User login
Common Ground: Primary Care and Specialty Clinicians’ Perceptions of E-Consults in the Veterans Health Administration
Electronic consultation (e-consult) is designed to increase access to specialty care by facilitating communication between primary care and specialty clinicians without the need for outpatient face-to-face encounters.1–4 In 2011, the US Department of Veterans Affairs (VA) implemented an e-consult program as a component of its overall strategy to increase access to specialty services, reduce costs of care, and reduce appointment travel burden on patients.
E-consult has substantially increased within the VA since its implementation.5,6 Consistent with limited evaluations from other health care systems, evaluations of the VA e-consult program demonstrated reduced costs, reduced travel time for patients, and improved access to specialty care.2,5–11 However, there is wide variation in e-consult use across VA specialties, facilities, and regions.5,6,12,13 For example, hematology, preoperative evaluation, neurosurgery, endocrinology, and infectious diseases use e-consults more frequently when compared with in-person consults in the VA.6 Reasons for this variation or specific barriers and facilitators of using e-consults have not been described.
Prior qualitative studies report that primary care practitioners (PCPs) describe e-consults as convenient, educational, beneficial for patient care, and useful for improving patient access to specialty care.8,14,15 One study identified limited PCP knowledge of e-consults as a barrier to use.16 Specialists have reported that e-consult improves clinical communication, but increases their workload.1,14,17,18 These studies did not assess perspectives from both clinicians who initiate e-consults and those who respond to them. This is the first qualitative study to assess e-consult perceptions from perspectives of both PCPs and specialists among a large, national sample of VA clinicians who use e-consults. The objective of this study was to understand perspectives of e-consults between PCPs and specialists that may be relevant to increasing adoption in the VA.
Methods
The team (CL, ML, PG, 2 analysts under the guidance of GS and JS and support from RRK, and a biostatistician) conducted semistructured interviews with PCPs, specialists, and specialty division leaders who were employed by VA in 2016 and 2017. Specialties of interest were identified by the VA Office of Specialty Care and included cardiology, endocrinology, gastroenterology, and hematology.
E-Consult Procedures
Within the VA, the specific procedures used to initiate, triage and manage e-consults are coordinated at VA medical centers (VAMCs) and at the Veterans Integrated Service Network (VISN) regional level. E-consult can be requested by any clinician. Generally, e-consults are initiated by PCPs through standardized, specialty-specific templates. Recipients, typically specialists, respond by answering questions, suggesting additional testing and evaluation, or requesting an in-person visit. Communication is documented in the patient’s electronic health record (EHR). Specialists receive different levels of workload credit for responding to e-consults similar to a relative value unit reimbursement model. Training in the use of e-consults is available to practitioners but may vary at local and regional levels.
Recruitment
Our sample included PCPs, specialists, and specialty care division leaders. We first quantified e-consult rates (e-consults per 100 patient visits) between July 2016 and June 2017 at VA facilities within primary care and the 4 priority specialties and identified the 30 sites with the highest e-consult rates and 30 sites with the lowest e-consult rates. Sites with < 500 total visits, < 3 specialties, or without any e-consult visit during the study period were excluded. E-consult rates at community-based outpatient clinics were included with associated VAMCs. We then stratified PCPs by whether they were high or low users of e-consults (determined by the top and bottom users within each site) and credentials (MD vs nurse practitioner [NP] or physician assistant [PA]). Specialists were sampled based on their rate of use relative to colleagues within their site and the use rate of their division. We sampled division chiefs and individuals who had > 300 total visits and 1 e-consult during the study period. To recruit participants, the primary investigator sent an initial email and 2 reminder emails. The team followed up with respondents to schedule an interview.
Interview guides were designed to elicit rich descriptions of barriers and facilitators to e-consult use (eAppendix available at doi:10.12788/fp.0214). The team used the Practical Robust Implementation and Sustainability Model (PRISM), which considers factors along 6 domains for intervention planning, implementation, and sustainment.19 Telephone interviews lasted about 20 minutes and were conducted between September 2017 and March 2018. Interviews were recorded and transcribed verbatim.
Analysis
The team used an iterative, team-based, inductive/deductive approach to conventional content analysis.20,21 Initial code categories were created so that we could identify e-consult best practices—facilitators of e-consult that were recommended by both PCPs and specialists. Inductive codes or labels applied to identify meaningful quotations, phrases, or key terms were used to identify emergent ideas and were added throughout coding after discussion among team members. Consensus was reached using a team-based approach.21 Four analysts independently coded the same 3 transcripts and met to discuss points of divergence and convergence. Analyses continued with emergent themes, categories, and conclusions. Atlas.ti. v.7 was used for coding and data management.22
Results
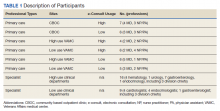
We conducted 34 interviews with clinicians (Table 1) from 13 VISNs. Four best-practice themes emerged among both PCPs and specialists, including that e-consults (1) are best suited for certain clinical questions and patients; (2) require relevant background information from requesting clinicians and clear recommendations from responding clinicians; (3) are a novel opportunity to provide efficient, transparent care; and (4) may not be fully adopted due to low awareness. Supporting quotations for the following findings are provided in Table 2.
Specific Clinical Questions and Patients
PCPs described specific patients and questions for which they most frequently used e-consults, such as for medication changes (Q1), determining treatment steps (Q2,3), and or clarifying laboratory or imaging findings. PCPs frequently used e-consults for patients who did not require a physical examination or when specialists could make recommendations without seeing patients face-to-face (Q3). An important use of e-consults described by PCPs was for treating conditions they could manage within primary care if additional guidance were available (Q4). Several PCPs and specialists also noted that e-consults were particularly useful for patients who were unable to travel or did not want face-to-face appointments (Q5). Notably, PCPs and specialists mentioned situations for which e-consults were inappropriate, including when a detailed history or physical examination was needed, or if a complex condition was suspected (Q6).
Background Data and Clear Recommendations
Participants described necessary data that should be included in high-quality e-consults. Specialists voiced frustration in time-consuming chart reviews that were often necessary when these data were not provided by the requestor. In some cases, specialists were unable to access necessary EHR data, which delayed responses (Q7). PCPs noted that the most useful responses carefully considered the question, used current patient information to determine treatments, provided clear recommendations, and defined who was responsible for next steps (Q8). PCPs and specialists stated that e-consult templates that required relevant information facilitated high-quality e-consults. Neither wanted to waste the other clinician's time (Q8).
A Novel Opportunity
Many PCPs felt that e-consults improved communication (eg, efficiency, response time), established new communication between clinicians, and reduced patients’ appointment burden (Q10, Q11). Many specialists felt that e-consults improved documentation of communication between clinicians and increased transparency of clinical decisions (Q12). Additionally, many specialists mentioned that e-consults capture previously informal curbside consults, enabling them to receive workload credit (Q13).
Lack of Awareness
Some noted that the biggest barrier to e-consults was not being aware of them generally, or which specialties offer e-consults (Q14). One PCP described e-consults as the best kept secret and found value in sharing the utility of e-consults with colleagues (Q15). All participants, including those who did not frequently use e-consults, felt that e-consults improved the quality of care by providing more timely care or better answers to clinical questions (Q16). Several practitioners also felt that e-consults increased access to specialty care. For example, specialists reported that e-consults enabled them to better manage patient load by using e-consults to answer relatively simple questions, reserving face-to-face consults for more complex patients (Q17).
Discussion
The objective of this study was to identify potential best practices for e-consults that may help increase their quality and use within the VA. We built on prior studies that offered insights on PCP and specialists’ overall satisfaction with e-consult by identifying several themes relevant to the further adoption of e-consults in the VA and elsewhere without a face-to-face visit.8,13,14,16–18 Future work may be beneficial in identifying whether the study themes identified can explain variation in e-consult use or whether addressing these factors might lead to increased or higher quality e-consult use. We are unaware of any qualitative study of comparable scale in a different health care system. Further, this is the first study to assess perspectives on e-consults among those who initiate and respond to them within the same health care system. Perhaps the most important finding from this study is that e-consults are generally viewed favorably, which is a necessary leverage point to increase their adoption within the system.
Clinicians reported several benefits to e-consults, including timely responses to clinical questions, efficient communication, allow for documentation of specialist recommendations, and help capture workload. These benefits are consistent with prior literature that indicates both PCPs and specialists in the VA and other health care systems feel that e-consults improves communication, decreases unnecessary visits, and improves quality of care.1,14,17,18 In particular, clinicians reported that e-consults improve their practice efficiency and efficacy. This is of critical importance given the pressures of providing timely access to primary and specialty care within the VA. Interestingly, many VA practitioners were unaware which specialties offered e-consults within their facilities, reflecting previous work showing that PCPs are often unaware of e-consult options.16 This may partially explain variation in e-consult use. Increasing awareness and educating clinicians on the benefits of e-consults may help promote use among non- and low users.
A common theme reported by both groups was the importance of providing necessary information within e-consult questions and responses. Specialists felt there was a need to ensure that PCPs provide relevant and patient-specific information that would enable them to efficiently and accurately answer questions without the need for extensive EHR review. This reflects previous work showing that specialists are often unable to respond to e-consult requests because they do not contain sufficient information.22 PCPs described a need to ensure that specialists’ responses included information that was detailed enough to make clinical decisions without the need for a reconsult. This highlights a common challenge to medical consultation, in that necessary or relevant information may not be apparent to all clinicians. To address this, there may be a role in developing enhanced, flexible templating that elicits necessary patient-specific information. Such a template may automatically pull relevant data from the EHR and prompt clinicians to provide important information. We did not assess how perspectives of templates varied, and further work could help define precisely what constitutes an effective template, including how it should capture appropriate patient data and how this impacts acceptability or use of e-consults generally. Collaboratively developed service agreements and e-consult templates could help guide PCPs and specialists to engage in efficient communication.
Another theme among both groups was that e-consult is most appropriate within specific clinical scenarios. Examples included review of laboratory results, questions about medication changes, or for patients who were reluctant to travel to appointments. Identifying and promoting specific opportunities for e-consults may help increase their use and align e-consult practices with scenarios that are likely to provide the most benefit to patients. For example, it could be helpful to understand the distance patients must travel for specialty care. Providing that information during clinical encounters could trigger clinicians to consider e-consults as an option. Future work might aim to identify clinical scenarios that clinicians feel are not well suited for e-consults and determine how to adapt them for those scenarios.
Limitations
Generalizability of these findings is limited given the qualitative study design. Participants’ descriptions of experiences with e-consults reflect the experiences of clinicians in the VA and may not reflect clinicians in other settings. We also interviewed a sample of clinicians who were already using e-consults. Important information could be learned from future work with those who have not yet adopted e-consult procedures or adopted and abandoned them.
Conclusions
E-consult is perceived as beneficial by VA PCPs and specialists. Participants suggested using e-consults for appropriate questions or patients and including necessary information and next steps in both the initial e-consult and response. Finding ways to facilitate e-consults with these suggestions in mind may increase delivery of high-quality e-consults. Future work could compare the findings of this work to similar work assessing clinicians perceptions of e-consults outside of the VA.
1. Battaglia C, Lambert-Kerzner A, Aron DC, et al. Evaluation of e-consults in the VHA: provider perspectives. Fed Pract. 2015;32(7):42-48.
2. Haverhals LM, Sayre G, Helfrich CD, et al. E-consult implementation: lessons learned using consolidated framework for implementation research. Am J Manag Care. 2015;21(12):e640-e647. Published 2015 Dec 1.
3. Sewell JL, Telischak KS, Day LW, Kirschner N, Weissman A. Preconsultation exchange in the United States: use, awareness, and attitudes. Am J Manag Care. 2014;20(12):e556-e564. Published 2014 Dec 1.
4. Horner K, Wagner E, Tufano J. Electronic consultations between primary and specialty care clinicians: early insights. Issue Brief (Commonw Fund). 2011;23:1-14.
5. Kirsh S, Carey E, Aron DC, et al. Impact of a national specialty e-consultation implementation project on access. Am J Manag Care. 2015;21(12):e648-654. Published 2015 Dec 1.
6. Saxon DR, Kaboli PJ, Haraldsson B, Wilson C, Ohl M, Augustine MR. Growth of electronic consultations in the Veterans Health Administration. Am J Manag Care. 2021;27(1):12-19. doi:10.37765/ajmc.2021.88572
7. Olayiwola JN, Anderson D, Jepeal N, et al. Electronic consultations to improve the primary care-specialty care interface for cardiology in the medically underserved: a cluster-randomized controlled trial. Ann Fam Med. 2016;14(2):133-140. doi:10.1370/afm.1869
8. Schettini P, Shah KP, O’Leary CP, et al. Keeping care connected: e-Consultation program improves access to nephrology care. J Telemed Telecare. 2019;25(3):142-150. doi:10.1177/1357633X17748350
9. Whittington MD, Ho PM, Kirsh SR, et al. Cost savings associated with electronic specialty consultations. Am J Manag Care. 2021;27(1):e16-e23. Published 2021 Jan 1. doi:10.37765/ajmc.2021.88579
10. Shipherd JC, Kauth MR, Matza A. Nationwide interdisciplinary e-consultation on transgender care in the Veterans Health Administration. Telemed J E Health. 2016;22(12):1008-1012. doi:10.1089/tmj.2016.0013
11. Strymish J, Gupte G, Afable MK, et al. Electronic consultations (E-consults): advancing infectious disease care in a large Veterans Affairs Healthcare System. Clin Infect Dis. 2017;64(8):1123-1125. doi:10.1093/cid/cix058
12. Williams KM, Kirsh S, Aron D, et al. Evaluation of the Veterans Health Administration’s Specialty Care Transformational Initiatives to promote patient-centered delivery of specialty care: a mixed-methods approach. Telemed J E-Health. 2017;23(7):577-589. doi:10.1089/tmj.2016.0166
13. US Department of Veterans Affairs, Veterans Health Administration, Specialty Care Transformational Initiative Evaluation Center. Evaluation of specialty care initiatives. Published 2013.
14. Vimalananda VG, Gupte G, Seraj SM, et al. Electronic consultations (e-consults) to improve access to specialty care: a systematic review and narrative synthesis. J Telemed Telecare. 2015;21(6):323-330. doi:10.1177/1357633X15582108
15. Lee M, Leonard C, Greene P, et al. Perspectives of VA primary care clinicians toward electronic consultation-related workload burden. JAMA Netw Open. 2020;3(10):e2018104. Published 2020 Oct 1. doi:10.1001/jamanetworkopen.2020.18104
16. Deeds SA, Dowdell KJ, Chew LD, Ackerman SL. Implementing an opt-in eConsult program at seven academic medical centers: a qualitative analysis of primary care provider experiences. J Gen Intern Med. 2019;34(8):1427-1433. doi:10.1007/s11606-019-05067-7
17. Rodriguez KL, Burkitt KH, Bayliss NK, et al. Veteran, primary care provider, and specialist satisfaction with electronic consultation. JMIR Med Inform. 2015;3(1):e5. Published 2015 Jan 14. doi:10.2196/medinform.3725
18. Gupte G, Vimalananda V, Simon SR, DeVito K, Clark J, Orlander JD. Disruptive innovation: implementation of electronic consultations in a Veterans Affairs Health Care System. JMIR Med Inform. 2016;4(1):e6. Published 2016 Feb 12. doi:10.2196/medinform.4801
19. Feldstein AC, Glasgow RE. A practical, robust implementation and sustainability model (PRISM) for integrating research findings into practice. Jt Comm J Qual Patient Saf. 2008;34(4):228-243. doi:10.1016/s1553-7250(08)34030-6
20. Patton MQ. Qualitative Research and Evaluation Methods. 3rd ed. Sage Publications; 2002.
21. Bradley EH, Curry LA, Devers KJ. Qualitative data analysis for health services research: developing taxonomy, themes, and theory. Health Serv Res. 2007;42(4):1758-1772. doi:10.1111/j.1475-6773.2006.00684.x
22. Kim EJ, Orlander JD, Afable M, et al. Cardiology electronic consultation (e-consult) use by primary care providers at VA medical centres in New England. J Telemed Telecare. 2019;25(6):370-377. doi:10.1177/1357633X18774468
Electronic consultation (e-consult) is designed to increase access to specialty care by facilitating communication between primary care and specialty clinicians without the need for outpatient face-to-face encounters.1–4 In 2011, the US Department of Veterans Affairs (VA) implemented an e-consult program as a component of its overall strategy to increase access to specialty services, reduce costs of care, and reduce appointment travel burden on patients.
E-consult has substantially increased within the VA since its implementation.5,6 Consistent with limited evaluations from other health care systems, evaluations of the VA e-consult program demonstrated reduced costs, reduced travel time for patients, and improved access to specialty care.2,5–11 However, there is wide variation in e-consult use across VA specialties, facilities, and regions.5,6,12,13 For example, hematology, preoperative evaluation, neurosurgery, endocrinology, and infectious diseases use e-consults more frequently when compared with in-person consults in the VA.6 Reasons for this variation or specific barriers and facilitators of using e-consults have not been described.
Prior qualitative studies report that primary care practitioners (PCPs) describe e-consults as convenient, educational, beneficial for patient care, and useful for improving patient access to specialty care.8,14,15 One study identified limited PCP knowledge of e-consults as a barrier to use.16 Specialists have reported that e-consult improves clinical communication, but increases their workload.1,14,17,18 These studies did not assess perspectives from both clinicians who initiate e-consults and those who respond to them. This is the first qualitative study to assess e-consult perceptions from perspectives of both PCPs and specialists among a large, national sample of VA clinicians who use e-consults. The objective of this study was to understand perspectives of e-consults between PCPs and specialists that may be relevant to increasing adoption in the VA.
Methods
The team (CL, ML, PG, 2 analysts under the guidance of GS and JS and support from RRK, and a biostatistician) conducted semistructured interviews with PCPs, specialists, and specialty division leaders who were employed by VA in 2016 and 2017. Specialties of interest were identified by the VA Office of Specialty Care and included cardiology, endocrinology, gastroenterology, and hematology.
E-Consult Procedures
Within the VA, the specific procedures used to initiate, triage and manage e-consults are coordinated at VA medical centers (VAMCs) and at the Veterans Integrated Service Network (VISN) regional level. E-consult can be requested by any clinician. Generally, e-consults are initiated by PCPs through standardized, specialty-specific templates. Recipients, typically specialists, respond by answering questions, suggesting additional testing and evaluation, or requesting an in-person visit. Communication is documented in the patient’s electronic health record (EHR). Specialists receive different levels of workload credit for responding to e-consults similar to a relative value unit reimbursement model. Training in the use of e-consults is available to practitioners but may vary at local and regional levels.
Recruitment
Our sample included PCPs, specialists, and specialty care division leaders. We first quantified e-consult rates (e-consults per 100 patient visits) between July 2016 and June 2017 at VA facilities within primary care and the 4 priority specialties and identified the 30 sites with the highest e-consult rates and 30 sites with the lowest e-consult rates. Sites with < 500 total visits, < 3 specialties, or without any e-consult visit during the study period were excluded. E-consult rates at community-based outpatient clinics were included with associated VAMCs. We then stratified PCPs by whether they were high or low users of e-consults (determined by the top and bottom users within each site) and credentials (MD vs nurse practitioner [NP] or physician assistant [PA]). Specialists were sampled based on their rate of use relative to colleagues within their site and the use rate of their division. We sampled division chiefs and individuals who had > 300 total visits and 1 e-consult during the study period. To recruit participants, the primary investigator sent an initial email and 2 reminder emails. The team followed up with respondents to schedule an interview.
Interview guides were designed to elicit rich descriptions of barriers and facilitators to e-consult use (eAppendix available at doi:10.12788/fp.0214). The team used the Practical Robust Implementation and Sustainability Model (PRISM), which considers factors along 6 domains for intervention planning, implementation, and sustainment.19 Telephone interviews lasted about 20 minutes and were conducted between September 2017 and March 2018. Interviews were recorded and transcribed verbatim.
Analysis
The team used an iterative, team-based, inductive/deductive approach to conventional content analysis.20,21 Initial code categories were created so that we could identify e-consult best practices—facilitators of e-consult that were recommended by both PCPs and specialists. Inductive codes or labels applied to identify meaningful quotations, phrases, or key terms were used to identify emergent ideas and were added throughout coding after discussion among team members. Consensus was reached using a team-based approach.21 Four analysts independently coded the same 3 transcripts and met to discuss points of divergence and convergence. Analyses continued with emergent themes, categories, and conclusions. Atlas.ti. v.7 was used for coding and data management.22
Results
We conducted 34 interviews with clinicians (Table 1) from 13 VISNs. Four best-practice themes emerged among both PCPs and specialists, including that e-consults (1) are best suited for certain clinical questions and patients; (2) require relevant background information from requesting clinicians and clear recommendations from responding clinicians; (3) are a novel opportunity to provide efficient, transparent care; and (4) may not be fully adopted due to low awareness. Supporting quotations for the following findings are provided in Table 2.
Specific Clinical Questions and Patients
PCPs described specific patients and questions for which they most frequently used e-consults, such as for medication changes (Q1), determining treatment steps (Q2,3), and or clarifying laboratory or imaging findings. PCPs frequently used e-consults for patients who did not require a physical examination or when specialists could make recommendations without seeing patients face-to-face (Q3). An important use of e-consults described by PCPs was for treating conditions they could manage within primary care if additional guidance were available (Q4). Several PCPs and specialists also noted that e-consults were particularly useful for patients who were unable to travel or did not want face-to-face appointments (Q5). Notably, PCPs and specialists mentioned situations for which e-consults were inappropriate, including when a detailed history or physical examination was needed, or if a complex condition was suspected (Q6).
Background Data and Clear Recommendations
Participants described necessary data that should be included in high-quality e-consults. Specialists voiced frustration in time-consuming chart reviews that were often necessary when these data were not provided by the requestor. In some cases, specialists were unable to access necessary EHR data, which delayed responses (Q7). PCPs noted that the most useful responses carefully considered the question, used current patient information to determine treatments, provided clear recommendations, and defined who was responsible for next steps (Q8). PCPs and specialists stated that e-consult templates that required relevant information facilitated high-quality e-consults. Neither wanted to waste the other clinician's time (Q8).
A Novel Opportunity
Many PCPs felt that e-consults improved communication (eg, efficiency, response time), established new communication between clinicians, and reduced patients’ appointment burden (Q10, Q11). Many specialists felt that e-consults improved documentation of communication between clinicians and increased transparency of clinical decisions (Q12). Additionally, many specialists mentioned that e-consults capture previously informal curbside consults, enabling them to receive workload credit (Q13).
Lack of Awareness
Some noted that the biggest barrier to e-consults was not being aware of them generally, or which specialties offer e-consults (Q14). One PCP described e-consults as the best kept secret and found value in sharing the utility of e-consults with colleagues (Q15). All participants, including those who did not frequently use e-consults, felt that e-consults improved the quality of care by providing more timely care or better answers to clinical questions (Q16). Several practitioners also felt that e-consults increased access to specialty care. For example, specialists reported that e-consults enabled them to better manage patient load by using e-consults to answer relatively simple questions, reserving face-to-face consults for more complex patients (Q17).
Discussion
The objective of this study was to identify potential best practices for e-consults that may help increase their quality and use within the VA. We built on prior studies that offered insights on PCP and specialists’ overall satisfaction with e-consult by identifying several themes relevant to the further adoption of e-consults in the VA and elsewhere without a face-to-face visit.8,13,14,16–18 Future work may be beneficial in identifying whether the study themes identified can explain variation in e-consult use or whether addressing these factors might lead to increased or higher quality e-consult use. We are unaware of any qualitative study of comparable scale in a different health care system. Further, this is the first study to assess perspectives on e-consults among those who initiate and respond to them within the same health care system. Perhaps the most important finding from this study is that e-consults are generally viewed favorably, which is a necessary leverage point to increase their adoption within the system.
Clinicians reported several benefits to e-consults, including timely responses to clinical questions, efficient communication, allow for documentation of specialist recommendations, and help capture workload. These benefits are consistent with prior literature that indicates both PCPs and specialists in the VA and other health care systems feel that e-consults improves communication, decreases unnecessary visits, and improves quality of care.1,14,17,18 In particular, clinicians reported that e-consults improve their practice efficiency and efficacy. This is of critical importance given the pressures of providing timely access to primary and specialty care within the VA. Interestingly, many VA practitioners were unaware which specialties offered e-consults within their facilities, reflecting previous work showing that PCPs are often unaware of e-consult options.16 This may partially explain variation in e-consult use. Increasing awareness and educating clinicians on the benefits of e-consults may help promote use among non- and low users.
A common theme reported by both groups was the importance of providing necessary information within e-consult questions and responses. Specialists felt there was a need to ensure that PCPs provide relevant and patient-specific information that would enable them to efficiently and accurately answer questions without the need for extensive EHR review. This reflects previous work showing that specialists are often unable to respond to e-consult requests because they do not contain sufficient information.22 PCPs described a need to ensure that specialists’ responses included information that was detailed enough to make clinical decisions without the need for a reconsult. This highlights a common challenge to medical consultation, in that necessary or relevant information may not be apparent to all clinicians. To address this, there may be a role in developing enhanced, flexible templating that elicits necessary patient-specific information. Such a template may automatically pull relevant data from the EHR and prompt clinicians to provide important information. We did not assess how perspectives of templates varied, and further work could help define precisely what constitutes an effective template, including how it should capture appropriate patient data and how this impacts acceptability or use of e-consults generally. Collaboratively developed service agreements and e-consult templates could help guide PCPs and specialists to engage in efficient communication.
Another theme among both groups was that e-consult is most appropriate within specific clinical scenarios. Examples included review of laboratory results, questions about medication changes, or for patients who were reluctant to travel to appointments. Identifying and promoting specific opportunities for e-consults may help increase their use and align e-consult practices with scenarios that are likely to provide the most benefit to patients. For example, it could be helpful to understand the distance patients must travel for specialty care. Providing that information during clinical encounters could trigger clinicians to consider e-consults as an option. Future work might aim to identify clinical scenarios that clinicians feel are not well suited for e-consults and determine how to adapt them for those scenarios.
Limitations
Generalizability of these findings is limited given the qualitative study design. Participants’ descriptions of experiences with e-consults reflect the experiences of clinicians in the VA and may not reflect clinicians in other settings. We also interviewed a sample of clinicians who were already using e-consults. Important information could be learned from future work with those who have not yet adopted e-consult procedures or adopted and abandoned them.
Conclusions
E-consult is perceived as beneficial by VA PCPs and specialists. Participants suggested using e-consults for appropriate questions or patients and including necessary information and next steps in both the initial e-consult and response. Finding ways to facilitate e-consults with these suggestions in mind may increase delivery of high-quality e-consults. Future work could compare the findings of this work to similar work assessing clinicians perceptions of e-consults outside of the VA.
Electronic consultation (e-consult) is designed to increase access to specialty care by facilitating communication between primary care and specialty clinicians without the need for outpatient face-to-face encounters.1–4 In 2011, the US Department of Veterans Affairs (VA) implemented an e-consult program as a component of its overall strategy to increase access to specialty services, reduce costs of care, and reduce appointment travel burden on patients.
E-consult has substantially increased within the VA since its implementation.5,6 Consistent with limited evaluations from other health care systems, evaluations of the VA e-consult program demonstrated reduced costs, reduced travel time for patients, and improved access to specialty care.2,5–11 However, there is wide variation in e-consult use across VA specialties, facilities, and regions.5,6,12,13 For example, hematology, preoperative evaluation, neurosurgery, endocrinology, and infectious diseases use e-consults more frequently when compared with in-person consults in the VA.6 Reasons for this variation or specific barriers and facilitators of using e-consults have not been described.
Prior qualitative studies report that primary care practitioners (PCPs) describe e-consults as convenient, educational, beneficial for patient care, and useful for improving patient access to specialty care.8,14,15 One study identified limited PCP knowledge of e-consults as a barrier to use.16 Specialists have reported that e-consult improves clinical communication, but increases their workload.1,14,17,18 These studies did not assess perspectives from both clinicians who initiate e-consults and those who respond to them. This is the first qualitative study to assess e-consult perceptions from perspectives of both PCPs and specialists among a large, national sample of VA clinicians who use e-consults. The objective of this study was to understand perspectives of e-consults between PCPs and specialists that may be relevant to increasing adoption in the VA.
Methods
The team (CL, ML, PG, 2 analysts under the guidance of GS and JS and support from RRK, and a biostatistician) conducted semistructured interviews with PCPs, specialists, and specialty division leaders who were employed by VA in 2016 and 2017. Specialties of interest were identified by the VA Office of Specialty Care and included cardiology, endocrinology, gastroenterology, and hematology.
E-Consult Procedures
Within the VA, the specific procedures used to initiate, triage and manage e-consults are coordinated at VA medical centers (VAMCs) and at the Veterans Integrated Service Network (VISN) regional level. E-consult can be requested by any clinician. Generally, e-consults are initiated by PCPs through standardized, specialty-specific templates. Recipients, typically specialists, respond by answering questions, suggesting additional testing and evaluation, or requesting an in-person visit. Communication is documented in the patient’s electronic health record (EHR). Specialists receive different levels of workload credit for responding to e-consults similar to a relative value unit reimbursement model. Training in the use of e-consults is available to practitioners but may vary at local and regional levels.
Recruitment
Our sample included PCPs, specialists, and specialty care division leaders. We first quantified e-consult rates (e-consults per 100 patient visits) between July 2016 and June 2017 at VA facilities within primary care and the 4 priority specialties and identified the 30 sites with the highest e-consult rates and 30 sites with the lowest e-consult rates. Sites with < 500 total visits, < 3 specialties, or without any e-consult visit during the study period were excluded. E-consult rates at community-based outpatient clinics were included with associated VAMCs. We then stratified PCPs by whether they were high or low users of e-consults (determined by the top and bottom users within each site) and credentials (MD vs nurse practitioner [NP] or physician assistant [PA]). Specialists were sampled based on their rate of use relative to colleagues within their site and the use rate of their division. We sampled division chiefs and individuals who had > 300 total visits and 1 e-consult during the study period. To recruit participants, the primary investigator sent an initial email and 2 reminder emails. The team followed up with respondents to schedule an interview.
Interview guides were designed to elicit rich descriptions of barriers and facilitators to e-consult use (eAppendix available at doi:10.12788/fp.0214). The team used the Practical Robust Implementation and Sustainability Model (PRISM), which considers factors along 6 domains for intervention planning, implementation, and sustainment.19 Telephone interviews lasted about 20 minutes and were conducted between September 2017 and March 2018. Interviews were recorded and transcribed verbatim.
Analysis
The team used an iterative, team-based, inductive/deductive approach to conventional content analysis.20,21 Initial code categories were created so that we could identify e-consult best practices—facilitators of e-consult that were recommended by both PCPs and specialists. Inductive codes or labels applied to identify meaningful quotations, phrases, or key terms were used to identify emergent ideas and were added throughout coding after discussion among team members. Consensus was reached using a team-based approach.21 Four analysts independently coded the same 3 transcripts and met to discuss points of divergence and convergence. Analyses continued with emergent themes, categories, and conclusions. Atlas.ti. v.7 was used for coding and data management.22
Results
We conducted 34 interviews with clinicians (Table 1) from 13 VISNs. Four best-practice themes emerged among both PCPs and specialists, including that e-consults (1) are best suited for certain clinical questions and patients; (2) require relevant background information from requesting clinicians and clear recommendations from responding clinicians; (3) are a novel opportunity to provide efficient, transparent care; and (4) may not be fully adopted due to low awareness. Supporting quotations for the following findings are provided in Table 2.
Specific Clinical Questions and Patients
PCPs described specific patients and questions for which they most frequently used e-consults, such as for medication changes (Q1), determining treatment steps (Q2,3), and or clarifying laboratory or imaging findings. PCPs frequently used e-consults for patients who did not require a physical examination or when specialists could make recommendations without seeing patients face-to-face (Q3). An important use of e-consults described by PCPs was for treating conditions they could manage within primary care if additional guidance were available (Q4). Several PCPs and specialists also noted that e-consults were particularly useful for patients who were unable to travel or did not want face-to-face appointments (Q5). Notably, PCPs and specialists mentioned situations for which e-consults were inappropriate, including when a detailed history or physical examination was needed, or if a complex condition was suspected (Q6).
Background Data and Clear Recommendations
Participants described necessary data that should be included in high-quality e-consults. Specialists voiced frustration in time-consuming chart reviews that were often necessary when these data were not provided by the requestor. In some cases, specialists were unable to access necessary EHR data, which delayed responses (Q7). PCPs noted that the most useful responses carefully considered the question, used current patient information to determine treatments, provided clear recommendations, and defined who was responsible for next steps (Q8). PCPs and specialists stated that e-consult templates that required relevant information facilitated high-quality e-consults. Neither wanted to waste the other clinician's time (Q8).
A Novel Opportunity
Many PCPs felt that e-consults improved communication (eg, efficiency, response time), established new communication between clinicians, and reduced patients’ appointment burden (Q10, Q11). Many specialists felt that e-consults improved documentation of communication between clinicians and increased transparency of clinical decisions (Q12). Additionally, many specialists mentioned that e-consults capture previously informal curbside consults, enabling them to receive workload credit (Q13).
Lack of Awareness
Some noted that the biggest barrier to e-consults was not being aware of them generally, or which specialties offer e-consults (Q14). One PCP described e-consults as the best kept secret and found value in sharing the utility of e-consults with colleagues (Q15). All participants, including those who did not frequently use e-consults, felt that e-consults improved the quality of care by providing more timely care or better answers to clinical questions (Q16). Several practitioners also felt that e-consults increased access to specialty care. For example, specialists reported that e-consults enabled them to better manage patient load by using e-consults to answer relatively simple questions, reserving face-to-face consults for more complex patients (Q17).
Discussion
The objective of this study was to identify potential best practices for e-consults that may help increase their quality and use within the VA. We built on prior studies that offered insights on PCP and specialists’ overall satisfaction with e-consult by identifying several themes relevant to the further adoption of e-consults in the VA and elsewhere without a face-to-face visit.8,13,14,16–18 Future work may be beneficial in identifying whether the study themes identified can explain variation in e-consult use or whether addressing these factors might lead to increased or higher quality e-consult use. We are unaware of any qualitative study of comparable scale in a different health care system. Further, this is the first study to assess perspectives on e-consults among those who initiate and respond to them within the same health care system. Perhaps the most important finding from this study is that e-consults are generally viewed favorably, which is a necessary leverage point to increase their adoption within the system.
Clinicians reported several benefits to e-consults, including timely responses to clinical questions, efficient communication, allow for documentation of specialist recommendations, and help capture workload. These benefits are consistent with prior literature that indicates both PCPs and specialists in the VA and other health care systems feel that e-consults improves communication, decreases unnecessary visits, and improves quality of care.1,14,17,18 In particular, clinicians reported that e-consults improve their practice efficiency and efficacy. This is of critical importance given the pressures of providing timely access to primary and specialty care within the VA. Interestingly, many VA practitioners were unaware which specialties offered e-consults within their facilities, reflecting previous work showing that PCPs are often unaware of e-consult options.16 This may partially explain variation in e-consult use. Increasing awareness and educating clinicians on the benefits of e-consults may help promote use among non- and low users.
A common theme reported by both groups was the importance of providing necessary information within e-consult questions and responses. Specialists felt there was a need to ensure that PCPs provide relevant and patient-specific information that would enable them to efficiently and accurately answer questions without the need for extensive EHR review. This reflects previous work showing that specialists are often unable to respond to e-consult requests because they do not contain sufficient information.22 PCPs described a need to ensure that specialists’ responses included information that was detailed enough to make clinical decisions without the need for a reconsult. This highlights a common challenge to medical consultation, in that necessary or relevant information may not be apparent to all clinicians. To address this, there may be a role in developing enhanced, flexible templating that elicits necessary patient-specific information. Such a template may automatically pull relevant data from the EHR and prompt clinicians to provide important information. We did not assess how perspectives of templates varied, and further work could help define precisely what constitutes an effective template, including how it should capture appropriate patient data and how this impacts acceptability or use of e-consults generally. Collaboratively developed service agreements and e-consult templates could help guide PCPs and specialists to engage in efficient communication.
Another theme among both groups was that e-consult is most appropriate within specific clinical scenarios. Examples included review of laboratory results, questions about medication changes, or for patients who were reluctant to travel to appointments. Identifying and promoting specific opportunities for e-consults may help increase their use and align e-consult practices with scenarios that are likely to provide the most benefit to patients. For example, it could be helpful to understand the distance patients must travel for specialty care. Providing that information during clinical encounters could trigger clinicians to consider e-consults as an option. Future work might aim to identify clinical scenarios that clinicians feel are not well suited for e-consults and determine how to adapt them for those scenarios.
Limitations
Generalizability of these findings is limited given the qualitative study design. Participants’ descriptions of experiences with e-consults reflect the experiences of clinicians in the VA and may not reflect clinicians in other settings. We also interviewed a sample of clinicians who were already using e-consults. Important information could be learned from future work with those who have not yet adopted e-consult procedures or adopted and abandoned them.
Conclusions
E-consult is perceived as beneficial by VA PCPs and specialists. Participants suggested using e-consults for appropriate questions or patients and including necessary information and next steps in both the initial e-consult and response. Finding ways to facilitate e-consults with these suggestions in mind may increase delivery of high-quality e-consults. Future work could compare the findings of this work to similar work assessing clinicians perceptions of e-consults outside of the VA.
1. Battaglia C, Lambert-Kerzner A, Aron DC, et al. Evaluation of e-consults in the VHA: provider perspectives. Fed Pract. 2015;32(7):42-48.
2. Haverhals LM, Sayre G, Helfrich CD, et al. E-consult implementation: lessons learned using consolidated framework for implementation research. Am J Manag Care. 2015;21(12):e640-e647. Published 2015 Dec 1.
3. Sewell JL, Telischak KS, Day LW, Kirschner N, Weissman A. Preconsultation exchange in the United States: use, awareness, and attitudes. Am J Manag Care. 2014;20(12):e556-e564. Published 2014 Dec 1.
4. Horner K, Wagner E, Tufano J. Electronic consultations between primary and specialty care clinicians: early insights. Issue Brief (Commonw Fund). 2011;23:1-14.
5. Kirsh S, Carey E, Aron DC, et al. Impact of a national specialty e-consultation implementation project on access. Am J Manag Care. 2015;21(12):e648-654. Published 2015 Dec 1.
6. Saxon DR, Kaboli PJ, Haraldsson B, Wilson C, Ohl M, Augustine MR. Growth of electronic consultations in the Veterans Health Administration. Am J Manag Care. 2021;27(1):12-19. doi:10.37765/ajmc.2021.88572
7. Olayiwola JN, Anderson D, Jepeal N, et al. Electronic consultations to improve the primary care-specialty care interface for cardiology in the medically underserved: a cluster-randomized controlled trial. Ann Fam Med. 2016;14(2):133-140. doi:10.1370/afm.1869
8. Schettini P, Shah KP, O’Leary CP, et al. Keeping care connected: e-Consultation program improves access to nephrology care. J Telemed Telecare. 2019;25(3):142-150. doi:10.1177/1357633X17748350
9. Whittington MD, Ho PM, Kirsh SR, et al. Cost savings associated with electronic specialty consultations. Am J Manag Care. 2021;27(1):e16-e23. Published 2021 Jan 1. doi:10.37765/ajmc.2021.88579
10. Shipherd JC, Kauth MR, Matza A. Nationwide interdisciplinary e-consultation on transgender care in the Veterans Health Administration. Telemed J E Health. 2016;22(12):1008-1012. doi:10.1089/tmj.2016.0013
11. Strymish J, Gupte G, Afable MK, et al. Electronic consultations (E-consults): advancing infectious disease care in a large Veterans Affairs Healthcare System. Clin Infect Dis. 2017;64(8):1123-1125. doi:10.1093/cid/cix058
12. Williams KM, Kirsh S, Aron D, et al. Evaluation of the Veterans Health Administration’s Specialty Care Transformational Initiatives to promote patient-centered delivery of specialty care: a mixed-methods approach. Telemed J E-Health. 2017;23(7):577-589. doi:10.1089/tmj.2016.0166
13. US Department of Veterans Affairs, Veterans Health Administration, Specialty Care Transformational Initiative Evaluation Center. Evaluation of specialty care initiatives. Published 2013.
14. Vimalananda VG, Gupte G, Seraj SM, et al. Electronic consultations (e-consults) to improve access to specialty care: a systematic review and narrative synthesis. J Telemed Telecare. 2015;21(6):323-330. doi:10.1177/1357633X15582108
15. Lee M, Leonard C, Greene P, et al. Perspectives of VA primary care clinicians toward electronic consultation-related workload burden. JAMA Netw Open. 2020;3(10):e2018104. Published 2020 Oct 1. doi:10.1001/jamanetworkopen.2020.18104
16. Deeds SA, Dowdell KJ, Chew LD, Ackerman SL. Implementing an opt-in eConsult program at seven academic medical centers: a qualitative analysis of primary care provider experiences. J Gen Intern Med. 2019;34(8):1427-1433. doi:10.1007/s11606-019-05067-7
17. Rodriguez KL, Burkitt KH, Bayliss NK, et al. Veteran, primary care provider, and specialist satisfaction with electronic consultation. JMIR Med Inform. 2015;3(1):e5. Published 2015 Jan 14. doi:10.2196/medinform.3725
18. Gupte G, Vimalananda V, Simon SR, DeVito K, Clark J, Orlander JD. Disruptive innovation: implementation of electronic consultations in a Veterans Affairs Health Care System. JMIR Med Inform. 2016;4(1):e6. Published 2016 Feb 12. doi:10.2196/medinform.4801
19. Feldstein AC, Glasgow RE. A practical, robust implementation and sustainability model (PRISM) for integrating research findings into practice. Jt Comm J Qual Patient Saf. 2008;34(4):228-243. doi:10.1016/s1553-7250(08)34030-6
20. Patton MQ. Qualitative Research and Evaluation Methods. 3rd ed. Sage Publications; 2002.
21. Bradley EH, Curry LA, Devers KJ. Qualitative data analysis for health services research: developing taxonomy, themes, and theory. Health Serv Res. 2007;42(4):1758-1772. doi:10.1111/j.1475-6773.2006.00684.x
22. Kim EJ, Orlander JD, Afable M, et al. Cardiology electronic consultation (e-consult) use by primary care providers at VA medical centres in New England. J Telemed Telecare. 2019;25(6):370-377. doi:10.1177/1357633X18774468
1. Battaglia C, Lambert-Kerzner A, Aron DC, et al. Evaluation of e-consults in the VHA: provider perspectives. Fed Pract. 2015;32(7):42-48.
2. Haverhals LM, Sayre G, Helfrich CD, et al. E-consult implementation: lessons learned using consolidated framework for implementation research. Am J Manag Care. 2015;21(12):e640-e647. Published 2015 Dec 1.
3. Sewell JL, Telischak KS, Day LW, Kirschner N, Weissman A. Preconsultation exchange in the United States: use, awareness, and attitudes. Am J Manag Care. 2014;20(12):e556-e564. Published 2014 Dec 1.
4. Horner K, Wagner E, Tufano J. Electronic consultations between primary and specialty care clinicians: early insights. Issue Brief (Commonw Fund). 2011;23:1-14.
5. Kirsh S, Carey E, Aron DC, et al. Impact of a national specialty e-consultation implementation project on access. Am J Manag Care. 2015;21(12):e648-654. Published 2015 Dec 1.
6. Saxon DR, Kaboli PJ, Haraldsson B, Wilson C, Ohl M, Augustine MR. Growth of electronic consultations in the Veterans Health Administration. Am J Manag Care. 2021;27(1):12-19. doi:10.37765/ajmc.2021.88572
7. Olayiwola JN, Anderson D, Jepeal N, et al. Electronic consultations to improve the primary care-specialty care interface for cardiology in the medically underserved: a cluster-randomized controlled trial. Ann Fam Med. 2016;14(2):133-140. doi:10.1370/afm.1869
8. Schettini P, Shah KP, O’Leary CP, et al. Keeping care connected: e-Consultation program improves access to nephrology care. J Telemed Telecare. 2019;25(3):142-150. doi:10.1177/1357633X17748350
9. Whittington MD, Ho PM, Kirsh SR, et al. Cost savings associated with electronic specialty consultations. Am J Manag Care. 2021;27(1):e16-e23. Published 2021 Jan 1. doi:10.37765/ajmc.2021.88579
10. Shipherd JC, Kauth MR, Matza A. Nationwide interdisciplinary e-consultation on transgender care in the Veterans Health Administration. Telemed J E Health. 2016;22(12):1008-1012. doi:10.1089/tmj.2016.0013
11. Strymish J, Gupte G, Afable MK, et al. Electronic consultations (E-consults): advancing infectious disease care in a large Veterans Affairs Healthcare System. Clin Infect Dis. 2017;64(8):1123-1125. doi:10.1093/cid/cix058
12. Williams KM, Kirsh S, Aron D, et al. Evaluation of the Veterans Health Administration’s Specialty Care Transformational Initiatives to promote patient-centered delivery of specialty care: a mixed-methods approach. Telemed J E-Health. 2017;23(7):577-589. doi:10.1089/tmj.2016.0166
13. US Department of Veterans Affairs, Veterans Health Administration, Specialty Care Transformational Initiative Evaluation Center. Evaluation of specialty care initiatives. Published 2013.
14. Vimalananda VG, Gupte G, Seraj SM, et al. Electronic consultations (e-consults) to improve access to specialty care: a systematic review and narrative synthesis. J Telemed Telecare. 2015;21(6):323-330. doi:10.1177/1357633X15582108
15. Lee M, Leonard C, Greene P, et al. Perspectives of VA primary care clinicians toward electronic consultation-related workload burden. JAMA Netw Open. 2020;3(10):e2018104. Published 2020 Oct 1. doi:10.1001/jamanetworkopen.2020.18104
16. Deeds SA, Dowdell KJ, Chew LD, Ackerman SL. Implementing an opt-in eConsult program at seven academic medical centers: a qualitative analysis of primary care provider experiences. J Gen Intern Med. 2019;34(8):1427-1433. doi:10.1007/s11606-019-05067-7
17. Rodriguez KL, Burkitt KH, Bayliss NK, et al. Veteran, primary care provider, and specialist satisfaction with electronic consultation. JMIR Med Inform. 2015;3(1):e5. Published 2015 Jan 14. doi:10.2196/medinform.3725
18. Gupte G, Vimalananda V, Simon SR, DeVito K, Clark J, Orlander JD. Disruptive innovation: implementation of electronic consultations in a Veterans Affairs Health Care System. JMIR Med Inform. 2016;4(1):e6. Published 2016 Feb 12. doi:10.2196/medinform.4801
19. Feldstein AC, Glasgow RE. A practical, robust implementation and sustainability model (PRISM) for integrating research findings into practice. Jt Comm J Qual Patient Saf. 2008;34(4):228-243. doi:10.1016/s1553-7250(08)34030-6
20. Patton MQ. Qualitative Research and Evaluation Methods. 3rd ed. Sage Publications; 2002.
21. Bradley EH, Curry LA, Devers KJ. Qualitative data analysis for health services research: developing taxonomy, themes, and theory. Health Serv Res. 2007;42(4):1758-1772. doi:10.1111/j.1475-6773.2006.00684.x
22. Kim EJ, Orlander JD, Afable M, et al. Cardiology electronic consultation (e-consult) use by primary care providers at VA medical centres in New England. J Telemed Telecare. 2019;25(6):370-377. doi:10.1177/1357633X18774468
Therapeutic aquatic exercise superior to physical therapy for back pain in study
“This is the first study to compare the efficacy of therapeutic aquatic exercise and physical therapy modalities in the treatment of chronic low back pain,” senior coauthors Pei-Jie Chen, PhD and Xue-Qiang Wang, PhD, both of the department of sport rehabilitation, Shanghai (China) University of Sport, wrote in JAMA Network Open. “Therapeutic aquatic exercise is a safe treatment for chronic low back pain and most participants who received it were willing to recommend it to other patients with chronic low back pain.”
As compared with individuals in the physical therapy modalities arm, the therapeutic aquatic exercise experienced greater relief of disability at all time points assessed: after the 3-month intervention, at the 6-month follow-up, and at the 12-month follow-up.
Commenting on the study, Linda Girgis, MD, FAAFP, a family physician in private practice in South River, N.J., agreed that aquatic therapy is a great tool for many chronic low back patients. “It helps them get active for one and do things that may exacerbate their symptoms doing the same exercises on land,” noted Dr. Girgis, who also is a clinical assistant professor at Robert Wood Johnson Medical School, New Brunswick.
She pointed out that access to a pool can be a problem. “But I have found a few physical therapy places in my area that do have access to a pool, and I refer appropriate patients there,” added Dr. Girgis, who was not involved with the study. “I have also found it works well for other types of pain, such as knee and hip pain. It is not for everyone but I have seen some patients get great benefit from it when they didn’t get any with traditional physical therapy.”
Aquatic therapy was more beneficial
Low back pain is a common condition, and clinical practice guidelines currently recommend therapeutic exercise and physical therapy modalities. Among the modalities that are available, therapeutic aquatic exercise is often prescribed for chronic low back pain, and it is becoming increasingly popular for treatment of chronic low back pain, the authors stated in their paper. The authors noted that water is an ideal environment for conducting an exercise program given its various properties, including buoyancy pressure, density, thermal capacity, and conductivity.
Two previously published systematic reviews have suggested that therapeutic aquatic exercise may be able to reduce the intensity of back pain and improve function in this population. But to date, evidence regarding long-term benefits in patients with chronic low back pain is very limited and there haven’t been any studies comparing the efficacy of therapeutic aquatic exercise and physical therapy modalities for chronic low back pain, according to the authors.
In this study, 113 individuals with chronic low back pain were randomized to either therapeutic aquatic exercise or to physical therapy, with an endpoint of efficacy regarding disability. This was measured using the Roland-Morris Disability Questionnaire.
Scores ranged from 0 to 24, with higher scores indicating more severe disability. Secondary endpoints included pain intensity, quality of life, sleep quality, and recommendation of intervention, and these were rated using various standardized tools.
Those randomized to the therapeutic aquatic exercise group had about an hour of therapy, beginning with a 10-minute active warm-up session to enhance neuromuscular activation, then an exercise session for 40 minutes followed by a 10-minute cooldown.
The physical therapy group received transcutaneous electrical nerve stimulation and infrared ray thermal therapy, also for 60 minutes. Both groups received these interventions twice a week for 3 months.
The overall mean age of the cohort was 31.0 years, and they were almost evenly divided by gender; 54 were men (47.8%), and 59 were women (52.2%).
As compared with the physical therapy group, individuals participating in therapeutic aquatic exercise group showed improvement in disability by an additional −1.77 points (95% confidence interval, −3.02 to −0.51) at the end of the 3-month intervention; at 6 months it was −2.42 points (95% CI, −4.13 to −0.70) and −3.61 points (95% CI, −5.63 to −1.58) at the 12-month follow-up (P < .001 for overall group x time interaction).
Functional improvement did not appear to be significantly affected by confounders that included age, sex, body mass index, low back pain duration, educational level, or pain level.
For secondary outcomes, those in the therapeutic aquatic exercise group demonstrated improvement in the most severe pain by an additional −0.79 points (95% CI, −1.31 to −0.27) after the 3-month intervention, −1.34 points (95% CI, −2.06 to −0.62) at 6 months, and −2.04 points (95% CI, −2.75 to −1.34) at the 12-month follow-up (P < .001 for overall group x time interaction), as compared with the physical therapy group. All pain scores differed significantly between the two groups at every time point.
In addition, individuals in the therapeutic aquatic exercise group showed more improvements on the 36-item Short-form Health Survey (overall group x time interaction, P = .003), Pittsburgh Sleep Quality Index (overall group x time interaction, P = .02), Tampa Scale for Kinesiophobia (overall group x time interaction, P < .001), and Fear-Avoidance Beliefs Questionnaire (physical activity subscale overall group x time interaction, P = .04), as compared with the physical therapy group. These improvements were also not influenced by confounders.
Finally, at the 12-month follow-up point, those in the aquatic therapy group had significantly greater improvements in the number of participants who met the minimal clinically important difference in pain (at least a 2-point improvement on the numeric rating scale).
More outside experts’ takes
“The current research evidence does suggest indeed that aquatic exercise therapy is suitable and often better than land exercise, passive relaxation, or other treatments for many people with low back pain,” commented Stelios Psycharakis PhD, senior lecturer in biomechanics, Institute for Sport, Physical Education and Health Sciences, University of Edinburgh.
He also noted that since low back pain is an issue affecting about 80% of all people at some stage of their life, it is “improbable that one could identify a single type of treatment or exercise therapy that would be suitable for every person with this problem.”
Dr. Psycharakis pointed out that there are also some contraindications for aquatic therapy, such as incontinence and skin conditions. “Other than that though, clinicians should definitely consider aquatic exercise therapy when advising people with chronic low back pain,” he said.
Justin M. Lantz, DPT, agreed that the study showed therapeutic aquatic exercise appears to be safe and beneficial in some patients with chronic low back pain, but he also shared limitations of the new research.
“The study has notable limitations as it did not include patients above 65 years old, pain levels were generally low for the subjects involved, and it did not include a treatment group with land therapeutic exercise – so it is difficult to determine if the beneficial effects reported were due to active exercise or because the exercises were performed in water,” said Dr. Lantz, director of the spine physical therapy fellowship program at the University of Southern California, Los Angeles, and an assistant professor of clinical physical therapy.
He also pointed out that, since active exercise has been shown to be beneficial and is advocated in multiple clinical practice guidelines for chronic low back pain, “it would be helpful to determine if the true effects on pain and disability were due to the water environment or the effect of active exercise itself.”
“Due to the significant positive long-term effects and limited adverse events reported, I believe this study supports the use of therapeutic aquatic exercise in select patient populations with chronic low back pain and should be considered as a part of a rehabilitation treatment plan if accessibility is feasible,” Dr. Lantz said.
The authors of the paper, Dr. Girgis, and Dr. Psycharakis had no conflicts of interest. Justin Lantz is a physical therapy consultant to SI-Bone.
“This is the first study to compare the efficacy of therapeutic aquatic exercise and physical therapy modalities in the treatment of chronic low back pain,” senior coauthors Pei-Jie Chen, PhD and Xue-Qiang Wang, PhD, both of the department of sport rehabilitation, Shanghai (China) University of Sport, wrote in JAMA Network Open. “Therapeutic aquatic exercise is a safe treatment for chronic low back pain and most participants who received it were willing to recommend it to other patients with chronic low back pain.”
As compared with individuals in the physical therapy modalities arm, the therapeutic aquatic exercise experienced greater relief of disability at all time points assessed: after the 3-month intervention, at the 6-month follow-up, and at the 12-month follow-up.
Commenting on the study, Linda Girgis, MD, FAAFP, a family physician in private practice in South River, N.J., agreed that aquatic therapy is a great tool for many chronic low back patients. “It helps them get active for one and do things that may exacerbate their symptoms doing the same exercises on land,” noted Dr. Girgis, who also is a clinical assistant professor at Robert Wood Johnson Medical School, New Brunswick.
She pointed out that access to a pool can be a problem. “But I have found a few physical therapy places in my area that do have access to a pool, and I refer appropriate patients there,” added Dr. Girgis, who was not involved with the study. “I have also found it works well for other types of pain, such as knee and hip pain. It is not for everyone but I have seen some patients get great benefit from it when they didn’t get any with traditional physical therapy.”
Aquatic therapy was more beneficial
Low back pain is a common condition, and clinical practice guidelines currently recommend therapeutic exercise and physical therapy modalities. Among the modalities that are available, therapeutic aquatic exercise is often prescribed for chronic low back pain, and it is becoming increasingly popular for treatment of chronic low back pain, the authors stated in their paper. The authors noted that water is an ideal environment for conducting an exercise program given its various properties, including buoyancy pressure, density, thermal capacity, and conductivity.
Two previously published systematic reviews have suggested that therapeutic aquatic exercise may be able to reduce the intensity of back pain and improve function in this population. But to date, evidence regarding long-term benefits in patients with chronic low back pain is very limited and there haven’t been any studies comparing the efficacy of therapeutic aquatic exercise and physical therapy modalities for chronic low back pain, according to the authors.
In this study, 113 individuals with chronic low back pain were randomized to either therapeutic aquatic exercise or to physical therapy, with an endpoint of efficacy regarding disability. This was measured using the Roland-Morris Disability Questionnaire.
Scores ranged from 0 to 24, with higher scores indicating more severe disability. Secondary endpoints included pain intensity, quality of life, sleep quality, and recommendation of intervention, and these were rated using various standardized tools.
Those randomized to the therapeutic aquatic exercise group had about an hour of therapy, beginning with a 10-minute active warm-up session to enhance neuromuscular activation, then an exercise session for 40 minutes followed by a 10-minute cooldown.
The physical therapy group received transcutaneous electrical nerve stimulation and infrared ray thermal therapy, also for 60 minutes. Both groups received these interventions twice a week for 3 months.
The overall mean age of the cohort was 31.0 years, and they were almost evenly divided by gender; 54 were men (47.8%), and 59 were women (52.2%).
As compared with the physical therapy group, individuals participating in therapeutic aquatic exercise group showed improvement in disability by an additional −1.77 points (95% confidence interval, −3.02 to −0.51) at the end of the 3-month intervention; at 6 months it was −2.42 points (95% CI, −4.13 to −0.70) and −3.61 points (95% CI, −5.63 to −1.58) at the 12-month follow-up (P < .001 for overall group x time interaction).
Functional improvement did not appear to be significantly affected by confounders that included age, sex, body mass index, low back pain duration, educational level, or pain level.
For secondary outcomes, those in the therapeutic aquatic exercise group demonstrated improvement in the most severe pain by an additional −0.79 points (95% CI, −1.31 to −0.27) after the 3-month intervention, −1.34 points (95% CI, −2.06 to −0.62) at 6 months, and −2.04 points (95% CI, −2.75 to −1.34) at the 12-month follow-up (P < .001 for overall group x time interaction), as compared with the physical therapy group. All pain scores differed significantly between the two groups at every time point.
In addition, individuals in the therapeutic aquatic exercise group showed more improvements on the 36-item Short-form Health Survey (overall group x time interaction, P = .003), Pittsburgh Sleep Quality Index (overall group x time interaction, P = .02), Tampa Scale for Kinesiophobia (overall group x time interaction, P < .001), and Fear-Avoidance Beliefs Questionnaire (physical activity subscale overall group x time interaction, P = .04), as compared with the physical therapy group. These improvements were also not influenced by confounders.
Finally, at the 12-month follow-up point, those in the aquatic therapy group had significantly greater improvements in the number of participants who met the minimal clinically important difference in pain (at least a 2-point improvement on the numeric rating scale).
More outside experts’ takes
“The current research evidence does suggest indeed that aquatic exercise therapy is suitable and often better than land exercise, passive relaxation, or other treatments for many people with low back pain,” commented Stelios Psycharakis PhD, senior lecturer in biomechanics, Institute for Sport, Physical Education and Health Sciences, University of Edinburgh.
He also noted that since low back pain is an issue affecting about 80% of all people at some stage of their life, it is “improbable that one could identify a single type of treatment or exercise therapy that would be suitable for every person with this problem.”
Dr. Psycharakis pointed out that there are also some contraindications for aquatic therapy, such as incontinence and skin conditions. “Other than that though, clinicians should definitely consider aquatic exercise therapy when advising people with chronic low back pain,” he said.
Justin M. Lantz, DPT, agreed that the study showed therapeutic aquatic exercise appears to be safe and beneficial in some patients with chronic low back pain, but he also shared limitations of the new research.
“The study has notable limitations as it did not include patients above 65 years old, pain levels were generally low for the subjects involved, and it did not include a treatment group with land therapeutic exercise – so it is difficult to determine if the beneficial effects reported were due to active exercise or because the exercises were performed in water,” said Dr. Lantz, director of the spine physical therapy fellowship program at the University of Southern California, Los Angeles, and an assistant professor of clinical physical therapy.
He also pointed out that, since active exercise has been shown to be beneficial and is advocated in multiple clinical practice guidelines for chronic low back pain, “it would be helpful to determine if the true effects on pain and disability were due to the water environment or the effect of active exercise itself.”
“Due to the significant positive long-term effects and limited adverse events reported, I believe this study supports the use of therapeutic aquatic exercise in select patient populations with chronic low back pain and should be considered as a part of a rehabilitation treatment plan if accessibility is feasible,” Dr. Lantz said.
The authors of the paper, Dr. Girgis, and Dr. Psycharakis had no conflicts of interest. Justin Lantz is a physical therapy consultant to SI-Bone.
“This is the first study to compare the efficacy of therapeutic aquatic exercise and physical therapy modalities in the treatment of chronic low back pain,” senior coauthors Pei-Jie Chen, PhD and Xue-Qiang Wang, PhD, both of the department of sport rehabilitation, Shanghai (China) University of Sport, wrote in JAMA Network Open. “Therapeutic aquatic exercise is a safe treatment for chronic low back pain and most participants who received it were willing to recommend it to other patients with chronic low back pain.”
As compared with individuals in the physical therapy modalities arm, the therapeutic aquatic exercise experienced greater relief of disability at all time points assessed: after the 3-month intervention, at the 6-month follow-up, and at the 12-month follow-up.
Commenting on the study, Linda Girgis, MD, FAAFP, a family physician in private practice in South River, N.J., agreed that aquatic therapy is a great tool for many chronic low back patients. “It helps them get active for one and do things that may exacerbate their symptoms doing the same exercises on land,” noted Dr. Girgis, who also is a clinical assistant professor at Robert Wood Johnson Medical School, New Brunswick.
She pointed out that access to a pool can be a problem. “But I have found a few physical therapy places in my area that do have access to a pool, and I refer appropriate patients there,” added Dr. Girgis, who was not involved with the study. “I have also found it works well for other types of pain, such as knee and hip pain. It is not for everyone but I have seen some patients get great benefit from it when they didn’t get any with traditional physical therapy.”
Aquatic therapy was more beneficial
Low back pain is a common condition, and clinical practice guidelines currently recommend therapeutic exercise and physical therapy modalities. Among the modalities that are available, therapeutic aquatic exercise is often prescribed for chronic low back pain, and it is becoming increasingly popular for treatment of chronic low back pain, the authors stated in their paper. The authors noted that water is an ideal environment for conducting an exercise program given its various properties, including buoyancy pressure, density, thermal capacity, and conductivity.
Two previously published systematic reviews have suggested that therapeutic aquatic exercise may be able to reduce the intensity of back pain and improve function in this population. But to date, evidence regarding long-term benefits in patients with chronic low back pain is very limited and there haven’t been any studies comparing the efficacy of therapeutic aquatic exercise and physical therapy modalities for chronic low back pain, according to the authors.
In this study, 113 individuals with chronic low back pain were randomized to either therapeutic aquatic exercise or to physical therapy, with an endpoint of efficacy regarding disability. This was measured using the Roland-Morris Disability Questionnaire.
Scores ranged from 0 to 24, with higher scores indicating more severe disability. Secondary endpoints included pain intensity, quality of life, sleep quality, and recommendation of intervention, and these were rated using various standardized tools.
Those randomized to the therapeutic aquatic exercise group had about an hour of therapy, beginning with a 10-minute active warm-up session to enhance neuromuscular activation, then an exercise session for 40 minutes followed by a 10-minute cooldown.
The physical therapy group received transcutaneous electrical nerve stimulation and infrared ray thermal therapy, also for 60 minutes. Both groups received these interventions twice a week for 3 months.
The overall mean age of the cohort was 31.0 years, and they were almost evenly divided by gender; 54 were men (47.8%), and 59 were women (52.2%).
As compared with the physical therapy group, individuals participating in therapeutic aquatic exercise group showed improvement in disability by an additional −1.77 points (95% confidence interval, −3.02 to −0.51) at the end of the 3-month intervention; at 6 months it was −2.42 points (95% CI, −4.13 to −0.70) and −3.61 points (95% CI, −5.63 to −1.58) at the 12-month follow-up (P < .001 for overall group x time interaction).
Functional improvement did not appear to be significantly affected by confounders that included age, sex, body mass index, low back pain duration, educational level, or pain level.
For secondary outcomes, those in the therapeutic aquatic exercise group demonstrated improvement in the most severe pain by an additional −0.79 points (95% CI, −1.31 to −0.27) after the 3-month intervention, −1.34 points (95% CI, −2.06 to −0.62) at 6 months, and −2.04 points (95% CI, −2.75 to −1.34) at the 12-month follow-up (P < .001 for overall group x time interaction), as compared with the physical therapy group. All pain scores differed significantly between the two groups at every time point.
In addition, individuals in the therapeutic aquatic exercise group showed more improvements on the 36-item Short-form Health Survey (overall group x time interaction, P = .003), Pittsburgh Sleep Quality Index (overall group x time interaction, P = .02), Tampa Scale for Kinesiophobia (overall group x time interaction, P < .001), and Fear-Avoidance Beliefs Questionnaire (physical activity subscale overall group x time interaction, P = .04), as compared with the physical therapy group. These improvements were also not influenced by confounders.
Finally, at the 12-month follow-up point, those in the aquatic therapy group had significantly greater improvements in the number of participants who met the minimal clinically important difference in pain (at least a 2-point improvement on the numeric rating scale).
More outside experts’ takes
“The current research evidence does suggest indeed that aquatic exercise therapy is suitable and often better than land exercise, passive relaxation, or other treatments for many people with low back pain,” commented Stelios Psycharakis PhD, senior lecturer in biomechanics, Institute for Sport, Physical Education and Health Sciences, University of Edinburgh.
He also noted that since low back pain is an issue affecting about 80% of all people at some stage of their life, it is “improbable that one could identify a single type of treatment or exercise therapy that would be suitable for every person with this problem.”
Dr. Psycharakis pointed out that there are also some contraindications for aquatic therapy, such as incontinence and skin conditions. “Other than that though, clinicians should definitely consider aquatic exercise therapy when advising people with chronic low back pain,” he said.
Justin M. Lantz, DPT, agreed that the study showed therapeutic aquatic exercise appears to be safe and beneficial in some patients with chronic low back pain, but he also shared limitations of the new research.
“The study has notable limitations as it did not include patients above 65 years old, pain levels were generally low for the subjects involved, and it did not include a treatment group with land therapeutic exercise – so it is difficult to determine if the beneficial effects reported were due to active exercise or because the exercises were performed in water,” said Dr. Lantz, director of the spine physical therapy fellowship program at the University of Southern California, Los Angeles, and an assistant professor of clinical physical therapy.
He also pointed out that, since active exercise has been shown to be beneficial and is advocated in multiple clinical practice guidelines for chronic low back pain, “it would be helpful to determine if the true effects on pain and disability were due to the water environment or the effect of active exercise itself.”
“Due to the significant positive long-term effects and limited adverse events reported, I believe this study supports the use of therapeutic aquatic exercise in select patient populations with chronic low back pain and should be considered as a part of a rehabilitation treatment plan if accessibility is feasible,” Dr. Lantz said.
The authors of the paper, Dr. Girgis, and Dr. Psycharakis had no conflicts of interest. Justin Lantz is a physical therapy consultant to SI-Bone.
FROM JAMA NETWORK OPEN
A Simple Message
I do not usually have difficulty writing editorials. However, this month was different. I kept coming up with grand ideas that flopped. First, I thought I would write a column entitled, “For What Should We Hope For?” When I started exploring the concept of hope, I quickly learned that there was extensive literature from multiple disciplines and even several centers and research projects dedicated to studying it.1 It seemed unlikely that I would have anything worthwhile to add to that literature. Then I thought I would discuss new year’s resolutions for federal practitioners. There was not much written about that topic, yet it seemed to be overly self-indulgent and superficial to discuss eating less and exercising more amid a pandemic and a climate change crisis. Finally, I wanted to opine on the futility of telling people to be resilient when we are all exhausted and demoralized, and yet that seemed too ponderous and paradoxical for our beleaguered state. With the third strike, I finally realized I was trying too hard. And perhaps that was exactly what I needed to say, at least to myself, and maybe some readers would benefit from reading that simple message as well.
I was surprised—though I probably should not have been given the explosion of media—to find that Americans were surveyed about what months they hate most. A 2021 poll of more than 15,000 adults found that January was the most disliked month.2 It’s not hard to figure out why. Characterized by a postholiday let down, these months in the middle of winter marked by either too much precipitation or if you live in the West not enough; short days and gray nights that are dark and cold. It is a long time to wait before spring with few holidays to break up the quotidian routine of work and school. January is a hard enough month in a good or even ordinary year. And 2022 is shaping up to be neither. We are entering the third year of a prolonged pandemic. Every time we have hope we are coming to the end of this long ordeal or at least things are moving toward normality, a new variant emerges, and we are back to living in fear and uncertainty.
COVID-19 is only the most relentless and deadly of our current disasters: There are rumors of wars, tornadoes, droughts, floods, shootings in schools and churches, political turmoil, and police violence. American society and the very planet seem to be in a perilous situation more than ever. No wonder then, that in the last month, several people have asked me, “Do you think this is the end of the world?” I suppose they think I am so old that I have become wise. And though I should cite a brilliant philosopher or renowned theologian: I am going to revert to my youth as a rock musician and quote R.E.M.: “It is the end of the world as we know it.” And “most of us do not feel fine!”
The world of 2022 is far more constricted and confined than it was before we heard the word COVID-19. We have less freedom of movement and fewer opportunities for companionship and gathering, for advancement and enjoyment. To thrive, and even to survive, in this cramped existence of limited possibilities, we need different values and attitudes than those that made us happy and successful in the open, hurried world before 2019. No generation since World War II has confronted such shortages of automobiles, paper goods, food, and even medicines as we have.
That is the first of the important simple messages I want to convey. Find something to be grateful for: your loved ones, your companion animals, your friends. Cherish the rainy or sunny day depending on how your climate has changed. Treasure the most basic and enduring pleasures, homemade cookies, favorite music, talking to a good friend even virtually, reading an actual book on a Sunday afternoon. These are things even the pandemic cannot take away from us unless we let our own inability to accept the conditions of our time ruin even what the meager, harsh Master of History has spared us.
The second of these simple messages is even more essential to finding any peace or joy in our current tense and somber existence: to show compassion for others and kindness to yourself. The most consistent report I have heard from people all over the country is that their fellow citizens are angry and selfish. We all understand, and even in some measure empathize with this the frustration and impatience with all the extraordinary pressure of having to function under these challenging conditions. Though we can take it out on the stranger at the grocery store or the family of the patient who has different views of masks and vaccines; it likely will not make the line shorter, the family any less demanding or seemingly unreasonable and probably will waste the little energy we have left to get home with the groceries or take care of the patient.
You never know what burden the person annoying you is carrying; it may perhaps be heavier than yours. And how we react to each other makes the weight of world weariness we all bear either easier or harder to shoulder. It sounds trite and trivial to say, yet tell people you care, and value, and love them. Although no less than Pope Francis in a Christmas present to marriages under strain from the stress of the pandemic that the 3 key words to remember are please, sorry, and thank you.3 I am applying that sage advice liberally to all relationships and interactions in the daily grind of work and home. The cost is little, the reward priceless.
It is good and right to have high hopes. We all need to take care of ourselves, whether we make resolutions to do so or not. Though more than anything else what we need is to be kind to ourselves. It is presumptuous of me to tell you what wellness means for your individual struggle, as it is inhuman of me to deign to tell you to be resilient when many of you face intolerable working conditions.4 As Jackson Browne sang in “Rock Me on the Water”, “Everyone must have some thought that’s going to pull them through somehow. Find your own thought, the reason you keep getting up and going to care for patients who increasingly respond with the rage of denial and resentment. Amid what morally distressed public health professionals have called so many unnecessary deaths,choose what gives you reason to keep serving that other side of this life full of healing.5 And if like so many of my fellow health care professionals, you are so spent and bent, that you feel that you can no longer practice without becoming someone you do not want to be, then let go with grace, get the help you deserve and perhaps one day when rested and mended, find another way to give.6
I rarely self-disclose but I want to end this column with a personal story that exemplifies more than all these words living this simple message. My spouse is a health care practitioner at a Veterans Affairs medical center. Like all of you on the front lines they work far too long hours in difficult conditions, with challenging patients and not enough staff to care for them. My partner had not an hour to get any gifts for me or our furry children. On Christmas Eve, before a long shift, they went to a packed Walgreens to buy our huskies each a toy and me a pair of fuzzy slippers. We sat by the tree and opened the hastily wrapped packages, and nothing could have been more memorable or meaningful. All of us at Federal Practitioner wish you, our readers, find in 2022 many such moments to sustain you.
1. The Center for the Advanced Study and Practice of Hope. T Denny Sanford School of Social and Family Dynamics, Arizona State University. Accessed January 3, 2022. https://thesanfordschool.asu.edu/research/centers-initiatives/hope-center
2. Ballard J. What is America’s favorite (and least favorite) month?” Published March 1, 2021. Accessed January 3, 2022. https://today.yougov.com/topics/lifestyle/articles-reports/2021/03/01/favorite-least-favorite-month-poll
3. Winlfield N. Pope’s 3 key words for a marriage: ‘please, thanks sorry.’ Associated Press. December 26, 2021. Accessed January 3, 2022. https://apnews.com/article/pope-francis-lifestyle-religion-relationships-couples-23c81169982e50c35d1c1fc7bfef8cbc
4. Dineen K. Why resilience isn’t always the answer to coping with challenging times. Published September 29, 2020. Accessed January 3, 2022. https://theconversation.com/why-resilience-isnt-always-the-answer-to-coping-with-challenging-times-145796
5. Caldwell T. ‘Everyone of those deaths is unnecessary,’ expert says of rising COVID-19 U.S. death toll as tens of millions remain unvaccinated. Published October 3, 2021. Accessed December 29, 2021. https://www.cnn.com/2021/10/03/health/us-coronavirus-sunday/index.html 6. Yong E. Why healthcare professionals are quitting in droves. The Atlantic. November 16, 2021. Accessed December 29, 2021. https://www.theatlantic.com/health/archive/2021/11/the-mass-exodus-of-americas-health-care-workers/620713/
I do not usually have difficulty writing editorials. However, this month was different. I kept coming up with grand ideas that flopped. First, I thought I would write a column entitled, “For What Should We Hope For?” When I started exploring the concept of hope, I quickly learned that there was extensive literature from multiple disciplines and even several centers and research projects dedicated to studying it.1 It seemed unlikely that I would have anything worthwhile to add to that literature. Then I thought I would discuss new year’s resolutions for federal practitioners. There was not much written about that topic, yet it seemed to be overly self-indulgent and superficial to discuss eating less and exercising more amid a pandemic and a climate change crisis. Finally, I wanted to opine on the futility of telling people to be resilient when we are all exhausted and demoralized, and yet that seemed too ponderous and paradoxical for our beleaguered state. With the third strike, I finally realized I was trying too hard. And perhaps that was exactly what I needed to say, at least to myself, and maybe some readers would benefit from reading that simple message as well.
I was surprised—though I probably should not have been given the explosion of media—to find that Americans were surveyed about what months they hate most. A 2021 poll of more than 15,000 adults found that January was the most disliked month.2 It’s not hard to figure out why. Characterized by a postholiday let down, these months in the middle of winter marked by either too much precipitation or if you live in the West not enough; short days and gray nights that are dark and cold. It is a long time to wait before spring with few holidays to break up the quotidian routine of work and school. January is a hard enough month in a good or even ordinary year. And 2022 is shaping up to be neither. We are entering the third year of a prolonged pandemic. Every time we have hope we are coming to the end of this long ordeal or at least things are moving toward normality, a new variant emerges, and we are back to living in fear and uncertainty.
COVID-19 is only the most relentless and deadly of our current disasters: There are rumors of wars, tornadoes, droughts, floods, shootings in schools and churches, political turmoil, and police violence. American society and the very planet seem to be in a perilous situation more than ever. No wonder then, that in the last month, several people have asked me, “Do you think this is the end of the world?” I suppose they think I am so old that I have become wise. And though I should cite a brilliant philosopher or renowned theologian: I am going to revert to my youth as a rock musician and quote R.E.M.: “It is the end of the world as we know it.” And “most of us do not feel fine!”
The world of 2022 is far more constricted and confined than it was before we heard the word COVID-19. We have less freedom of movement and fewer opportunities for companionship and gathering, for advancement and enjoyment. To thrive, and even to survive, in this cramped existence of limited possibilities, we need different values and attitudes than those that made us happy and successful in the open, hurried world before 2019. No generation since World War II has confronted such shortages of automobiles, paper goods, food, and even medicines as we have.
That is the first of the important simple messages I want to convey. Find something to be grateful for: your loved ones, your companion animals, your friends. Cherish the rainy or sunny day depending on how your climate has changed. Treasure the most basic and enduring pleasures, homemade cookies, favorite music, talking to a good friend even virtually, reading an actual book on a Sunday afternoon. These are things even the pandemic cannot take away from us unless we let our own inability to accept the conditions of our time ruin even what the meager, harsh Master of History has spared us.
The second of these simple messages is even more essential to finding any peace or joy in our current tense and somber existence: to show compassion for others and kindness to yourself. The most consistent report I have heard from people all over the country is that their fellow citizens are angry and selfish. We all understand, and even in some measure empathize with this the frustration and impatience with all the extraordinary pressure of having to function under these challenging conditions. Though we can take it out on the stranger at the grocery store or the family of the patient who has different views of masks and vaccines; it likely will not make the line shorter, the family any less demanding or seemingly unreasonable and probably will waste the little energy we have left to get home with the groceries or take care of the patient.
You never know what burden the person annoying you is carrying; it may perhaps be heavier than yours. And how we react to each other makes the weight of world weariness we all bear either easier or harder to shoulder. It sounds trite and trivial to say, yet tell people you care, and value, and love them. Although no less than Pope Francis in a Christmas present to marriages under strain from the stress of the pandemic that the 3 key words to remember are please, sorry, and thank you.3 I am applying that sage advice liberally to all relationships and interactions in the daily grind of work and home. The cost is little, the reward priceless.
It is good and right to have high hopes. We all need to take care of ourselves, whether we make resolutions to do so or not. Though more than anything else what we need is to be kind to ourselves. It is presumptuous of me to tell you what wellness means for your individual struggle, as it is inhuman of me to deign to tell you to be resilient when many of you face intolerable working conditions.4 As Jackson Browne sang in “Rock Me on the Water”, “Everyone must have some thought that’s going to pull them through somehow. Find your own thought, the reason you keep getting up and going to care for patients who increasingly respond with the rage of denial and resentment. Amid what morally distressed public health professionals have called so many unnecessary deaths,choose what gives you reason to keep serving that other side of this life full of healing.5 And if like so many of my fellow health care professionals, you are so spent and bent, that you feel that you can no longer practice without becoming someone you do not want to be, then let go with grace, get the help you deserve and perhaps one day when rested and mended, find another way to give.6
I rarely self-disclose but I want to end this column with a personal story that exemplifies more than all these words living this simple message. My spouse is a health care practitioner at a Veterans Affairs medical center. Like all of you on the front lines they work far too long hours in difficult conditions, with challenging patients and not enough staff to care for them. My partner had not an hour to get any gifts for me or our furry children. On Christmas Eve, before a long shift, they went to a packed Walgreens to buy our huskies each a toy and me a pair of fuzzy slippers. We sat by the tree and opened the hastily wrapped packages, and nothing could have been more memorable or meaningful. All of us at Federal Practitioner wish you, our readers, find in 2022 many such moments to sustain you.
I do not usually have difficulty writing editorials. However, this month was different. I kept coming up with grand ideas that flopped. First, I thought I would write a column entitled, “For What Should We Hope For?” When I started exploring the concept of hope, I quickly learned that there was extensive literature from multiple disciplines and even several centers and research projects dedicated to studying it.1 It seemed unlikely that I would have anything worthwhile to add to that literature. Then I thought I would discuss new year’s resolutions for federal practitioners. There was not much written about that topic, yet it seemed to be overly self-indulgent and superficial to discuss eating less and exercising more amid a pandemic and a climate change crisis. Finally, I wanted to opine on the futility of telling people to be resilient when we are all exhausted and demoralized, and yet that seemed too ponderous and paradoxical for our beleaguered state. With the third strike, I finally realized I was trying too hard. And perhaps that was exactly what I needed to say, at least to myself, and maybe some readers would benefit from reading that simple message as well.
I was surprised—though I probably should not have been given the explosion of media—to find that Americans were surveyed about what months they hate most. A 2021 poll of more than 15,000 adults found that January was the most disliked month.2 It’s not hard to figure out why. Characterized by a postholiday let down, these months in the middle of winter marked by either too much precipitation or if you live in the West not enough; short days and gray nights that are dark and cold. It is a long time to wait before spring with few holidays to break up the quotidian routine of work and school. January is a hard enough month in a good or even ordinary year. And 2022 is shaping up to be neither. We are entering the third year of a prolonged pandemic. Every time we have hope we are coming to the end of this long ordeal or at least things are moving toward normality, a new variant emerges, and we are back to living in fear and uncertainty.
COVID-19 is only the most relentless and deadly of our current disasters: There are rumors of wars, tornadoes, droughts, floods, shootings in schools and churches, political turmoil, and police violence. American society and the very planet seem to be in a perilous situation more than ever. No wonder then, that in the last month, several people have asked me, “Do you think this is the end of the world?” I suppose they think I am so old that I have become wise. And though I should cite a brilliant philosopher or renowned theologian: I am going to revert to my youth as a rock musician and quote R.E.M.: “It is the end of the world as we know it.” And “most of us do not feel fine!”
The world of 2022 is far more constricted and confined than it was before we heard the word COVID-19. We have less freedom of movement and fewer opportunities for companionship and gathering, for advancement and enjoyment. To thrive, and even to survive, in this cramped existence of limited possibilities, we need different values and attitudes than those that made us happy and successful in the open, hurried world before 2019. No generation since World War II has confronted such shortages of automobiles, paper goods, food, and even medicines as we have.
That is the first of the important simple messages I want to convey. Find something to be grateful for: your loved ones, your companion animals, your friends. Cherish the rainy or sunny day depending on how your climate has changed. Treasure the most basic and enduring pleasures, homemade cookies, favorite music, talking to a good friend even virtually, reading an actual book on a Sunday afternoon. These are things even the pandemic cannot take away from us unless we let our own inability to accept the conditions of our time ruin even what the meager, harsh Master of History has spared us.
The second of these simple messages is even more essential to finding any peace or joy in our current tense and somber existence: to show compassion for others and kindness to yourself. The most consistent report I have heard from people all over the country is that their fellow citizens are angry and selfish. We all understand, and even in some measure empathize with this the frustration and impatience with all the extraordinary pressure of having to function under these challenging conditions. Though we can take it out on the stranger at the grocery store or the family of the patient who has different views of masks and vaccines; it likely will not make the line shorter, the family any less demanding or seemingly unreasonable and probably will waste the little energy we have left to get home with the groceries or take care of the patient.
You never know what burden the person annoying you is carrying; it may perhaps be heavier than yours. And how we react to each other makes the weight of world weariness we all bear either easier or harder to shoulder. It sounds trite and trivial to say, yet tell people you care, and value, and love them. Although no less than Pope Francis in a Christmas present to marriages under strain from the stress of the pandemic that the 3 key words to remember are please, sorry, and thank you.3 I am applying that sage advice liberally to all relationships and interactions in the daily grind of work and home. The cost is little, the reward priceless.
It is good and right to have high hopes. We all need to take care of ourselves, whether we make resolutions to do so or not. Though more than anything else what we need is to be kind to ourselves. It is presumptuous of me to tell you what wellness means for your individual struggle, as it is inhuman of me to deign to tell you to be resilient when many of you face intolerable working conditions.4 As Jackson Browne sang in “Rock Me on the Water”, “Everyone must have some thought that’s going to pull them through somehow. Find your own thought, the reason you keep getting up and going to care for patients who increasingly respond with the rage of denial and resentment. Amid what morally distressed public health professionals have called so many unnecessary deaths,choose what gives you reason to keep serving that other side of this life full of healing.5 And if like so many of my fellow health care professionals, you are so spent and bent, that you feel that you can no longer practice without becoming someone you do not want to be, then let go with grace, get the help you deserve and perhaps one day when rested and mended, find another way to give.6
I rarely self-disclose but I want to end this column with a personal story that exemplifies more than all these words living this simple message. My spouse is a health care practitioner at a Veterans Affairs medical center. Like all of you on the front lines they work far too long hours in difficult conditions, with challenging patients and not enough staff to care for them. My partner had not an hour to get any gifts for me or our furry children. On Christmas Eve, before a long shift, they went to a packed Walgreens to buy our huskies each a toy and me a pair of fuzzy slippers. We sat by the tree and opened the hastily wrapped packages, and nothing could have been more memorable or meaningful. All of us at Federal Practitioner wish you, our readers, find in 2022 many such moments to sustain you.
1. The Center for the Advanced Study and Practice of Hope. T Denny Sanford School of Social and Family Dynamics, Arizona State University. Accessed January 3, 2022. https://thesanfordschool.asu.edu/research/centers-initiatives/hope-center
2. Ballard J. What is America’s favorite (and least favorite) month?” Published March 1, 2021. Accessed January 3, 2022. https://today.yougov.com/topics/lifestyle/articles-reports/2021/03/01/favorite-least-favorite-month-poll
3. Winlfield N. Pope’s 3 key words for a marriage: ‘please, thanks sorry.’ Associated Press. December 26, 2021. Accessed January 3, 2022. https://apnews.com/article/pope-francis-lifestyle-religion-relationships-couples-23c81169982e50c35d1c1fc7bfef8cbc
4. Dineen K. Why resilience isn’t always the answer to coping with challenging times. Published September 29, 2020. Accessed January 3, 2022. https://theconversation.com/why-resilience-isnt-always-the-answer-to-coping-with-challenging-times-145796
5. Caldwell T. ‘Everyone of those deaths is unnecessary,’ expert says of rising COVID-19 U.S. death toll as tens of millions remain unvaccinated. Published October 3, 2021. Accessed December 29, 2021. https://www.cnn.com/2021/10/03/health/us-coronavirus-sunday/index.html 6. Yong E. Why healthcare professionals are quitting in droves. The Atlantic. November 16, 2021. Accessed December 29, 2021. https://www.theatlantic.com/health/archive/2021/11/the-mass-exodus-of-americas-health-care-workers/620713/
1. The Center for the Advanced Study and Practice of Hope. T Denny Sanford School of Social and Family Dynamics, Arizona State University. Accessed January 3, 2022. https://thesanfordschool.asu.edu/research/centers-initiatives/hope-center
2. Ballard J. What is America’s favorite (and least favorite) month?” Published March 1, 2021. Accessed January 3, 2022. https://today.yougov.com/topics/lifestyle/articles-reports/2021/03/01/favorite-least-favorite-month-poll
3. Winlfield N. Pope’s 3 key words for a marriage: ‘please, thanks sorry.’ Associated Press. December 26, 2021. Accessed January 3, 2022. https://apnews.com/article/pope-francis-lifestyle-religion-relationships-couples-23c81169982e50c35d1c1fc7bfef8cbc
4. Dineen K. Why resilience isn’t always the answer to coping with challenging times. Published September 29, 2020. Accessed January 3, 2022. https://theconversation.com/why-resilience-isnt-always-the-answer-to-coping-with-challenging-times-145796
5. Caldwell T. ‘Everyone of those deaths is unnecessary,’ expert says of rising COVID-19 U.S. death toll as tens of millions remain unvaccinated. Published October 3, 2021. Accessed December 29, 2021. https://www.cnn.com/2021/10/03/health/us-coronavirus-sunday/index.html 6. Yong E. Why healthcare professionals are quitting in droves. The Atlantic. November 16, 2021. Accessed December 29, 2021. https://www.theatlantic.com/health/archive/2021/11/the-mass-exodus-of-americas-health-care-workers/620713/
Surgical groups push back against new revascularization guidelines
The new 2021 coronary revascularization guidelines are spurring controversy, as surgical associations raise concerns about the interpretation of the evidence behind key recommendations and the makeup of the writing committee.
The guideline was published in December by the American College of Cardiology, American Heart Association, and Society for Cardiovascular Angiography and Interventions (ACC/AHA/SCAI), and replaces the 2011 coronary artery bypass surgery (CABG) and the 2011 and 2015 percutaneous coronary intervention (PCI) guidelines.
The American Association for Thoracic Surgery (AATS) and Society of Thoracic Surgeons (STS) were part of the development of the document but have withdrawn their support, citing three areas of concern in a recent editorial in Annals of Thoracic Surgery.
“I do have to emphasize this is not just the AATS and STS – the European societies, Latin American societies, Asian societies, and even cardiologists are all coming out against these guidelines,” Joseph F. Sabik III, MD, University Hospitals Cleveland Medical Center, lead author of the editorial, said in an interview. “So, I think that tells us that something didn’t go right here.”
The main objection is the downgrading of CABG surgery from a class 1 to weak 2b recommendation to improve survival in patients with three-vessel coronary artery disease (CAD) and normal left ventricular function.
The ISCHEMIA trial was used to support this two-level downgrade and a class 1 to 2a downgrade for CABG in three-vessel CAD with mild to moderate left ventricular dysfunction. But the trial wasn’t powered for survival, only 20% of patients underwent CABG as the initial invasive strategy, and patients were followed for less than 5 years, the editorialists observed.
At the same time, there’s plenty of observational and randomized studies such as SYNTAX, EXCEL, and FAME 3 showing a clear survival benefit of CABG over PCI, Dr. Sabik said. “The criticism is that these are old studies and aren’t applicable today, but we don’t understand downgrading without any evidence suggesting it [CABG] isn’t effective anymore.”
CABG and PCI treated as equal
AATS and STS also object to the new guidelines treating PCI and CABG as equivalent revascularization strategies in decreasing ischemic events. Both were given a 2b recommendation for survival with triple-vessel disease, but randomized trials have demonstrated not only lower mortality with surgery but fewer reinterventions and myocardial infarctions.
“None of that gets acknowledged in the guidelines; they are treated equally,” Dr. Sabik said. “So if you’re going to say that CABG isn’t any better than medical therapy, in our mind, you have to say that PCI is worse than medical therapy. And we don’t believe that, I want you to know. We just think that the logic doesn’t make any sense. The committee used what it wanted to but didn’t use many things that committees have used in the past to give CABG a level 1 recommendation.”
The downgrade is also at odds with the 2018 European Society of Cardiology (ESC)/ European Association for Cardio-Thoracic Surgery (EACTS) guidelines, which give CABG a class 1 recommendation in three-vessel CAD as well as one- or two-vessel CAD with proximal left atrial descending artery stenosis.
In a Dec. 14 letter to the ACC/AHA Joint Committee, the Latin American Association of Cardiac and Endovascular Surgery (LACES) also called out the guideline committee for the 2b class of recommendation (COR) for PCI and CABG, saying it contradicts the text, which “clearly considers” the need to give a weaker endorsement for PCI than for CABG in patients with multivessel CAD.
“Considering that this section has the most significant impact due to the prevalence of stable ischemic heart disease in patients with multivessel CAD, such a contradiction may affect the lives and survival of millions of patients worldwide and have a major socioeconomic impact,” the letter states.
“Therefore, LACES respectfully but vehemently believes the Task Force should seriously reconsider the wording and recommendations in this specific large group of patients.”
Class I for radial conduit
AATS and STS also express concern about the new class 1 recommendation for the radial artery as a conduit in CABG. They note this is higher than bilateral internal mammary artery grafting and based on a meta-analysis of six relatively small studies with very strict inclusion criteria favorable for radial artery usage and patency.
“There’s a lot of studies that showed if you use the radial artery incorrectly, you have worse outcomes, and that’s what scares us a bit,” Dr. Sabik said. “If they’re giving it a class 1 recommendation, does that mean that becomes standard of care and could that cause patient harm? We think that level 1 is too high and that a [class] 2a with qualifications would be appropriate.”
Unequal footing
In a Dec. 23 letter, EACTS said it is “extremely concerned” about downgrading the COR for CABG without new randomized controlled trials to support the decision or to reject previously held evidence.
“The downgrading of CABG, and placing PCI at the same COR, does not meet our interpretation of the evidence, and may lead to avoidable loss of life,” EACTS officials said. “These guidelines also have implications on patient care: A COR IIb entails that CABG may not be reimbursable in some countries.”
EACTS called on AHA, ACC, and SCAI to review the evidence and called out the makeup of the guideline writing committee. “It is astonishing that no surgical association was involved, coauthored, or endorsed these guidelines.”
The AATS and STS each had a single representative on the guidelines’ writing committee but note that the six remaining surgeons were chosen by the ACC and AHA. Surgeons were also in the minority and only a majority was needed to approve the guidelines, highlighting the need to revisit the guideline development process to ensure equal representation by multidisciplinary experts across specialties.
“I hope the cardiology and surgical societies can come together and figure out how we do this better in the future, and we take a look again at these guidelines and come up with what we think is appropriate, especially since this is not just AATS and STS,” Dr. Sabik said.
In an emailed statement, the ACC/AHA said the AATS and STS representatives “actively participated throughout the writing process the past 3 years” and that the AATS and STS were involved in the “extensive peer review process” for the document with a reviewer from each organization. Nevertheless, AATS and STS both elected not to endorse the guidelines when at the organizational approval stage.
“Consequently, the AATS representative chose to stay with the committee and be recognized as having been appointed on behalf of the ACC and the AHA,” according to the statement. “The STS representative chose to withdraw from the committee and is not listed as a writing committee member on the final guideline. The final guideline reflects the latest evidence-based recommendations for coronary artery revascularization, as agreed by the ACC, AHA, SCAI, and the full writing committee.”
Despite pleas from the surgical groups to reconsider the evidence, “there is no further review process for the revascularization guideline,” the ACC/AHA spokesperson noted.
Jennifer S. Lawton, MD, chief of cardiac surgery at Johns Hopkins University, Baltimore, and guideline writing committee chair, did not respond to numerous requests for comment.
A version of this article first appeared on Medscape.com.
The new 2021 coronary revascularization guidelines are spurring controversy, as surgical associations raise concerns about the interpretation of the evidence behind key recommendations and the makeup of the writing committee.
The guideline was published in December by the American College of Cardiology, American Heart Association, and Society for Cardiovascular Angiography and Interventions (ACC/AHA/SCAI), and replaces the 2011 coronary artery bypass surgery (CABG) and the 2011 and 2015 percutaneous coronary intervention (PCI) guidelines.
The American Association for Thoracic Surgery (AATS) and Society of Thoracic Surgeons (STS) were part of the development of the document but have withdrawn their support, citing three areas of concern in a recent editorial in Annals of Thoracic Surgery.
“I do have to emphasize this is not just the AATS and STS – the European societies, Latin American societies, Asian societies, and even cardiologists are all coming out against these guidelines,” Joseph F. Sabik III, MD, University Hospitals Cleveland Medical Center, lead author of the editorial, said in an interview. “So, I think that tells us that something didn’t go right here.”
The main objection is the downgrading of CABG surgery from a class 1 to weak 2b recommendation to improve survival in patients with three-vessel coronary artery disease (CAD) and normal left ventricular function.
The ISCHEMIA trial was used to support this two-level downgrade and a class 1 to 2a downgrade for CABG in three-vessel CAD with mild to moderate left ventricular dysfunction. But the trial wasn’t powered for survival, only 20% of patients underwent CABG as the initial invasive strategy, and patients were followed for less than 5 years, the editorialists observed.
At the same time, there’s plenty of observational and randomized studies such as SYNTAX, EXCEL, and FAME 3 showing a clear survival benefit of CABG over PCI, Dr. Sabik said. “The criticism is that these are old studies and aren’t applicable today, but we don’t understand downgrading without any evidence suggesting it [CABG] isn’t effective anymore.”
CABG and PCI treated as equal
AATS and STS also object to the new guidelines treating PCI and CABG as equivalent revascularization strategies in decreasing ischemic events. Both were given a 2b recommendation for survival with triple-vessel disease, but randomized trials have demonstrated not only lower mortality with surgery but fewer reinterventions and myocardial infarctions.
“None of that gets acknowledged in the guidelines; they are treated equally,” Dr. Sabik said. “So if you’re going to say that CABG isn’t any better than medical therapy, in our mind, you have to say that PCI is worse than medical therapy. And we don’t believe that, I want you to know. We just think that the logic doesn’t make any sense. The committee used what it wanted to but didn’t use many things that committees have used in the past to give CABG a level 1 recommendation.”
The downgrade is also at odds with the 2018 European Society of Cardiology (ESC)/ European Association for Cardio-Thoracic Surgery (EACTS) guidelines, which give CABG a class 1 recommendation in three-vessel CAD as well as one- or two-vessel CAD with proximal left atrial descending artery stenosis.
In a Dec. 14 letter to the ACC/AHA Joint Committee, the Latin American Association of Cardiac and Endovascular Surgery (LACES) also called out the guideline committee for the 2b class of recommendation (COR) for PCI and CABG, saying it contradicts the text, which “clearly considers” the need to give a weaker endorsement for PCI than for CABG in patients with multivessel CAD.
“Considering that this section has the most significant impact due to the prevalence of stable ischemic heart disease in patients with multivessel CAD, such a contradiction may affect the lives and survival of millions of patients worldwide and have a major socioeconomic impact,” the letter states.
“Therefore, LACES respectfully but vehemently believes the Task Force should seriously reconsider the wording and recommendations in this specific large group of patients.”
Class I for radial conduit
AATS and STS also express concern about the new class 1 recommendation for the radial artery as a conduit in CABG. They note this is higher than bilateral internal mammary artery grafting and based on a meta-analysis of six relatively small studies with very strict inclusion criteria favorable for radial artery usage and patency.
“There’s a lot of studies that showed if you use the radial artery incorrectly, you have worse outcomes, and that’s what scares us a bit,” Dr. Sabik said. “If they’re giving it a class 1 recommendation, does that mean that becomes standard of care and could that cause patient harm? We think that level 1 is too high and that a [class] 2a with qualifications would be appropriate.”
Unequal footing
In a Dec. 23 letter, EACTS said it is “extremely concerned” about downgrading the COR for CABG without new randomized controlled trials to support the decision or to reject previously held evidence.
“The downgrading of CABG, and placing PCI at the same COR, does not meet our interpretation of the evidence, and may lead to avoidable loss of life,” EACTS officials said. “These guidelines also have implications on patient care: A COR IIb entails that CABG may not be reimbursable in some countries.”
EACTS called on AHA, ACC, and SCAI to review the evidence and called out the makeup of the guideline writing committee. “It is astonishing that no surgical association was involved, coauthored, or endorsed these guidelines.”
The AATS and STS each had a single representative on the guidelines’ writing committee but note that the six remaining surgeons were chosen by the ACC and AHA. Surgeons were also in the minority and only a majority was needed to approve the guidelines, highlighting the need to revisit the guideline development process to ensure equal representation by multidisciplinary experts across specialties.
“I hope the cardiology and surgical societies can come together and figure out how we do this better in the future, and we take a look again at these guidelines and come up with what we think is appropriate, especially since this is not just AATS and STS,” Dr. Sabik said.
In an emailed statement, the ACC/AHA said the AATS and STS representatives “actively participated throughout the writing process the past 3 years” and that the AATS and STS were involved in the “extensive peer review process” for the document with a reviewer from each organization. Nevertheless, AATS and STS both elected not to endorse the guidelines when at the organizational approval stage.
“Consequently, the AATS representative chose to stay with the committee and be recognized as having been appointed on behalf of the ACC and the AHA,” according to the statement. “The STS representative chose to withdraw from the committee and is not listed as a writing committee member on the final guideline. The final guideline reflects the latest evidence-based recommendations for coronary artery revascularization, as agreed by the ACC, AHA, SCAI, and the full writing committee.”
Despite pleas from the surgical groups to reconsider the evidence, “there is no further review process for the revascularization guideline,” the ACC/AHA spokesperson noted.
Jennifer S. Lawton, MD, chief of cardiac surgery at Johns Hopkins University, Baltimore, and guideline writing committee chair, did not respond to numerous requests for comment.
A version of this article first appeared on Medscape.com.
The new 2021 coronary revascularization guidelines are spurring controversy, as surgical associations raise concerns about the interpretation of the evidence behind key recommendations and the makeup of the writing committee.
The guideline was published in December by the American College of Cardiology, American Heart Association, and Society for Cardiovascular Angiography and Interventions (ACC/AHA/SCAI), and replaces the 2011 coronary artery bypass surgery (CABG) and the 2011 and 2015 percutaneous coronary intervention (PCI) guidelines.
The American Association for Thoracic Surgery (AATS) and Society of Thoracic Surgeons (STS) were part of the development of the document but have withdrawn their support, citing three areas of concern in a recent editorial in Annals of Thoracic Surgery.
“I do have to emphasize this is not just the AATS and STS – the European societies, Latin American societies, Asian societies, and even cardiologists are all coming out against these guidelines,” Joseph F. Sabik III, MD, University Hospitals Cleveland Medical Center, lead author of the editorial, said in an interview. “So, I think that tells us that something didn’t go right here.”
The main objection is the downgrading of CABG surgery from a class 1 to weak 2b recommendation to improve survival in patients with three-vessel coronary artery disease (CAD) and normal left ventricular function.
The ISCHEMIA trial was used to support this two-level downgrade and a class 1 to 2a downgrade for CABG in three-vessel CAD with mild to moderate left ventricular dysfunction. But the trial wasn’t powered for survival, only 20% of patients underwent CABG as the initial invasive strategy, and patients were followed for less than 5 years, the editorialists observed.
At the same time, there’s plenty of observational and randomized studies such as SYNTAX, EXCEL, and FAME 3 showing a clear survival benefit of CABG over PCI, Dr. Sabik said. “The criticism is that these are old studies and aren’t applicable today, but we don’t understand downgrading without any evidence suggesting it [CABG] isn’t effective anymore.”
CABG and PCI treated as equal
AATS and STS also object to the new guidelines treating PCI and CABG as equivalent revascularization strategies in decreasing ischemic events. Both were given a 2b recommendation for survival with triple-vessel disease, but randomized trials have demonstrated not only lower mortality with surgery but fewer reinterventions and myocardial infarctions.
“None of that gets acknowledged in the guidelines; they are treated equally,” Dr. Sabik said. “So if you’re going to say that CABG isn’t any better than medical therapy, in our mind, you have to say that PCI is worse than medical therapy. And we don’t believe that, I want you to know. We just think that the logic doesn’t make any sense. The committee used what it wanted to but didn’t use many things that committees have used in the past to give CABG a level 1 recommendation.”
The downgrade is also at odds with the 2018 European Society of Cardiology (ESC)/ European Association for Cardio-Thoracic Surgery (EACTS) guidelines, which give CABG a class 1 recommendation in three-vessel CAD as well as one- or two-vessel CAD with proximal left atrial descending artery stenosis.
In a Dec. 14 letter to the ACC/AHA Joint Committee, the Latin American Association of Cardiac and Endovascular Surgery (LACES) also called out the guideline committee for the 2b class of recommendation (COR) for PCI and CABG, saying it contradicts the text, which “clearly considers” the need to give a weaker endorsement for PCI than for CABG in patients with multivessel CAD.
“Considering that this section has the most significant impact due to the prevalence of stable ischemic heart disease in patients with multivessel CAD, such a contradiction may affect the lives and survival of millions of patients worldwide and have a major socioeconomic impact,” the letter states.
“Therefore, LACES respectfully but vehemently believes the Task Force should seriously reconsider the wording and recommendations in this specific large group of patients.”
Class I for radial conduit
AATS and STS also express concern about the new class 1 recommendation for the radial artery as a conduit in CABG. They note this is higher than bilateral internal mammary artery grafting and based on a meta-analysis of six relatively small studies with very strict inclusion criteria favorable for radial artery usage and patency.
“There’s a lot of studies that showed if you use the radial artery incorrectly, you have worse outcomes, and that’s what scares us a bit,” Dr. Sabik said. “If they’re giving it a class 1 recommendation, does that mean that becomes standard of care and could that cause patient harm? We think that level 1 is too high and that a [class] 2a with qualifications would be appropriate.”
Unequal footing
In a Dec. 23 letter, EACTS said it is “extremely concerned” about downgrading the COR for CABG without new randomized controlled trials to support the decision or to reject previously held evidence.
“The downgrading of CABG, and placing PCI at the same COR, does not meet our interpretation of the evidence, and may lead to avoidable loss of life,” EACTS officials said. “These guidelines also have implications on patient care: A COR IIb entails that CABG may not be reimbursable in some countries.”
EACTS called on AHA, ACC, and SCAI to review the evidence and called out the makeup of the guideline writing committee. “It is astonishing that no surgical association was involved, coauthored, or endorsed these guidelines.”
The AATS and STS each had a single representative on the guidelines’ writing committee but note that the six remaining surgeons were chosen by the ACC and AHA. Surgeons were also in the minority and only a majority was needed to approve the guidelines, highlighting the need to revisit the guideline development process to ensure equal representation by multidisciplinary experts across specialties.
“I hope the cardiology and surgical societies can come together and figure out how we do this better in the future, and we take a look again at these guidelines and come up with what we think is appropriate, especially since this is not just AATS and STS,” Dr. Sabik said.
In an emailed statement, the ACC/AHA said the AATS and STS representatives “actively participated throughout the writing process the past 3 years” and that the AATS and STS were involved in the “extensive peer review process” for the document with a reviewer from each organization. Nevertheless, AATS and STS both elected not to endorse the guidelines when at the organizational approval stage.
“Consequently, the AATS representative chose to stay with the committee and be recognized as having been appointed on behalf of the ACC and the AHA,” according to the statement. “The STS representative chose to withdraw from the committee and is not listed as a writing committee member on the final guideline. The final guideline reflects the latest evidence-based recommendations for coronary artery revascularization, as agreed by the ACC, AHA, SCAI, and the full writing committee.”
Despite pleas from the surgical groups to reconsider the evidence, “there is no further review process for the revascularization guideline,” the ACC/AHA spokesperson noted.
Jennifer S. Lawton, MD, chief of cardiac surgery at Johns Hopkins University, Baltimore, and guideline writing committee chair, did not respond to numerous requests for comment.
A version of this article first appeared on Medscape.com.
Midlife cardiovascular conditions tied to greater cognitive decline in women
Even though men in midlife have more cardiovascular (CV) conditions and risk factors than women of the same age, women are more affected by these conditions in terms of cognitive decline, new research suggests.
Analyses of almost 1,400 participants in the population-based Mayo Clinic Study of Aging showed that diabetes, dyslipidemia, and coronary heart disease (CHD) all had stronger associations with global cognitive decline in women than in men.
“All men and women should be treated for cardiovascular risk factors and conditions, but this study really highlights the importance of very early and perhaps more aggressive treatment in women with these conditions,” co-investigator Michelle M. Mielke, PhD, professor of epidemiology and neurology, Mayo Clinic, Rochester, Minn., told this news organization.
The findings were published online Jan. 5 in Neurology.
Assessing sex differences
Most previous studies in this area have focused on CV risk factors in midlife in relation to late-life dementia (after age 75) or on late-life vascular risk factors and late-life dementia, Dr. Mielke noted.
However, a few recent studies have suggested vascular risk factors can affect cognition even in midlife. The current investigators sought to determine whether there are sex differences in these associations.
They assessed 1,857 nondemented participants aged 50 to 69 years from the Mayo Clinic Study on Aging. The mean education level was 14.9 years, and the mean body mass index (BMI) was 29.7.
Among the participants, 78.9% had at least one CV condition or risk factor, and the proportion was higher in men than women (83.4% vs. 74.5%; P < .0001).
Frequency of each individual CV condition or risk factor was also higher in men than women, and they had more years of education and higher BMI but took fewer medications.
Every 15 months, participants had an in-person interview and physical examination that included a neurologic assessment and short test of memory.
The neuropsychological battery included nine tests across four domains: memory, language, executive function, and visuospatial skills. Researchers calculated z-scores for these domains and for global cognition.
Multiple cognitive domains
Whereas this study evaluated multiple cognitive domains, most previous research has focused on global cognitive decline and/or decline in only one or two cognitive domains, the investigators note.
They collected information from medical records on CV conditions such as CHD, arrhythmias, congestive heart failure, peripheral vascular disease (PVD), and stroke; and CV risk factors such as hypertension, diabetes, dyslipidemia, smoking status, and BMI.
Because of the small number of patients with stroke and PVD, these were classified as “other cardiovascular conditions” in the statistical analysis.
Researchers adjusted for sex, age, years of education, depressive symptoms, comorbidities, medications, and apolipoprotein E (APOE) genotyping. The mean follow-up was 3 years and did not differ by sex.
As some participants didn’t have a follow-up visit, the current analysis included 1,394 individuals. Those without follow-up visits were younger, had less education and more comorbidities, and took more medications compared with those with a follow-up.
Results showed most CV conditions were more strongly associated with cognitive function among women than men. For example, CHD was associated with global decline only in women (P < .05).
CHD, diabetes, and dyslipidemia were associated with language decline in women only (all, P < .05), but congestive heart failure was significantly associated with language decline in men only.
Dr. Mielke cautioned about reading too much into the language results for women.
“It’s an intriguing finding and definitely we need to follow up on it,” she said. However, “more studies are needed to examine sex differences before we start saying it only has an effect on language.”
‘Treat aggressively and right away’
The researchers were somewhat surprised by the study findings. Because there is a higher prevalence of CV conditions and risk factors in men, they presumed men would be more affected by these conditions, said Dr. Mielke.
“But that’s not what we saw; we saw the reverse. It was actually the women who were affected more by these cardiovascular risk factors and conditions,” she said.
As midlife is when women enter menopause, fluctuating estrogen levels may help explain the differential impact on cognition among women. But Dr. Mielke said she wants to “move beyond” just looking at hormones.
She pointed out there are a variety of psychosocial factors that may also contribute to an imbalance in the cognitive impact of CV conditions on women.
“Midlife is when many women are still taking care of their children at home, are also taking care of their adult parents, and may be undergoing more stress while continuing to do a job,” Dr. Miekle said.
Structural brain development and genetics may also contribute to the greater effect on cognition in women, the investigators note.
Dr. Mielke stressed that the current study only identifies associations. “The next steps are to understand what some of the underlying mechanisms for this are,” she said.
In the meantime, these new results suggest middle-aged women with high blood pressure, cholesterol, or glucose measures “should be treated aggressively and right away” said Dr. Mielke.
“For example, for women who are just starting to become hypertensive, clinicians should treat them right away and not watch and wait.”
Study limitations cited include that its sample was limited to Olmsted County, Minnesota – so results may not be generalized to other populations. Also, as researchers combined PVD and stroke into one group, larger sample sizes are needed, especially for stroke. Another limitation was the study did not have information on duration of all CV conditions or risk factors.
Helpful for tailoring interventions?
Commenting on the study, Glen R. Finney, MD, director, Memory and Cognition Program, Geisinger Health Clinic, Wilkes-Barre, Pennsylvania, said the results are important.
“The more we understand about risk factors for the development of Alzheimer’s disease and related dementias, the better we understand how we can reduce the risks,” said Dr. Finney, who was not involved with the research.
Awareness that CV conditions are major risk factors in midlife has been “definitely rising,” said Dr. Finney. “Many studies originally were looking at late life and are now looking more at earlier in the disease process, and I think that’s important.”
Understanding how sex, ethnicity, and other demographic variables affect risks can help to “tailor interventions” for individual patients, he said.
The study was supported by the National Institutes of Health, the GHR Foundation, and the Rochester Epidemiology Project. Dr. Mielke is a consultant for Biogen and Brain Protection Company and is on the editorial boards of Neurology and Alzheimer’s and Dementia. Dr. Finney has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Even though men in midlife have more cardiovascular (CV) conditions and risk factors than women of the same age, women are more affected by these conditions in terms of cognitive decline, new research suggests.
Analyses of almost 1,400 participants in the population-based Mayo Clinic Study of Aging showed that diabetes, dyslipidemia, and coronary heart disease (CHD) all had stronger associations with global cognitive decline in women than in men.
“All men and women should be treated for cardiovascular risk factors and conditions, but this study really highlights the importance of very early and perhaps more aggressive treatment in women with these conditions,” co-investigator Michelle M. Mielke, PhD, professor of epidemiology and neurology, Mayo Clinic, Rochester, Minn., told this news organization.
The findings were published online Jan. 5 in Neurology.
Assessing sex differences
Most previous studies in this area have focused on CV risk factors in midlife in relation to late-life dementia (after age 75) or on late-life vascular risk factors and late-life dementia, Dr. Mielke noted.
However, a few recent studies have suggested vascular risk factors can affect cognition even in midlife. The current investigators sought to determine whether there are sex differences in these associations.
They assessed 1,857 nondemented participants aged 50 to 69 years from the Mayo Clinic Study on Aging. The mean education level was 14.9 years, and the mean body mass index (BMI) was 29.7.
Among the participants, 78.9% had at least one CV condition or risk factor, and the proportion was higher in men than women (83.4% vs. 74.5%; P < .0001).
Frequency of each individual CV condition or risk factor was also higher in men than women, and they had more years of education and higher BMI but took fewer medications.
Every 15 months, participants had an in-person interview and physical examination that included a neurologic assessment and short test of memory.
The neuropsychological battery included nine tests across four domains: memory, language, executive function, and visuospatial skills. Researchers calculated z-scores for these domains and for global cognition.
Multiple cognitive domains
Whereas this study evaluated multiple cognitive domains, most previous research has focused on global cognitive decline and/or decline in only one or two cognitive domains, the investigators note.
They collected information from medical records on CV conditions such as CHD, arrhythmias, congestive heart failure, peripheral vascular disease (PVD), and stroke; and CV risk factors such as hypertension, diabetes, dyslipidemia, smoking status, and BMI.
Because of the small number of patients with stroke and PVD, these were classified as “other cardiovascular conditions” in the statistical analysis.
Researchers adjusted for sex, age, years of education, depressive symptoms, comorbidities, medications, and apolipoprotein E (APOE) genotyping. The mean follow-up was 3 years and did not differ by sex.
As some participants didn’t have a follow-up visit, the current analysis included 1,394 individuals. Those without follow-up visits were younger, had less education and more comorbidities, and took more medications compared with those with a follow-up.
Results showed most CV conditions were more strongly associated with cognitive function among women than men. For example, CHD was associated with global decline only in women (P < .05).
CHD, diabetes, and dyslipidemia were associated with language decline in women only (all, P < .05), but congestive heart failure was significantly associated with language decline in men only.
Dr. Mielke cautioned about reading too much into the language results for women.
“It’s an intriguing finding and definitely we need to follow up on it,” she said. However, “more studies are needed to examine sex differences before we start saying it only has an effect on language.”
‘Treat aggressively and right away’
The researchers were somewhat surprised by the study findings. Because there is a higher prevalence of CV conditions and risk factors in men, they presumed men would be more affected by these conditions, said Dr. Mielke.
“But that’s not what we saw; we saw the reverse. It was actually the women who were affected more by these cardiovascular risk factors and conditions,” she said.
As midlife is when women enter menopause, fluctuating estrogen levels may help explain the differential impact on cognition among women. But Dr. Mielke said she wants to “move beyond” just looking at hormones.
She pointed out there are a variety of psychosocial factors that may also contribute to an imbalance in the cognitive impact of CV conditions on women.
“Midlife is when many women are still taking care of their children at home, are also taking care of their adult parents, and may be undergoing more stress while continuing to do a job,” Dr. Miekle said.
Structural brain development and genetics may also contribute to the greater effect on cognition in women, the investigators note.
Dr. Mielke stressed that the current study only identifies associations. “The next steps are to understand what some of the underlying mechanisms for this are,” she said.
In the meantime, these new results suggest middle-aged women with high blood pressure, cholesterol, or glucose measures “should be treated aggressively and right away” said Dr. Mielke.
“For example, for women who are just starting to become hypertensive, clinicians should treat them right away and not watch and wait.”
Study limitations cited include that its sample was limited to Olmsted County, Minnesota – so results may not be generalized to other populations. Also, as researchers combined PVD and stroke into one group, larger sample sizes are needed, especially for stroke. Another limitation was the study did not have information on duration of all CV conditions or risk factors.
Helpful for tailoring interventions?
Commenting on the study, Glen R. Finney, MD, director, Memory and Cognition Program, Geisinger Health Clinic, Wilkes-Barre, Pennsylvania, said the results are important.
“The more we understand about risk factors for the development of Alzheimer’s disease and related dementias, the better we understand how we can reduce the risks,” said Dr. Finney, who was not involved with the research.
Awareness that CV conditions are major risk factors in midlife has been “definitely rising,” said Dr. Finney. “Many studies originally were looking at late life and are now looking more at earlier in the disease process, and I think that’s important.”
Understanding how sex, ethnicity, and other demographic variables affect risks can help to “tailor interventions” for individual patients, he said.
The study was supported by the National Institutes of Health, the GHR Foundation, and the Rochester Epidemiology Project. Dr. Mielke is a consultant for Biogen and Brain Protection Company and is on the editorial boards of Neurology and Alzheimer’s and Dementia. Dr. Finney has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Even though men in midlife have more cardiovascular (CV) conditions and risk factors than women of the same age, women are more affected by these conditions in terms of cognitive decline, new research suggests.
Analyses of almost 1,400 participants in the population-based Mayo Clinic Study of Aging showed that diabetes, dyslipidemia, and coronary heart disease (CHD) all had stronger associations with global cognitive decline in women than in men.
“All men and women should be treated for cardiovascular risk factors and conditions, but this study really highlights the importance of very early and perhaps more aggressive treatment in women with these conditions,” co-investigator Michelle M. Mielke, PhD, professor of epidemiology and neurology, Mayo Clinic, Rochester, Minn., told this news organization.
The findings were published online Jan. 5 in Neurology.
Assessing sex differences
Most previous studies in this area have focused on CV risk factors in midlife in relation to late-life dementia (after age 75) or on late-life vascular risk factors and late-life dementia, Dr. Mielke noted.
However, a few recent studies have suggested vascular risk factors can affect cognition even in midlife. The current investigators sought to determine whether there are sex differences in these associations.
They assessed 1,857 nondemented participants aged 50 to 69 years from the Mayo Clinic Study on Aging. The mean education level was 14.9 years, and the mean body mass index (BMI) was 29.7.
Among the participants, 78.9% had at least one CV condition or risk factor, and the proportion was higher in men than women (83.4% vs. 74.5%; P < .0001).
Frequency of each individual CV condition or risk factor was also higher in men than women, and they had more years of education and higher BMI but took fewer medications.
Every 15 months, participants had an in-person interview and physical examination that included a neurologic assessment and short test of memory.
The neuropsychological battery included nine tests across four domains: memory, language, executive function, and visuospatial skills. Researchers calculated z-scores for these domains and for global cognition.
Multiple cognitive domains
Whereas this study evaluated multiple cognitive domains, most previous research has focused on global cognitive decline and/or decline in only one or two cognitive domains, the investigators note.
They collected information from medical records on CV conditions such as CHD, arrhythmias, congestive heart failure, peripheral vascular disease (PVD), and stroke; and CV risk factors such as hypertension, diabetes, dyslipidemia, smoking status, and BMI.
Because of the small number of patients with stroke and PVD, these were classified as “other cardiovascular conditions” in the statistical analysis.
Researchers adjusted for sex, age, years of education, depressive symptoms, comorbidities, medications, and apolipoprotein E (APOE) genotyping. The mean follow-up was 3 years and did not differ by sex.
As some participants didn’t have a follow-up visit, the current analysis included 1,394 individuals. Those without follow-up visits were younger, had less education and more comorbidities, and took more medications compared with those with a follow-up.
Results showed most CV conditions were more strongly associated with cognitive function among women than men. For example, CHD was associated with global decline only in women (P < .05).
CHD, diabetes, and dyslipidemia were associated with language decline in women only (all, P < .05), but congestive heart failure was significantly associated with language decline in men only.
Dr. Mielke cautioned about reading too much into the language results for women.
“It’s an intriguing finding and definitely we need to follow up on it,” she said. However, “more studies are needed to examine sex differences before we start saying it only has an effect on language.”
‘Treat aggressively and right away’
The researchers were somewhat surprised by the study findings. Because there is a higher prevalence of CV conditions and risk factors in men, they presumed men would be more affected by these conditions, said Dr. Mielke.
“But that’s not what we saw; we saw the reverse. It was actually the women who were affected more by these cardiovascular risk factors and conditions,” she said.
As midlife is when women enter menopause, fluctuating estrogen levels may help explain the differential impact on cognition among women. But Dr. Mielke said she wants to “move beyond” just looking at hormones.
She pointed out there are a variety of psychosocial factors that may also contribute to an imbalance in the cognitive impact of CV conditions on women.
“Midlife is when many women are still taking care of their children at home, are also taking care of their adult parents, and may be undergoing more stress while continuing to do a job,” Dr. Miekle said.
Structural brain development and genetics may also contribute to the greater effect on cognition in women, the investigators note.
Dr. Mielke stressed that the current study only identifies associations. “The next steps are to understand what some of the underlying mechanisms for this are,” she said.
In the meantime, these new results suggest middle-aged women with high blood pressure, cholesterol, or glucose measures “should be treated aggressively and right away” said Dr. Mielke.
“For example, for women who are just starting to become hypertensive, clinicians should treat them right away and not watch and wait.”
Study limitations cited include that its sample was limited to Olmsted County, Minnesota – so results may not be generalized to other populations. Also, as researchers combined PVD and stroke into one group, larger sample sizes are needed, especially for stroke. Another limitation was the study did not have information on duration of all CV conditions or risk factors.
Helpful for tailoring interventions?
Commenting on the study, Glen R. Finney, MD, director, Memory and Cognition Program, Geisinger Health Clinic, Wilkes-Barre, Pennsylvania, said the results are important.
“The more we understand about risk factors for the development of Alzheimer’s disease and related dementias, the better we understand how we can reduce the risks,” said Dr. Finney, who was not involved with the research.
Awareness that CV conditions are major risk factors in midlife has been “definitely rising,” said Dr. Finney. “Many studies originally were looking at late life and are now looking more at earlier in the disease process, and I think that’s important.”
Understanding how sex, ethnicity, and other demographic variables affect risks can help to “tailor interventions” for individual patients, he said.
The study was supported by the National Institutes of Health, the GHR Foundation, and the Rochester Epidemiology Project. Dr. Mielke is a consultant for Biogen and Brain Protection Company and is on the editorial boards of Neurology and Alzheimer’s and Dementia. Dr. Finney has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SGLT2 inhibitors improve cardiovascular outcomes across groups
Sodium-glucose cotransporter 2 (SGLT2) inhibitors show “remarkable consistency of class benefit” for improving cardiovascular outcomes in high-risk people across age, sex, and race/ethnicity categories.
The findings, from a meta-analysis of 10 major randomized clinical trials, were published online Jan. 5, 2021, in JAMA Network Open by Mukul Bhattarai, MD, a cardiology fellow at Southern Illinois University, Springfield, and colleagues.
“Our meta-analysis evaluated a wide spectrum of efficacy outcomes, further characterizing the primary outcome in different subgroups from several well-designed large clinical trials. It supports that SGLT2 inhibitors have emerged as an effective class of drugs for improving cardiovascular morbidity and mortality, including the prevention of [hospitalization for heart failure] and reducing all-cause mortality in selected patients,” Dr. Bhattarai and colleagues wrote.
The cardiovascular outcomes of SGLT2 inhibitor therapy, they noted, “can be compared across all trials, and it demonstrates remarkable consistency of class benefit, despite the variations in populations enrolled.”
However, they also noted that SGLT inhibitors did not reduce the risk of acute MIn overall, and that most of the trials were short term, with a mean follow-up of just 2.3 years.
Ten trials, consistent cardiovascular benefits
Dr. Bhattarai and colleagues searched the literature through Jan. 10, 2021, as well as meeting presentations and other sources. They identified 10 placebo-controlled, randomized clinical trials in which participants had atherosclerotic cardiovascular disease or ASCVD risk factors, diabetes, or heart failure. Among a total of 71,553 high-risk patients, 39,053 received an SGLT2 inhibitor and 32,500 received a placebo.
The primary outcome of cardiovascular death or hospitalization for heart failure occurred in 8.10% randomized to SGLT2 inhibitors, compared with 11.56% in the placebo group, a significant difference with odds ratio 0.67 (P < .001). Both individual outcomes were lower in the SGLT2-inhibitor group, with a number needed to treat of 5.7 (P < .001).
Patients receiving SGLT2 inhibitors also had significantly lower rates of major adverse cardiovascular events, defined as death due to cardiovascular causes, nonfatal MI, or nonfatal stroke. Those events occurred in 9.82% versus 10.22%(OR, 0.90; P = .03).
Hospitalizations and ED visits with heart failure were also reduced with SGLT2 inhibitors (4.37% vs. 6.81%; OR, 0.67; P < .001), as was cardiovascular death (4.65% vs. 5.14%; OR, 0.87; P = .009). The reduction in heart failure is likely caused by a combination of a natriuretic effect and reduced interstitial fluid, along with inhibition of cardiac fibrosis, the authors said.
On the other hand, no reductions were seen in acute MI, evaluated in five of the studies. That event occurred in 4.66% taking SGLT2 inhibitors, compared with 4.70% of the placebo group, a nonsignificant difference with an OR of 0.95 (P = 0.22). This is likely because of the fact that SGLT2 inhibitors don’t have known antianginal properties or vasodilatory effects, they don’t reduce myocardial oxygen consumption, and they don’t prevent cardiac muscle remodeling, they noted.
All-cause mortality was significantly lower with SGLT2 inhibitors, though, at 7.09% versus 7.86% (odds ratio, 0.87; P = .004).
Benefits seen across age, sex, and race/ethnicity subgroups
While no differences in benefit were found between men and women when compared with placebo groups, the rates of cardiovascular death or heart failure hospitalizations were slightly higher in men than in women (9.01% [OR, 0.75; P < .001] vs. 5.34% [OR, 0.78; P = .002]).
By age, SGLT2 inhibitors benefited people both those younger than 65 years and those aged 65 years and older, although the primary outcome was slightly lower in the younger group (6.94% [OR, 0.79; P < 0.001] vs. 10.47% [OR, 0.78; P < .001]).
And by race, similar benefits from SGLT2 inhibitors were seen among individuals who were White, compared with those who were Asian, Black, or of other race/ethnicity, with event rates of 8.77% (OR, 0.82; P < .001) and 8.75% (OR, 0.66; P = .06), respectively.
“Owing to the short-term trial durations, future long-term prospective studies and postmarketing surveillance studies are warranted to discover the rate of cardiovascular outcomes,” Dr. Bhattarai and colleagues concluded.
The authors have no disclosures.
A version of this article first appeared on Medscape.com.
Sodium-glucose cotransporter 2 (SGLT2) inhibitors show “remarkable consistency of class benefit” for improving cardiovascular outcomes in high-risk people across age, sex, and race/ethnicity categories.
The findings, from a meta-analysis of 10 major randomized clinical trials, were published online Jan. 5, 2021, in JAMA Network Open by Mukul Bhattarai, MD, a cardiology fellow at Southern Illinois University, Springfield, and colleagues.
“Our meta-analysis evaluated a wide spectrum of efficacy outcomes, further characterizing the primary outcome in different subgroups from several well-designed large clinical trials. It supports that SGLT2 inhibitors have emerged as an effective class of drugs for improving cardiovascular morbidity and mortality, including the prevention of [hospitalization for heart failure] and reducing all-cause mortality in selected patients,” Dr. Bhattarai and colleagues wrote.
The cardiovascular outcomes of SGLT2 inhibitor therapy, they noted, “can be compared across all trials, and it demonstrates remarkable consistency of class benefit, despite the variations in populations enrolled.”
However, they also noted that SGLT inhibitors did not reduce the risk of acute MIn overall, and that most of the trials were short term, with a mean follow-up of just 2.3 years.
Ten trials, consistent cardiovascular benefits
Dr. Bhattarai and colleagues searched the literature through Jan. 10, 2021, as well as meeting presentations and other sources. They identified 10 placebo-controlled, randomized clinical trials in which participants had atherosclerotic cardiovascular disease or ASCVD risk factors, diabetes, or heart failure. Among a total of 71,553 high-risk patients, 39,053 received an SGLT2 inhibitor and 32,500 received a placebo.
The primary outcome of cardiovascular death or hospitalization for heart failure occurred in 8.10% randomized to SGLT2 inhibitors, compared with 11.56% in the placebo group, a significant difference with odds ratio 0.67 (P < .001). Both individual outcomes were lower in the SGLT2-inhibitor group, with a number needed to treat of 5.7 (P < .001).
Patients receiving SGLT2 inhibitors also had significantly lower rates of major adverse cardiovascular events, defined as death due to cardiovascular causes, nonfatal MI, or nonfatal stroke. Those events occurred in 9.82% versus 10.22%(OR, 0.90; P = .03).
Hospitalizations and ED visits with heart failure were also reduced with SGLT2 inhibitors (4.37% vs. 6.81%; OR, 0.67; P < .001), as was cardiovascular death (4.65% vs. 5.14%; OR, 0.87; P = .009). The reduction in heart failure is likely caused by a combination of a natriuretic effect and reduced interstitial fluid, along with inhibition of cardiac fibrosis, the authors said.
On the other hand, no reductions were seen in acute MI, evaluated in five of the studies. That event occurred in 4.66% taking SGLT2 inhibitors, compared with 4.70% of the placebo group, a nonsignificant difference with an OR of 0.95 (P = 0.22). This is likely because of the fact that SGLT2 inhibitors don’t have known antianginal properties or vasodilatory effects, they don’t reduce myocardial oxygen consumption, and they don’t prevent cardiac muscle remodeling, they noted.
All-cause mortality was significantly lower with SGLT2 inhibitors, though, at 7.09% versus 7.86% (odds ratio, 0.87; P = .004).
Benefits seen across age, sex, and race/ethnicity subgroups
While no differences in benefit were found between men and women when compared with placebo groups, the rates of cardiovascular death or heart failure hospitalizations were slightly higher in men than in women (9.01% [OR, 0.75; P < .001] vs. 5.34% [OR, 0.78; P = .002]).
By age, SGLT2 inhibitors benefited people both those younger than 65 years and those aged 65 years and older, although the primary outcome was slightly lower in the younger group (6.94% [OR, 0.79; P < 0.001] vs. 10.47% [OR, 0.78; P < .001]).
And by race, similar benefits from SGLT2 inhibitors were seen among individuals who were White, compared with those who were Asian, Black, or of other race/ethnicity, with event rates of 8.77% (OR, 0.82; P < .001) and 8.75% (OR, 0.66; P = .06), respectively.
“Owing to the short-term trial durations, future long-term prospective studies and postmarketing surveillance studies are warranted to discover the rate of cardiovascular outcomes,” Dr. Bhattarai and colleagues concluded.
The authors have no disclosures.
A version of this article first appeared on Medscape.com.
Sodium-glucose cotransporter 2 (SGLT2) inhibitors show “remarkable consistency of class benefit” for improving cardiovascular outcomes in high-risk people across age, sex, and race/ethnicity categories.
The findings, from a meta-analysis of 10 major randomized clinical trials, were published online Jan. 5, 2021, in JAMA Network Open by Mukul Bhattarai, MD, a cardiology fellow at Southern Illinois University, Springfield, and colleagues.
“Our meta-analysis evaluated a wide spectrum of efficacy outcomes, further characterizing the primary outcome in different subgroups from several well-designed large clinical trials. It supports that SGLT2 inhibitors have emerged as an effective class of drugs for improving cardiovascular morbidity and mortality, including the prevention of [hospitalization for heart failure] and reducing all-cause mortality in selected patients,” Dr. Bhattarai and colleagues wrote.
The cardiovascular outcomes of SGLT2 inhibitor therapy, they noted, “can be compared across all trials, and it demonstrates remarkable consistency of class benefit, despite the variations in populations enrolled.”
However, they also noted that SGLT inhibitors did not reduce the risk of acute MIn overall, and that most of the trials were short term, with a mean follow-up of just 2.3 years.
Ten trials, consistent cardiovascular benefits
Dr. Bhattarai and colleagues searched the literature through Jan. 10, 2021, as well as meeting presentations and other sources. They identified 10 placebo-controlled, randomized clinical trials in which participants had atherosclerotic cardiovascular disease or ASCVD risk factors, diabetes, or heart failure. Among a total of 71,553 high-risk patients, 39,053 received an SGLT2 inhibitor and 32,500 received a placebo.
The primary outcome of cardiovascular death or hospitalization for heart failure occurred in 8.10% randomized to SGLT2 inhibitors, compared with 11.56% in the placebo group, a significant difference with odds ratio 0.67 (P < .001). Both individual outcomes were lower in the SGLT2-inhibitor group, with a number needed to treat of 5.7 (P < .001).
Patients receiving SGLT2 inhibitors also had significantly lower rates of major adverse cardiovascular events, defined as death due to cardiovascular causes, nonfatal MI, or nonfatal stroke. Those events occurred in 9.82% versus 10.22%(OR, 0.90; P = .03).
Hospitalizations and ED visits with heart failure were also reduced with SGLT2 inhibitors (4.37% vs. 6.81%; OR, 0.67; P < .001), as was cardiovascular death (4.65% vs. 5.14%; OR, 0.87; P = .009). The reduction in heart failure is likely caused by a combination of a natriuretic effect and reduced interstitial fluid, along with inhibition of cardiac fibrosis, the authors said.
On the other hand, no reductions were seen in acute MI, evaluated in five of the studies. That event occurred in 4.66% taking SGLT2 inhibitors, compared with 4.70% of the placebo group, a nonsignificant difference with an OR of 0.95 (P = 0.22). This is likely because of the fact that SGLT2 inhibitors don’t have known antianginal properties or vasodilatory effects, they don’t reduce myocardial oxygen consumption, and they don’t prevent cardiac muscle remodeling, they noted.
All-cause mortality was significantly lower with SGLT2 inhibitors, though, at 7.09% versus 7.86% (odds ratio, 0.87; P = .004).
Benefits seen across age, sex, and race/ethnicity subgroups
While no differences in benefit were found between men and women when compared with placebo groups, the rates of cardiovascular death or heart failure hospitalizations were slightly higher in men than in women (9.01% [OR, 0.75; P < .001] vs. 5.34% [OR, 0.78; P = .002]).
By age, SGLT2 inhibitors benefited people both those younger than 65 years and those aged 65 years and older, although the primary outcome was slightly lower in the younger group (6.94% [OR, 0.79; P < 0.001] vs. 10.47% [OR, 0.78; P < .001]).
And by race, similar benefits from SGLT2 inhibitors were seen among individuals who were White, compared with those who were Asian, Black, or of other race/ethnicity, with event rates of 8.77% (OR, 0.82; P < .001) and 8.75% (OR, 0.66; P = .06), respectively.
“Owing to the short-term trial durations, future long-term prospective studies and postmarketing surveillance studies are warranted to discover the rate of cardiovascular outcomes,” Dr. Bhattarai and colleagues concluded.
The authors have no disclosures.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Fossilized blood proteins from child illness may cause chalky teeth
FROM FRONTIERS IN PHYSIOLOGY
Researchers have identified a potential cause of molar hypomineralization (MH), or “chalky teeth,” an underrecognized condition affecting one in five children worldwide. The discovery could lead to preventive medical therapies to reduce dental caries and extractions, they said.
According to a team led by biochemist Michael J. Hubbard, BDS, PhD, professor in the department of medicine, dentistry, and health sciences at the University of Melbourne, the “groundbreaking” research found that the failure of enamel to adequately harden is associated with exposure to serum albumin while teeth are developing. The blood protein “poisons” the growth of mineral crystals rather than injure the cells that deposit enamel, they reported.
The investigators, including researchers from Chile, said their findings hold promise for better clinical management of MH and open a new door into research on the broader pathogenesis and causes of the condition.
“We hope this breakthrough will eventually lead to medical prevention of MH, prompting global health benefits including major reductions in childhood tooth decay,” they wrote in an article published online Dec. 21 in Frontiers in Physiology.
More than cosmetic
Chalky teeth, characterized by discolored enamel spots, are not merely a cosmetic problem. The condition can lead to severe toothache, painful eating, tooth decay, and even abscesses and extractions. Although its triggers have eluded dental research for a century, Dr. Hubbard’s group said fossilized blood proteins such as albumin in the tooth appear to be at least one cause.
Biochemical evidence indicates that serum albumin surrounding developing teeth is normally excluded from enamel, Dr. Hubbard said in an interview. “Given that albumin binds strongly to hydroxyapatite-based mineral and blocks its growth, we infer that the epithelial barrier – the enamel-forming cells termed ameloblasts and normally responsible for excluding albumin – must break down in places in response to medical triggers.”
This breach enables localized infiltration of albumin, which then blocks further hardening of soft, immature enamel, leading to residual spots or patches of chalky enamel once the tooth eventually erupts into the mouth. “In other words, we infer that chalky enamel spots coincide with localized breaches of an epithelial barrier that are triggered by yet-to-be determined systemic insults,” he said.
Joseph Brofsky, DMD, section head of pediatric dentistry at North Shore LIJ Cohen Children’s Medical Center of New York, in Queens, agreed that that the definitive cause of MH has evaded identification for a hundred years. However, he expressed skepticism about the fossilized blood protein hypothesis.
“That’s a long shot. It’s a possibility, and I’m not ruling it out, but we’re not 100% sure,” said Dr. Brofsky, who was not involved in the research.
In his experience, MH is somewhat less prevalent in the United States, affecting about 1 in 10 children here, which is about half the global rate. “But it’s a problem, and we wish it would go away, but before we know beyond a reasonable doubt what causes this condition, it’s going to be hard to stop it.”
Most cases of MH involve hypomineralization of the 6-year molars, the first adult molars to erupt, but the process starts at birth. “For 6-year molars, normal hardening of dental enamel takes place from the early postnatal period through infancy,” Dr. Hubbard said.
The 2-year and 12-year molars are affected about half as frequently as their 6-year counterparts, “so this extends the medical-risk window out to early school days, and slightly back to the perinatal period for the 12-year and 2-year molars, respectively,” he said.
A critical question is which childhood illnesses are most likely to set the stage for MH, he added. “Forty-plus years of epidemiology have failed to nail a specific cause or causal association. But given the high prevalence of MH – 20% in otherwise healthy kids – naturally we suspect some common illnesses are the culprits,” he said. “But which diseases, which medications, and which combinations?”
Dr. Hubbard’s advice to pediatricians is to be alert to MH: “If you’re inspecting a child’s throat, then why not look at their back teeth, too – particularly when they’re getting their new molars at 2, 6, and 12 years?”
The study was supported by the Melbourne Research Unit for Facial Disorders Department of Pharmacology & Therapeutics, Department of Paediatrics, and Faculty of Medicine, Dentistry, and Health Sciences at the University of Melbourne. The authors and Dr. Brofsky have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM FRONTIERS IN PHYSIOLOGY
Researchers have identified a potential cause of molar hypomineralization (MH), or “chalky teeth,” an underrecognized condition affecting one in five children worldwide. The discovery could lead to preventive medical therapies to reduce dental caries and extractions, they said.
According to a team led by biochemist Michael J. Hubbard, BDS, PhD, professor in the department of medicine, dentistry, and health sciences at the University of Melbourne, the “groundbreaking” research found that the failure of enamel to adequately harden is associated with exposure to serum albumin while teeth are developing. The blood protein “poisons” the growth of mineral crystals rather than injure the cells that deposit enamel, they reported.
The investigators, including researchers from Chile, said their findings hold promise for better clinical management of MH and open a new door into research on the broader pathogenesis and causes of the condition.
“We hope this breakthrough will eventually lead to medical prevention of MH, prompting global health benefits including major reductions in childhood tooth decay,” they wrote in an article published online Dec. 21 in Frontiers in Physiology.
More than cosmetic
Chalky teeth, characterized by discolored enamel spots, are not merely a cosmetic problem. The condition can lead to severe toothache, painful eating, tooth decay, and even abscesses and extractions. Although its triggers have eluded dental research for a century, Dr. Hubbard’s group said fossilized blood proteins such as albumin in the tooth appear to be at least one cause.
Biochemical evidence indicates that serum albumin surrounding developing teeth is normally excluded from enamel, Dr. Hubbard said in an interview. “Given that albumin binds strongly to hydroxyapatite-based mineral and blocks its growth, we infer that the epithelial barrier – the enamel-forming cells termed ameloblasts and normally responsible for excluding albumin – must break down in places in response to medical triggers.”
This breach enables localized infiltration of albumin, which then blocks further hardening of soft, immature enamel, leading to residual spots or patches of chalky enamel once the tooth eventually erupts into the mouth. “In other words, we infer that chalky enamel spots coincide with localized breaches of an epithelial barrier that are triggered by yet-to-be determined systemic insults,” he said.
Joseph Brofsky, DMD, section head of pediatric dentistry at North Shore LIJ Cohen Children’s Medical Center of New York, in Queens, agreed that that the definitive cause of MH has evaded identification for a hundred years. However, he expressed skepticism about the fossilized blood protein hypothesis.
“That’s a long shot. It’s a possibility, and I’m not ruling it out, but we’re not 100% sure,” said Dr. Brofsky, who was not involved in the research.
In his experience, MH is somewhat less prevalent in the United States, affecting about 1 in 10 children here, which is about half the global rate. “But it’s a problem, and we wish it would go away, but before we know beyond a reasonable doubt what causes this condition, it’s going to be hard to stop it.”
Most cases of MH involve hypomineralization of the 6-year molars, the first adult molars to erupt, but the process starts at birth. “For 6-year molars, normal hardening of dental enamel takes place from the early postnatal period through infancy,” Dr. Hubbard said.
The 2-year and 12-year molars are affected about half as frequently as their 6-year counterparts, “so this extends the medical-risk window out to early school days, and slightly back to the perinatal period for the 12-year and 2-year molars, respectively,” he said.
A critical question is which childhood illnesses are most likely to set the stage for MH, he added. “Forty-plus years of epidemiology have failed to nail a specific cause or causal association. But given the high prevalence of MH – 20% in otherwise healthy kids – naturally we suspect some common illnesses are the culprits,” he said. “But which diseases, which medications, and which combinations?”
Dr. Hubbard’s advice to pediatricians is to be alert to MH: “If you’re inspecting a child’s throat, then why not look at their back teeth, too – particularly when they’re getting their new molars at 2, 6, and 12 years?”
The study was supported by the Melbourne Research Unit for Facial Disorders Department of Pharmacology & Therapeutics, Department of Paediatrics, and Faculty of Medicine, Dentistry, and Health Sciences at the University of Melbourne. The authors and Dr. Brofsky have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM FRONTIERS IN PHYSIOLOGY
Researchers have identified a potential cause of molar hypomineralization (MH), or “chalky teeth,” an underrecognized condition affecting one in five children worldwide. The discovery could lead to preventive medical therapies to reduce dental caries and extractions, they said.
According to a team led by biochemist Michael J. Hubbard, BDS, PhD, professor in the department of medicine, dentistry, and health sciences at the University of Melbourne, the “groundbreaking” research found that the failure of enamel to adequately harden is associated with exposure to serum albumin while teeth are developing. The blood protein “poisons” the growth of mineral crystals rather than injure the cells that deposit enamel, they reported.
The investigators, including researchers from Chile, said their findings hold promise for better clinical management of MH and open a new door into research on the broader pathogenesis and causes of the condition.
“We hope this breakthrough will eventually lead to medical prevention of MH, prompting global health benefits including major reductions in childhood tooth decay,” they wrote in an article published online Dec. 21 in Frontiers in Physiology.
More than cosmetic
Chalky teeth, characterized by discolored enamel spots, are not merely a cosmetic problem. The condition can lead to severe toothache, painful eating, tooth decay, and even abscesses and extractions. Although its triggers have eluded dental research for a century, Dr. Hubbard’s group said fossilized blood proteins such as albumin in the tooth appear to be at least one cause.
Biochemical evidence indicates that serum albumin surrounding developing teeth is normally excluded from enamel, Dr. Hubbard said in an interview. “Given that albumin binds strongly to hydroxyapatite-based mineral and blocks its growth, we infer that the epithelial barrier – the enamel-forming cells termed ameloblasts and normally responsible for excluding albumin – must break down in places in response to medical triggers.”
This breach enables localized infiltration of albumin, which then blocks further hardening of soft, immature enamel, leading to residual spots or patches of chalky enamel once the tooth eventually erupts into the mouth. “In other words, we infer that chalky enamel spots coincide with localized breaches of an epithelial barrier that are triggered by yet-to-be determined systemic insults,” he said.
Joseph Brofsky, DMD, section head of pediatric dentistry at North Shore LIJ Cohen Children’s Medical Center of New York, in Queens, agreed that that the definitive cause of MH has evaded identification for a hundred years. However, he expressed skepticism about the fossilized blood protein hypothesis.
“That’s a long shot. It’s a possibility, and I’m not ruling it out, but we’re not 100% sure,” said Dr. Brofsky, who was not involved in the research.
In his experience, MH is somewhat less prevalent in the United States, affecting about 1 in 10 children here, which is about half the global rate. “But it’s a problem, and we wish it would go away, but before we know beyond a reasonable doubt what causes this condition, it’s going to be hard to stop it.”
Most cases of MH involve hypomineralization of the 6-year molars, the first adult molars to erupt, but the process starts at birth. “For 6-year molars, normal hardening of dental enamel takes place from the early postnatal period through infancy,” Dr. Hubbard said.
The 2-year and 12-year molars are affected about half as frequently as their 6-year counterparts, “so this extends the medical-risk window out to early school days, and slightly back to the perinatal period for the 12-year and 2-year molars, respectively,” he said.
A critical question is which childhood illnesses are most likely to set the stage for MH, he added. “Forty-plus years of epidemiology have failed to nail a specific cause or causal association. But given the high prevalence of MH – 20% in otherwise healthy kids – naturally we suspect some common illnesses are the culprits,” he said. “But which diseases, which medications, and which combinations?”
Dr. Hubbard’s advice to pediatricians is to be alert to MH: “If you’re inspecting a child’s throat, then why not look at their back teeth, too – particularly when they’re getting their new molars at 2, 6, and 12 years?”
The study was supported by the Melbourne Research Unit for Facial Disorders Department of Pharmacology & Therapeutics, Department of Paediatrics, and Faculty of Medicine, Dentistry, and Health Sciences at the University of Melbourne. The authors and Dr. Brofsky have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID-19 vaccination has little impact on menstrual cycle
Women may rest a bit easier thanks to results from a study showing that vaccination against the SARS-CoV-2 virus has almost no impact on a woman’s menstrual cycle. The issue is significant, as regular menstruation is a sign of health and fertility, and fears of disturbances might increase vaccination hesitancy as COVID-19 cases continue to surge.
Alison Edelman, MD, MPH, a professor of obstetrics and gynecology at Oregon Health & Science University, Portland, led a group studying prospective data on almost 24,000 menstrual cycles reported by almost 4,000 U.S. women.
The investigators found that COVID-19 vaccination was associated with a less than 1-day change in cycle length for the menstrual cycles after the first and second inoculations, compared with prevaccine cycles. Vaccination had no effect on the actual number of days menstrual bleeding lasted.
The study looked at the menstrual patterns of women aged 18-45 years with normal cycle lengths of 24-38 days for the three consecutive cycles before the first vaccine dose and for three consecutive postvaccine cycles. The final sample included 2,403 vaccinated and 1,556 unvaccinated individuals.
In vaccinated women, the study initially found a slight average increase in cycle length after dose one of 71% of a day and 91% of a day after dose two. Following adjustments, those increases dropped to 64% of a day after the first dose and 79% of a day after the second dose.
In unvaccinated women, the study looked at six cycles over a similar time period and found no significant changes from baseline.
“Coronavirus disease 2019 vaccination is associated with a small change in cycle length but not menses length,” Dr. Edelman’s group concluded in Obstetrics and Gynecology.
In the rare instance that a woman received two vaccine doses within the same menstrual cycle, the change in length could increase to 2 days. These variations appear to resolve quickly, possibly as soon as the next cycle after vaccination and do not indicate any cause for long-term physical or reproductive health concern, according to the authors.
Reports by women on social media, however, have suggested that postvaccine menstrual disruptions are more common with, for example, heavier and breakthrough bleeding. But it appears such changes are temporary and resolve quickly.
“These findings are reassuring and validating,” Dr. Edelman said in an interview. On a population level, the changes indicate no cause for concern for long-term physical or reproductive health and no reason to avoid vaccination. “On a personal level, people want this information so they know what to expect when they get vaccinated, and not worry about a pregnancy scare or be disappointed if they were trying for pregnancy.”
According to the International Federation of Gynecologists and Obstetricians, variations in cycle length of fewer than 8 days are considered normal, said Christine Metz, PhD, a research biologist and a professor of molecular medicine at the Feinstein Institutes for Medical Research in Manhasset, N.Y. “Thus, the extra 17 hours added to the menstrual cycle length in the vaccination group in this study is well within the ‘normal’ range.”
In a group of about 1,600 menstruating women being studied at Dr. Metz’s center, some have anecdotally reported transient cycle changes post vaccination for COVID-19, including delays in menstruation onset and changes in bleeding patterns.
Exactly how vaccination might alter menstrual cycle length is not known and has not been studied with vaccination against other infections such as influenza and meningococcal disease.
“Many factors are known to affect menstrual cycle length including changes in diet, sleep, and exercise, as well as sickness, travel, and stress,” Dr. Metz said. The COVID-19 vaccines have affected people in different ways, with side effects ranging from injection-site pain to nausea, aches, fever, and fatigue. “Vaccination side effects, particularly if severe, could lead to changes in diet, exercise, and sleep, and feelings of sickness and/or stress.”
These stressors can alter hormone production and stability, as well as the body’s response to hormones such as estrogen, progesterone, follicle-stimulating hormone, luteinizing hormone, and other hormones associated with female reproduction. “Because these hormones regulate the menstrual cycle, variations in these hormones can either shorten or lengthen the cycle,” Dr. Metz explained.
More research needs to be done at the global level, according to the authors. “Questions remain about other possible changes in menstrual cycles, such as menstrual symptoms, unscheduled bleeding, and changes in the quality and quantity of menstrual bleeding.”
This research was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institutes of Health’s Office of Research on Women’s Health. Dr. Edelman reported support from the American College of Obstetrics and Gynecology, the World Health Organization, Gynuity, and the Karolinska Institute as well as royalties from UpToDate. Other study authors reported similar relationships with not-for-profit and private-sector companies. Three coauthors are employees of Natural Cycles, a fertility tracking device that was used in the study. Dr. Metz disclosed no conflicts of interest with regard to her comments.
Women may rest a bit easier thanks to results from a study showing that vaccination against the SARS-CoV-2 virus has almost no impact on a woman’s menstrual cycle. The issue is significant, as regular menstruation is a sign of health and fertility, and fears of disturbances might increase vaccination hesitancy as COVID-19 cases continue to surge.
Alison Edelman, MD, MPH, a professor of obstetrics and gynecology at Oregon Health & Science University, Portland, led a group studying prospective data on almost 24,000 menstrual cycles reported by almost 4,000 U.S. women.
The investigators found that COVID-19 vaccination was associated with a less than 1-day change in cycle length for the menstrual cycles after the first and second inoculations, compared with prevaccine cycles. Vaccination had no effect on the actual number of days menstrual bleeding lasted.
The study looked at the menstrual patterns of women aged 18-45 years with normal cycle lengths of 24-38 days for the three consecutive cycles before the first vaccine dose and for three consecutive postvaccine cycles. The final sample included 2,403 vaccinated and 1,556 unvaccinated individuals.
In vaccinated women, the study initially found a slight average increase in cycle length after dose one of 71% of a day and 91% of a day after dose two. Following adjustments, those increases dropped to 64% of a day after the first dose and 79% of a day after the second dose.
In unvaccinated women, the study looked at six cycles over a similar time period and found no significant changes from baseline.
“Coronavirus disease 2019 vaccination is associated with a small change in cycle length but not menses length,” Dr. Edelman’s group concluded in Obstetrics and Gynecology.
In the rare instance that a woman received two vaccine doses within the same menstrual cycle, the change in length could increase to 2 days. These variations appear to resolve quickly, possibly as soon as the next cycle after vaccination and do not indicate any cause for long-term physical or reproductive health concern, according to the authors.
Reports by women on social media, however, have suggested that postvaccine menstrual disruptions are more common with, for example, heavier and breakthrough bleeding. But it appears such changes are temporary and resolve quickly.
“These findings are reassuring and validating,” Dr. Edelman said in an interview. On a population level, the changes indicate no cause for concern for long-term physical or reproductive health and no reason to avoid vaccination. “On a personal level, people want this information so they know what to expect when they get vaccinated, and not worry about a pregnancy scare or be disappointed if they were trying for pregnancy.”
According to the International Federation of Gynecologists and Obstetricians, variations in cycle length of fewer than 8 days are considered normal, said Christine Metz, PhD, a research biologist and a professor of molecular medicine at the Feinstein Institutes for Medical Research in Manhasset, N.Y. “Thus, the extra 17 hours added to the menstrual cycle length in the vaccination group in this study is well within the ‘normal’ range.”
In a group of about 1,600 menstruating women being studied at Dr. Metz’s center, some have anecdotally reported transient cycle changes post vaccination for COVID-19, including delays in menstruation onset and changes in bleeding patterns.
Exactly how vaccination might alter menstrual cycle length is not known and has not been studied with vaccination against other infections such as influenza and meningococcal disease.
“Many factors are known to affect menstrual cycle length including changes in diet, sleep, and exercise, as well as sickness, travel, and stress,” Dr. Metz said. The COVID-19 vaccines have affected people in different ways, with side effects ranging from injection-site pain to nausea, aches, fever, and fatigue. “Vaccination side effects, particularly if severe, could lead to changes in diet, exercise, and sleep, and feelings of sickness and/or stress.”
These stressors can alter hormone production and stability, as well as the body’s response to hormones such as estrogen, progesterone, follicle-stimulating hormone, luteinizing hormone, and other hormones associated with female reproduction. “Because these hormones regulate the menstrual cycle, variations in these hormones can either shorten or lengthen the cycle,” Dr. Metz explained.
More research needs to be done at the global level, according to the authors. “Questions remain about other possible changes in menstrual cycles, such as menstrual symptoms, unscheduled bleeding, and changes in the quality and quantity of menstrual bleeding.”
This research was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institutes of Health’s Office of Research on Women’s Health. Dr. Edelman reported support from the American College of Obstetrics and Gynecology, the World Health Organization, Gynuity, and the Karolinska Institute as well as royalties from UpToDate. Other study authors reported similar relationships with not-for-profit and private-sector companies. Three coauthors are employees of Natural Cycles, a fertility tracking device that was used in the study. Dr. Metz disclosed no conflicts of interest with regard to her comments.
Women may rest a bit easier thanks to results from a study showing that vaccination against the SARS-CoV-2 virus has almost no impact on a woman’s menstrual cycle. The issue is significant, as regular menstruation is a sign of health and fertility, and fears of disturbances might increase vaccination hesitancy as COVID-19 cases continue to surge.
Alison Edelman, MD, MPH, a professor of obstetrics and gynecology at Oregon Health & Science University, Portland, led a group studying prospective data on almost 24,000 menstrual cycles reported by almost 4,000 U.S. women.
The investigators found that COVID-19 vaccination was associated with a less than 1-day change in cycle length for the menstrual cycles after the first and second inoculations, compared with prevaccine cycles. Vaccination had no effect on the actual number of days menstrual bleeding lasted.
The study looked at the menstrual patterns of women aged 18-45 years with normal cycle lengths of 24-38 days for the three consecutive cycles before the first vaccine dose and for three consecutive postvaccine cycles. The final sample included 2,403 vaccinated and 1,556 unvaccinated individuals.
In vaccinated women, the study initially found a slight average increase in cycle length after dose one of 71% of a day and 91% of a day after dose two. Following adjustments, those increases dropped to 64% of a day after the first dose and 79% of a day after the second dose.
In unvaccinated women, the study looked at six cycles over a similar time period and found no significant changes from baseline.
“Coronavirus disease 2019 vaccination is associated with a small change in cycle length but not menses length,” Dr. Edelman’s group concluded in Obstetrics and Gynecology.
In the rare instance that a woman received two vaccine doses within the same menstrual cycle, the change in length could increase to 2 days. These variations appear to resolve quickly, possibly as soon as the next cycle after vaccination and do not indicate any cause for long-term physical or reproductive health concern, according to the authors.
Reports by women on social media, however, have suggested that postvaccine menstrual disruptions are more common with, for example, heavier and breakthrough bleeding. But it appears such changes are temporary and resolve quickly.
“These findings are reassuring and validating,” Dr. Edelman said in an interview. On a population level, the changes indicate no cause for concern for long-term physical or reproductive health and no reason to avoid vaccination. “On a personal level, people want this information so they know what to expect when they get vaccinated, and not worry about a pregnancy scare or be disappointed if they were trying for pregnancy.”
According to the International Federation of Gynecologists and Obstetricians, variations in cycle length of fewer than 8 days are considered normal, said Christine Metz, PhD, a research biologist and a professor of molecular medicine at the Feinstein Institutes for Medical Research in Manhasset, N.Y. “Thus, the extra 17 hours added to the menstrual cycle length in the vaccination group in this study is well within the ‘normal’ range.”
In a group of about 1,600 menstruating women being studied at Dr. Metz’s center, some have anecdotally reported transient cycle changes post vaccination for COVID-19, including delays in menstruation onset and changes in bleeding patterns.
Exactly how vaccination might alter menstrual cycle length is not known and has not been studied with vaccination against other infections such as influenza and meningococcal disease.
“Many factors are known to affect menstrual cycle length including changes in diet, sleep, and exercise, as well as sickness, travel, and stress,” Dr. Metz said. The COVID-19 vaccines have affected people in different ways, with side effects ranging from injection-site pain to nausea, aches, fever, and fatigue. “Vaccination side effects, particularly if severe, could lead to changes in diet, exercise, and sleep, and feelings of sickness and/or stress.”
These stressors can alter hormone production and stability, as well as the body’s response to hormones such as estrogen, progesterone, follicle-stimulating hormone, luteinizing hormone, and other hormones associated with female reproduction. “Because these hormones regulate the menstrual cycle, variations in these hormones can either shorten or lengthen the cycle,” Dr. Metz explained.
More research needs to be done at the global level, according to the authors. “Questions remain about other possible changes in menstrual cycles, such as menstrual symptoms, unscheduled bleeding, and changes in the quality and quantity of menstrual bleeding.”
This research was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institutes of Health’s Office of Research on Women’s Health. Dr. Edelman reported support from the American College of Obstetrics and Gynecology, the World Health Organization, Gynuity, and the Karolinska Institute as well as royalties from UpToDate. Other study authors reported similar relationships with not-for-profit and private-sector companies. Three coauthors are employees of Natural Cycles, a fertility tracking device that was used in the study. Dr. Metz disclosed no conflicts of interest with regard to her comments.
FROM OBSTETRICS & GYNECOLOGY
Medicare expands coverage of continuous glucose monitoring devices for diabetes
Proposed in November 2020, the final CMS rule applies primarily to CGMs that integrate with Medtronic insulin pumps. Those CGMs have not been approved by the Food and Drug Administration to replace the need for fingerstick blood glucose measurements in determining insulin or other glucose-lowering medication dosing.
Other CGM systems, Dexcom G6 and Abbott Libre, have “therapeutic” indications and were already covered under Medicare, as was the combined insulin pump–CGM Tandem Diabetes Care Control-IQ technology system.
The expanded coverage means that people using the Medtronic 770G or 630G hybrid closed-loop insulin delivery systems will receive coverage for all the systems’ components, and that people aging into Medicare won’t lose any coverage for those devices.
Medtronic will continue to offer its CGM Access Discount to all Medicare customers until the ruling takes effect. The proposed rule was finalized on Dec. 21, 2021, and will be effective starting 60 days after official publication.
A version of this article first appeared on Medscape.com.
Proposed in November 2020, the final CMS rule applies primarily to CGMs that integrate with Medtronic insulin pumps. Those CGMs have not been approved by the Food and Drug Administration to replace the need for fingerstick blood glucose measurements in determining insulin or other glucose-lowering medication dosing.
Other CGM systems, Dexcom G6 and Abbott Libre, have “therapeutic” indications and were already covered under Medicare, as was the combined insulin pump–CGM Tandem Diabetes Care Control-IQ technology system.
The expanded coverage means that people using the Medtronic 770G or 630G hybrid closed-loop insulin delivery systems will receive coverage for all the systems’ components, and that people aging into Medicare won’t lose any coverage for those devices.
Medtronic will continue to offer its CGM Access Discount to all Medicare customers until the ruling takes effect. The proposed rule was finalized on Dec. 21, 2021, and will be effective starting 60 days after official publication.
A version of this article first appeared on Medscape.com.
Proposed in November 2020, the final CMS rule applies primarily to CGMs that integrate with Medtronic insulin pumps. Those CGMs have not been approved by the Food and Drug Administration to replace the need for fingerstick blood glucose measurements in determining insulin or other glucose-lowering medication dosing.
Other CGM systems, Dexcom G6 and Abbott Libre, have “therapeutic” indications and were already covered under Medicare, as was the combined insulin pump–CGM Tandem Diabetes Care Control-IQ technology system.
The expanded coverage means that people using the Medtronic 770G or 630G hybrid closed-loop insulin delivery systems will receive coverage for all the systems’ components, and that people aging into Medicare won’t lose any coverage for those devices.
Medtronic will continue to offer its CGM Access Discount to all Medicare customers until the ruling takes effect. The proposed rule was finalized on Dec. 21, 2021, and will be effective starting 60 days after official publication.
A version of this article first appeared on Medscape.com.
Effective alternatives to psychotherapy for borderline personality disorder
Early interventions that focus on clinical case management and psychiatric care, and not necessarily on individual psychotherapy, are effective for young patients with borderline personality disorder (BPD), new research suggests.
Findings from the Monitoring Outcomes of Borderline Personality Disorder in Youth (MOBY) trial also showed improved psychosocial functioning and reduced suicide ideation with these therapies.
The results suggest that, contrary to common belief, psychotherapy is not the only effective approach for early BPD, lead author Andrew M. Chanen, PhD, director of clinical programs and services and head of personality disorder research at Orygen, Melbourne, told this news organization.
“We can say that early diagnosis and early treatment is effective, and the treatment doesn’t need to involve individual psychotherapy but does need to involve clinical case management and psychiatric care,” said Dr. Chanen, a professorial fellow at the Centre for Youth Mental Health, University of Melbourne.
The findings were published online in JAMA Psychiatry.
Extreme sensitivity
Patients with BPD have “extreme sensitivity to interpersonal slights” and often exhibit intense and volatile emotions and impulsive behavior, Dr. Chanen noted. Many will self-harm, abuse drugs, or attempt suicide; the suicide rate among patients with BPD is 8%-10%.
The condition is typically diagnosed in puberty or early adulthood, affecting about 3% of young people and a little more than 1% of adults.
Because of their aggression and interpersonal difficulties, patients with BPD are often discriminated against by health professionals and end up not getting treated, said Dr. Chanen.
Those who are treated often receive individual psychotherapy, such as dialectical behavior therapy (DBT). That type of therapy, which teaches healthy ways to cope with stress and regulate emotions, is very effective, Dr. Chanen said.
The MOBY trial examined three treatment approaches: the Helping Young People Early (HYPE) model, HYPE combined with weekly “befriending,” and a general youth mental health service (YMHS) model combined with befriending.
A key element of HYPE is cognitive analytic therapy, a psychotherapy program focused on understanding problematic self-management and interpersonal relationship patterns. The model includes clinical case management, such as attending to housing, vocational and educational issues, other mental health needs, and physical health needs.
In the second model, the psychotherapy of the HYPE program was replaced with befriending, which involves chatting with a patient about neutral topics such as sports and avoiding emotionally loaded topics such as interpersonal problems.
For YMHS plus befriending, experts trained in treating young people, but not specialized in treating BPD, were involved in managing patients.
‘High satisfaction’
Researchers randomly assigned 139 participants aged 15-25 years (80.6% women; mean age, 19.1 years) with BPD to one of the treatment arms. Of these, 128 (92.1%) were included in the intent-to-treat analysis.
The primary endpoint was psychosocial functioning, as measured by the Inventory of Interpersonal Problems Circumplex Version and the Social Adjustment Scale–Self-Report. Secondary endpoints included suicidal ideation, suicide attempts, nonsuicidal self-injury, depression, substance use, and treatment satisfaction.
The investigators reported group averages, but the study’s noninferiority design did not allow for determining if one treatment had superior efficacy.
All groups improved significantly on the primary endpoint. At 12 months, there was a mean 28.91-point (23.8%) drop in interpersonal problems and a mean 0.55-point (19.3%) drop in social adjustment scores.
For secondary outcomes, mean improvements at 12 months ranged from 40.7% (17.64 points) on the depression scale to 52.7% (6.22 points) for suicide ideation.
“The only area where the treatment didn’t really have an impact was substance use,” said Dr. Chanen. “Satisfaction was high for all three interventions throughout the study, and it’s hard to improve on high satisfaction.”
‘Turns things upside down’
That patients across all groups had marked and sustained improvements “in ways you wouldn’t expect for BPD” supports the conclusion that the interventions had a true effect, Dr. Chanen said.
he added.
They also imply there are effective alternatives to psychotherapy, which many individuals in the field insist is the only way to treat BPD. “This study turns things upside down and says actually it’s not. It’s the basics of treatment that are important,” Dr. Chanen said.
When a patient presents at the emergency department following a severe overdose, “it’s a reflex” for clinicians to refer that person to a psychotherapy program. “The problem is, these programs are not plentiful enough to be able to service the needs of this group,” Dr. Chanen noted.
On the other hand, the skills for clinical case management and psychiatric care “are available throughout the mental health systems,” he added.
The researchers are planning another analysis to determine whether age and sex predict better outcomes in these patients with BPD.
Unique contribution
Commenting for this news organization, John M. Oldham, MD, distinguished emeritus professor, Baylor College of Medicine, Houston, said a “unique and important contribution” of the study is the focus on early intervention.
“The general standard approach in psychiatry and the diagnostic world has been to not even consider anything until after somebody is 18 years of age, which is a mistake because these kids can become quite impaired earlier than that,” he said.
Dr. Oldham, who was not involved with the research, chaired the American Psychiatric Association workgroup that developed the 2001 evidence-based practice guideline for treating BPD, which recommended psychotherapy as the primary treatment. The guideline was last updated in 2005 – and another update is currently being developed, he noted.
There is an emerging trend toward “good psychiatric management” that focuses on level of functioning rather than on a specific strategy requiring a certificate of training that “not many people out there have,” said Dr. Oldham.
“You’re not going to make much headway with these kids if you’re going to be searching around for a DBT-certified therapist. What you need is to bring them in, get them to trust you, and in a sense be a kind of overall behavioral medicine navigator for them,” he added.
Dr. Oldham noted that, although the primary study outcome improved between 19% and 24%, “that means three-quarters of the people didn’t improve.”
He also pointed out this was only a 1-year trial. “Sometimes treatment for people with a personality disorder such as borderline takes a lot longer than that,” Dr. Oldham concluded.
The trial was funded by the National Health and Medical Research Council. Dr. Chanen reports receiving grants from the Australian government’s National Health and Medical Research Council during the conduct of the study and other support from the Helping Young People Early (HYPE) translational program outside the submitted work. He and another investigator cofounded and lead the HYPE clinical program, a government-funded program with continuous support, and the HYPE translational program, a not-for-profit training program. Dr. Oldham reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Early interventions that focus on clinical case management and psychiatric care, and not necessarily on individual psychotherapy, are effective for young patients with borderline personality disorder (BPD), new research suggests.
Findings from the Monitoring Outcomes of Borderline Personality Disorder in Youth (MOBY) trial also showed improved psychosocial functioning and reduced suicide ideation with these therapies.
The results suggest that, contrary to common belief, psychotherapy is not the only effective approach for early BPD, lead author Andrew M. Chanen, PhD, director of clinical programs and services and head of personality disorder research at Orygen, Melbourne, told this news organization.
“We can say that early diagnosis and early treatment is effective, and the treatment doesn’t need to involve individual psychotherapy but does need to involve clinical case management and psychiatric care,” said Dr. Chanen, a professorial fellow at the Centre for Youth Mental Health, University of Melbourne.
The findings were published online in JAMA Psychiatry.
Extreme sensitivity
Patients with BPD have “extreme sensitivity to interpersonal slights” and often exhibit intense and volatile emotions and impulsive behavior, Dr. Chanen noted. Many will self-harm, abuse drugs, or attempt suicide; the suicide rate among patients with BPD is 8%-10%.
The condition is typically diagnosed in puberty or early adulthood, affecting about 3% of young people and a little more than 1% of adults.
Because of their aggression and interpersonal difficulties, patients with BPD are often discriminated against by health professionals and end up not getting treated, said Dr. Chanen.
Those who are treated often receive individual psychotherapy, such as dialectical behavior therapy (DBT). That type of therapy, which teaches healthy ways to cope with stress and regulate emotions, is very effective, Dr. Chanen said.
The MOBY trial examined three treatment approaches: the Helping Young People Early (HYPE) model, HYPE combined with weekly “befriending,” and a general youth mental health service (YMHS) model combined with befriending.
A key element of HYPE is cognitive analytic therapy, a psychotherapy program focused on understanding problematic self-management and interpersonal relationship patterns. The model includes clinical case management, such as attending to housing, vocational and educational issues, other mental health needs, and physical health needs.
In the second model, the psychotherapy of the HYPE program was replaced with befriending, which involves chatting with a patient about neutral topics such as sports and avoiding emotionally loaded topics such as interpersonal problems.
For YMHS plus befriending, experts trained in treating young people, but not specialized in treating BPD, were involved in managing patients.
‘High satisfaction’
Researchers randomly assigned 139 participants aged 15-25 years (80.6% women; mean age, 19.1 years) with BPD to one of the treatment arms. Of these, 128 (92.1%) were included in the intent-to-treat analysis.
The primary endpoint was psychosocial functioning, as measured by the Inventory of Interpersonal Problems Circumplex Version and the Social Adjustment Scale–Self-Report. Secondary endpoints included suicidal ideation, suicide attempts, nonsuicidal self-injury, depression, substance use, and treatment satisfaction.
The investigators reported group averages, but the study’s noninferiority design did not allow for determining if one treatment had superior efficacy.
All groups improved significantly on the primary endpoint. At 12 months, there was a mean 28.91-point (23.8%) drop in interpersonal problems and a mean 0.55-point (19.3%) drop in social adjustment scores.
For secondary outcomes, mean improvements at 12 months ranged from 40.7% (17.64 points) on the depression scale to 52.7% (6.22 points) for suicide ideation.
“The only area where the treatment didn’t really have an impact was substance use,” said Dr. Chanen. “Satisfaction was high for all three interventions throughout the study, and it’s hard to improve on high satisfaction.”
‘Turns things upside down’
That patients across all groups had marked and sustained improvements “in ways you wouldn’t expect for BPD” supports the conclusion that the interventions had a true effect, Dr. Chanen said.
he added.
They also imply there are effective alternatives to psychotherapy, which many individuals in the field insist is the only way to treat BPD. “This study turns things upside down and says actually it’s not. It’s the basics of treatment that are important,” Dr. Chanen said.
When a patient presents at the emergency department following a severe overdose, “it’s a reflex” for clinicians to refer that person to a psychotherapy program. “The problem is, these programs are not plentiful enough to be able to service the needs of this group,” Dr. Chanen noted.
On the other hand, the skills for clinical case management and psychiatric care “are available throughout the mental health systems,” he added.
The researchers are planning another analysis to determine whether age and sex predict better outcomes in these patients with BPD.
Unique contribution
Commenting for this news organization, John M. Oldham, MD, distinguished emeritus professor, Baylor College of Medicine, Houston, said a “unique and important contribution” of the study is the focus on early intervention.
“The general standard approach in psychiatry and the diagnostic world has been to not even consider anything until after somebody is 18 years of age, which is a mistake because these kids can become quite impaired earlier than that,” he said.
Dr. Oldham, who was not involved with the research, chaired the American Psychiatric Association workgroup that developed the 2001 evidence-based practice guideline for treating BPD, which recommended psychotherapy as the primary treatment. The guideline was last updated in 2005 – and another update is currently being developed, he noted.
There is an emerging trend toward “good psychiatric management” that focuses on level of functioning rather than on a specific strategy requiring a certificate of training that “not many people out there have,” said Dr. Oldham.
“You’re not going to make much headway with these kids if you’re going to be searching around for a DBT-certified therapist. What you need is to bring them in, get them to trust you, and in a sense be a kind of overall behavioral medicine navigator for them,” he added.
Dr. Oldham noted that, although the primary study outcome improved between 19% and 24%, “that means three-quarters of the people didn’t improve.”
He also pointed out this was only a 1-year trial. “Sometimes treatment for people with a personality disorder such as borderline takes a lot longer than that,” Dr. Oldham concluded.
The trial was funded by the National Health and Medical Research Council. Dr. Chanen reports receiving grants from the Australian government’s National Health and Medical Research Council during the conduct of the study and other support from the Helping Young People Early (HYPE) translational program outside the submitted work. He and another investigator cofounded and lead the HYPE clinical program, a government-funded program with continuous support, and the HYPE translational program, a not-for-profit training program. Dr. Oldham reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Early interventions that focus on clinical case management and psychiatric care, and not necessarily on individual psychotherapy, are effective for young patients with borderline personality disorder (BPD), new research suggests.
Findings from the Monitoring Outcomes of Borderline Personality Disorder in Youth (MOBY) trial also showed improved psychosocial functioning and reduced suicide ideation with these therapies.
The results suggest that, contrary to common belief, psychotherapy is not the only effective approach for early BPD, lead author Andrew M. Chanen, PhD, director of clinical programs and services and head of personality disorder research at Orygen, Melbourne, told this news organization.
“We can say that early diagnosis and early treatment is effective, and the treatment doesn’t need to involve individual psychotherapy but does need to involve clinical case management and psychiatric care,” said Dr. Chanen, a professorial fellow at the Centre for Youth Mental Health, University of Melbourne.
The findings were published online in JAMA Psychiatry.
Extreme sensitivity
Patients with BPD have “extreme sensitivity to interpersonal slights” and often exhibit intense and volatile emotions and impulsive behavior, Dr. Chanen noted. Many will self-harm, abuse drugs, or attempt suicide; the suicide rate among patients with BPD is 8%-10%.
The condition is typically diagnosed in puberty or early adulthood, affecting about 3% of young people and a little more than 1% of adults.
Because of their aggression and interpersonal difficulties, patients with BPD are often discriminated against by health professionals and end up not getting treated, said Dr. Chanen.
Those who are treated often receive individual psychotherapy, such as dialectical behavior therapy (DBT). That type of therapy, which teaches healthy ways to cope with stress and regulate emotions, is very effective, Dr. Chanen said.
The MOBY trial examined three treatment approaches: the Helping Young People Early (HYPE) model, HYPE combined with weekly “befriending,” and a general youth mental health service (YMHS) model combined with befriending.
A key element of HYPE is cognitive analytic therapy, a psychotherapy program focused on understanding problematic self-management and interpersonal relationship patterns. The model includes clinical case management, such as attending to housing, vocational and educational issues, other mental health needs, and physical health needs.
In the second model, the psychotherapy of the HYPE program was replaced with befriending, which involves chatting with a patient about neutral topics such as sports and avoiding emotionally loaded topics such as interpersonal problems.
For YMHS plus befriending, experts trained in treating young people, but not specialized in treating BPD, were involved in managing patients.
‘High satisfaction’
Researchers randomly assigned 139 participants aged 15-25 years (80.6% women; mean age, 19.1 years) with BPD to one of the treatment arms. Of these, 128 (92.1%) were included in the intent-to-treat analysis.
The primary endpoint was psychosocial functioning, as measured by the Inventory of Interpersonal Problems Circumplex Version and the Social Adjustment Scale–Self-Report. Secondary endpoints included suicidal ideation, suicide attempts, nonsuicidal self-injury, depression, substance use, and treatment satisfaction.
The investigators reported group averages, but the study’s noninferiority design did not allow for determining if one treatment had superior efficacy.
All groups improved significantly on the primary endpoint. At 12 months, there was a mean 28.91-point (23.8%) drop in interpersonal problems and a mean 0.55-point (19.3%) drop in social adjustment scores.
For secondary outcomes, mean improvements at 12 months ranged from 40.7% (17.64 points) on the depression scale to 52.7% (6.22 points) for suicide ideation.
“The only area where the treatment didn’t really have an impact was substance use,” said Dr. Chanen. “Satisfaction was high for all three interventions throughout the study, and it’s hard to improve on high satisfaction.”
‘Turns things upside down’
That patients across all groups had marked and sustained improvements “in ways you wouldn’t expect for BPD” supports the conclusion that the interventions had a true effect, Dr. Chanen said.
he added.
They also imply there are effective alternatives to psychotherapy, which many individuals in the field insist is the only way to treat BPD. “This study turns things upside down and says actually it’s not. It’s the basics of treatment that are important,” Dr. Chanen said.
When a patient presents at the emergency department following a severe overdose, “it’s a reflex” for clinicians to refer that person to a psychotherapy program. “The problem is, these programs are not plentiful enough to be able to service the needs of this group,” Dr. Chanen noted.
On the other hand, the skills for clinical case management and psychiatric care “are available throughout the mental health systems,” he added.
The researchers are planning another analysis to determine whether age and sex predict better outcomes in these patients with BPD.
Unique contribution
Commenting for this news organization, John M. Oldham, MD, distinguished emeritus professor, Baylor College of Medicine, Houston, said a “unique and important contribution” of the study is the focus on early intervention.
“The general standard approach in psychiatry and the diagnostic world has been to not even consider anything until after somebody is 18 years of age, which is a mistake because these kids can become quite impaired earlier than that,” he said.
Dr. Oldham, who was not involved with the research, chaired the American Psychiatric Association workgroup that developed the 2001 evidence-based practice guideline for treating BPD, which recommended psychotherapy as the primary treatment. The guideline was last updated in 2005 – and another update is currently being developed, he noted.
There is an emerging trend toward “good psychiatric management” that focuses on level of functioning rather than on a specific strategy requiring a certificate of training that “not many people out there have,” said Dr. Oldham.
“You’re not going to make much headway with these kids if you’re going to be searching around for a DBT-certified therapist. What you need is to bring them in, get them to trust you, and in a sense be a kind of overall behavioral medicine navigator for them,” he added.
Dr. Oldham noted that, although the primary study outcome improved between 19% and 24%, “that means three-quarters of the people didn’t improve.”
He also pointed out this was only a 1-year trial. “Sometimes treatment for people with a personality disorder such as borderline takes a lot longer than that,” Dr. Oldham concluded.
The trial was funded by the National Health and Medical Research Council. Dr. Chanen reports receiving grants from the Australian government’s National Health and Medical Research Council during the conduct of the study and other support from the Helping Young People Early (HYPE) translational program outside the submitted work. He and another investigator cofounded and lead the HYPE clinical program, a government-funded program with continuous support, and the HYPE translational program, a not-for-profit training program. Dr. Oldham reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA PSYCHIATRY