User login
Low HER2 expression has no prognostic significance in metastatic breast cancer
Key clinical point: In this real-world population, low expression of human epidermal growth factor receptor 2 (HER2) did not affect the prognosis of metastatic breast cancer (BC).
Major finding: HER2-low vs HER2-negative disease had no significant effect on the overall survival, neither in hormone receptor-positive (hazard ratio [HR], 0.89; P = .171) nor in triple-negative (HR, 0.92; P = .585) subgroups.
Study details: Findings are from an analysis of 1,973 patients with metastatic BC from the metastatic BC-Registry of the Austrian Study Group of Medical Tumor Therapy (AGMT). Among evaluable patients, 20.3% were HER2-positive, 35.2% were HER2-low, and 44.5% were completely HER2-negative.
Disclosures: The AGMT-MBC-registry is supported by grants from Roche, Daiichi Sankyo, Pfizer, and AstraZeneca. The authors declared expert testimony and/or receiving honoraria, research grants, and travel support from several sources.
Source: Gampenrieder SP et al. Breast Cancer Res. 2021 Dec 14. doi: 10.1186/s13058-021-01492-x.
Key clinical point: In this real-world population, low expression of human epidermal growth factor receptor 2 (HER2) did not affect the prognosis of metastatic breast cancer (BC).
Major finding: HER2-low vs HER2-negative disease had no significant effect on the overall survival, neither in hormone receptor-positive (hazard ratio [HR], 0.89; P = .171) nor in triple-negative (HR, 0.92; P = .585) subgroups.
Study details: Findings are from an analysis of 1,973 patients with metastatic BC from the metastatic BC-Registry of the Austrian Study Group of Medical Tumor Therapy (AGMT). Among evaluable patients, 20.3% were HER2-positive, 35.2% were HER2-low, and 44.5% were completely HER2-negative.
Disclosures: The AGMT-MBC-registry is supported by grants from Roche, Daiichi Sankyo, Pfizer, and AstraZeneca. The authors declared expert testimony and/or receiving honoraria, research grants, and travel support from several sources.
Source: Gampenrieder SP et al. Breast Cancer Res. 2021 Dec 14. doi: 10.1186/s13058-021-01492-x.
Key clinical point: In this real-world population, low expression of human epidermal growth factor receptor 2 (HER2) did not affect the prognosis of metastatic breast cancer (BC).
Major finding: HER2-low vs HER2-negative disease had no significant effect on the overall survival, neither in hormone receptor-positive (hazard ratio [HR], 0.89; P = .171) nor in triple-negative (HR, 0.92; P = .585) subgroups.
Study details: Findings are from an analysis of 1,973 patients with metastatic BC from the metastatic BC-Registry of the Austrian Study Group of Medical Tumor Therapy (AGMT). Among evaluable patients, 20.3% were HER2-positive, 35.2% were HER2-low, and 44.5% were completely HER2-negative.
Disclosures: The AGMT-MBC-registry is supported by grants from Roche, Daiichi Sankyo, Pfizer, and AstraZeneca. The authors declared expert testimony and/or receiving honoraria, research grants, and travel support from several sources.
Source: Gampenrieder SP et al. Breast Cancer Res. 2021 Dec 14. doi: 10.1186/s13058-021-01492-x.
HER2-positive early BC: Trastuzumab+pertuzumab+chemotherapy remains the standard care
Key clinical point: Replacing trastuzumab+taxane with trastuzumab emtansine (T-DM1) did not improve survival in high-risk human epidermal growth factor receptor 2 (HER2)-positive early breast cancer (BC).
Major finding: The risk for invasive disease-free survival was not significantly different between trastuzumab+taxane+pertuzumab and T-DM1+pertuzumab arms in the overall population (stratified hazard ratio [sHR], 0.98; 95% CI, 0.72-1.32) and the node-positive subpopulation (sHR, 0.97; 95% CI, 0.71-1.32) along with similar rates of grade 3 or higher and serious adverse events.
Study details: Findings are from the phase 3 KAITLIN study, including 1,846 patients with HER2-positive early BC who were randomly assigned to receive T-DM1+pertuzumab or trastuzumab+taxane+pertuzumab after surgery and anthracycline chemotherapy.
Disclosures: This study was supported by F. Hoffmann-La Roche Ltd. The authors declared serving as consultant, speaker, and/or receiving honorarium, funding, and travel expense from several sources. Some authors were employed and/or held stocks in various sources.
Source: Krop IE et al. J Clin Oncol. 2021 Dec 10. doi: 10.1200/JCO.21.00896.
Key clinical point: Replacing trastuzumab+taxane with trastuzumab emtansine (T-DM1) did not improve survival in high-risk human epidermal growth factor receptor 2 (HER2)-positive early breast cancer (BC).
Major finding: The risk for invasive disease-free survival was not significantly different between trastuzumab+taxane+pertuzumab and T-DM1+pertuzumab arms in the overall population (stratified hazard ratio [sHR], 0.98; 95% CI, 0.72-1.32) and the node-positive subpopulation (sHR, 0.97; 95% CI, 0.71-1.32) along with similar rates of grade 3 or higher and serious adverse events.
Study details: Findings are from the phase 3 KAITLIN study, including 1,846 patients with HER2-positive early BC who were randomly assigned to receive T-DM1+pertuzumab or trastuzumab+taxane+pertuzumab after surgery and anthracycline chemotherapy.
Disclosures: This study was supported by F. Hoffmann-La Roche Ltd. The authors declared serving as consultant, speaker, and/or receiving honorarium, funding, and travel expense from several sources. Some authors were employed and/or held stocks in various sources.
Source: Krop IE et al. J Clin Oncol. 2021 Dec 10. doi: 10.1200/JCO.21.00896.
Key clinical point: Replacing trastuzumab+taxane with trastuzumab emtansine (T-DM1) did not improve survival in high-risk human epidermal growth factor receptor 2 (HER2)-positive early breast cancer (BC).
Major finding: The risk for invasive disease-free survival was not significantly different between trastuzumab+taxane+pertuzumab and T-DM1+pertuzumab arms in the overall population (stratified hazard ratio [sHR], 0.98; 95% CI, 0.72-1.32) and the node-positive subpopulation (sHR, 0.97; 95% CI, 0.71-1.32) along with similar rates of grade 3 or higher and serious adverse events.
Study details: Findings are from the phase 3 KAITLIN study, including 1,846 patients with HER2-positive early BC who were randomly assigned to receive T-DM1+pertuzumab or trastuzumab+taxane+pertuzumab after surgery and anthracycline chemotherapy.
Disclosures: This study was supported by F. Hoffmann-La Roche Ltd. The authors declared serving as consultant, speaker, and/or receiving honorarium, funding, and travel expense from several sources. Some authors were employed and/or held stocks in various sources.
Source: Krop IE et al. J Clin Oncol. 2021 Dec 10. doi: 10.1200/JCO.21.00896.
HR+/HER2-negative breast cancer: Adding palbociclib to fulvestrant prolongs PFS in phase 2
Key clinical point: Palbociclib+fulvestrant prolonged progression-free survival (PFS) compared with placebo+fulvestrant in patients with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative, endocrine-sensitive advanced breast cancer (BC).
Major finding: Palbociclib+fulvestrant vs placebo+fulvestrant improved 1-year PFS (83.5% vs 71.9%) and reduced the risk for progressive disease by 45% (hazard ratio [HR], 0.55; 80% CI, 0.36-0.83). Grade 3 or higher adverse events were reported by 80.9% vs 37.9% of patients in palbociclib/fulvestrant vs placebo/fulvestrant arms.
Study details: This was a phase 2 FLIPPER study of 189 postmenopausal women with HR-positive, HER2-negative, endocrine-sensitive advanced BC who were randomly assigned to palbociclib+fulvestrant or placebo+fulvestrant.
Disclosures: This study was funded by GEICAM Spanish Breast Cancer Group, AstraZeneca, and Pfizer. The authors reported receiving research grants, advisory board fees, consulting fees, honoraria, and travel and accommodation support from several sources.
Source: Albanell J et al. Eur J Cancer. 2021 Dec 11. doi: 10.1016/j.ejca.2021.11.010.
Key clinical point: Palbociclib+fulvestrant prolonged progression-free survival (PFS) compared with placebo+fulvestrant in patients with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative, endocrine-sensitive advanced breast cancer (BC).
Major finding: Palbociclib+fulvestrant vs placebo+fulvestrant improved 1-year PFS (83.5% vs 71.9%) and reduced the risk for progressive disease by 45% (hazard ratio [HR], 0.55; 80% CI, 0.36-0.83). Grade 3 or higher adverse events were reported by 80.9% vs 37.9% of patients in palbociclib/fulvestrant vs placebo/fulvestrant arms.
Study details: This was a phase 2 FLIPPER study of 189 postmenopausal women with HR-positive, HER2-negative, endocrine-sensitive advanced BC who were randomly assigned to palbociclib+fulvestrant or placebo+fulvestrant.
Disclosures: This study was funded by GEICAM Spanish Breast Cancer Group, AstraZeneca, and Pfizer. The authors reported receiving research grants, advisory board fees, consulting fees, honoraria, and travel and accommodation support from several sources.
Source: Albanell J et al. Eur J Cancer. 2021 Dec 11. doi: 10.1016/j.ejca.2021.11.010.
Key clinical point: Palbociclib+fulvestrant prolonged progression-free survival (PFS) compared with placebo+fulvestrant in patients with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative, endocrine-sensitive advanced breast cancer (BC).
Major finding: Palbociclib+fulvestrant vs placebo+fulvestrant improved 1-year PFS (83.5% vs 71.9%) and reduced the risk for progressive disease by 45% (hazard ratio [HR], 0.55; 80% CI, 0.36-0.83). Grade 3 or higher adverse events were reported by 80.9% vs 37.9% of patients in palbociclib/fulvestrant vs placebo/fulvestrant arms.
Study details: This was a phase 2 FLIPPER study of 189 postmenopausal women with HR-positive, HER2-negative, endocrine-sensitive advanced BC who were randomly assigned to palbociclib+fulvestrant or placebo+fulvestrant.
Disclosures: This study was funded by GEICAM Spanish Breast Cancer Group, AstraZeneca, and Pfizer. The authors reported receiving research grants, advisory board fees, consulting fees, honoraria, and travel and accommodation support from several sources.
Source: Albanell J et al. Eur J Cancer. 2021 Dec 11. doi: 10.1016/j.ejca.2021.11.010.
Metastatic BC: Improved survival in long-term responders with no evidence of disease vs residual disease
Key clinical point: Women with metastatic breast cancer (BC) with long-term response to first-line human epidermal growth factor receptor 2 (HER2)-targeted therapy who achieved no evidence of disease (NED) showed improved survival than those with residual disease (RES).
Major finding: Women with NED vs RES achieved longer median progression-free survival (not reached vs 3.08 years; P < .001) and superior overall survival (not reached vs 5.38 years; P < .001) with premenopausal status (P = .006) and de novo metastases (P = .002) associated with higher chances of achieving NED.
Study details: Findings are from a retrospective study including 103 women with HER2-positive metastatic BC who received first-line chemotherapy+trastuzumab or taxane+trastuzumab+pertuzumab and showed a response duration ≥2-fold higher than those observed in pivotal trials.
Disclosures: This study did not report any source of funding. The authors declared serving as a member of a trial steering committee and/or receiving honoraria, funding, consultancy, and advisory fees from several sources.
Source: Veitch Z et al. Br J Cancer. 2021 Dec 20. doi: 10.1038/s41416-021-01676-4.
Key clinical point: Women with metastatic breast cancer (BC) with long-term response to first-line human epidermal growth factor receptor 2 (HER2)-targeted therapy who achieved no evidence of disease (NED) showed improved survival than those with residual disease (RES).
Major finding: Women with NED vs RES achieved longer median progression-free survival (not reached vs 3.08 years; P < .001) and superior overall survival (not reached vs 5.38 years; P < .001) with premenopausal status (P = .006) and de novo metastases (P = .002) associated with higher chances of achieving NED.
Study details: Findings are from a retrospective study including 103 women with HER2-positive metastatic BC who received first-line chemotherapy+trastuzumab or taxane+trastuzumab+pertuzumab and showed a response duration ≥2-fold higher than those observed in pivotal trials.
Disclosures: This study did not report any source of funding. The authors declared serving as a member of a trial steering committee and/or receiving honoraria, funding, consultancy, and advisory fees from several sources.
Source: Veitch Z et al. Br J Cancer. 2021 Dec 20. doi: 10.1038/s41416-021-01676-4.
Key clinical point: Women with metastatic breast cancer (BC) with long-term response to first-line human epidermal growth factor receptor 2 (HER2)-targeted therapy who achieved no evidence of disease (NED) showed improved survival than those with residual disease (RES).
Major finding: Women with NED vs RES achieved longer median progression-free survival (not reached vs 3.08 years; P < .001) and superior overall survival (not reached vs 5.38 years; P < .001) with premenopausal status (P = .006) and de novo metastases (P = .002) associated with higher chances of achieving NED.
Study details: Findings are from a retrospective study including 103 women with HER2-positive metastatic BC who received first-line chemotherapy+trastuzumab or taxane+trastuzumab+pertuzumab and showed a response duration ≥2-fold higher than those observed in pivotal trials.
Disclosures: This study did not report any source of funding. The authors declared serving as a member of a trial steering committee and/or receiving honoraria, funding, consultancy, and advisory fees from several sources.
Source: Veitch Z et al. Br J Cancer. 2021 Dec 20. doi: 10.1038/s41416-021-01676-4.
Tucatinib and trastuzumab+capecitabine combo offers survival benefit in HER2+ metastatic breast cancer
Key clinical point: Addition of tucatinib to trastuzumab and capecitabine continued to improve survival along with good tolerability in patients with human epidermal growth factor receptor 2 (HER2)-positive metastatic breast cancer (BC) who progressed on HER2-targeted therapies.
Major finding: Addition of tucatinib to trastuzumab and capecitabine significantly improved overall survival (hazard ratio [HR] for death, 0.73; P = .004) and progression-free survival (HR for disease-progression or death, 0.57; P < .00001) compared with placebo. Rates of grade 3 or higher adverse events (AEs) were similar between treatment arms, with only 5.9% of patients discontinuing treatment because of AEs.
Study details: These are the final outcomes from the phase 2 HER2CLIMB study including 612 patients with HER2-positive metastatic BC who progressed on trastuzumab, pertuzumab, and trastuzumab emtansine and were randomly assigned to tucatinib or placebo, each in combination with trastuzumab and capecitabine.
Disclosures: This work was supported by Seagen Inc. and Merck Sharp & Dohme Corp. Four authors declared being employees of Seagen and other sources, and other authors reported ties with various sources.
Source: Curigliano G et al. Ann Oncol. 2021 Dec 22. doi: 10.1016/j.annonc.2021.12.005.
Key clinical point: Addition of tucatinib to trastuzumab and capecitabine continued to improve survival along with good tolerability in patients with human epidermal growth factor receptor 2 (HER2)-positive metastatic breast cancer (BC) who progressed on HER2-targeted therapies.
Major finding: Addition of tucatinib to trastuzumab and capecitabine significantly improved overall survival (hazard ratio [HR] for death, 0.73; P = .004) and progression-free survival (HR for disease-progression or death, 0.57; P < .00001) compared with placebo. Rates of grade 3 or higher adverse events (AEs) were similar between treatment arms, with only 5.9% of patients discontinuing treatment because of AEs.
Study details: These are the final outcomes from the phase 2 HER2CLIMB study including 612 patients with HER2-positive metastatic BC who progressed on trastuzumab, pertuzumab, and trastuzumab emtansine and were randomly assigned to tucatinib or placebo, each in combination with trastuzumab and capecitabine.
Disclosures: This work was supported by Seagen Inc. and Merck Sharp & Dohme Corp. Four authors declared being employees of Seagen and other sources, and other authors reported ties with various sources.
Source: Curigliano G et al. Ann Oncol. 2021 Dec 22. doi: 10.1016/j.annonc.2021.12.005.
Key clinical point: Addition of tucatinib to trastuzumab and capecitabine continued to improve survival along with good tolerability in patients with human epidermal growth factor receptor 2 (HER2)-positive metastatic breast cancer (BC) who progressed on HER2-targeted therapies.
Major finding: Addition of tucatinib to trastuzumab and capecitabine significantly improved overall survival (hazard ratio [HR] for death, 0.73; P = .004) and progression-free survival (HR for disease-progression or death, 0.57; P < .00001) compared with placebo. Rates of grade 3 or higher adverse events (AEs) were similar between treatment arms, with only 5.9% of patients discontinuing treatment because of AEs.
Study details: These are the final outcomes from the phase 2 HER2CLIMB study including 612 patients with HER2-positive metastatic BC who progressed on trastuzumab, pertuzumab, and trastuzumab emtansine and were randomly assigned to tucatinib or placebo, each in combination with trastuzumab and capecitabine.
Disclosures: This work was supported by Seagen Inc. and Merck Sharp & Dohme Corp. Four authors declared being employees of Seagen and other sources, and other authors reported ties with various sources.
Source: Curigliano G et al. Ann Oncol. 2021 Dec 22. doi: 10.1016/j.annonc.2021.12.005.
GnRHa protects ovarian function in premenopausal women receiving chemotherapy for breast cancer
Key clinical point: Administration of gonadotropin-releasing hormone analogs (GnRHa) with chemotherapy reduced the risk for premature ovarian insufficiency (POI) in premenopausal women with breast cancer.
Major finding: At 12 months after chemotherapy, POI was reported by 10.3% vs 44.5% of patients in the GnRHa + chemotherapy vs chemotherapy-only group (odds ratio, 0.23; P < .001). No serious adverse events were reported.
Study details: Findings are from a phase 3 superiority trial including 330 premenopausal women with stages I-III operable breast cancer who were randomly assigned to GnRHa (3.6 mg of goserelin or 3.75 mg of leuprorelin) + chemotherapy or chemotherapy alone.
Disclosures: This study was funded by the Science and Technology Commission, Shanghai, and Zhejiang Medical Association. Dr. Zong reported receiving grants from the Science and Technology Commission of Shanghai Municipality.
Source: Zong X et al. JAMA Oncol. 2021 Dec 30. doi: 10.1001/jamaoncol.2021.6214.
Key clinical point: Administration of gonadotropin-releasing hormone analogs (GnRHa) with chemotherapy reduced the risk for premature ovarian insufficiency (POI) in premenopausal women with breast cancer.
Major finding: At 12 months after chemotherapy, POI was reported by 10.3% vs 44.5% of patients in the GnRHa + chemotherapy vs chemotherapy-only group (odds ratio, 0.23; P < .001). No serious adverse events were reported.
Study details: Findings are from a phase 3 superiority trial including 330 premenopausal women with stages I-III operable breast cancer who were randomly assigned to GnRHa (3.6 mg of goserelin or 3.75 mg of leuprorelin) + chemotherapy or chemotherapy alone.
Disclosures: This study was funded by the Science and Technology Commission, Shanghai, and Zhejiang Medical Association. Dr. Zong reported receiving grants from the Science and Technology Commission of Shanghai Municipality.
Source: Zong X et al. JAMA Oncol. 2021 Dec 30. doi: 10.1001/jamaoncol.2021.6214.
Key clinical point: Administration of gonadotropin-releasing hormone analogs (GnRHa) with chemotherapy reduced the risk for premature ovarian insufficiency (POI) in premenopausal women with breast cancer.
Major finding: At 12 months after chemotherapy, POI was reported by 10.3% vs 44.5% of patients in the GnRHa + chemotherapy vs chemotherapy-only group (odds ratio, 0.23; P < .001). No serious adverse events were reported.
Study details: Findings are from a phase 3 superiority trial including 330 premenopausal women with stages I-III operable breast cancer who were randomly assigned to GnRHa (3.6 mg of goserelin or 3.75 mg of leuprorelin) + chemotherapy or chemotherapy alone.
Disclosures: This study was funded by the Science and Technology Commission, Shanghai, and Zhejiang Medical Association. Dr. Zong reported receiving grants from the Science and Technology Commission of Shanghai Municipality.
Source: Zong X et al. JAMA Oncol. 2021 Dec 30. doi: 10.1001/jamaoncol.2021.6214.
Residual cancer burden prognostic across all breast cancer subtypes
Key clinical point: Residual cancer burden (RCB) after neoadjuvant chemotherapy was prognostic for event-free survival (EFS) in each hormone receptor (HR) and human epidermal growth factor receptor 2 (HER2) subtype of breast cancer.
Major finding: RCB was prognostic for EFS with the hazard ratio associated with each unit increase in RCB being 1.69 (P < .0001) for the overall population and ranging from 1.52 in HR-positive/HER2-negative group to 2.09 in HR-negative/HER2-positive group (P < .0001 for all subtypes).
Study details: Findings are pooled analysis of 4 trials and 8 clinical cohorts, including 5,161 adult patients with primary stage I-III breast cancer treated with neoadjuvant chemotherapy followed by surgery.
Disclosures: This study was funded by the National Cancer Institute, USA. Some of the authors declared serving as a consultant, data and safety monitoring advisor, and/or receiving grants, funding, personal fees, travel support, and honoraria from several sources.
Source: Yau C et al. Lancet Oncol. 2021 Dec 10. doi: 10.1016/S1470-2045(21)00589-1.
Key clinical point: Residual cancer burden (RCB) after neoadjuvant chemotherapy was prognostic for event-free survival (EFS) in each hormone receptor (HR) and human epidermal growth factor receptor 2 (HER2) subtype of breast cancer.
Major finding: RCB was prognostic for EFS with the hazard ratio associated with each unit increase in RCB being 1.69 (P < .0001) for the overall population and ranging from 1.52 in HR-positive/HER2-negative group to 2.09 in HR-negative/HER2-positive group (P < .0001 for all subtypes).
Study details: Findings are pooled analysis of 4 trials and 8 clinical cohorts, including 5,161 adult patients with primary stage I-III breast cancer treated with neoadjuvant chemotherapy followed by surgery.
Disclosures: This study was funded by the National Cancer Institute, USA. Some of the authors declared serving as a consultant, data and safety monitoring advisor, and/or receiving grants, funding, personal fees, travel support, and honoraria from several sources.
Source: Yau C et al. Lancet Oncol. 2021 Dec 10. doi: 10.1016/S1470-2045(21)00589-1.
Key clinical point: Residual cancer burden (RCB) after neoadjuvant chemotherapy was prognostic for event-free survival (EFS) in each hormone receptor (HR) and human epidermal growth factor receptor 2 (HER2) subtype of breast cancer.
Major finding: RCB was prognostic for EFS with the hazard ratio associated with each unit increase in RCB being 1.69 (P < .0001) for the overall population and ranging from 1.52 in HR-positive/HER2-negative group to 2.09 in HR-negative/HER2-positive group (P < .0001 for all subtypes).
Study details: Findings are pooled analysis of 4 trials and 8 clinical cohorts, including 5,161 adult patients with primary stage I-III breast cancer treated with neoadjuvant chemotherapy followed by surgery.
Disclosures: This study was funded by the National Cancer Institute, USA. Some of the authors declared serving as a consultant, data and safety monitoring advisor, and/or receiving grants, funding, personal fees, travel support, and honoraria from several sources.
Source: Yau C et al. Lancet Oncol. 2021 Dec 10. doi: 10.1016/S1470-2045(21)00589-1.
Study finds genetic factor for COVID smell and taste loss
, according to a new study published in the journal Nature Genetics
The finding could eventually help the 1.6 million people in the United States who still can’t smell or have had a change in their ability to smell more than 6 months after getting the coronavirus. The exact cause related to COVID-19 is still unknown, but researchers believe it could be because of damage in a part of the nose called the olfactory epithelium.
“How we get from infection to smell loss remains unclear,” Justin Turner, MD, an associate professor of otolaryngology at Vanderbilt University, Nashville, Tenn., told NBC News. Dr. Turner was not part of the research team.
“Early data suggest that supporting cells of the olfactory epithelium are the ones mostly being infected by the virus, and presumably this leads to the death of the neurons themselves,” he said. “But we don’t really, really know why and when that happens, and why it seems to preferentially happen in certain individuals.”
Researchers at 23andMe, a genomics and biotechnology company, did the study as part of a larger COVID-19 project, which includes people in the United States and the United Kingdom. They analyzed data from nearly 70,000 people who took online surveys after receiving a positive coronavirus test. Among those, 68% reported a loss of smell or taste as a symptom.
The study team compared the genetic differences between those who lost their sense of smell and taste and those who didn’t. They found that a location near two olfactory genes – UGT2A1 and UGT2A2 – is associated with COVID-19 loss of smell and taste. The genetic risk factor makes it 11% more likely for a person with COVID-19 to lose their sense of smell or taste.
The research team also found that women were 11% more likely than men to report a loss of smell and taste. About 73% of those who reported a loss of smell and taste were ages 26-35.
The researchers aren’t sure how the genes are involved, though they suspect that infected cells could lead to smell loss. Typically, the genes are expressed in tissue inside the nose involved with smell and play a role in processing things that have an odor. To use the findings, researchers need to learn more about the genes, how they are expressed, and what their functions are, NBC News reported.
The findings could help lead to treatments. Other research has shown that the loss of taste and smell is related to a “failure to protect the sensory cells of the nose and tongue from viral infection,” Danielle Reed, PhD, associate director of the Monell Chemical Senses Center in Philadelphia, told NBC News. She was not part of the research team but studies person-to-person differences in the loss of these senses because of COVID-19.
“This study suggests a different direction,” she said. “The pathways that break down the chemicals that cause taste and smell in the first place might be over or underactive, reducing or distorting the ability to taste and smell.”
A version of this article first appeared on WebMD.com.
, according to a new study published in the journal Nature Genetics
The finding could eventually help the 1.6 million people in the United States who still can’t smell or have had a change in their ability to smell more than 6 months after getting the coronavirus. The exact cause related to COVID-19 is still unknown, but researchers believe it could be because of damage in a part of the nose called the olfactory epithelium.
“How we get from infection to smell loss remains unclear,” Justin Turner, MD, an associate professor of otolaryngology at Vanderbilt University, Nashville, Tenn., told NBC News. Dr. Turner was not part of the research team.
“Early data suggest that supporting cells of the olfactory epithelium are the ones mostly being infected by the virus, and presumably this leads to the death of the neurons themselves,” he said. “But we don’t really, really know why and when that happens, and why it seems to preferentially happen in certain individuals.”
Researchers at 23andMe, a genomics and biotechnology company, did the study as part of a larger COVID-19 project, which includes people in the United States and the United Kingdom. They analyzed data from nearly 70,000 people who took online surveys after receiving a positive coronavirus test. Among those, 68% reported a loss of smell or taste as a symptom.
The study team compared the genetic differences between those who lost their sense of smell and taste and those who didn’t. They found that a location near two olfactory genes – UGT2A1 and UGT2A2 – is associated with COVID-19 loss of smell and taste. The genetic risk factor makes it 11% more likely for a person with COVID-19 to lose their sense of smell or taste.
The research team also found that women were 11% more likely than men to report a loss of smell and taste. About 73% of those who reported a loss of smell and taste were ages 26-35.
The researchers aren’t sure how the genes are involved, though they suspect that infected cells could lead to smell loss. Typically, the genes are expressed in tissue inside the nose involved with smell and play a role in processing things that have an odor. To use the findings, researchers need to learn more about the genes, how they are expressed, and what their functions are, NBC News reported.
The findings could help lead to treatments. Other research has shown that the loss of taste and smell is related to a “failure to protect the sensory cells of the nose and tongue from viral infection,” Danielle Reed, PhD, associate director of the Monell Chemical Senses Center in Philadelphia, told NBC News. She was not part of the research team but studies person-to-person differences in the loss of these senses because of COVID-19.
“This study suggests a different direction,” she said. “The pathways that break down the chemicals that cause taste and smell in the first place might be over or underactive, reducing or distorting the ability to taste and smell.”
A version of this article first appeared on WebMD.com.
, according to a new study published in the journal Nature Genetics
The finding could eventually help the 1.6 million people in the United States who still can’t smell or have had a change in their ability to smell more than 6 months after getting the coronavirus. The exact cause related to COVID-19 is still unknown, but researchers believe it could be because of damage in a part of the nose called the olfactory epithelium.
“How we get from infection to smell loss remains unclear,” Justin Turner, MD, an associate professor of otolaryngology at Vanderbilt University, Nashville, Tenn., told NBC News. Dr. Turner was not part of the research team.
“Early data suggest that supporting cells of the olfactory epithelium are the ones mostly being infected by the virus, and presumably this leads to the death of the neurons themselves,” he said. “But we don’t really, really know why and when that happens, and why it seems to preferentially happen in certain individuals.”
Researchers at 23andMe, a genomics and biotechnology company, did the study as part of a larger COVID-19 project, which includes people in the United States and the United Kingdom. They analyzed data from nearly 70,000 people who took online surveys after receiving a positive coronavirus test. Among those, 68% reported a loss of smell or taste as a symptom.
The study team compared the genetic differences between those who lost their sense of smell and taste and those who didn’t. They found that a location near two olfactory genes – UGT2A1 and UGT2A2 – is associated with COVID-19 loss of smell and taste. The genetic risk factor makes it 11% more likely for a person with COVID-19 to lose their sense of smell or taste.
The research team also found that women were 11% more likely than men to report a loss of smell and taste. About 73% of those who reported a loss of smell and taste were ages 26-35.
The researchers aren’t sure how the genes are involved, though they suspect that infected cells could lead to smell loss. Typically, the genes are expressed in tissue inside the nose involved with smell and play a role in processing things that have an odor. To use the findings, researchers need to learn more about the genes, how they are expressed, and what their functions are, NBC News reported.
The findings could help lead to treatments. Other research has shown that the loss of taste and smell is related to a “failure to protect the sensory cells of the nose and tongue from viral infection,” Danielle Reed, PhD, associate director of the Monell Chemical Senses Center in Philadelphia, told NBC News. She was not part of the research team but studies person-to-person differences in the loss of these senses because of COVID-19.
“This study suggests a different direction,” she said. “The pathways that break down the chemicals that cause taste and smell in the first place might be over or underactive, reducing or distorting the ability to taste and smell.”
A version of this article first appeared on WebMD.com.
FROM NATURE GENETICS
Fourth vaccine shot less effective against Omicron, Israeli study says
, according to new research at an Israeli hospital.
The preliminary results, released on Jan. 17, challenge the idea of giving a second booster dose to slow the spread of the coronavirus, according to USA Today.
“Despite increased antibody levels, the fourth vaccine only offers a partial defense against the virus,” Gili Regev-Yochay, MD, director of the hospital’s infection prevention and control units, told reporters.
“The vaccines, which were more effective against previous variants, offer less protection versus Omicron,” she said.
In a clinical trial, 274 medical workers at Sheba Medical Center near Tel Aviv received a fourth vaccine dose in December – 154 got the Pfizer vaccine and 120 got the Moderna vaccine – after previously getting three Pfizer shots.
Both groups received a boost in antibodies that was “slightly higher” than after the third shot, Dr. Regev-Yochay said. But when compared with a control group that didn’t receive the fourth dose, the extra boost didn’t prevent the spread of Omicron.
“We see many infected with Omicron who received the fourth dose,” Dr. Regev-Yochay said. “Granted, a bit less than in the control group, but still a lot of infections.”
Some public health officials in Israel say the campaign for fourth doses is still worthwhile, according to The Times of Israel. The vaccine still works well against the Alpha and Delta variants, Dr. Regev-Yochay said, and a fourth shot should go to older adults and those who face higher risks for severe COVID-19.
Hours after releasing the preliminary results, Sheba Medical Center published a statement calling for “continuing the vaccination drive for risk groups at this time, even though the vaccine doesn’t provide optimal protection against getting infected with the variant.” News outlets reported that the hospital was pressured into issuing the statement after Israel’s Health Ministry didn’t like the release of the early study results, The Times of Israel reported.
The second booster “returns the level of antibodies to what it was at the beginning of the third booster,” Nachman Ash, MD, director of Israel’s Health Ministry, told Channel 13 TV in Israel, according to The Associated Press.
“That has great importance, especially among the older population,” he said.
As of Sunday, more than 500,000 people in Israel had received fourth doses since the country began offering them last month to medical workers, immunocompromised patients, and people ages 60 years and older, the AP reported. At the same time, the country has faced a recent coronavirus surge that has led to record-breaking numbers of cases and rising hospitalizations.
On Tuesday, the Israeli government said it would shorten the mandatory quarantine period from 7 days to 5 days, the AP reported.
“This decision will enable us to continue safeguarding public health on the one hand and to keep the economy going at this time on the other, even though it is difficult, so that we can get through this wave safely,” Prime Minister Naftali Bennett said.
A version of this article first appeared on WebMD.com.
, according to new research at an Israeli hospital.
The preliminary results, released on Jan. 17, challenge the idea of giving a second booster dose to slow the spread of the coronavirus, according to USA Today.
“Despite increased antibody levels, the fourth vaccine only offers a partial defense against the virus,” Gili Regev-Yochay, MD, director of the hospital’s infection prevention and control units, told reporters.
“The vaccines, which were more effective against previous variants, offer less protection versus Omicron,” she said.
In a clinical trial, 274 medical workers at Sheba Medical Center near Tel Aviv received a fourth vaccine dose in December – 154 got the Pfizer vaccine and 120 got the Moderna vaccine – after previously getting three Pfizer shots.
Both groups received a boost in antibodies that was “slightly higher” than after the third shot, Dr. Regev-Yochay said. But when compared with a control group that didn’t receive the fourth dose, the extra boost didn’t prevent the spread of Omicron.
“We see many infected with Omicron who received the fourth dose,” Dr. Regev-Yochay said. “Granted, a bit less than in the control group, but still a lot of infections.”
Some public health officials in Israel say the campaign for fourth doses is still worthwhile, according to The Times of Israel. The vaccine still works well against the Alpha and Delta variants, Dr. Regev-Yochay said, and a fourth shot should go to older adults and those who face higher risks for severe COVID-19.
Hours after releasing the preliminary results, Sheba Medical Center published a statement calling for “continuing the vaccination drive for risk groups at this time, even though the vaccine doesn’t provide optimal protection against getting infected with the variant.” News outlets reported that the hospital was pressured into issuing the statement after Israel’s Health Ministry didn’t like the release of the early study results, The Times of Israel reported.
The second booster “returns the level of antibodies to what it was at the beginning of the third booster,” Nachman Ash, MD, director of Israel’s Health Ministry, told Channel 13 TV in Israel, according to The Associated Press.
“That has great importance, especially among the older population,” he said.
As of Sunday, more than 500,000 people in Israel had received fourth doses since the country began offering them last month to medical workers, immunocompromised patients, and people ages 60 years and older, the AP reported. At the same time, the country has faced a recent coronavirus surge that has led to record-breaking numbers of cases and rising hospitalizations.
On Tuesday, the Israeli government said it would shorten the mandatory quarantine period from 7 days to 5 days, the AP reported.
“This decision will enable us to continue safeguarding public health on the one hand and to keep the economy going at this time on the other, even though it is difficult, so that we can get through this wave safely,” Prime Minister Naftali Bennett said.
A version of this article first appeared on WebMD.com.
, according to new research at an Israeli hospital.
The preliminary results, released on Jan. 17, challenge the idea of giving a second booster dose to slow the spread of the coronavirus, according to USA Today.
“Despite increased antibody levels, the fourth vaccine only offers a partial defense against the virus,” Gili Regev-Yochay, MD, director of the hospital’s infection prevention and control units, told reporters.
“The vaccines, which were more effective against previous variants, offer less protection versus Omicron,” she said.
In a clinical trial, 274 medical workers at Sheba Medical Center near Tel Aviv received a fourth vaccine dose in December – 154 got the Pfizer vaccine and 120 got the Moderna vaccine – after previously getting three Pfizer shots.
Both groups received a boost in antibodies that was “slightly higher” than after the third shot, Dr. Regev-Yochay said. But when compared with a control group that didn’t receive the fourth dose, the extra boost didn’t prevent the spread of Omicron.
“We see many infected with Omicron who received the fourth dose,” Dr. Regev-Yochay said. “Granted, a bit less than in the control group, but still a lot of infections.”
Some public health officials in Israel say the campaign for fourth doses is still worthwhile, according to The Times of Israel. The vaccine still works well against the Alpha and Delta variants, Dr. Regev-Yochay said, and a fourth shot should go to older adults and those who face higher risks for severe COVID-19.
Hours after releasing the preliminary results, Sheba Medical Center published a statement calling for “continuing the vaccination drive for risk groups at this time, even though the vaccine doesn’t provide optimal protection against getting infected with the variant.” News outlets reported that the hospital was pressured into issuing the statement after Israel’s Health Ministry didn’t like the release of the early study results, The Times of Israel reported.
The second booster “returns the level of antibodies to what it was at the beginning of the third booster,” Nachman Ash, MD, director of Israel’s Health Ministry, told Channel 13 TV in Israel, according to The Associated Press.
“That has great importance, especially among the older population,” he said.
As of Sunday, more than 500,000 people in Israel had received fourth doses since the country began offering them last month to medical workers, immunocompromised patients, and people ages 60 years and older, the AP reported. At the same time, the country has faced a recent coronavirus surge that has led to record-breaking numbers of cases and rising hospitalizations.
On Tuesday, the Israeli government said it would shorten the mandatory quarantine period from 7 days to 5 days, the AP reported.
“This decision will enable us to continue safeguarding public health on the one hand and to keep the economy going at this time on the other, even though it is difficult, so that we can get through this wave safely,” Prime Minister Naftali Bennett said.
A version of this article first appeared on WebMD.com.
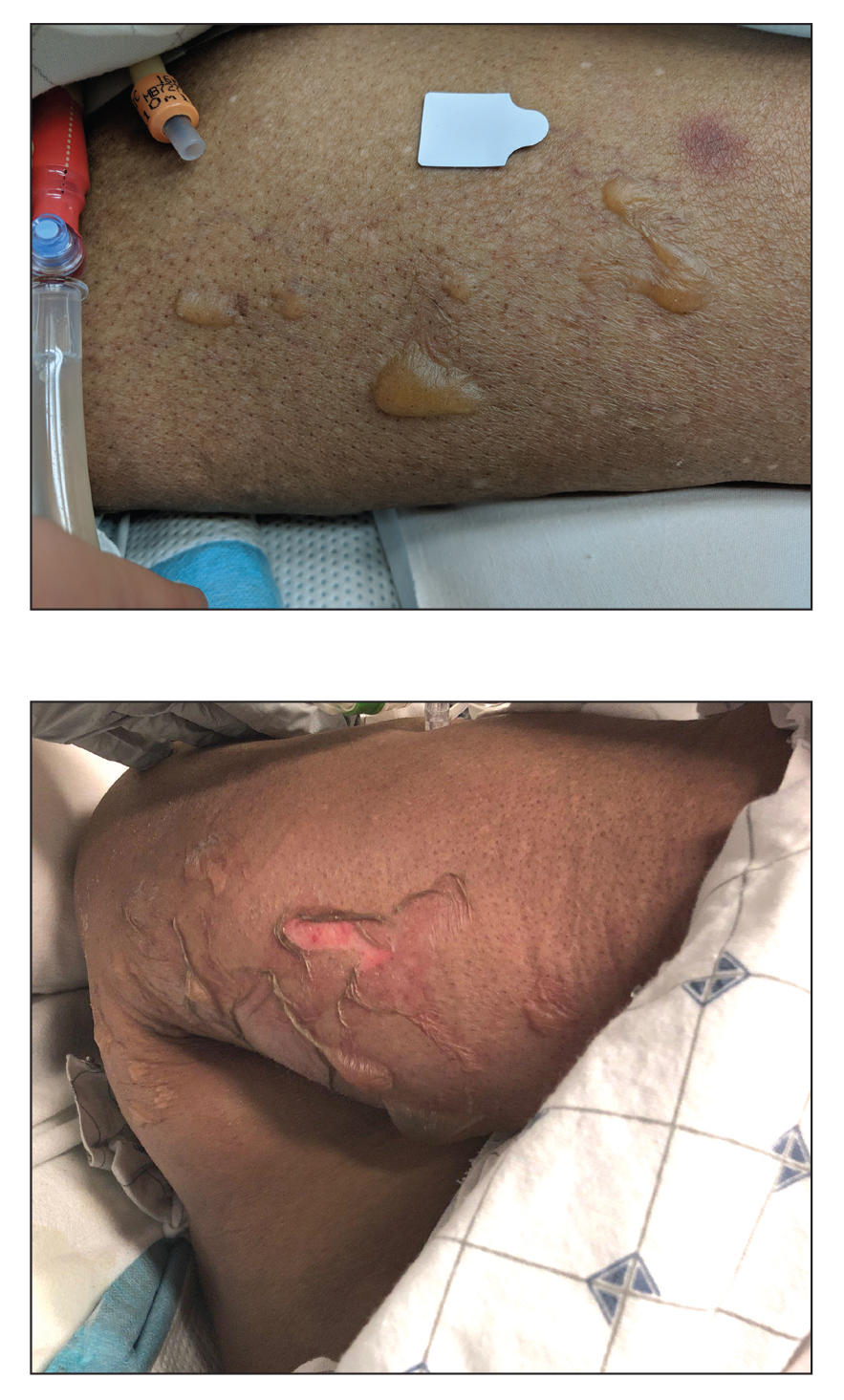
Blisters in a Comatose Elderly Woman
The Diagnosis: Coma Blisters
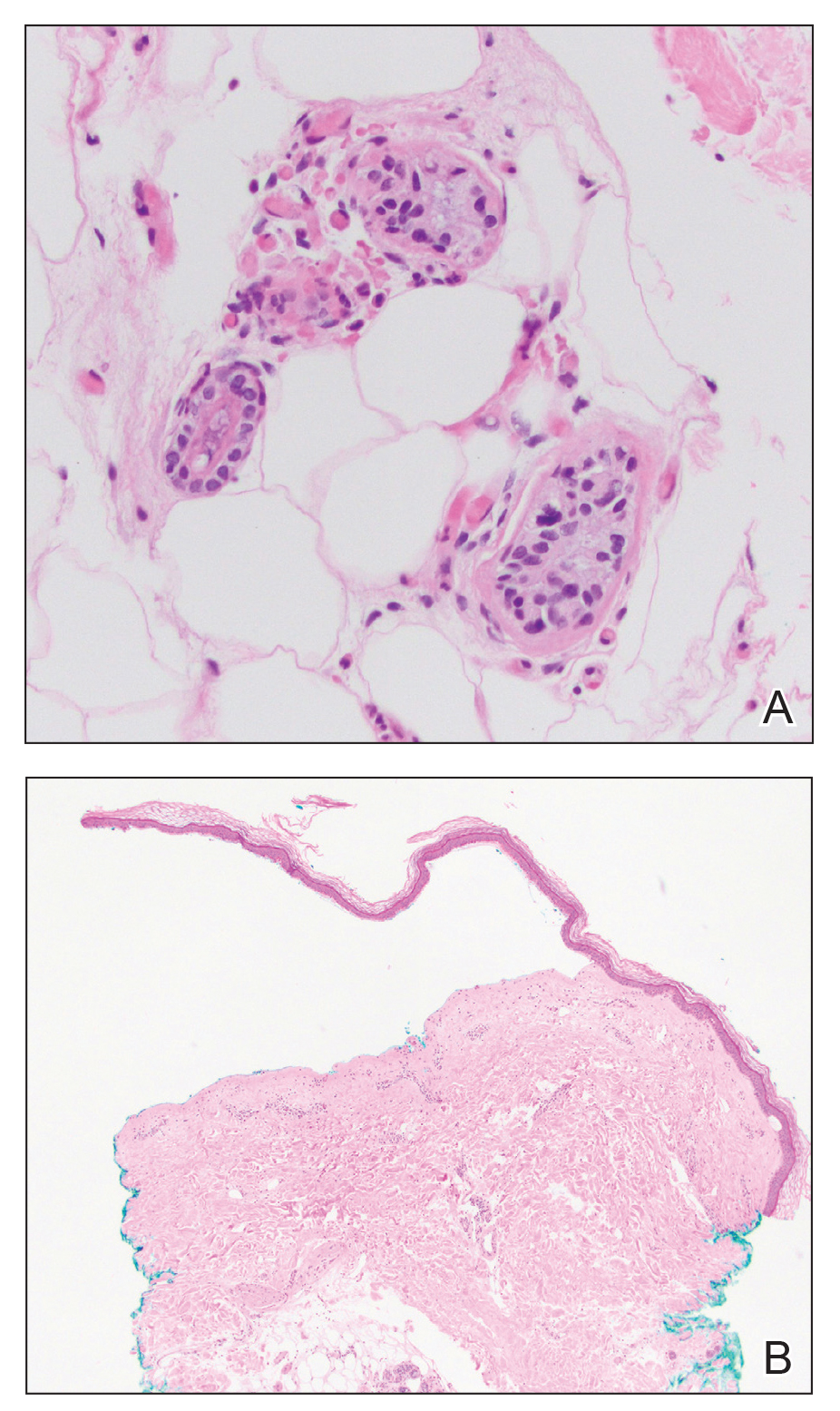
Histologic examination revealed pauci-inflammatory subepidermal blisters with swelling of eccrine cells, signaling impending gland necrosis (Figure). Direct immunofluorescence testing on perilesional skin was negative. These findings would be inconsistent for diagnoses of edema blisters (most commonly seen in patients with an acute exacerbation of chronic lower extremity edema), friction blisters (intraepidermal blisters seen on histopathology), and bullous pemphigoid (linear IgG and/or C3 staining along the basement membrane zone on direct immunofluorescence testing is characteristic). Although eccrine gland alterations have been seen in toxic epidermal necrolysis,1 the mucous membranes are involved in more than 90% of cases, making the diagnosis less likely. Furthermore, interface changes including prominent keratinocyte necrosis were not seen on histology.
Given the localized nature of the lesions in our patient and negative direct immunofluorescence studies, a diagnosis of coma blisters was made. Gentle wound care practices to the areas of denuded skin were implemented with complete resolution. The patient’s condition gradually improved, and she was extubated and discharged home.
Coma blisters are self-limited bullous lesions that have been reported in comatose patients as early as 1812 when Napoleon’s surgeon first noticed cutaneous blisters in comatose French soldiers being treated for carbon monoxide intoxication.2 Since then, barbiturate overdose has remained the most common association, but coma blisters have occurred in the absence of specific drug exposures. Clinically, erythematous or violaceous plaques typically appear within 24 hours of drug ingestion, and progression to large tense bullae usually occurs within 48 to 72 hours of unconsciousness.3 They characteristically occur in pressure-dependent areas, but reports have shown lesions in non–pressure-dependent areas, including the penis and mouth.1,4 Spontaneous resolution within 1 to 2 weeks is typical.5
The underlying pathogenesis remains controversial, as multiple mechanisms have been suggested, but clear causal evidence is lacking. The original proposition that direct effects of drug toxicity caused the cutaneous observations was later refuted after similar bullous lesions with eccrine gland necrosis were reported in comatose patients with neurologic conditions.6 It is largely accepted that pressure-induced local ischemia—proportional to the duration and amount of pressure—leads to tissue injury and is critical to the pathogenesis. During periods of ischemia, the most metabolically active tissues will undergo necrosis first; however, in eccrine glands, the earliest and most severe damage does not seem to occur in the most metabolically active cells.7 Additionally, this would not provide a viable explanation for coma blisters with eccrine gland necrosis developing in variable non–pressuredependent areas.
Moreover, drug- and non–drug-induced coma blisters can appear identically, but specific histopathologic differences have been reported. The most notable markers of non–drug-induced coma blisters are the absence of an inflammatory infiltrate in the epidermis and the presence of thrombosis in dermal vessels.8 Demonstration of necrotic changes in the secretory portion of the eccrine gland is considered the histopathologic hallmark for drug-induced coma blisters, but other findings can include subepidermal or intraepidermal bullae; perivascular infiltrates; and focal necrosis of the epidermis, dermis, subcutis, or epidermal appendages.6 Arteriolar wall necrosis and dermal inflammatory infiltrates also have been observed.7
Benzodiazepines have been widely prescribed and abused since their development, and overdose is much more common today than with barbiturates.9 Coma blisters rarely have been documented in the setting of isolated benzodiazepine overdose, and of the few cases, only one report implicated lorazepam as the causative agent.4,7 The characteristic finding of eccrine gland necrosis consistently was seen in our patient. This case not only emphasizes the need for greater awareness of the association between benzodiazepine overdose and coma blisters but also the importance of clinical context when considering diagnoses. It is essential to note that coma blisters themselves are nonspecific, and the diagnosis of drug-induced coma blisters warrants confirmatory toxicologic analysis.
- Ferreli C, Sulica VI, Aste N, et al. Drug-induced sweat gland necrosis in a non-comatose patient: a case presentation. J Eur Acad Dermatol Venereol. 2003;17:443-445.
- Larrey DJ. Memoires de Chirurgie Militaire et Campagnes. Smith and Buisson; 1812.
- Agarwal A, Bansal M, Conner K. Coma blisters with hypoxemic respiratory failure. Dermatol Online J. 2012;18:10.
- Varma AJ, Fisher BK, Sarin MK. Diazepam-induced coma with bullae and eccrine sweat gland necrosis. Arch Intern Med. 1977;137:1207-1210.
- Rocha J, Pereira T, Ventura F, et al. Coma blisters. Case Rep Dermatol. 2009;1:66-70.
- Arndt KA, Mihm MC, Parrish JA. Bullae: a cutaneous sign of a variety of neurologic diseases. J Invest Dermatol. 1973;60:312-320.
- Sánchez Yus E, Requena L, Simón P. Histopathology of cutaneous changes in drug-induced coma. Am J Dermatopathol. 1993;15:208-216.
- Kato N, Ueno H, Mimura M. Histopathology of cutaneous changes in non-drug-induced coma. Am J Dermatopathol. 1996;18:344-350.
- Kang M, Ghassemzadeh S. Benzodiazepine Toxicity. StatPearls Publishing; 2018.
The Diagnosis: Coma Blisters
Histologic examination revealed pauci-inflammatory subepidermal blisters with swelling of eccrine cells, signaling impending gland necrosis (Figure). Direct immunofluorescence testing on perilesional skin was negative. These findings would be inconsistent for diagnoses of edema blisters (most commonly seen in patients with an acute exacerbation of chronic lower extremity edema), friction blisters (intraepidermal blisters seen on histopathology), and bullous pemphigoid (linear IgG and/or C3 staining along the basement membrane zone on direct immunofluorescence testing is characteristic). Although eccrine gland alterations have been seen in toxic epidermal necrolysis,1 the mucous membranes are involved in more than 90% of cases, making the diagnosis less likely. Furthermore, interface changes including prominent keratinocyte necrosis were not seen on histology.

Given the localized nature of the lesions in our patient and negative direct immunofluorescence studies, a diagnosis of coma blisters was made. Gentle wound care practices to the areas of denuded skin were implemented with complete resolution. The patient’s condition gradually improved, and she was extubated and discharged home.
Coma blisters are self-limited bullous lesions that have been reported in comatose patients as early as 1812 when Napoleon’s surgeon first noticed cutaneous blisters in comatose French soldiers being treated for carbon monoxide intoxication.2 Since then, barbiturate overdose has remained the most common association, but coma blisters have occurred in the absence of specific drug exposures. Clinically, erythematous or violaceous plaques typically appear within 24 hours of drug ingestion, and progression to large tense bullae usually occurs within 48 to 72 hours of unconsciousness.3 They characteristically occur in pressure-dependent areas, but reports have shown lesions in non–pressure-dependent areas, including the penis and mouth.1,4 Spontaneous resolution within 1 to 2 weeks is typical.5
The underlying pathogenesis remains controversial, as multiple mechanisms have been suggested, but clear causal evidence is lacking. The original proposition that direct effects of drug toxicity caused the cutaneous observations was later refuted after similar bullous lesions with eccrine gland necrosis were reported in comatose patients with neurologic conditions.6 It is largely accepted that pressure-induced local ischemia—proportional to the duration and amount of pressure—leads to tissue injury and is critical to the pathogenesis. During periods of ischemia, the most metabolically active tissues will undergo necrosis first; however, in eccrine glands, the earliest and most severe damage does not seem to occur in the most metabolically active cells.7 Additionally, this would not provide a viable explanation for coma blisters with eccrine gland necrosis developing in variable non–pressuredependent areas.
Moreover, drug- and non–drug-induced coma blisters can appear identically, but specific histopathologic differences have been reported. The most notable markers of non–drug-induced coma blisters are the absence of an inflammatory infiltrate in the epidermis and the presence of thrombosis in dermal vessels.8 Demonstration of necrotic changes in the secretory portion of the eccrine gland is considered the histopathologic hallmark for drug-induced coma blisters, but other findings can include subepidermal or intraepidermal bullae; perivascular infiltrates; and focal necrosis of the epidermis, dermis, subcutis, or epidermal appendages.6 Arteriolar wall necrosis and dermal inflammatory infiltrates also have been observed.7
Benzodiazepines have been widely prescribed and abused since their development, and overdose is much more common today than with barbiturates.9 Coma blisters rarely have been documented in the setting of isolated benzodiazepine overdose, and of the few cases, only one report implicated lorazepam as the causative agent.4,7 The characteristic finding of eccrine gland necrosis consistently was seen in our patient. This case not only emphasizes the need for greater awareness of the association between benzodiazepine overdose and coma blisters but also the importance of clinical context when considering diagnoses. It is essential to note that coma blisters themselves are nonspecific, and the diagnosis of drug-induced coma blisters warrants confirmatory toxicologic analysis.
The Diagnosis: Coma Blisters
Histologic examination revealed pauci-inflammatory subepidermal blisters with swelling of eccrine cells, signaling impending gland necrosis (Figure). Direct immunofluorescence testing on perilesional skin was negative. These findings would be inconsistent for diagnoses of edema blisters (most commonly seen in patients with an acute exacerbation of chronic lower extremity edema), friction blisters (intraepidermal blisters seen on histopathology), and bullous pemphigoid (linear IgG and/or C3 staining along the basement membrane zone on direct immunofluorescence testing is characteristic). Although eccrine gland alterations have been seen in toxic epidermal necrolysis,1 the mucous membranes are involved in more than 90% of cases, making the diagnosis less likely. Furthermore, interface changes including prominent keratinocyte necrosis were not seen on histology.

Given the localized nature of the lesions in our patient and negative direct immunofluorescence studies, a diagnosis of coma blisters was made. Gentle wound care practices to the areas of denuded skin were implemented with complete resolution. The patient’s condition gradually improved, and she was extubated and discharged home.
Coma blisters are self-limited bullous lesions that have been reported in comatose patients as early as 1812 when Napoleon’s surgeon first noticed cutaneous blisters in comatose French soldiers being treated for carbon monoxide intoxication.2 Since then, barbiturate overdose has remained the most common association, but coma blisters have occurred in the absence of specific drug exposures. Clinically, erythematous or violaceous plaques typically appear within 24 hours of drug ingestion, and progression to large tense bullae usually occurs within 48 to 72 hours of unconsciousness.3 They characteristically occur in pressure-dependent areas, but reports have shown lesions in non–pressure-dependent areas, including the penis and mouth.1,4 Spontaneous resolution within 1 to 2 weeks is typical.5
The underlying pathogenesis remains controversial, as multiple mechanisms have been suggested, but clear causal evidence is lacking. The original proposition that direct effects of drug toxicity caused the cutaneous observations was later refuted after similar bullous lesions with eccrine gland necrosis were reported in comatose patients with neurologic conditions.6 It is largely accepted that pressure-induced local ischemia—proportional to the duration and amount of pressure—leads to tissue injury and is critical to the pathogenesis. During periods of ischemia, the most metabolically active tissues will undergo necrosis first; however, in eccrine glands, the earliest and most severe damage does not seem to occur in the most metabolically active cells.7 Additionally, this would not provide a viable explanation for coma blisters with eccrine gland necrosis developing in variable non–pressuredependent areas.
Moreover, drug- and non–drug-induced coma blisters can appear identically, but specific histopathologic differences have been reported. The most notable markers of non–drug-induced coma blisters are the absence of an inflammatory infiltrate in the epidermis and the presence of thrombosis in dermal vessels.8 Demonstration of necrotic changes in the secretory portion of the eccrine gland is considered the histopathologic hallmark for drug-induced coma blisters, but other findings can include subepidermal or intraepidermal bullae; perivascular infiltrates; and focal necrosis of the epidermis, dermis, subcutis, or epidermal appendages.6 Arteriolar wall necrosis and dermal inflammatory infiltrates also have been observed.7
Benzodiazepines have been widely prescribed and abused since their development, and overdose is much more common today than with barbiturates.9 Coma blisters rarely have been documented in the setting of isolated benzodiazepine overdose, and of the few cases, only one report implicated lorazepam as the causative agent.4,7 The characteristic finding of eccrine gland necrosis consistently was seen in our patient. This case not only emphasizes the need for greater awareness of the association between benzodiazepine overdose and coma blisters but also the importance of clinical context when considering diagnoses. It is essential to note that coma blisters themselves are nonspecific, and the diagnosis of drug-induced coma blisters warrants confirmatory toxicologic analysis.
- Ferreli C, Sulica VI, Aste N, et al. Drug-induced sweat gland necrosis in a non-comatose patient: a case presentation. J Eur Acad Dermatol Venereol. 2003;17:443-445.
- Larrey DJ. Memoires de Chirurgie Militaire et Campagnes. Smith and Buisson; 1812.
- Agarwal A, Bansal M, Conner K. Coma blisters with hypoxemic respiratory failure. Dermatol Online J. 2012;18:10.
- Varma AJ, Fisher BK, Sarin MK. Diazepam-induced coma with bullae and eccrine sweat gland necrosis. Arch Intern Med. 1977;137:1207-1210.
- Rocha J, Pereira T, Ventura F, et al. Coma blisters. Case Rep Dermatol. 2009;1:66-70.
- Arndt KA, Mihm MC, Parrish JA. Bullae: a cutaneous sign of a variety of neurologic diseases. J Invest Dermatol. 1973;60:312-320.
- Sánchez Yus E, Requena L, Simón P. Histopathology of cutaneous changes in drug-induced coma. Am J Dermatopathol. 1993;15:208-216.
- Kato N, Ueno H, Mimura M. Histopathology of cutaneous changes in non-drug-induced coma. Am J Dermatopathol. 1996;18:344-350.
- Kang M, Ghassemzadeh S. Benzodiazepine Toxicity. StatPearls Publishing; 2018.
- Ferreli C, Sulica VI, Aste N, et al. Drug-induced sweat gland necrosis in a non-comatose patient: a case presentation. J Eur Acad Dermatol Venereol. 2003;17:443-445.
- Larrey DJ. Memoires de Chirurgie Militaire et Campagnes. Smith and Buisson; 1812.
- Agarwal A, Bansal M, Conner K. Coma blisters with hypoxemic respiratory failure. Dermatol Online J. 2012;18:10.
- Varma AJ, Fisher BK, Sarin MK. Diazepam-induced coma with bullae and eccrine sweat gland necrosis. Arch Intern Med. 1977;137:1207-1210.
- Rocha J, Pereira T, Ventura F, et al. Coma blisters. Case Rep Dermatol. 2009;1:66-70.
- Arndt KA, Mihm MC, Parrish JA. Bullae: a cutaneous sign of a variety of neurologic diseases. J Invest Dermatol. 1973;60:312-320.
- Sánchez Yus E, Requena L, Simón P. Histopathology of cutaneous changes in drug-induced coma. Am J Dermatopathol. 1993;15:208-216.
- Kato N, Ueno H, Mimura M. Histopathology of cutaneous changes in non-drug-induced coma. Am J Dermatopathol. 1996;18:344-350.
- Kang M, Ghassemzadeh S. Benzodiazepine Toxicity. StatPearls Publishing; 2018.
An 82-year-old woman presented to the emergency department after her daughter found her unconscious in the bathroom laying on her right side. Her medical history was notable for hypertension and asthma for which she was on losartan, furosemide, diltiazem, and albuterol. She recently had been prescribed lorazepam for insomnia and had started taking the medication 2 days prior. She underwent intubation and was noted to have flaccid, fluid-filled bullae on the right thigh (top) along with large areas of desquamation on the right lateral arm (bottom) with minimal surrounding erythema. There was no mucous membrane involvement. Urine toxicology was positive for benzodiazepines and negative for all other drugs, including barbiturates.