User login
Seladelpar Shows Clinically Meaningful Improvements in PBC
MILAN — according to two interim analyses of the ASSURE long-term extension study.
The first analysis of 337 patients with PBC, with and without cirrhosis, showed that treatment with seladelpar had a durable effect up to 2 years on cholestasis and markers of liver injury, as well as a sustained reduction in pruritus, Palak Trivedi, MD, associate professor at the National Institute for Health Research Birmingham Biomedical Research Centre, University of Birmingham, Birmingham, England, reported in a poster presented at the European Association for the Study of the Liver (EASL) Congress 2024.
The 2-year analysis also showed that seladelpar, a first-in-class, orally active agent, was safe and well tolerated in this patient population, he added.
These “results are consistent with the pivotal phase 3 RESPONSE study,” Dr. Trivedi noted. The RESPONSE study showed that seladelpar significantly improved liver biomarkers of disease activity and symptoms of pruritus at 12 months in patients with PBC who had an inadequate response or intolerance to ursodeoxycholic acid (UDCA), the standard of care, and had no history of hepatic decompensation. Patients with cirrhosis were allowed to enroll.
A total of 158 patients from the RESPONSE trial, both from the placebo and from the active treatment arm, were rolled over into the ASSURE trial. Another subset of 179 patients were drawn from prior seladelpar placebo-controlled studies (referred to as “legacy studies”), including the ENHANCE study. All participants in the current analysis received 10 mg of seladelpar, once daily, for up to 155 weeks.
Of the participants from the legacy studies, 99 completed 24 months of treatment with seladelpar, and 164 completed 12 months of treatment. In the 24-month treatment group, 70% met the composite response endpoint, which included alkaline phosphatase (ALP) levels below 1.67 times the upper limit of normal, a decrease in ALP levels of at least 15%, and total bilirubin levels at or below the upper limit of normal, according to a press release of the study findings. In addition, 42% of these participants achieved ALP normalization at 24 months, a marker of liver disease progression. In the 12-month treatment group, 73% achieved the clinically meaningful composite response endpoint, with 42% experiencing ALP normalization.
For patients rolled over from RESPONSE, 102 received 18 months of treatment with seladelpar, and 29 received 24 months of treatment. A total of 62% of patients in the 18-month group achieved the composite endpoint, and 33% achieved ALP normalization, while 72% of the 24-month group reached the composite endpoint, and 17% had ALP normalization.
Of patients who had received a placebo in the RESPONSE trial and went on to receive treatment with seladelpar, 75% achieved the composite endpoint, 27% had ALP normalization at 6 months, and 94% achieved the composite endpoint and 50% reached ALP normalization at 12 months.
Key secondary endpoints included ALP normalization and changes in liver enzymes (ALP, total bilirubin, gamma-glutamyl transferase [GGT], alanine transaminase [ALT], and aspartate aminotransferase [AST]).
Pruritus Relief Important for Quality of Life
Among study participants who reported a four or more at baseline on the numerical rating scale (NRS) for pruritus, legacy patients at 12 months and 24 months of treatment reported a mean reduction of 3.8 and 3.1, respectively. Participants from RESPONSE also reported a mean reduction of 3.8.
This level of reduction in NRS is “considered clinically significant” and takes patients from a level of moderate to severe itching down to mild, said Carrie Frenette, MD, executive director, Global Medical Affairs, Liver Diseases, Gilead Sciences, Foster City, California, and a former hepatologist of 20 years with a special interest in liver transplantation.
This “is a huge benefit in quality of life for these patients,” Dr. Frenette said in an interview.
Dr. Frenette also noted that UDCA, the current first-line treatment for PBC, is inadequate in up to 40% of patients, and second-line treatments, notably obeticholic acid, can cause itching.
Eleonora De Martin, MD, transplant hepatologist at Centre Hépato-Biliaire, Paul Brousse Hospital, Paris, France, who comoderated the session, pointed out that PBC is a complex disease.
“We need both disease control and symptom control, and they’re not always compatible,” she said. “Sometimes you can control the disease but not the symptoms, and symptomatic control is so important,” especially with pruritus.
Patients With PBC and Cirrhosis
A separate analysis from ASSURE looked at a subset of 17 patients with PBC and cirrhosis who completed 24 months of treatment. The findings were presented by Stuart Gordon, MD, professor of medicine, Wayne State University School of Medicine, and hepatologist at Henry Ford Hospital, both in Detroit.
In this analysis, the mean patient age was 60.8 years, 91.4% were female, 88.6% were Child-Pugh A, and 22.9% had portal hypertension, while the mean baseline liver stiffness by FibroScan was 19.9 kPa.
Baseline biochemical measures were mean ALP of 245.4 U/L, mean total bilirubin of 0.995 mg/dL, mean GGT of 216.1 U/L, and mean ALT of 36.6 U/L.
A total of 11 participants (65%) met the composite endpoint at 24 months, with ALP normalization in 4 patients (24%). The overall mean percent change from baseline in ALP was approximately −30% and in total bilirubin was around −14%. Other changes in biochemical markers included reductions from baseline in GGT and ALT of approximately −30% and −10%, respectively. No change was observed in AST.
While 80% of patients with cirrhosis “had an adverse event of some form,” there were no treatment-related serious adverse events.
“It’s interesting to see results in these patients who have advanced disease and are cirrhotic because it might stabilize disease or even provide improvement,” Dr. De Martin commented. “However, the numbers in the study are very small, so it’s hard to draw firm conclusions yet, but it is a first step in showing that this drug is safe.”
Seladelpar is an “important step forward in PBC because we’ve been stuck with ursodeoxycholic acid for so many years,” Dr. De Martin added. “We’ve seen in liver disease with other etiologies that sometimes just one drug can make a difference, and you can change the natural history of the disease.”
Dr. Frenette is an employee and stockholder of Gilead Sciences. Dr. Gordon declared grants and support from AbbVie, Arbutus, CymaBay, Cour Pharmaceuticals, GlaxoSmithKline (GSK), Ipsen, and Mirum Pharmaceuticals; and advisory board activity from CymaBay, GSK, and Ipsen Pharmaceuticals. Dr. De Martin had no disclosures of relevance to seladelpar but has received speaker fees from other companies, including GSK, Ipsen, and Astellas. Dr. Trivedi reports institutional funding support from National Institute for Health Research Birmingham (UK); lecture fees from Advanz Pharma/Intercept Pharmaceuticals, Albireo/Ipsen, and Dr. Falk Pharma; advisory board/consulting fees from Advanz Pharma/Intercept Pharmaceuticals, Albireo/Ipsen, Chemomab Therapeutics, CymaBay, Dr. Falk Pharma, Gilead Sciences, Perspectum, and Pliant Therapeutics; and grant support from Advanz Pharma/Intercept Pharmaceuticals, Albireo/Ipsen, Bristol-Myers Squibb, Core (Guts UK), EASL, Gilead Sciences, GSK, LifeArc, NIHR, Mirum Pharma, PSC Support, The Wellcome Trust, The Medical Research Foundation (UK), and Regeneron.
A version of this article first appeared on Medscape.com.
MILAN — according to two interim analyses of the ASSURE long-term extension study.
The first analysis of 337 patients with PBC, with and without cirrhosis, showed that treatment with seladelpar had a durable effect up to 2 years on cholestasis and markers of liver injury, as well as a sustained reduction in pruritus, Palak Trivedi, MD, associate professor at the National Institute for Health Research Birmingham Biomedical Research Centre, University of Birmingham, Birmingham, England, reported in a poster presented at the European Association for the Study of the Liver (EASL) Congress 2024.
The 2-year analysis also showed that seladelpar, a first-in-class, orally active agent, was safe and well tolerated in this patient population, he added.
These “results are consistent with the pivotal phase 3 RESPONSE study,” Dr. Trivedi noted. The RESPONSE study showed that seladelpar significantly improved liver biomarkers of disease activity and symptoms of pruritus at 12 months in patients with PBC who had an inadequate response or intolerance to ursodeoxycholic acid (UDCA), the standard of care, and had no history of hepatic decompensation. Patients with cirrhosis were allowed to enroll.
A total of 158 patients from the RESPONSE trial, both from the placebo and from the active treatment arm, were rolled over into the ASSURE trial. Another subset of 179 patients were drawn from prior seladelpar placebo-controlled studies (referred to as “legacy studies”), including the ENHANCE study. All participants in the current analysis received 10 mg of seladelpar, once daily, for up to 155 weeks.
Of the participants from the legacy studies, 99 completed 24 months of treatment with seladelpar, and 164 completed 12 months of treatment. In the 24-month treatment group, 70% met the composite response endpoint, which included alkaline phosphatase (ALP) levels below 1.67 times the upper limit of normal, a decrease in ALP levels of at least 15%, and total bilirubin levels at or below the upper limit of normal, according to a press release of the study findings. In addition, 42% of these participants achieved ALP normalization at 24 months, a marker of liver disease progression. In the 12-month treatment group, 73% achieved the clinically meaningful composite response endpoint, with 42% experiencing ALP normalization.
For patients rolled over from RESPONSE, 102 received 18 months of treatment with seladelpar, and 29 received 24 months of treatment. A total of 62% of patients in the 18-month group achieved the composite endpoint, and 33% achieved ALP normalization, while 72% of the 24-month group reached the composite endpoint, and 17% had ALP normalization.
Of patients who had received a placebo in the RESPONSE trial and went on to receive treatment with seladelpar, 75% achieved the composite endpoint, 27% had ALP normalization at 6 months, and 94% achieved the composite endpoint and 50% reached ALP normalization at 12 months.
Key secondary endpoints included ALP normalization and changes in liver enzymes (ALP, total bilirubin, gamma-glutamyl transferase [GGT], alanine transaminase [ALT], and aspartate aminotransferase [AST]).
Pruritus Relief Important for Quality of Life
Among study participants who reported a four or more at baseline on the numerical rating scale (NRS) for pruritus, legacy patients at 12 months and 24 months of treatment reported a mean reduction of 3.8 and 3.1, respectively. Participants from RESPONSE also reported a mean reduction of 3.8.
This level of reduction in NRS is “considered clinically significant” and takes patients from a level of moderate to severe itching down to mild, said Carrie Frenette, MD, executive director, Global Medical Affairs, Liver Diseases, Gilead Sciences, Foster City, California, and a former hepatologist of 20 years with a special interest in liver transplantation.
This “is a huge benefit in quality of life for these patients,” Dr. Frenette said in an interview.
Dr. Frenette also noted that UDCA, the current first-line treatment for PBC, is inadequate in up to 40% of patients, and second-line treatments, notably obeticholic acid, can cause itching.
Eleonora De Martin, MD, transplant hepatologist at Centre Hépato-Biliaire, Paul Brousse Hospital, Paris, France, who comoderated the session, pointed out that PBC is a complex disease.
“We need both disease control and symptom control, and they’re not always compatible,” she said. “Sometimes you can control the disease but not the symptoms, and symptomatic control is so important,” especially with pruritus.
Patients With PBC and Cirrhosis
A separate analysis from ASSURE looked at a subset of 17 patients with PBC and cirrhosis who completed 24 months of treatment. The findings were presented by Stuart Gordon, MD, professor of medicine, Wayne State University School of Medicine, and hepatologist at Henry Ford Hospital, both in Detroit.
In this analysis, the mean patient age was 60.8 years, 91.4% were female, 88.6% were Child-Pugh A, and 22.9% had portal hypertension, while the mean baseline liver stiffness by FibroScan was 19.9 kPa.
Baseline biochemical measures were mean ALP of 245.4 U/L, mean total bilirubin of 0.995 mg/dL, mean GGT of 216.1 U/L, and mean ALT of 36.6 U/L.
A total of 11 participants (65%) met the composite endpoint at 24 months, with ALP normalization in 4 patients (24%). The overall mean percent change from baseline in ALP was approximately −30% and in total bilirubin was around −14%. Other changes in biochemical markers included reductions from baseline in GGT and ALT of approximately −30% and −10%, respectively. No change was observed in AST.
While 80% of patients with cirrhosis “had an adverse event of some form,” there were no treatment-related serious adverse events.
“It’s interesting to see results in these patients who have advanced disease and are cirrhotic because it might stabilize disease or even provide improvement,” Dr. De Martin commented. “However, the numbers in the study are very small, so it’s hard to draw firm conclusions yet, but it is a first step in showing that this drug is safe.”
Seladelpar is an “important step forward in PBC because we’ve been stuck with ursodeoxycholic acid for so many years,” Dr. De Martin added. “We’ve seen in liver disease with other etiologies that sometimes just one drug can make a difference, and you can change the natural history of the disease.”
Dr. Frenette is an employee and stockholder of Gilead Sciences. Dr. Gordon declared grants and support from AbbVie, Arbutus, CymaBay, Cour Pharmaceuticals, GlaxoSmithKline (GSK), Ipsen, and Mirum Pharmaceuticals; and advisory board activity from CymaBay, GSK, and Ipsen Pharmaceuticals. Dr. De Martin had no disclosures of relevance to seladelpar but has received speaker fees from other companies, including GSK, Ipsen, and Astellas. Dr. Trivedi reports institutional funding support from National Institute for Health Research Birmingham (UK); lecture fees from Advanz Pharma/Intercept Pharmaceuticals, Albireo/Ipsen, and Dr. Falk Pharma; advisory board/consulting fees from Advanz Pharma/Intercept Pharmaceuticals, Albireo/Ipsen, Chemomab Therapeutics, CymaBay, Dr. Falk Pharma, Gilead Sciences, Perspectum, and Pliant Therapeutics; and grant support from Advanz Pharma/Intercept Pharmaceuticals, Albireo/Ipsen, Bristol-Myers Squibb, Core (Guts UK), EASL, Gilead Sciences, GSK, LifeArc, NIHR, Mirum Pharma, PSC Support, The Wellcome Trust, The Medical Research Foundation (UK), and Regeneron.
A version of this article first appeared on Medscape.com.
MILAN — according to two interim analyses of the ASSURE long-term extension study.
The first analysis of 337 patients with PBC, with and without cirrhosis, showed that treatment with seladelpar had a durable effect up to 2 years on cholestasis and markers of liver injury, as well as a sustained reduction in pruritus, Palak Trivedi, MD, associate professor at the National Institute for Health Research Birmingham Biomedical Research Centre, University of Birmingham, Birmingham, England, reported in a poster presented at the European Association for the Study of the Liver (EASL) Congress 2024.
The 2-year analysis also showed that seladelpar, a first-in-class, orally active agent, was safe and well tolerated in this patient population, he added.
These “results are consistent with the pivotal phase 3 RESPONSE study,” Dr. Trivedi noted. The RESPONSE study showed that seladelpar significantly improved liver biomarkers of disease activity and symptoms of pruritus at 12 months in patients with PBC who had an inadequate response or intolerance to ursodeoxycholic acid (UDCA), the standard of care, and had no history of hepatic decompensation. Patients with cirrhosis were allowed to enroll.
A total of 158 patients from the RESPONSE trial, both from the placebo and from the active treatment arm, were rolled over into the ASSURE trial. Another subset of 179 patients were drawn from prior seladelpar placebo-controlled studies (referred to as “legacy studies”), including the ENHANCE study. All participants in the current analysis received 10 mg of seladelpar, once daily, for up to 155 weeks.
Of the participants from the legacy studies, 99 completed 24 months of treatment with seladelpar, and 164 completed 12 months of treatment. In the 24-month treatment group, 70% met the composite response endpoint, which included alkaline phosphatase (ALP) levels below 1.67 times the upper limit of normal, a decrease in ALP levels of at least 15%, and total bilirubin levels at or below the upper limit of normal, according to a press release of the study findings. In addition, 42% of these participants achieved ALP normalization at 24 months, a marker of liver disease progression. In the 12-month treatment group, 73% achieved the clinically meaningful composite response endpoint, with 42% experiencing ALP normalization.
For patients rolled over from RESPONSE, 102 received 18 months of treatment with seladelpar, and 29 received 24 months of treatment. A total of 62% of patients in the 18-month group achieved the composite endpoint, and 33% achieved ALP normalization, while 72% of the 24-month group reached the composite endpoint, and 17% had ALP normalization.
Of patients who had received a placebo in the RESPONSE trial and went on to receive treatment with seladelpar, 75% achieved the composite endpoint, 27% had ALP normalization at 6 months, and 94% achieved the composite endpoint and 50% reached ALP normalization at 12 months.
Key secondary endpoints included ALP normalization and changes in liver enzymes (ALP, total bilirubin, gamma-glutamyl transferase [GGT], alanine transaminase [ALT], and aspartate aminotransferase [AST]).
Pruritus Relief Important for Quality of Life
Among study participants who reported a four or more at baseline on the numerical rating scale (NRS) for pruritus, legacy patients at 12 months and 24 months of treatment reported a mean reduction of 3.8 and 3.1, respectively. Participants from RESPONSE also reported a mean reduction of 3.8.
This level of reduction in NRS is “considered clinically significant” and takes patients from a level of moderate to severe itching down to mild, said Carrie Frenette, MD, executive director, Global Medical Affairs, Liver Diseases, Gilead Sciences, Foster City, California, and a former hepatologist of 20 years with a special interest in liver transplantation.
This “is a huge benefit in quality of life for these patients,” Dr. Frenette said in an interview.
Dr. Frenette also noted that UDCA, the current first-line treatment for PBC, is inadequate in up to 40% of patients, and second-line treatments, notably obeticholic acid, can cause itching.
Eleonora De Martin, MD, transplant hepatologist at Centre Hépato-Biliaire, Paul Brousse Hospital, Paris, France, who comoderated the session, pointed out that PBC is a complex disease.
“We need both disease control and symptom control, and they’re not always compatible,” she said. “Sometimes you can control the disease but not the symptoms, and symptomatic control is so important,” especially with pruritus.
Patients With PBC and Cirrhosis
A separate analysis from ASSURE looked at a subset of 17 patients with PBC and cirrhosis who completed 24 months of treatment. The findings were presented by Stuart Gordon, MD, professor of medicine, Wayne State University School of Medicine, and hepatologist at Henry Ford Hospital, both in Detroit.
In this analysis, the mean patient age was 60.8 years, 91.4% were female, 88.6% were Child-Pugh A, and 22.9% had portal hypertension, while the mean baseline liver stiffness by FibroScan was 19.9 kPa.
Baseline biochemical measures were mean ALP of 245.4 U/L, mean total bilirubin of 0.995 mg/dL, mean GGT of 216.1 U/L, and mean ALT of 36.6 U/L.
A total of 11 participants (65%) met the composite endpoint at 24 months, with ALP normalization in 4 patients (24%). The overall mean percent change from baseline in ALP was approximately −30% and in total bilirubin was around −14%. Other changes in biochemical markers included reductions from baseline in GGT and ALT of approximately −30% and −10%, respectively. No change was observed in AST.
While 80% of patients with cirrhosis “had an adverse event of some form,” there were no treatment-related serious adverse events.
“It’s interesting to see results in these patients who have advanced disease and are cirrhotic because it might stabilize disease or even provide improvement,” Dr. De Martin commented. “However, the numbers in the study are very small, so it’s hard to draw firm conclusions yet, but it is a first step in showing that this drug is safe.”
Seladelpar is an “important step forward in PBC because we’ve been stuck with ursodeoxycholic acid for so many years,” Dr. De Martin added. “We’ve seen in liver disease with other etiologies that sometimes just one drug can make a difference, and you can change the natural history of the disease.”
Dr. Frenette is an employee and stockholder of Gilead Sciences. Dr. Gordon declared grants and support from AbbVie, Arbutus, CymaBay, Cour Pharmaceuticals, GlaxoSmithKline (GSK), Ipsen, and Mirum Pharmaceuticals; and advisory board activity from CymaBay, GSK, and Ipsen Pharmaceuticals. Dr. De Martin had no disclosures of relevance to seladelpar but has received speaker fees from other companies, including GSK, Ipsen, and Astellas. Dr. Trivedi reports institutional funding support from National Institute for Health Research Birmingham (UK); lecture fees from Advanz Pharma/Intercept Pharmaceuticals, Albireo/Ipsen, and Dr. Falk Pharma; advisory board/consulting fees from Advanz Pharma/Intercept Pharmaceuticals, Albireo/Ipsen, Chemomab Therapeutics, CymaBay, Dr. Falk Pharma, Gilead Sciences, Perspectum, and Pliant Therapeutics; and grant support from Advanz Pharma/Intercept Pharmaceuticals, Albireo/Ipsen, Bristol-Myers Squibb, Core (Guts UK), EASL, Gilead Sciences, GSK, LifeArc, NIHR, Mirum Pharma, PSC Support, The Wellcome Trust, The Medical Research Foundation (UK), and Regeneron.
A version of this article first appeared on Medscape.com.
FROM EASL 2024
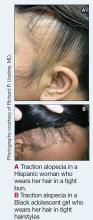
Act Fast With Traction Alopecia to Avoid Permanent Hair Loss
Traction alopecia (TA) is a common type of alopecia that ultimately can result in permanent hair loss. It often is caused or worsened by repetitive and prolonged hairstyling practices such as tight ponytails, braids, or locs, or use of wigs or weaves.1 Use of headwear, as in certain religious or ethnic groups, also can be contributory.2 Individuals participating in or training for occupations involving military service or ballet are at risk for TA due to hairstyling-specific policies. Early stages of TA are reversible with proper treatment and avoidance of exacerbating factors, emphasizing the importance of prompt recognition.3
Epidemiology
Data on the true prevalence of TA are lacking. It can occur in individuals of any race or any hair type. However, it is most common in women of African descent, affecting approximately one-third of this population.4 Other commonly affected groups include ballerinas and active-duty service members due to tight ponytails and buns, as well as the Sikh population due to the use of turbans as a part of their religious practice.2,5,6
Traction alopecia also impacts children, particularly those of African descent. A 2007 study of schoolchildren in South Africa determined that more than 17% of young African girls had evidence of TA—even some as young as 6 years of age.7
Traction alopecia can be caused or exacerbated by the use of hair clips and bobby pins that aid holding styles in place.8 Hair shaft morphology may contribute to the risk for TA, with more tightly coiled hair types being more susceptible.8 Variables such as use of chemical relaxers also increase the risk for disease, especially when combined with high-tension styling methods such as braids.9
Key clinical features
Patients with TA clinically present with hair loss and breakage in areas with tension, most commonly the marginal areas of the scalp as well as the frontal hairline and temporal scalp. Hair loss can result in a “fringe sign,” in which a patient may have preservation of a thin line of hairs at the frontal aspect of the hairline with a band of hair loss behind.10 This presentation may be used to differentiate TA from other forms of alopecia, including frontal fibrosing alopecia and female pattern hair loss. When the hair loss is not marginal, it may mimic other forms of patchy hair loss including alopecia areata and trichotillomania. Other clinical findings in TA may include broken hairs, pustules, and follicular papules.10 Patients also may describe symptoms such as scalp tenderness with specific hairstyles or headaches,11 or they may be completely asymptomatic.
Trichoscopy can be helpful in guiding diagnosis and treatment. Patients with TA often have perifollicular erythema and hair casts (cylindrical structures that encircle the proximal hair shafts) in the earlier stages of the disease, with eventual loss of follicular ostia in the later stages.10,12 Hair casts also may indicate ongoing traction.12 The flambeau sign—white tracks seen on trichoscopy in the direction the hair is pulled—resembles a lit torch.13
Worth noting
Early-stage TA can be reversed by avoiding hair tension. However, patients may not be amenable to this due to personal hairstyling preferences, job duties, or religious practices. Treatment with topical or intralesional steroids or even oral antibiotics such as doxycycline for its anti-inflammatory ability may result in regrowth of lost hair if the follicles are not permanently lost and exacerbating factors are avoided.3,14 Both topical and oral minoxidil have been used with success, with minoxidil thought to increase hair density by extending the anagen (growth) phase of hair follicles.3,15 Culturally sensitive patient counseling on the condition and potential exacerbating factors is critical.16
At later stages of the disease—after loss of follicular ostia has occurred—surgical interventions should be considered,17 such as hair transplantation, which can be successful but remains a technical challenge due to variability in hair shaft curvature.18 Additionally, the cost of the procedure can limit use, and some patients may not be optimal candidates due to the extent of their hair loss. Traction alopecia may not be the only hair loss condition present. Examining the scalp is important even if the chief area of concern is the marginal scalp.
Health disparity highlight
Prevention, early identification, and treatment initiated in a timely fashion are crucial to prevent permanent hair loss. There are added societal and cultural pressures that impact hairstyle and hair care practices, especially for those with tightly coiled hair.19 Historically, tightly coiled hair has been unfairly viewed as “unprofessional,” “unkempt,” and a challenge to “manage” by some. Thus, heat, chemical relaxers, and tight hairstyles holding hair in one position have been used to straighten the hair permanently or temporarily or to keep it maintained in a style that did not necessitate excessive manipulation—often contributing to further tension on the hair.
Military service branches have evaluated and changed some hair-related policies to reflect the diverse hair types of military personnel.20 The CROWN Act (www.thecrownact.com/about)—“Creating a Respectful and Open World for Natural Hair”—is a model law passed by 26 states that prohibits race-based hair discrimination, which is the denial of employment and educational opportunities because of hair texture. Although the law has not been passed in every state, it may help individuals with tightly coiled hair to embrace natural hairstyles. However, even hairstyles with one’s own natural curl pattern can contribute to tension and thus potential development of TA.
1. Larrondo J, McMichael AJ. Traction alopecia. JAMA Dermatol. 2023;159:676. doi:10.1001/jamadermatol.2022.6298
2. James J, Saladi RN, Fox JL. Traction alopecia in Sikh male patients. J Am Board Fam Med. 2007;20:497-498. doi:10.3122/jabfm.2007.05.070076
3. Callender VD, McMichael AJ, Cohen GF. Medical and surgical therapies for alopecias in black women. Dermatol Ther. 2004;17:164-176.
4. Loussouarn G, El Rawadi C, Genain G. Diversity of hair growth profiles. Int J Dermatol. 2005;44(suppl 1):6-9.
5. Samrao AChen CZedek Det al. Traction alopecia in a ballerina: clinicopathologic features. Arch Dermatol. 2010;146:918-935. doi:10.1001/archdermatol.2010.183
6. Korona-Bailey J, Banaag A, Nguyen DR, et al. Free the bun: prevalence of alopecia among active duty service women, fiscal years 2010-2019. Mil Med. 2023;188:e492-e496. doi:10.1093/milmed/usab274
7. Khumalo NP, Jessop S, Gumedze F, et al. Hairdressing is associated with scalp disease in African schoolchildren. Br J Dermatol. 2007;157:106-110. doi:10.1111/j.1365-2133.2007.07987.x
8. Billero V, Miteva M. Traction alopecia: the root of the problem. Clin Cosmet Investig Dermatol. 2018;11:149-159. doi:10.2147/CCID.S137296
9. Haskin A, Aguh C. All hairstyles are not created equal: what the dermatologist needs to know about black hairstyling practices and the risk of traction alopecia (TA). J Am Acad Dermatol. 2016;75:606-611. doi:10.1016/j.jaad.2016.02.1162
10. Samrao A, Price VH, Zedek D, et al. The “fringe sign”—a useful clinical finding in traction alopecia of the marginal hair line. Dermatol Online J. 2011;17:1.
11. Kararizou E, Bougea AM, Giotopoulou D, et al. An update on the less-known group of other primary headaches—a review. Eur Neurol Rev. 2014;9:71-77. doi:10.17925/ENR.2014.09.01.71
12. Tosti A, Miteva M, Torres F, et al. Hair casts are a dermoscopic clue for the diagnosis of traction alopecia. Br J Dermatol. 2010;163:1353-1355.
13. Agrawal S, Daruwalla SB, Dhurat RS. The flambeau sign—a new dermoscopy finding in a case of marginal traction alopecia. Australas J Dermatol. 2020;61:49-50. doi:10.1111/ajd.13187
14. Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Womens Dermatol. 2017;3:S21-S37.
15. Awad A, Chim I, Sharma P, et al. Low-dose oral minoxidil improves hair density in traction alopecia. J Am Acad Dermatol. 2023;89:157-159. doi:10.1016/j.jaad.2023.02.024
16. Grayson C, Heath CR. Counseling about traction alopecia: a “compliment, discuss, and suggest” method. Cutis. 2021;108:20-22.
17. Ozçelik D. Extensive traction alopecia attributable to ponytail hairstyle and its treatment with hair transplantation. Aesthetic Plast Surg. 2005;29:325-327. doi:10.1007/s00266-005-0004-5
18. Singh MK, Avram MR. Technical considerations for follicular unit extraction in African-American hair. Dermatol Surg. 2013;39:1282-1284. doi:10.1111/dsu.12229
19. Jones NL, Heath CR. Hair at the intersection of dermatology and anthropology: a conversation on race and relationships. Pediatr Dermatol. 2021;38(suppl 2):158-160.
20. Franklin JMM, Wohltmann WE, Wong EB. From buns to braids and ponytails: entering a new era of female military hair-grooming standards. Cutis. 2021;108:31-35. doi:10.12788/cutis.0296
Traction alopecia (TA) is a common type of alopecia that ultimately can result in permanent hair loss. It often is caused or worsened by repetitive and prolonged hairstyling practices such as tight ponytails, braids, or locs, or use of wigs or weaves.1 Use of headwear, as in certain religious or ethnic groups, also can be contributory.2 Individuals participating in or training for occupations involving military service or ballet are at risk for TA due to hairstyling-specific policies. Early stages of TA are reversible with proper treatment and avoidance of exacerbating factors, emphasizing the importance of prompt recognition.3
Epidemiology
Data on the true prevalence of TA are lacking. It can occur in individuals of any race or any hair type. However, it is most common in women of African descent, affecting approximately one-third of this population.4 Other commonly affected groups include ballerinas and active-duty service members due to tight ponytails and buns, as well as the Sikh population due to the use of turbans as a part of their religious practice.2,5,6
Traction alopecia also impacts children, particularly those of African descent. A 2007 study of schoolchildren in South Africa determined that more than 17% of young African girls had evidence of TA—even some as young as 6 years of age.7
Traction alopecia can be caused or exacerbated by the use of hair clips and bobby pins that aid holding styles in place.8 Hair shaft morphology may contribute to the risk for TA, with more tightly coiled hair types being more susceptible.8 Variables such as use of chemical relaxers also increase the risk for disease, especially when combined with high-tension styling methods such as braids.9
Key clinical features
Patients with TA clinically present with hair loss and breakage in areas with tension, most commonly the marginal areas of the scalp as well as the frontal hairline and temporal scalp. Hair loss can result in a “fringe sign,” in which a patient may have preservation of a thin line of hairs at the frontal aspect of the hairline with a band of hair loss behind.10 This presentation may be used to differentiate TA from other forms of alopecia, including frontal fibrosing alopecia and female pattern hair loss. When the hair loss is not marginal, it may mimic other forms of patchy hair loss including alopecia areata and trichotillomania. Other clinical findings in TA may include broken hairs, pustules, and follicular papules.10 Patients also may describe symptoms such as scalp tenderness with specific hairstyles or headaches,11 or they may be completely asymptomatic.
Trichoscopy can be helpful in guiding diagnosis and treatment. Patients with TA often have perifollicular erythema and hair casts (cylindrical structures that encircle the proximal hair shafts) in the earlier stages of the disease, with eventual loss of follicular ostia in the later stages.10,12 Hair casts also may indicate ongoing traction.12 The flambeau sign—white tracks seen on trichoscopy in the direction the hair is pulled—resembles a lit torch.13
Worth noting
Early-stage TA can be reversed by avoiding hair tension. However, patients may not be amenable to this due to personal hairstyling preferences, job duties, or religious practices. Treatment with topical or intralesional steroids or even oral antibiotics such as doxycycline for its anti-inflammatory ability may result in regrowth of lost hair if the follicles are not permanently lost and exacerbating factors are avoided.3,14 Both topical and oral minoxidil have been used with success, with minoxidil thought to increase hair density by extending the anagen (growth) phase of hair follicles.3,15 Culturally sensitive patient counseling on the condition and potential exacerbating factors is critical.16
At later stages of the disease—after loss of follicular ostia has occurred—surgical interventions should be considered,17 such as hair transplantation, which can be successful but remains a technical challenge due to variability in hair shaft curvature.18 Additionally, the cost of the procedure can limit use, and some patients may not be optimal candidates due to the extent of their hair loss. Traction alopecia may not be the only hair loss condition present. Examining the scalp is important even if the chief area of concern is the marginal scalp.
Health disparity highlight
Prevention, early identification, and treatment initiated in a timely fashion are crucial to prevent permanent hair loss. There are added societal and cultural pressures that impact hairstyle and hair care practices, especially for those with tightly coiled hair.19 Historically, tightly coiled hair has been unfairly viewed as “unprofessional,” “unkempt,” and a challenge to “manage” by some. Thus, heat, chemical relaxers, and tight hairstyles holding hair in one position have been used to straighten the hair permanently or temporarily or to keep it maintained in a style that did not necessitate excessive manipulation—often contributing to further tension on the hair.
Military service branches have evaluated and changed some hair-related policies to reflect the diverse hair types of military personnel.20 The CROWN Act (www.thecrownact.com/about)—“Creating a Respectful and Open World for Natural Hair”—is a model law passed by 26 states that prohibits race-based hair discrimination, which is the denial of employment and educational opportunities because of hair texture. Although the law has not been passed in every state, it may help individuals with tightly coiled hair to embrace natural hairstyles. However, even hairstyles with one’s own natural curl pattern can contribute to tension and thus potential development of TA.
Traction alopecia (TA) is a common type of alopecia that ultimately can result in permanent hair loss. It often is caused or worsened by repetitive and prolonged hairstyling practices such as tight ponytails, braids, or locs, or use of wigs or weaves.1 Use of headwear, as in certain religious or ethnic groups, also can be contributory.2 Individuals participating in or training for occupations involving military service or ballet are at risk for TA due to hairstyling-specific policies. Early stages of TA are reversible with proper treatment and avoidance of exacerbating factors, emphasizing the importance of prompt recognition.3
Epidemiology
Data on the true prevalence of TA are lacking. It can occur in individuals of any race or any hair type. However, it is most common in women of African descent, affecting approximately one-third of this population.4 Other commonly affected groups include ballerinas and active-duty service members due to tight ponytails and buns, as well as the Sikh population due to the use of turbans as a part of their religious practice.2,5,6
Traction alopecia also impacts children, particularly those of African descent. A 2007 study of schoolchildren in South Africa determined that more than 17% of young African girls had evidence of TA—even some as young as 6 years of age.7
Traction alopecia can be caused or exacerbated by the use of hair clips and bobby pins that aid holding styles in place.8 Hair shaft morphology may contribute to the risk for TA, with more tightly coiled hair types being more susceptible.8 Variables such as use of chemical relaxers also increase the risk for disease, especially when combined with high-tension styling methods such as braids.9
Key clinical features
Patients with TA clinically present with hair loss and breakage in areas with tension, most commonly the marginal areas of the scalp as well as the frontal hairline and temporal scalp. Hair loss can result in a “fringe sign,” in which a patient may have preservation of a thin line of hairs at the frontal aspect of the hairline with a band of hair loss behind.10 This presentation may be used to differentiate TA from other forms of alopecia, including frontal fibrosing alopecia and female pattern hair loss. When the hair loss is not marginal, it may mimic other forms of patchy hair loss including alopecia areata and trichotillomania. Other clinical findings in TA may include broken hairs, pustules, and follicular papules.10 Patients also may describe symptoms such as scalp tenderness with specific hairstyles or headaches,11 or they may be completely asymptomatic.
Trichoscopy can be helpful in guiding diagnosis and treatment. Patients with TA often have perifollicular erythema and hair casts (cylindrical structures that encircle the proximal hair shafts) in the earlier stages of the disease, with eventual loss of follicular ostia in the later stages.10,12 Hair casts also may indicate ongoing traction.12 The flambeau sign—white tracks seen on trichoscopy in the direction the hair is pulled—resembles a lit torch.13
Worth noting
Early-stage TA can be reversed by avoiding hair tension. However, patients may not be amenable to this due to personal hairstyling preferences, job duties, or religious practices. Treatment with topical or intralesional steroids or even oral antibiotics such as doxycycline for its anti-inflammatory ability may result in regrowth of lost hair if the follicles are not permanently lost and exacerbating factors are avoided.3,14 Both topical and oral minoxidil have been used with success, with minoxidil thought to increase hair density by extending the anagen (growth) phase of hair follicles.3,15 Culturally sensitive patient counseling on the condition and potential exacerbating factors is critical.16
At later stages of the disease—after loss of follicular ostia has occurred—surgical interventions should be considered,17 such as hair transplantation, which can be successful but remains a technical challenge due to variability in hair shaft curvature.18 Additionally, the cost of the procedure can limit use, and some patients may not be optimal candidates due to the extent of their hair loss. Traction alopecia may not be the only hair loss condition present. Examining the scalp is important even if the chief area of concern is the marginal scalp.
Health disparity highlight
Prevention, early identification, and treatment initiated in a timely fashion are crucial to prevent permanent hair loss. There are added societal and cultural pressures that impact hairstyle and hair care practices, especially for those with tightly coiled hair.19 Historically, tightly coiled hair has been unfairly viewed as “unprofessional,” “unkempt,” and a challenge to “manage” by some. Thus, heat, chemical relaxers, and tight hairstyles holding hair in one position have been used to straighten the hair permanently or temporarily or to keep it maintained in a style that did not necessitate excessive manipulation—often contributing to further tension on the hair.
Military service branches have evaluated and changed some hair-related policies to reflect the diverse hair types of military personnel.20 The CROWN Act (www.thecrownact.com/about)—“Creating a Respectful and Open World for Natural Hair”—is a model law passed by 26 states that prohibits race-based hair discrimination, which is the denial of employment and educational opportunities because of hair texture. Although the law has not been passed in every state, it may help individuals with tightly coiled hair to embrace natural hairstyles. However, even hairstyles with one’s own natural curl pattern can contribute to tension and thus potential development of TA.
1. Larrondo J, McMichael AJ. Traction alopecia. JAMA Dermatol. 2023;159:676. doi:10.1001/jamadermatol.2022.6298
2. James J, Saladi RN, Fox JL. Traction alopecia in Sikh male patients. J Am Board Fam Med. 2007;20:497-498. doi:10.3122/jabfm.2007.05.070076
3. Callender VD, McMichael AJ, Cohen GF. Medical and surgical therapies for alopecias in black women. Dermatol Ther. 2004;17:164-176.
4. Loussouarn G, El Rawadi C, Genain G. Diversity of hair growth profiles. Int J Dermatol. 2005;44(suppl 1):6-9.
5. Samrao AChen CZedek Det al. Traction alopecia in a ballerina: clinicopathologic features. Arch Dermatol. 2010;146:918-935. doi:10.1001/archdermatol.2010.183
6. Korona-Bailey J, Banaag A, Nguyen DR, et al. Free the bun: prevalence of alopecia among active duty service women, fiscal years 2010-2019. Mil Med. 2023;188:e492-e496. doi:10.1093/milmed/usab274
7. Khumalo NP, Jessop S, Gumedze F, et al. Hairdressing is associated with scalp disease in African schoolchildren. Br J Dermatol. 2007;157:106-110. doi:10.1111/j.1365-2133.2007.07987.x
8. Billero V, Miteva M. Traction alopecia: the root of the problem. Clin Cosmet Investig Dermatol. 2018;11:149-159. doi:10.2147/CCID.S137296
9. Haskin A, Aguh C. All hairstyles are not created equal: what the dermatologist needs to know about black hairstyling practices and the risk of traction alopecia (TA). J Am Acad Dermatol. 2016;75:606-611. doi:10.1016/j.jaad.2016.02.1162
10. Samrao A, Price VH, Zedek D, et al. The “fringe sign”—a useful clinical finding in traction alopecia of the marginal hair line. Dermatol Online J. 2011;17:1.
11. Kararizou E, Bougea AM, Giotopoulou D, et al. An update on the less-known group of other primary headaches—a review. Eur Neurol Rev. 2014;9:71-77. doi:10.17925/ENR.2014.09.01.71
12. Tosti A, Miteva M, Torres F, et al. Hair casts are a dermoscopic clue for the diagnosis of traction alopecia. Br J Dermatol. 2010;163:1353-1355.
13. Agrawal S, Daruwalla SB, Dhurat RS. The flambeau sign—a new dermoscopy finding in a case of marginal traction alopecia. Australas J Dermatol. 2020;61:49-50. doi:10.1111/ajd.13187
14. Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Womens Dermatol. 2017;3:S21-S37.
15. Awad A, Chim I, Sharma P, et al. Low-dose oral minoxidil improves hair density in traction alopecia. J Am Acad Dermatol. 2023;89:157-159. doi:10.1016/j.jaad.2023.02.024
16. Grayson C, Heath CR. Counseling about traction alopecia: a “compliment, discuss, and suggest” method. Cutis. 2021;108:20-22.
17. Ozçelik D. Extensive traction alopecia attributable to ponytail hairstyle and its treatment with hair transplantation. Aesthetic Plast Surg. 2005;29:325-327. doi:10.1007/s00266-005-0004-5
18. Singh MK, Avram MR. Technical considerations for follicular unit extraction in African-American hair. Dermatol Surg. 2013;39:1282-1284. doi:10.1111/dsu.12229
19. Jones NL, Heath CR. Hair at the intersection of dermatology and anthropology: a conversation on race and relationships. Pediatr Dermatol. 2021;38(suppl 2):158-160.
20. Franklin JMM, Wohltmann WE, Wong EB. From buns to braids and ponytails: entering a new era of female military hair-grooming standards. Cutis. 2021;108:31-35. doi:10.12788/cutis.0296
1. Larrondo J, McMichael AJ. Traction alopecia. JAMA Dermatol. 2023;159:676. doi:10.1001/jamadermatol.2022.6298
2. James J, Saladi RN, Fox JL. Traction alopecia in Sikh male patients. J Am Board Fam Med. 2007;20:497-498. doi:10.3122/jabfm.2007.05.070076
3. Callender VD, McMichael AJ, Cohen GF. Medical and surgical therapies for alopecias in black women. Dermatol Ther. 2004;17:164-176.
4. Loussouarn G, El Rawadi C, Genain G. Diversity of hair growth profiles. Int J Dermatol. 2005;44(suppl 1):6-9.
5. Samrao AChen CZedek Det al. Traction alopecia in a ballerina: clinicopathologic features. Arch Dermatol. 2010;146:918-935. doi:10.1001/archdermatol.2010.183
6. Korona-Bailey J, Banaag A, Nguyen DR, et al. Free the bun: prevalence of alopecia among active duty service women, fiscal years 2010-2019. Mil Med. 2023;188:e492-e496. doi:10.1093/milmed/usab274
7. Khumalo NP, Jessop S, Gumedze F, et al. Hairdressing is associated with scalp disease in African schoolchildren. Br J Dermatol. 2007;157:106-110. doi:10.1111/j.1365-2133.2007.07987.x
8. Billero V, Miteva M. Traction alopecia: the root of the problem. Clin Cosmet Investig Dermatol. 2018;11:149-159. doi:10.2147/CCID.S137296
9. Haskin A, Aguh C. All hairstyles are not created equal: what the dermatologist needs to know about black hairstyling practices and the risk of traction alopecia (TA). J Am Acad Dermatol. 2016;75:606-611. doi:10.1016/j.jaad.2016.02.1162
10. Samrao A, Price VH, Zedek D, et al. The “fringe sign”—a useful clinical finding in traction alopecia of the marginal hair line. Dermatol Online J. 2011;17:1.
11. Kararizou E, Bougea AM, Giotopoulou D, et al. An update on the less-known group of other primary headaches—a review. Eur Neurol Rev. 2014;9:71-77. doi:10.17925/ENR.2014.09.01.71
12. Tosti A, Miteva M, Torres F, et al. Hair casts are a dermoscopic clue for the diagnosis of traction alopecia. Br J Dermatol. 2010;163:1353-1355.
13. Agrawal S, Daruwalla SB, Dhurat RS. The flambeau sign—a new dermoscopy finding in a case of marginal traction alopecia. Australas J Dermatol. 2020;61:49-50. doi:10.1111/ajd.13187
14. Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Womens Dermatol. 2017;3:S21-S37.
15. Awad A, Chim I, Sharma P, et al. Low-dose oral minoxidil improves hair density in traction alopecia. J Am Acad Dermatol. 2023;89:157-159. doi:10.1016/j.jaad.2023.02.024
16. Grayson C, Heath CR. Counseling about traction alopecia: a “compliment, discuss, and suggest” method. Cutis. 2021;108:20-22.
17. Ozçelik D. Extensive traction alopecia attributable to ponytail hairstyle and its treatment with hair transplantation. Aesthetic Plast Surg. 2005;29:325-327. doi:10.1007/s00266-005-0004-5
18. Singh MK, Avram MR. Technical considerations for follicular unit extraction in African-American hair. Dermatol Surg. 2013;39:1282-1284. doi:10.1111/dsu.12229
19. Jones NL, Heath CR. Hair at the intersection of dermatology and anthropology: a conversation on race and relationships. Pediatr Dermatol. 2021;38(suppl 2):158-160.
20. Franklin JMM, Wohltmann WE, Wong EB. From buns to braids and ponytails: entering a new era of female military hair-grooming standards. Cutis. 2021;108:31-35. doi:10.12788/cutis.0296
In Prostate Cancer, Most Roads Lead to VA Pathway
The newly updated US Department of Veterans Affairs (VA) prostate cancer clinical pathway looks like a set of guidelines, but it’s really something unique. As attendees learned at an Association of VA Hematology/Oncology (AVAHO) regional meeting in Detroit in June, the clinical pathways are designed to point the way toward a standard ideal treatment for the majority of cases, not just to suggest a number of possible options.
“Pathways will always offer one scenario. They try to get oncologists to practice in a similar fashion so things can be managed more uniformly,” Michael M. Goodman, MD, told Federal Practitioner prior to the AVAHO meeting that was focused on prostate cancer care. Goodman is an associate professor of medicine with Atrium Health Wake Forest Baptist Medical Center and helped develop the VA genitourinary oncology pathways.
“The overall goal is not just to standardize care as much as possible but also to synthesize the best and most cost-effective practices,” Goodman said. For example, “If you have 5 different therapies, and they all have about the same efficacy and safety, and 1 is less costly than the other 4, then it would make sense to choose that.”
The VA has offered pathways for multiple types of cancer since 2021, and the pathway for prostate cancer is among the most comprehensive. The VA system updated the pathway in March 2024, is available online both via SharePoint and externally.
“It goes through the entire gamut from screening, diagnosis, and management to end of life,” Goodman explained. Multiple disciplines, from primary care and surgery to genetics and imaging, can rely on the pathway to assist decision-making.
In terms of screening, the pathway offers a flow map guiding the screening choices. In patients aged ≤ 54 years, only certain high-risk groups, such as African Americans and those with a family history of prostate cancer, should be screened. From ages 54 to 69 years, patients should be consulted as part of a shared decision making process, while screening is not recommended for patients aged ≥ 70 years.
Pathway flow maps also provide information about diagnostic standards, evaluation of the newly diagnosed, risk stratification, molecular testing, and end-of-life care.
Goodman says the pathway is now integrated into the VA electronic health record system via a template so clinicians can easily document pathway use. This allows the VA to track the use of the pathways locally, regionally, and nationally track the use of the pathways.
Clinicians are not mandated to follow every step in the pathway, but Goodman said the goal is > 80% adherence. If clinicians follow the standards, he said, “you’re considering efficacy, safety, and cost for that veteran.”
Prospective data suggests that adherence to the pathway eliminates certain disparities. African American veterans, for example, are as well-represented or even better represented than White veterans in prostate cancer care when pathways are followed.
Why might clinicians veer from the pathway? “If you’re seeing a patient who was treated in the community with drug X, but drug Y is chosen by the pathway, you can carry on with the previous care.” Alternatively, in some cases, patients may not tolerate the pathway standard, Goodman noted.
Goodman reports that he consults the pathway every day. “It’s helped standardize the care I provide to ensure there’s no gaps in how I’m treating patients.”
The newly updated US Department of Veterans Affairs (VA) prostate cancer clinical pathway looks like a set of guidelines, but it’s really something unique. As attendees learned at an Association of VA Hematology/Oncology (AVAHO) regional meeting in Detroit in June, the clinical pathways are designed to point the way toward a standard ideal treatment for the majority of cases, not just to suggest a number of possible options.
“Pathways will always offer one scenario. They try to get oncologists to practice in a similar fashion so things can be managed more uniformly,” Michael M. Goodman, MD, told Federal Practitioner prior to the AVAHO meeting that was focused on prostate cancer care. Goodman is an associate professor of medicine with Atrium Health Wake Forest Baptist Medical Center and helped develop the VA genitourinary oncology pathways.
“The overall goal is not just to standardize care as much as possible but also to synthesize the best and most cost-effective practices,” Goodman said. For example, “If you have 5 different therapies, and they all have about the same efficacy and safety, and 1 is less costly than the other 4, then it would make sense to choose that.”
The VA has offered pathways for multiple types of cancer since 2021, and the pathway for prostate cancer is among the most comprehensive. The VA system updated the pathway in March 2024, is available online both via SharePoint and externally.
“It goes through the entire gamut from screening, diagnosis, and management to end of life,” Goodman explained. Multiple disciplines, from primary care and surgery to genetics and imaging, can rely on the pathway to assist decision-making.
In terms of screening, the pathway offers a flow map guiding the screening choices. In patients aged ≤ 54 years, only certain high-risk groups, such as African Americans and those with a family history of prostate cancer, should be screened. From ages 54 to 69 years, patients should be consulted as part of a shared decision making process, while screening is not recommended for patients aged ≥ 70 years.
Pathway flow maps also provide information about diagnostic standards, evaluation of the newly diagnosed, risk stratification, molecular testing, and end-of-life care.
Goodman says the pathway is now integrated into the VA electronic health record system via a template so clinicians can easily document pathway use. This allows the VA to track the use of the pathways locally, regionally, and nationally track the use of the pathways.
Clinicians are not mandated to follow every step in the pathway, but Goodman said the goal is > 80% adherence. If clinicians follow the standards, he said, “you’re considering efficacy, safety, and cost for that veteran.”
Prospective data suggests that adherence to the pathway eliminates certain disparities. African American veterans, for example, are as well-represented or even better represented than White veterans in prostate cancer care when pathways are followed.
Why might clinicians veer from the pathway? “If you’re seeing a patient who was treated in the community with drug X, but drug Y is chosen by the pathway, you can carry on with the previous care.” Alternatively, in some cases, patients may not tolerate the pathway standard, Goodman noted.
Goodman reports that he consults the pathway every day. “It’s helped standardize the care I provide to ensure there’s no gaps in how I’m treating patients.”
The newly updated US Department of Veterans Affairs (VA) prostate cancer clinical pathway looks like a set of guidelines, but it’s really something unique. As attendees learned at an Association of VA Hematology/Oncology (AVAHO) regional meeting in Detroit in June, the clinical pathways are designed to point the way toward a standard ideal treatment for the majority of cases, not just to suggest a number of possible options.
“Pathways will always offer one scenario. They try to get oncologists to practice in a similar fashion so things can be managed more uniformly,” Michael M. Goodman, MD, told Federal Practitioner prior to the AVAHO meeting that was focused on prostate cancer care. Goodman is an associate professor of medicine with Atrium Health Wake Forest Baptist Medical Center and helped develop the VA genitourinary oncology pathways.
“The overall goal is not just to standardize care as much as possible but also to synthesize the best and most cost-effective practices,” Goodman said. For example, “If you have 5 different therapies, and they all have about the same efficacy and safety, and 1 is less costly than the other 4, then it would make sense to choose that.”
The VA has offered pathways for multiple types of cancer since 2021, and the pathway for prostate cancer is among the most comprehensive. The VA system updated the pathway in March 2024, is available online both via SharePoint and externally.
“It goes through the entire gamut from screening, diagnosis, and management to end of life,” Goodman explained. Multiple disciplines, from primary care and surgery to genetics and imaging, can rely on the pathway to assist decision-making.
In terms of screening, the pathway offers a flow map guiding the screening choices. In patients aged ≤ 54 years, only certain high-risk groups, such as African Americans and those with a family history of prostate cancer, should be screened. From ages 54 to 69 years, patients should be consulted as part of a shared decision making process, while screening is not recommended for patients aged ≥ 70 years.
Pathway flow maps also provide information about diagnostic standards, evaluation of the newly diagnosed, risk stratification, molecular testing, and end-of-life care.
Goodman says the pathway is now integrated into the VA electronic health record system via a template so clinicians can easily document pathway use. This allows the VA to track the use of the pathways locally, regionally, and nationally track the use of the pathways.
Clinicians are not mandated to follow every step in the pathway, but Goodman said the goal is > 80% adherence. If clinicians follow the standards, he said, “you’re considering efficacy, safety, and cost for that veteran.”
Prospective data suggests that adherence to the pathway eliminates certain disparities. African American veterans, for example, are as well-represented or even better represented than White veterans in prostate cancer care when pathways are followed.
Why might clinicians veer from the pathway? “If you’re seeing a patient who was treated in the community with drug X, but drug Y is chosen by the pathway, you can carry on with the previous care.” Alternatively, in some cases, patients may not tolerate the pathway standard, Goodman noted.
Goodman reports that he consults the pathway every day. “It’s helped standardize the care I provide to ensure there’s no gaps in how I’m treating patients.”
Fit for Promotion: Navy Changes the Policy
Time was—recent time, that is—sailors had two chances to pass a physical fitness assessment (PFA). Failing the first meant no promotion. Failing the second: No career. They could neither be promoted nor reenlist.
That’s changed; as of this month, the Navy now allows the sailor’s commanding officer to decide whether the sailor gets to go on, even after failing a second test.
In an administrative letter, Vice Adm. Rick Cheeseman, chief of naval personnel, said, "Commanding officers can now evaluate a sailor's physical readiness progress or lack of progress in performance evaluations, giving them the ability to manage risk, recognize earnest effort, and best take care of their people.”
According to the new policy, sailors who fail any PFA no longer need to have it noted on their annual evaluation (although they still may not advance until they pass another test). Enlisted sailors who fail a second consecutive PFA are no longer required to receive the lowest possible score in the "Military Bearing/Professionalism" category and are not denied the ability to reenlist.
In assessing eligibility for enlisted members, the memo states that commanders should consider a sailor’s ability to perform the functions of their rate without physical or medical limitation at sea, shore or isolated duty; their overall ability to contribute to Navy missions; and the likelihood of improvement in meeting PFA standards within the next 12 months.
“Building the bodies of great people,” Cheeseman wrote, “is more than annual (or biannual) testing and includes ensuring healthy food, adequate sleep, opportunities to exercise (especially outside), and medical readiness.”
According to a report by Military.com, “critics have argued that many of the changes were the Navy relaxing its standards in the face of a challenging recruiting environment and an increasingly overweight population of Americans.” However, Navy data provided in November indicate that the number of sailors failing PFAs has remained very low. In 2017, nearly 98% of sailors passed the PFA, and 95.1% passed the first post-pandemic PFA in 2022.
The PFA policy changes are part of the Navy’s Culture of Excellence 2.0, initiated earlier this year, Cheeseman says. This initiative “charges our leaders to build great people, great leaders, and great teams: their minds, bodies, and spirits, eliminating barriers wherever possible. In response, we are modernizing our PFA policy to acknowledge our diverse population, increase sailor trust, and enhance quality of service.”
Time was—recent time, that is—sailors had two chances to pass a physical fitness assessment (PFA). Failing the first meant no promotion. Failing the second: No career. They could neither be promoted nor reenlist.
That’s changed; as of this month, the Navy now allows the sailor’s commanding officer to decide whether the sailor gets to go on, even after failing a second test.
In an administrative letter, Vice Adm. Rick Cheeseman, chief of naval personnel, said, "Commanding officers can now evaluate a sailor's physical readiness progress or lack of progress in performance evaluations, giving them the ability to manage risk, recognize earnest effort, and best take care of their people.”
According to the new policy, sailors who fail any PFA no longer need to have it noted on their annual evaluation (although they still may not advance until they pass another test). Enlisted sailors who fail a second consecutive PFA are no longer required to receive the lowest possible score in the "Military Bearing/Professionalism" category and are not denied the ability to reenlist.
In assessing eligibility for enlisted members, the memo states that commanders should consider a sailor’s ability to perform the functions of their rate without physical or medical limitation at sea, shore or isolated duty; their overall ability to contribute to Navy missions; and the likelihood of improvement in meeting PFA standards within the next 12 months.
“Building the bodies of great people,” Cheeseman wrote, “is more than annual (or biannual) testing and includes ensuring healthy food, adequate sleep, opportunities to exercise (especially outside), and medical readiness.”
According to a report by Military.com, “critics have argued that many of the changes were the Navy relaxing its standards in the face of a challenging recruiting environment and an increasingly overweight population of Americans.” However, Navy data provided in November indicate that the number of sailors failing PFAs has remained very low. In 2017, nearly 98% of sailors passed the PFA, and 95.1% passed the first post-pandemic PFA in 2022.
The PFA policy changes are part of the Navy’s Culture of Excellence 2.0, initiated earlier this year, Cheeseman says. This initiative “charges our leaders to build great people, great leaders, and great teams: their minds, bodies, and spirits, eliminating barriers wherever possible. In response, we are modernizing our PFA policy to acknowledge our diverse population, increase sailor trust, and enhance quality of service.”
Time was—recent time, that is—sailors had two chances to pass a physical fitness assessment (PFA). Failing the first meant no promotion. Failing the second: No career. They could neither be promoted nor reenlist.
That’s changed; as of this month, the Navy now allows the sailor’s commanding officer to decide whether the sailor gets to go on, even after failing a second test.
In an administrative letter, Vice Adm. Rick Cheeseman, chief of naval personnel, said, "Commanding officers can now evaluate a sailor's physical readiness progress or lack of progress in performance evaluations, giving them the ability to manage risk, recognize earnest effort, and best take care of their people.”
According to the new policy, sailors who fail any PFA no longer need to have it noted on their annual evaluation (although they still may not advance until they pass another test). Enlisted sailors who fail a second consecutive PFA are no longer required to receive the lowest possible score in the "Military Bearing/Professionalism" category and are not denied the ability to reenlist.
In assessing eligibility for enlisted members, the memo states that commanders should consider a sailor’s ability to perform the functions of their rate without physical or medical limitation at sea, shore or isolated duty; their overall ability to contribute to Navy missions; and the likelihood of improvement in meeting PFA standards within the next 12 months.
“Building the bodies of great people,” Cheeseman wrote, “is more than annual (or biannual) testing and includes ensuring healthy food, adequate sleep, opportunities to exercise (especially outside), and medical readiness.”
According to a report by Military.com, “critics have argued that many of the changes were the Navy relaxing its standards in the face of a challenging recruiting environment and an increasingly overweight population of Americans.” However, Navy data provided in November indicate that the number of sailors failing PFAs has remained very low. In 2017, nearly 98% of sailors passed the PFA, and 95.1% passed the first post-pandemic PFA in 2022.
The PFA policy changes are part of the Navy’s Culture of Excellence 2.0, initiated earlier this year, Cheeseman says. This initiative “charges our leaders to build great people, great leaders, and great teams: their minds, bodies, and spirits, eliminating barriers wherever possible. In response, we are modernizing our PFA policy to acknowledge our diverse population, increase sailor trust, and enhance quality of service.”
Tirzepatide Shows Improvements in MASH Resolution, Fibrosis
MILAN — , according to the results of the phase 2 SYNERGY-NASH trial.
Specifically, 44%-62% of participants with MASH and moderate or severe fibrosis treated with 5-15 mg of tirzepatide achieved MASH resolution without worsening of fibrosis compared with 10% on placebo; 51%-55% of those on tirzepatide achieved at least one stage of fibrosis improvement without worsening of MASH compared with 30% on placebo. Tirzepatide also led to weight loss.
The study (Abstract LBO-001) was presented at the European Association for the Study of the Liver (EASL) Congress 2024 by Rohit Loomba, MD, professor of medicine, NAFLD Research Center, University of California at San Diego in La Jolla, and published simultaneously in The New England Journal of Medicine.
“The results are clinically meaningful,” Dr. Loomba said in an interview.
Both of the endpoints — improvements in MASH resolution and fibrosis — are considered approvable endpoints for MASH therapeutic development, and therefore, increase the likelihood of success of using such a strategy in a phase 3 setting, Dr. Loomba said.
MASH Resolution, No Worsening of Fibrosis
The dose-finding, multicenter, double-blind, placebo-controlled trial randomly assigned a total of 190 participants to receive once-weekly subcutaneous tirzepatide (5 mg, 10 mg, or 15 mg) or placebo for 52 weeks. Participants had biopsy-confirmed MASH and stage F2 or F3 (moderate or severe) fibrosis.
Overall, approximately 42% of participants had F2 fibrosis and over 57% had F3 fibrosis. The proportion of F3 fibrosis was numerically higher in the placebo (64.6%) and 5-mg tirzepatide (63.8%) groups.
The mean age of the study cohort was 54 years; 57% were female, 86% were White, and 36% were Hispanic; the mean body mass index was 36; 58% had type 2 diabetes; and A1c was 6.5. NAFLD activity score (NAS) was 5.3. Baseline noninvasive test results were consistent with the study population of MASH with F2/F3 fibrosis and NAS ≥ 4.
The primary endpoint was resolution of MASH without worsening of fibrosis at 52 weeks, and the key secondary endpoint was an improvement (decrease) of at least one fibrosis stage without worsening of MASH. Other secondary endpoints included a ≥ 2-point decrease in NAS with ≤ 1-point decrease in two or more NAS components.
A total of 157 participants (83%) underwent liver biopsies at week 52, providing results for the current analysis.
Among tirzepatide-treated patients, 43.6% in the 5-mg group, 55.5% in the 10-mg group, and 62.4% in the 15-mg group met the criteria for resolution of MASH without worsening of fibrosis compared with 10% in the placebo group (P < .001 for all three comparisons).
Fibrosis improved by at least one stage without worsening of MASH in 54.9% of participants in the 5-mg tirzepatide group, 51.3% in the 10-mg tirzepatide group, and 51.0% in the 15-mg tirzepatide group compared with 29.7% in the placebo group (P < .001 for all risk differences with placebo).
Changes in NAS and subscores for the individual components of NAS, including steatosis, lobular inflammation, and hepatocellular ballooning, were also seen in participants on tirzepatide.
The researchers used a composite endpoint of a ≥ 2-point decrease in NAS with a ≥ 1-point decrease in at least two NAS components. Of the tirzepatide-treated groups, 71.7%,78.3%, and 76.6% in the 5-mg, 10-mg, and 15-mg groups, respectively, met this endpoint compared with 36.7% in placebo.
Imaging of liver fat with MRI-based proton density fat fraction (MRI-PDFF) showed reductions from baseline of -45.7, -41.3, -57.0 in participants on 5-mg, 10-mg, and 15-mg tirzepatide, respectively. Differences from placebo were all statistically significant.
Percentage of body weight change from baseline was -10.7%, -13.3%, and -15.6% in the 5-mg, 10-mg, and 15-mg tirzepatide groups, respectively, compared with weight loss of -0.8% in the placebo group.
“Tirzepatide led to significant weight loss in both patients with diabetes and those without diabetes,” reported Dr. Loomba.
There were more adverse events in patients on tirzepatide (92.3%) compared with patients on placebo (83.3%).
“The most common adverse events were gastrointestinal in nature, with 96% of them mild to moderate in severity,” said Dr. Loomba. “Discontinuations occurred in 4.2% of participants, which was similar between patients on tirzepatide and those on placebo.”
He pointed out that the safety profile of tirzepatide in a MASH population “was generally similar to that observed in the phase 3 trials of type 2 diabetes and obesity.”
Incidence of serious adverse events was also similar at 6.3% for participants on tirzepatide vs 6.2% for those on placebo; 2.8% on tirzepatide and 4.2% on placebo progressed to cirrhosis. There was no evidence of drug-induced liver injury.
‘Convincing Results’
Commenting on the study, co-moderator Sven Francque, MD, hepatologist and head of department at the University Hospital of Antwerp, Belgium, said that the study was in a relatively “severe” patient population, which was one of its strengths.
“These are convincing results in terms of MASH resolution, showing a strong response and dose-dependence,” he said.
“In terms of fibrosis, the results look numerically strong but are somewhat more puzzling to interpret, as there was no dose-response relationship and no data on NITs [noninvasive tests] that could support the results,” he added.
“Patients with no-end-of-treatment biopsies were handled differently than in previous trials, which makes it difficult to appreciate antifibrotic potency,” he said. But “such a strong effect on MASH should translate into a reduction in fibrosis even in the absence of direct antifibrotic effects.”
Given that “about one third of patients in the active treatment arms” did not have end-of-treatment biopsy, these “are rather small numbers precluding firm conclusions,” he added.
However, Dr. Francque said that he believes the findings are compelling enough for the drug to go into phase 3 trials.
Dr. Francque has no disclosures of relevance to this study. Dr. Loomba serves as a consultant to Aardvark Therapeutics, Altimmune, Anylam/Regeneron, Amgen, Arrowhead Pharmaceuticals, AstraZeneca, Bristol Myers Squibb, CohBar, Eli Lilly, Galmed, Gilead, Glympse Bio, Hightide, Inipharma, Intercept, Inventiva, Ionis, Janssen, Madrigal, Metacrine, NGM Biopharmaceuticals, Novartis, Novo Nordisk, Merck, Pfizer, Sagimet, Theratechnologies, 89 bio, Terns Pharmaceuticals and Viking Therapeutics. In addition, his institutions received research grants from Arrowhead Pharmaceuticals, AstraZeneca, Boehringer-Ingelheim, Bristol Myers Squibb, Eli Lilly, Galectin Therapeutics, Galmed Pharmaceuticals, Gilead, Intercept, Hanmi, Intercept, Inventiva, Ionis, Janssen, Madrigal Pharmaceuticals, Merck, NGM Biopharmaceuticals, Novo Nordisk, Merck, Pfizer, Sonic Incytes, and Terns Pharmaceuticals. Dr. Loomba is a co-founder of LipoNexus.
A version of this article first appeared on Medscape.com.
MILAN — , according to the results of the phase 2 SYNERGY-NASH trial.
Specifically, 44%-62% of participants with MASH and moderate or severe fibrosis treated with 5-15 mg of tirzepatide achieved MASH resolution without worsening of fibrosis compared with 10% on placebo; 51%-55% of those on tirzepatide achieved at least one stage of fibrosis improvement without worsening of MASH compared with 30% on placebo. Tirzepatide also led to weight loss.
The study (Abstract LBO-001) was presented at the European Association for the Study of the Liver (EASL) Congress 2024 by Rohit Loomba, MD, professor of medicine, NAFLD Research Center, University of California at San Diego in La Jolla, and published simultaneously in The New England Journal of Medicine.
“The results are clinically meaningful,” Dr. Loomba said in an interview.
Both of the endpoints — improvements in MASH resolution and fibrosis — are considered approvable endpoints for MASH therapeutic development, and therefore, increase the likelihood of success of using such a strategy in a phase 3 setting, Dr. Loomba said.
MASH Resolution, No Worsening of Fibrosis
The dose-finding, multicenter, double-blind, placebo-controlled trial randomly assigned a total of 190 participants to receive once-weekly subcutaneous tirzepatide (5 mg, 10 mg, or 15 mg) or placebo for 52 weeks. Participants had biopsy-confirmed MASH and stage F2 or F3 (moderate or severe) fibrosis.
Overall, approximately 42% of participants had F2 fibrosis and over 57% had F3 fibrosis. The proportion of F3 fibrosis was numerically higher in the placebo (64.6%) and 5-mg tirzepatide (63.8%) groups.
The mean age of the study cohort was 54 years; 57% were female, 86% were White, and 36% were Hispanic; the mean body mass index was 36; 58% had type 2 diabetes; and A1c was 6.5. NAFLD activity score (NAS) was 5.3. Baseline noninvasive test results were consistent with the study population of MASH with F2/F3 fibrosis and NAS ≥ 4.
The primary endpoint was resolution of MASH without worsening of fibrosis at 52 weeks, and the key secondary endpoint was an improvement (decrease) of at least one fibrosis stage without worsening of MASH. Other secondary endpoints included a ≥ 2-point decrease in NAS with ≤ 1-point decrease in two or more NAS components.
A total of 157 participants (83%) underwent liver biopsies at week 52, providing results for the current analysis.
Among tirzepatide-treated patients, 43.6% in the 5-mg group, 55.5% in the 10-mg group, and 62.4% in the 15-mg group met the criteria for resolution of MASH without worsening of fibrosis compared with 10% in the placebo group (P < .001 for all three comparisons).
Fibrosis improved by at least one stage without worsening of MASH in 54.9% of participants in the 5-mg tirzepatide group, 51.3% in the 10-mg tirzepatide group, and 51.0% in the 15-mg tirzepatide group compared with 29.7% in the placebo group (P < .001 for all risk differences with placebo).
Changes in NAS and subscores for the individual components of NAS, including steatosis, lobular inflammation, and hepatocellular ballooning, were also seen in participants on tirzepatide.
The researchers used a composite endpoint of a ≥ 2-point decrease in NAS with a ≥ 1-point decrease in at least two NAS components. Of the tirzepatide-treated groups, 71.7%,78.3%, and 76.6% in the 5-mg, 10-mg, and 15-mg groups, respectively, met this endpoint compared with 36.7% in placebo.
Imaging of liver fat with MRI-based proton density fat fraction (MRI-PDFF) showed reductions from baseline of -45.7, -41.3, -57.0 in participants on 5-mg, 10-mg, and 15-mg tirzepatide, respectively. Differences from placebo were all statistically significant.
Percentage of body weight change from baseline was -10.7%, -13.3%, and -15.6% in the 5-mg, 10-mg, and 15-mg tirzepatide groups, respectively, compared with weight loss of -0.8% in the placebo group.
“Tirzepatide led to significant weight loss in both patients with diabetes and those without diabetes,” reported Dr. Loomba.
There were more adverse events in patients on tirzepatide (92.3%) compared with patients on placebo (83.3%).
“The most common adverse events were gastrointestinal in nature, with 96% of them mild to moderate in severity,” said Dr. Loomba. “Discontinuations occurred in 4.2% of participants, which was similar between patients on tirzepatide and those on placebo.”
He pointed out that the safety profile of tirzepatide in a MASH population “was generally similar to that observed in the phase 3 trials of type 2 diabetes and obesity.”
Incidence of serious adverse events was also similar at 6.3% for participants on tirzepatide vs 6.2% for those on placebo; 2.8% on tirzepatide and 4.2% on placebo progressed to cirrhosis. There was no evidence of drug-induced liver injury.
‘Convincing Results’
Commenting on the study, co-moderator Sven Francque, MD, hepatologist and head of department at the University Hospital of Antwerp, Belgium, said that the study was in a relatively “severe” patient population, which was one of its strengths.
“These are convincing results in terms of MASH resolution, showing a strong response and dose-dependence,” he said.
“In terms of fibrosis, the results look numerically strong but are somewhat more puzzling to interpret, as there was no dose-response relationship and no data on NITs [noninvasive tests] that could support the results,” he added.
“Patients with no-end-of-treatment biopsies were handled differently than in previous trials, which makes it difficult to appreciate antifibrotic potency,” he said. But “such a strong effect on MASH should translate into a reduction in fibrosis even in the absence of direct antifibrotic effects.”
Given that “about one third of patients in the active treatment arms” did not have end-of-treatment biopsy, these “are rather small numbers precluding firm conclusions,” he added.
However, Dr. Francque said that he believes the findings are compelling enough for the drug to go into phase 3 trials.
Dr. Francque has no disclosures of relevance to this study. Dr. Loomba serves as a consultant to Aardvark Therapeutics, Altimmune, Anylam/Regeneron, Amgen, Arrowhead Pharmaceuticals, AstraZeneca, Bristol Myers Squibb, CohBar, Eli Lilly, Galmed, Gilead, Glympse Bio, Hightide, Inipharma, Intercept, Inventiva, Ionis, Janssen, Madrigal, Metacrine, NGM Biopharmaceuticals, Novartis, Novo Nordisk, Merck, Pfizer, Sagimet, Theratechnologies, 89 bio, Terns Pharmaceuticals and Viking Therapeutics. In addition, his institutions received research grants from Arrowhead Pharmaceuticals, AstraZeneca, Boehringer-Ingelheim, Bristol Myers Squibb, Eli Lilly, Galectin Therapeutics, Galmed Pharmaceuticals, Gilead, Intercept, Hanmi, Intercept, Inventiva, Ionis, Janssen, Madrigal Pharmaceuticals, Merck, NGM Biopharmaceuticals, Novo Nordisk, Merck, Pfizer, Sonic Incytes, and Terns Pharmaceuticals. Dr. Loomba is a co-founder of LipoNexus.
A version of this article first appeared on Medscape.com.
MILAN — , according to the results of the phase 2 SYNERGY-NASH trial.
Specifically, 44%-62% of participants with MASH and moderate or severe fibrosis treated with 5-15 mg of tirzepatide achieved MASH resolution without worsening of fibrosis compared with 10% on placebo; 51%-55% of those on tirzepatide achieved at least one stage of fibrosis improvement without worsening of MASH compared with 30% on placebo. Tirzepatide also led to weight loss.
The study (Abstract LBO-001) was presented at the European Association for the Study of the Liver (EASL) Congress 2024 by Rohit Loomba, MD, professor of medicine, NAFLD Research Center, University of California at San Diego in La Jolla, and published simultaneously in The New England Journal of Medicine.
“The results are clinically meaningful,” Dr. Loomba said in an interview.
Both of the endpoints — improvements in MASH resolution and fibrosis — are considered approvable endpoints for MASH therapeutic development, and therefore, increase the likelihood of success of using such a strategy in a phase 3 setting, Dr. Loomba said.
MASH Resolution, No Worsening of Fibrosis
The dose-finding, multicenter, double-blind, placebo-controlled trial randomly assigned a total of 190 participants to receive once-weekly subcutaneous tirzepatide (5 mg, 10 mg, or 15 mg) or placebo for 52 weeks. Participants had biopsy-confirmed MASH and stage F2 or F3 (moderate or severe) fibrosis.
Overall, approximately 42% of participants had F2 fibrosis and over 57% had F3 fibrosis. The proportion of F3 fibrosis was numerically higher in the placebo (64.6%) and 5-mg tirzepatide (63.8%) groups.
The mean age of the study cohort was 54 years; 57% were female, 86% were White, and 36% were Hispanic; the mean body mass index was 36; 58% had type 2 diabetes; and A1c was 6.5. NAFLD activity score (NAS) was 5.3. Baseline noninvasive test results were consistent with the study population of MASH with F2/F3 fibrosis and NAS ≥ 4.
The primary endpoint was resolution of MASH without worsening of fibrosis at 52 weeks, and the key secondary endpoint was an improvement (decrease) of at least one fibrosis stage without worsening of MASH. Other secondary endpoints included a ≥ 2-point decrease in NAS with ≤ 1-point decrease in two or more NAS components.
A total of 157 participants (83%) underwent liver biopsies at week 52, providing results for the current analysis.
Among tirzepatide-treated patients, 43.6% in the 5-mg group, 55.5% in the 10-mg group, and 62.4% in the 15-mg group met the criteria for resolution of MASH without worsening of fibrosis compared with 10% in the placebo group (P < .001 for all three comparisons).
Fibrosis improved by at least one stage without worsening of MASH in 54.9% of participants in the 5-mg tirzepatide group, 51.3% in the 10-mg tirzepatide group, and 51.0% in the 15-mg tirzepatide group compared with 29.7% in the placebo group (P < .001 for all risk differences with placebo).
Changes in NAS and subscores for the individual components of NAS, including steatosis, lobular inflammation, and hepatocellular ballooning, were also seen in participants on tirzepatide.
The researchers used a composite endpoint of a ≥ 2-point decrease in NAS with a ≥ 1-point decrease in at least two NAS components. Of the tirzepatide-treated groups, 71.7%,78.3%, and 76.6% in the 5-mg, 10-mg, and 15-mg groups, respectively, met this endpoint compared with 36.7% in placebo.
Imaging of liver fat with MRI-based proton density fat fraction (MRI-PDFF) showed reductions from baseline of -45.7, -41.3, -57.0 in participants on 5-mg, 10-mg, and 15-mg tirzepatide, respectively. Differences from placebo were all statistically significant.
Percentage of body weight change from baseline was -10.7%, -13.3%, and -15.6% in the 5-mg, 10-mg, and 15-mg tirzepatide groups, respectively, compared with weight loss of -0.8% in the placebo group.
“Tirzepatide led to significant weight loss in both patients with diabetes and those without diabetes,” reported Dr. Loomba.
There were more adverse events in patients on tirzepatide (92.3%) compared with patients on placebo (83.3%).
“The most common adverse events were gastrointestinal in nature, with 96% of them mild to moderate in severity,” said Dr. Loomba. “Discontinuations occurred in 4.2% of participants, which was similar between patients on tirzepatide and those on placebo.”
He pointed out that the safety profile of tirzepatide in a MASH population “was generally similar to that observed in the phase 3 trials of type 2 diabetes and obesity.”
Incidence of serious adverse events was also similar at 6.3% for participants on tirzepatide vs 6.2% for those on placebo; 2.8% on tirzepatide and 4.2% on placebo progressed to cirrhosis. There was no evidence of drug-induced liver injury.
‘Convincing Results’
Commenting on the study, co-moderator Sven Francque, MD, hepatologist and head of department at the University Hospital of Antwerp, Belgium, said that the study was in a relatively “severe” patient population, which was one of its strengths.
“These are convincing results in terms of MASH resolution, showing a strong response and dose-dependence,” he said.
“In terms of fibrosis, the results look numerically strong but are somewhat more puzzling to interpret, as there was no dose-response relationship and no data on NITs [noninvasive tests] that could support the results,” he added.
“Patients with no-end-of-treatment biopsies were handled differently than in previous trials, which makes it difficult to appreciate antifibrotic potency,” he said. But “such a strong effect on MASH should translate into a reduction in fibrosis even in the absence of direct antifibrotic effects.”
Given that “about one third of patients in the active treatment arms” did not have end-of-treatment biopsy, these “are rather small numbers precluding firm conclusions,” he added.
However, Dr. Francque said that he believes the findings are compelling enough for the drug to go into phase 3 trials.
Dr. Francque has no disclosures of relevance to this study. Dr. Loomba serves as a consultant to Aardvark Therapeutics, Altimmune, Anylam/Regeneron, Amgen, Arrowhead Pharmaceuticals, AstraZeneca, Bristol Myers Squibb, CohBar, Eli Lilly, Galmed, Gilead, Glympse Bio, Hightide, Inipharma, Intercept, Inventiva, Ionis, Janssen, Madrigal, Metacrine, NGM Biopharmaceuticals, Novartis, Novo Nordisk, Merck, Pfizer, Sagimet, Theratechnologies, 89 bio, Terns Pharmaceuticals and Viking Therapeutics. In addition, his institutions received research grants from Arrowhead Pharmaceuticals, AstraZeneca, Boehringer-Ingelheim, Bristol Myers Squibb, Eli Lilly, Galectin Therapeutics, Galmed Pharmaceuticals, Gilead, Intercept, Hanmi, Intercept, Inventiva, Ionis, Janssen, Madrigal Pharmaceuticals, Merck, NGM Biopharmaceuticals, Novo Nordisk, Merck, Pfizer, Sonic Incytes, and Terns Pharmaceuticals. Dr. Loomba is a co-founder of LipoNexus.
A version of this article first appeared on Medscape.com.
FROM EASL 2024
AGA Research Scholar Awards Advance the GI Field
The AGA Research Foundation plays an important role in medical research by providing grants to talented scientists at a critical time in their career.
“The AGA Research Scholar Award will have a significant impact on my career,” said Dr. Jason (Yanjia) Zhang, 2024 AGA Research Scholar Award grant recipient, and a gastroenterologist at Boston Children’s Hospital. “I aspire to lead a laboratory studying the impact of the microbiome on human gastroenterological diseases. Our lab will focus on the molecular mechanisms underlying how microbes activate gut signaling. The AGA Research Foundation grant will support my transition to independence and build key capacities that will be the foundation of my future lab.”
Funded by the generosity of donors, the AGA Research Foundation’s research award program ensures that AGA is building a community of researchers whose work serves the greater community and benefits all our patients.
By joining other AGA members in supporting the AGA Research Foundation, you will ensure that young researchers have opportunities to continue their life-saving work. Your tax-deductible contribution supports the foundation’s research award program, including the RSA, which ensures that studies are funded, discoveries are made and patients are treated.
Learn more or make a contribution at www.foundation.gastro.org.
The AGA Research Foundation plays an important role in medical research by providing grants to talented scientists at a critical time in their career.
“The AGA Research Scholar Award will have a significant impact on my career,” said Dr. Jason (Yanjia) Zhang, 2024 AGA Research Scholar Award grant recipient, and a gastroenterologist at Boston Children’s Hospital. “I aspire to lead a laboratory studying the impact of the microbiome on human gastroenterological diseases. Our lab will focus on the molecular mechanisms underlying how microbes activate gut signaling. The AGA Research Foundation grant will support my transition to independence and build key capacities that will be the foundation of my future lab.”
Funded by the generosity of donors, the AGA Research Foundation’s research award program ensures that AGA is building a community of researchers whose work serves the greater community and benefits all our patients.
By joining other AGA members in supporting the AGA Research Foundation, you will ensure that young researchers have opportunities to continue their life-saving work. Your tax-deductible contribution supports the foundation’s research award program, including the RSA, which ensures that studies are funded, discoveries are made and patients are treated.
Learn more or make a contribution at www.foundation.gastro.org.
The AGA Research Foundation plays an important role in medical research by providing grants to talented scientists at a critical time in their career.
“The AGA Research Scholar Award will have a significant impact on my career,” said Dr. Jason (Yanjia) Zhang, 2024 AGA Research Scholar Award grant recipient, and a gastroenterologist at Boston Children’s Hospital. “I aspire to lead a laboratory studying the impact of the microbiome on human gastroenterological diseases. Our lab will focus on the molecular mechanisms underlying how microbes activate gut signaling. The AGA Research Foundation grant will support my transition to independence and build key capacities that will be the foundation of my future lab.”
Funded by the generosity of donors, the AGA Research Foundation’s research award program ensures that AGA is building a community of researchers whose work serves the greater community and benefits all our patients.
By joining other AGA members in supporting the AGA Research Foundation, you will ensure that young researchers have opportunities to continue their life-saving work. Your tax-deductible contribution supports the foundation’s research award program, including the RSA, which ensures that studies are funded, discoveries are made and patients are treated.
Learn more or make a contribution at www.foundation.gastro.org.
New Therapy May Provide COPD Patients With Relief, Convenience
, according to manufacturer Verona Pharma.
Ensifentrine offers a new medication and a new delivery method, according to a company press release. Ensifentrine is the first-in-class selective dual inhibitor of both phosphodiesterase 3 (PDE 3) and PDE 4, combining both bronchodilator and nonsteroidal anti-inflammatory effects in a single molecule. The drug is delivered through a standard jet nebulizer.
Disease Management Made Easier
Although currently approved therapies for COPD, such as bronchodilators and inhaled corticosteroids (ICS), have benefited many patients, additional treatment options are still needed to help those who remain symptomatic and suffer from frequent exacerbations, said Diego J. Maselli, MD, of the University of Texas Health Science Center, San Antonio.
“Ensifentrine is a new class of medication that inhibits both PDE 3 and PDE 4; this results in both bronchodilation and suppression of the inflammatory response in COPD,” said Dr. Maselli, who was not involved in studies of ensifentrine.
“Large phase III, double-blind, randomized, placebo-controlled studies have demonstrated that ensifentrine improved lung function and reduced the risk of exacerbations in patients with symptomatic moderate to severe COPD,” he said. The study participants were on no long-acting maintenance therapy, or they were receiving long-acting beta agonist (LABA) or long-acting muscarinic antagonist (LAMA) with or without inhaled corticosteroids, he noted.
The FDA approval was supported by data from the phase 3 ENHANCE 1 and 2 trials, which included 760 and 789 adults aged 40-80 years with moderate to severe symptomatic COPD, respectively. Participants were randomized to 3 mg ensifentrine delivered via nebulizer or a placebo twice daily.
In the studies, ensifentrine significantly improved lung function based on the primary outcome of average forced expiratory volume per second within 0-12 hours of administration compared with placebo in both studies. In ENHANCE 1, ensifentrine significantly improved symptoms and quality of life compared with placebo at 24 weeks. The ENHANCE 2 results showed similar trends in favor of ensifentrine, although the differences were not significant at 24 weeks. However, the effects of ensifentrine vs placebo were consistent overall across all symptom and quality of life endpoints at all assessments during the study period, the researchers wrote.
In addition, the inhaled drug was well tolerated, with similar proportions of ensifentrine and placebo patients reporting treatment-emergent adverse events (38.4% and 36.4%, respectively, in ENHANCE 1 and 35.3% and 35.4%, respectively, in ENHANCE 2). The most common treatment-emergent adverse events were nasopharyngitis, hypertension, and back pain, reported in < 3% of the ensifentrine group.
The safety profile of ensifentrine is a plus for patients, said Dr. Maselli. “Ensifentrine was well tolerated in these studies, and the side effect profile was similar to placebo,” he said. The “ensifentrine is delivered via nebulizer and dosed every 12 hours. Some patients may still prefer the use of inhalers, while others may feel more comfortable with this mode of delivery,” he said.
In clinical practice, “ensifentrine is a welcome addition to the current armamentarium of therapies for COPD as an option for patients who are symptomatic or who have frequent exacerbations,” Dr. Maselli emphasized.
Looking ahead, more studies are needed to evaluate ensifentrine in broader populations of COPD patients, Dr. Maselli said. For example, ensifentrine could be used as an add-on therapy for patients receiving triple therapy (ICS/LABA/LAMA) and for patients with other obstructive inflammatory diseases such as asthma, bronchiectasis, and cystic fibrosis, he noted.
Dr. Maselli disclosed serving as a consultant for GlaxoSmithKline, AstraZeneca, Amgen, and Sanofi/Regeneron; he also serves on the Editorial Board of CHEST Physician.
A version of this article appeared on Medscape.com.
, according to manufacturer Verona Pharma.
Ensifentrine offers a new medication and a new delivery method, according to a company press release. Ensifentrine is the first-in-class selective dual inhibitor of both phosphodiesterase 3 (PDE 3) and PDE 4, combining both bronchodilator and nonsteroidal anti-inflammatory effects in a single molecule. The drug is delivered through a standard jet nebulizer.
Disease Management Made Easier
Although currently approved therapies for COPD, such as bronchodilators and inhaled corticosteroids (ICS), have benefited many patients, additional treatment options are still needed to help those who remain symptomatic and suffer from frequent exacerbations, said Diego J. Maselli, MD, of the University of Texas Health Science Center, San Antonio.
“Ensifentrine is a new class of medication that inhibits both PDE 3 and PDE 4; this results in both bronchodilation and suppression of the inflammatory response in COPD,” said Dr. Maselli, who was not involved in studies of ensifentrine.
“Large phase III, double-blind, randomized, placebo-controlled studies have demonstrated that ensifentrine improved lung function and reduced the risk of exacerbations in patients with symptomatic moderate to severe COPD,” he said. The study participants were on no long-acting maintenance therapy, or they were receiving long-acting beta agonist (LABA) or long-acting muscarinic antagonist (LAMA) with or without inhaled corticosteroids, he noted.
The FDA approval was supported by data from the phase 3 ENHANCE 1 and 2 trials, which included 760 and 789 adults aged 40-80 years with moderate to severe symptomatic COPD, respectively. Participants were randomized to 3 mg ensifentrine delivered via nebulizer or a placebo twice daily.
In the studies, ensifentrine significantly improved lung function based on the primary outcome of average forced expiratory volume per second within 0-12 hours of administration compared with placebo in both studies. In ENHANCE 1, ensifentrine significantly improved symptoms and quality of life compared with placebo at 24 weeks. The ENHANCE 2 results showed similar trends in favor of ensifentrine, although the differences were not significant at 24 weeks. However, the effects of ensifentrine vs placebo were consistent overall across all symptom and quality of life endpoints at all assessments during the study period, the researchers wrote.
In addition, the inhaled drug was well tolerated, with similar proportions of ensifentrine and placebo patients reporting treatment-emergent adverse events (38.4% and 36.4%, respectively, in ENHANCE 1 and 35.3% and 35.4%, respectively, in ENHANCE 2). The most common treatment-emergent adverse events were nasopharyngitis, hypertension, and back pain, reported in < 3% of the ensifentrine group.
The safety profile of ensifentrine is a plus for patients, said Dr. Maselli. “Ensifentrine was well tolerated in these studies, and the side effect profile was similar to placebo,” he said. The “ensifentrine is delivered via nebulizer and dosed every 12 hours. Some patients may still prefer the use of inhalers, while others may feel more comfortable with this mode of delivery,” he said.
In clinical practice, “ensifentrine is a welcome addition to the current armamentarium of therapies for COPD as an option for patients who are symptomatic or who have frequent exacerbations,” Dr. Maselli emphasized.
Looking ahead, more studies are needed to evaluate ensifentrine in broader populations of COPD patients, Dr. Maselli said. For example, ensifentrine could be used as an add-on therapy for patients receiving triple therapy (ICS/LABA/LAMA) and for patients with other obstructive inflammatory diseases such as asthma, bronchiectasis, and cystic fibrosis, he noted.
Dr. Maselli disclosed serving as a consultant for GlaxoSmithKline, AstraZeneca, Amgen, and Sanofi/Regeneron; he also serves on the Editorial Board of CHEST Physician.
A version of this article appeared on Medscape.com.
, according to manufacturer Verona Pharma.
Ensifentrine offers a new medication and a new delivery method, according to a company press release. Ensifentrine is the first-in-class selective dual inhibitor of both phosphodiesterase 3 (PDE 3) and PDE 4, combining both bronchodilator and nonsteroidal anti-inflammatory effects in a single molecule. The drug is delivered through a standard jet nebulizer.
Disease Management Made Easier
Although currently approved therapies for COPD, such as bronchodilators and inhaled corticosteroids (ICS), have benefited many patients, additional treatment options are still needed to help those who remain symptomatic and suffer from frequent exacerbations, said Diego J. Maselli, MD, of the University of Texas Health Science Center, San Antonio.
“Ensifentrine is a new class of medication that inhibits both PDE 3 and PDE 4; this results in both bronchodilation and suppression of the inflammatory response in COPD,” said Dr. Maselli, who was not involved in studies of ensifentrine.
“Large phase III, double-blind, randomized, placebo-controlled studies have demonstrated that ensifentrine improved lung function and reduced the risk of exacerbations in patients with symptomatic moderate to severe COPD,” he said. The study participants were on no long-acting maintenance therapy, or they were receiving long-acting beta agonist (LABA) or long-acting muscarinic antagonist (LAMA) with or without inhaled corticosteroids, he noted.
The FDA approval was supported by data from the phase 3 ENHANCE 1 and 2 trials, which included 760 and 789 adults aged 40-80 years with moderate to severe symptomatic COPD, respectively. Participants were randomized to 3 mg ensifentrine delivered via nebulizer or a placebo twice daily.
In the studies, ensifentrine significantly improved lung function based on the primary outcome of average forced expiratory volume per second within 0-12 hours of administration compared with placebo in both studies. In ENHANCE 1, ensifentrine significantly improved symptoms and quality of life compared with placebo at 24 weeks. The ENHANCE 2 results showed similar trends in favor of ensifentrine, although the differences were not significant at 24 weeks. However, the effects of ensifentrine vs placebo were consistent overall across all symptom and quality of life endpoints at all assessments during the study period, the researchers wrote.
In addition, the inhaled drug was well tolerated, with similar proportions of ensifentrine and placebo patients reporting treatment-emergent adverse events (38.4% and 36.4%, respectively, in ENHANCE 1 and 35.3% and 35.4%, respectively, in ENHANCE 2). The most common treatment-emergent adverse events were nasopharyngitis, hypertension, and back pain, reported in < 3% of the ensifentrine group.
The safety profile of ensifentrine is a plus for patients, said Dr. Maselli. “Ensifentrine was well tolerated in these studies, and the side effect profile was similar to placebo,” he said. The “ensifentrine is delivered via nebulizer and dosed every 12 hours. Some patients may still prefer the use of inhalers, while others may feel more comfortable with this mode of delivery,” he said.
In clinical practice, “ensifentrine is a welcome addition to the current armamentarium of therapies for COPD as an option for patients who are symptomatic or who have frequent exacerbations,” Dr. Maselli emphasized.
Looking ahead, more studies are needed to evaluate ensifentrine in broader populations of COPD patients, Dr. Maselli said. For example, ensifentrine could be used as an add-on therapy for patients receiving triple therapy (ICS/LABA/LAMA) and for patients with other obstructive inflammatory diseases such as asthma, bronchiectasis, and cystic fibrosis, he noted.
Dr. Maselli disclosed serving as a consultant for GlaxoSmithKline, AstraZeneca, Amgen, and Sanofi/Regeneron; he also serves on the Editorial Board of CHEST Physician.
A version of this article appeared on Medscape.com.
Philips Respironics Issues Update on Ventilator Alarm Failure
, according to a recall statement from the US Food and Drug Administration (FDA).
The OLA+ Ventilator is designed for use by individuals with obstructive sleep apnea, breathing problems, and mixed apnea and is approved for children aged 7 years and older, as well as adults.
The recall does not involve removal of the devices from where they are used or sold but does update the instructions for use, and its use without following the updated instructions could result in serious injury or death, according to the statement.
Following an alarm failure, the device may fail in one of two ways: By entering a ventilator inoperative state after three reboots within 24 hours (with no therapy and audible and visual alarms present) or by entering a ventilator inoperative state without rebooting first.
According to the statement, the alarm issue may be corrected with a software patch, available from Philips, or the company will offer a replacement device for patients until the affected devices are repaired. The statement updates an April 1, 2024, urgent recall from Philips urging the immediate removal of a patient from an OLA+ Ventilator and connecting them to alternative ventilation if possible if the ventilator’s inoperative alarm occurs.
The device failures may cause interruption or loss of therapy with effects including anxiety, confusion/disorientation, changes in respiratory rate, dyspnea, tachycardia, respiratory failure, and even death in especially vulnerable individuals. One death and 15 injuries have been reported as a result of the alarm failure, according to the FDA.
US customers can contact Philips Respironics Inc. at 1-800-345-6443 or [email protected] with questions, according to the FDA, and clinicians and patients may report adverse reactions or other problems with the devices to MedWatch: The FDA Safety Information and Adverse Event Reporting Program.
A version of this article appeared on Medscape.com.
, according to a recall statement from the US Food and Drug Administration (FDA).
The OLA+ Ventilator is designed for use by individuals with obstructive sleep apnea, breathing problems, and mixed apnea and is approved for children aged 7 years and older, as well as adults.
The recall does not involve removal of the devices from where they are used or sold but does update the instructions for use, and its use without following the updated instructions could result in serious injury or death, according to the statement.
Following an alarm failure, the device may fail in one of two ways: By entering a ventilator inoperative state after three reboots within 24 hours (with no therapy and audible and visual alarms present) or by entering a ventilator inoperative state without rebooting first.
According to the statement, the alarm issue may be corrected with a software patch, available from Philips, or the company will offer a replacement device for patients until the affected devices are repaired. The statement updates an April 1, 2024, urgent recall from Philips urging the immediate removal of a patient from an OLA+ Ventilator and connecting them to alternative ventilation if possible if the ventilator’s inoperative alarm occurs.
The device failures may cause interruption or loss of therapy with effects including anxiety, confusion/disorientation, changes in respiratory rate, dyspnea, tachycardia, respiratory failure, and even death in especially vulnerable individuals. One death and 15 injuries have been reported as a result of the alarm failure, according to the FDA.
US customers can contact Philips Respironics Inc. at 1-800-345-6443 or [email protected] with questions, according to the FDA, and clinicians and patients may report adverse reactions or other problems with the devices to MedWatch: The FDA Safety Information and Adverse Event Reporting Program.
A version of this article appeared on Medscape.com.
, according to a recall statement from the US Food and Drug Administration (FDA).
The OLA+ Ventilator is designed for use by individuals with obstructive sleep apnea, breathing problems, and mixed apnea and is approved for children aged 7 years and older, as well as adults.
The recall does not involve removal of the devices from where they are used or sold but does update the instructions for use, and its use without following the updated instructions could result in serious injury or death, according to the statement.
Following an alarm failure, the device may fail in one of two ways: By entering a ventilator inoperative state after three reboots within 24 hours (with no therapy and audible and visual alarms present) or by entering a ventilator inoperative state without rebooting first.
According to the statement, the alarm issue may be corrected with a software patch, available from Philips, or the company will offer a replacement device for patients until the affected devices are repaired. The statement updates an April 1, 2024, urgent recall from Philips urging the immediate removal of a patient from an OLA+ Ventilator and connecting them to alternative ventilation if possible if the ventilator’s inoperative alarm occurs.
The device failures may cause interruption or loss of therapy with effects including anxiety, confusion/disorientation, changes in respiratory rate, dyspnea, tachycardia, respiratory failure, and even death in especially vulnerable individuals. One death and 15 injuries have been reported as a result of the alarm failure, according to the FDA.
US customers can contact Philips Respironics Inc. at 1-800-345-6443 or [email protected] with questions, according to the FDA, and clinicians and patients may report adverse reactions or other problems with the devices to MedWatch: The FDA Safety Information and Adverse Event Reporting Program.
A version of this article appeared on Medscape.com.
What Is a Blue Zone Certified Clinician?
It is a great day when a patient shows up at clinical appointment already motivated to make lifestyle behavior changes. Often, they have been inspired by health information they consumed elsewhere, such as from a book, movie, documentary, TV show, a friend, or something out in the community.
Currently, one of the more public representations of health and longevity promotion is Blue Zones. The organization, named for specific areas of the world — the so-called blue zones, where people experience less disease and live longer lives — has created considerable public awareness for healthy living. Today, there are more than 75 Blue Zones Project communities across the United States, where community leaders, businesses, organizations, and citizens collaborate to make healthier choices the easier choices. A recent Netflix special, Live to 100: Secrets of the Blue Zones, further propelled blue zones into the public consciousness.
The Blue Zones emphasis on “plant-slant” diet, natural movement, purpose and contribution, downshifting, and family and community intersect with the lifestyle medicine pillars of whole-food, plant-predominant eating patterns, regular physical activity, stress management, restorative sleep, and positive social connections. Both Blue Zones and lifestyle medicine share a goal of creating healthier and stronger individuals and communities.
For those reasons, it made perfect sense that Blue Zones and the American College of Lifestyle Medicine (ACLM) recently announced a partnership to synergize both organizations’ strengths and resources. Among other things, the collaboration will establish a new certification status of Blue Zones–Certified Physician or Blue Zones–Certified Healthcare Professional, available in 2025 exclusively to clinicians who already are or become certified in lifestyle medicine.
Because of Blue Zones’ considerable consumer awareness, physicians and other health professionals who earn the certification will stand out to potential patients as clinicians with the training and knowledge to help them make sustainable lifestyle behavior changes. A challenging part of any clinician’s job is educating and convincing patients on the proven health benefits of lifestyle behavior change within the time restraints of a routine clinical visit. Patients familiar with Blue Zones are more likely to arrive already interested in changing lifestyle behavior, and clinicians should have the skills to help them achieve their goals.
In addition, community infrastructure developed through Blue Zones that supports healthful lifestyle choices is significant for patients. Lack of resources in their home, work, and community environments is a common obstacle that patients cite when discussing lifestyle change with a clinician. Bicycle lanes for commuting, parks with exercise equipment, accessible healthy food options, and community events to facilitate positive social connections enhance lifestyle-medicine prescriptions. Workplaces, restaurants, places of worship, and grocery stores are examples of community stakeholders that collaborate in Blue Zones communities to promote healthy lifestyle decisions. Although lifestyle medicine clinicians can and do identify creative ways to support patients in communities without strong healthy choice infrastructure, the Blue Zones road map is a welcome companion.
The timing is right for this synthesis of Blue Zones and lifestyle medicine. As consumer interest in Blue Zones has risen, so has clinician interest in evidence-based lifestyle medicine. Since certification in lifestyle medicine began in 2017, almost 6700 physicians and other health professionals have become certified worldwide. More than 43,000 health care professionals have registered for ACLM’s complimentary lifestyle and food-as-medicine courses highlighted by the White House Conference on Hunger, Nutrition, and Health.
What if more patients came to us motivated to make lifestyle changes because of awareness infused in their work and supported in their surrounding community? Matching lifestyle medicine certification with Blue Zone communities equips clinicians to help these patients achieve what they really want: to live longer and better.
Dr. Collings is Director of Lifestyle Medicine, Silicon Valley Medical Development, and Past President, American College of Lifestyle Medicine, Mountain View, California. She has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
It is a great day when a patient shows up at clinical appointment already motivated to make lifestyle behavior changes. Often, they have been inspired by health information they consumed elsewhere, such as from a book, movie, documentary, TV show, a friend, or something out in the community.
Currently, one of the more public representations of health and longevity promotion is Blue Zones. The organization, named for specific areas of the world — the so-called blue zones, where people experience less disease and live longer lives — has created considerable public awareness for healthy living. Today, there are more than 75 Blue Zones Project communities across the United States, where community leaders, businesses, organizations, and citizens collaborate to make healthier choices the easier choices. A recent Netflix special, Live to 100: Secrets of the Blue Zones, further propelled blue zones into the public consciousness.
The Blue Zones emphasis on “plant-slant” diet, natural movement, purpose and contribution, downshifting, and family and community intersect with the lifestyle medicine pillars of whole-food, plant-predominant eating patterns, regular physical activity, stress management, restorative sleep, and positive social connections. Both Blue Zones and lifestyle medicine share a goal of creating healthier and stronger individuals and communities.
For those reasons, it made perfect sense that Blue Zones and the American College of Lifestyle Medicine (ACLM) recently announced a partnership to synergize both organizations’ strengths and resources. Among other things, the collaboration will establish a new certification status of Blue Zones–Certified Physician or Blue Zones–Certified Healthcare Professional, available in 2025 exclusively to clinicians who already are or become certified in lifestyle medicine.
Because of Blue Zones’ considerable consumer awareness, physicians and other health professionals who earn the certification will stand out to potential patients as clinicians with the training and knowledge to help them make sustainable lifestyle behavior changes. A challenging part of any clinician’s job is educating and convincing patients on the proven health benefits of lifestyle behavior change within the time restraints of a routine clinical visit. Patients familiar with Blue Zones are more likely to arrive already interested in changing lifestyle behavior, and clinicians should have the skills to help them achieve their goals.
In addition, community infrastructure developed through Blue Zones that supports healthful lifestyle choices is significant for patients. Lack of resources in their home, work, and community environments is a common obstacle that patients cite when discussing lifestyle change with a clinician. Bicycle lanes for commuting, parks with exercise equipment, accessible healthy food options, and community events to facilitate positive social connections enhance lifestyle-medicine prescriptions. Workplaces, restaurants, places of worship, and grocery stores are examples of community stakeholders that collaborate in Blue Zones communities to promote healthy lifestyle decisions. Although lifestyle medicine clinicians can and do identify creative ways to support patients in communities without strong healthy choice infrastructure, the Blue Zones road map is a welcome companion.
The timing is right for this synthesis of Blue Zones and lifestyle medicine. As consumer interest in Blue Zones has risen, so has clinician interest in evidence-based lifestyle medicine. Since certification in lifestyle medicine began in 2017, almost 6700 physicians and other health professionals have become certified worldwide. More than 43,000 health care professionals have registered for ACLM’s complimentary lifestyle and food-as-medicine courses highlighted by the White House Conference on Hunger, Nutrition, and Health.
What if more patients came to us motivated to make lifestyle changes because of awareness infused in their work and supported in their surrounding community? Matching lifestyle medicine certification with Blue Zone communities equips clinicians to help these patients achieve what they really want: to live longer and better.
Dr. Collings is Director of Lifestyle Medicine, Silicon Valley Medical Development, and Past President, American College of Lifestyle Medicine, Mountain View, California. She has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
It is a great day when a patient shows up at clinical appointment already motivated to make lifestyle behavior changes. Often, they have been inspired by health information they consumed elsewhere, such as from a book, movie, documentary, TV show, a friend, or something out in the community.
Currently, one of the more public representations of health and longevity promotion is Blue Zones. The organization, named for specific areas of the world — the so-called blue zones, where people experience less disease and live longer lives — has created considerable public awareness for healthy living. Today, there are more than 75 Blue Zones Project communities across the United States, where community leaders, businesses, organizations, and citizens collaborate to make healthier choices the easier choices. A recent Netflix special, Live to 100: Secrets of the Blue Zones, further propelled blue zones into the public consciousness.
The Blue Zones emphasis on “plant-slant” diet, natural movement, purpose and contribution, downshifting, and family and community intersect with the lifestyle medicine pillars of whole-food, plant-predominant eating patterns, regular physical activity, stress management, restorative sleep, and positive social connections. Both Blue Zones and lifestyle medicine share a goal of creating healthier and stronger individuals and communities.
For those reasons, it made perfect sense that Blue Zones and the American College of Lifestyle Medicine (ACLM) recently announced a partnership to synergize both organizations’ strengths and resources. Among other things, the collaboration will establish a new certification status of Blue Zones–Certified Physician or Blue Zones–Certified Healthcare Professional, available in 2025 exclusively to clinicians who already are or become certified in lifestyle medicine.
Because of Blue Zones’ considerable consumer awareness, physicians and other health professionals who earn the certification will stand out to potential patients as clinicians with the training and knowledge to help them make sustainable lifestyle behavior changes. A challenging part of any clinician’s job is educating and convincing patients on the proven health benefits of lifestyle behavior change within the time restraints of a routine clinical visit. Patients familiar with Blue Zones are more likely to arrive already interested in changing lifestyle behavior, and clinicians should have the skills to help them achieve their goals.
In addition, community infrastructure developed through Blue Zones that supports healthful lifestyle choices is significant for patients. Lack of resources in their home, work, and community environments is a common obstacle that patients cite when discussing lifestyle change with a clinician. Bicycle lanes for commuting, parks with exercise equipment, accessible healthy food options, and community events to facilitate positive social connections enhance lifestyle-medicine prescriptions. Workplaces, restaurants, places of worship, and grocery stores are examples of community stakeholders that collaborate in Blue Zones communities to promote healthy lifestyle decisions. Although lifestyle medicine clinicians can and do identify creative ways to support patients in communities without strong healthy choice infrastructure, the Blue Zones road map is a welcome companion.
The timing is right for this synthesis of Blue Zones and lifestyle medicine. As consumer interest in Blue Zones has risen, so has clinician interest in evidence-based lifestyle medicine. Since certification in lifestyle medicine began in 2017, almost 6700 physicians and other health professionals have become certified worldwide. More than 43,000 health care professionals have registered for ACLM’s complimentary lifestyle and food-as-medicine courses highlighted by the White House Conference on Hunger, Nutrition, and Health.
What if more patients came to us motivated to make lifestyle changes because of awareness infused in their work and supported in their surrounding community? Matching lifestyle medicine certification with Blue Zone communities equips clinicians to help these patients achieve what they really want: to live longer and better.
Dr. Collings is Director of Lifestyle Medicine, Silicon Valley Medical Development, and Past President, American College of Lifestyle Medicine, Mountain View, California. She has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Concierge Catch: Improved Access for Some Patients Disrupts Care for Many
“You had to pay the fee, or the doctor wasn’t going to see you anymore.”
That was the takeaway for Terri Marroquin of Midland, Texas, when her longtime physician began charging a membership fee in 2019. She found out about the change when someone at the physician’s front desk pointed to a posted notice.
At first, she stuck with the practice; in her area, she said, it is now tough to find a primary care doctor who doesn’t charge an annual membership fee from $350 to $500.
But last year, Ms. Marroquin finally left to join a practice with no membership fee where she sees a physician assistant rather than a doctor. “I had had enough. The concierge fee kept going up, and the doctor’s office kept getting nicer and nicer,” she said, referring to the décor.
Many people seeking a new doctor are calling a long list of primary care practices only to be told they’re not taking new patients.
“Concierge medicine potentially leads to disproportionately richer people being able to pay for the scarce resource of physician time and crowding out people who have lower incomes and are sicker,” said Adam Leive, PhD, lead author of a 2023 study on concierge medicine and researcher at the University of California, Berkeley.
Dr. Leive’s research showed no decrease in mortality for concierge patients compared with similar patients who saw nonconcierge physicians, suggesting concierge care may not notably improve some health outcomes.
A 2005 study showed concierge physicians had smaller proportions of patients with diabetes than their nonconcierge counterparts and provided care for fewer Black and Hispanic patients.
There’s little reliable data available on the size of the concierge medicine market. But one market research firm projects that concierge medicine revenue will grow about 10.4% annually through 2030. About 5,000 to 7,000 physicians and practices provide concierge care in the United States, most of whom are primary care providers, according to Concierge Medicine Today. (Yes, the burgeoning field already has a trade publication.)
The concierge pitch is simple: More time with your doctor, in-person or remotely, promptly and at your convenience. With many primary care physicians caring for thousands of patients each in appointments of 15 minutes or less, some people who can afford the fee say they feel forced to pay it just to maintain adequate access to their doctor.
As primary care providers convert to concierge medicine, many patients could face the financial and health consequences of a potentially lengthy search for a new provider. With fewer physicians in nonconcierge practices, the pool available to people who can’t or won’t pay is smaller. For them, it is harder to find a doctor.
Concierge care models vary widely, but all involve paying a periodic fee to be a patient of the practice.
These fees are generally not covered by insurance nor payable with a tax-advantaged flexible spending account or health savings account. Annual fees range from $199 for Amazon’s One Medical (with a discount available for Prime members) to low four figures for companies like MDVIP and SignatureMD that partner with physicians, to $10,000 or more for top-branded practices like Massachusetts General Hospital’s.
Many patients are exasperated with the prospect of pay-to-play primary care. For one thing, under the Affordable Care Act, insurers are required to cover a variety of preventive services without a patient paying out of pocket. “Your annual physical should be free,” said Caitlin Donovan, a spokesperson for the National Patient Advocate Foundation. “Why are you paying $2,000 for it?”
Liz Glatzer felt her doctor in Providence, Rhode Island, was competent but didn’t have time to absorb her full health history. “I had double mastectomy 25 years ago,” she said. “At my first physical, the doctor ran through my meds and whatever else, and she said, ‘Oh, you haven’t had a mammogram.’ I said, ‘I don’t have breasts to have mammography.’ ”
In 2023, after repeating that same exchange during her next two physicals, Ms. Glatzer signed up to pay $1,900 a year for MDVIP, a concierge staffing service that contracts with her new doctor, who is also a friend’s husband. In her first couple of visits, Ms. Glatzer’s new physician took hours to get to know her, she said.
For the growing numbers of Americans who can’t or won’t pay when their doctor switches to concierge care, finding new primary care can mean frustration, delayed or missed tests or treatments, and fragmented health care.
“I’ve met so many patients who couldn’t afford the concierge services and needed to look for a new primary care physician,” said Yalda Jabbarpour, director of the Robert Graham Center and a practicing family physician. Separating from a doctor who’s transitioning to concierge care “breaks the continuity with the provider that we know is so important for good health outcomes.” .
That disruption has consequences. “People don’t get the preventive services that they should, and they use more expensive and inefficient avenues for care that could have otherwise been provided by their doctor,” said Abbie Leibowitz, chief medical officer at Health Advocate, a company that helps patients find care and resolve insurance issues.
What happens to patients who find themselves at loose ends when a physician transitions to concierge practice?
Patients who lose their doctors often give up on having an ongoing relationship with a primary care clinician. They may rely solely on a pharmacy-based clinic or urgent care center or even a hospital emergency department for primary care.
Some concierge providers say they are responding to concerns about access and equity by allowing patients to opt out of concierge care but stay with the practice group at a lower tier of service. This might entail longer waits for shorter appointments, fewer visits with a physician, and more visits with mid-level providers, for example.
Deb Gordon of Cambridge, Massachusetts, said she is searching for a new primary care doctor after hers switched to concierge medicine — a challenge that involves finding someone in her network who has admitting privileges at her preferred hospitals and is accepting new patients.
Ms. Gordon, who is codirector of the Alliance of Professional Health Advocates, which provides support services to patient advocates, said the practice that her doctor left has not assigned her a new provider, and her health plan said it was OK if she went without one. “I was shocked that they literally said, ‘You can go to urgent care.’ ”
Some patients find themselves turning to physician assistants and other mid-level providers. But those clinicians have much less training than physicians with board certification in family medicine or internal medicine and so may not be fully qualified to treat patients with complex health problems. “The expertise of physician assistants and nurse practitioners can really vary widely,” said Russell Phillips, director of the Harvard Medical School Center for Primary Care.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF—an independent source of health policy research, polling, and journalism. Learn more about KFF.
“You had to pay the fee, or the doctor wasn’t going to see you anymore.”
That was the takeaway for Terri Marroquin of Midland, Texas, when her longtime physician began charging a membership fee in 2019. She found out about the change when someone at the physician’s front desk pointed to a posted notice.
At first, she stuck with the practice; in her area, she said, it is now tough to find a primary care doctor who doesn’t charge an annual membership fee from $350 to $500.
But last year, Ms. Marroquin finally left to join a practice with no membership fee where she sees a physician assistant rather than a doctor. “I had had enough. The concierge fee kept going up, and the doctor’s office kept getting nicer and nicer,” she said, referring to the décor.
Many people seeking a new doctor are calling a long list of primary care practices only to be told they’re not taking new patients.
“Concierge medicine potentially leads to disproportionately richer people being able to pay for the scarce resource of physician time and crowding out people who have lower incomes and are sicker,” said Adam Leive, PhD, lead author of a 2023 study on concierge medicine and researcher at the University of California, Berkeley.
Dr. Leive’s research showed no decrease in mortality for concierge patients compared with similar patients who saw nonconcierge physicians, suggesting concierge care may not notably improve some health outcomes.
A 2005 study showed concierge physicians had smaller proportions of patients with diabetes than their nonconcierge counterparts and provided care for fewer Black and Hispanic patients.
There’s little reliable data available on the size of the concierge medicine market. But one market research firm projects that concierge medicine revenue will grow about 10.4% annually through 2030. About 5,000 to 7,000 physicians and practices provide concierge care in the United States, most of whom are primary care providers, according to Concierge Medicine Today. (Yes, the burgeoning field already has a trade publication.)
The concierge pitch is simple: More time with your doctor, in-person or remotely, promptly and at your convenience. With many primary care physicians caring for thousands of patients each in appointments of 15 minutes or less, some people who can afford the fee say they feel forced to pay it just to maintain adequate access to their doctor.
As primary care providers convert to concierge medicine, many patients could face the financial and health consequences of a potentially lengthy search for a new provider. With fewer physicians in nonconcierge practices, the pool available to people who can’t or won’t pay is smaller. For them, it is harder to find a doctor.
Concierge care models vary widely, but all involve paying a periodic fee to be a patient of the practice.
These fees are generally not covered by insurance nor payable with a tax-advantaged flexible spending account or health savings account. Annual fees range from $199 for Amazon’s One Medical (with a discount available for Prime members) to low four figures for companies like MDVIP and SignatureMD that partner with physicians, to $10,000 or more for top-branded practices like Massachusetts General Hospital’s.
Many patients are exasperated with the prospect of pay-to-play primary care. For one thing, under the Affordable Care Act, insurers are required to cover a variety of preventive services without a patient paying out of pocket. “Your annual physical should be free,” said Caitlin Donovan, a spokesperson for the National Patient Advocate Foundation. “Why are you paying $2,000 for it?”
Liz Glatzer felt her doctor in Providence, Rhode Island, was competent but didn’t have time to absorb her full health history. “I had double mastectomy 25 years ago,” she said. “At my first physical, the doctor ran through my meds and whatever else, and she said, ‘Oh, you haven’t had a mammogram.’ I said, ‘I don’t have breasts to have mammography.’ ”
In 2023, after repeating that same exchange during her next two physicals, Ms. Glatzer signed up to pay $1,900 a year for MDVIP, a concierge staffing service that contracts with her new doctor, who is also a friend’s husband. In her first couple of visits, Ms. Glatzer’s new physician took hours to get to know her, she said.
For the growing numbers of Americans who can’t or won’t pay when their doctor switches to concierge care, finding new primary care can mean frustration, delayed or missed tests or treatments, and fragmented health care.
“I’ve met so many patients who couldn’t afford the concierge services and needed to look for a new primary care physician,” said Yalda Jabbarpour, director of the Robert Graham Center and a practicing family physician. Separating from a doctor who’s transitioning to concierge care “breaks the continuity with the provider that we know is so important for good health outcomes.” .
That disruption has consequences. “People don’t get the preventive services that they should, and they use more expensive and inefficient avenues for care that could have otherwise been provided by their doctor,” said Abbie Leibowitz, chief medical officer at Health Advocate, a company that helps patients find care and resolve insurance issues.
What happens to patients who find themselves at loose ends when a physician transitions to concierge practice?
Patients who lose their doctors often give up on having an ongoing relationship with a primary care clinician. They may rely solely on a pharmacy-based clinic or urgent care center or even a hospital emergency department for primary care.
Some concierge providers say they are responding to concerns about access and equity by allowing patients to opt out of concierge care but stay with the practice group at a lower tier of service. This might entail longer waits for shorter appointments, fewer visits with a physician, and more visits with mid-level providers, for example.
Deb Gordon of Cambridge, Massachusetts, said she is searching for a new primary care doctor after hers switched to concierge medicine — a challenge that involves finding someone in her network who has admitting privileges at her preferred hospitals and is accepting new patients.
Ms. Gordon, who is codirector of the Alliance of Professional Health Advocates, which provides support services to patient advocates, said the practice that her doctor left has not assigned her a new provider, and her health plan said it was OK if she went without one. “I was shocked that they literally said, ‘You can go to urgent care.’ ”
Some patients find themselves turning to physician assistants and other mid-level providers. But those clinicians have much less training than physicians with board certification in family medicine or internal medicine and so may not be fully qualified to treat patients with complex health problems. “The expertise of physician assistants and nurse practitioners can really vary widely,” said Russell Phillips, director of the Harvard Medical School Center for Primary Care.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF—an independent source of health policy research, polling, and journalism. Learn more about KFF.
“You had to pay the fee, or the doctor wasn’t going to see you anymore.”
That was the takeaway for Terri Marroquin of Midland, Texas, when her longtime physician began charging a membership fee in 2019. She found out about the change when someone at the physician’s front desk pointed to a posted notice.
At first, she stuck with the practice; in her area, she said, it is now tough to find a primary care doctor who doesn’t charge an annual membership fee from $350 to $500.
But last year, Ms. Marroquin finally left to join a practice with no membership fee where she sees a physician assistant rather than a doctor. “I had had enough. The concierge fee kept going up, and the doctor’s office kept getting nicer and nicer,” she said, referring to the décor.
Many people seeking a new doctor are calling a long list of primary care practices only to be told they’re not taking new patients.
“Concierge medicine potentially leads to disproportionately richer people being able to pay for the scarce resource of physician time and crowding out people who have lower incomes and are sicker,” said Adam Leive, PhD, lead author of a 2023 study on concierge medicine and researcher at the University of California, Berkeley.
Dr. Leive’s research showed no decrease in mortality for concierge patients compared with similar patients who saw nonconcierge physicians, suggesting concierge care may not notably improve some health outcomes.
A 2005 study showed concierge physicians had smaller proportions of patients with diabetes than their nonconcierge counterparts and provided care for fewer Black and Hispanic patients.
There’s little reliable data available on the size of the concierge medicine market. But one market research firm projects that concierge medicine revenue will grow about 10.4% annually through 2030. About 5,000 to 7,000 physicians and practices provide concierge care in the United States, most of whom are primary care providers, according to Concierge Medicine Today. (Yes, the burgeoning field already has a trade publication.)
The concierge pitch is simple: More time with your doctor, in-person or remotely, promptly and at your convenience. With many primary care physicians caring for thousands of patients each in appointments of 15 minutes or less, some people who can afford the fee say they feel forced to pay it just to maintain adequate access to their doctor.
As primary care providers convert to concierge medicine, many patients could face the financial and health consequences of a potentially lengthy search for a new provider. With fewer physicians in nonconcierge practices, the pool available to people who can’t or won’t pay is smaller. For them, it is harder to find a doctor.
Concierge care models vary widely, but all involve paying a periodic fee to be a patient of the practice.
These fees are generally not covered by insurance nor payable with a tax-advantaged flexible spending account or health savings account. Annual fees range from $199 for Amazon’s One Medical (with a discount available for Prime members) to low four figures for companies like MDVIP and SignatureMD that partner with physicians, to $10,000 or more for top-branded practices like Massachusetts General Hospital’s.
Many patients are exasperated with the prospect of pay-to-play primary care. For one thing, under the Affordable Care Act, insurers are required to cover a variety of preventive services without a patient paying out of pocket. “Your annual physical should be free,” said Caitlin Donovan, a spokesperson for the National Patient Advocate Foundation. “Why are you paying $2,000 for it?”
Liz Glatzer felt her doctor in Providence, Rhode Island, was competent but didn’t have time to absorb her full health history. “I had double mastectomy 25 years ago,” she said. “At my first physical, the doctor ran through my meds and whatever else, and she said, ‘Oh, you haven’t had a mammogram.’ I said, ‘I don’t have breasts to have mammography.’ ”
In 2023, after repeating that same exchange during her next two physicals, Ms. Glatzer signed up to pay $1,900 a year for MDVIP, a concierge staffing service that contracts with her new doctor, who is also a friend’s husband. In her first couple of visits, Ms. Glatzer’s new physician took hours to get to know her, she said.
For the growing numbers of Americans who can’t or won’t pay when their doctor switches to concierge care, finding new primary care can mean frustration, delayed or missed tests or treatments, and fragmented health care.
“I’ve met so many patients who couldn’t afford the concierge services and needed to look for a new primary care physician,” said Yalda Jabbarpour, director of the Robert Graham Center and a practicing family physician. Separating from a doctor who’s transitioning to concierge care “breaks the continuity with the provider that we know is so important for good health outcomes.” .
That disruption has consequences. “People don’t get the preventive services that they should, and they use more expensive and inefficient avenues for care that could have otherwise been provided by their doctor,” said Abbie Leibowitz, chief medical officer at Health Advocate, a company that helps patients find care and resolve insurance issues.
What happens to patients who find themselves at loose ends when a physician transitions to concierge practice?
Patients who lose their doctors often give up on having an ongoing relationship with a primary care clinician. They may rely solely on a pharmacy-based clinic or urgent care center or even a hospital emergency department for primary care.
Some concierge providers say they are responding to concerns about access and equity by allowing patients to opt out of concierge care but stay with the practice group at a lower tier of service. This might entail longer waits for shorter appointments, fewer visits with a physician, and more visits with mid-level providers, for example.
Deb Gordon of Cambridge, Massachusetts, said she is searching for a new primary care doctor after hers switched to concierge medicine — a challenge that involves finding someone in her network who has admitting privileges at her preferred hospitals and is accepting new patients.
Ms. Gordon, who is codirector of the Alliance of Professional Health Advocates, which provides support services to patient advocates, said the practice that her doctor left has not assigned her a new provider, and her health plan said it was OK if she went without one. “I was shocked that they literally said, ‘You can go to urgent care.’ ”
Some patients find themselves turning to physician assistants and other mid-level providers. But those clinicians have much less training than physicians with board certification in family medicine or internal medicine and so may not be fully qualified to treat patients with complex health problems. “The expertise of physician assistants and nurse practitioners can really vary widely,” said Russell Phillips, director of the Harvard Medical School Center for Primary Care.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF—an independent source of health policy research, polling, and journalism. Learn more about KFF.