User login
Shortness of breath and abdominal pain
On the basis of the patient's history and clinical presentation, the likely diagnosis is ketosis-prone diabetes (KPD) type 2. KPD is widely thought of as an atypical diabetes syndrome, though some groups consider it to be a common clinical presentation in newly diagnosed patients with type 2 diabetes (T2D) rather than a subtype of disease. The condition is more prevalent in males and among Black and Hispanic populations.
Definitive diagnosis of the type of diabetes can present a clinical challenge during acute presentation. Although the majority of diabetic ketoacidosis (DKA) episodes occur in patients previously diagnosed with type 1 diabetes, an estimated 34% occur in patients with T2D. For patients who are obese or have a family history of diabetes, there should be a high index of suspicion for new-onset DKA in type 2 diabetes.
Patients with KPD typically present in a state of ketoacidosis with a brief but acute history of hyperglycemic symptoms. Hyperglycemia, elevated anion gap acidosis, and ketonemia form the expected constellation of DKA symptoms. A key consideration in the differential diagnosis is hyperosmolar hyperglycemic state (HHS). Patients with HHS are much more likely to have altered mental status than are patients with DKA. Metabolic acidosis and ketonemia are absent or mild, and anion gap is either normal or slightly elevated. In HHS, extreme elevations of glucose are seen, with a lack of significant ketoacidosis. Glucose levels tend to be higher in HHS than in DKA; they are almost always > 600 mg/dL, and levels > 1000 mg/dL are not uncommon. In DKA, glucose levels are still markedly high — generally 500-800 mg/dL but rarely exceeding 900 mg/dL. Patients with DKA also usually present with an A1c > 10% and a blood pH < 7.30.
Treatment for KPD is initially acute, beginning with aggressive intravenous fluid and insulin therapy A. Once this state resolves, insulin requirements typically decrease for patients with T2D, and they are able to maintain adequate glycemic control with an oral therapy regimen.
Romesh K. Khardori, MD, PhD, Professor, Department of Internal Medicine, Division of Diabetes, Endocrine, and Metabolic Disorders, Eastern Virginia Medical School; EVMS Medical Group, Norfolk, Virginia.
Romesh K. Khardori, MD, PhD, has disclosed no relevant financial relationships.
On the basis of the patient's history and clinical presentation, the likely diagnosis is ketosis-prone diabetes (KPD) type 2. KPD is widely thought of as an atypical diabetes syndrome, though some groups consider it to be a common clinical presentation in newly diagnosed patients with type 2 diabetes (T2D) rather than a subtype of disease. The condition is more prevalent in males and among Black and Hispanic populations.
Definitive diagnosis of the type of diabetes can present a clinical challenge during acute presentation. Although the majority of diabetic ketoacidosis (DKA) episodes occur in patients previously diagnosed with type 1 diabetes, an estimated 34% occur in patients with T2D. For patients who are obese or have a family history of diabetes, there should be a high index of suspicion for new-onset DKA in type 2 diabetes.
Patients with KPD typically present in a state of ketoacidosis with a brief but acute history of hyperglycemic symptoms. Hyperglycemia, elevated anion gap acidosis, and ketonemia form the expected constellation of DKA symptoms. A key consideration in the differential diagnosis is hyperosmolar hyperglycemic state (HHS). Patients with HHS are much more likely to have altered mental status than are patients with DKA. Metabolic acidosis and ketonemia are absent or mild, and anion gap is either normal or slightly elevated. In HHS, extreme elevations of glucose are seen, with a lack of significant ketoacidosis. Glucose levels tend to be higher in HHS than in DKA; they are almost always > 600 mg/dL, and levels > 1000 mg/dL are not uncommon. In DKA, glucose levels are still markedly high — generally 500-800 mg/dL but rarely exceeding 900 mg/dL. Patients with DKA also usually present with an A1c > 10% and a blood pH < 7.30.
Treatment for KPD is initially acute, beginning with aggressive intravenous fluid and insulin therapy A. Once this state resolves, insulin requirements typically decrease for patients with T2D, and they are able to maintain adequate glycemic control with an oral therapy regimen.
Romesh K. Khardori, MD, PhD, Professor, Department of Internal Medicine, Division of Diabetes, Endocrine, and Metabolic Disorders, Eastern Virginia Medical School; EVMS Medical Group, Norfolk, Virginia.
Romesh K. Khardori, MD, PhD, has disclosed no relevant financial relationships.
On the basis of the patient's history and clinical presentation, the likely diagnosis is ketosis-prone diabetes (KPD) type 2. KPD is widely thought of as an atypical diabetes syndrome, though some groups consider it to be a common clinical presentation in newly diagnosed patients with type 2 diabetes (T2D) rather than a subtype of disease. The condition is more prevalent in males and among Black and Hispanic populations.
Definitive diagnosis of the type of diabetes can present a clinical challenge during acute presentation. Although the majority of diabetic ketoacidosis (DKA) episodes occur in patients previously diagnosed with type 1 diabetes, an estimated 34% occur in patients with T2D. For patients who are obese or have a family history of diabetes, there should be a high index of suspicion for new-onset DKA in type 2 diabetes.
Patients with KPD typically present in a state of ketoacidosis with a brief but acute history of hyperglycemic symptoms. Hyperglycemia, elevated anion gap acidosis, and ketonemia form the expected constellation of DKA symptoms. A key consideration in the differential diagnosis is hyperosmolar hyperglycemic state (HHS). Patients with HHS are much more likely to have altered mental status than are patients with DKA. Metabolic acidosis and ketonemia are absent or mild, and anion gap is either normal or slightly elevated. In HHS, extreme elevations of glucose are seen, with a lack of significant ketoacidosis. Glucose levels tend to be higher in HHS than in DKA; they are almost always > 600 mg/dL, and levels > 1000 mg/dL are not uncommon. In DKA, glucose levels are still markedly high — generally 500-800 mg/dL but rarely exceeding 900 mg/dL. Patients with DKA also usually present with an A1c > 10% and a blood pH < 7.30.
Treatment for KPD is initially acute, beginning with aggressive intravenous fluid and insulin therapy A. Once this state resolves, insulin requirements typically decrease for patients with T2D, and they are able to maintain adequate glycemic control with an oral therapy regimen.
Romesh K. Khardori, MD, PhD, Professor, Department of Internal Medicine, Division of Diabetes, Endocrine, and Metabolic Disorders, Eastern Virginia Medical School; EVMS Medical Group, Norfolk, Virginia.
Romesh K. Khardori, MD, PhD, has disclosed no relevant financial relationships.
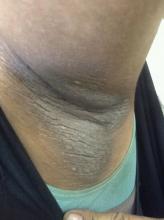
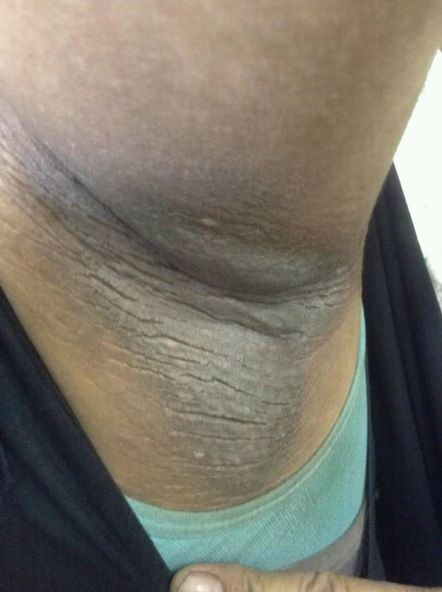
A 21-year-old man presents with shortness of breath and abdominal pain. He has a BMI of 34.6 and explains that he has had asthma for several years, using an inhaler when needed. He reports a few weeks of polydipsia and polyuria. The patient notes that his father has kidney disease. He believes other close relatives are managing chronic metabolic conditions but does not know any further detail regarding their diagnoses. On laboratory testing, blood pH is 6.30 and bicarbonate level is 11.1 mmol/L. A1c is 12.0%. Acanthosis nigricans are noted on the neck and in the axilla bilaterally.
2022 Update on obstetrics
Obstetrical practice saw updates in 2021 to 3 major areas of pregnancy management: preterm birth prevention, antepartum fetal surveillance, and the use of antenatal corticosteroids.
Updated guidance on predicting and preventing spontaneous PTB
American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. Prediction and prevention of spontaneous preterm birth: ACOG practice bulletin, number 234. Obstet Gynecol. 2021;138:e65-e90.
Preterm birth (PTB) continues to pose a challenge in clinical obstetrics, with the most recently reported rate of 10.2% in the United States.1 This accounts for almost 75% of perinatal mortality and more than half of neonatal morbidity, in which effects last well past the neonatal period. PTB is classified as spontaneous (following preterm labor, preterm prelabor rupture of membranes, or cervical insufficiency) or iatrogenic (indicated due to maternal and/or fetal complications).
Assessing risk for PTB
The single strongest predictor of subsequent PTB is a history of spontaneous PTB. Recurrence risk is further increased by the number of prior PTBs and the gestational age at prior PTB. Identification of and intervention for a short cervix has been shown to prolong gestation. Transvaginal ultrasonography of the cervix is the most accurate method for evaluating cervical length (CL). Specific examination criteria exist to ensure that CL measurements are reproducible and reliable.2 A short CL is generally defined as a measurement of less than 25 mm between 16 and 24 weeks’ gestation.
Screening strategies
The American College of Obstetricians and Gynecologists (ACOG), with an endorsement from the Society for Maternal-Fetal Medicine (SMFM), recommends cervical evaluation during the anatomy ultrasound exam between 18 0/7 and 22 6/7 weeks’ gestation in all pregnant patients regardless of prior PTB.3 If transabdominal imaging is concerning for a shortened cervix, transvaginal ultrasonography should be performed to assess the CL.
Serial transvaginal CL measurements are recommended between 16 0/7 and 24 0/7 weeks’ gestation for patients with a current singleton pregnancy and history of a spontaneous PTB, but not for patients with a history of iatrogenic or indicated PTB.
Interventions: Mind your p’s and c’s
Interventions to reduce the risk of spontaneous PTB depend on whether the current pregnancy is a singleton, twins, or higher-order multiples; CL measurement; and history of spontaneous PTB. Preconception optimization of underlying medical conditions also is important to reduce the risk of recurrent indicated PTB.
Continue to: Progesterone...
Progesterone
Vaginal administration. Several trials have shown that vaginal progesterone can be used to reduce the risk of spontaneous PTB in asymptomatic patients with a singleton pregnancy, incidental finding of a short cervix (<25 mm), and no history of spontaneous PTB. This is a change from the prior recommendation of CL of less than 20 mm. In the setting of a twin pregnancy, regardless of CL, data do not definitively support the use of vaginal progesterone.
Intramuscular administration.4,5 The popularity of intramuscular progesterone has waxed and waned. At present, ACOG recommends that all patients with a singleton pregnancy and history of spontaneous PTB be offered progesterone beginning at 16 0/7 weeks’ gestation following a shared decision-making process that includes the limited data of efficacy noted in existing studies.
In a twin pregnancy with no history of spontaneous PTB, the use of intramuscular progesterone has been shown to potentially increase the risk of PTB and admission to the neonatal intensive care unit. As such, intramuscular progesterone in the setting of a twin gestation without a history of spontaneous PTB is not recommended. When a prior spontaneous PTB has occurred, there may be some benefit to intramuscular progesterone in twin gestations.
Cerclage
Ultrasound indicated. In a singleton pregnancy with an incidental finding of short cervix (<25 mm) and no history of PTB, the use of cerclage is of uncertain benefit. Effectiveness may be seen if the cervix is less than 10 mm. Ultrasound-indicated cerclage should be considered in a singleton pregnancy with a CL less than 25 mm and a history of spontaneous PTB.
Possibly one of the most controversial topics is ultrasound-indicated cerclage placement in twin gestation. As with many situations in obstetrics, data regarding ultrasound-indicated cerclage in twin gestation is based on small retrospective studies fraught with bias. Results from these studies range from no benefit, to potential benefit, to even possible increased risk of PTB. Since data are limited, as we await more evidence, it is recommended that the clinician and patient use shared decision making to decide on cerclage placement in a twin gestation.
Exam indicated. In a singleton pregnancy with a dilated cervix on digital or speculum exam between 16 0/7 to 23 6/7 weeks’ gestation, a physical exam–indicated cerclage should be offered. Exam-indicated cerclage also may reduce the incidence of PTB in twin gestations with cervical dilation between 16 0/7 and 23 6/7 weeks’ gestation. Indomethacin tocolysis and perioperative antibiotics should be considered when an exam-indicated cerclage is placed.
As the limits of viability are continually pushed earlier, more in-depth conversation is needed with patients who are considering an exam-indicated cerclage. The nuances of periviability and the likelihood that an exam-indicated cerclage will commit a pregnancy to a periviable or extremely preterm birth should be discussed in detail using a shared decision making model.
Regardless of whether the cerclage is ultrasound or exam indicated, once it is placed there is no utility in additional CL ultrasound monitoring.
Pessary
Vaginal pessaries for prevention of PTB have not gained popularity in the United States as they have in other countries. Trials are being conducted to determine the utility of vaginal pessary, but current data have not proven its effectiveness in preventing PTB in the setting of singleton pregnancy, short cervix, and no history of spontaneous PTB. So for now, pessary is not recommended. The same can be said for use in the twin gestation.
- All patients should have cervical evaluation during pregnancy. Serial imaging is reserved for those with a history of spontaneous PTB.
- Progesterone supplementation should be offered to patients with a singleton pregnancy and a history of spontaneous PTB or to patients with a singleton pregnancy and no history of spontaneous PTB who have cervical shortening at less than 24 weeks.
- Cerclage may be offered between 16 and 24 weeks for a cervical length less than 25 mm in a patient with a singleton gestation who has a history of spontaneous PTB (<10 mm if no history of spontaneous PTB) or for a dilated cervix on exam regardless of history.
- Women who have a twin gestation with cervical dilation may be offered physical exam–indicated cerclage.
Which patients may benefit from antepartum fetal surveillance and when to initiate it
American College of Obstetricians and Gynecologists’ Committee on Obstetrics Practice, Society for Maternal-Fetal Medicine. Indications for outpatient antenatal surveillance: ACOG committee opinion, number 828. Obstet Gynecol. 2021;137:e177-e197.
American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics. Antepartum fetal surveillance: ACOG practice bulletin, number 229. Obstet Gynecol. 2021;137:e116-e127.
The ultimate purpose of antenatal fetal surveillance is to prevent stillbirth. However, stillbirth has multiple etiologies, not all of which are preventable with testing. In June 2021, ACOG released a new Committee Opinion containing guidelines for fetal surveillance, including suggested gestational age at initiation and frequency of testing, for the most common high-risk conditions. ACOG also released an update to the Practice Bulletin on antepartum fetal surveillance; additions include randomized controlled trial level data on the utility of fetal kick counts (FKCs) and recommendations that align with the new Committee Opinion.
Data for the efficacy of antepartum fetal surveillance are lacking, mainly due to the difficulty of performing prospective studies in stillbirth. The existing evidence is subject to intervention bias, as deliveries increase in tested patients, and recommendations rely heavily on expert consensus and nonrandomized studies. Antenatal testing is also time, cost, and labor intensive, with the risk of intervention for a false-positive result. Despite these limitations, obstetrical practices routinely perform antenatal fetal surveillance.
The new guidelines: The why, when, and how often
Why. Antepartum fetal surveillance is suggested for conditions that have a risk of stillbirth greater than 0.8 per 1,000 (that is, the false-negative rate of a biophysical profile or a modified biophysical profile) and the relative risk or odds ratio is greater than 2.0 for stillbirth compared with unaffected pregnancies.
When. For most conditions, ACOG recommends initiation of testing at 32 weeks or later, with notable earlier exceptions for some of the highest-risk patients. For certain conditions, such as fetal growth restriction and hypertensive disorders of pregnancy, the recommendation is to start “at diagnosis,” with the corollary “or at a gestational age when delivery would be considered because of abnormal results.” Shared decision making with the patient about pregnancy goals therefore is required, particularly in cases of fetal anomalies, genetic conditions, or at very early gestational ages.
How often. The recommended frequency of testing is at least weekly. Testing frequency should be increased to twice-weekly outpatient or daily inpatient for the most complicated pregnancies (for example, fetal growth restriction with abnormal umbilical artery Doppler studies, preeclampsia with severe features).
Once or twice weekly is an option for many conditions, which gives the clinician the opportunity to assess clinical stability as well as the patient’s input in terms of logistics and anxiety.
Patients with multiple conditions may fall into the “individualized” category, as do patients with suboptimal control of conditions (for example, diabetes, hypertension) that may affect the fetus as the pregnancy progresses.
New diagnoses included for surveillance
Several diagnoses not previously included now qualify for antepartum fetal surveillance under the new guidelines, most notably:
- history of obstetrical complications in the immediate preceding pregnancy
—history of prior fetal growth restriction requiring preterm delivery
—history of prior preeclampsia requiring delivery
- alcohol use of 5 or more drinks per day
- in vitro fertilization
- abnormal serum markers
—pregnancy-associated plasma protein A (PAPP-A) in the fifth or lower percentile or 0.4 multiples of the median (MoM)
—second trimester inhibin A of 2 or greater MoM
- prepregnancy body mass index (BMI)
—this is divided into 2 categories for timing of initiation of testing:
- 37 weeks for BMI of 35 to 39.9 kg/m2
- 34 weeks for BMI of or greater than 40 kg/m2.
Fetal kick counts
The major change to the updated Practice Bulletin on antenatal surveillance is the inclusion of data on FKCs, a simple modality of fetal surveillance that does not require a clinical visit. For FKCs, a meta-analysis of more than 450,000 patients did not demonstrate a difference in perinatal death between the FKC intervention group (0.54%) and the control group (0.59%). Of note, there were small but statistically significant increases in the rates of induction of labor, cesarean delivery, and preterm delivery in the FKC intervention group. Therefore, this update does not recommend a formal program of FKCs for all patients.
- The antenatal fetal surveillance guidelines are just that—guidelines, not mandates. Their use will need to be adapted for specific patient populations and practice management patterns.
- Many conditions qualify for “individualized” surveillance, which offers the opportunity for detailed discussions on the patient’s care. This includes shared decision making with patients to meet their goals for the pregnancy.
- Although patient-perceived decreased fetal movement always warrants clinical evaluation, a regular program of fetal kick count monitoring is not recommended for all patients due to lack of data supporting its benefit in reducing perinatal death.
- As with any change, new guidelines potentially are a source of frustration, so a concerted effort by obstetrical clinicians to agree on adoption of the guidelines is needed. Additional clinical resources and both clinician and patient education may be required depending on current practice style, as the new strategy may increase the number of appointments and ultrasound exams required.
Continue to: Use of antenatal corticosteroids now may be considered at 22 weeks’ gestation...
Use of antenatal corticosteroids now may be considered at 22 weeks’ gestation
American College of Obstetricians and Gynecologists. Use of antenatal corticosteroids at 22 weeks of gestation: ACOG practice advisory. September 2021. https://www .acog.org/clinical/clinical-guidance/practice-advisory /articles/2021/09/use-of-antenatal-corticosteroids-at -22-weeks-of-gestation. Accessed December 11, 2021.
In September 2021, ACOG and SMFM released a Practice Advisory updating the current recommendations for the administration of antenatal corticosteroids in the periviable period (22 to 25 6/7 weeks’ gestation). Whereas the prior lower limit of gestational age for consideration of steroids was 23 weeks, the new recommendation now extends this consideration down to 22 weeks.
The cited data include a meta-analysis of more than 2,200 patients in which the survival rate of infants born between 22 and 22 6/7 weeks who were exposed to antenatal steroids was 39% compared with 19.5% in the unexposed group. Another study of more than 1,000 patients demonstrated a statistically significant increase in overall survival in patients treated with antenatal steroids plus life support compared with life support only (38.5% vs 17.7%). Survival without major morbidity in this study, although increased from 1% to 4.4%, was still low.
Recommendation carries caveats
Given this information, the Practice Advisory offers a 2C level recommendation (weak recommendation, low quality of evidence) for antenatal steroids at 22 to 22 6/7 weeks’ gestation if neonatal resuscitation is planned, acknowledging the limitations and potential bias of the available data.
The Practice Advisory emphasizes the importance of counseling and patient involvement in the decision making. This requires a multidisciplinary collaboration among the neonatology and obstetrical teams, flexibility in the plan after birth depending on the infant’s condition, and redirection of care if appropriate. Estimated fetal weight, the presence of multiple gestations, fetal biologic sex, and any anomalies are also important in helping families make an informed decision for their particular pregnancy. As described in the Obstetric Care Consensus on periviable birth,6 it is important to remember that considerations and recommendations are not the same as requirements, and redirection of care to comfort and family memory making is not the same as withholding care.
The rest of the recommendations for the administration of antenatal steroids remain the same: Antenatal steroids are not recommended at less than 22 weeks due to lack of evidence of benefit, and they continue to be recommended at 24 weeks and beyond. ●
- Antenatal corticosteroids may be considered at 22 to 22 6/7 weeks’ gestation if, after thorough patient counseling, neonatal resuscitation is desired and planned by the family.
- The overall likelihood of survival and survival without major morbidities continues to be very low in the periviable period, especially at 22 weeks. Gestational age is only one of the many factors that must be considered in the shared decision making for this very difficult decision.
- Palliative care is a valid and appropriate option for patients facing a periviable delivery after appropriate counseling or after evaluation of the infant has occurred after birth.
- Martin JA, Hamilton BE, Osterman MJK. Births in the United States, 2019. NCHS Data Brief, no 387. Hyattsville, MD: National Center for Health Statistics. October 2020. www.cdc.gov/nchs /data/databriefs/db387-H.pdf. Accessed December 20, 2021.
- To MS, Skentou C, Chan C, et al. Cervical assessment at the routine 23-week scan: standardizing techniques. Ultrasound Obstet Gynecol. 2001;17:217-219.
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. Prediction and prevention of spontaneous preterm birth: ACOG practice bulletin, number 234. Obstet Gynecol. 2021;138:e65-e90.
- Society for Maternal-Fetal Medicine (SMFM) Publications Committee. SMFM statement: use of 17-alpha hydroxyprogesterone caproate for prevention of recurrent preterm birth. Am J Obstet Gynecol. 2020;223:B16-B18.
- Blackwell SC, Gyamfi-Bannerman C, Biggio JR Jr, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am J Perinatol. 2020;37:127-136.
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. Obstetric care consensus no. 6: Periviable birth. Obstet Gynecol. 2017;130:e187-e199.
Obstetrical practice saw updates in 2021 to 3 major areas of pregnancy management: preterm birth prevention, antepartum fetal surveillance, and the use of antenatal corticosteroids.
Updated guidance on predicting and preventing spontaneous PTB
American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. Prediction and prevention of spontaneous preterm birth: ACOG practice bulletin, number 234. Obstet Gynecol. 2021;138:e65-e90.
Preterm birth (PTB) continues to pose a challenge in clinical obstetrics, with the most recently reported rate of 10.2% in the United States.1 This accounts for almost 75% of perinatal mortality and more than half of neonatal morbidity, in which effects last well past the neonatal period. PTB is classified as spontaneous (following preterm labor, preterm prelabor rupture of membranes, or cervical insufficiency) or iatrogenic (indicated due to maternal and/or fetal complications).
Assessing risk for PTB
The single strongest predictor of subsequent PTB is a history of spontaneous PTB. Recurrence risk is further increased by the number of prior PTBs and the gestational age at prior PTB. Identification of and intervention for a short cervix has been shown to prolong gestation. Transvaginal ultrasonography of the cervix is the most accurate method for evaluating cervical length (CL). Specific examination criteria exist to ensure that CL measurements are reproducible and reliable.2 A short CL is generally defined as a measurement of less than 25 mm between 16 and 24 weeks’ gestation.
Screening strategies
The American College of Obstetricians and Gynecologists (ACOG), with an endorsement from the Society for Maternal-Fetal Medicine (SMFM), recommends cervical evaluation during the anatomy ultrasound exam between 18 0/7 and 22 6/7 weeks’ gestation in all pregnant patients regardless of prior PTB.3 If transabdominal imaging is concerning for a shortened cervix, transvaginal ultrasonography should be performed to assess the CL.
Serial transvaginal CL measurements are recommended between 16 0/7 and 24 0/7 weeks’ gestation for patients with a current singleton pregnancy and history of a spontaneous PTB, but not for patients with a history of iatrogenic or indicated PTB.
Interventions: Mind your p’s and c’s
Interventions to reduce the risk of spontaneous PTB depend on whether the current pregnancy is a singleton, twins, or higher-order multiples; CL measurement; and history of spontaneous PTB. Preconception optimization of underlying medical conditions also is important to reduce the risk of recurrent indicated PTB.
Continue to: Progesterone...
Progesterone
Vaginal administration. Several trials have shown that vaginal progesterone can be used to reduce the risk of spontaneous PTB in asymptomatic patients with a singleton pregnancy, incidental finding of a short cervix (<25 mm), and no history of spontaneous PTB. This is a change from the prior recommendation of CL of less than 20 mm. In the setting of a twin pregnancy, regardless of CL, data do not definitively support the use of vaginal progesterone.
Intramuscular administration.4,5 The popularity of intramuscular progesterone has waxed and waned. At present, ACOG recommends that all patients with a singleton pregnancy and history of spontaneous PTB be offered progesterone beginning at 16 0/7 weeks’ gestation following a shared decision-making process that includes the limited data of efficacy noted in existing studies.
In a twin pregnancy with no history of spontaneous PTB, the use of intramuscular progesterone has been shown to potentially increase the risk of PTB and admission to the neonatal intensive care unit. As such, intramuscular progesterone in the setting of a twin gestation without a history of spontaneous PTB is not recommended. When a prior spontaneous PTB has occurred, there may be some benefit to intramuscular progesterone in twin gestations.
Cerclage
Ultrasound indicated. In a singleton pregnancy with an incidental finding of short cervix (<25 mm) and no history of PTB, the use of cerclage is of uncertain benefit. Effectiveness may be seen if the cervix is less than 10 mm. Ultrasound-indicated cerclage should be considered in a singleton pregnancy with a CL less than 25 mm and a history of spontaneous PTB.
Possibly one of the most controversial topics is ultrasound-indicated cerclage placement in twin gestation. As with many situations in obstetrics, data regarding ultrasound-indicated cerclage in twin gestation is based on small retrospective studies fraught with bias. Results from these studies range from no benefit, to potential benefit, to even possible increased risk of PTB. Since data are limited, as we await more evidence, it is recommended that the clinician and patient use shared decision making to decide on cerclage placement in a twin gestation.
Exam indicated. In a singleton pregnancy with a dilated cervix on digital or speculum exam between 16 0/7 to 23 6/7 weeks’ gestation, a physical exam–indicated cerclage should be offered. Exam-indicated cerclage also may reduce the incidence of PTB in twin gestations with cervical dilation between 16 0/7 and 23 6/7 weeks’ gestation. Indomethacin tocolysis and perioperative antibiotics should be considered when an exam-indicated cerclage is placed.
As the limits of viability are continually pushed earlier, more in-depth conversation is needed with patients who are considering an exam-indicated cerclage. The nuances of periviability and the likelihood that an exam-indicated cerclage will commit a pregnancy to a periviable or extremely preterm birth should be discussed in detail using a shared decision making model.
Regardless of whether the cerclage is ultrasound or exam indicated, once it is placed there is no utility in additional CL ultrasound monitoring.
Pessary
Vaginal pessaries for prevention of PTB have not gained popularity in the United States as they have in other countries. Trials are being conducted to determine the utility of vaginal pessary, but current data have not proven its effectiveness in preventing PTB in the setting of singleton pregnancy, short cervix, and no history of spontaneous PTB. So for now, pessary is not recommended. The same can be said for use in the twin gestation.
- All patients should have cervical evaluation during pregnancy. Serial imaging is reserved for those with a history of spontaneous PTB.
- Progesterone supplementation should be offered to patients with a singleton pregnancy and a history of spontaneous PTB or to patients with a singleton pregnancy and no history of spontaneous PTB who have cervical shortening at less than 24 weeks.
- Cerclage may be offered between 16 and 24 weeks for a cervical length less than 25 mm in a patient with a singleton gestation who has a history of spontaneous PTB (<10 mm if no history of spontaneous PTB) or for a dilated cervix on exam regardless of history.
- Women who have a twin gestation with cervical dilation may be offered physical exam–indicated cerclage.
Which patients may benefit from antepartum fetal surveillance and when to initiate it
American College of Obstetricians and Gynecologists’ Committee on Obstetrics Practice, Society for Maternal-Fetal Medicine. Indications for outpatient antenatal surveillance: ACOG committee opinion, number 828. Obstet Gynecol. 2021;137:e177-e197.
American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics. Antepartum fetal surveillance: ACOG practice bulletin, number 229. Obstet Gynecol. 2021;137:e116-e127.
The ultimate purpose of antenatal fetal surveillance is to prevent stillbirth. However, stillbirth has multiple etiologies, not all of which are preventable with testing. In June 2021, ACOG released a new Committee Opinion containing guidelines for fetal surveillance, including suggested gestational age at initiation and frequency of testing, for the most common high-risk conditions. ACOG also released an update to the Practice Bulletin on antepartum fetal surveillance; additions include randomized controlled trial level data on the utility of fetal kick counts (FKCs) and recommendations that align with the new Committee Opinion.
Data for the efficacy of antepartum fetal surveillance are lacking, mainly due to the difficulty of performing prospective studies in stillbirth. The existing evidence is subject to intervention bias, as deliveries increase in tested patients, and recommendations rely heavily on expert consensus and nonrandomized studies. Antenatal testing is also time, cost, and labor intensive, with the risk of intervention for a false-positive result. Despite these limitations, obstetrical practices routinely perform antenatal fetal surveillance.
The new guidelines: The why, when, and how often
Why. Antepartum fetal surveillance is suggested for conditions that have a risk of stillbirth greater than 0.8 per 1,000 (that is, the false-negative rate of a biophysical profile or a modified biophysical profile) and the relative risk or odds ratio is greater than 2.0 for stillbirth compared with unaffected pregnancies.
When. For most conditions, ACOG recommends initiation of testing at 32 weeks or later, with notable earlier exceptions for some of the highest-risk patients. For certain conditions, such as fetal growth restriction and hypertensive disorders of pregnancy, the recommendation is to start “at diagnosis,” with the corollary “or at a gestational age when delivery would be considered because of abnormal results.” Shared decision making with the patient about pregnancy goals therefore is required, particularly in cases of fetal anomalies, genetic conditions, or at very early gestational ages.
How often. The recommended frequency of testing is at least weekly. Testing frequency should be increased to twice-weekly outpatient or daily inpatient for the most complicated pregnancies (for example, fetal growth restriction with abnormal umbilical artery Doppler studies, preeclampsia with severe features).
Once or twice weekly is an option for many conditions, which gives the clinician the opportunity to assess clinical stability as well as the patient’s input in terms of logistics and anxiety.
Patients with multiple conditions may fall into the “individualized” category, as do patients with suboptimal control of conditions (for example, diabetes, hypertension) that may affect the fetus as the pregnancy progresses.
New diagnoses included for surveillance
Several diagnoses not previously included now qualify for antepartum fetal surveillance under the new guidelines, most notably:
- history of obstetrical complications in the immediate preceding pregnancy
—history of prior fetal growth restriction requiring preterm delivery
—history of prior preeclampsia requiring delivery
- alcohol use of 5 or more drinks per day
- in vitro fertilization
- abnormal serum markers
—pregnancy-associated plasma protein A (PAPP-A) in the fifth or lower percentile or 0.4 multiples of the median (MoM)
—second trimester inhibin A of 2 or greater MoM
- prepregnancy body mass index (BMI)
—this is divided into 2 categories for timing of initiation of testing:
- 37 weeks for BMI of 35 to 39.9 kg/m2
- 34 weeks for BMI of or greater than 40 kg/m2.
Fetal kick counts
The major change to the updated Practice Bulletin on antenatal surveillance is the inclusion of data on FKCs, a simple modality of fetal surveillance that does not require a clinical visit. For FKCs, a meta-analysis of more than 450,000 patients did not demonstrate a difference in perinatal death between the FKC intervention group (0.54%) and the control group (0.59%). Of note, there were small but statistically significant increases in the rates of induction of labor, cesarean delivery, and preterm delivery in the FKC intervention group. Therefore, this update does not recommend a formal program of FKCs for all patients.
- The antenatal fetal surveillance guidelines are just that—guidelines, not mandates. Their use will need to be adapted for specific patient populations and practice management patterns.
- Many conditions qualify for “individualized” surveillance, which offers the opportunity for detailed discussions on the patient’s care. This includes shared decision making with patients to meet their goals for the pregnancy.
- Although patient-perceived decreased fetal movement always warrants clinical evaluation, a regular program of fetal kick count monitoring is not recommended for all patients due to lack of data supporting its benefit in reducing perinatal death.
- As with any change, new guidelines potentially are a source of frustration, so a concerted effort by obstetrical clinicians to agree on adoption of the guidelines is needed. Additional clinical resources and both clinician and patient education may be required depending on current practice style, as the new strategy may increase the number of appointments and ultrasound exams required.
Continue to: Use of antenatal corticosteroids now may be considered at 22 weeks’ gestation...
Use of antenatal corticosteroids now may be considered at 22 weeks’ gestation
American College of Obstetricians and Gynecologists. Use of antenatal corticosteroids at 22 weeks of gestation: ACOG practice advisory. September 2021. https://www .acog.org/clinical/clinical-guidance/practice-advisory /articles/2021/09/use-of-antenatal-corticosteroids-at -22-weeks-of-gestation. Accessed December 11, 2021.
In September 2021, ACOG and SMFM released a Practice Advisory updating the current recommendations for the administration of antenatal corticosteroids in the periviable period (22 to 25 6/7 weeks’ gestation). Whereas the prior lower limit of gestational age for consideration of steroids was 23 weeks, the new recommendation now extends this consideration down to 22 weeks.
The cited data include a meta-analysis of more than 2,200 patients in which the survival rate of infants born between 22 and 22 6/7 weeks who were exposed to antenatal steroids was 39% compared with 19.5% in the unexposed group. Another study of more than 1,000 patients demonstrated a statistically significant increase in overall survival in patients treated with antenatal steroids plus life support compared with life support only (38.5% vs 17.7%). Survival without major morbidity in this study, although increased from 1% to 4.4%, was still low.
Recommendation carries caveats
Given this information, the Practice Advisory offers a 2C level recommendation (weak recommendation, low quality of evidence) for antenatal steroids at 22 to 22 6/7 weeks’ gestation if neonatal resuscitation is planned, acknowledging the limitations and potential bias of the available data.
The Practice Advisory emphasizes the importance of counseling and patient involvement in the decision making. This requires a multidisciplinary collaboration among the neonatology and obstetrical teams, flexibility in the plan after birth depending on the infant’s condition, and redirection of care if appropriate. Estimated fetal weight, the presence of multiple gestations, fetal biologic sex, and any anomalies are also important in helping families make an informed decision for their particular pregnancy. As described in the Obstetric Care Consensus on periviable birth,6 it is important to remember that considerations and recommendations are not the same as requirements, and redirection of care to comfort and family memory making is not the same as withholding care.
The rest of the recommendations for the administration of antenatal steroids remain the same: Antenatal steroids are not recommended at less than 22 weeks due to lack of evidence of benefit, and they continue to be recommended at 24 weeks and beyond. ●
- Antenatal corticosteroids may be considered at 22 to 22 6/7 weeks’ gestation if, after thorough patient counseling, neonatal resuscitation is desired and planned by the family.
- The overall likelihood of survival and survival without major morbidities continues to be very low in the periviable period, especially at 22 weeks. Gestational age is only one of the many factors that must be considered in the shared decision making for this very difficult decision.
- Palliative care is a valid and appropriate option for patients facing a periviable delivery after appropriate counseling or after evaluation of the infant has occurred after birth.
Obstetrical practice saw updates in 2021 to 3 major areas of pregnancy management: preterm birth prevention, antepartum fetal surveillance, and the use of antenatal corticosteroids.
Updated guidance on predicting and preventing spontaneous PTB
American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. Prediction and prevention of spontaneous preterm birth: ACOG practice bulletin, number 234. Obstet Gynecol. 2021;138:e65-e90.
Preterm birth (PTB) continues to pose a challenge in clinical obstetrics, with the most recently reported rate of 10.2% in the United States.1 This accounts for almost 75% of perinatal mortality and more than half of neonatal morbidity, in which effects last well past the neonatal period. PTB is classified as spontaneous (following preterm labor, preterm prelabor rupture of membranes, or cervical insufficiency) or iatrogenic (indicated due to maternal and/or fetal complications).
Assessing risk for PTB
The single strongest predictor of subsequent PTB is a history of spontaneous PTB. Recurrence risk is further increased by the number of prior PTBs and the gestational age at prior PTB. Identification of and intervention for a short cervix has been shown to prolong gestation. Transvaginal ultrasonography of the cervix is the most accurate method for evaluating cervical length (CL). Specific examination criteria exist to ensure that CL measurements are reproducible and reliable.2 A short CL is generally defined as a measurement of less than 25 mm between 16 and 24 weeks’ gestation.
Screening strategies
The American College of Obstetricians and Gynecologists (ACOG), with an endorsement from the Society for Maternal-Fetal Medicine (SMFM), recommends cervical evaluation during the anatomy ultrasound exam between 18 0/7 and 22 6/7 weeks’ gestation in all pregnant patients regardless of prior PTB.3 If transabdominal imaging is concerning for a shortened cervix, transvaginal ultrasonography should be performed to assess the CL.
Serial transvaginal CL measurements are recommended between 16 0/7 and 24 0/7 weeks’ gestation for patients with a current singleton pregnancy and history of a spontaneous PTB, but not for patients with a history of iatrogenic or indicated PTB.
Interventions: Mind your p’s and c’s
Interventions to reduce the risk of spontaneous PTB depend on whether the current pregnancy is a singleton, twins, or higher-order multiples; CL measurement; and history of spontaneous PTB. Preconception optimization of underlying medical conditions also is important to reduce the risk of recurrent indicated PTB.
Continue to: Progesterone...
Progesterone
Vaginal administration. Several trials have shown that vaginal progesterone can be used to reduce the risk of spontaneous PTB in asymptomatic patients with a singleton pregnancy, incidental finding of a short cervix (<25 mm), and no history of spontaneous PTB. This is a change from the prior recommendation of CL of less than 20 mm. In the setting of a twin pregnancy, regardless of CL, data do not definitively support the use of vaginal progesterone.
Intramuscular administration.4,5 The popularity of intramuscular progesterone has waxed and waned. At present, ACOG recommends that all patients with a singleton pregnancy and history of spontaneous PTB be offered progesterone beginning at 16 0/7 weeks’ gestation following a shared decision-making process that includes the limited data of efficacy noted in existing studies.
In a twin pregnancy with no history of spontaneous PTB, the use of intramuscular progesterone has been shown to potentially increase the risk of PTB and admission to the neonatal intensive care unit. As such, intramuscular progesterone in the setting of a twin gestation without a history of spontaneous PTB is not recommended. When a prior spontaneous PTB has occurred, there may be some benefit to intramuscular progesterone in twin gestations.
Cerclage
Ultrasound indicated. In a singleton pregnancy with an incidental finding of short cervix (<25 mm) and no history of PTB, the use of cerclage is of uncertain benefit. Effectiveness may be seen if the cervix is less than 10 mm. Ultrasound-indicated cerclage should be considered in a singleton pregnancy with a CL less than 25 mm and a history of spontaneous PTB.
Possibly one of the most controversial topics is ultrasound-indicated cerclage placement in twin gestation. As with many situations in obstetrics, data regarding ultrasound-indicated cerclage in twin gestation is based on small retrospective studies fraught with bias. Results from these studies range from no benefit, to potential benefit, to even possible increased risk of PTB. Since data are limited, as we await more evidence, it is recommended that the clinician and patient use shared decision making to decide on cerclage placement in a twin gestation.
Exam indicated. In a singleton pregnancy with a dilated cervix on digital or speculum exam between 16 0/7 to 23 6/7 weeks’ gestation, a physical exam–indicated cerclage should be offered. Exam-indicated cerclage also may reduce the incidence of PTB in twin gestations with cervical dilation between 16 0/7 and 23 6/7 weeks’ gestation. Indomethacin tocolysis and perioperative antibiotics should be considered when an exam-indicated cerclage is placed.
As the limits of viability are continually pushed earlier, more in-depth conversation is needed with patients who are considering an exam-indicated cerclage. The nuances of periviability and the likelihood that an exam-indicated cerclage will commit a pregnancy to a periviable or extremely preterm birth should be discussed in detail using a shared decision making model.
Regardless of whether the cerclage is ultrasound or exam indicated, once it is placed there is no utility in additional CL ultrasound monitoring.
Pessary
Vaginal pessaries for prevention of PTB have not gained popularity in the United States as they have in other countries. Trials are being conducted to determine the utility of vaginal pessary, but current data have not proven its effectiveness in preventing PTB in the setting of singleton pregnancy, short cervix, and no history of spontaneous PTB. So for now, pessary is not recommended. The same can be said for use in the twin gestation.
- All patients should have cervical evaluation during pregnancy. Serial imaging is reserved for those with a history of spontaneous PTB.
- Progesterone supplementation should be offered to patients with a singleton pregnancy and a history of spontaneous PTB or to patients with a singleton pregnancy and no history of spontaneous PTB who have cervical shortening at less than 24 weeks.
- Cerclage may be offered between 16 and 24 weeks for a cervical length less than 25 mm in a patient with a singleton gestation who has a history of spontaneous PTB (<10 mm if no history of spontaneous PTB) or for a dilated cervix on exam regardless of history.
- Women who have a twin gestation with cervical dilation may be offered physical exam–indicated cerclage.
Which patients may benefit from antepartum fetal surveillance and when to initiate it
American College of Obstetricians and Gynecologists’ Committee on Obstetrics Practice, Society for Maternal-Fetal Medicine. Indications for outpatient antenatal surveillance: ACOG committee opinion, number 828. Obstet Gynecol. 2021;137:e177-e197.
American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics. Antepartum fetal surveillance: ACOG practice bulletin, number 229. Obstet Gynecol. 2021;137:e116-e127.
The ultimate purpose of antenatal fetal surveillance is to prevent stillbirth. However, stillbirth has multiple etiologies, not all of which are preventable with testing. In June 2021, ACOG released a new Committee Opinion containing guidelines for fetal surveillance, including suggested gestational age at initiation and frequency of testing, for the most common high-risk conditions. ACOG also released an update to the Practice Bulletin on antepartum fetal surveillance; additions include randomized controlled trial level data on the utility of fetal kick counts (FKCs) and recommendations that align with the new Committee Opinion.
Data for the efficacy of antepartum fetal surveillance are lacking, mainly due to the difficulty of performing prospective studies in stillbirth. The existing evidence is subject to intervention bias, as deliveries increase in tested patients, and recommendations rely heavily on expert consensus and nonrandomized studies. Antenatal testing is also time, cost, and labor intensive, with the risk of intervention for a false-positive result. Despite these limitations, obstetrical practices routinely perform antenatal fetal surveillance.
The new guidelines: The why, when, and how often
Why. Antepartum fetal surveillance is suggested for conditions that have a risk of stillbirth greater than 0.8 per 1,000 (that is, the false-negative rate of a biophysical profile or a modified biophysical profile) and the relative risk or odds ratio is greater than 2.0 for stillbirth compared with unaffected pregnancies.
When. For most conditions, ACOG recommends initiation of testing at 32 weeks or later, with notable earlier exceptions for some of the highest-risk patients. For certain conditions, such as fetal growth restriction and hypertensive disorders of pregnancy, the recommendation is to start “at diagnosis,” with the corollary “or at a gestational age when delivery would be considered because of abnormal results.” Shared decision making with the patient about pregnancy goals therefore is required, particularly in cases of fetal anomalies, genetic conditions, or at very early gestational ages.
How often. The recommended frequency of testing is at least weekly. Testing frequency should be increased to twice-weekly outpatient or daily inpatient for the most complicated pregnancies (for example, fetal growth restriction with abnormal umbilical artery Doppler studies, preeclampsia with severe features).
Once or twice weekly is an option for many conditions, which gives the clinician the opportunity to assess clinical stability as well as the patient’s input in terms of logistics and anxiety.
Patients with multiple conditions may fall into the “individualized” category, as do patients with suboptimal control of conditions (for example, diabetes, hypertension) that may affect the fetus as the pregnancy progresses.
New diagnoses included for surveillance
Several diagnoses not previously included now qualify for antepartum fetal surveillance under the new guidelines, most notably:
- history of obstetrical complications in the immediate preceding pregnancy
—history of prior fetal growth restriction requiring preterm delivery
—history of prior preeclampsia requiring delivery
- alcohol use of 5 or more drinks per day
- in vitro fertilization
- abnormal serum markers
—pregnancy-associated plasma protein A (PAPP-A) in the fifth or lower percentile or 0.4 multiples of the median (MoM)
—second trimester inhibin A of 2 or greater MoM
- prepregnancy body mass index (BMI)
—this is divided into 2 categories for timing of initiation of testing:
- 37 weeks for BMI of 35 to 39.9 kg/m2
- 34 weeks for BMI of or greater than 40 kg/m2.
Fetal kick counts
The major change to the updated Practice Bulletin on antenatal surveillance is the inclusion of data on FKCs, a simple modality of fetal surveillance that does not require a clinical visit. For FKCs, a meta-analysis of more than 450,000 patients did not demonstrate a difference in perinatal death between the FKC intervention group (0.54%) and the control group (0.59%). Of note, there were small but statistically significant increases in the rates of induction of labor, cesarean delivery, and preterm delivery in the FKC intervention group. Therefore, this update does not recommend a formal program of FKCs for all patients.
- The antenatal fetal surveillance guidelines are just that—guidelines, not mandates. Their use will need to be adapted for specific patient populations and practice management patterns.
- Many conditions qualify for “individualized” surveillance, which offers the opportunity for detailed discussions on the patient’s care. This includes shared decision making with patients to meet their goals for the pregnancy.
- Although patient-perceived decreased fetal movement always warrants clinical evaluation, a regular program of fetal kick count monitoring is not recommended for all patients due to lack of data supporting its benefit in reducing perinatal death.
- As with any change, new guidelines potentially are a source of frustration, so a concerted effort by obstetrical clinicians to agree on adoption of the guidelines is needed. Additional clinical resources and both clinician and patient education may be required depending on current practice style, as the new strategy may increase the number of appointments and ultrasound exams required.
Continue to: Use of antenatal corticosteroids now may be considered at 22 weeks’ gestation...
Use of antenatal corticosteroids now may be considered at 22 weeks’ gestation
American College of Obstetricians and Gynecologists. Use of antenatal corticosteroids at 22 weeks of gestation: ACOG practice advisory. September 2021. https://www .acog.org/clinical/clinical-guidance/practice-advisory /articles/2021/09/use-of-antenatal-corticosteroids-at -22-weeks-of-gestation. Accessed December 11, 2021.
In September 2021, ACOG and SMFM released a Practice Advisory updating the current recommendations for the administration of antenatal corticosteroids in the periviable period (22 to 25 6/7 weeks’ gestation). Whereas the prior lower limit of gestational age for consideration of steroids was 23 weeks, the new recommendation now extends this consideration down to 22 weeks.
The cited data include a meta-analysis of more than 2,200 patients in which the survival rate of infants born between 22 and 22 6/7 weeks who were exposed to antenatal steroids was 39% compared with 19.5% in the unexposed group. Another study of more than 1,000 patients demonstrated a statistically significant increase in overall survival in patients treated with antenatal steroids plus life support compared with life support only (38.5% vs 17.7%). Survival without major morbidity in this study, although increased from 1% to 4.4%, was still low.
Recommendation carries caveats
Given this information, the Practice Advisory offers a 2C level recommendation (weak recommendation, low quality of evidence) for antenatal steroids at 22 to 22 6/7 weeks’ gestation if neonatal resuscitation is planned, acknowledging the limitations and potential bias of the available data.
The Practice Advisory emphasizes the importance of counseling and patient involvement in the decision making. This requires a multidisciplinary collaboration among the neonatology and obstetrical teams, flexibility in the plan after birth depending on the infant’s condition, and redirection of care if appropriate. Estimated fetal weight, the presence of multiple gestations, fetal biologic sex, and any anomalies are also important in helping families make an informed decision for their particular pregnancy. As described in the Obstetric Care Consensus on periviable birth,6 it is important to remember that considerations and recommendations are not the same as requirements, and redirection of care to comfort and family memory making is not the same as withholding care.
The rest of the recommendations for the administration of antenatal steroids remain the same: Antenatal steroids are not recommended at less than 22 weeks due to lack of evidence of benefit, and they continue to be recommended at 24 weeks and beyond. ●
- Antenatal corticosteroids may be considered at 22 to 22 6/7 weeks’ gestation if, after thorough patient counseling, neonatal resuscitation is desired and planned by the family.
- The overall likelihood of survival and survival without major morbidities continues to be very low in the periviable period, especially at 22 weeks. Gestational age is only one of the many factors that must be considered in the shared decision making for this very difficult decision.
- Palliative care is a valid and appropriate option for patients facing a periviable delivery after appropriate counseling or after evaluation of the infant has occurred after birth.
- Martin JA, Hamilton BE, Osterman MJK. Births in the United States, 2019. NCHS Data Brief, no 387. Hyattsville, MD: National Center for Health Statistics. October 2020. www.cdc.gov/nchs /data/databriefs/db387-H.pdf. Accessed December 20, 2021.
- To MS, Skentou C, Chan C, et al. Cervical assessment at the routine 23-week scan: standardizing techniques. Ultrasound Obstet Gynecol. 2001;17:217-219.
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. Prediction and prevention of spontaneous preterm birth: ACOG practice bulletin, number 234. Obstet Gynecol. 2021;138:e65-e90.
- Society for Maternal-Fetal Medicine (SMFM) Publications Committee. SMFM statement: use of 17-alpha hydroxyprogesterone caproate for prevention of recurrent preterm birth. Am J Obstet Gynecol. 2020;223:B16-B18.
- Blackwell SC, Gyamfi-Bannerman C, Biggio JR Jr, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am J Perinatol. 2020;37:127-136.
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. Obstetric care consensus no. 6: Periviable birth. Obstet Gynecol. 2017;130:e187-e199.
- Martin JA, Hamilton BE, Osterman MJK. Births in the United States, 2019. NCHS Data Brief, no 387. Hyattsville, MD: National Center for Health Statistics. October 2020. www.cdc.gov/nchs /data/databriefs/db387-H.pdf. Accessed December 20, 2021.
- To MS, Skentou C, Chan C, et al. Cervical assessment at the routine 23-week scan: standardizing techniques. Ultrasound Obstet Gynecol. 2001;17:217-219.
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. Prediction and prevention of spontaneous preterm birth: ACOG practice bulletin, number 234. Obstet Gynecol. 2021;138:e65-e90.
- Society for Maternal-Fetal Medicine (SMFM) Publications Committee. SMFM statement: use of 17-alpha hydroxyprogesterone caproate for prevention of recurrent preterm birth. Am J Obstet Gynecol. 2020;223:B16-B18.
- Blackwell SC, Gyamfi-Bannerman C, Biggio JR Jr, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am J Perinatol. 2020;37:127-136.
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. Obstetric care consensus no. 6: Periviable birth. Obstet Gynecol. 2017;130:e187-e199.
Learning to coexist
There’s another doctor with whom I’ve referred patients, back and forth, for the last 20 years. I think he’s good at his job and assume he feels the same way about me. We aren’t social friends, but chat briefly when we run into each other at the hospital, or store, or local restaurants.
Last week I was at the hospital to read EEGs, and happened to see him in the doctor’s parking lot. We wished each other a happy new year, talked briefly about a few mutual patients, and then went our separate ways.
As he pulled out, I noticed his car had a bumper sticker for a cause I strongly disagree with. I mean, 180 degrees opposed.
Suddenly, I didn’t want to ever refer to him again. Why should I support him? He’s the enemy.
Why should I help him out by referring patients?
But then I had to stop. Isn’t this 2022? Aren’t we supposed to be in a civilized world? This isn’t my tribe versus your tribe, my cave versus your cave. The closest we’re supposed to come to direct conflict with others is the “us versus them” world of professional and college sports.
I hope.
Aren’t I supposed to be better than this? Isn’t learning to coexist the whole point of the playground as a kid (besides burning off energy and giving the teacher a break)? Like Hamilton and Jefferson, or Ronald Reagan and Tip O’Neill?
Refusing to work with another competent physician because I disagree with their personal, religious, or political beliefs is just plain stupid.
Politicians and pundits try to convince us that people who disagree with us are the enemy, but that’s horse hockey. The truth is that the majority of people out there, regardless of personal beliefs, are decent, hardworking, and just trying to support their families like I am mine.
Later that week I had a patient who clearly needed the other doctor’s expertise, and I gave her his name and phone number. She asked if I’d send my own family to him, and I said, unequivocally, “yes” (actually I have).
Because, at the end of the day, we’re all people, along on the same ride. To not send a patient to him wouldn’t be in their best interest, which is what I’m supposed to be watching out for.
Not only that, but if I don’t refer just because I disagree with him as a person, then I’ve become the problem and not the solution.
Because I, and everyone else, have to try to be better than that.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
There’s another doctor with whom I’ve referred patients, back and forth, for the last 20 years. I think he’s good at his job and assume he feels the same way about me. We aren’t social friends, but chat briefly when we run into each other at the hospital, or store, or local restaurants.
Last week I was at the hospital to read EEGs, and happened to see him in the doctor’s parking lot. We wished each other a happy new year, talked briefly about a few mutual patients, and then went our separate ways.
As he pulled out, I noticed his car had a bumper sticker for a cause I strongly disagree with. I mean, 180 degrees opposed.
Suddenly, I didn’t want to ever refer to him again. Why should I support him? He’s the enemy.
Why should I help him out by referring patients?
But then I had to stop. Isn’t this 2022? Aren’t we supposed to be in a civilized world? This isn’t my tribe versus your tribe, my cave versus your cave. The closest we’re supposed to come to direct conflict with others is the “us versus them” world of professional and college sports.
I hope.
Aren’t I supposed to be better than this? Isn’t learning to coexist the whole point of the playground as a kid (besides burning off energy and giving the teacher a break)? Like Hamilton and Jefferson, or Ronald Reagan and Tip O’Neill?
Refusing to work with another competent physician because I disagree with their personal, religious, or political beliefs is just plain stupid.
Politicians and pundits try to convince us that people who disagree with us are the enemy, but that’s horse hockey. The truth is that the majority of people out there, regardless of personal beliefs, are decent, hardworking, and just trying to support their families like I am mine.
Later that week I had a patient who clearly needed the other doctor’s expertise, and I gave her his name and phone number. She asked if I’d send my own family to him, and I said, unequivocally, “yes” (actually I have).
Because, at the end of the day, we’re all people, along on the same ride. To not send a patient to him wouldn’t be in their best interest, which is what I’m supposed to be watching out for.
Not only that, but if I don’t refer just because I disagree with him as a person, then I’ve become the problem and not the solution.
Because I, and everyone else, have to try to be better than that.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
There’s another doctor with whom I’ve referred patients, back and forth, for the last 20 years. I think he’s good at his job and assume he feels the same way about me. We aren’t social friends, but chat briefly when we run into each other at the hospital, or store, or local restaurants.
Last week I was at the hospital to read EEGs, and happened to see him in the doctor’s parking lot. We wished each other a happy new year, talked briefly about a few mutual patients, and then went our separate ways.
As he pulled out, I noticed his car had a bumper sticker for a cause I strongly disagree with. I mean, 180 degrees opposed.
Suddenly, I didn’t want to ever refer to him again. Why should I support him? He’s the enemy.
Why should I help him out by referring patients?
But then I had to stop. Isn’t this 2022? Aren’t we supposed to be in a civilized world? This isn’t my tribe versus your tribe, my cave versus your cave. The closest we’re supposed to come to direct conflict with others is the “us versus them” world of professional and college sports.
I hope.
Aren’t I supposed to be better than this? Isn’t learning to coexist the whole point of the playground as a kid (besides burning off energy and giving the teacher a break)? Like Hamilton and Jefferson, or Ronald Reagan and Tip O’Neill?
Refusing to work with another competent physician because I disagree with their personal, religious, or political beliefs is just plain stupid.
Politicians and pundits try to convince us that people who disagree with us are the enemy, but that’s horse hockey. The truth is that the majority of people out there, regardless of personal beliefs, are decent, hardworking, and just trying to support their families like I am mine.
Later that week I had a patient who clearly needed the other doctor’s expertise, and I gave her his name and phone number. She asked if I’d send my own family to him, and I said, unequivocally, “yes” (actually I have).
Because, at the end of the day, we’re all people, along on the same ride. To not send a patient to him wouldn’t be in their best interest, which is what I’m supposed to be watching out for.
Not only that, but if I don’t refer just because I disagree with him as a person, then I’ve become the problem and not the solution.
Because I, and everyone else, have to try to be better than that.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Family-oriented care in adult psychiatric residency
The Group for the Advancement of Psychiatry’s Committee on the Family published an updated curriculum in October 2021 on family-oriented care. The first curriculum, published in 2006, was nominated as the American Association of Directors of Psychiatric Residency Training model curriculum for family-oriented care. The updated curriculum, produced by the GAP family committee and guests, is shorter and more focused.
The following is a summary of the introduction and the highlights.
Introduction
Use of family systems–based techniques in the diagnosis and care of patients is a key evidence-based tool for psychiatric disorders. However, it is not a current Accreditation Council for Graduate Medical Education Training training requirement, and it is possible to complete psychiatry residency without exposure to this key framework.
Here, we highlight the importance of considering patients through a “family systems” lens and the incorporation of multiple individuals from an individual patient’s identified system in their care.
Current medicine curricula emphasize patient autonomy, one of the core pillars of ethics. Autonomy is the cornerstone of the everyday practice within medicine of communicating all risks, benefits, and alternatives of a proposed treatment to a patient making decisions about desired paths forward. This prevents paternalistic care in which the doctor “knows best” and makes decisions for the patient. Unfortunately, the emphasis of this pillar has morphed over time into the idea that the individual patient is the only person to whom this information should be provided or from whom information should be obtained.
Extensive research proves conclusively that family support, education, and psychoeducation improve both patient and family functioning in medical and psychiatric illness. as well as overlook the opportunity to use the structure and support system around a patient to strengthen their care and improve treatment outcomes.
The network and family dynamics around a patient can be critical to providing accurate information on medication adherence and symptoms, supporting recovery, and handling emergencies. Markedly improved patient outcomes occur when family members are seen as allies and offered support, assessment, and psychoeducation. In fact, the American Psychiatric Association’s Practice Guidelines on the treatment of schizophrenia (2020), major depressive disorder (2010), and bipolar disorder (2002) include the expectation that patients’ family members will be involved in the assessment and treatment of patients. Yet, training in how to incorporate these practices is often minimal or nonexistent during residency.
A family systems orientation is distinguished by its view of the family as a transactional system. Stressful events and problems of an individual member affect the whole family as a functional unit, with ripple effects for all members and their relationships. In turn, the family response – how the family handles problems – contributes significantly to positive adaptation or to individual and relational dysfunction. Thus, individual problems are assessed and addressed in the context of the family, with attention to socioeconomic and other environmental stressors.
A family systems approach is distinguished less by who is in the room and more by the clinician’s attention to relationship systems in assessment and treatment planning. We need to consider how family members may contribute to – and be affected by – problem situations. Most importantly, regardless of the source of difficulties, we involve key family members who can contribute to needed changes. Interventions are aimed at modifying dysfunctional patterns, tapping family resources, and strengthening both individual and family functioning.
A family systemic lens is useful for working with all types of families, for example: refugee families, thinking through child adoption processes, working with families with specific social disadvantages, etc. Incorporating issues of race, gender, and sexual orientation is important in this work, as is working with larger systems such as schools, workplaces, and health care settings.
As opposed to previous viewpoints that family therapy is the only “family” skill to be taught during residency, the GAP committee proposes that psychiatric residents should be trained in skills of family inclusion, support, and psychoeducation, and that these skills should be taught throughout the residency. Our goal is to have residents be able to consider any case through a family systems lens, to understand how patients’ illnesses and their family systems have bidirectional effects on each other, to perform a basic assessment of family system functioning, and to use this information in diagnostic and treatment planning.
Training goals
Systems-based thinking will enable trainees to:
1. Ally with family members to work with the patient to comply with goals of care (for example, taking medications, complying with lifestyle changes, and maintaining sobriety).
- Teachers focus on engagement, joining with families.
2. Help patients understand the influences of their families in their own lives, such as intergenerational transmission of trauma and resilience.
- Teachers focus on the creation of a genogram, and the location of the individual within their family system.
3. Understand that mental health includes the creation and maintenance of healthy relationships.
- Teachers focus on assessing a willingness to listen to others’ points of view and the cocreation of a shared reality and belief system: a belief that relationships can change over time and how to create new family narratives.
4. Understand the impact of illness on the family unit and the impact of the family unit on illness.
- Teachers focus on the concept of a family system, clarifying the roles within the family, including caregiving responsibilities.
5. Assess the family for strengths and weaknesses.
- Teachers focus on how families maintain a healthy emotional climate, allocate roles, decide on rules, problem-solving abilities, and so on.
6. Gather information from multiple informants in the same room.
- Teachers focus on using communication techniques to elicit, guide, and redirect information from multiple individuals of a system with varying perspectives in the same room. Teachers help students understand that there are multiple realities in families and learn how to maintain multidirectional partiality.
Knowledge, skills, and attitudes across all treatment settings
Knowledge: Beginning level
- Healthy family functioning at the various phases of the family life cycle. Systems concepts are applicable to families, multidisciplinary teams in clinical settings, and medical/government organizations. However, family systems are distinguished by deep attachment bonds, specific generational hierarchy, goals of emotional safety and, for many families, child rearing.
- Systemic thinking, unlike a linear cause and effect model, examines the feedback loops by which multiple persons or groups arrive at a specific way of functioning.
- Understanding boundaries, subsystems, and feedback loops is critical to understanding interpersonal connections. Understand how the family affects and is affected by psychiatric and medical illnesses. Impact of interpersonal stress on biological systems. The role of expressed emotion (EE) in psychiatric illness. EE describes the level of criticism, hostility, and emotional overinvolvement in families. It has been studied extensively across the health care spectrum, and cultural variance is significant.
- The components of family psychoeducation, and its associated research in improving patient and family outcomes.
Knowledge: Advanced level
- Principles of adaptive and maladaptive relational functioning in family life and family organization, communication, problem solving, and emotional regulation. Role of family strengths, resilience in reducing vulnerability.
- Couple and family development over the life cycle.
- Understanding multigenerational patterns.
- How age, gender, class, culture, and spirituality affect family life.
- The variety of family forms (for example, single parent, stepfamilies, same-sex parents).
- Special issues in couples and families, including loss, divorce and remarriage, immigration, illness, secrets, affairs, violence, alcohol and substance abuse, sexuality, including LGBTQi. Relationship of families to larger systems, for example, schools, work, health care systems, government agencies.
Skills
- Family-interviewing skills, especially managing high levels of emotion and making room for multiple points of view.
- Promoting resilience, hope, and strength.
- Basic psychoeducation techniques, which includes providing a therapeutic space for emotional processing, providing information about the illness, skills such as better communication, problem-solving, and relapse drill and support.
- Collaborative treatment planning with family members and other helping professionals. Treatment planning should include all members of the system: patient, family members, and members of the treatment team. Good planning establishes a role for family members, helps define criteria for managing emergencies, looks for areas of strength and resilience and provides clear and realistic goals for treatment.
- Knowledge of, and referral to, local and national resources, both in the community and online.
Attitudes
- Appreciate the multiple points of view in a family.
- Interest in family members as people with their own needs and history.
- Including family members as a resource in recovery.
- Understand caregiver burden and rewards and that stress extends to all family members.
Training techniques
Most learning takes place at the level of patient, supervisor, and resident. It is critical that the resident sees faculty members dealing with patients in observed or shared family sessions, and /or sees videos made by faculty or professionally made videos. Attitudes are best learned by modeling.
Areas of focus can include time management, addressing the fear that family sessions may get out of control, and the influence of the residents’ own life experiences and background including potential generational or cultural differences on their assessment and interactions with patient family dynamics. In skill development, our goal is efficient interviewing, history taking, and support in controlling sessions.
It is difficult to specify which techniques are most useful in didactic sessions as each presenter will have a different skill set for engaging the class. The techniques that work best are the ones most comfortable to the presenter. Any technique that gets emotions involved, such as role play, sculpting, discussing movie clips, bringing in family members to discuss their experiences, or self-exploration, will generate the most powerful learning. If time permits, exploration of the resident’s own family, including a genogram, is an exceptionally helpful technique, especially if accompanied by asking the residents to interview their own families.
Adult didactic curriculum
The curriculum represents basic concepts. We have vignettes by the authors, if needed, but it is best if the class, including the supervisor, uses vignettes from their own experiences. Material for use in class is in references, but the class is urged to draw on their own experiences as this supports strength-based teaching. The following are key topics and concepts for each of the training years.
Basic concepts for PGY1 and PGY2
1. Where are you in the family and individual life cycles? What are your experiences with psychiatric illness in family/friends? Open discussion about how individual and family life cycles interact. Draw genograms of s/o in the class or with the supervisor.
2. Healthy family functioning and family resilience. Recommend asking residents to talk to their parents/elders about their lives and family life cycle when they were your age. Open discussion about what makes a healthy resilient family.
3. How do I connect with the family rather than just one person? How do you learn to hold multiple perspectives? How do I try not to take sides/multidirectional partiality? How do I see each person in a positive way? How do I focus on family strengths, rather than focusing on someone behaving badly (which is really hard because it is overlearned in individual therapy).
4. What are the common factors used across all therapies, both individual and family? When is it best to use an individual relational approach versus a family systemic approach?
5. How do I decide if a family needs support or education or family therapy?
6. Psychoeducation: Research, current use and cultural adaptations.
7. Attachment styles and couples therapy.
8. What is the evidence base behind our work?
System practice for PGY 3 and 4
These seminars follow the basic seminars. The focus is on clarification of what systems thinking means. Systems thinking or relational thinking is to be differentiated from systems-based practice. These lectures require knowledge of systemic practice. If there are no local experts, residency programs can reach out to national experts at the Association of Family Psychiatrists, for help with virtual/remote or in-person teaching.
Here is a list of other topics that should be covered:
- Relational formulation, nested subsystems, boundaries, history of these concepts, contributions to the development of family therapy.
- How to define and identify common systems concepts, such as circular patterns, feedback loops, and triangulation. Teach circular questioning. Framing. This concept is the family systems equivalent of insight. How to intervene to effect communication change and behavior change?
- Working at interfaces: community, legal, government, agencies, and so on, and other treaters, consultation. Include systemic and individual racism.
- Understanding the complexity of intimacy.
- Emergency situations. When to report regarding abuse. Dealing with family trauma.
- Varieties of family therapy; assumptions and major concepts.
*The new curriculum was written by The GAP Committee on the Family: Ellen Berman, MD; John Rolland, MD, MPH; John Sargent, MD; and me, and with guests Chayanin Foongsathaporn, MD; Sarah Nguyen, MD, MPH; Neha Sharma, DO; and Jodi Zik, MD. For the full curriculum, which includes residency milestones, site-specific training goals, references, and case studies, please access the Association of Family Psychiatry’s website: www.familypsychiatrists.org.Dr. Heru is professor of psychiatry at the University of Colorado Denver, Aurora. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013). She has no conflicts of interest to disclose. Contact Dr. Heru at [email protected].
The Group for the Advancement of Psychiatry’s Committee on the Family published an updated curriculum in October 2021 on family-oriented care. The first curriculum, published in 2006, was nominated as the American Association of Directors of Psychiatric Residency Training model curriculum for family-oriented care. The updated curriculum, produced by the GAP family committee and guests, is shorter and more focused.
The following is a summary of the introduction and the highlights.
Introduction
Use of family systems–based techniques in the diagnosis and care of patients is a key evidence-based tool for psychiatric disorders. However, it is not a current Accreditation Council for Graduate Medical Education Training training requirement, and it is possible to complete psychiatry residency without exposure to this key framework.
Here, we highlight the importance of considering patients through a “family systems” lens and the incorporation of multiple individuals from an individual patient’s identified system in their care.
Current medicine curricula emphasize patient autonomy, one of the core pillars of ethics. Autonomy is the cornerstone of the everyday practice within medicine of communicating all risks, benefits, and alternatives of a proposed treatment to a patient making decisions about desired paths forward. This prevents paternalistic care in which the doctor “knows best” and makes decisions for the patient. Unfortunately, the emphasis of this pillar has morphed over time into the idea that the individual patient is the only person to whom this information should be provided or from whom information should be obtained.
Extensive research proves conclusively that family support, education, and psychoeducation improve both patient and family functioning in medical and psychiatric illness. as well as overlook the opportunity to use the structure and support system around a patient to strengthen their care and improve treatment outcomes.
The network and family dynamics around a patient can be critical to providing accurate information on medication adherence and symptoms, supporting recovery, and handling emergencies. Markedly improved patient outcomes occur when family members are seen as allies and offered support, assessment, and psychoeducation. In fact, the American Psychiatric Association’s Practice Guidelines on the treatment of schizophrenia (2020), major depressive disorder (2010), and bipolar disorder (2002) include the expectation that patients’ family members will be involved in the assessment and treatment of patients. Yet, training in how to incorporate these practices is often minimal or nonexistent during residency.
A family systems orientation is distinguished by its view of the family as a transactional system. Stressful events and problems of an individual member affect the whole family as a functional unit, with ripple effects for all members and their relationships. In turn, the family response – how the family handles problems – contributes significantly to positive adaptation or to individual and relational dysfunction. Thus, individual problems are assessed and addressed in the context of the family, with attention to socioeconomic and other environmental stressors.
A family systems approach is distinguished less by who is in the room and more by the clinician’s attention to relationship systems in assessment and treatment planning. We need to consider how family members may contribute to – and be affected by – problem situations. Most importantly, regardless of the source of difficulties, we involve key family members who can contribute to needed changes. Interventions are aimed at modifying dysfunctional patterns, tapping family resources, and strengthening both individual and family functioning.
A family systemic lens is useful for working with all types of families, for example: refugee families, thinking through child adoption processes, working with families with specific social disadvantages, etc. Incorporating issues of race, gender, and sexual orientation is important in this work, as is working with larger systems such as schools, workplaces, and health care settings.
As opposed to previous viewpoints that family therapy is the only “family” skill to be taught during residency, the GAP committee proposes that psychiatric residents should be trained in skills of family inclusion, support, and psychoeducation, and that these skills should be taught throughout the residency. Our goal is to have residents be able to consider any case through a family systems lens, to understand how patients’ illnesses and their family systems have bidirectional effects on each other, to perform a basic assessment of family system functioning, and to use this information in diagnostic and treatment planning.
Training goals
Systems-based thinking will enable trainees to:
1. Ally with family members to work with the patient to comply with goals of care (for example, taking medications, complying with lifestyle changes, and maintaining sobriety).
- Teachers focus on engagement, joining with families.
2. Help patients understand the influences of their families in their own lives, such as intergenerational transmission of trauma and resilience.
- Teachers focus on the creation of a genogram, and the location of the individual within their family system.
3. Understand that mental health includes the creation and maintenance of healthy relationships.
- Teachers focus on assessing a willingness to listen to others’ points of view and the cocreation of a shared reality and belief system: a belief that relationships can change over time and how to create new family narratives.
4. Understand the impact of illness on the family unit and the impact of the family unit on illness.
- Teachers focus on the concept of a family system, clarifying the roles within the family, including caregiving responsibilities.
5. Assess the family for strengths and weaknesses.
- Teachers focus on how families maintain a healthy emotional climate, allocate roles, decide on rules, problem-solving abilities, and so on.
6. Gather information from multiple informants in the same room.
- Teachers focus on using communication techniques to elicit, guide, and redirect information from multiple individuals of a system with varying perspectives in the same room. Teachers help students understand that there are multiple realities in families and learn how to maintain multidirectional partiality.
Knowledge, skills, and attitudes across all treatment settings
Knowledge: Beginning level
- Healthy family functioning at the various phases of the family life cycle. Systems concepts are applicable to families, multidisciplinary teams in clinical settings, and medical/government organizations. However, family systems are distinguished by deep attachment bonds, specific generational hierarchy, goals of emotional safety and, for many families, child rearing.
- Systemic thinking, unlike a linear cause and effect model, examines the feedback loops by which multiple persons or groups arrive at a specific way of functioning.
- Understanding boundaries, subsystems, and feedback loops is critical to understanding interpersonal connections. Understand how the family affects and is affected by psychiatric and medical illnesses. Impact of interpersonal stress on biological systems. The role of expressed emotion (EE) in psychiatric illness. EE describes the level of criticism, hostility, and emotional overinvolvement in families. It has been studied extensively across the health care spectrum, and cultural variance is significant.
- The components of family psychoeducation, and its associated research in improving patient and family outcomes.
Knowledge: Advanced level
- Principles of adaptive and maladaptive relational functioning in family life and family organization, communication, problem solving, and emotional regulation. Role of family strengths, resilience in reducing vulnerability.
- Couple and family development over the life cycle.
- Understanding multigenerational patterns.
- How age, gender, class, culture, and spirituality affect family life.
- The variety of family forms (for example, single parent, stepfamilies, same-sex parents).
- Special issues in couples and families, including loss, divorce and remarriage, immigration, illness, secrets, affairs, violence, alcohol and substance abuse, sexuality, including LGBTQi. Relationship of families to larger systems, for example, schools, work, health care systems, government agencies.
Skills
- Family-interviewing skills, especially managing high levels of emotion and making room for multiple points of view.
- Promoting resilience, hope, and strength.
- Basic psychoeducation techniques, which includes providing a therapeutic space for emotional processing, providing information about the illness, skills such as better communication, problem-solving, and relapse drill and support.
- Collaborative treatment planning with family members and other helping professionals. Treatment planning should include all members of the system: patient, family members, and members of the treatment team. Good planning establishes a role for family members, helps define criteria for managing emergencies, looks for areas of strength and resilience and provides clear and realistic goals for treatment.
- Knowledge of, and referral to, local and national resources, both in the community and online.
Attitudes
- Appreciate the multiple points of view in a family.
- Interest in family members as people with their own needs and history.
- Including family members as a resource in recovery.
- Understand caregiver burden and rewards and that stress extends to all family members.
Training techniques
Most learning takes place at the level of patient, supervisor, and resident. It is critical that the resident sees faculty members dealing with patients in observed or shared family sessions, and /or sees videos made by faculty or professionally made videos. Attitudes are best learned by modeling.
Areas of focus can include time management, addressing the fear that family sessions may get out of control, and the influence of the residents’ own life experiences and background including potential generational or cultural differences on their assessment and interactions with patient family dynamics. In skill development, our goal is efficient interviewing, history taking, and support in controlling sessions.
It is difficult to specify which techniques are most useful in didactic sessions as each presenter will have a different skill set for engaging the class. The techniques that work best are the ones most comfortable to the presenter. Any technique that gets emotions involved, such as role play, sculpting, discussing movie clips, bringing in family members to discuss their experiences, or self-exploration, will generate the most powerful learning. If time permits, exploration of the resident’s own family, including a genogram, is an exceptionally helpful technique, especially if accompanied by asking the residents to interview their own families.
Adult didactic curriculum
The curriculum represents basic concepts. We have vignettes by the authors, if needed, but it is best if the class, including the supervisor, uses vignettes from their own experiences. Material for use in class is in references, but the class is urged to draw on their own experiences as this supports strength-based teaching. The following are key topics and concepts for each of the training years.
Basic concepts for PGY1 and PGY2
1. Where are you in the family and individual life cycles? What are your experiences with psychiatric illness in family/friends? Open discussion about how individual and family life cycles interact. Draw genograms of s/o in the class or with the supervisor.
2. Healthy family functioning and family resilience. Recommend asking residents to talk to their parents/elders about their lives and family life cycle when they were your age. Open discussion about what makes a healthy resilient family.
3. How do I connect with the family rather than just one person? How do you learn to hold multiple perspectives? How do I try not to take sides/multidirectional partiality? How do I see each person in a positive way? How do I focus on family strengths, rather than focusing on someone behaving badly (which is really hard because it is overlearned in individual therapy).
4. What are the common factors used across all therapies, both individual and family? When is it best to use an individual relational approach versus a family systemic approach?
5. How do I decide if a family needs support or education or family therapy?
6. Psychoeducation: Research, current use and cultural adaptations.
7. Attachment styles and couples therapy.
8. What is the evidence base behind our work?
System practice for PGY 3 and 4
These seminars follow the basic seminars. The focus is on clarification of what systems thinking means. Systems thinking or relational thinking is to be differentiated from systems-based practice. These lectures require knowledge of systemic practice. If there are no local experts, residency programs can reach out to national experts at the Association of Family Psychiatrists, for help with virtual/remote or in-person teaching.
Here is a list of other topics that should be covered:
- Relational formulation, nested subsystems, boundaries, history of these concepts, contributions to the development of family therapy.
- How to define and identify common systems concepts, such as circular patterns, feedback loops, and triangulation. Teach circular questioning. Framing. This concept is the family systems equivalent of insight. How to intervene to effect communication change and behavior change?
- Working at interfaces: community, legal, government, agencies, and so on, and other treaters, consultation. Include systemic and individual racism.
- Understanding the complexity of intimacy.
- Emergency situations. When to report regarding abuse. Dealing with family trauma.
- Varieties of family therapy; assumptions and major concepts.
*The new curriculum was written by The GAP Committee on the Family: Ellen Berman, MD; John Rolland, MD, MPH; John Sargent, MD; and me, and with guests Chayanin Foongsathaporn, MD; Sarah Nguyen, MD, MPH; Neha Sharma, DO; and Jodi Zik, MD. For the full curriculum, which includes residency milestones, site-specific training goals, references, and case studies, please access the Association of Family Psychiatry’s website: www.familypsychiatrists.org.Dr. Heru is professor of psychiatry at the University of Colorado Denver, Aurora. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013). She has no conflicts of interest to disclose. Contact Dr. Heru at [email protected].
The Group for the Advancement of Psychiatry’s Committee on the Family published an updated curriculum in October 2021 on family-oriented care. The first curriculum, published in 2006, was nominated as the American Association of Directors of Psychiatric Residency Training model curriculum for family-oriented care. The updated curriculum, produced by the GAP family committee and guests, is shorter and more focused.
The following is a summary of the introduction and the highlights.
Introduction
Use of family systems–based techniques in the diagnosis and care of patients is a key evidence-based tool for psychiatric disorders. However, it is not a current Accreditation Council for Graduate Medical Education Training training requirement, and it is possible to complete psychiatry residency without exposure to this key framework.
Here, we highlight the importance of considering patients through a “family systems” lens and the incorporation of multiple individuals from an individual patient’s identified system in their care.
Current medicine curricula emphasize patient autonomy, one of the core pillars of ethics. Autonomy is the cornerstone of the everyday practice within medicine of communicating all risks, benefits, and alternatives of a proposed treatment to a patient making decisions about desired paths forward. This prevents paternalistic care in which the doctor “knows best” and makes decisions for the patient. Unfortunately, the emphasis of this pillar has morphed over time into the idea that the individual patient is the only person to whom this information should be provided or from whom information should be obtained.
Extensive research proves conclusively that family support, education, and psychoeducation improve both patient and family functioning in medical and psychiatric illness. as well as overlook the opportunity to use the structure and support system around a patient to strengthen their care and improve treatment outcomes.
The network and family dynamics around a patient can be critical to providing accurate information on medication adherence and symptoms, supporting recovery, and handling emergencies. Markedly improved patient outcomes occur when family members are seen as allies and offered support, assessment, and psychoeducation. In fact, the American Psychiatric Association’s Practice Guidelines on the treatment of schizophrenia (2020), major depressive disorder (2010), and bipolar disorder (2002) include the expectation that patients’ family members will be involved in the assessment and treatment of patients. Yet, training in how to incorporate these practices is often minimal or nonexistent during residency.
A family systems orientation is distinguished by its view of the family as a transactional system. Stressful events and problems of an individual member affect the whole family as a functional unit, with ripple effects for all members and their relationships. In turn, the family response – how the family handles problems – contributes significantly to positive adaptation or to individual and relational dysfunction. Thus, individual problems are assessed and addressed in the context of the family, with attention to socioeconomic and other environmental stressors.
A family systems approach is distinguished less by who is in the room and more by the clinician’s attention to relationship systems in assessment and treatment planning. We need to consider how family members may contribute to – and be affected by – problem situations. Most importantly, regardless of the source of difficulties, we involve key family members who can contribute to needed changes. Interventions are aimed at modifying dysfunctional patterns, tapping family resources, and strengthening both individual and family functioning.
A family systemic lens is useful for working with all types of families, for example: refugee families, thinking through child adoption processes, working with families with specific social disadvantages, etc. Incorporating issues of race, gender, and sexual orientation is important in this work, as is working with larger systems such as schools, workplaces, and health care settings.
As opposed to previous viewpoints that family therapy is the only “family” skill to be taught during residency, the GAP committee proposes that psychiatric residents should be trained in skills of family inclusion, support, and psychoeducation, and that these skills should be taught throughout the residency. Our goal is to have residents be able to consider any case through a family systems lens, to understand how patients’ illnesses and their family systems have bidirectional effects on each other, to perform a basic assessment of family system functioning, and to use this information in diagnostic and treatment planning.
Training goals
Systems-based thinking will enable trainees to:
1. Ally with family members to work with the patient to comply with goals of care (for example, taking medications, complying with lifestyle changes, and maintaining sobriety).
- Teachers focus on engagement, joining with families.
2. Help patients understand the influences of their families in their own lives, such as intergenerational transmission of trauma and resilience.
- Teachers focus on the creation of a genogram, and the location of the individual within their family system.
3. Understand that mental health includes the creation and maintenance of healthy relationships.
- Teachers focus on assessing a willingness to listen to others’ points of view and the cocreation of a shared reality and belief system: a belief that relationships can change over time and how to create new family narratives.
4. Understand the impact of illness on the family unit and the impact of the family unit on illness.
- Teachers focus on the concept of a family system, clarifying the roles within the family, including caregiving responsibilities.
5. Assess the family for strengths and weaknesses.
- Teachers focus on how families maintain a healthy emotional climate, allocate roles, decide on rules, problem-solving abilities, and so on.
6. Gather information from multiple informants in the same room.
- Teachers focus on using communication techniques to elicit, guide, and redirect information from multiple individuals of a system with varying perspectives in the same room. Teachers help students understand that there are multiple realities in families and learn how to maintain multidirectional partiality.
Knowledge, skills, and attitudes across all treatment settings
Knowledge: Beginning level
- Healthy family functioning at the various phases of the family life cycle. Systems concepts are applicable to families, multidisciplinary teams in clinical settings, and medical/government organizations. However, family systems are distinguished by deep attachment bonds, specific generational hierarchy, goals of emotional safety and, for many families, child rearing.
- Systemic thinking, unlike a linear cause and effect model, examines the feedback loops by which multiple persons or groups arrive at a specific way of functioning.
- Understanding boundaries, subsystems, and feedback loops is critical to understanding interpersonal connections. Understand how the family affects and is affected by psychiatric and medical illnesses. Impact of interpersonal stress on biological systems. The role of expressed emotion (EE) in psychiatric illness. EE describes the level of criticism, hostility, and emotional overinvolvement in families. It has been studied extensively across the health care spectrum, and cultural variance is significant.
- The components of family psychoeducation, and its associated research in improving patient and family outcomes.
Knowledge: Advanced level
- Principles of adaptive and maladaptive relational functioning in family life and family organization, communication, problem solving, and emotional regulation. Role of family strengths, resilience in reducing vulnerability.
- Couple and family development over the life cycle.
- Understanding multigenerational patterns.
- How age, gender, class, culture, and spirituality affect family life.
- The variety of family forms (for example, single parent, stepfamilies, same-sex parents).
- Special issues in couples and families, including loss, divorce and remarriage, immigration, illness, secrets, affairs, violence, alcohol and substance abuse, sexuality, including LGBTQi. Relationship of families to larger systems, for example, schools, work, health care systems, government agencies.
Skills
- Family-interviewing skills, especially managing high levels of emotion and making room for multiple points of view.
- Promoting resilience, hope, and strength.
- Basic psychoeducation techniques, which includes providing a therapeutic space for emotional processing, providing information about the illness, skills such as better communication, problem-solving, and relapse drill and support.
- Collaborative treatment planning with family members and other helping professionals. Treatment planning should include all members of the system: patient, family members, and members of the treatment team. Good planning establishes a role for family members, helps define criteria for managing emergencies, looks for areas of strength and resilience and provides clear and realistic goals for treatment.
- Knowledge of, and referral to, local and national resources, both in the community and online.
Attitudes
- Appreciate the multiple points of view in a family.
- Interest in family members as people with their own needs and history.
- Including family members as a resource in recovery.
- Understand caregiver burden and rewards and that stress extends to all family members.
Training techniques
Most learning takes place at the level of patient, supervisor, and resident. It is critical that the resident sees faculty members dealing with patients in observed or shared family sessions, and /or sees videos made by faculty or professionally made videos. Attitudes are best learned by modeling.
Areas of focus can include time management, addressing the fear that family sessions may get out of control, and the influence of the residents’ own life experiences and background including potential generational or cultural differences on their assessment and interactions with patient family dynamics. In skill development, our goal is efficient interviewing, history taking, and support in controlling sessions.
It is difficult to specify which techniques are most useful in didactic sessions as each presenter will have a different skill set for engaging the class. The techniques that work best are the ones most comfortable to the presenter. Any technique that gets emotions involved, such as role play, sculpting, discussing movie clips, bringing in family members to discuss their experiences, or self-exploration, will generate the most powerful learning. If time permits, exploration of the resident’s own family, including a genogram, is an exceptionally helpful technique, especially if accompanied by asking the residents to interview their own families.
Adult didactic curriculum
The curriculum represents basic concepts. We have vignettes by the authors, if needed, but it is best if the class, including the supervisor, uses vignettes from their own experiences. Material for use in class is in references, but the class is urged to draw on their own experiences as this supports strength-based teaching. The following are key topics and concepts for each of the training years.
Basic concepts for PGY1 and PGY2
1. Where are you in the family and individual life cycles? What are your experiences with psychiatric illness in family/friends? Open discussion about how individual and family life cycles interact. Draw genograms of s/o in the class or with the supervisor.
2. Healthy family functioning and family resilience. Recommend asking residents to talk to their parents/elders about their lives and family life cycle when they were your age. Open discussion about what makes a healthy resilient family.
3. How do I connect with the family rather than just one person? How do you learn to hold multiple perspectives? How do I try not to take sides/multidirectional partiality? How do I see each person in a positive way? How do I focus on family strengths, rather than focusing on someone behaving badly (which is really hard because it is overlearned in individual therapy).
4. What are the common factors used across all therapies, both individual and family? When is it best to use an individual relational approach versus a family systemic approach?
5. How do I decide if a family needs support or education or family therapy?
6. Psychoeducation: Research, current use and cultural adaptations.
7. Attachment styles and couples therapy.
8. What is the evidence base behind our work?
System practice for PGY 3 and 4
These seminars follow the basic seminars. The focus is on clarification of what systems thinking means. Systems thinking or relational thinking is to be differentiated from systems-based practice. These lectures require knowledge of systemic practice. If there are no local experts, residency programs can reach out to national experts at the Association of Family Psychiatrists, for help with virtual/remote or in-person teaching.
Here is a list of other topics that should be covered:
- Relational formulation, nested subsystems, boundaries, history of these concepts, contributions to the development of family therapy.
- How to define and identify common systems concepts, such as circular patterns, feedback loops, and triangulation. Teach circular questioning. Framing. This concept is the family systems equivalent of insight. How to intervene to effect communication change and behavior change?
- Working at interfaces: community, legal, government, agencies, and so on, and other treaters, consultation. Include systemic and individual racism.
- Understanding the complexity of intimacy.
- Emergency situations. When to report regarding abuse. Dealing with family trauma.
- Varieties of family therapy; assumptions and major concepts.
*The new curriculum was written by The GAP Committee on the Family: Ellen Berman, MD; John Rolland, MD, MPH; John Sargent, MD; and me, and with guests Chayanin Foongsathaporn, MD; Sarah Nguyen, MD, MPH; Neha Sharma, DO; and Jodi Zik, MD. For the full curriculum, which includes residency milestones, site-specific training goals, references, and case studies, please access the Association of Family Psychiatry’s website: www.familypsychiatrists.org.Dr. Heru is professor of psychiatry at the University of Colorado Denver, Aurora. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013). She has no conflicts of interest to disclose. Contact Dr. Heru at [email protected].
Do recent data on use of menopausal HT and subsequent risk of dementia indicate an association?
Vinogradova Y, Dening T, Hippisley-Cox J, et al. Use of menopausal hormone therapy and risk of dementia: nested case-control studies using QResearch and CPRD databases. BMJ. 2021;374:n2182. doi: 10.1136/bmj.n2182.
Much interest has surrounded whether the use of menopausal HT impacts future risk of cognitive decline. Recently, Vinogradova and colleagues conducted an observational study using data from 2 large primary care databases, QResearch and the Clinical Practice Research Datalink (CPRD), in the United Kingdom.1 The investigators conducted case-control studies that included women aged 55 and older diagnosed with dementia and up to 5 controls without dementia. Only cases and controls with at least 10 years of medical records prior to the index date (that is, the time of dementia diagnosis in cases) were included. Since early symptoms of dementia prior to diagnosis may cause sleep problems and dysphoria (which also may be symptoms of menopause), HT prescriptions during the 3 years prior to the index date were excluded.
Details of the study
Among 16,291 cases and 68,726 controls, the women’s mean age was approximately 83 years. Cases were identified by using codes for dementia from patients’ clinical records or records of prescriptions for drugs used to treat dementia.
More than half the women were being treated for hypertension, and 14% of women in both groups had used HT. Women were considered users of estrogen-only therapy if they had no prescriptions containing a progestogen after their first prescription for systemic estrogen as the start of exposure to HT. Those with any subsequent prescription containing a progestogen were classified as combined HT users.
Results. In an analysis adjusted for all available potential confounders—including lifestyle factors, ethnicity, family history of dementia, early menopause, oophorectomy/ hysterectomy, comorbidities, and use of other relevant drugs—the use of HT was not associated with risk of dementia.
A reduced risk of dementia was noted among women who had been taking estrogen-only HT for 10 years or more (odds ratio [OR], 0.85; 95% confidence interval [CI], 0.76–0.94). An elevated risk of Alzheimer disease was noted among women who had used estrogen-progestin HT for 5 to 9 years (OR, 1.19; CI, 1.06–1.33).1
Study strengths and limitations
The authors pointed out that this study’s main strengths were that it had a very large sample size representative of the general population and that its design permitted capture of all known cases as well as precision recording for prescribed drugs. On the other hand, the study is limited by the possible lack of data for some older women before the index date; that is, menopause in this latter group started before their registration or before these data were gathered electronically by their practice. ●
The authors of this British large observational study took pains to minimize potential bias. The finding that long-term use of estrogen-only HT may be neuroprotective is consistent with results of recent studies in the United States and Finland,2-4 as well as with the Women’s Health Initiative randomized trial, which found that with 18 years of follow-up, treatment with conjugated estrogen alone was associated with a 26% reduced risk of death from Alzheimer disease.5 Overall, however, the main message we should glean from this important study by Vinogradova and colleagues is that women with bothersome vasomotor symptoms considering use of menopausal HT can be reassured that such therapy has little if any impact on future risk of cognitive decline.
ANDREW M. KAUNITZ, MD, NCMP
- Vinogradova Y, Dening T, Hippisley-Cox J, et al. Use of menopausal hormone therapy and risk of dementia: nested case-control studies using QResearch and CPRD databases. BMJ. 2021;374:n2182. doi: 10.1136/bmj.n2182.
- Matyi JM, Rattinger GB, Schwartz S, et al. Lifetime estrogen exposure and cognition in late life: the Cache County study. Menopause. 2019;26:1366-1374. doi: 10.1097 /GME.0000000000001405.
- Liu JH. Does estrogen provide “neuroprotection” for postmenopausal women? Menopause. 2019;26:1361-1362. doi: 10.1097/GME.0000000000001459.
- Imtiaz B, Tuppurainen M, Rikkonen T, et al. Postmenopausal hormone therapy and Alzheimer disease: a prospective cohort study. Neurology. 2017;88:1062-1068. doi: 10.1212 /WNL.0000000000003696.
- Manson JE, Aragaki AK, Rossouw JE, et al; WHI Investigators. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318:927-938. doi: 10.1001 /jama.2017.11217.
Vinogradova Y, Dening T, Hippisley-Cox J, et al. Use of menopausal hormone therapy and risk of dementia: nested case-control studies using QResearch and CPRD databases. BMJ. 2021;374:n2182. doi: 10.1136/bmj.n2182.
Much interest has surrounded whether the use of menopausal HT impacts future risk of cognitive decline. Recently, Vinogradova and colleagues conducted an observational study using data from 2 large primary care databases, QResearch and the Clinical Practice Research Datalink (CPRD), in the United Kingdom.1 The investigators conducted case-control studies that included women aged 55 and older diagnosed with dementia and up to 5 controls without dementia. Only cases and controls with at least 10 years of medical records prior to the index date (that is, the time of dementia diagnosis in cases) were included. Since early symptoms of dementia prior to diagnosis may cause sleep problems and dysphoria (which also may be symptoms of menopause), HT prescriptions during the 3 years prior to the index date were excluded.
Details of the study
Among 16,291 cases and 68,726 controls, the women’s mean age was approximately 83 years. Cases were identified by using codes for dementia from patients’ clinical records or records of prescriptions for drugs used to treat dementia.
More than half the women were being treated for hypertension, and 14% of women in both groups had used HT. Women were considered users of estrogen-only therapy if they had no prescriptions containing a progestogen after their first prescription for systemic estrogen as the start of exposure to HT. Those with any subsequent prescription containing a progestogen were classified as combined HT users.
Results. In an analysis adjusted for all available potential confounders—including lifestyle factors, ethnicity, family history of dementia, early menopause, oophorectomy/ hysterectomy, comorbidities, and use of other relevant drugs—the use of HT was not associated with risk of dementia.
A reduced risk of dementia was noted among women who had been taking estrogen-only HT for 10 years or more (odds ratio [OR], 0.85; 95% confidence interval [CI], 0.76–0.94). An elevated risk of Alzheimer disease was noted among women who had used estrogen-progestin HT for 5 to 9 years (OR, 1.19; CI, 1.06–1.33).1
Study strengths and limitations
The authors pointed out that this study’s main strengths were that it had a very large sample size representative of the general population and that its design permitted capture of all known cases as well as precision recording for prescribed drugs. On the other hand, the study is limited by the possible lack of data for some older women before the index date; that is, menopause in this latter group started before their registration or before these data were gathered electronically by their practice. ●
The authors of this British large observational study took pains to minimize potential bias. The finding that long-term use of estrogen-only HT may be neuroprotective is consistent with results of recent studies in the United States and Finland,2-4 as well as with the Women’s Health Initiative randomized trial, which found that with 18 years of follow-up, treatment with conjugated estrogen alone was associated with a 26% reduced risk of death from Alzheimer disease.5 Overall, however, the main message we should glean from this important study by Vinogradova and colleagues is that women with bothersome vasomotor symptoms considering use of menopausal HT can be reassured that such therapy has little if any impact on future risk of cognitive decline.
ANDREW M. KAUNITZ, MD, NCMP
Vinogradova Y, Dening T, Hippisley-Cox J, et al. Use of menopausal hormone therapy and risk of dementia: nested case-control studies using QResearch and CPRD databases. BMJ. 2021;374:n2182. doi: 10.1136/bmj.n2182.
Much interest has surrounded whether the use of menopausal HT impacts future risk of cognitive decline. Recently, Vinogradova and colleagues conducted an observational study using data from 2 large primary care databases, QResearch and the Clinical Practice Research Datalink (CPRD), in the United Kingdom.1 The investigators conducted case-control studies that included women aged 55 and older diagnosed with dementia and up to 5 controls without dementia. Only cases and controls with at least 10 years of medical records prior to the index date (that is, the time of dementia diagnosis in cases) were included. Since early symptoms of dementia prior to diagnosis may cause sleep problems and dysphoria (which also may be symptoms of menopause), HT prescriptions during the 3 years prior to the index date were excluded.
Details of the study
Among 16,291 cases and 68,726 controls, the women’s mean age was approximately 83 years. Cases were identified by using codes for dementia from patients’ clinical records or records of prescriptions for drugs used to treat dementia.
More than half the women were being treated for hypertension, and 14% of women in both groups had used HT. Women were considered users of estrogen-only therapy if they had no prescriptions containing a progestogen after their first prescription for systemic estrogen as the start of exposure to HT. Those with any subsequent prescription containing a progestogen were classified as combined HT users.
Results. In an analysis adjusted for all available potential confounders—including lifestyle factors, ethnicity, family history of dementia, early menopause, oophorectomy/ hysterectomy, comorbidities, and use of other relevant drugs—the use of HT was not associated with risk of dementia.
A reduced risk of dementia was noted among women who had been taking estrogen-only HT for 10 years or more (odds ratio [OR], 0.85; 95% confidence interval [CI], 0.76–0.94). An elevated risk of Alzheimer disease was noted among women who had used estrogen-progestin HT for 5 to 9 years (OR, 1.19; CI, 1.06–1.33).1
Study strengths and limitations
The authors pointed out that this study’s main strengths were that it had a very large sample size representative of the general population and that its design permitted capture of all known cases as well as precision recording for prescribed drugs. On the other hand, the study is limited by the possible lack of data for some older women before the index date; that is, menopause in this latter group started before their registration or before these data were gathered electronically by their practice. ●
The authors of this British large observational study took pains to minimize potential bias. The finding that long-term use of estrogen-only HT may be neuroprotective is consistent with results of recent studies in the United States and Finland,2-4 as well as with the Women’s Health Initiative randomized trial, which found that with 18 years of follow-up, treatment with conjugated estrogen alone was associated with a 26% reduced risk of death from Alzheimer disease.5 Overall, however, the main message we should glean from this important study by Vinogradova and colleagues is that women with bothersome vasomotor symptoms considering use of menopausal HT can be reassured that such therapy has little if any impact on future risk of cognitive decline.
ANDREW M. KAUNITZ, MD, NCMP
- Vinogradova Y, Dening T, Hippisley-Cox J, et al. Use of menopausal hormone therapy and risk of dementia: nested case-control studies using QResearch and CPRD databases. BMJ. 2021;374:n2182. doi: 10.1136/bmj.n2182.
- Matyi JM, Rattinger GB, Schwartz S, et al. Lifetime estrogen exposure and cognition in late life: the Cache County study. Menopause. 2019;26:1366-1374. doi: 10.1097 /GME.0000000000001405.
- Liu JH. Does estrogen provide “neuroprotection” for postmenopausal women? Menopause. 2019;26:1361-1362. doi: 10.1097/GME.0000000000001459.
- Imtiaz B, Tuppurainen M, Rikkonen T, et al. Postmenopausal hormone therapy and Alzheimer disease: a prospective cohort study. Neurology. 2017;88:1062-1068. doi: 10.1212 /WNL.0000000000003696.
- Manson JE, Aragaki AK, Rossouw JE, et al; WHI Investigators. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318:927-938. doi: 10.1001 /jama.2017.11217.
- Vinogradova Y, Dening T, Hippisley-Cox J, et al. Use of menopausal hormone therapy and risk of dementia: nested case-control studies using QResearch and CPRD databases. BMJ. 2021;374:n2182. doi: 10.1136/bmj.n2182.
- Matyi JM, Rattinger GB, Schwartz S, et al. Lifetime estrogen exposure and cognition in late life: the Cache County study. Menopause. 2019;26:1366-1374. doi: 10.1097 /GME.0000000000001405.
- Liu JH. Does estrogen provide “neuroprotection” for postmenopausal women? Menopause. 2019;26:1361-1362. doi: 10.1097/GME.0000000000001459.
- Imtiaz B, Tuppurainen M, Rikkonen T, et al. Postmenopausal hormone therapy and Alzheimer disease: a prospective cohort study. Neurology. 2017;88:1062-1068. doi: 10.1212 /WNL.0000000000003696.
- Manson JE, Aragaki AK, Rossouw JE, et al; WHI Investigators. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318:927-938. doi: 10.1001 /jama.2017.11217.
Children and COVID: U.S. sees almost 1 million new cases
Another record week for COVID-19 brought almost 1 million new cases to the children of the United States, according to new data from the American Academy of Pediatrics and the Children’s Hospital Association.
The pre-Omicron high for new cases in a week – 252,000 during the Delta surge of the late summer and early fall – has been surpassed each of the last 3 weeks and now stands at 981,000 (Jan. 7-13), according to the AAP/CHA weekly COVID-19 report. Over the 3-week stretch from Dec. 17 to Jan. 13, weekly cases increased by 394%.
Hospitalizations also climbed to new heights, as daily admissions reached 1.23 per 100,000 children on Jan. 14, an increase of 547% since Nov. 30, when the rate was 0.19 per 100,000. Before Omicron, the highest rate for children was 0.47 per 100,000, based on data from the Centers for Disease Control and Prevention.
The inpatient population count, meanwhile, has followed suit. On Jan. 16, there were 3,822 children hospitalized in pediatric inpatient beds with laboratory-confirmed COVID-19, which is 523% higher than the 613 children who were hospitalized on Nov. 14, according to the Department of Health & Human Services. In the last week, though, the population was up by just 10%.
The one thing that has not surged in the last few weeks is vaccination. Among children aged 5-11 years, the weekly count of those who have received at least one dose dropped by 34% over the last 5 weeks, falling from 527,000 for Dec.11-17 to 347,000 during Jan. 8-14, the CDC said on the COVID Data Tracker, which also noted that just 18.4% of this age group is fully vaccinated.
The situation was reversed in children aged 12-15, who were up by 36% over that same time, but their numbers were much smaller: 78,000 for the week of Dec. 11-17 and 106,000 for Jan. 8-14. Those aged 16-17 were up by just 4% over that 5-week span, the CDC data show.
Over the course of the entire pandemic, almost 9.5 million cases of COVID-19 in children have been reported, and children represent 17.8% of all cases reported in 49 states (excluding New York but including New York City), the District of Columbia, Puerto Rico, and Guam, the AAP and CHA said in their report.
Three states (Alabama, Nebraska, and Texas) stopped public reporting over the summer, but many states count individuals up to age 19 as children, and others (South Carolina, Tennessee, and West Virginia) go up to age 20, the AAP and CHA noted. The CDC, by comparison, puts the number of cases for those aged 0-17 at 8.3 million, but that estimate is based on only 51 million of the nearly 67 million U.S. cases as of Jan. 18.
Another record week for COVID-19 brought almost 1 million new cases to the children of the United States, according to new data from the American Academy of Pediatrics and the Children’s Hospital Association.
The pre-Omicron high for new cases in a week – 252,000 during the Delta surge of the late summer and early fall – has been surpassed each of the last 3 weeks and now stands at 981,000 (Jan. 7-13), according to the AAP/CHA weekly COVID-19 report. Over the 3-week stretch from Dec. 17 to Jan. 13, weekly cases increased by 394%.
Hospitalizations also climbed to new heights, as daily admissions reached 1.23 per 100,000 children on Jan. 14, an increase of 547% since Nov. 30, when the rate was 0.19 per 100,000. Before Omicron, the highest rate for children was 0.47 per 100,000, based on data from the Centers for Disease Control and Prevention.
The inpatient population count, meanwhile, has followed suit. On Jan. 16, there were 3,822 children hospitalized in pediatric inpatient beds with laboratory-confirmed COVID-19, which is 523% higher than the 613 children who were hospitalized on Nov. 14, according to the Department of Health & Human Services. In the last week, though, the population was up by just 10%.
The one thing that has not surged in the last few weeks is vaccination. Among children aged 5-11 years, the weekly count of those who have received at least one dose dropped by 34% over the last 5 weeks, falling from 527,000 for Dec.11-17 to 347,000 during Jan. 8-14, the CDC said on the COVID Data Tracker, which also noted that just 18.4% of this age group is fully vaccinated.
The situation was reversed in children aged 12-15, who were up by 36% over that same time, but their numbers were much smaller: 78,000 for the week of Dec. 11-17 and 106,000 for Jan. 8-14. Those aged 16-17 were up by just 4% over that 5-week span, the CDC data show.
Over the course of the entire pandemic, almost 9.5 million cases of COVID-19 in children have been reported, and children represent 17.8% of all cases reported in 49 states (excluding New York but including New York City), the District of Columbia, Puerto Rico, and Guam, the AAP and CHA said in their report.
Three states (Alabama, Nebraska, and Texas) stopped public reporting over the summer, but many states count individuals up to age 19 as children, and others (South Carolina, Tennessee, and West Virginia) go up to age 20, the AAP and CHA noted. The CDC, by comparison, puts the number of cases for those aged 0-17 at 8.3 million, but that estimate is based on only 51 million of the nearly 67 million U.S. cases as of Jan. 18.
Another record week for COVID-19 brought almost 1 million new cases to the children of the United States, according to new data from the American Academy of Pediatrics and the Children’s Hospital Association.
The pre-Omicron high for new cases in a week – 252,000 during the Delta surge of the late summer and early fall – has been surpassed each of the last 3 weeks and now stands at 981,000 (Jan. 7-13), according to the AAP/CHA weekly COVID-19 report. Over the 3-week stretch from Dec. 17 to Jan. 13, weekly cases increased by 394%.
Hospitalizations also climbed to new heights, as daily admissions reached 1.23 per 100,000 children on Jan. 14, an increase of 547% since Nov. 30, when the rate was 0.19 per 100,000. Before Omicron, the highest rate for children was 0.47 per 100,000, based on data from the Centers for Disease Control and Prevention.
The inpatient population count, meanwhile, has followed suit. On Jan. 16, there were 3,822 children hospitalized in pediatric inpatient beds with laboratory-confirmed COVID-19, which is 523% higher than the 613 children who were hospitalized on Nov. 14, according to the Department of Health & Human Services. In the last week, though, the population was up by just 10%.
The one thing that has not surged in the last few weeks is vaccination. Among children aged 5-11 years, the weekly count of those who have received at least one dose dropped by 34% over the last 5 weeks, falling from 527,000 for Dec.11-17 to 347,000 during Jan. 8-14, the CDC said on the COVID Data Tracker, which also noted that just 18.4% of this age group is fully vaccinated.
The situation was reversed in children aged 12-15, who were up by 36% over that same time, but their numbers were much smaller: 78,000 for the week of Dec. 11-17 and 106,000 for Jan. 8-14. Those aged 16-17 were up by just 4% over that 5-week span, the CDC data show.
Over the course of the entire pandemic, almost 9.5 million cases of COVID-19 in children have been reported, and children represent 17.8% of all cases reported in 49 states (excluding New York but including New York City), the District of Columbia, Puerto Rico, and Guam, the AAP and CHA said in their report.
Three states (Alabama, Nebraska, and Texas) stopped public reporting over the summer, but many states count individuals up to age 19 as children, and others (South Carolina, Tennessee, and West Virginia) go up to age 20, the AAP and CHA noted. The CDC, by comparison, puts the number of cases for those aged 0-17 at 8.3 million, but that estimate is based on only 51 million of the nearly 67 million U.S. cases as of Jan. 18.
Negative home COVID test no ‘free pass’ for kids, study finds
With the country looking increasingly to rapid testing as an off-ramp from the COVID-19 pandemic, a new study shows that the performance of the tests in children falls below standards set by regulatory agencies in the United States and elsewhere for diagnostic accuracy.
Experts said the findings, from a meta-analysis by researchers in the United Kingdom and Germany, underscore that, while a positive result on a rapid test is almost certainly an indicator of infection, negative results often are unreliable and can lead to a false sense of security.
“Real-life performance of current antigen tests for professional use in pediatric populations is below the minimum performance criteria set by WHO, the United States Food and Drug Administration, or the Medicines and Healthcare products Regulatory Agency (U.K.),” according to Naomi Fujita-Rohwerder, PhD, a research associate at the Cologne-based German Institute for Quality and Efficiency in Health Care (IQWiG), and her colleagues, whose study appears in BMJ Evidence-Based Medicine.
The researchers said that the study suggests that performance of rapid testing in a pediatric population is comparable to that in adults. However, they said they could not identify any studies investigating self-testing in children, which also could affect test performance.
Egon Ozer, MD, PhD, director of the center for pathogen genomics and microbial evolution at Northwestern University in Chicago, said the finding that specificity was high but sensitivity was middling “suggests that we should be very careful about interpreting negative antigen test results in children and recognize that there is a fair amount of uncertainty in the tests in this situation.”
Researchers from IQWiG, which examines the advantages and disadvantages of medical interventions, and the University of Manchester (England), conducted the systematic review and meta-analysis, which they described as the first of its kind to evaluate the diagnostic accuracy of rapid point-of-care tests for current SARS-CoV-2 infections in children.
They compiled information from 17 studies with a total 6,355 participants. They compared all antigen tests to reverse-transcription polymerase chain reaction (PCR). The studies compared eight antigen tests from six different brands. The rapid antigen tests, available from pharmacies and online stores, are widely used for self-testing in schools and testing toddlers before kindergarten.
The pooled diagnostic sensitivity of antigen tests was 64.2% and specificity was 99.1%.
Dr. Ozer noted that the analysis “was not able to address important outstanding questions such as the likelihood of transmitting infection with a false-negative antigen test versus a true-negative antigen test or how much repeated testing can increase the sensitivity.”
“In Europe, we don’t know how most tests perform in real life,” Dr. Fujita-Rohwerder said. “And even in countries like the United States, where market access is more stringent, we don’t know whether self-testing performed by children or sample collection in toddlers by laypersons has a significant impact on the diagnostic accuracy. Also, diagnostic accuracy estimates reported in our study may not apply to the current omicron or future variants of SARS-CoV-2 or vaccinated children. Hopefully, these essential gaps in the evidence will get addressed soon.”
Dr. Ozer said one takeaway from this study is negative antigen tests should not be considered a “free pass” in children, especially if the child is symptomatic, has been recently exposed to COVID-19, or is planning to spend time with individuals with conditions that place them at high risk for complications of COVID-19 infection. “In such cases, consider getting PCR testing or at least performing a repeat antigen test 36-48 hours after the first negative,” he said.
Dr. Fujita-Rohwerder said the low diagnostic sensitivity may affect the use of the tests. The gaps in evidence her group found in their study point to research needed to support evidence-based decision-making. “In particular, evidence is needed on real-life performance of tests in schools, self-testing performed by children, and kindergarten, [particularly] sample collection in toddlers by laypersons,” she said.
However, she stressed, testing is only a single measure. “Effectively reducing the spread of SARS-CoV-2 during the current pandemic requires multilayered mitigation measures,” she said. “Rapid testing represents one single layer. It can have its use at the population level, even though the sensitivity of antigen tests is lower than expected. However, antigen-based rapid testing is not a magic bullet: If your kid tests negative, do not disregard other mitigation measures.”
Edward Campbell, PhD, a virologist at Loyola University of Chicago, who serves on the board of LaGrange Elementary School District 102 outside Chicago, said the findings were unsurprising.
“This study generally looks consistent with what is known for adults. These rapid antigen tests are less sensitive than other tests,” said Dr. Campbell, who also runs a testing company for private schools in the Chicago area using reverse transcription-loop-mediated isothermal amplification technology. Even so, he said, “These tests are still effective at identifying people who are infectious to some degree. Never miss an opportunity to test.”
Dr. Fujita-Rohwerder disclosed no relevant financial conflicts of interest. Dr. Campbell owns Safeguard Surveillance.
With the country looking increasingly to rapid testing as an off-ramp from the COVID-19 pandemic, a new study shows that the performance of the tests in children falls below standards set by regulatory agencies in the United States and elsewhere for diagnostic accuracy.
Experts said the findings, from a meta-analysis by researchers in the United Kingdom and Germany, underscore that, while a positive result on a rapid test is almost certainly an indicator of infection, negative results often are unreliable and can lead to a false sense of security.
“Real-life performance of current antigen tests for professional use in pediatric populations is below the minimum performance criteria set by WHO, the United States Food and Drug Administration, or the Medicines and Healthcare products Regulatory Agency (U.K.),” according to Naomi Fujita-Rohwerder, PhD, a research associate at the Cologne-based German Institute for Quality and Efficiency in Health Care (IQWiG), and her colleagues, whose study appears in BMJ Evidence-Based Medicine.
The researchers said that the study suggests that performance of rapid testing in a pediatric population is comparable to that in adults. However, they said they could not identify any studies investigating self-testing in children, which also could affect test performance.
Egon Ozer, MD, PhD, director of the center for pathogen genomics and microbial evolution at Northwestern University in Chicago, said the finding that specificity was high but sensitivity was middling “suggests that we should be very careful about interpreting negative antigen test results in children and recognize that there is a fair amount of uncertainty in the tests in this situation.”
Researchers from IQWiG, which examines the advantages and disadvantages of medical interventions, and the University of Manchester (England), conducted the systematic review and meta-analysis, which they described as the first of its kind to evaluate the diagnostic accuracy of rapid point-of-care tests for current SARS-CoV-2 infections in children.
They compiled information from 17 studies with a total 6,355 participants. They compared all antigen tests to reverse-transcription polymerase chain reaction (PCR). The studies compared eight antigen tests from six different brands. The rapid antigen tests, available from pharmacies and online stores, are widely used for self-testing in schools and testing toddlers before kindergarten.
The pooled diagnostic sensitivity of antigen tests was 64.2% and specificity was 99.1%.
Dr. Ozer noted that the analysis “was not able to address important outstanding questions such as the likelihood of transmitting infection with a false-negative antigen test versus a true-negative antigen test or how much repeated testing can increase the sensitivity.”
“In Europe, we don’t know how most tests perform in real life,” Dr. Fujita-Rohwerder said. “And even in countries like the United States, where market access is more stringent, we don’t know whether self-testing performed by children or sample collection in toddlers by laypersons has a significant impact on the diagnostic accuracy. Also, diagnostic accuracy estimates reported in our study may not apply to the current omicron or future variants of SARS-CoV-2 or vaccinated children. Hopefully, these essential gaps in the evidence will get addressed soon.”
Dr. Ozer said one takeaway from this study is negative antigen tests should not be considered a “free pass” in children, especially if the child is symptomatic, has been recently exposed to COVID-19, or is planning to spend time with individuals with conditions that place them at high risk for complications of COVID-19 infection. “In such cases, consider getting PCR testing or at least performing a repeat antigen test 36-48 hours after the first negative,” he said.
Dr. Fujita-Rohwerder said the low diagnostic sensitivity may affect the use of the tests. The gaps in evidence her group found in their study point to research needed to support evidence-based decision-making. “In particular, evidence is needed on real-life performance of tests in schools, self-testing performed by children, and kindergarten, [particularly] sample collection in toddlers by laypersons,” she said.
However, she stressed, testing is only a single measure. “Effectively reducing the spread of SARS-CoV-2 during the current pandemic requires multilayered mitigation measures,” she said. “Rapid testing represents one single layer. It can have its use at the population level, even though the sensitivity of antigen tests is lower than expected. However, antigen-based rapid testing is not a magic bullet: If your kid tests negative, do not disregard other mitigation measures.”
Edward Campbell, PhD, a virologist at Loyola University of Chicago, who serves on the board of LaGrange Elementary School District 102 outside Chicago, said the findings were unsurprising.
“This study generally looks consistent with what is known for adults. These rapid antigen tests are less sensitive than other tests,” said Dr. Campbell, who also runs a testing company for private schools in the Chicago area using reverse transcription-loop-mediated isothermal amplification technology. Even so, he said, “These tests are still effective at identifying people who are infectious to some degree. Never miss an opportunity to test.”
Dr. Fujita-Rohwerder disclosed no relevant financial conflicts of interest. Dr. Campbell owns Safeguard Surveillance.
With the country looking increasingly to rapid testing as an off-ramp from the COVID-19 pandemic, a new study shows that the performance of the tests in children falls below standards set by regulatory agencies in the United States and elsewhere for diagnostic accuracy.
Experts said the findings, from a meta-analysis by researchers in the United Kingdom and Germany, underscore that, while a positive result on a rapid test is almost certainly an indicator of infection, negative results often are unreliable and can lead to a false sense of security.
“Real-life performance of current antigen tests for professional use in pediatric populations is below the minimum performance criteria set by WHO, the United States Food and Drug Administration, or the Medicines and Healthcare products Regulatory Agency (U.K.),” according to Naomi Fujita-Rohwerder, PhD, a research associate at the Cologne-based German Institute for Quality and Efficiency in Health Care (IQWiG), and her colleagues, whose study appears in BMJ Evidence-Based Medicine.
The researchers said that the study suggests that performance of rapid testing in a pediatric population is comparable to that in adults. However, they said they could not identify any studies investigating self-testing in children, which also could affect test performance.
Egon Ozer, MD, PhD, director of the center for pathogen genomics and microbial evolution at Northwestern University in Chicago, said the finding that specificity was high but sensitivity was middling “suggests that we should be very careful about interpreting negative antigen test results in children and recognize that there is a fair amount of uncertainty in the tests in this situation.”
Researchers from IQWiG, which examines the advantages and disadvantages of medical interventions, and the University of Manchester (England), conducted the systematic review and meta-analysis, which they described as the first of its kind to evaluate the diagnostic accuracy of rapid point-of-care tests for current SARS-CoV-2 infections in children.
They compiled information from 17 studies with a total 6,355 participants. They compared all antigen tests to reverse-transcription polymerase chain reaction (PCR). The studies compared eight antigen tests from six different brands. The rapid antigen tests, available from pharmacies and online stores, are widely used for self-testing in schools and testing toddlers before kindergarten.
The pooled diagnostic sensitivity of antigen tests was 64.2% and specificity was 99.1%.
Dr. Ozer noted that the analysis “was not able to address important outstanding questions such as the likelihood of transmitting infection with a false-negative antigen test versus a true-negative antigen test or how much repeated testing can increase the sensitivity.”
“In Europe, we don’t know how most tests perform in real life,” Dr. Fujita-Rohwerder said. “And even in countries like the United States, where market access is more stringent, we don’t know whether self-testing performed by children or sample collection in toddlers by laypersons has a significant impact on the diagnostic accuracy. Also, diagnostic accuracy estimates reported in our study may not apply to the current omicron or future variants of SARS-CoV-2 or vaccinated children. Hopefully, these essential gaps in the evidence will get addressed soon.”
Dr. Ozer said one takeaway from this study is negative antigen tests should not be considered a “free pass” in children, especially if the child is symptomatic, has been recently exposed to COVID-19, or is planning to spend time with individuals with conditions that place them at high risk for complications of COVID-19 infection. “In such cases, consider getting PCR testing or at least performing a repeat antigen test 36-48 hours after the first negative,” he said.
Dr. Fujita-Rohwerder said the low diagnostic sensitivity may affect the use of the tests. The gaps in evidence her group found in their study point to research needed to support evidence-based decision-making. “In particular, evidence is needed on real-life performance of tests in schools, self-testing performed by children, and kindergarten, [particularly] sample collection in toddlers by laypersons,” she said.
However, she stressed, testing is only a single measure. “Effectively reducing the spread of SARS-CoV-2 during the current pandemic requires multilayered mitigation measures,” she said. “Rapid testing represents one single layer. It can have its use at the population level, even though the sensitivity of antigen tests is lower than expected. However, antigen-based rapid testing is not a magic bullet: If your kid tests negative, do not disregard other mitigation measures.”
Edward Campbell, PhD, a virologist at Loyola University of Chicago, who serves on the board of LaGrange Elementary School District 102 outside Chicago, said the findings were unsurprising.
“This study generally looks consistent with what is known for adults. These rapid antigen tests are less sensitive than other tests,” said Dr. Campbell, who also runs a testing company for private schools in the Chicago area using reverse transcription-loop-mediated isothermal amplification technology. Even so, he said, “These tests are still effective at identifying people who are infectious to some degree. Never miss an opportunity to test.”
Dr. Fujita-Rohwerder disclosed no relevant financial conflicts of interest. Dr. Campbell owns Safeguard Surveillance.
BMJ EVIDENCE-BASED MEDICINE
Feds’ website for free at-home COVID tests launches day early
The Biden administration’s new no-cost, at-home testing program launched Jan. 18, a day ahead of schedule.
The administration said 500 million tests are available to be delivered to homes across the country. This accounts for half of the president’s recent pledge to purchase 1 billion free at-home COVID-19 tests to distribute to the American public.
On a Jan. 14 call with reporters, senior White House officials offered some details about the new program.
Here’s what we know so far.
How do I order my free tests?
Americans can visit COVIDtests.gov to order their rapid at-home tests. You can also order directly from the U.S. Postal Service website. After you order, you’ll receive a confirmation email that promises to send tracking information once your order ships.
What information do I need to order the tests?
You only need your name and home mailing address.
There is also an option to provide your email address to get updates on the status of your order.
What if someone needs help ordering the tests?
There will be a free call-in line for people needing more help, including those having trouble accessing the internet, according to White House officials.
What tests will be available?
There are nine at-home tests available through FDA emergency use authorization. According to the Frequently Asked Questions section of COVIDtests.gov, "You will not be able to choose the brand you order as part of this program.”
How long will it take to get the tests once I order them?
Tests are expected to ship 7 to 12 days after you order them.
But White House officials say that the time frame will likely shorten as the program gains steam.
How many can I order?
There’s a limit of four tests per residential mailing address.
For larger families, White House officials suggest trying other free testing options, like visiting COVID-19 testing sites or your local health center.
Is this a one-time opportunity?
The White House doesn’t say, but officials did mention that if you run out of your four free tests, there are many other ways to access free at-home tests, such as COVID-19 testing sites, pharmacies, and community health centers.
The free tests available through COVIDtests.gov are in addition to an estimated 375 million at-home rapid tests on the market in the U.S. this month.
When should people use a rapid at-home test?
The CDC and experts with other public health groups agree that Americans should consider using at-home rapid tests in the following situations:
- If they begin to have symptoms consistent with COVID-19;
- At least 5 days after close contact with someone who has COVID;
- If someone is indoors with a group of people who are at risk of severe disease or are unvaccinated.
Are at-home rapid tests accurate?
The U.S. Department of Health and Human Services and other federal officials confirmed through studies that all tests distributed through this program can detect the Omicron variant. These agencies also confirmed that their performance is consistent with the FDA’s emergency use authorization.
Is the website designed to handle high demand?
After the original website to sign up for health insurance under the Affordable Care Act crashed repeatedly at launch, the government says it has prepared for high demand for ordering at-home rapid tests.
The U.S. Digital Service (USDS), an organization founded after Healthcare.gov, has partnered with the Postal Service to plan for the launch.
The Postal Service has expanded its staffing, similar to what’s done during the holidays.
All orders in the continental United States will be shipped through first-class mail, with shipments to Alaska, Hawaii, U.S. territories, and military and overseas addresses sent through priority mail.
A version of this article first appeared on WebMD.com.
The Biden administration’s new no-cost, at-home testing program launched Jan. 18, a day ahead of schedule.
The administration said 500 million tests are available to be delivered to homes across the country. This accounts for half of the president’s recent pledge to purchase 1 billion free at-home COVID-19 tests to distribute to the American public.
On a Jan. 14 call with reporters, senior White House officials offered some details about the new program.
Here’s what we know so far.
How do I order my free tests?
Americans can visit COVIDtests.gov to order their rapid at-home tests. You can also order directly from the U.S. Postal Service website. After you order, you’ll receive a confirmation email that promises to send tracking information once your order ships.
What information do I need to order the tests?
You only need your name and home mailing address.
There is also an option to provide your email address to get updates on the status of your order.
What if someone needs help ordering the tests?
There will be a free call-in line for people needing more help, including those having trouble accessing the internet, according to White House officials.
What tests will be available?
There are nine at-home tests available through FDA emergency use authorization. According to the Frequently Asked Questions section of COVIDtests.gov, "You will not be able to choose the brand you order as part of this program.”
How long will it take to get the tests once I order them?
Tests are expected to ship 7 to 12 days after you order them.
But White House officials say that the time frame will likely shorten as the program gains steam.
How many can I order?
There’s a limit of four tests per residential mailing address.
For larger families, White House officials suggest trying other free testing options, like visiting COVID-19 testing sites or your local health center.
Is this a one-time opportunity?
The White House doesn’t say, but officials did mention that if you run out of your four free tests, there are many other ways to access free at-home tests, such as COVID-19 testing sites, pharmacies, and community health centers.
The free tests available through COVIDtests.gov are in addition to an estimated 375 million at-home rapid tests on the market in the U.S. this month.
When should people use a rapid at-home test?
The CDC and experts with other public health groups agree that Americans should consider using at-home rapid tests in the following situations:
- If they begin to have symptoms consistent with COVID-19;
- At least 5 days after close contact with someone who has COVID;
- If someone is indoors with a group of people who are at risk of severe disease or are unvaccinated.
Are at-home rapid tests accurate?
The U.S. Department of Health and Human Services and other federal officials confirmed through studies that all tests distributed through this program can detect the Omicron variant. These agencies also confirmed that their performance is consistent with the FDA’s emergency use authorization.
Is the website designed to handle high demand?
After the original website to sign up for health insurance under the Affordable Care Act crashed repeatedly at launch, the government says it has prepared for high demand for ordering at-home rapid tests.
The U.S. Digital Service (USDS), an organization founded after Healthcare.gov, has partnered with the Postal Service to plan for the launch.
The Postal Service has expanded its staffing, similar to what’s done during the holidays.
All orders in the continental United States will be shipped through first-class mail, with shipments to Alaska, Hawaii, U.S. territories, and military and overseas addresses sent through priority mail.
A version of this article first appeared on WebMD.com.
The Biden administration’s new no-cost, at-home testing program launched Jan. 18, a day ahead of schedule.
The administration said 500 million tests are available to be delivered to homes across the country. This accounts for half of the president’s recent pledge to purchase 1 billion free at-home COVID-19 tests to distribute to the American public.
On a Jan. 14 call with reporters, senior White House officials offered some details about the new program.
Here’s what we know so far.
How do I order my free tests?
Americans can visit COVIDtests.gov to order their rapid at-home tests. You can also order directly from the U.S. Postal Service website. After you order, you’ll receive a confirmation email that promises to send tracking information once your order ships.
What information do I need to order the tests?
You only need your name and home mailing address.
There is also an option to provide your email address to get updates on the status of your order.
What if someone needs help ordering the tests?
There will be a free call-in line for people needing more help, including those having trouble accessing the internet, according to White House officials.
What tests will be available?
There are nine at-home tests available through FDA emergency use authorization. According to the Frequently Asked Questions section of COVIDtests.gov, "You will not be able to choose the brand you order as part of this program.”
How long will it take to get the tests once I order them?
Tests are expected to ship 7 to 12 days after you order them.
But White House officials say that the time frame will likely shorten as the program gains steam.
How many can I order?
There’s a limit of four tests per residential mailing address.
For larger families, White House officials suggest trying other free testing options, like visiting COVID-19 testing sites or your local health center.
Is this a one-time opportunity?
The White House doesn’t say, but officials did mention that if you run out of your four free tests, there are many other ways to access free at-home tests, such as COVID-19 testing sites, pharmacies, and community health centers.
The free tests available through COVIDtests.gov are in addition to an estimated 375 million at-home rapid tests on the market in the U.S. this month.
When should people use a rapid at-home test?
The CDC and experts with other public health groups agree that Americans should consider using at-home rapid tests in the following situations:
- If they begin to have symptoms consistent with COVID-19;
- At least 5 days after close contact with someone who has COVID;
- If someone is indoors with a group of people who are at risk of severe disease or are unvaccinated.
Are at-home rapid tests accurate?
The U.S. Department of Health and Human Services and other federal officials confirmed through studies that all tests distributed through this program can detect the Omicron variant. These agencies also confirmed that their performance is consistent with the FDA’s emergency use authorization.
Is the website designed to handle high demand?
After the original website to sign up for health insurance under the Affordable Care Act crashed repeatedly at launch, the government says it has prepared for high demand for ordering at-home rapid tests.
The U.S. Digital Service (USDS), an organization founded after Healthcare.gov, has partnered with the Postal Service to plan for the launch.
The Postal Service has expanded its staffing, similar to what’s done during the holidays.
All orders in the continental United States will be shipped through first-class mail, with shipments to Alaska, Hawaii, U.S. territories, and military and overseas addresses sent through priority mail.
A version of this article first appeared on WebMD.com.
Survey: Medical cannabis use for skin conditions lags behind interest, acceptance
A , according to the results of a recent survey.
Almost 89% of respondents were in favor of medical cannabis use for dermatologic diseases, and 73% said that they would be comfortable seeing a dermatologist who recommended such products to them, Samuel Yeroushalmi, a 4th-year medical student at George Washington University, Washington, and associates reported.
“Consumers and patients are already using MCPs [medical cannabis products] to treat inflammatory skin conditions, such as acne, rosacea, atopic dermatitis, and psoriasis, even without guidance from a dermatologist. While acceptance was high, there were clear barriers reported limiting use and uptake, such as patient skepticism and a lack of understanding,” Adam Friedman, MD, senior author and chair of the department of dermatology at the university, said in a separate statement.
Dermatologic use of OTC cannabis products without the recommendation of a dermatologist was reported by 18% of the 504 of 700 adults who responded in the SurveyMonkey online panel. Of the two-thirds who had seen a dermatologist, 20% received a recommendation for an OTC product and 11% were recommended a product that required a department of health medical card, the investigators said.
Uptake among the patients who did receive a recommendation, however, was high: 76% for OTC products and 72% for those that required a medical card. Among those who had received an OTC recommendation, 32% used the cannabis product for psoriasis and 30% each for acne and rosacea, Mr. Yeroushalmi and his coauthors said.
The most common indication among the respondents with dermatologist recommendations for products requiring a medical card was for acne (68%), followed by psoriasis and rosacea (28% each). Cost was the main deterrent (60%) for those who declined to use the recommended cannabis product, with skepticism, limited understanding, and product illegality in their state each at 50%, the researchers said.
“Though cost and legality concerns are nonmodifiable barriers, dermatologists have an opportunity to educate those who know little in the way of medical cannabis or are skeptic[s],” they wrote. The survey results show that many patients are interested, and “the future should be bright for MCPs; we just need to show and disseminate the science,” Dr. Friedman commented in the statement.
One of the authors was from the University of Maryland, College Park. The authors had no disclosures to report.
A , according to the results of a recent survey.
Almost 89% of respondents were in favor of medical cannabis use for dermatologic diseases, and 73% said that they would be comfortable seeing a dermatologist who recommended such products to them, Samuel Yeroushalmi, a 4th-year medical student at George Washington University, Washington, and associates reported.
“Consumers and patients are already using MCPs [medical cannabis products] to treat inflammatory skin conditions, such as acne, rosacea, atopic dermatitis, and psoriasis, even without guidance from a dermatologist. While acceptance was high, there were clear barriers reported limiting use and uptake, such as patient skepticism and a lack of understanding,” Adam Friedman, MD, senior author and chair of the department of dermatology at the university, said in a separate statement.
Dermatologic use of OTC cannabis products without the recommendation of a dermatologist was reported by 18% of the 504 of 700 adults who responded in the SurveyMonkey online panel. Of the two-thirds who had seen a dermatologist, 20% received a recommendation for an OTC product and 11% were recommended a product that required a department of health medical card, the investigators said.
Uptake among the patients who did receive a recommendation, however, was high: 76% for OTC products and 72% for those that required a medical card. Among those who had received an OTC recommendation, 32% used the cannabis product for psoriasis and 30% each for acne and rosacea, Mr. Yeroushalmi and his coauthors said.
The most common indication among the respondents with dermatologist recommendations for products requiring a medical card was for acne (68%), followed by psoriasis and rosacea (28% each). Cost was the main deterrent (60%) for those who declined to use the recommended cannabis product, with skepticism, limited understanding, and product illegality in their state each at 50%, the researchers said.
“Though cost and legality concerns are nonmodifiable barriers, dermatologists have an opportunity to educate those who know little in the way of medical cannabis or are skeptic[s],” they wrote. The survey results show that many patients are interested, and “the future should be bright for MCPs; we just need to show and disseminate the science,” Dr. Friedman commented in the statement.
One of the authors was from the University of Maryland, College Park. The authors had no disclosures to report.
A , according to the results of a recent survey.
Almost 89% of respondents were in favor of medical cannabis use for dermatologic diseases, and 73% said that they would be comfortable seeing a dermatologist who recommended such products to them, Samuel Yeroushalmi, a 4th-year medical student at George Washington University, Washington, and associates reported.
“Consumers and patients are already using MCPs [medical cannabis products] to treat inflammatory skin conditions, such as acne, rosacea, atopic dermatitis, and psoriasis, even without guidance from a dermatologist. While acceptance was high, there were clear barriers reported limiting use and uptake, such as patient skepticism and a lack of understanding,” Adam Friedman, MD, senior author and chair of the department of dermatology at the university, said in a separate statement.
Dermatologic use of OTC cannabis products without the recommendation of a dermatologist was reported by 18% of the 504 of 700 adults who responded in the SurveyMonkey online panel. Of the two-thirds who had seen a dermatologist, 20% received a recommendation for an OTC product and 11% were recommended a product that required a department of health medical card, the investigators said.
Uptake among the patients who did receive a recommendation, however, was high: 76% for OTC products and 72% for those that required a medical card. Among those who had received an OTC recommendation, 32% used the cannabis product for psoriasis and 30% each for acne and rosacea, Mr. Yeroushalmi and his coauthors said.
The most common indication among the respondents with dermatologist recommendations for products requiring a medical card was for acne (68%), followed by psoriasis and rosacea (28% each). Cost was the main deterrent (60%) for those who declined to use the recommended cannabis product, with skepticism, limited understanding, and product illegality in their state each at 50%, the researchers said.
“Though cost and legality concerns are nonmodifiable barriers, dermatologists have an opportunity to educate those who know little in the way of medical cannabis or are skeptic[s],” they wrote. The survey results show that many patients are interested, and “the future should be bright for MCPs; we just need to show and disseminate the science,” Dr. Friedman commented in the statement.
One of the authors was from the University of Maryland, College Park. The authors had no disclosures to report.
FROM JOURNAL OF DRUGS IN DERMATOLOGY