User login
As constituents clamor for ivermectin, Republican politicians embrace the cause
When state senators in South Carolina held two hearings in September about COVID-19 treatments, they got an earful on the benefits of ivermectin — which many of the lawmakers echoed, sharing experiences of their own loved ones.
The demands for access to the drug were loud and insistent, despite federal regulators’ recent warning against using the drug to treat COVID.
Ivermectin is a generic drug that has been used for decades to treat river blindness, scabies, and even head lice. Veterinarians also use it, in different formulations and dosages, to treat animals for parasites like worms.
At one of the South Carolina hearings, Pressley Stutts III reminded the panel that his father, a prominent GOP leader in the state, had died of COVID a month earlier. He believed ivermectin could have helped him. But doctors at the hospital wouldn’t discuss it.
“I went every bit as far as I could without getting myself thrown in jail trying to save my father’s life,” he told the panel, as lawmakers offered condolences.
“What is going on here?” he asked, with the passion in his voice growing. “My dad’s dead!”
After the pandemic began, scientists launched clinical trials to see if ivermectin could help as a treatment for COVID. Some are still ongoing. But providers in mainstream medicine have rejected it as a COVID treatment, citing the poor quality of the studies to date, and two notorious “preprint” studies that were circulated before they were peer-reviewed, and later taken off the internet because of inaccurate and flawed data.
On Aug. 26, the Centers for Disease Control and Prevention advised clinicians not to use ivermectin, citing insufficient evidence of benefit and pointing out that unauthorized use had led to accidental poisonings. Vaccination, the CDC reiterated, is still the best way to avoid serious illness and death from the coronavirus.
But many Americans remain convinced ivermectin could be beneficial, and some politicians appear to be listening to them.
“If we have medications out here that are working — or seem to be working — I think it’s absolutely horrible that we’re not trying them,” said Republican state Sen. Tom Corbin in South Carolina. He questioned doctors who had come to the Statehouse to counter efforts to move ivermectin into mainstream use.
The doctors challenged the implied insult that they weren’t following best practices: “Any implication that any of us would do anything to withhold effective treatments from our patients is really insulting to our profession,” said Dr. Annie Andrews, a professor at the Medical University of South Carolina who has cared for COVID patients throughout the pandemic.
Instead of listening to the medical consensus, some politicians in states like South Carolina seem to be taking cues from doctors on the fringe. During one September hearing, state senators patched in a call from Dr. Pierre Kory.
Last year, Dr. Kory started a nonprofit called the Front Line COVID-19 Critical Care Alliance, which promotes ivermectin. He said he’s not making money by prescribing the drug, though the nonprofit does solicit donations and has not yet filed required financial documents with the IRS.
Dr. Kory acknowledged his medical opinions have landed him on “an island.”
He first testified about ivermectin to a U.S. Senate committee in December. That video went viral. Although it was taken down by YouTube, his Senate testimony prompted patients across the country to ask for ivermectin when they fell ill.
By late August, outpatient prescriptions had jumped 24-fold. Calls to poison control hotlines had tripled, mostly related to people taking ivermectin formulations meant for livestock.
Dr. Kory said he has effectively lost two jobs over his views on ivermectin. At his current hospital in Wisconsin, where he runs the intensive care unit two weeks a month, managers called him to a meeting in September, where he was informed he could no longer prescribe ivermectin. He’d been giving it to “every patient with COVID,” he said.
“After the pharma-geddon that was unleashed, yeah, they shut it down,” he told the South Carolina lawmakers. “And I will tell you that many hospitals across the country had already shut it down months ago.”
Framing the ivermectin fight as a battle against faceless federal agencies and big pharmaceutical corporations appealed to Americans already suspicious of the science behind the pandemic and the approved COVID vaccines.
Dr. Kory suggests success stories with COVID treatments in other parts of the world have been suppressed to instead promote the vaccines.
In an interview with NPR, Dr. Kory said he regrets the flashpoint he helped ignite.
“I feel really bad for the patients, and I feel really bad for the doctors,” he told NPR. “Both of them — both the patients and doctors — are trapped.”
Patients are still demanding the treatment, but doctors sympathetic to their wishes are being told by their health systems not to try it.
Now conservatives in elected office are sensing political payoff if they step in to help patients get the drug. State legislatures, including those in Tennessee and Alaska, are debating various ways to increase access to ivermectin — with proposals such as shielding doctors from repercussions for prescribing it, or forcing pharmacists to fill questionable prescriptions.
The Montana State News Bureau reported that the state’s Republican attorney general dispatched a state trooper to a hospital in Helena where a politically connected patient was dying of COVID. Her family was asking for ivermectin.
In a statement, St. Peter’s Hospital said doctors and nurses were “harassed and threatened by three public officials.”
“These officials have no medical training or experience, yet they were insisting our providers give treatments for COVID-19 that are not authorized, clinically approved, or within the guidelines established by the FDA and the CDC,” the statement added.
On Oct. 14, the Republican attorney general in Nebraska addressed the controversy, issuing a nearly 50-page legal opinion arguing that doctors who consider the “off-label” use of ivermectin and hydroxychloroquine for COVID are acting within the parameters of their state medical licenses, as long as the physician obtains appropriate informed consent from a patient.
Some patients have filed lawsuits to obtain ivermectin, with mixed success. A patient in Illinois was denied. But other hospitals, including one in Ohio, have been forced to administer the drug against the objections of their physicians.
Even as they gain powerful political supporters, some ivermectin fans say they’re now avoiding the health care system — because they’ve lost faith in it.
Lesa Berry, of Richmond, Va., had a friend who died earlier this year of COVID. The doctors refused to use ivermectin, despite requests from Ms. Berry and the patient’s daughter.
They know better now, she said.
“My first attempt would have been to keep her out of the hospital,” Ms. Berry said. “Because right now when you go to the hospital, they only give you what’s on the CDC protocol.”
Ms. Berry and her husband have purchased their own supply of ivermectin, which they keep at home.
This story is from a partnership that includes NPR, Nashville Public Radio and KHN. KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
When state senators in South Carolina held two hearings in September about COVID-19 treatments, they got an earful on the benefits of ivermectin — which many of the lawmakers echoed, sharing experiences of their own loved ones.
The demands for access to the drug were loud and insistent, despite federal regulators’ recent warning against using the drug to treat COVID.
Ivermectin is a generic drug that has been used for decades to treat river blindness, scabies, and even head lice. Veterinarians also use it, in different formulations and dosages, to treat animals for parasites like worms.
At one of the South Carolina hearings, Pressley Stutts III reminded the panel that his father, a prominent GOP leader in the state, had died of COVID a month earlier. He believed ivermectin could have helped him. But doctors at the hospital wouldn’t discuss it.
“I went every bit as far as I could without getting myself thrown in jail trying to save my father’s life,” he told the panel, as lawmakers offered condolences.
“What is going on here?” he asked, with the passion in his voice growing. “My dad’s dead!”
After the pandemic began, scientists launched clinical trials to see if ivermectin could help as a treatment for COVID. Some are still ongoing. But providers in mainstream medicine have rejected it as a COVID treatment, citing the poor quality of the studies to date, and two notorious “preprint” studies that were circulated before they were peer-reviewed, and later taken off the internet because of inaccurate and flawed data.
On Aug. 26, the Centers for Disease Control and Prevention advised clinicians not to use ivermectin, citing insufficient evidence of benefit and pointing out that unauthorized use had led to accidental poisonings. Vaccination, the CDC reiterated, is still the best way to avoid serious illness and death from the coronavirus.
But many Americans remain convinced ivermectin could be beneficial, and some politicians appear to be listening to them.
“If we have medications out here that are working — or seem to be working — I think it’s absolutely horrible that we’re not trying them,” said Republican state Sen. Tom Corbin in South Carolina. He questioned doctors who had come to the Statehouse to counter efforts to move ivermectin into mainstream use.
The doctors challenged the implied insult that they weren’t following best practices: “Any implication that any of us would do anything to withhold effective treatments from our patients is really insulting to our profession,” said Dr. Annie Andrews, a professor at the Medical University of South Carolina who has cared for COVID patients throughout the pandemic.
Instead of listening to the medical consensus, some politicians in states like South Carolina seem to be taking cues from doctors on the fringe. During one September hearing, state senators patched in a call from Dr. Pierre Kory.
Last year, Dr. Kory started a nonprofit called the Front Line COVID-19 Critical Care Alliance, which promotes ivermectin. He said he’s not making money by prescribing the drug, though the nonprofit does solicit donations and has not yet filed required financial documents with the IRS.
Dr. Kory acknowledged his medical opinions have landed him on “an island.”
He first testified about ivermectin to a U.S. Senate committee in December. That video went viral. Although it was taken down by YouTube, his Senate testimony prompted patients across the country to ask for ivermectin when they fell ill.
By late August, outpatient prescriptions had jumped 24-fold. Calls to poison control hotlines had tripled, mostly related to people taking ivermectin formulations meant for livestock.
Dr. Kory said he has effectively lost two jobs over his views on ivermectin. At his current hospital in Wisconsin, where he runs the intensive care unit two weeks a month, managers called him to a meeting in September, where he was informed he could no longer prescribe ivermectin. He’d been giving it to “every patient with COVID,” he said.
“After the pharma-geddon that was unleashed, yeah, they shut it down,” he told the South Carolina lawmakers. “And I will tell you that many hospitals across the country had already shut it down months ago.”
Framing the ivermectin fight as a battle against faceless federal agencies and big pharmaceutical corporations appealed to Americans already suspicious of the science behind the pandemic and the approved COVID vaccines.
Dr. Kory suggests success stories with COVID treatments in other parts of the world have been suppressed to instead promote the vaccines.
In an interview with NPR, Dr. Kory said he regrets the flashpoint he helped ignite.
“I feel really bad for the patients, and I feel really bad for the doctors,” he told NPR. “Both of them — both the patients and doctors — are trapped.”
Patients are still demanding the treatment, but doctors sympathetic to their wishes are being told by their health systems not to try it.
Now conservatives in elected office are sensing political payoff if they step in to help patients get the drug. State legislatures, including those in Tennessee and Alaska, are debating various ways to increase access to ivermectin — with proposals such as shielding doctors from repercussions for prescribing it, or forcing pharmacists to fill questionable prescriptions.
The Montana State News Bureau reported that the state’s Republican attorney general dispatched a state trooper to a hospital in Helena where a politically connected patient was dying of COVID. Her family was asking for ivermectin.
In a statement, St. Peter’s Hospital said doctors and nurses were “harassed and threatened by three public officials.”
“These officials have no medical training or experience, yet they were insisting our providers give treatments for COVID-19 that are not authorized, clinically approved, or within the guidelines established by the FDA and the CDC,” the statement added.
On Oct. 14, the Republican attorney general in Nebraska addressed the controversy, issuing a nearly 50-page legal opinion arguing that doctors who consider the “off-label” use of ivermectin and hydroxychloroquine for COVID are acting within the parameters of their state medical licenses, as long as the physician obtains appropriate informed consent from a patient.
Some patients have filed lawsuits to obtain ivermectin, with mixed success. A patient in Illinois was denied. But other hospitals, including one in Ohio, have been forced to administer the drug against the objections of their physicians.
Even as they gain powerful political supporters, some ivermectin fans say they’re now avoiding the health care system — because they’ve lost faith in it.
Lesa Berry, of Richmond, Va., had a friend who died earlier this year of COVID. The doctors refused to use ivermectin, despite requests from Ms. Berry and the patient’s daughter.
They know better now, she said.
“My first attempt would have been to keep her out of the hospital,” Ms. Berry said. “Because right now when you go to the hospital, they only give you what’s on the CDC protocol.”
Ms. Berry and her husband have purchased their own supply of ivermectin, which they keep at home.
This story is from a partnership that includes NPR, Nashville Public Radio and KHN. KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
When state senators in South Carolina held two hearings in September about COVID-19 treatments, they got an earful on the benefits of ivermectin — which many of the lawmakers echoed, sharing experiences of their own loved ones.
The demands for access to the drug were loud and insistent, despite federal regulators’ recent warning against using the drug to treat COVID.
Ivermectin is a generic drug that has been used for decades to treat river blindness, scabies, and even head lice. Veterinarians also use it, in different formulations and dosages, to treat animals for parasites like worms.
At one of the South Carolina hearings, Pressley Stutts III reminded the panel that his father, a prominent GOP leader in the state, had died of COVID a month earlier. He believed ivermectin could have helped him. But doctors at the hospital wouldn’t discuss it.
“I went every bit as far as I could without getting myself thrown in jail trying to save my father’s life,” he told the panel, as lawmakers offered condolences.
“What is going on here?” he asked, with the passion in his voice growing. “My dad’s dead!”
After the pandemic began, scientists launched clinical trials to see if ivermectin could help as a treatment for COVID. Some are still ongoing. But providers in mainstream medicine have rejected it as a COVID treatment, citing the poor quality of the studies to date, and two notorious “preprint” studies that were circulated before they were peer-reviewed, and later taken off the internet because of inaccurate and flawed data.
On Aug. 26, the Centers for Disease Control and Prevention advised clinicians not to use ivermectin, citing insufficient evidence of benefit and pointing out that unauthorized use had led to accidental poisonings. Vaccination, the CDC reiterated, is still the best way to avoid serious illness and death from the coronavirus.
But many Americans remain convinced ivermectin could be beneficial, and some politicians appear to be listening to them.
“If we have medications out here that are working — or seem to be working — I think it’s absolutely horrible that we’re not trying them,” said Republican state Sen. Tom Corbin in South Carolina. He questioned doctors who had come to the Statehouse to counter efforts to move ivermectin into mainstream use.
The doctors challenged the implied insult that they weren’t following best practices: “Any implication that any of us would do anything to withhold effective treatments from our patients is really insulting to our profession,” said Dr. Annie Andrews, a professor at the Medical University of South Carolina who has cared for COVID patients throughout the pandemic.
Instead of listening to the medical consensus, some politicians in states like South Carolina seem to be taking cues from doctors on the fringe. During one September hearing, state senators patched in a call from Dr. Pierre Kory.
Last year, Dr. Kory started a nonprofit called the Front Line COVID-19 Critical Care Alliance, which promotes ivermectin. He said he’s not making money by prescribing the drug, though the nonprofit does solicit donations and has not yet filed required financial documents with the IRS.
Dr. Kory acknowledged his medical opinions have landed him on “an island.”
He first testified about ivermectin to a U.S. Senate committee in December. That video went viral. Although it was taken down by YouTube, his Senate testimony prompted patients across the country to ask for ivermectin when they fell ill.
By late August, outpatient prescriptions had jumped 24-fold. Calls to poison control hotlines had tripled, mostly related to people taking ivermectin formulations meant for livestock.
Dr. Kory said he has effectively lost two jobs over his views on ivermectin. At his current hospital in Wisconsin, where he runs the intensive care unit two weeks a month, managers called him to a meeting in September, where he was informed he could no longer prescribe ivermectin. He’d been giving it to “every patient with COVID,” he said.
“After the pharma-geddon that was unleashed, yeah, they shut it down,” he told the South Carolina lawmakers. “And I will tell you that many hospitals across the country had already shut it down months ago.”
Framing the ivermectin fight as a battle against faceless federal agencies and big pharmaceutical corporations appealed to Americans already suspicious of the science behind the pandemic and the approved COVID vaccines.
Dr. Kory suggests success stories with COVID treatments in other parts of the world have been suppressed to instead promote the vaccines.
In an interview with NPR, Dr. Kory said he regrets the flashpoint he helped ignite.
“I feel really bad for the patients, and I feel really bad for the doctors,” he told NPR. “Both of them — both the patients and doctors — are trapped.”
Patients are still demanding the treatment, but doctors sympathetic to their wishes are being told by their health systems not to try it.
Now conservatives in elected office are sensing political payoff if they step in to help patients get the drug. State legislatures, including those in Tennessee and Alaska, are debating various ways to increase access to ivermectin — with proposals such as shielding doctors from repercussions for prescribing it, or forcing pharmacists to fill questionable prescriptions.
The Montana State News Bureau reported that the state’s Republican attorney general dispatched a state trooper to a hospital in Helena where a politically connected patient was dying of COVID. Her family was asking for ivermectin.
In a statement, St. Peter’s Hospital said doctors and nurses were “harassed and threatened by three public officials.”
“These officials have no medical training or experience, yet they were insisting our providers give treatments for COVID-19 that are not authorized, clinically approved, or within the guidelines established by the FDA and the CDC,” the statement added.
On Oct. 14, the Republican attorney general in Nebraska addressed the controversy, issuing a nearly 50-page legal opinion arguing that doctors who consider the “off-label” use of ivermectin and hydroxychloroquine for COVID are acting within the parameters of their state medical licenses, as long as the physician obtains appropriate informed consent from a patient.
Some patients have filed lawsuits to obtain ivermectin, with mixed success. A patient in Illinois was denied. But other hospitals, including one in Ohio, have been forced to administer the drug against the objections of their physicians.
Even as they gain powerful political supporters, some ivermectin fans say they’re now avoiding the health care system — because they’ve lost faith in it.
Lesa Berry, of Richmond, Va., had a friend who died earlier this year of COVID. The doctors refused to use ivermectin, despite requests from Ms. Berry and the patient’s daughter.
They know better now, she said.
“My first attempt would have been to keep her out of the hospital,” Ms. Berry said. “Because right now when you go to the hospital, they only give you what’s on the CDC protocol.”
Ms. Berry and her husband have purchased their own supply of ivermectin, which they keep at home.
This story is from a partnership that includes NPR, Nashville Public Radio and KHN. KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Breast milk of COVID-19–infected mothers helps build infant’s immune defenses
It’s rare for mothers with COVID-19 to transfer the infection to their newborns, according to a new small study.
The research, published in JAMA Network Open, found that newborns of mothers infected with the COVID-19 virus were able to develop their own immune defenses via their mother’s breast milk. Researchers detected antibodies in the infants’ saliva.
“It is the first time that this mechanism has been demonstrated,” said study author Rita Carsetti, MD, head of immunology diagnostics for Bambino Gesù Children’s Hospital in Rome. “We now know how breast milk can help babies develop their immune defenses. The system could work the same way for many other pathogens, which are present in the mother during breastfeeding.”
Dr. Carsetti and colleagues examined data from 28 pregnant women who tested positive for COVID-19 and who gave birth at Policlinico Umberto I in Rome between November 2020 and May 2021, and their newborns. They investigated the immune responses of the mothers and their newborns by detecting spike-specific antibodies in serum, and the mucosal immune response was assessed by measuring specific antibodies in maternal breast milk and infant saliva 48 hours after delivery and 2 months later.
Twenty-one mothers and their newborns completed the 2 months of follow-up. Researchers found that the majority of the mothers had mild symptoms of COVID-19, while only three of them were admitted for worsening condition. There was only one reported case of a possible vertical transmission – transmitted in utero – and one case of a horizontal infection through droplets or respiratory secretions, which occurred when the newborn was taken home.
The results of the study showed that antibodies specific to the virus were present in the mothers’ blood at 2 months after delivery, but not at 48 hours. However, in milk, specific antibodies were already present 48 hours after delivery.
Therefore, after 48 hours, the breastfed babies had specific mucosal antibodies against COVID-19 in their saliva that the other newborns did not have. Two months later, these antibodies continued to be present even though the mothers had stopped producing them.
The findings suggest that breast milk offers protection by transferring the antibodies produced by the mother to the baby, but also by helping them to produce their own immune defenses.
“I am not surprised that infants of mothers who had COVID-19 infection in the peripartum period pass anti-spike protein IgA to their infants,” J. Howard Smart, MD, FAAP, who was not involved with the study, said in an interview. “This confirmation is good news for breastfeeding mothers.
“I wonder whether we really know these infants did not become infected, and produce their own antibodies,” said Dr. Smart, chairman of the department of pediatrics at Sharp Rees-Stealy Medical Group in San Diego.
The American College of Obstetricians and Gynecologists said having COVID-19 should not stop mothers from giving their children breast milk. The organization also said that the chance of COVID-19 passing through the breast milk and causing infection in the newborn infant is slim.
“Breast milk also helps protect babies from infections, including infections of the ears, lungs, and digestive system. For these reasons, having COVID-19 should not stop you from giving your baby breast milk,” according to ACOG’s website.
Similar studies on mothers who received the COVID-19 vaccination rather than being infected would be interesting, Dr. Smart added.
The authors of the current study plan to broaden their research by evaluating the response of pregnant mothers vaccinated against SARS-CoV-2 for the presence of antibodies in the milk and the immunity of their newborns. Dr. Carsetti said her team plans to expand the study to other infections, such as cytomegalovirus and respiratory syncytial virus.
None of the researchers or commentators had financial disclosures.
It’s rare for mothers with COVID-19 to transfer the infection to their newborns, according to a new small study.
The research, published in JAMA Network Open, found that newborns of mothers infected with the COVID-19 virus were able to develop their own immune defenses via their mother’s breast milk. Researchers detected antibodies in the infants’ saliva.
“It is the first time that this mechanism has been demonstrated,” said study author Rita Carsetti, MD, head of immunology diagnostics for Bambino Gesù Children’s Hospital in Rome. “We now know how breast milk can help babies develop their immune defenses. The system could work the same way for many other pathogens, which are present in the mother during breastfeeding.”
Dr. Carsetti and colleagues examined data from 28 pregnant women who tested positive for COVID-19 and who gave birth at Policlinico Umberto I in Rome between November 2020 and May 2021, and their newborns. They investigated the immune responses of the mothers and their newborns by detecting spike-specific antibodies in serum, and the mucosal immune response was assessed by measuring specific antibodies in maternal breast milk and infant saliva 48 hours after delivery and 2 months later.
Twenty-one mothers and their newborns completed the 2 months of follow-up. Researchers found that the majority of the mothers had mild symptoms of COVID-19, while only three of them were admitted for worsening condition. There was only one reported case of a possible vertical transmission – transmitted in utero – and one case of a horizontal infection through droplets or respiratory secretions, which occurred when the newborn was taken home.
The results of the study showed that antibodies specific to the virus were present in the mothers’ blood at 2 months after delivery, but not at 48 hours. However, in milk, specific antibodies were already present 48 hours after delivery.
Therefore, after 48 hours, the breastfed babies had specific mucosal antibodies against COVID-19 in their saliva that the other newborns did not have. Two months later, these antibodies continued to be present even though the mothers had stopped producing them.
The findings suggest that breast milk offers protection by transferring the antibodies produced by the mother to the baby, but also by helping them to produce their own immune defenses.
“I am not surprised that infants of mothers who had COVID-19 infection in the peripartum period pass anti-spike protein IgA to their infants,” J. Howard Smart, MD, FAAP, who was not involved with the study, said in an interview. “This confirmation is good news for breastfeeding mothers.
“I wonder whether we really know these infants did not become infected, and produce their own antibodies,” said Dr. Smart, chairman of the department of pediatrics at Sharp Rees-Stealy Medical Group in San Diego.
The American College of Obstetricians and Gynecologists said having COVID-19 should not stop mothers from giving their children breast milk. The organization also said that the chance of COVID-19 passing through the breast milk and causing infection in the newborn infant is slim.
“Breast milk also helps protect babies from infections, including infections of the ears, lungs, and digestive system. For these reasons, having COVID-19 should not stop you from giving your baby breast milk,” according to ACOG’s website.
Similar studies on mothers who received the COVID-19 vaccination rather than being infected would be interesting, Dr. Smart added.
The authors of the current study plan to broaden their research by evaluating the response of pregnant mothers vaccinated against SARS-CoV-2 for the presence of antibodies in the milk and the immunity of their newborns. Dr. Carsetti said her team plans to expand the study to other infections, such as cytomegalovirus and respiratory syncytial virus.
None of the researchers or commentators had financial disclosures.
It’s rare for mothers with COVID-19 to transfer the infection to their newborns, according to a new small study.
The research, published in JAMA Network Open, found that newborns of mothers infected with the COVID-19 virus were able to develop their own immune defenses via their mother’s breast milk. Researchers detected antibodies in the infants’ saliva.
“It is the first time that this mechanism has been demonstrated,” said study author Rita Carsetti, MD, head of immunology diagnostics for Bambino Gesù Children’s Hospital in Rome. “We now know how breast milk can help babies develop their immune defenses. The system could work the same way for many other pathogens, which are present in the mother during breastfeeding.”
Dr. Carsetti and colleagues examined data from 28 pregnant women who tested positive for COVID-19 and who gave birth at Policlinico Umberto I in Rome between November 2020 and May 2021, and their newborns. They investigated the immune responses of the mothers and their newborns by detecting spike-specific antibodies in serum, and the mucosal immune response was assessed by measuring specific antibodies in maternal breast milk and infant saliva 48 hours after delivery and 2 months later.
Twenty-one mothers and their newborns completed the 2 months of follow-up. Researchers found that the majority of the mothers had mild symptoms of COVID-19, while only three of them were admitted for worsening condition. There was only one reported case of a possible vertical transmission – transmitted in utero – and one case of a horizontal infection through droplets or respiratory secretions, which occurred when the newborn was taken home.
The results of the study showed that antibodies specific to the virus were present in the mothers’ blood at 2 months after delivery, but not at 48 hours. However, in milk, specific antibodies were already present 48 hours after delivery.
Therefore, after 48 hours, the breastfed babies had specific mucosal antibodies against COVID-19 in their saliva that the other newborns did not have. Two months later, these antibodies continued to be present even though the mothers had stopped producing them.
The findings suggest that breast milk offers protection by transferring the antibodies produced by the mother to the baby, but also by helping them to produce their own immune defenses.
“I am not surprised that infants of mothers who had COVID-19 infection in the peripartum period pass anti-spike protein IgA to their infants,” J. Howard Smart, MD, FAAP, who was not involved with the study, said in an interview. “This confirmation is good news for breastfeeding mothers.
“I wonder whether we really know these infants did not become infected, and produce their own antibodies,” said Dr. Smart, chairman of the department of pediatrics at Sharp Rees-Stealy Medical Group in San Diego.
The American College of Obstetricians and Gynecologists said having COVID-19 should not stop mothers from giving their children breast milk. The organization also said that the chance of COVID-19 passing through the breast milk and causing infection in the newborn infant is slim.
“Breast milk also helps protect babies from infections, including infections of the ears, lungs, and digestive system. For these reasons, having COVID-19 should not stop you from giving your baby breast milk,” according to ACOG’s website.
Similar studies on mothers who received the COVID-19 vaccination rather than being infected would be interesting, Dr. Smart added.
The authors of the current study plan to broaden their research by evaluating the response of pregnant mothers vaccinated against SARS-CoV-2 for the presence of antibodies in the milk and the immunity of their newborns. Dr. Carsetti said her team plans to expand the study to other infections, such as cytomegalovirus and respiratory syncytial virus.
None of the researchers or commentators had financial disclosures.
FROM JAMA NETWORK OPEN
Genotype, need for transfusion predict death in VEXAS syndrome
Among patients with the recently defined severe autoinflammatory syndrome VEXAS, those who are transfusion dependent or have a specific amino acid substitution are at highest risk for death, whereas those with ear chondritis are at significantly lower risk, a multinational team of investigators has found.
Their study of mortality and predictors of survival among patients with genetically confirmed VEXAS showed that patients with a VEXAS variant resulting in an amino acid substitution of a methionine for a valine had a 3.5-fold higher risk for death, compared with patients with either a methionine-to-threonine substitution or a methionine-to-leucine swap.
Transfusion dependence was an independent predictor of mortality. Patients who became dependent on transfusions after symptom onset had a nearly threefold higher risk for death, reported Marcela A. Ferrada, MD, a clinical fellow at the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
“These findings should inform risk assessment and clinical management in patients with VEXAS syndrome,” she said in an oral abstract presentation during the virtual annual meeting of the American College of Rheumatology.
“These genetic findings have proven right now to be not only diagnostic, but we have shown that they’re also prognostic, and we hope that this is going to help us identify patients who could have more aggressive treatment,” Dr. Ferrada said.
She also discussed her findings in a media briefing held 2 days prior to her plenary presentation. At that briefing, this news organization asked participating clinicians whether they had patients who they suspected may have had undiagnosed VEXAS.
“My answer to that is interesting,” replied moderator Vaneet Sandhu, MD, from Loma Linda (Calif.) University and Riverside University Health System.
“In the last couple of days, I’ve been reading about VEXAS, and actually texted one of my colleagues yesterday and said, ‘Hey, you know these patients we’ve been seeing who have these strange rashes and chondritis and have maybe a diagnosis of leukocytoclastic vasculitis or something else – are we not diagnosing these patients?’ ” she said.
“I think we are looking at every patient with chondritis and reexamining their phenotype. We had dismissed certain symptoms because they didn’t fit the archetype for relapsing polychondritis, for example, but it could be VEXAS,” said Alfred Kim, MD, PhD, of Washington University in St. Louis, who also presented data during the briefing.
Three variants
VEXAS is caused by somatic mutations in UBA1, a gene that initiates cytoplasmic ubiquitylation, a process by which misfolded proteins are tagged for degradation.
The syndrome’s name is an acronym descriptive of the major features:
- Vacuoles in bone marrow cells.
- E-1 activating enzyme that UBA1 encodes for.
- X-linked.
- Autoinflammatory.
- Somatic mutation featuring hematologic mosaicism.
VEXAS results in rheumatologic, dermatologic, and hematologic symptoms that are often misdiagnosed as being caused by treatment-refractory relapsing polychondritis, polyarteritis nodosa, Sweet syndrome, giant cell arteritis, or myelodysplastic syndrome (MDS).
VEXAS was identified as a distinct syndrome within the past year by Dr. Ferrada and other investigators at NIAMS, the National Human Genome Research Institute, and other institutions.
In the study reported at ACR 2021, Dr. Ferrada and colleagues assessed 83 men who had been referred for genetic testing for VEXAS at the National Institutes of Health, in Bethesda, Md., and at Leeds (England) Teaching Hospitals NHS Trust.
All patients were confirmed to have VEXAS-defining genetic mutations in UBA1 by Sanger sequencing of peripheral blood samples. Only those patients with mutations at codon p.Met41 were included in the investigators’ analysis. Mutations at that site account for nearly all cases of VEXAS that have been identified to date.
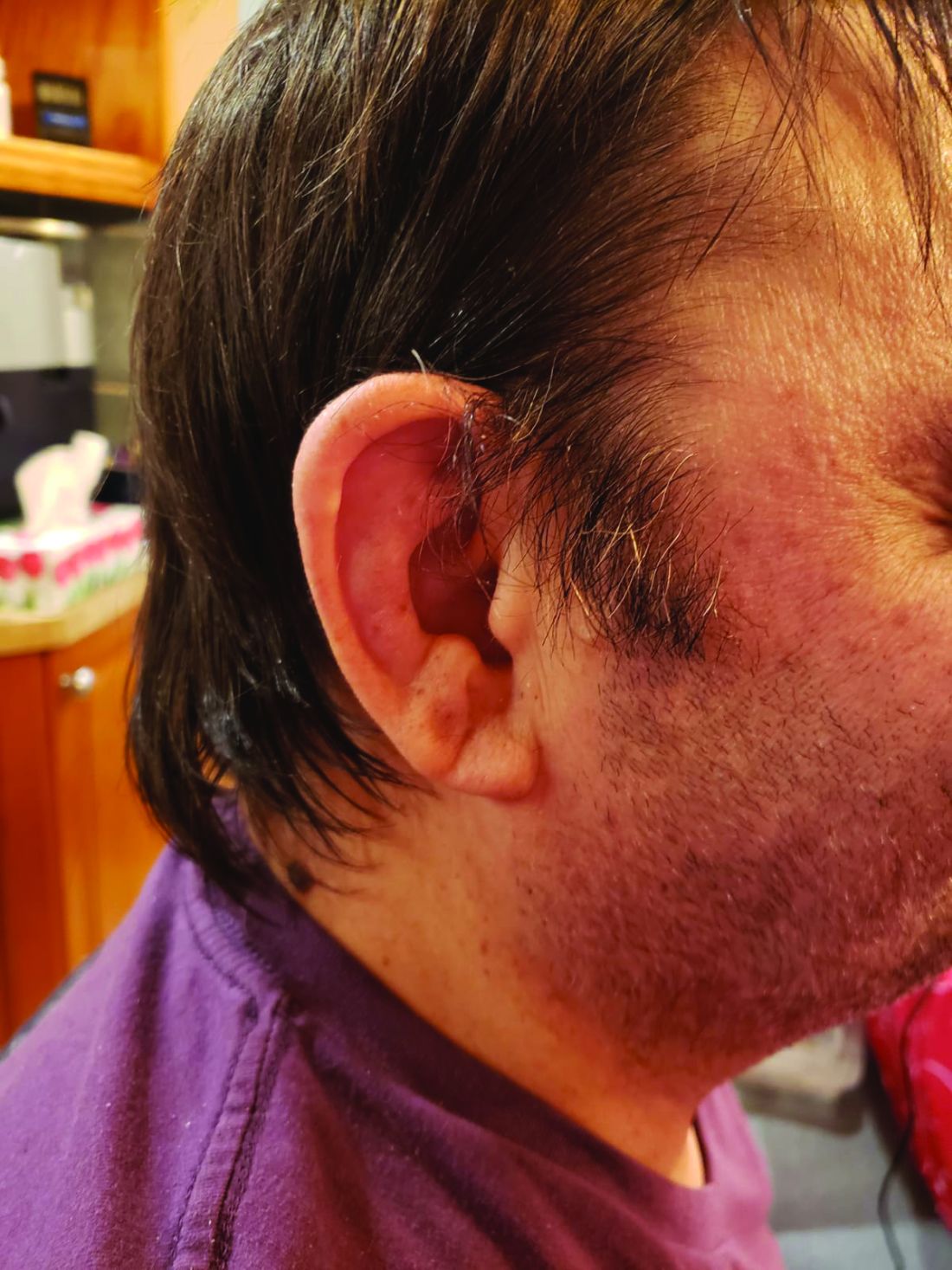
The most common clinical manifestation of VEXAS was skin involvement, which occurred in all but one of the 83 patients. Other common manifestations included arthritis (58 patients), pulmonary infiltrates (57 patients), and ear chondritis (54 patients).
Fifteen patients were found to have the leucine variant, 18 had the valine variant, and 50 had the threonine variant. The median age at disease onset was 66 years in the leucine and threonine variant groups and 65 in the valine variant group.
The clinical diagnosis differed according to genotype: 4 of 18 patients (22%) with the valine variant were diagnosed with relapsing polychondritis, compared with 8 of 15 (53%) with the leucine variant and 31 of 50 (62%) with the threonine variant (P = .01).
In contrast, 55% of patients with valine genotype were diagnosed with undifferentiated fever, compared with 6% of those with the leucine and 16% with the threonine genotypes (P = .001). More patients with the leucine variant (60%) were diagnosed with Sweet syndrome, compared with 11% and 14% of patients with the valine and threonine variants, respectively (P = .001).
There was no significant difference among the three genotypes in the percentage of patients diagnosed with MDS.
The follow-up period ranged from 1 to 18 years (median, 4.7 years). The median survival time from disease onset for all patients was 10 years.
Among patients with the valine variant, median survival was 9 years, which was significantly less than among patients with the other two variants (P = .01).
In univariable analysis, independent predictors of mortality were ear chondritis (hazard ratio, 0.26; P = .005), transfusion dependence, a time-dependent variable (HR, 2.59; P = .03), and the valine variant (HR, 3.5; P = .008).
The association between VEXAS genotype and phenotype could be explained by the finding that, among patients with the valine variant, there was significantly less translation of the catalytically proficient UBA1b isoform than in patients with the other two variants, Dr. Ferrada said.
Therapeutic options
Dr. Ferrada noted that to date no drugs have been shown to provide consistent therapeutic benefits for patients with VEXAS, but evidence as to the etiology of the syndrome points to possible treatment approaches.
“All of these findings I think are extremely important to help us guide management of these patients, as we know that the mutation is located in the stem cells in the bone marrow. So we suspect that doing a bone marrow transplant in these patients is going to be curative,” Dr. Ferrada said during the briefing.
Investigators are planning a phase 2 trial of allogeneic hematopoietic stem cell transplant for patients with VEXAS.
The study was supported by the National Institutes of Health. Dr. Ferrada, Dr. Sandhu, and Dr. Kim have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Among patients with the recently defined severe autoinflammatory syndrome VEXAS, those who are transfusion dependent or have a specific amino acid substitution are at highest risk for death, whereas those with ear chondritis are at significantly lower risk, a multinational team of investigators has found.
Their study of mortality and predictors of survival among patients with genetically confirmed VEXAS showed that patients with a VEXAS variant resulting in an amino acid substitution of a methionine for a valine had a 3.5-fold higher risk for death, compared with patients with either a methionine-to-threonine substitution or a methionine-to-leucine swap.
Transfusion dependence was an independent predictor of mortality. Patients who became dependent on transfusions after symptom onset had a nearly threefold higher risk for death, reported Marcela A. Ferrada, MD, a clinical fellow at the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
“These findings should inform risk assessment and clinical management in patients with VEXAS syndrome,” she said in an oral abstract presentation during the virtual annual meeting of the American College of Rheumatology.
“These genetic findings have proven right now to be not only diagnostic, but we have shown that they’re also prognostic, and we hope that this is going to help us identify patients who could have more aggressive treatment,” Dr. Ferrada said.
She also discussed her findings in a media briefing held 2 days prior to her plenary presentation. At that briefing, this news organization asked participating clinicians whether they had patients who they suspected may have had undiagnosed VEXAS.
“My answer to that is interesting,” replied moderator Vaneet Sandhu, MD, from Loma Linda (Calif.) University and Riverside University Health System.
“In the last couple of days, I’ve been reading about VEXAS, and actually texted one of my colleagues yesterday and said, ‘Hey, you know these patients we’ve been seeing who have these strange rashes and chondritis and have maybe a diagnosis of leukocytoclastic vasculitis or something else – are we not diagnosing these patients?’ ” she said.
“I think we are looking at every patient with chondritis and reexamining their phenotype. We had dismissed certain symptoms because they didn’t fit the archetype for relapsing polychondritis, for example, but it could be VEXAS,” said Alfred Kim, MD, PhD, of Washington University in St. Louis, who also presented data during the briefing.
Three variants
VEXAS is caused by somatic mutations in UBA1, a gene that initiates cytoplasmic ubiquitylation, a process by which misfolded proteins are tagged for degradation.
The syndrome’s name is an acronym descriptive of the major features:
- Vacuoles in bone marrow cells.
- E-1 activating enzyme that UBA1 encodes for.
- X-linked.
- Autoinflammatory.
- Somatic mutation featuring hematologic mosaicism.
VEXAS results in rheumatologic, dermatologic, and hematologic symptoms that are often misdiagnosed as being caused by treatment-refractory relapsing polychondritis, polyarteritis nodosa, Sweet syndrome, giant cell arteritis, or myelodysplastic syndrome (MDS).
VEXAS was identified as a distinct syndrome within the past year by Dr. Ferrada and other investigators at NIAMS, the National Human Genome Research Institute, and other institutions.
In the study reported at ACR 2021, Dr. Ferrada and colleagues assessed 83 men who had been referred for genetic testing for VEXAS at the National Institutes of Health, in Bethesda, Md., and at Leeds (England) Teaching Hospitals NHS Trust.
All patients were confirmed to have VEXAS-defining genetic mutations in UBA1 by Sanger sequencing of peripheral blood samples. Only those patients with mutations at codon p.Met41 were included in the investigators’ analysis. Mutations at that site account for nearly all cases of VEXAS that have been identified to date.
The most common clinical manifestation of VEXAS was skin involvement, which occurred in all but one of the 83 patients. Other common manifestations included arthritis (58 patients), pulmonary infiltrates (57 patients), and ear chondritis (54 patients).
Fifteen patients were found to have the leucine variant, 18 had the valine variant, and 50 had the threonine variant. The median age at disease onset was 66 years in the leucine and threonine variant groups and 65 in the valine variant group.
The clinical diagnosis differed according to genotype: 4 of 18 patients (22%) with the valine variant were diagnosed with relapsing polychondritis, compared with 8 of 15 (53%) with the leucine variant and 31 of 50 (62%) with the threonine variant (P = .01).
In contrast, 55% of patients with valine genotype were diagnosed with undifferentiated fever, compared with 6% of those with the leucine and 16% with the threonine genotypes (P = .001). More patients with the leucine variant (60%) were diagnosed with Sweet syndrome, compared with 11% and 14% of patients with the valine and threonine variants, respectively (P = .001).
There was no significant difference among the three genotypes in the percentage of patients diagnosed with MDS.
The follow-up period ranged from 1 to 18 years (median, 4.7 years). The median survival time from disease onset for all patients was 10 years.
Among patients with the valine variant, median survival was 9 years, which was significantly less than among patients with the other two variants (P = .01).
In univariable analysis, independent predictors of mortality were ear chondritis (hazard ratio, 0.26; P = .005), transfusion dependence, a time-dependent variable (HR, 2.59; P = .03), and the valine variant (HR, 3.5; P = .008).
The association between VEXAS genotype and phenotype could be explained by the finding that, among patients with the valine variant, there was significantly less translation of the catalytically proficient UBA1b isoform than in patients with the other two variants, Dr. Ferrada said.
Therapeutic options
Dr. Ferrada noted that to date no drugs have been shown to provide consistent therapeutic benefits for patients with VEXAS, but evidence as to the etiology of the syndrome points to possible treatment approaches.
“All of these findings I think are extremely important to help us guide management of these patients, as we know that the mutation is located in the stem cells in the bone marrow. So we suspect that doing a bone marrow transplant in these patients is going to be curative,” Dr. Ferrada said during the briefing.
Investigators are planning a phase 2 trial of allogeneic hematopoietic stem cell transplant for patients with VEXAS.
The study was supported by the National Institutes of Health. Dr. Ferrada, Dr. Sandhu, and Dr. Kim have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Among patients with the recently defined severe autoinflammatory syndrome VEXAS, those who are transfusion dependent or have a specific amino acid substitution are at highest risk for death, whereas those with ear chondritis are at significantly lower risk, a multinational team of investigators has found.
Their study of mortality and predictors of survival among patients with genetically confirmed VEXAS showed that patients with a VEXAS variant resulting in an amino acid substitution of a methionine for a valine had a 3.5-fold higher risk for death, compared with patients with either a methionine-to-threonine substitution or a methionine-to-leucine swap.
Transfusion dependence was an independent predictor of mortality. Patients who became dependent on transfusions after symptom onset had a nearly threefold higher risk for death, reported Marcela A. Ferrada, MD, a clinical fellow at the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
“These findings should inform risk assessment and clinical management in patients with VEXAS syndrome,” she said in an oral abstract presentation during the virtual annual meeting of the American College of Rheumatology.
“These genetic findings have proven right now to be not only diagnostic, but we have shown that they’re also prognostic, and we hope that this is going to help us identify patients who could have more aggressive treatment,” Dr. Ferrada said.
She also discussed her findings in a media briefing held 2 days prior to her plenary presentation. At that briefing, this news organization asked participating clinicians whether they had patients who they suspected may have had undiagnosed VEXAS.
“My answer to that is interesting,” replied moderator Vaneet Sandhu, MD, from Loma Linda (Calif.) University and Riverside University Health System.
“In the last couple of days, I’ve been reading about VEXAS, and actually texted one of my colleagues yesterday and said, ‘Hey, you know these patients we’ve been seeing who have these strange rashes and chondritis and have maybe a diagnosis of leukocytoclastic vasculitis or something else – are we not diagnosing these patients?’ ” she said.
“I think we are looking at every patient with chondritis and reexamining their phenotype. We had dismissed certain symptoms because they didn’t fit the archetype for relapsing polychondritis, for example, but it could be VEXAS,” said Alfred Kim, MD, PhD, of Washington University in St. Louis, who also presented data during the briefing.
Three variants
VEXAS is caused by somatic mutations in UBA1, a gene that initiates cytoplasmic ubiquitylation, a process by which misfolded proteins are tagged for degradation.
The syndrome’s name is an acronym descriptive of the major features:
- Vacuoles in bone marrow cells.
- E-1 activating enzyme that UBA1 encodes for.
- X-linked.
- Autoinflammatory.
- Somatic mutation featuring hematologic mosaicism.
VEXAS results in rheumatologic, dermatologic, and hematologic symptoms that are often misdiagnosed as being caused by treatment-refractory relapsing polychondritis, polyarteritis nodosa, Sweet syndrome, giant cell arteritis, or myelodysplastic syndrome (MDS).
VEXAS was identified as a distinct syndrome within the past year by Dr. Ferrada and other investigators at NIAMS, the National Human Genome Research Institute, and other institutions.
In the study reported at ACR 2021, Dr. Ferrada and colleagues assessed 83 men who had been referred for genetic testing for VEXAS at the National Institutes of Health, in Bethesda, Md., and at Leeds (England) Teaching Hospitals NHS Trust.
All patients were confirmed to have VEXAS-defining genetic mutations in UBA1 by Sanger sequencing of peripheral blood samples. Only those patients with mutations at codon p.Met41 were included in the investigators’ analysis. Mutations at that site account for nearly all cases of VEXAS that have been identified to date.
The most common clinical manifestation of VEXAS was skin involvement, which occurred in all but one of the 83 patients. Other common manifestations included arthritis (58 patients), pulmonary infiltrates (57 patients), and ear chondritis (54 patients).
Fifteen patients were found to have the leucine variant, 18 had the valine variant, and 50 had the threonine variant. The median age at disease onset was 66 years in the leucine and threonine variant groups and 65 in the valine variant group.
The clinical diagnosis differed according to genotype: 4 of 18 patients (22%) with the valine variant were diagnosed with relapsing polychondritis, compared with 8 of 15 (53%) with the leucine variant and 31 of 50 (62%) with the threonine variant (P = .01).
In contrast, 55% of patients with valine genotype were diagnosed with undifferentiated fever, compared with 6% of those with the leucine and 16% with the threonine genotypes (P = .001). More patients with the leucine variant (60%) were diagnosed with Sweet syndrome, compared with 11% and 14% of patients with the valine and threonine variants, respectively (P = .001).
There was no significant difference among the three genotypes in the percentage of patients diagnosed with MDS.
The follow-up period ranged from 1 to 18 years (median, 4.7 years). The median survival time from disease onset for all patients was 10 years.
Among patients with the valine variant, median survival was 9 years, which was significantly less than among patients with the other two variants (P = .01).
In univariable analysis, independent predictors of mortality were ear chondritis (hazard ratio, 0.26; P = .005), transfusion dependence, a time-dependent variable (HR, 2.59; P = .03), and the valine variant (HR, 3.5; P = .008).
The association between VEXAS genotype and phenotype could be explained by the finding that, among patients with the valine variant, there was significantly less translation of the catalytically proficient UBA1b isoform than in patients with the other two variants, Dr. Ferrada said.
Therapeutic options
Dr. Ferrada noted that to date no drugs have been shown to provide consistent therapeutic benefits for patients with VEXAS, but evidence as to the etiology of the syndrome points to possible treatment approaches.
“All of these findings I think are extremely important to help us guide management of these patients, as we know that the mutation is located in the stem cells in the bone marrow. So we suspect that doing a bone marrow transplant in these patients is going to be curative,” Dr. Ferrada said during the briefing.
Investigators are planning a phase 2 trial of allogeneic hematopoietic stem cell transplant for patients with VEXAS.
The study was supported by the National Institutes of Health. Dr. Ferrada, Dr. Sandhu, and Dr. Kim have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ACR 2021
Lupus patients in remission see more flares with HCQ reduction, discontinuation
Continuation of hydroxychloroquine (HCQ) when a patient’s systemic lupus erythematosus (SLE) is in remission or has very low disease activity is linked to a lower risk of flares than is reducing or stopping the antimalarial drug, according to new research presented at the virtual annual meeting of the American College of Rheumatology.
“Though HCQ is a cornerstone SLE drug, physicians and patients often consider lowering or stopping the drug during remission or low disease activity in order to limit long-term toxicity,” Sasha Bernatsky, MD, PhD, a professor of rheumatology at McGill University in Montreal, told attendees. Her group’s findings revealed a 20% increased risk of flares in those who reduced their HCQ dose and a 56% greater risk of flares in those who discontinued HCQ, compared with those who continued on a maintenance dose.
“I’m going to be using these results in discussions with my patients regarding what the potential implications are of lowering or stopping hydroxychloroquine,” Dr. Bernatsky told attendees. “I think, in the end, this information should be in their hands so that they can be the ones to make these decisions with us, and, of course, given the significant flare rates even in remission, we need to keep on working on optimizing lupus treatments.”
Study details
The researchers analyzed prospective data from 1,460 patients enrolled in the Systemic Lupus Erythematosus International Collaborating Clinics (SLICC) cohort, which includes 33 sites across Europe, Asia, and North America. Patients in this cohort undergo annual follow-ups after enrollment within 15 months of their diagnosis. The study population was 89% female and 52% white. All participants either had low disease activity, defined as a score of 4 or lower on the Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) and/or as a prednisone dose no greater than 7.5 mg/day, or were in complete remission, defined as a 0 on SLEDAI-2K while receiving no therapy, including no prednisone or immunosuppressives in the past year.
In addition to adjusting for sex, race/ethnicity, age, education, and geographic residence, the researchers took into account baseline SLE duration, renal damage, body mass index, smoking status, and use of prednisone, immunosuppressives, and biologics. For the outcome of time to first flare, the researchers analyzed those who discontinued HCQ separately from those who reduced the dose, comparing each to those who continued HCQ maintenance therapy. The researchers defined first flare as either hospitalization because of SLE, increased disease activity (at least 4 points on the SLEDAI-2K), or therapy augmentation with steroids, immunosuppressives, antimalarials, or biologics.
Within each cohort, patients who reduced or stopped HCQ therapy were matched to patients who continued HCQ maintenance therapy based on duration of HCQ since time zero, the point at which participants were considered at risk for SLE flares. In the reduction cohort, time zero was the date of a participant’s first HCQ reduction; in the discontinuation cohort, time zero was the date a participant stopped the therapy. Because of the study’s design and reliance on person-years of exposure, it was possible for a single participant to contribute data to more than one cohort.
Results
The overall cohort examining reduction of HCQ dose included 564 patients who reduced their dose, contributing 1,063 person-years of data, and 778 matched patients who started HCQ at the same time but continued HCQ maintenance therapy without a dose reduction, contributing 1,242 person-years. The average duration of HCQ use since time zero in this cohort was 3.4 years.
Before stratifying for disease activity, the group who reduced their therapy experienced 40 first flares per 100 person-years, compared with 31.9 first flares per 100 person-years on maintenance therapy. Those who reduced HCQ had a 20% greater risk of flares than did those who continued it (adjusted hazard ratio, 1.2). However, when those in remission were compared with those not in remission – independent of disease activity level – patients in remission were twice as likely to experience a flare if they reduced their HCQ dose (aHR, 2.14).
In the discontinuation cohort, 389 patients who stopped HCQ therapy contributed 657 person-years, and 577 matched patients who continued HCQ maintenance therapy contributed 924 person-years. The average duration of HCQ use since time zero in this cohort was 4.2 years. Before stratifying for disease activity, the average number of first flares per 100 person-years was 41.3 in the HCQ discontinuation group and 30 in the HCQ maintenance group, resulting in a 56% higher risk of flares for those who stopped HCQ, compared with patients who continued HCQ (aHR, 1.56). Looking only at those in remission, patients were nearly three times more likely to experience a flare if they stopped HCQ than were patients not in remission who continued a maintenance dose (aHR, 2.77).
Patient age is an important consideration
Overall, these findings are not surprising, said Jill P. Buyon, MD, director of the division of rheumatology and of the Lupus Center at NYU-Langone Health in New York. Dr. Buyon is not involved in the current study but is studying discontinuation of HCQ in older adults with lupus.
“It has been already shown that when lupus patients discontinue HCQ, flares are more likely, but does this apply to all age groups?” Dr. Buyon asked in an interview. “Data are essential to more accurately weigh the balance between accumulating ocular exposure, the explosion of new tools to assess retinal injury, and the risk of disease flare in a population that may have more stable/quiescent disease than younger patients.”
Although HCQ’s track record with infection risk is consistently better than that of more immunosuppressive drugs and is very safe during pregnancy, Dr. Buyon said her “ophthalmology colleagues persistently emphasize the risk of retinal accumulation of drug and ocular toxicity over time.” She referenced a recent case-control study in which overall prevalence of HCQ retinopathy was 7.5%, and greater for patients taking more than 5 mg/kg of HCQ or who used HCQ for more than 10 years.
”Risk escalates with continued use, and evaluation by sensitive approaches such as multifocal electroretinography suggests nearly a third of patients accrue retinal damage,” Dr. Buyon said. “As the longevity of patients improves and comorbidities such as renal insufficiency (which affects HCQ clearance) may increase, the ratio of efficacy to toxicity would be expected to decrease.” Further, the fact that disease activity may wane as people age means that rheumatologists treating older adults need to address a critical question, she said: “Can HCQ be safely withdrawn? This question is important in the context of an even broader concern regarding management of SLE in the elderly population, a topic which has received minimal attention.”
The study is limited by its observational design and the fact that the intervention was not randomly allocated, although the researchers attempted to adjust for confounders. Dr. Bernatsky also noted that mild flares might have been missed, and the researchers did not evaluate HCQ levels or adherence, nor did the data set include physicians’ or patients’ explicitly stated reasons for HCQ reduction or discontinuation.
”We estimated that 5% of patients may have reduced HCQ therapy as result of the AAO [American Academy of Ophthalmology] guidelines, 55% because of low disease activity state, and the remainder (40%) for other reasons, possibly intolerance or patient preference,” the researchers noted in their abstract. “Among those who discontinued HCQ, 4% had retinal changes of concern, 15% were in clinical remission, and the remainder stopped for unknown reasons, possibly intolerance or patient preference.”
Dr. Buyon also pointed out that the cohort was initially intended for studying cardiovascular risk and not designed to capture all visits during each year of follow-up.
“Thus, while hospitalizations would be well captured, not all flares, particularly those not severe, would be captured, and thus we may not have the complete picture,” she said, reiterating Dr. Bernatsky’s point that mild flares may have been missed.
”Clearly, this is a very important topic for the management of our patients, particularly those who are elderly and may have already reaped the benefits of hydroxychloroquine,” Dr. Buyon said. “Of course, we have to be mindful of the potential benefit with regard to blood clotting and lipid lowering. Nevertheless, accumulated ocular toxicity and cardiac issues such as cardiomyopathy may emerge to tip the balance after years of accumulated drug exposure.”
The research was funded by the Canadian Institute of Health Research, the Singer Family Fund for Lupus Research, and the SLICC Group. Dr. Bernatsky had no disclosures. Dr. Buyon noted that she has an R34 NIH planning grant to study the safety of withdrawal of hydroxychloroquine in elderly lupus patients that is relevant to this study.
Continuation of hydroxychloroquine (HCQ) when a patient’s systemic lupus erythematosus (SLE) is in remission or has very low disease activity is linked to a lower risk of flares than is reducing or stopping the antimalarial drug, according to new research presented at the virtual annual meeting of the American College of Rheumatology.
“Though HCQ is a cornerstone SLE drug, physicians and patients often consider lowering or stopping the drug during remission or low disease activity in order to limit long-term toxicity,” Sasha Bernatsky, MD, PhD, a professor of rheumatology at McGill University in Montreal, told attendees. Her group’s findings revealed a 20% increased risk of flares in those who reduced their HCQ dose and a 56% greater risk of flares in those who discontinued HCQ, compared with those who continued on a maintenance dose.
“I’m going to be using these results in discussions with my patients regarding what the potential implications are of lowering or stopping hydroxychloroquine,” Dr. Bernatsky told attendees. “I think, in the end, this information should be in their hands so that they can be the ones to make these decisions with us, and, of course, given the significant flare rates even in remission, we need to keep on working on optimizing lupus treatments.”
Study details
The researchers analyzed prospective data from 1,460 patients enrolled in the Systemic Lupus Erythematosus International Collaborating Clinics (SLICC) cohort, which includes 33 sites across Europe, Asia, and North America. Patients in this cohort undergo annual follow-ups after enrollment within 15 months of their diagnosis. The study population was 89% female and 52% white. All participants either had low disease activity, defined as a score of 4 or lower on the Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) and/or as a prednisone dose no greater than 7.5 mg/day, or were in complete remission, defined as a 0 on SLEDAI-2K while receiving no therapy, including no prednisone or immunosuppressives in the past year.
In addition to adjusting for sex, race/ethnicity, age, education, and geographic residence, the researchers took into account baseline SLE duration, renal damage, body mass index, smoking status, and use of prednisone, immunosuppressives, and biologics. For the outcome of time to first flare, the researchers analyzed those who discontinued HCQ separately from those who reduced the dose, comparing each to those who continued HCQ maintenance therapy. The researchers defined first flare as either hospitalization because of SLE, increased disease activity (at least 4 points on the SLEDAI-2K), or therapy augmentation with steroids, immunosuppressives, antimalarials, or biologics.
Within each cohort, patients who reduced or stopped HCQ therapy were matched to patients who continued HCQ maintenance therapy based on duration of HCQ since time zero, the point at which participants were considered at risk for SLE flares. In the reduction cohort, time zero was the date of a participant’s first HCQ reduction; in the discontinuation cohort, time zero was the date a participant stopped the therapy. Because of the study’s design and reliance on person-years of exposure, it was possible for a single participant to contribute data to more than one cohort.
Results
The overall cohort examining reduction of HCQ dose included 564 patients who reduced their dose, contributing 1,063 person-years of data, and 778 matched patients who started HCQ at the same time but continued HCQ maintenance therapy without a dose reduction, contributing 1,242 person-years. The average duration of HCQ use since time zero in this cohort was 3.4 years.
Before stratifying for disease activity, the group who reduced their therapy experienced 40 first flares per 100 person-years, compared with 31.9 first flares per 100 person-years on maintenance therapy. Those who reduced HCQ had a 20% greater risk of flares than did those who continued it (adjusted hazard ratio, 1.2). However, when those in remission were compared with those not in remission – independent of disease activity level – patients in remission were twice as likely to experience a flare if they reduced their HCQ dose (aHR, 2.14).
In the discontinuation cohort, 389 patients who stopped HCQ therapy contributed 657 person-years, and 577 matched patients who continued HCQ maintenance therapy contributed 924 person-years. The average duration of HCQ use since time zero in this cohort was 4.2 years. Before stratifying for disease activity, the average number of first flares per 100 person-years was 41.3 in the HCQ discontinuation group and 30 in the HCQ maintenance group, resulting in a 56% higher risk of flares for those who stopped HCQ, compared with patients who continued HCQ (aHR, 1.56). Looking only at those in remission, patients were nearly three times more likely to experience a flare if they stopped HCQ than were patients not in remission who continued a maintenance dose (aHR, 2.77).
Patient age is an important consideration
Overall, these findings are not surprising, said Jill P. Buyon, MD, director of the division of rheumatology and of the Lupus Center at NYU-Langone Health in New York. Dr. Buyon is not involved in the current study but is studying discontinuation of HCQ in older adults with lupus.
“It has been already shown that when lupus patients discontinue HCQ, flares are more likely, but does this apply to all age groups?” Dr. Buyon asked in an interview. “Data are essential to more accurately weigh the balance between accumulating ocular exposure, the explosion of new tools to assess retinal injury, and the risk of disease flare in a population that may have more stable/quiescent disease than younger patients.”
Although HCQ’s track record with infection risk is consistently better than that of more immunosuppressive drugs and is very safe during pregnancy, Dr. Buyon said her “ophthalmology colleagues persistently emphasize the risk of retinal accumulation of drug and ocular toxicity over time.” She referenced a recent case-control study in which overall prevalence of HCQ retinopathy was 7.5%, and greater for patients taking more than 5 mg/kg of HCQ or who used HCQ for more than 10 years.
”Risk escalates with continued use, and evaluation by sensitive approaches such as multifocal electroretinography suggests nearly a third of patients accrue retinal damage,” Dr. Buyon said. “As the longevity of patients improves and comorbidities such as renal insufficiency (which affects HCQ clearance) may increase, the ratio of efficacy to toxicity would be expected to decrease.” Further, the fact that disease activity may wane as people age means that rheumatologists treating older adults need to address a critical question, she said: “Can HCQ be safely withdrawn? This question is important in the context of an even broader concern regarding management of SLE in the elderly population, a topic which has received minimal attention.”
The study is limited by its observational design and the fact that the intervention was not randomly allocated, although the researchers attempted to adjust for confounders. Dr. Bernatsky also noted that mild flares might have been missed, and the researchers did not evaluate HCQ levels or adherence, nor did the data set include physicians’ or patients’ explicitly stated reasons for HCQ reduction or discontinuation.
”We estimated that 5% of patients may have reduced HCQ therapy as result of the AAO [American Academy of Ophthalmology] guidelines, 55% because of low disease activity state, and the remainder (40%) for other reasons, possibly intolerance or patient preference,” the researchers noted in their abstract. “Among those who discontinued HCQ, 4% had retinal changes of concern, 15% were in clinical remission, and the remainder stopped for unknown reasons, possibly intolerance or patient preference.”
Dr. Buyon also pointed out that the cohort was initially intended for studying cardiovascular risk and not designed to capture all visits during each year of follow-up.
“Thus, while hospitalizations would be well captured, not all flares, particularly those not severe, would be captured, and thus we may not have the complete picture,” she said, reiterating Dr. Bernatsky’s point that mild flares may have been missed.
”Clearly, this is a very important topic for the management of our patients, particularly those who are elderly and may have already reaped the benefits of hydroxychloroquine,” Dr. Buyon said. “Of course, we have to be mindful of the potential benefit with regard to blood clotting and lipid lowering. Nevertheless, accumulated ocular toxicity and cardiac issues such as cardiomyopathy may emerge to tip the balance after years of accumulated drug exposure.”
The research was funded by the Canadian Institute of Health Research, the Singer Family Fund for Lupus Research, and the SLICC Group. Dr. Bernatsky had no disclosures. Dr. Buyon noted that she has an R34 NIH planning grant to study the safety of withdrawal of hydroxychloroquine in elderly lupus patients that is relevant to this study.
Continuation of hydroxychloroquine (HCQ) when a patient’s systemic lupus erythematosus (SLE) is in remission or has very low disease activity is linked to a lower risk of flares than is reducing or stopping the antimalarial drug, according to new research presented at the virtual annual meeting of the American College of Rheumatology.
“Though HCQ is a cornerstone SLE drug, physicians and patients often consider lowering or stopping the drug during remission or low disease activity in order to limit long-term toxicity,” Sasha Bernatsky, MD, PhD, a professor of rheumatology at McGill University in Montreal, told attendees. Her group’s findings revealed a 20% increased risk of flares in those who reduced their HCQ dose and a 56% greater risk of flares in those who discontinued HCQ, compared with those who continued on a maintenance dose.
“I’m going to be using these results in discussions with my patients regarding what the potential implications are of lowering or stopping hydroxychloroquine,” Dr. Bernatsky told attendees. “I think, in the end, this information should be in their hands so that they can be the ones to make these decisions with us, and, of course, given the significant flare rates even in remission, we need to keep on working on optimizing lupus treatments.”
Study details
The researchers analyzed prospective data from 1,460 patients enrolled in the Systemic Lupus Erythematosus International Collaborating Clinics (SLICC) cohort, which includes 33 sites across Europe, Asia, and North America. Patients in this cohort undergo annual follow-ups after enrollment within 15 months of their diagnosis. The study population was 89% female and 52% white. All participants either had low disease activity, defined as a score of 4 or lower on the Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) and/or as a prednisone dose no greater than 7.5 mg/day, or were in complete remission, defined as a 0 on SLEDAI-2K while receiving no therapy, including no prednisone or immunosuppressives in the past year.
In addition to adjusting for sex, race/ethnicity, age, education, and geographic residence, the researchers took into account baseline SLE duration, renal damage, body mass index, smoking status, and use of prednisone, immunosuppressives, and biologics. For the outcome of time to first flare, the researchers analyzed those who discontinued HCQ separately from those who reduced the dose, comparing each to those who continued HCQ maintenance therapy. The researchers defined first flare as either hospitalization because of SLE, increased disease activity (at least 4 points on the SLEDAI-2K), or therapy augmentation with steroids, immunosuppressives, antimalarials, or biologics.
Within each cohort, patients who reduced or stopped HCQ therapy were matched to patients who continued HCQ maintenance therapy based on duration of HCQ since time zero, the point at which participants were considered at risk for SLE flares. In the reduction cohort, time zero was the date of a participant’s first HCQ reduction; in the discontinuation cohort, time zero was the date a participant stopped the therapy. Because of the study’s design and reliance on person-years of exposure, it was possible for a single participant to contribute data to more than one cohort.
Results
The overall cohort examining reduction of HCQ dose included 564 patients who reduced their dose, contributing 1,063 person-years of data, and 778 matched patients who started HCQ at the same time but continued HCQ maintenance therapy without a dose reduction, contributing 1,242 person-years. The average duration of HCQ use since time zero in this cohort was 3.4 years.
Before stratifying for disease activity, the group who reduced their therapy experienced 40 first flares per 100 person-years, compared with 31.9 first flares per 100 person-years on maintenance therapy. Those who reduced HCQ had a 20% greater risk of flares than did those who continued it (adjusted hazard ratio, 1.2). However, when those in remission were compared with those not in remission – independent of disease activity level – patients in remission were twice as likely to experience a flare if they reduced their HCQ dose (aHR, 2.14).
In the discontinuation cohort, 389 patients who stopped HCQ therapy contributed 657 person-years, and 577 matched patients who continued HCQ maintenance therapy contributed 924 person-years. The average duration of HCQ use since time zero in this cohort was 4.2 years. Before stratifying for disease activity, the average number of first flares per 100 person-years was 41.3 in the HCQ discontinuation group and 30 in the HCQ maintenance group, resulting in a 56% higher risk of flares for those who stopped HCQ, compared with patients who continued HCQ (aHR, 1.56). Looking only at those in remission, patients were nearly three times more likely to experience a flare if they stopped HCQ than were patients not in remission who continued a maintenance dose (aHR, 2.77).
Patient age is an important consideration
Overall, these findings are not surprising, said Jill P. Buyon, MD, director of the division of rheumatology and of the Lupus Center at NYU-Langone Health in New York. Dr. Buyon is not involved in the current study but is studying discontinuation of HCQ in older adults with lupus.
“It has been already shown that when lupus patients discontinue HCQ, flares are more likely, but does this apply to all age groups?” Dr. Buyon asked in an interview. “Data are essential to more accurately weigh the balance between accumulating ocular exposure, the explosion of new tools to assess retinal injury, and the risk of disease flare in a population that may have more stable/quiescent disease than younger patients.”
Although HCQ’s track record with infection risk is consistently better than that of more immunosuppressive drugs and is very safe during pregnancy, Dr. Buyon said her “ophthalmology colleagues persistently emphasize the risk of retinal accumulation of drug and ocular toxicity over time.” She referenced a recent case-control study in which overall prevalence of HCQ retinopathy was 7.5%, and greater for patients taking more than 5 mg/kg of HCQ or who used HCQ for more than 10 years.
”Risk escalates with continued use, and evaluation by sensitive approaches such as multifocal electroretinography suggests nearly a third of patients accrue retinal damage,” Dr. Buyon said. “As the longevity of patients improves and comorbidities such as renal insufficiency (which affects HCQ clearance) may increase, the ratio of efficacy to toxicity would be expected to decrease.” Further, the fact that disease activity may wane as people age means that rheumatologists treating older adults need to address a critical question, she said: “Can HCQ be safely withdrawn? This question is important in the context of an even broader concern regarding management of SLE in the elderly population, a topic which has received minimal attention.”
The study is limited by its observational design and the fact that the intervention was not randomly allocated, although the researchers attempted to adjust for confounders. Dr. Bernatsky also noted that mild flares might have been missed, and the researchers did not evaluate HCQ levels or adherence, nor did the data set include physicians’ or patients’ explicitly stated reasons for HCQ reduction or discontinuation.
”We estimated that 5% of patients may have reduced HCQ therapy as result of the AAO [American Academy of Ophthalmology] guidelines, 55% because of low disease activity state, and the remainder (40%) for other reasons, possibly intolerance or patient preference,” the researchers noted in their abstract. “Among those who discontinued HCQ, 4% had retinal changes of concern, 15% were in clinical remission, and the remainder stopped for unknown reasons, possibly intolerance or patient preference.”
Dr. Buyon also pointed out that the cohort was initially intended for studying cardiovascular risk and not designed to capture all visits during each year of follow-up.
“Thus, while hospitalizations would be well captured, not all flares, particularly those not severe, would be captured, and thus we may not have the complete picture,” she said, reiterating Dr. Bernatsky’s point that mild flares may have been missed.
”Clearly, this is a very important topic for the management of our patients, particularly those who are elderly and may have already reaped the benefits of hydroxychloroquine,” Dr. Buyon said. “Of course, we have to be mindful of the potential benefit with regard to blood clotting and lipid lowering. Nevertheless, accumulated ocular toxicity and cardiac issues such as cardiomyopathy may emerge to tip the balance after years of accumulated drug exposure.”
The research was funded by the Canadian Institute of Health Research, the Singer Family Fund for Lupus Research, and the SLICC Group. Dr. Bernatsky had no disclosures. Dr. Buyon noted that she has an R34 NIH planning grant to study the safety of withdrawal of hydroxychloroquine in elderly lupus patients that is relevant to this study.
FROM ACR 2021
New trials in leukemia: Could your patient benefit?
A number of late-phase clinical trials in leukemia have opened in recent months. Maybe one of your patients could benefit from being enrolled.
Adults and children with acute or chronic leukemias
A phase 2 study partnering with the National Marrow Donor Program is seeking individuals aged 1-65 years with lymphoma or one of the following leukemias: “acute leukemia”, acute lymphoblastic (ALL), acute myelogenous (AML), mixed-phenotype acute, chronic myelogenous (CML), and chronic lymphocytic (CLL). Researchers hope to find a way to improve outcomes of hematopoietic-cell transplantation from mismatched, unrelated donors. Participants will receive the transplant and one of seven drug regimens and will be followed for a year. The trial plans to enroll 180 people and began recruiting on Sept. 30 in California, New York, and Virginia. The primary outcome is overall survival (OS). Quality of life (QoL) will not be measured.
Mast-cell leukemia (MCL)
Adults with MCL are sought for a phase 2 study of bezuclastinib, an experimental tyrosine-kinase inhibitor (TKI) called CGT9486. CGT9486 blocks the activity of a mutated version of tyrosine-kinase receptor KIT, called KIT D816V, which is known to cause systemic mastocytosis. Participants will receive oral CGT9486 daily for up to 18 months. The study opened in October, aiming for 140 participants with any advanced systemic mastocytoses (including MCL) at sites in California, Florida, Massachusetts, New York, Ohio, Texas, and Utah. OS and QoL will be tracked.
Previously Treated CLL/Small Lymphocytic Lymphoma (SLL)
Patients with CLL/SLL who have progressed on previous therapy can join a phase 3 study of another experimental oral TIK, pirtobrutinib, this time targeting Bruton’s tyrosine kinase (BTK). BTK plays a key role in the lifecycle of white blood cells. Participants will receive either “fixed-duration” pirtobrutinib plus venetoclax (Venclexta) and rituximab (Ruxience, Riabni, Truxima, Rituxan, MabThera) or the venetoclax-rituximab combo only, for up to 5 years. Investigators started recruiting in September, aiming for 600 participants across Florida, Louisiana, Missouri, New York, and Tennessee. Progression-free survival is the primary outcome; OS is a secondary outcome and QoL will not be tracked.
High-grade myeloid cancers with measurable residual disease
Patients with AML, myelodysplastic syndrome with excess blasts-2 or myeloid neoplasm, and whose original disease is still present, are eligible for a phase 2 study of CPX-351 (daunorubicin-cytarabine, Vyxeos). The intravenous chemotherapy was approved in 2017 for certain types of AML. The goal of this study is to determine if pretreatment with CPX-351 improves the outcome of donor stem-cell transplantation. Patients will either undergo immediate transplantation or receive CPX-351 for up to 10 days followed 60 days later by the transplant. The study, being conducted at the Fred Hutchinson Cancer Research Center in Seattle, started recruiting 130 patients in August. The primary outcome is OS; QoL will not be tracked.
Newly diagnosed Philadelphia-negative ALL
Patients aged 22 or older with Philadelphia-negative ALL who have not received chemotherapy or radiation therapy are invited to join a trial of calaspargase pegol (Asparlas). The therapy was approved in 2018 for ALL in children and young adults (1 month to 21 years). The aim of this study is to confirm the recommended doses and evaluate the drug’s safety and pharmacodynamics in adults over aged 21. Each participant will receive six 2-hour infusions of calaspargase pegol over several months. The primary outcomes are safety and drug activity; OS is a secondary outcome and QoL will not be measured. The study opened on July 7 and aims to recruit 122 participants in 11 states.
Untreated adults with TP53-mutant AML
Adult patients with previously untreated AML who have at least one TP53 gene mutation are sought for a phase 3 study of magrolimab, an investigational anti-CD47 monoclonal antibody. Participants will be treated for up to 27 months with either magrolimab plus azacytidine (Vidaza), venetoclax plus azacytidine (patients deemed “appropriate for nonintensive therapy”), or standard chemotherapy (those “appropriate for intensive therapy”). In patients who received nonintensive therapy, OS is the primary outcome; OS in all participants is a secondary outcome, and QoL won’t be assessed. The trial opened in July and aims to recruit 346 individuals in Hong Kong, Australia, and the United States (California, Missouri, Oklahoma, Pennsylvania, South Carolina, and Texas).
All trial information is from the U.S. National Library of Medicine, National Institutes of Health.
A version of this article first appeared on Medscape.com.
A number of late-phase clinical trials in leukemia have opened in recent months. Maybe one of your patients could benefit from being enrolled.
Adults and children with acute or chronic leukemias
A phase 2 study partnering with the National Marrow Donor Program is seeking individuals aged 1-65 years with lymphoma or one of the following leukemias: “acute leukemia”, acute lymphoblastic (ALL), acute myelogenous (AML), mixed-phenotype acute, chronic myelogenous (CML), and chronic lymphocytic (CLL). Researchers hope to find a way to improve outcomes of hematopoietic-cell transplantation from mismatched, unrelated donors. Participants will receive the transplant and one of seven drug regimens and will be followed for a year. The trial plans to enroll 180 people and began recruiting on Sept. 30 in California, New York, and Virginia. The primary outcome is overall survival (OS). Quality of life (QoL) will not be measured.
Mast-cell leukemia (MCL)
Adults with MCL are sought for a phase 2 study of bezuclastinib, an experimental tyrosine-kinase inhibitor (TKI) called CGT9486. CGT9486 blocks the activity of a mutated version of tyrosine-kinase receptor KIT, called KIT D816V, which is known to cause systemic mastocytosis. Participants will receive oral CGT9486 daily for up to 18 months. The study opened in October, aiming for 140 participants with any advanced systemic mastocytoses (including MCL) at sites in California, Florida, Massachusetts, New York, Ohio, Texas, and Utah. OS and QoL will be tracked.
Previously Treated CLL/Small Lymphocytic Lymphoma (SLL)
Patients with CLL/SLL who have progressed on previous therapy can join a phase 3 study of another experimental oral TIK, pirtobrutinib, this time targeting Bruton’s tyrosine kinase (BTK). BTK plays a key role in the lifecycle of white blood cells. Participants will receive either “fixed-duration” pirtobrutinib plus venetoclax (Venclexta) and rituximab (Ruxience, Riabni, Truxima, Rituxan, MabThera) or the venetoclax-rituximab combo only, for up to 5 years. Investigators started recruiting in September, aiming for 600 participants across Florida, Louisiana, Missouri, New York, and Tennessee. Progression-free survival is the primary outcome; OS is a secondary outcome and QoL will not be tracked.
High-grade myeloid cancers with measurable residual disease
Patients with AML, myelodysplastic syndrome with excess blasts-2 or myeloid neoplasm, and whose original disease is still present, are eligible for a phase 2 study of CPX-351 (daunorubicin-cytarabine, Vyxeos). The intravenous chemotherapy was approved in 2017 for certain types of AML. The goal of this study is to determine if pretreatment with CPX-351 improves the outcome of donor stem-cell transplantation. Patients will either undergo immediate transplantation or receive CPX-351 for up to 10 days followed 60 days later by the transplant. The study, being conducted at the Fred Hutchinson Cancer Research Center in Seattle, started recruiting 130 patients in August. The primary outcome is OS; QoL will not be tracked.
Newly diagnosed Philadelphia-negative ALL
Patients aged 22 or older with Philadelphia-negative ALL who have not received chemotherapy or radiation therapy are invited to join a trial of calaspargase pegol (Asparlas). The therapy was approved in 2018 for ALL in children and young adults (1 month to 21 years). The aim of this study is to confirm the recommended doses and evaluate the drug’s safety and pharmacodynamics in adults over aged 21. Each participant will receive six 2-hour infusions of calaspargase pegol over several months. The primary outcomes are safety and drug activity; OS is a secondary outcome and QoL will not be measured. The study opened on July 7 and aims to recruit 122 participants in 11 states.
Untreated adults with TP53-mutant AML
Adult patients with previously untreated AML who have at least one TP53 gene mutation are sought for a phase 3 study of magrolimab, an investigational anti-CD47 monoclonal antibody. Participants will be treated for up to 27 months with either magrolimab plus azacytidine (Vidaza), venetoclax plus azacytidine (patients deemed “appropriate for nonintensive therapy”), or standard chemotherapy (those “appropriate for intensive therapy”). In patients who received nonintensive therapy, OS is the primary outcome; OS in all participants is a secondary outcome, and QoL won’t be assessed. The trial opened in July and aims to recruit 346 individuals in Hong Kong, Australia, and the United States (California, Missouri, Oklahoma, Pennsylvania, South Carolina, and Texas).
All trial information is from the U.S. National Library of Medicine, National Institutes of Health.
A version of this article first appeared on Medscape.com.
A number of late-phase clinical trials in leukemia have opened in recent months. Maybe one of your patients could benefit from being enrolled.
Adults and children with acute or chronic leukemias
A phase 2 study partnering with the National Marrow Donor Program is seeking individuals aged 1-65 years with lymphoma or one of the following leukemias: “acute leukemia”, acute lymphoblastic (ALL), acute myelogenous (AML), mixed-phenotype acute, chronic myelogenous (CML), and chronic lymphocytic (CLL). Researchers hope to find a way to improve outcomes of hematopoietic-cell transplantation from mismatched, unrelated donors. Participants will receive the transplant and one of seven drug regimens and will be followed for a year. The trial plans to enroll 180 people and began recruiting on Sept. 30 in California, New York, and Virginia. The primary outcome is overall survival (OS). Quality of life (QoL) will not be measured.
Mast-cell leukemia (MCL)
Adults with MCL are sought for a phase 2 study of bezuclastinib, an experimental tyrosine-kinase inhibitor (TKI) called CGT9486. CGT9486 blocks the activity of a mutated version of tyrosine-kinase receptor KIT, called KIT D816V, which is known to cause systemic mastocytosis. Participants will receive oral CGT9486 daily for up to 18 months. The study opened in October, aiming for 140 participants with any advanced systemic mastocytoses (including MCL) at sites in California, Florida, Massachusetts, New York, Ohio, Texas, and Utah. OS and QoL will be tracked.
Previously Treated CLL/Small Lymphocytic Lymphoma (SLL)
Patients with CLL/SLL who have progressed on previous therapy can join a phase 3 study of another experimental oral TIK, pirtobrutinib, this time targeting Bruton’s tyrosine kinase (BTK). BTK plays a key role in the lifecycle of white blood cells. Participants will receive either “fixed-duration” pirtobrutinib plus venetoclax (Venclexta) and rituximab (Ruxience, Riabni, Truxima, Rituxan, MabThera) or the venetoclax-rituximab combo only, for up to 5 years. Investigators started recruiting in September, aiming for 600 participants across Florida, Louisiana, Missouri, New York, and Tennessee. Progression-free survival is the primary outcome; OS is a secondary outcome and QoL will not be tracked.
High-grade myeloid cancers with measurable residual disease
Patients with AML, myelodysplastic syndrome with excess blasts-2 or myeloid neoplasm, and whose original disease is still present, are eligible for a phase 2 study of CPX-351 (daunorubicin-cytarabine, Vyxeos). The intravenous chemotherapy was approved in 2017 for certain types of AML. The goal of this study is to determine if pretreatment with CPX-351 improves the outcome of donor stem-cell transplantation. Patients will either undergo immediate transplantation or receive CPX-351 for up to 10 days followed 60 days later by the transplant. The study, being conducted at the Fred Hutchinson Cancer Research Center in Seattle, started recruiting 130 patients in August. The primary outcome is OS; QoL will not be tracked.
Newly diagnosed Philadelphia-negative ALL
Patients aged 22 or older with Philadelphia-negative ALL who have not received chemotherapy or radiation therapy are invited to join a trial of calaspargase pegol (Asparlas). The therapy was approved in 2018 for ALL in children and young adults (1 month to 21 years). The aim of this study is to confirm the recommended doses and evaluate the drug’s safety and pharmacodynamics in adults over aged 21. Each participant will receive six 2-hour infusions of calaspargase pegol over several months. The primary outcomes are safety and drug activity; OS is a secondary outcome and QoL will not be measured. The study opened on July 7 and aims to recruit 122 participants in 11 states.
Untreated adults with TP53-mutant AML
Adult patients with previously untreated AML who have at least one TP53 gene mutation are sought for a phase 3 study of magrolimab, an investigational anti-CD47 monoclonal antibody. Participants will be treated for up to 27 months with either magrolimab plus azacytidine (Vidaza), venetoclax plus azacytidine (patients deemed “appropriate for nonintensive therapy”), or standard chemotherapy (those “appropriate for intensive therapy”). In patients who received nonintensive therapy, OS is the primary outcome; OS in all participants is a secondary outcome, and QoL won’t be assessed. The trial opened in July and aims to recruit 346 individuals in Hong Kong, Australia, and the United States (California, Missouri, Oklahoma, Pennsylvania, South Carolina, and Texas).
All trial information is from the U.S. National Library of Medicine, National Institutes of Health.
A version of this article first appeared on Medscape.com.
More eczema in children exposed to toxic metals in utero
published Oct. 27, 2021, in JAMA Network Open.
In this multicenter cohort study, led by epidemiologist Shu-Li Wang, PhD, of the National Institute of Environmental Health Sciences, in Taiwan, each twofold increase in prenatal arsenic level correlated with a 2.4-fold higher rate of atopic dermatitis in 4-year-olds.
Atopic diseases have been on the rise. Eczema (atopic dermatitis) is the first stage of the so-called atopic march, followed by food allergies, allergic rhinitis, and asthma later in childhood. Previous research has linked heavy metal exposure to allergic diseases in adults. In another study by Dr. Wang and colleagues that was published in 2021, prenatal and early-life arsenic exposure was found to correlate with higher rates of allergic rhinitis and asthma in children. In that study, the participants were followed every 2-3 years through the age of 14 as part of the Taiwan Maternal and Infant Cohort Study.
The new study included 370 mother and child pairs who were enrolled in that birth cohort study between October 2012 and May 2015. During their third trimester of pregnancy, women completed questionnaires about their lifestyle, diet, and living environment. In addition, their height, weight, and blood pressure were recorded, and urine samples were taken. In follow-up interviews 3-4 years later, the mothers were asked whether their child had ever been diagnosed with atopic dermatitis.
The researchers used an inductively coupled plasma mass spectrometer to analyze the participants’ urine samples. They assessed for exposures in utero to eight metals: arsenic, cadmium, lead, cobalt, copper, nickel, thallium, and zinc.
Each unit increase of an index that estimates the combined exposure to these metals during pregnancy was associated with 63% higher odds of atopic dermatitis in the children by age 4. The researchers adjusted for parental allergies (yes or no), mother’s educational level (<12 years, 13-16 years, or >16 years), geographic area (central or eastern Taiwan), exposure to tobacco smoke during pregnancy, and the child’s gender. Arsenic (40.1%) and cadmium (20.5%) accounted for most of the metal coexposure index.
A wealth of previous research links arsenic exposure during adulthood to skin disease and immune dysfunction. Early-life arsenic exposure has been linked with elevated risk for various adult disorders, including cancer, diabetes, and heart disease, years later. In light of such research, “the findings in this paper are not surprising,” J. Christopher States, PhD, director of the Center for Integrative Environmental Health Science at the University of Louisville (Ky.), told this news organization. “Low-level arsenic exposure does not cause disease immediately, but it does appear to have long-lasting effects, making individuals susceptible to ‘second hits’ with another environmental agent.”
Research into the molecular mechanisms for these links has shown that arsenic and cadmium exposure can promote allergic phenotypes in immune cells. “We think the toxic metals activate the alarmin pathway, thus inducing innate lymphoid cells, then activating T-helper 2 cells, which drive immunoglobulin E production and breakdown of the epithelium and promotion of allergies,” said Kari Nadeau, MD, PhD, director of the Sean N. Parker Center for Allergy and Asthma Research at Stanford University. Dr. Nadeau led that study, published in 2017 in PLOS One, along with epidemiologist Margaret Karagas, PhD, of Geisel School of Medicine at Dartmouth, Hanover, N.H.
As for what pregnant women can do to minimize their exposure to heavy metals, “that is a difficult problem and primarily a function of where one lives,” said Dr. States.
Drinking water and food are major sources of arsenic exposure. Groundwater is naturally contaminated with arsenic deposits that seep in from bedrock, said Dr. States. The U.S. Environmental Protection Agency regulates arsenic levels in public drinking water that is supplied to more than a few thousand people. However, small water supplies and private wells are unregulated, he said, and having these water sources tested for arsenic or fitted with systems to reduce arsenic can be very expensive.
Among foods, rice can have high concentrations of arsenic, Dr. Karagas told this news organization. To minimize arsenic exposure through the diet, women can limit rice-based foods, according to a web-based tool developed by her and coworkers.
In addition, tobacco smoke is a major source of cadmium exposure and a moderate source of arsenic exposure, Dr. States noted. Women can reduce their exposure to these metals by avoiding tobacco and secondhand smoke.
The study was supported by grants from the National Health Research Institutes, Chung Shan Medical University Hospital, Taiwan Ministry of Science and Technology, and the Taiwan Environmental Protection Administration. The authors and quoted experts report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
published Oct. 27, 2021, in JAMA Network Open.
In this multicenter cohort study, led by epidemiologist Shu-Li Wang, PhD, of the National Institute of Environmental Health Sciences, in Taiwan, each twofold increase in prenatal arsenic level correlated with a 2.4-fold higher rate of atopic dermatitis in 4-year-olds.
Atopic diseases have been on the rise. Eczema (atopic dermatitis) is the first stage of the so-called atopic march, followed by food allergies, allergic rhinitis, and asthma later in childhood. Previous research has linked heavy metal exposure to allergic diseases in adults. In another study by Dr. Wang and colleagues that was published in 2021, prenatal and early-life arsenic exposure was found to correlate with higher rates of allergic rhinitis and asthma in children. In that study, the participants were followed every 2-3 years through the age of 14 as part of the Taiwan Maternal and Infant Cohort Study.
The new study included 370 mother and child pairs who were enrolled in that birth cohort study between October 2012 and May 2015. During their third trimester of pregnancy, women completed questionnaires about their lifestyle, diet, and living environment. In addition, their height, weight, and blood pressure were recorded, and urine samples were taken. In follow-up interviews 3-4 years later, the mothers were asked whether their child had ever been diagnosed with atopic dermatitis.
The researchers used an inductively coupled plasma mass spectrometer to analyze the participants’ urine samples. They assessed for exposures in utero to eight metals: arsenic, cadmium, lead, cobalt, copper, nickel, thallium, and zinc.
Each unit increase of an index that estimates the combined exposure to these metals during pregnancy was associated with 63% higher odds of atopic dermatitis in the children by age 4. The researchers adjusted for parental allergies (yes or no), mother’s educational level (<12 years, 13-16 years, or >16 years), geographic area (central or eastern Taiwan), exposure to tobacco smoke during pregnancy, and the child’s gender. Arsenic (40.1%) and cadmium (20.5%) accounted for most of the metal coexposure index.
A wealth of previous research links arsenic exposure during adulthood to skin disease and immune dysfunction. Early-life arsenic exposure has been linked with elevated risk for various adult disorders, including cancer, diabetes, and heart disease, years later. In light of such research, “the findings in this paper are not surprising,” J. Christopher States, PhD, director of the Center for Integrative Environmental Health Science at the University of Louisville (Ky.), told this news organization. “Low-level arsenic exposure does not cause disease immediately, but it does appear to have long-lasting effects, making individuals susceptible to ‘second hits’ with another environmental agent.”
Research into the molecular mechanisms for these links has shown that arsenic and cadmium exposure can promote allergic phenotypes in immune cells. “We think the toxic metals activate the alarmin pathway, thus inducing innate lymphoid cells, then activating T-helper 2 cells, which drive immunoglobulin E production and breakdown of the epithelium and promotion of allergies,” said Kari Nadeau, MD, PhD, director of the Sean N. Parker Center for Allergy and Asthma Research at Stanford University. Dr. Nadeau led that study, published in 2017 in PLOS One, along with epidemiologist Margaret Karagas, PhD, of Geisel School of Medicine at Dartmouth, Hanover, N.H.
As for what pregnant women can do to minimize their exposure to heavy metals, “that is a difficult problem and primarily a function of where one lives,” said Dr. States.
Drinking water and food are major sources of arsenic exposure. Groundwater is naturally contaminated with arsenic deposits that seep in from bedrock, said Dr. States. The U.S. Environmental Protection Agency regulates arsenic levels in public drinking water that is supplied to more than a few thousand people. However, small water supplies and private wells are unregulated, he said, and having these water sources tested for arsenic or fitted with systems to reduce arsenic can be very expensive.
Among foods, rice can have high concentrations of arsenic, Dr. Karagas told this news organization. To minimize arsenic exposure through the diet, women can limit rice-based foods, according to a web-based tool developed by her and coworkers.
In addition, tobacco smoke is a major source of cadmium exposure and a moderate source of arsenic exposure, Dr. States noted. Women can reduce their exposure to these metals by avoiding tobacco and secondhand smoke.
The study was supported by grants from the National Health Research Institutes, Chung Shan Medical University Hospital, Taiwan Ministry of Science and Technology, and the Taiwan Environmental Protection Administration. The authors and quoted experts report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
published Oct. 27, 2021, in JAMA Network Open.
In this multicenter cohort study, led by epidemiologist Shu-Li Wang, PhD, of the National Institute of Environmental Health Sciences, in Taiwan, each twofold increase in prenatal arsenic level correlated with a 2.4-fold higher rate of atopic dermatitis in 4-year-olds.
Atopic diseases have been on the rise. Eczema (atopic dermatitis) is the first stage of the so-called atopic march, followed by food allergies, allergic rhinitis, and asthma later in childhood. Previous research has linked heavy metal exposure to allergic diseases in adults. In another study by Dr. Wang and colleagues that was published in 2021, prenatal and early-life arsenic exposure was found to correlate with higher rates of allergic rhinitis and asthma in children. In that study, the participants were followed every 2-3 years through the age of 14 as part of the Taiwan Maternal and Infant Cohort Study.
The new study included 370 mother and child pairs who were enrolled in that birth cohort study between October 2012 and May 2015. During their third trimester of pregnancy, women completed questionnaires about their lifestyle, diet, and living environment. In addition, their height, weight, and blood pressure were recorded, and urine samples were taken. In follow-up interviews 3-4 years later, the mothers were asked whether their child had ever been diagnosed with atopic dermatitis.
The researchers used an inductively coupled plasma mass spectrometer to analyze the participants’ urine samples. They assessed for exposures in utero to eight metals: arsenic, cadmium, lead, cobalt, copper, nickel, thallium, and zinc.
Each unit increase of an index that estimates the combined exposure to these metals during pregnancy was associated with 63% higher odds of atopic dermatitis in the children by age 4. The researchers adjusted for parental allergies (yes or no), mother’s educational level (<12 years, 13-16 years, or >16 years), geographic area (central or eastern Taiwan), exposure to tobacco smoke during pregnancy, and the child’s gender. Arsenic (40.1%) and cadmium (20.5%) accounted for most of the metal coexposure index.
A wealth of previous research links arsenic exposure during adulthood to skin disease and immune dysfunction. Early-life arsenic exposure has been linked with elevated risk for various adult disorders, including cancer, diabetes, and heart disease, years later. In light of such research, “the findings in this paper are not surprising,” J. Christopher States, PhD, director of the Center for Integrative Environmental Health Science at the University of Louisville (Ky.), told this news organization. “Low-level arsenic exposure does not cause disease immediately, but it does appear to have long-lasting effects, making individuals susceptible to ‘second hits’ with another environmental agent.”
Research into the molecular mechanisms for these links has shown that arsenic and cadmium exposure can promote allergic phenotypes in immune cells. “We think the toxic metals activate the alarmin pathway, thus inducing innate lymphoid cells, then activating T-helper 2 cells, which drive immunoglobulin E production and breakdown of the epithelium and promotion of allergies,” said Kari Nadeau, MD, PhD, director of the Sean N. Parker Center for Allergy and Asthma Research at Stanford University. Dr. Nadeau led that study, published in 2017 in PLOS One, along with epidemiologist Margaret Karagas, PhD, of Geisel School of Medicine at Dartmouth, Hanover, N.H.
As for what pregnant women can do to minimize their exposure to heavy metals, “that is a difficult problem and primarily a function of where one lives,” said Dr. States.
Drinking water and food are major sources of arsenic exposure. Groundwater is naturally contaminated with arsenic deposits that seep in from bedrock, said Dr. States. The U.S. Environmental Protection Agency regulates arsenic levels in public drinking water that is supplied to more than a few thousand people. However, small water supplies and private wells are unregulated, he said, and having these water sources tested for arsenic or fitted with systems to reduce arsenic can be very expensive.
Among foods, rice can have high concentrations of arsenic, Dr. Karagas told this news organization. To minimize arsenic exposure through the diet, women can limit rice-based foods, according to a web-based tool developed by her and coworkers.
In addition, tobacco smoke is a major source of cadmium exposure and a moderate source of arsenic exposure, Dr. States noted. Women can reduce their exposure to these metals by avoiding tobacco and secondhand smoke.
The study was supported by grants from the National Health Research Institutes, Chung Shan Medical University Hospital, Taiwan Ministry of Science and Technology, and the Taiwan Environmental Protection Administration. The authors and quoted experts report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
‘If obesity were diabetes or cancer, how would you approach it?’
“When considering the challenges of obesity, ask yourself: ‘If it were diabetes, cancer, HIV, or Alzheimer’s, how would you discuss it, approach it, assess it, treat it?’” Lee M. Kaplan, MD, PhD, asked the audience of health care professionals during ObesityWeek®, the annual meeting of The Obesity Society.
“And then do it for obesity, using the full spectrum of tools at our disposal,” he advised.
This was the takeaway that Dr. Kaplan, director of the Obesity, Metabolism, and Nutrition Institute at Massachusetts General Hospital and associate professor, Harvard Medical School, Boston, left the audience with at the end of his lecture entitled, “What does the future of obesity care look like?”
Invited to summarize his main points, Dr. Kaplan told this news organization in an interview that practitioners caring for patients with obesity need to first “recognize that obesity is a disease” caused by dysfunction of the metabolic system that regulates body fat – in the same way immune dysregulation can lead to asthma.
Second, “we are finally developing noninvasive therapies that are more effective,” he noted, referring to the recently approved semaglutide, and even more potent weight-loss therapies that could be on the market within 3 years, so that weight-loss outcomes with pharmacotherapy are approaching those with bariatric surgery.
Third, it is important that patients with obesity get “broad and equitable access” to treatment, and health care practitioners need to be on the same page and have a “shared understanding” of which treatments are appropriate for individual patients, “just as we do for other diseases.”
Need for a shared understanding
“Dr. Kaplan really brought home the idea that we all need a shared understanding of what obesity is – and what it is not,” agreed symposium moderator Donna H. Ryan, MD, in an email.
“He underscored the biologic basis of obesity,” noted Dr. Ryan, professor emerita at Pennington Biomedical Research Center in Baton Rouge, Louisiana, and associate editor-in-chief of Obesity, the official journal of The Obesity Society.
“It is a dysregulation of the body’s weight (especially adipose tissue) regulatory system,” she continued. “The body responds to powerful environmental pressures that produce excess energy balance, and we store that as fat and defend our highest fat mass. This makes obesity a disease, a chronic disease that requires a medical approach to reverse. It’s not a cosmetic problem, it’s a medical problem,” she emphasized.
There is so much misinformation out there about obesity, according to Dr. Ryan.
“People think it’s a lack of willpower, and even patients blame themselves for not being able to lose weight and keep it off. It’s not their fault! It’s biology.”
Although the supplement industry and fad diets falsely promise fast results, there is no magic diet, she continued.
“But we have made progress based on understanding the biologic basis of obesity and have new medications that offer real hope for patients.”
“With 42% of U.S. adults having a BMI that qualifies as obesity, we need a concerted and broad effort to address this problem, and that starts with everybody on the same page as to what obesity is ... a shared understanding of the biologic basis of obesity. It’s time to take obesity seriously,” she summarized, echoing Dr. Kaplan.
A question of biology
“Obesity results from inappropriate pathophysiological regulation of body fat mass,” when the body defends adiposity, Dr. Kaplan explained at the start of his lecture.
The treatment strategy for obesity has always been a stepwise approach starting with lifestyle changes, then pharmacotherapy, then possibly bariatric surgery – each step with a potentially greater chance of weight loss. But now, he explained, medicine is on the verge of having an armamentarium of more potent weight-loss medications.
Compared with phentermine/topiramate, orlistat, naltrexone/bupropion, and liraglutide – which roughly might provide 5% to 10% weight loss, the glucagon-like peptide-1 (GLP-1) agonist semaglutide 2.4 mg/week (Wegovy, Novo Nordisk), approved by the U.S. Food and Drug Association in June, provides almost double this potential weight loss.
And two new agents that could provide “never seen before weight loss” of 25% could potentially enter the marketplace by 2025: the amylin agonist cagrilintide (Novo Nordisk) and the twincretin tirzepatide (Eli Lilly) (a combined glucose-dependent insulinotropic polypeptide [GIP] and GLP-1 receptor agonist).
In addition, when liraglutide comes off patent, a generic version could potentially be introduced, and combined generic liraglutide plus generic phentermine/topiramate could be a less expensive weight-loss treatment option in the future, he noted.
One size does not fit all
Importantly, weight loss varies widely among individual patients.
A graph of potential weight loss with different treatments (for example, bariatric surgery or liraglutide) versus the percentage of patients that attain the weight losses is roughly bell-shaped, Dr. Kaplan explained. For example, in the STEP1 trial of semaglutide, roughly 7.1% of patients lost less than 5% of their initial weight, 25% of patients lost 20% to 30%, and 10.8% of patients lost 30% or more; that is, patients at the higher end had weight loss comparable to that seen with bariatric surgery
Adding pharmacotherapy after bariatric surgery could be synergistic. For example, in the GRAVITAS study of patients with type 2 diabetes who had gastric bypass surgery, those who received liraglutide after surgery had augmented weight loss compared with those who received placebo.
People at a cocktail party might come up to him and say, “I’d like to lose 5 pounds, 10 pounds,” Dr. Kaplan related in the Q&A session.
“That’s not obesity,” he emphasized. Obesity is excess body fat that poses a risk to health. A person with obesity may have 50 or more excess pounds, and the body is trying to defend this weight.
“If we want to treat obesity more effectively, we have to fully understand why it is a disease and how that disease differs from the cultural desire for thinness,” he reiterated.
“We have to keep the needs and goals of all people living with obesity foremost in our minds, even if many of them have been previously misled by the bias, stigma, blame, and discrimination that surrounds them.”
“We need to re-evaluate what we think we know about obesity and open our minds to new ideas,” he added.
Dr. Kaplan has reported financial ties to Eli Lilly, Gelesis, GI Dynamics, IntelliHealth, Johnson & Johnson, Novo Nordisk, Pfizer, and Rhythm Pharmaceuticals. Dr. Ryan has ties to numerous Novo Nordisk, Pfizer, and several other pharmaceutical companies, including having an ownership interest in Gila Therapeutics, Xeno Biosciences, Epitomee, Calibrate, Roman, and Scientific Intake.
A version of this article first appeared on Medscape.com.
“When considering the challenges of obesity, ask yourself: ‘If it were diabetes, cancer, HIV, or Alzheimer’s, how would you discuss it, approach it, assess it, treat it?’” Lee M. Kaplan, MD, PhD, asked the audience of health care professionals during ObesityWeek®, the annual meeting of The Obesity Society.
“And then do it for obesity, using the full spectrum of tools at our disposal,” he advised.
This was the takeaway that Dr. Kaplan, director of the Obesity, Metabolism, and Nutrition Institute at Massachusetts General Hospital and associate professor, Harvard Medical School, Boston, left the audience with at the end of his lecture entitled, “What does the future of obesity care look like?”
Invited to summarize his main points, Dr. Kaplan told this news organization in an interview that practitioners caring for patients with obesity need to first “recognize that obesity is a disease” caused by dysfunction of the metabolic system that regulates body fat – in the same way immune dysregulation can lead to asthma.
Second, “we are finally developing noninvasive therapies that are more effective,” he noted, referring to the recently approved semaglutide, and even more potent weight-loss therapies that could be on the market within 3 years, so that weight-loss outcomes with pharmacotherapy are approaching those with bariatric surgery.
Third, it is important that patients with obesity get “broad and equitable access” to treatment, and health care practitioners need to be on the same page and have a “shared understanding” of which treatments are appropriate for individual patients, “just as we do for other diseases.”
Need for a shared understanding
“Dr. Kaplan really brought home the idea that we all need a shared understanding of what obesity is – and what it is not,” agreed symposium moderator Donna H. Ryan, MD, in an email.
“He underscored the biologic basis of obesity,” noted Dr. Ryan, professor emerita at Pennington Biomedical Research Center in Baton Rouge, Louisiana, and associate editor-in-chief of Obesity, the official journal of The Obesity Society.
“It is a dysregulation of the body’s weight (especially adipose tissue) regulatory system,” she continued. “The body responds to powerful environmental pressures that produce excess energy balance, and we store that as fat and defend our highest fat mass. This makes obesity a disease, a chronic disease that requires a medical approach to reverse. It’s not a cosmetic problem, it’s a medical problem,” she emphasized.
There is so much misinformation out there about obesity, according to Dr. Ryan.
“People think it’s a lack of willpower, and even patients blame themselves for not being able to lose weight and keep it off. It’s not their fault! It’s biology.”
Although the supplement industry and fad diets falsely promise fast results, there is no magic diet, she continued.
“But we have made progress based on understanding the biologic basis of obesity and have new medications that offer real hope for patients.”
“With 42% of U.S. adults having a BMI that qualifies as obesity, we need a concerted and broad effort to address this problem, and that starts with everybody on the same page as to what obesity is ... a shared understanding of the biologic basis of obesity. It’s time to take obesity seriously,” she summarized, echoing Dr. Kaplan.
A question of biology
“Obesity results from inappropriate pathophysiological regulation of body fat mass,” when the body defends adiposity, Dr. Kaplan explained at the start of his lecture.
The treatment strategy for obesity has always been a stepwise approach starting with lifestyle changes, then pharmacotherapy, then possibly bariatric surgery – each step with a potentially greater chance of weight loss. But now, he explained, medicine is on the verge of having an armamentarium of more potent weight-loss medications.
Compared with phentermine/topiramate, orlistat, naltrexone/bupropion, and liraglutide – which roughly might provide 5% to 10% weight loss, the glucagon-like peptide-1 (GLP-1) agonist semaglutide 2.4 mg/week (Wegovy, Novo Nordisk), approved by the U.S. Food and Drug Association in June, provides almost double this potential weight loss.
And two new agents that could provide “never seen before weight loss” of 25% could potentially enter the marketplace by 2025: the amylin agonist cagrilintide (Novo Nordisk) and the twincretin tirzepatide (Eli Lilly) (a combined glucose-dependent insulinotropic polypeptide [GIP] and GLP-1 receptor agonist).
In addition, when liraglutide comes off patent, a generic version could potentially be introduced, and combined generic liraglutide plus generic phentermine/topiramate could be a less expensive weight-loss treatment option in the future, he noted.
One size does not fit all
Importantly, weight loss varies widely among individual patients.
A graph of potential weight loss with different treatments (for example, bariatric surgery or liraglutide) versus the percentage of patients that attain the weight losses is roughly bell-shaped, Dr. Kaplan explained. For example, in the STEP1 trial of semaglutide, roughly 7.1% of patients lost less than 5% of their initial weight, 25% of patients lost 20% to 30%, and 10.8% of patients lost 30% or more; that is, patients at the higher end had weight loss comparable to that seen with bariatric surgery
Adding pharmacotherapy after bariatric surgery could be synergistic. For example, in the GRAVITAS study of patients with type 2 diabetes who had gastric bypass surgery, those who received liraglutide after surgery had augmented weight loss compared with those who received placebo.
People at a cocktail party might come up to him and say, “I’d like to lose 5 pounds, 10 pounds,” Dr. Kaplan related in the Q&A session.
“That’s not obesity,” he emphasized. Obesity is excess body fat that poses a risk to health. A person with obesity may have 50 or more excess pounds, and the body is trying to defend this weight.
“If we want to treat obesity more effectively, we have to fully understand why it is a disease and how that disease differs from the cultural desire for thinness,” he reiterated.
“We have to keep the needs and goals of all people living with obesity foremost in our minds, even if many of them have been previously misled by the bias, stigma, blame, and discrimination that surrounds them.”
“We need to re-evaluate what we think we know about obesity and open our minds to new ideas,” he added.
Dr. Kaplan has reported financial ties to Eli Lilly, Gelesis, GI Dynamics, IntelliHealth, Johnson & Johnson, Novo Nordisk, Pfizer, and Rhythm Pharmaceuticals. Dr. Ryan has ties to numerous Novo Nordisk, Pfizer, and several other pharmaceutical companies, including having an ownership interest in Gila Therapeutics, Xeno Biosciences, Epitomee, Calibrate, Roman, and Scientific Intake.
A version of this article first appeared on Medscape.com.
“When considering the challenges of obesity, ask yourself: ‘If it were diabetes, cancer, HIV, or Alzheimer’s, how would you discuss it, approach it, assess it, treat it?’” Lee M. Kaplan, MD, PhD, asked the audience of health care professionals during ObesityWeek®, the annual meeting of The Obesity Society.
“And then do it for obesity, using the full spectrum of tools at our disposal,” he advised.
This was the takeaway that Dr. Kaplan, director of the Obesity, Metabolism, and Nutrition Institute at Massachusetts General Hospital and associate professor, Harvard Medical School, Boston, left the audience with at the end of his lecture entitled, “What does the future of obesity care look like?”
Invited to summarize his main points, Dr. Kaplan told this news organization in an interview that practitioners caring for patients with obesity need to first “recognize that obesity is a disease” caused by dysfunction of the metabolic system that regulates body fat – in the same way immune dysregulation can lead to asthma.
Second, “we are finally developing noninvasive therapies that are more effective,” he noted, referring to the recently approved semaglutide, and even more potent weight-loss therapies that could be on the market within 3 years, so that weight-loss outcomes with pharmacotherapy are approaching those with bariatric surgery.
Third, it is important that patients with obesity get “broad and equitable access” to treatment, and health care practitioners need to be on the same page and have a “shared understanding” of which treatments are appropriate for individual patients, “just as we do for other diseases.”
Need for a shared understanding
“Dr. Kaplan really brought home the idea that we all need a shared understanding of what obesity is – and what it is not,” agreed symposium moderator Donna H. Ryan, MD, in an email.
“He underscored the biologic basis of obesity,” noted Dr. Ryan, professor emerita at Pennington Biomedical Research Center in Baton Rouge, Louisiana, and associate editor-in-chief of Obesity, the official journal of The Obesity Society.
“It is a dysregulation of the body’s weight (especially adipose tissue) regulatory system,” she continued. “The body responds to powerful environmental pressures that produce excess energy balance, and we store that as fat and defend our highest fat mass. This makes obesity a disease, a chronic disease that requires a medical approach to reverse. It’s not a cosmetic problem, it’s a medical problem,” she emphasized.
There is so much misinformation out there about obesity, according to Dr. Ryan.
“People think it’s a lack of willpower, and even patients blame themselves for not being able to lose weight and keep it off. It’s not their fault! It’s biology.”
Although the supplement industry and fad diets falsely promise fast results, there is no magic diet, she continued.
“But we have made progress based on understanding the biologic basis of obesity and have new medications that offer real hope for patients.”
“With 42% of U.S. adults having a BMI that qualifies as obesity, we need a concerted and broad effort to address this problem, and that starts with everybody on the same page as to what obesity is ... a shared understanding of the biologic basis of obesity. It’s time to take obesity seriously,” she summarized, echoing Dr. Kaplan.
A question of biology
“Obesity results from inappropriate pathophysiological regulation of body fat mass,” when the body defends adiposity, Dr. Kaplan explained at the start of his lecture.
The treatment strategy for obesity has always been a stepwise approach starting with lifestyle changes, then pharmacotherapy, then possibly bariatric surgery – each step with a potentially greater chance of weight loss. But now, he explained, medicine is on the verge of having an armamentarium of more potent weight-loss medications.
Compared with phentermine/topiramate, orlistat, naltrexone/bupropion, and liraglutide – which roughly might provide 5% to 10% weight loss, the glucagon-like peptide-1 (GLP-1) agonist semaglutide 2.4 mg/week (Wegovy, Novo Nordisk), approved by the U.S. Food and Drug Association in June, provides almost double this potential weight loss.
And two new agents that could provide “never seen before weight loss” of 25% could potentially enter the marketplace by 2025: the amylin agonist cagrilintide (Novo Nordisk) and the twincretin tirzepatide (Eli Lilly) (a combined glucose-dependent insulinotropic polypeptide [GIP] and GLP-1 receptor agonist).
In addition, when liraglutide comes off patent, a generic version could potentially be introduced, and combined generic liraglutide plus generic phentermine/topiramate could be a less expensive weight-loss treatment option in the future, he noted.
One size does not fit all
Importantly, weight loss varies widely among individual patients.
A graph of potential weight loss with different treatments (for example, bariatric surgery or liraglutide) versus the percentage of patients that attain the weight losses is roughly bell-shaped, Dr. Kaplan explained. For example, in the STEP1 trial of semaglutide, roughly 7.1% of patients lost less than 5% of their initial weight, 25% of patients lost 20% to 30%, and 10.8% of patients lost 30% or more; that is, patients at the higher end had weight loss comparable to that seen with bariatric surgery
Adding pharmacotherapy after bariatric surgery could be synergistic. For example, in the GRAVITAS study of patients with type 2 diabetes who had gastric bypass surgery, those who received liraglutide after surgery had augmented weight loss compared with those who received placebo.
People at a cocktail party might come up to him and say, “I’d like to lose 5 pounds, 10 pounds,” Dr. Kaplan related in the Q&A session.
“That’s not obesity,” he emphasized. Obesity is excess body fat that poses a risk to health. A person with obesity may have 50 or more excess pounds, and the body is trying to defend this weight.
“If we want to treat obesity more effectively, we have to fully understand why it is a disease and how that disease differs from the cultural desire for thinness,” he reiterated.
“We have to keep the needs and goals of all people living with obesity foremost in our minds, even if many of them have been previously misled by the bias, stigma, blame, and discrimination that surrounds them.”
“We need to re-evaluate what we think we know about obesity and open our minds to new ideas,” he added.
Dr. Kaplan has reported financial ties to Eli Lilly, Gelesis, GI Dynamics, IntelliHealth, Johnson & Johnson, Novo Nordisk, Pfizer, and Rhythm Pharmaceuticals. Dr. Ryan has ties to numerous Novo Nordisk, Pfizer, and several other pharmaceutical companies, including having an ownership interest in Gila Therapeutics, Xeno Biosciences, Epitomee, Calibrate, Roman, and Scientific Intake.
A version of this article first appeared on Medscape.com.
FROM OBESITY WEEK 2020
Does the use of frankincense make sense in dermatology?
The Boswellia serrata exudate or gum (known in India as “guggulu”) that forms an aromatic resin traditionally used as incense – and known as frankincense (especially when retrieved from Boswellia species found in Eritrea and Somalia but also from the Indian variety) – has been considered for thousands of years to possess therapeutic properties. It is used in Ayurvedic medicine, as well as in traditional medicine in China and the Middle East, particularly for its anti-inflammatory effects to treat chronic conditions.1-8 In fact, such essential oils have been used since 2800 BC to treat various inflammatory conditions, including skin sores and wounds, as well as in perfumes and incense.2,9 In the West, use of frankincense dates back to thousands of years as well, more often found in the form of incense for religious and cultural ceremonies.7 Over the past 2 decades, .3 This column focuses on some of the emerging data on this ancient botanical agent.
Chemical constituents
Terpenoids and essential oils are the primary components of frankincense and are known to impart anti-inflammatory and anticancer activity. The same is true for myrrh, which has been combined with frankincense in traditional Chinese medicine as a single medication for millennia, with the two acting synergistically and considered still to be a potent combination in conferring various biological benefits.7
In 2010, in a systematic review of the anti-inflammatory and anticancer activities of Boswellia species and their chemical ingredients, Efferth and Oesch found that frankincense blocks the production of leukotrienes, cyclooxygenase (COX) 1 and 2, as well as 5-lipoxygenase; and oxidative stress. It also contributes to regulation of immune cells from the innate and acquired immune systems and exerts anticancer activity by influencing signaling transduction responsible for cell cycle arrest, as well as inhibition of proliferation, angiogenesis, invasion, and metastasis. The investigators also reported on clinical trial results that have found efficacy of frankincense and its constituents in ameliorating symptoms of psoriasis and erythematous eczema, among other disorders.3
Anti-inflammatory activity
Li et al. completed a study in 2016 to identify the active ingredients responsible for the anti-inflammatory and analgesic effects of frankincense. They found that alpha-pinene, linalool, and 1-octanol were key contributors. These constituents were noted for suppressing COX-2 overexpression in mice, as well as nociceptive stimulus-induced inflammatory infiltrates.10
Noting the increasing popularity of frankincense essential oil in skin care, despite a paucity of data, in 2017, Han et al. evaluated the biological activities of the essential oil in pre-inflamed human dermal fibroblasts using 17 key protein biomarkers. Frankincense essential oil displayed significant antiproliferative activity and suppressed collagen III, interferon gamma-induced protein 10, and intracellular adhesion molecule 1. The investigators referred to the overall encouraging potential of frankincense essential oil to exert influence over inflammation and tissue remodeling in human skin and called for additional research into its mechanisms of action and active constituents.11
Anticancer activity
The main active ingredient in frankincense, boswellic acid, has been shown to promote apoptosis, suppress matrix metalloproteinase secretion, and hinder migration in metastatic melanoma cell lines in mice.6,12
In 2019, Hakkim et al. demonstrated that frankincense essential oil yielded substantial antimelanoma activity in vitro and in vivo and ameliorated hepatotoxicity caused by acetaminophen.13
There is one case report in the literature on the use of frankincense as a treatment for skin cancer. A 56-year-old man received frankincense oil multiple times a day for 4 months to treat a nodular basal cell carcinoma on one arm (which resolved) and an infiltrative BCC on the chest (some focal residual tumor remained).6,14 Topical frankincense or boswellic acid has been given a grade D recommendation for treating skin cancer, however, because of only one level-of-evidence-5 study.6
Antimicrobial activity
In 2012, de Rapper et al. collected samples of three essential oils of frankincense (Boswellia rivae, Boswellia neglecta, and Boswellia papyrifera) and two essential oil samples of myrrh and sweet myrrh from different regions of Ethiopia to study their anti-infective properties alone and in combination. The investigators observed synergistic and additive effects, particularly between B. papyrifera and Commiphora myrrha. While noting the long history of the combined use of frankincense and myrrh essential oils since 1500 BC, the investigators highlighted their study as the first antimicrobial work to verify the effectiveness of this combination, validating the use of this combination to thwart particular pathogens.15
Just 2 years ago, Ljaljević Grbić et al. evaluated the in vitro antimicrobial potential of the liquid and vapor phases of B. carteri and C. myrrha (frankincense and myrrh, respectively) essential oils, finding that frankincense demonstrated marked capacity to act as a natural antimicrobial agent.9
Transdermal delivery
In 2017, Zhu et al. showed that frankincense and myrrh essential oils promoted the permeability of the Chinese herb Chuanxiong and may facilitate drug elimination from the epidermis via dermal capillaries by dint of improved cutaneous blood flow, thereby augmenting transdermal drug delivery.16 The same team also showed that frankincense and myrrh essential oils, by fostering permeation by enhancing drug delivery across the stratum corneum, can also alter the structure of the stratum corneum.17
Conclusion
The use of frankincense in traditional medicine has a long and impressive track record. Recent research provides reason for optimism, and further investigating the possible incorporation of this botanical agent into modern dermatologic therapies appears warranted. Clearly, however, much more research is needed.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Kimmatkar N et al. Phytomedicine. 2003 Jan;10(1):3-7.
2. Ammon HP. Wien Med Wochenschr. 2002;152(15-16):373-8.
3. Efferth T & Oesch F. Semin Cancer Biol. 2020 Feb 4;S1044-579X(20)30034-1.
4. Banno N et al. J Ethnopharmacol. 2006 Sep 19;107(2):249-53.
5. Poeckel D & Werz O. Curr Med Chem. 2006;13(28):3359-69.
6. Li JY, Kampp JT. Dermatol Surg. 2019 Jan;45(1):58-67.
7. Cao B et al. Molecules. 2019 Aug 24;24(17): 3076.
8. Mertens M et al. Flavour Fragr J. 2009;24:279-300.
9. Ljaljević Grbić M et al. J Ethnopharmacol. 2018 Jun 12;219:1-14.
10. Li XJ et al. J Ethnopharmacol. 2016 Feb 17;179:22-6.
11. Han X et al. Biochim Open. 2017 Feb 3;4:31-5.
12. Zhao W et al. Cancer Detect Prev. 2003;27:67-75.
13. Hakkim FL et al. Oncotarget. 2019 May 28;10(37):3472-90.
14. Fung K et al. OA Altern Med 2013;1:14.
15. de Rapper S et al. Lett Appl Microbiol. 2012 Apr;54(4):352-8.
16. Zhu XF et al. Zhongguo Zhong Yao Za Zhi. 2017 Feb;42(4):680-5.
17. Guan YM et al. Zhongguo Zhong Yao Za Zhi. 2017 Sep;42(17):3350-5.
The Boswellia serrata exudate or gum (known in India as “guggulu”) that forms an aromatic resin traditionally used as incense – and known as frankincense (especially when retrieved from Boswellia species found in Eritrea and Somalia but also from the Indian variety) – has been considered for thousands of years to possess therapeutic properties. It is used in Ayurvedic medicine, as well as in traditional medicine in China and the Middle East, particularly for its anti-inflammatory effects to treat chronic conditions.1-8 In fact, such essential oils have been used since 2800 BC to treat various inflammatory conditions, including skin sores and wounds, as well as in perfumes and incense.2,9 In the West, use of frankincense dates back to thousands of years as well, more often found in the form of incense for religious and cultural ceremonies.7 Over the past 2 decades, .3 This column focuses on some of the emerging data on this ancient botanical agent.
Chemical constituents
Terpenoids and essential oils are the primary components of frankincense and are known to impart anti-inflammatory and anticancer activity. The same is true for myrrh, which has been combined with frankincense in traditional Chinese medicine as a single medication for millennia, with the two acting synergistically and considered still to be a potent combination in conferring various biological benefits.7
In 2010, in a systematic review of the anti-inflammatory and anticancer activities of Boswellia species and their chemical ingredients, Efferth and Oesch found that frankincense blocks the production of leukotrienes, cyclooxygenase (COX) 1 and 2, as well as 5-lipoxygenase; and oxidative stress. It also contributes to regulation of immune cells from the innate and acquired immune systems and exerts anticancer activity by influencing signaling transduction responsible for cell cycle arrest, as well as inhibition of proliferation, angiogenesis, invasion, and metastasis. The investigators also reported on clinical trial results that have found efficacy of frankincense and its constituents in ameliorating symptoms of psoriasis and erythematous eczema, among other disorders.3
Anti-inflammatory activity
Li et al. completed a study in 2016 to identify the active ingredients responsible for the anti-inflammatory and analgesic effects of frankincense. They found that alpha-pinene, linalool, and 1-octanol were key contributors. These constituents were noted for suppressing COX-2 overexpression in mice, as well as nociceptive stimulus-induced inflammatory infiltrates.10
Noting the increasing popularity of frankincense essential oil in skin care, despite a paucity of data, in 2017, Han et al. evaluated the biological activities of the essential oil in pre-inflamed human dermal fibroblasts using 17 key protein biomarkers. Frankincense essential oil displayed significant antiproliferative activity and suppressed collagen III, interferon gamma-induced protein 10, and intracellular adhesion molecule 1. The investigators referred to the overall encouraging potential of frankincense essential oil to exert influence over inflammation and tissue remodeling in human skin and called for additional research into its mechanisms of action and active constituents.11
Anticancer activity
The main active ingredient in frankincense, boswellic acid, has been shown to promote apoptosis, suppress matrix metalloproteinase secretion, and hinder migration in metastatic melanoma cell lines in mice.6,12
In 2019, Hakkim et al. demonstrated that frankincense essential oil yielded substantial antimelanoma activity in vitro and in vivo and ameliorated hepatotoxicity caused by acetaminophen.13
There is one case report in the literature on the use of frankincense as a treatment for skin cancer. A 56-year-old man received frankincense oil multiple times a day for 4 months to treat a nodular basal cell carcinoma on one arm (which resolved) and an infiltrative BCC on the chest (some focal residual tumor remained).6,14 Topical frankincense or boswellic acid has been given a grade D recommendation for treating skin cancer, however, because of only one level-of-evidence-5 study.6
Antimicrobial activity
In 2012, de Rapper et al. collected samples of three essential oils of frankincense (Boswellia rivae, Boswellia neglecta, and Boswellia papyrifera) and two essential oil samples of myrrh and sweet myrrh from different regions of Ethiopia to study their anti-infective properties alone and in combination. The investigators observed synergistic and additive effects, particularly between B. papyrifera and Commiphora myrrha. While noting the long history of the combined use of frankincense and myrrh essential oils since 1500 BC, the investigators highlighted their study as the first antimicrobial work to verify the effectiveness of this combination, validating the use of this combination to thwart particular pathogens.15
Just 2 years ago, Ljaljević Grbić et al. evaluated the in vitro antimicrobial potential of the liquid and vapor phases of B. carteri and C. myrrha (frankincense and myrrh, respectively) essential oils, finding that frankincense demonstrated marked capacity to act as a natural antimicrobial agent.9
Transdermal delivery
In 2017, Zhu et al. showed that frankincense and myrrh essential oils promoted the permeability of the Chinese herb Chuanxiong and may facilitate drug elimination from the epidermis via dermal capillaries by dint of improved cutaneous blood flow, thereby augmenting transdermal drug delivery.16 The same team also showed that frankincense and myrrh essential oils, by fostering permeation by enhancing drug delivery across the stratum corneum, can also alter the structure of the stratum corneum.17
Conclusion
The use of frankincense in traditional medicine has a long and impressive track record. Recent research provides reason for optimism, and further investigating the possible incorporation of this botanical agent into modern dermatologic therapies appears warranted. Clearly, however, much more research is needed.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Kimmatkar N et al. Phytomedicine. 2003 Jan;10(1):3-7.
2. Ammon HP. Wien Med Wochenschr. 2002;152(15-16):373-8.
3. Efferth T & Oesch F. Semin Cancer Biol. 2020 Feb 4;S1044-579X(20)30034-1.
4. Banno N et al. J Ethnopharmacol. 2006 Sep 19;107(2):249-53.
5. Poeckel D & Werz O. Curr Med Chem. 2006;13(28):3359-69.
6. Li JY, Kampp JT. Dermatol Surg. 2019 Jan;45(1):58-67.
7. Cao B et al. Molecules. 2019 Aug 24;24(17): 3076.
8. Mertens M et al. Flavour Fragr J. 2009;24:279-300.
9. Ljaljević Grbić M et al. J Ethnopharmacol. 2018 Jun 12;219:1-14.
10. Li XJ et al. J Ethnopharmacol. 2016 Feb 17;179:22-6.
11. Han X et al. Biochim Open. 2017 Feb 3;4:31-5.
12. Zhao W et al. Cancer Detect Prev. 2003;27:67-75.
13. Hakkim FL et al. Oncotarget. 2019 May 28;10(37):3472-90.
14. Fung K et al. OA Altern Med 2013;1:14.
15. de Rapper S et al. Lett Appl Microbiol. 2012 Apr;54(4):352-8.
16. Zhu XF et al. Zhongguo Zhong Yao Za Zhi. 2017 Feb;42(4):680-5.
17. Guan YM et al. Zhongguo Zhong Yao Za Zhi. 2017 Sep;42(17):3350-5.
The Boswellia serrata exudate or gum (known in India as “guggulu”) that forms an aromatic resin traditionally used as incense – and known as frankincense (especially when retrieved from Boswellia species found in Eritrea and Somalia but also from the Indian variety) – has been considered for thousands of years to possess therapeutic properties. It is used in Ayurvedic medicine, as well as in traditional medicine in China and the Middle East, particularly for its anti-inflammatory effects to treat chronic conditions.1-8 In fact, such essential oils have been used since 2800 BC to treat various inflammatory conditions, including skin sores and wounds, as well as in perfumes and incense.2,9 In the West, use of frankincense dates back to thousands of years as well, more often found in the form of incense for religious and cultural ceremonies.7 Over the past 2 decades, .3 This column focuses on some of the emerging data on this ancient botanical agent.
Chemical constituents
Terpenoids and essential oils are the primary components of frankincense and are known to impart anti-inflammatory and anticancer activity. The same is true for myrrh, which has been combined with frankincense in traditional Chinese medicine as a single medication for millennia, with the two acting synergistically and considered still to be a potent combination in conferring various biological benefits.7
In 2010, in a systematic review of the anti-inflammatory and anticancer activities of Boswellia species and their chemical ingredients, Efferth and Oesch found that frankincense blocks the production of leukotrienes, cyclooxygenase (COX) 1 and 2, as well as 5-lipoxygenase; and oxidative stress. It also contributes to regulation of immune cells from the innate and acquired immune systems and exerts anticancer activity by influencing signaling transduction responsible for cell cycle arrest, as well as inhibition of proliferation, angiogenesis, invasion, and metastasis. The investigators also reported on clinical trial results that have found efficacy of frankincense and its constituents in ameliorating symptoms of psoriasis and erythematous eczema, among other disorders.3
Anti-inflammatory activity
Li et al. completed a study in 2016 to identify the active ingredients responsible for the anti-inflammatory and analgesic effects of frankincense. They found that alpha-pinene, linalool, and 1-octanol were key contributors. These constituents were noted for suppressing COX-2 overexpression in mice, as well as nociceptive stimulus-induced inflammatory infiltrates.10
Noting the increasing popularity of frankincense essential oil in skin care, despite a paucity of data, in 2017, Han et al. evaluated the biological activities of the essential oil in pre-inflamed human dermal fibroblasts using 17 key protein biomarkers. Frankincense essential oil displayed significant antiproliferative activity and suppressed collagen III, interferon gamma-induced protein 10, and intracellular adhesion molecule 1. The investigators referred to the overall encouraging potential of frankincense essential oil to exert influence over inflammation and tissue remodeling in human skin and called for additional research into its mechanisms of action and active constituents.11
Anticancer activity
The main active ingredient in frankincense, boswellic acid, has been shown to promote apoptosis, suppress matrix metalloproteinase secretion, and hinder migration in metastatic melanoma cell lines in mice.6,12
In 2019, Hakkim et al. demonstrated that frankincense essential oil yielded substantial antimelanoma activity in vitro and in vivo and ameliorated hepatotoxicity caused by acetaminophen.13
There is one case report in the literature on the use of frankincense as a treatment for skin cancer. A 56-year-old man received frankincense oil multiple times a day for 4 months to treat a nodular basal cell carcinoma on one arm (which resolved) and an infiltrative BCC on the chest (some focal residual tumor remained).6,14 Topical frankincense or boswellic acid has been given a grade D recommendation for treating skin cancer, however, because of only one level-of-evidence-5 study.6
Antimicrobial activity
In 2012, de Rapper et al. collected samples of three essential oils of frankincense (Boswellia rivae, Boswellia neglecta, and Boswellia papyrifera) and two essential oil samples of myrrh and sweet myrrh from different regions of Ethiopia to study their anti-infective properties alone and in combination. The investigators observed synergistic and additive effects, particularly between B. papyrifera and Commiphora myrrha. While noting the long history of the combined use of frankincense and myrrh essential oils since 1500 BC, the investigators highlighted their study as the first antimicrobial work to verify the effectiveness of this combination, validating the use of this combination to thwart particular pathogens.15
Just 2 years ago, Ljaljević Grbić et al. evaluated the in vitro antimicrobial potential of the liquid and vapor phases of B. carteri and C. myrrha (frankincense and myrrh, respectively) essential oils, finding that frankincense demonstrated marked capacity to act as a natural antimicrobial agent.9
Transdermal delivery
In 2017, Zhu et al. showed that frankincense and myrrh essential oils promoted the permeability of the Chinese herb Chuanxiong and may facilitate drug elimination from the epidermis via dermal capillaries by dint of improved cutaneous blood flow, thereby augmenting transdermal drug delivery.16 The same team also showed that frankincense and myrrh essential oils, by fostering permeation by enhancing drug delivery across the stratum corneum, can also alter the structure of the stratum corneum.17
Conclusion
The use of frankincense in traditional medicine has a long and impressive track record. Recent research provides reason for optimism, and further investigating the possible incorporation of this botanical agent into modern dermatologic therapies appears warranted. Clearly, however, much more research is needed.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Kimmatkar N et al. Phytomedicine. 2003 Jan;10(1):3-7.
2. Ammon HP. Wien Med Wochenschr. 2002;152(15-16):373-8.
3. Efferth T & Oesch F. Semin Cancer Biol. 2020 Feb 4;S1044-579X(20)30034-1.
4. Banno N et al. J Ethnopharmacol. 2006 Sep 19;107(2):249-53.
5. Poeckel D & Werz O. Curr Med Chem. 2006;13(28):3359-69.
6. Li JY, Kampp JT. Dermatol Surg. 2019 Jan;45(1):58-67.
7. Cao B et al. Molecules. 2019 Aug 24;24(17): 3076.
8. Mertens M et al. Flavour Fragr J. 2009;24:279-300.
9. Ljaljević Grbić M et al. J Ethnopharmacol. 2018 Jun 12;219:1-14.
10. Li XJ et al. J Ethnopharmacol. 2016 Feb 17;179:22-6.
11. Han X et al. Biochim Open. 2017 Feb 3;4:31-5.
12. Zhao W et al. Cancer Detect Prev. 2003;27:67-75.
13. Hakkim FL et al. Oncotarget. 2019 May 28;10(37):3472-90.
14. Fung K et al. OA Altern Med 2013;1:14.
15. de Rapper S et al. Lett Appl Microbiol. 2012 Apr;54(4):352-8.
16. Zhu XF et al. Zhongguo Zhong Yao Za Zhi. 2017 Feb;42(4):680-5.
17. Guan YM et al. Zhongguo Zhong Yao Za Zhi. 2017 Sep;42(17):3350-5.
The Supreme Court 2020‒2021: What will affect ObGyns?
The Supreme Court’s usual processes were disrupted this term. The COVID-19 pandemic required audio hearings rather than in-person, and it resulted in a number of emergency legal appeals. As the Court began its regular sessions on October 5, 2020, there were only 8 justices—Justice Ruth Bader Ginsburg had passed away and Amy Coney Barrett had not yet been confirmed by the Senate. The Court decided many important cases this term, including dealing with the delivery of drugs to induce abortions, a Centers for Disease Control and Prevention (CDC) moratorium on housing evictions, yet another case on the Affordable Care Act, state laws concerning pharmacy benefit managers, and the Hologic and Minerva endometrial ablation systems patents. After considering these cases, we also will briefly look at other cases of general interest.
Abortion
Patient access to mifepristone
In May 2020, the American College of Obstetricians and Gynecologists (ACOG) was the named plaintiff in a lawsuit against the US Food and Drug Administration (FDA) regarding the drugs mifepristone and misoprostol that are used to induce medical abortions.1 The case was filed by the American Civil Liberties Union on behalf of ACOG and others2,3 and raised the issue of patients’ access to these medications. The basic claim of the case was that during the pandemic, the FDA’s regulation of mifepristone was unconstitutional in that they imposed an undue burden on the decision of women to have an abortion.4 (Although misoprostol is a part of the medical abortion regimen, it is not subject to special regulation and was not part of the litigation.)
The FDA regulation of mifepristone, begun in 2000 but modified since then, includes 3 elements to assure safe use:
- prescribers must have special training or certification
- the drug can be dispensed to patients only in a hospital, clinic, or medical office under the supervision of a certified health care provider (known as the “in-person dispensing requirement” because retail pharmacy or mail distribution are prohibited)
- the health care provider must review a “patient agreement form” with the patient and have the patient sign the consent form in the provider’s presence.5
The pandemic made fulfilling these requirements substantially more burdensome and difficult. The question was whether the FDA was constitutionally required to modify its regulations during a pandemic to take account of the undue burden of the regulation created by the pandemic. That is, the question was not whether the FDA could have or should have chosen to make the modification, but whether it was required to do so.
In July 2020, a federal district court in Maryland held that the FDA regulation was an unconstitutional burden on the abortion rights of women during the pandemic and issued a preliminary injunction to stop the FDA from enforcing the in-person dispensing and signature rules. The district judge applied the injunction to Maryland, but also made it a nationwide injunction. (The issue of district court nationwide injunctions is considered in, “District court ‘nationwide injunctions’”).
The FDA asked the Fourth Circuit Court of Appeals to stay the enforcement of the injunction, which the appeals court denied. The FDA then appealed to the Supreme Court, asking it to stay the injunction. In October 2020, the Court announced that it was holding the FDA’s request “in abeyance” to allow the district court to consider a motion by the FDA to dissolve or change the injunction. It gave the district court 40 days in which to act. That decision by the Court was in the “Shadow Docket” (see sidebar on page XX), so the exact vote of the Court in October is not clear, but 2 Justices (Alito and Thomas) dissented and would have stayed the injunction.6 Over the next 40 days, the district court did not withdraw its nationwide injunction.
Thus, on January 12, 2021, the case was again before the Supreme Court, which let the FDA’s regulations regarding mifepristone remain in place by lifting the district court’s injunction. Most of the justices supporting the stay did not write to explain their decision, although their dissent in the earlier cases may have served that purpose. (Maryland was permitting many kinds of activity that were more risky than visiting a clinic—indoor dining, with open hair salons, gyms, and casinos.)7 Chief Justice Roberts wrote a concurrence to indicate that, in his view, the issue was not whether the FDA’s regulations placed an undue burden on a right to an abortion generally, but that “My view is that courts owe significant deference” to the public health authorities (here meaning the FDA). Justices Sotomayor and Kagan dissented, saying that the issue was the undue burden on women, given the difficulties of the pandemic, particularly going to medical facilities during the COVID-19 pandemic.8
The injunction, sought by ACOG and others, was issued by the district court and was in effect for several months before it was dissolved by the Supreme Court. Following the change in presidential administrations, in April 2021 the FDA announced that it was going to “exercise enforcement discretion with respect to the in-person dispensing requirement…during the COVID-19 public health emergency.”9
Continue to: The Texas abortion case...
The Texas abortion case
The Court, on September 1, 2021, declined to block a Texas abortion statute from taking effect.10 This law precludes abortions after a fetal heartbeat is present at about 6 weeks of gestation. The Fifth Circuit declined to grant an injunction delaying implementation of the Texas law, and the Court did not reverse that decision.
Over the years, a variety of states have placed limitations on abortion, and those almost always have been enjoined by federal courts before they went into effect. However, the Texas statute, which undoubtedly is unconstitutional, was creatively constructed to avoid an early injunction.11 The statute does not allow state officials to enforce the new law, but rather it allows almost any private citizen to seek monetary damages from anyone performing an abortion or who “aids and abets” an abortion. Thus, it is difficult to tailor a lawsuit before this law is enforced. First, courts do not enjoin laws; they usually enjoin individuals from enforcing the law, and in this case it is difficult to know which individuals will be enforcing the laws and what their decisions might be. There also are some questions about the degree to which federal courts can enjoin state courts from deciding lawsuits under state law. For these procedural reasons, the majority of the Court found that those attacking the Texas law had not met their burden of showing that that they would win their case.
Even 3 of the dissenting justices said the defendants may be right that “existing doctrines preclude judicial intervention,” but that the consequences are such that the Court should delay the law until there is time for briefing and argument. The other 3 dissenting justices thought there would be ways of getting around the clever roadblock Texas had erected for the federal courts.
There has been some commentary that this case portends the abandonment of Roe v Wade and Casey,12 but that conclusion does not seem warranted by this case. The Court has accepted a Mississippi abortion law to be heard next term.13 In addition, the Texas statute is likely to be back in federal court once a private individual has filed a claim for money from an abortion provider (and likely even before that).
COVID-19 cases
The Supreme Court decided several cases related to COVID-19, including adjustments to election procedures, church services, and CDC eviction moratoria. As a general matter early in the pandemic, the Court deferred to government authorities, generally upholding government actions. Chief Justice Roberts emphasized the importance of the Court deferring to government officials in emergencies. As the pandemic progressed into 2021, however, the Court became less and less sympathetic to government actions that were not consistent, permitted by existing law, or reasonably necessary. For example, regulations of churches that were inconsistent with the regulation of similar organizations were struck down.14
Among the most interesting of the summer 2021 cases was the CDC eviction moratorium that essentially prohibited landlords nationwide from evicting tenants for nonpayment of rent. When the challenges to these CDC regulations first reached the Court, the moratorium was about to expire; in a 5-4 decision, the Court did not enjoin the CDC from continuing that policy. Justice Kavanaugh (the fifth vote) warned that “clear and specific congressional authorization…would be necessary to extend the moratorium past July 31.”15 Despite telling the Court that the moratorium would expire on July 31, just 3 days after the expiration and without any congressional authorization, the CDC reinstated what was practically the same moratorium.16 On August 26, the Court struck down the reinstated regulation, probably by a 6-3 margin. (Because this case arose in the “Shadow Docket,” the vote of some justices is not certain).17
Continue to: The Affordable Care Act...
The Affordable Care Act
The Affordable Care Act was challenged in the Court for the third time.18 In this term’s case, several states argued that when Congress essentially eliminated the penalty/tax for not purchasing insurance coverage, there was no longer a constitutional basis for the individual mandate. With that centerpiece gone, they claimed, the whole statute should be declared unconstitutional.
Along with many other specialty groups, ACOG joined an amicus curiae brief sponsored by the American Medical Association (AMA).19 An amicus brief is one not filed by the parties to the case, but by organizations or individuals who have information that may be of use to the Court in considering the case. Among other things, the filing of an amicus brief indicates the interest of the organization in the outcome of the case. In this case, the crux of the amicus was that even if the individual mandate currently is not constitutional, the Court should sever that provision and retain the rest of the ACA.
Despite some wild predictions about what the Court might do, it did not decide any substantive issue. Rather, it found that none of the parties to the case had “standing” to challenge the constitutionality of the ACA. Therefore, in effect, the Court dismissed the case without deciding the substantive legal issues.
Pharmacy Benefit Managers
The powerful Pharmacy Benefit Managers (PBMs) are a hidden part of the health care system; however, in recent years there has been increasing regulatory attention paid to them. Some states have begun regulating aspects of PBMs. In this term, the Court considered an Arkansas law that sought to protect local pharmacies from PBM pricing practices.20 The AMA filed an amicus brief in the case which made legal arguments, most of which had been made by the parties to the litigation.21
PBMs generally tell pharmacies how much they will reimburse the pharmacy for filling a prescription for a particular drug. In some instances, PBMs will set a reimbursement price that is lower than the wholesale price at which local pharmacies can purchase the drug. The Arkansas law prohibited PBMs in the state from reimbursing pharmacies for less than the wholesale cost the pharmacy paid for the drug.
The claim of the PBMs was that the Arkansas law violated the Employee Retirement Income Security Act (ERISA). In part, this act preempts state law that relates to fringe benefit plans. States have the authority to regulate insurance, but ERISA limits what they can do when the insurance relates to fringe benefits. The Court held that ERISA does not preempt the Arkansas law or similar state laws in other states. Because the state law was not preempted by the state law, the Arkansas regulation was upheld. The fact that this was a unanimous decision (8-0, because Justice Barrett was not on the Court when the case was heard) suggests that states may have leeway in additional regulations of PBMs, and it would not be surprising to see more of that state regulation in the future.
Continue to: Patent uncertainty...
Patent uncertainty
Csaba Truckai invented and patented the NovaSure System ablation device with a “moisture permeable” head. He sold his company and the related patents, which eventually were purchased by Hologic. Over time, Hologic added claims to the original patent. In the meantime, Truckai went on to invent another device, the Minerva Endometrial Ablation System (MEAS), which had a “moisture impermeable” head. (Note that the “Minerva Surgical, Inc.” involved in this case is not related to the company “Minerva Industries,” which some identified as a “patent troll.”)22
Hologic sued Minerva, claiming that Truckai’s second device (MEAS) infringed on its patent for the first device (NovaSure). Truckai’s defense was that the patent on NovaSure was invalid. Hologic felt that since Truckai had obtained that patent and then sold it, it was improper for him now to claim it was invalid. There is a doctrine for that: assignor estoppel—the person who sold (assigned) the patent is prevented from later claiming it was invalid. The question in this case was whether assignor estoppel is part of the patent law of the United States. It is not in the patent statutes, so it is a court-determined part of the law.
In a 5-4 decision this Term, the Court held that assignor estoppel is recognized, but that it is narrow.23 The Court identified several exceptions to assignor estoppel, notably for this case, including the situation in which the purchaser of the patent, after the purchase, returns to the Patent and Trademark Office to expand (amend) the patent’s claims. In that case, the seller could not be estopped by the amended terms of the patent. Minerva claimed that it was attacking the expanded patent that included changes made after it sold the patent. The Court, therefore, returned the case to the Federal Circuit to apply the principles it laid out about assignor estoppel.
Biotech and other fast-moving fields frequently have new technology building on slightly earlier technology. The current patent system often leaves uncertainty about who owns which part of a valid patent. This uncertainty is a drag on innovation, and the patent system is supposed to spur innovation. Assignor estoppel is likely to create additional complexity and uncertainty in some patents, which is regrettable.
Review of the Term
In addition to the other disruptions of the Term, during the first part of the Term, Amy Coney Barrett was not yet confirmed by the Senate, so there were only 8 justices until October 27. She did not participate in those cases that were heard before she joined the Court. The consensus is that the Court heard 67 cases: 57 were formally briefed and argued along with 8 summary reversals and 2 religious cases in the Shadow Docket. In my opinion, this undercounts both the number and the importance of the Shadow Docket cases, but the following data use the 67 case convention.24
The Court was unanimous in 43% of the cases, including some of the most divisive issues. That unanimity reflects very narrow decisions. There were (by conventional count) only eight 5-4 opinions (12%), an unusually low number. Justice Kavanaugh is viewed as the “median” justice. He was in the majority in 97% of all cases. Chief Justice Roberts and Justice Barrett were in the majority 91%, and Justice Gorsuch 90%. As for the other justices, they were in the majority (all cases) most of the time: Justice Alito, 83%; Justice Thomas, 81%; Justice Breyer, 76%; Justice Kagan, 75%; and Justice Sotomayor, 69%. In “divided cases” (when unanimous cases are removed), the percentages are: Justice Kavanaugh, 95%; Chief Justice Roberts and Justice Barrett, 84%; Justice Gorsuch, 82%; Justice Alito, 70%; Justice Thomas, 66%; Justice Breyer, 58%; Justice Kagan, 55%; and Justice Sotomayor, 45%.
When the term began, many Court watchers expected a relatively uninteresting term, dealing with many technical legal details. In fact, it turned out to be more interesting and important than expected, even with narrow holdings in important cases. Part of the secret of the term was that a lot of the real action was in the Shadow Docket. The end of the term is sometimes the moment when a justice announces a plan to retire. Many commentators expected Justice Breyer might announce—he has been under pressure to do so, to allow President Biden to nominate and a Democratic Senate to confirm a progressive justice. However, he did not do so. It is possible that he will announce his retirement to be effective when his successor is confirmed, but that is pure speculation.
Continue to: Next Term...
Next Term
The next term began on Monday, October 4, 2021. With the considerable current activity in the Shadow Docket, there was not much of a summer break. The coming term looks extraordinary. The headline case is an abortion case from Mississippi, Dobbs v Jackson Women’s Health Organization.25 The legal question is the constitutionality of Mississippi law that prohibits most abortions after 15 weeks of gestation. The Texas abortion law will also be back before the Court. As we saw this term, big cases may produce very narrow results, but this case has the potential for being a notable abortion decision.
In a different case the Court will decide whether a state attorney general can step in to defend an abortion law when the state health secretary does not do so.26
The Court also has accepted 3 cases dealing with reimbursement for health services. One deals with whether or not the Department of Health and Human Services can set reimbursement rates without good survey data regarding costs,27 another involves the calculation of additional payments for hospitals that serve a “disproportionate number of low-income patients,”28 and the third whether state Medicaid programs can take funds from an injured beneficiary’s tort recovery to cover future Medicaid costs.29
In other cases, the Court will review a gun control law from New York. The Court’s earlier Second Amendment cases involved guns in the home used for self-defense, but this case raises the question of whether a state can practically preclude “concealed-carry licenses.”30 Many experts believe the Court will accept a case dealing with racial preferences in college admissions, perhaps the Harvard case in which the claim is discrimination against Asian Americans.31
The ACOG mifepristone case was interesting, in part because the federal district court issued a nationwide injunction against the Americans with Disabilities Act, enforcing its rules anywhere in the country. The effect of these orders is for a single district judge to create the “law of the land,” at least until that is reviewed—which can take months. The advantage of the nationwide injunction is that it avoids having to repeatedly litigate the same issues in multiple courts around the country. The downside is that plaintiffs can seek out a nonrepresentative judge or circuit and receive an injunction that would be granted by few other circuits. In addition, a nationwide injunction can apply to specific circumstances that are not before the court issuing the injunction. In the mifepristone case, for example, 10 states requested to intervene in the ACOG case. The court rejected the request, but the nationwide injunction applied to those states.1
Although federal judges have had the authority to issue nationwide injunctions for years, they are becoming much more common. One reason is the ease of forum shopping noted earlier—organizations can cherry-pick district courts and circuits sympathetic to their views. Both left- and right-leaning organizations have learned this lesson, so left-leaning groups are likely to file in specific districts in the Ninth Circuit, and right-leaning groups to districts in the Fifth Circuit.
If the current trend of increasing nationwide injunctions continues, either the rules for the federal courts or congressional action may be required to reduce some of the abuses by both sides of the political spectrum.
The ACOG mifepristone case was interesting, in part because the federal district court issued a nationwide injunction against the Americans with Disabilities Act, enforcing its rules anywhere in the country. The effect of these orders is for a single district judge to create the “law of the land,” at least until that is reviewed—which can take months. The advantage of the nationwide injunction is that it avoids having to repeatedly litigate the same issues in multiple courts around the country. The downside is that plaintiffs can seek out a nonrepresentative judge or circuit and receive an injunction that would be granted by few other circuits. In addition, a nationwide injunction can apply to specific circumstances that are not before the court issuing the injunction. In the mifepristone case, for example, 10 states requested to intervene in the ACOG case. The court rejected the request, but the nationwide injunction applied to those states.1
Although federal judges have had the authority to issue nationwide injunctions for years, they are becoming much more common. One reason is the ease of forum shopping noted earlier—organizations can cherry-pick district courts and circuits sympathetic to their views. Both left- and right-leaning organizations have learned this lesson, so left-leaning groups are likely to file in specific districts in the Ninth Circuit, and right-leaning groups to districts in the Fifth Circuit.
If the current trend of increasing nationwide injunctions continues, either the rules for the federal courts or congressional action may be required to reduce some of the abuses by both sides of the political spectrum. Reference Am. Coll. of Obstetricians & Gynecologists v. United States FDA, 467 F. Supp. 3d 282, 284 (D. Md. 2020).
Reference
1. Am. Coll. of Obstetricians & Gynecologists v. United States FDA, 467 F. Supp. 3d 282, 284 (D. Md. 2020).
The “Shadow Docket”
The ACOG mifepristone decisions do not appear on the Supreme Court’s “Court Opinions” website.1 They appear in what has become known in recent years as “The Shadow Docket,” an informal term that includes many orders of the Court and statements of individual justices regarding some cases.2 There are hundreds of orders by the Court each Term, there is nothing particularly shadowy about any of these items—they are all publicly available on the Court’s website and later in paper format. It is, however, a little harder to find and much harder to sort through than the major opinions. In some cases, it is not possible to tell what the vote was, how each justice voted, and what the reasoning of the Court was. In a few cases it is difficult to know exactly what the Court was holding or otherwise leaves some confusion about what the law actually is.3
The part of the Shadow Docket that is most intriguing for commentators, and where the ACOG cases appear, is the “Opinions Relating to Orders.”4 These are a variety of opinions, some written by the Court and many by individual justices. It also includes the action of the Court in some cases in which there was not full briefing or oral argument. The statements by justices often are to dissent from the denial of cert of decisions of the Court. These opinions have become much more common over the years. In this past term, there were approximately 60 such opinions related to about 50 cases. In part, this relates to the number of pandemic cases that could not wait for a Court decision going through the extended ordinary process. Although the Shadow Docket has been of interest to academic observers and Court watchers for years, this year it has attracted the attention of Congress.5
References
1. Opinions of the Court. Supreme Court website. https://www.supremecourt.gov/opinions/slipopinion/20#list. Accessed October 10, 2021.
2. Baude W. Foreword: the Supreme Court’s Shadow Docket, 9 N.Y.U. J.L. & Liberty 1 (2015).
3. Vladeck SI. The Solicitor General and the Shadow Docket, 133 Harvard Law Review. 123 (2019).
4. Opinions relating to orders. Supreme Court website. https://www.supremecourt.gov/opinions/relatingtoorders/20#list. Accessed October 10, 2021.
5. The Supreme Court’s Shadow Docket: Hearing Before the Subcommittee on Courts, Intellectual Property and the Internet of the H. Committee on the Judiciary, 117th Congress (2021).
- American College of Obstetricians & Gynecologists v. United States FDA, 472 F. Supp. 3d 183 (D. Md. 2020).
- Michael Kunzelman, Doctors Sue to Block FDA Abortion Pill Rule During Pandemic, (May 29, 2020).
- ACLU, American College Of Obstetricians And Gynecologists V. U.S. Food And Drug Administration, https://www.aclu.org/cases/american-college-obstetricians-and-gynecologists-v-us-food-and-drug-administration. Updated February 12, 2021. Accessed August 27, 2021.
- Whole Woman’s Health v Hellerstedt, 579 US ___ (2016), 136 S Ct 2292.
- 2016 Clinical Review at 39, 47, 49, Opp’n Mot. PI Ex. 19, ECF No. 62-11.
- American College of Obstetricians and Gynecologists v FDA (I), decided October 8, 2020.
- October 8, 2020, dissenting opinion by Justice Alito.
- January 12, 2021, dissenting opinion by Justice Sotomayor.
- Questions and answers on Mifeprex. U.S. Food and Drug Administration website. Published April 13, 2021. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/questions-and-answers-mifeprex. Accessed October 9, 2021.
- Whole Woman’s Health v Jackson, decided September 1, 2021.
- Texas Senate Bill 8, relating to abortion, including abortions after detection of unborn child’s heartbeat; authorizing a private civil right of action. LegiScan website. https://legiscan.com/TX/text/SB8/id/2395961. Accessed October 9, 2021.
- Planned Parenthood of Southeastern Pennsylvania v Casey, 505 U. S. 833 (1992); Roe v Wade, 410 U. S. 113 (1973).
- Dobbs v Jackson Women’s Health Organization, No. 19-1392.
- Roman Catholic Diocese of Brooklyn v Cuomo, decided November 25, 2020.
- Alabama Association of Realtors v Department of Health and Human Services, decided June 29, 2021.
- Temporary halt in residential evictions in communities with substantial or high levels of community transmission of COVID-19 to prevent the further spread of COVID-19. August 6, 2021. https://www.federalregister.gov/documents/2021/08/06/2021-16945/temporary-halt-in-residential-evictions-in-communities-with-substantial-or-high-transmission-of.
- Alabama Association of Realtors v Department of Health and Human Services, decided August 26, 2021.
- California v Texas, decided June 17, 2021.
- Brief of Amici Curiae American Medical Association, American Academy of Allergy, Asthma and Immunology, Aerospace Medical Association, American Academy of Family Physicians, American Academy of Pediatrics, American College of Cardiology, American College of Emergency Physicians, American College of Medical Genetics and Genomics, American College of Obstetricians and Gynecologists, American College of Physicians, American College of Radiation Oncology, American College of Radiology, American Psychiatric Association, American Society of Gastrointestinal Endoscopy, American Society of Hematology, American Society of Metabolic and Bariatric Surgery, Endocrine Society, GLMA: Health Professionals Advancing LGBTQ Equality, Renal Physicians Association, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology in Support of Petitioners, in California v. Texas. May 13, 2020. https://www.supremecourt.gov/DocketPDF/19/19-840/143469/20200513150051995_19-840%20Amici%20Brief%20AMA.pdf. Accessed October 9, 2021.
- Rutledge v Pharmaceutical Care Management Association, decided December 10, 2020.
- Brief of the American Medical Association, The Arkansas Medical Society, and The Litigation Center of the American Medical Association and the State Medical Societies as Amici Curiae in Support of Petitioner in Rutledge v Pharmaceutical Care Management Association. March 2, 2020. https://www.supremecourt.gov/DocketPDF/18/18-540/134670/20200302163622018_Rutledge%20v.%20PCMA%20Amicus%20Brief%20of%20AMA%20et%20al.pdf. Accessed October 9, 2021.
- Apple quietly settles patent lawsuit, promptly gets hit with another one. TechCrunch website. Published July 30, 2010. https://techcrunch.com/2010/07/30/apple-minerva-emblaze/. Accessed October 9, 2021.
- Minerva Surgical, Inc. v Hologic, Inc., decided June 29, 2021.
- Stat pack. SCOTUS Blog website. Published July 6, 2021. https://www.scotusblog.com/wp-content/uploads/2021/07/Final-Stat-Pack-7.6.21.pdf. Accessed October 9, 2021.
- Dobbs v Jackson Women’s Health Organization, No. 19-1392.
- Cameron v. EMW Women’s Surgical Center, https://www.scotusblog.com/case-files/cases/cameron-v-emw-womens-surgical-center-p-s-c/. Accessed August 28, 2021.
- American Hospital Association v Becerra, No. 20-1114.
- Becerra v Empire Health Foundation, No. 20-1312.
- Gallardo v Marstiller, No. 20-1263.
- New York State Rifle & Pistol Association Inc. v Corlett, No. 20-843.
- Students for Fair Admissions v President & Fellows of Harvard College, No. 20-1199.
The Supreme Court’s usual processes were disrupted this term. The COVID-19 pandemic required audio hearings rather than in-person, and it resulted in a number of emergency legal appeals. As the Court began its regular sessions on October 5, 2020, there were only 8 justices—Justice Ruth Bader Ginsburg had passed away and Amy Coney Barrett had not yet been confirmed by the Senate. The Court decided many important cases this term, including dealing with the delivery of drugs to induce abortions, a Centers for Disease Control and Prevention (CDC) moratorium on housing evictions, yet another case on the Affordable Care Act, state laws concerning pharmacy benefit managers, and the Hologic and Minerva endometrial ablation systems patents. After considering these cases, we also will briefly look at other cases of general interest.
Abortion
Patient access to mifepristone
In May 2020, the American College of Obstetricians and Gynecologists (ACOG) was the named plaintiff in a lawsuit against the US Food and Drug Administration (FDA) regarding the drugs mifepristone and misoprostol that are used to induce medical abortions.1 The case was filed by the American Civil Liberties Union on behalf of ACOG and others2,3 and raised the issue of patients’ access to these medications. The basic claim of the case was that during the pandemic, the FDA’s regulation of mifepristone was unconstitutional in that they imposed an undue burden on the decision of women to have an abortion.4 (Although misoprostol is a part of the medical abortion regimen, it is not subject to special regulation and was not part of the litigation.)
The FDA regulation of mifepristone, begun in 2000 but modified since then, includes 3 elements to assure safe use:
- prescribers must have special training or certification
- the drug can be dispensed to patients only in a hospital, clinic, or medical office under the supervision of a certified health care provider (known as the “in-person dispensing requirement” because retail pharmacy or mail distribution are prohibited)
- the health care provider must review a “patient agreement form” with the patient and have the patient sign the consent form in the provider’s presence.5
The pandemic made fulfilling these requirements substantially more burdensome and difficult. The question was whether the FDA was constitutionally required to modify its regulations during a pandemic to take account of the undue burden of the regulation created by the pandemic. That is, the question was not whether the FDA could have or should have chosen to make the modification, but whether it was required to do so.
In July 2020, a federal district court in Maryland held that the FDA regulation was an unconstitutional burden on the abortion rights of women during the pandemic and issued a preliminary injunction to stop the FDA from enforcing the in-person dispensing and signature rules. The district judge applied the injunction to Maryland, but also made it a nationwide injunction. (The issue of district court nationwide injunctions is considered in, “District court ‘nationwide injunctions’”).
The FDA asked the Fourth Circuit Court of Appeals to stay the enforcement of the injunction, which the appeals court denied. The FDA then appealed to the Supreme Court, asking it to stay the injunction. In October 2020, the Court announced that it was holding the FDA’s request “in abeyance” to allow the district court to consider a motion by the FDA to dissolve or change the injunction. It gave the district court 40 days in which to act. That decision by the Court was in the “Shadow Docket” (see sidebar on page XX), so the exact vote of the Court in October is not clear, but 2 Justices (Alito and Thomas) dissented and would have stayed the injunction.6 Over the next 40 days, the district court did not withdraw its nationwide injunction.
Thus, on January 12, 2021, the case was again before the Supreme Court, which let the FDA’s regulations regarding mifepristone remain in place by lifting the district court’s injunction. Most of the justices supporting the stay did not write to explain their decision, although their dissent in the earlier cases may have served that purpose. (Maryland was permitting many kinds of activity that were more risky than visiting a clinic—indoor dining, with open hair salons, gyms, and casinos.)7 Chief Justice Roberts wrote a concurrence to indicate that, in his view, the issue was not whether the FDA’s regulations placed an undue burden on a right to an abortion generally, but that “My view is that courts owe significant deference” to the public health authorities (here meaning the FDA). Justices Sotomayor and Kagan dissented, saying that the issue was the undue burden on women, given the difficulties of the pandemic, particularly going to medical facilities during the COVID-19 pandemic.8
The injunction, sought by ACOG and others, was issued by the district court and was in effect for several months before it was dissolved by the Supreme Court. Following the change in presidential administrations, in April 2021 the FDA announced that it was going to “exercise enforcement discretion with respect to the in-person dispensing requirement…during the COVID-19 public health emergency.”9
Continue to: The Texas abortion case...
The Texas abortion case
The Court, on September 1, 2021, declined to block a Texas abortion statute from taking effect.10 This law precludes abortions after a fetal heartbeat is present at about 6 weeks of gestation. The Fifth Circuit declined to grant an injunction delaying implementation of the Texas law, and the Court did not reverse that decision.
Over the years, a variety of states have placed limitations on abortion, and those almost always have been enjoined by federal courts before they went into effect. However, the Texas statute, which undoubtedly is unconstitutional, was creatively constructed to avoid an early injunction.11 The statute does not allow state officials to enforce the new law, but rather it allows almost any private citizen to seek monetary damages from anyone performing an abortion or who “aids and abets” an abortion. Thus, it is difficult to tailor a lawsuit before this law is enforced. First, courts do not enjoin laws; they usually enjoin individuals from enforcing the law, and in this case it is difficult to know which individuals will be enforcing the laws and what their decisions might be. There also are some questions about the degree to which federal courts can enjoin state courts from deciding lawsuits under state law. For these procedural reasons, the majority of the Court found that those attacking the Texas law had not met their burden of showing that that they would win their case.
Even 3 of the dissenting justices said the defendants may be right that “existing doctrines preclude judicial intervention,” but that the consequences are such that the Court should delay the law until there is time for briefing and argument. The other 3 dissenting justices thought there would be ways of getting around the clever roadblock Texas had erected for the federal courts.
There has been some commentary that this case portends the abandonment of Roe v Wade and Casey,12 but that conclusion does not seem warranted by this case. The Court has accepted a Mississippi abortion law to be heard next term.13 In addition, the Texas statute is likely to be back in federal court once a private individual has filed a claim for money from an abortion provider (and likely even before that).
COVID-19 cases
The Supreme Court decided several cases related to COVID-19, including adjustments to election procedures, church services, and CDC eviction moratoria. As a general matter early in the pandemic, the Court deferred to government authorities, generally upholding government actions. Chief Justice Roberts emphasized the importance of the Court deferring to government officials in emergencies. As the pandemic progressed into 2021, however, the Court became less and less sympathetic to government actions that were not consistent, permitted by existing law, or reasonably necessary. For example, regulations of churches that were inconsistent with the regulation of similar organizations were struck down.14
Among the most interesting of the summer 2021 cases was the CDC eviction moratorium that essentially prohibited landlords nationwide from evicting tenants for nonpayment of rent. When the challenges to these CDC regulations first reached the Court, the moratorium was about to expire; in a 5-4 decision, the Court did not enjoin the CDC from continuing that policy. Justice Kavanaugh (the fifth vote) warned that “clear and specific congressional authorization…would be necessary to extend the moratorium past July 31.”15 Despite telling the Court that the moratorium would expire on July 31, just 3 days after the expiration and without any congressional authorization, the CDC reinstated what was practically the same moratorium.16 On August 26, the Court struck down the reinstated regulation, probably by a 6-3 margin. (Because this case arose in the “Shadow Docket,” the vote of some justices is not certain).17
Continue to: The Affordable Care Act...
The Affordable Care Act
The Affordable Care Act was challenged in the Court for the third time.18 In this term’s case, several states argued that when Congress essentially eliminated the penalty/tax for not purchasing insurance coverage, there was no longer a constitutional basis for the individual mandate. With that centerpiece gone, they claimed, the whole statute should be declared unconstitutional.
Along with many other specialty groups, ACOG joined an amicus curiae brief sponsored by the American Medical Association (AMA).19 An amicus brief is one not filed by the parties to the case, but by organizations or individuals who have information that may be of use to the Court in considering the case. Among other things, the filing of an amicus brief indicates the interest of the organization in the outcome of the case. In this case, the crux of the amicus was that even if the individual mandate currently is not constitutional, the Court should sever that provision and retain the rest of the ACA.
Despite some wild predictions about what the Court might do, it did not decide any substantive issue. Rather, it found that none of the parties to the case had “standing” to challenge the constitutionality of the ACA. Therefore, in effect, the Court dismissed the case without deciding the substantive legal issues.
Pharmacy Benefit Managers
The powerful Pharmacy Benefit Managers (PBMs) are a hidden part of the health care system; however, in recent years there has been increasing regulatory attention paid to them. Some states have begun regulating aspects of PBMs. In this term, the Court considered an Arkansas law that sought to protect local pharmacies from PBM pricing practices.20 The AMA filed an amicus brief in the case which made legal arguments, most of which had been made by the parties to the litigation.21
PBMs generally tell pharmacies how much they will reimburse the pharmacy for filling a prescription for a particular drug. In some instances, PBMs will set a reimbursement price that is lower than the wholesale price at which local pharmacies can purchase the drug. The Arkansas law prohibited PBMs in the state from reimbursing pharmacies for less than the wholesale cost the pharmacy paid for the drug.
The claim of the PBMs was that the Arkansas law violated the Employee Retirement Income Security Act (ERISA). In part, this act preempts state law that relates to fringe benefit plans. States have the authority to regulate insurance, but ERISA limits what they can do when the insurance relates to fringe benefits. The Court held that ERISA does not preempt the Arkansas law or similar state laws in other states. Because the state law was not preempted by the state law, the Arkansas regulation was upheld. The fact that this was a unanimous decision (8-0, because Justice Barrett was not on the Court when the case was heard) suggests that states may have leeway in additional regulations of PBMs, and it would not be surprising to see more of that state regulation in the future.
Continue to: Patent uncertainty...
Patent uncertainty
Csaba Truckai invented and patented the NovaSure System ablation device with a “moisture permeable” head. He sold his company and the related patents, which eventually were purchased by Hologic. Over time, Hologic added claims to the original patent. In the meantime, Truckai went on to invent another device, the Minerva Endometrial Ablation System (MEAS), which had a “moisture impermeable” head. (Note that the “Minerva Surgical, Inc.” involved in this case is not related to the company “Minerva Industries,” which some identified as a “patent troll.”)22
Hologic sued Minerva, claiming that Truckai’s second device (MEAS) infringed on its patent for the first device (NovaSure). Truckai’s defense was that the patent on NovaSure was invalid. Hologic felt that since Truckai had obtained that patent and then sold it, it was improper for him now to claim it was invalid. There is a doctrine for that: assignor estoppel—the person who sold (assigned) the patent is prevented from later claiming it was invalid. The question in this case was whether assignor estoppel is part of the patent law of the United States. It is not in the patent statutes, so it is a court-determined part of the law.
In a 5-4 decision this Term, the Court held that assignor estoppel is recognized, but that it is narrow.23 The Court identified several exceptions to assignor estoppel, notably for this case, including the situation in which the purchaser of the patent, after the purchase, returns to the Patent and Trademark Office to expand (amend) the patent’s claims. In that case, the seller could not be estopped by the amended terms of the patent. Minerva claimed that it was attacking the expanded patent that included changes made after it sold the patent. The Court, therefore, returned the case to the Federal Circuit to apply the principles it laid out about assignor estoppel.
Biotech and other fast-moving fields frequently have new technology building on slightly earlier technology. The current patent system often leaves uncertainty about who owns which part of a valid patent. This uncertainty is a drag on innovation, and the patent system is supposed to spur innovation. Assignor estoppel is likely to create additional complexity and uncertainty in some patents, which is regrettable.
Review of the Term
In addition to the other disruptions of the Term, during the first part of the Term, Amy Coney Barrett was not yet confirmed by the Senate, so there were only 8 justices until October 27. She did not participate in those cases that were heard before she joined the Court. The consensus is that the Court heard 67 cases: 57 were formally briefed and argued along with 8 summary reversals and 2 religious cases in the Shadow Docket. In my opinion, this undercounts both the number and the importance of the Shadow Docket cases, but the following data use the 67 case convention.24
The Court was unanimous in 43% of the cases, including some of the most divisive issues. That unanimity reflects very narrow decisions. There were (by conventional count) only eight 5-4 opinions (12%), an unusually low number. Justice Kavanaugh is viewed as the “median” justice. He was in the majority in 97% of all cases. Chief Justice Roberts and Justice Barrett were in the majority 91%, and Justice Gorsuch 90%. As for the other justices, they were in the majority (all cases) most of the time: Justice Alito, 83%; Justice Thomas, 81%; Justice Breyer, 76%; Justice Kagan, 75%; and Justice Sotomayor, 69%. In “divided cases” (when unanimous cases are removed), the percentages are: Justice Kavanaugh, 95%; Chief Justice Roberts and Justice Barrett, 84%; Justice Gorsuch, 82%; Justice Alito, 70%; Justice Thomas, 66%; Justice Breyer, 58%; Justice Kagan, 55%; and Justice Sotomayor, 45%.
When the term began, many Court watchers expected a relatively uninteresting term, dealing with many technical legal details. In fact, it turned out to be more interesting and important than expected, even with narrow holdings in important cases. Part of the secret of the term was that a lot of the real action was in the Shadow Docket. The end of the term is sometimes the moment when a justice announces a plan to retire. Many commentators expected Justice Breyer might announce—he has been under pressure to do so, to allow President Biden to nominate and a Democratic Senate to confirm a progressive justice. However, he did not do so. It is possible that he will announce his retirement to be effective when his successor is confirmed, but that is pure speculation.
Continue to: Next Term...
Next Term
The next term began on Monday, October 4, 2021. With the considerable current activity in the Shadow Docket, there was not much of a summer break. The coming term looks extraordinary. The headline case is an abortion case from Mississippi, Dobbs v Jackson Women’s Health Organization.25 The legal question is the constitutionality of Mississippi law that prohibits most abortions after 15 weeks of gestation. The Texas abortion law will also be back before the Court. As we saw this term, big cases may produce very narrow results, but this case has the potential for being a notable abortion decision.
In a different case the Court will decide whether a state attorney general can step in to defend an abortion law when the state health secretary does not do so.26
The Court also has accepted 3 cases dealing with reimbursement for health services. One deals with whether or not the Department of Health and Human Services can set reimbursement rates without good survey data regarding costs,27 another involves the calculation of additional payments for hospitals that serve a “disproportionate number of low-income patients,”28 and the third whether state Medicaid programs can take funds from an injured beneficiary’s tort recovery to cover future Medicaid costs.29
In other cases, the Court will review a gun control law from New York. The Court’s earlier Second Amendment cases involved guns in the home used for self-defense, but this case raises the question of whether a state can practically preclude “concealed-carry licenses.”30 Many experts believe the Court will accept a case dealing with racial preferences in college admissions, perhaps the Harvard case in which the claim is discrimination against Asian Americans.31
The ACOG mifepristone case was interesting, in part because the federal district court issued a nationwide injunction against the Americans with Disabilities Act, enforcing its rules anywhere in the country. The effect of these orders is for a single district judge to create the “law of the land,” at least until that is reviewed—which can take months. The advantage of the nationwide injunction is that it avoids having to repeatedly litigate the same issues in multiple courts around the country. The downside is that plaintiffs can seek out a nonrepresentative judge or circuit and receive an injunction that would be granted by few other circuits. In addition, a nationwide injunction can apply to specific circumstances that are not before the court issuing the injunction. In the mifepristone case, for example, 10 states requested to intervene in the ACOG case. The court rejected the request, but the nationwide injunction applied to those states.1
Although federal judges have had the authority to issue nationwide injunctions for years, they are becoming much more common. One reason is the ease of forum shopping noted earlier—organizations can cherry-pick district courts and circuits sympathetic to their views. Both left- and right-leaning organizations have learned this lesson, so left-leaning groups are likely to file in specific districts in the Ninth Circuit, and right-leaning groups to districts in the Fifth Circuit.
If the current trend of increasing nationwide injunctions continues, either the rules for the federal courts or congressional action may be required to reduce some of the abuses by both sides of the political spectrum.
The ACOG mifepristone case was interesting, in part because the federal district court issued a nationwide injunction against the Americans with Disabilities Act, enforcing its rules anywhere in the country. The effect of these orders is for a single district judge to create the “law of the land,” at least until that is reviewed—which can take months. The advantage of the nationwide injunction is that it avoids having to repeatedly litigate the same issues in multiple courts around the country. The downside is that plaintiffs can seek out a nonrepresentative judge or circuit and receive an injunction that would be granted by few other circuits. In addition, a nationwide injunction can apply to specific circumstances that are not before the court issuing the injunction. In the mifepristone case, for example, 10 states requested to intervene in the ACOG case. The court rejected the request, but the nationwide injunction applied to those states.1
Although federal judges have had the authority to issue nationwide injunctions for years, they are becoming much more common. One reason is the ease of forum shopping noted earlier—organizations can cherry-pick district courts and circuits sympathetic to their views. Both left- and right-leaning organizations have learned this lesson, so left-leaning groups are likely to file in specific districts in the Ninth Circuit, and right-leaning groups to districts in the Fifth Circuit.
If the current trend of increasing nationwide injunctions continues, either the rules for the federal courts or congressional action may be required to reduce some of the abuses by both sides of the political spectrum. Reference Am. Coll. of Obstetricians & Gynecologists v. United States FDA, 467 F. Supp. 3d 282, 284 (D. Md. 2020).
Reference
1. Am. Coll. of Obstetricians & Gynecologists v. United States FDA, 467 F. Supp. 3d 282, 284 (D. Md. 2020).
The “Shadow Docket”
The ACOG mifepristone decisions do not appear on the Supreme Court’s “Court Opinions” website.1 They appear in what has become known in recent years as “The Shadow Docket,” an informal term that includes many orders of the Court and statements of individual justices regarding some cases.2 There are hundreds of orders by the Court each Term, there is nothing particularly shadowy about any of these items—they are all publicly available on the Court’s website and later in paper format. It is, however, a little harder to find and much harder to sort through than the major opinions. In some cases, it is not possible to tell what the vote was, how each justice voted, and what the reasoning of the Court was. In a few cases it is difficult to know exactly what the Court was holding or otherwise leaves some confusion about what the law actually is.3
The part of the Shadow Docket that is most intriguing for commentators, and where the ACOG cases appear, is the “Opinions Relating to Orders.”4 These are a variety of opinions, some written by the Court and many by individual justices. It also includes the action of the Court in some cases in which there was not full briefing or oral argument. The statements by justices often are to dissent from the denial of cert of decisions of the Court. These opinions have become much more common over the years. In this past term, there were approximately 60 such opinions related to about 50 cases. In part, this relates to the number of pandemic cases that could not wait for a Court decision going through the extended ordinary process. Although the Shadow Docket has been of interest to academic observers and Court watchers for years, this year it has attracted the attention of Congress.5
References
1. Opinions of the Court. Supreme Court website. https://www.supremecourt.gov/opinions/slipopinion/20#list. Accessed October 10, 2021.
2. Baude W. Foreword: the Supreme Court’s Shadow Docket, 9 N.Y.U. J.L. & Liberty 1 (2015).
3. Vladeck SI. The Solicitor General and the Shadow Docket, 133 Harvard Law Review. 123 (2019).
4. Opinions relating to orders. Supreme Court website. https://www.supremecourt.gov/opinions/relatingtoorders/20#list. Accessed October 10, 2021.
5. The Supreme Court’s Shadow Docket: Hearing Before the Subcommittee on Courts, Intellectual Property and the Internet of the H. Committee on the Judiciary, 117th Congress (2021).
The Supreme Court’s usual processes were disrupted this term. The COVID-19 pandemic required audio hearings rather than in-person, and it resulted in a number of emergency legal appeals. As the Court began its regular sessions on October 5, 2020, there were only 8 justices—Justice Ruth Bader Ginsburg had passed away and Amy Coney Barrett had not yet been confirmed by the Senate. The Court decided many important cases this term, including dealing with the delivery of drugs to induce abortions, a Centers for Disease Control and Prevention (CDC) moratorium on housing evictions, yet another case on the Affordable Care Act, state laws concerning pharmacy benefit managers, and the Hologic and Minerva endometrial ablation systems patents. After considering these cases, we also will briefly look at other cases of general interest.
Abortion
Patient access to mifepristone
In May 2020, the American College of Obstetricians and Gynecologists (ACOG) was the named plaintiff in a lawsuit against the US Food and Drug Administration (FDA) regarding the drugs mifepristone and misoprostol that are used to induce medical abortions.1 The case was filed by the American Civil Liberties Union on behalf of ACOG and others2,3 and raised the issue of patients’ access to these medications. The basic claim of the case was that during the pandemic, the FDA’s regulation of mifepristone was unconstitutional in that they imposed an undue burden on the decision of women to have an abortion.4 (Although misoprostol is a part of the medical abortion regimen, it is not subject to special regulation and was not part of the litigation.)
The FDA regulation of mifepristone, begun in 2000 but modified since then, includes 3 elements to assure safe use:
- prescribers must have special training or certification
- the drug can be dispensed to patients only in a hospital, clinic, or medical office under the supervision of a certified health care provider (known as the “in-person dispensing requirement” because retail pharmacy or mail distribution are prohibited)
- the health care provider must review a “patient agreement form” with the patient and have the patient sign the consent form in the provider’s presence.5
The pandemic made fulfilling these requirements substantially more burdensome and difficult. The question was whether the FDA was constitutionally required to modify its regulations during a pandemic to take account of the undue burden of the regulation created by the pandemic. That is, the question was not whether the FDA could have or should have chosen to make the modification, but whether it was required to do so.
In July 2020, a federal district court in Maryland held that the FDA regulation was an unconstitutional burden on the abortion rights of women during the pandemic and issued a preliminary injunction to stop the FDA from enforcing the in-person dispensing and signature rules. The district judge applied the injunction to Maryland, but also made it a nationwide injunction. (The issue of district court nationwide injunctions is considered in, “District court ‘nationwide injunctions’”).
The FDA asked the Fourth Circuit Court of Appeals to stay the enforcement of the injunction, which the appeals court denied. The FDA then appealed to the Supreme Court, asking it to stay the injunction. In October 2020, the Court announced that it was holding the FDA’s request “in abeyance” to allow the district court to consider a motion by the FDA to dissolve or change the injunction. It gave the district court 40 days in which to act. That decision by the Court was in the “Shadow Docket” (see sidebar on page XX), so the exact vote of the Court in October is not clear, but 2 Justices (Alito and Thomas) dissented and would have stayed the injunction.6 Over the next 40 days, the district court did not withdraw its nationwide injunction.
Thus, on January 12, 2021, the case was again before the Supreme Court, which let the FDA’s regulations regarding mifepristone remain in place by lifting the district court’s injunction. Most of the justices supporting the stay did not write to explain their decision, although their dissent in the earlier cases may have served that purpose. (Maryland was permitting many kinds of activity that were more risky than visiting a clinic—indoor dining, with open hair salons, gyms, and casinos.)7 Chief Justice Roberts wrote a concurrence to indicate that, in his view, the issue was not whether the FDA’s regulations placed an undue burden on a right to an abortion generally, but that “My view is that courts owe significant deference” to the public health authorities (here meaning the FDA). Justices Sotomayor and Kagan dissented, saying that the issue was the undue burden on women, given the difficulties of the pandemic, particularly going to medical facilities during the COVID-19 pandemic.8
The injunction, sought by ACOG and others, was issued by the district court and was in effect for several months before it was dissolved by the Supreme Court. Following the change in presidential administrations, in April 2021 the FDA announced that it was going to “exercise enforcement discretion with respect to the in-person dispensing requirement…during the COVID-19 public health emergency.”9
Continue to: The Texas abortion case...
The Texas abortion case
The Court, on September 1, 2021, declined to block a Texas abortion statute from taking effect.10 This law precludes abortions after a fetal heartbeat is present at about 6 weeks of gestation. The Fifth Circuit declined to grant an injunction delaying implementation of the Texas law, and the Court did not reverse that decision.
Over the years, a variety of states have placed limitations on abortion, and those almost always have been enjoined by federal courts before they went into effect. However, the Texas statute, which undoubtedly is unconstitutional, was creatively constructed to avoid an early injunction.11 The statute does not allow state officials to enforce the new law, but rather it allows almost any private citizen to seek monetary damages from anyone performing an abortion or who “aids and abets” an abortion. Thus, it is difficult to tailor a lawsuit before this law is enforced. First, courts do not enjoin laws; they usually enjoin individuals from enforcing the law, and in this case it is difficult to know which individuals will be enforcing the laws and what their decisions might be. There also are some questions about the degree to which federal courts can enjoin state courts from deciding lawsuits under state law. For these procedural reasons, the majority of the Court found that those attacking the Texas law had not met their burden of showing that that they would win their case.
Even 3 of the dissenting justices said the defendants may be right that “existing doctrines preclude judicial intervention,” but that the consequences are such that the Court should delay the law until there is time for briefing and argument. The other 3 dissenting justices thought there would be ways of getting around the clever roadblock Texas had erected for the federal courts.
There has been some commentary that this case portends the abandonment of Roe v Wade and Casey,12 but that conclusion does not seem warranted by this case. The Court has accepted a Mississippi abortion law to be heard next term.13 In addition, the Texas statute is likely to be back in federal court once a private individual has filed a claim for money from an abortion provider (and likely even before that).
COVID-19 cases
The Supreme Court decided several cases related to COVID-19, including adjustments to election procedures, church services, and CDC eviction moratoria. As a general matter early in the pandemic, the Court deferred to government authorities, generally upholding government actions. Chief Justice Roberts emphasized the importance of the Court deferring to government officials in emergencies. As the pandemic progressed into 2021, however, the Court became less and less sympathetic to government actions that were not consistent, permitted by existing law, or reasonably necessary. For example, regulations of churches that were inconsistent with the regulation of similar organizations were struck down.14
Among the most interesting of the summer 2021 cases was the CDC eviction moratorium that essentially prohibited landlords nationwide from evicting tenants for nonpayment of rent. When the challenges to these CDC regulations first reached the Court, the moratorium was about to expire; in a 5-4 decision, the Court did not enjoin the CDC from continuing that policy. Justice Kavanaugh (the fifth vote) warned that “clear and specific congressional authorization…would be necessary to extend the moratorium past July 31.”15 Despite telling the Court that the moratorium would expire on July 31, just 3 days after the expiration and without any congressional authorization, the CDC reinstated what was practically the same moratorium.16 On August 26, the Court struck down the reinstated regulation, probably by a 6-3 margin. (Because this case arose in the “Shadow Docket,” the vote of some justices is not certain).17
Continue to: The Affordable Care Act...
The Affordable Care Act
The Affordable Care Act was challenged in the Court for the third time.18 In this term’s case, several states argued that when Congress essentially eliminated the penalty/tax for not purchasing insurance coverage, there was no longer a constitutional basis for the individual mandate. With that centerpiece gone, they claimed, the whole statute should be declared unconstitutional.
Along with many other specialty groups, ACOG joined an amicus curiae brief sponsored by the American Medical Association (AMA).19 An amicus brief is one not filed by the parties to the case, but by organizations or individuals who have information that may be of use to the Court in considering the case. Among other things, the filing of an amicus brief indicates the interest of the organization in the outcome of the case. In this case, the crux of the amicus was that even if the individual mandate currently is not constitutional, the Court should sever that provision and retain the rest of the ACA.
Despite some wild predictions about what the Court might do, it did not decide any substantive issue. Rather, it found that none of the parties to the case had “standing” to challenge the constitutionality of the ACA. Therefore, in effect, the Court dismissed the case without deciding the substantive legal issues.
Pharmacy Benefit Managers
The powerful Pharmacy Benefit Managers (PBMs) are a hidden part of the health care system; however, in recent years there has been increasing regulatory attention paid to them. Some states have begun regulating aspects of PBMs. In this term, the Court considered an Arkansas law that sought to protect local pharmacies from PBM pricing practices.20 The AMA filed an amicus brief in the case which made legal arguments, most of which had been made by the parties to the litigation.21
PBMs generally tell pharmacies how much they will reimburse the pharmacy for filling a prescription for a particular drug. In some instances, PBMs will set a reimbursement price that is lower than the wholesale price at which local pharmacies can purchase the drug. The Arkansas law prohibited PBMs in the state from reimbursing pharmacies for less than the wholesale cost the pharmacy paid for the drug.
The claim of the PBMs was that the Arkansas law violated the Employee Retirement Income Security Act (ERISA). In part, this act preempts state law that relates to fringe benefit plans. States have the authority to regulate insurance, but ERISA limits what they can do when the insurance relates to fringe benefits. The Court held that ERISA does not preempt the Arkansas law or similar state laws in other states. Because the state law was not preempted by the state law, the Arkansas regulation was upheld. The fact that this was a unanimous decision (8-0, because Justice Barrett was not on the Court when the case was heard) suggests that states may have leeway in additional regulations of PBMs, and it would not be surprising to see more of that state regulation in the future.
Continue to: Patent uncertainty...
Patent uncertainty
Csaba Truckai invented and patented the NovaSure System ablation device with a “moisture permeable” head. He sold his company and the related patents, which eventually were purchased by Hologic. Over time, Hologic added claims to the original patent. In the meantime, Truckai went on to invent another device, the Minerva Endometrial Ablation System (MEAS), which had a “moisture impermeable” head. (Note that the “Minerva Surgical, Inc.” involved in this case is not related to the company “Minerva Industries,” which some identified as a “patent troll.”)22
Hologic sued Minerva, claiming that Truckai’s second device (MEAS) infringed on its patent for the first device (NovaSure). Truckai’s defense was that the patent on NovaSure was invalid. Hologic felt that since Truckai had obtained that patent and then sold it, it was improper for him now to claim it was invalid. There is a doctrine for that: assignor estoppel—the person who sold (assigned) the patent is prevented from later claiming it was invalid. The question in this case was whether assignor estoppel is part of the patent law of the United States. It is not in the patent statutes, so it is a court-determined part of the law.
In a 5-4 decision this Term, the Court held that assignor estoppel is recognized, but that it is narrow.23 The Court identified several exceptions to assignor estoppel, notably for this case, including the situation in which the purchaser of the patent, after the purchase, returns to the Patent and Trademark Office to expand (amend) the patent’s claims. In that case, the seller could not be estopped by the amended terms of the patent. Minerva claimed that it was attacking the expanded patent that included changes made after it sold the patent. The Court, therefore, returned the case to the Federal Circuit to apply the principles it laid out about assignor estoppel.
Biotech and other fast-moving fields frequently have new technology building on slightly earlier technology. The current patent system often leaves uncertainty about who owns which part of a valid patent. This uncertainty is a drag on innovation, and the patent system is supposed to spur innovation. Assignor estoppel is likely to create additional complexity and uncertainty in some patents, which is regrettable.
Review of the Term
In addition to the other disruptions of the Term, during the first part of the Term, Amy Coney Barrett was not yet confirmed by the Senate, so there were only 8 justices until October 27. She did not participate in those cases that were heard before she joined the Court. The consensus is that the Court heard 67 cases: 57 were formally briefed and argued along with 8 summary reversals and 2 religious cases in the Shadow Docket. In my opinion, this undercounts both the number and the importance of the Shadow Docket cases, but the following data use the 67 case convention.24
The Court was unanimous in 43% of the cases, including some of the most divisive issues. That unanimity reflects very narrow decisions. There were (by conventional count) only eight 5-4 opinions (12%), an unusually low number. Justice Kavanaugh is viewed as the “median” justice. He was in the majority in 97% of all cases. Chief Justice Roberts and Justice Barrett were in the majority 91%, and Justice Gorsuch 90%. As for the other justices, they were in the majority (all cases) most of the time: Justice Alito, 83%; Justice Thomas, 81%; Justice Breyer, 76%; Justice Kagan, 75%; and Justice Sotomayor, 69%. In “divided cases” (when unanimous cases are removed), the percentages are: Justice Kavanaugh, 95%; Chief Justice Roberts and Justice Barrett, 84%; Justice Gorsuch, 82%; Justice Alito, 70%; Justice Thomas, 66%; Justice Breyer, 58%; Justice Kagan, 55%; and Justice Sotomayor, 45%.
When the term began, many Court watchers expected a relatively uninteresting term, dealing with many technical legal details. In fact, it turned out to be more interesting and important than expected, even with narrow holdings in important cases. Part of the secret of the term was that a lot of the real action was in the Shadow Docket. The end of the term is sometimes the moment when a justice announces a plan to retire. Many commentators expected Justice Breyer might announce—he has been under pressure to do so, to allow President Biden to nominate and a Democratic Senate to confirm a progressive justice. However, he did not do so. It is possible that he will announce his retirement to be effective when his successor is confirmed, but that is pure speculation.
Continue to: Next Term...
Next Term
The next term began on Monday, October 4, 2021. With the considerable current activity in the Shadow Docket, there was not much of a summer break. The coming term looks extraordinary. The headline case is an abortion case from Mississippi, Dobbs v Jackson Women’s Health Organization.25 The legal question is the constitutionality of Mississippi law that prohibits most abortions after 15 weeks of gestation. The Texas abortion law will also be back before the Court. As we saw this term, big cases may produce very narrow results, but this case has the potential for being a notable abortion decision.
In a different case the Court will decide whether a state attorney general can step in to defend an abortion law when the state health secretary does not do so.26
The Court also has accepted 3 cases dealing with reimbursement for health services. One deals with whether or not the Department of Health and Human Services can set reimbursement rates without good survey data regarding costs,27 another involves the calculation of additional payments for hospitals that serve a “disproportionate number of low-income patients,”28 and the third whether state Medicaid programs can take funds from an injured beneficiary’s tort recovery to cover future Medicaid costs.29
In other cases, the Court will review a gun control law from New York. The Court’s earlier Second Amendment cases involved guns in the home used for self-defense, but this case raises the question of whether a state can practically preclude “concealed-carry licenses.”30 Many experts believe the Court will accept a case dealing with racial preferences in college admissions, perhaps the Harvard case in which the claim is discrimination against Asian Americans.31
The ACOG mifepristone case was interesting, in part because the federal district court issued a nationwide injunction against the Americans with Disabilities Act, enforcing its rules anywhere in the country. The effect of these orders is for a single district judge to create the “law of the land,” at least until that is reviewed—which can take months. The advantage of the nationwide injunction is that it avoids having to repeatedly litigate the same issues in multiple courts around the country. The downside is that plaintiffs can seek out a nonrepresentative judge or circuit and receive an injunction that would be granted by few other circuits. In addition, a nationwide injunction can apply to specific circumstances that are not before the court issuing the injunction. In the mifepristone case, for example, 10 states requested to intervene in the ACOG case. The court rejected the request, but the nationwide injunction applied to those states.1
Although federal judges have had the authority to issue nationwide injunctions for years, they are becoming much more common. One reason is the ease of forum shopping noted earlier—organizations can cherry-pick district courts and circuits sympathetic to their views. Both left- and right-leaning organizations have learned this lesson, so left-leaning groups are likely to file in specific districts in the Ninth Circuit, and right-leaning groups to districts in the Fifth Circuit.
If the current trend of increasing nationwide injunctions continues, either the rules for the federal courts or congressional action may be required to reduce some of the abuses by both sides of the political spectrum.
The ACOG mifepristone case was interesting, in part because the federal district court issued a nationwide injunction against the Americans with Disabilities Act, enforcing its rules anywhere in the country. The effect of these orders is for a single district judge to create the “law of the land,” at least until that is reviewed—which can take months. The advantage of the nationwide injunction is that it avoids having to repeatedly litigate the same issues in multiple courts around the country. The downside is that plaintiffs can seek out a nonrepresentative judge or circuit and receive an injunction that would be granted by few other circuits. In addition, a nationwide injunction can apply to specific circumstances that are not before the court issuing the injunction. In the mifepristone case, for example, 10 states requested to intervene in the ACOG case. The court rejected the request, but the nationwide injunction applied to those states.1
Although federal judges have had the authority to issue nationwide injunctions for years, they are becoming much more common. One reason is the ease of forum shopping noted earlier—organizations can cherry-pick district courts and circuits sympathetic to their views. Both left- and right-leaning organizations have learned this lesson, so left-leaning groups are likely to file in specific districts in the Ninth Circuit, and right-leaning groups to districts in the Fifth Circuit.
If the current trend of increasing nationwide injunctions continues, either the rules for the federal courts or congressional action may be required to reduce some of the abuses by both sides of the political spectrum. Reference Am. Coll. of Obstetricians & Gynecologists v. United States FDA, 467 F. Supp. 3d 282, 284 (D. Md. 2020).
Reference
1. Am. Coll. of Obstetricians & Gynecologists v. United States FDA, 467 F. Supp. 3d 282, 284 (D. Md. 2020).
The “Shadow Docket”
The ACOG mifepristone decisions do not appear on the Supreme Court’s “Court Opinions” website.1 They appear in what has become known in recent years as “The Shadow Docket,” an informal term that includes many orders of the Court and statements of individual justices regarding some cases.2 There are hundreds of orders by the Court each Term, there is nothing particularly shadowy about any of these items—they are all publicly available on the Court’s website and later in paper format. It is, however, a little harder to find and much harder to sort through than the major opinions. In some cases, it is not possible to tell what the vote was, how each justice voted, and what the reasoning of the Court was. In a few cases it is difficult to know exactly what the Court was holding or otherwise leaves some confusion about what the law actually is.3
The part of the Shadow Docket that is most intriguing for commentators, and where the ACOG cases appear, is the “Opinions Relating to Orders.”4 These are a variety of opinions, some written by the Court and many by individual justices. It also includes the action of the Court in some cases in which there was not full briefing or oral argument. The statements by justices often are to dissent from the denial of cert of decisions of the Court. These opinions have become much more common over the years. In this past term, there were approximately 60 such opinions related to about 50 cases. In part, this relates to the number of pandemic cases that could not wait for a Court decision going through the extended ordinary process. Although the Shadow Docket has been of interest to academic observers and Court watchers for years, this year it has attracted the attention of Congress.5
References
1. Opinions of the Court. Supreme Court website. https://www.supremecourt.gov/opinions/slipopinion/20#list. Accessed October 10, 2021.
2. Baude W. Foreword: the Supreme Court’s Shadow Docket, 9 N.Y.U. J.L. & Liberty 1 (2015).
3. Vladeck SI. The Solicitor General and the Shadow Docket, 133 Harvard Law Review. 123 (2019).
4. Opinions relating to orders. Supreme Court website. https://www.supremecourt.gov/opinions/relatingtoorders/20#list. Accessed October 10, 2021.
5. The Supreme Court’s Shadow Docket: Hearing Before the Subcommittee on Courts, Intellectual Property and the Internet of the H. Committee on the Judiciary, 117th Congress (2021).
- American College of Obstetricians & Gynecologists v. United States FDA, 472 F. Supp. 3d 183 (D. Md. 2020).
- Michael Kunzelman, Doctors Sue to Block FDA Abortion Pill Rule During Pandemic, (May 29, 2020).
- ACLU, American College Of Obstetricians And Gynecologists V. U.S. Food And Drug Administration, https://www.aclu.org/cases/american-college-obstetricians-and-gynecologists-v-us-food-and-drug-administration. Updated February 12, 2021. Accessed August 27, 2021.
- Whole Woman’s Health v Hellerstedt, 579 US ___ (2016), 136 S Ct 2292.
- 2016 Clinical Review at 39, 47, 49, Opp’n Mot. PI Ex. 19, ECF No. 62-11.
- American College of Obstetricians and Gynecologists v FDA (I), decided October 8, 2020.
- October 8, 2020, dissenting opinion by Justice Alito.
- January 12, 2021, dissenting opinion by Justice Sotomayor.
- Questions and answers on Mifeprex. U.S. Food and Drug Administration website. Published April 13, 2021. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/questions-and-answers-mifeprex. Accessed October 9, 2021.
- Whole Woman’s Health v Jackson, decided September 1, 2021.
- Texas Senate Bill 8, relating to abortion, including abortions after detection of unborn child’s heartbeat; authorizing a private civil right of action. LegiScan website. https://legiscan.com/TX/text/SB8/id/2395961. Accessed October 9, 2021.
- Planned Parenthood of Southeastern Pennsylvania v Casey, 505 U. S. 833 (1992); Roe v Wade, 410 U. S. 113 (1973).
- Dobbs v Jackson Women’s Health Organization, No. 19-1392.
- Roman Catholic Diocese of Brooklyn v Cuomo, decided November 25, 2020.
- Alabama Association of Realtors v Department of Health and Human Services, decided June 29, 2021.
- Temporary halt in residential evictions in communities with substantial or high levels of community transmission of COVID-19 to prevent the further spread of COVID-19. August 6, 2021. https://www.federalregister.gov/documents/2021/08/06/2021-16945/temporary-halt-in-residential-evictions-in-communities-with-substantial-or-high-transmission-of.
- Alabama Association of Realtors v Department of Health and Human Services, decided August 26, 2021.
- California v Texas, decided June 17, 2021.
- Brief of Amici Curiae American Medical Association, American Academy of Allergy, Asthma and Immunology, Aerospace Medical Association, American Academy of Family Physicians, American Academy of Pediatrics, American College of Cardiology, American College of Emergency Physicians, American College of Medical Genetics and Genomics, American College of Obstetricians and Gynecologists, American College of Physicians, American College of Radiation Oncology, American College of Radiology, American Psychiatric Association, American Society of Gastrointestinal Endoscopy, American Society of Hematology, American Society of Metabolic and Bariatric Surgery, Endocrine Society, GLMA: Health Professionals Advancing LGBTQ Equality, Renal Physicians Association, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology in Support of Petitioners, in California v. Texas. May 13, 2020. https://www.supremecourt.gov/DocketPDF/19/19-840/143469/20200513150051995_19-840%20Amici%20Brief%20AMA.pdf. Accessed October 9, 2021.
- Rutledge v Pharmaceutical Care Management Association, decided December 10, 2020.
- Brief of the American Medical Association, The Arkansas Medical Society, and The Litigation Center of the American Medical Association and the State Medical Societies as Amici Curiae in Support of Petitioner in Rutledge v Pharmaceutical Care Management Association. March 2, 2020. https://www.supremecourt.gov/DocketPDF/18/18-540/134670/20200302163622018_Rutledge%20v.%20PCMA%20Amicus%20Brief%20of%20AMA%20et%20al.pdf. Accessed October 9, 2021.
- Apple quietly settles patent lawsuit, promptly gets hit with another one. TechCrunch website. Published July 30, 2010. https://techcrunch.com/2010/07/30/apple-minerva-emblaze/. Accessed October 9, 2021.
- Minerva Surgical, Inc. v Hologic, Inc., decided June 29, 2021.
- Stat pack. SCOTUS Blog website. Published July 6, 2021. https://www.scotusblog.com/wp-content/uploads/2021/07/Final-Stat-Pack-7.6.21.pdf. Accessed October 9, 2021.
- Dobbs v Jackson Women’s Health Organization, No. 19-1392.
- Cameron v. EMW Women’s Surgical Center, https://www.scotusblog.com/case-files/cases/cameron-v-emw-womens-surgical-center-p-s-c/. Accessed August 28, 2021.
- American Hospital Association v Becerra, No. 20-1114.
- Becerra v Empire Health Foundation, No. 20-1312.
- Gallardo v Marstiller, No. 20-1263.
- New York State Rifle & Pistol Association Inc. v Corlett, No. 20-843.
- Students for Fair Admissions v President & Fellows of Harvard College, No. 20-1199.
- American College of Obstetricians & Gynecologists v. United States FDA, 472 F. Supp. 3d 183 (D. Md. 2020).
- Michael Kunzelman, Doctors Sue to Block FDA Abortion Pill Rule During Pandemic, (May 29, 2020).
- ACLU, American College Of Obstetricians And Gynecologists V. U.S. Food And Drug Administration, https://www.aclu.org/cases/american-college-obstetricians-and-gynecologists-v-us-food-and-drug-administration. Updated February 12, 2021. Accessed August 27, 2021.
- Whole Woman’s Health v Hellerstedt, 579 US ___ (2016), 136 S Ct 2292.
- 2016 Clinical Review at 39, 47, 49, Opp’n Mot. PI Ex. 19, ECF No. 62-11.
- American College of Obstetricians and Gynecologists v FDA (I), decided October 8, 2020.
- October 8, 2020, dissenting opinion by Justice Alito.
- January 12, 2021, dissenting opinion by Justice Sotomayor.
- Questions and answers on Mifeprex. U.S. Food and Drug Administration website. Published April 13, 2021. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/questions-and-answers-mifeprex. Accessed October 9, 2021.
- Whole Woman’s Health v Jackson, decided September 1, 2021.
- Texas Senate Bill 8, relating to abortion, including abortions after detection of unborn child’s heartbeat; authorizing a private civil right of action. LegiScan website. https://legiscan.com/TX/text/SB8/id/2395961. Accessed October 9, 2021.
- Planned Parenthood of Southeastern Pennsylvania v Casey, 505 U. S. 833 (1992); Roe v Wade, 410 U. S. 113 (1973).
- Dobbs v Jackson Women’s Health Organization, No. 19-1392.
- Roman Catholic Diocese of Brooklyn v Cuomo, decided November 25, 2020.
- Alabama Association of Realtors v Department of Health and Human Services, decided June 29, 2021.
- Temporary halt in residential evictions in communities with substantial or high levels of community transmission of COVID-19 to prevent the further spread of COVID-19. August 6, 2021. https://www.federalregister.gov/documents/2021/08/06/2021-16945/temporary-halt-in-residential-evictions-in-communities-with-substantial-or-high-transmission-of.
- Alabama Association of Realtors v Department of Health and Human Services, decided August 26, 2021.
- California v Texas, decided June 17, 2021.
- Brief of Amici Curiae American Medical Association, American Academy of Allergy, Asthma and Immunology, Aerospace Medical Association, American Academy of Family Physicians, American Academy of Pediatrics, American College of Cardiology, American College of Emergency Physicians, American College of Medical Genetics and Genomics, American College of Obstetricians and Gynecologists, American College of Physicians, American College of Radiation Oncology, American College of Radiology, American Psychiatric Association, American Society of Gastrointestinal Endoscopy, American Society of Hematology, American Society of Metabolic and Bariatric Surgery, Endocrine Society, GLMA: Health Professionals Advancing LGBTQ Equality, Renal Physicians Association, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology in Support of Petitioners, in California v. Texas. May 13, 2020. https://www.supremecourt.gov/DocketPDF/19/19-840/143469/20200513150051995_19-840%20Amici%20Brief%20AMA.pdf. Accessed October 9, 2021.
- Rutledge v Pharmaceutical Care Management Association, decided December 10, 2020.
- Brief of the American Medical Association, The Arkansas Medical Society, and The Litigation Center of the American Medical Association and the State Medical Societies as Amici Curiae in Support of Petitioner in Rutledge v Pharmaceutical Care Management Association. March 2, 2020. https://www.supremecourt.gov/DocketPDF/18/18-540/134670/20200302163622018_Rutledge%20v.%20PCMA%20Amicus%20Brief%20of%20AMA%20et%20al.pdf. Accessed October 9, 2021.
- Apple quietly settles patent lawsuit, promptly gets hit with another one. TechCrunch website. Published July 30, 2010. https://techcrunch.com/2010/07/30/apple-minerva-emblaze/. Accessed October 9, 2021.
- Minerva Surgical, Inc. v Hologic, Inc., decided June 29, 2021.
- Stat pack. SCOTUS Blog website. Published July 6, 2021. https://www.scotusblog.com/wp-content/uploads/2021/07/Final-Stat-Pack-7.6.21.pdf. Accessed October 9, 2021.
- Dobbs v Jackson Women’s Health Organization, No. 19-1392.
- Cameron v. EMW Women’s Surgical Center, https://www.scotusblog.com/case-files/cases/cameron-v-emw-womens-surgical-center-p-s-c/. Accessed August 28, 2021.
- American Hospital Association v Becerra, No. 20-1114.
- Becerra v Empire Health Foundation, No. 20-1312.
- Gallardo v Marstiller, No. 20-1263.
- New York State Rifle & Pistol Association Inc. v Corlett, No. 20-843.
- Students for Fair Admissions v President & Fellows of Harvard College, No. 20-1199.
Newly discovered vascular barrier in the brain may explain IBD-related anxiety, depression
A newly discovered vascular brain barrier that blocks the passage of inflammatory molecules triggered by gut bacteria may be why patients with inflammatory bowel disease (IBD) are at increased risk for certain mental health disorders, including anxiety and depression, early research suggests.
The discovery, which was based on a preclinical model, could lead to new therapeutic targets that could have applications for both gastrointestinal and psychiatric conditions, investigators note.
The research team, which was led by immunologist Maria Rescigno, PhD, and neuroscientist Simona Lodato, PhD, both from Humanitas University, Milan, notes that the barrier resides in the choroid plexus, a region of the brain that is involved in filtering cerebrospinal fluid. The researchers found that the region closes in response to inflammatory molecules produced in reaction to the presence of intestinal bacteria in patients with gut disorders.
Dr. Lodato said in an interview that the brain’s choroid plexus vascular barrier, along with another barrier between the gut and liver, known as the gut vascular barrier, appear to control the movement of molecules along the gut-brain axis.
“We show that in addition to the epithelial barrier in the choroid plexus, there is a functional vascular barrier that only becomes evident in blocking entry of various inflammatory molecules under conditions of systemic inflammation,” Dr. Lodato said.
“This interruption of the gut-brain interaction has developed to protect the brain from inflammation. Why this happens is not yet known, but it is likely to prevent epileptic seizures and imbalanced neuronal activity,” added Dr. Rescigno.
The study was published online October 22 in Science.
The gut a root cause of mental illness?
Nearly 40% of patients with IBD also experience depression and anxiety. It was once thought that these conditions arose because of patients’ difficulties in coping with their disease, said Dr. Rescigno.
“People with these disorders conventionally thought to be caused by an imbalance in the brain may actually find the root cause is located in the intestine. This is the first time these symptoms have been associated with the choroid plexus vascular brain barrier and its closure,” she noted.
Dr. Rescigno added that subtle, rather than overt, inflammation may be all that’s required for closure of the choroid plexus and the subsequent effects on mental health.
In 2015, Dr. Rescigno’s group first described the gut vascular barrier that protects the systemic circulation from gut bacteria or associated bacteria-derived molecules. During intestinal inflammation, such as occurs in IBD, this barrier is compromised and becomes more permeable. This allows microbes to pass across the epithelium of the gut barrier and enter the systemic circulation, including the liver and spleen, explained Dr. Rescigno.
Dr. Rescigno and Dr. Lodato then explored whether this systemic inflammatory condition was connected to the brain along a gut-brain axis and found that it was.
The researchers tested the hypothesis that central nervous system symptoms may be due to vascular changes at the interface between the gut or the brain and elsewhere in the body.
“We set out to test whether opening of the gut vascular barrier would allow gut bacteria to trigger the release of inflammatory molecules that spread to more distant areas, possibly leading to a deficiency of certain nutrients and precipitating mental disorders,” they said.
An experimental preclinical model of the choroid plexus vascular barrier closure led to anxiety-like behavior, as well as short-term memory loss. That this behavior occurred independently of inflammation suggested that it was likely a response to closure itself, they note.
In the noninflammatory state, the epithelium of the choroid plexus filters molecules. Those that are ≤70 kDa are allowed to pass through to the brain. However, the investigators found that during systemic inflammation, this filtration stops, and the blood capillaries of the choroid plexus prevent entry of inflammatory molecules such as cytokines.
Dr. Lodato speculated that when the vascular barrier of the choroid plexus shuts off during the systemic inflammatory state, it responds by bathing the brain in cerebrospinal fluid.
“When the choroid plexus closes, like a door slamming shut, then communication between the brain and the rest of the body is halted. This means that the brain is deprived of certain nutrients and other beneficial molecules that usually enter via the cerebrospinal fluid or enriched of potentially dangerous ones, as drainage could also be affected,” she said.
If confirmed in further studies, these results may open the way to new interventions.
‘A significant leap forward’
Commenting on the findings, David T. Rubin, MD, professor of medicine at the University of Chicago, noted that the study’s results represent “a significant leap forward” and that it highlights “another important cost to uncontrolled gut inflammation that is the potential for worsened mental health disorders.”
Dr. Rubin, whose research involves measuring metabolites of the dietary amino acid tryptophan, including melatonin and serotonin, in patients with IBD, added that the findings offer a possible explanation for the association of both Crohn’s disease and ulcerative colitis with anxiety and depressive disorders.
“There was a belief that the association was in the opposite direction, that the mental health disorder was causing or worsening the gut inflammation, but this has been disavowed,” Dr. Rubin said.
“Most recently, the recognition that the major sources of serotonin and other metabolites of tryptophan that come from the gut microbiome has led to the hypothesis that the inflamed bowel and dysbiotic gut biome may in fact be driving the mental health disorders due to the effect of neurotransmitter imbalance,” he added. Dr. Rubin also suggested that the shutdown of the choroid plexus vascular barrier may contribute to this imbalance but that this needs additional study.
“This further supports my ongoing contention that the gut really is the center of the universe,” said Dr. Rubin.
Also commenting on the findings, Miguel Rigueiro, MD, professor in the department of medicine in the Cleveland Clinic Lerner College of Medicine of Case Western Reserve University, Cleveland, said, “There’s an implication that anxiety and depression and other behavioral health disorders may be explained by this mechanism. If that is the case, there may be a way to target medications against the choroid plexus and potentially treat depression or anxiety.”
This prospect was echoed by Dr. Rubin, who said, “The clinical implication is that treatment of gut inflammation may restore a balance to the neurotransmitters and resolve anxiety or depressive disorders.”
To identify new therapeutic targets, investigators will study the regions and circuits of the brain that are more susceptible to this closure of the choroid plexus, said Dr. Lodato.
“If these regions are associated with depression or other psychosocial disorders, then this new understanding around the choroid plexus vascular barrier might eventually have implications for helping treat such disorders,” she noted.
Reflecting a general shift from a brain-centric view of some psychosocial disorders to an intestinal-centric one, Dr. Lodato added, “The brain cannot be considered in isolation. It is part of a much larger body, and we need to think this way.”
Dr. Rescigno, Dr. Lodato, and Dr. Rubin report no relevant financial relationships. Dr. Rigueiro has served on advisory boards and as consultant for AbbVie, Janssen, UCB, Takeda, Pfizer, Miraca Labs, Amgen, Celgene, Seres, Allergan, Genentech, Gilead, Salix, Prometheus, Lilly, TARGET Pharma Solutions, ALFASIGMA, SpA, and Bristol-Meyer Squibb.
A version of this article first appeared on Medscape.com.
A newly discovered vascular brain barrier that blocks the passage of inflammatory molecules triggered by gut bacteria may be why patients with inflammatory bowel disease (IBD) are at increased risk for certain mental health disorders, including anxiety and depression, early research suggests.
The discovery, which was based on a preclinical model, could lead to new therapeutic targets that could have applications for both gastrointestinal and psychiatric conditions, investigators note.
The research team, which was led by immunologist Maria Rescigno, PhD, and neuroscientist Simona Lodato, PhD, both from Humanitas University, Milan, notes that the barrier resides in the choroid plexus, a region of the brain that is involved in filtering cerebrospinal fluid. The researchers found that the region closes in response to inflammatory molecules produced in reaction to the presence of intestinal bacteria in patients with gut disorders.
Dr. Lodato said in an interview that the brain’s choroid plexus vascular barrier, along with another barrier between the gut and liver, known as the gut vascular barrier, appear to control the movement of molecules along the gut-brain axis.
“We show that in addition to the epithelial barrier in the choroid plexus, there is a functional vascular barrier that only becomes evident in blocking entry of various inflammatory molecules under conditions of systemic inflammation,” Dr. Lodato said.
“This interruption of the gut-brain interaction has developed to protect the brain from inflammation. Why this happens is not yet known, but it is likely to prevent epileptic seizures and imbalanced neuronal activity,” added Dr. Rescigno.
The study was published online October 22 in Science.
The gut a root cause of mental illness?
Nearly 40% of patients with IBD also experience depression and anxiety. It was once thought that these conditions arose because of patients’ difficulties in coping with their disease, said Dr. Rescigno.
“People with these disorders conventionally thought to be caused by an imbalance in the brain may actually find the root cause is located in the intestine. This is the first time these symptoms have been associated with the choroid plexus vascular brain barrier and its closure,” she noted.
Dr. Rescigno added that subtle, rather than overt, inflammation may be all that’s required for closure of the choroid plexus and the subsequent effects on mental health.
In 2015, Dr. Rescigno’s group first described the gut vascular barrier that protects the systemic circulation from gut bacteria or associated bacteria-derived molecules. During intestinal inflammation, such as occurs in IBD, this barrier is compromised and becomes more permeable. This allows microbes to pass across the epithelium of the gut barrier and enter the systemic circulation, including the liver and spleen, explained Dr. Rescigno.
Dr. Rescigno and Dr. Lodato then explored whether this systemic inflammatory condition was connected to the brain along a gut-brain axis and found that it was.
The researchers tested the hypothesis that central nervous system symptoms may be due to vascular changes at the interface between the gut or the brain and elsewhere in the body.
“We set out to test whether opening of the gut vascular barrier would allow gut bacteria to trigger the release of inflammatory molecules that spread to more distant areas, possibly leading to a deficiency of certain nutrients and precipitating mental disorders,” they said.
An experimental preclinical model of the choroid plexus vascular barrier closure led to anxiety-like behavior, as well as short-term memory loss. That this behavior occurred independently of inflammation suggested that it was likely a response to closure itself, they note.
In the noninflammatory state, the epithelium of the choroid plexus filters molecules. Those that are ≤70 kDa are allowed to pass through to the brain. However, the investigators found that during systemic inflammation, this filtration stops, and the blood capillaries of the choroid plexus prevent entry of inflammatory molecules such as cytokines.
Dr. Lodato speculated that when the vascular barrier of the choroid plexus shuts off during the systemic inflammatory state, it responds by bathing the brain in cerebrospinal fluid.
“When the choroid plexus closes, like a door slamming shut, then communication between the brain and the rest of the body is halted. This means that the brain is deprived of certain nutrients and other beneficial molecules that usually enter via the cerebrospinal fluid or enriched of potentially dangerous ones, as drainage could also be affected,” she said.
If confirmed in further studies, these results may open the way to new interventions.
‘A significant leap forward’
Commenting on the findings, David T. Rubin, MD, professor of medicine at the University of Chicago, noted that the study’s results represent “a significant leap forward” and that it highlights “another important cost to uncontrolled gut inflammation that is the potential for worsened mental health disorders.”
Dr. Rubin, whose research involves measuring metabolites of the dietary amino acid tryptophan, including melatonin and serotonin, in patients with IBD, added that the findings offer a possible explanation for the association of both Crohn’s disease and ulcerative colitis with anxiety and depressive disorders.
“There was a belief that the association was in the opposite direction, that the mental health disorder was causing or worsening the gut inflammation, but this has been disavowed,” Dr. Rubin said.
“Most recently, the recognition that the major sources of serotonin and other metabolites of tryptophan that come from the gut microbiome has led to the hypothesis that the inflamed bowel and dysbiotic gut biome may in fact be driving the mental health disorders due to the effect of neurotransmitter imbalance,” he added. Dr. Rubin also suggested that the shutdown of the choroid plexus vascular barrier may contribute to this imbalance but that this needs additional study.
“This further supports my ongoing contention that the gut really is the center of the universe,” said Dr. Rubin.
Also commenting on the findings, Miguel Rigueiro, MD, professor in the department of medicine in the Cleveland Clinic Lerner College of Medicine of Case Western Reserve University, Cleveland, said, “There’s an implication that anxiety and depression and other behavioral health disorders may be explained by this mechanism. If that is the case, there may be a way to target medications against the choroid plexus and potentially treat depression or anxiety.”
This prospect was echoed by Dr. Rubin, who said, “The clinical implication is that treatment of gut inflammation may restore a balance to the neurotransmitters and resolve anxiety or depressive disorders.”
To identify new therapeutic targets, investigators will study the regions and circuits of the brain that are more susceptible to this closure of the choroid plexus, said Dr. Lodato.
“If these regions are associated with depression or other psychosocial disorders, then this new understanding around the choroid plexus vascular barrier might eventually have implications for helping treat such disorders,” she noted.
Reflecting a general shift from a brain-centric view of some psychosocial disorders to an intestinal-centric one, Dr. Lodato added, “The brain cannot be considered in isolation. It is part of a much larger body, and we need to think this way.”
Dr. Rescigno, Dr. Lodato, and Dr. Rubin report no relevant financial relationships. Dr. Rigueiro has served on advisory boards and as consultant for AbbVie, Janssen, UCB, Takeda, Pfizer, Miraca Labs, Amgen, Celgene, Seres, Allergan, Genentech, Gilead, Salix, Prometheus, Lilly, TARGET Pharma Solutions, ALFASIGMA, SpA, and Bristol-Meyer Squibb.
A version of this article first appeared on Medscape.com.
A newly discovered vascular brain barrier that blocks the passage of inflammatory molecules triggered by gut bacteria may be why patients with inflammatory bowel disease (IBD) are at increased risk for certain mental health disorders, including anxiety and depression, early research suggests.
The discovery, which was based on a preclinical model, could lead to new therapeutic targets that could have applications for both gastrointestinal and psychiatric conditions, investigators note.
The research team, which was led by immunologist Maria Rescigno, PhD, and neuroscientist Simona Lodato, PhD, both from Humanitas University, Milan, notes that the barrier resides in the choroid plexus, a region of the brain that is involved in filtering cerebrospinal fluid. The researchers found that the region closes in response to inflammatory molecules produced in reaction to the presence of intestinal bacteria in patients with gut disorders.
Dr. Lodato said in an interview that the brain’s choroid plexus vascular barrier, along with another barrier between the gut and liver, known as the gut vascular barrier, appear to control the movement of molecules along the gut-brain axis.
“We show that in addition to the epithelial barrier in the choroid plexus, there is a functional vascular barrier that only becomes evident in blocking entry of various inflammatory molecules under conditions of systemic inflammation,” Dr. Lodato said.
“This interruption of the gut-brain interaction has developed to protect the brain from inflammation. Why this happens is not yet known, but it is likely to prevent epileptic seizures and imbalanced neuronal activity,” added Dr. Rescigno.
The study was published online October 22 in Science.
The gut a root cause of mental illness?
Nearly 40% of patients with IBD also experience depression and anxiety. It was once thought that these conditions arose because of patients’ difficulties in coping with their disease, said Dr. Rescigno.
“People with these disorders conventionally thought to be caused by an imbalance in the brain may actually find the root cause is located in the intestine. This is the first time these symptoms have been associated with the choroid plexus vascular brain barrier and its closure,” she noted.
Dr. Rescigno added that subtle, rather than overt, inflammation may be all that’s required for closure of the choroid plexus and the subsequent effects on mental health.
In 2015, Dr. Rescigno’s group first described the gut vascular barrier that protects the systemic circulation from gut bacteria or associated bacteria-derived molecules. During intestinal inflammation, such as occurs in IBD, this barrier is compromised and becomes more permeable. This allows microbes to pass across the epithelium of the gut barrier and enter the systemic circulation, including the liver and spleen, explained Dr. Rescigno.
Dr. Rescigno and Dr. Lodato then explored whether this systemic inflammatory condition was connected to the brain along a gut-brain axis and found that it was.
The researchers tested the hypothesis that central nervous system symptoms may be due to vascular changes at the interface between the gut or the brain and elsewhere in the body.
“We set out to test whether opening of the gut vascular barrier would allow gut bacteria to trigger the release of inflammatory molecules that spread to more distant areas, possibly leading to a deficiency of certain nutrients and precipitating mental disorders,” they said.
An experimental preclinical model of the choroid plexus vascular barrier closure led to anxiety-like behavior, as well as short-term memory loss. That this behavior occurred independently of inflammation suggested that it was likely a response to closure itself, they note.
In the noninflammatory state, the epithelium of the choroid plexus filters molecules. Those that are ≤70 kDa are allowed to pass through to the brain. However, the investigators found that during systemic inflammation, this filtration stops, and the blood capillaries of the choroid plexus prevent entry of inflammatory molecules such as cytokines.
Dr. Lodato speculated that when the vascular barrier of the choroid plexus shuts off during the systemic inflammatory state, it responds by bathing the brain in cerebrospinal fluid.
“When the choroid plexus closes, like a door slamming shut, then communication between the brain and the rest of the body is halted. This means that the brain is deprived of certain nutrients and other beneficial molecules that usually enter via the cerebrospinal fluid or enriched of potentially dangerous ones, as drainage could also be affected,” she said.
If confirmed in further studies, these results may open the way to new interventions.
‘A significant leap forward’
Commenting on the findings, David T. Rubin, MD, professor of medicine at the University of Chicago, noted that the study’s results represent “a significant leap forward” and that it highlights “another important cost to uncontrolled gut inflammation that is the potential for worsened mental health disorders.”
Dr. Rubin, whose research involves measuring metabolites of the dietary amino acid tryptophan, including melatonin and serotonin, in patients with IBD, added that the findings offer a possible explanation for the association of both Crohn’s disease and ulcerative colitis with anxiety and depressive disorders.
“There was a belief that the association was in the opposite direction, that the mental health disorder was causing or worsening the gut inflammation, but this has been disavowed,” Dr. Rubin said.
“Most recently, the recognition that the major sources of serotonin and other metabolites of tryptophan that come from the gut microbiome has led to the hypothesis that the inflamed bowel and dysbiotic gut biome may in fact be driving the mental health disorders due to the effect of neurotransmitter imbalance,” he added. Dr. Rubin also suggested that the shutdown of the choroid plexus vascular barrier may contribute to this imbalance but that this needs additional study.
“This further supports my ongoing contention that the gut really is the center of the universe,” said Dr. Rubin.
Also commenting on the findings, Miguel Rigueiro, MD, professor in the department of medicine in the Cleveland Clinic Lerner College of Medicine of Case Western Reserve University, Cleveland, said, “There’s an implication that anxiety and depression and other behavioral health disorders may be explained by this mechanism. If that is the case, there may be a way to target medications against the choroid plexus and potentially treat depression or anxiety.”
This prospect was echoed by Dr. Rubin, who said, “The clinical implication is that treatment of gut inflammation may restore a balance to the neurotransmitters and resolve anxiety or depressive disorders.”
To identify new therapeutic targets, investigators will study the regions and circuits of the brain that are more susceptible to this closure of the choroid plexus, said Dr. Lodato.
“If these regions are associated with depression or other psychosocial disorders, then this new understanding around the choroid plexus vascular barrier might eventually have implications for helping treat such disorders,” she noted.
Reflecting a general shift from a brain-centric view of some psychosocial disorders to an intestinal-centric one, Dr. Lodato added, “The brain cannot be considered in isolation. It is part of a much larger body, and we need to think this way.”
Dr. Rescigno, Dr. Lodato, and Dr. Rubin report no relevant financial relationships. Dr. Rigueiro has served on advisory boards and as consultant for AbbVie, Janssen, UCB, Takeda, Pfizer, Miraca Labs, Amgen, Celgene, Seres, Allergan, Genentech, Gilead, Salix, Prometheus, Lilly, TARGET Pharma Solutions, ALFASIGMA, SpA, and Bristol-Meyer Squibb.
A version of this article first appeared on Medscape.com.
FROM SCIENCE