User login
Does imaging surveillance intensity govern clinical outcomes in HCC?
Key clinical point: Compared with the standard ultrasonography (US)-based imaging surveillance for hepatocellular carcinoma (HCC), intensive surveillance using alternative computed tomography (CT)/magnetic resonance imaging (MRI) in addition to US may facilitate the diagnosis of very early-stage HCC without providing any survival advantage.
Major finding: Diagnosis of very early-stage HCC was better in the low- (adjusted odds ratio [aOR] 0.44; P = .034) and high- (aOR 0.40; P = .014) intensive surveillance groups than in the standard surveillance group. However, overall survival remained unaffected by the surveillance intensity (P > .05).
Study details: This was a retrospective cohort study including 529 patients with newly diagnosed HCC who were on regular surveillance and were monitored using only US (standard group; n = 62) or CT/MRI plus US (categorized into low-intensive group [n = 232] and high-intensive group [n = 235] based on the median percentage of CT/MRI investigations [cut-off, 27%]).
Disclosures: The study did not receive any funding. The authors disclosed no conflicts of interest.
Source: Hwang JA et al. Association between intensity of imaging surveillance and clinical outcomes in patients with hepatocellular carcinoma. Eur J Radiol. 2022;151:110328 (Apr 21). Doi: 10.1016/j.ejrad.2022.110328
Key clinical point: Compared with the standard ultrasonography (US)-based imaging surveillance for hepatocellular carcinoma (HCC), intensive surveillance using alternative computed tomography (CT)/magnetic resonance imaging (MRI) in addition to US may facilitate the diagnosis of very early-stage HCC without providing any survival advantage.
Major finding: Diagnosis of very early-stage HCC was better in the low- (adjusted odds ratio [aOR] 0.44; P = .034) and high- (aOR 0.40; P = .014) intensive surveillance groups than in the standard surveillance group. However, overall survival remained unaffected by the surveillance intensity (P > .05).
Study details: This was a retrospective cohort study including 529 patients with newly diagnosed HCC who were on regular surveillance and were monitored using only US (standard group; n = 62) or CT/MRI plus US (categorized into low-intensive group [n = 232] and high-intensive group [n = 235] based on the median percentage of CT/MRI investigations [cut-off, 27%]).
Disclosures: The study did not receive any funding. The authors disclosed no conflicts of interest.
Source: Hwang JA et al. Association between intensity of imaging surveillance and clinical outcomes in patients with hepatocellular carcinoma. Eur J Radiol. 2022;151:110328 (Apr 21). Doi: 10.1016/j.ejrad.2022.110328
Key clinical point: Compared with the standard ultrasonography (US)-based imaging surveillance for hepatocellular carcinoma (HCC), intensive surveillance using alternative computed tomography (CT)/magnetic resonance imaging (MRI) in addition to US may facilitate the diagnosis of very early-stage HCC without providing any survival advantage.
Major finding: Diagnosis of very early-stage HCC was better in the low- (adjusted odds ratio [aOR] 0.44; P = .034) and high- (aOR 0.40; P = .014) intensive surveillance groups than in the standard surveillance group. However, overall survival remained unaffected by the surveillance intensity (P > .05).
Study details: This was a retrospective cohort study including 529 patients with newly diagnosed HCC who were on regular surveillance and were monitored using only US (standard group; n = 62) or CT/MRI plus US (categorized into low-intensive group [n = 232] and high-intensive group [n = 235] based on the median percentage of CT/MRI investigations [cut-off, 27%]).
Disclosures: The study did not receive any funding. The authors disclosed no conflicts of interest.
Source: Hwang JA et al. Association between intensity of imaging surveillance and clinical outcomes in patients with hepatocellular carcinoma. Eur J Radiol. 2022;151:110328 (Apr 21). Doi: 10.1016/j.ejrad.2022.110328
Subsequent anticancer therapy after ICI treatment prolongs survival in HCC
Key clinical point: Compared with best supportive care (BSC), treatment with any type of anticancer therapy after immune checkpoint inhibitor (ICI) therapy discontinuation in hepatocellular carcinoma (HCC) is associated with a significant improvement in overall survival.
Major finding: After ICI therapy discontinuation, patients who received anticancer therapy vs. BSC showed a significantly improved median overall survival (12.2 vs 3.2 months; hazard ratio 0.4; P < .001).
Study details: This was a retrospective, multicenter study that included 420 patients with HCC who were treated with ICI followed by subsequent anticancer treatment (n = 163) or BSC (n = 152).
Disclosures: The study was supported by the Wellcome Trust Strategic Fund, UK. Some authors declared serving as advisors, consultants, or speakers for and receiving grants from various sources.
Source: Sharma R et al. Patterns and outcomes of subsequent therapy after immune checkpoint inhibitor discontinuation in HCC. Hepatol Commun. 2022 (Apr 28). Doi: 10.1002/hep4.1927
Key clinical point: Compared with best supportive care (BSC), treatment with any type of anticancer therapy after immune checkpoint inhibitor (ICI) therapy discontinuation in hepatocellular carcinoma (HCC) is associated with a significant improvement in overall survival.
Major finding: After ICI therapy discontinuation, patients who received anticancer therapy vs. BSC showed a significantly improved median overall survival (12.2 vs 3.2 months; hazard ratio 0.4; P < .001).
Study details: This was a retrospective, multicenter study that included 420 patients with HCC who were treated with ICI followed by subsequent anticancer treatment (n = 163) or BSC (n = 152).
Disclosures: The study was supported by the Wellcome Trust Strategic Fund, UK. Some authors declared serving as advisors, consultants, or speakers for and receiving grants from various sources.
Source: Sharma R et al. Patterns and outcomes of subsequent therapy after immune checkpoint inhibitor discontinuation in HCC. Hepatol Commun. 2022 (Apr 28). Doi: 10.1002/hep4.1927
Key clinical point: Compared with best supportive care (BSC), treatment with any type of anticancer therapy after immune checkpoint inhibitor (ICI) therapy discontinuation in hepatocellular carcinoma (HCC) is associated with a significant improvement in overall survival.
Major finding: After ICI therapy discontinuation, patients who received anticancer therapy vs. BSC showed a significantly improved median overall survival (12.2 vs 3.2 months; hazard ratio 0.4; P < .001).
Study details: This was a retrospective, multicenter study that included 420 patients with HCC who were treated with ICI followed by subsequent anticancer treatment (n = 163) or BSC (n = 152).
Disclosures: The study was supported by the Wellcome Trust Strategic Fund, UK. Some authors declared serving as advisors, consultants, or speakers for and receiving grants from various sources.
Source: Sharma R et al. Patterns and outcomes of subsequent therapy after immune checkpoint inhibitor discontinuation in HCC. Hepatol Commun. 2022 (Apr 28). Doi: 10.1002/hep4.1927
Pembrolizumab monotherapy shows promise for untreated advanced HCC in phase 2
Key clinical point: Pembrolizumab monotherapy showed favorable efficacy in systemic therapy-naive patients with advanced hepatocellular carcinoma (aHCC), with no new safety signals in addition to those observed for pembrolizumab in aHCC in a second-line setting.
Major finding: The objective response rate was 16% (95% CI 7%-29%), and the median duration of response was 16 months. The median progression-free and overall survival were 4 (95% CI 2-8) and 17 (95% CI 8-23) months, respectively. Only 16% of patients experienced grade ≥3 treatment-related adverse events.
Study details: Findings are from the phase 2 KEYNOTE-224 trial including 51 adult, systemic therapy-naive patients with aHCC who received 200 mg pembrolizumab intravenously every 3 weeks for up to 35 cycles.
Disclosures: The study was sponsored by Merck Sharp & Dohme Corp. (MSD), a subsidiary of Merck & Co., Inc., USA. Some authors reported being advisory board members, consultants, or advisors or receiving research grants, speaker honoraria, or travel and accommodation expenses from various sources, including MSD. The other authors are employees and stock owners of MSD.
Source: Verset G et al. Pembrolizumab monotherapy for previously untreated advanced hepatocellular carcinoma: Data from the open-label, phase II KEYNOTE-224 trial. Clin Cancer Res. 2022 (Apr 14). Doi: 10.1158/1078-0432.CCR-21-3807
Key clinical point: Pembrolizumab monotherapy showed favorable efficacy in systemic therapy-naive patients with advanced hepatocellular carcinoma (aHCC), with no new safety signals in addition to those observed for pembrolizumab in aHCC in a second-line setting.
Major finding: The objective response rate was 16% (95% CI 7%-29%), and the median duration of response was 16 months. The median progression-free and overall survival were 4 (95% CI 2-8) and 17 (95% CI 8-23) months, respectively. Only 16% of patients experienced grade ≥3 treatment-related adverse events.
Study details: Findings are from the phase 2 KEYNOTE-224 trial including 51 adult, systemic therapy-naive patients with aHCC who received 200 mg pembrolizumab intravenously every 3 weeks for up to 35 cycles.
Disclosures: The study was sponsored by Merck Sharp & Dohme Corp. (MSD), a subsidiary of Merck & Co., Inc., USA. Some authors reported being advisory board members, consultants, or advisors or receiving research grants, speaker honoraria, or travel and accommodation expenses from various sources, including MSD. The other authors are employees and stock owners of MSD.
Source: Verset G et al. Pembrolizumab monotherapy for previously untreated advanced hepatocellular carcinoma: Data from the open-label, phase II KEYNOTE-224 trial. Clin Cancer Res. 2022 (Apr 14). Doi: 10.1158/1078-0432.CCR-21-3807
Key clinical point: Pembrolizumab monotherapy showed favorable efficacy in systemic therapy-naive patients with advanced hepatocellular carcinoma (aHCC), with no new safety signals in addition to those observed for pembrolizumab in aHCC in a second-line setting.
Major finding: The objective response rate was 16% (95% CI 7%-29%), and the median duration of response was 16 months. The median progression-free and overall survival were 4 (95% CI 2-8) and 17 (95% CI 8-23) months, respectively. Only 16% of patients experienced grade ≥3 treatment-related adverse events.
Study details: Findings are from the phase 2 KEYNOTE-224 trial including 51 adult, systemic therapy-naive patients with aHCC who received 200 mg pembrolizumab intravenously every 3 weeks for up to 35 cycles.
Disclosures: The study was sponsored by Merck Sharp & Dohme Corp. (MSD), a subsidiary of Merck & Co., Inc., USA. Some authors reported being advisory board members, consultants, or advisors or receiving research grants, speaker honoraria, or travel and accommodation expenses from various sources, including MSD. The other authors are employees and stock owners of MSD.
Source: Verset G et al. Pembrolizumab monotherapy for previously untreated advanced hepatocellular carcinoma: Data from the open-label, phase II KEYNOTE-224 trial. Clin Cancer Res. 2022 (Apr 14). Doi: 10.1158/1078-0432.CCR-21-3807
Early-stage HCC: OLT offers better survival outcomes than ablative therapies in the elderly
Key clinical point: Compared with liver-directed ablative therapies, orthotopic liver transplantation (OLT) offers a survival advantage for elderly patients with early-stage hepatocellular carcinoma (HCC) and alpha-fetoprotein (AFP) levels <500 ng/mL.
Major finding: Multivariable analysis revealed a significant survival benefit of OLT compared with ablative therapy alone (adjusted hazard ratio [aHR] 0.31; P < .001), with OLT being associated with better survival even after adjusting for imbalanced factors after propensity matching (aHR 0.35; P < .001).
Study details: The data come from a retrospective review study that propensity score matched patients aged ≥70 years with stage I-II HCC and AFP levels of <500 ng/mL receiving OLT (n = 170) with those undergoing liver-directed ablative therapy (n = 170).
Disclosures: No source of funding or conflicts of interest was declared by the authors.
Source: Shah MB et al. Outcomes in elderly patients undergoing liver transplantation compared with liver-directed ablative therapy in early-stage hepatocellular carcinoma. J Am Coll Surg. 2022;234(5):892-899 (Apr 15). Doi: 10.1097/XCS.0000000000000135
Key clinical point: Compared with liver-directed ablative therapies, orthotopic liver transplantation (OLT) offers a survival advantage for elderly patients with early-stage hepatocellular carcinoma (HCC) and alpha-fetoprotein (AFP) levels <500 ng/mL.
Major finding: Multivariable analysis revealed a significant survival benefit of OLT compared with ablative therapy alone (adjusted hazard ratio [aHR] 0.31; P < .001), with OLT being associated with better survival even after adjusting for imbalanced factors after propensity matching (aHR 0.35; P < .001).
Study details: The data come from a retrospective review study that propensity score matched patients aged ≥70 years with stage I-II HCC and AFP levels of <500 ng/mL receiving OLT (n = 170) with those undergoing liver-directed ablative therapy (n = 170).
Disclosures: No source of funding or conflicts of interest was declared by the authors.
Source: Shah MB et al. Outcomes in elderly patients undergoing liver transplantation compared with liver-directed ablative therapy in early-stage hepatocellular carcinoma. J Am Coll Surg. 2022;234(5):892-899 (Apr 15). Doi: 10.1097/XCS.0000000000000135
Key clinical point: Compared with liver-directed ablative therapies, orthotopic liver transplantation (OLT) offers a survival advantage for elderly patients with early-stage hepatocellular carcinoma (HCC) and alpha-fetoprotein (AFP) levels <500 ng/mL.
Major finding: Multivariable analysis revealed a significant survival benefit of OLT compared with ablative therapy alone (adjusted hazard ratio [aHR] 0.31; P < .001), with OLT being associated with better survival even after adjusting for imbalanced factors after propensity matching (aHR 0.35; P < .001).
Study details: The data come from a retrospective review study that propensity score matched patients aged ≥70 years with stage I-II HCC and AFP levels of <500 ng/mL receiving OLT (n = 170) with those undergoing liver-directed ablative therapy (n = 170).
Disclosures: No source of funding or conflicts of interest was declared by the authors.
Source: Shah MB et al. Outcomes in elderly patients undergoing liver transplantation compared with liver-directed ablative therapy in early-stage hepatocellular carcinoma. J Am Coll Surg. 2022;234(5):892-899 (Apr 15). Doi: 10.1097/XCS.0000000000000135
Four mental health trajectories in youth: Predicting persistent psychopathology
A study that tracked psychopathology in 13,000 children and adolescents found that
Investigators also found a strong correlation between new incidence of high psychopathology and externalizing problems such as hyperactivity. “It is of paramount importance to identify factors that distinguish those with persisting problems and escalating trajectories so that resources can be appropriately directed,” wrote the authors of the study published online in JAMA Network Open.
Recent studies have shown that concurrent and sequential comorbidity of psychiatric disorders are very common in adult populations, lead author Colm Healy, PhD, a postdoctoral researcher for psychiatry with the University of Medicine and Health Sciences, Ireland, said in an interview.
The speculation is that this occurs in early life when psychiatry symptoms experience high fluidity. “This presents a complex scenario to model, where young people’s mental health appears to shift and change across development. Few investigations to date have had the data available to examine these trajectories over the full range of child development,” said Dr. Healy.
He and his colleagues attempted to map the profiles and trajectories of psychopathology in children and adolescents, using latent profile transition analysis (LPTA), a person-centered method, to assess comorbidity and movement in the various phases of childhood development.
“The idea behind person-centered methods such as LTPA is that it identifies unobserved subgroups of participants who respond similarly to specific variables – in this case responses to a broad measure of psychopathology,” explained Dr. Healy.
The study included 7,507 children from the child sample (ages 3, 5, and 9 years) and 6,039 children from the adolescent sample (ages 9, 13, and 17 or 18 years). Data analysis took place from October 2020 to September 2021.
Dr. Healy and colleagues in a supplementary investigation compared cohorts at age 9 years to look for sex and generational differences.
Four developmental profiles
Researchers identified 4 distinct developmental profies for person-centered psychopathological trajectories: no psychopathology (incidence range, 60%-70%), high psychopathology (incidence range, 3%-5%), externalizing problems (incidence range, 15%-25%), and internalizing problems (incidence range, 7%-12%).
Internalizing problems reflect issues with peers and emotional problems whereas externalizing problems more closely associate with hyperactivity and conduct.
Less than 5% of the youth studied experienced persistent symptoms. However, 48.6% in the child cohort and 44.1% in the adolescent cohort moved into one of the 3 psychopathology profiles (high psychopathology, externalizing, internalizing problems) at some point in development.
The spread of trajectories was more diverse in the child cohort, said Dr. Healy. “Children ebbed and flowed between the different profiles over time with a large proportion falling into one of the psychopathology categories and then switching between these profiles.” Switching was also evident in the adolescent cohort but to a lesser extent, he said.
Externalizing problems link to high psychopathology
Rates of remittance were higher among individuals in both cohorts for internalizing problems, compared with externalizing problems.
It’s possible that for some of these young people, internalizing problems are a reaction to environmental stressors such as bullying,” said Dr. Healy. “When that stress is relieved, the internalizing problems may dissipate.”
In a clinically relevant finding, children with externalizing problems (age 5, 129 [61.3%] and age 9, 95 [74.3%]) were more likely to present with new incidents of high psychopathology. This was also true in the adolescent group (age 13, 129 [91.1%] and age 17, 146 [89.9%]).
This suggests that a proportion of youth with externalizing problems have an escalating trajectory of psychopathology. “Thus, it may be possible to distinguish those with an escalating trajectory from a stable or remitting trajectory. The specific distinguishing factors require further investigation, but it has been observed before that some of those reporting externalizing problems in early life continue to have difficulties into later life,” noted Dr. Healy.
A combination of environmental or biological factors may explain this escalation, which could respond to early intervention, he said.
Overall, few children in the study transitioned directly from no psychopathology to high psychopathology.
Differences between boys, girls
In both cohorts, investigators noticed significant differences between the sexes.
Boys in childhood made up a larger proportion of the three psychopathology profiles. But by late adolescence, girls made up a larger proportion of the internalizing profile whereas boys made up a larger proportion of the externalizing profile. “These differences were in line with our expectations,” said Dr. Healy.
Trajectories also differed among boys and girls. In childhood, girls had a higher percentage of de-escalating trajectories relative to boys. “More girls than boys in the psychopathology profiles switched to a non or less severe profile. In adolescence, differences in trajectories were less obvious, with the exception that girls were more likely than boys to transition to internalizing problems from all of the other profiles at age 17,” said Dr. Healy.
Most young people who experience psychopathology will eventually see an improvement in symptoms, noted Dr. Healy. Next steps are to identify markers that distinguish individuals with persistent trajectories from remitting trajectories at the different phases of development, he said.
Study draws mixed reviews
Clinical psychiatrists not involved in the study had varying reactions to the results.
“This study is notable for its data-driven and powerful illustration of how childhood and adolescence are dynamic periods during which psychiatric symptoms can emerge and evolve,” said Sunny X. Tang, MD, a psychiatrist and an assistant professor at the Institute of Behavioral Science and the Feinstein Institutes for Medical Research, Manhasset, New York.
The clinical call for action is for person-centered mental health screening to be a routine part of pediatric and adolescent primary care or school-based services, noted Dr. Tang.
Paul S. Nestadt, MD, an assistant professor and public mental health researcher at Johns Hopkins University, Baltimore, did not think the study would have a significant impact on clinical practice.
He noted that Dr. Healy and coauthors found that some children stayed true to type, but many fluctuated between the four profile groups. The finding that fluctuation occurred more frequently in younger children is not surprising “and is consistent with what we know about the ‘moving targets’ that make diagnosing children so difficult,” said Dr. Nestadt.
“It would have been helpful to have identified clinical indicators of likely persistence in psychopathology, but the measure employed here did not allow that. It is also frustrating to not have any information on treatment, such that we cannot know whether the children who shifted to ‘no psychopathology’ did so because of treatment or spontaneously,” he added.
Victor M. Fornari, MD, MS, director of the Division of Child & Adolescent Psychiatry at The Zucker Hillside Hospital and Cohen’s Children’s Medical Center, New York, said the study is an important contribution to understanding the development of psychopathology during childhood.
“Generally, it is felt that nearly one in five youth will meet criteria for at least one psychiatric disorder by the age of 18. It is well known that externalizing disorders like ADHD manifest earlier in childhood and that depression often manifests later in adolescence,” he said.
No disclosures were reported.
A study that tracked psychopathology in 13,000 children and adolescents found that
Investigators also found a strong correlation between new incidence of high psychopathology and externalizing problems such as hyperactivity. “It is of paramount importance to identify factors that distinguish those with persisting problems and escalating trajectories so that resources can be appropriately directed,” wrote the authors of the study published online in JAMA Network Open.
Recent studies have shown that concurrent and sequential comorbidity of psychiatric disorders are very common in adult populations, lead author Colm Healy, PhD, a postdoctoral researcher for psychiatry with the University of Medicine and Health Sciences, Ireland, said in an interview.
The speculation is that this occurs in early life when psychiatry symptoms experience high fluidity. “This presents a complex scenario to model, where young people’s mental health appears to shift and change across development. Few investigations to date have had the data available to examine these trajectories over the full range of child development,” said Dr. Healy.
He and his colleagues attempted to map the profiles and trajectories of psychopathology in children and adolescents, using latent profile transition analysis (LPTA), a person-centered method, to assess comorbidity and movement in the various phases of childhood development.
“The idea behind person-centered methods such as LTPA is that it identifies unobserved subgroups of participants who respond similarly to specific variables – in this case responses to a broad measure of psychopathology,” explained Dr. Healy.
The study included 7,507 children from the child sample (ages 3, 5, and 9 years) and 6,039 children from the adolescent sample (ages 9, 13, and 17 or 18 years). Data analysis took place from October 2020 to September 2021.
Dr. Healy and colleagues in a supplementary investigation compared cohorts at age 9 years to look for sex and generational differences.
Four developmental profiles
Researchers identified 4 distinct developmental profies for person-centered psychopathological trajectories: no psychopathology (incidence range, 60%-70%), high psychopathology (incidence range, 3%-5%), externalizing problems (incidence range, 15%-25%), and internalizing problems (incidence range, 7%-12%).
Internalizing problems reflect issues with peers and emotional problems whereas externalizing problems more closely associate with hyperactivity and conduct.
Less than 5% of the youth studied experienced persistent symptoms. However, 48.6% in the child cohort and 44.1% in the adolescent cohort moved into one of the 3 psychopathology profiles (high psychopathology, externalizing, internalizing problems) at some point in development.
The spread of trajectories was more diverse in the child cohort, said Dr. Healy. “Children ebbed and flowed between the different profiles over time with a large proportion falling into one of the psychopathology categories and then switching between these profiles.” Switching was also evident in the adolescent cohort but to a lesser extent, he said.
Externalizing problems link to high psychopathology
Rates of remittance were higher among individuals in both cohorts for internalizing problems, compared with externalizing problems.
It’s possible that for some of these young people, internalizing problems are a reaction to environmental stressors such as bullying,” said Dr. Healy. “When that stress is relieved, the internalizing problems may dissipate.”
In a clinically relevant finding, children with externalizing problems (age 5, 129 [61.3%] and age 9, 95 [74.3%]) were more likely to present with new incidents of high psychopathology. This was also true in the adolescent group (age 13, 129 [91.1%] and age 17, 146 [89.9%]).
This suggests that a proportion of youth with externalizing problems have an escalating trajectory of psychopathology. “Thus, it may be possible to distinguish those with an escalating trajectory from a stable or remitting trajectory. The specific distinguishing factors require further investigation, but it has been observed before that some of those reporting externalizing problems in early life continue to have difficulties into later life,” noted Dr. Healy.
A combination of environmental or biological factors may explain this escalation, which could respond to early intervention, he said.
Overall, few children in the study transitioned directly from no psychopathology to high psychopathology.
Differences between boys, girls
In both cohorts, investigators noticed significant differences between the sexes.
Boys in childhood made up a larger proportion of the three psychopathology profiles. But by late adolescence, girls made up a larger proportion of the internalizing profile whereas boys made up a larger proportion of the externalizing profile. “These differences were in line with our expectations,” said Dr. Healy.
Trajectories also differed among boys and girls. In childhood, girls had a higher percentage of de-escalating trajectories relative to boys. “More girls than boys in the psychopathology profiles switched to a non or less severe profile. In adolescence, differences in trajectories were less obvious, with the exception that girls were more likely than boys to transition to internalizing problems from all of the other profiles at age 17,” said Dr. Healy.
Most young people who experience psychopathology will eventually see an improvement in symptoms, noted Dr. Healy. Next steps are to identify markers that distinguish individuals with persistent trajectories from remitting trajectories at the different phases of development, he said.
Study draws mixed reviews
Clinical psychiatrists not involved in the study had varying reactions to the results.
“This study is notable for its data-driven and powerful illustration of how childhood and adolescence are dynamic periods during which psychiatric symptoms can emerge and evolve,” said Sunny X. Tang, MD, a psychiatrist and an assistant professor at the Institute of Behavioral Science and the Feinstein Institutes for Medical Research, Manhasset, New York.
The clinical call for action is for person-centered mental health screening to be a routine part of pediatric and adolescent primary care or school-based services, noted Dr. Tang.
Paul S. Nestadt, MD, an assistant professor and public mental health researcher at Johns Hopkins University, Baltimore, did not think the study would have a significant impact on clinical practice.
He noted that Dr. Healy and coauthors found that some children stayed true to type, but many fluctuated between the four profile groups. The finding that fluctuation occurred more frequently in younger children is not surprising “and is consistent with what we know about the ‘moving targets’ that make diagnosing children so difficult,” said Dr. Nestadt.
“It would have been helpful to have identified clinical indicators of likely persistence in psychopathology, but the measure employed here did not allow that. It is also frustrating to not have any information on treatment, such that we cannot know whether the children who shifted to ‘no psychopathology’ did so because of treatment or spontaneously,” he added.
Victor M. Fornari, MD, MS, director of the Division of Child & Adolescent Psychiatry at The Zucker Hillside Hospital and Cohen’s Children’s Medical Center, New York, said the study is an important contribution to understanding the development of psychopathology during childhood.
“Generally, it is felt that nearly one in five youth will meet criteria for at least one psychiatric disorder by the age of 18. It is well known that externalizing disorders like ADHD manifest earlier in childhood and that depression often manifests later in adolescence,” he said.
No disclosures were reported.
A study that tracked psychopathology in 13,000 children and adolescents found that
Investigators also found a strong correlation between new incidence of high psychopathology and externalizing problems such as hyperactivity. “It is of paramount importance to identify factors that distinguish those with persisting problems and escalating trajectories so that resources can be appropriately directed,” wrote the authors of the study published online in JAMA Network Open.
Recent studies have shown that concurrent and sequential comorbidity of psychiatric disorders are very common in adult populations, lead author Colm Healy, PhD, a postdoctoral researcher for psychiatry with the University of Medicine and Health Sciences, Ireland, said in an interview.
The speculation is that this occurs in early life when psychiatry symptoms experience high fluidity. “This presents a complex scenario to model, where young people’s mental health appears to shift and change across development. Few investigations to date have had the data available to examine these trajectories over the full range of child development,” said Dr. Healy.
He and his colleagues attempted to map the profiles and trajectories of psychopathology in children and adolescents, using latent profile transition analysis (LPTA), a person-centered method, to assess comorbidity and movement in the various phases of childhood development.
“The idea behind person-centered methods such as LTPA is that it identifies unobserved subgroups of participants who respond similarly to specific variables – in this case responses to a broad measure of psychopathology,” explained Dr. Healy.
The study included 7,507 children from the child sample (ages 3, 5, and 9 years) and 6,039 children from the adolescent sample (ages 9, 13, and 17 or 18 years). Data analysis took place from October 2020 to September 2021.
Dr. Healy and colleagues in a supplementary investigation compared cohorts at age 9 years to look for sex and generational differences.
Four developmental profiles
Researchers identified 4 distinct developmental profies for person-centered psychopathological trajectories: no psychopathology (incidence range, 60%-70%), high psychopathology (incidence range, 3%-5%), externalizing problems (incidence range, 15%-25%), and internalizing problems (incidence range, 7%-12%).
Internalizing problems reflect issues with peers and emotional problems whereas externalizing problems more closely associate with hyperactivity and conduct.
Less than 5% of the youth studied experienced persistent symptoms. However, 48.6% in the child cohort and 44.1% in the adolescent cohort moved into one of the 3 psychopathology profiles (high psychopathology, externalizing, internalizing problems) at some point in development.
The spread of trajectories was more diverse in the child cohort, said Dr. Healy. “Children ebbed and flowed between the different profiles over time with a large proportion falling into one of the psychopathology categories and then switching between these profiles.” Switching was also evident in the adolescent cohort but to a lesser extent, he said.
Externalizing problems link to high psychopathology
Rates of remittance were higher among individuals in both cohorts for internalizing problems, compared with externalizing problems.
It’s possible that for some of these young people, internalizing problems are a reaction to environmental stressors such as bullying,” said Dr. Healy. “When that stress is relieved, the internalizing problems may dissipate.”
In a clinically relevant finding, children with externalizing problems (age 5, 129 [61.3%] and age 9, 95 [74.3%]) were more likely to present with new incidents of high psychopathology. This was also true in the adolescent group (age 13, 129 [91.1%] and age 17, 146 [89.9%]).
This suggests that a proportion of youth with externalizing problems have an escalating trajectory of psychopathology. “Thus, it may be possible to distinguish those with an escalating trajectory from a stable or remitting trajectory. The specific distinguishing factors require further investigation, but it has been observed before that some of those reporting externalizing problems in early life continue to have difficulties into later life,” noted Dr. Healy.
A combination of environmental or biological factors may explain this escalation, which could respond to early intervention, he said.
Overall, few children in the study transitioned directly from no psychopathology to high psychopathology.
Differences between boys, girls
In both cohorts, investigators noticed significant differences between the sexes.
Boys in childhood made up a larger proportion of the three psychopathology profiles. But by late adolescence, girls made up a larger proportion of the internalizing profile whereas boys made up a larger proportion of the externalizing profile. “These differences were in line with our expectations,” said Dr. Healy.
Trajectories also differed among boys and girls. In childhood, girls had a higher percentage of de-escalating trajectories relative to boys. “More girls than boys in the psychopathology profiles switched to a non or less severe profile. In adolescence, differences in trajectories were less obvious, with the exception that girls were more likely than boys to transition to internalizing problems from all of the other profiles at age 17,” said Dr. Healy.
Most young people who experience psychopathology will eventually see an improvement in symptoms, noted Dr. Healy. Next steps are to identify markers that distinguish individuals with persistent trajectories from remitting trajectories at the different phases of development, he said.
Study draws mixed reviews
Clinical psychiatrists not involved in the study had varying reactions to the results.
“This study is notable for its data-driven and powerful illustration of how childhood and adolescence are dynamic periods during which psychiatric symptoms can emerge and evolve,” said Sunny X. Tang, MD, a psychiatrist and an assistant professor at the Institute of Behavioral Science and the Feinstein Institutes for Medical Research, Manhasset, New York.
The clinical call for action is for person-centered mental health screening to be a routine part of pediatric and adolescent primary care or school-based services, noted Dr. Tang.
Paul S. Nestadt, MD, an assistant professor and public mental health researcher at Johns Hopkins University, Baltimore, did not think the study would have a significant impact on clinical practice.
He noted that Dr. Healy and coauthors found that some children stayed true to type, but many fluctuated between the four profile groups. The finding that fluctuation occurred more frequently in younger children is not surprising “and is consistent with what we know about the ‘moving targets’ that make diagnosing children so difficult,” said Dr. Nestadt.
“It would have been helpful to have identified clinical indicators of likely persistence in psychopathology, but the measure employed here did not allow that. It is also frustrating to not have any information on treatment, such that we cannot know whether the children who shifted to ‘no psychopathology’ did so because of treatment or spontaneously,” he added.
Victor M. Fornari, MD, MS, director of the Division of Child & Adolescent Psychiatry at The Zucker Hillside Hospital and Cohen’s Children’s Medical Center, New York, said the study is an important contribution to understanding the development of psychopathology during childhood.
“Generally, it is felt that nearly one in five youth will meet criteria for at least one psychiatric disorder by the age of 18. It is well known that externalizing disorders like ADHD manifest earlier in childhood and that depression often manifests later in adolescence,” he said.
No disclosures were reported.
FROM JAMA NETWORK OPEN
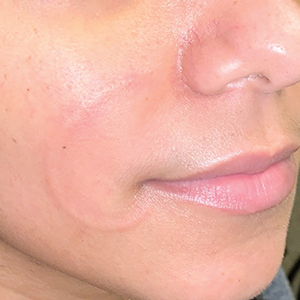
Recurrent Arciform Plaque on the Face
The Diagnosis: Lupus Erythematosus Tumidus
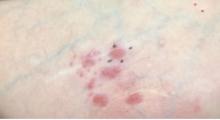
Histopathologic evaluation of a punch biopsy revealed focal dermal mucin deposition and CD123+ discrete clusters of plasmacytoid dendritic cells without interface changes (Figure 1), favoring a diagnosis of lupus erythematosus tumidus (LET) in our patient. There was no clinical improvement in symptoms when she previously was treated with topical antifungals or class III corticosteroid creams. Tacrolimus ointment 0.1% twice daily for 1 month did not result in substantial improvement in the appearance of the plaque, and it spontaneously resolved after 2 to 3 months. She declined treatment with hydroxychloroquine.
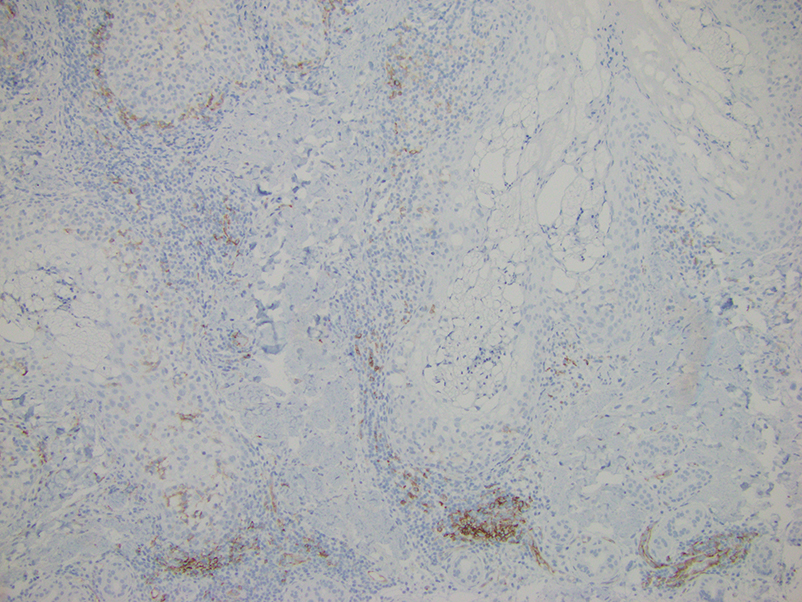
Lupus erythematosus tumidus is an uncommon subtype of chronic cutaneous lupus erythematosus with no distinct etiology. It is clinically characterized by edematous, urticarial, single or multiple plaques with a smooth surface affecting sun-exposed areas that can last for months to years.1 In contrast to other variations of chronic cutaneous lupus such as discoid lupus erythematosus, LET lesions lack surface papulosquamous features such as scaling, atrophy, and follicular plugging.1-4 Based solely on histologic findings, LET may be indistinguishable from reticular erythematous mucinosis and Jessner lymphocytic infiltration of the skin (JLIS) due to a similar lack of epidermal involvement and presence of a perivascular lymphocytic infiltrate (Figure 2).
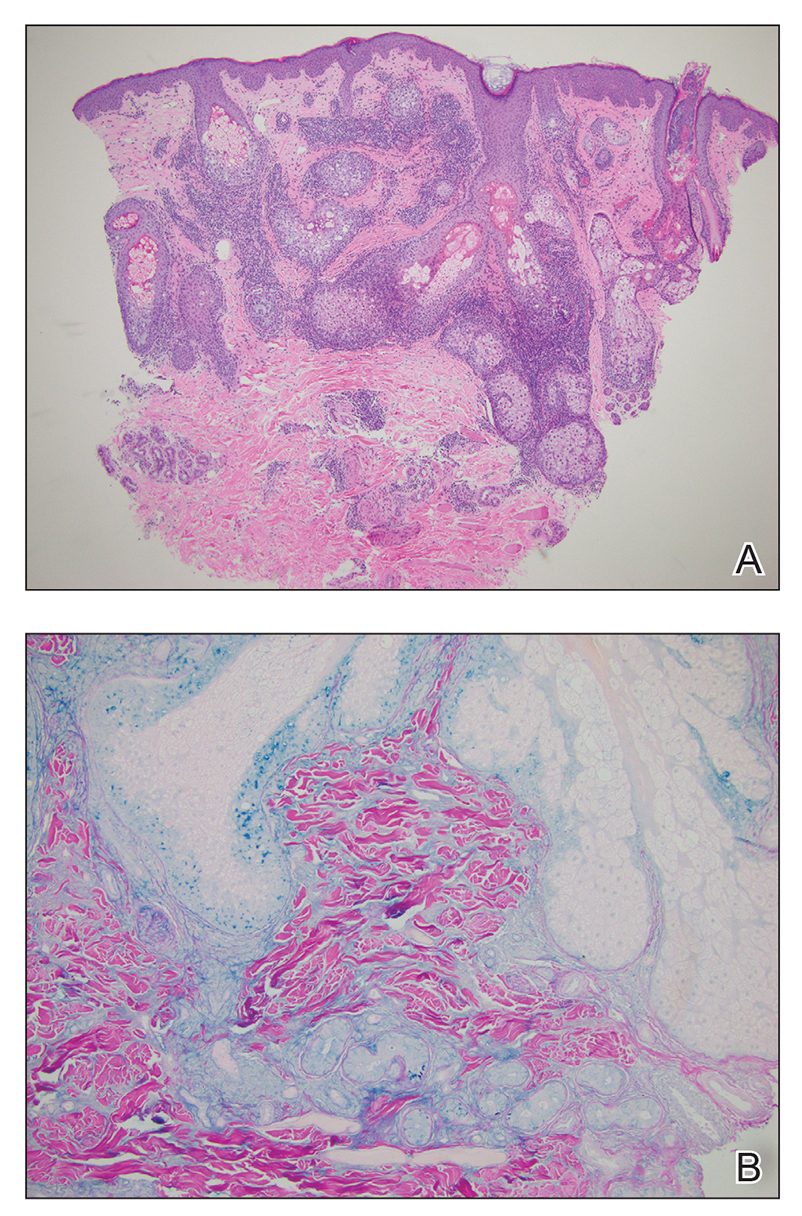
The average age at disease onset is 36 years, nearly the same as that described in discoid lupus erythematosus.4 Lupus erythematosus tumidus has a favorable prognosis and commonly presents without other autoimmune signs, serologic abnormalities, or gender preference, with concomitant systemic lupus erythematosus sometimes reported.5
The absence of clinical and histological epidermal involvement are the most important clues to aid in the diagnosis. It has been postulated that JLIS could be an early cutaneous manifestation of LET.6 The differential diagnosis also may include erythema annulare centrifugum, granuloma annulare, and urticarial vasculitis. Lesions typically respond well to photoprotection, topical corticosteroids, and/or antimalarials. The addition of tacrolimus ointment 0.1% may result in complete regression without recurrence.7
Erythema annulare centrifugum is a reactive erythema that classically begins as a pink papule that gradually enlarges to form an annular erythematous plaque with a fine trailing scale that may recur.8 The histopathology of erythema annulare centrifugum shares features seen in LET, making the diagnosis difficult; however, secondary changes to the epidermis (eg, spongiosis, hyperkeratosis) may be seen. This condition has been associated with lymphoproliferative malignancies.8
Reticular erythematous mucinosis is clinically distinguished from LET, as it presents as reticular, rather than arciform, erythematous macules, papules, or plaques that may be asymptomatic or pruritic.9 Histopathology typically shows more superficial mucin deposition than in LET as well as superficial to mid-dermal perivascular and periadnexal lymphocytic infiltrates. Reticular erythematous mucinosis more frequently is reported in women in their 30s and 40s and has been associated with UV exposure and hormonal triggers, such as oral contraceptive medications and pregnancy.9
Granuloma annulare typically presents as asymptomatic, erythematous, annular plaques or papules in young women.10 There are several histologic subtypes that show focal collagen degeneration, inflammation with palisaded or interstitial histiocytes, and mucin deposition, regardless of clinical presentation. Granuloma annulare has been associated with systemic diseases including type 2 diabetes mellitus and thyroid disease. Localized granuloma annulare most commonly presents on the dorsal aspects of the hands or feet.10
We present a case of LET on the face. Although histologically similar to other dermatoses, LET often lacks epidermal involvement and presents on sun-exposed areas of the body. Jessner lymphocytic infiltration of the skin also should be considered in the differential, as there is an overlap of clinical and histopathological features; JLIS lacks mucin deposits.6 This case reinforces the importance of correlating clinical with histopathologic findings. Our patient was treated with tacrolimus ointment 0.1%, and the plaque eventually resolved in 2 to 3 months without recurrence. This condition should be included in the differential diagnosis of recurring annular plaques on sunexposed areas, particularly in middle-aged adults, even in the absence of systemic involvement.
- Liu E, Daze RP, Moon S. Tumid lupus erythematosus: a rare and distinctive variant of cutaneous lupus erythematosus masquerading as urticarial vasculitis. Cureus. 2020;12:E8305. doi:10.7759/cureus.8305
- Saleh D, Crane JS. Tumid lupus erythematosus. In: StatPearls. StatPearls Publishing; 2020.
- Verma P, Sharma S, Yadav P, et al. Tumid lupus erythematosus: an intriguing dermatopathological connotation treated successfully with topical tacrolimus and hydroxychloroquine combination. Indian J Dermatol. 2014;59:210. doi:10.4103/0019-5154.127716
- Kuhn A, Bein D, Bonsmann G. The 100th anniversary of lupus erythematosus tumidus. Autoimmun Rev. 2009;8:441-448. doi:10.1016/j. autrev.2008.12.010
- Jatwani K, Chugh K, Osholowu OS, et al. Tumid lupus erythematosus and systemic lupus erythematosus: a report on their rare coexistence. Cureus. 2020;12:E7545. doi:10.7759/cureus.7545
- Tomasini D, Mentzel T, Hantschke M, et al. Plasmacytoid dendritic cells: an overview of their presence and distribution in different inflammatory skin diseases, with special emphasis on Jessner’s lymphocytic infiltrate of the skin and cutaneous lupus erythematosus. J Cutan Pathol. 2010;37:1132-1139. doi:10.1111/j.1600-0560.2010.01587.x
- Patsinakidis N, Kautz O, Gibbs BF, et al. Lupus erythematosus tumidus: clinical perspectives. Clin Cosmet Investig Dermatol. 2019;12:707-719. doi:10.2147/CCID.S166723
- Mu EW, Sanchez M, Mir A, et al. Paraneoplastic erythema annulare centrifugum eruption (PEACE). Dermatol Online J. 2015;21:13030/ qt6053h29n.
- Ocanha-Xavier JP, Cola-Senra CO, Xavier-Junior JCC. Reticular erythematous mucinosis: literature review and case report of a 24-year-old patient with systemic erythematosus lupus. Lupus. 2021;30:325-335. doi:10.1177/0961203320965702 10. Keimig EL. Granuloma annulare. Dermatol Clin. 2015;33:315-329. doi:10.1016/j.det.2015.03.001
The Diagnosis: Lupus Erythematosus Tumidus
Histopathologic evaluation of a punch biopsy revealed focal dermal mucin deposition and CD123+ discrete clusters of plasmacytoid dendritic cells without interface changes (Figure 1), favoring a diagnosis of lupus erythematosus tumidus (LET) in our patient. There was no clinical improvement in symptoms when she previously was treated with topical antifungals or class III corticosteroid creams. Tacrolimus ointment 0.1% twice daily for 1 month did not result in substantial improvement in the appearance of the plaque, and it spontaneously resolved after 2 to 3 months. She declined treatment with hydroxychloroquine.

Lupus erythematosus tumidus is an uncommon subtype of chronic cutaneous lupus erythematosus with no distinct etiology. It is clinically characterized by edematous, urticarial, single or multiple plaques with a smooth surface affecting sun-exposed areas that can last for months to years.1 In contrast to other variations of chronic cutaneous lupus such as discoid lupus erythematosus, LET lesions lack surface papulosquamous features such as scaling, atrophy, and follicular plugging.1-4 Based solely on histologic findings, LET may be indistinguishable from reticular erythematous mucinosis and Jessner lymphocytic infiltration of the skin (JLIS) due to a similar lack of epidermal involvement and presence of a perivascular lymphocytic infiltrate (Figure 2).

The average age at disease onset is 36 years, nearly the same as that described in discoid lupus erythematosus.4 Lupus erythematosus tumidus has a favorable prognosis and commonly presents without other autoimmune signs, serologic abnormalities, or gender preference, with concomitant systemic lupus erythematosus sometimes reported.5
The absence of clinical and histological epidermal involvement are the most important clues to aid in the diagnosis. It has been postulated that JLIS could be an early cutaneous manifestation of LET.6 The differential diagnosis also may include erythema annulare centrifugum, granuloma annulare, and urticarial vasculitis. Lesions typically respond well to photoprotection, topical corticosteroids, and/or antimalarials. The addition of tacrolimus ointment 0.1% may result in complete regression without recurrence.7
Erythema annulare centrifugum is a reactive erythema that classically begins as a pink papule that gradually enlarges to form an annular erythematous plaque with a fine trailing scale that may recur.8 The histopathology of erythema annulare centrifugum shares features seen in LET, making the diagnosis difficult; however, secondary changes to the epidermis (eg, spongiosis, hyperkeratosis) may be seen. This condition has been associated with lymphoproliferative malignancies.8
Reticular erythematous mucinosis is clinically distinguished from LET, as it presents as reticular, rather than arciform, erythematous macules, papules, or plaques that may be asymptomatic or pruritic.9 Histopathology typically shows more superficial mucin deposition than in LET as well as superficial to mid-dermal perivascular and periadnexal lymphocytic infiltrates. Reticular erythematous mucinosis more frequently is reported in women in their 30s and 40s and has been associated with UV exposure and hormonal triggers, such as oral contraceptive medications and pregnancy.9
Granuloma annulare typically presents as asymptomatic, erythematous, annular plaques or papules in young women.10 There are several histologic subtypes that show focal collagen degeneration, inflammation with palisaded or interstitial histiocytes, and mucin deposition, regardless of clinical presentation. Granuloma annulare has been associated with systemic diseases including type 2 diabetes mellitus and thyroid disease. Localized granuloma annulare most commonly presents on the dorsal aspects of the hands or feet.10
We present a case of LET on the face. Although histologically similar to other dermatoses, LET often lacks epidermal involvement and presents on sun-exposed areas of the body. Jessner lymphocytic infiltration of the skin also should be considered in the differential, as there is an overlap of clinical and histopathological features; JLIS lacks mucin deposits.6 This case reinforces the importance of correlating clinical with histopathologic findings. Our patient was treated with tacrolimus ointment 0.1%, and the plaque eventually resolved in 2 to 3 months without recurrence. This condition should be included in the differential diagnosis of recurring annular plaques on sunexposed areas, particularly in middle-aged adults, even in the absence of systemic involvement.
The Diagnosis: Lupus Erythematosus Tumidus
Histopathologic evaluation of a punch biopsy revealed focal dermal mucin deposition and CD123+ discrete clusters of plasmacytoid dendritic cells without interface changes (Figure 1), favoring a diagnosis of lupus erythematosus tumidus (LET) in our patient. There was no clinical improvement in symptoms when she previously was treated with topical antifungals or class III corticosteroid creams. Tacrolimus ointment 0.1% twice daily for 1 month did not result in substantial improvement in the appearance of the plaque, and it spontaneously resolved after 2 to 3 months. She declined treatment with hydroxychloroquine.

Lupus erythematosus tumidus is an uncommon subtype of chronic cutaneous lupus erythematosus with no distinct etiology. It is clinically characterized by edematous, urticarial, single or multiple plaques with a smooth surface affecting sun-exposed areas that can last for months to years.1 In contrast to other variations of chronic cutaneous lupus such as discoid lupus erythematosus, LET lesions lack surface papulosquamous features such as scaling, atrophy, and follicular plugging.1-4 Based solely on histologic findings, LET may be indistinguishable from reticular erythematous mucinosis and Jessner lymphocytic infiltration of the skin (JLIS) due to a similar lack of epidermal involvement and presence of a perivascular lymphocytic infiltrate (Figure 2).

The average age at disease onset is 36 years, nearly the same as that described in discoid lupus erythematosus.4 Lupus erythematosus tumidus has a favorable prognosis and commonly presents without other autoimmune signs, serologic abnormalities, or gender preference, with concomitant systemic lupus erythematosus sometimes reported.5
The absence of clinical and histological epidermal involvement are the most important clues to aid in the diagnosis. It has been postulated that JLIS could be an early cutaneous manifestation of LET.6 The differential diagnosis also may include erythema annulare centrifugum, granuloma annulare, and urticarial vasculitis. Lesions typically respond well to photoprotection, topical corticosteroids, and/or antimalarials. The addition of tacrolimus ointment 0.1% may result in complete regression without recurrence.7
Erythema annulare centrifugum is a reactive erythema that classically begins as a pink papule that gradually enlarges to form an annular erythematous plaque with a fine trailing scale that may recur.8 The histopathology of erythema annulare centrifugum shares features seen in LET, making the diagnosis difficult; however, secondary changes to the epidermis (eg, spongiosis, hyperkeratosis) may be seen. This condition has been associated with lymphoproliferative malignancies.8
Reticular erythematous mucinosis is clinically distinguished from LET, as it presents as reticular, rather than arciform, erythematous macules, papules, or plaques that may be asymptomatic or pruritic.9 Histopathology typically shows more superficial mucin deposition than in LET as well as superficial to mid-dermal perivascular and periadnexal lymphocytic infiltrates. Reticular erythematous mucinosis more frequently is reported in women in their 30s and 40s and has been associated with UV exposure and hormonal triggers, such as oral contraceptive medications and pregnancy.9
Granuloma annulare typically presents as asymptomatic, erythematous, annular plaques or papules in young women.10 There are several histologic subtypes that show focal collagen degeneration, inflammation with palisaded or interstitial histiocytes, and mucin deposition, regardless of clinical presentation. Granuloma annulare has been associated with systemic diseases including type 2 diabetes mellitus and thyroid disease. Localized granuloma annulare most commonly presents on the dorsal aspects of the hands or feet.10
We present a case of LET on the face. Although histologically similar to other dermatoses, LET often lacks epidermal involvement and presents on sun-exposed areas of the body. Jessner lymphocytic infiltration of the skin also should be considered in the differential, as there is an overlap of clinical and histopathological features; JLIS lacks mucin deposits.6 This case reinforces the importance of correlating clinical with histopathologic findings. Our patient was treated with tacrolimus ointment 0.1%, and the plaque eventually resolved in 2 to 3 months without recurrence. This condition should be included in the differential diagnosis of recurring annular plaques on sunexposed areas, particularly in middle-aged adults, even in the absence of systemic involvement.
- Liu E, Daze RP, Moon S. Tumid lupus erythematosus: a rare and distinctive variant of cutaneous lupus erythematosus masquerading as urticarial vasculitis. Cureus. 2020;12:E8305. doi:10.7759/cureus.8305
- Saleh D, Crane JS. Tumid lupus erythematosus. In: StatPearls. StatPearls Publishing; 2020.
- Verma P, Sharma S, Yadav P, et al. Tumid lupus erythematosus: an intriguing dermatopathological connotation treated successfully with topical tacrolimus and hydroxychloroquine combination. Indian J Dermatol. 2014;59:210. doi:10.4103/0019-5154.127716
- Kuhn A, Bein D, Bonsmann G. The 100th anniversary of lupus erythematosus tumidus. Autoimmun Rev. 2009;8:441-448. doi:10.1016/j. autrev.2008.12.010
- Jatwani K, Chugh K, Osholowu OS, et al. Tumid lupus erythematosus and systemic lupus erythematosus: a report on their rare coexistence. Cureus. 2020;12:E7545. doi:10.7759/cureus.7545
- Tomasini D, Mentzel T, Hantschke M, et al. Plasmacytoid dendritic cells: an overview of their presence and distribution in different inflammatory skin diseases, with special emphasis on Jessner’s lymphocytic infiltrate of the skin and cutaneous lupus erythematosus. J Cutan Pathol. 2010;37:1132-1139. doi:10.1111/j.1600-0560.2010.01587.x
- Patsinakidis N, Kautz O, Gibbs BF, et al. Lupus erythematosus tumidus: clinical perspectives. Clin Cosmet Investig Dermatol. 2019;12:707-719. doi:10.2147/CCID.S166723
- Mu EW, Sanchez M, Mir A, et al. Paraneoplastic erythema annulare centrifugum eruption (PEACE). Dermatol Online J. 2015;21:13030/ qt6053h29n.
- Ocanha-Xavier JP, Cola-Senra CO, Xavier-Junior JCC. Reticular erythematous mucinosis: literature review and case report of a 24-year-old patient with systemic erythematosus lupus. Lupus. 2021;30:325-335. doi:10.1177/0961203320965702 10. Keimig EL. Granuloma annulare. Dermatol Clin. 2015;33:315-329. doi:10.1016/j.det.2015.03.001
- Liu E, Daze RP, Moon S. Tumid lupus erythematosus: a rare and distinctive variant of cutaneous lupus erythematosus masquerading as urticarial vasculitis. Cureus. 2020;12:E8305. doi:10.7759/cureus.8305
- Saleh D, Crane JS. Tumid lupus erythematosus. In: StatPearls. StatPearls Publishing; 2020.
- Verma P, Sharma S, Yadav P, et al. Tumid lupus erythematosus: an intriguing dermatopathological connotation treated successfully with topical tacrolimus and hydroxychloroquine combination. Indian J Dermatol. 2014;59:210. doi:10.4103/0019-5154.127716
- Kuhn A, Bein D, Bonsmann G. The 100th anniversary of lupus erythematosus tumidus. Autoimmun Rev. 2009;8:441-448. doi:10.1016/j. autrev.2008.12.010
- Jatwani K, Chugh K, Osholowu OS, et al. Tumid lupus erythematosus and systemic lupus erythematosus: a report on their rare coexistence. Cureus. 2020;12:E7545. doi:10.7759/cureus.7545
- Tomasini D, Mentzel T, Hantschke M, et al. Plasmacytoid dendritic cells: an overview of their presence and distribution in different inflammatory skin diseases, with special emphasis on Jessner’s lymphocytic infiltrate of the skin and cutaneous lupus erythematosus. J Cutan Pathol. 2010;37:1132-1139. doi:10.1111/j.1600-0560.2010.01587.x
- Patsinakidis N, Kautz O, Gibbs BF, et al. Lupus erythematosus tumidus: clinical perspectives. Clin Cosmet Investig Dermatol. 2019;12:707-719. doi:10.2147/CCID.S166723
- Mu EW, Sanchez M, Mir A, et al. Paraneoplastic erythema annulare centrifugum eruption (PEACE). Dermatol Online J. 2015;21:13030/ qt6053h29n.
- Ocanha-Xavier JP, Cola-Senra CO, Xavier-Junior JCC. Reticular erythematous mucinosis: literature review and case report of a 24-year-old patient with systemic erythematosus lupus. Lupus. 2021;30:325-335. doi:10.1177/0961203320965702 10. Keimig EL. Granuloma annulare. Dermatol Clin. 2015;33:315-329. doi:10.1016/j.det.2015.03.001
An otherwise healthy 31-year-old woman presented with a gradual growth of a semiannular, arciform, mildly pruritic plaque around the mouth of 10 years’ duration that recurred biannually, persisted for a few months, and spontaneously remitted without residual scarring. She denied joint pain, muscle aches, sores in the mouth, personal or family history of autoimmune diseases, or other remarkable review of systems. Physical examination revealed a welldefined, edematous, smooth, arciform plaque on the face with no mucous membrane involvement. Laboratory evaluation, including complete blood cell count, comprehensive metabolic panel, and antinuclear antibody titer, was unremarkable. A punch biopsy was obtained.

IBD after age 60: More evidence antibiotics play a role
Most studies to date have assessed a link between antibiotics and IBD in younger patients, lead researcher Adam S. Faye, MD, said during a media briefing that previewed select research for the annual Digestive Disease Week® (DDW).
The impact of antibiotic use on the incidence of IBD in older adults is really unknown, he added.
In contrast to younger people with IBD, who tend to have a strong family history or genetic predisposition to developing Crohn’s disease or ulcerative colitis, the cause is likely different in older populations.
“There’s clearly something in the environment that’s driving this new older-onset IBD,” said Dr. Faye, who is an assistant professor of medicine and population health at New York University.
Antibiotics as a contributing link
Dr. Faye and colleagues took a closer look at antibiotics as contributing to this link. They studied 2.3 million patient records in Denmark’s national medical registry from 2000 to 2018. They identified people aged 60 years and older who were newly diagnosed with IBD, and they then assessed the number, frequency, and timing of any antibiotic prescriptions.
They found that IBD was 27% more likely in this age group if the patients had received any antibiotic prescription.
They also found that the chance of developing IBD was higher as the number of antibiotic prescriptions increased. For example, IBD was 55% more likely if a person had received two prescriptions, and it was 96% more likely with four prescriptions. The risk really jumped with five or more antibiotic prescriptions – a person with this many prescriptions was more than 2.3 times (236%) more likely to be diagnosed with IBD than those who had not been prescribed antibiotics in the prior 5 years.
Not all antibiotics were equal, however. For example, the investigators found no link with nitrofurantoin, an antibiotic commonly prescribed for urinary tract infections. In contrast, all other antibiotic agents that were evaluated, and especially fluoroquinolones, nitroimidazoles, and macrolides, were associated with IBD.
Timing made some difference.
“The risk was highest if antibiotics were prescribed within the 1- to 2-year period before diagnosis, and it declined as you go farther out. But the risks persist,” Dr. Faye said. He noted that they even found that risk was elevated 10 years out.
The investigators also considered whether the antibiotic agent or infection was behind the association.
Dr. Faye cited previous research, again in younger people with IBD, that revealed that “infections plus antibiotics substantially increase the odds or risk of developing IBD more than the infection alone.
“So there really does seem to be something that the antibiotics are doing here,” Dr. Faye said.
A leading theory is that antibiotics disrupt the gut microbiota and increase the risk for developing IBD. “But, obviously, it’s quite complicated,” he said.
Clinical implications
The findings suggest that older people who may have IBD should be screened for prior antibiotic use, Dr. Faye said.
“This is a result that really has important implications for diagnosing older adults with new gastrointestinal symptoms,” he said. “Inflammatory bowel disease often can be overlooked in older adults because there’s a lot of different diagnoses you’re thinking of.”
IBD “should be considered, especially if you have a patient who’s reporting multiple courses of antibiotics in the last few years,” he added.
The results suggest another reason that antimicrobial stewardship programs should promote judicial use of these agents beyond concerns about resistance.
“We think of antibiotic stewardship to prevent the development of multidrug-resistant organisms, but we should be thinking about it to also prevent the development of inflammatory bowel disease,” Dr. Faye said.
Although this study adds to the evidence implicating antibiotics and expands the concept to an older population, “we really don’t have a great handle on what all of the environmental and other factors are,” he said.
Some researchers point to smoking and diet, among other factors, but the interplay remains unknown, Dr. Faye added.
The study is important because the incidence of IBD is increasing within the older population, “and this is one of the first studies to look at it,” he said.
Dr. Faye and colleagues plan to start a new study to evaluate other environmental factors.
“Hopefully, we’ll have more within the next few years to report,” he said.
Shedding more light on older-onset IBD worldwide
“It’s a well-done study,” Aline Charabaty, MD, said in a comment. “We are seeing that there’s an increase of incidence of IBD in the entire population, but even more so in the elderly.”
IBD is likely caused by a combination of factors, including genetics, environmental influences, and dysfunction of the gut immune system, agreed Dr. Charabaty, who is an assistant clinical professor in the division of gastroenterology at Johns Hopkins University, Baltimore.
The research “goes along with other studies that we’ve done in the pediatric and adult populations that show antibiotics exposure increases the risk of developing inflammatory bowel disease,” she said.
For a broader perspective of the study’s findings, Dr. Faye was asked during the media briefing if the results of this Danish registry study would be generalizable to the U.S. population.
“The simplest answer is we’ll need to redo this study within the U.S. to make absolutely sure,” Dr. Faye said. She noted that prior studies in the United States and elsewhere have found a risk associated with antibiotics, although again these studies focused on younger patients.
Dr. Charabaty was more certain that the findings were meaningful outside of Denmark.
“I definitely think this will apply to our U.S. population,” added Dr. Charabaty, who is also the clinical director of the IBD Center at Johns Hopkins–Sibley Memorial Hospital, Washington. “We have very similar practices in terms of how we approach antibiotic use.
“This could be one of the risk factors that’s promoting an increase in IBD everywhere,” she added.
The study was conducted in partnership with the Danish National Center of Excellence PREDICT Program. Dr. Faye and Dr. Charabaty did not report any conflicts of interest related to this study.
A version of this article first appeared on Medscape.com.
Most studies to date have assessed a link between antibiotics and IBD in younger patients, lead researcher Adam S. Faye, MD, said during a media briefing that previewed select research for the annual Digestive Disease Week® (DDW).
The impact of antibiotic use on the incidence of IBD in older adults is really unknown, he added.
In contrast to younger people with IBD, who tend to have a strong family history or genetic predisposition to developing Crohn’s disease or ulcerative colitis, the cause is likely different in older populations.
“There’s clearly something in the environment that’s driving this new older-onset IBD,” said Dr. Faye, who is an assistant professor of medicine and population health at New York University.
Antibiotics as a contributing link
Dr. Faye and colleagues took a closer look at antibiotics as contributing to this link. They studied 2.3 million patient records in Denmark’s national medical registry from 2000 to 2018. They identified people aged 60 years and older who were newly diagnosed with IBD, and they then assessed the number, frequency, and timing of any antibiotic prescriptions.
They found that IBD was 27% more likely in this age group if the patients had received any antibiotic prescription.
They also found that the chance of developing IBD was higher as the number of antibiotic prescriptions increased. For example, IBD was 55% more likely if a person had received two prescriptions, and it was 96% more likely with four prescriptions. The risk really jumped with five or more antibiotic prescriptions – a person with this many prescriptions was more than 2.3 times (236%) more likely to be diagnosed with IBD than those who had not been prescribed antibiotics in the prior 5 years.
Not all antibiotics were equal, however. For example, the investigators found no link with nitrofurantoin, an antibiotic commonly prescribed for urinary tract infections. In contrast, all other antibiotic agents that were evaluated, and especially fluoroquinolones, nitroimidazoles, and macrolides, were associated with IBD.
Timing made some difference.
“The risk was highest if antibiotics were prescribed within the 1- to 2-year period before diagnosis, and it declined as you go farther out. But the risks persist,” Dr. Faye said. He noted that they even found that risk was elevated 10 years out.
The investigators also considered whether the antibiotic agent or infection was behind the association.
Dr. Faye cited previous research, again in younger people with IBD, that revealed that “infections plus antibiotics substantially increase the odds or risk of developing IBD more than the infection alone.
“So there really does seem to be something that the antibiotics are doing here,” Dr. Faye said.
A leading theory is that antibiotics disrupt the gut microbiota and increase the risk for developing IBD. “But, obviously, it’s quite complicated,” he said.
Clinical implications
The findings suggest that older people who may have IBD should be screened for prior antibiotic use, Dr. Faye said.
“This is a result that really has important implications for diagnosing older adults with new gastrointestinal symptoms,” he said. “Inflammatory bowel disease often can be overlooked in older adults because there’s a lot of different diagnoses you’re thinking of.”
IBD “should be considered, especially if you have a patient who’s reporting multiple courses of antibiotics in the last few years,” he added.
The results suggest another reason that antimicrobial stewardship programs should promote judicial use of these agents beyond concerns about resistance.
“We think of antibiotic stewardship to prevent the development of multidrug-resistant organisms, but we should be thinking about it to also prevent the development of inflammatory bowel disease,” Dr. Faye said.
Although this study adds to the evidence implicating antibiotics and expands the concept to an older population, “we really don’t have a great handle on what all of the environmental and other factors are,” he said.
Some researchers point to smoking and diet, among other factors, but the interplay remains unknown, Dr. Faye added.
The study is important because the incidence of IBD is increasing within the older population, “and this is one of the first studies to look at it,” he said.
Dr. Faye and colleagues plan to start a new study to evaluate other environmental factors.
“Hopefully, we’ll have more within the next few years to report,” he said.
Shedding more light on older-onset IBD worldwide
“It’s a well-done study,” Aline Charabaty, MD, said in a comment. “We are seeing that there’s an increase of incidence of IBD in the entire population, but even more so in the elderly.”
IBD is likely caused by a combination of factors, including genetics, environmental influences, and dysfunction of the gut immune system, agreed Dr. Charabaty, who is an assistant clinical professor in the division of gastroenterology at Johns Hopkins University, Baltimore.
The research “goes along with other studies that we’ve done in the pediatric and adult populations that show antibiotics exposure increases the risk of developing inflammatory bowel disease,” she said.
For a broader perspective of the study’s findings, Dr. Faye was asked during the media briefing if the results of this Danish registry study would be generalizable to the U.S. population.
“The simplest answer is we’ll need to redo this study within the U.S. to make absolutely sure,” Dr. Faye said. She noted that prior studies in the United States and elsewhere have found a risk associated with antibiotics, although again these studies focused on younger patients.
Dr. Charabaty was more certain that the findings were meaningful outside of Denmark.
“I definitely think this will apply to our U.S. population,” added Dr. Charabaty, who is also the clinical director of the IBD Center at Johns Hopkins–Sibley Memorial Hospital, Washington. “We have very similar practices in terms of how we approach antibiotic use.
“This could be one of the risk factors that’s promoting an increase in IBD everywhere,” she added.
The study was conducted in partnership with the Danish National Center of Excellence PREDICT Program. Dr. Faye and Dr. Charabaty did not report any conflicts of interest related to this study.
A version of this article first appeared on Medscape.com.
Most studies to date have assessed a link between antibiotics and IBD in younger patients, lead researcher Adam S. Faye, MD, said during a media briefing that previewed select research for the annual Digestive Disease Week® (DDW).
The impact of antibiotic use on the incidence of IBD in older adults is really unknown, he added.
In contrast to younger people with IBD, who tend to have a strong family history or genetic predisposition to developing Crohn’s disease or ulcerative colitis, the cause is likely different in older populations.
“There’s clearly something in the environment that’s driving this new older-onset IBD,” said Dr. Faye, who is an assistant professor of medicine and population health at New York University.
Antibiotics as a contributing link
Dr. Faye and colleagues took a closer look at antibiotics as contributing to this link. They studied 2.3 million patient records in Denmark’s national medical registry from 2000 to 2018. They identified people aged 60 years and older who were newly diagnosed with IBD, and they then assessed the number, frequency, and timing of any antibiotic prescriptions.
They found that IBD was 27% more likely in this age group if the patients had received any antibiotic prescription.
They also found that the chance of developing IBD was higher as the number of antibiotic prescriptions increased. For example, IBD was 55% more likely if a person had received two prescriptions, and it was 96% more likely with four prescriptions. The risk really jumped with five or more antibiotic prescriptions – a person with this many prescriptions was more than 2.3 times (236%) more likely to be diagnosed with IBD than those who had not been prescribed antibiotics in the prior 5 years.
Not all antibiotics were equal, however. For example, the investigators found no link with nitrofurantoin, an antibiotic commonly prescribed for urinary tract infections. In contrast, all other antibiotic agents that were evaluated, and especially fluoroquinolones, nitroimidazoles, and macrolides, were associated with IBD.
Timing made some difference.
“The risk was highest if antibiotics were prescribed within the 1- to 2-year period before diagnosis, and it declined as you go farther out. But the risks persist,” Dr. Faye said. He noted that they even found that risk was elevated 10 years out.
The investigators also considered whether the antibiotic agent or infection was behind the association.
Dr. Faye cited previous research, again in younger people with IBD, that revealed that “infections plus antibiotics substantially increase the odds or risk of developing IBD more than the infection alone.
“So there really does seem to be something that the antibiotics are doing here,” Dr. Faye said.
A leading theory is that antibiotics disrupt the gut microbiota and increase the risk for developing IBD. “But, obviously, it’s quite complicated,” he said.
Clinical implications
The findings suggest that older people who may have IBD should be screened for prior antibiotic use, Dr. Faye said.
“This is a result that really has important implications for diagnosing older adults with new gastrointestinal symptoms,” he said. “Inflammatory bowel disease often can be overlooked in older adults because there’s a lot of different diagnoses you’re thinking of.”
IBD “should be considered, especially if you have a patient who’s reporting multiple courses of antibiotics in the last few years,” he added.
The results suggest another reason that antimicrobial stewardship programs should promote judicial use of these agents beyond concerns about resistance.
“We think of antibiotic stewardship to prevent the development of multidrug-resistant organisms, but we should be thinking about it to also prevent the development of inflammatory bowel disease,” Dr. Faye said.
Although this study adds to the evidence implicating antibiotics and expands the concept to an older population, “we really don’t have a great handle on what all of the environmental and other factors are,” he said.
Some researchers point to smoking and diet, among other factors, but the interplay remains unknown, Dr. Faye added.
The study is important because the incidence of IBD is increasing within the older population, “and this is one of the first studies to look at it,” he said.
Dr. Faye and colleagues plan to start a new study to evaluate other environmental factors.
“Hopefully, we’ll have more within the next few years to report,” he said.
Shedding more light on older-onset IBD worldwide
“It’s a well-done study,” Aline Charabaty, MD, said in a comment. “We are seeing that there’s an increase of incidence of IBD in the entire population, but even more so in the elderly.”
IBD is likely caused by a combination of factors, including genetics, environmental influences, and dysfunction of the gut immune system, agreed Dr. Charabaty, who is an assistant clinical professor in the division of gastroenterology at Johns Hopkins University, Baltimore.
The research “goes along with other studies that we’ve done in the pediatric and adult populations that show antibiotics exposure increases the risk of developing inflammatory bowel disease,” she said.
For a broader perspective of the study’s findings, Dr. Faye was asked during the media briefing if the results of this Danish registry study would be generalizable to the U.S. population.
“The simplest answer is we’ll need to redo this study within the U.S. to make absolutely sure,” Dr. Faye said. She noted that prior studies in the United States and elsewhere have found a risk associated with antibiotics, although again these studies focused on younger patients.
Dr. Charabaty was more certain that the findings were meaningful outside of Denmark.
“I definitely think this will apply to our U.S. population,” added Dr. Charabaty, who is also the clinical director of the IBD Center at Johns Hopkins–Sibley Memorial Hospital, Washington. “We have very similar practices in terms of how we approach antibiotic use.
“This could be one of the risk factors that’s promoting an increase in IBD everywhere,” she added.
The study was conducted in partnership with the Danish National Center of Excellence PREDICT Program. Dr. Faye and Dr. Charabaty did not report any conflicts of interest related to this study.
A version of this article first appeared on Medscape.com.
FROM DDW 2022
Measles outbreaks: Protecting your patients during international travel
The U.S. immunization program is one of the best public health success stories. Physicians who provide care for children are familiar with the routine childhood immunization schedule and administer a measles-containing vaccine at age-appropriate times. Thanks to its rigorous implementation and acceptance, endemic measles (absence of continuous virus transmission for > 1 year) was eliminated in the U.S. in 2000. Loss of this status was in jeopardy in 2019 when 22 measles outbreaks occurred in 17 states (7 were multistate outbreaks). That year, 1,163 cases were reported.1 Most cases occurred in unvaccinated persons (89%) and 81 cases were imported of which 54 were in U.S. citizens returning from international travel. All outbreaks were linked to travel. Fortunately, the outbreaks were controlled prior to the elimination deadline, or the United States would have lost its measles elimination status. Restrictions on travel because of COVID-19 have relaxed significantly since the introduction of COVID-19 vaccines, resulting in increased regional and international travel. Multiple countries, including the United States noted a decline in routine immunizations rates during the last 2 years. Recent U.S. data for the 2020-2021 school year indicates that MMR immunizations rates (two doses) for kindergarteners declined to 93.9% (range 78.9% to > 98.9%), while the overall percentage of those students with an exemption remained low at 2.2%. Vaccine coverage greater than 95% was reported in only 16 states. Coverage of less than 90% was reported in seven states and the District of Columbia (Georgia, Idaho, Kentucky, Maryland, Minnesota, Ohio, and Wisconsin).2 Vaccine coverage should be 95% or higher to maintain herd immunity and control outbreaks.
Why is measles prevention so important? Many physicians practicing in the United States today have never seen a case or know its potential complications. I saw my first case as a resident in an immigrant child. It took our training director to point out the subtle signs and symptoms. It was the first time I saw Kolpik spots. Measles is transmitted person to person via large respiratory droplets and less often by airborne spread. It is highly contagious for susceptible individuals with an attack rate of 90%. In this case, a medical student on the team developed symptoms about 10 days later. Six years would pass before I diagnosed my next case of measles. An HIV patient acquired it after close contact with someone who was in the prodromal stage. He presented with the 3 C’s: Cough, coryza, and conjunctivitis, in addition to fever and an erythematous rash. He did not recover from complications of the disease.
Prior to the routine administration of a measles vaccine, 3-4 million cases with almost 500 deaths occurred annually in the United States. Worldwide, 35 million cases and more than 6 million deaths occurred each year. Here, most patients recover completely; however, complications including otitis media, pneumonia, croup, and encephalitis can develop. Complications commonly occur in immunocompromised individuals and young children. Groups with the highest fatality rates include children aged less than 5 years, immunocompromised persons, and pregnant women. Worldwide, fatality rates are dependent on the patients underlying nutritional and health status in addition to the quality of health care available.3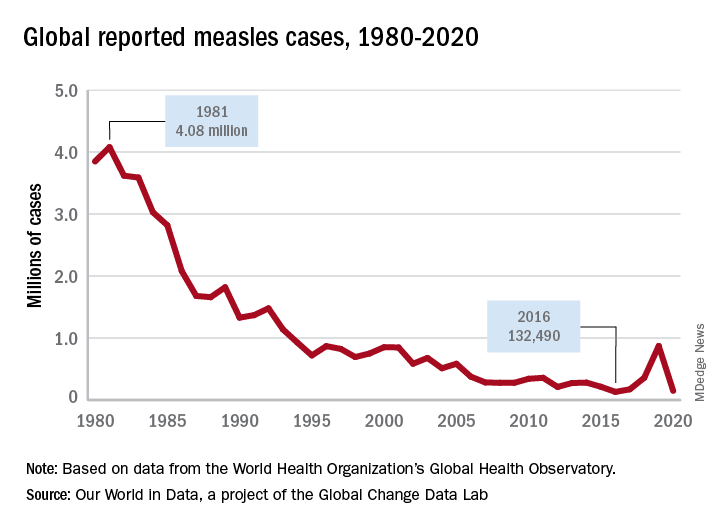
Measles vaccine was licensed in 1963 and cases began to decline (Figure 1). There was a resurgence in 1989 but it was not limited to the United States. The cause of the U.S. resurgence was multifactorial: Widespread viral transmission among unvaccinated preschool-age children residing in inner cities, outbreaks in vaccinated school-age children, outbreaks in students and personnel on college campuses, and primary vaccine failure (2%-5% of recipients failed to have an adequate response). In 1989, to help prevent future outbreaks, the United States recommended a two-dose schedule for measles and in 1993, the Vaccines for Children Program, a federally funded program, was established to improve access to vaccines for all children.
What is going on internationally?
Figure 2 lists the top 10 countries with current measles outbreaks.

Most countries on the list may not be typical travel destinations for tourists; however, they are common destinations for individuals visiting friends and relatives after immigrating to the United States. In contrast to the United States, most countries with limited resources and infrastructure have mass-vaccination campaigns to ensure vaccine administration to large segments of the population. They too have been affected by the COVID-19 pandemic. By report, at least 41 countries delayed implementation of their measles campaign in 2020 and 2021, thus, leading to the potential for even larger outbreaks.4
Progress toward the global elimination of measles is evidenced by the following: All 194 countries now include one dose of measles in their routine schedules; between 2000 and 2019 coverage of one dose of measles increased from 72% to 85% and countries with more than 90% coverage increased from 45% to 63%. Finally, the number of countries offering two doses of measles increased from 50% to 91% and vaccine coverage increased from 18% to 71% over the same time period.3
What can you do for your patients and their parents before they travel abroad?
- Inform all staff that the MMR vaccine can be administered to children as young as 6 months and at times other than those listed on the routine immunization schedule. This will help avoid parents seeking vaccine being denied an appointment.
- Children 6-11 months need 1 dose of MMR. Two additional doses will still need to be administered at the routine time.
- Children 12 months or older need 2 doses of MMR at least 4 weeks apart.
- If yellow fever vaccine is needed, coordinate administration with a travel medicine clinic since both are live vaccines and must be given on the same day.
- Any person born after 1956 should have 2 doses of MMR at least 4 weeks apart if they have no evidence of immunity.
- Encourage parents to always inform you and your staff of any international travel plans.
Moving forward, remember this increased global activity and the presence of inadequately vaccinated individuals/communities keeps the United States at continued risk for measles outbreaks. The source of the next outbreak may only be one plane ride away.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
This article was updated 6/29/22.
References
1. Patel M et al. MMWR. 2019 Oct 11; 68(40):893-6.
2. Seither R et al. MMWR. 2022 Apr 22;71(16):561-8.
3. Gastañaduy PA et al. J Infect Dis. 2021 Sep 30;224(12 Suppl 2):S420-8. doi: 10.1093/infdis/jiaa793.
4. Centers for Disease Control and Prevention. Measles (Rubeola). http://www.CDC.gov/Measles.
The U.S. immunization program is one of the best public health success stories. Physicians who provide care for children are familiar with the routine childhood immunization schedule and administer a measles-containing vaccine at age-appropriate times. Thanks to its rigorous implementation and acceptance, endemic measles (absence of continuous virus transmission for > 1 year) was eliminated in the U.S. in 2000. Loss of this status was in jeopardy in 2019 when 22 measles outbreaks occurred in 17 states (7 were multistate outbreaks). That year, 1,163 cases were reported.1 Most cases occurred in unvaccinated persons (89%) and 81 cases were imported of which 54 were in U.S. citizens returning from international travel. All outbreaks were linked to travel. Fortunately, the outbreaks were controlled prior to the elimination deadline, or the United States would have lost its measles elimination status. Restrictions on travel because of COVID-19 have relaxed significantly since the introduction of COVID-19 vaccines, resulting in increased regional and international travel. Multiple countries, including the United States noted a decline in routine immunizations rates during the last 2 years. Recent U.S. data for the 2020-2021 school year indicates that MMR immunizations rates (two doses) for kindergarteners declined to 93.9% (range 78.9% to > 98.9%), while the overall percentage of those students with an exemption remained low at 2.2%. Vaccine coverage greater than 95% was reported in only 16 states. Coverage of less than 90% was reported in seven states and the District of Columbia (Georgia, Idaho, Kentucky, Maryland, Minnesota, Ohio, and Wisconsin).2 Vaccine coverage should be 95% or higher to maintain herd immunity and control outbreaks.
Why is measles prevention so important? Many physicians practicing in the United States today have never seen a case or know its potential complications. I saw my first case as a resident in an immigrant child. It took our training director to point out the subtle signs and symptoms. It was the first time I saw Kolpik spots. Measles is transmitted person to person via large respiratory droplets and less often by airborne spread. It is highly contagious for susceptible individuals with an attack rate of 90%. In this case, a medical student on the team developed symptoms about 10 days later. Six years would pass before I diagnosed my next case of measles. An HIV patient acquired it after close contact with someone who was in the prodromal stage. He presented with the 3 C’s: Cough, coryza, and conjunctivitis, in addition to fever and an erythematous rash. He did not recover from complications of the disease.
Prior to the routine administration of a measles vaccine, 3-4 million cases with almost 500 deaths occurred annually in the United States. Worldwide, 35 million cases and more than 6 million deaths occurred each year. Here, most patients recover completely; however, complications including otitis media, pneumonia, croup, and encephalitis can develop. Complications commonly occur in immunocompromised individuals and young children. Groups with the highest fatality rates include children aged less than 5 years, immunocompromised persons, and pregnant women. Worldwide, fatality rates are dependent on the patients underlying nutritional and health status in addition to the quality of health care available.3
Measles vaccine was licensed in 1963 and cases began to decline (Figure 1). There was a resurgence in 1989 but it was not limited to the United States. The cause of the U.S. resurgence was multifactorial: Widespread viral transmission among unvaccinated preschool-age children residing in inner cities, outbreaks in vaccinated school-age children, outbreaks in students and personnel on college campuses, and primary vaccine failure (2%-5% of recipients failed to have an adequate response). In 1989, to help prevent future outbreaks, the United States recommended a two-dose schedule for measles and in 1993, the Vaccines for Children Program, a federally funded program, was established to improve access to vaccines for all children.
What is going on internationally?
Figure 2 lists the top 10 countries with current measles outbreaks.

Most countries on the list may not be typical travel destinations for tourists; however, they are common destinations for individuals visiting friends and relatives after immigrating to the United States. In contrast to the United States, most countries with limited resources and infrastructure have mass-vaccination campaigns to ensure vaccine administration to large segments of the population. They too have been affected by the COVID-19 pandemic. By report, at least 41 countries delayed implementation of their measles campaign in 2020 and 2021, thus, leading to the potential for even larger outbreaks.4
Progress toward the global elimination of measles is evidenced by the following: All 194 countries now include one dose of measles in their routine schedules; between 2000 and 2019 coverage of one dose of measles increased from 72% to 85% and countries with more than 90% coverage increased from 45% to 63%. Finally, the number of countries offering two doses of measles increased from 50% to 91% and vaccine coverage increased from 18% to 71% over the same time period.3
What can you do for your patients and their parents before they travel abroad?
- Inform all staff that the MMR vaccine can be administered to children as young as 6 months and at times other than those listed on the routine immunization schedule. This will help avoid parents seeking vaccine being denied an appointment.
- Children 6-11 months need 1 dose of MMR. Two additional doses will still need to be administered at the routine time.
- Children 12 months or older need 2 doses of MMR at least 4 weeks apart.
- If yellow fever vaccine is needed, coordinate administration with a travel medicine clinic since both are live vaccines and must be given on the same day.
- Any person born after 1956 should have 2 doses of MMR at least 4 weeks apart if they have no evidence of immunity.
- Encourage parents to always inform you and your staff of any international travel plans.
Moving forward, remember this increased global activity and the presence of inadequately vaccinated individuals/communities keeps the United States at continued risk for measles outbreaks. The source of the next outbreak may only be one plane ride away.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
This article was updated 6/29/22.
References
1. Patel M et al. MMWR. 2019 Oct 11; 68(40):893-6.
2. Seither R et al. MMWR. 2022 Apr 22;71(16):561-8.
3. Gastañaduy PA et al. J Infect Dis. 2021 Sep 30;224(12 Suppl 2):S420-8. doi: 10.1093/infdis/jiaa793.
4. Centers for Disease Control and Prevention. Measles (Rubeola). http://www.CDC.gov/Measles.
The U.S. immunization program is one of the best public health success stories. Physicians who provide care for children are familiar with the routine childhood immunization schedule and administer a measles-containing vaccine at age-appropriate times. Thanks to its rigorous implementation and acceptance, endemic measles (absence of continuous virus transmission for > 1 year) was eliminated in the U.S. in 2000. Loss of this status was in jeopardy in 2019 when 22 measles outbreaks occurred in 17 states (7 were multistate outbreaks). That year, 1,163 cases were reported.1 Most cases occurred in unvaccinated persons (89%) and 81 cases were imported of which 54 were in U.S. citizens returning from international travel. All outbreaks were linked to travel. Fortunately, the outbreaks were controlled prior to the elimination deadline, or the United States would have lost its measles elimination status. Restrictions on travel because of COVID-19 have relaxed significantly since the introduction of COVID-19 vaccines, resulting in increased regional and international travel. Multiple countries, including the United States noted a decline in routine immunizations rates during the last 2 years. Recent U.S. data for the 2020-2021 school year indicates that MMR immunizations rates (two doses) for kindergarteners declined to 93.9% (range 78.9% to > 98.9%), while the overall percentage of those students with an exemption remained low at 2.2%. Vaccine coverage greater than 95% was reported in only 16 states. Coverage of less than 90% was reported in seven states and the District of Columbia (Georgia, Idaho, Kentucky, Maryland, Minnesota, Ohio, and Wisconsin).2 Vaccine coverage should be 95% or higher to maintain herd immunity and control outbreaks.
Why is measles prevention so important? Many physicians practicing in the United States today have never seen a case or know its potential complications. I saw my first case as a resident in an immigrant child. It took our training director to point out the subtle signs and symptoms. It was the first time I saw Kolpik spots. Measles is transmitted person to person via large respiratory droplets and less often by airborne spread. It is highly contagious for susceptible individuals with an attack rate of 90%. In this case, a medical student on the team developed symptoms about 10 days later. Six years would pass before I diagnosed my next case of measles. An HIV patient acquired it after close contact with someone who was in the prodromal stage. He presented with the 3 C’s: Cough, coryza, and conjunctivitis, in addition to fever and an erythematous rash. He did not recover from complications of the disease.
Prior to the routine administration of a measles vaccine, 3-4 million cases with almost 500 deaths occurred annually in the United States. Worldwide, 35 million cases and more than 6 million deaths occurred each year. Here, most patients recover completely; however, complications including otitis media, pneumonia, croup, and encephalitis can develop. Complications commonly occur in immunocompromised individuals and young children. Groups with the highest fatality rates include children aged less than 5 years, immunocompromised persons, and pregnant women. Worldwide, fatality rates are dependent on the patients underlying nutritional and health status in addition to the quality of health care available.3
Measles vaccine was licensed in 1963 and cases began to decline (Figure 1). There was a resurgence in 1989 but it was not limited to the United States. The cause of the U.S. resurgence was multifactorial: Widespread viral transmission among unvaccinated preschool-age children residing in inner cities, outbreaks in vaccinated school-age children, outbreaks in students and personnel on college campuses, and primary vaccine failure (2%-5% of recipients failed to have an adequate response). In 1989, to help prevent future outbreaks, the United States recommended a two-dose schedule for measles and in 1993, the Vaccines for Children Program, a federally funded program, was established to improve access to vaccines for all children.
What is going on internationally?
Figure 2 lists the top 10 countries with current measles outbreaks.

Most countries on the list may not be typical travel destinations for tourists; however, they are common destinations for individuals visiting friends and relatives after immigrating to the United States. In contrast to the United States, most countries with limited resources and infrastructure have mass-vaccination campaigns to ensure vaccine administration to large segments of the population. They too have been affected by the COVID-19 pandemic. By report, at least 41 countries delayed implementation of their measles campaign in 2020 and 2021, thus, leading to the potential for even larger outbreaks.4
Progress toward the global elimination of measles is evidenced by the following: All 194 countries now include one dose of measles in their routine schedules; between 2000 and 2019 coverage of one dose of measles increased from 72% to 85% and countries with more than 90% coverage increased from 45% to 63%. Finally, the number of countries offering two doses of measles increased from 50% to 91% and vaccine coverage increased from 18% to 71% over the same time period.3
What can you do for your patients and their parents before they travel abroad?
- Inform all staff that the MMR vaccine can be administered to children as young as 6 months and at times other than those listed on the routine immunization schedule. This will help avoid parents seeking vaccine being denied an appointment.
- Children 6-11 months need 1 dose of MMR. Two additional doses will still need to be administered at the routine time.
- Children 12 months or older need 2 doses of MMR at least 4 weeks apart.
- If yellow fever vaccine is needed, coordinate administration with a travel medicine clinic since both are live vaccines and must be given on the same day.
- Any person born after 1956 should have 2 doses of MMR at least 4 weeks apart if they have no evidence of immunity.
- Encourage parents to always inform you and your staff of any international travel plans.
Moving forward, remember this increased global activity and the presence of inadequately vaccinated individuals/communities keeps the United States at continued risk for measles outbreaks. The source of the next outbreak may only be one plane ride away.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
This article was updated 6/29/22.
References
1. Patel M et al. MMWR. 2019 Oct 11; 68(40):893-6.
2. Seither R et al. MMWR. 2022 Apr 22;71(16):561-8.
3. Gastañaduy PA et al. J Infect Dis. 2021 Sep 30;224(12 Suppl 2):S420-8. doi: 10.1093/infdis/jiaa793.
4. Centers for Disease Control and Prevention. Measles (Rubeola). http://www.CDC.gov/Measles.
Severe infections often accompany severe psoriasis
of nearly 95,000 patients.
Although previous studies have shown a higher risk for comorbid conditions in people with psoriasis, compared with those without psoriasis, data on the occurrence of severe and rare infections in patients with psoriasis are limited, wrote Nikolai Loft, MD, of the department of dermatology and allergy, Copenhagen University Hospital, Gentofte, and colleagues.
Psoriasis patients are often treated with immunosuppressive therapies that may promote or aggravate infections; therefore, a better understanding of psoriasis and risk of infections is needed, they said. In a study published in the British Journal of Dermatology, Dr. Loft and his coinvestigators reviewed data on adults aged 18 years and older from the Danish National Patient Register between Jan. 1, 1997 and Dec. 31, 2018. The study population included 94,450 adults with psoriasis and 566,700 matched controls. Patients with any type of psoriasis and any degree of severity were included.
The primary outcome was the occurrence of severe infections, defined as those requiring assessment at a hospital, and rare infections, defined as HIV, TB, HBV, and HCV. The median age of the participants was 52.3 years, and slightly more than half were women.
Overall, the incidence rate of severe and rare infections among patients with any type of psoriasis was 3,104.9 per 100,000 person-years, compared with 2,381.1 for controls, with a hazard ratio, adjusted for gender, age, ethnicity, socioeconomic status, alcohol-related conditions, and Charlson comorbidity index (aHR) of 1.29.
For any infections resulting in hospitalization, the incidence rate was 2,005.1 vs. 1,531.8 per 100,000 person-years for patients with any type of psoriasis and controls, respectively.
The results were similar when severe infections and rare infections were analyzed separately. The incidence rate of severe infections was 3,080.6 and 2,364.4 per 100,000 person-years for patients with any psoriasis, compared with controls; the incidence rate for rare infections was 42.9 and 31.8 for all psoriasis patients and controls, respectively.
When the data were examined by psoriasis severity, the incidence rate of severe and rare infections among patients with severe psoriasis was 3,847.7 per 100,000 person-years, compared with 2,351.9 per 100,000 person years among controls (aHR, 1.58) and also higher than in patients with mild psoriasis. The incidence rate of severe and rare infections in patients with mild psoriasis (2,979.1 per 100,000 person-years) also was higher than in controls (aHR, 1.26).
Factors that might explain the increased infection risk with severe psoriasis include the altered immune environment in these patients, the researchers wrote in their discussion of the findings. Also, “patients with severe psoriasis are defined by their eligibility for systemics, either conventional or biologic,” and their increased infection risk may stem from these treatments, rather than disease severity itself, they noted.
The study findings were limited by several factors including the lack of data on such confounders as weight, body mass index, and smoking status, they added. Other limitations included potential surveillance bias because of greater TB screening, and the use of prescriptions, rather than the Psoriasis Area Severity Index, to define severity. However, the results were strengthened by the large sample size, and suggest that patients with any type of psoriasis have higher rates of any infection, severe or rare, than the general population, the researchers concluded.
Data show need for clinician vigilance
Based on the 2020 Census data, an estimated 7.55 million adults in the United States have psoriasis, David Robles, MD, said in an interview. “Patients with psoriasis have a high risk for multiple comorbid conditions including metabolic syndrome, which is characterized by obesity, hypertension, and dyslipidemia,” said Dr. Robles, a dermatologist in private practice in Pomona, Calif., who was not involved in the study. “Although these complications were previously attributed to diet and obesity, it has become clear that the proinflammatory cytokines associated with psoriasis may be playing an important role underlying the pathologic basis of these other comorbidities.”
There is an emerging body of literature “indicating that psoriasis is associated with an increased risk of infections,” he added. Research in this area is particularly important because of the increased risk of infections associated with many biologic and immune-modulating treatments for psoriasis, Dr. Robles noted.
The study findings “indicate that, as the severity of psoriasis increases, so does the risk of severe and rare infections,” he said. “This makes it imperative for clinicians to be alert to the possibility of severe or rare infections in patients with psoriasis, especially those with severe psoriasis, so that early intervention can be initiated.”
As for additional research, “as an immunologist and dermatologist, I cannot help but think about the possible role the genetic and cytokine pathways involved in psoriasis may be playing in modulating the immune system and/or microbiome, and whether this contributes to a higher risk of infections,” Dr. Robles said. “Just as it was discovered that patients with atopic dermatitis have decreased levels of antimicrobial peptides in their skin, making them susceptible to recurrent bacterial skin infections, we may find that the genetic and immunological changes associated with psoriasis may independently contribute to infection susceptibility,” he noted. “More basic immunology and virology research may one day shed light on this observation.”
The study was supported by Novartis. Lead author Dr. Loft disclosed serving as a speaker for Eli Lilly and Janssen Cilag, other authors disclosed relationships with multiple companies including Novartis, and two authors are Novartis employees. Dr. Robles had no relevant financial disclosures.
of nearly 95,000 patients.
Although previous studies have shown a higher risk for comorbid conditions in people with psoriasis, compared with those without psoriasis, data on the occurrence of severe and rare infections in patients with psoriasis are limited, wrote Nikolai Loft, MD, of the department of dermatology and allergy, Copenhagen University Hospital, Gentofte, and colleagues.
Psoriasis patients are often treated with immunosuppressive therapies that may promote or aggravate infections; therefore, a better understanding of psoriasis and risk of infections is needed, they said. In a study published in the British Journal of Dermatology, Dr. Loft and his coinvestigators reviewed data on adults aged 18 years and older from the Danish National Patient Register between Jan. 1, 1997 and Dec. 31, 2018. The study population included 94,450 adults with psoriasis and 566,700 matched controls. Patients with any type of psoriasis and any degree of severity were included.
The primary outcome was the occurrence of severe infections, defined as those requiring assessment at a hospital, and rare infections, defined as HIV, TB, HBV, and HCV. The median age of the participants was 52.3 years, and slightly more than half were women.
Overall, the incidence rate of severe and rare infections among patients with any type of psoriasis was 3,104.9 per 100,000 person-years, compared with 2,381.1 for controls, with a hazard ratio, adjusted for gender, age, ethnicity, socioeconomic status, alcohol-related conditions, and Charlson comorbidity index (aHR) of 1.29.
For any infections resulting in hospitalization, the incidence rate was 2,005.1 vs. 1,531.8 per 100,000 person-years for patients with any type of psoriasis and controls, respectively.
The results were similar when severe infections and rare infections were analyzed separately. The incidence rate of severe infections was 3,080.6 and 2,364.4 per 100,000 person-years for patients with any psoriasis, compared with controls; the incidence rate for rare infections was 42.9 and 31.8 for all psoriasis patients and controls, respectively.
When the data were examined by psoriasis severity, the incidence rate of severe and rare infections among patients with severe psoriasis was 3,847.7 per 100,000 person-years, compared with 2,351.9 per 100,000 person years among controls (aHR, 1.58) and also higher than in patients with mild psoriasis. The incidence rate of severe and rare infections in patients with mild psoriasis (2,979.1 per 100,000 person-years) also was higher than in controls (aHR, 1.26).
Factors that might explain the increased infection risk with severe psoriasis include the altered immune environment in these patients, the researchers wrote in their discussion of the findings. Also, “patients with severe psoriasis are defined by their eligibility for systemics, either conventional or biologic,” and their increased infection risk may stem from these treatments, rather than disease severity itself, they noted.
The study findings were limited by several factors including the lack of data on such confounders as weight, body mass index, and smoking status, they added. Other limitations included potential surveillance bias because of greater TB screening, and the use of prescriptions, rather than the Psoriasis Area Severity Index, to define severity. However, the results were strengthened by the large sample size, and suggest that patients with any type of psoriasis have higher rates of any infection, severe or rare, than the general population, the researchers concluded.
Data show need for clinician vigilance
Based on the 2020 Census data, an estimated 7.55 million adults in the United States have psoriasis, David Robles, MD, said in an interview. “Patients with psoriasis have a high risk for multiple comorbid conditions including metabolic syndrome, which is characterized by obesity, hypertension, and dyslipidemia,” said Dr. Robles, a dermatologist in private practice in Pomona, Calif., who was not involved in the study. “Although these complications were previously attributed to diet and obesity, it has become clear that the proinflammatory cytokines associated with psoriasis may be playing an important role underlying the pathologic basis of these other comorbidities.”
There is an emerging body of literature “indicating that psoriasis is associated with an increased risk of infections,” he added. Research in this area is particularly important because of the increased risk of infections associated with many biologic and immune-modulating treatments for psoriasis, Dr. Robles noted.
The study findings “indicate that, as the severity of psoriasis increases, so does the risk of severe and rare infections,” he said. “This makes it imperative for clinicians to be alert to the possibility of severe or rare infections in patients with psoriasis, especially those with severe psoriasis, so that early intervention can be initiated.”
As for additional research, “as an immunologist and dermatologist, I cannot help but think about the possible role the genetic and cytokine pathways involved in psoriasis may be playing in modulating the immune system and/or microbiome, and whether this contributes to a higher risk of infections,” Dr. Robles said. “Just as it was discovered that patients with atopic dermatitis have decreased levels of antimicrobial peptides in their skin, making them susceptible to recurrent bacterial skin infections, we may find that the genetic and immunological changes associated with psoriasis may independently contribute to infection susceptibility,” he noted. “More basic immunology and virology research may one day shed light on this observation.”
The study was supported by Novartis. Lead author Dr. Loft disclosed serving as a speaker for Eli Lilly and Janssen Cilag, other authors disclosed relationships with multiple companies including Novartis, and two authors are Novartis employees. Dr. Robles had no relevant financial disclosures.
of nearly 95,000 patients.
Although previous studies have shown a higher risk for comorbid conditions in people with psoriasis, compared with those without psoriasis, data on the occurrence of severe and rare infections in patients with psoriasis are limited, wrote Nikolai Loft, MD, of the department of dermatology and allergy, Copenhagen University Hospital, Gentofte, and colleagues.
Psoriasis patients are often treated with immunosuppressive therapies that may promote or aggravate infections; therefore, a better understanding of psoriasis and risk of infections is needed, they said. In a study published in the British Journal of Dermatology, Dr. Loft and his coinvestigators reviewed data on adults aged 18 years and older from the Danish National Patient Register between Jan. 1, 1997 and Dec. 31, 2018. The study population included 94,450 adults with psoriasis and 566,700 matched controls. Patients with any type of psoriasis and any degree of severity were included.
The primary outcome was the occurrence of severe infections, defined as those requiring assessment at a hospital, and rare infections, defined as HIV, TB, HBV, and HCV. The median age of the participants was 52.3 years, and slightly more than half were women.
Overall, the incidence rate of severe and rare infections among patients with any type of psoriasis was 3,104.9 per 100,000 person-years, compared with 2,381.1 for controls, with a hazard ratio, adjusted for gender, age, ethnicity, socioeconomic status, alcohol-related conditions, and Charlson comorbidity index (aHR) of 1.29.
For any infections resulting in hospitalization, the incidence rate was 2,005.1 vs. 1,531.8 per 100,000 person-years for patients with any type of psoriasis and controls, respectively.
The results were similar when severe infections and rare infections were analyzed separately. The incidence rate of severe infections was 3,080.6 and 2,364.4 per 100,000 person-years for patients with any psoriasis, compared with controls; the incidence rate for rare infections was 42.9 and 31.8 for all psoriasis patients and controls, respectively.
When the data were examined by psoriasis severity, the incidence rate of severe and rare infections among patients with severe psoriasis was 3,847.7 per 100,000 person-years, compared with 2,351.9 per 100,000 person years among controls (aHR, 1.58) and also higher than in patients with mild psoriasis. The incidence rate of severe and rare infections in patients with mild psoriasis (2,979.1 per 100,000 person-years) also was higher than in controls (aHR, 1.26).
Factors that might explain the increased infection risk with severe psoriasis include the altered immune environment in these patients, the researchers wrote in their discussion of the findings. Also, “patients with severe psoriasis are defined by their eligibility for systemics, either conventional or biologic,” and their increased infection risk may stem from these treatments, rather than disease severity itself, they noted.
The study findings were limited by several factors including the lack of data on such confounders as weight, body mass index, and smoking status, they added. Other limitations included potential surveillance bias because of greater TB screening, and the use of prescriptions, rather than the Psoriasis Area Severity Index, to define severity. However, the results were strengthened by the large sample size, and suggest that patients with any type of psoriasis have higher rates of any infection, severe or rare, than the general population, the researchers concluded.
Data show need for clinician vigilance
Based on the 2020 Census data, an estimated 7.55 million adults in the United States have psoriasis, David Robles, MD, said in an interview. “Patients with psoriasis have a high risk for multiple comorbid conditions including metabolic syndrome, which is characterized by obesity, hypertension, and dyslipidemia,” said Dr. Robles, a dermatologist in private practice in Pomona, Calif., who was not involved in the study. “Although these complications were previously attributed to diet and obesity, it has become clear that the proinflammatory cytokines associated with psoriasis may be playing an important role underlying the pathologic basis of these other comorbidities.”
There is an emerging body of literature “indicating that psoriasis is associated with an increased risk of infections,” he added. Research in this area is particularly important because of the increased risk of infections associated with many biologic and immune-modulating treatments for psoriasis, Dr. Robles noted.
The study findings “indicate that, as the severity of psoriasis increases, so does the risk of severe and rare infections,” he said. “This makes it imperative for clinicians to be alert to the possibility of severe or rare infections in patients with psoriasis, especially those with severe psoriasis, so that early intervention can be initiated.”
As for additional research, “as an immunologist and dermatologist, I cannot help but think about the possible role the genetic and cytokine pathways involved in psoriasis may be playing in modulating the immune system and/or microbiome, and whether this contributes to a higher risk of infections,” Dr. Robles said. “Just as it was discovered that patients with atopic dermatitis have decreased levels of antimicrobial peptides in their skin, making them susceptible to recurrent bacterial skin infections, we may find that the genetic and immunological changes associated with psoriasis may independently contribute to infection susceptibility,” he noted. “More basic immunology and virology research may one day shed light on this observation.”
The study was supported by Novartis. Lead author Dr. Loft disclosed serving as a speaker for Eli Lilly and Janssen Cilag, other authors disclosed relationships with multiple companies including Novartis, and two authors are Novartis employees. Dr. Robles had no relevant financial disclosures.
FROM BRITISH JOURNAL OF DERMATOLOGY
A 64-year-old woman presents with a history of asymptomatic erythematous grouped papules on the right breast
. Recurrences may occur. Rarely, lymph nodes, the gastrointestinal system, lung, bone and bone marrow may be involved as extracutaneous sites.
Primary cutaneous B-cell lymphomas account for approximately 25% of all cutaneous lymphomas. Clinically, patients present with either solitary or multiple papules or plaques, typically on the upper extremities or trunk.
Histopathology is vital for the correct diagnosis. In this patient, the histologic report was written as follows: “The findings are those of a well-differentiated but atypical diffuse mixed small lymphocytic infiltrate representing a mixture of T-cells and B-cells. The minor component of the infiltrate is of T-cell lineage, whereby the cells do not show any phenotypic abnormalities. The background cell population is interpreted as reactive. However, the dominant cell population is in fact of B-cell lineage. It is extensively highlighted by CD20. Only a minor component of the B cell infiltrate appeared to be in the context of representing germinal centers as characterized by small foci of centrocytic and centroblastic infiltration highlighted by BCL6 and CD10. The overwhelming B-cell component is a non–germinal center small B cell that does demonstrate BCL2 positivity and significant immunoreactivity for CD23. This small lymphocytic infiltrate obscures the germinal centers. There are only a few plasma cells; they do not show light chain restriction.”
The pathologist remarked that “this type of morphology of a diffuse small B-cell lymphocytic infiltrate that is without any evidence of light chain restriction amidst plasma cells, whereby the B cell component is dominant over the T-cell component would in fact be consistent with a unique variant of marginal zone lymphoma derived from a naive mantle zone.”
PCMZL has an excellent prognosis. When limited to the skin, local radiation or excision are effective treatments. Intravenous rituximab has been used to treat multifocal PCMZL. This patient was found to have no extracutaneous involvement and was treated with radiation.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Virmani P et al. JAAD Case Rep. 2017 Jun 14;3(4):269-72.
Magro CM and Olson LC. Ann Diagn Pathol. 2018 Jun;34:116-21.
. Recurrences may occur. Rarely, lymph nodes, the gastrointestinal system, lung, bone and bone marrow may be involved as extracutaneous sites.
Primary cutaneous B-cell lymphomas account for approximately 25% of all cutaneous lymphomas. Clinically, patients present with either solitary or multiple papules or plaques, typically on the upper extremities or trunk.
Histopathology is vital for the correct diagnosis. In this patient, the histologic report was written as follows: “The findings are those of a well-differentiated but atypical diffuse mixed small lymphocytic infiltrate representing a mixture of T-cells and B-cells. The minor component of the infiltrate is of T-cell lineage, whereby the cells do not show any phenotypic abnormalities. The background cell population is interpreted as reactive. However, the dominant cell population is in fact of B-cell lineage. It is extensively highlighted by CD20. Only a minor component of the B cell infiltrate appeared to be in the context of representing germinal centers as characterized by small foci of centrocytic and centroblastic infiltration highlighted by BCL6 and CD10. The overwhelming B-cell component is a non–germinal center small B cell that does demonstrate BCL2 positivity and significant immunoreactivity for CD23. This small lymphocytic infiltrate obscures the germinal centers. There are only a few plasma cells; they do not show light chain restriction.”
The pathologist remarked that “this type of morphology of a diffuse small B-cell lymphocytic infiltrate that is without any evidence of light chain restriction amidst plasma cells, whereby the B cell component is dominant over the T-cell component would in fact be consistent with a unique variant of marginal zone lymphoma derived from a naive mantle zone.”
PCMZL has an excellent prognosis. When limited to the skin, local radiation or excision are effective treatments. Intravenous rituximab has been used to treat multifocal PCMZL. This patient was found to have no extracutaneous involvement and was treated with radiation.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Virmani P et al. JAAD Case Rep. 2017 Jun 14;3(4):269-72.
Magro CM and Olson LC. Ann Diagn Pathol. 2018 Jun;34:116-21.
. Recurrences may occur. Rarely, lymph nodes, the gastrointestinal system, lung, bone and bone marrow may be involved as extracutaneous sites.
Primary cutaneous B-cell lymphomas account for approximately 25% of all cutaneous lymphomas. Clinically, patients present with either solitary or multiple papules or plaques, typically on the upper extremities or trunk.
Histopathology is vital for the correct diagnosis. In this patient, the histologic report was written as follows: “The findings are those of a well-differentiated but atypical diffuse mixed small lymphocytic infiltrate representing a mixture of T-cells and B-cells. The minor component of the infiltrate is of T-cell lineage, whereby the cells do not show any phenotypic abnormalities. The background cell population is interpreted as reactive. However, the dominant cell population is in fact of B-cell lineage. It is extensively highlighted by CD20. Only a minor component of the B cell infiltrate appeared to be in the context of representing germinal centers as characterized by small foci of centrocytic and centroblastic infiltration highlighted by BCL6 and CD10. The overwhelming B-cell component is a non–germinal center small B cell that does demonstrate BCL2 positivity and significant immunoreactivity for CD23. This small lymphocytic infiltrate obscures the germinal centers. There are only a few plasma cells; they do not show light chain restriction.”
The pathologist remarked that “this type of morphology of a diffuse small B-cell lymphocytic infiltrate that is without any evidence of light chain restriction amidst plasma cells, whereby the B cell component is dominant over the T-cell component would in fact be consistent with a unique variant of marginal zone lymphoma derived from a naive mantle zone.”
PCMZL has an excellent prognosis. When limited to the skin, local radiation or excision are effective treatments. Intravenous rituximab has been used to treat multifocal PCMZL. This patient was found to have no extracutaneous involvement and was treated with radiation.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Virmani P et al. JAAD Case Rep. 2017 Jun 14;3(4):269-72.
Magro CM and Olson LC. Ann Diagn Pathol. 2018 Jun;34:116-21.