User login
NeoChemo preserves rectum in half of patients with rectal cancer
Among patients with stage II or stage III rectal adenocarcinoma, organ preservation is achievable in up to half of patients who undergo total neoadjuvant chemotherapy (TNT), according to the results from a new randomized phase 2 trial.
The study included 324 patients from 18 centers who were randomized into one of two groups: induction chemotherapy followed by chemoradiotherapy (INCT-CRT) or chemoradiotherapy followed by consolidation chemotherapy (CRT-CNCT). Patients in both groups then underwent either total mesorectal excision (TME) or a watch-and-wait strategy, depending on tumor response.
“What the study shows is that the order of the chemo and the radiation dose doesn’t affect survival, but it seems to affect the probability of preserving the rectum. That data is consistent with other studies that have compared head-to-head chemotherapy followed by radiation versus radiation followed by chemotherapy. In addition, the survival rate for this study is no different from other prospective studies that included patients with similar-stage tumors selected by MRI. So the data suggest that you can probably avoid surgery in half of the patients with locally advanced rectal cancer and still achieve similar survival compared to patients treated with more conventional neoadjuvant treatments and mandatory surgery,” said lead author Julio Garcia-Aguilar, MD, PhD, in an interview.
“It is a significant shift in the treatment paradigm, that can potentially benefit half of the 50,000 rectal cancer patients diagnosed every year in the United States,” said Dr. Garcia-Aguilar, chief of colorectal surgery at Memorial Sloan Kettering Cancer Center, New York.
The study was published online in the Journal of Clinical Oncology.
Neoadjuvant CRT, TME, and adjuvant chemotherapy is an effective treatment strategy for locally advanced rectal adenocarcinoma, but the regimen can cause bowel, urinary, and sexual dysfunction. The majority of adverse effects from the therapy can be traced to surgery. In addition, some patients with distal rectal cancer often require a permanent colostomy.
TNT is a newer approach that delivers chemotherapy plus radiotherapy before surgery. It is designed to improve treatment compliance and eradicate micrometastases in advance of surgery.
After a median follow-up of 3 years, disease-free survival (76% in both groups) was similar to historical controls (75%). Both groups had similar rates of local recurrence-free survival (94% each) and distant metastasis–free survival (84% for INCT-CRT and 82% for CRT-CNCT).
Following TNT, 26% of patients were recommended for TME, including 28% in the INCT-CRT group and 24% in the CRT-CNCT group, and the rest offered watchful-waiting. Forty percent of those in the INCT-CRT group and 27% in the CRT-CNCT group who went on to watchful waiting had tumor regrowth. Of these combined 75 patients, 67 underwent successful salvage surgery.
In the intention-to-treat analysis, 53% of patients had a preserved rectum at 3 years (95% confidence interval, 45%-62%) in the CRT-CNCT group versus 41% in the INCT-CRT group (95% CI, 33%-50%; P = .01).
The new results reinforce other results and should contribute to shifting clinical practice, according to Dr. Garcia-Aguilar. “I think what we have learned is that rectal cancers respond to chemotherapy and radiation at a higher rate that we thought previously, but that the response takes time. That’s something that we use currently in an adaptive way to modify the treatment as we observe the tumor response,” he said.
The slow regrowth means that patients can be closely monitored without undue risk, but such an approach demands buy-in from the patient. “The patient needs to be compliant with a close surveillance protocol, because otherwise it can be a disaster. I think that’s really part of the message,” Dr. Garcia-Aguilar said.
Dr. Garcia-Aguilar has an ownership interest in Intuitive Surgical and has advised or consulted for Medtronic, Intuitive Surgical, and Johnson & Johnson.
Among patients with stage II or stage III rectal adenocarcinoma, organ preservation is achievable in up to half of patients who undergo total neoadjuvant chemotherapy (TNT), according to the results from a new randomized phase 2 trial.
The study included 324 patients from 18 centers who were randomized into one of two groups: induction chemotherapy followed by chemoradiotherapy (INCT-CRT) or chemoradiotherapy followed by consolidation chemotherapy (CRT-CNCT). Patients in both groups then underwent either total mesorectal excision (TME) or a watch-and-wait strategy, depending on tumor response.
“What the study shows is that the order of the chemo and the radiation dose doesn’t affect survival, but it seems to affect the probability of preserving the rectum. That data is consistent with other studies that have compared head-to-head chemotherapy followed by radiation versus radiation followed by chemotherapy. In addition, the survival rate for this study is no different from other prospective studies that included patients with similar-stage tumors selected by MRI. So the data suggest that you can probably avoid surgery in half of the patients with locally advanced rectal cancer and still achieve similar survival compared to patients treated with more conventional neoadjuvant treatments and mandatory surgery,” said lead author Julio Garcia-Aguilar, MD, PhD, in an interview.
“It is a significant shift in the treatment paradigm, that can potentially benefit half of the 50,000 rectal cancer patients diagnosed every year in the United States,” said Dr. Garcia-Aguilar, chief of colorectal surgery at Memorial Sloan Kettering Cancer Center, New York.
The study was published online in the Journal of Clinical Oncology.
Neoadjuvant CRT, TME, and adjuvant chemotherapy is an effective treatment strategy for locally advanced rectal adenocarcinoma, but the regimen can cause bowel, urinary, and sexual dysfunction. The majority of adverse effects from the therapy can be traced to surgery. In addition, some patients with distal rectal cancer often require a permanent colostomy.
TNT is a newer approach that delivers chemotherapy plus radiotherapy before surgery. It is designed to improve treatment compliance and eradicate micrometastases in advance of surgery.
After a median follow-up of 3 years, disease-free survival (76% in both groups) was similar to historical controls (75%). Both groups had similar rates of local recurrence-free survival (94% each) and distant metastasis–free survival (84% for INCT-CRT and 82% for CRT-CNCT).
Following TNT, 26% of patients were recommended for TME, including 28% in the INCT-CRT group and 24% in the CRT-CNCT group, and the rest offered watchful-waiting. Forty percent of those in the INCT-CRT group and 27% in the CRT-CNCT group who went on to watchful waiting had tumor regrowth. Of these combined 75 patients, 67 underwent successful salvage surgery.
In the intention-to-treat analysis, 53% of patients had a preserved rectum at 3 years (95% confidence interval, 45%-62%) in the CRT-CNCT group versus 41% in the INCT-CRT group (95% CI, 33%-50%; P = .01).
The new results reinforce other results and should contribute to shifting clinical practice, according to Dr. Garcia-Aguilar. “I think what we have learned is that rectal cancers respond to chemotherapy and radiation at a higher rate that we thought previously, but that the response takes time. That’s something that we use currently in an adaptive way to modify the treatment as we observe the tumor response,” he said.
The slow regrowth means that patients can be closely monitored without undue risk, but such an approach demands buy-in from the patient. “The patient needs to be compliant with a close surveillance protocol, because otherwise it can be a disaster. I think that’s really part of the message,” Dr. Garcia-Aguilar said.
Dr. Garcia-Aguilar has an ownership interest in Intuitive Surgical and has advised or consulted for Medtronic, Intuitive Surgical, and Johnson & Johnson.
Among patients with stage II or stage III rectal adenocarcinoma, organ preservation is achievable in up to half of patients who undergo total neoadjuvant chemotherapy (TNT), according to the results from a new randomized phase 2 trial.
The study included 324 patients from 18 centers who were randomized into one of two groups: induction chemotherapy followed by chemoradiotherapy (INCT-CRT) or chemoradiotherapy followed by consolidation chemotherapy (CRT-CNCT). Patients in both groups then underwent either total mesorectal excision (TME) or a watch-and-wait strategy, depending on tumor response.
“What the study shows is that the order of the chemo and the radiation dose doesn’t affect survival, but it seems to affect the probability of preserving the rectum. That data is consistent with other studies that have compared head-to-head chemotherapy followed by radiation versus radiation followed by chemotherapy. In addition, the survival rate for this study is no different from other prospective studies that included patients with similar-stage tumors selected by MRI. So the data suggest that you can probably avoid surgery in half of the patients with locally advanced rectal cancer and still achieve similar survival compared to patients treated with more conventional neoadjuvant treatments and mandatory surgery,” said lead author Julio Garcia-Aguilar, MD, PhD, in an interview.
“It is a significant shift in the treatment paradigm, that can potentially benefit half of the 50,000 rectal cancer patients diagnosed every year in the United States,” said Dr. Garcia-Aguilar, chief of colorectal surgery at Memorial Sloan Kettering Cancer Center, New York.
The study was published online in the Journal of Clinical Oncology.
Neoadjuvant CRT, TME, and adjuvant chemotherapy is an effective treatment strategy for locally advanced rectal adenocarcinoma, but the regimen can cause bowel, urinary, and sexual dysfunction. The majority of adverse effects from the therapy can be traced to surgery. In addition, some patients with distal rectal cancer often require a permanent colostomy.
TNT is a newer approach that delivers chemotherapy plus radiotherapy before surgery. It is designed to improve treatment compliance and eradicate micrometastases in advance of surgery.
After a median follow-up of 3 years, disease-free survival (76% in both groups) was similar to historical controls (75%). Both groups had similar rates of local recurrence-free survival (94% each) and distant metastasis–free survival (84% for INCT-CRT and 82% for CRT-CNCT).
Following TNT, 26% of patients were recommended for TME, including 28% in the INCT-CRT group and 24% in the CRT-CNCT group, and the rest offered watchful-waiting. Forty percent of those in the INCT-CRT group and 27% in the CRT-CNCT group who went on to watchful waiting had tumor regrowth. Of these combined 75 patients, 67 underwent successful salvage surgery.
In the intention-to-treat analysis, 53% of patients had a preserved rectum at 3 years (95% confidence interval, 45%-62%) in the CRT-CNCT group versus 41% in the INCT-CRT group (95% CI, 33%-50%; P = .01).
The new results reinforce other results and should contribute to shifting clinical practice, according to Dr. Garcia-Aguilar. “I think what we have learned is that rectal cancers respond to chemotherapy and radiation at a higher rate that we thought previously, but that the response takes time. That’s something that we use currently in an adaptive way to modify the treatment as we observe the tumor response,” he said.
The slow regrowth means that patients can be closely monitored without undue risk, but such an approach demands buy-in from the patient. “The patient needs to be compliant with a close surveillance protocol, because otherwise it can be a disaster. I think that’s really part of the message,” Dr. Garcia-Aguilar said.
Dr. Garcia-Aguilar has an ownership interest in Intuitive Surgical and has advised or consulted for Medtronic, Intuitive Surgical, and Johnson & Johnson.
FROM JOURNAL OF CLINICAL ONCOLOGY
Bleeding Nodule on the Lip
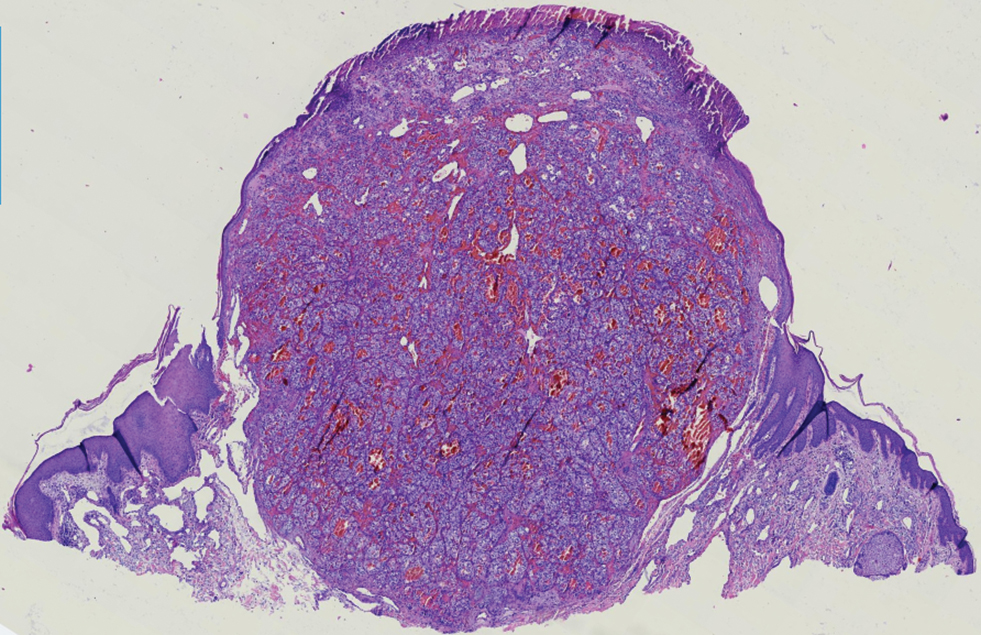
The Diagnosis: Metastatic Clear Cell Renal Cell Carcinoma
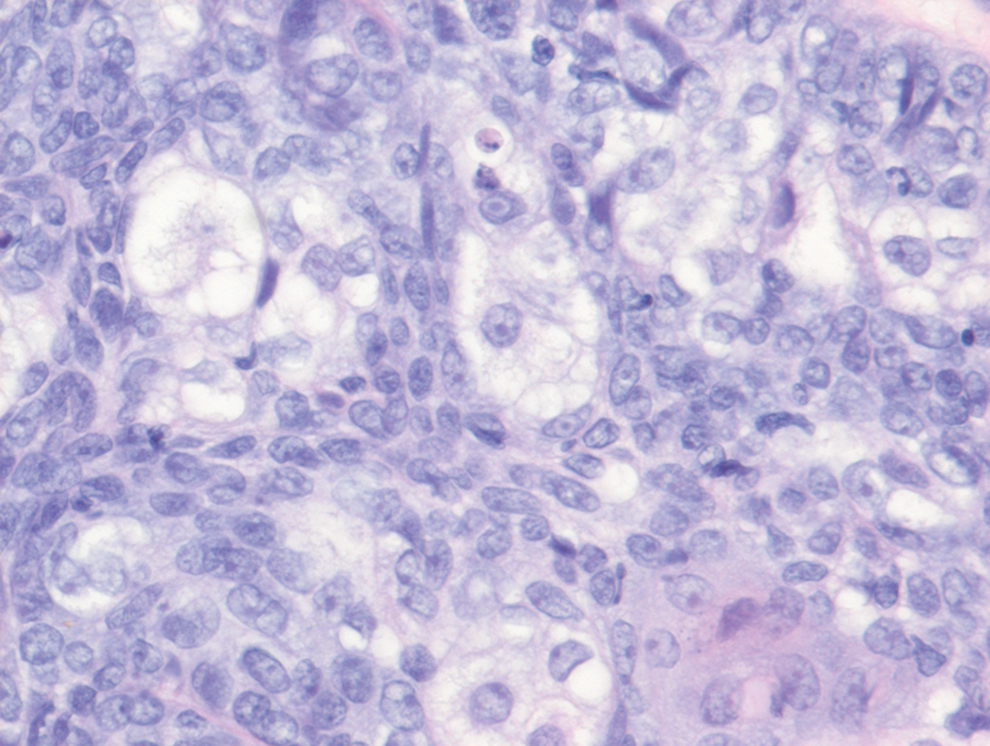
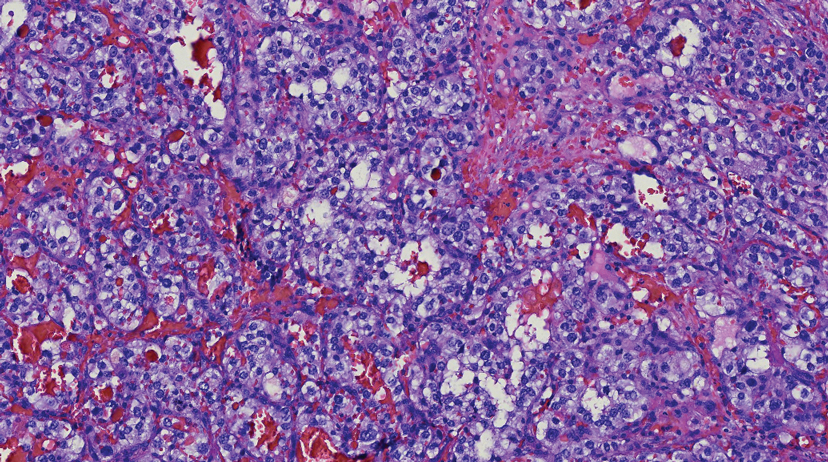
Renal cell carcinoma (RCC) is a common genitourinary system malignancy with incidence peaking between 50 and 70 years of age and a male predominance.1 The clear cell variant is the most common subtype of RCC, accounting for 70% to 75% of all cases. It is known to be a highly aggressive malignancy that frequently metastasizes to the lungs, lymphatics, bones, liver, and brain.2,3 Approximately 20% to 50% of patients with RCC eventually will develop metastasis after nephrectomy.4 Survival with metastatic RCC to any site typically is in the range of 10 to 22 months.5,6 Cutaneous metastases of RCC rarely have been reported in the literature (3%–6% of cases7) and most commonly are found on the scalp, followed by the chest or abdomen. 8 Cutaneous metastases generally are regarded as a late manifestation of the disease with a very poor prognosis. 9 It is unusual to identify cutaneous RCC metastasis without known RCC or other symptoms consistent with advanced RCC, such as hematuria or abdominal/flank pain. Renal cell carcinoma accounts for an estimated 6% to 7% of all cutaneous metastatic lesions.10 Cutaneous metastatic lesions of RCC often are solitary and grow rapidly, with the clinical appearance of an erythematous or violaceous, nodular, highly vascular, and often hemorrhagic growth.9,11,12
Following the histologic diagnosis of metastatic clear cell RCC, our patient was referred to medical oncology for further workup. Magnetic resonance imaging and a positron emission tomography scan demonstrated widespread disease with a 7-cm left renal mass, liver and lung metastases, and bilateral mediastinal lymphadenopathy. The patient was started on combination immunotherapy as a palliative treatment given the widespread disease.
Histologically, clear cell RCC is characterized by lipid and glycogen-rich cells with ample cytoplasm and a well-developed vascular network, which often is thin walled with a chicken wire–like architecture. Metastatic clear cell RCC tumor cells may form glandular, acinar, or papillary structures with variable lymphocytic inflammatory infiltrates and abundant capillary formation. Immunohistochemically, the tumor cells should demonstrate positivity for paired box gene 8, PAX8, and RCC marker antigen.13 Vimentin and carcinoembryonic antigen may be utilized to distinguish from hidradenoma as carcinoembryonic antigen will be positive in hidradenoma and vimentin will be negative.14 Renal cell carcinoma also has a common molecular signature of von Hippel-Lindau tumor suppressor gene inactivation as well as upregulation of hypoxia inducible factor and vascular endothelial growth factor.15
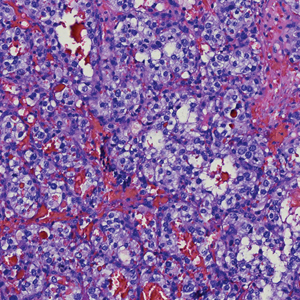
Balloon cell nevi often clinically present in young patients as bicolored nevi that sometimes are polypoid or verrucous in appearance with central yellow globules surrounded by a peripheral reticular pattern on dermoscopy. Histologically, balloon cell nevi are characterized by large cells with small, round, centrally located basophilic nuclei and clear foamy cytoplasm (Figure 1), which are thought to be formed by progressive vacuolization of melanocytes due to the enlargement and disintegration of melanosomes. This ballooning change reflects an seen in malignant melanoma, in which case nuclear pleomorphism, atypia, and increased mitotic activity also are observed. The prominent vascular network characteristic of RCC typically is not present.16
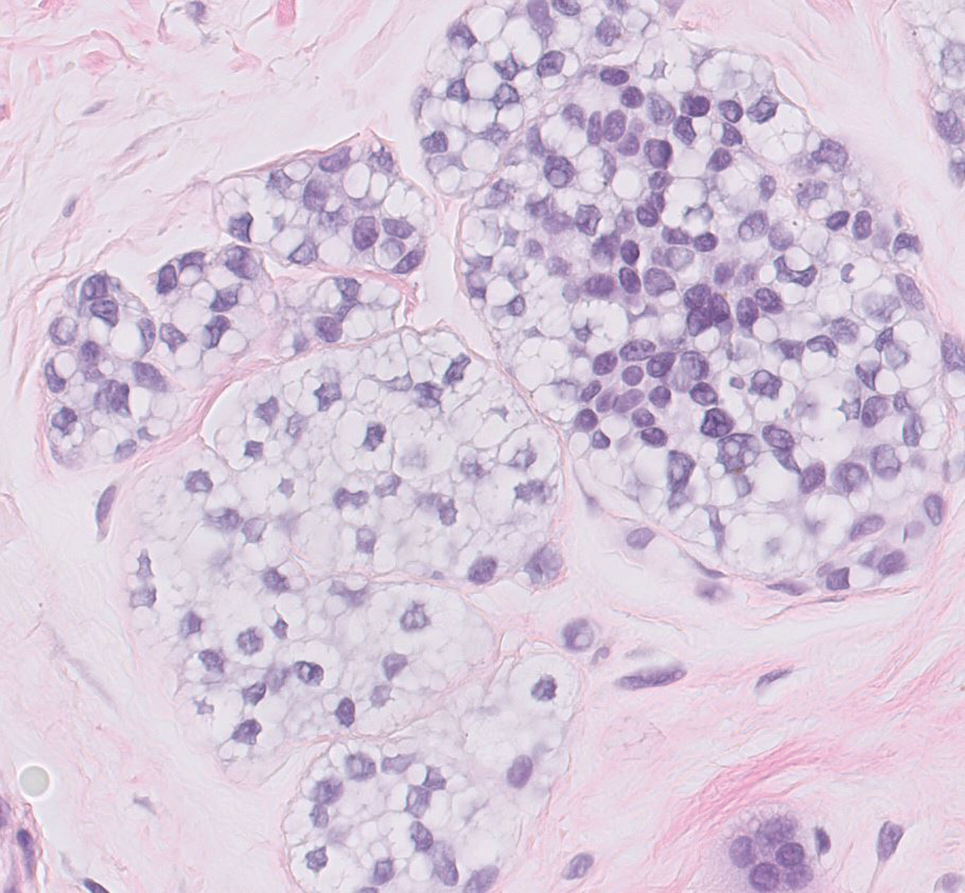
Clear cell hidradenomas are benign skin appendage tumors that often present as small, firm, solitary dermal nodules that may extend into the subcutaneous fat. They have a predilection for the head, face, and arms and demonstrate 2 predominant cell types, including a polyhedral cell with a rounded nucleus and slightly basophilic cytoplasm as well as a round cell with clear cytoplasm and bland nuclei (Figure 2). The latter cell type is less common, representing the predominant cell type in less than one-third of hidradenomas, and can present a diagnostic quandary based on histologic similarity to other clear cell neoplasms. The clear cells contain glycogen but no lipid. Ductlike structures often are present, and the intervening stroma varies from delicate vascularized cords of fibrous tissue to dense hyalinized collagen. Immunohistochemistry may be required for definitive diagnosis, and clear cell hidradenomas should react with monoclonal antibodies that label both eccrine and apocrine secretory elements, such as cytokeratins 6/18, 7, and 8/18.17
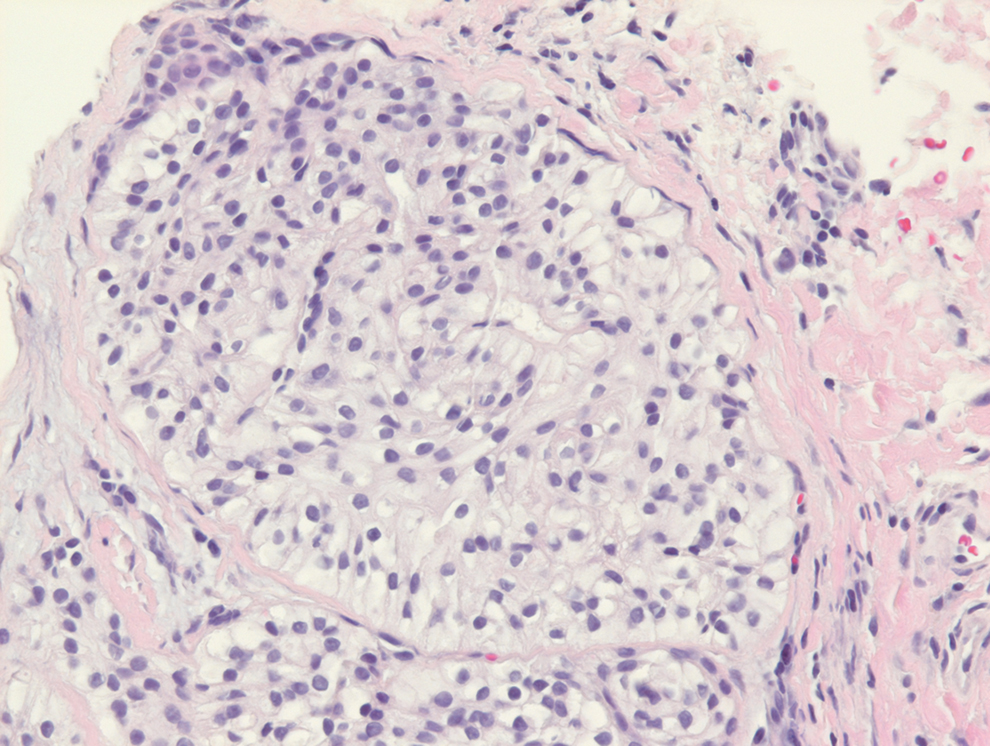
Pyogenic granulomas (also referred to as lobular capillary hemangiomas) are common and present clinically as rapidly growing, polypoid, red masses surrounded by a thickened epidermis that often are found on the fingers or lips. This entity is benign and often regresses spontaneously. Histologically, pyogenic granulomas are characterized by a lobular pattern of vascular proliferation associated with edema and inflammation resembling granulation tissue, with acanthosis and hyperkeratosis at the edges of the lesion (Figure 3).18
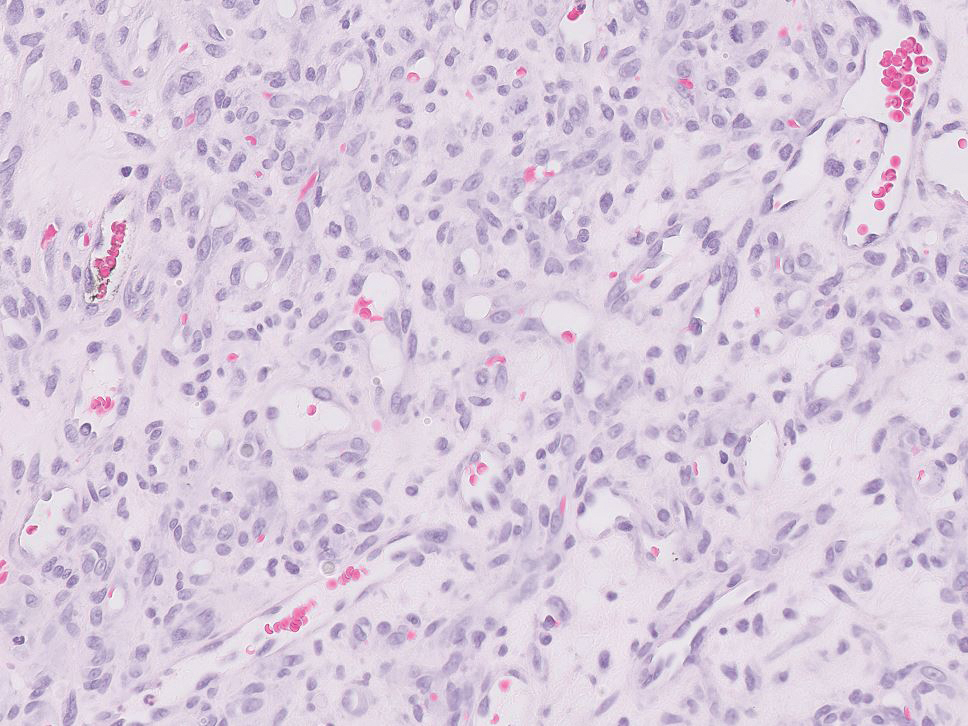
Sebaceous carcinoma is a locally aggressive malignant neoplasm arising from the cells of the sebaceous glands and occurring most commonly in the periorbital area. This neoplasm most often affects older adults, with a mean age at diagnosis of 63 to 77 years. It commonly presents as a solitary nodule with yellowish discoloration and madarosis, which is a key distinguishing feature to differentiate this entity from a chalazion or hordeolum. Histologically, sebaceous carcinoma is a dermal-based infiltrative, nodular tumor with varying degrees of clear cell changes—well-differentiated tumors show more clear cell change as compared to more poorly differentiated variants—along with basaloid or squamous features and abundant mitotic activity (Figure 4), which may be useful in distinguishing it from the other entities in the clear cell neoplasm differential.19-22
- Alves de Paula T, Lopes da Silva P, Sueth Berriel LG. Renal cell carcinoma with cutaneous metastasis: case report. J Bras Nefrol. 2010;32:213-215.
- Amaadour L, Atreche L, Azegrar M, et al. Cutaneous metastasis of renal cell carcinoma: a case report. J Cancer Ther. 2017;8:603-607.
- Weiss L, Harlos JP, Torhorst J, et al. Metastatic patterns of renal carcinoma: an analysis of 687 necropsies. J Cancer Res Clin Oncol. 1988;114:605-612.
- Flamigan RC, Campbell SC, Clark JI, et al. Metastatic renal cell carcinoma. Curr Treat Options Oncol. 2003;4:385-390.
- Motzer RJ, Bacik J, Schwarz LH, et al. Prognostic factors for survival in previously treated patients with metastatic renal cell carcinoma. J Clin Oncol. 2004;22:453-463.
- Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor–targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27:5694-5799.
- Smyth LG, Rowan GC, David MQ. Renal cell carcinoma presenting as an ominous metachronous scalp metastasis. Can Urol Assoc J. 2010;4:E64-E66.
- Dorairajan LN, Hemal AK, Aron M, et al. Cutaneous metastases in renal cell carcinoma. Urol Int. 1999;63:164-167.
- Koga S, Tsuda S, Nishikido M, et al. Renal cell carcinoma metastatic to the skin. Anticancer Res. 2000;20:1939-1940.
- Krathen RA, Orengo IF, Rosen T. Cutaneous metastasis: a metaanalysis of the data. South Med J. 2003;96:164-167.
- Amano Y, Ohni S, Ishige T, et al. A case of cutaneous metastasis from a clear cell renal cell carcinoma with an eosinophilic cell component to the submandibular region. J Nihon Univ Med Assoc. 2015;74:73-77.
- Arrabal-Polo MA, Arias-Santiago SA, Aneiros-Fernandez J, et al. Cutaneous metastases in renal cell carcinoma: a case report. Cases J. 2009;2:7948.
- Sangoi AR, Karamchandani J, Kim J, et al. The use of immunohistochemistry in the diagnosis of metastatic clear cell renal cell carcinoma: a review of PAX-8, PAX-2, hKIM-1, RCCma, and CD10. Adv Anat Pathol. 2010;17:377-393.
- Velez MJ, Thomas CL, Stratton J, et al. The utility of using immunohistochemistry in the differentiation of metastatic, cutaneous clear cell renal cell carcinoma and clear cell hidradenoma. J Cutan Pathol. 2017;44:612-615.
- Nezami BG, MacLennan G. Clear cell. PathologyOutlines website. Published April 20, 2021. Updated March 2, 2022. Accessed April 22, 2022. https://www.pathologyoutlines.com/topic/kidneytumormalignantrccclear.html
- Dhaille F, Courville P, Joly P, et al. Balloon cell nevus: histologic and dermoscopic features. J Am Acad Dermatol. 2015;72:E55-E56.
- Volmar KE, Cummings TJ, Wang WH, et al. Clear cell hidradenoma: a mimic of metastatic clear cell tumors. Arch Pathol Lab Med. 2005;129:E113-E116.
- Hale CS. Capillary/pyogenic granuloma. Pathology Outlines website. Published August 1, 2012. Updated March 10, 2022. Accessed April 20, 2022. https://www.pathologyoutlines.com/topic/skintumornonmelanocyticpyogenicgranuloma.html
- Zada S, Lee BA. Sebaceous carcinoma. Pathology Outlines website. Published August 11, 2021. Accessed April 20, 2022. https://www.pathologyoutlines.com/topic/skintumornonmelanocyticsebaceouscarcinoma.html
- Kahana A, Pribila, JT, Nelson CC, et al. Sebaceous cell carcinoma. In: Levin LA, Albert DM, eds. Ocular Disease: Mechanisms and Management. Elsevier; 2010:396-407.
- Wick MR. Cutaneous tumors and pseudotumors of the head and neck. In: Gnepp DR, ed. Diagnostic Surgical Pathology of the Head and Neck. 2nd ed. Saunders Elsevier; 2009:975-1068.
- Cassarino DS, Dadras SS, Lindberg MR, et al. Sebaceous carcinoma. In: Cassarino DS, Dadras SS, Lindberg MR, et al, eds. Diagnostic Pathology: Neoplastic Dermatopathology. 2nd ed. Elsevier; 2017:174-179.
The Diagnosis: Metastatic Clear Cell Renal Cell Carcinoma
Renal cell carcinoma (RCC) is a common genitourinary system malignancy with incidence peaking between 50 and 70 years of age and a male predominance.1 The clear cell variant is the most common subtype of RCC, accounting for 70% to 75% of all cases. It is known to be a highly aggressive malignancy that frequently metastasizes to the lungs, lymphatics, bones, liver, and brain.2,3 Approximately 20% to 50% of patients with RCC eventually will develop metastasis after nephrectomy.4 Survival with metastatic RCC to any site typically is in the range of 10 to 22 months.5,6 Cutaneous metastases of RCC rarely have been reported in the literature (3%–6% of cases7) and most commonly are found on the scalp, followed by the chest or abdomen. 8 Cutaneous metastases generally are regarded as a late manifestation of the disease with a very poor prognosis. 9 It is unusual to identify cutaneous RCC metastasis without known RCC or other symptoms consistent with advanced RCC, such as hematuria or abdominal/flank pain. Renal cell carcinoma accounts for an estimated 6% to 7% of all cutaneous metastatic lesions.10 Cutaneous metastatic lesions of RCC often are solitary and grow rapidly, with the clinical appearance of an erythematous or violaceous, nodular, highly vascular, and often hemorrhagic growth.9,11,12
Following the histologic diagnosis of metastatic clear cell RCC, our patient was referred to medical oncology for further workup. Magnetic resonance imaging and a positron emission tomography scan demonstrated widespread disease with a 7-cm left renal mass, liver and lung metastases, and bilateral mediastinal lymphadenopathy. The patient was started on combination immunotherapy as a palliative treatment given the widespread disease.
Histologically, clear cell RCC is characterized by lipid and glycogen-rich cells with ample cytoplasm and a well-developed vascular network, which often is thin walled with a chicken wire–like architecture. Metastatic clear cell RCC tumor cells may form glandular, acinar, or papillary structures with variable lymphocytic inflammatory infiltrates and abundant capillary formation. Immunohistochemically, the tumor cells should demonstrate positivity for paired box gene 8, PAX8, and RCC marker antigen.13 Vimentin and carcinoembryonic antigen may be utilized to distinguish from hidradenoma as carcinoembryonic antigen will be positive in hidradenoma and vimentin will be negative.14 Renal cell carcinoma also has a common molecular signature of von Hippel-Lindau tumor suppressor gene inactivation as well as upregulation of hypoxia inducible factor and vascular endothelial growth factor.15
Balloon cell nevi often clinically present in young patients as bicolored nevi that sometimes are polypoid or verrucous in appearance with central yellow globules surrounded by a peripheral reticular pattern on dermoscopy. Histologically, balloon cell nevi are characterized by large cells with small, round, centrally located basophilic nuclei and clear foamy cytoplasm (Figure 1), which are thought to be formed by progressive vacuolization of melanocytes due to the enlargement and disintegration of melanosomes. This ballooning change reflects an seen in malignant melanoma, in which case nuclear pleomorphism, atypia, and increased mitotic activity also are observed. The prominent vascular network characteristic of RCC typically is not present.16

Clear cell hidradenomas are benign skin appendage tumors that often present as small, firm, solitary dermal nodules that may extend into the subcutaneous fat. They have a predilection for the head, face, and arms and demonstrate 2 predominant cell types, including a polyhedral cell with a rounded nucleus and slightly basophilic cytoplasm as well as a round cell with clear cytoplasm and bland nuclei (Figure 2). The latter cell type is less common, representing the predominant cell type in less than one-third of hidradenomas, and can present a diagnostic quandary based on histologic similarity to other clear cell neoplasms. The clear cells contain glycogen but no lipid. Ductlike structures often are present, and the intervening stroma varies from delicate vascularized cords of fibrous tissue to dense hyalinized collagen. Immunohistochemistry may be required for definitive diagnosis, and clear cell hidradenomas should react with monoclonal antibodies that label both eccrine and apocrine secretory elements, such as cytokeratins 6/18, 7, and 8/18.17

Pyogenic granulomas (also referred to as lobular capillary hemangiomas) are common and present clinically as rapidly growing, polypoid, red masses surrounded by a thickened epidermis that often are found on the fingers or lips. This entity is benign and often regresses spontaneously. Histologically, pyogenic granulomas are characterized by a lobular pattern of vascular proliferation associated with edema and inflammation resembling granulation tissue, with acanthosis and hyperkeratosis at the edges of the lesion (Figure 3).18

Sebaceous carcinoma is a locally aggressive malignant neoplasm arising from the cells of the sebaceous glands and occurring most commonly in the periorbital area. This neoplasm most often affects older adults, with a mean age at diagnosis of 63 to 77 years. It commonly presents as a solitary nodule with yellowish discoloration and madarosis, which is a key distinguishing feature to differentiate this entity from a chalazion or hordeolum. Histologically, sebaceous carcinoma is a dermal-based infiltrative, nodular tumor with varying degrees of clear cell changes—well-differentiated tumors show more clear cell change as compared to more poorly differentiated variants—along with basaloid or squamous features and abundant mitotic activity (Figure 4), which may be useful in distinguishing it from the other entities in the clear cell neoplasm differential.19-22

The Diagnosis: Metastatic Clear Cell Renal Cell Carcinoma
Renal cell carcinoma (RCC) is a common genitourinary system malignancy with incidence peaking between 50 and 70 years of age and a male predominance.1 The clear cell variant is the most common subtype of RCC, accounting for 70% to 75% of all cases. It is known to be a highly aggressive malignancy that frequently metastasizes to the lungs, lymphatics, bones, liver, and brain.2,3 Approximately 20% to 50% of patients with RCC eventually will develop metastasis after nephrectomy.4 Survival with metastatic RCC to any site typically is in the range of 10 to 22 months.5,6 Cutaneous metastases of RCC rarely have been reported in the literature (3%–6% of cases7) and most commonly are found on the scalp, followed by the chest or abdomen. 8 Cutaneous metastases generally are regarded as a late manifestation of the disease with a very poor prognosis. 9 It is unusual to identify cutaneous RCC metastasis without known RCC or other symptoms consistent with advanced RCC, such as hematuria or abdominal/flank pain. Renal cell carcinoma accounts for an estimated 6% to 7% of all cutaneous metastatic lesions.10 Cutaneous metastatic lesions of RCC often are solitary and grow rapidly, with the clinical appearance of an erythematous or violaceous, nodular, highly vascular, and often hemorrhagic growth.9,11,12
Following the histologic diagnosis of metastatic clear cell RCC, our patient was referred to medical oncology for further workup. Magnetic resonance imaging and a positron emission tomography scan demonstrated widespread disease with a 7-cm left renal mass, liver and lung metastases, and bilateral mediastinal lymphadenopathy. The patient was started on combination immunotherapy as a palliative treatment given the widespread disease.
Histologically, clear cell RCC is characterized by lipid and glycogen-rich cells with ample cytoplasm and a well-developed vascular network, which often is thin walled with a chicken wire–like architecture. Metastatic clear cell RCC tumor cells may form glandular, acinar, or papillary structures with variable lymphocytic inflammatory infiltrates and abundant capillary formation. Immunohistochemically, the tumor cells should demonstrate positivity for paired box gene 8, PAX8, and RCC marker antigen.13 Vimentin and carcinoembryonic antigen may be utilized to distinguish from hidradenoma as carcinoembryonic antigen will be positive in hidradenoma and vimentin will be negative.14 Renal cell carcinoma also has a common molecular signature of von Hippel-Lindau tumor suppressor gene inactivation as well as upregulation of hypoxia inducible factor and vascular endothelial growth factor.15
Balloon cell nevi often clinically present in young patients as bicolored nevi that sometimes are polypoid or verrucous in appearance with central yellow globules surrounded by a peripheral reticular pattern on dermoscopy. Histologically, balloon cell nevi are characterized by large cells with small, round, centrally located basophilic nuclei and clear foamy cytoplasm (Figure 1), which are thought to be formed by progressive vacuolization of melanocytes due to the enlargement and disintegration of melanosomes. This ballooning change reflects an seen in malignant melanoma, in which case nuclear pleomorphism, atypia, and increased mitotic activity also are observed. The prominent vascular network characteristic of RCC typically is not present.16

Clear cell hidradenomas are benign skin appendage tumors that often present as small, firm, solitary dermal nodules that may extend into the subcutaneous fat. They have a predilection for the head, face, and arms and demonstrate 2 predominant cell types, including a polyhedral cell with a rounded nucleus and slightly basophilic cytoplasm as well as a round cell with clear cytoplasm and bland nuclei (Figure 2). The latter cell type is less common, representing the predominant cell type in less than one-third of hidradenomas, and can present a diagnostic quandary based on histologic similarity to other clear cell neoplasms. The clear cells contain glycogen but no lipid. Ductlike structures often are present, and the intervening stroma varies from delicate vascularized cords of fibrous tissue to dense hyalinized collagen. Immunohistochemistry may be required for definitive diagnosis, and clear cell hidradenomas should react with monoclonal antibodies that label both eccrine and apocrine secretory elements, such as cytokeratins 6/18, 7, and 8/18.17

Pyogenic granulomas (also referred to as lobular capillary hemangiomas) are common and present clinically as rapidly growing, polypoid, red masses surrounded by a thickened epidermis that often are found on the fingers or lips. This entity is benign and often regresses spontaneously. Histologically, pyogenic granulomas are characterized by a lobular pattern of vascular proliferation associated with edema and inflammation resembling granulation tissue, with acanthosis and hyperkeratosis at the edges of the lesion (Figure 3).18

Sebaceous carcinoma is a locally aggressive malignant neoplasm arising from the cells of the sebaceous glands and occurring most commonly in the periorbital area. This neoplasm most often affects older adults, with a mean age at diagnosis of 63 to 77 years. It commonly presents as a solitary nodule with yellowish discoloration and madarosis, which is a key distinguishing feature to differentiate this entity from a chalazion or hordeolum. Histologically, sebaceous carcinoma is a dermal-based infiltrative, nodular tumor with varying degrees of clear cell changes—well-differentiated tumors show more clear cell change as compared to more poorly differentiated variants—along with basaloid or squamous features and abundant mitotic activity (Figure 4), which may be useful in distinguishing it from the other entities in the clear cell neoplasm differential.19-22

- Alves de Paula T, Lopes da Silva P, Sueth Berriel LG. Renal cell carcinoma with cutaneous metastasis: case report. J Bras Nefrol. 2010;32:213-215.
- Amaadour L, Atreche L, Azegrar M, et al. Cutaneous metastasis of renal cell carcinoma: a case report. J Cancer Ther. 2017;8:603-607.
- Weiss L, Harlos JP, Torhorst J, et al. Metastatic patterns of renal carcinoma: an analysis of 687 necropsies. J Cancer Res Clin Oncol. 1988;114:605-612.
- Flamigan RC, Campbell SC, Clark JI, et al. Metastatic renal cell carcinoma. Curr Treat Options Oncol. 2003;4:385-390.
- Motzer RJ, Bacik J, Schwarz LH, et al. Prognostic factors for survival in previously treated patients with metastatic renal cell carcinoma. J Clin Oncol. 2004;22:453-463.
- Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor–targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27:5694-5799.
- Smyth LG, Rowan GC, David MQ. Renal cell carcinoma presenting as an ominous metachronous scalp metastasis. Can Urol Assoc J. 2010;4:E64-E66.
- Dorairajan LN, Hemal AK, Aron M, et al. Cutaneous metastases in renal cell carcinoma. Urol Int. 1999;63:164-167.
- Koga S, Tsuda S, Nishikido M, et al. Renal cell carcinoma metastatic to the skin. Anticancer Res. 2000;20:1939-1940.
- Krathen RA, Orengo IF, Rosen T. Cutaneous metastasis: a metaanalysis of the data. South Med J. 2003;96:164-167.
- Amano Y, Ohni S, Ishige T, et al. A case of cutaneous metastasis from a clear cell renal cell carcinoma with an eosinophilic cell component to the submandibular region. J Nihon Univ Med Assoc. 2015;74:73-77.
- Arrabal-Polo MA, Arias-Santiago SA, Aneiros-Fernandez J, et al. Cutaneous metastases in renal cell carcinoma: a case report. Cases J. 2009;2:7948.
- Sangoi AR, Karamchandani J, Kim J, et al. The use of immunohistochemistry in the diagnosis of metastatic clear cell renal cell carcinoma: a review of PAX-8, PAX-2, hKIM-1, RCCma, and CD10. Adv Anat Pathol. 2010;17:377-393.
- Velez MJ, Thomas CL, Stratton J, et al. The utility of using immunohistochemistry in the differentiation of metastatic, cutaneous clear cell renal cell carcinoma and clear cell hidradenoma. J Cutan Pathol. 2017;44:612-615.
- Nezami BG, MacLennan G. Clear cell. PathologyOutlines website. Published April 20, 2021. Updated March 2, 2022. Accessed April 22, 2022. https://www.pathologyoutlines.com/topic/kidneytumormalignantrccclear.html
- Dhaille F, Courville P, Joly P, et al. Balloon cell nevus: histologic and dermoscopic features. J Am Acad Dermatol. 2015;72:E55-E56.
- Volmar KE, Cummings TJ, Wang WH, et al. Clear cell hidradenoma: a mimic of metastatic clear cell tumors. Arch Pathol Lab Med. 2005;129:E113-E116.
- Hale CS. Capillary/pyogenic granuloma. Pathology Outlines website. Published August 1, 2012. Updated March 10, 2022. Accessed April 20, 2022. https://www.pathologyoutlines.com/topic/skintumornonmelanocyticpyogenicgranuloma.html
- Zada S, Lee BA. Sebaceous carcinoma. Pathology Outlines website. Published August 11, 2021. Accessed April 20, 2022. https://www.pathologyoutlines.com/topic/skintumornonmelanocyticsebaceouscarcinoma.html
- Kahana A, Pribila, JT, Nelson CC, et al. Sebaceous cell carcinoma. In: Levin LA, Albert DM, eds. Ocular Disease: Mechanisms and Management. Elsevier; 2010:396-407.
- Wick MR. Cutaneous tumors and pseudotumors of the head and neck. In: Gnepp DR, ed. Diagnostic Surgical Pathology of the Head and Neck. 2nd ed. Saunders Elsevier; 2009:975-1068.
- Cassarino DS, Dadras SS, Lindberg MR, et al. Sebaceous carcinoma. In: Cassarino DS, Dadras SS, Lindberg MR, et al, eds. Diagnostic Pathology: Neoplastic Dermatopathology. 2nd ed. Elsevier; 2017:174-179.
- Alves de Paula T, Lopes da Silva P, Sueth Berriel LG. Renal cell carcinoma with cutaneous metastasis: case report. J Bras Nefrol. 2010;32:213-215.
- Amaadour L, Atreche L, Azegrar M, et al. Cutaneous metastasis of renal cell carcinoma: a case report. J Cancer Ther. 2017;8:603-607.
- Weiss L, Harlos JP, Torhorst J, et al. Metastatic patterns of renal carcinoma: an analysis of 687 necropsies. J Cancer Res Clin Oncol. 1988;114:605-612.
- Flamigan RC, Campbell SC, Clark JI, et al. Metastatic renal cell carcinoma. Curr Treat Options Oncol. 2003;4:385-390.
- Motzer RJ, Bacik J, Schwarz LH, et al. Prognostic factors for survival in previously treated patients with metastatic renal cell carcinoma. J Clin Oncol. 2004;22:453-463.
- Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor–targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27:5694-5799.
- Smyth LG, Rowan GC, David MQ. Renal cell carcinoma presenting as an ominous metachronous scalp metastasis. Can Urol Assoc J. 2010;4:E64-E66.
- Dorairajan LN, Hemal AK, Aron M, et al. Cutaneous metastases in renal cell carcinoma. Urol Int. 1999;63:164-167.
- Koga S, Tsuda S, Nishikido M, et al. Renal cell carcinoma metastatic to the skin. Anticancer Res. 2000;20:1939-1940.
- Krathen RA, Orengo IF, Rosen T. Cutaneous metastasis: a metaanalysis of the data. South Med J. 2003;96:164-167.
- Amano Y, Ohni S, Ishige T, et al. A case of cutaneous metastasis from a clear cell renal cell carcinoma with an eosinophilic cell component to the submandibular region. J Nihon Univ Med Assoc. 2015;74:73-77.
- Arrabal-Polo MA, Arias-Santiago SA, Aneiros-Fernandez J, et al. Cutaneous metastases in renal cell carcinoma: a case report. Cases J. 2009;2:7948.
- Sangoi AR, Karamchandani J, Kim J, et al. The use of immunohistochemistry in the diagnosis of metastatic clear cell renal cell carcinoma: a review of PAX-8, PAX-2, hKIM-1, RCCma, and CD10. Adv Anat Pathol. 2010;17:377-393.
- Velez MJ, Thomas CL, Stratton J, et al. The utility of using immunohistochemistry in the differentiation of metastatic, cutaneous clear cell renal cell carcinoma and clear cell hidradenoma. J Cutan Pathol. 2017;44:612-615.
- Nezami BG, MacLennan G. Clear cell. PathologyOutlines website. Published April 20, 2021. Updated March 2, 2022. Accessed April 22, 2022. https://www.pathologyoutlines.com/topic/kidneytumormalignantrccclear.html
- Dhaille F, Courville P, Joly P, et al. Balloon cell nevus: histologic and dermoscopic features. J Am Acad Dermatol. 2015;72:E55-E56.
- Volmar KE, Cummings TJ, Wang WH, et al. Clear cell hidradenoma: a mimic of metastatic clear cell tumors. Arch Pathol Lab Med. 2005;129:E113-E116.
- Hale CS. Capillary/pyogenic granuloma. Pathology Outlines website. Published August 1, 2012. Updated March 10, 2022. Accessed April 20, 2022. https://www.pathologyoutlines.com/topic/skintumornonmelanocyticpyogenicgranuloma.html
- Zada S, Lee BA. Sebaceous carcinoma. Pathology Outlines website. Published August 11, 2021. Accessed April 20, 2022. https://www.pathologyoutlines.com/topic/skintumornonmelanocyticsebaceouscarcinoma.html
- Kahana A, Pribila, JT, Nelson CC, et al. Sebaceous cell carcinoma. In: Levin LA, Albert DM, eds. Ocular Disease: Mechanisms and Management. Elsevier; 2010:396-407.
- Wick MR. Cutaneous tumors and pseudotumors of the head and neck. In: Gnepp DR, ed. Diagnostic Surgical Pathology of the Head and Neck. 2nd ed. Saunders Elsevier; 2009:975-1068.
- Cassarino DS, Dadras SS, Lindberg MR, et al. Sebaceous carcinoma. In: Cassarino DS, Dadras SS, Lindberg MR, et al, eds. Diagnostic Pathology: Neoplastic Dermatopathology. 2nd ed. Elsevier; 2017:174-179.
A 71-year-old man with no notable medical history presented with a bleeding nodule on the right lower cutaneous lip of 9 weeks’ duration. The patient denied any systemic symptoms. A shave biopsy was performed.
CDC updates guidelines for hepatitis outbreak among children
The Centers for Disease Control and Prevention updated its recommendations for doctors and public health officials regarding the unusual outbreak of acute hepatitis among children.
As of May 5, the CDC and state health departments are investigating 109 children with hepatitis of unknown origin across 25 states and territories.
More than half have tested positive for adenovirus, the CDC said. More than 90% have been hospitalized, and 14% have had liver transplants. Five deaths are under investigation.
This week’s CDC alert provides updated recommendations for testing, given the potential association between adenovirus infection and pediatric hepatitis, or liver inflammation.
“Clinicians are recommended to consider adenovirus testing for patients with hepatitis of unknown etiology and to report such cases to their state or jurisdictional public health authorities,” the CDC said.
Doctors should also consider collecting a blood sample, respiratory sample, and stool sample. They may also collect liver tissue if a biopsy occurred or an autopsy is available.
In November 2021, clinicians at a large children’s hospital in Alabama notified the CDC about five pediatric patients with significant liver injury, including three with acute liver failure, who also tested positive for adenovirus. All children were previously healthy, and none had COVID-19, according to a CDC alert in April.
Four additional pediatric patients with hepatitis and adenovirus infection were identified. After lab testing found adenovirus infection in all nine patients in the initial cluster, public health officials began investigating a possible association between pediatric hepatitis and adenovirus. Among the five specimens that could be sequenced, they were all adenovirus type 41.
Unexplained hepatitis cases have been reported in children worldwide, reaching 450 cases and 11 deaths, according to the latest update from the European Centre for Disease Prevention and Control.
The cases have been reported in more than two dozen countries around the world, with 14 countries reporting more than five cases. The United Kingdom and the United States have reported the largest case counts so far.
In the United Kingdom, officials have identified 163 cases in children under age 16 years, including 11 that required liver transplants.
In the European Union, 14 countries have reported 106 cases collectively, with Italy reporting 35 cases and Spain reporting 22 cases. Outside of the European Union, Brazil has reported 16, Indonesia has reported 15, and Israel has reported 12.
Among the 11 deaths reported globally, the Uniyed States has reported five, Indonesia has reported five, and Palestine has reported one.
The cause of severe hepatitis remains a mystery, according to Ars Technica. Some cases have been identified retrospectively, dating back to the beginning of October 2021.
About 70% of the cases that have been tested for an adenovirus have tested positive, and subtype testing continues to show adenovirus type 41. The cases don’t appear to be linked to common causes, such as hepatitis viruses A, B, C, D, or E, which can cause liver inflammation and injury.
Adenoviruses aren’t known to cause hepatitis in healthy children, though the viruses have been linked to liver damage in children with compromised immune systems, according to Ars Technica. Adenoviruses typically cause respiratory infections in children, although type 41 tends to cause gastrointestinal illness.
“At present, the leading hypotheses remain those which involve adenovirus,” Philippa Easterbrook, a senior scientist at the WHO, said May 10 during a press briefing.
“I think [there’s] also still an important consideration about the role of COVID as well, either as a co-infection or as a past infection,” she said.
WHO officials expect data within a week from U.K. cases, Ms. Easterbrook said, which may indicate whether the adenovirus is an incidental infection or a more direct cause.
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention updated its recommendations for doctors and public health officials regarding the unusual outbreak of acute hepatitis among children.
As of May 5, the CDC and state health departments are investigating 109 children with hepatitis of unknown origin across 25 states and territories.
More than half have tested positive for adenovirus, the CDC said. More than 90% have been hospitalized, and 14% have had liver transplants. Five deaths are under investigation.
This week’s CDC alert provides updated recommendations for testing, given the potential association between adenovirus infection and pediatric hepatitis, or liver inflammation.
“Clinicians are recommended to consider adenovirus testing for patients with hepatitis of unknown etiology and to report such cases to their state or jurisdictional public health authorities,” the CDC said.
Doctors should also consider collecting a blood sample, respiratory sample, and stool sample. They may also collect liver tissue if a biopsy occurred or an autopsy is available.
In November 2021, clinicians at a large children’s hospital in Alabama notified the CDC about five pediatric patients with significant liver injury, including three with acute liver failure, who also tested positive for adenovirus. All children were previously healthy, and none had COVID-19, according to a CDC alert in April.
Four additional pediatric patients with hepatitis and adenovirus infection were identified. After lab testing found adenovirus infection in all nine patients in the initial cluster, public health officials began investigating a possible association between pediatric hepatitis and adenovirus. Among the five specimens that could be sequenced, they were all adenovirus type 41.
Unexplained hepatitis cases have been reported in children worldwide, reaching 450 cases and 11 deaths, according to the latest update from the European Centre for Disease Prevention and Control.
The cases have been reported in more than two dozen countries around the world, with 14 countries reporting more than five cases. The United Kingdom and the United States have reported the largest case counts so far.
In the United Kingdom, officials have identified 163 cases in children under age 16 years, including 11 that required liver transplants.
In the European Union, 14 countries have reported 106 cases collectively, with Italy reporting 35 cases and Spain reporting 22 cases. Outside of the European Union, Brazil has reported 16, Indonesia has reported 15, and Israel has reported 12.
Among the 11 deaths reported globally, the Uniyed States has reported five, Indonesia has reported five, and Palestine has reported one.
The cause of severe hepatitis remains a mystery, according to Ars Technica. Some cases have been identified retrospectively, dating back to the beginning of October 2021.
About 70% of the cases that have been tested for an adenovirus have tested positive, and subtype testing continues to show adenovirus type 41. The cases don’t appear to be linked to common causes, such as hepatitis viruses A, B, C, D, or E, which can cause liver inflammation and injury.
Adenoviruses aren’t known to cause hepatitis in healthy children, though the viruses have been linked to liver damage in children with compromised immune systems, according to Ars Technica. Adenoviruses typically cause respiratory infections in children, although type 41 tends to cause gastrointestinal illness.
“At present, the leading hypotheses remain those which involve adenovirus,” Philippa Easterbrook, a senior scientist at the WHO, said May 10 during a press briefing.
“I think [there’s] also still an important consideration about the role of COVID as well, either as a co-infection or as a past infection,” she said.
WHO officials expect data within a week from U.K. cases, Ms. Easterbrook said, which may indicate whether the adenovirus is an incidental infection or a more direct cause.
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention updated its recommendations for doctors and public health officials regarding the unusual outbreak of acute hepatitis among children.
As of May 5, the CDC and state health departments are investigating 109 children with hepatitis of unknown origin across 25 states and territories.
More than half have tested positive for adenovirus, the CDC said. More than 90% have been hospitalized, and 14% have had liver transplants. Five deaths are under investigation.
This week’s CDC alert provides updated recommendations for testing, given the potential association between adenovirus infection and pediatric hepatitis, or liver inflammation.
“Clinicians are recommended to consider adenovirus testing for patients with hepatitis of unknown etiology and to report such cases to their state or jurisdictional public health authorities,” the CDC said.
Doctors should also consider collecting a blood sample, respiratory sample, and stool sample. They may also collect liver tissue if a biopsy occurred or an autopsy is available.
In November 2021, clinicians at a large children’s hospital in Alabama notified the CDC about five pediatric patients with significant liver injury, including three with acute liver failure, who also tested positive for adenovirus. All children were previously healthy, and none had COVID-19, according to a CDC alert in April.
Four additional pediatric patients with hepatitis and adenovirus infection were identified. After lab testing found adenovirus infection in all nine patients in the initial cluster, public health officials began investigating a possible association between pediatric hepatitis and adenovirus. Among the five specimens that could be sequenced, they were all adenovirus type 41.
Unexplained hepatitis cases have been reported in children worldwide, reaching 450 cases and 11 deaths, according to the latest update from the European Centre for Disease Prevention and Control.
The cases have been reported in more than two dozen countries around the world, with 14 countries reporting more than five cases. The United Kingdom and the United States have reported the largest case counts so far.
In the United Kingdom, officials have identified 163 cases in children under age 16 years, including 11 that required liver transplants.
In the European Union, 14 countries have reported 106 cases collectively, with Italy reporting 35 cases and Spain reporting 22 cases. Outside of the European Union, Brazil has reported 16, Indonesia has reported 15, and Israel has reported 12.
Among the 11 deaths reported globally, the Uniyed States has reported five, Indonesia has reported five, and Palestine has reported one.
The cause of severe hepatitis remains a mystery, according to Ars Technica. Some cases have been identified retrospectively, dating back to the beginning of October 2021.
About 70% of the cases that have been tested for an adenovirus have tested positive, and subtype testing continues to show adenovirus type 41. The cases don’t appear to be linked to common causes, such as hepatitis viruses A, B, C, D, or E, which can cause liver inflammation and injury.
Adenoviruses aren’t known to cause hepatitis in healthy children, though the viruses have been linked to liver damage in children with compromised immune systems, according to Ars Technica. Adenoviruses typically cause respiratory infections in children, although type 41 tends to cause gastrointestinal illness.
“At present, the leading hypotheses remain those which involve adenovirus,” Philippa Easterbrook, a senior scientist at the WHO, said May 10 during a press briefing.
“I think [there’s] also still an important consideration about the role of COVID as well, either as a co-infection or as a past infection,” she said.
WHO officials expect data within a week from U.K. cases, Ms. Easterbrook said, which may indicate whether the adenovirus is an incidental infection or a more direct cause.
A version of this article first appeared on Medscape.com.
TIPS plus sequential systemic therapy shows promise in advanced HCC with tumor thrombus-related SPH
Key clinical point: Transjugular intrahepatic portosystemic shunt (TIPS) plus sequential systemic therapy is safe and feasible for treating portal vein tumor thrombus (PVTT)-related symptomatic portal hypertension (SPH) in advanced hepatocellular carcinoma (aHCC) and may supplement current aHCC treatments.
Major finding: TIPS plus sequential systemic therapy vs only symptomatic and supportive treatment showed a significantly lower variceal rebleeding rate (5.0% vs 73.7%; P < .001) and a significantly higher median overall survival (9.6 vs 4.9 months; P < .001).
Study details: This retrospective study propensity score matched patients with aHCC and PVTT-related SPH who received TIPS plus sequential systemic therapy (n = 42) with those who received only symptomatic and supportive treatment (n = 42).
Disclosures: This study was funded by the National Natural Science Foundation of China, Science and Technology Planning Project of Guangdong Province, and Medical Science and Technology Foundation of Guangdong Province. The authors declared no conflicts of interest.
Source: Qiu Z et al. TIPS plus sequential systemic therapy of advanced HCC patients with tumour thrombus-related symptomatic portal hypertension. Eur Radiol. 2022 (Apr 20). Doi: 10.1007/s00330-022-08705-7
Key clinical point: Transjugular intrahepatic portosystemic shunt (TIPS) plus sequential systemic therapy is safe and feasible for treating portal vein tumor thrombus (PVTT)-related symptomatic portal hypertension (SPH) in advanced hepatocellular carcinoma (aHCC) and may supplement current aHCC treatments.
Major finding: TIPS plus sequential systemic therapy vs only symptomatic and supportive treatment showed a significantly lower variceal rebleeding rate (5.0% vs 73.7%; P < .001) and a significantly higher median overall survival (9.6 vs 4.9 months; P < .001).
Study details: This retrospective study propensity score matched patients with aHCC and PVTT-related SPH who received TIPS plus sequential systemic therapy (n = 42) with those who received only symptomatic and supportive treatment (n = 42).
Disclosures: This study was funded by the National Natural Science Foundation of China, Science and Technology Planning Project of Guangdong Province, and Medical Science and Technology Foundation of Guangdong Province. The authors declared no conflicts of interest.
Source: Qiu Z et al. TIPS plus sequential systemic therapy of advanced HCC patients with tumour thrombus-related symptomatic portal hypertension. Eur Radiol. 2022 (Apr 20). Doi: 10.1007/s00330-022-08705-7
Key clinical point: Transjugular intrahepatic portosystemic shunt (TIPS) plus sequential systemic therapy is safe and feasible for treating portal vein tumor thrombus (PVTT)-related symptomatic portal hypertension (SPH) in advanced hepatocellular carcinoma (aHCC) and may supplement current aHCC treatments.
Major finding: TIPS plus sequential systemic therapy vs only symptomatic and supportive treatment showed a significantly lower variceal rebleeding rate (5.0% vs 73.7%; P < .001) and a significantly higher median overall survival (9.6 vs 4.9 months; P < .001).
Study details: This retrospective study propensity score matched patients with aHCC and PVTT-related SPH who received TIPS plus sequential systemic therapy (n = 42) with those who received only symptomatic and supportive treatment (n = 42).
Disclosures: This study was funded by the National Natural Science Foundation of China, Science and Technology Planning Project of Guangdong Province, and Medical Science and Technology Foundation of Guangdong Province. The authors declared no conflicts of interest.
Source: Qiu Z et al. TIPS plus sequential systemic therapy of advanced HCC patients with tumour thrombus-related symptomatic portal hypertension. Eur Radiol. 2022 (Apr 20). Doi: 10.1007/s00330-022-08705-7
HCC incidence after successful DAA therapy for hepatitis C varies with cirrhosis status
Key clinical point: Among patients with hepatitis C virus (HCV) who achieved a sustained virologic response (SVR) after direct-acting antiviral (DAA) therapy, those with cirrhosis showed an extremely high incidence of hepatocellular carcinoma (HCC).
Major finding: The incidence of HCC in patients with cirrhosis was 2.99 per 100 person-years (95% CI 2.52-3.54), whereas that in patients without cirrhosis was 0.47 per 100 person-years (95% CI 0.32-0.70).
Study details: This was a meta-analysis of 42 studies including 59,834 adult patients with HCV who achieved SVR after DAA therapy and were categorized into those with (n = 27,711; 31 studies) or without (n = 32,123; 11 studies) cirrhosis.
Disclosures: This study was funded by the US National Institutes of Health. The authors declared no conflict of interests.
Source: Kim NJ et al. Fibrosis-stage specific incidence of hepatocellular cancer after hepatitis C cure with direct-acting antivirals: A systematic review & meta-analysis. Clin Gastroenterol Hepatol. 2022 (May 4). Doi: 10.1016/j.cgh.2022.04.013
Key clinical point: Among patients with hepatitis C virus (HCV) who achieved a sustained virologic response (SVR) after direct-acting antiviral (DAA) therapy, those with cirrhosis showed an extremely high incidence of hepatocellular carcinoma (HCC).
Major finding: The incidence of HCC in patients with cirrhosis was 2.99 per 100 person-years (95% CI 2.52-3.54), whereas that in patients without cirrhosis was 0.47 per 100 person-years (95% CI 0.32-0.70).
Study details: This was a meta-analysis of 42 studies including 59,834 adult patients with HCV who achieved SVR after DAA therapy and were categorized into those with (n = 27,711; 31 studies) or without (n = 32,123; 11 studies) cirrhosis.
Disclosures: This study was funded by the US National Institutes of Health. The authors declared no conflict of interests.
Source: Kim NJ et al. Fibrosis-stage specific incidence of hepatocellular cancer after hepatitis C cure with direct-acting antivirals: A systematic review & meta-analysis. Clin Gastroenterol Hepatol. 2022 (May 4). Doi: 10.1016/j.cgh.2022.04.013
Key clinical point: Among patients with hepatitis C virus (HCV) who achieved a sustained virologic response (SVR) after direct-acting antiviral (DAA) therapy, those with cirrhosis showed an extremely high incidence of hepatocellular carcinoma (HCC).
Major finding: The incidence of HCC in patients with cirrhosis was 2.99 per 100 person-years (95% CI 2.52-3.54), whereas that in patients without cirrhosis was 0.47 per 100 person-years (95% CI 0.32-0.70).
Study details: This was a meta-analysis of 42 studies including 59,834 adult patients with HCV who achieved SVR after DAA therapy and were categorized into those with (n = 27,711; 31 studies) or without (n = 32,123; 11 studies) cirrhosis.
Disclosures: This study was funded by the US National Institutes of Health. The authors declared no conflict of interests.
Source: Kim NJ et al. Fibrosis-stage specific incidence of hepatocellular cancer after hepatitis C cure with direct-acting antivirals: A systematic review & meta-analysis. Clin Gastroenterol Hepatol. 2022 (May 4). Doi: 10.1016/j.cgh.2022.04.013
Efficacy and safety of tumor vaccines in HCC: Lessons from two decades of research
Key clinical point: Tumor vaccines are effective and safe in patients with hepatocellular carcinoma (HCC).
Major finding: Tumor vaccines effectuated a pooled objective response rate, median overall survival, and median progression-free survival of 7% (95% CI 3%-14%), 13.7 (95% CI 8.2-22.8) months, and 6.2 (95% CI 3.0-12.9) months, respectively. The pooled rate of severe adverse events (AE; grades 3-5) was only 7.9%, and the most prevalent AE was grade 1-2 injection site reaction.
Study details: This was a meta-analysis of 35 cohorts in 31 studies (published between 2001 and 2021) that included 932 patients with HCC who received tumor vaccines.
Disclosures: The study was supported by the Taishan Scholars Program for Young Expert of Shandong Province and National Natural Science Foundation of China, among others. The authors declared no conflicts of interest.
Source: Han CL et al. Efficacy and security of tumor vaccines for hepatocellular carcinoma: A systemic review and meta-analysis of the last 2 decades. J Cancer Res Clin Oncol. 2022 (Apr 28). Doi: 10.1007/s00432-022-04008-y
Key clinical point: Tumor vaccines are effective and safe in patients with hepatocellular carcinoma (HCC).
Major finding: Tumor vaccines effectuated a pooled objective response rate, median overall survival, and median progression-free survival of 7% (95% CI 3%-14%), 13.7 (95% CI 8.2-22.8) months, and 6.2 (95% CI 3.0-12.9) months, respectively. The pooled rate of severe adverse events (AE; grades 3-5) was only 7.9%, and the most prevalent AE was grade 1-2 injection site reaction.
Study details: This was a meta-analysis of 35 cohorts in 31 studies (published between 2001 and 2021) that included 932 patients with HCC who received tumor vaccines.
Disclosures: The study was supported by the Taishan Scholars Program for Young Expert of Shandong Province and National Natural Science Foundation of China, among others. The authors declared no conflicts of interest.
Source: Han CL et al. Efficacy and security of tumor vaccines for hepatocellular carcinoma: A systemic review and meta-analysis of the last 2 decades. J Cancer Res Clin Oncol. 2022 (Apr 28). Doi: 10.1007/s00432-022-04008-y
Key clinical point: Tumor vaccines are effective and safe in patients with hepatocellular carcinoma (HCC).
Major finding: Tumor vaccines effectuated a pooled objective response rate, median overall survival, and median progression-free survival of 7% (95% CI 3%-14%), 13.7 (95% CI 8.2-22.8) months, and 6.2 (95% CI 3.0-12.9) months, respectively. The pooled rate of severe adverse events (AE; grades 3-5) was only 7.9%, and the most prevalent AE was grade 1-2 injection site reaction.
Study details: This was a meta-analysis of 35 cohorts in 31 studies (published between 2001 and 2021) that included 932 patients with HCC who received tumor vaccines.
Disclosures: The study was supported by the Taishan Scholars Program for Young Expert of Shandong Province and National Natural Science Foundation of China, among others. The authors declared no conflicts of interest.
Source: Han CL et al. Efficacy and security of tumor vaccines for hepatocellular carcinoma: A systemic review and meta-analysis of the last 2 decades. J Cancer Res Clin Oncol. 2022 (Apr 28). Doi: 10.1007/s00432-022-04008-y
Advanced HCC: Lenvatinib plus nivolumab yields promising results in real-world
Key clinical point: Lenvatinib plus nivolumab shows a promising efficacy and safety profile against advanced hepatocellular carcinoma (HCC) in the real-world setting.
Major finding: The lenvatinib plus nivolumab vs lenvatinib group showed a higher objective response rate (45.0% vs 23.4%; P = .03) and longer progression-free survival (7.5 vs 4.8 months; P = .05) and overall survival (22.9 vs 10.3 months; P = .01). Only a few patients developed grade 3/4 toxicities, such as dermatitis (15.0%), gastrointestinal bleeding (7.5%), and hypertension (5.0%).
Study details: This was a retrospective study including 87 patients aged ≥20 years with advanced HCC who received lenvatinib plus nivolumab (n = 40) or lenvatinib alone (n = 47).
Disclosures: The study was funded by the Ministry of Health and Welfare and the Center of Excellence for Cancer Research and Taipei Veterans General Hospital. The authors declared no conflicts if interest.
Source: Wu W-C et al. Lenvatinib combined with nivolumab in advanced hepatocellular carcinoma-real-world experience. Invest New Drugs. 2022 (Apr 28). Doi: 10.1007/s10637-022-01248-0
Key clinical point: Lenvatinib plus nivolumab shows a promising efficacy and safety profile against advanced hepatocellular carcinoma (HCC) in the real-world setting.
Major finding: The lenvatinib plus nivolumab vs lenvatinib group showed a higher objective response rate (45.0% vs 23.4%; P = .03) and longer progression-free survival (7.5 vs 4.8 months; P = .05) and overall survival (22.9 vs 10.3 months; P = .01). Only a few patients developed grade 3/4 toxicities, such as dermatitis (15.0%), gastrointestinal bleeding (7.5%), and hypertension (5.0%).
Study details: This was a retrospective study including 87 patients aged ≥20 years with advanced HCC who received lenvatinib plus nivolumab (n = 40) or lenvatinib alone (n = 47).
Disclosures: The study was funded by the Ministry of Health and Welfare and the Center of Excellence for Cancer Research and Taipei Veterans General Hospital. The authors declared no conflicts if interest.
Source: Wu W-C et al. Lenvatinib combined with nivolumab in advanced hepatocellular carcinoma-real-world experience. Invest New Drugs. 2022 (Apr 28). Doi: 10.1007/s10637-022-01248-0
Key clinical point: Lenvatinib plus nivolumab shows a promising efficacy and safety profile against advanced hepatocellular carcinoma (HCC) in the real-world setting.
Major finding: The lenvatinib plus nivolumab vs lenvatinib group showed a higher objective response rate (45.0% vs 23.4%; P = .03) and longer progression-free survival (7.5 vs 4.8 months; P = .05) and overall survival (22.9 vs 10.3 months; P = .01). Only a few patients developed grade 3/4 toxicities, such as dermatitis (15.0%), gastrointestinal bleeding (7.5%), and hypertension (5.0%).
Study details: This was a retrospective study including 87 patients aged ≥20 years with advanced HCC who received lenvatinib plus nivolumab (n = 40) or lenvatinib alone (n = 47).
Disclosures: The study was funded by the Ministry of Health and Welfare and the Center of Excellence for Cancer Research and Taipei Veterans General Hospital. The authors declared no conflicts if interest.
Source: Wu W-C et al. Lenvatinib combined with nivolumab in advanced hepatocellular carcinoma-real-world experience. Invest New Drugs. 2022 (Apr 28). Doi: 10.1007/s10637-022-01248-0
Advanced HCC: Immunotherapy vs chemotherapy improves survival
Key clinical point: Immunotherapy was associated with prolonged survival compared with chemotherapy in patients with advanced hepatocellular carcinoma (HCC).
Major finding: After adjusting for confounding variables, immunotherapy was independently associated with improved overall survival (adjusted hazard ratio 0.76; 95% CI 0.65-0.88) compared with chemotherapy.
Study details: Findings are from a retrospective cohort study that included 3990 patients with advanced HCC (tumor-node-metastasis stage III or IV) from the National Cancer Database who received chemotherapy (n = 3248) or immunotherapy (n = 742) as the first-line systemic treatment.
Disclosures: No funding source was reported. Some authors declared serving as consultants or advisors or receiving institutional research support from various organizations.
Source: Ahn JC et al. Racial and ethnic disparities in early treatment with immunotherapy for advanced HCC in the United States. Hepatology. 2022 (Apr 16). Doi: 10.1002/hep.32527
Key clinical point: Immunotherapy was associated with prolonged survival compared with chemotherapy in patients with advanced hepatocellular carcinoma (HCC).
Major finding: After adjusting for confounding variables, immunotherapy was independently associated with improved overall survival (adjusted hazard ratio 0.76; 95% CI 0.65-0.88) compared with chemotherapy.
Study details: Findings are from a retrospective cohort study that included 3990 patients with advanced HCC (tumor-node-metastasis stage III or IV) from the National Cancer Database who received chemotherapy (n = 3248) or immunotherapy (n = 742) as the first-line systemic treatment.
Disclosures: No funding source was reported. Some authors declared serving as consultants or advisors or receiving institutional research support from various organizations.
Source: Ahn JC et al. Racial and ethnic disparities in early treatment with immunotherapy for advanced HCC in the United States. Hepatology. 2022 (Apr 16). Doi: 10.1002/hep.32527
Key clinical point: Immunotherapy was associated with prolonged survival compared with chemotherapy in patients with advanced hepatocellular carcinoma (HCC).
Major finding: After adjusting for confounding variables, immunotherapy was independently associated with improved overall survival (adjusted hazard ratio 0.76; 95% CI 0.65-0.88) compared with chemotherapy.
Study details: Findings are from a retrospective cohort study that included 3990 patients with advanced HCC (tumor-node-metastasis stage III or IV) from the National Cancer Database who received chemotherapy (n = 3248) or immunotherapy (n = 742) as the first-line systemic treatment.
Disclosures: No funding source was reported. Some authors declared serving as consultants or advisors or receiving institutional research support from various organizations.
Source: Ahn JC et al. Racial and ethnic disparities in early treatment with immunotherapy for advanced HCC in the United States. Hepatology. 2022 (Apr 16). Doi: 10.1002/hep.32527
Race-based spirometry may lead to missed diagnoses
SAN FRANCISCO – It may be time to move beyond relying largely on spirometry to distinguish between healthy and abnormal lung function in diverse populations.
That conclusion comes from investigators who looked at patients with ostensibly normal spirometry values in a large population-based study and found that using standard equations to adjust for racial differences in lung-function measures appeared to miss emphysema in a significant proportion of Black patients.
“Our traditional measures of lung health based on spirometry may be under-recognizing impaired respiratory health in Black adults and particularly Black men,” said lead author Gabrielle Liu, MD, a fellow in the division of pulmonary and critical care medicine at the Northwestern University Feinberg School of Medicine, Chicago.
“CT imaging may be useful in the evaluation of those with suspected impaired respiratory health and normal spirometry,” she said in an oral abstract session at the American Thoracic Society International Conference 2022.
Dr. Liu and colleagues studied the association between self-identified race and visually identified emphysema among 2,674 participants in the Coronary Artery Risk Development in Young Adults (CARDIA) study. The patients had CT scans at a mean age of 50 and spirometry at a mean age of 55.
Racial differences
The investigators found that among men with forced expiratory volume in 1 second (FEV1) ranging from 100% to 120% of predicted according to race-adjusted formulas, 14.6% of Black men had emphysema, compared with only 1.7% of White men (P < .001). Respective emphysema rates in Black women and White women were 3.8% and 1.9%; this difference was not statistically significant.
Among patients with FEV1 80% to 99% of predicted according to race-specific measures, 15.5% of Black men had emphysema, compared with 4% of White men (P < .001). Respective rates of emphysema were 6.9% for Black women versus 3.2% for White women (P = .025).
When the investigators applied race-neutral spirometry reference equations to the same population, they found that it attenuated but did not completely eliminate the racial disparity in emphysema prevalence among patients with FEV1, ranging from 80% to 120% of predicted.
Relic of the past
The results suggest that race-based adjustments of spirometry measures are a relic of less enlightened times, said Adam Gaffney, MD, MPH, assistant professor of medicine at Harvard Medical School, Boston, and a pulmonologist and critical care physician at Cambridge Health Alliance, Massachusetts.
“If the average lower lung function of Black people is being driven by adversity, structural racism, and deprivation, that means that race-specific equations are normalizing that adversity,” he said in an interview.
“In my opinion, it is time to move beyond race-based equations in clinical pulmonary medicine, particularly in the context of patients with established lung disease in whom use of race-based equations might actually lead to undertreatment,” said Dr. Gaffney, who was not involved in the study.
Dr. Liu agreed that it’s time to move to race-neutral measures and that the whole concept of race-based differences is flawed.
“The long-standing structural inequities in health likely made the reference populations have lower lung function than among Whites,” she told this news organization.
Dr. Liu said that evaluation of lung function should not rely on spirometry alone, but should also include – when appropriate – CT scans, as well as improved understanding of how symptoms may be predictive for poor outcomes.
The study was supported by grants from the National Institutes of Health. Dr. Liu and Dr. Gaffney have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO – It may be time to move beyond relying largely on spirometry to distinguish between healthy and abnormal lung function in diverse populations.
That conclusion comes from investigators who looked at patients with ostensibly normal spirometry values in a large population-based study and found that using standard equations to adjust for racial differences in lung-function measures appeared to miss emphysema in a significant proportion of Black patients.
“Our traditional measures of lung health based on spirometry may be under-recognizing impaired respiratory health in Black adults and particularly Black men,” said lead author Gabrielle Liu, MD, a fellow in the division of pulmonary and critical care medicine at the Northwestern University Feinberg School of Medicine, Chicago.
“CT imaging may be useful in the evaluation of those with suspected impaired respiratory health and normal spirometry,” she said in an oral abstract session at the American Thoracic Society International Conference 2022.
Dr. Liu and colleagues studied the association between self-identified race and visually identified emphysema among 2,674 participants in the Coronary Artery Risk Development in Young Adults (CARDIA) study. The patients had CT scans at a mean age of 50 and spirometry at a mean age of 55.
Racial differences
The investigators found that among men with forced expiratory volume in 1 second (FEV1) ranging from 100% to 120% of predicted according to race-adjusted formulas, 14.6% of Black men had emphysema, compared with only 1.7% of White men (P < .001). Respective emphysema rates in Black women and White women were 3.8% and 1.9%; this difference was not statistically significant.
Among patients with FEV1 80% to 99% of predicted according to race-specific measures, 15.5% of Black men had emphysema, compared with 4% of White men (P < .001). Respective rates of emphysema were 6.9% for Black women versus 3.2% for White women (P = .025).
When the investigators applied race-neutral spirometry reference equations to the same population, they found that it attenuated but did not completely eliminate the racial disparity in emphysema prevalence among patients with FEV1, ranging from 80% to 120% of predicted.
Relic of the past
The results suggest that race-based adjustments of spirometry measures are a relic of less enlightened times, said Adam Gaffney, MD, MPH, assistant professor of medicine at Harvard Medical School, Boston, and a pulmonologist and critical care physician at Cambridge Health Alliance, Massachusetts.
“If the average lower lung function of Black people is being driven by adversity, structural racism, and deprivation, that means that race-specific equations are normalizing that adversity,” he said in an interview.
“In my opinion, it is time to move beyond race-based equations in clinical pulmonary medicine, particularly in the context of patients with established lung disease in whom use of race-based equations might actually lead to undertreatment,” said Dr. Gaffney, who was not involved in the study.
Dr. Liu agreed that it’s time to move to race-neutral measures and that the whole concept of race-based differences is flawed.
“The long-standing structural inequities in health likely made the reference populations have lower lung function than among Whites,” she told this news organization.
Dr. Liu said that evaluation of lung function should not rely on spirometry alone, but should also include – when appropriate – CT scans, as well as improved understanding of how symptoms may be predictive for poor outcomes.
The study was supported by grants from the National Institutes of Health. Dr. Liu and Dr. Gaffney have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO – It may be time to move beyond relying largely on spirometry to distinguish between healthy and abnormal lung function in diverse populations.
That conclusion comes from investigators who looked at patients with ostensibly normal spirometry values in a large population-based study and found that using standard equations to adjust for racial differences in lung-function measures appeared to miss emphysema in a significant proportion of Black patients.
“Our traditional measures of lung health based on spirometry may be under-recognizing impaired respiratory health in Black adults and particularly Black men,” said lead author Gabrielle Liu, MD, a fellow in the division of pulmonary and critical care medicine at the Northwestern University Feinberg School of Medicine, Chicago.
“CT imaging may be useful in the evaluation of those with suspected impaired respiratory health and normal spirometry,” she said in an oral abstract session at the American Thoracic Society International Conference 2022.
Dr. Liu and colleagues studied the association between self-identified race and visually identified emphysema among 2,674 participants in the Coronary Artery Risk Development in Young Adults (CARDIA) study. The patients had CT scans at a mean age of 50 and spirometry at a mean age of 55.
Racial differences
The investigators found that among men with forced expiratory volume in 1 second (FEV1) ranging from 100% to 120% of predicted according to race-adjusted formulas, 14.6% of Black men had emphysema, compared with only 1.7% of White men (P < .001). Respective emphysema rates in Black women and White women were 3.8% and 1.9%; this difference was not statistically significant.
Among patients with FEV1 80% to 99% of predicted according to race-specific measures, 15.5% of Black men had emphysema, compared with 4% of White men (P < .001). Respective rates of emphysema were 6.9% for Black women versus 3.2% for White women (P = .025).
When the investigators applied race-neutral spirometry reference equations to the same population, they found that it attenuated but did not completely eliminate the racial disparity in emphysema prevalence among patients with FEV1, ranging from 80% to 120% of predicted.
Relic of the past
The results suggest that race-based adjustments of spirometry measures are a relic of less enlightened times, said Adam Gaffney, MD, MPH, assistant professor of medicine at Harvard Medical School, Boston, and a pulmonologist and critical care physician at Cambridge Health Alliance, Massachusetts.
“If the average lower lung function of Black people is being driven by adversity, structural racism, and deprivation, that means that race-specific equations are normalizing that adversity,” he said in an interview.
“In my opinion, it is time to move beyond race-based equations in clinical pulmonary medicine, particularly in the context of patients with established lung disease in whom use of race-based equations might actually lead to undertreatment,” said Dr. Gaffney, who was not involved in the study.
Dr. Liu agreed that it’s time to move to race-neutral measures and that the whole concept of race-based differences is flawed.
“The long-standing structural inequities in health likely made the reference populations have lower lung function than among Whites,” she told this news organization.
Dr. Liu said that evaluation of lung function should not rely on spirometry alone, but should also include – when appropriate – CT scans, as well as improved understanding of how symptoms may be predictive for poor outcomes.
The study was supported by grants from the National Institutes of Health. Dr. Liu and Dr. Gaffney have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ATS 2022
Laparoscopic anatomic hepatectomy achieves better follow-up outcomes than non-anatomical hepatectomy in HCC
Key clinical point: As laparoscopic anatomical hepatectomy (LAH) is associated with increased disease-free survival (DFS) and comparable long-term overall survival (OS) and postoperative complications compared with non-anatomical hepatectomy (LNAH), it is recommended over LNAH for selected patients with HCC.
Major finding: Patients who underwent LAH vs LNAH showed significantly higher 5-year DFS rate (33.9% vs 30.1%; P = .009) and comparable long-term OS (43.2% vs 35.2%; P = .054) and postoperative complication (8.9% vs 12.4%; P = .255) rates.
Study details: Findings are from a single-center, prospective randomized controlled trial including 385 adult patients with HCC (single tumor ≤10 cm in size) who were randomly assigned to undergo LAH (n = 192) or LNAH (n = 193).
Disclosures: The study was sponsored by the National Natural Science Foundation of China and Project of Chongqing Municipality. The authors reported no conflicts of interest.
Source: Liao K et al. Laparoscopic anatomical versus non-anatomical hepatectomy in the treatment of hepatocellular carcinoma: A randomised controlled trial. Int J Surg. 2022;102:106652 (May 4). Doi: 10.1016/j.ijsu.2022.106652
Key clinical point: As laparoscopic anatomical hepatectomy (LAH) is associated with increased disease-free survival (DFS) and comparable long-term overall survival (OS) and postoperative complications compared with non-anatomical hepatectomy (LNAH), it is recommended over LNAH for selected patients with HCC.
Major finding: Patients who underwent LAH vs LNAH showed significantly higher 5-year DFS rate (33.9% vs 30.1%; P = .009) and comparable long-term OS (43.2% vs 35.2%; P = .054) and postoperative complication (8.9% vs 12.4%; P = .255) rates.
Study details: Findings are from a single-center, prospective randomized controlled trial including 385 adult patients with HCC (single tumor ≤10 cm in size) who were randomly assigned to undergo LAH (n = 192) or LNAH (n = 193).
Disclosures: The study was sponsored by the National Natural Science Foundation of China and Project of Chongqing Municipality. The authors reported no conflicts of interest.
Source: Liao K et al. Laparoscopic anatomical versus non-anatomical hepatectomy in the treatment of hepatocellular carcinoma: A randomised controlled trial. Int J Surg. 2022;102:106652 (May 4). Doi: 10.1016/j.ijsu.2022.106652
Key clinical point: As laparoscopic anatomical hepatectomy (LAH) is associated with increased disease-free survival (DFS) and comparable long-term overall survival (OS) and postoperative complications compared with non-anatomical hepatectomy (LNAH), it is recommended over LNAH for selected patients with HCC.
Major finding: Patients who underwent LAH vs LNAH showed significantly higher 5-year DFS rate (33.9% vs 30.1%; P = .009) and comparable long-term OS (43.2% vs 35.2%; P = .054) and postoperative complication (8.9% vs 12.4%; P = .255) rates.
Study details: Findings are from a single-center, prospective randomized controlled trial including 385 adult patients with HCC (single tumor ≤10 cm in size) who were randomly assigned to undergo LAH (n = 192) or LNAH (n = 193).
Disclosures: The study was sponsored by the National Natural Science Foundation of China and Project of Chongqing Municipality. The authors reported no conflicts of interest.
Source: Liao K et al. Laparoscopic anatomical versus non-anatomical hepatectomy in the treatment of hepatocellular carcinoma: A randomised controlled trial. Int J Surg. 2022;102:106652 (May 4). Doi: 10.1016/j.ijsu.2022.106652