User login
A COVID-19 Clinical Management Committee to Standardize Care in a 2-Hospital System
From the Department of Medicine (Drs. Meisenberg, Muganlinskaya, Sharma, Amjadi, Arnold, Barnes, Clance, Khalil, Miller, Mooradian, O’Connell, Patel, Press, Samaras, Shanmugam, Tavadze, and Thompson), Department of Pharmacy (Drs. Jiang, Jarawan, Sheth, and Trinh), Department of Nursing (Dr. Ohnmacht), and Department of Women and Children’s Services (Dr. Raji), Luminis Health, Annapolis, MD, and Lanham, MD.
Objective: The COVID-19 pandemic has been a challenge for hospital medical staffs worldwide due to high volumes of patients acutely ill with novel syndromes and prevailing uncertainty regarding optimum supportive and therapeutic interventions. Additionally, the response to this crisis was driven by a plethora of nontraditional information sources, such as email chains, websites, non–peer-reviewed preprints, and press releases. Care patterns became idiosyncratic and often incorporated unproven interventions driven by these nontraditional information sources. This report evaluates the efforts of a health system to create and empower a multidisciplinary committee to develop, implement, and monitor evidence-based, standardized protocols for patients with COVID-19.
Methods: This report describes the composition of the committee, its scope, and its important interactions with the health system pharmacy and therapeutics committee, research teams, and other work groups planning other aspects of COVID-19 management. It illustrates how the committee was used to demonstrate for trainees the process and value of critically examining evidence, even in a chaotic environment.
Results: Data show successful interventions in reducing excessive ordering of certain laboratory tests, reduction of nonrecommended therapies, and rapid uptake of evidence-based or guidelines-supported interventions.
Conclusions: A multidisciplinary committee dedicated solely to planning, implementing, and monitoring standard approaches that eventually became evidence-based decision-making led to an improved focus on treatment options and outcomes for COVID-19 patients. Data presented illustrate the attainable success that is both adaptable and suitable for similar emergencies in the future.
Keywords: COVID-19; clinical management; pharmacy and therapeutics; treatment; therapy.
The COVID-19 pandemic has spread to nearly all countries, carrying with it high morbidity, mortality, and severe impacts on both well-developed and less-well-developed health systems. Media reports of chaos within overwhelmed hospitals have been prominent.1,2 As of January 5, 2022, SARS-CoV-2 has infected more than 295 million people globally and directly caused the death of more than 5.4 million,3 though this number is likely an undercount even in countries with well-developed mortality tracking.4
Throughout the COVID-19 pandemic, hospital-based medical teams have been confronted with a flood of severely ill patients with novel syndromes. Initially, there were no standards for therapy or supportive care except for treatments borrowed from similar syndromes. In the setting of high volumes, high acuity, and public dismay, it is unsurprising that the usual deliberative methods for weighing evidence and initiating interventions were often pushed aside in favor of the solace of active intervention.5 In this milieu of limited evidence, there was a lamentable, if understandable, tendency to seek guidance from “nontraditional” sources,6 including email chains from colleagues, hospital websites, non–peer-reviewed manuscripts, advanced publication by medical journals,7 and nonscientific media presentations. In many localities, practitioners responded in idiosyncratic ways. For example, findings of high cytokine levels in COVID-19,8 along with reports of in-vitro antiviral activity with drugs like hydroxychloroquine against both SARS9 and SARS-CoV-2,10 drove laboratory test ordering and therapeutic interventions, respectively, carving shortcuts into the traditional clinical trial–dependent standards. Clinical trial results eventually emerged.11COVID-19 created a clinical dilemma for hospital medical staffs in terms of how to organize, standardize, and rapidly adapt to a flood of new information. In this report, we describe how 1 health system responded to these challenges by forming a COVID-19 Clinical Management Committee (CCMC) and empowering this interdisciplinary team to review evidence, create and adjust order sets, educate practitioners, oversee care, and collaborate across teams addressing other aspects of the COVID-19 response.
Program Overview
Health System Description
Luminis Health is a health system with 2 acute care hospitals that was formed in 2019 just before the start of the pandemic. Anne Arundel Medical Center (hospital A) is a 385-bed teaching hospital in Annapolis, MD. It has more than 23 000 discharges annually. Patients with COVID-19 were cared for by either an internal medicine teaching service or nonteaching hospitalist services on cohorted nursing units. Doctor’s Community Medical Center, in Lanham, MD (hospital B), is a 206-bed acute care hospital with more than 10 350 annual discharges. COVID-19 patients were cared for by hospitalist groups, initially in noncohorted units with transition to cohorted nursing units after a few months. The medical staffs are generally distinct, with different leadership structures, though the Luminis Health Department of Medicine has oversight responsibilities at both hospitals. More than 47 physicians attended COVID-19 patients at hospital A (with medical residents) and 30 individual physicians at hospital B, respectively, including intensivists. The nursing and pharmacy staffs are distinct, but there is a shared oversight Pharmacy and Therapeutics (P&T) Committee.
The 2 hospitals had distinct electronic medical records (EMR) until January 2021, when hospital B adopted the same EMR as hospital A (Epic).
Mission and Formation of CCMC
In order to coordinate the therapeutic approach across the health system, it was important for the CCMC to be designated by the health system P&T committee as an official subcommittee so that decisions on restrictions of medications and/or new or revised order sets could be rapidly initiated across the system without waiting for the subsequent P&T meetings. The full committee retained oversight of the CCMC. Some P&T members were also on the CCMC.
The committee reviewed new reports in medical journals and prepublication servers and consulted recommendations of professional societies, such as the National Institutes of Health (NIH) COVID-19 guidelines, Infectious Diseases Society of America, Society of Critical Care Medicine, and US Food and Drug Administration (FDA) Emergency Use Authorizations (EUA), among other sources.
Composition of the CCMC
Physician leaders from both hospitals in the following specialties were solicited for participation: critical care, epidemiology, hospital medicine (internal medicine), emergency medicine, infectious diseases, nephrology, women and children’s services, and medical informatics. Specialists in other areas, such as hematology, were invited for topic-specific discussions. Hospital pharmacists with different specialties and nursing leadership were essential contributors. The committee members were expected to use various communication channels to inform frontline clinicians of new care standards and the existence of new order sets, which were embedded in the EMR.
Clinical Research
An important connection for the CCMC was with theCOVID-19 clinical research team. Three members of the research team were also members of the CCMC. All new study proposals for therapeutics were discussed with the CCMC as they were being considered by the research team. In this way, feedback on the feasibility and acceptance of new study opportunities could be discussed with the CCMC. Occasionally, CCMC decisions impacted clinical research accrual strategies. For example, new data from randomized trials about tocilizumab1,2 demonstrated benefits in some subsets of patients and resulted in a recommendation for use by the NIH guideline committee in these populations.1 The CCMC quickly adopted this recommendation, which required a reprioritization of clinical research enrollment for studies testing other immune-modulating agents. This important dialogue was mediated within the CCMC.
Guideline Distribution, Reinforcement, and Platform for Feedback
New guidelines were disseminated to clinicians via daily brief patient huddles held on COVID units, with participation by nursing and pharmacy, and by weekly meetings with hospitalist leaders and frontline hospital physicians. Order sets and guidelines were maintained on the intranet. Adherence was reinforced by unit-based and central pharmacists. Order sets, including admission order sets, could be created only by designated informatics personnel, thus enforcing standardization. Feedback on the utility of the order sets was obtained during the weekly meetings or huddles, as described above. To ensure a sense of transparency, physicians who had interest in commenting on a particular therapy, or who wished to discuss a particular manuscript, news article, or website, were invited to attend CCMC meetings.
Scope of CCMC
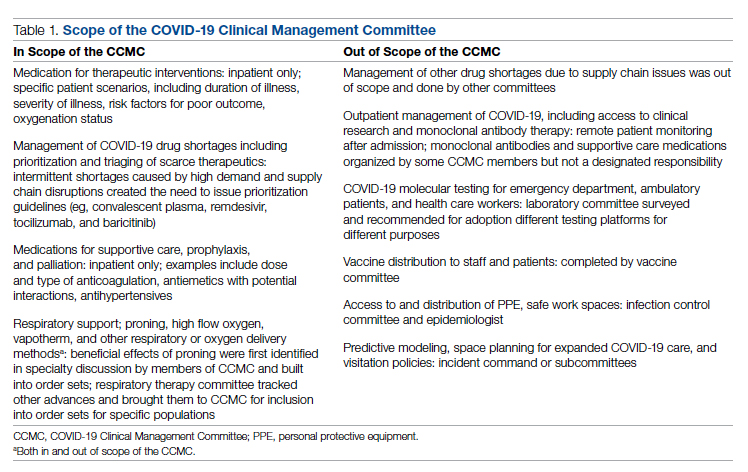
In order to be effective and timely, we limited the scope of our work to the report to the inpatient therapeutic environment, allowing other committees to work on other aspects of the pandemic response. In addition to issuing guidance and creating order sets to direct clinical practice, the CCMC also monitored COVID-19 therapeutic shortages15,16 and advised on prioritization of such treatments as convalescent plasma, remdesivir (prioritization and duration of therapy, 5 vs 10 days), baricitinib, and tocilizumab, depending upon the location of the patient (critical care or not). The CCMC was not involved in the management of non–COVID-19 shortages brought about by supply chain deficiencies.
Table 1 shows some aspects of the health system pandemic-response planning and the committee workforce that undertook that work. Though many items were out of scope for the CCMC, members of the CCMC did participate in the planning work of these other committees and therefore stayed connected to this complementary work.
A Teaching Opportunity About Making Thoughtful Choices
Another important feature of the CCMC was the contributions of residents from both pharmacy and internal medicine. The purpose and operations of the committee were recognized as an opportunity to involve learners in a curriculum based on Kern’s 6-step approach.17 Though the problem identification and general needs assessment were easily defined, the targeted needs assessment, extracted from individual and group interviews with learners and the committee members, pointed at the need to learn how to assess and analyze a rapidly growing body of literature on several relevant clinical aspects of SARS-CoV-2 and COVID-19. To achieve goals and objectives, residents were assigned to present current literature on a particular intervention during a committee meeting, specifically commenting on the merit or deficiencies of the study design, the strength of the data, and applicability to the local context with a recommendation. Prior to the presentations, the residents worked with faculty to identify the best studies or systematic analyses with potential to alter current practices. We thus used the CCMC process as a teaching tool about evidence-based medicine and the dilemma of clinical equipoise. This was imperative, since trainees thrust into the COVID-19 response have often keenly observed a movement away from deliberative decision-making.18 Indeed, including residents in the process of deliberative responses to COVID-19 addresses a recent call to adjust medical education during COVID-19 to “adapt curriculum to current issues in real time.”19
Interventions and Therapies Considered
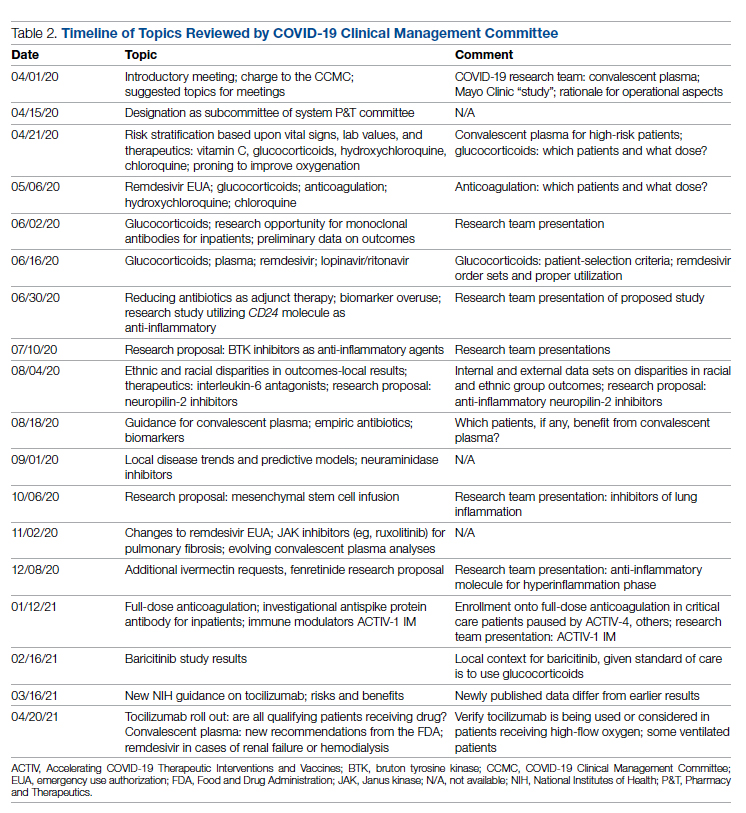
Table 2 shows the topics reviewed by the CCMC. By the time of the first meeting, nonstandardization of care was already a source of concern for clinicians. Dialogue often continued outside of the formal meetings. Many topics were considered more than once as new guidance developed, changes to EUAs occurred, and new data or new publicity arose.
Methods
The Human Protections Administrator determined that this work constituted “quality improvement, and not research” and was therefore exempt from institutional review board review.
Quantitative Analysis
All admitted patients from March 10, 2020, through April 20, 2021, were considered in the quantitative aspects of this report except as noted. Patients diagnosed with COVID-19 were identified by searching our internal data base using diagnostic codes. Patient admissions with the following diagnostic codes were included (prior to April 1, 2020): J12.89, J20.8, J40, J22, J98.8, J80, each with the additional code of B97.29. After April 1, 2020, the guideline for coding COVID-19 was U07.1.
Descriptive statistics were used to measure utilization rates of certain medications and laboratory tests of interest over time. These data were adjusted for number of unique admissions. In a few cases, not all data elements were available from both hospitals due to differences in the EMR.
Case fatality rate was calculated based upon whether the patient died or was admitted to inpatient hospice as a result of COVID-19. Four patients transferred out of hospital A and 18 transferred out of hospital B were censored from case-fatality-rate determination.
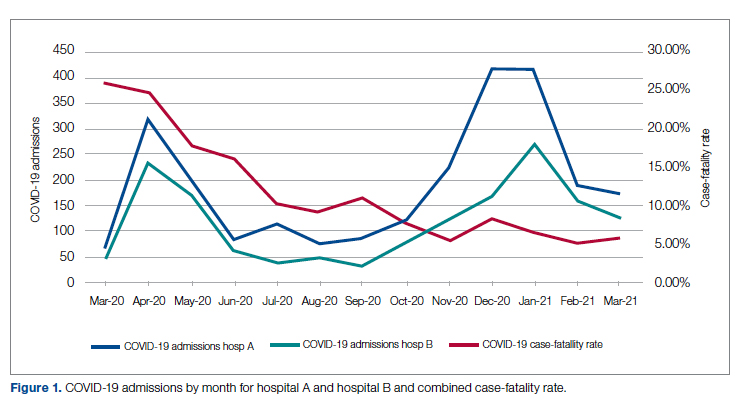
Figure 1 shows the number of admissions for each acute care hospital in the health system and the combined COVID-19 case-fatality rate over time.
Results
A total of 5955 consecutive COVID-19 patients admitted from March 10, 2020, through April 30, 2021, were analyzed. Patients with International Statistical Classification of Diseases, Tenth Revision codes J12.89. J20.8, J40, J22, J98.8, J80, each with the additional code of B97.29 (or the code UO7.1 after April 1, 2020), were included in the analysis. The median age of admitted patients was 65 years (range 19-91 years). Using the NIH classification system for severity,20 the distribution of severity during the first 24 hours after the time of hospital admission was as follows: asymptomatic/presymptomatic, 0.5%; mild illness, 5.3%; moderate illness, 37.1%; severe illness, 55.5%; and critical illness, 1.1%.
The impact of the CCMC can be estimated by looking at care patterns over time. Since the work of the CCMC was aimed at influencing and standardizing physician ordering and therapy choices through order set creation and other forms of oversight, we measured the use of the CCMC-approved order sets at both hospitals and the use of certain laboratory tests and therapies that the CCMC sought either to limit or increase. These counts were adjusted for number of unique COVID-19 admissions. But the limits of the case collection tool meant it also collected cases that were not eligible for some of the interventions. For example, COVID-19 admissions without hypoxemia would not have been eligible for remdesivir or glucocorticoids. When admitted, some patients were already on steroids for other medical indications and did not receive the prescribed dexamethasone dose that we measured in pharmacy databases. Similarly, a few patients were hospitalized for indications unrelated to COVID-19, such as surgery or childbirth, and were found to be SARS-CoV-2-positive on routine screening.
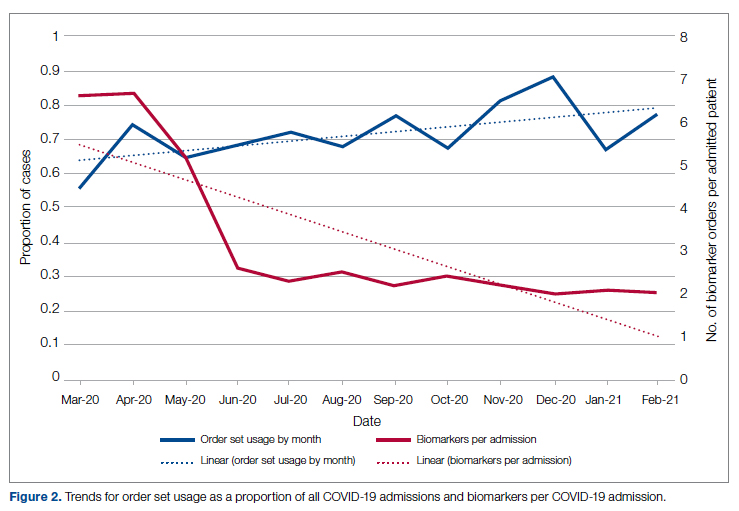
Figure 2 shows the utilization of CCMC-approved standard COVID-19 admission order sets as a proportion of all COVID-19 admissions over time. The trend reveals a modest increase in usage (R2 = 0.34), but these data do not reflect the progressive build of content into order sets over time. One of the goals of the order sets was to standardize and reduce the ordering of certain biomarkers: C-reactive protein, serum ferritin, and D-dimer, which were ordered frequently in many early patients. Orders for these 3 laboratory tests are combined and expressed as an average number of labs per COVID-19 admission in Figure 2. A downward trend, with an R2 value of 0.65, is suggestive of impact from the order sets, though other explanations are possible.
Medication guidance was also a goal of the CCMC, simultaneously discouraging poorly supported interventions and driving uptake of the recommended evidence-based interventions in appropriate patients. Figure 3 shows the utilization pattern for some drugs of interest over the course of the pandemic, specifically the proportion of patients receiving at least 1 dose of medication among all COVID-19 admissions by month. (Data for hospital B was excluded from this analysis because it did not include all admitted patients.)
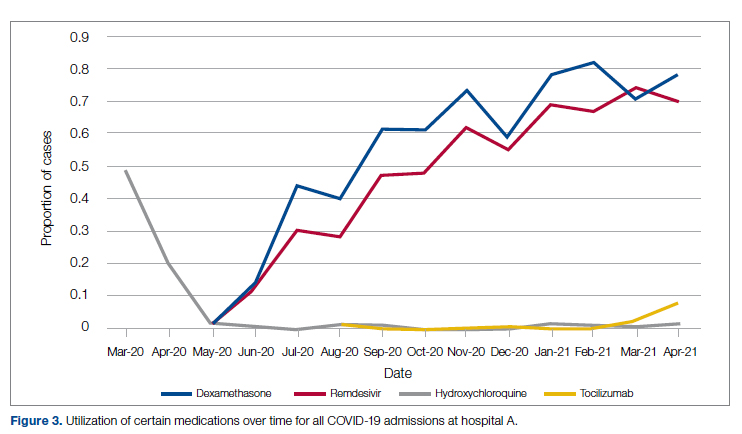
Hydroxychloroquine, which enjoyed a wave of popularity early on during the pandemic, was a target of successful order stewardship through the CCMC. Use of hydroxychloroquine as a COVID-19 therapeutic option after the first 2 months of the pandemic stopped, and subsequent use at low levels likely represented continuation therapy for outpatients who took hydroxychloroquine for rheumatologic indications.
Dexamethasone, as used in the RECOVERY trial,21 had a swift uptake among physicians after it was incorporated into order sets and its use encouraged. Similarly, uptake was immediate for remdesivir when, in May 2020, preliminary reports showed at least some benefits, confirmed by later analysis,22 and it received an FDA EUA.
Our data also show successful stewardship of the interleukin-6 antagonist toclilizumab, which was discouraged early on by the CCMC due to lack of data or negative results. But in March 2021, with new studies releasing data12,13 and new recommendations14 for its use in some subsets of patients with COVID-19, this drug was encouraged in appropriate subsets. A new order set with qualifying indications was prepared by the CCMC and new educational efforts made to encourage its use in appropriate patients.
Ivermectin was nonformulary at the start of the pandemic. This drug enjoyed much publicity from media sources and was promoted by certain physicians and on websites,23 based on in-vitro activity against coronaviruses. Eventually, the World Health Organization24 and the FDA25 found it necessary to issue advisory statements to the public against its use outside of clinical trials. The CCMC had requests from physicians to incorporate ivermectin but declined to add it to the formulary and recommended not approving nonformulary requests due to lack of data. As a result, ivermectin was not used at either hospital.
Discussion
COVID-19 represents many challenges to health systems all over the world. For Luminis Health, the high volume of acutely ill patients with novel syndromes was a particular challenge for the hospital-based care teams. A flood of information from preprints, press releases, preliminary reports, and many other nontraditional sources made deliberative management decisions difficult for individual physicians. Much commentary has appeared around the phenomenon but with less practical advice about how to make day-to-day care decisions at a time of scientific uncertainty and intense pressure to intervene.26,27 The CCMC was designed to overcome the information management dilemma. The need to coordinate, standardize, and oversee care was necessary given the large number of physicians who cared for COVID-19 patients on inpatient services.
It should be noted that creating order sets and issuing guidance is necessary, but not sufficient, to achieve our goals of being updated and consistent. This is especially true with large cadres of health care workers attending COVID-19 patients. Guidelines and recommendations were reinforced by unit-based oversight and stewardship from pharmacy and other leaders who constituted the CCMC.
The reduction in COVID-19 mortality over time experienced in this health care system was not unique and cannot necessarily be attributed to standardization of care. Similar improvements in mortality have been reported at many US hospitals in aggregate.28 Many other factors, including changes in patient characteristics, may be responsible for reduction in mortality over time.
Throughout this report we have relied upon an implicit assumption that standardization of medical therapeutics is desirable and leads to better outcomes as compared with allowing unlimited empiricism by individual physicians, either consultants or hospitalists. Our program represents a single health system with 2 acute care hospitals located 25 miles apart and which thus were similarly impacted by the different phases of the pandemic. Generalizability to health systems either smaller or larger, or in different geographical areas, has not been established. Data limitations have already been discussed.
We did not measure user satisfaction with the program either from physicians or nurses. However, the high rate of compliance suggests general agreement with the content and process.
We cannot definitively ascribe reduction in utilization of some nonrecommended treatments and increased utilization of the recommended therapies to the work of the CCMC. Individual physicians may have made these adjustments on their own or under the influence of other sources.
Finally, it should be noted that the mission to rapidly respond to data from well-conducted trials might be thwarted by too rigid a process or a committee’s lack of a sense of urgency. Organizing a committee and then empowering it to act is no guarantee of success; commitment to the mission is.
Conclusion
COVID-19 represented a challenge to medical staffs everywhere, inundating them with high volumes of acutely ill patients presenting with unfamiliar syndromes. Initial responses were characterized by idiosyncratic management approaches based on nontraditional sources of opinion and influences.
This report describes how a complex medical system brought order and standardization through a deliberative, but urgent, multidisciplinary committee with responsibility for planning, implementing, and monitoring standard approaches that eventually became evidence based. The composition of the committee and its scope of influence, limited to inpatient management, were important elements of success, allowing for better focus on the many treatment decisions. The important connection between the management committee and the system P&T committee, the clinical research effort, and teaching programs in both medicine and pharmacy are offered as exemplars of coordination. The data presented show success in achieving standardized, guideline-directed care. The approach is adoptable and suitable for similar emergencies in the future.
Acknowledgments: The authors thank Gary Scabis, Kip Waite, John Moxley, Angela Clubb, and David Woodley for their assistance in gathering data. We express appreciation and admiration for all our colleagues at the bedside.
Corresponding author: Barry R. Meisenberg, MD, Department of Medicine, Luminis Health, 2001 Medical Pkwy, Annapolis, MD 21401; [email protected].
Financial disclosures: None.
1. Gettleman J, Raj S, Kumar H. India’s health system cracks under the strain as coronavirus cases surge. The New York Times. April 22, 2021. https://www.nytimes.com/2021/04/21/world/asia/india-coronavirus-oxygen.html
2. Rappleye H, Lehren AW, Strickler L, Fitzpatrick S. ‘This system is doomed’: doctors, nurses sound off in NBC News coronavirus survey. NBC News. March 20, 2020. https://www.nbcnews.com/news/us-news/system-doomed-doctors-nurses-sound-nbc-news-coronavirus-survey-n1164841
3. Johns Hopkins Coronavirus Resource Center. Accessed January 5, 2022. https://coronavirus.jhu.edu/map.html
4. Fineberg HV. The toll of COVID-19. JAMA. 2020;324(15):1502-1503. doi:10.1001/jama.2020.20019
5. Meisenberg BR. Medical staffs response to COVID-19 ‘data’: have we misplaced our skeptic’s eye? Am J Med. 2021;134(2):151-152. doi:10.1016/j.amjmed.2020.09.013
6. McMahon JH, Lydeamore MH, Stewardson AJ. Bringing evidence from press release to the clinic in the era of COVID-19. J Antimicrob Chemother. 2021;76(3):547-549. doi:10.1093/jac/dkaa506
7. Rubin EJ, Baden LR, Morrissey S, Campion EW. Medical journals and the 2019-nCoV outbreak. N Engl J Med. 2020;382(9):866. doi:10.1056/NEJMe2001329
8. Liu F, Li L, Xu M, et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol. 2020;127:104370. doi:10.1016/j.jcv.2020.104370
9. Vincent MJ, Bergeron E, Benjannet S, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. doi:10.1186/1743-422X-2-69
10. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269-271. doi:10.1038/s41422-020-0282-0
11. RECOVERY Collaborative Group. Effect of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;383:2030-2040. doi:10.1056/NEJMoa2022926
12. RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): preliminary results of a randomised, controlled, open-label, platform trial [preprint]. February 11, 2021. doi:10.1101/2021.02.11.21249258 https://www.medrxiv.org/content/10.1101/2021.02.11.21249258v1
13. REMAP-CAP Investigators. Interleukin-6 receptor antagonists in critically ill patients with COVID-19. N Engl J Med. 2021;384(16):1491-1502. doi:10.1056/NEJMoa2100433
14. National Institutes of Health. COVID-19 treatment guidelines: interleukin-6 inhibitors. https://www.covid19treatmentguidelines.nih.gov/immunomodulators/interleukin-6-inhibitors/
15. Deana C, Vetrugno L, Tonizzo A, et al. Drug supply during COVID-19 pandemic: remember not to run with your tank empty. Hosp Pharm. 2021;56(5):405-407. doi:10.1177/0018578720931749
16. Choe J, Crane M, Greene J, et al. The Pandemic and the Supply Chain: Addressing Gaps in Pharmaceutical Production and Distribution. Johns Hopkins University, November 2020. https://www.jhsph.edu/research/affiliated-programs/johns-hopkins-drug-access-and-affordability-initiative/publications/Pandemic_Supply_Chain.pdf
17. Kern DE. Overview: a six-step approach to curriculum development. In: Kern DE, Thornton PA, Hughes MT, eds. Curriculum Development for Medical Education: A Six-Step Approach. 3rd ed. Johns Hopkins University Press; 2016.
18. Rice TW, Janz DR. In defense of evidence-based medicine for the treatment of COVID-19 acute respiratory distress syndrome. Ann Am Thorac Soc. 2020;17(7):787-789. doi:10.1513/AnnalsATS.202004-325IP
19. Lucey CR, Johnston SC. The transformational effects of COVID-19 on medical education. JAMA. 2020;324(11):1033-1034. doi:10.1001/jama.2020.14136
20. National Institutes of Health. COVID-19 treatment guidelines: clinical spectrum of SARS-CoV-2 infection. https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/
21. RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693-704. doi:10.1056/NEJMoa2021436
22. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19—final report. N Engl J Med. 2020;383:1813-1826. doi:10.1056/NEJMoa2007764
23. Jiminez D. Ivermectin and Covid-19: how a cheap antiparasitic became political. April 19, 2021. https://www.pharmaceutical-technology.com/features/ivermectin-covid-19-antiparasitic-political/
24. World Health Organization. WHO advises that ivermectin only be used to treat COVID-19 within clinical trials. March 31, 2021. https://www.who.int/news-room/feature-stories/detail/who-advises-that-ivermectin-only-be-used-to-treat-covid-19-within-clinical-trials
25. U.S. Food and Drug Administration. Why you should not use ivermectin to treat or prevent COVID-19. March 5, 2021. https://www.fda.gov/consumers/consumer-updates/why-you-should-not-use-ivermectin-treat-or-prevent-covid-19
26. Seymour CW, McCreary EK, Stegenga J. Sensible medicine-balancing intervention and inaction during the COVID-19 pandemic. JAMA. 2020;324(18):1827-1828. doi:10.1001/jama.2020.20271
27. Flanagin A, Fontanarosa PB, Bauchner H. Preprints involving medical research—do the benefits outweigh the challenges? JAMA. 2020;324(18):1840-1843. doi:10.1001/jama.2020.20674
28. Asch DA, Shells NE, Islam N, et al. Variation in US hospital mortality rates for patients admitted with COVID-19 during the first 6 months of the pandemic. JAMA Intern Med. 2021;181(4):471-478. doi:10.1001/jamainternmed.2020.8193
From the Department of Medicine (Drs. Meisenberg, Muganlinskaya, Sharma, Amjadi, Arnold, Barnes, Clance, Khalil, Miller, Mooradian, O’Connell, Patel, Press, Samaras, Shanmugam, Tavadze, and Thompson), Department of Pharmacy (Drs. Jiang, Jarawan, Sheth, and Trinh), Department of Nursing (Dr. Ohnmacht), and Department of Women and Children’s Services (Dr. Raji), Luminis Health, Annapolis, MD, and Lanham, MD.
Objective: The COVID-19 pandemic has been a challenge for hospital medical staffs worldwide due to high volumes of patients acutely ill with novel syndromes and prevailing uncertainty regarding optimum supportive and therapeutic interventions. Additionally, the response to this crisis was driven by a plethora of nontraditional information sources, such as email chains, websites, non–peer-reviewed preprints, and press releases. Care patterns became idiosyncratic and often incorporated unproven interventions driven by these nontraditional information sources. This report evaluates the efforts of a health system to create and empower a multidisciplinary committee to develop, implement, and monitor evidence-based, standardized protocols for patients with COVID-19.
Methods: This report describes the composition of the committee, its scope, and its important interactions with the health system pharmacy and therapeutics committee, research teams, and other work groups planning other aspects of COVID-19 management. It illustrates how the committee was used to demonstrate for trainees the process and value of critically examining evidence, even in a chaotic environment.
Results: Data show successful interventions in reducing excessive ordering of certain laboratory tests, reduction of nonrecommended therapies, and rapid uptake of evidence-based or guidelines-supported interventions.
Conclusions: A multidisciplinary committee dedicated solely to planning, implementing, and monitoring standard approaches that eventually became evidence-based decision-making led to an improved focus on treatment options and outcomes for COVID-19 patients. Data presented illustrate the attainable success that is both adaptable and suitable for similar emergencies in the future.
Keywords: COVID-19; clinical management; pharmacy and therapeutics; treatment; therapy.
The COVID-19 pandemic has spread to nearly all countries, carrying with it high morbidity, mortality, and severe impacts on both well-developed and less-well-developed health systems. Media reports of chaos within overwhelmed hospitals have been prominent.1,2 As of January 5, 2022, SARS-CoV-2 has infected more than 295 million people globally and directly caused the death of more than 5.4 million,3 though this number is likely an undercount even in countries with well-developed mortality tracking.4
Throughout the COVID-19 pandemic, hospital-based medical teams have been confronted with a flood of severely ill patients with novel syndromes. Initially, there were no standards for therapy or supportive care except for treatments borrowed from similar syndromes. In the setting of high volumes, high acuity, and public dismay, it is unsurprising that the usual deliberative methods for weighing evidence and initiating interventions were often pushed aside in favor of the solace of active intervention.5 In this milieu of limited evidence, there was a lamentable, if understandable, tendency to seek guidance from “nontraditional” sources,6 including email chains from colleagues, hospital websites, non–peer-reviewed manuscripts, advanced publication by medical journals,7 and nonscientific media presentations. In many localities, practitioners responded in idiosyncratic ways. For example, findings of high cytokine levels in COVID-19,8 along with reports of in-vitro antiviral activity with drugs like hydroxychloroquine against both SARS9 and SARS-CoV-2,10 drove laboratory test ordering and therapeutic interventions, respectively, carving shortcuts into the traditional clinical trial–dependent standards. Clinical trial results eventually emerged.11COVID-19 created a clinical dilemma for hospital medical staffs in terms of how to organize, standardize, and rapidly adapt to a flood of new information. In this report, we describe how 1 health system responded to these challenges by forming a COVID-19 Clinical Management Committee (CCMC) and empowering this interdisciplinary team to review evidence, create and adjust order sets, educate practitioners, oversee care, and collaborate across teams addressing other aspects of the COVID-19 response.
Program Overview
Health System Description
Luminis Health is a health system with 2 acute care hospitals that was formed in 2019 just before the start of the pandemic. Anne Arundel Medical Center (hospital A) is a 385-bed teaching hospital in Annapolis, MD. It has more than 23 000 discharges annually. Patients with COVID-19 were cared for by either an internal medicine teaching service or nonteaching hospitalist services on cohorted nursing units. Doctor’s Community Medical Center, in Lanham, MD (hospital B), is a 206-bed acute care hospital with more than 10 350 annual discharges. COVID-19 patients were cared for by hospitalist groups, initially in noncohorted units with transition to cohorted nursing units after a few months. The medical staffs are generally distinct, with different leadership structures, though the Luminis Health Department of Medicine has oversight responsibilities at both hospitals. More than 47 physicians attended COVID-19 patients at hospital A (with medical residents) and 30 individual physicians at hospital B, respectively, including intensivists. The nursing and pharmacy staffs are distinct, but there is a shared oversight Pharmacy and Therapeutics (P&T) Committee.
The 2 hospitals had distinct electronic medical records (EMR) until January 2021, when hospital B adopted the same EMR as hospital A (Epic).
Mission and Formation of CCMC
In order to coordinate the therapeutic approach across the health system, it was important for the CCMC to be designated by the health system P&T committee as an official subcommittee so that decisions on restrictions of medications and/or new or revised order sets could be rapidly initiated across the system without waiting for the subsequent P&T meetings. The full committee retained oversight of the CCMC. Some P&T members were also on the CCMC.
The committee reviewed new reports in medical journals and prepublication servers and consulted recommendations of professional societies, such as the National Institutes of Health (NIH) COVID-19 guidelines, Infectious Diseases Society of America, Society of Critical Care Medicine, and US Food and Drug Administration (FDA) Emergency Use Authorizations (EUA), among other sources.
Composition of the CCMC
Physician leaders from both hospitals in the following specialties were solicited for participation: critical care, epidemiology, hospital medicine (internal medicine), emergency medicine, infectious diseases, nephrology, women and children’s services, and medical informatics. Specialists in other areas, such as hematology, were invited for topic-specific discussions. Hospital pharmacists with different specialties and nursing leadership were essential contributors. The committee members were expected to use various communication channels to inform frontline clinicians of new care standards and the existence of new order sets, which were embedded in the EMR.
Clinical Research
An important connection for the CCMC was with theCOVID-19 clinical research team. Three members of the research team were also members of the CCMC. All new study proposals for therapeutics were discussed with the CCMC as they were being considered by the research team. In this way, feedback on the feasibility and acceptance of new study opportunities could be discussed with the CCMC. Occasionally, CCMC decisions impacted clinical research accrual strategies. For example, new data from randomized trials about tocilizumab1,2 demonstrated benefits in some subsets of patients and resulted in a recommendation for use by the NIH guideline committee in these populations.1 The CCMC quickly adopted this recommendation, which required a reprioritization of clinical research enrollment for studies testing other immune-modulating agents. This important dialogue was mediated within the CCMC.
Guideline Distribution, Reinforcement, and Platform for Feedback
New guidelines were disseminated to clinicians via daily brief patient huddles held on COVID units, with participation by nursing and pharmacy, and by weekly meetings with hospitalist leaders and frontline hospital physicians. Order sets and guidelines were maintained on the intranet. Adherence was reinforced by unit-based and central pharmacists. Order sets, including admission order sets, could be created only by designated informatics personnel, thus enforcing standardization. Feedback on the utility of the order sets was obtained during the weekly meetings or huddles, as described above. To ensure a sense of transparency, physicians who had interest in commenting on a particular therapy, or who wished to discuss a particular manuscript, news article, or website, were invited to attend CCMC meetings.
Scope of CCMC
In order to be effective and timely, we limited the scope of our work to the report to the inpatient therapeutic environment, allowing other committees to work on other aspects of the pandemic response. In addition to issuing guidance and creating order sets to direct clinical practice, the CCMC also monitored COVID-19 therapeutic shortages15,16 and advised on prioritization of such treatments as convalescent plasma, remdesivir (prioritization and duration of therapy, 5 vs 10 days), baricitinib, and tocilizumab, depending upon the location of the patient (critical care or not). The CCMC was not involved in the management of non–COVID-19 shortages brought about by supply chain deficiencies.
Table 1 shows some aspects of the health system pandemic-response planning and the committee workforce that undertook that work. Though many items were out of scope for the CCMC, members of the CCMC did participate in the planning work of these other committees and therefore stayed connected to this complementary work.

A Teaching Opportunity About Making Thoughtful Choices
Another important feature of the CCMC was the contributions of residents from both pharmacy and internal medicine. The purpose and operations of the committee were recognized as an opportunity to involve learners in a curriculum based on Kern’s 6-step approach.17 Though the problem identification and general needs assessment were easily defined, the targeted needs assessment, extracted from individual and group interviews with learners and the committee members, pointed at the need to learn how to assess and analyze a rapidly growing body of literature on several relevant clinical aspects of SARS-CoV-2 and COVID-19. To achieve goals and objectives, residents were assigned to present current literature on a particular intervention during a committee meeting, specifically commenting on the merit or deficiencies of the study design, the strength of the data, and applicability to the local context with a recommendation. Prior to the presentations, the residents worked with faculty to identify the best studies or systematic analyses with potential to alter current practices. We thus used the CCMC process as a teaching tool about evidence-based medicine and the dilemma of clinical equipoise. This was imperative, since trainees thrust into the COVID-19 response have often keenly observed a movement away from deliberative decision-making.18 Indeed, including residents in the process of deliberative responses to COVID-19 addresses a recent call to adjust medical education during COVID-19 to “adapt curriculum to current issues in real time.”19
Interventions and Therapies Considered
Table 2 shows the topics reviewed by the CCMC. By the time of the first meeting, nonstandardization of care was already a source of concern for clinicians. Dialogue often continued outside of the formal meetings. Many topics were considered more than once as new guidance developed, changes to EUAs occurred, and new data or new publicity arose.

Methods
The Human Protections Administrator determined that this work constituted “quality improvement, and not research” and was therefore exempt from institutional review board review.
Quantitative Analysis
All admitted patients from March 10, 2020, through April 20, 2021, were considered in the quantitative aspects of this report except as noted. Patients diagnosed with COVID-19 were identified by searching our internal data base using diagnostic codes. Patient admissions with the following diagnostic codes were included (prior to April 1, 2020): J12.89, J20.8, J40, J22, J98.8, J80, each with the additional code of B97.29. After April 1, 2020, the guideline for coding COVID-19 was U07.1.
Descriptive statistics were used to measure utilization rates of certain medications and laboratory tests of interest over time. These data were adjusted for number of unique admissions. In a few cases, not all data elements were available from both hospitals due to differences in the EMR.
Case fatality rate was calculated based upon whether the patient died or was admitted to inpatient hospice as a result of COVID-19. Four patients transferred out of hospital A and 18 transferred out of hospital B were censored from case-fatality-rate determination.
Figure 1 shows the number of admissions for each acute care hospital in the health system and the combined COVID-19 case-fatality rate over time.

Results
A total of 5955 consecutive COVID-19 patients admitted from March 10, 2020, through April 30, 2021, were analyzed. Patients with International Statistical Classification of Diseases, Tenth Revision codes J12.89. J20.8, J40, J22, J98.8, J80, each with the additional code of B97.29 (or the code UO7.1 after April 1, 2020), were included in the analysis. The median age of admitted patients was 65 years (range 19-91 years). Using the NIH classification system for severity,20 the distribution of severity during the first 24 hours after the time of hospital admission was as follows: asymptomatic/presymptomatic, 0.5%; mild illness, 5.3%; moderate illness, 37.1%; severe illness, 55.5%; and critical illness, 1.1%.
The impact of the CCMC can be estimated by looking at care patterns over time. Since the work of the CCMC was aimed at influencing and standardizing physician ordering and therapy choices through order set creation and other forms of oversight, we measured the use of the CCMC-approved order sets at both hospitals and the use of certain laboratory tests and therapies that the CCMC sought either to limit or increase. These counts were adjusted for number of unique COVID-19 admissions. But the limits of the case collection tool meant it also collected cases that were not eligible for some of the interventions. For example, COVID-19 admissions without hypoxemia would not have been eligible for remdesivir or glucocorticoids. When admitted, some patients were already on steroids for other medical indications and did not receive the prescribed dexamethasone dose that we measured in pharmacy databases. Similarly, a few patients were hospitalized for indications unrelated to COVID-19, such as surgery or childbirth, and were found to be SARS-CoV-2-positive on routine screening.
Figure 2 shows the utilization of CCMC-approved standard COVID-19 admission order sets as a proportion of all COVID-19 admissions over time. The trend reveals a modest increase in usage (R2 = 0.34), but these data do not reflect the progressive build of content into order sets over time. One of the goals of the order sets was to standardize and reduce the ordering of certain biomarkers: C-reactive protein, serum ferritin, and D-dimer, which were ordered frequently in many early patients. Orders for these 3 laboratory tests are combined and expressed as an average number of labs per COVID-19 admission in Figure 2. A downward trend, with an R2 value of 0.65, is suggestive of impact from the order sets, though other explanations are possible.

Medication guidance was also a goal of the CCMC, simultaneously discouraging poorly supported interventions and driving uptake of the recommended evidence-based interventions in appropriate patients. Figure 3 shows the utilization pattern for some drugs of interest over the course of the pandemic, specifically the proportion of patients receiving at least 1 dose of medication among all COVID-19 admissions by month. (Data for hospital B was excluded from this analysis because it did not include all admitted patients.)

Hydroxychloroquine, which enjoyed a wave of popularity early on during the pandemic, was a target of successful order stewardship through the CCMC. Use of hydroxychloroquine as a COVID-19 therapeutic option after the first 2 months of the pandemic stopped, and subsequent use at low levels likely represented continuation therapy for outpatients who took hydroxychloroquine for rheumatologic indications.
Dexamethasone, as used in the RECOVERY trial,21 had a swift uptake among physicians after it was incorporated into order sets and its use encouraged. Similarly, uptake was immediate for remdesivir when, in May 2020, preliminary reports showed at least some benefits, confirmed by later analysis,22 and it received an FDA EUA.
Our data also show successful stewardship of the interleukin-6 antagonist toclilizumab, which was discouraged early on by the CCMC due to lack of data or negative results. But in March 2021, with new studies releasing data12,13 and new recommendations14 for its use in some subsets of patients with COVID-19, this drug was encouraged in appropriate subsets. A new order set with qualifying indications was prepared by the CCMC and new educational efforts made to encourage its use in appropriate patients.
Ivermectin was nonformulary at the start of the pandemic. This drug enjoyed much publicity from media sources and was promoted by certain physicians and on websites,23 based on in-vitro activity against coronaviruses. Eventually, the World Health Organization24 and the FDA25 found it necessary to issue advisory statements to the public against its use outside of clinical trials. The CCMC had requests from physicians to incorporate ivermectin but declined to add it to the formulary and recommended not approving nonformulary requests due to lack of data. As a result, ivermectin was not used at either hospital.
Discussion
COVID-19 represents many challenges to health systems all over the world. For Luminis Health, the high volume of acutely ill patients with novel syndromes was a particular challenge for the hospital-based care teams. A flood of information from preprints, press releases, preliminary reports, and many other nontraditional sources made deliberative management decisions difficult for individual physicians. Much commentary has appeared around the phenomenon but with less practical advice about how to make day-to-day care decisions at a time of scientific uncertainty and intense pressure to intervene.26,27 The CCMC was designed to overcome the information management dilemma. The need to coordinate, standardize, and oversee care was necessary given the large number of physicians who cared for COVID-19 patients on inpatient services.
It should be noted that creating order sets and issuing guidance is necessary, but not sufficient, to achieve our goals of being updated and consistent. This is especially true with large cadres of health care workers attending COVID-19 patients. Guidelines and recommendations were reinforced by unit-based oversight and stewardship from pharmacy and other leaders who constituted the CCMC.
The reduction in COVID-19 mortality over time experienced in this health care system was not unique and cannot necessarily be attributed to standardization of care. Similar improvements in mortality have been reported at many US hospitals in aggregate.28 Many other factors, including changes in patient characteristics, may be responsible for reduction in mortality over time.
Throughout this report we have relied upon an implicit assumption that standardization of medical therapeutics is desirable and leads to better outcomes as compared with allowing unlimited empiricism by individual physicians, either consultants or hospitalists. Our program represents a single health system with 2 acute care hospitals located 25 miles apart and which thus were similarly impacted by the different phases of the pandemic. Generalizability to health systems either smaller or larger, or in different geographical areas, has not been established. Data limitations have already been discussed.
We did not measure user satisfaction with the program either from physicians or nurses. However, the high rate of compliance suggests general agreement with the content and process.
We cannot definitively ascribe reduction in utilization of some nonrecommended treatments and increased utilization of the recommended therapies to the work of the CCMC. Individual physicians may have made these adjustments on their own or under the influence of other sources.
Finally, it should be noted that the mission to rapidly respond to data from well-conducted trials might be thwarted by too rigid a process or a committee’s lack of a sense of urgency. Organizing a committee and then empowering it to act is no guarantee of success; commitment to the mission is.
Conclusion
COVID-19 represented a challenge to medical staffs everywhere, inundating them with high volumes of acutely ill patients presenting with unfamiliar syndromes. Initial responses were characterized by idiosyncratic management approaches based on nontraditional sources of opinion and influences.
This report describes how a complex medical system brought order and standardization through a deliberative, but urgent, multidisciplinary committee with responsibility for planning, implementing, and monitoring standard approaches that eventually became evidence based. The composition of the committee and its scope of influence, limited to inpatient management, were important elements of success, allowing for better focus on the many treatment decisions. The important connection between the management committee and the system P&T committee, the clinical research effort, and teaching programs in both medicine and pharmacy are offered as exemplars of coordination. The data presented show success in achieving standardized, guideline-directed care. The approach is adoptable and suitable for similar emergencies in the future.
Acknowledgments: The authors thank Gary Scabis, Kip Waite, John Moxley, Angela Clubb, and David Woodley for their assistance in gathering data. We express appreciation and admiration for all our colleagues at the bedside.
Corresponding author: Barry R. Meisenberg, MD, Department of Medicine, Luminis Health, 2001 Medical Pkwy, Annapolis, MD 21401; [email protected].
Financial disclosures: None.
From the Department of Medicine (Drs. Meisenberg, Muganlinskaya, Sharma, Amjadi, Arnold, Barnes, Clance, Khalil, Miller, Mooradian, O’Connell, Patel, Press, Samaras, Shanmugam, Tavadze, and Thompson), Department of Pharmacy (Drs. Jiang, Jarawan, Sheth, and Trinh), Department of Nursing (Dr. Ohnmacht), and Department of Women and Children’s Services (Dr. Raji), Luminis Health, Annapolis, MD, and Lanham, MD.
Objective: The COVID-19 pandemic has been a challenge for hospital medical staffs worldwide due to high volumes of patients acutely ill with novel syndromes and prevailing uncertainty regarding optimum supportive and therapeutic interventions. Additionally, the response to this crisis was driven by a plethora of nontraditional information sources, such as email chains, websites, non–peer-reviewed preprints, and press releases. Care patterns became idiosyncratic and often incorporated unproven interventions driven by these nontraditional information sources. This report evaluates the efforts of a health system to create and empower a multidisciplinary committee to develop, implement, and monitor evidence-based, standardized protocols for patients with COVID-19.
Methods: This report describes the composition of the committee, its scope, and its important interactions with the health system pharmacy and therapeutics committee, research teams, and other work groups planning other aspects of COVID-19 management. It illustrates how the committee was used to demonstrate for trainees the process and value of critically examining evidence, even in a chaotic environment.
Results: Data show successful interventions in reducing excessive ordering of certain laboratory tests, reduction of nonrecommended therapies, and rapid uptake of evidence-based or guidelines-supported interventions.
Conclusions: A multidisciplinary committee dedicated solely to planning, implementing, and monitoring standard approaches that eventually became evidence-based decision-making led to an improved focus on treatment options and outcomes for COVID-19 patients. Data presented illustrate the attainable success that is both adaptable and suitable for similar emergencies in the future.
Keywords: COVID-19; clinical management; pharmacy and therapeutics; treatment; therapy.
The COVID-19 pandemic has spread to nearly all countries, carrying with it high morbidity, mortality, and severe impacts on both well-developed and less-well-developed health systems. Media reports of chaos within overwhelmed hospitals have been prominent.1,2 As of January 5, 2022, SARS-CoV-2 has infected more than 295 million people globally and directly caused the death of more than 5.4 million,3 though this number is likely an undercount even in countries with well-developed mortality tracking.4
Throughout the COVID-19 pandemic, hospital-based medical teams have been confronted with a flood of severely ill patients with novel syndromes. Initially, there were no standards for therapy or supportive care except for treatments borrowed from similar syndromes. In the setting of high volumes, high acuity, and public dismay, it is unsurprising that the usual deliberative methods for weighing evidence and initiating interventions were often pushed aside in favor of the solace of active intervention.5 In this milieu of limited evidence, there was a lamentable, if understandable, tendency to seek guidance from “nontraditional” sources,6 including email chains from colleagues, hospital websites, non–peer-reviewed manuscripts, advanced publication by medical journals,7 and nonscientific media presentations. In many localities, practitioners responded in idiosyncratic ways. For example, findings of high cytokine levels in COVID-19,8 along with reports of in-vitro antiviral activity with drugs like hydroxychloroquine against both SARS9 and SARS-CoV-2,10 drove laboratory test ordering and therapeutic interventions, respectively, carving shortcuts into the traditional clinical trial–dependent standards. Clinical trial results eventually emerged.11COVID-19 created a clinical dilemma for hospital medical staffs in terms of how to organize, standardize, and rapidly adapt to a flood of new information. In this report, we describe how 1 health system responded to these challenges by forming a COVID-19 Clinical Management Committee (CCMC) and empowering this interdisciplinary team to review evidence, create and adjust order sets, educate practitioners, oversee care, and collaborate across teams addressing other aspects of the COVID-19 response.
Program Overview
Health System Description
Luminis Health is a health system with 2 acute care hospitals that was formed in 2019 just before the start of the pandemic. Anne Arundel Medical Center (hospital A) is a 385-bed teaching hospital in Annapolis, MD. It has more than 23 000 discharges annually. Patients with COVID-19 were cared for by either an internal medicine teaching service or nonteaching hospitalist services on cohorted nursing units. Doctor’s Community Medical Center, in Lanham, MD (hospital B), is a 206-bed acute care hospital with more than 10 350 annual discharges. COVID-19 patients were cared for by hospitalist groups, initially in noncohorted units with transition to cohorted nursing units after a few months. The medical staffs are generally distinct, with different leadership structures, though the Luminis Health Department of Medicine has oversight responsibilities at both hospitals. More than 47 physicians attended COVID-19 patients at hospital A (with medical residents) and 30 individual physicians at hospital B, respectively, including intensivists. The nursing and pharmacy staffs are distinct, but there is a shared oversight Pharmacy and Therapeutics (P&T) Committee.
The 2 hospitals had distinct electronic medical records (EMR) until January 2021, when hospital B adopted the same EMR as hospital A (Epic).
Mission and Formation of CCMC
In order to coordinate the therapeutic approach across the health system, it was important for the CCMC to be designated by the health system P&T committee as an official subcommittee so that decisions on restrictions of medications and/or new or revised order sets could be rapidly initiated across the system without waiting for the subsequent P&T meetings. The full committee retained oversight of the CCMC. Some P&T members were also on the CCMC.
The committee reviewed new reports in medical journals and prepublication servers and consulted recommendations of professional societies, such as the National Institutes of Health (NIH) COVID-19 guidelines, Infectious Diseases Society of America, Society of Critical Care Medicine, and US Food and Drug Administration (FDA) Emergency Use Authorizations (EUA), among other sources.
Composition of the CCMC
Physician leaders from both hospitals in the following specialties were solicited for participation: critical care, epidemiology, hospital medicine (internal medicine), emergency medicine, infectious diseases, nephrology, women and children’s services, and medical informatics. Specialists in other areas, such as hematology, were invited for topic-specific discussions. Hospital pharmacists with different specialties and nursing leadership were essential contributors. The committee members were expected to use various communication channels to inform frontline clinicians of new care standards and the existence of new order sets, which were embedded in the EMR.
Clinical Research
An important connection for the CCMC was with theCOVID-19 clinical research team. Three members of the research team were also members of the CCMC. All new study proposals for therapeutics were discussed with the CCMC as they were being considered by the research team. In this way, feedback on the feasibility and acceptance of new study opportunities could be discussed with the CCMC. Occasionally, CCMC decisions impacted clinical research accrual strategies. For example, new data from randomized trials about tocilizumab1,2 demonstrated benefits in some subsets of patients and resulted in a recommendation for use by the NIH guideline committee in these populations.1 The CCMC quickly adopted this recommendation, which required a reprioritization of clinical research enrollment for studies testing other immune-modulating agents. This important dialogue was mediated within the CCMC.
Guideline Distribution, Reinforcement, and Platform for Feedback
New guidelines were disseminated to clinicians via daily brief patient huddles held on COVID units, with participation by nursing and pharmacy, and by weekly meetings with hospitalist leaders and frontline hospital physicians. Order sets and guidelines were maintained on the intranet. Adherence was reinforced by unit-based and central pharmacists. Order sets, including admission order sets, could be created only by designated informatics personnel, thus enforcing standardization. Feedback on the utility of the order sets was obtained during the weekly meetings or huddles, as described above. To ensure a sense of transparency, physicians who had interest in commenting on a particular therapy, or who wished to discuss a particular manuscript, news article, or website, were invited to attend CCMC meetings.
Scope of CCMC
In order to be effective and timely, we limited the scope of our work to the report to the inpatient therapeutic environment, allowing other committees to work on other aspects of the pandemic response. In addition to issuing guidance and creating order sets to direct clinical practice, the CCMC also monitored COVID-19 therapeutic shortages15,16 and advised on prioritization of such treatments as convalescent plasma, remdesivir (prioritization and duration of therapy, 5 vs 10 days), baricitinib, and tocilizumab, depending upon the location of the patient (critical care or not). The CCMC was not involved in the management of non–COVID-19 shortages brought about by supply chain deficiencies.
Table 1 shows some aspects of the health system pandemic-response planning and the committee workforce that undertook that work. Though many items were out of scope for the CCMC, members of the CCMC did participate in the planning work of these other committees and therefore stayed connected to this complementary work.

A Teaching Opportunity About Making Thoughtful Choices
Another important feature of the CCMC was the contributions of residents from both pharmacy and internal medicine. The purpose and operations of the committee were recognized as an opportunity to involve learners in a curriculum based on Kern’s 6-step approach.17 Though the problem identification and general needs assessment were easily defined, the targeted needs assessment, extracted from individual and group interviews with learners and the committee members, pointed at the need to learn how to assess and analyze a rapidly growing body of literature on several relevant clinical aspects of SARS-CoV-2 and COVID-19. To achieve goals and objectives, residents were assigned to present current literature on a particular intervention during a committee meeting, specifically commenting on the merit or deficiencies of the study design, the strength of the data, and applicability to the local context with a recommendation. Prior to the presentations, the residents worked with faculty to identify the best studies or systematic analyses with potential to alter current practices. We thus used the CCMC process as a teaching tool about evidence-based medicine and the dilemma of clinical equipoise. This was imperative, since trainees thrust into the COVID-19 response have often keenly observed a movement away from deliberative decision-making.18 Indeed, including residents in the process of deliberative responses to COVID-19 addresses a recent call to adjust medical education during COVID-19 to “adapt curriculum to current issues in real time.”19
Interventions and Therapies Considered
Table 2 shows the topics reviewed by the CCMC. By the time of the first meeting, nonstandardization of care was already a source of concern for clinicians. Dialogue often continued outside of the formal meetings. Many topics were considered more than once as new guidance developed, changes to EUAs occurred, and new data or new publicity arose.

Methods
The Human Protections Administrator determined that this work constituted “quality improvement, and not research” and was therefore exempt from institutional review board review.
Quantitative Analysis
All admitted patients from March 10, 2020, through April 20, 2021, were considered in the quantitative aspects of this report except as noted. Patients diagnosed with COVID-19 were identified by searching our internal data base using diagnostic codes. Patient admissions with the following diagnostic codes were included (prior to April 1, 2020): J12.89, J20.8, J40, J22, J98.8, J80, each with the additional code of B97.29. After April 1, 2020, the guideline for coding COVID-19 was U07.1.
Descriptive statistics were used to measure utilization rates of certain medications and laboratory tests of interest over time. These data were adjusted for number of unique admissions. In a few cases, not all data elements were available from both hospitals due to differences in the EMR.
Case fatality rate was calculated based upon whether the patient died or was admitted to inpatient hospice as a result of COVID-19. Four patients transferred out of hospital A and 18 transferred out of hospital B were censored from case-fatality-rate determination.
Figure 1 shows the number of admissions for each acute care hospital in the health system and the combined COVID-19 case-fatality rate over time.

Results
A total of 5955 consecutive COVID-19 patients admitted from March 10, 2020, through April 30, 2021, were analyzed. Patients with International Statistical Classification of Diseases, Tenth Revision codes J12.89. J20.8, J40, J22, J98.8, J80, each with the additional code of B97.29 (or the code UO7.1 after April 1, 2020), were included in the analysis. The median age of admitted patients was 65 years (range 19-91 years). Using the NIH classification system for severity,20 the distribution of severity during the first 24 hours after the time of hospital admission was as follows: asymptomatic/presymptomatic, 0.5%; mild illness, 5.3%; moderate illness, 37.1%; severe illness, 55.5%; and critical illness, 1.1%.
The impact of the CCMC can be estimated by looking at care patterns over time. Since the work of the CCMC was aimed at influencing and standardizing physician ordering and therapy choices through order set creation and other forms of oversight, we measured the use of the CCMC-approved order sets at both hospitals and the use of certain laboratory tests and therapies that the CCMC sought either to limit or increase. These counts were adjusted for number of unique COVID-19 admissions. But the limits of the case collection tool meant it also collected cases that were not eligible for some of the interventions. For example, COVID-19 admissions without hypoxemia would not have been eligible for remdesivir or glucocorticoids. When admitted, some patients were already on steroids for other medical indications and did not receive the prescribed dexamethasone dose that we measured in pharmacy databases. Similarly, a few patients were hospitalized for indications unrelated to COVID-19, such as surgery or childbirth, and were found to be SARS-CoV-2-positive on routine screening.
Figure 2 shows the utilization of CCMC-approved standard COVID-19 admission order sets as a proportion of all COVID-19 admissions over time. The trend reveals a modest increase in usage (R2 = 0.34), but these data do not reflect the progressive build of content into order sets over time. One of the goals of the order sets was to standardize and reduce the ordering of certain biomarkers: C-reactive protein, serum ferritin, and D-dimer, which were ordered frequently in many early patients. Orders for these 3 laboratory tests are combined and expressed as an average number of labs per COVID-19 admission in Figure 2. A downward trend, with an R2 value of 0.65, is suggestive of impact from the order sets, though other explanations are possible.

Medication guidance was also a goal of the CCMC, simultaneously discouraging poorly supported interventions and driving uptake of the recommended evidence-based interventions in appropriate patients. Figure 3 shows the utilization pattern for some drugs of interest over the course of the pandemic, specifically the proportion of patients receiving at least 1 dose of medication among all COVID-19 admissions by month. (Data for hospital B was excluded from this analysis because it did not include all admitted patients.)

Hydroxychloroquine, which enjoyed a wave of popularity early on during the pandemic, was a target of successful order stewardship through the CCMC. Use of hydroxychloroquine as a COVID-19 therapeutic option after the first 2 months of the pandemic stopped, and subsequent use at low levels likely represented continuation therapy for outpatients who took hydroxychloroquine for rheumatologic indications.
Dexamethasone, as used in the RECOVERY trial,21 had a swift uptake among physicians after it was incorporated into order sets and its use encouraged. Similarly, uptake was immediate for remdesivir when, in May 2020, preliminary reports showed at least some benefits, confirmed by later analysis,22 and it received an FDA EUA.
Our data also show successful stewardship of the interleukin-6 antagonist toclilizumab, which was discouraged early on by the CCMC due to lack of data or negative results. But in March 2021, with new studies releasing data12,13 and new recommendations14 for its use in some subsets of patients with COVID-19, this drug was encouraged in appropriate subsets. A new order set with qualifying indications was prepared by the CCMC and new educational efforts made to encourage its use in appropriate patients.
Ivermectin was nonformulary at the start of the pandemic. This drug enjoyed much publicity from media sources and was promoted by certain physicians and on websites,23 based on in-vitro activity against coronaviruses. Eventually, the World Health Organization24 and the FDA25 found it necessary to issue advisory statements to the public against its use outside of clinical trials. The CCMC had requests from physicians to incorporate ivermectin but declined to add it to the formulary and recommended not approving nonformulary requests due to lack of data. As a result, ivermectin was not used at either hospital.
Discussion
COVID-19 represents many challenges to health systems all over the world. For Luminis Health, the high volume of acutely ill patients with novel syndromes was a particular challenge for the hospital-based care teams. A flood of information from preprints, press releases, preliminary reports, and many other nontraditional sources made deliberative management decisions difficult for individual physicians. Much commentary has appeared around the phenomenon but with less practical advice about how to make day-to-day care decisions at a time of scientific uncertainty and intense pressure to intervene.26,27 The CCMC was designed to overcome the information management dilemma. The need to coordinate, standardize, and oversee care was necessary given the large number of physicians who cared for COVID-19 patients on inpatient services.
It should be noted that creating order sets and issuing guidance is necessary, but not sufficient, to achieve our goals of being updated and consistent. This is especially true with large cadres of health care workers attending COVID-19 patients. Guidelines and recommendations were reinforced by unit-based oversight and stewardship from pharmacy and other leaders who constituted the CCMC.
The reduction in COVID-19 mortality over time experienced in this health care system was not unique and cannot necessarily be attributed to standardization of care. Similar improvements in mortality have been reported at many US hospitals in aggregate.28 Many other factors, including changes in patient characteristics, may be responsible for reduction in mortality over time.
Throughout this report we have relied upon an implicit assumption that standardization of medical therapeutics is desirable and leads to better outcomes as compared with allowing unlimited empiricism by individual physicians, either consultants or hospitalists. Our program represents a single health system with 2 acute care hospitals located 25 miles apart and which thus were similarly impacted by the different phases of the pandemic. Generalizability to health systems either smaller or larger, or in different geographical areas, has not been established. Data limitations have already been discussed.
We did not measure user satisfaction with the program either from physicians or nurses. However, the high rate of compliance suggests general agreement with the content and process.
We cannot definitively ascribe reduction in utilization of some nonrecommended treatments and increased utilization of the recommended therapies to the work of the CCMC. Individual physicians may have made these adjustments on their own or under the influence of other sources.
Finally, it should be noted that the mission to rapidly respond to data from well-conducted trials might be thwarted by too rigid a process or a committee’s lack of a sense of urgency. Organizing a committee and then empowering it to act is no guarantee of success; commitment to the mission is.
Conclusion
COVID-19 represented a challenge to medical staffs everywhere, inundating them with high volumes of acutely ill patients presenting with unfamiliar syndromes. Initial responses were characterized by idiosyncratic management approaches based on nontraditional sources of opinion and influences.
This report describes how a complex medical system brought order and standardization through a deliberative, but urgent, multidisciplinary committee with responsibility for planning, implementing, and monitoring standard approaches that eventually became evidence based. The composition of the committee and its scope of influence, limited to inpatient management, were important elements of success, allowing for better focus on the many treatment decisions. The important connection between the management committee and the system P&T committee, the clinical research effort, and teaching programs in both medicine and pharmacy are offered as exemplars of coordination. The data presented show success in achieving standardized, guideline-directed care. The approach is adoptable and suitable for similar emergencies in the future.
Acknowledgments: The authors thank Gary Scabis, Kip Waite, John Moxley, Angela Clubb, and David Woodley for their assistance in gathering data. We express appreciation and admiration for all our colleagues at the bedside.
Corresponding author: Barry R. Meisenberg, MD, Department of Medicine, Luminis Health, 2001 Medical Pkwy, Annapolis, MD 21401; [email protected].
Financial disclosures: None.
1. Gettleman J, Raj S, Kumar H. India’s health system cracks under the strain as coronavirus cases surge. The New York Times. April 22, 2021. https://www.nytimes.com/2021/04/21/world/asia/india-coronavirus-oxygen.html
2. Rappleye H, Lehren AW, Strickler L, Fitzpatrick S. ‘This system is doomed’: doctors, nurses sound off in NBC News coronavirus survey. NBC News. March 20, 2020. https://www.nbcnews.com/news/us-news/system-doomed-doctors-nurses-sound-nbc-news-coronavirus-survey-n1164841
3. Johns Hopkins Coronavirus Resource Center. Accessed January 5, 2022. https://coronavirus.jhu.edu/map.html
4. Fineberg HV. The toll of COVID-19. JAMA. 2020;324(15):1502-1503. doi:10.1001/jama.2020.20019
5. Meisenberg BR. Medical staffs response to COVID-19 ‘data’: have we misplaced our skeptic’s eye? Am J Med. 2021;134(2):151-152. doi:10.1016/j.amjmed.2020.09.013
6. McMahon JH, Lydeamore MH, Stewardson AJ. Bringing evidence from press release to the clinic in the era of COVID-19. J Antimicrob Chemother. 2021;76(3):547-549. doi:10.1093/jac/dkaa506
7. Rubin EJ, Baden LR, Morrissey S, Campion EW. Medical journals and the 2019-nCoV outbreak. N Engl J Med. 2020;382(9):866. doi:10.1056/NEJMe2001329
8. Liu F, Li L, Xu M, et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol. 2020;127:104370. doi:10.1016/j.jcv.2020.104370
9. Vincent MJ, Bergeron E, Benjannet S, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. doi:10.1186/1743-422X-2-69
10. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269-271. doi:10.1038/s41422-020-0282-0
11. RECOVERY Collaborative Group. Effect of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;383:2030-2040. doi:10.1056/NEJMoa2022926
12. RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): preliminary results of a randomised, controlled, open-label, platform trial [preprint]. February 11, 2021. doi:10.1101/2021.02.11.21249258 https://www.medrxiv.org/content/10.1101/2021.02.11.21249258v1
13. REMAP-CAP Investigators. Interleukin-6 receptor antagonists in critically ill patients with COVID-19. N Engl J Med. 2021;384(16):1491-1502. doi:10.1056/NEJMoa2100433
14. National Institutes of Health. COVID-19 treatment guidelines: interleukin-6 inhibitors. https://www.covid19treatmentguidelines.nih.gov/immunomodulators/interleukin-6-inhibitors/
15. Deana C, Vetrugno L, Tonizzo A, et al. Drug supply during COVID-19 pandemic: remember not to run with your tank empty. Hosp Pharm. 2021;56(5):405-407. doi:10.1177/0018578720931749
16. Choe J, Crane M, Greene J, et al. The Pandemic and the Supply Chain: Addressing Gaps in Pharmaceutical Production and Distribution. Johns Hopkins University, November 2020. https://www.jhsph.edu/research/affiliated-programs/johns-hopkins-drug-access-and-affordability-initiative/publications/Pandemic_Supply_Chain.pdf
17. Kern DE. Overview: a six-step approach to curriculum development. In: Kern DE, Thornton PA, Hughes MT, eds. Curriculum Development for Medical Education: A Six-Step Approach. 3rd ed. Johns Hopkins University Press; 2016.
18. Rice TW, Janz DR. In defense of evidence-based medicine for the treatment of COVID-19 acute respiratory distress syndrome. Ann Am Thorac Soc. 2020;17(7):787-789. doi:10.1513/AnnalsATS.202004-325IP
19. Lucey CR, Johnston SC. The transformational effects of COVID-19 on medical education. JAMA. 2020;324(11):1033-1034. doi:10.1001/jama.2020.14136
20. National Institutes of Health. COVID-19 treatment guidelines: clinical spectrum of SARS-CoV-2 infection. https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/
21. RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693-704. doi:10.1056/NEJMoa2021436
22. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19—final report. N Engl J Med. 2020;383:1813-1826. doi:10.1056/NEJMoa2007764
23. Jiminez D. Ivermectin and Covid-19: how a cheap antiparasitic became political. April 19, 2021. https://www.pharmaceutical-technology.com/features/ivermectin-covid-19-antiparasitic-political/
24. World Health Organization. WHO advises that ivermectin only be used to treat COVID-19 within clinical trials. March 31, 2021. https://www.who.int/news-room/feature-stories/detail/who-advises-that-ivermectin-only-be-used-to-treat-covid-19-within-clinical-trials
25. U.S. Food and Drug Administration. Why you should not use ivermectin to treat or prevent COVID-19. March 5, 2021. https://www.fda.gov/consumers/consumer-updates/why-you-should-not-use-ivermectin-treat-or-prevent-covid-19
26. Seymour CW, McCreary EK, Stegenga J. Sensible medicine-balancing intervention and inaction during the COVID-19 pandemic. JAMA. 2020;324(18):1827-1828. doi:10.1001/jama.2020.20271
27. Flanagin A, Fontanarosa PB, Bauchner H. Preprints involving medical research—do the benefits outweigh the challenges? JAMA. 2020;324(18):1840-1843. doi:10.1001/jama.2020.20674
28. Asch DA, Shells NE, Islam N, et al. Variation in US hospital mortality rates for patients admitted with COVID-19 during the first 6 months of the pandemic. JAMA Intern Med. 2021;181(4):471-478. doi:10.1001/jamainternmed.2020.8193
1. Gettleman J, Raj S, Kumar H. India’s health system cracks under the strain as coronavirus cases surge. The New York Times. April 22, 2021. https://www.nytimes.com/2021/04/21/world/asia/india-coronavirus-oxygen.html
2. Rappleye H, Lehren AW, Strickler L, Fitzpatrick S. ‘This system is doomed’: doctors, nurses sound off in NBC News coronavirus survey. NBC News. March 20, 2020. https://www.nbcnews.com/news/us-news/system-doomed-doctors-nurses-sound-nbc-news-coronavirus-survey-n1164841
3. Johns Hopkins Coronavirus Resource Center. Accessed January 5, 2022. https://coronavirus.jhu.edu/map.html
4. Fineberg HV. The toll of COVID-19. JAMA. 2020;324(15):1502-1503. doi:10.1001/jama.2020.20019
5. Meisenberg BR. Medical staffs response to COVID-19 ‘data’: have we misplaced our skeptic’s eye? Am J Med. 2021;134(2):151-152. doi:10.1016/j.amjmed.2020.09.013
6. McMahon JH, Lydeamore MH, Stewardson AJ. Bringing evidence from press release to the clinic in the era of COVID-19. J Antimicrob Chemother. 2021;76(3):547-549. doi:10.1093/jac/dkaa506
7. Rubin EJ, Baden LR, Morrissey S, Campion EW. Medical journals and the 2019-nCoV outbreak. N Engl J Med. 2020;382(9):866. doi:10.1056/NEJMe2001329
8. Liu F, Li L, Xu M, et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol. 2020;127:104370. doi:10.1016/j.jcv.2020.104370
9. Vincent MJ, Bergeron E, Benjannet S, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. doi:10.1186/1743-422X-2-69
10. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269-271. doi:10.1038/s41422-020-0282-0
11. RECOVERY Collaborative Group. Effect of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;383:2030-2040. doi:10.1056/NEJMoa2022926
12. RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): preliminary results of a randomised, controlled, open-label, platform trial [preprint]. February 11, 2021. doi:10.1101/2021.02.11.21249258 https://www.medrxiv.org/content/10.1101/2021.02.11.21249258v1
13. REMAP-CAP Investigators. Interleukin-6 receptor antagonists in critically ill patients with COVID-19. N Engl J Med. 2021;384(16):1491-1502. doi:10.1056/NEJMoa2100433
14. National Institutes of Health. COVID-19 treatment guidelines: interleukin-6 inhibitors. https://www.covid19treatmentguidelines.nih.gov/immunomodulators/interleukin-6-inhibitors/
15. Deana C, Vetrugno L, Tonizzo A, et al. Drug supply during COVID-19 pandemic: remember not to run with your tank empty. Hosp Pharm. 2021;56(5):405-407. doi:10.1177/0018578720931749
16. Choe J, Crane M, Greene J, et al. The Pandemic and the Supply Chain: Addressing Gaps in Pharmaceutical Production and Distribution. Johns Hopkins University, November 2020. https://www.jhsph.edu/research/affiliated-programs/johns-hopkins-drug-access-and-affordability-initiative/publications/Pandemic_Supply_Chain.pdf
17. Kern DE. Overview: a six-step approach to curriculum development. In: Kern DE, Thornton PA, Hughes MT, eds. Curriculum Development for Medical Education: A Six-Step Approach. 3rd ed. Johns Hopkins University Press; 2016.
18. Rice TW, Janz DR. In defense of evidence-based medicine for the treatment of COVID-19 acute respiratory distress syndrome. Ann Am Thorac Soc. 2020;17(7):787-789. doi:10.1513/AnnalsATS.202004-325IP
19. Lucey CR, Johnston SC. The transformational effects of COVID-19 on medical education. JAMA. 2020;324(11):1033-1034. doi:10.1001/jama.2020.14136
20. National Institutes of Health. COVID-19 treatment guidelines: clinical spectrum of SARS-CoV-2 infection. https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/
21. RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693-704. doi:10.1056/NEJMoa2021436
22. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19—final report. N Engl J Med. 2020;383:1813-1826. doi:10.1056/NEJMoa2007764
23. Jiminez D. Ivermectin and Covid-19: how a cheap antiparasitic became political. April 19, 2021. https://www.pharmaceutical-technology.com/features/ivermectin-covid-19-antiparasitic-political/
24. World Health Organization. WHO advises that ivermectin only be used to treat COVID-19 within clinical trials. March 31, 2021. https://www.who.int/news-room/feature-stories/detail/who-advises-that-ivermectin-only-be-used-to-treat-covid-19-within-clinical-trials
25. U.S. Food and Drug Administration. Why you should not use ivermectin to treat or prevent COVID-19. March 5, 2021. https://www.fda.gov/consumers/consumer-updates/why-you-should-not-use-ivermectin-treat-or-prevent-covid-19
26. Seymour CW, McCreary EK, Stegenga J. Sensible medicine-balancing intervention and inaction during the COVID-19 pandemic. JAMA. 2020;324(18):1827-1828. doi:10.1001/jama.2020.20271
27. Flanagin A, Fontanarosa PB, Bauchner H. Preprints involving medical research—do the benefits outweigh the challenges? JAMA. 2020;324(18):1840-1843. doi:10.1001/jama.2020.20674
28. Asch DA, Shells NE, Islam N, et al. Variation in US hospital mortality rates for patients admitted with COVID-19 during the first 6 months of the pandemic. JAMA Intern Med. 2021;181(4):471-478. doi:10.1001/jamainternmed.2020.8193
Experimental plasma exchange shows promise for IPF flares in preliminary study
Acute flares of idiopathic pulmonary fibrosis have a mortality rate as high as 90% or more, depending on their severity. But an experimental regimen that includes autoantibody reduction was found to improve survival significantly, as well as oxygen levels and walk distances, according to a small preliminary study published in PLOS ONE.
“It’s a preliminary study, but it’s very exciting,” Amit Gaggar, MD, PhD, an endowed professor of medicine at the University of Alabama at Birmingham (UAB), said in an interview. “We don’t really have a treatment for acute exacerbations of pulmonary fibrosis, and the mortality is extremely high, so it’s really critical that we start thinking outside the box a little bit for therapeutics.” Dr. Gaggar isn’t affiliated with the study.
Study leader Steven R. Duncan, MD, also of UAB, acknowledged that the experimental therapy has its detractors. “There’s been a tremendous bias against the role of immunologic therapy in idiopathic fibrosis, although it seems to be lessening,” he said.
The preliminary study treated 24 patients who had acute exacerbations of idiopathic pulmonary fibrosis (AE-IPF) with a 19-day regimen called triple-modality autoantibody reduction. The three contributing modalities are therapeutic plasma exchange (TPE), rituximab, and intravenous immunoglobulin treatments. The standard treatment for AE-IPF consists of antibiotics and corticosteroids.
Dr. Duncan led the only other study of autoantibody reduction for AE-IPF, published in PLOS ONE in 2015. The latest preliminary study is a precursor to a National Heart, Lung, and Blood Institute–funded phase 2 randomized clinical trial, called STRIVE-IPF, currently enrolling AE-IPF patients at six sites.
Overall survival rates at 1, 3, and 6 months were 67%, 63%, and 46%. The study couldn’t identify characteristics of survivors versus nonsurvivors, although the latter had a trend toward greater initial oxygen requirements. Among the 10 patients who needed less than 25 L/min supplemental O2, the survival rate was 57%. In patients who needed more than 25 L/min, the survival rate was 20% (P = .07). Only 1 of 5 patients who needed greater than 40 L/min survived a year (P = .36).
After the 19-day regimen, 15 patients, or 63%, had significant drops in supplemental O2 requirements, from an average of 15 L/min to 3 L/min (P = .0007). Thirteen (87%) of the patients who were taking an antifibrotic medication (either pirfenidone or nintedanib) at baseline needed less O2 and/or had increased walking distances, compared with five who weren’t prescribed either of the agents (P = .15), although 1-year survival didn’t vary significantly with antifibrotic use.
The mechanism of antibody reduction is to filter out B-cells, infiltrates of which are typically found in lungs of AE-IPF patients, Dr. Duncan said. The regimen involves nine TPEs over 15 days, two IV rituximab 1-gm treatments over that course, and IV Ig 0.5-gm/kg treatments daily on days 16 through 19.
“Plasma exchange rapidly gets rid of the antibodies,” Dr. Duncan said in an interview. “It’s the basis for a number of autoantibody-mediated diseases, such as myasthenia gravis.”
While the TPE removes the B-cells, they have a proclivity to re-emerge, hence the rituximab treatment, he said. IV Ig further inhibits B-cell activity. “The IV Ig probably works in large part by feedback inhibition of the B-cells that have survived the rituximab,” Dr. Duncan said.
He added that with the TPE and rituximab patients had “sometimes amazing response” but then would relapse. “Since we added IV Ig, we see far fewer relapses,” he said. “And interestingly, if they do relapse, we can salvage them by giving them this treatment again.”
The preliminary study doesn’t make clear what patients would benefit most from the triple-modality therapy, but it did provide some clues. “We found that patients who have higher levels of antibodies against epithelial cells tend to do the best, and patients who had less severe disease – that is, less disturbance of gas exchange requiring less O2 – tend to do better,” Dr. Duncan said. The STRIVE trial should serve to identify specific biomarkers, he said.
Dr. Gaggar, the UAB professor who’s not affiliated with the study, concurred that it’s “too early to tell” which patients would benefit. “Certainly, these patients that undergo exacerbations would be of high interest,” he said, “but the potential is there that the other chronic lung diseases that have exacerbations may also benefit from this kind of therapy.”
He noted that the preliminary study focused on one type of autoantibody generating from epithelial cells. “In many of these studies where we limit ourselves to a single autoantibody population, we might be at the tip of iceberg,” Dr. Gaggar said. “There might be autoantibodies generated from other cells in the lung or the body that might be also pathogenic. This is really powerful because this is a subgroup of autoantibodies, but they still had that kind of impact in this small study.”
The STRIVE study is scheduled for completion in September 2022.
Dr. Duncan disclosed relationships with Novartis and Tyr Pharma outside the study subject. Dr. Gaggar has no relevant disclosures.
A version of this article first appeared on Medscape.com.
Acute flares of idiopathic pulmonary fibrosis have a mortality rate as high as 90% or more, depending on their severity. But an experimental regimen that includes autoantibody reduction was found to improve survival significantly, as well as oxygen levels and walk distances, according to a small preliminary study published in PLOS ONE.
“It’s a preliminary study, but it’s very exciting,” Amit Gaggar, MD, PhD, an endowed professor of medicine at the University of Alabama at Birmingham (UAB), said in an interview. “We don’t really have a treatment for acute exacerbations of pulmonary fibrosis, and the mortality is extremely high, so it’s really critical that we start thinking outside the box a little bit for therapeutics.” Dr. Gaggar isn’t affiliated with the study.
Study leader Steven R. Duncan, MD, also of UAB, acknowledged that the experimental therapy has its detractors. “There’s been a tremendous bias against the role of immunologic therapy in idiopathic fibrosis, although it seems to be lessening,” he said.
The preliminary study treated 24 patients who had acute exacerbations of idiopathic pulmonary fibrosis (AE-IPF) with a 19-day regimen called triple-modality autoantibody reduction. The three contributing modalities are therapeutic plasma exchange (TPE), rituximab, and intravenous immunoglobulin treatments. The standard treatment for AE-IPF consists of antibiotics and corticosteroids.
Dr. Duncan led the only other study of autoantibody reduction for AE-IPF, published in PLOS ONE in 2015. The latest preliminary study is a precursor to a National Heart, Lung, and Blood Institute–funded phase 2 randomized clinical trial, called STRIVE-IPF, currently enrolling AE-IPF patients at six sites.
Overall survival rates at 1, 3, and 6 months were 67%, 63%, and 46%. The study couldn’t identify characteristics of survivors versus nonsurvivors, although the latter had a trend toward greater initial oxygen requirements. Among the 10 patients who needed less than 25 L/min supplemental O2, the survival rate was 57%. In patients who needed more than 25 L/min, the survival rate was 20% (P = .07). Only 1 of 5 patients who needed greater than 40 L/min survived a year (P = .36).
After the 19-day regimen, 15 patients, or 63%, had significant drops in supplemental O2 requirements, from an average of 15 L/min to 3 L/min (P = .0007). Thirteen (87%) of the patients who were taking an antifibrotic medication (either pirfenidone or nintedanib) at baseline needed less O2 and/or had increased walking distances, compared with five who weren’t prescribed either of the agents (P = .15), although 1-year survival didn’t vary significantly with antifibrotic use.
The mechanism of antibody reduction is to filter out B-cells, infiltrates of which are typically found in lungs of AE-IPF patients, Dr. Duncan said. The regimen involves nine TPEs over 15 days, two IV rituximab 1-gm treatments over that course, and IV Ig 0.5-gm/kg treatments daily on days 16 through 19.
“Plasma exchange rapidly gets rid of the antibodies,” Dr. Duncan said in an interview. “It’s the basis for a number of autoantibody-mediated diseases, such as myasthenia gravis.”
While the TPE removes the B-cells, they have a proclivity to re-emerge, hence the rituximab treatment, he said. IV Ig further inhibits B-cell activity. “The IV Ig probably works in large part by feedback inhibition of the B-cells that have survived the rituximab,” Dr. Duncan said.
He added that with the TPE and rituximab patients had “sometimes amazing response” but then would relapse. “Since we added IV Ig, we see far fewer relapses,” he said. “And interestingly, if they do relapse, we can salvage them by giving them this treatment again.”
The preliminary study doesn’t make clear what patients would benefit most from the triple-modality therapy, but it did provide some clues. “We found that patients who have higher levels of antibodies against epithelial cells tend to do the best, and patients who had less severe disease – that is, less disturbance of gas exchange requiring less O2 – tend to do better,” Dr. Duncan said. The STRIVE trial should serve to identify specific biomarkers, he said.
Dr. Gaggar, the UAB professor who’s not affiliated with the study, concurred that it’s “too early to tell” which patients would benefit. “Certainly, these patients that undergo exacerbations would be of high interest,” he said, “but the potential is there that the other chronic lung diseases that have exacerbations may also benefit from this kind of therapy.”
He noted that the preliminary study focused on one type of autoantibody generating from epithelial cells. “In many of these studies where we limit ourselves to a single autoantibody population, we might be at the tip of iceberg,” Dr. Gaggar said. “There might be autoantibodies generated from other cells in the lung or the body that might be also pathogenic. This is really powerful because this is a subgroup of autoantibodies, but they still had that kind of impact in this small study.”
The STRIVE study is scheduled for completion in September 2022.
Dr. Duncan disclosed relationships with Novartis and Tyr Pharma outside the study subject. Dr. Gaggar has no relevant disclosures.
A version of this article first appeared on Medscape.com.
Acute flares of idiopathic pulmonary fibrosis have a mortality rate as high as 90% or more, depending on their severity. But an experimental regimen that includes autoantibody reduction was found to improve survival significantly, as well as oxygen levels and walk distances, according to a small preliminary study published in PLOS ONE.
“It’s a preliminary study, but it’s very exciting,” Amit Gaggar, MD, PhD, an endowed professor of medicine at the University of Alabama at Birmingham (UAB), said in an interview. “We don’t really have a treatment for acute exacerbations of pulmonary fibrosis, and the mortality is extremely high, so it’s really critical that we start thinking outside the box a little bit for therapeutics.” Dr. Gaggar isn’t affiliated with the study.
Study leader Steven R. Duncan, MD, also of UAB, acknowledged that the experimental therapy has its detractors. “There’s been a tremendous bias against the role of immunologic therapy in idiopathic fibrosis, although it seems to be lessening,” he said.
The preliminary study treated 24 patients who had acute exacerbations of idiopathic pulmonary fibrosis (AE-IPF) with a 19-day regimen called triple-modality autoantibody reduction. The three contributing modalities are therapeutic plasma exchange (TPE), rituximab, and intravenous immunoglobulin treatments. The standard treatment for AE-IPF consists of antibiotics and corticosteroids.
Dr. Duncan led the only other study of autoantibody reduction for AE-IPF, published in PLOS ONE in 2015. The latest preliminary study is a precursor to a National Heart, Lung, and Blood Institute–funded phase 2 randomized clinical trial, called STRIVE-IPF, currently enrolling AE-IPF patients at six sites.
Overall survival rates at 1, 3, and 6 months were 67%, 63%, and 46%. The study couldn’t identify characteristics of survivors versus nonsurvivors, although the latter had a trend toward greater initial oxygen requirements. Among the 10 patients who needed less than 25 L/min supplemental O2, the survival rate was 57%. In patients who needed more than 25 L/min, the survival rate was 20% (P = .07). Only 1 of 5 patients who needed greater than 40 L/min survived a year (P = .36).
After the 19-day regimen, 15 patients, or 63%, had significant drops in supplemental O2 requirements, from an average of 15 L/min to 3 L/min (P = .0007). Thirteen (87%) of the patients who were taking an antifibrotic medication (either pirfenidone or nintedanib) at baseline needed less O2 and/or had increased walking distances, compared with five who weren’t prescribed either of the agents (P = .15), although 1-year survival didn’t vary significantly with antifibrotic use.
The mechanism of antibody reduction is to filter out B-cells, infiltrates of which are typically found in lungs of AE-IPF patients, Dr. Duncan said. The regimen involves nine TPEs over 15 days, two IV rituximab 1-gm treatments over that course, and IV Ig 0.5-gm/kg treatments daily on days 16 through 19.
“Plasma exchange rapidly gets rid of the antibodies,” Dr. Duncan said in an interview. “It’s the basis for a number of autoantibody-mediated diseases, such as myasthenia gravis.”
While the TPE removes the B-cells, they have a proclivity to re-emerge, hence the rituximab treatment, he said. IV Ig further inhibits B-cell activity. “The IV Ig probably works in large part by feedback inhibition of the B-cells that have survived the rituximab,” Dr. Duncan said.
He added that with the TPE and rituximab patients had “sometimes amazing response” but then would relapse. “Since we added IV Ig, we see far fewer relapses,” he said. “And interestingly, if they do relapse, we can salvage them by giving them this treatment again.”
The preliminary study doesn’t make clear what patients would benefit most from the triple-modality therapy, but it did provide some clues. “We found that patients who have higher levels of antibodies against epithelial cells tend to do the best, and patients who had less severe disease – that is, less disturbance of gas exchange requiring less O2 – tend to do better,” Dr. Duncan said. The STRIVE trial should serve to identify specific biomarkers, he said.
Dr. Gaggar, the UAB professor who’s not affiliated with the study, concurred that it’s “too early to tell” which patients would benefit. “Certainly, these patients that undergo exacerbations would be of high interest,” he said, “but the potential is there that the other chronic lung diseases that have exacerbations may also benefit from this kind of therapy.”
He noted that the preliminary study focused on one type of autoantibody generating from epithelial cells. “In many of these studies where we limit ourselves to a single autoantibody population, we might be at the tip of iceberg,” Dr. Gaggar said. “There might be autoantibodies generated from other cells in the lung or the body that might be also pathogenic. This is really powerful because this is a subgroup of autoantibodies, but they still had that kind of impact in this small study.”
The STRIVE study is scheduled for completion in September 2022.
Dr. Duncan disclosed relationships with Novartis and Tyr Pharma outside the study subject. Dr. Gaggar has no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM PLOS ONE
Neonatal sepsis: WHO-recommended Rx needs a major rethink
First-line treatment of neonatal sepsis in low- and middle-income countries (LMICs) with ampicillin-gentamicin – as recommended by the World Health Organization – needs to be reassessed, a retrospective, observational cohort study suggests. Rates of resistance to this particular antibiotic combination are extremely high in LMICs, and this treatment is unlikely to save many neonatal patients, according to the study’s results.
“The WHO guidelines are over 10 years old, and they are actually based on high-income country data, whereas data reported from low-income countries are reported by private labs, and they do not cater to the lower socioeconomic groups within these countries, which is important data to capture,” Timothy Walsh, MD, University of Oxford, United Kingdom, told this news organization.
“The main take-home message from our data is that ampicillin-gentamicin doesn’t work for most of the Gram-negative isolates we tested, and while there are alternatives, their use is confounded by [a lack of] financial support,” he added.
The study was published online in The Lancet Infectious Diseases.
BARNARDS study
In this substudy of the Burden of Antibiotic Resistance in Neonates from Developing Societies (BARNARDS) study, investigators focused on the effectiveness of antibiotic therapies after taking into account the high prevalence of pathogen resistance to ampicillin-gentamicin. Participating countries included Bangladesh, Ethiopia, India, Nigeria, Pakistan, Rwanda, and South Africa.
“Blood samples were obtained from neonates presenting with clinical signs of sepsis,” the authors note, “and WGS [whole-genome sequencing] and MICs [minimum inhibitory concentrations] for antibiotic treatment were determined for bacterial isolates from culture-confirmed sepsis.” Between Nov. 2015 and Feb. 2018, 36,285 neonates were enrolled into the main BARNARDS study, of whom 9,874 had clinically diagnosed sepsis and 5,749 had antibiotic data.
A total of 2,483 neonates had culture-confirmed sepsis, and WGS data were available for 457 isolates taken from 442 neonates. Slightly over three-quarters of the 5,749 neonates who had antibiotic data received first-line ampicillin-gentamicin. The other three most commonly prescribed antibiotic combinations were ceftazidime-amikacin, piperacillin-tazobactam-amikacin, and amoxicillin-clavulanate-amikacin.
Neonates treated with ceftazidime-amikacin had a 68% lower reported mortality than those treated with ampicillin-gentamicin at an adjusted hazard ratio of 0.32 (95% confidence interval, 0.14-0.72; P = .006), the investigators report. In contrast, no significant differences in mortality rates were reported for neonates treated with amoxicillin-clavulanate-amikacin or piperacillin-tazobactam-amikacin compared to those treated with ampicillin-gentamicin.
Investigators were careful to suggest that mortality effects associated with the different antibiotic combinations might have been confounded by either country-specific effects or underreporting of mortality, as a large proportion of neonates who were treated with ampicillin-gentamicin were followed for fewer than 10 days. However, in an unreported aspect of the same study, neonatal mortality from sepsis dropped by over 50% in two federally funded sites in Nigeria that changed their treatment from the WHO-recommended ampicillin-gentamicin regimen to ceftazidime-amikacin – which Dr. Walsh suggested was an endorsement of ceftazidime-amikacin over ampicillin-gentamicin if ever there was one.
Gram-negative resistance
In looking at resistance patterns to the antibiotic combinations used in these countries, investigators found that almost all Gram-negative isolates tested were “overwhelmingly resistant” to ampicillin, and over 70% of them were resistant to gentamicin as well. Extremely high resistance rates were also found against Staphylococcus spp, which are regarded as intrinsically resistant to ampicillin, rendering it basically useless in this particular treatment setting.
Amikacin had much lower level of resistance, with only about 26% of Gram-negative isolates showing resistance. In terms of coverage against Gram-negative isolates, the lowest level of coverage was provided by ampicillin-gentamicin at slightly over 28%, compared with about 73% for amoxicillin-clavulanate-amikacin, 77% for ceftazidime-amikacin, and 80% for piperacillin-tazobactam-amikacin.
In contrast, “Gram-positive isolates generally had reduced levels of resistance,” the authors state. As Dr. Walsh noted, the consortium also did an analysis assessing how much the antibiotic combinations cost and how much payment was deferred to the parents. For example, in Nigeria, the entire cost of treatment is passed down to the parents, “so if they are earning, say, $5.00 a day and the infant needs ceftazidime-amikacin, where the cost per dose is about $6.00 or $7.00 a day, parents can’t afford it,” Dr. Walsh observed.
This part of the conversation, he added, tends to get lost in many studies of antibiotic resistance in LMICs, which is a critical omission, because in many instances, the choice of treatment does come down to affordability. “It’s all very well for the WHO to sit there and say, ampicillin-gentamicin is perfect, but the combination actually doesn’t work in over 70% of the Gram-negative bacteria we looked at in these countries,” Dr. Walsh emphasized.
“The fact is that we have to be a lot more internationally engaged as to what’s actually happening in poorer populations, because unless we do, neonates are going to continue to die,” he said.
Editorial commentary
Commenting on the findings, lead editorialist Luregn Schlapbach, MD, PhD, of University Children’s Hospital Zurich, Switzerland, pointed out that the study has a number of limitations, including a high rate of dropouts from follow-up. This could possibly result in underestimation of neonatal mortality as well as country-specific biases. Nevertheless, Dr. Schlapbach feels that the integration of sequential clinical, genomic, microbiologic, drug, and cost data across a large network in LMIC settings is “exceptional” and will serve to inform “urgently needed” clinical trials in the field of neonatal sepsis.
“At present, increasing global antibiotic resistance is threatening progress against neonatal sepsis, prompting urgency to develop improved measures to effectively prevent and treat life-threatening infections in this high-risk group,” Dr. Schlapbach and colleagues write.
“The findings from the BARNARDS study call for randomized trials comparing mortality benefit and cost efficiency of different antibiotic combinations and management algorithms to safely reduce unnecessary antibiotic exposure for neonatal sepsis,” the editorialists concluded.
The authors and editorialists have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
First-line treatment of neonatal sepsis in low- and middle-income countries (LMICs) with ampicillin-gentamicin – as recommended by the World Health Organization – needs to be reassessed, a retrospective, observational cohort study suggests. Rates of resistance to this particular antibiotic combination are extremely high in LMICs, and this treatment is unlikely to save many neonatal patients, according to the study’s results.
“The WHO guidelines are over 10 years old, and they are actually based on high-income country data, whereas data reported from low-income countries are reported by private labs, and they do not cater to the lower socioeconomic groups within these countries, which is important data to capture,” Timothy Walsh, MD, University of Oxford, United Kingdom, told this news organization.
“The main take-home message from our data is that ampicillin-gentamicin doesn’t work for most of the Gram-negative isolates we tested, and while there are alternatives, their use is confounded by [a lack of] financial support,” he added.
The study was published online in The Lancet Infectious Diseases.
BARNARDS study
In this substudy of the Burden of Antibiotic Resistance in Neonates from Developing Societies (BARNARDS) study, investigators focused on the effectiveness of antibiotic therapies after taking into account the high prevalence of pathogen resistance to ampicillin-gentamicin. Participating countries included Bangladesh, Ethiopia, India, Nigeria, Pakistan, Rwanda, and South Africa.
“Blood samples were obtained from neonates presenting with clinical signs of sepsis,” the authors note, “and WGS [whole-genome sequencing] and MICs [minimum inhibitory concentrations] for antibiotic treatment were determined for bacterial isolates from culture-confirmed sepsis.” Between Nov. 2015 and Feb. 2018, 36,285 neonates were enrolled into the main BARNARDS study, of whom 9,874 had clinically diagnosed sepsis and 5,749 had antibiotic data.
A total of 2,483 neonates had culture-confirmed sepsis, and WGS data were available for 457 isolates taken from 442 neonates. Slightly over three-quarters of the 5,749 neonates who had antibiotic data received first-line ampicillin-gentamicin. The other three most commonly prescribed antibiotic combinations were ceftazidime-amikacin, piperacillin-tazobactam-amikacin, and amoxicillin-clavulanate-amikacin.
Neonates treated with ceftazidime-amikacin had a 68% lower reported mortality than those treated with ampicillin-gentamicin at an adjusted hazard ratio of 0.32 (95% confidence interval, 0.14-0.72; P = .006), the investigators report. In contrast, no significant differences in mortality rates were reported for neonates treated with amoxicillin-clavulanate-amikacin or piperacillin-tazobactam-amikacin compared to those treated with ampicillin-gentamicin.
Investigators were careful to suggest that mortality effects associated with the different antibiotic combinations might have been confounded by either country-specific effects or underreporting of mortality, as a large proportion of neonates who were treated with ampicillin-gentamicin were followed for fewer than 10 days. However, in an unreported aspect of the same study, neonatal mortality from sepsis dropped by over 50% in two federally funded sites in Nigeria that changed their treatment from the WHO-recommended ampicillin-gentamicin regimen to ceftazidime-amikacin – which Dr. Walsh suggested was an endorsement of ceftazidime-amikacin over ampicillin-gentamicin if ever there was one.
Gram-negative resistance
In looking at resistance patterns to the antibiotic combinations used in these countries, investigators found that almost all Gram-negative isolates tested were “overwhelmingly resistant” to ampicillin, and over 70% of them were resistant to gentamicin as well. Extremely high resistance rates were also found against Staphylococcus spp, which are regarded as intrinsically resistant to ampicillin, rendering it basically useless in this particular treatment setting.
Amikacin had much lower level of resistance, with only about 26% of Gram-negative isolates showing resistance. In terms of coverage against Gram-negative isolates, the lowest level of coverage was provided by ampicillin-gentamicin at slightly over 28%, compared with about 73% for amoxicillin-clavulanate-amikacin, 77% for ceftazidime-amikacin, and 80% for piperacillin-tazobactam-amikacin.
In contrast, “Gram-positive isolates generally had reduced levels of resistance,” the authors state. As Dr. Walsh noted, the consortium also did an analysis assessing how much the antibiotic combinations cost and how much payment was deferred to the parents. For example, in Nigeria, the entire cost of treatment is passed down to the parents, “so if they are earning, say, $5.00 a day and the infant needs ceftazidime-amikacin, where the cost per dose is about $6.00 or $7.00 a day, parents can’t afford it,” Dr. Walsh observed.
This part of the conversation, he added, tends to get lost in many studies of antibiotic resistance in LMICs, which is a critical omission, because in many instances, the choice of treatment does come down to affordability. “It’s all very well for the WHO to sit there and say, ampicillin-gentamicin is perfect, but the combination actually doesn’t work in over 70% of the Gram-negative bacteria we looked at in these countries,” Dr. Walsh emphasized.
“The fact is that we have to be a lot more internationally engaged as to what’s actually happening in poorer populations, because unless we do, neonates are going to continue to die,” he said.
Editorial commentary
Commenting on the findings, lead editorialist Luregn Schlapbach, MD, PhD, of University Children’s Hospital Zurich, Switzerland, pointed out that the study has a number of limitations, including a high rate of dropouts from follow-up. This could possibly result in underestimation of neonatal mortality as well as country-specific biases. Nevertheless, Dr. Schlapbach feels that the integration of sequential clinical, genomic, microbiologic, drug, and cost data across a large network in LMIC settings is “exceptional” and will serve to inform “urgently needed” clinical trials in the field of neonatal sepsis.
“At present, increasing global antibiotic resistance is threatening progress against neonatal sepsis, prompting urgency to develop improved measures to effectively prevent and treat life-threatening infections in this high-risk group,” Dr. Schlapbach and colleagues write.
“The findings from the BARNARDS study call for randomized trials comparing mortality benefit and cost efficiency of different antibiotic combinations and management algorithms to safely reduce unnecessary antibiotic exposure for neonatal sepsis,” the editorialists concluded.
The authors and editorialists have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
First-line treatment of neonatal sepsis in low- and middle-income countries (LMICs) with ampicillin-gentamicin – as recommended by the World Health Organization – needs to be reassessed, a retrospective, observational cohort study suggests. Rates of resistance to this particular antibiotic combination are extremely high in LMICs, and this treatment is unlikely to save many neonatal patients, according to the study’s results.
“The WHO guidelines are over 10 years old, and they are actually based on high-income country data, whereas data reported from low-income countries are reported by private labs, and they do not cater to the lower socioeconomic groups within these countries, which is important data to capture,” Timothy Walsh, MD, University of Oxford, United Kingdom, told this news organization.
“The main take-home message from our data is that ampicillin-gentamicin doesn’t work for most of the Gram-negative isolates we tested, and while there are alternatives, their use is confounded by [a lack of] financial support,” he added.
The study was published online in The Lancet Infectious Diseases.
BARNARDS study
In this substudy of the Burden of Antibiotic Resistance in Neonates from Developing Societies (BARNARDS) study, investigators focused on the effectiveness of antibiotic therapies after taking into account the high prevalence of pathogen resistance to ampicillin-gentamicin. Participating countries included Bangladesh, Ethiopia, India, Nigeria, Pakistan, Rwanda, and South Africa.
“Blood samples were obtained from neonates presenting with clinical signs of sepsis,” the authors note, “and WGS [whole-genome sequencing] and MICs [minimum inhibitory concentrations] for antibiotic treatment were determined for bacterial isolates from culture-confirmed sepsis.” Between Nov. 2015 and Feb. 2018, 36,285 neonates were enrolled into the main BARNARDS study, of whom 9,874 had clinically diagnosed sepsis and 5,749 had antibiotic data.
A total of 2,483 neonates had culture-confirmed sepsis, and WGS data were available for 457 isolates taken from 442 neonates. Slightly over three-quarters of the 5,749 neonates who had antibiotic data received first-line ampicillin-gentamicin. The other three most commonly prescribed antibiotic combinations were ceftazidime-amikacin, piperacillin-tazobactam-amikacin, and amoxicillin-clavulanate-amikacin.
Neonates treated with ceftazidime-amikacin had a 68% lower reported mortality than those treated with ampicillin-gentamicin at an adjusted hazard ratio of 0.32 (95% confidence interval, 0.14-0.72; P = .006), the investigators report. In contrast, no significant differences in mortality rates were reported for neonates treated with amoxicillin-clavulanate-amikacin or piperacillin-tazobactam-amikacin compared to those treated with ampicillin-gentamicin.
Investigators were careful to suggest that mortality effects associated with the different antibiotic combinations might have been confounded by either country-specific effects or underreporting of mortality, as a large proportion of neonates who were treated with ampicillin-gentamicin were followed for fewer than 10 days. However, in an unreported aspect of the same study, neonatal mortality from sepsis dropped by over 50% in two federally funded sites in Nigeria that changed their treatment from the WHO-recommended ampicillin-gentamicin regimen to ceftazidime-amikacin – which Dr. Walsh suggested was an endorsement of ceftazidime-amikacin over ampicillin-gentamicin if ever there was one.
Gram-negative resistance
In looking at resistance patterns to the antibiotic combinations used in these countries, investigators found that almost all Gram-negative isolates tested were “overwhelmingly resistant” to ampicillin, and over 70% of them were resistant to gentamicin as well. Extremely high resistance rates were also found against Staphylococcus spp, which are regarded as intrinsically resistant to ampicillin, rendering it basically useless in this particular treatment setting.
Amikacin had much lower level of resistance, with only about 26% of Gram-negative isolates showing resistance. In terms of coverage against Gram-negative isolates, the lowest level of coverage was provided by ampicillin-gentamicin at slightly over 28%, compared with about 73% for amoxicillin-clavulanate-amikacin, 77% for ceftazidime-amikacin, and 80% for piperacillin-tazobactam-amikacin.
In contrast, “Gram-positive isolates generally had reduced levels of resistance,” the authors state. As Dr. Walsh noted, the consortium also did an analysis assessing how much the antibiotic combinations cost and how much payment was deferred to the parents. For example, in Nigeria, the entire cost of treatment is passed down to the parents, “so if they are earning, say, $5.00 a day and the infant needs ceftazidime-amikacin, where the cost per dose is about $6.00 or $7.00 a day, parents can’t afford it,” Dr. Walsh observed.
This part of the conversation, he added, tends to get lost in many studies of antibiotic resistance in LMICs, which is a critical omission, because in many instances, the choice of treatment does come down to affordability. “It’s all very well for the WHO to sit there and say, ampicillin-gentamicin is perfect, but the combination actually doesn’t work in over 70% of the Gram-negative bacteria we looked at in these countries,” Dr. Walsh emphasized.
“The fact is that we have to be a lot more internationally engaged as to what’s actually happening in poorer populations, because unless we do, neonates are going to continue to die,” he said.
Editorial commentary
Commenting on the findings, lead editorialist Luregn Schlapbach, MD, PhD, of University Children’s Hospital Zurich, Switzerland, pointed out that the study has a number of limitations, including a high rate of dropouts from follow-up. This could possibly result in underestimation of neonatal mortality as well as country-specific biases. Nevertheless, Dr. Schlapbach feels that the integration of sequential clinical, genomic, microbiologic, drug, and cost data across a large network in LMIC settings is “exceptional” and will serve to inform “urgently needed” clinical trials in the field of neonatal sepsis.
“At present, increasing global antibiotic resistance is threatening progress against neonatal sepsis, prompting urgency to develop improved measures to effectively prevent and treat life-threatening infections in this high-risk group,” Dr. Schlapbach and colleagues write.
“The findings from the BARNARDS study call for randomized trials comparing mortality benefit and cost efficiency of different antibiotic combinations and management algorithms to safely reduce unnecessary antibiotic exposure for neonatal sepsis,” the editorialists concluded.
The authors and editorialists have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Enlarging Nodule on the Back
The Diagnosis: Cutaneous Myxoma
Microscopic analysis showed features of cutaneous myxoma (quiz images). The epidermis was essentially unremarkable. Stellate to spindle cells with bland nuclear chromatin were present in the dermis with abundant pools of myxoid stroma. Colloidal iron staining highlighted the markedly increased dermal mucin.
Cutaneous myxomas (also referred to as superficial angiomyxomas) are rare, well-demarcated tumors of the dermis and subcutis.1,2 They can present as solitary, fleshcolored nodules on the trunk, lower extremities, head, or neck, and they often measure between 1 and 5 cm.2,3 Histologically, cutaneous myxomas are hypocellular with some stellate fibroblasts, occasional epithelial structures, and an abundant myxoid stroma, with notable thinwalled small blood vessels.2,4 These lesions contain pools of mucin and are positive for mesenchymal mucin stains such as colloidal iron and Alcian blue.1 Moreover, perivascular neutrophils are a distinguishing characteristic of cutaneous myxomas.4
Multiple cutaneous myxomas should raise concern for Carney complex,1,5 a genodermatologic syndrome that arises due to a mutation in the protein kinase CAMP-dependent type I regulatory subunit alpha gene, PRKAR1A, on chromosome 2.1,5 Additional cutaneous manifestations include blue nevi, lentigines, and café-aulait macules.5 Carney complex also is known for endocrine overactivity and cardiac myxomas, which can cause serious embolic complications.1
Recommended management is complete excision with close follow-up, as these lesions may recur in up to one-third of cases. Although there is a potential for recurrence, metastases are uncommon.3 Even without recurrence in the presenting location, follow-up should include screening for manifestations of Carney complex.1,3
The clinical and histological differential for cutaneous myxoma may include nerve sheath myxoma or neurofibroma. A nerve sheath myxoma is a dermal tumor that manifests as a solitary, flesh-colored nodule, measuring less than 2 cm. These lesions commonly present on the head, neck, and upper body.6 Cutaneous myxomas can grow larger than 2 cm, but these two lesions have a great deal of overlap in their other features.3,6 Thus, histology can be used to distinguish them.
Nerve sheath myxomas are circumscribed nonencapsulated tumors of the dermis composed of multilobular aggregates of spindle to epithelioid cells in a mucinous matrix (Figure 1). Clefts often are present around the cell aggregates. Despite previously being termed myxoid neurothekeomas, nerve sheath myxomas are S-100 positive, whereas cellular neurothekeomas are S-100 negative and likely not of neural origin. Cutaneous myxomas, in contrast to nerve sheath myxomas, are S-100 negative. Nerve sheath myxomas are more cellular and lack the characteristic mucin pools compared with cutaneous myxomas.1,2,6 Neurofibromas frequently are flesh colored and pedunculated, as was the lesion in our patient, yet they are vastly different microscopically. The stroma of neurofibromas can vary, but cellularity typically is greater than a cutaneous myxoma and consists of increased numbers of bland spindle cells with wavy nuclei (Schwann cells) and fibrillar cytoplasm as well as mast cells and fibroblasts (Figure 2). Neurofibromas stain positively for S-100 and SOX-10 (Sry-related HMg-box 10).2,7 In addition to café-au-lait macules, axillary freckling, optic gliomas, and positive family history, neurofibromas are associated with neurofibromatosis type 1, which is linked to a defect in a tumor suppressor gene that codes for neurofibromin.7
Nodular fasciitis is a self-limited myofibroblastic neoplasm that contains fusion genes, with the most common being myosin-9–ubiquitin specific peptidase 6, MYH9-USP6, which leads to overexpression of USP6. Nodular fasciitis presents as a solitary, rapidly enlarging nodule affecting the subcutaneous tissue, muscles, or fascia.8,9 It usually presents in the third or fourth decades of life.8 The arms are the most common location in adults, while the most commonly affected site in children is the head or neck. Histopathology reveals a characteristic tissue culture pattern with a proliferation of plump spindle and stellate fibroblasts as well as myofibroblasts (Figure 3). Early lesions have haphazard spindle cells with a proliferation of small blood vessels and extravasated erythrocytes. Despite increased mitotic figures, cellular atypia is rare. The fibroblasts and myofibroblasts react positively for vimentin and muscle-specific actin.8 This lesion is highly cellular comparatively and notably lacks the perivascular neutrophils and epithelial structures that would be expected in a cutaneous myxoma.4,8
Spindle cell lipomas, solitary subcutaneous masses commonly presenting on the upper back in middle-aged men, also can mimic cutaneous myxomas.4 Histologically, these lesions may contain short bundles of spindle cells arranged in a school of fish–like pattern, mature adipocytes, or myxoid stroma and characteristic CD34 positivity (Figure 4). Spindle cell lipomas often will present with ropey collagen, which can easily distinguish them from cutaneous myxomas.4
- Lanjewar DN, Bhatia VO, Lanjewar SD, et al. Cutaneous myxoma: an important clue to Carney complex. Indian J Pathol Microbiol. 2014;57:460-462.
- Choi HJ, Kim YJ, Yim JH, et al. Unusual presentation of solitary cutaneous myxoma. J Eur Acad Dermatol Venereol. 2007;21:403-404. doi:10.1111/j.1468-3083.2006.01881.x
- Kura MM, Jindal SR. Solitary superficial acral angiomyxoma: an infrequently reported soft tissue tumor. Indian J Dermatol. 2014;59:1-3. doi:10.4103/0019-5154.139893
- Zou Y, Billings SD. Myxoid cutaneous tumors: a review. J Cutan Pathol. 2016;43:903-918.
- Sarfo A, Helm K, Flamm A. Cutaneous myxomas and a psammomatous melanotic schwannoma in a patient with Carney complex. J Cutan Pathol. 2019;46:93-96. doi:10.1111/cup.13385
- Gill P, Abi Daoud MS. Multiple cellular neurothekeomas in a middleaged woman including the lower extremity: a case report and review of the current literature. J Cutan Pathol. 2019;46:67-73. doi:10.1111/ cup.13366
- Ohgaki H, Kim Y, Steinbach JP. Nervous system tumors associated with familial tumor syndromes. Curr Opin Neurol. 2010;23:583-591. doi:10.1097/WCO.0b013e3283405b5f
- Luna A, Molinari L, Bollea Garlatti LA, et al. Nodular fasciitis, a forgotten entity. Int J Dermatol. 2019;58:190-193. doi:10.1111/ijd.14219
- Patel N, Chrisinger J, Demicco E, et al. USP6 activation in nodular fasciitis by promoter-swapping gene fusions. Mod Pathol. 2017; 30:1577-1588.
The Diagnosis: Cutaneous Myxoma
Microscopic analysis showed features of cutaneous myxoma (quiz images). The epidermis was essentially unremarkable. Stellate to spindle cells with bland nuclear chromatin were present in the dermis with abundant pools of myxoid stroma. Colloidal iron staining highlighted the markedly increased dermal mucin.
Cutaneous myxomas (also referred to as superficial angiomyxomas) are rare, well-demarcated tumors of the dermis and subcutis.1,2 They can present as solitary, fleshcolored nodules on the trunk, lower extremities, head, or neck, and they often measure between 1 and 5 cm.2,3 Histologically, cutaneous myxomas are hypocellular with some stellate fibroblasts, occasional epithelial structures, and an abundant myxoid stroma, with notable thinwalled small blood vessels.2,4 These lesions contain pools of mucin and are positive for mesenchymal mucin stains such as colloidal iron and Alcian blue.1 Moreover, perivascular neutrophils are a distinguishing characteristic of cutaneous myxomas.4
Multiple cutaneous myxomas should raise concern for Carney complex,1,5 a genodermatologic syndrome that arises due to a mutation in the protein kinase CAMP-dependent type I regulatory subunit alpha gene, PRKAR1A, on chromosome 2.1,5 Additional cutaneous manifestations include blue nevi, lentigines, and café-aulait macules.5 Carney complex also is known for endocrine overactivity and cardiac myxomas, which can cause serious embolic complications.1
Recommended management is complete excision with close follow-up, as these lesions may recur in up to one-third of cases. Although there is a potential for recurrence, metastases are uncommon.3 Even without recurrence in the presenting location, follow-up should include screening for manifestations of Carney complex.1,3
The clinical and histological differential for cutaneous myxoma may include nerve sheath myxoma or neurofibroma. A nerve sheath myxoma is a dermal tumor that manifests as a solitary, flesh-colored nodule, measuring less than 2 cm. These lesions commonly present on the head, neck, and upper body.6 Cutaneous myxomas can grow larger than 2 cm, but these two lesions have a great deal of overlap in their other features.3,6 Thus, histology can be used to distinguish them.

Nerve sheath myxomas are circumscribed nonencapsulated tumors of the dermis composed of multilobular aggregates of spindle to epithelioid cells in a mucinous matrix (Figure 1). Clefts often are present around the cell aggregates. Despite previously being termed myxoid neurothekeomas, nerve sheath myxomas are S-100 positive, whereas cellular neurothekeomas are S-100 negative and likely not of neural origin. Cutaneous myxomas, in contrast to nerve sheath myxomas, are S-100 negative. Nerve sheath myxomas are more cellular and lack the characteristic mucin pools compared with cutaneous myxomas.1,2,6 Neurofibromas frequently are flesh colored and pedunculated, as was the lesion in our patient, yet they are vastly different microscopically. The stroma of neurofibromas can vary, but cellularity typically is greater than a cutaneous myxoma and consists of increased numbers of bland spindle cells with wavy nuclei (Schwann cells) and fibrillar cytoplasm as well as mast cells and fibroblasts (Figure 2). Neurofibromas stain positively for S-100 and SOX-10 (Sry-related HMg-box 10).2,7 In addition to café-au-lait macules, axillary freckling, optic gliomas, and positive family history, neurofibromas are associated with neurofibromatosis type 1, which is linked to a defect in a tumor suppressor gene that codes for neurofibromin.7

Nodular fasciitis is a self-limited myofibroblastic neoplasm that contains fusion genes, with the most common being myosin-9–ubiquitin specific peptidase 6, MYH9-USP6, which leads to overexpression of USP6. Nodular fasciitis presents as a solitary, rapidly enlarging nodule affecting the subcutaneous tissue, muscles, or fascia.8,9 It usually presents in the third or fourth decades of life.8 The arms are the most common location in adults, while the most commonly affected site in children is the head or neck. Histopathology reveals a characteristic tissue culture pattern with a proliferation of plump spindle and stellate fibroblasts as well as myofibroblasts (Figure 3). Early lesions have haphazard spindle cells with a proliferation of small blood vessels and extravasated erythrocytes. Despite increased mitotic figures, cellular atypia is rare. The fibroblasts and myofibroblasts react positively for vimentin and muscle-specific actin.8 This lesion is highly cellular comparatively and notably lacks the perivascular neutrophils and epithelial structures that would be expected in a cutaneous myxoma.4,8

Spindle cell lipomas, solitary subcutaneous masses commonly presenting on the upper back in middle-aged men, also can mimic cutaneous myxomas.4 Histologically, these lesions may contain short bundles of spindle cells arranged in a school of fish–like pattern, mature adipocytes, or myxoid stroma and characteristic CD34 positivity (Figure 4). Spindle cell lipomas often will present with ropey collagen, which can easily distinguish them from cutaneous myxomas.4

The Diagnosis: Cutaneous Myxoma
Microscopic analysis showed features of cutaneous myxoma (quiz images). The epidermis was essentially unremarkable. Stellate to spindle cells with bland nuclear chromatin were present in the dermis with abundant pools of myxoid stroma. Colloidal iron staining highlighted the markedly increased dermal mucin.
Cutaneous myxomas (also referred to as superficial angiomyxomas) are rare, well-demarcated tumors of the dermis and subcutis.1,2 They can present as solitary, fleshcolored nodules on the trunk, lower extremities, head, or neck, and they often measure between 1 and 5 cm.2,3 Histologically, cutaneous myxomas are hypocellular with some stellate fibroblasts, occasional epithelial structures, and an abundant myxoid stroma, with notable thinwalled small blood vessels.2,4 These lesions contain pools of mucin and are positive for mesenchymal mucin stains such as colloidal iron and Alcian blue.1 Moreover, perivascular neutrophils are a distinguishing characteristic of cutaneous myxomas.4
Multiple cutaneous myxomas should raise concern for Carney complex,1,5 a genodermatologic syndrome that arises due to a mutation in the protein kinase CAMP-dependent type I regulatory subunit alpha gene, PRKAR1A, on chromosome 2.1,5 Additional cutaneous manifestations include blue nevi, lentigines, and café-aulait macules.5 Carney complex also is known for endocrine overactivity and cardiac myxomas, which can cause serious embolic complications.1
Recommended management is complete excision with close follow-up, as these lesions may recur in up to one-third of cases. Although there is a potential for recurrence, metastases are uncommon.3 Even without recurrence in the presenting location, follow-up should include screening for manifestations of Carney complex.1,3
The clinical and histological differential for cutaneous myxoma may include nerve sheath myxoma or neurofibroma. A nerve sheath myxoma is a dermal tumor that manifests as a solitary, flesh-colored nodule, measuring less than 2 cm. These lesions commonly present on the head, neck, and upper body.6 Cutaneous myxomas can grow larger than 2 cm, but these two lesions have a great deal of overlap in their other features.3,6 Thus, histology can be used to distinguish them.

Nerve sheath myxomas are circumscribed nonencapsulated tumors of the dermis composed of multilobular aggregates of spindle to epithelioid cells in a mucinous matrix (Figure 1). Clefts often are present around the cell aggregates. Despite previously being termed myxoid neurothekeomas, nerve sheath myxomas are S-100 positive, whereas cellular neurothekeomas are S-100 negative and likely not of neural origin. Cutaneous myxomas, in contrast to nerve sheath myxomas, are S-100 negative. Nerve sheath myxomas are more cellular and lack the characteristic mucin pools compared with cutaneous myxomas.1,2,6 Neurofibromas frequently are flesh colored and pedunculated, as was the lesion in our patient, yet they are vastly different microscopically. The stroma of neurofibromas can vary, but cellularity typically is greater than a cutaneous myxoma and consists of increased numbers of bland spindle cells with wavy nuclei (Schwann cells) and fibrillar cytoplasm as well as mast cells and fibroblasts (Figure 2). Neurofibromas stain positively for S-100 and SOX-10 (Sry-related HMg-box 10).2,7 In addition to café-au-lait macules, axillary freckling, optic gliomas, and positive family history, neurofibromas are associated with neurofibromatosis type 1, which is linked to a defect in a tumor suppressor gene that codes for neurofibromin.7

Nodular fasciitis is a self-limited myofibroblastic neoplasm that contains fusion genes, with the most common being myosin-9–ubiquitin specific peptidase 6, MYH9-USP6, which leads to overexpression of USP6. Nodular fasciitis presents as a solitary, rapidly enlarging nodule affecting the subcutaneous tissue, muscles, or fascia.8,9 It usually presents in the third or fourth decades of life.8 The arms are the most common location in adults, while the most commonly affected site in children is the head or neck. Histopathology reveals a characteristic tissue culture pattern with a proliferation of plump spindle and stellate fibroblasts as well as myofibroblasts (Figure 3). Early lesions have haphazard spindle cells with a proliferation of small blood vessels and extravasated erythrocytes. Despite increased mitotic figures, cellular atypia is rare. The fibroblasts and myofibroblasts react positively for vimentin and muscle-specific actin.8 This lesion is highly cellular comparatively and notably lacks the perivascular neutrophils and epithelial structures that would be expected in a cutaneous myxoma.4,8

Spindle cell lipomas, solitary subcutaneous masses commonly presenting on the upper back in middle-aged men, also can mimic cutaneous myxomas.4 Histologically, these lesions may contain short bundles of spindle cells arranged in a school of fish–like pattern, mature adipocytes, or myxoid stroma and characteristic CD34 positivity (Figure 4). Spindle cell lipomas often will present with ropey collagen, which can easily distinguish them from cutaneous myxomas.4

- Lanjewar DN, Bhatia VO, Lanjewar SD, et al. Cutaneous myxoma: an important clue to Carney complex. Indian J Pathol Microbiol. 2014;57:460-462.
- Choi HJ, Kim YJ, Yim JH, et al. Unusual presentation of solitary cutaneous myxoma. J Eur Acad Dermatol Venereol. 2007;21:403-404. doi:10.1111/j.1468-3083.2006.01881.x
- Kura MM, Jindal SR. Solitary superficial acral angiomyxoma: an infrequently reported soft tissue tumor. Indian J Dermatol. 2014;59:1-3. doi:10.4103/0019-5154.139893
- Zou Y, Billings SD. Myxoid cutaneous tumors: a review. J Cutan Pathol. 2016;43:903-918.
- Sarfo A, Helm K, Flamm A. Cutaneous myxomas and a psammomatous melanotic schwannoma in a patient with Carney complex. J Cutan Pathol. 2019;46:93-96. doi:10.1111/cup.13385
- Gill P, Abi Daoud MS. Multiple cellular neurothekeomas in a middleaged woman including the lower extremity: a case report and review of the current literature. J Cutan Pathol. 2019;46:67-73. doi:10.1111/ cup.13366
- Ohgaki H, Kim Y, Steinbach JP. Nervous system tumors associated with familial tumor syndromes. Curr Opin Neurol. 2010;23:583-591. doi:10.1097/WCO.0b013e3283405b5f
- Luna A, Molinari L, Bollea Garlatti LA, et al. Nodular fasciitis, a forgotten entity. Int J Dermatol. 2019;58:190-193. doi:10.1111/ijd.14219
- Patel N, Chrisinger J, Demicco E, et al. USP6 activation in nodular fasciitis by promoter-swapping gene fusions. Mod Pathol. 2017; 30:1577-1588.
- Lanjewar DN, Bhatia VO, Lanjewar SD, et al. Cutaneous myxoma: an important clue to Carney complex. Indian J Pathol Microbiol. 2014;57:460-462.
- Choi HJ, Kim YJ, Yim JH, et al. Unusual presentation of solitary cutaneous myxoma. J Eur Acad Dermatol Venereol. 2007;21:403-404. doi:10.1111/j.1468-3083.2006.01881.x
- Kura MM, Jindal SR. Solitary superficial acral angiomyxoma: an infrequently reported soft tissue tumor. Indian J Dermatol. 2014;59:1-3. doi:10.4103/0019-5154.139893
- Zou Y, Billings SD. Myxoid cutaneous tumors: a review. J Cutan Pathol. 2016;43:903-918.
- Sarfo A, Helm K, Flamm A. Cutaneous myxomas and a psammomatous melanotic schwannoma in a patient with Carney complex. J Cutan Pathol. 2019;46:93-96. doi:10.1111/cup.13385
- Gill P, Abi Daoud MS. Multiple cellular neurothekeomas in a middleaged woman including the lower extremity: a case report and review of the current literature. J Cutan Pathol. 2019;46:67-73. doi:10.1111/ cup.13366
- Ohgaki H, Kim Y, Steinbach JP. Nervous system tumors associated with familial tumor syndromes. Curr Opin Neurol. 2010;23:583-591. doi:10.1097/WCO.0b013e3283405b5f
- Luna A, Molinari L, Bollea Garlatti LA, et al. Nodular fasciitis, a forgotten entity. Int J Dermatol. 2019;58:190-193. doi:10.1111/ijd.14219
- Patel N, Chrisinger J, Demicco E, et al. USP6 activation in nodular fasciitis by promoter-swapping gene fusions. Mod Pathol. 2017; 30:1577-1588.
A 43-year-old man with an unremarkable medical history presented to our clinic with an enlarging painful nodule on the upper back that was present for years without bleeding or ulceration. He denied prior treatment or any similar lesions. Physical examination was notable for a 2×1.5-cm, pedunculated, flesh-colored nodule on the left upper back. A shave excision of the lesion was performed.
Erythematous Indurated Nodule on the Forehead
The Diagnosis: Dermatofibrosarcoma Protuberans
Histopathologic examination showed a dermal tumor composed of spindle cells in a storiform arrangement (Figure 1). Immunohistochemistry demonstrated positive CD34 staining of the tumoral cells (Figure 2). Clinical review, histopathologic examination, and immunohistochemistry confirmed a diagnosis of dermatofibrosarcoma protuberans (DFSP). The patient underwent Mohs micrographic surgery (MMS) with clear margins after 3 stages, followed by repair with a rotation flap. No evidence of recurrence was found at 4-year follow-up.
Dermatofibrosarcoma protuberans is a rare low-grade sarcoma of fibroblast origin with an annual incidence of 0.8 to 5 cases per million individuals.1 It typically presents in patients aged 30 to 50 years on the trunk, scalp, or proximal extremities as an asymptomatic, flesh-colored, erythematous or brown, indurated plaque or nodule.2 Due to its variable presentation, these lesions often may be misdiagnosed as lipomas or epidermoid cysts, preventing proper targeted treatment. Therefore, suspicious enlarging indurated nodules require a lower threshold for biopsy.1
A definitive diagnosis of DFSP is achieved after a biopsy and histopathologic evaluation. Hematoxylin and eosin staining typically shows diffuse infiltration of the dermis and the subcutaneous fat by densely packed, cytologic, relatively uniform, spindle-shaped tumor cells arranged in a characteristic storiform shape. Tumor cells are spread along the septae of the subcutaneous fatty tissue.3 Immunohistochemistry is characterized by positive CD34 and negative factor XIIIa, with rare exceptions.
The differential diagnosis includes lipoma, epidermoid cyst, plexiform fibrohistiocytic tumor, and malignant peripheral nerve sheath tumor.3 Positive CD34 immunostaining, negative S-100 staining, and a storiform pattern of spindle cells can assist in differentiating DFSP from these possible differential diagnoses; lesions of these other entities are characterized by different pathologic findings. Lipomas are composed of fat tissue, epidermoid cysts have epithelial-lined cysts filled with keratin, plexiform fibrohistiocytic tumors have plexiform rays of fibrous tissue extending into fat with negative CD34 staining, and malignant peripheral nerve sheath tumors have fleshy variegated masses involving the peripheral nerve trunks with partial S-100 staining.4-7 Additional evaluation to confirm DFSP can be accomplished by analysis of tumor samples by fluorescence in situ hybridization or reverse transcriptase–polymerase chain reaction to detect chromosomal translocations and fusion gene transcripts, as chromosomal translocations may be found in more than 90% of cases.3
Early diagnosis of DFSP is beneficial, as it can help prevent recurrence as well as metastasis. Studies have attempted to document the risk for recurrence as well as metastasis based on characteristic features and treatment strategies of DFSP. In a study of 186 patients, 3 had metastatic disease to the lungs, the most common site of metastasis.8 These 3 patients had fibrosarcomatous transformation within DFSP, emphasizing the importance of detailing this finding early in the diagnosis, as it was characterized by a higher degree of cellularity, cytologic atypia, mitotic activity, and negative CD34 immunostaining.9 In patients with suspected metastasis, lymph node ultrasonography, chest radiography, and computed tomography may be utilized.3
When treating DFSP, the goal is complete removal of the tumor with clear margins. Mohs micrographic surgery, modified MMS, and wide local excision (WLE) with 2- to 4-cm margins are appropriate treatment options, though MMS is the treatment of choice. A study comparing MMS and WLE demonstrated 3% and 30.8% recurrence rates, respectively.8 In MMS, complete margin evaluation on microscopy is performed after each stage to ensure negative surgical margins. The presence of positive surgical margins elicits continued resection until the margins are clear.10,11
Other treatment modalities may be considered for patients with DFSP. Molecular therapy with imatinib, an oral tyrosine kinase inhibitor targeting platelet-derived growth factor–regulated expression, can be utilized for inoperable tumors; however, additional clinical trials are required to ensure efficacy.3 Surgical removal of the possible remaining tumor is still recommended after molecular therapy. Radiotherapy is an additional method of treatment that may be used for inoperable tumors.3
Dermatofibrosarcoma protuberans is a rare lowgrade sarcoma of fibroblast origin that typically does not metastasize but often has notable subclinical extension and recurrence. Differentiating DFSP from other tumors often may be difficult. A protuberant, flesh-colored, slowgrowing, and asymptomatic lesion often may be confused with lipomas or epidermoid cysts; therefore, biopsies with immunohistostaining for suspicious lesions is required.12 Mohs micrographic surgery has evolved as the treatment of choice for this tumor, though WLE and new targeted molecular therapies still are considered. Proper diagnosis and treatment of DFSP is paramount in preventing future morbidity.
- Benoit A, Aycock J, Milam D, et al. Dermatofibrosarcoma protuberans of the forehead with extensive subclinical spread. Dermatol Surg. 2016;42:261-264. doi:10.1097/DSS.0000000000000604
- Khachemoune A, Barkoe D, Braun M, et al. Dermatofibrosarcoma protuberans of the forehead and scalp with involvement of the outer calvarial plate: multistaged repair with the use of skin expanders. Dermatol Surg. 2005;31:115-119. doi:10.1111/j.1524-4725.2005.31021
- Saiag P, Grob J-J, Lebbe C, et al. Diagnosis and treatment of dermatofibrosarcoma protuberans. European consensus-based interdisciplinary guideline. Eur J Cancer. 2015;51:2604-2608. doi:10.1016/j.ejca.2015.06.108
- Charifa A, Badri T. Lipomas, pathology. StatPearls. StatPearls Publishing; 2020.
- Zito PM, Scharf R. Cyst, epidermoid (sebaceous cyst). StatPearls. StatPearls Publishing; 2020.
- Taher A, Pushpanathan C. Plexiform fibrohistiocytic tumor: a brief review. Arch Pathol Lab Med. 2007;131:1135-1138. doi:10.5858 /2007-131-1135-PFTABR
- Rodriguez FJ, Folpe AL, Giannini C, et al. Pathology of peripheral nerve sheath tumors: diagnostic overview and update on selected diagnostic problems. Acta Neuropathol. 2012;123:295-319. doi:10.1007 /s00401-012-0954-z
- Lowe GC, Onajin O, Baum CL, et al. A comparison of Mohs micrographic surgery and wide local excision for treatment of dermatofibrosarcoma protuberans with long-term follow-up: the Mayo Clinic experience. Dermatol Surg. 2017;43:98-106. doi:10.1097/DSS.0000000000000910
- Rouhani P, Fletcher CDM, Devesa SS, et al. Cutaneous soft tissue sarcoma incidence patterns in the U.S.: an analysis of 12,114 cases. Cancer. 2008;113:616-627. doi:10.1002/cncr.23571
- Ratner D, Thomas CO, Johnson TM, et al. Mohs micrographic surgery for the treatment of dermatofibrosarcoma protuberans. results of a multiinstitutional series with an analysis of the extent of microscopic spread. J Am Acad Dermatol. 1997;37:600-613. doi:10.1016/s0190 -9622(97)70179-8
- Buck DW, Kim JYS, Alam M, et al. Multidisciplinary approach to the management of dermatofibrosarcoma protuberans. J Am Acad Dermatol. 2012;67:861-866. doi:10.1016/j.jaad.2012.01.039
- Shih P-Y, Chen C-H, Kuo T-T, et al. Deep dermatofibrosarcoma protuberans: a pitfall in the ultrasonographic diagnosis of lipoma -like subcutaneous lesions. Dermatologica Sinica. 2010;28:32-35. doi:10.1016/S1027-8117(10)60005-5
The Diagnosis: Dermatofibrosarcoma Protuberans
Histopathologic examination showed a dermal tumor composed of spindle cells in a storiform arrangement (Figure 1). Immunohistochemistry demonstrated positive CD34 staining of the tumoral cells (Figure 2). Clinical review, histopathologic examination, and immunohistochemistry confirmed a diagnosis of dermatofibrosarcoma protuberans (DFSP). The patient underwent Mohs micrographic surgery (MMS) with clear margins after 3 stages, followed by repair with a rotation flap. No evidence of recurrence was found at 4-year follow-up.

Dermatofibrosarcoma protuberans is a rare low-grade sarcoma of fibroblast origin with an annual incidence of 0.8 to 5 cases per million individuals.1 It typically presents in patients aged 30 to 50 years on the trunk, scalp, or proximal extremities as an asymptomatic, flesh-colored, erythematous or brown, indurated plaque or nodule.2 Due to its variable presentation, these lesions often may be misdiagnosed as lipomas or epidermoid cysts, preventing proper targeted treatment. Therefore, suspicious enlarging indurated nodules require a lower threshold for biopsy.1

A definitive diagnosis of DFSP is achieved after a biopsy and histopathologic evaluation. Hematoxylin and eosin staining typically shows diffuse infiltration of the dermis and the subcutaneous fat by densely packed, cytologic, relatively uniform, spindle-shaped tumor cells arranged in a characteristic storiform shape. Tumor cells are spread along the septae of the subcutaneous fatty tissue.3 Immunohistochemistry is characterized by positive CD34 and negative factor XIIIa, with rare exceptions.
The differential diagnosis includes lipoma, epidermoid cyst, plexiform fibrohistiocytic tumor, and malignant peripheral nerve sheath tumor.3 Positive CD34 immunostaining, negative S-100 staining, and a storiform pattern of spindle cells can assist in differentiating DFSP from these possible differential diagnoses; lesions of these other entities are characterized by different pathologic findings. Lipomas are composed of fat tissue, epidermoid cysts have epithelial-lined cysts filled with keratin, plexiform fibrohistiocytic tumors have plexiform rays of fibrous tissue extending into fat with negative CD34 staining, and malignant peripheral nerve sheath tumors have fleshy variegated masses involving the peripheral nerve trunks with partial S-100 staining.4-7 Additional evaluation to confirm DFSP can be accomplished by analysis of tumor samples by fluorescence in situ hybridization or reverse transcriptase–polymerase chain reaction to detect chromosomal translocations and fusion gene transcripts, as chromosomal translocations may be found in more than 90% of cases.3
Early diagnosis of DFSP is beneficial, as it can help prevent recurrence as well as metastasis. Studies have attempted to document the risk for recurrence as well as metastasis based on characteristic features and treatment strategies of DFSP. In a study of 186 patients, 3 had metastatic disease to the lungs, the most common site of metastasis.8 These 3 patients had fibrosarcomatous transformation within DFSP, emphasizing the importance of detailing this finding early in the diagnosis, as it was characterized by a higher degree of cellularity, cytologic atypia, mitotic activity, and negative CD34 immunostaining.9 In patients with suspected metastasis, lymph node ultrasonography, chest radiography, and computed tomography may be utilized.3
When treating DFSP, the goal is complete removal of the tumor with clear margins. Mohs micrographic surgery, modified MMS, and wide local excision (WLE) with 2- to 4-cm margins are appropriate treatment options, though MMS is the treatment of choice. A study comparing MMS and WLE demonstrated 3% and 30.8% recurrence rates, respectively.8 In MMS, complete margin evaluation on microscopy is performed after each stage to ensure negative surgical margins. The presence of positive surgical margins elicits continued resection until the margins are clear.10,11
Other treatment modalities may be considered for patients with DFSP. Molecular therapy with imatinib, an oral tyrosine kinase inhibitor targeting platelet-derived growth factor–regulated expression, can be utilized for inoperable tumors; however, additional clinical trials are required to ensure efficacy.3 Surgical removal of the possible remaining tumor is still recommended after molecular therapy. Radiotherapy is an additional method of treatment that may be used for inoperable tumors.3
Dermatofibrosarcoma protuberans is a rare lowgrade sarcoma of fibroblast origin that typically does not metastasize but often has notable subclinical extension and recurrence. Differentiating DFSP from other tumors often may be difficult. A protuberant, flesh-colored, slowgrowing, and asymptomatic lesion often may be confused with lipomas or epidermoid cysts; therefore, biopsies with immunohistostaining for suspicious lesions is required.12 Mohs micrographic surgery has evolved as the treatment of choice for this tumor, though WLE and new targeted molecular therapies still are considered. Proper diagnosis and treatment of DFSP is paramount in preventing future morbidity.
The Diagnosis: Dermatofibrosarcoma Protuberans
Histopathologic examination showed a dermal tumor composed of spindle cells in a storiform arrangement (Figure 1). Immunohistochemistry demonstrated positive CD34 staining of the tumoral cells (Figure 2). Clinical review, histopathologic examination, and immunohistochemistry confirmed a diagnosis of dermatofibrosarcoma protuberans (DFSP). The patient underwent Mohs micrographic surgery (MMS) with clear margins after 3 stages, followed by repair with a rotation flap. No evidence of recurrence was found at 4-year follow-up.

Dermatofibrosarcoma protuberans is a rare low-grade sarcoma of fibroblast origin with an annual incidence of 0.8 to 5 cases per million individuals.1 It typically presents in patients aged 30 to 50 years on the trunk, scalp, or proximal extremities as an asymptomatic, flesh-colored, erythematous or brown, indurated plaque or nodule.2 Due to its variable presentation, these lesions often may be misdiagnosed as lipomas or epidermoid cysts, preventing proper targeted treatment. Therefore, suspicious enlarging indurated nodules require a lower threshold for biopsy.1

A definitive diagnosis of DFSP is achieved after a biopsy and histopathologic evaluation. Hematoxylin and eosin staining typically shows diffuse infiltration of the dermis and the subcutaneous fat by densely packed, cytologic, relatively uniform, spindle-shaped tumor cells arranged in a characteristic storiform shape. Tumor cells are spread along the septae of the subcutaneous fatty tissue.3 Immunohistochemistry is characterized by positive CD34 and negative factor XIIIa, with rare exceptions.
The differential diagnosis includes lipoma, epidermoid cyst, plexiform fibrohistiocytic tumor, and malignant peripheral nerve sheath tumor.3 Positive CD34 immunostaining, negative S-100 staining, and a storiform pattern of spindle cells can assist in differentiating DFSP from these possible differential diagnoses; lesions of these other entities are characterized by different pathologic findings. Lipomas are composed of fat tissue, epidermoid cysts have epithelial-lined cysts filled with keratin, plexiform fibrohistiocytic tumors have plexiform rays of fibrous tissue extending into fat with negative CD34 staining, and malignant peripheral nerve sheath tumors have fleshy variegated masses involving the peripheral nerve trunks with partial S-100 staining.4-7 Additional evaluation to confirm DFSP can be accomplished by analysis of tumor samples by fluorescence in situ hybridization or reverse transcriptase–polymerase chain reaction to detect chromosomal translocations and fusion gene transcripts, as chromosomal translocations may be found in more than 90% of cases.3
Early diagnosis of DFSP is beneficial, as it can help prevent recurrence as well as metastasis. Studies have attempted to document the risk for recurrence as well as metastasis based on characteristic features and treatment strategies of DFSP. In a study of 186 patients, 3 had metastatic disease to the lungs, the most common site of metastasis.8 These 3 patients had fibrosarcomatous transformation within DFSP, emphasizing the importance of detailing this finding early in the diagnosis, as it was characterized by a higher degree of cellularity, cytologic atypia, mitotic activity, and negative CD34 immunostaining.9 In patients with suspected metastasis, lymph node ultrasonography, chest radiography, and computed tomography may be utilized.3
When treating DFSP, the goal is complete removal of the tumor with clear margins. Mohs micrographic surgery, modified MMS, and wide local excision (WLE) with 2- to 4-cm margins are appropriate treatment options, though MMS is the treatment of choice. A study comparing MMS and WLE demonstrated 3% and 30.8% recurrence rates, respectively.8 In MMS, complete margin evaluation on microscopy is performed after each stage to ensure negative surgical margins. The presence of positive surgical margins elicits continued resection until the margins are clear.10,11
Other treatment modalities may be considered for patients with DFSP. Molecular therapy with imatinib, an oral tyrosine kinase inhibitor targeting platelet-derived growth factor–regulated expression, can be utilized for inoperable tumors; however, additional clinical trials are required to ensure efficacy.3 Surgical removal of the possible remaining tumor is still recommended after molecular therapy. Radiotherapy is an additional method of treatment that may be used for inoperable tumors.3
Dermatofibrosarcoma protuberans is a rare lowgrade sarcoma of fibroblast origin that typically does not metastasize but often has notable subclinical extension and recurrence. Differentiating DFSP from other tumors often may be difficult. A protuberant, flesh-colored, slowgrowing, and asymptomatic lesion often may be confused with lipomas or epidermoid cysts; therefore, biopsies with immunohistostaining for suspicious lesions is required.12 Mohs micrographic surgery has evolved as the treatment of choice for this tumor, though WLE and new targeted molecular therapies still are considered. Proper diagnosis and treatment of DFSP is paramount in preventing future morbidity.
- Benoit A, Aycock J, Milam D, et al. Dermatofibrosarcoma protuberans of the forehead with extensive subclinical spread. Dermatol Surg. 2016;42:261-264. doi:10.1097/DSS.0000000000000604
- Khachemoune A, Barkoe D, Braun M, et al. Dermatofibrosarcoma protuberans of the forehead and scalp with involvement of the outer calvarial plate: multistaged repair with the use of skin expanders. Dermatol Surg. 2005;31:115-119. doi:10.1111/j.1524-4725.2005.31021
- Saiag P, Grob J-J, Lebbe C, et al. Diagnosis and treatment of dermatofibrosarcoma protuberans. European consensus-based interdisciplinary guideline. Eur J Cancer. 2015;51:2604-2608. doi:10.1016/j.ejca.2015.06.108
- Charifa A, Badri T. Lipomas, pathology. StatPearls. StatPearls Publishing; 2020.
- Zito PM, Scharf R. Cyst, epidermoid (sebaceous cyst). StatPearls. StatPearls Publishing; 2020.
- Taher A, Pushpanathan C. Plexiform fibrohistiocytic tumor: a brief review. Arch Pathol Lab Med. 2007;131:1135-1138. doi:10.5858 /2007-131-1135-PFTABR
- Rodriguez FJ, Folpe AL, Giannini C, et al. Pathology of peripheral nerve sheath tumors: diagnostic overview and update on selected diagnostic problems. Acta Neuropathol. 2012;123:295-319. doi:10.1007 /s00401-012-0954-z
- Lowe GC, Onajin O, Baum CL, et al. A comparison of Mohs micrographic surgery and wide local excision for treatment of dermatofibrosarcoma protuberans with long-term follow-up: the Mayo Clinic experience. Dermatol Surg. 2017;43:98-106. doi:10.1097/DSS.0000000000000910
- Rouhani P, Fletcher CDM, Devesa SS, et al. Cutaneous soft tissue sarcoma incidence patterns in the U.S.: an analysis of 12,114 cases. Cancer. 2008;113:616-627. doi:10.1002/cncr.23571
- Ratner D, Thomas CO, Johnson TM, et al. Mohs micrographic surgery for the treatment of dermatofibrosarcoma protuberans. results of a multiinstitutional series with an analysis of the extent of microscopic spread. J Am Acad Dermatol. 1997;37:600-613. doi:10.1016/s0190 -9622(97)70179-8
- Buck DW, Kim JYS, Alam M, et al. Multidisciplinary approach to the management of dermatofibrosarcoma protuberans. J Am Acad Dermatol. 2012;67:861-866. doi:10.1016/j.jaad.2012.01.039
- Shih P-Y, Chen C-H, Kuo T-T, et al. Deep dermatofibrosarcoma protuberans: a pitfall in the ultrasonographic diagnosis of lipoma -like subcutaneous lesions. Dermatologica Sinica. 2010;28:32-35. doi:10.1016/S1027-8117(10)60005-5
- Benoit A, Aycock J, Milam D, et al. Dermatofibrosarcoma protuberans of the forehead with extensive subclinical spread. Dermatol Surg. 2016;42:261-264. doi:10.1097/DSS.0000000000000604
- Khachemoune A, Barkoe D, Braun M, et al. Dermatofibrosarcoma protuberans of the forehead and scalp with involvement of the outer calvarial plate: multistaged repair with the use of skin expanders. Dermatol Surg. 2005;31:115-119. doi:10.1111/j.1524-4725.2005.31021
- Saiag P, Grob J-J, Lebbe C, et al. Diagnosis and treatment of dermatofibrosarcoma protuberans. European consensus-based interdisciplinary guideline. Eur J Cancer. 2015;51:2604-2608. doi:10.1016/j.ejca.2015.06.108
- Charifa A, Badri T. Lipomas, pathology. StatPearls. StatPearls Publishing; 2020.
- Zito PM, Scharf R. Cyst, epidermoid (sebaceous cyst). StatPearls. StatPearls Publishing; 2020.
- Taher A, Pushpanathan C. Plexiform fibrohistiocytic tumor: a brief review. Arch Pathol Lab Med. 2007;131:1135-1138. doi:10.5858 /2007-131-1135-PFTABR
- Rodriguez FJ, Folpe AL, Giannini C, et al. Pathology of peripheral nerve sheath tumors: diagnostic overview and update on selected diagnostic problems. Acta Neuropathol. 2012;123:295-319. doi:10.1007 /s00401-012-0954-z
- Lowe GC, Onajin O, Baum CL, et al. A comparison of Mohs micrographic surgery and wide local excision for treatment of dermatofibrosarcoma protuberans with long-term follow-up: the Mayo Clinic experience. Dermatol Surg. 2017;43:98-106. doi:10.1097/DSS.0000000000000910
- Rouhani P, Fletcher CDM, Devesa SS, et al. Cutaneous soft tissue sarcoma incidence patterns in the U.S.: an analysis of 12,114 cases. Cancer. 2008;113:616-627. doi:10.1002/cncr.23571
- Ratner D, Thomas CO, Johnson TM, et al. Mohs micrographic surgery for the treatment of dermatofibrosarcoma protuberans. results of a multiinstitutional series with an analysis of the extent of microscopic spread. J Am Acad Dermatol. 1997;37:600-613. doi:10.1016/s0190 -9622(97)70179-8
- Buck DW, Kim JYS, Alam M, et al. Multidisciplinary approach to the management of dermatofibrosarcoma protuberans. J Am Acad Dermatol. 2012;67:861-866. doi:10.1016/j.jaad.2012.01.039
- Shih P-Y, Chen C-H, Kuo T-T, et al. Deep dermatofibrosarcoma protuberans: a pitfall in the ultrasonographic diagnosis of lipoma -like subcutaneous lesions. Dermatologica Sinica. 2010;28:32-35. doi:10.1016/S1027-8117(10)60005-5
A 39-year-old man presented with an enlarging asymptomatic nodule on the forehead of more than 3 years’ duration. Physical examination revealed a 3.4×2.3-cm, indurated, firm, erythematous nodule on the frontotemporal scalp. The patient denied any history of trauma to the area.

Smoking and alcohol raise risk of second cancer in squamous cell carcinoma
Field cancerization and subsequent second cancer in squamous cell carcinoma (SCC) patients was significantly associated with cigarette and alcohol use, based on data from more than 300 individuals.
Cigarette and alcohol use are established risk factors for SCCs of the esophagus, head, and neck, Manabu Moto, MD, of Kyoto University and colleagues wrote. “In addition, squamous cell carcinoma and squamous dysplastic epithelium develop multifocally in these organs,” in a phenomenon known as field cancerization, but the interaction of multiple dysplastic epithelium with other factors, notably whether cessation of cigarette and alcohol use would reduce risk of SCC, has not been well studied.
In a study published in Gastro Hep Advances, the researchers identified 331 adults with newly diagnosed superficial esophageal SCC who underwent endoscopic resection, and 1,022 healthy controls. Field cancerization was based on the number of Lugol-voiding lesions (LVLs) per endoscopic view according to three groups: grade A, 0 LVLs; grade B, 1-9; or grade C, at least 10. The primary study outcome was a measure of risk factors for the development of LVLs.
“Multiple LVLs are closely associated with inactive aldehyde dehydrogenase 2 (ALDH2) and field cancerization,” the researchers wrote. Before assessing their human subjects, they used a mouse model to investigate whether alcohol intake and abstinence would affect acetaldehyde-induced DNA damage to the esophageal epithelium among individuals with ALDH2 dysfunction.
The researchers found that DNA damage, measured by acetaldehyde-derived DNA adduct levels (via N2-ethylidene-dG), accumulated with alcohol consumption over time, but decreased with alcohol cessation in the mouse model.
For the human part of the study, participants completed a lifestyle survey at entry, with questions about alcohol consumption history, alcohol flushing response, smoking, consumption of high-temperature foods, and consumption of green and yellow vegetables and fruit. Drinking status was divided into five groups: never/rarely (of less than 1 unit/week), light (1-8.9 units/week), moderate (9-17.9 units/week), heavy (18 or more units/week), and ex-drinker, with 1 unit defined as 22 g of ethanol. Smoking was divided into three groups: never (0 pack-years), light (less than 30 pack-years), and heavy (30 or more pack-years). Patients were given educational materials at study entry about the importance of alcohol and smoking cessation, as well as verbal advice to cease these behaviors.
Participants underwent endoscopic surveillance at 3-month intervals for up to 6 months following endoscopic resection.
Overall, increased alcohol consumption was associated with increased risk in development of LVL across all LVL grades; higher grades of LVLs were positively associated with high-intensity alcohol consumption, smoking, flushing, and high-temperature foods, and negatively associated with eating vegetables and fruit.
The risk of LVL grade progression was most strongly associated with increased alcohol consumption and with reported flushing. “The greatest risk was observed in the patients with flushing reactions who consumed an average of 30 units per week in grade C LVL,” with an odds ratio of 534, compared with healthy controls. “Since flushing reaction is caused by accumulation of acetaldehyde due to ALDH2 deficient, our result also means that acetaldehyde is a strong carcinogen in field cancerization.”
Secondary outcomes included the incidence of second primary esophageal SCC and head/neck SCC; these were significantly more prevalent in patients with grade C LVL (cumulative 5-year incidence of 47.1% for ESCC and 13.3% for head and neck SCC). However, alcohol and smoking cessation significantly reduced the development of second primary esophageal SCC (adjusted hazard ratios, 0.47 for alcohol and 0.49 for smoking).
The study findings were limited by several factors including the lack of randomization to noncessation and cessation groups and the inclusion of cancer patients, but not long-term cancer survivors, the researchers noted.
“We believe that our data will be useful to establish a prevention and surveillance strategy for cancer survivors, because the overall prognosis of esophageal cancer and head and neck cancer is still poor,” with a 5-year survival rate of less than 20%, and the results highlight the need to educate cancer survivors on the value of smoking and alcohol cessation, they added.
The study was supported by the National Cancer Center Research and Development Fund 36 by the Ministry of Health, Labour, and Welfare of Japan. The researchers had no financial conflicts to disclose.
In this large, prospective, multicenter Japanese study published in the December 2021 issue of Gastro Hep Advances, alcohol and/or smoking cessation for 5 or more years was found to reduce the risk of field cancerization in patients with superficial esophageal squamous cell carcinoma (ESCC). Multiple lesions that are identified by lack of staining of squamous epithelium of the esophagus with Lugol iodine (Lugol-voiding lesion) are known as field cancerization effect. The investigators found that, following endoscopic resection of first primary ESCC (n = 331), alcohol cessation (adjusted hazard-ratio, 0.47; 95% confidence interval, 0.26-0.85) and cigarette smoking cessation (AHR 0.49, 95% CI, 0.26-0.91) reduced the rate of development of second primary ESCC.
The take-home message from this study is that alcohol and tobacco cessation for 5 years can significantly reduce the risk of second primary ESCC. Practitioners should be vigilant in counseling patients, particularly those with Lugol-voiding lesions grades B or C or those who have a flushing reaction.
Anand Jain, MD, is with the division of digestive diseases at Emory University, Atlanta. Ravinder Mittal, MD, is with the division of digestive diseases at University of California, San Diego. They declared having no relevant conflicts of interest.
In this large, prospective, multicenter Japanese study published in the December 2021 issue of Gastro Hep Advances, alcohol and/or smoking cessation for 5 or more years was found to reduce the risk of field cancerization in patients with superficial esophageal squamous cell carcinoma (ESCC). Multiple lesions that are identified by lack of staining of squamous epithelium of the esophagus with Lugol iodine (Lugol-voiding lesion) are known as field cancerization effect. The investigators found that, following endoscopic resection of first primary ESCC (n = 331), alcohol cessation (adjusted hazard-ratio, 0.47; 95% confidence interval, 0.26-0.85) and cigarette smoking cessation (AHR 0.49, 95% CI, 0.26-0.91) reduced the rate of development of second primary ESCC.
The take-home message from this study is that alcohol and tobacco cessation for 5 years can significantly reduce the risk of second primary ESCC. Practitioners should be vigilant in counseling patients, particularly those with Lugol-voiding lesions grades B or C or those who have a flushing reaction.
Anand Jain, MD, is with the division of digestive diseases at Emory University, Atlanta. Ravinder Mittal, MD, is with the division of digestive diseases at University of California, San Diego. They declared having no relevant conflicts of interest.
In this large, prospective, multicenter Japanese study published in the December 2021 issue of Gastro Hep Advances, alcohol and/or smoking cessation for 5 or more years was found to reduce the risk of field cancerization in patients with superficial esophageal squamous cell carcinoma (ESCC). Multiple lesions that are identified by lack of staining of squamous epithelium of the esophagus with Lugol iodine (Lugol-voiding lesion) are known as field cancerization effect. The investigators found that, following endoscopic resection of first primary ESCC (n = 331), alcohol cessation (adjusted hazard-ratio, 0.47; 95% confidence interval, 0.26-0.85) and cigarette smoking cessation (AHR 0.49, 95% CI, 0.26-0.91) reduced the rate of development of second primary ESCC.
The take-home message from this study is that alcohol and tobacco cessation for 5 years can significantly reduce the risk of second primary ESCC. Practitioners should be vigilant in counseling patients, particularly those with Lugol-voiding lesions grades B or C or those who have a flushing reaction.
Anand Jain, MD, is with the division of digestive diseases at Emory University, Atlanta. Ravinder Mittal, MD, is with the division of digestive diseases at University of California, San Diego. They declared having no relevant conflicts of interest.
Field cancerization and subsequent second cancer in squamous cell carcinoma (SCC) patients was significantly associated with cigarette and alcohol use, based on data from more than 300 individuals.
Cigarette and alcohol use are established risk factors for SCCs of the esophagus, head, and neck, Manabu Moto, MD, of Kyoto University and colleagues wrote. “In addition, squamous cell carcinoma and squamous dysplastic epithelium develop multifocally in these organs,” in a phenomenon known as field cancerization, but the interaction of multiple dysplastic epithelium with other factors, notably whether cessation of cigarette and alcohol use would reduce risk of SCC, has not been well studied.
In a study published in Gastro Hep Advances, the researchers identified 331 adults with newly diagnosed superficial esophageal SCC who underwent endoscopic resection, and 1,022 healthy controls. Field cancerization was based on the number of Lugol-voiding lesions (LVLs) per endoscopic view according to three groups: grade A, 0 LVLs; grade B, 1-9; or grade C, at least 10. The primary study outcome was a measure of risk factors for the development of LVLs.
“Multiple LVLs are closely associated with inactive aldehyde dehydrogenase 2 (ALDH2) and field cancerization,” the researchers wrote. Before assessing their human subjects, they used a mouse model to investigate whether alcohol intake and abstinence would affect acetaldehyde-induced DNA damage to the esophageal epithelium among individuals with ALDH2 dysfunction.
The researchers found that DNA damage, measured by acetaldehyde-derived DNA adduct levels (via N2-ethylidene-dG), accumulated with alcohol consumption over time, but decreased with alcohol cessation in the mouse model.
For the human part of the study, participants completed a lifestyle survey at entry, with questions about alcohol consumption history, alcohol flushing response, smoking, consumption of high-temperature foods, and consumption of green and yellow vegetables and fruit. Drinking status was divided into five groups: never/rarely (of less than 1 unit/week), light (1-8.9 units/week), moderate (9-17.9 units/week), heavy (18 or more units/week), and ex-drinker, with 1 unit defined as 22 g of ethanol. Smoking was divided into three groups: never (0 pack-years), light (less than 30 pack-years), and heavy (30 or more pack-years). Patients were given educational materials at study entry about the importance of alcohol and smoking cessation, as well as verbal advice to cease these behaviors.
Participants underwent endoscopic surveillance at 3-month intervals for up to 6 months following endoscopic resection.
Overall, increased alcohol consumption was associated with increased risk in development of LVL across all LVL grades; higher grades of LVLs were positively associated with high-intensity alcohol consumption, smoking, flushing, and high-temperature foods, and negatively associated with eating vegetables and fruit.
The risk of LVL grade progression was most strongly associated with increased alcohol consumption and with reported flushing. “The greatest risk was observed in the patients with flushing reactions who consumed an average of 30 units per week in grade C LVL,” with an odds ratio of 534, compared with healthy controls. “Since flushing reaction is caused by accumulation of acetaldehyde due to ALDH2 deficient, our result also means that acetaldehyde is a strong carcinogen in field cancerization.”
Secondary outcomes included the incidence of second primary esophageal SCC and head/neck SCC; these were significantly more prevalent in patients with grade C LVL (cumulative 5-year incidence of 47.1% for ESCC and 13.3% for head and neck SCC). However, alcohol and smoking cessation significantly reduced the development of second primary esophageal SCC (adjusted hazard ratios, 0.47 for alcohol and 0.49 for smoking).
The study findings were limited by several factors including the lack of randomization to noncessation and cessation groups and the inclusion of cancer patients, but not long-term cancer survivors, the researchers noted.
“We believe that our data will be useful to establish a prevention and surveillance strategy for cancer survivors, because the overall prognosis of esophageal cancer and head and neck cancer is still poor,” with a 5-year survival rate of less than 20%, and the results highlight the need to educate cancer survivors on the value of smoking and alcohol cessation, they added.
The study was supported by the National Cancer Center Research and Development Fund 36 by the Ministry of Health, Labour, and Welfare of Japan. The researchers had no financial conflicts to disclose.
Field cancerization and subsequent second cancer in squamous cell carcinoma (SCC) patients was significantly associated with cigarette and alcohol use, based on data from more than 300 individuals.
Cigarette and alcohol use are established risk factors for SCCs of the esophagus, head, and neck, Manabu Moto, MD, of Kyoto University and colleagues wrote. “In addition, squamous cell carcinoma and squamous dysplastic epithelium develop multifocally in these organs,” in a phenomenon known as field cancerization, but the interaction of multiple dysplastic epithelium with other factors, notably whether cessation of cigarette and alcohol use would reduce risk of SCC, has not been well studied.
In a study published in Gastro Hep Advances, the researchers identified 331 adults with newly diagnosed superficial esophageal SCC who underwent endoscopic resection, and 1,022 healthy controls. Field cancerization was based on the number of Lugol-voiding lesions (LVLs) per endoscopic view according to three groups: grade A, 0 LVLs; grade B, 1-9; or grade C, at least 10. The primary study outcome was a measure of risk factors for the development of LVLs.
“Multiple LVLs are closely associated with inactive aldehyde dehydrogenase 2 (ALDH2) and field cancerization,” the researchers wrote. Before assessing their human subjects, they used a mouse model to investigate whether alcohol intake and abstinence would affect acetaldehyde-induced DNA damage to the esophageal epithelium among individuals with ALDH2 dysfunction.
The researchers found that DNA damage, measured by acetaldehyde-derived DNA adduct levels (via N2-ethylidene-dG), accumulated with alcohol consumption over time, but decreased with alcohol cessation in the mouse model.
For the human part of the study, participants completed a lifestyle survey at entry, with questions about alcohol consumption history, alcohol flushing response, smoking, consumption of high-temperature foods, and consumption of green and yellow vegetables and fruit. Drinking status was divided into five groups: never/rarely (of less than 1 unit/week), light (1-8.9 units/week), moderate (9-17.9 units/week), heavy (18 or more units/week), and ex-drinker, with 1 unit defined as 22 g of ethanol. Smoking was divided into three groups: never (0 pack-years), light (less than 30 pack-years), and heavy (30 or more pack-years). Patients were given educational materials at study entry about the importance of alcohol and smoking cessation, as well as verbal advice to cease these behaviors.
Participants underwent endoscopic surveillance at 3-month intervals for up to 6 months following endoscopic resection.
Overall, increased alcohol consumption was associated with increased risk in development of LVL across all LVL grades; higher grades of LVLs were positively associated with high-intensity alcohol consumption, smoking, flushing, and high-temperature foods, and negatively associated with eating vegetables and fruit.
The risk of LVL grade progression was most strongly associated with increased alcohol consumption and with reported flushing. “The greatest risk was observed in the patients with flushing reactions who consumed an average of 30 units per week in grade C LVL,” with an odds ratio of 534, compared with healthy controls. “Since flushing reaction is caused by accumulation of acetaldehyde due to ALDH2 deficient, our result also means that acetaldehyde is a strong carcinogen in field cancerization.”
Secondary outcomes included the incidence of second primary esophageal SCC and head/neck SCC; these were significantly more prevalent in patients with grade C LVL (cumulative 5-year incidence of 47.1% for ESCC and 13.3% for head and neck SCC). However, alcohol and smoking cessation significantly reduced the development of second primary esophageal SCC (adjusted hazard ratios, 0.47 for alcohol and 0.49 for smoking).
The study findings were limited by several factors including the lack of randomization to noncessation and cessation groups and the inclusion of cancer patients, but not long-term cancer survivors, the researchers noted.
“We believe that our data will be useful to establish a prevention and surveillance strategy for cancer survivors, because the overall prognosis of esophageal cancer and head and neck cancer is still poor,” with a 5-year survival rate of less than 20%, and the results highlight the need to educate cancer survivors on the value of smoking and alcohol cessation, they added.
The study was supported by the National Cancer Center Research and Development Fund 36 by the Ministry of Health, Labour, and Welfare of Japan. The researchers had no financial conflicts to disclose.
FROM GASTRO HEP ADVANCES
Sarcoidosis

THE COMPARISON
A Pink, elevated, granulomatous, indurated plaques on the face, including the nasal alae, of a 52-year-old woman with a darker skin tone.
B Orange and pink, elevated, granulomatous, indurated plaques on the face of a 55-year-old woman with a lighter skin tone.
Sarcoidosis is a granulomatous disease that may affect the skin in addition to multiple body organ systems, including the lungs. Bilateral hilar adenopathy on a chest radiograph is the most common finding. Sarcoidosis also has a variety of cutaneous manifestations. Early diagnosis is vital, as patients with with sarcoidosis and pulmonary fibrosis have a shortened life span compared to the overall population.1 With a growing skin of color population, it is important to recognize sarcoidosis as soon as possible.2
Epidemiology
People of African descent have the highest sarcoidosis prevalence in the United States.3 In the United States, the incidence of sarcoidosis in Black individuals peaks in the fourth decade of life. A 5-year study in a US health maintenance organization found that the age-adjusted annual incidence was 10.9 per 100,000 cases among Whites and 35.5 per 100,000 cases among Blacks.4
Key clinical features in people with darker skin tones:
• Papules are seen in sarcoidosis, primarily on the face, and may start as orange hued or yellow-brown and then become brown-red or pink to violaceous before involuting into faint macules.5-7
• When round or oval sarcoid plaques appear, they often are more erythematous. In skin of color, plaques may become hypopigmented.8
• Erythema nodosum, the most common nonspecific cutaneous lesion seen in sarcoidosis, is less commonly seen in those of African and Asian descent.9-11 This is in contrast to distinctive forms of specific sarcoid skin lesions such as lupus pernio and scar sarcoidosis, as well as papules and plaques and minor forms of specific sarcoid skin lesions including subcutaneous nodules; hypopigmented macules; psoriasiform lesions; and ulcerative, localized erythrodermic, ichthyosiform, scalp, and nail lesions.
• Lupus pernio is a cutaneous manifestation of sarcoidosis that appears on the face. It looks similar to lupus erythematosus and occurs most commonly in women of African descent.8,12
• Hypopigmented lesions are more common in those with darker skin tones.9
• Ulcerative lesions are more common in those of African descent and women.13
• Scalp sarcoidosis is more common in patients of African descent.14
• Sarcoidosis may develop at sites of trauma, such as scars and tattoos.15-17
Worth noting
The cutaneous lesions seen in sarcoidosis may be emotionally devastating and disfiguring. Due to the variety of clinical manifestations, sarcoidosis may be misdiagnosed, leading to delays in treatment.18
Health disparity highlight
Patients older than 40 years presenting with sarcoidosis and those of African descent have a worse prognosis.19 Despite adjusting for race, ethnic group, age, and sex, patients with low income and financial barriers present with more severe sarcoidosis.20
- Nardi A, Brillet P-Y, Letoumelin P, et al. Stage IV sarcoidosis: comparison of survival with the general population and causes of death. Eur Respir J. 2011;38:1368-1373.
- Heath CR, David J, Taylor SC. Sarcoidosis: are there differences in your skin of color patients? J Am Acad Dermatol. 2012;66:121.e1-121.e14.
- Sève P, Pacheco Y, Durupt F, et al. Sarcoidosis: a clinical overview from symptoms to diagnosis. Cells. 2021;10:766. doi:10.3390/cells10040766
- Rybicki BA, Major M, Popovich J Jr, et al. Racial differences in sarcoidosis incidence: a 5-year study in a health maintenance organization. Am J Epidemiol. 1997;145:234-241. doi:10.1093/oxfordjournals.aje.a009096
- Mahajan VK, Sharma NL, Sharma RC, et al. Cutaneous sarcoidosis: clinical profile of 23 Indian patients. Indian J Dermatol Venerol Leprol. 2007;73:16-21.
- Yanardag H, Pamuk ON, Karayel T. Cutaneous involvement in sarcoidosis: analysis if the features in 170 patients. Respir Med. 2003;97:978-982.
- Olive KE, Kartaria YP. Cutaneous manifestations of sarcoidosis to other organ system involvement, abnormal laboratory measurements, and disease course. Arch Intern Med. 1985;145:1811-1814.
- Mañá J, Marcoval J, Graells J, et al. Cutaneous involvement in sarcoidosis. relationship to systemic disease. Arch Dermatol. 1997;133:882-888. doi:10.1001/archderm.1997.03890430098013
- Minus HR, Grimes PE. Cutaneous manifestations of sarcoidosis in blacks. Cutis. 1983;32:361-364.
- Edmondstone WM, Wilson AG. Sarcoidosis in Caucasians, blacks and Asians in London. Br J Dis Chest. 1985;79:27-36.
- James DG, Neville E, Siltzbach LE. Worldwide review of sarcoidosis. Ann N Y Acad Sci. 1976;278:321-334.
- Hunninghake GW, Costabel U, Ando M, et al. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc Diffuse Lung Dis. 1999;16:149-173.
- Albertini JG, Tyler W, Miller OF III. Ulcerative sarcoidosis: case report and review of literature. Arch Dermatol. 1997;133:215-219.
- Marchell RM, Judson MA. Chronic cutaneous lesions of sarcoidosis. Clin Dermatol. 2007;25:295-302.
- Nayar M. Sarcoidosis on ritual scarification. Int J Dermatol. 1993;32:116-118.
- Chudomirova K, Velichkva L, Anavi B. Recurrent sarcoidosis in skin scars accompanying systemic sarcoidosis. J Eur Acad Dermatol Venerol. 2003;17:360-361.
- Kim YC, Triffet MK, Gibson LE. Foreign bodies in sarcoidosis. Am J Dermatopathol. 2000;22:408-412.
- Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007; 357:2153-2165.
- Nunes H, Bouvry D, Soler P, et al. Sarcoidosis. Orphanet J Rare Dis. 2007;2:46. doi:10.1186/1750-1172-2-46
- Baughman RP, Teirstein AS, Judson MA, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001;164:1885-1889.

THE COMPARISON
A Pink, elevated, granulomatous, indurated plaques on the face, including the nasal alae, of a 52-year-old woman with a darker skin tone.
B Orange and pink, elevated, granulomatous, indurated plaques on the face of a 55-year-old woman with a lighter skin tone.
Sarcoidosis is a granulomatous disease that may affect the skin in addition to multiple body organ systems, including the lungs. Bilateral hilar adenopathy on a chest radiograph is the most common finding. Sarcoidosis also has a variety of cutaneous manifestations. Early diagnosis is vital, as patients with with sarcoidosis and pulmonary fibrosis have a shortened life span compared to the overall population.1 With a growing skin of color population, it is important to recognize sarcoidosis as soon as possible.2
Epidemiology
People of African descent have the highest sarcoidosis prevalence in the United States.3 In the United States, the incidence of sarcoidosis in Black individuals peaks in the fourth decade of life. A 5-year study in a US health maintenance organization found that the age-adjusted annual incidence was 10.9 per 100,000 cases among Whites and 35.5 per 100,000 cases among Blacks.4
Key clinical features in people with darker skin tones:
• Papules are seen in sarcoidosis, primarily on the face, and may start as orange hued or yellow-brown and then become brown-red or pink to violaceous before involuting into faint macules.5-7
• When round or oval sarcoid plaques appear, they often are more erythematous. In skin of color, plaques may become hypopigmented.8
• Erythema nodosum, the most common nonspecific cutaneous lesion seen in sarcoidosis, is less commonly seen in those of African and Asian descent.9-11 This is in contrast to distinctive forms of specific sarcoid skin lesions such as lupus pernio and scar sarcoidosis, as well as papules and plaques and minor forms of specific sarcoid skin lesions including subcutaneous nodules; hypopigmented macules; psoriasiform lesions; and ulcerative, localized erythrodermic, ichthyosiform, scalp, and nail lesions.
• Lupus pernio is a cutaneous manifestation of sarcoidosis that appears on the face. It looks similar to lupus erythematosus and occurs most commonly in women of African descent.8,12
• Hypopigmented lesions are more common in those with darker skin tones.9
• Ulcerative lesions are more common in those of African descent and women.13
• Scalp sarcoidosis is more common in patients of African descent.14
• Sarcoidosis may develop at sites of trauma, such as scars and tattoos.15-17
Worth noting
The cutaneous lesions seen in sarcoidosis may be emotionally devastating and disfiguring. Due to the variety of clinical manifestations, sarcoidosis may be misdiagnosed, leading to delays in treatment.18
Health disparity highlight
Patients older than 40 years presenting with sarcoidosis and those of African descent have a worse prognosis.19 Despite adjusting for race, ethnic group, age, and sex, patients with low income and financial barriers present with more severe sarcoidosis.20

THE COMPARISON
A Pink, elevated, granulomatous, indurated plaques on the face, including the nasal alae, of a 52-year-old woman with a darker skin tone.
B Orange and pink, elevated, granulomatous, indurated plaques on the face of a 55-year-old woman with a lighter skin tone.
Sarcoidosis is a granulomatous disease that may affect the skin in addition to multiple body organ systems, including the lungs. Bilateral hilar adenopathy on a chest radiograph is the most common finding. Sarcoidosis also has a variety of cutaneous manifestations. Early diagnosis is vital, as patients with with sarcoidosis and pulmonary fibrosis have a shortened life span compared to the overall population.1 With a growing skin of color population, it is important to recognize sarcoidosis as soon as possible.2
Epidemiology
People of African descent have the highest sarcoidosis prevalence in the United States.3 In the United States, the incidence of sarcoidosis in Black individuals peaks in the fourth decade of life. A 5-year study in a US health maintenance organization found that the age-adjusted annual incidence was 10.9 per 100,000 cases among Whites and 35.5 per 100,000 cases among Blacks.4
Key clinical features in people with darker skin tones:
• Papules are seen in sarcoidosis, primarily on the face, and may start as orange hued or yellow-brown and then become brown-red or pink to violaceous before involuting into faint macules.5-7
• When round or oval sarcoid plaques appear, they often are more erythematous. In skin of color, plaques may become hypopigmented.8
• Erythema nodosum, the most common nonspecific cutaneous lesion seen in sarcoidosis, is less commonly seen in those of African and Asian descent.9-11 This is in contrast to distinctive forms of specific sarcoid skin lesions such as lupus pernio and scar sarcoidosis, as well as papules and plaques and minor forms of specific sarcoid skin lesions including subcutaneous nodules; hypopigmented macules; psoriasiform lesions; and ulcerative, localized erythrodermic, ichthyosiform, scalp, and nail lesions.
• Lupus pernio is a cutaneous manifestation of sarcoidosis that appears on the face. It looks similar to lupus erythematosus and occurs most commonly in women of African descent.8,12
• Hypopigmented lesions are more common in those with darker skin tones.9
• Ulcerative lesions are more common in those of African descent and women.13
• Scalp sarcoidosis is more common in patients of African descent.14
• Sarcoidosis may develop at sites of trauma, such as scars and tattoos.15-17
Worth noting
The cutaneous lesions seen in sarcoidosis may be emotionally devastating and disfiguring. Due to the variety of clinical manifestations, sarcoidosis may be misdiagnosed, leading to delays in treatment.18
Health disparity highlight
Patients older than 40 years presenting with sarcoidosis and those of African descent have a worse prognosis.19 Despite adjusting for race, ethnic group, age, and sex, patients with low income and financial barriers present with more severe sarcoidosis.20
- Nardi A, Brillet P-Y, Letoumelin P, et al. Stage IV sarcoidosis: comparison of survival with the general population and causes of death. Eur Respir J. 2011;38:1368-1373.
- Heath CR, David J, Taylor SC. Sarcoidosis: are there differences in your skin of color patients? J Am Acad Dermatol. 2012;66:121.e1-121.e14.
- Sève P, Pacheco Y, Durupt F, et al. Sarcoidosis: a clinical overview from symptoms to diagnosis. Cells. 2021;10:766. doi:10.3390/cells10040766
- Rybicki BA, Major M, Popovich J Jr, et al. Racial differences in sarcoidosis incidence: a 5-year study in a health maintenance organization. Am J Epidemiol. 1997;145:234-241. doi:10.1093/oxfordjournals.aje.a009096
- Mahajan VK, Sharma NL, Sharma RC, et al. Cutaneous sarcoidosis: clinical profile of 23 Indian patients. Indian J Dermatol Venerol Leprol. 2007;73:16-21.
- Yanardag H, Pamuk ON, Karayel T. Cutaneous involvement in sarcoidosis: analysis if the features in 170 patients. Respir Med. 2003;97:978-982.
- Olive KE, Kartaria YP. Cutaneous manifestations of sarcoidosis to other organ system involvement, abnormal laboratory measurements, and disease course. Arch Intern Med. 1985;145:1811-1814.
- Mañá J, Marcoval J, Graells J, et al. Cutaneous involvement in sarcoidosis. relationship to systemic disease. Arch Dermatol. 1997;133:882-888. doi:10.1001/archderm.1997.03890430098013
- Minus HR, Grimes PE. Cutaneous manifestations of sarcoidosis in blacks. Cutis. 1983;32:361-364.
- Edmondstone WM, Wilson AG. Sarcoidosis in Caucasians, blacks and Asians in London. Br J Dis Chest. 1985;79:27-36.
- James DG, Neville E, Siltzbach LE. Worldwide review of sarcoidosis. Ann N Y Acad Sci. 1976;278:321-334.
- Hunninghake GW, Costabel U, Ando M, et al. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc Diffuse Lung Dis. 1999;16:149-173.
- Albertini JG, Tyler W, Miller OF III. Ulcerative sarcoidosis: case report and review of literature. Arch Dermatol. 1997;133:215-219.
- Marchell RM, Judson MA. Chronic cutaneous lesions of sarcoidosis. Clin Dermatol. 2007;25:295-302.
- Nayar M. Sarcoidosis on ritual scarification. Int J Dermatol. 1993;32:116-118.
- Chudomirova K, Velichkva L, Anavi B. Recurrent sarcoidosis in skin scars accompanying systemic sarcoidosis. J Eur Acad Dermatol Venerol. 2003;17:360-361.
- Kim YC, Triffet MK, Gibson LE. Foreign bodies in sarcoidosis. Am J Dermatopathol. 2000;22:408-412.
- Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007; 357:2153-2165.
- Nunes H, Bouvry D, Soler P, et al. Sarcoidosis. Orphanet J Rare Dis. 2007;2:46. doi:10.1186/1750-1172-2-46
- Baughman RP, Teirstein AS, Judson MA, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001;164:1885-1889.
- Nardi A, Brillet P-Y, Letoumelin P, et al. Stage IV sarcoidosis: comparison of survival with the general population and causes of death. Eur Respir J. 2011;38:1368-1373.
- Heath CR, David J, Taylor SC. Sarcoidosis: are there differences in your skin of color patients? J Am Acad Dermatol. 2012;66:121.e1-121.e14.
- Sève P, Pacheco Y, Durupt F, et al. Sarcoidosis: a clinical overview from symptoms to diagnosis. Cells. 2021;10:766. doi:10.3390/cells10040766
- Rybicki BA, Major M, Popovich J Jr, et al. Racial differences in sarcoidosis incidence: a 5-year study in a health maintenance organization. Am J Epidemiol. 1997;145:234-241. doi:10.1093/oxfordjournals.aje.a009096
- Mahajan VK, Sharma NL, Sharma RC, et al. Cutaneous sarcoidosis: clinical profile of 23 Indian patients. Indian J Dermatol Venerol Leprol. 2007;73:16-21.
- Yanardag H, Pamuk ON, Karayel T. Cutaneous involvement in sarcoidosis: analysis if the features in 170 patients. Respir Med. 2003;97:978-982.
- Olive KE, Kartaria YP. Cutaneous manifestations of sarcoidosis to other organ system involvement, abnormal laboratory measurements, and disease course. Arch Intern Med. 1985;145:1811-1814.
- Mañá J, Marcoval J, Graells J, et al. Cutaneous involvement in sarcoidosis. relationship to systemic disease. Arch Dermatol. 1997;133:882-888. doi:10.1001/archderm.1997.03890430098013
- Minus HR, Grimes PE. Cutaneous manifestations of sarcoidosis in blacks. Cutis. 1983;32:361-364.
- Edmondstone WM, Wilson AG. Sarcoidosis in Caucasians, blacks and Asians in London. Br J Dis Chest. 1985;79:27-36.
- James DG, Neville E, Siltzbach LE. Worldwide review of sarcoidosis. Ann N Y Acad Sci. 1976;278:321-334.
- Hunninghake GW, Costabel U, Ando M, et al. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc Diffuse Lung Dis. 1999;16:149-173.
- Albertini JG, Tyler W, Miller OF III. Ulcerative sarcoidosis: case report and review of literature. Arch Dermatol. 1997;133:215-219.
- Marchell RM, Judson MA. Chronic cutaneous lesions of sarcoidosis. Clin Dermatol. 2007;25:295-302.
- Nayar M. Sarcoidosis on ritual scarification. Int J Dermatol. 1993;32:116-118.
- Chudomirova K, Velichkva L, Anavi B. Recurrent sarcoidosis in skin scars accompanying systemic sarcoidosis. J Eur Acad Dermatol Venerol. 2003;17:360-361.
- Kim YC, Triffet MK, Gibson LE. Foreign bodies in sarcoidosis. Am J Dermatopathol. 2000;22:408-412.
- Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007; 357:2153-2165.
- Nunes H, Bouvry D, Soler P, et al. Sarcoidosis. Orphanet J Rare Dis. 2007;2:46. doi:10.1186/1750-1172-2-46
- Baughman RP, Teirstein AS, Judson MA, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001;164:1885-1889.
Contact Allergy to Topical Medicaments, Part 2: Steroids, Immunomodulators, and Anesthetics, Oh My!
In the first part of this 2-part series (Cutis. 2021;108:271-275), we discussed topical medicament allergic contact dermatitis (ACD) from acne and rosacea medications, antimicrobials, antihistamines, and topical pain preparations. In part 2 of this series, we focus on topical corticosteroids, immunomodulators, and anesthetics.
Corticosteroids
Given their anti-inflammatory and immune-modulating effects, topical corticosteroids are utilized for the treatment of contact dermatitis and yet also are frequent culprits of ACD. The North American Contact Dermatitis Group (NACDG) demonstrated a 4% frequency of positive patch tests to at least one corticosteroid from 2007 to 2014; the relevant allergens were tixocortol pivalate (TP)(2.3%), budesonide (0.9%), hydrocortisone-17-butyrate (0.4%), clobetasol-17-propionate (0.3%), and desoximetasone (0.2%).1 Corticosteroid contact allergy can be difficult to recognize and may present as a flare of the underlying condition being treated. Clinically, these rashes may demonstrate an edge effect, characterized by pronounced dermatitis adjacent to and surrounding the treatment area due to concentrated anti-inflammatory effects in the center.
Traditionally, corticosteroids are divided into 4 basic structural groups—classes A, B, C, and D—based on the Coopman et al2 classification (Table). The class D corticosteroids were further subdivided into classes D1, defined by C16-methyl substitution and halogenation of the B ring, and D2, which lacks the aforementioned substitutions.4 However, more recently Baeck et al5 simplified this classification into 3 main groups of steroids based on molecular modeling in combination with patch test results. Group 1 combines the nonmethylated and (mostly) nonhalogenated class A and D2 molecules plus budesonide; group 2 accounts for some halogenated class B molecules with the C16, C17 cis ketal or diol structure; and group 3 includes halogenated and C16-methylated molecules from classes C and D1.4 For the purposes of this review, discussion of classes A through D refers to the Coopman et al2 classification, and groups 1 through 3 refers to Baeck et al.5
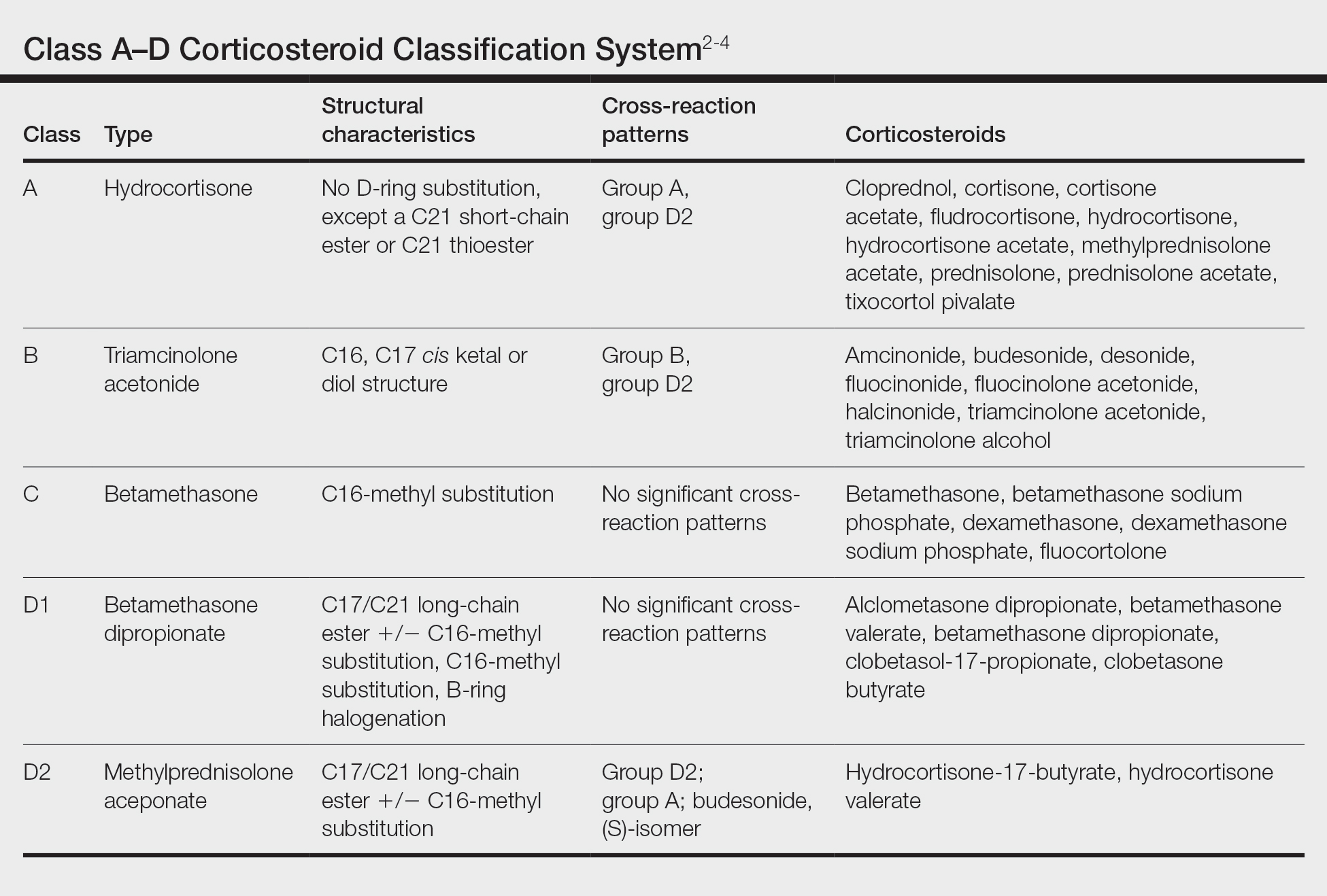
Tixocortol pivalate is used as a surrogate marker for hydrocortisone allergy and other class A corticosteroids and is part of the group 1 steroid classification. Interestingly, patients with TP-positive patch tests may not exhibit signs or symptoms of ACD from the use of hydrocortisone products. Repeat open application testing (ROAT) or provocative use testing may elicit a positive response in these patients, especially with the use of hydrocortisone cream (vs ointment), likely due to greater transepidermal penetration.6 There is little consensus on the optimal concentration of TP for patch testing. Although TP 1% often is recommended, studies have shown mixed findings of notable differences between high (1% petrolatum) and low (0.1% petrolatum) concentrations of TP.7,8
Budesonide also is part of group 1 and is a marker for contact allergy to class B corticosteroids, such as triamcinolone and fluocinonide. Cross-reactions between budesonide and other corticosteroids traditionally classified as group B may be explained by structural similarities, whereas cross-reactions with certain class D corticosteroids, such as hydrocortisone-17-butyrate, may be better explained by the diastereomer composition of budesonide.9,10 In a European study, budesonide 0.01% and TP 0.1% included in the European Baseline Series detected 85% (23/27) of cases of corticosteroid allergies.11 Use of inhaled budesonide can provoke recall dermatitis and therefore should be avoided in allergic patients.12
Testing for ACD to topical steroids is complex, as the potent anti-inflammatory properties of these medications can complicate results. Selecting the appropriate test, vehicle, and concentration can help avoid false negatives. Although intradermal testing previously was thought to be superior to patch testing in detecting topical corticosteroid contact allergy, newer data have demonstrated strong concordance between the two methods.13,14 The risk for skin atrophy, particularly with the use of suspensions, limits the use of intradermal testing.14 An ethanol vehicle is recommended for patch testing, except when testing with TP or budesonide when petrolatum provides greater corticosteroid stability.14-16 An irritant pattern or a rim effect on patch testing often is considered positive when testing corticosteroids, as the effect of the steroid itself can diminish a positive reaction. As a result, 0.1% dilutions sometimes are favored over 1% test concentrations.14,15,17 Late readings (>7 days) may be necessary to detect positive reactions in both adults and children.18,19
The authors (M.R., A.R.A.) find these varied classifications of steroids daunting (and somewhat confusing!). In general, when ACD to topical steroids is suspected, in addition to standard patch testing with a corticosteroid series, ROAT of the suspected steroid may be necessary, as the rules of steroid classification may not be reproducible in the real world. For patients with only corticosteroid allergy, calcineurin inhibitors are a safe alternative.
Immunomodulators
Calcipotriol is a vitamin D analogue commonly used to treat psoriasis. Although it is a well-known irritant, ACD to topical calcipotriol rarely has been reported.20-23 Topical calcipotriol does not seem to cross-react with other vitamin D analogues, including tacalcitol and calcitriol.21,24 Based on the literature and the nonirritant reactive thresholds described by Fullerton et al,25 recommended patch test concentrations of calcipotriol in isopropanol are 2 to 10 µg/mL. Given its immunomodulating effects, calcipotriol may suppress contact hypersensitization from other allergens, similar to the effects seen with UV radiation.26
Calcineurin inhibitors act on the nuclear factor of activated T cells signaling pathway, resulting in downstream suppression of proinflammatory cytokines. Contact allergy to these topical medications is rare and mainly has involved pimecrolimus.27-30 In one case, a patient with a previously documented topical tacrolimus contact allergy demonstrated cross-reactivity with pimecrolimus on a double-blinded, right-vs-left ROAT, as well as by patch testing with pimecrolimus cream 1%, which was only weakly positive (+).27 Patch test concentrations of 2.5% or higher may be required to elicit positive reactions to tacrolimus, as shown in one case where this was attributed to high molecular weight and poor extrafacial skin absorption of tacrolimus.30 In an unusual case, a patient reacted positively to patch testing and ROAT using pimecrolimus cream 1% but not pimecrolimus 1% to 5% in petrolatum or alcohol nor the individual excipients, illustrating the importance of testing with both active and inactive ingredients.29
Anesthetics
Local anesthetics can be separated into 2 main groups—amides and esters—based on their chemical structures. From 2001 to 2004, the NACDG patch tested 10,061 patients and found 344 (3.4%) with a positive reaction to at least one topical anesthetic.31 We will discuss some of the allergic cutaneous reactions associated with topical benzocaine (an ester) and lidocaine and prilocaine (amides).
According to the NACDG, the estimated prevalence of topical benzocaine allergy from 2001 to 2018 was roughly 3%.32 Allergic contact dermatitis has been reported in patients who used topical benzocaine to treat localized pain disorders, including herpes zoster and dental pain.33,34 Benzocaine may be used in the anogenital region in the form of antihemorrhoidal creams and in condoms and is a considerably more common allergen in those with anogenital dermatitis compared to those without.35-38 Although cross-reactions within the same anesthetic group are common, clinicians also should be aware of the potential for concomitant sensitivity between unrelated local anesthetics.39-41
From 2001 to 2018, the prevalence of ACD to topical lidocaine was estimated to be 7.9%, according to the NACDG.32 A topical anesthetic containing both lidocaine and prilocaine often is used preprocedurally and can be a source of ACD. Interestingly, several cases of ACD to combination lidocaine/prilocaine cream demonstrated positive patch tests to prilocaine but not lidocaine, despite their structural similarities.42-44 One case report described simultaneous positive reactions to both prilocaine 5% and lidocaine 1%.45
There are a few key points to consider when working up contact allergy to local anesthetics. Patients who develop positive patch test reactions to a local anesthetic should undergo further testing to better understand alternatives and future use. As previously mentioned, ACD to one anesthetic does not necessarily preclude the use of other related anesthetics. Intradermal testing may help differentiate immediate and delayed-type allergic reactions to local anesthetics and should therefore follow positive patch tests.46 Importantly, a delayed reading (ie, after day 6 or 7) also should be performed as part of intradermal testing. Patients with positive patch tests but negative intradermal test results may be able to tolerate systemic anesthetic use.47
Patch Testing for Potential Medicament ACD
In this article, we touched on several topical medications that have nuanced patch testing specifications given their immunomodulating effects. A simplified outline of recommended patch test concentrations is provided in the eTable, and we encourage you to revisit these useful resources as needed. In many cases, referral to a specialized patch test clinic may be necessary. Although they are not reviewed in this article, always consider inactive ingredients such as preservatives, softening agents, and emulsifiers in the setting of medicament dermatitis, as they also may be culprits of ACD.
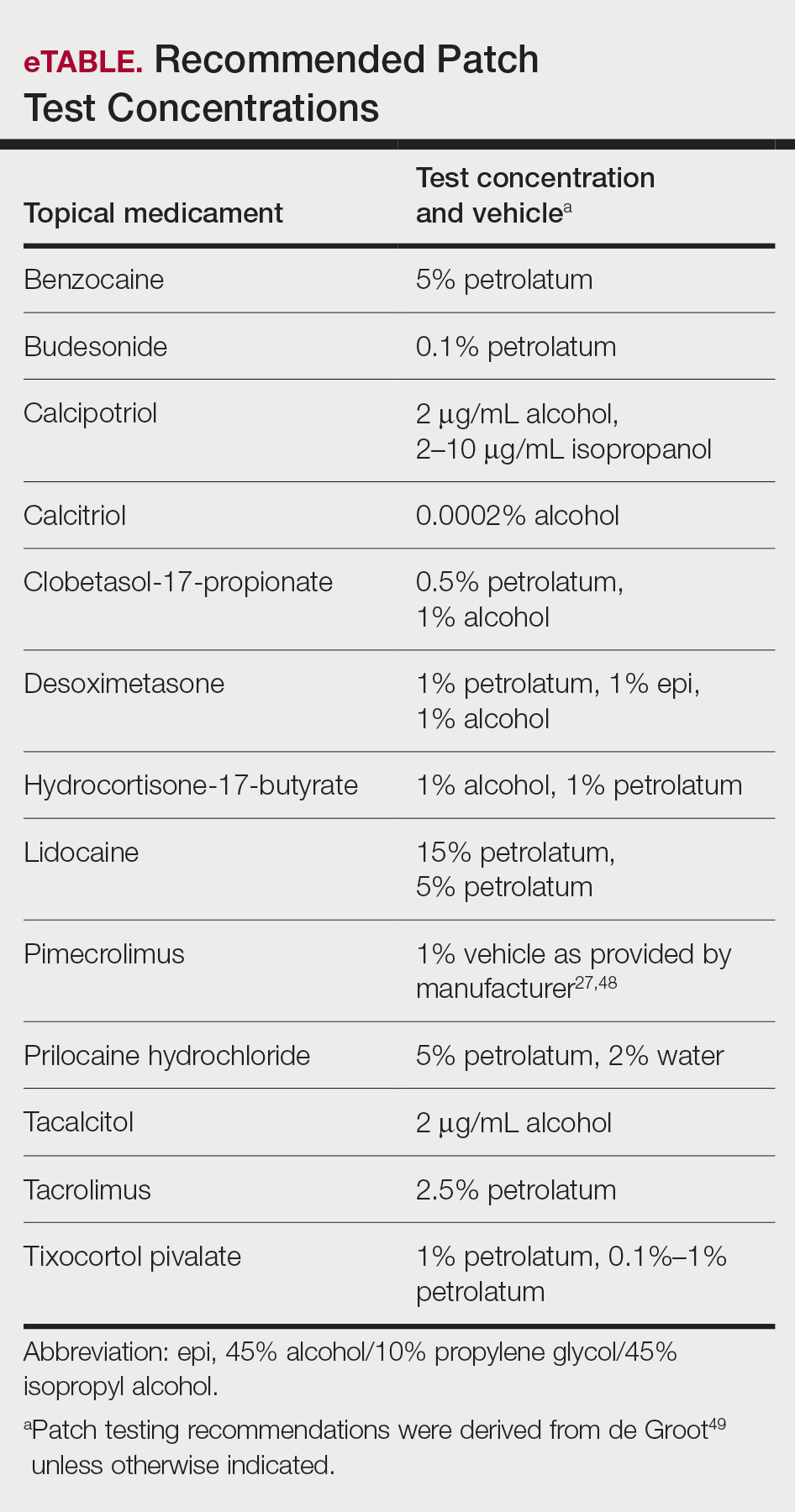
Final Interpretation
In this 2-part series, we covered ACD to several common topical drugs with a focus on active ingredients as the source of allergy, and yet this is just the tip of the iceberg. Topical medicaments are prevalent in the field of dermatology, and associated cases of ACD have been reported proportionately. Consider ACD when topical medication efficacy plateaus, triggers new-onset dermatitis, or seems to exacerbate an underlying dermatitis.
- Pratt MD, Mufti A, Lipson J, et al. Patch test reactions to corticosteroids: retrospective analysis from the North American Contact Dermatitis Group 2007-2014. Dermatitis. 2017;28:58-63. doi:10.1097/DER.0000000000000251
- Coopman S, Degreef H, Dooms-Goossens A. Identification of cross-reaction patterns in allergic contact dermatitis from topical corticosteroids. Br J Dermatol. 1989;121:27-34. doi:10.1111/j.1365-2133.1989.tb01396.x
- Jacob SE, Steele T. Corticosteroid classes: a quick reference guide including patch test substances and cross-reactivity. J Am Acad Dermatol. 2006;54:723-727. doi:10.1016/j.jaad.2005.12.028
- Matura M, Goossens A. Contact allergy to corticosteroids. Allergy. 2000;55:698-704. doi:10.1034/j.1398-9995.2000.00121.x
- Baeck M, Chemelle JA, Goossens A, et al. Corticosteroid cross-reactivity: clinical and molecular modelling tools. Allergy. 2011;66:1367-1374. doi:10.1111/j.1398-9995.2011.02666.x
- Shaw DW, Maibach HI. Clinical relevance of tixocortol pivalate-positive patch tests and questionable bioequivalence of different hydrocortisone preparations. Contact Dermatitis. 2013;68:369-375. doi:10.1111/cod.12066
- Kalavala M, Statham BN, Green CM, et al. Tixocortol pivalate: what is the right concentration? Contact Dermatitis. 2007;57:44-46. doi:10.1111/j.1600-0536.2007.01136.x
- Chowdhury MM, Statham BN, Sansom JE, et al. Patch testing for corticosteroid allergy with low and high concentrations of tixocortol pivalate and budesonide. Contact Dermatitis. 2002;46:311-312. doi:10.1034/j.1600-0536.2002.460519.x
- Isaksson M, Bruze M, Lepoittevin JP, et al. Patch testing with serial dilutions of budesonide, its R and S diastereomers, and potentially cross-reacting substances. Am J Contact Dermat. 2001;12:170-176.
- Ferguson AD, Emerson RM, English JS. Cross-reactivity patterns to budesonide. Contact Dermatitis. 2002;47:337-340. doi:10.1034/j.1600-0536.2002.470604.x
- Kot M, Bogaczewicz J, Kre˛cisz B, et al. Contact allergy in the population of patients with chronic inflammatory dermatoses and contact hypersensitivity to corticosteroids. Postepy Dermatol Alergol. 2017;34:253-259. doi:10.5114/ada.2017.67848
- Isaksson M, Bruze M. Allergic contact dermatitis in response to budesonide reactivated by inhalation of the allergen. J Am Acad Dermatol. 2002;46:880-885. doi:10.1067/mjd.2002.120464
- Mimesh S, Pratt M. Allergic contact dermatitis from corticosteroids: reproducibility of patch testing and correlation with intradermal testing. Dermatitis. 2006;17:137-142. doi:10.2310/6620.2006.05048
- Soria A, Baeck M, Goossens A, et al. Patch, prick or intradermal tests to detect delayed hypersensitivity to corticosteroids?. Contact Dermatitis. 2011;64:313-324. doi:10.1111/j.1600-0536.2011.01888.x
- Wilkinson SM, Beck MH. Corticosteroid contact hypersensitivity: what vehicle and concentration? Contact Dermatitis. 1996;34:305-308. doi:10.1111/j.1600-0536.1996.tb02212.x
- Isaksson M, Beck MH, Wilkinson SM. Comparative testing with budesonide in petrolatum and ethanol in a standard series. Contact Dermatitis. 2002;47:123-124. doi:10.1034/j.1600-0536.2002.470210_16.x
- Baeck M, Goossens A. Immediate and delayed allergic hypersensitivity to corticosteroids: practical guidelines. Contact Dermatitis. 2012;66:38-45. doi:10.1111/j.1600-0536.2011.01967.x
- Isaksson M. Corticosteroid contact allergy—the importance of late readings and testing with corticosteroids used by the patients. Contact Dermatitis. 2007;56:56-57. doi:10.1111/j.1600-0536.2007.00959.x
- Tam I, Yu J. Delayed patch test reaction to budesonide in an 8-year-old. Pediatr Dermatol. 2020;37:690-691. doi:10.1111/pde.14168
- Garcia-Bravo B, Camacho F. Two cases of contact dermatitis caused by calcipotriol cream. Am J Contact Dermat. 1996;7:118-119.
- Zollner TM, Ochsendorf FR, Hensel O, et al. Delayed-type reactivity to calcipotriol without cross-sensitization to tacalcitol. Contact Dermatitis. 1997;37:251. doi:10.1111/j.1600-0536.1997.tb02457.x
- Frosch PJ, Rustemeyer T. Contact allergy to calcipotriol does exist. report of an unequivocal case and review of the literature. Contact Dermatitis. 1999;40:66-71. doi:10.1111/j.1600-0536.1999.tb05993.x
- Gilissen L, Huygens S, Goossens A. Allergic contact dermatitis caused by calcipotriol. Contact Dermatitis. 2018;78:139-142. doi:10.1111/cod.12910
- Foti C, Carnimeo L, Bonamonte D, et al. Tolerance to calcitriol and tacalcitol in three patients with allergic contact dermatitis to calcipotriol. J Drugs Dermatol. 2005;4:756-759.
- Fullerton A, Benfeldt E, Petersen JR, et al. The calcipotriol dose-irritation relationship: 48-hour occlusive testing in healthy volunteers using Finn Chambers. Br J Dermatol. 1998;138:259-265. doi:10.1046/j.1365-2133.1998.02071.x
- Hanneman KK, Scull HM, Cooper KD, et al. Effect of topical vitamin D analogue on in vivo contact sensitization. Arch Dermatol. 2006;142:1332-1334. doi:10.1001/archderm.142.10.1332
- Shaw DW, Maibach HI, Eichenfield LF. Allergic contact dermatitis from pimecrolimus in a patient with tacrolimus allergy. J Am Acad Dermatol. 2007;56:342-345. doi:10.1016/j.jaad.2006.09.033
- Saitta P, Brancaccio R. Allergic contact dermatitis to pimecrolimus. Contact Dermatitis. 2007;56:43-44. doi:10.1111/j.1600-0536.2007.00822.x
- Neczyporenko F, Blondeel A. Allergic contact dermatitis to Elidel cream itself? Contact Dermatitis. 2010;63:171-172. doi:10.1111/j.1600-0536.2010.01764.x
- Shaw DW, Eichenfield LF, Shainhouse T, et al. Allergic contact dermatitis from tacrolimus. J Am Acad Dermatol. 2004;50:962-965. doi:10.1016/j.jaad.2003.09.013
- Warshaw EM, Schram SE, Belsito DV, et al. Patch-test reactions to topical anesthetics: retrospective analysis of cross-sectional data, 2001 to 2004. Dermatitis. 2008;19:81-85.
- Warshaw EM, Shaver RL, DeKoven JG, et al. Patch test reactions associated with topical medications: a retrospective analysis of the North American Contact Dermatitis Group data (2001-2018)[published online September 1, 2021]. Dermatitis. doi:10.1097/DER.0000000000000777
- Roos TC, Merk HF. Allergic contact dermatitis from benzocaine ointment during treatment of herpes zoster. Contact Dermatitis. 2001;44:104. doi:10.1034/j.1600-0536.2001.4402097.x
- González-Rodríguez AJ, Gutiérrez-Paredes EM, Revert Fernández Á, et al. Allergic contact dermatitis to benzocaine: the importance of concomitant positive patch test results. Actas Dermosifiliogr. 2013;104:156-158. doi:10.1016/j.ad.2011.07.023
- Muratore L, Calogiuri G, Foti C, et al. Contact allergy to benzocaine in a condom. Contact Dermatitis. 2008;59:173-174. doi:10.1111/j.1600-0536.2008.01359.x
- Sharma A, Agarwal S, Garg G, et al. Desire for lasting long in bed led to contact allergic dermatitis and subsequent superficial penile gangrene: a dreadful complication of benzocaine-containing extended-pleasure condom [published online September 27, 2018]. BMJ Case Rep. 2018;2018:bcr2018227351. doi:10.1136/bcr-2018-227351
- Bauer A, Geier J, Elsner P. Allergic contact dermatitis in patients with anogenital complaints. J Reprod Med. 2000;45:649-654.
- Warshaw EM, Kimyon RS, Silverberg JI, et al. Evaluation of patch test findings in patients with anogenital dermatitis. JAMA Dermatol. 2020;156:85-91. doi:10.1001/jamadermatol.2019.3844
- Weightman W, Turner T. Allergic contact dermatitis from lignocaine: report of 29 cases and review of the literature. Contact Dermatitis. 1998;39:265-266. doi:10.1111/j.1600-0536.1998.tb05928.x
- Jovanovic´ M, Karadaglic´ D, Brkic´ S. Contact urticaria and allergic contact dermatitis to lidocaine in a patient sensitive to benzocaine and propolis. Contact Dermatitis. 2006;54:124-126. doi:10.1111/j.0105-1873.2006.0560f.x
- Carazo JL, Morera BS, Colom LP, et al. Allergic contact dermatitis from ethyl chloride and benzocaine. Dermatitis. 2009;20:E13-E15.
- le Coz CJ, Cribier BJ, Heid E. Patch testing in suspected allergic contact dermatitis due to EMLA cream in haemodialyzed patients. Contact Dermatitis. 1996;35:316-317. doi:10.1111/j.1600-0536.1996.tb02407.x
- Ismail F, Goldsmith PC. EMLA cream-induced allergic contact dermatitis in a child with thalassaemia major. Contact Dermatitis. 2005;52:111. doi:10.1111/j.0105-1873.2005.00498e.x
- Pérez-Pérez LC, Fernández-Redondo V, Ginarte-Val M, et al. Allergic contact dermatitis from EMLA cream in a hemodialyzed patient. Dermatitis. 2006;17:85-87.
- Timmermans MW, Bruynzeel DP, Rustemeyer T. Allergic contact dermatitis from EMLA cream: concomitant sensitization to both local anesthetics lidocaine and prilocaine. J Dtsch Dermatol Ges. 2009;7:237-238. doi:10.1111/j.1610-0387.2008.06932.x
- Fuzier R, Lapeyre-Mestre M, Mertes PM, et al. Immediate- and delayed-type allergic reactions to amide local anesthetics: clinical features and skin testing. Pharmacoepidemiol Drug Saf. 2009;18:595-601. doi:10.1002/pds.1758
- Ruzicka T, Gerstmeier M, Przybilla B, et al. Allergy to local anesthetics: comparison of patch test with prick and intradermal test results. J Am Acad Dermatol. 1987;16:1202-1208. doi:10.1016/s0190-9622(87)70158-3
- Fowler JF Jr, Fowler L, Douglas JL, et al. Skin reactions to pimecrolimus cream 1% in patients allergic to propylene glycol: a double-blind randomized study. Dermatitis. 2007;18:134-139. doi:10.2310/6620.2007.06028
- de Groot A. Patch Testing. 3rd ed. acdegroot publishing; 2008.
In the first part of this 2-part series (Cutis. 2021;108:271-275), we discussed topical medicament allergic contact dermatitis (ACD) from acne and rosacea medications, antimicrobials, antihistamines, and topical pain preparations. In part 2 of this series, we focus on topical corticosteroids, immunomodulators, and anesthetics.
Corticosteroids
Given their anti-inflammatory and immune-modulating effects, topical corticosteroids are utilized for the treatment of contact dermatitis and yet also are frequent culprits of ACD. The North American Contact Dermatitis Group (NACDG) demonstrated a 4% frequency of positive patch tests to at least one corticosteroid from 2007 to 2014; the relevant allergens were tixocortol pivalate (TP)(2.3%), budesonide (0.9%), hydrocortisone-17-butyrate (0.4%), clobetasol-17-propionate (0.3%), and desoximetasone (0.2%).1 Corticosteroid contact allergy can be difficult to recognize and may present as a flare of the underlying condition being treated. Clinically, these rashes may demonstrate an edge effect, characterized by pronounced dermatitis adjacent to and surrounding the treatment area due to concentrated anti-inflammatory effects in the center.
Traditionally, corticosteroids are divided into 4 basic structural groups—classes A, B, C, and D—based on the Coopman et al2 classification (Table). The class D corticosteroids were further subdivided into classes D1, defined by C16-methyl substitution and halogenation of the B ring, and D2, which lacks the aforementioned substitutions.4 However, more recently Baeck et al5 simplified this classification into 3 main groups of steroids based on molecular modeling in combination with patch test results. Group 1 combines the nonmethylated and (mostly) nonhalogenated class A and D2 molecules plus budesonide; group 2 accounts for some halogenated class B molecules with the C16, C17 cis ketal or diol structure; and group 3 includes halogenated and C16-methylated molecules from classes C and D1.4 For the purposes of this review, discussion of classes A through D refers to the Coopman et al2 classification, and groups 1 through 3 refers to Baeck et al.5

Tixocortol pivalate is used as a surrogate marker for hydrocortisone allergy and other class A corticosteroids and is part of the group 1 steroid classification. Interestingly, patients with TP-positive patch tests may not exhibit signs or symptoms of ACD from the use of hydrocortisone products. Repeat open application testing (ROAT) or provocative use testing may elicit a positive response in these patients, especially with the use of hydrocortisone cream (vs ointment), likely due to greater transepidermal penetration.6 There is little consensus on the optimal concentration of TP for patch testing. Although TP 1% often is recommended, studies have shown mixed findings of notable differences between high (1% petrolatum) and low (0.1% petrolatum) concentrations of TP.7,8
Budesonide also is part of group 1 and is a marker for contact allergy to class B corticosteroids, such as triamcinolone and fluocinonide. Cross-reactions between budesonide and other corticosteroids traditionally classified as group B may be explained by structural similarities, whereas cross-reactions with certain class D corticosteroids, such as hydrocortisone-17-butyrate, may be better explained by the diastereomer composition of budesonide.9,10 In a European study, budesonide 0.01% and TP 0.1% included in the European Baseline Series detected 85% (23/27) of cases of corticosteroid allergies.11 Use of inhaled budesonide can provoke recall dermatitis and therefore should be avoided in allergic patients.12
Testing for ACD to topical steroids is complex, as the potent anti-inflammatory properties of these medications can complicate results. Selecting the appropriate test, vehicle, and concentration can help avoid false negatives. Although intradermal testing previously was thought to be superior to patch testing in detecting topical corticosteroid contact allergy, newer data have demonstrated strong concordance between the two methods.13,14 The risk for skin atrophy, particularly with the use of suspensions, limits the use of intradermal testing.14 An ethanol vehicle is recommended for patch testing, except when testing with TP or budesonide when petrolatum provides greater corticosteroid stability.14-16 An irritant pattern or a rim effect on patch testing often is considered positive when testing corticosteroids, as the effect of the steroid itself can diminish a positive reaction. As a result, 0.1% dilutions sometimes are favored over 1% test concentrations.14,15,17 Late readings (>7 days) may be necessary to detect positive reactions in both adults and children.18,19
The authors (M.R., A.R.A.) find these varied classifications of steroids daunting (and somewhat confusing!). In general, when ACD to topical steroids is suspected, in addition to standard patch testing with a corticosteroid series, ROAT of the suspected steroid may be necessary, as the rules of steroid classification may not be reproducible in the real world. For patients with only corticosteroid allergy, calcineurin inhibitors are a safe alternative.
Immunomodulators
Calcipotriol is a vitamin D analogue commonly used to treat psoriasis. Although it is a well-known irritant, ACD to topical calcipotriol rarely has been reported.20-23 Topical calcipotriol does not seem to cross-react with other vitamin D analogues, including tacalcitol and calcitriol.21,24 Based on the literature and the nonirritant reactive thresholds described by Fullerton et al,25 recommended patch test concentrations of calcipotriol in isopropanol are 2 to 10 µg/mL. Given its immunomodulating effects, calcipotriol may suppress contact hypersensitization from other allergens, similar to the effects seen with UV radiation.26
Calcineurin inhibitors act on the nuclear factor of activated T cells signaling pathway, resulting in downstream suppression of proinflammatory cytokines. Contact allergy to these topical medications is rare and mainly has involved pimecrolimus.27-30 In one case, a patient with a previously documented topical tacrolimus contact allergy demonstrated cross-reactivity with pimecrolimus on a double-blinded, right-vs-left ROAT, as well as by patch testing with pimecrolimus cream 1%, which was only weakly positive (+).27 Patch test concentrations of 2.5% or higher may be required to elicit positive reactions to tacrolimus, as shown in one case where this was attributed to high molecular weight and poor extrafacial skin absorption of tacrolimus.30 In an unusual case, a patient reacted positively to patch testing and ROAT using pimecrolimus cream 1% but not pimecrolimus 1% to 5% in petrolatum or alcohol nor the individual excipients, illustrating the importance of testing with both active and inactive ingredients.29
Anesthetics
Local anesthetics can be separated into 2 main groups—amides and esters—based on their chemical structures. From 2001 to 2004, the NACDG patch tested 10,061 patients and found 344 (3.4%) with a positive reaction to at least one topical anesthetic.31 We will discuss some of the allergic cutaneous reactions associated with topical benzocaine (an ester) and lidocaine and prilocaine (amides).
According to the NACDG, the estimated prevalence of topical benzocaine allergy from 2001 to 2018 was roughly 3%.32 Allergic contact dermatitis has been reported in patients who used topical benzocaine to treat localized pain disorders, including herpes zoster and dental pain.33,34 Benzocaine may be used in the anogenital region in the form of antihemorrhoidal creams and in condoms and is a considerably more common allergen in those with anogenital dermatitis compared to those without.35-38 Although cross-reactions within the same anesthetic group are common, clinicians also should be aware of the potential for concomitant sensitivity between unrelated local anesthetics.39-41
From 2001 to 2018, the prevalence of ACD to topical lidocaine was estimated to be 7.9%, according to the NACDG.32 A topical anesthetic containing both lidocaine and prilocaine often is used preprocedurally and can be a source of ACD. Interestingly, several cases of ACD to combination lidocaine/prilocaine cream demonstrated positive patch tests to prilocaine but not lidocaine, despite their structural similarities.42-44 One case report described simultaneous positive reactions to both prilocaine 5% and lidocaine 1%.45
There are a few key points to consider when working up contact allergy to local anesthetics. Patients who develop positive patch test reactions to a local anesthetic should undergo further testing to better understand alternatives and future use. As previously mentioned, ACD to one anesthetic does not necessarily preclude the use of other related anesthetics. Intradermal testing may help differentiate immediate and delayed-type allergic reactions to local anesthetics and should therefore follow positive patch tests.46 Importantly, a delayed reading (ie, after day 6 or 7) also should be performed as part of intradermal testing. Patients with positive patch tests but negative intradermal test results may be able to tolerate systemic anesthetic use.47
Patch Testing for Potential Medicament ACD
In this article, we touched on several topical medications that have nuanced patch testing specifications given their immunomodulating effects. A simplified outline of recommended patch test concentrations is provided in the eTable, and we encourage you to revisit these useful resources as needed. In many cases, referral to a specialized patch test clinic may be necessary. Although they are not reviewed in this article, always consider inactive ingredients such as preservatives, softening agents, and emulsifiers in the setting of medicament dermatitis, as they also may be culprits of ACD.

Final Interpretation
In this 2-part series, we covered ACD to several common topical drugs with a focus on active ingredients as the source of allergy, and yet this is just the tip of the iceberg. Topical medicaments are prevalent in the field of dermatology, and associated cases of ACD have been reported proportionately. Consider ACD when topical medication efficacy plateaus, triggers new-onset dermatitis, or seems to exacerbate an underlying dermatitis.
In the first part of this 2-part series (Cutis. 2021;108:271-275), we discussed topical medicament allergic contact dermatitis (ACD) from acne and rosacea medications, antimicrobials, antihistamines, and topical pain preparations. In part 2 of this series, we focus on topical corticosteroids, immunomodulators, and anesthetics.
Corticosteroids
Given their anti-inflammatory and immune-modulating effects, topical corticosteroids are utilized for the treatment of contact dermatitis and yet also are frequent culprits of ACD. The North American Contact Dermatitis Group (NACDG) demonstrated a 4% frequency of positive patch tests to at least one corticosteroid from 2007 to 2014; the relevant allergens were tixocortol pivalate (TP)(2.3%), budesonide (0.9%), hydrocortisone-17-butyrate (0.4%), clobetasol-17-propionate (0.3%), and desoximetasone (0.2%).1 Corticosteroid contact allergy can be difficult to recognize and may present as a flare of the underlying condition being treated. Clinically, these rashes may demonstrate an edge effect, characterized by pronounced dermatitis adjacent to and surrounding the treatment area due to concentrated anti-inflammatory effects in the center.
Traditionally, corticosteroids are divided into 4 basic structural groups—classes A, B, C, and D—based on the Coopman et al2 classification (Table). The class D corticosteroids were further subdivided into classes D1, defined by C16-methyl substitution and halogenation of the B ring, and D2, which lacks the aforementioned substitutions.4 However, more recently Baeck et al5 simplified this classification into 3 main groups of steroids based on molecular modeling in combination with patch test results. Group 1 combines the nonmethylated and (mostly) nonhalogenated class A and D2 molecules plus budesonide; group 2 accounts for some halogenated class B molecules with the C16, C17 cis ketal or diol structure; and group 3 includes halogenated and C16-methylated molecules from classes C and D1.4 For the purposes of this review, discussion of classes A through D refers to the Coopman et al2 classification, and groups 1 through 3 refers to Baeck et al.5

Tixocortol pivalate is used as a surrogate marker for hydrocortisone allergy and other class A corticosteroids and is part of the group 1 steroid classification. Interestingly, patients with TP-positive patch tests may not exhibit signs or symptoms of ACD from the use of hydrocortisone products. Repeat open application testing (ROAT) or provocative use testing may elicit a positive response in these patients, especially with the use of hydrocortisone cream (vs ointment), likely due to greater transepidermal penetration.6 There is little consensus on the optimal concentration of TP for patch testing. Although TP 1% often is recommended, studies have shown mixed findings of notable differences between high (1% petrolatum) and low (0.1% petrolatum) concentrations of TP.7,8
Budesonide also is part of group 1 and is a marker for contact allergy to class B corticosteroids, such as triamcinolone and fluocinonide. Cross-reactions between budesonide and other corticosteroids traditionally classified as group B may be explained by structural similarities, whereas cross-reactions with certain class D corticosteroids, such as hydrocortisone-17-butyrate, may be better explained by the diastereomer composition of budesonide.9,10 In a European study, budesonide 0.01% and TP 0.1% included in the European Baseline Series detected 85% (23/27) of cases of corticosteroid allergies.11 Use of inhaled budesonide can provoke recall dermatitis and therefore should be avoided in allergic patients.12
Testing for ACD to topical steroids is complex, as the potent anti-inflammatory properties of these medications can complicate results. Selecting the appropriate test, vehicle, and concentration can help avoid false negatives. Although intradermal testing previously was thought to be superior to patch testing in detecting topical corticosteroid contact allergy, newer data have demonstrated strong concordance between the two methods.13,14 The risk for skin atrophy, particularly with the use of suspensions, limits the use of intradermal testing.14 An ethanol vehicle is recommended for patch testing, except when testing with TP or budesonide when petrolatum provides greater corticosteroid stability.14-16 An irritant pattern or a rim effect on patch testing often is considered positive when testing corticosteroids, as the effect of the steroid itself can diminish a positive reaction. As a result, 0.1% dilutions sometimes are favored over 1% test concentrations.14,15,17 Late readings (>7 days) may be necessary to detect positive reactions in both adults and children.18,19
The authors (M.R., A.R.A.) find these varied classifications of steroids daunting (and somewhat confusing!). In general, when ACD to topical steroids is suspected, in addition to standard patch testing with a corticosteroid series, ROAT of the suspected steroid may be necessary, as the rules of steroid classification may not be reproducible in the real world. For patients with only corticosteroid allergy, calcineurin inhibitors are a safe alternative.
Immunomodulators
Calcipotriol is a vitamin D analogue commonly used to treat psoriasis. Although it is a well-known irritant, ACD to topical calcipotriol rarely has been reported.20-23 Topical calcipotriol does not seem to cross-react with other vitamin D analogues, including tacalcitol and calcitriol.21,24 Based on the literature and the nonirritant reactive thresholds described by Fullerton et al,25 recommended patch test concentrations of calcipotriol in isopropanol are 2 to 10 µg/mL. Given its immunomodulating effects, calcipotriol may suppress contact hypersensitization from other allergens, similar to the effects seen with UV radiation.26
Calcineurin inhibitors act on the nuclear factor of activated T cells signaling pathway, resulting in downstream suppression of proinflammatory cytokines. Contact allergy to these topical medications is rare and mainly has involved pimecrolimus.27-30 In one case, a patient with a previously documented topical tacrolimus contact allergy demonstrated cross-reactivity with pimecrolimus on a double-blinded, right-vs-left ROAT, as well as by patch testing with pimecrolimus cream 1%, which was only weakly positive (+).27 Patch test concentrations of 2.5% or higher may be required to elicit positive reactions to tacrolimus, as shown in one case where this was attributed to high molecular weight and poor extrafacial skin absorption of tacrolimus.30 In an unusual case, a patient reacted positively to patch testing and ROAT using pimecrolimus cream 1% but not pimecrolimus 1% to 5% in petrolatum or alcohol nor the individual excipients, illustrating the importance of testing with both active and inactive ingredients.29
Anesthetics
Local anesthetics can be separated into 2 main groups—amides and esters—based on their chemical structures. From 2001 to 2004, the NACDG patch tested 10,061 patients and found 344 (3.4%) with a positive reaction to at least one topical anesthetic.31 We will discuss some of the allergic cutaneous reactions associated with topical benzocaine (an ester) and lidocaine and prilocaine (amides).
According to the NACDG, the estimated prevalence of topical benzocaine allergy from 2001 to 2018 was roughly 3%.32 Allergic contact dermatitis has been reported in patients who used topical benzocaine to treat localized pain disorders, including herpes zoster and dental pain.33,34 Benzocaine may be used in the anogenital region in the form of antihemorrhoidal creams and in condoms and is a considerably more common allergen in those with anogenital dermatitis compared to those without.35-38 Although cross-reactions within the same anesthetic group are common, clinicians also should be aware of the potential for concomitant sensitivity between unrelated local anesthetics.39-41
From 2001 to 2018, the prevalence of ACD to topical lidocaine was estimated to be 7.9%, according to the NACDG.32 A topical anesthetic containing both lidocaine and prilocaine often is used preprocedurally and can be a source of ACD. Interestingly, several cases of ACD to combination lidocaine/prilocaine cream demonstrated positive patch tests to prilocaine but not lidocaine, despite their structural similarities.42-44 One case report described simultaneous positive reactions to both prilocaine 5% and lidocaine 1%.45
There are a few key points to consider when working up contact allergy to local anesthetics. Patients who develop positive patch test reactions to a local anesthetic should undergo further testing to better understand alternatives and future use. As previously mentioned, ACD to one anesthetic does not necessarily preclude the use of other related anesthetics. Intradermal testing may help differentiate immediate and delayed-type allergic reactions to local anesthetics and should therefore follow positive patch tests.46 Importantly, a delayed reading (ie, after day 6 or 7) also should be performed as part of intradermal testing. Patients with positive patch tests but negative intradermal test results may be able to tolerate systemic anesthetic use.47
Patch Testing for Potential Medicament ACD
In this article, we touched on several topical medications that have nuanced patch testing specifications given their immunomodulating effects. A simplified outline of recommended patch test concentrations is provided in the eTable, and we encourage you to revisit these useful resources as needed. In many cases, referral to a specialized patch test clinic may be necessary. Although they are not reviewed in this article, always consider inactive ingredients such as preservatives, softening agents, and emulsifiers in the setting of medicament dermatitis, as they also may be culprits of ACD.

Final Interpretation
In this 2-part series, we covered ACD to several common topical drugs with a focus on active ingredients as the source of allergy, and yet this is just the tip of the iceberg. Topical medicaments are prevalent in the field of dermatology, and associated cases of ACD have been reported proportionately. Consider ACD when topical medication efficacy plateaus, triggers new-onset dermatitis, or seems to exacerbate an underlying dermatitis.
- Pratt MD, Mufti A, Lipson J, et al. Patch test reactions to corticosteroids: retrospective analysis from the North American Contact Dermatitis Group 2007-2014. Dermatitis. 2017;28:58-63. doi:10.1097/DER.0000000000000251
- Coopman S, Degreef H, Dooms-Goossens A. Identification of cross-reaction patterns in allergic contact dermatitis from topical corticosteroids. Br J Dermatol. 1989;121:27-34. doi:10.1111/j.1365-2133.1989.tb01396.x
- Jacob SE, Steele T. Corticosteroid classes: a quick reference guide including patch test substances and cross-reactivity. J Am Acad Dermatol. 2006;54:723-727. doi:10.1016/j.jaad.2005.12.028
- Matura M, Goossens A. Contact allergy to corticosteroids. Allergy. 2000;55:698-704. doi:10.1034/j.1398-9995.2000.00121.x
- Baeck M, Chemelle JA, Goossens A, et al. Corticosteroid cross-reactivity: clinical and molecular modelling tools. Allergy. 2011;66:1367-1374. doi:10.1111/j.1398-9995.2011.02666.x
- Shaw DW, Maibach HI. Clinical relevance of tixocortol pivalate-positive patch tests and questionable bioequivalence of different hydrocortisone preparations. Contact Dermatitis. 2013;68:369-375. doi:10.1111/cod.12066
- Kalavala M, Statham BN, Green CM, et al. Tixocortol pivalate: what is the right concentration? Contact Dermatitis. 2007;57:44-46. doi:10.1111/j.1600-0536.2007.01136.x
- Chowdhury MM, Statham BN, Sansom JE, et al. Patch testing for corticosteroid allergy with low and high concentrations of tixocortol pivalate and budesonide. Contact Dermatitis. 2002;46:311-312. doi:10.1034/j.1600-0536.2002.460519.x
- Isaksson M, Bruze M, Lepoittevin JP, et al. Patch testing with serial dilutions of budesonide, its R and S diastereomers, and potentially cross-reacting substances. Am J Contact Dermat. 2001;12:170-176.
- Ferguson AD, Emerson RM, English JS. Cross-reactivity patterns to budesonide. Contact Dermatitis. 2002;47:337-340. doi:10.1034/j.1600-0536.2002.470604.x
- Kot M, Bogaczewicz J, Kre˛cisz B, et al. Contact allergy in the population of patients with chronic inflammatory dermatoses and contact hypersensitivity to corticosteroids. Postepy Dermatol Alergol. 2017;34:253-259. doi:10.5114/ada.2017.67848
- Isaksson M, Bruze M. Allergic contact dermatitis in response to budesonide reactivated by inhalation of the allergen. J Am Acad Dermatol. 2002;46:880-885. doi:10.1067/mjd.2002.120464
- Mimesh S, Pratt M. Allergic contact dermatitis from corticosteroids: reproducibility of patch testing and correlation with intradermal testing. Dermatitis. 2006;17:137-142. doi:10.2310/6620.2006.05048
- Soria A, Baeck M, Goossens A, et al. Patch, prick or intradermal tests to detect delayed hypersensitivity to corticosteroids?. Contact Dermatitis. 2011;64:313-324. doi:10.1111/j.1600-0536.2011.01888.x
- Wilkinson SM, Beck MH. Corticosteroid contact hypersensitivity: what vehicle and concentration? Contact Dermatitis. 1996;34:305-308. doi:10.1111/j.1600-0536.1996.tb02212.x
- Isaksson M, Beck MH, Wilkinson SM. Comparative testing with budesonide in petrolatum and ethanol in a standard series. Contact Dermatitis. 2002;47:123-124. doi:10.1034/j.1600-0536.2002.470210_16.x
- Baeck M, Goossens A. Immediate and delayed allergic hypersensitivity to corticosteroids: practical guidelines. Contact Dermatitis. 2012;66:38-45. doi:10.1111/j.1600-0536.2011.01967.x
- Isaksson M. Corticosteroid contact allergy—the importance of late readings and testing with corticosteroids used by the patients. Contact Dermatitis. 2007;56:56-57. doi:10.1111/j.1600-0536.2007.00959.x
- Tam I, Yu J. Delayed patch test reaction to budesonide in an 8-year-old. Pediatr Dermatol. 2020;37:690-691. doi:10.1111/pde.14168
- Garcia-Bravo B, Camacho F. Two cases of contact dermatitis caused by calcipotriol cream. Am J Contact Dermat. 1996;7:118-119.
- Zollner TM, Ochsendorf FR, Hensel O, et al. Delayed-type reactivity to calcipotriol without cross-sensitization to tacalcitol. Contact Dermatitis. 1997;37:251. doi:10.1111/j.1600-0536.1997.tb02457.x
- Frosch PJ, Rustemeyer T. Contact allergy to calcipotriol does exist. report of an unequivocal case and review of the literature. Contact Dermatitis. 1999;40:66-71. doi:10.1111/j.1600-0536.1999.tb05993.x
- Gilissen L, Huygens S, Goossens A. Allergic contact dermatitis caused by calcipotriol. Contact Dermatitis. 2018;78:139-142. doi:10.1111/cod.12910
- Foti C, Carnimeo L, Bonamonte D, et al. Tolerance to calcitriol and tacalcitol in three patients with allergic contact dermatitis to calcipotriol. J Drugs Dermatol. 2005;4:756-759.
- Fullerton A, Benfeldt E, Petersen JR, et al. The calcipotriol dose-irritation relationship: 48-hour occlusive testing in healthy volunteers using Finn Chambers. Br J Dermatol. 1998;138:259-265. doi:10.1046/j.1365-2133.1998.02071.x
- Hanneman KK, Scull HM, Cooper KD, et al. Effect of topical vitamin D analogue on in vivo contact sensitization. Arch Dermatol. 2006;142:1332-1334. doi:10.1001/archderm.142.10.1332
- Shaw DW, Maibach HI, Eichenfield LF. Allergic contact dermatitis from pimecrolimus in a patient with tacrolimus allergy. J Am Acad Dermatol. 2007;56:342-345. doi:10.1016/j.jaad.2006.09.033
- Saitta P, Brancaccio R. Allergic contact dermatitis to pimecrolimus. Contact Dermatitis. 2007;56:43-44. doi:10.1111/j.1600-0536.2007.00822.x
- Neczyporenko F, Blondeel A. Allergic contact dermatitis to Elidel cream itself? Contact Dermatitis. 2010;63:171-172. doi:10.1111/j.1600-0536.2010.01764.x
- Shaw DW, Eichenfield LF, Shainhouse T, et al. Allergic contact dermatitis from tacrolimus. J Am Acad Dermatol. 2004;50:962-965. doi:10.1016/j.jaad.2003.09.013
- Warshaw EM, Schram SE, Belsito DV, et al. Patch-test reactions to topical anesthetics: retrospective analysis of cross-sectional data, 2001 to 2004. Dermatitis. 2008;19:81-85.
- Warshaw EM, Shaver RL, DeKoven JG, et al. Patch test reactions associated with topical medications: a retrospective analysis of the North American Contact Dermatitis Group data (2001-2018)[published online September 1, 2021]. Dermatitis. doi:10.1097/DER.0000000000000777
- Roos TC, Merk HF. Allergic contact dermatitis from benzocaine ointment during treatment of herpes zoster. Contact Dermatitis. 2001;44:104. doi:10.1034/j.1600-0536.2001.4402097.x
- González-Rodríguez AJ, Gutiérrez-Paredes EM, Revert Fernández Á, et al. Allergic contact dermatitis to benzocaine: the importance of concomitant positive patch test results. Actas Dermosifiliogr. 2013;104:156-158. doi:10.1016/j.ad.2011.07.023
- Muratore L, Calogiuri G, Foti C, et al. Contact allergy to benzocaine in a condom. Contact Dermatitis. 2008;59:173-174. doi:10.1111/j.1600-0536.2008.01359.x
- Sharma A, Agarwal S, Garg G, et al. Desire for lasting long in bed led to contact allergic dermatitis and subsequent superficial penile gangrene: a dreadful complication of benzocaine-containing extended-pleasure condom [published online September 27, 2018]. BMJ Case Rep. 2018;2018:bcr2018227351. doi:10.1136/bcr-2018-227351
- Bauer A, Geier J, Elsner P. Allergic contact dermatitis in patients with anogenital complaints. J Reprod Med. 2000;45:649-654.
- Warshaw EM, Kimyon RS, Silverberg JI, et al. Evaluation of patch test findings in patients with anogenital dermatitis. JAMA Dermatol. 2020;156:85-91. doi:10.1001/jamadermatol.2019.3844
- Weightman W, Turner T. Allergic contact dermatitis from lignocaine: report of 29 cases and review of the literature. Contact Dermatitis. 1998;39:265-266. doi:10.1111/j.1600-0536.1998.tb05928.x
- Jovanovic´ M, Karadaglic´ D, Brkic´ S. Contact urticaria and allergic contact dermatitis to lidocaine in a patient sensitive to benzocaine and propolis. Contact Dermatitis. 2006;54:124-126. doi:10.1111/j.0105-1873.2006.0560f.x
- Carazo JL, Morera BS, Colom LP, et al. Allergic contact dermatitis from ethyl chloride and benzocaine. Dermatitis. 2009;20:E13-E15.
- le Coz CJ, Cribier BJ, Heid E. Patch testing in suspected allergic contact dermatitis due to EMLA cream in haemodialyzed patients. Contact Dermatitis. 1996;35:316-317. doi:10.1111/j.1600-0536.1996.tb02407.x
- Ismail F, Goldsmith PC. EMLA cream-induced allergic contact dermatitis in a child with thalassaemia major. Contact Dermatitis. 2005;52:111. doi:10.1111/j.0105-1873.2005.00498e.x
- Pérez-Pérez LC, Fernández-Redondo V, Ginarte-Val M, et al. Allergic contact dermatitis from EMLA cream in a hemodialyzed patient. Dermatitis. 2006;17:85-87.
- Timmermans MW, Bruynzeel DP, Rustemeyer T. Allergic contact dermatitis from EMLA cream: concomitant sensitization to both local anesthetics lidocaine and prilocaine. J Dtsch Dermatol Ges. 2009;7:237-238. doi:10.1111/j.1610-0387.2008.06932.x
- Fuzier R, Lapeyre-Mestre M, Mertes PM, et al. Immediate- and delayed-type allergic reactions to amide local anesthetics: clinical features and skin testing. Pharmacoepidemiol Drug Saf. 2009;18:595-601. doi:10.1002/pds.1758
- Ruzicka T, Gerstmeier M, Przybilla B, et al. Allergy to local anesthetics: comparison of patch test with prick and intradermal test results. J Am Acad Dermatol. 1987;16:1202-1208. doi:10.1016/s0190-9622(87)70158-3
- Fowler JF Jr, Fowler L, Douglas JL, et al. Skin reactions to pimecrolimus cream 1% in patients allergic to propylene glycol: a double-blind randomized study. Dermatitis. 2007;18:134-139. doi:10.2310/6620.2007.06028
- de Groot A. Patch Testing. 3rd ed. acdegroot publishing; 2008.
- Pratt MD, Mufti A, Lipson J, et al. Patch test reactions to corticosteroids: retrospective analysis from the North American Contact Dermatitis Group 2007-2014. Dermatitis. 2017;28:58-63. doi:10.1097/DER.0000000000000251
- Coopman S, Degreef H, Dooms-Goossens A. Identification of cross-reaction patterns in allergic contact dermatitis from topical corticosteroids. Br J Dermatol. 1989;121:27-34. doi:10.1111/j.1365-2133.1989.tb01396.x
- Jacob SE, Steele T. Corticosteroid classes: a quick reference guide including patch test substances and cross-reactivity. J Am Acad Dermatol. 2006;54:723-727. doi:10.1016/j.jaad.2005.12.028
- Matura M, Goossens A. Contact allergy to corticosteroids. Allergy. 2000;55:698-704. doi:10.1034/j.1398-9995.2000.00121.x
- Baeck M, Chemelle JA, Goossens A, et al. Corticosteroid cross-reactivity: clinical and molecular modelling tools. Allergy. 2011;66:1367-1374. doi:10.1111/j.1398-9995.2011.02666.x
- Shaw DW, Maibach HI. Clinical relevance of tixocortol pivalate-positive patch tests and questionable bioequivalence of different hydrocortisone preparations. Contact Dermatitis. 2013;68:369-375. doi:10.1111/cod.12066
- Kalavala M, Statham BN, Green CM, et al. Tixocortol pivalate: what is the right concentration? Contact Dermatitis. 2007;57:44-46. doi:10.1111/j.1600-0536.2007.01136.x
- Chowdhury MM, Statham BN, Sansom JE, et al. Patch testing for corticosteroid allergy with low and high concentrations of tixocortol pivalate and budesonide. Contact Dermatitis. 2002;46:311-312. doi:10.1034/j.1600-0536.2002.460519.x
- Isaksson M, Bruze M, Lepoittevin JP, et al. Patch testing with serial dilutions of budesonide, its R and S diastereomers, and potentially cross-reacting substances. Am J Contact Dermat. 2001;12:170-176.
- Ferguson AD, Emerson RM, English JS. Cross-reactivity patterns to budesonide. Contact Dermatitis. 2002;47:337-340. doi:10.1034/j.1600-0536.2002.470604.x
- Kot M, Bogaczewicz J, Kre˛cisz B, et al. Contact allergy in the population of patients with chronic inflammatory dermatoses and contact hypersensitivity to corticosteroids. Postepy Dermatol Alergol. 2017;34:253-259. doi:10.5114/ada.2017.67848
- Isaksson M, Bruze M. Allergic contact dermatitis in response to budesonide reactivated by inhalation of the allergen. J Am Acad Dermatol. 2002;46:880-885. doi:10.1067/mjd.2002.120464
- Mimesh S, Pratt M. Allergic contact dermatitis from corticosteroids: reproducibility of patch testing and correlation with intradermal testing. Dermatitis. 2006;17:137-142. doi:10.2310/6620.2006.05048
- Soria A, Baeck M, Goossens A, et al. Patch, prick or intradermal tests to detect delayed hypersensitivity to corticosteroids?. Contact Dermatitis. 2011;64:313-324. doi:10.1111/j.1600-0536.2011.01888.x
- Wilkinson SM, Beck MH. Corticosteroid contact hypersensitivity: what vehicle and concentration? Contact Dermatitis. 1996;34:305-308. doi:10.1111/j.1600-0536.1996.tb02212.x
- Isaksson M, Beck MH, Wilkinson SM. Comparative testing with budesonide in petrolatum and ethanol in a standard series. Contact Dermatitis. 2002;47:123-124. doi:10.1034/j.1600-0536.2002.470210_16.x
- Baeck M, Goossens A. Immediate and delayed allergic hypersensitivity to corticosteroids: practical guidelines. Contact Dermatitis. 2012;66:38-45. doi:10.1111/j.1600-0536.2011.01967.x
- Isaksson M. Corticosteroid contact allergy—the importance of late readings and testing with corticosteroids used by the patients. Contact Dermatitis. 2007;56:56-57. doi:10.1111/j.1600-0536.2007.00959.x
- Tam I, Yu J. Delayed patch test reaction to budesonide in an 8-year-old. Pediatr Dermatol. 2020;37:690-691. doi:10.1111/pde.14168
- Garcia-Bravo B, Camacho F. Two cases of contact dermatitis caused by calcipotriol cream. Am J Contact Dermat. 1996;7:118-119.
- Zollner TM, Ochsendorf FR, Hensel O, et al. Delayed-type reactivity to calcipotriol without cross-sensitization to tacalcitol. Contact Dermatitis. 1997;37:251. doi:10.1111/j.1600-0536.1997.tb02457.x
- Frosch PJ, Rustemeyer T. Contact allergy to calcipotriol does exist. report of an unequivocal case and review of the literature. Contact Dermatitis. 1999;40:66-71. doi:10.1111/j.1600-0536.1999.tb05993.x
- Gilissen L, Huygens S, Goossens A. Allergic contact dermatitis caused by calcipotriol. Contact Dermatitis. 2018;78:139-142. doi:10.1111/cod.12910
- Foti C, Carnimeo L, Bonamonte D, et al. Tolerance to calcitriol and tacalcitol in three patients with allergic contact dermatitis to calcipotriol. J Drugs Dermatol. 2005;4:756-759.
- Fullerton A, Benfeldt E, Petersen JR, et al. The calcipotriol dose-irritation relationship: 48-hour occlusive testing in healthy volunteers using Finn Chambers. Br J Dermatol. 1998;138:259-265. doi:10.1046/j.1365-2133.1998.02071.x
- Hanneman KK, Scull HM, Cooper KD, et al. Effect of topical vitamin D analogue on in vivo contact sensitization. Arch Dermatol. 2006;142:1332-1334. doi:10.1001/archderm.142.10.1332
- Shaw DW, Maibach HI, Eichenfield LF. Allergic contact dermatitis from pimecrolimus in a patient with tacrolimus allergy. J Am Acad Dermatol. 2007;56:342-345. doi:10.1016/j.jaad.2006.09.033
- Saitta P, Brancaccio R. Allergic contact dermatitis to pimecrolimus. Contact Dermatitis. 2007;56:43-44. doi:10.1111/j.1600-0536.2007.00822.x
- Neczyporenko F, Blondeel A. Allergic contact dermatitis to Elidel cream itself? Contact Dermatitis. 2010;63:171-172. doi:10.1111/j.1600-0536.2010.01764.x
- Shaw DW, Eichenfield LF, Shainhouse T, et al. Allergic contact dermatitis from tacrolimus. J Am Acad Dermatol. 2004;50:962-965. doi:10.1016/j.jaad.2003.09.013
- Warshaw EM, Schram SE, Belsito DV, et al. Patch-test reactions to topical anesthetics: retrospective analysis of cross-sectional data, 2001 to 2004. Dermatitis. 2008;19:81-85.
- Warshaw EM, Shaver RL, DeKoven JG, et al. Patch test reactions associated with topical medications: a retrospective analysis of the North American Contact Dermatitis Group data (2001-2018)[published online September 1, 2021]. Dermatitis. doi:10.1097/DER.0000000000000777
- Roos TC, Merk HF. Allergic contact dermatitis from benzocaine ointment during treatment of herpes zoster. Contact Dermatitis. 2001;44:104. doi:10.1034/j.1600-0536.2001.4402097.x
- González-Rodríguez AJ, Gutiérrez-Paredes EM, Revert Fernández Á, et al. Allergic contact dermatitis to benzocaine: the importance of concomitant positive patch test results. Actas Dermosifiliogr. 2013;104:156-158. doi:10.1016/j.ad.2011.07.023
- Muratore L, Calogiuri G, Foti C, et al. Contact allergy to benzocaine in a condom. Contact Dermatitis. 2008;59:173-174. doi:10.1111/j.1600-0536.2008.01359.x
- Sharma A, Agarwal S, Garg G, et al. Desire for lasting long in bed led to contact allergic dermatitis and subsequent superficial penile gangrene: a dreadful complication of benzocaine-containing extended-pleasure condom [published online September 27, 2018]. BMJ Case Rep. 2018;2018:bcr2018227351. doi:10.1136/bcr-2018-227351
- Bauer A, Geier J, Elsner P. Allergic contact dermatitis in patients with anogenital complaints. J Reprod Med. 2000;45:649-654.
- Warshaw EM, Kimyon RS, Silverberg JI, et al. Evaluation of patch test findings in patients with anogenital dermatitis. JAMA Dermatol. 2020;156:85-91. doi:10.1001/jamadermatol.2019.3844
- Weightman W, Turner T. Allergic contact dermatitis from lignocaine: report of 29 cases and review of the literature. Contact Dermatitis. 1998;39:265-266. doi:10.1111/j.1600-0536.1998.tb05928.x
- Jovanovic´ M, Karadaglic´ D, Brkic´ S. Contact urticaria and allergic contact dermatitis to lidocaine in a patient sensitive to benzocaine and propolis. Contact Dermatitis. 2006;54:124-126. doi:10.1111/j.0105-1873.2006.0560f.x
- Carazo JL, Morera BS, Colom LP, et al. Allergic contact dermatitis from ethyl chloride and benzocaine. Dermatitis. 2009;20:E13-E15.
- le Coz CJ, Cribier BJ, Heid E. Patch testing in suspected allergic contact dermatitis due to EMLA cream in haemodialyzed patients. Contact Dermatitis. 1996;35:316-317. doi:10.1111/j.1600-0536.1996.tb02407.x
- Ismail F, Goldsmith PC. EMLA cream-induced allergic contact dermatitis in a child with thalassaemia major. Contact Dermatitis. 2005;52:111. doi:10.1111/j.0105-1873.2005.00498e.x
- Pérez-Pérez LC, Fernández-Redondo V, Ginarte-Val M, et al. Allergic contact dermatitis from EMLA cream in a hemodialyzed patient. Dermatitis. 2006;17:85-87.
- Timmermans MW, Bruynzeel DP, Rustemeyer T. Allergic contact dermatitis from EMLA cream: concomitant sensitization to both local anesthetics lidocaine and prilocaine. J Dtsch Dermatol Ges. 2009;7:237-238. doi:10.1111/j.1610-0387.2008.06932.x
- Fuzier R, Lapeyre-Mestre M, Mertes PM, et al. Immediate- and delayed-type allergic reactions to amide local anesthetics: clinical features and skin testing. Pharmacoepidemiol Drug Saf. 2009;18:595-601. doi:10.1002/pds.1758
- Ruzicka T, Gerstmeier M, Przybilla B, et al. Allergy to local anesthetics: comparison of patch test with prick and intradermal test results. J Am Acad Dermatol. 1987;16:1202-1208. doi:10.1016/s0190-9622(87)70158-3
- Fowler JF Jr, Fowler L, Douglas JL, et al. Skin reactions to pimecrolimus cream 1% in patients allergic to propylene glycol: a double-blind randomized study. Dermatitis. 2007;18:134-139. doi:10.2310/6620.2007.06028
- de Groot A. Patch Testing. 3rd ed. acdegroot publishing; 2008.
Practice Points
- Allergic contact dermatitis (ACD) should be suspected in patients with persistent or worsening dermatitis after use of topical medications.
- Cross-reactions commonly occur between structurally similar compounds and occasionally between molecules from different drug classes.
- Some cases of topical medicament ACD remain elusive after patch testing, particularly drugs with potent immunomodulating effects.
Buccal Fat Pad Reduction With Intraoperative Fat Transfer to the Temple
The buccal fat pad (Bichat fat pad) is a tubular-shaped collection of adipose tissue that occupies a prominent position in the midface. The buccal fat pad has been described as having 3 lobes: an anterior lobe, which is anterior to the masseter muscle; an intermediate lobe between the masseter and buccinator muscles; and a posterior lobe between the temporal masticatory space.1 There are 4 extensions from the body of the buccal fat pad: the buccal, the sublevator, the melolabial, and the pterygoid. It is the buccal extension and main body that are removed intraorally to achieve midfacial and lower facial contouring, as these support the contours of the cheeks. The deep fat pad within the temporal fossa is a true extension of the buccal fat pad (Figure).2 It has a complex relationship to the facial structures, with known variability in the positions of the buccal branch of the facial nerve and the parotid duct.3 The parotid duct travels over, superior to, or through the buccal extension 42%, 32%, and 26% of the time, respectively. The duct travels along the surface of the masseter, then pierces the buccinator to drain into the vestibule of the mouth at the second superior molar tooth. The buccal branch of the facial nerve travels on the surface of the buccal fat pad 73% of the time, whereas 27% of the time it travels deeper through the buccal extension.4 A study that used ultrasonography to map the surface anatomy path of the parotid duct in 50 healthy patients showed that the duct was within 1.5 cm of the middle half of a line between the lower border of the tragus and the oral commissure in 93% of individuals.5 We describe a technique in which part of the buccal fat pad is removed and the fat is transferred to the temple to achieve aesthetically pleasing facial contouring. We used a vertical line from the lateral canthus as a surface anatomy landmark to determine when the duct emerges from the gland and is most susceptible to injury.
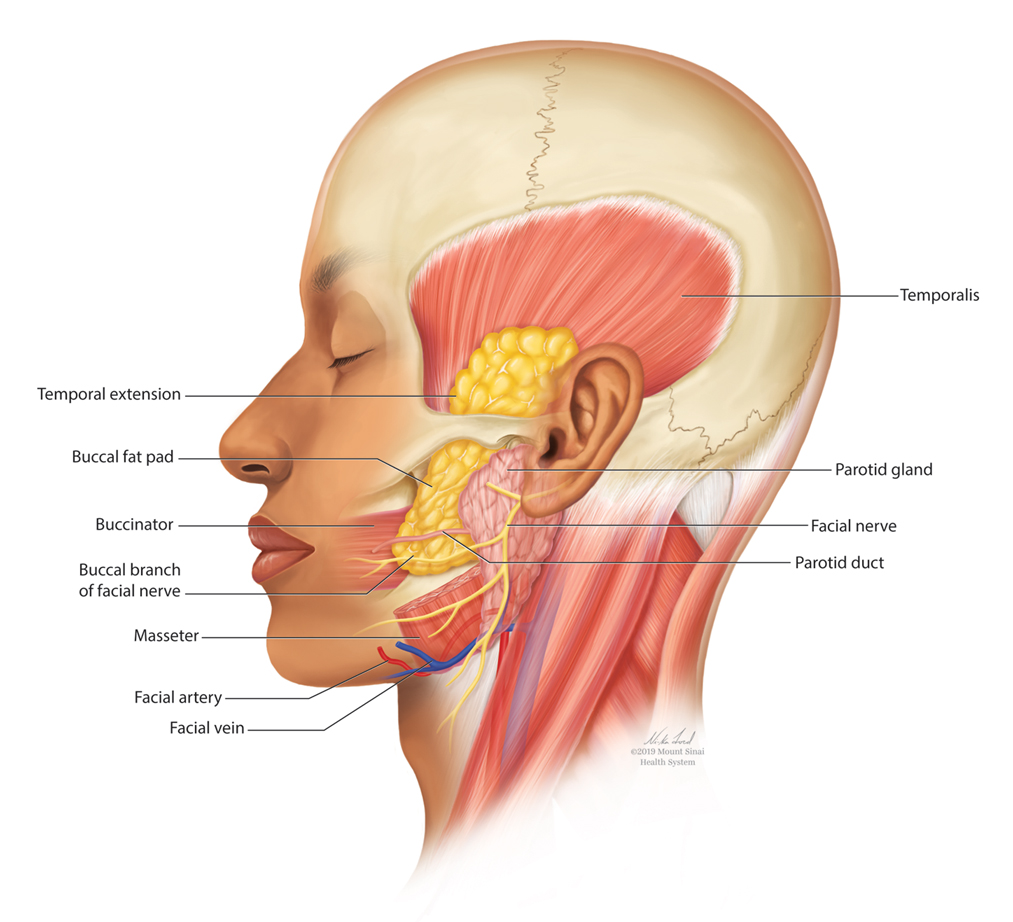
Operative Technique
Correct instrumentation is important to obtain appropriate anatomic exposure for this procedure. The surgical tray should include 4-0 poliglecaprone 25 suture, bite guards, a needle driver, a hemostat, surgical scissors, toothed forceps, a Beaver surgical handle with #15 blade, a protected diathermy needle, cotton tip applicators, and gauze.
Fat Harvest—With the patient supine, bite blocks are placed, and the buccal fat pad incision line is marked with a surgical marker. A 1-cm line is drawn approximately 4 cm posterior to the oral commissure by the buccal bite marks. The location is verified by balloting externally on the buccal fat pad on the cheek. The incision line is then anesthetized transorally with lidocaine and epinephrine-containing solution. The cheek is retracted laterally with Caldwell-Luc retractors, and a 1-cm incision is made and carried through the mucosa and superficial muscle using the Colorado needle. Scissors are then used to spread the deeper muscle fibers to expose the deeper fascia and fat pads. Metzenbaum scissors are used to gently spread the fat while the surgeon places pressure on the external cheek, manipulating the fat into the wound. Without excess traction, the walnut-sized portion of the fat pad that protrudes is grasped with Debakey forceps, gently teased into the field, clamped at its base with a curved hemostat, and excised. The stump is electrocoagulated with an extendable protected Colorado needle, with care to prevent inadvertent cauterization of the lips. The wound is closed with a single 4-0 poliglecaprone-25 suture.
A 5-cc Luer lock syringe is preloaded with 2 cc of normal saline and attached to another 5-cc Luer lock syringe via a female-female attachment. The excised fat is then placed in a 5-cc Luer lock syringe by removing the plunger. The plunger is then reinstalled, and the fat is injected back and forth approximately 30 times. The fat is centrifuged at 3500 rpm for 3 minutes. The purified fat is then transferred to a 1-cc Luer lock syringe attached to an 18-gauge needle.
Fat Injection—The authors use an 18-gauge needle to perform depot injections into the temporal fossae above the periosteum. This is a relatively safe area of the face to inject, but care must be taken to avoid injury to the superficial temporal artery. Between 1.5 and 3 cc of high-quality fat usually are administered to each temple.
Aftercare Instructions—The patient is instructed to have a soft diet for 24 to 48 hours and can return to work the next day. The patient also is given prophylactic antibiotics with Gram-negative coverage for 7 days (amoxicillin-clavulanate 875 mg/125 mg orally twice daily for 7 days).
Candidates for Buccal Fat Pad Reduction
Buccal fat pad reduction has become an increasingly popular technique for midface and lower face shaping to decrease the appearance of a round face. To achieve an aesthetically pleasing midface, surgeons should consider enhancing zygomatic eminences while emphasizing the border between the zygomatic prominence and cheek hollow.6 Selection criteria for buccal fat pad reduction are not well established. One study recommended avoiding the procedure in pregnant or lactating patients, patients with chronic illnesses, patients on blood-thinning agents, and patients younger than 18 years. In addition, this study suggested ensuring the malar fullness is in the anteromedial portion of the face, as posterolateral fullness may be due to masseter hypertrophy.6
Complications From Buccal Fat Pad Reduction
Complications associated with buccal fat pad reduction include inadvertent damage to surrounding structures, including the buccal branch of the facial nerve and parotid duct. Because the location of the facial nerve in relation to the parotid duct is highly variable, surgeons must be aware of its anatomy to avoid unintentional damage. Hwang et al7 reported that the parotid duct and buccal branches of the facial nerves passed through the buccal extension in 26.3% of cadavers. The transbuccal approach is preferred over the sub–superficial muscular aponeurotic system approach largely because it avoids these structures. In addition, blunt dissection may further decrease chances of injury. Although the long-term effects are unknown, there is a potential risk for facial hollowing.3 The use of preprocedure ultrasonography to quantify the buccal fat pad may avoid overresection and enhanced potential for facial hollowing.6
Avoidance of Temporal Hollowing
Because the buccal fat pad extends into the temporal space, buccal fat pad reduction may lead to further temporal hollowing, contributing to an aged appearance. The authors’ technique addresses both midface and upper face contouring in one minimally invasive procedure. Temporal hollowing commonly has been corrected with autologous fat grafting from the thigh or abdomen, which leads to an additional scar at the donor site. Our technique relies on autologous adjacent fat transfer from previously removed buccal fat. In addition, compared with the use of hyaluronic acid fillers for temple reflation, fat transfer largely is safe and biocompatible. Major complications of autologous fat transfer to the temples include nodularity or fat clumping, fat necrosis, sensory or motor nerve damage, and edema or ecchymosis.4 Also, with time there will be ongoing hollowing of the temples as part of the aging process with soft tissue and bone resorption. Therefore, further volume restoration procedures may be required in the future to address these dynamic changes.
Conclusion
The buccal fat pad has been extensively used to reconstruct oral defects, including oroantral and cranial base defects, owing to its high vascularity.6 However, there also is great potential to utilize buccal fat for autologous fat transfer to improve temporal wasting. Further studies are needed to determine optimal technique as well as longer-term safety and efficacy of this procedure.
- Zhang HM, Yan YP, Qi KM, et al. Anatomical structure of the buccal fat pad and its clinical adaptations. Plast Reconstr Surg. 2002;109:2509-2518.
- Yousuf S, Tubbs RS, Wartmann CT, et al. A review of the gross anatomy, functions, pathology, and clinical uses of the buccal fat pad. Surg Radiol Anat. 2010;32:427-436.
- Benjamin M, Reish RG. Buccal fat pad excision: proceed with caution. Plast Reconstr Surg Glob Open. 2018;6:E1970.
- Tzikas TL. Fat grafting volume restoration to the brow and temporal regions. Facial Plast Surg. 2018;34:164-172.
- Stringer MD, Mirjalili SA, Meredith SJ, et al. Redefining the surface anatomy of the parotid duct: an in vivo ultrasound study. Plast Reconstr Surg. 2012;130:1032-1037.
- Sezgin B, Tatar S, Boge M, et al. The excision of the buccal fat pad for cheek refinement: volumetric considerations. Aesthet Surg J. 2019;39:585-592.
- Hwang K, Cho HJ, Battuvshin D, et al. Interrelated buccal fat pad with facial buccal branches and parotid duct. J Craniofac Surg. 2005;16:658-660.
The buccal fat pad (Bichat fat pad) is a tubular-shaped collection of adipose tissue that occupies a prominent position in the midface. The buccal fat pad has been described as having 3 lobes: an anterior lobe, which is anterior to the masseter muscle; an intermediate lobe between the masseter and buccinator muscles; and a posterior lobe between the temporal masticatory space.1 There are 4 extensions from the body of the buccal fat pad: the buccal, the sublevator, the melolabial, and the pterygoid. It is the buccal extension and main body that are removed intraorally to achieve midfacial and lower facial contouring, as these support the contours of the cheeks. The deep fat pad within the temporal fossa is a true extension of the buccal fat pad (Figure).2 It has a complex relationship to the facial structures, with known variability in the positions of the buccal branch of the facial nerve and the parotid duct.3 The parotid duct travels over, superior to, or through the buccal extension 42%, 32%, and 26% of the time, respectively. The duct travels along the surface of the masseter, then pierces the buccinator to drain into the vestibule of the mouth at the second superior molar tooth. The buccal branch of the facial nerve travels on the surface of the buccal fat pad 73% of the time, whereas 27% of the time it travels deeper through the buccal extension.4 A study that used ultrasonography to map the surface anatomy path of the parotid duct in 50 healthy patients showed that the duct was within 1.5 cm of the middle half of a line between the lower border of the tragus and the oral commissure in 93% of individuals.5 We describe a technique in which part of the buccal fat pad is removed and the fat is transferred to the temple to achieve aesthetically pleasing facial contouring. We used a vertical line from the lateral canthus as a surface anatomy landmark to determine when the duct emerges from the gland and is most susceptible to injury.

Operative Technique
Correct instrumentation is important to obtain appropriate anatomic exposure for this procedure. The surgical tray should include 4-0 poliglecaprone 25 suture, bite guards, a needle driver, a hemostat, surgical scissors, toothed forceps, a Beaver surgical handle with #15 blade, a protected diathermy needle, cotton tip applicators, and gauze.
Fat Harvest—With the patient supine, bite blocks are placed, and the buccal fat pad incision line is marked with a surgical marker. A 1-cm line is drawn approximately 4 cm posterior to the oral commissure by the buccal bite marks. The location is verified by balloting externally on the buccal fat pad on the cheek. The incision line is then anesthetized transorally with lidocaine and epinephrine-containing solution. The cheek is retracted laterally with Caldwell-Luc retractors, and a 1-cm incision is made and carried through the mucosa and superficial muscle using the Colorado needle. Scissors are then used to spread the deeper muscle fibers to expose the deeper fascia and fat pads. Metzenbaum scissors are used to gently spread the fat while the surgeon places pressure on the external cheek, manipulating the fat into the wound. Without excess traction, the walnut-sized portion of the fat pad that protrudes is grasped with Debakey forceps, gently teased into the field, clamped at its base with a curved hemostat, and excised. The stump is electrocoagulated with an extendable protected Colorado needle, with care to prevent inadvertent cauterization of the lips. The wound is closed with a single 4-0 poliglecaprone-25 suture.
A 5-cc Luer lock syringe is preloaded with 2 cc of normal saline and attached to another 5-cc Luer lock syringe via a female-female attachment. The excised fat is then placed in a 5-cc Luer lock syringe by removing the plunger. The plunger is then reinstalled, and the fat is injected back and forth approximately 30 times. The fat is centrifuged at 3500 rpm for 3 minutes. The purified fat is then transferred to a 1-cc Luer lock syringe attached to an 18-gauge needle.
Fat Injection—The authors use an 18-gauge needle to perform depot injections into the temporal fossae above the periosteum. This is a relatively safe area of the face to inject, but care must be taken to avoid injury to the superficial temporal artery. Between 1.5 and 3 cc of high-quality fat usually are administered to each temple.
Aftercare Instructions—The patient is instructed to have a soft diet for 24 to 48 hours and can return to work the next day. The patient also is given prophylactic antibiotics with Gram-negative coverage for 7 days (amoxicillin-clavulanate 875 mg/125 mg orally twice daily for 7 days).
Candidates for Buccal Fat Pad Reduction
Buccal fat pad reduction has become an increasingly popular technique for midface and lower face shaping to decrease the appearance of a round face. To achieve an aesthetically pleasing midface, surgeons should consider enhancing zygomatic eminences while emphasizing the border between the zygomatic prominence and cheek hollow.6 Selection criteria for buccal fat pad reduction are not well established. One study recommended avoiding the procedure in pregnant or lactating patients, patients with chronic illnesses, patients on blood-thinning agents, and patients younger than 18 years. In addition, this study suggested ensuring the malar fullness is in the anteromedial portion of the face, as posterolateral fullness may be due to masseter hypertrophy.6
Complications From Buccal Fat Pad Reduction
Complications associated with buccal fat pad reduction include inadvertent damage to surrounding structures, including the buccal branch of the facial nerve and parotid duct. Because the location of the facial nerve in relation to the parotid duct is highly variable, surgeons must be aware of its anatomy to avoid unintentional damage. Hwang et al7 reported that the parotid duct and buccal branches of the facial nerves passed through the buccal extension in 26.3% of cadavers. The transbuccal approach is preferred over the sub–superficial muscular aponeurotic system approach largely because it avoids these structures. In addition, blunt dissection may further decrease chances of injury. Although the long-term effects are unknown, there is a potential risk for facial hollowing.3 The use of preprocedure ultrasonography to quantify the buccal fat pad may avoid overresection and enhanced potential for facial hollowing.6
Avoidance of Temporal Hollowing
Because the buccal fat pad extends into the temporal space, buccal fat pad reduction may lead to further temporal hollowing, contributing to an aged appearance. The authors’ technique addresses both midface and upper face contouring in one minimally invasive procedure. Temporal hollowing commonly has been corrected with autologous fat grafting from the thigh or abdomen, which leads to an additional scar at the donor site. Our technique relies on autologous adjacent fat transfer from previously removed buccal fat. In addition, compared with the use of hyaluronic acid fillers for temple reflation, fat transfer largely is safe and biocompatible. Major complications of autologous fat transfer to the temples include nodularity or fat clumping, fat necrosis, sensory or motor nerve damage, and edema or ecchymosis.4 Also, with time there will be ongoing hollowing of the temples as part of the aging process with soft tissue and bone resorption. Therefore, further volume restoration procedures may be required in the future to address these dynamic changes.
Conclusion
The buccal fat pad has been extensively used to reconstruct oral defects, including oroantral and cranial base defects, owing to its high vascularity.6 However, there also is great potential to utilize buccal fat for autologous fat transfer to improve temporal wasting. Further studies are needed to determine optimal technique as well as longer-term safety and efficacy of this procedure.
The buccal fat pad (Bichat fat pad) is a tubular-shaped collection of adipose tissue that occupies a prominent position in the midface. The buccal fat pad has been described as having 3 lobes: an anterior lobe, which is anterior to the masseter muscle; an intermediate lobe between the masseter and buccinator muscles; and a posterior lobe between the temporal masticatory space.1 There are 4 extensions from the body of the buccal fat pad: the buccal, the sublevator, the melolabial, and the pterygoid. It is the buccal extension and main body that are removed intraorally to achieve midfacial and lower facial contouring, as these support the contours of the cheeks. The deep fat pad within the temporal fossa is a true extension of the buccal fat pad (Figure).2 It has a complex relationship to the facial structures, with known variability in the positions of the buccal branch of the facial nerve and the parotid duct.3 The parotid duct travels over, superior to, or through the buccal extension 42%, 32%, and 26% of the time, respectively. The duct travels along the surface of the masseter, then pierces the buccinator to drain into the vestibule of the mouth at the second superior molar tooth. The buccal branch of the facial nerve travels on the surface of the buccal fat pad 73% of the time, whereas 27% of the time it travels deeper through the buccal extension.4 A study that used ultrasonography to map the surface anatomy path of the parotid duct in 50 healthy patients showed that the duct was within 1.5 cm of the middle half of a line between the lower border of the tragus and the oral commissure in 93% of individuals.5 We describe a technique in which part of the buccal fat pad is removed and the fat is transferred to the temple to achieve aesthetically pleasing facial contouring. We used a vertical line from the lateral canthus as a surface anatomy landmark to determine when the duct emerges from the gland and is most susceptible to injury.

Operative Technique
Correct instrumentation is important to obtain appropriate anatomic exposure for this procedure. The surgical tray should include 4-0 poliglecaprone 25 suture, bite guards, a needle driver, a hemostat, surgical scissors, toothed forceps, a Beaver surgical handle with #15 blade, a protected diathermy needle, cotton tip applicators, and gauze.
Fat Harvest—With the patient supine, bite blocks are placed, and the buccal fat pad incision line is marked with a surgical marker. A 1-cm line is drawn approximately 4 cm posterior to the oral commissure by the buccal bite marks. The location is verified by balloting externally on the buccal fat pad on the cheek. The incision line is then anesthetized transorally with lidocaine and epinephrine-containing solution. The cheek is retracted laterally with Caldwell-Luc retractors, and a 1-cm incision is made and carried through the mucosa and superficial muscle using the Colorado needle. Scissors are then used to spread the deeper muscle fibers to expose the deeper fascia and fat pads. Metzenbaum scissors are used to gently spread the fat while the surgeon places pressure on the external cheek, manipulating the fat into the wound. Without excess traction, the walnut-sized portion of the fat pad that protrudes is grasped with Debakey forceps, gently teased into the field, clamped at its base with a curved hemostat, and excised. The stump is electrocoagulated with an extendable protected Colorado needle, with care to prevent inadvertent cauterization of the lips. The wound is closed with a single 4-0 poliglecaprone-25 suture.
A 5-cc Luer lock syringe is preloaded with 2 cc of normal saline and attached to another 5-cc Luer lock syringe via a female-female attachment. The excised fat is then placed in a 5-cc Luer lock syringe by removing the plunger. The plunger is then reinstalled, and the fat is injected back and forth approximately 30 times. The fat is centrifuged at 3500 rpm for 3 minutes. The purified fat is then transferred to a 1-cc Luer lock syringe attached to an 18-gauge needle.
Fat Injection—The authors use an 18-gauge needle to perform depot injections into the temporal fossae above the periosteum. This is a relatively safe area of the face to inject, but care must be taken to avoid injury to the superficial temporal artery. Between 1.5 and 3 cc of high-quality fat usually are administered to each temple.
Aftercare Instructions—The patient is instructed to have a soft diet for 24 to 48 hours and can return to work the next day. The patient also is given prophylactic antibiotics with Gram-negative coverage for 7 days (amoxicillin-clavulanate 875 mg/125 mg orally twice daily for 7 days).
Candidates for Buccal Fat Pad Reduction
Buccal fat pad reduction has become an increasingly popular technique for midface and lower face shaping to decrease the appearance of a round face. To achieve an aesthetically pleasing midface, surgeons should consider enhancing zygomatic eminences while emphasizing the border between the zygomatic prominence and cheek hollow.6 Selection criteria for buccal fat pad reduction are not well established. One study recommended avoiding the procedure in pregnant or lactating patients, patients with chronic illnesses, patients on blood-thinning agents, and patients younger than 18 years. In addition, this study suggested ensuring the malar fullness is in the anteromedial portion of the face, as posterolateral fullness may be due to masseter hypertrophy.6
Complications From Buccal Fat Pad Reduction
Complications associated with buccal fat pad reduction include inadvertent damage to surrounding structures, including the buccal branch of the facial nerve and parotid duct. Because the location of the facial nerve in relation to the parotid duct is highly variable, surgeons must be aware of its anatomy to avoid unintentional damage. Hwang et al7 reported that the parotid duct and buccal branches of the facial nerves passed through the buccal extension in 26.3% of cadavers. The transbuccal approach is preferred over the sub–superficial muscular aponeurotic system approach largely because it avoids these structures. In addition, blunt dissection may further decrease chances of injury. Although the long-term effects are unknown, there is a potential risk for facial hollowing.3 The use of preprocedure ultrasonography to quantify the buccal fat pad may avoid overresection and enhanced potential for facial hollowing.6
Avoidance of Temporal Hollowing
Because the buccal fat pad extends into the temporal space, buccal fat pad reduction may lead to further temporal hollowing, contributing to an aged appearance. The authors’ technique addresses both midface and upper face contouring in one minimally invasive procedure. Temporal hollowing commonly has been corrected with autologous fat grafting from the thigh or abdomen, which leads to an additional scar at the donor site. Our technique relies on autologous adjacent fat transfer from previously removed buccal fat. In addition, compared with the use of hyaluronic acid fillers for temple reflation, fat transfer largely is safe and biocompatible. Major complications of autologous fat transfer to the temples include nodularity or fat clumping, fat necrosis, sensory or motor nerve damage, and edema or ecchymosis.4 Also, with time there will be ongoing hollowing of the temples as part of the aging process with soft tissue and bone resorption. Therefore, further volume restoration procedures may be required in the future to address these dynamic changes.
Conclusion
The buccal fat pad has been extensively used to reconstruct oral defects, including oroantral and cranial base defects, owing to its high vascularity.6 However, there also is great potential to utilize buccal fat for autologous fat transfer to improve temporal wasting. Further studies are needed to determine optimal technique as well as longer-term safety and efficacy of this procedure.
- Zhang HM, Yan YP, Qi KM, et al. Anatomical structure of the buccal fat pad and its clinical adaptations. Plast Reconstr Surg. 2002;109:2509-2518.
- Yousuf S, Tubbs RS, Wartmann CT, et al. A review of the gross anatomy, functions, pathology, and clinical uses of the buccal fat pad. Surg Radiol Anat. 2010;32:427-436.
- Benjamin M, Reish RG. Buccal fat pad excision: proceed with caution. Plast Reconstr Surg Glob Open. 2018;6:E1970.
- Tzikas TL. Fat grafting volume restoration to the brow and temporal regions. Facial Plast Surg. 2018;34:164-172.
- Stringer MD, Mirjalili SA, Meredith SJ, et al. Redefining the surface anatomy of the parotid duct: an in vivo ultrasound study. Plast Reconstr Surg. 2012;130:1032-1037.
- Sezgin B, Tatar S, Boge M, et al. The excision of the buccal fat pad for cheek refinement: volumetric considerations. Aesthet Surg J. 2019;39:585-592.
- Hwang K, Cho HJ, Battuvshin D, et al. Interrelated buccal fat pad with facial buccal branches and parotid duct. J Craniofac Surg. 2005;16:658-660.
- Zhang HM, Yan YP, Qi KM, et al. Anatomical structure of the buccal fat pad and its clinical adaptations. Plast Reconstr Surg. 2002;109:2509-2518.
- Yousuf S, Tubbs RS, Wartmann CT, et al. A review of the gross anatomy, functions, pathology, and clinical uses of the buccal fat pad. Surg Radiol Anat. 2010;32:427-436.
- Benjamin M, Reish RG. Buccal fat pad excision: proceed with caution. Plast Reconstr Surg Glob Open. 2018;6:E1970.
- Tzikas TL. Fat grafting volume restoration to the brow and temporal regions. Facial Plast Surg. 2018;34:164-172.
- Stringer MD, Mirjalili SA, Meredith SJ, et al. Redefining the surface anatomy of the parotid duct: an in vivo ultrasound study. Plast Reconstr Surg. 2012;130:1032-1037.
- Sezgin B, Tatar S, Boge M, et al. The excision of the buccal fat pad for cheek refinement: volumetric considerations. Aesthet Surg J. 2019;39:585-592.
- Hwang K, Cho HJ, Battuvshin D, et al. Interrelated buccal fat pad with facial buccal branches and parotid duct. J Craniofac Surg. 2005;16:658-660.
Practice Points
- Buccal fat pad reduction is an increasingly popular procedure for facial shaping.
- Buccal fat pad reduction in addition to natural aging can result in volume depletion of the temporal fossae.
- Removed buccal fat can be transferred to the temples for increased volume.
Behavioral factors are important in migraine management
Consider the following clinical scenarios.
Ellen, a 42-year-old married woman, presents to Dr. H’s office with a recent increase in her migraines. She looks sad and worried.
Dr. H. walks into the room, introduces himself, and immediately opens the electronic record to review her medical history forms. Her migraine episodes have increased from once biweekly to 1 to 2 times weekly; with additional less intense headaches on many other days. She uses both a triptan and an over-the-counter medication to control the pain–she gets a limited number of sumatriptan each month and is beginning to escalate her OTC usage. Dr. H. asks her about the intensity and duration of her headaches, reviews her medication use, and questions her about associated symptoms such as nausea or light and sound sensitivity? Ellen responds with yes and no answers. Dr. H. reviews different medication options, prescribes an older preventative medication and renews her sumatriptan.
In the second scenario, Ellen is in Dr. J’s office. When Dr. J enters the doorway to her office, she introduces herself and is welcoming and seated in a less formal manner. Dr. J is making eye contact with Ellen and not looking at her computer.
Instead of asking her questions that require a yes or no reply, she asks Ellen to walk her through her migraine experiences. She learns that the patient has been under much stress with work, and hears about troubling family issues, and that she is worried about her increased number of headaches and decreased functionality. Dr. J says, let’s talk about options. She tells her the first thing is to optimize acute care in order treat the acute attacks effectively. Simply “taking” a medication is insufficient to know whether a patient is taking that medication optimally. She asks Ellen to take her through her process in treating a migraine.
Ellen, Dr. J surmises, has a penchant for treating any sensation associated with a possible approaching headache with OTCs, which needs to be curtailed. Her use of OTCs could be at medication overuse levels thereby contributing to her headaches. Dr. J explains and shows Ellen a simple headache diary. Dr. J then discusses the future: the two of them will develop a plan to control the migraine frequency for the long term. The plan will include ways to control the stressors in Ellen’s life. Dr. J provides Ellen with names of psychologists with expertise in cognitive behavior therapies and relaxation-based treatments; they can help Ellen manage stressors that could be impacting her headaches. Dr. J communicates that migraine management requires a comprehensive approach that can involve behavioral as well as pharmacological therapies to maximize both headache relief and reduce disability.
Migraine is a brain disease that can often be fueled by behavioral issues. Psychological stress, sleep problems, mood and anxiety issues can transform migraine from episodic to chronic. The operative word here is can. Patients with migraine who learn to better manage stress, employ simple relaxation strategies, and identify and treat comorbid psychiatric issues may show significant improvement. Migraine treatment can require more than one health care professional asheadache specialists, psychologists, perhaps psychiatrists, and sleep specialists may all be involved
Getting migraine under control often cannot be accomplished in just one visit; it can take time, as medications might need to be added or adjusted, sleep, diet, and physical activity modified along with stressors identified and managed. Helping patients optimize their acute treatment regimen is critical so they get quick relief while limiting overuse. Overuse of either prescription or OTC medications can lead to medication overuse headache (MOH). MOH can increase headache frequency and reduce the effectiveness of some preventive medications as well as other therapies.
All these steps require good communication strategies by the physician and an understanding of the benefit of comprehensive treatment strategies that include behavioral therapies.
Helping motivation to change
Readiness to change will vary with different patients. Some people will be open to treating stress-related issues in an initial session while others will require many sessions in which the physician gently explores these concerns. It is helpful for the physician to ask open-ended questions, helping patients to “tell their stories.” The clinician needs to actively listen and accurately reflect patient’s thoughts and feelings (“it sounds like you…”) Avoiding overinterpretation and occasionally summarizing ensures clear communication. Both patients and physicians have identified high quality communication in the patient-physician relationship to be a key factor in adherence with acute headache medications.
Anxiety is common in migraine sufferers and predicts long-term migraine persistence. Some individuals with high levels of anxiety may overuse immediate relief medications because of worry about getting a migraine. Many migraineurs have a significant amount of fear about any sensation that may herald a migraine. Consequently, some medicate fear, preemptively. Patients also can fear side effects to new medications, thereby reducing their willingness to change existing therapy for a potentially more effective treatment.
Biological rhythms, sleep and coping skills
Managing migraine also includes managing consistent biological rhythms. The literature has shown that chronobiological issues can be a driver of headache frequency and may also contribute to mood and anxiety disorders. Studies have shown that a simple cognitive-behavioral treatment for insomnia has transformed many migraineurs from chronic migraine to episodic migraine.
Studies have demonstrated that a combination of optimal medication and cognitive behavioral therapy can be very effective. Behavioral therapies increase self-efficacy, a belief that patients have the requisite skills to manage a complicated disorder like migraine. A few sessions of stress management training combined with preventive medications and maximizing acute care options may have significant added value—reducing migraine frequency and related disability and ensuring better disease-coping mechanisms.
Final notes
Migraine is a biobehavioral disorder and it is important for the clinician to evaluate a diverse set of factors and come up with a comprehensive plan. This is particularly important for the patient with high frequency migraine who exhibits stress-related factors and possible psychiatric comorbidities. There are numerous cognitive behavioral therapies incorporating relaxation strategies and stress management techniques that can be very effective in caring for these complicated patients.
- Buse DC, Lipton RB. Facilitating communication with patients for improved migraine outcomes. Curr Pain Headache Rep. 2008 Jun;12(3):230-6.
- Torres-Ferrús M, Ursitti F, Alpuente A, et al. School of Advanced Studies of European Headache Federation (EHF-SAS). From transformation to chronification of migraine: pathophysiological and clinical aspects. J Headache Pain. 2020 Apr 29;21(1):42.
- Maleki N, Becerra L, Borsook D. Migraine: maladaptive brain responses to stress. Headache. 2012 Oct;52 Suppl 2(Suppl 2):102-6.
- Stubberud A, Buse DC, Kristoffersen ES, Linde M, Tronvik E. Is there a causal relationship between stress and migraine? Current evidence and implications for management. J Headache Pain. 2021 Dec 20;22(1):155.
- Langenbahn D, Matsuzawa Y, et al.. Underuse of Behavioral Treatments for Headache: a Narrative Review Examining Societal and Cultural Factors. J Gen Intern Med. 2021 Oct;36(10):3103-3112.
- Minen MT, Azarchi S, Sobolev R, et al. Factors Related to Migraine Patients' Decisions to Initiate Behavioral Migraine Treatment Following a Headache Specialist's Recommendation: A Prospective Observational Study. Pain Med. 2018 Nov 1;19(11):2274-2282.
- Penzien DB, Irby MB, Smitherman TA, Rains JC, Houle TT. Well-Established and Empirically Supported Behavioral Treatments for Migraine. Curr Pain Headache Rep. 2015 Jul;19(7):34.
- Seng EK, Conway AB, Grinberg AS, et al. Response to Mindfulness-Based Cognitive Therapy Differs Between Chronic and Episodic Migraine. Neurol Clin Pract. 2021 Jun;11(3):194-205.
- Smitherman TA, Kuka AJ, et al. Cognitive-Behavioral Therapy for Insomnia to Reduce Chronic Migraine: A Sequential Bayesian Analysis. Headache. 2018 Jul;58(7):1052-1059.
- Baskin SM, Smitherman TA. Migraine and psychiatric disorders: comorbidities, mechanisms, and clinical applications. Neurol Sci. 2009 May;30 Suppl 1:S61-5.
- Smitherman TA, Davis RE, et al. Anxiety sensitivity and headache: diagnostic differences, impact, and relations with perceived headache triggers. Cephalalgia. 2015 Jul;35(8):710-21.
Consider the following clinical scenarios.
Ellen, a 42-year-old married woman, presents to Dr. H’s office with a recent increase in her migraines. She looks sad and worried.
Dr. H. walks into the room, introduces himself, and immediately opens the electronic record to review her medical history forms. Her migraine episodes have increased from once biweekly to 1 to 2 times weekly; with additional less intense headaches on many other days. She uses both a triptan and an over-the-counter medication to control the pain–she gets a limited number of sumatriptan each month and is beginning to escalate her OTC usage. Dr. H. asks her about the intensity and duration of her headaches, reviews her medication use, and questions her about associated symptoms such as nausea or light and sound sensitivity? Ellen responds with yes and no answers. Dr. H. reviews different medication options, prescribes an older preventative medication and renews her sumatriptan.
In the second scenario, Ellen is in Dr. J’s office. When Dr. J enters the doorway to her office, she introduces herself and is welcoming and seated in a less formal manner. Dr. J is making eye contact with Ellen and not looking at her computer.
Instead of asking her questions that require a yes or no reply, she asks Ellen to walk her through her migraine experiences. She learns that the patient has been under much stress with work, and hears about troubling family issues, and that she is worried about her increased number of headaches and decreased functionality. Dr. J says, let’s talk about options. She tells her the first thing is to optimize acute care in order treat the acute attacks effectively. Simply “taking” a medication is insufficient to know whether a patient is taking that medication optimally. She asks Ellen to take her through her process in treating a migraine.
Ellen, Dr. J surmises, has a penchant for treating any sensation associated with a possible approaching headache with OTCs, which needs to be curtailed. Her use of OTCs could be at medication overuse levels thereby contributing to her headaches. Dr. J explains and shows Ellen a simple headache diary. Dr. J then discusses the future: the two of them will develop a plan to control the migraine frequency for the long term. The plan will include ways to control the stressors in Ellen’s life. Dr. J provides Ellen with names of psychologists with expertise in cognitive behavior therapies and relaxation-based treatments; they can help Ellen manage stressors that could be impacting her headaches. Dr. J communicates that migraine management requires a comprehensive approach that can involve behavioral as well as pharmacological therapies to maximize both headache relief and reduce disability.
Migraine is a brain disease that can often be fueled by behavioral issues. Psychological stress, sleep problems, mood and anxiety issues can transform migraine from episodic to chronic. The operative word here is can. Patients with migraine who learn to better manage stress, employ simple relaxation strategies, and identify and treat comorbid psychiatric issues may show significant improvement. Migraine treatment can require more than one health care professional asheadache specialists, psychologists, perhaps psychiatrists, and sleep specialists may all be involved
Getting migraine under control often cannot be accomplished in just one visit; it can take time, as medications might need to be added or adjusted, sleep, diet, and physical activity modified along with stressors identified and managed. Helping patients optimize their acute treatment regimen is critical so they get quick relief while limiting overuse. Overuse of either prescription or OTC medications can lead to medication overuse headache (MOH). MOH can increase headache frequency and reduce the effectiveness of some preventive medications as well as other therapies.
All these steps require good communication strategies by the physician and an understanding of the benefit of comprehensive treatment strategies that include behavioral therapies.
Helping motivation to change
Readiness to change will vary with different patients. Some people will be open to treating stress-related issues in an initial session while others will require many sessions in which the physician gently explores these concerns. It is helpful for the physician to ask open-ended questions, helping patients to “tell their stories.” The clinician needs to actively listen and accurately reflect patient’s thoughts and feelings (“it sounds like you…”) Avoiding overinterpretation and occasionally summarizing ensures clear communication. Both patients and physicians have identified high quality communication in the patient-physician relationship to be a key factor in adherence with acute headache medications.
Anxiety is common in migraine sufferers and predicts long-term migraine persistence. Some individuals with high levels of anxiety may overuse immediate relief medications because of worry about getting a migraine. Many migraineurs have a significant amount of fear about any sensation that may herald a migraine. Consequently, some medicate fear, preemptively. Patients also can fear side effects to new medications, thereby reducing their willingness to change existing therapy for a potentially more effective treatment.
Biological rhythms, sleep and coping skills
Managing migraine also includes managing consistent biological rhythms. The literature has shown that chronobiological issues can be a driver of headache frequency and may also contribute to mood and anxiety disorders. Studies have shown that a simple cognitive-behavioral treatment for insomnia has transformed many migraineurs from chronic migraine to episodic migraine.
Studies have demonstrated that a combination of optimal medication and cognitive behavioral therapy can be very effective. Behavioral therapies increase self-efficacy, a belief that patients have the requisite skills to manage a complicated disorder like migraine. A few sessions of stress management training combined with preventive medications and maximizing acute care options may have significant added value—reducing migraine frequency and related disability and ensuring better disease-coping mechanisms.
Final notes
Migraine is a biobehavioral disorder and it is important for the clinician to evaluate a diverse set of factors and come up with a comprehensive plan. This is particularly important for the patient with high frequency migraine who exhibits stress-related factors and possible psychiatric comorbidities. There are numerous cognitive behavioral therapies incorporating relaxation strategies and stress management techniques that can be very effective in caring for these complicated patients.
Consider the following clinical scenarios.
Ellen, a 42-year-old married woman, presents to Dr. H’s office with a recent increase in her migraines. She looks sad and worried.
Dr. H. walks into the room, introduces himself, and immediately opens the electronic record to review her medical history forms. Her migraine episodes have increased from once biweekly to 1 to 2 times weekly; with additional less intense headaches on many other days. She uses both a triptan and an over-the-counter medication to control the pain–she gets a limited number of sumatriptan each month and is beginning to escalate her OTC usage. Dr. H. asks her about the intensity and duration of her headaches, reviews her medication use, and questions her about associated symptoms such as nausea or light and sound sensitivity? Ellen responds with yes and no answers. Dr. H. reviews different medication options, prescribes an older preventative medication and renews her sumatriptan.
In the second scenario, Ellen is in Dr. J’s office. When Dr. J enters the doorway to her office, she introduces herself and is welcoming and seated in a less formal manner. Dr. J is making eye contact with Ellen and not looking at her computer.
Instead of asking her questions that require a yes or no reply, she asks Ellen to walk her through her migraine experiences. She learns that the patient has been under much stress with work, and hears about troubling family issues, and that she is worried about her increased number of headaches and decreased functionality. Dr. J says, let’s talk about options. She tells her the first thing is to optimize acute care in order treat the acute attacks effectively. Simply “taking” a medication is insufficient to know whether a patient is taking that medication optimally. She asks Ellen to take her through her process in treating a migraine.
Ellen, Dr. J surmises, has a penchant for treating any sensation associated with a possible approaching headache with OTCs, which needs to be curtailed. Her use of OTCs could be at medication overuse levels thereby contributing to her headaches. Dr. J explains and shows Ellen a simple headache diary. Dr. J then discusses the future: the two of them will develop a plan to control the migraine frequency for the long term. The plan will include ways to control the stressors in Ellen’s life. Dr. J provides Ellen with names of psychologists with expertise in cognitive behavior therapies and relaxation-based treatments; they can help Ellen manage stressors that could be impacting her headaches. Dr. J communicates that migraine management requires a comprehensive approach that can involve behavioral as well as pharmacological therapies to maximize both headache relief and reduce disability.
Migraine is a brain disease that can often be fueled by behavioral issues. Psychological stress, sleep problems, mood and anxiety issues can transform migraine from episodic to chronic. The operative word here is can. Patients with migraine who learn to better manage stress, employ simple relaxation strategies, and identify and treat comorbid psychiatric issues may show significant improvement. Migraine treatment can require more than one health care professional asheadache specialists, psychologists, perhaps psychiatrists, and sleep specialists may all be involved
Getting migraine under control often cannot be accomplished in just one visit; it can take time, as medications might need to be added or adjusted, sleep, diet, and physical activity modified along with stressors identified and managed. Helping patients optimize their acute treatment regimen is critical so they get quick relief while limiting overuse. Overuse of either prescription or OTC medications can lead to medication overuse headache (MOH). MOH can increase headache frequency and reduce the effectiveness of some preventive medications as well as other therapies.
All these steps require good communication strategies by the physician and an understanding of the benefit of comprehensive treatment strategies that include behavioral therapies.
Helping motivation to change
Readiness to change will vary with different patients. Some people will be open to treating stress-related issues in an initial session while others will require many sessions in which the physician gently explores these concerns. It is helpful for the physician to ask open-ended questions, helping patients to “tell their stories.” The clinician needs to actively listen and accurately reflect patient’s thoughts and feelings (“it sounds like you…”) Avoiding overinterpretation and occasionally summarizing ensures clear communication. Both patients and physicians have identified high quality communication in the patient-physician relationship to be a key factor in adherence with acute headache medications.
Anxiety is common in migraine sufferers and predicts long-term migraine persistence. Some individuals with high levels of anxiety may overuse immediate relief medications because of worry about getting a migraine. Many migraineurs have a significant amount of fear about any sensation that may herald a migraine. Consequently, some medicate fear, preemptively. Patients also can fear side effects to new medications, thereby reducing their willingness to change existing therapy for a potentially more effective treatment.
Biological rhythms, sleep and coping skills
Managing migraine also includes managing consistent biological rhythms. The literature has shown that chronobiological issues can be a driver of headache frequency and may also contribute to mood and anxiety disorders. Studies have shown that a simple cognitive-behavioral treatment for insomnia has transformed many migraineurs from chronic migraine to episodic migraine.
Studies have demonstrated that a combination of optimal medication and cognitive behavioral therapy can be very effective. Behavioral therapies increase self-efficacy, a belief that patients have the requisite skills to manage a complicated disorder like migraine. A few sessions of stress management training combined with preventive medications and maximizing acute care options may have significant added value—reducing migraine frequency and related disability and ensuring better disease-coping mechanisms.
Final notes
Migraine is a biobehavioral disorder and it is important for the clinician to evaluate a diverse set of factors and come up with a comprehensive plan. This is particularly important for the patient with high frequency migraine who exhibits stress-related factors and possible psychiatric comorbidities. There are numerous cognitive behavioral therapies incorporating relaxation strategies and stress management techniques that can be very effective in caring for these complicated patients.
- Buse DC, Lipton RB. Facilitating communication with patients for improved migraine outcomes. Curr Pain Headache Rep. 2008 Jun;12(3):230-6.
- Torres-Ferrús M, Ursitti F, Alpuente A, et al. School of Advanced Studies of European Headache Federation (EHF-SAS). From transformation to chronification of migraine: pathophysiological and clinical aspects. J Headache Pain. 2020 Apr 29;21(1):42.
- Maleki N, Becerra L, Borsook D. Migraine: maladaptive brain responses to stress. Headache. 2012 Oct;52 Suppl 2(Suppl 2):102-6.
- Stubberud A, Buse DC, Kristoffersen ES, Linde M, Tronvik E. Is there a causal relationship between stress and migraine? Current evidence and implications for management. J Headache Pain. 2021 Dec 20;22(1):155.
- Langenbahn D, Matsuzawa Y, et al.. Underuse of Behavioral Treatments for Headache: a Narrative Review Examining Societal and Cultural Factors. J Gen Intern Med. 2021 Oct;36(10):3103-3112.
- Minen MT, Azarchi S, Sobolev R, et al. Factors Related to Migraine Patients' Decisions to Initiate Behavioral Migraine Treatment Following a Headache Specialist's Recommendation: A Prospective Observational Study. Pain Med. 2018 Nov 1;19(11):2274-2282.
- Penzien DB, Irby MB, Smitherman TA, Rains JC, Houle TT. Well-Established and Empirically Supported Behavioral Treatments for Migraine. Curr Pain Headache Rep. 2015 Jul;19(7):34.
- Seng EK, Conway AB, Grinberg AS, et al. Response to Mindfulness-Based Cognitive Therapy Differs Between Chronic and Episodic Migraine. Neurol Clin Pract. 2021 Jun;11(3):194-205.
- Smitherman TA, Kuka AJ, et al. Cognitive-Behavioral Therapy for Insomnia to Reduce Chronic Migraine: A Sequential Bayesian Analysis. Headache. 2018 Jul;58(7):1052-1059.
- Baskin SM, Smitherman TA. Migraine and psychiatric disorders: comorbidities, mechanisms, and clinical applications. Neurol Sci. 2009 May;30 Suppl 1:S61-5.
- Smitherman TA, Davis RE, et al. Anxiety sensitivity and headache: diagnostic differences, impact, and relations with perceived headache triggers. Cephalalgia. 2015 Jul;35(8):710-21.
- Buse DC, Lipton RB. Facilitating communication with patients for improved migraine outcomes. Curr Pain Headache Rep. 2008 Jun;12(3):230-6.
- Torres-Ferrús M, Ursitti F, Alpuente A, et al. School of Advanced Studies of European Headache Federation (EHF-SAS). From transformation to chronification of migraine: pathophysiological and clinical aspects. J Headache Pain. 2020 Apr 29;21(1):42.
- Maleki N, Becerra L, Borsook D. Migraine: maladaptive brain responses to stress. Headache. 2012 Oct;52 Suppl 2(Suppl 2):102-6.
- Stubberud A, Buse DC, Kristoffersen ES, Linde M, Tronvik E. Is there a causal relationship between stress and migraine? Current evidence and implications for management. J Headache Pain. 2021 Dec 20;22(1):155.
- Langenbahn D, Matsuzawa Y, et al.. Underuse of Behavioral Treatments for Headache: a Narrative Review Examining Societal and Cultural Factors. J Gen Intern Med. 2021 Oct;36(10):3103-3112.
- Minen MT, Azarchi S, Sobolev R, et al. Factors Related to Migraine Patients' Decisions to Initiate Behavioral Migraine Treatment Following a Headache Specialist's Recommendation: A Prospective Observational Study. Pain Med. 2018 Nov 1;19(11):2274-2282.
- Penzien DB, Irby MB, Smitherman TA, Rains JC, Houle TT. Well-Established and Empirically Supported Behavioral Treatments for Migraine. Curr Pain Headache Rep. 2015 Jul;19(7):34.
- Seng EK, Conway AB, Grinberg AS, et al. Response to Mindfulness-Based Cognitive Therapy Differs Between Chronic and Episodic Migraine. Neurol Clin Pract. 2021 Jun;11(3):194-205.
- Smitherman TA, Kuka AJ, et al. Cognitive-Behavioral Therapy for Insomnia to Reduce Chronic Migraine: A Sequential Bayesian Analysis. Headache. 2018 Jul;58(7):1052-1059.
- Baskin SM, Smitherman TA. Migraine and psychiatric disorders: comorbidities, mechanisms, and clinical applications. Neurol Sci. 2009 May;30 Suppl 1:S61-5.
- Smitherman TA, Davis RE, et al. Anxiety sensitivity and headache: diagnostic differences, impact, and relations with perceived headache triggers. Cephalalgia. 2015 Jul;35(8):710-21.