User login
Polypectomy clipping success is based on anticoagulant type
A subanalysis of anticoagulant types reveals that, despite no overall benefit for prophylactic polypectomy clipping, there are differences by subgroup: There was a significantly lower bleeding in patients on direct oral anticoagulants (DOACs) and higher bleeding risk in patients on warfarin.
“In DOAC users, prophylactic clipping was associated with a 64% relative risk reduction in 30-day PPB [postpolypectomy bleeding],” versus no clipping, reported the authors of the study, published in Gastrointestinal Endoscopy.
The removal of colonic polyps is known to carry a high risk of hemorrhage, and the use of antithrombotic medications, including DOACs and warfarin, are well-established as key risk factors for the bleeding.
However, data on the effectiveness of prophylactic hemoclips in preventing PPB is inconsistent, with one meta-analysis only showing a benefit in colonic lesions that are larger than 20 mm and proximal to the hepatic flexure, and other studies failing to show any significant benefits.
To further investigate the effects among patients treated with anticoagulants, first author Louis H.S. Lau, MBChB, an assistant clinical professor in the department of medicine and therapeutics at the Chinese University of Hong Kong, and colleagues enrolled 547 patients with 1,485 polyps who underwent colonoscopic polypectomy while being treated with an oral anticoagulant between 2012 and 2020.
The percentages of warfarin and DOAC users were similar between the groups, at about 50% each.
Overall, PPB occurred in 30 out of the 285 patients (10.6%) who had clipping and 11 out of the 262 patients (4.2%) who did not have clipping. The mean polyp size among patients with PPB was about 8-9 mm, and the mean time to bleeding was between 7 and 9 days.
In the propensity-weighted analysis, there was no statistically significant difference in bleeding among those who did and did not receive clipping (odds ratio, 1.19; 95% confidence interval, 0.73-1.95; P = .497).
However, a subgroup per-patient analysis did show prophylactic clipping was associated with a significantly lower 30-day PPB risk among patients treated with DOACs (OR, 0.36; 95% CI, 0.16-0.82; P = .015), but a significantly higher bleeding risk in patients taking warfarin (OR, 2.98; P = .003), and in those with heparin bridging (OR, 1.69; P = .023).
The subanalysis showed no benefit of prophylactic clipping among the largest polyps, which differed in size across the subgroups (<10 mm vs. 10-20 mm vs. 20 mm).
Of note, the overall analysis showed a significantly higher risk of PPB with hot resection polypectomy using electrocautery (OR, 9.76; 95% CI, 3.94-32.60; P < .001), compared with cold biopsy or snare polypectomy.
The authors noted several limitations to their study, including the relatively high rate of bleeding overall (7.5%), which could be related to the more frequent use of hot snare in their cohort earlier in the study.
Effects caused by DOACs’ rapid onset?
In speculating on the reasons for the different risks observed between DOACs and warfarin, the authors suggested that “a possible explanation could be the rapid onset of action and steady pharmacokinetics of DOAC, reducing the necessity of heparin bridging in most cases.”
Meanwhile, the increased bleeding observed with warfarin despite clipping could be “related to the intrinsic properties of warfarin,” they added.
“Because of its slow onset of action, a larger proportion of patients will receive heparin bridging, which was previously reported to be a significant risk factor of PPB,” they noted. “Moreover, due to the substantial fluctuation in anticoagulation effect during warfarin titration, it may provoke delayed bleeding after the endoclips fall off subsequently.”
Unique focus on anticoagulant-treated patients
Senior author Raymond Shing-Yan Tang, MD, an assistant professor in the department of medicine and therapeutics, faculty of medicine, at the Chinese University of Hong Kong, noted that the study’s unique focus on patients treated with anticoagulants is important.
“Prior studies evaluating the effectiveness of prophylactic clipping in preventing postpolypectomy bleeding included a more heterogeneous patient population with both nonanticoagulated and anticoagulated patients,” Dr. Tang said in an interview.
“The strengths of our study were that it was a dedicated study that included only patients on oral anticoagulants, including warfarin and DOACs, and had a relatively larger sample size when compared to prior studies,” he said.
While most guidelines recommend prophylactic clipping in patients undergoing polypectomy for colonic lesions larger than 20 mm and proximal to the hepatic flexure, a variety of factors may ultimately guide decisions, Dr. Tang noted.
“In clinical practice, the decision to use prophylactic clipping after polypectomy in patients on anticoagulation is often individualized at the discretion of the endoscopist,” he said.
Anticoagulation question is important, but study has limitations
In commenting on the study, Heiko Pohl, MD, a professor of medicine at Geisel School of Medicine at Dartmouth, Hanover, N.H., noted that, while this study is important, it has some key limitations.
“The question the study raises is relevant – we really have no good idea whether this subset of patients that are anticoagulated should always be clipped,” he said in an interview.
However, he noted potential limitations in the methodology.
“It’s difficult to control for all important factors in a propensity trial,” he said, adding “there could be some unmeasured confounders that could not be accounted for due to the retrospective design.”
Nevertheless, Dr. Pohl agreed that the relatively rapid action of DOACs could help explain the effects.
“DOACs may have a high risk of bleeding sooner [than warfarin] to begin with, and therefore the clipping makes sense, so that may be the mechanistic idea,” he said. “But it’s difficult to generalize, because there have been no previous studies that have shown benefits from clipping for smaller polyps, even among patients on anticoagulants.”
The authors had no disclosures to report. Dr. Pohl has received grants from Steris and Cosmo Pharmaceuticals.
A subanalysis of anticoagulant types reveals that, despite no overall benefit for prophylactic polypectomy clipping, there are differences by subgroup: There was a significantly lower bleeding in patients on direct oral anticoagulants (DOACs) and higher bleeding risk in patients on warfarin.
“In DOAC users, prophylactic clipping was associated with a 64% relative risk reduction in 30-day PPB [postpolypectomy bleeding],” versus no clipping, reported the authors of the study, published in Gastrointestinal Endoscopy.
The removal of colonic polyps is known to carry a high risk of hemorrhage, and the use of antithrombotic medications, including DOACs and warfarin, are well-established as key risk factors for the bleeding.
However, data on the effectiveness of prophylactic hemoclips in preventing PPB is inconsistent, with one meta-analysis only showing a benefit in colonic lesions that are larger than 20 mm and proximal to the hepatic flexure, and other studies failing to show any significant benefits.
To further investigate the effects among patients treated with anticoagulants, first author Louis H.S. Lau, MBChB, an assistant clinical professor in the department of medicine and therapeutics at the Chinese University of Hong Kong, and colleagues enrolled 547 patients with 1,485 polyps who underwent colonoscopic polypectomy while being treated with an oral anticoagulant between 2012 and 2020.
The percentages of warfarin and DOAC users were similar between the groups, at about 50% each.
Overall, PPB occurred in 30 out of the 285 patients (10.6%) who had clipping and 11 out of the 262 patients (4.2%) who did not have clipping. The mean polyp size among patients with PPB was about 8-9 mm, and the mean time to bleeding was between 7 and 9 days.
In the propensity-weighted analysis, there was no statistically significant difference in bleeding among those who did and did not receive clipping (odds ratio, 1.19; 95% confidence interval, 0.73-1.95; P = .497).
However, a subgroup per-patient analysis did show prophylactic clipping was associated with a significantly lower 30-day PPB risk among patients treated with DOACs (OR, 0.36; 95% CI, 0.16-0.82; P = .015), but a significantly higher bleeding risk in patients taking warfarin (OR, 2.98; P = .003), and in those with heparin bridging (OR, 1.69; P = .023).
The subanalysis showed no benefit of prophylactic clipping among the largest polyps, which differed in size across the subgroups (<10 mm vs. 10-20 mm vs. 20 mm).
Of note, the overall analysis showed a significantly higher risk of PPB with hot resection polypectomy using electrocautery (OR, 9.76; 95% CI, 3.94-32.60; P < .001), compared with cold biopsy or snare polypectomy.
The authors noted several limitations to their study, including the relatively high rate of bleeding overall (7.5%), which could be related to the more frequent use of hot snare in their cohort earlier in the study.
Effects caused by DOACs’ rapid onset?
In speculating on the reasons for the different risks observed between DOACs and warfarin, the authors suggested that “a possible explanation could be the rapid onset of action and steady pharmacokinetics of DOAC, reducing the necessity of heparin bridging in most cases.”
Meanwhile, the increased bleeding observed with warfarin despite clipping could be “related to the intrinsic properties of warfarin,” they added.
“Because of its slow onset of action, a larger proportion of patients will receive heparin bridging, which was previously reported to be a significant risk factor of PPB,” they noted. “Moreover, due to the substantial fluctuation in anticoagulation effect during warfarin titration, it may provoke delayed bleeding after the endoclips fall off subsequently.”
Unique focus on anticoagulant-treated patients
Senior author Raymond Shing-Yan Tang, MD, an assistant professor in the department of medicine and therapeutics, faculty of medicine, at the Chinese University of Hong Kong, noted that the study’s unique focus on patients treated with anticoagulants is important.
“Prior studies evaluating the effectiveness of prophylactic clipping in preventing postpolypectomy bleeding included a more heterogeneous patient population with both nonanticoagulated and anticoagulated patients,” Dr. Tang said in an interview.
“The strengths of our study were that it was a dedicated study that included only patients on oral anticoagulants, including warfarin and DOACs, and had a relatively larger sample size when compared to prior studies,” he said.
While most guidelines recommend prophylactic clipping in patients undergoing polypectomy for colonic lesions larger than 20 mm and proximal to the hepatic flexure, a variety of factors may ultimately guide decisions, Dr. Tang noted.
“In clinical practice, the decision to use prophylactic clipping after polypectomy in patients on anticoagulation is often individualized at the discretion of the endoscopist,” he said.
Anticoagulation question is important, but study has limitations
In commenting on the study, Heiko Pohl, MD, a professor of medicine at Geisel School of Medicine at Dartmouth, Hanover, N.H., noted that, while this study is important, it has some key limitations.
“The question the study raises is relevant – we really have no good idea whether this subset of patients that are anticoagulated should always be clipped,” he said in an interview.
However, he noted potential limitations in the methodology.
“It’s difficult to control for all important factors in a propensity trial,” he said, adding “there could be some unmeasured confounders that could not be accounted for due to the retrospective design.”
Nevertheless, Dr. Pohl agreed that the relatively rapid action of DOACs could help explain the effects.
“DOACs may have a high risk of bleeding sooner [than warfarin] to begin with, and therefore the clipping makes sense, so that may be the mechanistic idea,” he said. “But it’s difficult to generalize, because there have been no previous studies that have shown benefits from clipping for smaller polyps, even among patients on anticoagulants.”
The authors had no disclosures to report. Dr. Pohl has received grants from Steris and Cosmo Pharmaceuticals.
A subanalysis of anticoagulant types reveals that, despite no overall benefit for prophylactic polypectomy clipping, there are differences by subgroup: There was a significantly lower bleeding in patients on direct oral anticoagulants (DOACs) and higher bleeding risk in patients on warfarin.
“In DOAC users, prophylactic clipping was associated with a 64% relative risk reduction in 30-day PPB [postpolypectomy bleeding],” versus no clipping, reported the authors of the study, published in Gastrointestinal Endoscopy.
The removal of colonic polyps is known to carry a high risk of hemorrhage, and the use of antithrombotic medications, including DOACs and warfarin, are well-established as key risk factors for the bleeding.
However, data on the effectiveness of prophylactic hemoclips in preventing PPB is inconsistent, with one meta-analysis only showing a benefit in colonic lesions that are larger than 20 mm and proximal to the hepatic flexure, and other studies failing to show any significant benefits.
To further investigate the effects among patients treated with anticoagulants, first author Louis H.S. Lau, MBChB, an assistant clinical professor in the department of medicine and therapeutics at the Chinese University of Hong Kong, and colleagues enrolled 547 patients with 1,485 polyps who underwent colonoscopic polypectomy while being treated with an oral anticoagulant between 2012 and 2020.
The percentages of warfarin and DOAC users were similar between the groups, at about 50% each.
Overall, PPB occurred in 30 out of the 285 patients (10.6%) who had clipping and 11 out of the 262 patients (4.2%) who did not have clipping. The mean polyp size among patients with PPB was about 8-9 mm, and the mean time to bleeding was between 7 and 9 days.
In the propensity-weighted analysis, there was no statistically significant difference in bleeding among those who did and did not receive clipping (odds ratio, 1.19; 95% confidence interval, 0.73-1.95; P = .497).
However, a subgroup per-patient analysis did show prophylactic clipping was associated with a significantly lower 30-day PPB risk among patients treated with DOACs (OR, 0.36; 95% CI, 0.16-0.82; P = .015), but a significantly higher bleeding risk in patients taking warfarin (OR, 2.98; P = .003), and in those with heparin bridging (OR, 1.69; P = .023).
The subanalysis showed no benefit of prophylactic clipping among the largest polyps, which differed in size across the subgroups (<10 mm vs. 10-20 mm vs. 20 mm).
Of note, the overall analysis showed a significantly higher risk of PPB with hot resection polypectomy using electrocautery (OR, 9.76; 95% CI, 3.94-32.60; P < .001), compared with cold biopsy or snare polypectomy.
The authors noted several limitations to their study, including the relatively high rate of bleeding overall (7.5%), which could be related to the more frequent use of hot snare in their cohort earlier in the study.
Effects caused by DOACs’ rapid onset?
In speculating on the reasons for the different risks observed between DOACs and warfarin, the authors suggested that “a possible explanation could be the rapid onset of action and steady pharmacokinetics of DOAC, reducing the necessity of heparin bridging in most cases.”
Meanwhile, the increased bleeding observed with warfarin despite clipping could be “related to the intrinsic properties of warfarin,” they added.
“Because of its slow onset of action, a larger proportion of patients will receive heparin bridging, which was previously reported to be a significant risk factor of PPB,” they noted. “Moreover, due to the substantial fluctuation in anticoagulation effect during warfarin titration, it may provoke delayed bleeding after the endoclips fall off subsequently.”
Unique focus on anticoagulant-treated patients
Senior author Raymond Shing-Yan Tang, MD, an assistant professor in the department of medicine and therapeutics, faculty of medicine, at the Chinese University of Hong Kong, noted that the study’s unique focus on patients treated with anticoagulants is important.
“Prior studies evaluating the effectiveness of prophylactic clipping in preventing postpolypectomy bleeding included a more heterogeneous patient population with both nonanticoagulated and anticoagulated patients,” Dr. Tang said in an interview.
“The strengths of our study were that it was a dedicated study that included only patients on oral anticoagulants, including warfarin and DOACs, and had a relatively larger sample size when compared to prior studies,” he said.
While most guidelines recommend prophylactic clipping in patients undergoing polypectomy for colonic lesions larger than 20 mm and proximal to the hepatic flexure, a variety of factors may ultimately guide decisions, Dr. Tang noted.
“In clinical practice, the decision to use prophylactic clipping after polypectomy in patients on anticoagulation is often individualized at the discretion of the endoscopist,” he said.
Anticoagulation question is important, but study has limitations
In commenting on the study, Heiko Pohl, MD, a professor of medicine at Geisel School of Medicine at Dartmouth, Hanover, N.H., noted that, while this study is important, it has some key limitations.
“The question the study raises is relevant – we really have no good idea whether this subset of patients that are anticoagulated should always be clipped,” he said in an interview.
However, he noted potential limitations in the methodology.
“It’s difficult to control for all important factors in a propensity trial,” he said, adding “there could be some unmeasured confounders that could not be accounted for due to the retrospective design.”
Nevertheless, Dr. Pohl agreed that the relatively rapid action of DOACs could help explain the effects.
“DOACs may have a high risk of bleeding sooner [than warfarin] to begin with, and therefore the clipping makes sense, so that may be the mechanistic idea,” he said. “But it’s difficult to generalize, because there have been no previous studies that have shown benefits from clipping for smaller polyps, even among patients on anticoagulants.”
The authors had no disclosures to report. Dr. Pohl has received grants from Steris and Cosmo Pharmaceuticals.
FROM GASTROINTESTINAL ENDOSCOPY
NAFLD vs. MAFLD: What’s in a name?
Non-alcoholic fatty liver disease (NAFLD) and metabolic associated fatty liver disease (MAFLD) demonstrate highly similar clinical courses and mortality rates, and a name change may not be clinically beneficial, based on data from more than 17,000 patients.
Instead, etiologic subcategorization of fatty liver disease (FLD) should be considered, reported lead author Zobair M. Younossi, MD, of Betty and Guy Beatty Center for Integrated Research, Inova Health System, Falls Church, Va., and colleagues.
“There is debate about whether NAFLD is an appropriate name as the term ‘non-alcoholic’ overemphasizes the absence of alcohol use and underemphasizes the importance of the metabolic risk factors which are the main drivers of disease progression,” the investigators wrote in Hepatology. “It has been suggested that MAFLD may better reflect these risk factors. However, such a recommendation is made despite a lack of a general consensus on the definition of ‘metabolic health’ and disagreements in endocrinology circles about the term ‘metabolic syndrome.’ Nevertheless, a few investigators have suggested that MAFLD but not NAFLD is associated with increased fibrosis and mortality.”
To look for clinical differences between the two disease entities, Dr. Younossi and colleagues turned to the National Health and Nutrition Examination Survey (NHANES). Specifically, the NHANES III and NHANES 2017-2018 cohorts were employed, including 12,878 and 4,328 participants, respectively.
MAFLD was defined as FLD with overweight/obesity, evidence of metabolic dysregulation, or type 2 diabetes mellitus. NAFLD was defined as FLD without excessive alcohol consumption or other causes of chronic liver disease. Patients were sorted into four groups: NAFLD, MAFLD, both disease types, or neither disease type. Since the categories were not mutually exclusive, the investigators compared clinical characteristics based on 95% confidence intervals. If no overlap was found, then differences were deemed statistically significant.
Diagnoses of NAFLD and MAFLD were highly concordant (kappa coefficient = 0.83-0.94). After a median of 22.8 years follow-up, no significant differences were found between groups for cause-specific mortality, all-cause mortality, or major clinical characteristics except those inherent to the disease definitions (for example, lack of alcohol use in NAFLD). Greatest risk factors for advanced fibrosis in both groups were obesity, high-risk fibrosis, and type 2 diabetes mellitus.
As anticipated, by definition, alcoholic liver disease and excess alcohol use were documented in approximately 15% of patients with MAFLD, but in no patients with NAFLD. As such, alcoholic liver disease predicted liver-specific mortality for MAFLD (hazard ratio, 4.50; 95% confidence interval, 1.89-10.75) but not NAFLD. Conversely, insulin resistance predicted liver-specific mortality in NAFLD (HR, 3.57; 95% CI, 1.35-9.42) but not MAFLD (HR, 0.84; 95% CI, 0.36-1.95).
“These data do not support the notion that a name change from NAFLD to MAFLD will better capture the risk for long-term outcomes of these patients or better define metabolically at-risk patients who present with FLD,” the investigators concluded. “On the other hand, enlarging the definition to FLD with subcategories of ‘alcoholic,’ ‘non-alcoholic,’ ‘drug-induced,’ etc. has merit and needs to be further considered. In this context, a true international consensus group of experts supported by liver and non-liver scientific societies must undertake an evidence-based and comprehensive approach to this issue and assess both the benefits and risks of changing the name.”
According to Rohit Loomba, MD, director of the NAFLD research center and professor of medicine in the division of gastroenterology and hepatology at University of California, San Diego, the study offers a preview of the consequences if NAFLD were changed to MAFLD, most notably by making alcohol a key driver of outcomes.
“If we change the name of a disease entity ... how does that impact natural history?” Dr. Loomba asked in an interview. “This paper gives you an idea. If you start calling it MAFLD, then people are dying from alcohol use, and they’re not dying from what we are currently seeing patients with NAFLD die of.”
He also noted that the name change could disrupt drug development and outcome measures since most drugs currently in development are directed at nonalcoholic steatohepatitis (NASH).
“Is it worth the headache?” Dr. Loomba asked. “How are we going to define NASH-related fibrosis? That probably will remain the same because the therapies that we will use to address that will remain consistent with what we are currently pursuing. ... It’s probably premature to change the nomenclature before assessing the impact on finding new treatment.”
Dr. Younossi disclosed relationships with BMS, Novartis, Gilead, and others. Dr. Loomba serves as a consultant to Aardvark Therapeutics, Altimmune, Anylam/Regeneron, Amgen, Arrowhead Pharmaceuticals, AstraZeneca, Bristol-Myer Squibb, CohBar, Eli Lilly, Galmed, Gilead, Glympse bio, Hightide, Inipharma, Intercept, Inventiva, Ionis, Janssen, Madrigal, Metacrine, NGM Biopharmaceuticals, Novartis, Novo Nordisk, Merck, Pfizer, Sagimet, Theratechnologies, 89 bio, Terns Pharmaceuticals, and Viking Therapeutics.
Non-alcoholic fatty liver disease (NAFLD) and metabolic associated fatty liver disease (MAFLD) demonstrate highly similar clinical courses and mortality rates, and a name change may not be clinically beneficial, based on data from more than 17,000 patients.
Instead, etiologic subcategorization of fatty liver disease (FLD) should be considered, reported lead author Zobair M. Younossi, MD, of Betty and Guy Beatty Center for Integrated Research, Inova Health System, Falls Church, Va., and colleagues.
“There is debate about whether NAFLD is an appropriate name as the term ‘non-alcoholic’ overemphasizes the absence of alcohol use and underemphasizes the importance of the metabolic risk factors which are the main drivers of disease progression,” the investigators wrote in Hepatology. “It has been suggested that MAFLD may better reflect these risk factors. However, such a recommendation is made despite a lack of a general consensus on the definition of ‘metabolic health’ and disagreements in endocrinology circles about the term ‘metabolic syndrome.’ Nevertheless, a few investigators have suggested that MAFLD but not NAFLD is associated with increased fibrosis and mortality.”
To look for clinical differences between the two disease entities, Dr. Younossi and colleagues turned to the National Health and Nutrition Examination Survey (NHANES). Specifically, the NHANES III and NHANES 2017-2018 cohorts were employed, including 12,878 and 4,328 participants, respectively.
MAFLD was defined as FLD with overweight/obesity, evidence of metabolic dysregulation, or type 2 diabetes mellitus. NAFLD was defined as FLD without excessive alcohol consumption or other causes of chronic liver disease. Patients were sorted into four groups: NAFLD, MAFLD, both disease types, or neither disease type. Since the categories were not mutually exclusive, the investigators compared clinical characteristics based on 95% confidence intervals. If no overlap was found, then differences were deemed statistically significant.
Diagnoses of NAFLD and MAFLD were highly concordant (kappa coefficient = 0.83-0.94). After a median of 22.8 years follow-up, no significant differences were found between groups for cause-specific mortality, all-cause mortality, or major clinical characteristics except those inherent to the disease definitions (for example, lack of alcohol use in NAFLD). Greatest risk factors for advanced fibrosis in both groups were obesity, high-risk fibrosis, and type 2 diabetes mellitus.
As anticipated, by definition, alcoholic liver disease and excess alcohol use were documented in approximately 15% of patients with MAFLD, but in no patients with NAFLD. As such, alcoholic liver disease predicted liver-specific mortality for MAFLD (hazard ratio, 4.50; 95% confidence interval, 1.89-10.75) but not NAFLD. Conversely, insulin resistance predicted liver-specific mortality in NAFLD (HR, 3.57; 95% CI, 1.35-9.42) but not MAFLD (HR, 0.84; 95% CI, 0.36-1.95).
“These data do not support the notion that a name change from NAFLD to MAFLD will better capture the risk for long-term outcomes of these patients or better define metabolically at-risk patients who present with FLD,” the investigators concluded. “On the other hand, enlarging the definition to FLD with subcategories of ‘alcoholic,’ ‘non-alcoholic,’ ‘drug-induced,’ etc. has merit and needs to be further considered. In this context, a true international consensus group of experts supported by liver and non-liver scientific societies must undertake an evidence-based and comprehensive approach to this issue and assess both the benefits and risks of changing the name.”
According to Rohit Loomba, MD, director of the NAFLD research center and professor of medicine in the division of gastroenterology and hepatology at University of California, San Diego, the study offers a preview of the consequences if NAFLD were changed to MAFLD, most notably by making alcohol a key driver of outcomes.
“If we change the name of a disease entity ... how does that impact natural history?” Dr. Loomba asked in an interview. “This paper gives you an idea. If you start calling it MAFLD, then people are dying from alcohol use, and they’re not dying from what we are currently seeing patients with NAFLD die of.”
He also noted that the name change could disrupt drug development and outcome measures since most drugs currently in development are directed at nonalcoholic steatohepatitis (NASH).
“Is it worth the headache?” Dr. Loomba asked. “How are we going to define NASH-related fibrosis? That probably will remain the same because the therapies that we will use to address that will remain consistent with what we are currently pursuing. ... It’s probably premature to change the nomenclature before assessing the impact on finding new treatment.”
Dr. Younossi disclosed relationships with BMS, Novartis, Gilead, and others. Dr. Loomba serves as a consultant to Aardvark Therapeutics, Altimmune, Anylam/Regeneron, Amgen, Arrowhead Pharmaceuticals, AstraZeneca, Bristol-Myer Squibb, CohBar, Eli Lilly, Galmed, Gilead, Glympse bio, Hightide, Inipharma, Intercept, Inventiva, Ionis, Janssen, Madrigal, Metacrine, NGM Biopharmaceuticals, Novartis, Novo Nordisk, Merck, Pfizer, Sagimet, Theratechnologies, 89 bio, Terns Pharmaceuticals, and Viking Therapeutics.
Non-alcoholic fatty liver disease (NAFLD) and metabolic associated fatty liver disease (MAFLD) demonstrate highly similar clinical courses and mortality rates, and a name change may not be clinically beneficial, based on data from more than 17,000 patients.
Instead, etiologic subcategorization of fatty liver disease (FLD) should be considered, reported lead author Zobair M. Younossi, MD, of Betty and Guy Beatty Center for Integrated Research, Inova Health System, Falls Church, Va., and colleagues.
“There is debate about whether NAFLD is an appropriate name as the term ‘non-alcoholic’ overemphasizes the absence of alcohol use and underemphasizes the importance of the metabolic risk factors which are the main drivers of disease progression,” the investigators wrote in Hepatology. “It has been suggested that MAFLD may better reflect these risk factors. However, such a recommendation is made despite a lack of a general consensus on the definition of ‘metabolic health’ and disagreements in endocrinology circles about the term ‘metabolic syndrome.’ Nevertheless, a few investigators have suggested that MAFLD but not NAFLD is associated with increased fibrosis and mortality.”
To look for clinical differences between the two disease entities, Dr. Younossi and colleagues turned to the National Health and Nutrition Examination Survey (NHANES). Specifically, the NHANES III and NHANES 2017-2018 cohorts were employed, including 12,878 and 4,328 participants, respectively.
MAFLD was defined as FLD with overweight/obesity, evidence of metabolic dysregulation, or type 2 diabetes mellitus. NAFLD was defined as FLD without excessive alcohol consumption or other causes of chronic liver disease. Patients were sorted into four groups: NAFLD, MAFLD, both disease types, or neither disease type. Since the categories were not mutually exclusive, the investigators compared clinical characteristics based on 95% confidence intervals. If no overlap was found, then differences were deemed statistically significant.
Diagnoses of NAFLD and MAFLD were highly concordant (kappa coefficient = 0.83-0.94). After a median of 22.8 years follow-up, no significant differences were found between groups for cause-specific mortality, all-cause mortality, or major clinical characteristics except those inherent to the disease definitions (for example, lack of alcohol use in NAFLD). Greatest risk factors for advanced fibrosis in both groups were obesity, high-risk fibrosis, and type 2 diabetes mellitus.
As anticipated, by definition, alcoholic liver disease and excess alcohol use were documented in approximately 15% of patients with MAFLD, but in no patients with NAFLD. As such, alcoholic liver disease predicted liver-specific mortality for MAFLD (hazard ratio, 4.50; 95% confidence interval, 1.89-10.75) but not NAFLD. Conversely, insulin resistance predicted liver-specific mortality in NAFLD (HR, 3.57; 95% CI, 1.35-9.42) but not MAFLD (HR, 0.84; 95% CI, 0.36-1.95).
“These data do not support the notion that a name change from NAFLD to MAFLD will better capture the risk for long-term outcomes of these patients or better define metabolically at-risk patients who present with FLD,” the investigators concluded. “On the other hand, enlarging the definition to FLD with subcategories of ‘alcoholic,’ ‘non-alcoholic,’ ‘drug-induced,’ etc. has merit and needs to be further considered. In this context, a true international consensus group of experts supported by liver and non-liver scientific societies must undertake an evidence-based and comprehensive approach to this issue and assess both the benefits and risks of changing the name.”
According to Rohit Loomba, MD, director of the NAFLD research center and professor of medicine in the division of gastroenterology and hepatology at University of California, San Diego, the study offers a preview of the consequences if NAFLD were changed to MAFLD, most notably by making alcohol a key driver of outcomes.
“If we change the name of a disease entity ... how does that impact natural history?” Dr. Loomba asked in an interview. “This paper gives you an idea. If you start calling it MAFLD, then people are dying from alcohol use, and they’re not dying from what we are currently seeing patients with NAFLD die of.”
He also noted that the name change could disrupt drug development and outcome measures since most drugs currently in development are directed at nonalcoholic steatohepatitis (NASH).
“Is it worth the headache?” Dr. Loomba asked. “How are we going to define NASH-related fibrosis? That probably will remain the same because the therapies that we will use to address that will remain consistent with what we are currently pursuing. ... It’s probably premature to change the nomenclature before assessing the impact on finding new treatment.”
Dr. Younossi disclosed relationships with BMS, Novartis, Gilead, and others. Dr. Loomba serves as a consultant to Aardvark Therapeutics, Altimmune, Anylam/Regeneron, Amgen, Arrowhead Pharmaceuticals, AstraZeneca, Bristol-Myer Squibb, CohBar, Eli Lilly, Galmed, Gilead, Glympse bio, Hightide, Inipharma, Intercept, Inventiva, Ionis, Janssen, Madrigal, Metacrine, NGM Biopharmaceuticals, Novartis, Novo Nordisk, Merck, Pfizer, Sagimet, Theratechnologies, 89 bio, Terns Pharmaceuticals, and Viking Therapeutics.
FROM HEPATOLOGY
FDA approves Medtronic’s Onyx Frontier drug-eluting stent
The U.S. Food and Drug Administration has approved the Onyx Frontier drug-eluting stent (DES) to treat patients with coronary artery disease, the device manufacturer, Medtronic, announced today.
The Onyx Frontier shares the same stent platform and clinical indications as the previous-generation Resolute Onyx zotarolimus-eluting stent, including the most recent approval for patients at high risk of bleeding who may benefit from just 1 month dual-antiplatelet therapy.
“Meaningful design changes, including increased catheter flexibility, an innovative dual-layer balloon technology and a lower crossing profile led to a 16% improvement in deliverability with Onyx Frontier vs. the previous generation Resolute Onyx DES,” Medtronic said in a news release.
Onyx Frontier also offers a broad size matrix to treat more patients, and joins the Resolute Onyx as the only 2-mm DES available in the United States, the company noted. The stent is available in 4.5- to 5-mm sizes that can be expanded to 6 mm, specifically designed to support extra-large vessels.
The Onyx Frontier DES is pending CE Mark in Europe.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has approved the Onyx Frontier drug-eluting stent (DES) to treat patients with coronary artery disease, the device manufacturer, Medtronic, announced today.
The Onyx Frontier shares the same stent platform and clinical indications as the previous-generation Resolute Onyx zotarolimus-eluting stent, including the most recent approval for patients at high risk of bleeding who may benefit from just 1 month dual-antiplatelet therapy.
“Meaningful design changes, including increased catheter flexibility, an innovative dual-layer balloon technology and a lower crossing profile led to a 16% improvement in deliverability with Onyx Frontier vs. the previous generation Resolute Onyx DES,” Medtronic said in a news release.
Onyx Frontier also offers a broad size matrix to treat more patients, and joins the Resolute Onyx as the only 2-mm DES available in the United States, the company noted. The stent is available in 4.5- to 5-mm sizes that can be expanded to 6 mm, specifically designed to support extra-large vessels.
The Onyx Frontier DES is pending CE Mark in Europe.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has approved the Onyx Frontier drug-eluting stent (DES) to treat patients with coronary artery disease, the device manufacturer, Medtronic, announced today.
The Onyx Frontier shares the same stent platform and clinical indications as the previous-generation Resolute Onyx zotarolimus-eluting stent, including the most recent approval for patients at high risk of bleeding who may benefit from just 1 month dual-antiplatelet therapy.
“Meaningful design changes, including increased catheter flexibility, an innovative dual-layer balloon technology and a lower crossing profile led to a 16% improvement in deliverability with Onyx Frontier vs. the previous generation Resolute Onyx DES,” Medtronic said in a news release.
Onyx Frontier also offers a broad size matrix to treat more patients, and joins the Resolute Onyx as the only 2-mm DES available in the United States, the company noted. The stent is available in 4.5- to 5-mm sizes that can be expanded to 6 mm, specifically designed to support extra-large vessels.
The Onyx Frontier DES is pending CE Mark in Europe.
A version of this article first appeared on Medscape.com.
Study shows link between dairy consumption and cancer
A relationship between consumption of dairy products and risk of various cancers has been intensively investigated in the past but yielded inconclusive or conflicting results.
The study, by researchers from Oxford University’s department of population health, and Peking University and the Chinese Academy of Medical Sciences in Beijing, used data from the China Kadoorie Biobank Study, a long-term prospective study involving more than over 510,000 participants recruited from 10 geographically diverse areas across China, including both rural and urban regions. They compared this to data from the UK biobank.
Subjects were 59% female, 41% male, aged 30-79 years, and had no history of cancer at recruitment between 2004 and 2008. Food questionnaires were completed at the outset and participants followed for an average of 11 years, using national cancer and death registries and health insurance records to identify new cancer diagnoses, including both fatal and nonfatal events.
Participants were categorized into three groups according to how often they consumed dairy products (primarily milk):
- Regular consumers (at least once a week): 20.4% of the cohort.
- Monthly consumers: 11.1%.
- Nonconsumers who never or rarely consumed dairy products: 68.5%.
Average dairy consumption was 37.9 g/day overall and 80.8 g/day among regular consumers. This compares with an average consumption of around 300 g/day in participants in the UK Biobank cohort.
Over the course of the study, 29,277 new cancer cases were recorded, including 6,282 lung, 2,582 female breast, 3,577 stomach, 3,350 colorectal, and 3,191 liver cancer cases.
Analyses correlating cases with consumption took into account a range of other factors potentially affecting cancer risk, including age, sex, region, family history of cancer, socioeconomic status (education and income), lifestyle factors (alcohol intake, smoking, physical activity, soy consumption, and fresh fruit intake), body mass index, chronic hepatitis B virus infection, and female reproductive factors.
Higher dairy intakes linked with risk of liver and breast cancers
Results revealed that higher regular dairy intake was associated with significantly higher risks of liver cancer and female breast cancer, both common types of cancer in China. Analyses indicated that for each 50-g/day intake, the risks increased by 12% and 17%, respectively.
There was also an increase in total cancer diagnoses, and an increased risk of lymphoma, though this was not statistically significant after correction for confounders. No association was found between dairy products and colorectal cancer, prostate cancer, or any other site-specific cancer.
The research, published in BMC Medicine, is the first major study to investigate dairy consumption and cancer risk in Chinese adults. The results conflict with previous studies on Western populations, which have suggested that dairy products may be associated with a lower risk of colorectal cancer and a higher risk of prostate cancer but have found no clear link for breast or other types of cancer.
Lead researchers Maria Kakkoura, PhD, MSc, and associate professor Huaidong Du, MD, PhD, told this news organization that, although they don’t know the reason for the difference, “there is clear evidence that colorectal cancer has a different incidence pattern in China, compared with Western countries. Other risk factors, like adiposity, may have a stronger effect on the risk of colorectal cancer in Western countries than in China.” Notably, the mean body mass index in the study population was around 23 kg/m2, they said – by contrast in the United Kingdom it is 27.6 kg/m2.
Effects not necessarily causal
Ian Givens, PhD, professor of food chain nutrition at the University of Reading (England), said the study was “potentially very important for Chinese people, if it can be confirmed that dairy products affect the risk of breast and/or liver cancer differently in Chinese subjects to those in Western Societies, especially as dairy consumption in China is much lower than in most Western diets.”
He added: “As always it needs to be kept in mind that this type of study can only establish associations with disease risk, not cause.”
Dr. Kakkoura, nutritional epidemiologist at Oxford (England) University’s department of population health, said: “This was the first major study to investigate the link between dairy products and cancer risk in a Chinese population. Further studies are needed to validate these current findings, establish if these associations are causal, and investigate the potential underlying mechanisms involved.”
The researchers said that, while the results do not prove causation, “there are several plausible biological mechanisms that may explain these associations.” They pointed to higher dairy consumption potentially increasing levels of insulinlike growth factor-I, known to promote cell proliferation and associated with higher risks of several types of cancer.
In addition, estrogen and progesterone present in cows’ milk may play a role in increasing breast cancer risk, whilst saturated and trans-fatty acids from dairy products may increase the risk of liver cancer. As many Chinese people are lactase deficient, dairy products may also be broken down into products that affect cancer risk.
No justification for dietary change
Confounding factors may also have influenced the results, commented Duane Mellor, PhD, RD, RNutr, registered dietitian and senior teaching fellow at Aston University, Birmingham, England. “Those in the study who consumed dairy were more likely to live in cities and have other health conditions, including cardiovascular disease and diabetes – although some of these factors were considered in the analysis, not all of these covariates were, which could influence the findings.
“In my view this study alone does not provide strong evidence that reducing dairy intake would reduce cancer risk.”
He added: “Although the paper suggests a 12% increased relative risk for female breast cancer, this does not equate to 12 more cases per 100 individuals – in absolute terms this would be more like 1 or 2 cases per 1,000 people.”
Similarly, Kevin McConway, PhD, emeritus professor of applied statistics at the Open University, Milton Keynes, England, said: “An issue is that there were many differences between the people that consumed different amounts of dairy products, apart from their difference in dairy consumption. For instance, of those who never or rarely consumed dairy products, fewer than a third lived in urban areas, but of regular dairy consumers (at least once a week), 83% lived in urban areas. Regular consumers were considerably more likely to be well educated than those who never or rarely consumed dairy products, and there were other differences too.
“So if, as the researchers found, a greater proportion of the regular consumers than of the never or rare consumers had a cancer diagnosis, that could have been because of their different dairy consumption, or it could have been (in part or entirely) because of the different places they lived, or their different education levels, or any of the other factors on which the groups differed.
“One can never be sure that all the relevant factors have been adjusted for. That’s why the researchers rightly say that these results can’t establish whether the associations between dairy consumption and the risks of some cancers, that they found, are there because the dairy consumption differences change the cancer risks in a cause-and-effect way. They might, or they might not.”
He cautioned: “I don’t think anyone should decide to change their individual diet solely because of the results of this new study.”
Commenting on the study, Fiona Osgun, senior health information manager at Cancer Research UK, London, told this news organization: “This early-stage study found an association between dairy consumption and the risks of certain cancers, but that doesn’t mean that they’re causing them or that people need to avoid dairy. Dairy products can be part of a healthy balanced diet and, in the U.K., the Food Standards Agency regulates them to make sure they’re safe. There’s good evidence that dairy reduces the risk of bowel cancer, but no clear evidence for other cancer types, and this is no different for people who are lactose intolerant.”
A version of this article first appeared on Medscape UK.
A relationship between consumption of dairy products and risk of various cancers has been intensively investigated in the past but yielded inconclusive or conflicting results.
The study, by researchers from Oxford University’s department of population health, and Peking University and the Chinese Academy of Medical Sciences in Beijing, used data from the China Kadoorie Biobank Study, a long-term prospective study involving more than over 510,000 participants recruited from 10 geographically diverse areas across China, including both rural and urban regions. They compared this to data from the UK biobank.
Subjects were 59% female, 41% male, aged 30-79 years, and had no history of cancer at recruitment between 2004 and 2008. Food questionnaires were completed at the outset and participants followed for an average of 11 years, using national cancer and death registries and health insurance records to identify new cancer diagnoses, including both fatal and nonfatal events.
Participants were categorized into three groups according to how often they consumed dairy products (primarily milk):
- Regular consumers (at least once a week): 20.4% of the cohort.
- Monthly consumers: 11.1%.
- Nonconsumers who never or rarely consumed dairy products: 68.5%.
Average dairy consumption was 37.9 g/day overall and 80.8 g/day among regular consumers. This compares with an average consumption of around 300 g/day in participants in the UK Biobank cohort.
Over the course of the study, 29,277 new cancer cases were recorded, including 6,282 lung, 2,582 female breast, 3,577 stomach, 3,350 colorectal, and 3,191 liver cancer cases.
Analyses correlating cases with consumption took into account a range of other factors potentially affecting cancer risk, including age, sex, region, family history of cancer, socioeconomic status (education and income), lifestyle factors (alcohol intake, smoking, physical activity, soy consumption, and fresh fruit intake), body mass index, chronic hepatitis B virus infection, and female reproductive factors.
Higher dairy intakes linked with risk of liver and breast cancers
Results revealed that higher regular dairy intake was associated with significantly higher risks of liver cancer and female breast cancer, both common types of cancer in China. Analyses indicated that for each 50-g/day intake, the risks increased by 12% and 17%, respectively.
There was also an increase in total cancer diagnoses, and an increased risk of lymphoma, though this was not statistically significant after correction for confounders. No association was found between dairy products and colorectal cancer, prostate cancer, or any other site-specific cancer.
The research, published in BMC Medicine, is the first major study to investigate dairy consumption and cancer risk in Chinese adults. The results conflict with previous studies on Western populations, which have suggested that dairy products may be associated with a lower risk of colorectal cancer and a higher risk of prostate cancer but have found no clear link for breast or other types of cancer.
Lead researchers Maria Kakkoura, PhD, MSc, and associate professor Huaidong Du, MD, PhD, told this news organization that, although they don’t know the reason for the difference, “there is clear evidence that colorectal cancer has a different incidence pattern in China, compared with Western countries. Other risk factors, like adiposity, may have a stronger effect on the risk of colorectal cancer in Western countries than in China.” Notably, the mean body mass index in the study population was around 23 kg/m2, they said – by contrast in the United Kingdom it is 27.6 kg/m2.
Effects not necessarily causal
Ian Givens, PhD, professor of food chain nutrition at the University of Reading (England), said the study was “potentially very important for Chinese people, if it can be confirmed that dairy products affect the risk of breast and/or liver cancer differently in Chinese subjects to those in Western Societies, especially as dairy consumption in China is much lower than in most Western diets.”
He added: “As always it needs to be kept in mind that this type of study can only establish associations with disease risk, not cause.”
Dr. Kakkoura, nutritional epidemiologist at Oxford (England) University’s department of population health, said: “This was the first major study to investigate the link between dairy products and cancer risk in a Chinese population. Further studies are needed to validate these current findings, establish if these associations are causal, and investigate the potential underlying mechanisms involved.”
The researchers said that, while the results do not prove causation, “there are several plausible biological mechanisms that may explain these associations.” They pointed to higher dairy consumption potentially increasing levels of insulinlike growth factor-I, known to promote cell proliferation and associated with higher risks of several types of cancer.
In addition, estrogen and progesterone present in cows’ milk may play a role in increasing breast cancer risk, whilst saturated and trans-fatty acids from dairy products may increase the risk of liver cancer. As many Chinese people are lactase deficient, dairy products may also be broken down into products that affect cancer risk.
No justification for dietary change
Confounding factors may also have influenced the results, commented Duane Mellor, PhD, RD, RNutr, registered dietitian and senior teaching fellow at Aston University, Birmingham, England. “Those in the study who consumed dairy were more likely to live in cities and have other health conditions, including cardiovascular disease and diabetes – although some of these factors were considered in the analysis, not all of these covariates were, which could influence the findings.
“In my view this study alone does not provide strong evidence that reducing dairy intake would reduce cancer risk.”
He added: “Although the paper suggests a 12% increased relative risk for female breast cancer, this does not equate to 12 more cases per 100 individuals – in absolute terms this would be more like 1 or 2 cases per 1,000 people.”
Similarly, Kevin McConway, PhD, emeritus professor of applied statistics at the Open University, Milton Keynes, England, said: “An issue is that there were many differences between the people that consumed different amounts of dairy products, apart from their difference in dairy consumption. For instance, of those who never or rarely consumed dairy products, fewer than a third lived in urban areas, but of regular dairy consumers (at least once a week), 83% lived in urban areas. Regular consumers were considerably more likely to be well educated than those who never or rarely consumed dairy products, and there were other differences too.
“So if, as the researchers found, a greater proportion of the regular consumers than of the never or rare consumers had a cancer diagnosis, that could have been because of their different dairy consumption, or it could have been (in part or entirely) because of the different places they lived, or their different education levels, or any of the other factors on which the groups differed.
“One can never be sure that all the relevant factors have been adjusted for. That’s why the researchers rightly say that these results can’t establish whether the associations between dairy consumption and the risks of some cancers, that they found, are there because the dairy consumption differences change the cancer risks in a cause-and-effect way. They might, or they might not.”
He cautioned: “I don’t think anyone should decide to change their individual diet solely because of the results of this new study.”
Commenting on the study, Fiona Osgun, senior health information manager at Cancer Research UK, London, told this news organization: “This early-stage study found an association between dairy consumption and the risks of certain cancers, but that doesn’t mean that they’re causing them or that people need to avoid dairy. Dairy products can be part of a healthy balanced diet and, in the U.K., the Food Standards Agency regulates them to make sure they’re safe. There’s good evidence that dairy reduces the risk of bowel cancer, but no clear evidence for other cancer types, and this is no different for people who are lactose intolerant.”
A version of this article first appeared on Medscape UK.
A relationship between consumption of dairy products and risk of various cancers has been intensively investigated in the past but yielded inconclusive or conflicting results.
The study, by researchers from Oxford University’s department of population health, and Peking University and the Chinese Academy of Medical Sciences in Beijing, used data from the China Kadoorie Biobank Study, a long-term prospective study involving more than over 510,000 participants recruited from 10 geographically diverse areas across China, including both rural and urban regions. They compared this to data from the UK biobank.
Subjects were 59% female, 41% male, aged 30-79 years, and had no history of cancer at recruitment between 2004 and 2008. Food questionnaires were completed at the outset and participants followed for an average of 11 years, using national cancer and death registries and health insurance records to identify new cancer diagnoses, including both fatal and nonfatal events.
Participants were categorized into three groups according to how often they consumed dairy products (primarily milk):
- Regular consumers (at least once a week): 20.4% of the cohort.
- Monthly consumers: 11.1%.
- Nonconsumers who never or rarely consumed dairy products: 68.5%.
Average dairy consumption was 37.9 g/day overall and 80.8 g/day among regular consumers. This compares with an average consumption of around 300 g/day in participants in the UK Biobank cohort.
Over the course of the study, 29,277 new cancer cases were recorded, including 6,282 lung, 2,582 female breast, 3,577 stomach, 3,350 colorectal, and 3,191 liver cancer cases.
Analyses correlating cases with consumption took into account a range of other factors potentially affecting cancer risk, including age, sex, region, family history of cancer, socioeconomic status (education and income), lifestyle factors (alcohol intake, smoking, physical activity, soy consumption, and fresh fruit intake), body mass index, chronic hepatitis B virus infection, and female reproductive factors.
Higher dairy intakes linked with risk of liver and breast cancers
Results revealed that higher regular dairy intake was associated with significantly higher risks of liver cancer and female breast cancer, both common types of cancer in China. Analyses indicated that for each 50-g/day intake, the risks increased by 12% and 17%, respectively.
There was also an increase in total cancer diagnoses, and an increased risk of lymphoma, though this was not statistically significant after correction for confounders. No association was found between dairy products and colorectal cancer, prostate cancer, or any other site-specific cancer.
The research, published in BMC Medicine, is the first major study to investigate dairy consumption and cancer risk in Chinese adults. The results conflict with previous studies on Western populations, which have suggested that dairy products may be associated with a lower risk of colorectal cancer and a higher risk of prostate cancer but have found no clear link for breast or other types of cancer.
Lead researchers Maria Kakkoura, PhD, MSc, and associate professor Huaidong Du, MD, PhD, told this news organization that, although they don’t know the reason for the difference, “there is clear evidence that colorectal cancer has a different incidence pattern in China, compared with Western countries. Other risk factors, like adiposity, may have a stronger effect on the risk of colorectal cancer in Western countries than in China.” Notably, the mean body mass index in the study population was around 23 kg/m2, they said – by contrast in the United Kingdom it is 27.6 kg/m2.
Effects not necessarily causal
Ian Givens, PhD, professor of food chain nutrition at the University of Reading (England), said the study was “potentially very important for Chinese people, if it can be confirmed that dairy products affect the risk of breast and/or liver cancer differently in Chinese subjects to those in Western Societies, especially as dairy consumption in China is much lower than in most Western diets.”
He added: “As always it needs to be kept in mind that this type of study can only establish associations with disease risk, not cause.”
Dr. Kakkoura, nutritional epidemiologist at Oxford (England) University’s department of population health, said: “This was the first major study to investigate the link between dairy products and cancer risk in a Chinese population. Further studies are needed to validate these current findings, establish if these associations are causal, and investigate the potential underlying mechanisms involved.”
The researchers said that, while the results do not prove causation, “there are several plausible biological mechanisms that may explain these associations.” They pointed to higher dairy consumption potentially increasing levels of insulinlike growth factor-I, known to promote cell proliferation and associated with higher risks of several types of cancer.
In addition, estrogen and progesterone present in cows’ milk may play a role in increasing breast cancer risk, whilst saturated and trans-fatty acids from dairy products may increase the risk of liver cancer. As many Chinese people are lactase deficient, dairy products may also be broken down into products that affect cancer risk.
No justification for dietary change
Confounding factors may also have influenced the results, commented Duane Mellor, PhD, RD, RNutr, registered dietitian and senior teaching fellow at Aston University, Birmingham, England. “Those in the study who consumed dairy were more likely to live in cities and have other health conditions, including cardiovascular disease and diabetes – although some of these factors were considered in the analysis, not all of these covariates were, which could influence the findings.
“In my view this study alone does not provide strong evidence that reducing dairy intake would reduce cancer risk.”
He added: “Although the paper suggests a 12% increased relative risk for female breast cancer, this does not equate to 12 more cases per 100 individuals – in absolute terms this would be more like 1 or 2 cases per 1,000 people.”
Similarly, Kevin McConway, PhD, emeritus professor of applied statistics at the Open University, Milton Keynes, England, said: “An issue is that there were many differences between the people that consumed different amounts of dairy products, apart from their difference in dairy consumption. For instance, of those who never or rarely consumed dairy products, fewer than a third lived in urban areas, but of regular dairy consumers (at least once a week), 83% lived in urban areas. Regular consumers were considerably more likely to be well educated than those who never or rarely consumed dairy products, and there were other differences too.
“So if, as the researchers found, a greater proportion of the regular consumers than of the never or rare consumers had a cancer diagnosis, that could have been because of their different dairy consumption, or it could have been (in part or entirely) because of the different places they lived, or their different education levels, or any of the other factors on which the groups differed.
“One can never be sure that all the relevant factors have been adjusted for. That’s why the researchers rightly say that these results can’t establish whether the associations between dairy consumption and the risks of some cancers, that they found, are there because the dairy consumption differences change the cancer risks in a cause-and-effect way. They might, or they might not.”
He cautioned: “I don’t think anyone should decide to change their individual diet solely because of the results of this new study.”
Commenting on the study, Fiona Osgun, senior health information manager at Cancer Research UK, London, told this news organization: “This early-stage study found an association between dairy consumption and the risks of certain cancers, but that doesn’t mean that they’re causing them or that people need to avoid dairy. Dairy products can be part of a healthy balanced diet and, in the U.K., the Food Standards Agency regulates them to make sure they’re safe. There’s good evidence that dairy reduces the risk of bowel cancer, but no clear evidence for other cancer types, and this is no different for people who are lactose intolerant.”
A version of this article first appeared on Medscape UK.
FROM BMC MEDICINE
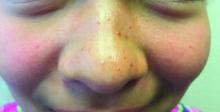
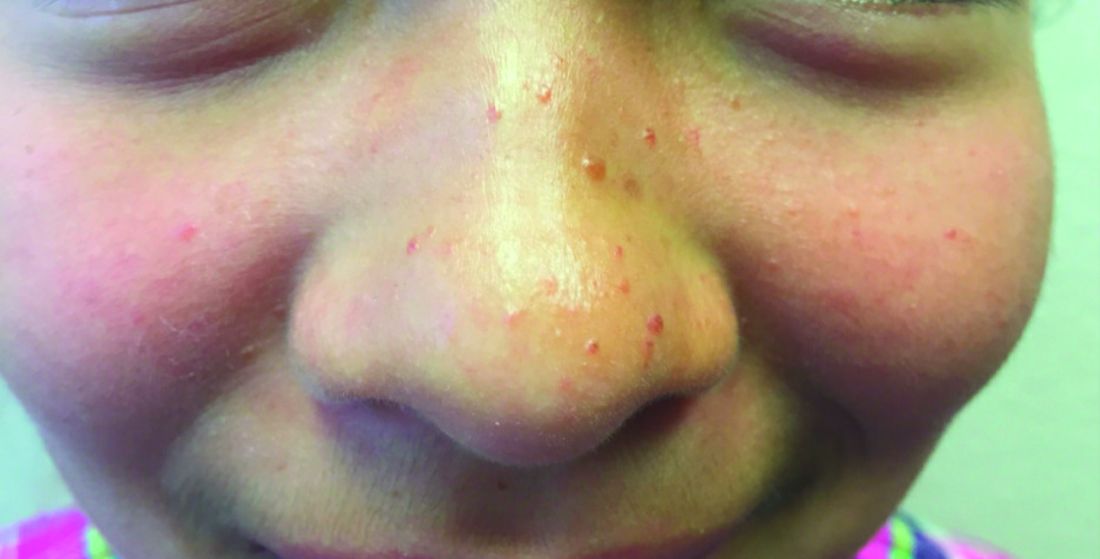
A 7-year-old with red bumps on her nose
The finding of individual, 1- to 4-mm firm, red papules depicted in the image are consistent with facial angiofibromas, which are most commonly seen in pediatric patients as a manifestation of tuberous sclerosis (TSC). Angiofibromas, previously called adenoma sebaceum, a misnomer, are seen in TSC as smooth papules, nodules, and occasionally plaques that typically involve the malar region of the face. These lesions usually develop in childhood and adolescence and can be misdiagnosed as lesions of acne. The number of lesions tend to increase with age, though there is no significant risk of malignant transformation. Ultraviolet-induced DNA damage is thought to play a role in the development of facial angiofibromas, so sun protection is called for.1 Patients may seek treatment to minimize deformity and the stigma of angiofibromas. Recently, the mammalian target of rapamycin inhibitor sirolimus (rapamycin) topical gel received Food and Drug Administration approval for the treatment of facial angiofibromas associated with TSC in patients age at least 6 years.2
The presence of angiofibromas should prompt consideration of TSC and as such, a thorough family history, medical history, and full-body skin examination. TSC is a rare autosomal-dominant genetic disorder, caused by a pathogenic variant in either the TSC1 or TSC2 gene. This neurocutaneous disorder is characterized by the development of multiple benign hamartomas across many organ systems including the brain, eyes, heart, lung, liver, kidney, and skin. The phenotypic expression of TSC is highly variable. Besides angiofibromas, some other characteristic dermatological findings in TSC include periungual fibromas, hypopigmented macules usually elliptical in shape (known as ash-leaf spots), and irregularly shaped elevated flesh-colored fibrous tissue most often found over the lower back (known as shagreen patches).3
What is on the differential?
Agminated spitz nevi refers to multiple spitz nevi in a localized area. Spitz nevi present as a well-circumscribed, dome-shaped, pink-red or brown papules, most commonly located on the face or lower extremities.4 The finding of agminated spitz nevi is very rare and less likely for this patient given the concomitant skin findings of dental pitting, renal cysts, and cortical tubers.
Juvenile xanthogranulomas are benign,proliferations of histiocytic cells that present as reddish or yellowish-to-brown papules, plaques, or nodules that typically develop in young children around the age of 1. With time, juvenile xanthogranulomas may flatten and become more yellow.
Basal cell carcinomas present as dome-shaped lesions with centralized erosions on sun-exposed areas of the skin. They are remarkably uncommon in children but are occasionally seen in basal cell nevus syndrome (also known as nevoid basal cell carcinoma syndrome or Gorlin syndrome). Affected patients may have other findings such as developmental anomalies, bifid ribs, palmar and plantar pitting, odontogenic keratocysts, and/or medulloblastomas.5
Flat warts commonly occur in children and occur by direct skin contact with human papillomavirus. Of the various types of warts, flat warts are smaller and tend to be smooth on top. The diagnosis of cutaneous warts is based on clinical appearance, showing thrombosed capillaries underneath the overlying hyperkeratotic debris. Our patient’s history of having a common wart on her hands raises suspicion for inoculation onto her face, but the morphology, distribution, and lack of response to tretinoin makes this diagnosis much less likely.
Disease workup and course
Our patient’s physical exam revealed dental pits but no evidence of hypopigmented macules, shagreen patches, or periungual lesions. Ultrasound of the kidney displayed renal cortical cysts and brain MRI showed cortical tubers, confirming extracutaneous TSC involvement. Over time, our patient developed angiofibromas on the forehead and was ultimately started on topical sirolimus, which led to marked improvement within months.
Ms. Kleinman is a pediatric dermatology research associate, division of pediatric and adolescent dermatology, University of California, San Diego, and Rady Children’s Hospital, also in San Diego. Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital. They have no relevant financial disclosures.
References
1. Tyburczy ME et al. Hum Molec Genet. 2014;23(8):2023-9.
2. Food & Drug Administration. New drug application (NDA) approval for Hyftor (sirolimus topical gel). https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2022/213478Orig1s000ltr.pdf.
3. Webb DW et al. Br J Dermatol. 1996;135(1):1-5.
4. Ricci F et al. Eur J Dermatol. 2017;27(1):59-62.
5. Evans DG and Farndon PA. Nevoid basal cell carcinoma syndrome, in “GeneReviews®.” Seattle: University of Washington, 2002.
The finding of individual, 1- to 4-mm firm, red papules depicted in the image are consistent with facial angiofibromas, which are most commonly seen in pediatric patients as a manifestation of tuberous sclerosis (TSC). Angiofibromas, previously called adenoma sebaceum, a misnomer, are seen in TSC as smooth papules, nodules, and occasionally plaques that typically involve the malar region of the face. These lesions usually develop in childhood and adolescence and can be misdiagnosed as lesions of acne. The number of lesions tend to increase with age, though there is no significant risk of malignant transformation. Ultraviolet-induced DNA damage is thought to play a role in the development of facial angiofibromas, so sun protection is called for.1 Patients may seek treatment to minimize deformity and the stigma of angiofibromas. Recently, the mammalian target of rapamycin inhibitor sirolimus (rapamycin) topical gel received Food and Drug Administration approval for the treatment of facial angiofibromas associated with TSC in patients age at least 6 years.2
The presence of angiofibromas should prompt consideration of TSC and as such, a thorough family history, medical history, and full-body skin examination. TSC is a rare autosomal-dominant genetic disorder, caused by a pathogenic variant in either the TSC1 or TSC2 gene. This neurocutaneous disorder is characterized by the development of multiple benign hamartomas across many organ systems including the brain, eyes, heart, lung, liver, kidney, and skin. The phenotypic expression of TSC is highly variable. Besides angiofibromas, some other characteristic dermatological findings in TSC include periungual fibromas, hypopigmented macules usually elliptical in shape (known as ash-leaf spots), and irregularly shaped elevated flesh-colored fibrous tissue most often found over the lower back (known as shagreen patches).3
What is on the differential?
Agminated spitz nevi refers to multiple spitz nevi in a localized area. Spitz nevi present as a well-circumscribed, dome-shaped, pink-red or brown papules, most commonly located on the face or lower extremities.4 The finding of agminated spitz nevi is very rare and less likely for this patient given the concomitant skin findings of dental pitting, renal cysts, and cortical tubers.
Juvenile xanthogranulomas are benign,proliferations of histiocytic cells that present as reddish or yellowish-to-brown papules, plaques, or nodules that typically develop in young children around the age of 1. With time, juvenile xanthogranulomas may flatten and become more yellow.
Basal cell carcinomas present as dome-shaped lesions with centralized erosions on sun-exposed areas of the skin. They are remarkably uncommon in children but are occasionally seen in basal cell nevus syndrome (also known as nevoid basal cell carcinoma syndrome or Gorlin syndrome). Affected patients may have other findings such as developmental anomalies, bifid ribs, palmar and plantar pitting, odontogenic keratocysts, and/or medulloblastomas.5
Flat warts commonly occur in children and occur by direct skin contact with human papillomavirus. Of the various types of warts, flat warts are smaller and tend to be smooth on top. The diagnosis of cutaneous warts is based on clinical appearance, showing thrombosed capillaries underneath the overlying hyperkeratotic debris. Our patient’s history of having a common wart on her hands raises suspicion for inoculation onto her face, but the morphology, distribution, and lack of response to tretinoin makes this diagnosis much less likely.
Disease workup and course
Our patient’s physical exam revealed dental pits but no evidence of hypopigmented macules, shagreen patches, or periungual lesions. Ultrasound of the kidney displayed renal cortical cysts and brain MRI showed cortical tubers, confirming extracutaneous TSC involvement. Over time, our patient developed angiofibromas on the forehead and was ultimately started on topical sirolimus, which led to marked improvement within months.
Ms. Kleinman is a pediatric dermatology research associate, division of pediatric and adolescent dermatology, University of California, San Diego, and Rady Children’s Hospital, also in San Diego. Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital. They have no relevant financial disclosures.
References
1. Tyburczy ME et al. Hum Molec Genet. 2014;23(8):2023-9.
2. Food & Drug Administration. New drug application (NDA) approval for Hyftor (sirolimus topical gel). https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2022/213478Orig1s000ltr.pdf.
3. Webb DW et al. Br J Dermatol. 1996;135(1):1-5.
4. Ricci F et al. Eur J Dermatol. 2017;27(1):59-62.
5. Evans DG and Farndon PA. Nevoid basal cell carcinoma syndrome, in “GeneReviews®.” Seattle: University of Washington, 2002.
The finding of individual, 1- to 4-mm firm, red papules depicted in the image are consistent with facial angiofibromas, which are most commonly seen in pediatric patients as a manifestation of tuberous sclerosis (TSC). Angiofibromas, previously called adenoma sebaceum, a misnomer, are seen in TSC as smooth papules, nodules, and occasionally plaques that typically involve the malar region of the face. These lesions usually develop in childhood and adolescence and can be misdiagnosed as lesions of acne. The number of lesions tend to increase with age, though there is no significant risk of malignant transformation. Ultraviolet-induced DNA damage is thought to play a role in the development of facial angiofibromas, so sun protection is called for.1 Patients may seek treatment to minimize deformity and the stigma of angiofibromas. Recently, the mammalian target of rapamycin inhibitor sirolimus (rapamycin) topical gel received Food and Drug Administration approval for the treatment of facial angiofibromas associated with TSC in patients age at least 6 years.2
The presence of angiofibromas should prompt consideration of TSC and as such, a thorough family history, medical history, and full-body skin examination. TSC is a rare autosomal-dominant genetic disorder, caused by a pathogenic variant in either the TSC1 or TSC2 gene. This neurocutaneous disorder is characterized by the development of multiple benign hamartomas across many organ systems including the brain, eyes, heart, lung, liver, kidney, and skin. The phenotypic expression of TSC is highly variable. Besides angiofibromas, some other characteristic dermatological findings in TSC include periungual fibromas, hypopigmented macules usually elliptical in shape (known as ash-leaf spots), and irregularly shaped elevated flesh-colored fibrous tissue most often found over the lower back (known as shagreen patches).3
What is on the differential?
Agminated spitz nevi refers to multiple spitz nevi in a localized area. Spitz nevi present as a well-circumscribed, dome-shaped, pink-red or brown papules, most commonly located on the face or lower extremities.4 The finding of agminated spitz nevi is very rare and less likely for this patient given the concomitant skin findings of dental pitting, renal cysts, and cortical tubers.
Juvenile xanthogranulomas are benign,proliferations of histiocytic cells that present as reddish or yellowish-to-brown papules, plaques, or nodules that typically develop in young children around the age of 1. With time, juvenile xanthogranulomas may flatten and become more yellow.
Basal cell carcinomas present as dome-shaped lesions with centralized erosions on sun-exposed areas of the skin. They are remarkably uncommon in children but are occasionally seen in basal cell nevus syndrome (also known as nevoid basal cell carcinoma syndrome or Gorlin syndrome). Affected patients may have other findings such as developmental anomalies, bifid ribs, palmar and plantar pitting, odontogenic keratocysts, and/or medulloblastomas.5
Flat warts commonly occur in children and occur by direct skin contact with human papillomavirus. Of the various types of warts, flat warts are smaller and tend to be smooth on top. The diagnosis of cutaneous warts is based on clinical appearance, showing thrombosed capillaries underneath the overlying hyperkeratotic debris. Our patient’s history of having a common wart on her hands raises suspicion for inoculation onto her face, but the morphology, distribution, and lack of response to tretinoin makes this diagnosis much less likely.
Disease workup and course
Our patient’s physical exam revealed dental pits but no evidence of hypopigmented macules, shagreen patches, or periungual lesions. Ultrasound of the kidney displayed renal cortical cysts and brain MRI showed cortical tubers, confirming extracutaneous TSC involvement. Over time, our patient developed angiofibromas on the forehead and was ultimately started on topical sirolimus, which led to marked improvement within months.
Ms. Kleinman is a pediatric dermatology research associate, division of pediatric and adolescent dermatology, University of California, San Diego, and Rady Children’s Hospital, also in San Diego. Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital. They have no relevant financial disclosures.
References
1. Tyburczy ME et al. Hum Molec Genet. 2014;23(8):2023-9.
2. Food & Drug Administration. New drug application (NDA) approval for Hyftor (sirolimus topical gel). https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2022/213478Orig1s000ltr.pdf.
3. Webb DW et al. Br J Dermatol. 1996;135(1):1-5.
4. Ricci F et al. Eur J Dermatol. 2017;27(1):59-62.
5. Evans DG and Farndon PA. Nevoid basal cell carcinoma syndrome, in “GeneReviews®.” Seattle: University of Washington, 2002.
A 7-year-old female presented with a bump on the bridge of her nose that was present for 10 months, with subsequent development of multiple papules on the nose and cheeks.
A 7-year-old, previously healthy female presented with a bump on the bridge of her nose that was present for 10 months, with subsequent development of multiple papules on the nose and cheeks. She has no significant medical history aside from a wart on her hand that was recently frozen with liquid nitrogen and resolved. She denied pruritus, bumps, or skin changes elsewhere on the body. The patient was prescribed tretinoin 0.1% cream applied nightly for several months without response.
First fatty liver guidelines for endocrinology, primary care
New clinical practice guidelines for the diagnosis and management of nonalcoholic fatty liver disease (NAFLD) are the first to be targeted specifically to primary care and endocrinology clinical settings.
They include 34 evidence-based clinical practice recommendations for screening, diagnosis, management, and referral, presented in a table and an algorithm flow chart as well as detailed text.
The new guidelines are by the American Association of Clinical Endocrinology and cosponsored by the American Association for the Study of Liver Diseases. They were presented at the annual scientific & clinical congress of the AACE and simultaneously published in Endocrine Practice.
These are “the first of this type for this field of medicine. The vast majority of patients with NAFLD are being seen in the primary care and endocrinology settings. Only when they get to the more advanced disease are they being referred to the liver specialists. So, we need to be the ones who are diagnosing and managing these patients because there just aren’t enough liver specialists to do that,” Scott Isaacs, MD, cochair of the writing panel for the guidelines, said in an interview.
80 million Americans have NAFLD, but very few are aware
The spectrum of NAFLD ranges from nonprogressive steatosis to the progressive conditions nonalcoholic steatohepatitis, fibrotic NASH, and end-stage NASH cirrhosis. And NASH, in turn, is a major cause of liver cancer. NAFLD is also strongly associated with insulin resistance, type 2 diabetes, atherogenesis, and myocardial dysfunction.
The global prevalence of NAFLD is about 25% and NASH, about 12%-14%. However, a recent study found that, among patients in endocrine and primary care clinics, more than 70% of patients with type 2 diabetes and more than 90% with type 2 diabetes who had a body mass index above 35 kg/m2 also had NAFLD, and more than 20% of those patients had significant liver fibrosis.
Problematically, very few people are aware they have either. “It’s so common. At least 80 million Americans have this but only about 6% know they have it. We talk about it a lot, but it’s not talked about enough,” said Dr. Isaacs, an endocrinologist who practices in Atlanta.
In fact, most cases of NAFLD are diagnosed incidentally when people undergo an ultrasound or a CT scan for another reason. And, in about 70% of cases the liver enzymes are normal, and those patients rarely undergo liver workups, Dr. Isaacs noted.
In an accompanying editorial, Suthat Liangpunsakul, MD, wrote: “In my perspective, as a hepatologist, this AACE guideline is very practical and easy to incorporate into routine practice in primary care and endocrinology settings. ... Early identification and risk stratification of patients with NAFLD, especially the degree of hepatic fibrosis, are required to reduce downstream health care costs and triage unwarranted specialty care referrals.”
And “an effective screening strategy may also identify those in primary care and endocrinology settings who may benefit from an appropriate referral to hepatologists before the development of portal hypertension complications, decompensated liver disease, and hepatocellular carcinoma,” added Dr. Liangpunsakul, professor of medicine in the division of gastroenterology and hepatology at Indiana University, Indianapolis.
Screening advised using new FIB-4 test
The guideline calls for screening all patients at high risk for NAFLD, including those with prediabetes, type 2 diabetes, obesity, and/or two or more cardiometabolic risk factors, or those with hepatic steatosis found on imaging, and/or persistently elevated plasma aminotransferase levels (that is, for more than 6 months).
The recommended screening test is the Fibrosis-4 (FIB-4) index, calculated using the patient’s age, AST level, platelet count, and ALT level: FIB-4 score = age (years) x AST (U/L)/PLT (109/L) x ALT ½ (U/L).
Recently approved by the Food and Drug Administration, the FIB-4 has been demonstrated to help identify liver disease in primary care settings.
“We really want to encourage clinicians to do the screening. The first step is the FIB-4 test. It’s a mathematical calculation using blood tests that we do anyway,” Dr. Isaacs said in an interview.
The FIB-4 stratifies patients as being low, intermediate, or high risk for liver fibrosis. Those at low risk can be managed in primary care or endocrinology settings with a focus on obesity management and cardiovascular disease prevention. “Those at low risk on FIB-4 still have a high cardiovascular disease risk. They still need to be managed,” Dr. Isaacs observed.
For those at intermediate risk, a second noninvasive test – either a liver stiffness measurement by elastography or an enhanced liver fibrosis test – is advised. If the patient is found to be at high risk or is still indeterminant after two noninvasive tests, referral to a liver specialist for further testing, including possible biopsy, is advised.
Those found to be at high risk with the FIB-4 should also be referred to hepatology. In both the intermediate- and high-risk groups, management should be multidisciplinary, including a hepatologist, endocrinologist, and other professionals to prevent both cardiovascular disease and progression to cirrhosis, the guidelines say.
“The diagnosis isn’t about diagnosing liver fat. It’s about diagnosing fibrosis, or the risk for clinically significant fibrosis. That’s really where the challenge lies,” Dr. Isaacs commented.
NAFLD treatment in endocrinology and primary care: CVD prevention
During the presentation at the AACE meeting, guideline panel cochair Kenneth Cusi, MD, chief of endocrinology, diabetes, and metabolism at the University of Florida, Gainesville, summarized current and future treatments for NAFLD.
Lifestyle intervention, cardiovascular risk reduction, and weight loss for those who are overweight or obese are recommended for all patients with NAFLD, including structured weight-loss programs, antiobesity medications, and bariatric surgery if indicated.
There are currently no FDA-approved medications specifically for NASH, but pioglitazone, approved for type 2 diabetes, and glucagonlike peptide–1 agonists, approved for type 2 diabetes and weight loss, have been shown to be effective in treating the condition and preventing progression. Other treatments are in development, Dr. Cusi said.
The guideline also includes a section on diagnosis and management of NAFLD in children and adolescents. Here, the FIB-4 is not recommended because it isn’t accurate due to the age part of the equation, so liver enzyme tests are used in pediatric patients considered at high risk because of clinical factors. Management is similar to adults, except not all medications used in adults are approved for use in children.
In the editorial, Dr. Liangpunsakul cautioned that “the level of uptake and usage of the guideline may be an obstacle.”
To remedy that, he advised that “the next effort should gear toward distributing this guideline to the targeted providers and developing the ‘feedback platforms’ on its execution in the real-world. ... The successful implementation of this AACE guideline by the primary care providers and endocrinologists, hopefully, will deescalate the future burden of NAFLD-related morbidity and mortality.”
Dr. Isaacs and Dr. Liangpunsakul have reported no relevant financial relationships. Dr. Cusi has reported receiving research support towards the University of Florida as principal investigator from the National Institute of Health, Echosens, Inventiva, Nordic Bioscience, Novo Nordisk, Poxel, Labcorp, and Zydus, and is a consultant for Altimmune, Akero, Arrowhead, AstraZeneca, 89Bio, Bristol-Myers Squibb, Coherus, Intercept, Lilly, Madrigal, Merck, Novo Nordisk, Quest, Sagimet, Sonic Incytes, Terns, and Thera Technologies.
A version of this article first appeared on Medscape.com.
New clinical practice guidelines for the diagnosis and management of nonalcoholic fatty liver disease (NAFLD) are the first to be targeted specifically to primary care and endocrinology clinical settings.
They include 34 evidence-based clinical practice recommendations for screening, diagnosis, management, and referral, presented in a table and an algorithm flow chart as well as detailed text.
The new guidelines are by the American Association of Clinical Endocrinology and cosponsored by the American Association for the Study of Liver Diseases. They were presented at the annual scientific & clinical congress of the AACE and simultaneously published in Endocrine Practice.
These are “the first of this type for this field of medicine. The vast majority of patients with NAFLD are being seen in the primary care and endocrinology settings. Only when they get to the more advanced disease are they being referred to the liver specialists. So, we need to be the ones who are diagnosing and managing these patients because there just aren’t enough liver specialists to do that,” Scott Isaacs, MD, cochair of the writing panel for the guidelines, said in an interview.
80 million Americans have NAFLD, but very few are aware
The spectrum of NAFLD ranges from nonprogressive steatosis to the progressive conditions nonalcoholic steatohepatitis, fibrotic NASH, and end-stage NASH cirrhosis. And NASH, in turn, is a major cause of liver cancer. NAFLD is also strongly associated with insulin resistance, type 2 diabetes, atherogenesis, and myocardial dysfunction.
The global prevalence of NAFLD is about 25% and NASH, about 12%-14%. However, a recent study found that, among patients in endocrine and primary care clinics, more than 70% of patients with type 2 diabetes and more than 90% with type 2 diabetes who had a body mass index above 35 kg/m2 also had NAFLD, and more than 20% of those patients had significant liver fibrosis.
Problematically, very few people are aware they have either. “It’s so common. At least 80 million Americans have this but only about 6% know they have it. We talk about it a lot, but it’s not talked about enough,” said Dr. Isaacs, an endocrinologist who practices in Atlanta.
In fact, most cases of NAFLD are diagnosed incidentally when people undergo an ultrasound or a CT scan for another reason. And, in about 70% of cases the liver enzymes are normal, and those patients rarely undergo liver workups, Dr. Isaacs noted.
In an accompanying editorial, Suthat Liangpunsakul, MD, wrote: “In my perspective, as a hepatologist, this AACE guideline is very practical and easy to incorporate into routine practice in primary care and endocrinology settings. ... Early identification and risk stratification of patients with NAFLD, especially the degree of hepatic fibrosis, are required to reduce downstream health care costs and triage unwarranted specialty care referrals.”
And “an effective screening strategy may also identify those in primary care and endocrinology settings who may benefit from an appropriate referral to hepatologists before the development of portal hypertension complications, decompensated liver disease, and hepatocellular carcinoma,” added Dr. Liangpunsakul, professor of medicine in the division of gastroenterology and hepatology at Indiana University, Indianapolis.
Screening advised using new FIB-4 test
The guideline calls for screening all patients at high risk for NAFLD, including those with prediabetes, type 2 diabetes, obesity, and/or two or more cardiometabolic risk factors, or those with hepatic steatosis found on imaging, and/or persistently elevated plasma aminotransferase levels (that is, for more than 6 months).
The recommended screening test is the Fibrosis-4 (FIB-4) index, calculated using the patient’s age, AST level, platelet count, and ALT level: FIB-4 score = age (years) x AST (U/L)/PLT (109/L) x ALT ½ (U/L).
Recently approved by the Food and Drug Administration, the FIB-4 has been demonstrated to help identify liver disease in primary care settings.
“We really want to encourage clinicians to do the screening. The first step is the FIB-4 test. It’s a mathematical calculation using blood tests that we do anyway,” Dr. Isaacs said in an interview.
The FIB-4 stratifies patients as being low, intermediate, or high risk for liver fibrosis. Those at low risk can be managed in primary care or endocrinology settings with a focus on obesity management and cardiovascular disease prevention. “Those at low risk on FIB-4 still have a high cardiovascular disease risk. They still need to be managed,” Dr. Isaacs observed.
For those at intermediate risk, a second noninvasive test – either a liver stiffness measurement by elastography or an enhanced liver fibrosis test – is advised. If the patient is found to be at high risk or is still indeterminant after two noninvasive tests, referral to a liver specialist for further testing, including possible biopsy, is advised.
Those found to be at high risk with the FIB-4 should also be referred to hepatology. In both the intermediate- and high-risk groups, management should be multidisciplinary, including a hepatologist, endocrinologist, and other professionals to prevent both cardiovascular disease and progression to cirrhosis, the guidelines say.
“The diagnosis isn’t about diagnosing liver fat. It’s about diagnosing fibrosis, or the risk for clinically significant fibrosis. That’s really where the challenge lies,” Dr. Isaacs commented.
NAFLD treatment in endocrinology and primary care: CVD prevention
During the presentation at the AACE meeting, guideline panel cochair Kenneth Cusi, MD, chief of endocrinology, diabetes, and metabolism at the University of Florida, Gainesville, summarized current and future treatments for NAFLD.
Lifestyle intervention, cardiovascular risk reduction, and weight loss for those who are overweight or obese are recommended for all patients with NAFLD, including structured weight-loss programs, antiobesity medications, and bariatric surgery if indicated.
There are currently no FDA-approved medications specifically for NASH, but pioglitazone, approved for type 2 diabetes, and glucagonlike peptide–1 agonists, approved for type 2 diabetes and weight loss, have been shown to be effective in treating the condition and preventing progression. Other treatments are in development, Dr. Cusi said.
The guideline also includes a section on diagnosis and management of NAFLD in children and adolescents. Here, the FIB-4 is not recommended because it isn’t accurate due to the age part of the equation, so liver enzyme tests are used in pediatric patients considered at high risk because of clinical factors. Management is similar to adults, except not all medications used in adults are approved for use in children.
In the editorial, Dr. Liangpunsakul cautioned that “the level of uptake and usage of the guideline may be an obstacle.”
To remedy that, he advised that “the next effort should gear toward distributing this guideline to the targeted providers and developing the ‘feedback platforms’ on its execution in the real-world. ... The successful implementation of this AACE guideline by the primary care providers and endocrinologists, hopefully, will deescalate the future burden of NAFLD-related morbidity and mortality.”
Dr. Isaacs and Dr. Liangpunsakul have reported no relevant financial relationships. Dr. Cusi has reported receiving research support towards the University of Florida as principal investigator from the National Institute of Health, Echosens, Inventiva, Nordic Bioscience, Novo Nordisk, Poxel, Labcorp, and Zydus, and is a consultant for Altimmune, Akero, Arrowhead, AstraZeneca, 89Bio, Bristol-Myers Squibb, Coherus, Intercept, Lilly, Madrigal, Merck, Novo Nordisk, Quest, Sagimet, Sonic Incytes, Terns, and Thera Technologies.
A version of this article first appeared on Medscape.com.
New clinical practice guidelines for the diagnosis and management of nonalcoholic fatty liver disease (NAFLD) are the first to be targeted specifically to primary care and endocrinology clinical settings.
They include 34 evidence-based clinical practice recommendations for screening, diagnosis, management, and referral, presented in a table and an algorithm flow chart as well as detailed text.
The new guidelines are by the American Association of Clinical Endocrinology and cosponsored by the American Association for the Study of Liver Diseases. They were presented at the annual scientific & clinical congress of the AACE and simultaneously published in Endocrine Practice.
These are “the first of this type for this field of medicine. The vast majority of patients with NAFLD are being seen in the primary care and endocrinology settings. Only when they get to the more advanced disease are they being referred to the liver specialists. So, we need to be the ones who are diagnosing and managing these patients because there just aren’t enough liver specialists to do that,” Scott Isaacs, MD, cochair of the writing panel for the guidelines, said in an interview.
80 million Americans have NAFLD, but very few are aware
The spectrum of NAFLD ranges from nonprogressive steatosis to the progressive conditions nonalcoholic steatohepatitis, fibrotic NASH, and end-stage NASH cirrhosis. And NASH, in turn, is a major cause of liver cancer. NAFLD is also strongly associated with insulin resistance, type 2 diabetes, atherogenesis, and myocardial dysfunction.
The global prevalence of NAFLD is about 25% and NASH, about 12%-14%. However, a recent study found that, among patients in endocrine and primary care clinics, more than 70% of patients with type 2 diabetes and more than 90% with type 2 diabetes who had a body mass index above 35 kg/m2 also had NAFLD, and more than 20% of those patients had significant liver fibrosis.
Problematically, very few people are aware they have either. “It’s so common. At least 80 million Americans have this but only about 6% know they have it. We talk about it a lot, but it’s not talked about enough,” said Dr. Isaacs, an endocrinologist who practices in Atlanta.
In fact, most cases of NAFLD are diagnosed incidentally when people undergo an ultrasound or a CT scan for another reason. And, in about 70% of cases the liver enzymes are normal, and those patients rarely undergo liver workups, Dr. Isaacs noted.
In an accompanying editorial, Suthat Liangpunsakul, MD, wrote: “In my perspective, as a hepatologist, this AACE guideline is very practical and easy to incorporate into routine practice in primary care and endocrinology settings. ... Early identification and risk stratification of patients with NAFLD, especially the degree of hepatic fibrosis, are required to reduce downstream health care costs and triage unwarranted specialty care referrals.”
And “an effective screening strategy may also identify those in primary care and endocrinology settings who may benefit from an appropriate referral to hepatologists before the development of portal hypertension complications, decompensated liver disease, and hepatocellular carcinoma,” added Dr. Liangpunsakul, professor of medicine in the division of gastroenterology and hepatology at Indiana University, Indianapolis.
Screening advised using new FIB-4 test
The guideline calls for screening all patients at high risk for NAFLD, including those with prediabetes, type 2 diabetes, obesity, and/or two or more cardiometabolic risk factors, or those with hepatic steatosis found on imaging, and/or persistently elevated plasma aminotransferase levels (that is, for more than 6 months).
The recommended screening test is the Fibrosis-4 (FIB-4) index, calculated using the patient’s age, AST level, platelet count, and ALT level: FIB-4 score = age (years) x AST (U/L)/PLT (109/L) x ALT ½ (U/L).
Recently approved by the Food and Drug Administration, the FIB-4 has been demonstrated to help identify liver disease in primary care settings.
“We really want to encourage clinicians to do the screening. The first step is the FIB-4 test. It’s a mathematical calculation using blood tests that we do anyway,” Dr. Isaacs said in an interview.
The FIB-4 stratifies patients as being low, intermediate, or high risk for liver fibrosis. Those at low risk can be managed in primary care or endocrinology settings with a focus on obesity management and cardiovascular disease prevention. “Those at low risk on FIB-4 still have a high cardiovascular disease risk. They still need to be managed,” Dr. Isaacs observed.
For those at intermediate risk, a second noninvasive test – either a liver stiffness measurement by elastography or an enhanced liver fibrosis test – is advised. If the patient is found to be at high risk or is still indeterminant after two noninvasive tests, referral to a liver specialist for further testing, including possible biopsy, is advised.
Those found to be at high risk with the FIB-4 should also be referred to hepatology. In both the intermediate- and high-risk groups, management should be multidisciplinary, including a hepatologist, endocrinologist, and other professionals to prevent both cardiovascular disease and progression to cirrhosis, the guidelines say.
“The diagnosis isn’t about diagnosing liver fat. It’s about diagnosing fibrosis, or the risk for clinically significant fibrosis. That’s really where the challenge lies,” Dr. Isaacs commented.
NAFLD treatment in endocrinology and primary care: CVD prevention
During the presentation at the AACE meeting, guideline panel cochair Kenneth Cusi, MD, chief of endocrinology, diabetes, and metabolism at the University of Florida, Gainesville, summarized current and future treatments for NAFLD.
Lifestyle intervention, cardiovascular risk reduction, and weight loss for those who are overweight or obese are recommended for all patients with NAFLD, including structured weight-loss programs, antiobesity medications, and bariatric surgery if indicated.
There are currently no FDA-approved medications specifically for NASH, but pioglitazone, approved for type 2 diabetes, and glucagonlike peptide–1 agonists, approved for type 2 diabetes and weight loss, have been shown to be effective in treating the condition and preventing progression. Other treatments are in development, Dr. Cusi said.
The guideline also includes a section on diagnosis and management of NAFLD in children and adolescents. Here, the FIB-4 is not recommended because it isn’t accurate due to the age part of the equation, so liver enzyme tests are used in pediatric patients considered at high risk because of clinical factors. Management is similar to adults, except not all medications used in adults are approved for use in children.
In the editorial, Dr. Liangpunsakul cautioned that “the level of uptake and usage of the guideline may be an obstacle.”
To remedy that, he advised that “the next effort should gear toward distributing this guideline to the targeted providers and developing the ‘feedback platforms’ on its execution in the real-world. ... The successful implementation of this AACE guideline by the primary care providers and endocrinologists, hopefully, will deescalate the future burden of NAFLD-related morbidity and mortality.”
Dr. Isaacs and Dr. Liangpunsakul have reported no relevant financial relationships. Dr. Cusi has reported receiving research support towards the University of Florida as principal investigator from the National Institute of Health, Echosens, Inventiva, Nordic Bioscience, Novo Nordisk, Poxel, Labcorp, and Zydus, and is a consultant for Altimmune, Akero, Arrowhead, AstraZeneca, 89Bio, Bristol-Myers Squibb, Coherus, Intercept, Lilly, Madrigal, Merck, Novo Nordisk, Quest, Sagimet, Sonic Incytes, Terns, and Thera Technologies.
A version of this article first appeared on Medscape.com.
FROM AACE 2022
Mixing BP meds with NSAID may be ‘triple whammy’ for kidneys
The study also looked at risk factors associated with the effect of triple therapy with these agents, which has been called “triple whammy” AKI.
“It’s not that everyone who happens to take this combination of drugs is going to have problems,” Anita Layton, PhD, University of Waterloo, Ontario, said in a statement. “But the research shows it’s enough of a problem that you should exercise caution.”
The study was published online in Mathematical Biosciences.
In an earlier study, triple therapy with a diuretic, RAS inhibitor, and NSAID was associated with a 31% increased risk for AKI, relative to diuretic and RAS inhibitor therapy only.
However, the factors that predispose some patients to develop “triple whammy” AKI are unclear.
To better understand the mechanism by which triple therapy increases risk for AKI, Dr. Layton and colleagues used computational models to gauge interactions between concurrent use of a diuretic, a RAS inhibitor, and an NSAID.
They identified dehydration and high sensitivity to drug treatment as key contributing factors to the development of triple whammy AKI.
Their model simulations suggested that low water intake, the myogenic response (that is, the reflex response of arteries and arterioles to changes in blood pressure to maintain consistent blood flow), and drug sensitivity “may predispose patients with hypertension to develop triple whammy-induced AKI,” they wrote.
“We hypothesize that individuals with an impaired myogenic response may be particularly susceptible to triple whammy AKI. Additionally, increased drug sensitivity or low water intake can predispose patients to triple whammy AKI,” they added.
In the absence of additional risk factors, there was no indication of an elevated risk for AKI when an angiotensin-converting enzyme (ACE) inhibitor and NSAID are combined, the study team said.
In contrast, when an ACE inhibitor, diuretic, and NSAID are combined, critical blood pressure and glomerular filtration rate (GFR) regulatory mechanisms are simultaneously interrupted, they reported.
“Perhaps not unexpectedly, model simulations indicate that triple treatment reduces GFR more than single or double treatments in all individuals. However, under triple treatment, urine volume and GFR have not been predicted to fall sufficiently far to indicate AKI,” they wrote. “This result is consistent with the fact that only a fraction of individuals develop AKI following triple treatment.”
They expect, therefore, that hypertensive patients who are otherwise healthy will be able to withstand triple treatment, in the absence of these aggravating factors, the researchers concluded.
Nonetheless, it’s wise to “always be careful when mixing medications,” Dr. Layton told this news organization.
She noted that “triple whammy AKI is known among kidney researchers and nephrologists. To what extent nonspecialists are aware, it isn’t clear.
“More importantly,” Dr. Layton said, “NSAIDs can be obtained over the counter, and triple whammy AKI isn’t common knowledge outside of the medical community.”
This research was supported by the Canada 150 Research Chair program and by the Natural Sciences and Engineering Research Council of Canada. The authors have declared no conflicts of interest.
A version of this article first appeared on Medscape.com.
The study also looked at risk factors associated with the effect of triple therapy with these agents, which has been called “triple whammy” AKI.
“It’s not that everyone who happens to take this combination of drugs is going to have problems,” Anita Layton, PhD, University of Waterloo, Ontario, said in a statement. “But the research shows it’s enough of a problem that you should exercise caution.”
The study was published online in Mathematical Biosciences.
In an earlier study, triple therapy with a diuretic, RAS inhibitor, and NSAID was associated with a 31% increased risk for AKI, relative to diuretic and RAS inhibitor therapy only.
However, the factors that predispose some patients to develop “triple whammy” AKI are unclear.
To better understand the mechanism by which triple therapy increases risk for AKI, Dr. Layton and colleagues used computational models to gauge interactions between concurrent use of a diuretic, a RAS inhibitor, and an NSAID.
They identified dehydration and high sensitivity to drug treatment as key contributing factors to the development of triple whammy AKI.
Their model simulations suggested that low water intake, the myogenic response (that is, the reflex response of arteries and arterioles to changes in blood pressure to maintain consistent blood flow), and drug sensitivity “may predispose patients with hypertension to develop triple whammy-induced AKI,” they wrote.
“We hypothesize that individuals with an impaired myogenic response may be particularly susceptible to triple whammy AKI. Additionally, increased drug sensitivity or low water intake can predispose patients to triple whammy AKI,” they added.
In the absence of additional risk factors, there was no indication of an elevated risk for AKI when an angiotensin-converting enzyme (ACE) inhibitor and NSAID are combined, the study team said.
In contrast, when an ACE inhibitor, diuretic, and NSAID are combined, critical blood pressure and glomerular filtration rate (GFR) regulatory mechanisms are simultaneously interrupted, they reported.
“Perhaps not unexpectedly, model simulations indicate that triple treatment reduces GFR more than single or double treatments in all individuals. However, under triple treatment, urine volume and GFR have not been predicted to fall sufficiently far to indicate AKI,” they wrote. “This result is consistent with the fact that only a fraction of individuals develop AKI following triple treatment.”
They expect, therefore, that hypertensive patients who are otherwise healthy will be able to withstand triple treatment, in the absence of these aggravating factors, the researchers concluded.
Nonetheless, it’s wise to “always be careful when mixing medications,” Dr. Layton told this news organization.
She noted that “triple whammy AKI is known among kidney researchers and nephrologists. To what extent nonspecialists are aware, it isn’t clear.
“More importantly,” Dr. Layton said, “NSAIDs can be obtained over the counter, and triple whammy AKI isn’t common knowledge outside of the medical community.”
This research was supported by the Canada 150 Research Chair program and by the Natural Sciences and Engineering Research Council of Canada. The authors have declared no conflicts of interest.
A version of this article first appeared on Medscape.com.
The study also looked at risk factors associated with the effect of triple therapy with these agents, which has been called “triple whammy” AKI.
“It’s not that everyone who happens to take this combination of drugs is going to have problems,” Anita Layton, PhD, University of Waterloo, Ontario, said in a statement. “But the research shows it’s enough of a problem that you should exercise caution.”
The study was published online in Mathematical Biosciences.
In an earlier study, triple therapy with a diuretic, RAS inhibitor, and NSAID was associated with a 31% increased risk for AKI, relative to diuretic and RAS inhibitor therapy only.
However, the factors that predispose some patients to develop “triple whammy” AKI are unclear.
To better understand the mechanism by which triple therapy increases risk for AKI, Dr. Layton and colleagues used computational models to gauge interactions between concurrent use of a diuretic, a RAS inhibitor, and an NSAID.
They identified dehydration and high sensitivity to drug treatment as key contributing factors to the development of triple whammy AKI.
Their model simulations suggested that low water intake, the myogenic response (that is, the reflex response of arteries and arterioles to changes in blood pressure to maintain consistent blood flow), and drug sensitivity “may predispose patients with hypertension to develop triple whammy-induced AKI,” they wrote.
“We hypothesize that individuals with an impaired myogenic response may be particularly susceptible to triple whammy AKI. Additionally, increased drug sensitivity or low water intake can predispose patients to triple whammy AKI,” they added.
In the absence of additional risk factors, there was no indication of an elevated risk for AKI when an angiotensin-converting enzyme (ACE) inhibitor and NSAID are combined, the study team said.
In contrast, when an ACE inhibitor, diuretic, and NSAID are combined, critical blood pressure and glomerular filtration rate (GFR) regulatory mechanisms are simultaneously interrupted, they reported.
“Perhaps not unexpectedly, model simulations indicate that triple treatment reduces GFR more than single or double treatments in all individuals. However, under triple treatment, urine volume and GFR have not been predicted to fall sufficiently far to indicate AKI,” they wrote. “This result is consistent with the fact that only a fraction of individuals develop AKI following triple treatment.”
They expect, therefore, that hypertensive patients who are otherwise healthy will be able to withstand triple treatment, in the absence of these aggravating factors, the researchers concluded.
Nonetheless, it’s wise to “always be careful when mixing medications,” Dr. Layton told this news organization.
She noted that “triple whammy AKI is known among kidney researchers and nephrologists. To what extent nonspecialists are aware, it isn’t clear.
“More importantly,” Dr. Layton said, “NSAIDs can be obtained over the counter, and triple whammy AKI isn’t common knowledge outside of the medical community.”
This research was supported by the Canada 150 Research Chair program and by the Natural Sciences and Engineering Research Council of Canada. The authors have declared no conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM MATHEMATICAL BIOSCIENCES
Myositis guidelines aim to standardize adult and pediatric care
All patients with idiopathic inflammatory myopathies (IIM) should be screened for swallowing difficulties, according to the first evidence-based guideline to be produced.
The guideline, which has been developed by a working group of the British Society for Rheumatology (BSR), also advises that all diagnosed patients should have their myositis antibody levels checked and have their overall well-being assessed. Other recommendations for all patients include the use of glucocorticoids to reduce muscle inflammation and conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) for long-term treatment.
“Finally, now, we’re able to standardize the way we treat adults and children with IIM,” senior guideline author Hector Chinoy, PhD, said at the society’s annual meeting.
It has been a long labor of love, however, taking 4 years to get the guideline published, said Dr. Chinoy, professor of rheumatology and neuromuscular disease at the University of Manchester (England), and a consultant at Salford (England) Royal Hospital.
“We’re not covering diagnosis, classification, or the investigation of suspected IIM,” said Dr. Chinoy. Inclusion body myositis also is not included.
Altogether, there are 13 recommendations that have been developed using a PICO (patient or population, intervention, comparison, outcome) format, graded based on the quality of the available evidence, and then voted on by the working group members to give a score of the strength of agreement. Dr. Chinoy noted that there was a checklist included in the Supplementary Data section of the guideline to help follow the recommendations.
“The target audience for the guideline reflects the variety of clinicians caring for patients with IIM,” Dr. Chinoy said. So that is not just pediatric and adult rheumatologists, but also neurologists, dermatologists, respiratory physicians, oncologists, gastroenterologists, cardiologists, and of course other health care professionals. This includes rheumatology and neurology nurses, psychologists, speech and language therapists, and podiatrists, as well as rheumatology specialist pharmacists, physiotherapists, and occupational therapists.
With reference to the latter, Liza McCann, MBBS, who co-led the development of the guideline, said in a statement released by the BSR that the guideline “highlights the importance of exercise, led and monitored by specialist physiotherapists and occupational therapists.”
Dr. McCann, a consultant pediatric rheumatologist at Alder Hey Hospital, Liverpool, England, and Honorary Clinical Lecturer at the University of Liverpool, added that the guidelines also cover “the need to address psychological wellbeing as an integral part of treatment, in parallel with pharmacological therapies.”
Recommendation highlights
Some of the highlights of the recommendations include the use of high-dose glucocorticoids to manage skeletal muscle inflammation at the time of treatment induction, with specific guidance on the different doses to use in adults and in children. There also is guidance on the use of csDMARDs in both populations and what to use if there is refractory disease – with the strongest evidence supporting the use of intravenous immunoglobulin (IVIG) or cyclophosphamide, and possibly rituximab and abatacept.
“There is insufficient evidence to recommend JAK inhibition,” Dr. Chinoy said. The data search used to develop the guideline had a cutoff of October 2020, but even now there is only anecdotal evidence from case studies, he added.
Importantly, the guidelines recognize that childhood IIM differs from adult disease and call for children to be managed by pediatric specialists.
“Routine assessment of dysphagia should be considered in all patients,” Dr. Chinoy said, “so ask the question.” The recommendation is that a swallowing assessment should involve a speech and language therapist or gastroenterologist, and that IVIG be considered for active disease and dysphagia that is resistant to other treatments.
There also are recommendations to screen adult patients for interstitial lung disease, consider fracture risk, and screen adult patients for cancer if they have specific risk factors that include older age at onset, male gender, dysphagia, and rapid disease onset, among others.
Separate cancer screening guidelines on cards
“Around one in four patients with myositis will develop cancer within the 3 years either before or after myositis onset,” Alexander Oldroyd, MBChB, PhD, said in a separate presentation at the BSR annual meeting.
“It’s a hugely increased risk compared to the general population, and a great worry for patients,” he added. Exactly why there is an increased risk is not known, but “there’s a big link between the biological onset of cancer and myositis.”
Dr. Oldroyd, who is an NIHR Academic Clinical Lecturer at the University of Manchester in England and a coauthor of the BSR myositis guideline, is part of a special interest group set up by the International Myositis Assessment and Clinical Studies Group (IMACS) that is in the process of developing separate guidelines for cancer screening in people newly diagnosed with IIM.
The aim was to produce evidence-based recommendations that were both “pragmatic and practical,” that could help clinicians answer patient’s questions on their risk and how best and how often to screen them, Dr. Oldroyd explained. Importantly, IMACS has endeavored to create recommendations that should be applicable across different countries and health care systems.
“We had to acknowledge that there’s not a lot of evidence base there,” Dr. Oldroyd said, noting that he and colleagues conducted a systematic literature review and meta-analysis and used a Delphi process to draft 20 recommendations. These cover identifying risk factors for cancer in people with myositis and categorizing people into low, medium, and high-risk categories. The recommendations also cover what should constitute basic and enhanced screening, and how often someone should be screened.
Moreover, the authors make recommendations on the use of imaging modalities such as PET and CT scans, as well as upper and lower gastrointestinal endoscopy and naso-endoscopy.
“As rheumatologists, we don’t talk about cancer a lot,” Dr. Oldroyd said. “We pick up a lot of incidental cancers, but we don’t usually talk about cancer screening with patients.” That’s something that needs to change, he said.
“It’s important – just get it out in the open, talk to people about it,” Dr. Oldroyd said.
“Tell them what you’re wanting to do, how you’re wanting to investigate for it, clearly communicate their risk,” he said. “But also acknowledge the limited evidence as well, and clearly communicate the results.”
Dr. Chinoy acknowledged he had received fees for presentations (UCB, Biogen), consultancy (Alexion, Novartis, Eli Lilly, Orphazyme, AstraZeneca), or grant support (Eli Lilly, UCB) that had been paid via his institution for the purpose of furthering myositis research. Dr. Oldroyd had no conflicts of interest to disclose.
All patients with idiopathic inflammatory myopathies (IIM) should be screened for swallowing difficulties, according to the first evidence-based guideline to be produced.
The guideline, which has been developed by a working group of the British Society for Rheumatology (BSR), also advises that all diagnosed patients should have their myositis antibody levels checked and have their overall well-being assessed. Other recommendations for all patients include the use of glucocorticoids to reduce muscle inflammation and conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) for long-term treatment.
“Finally, now, we’re able to standardize the way we treat adults and children with IIM,” senior guideline author Hector Chinoy, PhD, said at the society’s annual meeting.
It has been a long labor of love, however, taking 4 years to get the guideline published, said Dr. Chinoy, professor of rheumatology and neuromuscular disease at the University of Manchester (England), and a consultant at Salford (England) Royal Hospital.
“We’re not covering diagnosis, classification, or the investigation of suspected IIM,” said Dr. Chinoy. Inclusion body myositis also is not included.
Altogether, there are 13 recommendations that have been developed using a PICO (patient or population, intervention, comparison, outcome) format, graded based on the quality of the available evidence, and then voted on by the working group members to give a score of the strength of agreement. Dr. Chinoy noted that there was a checklist included in the Supplementary Data section of the guideline to help follow the recommendations.
“The target audience for the guideline reflects the variety of clinicians caring for patients with IIM,” Dr. Chinoy said. So that is not just pediatric and adult rheumatologists, but also neurologists, dermatologists, respiratory physicians, oncologists, gastroenterologists, cardiologists, and of course other health care professionals. This includes rheumatology and neurology nurses, psychologists, speech and language therapists, and podiatrists, as well as rheumatology specialist pharmacists, physiotherapists, and occupational therapists.
With reference to the latter, Liza McCann, MBBS, who co-led the development of the guideline, said in a statement released by the BSR that the guideline “highlights the importance of exercise, led and monitored by specialist physiotherapists and occupational therapists.”
Dr. McCann, a consultant pediatric rheumatologist at Alder Hey Hospital, Liverpool, England, and Honorary Clinical Lecturer at the University of Liverpool, added that the guidelines also cover “the need to address psychological wellbeing as an integral part of treatment, in parallel with pharmacological therapies.”
Recommendation highlights
Some of the highlights of the recommendations include the use of high-dose glucocorticoids to manage skeletal muscle inflammation at the time of treatment induction, with specific guidance on the different doses to use in adults and in children. There also is guidance on the use of csDMARDs in both populations and what to use if there is refractory disease – with the strongest evidence supporting the use of intravenous immunoglobulin (IVIG) or cyclophosphamide, and possibly rituximab and abatacept.
“There is insufficient evidence to recommend JAK inhibition,” Dr. Chinoy said. The data search used to develop the guideline had a cutoff of October 2020, but even now there is only anecdotal evidence from case studies, he added.
Importantly, the guidelines recognize that childhood IIM differs from adult disease and call for children to be managed by pediatric specialists.
“Routine assessment of dysphagia should be considered in all patients,” Dr. Chinoy said, “so ask the question.” The recommendation is that a swallowing assessment should involve a speech and language therapist or gastroenterologist, and that IVIG be considered for active disease and dysphagia that is resistant to other treatments.
There also are recommendations to screen adult patients for interstitial lung disease, consider fracture risk, and screen adult patients for cancer if they have specific risk factors that include older age at onset, male gender, dysphagia, and rapid disease onset, among others.
Separate cancer screening guidelines on cards
“Around one in four patients with myositis will develop cancer within the 3 years either before or after myositis onset,” Alexander Oldroyd, MBChB, PhD, said in a separate presentation at the BSR annual meeting.
“It’s a hugely increased risk compared to the general population, and a great worry for patients,” he added. Exactly why there is an increased risk is not known, but “there’s a big link between the biological onset of cancer and myositis.”
Dr. Oldroyd, who is an NIHR Academic Clinical Lecturer at the University of Manchester in England and a coauthor of the BSR myositis guideline, is part of a special interest group set up by the International Myositis Assessment and Clinical Studies Group (IMACS) that is in the process of developing separate guidelines for cancer screening in people newly diagnosed with IIM.
The aim was to produce evidence-based recommendations that were both “pragmatic and practical,” that could help clinicians answer patient’s questions on their risk and how best and how often to screen them, Dr. Oldroyd explained. Importantly, IMACS has endeavored to create recommendations that should be applicable across different countries and health care systems.
“We had to acknowledge that there’s not a lot of evidence base there,” Dr. Oldroyd said, noting that he and colleagues conducted a systematic literature review and meta-analysis and used a Delphi process to draft 20 recommendations. These cover identifying risk factors for cancer in people with myositis and categorizing people into low, medium, and high-risk categories. The recommendations also cover what should constitute basic and enhanced screening, and how often someone should be screened.
Moreover, the authors make recommendations on the use of imaging modalities such as PET and CT scans, as well as upper and lower gastrointestinal endoscopy and naso-endoscopy.
“As rheumatologists, we don’t talk about cancer a lot,” Dr. Oldroyd said. “We pick up a lot of incidental cancers, but we don’t usually talk about cancer screening with patients.” That’s something that needs to change, he said.
“It’s important – just get it out in the open, talk to people about it,” Dr. Oldroyd said.
“Tell them what you’re wanting to do, how you’re wanting to investigate for it, clearly communicate their risk,” he said. “But also acknowledge the limited evidence as well, and clearly communicate the results.”
Dr. Chinoy acknowledged he had received fees for presentations (UCB, Biogen), consultancy (Alexion, Novartis, Eli Lilly, Orphazyme, AstraZeneca), or grant support (Eli Lilly, UCB) that had been paid via his institution for the purpose of furthering myositis research. Dr. Oldroyd had no conflicts of interest to disclose.
All patients with idiopathic inflammatory myopathies (IIM) should be screened for swallowing difficulties, according to the first evidence-based guideline to be produced.
The guideline, which has been developed by a working group of the British Society for Rheumatology (BSR), also advises that all diagnosed patients should have their myositis antibody levels checked and have their overall well-being assessed. Other recommendations for all patients include the use of glucocorticoids to reduce muscle inflammation and conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) for long-term treatment.
“Finally, now, we’re able to standardize the way we treat adults and children with IIM,” senior guideline author Hector Chinoy, PhD, said at the society’s annual meeting.
It has been a long labor of love, however, taking 4 years to get the guideline published, said Dr. Chinoy, professor of rheumatology and neuromuscular disease at the University of Manchester (England), and a consultant at Salford (England) Royal Hospital.
“We’re not covering diagnosis, classification, or the investigation of suspected IIM,” said Dr. Chinoy. Inclusion body myositis also is not included.
Altogether, there are 13 recommendations that have been developed using a PICO (patient or population, intervention, comparison, outcome) format, graded based on the quality of the available evidence, and then voted on by the working group members to give a score of the strength of agreement. Dr. Chinoy noted that there was a checklist included in the Supplementary Data section of the guideline to help follow the recommendations.
“The target audience for the guideline reflects the variety of clinicians caring for patients with IIM,” Dr. Chinoy said. So that is not just pediatric and adult rheumatologists, but also neurologists, dermatologists, respiratory physicians, oncologists, gastroenterologists, cardiologists, and of course other health care professionals. This includes rheumatology and neurology nurses, psychologists, speech and language therapists, and podiatrists, as well as rheumatology specialist pharmacists, physiotherapists, and occupational therapists.
With reference to the latter, Liza McCann, MBBS, who co-led the development of the guideline, said in a statement released by the BSR that the guideline “highlights the importance of exercise, led and monitored by specialist physiotherapists and occupational therapists.”
Dr. McCann, a consultant pediatric rheumatologist at Alder Hey Hospital, Liverpool, England, and Honorary Clinical Lecturer at the University of Liverpool, added that the guidelines also cover “the need to address psychological wellbeing as an integral part of treatment, in parallel with pharmacological therapies.”
Recommendation highlights
Some of the highlights of the recommendations include the use of high-dose glucocorticoids to manage skeletal muscle inflammation at the time of treatment induction, with specific guidance on the different doses to use in adults and in children. There also is guidance on the use of csDMARDs in both populations and what to use if there is refractory disease – with the strongest evidence supporting the use of intravenous immunoglobulin (IVIG) or cyclophosphamide, and possibly rituximab and abatacept.
“There is insufficient evidence to recommend JAK inhibition,” Dr. Chinoy said. The data search used to develop the guideline had a cutoff of October 2020, but even now there is only anecdotal evidence from case studies, he added.
Importantly, the guidelines recognize that childhood IIM differs from adult disease and call for children to be managed by pediatric specialists.
“Routine assessment of dysphagia should be considered in all patients,” Dr. Chinoy said, “so ask the question.” The recommendation is that a swallowing assessment should involve a speech and language therapist or gastroenterologist, and that IVIG be considered for active disease and dysphagia that is resistant to other treatments.
There also are recommendations to screen adult patients for interstitial lung disease, consider fracture risk, and screen adult patients for cancer if they have specific risk factors that include older age at onset, male gender, dysphagia, and rapid disease onset, among others.
Separate cancer screening guidelines on cards
“Around one in four patients with myositis will develop cancer within the 3 years either before or after myositis onset,” Alexander Oldroyd, MBChB, PhD, said in a separate presentation at the BSR annual meeting.
“It’s a hugely increased risk compared to the general population, and a great worry for patients,” he added. Exactly why there is an increased risk is not known, but “there’s a big link between the biological onset of cancer and myositis.”
Dr. Oldroyd, who is an NIHR Academic Clinical Lecturer at the University of Manchester in England and a coauthor of the BSR myositis guideline, is part of a special interest group set up by the International Myositis Assessment and Clinical Studies Group (IMACS) that is in the process of developing separate guidelines for cancer screening in people newly diagnosed with IIM.
The aim was to produce evidence-based recommendations that were both “pragmatic and practical,” that could help clinicians answer patient’s questions on their risk and how best and how often to screen them, Dr. Oldroyd explained. Importantly, IMACS has endeavored to create recommendations that should be applicable across different countries and health care systems.
“We had to acknowledge that there’s not a lot of evidence base there,” Dr. Oldroyd said, noting that he and colleagues conducted a systematic literature review and meta-analysis and used a Delphi process to draft 20 recommendations. These cover identifying risk factors for cancer in people with myositis and categorizing people into low, medium, and high-risk categories. The recommendations also cover what should constitute basic and enhanced screening, and how often someone should be screened.
Moreover, the authors make recommendations on the use of imaging modalities such as PET and CT scans, as well as upper and lower gastrointestinal endoscopy and naso-endoscopy.
“As rheumatologists, we don’t talk about cancer a lot,” Dr. Oldroyd said. “We pick up a lot of incidental cancers, but we don’t usually talk about cancer screening with patients.” That’s something that needs to change, he said.
“It’s important – just get it out in the open, talk to people about it,” Dr. Oldroyd said.
“Tell them what you’re wanting to do, how you’re wanting to investigate for it, clearly communicate their risk,” he said. “But also acknowledge the limited evidence as well, and clearly communicate the results.”
Dr. Chinoy acknowledged he had received fees for presentations (UCB, Biogen), consultancy (Alexion, Novartis, Eli Lilly, Orphazyme, AstraZeneca), or grant support (Eli Lilly, UCB) that had been paid via his institution for the purpose of furthering myositis research. Dr. Oldroyd had no conflicts of interest to disclose.
FROM BSR 2022