on right before I was about to address a large group of people in a professional capacity.”
Addressing these systemic issues “needs to be done and amplified not only by women but also by men in medicine,” said Dr. Jain, founder and director of Women in Medicine, an organization consisting of women physicians whose goal is to “find and implement solutions to gender inequity.”
Dr. Jain said the organization offers an Inclusive Leadership Development Lab – a course specifically for men in health care leadership positions to learn how to be more equitable, inclusive leaders.
A personal decision
Dr. Pasricha hopes she “handled the patient’s misidentification graciously.” She explained to him that she would be the physician conducting the procedure. The patient was initially “a little embarrassed” that he had misidentified her, but she put him at ease and “we moved forward quickly.”
At this point, although some of her colleagues have continued to wear scrubs or have returned to wearing fleeces with hospital logos, Dr. Pasricha prefers to wear a white coat in both inpatient and outpatient settings because it reduces the likelihood of misidentification.
And white coats can be more convenient – for example, Dr. Jain likes the fact that the white coat has pockets where she can put her stethoscope and other items, while some of her professional clothes don’t always have pockets.
Dr. Jain noted that there are some institutions where everyone seems to wear white coats, not only the physician – “from the chaplain to the phlebotomist to the social worker.” In those settings, the white coat no longer distinguishes physicians from nonphysicians, and so wearing a white coat may not confer additional credibility as a physician.
Nevertheless, “if you want to wear a white coat, if you feel it gives you that added level of authority, if you feel it tells people more clearly that you’re a physician, by all means go ahead and do so,” she said. “There’s no ‘one-size-fits-all’ strategy or solution. What’s more important than your clothing is your professionalism.”
A version of this article first appeared on Medscape.com.
How to make visits run more smoothly and be more productive
We all feel pressure from not having enough time to care for our patients the way we want to.
Organ recital
Some of our patients need to share an update on all their symptoms each visit, old and new, including those that are minor or possibly concerning. I have learned over the years that, for many patients, this allows them to release the worry about symptoms.
Some symptoms are so distressing and severe that symptomatic treatment is needed, but most aren’t.
I am very honest with patients when I have no idea what is causing their symptoms. I tell them, we will watch for other clues to see if the symptom needs a workup.
One thing I don’t do, and I strongly recommend against, is doing a review of systems. This leads a patient to believe you are concerned about exploring each possible symptom, ones that they didn’t even bring up! The yield is very low, and the time commitment is great.
The angry patient
Imagine a scenario when you are running 15 minutes behind and, when you step into the room, your patient is angry. You are already behind, and helping the patient navigate their anger will be part of your clinic visit.
In these situations, I always address the patient’s anger immediately. Problems with getting appointments with specialists, delays in diagnostic tests, or a broken entry to the parking garage have all been sources of my patients’ frustrations.
When we have limited time, using much of the clinic visit to process frustration leads to empty clinic visits. I listen and work to empathize with the patient, often agreeing that there are so many messed up aspects of the health care system. I do not like to use the corporate “I am sad you feel that way” response, because I feel it is not helpful. Instead, I tell them how much I want to help them today in any way I can at this visit.
The Internet sleuth
When our patients have new symptoms, some of them will go to the Internet to try to self-diagnose. Sometimes they make a correct diagnosis, but other times consider scary diagnoses we would not consider based on their symptoms and risk factors.
In these scenarios, I always ask the patient why they think their diagnosis is accurate. Their response to this question gives me insight into where their beliefs come from and helps me understand what information I need to provide.
McMullan said physicians can be defensive, collaborative, and informative when they interact with patients about information they have found on the Internet. In the first model, the physician is authoritative. The second involves working with the patient and obtaining and analyzing information. In the third model, the physician provides reputable internet sites to patients for obtaining information.
‘Oh, by the way’
Patients frequently bring up sensitive topics or complicated requests after the visit has wrapped up. Topics such as insomnia, erectile dysfunction, and anxiety are often brought up with the assumption that a quick prescription is the answer. For many years, I would add time to the appointment and try to address these issues as quickly as I could. But I invariably did a poor job at helping with these problems. Now, I offer to see the patient back soon to spend an entire visit discussing the newly brought up concern. I tell them that the problem is too important to not have my full attention and focus.
Pearls
- Empathetically listen to descriptions of symptoms, but don’t focus on fixing them.
- Empathize with the angry patient, and move on to taking care of their medical problems.
- Avoid the urge to address newly raised problems at the end of the visit.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose.
We all feel pressure from not having enough time to care for our patients the way we want to.
Organ recital
Some of our patients need to share an update on all their symptoms each visit, old and new, including those that are minor or possibly concerning. I have learned over the years that, for many patients, this allows them to release the worry about symptoms.
Some symptoms are so distressing and severe that symptomatic treatment is needed, but most aren’t.
I am very honest with patients when I have no idea what is causing their symptoms. I tell them, we will watch for other clues to see if the symptom needs a workup.
One thing I don’t do, and I strongly recommend against, is doing a review of systems. This leads a patient to believe you are concerned about exploring each possible symptom, ones that they didn’t even bring up! The yield is very low, and the time commitment is great.
The angry patient
Imagine a scenario when you are running 15 minutes behind and, when you step into the room, your patient is angry. You are already behind, and helping the patient navigate their anger will be part of your clinic visit.
In these situations, I always address the patient’s anger immediately. Problems with getting appointments with specialists, delays in diagnostic tests, or a broken entry to the parking garage have all been sources of my patients’ frustrations.
When we have limited time, using much of the clinic visit to process frustration leads to empty clinic visits. I listen and work to empathize with the patient, often agreeing that there are so many messed up aspects of the health care system. I do not like to use the corporate “I am sad you feel that way” response, because I feel it is not helpful. Instead, I tell them how much I want to help them today in any way I can at this visit.
The Internet sleuth
When our patients have new symptoms, some of them will go to the Internet to try to self-diagnose. Sometimes they make a correct diagnosis, but other times consider scary diagnoses we would not consider based on their symptoms and risk factors.
In these scenarios, I always ask the patient why they think their diagnosis is accurate. Their response to this question gives me insight into where their beliefs come from and helps me understand what information I need to provide.
McMullan said physicians can be defensive, collaborative, and informative when they interact with patients about information they have found on the Internet. In the first model, the physician is authoritative. The second involves working with the patient and obtaining and analyzing information. In the third model, the physician provides reputable internet sites to patients for obtaining information.
‘Oh, by the way’
Patients frequently bring up sensitive topics or complicated requests after the visit has wrapped up. Topics such as insomnia, erectile dysfunction, and anxiety are often brought up with the assumption that a quick prescription is the answer. For many years, I would add time to the appointment and try to address these issues as quickly as I could. But I invariably did a poor job at helping with these problems. Now, I offer to see the patient back soon to spend an entire visit discussing the newly brought up concern. I tell them that the problem is too important to not have my full attention and focus.
Pearls
- Empathetically listen to descriptions of symptoms, but don’t focus on fixing them.
- Empathize with the angry patient, and move on to taking care of their medical problems.
- Avoid the urge to address newly raised problems at the end of the visit.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose.
We all feel pressure from not having enough time to care for our patients the way we want to.
Organ recital
Some of our patients need to share an update on all their symptoms each visit, old and new, including those that are minor or possibly concerning. I have learned over the years that, for many patients, this allows them to release the worry about symptoms.
Some symptoms are so distressing and severe that symptomatic treatment is needed, but most aren’t.
I am very honest with patients when I have no idea what is causing their symptoms. I tell them, we will watch for other clues to see if the symptom needs a workup.
One thing I don’t do, and I strongly recommend against, is doing a review of systems. This leads a patient to believe you are concerned about exploring each possible symptom, ones that they didn’t even bring up! The yield is very low, and the time commitment is great.
The angry patient
Imagine a scenario when you are running 15 minutes behind and, when you step into the room, your patient is angry. You are already behind, and helping the patient navigate their anger will be part of your clinic visit.
In these situations, I always address the patient’s anger immediately. Problems with getting appointments with specialists, delays in diagnostic tests, or a broken entry to the parking garage have all been sources of my patients’ frustrations.
When we have limited time, using much of the clinic visit to process frustration leads to empty clinic visits. I listen and work to empathize with the patient, often agreeing that there are so many messed up aspects of the health care system. I do not like to use the corporate “I am sad you feel that way” response, because I feel it is not helpful. Instead, I tell them how much I want to help them today in any way I can at this visit.
The Internet sleuth
When our patients have new symptoms, some of them will go to the Internet to try to self-diagnose. Sometimes they make a correct diagnosis, but other times consider scary diagnoses we would not consider based on their symptoms and risk factors.
In these scenarios, I always ask the patient why they think their diagnosis is accurate. Their response to this question gives me insight into where their beliefs come from and helps me understand what information I need to provide.
McMullan said physicians can be defensive, collaborative, and informative when they interact with patients about information they have found on the Internet. In the first model, the physician is authoritative. The second involves working with the patient and obtaining and analyzing information. In the third model, the physician provides reputable internet sites to patients for obtaining information.
‘Oh, by the way’
Patients frequently bring up sensitive topics or complicated requests after the visit has wrapped up. Topics such as insomnia, erectile dysfunction, and anxiety are often brought up with the assumption that a quick prescription is the answer. For many years, I would add time to the appointment and try to address these issues as quickly as I could. But I invariably did a poor job at helping with these problems. Now, I offer to see the patient back soon to spend an entire visit discussing the newly brought up concern. I tell them that the problem is too important to not have my full attention and focus.
Pearls
- Empathetically listen to descriptions of symptoms, but don’t focus on fixing them.
- Empathize with the angry patient, and move on to taking care of their medical problems.
- Avoid the urge to address newly raised problems at the end of the visit.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose.