User login
Specialty pharmacists may speed time to MS treatment
, new data suggest.
“As DMT management and treatment options for MS symptoms become more complex, clinical pharmacists can be utilized for medication education and management,” Jenelle Hall Montgomery, PharmD, a clinical pharmacist practitioner at the Multiple Sclerosis and Neuroimmunology Division, department of neurology, Duke University Hospital, Durham, N.C., told delegates attending the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
Since 2018, more than half a dozen DMTs have been approved for MS by the U.S. Food and Drug Administration. However, there is currently no established DMT selection algorithm, and because of this, there is a need for specialty pharmacists, she added.
“DMT approvals by the FDA have outpaced MS guideline recommendations. This can be overwhelming for patients, especially now that they have so many options to choose from,” she said.
Key services provided by specialty pharmacists include coordinating pretreatment requirements, as well as help with dosing, side effects, safety monitoring, and treatment adherence. In addition, pharmacists help with switching therapies, dispensing, and cost and authorization problems.
In reporting on improvements associated with specialty pharmacists, researchers from prominent MS centers around the country described specific outcomes.
Aids early intervention
A report on the Kaiser Permanente Washington (KPWA) MS Pharmacy Program detailed significant reductions in the time to address patients’ needs through the use of specialty pharmacists. In an assessment of 391 referrals to the program from 2019 to 2020, the average total time spent per patient per year dropped from 145 minutes in 2019 to 109 minutes in 2020.
Services included assessment of medication adherence, adverse drug reaction consultation, lab monitoring, patient counseling on initiation of a DMT, shared decision making, and follow-up visits.
“The KPWA MS Pharmacy Program plays an integral role in the care of patients with MS. The MS clinical pharmacists ensure patients are well informed about their DMT options and are fully educated about selected treatment,” the investigators noted.
A report on an outpatient MS clinic at Emory Healthcare, Atlanta, described how use of specialty pharmacist services resulted in a 49% reduction in time to treatment initiation with fingolimod. The time decreased from 83.9 days to 42.9 days following the introduction of specialty pharmacist services.
“Integration of a clinical pharmacy specialist in the therapeutic management of MS patients is crucial to early intervention with disease-modifying therapy,” the investigators noted.
A report on the specialty pharmacy services provided at Johns Hopkins MS Precision Medicine Center of Excellence, Baltimore, described an evaluation of 708 assessments between July 2019 and June 2020. Results showed that the vast majority (98%) of patients reported no missed days from work or school due to MS-related symptoms and that 99.3% reported no hospitalizations due to MS relapses, which are both key measures of MS treatment adherence.
High patient satisfaction
Patients reported high satisfaction with the in-house pharmacy on the National Association of Specialty Pharmacy’s patient satisfaction survey. In the survey, the average score was 82, compared with 79 for external specialty pharmacies.
“Moreover, patients were highly satisfied with the services provided at the pharmacy and were likely to continue receiving their comprehensive pharmacy care at our institution,” the researchers reported.
The study “highlights the value of pharmacists’ involvement in patient care and supports the need for continuation of integrated clinical services in health system specialty pharmacy,” the investigators noted.
CMSC President Scott D. Newsome, DO, director of the Neurosciences Consultation and Infusion Center at Green Spring Station, Lutherville, Maryland, and associate professor of neurology at Johns Hopkins University School of Medicine, said that as a clinician, he is highly satisfied with the specialty pharmacy services for MS at Johns Hopkins.
“Our pharmacists are fantastic in communicating with the prescriber if something comes up related to medication safety or they are concerned that the patient isn’t adhering to the medication,” Dr. Newsome said.
He noted that in addition to helping to alleviate the burden of a myriad of tasks associated with prescribing for patients with MS, specialty pharmacists may have an important impact on outcomes, although more data are needed.
“Having a specialty pharmacy involved in the care of our patients can help navigate the challenges associated with the process of obtaining approval for DMTs,” he said. “We know how important it is to expedite and shorten the time frame from writing the prescription to getting the person on their DMT.”
Telemedicine, other models
Although integrated specialty pharmacist services may seem out of reach for smaller MS clinics, the use of telemedicine and other models may help achieve similar results.
“A model I have seen is having pharmacists split their time between a specialty pharmacy and the MS clinic,” said Dr. Montgomery.
“A telemedicine model can also be utilized, in which a pharmacist can reach out to patients by telephone or through video visits. This would allow a pharmacist to be utilized for multiple clinics or as an MS specialist within a specialty pharmacy,” she added.
Whether provided in house or through telemedicine, a key benefit for clinicians is in freeing up valuable time, which has a domino effect in improving quality all around.
“In addition to improving safety outcomes, specialty pharmacists help with the allocation of clinic staff to other clinic responsibilities, and the utilization of services by patients results in more resources allocated for their care,” Dr. Montgomery said.
Dr. Montgomery is a nonpromotional speaker for Novartis and is on its advisory board.
A version of this article first appeared on Medscape.com.
, new data suggest.
“As DMT management and treatment options for MS symptoms become more complex, clinical pharmacists can be utilized for medication education and management,” Jenelle Hall Montgomery, PharmD, a clinical pharmacist practitioner at the Multiple Sclerosis and Neuroimmunology Division, department of neurology, Duke University Hospital, Durham, N.C., told delegates attending the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
Since 2018, more than half a dozen DMTs have been approved for MS by the U.S. Food and Drug Administration. However, there is currently no established DMT selection algorithm, and because of this, there is a need for specialty pharmacists, she added.
“DMT approvals by the FDA have outpaced MS guideline recommendations. This can be overwhelming for patients, especially now that they have so many options to choose from,” she said.
Key services provided by specialty pharmacists include coordinating pretreatment requirements, as well as help with dosing, side effects, safety monitoring, and treatment adherence. In addition, pharmacists help with switching therapies, dispensing, and cost and authorization problems.
In reporting on improvements associated with specialty pharmacists, researchers from prominent MS centers around the country described specific outcomes.
Aids early intervention
A report on the Kaiser Permanente Washington (KPWA) MS Pharmacy Program detailed significant reductions in the time to address patients’ needs through the use of specialty pharmacists. In an assessment of 391 referrals to the program from 2019 to 2020, the average total time spent per patient per year dropped from 145 minutes in 2019 to 109 minutes in 2020.
Services included assessment of medication adherence, adverse drug reaction consultation, lab monitoring, patient counseling on initiation of a DMT, shared decision making, and follow-up visits.
“The KPWA MS Pharmacy Program plays an integral role in the care of patients with MS. The MS clinical pharmacists ensure patients are well informed about their DMT options and are fully educated about selected treatment,” the investigators noted.
A report on an outpatient MS clinic at Emory Healthcare, Atlanta, described how use of specialty pharmacist services resulted in a 49% reduction in time to treatment initiation with fingolimod. The time decreased from 83.9 days to 42.9 days following the introduction of specialty pharmacist services.
“Integration of a clinical pharmacy specialist in the therapeutic management of MS patients is crucial to early intervention with disease-modifying therapy,” the investigators noted.
A report on the specialty pharmacy services provided at Johns Hopkins MS Precision Medicine Center of Excellence, Baltimore, described an evaluation of 708 assessments between July 2019 and June 2020. Results showed that the vast majority (98%) of patients reported no missed days from work or school due to MS-related symptoms and that 99.3% reported no hospitalizations due to MS relapses, which are both key measures of MS treatment adherence.
High patient satisfaction
Patients reported high satisfaction with the in-house pharmacy on the National Association of Specialty Pharmacy’s patient satisfaction survey. In the survey, the average score was 82, compared with 79 for external specialty pharmacies.
“Moreover, patients were highly satisfied with the services provided at the pharmacy and were likely to continue receiving their comprehensive pharmacy care at our institution,” the researchers reported.
The study “highlights the value of pharmacists’ involvement in patient care and supports the need for continuation of integrated clinical services in health system specialty pharmacy,” the investigators noted.
CMSC President Scott D. Newsome, DO, director of the Neurosciences Consultation and Infusion Center at Green Spring Station, Lutherville, Maryland, and associate professor of neurology at Johns Hopkins University School of Medicine, said that as a clinician, he is highly satisfied with the specialty pharmacy services for MS at Johns Hopkins.
“Our pharmacists are fantastic in communicating with the prescriber if something comes up related to medication safety or they are concerned that the patient isn’t adhering to the medication,” Dr. Newsome said.
He noted that in addition to helping to alleviate the burden of a myriad of tasks associated with prescribing for patients with MS, specialty pharmacists may have an important impact on outcomes, although more data are needed.
“Having a specialty pharmacy involved in the care of our patients can help navigate the challenges associated with the process of obtaining approval for DMTs,” he said. “We know how important it is to expedite and shorten the time frame from writing the prescription to getting the person on their DMT.”
Telemedicine, other models
Although integrated specialty pharmacist services may seem out of reach for smaller MS clinics, the use of telemedicine and other models may help achieve similar results.
“A model I have seen is having pharmacists split their time between a specialty pharmacy and the MS clinic,” said Dr. Montgomery.
“A telemedicine model can also be utilized, in which a pharmacist can reach out to patients by telephone or through video visits. This would allow a pharmacist to be utilized for multiple clinics or as an MS specialist within a specialty pharmacy,” she added.
Whether provided in house or through telemedicine, a key benefit for clinicians is in freeing up valuable time, which has a domino effect in improving quality all around.
“In addition to improving safety outcomes, specialty pharmacists help with the allocation of clinic staff to other clinic responsibilities, and the utilization of services by patients results in more resources allocated for their care,” Dr. Montgomery said.
Dr. Montgomery is a nonpromotional speaker for Novartis and is on its advisory board.
A version of this article first appeared on Medscape.com.
, new data suggest.
“As DMT management and treatment options for MS symptoms become more complex, clinical pharmacists can be utilized for medication education and management,” Jenelle Hall Montgomery, PharmD, a clinical pharmacist practitioner at the Multiple Sclerosis and Neuroimmunology Division, department of neurology, Duke University Hospital, Durham, N.C., told delegates attending the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
Since 2018, more than half a dozen DMTs have been approved for MS by the U.S. Food and Drug Administration. However, there is currently no established DMT selection algorithm, and because of this, there is a need for specialty pharmacists, she added.
“DMT approvals by the FDA have outpaced MS guideline recommendations. This can be overwhelming for patients, especially now that they have so many options to choose from,” she said.
Key services provided by specialty pharmacists include coordinating pretreatment requirements, as well as help with dosing, side effects, safety monitoring, and treatment adherence. In addition, pharmacists help with switching therapies, dispensing, and cost and authorization problems.
In reporting on improvements associated with specialty pharmacists, researchers from prominent MS centers around the country described specific outcomes.
Aids early intervention
A report on the Kaiser Permanente Washington (KPWA) MS Pharmacy Program detailed significant reductions in the time to address patients’ needs through the use of specialty pharmacists. In an assessment of 391 referrals to the program from 2019 to 2020, the average total time spent per patient per year dropped from 145 minutes in 2019 to 109 minutes in 2020.
Services included assessment of medication adherence, adverse drug reaction consultation, lab monitoring, patient counseling on initiation of a DMT, shared decision making, and follow-up visits.
“The KPWA MS Pharmacy Program plays an integral role in the care of patients with MS. The MS clinical pharmacists ensure patients are well informed about their DMT options and are fully educated about selected treatment,” the investigators noted.
A report on an outpatient MS clinic at Emory Healthcare, Atlanta, described how use of specialty pharmacist services resulted in a 49% reduction in time to treatment initiation with fingolimod. The time decreased from 83.9 days to 42.9 days following the introduction of specialty pharmacist services.
“Integration of a clinical pharmacy specialist in the therapeutic management of MS patients is crucial to early intervention with disease-modifying therapy,” the investigators noted.
A report on the specialty pharmacy services provided at Johns Hopkins MS Precision Medicine Center of Excellence, Baltimore, described an evaluation of 708 assessments between July 2019 and June 2020. Results showed that the vast majority (98%) of patients reported no missed days from work or school due to MS-related symptoms and that 99.3% reported no hospitalizations due to MS relapses, which are both key measures of MS treatment adherence.
High patient satisfaction
Patients reported high satisfaction with the in-house pharmacy on the National Association of Specialty Pharmacy’s patient satisfaction survey. In the survey, the average score was 82, compared with 79 for external specialty pharmacies.
“Moreover, patients were highly satisfied with the services provided at the pharmacy and were likely to continue receiving their comprehensive pharmacy care at our institution,” the researchers reported.
The study “highlights the value of pharmacists’ involvement in patient care and supports the need for continuation of integrated clinical services in health system specialty pharmacy,” the investigators noted.
CMSC President Scott D. Newsome, DO, director of the Neurosciences Consultation and Infusion Center at Green Spring Station, Lutherville, Maryland, and associate professor of neurology at Johns Hopkins University School of Medicine, said that as a clinician, he is highly satisfied with the specialty pharmacy services for MS at Johns Hopkins.
“Our pharmacists are fantastic in communicating with the prescriber if something comes up related to medication safety or they are concerned that the patient isn’t adhering to the medication,” Dr. Newsome said.
He noted that in addition to helping to alleviate the burden of a myriad of tasks associated with prescribing for patients with MS, specialty pharmacists may have an important impact on outcomes, although more data are needed.
“Having a specialty pharmacy involved in the care of our patients can help navigate the challenges associated with the process of obtaining approval for DMTs,” he said. “We know how important it is to expedite and shorten the time frame from writing the prescription to getting the person on their DMT.”
Telemedicine, other models
Although integrated specialty pharmacist services may seem out of reach for smaller MS clinics, the use of telemedicine and other models may help achieve similar results.
“A model I have seen is having pharmacists split their time between a specialty pharmacy and the MS clinic,” said Dr. Montgomery.
“A telemedicine model can also be utilized, in which a pharmacist can reach out to patients by telephone or through video visits. This would allow a pharmacist to be utilized for multiple clinics or as an MS specialist within a specialty pharmacy,” she added.
Whether provided in house or through telemedicine, a key benefit for clinicians is in freeing up valuable time, which has a domino effect in improving quality all around.
“In addition to improving safety outcomes, specialty pharmacists help with the allocation of clinic staff to other clinic responsibilities, and the utilization of services by patients results in more resources allocated for their care,” Dr. Montgomery said.
Dr. Montgomery is a nonpromotional speaker for Novartis and is on its advisory board.
A version of this article first appeared on Medscape.com.
FROM CMSC 2021
Evaluation of the Effectiveness and Safety of Alirocumab Use in Statin-Intolerant Veterans
In 2016, 17.6 million deaths occurred globally due to cardiovascular disease (CVD) with coronary artery disease (CAD) and ischemic stroke as top contributors.1 Elevated low-density lipoprotein cholesterol (LDL-C) has been linked to greater risk of atherosclerotic cardiovascular disease (ASCVD); therefore, LDL-C reduction is imperative to decrease risk of cardiovascular (CV) morbidity and mortality.2 Since 1987, statin therapy has been the mainstay of treatment for hypercholesterolemia, and current practice guidelines recommend statins as first-line therapy given demonstrated reductions in LDL-C and CV mortality reduction in robust clinical trials.2-4 Although generally safe and well tolerated, muscle-related adverse events (AEs) limit optimal use of statins in up to 20% of individuals who have an indication for statin therapy.5 As a consequence, these patients receive suboptimal statin doses or no statin therapy and are at a higher risk for ASCVD.5
Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors have been shown to significantly lower LDL-C when used as monotherapy or in combination with statins and/or other lipid-lowering therapies.5 These agents are currently approved by the US Food and Drug Administration as an adjunct to diet with or without other lipid-lowering therapies for the management of primary hypercholesterolemia (including heterozygous familial hypercholesterolemia), homozygous familial hypercholesterolemia (evolocumab only), and for use in patients with established CVD unable to achieve their lipid-lowering goals with maximally tolerated statin doses and ezetimibe.4 With the ability to reduce LDL-C by up to 65%, PCSK9 inhibitors offer an alternative option for LDL-C and potentially CV risk reduction in statin-intolerant patients.5
Alirocumab, the formulary preferred PCSK9 inhibitor at the Michael E. DeBakey Veterans Affairs Medical Center (MEDVAMC) in Houston, Texas, has been increasingly used in high-risk statin-intolerant veterans. The primary objective of this case series was to assess LDL-C reduction associated with alirocumab use in statin-intolerant veterans at the MEDVAMC. The secondary objective was to assess the incidence of CV events. This study was approved by the MEDVAMC Quality Assurance and Regulatory Affairs committee.
Methods
In this single-center case series, a retrospective chart review was conducted to identify statin-intolerant veterans who were initiated on treatment with alirocumab for LDL-C and/or CV risk reduction between June 2017 and May 2019. Adult veterans with a diagnosis of primary hypercholesterolemia (including heterozygous familial hypercholesterolemia) and/or CAD with documented statin intolerance were included in the study. Statin intolerance was defined in accordance with the National Lipid Association (NLA) definition as aninability to tolerate ≥ 2 statins with a trial of at least 1 statin at its lowest daily dose.5 Veterans who previously received treatment with evolocumab, those prescribed concurrent statin therapies, and those missing follow-up lipid panels at 24 weeks were excluded from the study. To assess LDL-C reduction, LDL-C at baseline was compared with LDL-C at 4 and 24 weeks. Incident CV events before and after alirocumab initiation were documented. The US Department of Veteran Affairs (VA) Computerized Patient Record System was used to collect patient data.
Data Collection, Measures, and Analysis
Electronic health records of all eligible patients who received alirocumab were reviewed, and basic demographics (patient age, sex, and race/ethnicity) as well as medical characteristics at baseline were collected. To confirm statin intolerance, each veteran’s history of statin use and use of additional lipid-lowering agents was documented. CV history was measured with an index of categorical measures for hypertension, confirmed CAD, hyperlipidemia, heart failure, arrhythmias, peripheral artery disease, stroke, diabetes mellitus, and hypothyroidism. Additionally, concomitant medications, such as aspirin, P2Y12 inhibitors, β-blockers, angiotensin-converting enzyme inhibitors, and angiotensin II receptor blockers that patients were taking also were collected. Each veteran’s lipid panel at baseline, and at 4 and 24 weeks posttreatment initiation, also was extracted. Continuous variables were summarized with means (SD), and categorical variables were summarized with frequencies and proportions. The paired Wilcoxon signed rank test was used to compare LDL-C at 4 and 24 weeks after alirocumab initiation with patients’ baseline LDL-C.
Results
Between June 2017 and May 2019, 122 veterans were initiated on alirocumab. Of these veterans, 98 were excluded: 35 concurrently received statin therapy, 33 missed follow-up lipid panels, 21 had previously received evolocumab, 6 failed to meet the NLA definition for statin intolerance, 2 did not fill active alirocumab prescriptions, and 1 had an incalculable LDL-C with a baseline triglyceride level of 3079 mg/dL. This resulted in 24 veterans included in the analysis.
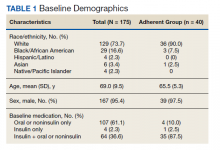
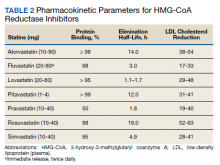
Most participants were male (87.5%) and White veterans (79.2%) with a mean (SD) age of 66.0 (8.4) years and mean (SD) baseline LDL-C of 161.9 (74.3) mg/dL. At baseline, 21 veterans had a history of primary hyperlipidemia, 19 had a history of CAD, and 2 had a history of heterozygous familial hypercholesterolemia. Of the 24 patients included, the most trialed statins before alirocumab initiation were atorvastatin (95.8%), simvastatin (79.2%), rosuvastatin (79.2%), and pravastatin (62.5%) (Table).
LDL-C Reduction
Veterans were initially treated with alirocumab 75 mg administered subcutaneously every 2 weeks; however, 11 veterans required a dose increase to 150 mg every 2 weeks. At treatment week 4, the median LDL-C reduction was 78.5 mg/dL (IQR, 28.0-107.3; P < .01), and at treatment week 24, the median LDL-C reduction was 55.6 mg/dL (IQR, 18.6-85.3; P < .01). This equated to median LDL-C reductions from baseline of 48.5% at week 4 and 34.3% at week 24. A total of 3 veterans experienced LDL-C increases following initiation of alirocumab. At week 4, 9 veterans were noted to have an LDL-C reduction > 50%, 7 veterans had an LDL-C reduction between 30% and 50%, and 5 veterans had an LDL-C reduction of < 30%. At week 24, 6 had an LDL-C reduction > 50%, 9 veterans had an LDL-C reduction between 30% and 50%, and 6 had a LDL-C reduction < 30%.
Cardiovascular Events
Before alirocumab initiation, 22 CV events and interventions were reported in 16 veterans: 12 percutaneous coronary interventions, 5 coronary artery bypass surgeries (CABG), 4 myocardial infarctions, and 1 transient ischemic attack. One month following alirocumab initiation, 1 veteran underwent a CABG after a non-ST-elevation myocardial infarction (NSTEMI).
Safety and Tolerability
Alirocumab was discontinued in 5 veterans due to 4 cases of intolerance (reported memory loss, lethargy, myalgias, and body aches with dyspnea) and 1 case of persistent LDL-C of < 40 mg/dL. Alirocumab was discontinued after 1 year in 2 patients (persistent LDL-C < 40 mg/dL and reported memory loss) and after 6 months in the veteran who reported lethargy. Alirocumab was discontinued after 4 months in the veteran with myalgias and within 2 months in the veteran with body aches and dyspnea. No other AEs were reported.
Discussion
The Efficacy and Safety of Alirocumab vs Ezetimibe in Statin-Intolerant Veterans With a Statin Rechallenge Arm trial is the first clinical trial to examine the efficacy and safety of alirocumab use in statin-intolerant patients. In the trial, 314 patients were randomized to receive alirocumab, ezetimibe, or an atorvastatin rechallenge.6 At 24 weeks, alirocumab reduced mean (SE) LDL-C by 45.0% (2.2%) vs 14.6% (2.2%) with ezetimibe (mean difference 30.4% [3.1%], P < .01).6 Fewer skeletal-muscle-related events also were noted with alirocumab vs atorvastatin (hazard ratio, 0.61; 95% CI, 0.38-0.99; P = .04).6
In this case series, an LDL-C reduction of > 50% was observed in 9 veterans (42.9%) following 4 weeks of treatment; however, LDL-C reduction of > 50% compared with baseline was sustained in only 6 veterans (28.6%) at week 24. Additionally, LDL-C increases from baseline were observed in 3 veterans; the reasoning for the observed increase was unclear, but this may have been due to nonadherence and dietary factors.4 Although a majority of patients saw a significant and clinically meaningful reduction in LDL-C, the group of patients with an increase in the same may have benefitted from targeted intervention to improve medication and dietary adherence. PCSK9 inhibitor resistance also may have contributed to an increase in LDL-C during treatment.7
Of the 24 patients included, 4 reported AEs resulted in therapy discontinuation. Memory impairment, a rare AE of alirocumab, was reported 1 year following alirocumab initiation. Additionally, lethargy was reported after 6 months of treatment. Myalgia also was reported in a veteran 4 months following treatment, and 1 veteran experienced body aches and dyspnea < 2 months following treatment. The most common AEs associated with alirocumab, as noted in previous safety and efficacy clinical trials, included: nasopharyngitis, injection site reaction, influenza, urinary tract infection, and myalgias.8 Many of these more common AEs may be subclinical and underreported. This small case series, however, detected 4 events severe enough to lead to therapy discontinuation. Although this sample is not representative of all statin-intolerant patients who receive treatment with alirocumab, our findings suggest the need for patient education on potential AEs before therapy initiation and clinician monitoring at follow-up visits.
The ODYSSEY OUTCOMES trial established a CV benefit associated with alirocumab; however, patients included had a recent acute coronary syndrome event and were receiving a high-intensity statin.9 This case series is unique in that before alirocumab initiation, 22 CV events/interventions were reported in the sample of 24 patients. After therapy initiation, 1 patient underwent a CABG after an NSTEMI in the month following initiation. This suggests that cardiac complications are possible after PCSK-9 initiation; however, little information can be gained from 1 patient. Nevertheless, early therapy failure should be investigated in the context of real-world use in statin-intolerant patients. This is a complex task, however, given the difficulties of achieving a balanced study design. Statin intolerance is a clear source of selection bias into treatment with alirocumab as patients in this population have already initiated and failed statin therapy. The prevalence of prior CV events and the time-dependent association between prior and future CV events stand as another complex confounder. Although there is a clear and pressing need to understand the risks and benefits of PCSK9 therapy in statin-intolerant patients, future research in this area will need to cautiously address these important sources of bias.
Overall, the results of this case series support LDL-C reduction associated with alirocumab in the absence of statin therapy. Despite favorable results, use of alirocumab may be limited by cost and its subcutaneous route of administration. Bempedoic acid, an oral, once-daily lipid-lowering agent poses an alternative to PCSK9 inhibitors, but further data regarding CV outcomes with this agent is needed.10,11 Robust randomized controlled trials also are needed to evaluate CV outcomes for alirocumab use in statin-intolerant veterans.
Limitations
Only 24 veterans were included in the study, reflecting 20% of the charts reviewed (80% exclusion rate), and in this small sample, only 1 CV event was observed. Both of these serve as threats to external validity. As the study information was extracted from chart review, the results may be limited by coding or historical bias. Medical information from outside institutions may be missing from medical records. Additionally, results may be skewed by possible documentation errors. Furthermore, the period between previous CV events and alirocumab initiation is unclear as event dates were often not recorded if treatment was received at an outside institution.
Due to the short follow-up period, the case series is limited in its assessment of CV outcomes and safety outcomes. Larger studies over an extended period are needed to assess CV outcomes and safety of alirocumab use in statin-intolerant patients. Also, medication adherence was not assessed. Given the impact of medication adherence on LDL-C reduction, it is unclear what role medication adherence played in the LDL-C reduction observed in this study.4
Conclusions
Alirocumab use in 24 statin-intolerant veterans resulted in a significant reduction in LDL-C at 4 and 24 weeks after initiation. In addition, 1 CV event/intervention was observed following alirocumab initiation, although this should be interpreted with caution due to the retrospective nature of this case series, small sample size, and short follow-up period. Large, long-term studies would better evaluate the CV benefit associated with alirocumab therapy in a veteran population.
1. Benjamin EJ, Munter P, Alonso A, et al; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2019 update: a report from the American Heart Association. Circulation. 2019;139(10):e56-e528. doi:10.1161/CIR.0000000000000659
2. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014 Jun 24;129(25)(suppl 2):S1-S45. doi:10.1016/j.jacc.2013.11.002
3. Hajar R. Statins: past and present. Heart Views. 2011;12(3): 121-127. doi:10.4103/1995-705X.95070
4. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;73(4):3168-3209. doi:10.1016/j.jacc.2018.11.002
5. Toth PH, Patti AM, Giglio RV, et al. Management of statin intolerance in 2018: still more questions than answers. Am J Cardiovasc Drugs. 2018;18(3):157-173. doi:10.1007/s40256-017-0259-7
6. Moriarty PM, Thompson PD, Cannon CP, et al; ODYSSEY ALTERNATIVE Investigators. Efficacy and safety of alirocumab vs ezetimibe in statin-intolerant patients, with a statin rechallenge arm: The ODYSSEY ALTERNATIVE randomized trial. J Clin Lipidol. 2015;9(6):758-769. doi:10.1016/j.jacl.2015.08.006
7. Shapiro MD, Miles J, Tavori H, Fazio S. Diagnosing resistance to a proprotein convertase subtilisin/kexin type 9 inhibitor. Ann Intern Med. 2018;168(5):376-379. doi:10.7326/M17-2485
8. Raedler LA. Praluent (alirocumab): first PCSK9 inhibitor approved by the FDA for hypercholesterolemia. Am Health Drug Benefits. 2016;9:123-126.
9. Schwartz GC, Steg PC, Szarek M, et al; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379(22):2097-2107. doi:10.1056/NEJMoa1801174
10. Nexletol. Package insert. Esperion Therapeutics Inc; 2020.
11. Laufs U, Banach M, Mancini GBJ, et al. Efficacy and safety of bempedoic acid in patients with hypercholesterolemia and statin intolerance. J Am Heart Assoc. 2019;8(7):e011662. doi:10.1161/JAHA.118.011662
In 2016, 17.6 million deaths occurred globally due to cardiovascular disease (CVD) with coronary artery disease (CAD) and ischemic stroke as top contributors.1 Elevated low-density lipoprotein cholesterol (LDL-C) has been linked to greater risk of atherosclerotic cardiovascular disease (ASCVD); therefore, LDL-C reduction is imperative to decrease risk of cardiovascular (CV) morbidity and mortality.2 Since 1987, statin therapy has been the mainstay of treatment for hypercholesterolemia, and current practice guidelines recommend statins as first-line therapy given demonstrated reductions in LDL-C and CV mortality reduction in robust clinical trials.2-4 Although generally safe and well tolerated, muscle-related adverse events (AEs) limit optimal use of statins in up to 20% of individuals who have an indication for statin therapy.5 As a consequence, these patients receive suboptimal statin doses or no statin therapy and are at a higher risk for ASCVD.5
Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors have been shown to significantly lower LDL-C when used as monotherapy or in combination with statins and/or other lipid-lowering therapies.5 These agents are currently approved by the US Food and Drug Administration as an adjunct to diet with or without other lipid-lowering therapies for the management of primary hypercholesterolemia (including heterozygous familial hypercholesterolemia), homozygous familial hypercholesterolemia (evolocumab only), and for use in patients with established CVD unable to achieve their lipid-lowering goals with maximally tolerated statin doses and ezetimibe.4 With the ability to reduce LDL-C by up to 65%, PCSK9 inhibitors offer an alternative option for LDL-C and potentially CV risk reduction in statin-intolerant patients.5
Alirocumab, the formulary preferred PCSK9 inhibitor at the Michael E. DeBakey Veterans Affairs Medical Center (MEDVAMC) in Houston, Texas, has been increasingly used in high-risk statin-intolerant veterans. The primary objective of this case series was to assess LDL-C reduction associated with alirocumab use in statin-intolerant veterans at the MEDVAMC. The secondary objective was to assess the incidence of CV events. This study was approved by the MEDVAMC Quality Assurance and Regulatory Affairs committee.
Methods
In this single-center case series, a retrospective chart review was conducted to identify statin-intolerant veterans who were initiated on treatment with alirocumab for LDL-C and/or CV risk reduction between June 2017 and May 2019. Adult veterans with a diagnosis of primary hypercholesterolemia (including heterozygous familial hypercholesterolemia) and/or CAD with documented statin intolerance were included in the study. Statin intolerance was defined in accordance with the National Lipid Association (NLA) definition as aninability to tolerate ≥ 2 statins with a trial of at least 1 statin at its lowest daily dose.5 Veterans who previously received treatment with evolocumab, those prescribed concurrent statin therapies, and those missing follow-up lipid panels at 24 weeks were excluded from the study. To assess LDL-C reduction, LDL-C at baseline was compared with LDL-C at 4 and 24 weeks. Incident CV events before and after alirocumab initiation were documented. The US Department of Veteran Affairs (VA) Computerized Patient Record System was used to collect patient data.
Data Collection, Measures, and Analysis
Electronic health records of all eligible patients who received alirocumab were reviewed, and basic demographics (patient age, sex, and race/ethnicity) as well as medical characteristics at baseline were collected. To confirm statin intolerance, each veteran’s history of statin use and use of additional lipid-lowering agents was documented. CV history was measured with an index of categorical measures for hypertension, confirmed CAD, hyperlipidemia, heart failure, arrhythmias, peripheral artery disease, stroke, diabetes mellitus, and hypothyroidism. Additionally, concomitant medications, such as aspirin, P2Y12 inhibitors, β-blockers, angiotensin-converting enzyme inhibitors, and angiotensin II receptor blockers that patients were taking also were collected. Each veteran’s lipid panel at baseline, and at 4 and 24 weeks posttreatment initiation, also was extracted. Continuous variables were summarized with means (SD), and categorical variables were summarized with frequencies and proportions. The paired Wilcoxon signed rank test was used to compare LDL-C at 4 and 24 weeks after alirocumab initiation with patients’ baseline LDL-C.
Results
Between June 2017 and May 2019, 122 veterans were initiated on alirocumab. Of these veterans, 98 were excluded: 35 concurrently received statin therapy, 33 missed follow-up lipid panels, 21 had previously received evolocumab, 6 failed to meet the NLA definition for statin intolerance, 2 did not fill active alirocumab prescriptions, and 1 had an incalculable LDL-C with a baseline triglyceride level of 3079 mg/dL. This resulted in 24 veterans included in the analysis.
Most participants were male (87.5%) and White veterans (79.2%) with a mean (SD) age of 66.0 (8.4) years and mean (SD) baseline LDL-C of 161.9 (74.3) mg/dL. At baseline, 21 veterans had a history of primary hyperlipidemia, 19 had a history of CAD, and 2 had a history of heterozygous familial hypercholesterolemia. Of the 24 patients included, the most trialed statins before alirocumab initiation were atorvastatin (95.8%), simvastatin (79.2%), rosuvastatin (79.2%), and pravastatin (62.5%) (Table).
LDL-C Reduction
Veterans were initially treated with alirocumab 75 mg administered subcutaneously every 2 weeks; however, 11 veterans required a dose increase to 150 mg every 2 weeks. At treatment week 4, the median LDL-C reduction was 78.5 mg/dL (IQR, 28.0-107.3; P < .01), and at treatment week 24, the median LDL-C reduction was 55.6 mg/dL (IQR, 18.6-85.3; P < .01). This equated to median LDL-C reductions from baseline of 48.5% at week 4 and 34.3% at week 24. A total of 3 veterans experienced LDL-C increases following initiation of alirocumab. At week 4, 9 veterans were noted to have an LDL-C reduction > 50%, 7 veterans had an LDL-C reduction between 30% and 50%, and 5 veterans had an LDL-C reduction of < 30%. At week 24, 6 had an LDL-C reduction > 50%, 9 veterans had an LDL-C reduction between 30% and 50%, and 6 had a LDL-C reduction < 30%.
Cardiovascular Events
Before alirocumab initiation, 22 CV events and interventions were reported in 16 veterans: 12 percutaneous coronary interventions, 5 coronary artery bypass surgeries (CABG), 4 myocardial infarctions, and 1 transient ischemic attack. One month following alirocumab initiation, 1 veteran underwent a CABG after a non-ST-elevation myocardial infarction (NSTEMI).
Safety and Tolerability
Alirocumab was discontinued in 5 veterans due to 4 cases of intolerance (reported memory loss, lethargy, myalgias, and body aches with dyspnea) and 1 case of persistent LDL-C of < 40 mg/dL. Alirocumab was discontinued after 1 year in 2 patients (persistent LDL-C < 40 mg/dL and reported memory loss) and after 6 months in the veteran who reported lethargy. Alirocumab was discontinued after 4 months in the veteran with myalgias and within 2 months in the veteran with body aches and dyspnea. No other AEs were reported.
Discussion
The Efficacy and Safety of Alirocumab vs Ezetimibe in Statin-Intolerant Veterans With a Statin Rechallenge Arm trial is the first clinical trial to examine the efficacy and safety of alirocumab use in statin-intolerant patients. In the trial, 314 patients were randomized to receive alirocumab, ezetimibe, or an atorvastatin rechallenge.6 At 24 weeks, alirocumab reduced mean (SE) LDL-C by 45.0% (2.2%) vs 14.6% (2.2%) with ezetimibe (mean difference 30.4% [3.1%], P < .01).6 Fewer skeletal-muscle-related events also were noted with alirocumab vs atorvastatin (hazard ratio, 0.61; 95% CI, 0.38-0.99; P = .04).6
In this case series, an LDL-C reduction of > 50% was observed in 9 veterans (42.9%) following 4 weeks of treatment; however, LDL-C reduction of > 50% compared with baseline was sustained in only 6 veterans (28.6%) at week 24. Additionally, LDL-C increases from baseline were observed in 3 veterans; the reasoning for the observed increase was unclear, but this may have been due to nonadherence and dietary factors.4 Although a majority of patients saw a significant and clinically meaningful reduction in LDL-C, the group of patients with an increase in the same may have benefitted from targeted intervention to improve medication and dietary adherence. PCSK9 inhibitor resistance also may have contributed to an increase in LDL-C during treatment.7
Of the 24 patients included, 4 reported AEs resulted in therapy discontinuation. Memory impairment, a rare AE of alirocumab, was reported 1 year following alirocumab initiation. Additionally, lethargy was reported after 6 months of treatment. Myalgia also was reported in a veteran 4 months following treatment, and 1 veteran experienced body aches and dyspnea < 2 months following treatment. The most common AEs associated with alirocumab, as noted in previous safety and efficacy clinical trials, included: nasopharyngitis, injection site reaction, influenza, urinary tract infection, and myalgias.8 Many of these more common AEs may be subclinical and underreported. This small case series, however, detected 4 events severe enough to lead to therapy discontinuation. Although this sample is not representative of all statin-intolerant patients who receive treatment with alirocumab, our findings suggest the need for patient education on potential AEs before therapy initiation and clinician monitoring at follow-up visits.
The ODYSSEY OUTCOMES trial established a CV benefit associated with alirocumab; however, patients included had a recent acute coronary syndrome event and were receiving a high-intensity statin.9 This case series is unique in that before alirocumab initiation, 22 CV events/interventions were reported in the sample of 24 patients. After therapy initiation, 1 patient underwent a CABG after an NSTEMI in the month following initiation. This suggests that cardiac complications are possible after PCSK-9 initiation; however, little information can be gained from 1 patient. Nevertheless, early therapy failure should be investigated in the context of real-world use in statin-intolerant patients. This is a complex task, however, given the difficulties of achieving a balanced study design. Statin intolerance is a clear source of selection bias into treatment with alirocumab as patients in this population have already initiated and failed statin therapy. The prevalence of prior CV events and the time-dependent association between prior and future CV events stand as another complex confounder. Although there is a clear and pressing need to understand the risks and benefits of PCSK9 therapy in statin-intolerant patients, future research in this area will need to cautiously address these important sources of bias.
Overall, the results of this case series support LDL-C reduction associated with alirocumab in the absence of statin therapy. Despite favorable results, use of alirocumab may be limited by cost and its subcutaneous route of administration. Bempedoic acid, an oral, once-daily lipid-lowering agent poses an alternative to PCSK9 inhibitors, but further data regarding CV outcomes with this agent is needed.10,11 Robust randomized controlled trials also are needed to evaluate CV outcomes for alirocumab use in statin-intolerant veterans.
Limitations
Only 24 veterans were included in the study, reflecting 20% of the charts reviewed (80% exclusion rate), and in this small sample, only 1 CV event was observed. Both of these serve as threats to external validity. As the study information was extracted from chart review, the results may be limited by coding or historical bias. Medical information from outside institutions may be missing from medical records. Additionally, results may be skewed by possible documentation errors. Furthermore, the period between previous CV events and alirocumab initiation is unclear as event dates were often not recorded if treatment was received at an outside institution.
Due to the short follow-up period, the case series is limited in its assessment of CV outcomes and safety outcomes. Larger studies over an extended period are needed to assess CV outcomes and safety of alirocumab use in statin-intolerant patients. Also, medication adherence was not assessed. Given the impact of medication adherence on LDL-C reduction, it is unclear what role medication adherence played in the LDL-C reduction observed in this study.4
Conclusions
Alirocumab use in 24 statin-intolerant veterans resulted in a significant reduction in LDL-C at 4 and 24 weeks after initiation. In addition, 1 CV event/intervention was observed following alirocumab initiation, although this should be interpreted with caution due to the retrospective nature of this case series, small sample size, and short follow-up period. Large, long-term studies would better evaluate the CV benefit associated with alirocumab therapy in a veteran population.
In 2016, 17.6 million deaths occurred globally due to cardiovascular disease (CVD) with coronary artery disease (CAD) and ischemic stroke as top contributors.1 Elevated low-density lipoprotein cholesterol (LDL-C) has been linked to greater risk of atherosclerotic cardiovascular disease (ASCVD); therefore, LDL-C reduction is imperative to decrease risk of cardiovascular (CV) morbidity and mortality.2 Since 1987, statin therapy has been the mainstay of treatment for hypercholesterolemia, and current practice guidelines recommend statins as first-line therapy given demonstrated reductions in LDL-C and CV mortality reduction in robust clinical trials.2-4 Although generally safe and well tolerated, muscle-related adverse events (AEs) limit optimal use of statins in up to 20% of individuals who have an indication for statin therapy.5 As a consequence, these patients receive suboptimal statin doses or no statin therapy and are at a higher risk for ASCVD.5
Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors have been shown to significantly lower LDL-C when used as monotherapy or in combination with statins and/or other lipid-lowering therapies.5 These agents are currently approved by the US Food and Drug Administration as an adjunct to diet with or without other lipid-lowering therapies for the management of primary hypercholesterolemia (including heterozygous familial hypercholesterolemia), homozygous familial hypercholesterolemia (evolocumab only), and for use in patients with established CVD unable to achieve their lipid-lowering goals with maximally tolerated statin doses and ezetimibe.4 With the ability to reduce LDL-C by up to 65%, PCSK9 inhibitors offer an alternative option for LDL-C and potentially CV risk reduction in statin-intolerant patients.5
Alirocumab, the formulary preferred PCSK9 inhibitor at the Michael E. DeBakey Veterans Affairs Medical Center (MEDVAMC) in Houston, Texas, has been increasingly used in high-risk statin-intolerant veterans. The primary objective of this case series was to assess LDL-C reduction associated with alirocumab use in statin-intolerant veterans at the MEDVAMC. The secondary objective was to assess the incidence of CV events. This study was approved by the MEDVAMC Quality Assurance and Regulatory Affairs committee.
Methods
In this single-center case series, a retrospective chart review was conducted to identify statin-intolerant veterans who were initiated on treatment with alirocumab for LDL-C and/or CV risk reduction between June 2017 and May 2019. Adult veterans with a diagnosis of primary hypercholesterolemia (including heterozygous familial hypercholesterolemia) and/or CAD with documented statin intolerance were included in the study. Statin intolerance was defined in accordance with the National Lipid Association (NLA) definition as aninability to tolerate ≥ 2 statins with a trial of at least 1 statin at its lowest daily dose.5 Veterans who previously received treatment with evolocumab, those prescribed concurrent statin therapies, and those missing follow-up lipid panels at 24 weeks were excluded from the study. To assess LDL-C reduction, LDL-C at baseline was compared with LDL-C at 4 and 24 weeks. Incident CV events before and after alirocumab initiation were documented. The US Department of Veteran Affairs (VA) Computerized Patient Record System was used to collect patient data.
Data Collection, Measures, and Analysis
Electronic health records of all eligible patients who received alirocumab were reviewed, and basic demographics (patient age, sex, and race/ethnicity) as well as medical characteristics at baseline were collected. To confirm statin intolerance, each veteran’s history of statin use and use of additional lipid-lowering agents was documented. CV history was measured with an index of categorical measures for hypertension, confirmed CAD, hyperlipidemia, heart failure, arrhythmias, peripheral artery disease, stroke, diabetes mellitus, and hypothyroidism. Additionally, concomitant medications, such as aspirin, P2Y12 inhibitors, β-blockers, angiotensin-converting enzyme inhibitors, and angiotensin II receptor blockers that patients were taking also were collected. Each veteran’s lipid panel at baseline, and at 4 and 24 weeks posttreatment initiation, also was extracted. Continuous variables were summarized with means (SD), and categorical variables were summarized with frequencies and proportions. The paired Wilcoxon signed rank test was used to compare LDL-C at 4 and 24 weeks after alirocumab initiation with patients’ baseline LDL-C.
Results
Between June 2017 and May 2019, 122 veterans were initiated on alirocumab. Of these veterans, 98 were excluded: 35 concurrently received statin therapy, 33 missed follow-up lipid panels, 21 had previously received evolocumab, 6 failed to meet the NLA definition for statin intolerance, 2 did not fill active alirocumab prescriptions, and 1 had an incalculable LDL-C with a baseline triglyceride level of 3079 mg/dL. This resulted in 24 veterans included in the analysis.
Most participants were male (87.5%) and White veterans (79.2%) with a mean (SD) age of 66.0 (8.4) years and mean (SD) baseline LDL-C of 161.9 (74.3) mg/dL. At baseline, 21 veterans had a history of primary hyperlipidemia, 19 had a history of CAD, and 2 had a history of heterozygous familial hypercholesterolemia. Of the 24 patients included, the most trialed statins before alirocumab initiation were atorvastatin (95.8%), simvastatin (79.2%), rosuvastatin (79.2%), and pravastatin (62.5%) (Table).
LDL-C Reduction
Veterans were initially treated with alirocumab 75 mg administered subcutaneously every 2 weeks; however, 11 veterans required a dose increase to 150 mg every 2 weeks. At treatment week 4, the median LDL-C reduction was 78.5 mg/dL (IQR, 28.0-107.3; P < .01), and at treatment week 24, the median LDL-C reduction was 55.6 mg/dL (IQR, 18.6-85.3; P < .01). This equated to median LDL-C reductions from baseline of 48.5% at week 4 and 34.3% at week 24. A total of 3 veterans experienced LDL-C increases following initiation of alirocumab. At week 4, 9 veterans were noted to have an LDL-C reduction > 50%, 7 veterans had an LDL-C reduction between 30% and 50%, and 5 veterans had an LDL-C reduction of < 30%. At week 24, 6 had an LDL-C reduction > 50%, 9 veterans had an LDL-C reduction between 30% and 50%, and 6 had a LDL-C reduction < 30%.
Cardiovascular Events
Before alirocumab initiation, 22 CV events and interventions were reported in 16 veterans: 12 percutaneous coronary interventions, 5 coronary artery bypass surgeries (CABG), 4 myocardial infarctions, and 1 transient ischemic attack. One month following alirocumab initiation, 1 veteran underwent a CABG after a non-ST-elevation myocardial infarction (NSTEMI).
Safety and Tolerability
Alirocumab was discontinued in 5 veterans due to 4 cases of intolerance (reported memory loss, lethargy, myalgias, and body aches with dyspnea) and 1 case of persistent LDL-C of < 40 mg/dL. Alirocumab was discontinued after 1 year in 2 patients (persistent LDL-C < 40 mg/dL and reported memory loss) and after 6 months in the veteran who reported lethargy. Alirocumab was discontinued after 4 months in the veteran with myalgias and within 2 months in the veteran with body aches and dyspnea. No other AEs were reported.
Discussion
The Efficacy and Safety of Alirocumab vs Ezetimibe in Statin-Intolerant Veterans With a Statin Rechallenge Arm trial is the first clinical trial to examine the efficacy and safety of alirocumab use in statin-intolerant patients. In the trial, 314 patients were randomized to receive alirocumab, ezetimibe, or an atorvastatin rechallenge.6 At 24 weeks, alirocumab reduced mean (SE) LDL-C by 45.0% (2.2%) vs 14.6% (2.2%) with ezetimibe (mean difference 30.4% [3.1%], P < .01).6 Fewer skeletal-muscle-related events also were noted with alirocumab vs atorvastatin (hazard ratio, 0.61; 95% CI, 0.38-0.99; P = .04).6
In this case series, an LDL-C reduction of > 50% was observed in 9 veterans (42.9%) following 4 weeks of treatment; however, LDL-C reduction of > 50% compared with baseline was sustained in only 6 veterans (28.6%) at week 24. Additionally, LDL-C increases from baseline were observed in 3 veterans; the reasoning for the observed increase was unclear, but this may have been due to nonadherence and dietary factors.4 Although a majority of patients saw a significant and clinically meaningful reduction in LDL-C, the group of patients with an increase in the same may have benefitted from targeted intervention to improve medication and dietary adherence. PCSK9 inhibitor resistance also may have contributed to an increase in LDL-C during treatment.7
Of the 24 patients included, 4 reported AEs resulted in therapy discontinuation. Memory impairment, a rare AE of alirocumab, was reported 1 year following alirocumab initiation. Additionally, lethargy was reported after 6 months of treatment. Myalgia also was reported in a veteran 4 months following treatment, and 1 veteran experienced body aches and dyspnea < 2 months following treatment. The most common AEs associated with alirocumab, as noted in previous safety and efficacy clinical trials, included: nasopharyngitis, injection site reaction, influenza, urinary tract infection, and myalgias.8 Many of these more common AEs may be subclinical and underreported. This small case series, however, detected 4 events severe enough to lead to therapy discontinuation. Although this sample is not representative of all statin-intolerant patients who receive treatment with alirocumab, our findings suggest the need for patient education on potential AEs before therapy initiation and clinician monitoring at follow-up visits.
The ODYSSEY OUTCOMES trial established a CV benefit associated with alirocumab; however, patients included had a recent acute coronary syndrome event and were receiving a high-intensity statin.9 This case series is unique in that before alirocumab initiation, 22 CV events/interventions were reported in the sample of 24 patients. After therapy initiation, 1 patient underwent a CABG after an NSTEMI in the month following initiation. This suggests that cardiac complications are possible after PCSK-9 initiation; however, little information can be gained from 1 patient. Nevertheless, early therapy failure should be investigated in the context of real-world use in statin-intolerant patients. This is a complex task, however, given the difficulties of achieving a balanced study design. Statin intolerance is a clear source of selection bias into treatment with alirocumab as patients in this population have already initiated and failed statin therapy. The prevalence of prior CV events and the time-dependent association between prior and future CV events stand as another complex confounder. Although there is a clear and pressing need to understand the risks and benefits of PCSK9 therapy in statin-intolerant patients, future research in this area will need to cautiously address these important sources of bias.
Overall, the results of this case series support LDL-C reduction associated with alirocumab in the absence of statin therapy. Despite favorable results, use of alirocumab may be limited by cost and its subcutaneous route of administration. Bempedoic acid, an oral, once-daily lipid-lowering agent poses an alternative to PCSK9 inhibitors, but further data regarding CV outcomes with this agent is needed.10,11 Robust randomized controlled trials also are needed to evaluate CV outcomes for alirocumab use in statin-intolerant veterans.
Limitations
Only 24 veterans were included in the study, reflecting 20% of the charts reviewed (80% exclusion rate), and in this small sample, only 1 CV event was observed. Both of these serve as threats to external validity. As the study information was extracted from chart review, the results may be limited by coding or historical bias. Medical information from outside institutions may be missing from medical records. Additionally, results may be skewed by possible documentation errors. Furthermore, the period between previous CV events and alirocumab initiation is unclear as event dates were often not recorded if treatment was received at an outside institution.
Due to the short follow-up period, the case series is limited in its assessment of CV outcomes and safety outcomes. Larger studies over an extended period are needed to assess CV outcomes and safety of alirocumab use in statin-intolerant patients. Also, medication adherence was not assessed. Given the impact of medication adherence on LDL-C reduction, it is unclear what role medication adherence played in the LDL-C reduction observed in this study.4
Conclusions
Alirocumab use in 24 statin-intolerant veterans resulted in a significant reduction in LDL-C at 4 and 24 weeks after initiation. In addition, 1 CV event/intervention was observed following alirocumab initiation, although this should be interpreted with caution due to the retrospective nature of this case series, small sample size, and short follow-up period. Large, long-term studies would better evaluate the CV benefit associated with alirocumab therapy in a veteran population.
1. Benjamin EJ, Munter P, Alonso A, et al; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2019 update: a report from the American Heart Association. Circulation. 2019;139(10):e56-e528. doi:10.1161/CIR.0000000000000659
2. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014 Jun 24;129(25)(suppl 2):S1-S45. doi:10.1016/j.jacc.2013.11.002
3. Hajar R. Statins: past and present. Heart Views. 2011;12(3): 121-127. doi:10.4103/1995-705X.95070
4. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;73(4):3168-3209. doi:10.1016/j.jacc.2018.11.002
5. Toth PH, Patti AM, Giglio RV, et al. Management of statin intolerance in 2018: still more questions than answers. Am J Cardiovasc Drugs. 2018;18(3):157-173. doi:10.1007/s40256-017-0259-7
6. Moriarty PM, Thompson PD, Cannon CP, et al; ODYSSEY ALTERNATIVE Investigators. Efficacy and safety of alirocumab vs ezetimibe in statin-intolerant patients, with a statin rechallenge arm: The ODYSSEY ALTERNATIVE randomized trial. J Clin Lipidol. 2015;9(6):758-769. doi:10.1016/j.jacl.2015.08.006
7. Shapiro MD, Miles J, Tavori H, Fazio S. Diagnosing resistance to a proprotein convertase subtilisin/kexin type 9 inhibitor. Ann Intern Med. 2018;168(5):376-379. doi:10.7326/M17-2485
8. Raedler LA. Praluent (alirocumab): first PCSK9 inhibitor approved by the FDA for hypercholesterolemia. Am Health Drug Benefits. 2016;9:123-126.
9. Schwartz GC, Steg PC, Szarek M, et al; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379(22):2097-2107. doi:10.1056/NEJMoa1801174
10. Nexletol. Package insert. Esperion Therapeutics Inc; 2020.
11. Laufs U, Banach M, Mancini GBJ, et al. Efficacy and safety of bempedoic acid in patients with hypercholesterolemia and statin intolerance. J Am Heart Assoc. 2019;8(7):e011662. doi:10.1161/JAHA.118.011662
1. Benjamin EJ, Munter P, Alonso A, et al; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2019 update: a report from the American Heart Association. Circulation. 2019;139(10):e56-e528. doi:10.1161/CIR.0000000000000659
2. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014 Jun 24;129(25)(suppl 2):S1-S45. doi:10.1016/j.jacc.2013.11.002
3. Hajar R. Statins: past and present. Heart Views. 2011;12(3): 121-127. doi:10.4103/1995-705X.95070
4. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;73(4):3168-3209. doi:10.1016/j.jacc.2018.11.002
5. Toth PH, Patti AM, Giglio RV, et al. Management of statin intolerance in 2018: still more questions than answers. Am J Cardiovasc Drugs. 2018;18(3):157-173. doi:10.1007/s40256-017-0259-7
6. Moriarty PM, Thompson PD, Cannon CP, et al; ODYSSEY ALTERNATIVE Investigators. Efficacy and safety of alirocumab vs ezetimibe in statin-intolerant patients, with a statin rechallenge arm: The ODYSSEY ALTERNATIVE randomized trial. J Clin Lipidol. 2015;9(6):758-769. doi:10.1016/j.jacl.2015.08.006
7. Shapiro MD, Miles J, Tavori H, Fazio S. Diagnosing resistance to a proprotein convertase subtilisin/kexin type 9 inhibitor. Ann Intern Med. 2018;168(5):376-379. doi:10.7326/M17-2485
8. Raedler LA. Praluent (alirocumab): first PCSK9 inhibitor approved by the FDA for hypercholesterolemia. Am Health Drug Benefits. 2016;9:123-126.
9. Schwartz GC, Steg PC, Szarek M, et al; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379(22):2097-2107. doi:10.1056/NEJMoa1801174
10. Nexletol. Package insert. Esperion Therapeutics Inc; 2020.
11. Laufs U, Banach M, Mancini GBJ, et al. Efficacy and safety of bempedoic acid in patients with hypercholesterolemia and statin intolerance. J Am Heart Assoc. 2019;8(7):e011662. doi:10.1161/JAHA.118.011662
Continuous Blood Glucose Monitoring Outcomes in Veterans With Type 2 Diabetes
Nearly 25% of patients served in the US Department of Veterans Affairs (VA) have been diagnosed with type 2 diabetes mellitus (T2DM), although the prevalence among adults in the United States is 9%.1 Patients with DM typically monitor their blood glucose using intermittent fingerstick self-testing. Continuous glucose monitoring (CGM) might offer a more comprehensive picture of glucose control to improve disease management. Within the VA, criteria for CGM use varies among facilities, but generally veterans prescribed at least 3 daily insulin injections and 4 daily blood glucose checks qualify.2
CGM therapy has been extensively researched for type 1 DM (T1DM); however, outcomes of CGM use among older adults with T2DM have not been fully evaluated. In a 2018 review of randomized clinical trials evaluating CGM use, 17 trials examined only patients with T1DM (2009 participants), 4 included only patients with T2DM patients (547 patients), 3 evaluated patients with T1DM or T2DM (655 patients), and 3 included women with gestational diabetes (585 patients).3 Of 27 studies that included change in hemoglobin A1c (HbA1c) as an endpoint, 15 found a statistically significant reduction in HbA1c for the CGM group. Four trials evaluated CGM use in adults with T2DM and 3 found no difference in HbA1c overall. However, 1 study found a difference in HbA1c only in individuals aged < 65 years, and another study found a greater improvement in the CGM group (approximately 0.5%).4,5 These mixed results indicate a need for further subgroup analysis in specific populations to determine the optimal use of CGM in adults with T2DM. Although this study was not designed to measure changes in hypoglycemic episodes or the relative efficacy of different CGM products, it establishes a baseline from which to conduct additional research.
Our primary objective was to determine change in HbA1c in each patient from the year before CGM initiation to the year after. Secondary objectives included changes in blood pressure (BP), weight, and diabetes-related hospital and clinic visits during the same time frame. We also completed subanalysis comparing primary outcomes in engaged or adherent patients compared with the entire study group. This study was completed as a quality improvement project with approval from the Lexington Veterans Affairs Health Care System in Kentucky information security office and was exempted from institutional review board review.
Methods
This project was a retrospective evaluation using the VA database of patient records. Rather than using a control group, our study used a pre–post model to determine the impact of CGM for each patient. For the primary outcome, average HbA1c values were calculated for the year before and year after CGM initiation. Hemoglobin and hematocrit values were included if reported within 3 months of the HbA1c values to ensure validity of HbA1c results. Average HbA1c was 13.37 g/dL (range, 10.5-17.3), and average hematocrit was 43.3% (range, 36-52). Change in average HbA1c was recorded for each patient. Based on research by Taylor and colleagues, a change in HbA1c of 0.8% was considered clinically significant for this project.6
Mean BP and weight were calculated for the years before and after CGM initiation. Only values for routine clinic visits were included; values taken during an acute health incident, inpatient stay, infusion clinic appointments, or home readings were excluded. Changes were recorded for each patient. Patient encounter notes were used to determine the number of DM-related hospital, emergency department, and clinic visits, such as nephrology, podiatry, vascular medicine, or infectious disease clinic or inpatient encounters during the study period. Routine endocrinology or primary care visits were not included, and patient care notes were consulted to ensure that the encounters were related to a DM complication. The change in number of visits was calculated for each patient.
Adherence was defined as patients receiving active medication management, documented treatment regimen adherence, and > 4 annual endocrinology clinic visits. Active medication management was defined as having > 1 dosage or medication change for oral or noninsulin antihyperglycemics, initiation, or adjustment of insulin dosages according to the patient records. Treatment adherence was determined based on medication reconciliation notes and refill request history. Only endocrinology clinic visits at VA outpatient clinics were included.
Study Population
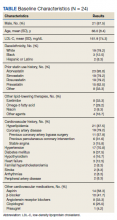
A sample of 166 patients was needed to detect an HbA1c change of 0.8 per power analysis. The normal approximation method using the z statistic was used, with 2-tailed α = 0.05, β = 0.05, E = 0.8, and S = 1.2. We randomly selected 175 patients among all individuals with an active prescription for CGM in 2018 and 2019, who had a diagnosis of T2DM, and were managed by VA endocrinology clinics (including endocrine clinics, diabetes clinics, and patient aligned care team clinics) at 87 VA medical centers. Patients with types of DM other than T2DM were excluded, as well as those with a diagnosed hemoglobinopathy or HbA1c < 10 g/dL. The adherent subgroup included 40 patients of the 175 sample population (Table 1).
Results
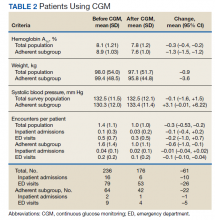
Both the total population and the adherent subgroup showed reduction in HbA1c, the primary endpoint. The complete population showed a HbA1c change of –0.3 (95% CI, –0.4 to –0.2), and the adherent subgroup had a change of –1.3 (95% CI, –1.5 to –1.2). The total survey population had a mean change in weight of –1.9 lb (–0.9 kg) (95% CI, –3.7 to –0.1) and the adherent subgroup had an average change of –8.0 lb (–3.6 kg) (95% CI, –12.3 to –3.8). Average systolic BP changes were –0.1 mm Hg (95% CI, –1.6 to 1.5) in the total population and +3.3 mm Hg (95% CI, –0.01 to 6.22) in the adherent subgroup. A decrease in total encounters for DM complications was observed in the population (–0.3 total encounters per patient, 95% CI, –0.5 to –0.2) and the adherent subgroup (–0.6 total encounters per patient, 95% CI, –1.0 to –0.1) (Table 2).
Before the study, 107 (61.1%) patients were taking oral or noninsulin DM medication only, 4 (2.3%) were on insulin only, and 64 (36.6%) were prescribed both insulin and oral/noninsulin antihyperglycemics. Noninsulin and oral antihyperglycemic regimens included combinations of biguanide, dipeptidyl peptidase- 4 inhibitor, sodium-glucose cotransporter-2 inhibitor, sulfonylurea, meglitinide, β-glucosidase inhibitor, glucagon-like peptide-1 (GLP-1) analog, and thiazolidinedione drug classes. Nearly 70% (122) had no reported changes in DM treatment beyond dosage titrations. Among these patients, 18 (10.3%) were on an insulin pump for the duration of the study. Among the 53 (30.3%) patients who had changes in treatment, 31 (17.7%) transitioned from insulin injections to an insulin pump, 13 (7.4%) changed from 1 insulin injection to another (ie, addition of long-acting insulin, transition to u500 insulin, changing from 1 insulin category or brand to another), 8 (4.6%) began an oral/noninsulin antihyperglycemic, 4 (2.3%) began insulin injections, 13 (7.4%) discontinued noninsulin or oral antihyperglycemics, and 2 (1.1%) discontinued insulin during the study period.
Data showed that 113 (64.5%) patients had no changes in antihypertensives. The remaining 62 (35.4%) had the following adjustments: 14 (8%) increased dose of current medication(s), 9 (5.1%) decreased dose of current medication(s), 8 (4.6%) discontinued all antihypertensive medications, 10 (5.7%) switched to a different antihypertensive class, and 16 (9.1%) added additional antihypertensive medication(s) to their existing regimen during the study period.
Patients in the study group used 7 different types of CGM sensors. Chart review revealed that 84 (47.7%) patients used Medtronic devices, with 26 (14.8%) using first-generation Guardian sensors, 50 (28.4%) using Enlite sensors, and 8 (4.5) using Guardian 3 sensors. We found that 81 (46.0%) veterans were prescribed Dexcom devices, with 5 (2.8%) using SEVEN PLUS sensors, 68 (38.6%) using G4-5 sensors, and 8 (4.5%) using G6 sensors. The remaining 10 (5.7%) patients were using Freestyle Libre sensors during the study period.
Discussion
CGM did not correspond with clinically significant reductions in HbA1c. However, veterans with increased health care engagement were likely to achieve clinically significant HbA1c improvements. The veterans in the adherent subgroup had a higher baseline HbA1c, which could be because of a variety of factors mentioned in patient care notes, including insulin resistance, poor dietary habits, and exercise regimen nonadherence. These patients might have had more room to improve their glycemic control without concern of hypoglycemia, and their higher baseline HbA1c could have provided increased motivation for improving their health during the study period.
Adherent patients also had a greater reduction in weight and hospital or clinic visits with CGM compared with the total population. These veterans’ increased involvement in their health care might have led to better dietary and exercise adherence, which would have decreased insulin dosing and contributed to weight loss. Only 1 patient in the adherent subgroup initiated a GLP-1 agonist during the study period, making it unlikely that medication changes had a significant impact on weight loss in the subgroup analysis. This improvement in overall health status might have contributed to the reduction in hospital or clinic visits observed in this population.
Average systolic BP data decreased minimally in the total survey population and increased in the adherent subgroup over the course of the study. These results were determined to be statistically significant. Changes in systolic BP readings were minimal, indicating that it is unlikely that these changes contributed meaningfully to the patients’ overall health status.
Although not related to the study objectives, the adherent population required less antihypertensive adjustments with similar BP control. This could be explained by improved overall health or better adherence and engagement in therapy. The results of this project show that despite limited medication changes, T2DM management improved among adherent patients using CGM. The general study population, which was more likely to have documented nonadherence with treatment or clinic appointments, had minimal benefit. CGM technology in the T2DM veteran population is more likely to have significant clinical benefit in patients who are adherent with their medication regimens and follow-up appointments compared with the larger study population.
The results of this study are in line with previous studies on CGM use in the T2DM patient population. We agree with the previously published research that CGM alone does not have a meaningful impact on HbA1c reduction. Our study population also was older than those in previous studies, adding to the Haak and colleagues conclusion that patients aged < 65 years might have better outcomes with CGM.4
Strengths of this study include specificity to the veteran population using VA resources, as well as including nondiabetes outcomes. This allows for specific application to the veteran population and could provide broader evidence for CGM use. Demonstrated decreases in HbA1c, weight, and clinic visits in the adherent population suggest that providing veterans with CGM therapy with frequent endocrinology follow-up improves health outcomes and could decrease overall health spending.
Limitations
Limitations of this study include retrospective design, a small sample size, and solely focusing on T2DM. As a retrospective study, we cannot rule out the influence of outside factors, such as participation in a non-VA weight loss program. This study lacked the power to assess the impact of the different CGM brands. The study did not include data on severe hypoglycemic or hyperglycemic episodes as veterans might have needed emergent care at non-VA facilities. Future research will evaluate the impact of CGM on symptomatic and severe hypoglycemic episodes and use of insulin vs oral or noninsulin antihyperglycemics and the comparative efficacy of different CGM brands among veterans.
Conclusions
CGM did not correspond with clinically significant reductions in HbA1c. However, veterans with increased health care engagement were likely to achieve clinically significant HbA1c improvements. Adherent patients also had more reduction in weight and hospital or clinic visits with CGM compared with the total population. These veterans’ increased involvement in their health care might have led to better dietary and exercise adherence, which would have decreased insulin dosing and contributed to weight loss.
1. Liu Y, Sayam S, Shao X, et al. Prevalence of and trends in diabetes among veterans, United States, 2005-2014. Prev Chronic Dis. 2017;14:E135. Published 2017 Dec 14. doi:10.5888/pcd14.170230
2. Hackett M. VA pharmacies now carry the Dexcom G6 CGM at no cost for qualifying patients. September 23, 2020. Accessed September 28, 2021. https://www.mobihealthnews.com/news/va-pharmacies-now-carry-dexcom-g6-cgm-no-cost-qualifying-patients
3. Peters AL. The evidence base for continuous glucose monitoring. In: Role of Continuous Glucose Monitoring in Diabetes Treatment. Arlington (VA): American Diabetes Association; August 2018.3-7. doi:10.2337/db20181-3
4. Haak T, Hanaire H, Ajjan R, Hermanns N, Riveline JP, Rayman G. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther. 2017;8(1):55-73. doi:10.1007/s13300-016-0223-6
5. Yoo HJ, An HG, Park SY, et al. Use of a real time continuous glucose monitoring system as a motivational device for poorly controlled type 2 diabetes. Diabetes Res Clin Pract. 2008;82(1):73-79. doi:10.1016/j.diabres.2008.06.015
6. Taylor PJ, Thompson CH, Brinkworth GD. Effectiveness and acceptability of continuous glucose monitoring for type 2 diabetes management: A narrative review. J Diabetes Investig. 2018;9(4):713-725. doi:10.1111/jdi.12807
Nearly 25% of patients served in the US Department of Veterans Affairs (VA) have been diagnosed with type 2 diabetes mellitus (T2DM), although the prevalence among adults in the United States is 9%.1 Patients with DM typically monitor their blood glucose using intermittent fingerstick self-testing. Continuous glucose monitoring (CGM) might offer a more comprehensive picture of glucose control to improve disease management. Within the VA, criteria for CGM use varies among facilities, but generally veterans prescribed at least 3 daily insulin injections and 4 daily blood glucose checks qualify.2
CGM therapy has been extensively researched for type 1 DM (T1DM); however, outcomes of CGM use among older adults with T2DM have not been fully evaluated. In a 2018 review of randomized clinical trials evaluating CGM use, 17 trials examined only patients with T1DM (2009 participants), 4 included only patients with T2DM patients (547 patients), 3 evaluated patients with T1DM or T2DM (655 patients), and 3 included women with gestational diabetes (585 patients).3 Of 27 studies that included change in hemoglobin A1c (HbA1c) as an endpoint, 15 found a statistically significant reduction in HbA1c for the CGM group. Four trials evaluated CGM use in adults with T2DM and 3 found no difference in HbA1c overall. However, 1 study found a difference in HbA1c only in individuals aged < 65 years, and another study found a greater improvement in the CGM group (approximately 0.5%).4,5 These mixed results indicate a need for further subgroup analysis in specific populations to determine the optimal use of CGM in adults with T2DM. Although this study was not designed to measure changes in hypoglycemic episodes or the relative efficacy of different CGM products, it establishes a baseline from which to conduct additional research.
Our primary objective was to determine change in HbA1c in each patient from the year before CGM initiation to the year after. Secondary objectives included changes in blood pressure (BP), weight, and diabetes-related hospital and clinic visits during the same time frame. We also completed subanalysis comparing primary outcomes in engaged or adherent patients compared with the entire study group. This study was completed as a quality improvement project with approval from the Lexington Veterans Affairs Health Care System in Kentucky information security office and was exempted from institutional review board review.
Methods
This project was a retrospective evaluation using the VA database of patient records. Rather than using a control group, our study used a pre–post model to determine the impact of CGM for each patient. For the primary outcome, average HbA1c values were calculated for the year before and year after CGM initiation. Hemoglobin and hematocrit values were included if reported within 3 months of the HbA1c values to ensure validity of HbA1c results. Average HbA1c was 13.37 g/dL (range, 10.5-17.3), and average hematocrit was 43.3% (range, 36-52). Change in average HbA1c was recorded for each patient. Based on research by Taylor and colleagues, a change in HbA1c of 0.8% was considered clinically significant for this project.6
Mean BP and weight were calculated for the years before and after CGM initiation. Only values for routine clinic visits were included; values taken during an acute health incident, inpatient stay, infusion clinic appointments, or home readings were excluded. Changes were recorded for each patient. Patient encounter notes were used to determine the number of DM-related hospital, emergency department, and clinic visits, such as nephrology, podiatry, vascular medicine, or infectious disease clinic or inpatient encounters during the study period. Routine endocrinology or primary care visits were not included, and patient care notes were consulted to ensure that the encounters were related to a DM complication. The change in number of visits was calculated for each patient.
Adherence was defined as patients receiving active medication management, documented treatment regimen adherence, and > 4 annual endocrinology clinic visits. Active medication management was defined as having > 1 dosage or medication change for oral or noninsulin antihyperglycemics, initiation, or adjustment of insulin dosages according to the patient records. Treatment adherence was determined based on medication reconciliation notes and refill request history. Only endocrinology clinic visits at VA outpatient clinics were included.
Study Population
A sample of 166 patients was needed to detect an HbA1c change of 0.8 per power analysis. The normal approximation method using the z statistic was used, with 2-tailed α = 0.05, β = 0.05, E = 0.8, and S = 1.2. We randomly selected 175 patients among all individuals with an active prescription for CGM in 2018 and 2019, who had a diagnosis of T2DM, and were managed by VA endocrinology clinics (including endocrine clinics, diabetes clinics, and patient aligned care team clinics) at 87 VA medical centers. Patients with types of DM other than T2DM were excluded, as well as those with a diagnosed hemoglobinopathy or HbA1c < 10 g/dL. The adherent subgroup included 40 patients of the 175 sample population (Table 1).
Results
Both the total population and the adherent subgroup showed reduction in HbA1c, the primary endpoint. The complete population showed a HbA1c change of –0.3 (95% CI, –0.4 to –0.2), and the adherent subgroup had a change of –1.3 (95% CI, –1.5 to –1.2). The total survey population had a mean change in weight of –1.9 lb (–0.9 kg) (95% CI, –3.7 to –0.1) and the adherent subgroup had an average change of –8.0 lb (–3.6 kg) (95% CI, –12.3 to –3.8). Average systolic BP changes were –0.1 mm Hg (95% CI, –1.6 to 1.5) in the total population and +3.3 mm Hg (95% CI, –0.01 to 6.22) in the adherent subgroup. A decrease in total encounters for DM complications was observed in the population (–0.3 total encounters per patient, 95% CI, –0.5 to –0.2) and the adherent subgroup (–0.6 total encounters per patient, 95% CI, –1.0 to –0.1) (Table 2).
Before the study, 107 (61.1%) patients were taking oral or noninsulin DM medication only, 4 (2.3%) were on insulin only, and 64 (36.6%) were prescribed both insulin and oral/noninsulin antihyperglycemics. Noninsulin and oral antihyperglycemic regimens included combinations of biguanide, dipeptidyl peptidase- 4 inhibitor, sodium-glucose cotransporter-2 inhibitor, sulfonylurea, meglitinide, β-glucosidase inhibitor, glucagon-like peptide-1 (GLP-1) analog, and thiazolidinedione drug classes. Nearly 70% (122) had no reported changes in DM treatment beyond dosage titrations. Among these patients, 18 (10.3%) were on an insulin pump for the duration of the study. Among the 53 (30.3%) patients who had changes in treatment, 31 (17.7%) transitioned from insulin injections to an insulin pump, 13 (7.4%) changed from 1 insulin injection to another (ie, addition of long-acting insulin, transition to u500 insulin, changing from 1 insulin category or brand to another), 8 (4.6%) began an oral/noninsulin antihyperglycemic, 4 (2.3%) began insulin injections, 13 (7.4%) discontinued noninsulin or oral antihyperglycemics, and 2 (1.1%) discontinued insulin during the study period.
Data showed that 113 (64.5%) patients had no changes in antihypertensives. The remaining 62 (35.4%) had the following adjustments: 14 (8%) increased dose of current medication(s), 9 (5.1%) decreased dose of current medication(s), 8 (4.6%) discontinued all antihypertensive medications, 10 (5.7%) switched to a different antihypertensive class, and 16 (9.1%) added additional antihypertensive medication(s) to their existing regimen during the study period.
Patients in the study group used 7 different types of CGM sensors. Chart review revealed that 84 (47.7%) patients used Medtronic devices, with 26 (14.8%) using first-generation Guardian sensors, 50 (28.4%) using Enlite sensors, and 8 (4.5) using Guardian 3 sensors. We found that 81 (46.0%) veterans were prescribed Dexcom devices, with 5 (2.8%) using SEVEN PLUS sensors, 68 (38.6%) using G4-5 sensors, and 8 (4.5%) using G6 sensors. The remaining 10 (5.7%) patients were using Freestyle Libre sensors during the study period.
Discussion
CGM did not correspond with clinically significant reductions in HbA1c. However, veterans with increased health care engagement were likely to achieve clinically significant HbA1c improvements. The veterans in the adherent subgroup had a higher baseline HbA1c, which could be because of a variety of factors mentioned in patient care notes, including insulin resistance, poor dietary habits, and exercise regimen nonadherence. These patients might have had more room to improve their glycemic control without concern of hypoglycemia, and their higher baseline HbA1c could have provided increased motivation for improving their health during the study period.
Adherent patients also had a greater reduction in weight and hospital or clinic visits with CGM compared with the total population. These veterans’ increased involvement in their health care might have led to better dietary and exercise adherence, which would have decreased insulin dosing and contributed to weight loss. Only 1 patient in the adherent subgroup initiated a GLP-1 agonist during the study period, making it unlikely that medication changes had a significant impact on weight loss in the subgroup analysis. This improvement in overall health status might have contributed to the reduction in hospital or clinic visits observed in this population.
Average systolic BP data decreased minimally in the total survey population and increased in the adherent subgroup over the course of the study. These results were determined to be statistically significant. Changes in systolic BP readings were minimal, indicating that it is unlikely that these changes contributed meaningfully to the patients’ overall health status.
Although not related to the study objectives, the adherent population required less antihypertensive adjustments with similar BP control. This could be explained by improved overall health or better adherence and engagement in therapy. The results of this project show that despite limited medication changes, T2DM management improved among adherent patients using CGM. The general study population, which was more likely to have documented nonadherence with treatment or clinic appointments, had minimal benefit. CGM technology in the T2DM veteran population is more likely to have significant clinical benefit in patients who are adherent with their medication regimens and follow-up appointments compared with the larger study population.
The results of this study are in line with previous studies on CGM use in the T2DM patient population. We agree with the previously published research that CGM alone does not have a meaningful impact on HbA1c reduction. Our study population also was older than those in previous studies, adding to the Haak and colleagues conclusion that patients aged < 65 years might have better outcomes with CGM.4
Strengths of this study include specificity to the veteran population using VA resources, as well as including nondiabetes outcomes. This allows for specific application to the veteran population and could provide broader evidence for CGM use. Demonstrated decreases in HbA1c, weight, and clinic visits in the adherent population suggest that providing veterans with CGM therapy with frequent endocrinology follow-up improves health outcomes and could decrease overall health spending.
Limitations
Limitations of this study include retrospective design, a small sample size, and solely focusing on T2DM. As a retrospective study, we cannot rule out the influence of outside factors, such as participation in a non-VA weight loss program. This study lacked the power to assess the impact of the different CGM brands. The study did not include data on severe hypoglycemic or hyperglycemic episodes as veterans might have needed emergent care at non-VA facilities. Future research will evaluate the impact of CGM on symptomatic and severe hypoglycemic episodes and use of insulin vs oral or noninsulin antihyperglycemics and the comparative efficacy of different CGM brands among veterans.
Conclusions
CGM did not correspond with clinically significant reductions in HbA1c. However, veterans with increased health care engagement were likely to achieve clinically significant HbA1c improvements. Adherent patients also had more reduction in weight and hospital or clinic visits with CGM compared with the total population. These veterans’ increased involvement in their health care might have led to better dietary and exercise adherence, which would have decreased insulin dosing and contributed to weight loss.
Nearly 25% of patients served in the US Department of Veterans Affairs (VA) have been diagnosed with type 2 diabetes mellitus (T2DM), although the prevalence among adults in the United States is 9%.1 Patients with DM typically monitor their blood glucose using intermittent fingerstick self-testing. Continuous glucose monitoring (CGM) might offer a more comprehensive picture of glucose control to improve disease management. Within the VA, criteria for CGM use varies among facilities, but generally veterans prescribed at least 3 daily insulin injections and 4 daily blood glucose checks qualify.2
CGM therapy has been extensively researched for type 1 DM (T1DM); however, outcomes of CGM use among older adults with T2DM have not been fully evaluated. In a 2018 review of randomized clinical trials evaluating CGM use, 17 trials examined only patients with T1DM (2009 participants), 4 included only patients with T2DM patients (547 patients), 3 evaluated patients with T1DM or T2DM (655 patients), and 3 included women with gestational diabetes (585 patients).3 Of 27 studies that included change in hemoglobin A1c (HbA1c) as an endpoint, 15 found a statistically significant reduction in HbA1c for the CGM group. Four trials evaluated CGM use in adults with T2DM and 3 found no difference in HbA1c overall. However, 1 study found a difference in HbA1c only in individuals aged < 65 years, and another study found a greater improvement in the CGM group (approximately 0.5%).4,5 These mixed results indicate a need for further subgroup analysis in specific populations to determine the optimal use of CGM in adults with T2DM. Although this study was not designed to measure changes in hypoglycemic episodes or the relative efficacy of different CGM products, it establishes a baseline from which to conduct additional research.
Our primary objective was to determine change in HbA1c in each patient from the year before CGM initiation to the year after. Secondary objectives included changes in blood pressure (BP), weight, and diabetes-related hospital and clinic visits during the same time frame. We also completed subanalysis comparing primary outcomes in engaged or adherent patients compared with the entire study group. This study was completed as a quality improvement project with approval from the Lexington Veterans Affairs Health Care System in Kentucky information security office and was exempted from institutional review board review.
Methods
This project was a retrospective evaluation using the VA database of patient records. Rather than using a control group, our study used a pre–post model to determine the impact of CGM for each patient. For the primary outcome, average HbA1c values were calculated for the year before and year after CGM initiation. Hemoglobin and hematocrit values were included if reported within 3 months of the HbA1c values to ensure validity of HbA1c results. Average HbA1c was 13.37 g/dL (range, 10.5-17.3), and average hematocrit was 43.3% (range, 36-52). Change in average HbA1c was recorded for each patient. Based on research by Taylor and colleagues, a change in HbA1c of 0.8% was considered clinically significant for this project.6
Mean BP and weight were calculated for the years before and after CGM initiation. Only values for routine clinic visits were included; values taken during an acute health incident, inpatient stay, infusion clinic appointments, or home readings were excluded. Changes were recorded for each patient. Patient encounter notes were used to determine the number of DM-related hospital, emergency department, and clinic visits, such as nephrology, podiatry, vascular medicine, or infectious disease clinic or inpatient encounters during the study period. Routine endocrinology or primary care visits were not included, and patient care notes were consulted to ensure that the encounters were related to a DM complication. The change in number of visits was calculated for each patient.
Adherence was defined as patients receiving active medication management, documented treatment regimen adherence, and > 4 annual endocrinology clinic visits. Active medication management was defined as having > 1 dosage or medication change for oral or noninsulin antihyperglycemics, initiation, or adjustment of insulin dosages according to the patient records. Treatment adherence was determined based on medication reconciliation notes and refill request history. Only endocrinology clinic visits at VA outpatient clinics were included.
Study Population
A sample of 166 patients was needed to detect an HbA1c change of 0.8 per power analysis. The normal approximation method using the z statistic was used, with 2-tailed α = 0.05, β = 0.05, E = 0.8, and S = 1.2. We randomly selected 175 patients among all individuals with an active prescription for CGM in 2018 and 2019, who had a diagnosis of T2DM, and were managed by VA endocrinology clinics (including endocrine clinics, diabetes clinics, and patient aligned care team clinics) at 87 VA medical centers. Patients with types of DM other than T2DM were excluded, as well as those with a diagnosed hemoglobinopathy or HbA1c < 10 g/dL. The adherent subgroup included 40 patients of the 175 sample population (Table 1).
Results
Both the total population and the adherent subgroup showed reduction in HbA1c, the primary endpoint. The complete population showed a HbA1c change of –0.3 (95% CI, –0.4 to –0.2), and the adherent subgroup had a change of –1.3 (95% CI, –1.5 to –1.2). The total survey population had a mean change in weight of –1.9 lb (–0.9 kg) (95% CI, –3.7 to –0.1) and the adherent subgroup had an average change of –8.0 lb (–3.6 kg) (95% CI, –12.3 to –3.8). Average systolic BP changes were –0.1 mm Hg (95% CI, –1.6 to 1.5) in the total population and +3.3 mm Hg (95% CI, –0.01 to 6.22) in the adherent subgroup. A decrease in total encounters for DM complications was observed in the population (–0.3 total encounters per patient, 95% CI, –0.5 to –0.2) and the adherent subgroup (–0.6 total encounters per patient, 95% CI, –1.0 to –0.1) (Table 2).
Before the study, 107 (61.1%) patients were taking oral or noninsulin DM medication only, 4 (2.3%) were on insulin only, and 64 (36.6%) were prescribed both insulin and oral/noninsulin antihyperglycemics. Noninsulin and oral antihyperglycemic regimens included combinations of biguanide, dipeptidyl peptidase- 4 inhibitor, sodium-glucose cotransporter-2 inhibitor, sulfonylurea, meglitinide, β-glucosidase inhibitor, glucagon-like peptide-1 (GLP-1) analog, and thiazolidinedione drug classes. Nearly 70% (122) had no reported changes in DM treatment beyond dosage titrations. Among these patients, 18 (10.3%) were on an insulin pump for the duration of the study. Among the 53 (30.3%) patients who had changes in treatment, 31 (17.7%) transitioned from insulin injections to an insulin pump, 13 (7.4%) changed from 1 insulin injection to another (ie, addition of long-acting insulin, transition to u500 insulin, changing from 1 insulin category or brand to another), 8 (4.6%) began an oral/noninsulin antihyperglycemic, 4 (2.3%) began insulin injections, 13 (7.4%) discontinued noninsulin or oral antihyperglycemics, and 2 (1.1%) discontinued insulin during the study period.
Data showed that 113 (64.5%) patients had no changes in antihypertensives. The remaining 62 (35.4%) had the following adjustments: 14 (8%) increased dose of current medication(s), 9 (5.1%) decreased dose of current medication(s), 8 (4.6%) discontinued all antihypertensive medications, 10 (5.7%) switched to a different antihypertensive class, and 16 (9.1%) added additional antihypertensive medication(s) to their existing regimen during the study period.
Patients in the study group used 7 different types of CGM sensors. Chart review revealed that 84 (47.7%) patients used Medtronic devices, with 26 (14.8%) using first-generation Guardian sensors, 50 (28.4%) using Enlite sensors, and 8 (4.5) using Guardian 3 sensors. We found that 81 (46.0%) veterans were prescribed Dexcom devices, with 5 (2.8%) using SEVEN PLUS sensors, 68 (38.6%) using G4-5 sensors, and 8 (4.5%) using G6 sensors. The remaining 10 (5.7%) patients were using Freestyle Libre sensors during the study period.
Discussion
CGM did not correspond with clinically significant reductions in HbA1c. However, veterans with increased health care engagement were likely to achieve clinically significant HbA1c improvements. The veterans in the adherent subgroup had a higher baseline HbA1c, which could be because of a variety of factors mentioned in patient care notes, including insulin resistance, poor dietary habits, and exercise regimen nonadherence. These patients might have had more room to improve their glycemic control without concern of hypoglycemia, and their higher baseline HbA1c could have provided increased motivation for improving their health during the study period.
Adherent patients also had a greater reduction in weight and hospital or clinic visits with CGM compared with the total population. These veterans’ increased involvement in their health care might have led to better dietary and exercise adherence, which would have decreased insulin dosing and contributed to weight loss. Only 1 patient in the adherent subgroup initiated a GLP-1 agonist during the study period, making it unlikely that medication changes had a significant impact on weight loss in the subgroup analysis. This improvement in overall health status might have contributed to the reduction in hospital or clinic visits observed in this population.
Average systolic BP data decreased minimally in the total survey population and increased in the adherent subgroup over the course of the study. These results were determined to be statistically significant. Changes in systolic BP readings were minimal, indicating that it is unlikely that these changes contributed meaningfully to the patients’ overall health status.
Although not related to the study objectives, the adherent population required less antihypertensive adjustments with similar BP control. This could be explained by improved overall health or better adherence and engagement in therapy. The results of this project show that despite limited medication changes, T2DM management improved among adherent patients using CGM. The general study population, which was more likely to have documented nonadherence with treatment or clinic appointments, had minimal benefit. CGM technology in the T2DM veteran population is more likely to have significant clinical benefit in patients who are adherent with their medication regimens and follow-up appointments compared with the larger study population.
The results of this study are in line with previous studies on CGM use in the T2DM patient population. We agree with the previously published research that CGM alone does not have a meaningful impact on HbA1c reduction. Our study population also was older than those in previous studies, adding to the Haak and colleagues conclusion that patients aged < 65 years might have better outcomes with CGM.4
Strengths of this study include specificity to the veteran population using VA resources, as well as including nondiabetes outcomes. This allows for specific application to the veteran population and could provide broader evidence for CGM use. Demonstrated decreases in HbA1c, weight, and clinic visits in the adherent population suggest that providing veterans with CGM therapy with frequent endocrinology follow-up improves health outcomes and could decrease overall health spending.
Limitations
Limitations of this study include retrospective design, a small sample size, and solely focusing on T2DM. As a retrospective study, we cannot rule out the influence of outside factors, such as participation in a non-VA weight loss program. This study lacked the power to assess the impact of the different CGM brands. The study did not include data on severe hypoglycemic or hyperglycemic episodes as veterans might have needed emergent care at non-VA facilities. Future research will evaluate the impact of CGM on symptomatic and severe hypoglycemic episodes and use of insulin vs oral or noninsulin antihyperglycemics and the comparative efficacy of different CGM brands among veterans.
Conclusions
CGM did not correspond with clinically significant reductions in HbA1c. However, veterans with increased health care engagement were likely to achieve clinically significant HbA1c improvements. Adherent patients also had more reduction in weight and hospital or clinic visits with CGM compared with the total population. These veterans’ increased involvement in their health care might have led to better dietary and exercise adherence, which would have decreased insulin dosing and contributed to weight loss.
1. Liu Y, Sayam S, Shao X, et al. Prevalence of and trends in diabetes among veterans, United States, 2005-2014. Prev Chronic Dis. 2017;14:E135. Published 2017 Dec 14. doi:10.5888/pcd14.170230
2. Hackett M. VA pharmacies now carry the Dexcom G6 CGM at no cost for qualifying patients. September 23, 2020. Accessed September 28, 2021. https://www.mobihealthnews.com/news/va-pharmacies-now-carry-dexcom-g6-cgm-no-cost-qualifying-patients
3. Peters AL. The evidence base for continuous glucose monitoring. In: Role of Continuous Glucose Monitoring in Diabetes Treatment. Arlington (VA): American Diabetes Association; August 2018.3-7. doi:10.2337/db20181-3
4. Haak T, Hanaire H, Ajjan R, Hermanns N, Riveline JP, Rayman G. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther. 2017;8(1):55-73. doi:10.1007/s13300-016-0223-6
5. Yoo HJ, An HG, Park SY, et al. Use of a real time continuous glucose monitoring system as a motivational device for poorly controlled type 2 diabetes. Diabetes Res Clin Pract. 2008;82(1):73-79. doi:10.1016/j.diabres.2008.06.015
6. Taylor PJ, Thompson CH, Brinkworth GD. Effectiveness and acceptability of continuous glucose monitoring for type 2 diabetes management: A narrative review. J Diabetes Investig. 2018;9(4):713-725. doi:10.1111/jdi.12807
1. Liu Y, Sayam S, Shao X, et al. Prevalence of and trends in diabetes among veterans, United States, 2005-2014. Prev Chronic Dis. 2017;14:E135. Published 2017 Dec 14. doi:10.5888/pcd14.170230
2. Hackett M. VA pharmacies now carry the Dexcom G6 CGM at no cost for qualifying patients. September 23, 2020. Accessed September 28, 2021. https://www.mobihealthnews.com/news/va-pharmacies-now-carry-dexcom-g6-cgm-no-cost-qualifying-patients
3. Peters AL. The evidence base for continuous glucose monitoring. In: Role of Continuous Glucose Monitoring in Diabetes Treatment. Arlington (VA): American Diabetes Association; August 2018.3-7. doi:10.2337/db20181-3
4. Haak T, Hanaire H, Ajjan R, Hermanns N, Riveline JP, Rayman G. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther. 2017;8(1):55-73. doi:10.1007/s13300-016-0223-6
5. Yoo HJ, An HG, Park SY, et al. Use of a real time continuous glucose monitoring system as a motivational device for poorly controlled type 2 diabetes. Diabetes Res Clin Pract. 2008;82(1):73-79. doi:10.1016/j.diabres.2008.06.015
6. Taylor PJ, Thompson CH, Brinkworth GD. Effectiveness and acceptability of continuous glucose monitoring for type 2 diabetes management: A narrative review. J Diabetes Investig. 2018;9(4):713-725. doi:10.1111/jdi.12807
Long QT and Cardiac Arrest After Symptomatic Improvement of Pulmonary Edema
A case of extreme QT prolongation induced following symptomatic resolution of acute pulmonary edema is both relatively unknown and poorly understood.
Abnormalities in the T-wave morphology of an electrocardiogram (ECG) are classically attributed to ischemic cardiac disease. However, these changes can be seen in a variety of other etiologies, including noncardiac pathology, which should be considered whenever reviewing an ECG: central nervous system disease, including stroke and subarachnoid hemorrhage; hypothermia; pulmonary disease, such as pulmonary embolism or chronic obstructive pulmonary disease; myopericarditis; drug effects; and electrolyte abnormalities.
Prolongation of the QT interval, on the other hand, can be precipitated by medications, metabolic derangements, or genetic phenotypes. The QT interval is measured from the beginning of the QRS complex to the termination of the T wave and represents the total time for ventricular depolarization and repolarization. The QT interval must be corrected based on the patient’s heart rate, known as the QTc. As the QTc interval lengthens, there is increased risk of R-on-T phenomena, which may result in Torsades de Pointes (TdP). Typical features of TdP include an antecedent prolonged QTc, cyclic polymorphic ventricular tachycardia on the surface ECG, and either a short-lived spontaneously terminating course or degeneration into ventricular fibrillation (VF) and sudden cardiac death.1 These dysrhythmias become more likely as the QTc interval exceeds 500 msec.2
The combination of new-onset global T-wave inversions with prolongation of the QT interval has been reported in only a few limited conditions. Some known causes of these QT T changes include cardiac ischemia, status epilepticus, pheochromocytoma, and acute cocaine intoxication.3 One uncommon and rarely reported cause of extreme QT prolongation and T-wave inversion is acute pulmonary edema. The ECG findings are not present on initial patient presentation; rather the dynamic changes occur after resolution of the pulmonary symptoms. Despite significant ECG changes, all prior reported cases describe ECG normalization without significant morbidity.4,5 We report a case of extreme QT prolongation following acute pulmonary edema that resulted in cardiac arrest secondary to VF.
Case Presentation
A 72-year-old male with medical history of combined systolic and diastolic heart failure, ischemic cardiomyopathy, coronary artery disease, cerebral vascular accident, hypertension, hyperlipidemia, type 2 diabetes mellitus, and tobacco dependence presented to the emergency department (ED) by emergency medical services after awaking with acute onset of dyspnea and diaphoresis. On arrival at the ED, the patient was noted to be in respiratory distress (ie, unable to speak single words) and was extremely diaphoretic. His initial vital signs included blood pressure, 186/113 mm Hg, heart rate, 104 beats per minute, respiratory rate, 40 breaths per minute, and temperature, 36.4 °C. The patient was quickly placed on bilevel positive airway pressure and given sublingual nitroglycerin followed by transdermal nitroglycerin with a single dose of 40 mg IV furosemide, which improved his respiratory status. A chest X-ray was consistent with pulmonary edema, and his brain natriuretic peptide was 1654 pg/mL. An ECG demonstrated new T-wave inversions, and his troponin increased from 0.04 to 0.24 ng/mL during his ED stay (Figure 1). He was started on a heparin infusion and admitted to the hospital for hypertensive emergency with presumed acute decompensated heart failure and non-ST-elevated myocardial infarction.
Throughout the patient’s first night, the troponin level started to down-trend after peaking at 0.24 ng/mL, and his oxygen requirements decreased allowing transition to nasal cannula. However, his repeat ECGs demonstrated significant T-wave abnormalities, new premature ventricular contractions, bradycardia, and a prolonging QTc interval to 703 msec (Figure 2). At this time, the patient’s electrolytes were normal, specifically a potassium level of 4.4 mEq/L, calcium 8.8 mg/dL, magnesium 2.0 mg/dL, and phosphorus 2.6 mg/dL. Given the worsening ECG changes, a computed tomography scan of his head was ordered to rule out intracranial pathology. While in the scanner, the patient went into pulseless VF, prompting defibrillation with 200 J. In addition, he was given 75 mg IV lidocaine, 2 g IV magnesium, and 1 ampule of both calcium chloride and sodium bicarbonate. With treatment, he had return of spontaneous circulation and was taken promptly to cardiac catheterization. The catheterization showed no significant obstructive coronary artery disease, and no interventions were performed. The patient was transferred to the cardiac intensive care unit for continued care.
During his course in the intensive care unit, the patient’s potassium and magnesium levels were maintained at high-normal levels. The patient was started on a dobutamine infusion to increase his heart rate and attempt to decrease his QTc. The patient also underwent cardiac magnetic resonance imaging (MRI) to evaluate for possible myocarditis, which showed no evidence of acute inflammation. Echocardiogram demonstrated an ejection fraction of 40% and global hypokinesis but no specific regional abnormalities and no change from prior echocardiogram performed 1 year earlier. Over the course of 3 days, his ECG normalized and his QTc shortened to 477 msec. Genetic testing was performed and did not reveal any mutations associated with long QT syndrome. Ultimately, an automated internal cardiac defibrillator (AICD) was placed, and the patient was discharged home.
Over the 2 years since his initial event, the patient has not experienced recurrent VF and his AICD has not fired. The patient continues to have ED presentations for heart-failure symptoms, though he has been stable from an electrophysiologic standpoint and his QTc remains less than 500 msec.
Discussion
Prolongation of the QT interval as a result of deep, global T-wave inversions after resolution of acute pulmonary edema has been minimally reported.4,5 This phenomenon has been described in the cardiology literature but has not been discussed in the emergency medicine literature and bears consideration in this case.4,5 As noted, an extensive evaluation did not reveal another cause of QTc prolongation. The patient had normal electrolytes and temperature, his neurologic examination and computed tomography were not remarkable. The patient had no obstructive coronary artery disease on catheterization, no evidence of acute myocarditis on cardiac MRI, no prescribed medications associated with QT prolongation, and no evidence of genetic mutations associated with QT prolongation on testing. The minimal troponin elevation was felt to represent a type II myocardial infarction related to ischemia due to supply-demand mismatch rather than acute plaque rupture.
Littmann published a case series of 9 cases of delayed onset T-wave inversion and extreme QTc prolongation in the 24 to 48 hours following treatment and symptomatic improvement in acute pulmonary edema.4 In each of his patients, an ischemic cardiac insult was ruled out as the etiology of the pulmonary edema by laboratory assessment, echocardiography, and left heart catheterization.All of the patients in this case series recovered without incident and with normalization of the QTc interval.4 Similarly, in our patient, significant QT T changes occurred approximately 22 hours after presentation and with resolution of symptoms of pulmonary edema. Pascale and colleagues also published a series of 3 patients developing similar ECG patterns following a hypertensive crisis with resolution of ECG findings and without any morbidity.5 In contrast, our patient experienced significant morbidity secondary to the extreme QTc prolongation.
Conclusions
We believe this is the first reported case of excessive prolongation of the QTc with VF arrest secondary to resolution of acute pulmonary edema. The pattern observed in our patient follows the patterns outlined in the previous case series—patients present with acute pulmonary edema and hypertensive crisis but develop significant ECG abnormalities about 24 hours after the resolution of the high catecholamine state. Our patient did have a history of prior cardiac insult, given the QTc changes developed acutely, with frequent premature ventricular contractions, and the cardiac arrest occurred at maximal QTc prolongation, yet after resolution of the high catecholamine state, the treatment team felt there was likely an uncaptured and short-lived episode of TdP that degenerated into VF. This theory is further supported by the lack of recurrent VF episodes, confirmed by AICD interrogation, after normalization of the QTc in our patient.
1. Passman R, Kadish A. Polymorphic ventricular tachycardia, long Q-T syndrome, and torsades de pointes. Med Clin North Am. 2001;85(2):321-341. doi:10.1016/s0025-7125(05)70318-7
2. Kallergis EM, Goudis CA, Simantirakis EN, Kochiadakis GE, Vardas PE. Mechanisms, risk factors, and management of acquired long QT syndrome: a comprehensive review. ScientificWorldJournal. 2012;2012:212178. doi:10.1100/2012/212178
3. Miller MA, Elmariah S, Fischer A. Giant T-wave inversions and extreme QT prolongation. Circ Arrhythm Electrophysiol. 2009;2(6):e42-e43. doi:10.1161/CIRCEP.108.825729
4. Littmann L. Large T wave inversion and QT prolongation associated with pulmonary edema: a report of nine cases. J Am Coll Cardiol. 1999;34(4):1106-1110. doi:10.1016/s0735-1097(99)00311-3
5. Pascale P, Quartenoud B, Stauffer JC. Isolated large inverted T wave in pulmonary edema due to hypertensive crisis: a novel electrocardiographic phenomenon mimicking ischemia?. Clin Res Cardiol. 2007;96(5):288-294. doi:10.1007/s00392-007-0504-1
A case of extreme QT prolongation induced following symptomatic resolution of acute pulmonary edema is both relatively unknown and poorly understood.
A case of extreme QT prolongation induced following symptomatic resolution of acute pulmonary edema is both relatively unknown and poorly understood.
Abnormalities in the T-wave morphology of an electrocardiogram (ECG) are classically attributed to ischemic cardiac disease. However, these changes can be seen in a variety of other etiologies, including noncardiac pathology, which should be considered whenever reviewing an ECG: central nervous system disease, including stroke and subarachnoid hemorrhage; hypothermia; pulmonary disease, such as pulmonary embolism or chronic obstructive pulmonary disease; myopericarditis; drug effects; and electrolyte abnormalities.
Prolongation of the QT interval, on the other hand, can be precipitated by medications, metabolic derangements, or genetic phenotypes. The QT interval is measured from the beginning of the QRS complex to the termination of the T wave and represents the total time for ventricular depolarization and repolarization. The QT interval must be corrected based on the patient’s heart rate, known as the QTc. As the QTc interval lengthens, there is increased risk of R-on-T phenomena, which may result in Torsades de Pointes (TdP). Typical features of TdP include an antecedent prolonged QTc, cyclic polymorphic ventricular tachycardia on the surface ECG, and either a short-lived spontaneously terminating course or degeneration into ventricular fibrillation (VF) and sudden cardiac death.1 These dysrhythmias become more likely as the QTc interval exceeds 500 msec.2
The combination of new-onset global T-wave inversions with prolongation of the QT interval has been reported in only a few limited conditions. Some known causes of these QT T changes include cardiac ischemia, status epilepticus, pheochromocytoma, and acute cocaine intoxication.3 One uncommon and rarely reported cause of extreme QT prolongation and T-wave inversion is acute pulmonary edema. The ECG findings are not present on initial patient presentation; rather the dynamic changes occur after resolution of the pulmonary symptoms. Despite significant ECG changes, all prior reported cases describe ECG normalization without significant morbidity.4,5 We report a case of extreme QT prolongation following acute pulmonary edema that resulted in cardiac arrest secondary to VF.
Case Presentation
A 72-year-old male with medical history of combined systolic and diastolic heart failure, ischemic cardiomyopathy, coronary artery disease, cerebral vascular accident, hypertension, hyperlipidemia, type 2 diabetes mellitus, and tobacco dependence presented to the emergency department (ED) by emergency medical services after awaking with acute onset of dyspnea and diaphoresis. On arrival at the ED, the patient was noted to be in respiratory distress (ie, unable to speak single words) and was extremely diaphoretic. His initial vital signs included blood pressure, 186/113 mm Hg, heart rate, 104 beats per minute, respiratory rate, 40 breaths per minute, and temperature, 36.4 °C. The patient was quickly placed on bilevel positive airway pressure and given sublingual nitroglycerin followed by transdermal nitroglycerin with a single dose of 40 mg IV furosemide, which improved his respiratory status. A chest X-ray was consistent with pulmonary edema, and his brain natriuretic peptide was 1654 pg/mL. An ECG demonstrated new T-wave inversions, and his troponin increased from 0.04 to 0.24 ng/mL during his ED stay (Figure 1). He was started on a heparin infusion and admitted to the hospital for hypertensive emergency with presumed acute decompensated heart failure and non-ST-elevated myocardial infarction.
Throughout the patient’s first night, the troponin level started to down-trend after peaking at 0.24 ng/mL, and his oxygen requirements decreased allowing transition to nasal cannula. However, his repeat ECGs demonstrated significant T-wave abnormalities, new premature ventricular contractions, bradycardia, and a prolonging QTc interval to 703 msec (Figure 2). At this time, the patient’s electrolytes were normal, specifically a potassium level of 4.4 mEq/L, calcium 8.8 mg/dL, magnesium 2.0 mg/dL, and phosphorus 2.6 mg/dL. Given the worsening ECG changes, a computed tomography scan of his head was ordered to rule out intracranial pathology. While in the scanner, the patient went into pulseless VF, prompting defibrillation with 200 J. In addition, he was given 75 mg IV lidocaine, 2 g IV magnesium, and 1 ampule of both calcium chloride and sodium bicarbonate. With treatment, he had return of spontaneous circulation and was taken promptly to cardiac catheterization. The catheterization showed no significant obstructive coronary artery disease, and no interventions were performed. The patient was transferred to the cardiac intensive care unit for continued care.
During his course in the intensive care unit, the patient’s potassium and magnesium levels were maintained at high-normal levels. The patient was started on a dobutamine infusion to increase his heart rate and attempt to decrease his QTc. The patient also underwent cardiac magnetic resonance imaging (MRI) to evaluate for possible myocarditis, which showed no evidence of acute inflammation. Echocardiogram demonstrated an ejection fraction of 40% and global hypokinesis but no specific regional abnormalities and no change from prior echocardiogram performed 1 year earlier. Over the course of 3 days, his ECG normalized and his QTc shortened to 477 msec. Genetic testing was performed and did not reveal any mutations associated with long QT syndrome. Ultimately, an automated internal cardiac defibrillator (AICD) was placed, and the patient was discharged home.
Over the 2 years since his initial event, the patient has not experienced recurrent VF and his AICD has not fired. The patient continues to have ED presentations for heart-failure symptoms, though he has been stable from an electrophysiologic standpoint and his QTc remains less than 500 msec.
Discussion
Prolongation of the QT interval as a result of deep, global T-wave inversions after resolution of acute pulmonary edema has been minimally reported.4,5 This phenomenon has been described in the cardiology literature but has not been discussed in the emergency medicine literature and bears consideration in this case.4,5 As noted, an extensive evaluation did not reveal another cause of QTc prolongation. The patient had normal electrolytes and temperature, his neurologic examination and computed tomography were not remarkable. The patient had no obstructive coronary artery disease on catheterization, no evidence of acute myocarditis on cardiac MRI, no prescribed medications associated with QT prolongation, and no evidence of genetic mutations associated with QT prolongation on testing. The minimal troponin elevation was felt to represent a type II myocardial infarction related to ischemia due to supply-demand mismatch rather than acute plaque rupture.
Littmann published a case series of 9 cases of delayed onset T-wave inversion and extreme QTc prolongation in the 24 to 48 hours following treatment and symptomatic improvement in acute pulmonary edema.4 In each of his patients, an ischemic cardiac insult was ruled out as the etiology of the pulmonary edema by laboratory assessment, echocardiography, and left heart catheterization.All of the patients in this case series recovered without incident and with normalization of the QTc interval.4 Similarly, in our patient, significant QT T changes occurred approximately 22 hours after presentation and with resolution of symptoms of pulmonary edema. Pascale and colleagues also published a series of 3 patients developing similar ECG patterns following a hypertensive crisis with resolution of ECG findings and without any morbidity.5 In contrast, our patient experienced significant morbidity secondary to the extreme QTc prolongation.
Conclusions
We believe this is the first reported case of excessive prolongation of the QTc with VF arrest secondary to resolution of acute pulmonary edema. The pattern observed in our patient follows the patterns outlined in the previous case series—patients present with acute pulmonary edema and hypertensive crisis but develop significant ECG abnormalities about 24 hours after the resolution of the high catecholamine state. Our patient did have a history of prior cardiac insult, given the QTc changes developed acutely, with frequent premature ventricular contractions, and the cardiac arrest occurred at maximal QTc prolongation, yet after resolution of the high catecholamine state, the treatment team felt there was likely an uncaptured and short-lived episode of TdP that degenerated into VF. This theory is further supported by the lack of recurrent VF episodes, confirmed by AICD interrogation, after normalization of the QTc in our patient.
Abnormalities in the T-wave morphology of an electrocardiogram (ECG) are classically attributed to ischemic cardiac disease. However, these changes can be seen in a variety of other etiologies, including noncardiac pathology, which should be considered whenever reviewing an ECG: central nervous system disease, including stroke and subarachnoid hemorrhage; hypothermia; pulmonary disease, such as pulmonary embolism or chronic obstructive pulmonary disease; myopericarditis; drug effects; and electrolyte abnormalities.
Prolongation of the QT interval, on the other hand, can be precipitated by medications, metabolic derangements, or genetic phenotypes. The QT interval is measured from the beginning of the QRS complex to the termination of the T wave and represents the total time for ventricular depolarization and repolarization. The QT interval must be corrected based on the patient’s heart rate, known as the QTc. As the QTc interval lengthens, there is increased risk of R-on-T phenomena, which may result in Torsades de Pointes (TdP). Typical features of TdP include an antecedent prolonged QTc, cyclic polymorphic ventricular tachycardia on the surface ECG, and either a short-lived spontaneously terminating course or degeneration into ventricular fibrillation (VF) and sudden cardiac death.1 These dysrhythmias become more likely as the QTc interval exceeds 500 msec.2
The combination of new-onset global T-wave inversions with prolongation of the QT interval has been reported in only a few limited conditions. Some known causes of these QT T changes include cardiac ischemia, status epilepticus, pheochromocytoma, and acute cocaine intoxication.3 One uncommon and rarely reported cause of extreme QT prolongation and T-wave inversion is acute pulmonary edema. The ECG findings are not present on initial patient presentation; rather the dynamic changes occur after resolution of the pulmonary symptoms. Despite significant ECG changes, all prior reported cases describe ECG normalization without significant morbidity.4,5 We report a case of extreme QT prolongation following acute pulmonary edema that resulted in cardiac arrest secondary to VF.
Case Presentation
A 72-year-old male with medical history of combined systolic and diastolic heart failure, ischemic cardiomyopathy, coronary artery disease, cerebral vascular accident, hypertension, hyperlipidemia, type 2 diabetes mellitus, and tobacco dependence presented to the emergency department (ED) by emergency medical services after awaking with acute onset of dyspnea and diaphoresis. On arrival at the ED, the patient was noted to be in respiratory distress (ie, unable to speak single words) and was extremely diaphoretic. His initial vital signs included blood pressure, 186/113 mm Hg, heart rate, 104 beats per minute, respiratory rate, 40 breaths per minute, and temperature, 36.4 °C. The patient was quickly placed on bilevel positive airway pressure and given sublingual nitroglycerin followed by transdermal nitroglycerin with a single dose of 40 mg IV furosemide, which improved his respiratory status. A chest X-ray was consistent with pulmonary edema, and his brain natriuretic peptide was 1654 pg/mL. An ECG demonstrated new T-wave inversions, and his troponin increased from 0.04 to 0.24 ng/mL during his ED stay (Figure 1). He was started on a heparin infusion and admitted to the hospital for hypertensive emergency with presumed acute decompensated heart failure and non-ST-elevated myocardial infarction.
Throughout the patient’s first night, the troponin level started to down-trend after peaking at 0.24 ng/mL, and his oxygen requirements decreased allowing transition to nasal cannula. However, his repeat ECGs demonstrated significant T-wave abnormalities, new premature ventricular contractions, bradycardia, and a prolonging QTc interval to 703 msec (Figure 2). At this time, the patient’s electrolytes were normal, specifically a potassium level of 4.4 mEq/L, calcium 8.8 mg/dL, magnesium 2.0 mg/dL, and phosphorus 2.6 mg/dL. Given the worsening ECG changes, a computed tomography scan of his head was ordered to rule out intracranial pathology. While in the scanner, the patient went into pulseless VF, prompting defibrillation with 200 J. In addition, he was given 75 mg IV lidocaine, 2 g IV magnesium, and 1 ampule of both calcium chloride and sodium bicarbonate. With treatment, he had return of spontaneous circulation and was taken promptly to cardiac catheterization. The catheterization showed no significant obstructive coronary artery disease, and no interventions were performed. The patient was transferred to the cardiac intensive care unit for continued care.
During his course in the intensive care unit, the patient’s potassium and magnesium levels were maintained at high-normal levels. The patient was started on a dobutamine infusion to increase his heart rate and attempt to decrease his QTc. The patient also underwent cardiac magnetic resonance imaging (MRI) to evaluate for possible myocarditis, which showed no evidence of acute inflammation. Echocardiogram demonstrated an ejection fraction of 40% and global hypokinesis but no specific regional abnormalities and no change from prior echocardiogram performed 1 year earlier. Over the course of 3 days, his ECG normalized and his QTc shortened to 477 msec. Genetic testing was performed and did not reveal any mutations associated with long QT syndrome. Ultimately, an automated internal cardiac defibrillator (AICD) was placed, and the patient was discharged home.
Over the 2 years since his initial event, the patient has not experienced recurrent VF and his AICD has not fired. The patient continues to have ED presentations for heart-failure symptoms, though he has been stable from an electrophysiologic standpoint and his QTc remains less than 500 msec.
Discussion
Prolongation of the QT interval as a result of deep, global T-wave inversions after resolution of acute pulmonary edema has been minimally reported.4,5 This phenomenon has been described in the cardiology literature but has not been discussed in the emergency medicine literature and bears consideration in this case.4,5 As noted, an extensive evaluation did not reveal another cause of QTc prolongation. The patient had normal electrolytes and temperature, his neurologic examination and computed tomography were not remarkable. The patient had no obstructive coronary artery disease on catheterization, no evidence of acute myocarditis on cardiac MRI, no prescribed medications associated with QT prolongation, and no evidence of genetic mutations associated with QT prolongation on testing. The minimal troponin elevation was felt to represent a type II myocardial infarction related to ischemia due to supply-demand mismatch rather than acute plaque rupture.
Littmann published a case series of 9 cases of delayed onset T-wave inversion and extreme QTc prolongation in the 24 to 48 hours following treatment and symptomatic improvement in acute pulmonary edema.4 In each of his patients, an ischemic cardiac insult was ruled out as the etiology of the pulmonary edema by laboratory assessment, echocardiography, and left heart catheterization.All of the patients in this case series recovered without incident and with normalization of the QTc interval.4 Similarly, in our patient, significant QT T changes occurred approximately 22 hours after presentation and with resolution of symptoms of pulmonary edema. Pascale and colleagues also published a series of 3 patients developing similar ECG patterns following a hypertensive crisis with resolution of ECG findings and without any morbidity.5 In contrast, our patient experienced significant morbidity secondary to the extreme QTc prolongation.
Conclusions
We believe this is the first reported case of excessive prolongation of the QTc with VF arrest secondary to resolution of acute pulmonary edema. The pattern observed in our patient follows the patterns outlined in the previous case series—patients present with acute pulmonary edema and hypertensive crisis but develop significant ECG abnormalities about 24 hours after the resolution of the high catecholamine state. Our patient did have a history of prior cardiac insult, given the QTc changes developed acutely, with frequent premature ventricular contractions, and the cardiac arrest occurred at maximal QTc prolongation, yet after resolution of the high catecholamine state, the treatment team felt there was likely an uncaptured and short-lived episode of TdP that degenerated into VF. This theory is further supported by the lack of recurrent VF episodes, confirmed by AICD interrogation, after normalization of the QTc in our patient.
1. Passman R, Kadish A. Polymorphic ventricular tachycardia, long Q-T syndrome, and torsades de pointes. Med Clin North Am. 2001;85(2):321-341. doi:10.1016/s0025-7125(05)70318-7
2. Kallergis EM, Goudis CA, Simantirakis EN, Kochiadakis GE, Vardas PE. Mechanisms, risk factors, and management of acquired long QT syndrome: a comprehensive review. ScientificWorldJournal. 2012;2012:212178. doi:10.1100/2012/212178
3. Miller MA, Elmariah S, Fischer A. Giant T-wave inversions and extreme QT prolongation. Circ Arrhythm Electrophysiol. 2009;2(6):e42-e43. doi:10.1161/CIRCEP.108.825729
4. Littmann L. Large T wave inversion and QT prolongation associated with pulmonary edema: a report of nine cases. J Am Coll Cardiol. 1999;34(4):1106-1110. doi:10.1016/s0735-1097(99)00311-3
5. Pascale P, Quartenoud B, Stauffer JC. Isolated large inverted T wave in pulmonary edema due to hypertensive crisis: a novel electrocardiographic phenomenon mimicking ischemia?. Clin Res Cardiol. 2007;96(5):288-294. doi:10.1007/s00392-007-0504-1
1. Passman R, Kadish A. Polymorphic ventricular tachycardia, long Q-T syndrome, and torsades de pointes. Med Clin North Am. 2001;85(2):321-341. doi:10.1016/s0025-7125(05)70318-7
2. Kallergis EM, Goudis CA, Simantirakis EN, Kochiadakis GE, Vardas PE. Mechanisms, risk factors, and management of acquired long QT syndrome: a comprehensive review. ScientificWorldJournal. 2012;2012:212178. doi:10.1100/2012/212178
3. Miller MA, Elmariah S, Fischer A. Giant T-wave inversions and extreme QT prolongation. Circ Arrhythm Electrophysiol. 2009;2(6):e42-e43. doi:10.1161/CIRCEP.108.825729
4. Littmann L. Large T wave inversion and QT prolongation associated with pulmonary edema: a report of nine cases. J Am Coll Cardiol. 1999;34(4):1106-1110. doi:10.1016/s0735-1097(99)00311-3
5. Pascale P, Quartenoud B, Stauffer JC. Isolated large inverted T wave in pulmonary edema due to hypertensive crisis: a novel electrocardiographic phenomenon mimicking ischemia?. Clin Res Cardiol. 2007;96(5):288-294. doi:10.1007/s00392-007-0504-1
Emphysematous Aortitis due to Klebsiella Pneumoniae in a Patient With Poorly Controlled Diabetes Mellitus
Patients with poorly controlled diabetes mellitus and an infectious source can be predisposed to infectious aortitis.
Aortitis is the all-encompassing term ascribed to the inflammatory process in the aortic wall that can be either infective or noninfective in origin, commonly autoimmune or inflammatory large-vessel vasculitis.1 Infectious aortitis, also known as bacterial, microbial, or cryptogenic aortitis, as well as mycotic or infected aneurysm, is a rare entity in the current antibiotic era but potentially a life-threatening disorder.2 The potential complications of infectious aortitis include emphysematous aortitis (EA), pseudoaneurysm, aortic rupture, septic emboli, and fistula formation (eg, aorto-enteric fistula).2,3
EA is a rare but serious inflammatory condition of the aorta with a nonspecific clinical presentation associated with high morbidity and mortality.2-6 The condition is characterized by a localized collection of gas and purulent exudate at the aortic wall.1,3 A few cases of EA have previously been reported; however, no known cases have been reported in the literature due to Klebsiella pneumoniae (K pneumoniae).
The pathophysiology of EA is the presence of underlying damage to the arterial wall caused by a hematogenously inoculated gas-producing organism.2,3 Most reported cases of EA are due to endovascular graft complications. Under normal circumstances, the aortic intima is highly resistant to infectious pathogens; however, certain risk factors, such as diabetes mellitus (DM), atherosclerotic disease, preexisting aneurysm, cystic medial necrosis, vascular malformation, presence of medical devices, surgery, or impaired immunity can alter the integrity of the aortic intimal layer and predispose the aortic intima to infection.1,4-7 Bacteria are the most common causative organisms that can infect the aorta, especially Staphylococcus, Enterococcus, Streptococcus, Salmonella, and spirochete Treponema pallidum (syphilis).1,2,4,8 The site of the primary infection remains unclear in some patients.2,3,5,6 Infection of the aorta can arise by several mechanisms: direct extension of a local infection to an existing intimal injury or atherosclerotic plaque (the most common mechanism), septic embolism from endocarditis, direct bacterial inoculation from traumatic contamination, contiguous infection extending to the aorta wall, or a distant source of bacteremia.2,3
Clinical manifestations of EA depend on the site and the extent of infection. The diagnosis should be considered in patients with atherosclerosis, fever, abdominal pain, and leukocytosis.2,4-8 The differential diagnosis for EA includes (1) noninfective causes of aortitis, including rheumatoid arthritis and systemic lupus erythematosus; (2) tuberculous aortitis; (3) syphilitic aortitis; and (4) idiopathic isolated aortitis. Establishing an early diagnosis of infectious aortitis is extremely important because this condition is associated with a high rate of morbidity and mortality secondary to aortic rupture.2-7
Imaging is critical for a reliable and quick diagnosis of acute aortic pathology. Noninvasive cross-sectional imaging modalities, such as contrast-enhanced computed tomography (CT), magnetic resonance imaging, nuclear medicine, or positron emission tomography, are used for both the initial diagnosis and follow-up of aortitis.1 CT is the primary imaging method in most medical centers because it is widely available with short acquisition time in critically ill patients.3 CT allows rapid detection of abnormalities in wall thickness, diameter, and density, and enhancement of periaortic structures, enabling reliable exclusion of other aortic pathologies that may resemble acute aortitis. Also, CT aids in planning the optimal therapeutic approach.1,3,5-8
This case illustrates EA associated with infection by K pneumoniae in a patient with poorly controlled type 2 DM (T2DM). In this single case, our patient presented to the Bay Pines Veterans Affairs Healthcare System (BPVAHS) in Florida with recent superficial soft tissue injury, severe hyperglycemia, worsening abdominal pain, and leukocytosis without fever or chills. The correct diagnosis of EA was confirmed by characteristic CT findings.
Case Presentation
A 72-year-old male with a history of T2DM, hypertension, atherosclerotic vascular disease, obstructive lung disease, and smoking 1.5 packs per day for 40 years presented with diabetic ketoacidosis, a urinary tract infection, and abdominal pain of 1-week duration that started to worsen the morning he arrived at the BPVAHS emergency department. He reported no nausea, vomiting, diarrhea, constipation, chest pain, shortness of breath, fever, chills, fatigue, or dysuria. He had a nonhealing laceration on his left medial foot that occurred 18 days before admission and was treated at an outside hospital.
The patient’s surgical history included a left common femoral endarterectomy and a left femoral popliteal above-knee reverse saphenous vein bypass 4 years ago for severe critical limb ischemia due to occlusion of his left superficial femoral artery with distal embolization to the first and fifth toes. About 6 months later, he developed disabling claudication in his left lower extremity due to distal popliteal artery occlusion and had another bypass surgery to the below-knee popliteal artery with a reverse saphenous vein graft harvested from the right thigh.
On initial examination, his vital signs were within normal limits except for a blood pressure of 177/87 mm Hg. His physical examination demonstrated a nondistended abdomen with normal bowel sounds, mild lower quadrant tenderness on the left more than on the right, intermittent abdominal pain located around umbilicus with radiation to the back, and a negative psoas sign. His left medial foot had a nonhealing laceration with black sutures in place, with minimal erythema in the surrounding tissue and scab formation. He also had mild costovertebral tenderness on the left.
Initial laboratory investigation results were notable for a glucose level of 609 mg/dL and a white blood cell count of 14.6 × 103 cells/mcL with 86.5% of neutrophils. A CT scan of his abdomen revealed extensive atherosclerosis of the abdominal aorta and periaortic aneurysmal fluid collection with multiple foci of gas (Figure 1). Additionally, the aneurysmal fluid collection involved the proximal segment of the left common femoral artery, suspicious for left femoral arteritis (Figure 2). The patient was started on broad-spectrum antibiotics, morphine, and an insulin drip. Both urine and blood cultures were positive for K pneumoniae susceptible to multiple antibiotics. He was transferred to a tertiary medical center and was referred for a vascular surgery consultation.
The patient underwent surgical resection of the infected infrarenal EA and infected left common femoral artery with right axillary-bifemoral bypass with an 8-mm PTFE (polytetrafluoroethylene) graft. During the surgery, excision of the wall of the left common femoral artery and infrarenal aorta revealed frank pus with purulent fluid, which was sent to cytology for analysis and culture. His intraoperative cultures grew K pneumoniae sensitive to multiple antibiotics, including ceftriaxone, sulfamethoxazole/trimethoprim, and ampicillin/sulbactam. The vascular surgery team recommended inpatient admission and administration of 6 weeks of IV antibiotics postoperatively with ceftriaxone, followed by outpatient oral suppression therapy after discharge. The patient tolerated the surgery well and was discharged after 6 weeks of IV ceftriaxone followed by outpatient oral suppression therapy. However, the patient was transferred back to BPVAHS for continued care and rehabilitation placement.
The patient’s subsequent course was complicated by multiple hospital admissions, including aspiration pneumonia, hypoglycemia, diarrhea, and anemia. On one of his CT abdomen/pelvic examinations, a cysticlike mass was noted in the pancreatic head with a possible pancreatic duodenal fistula (this mass was not mentioned on the initial presurgical CT, although it can be seen in retrospect (Figure 3). Gastroenterology was consulted.
An upper endoscopy was performed that confirmed the fistula at the second portion of the duodenum. Findings from an endoscopic ultrasonography performed at an outside institution were concerning for a main duct intraductal papillary mucinous neoplasm (IPMN) with fistula, with biopsy results pending.
Discussion
This case contributes to the evidence that poorly controlled T2DM can be a predisposing factor for multiple vascular complications, including the infection of the aortic wall with progression to EA. Klebsiella species are considered opportunistic, Gram-negative pathogens that may disseminate to other tissues, causing life-threatening infections, including pneumonia, UTIs, bacteremia, and sepsis.9K pneumoniae infections are particularly challenging in neonates, the elderly, and immunocompromised individuals.9 CT is sensitive and specific in the detection of this pathologic entity.1,3 In patients with a suspected infectious etiology, the presence of foci of gas on CT in solid organ tissues is usually associated with an anaerobic infection. Gas can also be produced by Gram-negative facultative anaerobes that can ferment glucose in necrotic tissues.9
Although any microorganism can infect the aorta, K pneumoniae cultured from the blood specimen, urine culture, and intraoperative specimens in our patient was responsible for the formed gas in the aortic wall. Occurrence of spontaneous gas by this microorganism is usually associated with conditions leading to either increased vulnerability to infections and/or enhanced bacterial virulence.9 Although a relationship between EA and T2DM has not been proved, it is well known that patients with T2DM have a defect in their host-defense mechanisms, making them more susceptible to infections such as EA. Furthermore, because patients with T2DM are prone to the development of Gram-negative sepsis, organisms such as K pneumoniae would tend to emerge. Patients with poorly controlled T2DM and the presence of an infectious source can be predisposed to infectious aortitis, eventually leading to a gas-forming infection of the aorta.5,7
We postulate that the hematogenous spread of bacteria from a laceration in the leg as well as the presence of the pancreaticoduodenal fistula was likely the cause of the infectious EA in this case, considering the patient’s underlying uncontrolled T2DM. The patient’s prior left lower extremity vascular graft also may have provided a nidus for spreading to the aorta. Other reported underlying diseases of EA include aortic atherosclerosis, T2DM, diverticulitis, colon cancer, underlying aneurysm, immune-compromised status, and the presence of a medical device or open surgery.4-7,9
To our knowledge, this is the first case of EA associated with a pancreaticoduodenal fistula related to intraductal papillary mucinous neoplasm (IPMN). Fistulation of a main duct IPMN is rare, occurring in just 6.6% of cases.10 It can occur both before and after malignant degeneration.
EA requires rapid diagnosis, antibiotic therapy, and consultation with a vascular surgeon for immediate resection of the infected artery and graft bypass. The initial treatment of suspected infectious aortitis is IV antibiotics with broad antimicrobial coverage of the most likely pathologic organisms, particularly staphylococcal species and Gram-negative rods. Surgical debridement and revascularization should be completed early because of the high mortality rate of this condition. The intent of surgery is to control sepsis and reconstruct the arterial vasculature. Patients should remain on parenteral or oral antibiotics for at least 6 weeks to ensure full clearance of the infection.8 They should be followed up closely with serial blood cultures and CT scans.8 The rarity of the disorder, low level of awareness, varying presentations, and lack of evidence delineating pathogenesis and causality contribute to the challenge of recognizing, diagnosing, and treating EA in patients with T2DM and inflammation.
Conclusions
This case report can help bring awareness of this rare and potentially life-threatening condition in patients with T2DM. Clinicians should be aware of the risk of AE, particularly in patients with several additional risk factors: recent skin/soft tissue trauma, prior vascular graft surgery, and an underlying pancreatic mass. CT is the imaging method of choice that helps to rapidly choose a necessary emergent treatment approach.
1. Litmanovich DE, Yıldırım A, Bankier AA. Insights into imaging of aortitis. Insights Imaging. 2012;3(6):545-560. doi:10.1007/s13244-012-0192-x
2. Lopes RJ, Almeida J, Dias PJ, Pinho P, Maciel MJ. Infectious thoracic aortitis: a literature review. Clin Cardiol. 2009;32(9):488-490. doi:10.1002/clc.20578
3. Murphy DJ, Keraliya AR, Agrawal MD, Aghayev A, Steigner ML. Cross-sectional imaging of aortic infections. Insights Imaging. 2016;7(6):801-818. doi:10.1007/s13244-016-0522-5
4. Md Noh MSF, Abdul Rashid AM, Ar A, B N, Mohammed Y, A RE. Emphysematous aortitis: report of two cases and CT imaging findings. BJR Case Rep. 2017;3(3):20170006. doi:10.1259/bjrcr.20170006
5. Harris C, Geffen J, Rizg K, et al. A rare report of infectious emphysematous aortitis secondary to Clostridium septicum without prior vascular intervention. Case Rep Vasc Med. 2017;2017:4984325. doi:10.1155/2017/4984325
6. Ito F, Inokuchi R, Matsumoto A, et al. Presence of periaortic gas in Clostridium septicum-infected aortic aneurysm aids in early diagnosis: a case report and systematic review of the literature. J Med Case Rep. 2017;11(1):268. doi:10.1186/s13256-017-1422-0
7. Urgiles S, Matos-Casano H, Win KZ, Berardo J, Bhatt U, Shah J. Emphysematous aortitis due to Clostridium septicum in an 89-year-old female with ileus. Case Rep Infect Dis. 2019;2019:1094837. doi:10.1155/2019/1094837
8. Foote EA, Postier RG, Greenfield RA, Bronze MS. Infectious aortitis. Curr Treat Options Cardiovasc Med. 2005;7(2):89-97. doi:10.1007/s11936-005-0010-6
9. Paczosa MK, Mecsas J. Klebsiella pneumoniae: going on the offense with a strong defense. Microbiol Mol Biol Rev. 2016;80(3):629-661. doi:10.1128/mmbr.00078-15
10. Kobayashi G, Fujita N, Noda Y, et al. Intraductal papillary mucinous neoplasms of the pancreas showing fistula formation into other organs. J Gastroenterol. 2010;45(10):1080-1089. doi:10.1007/s00535-010-0263-z
Patients with poorly controlled diabetes mellitus and an infectious source can be predisposed to infectious aortitis.
Patients with poorly controlled diabetes mellitus and an infectious source can be predisposed to infectious aortitis.
Aortitis is the all-encompassing term ascribed to the inflammatory process in the aortic wall that can be either infective or noninfective in origin, commonly autoimmune or inflammatory large-vessel vasculitis.1 Infectious aortitis, also known as bacterial, microbial, or cryptogenic aortitis, as well as mycotic or infected aneurysm, is a rare entity in the current antibiotic era but potentially a life-threatening disorder.2 The potential complications of infectious aortitis include emphysematous aortitis (EA), pseudoaneurysm, aortic rupture, septic emboli, and fistula formation (eg, aorto-enteric fistula).2,3
EA is a rare but serious inflammatory condition of the aorta with a nonspecific clinical presentation associated with high morbidity and mortality.2-6 The condition is characterized by a localized collection of gas and purulent exudate at the aortic wall.1,3 A few cases of EA have previously been reported; however, no known cases have been reported in the literature due to Klebsiella pneumoniae (K pneumoniae).
The pathophysiology of EA is the presence of underlying damage to the arterial wall caused by a hematogenously inoculated gas-producing organism.2,3 Most reported cases of EA are due to endovascular graft complications. Under normal circumstances, the aortic intima is highly resistant to infectious pathogens; however, certain risk factors, such as diabetes mellitus (DM), atherosclerotic disease, preexisting aneurysm, cystic medial necrosis, vascular malformation, presence of medical devices, surgery, or impaired immunity can alter the integrity of the aortic intimal layer and predispose the aortic intima to infection.1,4-7 Bacteria are the most common causative organisms that can infect the aorta, especially Staphylococcus, Enterococcus, Streptococcus, Salmonella, and spirochete Treponema pallidum (syphilis).1,2,4,8 The site of the primary infection remains unclear in some patients.2,3,5,6 Infection of the aorta can arise by several mechanisms: direct extension of a local infection to an existing intimal injury or atherosclerotic plaque (the most common mechanism), septic embolism from endocarditis, direct bacterial inoculation from traumatic contamination, contiguous infection extending to the aorta wall, or a distant source of bacteremia.2,3
Clinical manifestations of EA depend on the site and the extent of infection. The diagnosis should be considered in patients with atherosclerosis, fever, abdominal pain, and leukocytosis.2,4-8 The differential diagnosis for EA includes (1) noninfective causes of aortitis, including rheumatoid arthritis and systemic lupus erythematosus; (2) tuberculous aortitis; (3) syphilitic aortitis; and (4) idiopathic isolated aortitis. Establishing an early diagnosis of infectious aortitis is extremely important because this condition is associated with a high rate of morbidity and mortality secondary to aortic rupture.2-7
Imaging is critical for a reliable and quick diagnosis of acute aortic pathology. Noninvasive cross-sectional imaging modalities, such as contrast-enhanced computed tomography (CT), magnetic resonance imaging, nuclear medicine, or positron emission tomography, are used for both the initial diagnosis and follow-up of aortitis.1 CT is the primary imaging method in most medical centers because it is widely available with short acquisition time in critically ill patients.3 CT allows rapid detection of abnormalities in wall thickness, diameter, and density, and enhancement of periaortic structures, enabling reliable exclusion of other aortic pathologies that may resemble acute aortitis. Also, CT aids in planning the optimal therapeutic approach.1,3,5-8
This case illustrates EA associated with infection by K pneumoniae in a patient with poorly controlled type 2 DM (T2DM). In this single case, our patient presented to the Bay Pines Veterans Affairs Healthcare System (BPVAHS) in Florida with recent superficial soft tissue injury, severe hyperglycemia, worsening abdominal pain, and leukocytosis without fever or chills. The correct diagnosis of EA was confirmed by characteristic CT findings.
Case Presentation
A 72-year-old male with a history of T2DM, hypertension, atherosclerotic vascular disease, obstructive lung disease, and smoking 1.5 packs per day for 40 years presented with diabetic ketoacidosis, a urinary tract infection, and abdominal pain of 1-week duration that started to worsen the morning he arrived at the BPVAHS emergency department. He reported no nausea, vomiting, diarrhea, constipation, chest pain, shortness of breath, fever, chills, fatigue, or dysuria. He had a nonhealing laceration on his left medial foot that occurred 18 days before admission and was treated at an outside hospital.
The patient’s surgical history included a left common femoral endarterectomy and a left femoral popliteal above-knee reverse saphenous vein bypass 4 years ago for severe critical limb ischemia due to occlusion of his left superficial femoral artery with distal embolization to the first and fifth toes. About 6 months later, he developed disabling claudication in his left lower extremity due to distal popliteal artery occlusion and had another bypass surgery to the below-knee popliteal artery with a reverse saphenous vein graft harvested from the right thigh.
On initial examination, his vital signs were within normal limits except for a blood pressure of 177/87 mm Hg. His physical examination demonstrated a nondistended abdomen with normal bowel sounds, mild lower quadrant tenderness on the left more than on the right, intermittent abdominal pain located around umbilicus with radiation to the back, and a negative psoas sign. His left medial foot had a nonhealing laceration with black sutures in place, with minimal erythema in the surrounding tissue and scab formation. He also had mild costovertebral tenderness on the left.
Initial laboratory investigation results were notable for a glucose level of 609 mg/dL and a white blood cell count of 14.6 × 103 cells/mcL with 86.5% of neutrophils. A CT scan of his abdomen revealed extensive atherosclerosis of the abdominal aorta and periaortic aneurysmal fluid collection with multiple foci of gas (Figure 1). Additionally, the aneurysmal fluid collection involved the proximal segment of the left common femoral artery, suspicious for left femoral arteritis (Figure 2). The patient was started on broad-spectrum antibiotics, morphine, and an insulin drip. Both urine and blood cultures were positive for K pneumoniae susceptible to multiple antibiotics. He was transferred to a tertiary medical center and was referred for a vascular surgery consultation.
The patient underwent surgical resection of the infected infrarenal EA and infected left common femoral artery with right axillary-bifemoral bypass with an 8-mm PTFE (polytetrafluoroethylene) graft. During the surgery, excision of the wall of the left common femoral artery and infrarenal aorta revealed frank pus with purulent fluid, which was sent to cytology for analysis and culture. His intraoperative cultures grew K pneumoniae sensitive to multiple antibiotics, including ceftriaxone, sulfamethoxazole/trimethoprim, and ampicillin/sulbactam. The vascular surgery team recommended inpatient admission and administration of 6 weeks of IV antibiotics postoperatively with ceftriaxone, followed by outpatient oral suppression therapy after discharge. The patient tolerated the surgery well and was discharged after 6 weeks of IV ceftriaxone followed by outpatient oral suppression therapy. However, the patient was transferred back to BPVAHS for continued care and rehabilitation placement.
The patient’s subsequent course was complicated by multiple hospital admissions, including aspiration pneumonia, hypoglycemia, diarrhea, and anemia. On one of his CT abdomen/pelvic examinations, a cysticlike mass was noted in the pancreatic head with a possible pancreatic duodenal fistula (this mass was not mentioned on the initial presurgical CT, although it can be seen in retrospect (Figure 3). Gastroenterology was consulted.
An upper endoscopy was performed that confirmed the fistula at the second portion of the duodenum. Findings from an endoscopic ultrasonography performed at an outside institution were concerning for a main duct intraductal papillary mucinous neoplasm (IPMN) with fistula, with biopsy results pending.
Discussion
This case contributes to the evidence that poorly controlled T2DM can be a predisposing factor for multiple vascular complications, including the infection of the aortic wall with progression to EA. Klebsiella species are considered opportunistic, Gram-negative pathogens that may disseminate to other tissues, causing life-threatening infections, including pneumonia, UTIs, bacteremia, and sepsis.9K pneumoniae infections are particularly challenging in neonates, the elderly, and immunocompromised individuals.9 CT is sensitive and specific in the detection of this pathologic entity.1,3 In patients with a suspected infectious etiology, the presence of foci of gas on CT in solid organ tissues is usually associated with an anaerobic infection. Gas can also be produced by Gram-negative facultative anaerobes that can ferment glucose in necrotic tissues.9
Although any microorganism can infect the aorta, K pneumoniae cultured from the blood specimen, urine culture, and intraoperative specimens in our patient was responsible for the formed gas in the aortic wall. Occurrence of spontaneous gas by this microorganism is usually associated with conditions leading to either increased vulnerability to infections and/or enhanced bacterial virulence.9 Although a relationship between EA and T2DM has not been proved, it is well known that patients with T2DM have a defect in their host-defense mechanisms, making them more susceptible to infections such as EA. Furthermore, because patients with T2DM are prone to the development of Gram-negative sepsis, organisms such as K pneumoniae would tend to emerge. Patients with poorly controlled T2DM and the presence of an infectious source can be predisposed to infectious aortitis, eventually leading to a gas-forming infection of the aorta.5,7
We postulate that the hematogenous spread of bacteria from a laceration in the leg as well as the presence of the pancreaticoduodenal fistula was likely the cause of the infectious EA in this case, considering the patient’s underlying uncontrolled T2DM. The patient’s prior left lower extremity vascular graft also may have provided a nidus for spreading to the aorta. Other reported underlying diseases of EA include aortic atherosclerosis, T2DM, diverticulitis, colon cancer, underlying aneurysm, immune-compromised status, and the presence of a medical device or open surgery.4-7,9
To our knowledge, this is the first case of EA associated with a pancreaticoduodenal fistula related to intraductal papillary mucinous neoplasm (IPMN). Fistulation of a main duct IPMN is rare, occurring in just 6.6% of cases.10 It can occur both before and after malignant degeneration.
EA requires rapid diagnosis, antibiotic therapy, and consultation with a vascular surgeon for immediate resection of the infected artery and graft bypass. The initial treatment of suspected infectious aortitis is IV antibiotics with broad antimicrobial coverage of the most likely pathologic organisms, particularly staphylococcal species and Gram-negative rods. Surgical debridement and revascularization should be completed early because of the high mortality rate of this condition. The intent of surgery is to control sepsis and reconstruct the arterial vasculature. Patients should remain on parenteral or oral antibiotics for at least 6 weeks to ensure full clearance of the infection.8 They should be followed up closely with serial blood cultures and CT scans.8 The rarity of the disorder, low level of awareness, varying presentations, and lack of evidence delineating pathogenesis and causality contribute to the challenge of recognizing, diagnosing, and treating EA in patients with T2DM and inflammation.
Conclusions
This case report can help bring awareness of this rare and potentially life-threatening condition in patients with T2DM. Clinicians should be aware of the risk of AE, particularly in patients with several additional risk factors: recent skin/soft tissue trauma, prior vascular graft surgery, and an underlying pancreatic mass. CT is the imaging method of choice that helps to rapidly choose a necessary emergent treatment approach.
Aortitis is the all-encompassing term ascribed to the inflammatory process in the aortic wall that can be either infective or noninfective in origin, commonly autoimmune or inflammatory large-vessel vasculitis.1 Infectious aortitis, also known as bacterial, microbial, or cryptogenic aortitis, as well as mycotic or infected aneurysm, is a rare entity in the current antibiotic era but potentially a life-threatening disorder.2 The potential complications of infectious aortitis include emphysematous aortitis (EA), pseudoaneurysm, aortic rupture, septic emboli, and fistula formation (eg, aorto-enteric fistula).2,3
EA is a rare but serious inflammatory condition of the aorta with a nonspecific clinical presentation associated with high morbidity and mortality.2-6 The condition is characterized by a localized collection of gas and purulent exudate at the aortic wall.1,3 A few cases of EA have previously been reported; however, no known cases have been reported in the literature due to Klebsiella pneumoniae (K pneumoniae).
The pathophysiology of EA is the presence of underlying damage to the arterial wall caused by a hematogenously inoculated gas-producing organism.2,3 Most reported cases of EA are due to endovascular graft complications. Under normal circumstances, the aortic intima is highly resistant to infectious pathogens; however, certain risk factors, such as diabetes mellitus (DM), atherosclerotic disease, preexisting aneurysm, cystic medial necrosis, vascular malformation, presence of medical devices, surgery, or impaired immunity can alter the integrity of the aortic intimal layer and predispose the aortic intima to infection.1,4-7 Bacteria are the most common causative organisms that can infect the aorta, especially Staphylococcus, Enterococcus, Streptococcus, Salmonella, and spirochete Treponema pallidum (syphilis).1,2,4,8 The site of the primary infection remains unclear in some patients.2,3,5,6 Infection of the aorta can arise by several mechanisms: direct extension of a local infection to an existing intimal injury or atherosclerotic plaque (the most common mechanism), septic embolism from endocarditis, direct bacterial inoculation from traumatic contamination, contiguous infection extending to the aorta wall, or a distant source of bacteremia.2,3
Clinical manifestations of EA depend on the site and the extent of infection. The diagnosis should be considered in patients with atherosclerosis, fever, abdominal pain, and leukocytosis.2,4-8 The differential diagnosis for EA includes (1) noninfective causes of aortitis, including rheumatoid arthritis and systemic lupus erythematosus; (2) tuberculous aortitis; (3) syphilitic aortitis; and (4) idiopathic isolated aortitis. Establishing an early diagnosis of infectious aortitis is extremely important because this condition is associated with a high rate of morbidity and mortality secondary to aortic rupture.2-7
Imaging is critical for a reliable and quick diagnosis of acute aortic pathology. Noninvasive cross-sectional imaging modalities, such as contrast-enhanced computed tomography (CT), magnetic resonance imaging, nuclear medicine, or positron emission tomography, are used for both the initial diagnosis and follow-up of aortitis.1 CT is the primary imaging method in most medical centers because it is widely available with short acquisition time in critically ill patients.3 CT allows rapid detection of abnormalities in wall thickness, diameter, and density, and enhancement of periaortic structures, enabling reliable exclusion of other aortic pathologies that may resemble acute aortitis. Also, CT aids in planning the optimal therapeutic approach.1,3,5-8
This case illustrates EA associated with infection by K pneumoniae in a patient with poorly controlled type 2 DM (T2DM). In this single case, our patient presented to the Bay Pines Veterans Affairs Healthcare System (BPVAHS) in Florida with recent superficial soft tissue injury, severe hyperglycemia, worsening abdominal pain, and leukocytosis without fever or chills. The correct diagnosis of EA was confirmed by characteristic CT findings.
Case Presentation
A 72-year-old male with a history of T2DM, hypertension, atherosclerotic vascular disease, obstructive lung disease, and smoking 1.5 packs per day for 40 years presented with diabetic ketoacidosis, a urinary tract infection, and abdominal pain of 1-week duration that started to worsen the morning he arrived at the BPVAHS emergency department. He reported no nausea, vomiting, diarrhea, constipation, chest pain, shortness of breath, fever, chills, fatigue, or dysuria. He had a nonhealing laceration on his left medial foot that occurred 18 days before admission and was treated at an outside hospital.
The patient’s surgical history included a left common femoral endarterectomy and a left femoral popliteal above-knee reverse saphenous vein bypass 4 years ago for severe critical limb ischemia due to occlusion of his left superficial femoral artery with distal embolization to the first and fifth toes. About 6 months later, he developed disabling claudication in his left lower extremity due to distal popliteal artery occlusion and had another bypass surgery to the below-knee popliteal artery with a reverse saphenous vein graft harvested from the right thigh.
On initial examination, his vital signs were within normal limits except for a blood pressure of 177/87 mm Hg. His physical examination demonstrated a nondistended abdomen with normal bowel sounds, mild lower quadrant tenderness on the left more than on the right, intermittent abdominal pain located around umbilicus with radiation to the back, and a negative psoas sign. His left medial foot had a nonhealing laceration with black sutures in place, with minimal erythema in the surrounding tissue and scab formation. He also had mild costovertebral tenderness on the left.
Initial laboratory investigation results were notable for a glucose level of 609 mg/dL and a white blood cell count of 14.6 × 103 cells/mcL with 86.5% of neutrophils. A CT scan of his abdomen revealed extensive atherosclerosis of the abdominal aorta and periaortic aneurysmal fluid collection with multiple foci of gas (Figure 1). Additionally, the aneurysmal fluid collection involved the proximal segment of the left common femoral artery, suspicious for left femoral arteritis (Figure 2). The patient was started on broad-spectrum antibiotics, morphine, and an insulin drip. Both urine and blood cultures were positive for K pneumoniae susceptible to multiple antibiotics. He was transferred to a tertiary medical center and was referred for a vascular surgery consultation.
The patient underwent surgical resection of the infected infrarenal EA and infected left common femoral artery with right axillary-bifemoral bypass with an 8-mm PTFE (polytetrafluoroethylene) graft. During the surgery, excision of the wall of the left common femoral artery and infrarenal aorta revealed frank pus with purulent fluid, which was sent to cytology for analysis and culture. His intraoperative cultures grew K pneumoniae sensitive to multiple antibiotics, including ceftriaxone, sulfamethoxazole/trimethoprim, and ampicillin/sulbactam. The vascular surgery team recommended inpatient admission and administration of 6 weeks of IV antibiotics postoperatively with ceftriaxone, followed by outpatient oral suppression therapy after discharge. The patient tolerated the surgery well and was discharged after 6 weeks of IV ceftriaxone followed by outpatient oral suppression therapy. However, the patient was transferred back to BPVAHS for continued care and rehabilitation placement.
The patient’s subsequent course was complicated by multiple hospital admissions, including aspiration pneumonia, hypoglycemia, diarrhea, and anemia. On one of his CT abdomen/pelvic examinations, a cysticlike mass was noted in the pancreatic head with a possible pancreatic duodenal fistula (this mass was not mentioned on the initial presurgical CT, although it can be seen in retrospect (Figure 3). Gastroenterology was consulted.
An upper endoscopy was performed that confirmed the fistula at the second portion of the duodenum. Findings from an endoscopic ultrasonography performed at an outside institution were concerning for a main duct intraductal papillary mucinous neoplasm (IPMN) with fistula, with biopsy results pending.
Discussion
This case contributes to the evidence that poorly controlled T2DM can be a predisposing factor for multiple vascular complications, including the infection of the aortic wall with progression to EA. Klebsiella species are considered opportunistic, Gram-negative pathogens that may disseminate to other tissues, causing life-threatening infections, including pneumonia, UTIs, bacteremia, and sepsis.9K pneumoniae infections are particularly challenging in neonates, the elderly, and immunocompromised individuals.9 CT is sensitive and specific in the detection of this pathologic entity.1,3 In patients with a suspected infectious etiology, the presence of foci of gas on CT in solid organ tissues is usually associated with an anaerobic infection. Gas can also be produced by Gram-negative facultative anaerobes that can ferment glucose in necrotic tissues.9
Although any microorganism can infect the aorta, K pneumoniae cultured from the blood specimen, urine culture, and intraoperative specimens in our patient was responsible for the formed gas in the aortic wall. Occurrence of spontaneous gas by this microorganism is usually associated with conditions leading to either increased vulnerability to infections and/or enhanced bacterial virulence.9 Although a relationship between EA and T2DM has not been proved, it is well known that patients with T2DM have a defect in their host-defense mechanisms, making them more susceptible to infections such as EA. Furthermore, because patients with T2DM are prone to the development of Gram-negative sepsis, organisms such as K pneumoniae would tend to emerge. Patients with poorly controlled T2DM and the presence of an infectious source can be predisposed to infectious aortitis, eventually leading to a gas-forming infection of the aorta.5,7
We postulate that the hematogenous spread of bacteria from a laceration in the leg as well as the presence of the pancreaticoduodenal fistula was likely the cause of the infectious EA in this case, considering the patient’s underlying uncontrolled T2DM. The patient’s prior left lower extremity vascular graft also may have provided a nidus for spreading to the aorta. Other reported underlying diseases of EA include aortic atherosclerosis, T2DM, diverticulitis, colon cancer, underlying aneurysm, immune-compromised status, and the presence of a medical device or open surgery.4-7,9
To our knowledge, this is the first case of EA associated with a pancreaticoduodenal fistula related to intraductal papillary mucinous neoplasm (IPMN). Fistulation of a main duct IPMN is rare, occurring in just 6.6% of cases.10 It can occur both before and after malignant degeneration.
EA requires rapid diagnosis, antibiotic therapy, and consultation with a vascular surgeon for immediate resection of the infected artery and graft bypass. The initial treatment of suspected infectious aortitis is IV antibiotics with broad antimicrobial coverage of the most likely pathologic organisms, particularly staphylococcal species and Gram-negative rods. Surgical debridement and revascularization should be completed early because of the high mortality rate of this condition. The intent of surgery is to control sepsis and reconstruct the arterial vasculature. Patients should remain on parenteral or oral antibiotics for at least 6 weeks to ensure full clearance of the infection.8 They should be followed up closely with serial blood cultures and CT scans.8 The rarity of the disorder, low level of awareness, varying presentations, and lack of evidence delineating pathogenesis and causality contribute to the challenge of recognizing, diagnosing, and treating EA in patients with T2DM and inflammation.
Conclusions
This case report can help bring awareness of this rare and potentially life-threatening condition in patients with T2DM. Clinicians should be aware of the risk of AE, particularly in patients with several additional risk factors: recent skin/soft tissue trauma, prior vascular graft surgery, and an underlying pancreatic mass. CT is the imaging method of choice that helps to rapidly choose a necessary emergent treatment approach.
1. Litmanovich DE, Yıldırım A, Bankier AA. Insights into imaging of aortitis. Insights Imaging. 2012;3(6):545-560. doi:10.1007/s13244-012-0192-x
2. Lopes RJ, Almeida J, Dias PJ, Pinho P, Maciel MJ. Infectious thoracic aortitis: a literature review. Clin Cardiol. 2009;32(9):488-490. doi:10.1002/clc.20578
3. Murphy DJ, Keraliya AR, Agrawal MD, Aghayev A, Steigner ML. Cross-sectional imaging of aortic infections. Insights Imaging. 2016;7(6):801-818. doi:10.1007/s13244-016-0522-5
4. Md Noh MSF, Abdul Rashid AM, Ar A, B N, Mohammed Y, A RE. Emphysematous aortitis: report of two cases and CT imaging findings. BJR Case Rep. 2017;3(3):20170006. doi:10.1259/bjrcr.20170006
5. Harris C, Geffen J, Rizg K, et al. A rare report of infectious emphysematous aortitis secondary to Clostridium septicum without prior vascular intervention. Case Rep Vasc Med. 2017;2017:4984325. doi:10.1155/2017/4984325
6. Ito F, Inokuchi R, Matsumoto A, et al. Presence of periaortic gas in Clostridium septicum-infected aortic aneurysm aids in early diagnosis: a case report and systematic review of the literature. J Med Case Rep. 2017;11(1):268. doi:10.1186/s13256-017-1422-0
7. Urgiles S, Matos-Casano H, Win KZ, Berardo J, Bhatt U, Shah J. Emphysematous aortitis due to Clostridium septicum in an 89-year-old female with ileus. Case Rep Infect Dis. 2019;2019:1094837. doi:10.1155/2019/1094837
8. Foote EA, Postier RG, Greenfield RA, Bronze MS. Infectious aortitis. Curr Treat Options Cardiovasc Med. 2005;7(2):89-97. doi:10.1007/s11936-005-0010-6
9. Paczosa MK, Mecsas J. Klebsiella pneumoniae: going on the offense with a strong defense. Microbiol Mol Biol Rev. 2016;80(3):629-661. doi:10.1128/mmbr.00078-15
10. Kobayashi G, Fujita N, Noda Y, et al. Intraductal papillary mucinous neoplasms of the pancreas showing fistula formation into other organs. J Gastroenterol. 2010;45(10):1080-1089. doi:10.1007/s00535-010-0263-z
1. Litmanovich DE, Yıldırım A, Bankier AA. Insights into imaging of aortitis. Insights Imaging. 2012;3(6):545-560. doi:10.1007/s13244-012-0192-x
2. Lopes RJ, Almeida J, Dias PJ, Pinho P, Maciel MJ. Infectious thoracic aortitis: a literature review. Clin Cardiol. 2009;32(9):488-490. doi:10.1002/clc.20578
3. Murphy DJ, Keraliya AR, Agrawal MD, Aghayev A, Steigner ML. Cross-sectional imaging of aortic infections. Insights Imaging. 2016;7(6):801-818. doi:10.1007/s13244-016-0522-5
4. Md Noh MSF, Abdul Rashid AM, Ar A, B N, Mohammed Y, A RE. Emphysematous aortitis: report of two cases and CT imaging findings. BJR Case Rep. 2017;3(3):20170006. doi:10.1259/bjrcr.20170006
5. Harris C, Geffen J, Rizg K, et al. A rare report of infectious emphysematous aortitis secondary to Clostridium septicum without prior vascular intervention. Case Rep Vasc Med. 2017;2017:4984325. doi:10.1155/2017/4984325
6. Ito F, Inokuchi R, Matsumoto A, et al. Presence of periaortic gas in Clostridium septicum-infected aortic aneurysm aids in early diagnosis: a case report and systematic review of the literature. J Med Case Rep. 2017;11(1):268. doi:10.1186/s13256-017-1422-0
7. Urgiles S, Matos-Casano H, Win KZ, Berardo J, Bhatt U, Shah J. Emphysematous aortitis due to Clostridium septicum in an 89-year-old female with ileus. Case Rep Infect Dis. 2019;2019:1094837. doi:10.1155/2019/1094837
8. Foote EA, Postier RG, Greenfield RA, Bronze MS. Infectious aortitis. Curr Treat Options Cardiovasc Med. 2005;7(2):89-97. doi:10.1007/s11936-005-0010-6
9. Paczosa MK, Mecsas J. Klebsiella pneumoniae: going on the offense with a strong defense. Microbiol Mol Biol Rev. 2016;80(3):629-661. doi:10.1128/mmbr.00078-15
10. Kobayashi G, Fujita N, Noda Y, et al. Intraductal papillary mucinous neoplasms of the pancreas showing fistula formation into other organs. J Gastroenterol. 2010;45(10):1080-1089. doi:10.1007/s00535-010-0263-z
Rosuvastatin-Induced Rhabdomyolysis, Pancreatitis, Transaminitis, and Acute Kidney Injury
Changing medications within a drug class requires considering the indication and dosage, possible adverse effects, and drug-drug interactions.
Attention should be paid to changing a tolerated medication to another within its class. Many drugs approved by the US Food and Drug Administration (FDA), have equivalent therapeutic properties as existing drugs. Rarely do such medications share the same potency and adverse effect (AE) profile.
Case Presentation
A 77-year-old man presented to the emergency department (ED) at the Raymond G. Murphy Medical Center in Albuquerque, New Mexico, with a 1-month history of progressive muscle weakness, which was so severe that he required assistance rising from chairs. The symptoms began when he switched from atorvastatin 40 mg daily to rosuvastatin 40 mg daily. A nephrology consultation was requested for an elevated plasma creatinine.
The patient reported strict adherence to his prescribed medications. In the days following the switch to rosuvastatin, he noticed that his urine turned black. He described the color as “like burnt coffee.” The color gradually cleared before his ED presentation. The patient stopped taking rosuvastatin the day prior to presentation and noted improvement of his symptoms. Review of symptoms was significant for lower extremity paresthesia and numbness the day he started rosuvastatin. He had no symptoms of decompensated heart failure and no recent exacerbations requiring alteration of his diuretic regimen.
The patient’s medical history was significant for traumatic brain injury with complex partial seizures, carpal tunnel syndrome, dyslipidemia, coronary artery disease with percutaneous intervention to the right coronary artery in the late 1990s, atrial fibrillation and ventricular tachycardia, status post implantable cardioverter defibrillator, heart failure with reduced ejection fraction (25%) attributed to ischemic cardiomyopathy, hypertension, lower urinary tract symptoms/prostatism, and previous bladder cancer. In the mid-1960s, the patient served in the US Army and had been deployed to South Korea. After the service, he worked for the local city government. He was retired for about 15 years. He reported no tobacco, alcohol, or recreational drug use and no tattoos. He did not require prior blood or blood product transfusions. None of his family members—parents, siblings, or children—had any history of kidney disease.
The patient’s outpatient medications included levetiracetam 750 mg twice daily, melatonin 9 mg at night, menthol 16%/methyl-salicylate 30% topically up to 4 times per day as needed, aspirin 81 mg once daily, fish oil 1000 mg twice daily, amiodarone 400 mg twice daily, hydralazine 20 mg 3 times daily, isosorbide mononitrate 60 mg daily, metoprolol succinate 100 mg daily, and tamsulosin 0.4 mg at night. His vital signs were stable: afebrile (97.5 ºF), normocardic (74 beats per minute), normotensive (118/78 mm Hg), and normoxic (98% on room air). On examination, he appeared elderly, somewhat frail, and chronically ill but in no acute distress. Affect was pleasant and appropriate, attention was high, and his thought process was logical. He had sparse, grey scalp hair. Extraocular movements were intact. Oral mucosa was pink and moist. His back was nontender, and there was no costovertebral tenderness bilaterally. The patient was in no respiratory distress, with a slightly hyperresonant chest to percussion bilaterally, very faint inspiratory basilar crepitant rales (that cleared with repeat inspiration), and was otherwise clear to auscultation throughout. An outline of an implanted pacemaker was evident on the chest under his left clavicle, with a laterally displaced apical impulse. The rate was normal and the rhythm was regular. Upper extremities demonstrated papyraceous skin but without cyanosis, clubbing, or edema. Radial pulses were slightly diminished. He had no lower extremity edema.
His laboratory values are provided in Table 1. Kidney function was stable months prior to admission. Of note, the blood urea nitrogen and plasma creatinine were increased from his baseline up to 47 and 5.89 mg/dL, respectively. The serum glutamic-oxaloacetic transaminase and serum glutamic pyruvic transaminase were 1051 U/L and 408 U/L, respectively. Plasma amylase and lipase levels also were elevated, 230 U/L and 892 U/L, respectively. Creatine kinase was 41,099 U/L. Urinalysis demonstrated a specific gravity of 1.017, pH of 5, and a large amount of blood (92 red blood cells/high power field).
A 12-lead electrocardiogram demonstrated a sinus rhythm, PR interval of 0.20 ms, narrow QRS with a leftward frontal axis deviation, R-transition between precordial leads V1 and V2, and flattening of the ST segments in III, V1-V3 (Figure 1). A portable chest X-ray demonstrated clear lung fields, no evidence of effusion in the costophrenic area. Ultrasonography was conducted at the time of the examination (Figure 2). The kidneys were smoothly contoured, each measuring > 10 cm; there was an exophytic cyst on the left. Otherwise, the cortices, perhaps slightly echogenic, did not appear diminished. The bladder was not abnormally enlarged.
Rosuvastatin-induced rhabdomyolysis, pancreatitis, transaminitis, and drug-induced acute kidney injury were considered high among the diagnostic differentials. The 3-hydroxy-3-methyl-glutaryl-CoA (HMG-CoA) reductase inhibitor was stopped, and he was prescribed an acute renal insufficiency diet. All laboratory parameters improved with this change (Figure 3). Two months after presentation (and with rosuvastatin added to his list of adverse reactions), all symptoms resolved and his plasma creatinine reached a nadir of 1.22 mg/dL.
Discussion
Statin-class drugs inhibit the HMG-CoA reductase (Table 2). Upregulation of low-density lipoprotein cholesterol (LDL-C) receptors in the liver result in increased LDL-C uptake and cholesterol catabolism.1 Prescribed inhibitors of the HMG-CoA reductase—statins—are known to reduce mortality due to cardiovascular disease (CVD). Much like any other pharmaceutical agent with any measurable potency, HMG-CoA inhibitors can have AEs. Statin therapy has been associated with pancreatitis.2 Muscle toxicity is a complication of HMG-CoA reductase inhibitors, and statin-associated symptoms are a leading cause of nonadherence.3 Rosuvastatin had higher AE and drug reactions compared with that of atorvastatin and pitavastatin (35.6%, 8.7%, and 22.2%, respectively) in clinical trials for approval.4 We have reported concomitant adermatopathic dermatomyositis with statin-induced myopathy in a 48-year-old man from simvastatin (40 to 80 mg daily).1
Toxin-induced myopathy should be considered early in the differential diagnosis of weakness.5 All HMG-CoA inhibitors have been associated with acute kidney injury, particularly at high doses and also are known to induce myopathies, sometimes with inclusion bodies.1 Muscle-related AEs correlate with the potency of an HMG-CoA reductase inhibitor according to an analysis using the FDA AE Reporting System (AERS).6 Myalgia and rhabdomyolysis are well-known AEs of this class of medications. Furthermore, type II muscle atrophy—particularly in the proximal limb muscles—has been reported.5 Patients may have difficulty rising from chairs.1 Rosuvastatin had the strongest signal for muscular AEs (eg, myalgia, rhabdomyolysis, increased creatine phosphokinase level) from an FDA analysis of AERS.7
Rosuvastatin is the only HMG-CoA reductase inhibitor that causes dose-dependent increases in proteinuria and hematuria (Figure 4).8 Rosuvastatin at a 5-mg dose may induce 4 times the proteinuria as a placebo. Typically, other statins potentially reduce proteinuria (without hematuria). Proteinuria may be induced by rosuvastatin even at low doses.8 Proteinuria is attributed to how rosuvastatin impacts proximal tubular function.9 The drug is transported into the proximal tubule by the organic anion transporter-3. Acute kidney injury has been associated with several statins, including rosuvastatin.7,10 This may be associated with denuded tubular epithelia, active urinary sediment, acute tubular toxicity, vacuolated epithelial cells, and tubular cell casts. Unlike atorvastatin, the increase in proteinuria and hematuria also is dose dependent.
In patients with renal insufficiency (short of end-stage renal disease [ESRD]), most statins other than rosuvastatin are well tolerated and recommended for reduction of overall and CVD mortality risk. However, these benefits seem to diminish once ESRD is reached. Atorvastatin did not impact CVD mortality in patients with type 2 diabetes mellitus (T2DM) and ESRD (despite decreasing LDL-C).11 The AURORA study randomized 10 mg of statin vs placebo in 2776 maintenance dialysis patients aged 50 to 80 years. Rosuvastatin lowered the LDL-C but did not affect all-cause mortality (13.5 vs 14.0 events per 100 patient-years). Patients randomized to rosuvastatin had more than twice as many unclassified strokes (9 vs 4). Rosuvastatin, although efficacious in reducing LDL-C, had no impact on CVD mortality, nonfatal myocardial infarction, or nonfatal stroke.12 Post hoc analysis demonstrated that in patients with T2DM with ESRD the hazard ratio for hemorrhagic stroke was 5.2.13
Rosuvastatin ranked lower than lovastatin, pravastatin, simvastatin, atorvastatin, and fluvastatin with respect to reduction of all-cause mortality in trials of participants with or without prior coronary artery disease.14 AEs, such as rhabdomyolysis, proteinuria, nephropathy, renal failure, liver, and muscle toxicity are higher with rosuvastatin than other medications in its class.15
Conclusions
For patients with existing CVD, standard clinical practice is to encourage increased and regular physical activity, cholesterol-lowering diets, weight loss, and smoking cessation. Hypertension should be treated. Glycemia should be well controlled in the setting of T2DM. β-blockers may be beneficial in those with histories of myocardial infarction or heart failure with reduced systolic function. Statins are a valuable tool in the treatment of dyslipidemia.
Statin-induced muscle symptoms are a major reason for discontinuation and nonadherence.16 Statin-induced myalgia, myositis, and myopathy have been used interchangeably.17 Rhabdomyolysis, myalgia, increased creatine kinase, statin myopathy, and immune-mediated necrotizing myopathy are among the clinical phenotypes caused by statins.17 There are 33,695 serious cases—1808 deaths—reported with rosuvastatin in the FDA AERS as of June 30, 2021. Myalgia, pain in extremity, muscle spasms, pain, and arthralgia top the list of AEs. When statin-induced symptoms occur, adherence is rarely improved by dismissive clinicians.18
Drugs in the same class often have common therapeutic properties. Potencies and AE profiles are seldom uniform. The decision to add or change the brand of medication within a class should be balanced with considerations for the indication, duplications, simplification, AEs, appropriate dosage, and drug-drug interactions.
Acknowledgments
Brent Wagner is funded by a US Department of Veterans Affairs Merit Award (I01 BX001958), a National Institutes of Health R01 grant (DK-102085), Dialysis Clinic, Inc., and partially supported by the University of New Mexico Brain and Behavioral Health Institute (BBHI 2018-1008, 2020-21-002) and in part by the University of New Mexico’s Signature Program in Cardiovascular and Metabolic Disease (CVMD); and the University of New Mexico School of Medicine Research Allocation Committee (C-2459-RAC, New Mexico Medical Trust). Brent Wagner is an Associate Member to the University of New Mexico Health Sciences Center Autophagy, Inflammation, and Metabolism Center of Biomedical Research Excellence (AIM CoBRE) supported by NIH grant P20GM121176.
Funding
National Institutes of Health Grant R01 DK-102085, Dialysis Clinic Inc., VA Merit Award I01 BX001958, Center for Integrated Nanotechnologies User Agreement 2019AU0120, Brain & Behavioral Health Institute (grants 2018-1008, 2020-21-002), University of New Mexico’s Signature Program in Cardiovascular and Metabolic Disease (CVMD), the University of New Mexico School of Medicine Research Allocation Committee (C-2459-RAC, New Mexico Medical Trust) and a metabolomics voucher from the AIM Center (NIH P20GM121176).
1. Wagner B, Kagan-Hallet KS, Russell IJ. Concomitant presentation of adermatopathic dermatomyositis, statin myopathy, fibromyalgia syndrome, piriformis muscle myofascial pain and diabetic neuropathy. J Musculoskeletal Pain. 2003;11(2):25-30. doi:10.1300/J094v11n02_05
2. Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy [published correction appears in Lancet. 2017 Feb 11;389(10069):602]. Lancet. 2016;388(10059):2532-2561. doi:10.1016/S0140-6736(16)31357-5
3. Stroes ES, Thompson PD, Corsini A, et al. Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J. 2015;36(17):1012-1022. doi:10.1093/eurheartj/ehv043
4. Saku K, Zhang B, Noda K; PATROL Trial Investigators. Randomized head-to-head comparison of pitavastatin, atorvastatin, and rosuvastatin for safety and efficacy (quantity and quality of LDL): the PATROL trial. Circ J. 2011;75(6):1493-1505. doi:10.1253/circj.cj-10-1281
5. Wald JJ. The effects of toxins on muscle. Neurol Clin. 2000;18(3):695-718. doi:10.1016/s0733-8619(05)70219-x
6. Hoffman KB, Kraus C, Dimbil M, Golomb BA. A survey of the FDA’s AERS database regarding muscle and tendon adverse events linked to the statin drug class. PLoS One. 2012;7(8):e42866. doi:10.1371/journal.pone.0042866
7. Sakaeda T, Kadoyama K, Okuno Y. Statin-associated muscular and renal adverse events: data mining of the public version of the FDA adverse event reporting system. PLoS One. 2011;6(12):e28124. doi:10.1371/journal.pone.0028124
8. Tiwari A. An overview of statin-associated proteinuria. Drug Discov Today. 2006;11(9-10):458-464. doi:10.1016/j.drudis.2006.03.017
9. Verhulst A, Sayer R, De Broe ME, D’Haese PC, Brown CD. Human proximal tubular epithelium actively secretes but does not retain rosuvastatin. Mol Pharmacol. 2008;74(4):1084-1091. doi:10.1124/mol.108.047647
10. Jones PH, Davidson MH, Stein EA, et al. Comparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses (STELLAR* Trial). Am J Cardiol. 2003;92(2):152-160. doi:10.1016/s0002-9149(03)00530-7
11. Wanner C, Krane V, März W, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis [published correction appears in N Engl J Med. 2005 Oct 13;353(15):1640]. N Engl J Med. 2005;353(3):238-248. doi:10.1056/NEJMoa043545
12. Fellström BC, Jardine AG, Schmieder RE, et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis [published correction appears in N Engl J Med. 2010 Apr 15;362(15):1450]. N Engl J Med. 2009;360(14):1395-1407. doi:10.1056/NEJMoa0810177
13. Holdaas H, Holme I, Schmieder RE, et al. Rosuvastatin in diabetic hemodialysis patients. J Am Soc Nephrol. 2011;22(7):1335-1341. doi:10.1681/ASN.2010090987
14. Naci H, Brugts JJ, Fleurence R, Tsoi B, Toor H, Ades AE. Comparative benefits of statins in the primary and secondary prevention of major coronary events and all-cause mortality: a network meta-analysis of placebo-controlled and active-comparator trials. Eur J Prev Cardiol. 2013;20(4):641-657. doi:10.1177/2047487313480435
15. Alsheikh-Ali AA, Ambrose MS, Kuvin JT, Karas RH. The safety of rosuvastatin as used in common clinical practice: a postmarketing analysis. Circulation. 2005;111(23):3051-3057. doi:10.1161/CIRCULATIONAHA.105.555482
16. Ward NC, Watts GF, Eckel RH. Statin toxicity. Circ Res. 2019;124(2):328-350. doi:10.1161/CIRCRESAHA.118.312782
17. Selva-O’Callaghan A, Alvarado-Cardenas M, Pinal-Fernández I, et al. Statin-induced myalgia and myositis: an update on pathogenesis and clinical recommendations. Expert Rev Clin Immunol. 2018;14(3):215-224. doi:10.1080/1744666X.2018.1440206
18. Koslik HJ, Meskimen AH, Golomb BA. Physicians’ Experiences as patients with statin side effects: a case series. Drug Saf Case Rep. 2017;4(1):3. doi:10.1007/s40800-017-0045-0
Changing medications within a drug class requires considering the indication and dosage, possible adverse effects, and drug-drug interactions.
Changing medications within a drug class requires considering the indication and dosage, possible adverse effects, and drug-drug interactions.
Attention should be paid to changing a tolerated medication to another within its class. Many drugs approved by the US Food and Drug Administration (FDA), have equivalent therapeutic properties as existing drugs. Rarely do such medications share the same potency and adverse effect (AE) profile.
Case Presentation
A 77-year-old man presented to the emergency department (ED) at the Raymond G. Murphy Medical Center in Albuquerque, New Mexico, with a 1-month history of progressive muscle weakness, which was so severe that he required assistance rising from chairs. The symptoms began when he switched from atorvastatin 40 mg daily to rosuvastatin 40 mg daily. A nephrology consultation was requested for an elevated plasma creatinine.
The patient reported strict adherence to his prescribed medications. In the days following the switch to rosuvastatin, he noticed that his urine turned black. He described the color as “like burnt coffee.” The color gradually cleared before his ED presentation. The patient stopped taking rosuvastatin the day prior to presentation and noted improvement of his symptoms. Review of symptoms was significant for lower extremity paresthesia and numbness the day he started rosuvastatin. He had no symptoms of decompensated heart failure and no recent exacerbations requiring alteration of his diuretic regimen.
The patient’s medical history was significant for traumatic brain injury with complex partial seizures, carpal tunnel syndrome, dyslipidemia, coronary artery disease with percutaneous intervention to the right coronary artery in the late 1990s, atrial fibrillation and ventricular tachycardia, status post implantable cardioverter defibrillator, heart failure with reduced ejection fraction (25%) attributed to ischemic cardiomyopathy, hypertension, lower urinary tract symptoms/prostatism, and previous bladder cancer. In the mid-1960s, the patient served in the US Army and had been deployed to South Korea. After the service, he worked for the local city government. He was retired for about 15 years. He reported no tobacco, alcohol, or recreational drug use and no tattoos. He did not require prior blood or blood product transfusions. None of his family members—parents, siblings, or children—had any history of kidney disease.
The patient’s outpatient medications included levetiracetam 750 mg twice daily, melatonin 9 mg at night, menthol 16%/methyl-salicylate 30% topically up to 4 times per day as needed, aspirin 81 mg once daily, fish oil 1000 mg twice daily, amiodarone 400 mg twice daily, hydralazine 20 mg 3 times daily, isosorbide mononitrate 60 mg daily, metoprolol succinate 100 mg daily, and tamsulosin 0.4 mg at night. His vital signs were stable: afebrile (97.5 ºF), normocardic (74 beats per minute), normotensive (118/78 mm Hg), and normoxic (98% on room air). On examination, he appeared elderly, somewhat frail, and chronically ill but in no acute distress. Affect was pleasant and appropriate, attention was high, and his thought process was logical. He had sparse, grey scalp hair. Extraocular movements were intact. Oral mucosa was pink and moist. His back was nontender, and there was no costovertebral tenderness bilaterally. The patient was in no respiratory distress, with a slightly hyperresonant chest to percussion bilaterally, very faint inspiratory basilar crepitant rales (that cleared with repeat inspiration), and was otherwise clear to auscultation throughout. An outline of an implanted pacemaker was evident on the chest under his left clavicle, with a laterally displaced apical impulse. The rate was normal and the rhythm was regular. Upper extremities demonstrated papyraceous skin but without cyanosis, clubbing, or edema. Radial pulses were slightly diminished. He had no lower extremity edema.
His laboratory values are provided in Table 1. Kidney function was stable months prior to admission. Of note, the blood urea nitrogen and plasma creatinine were increased from his baseline up to 47 and 5.89 mg/dL, respectively. The serum glutamic-oxaloacetic transaminase and serum glutamic pyruvic transaminase were 1051 U/L and 408 U/L, respectively. Plasma amylase and lipase levels also were elevated, 230 U/L and 892 U/L, respectively. Creatine kinase was 41,099 U/L. Urinalysis demonstrated a specific gravity of 1.017, pH of 5, and a large amount of blood (92 red blood cells/high power field).
A 12-lead electrocardiogram demonstrated a sinus rhythm, PR interval of 0.20 ms, narrow QRS with a leftward frontal axis deviation, R-transition between precordial leads V1 and V2, and flattening of the ST segments in III, V1-V3 (Figure 1). A portable chest X-ray demonstrated clear lung fields, no evidence of effusion in the costophrenic area. Ultrasonography was conducted at the time of the examination (Figure 2). The kidneys were smoothly contoured, each measuring > 10 cm; there was an exophytic cyst on the left. Otherwise, the cortices, perhaps slightly echogenic, did not appear diminished. The bladder was not abnormally enlarged.
Rosuvastatin-induced rhabdomyolysis, pancreatitis, transaminitis, and drug-induced acute kidney injury were considered high among the diagnostic differentials. The 3-hydroxy-3-methyl-glutaryl-CoA (HMG-CoA) reductase inhibitor was stopped, and he was prescribed an acute renal insufficiency diet. All laboratory parameters improved with this change (Figure 3). Two months after presentation (and with rosuvastatin added to his list of adverse reactions), all symptoms resolved and his plasma creatinine reached a nadir of 1.22 mg/dL.
Discussion
Statin-class drugs inhibit the HMG-CoA reductase (Table 2). Upregulation of low-density lipoprotein cholesterol (LDL-C) receptors in the liver result in increased LDL-C uptake and cholesterol catabolism.1 Prescribed inhibitors of the HMG-CoA reductase—statins—are known to reduce mortality due to cardiovascular disease (CVD). Much like any other pharmaceutical agent with any measurable potency, HMG-CoA inhibitors can have AEs. Statin therapy has been associated with pancreatitis.2 Muscle toxicity is a complication of HMG-CoA reductase inhibitors, and statin-associated symptoms are a leading cause of nonadherence.3 Rosuvastatin had higher AE and drug reactions compared with that of atorvastatin and pitavastatin (35.6%, 8.7%, and 22.2%, respectively) in clinical trials for approval.4 We have reported concomitant adermatopathic dermatomyositis with statin-induced myopathy in a 48-year-old man from simvastatin (40 to 80 mg daily).1
Toxin-induced myopathy should be considered early in the differential diagnosis of weakness.5 All HMG-CoA inhibitors have been associated with acute kidney injury, particularly at high doses and also are known to induce myopathies, sometimes with inclusion bodies.1 Muscle-related AEs correlate with the potency of an HMG-CoA reductase inhibitor according to an analysis using the FDA AE Reporting System (AERS).6 Myalgia and rhabdomyolysis are well-known AEs of this class of medications. Furthermore, type II muscle atrophy—particularly in the proximal limb muscles—has been reported.5 Patients may have difficulty rising from chairs.1 Rosuvastatin had the strongest signal for muscular AEs (eg, myalgia, rhabdomyolysis, increased creatine phosphokinase level) from an FDA analysis of AERS.7
Rosuvastatin is the only HMG-CoA reductase inhibitor that causes dose-dependent increases in proteinuria and hematuria (Figure 4).8 Rosuvastatin at a 5-mg dose may induce 4 times the proteinuria as a placebo. Typically, other statins potentially reduce proteinuria (without hematuria). Proteinuria may be induced by rosuvastatin even at low doses.8 Proteinuria is attributed to how rosuvastatin impacts proximal tubular function.9 The drug is transported into the proximal tubule by the organic anion transporter-3. Acute kidney injury has been associated with several statins, including rosuvastatin.7,10 This may be associated with denuded tubular epithelia, active urinary sediment, acute tubular toxicity, vacuolated epithelial cells, and tubular cell casts. Unlike atorvastatin, the increase in proteinuria and hematuria also is dose dependent.
In patients with renal insufficiency (short of end-stage renal disease [ESRD]), most statins other than rosuvastatin are well tolerated and recommended for reduction of overall and CVD mortality risk. However, these benefits seem to diminish once ESRD is reached. Atorvastatin did not impact CVD mortality in patients with type 2 diabetes mellitus (T2DM) and ESRD (despite decreasing LDL-C).11 The AURORA study randomized 10 mg of statin vs placebo in 2776 maintenance dialysis patients aged 50 to 80 years. Rosuvastatin lowered the LDL-C but did not affect all-cause mortality (13.5 vs 14.0 events per 100 patient-years). Patients randomized to rosuvastatin had more than twice as many unclassified strokes (9 vs 4). Rosuvastatin, although efficacious in reducing LDL-C, had no impact on CVD mortality, nonfatal myocardial infarction, or nonfatal stroke.12 Post hoc analysis demonstrated that in patients with T2DM with ESRD the hazard ratio for hemorrhagic stroke was 5.2.13
Rosuvastatin ranked lower than lovastatin, pravastatin, simvastatin, atorvastatin, and fluvastatin with respect to reduction of all-cause mortality in trials of participants with or without prior coronary artery disease.14 AEs, such as rhabdomyolysis, proteinuria, nephropathy, renal failure, liver, and muscle toxicity are higher with rosuvastatin than other medications in its class.15
Conclusions
For patients with existing CVD, standard clinical practice is to encourage increased and regular physical activity, cholesterol-lowering diets, weight loss, and smoking cessation. Hypertension should be treated. Glycemia should be well controlled in the setting of T2DM. β-blockers may be beneficial in those with histories of myocardial infarction or heart failure with reduced systolic function. Statins are a valuable tool in the treatment of dyslipidemia.
Statin-induced muscle symptoms are a major reason for discontinuation and nonadherence.16 Statin-induced myalgia, myositis, and myopathy have been used interchangeably.17 Rhabdomyolysis, myalgia, increased creatine kinase, statin myopathy, and immune-mediated necrotizing myopathy are among the clinical phenotypes caused by statins.17 There are 33,695 serious cases—1808 deaths—reported with rosuvastatin in the FDA AERS as of June 30, 2021. Myalgia, pain in extremity, muscle spasms, pain, and arthralgia top the list of AEs. When statin-induced symptoms occur, adherence is rarely improved by dismissive clinicians.18
Drugs in the same class often have common therapeutic properties. Potencies and AE profiles are seldom uniform. The decision to add or change the brand of medication within a class should be balanced with considerations for the indication, duplications, simplification, AEs, appropriate dosage, and drug-drug interactions.
Acknowledgments
Brent Wagner is funded by a US Department of Veterans Affairs Merit Award (I01 BX001958), a National Institutes of Health R01 grant (DK-102085), Dialysis Clinic, Inc., and partially supported by the University of New Mexico Brain and Behavioral Health Institute (BBHI 2018-1008, 2020-21-002) and in part by the University of New Mexico’s Signature Program in Cardiovascular and Metabolic Disease (CVMD); and the University of New Mexico School of Medicine Research Allocation Committee (C-2459-RAC, New Mexico Medical Trust). Brent Wagner is an Associate Member to the University of New Mexico Health Sciences Center Autophagy, Inflammation, and Metabolism Center of Biomedical Research Excellence (AIM CoBRE) supported by NIH grant P20GM121176.
Funding
National Institutes of Health Grant R01 DK-102085, Dialysis Clinic Inc., VA Merit Award I01 BX001958, Center for Integrated Nanotechnologies User Agreement 2019AU0120, Brain & Behavioral Health Institute (grants 2018-1008, 2020-21-002), University of New Mexico’s Signature Program in Cardiovascular and Metabolic Disease (CVMD), the University of New Mexico School of Medicine Research Allocation Committee (C-2459-RAC, New Mexico Medical Trust) and a metabolomics voucher from the AIM Center (NIH P20GM121176).
Attention should be paid to changing a tolerated medication to another within its class. Many drugs approved by the US Food and Drug Administration (FDA), have equivalent therapeutic properties as existing drugs. Rarely do such medications share the same potency and adverse effect (AE) profile.
Case Presentation
A 77-year-old man presented to the emergency department (ED) at the Raymond G. Murphy Medical Center in Albuquerque, New Mexico, with a 1-month history of progressive muscle weakness, which was so severe that he required assistance rising from chairs. The symptoms began when he switched from atorvastatin 40 mg daily to rosuvastatin 40 mg daily. A nephrology consultation was requested for an elevated plasma creatinine.
The patient reported strict adherence to his prescribed medications. In the days following the switch to rosuvastatin, he noticed that his urine turned black. He described the color as “like burnt coffee.” The color gradually cleared before his ED presentation. The patient stopped taking rosuvastatin the day prior to presentation and noted improvement of his symptoms. Review of symptoms was significant for lower extremity paresthesia and numbness the day he started rosuvastatin. He had no symptoms of decompensated heart failure and no recent exacerbations requiring alteration of his diuretic regimen.
The patient’s medical history was significant for traumatic brain injury with complex partial seizures, carpal tunnel syndrome, dyslipidemia, coronary artery disease with percutaneous intervention to the right coronary artery in the late 1990s, atrial fibrillation and ventricular tachycardia, status post implantable cardioverter defibrillator, heart failure with reduced ejection fraction (25%) attributed to ischemic cardiomyopathy, hypertension, lower urinary tract symptoms/prostatism, and previous bladder cancer. In the mid-1960s, the patient served in the US Army and had been deployed to South Korea. After the service, he worked for the local city government. He was retired for about 15 years. He reported no tobacco, alcohol, or recreational drug use and no tattoos. He did not require prior blood or blood product transfusions. None of his family members—parents, siblings, or children—had any history of kidney disease.
The patient’s outpatient medications included levetiracetam 750 mg twice daily, melatonin 9 mg at night, menthol 16%/methyl-salicylate 30% topically up to 4 times per day as needed, aspirin 81 mg once daily, fish oil 1000 mg twice daily, amiodarone 400 mg twice daily, hydralazine 20 mg 3 times daily, isosorbide mononitrate 60 mg daily, metoprolol succinate 100 mg daily, and tamsulosin 0.4 mg at night. His vital signs were stable: afebrile (97.5 ºF), normocardic (74 beats per minute), normotensive (118/78 mm Hg), and normoxic (98% on room air). On examination, he appeared elderly, somewhat frail, and chronically ill but in no acute distress. Affect was pleasant and appropriate, attention was high, and his thought process was logical. He had sparse, grey scalp hair. Extraocular movements were intact. Oral mucosa was pink and moist. His back was nontender, and there was no costovertebral tenderness bilaterally. The patient was in no respiratory distress, with a slightly hyperresonant chest to percussion bilaterally, very faint inspiratory basilar crepitant rales (that cleared with repeat inspiration), and was otherwise clear to auscultation throughout. An outline of an implanted pacemaker was evident on the chest under his left clavicle, with a laterally displaced apical impulse. The rate was normal and the rhythm was regular. Upper extremities demonstrated papyraceous skin but without cyanosis, clubbing, or edema. Radial pulses were slightly diminished. He had no lower extremity edema.
His laboratory values are provided in Table 1. Kidney function was stable months prior to admission. Of note, the blood urea nitrogen and plasma creatinine were increased from his baseline up to 47 and 5.89 mg/dL, respectively. The serum glutamic-oxaloacetic transaminase and serum glutamic pyruvic transaminase were 1051 U/L and 408 U/L, respectively. Plasma amylase and lipase levels also were elevated, 230 U/L and 892 U/L, respectively. Creatine kinase was 41,099 U/L. Urinalysis demonstrated a specific gravity of 1.017, pH of 5, and a large amount of blood (92 red blood cells/high power field).
A 12-lead electrocardiogram demonstrated a sinus rhythm, PR interval of 0.20 ms, narrow QRS with a leftward frontal axis deviation, R-transition between precordial leads V1 and V2, and flattening of the ST segments in III, V1-V3 (Figure 1). A portable chest X-ray demonstrated clear lung fields, no evidence of effusion in the costophrenic area. Ultrasonography was conducted at the time of the examination (Figure 2). The kidneys were smoothly contoured, each measuring > 10 cm; there was an exophytic cyst on the left. Otherwise, the cortices, perhaps slightly echogenic, did not appear diminished. The bladder was not abnormally enlarged.
Rosuvastatin-induced rhabdomyolysis, pancreatitis, transaminitis, and drug-induced acute kidney injury were considered high among the diagnostic differentials. The 3-hydroxy-3-methyl-glutaryl-CoA (HMG-CoA) reductase inhibitor was stopped, and he was prescribed an acute renal insufficiency diet. All laboratory parameters improved with this change (Figure 3). Two months after presentation (and with rosuvastatin added to his list of adverse reactions), all symptoms resolved and his plasma creatinine reached a nadir of 1.22 mg/dL.
Discussion
Statin-class drugs inhibit the HMG-CoA reductase (Table 2). Upregulation of low-density lipoprotein cholesterol (LDL-C) receptors in the liver result in increased LDL-C uptake and cholesterol catabolism.1 Prescribed inhibitors of the HMG-CoA reductase—statins—are known to reduce mortality due to cardiovascular disease (CVD). Much like any other pharmaceutical agent with any measurable potency, HMG-CoA inhibitors can have AEs. Statin therapy has been associated with pancreatitis.2 Muscle toxicity is a complication of HMG-CoA reductase inhibitors, and statin-associated symptoms are a leading cause of nonadherence.3 Rosuvastatin had higher AE and drug reactions compared with that of atorvastatin and pitavastatin (35.6%, 8.7%, and 22.2%, respectively) in clinical trials for approval.4 We have reported concomitant adermatopathic dermatomyositis with statin-induced myopathy in a 48-year-old man from simvastatin (40 to 80 mg daily).1
Toxin-induced myopathy should be considered early in the differential diagnosis of weakness.5 All HMG-CoA inhibitors have been associated with acute kidney injury, particularly at high doses and also are known to induce myopathies, sometimes with inclusion bodies.1 Muscle-related AEs correlate with the potency of an HMG-CoA reductase inhibitor according to an analysis using the FDA AE Reporting System (AERS).6 Myalgia and rhabdomyolysis are well-known AEs of this class of medications. Furthermore, type II muscle atrophy—particularly in the proximal limb muscles—has been reported.5 Patients may have difficulty rising from chairs.1 Rosuvastatin had the strongest signal for muscular AEs (eg, myalgia, rhabdomyolysis, increased creatine phosphokinase level) from an FDA analysis of AERS.7
Rosuvastatin is the only HMG-CoA reductase inhibitor that causes dose-dependent increases in proteinuria and hematuria (Figure 4).8 Rosuvastatin at a 5-mg dose may induce 4 times the proteinuria as a placebo. Typically, other statins potentially reduce proteinuria (without hematuria). Proteinuria may be induced by rosuvastatin even at low doses.8 Proteinuria is attributed to how rosuvastatin impacts proximal tubular function.9 The drug is transported into the proximal tubule by the organic anion transporter-3. Acute kidney injury has been associated with several statins, including rosuvastatin.7,10 This may be associated with denuded tubular epithelia, active urinary sediment, acute tubular toxicity, vacuolated epithelial cells, and tubular cell casts. Unlike atorvastatin, the increase in proteinuria and hematuria also is dose dependent.
In patients with renal insufficiency (short of end-stage renal disease [ESRD]), most statins other than rosuvastatin are well tolerated and recommended for reduction of overall and CVD mortality risk. However, these benefits seem to diminish once ESRD is reached. Atorvastatin did not impact CVD mortality in patients with type 2 diabetes mellitus (T2DM) and ESRD (despite decreasing LDL-C).11 The AURORA study randomized 10 mg of statin vs placebo in 2776 maintenance dialysis patients aged 50 to 80 years. Rosuvastatin lowered the LDL-C but did not affect all-cause mortality (13.5 vs 14.0 events per 100 patient-years). Patients randomized to rosuvastatin had more than twice as many unclassified strokes (9 vs 4). Rosuvastatin, although efficacious in reducing LDL-C, had no impact on CVD mortality, nonfatal myocardial infarction, or nonfatal stroke.12 Post hoc analysis demonstrated that in patients with T2DM with ESRD the hazard ratio for hemorrhagic stroke was 5.2.13
Rosuvastatin ranked lower than lovastatin, pravastatin, simvastatin, atorvastatin, and fluvastatin with respect to reduction of all-cause mortality in trials of participants with or without prior coronary artery disease.14 AEs, such as rhabdomyolysis, proteinuria, nephropathy, renal failure, liver, and muscle toxicity are higher with rosuvastatin than other medications in its class.15
Conclusions
For patients with existing CVD, standard clinical practice is to encourage increased and regular physical activity, cholesterol-lowering diets, weight loss, and smoking cessation. Hypertension should be treated. Glycemia should be well controlled in the setting of T2DM. β-blockers may be beneficial in those with histories of myocardial infarction or heart failure with reduced systolic function. Statins are a valuable tool in the treatment of dyslipidemia.
Statin-induced muscle symptoms are a major reason for discontinuation and nonadherence.16 Statin-induced myalgia, myositis, and myopathy have been used interchangeably.17 Rhabdomyolysis, myalgia, increased creatine kinase, statin myopathy, and immune-mediated necrotizing myopathy are among the clinical phenotypes caused by statins.17 There are 33,695 serious cases—1808 deaths—reported with rosuvastatin in the FDA AERS as of June 30, 2021. Myalgia, pain in extremity, muscle spasms, pain, and arthralgia top the list of AEs. When statin-induced symptoms occur, adherence is rarely improved by dismissive clinicians.18
Drugs in the same class often have common therapeutic properties. Potencies and AE profiles are seldom uniform. The decision to add or change the brand of medication within a class should be balanced with considerations for the indication, duplications, simplification, AEs, appropriate dosage, and drug-drug interactions.
Acknowledgments
Brent Wagner is funded by a US Department of Veterans Affairs Merit Award (I01 BX001958), a National Institutes of Health R01 grant (DK-102085), Dialysis Clinic, Inc., and partially supported by the University of New Mexico Brain and Behavioral Health Institute (BBHI 2018-1008, 2020-21-002) and in part by the University of New Mexico’s Signature Program in Cardiovascular and Metabolic Disease (CVMD); and the University of New Mexico School of Medicine Research Allocation Committee (C-2459-RAC, New Mexico Medical Trust). Brent Wagner is an Associate Member to the University of New Mexico Health Sciences Center Autophagy, Inflammation, and Metabolism Center of Biomedical Research Excellence (AIM CoBRE) supported by NIH grant P20GM121176.
Funding
National Institutes of Health Grant R01 DK-102085, Dialysis Clinic Inc., VA Merit Award I01 BX001958, Center for Integrated Nanotechnologies User Agreement 2019AU0120, Brain & Behavioral Health Institute (grants 2018-1008, 2020-21-002), University of New Mexico’s Signature Program in Cardiovascular and Metabolic Disease (CVMD), the University of New Mexico School of Medicine Research Allocation Committee (C-2459-RAC, New Mexico Medical Trust) and a metabolomics voucher from the AIM Center (NIH P20GM121176).
1. Wagner B, Kagan-Hallet KS, Russell IJ. Concomitant presentation of adermatopathic dermatomyositis, statin myopathy, fibromyalgia syndrome, piriformis muscle myofascial pain and diabetic neuropathy. J Musculoskeletal Pain. 2003;11(2):25-30. doi:10.1300/J094v11n02_05
2. Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy [published correction appears in Lancet. 2017 Feb 11;389(10069):602]. Lancet. 2016;388(10059):2532-2561. doi:10.1016/S0140-6736(16)31357-5
3. Stroes ES, Thompson PD, Corsini A, et al. Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J. 2015;36(17):1012-1022. doi:10.1093/eurheartj/ehv043
4. Saku K, Zhang B, Noda K; PATROL Trial Investigators. Randomized head-to-head comparison of pitavastatin, atorvastatin, and rosuvastatin for safety and efficacy (quantity and quality of LDL): the PATROL trial. Circ J. 2011;75(6):1493-1505. doi:10.1253/circj.cj-10-1281
5. Wald JJ. The effects of toxins on muscle. Neurol Clin. 2000;18(3):695-718. doi:10.1016/s0733-8619(05)70219-x
6. Hoffman KB, Kraus C, Dimbil M, Golomb BA. A survey of the FDA’s AERS database regarding muscle and tendon adverse events linked to the statin drug class. PLoS One. 2012;7(8):e42866. doi:10.1371/journal.pone.0042866
7. Sakaeda T, Kadoyama K, Okuno Y. Statin-associated muscular and renal adverse events: data mining of the public version of the FDA adverse event reporting system. PLoS One. 2011;6(12):e28124. doi:10.1371/journal.pone.0028124
8. Tiwari A. An overview of statin-associated proteinuria. Drug Discov Today. 2006;11(9-10):458-464. doi:10.1016/j.drudis.2006.03.017
9. Verhulst A, Sayer R, De Broe ME, D’Haese PC, Brown CD. Human proximal tubular epithelium actively secretes but does not retain rosuvastatin. Mol Pharmacol. 2008;74(4):1084-1091. doi:10.1124/mol.108.047647
10. Jones PH, Davidson MH, Stein EA, et al. Comparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses (STELLAR* Trial). Am J Cardiol. 2003;92(2):152-160. doi:10.1016/s0002-9149(03)00530-7
11. Wanner C, Krane V, März W, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis [published correction appears in N Engl J Med. 2005 Oct 13;353(15):1640]. N Engl J Med. 2005;353(3):238-248. doi:10.1056/NEJMoa043545
12. Fellström BC, Jardine AG, Schmieder RE, et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis [published correction appears in N Engl J Med. 2010 Apr 15;362(15):1450]. N Engl J Med. 2009;360(14):1395-1407. doi:10.1056/NEJMoa0810177
13. Holdaas H, Holme I, Schmieder RE, et al. Rosuvastatin in diabetic hemodialysis patients. J Am Soc Nephrol. 2011;22(7):1335-1341. doi:10.1681/ASN.2010090987
14. Naci H, Brugts JJ, Fleurence R, Tsoi B, Toor H, Ades AE. Comparative benefits of statins in the primary and secondary prevention of major coronary events and all-cause mortality: a network meta-analysis of placebo-controlled and active-comparator trials. Eur J Prev Cardiol. 2013;20(4):641-657. doi:10.1177/2047487313480435
15. Alsheikh-Ali AA, Ambrose MS, Kuvin JT, Karas RH. The safety of rosuvastatin as used in common clinical practice: a postmarketing analysis. Circulation. 2005;111(23):3051-3057. doi:10.1161/CIRCULATIONAHA.105.555482
16. Ward NC, Watts GF, Eckel RH. Statin toxicity. Circ Res. 2019;124(2):328-350. doi:10.1161/CIRCRESAHA.118.312782
17. Selva-O’Callaghan A, Alvarado-Cardenas M, Pinal-Fernández I, et al. Statin-induced myalgia and myositis: an update on pathogenesis and clinical recommendations. Expert Rev Clin Immunol. 2018;14(3):215-224. doi:10.1080/1744666X.2018.1440206
18. Koslik HJ, Meskimen AH, Golomb BA. Physicians’ Experiences as patients with statin side effects: a case series. Drug Saf Case Rep. 2017;4(1):3. doi:10.1007/s40800-017-0045-0
1. Wagner B, Kagan-Hallet KS, Russell IJ. Concomitant presentation of adermatopathic dermatomyositis, statin myopathy, fibromyalgia syndrome, piriformis muscle myofascial pain and diabetic neuropathy. J Musculoskeletal Pain. 2003;11(2):25-30. doi:10.1300/J094v11n02_05
2. Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy [published correction appears in Lancet. 2017 Feb 11;389(10069):602]. Lancet. 2016;388(10059):2532-2561. doi:10.1016/S0140-6736(16)31357-5
3. Stroes ES, Thompson PD, Corsini A, et al. Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J. 2015;36(17):1012-1022. doi:10.1093/eurheartj/ehv043
4. Saku K, Zhang B, Noda K; PATROL Trial Investigators. Randomized head-to-head comparison of pitavastatin, atorvastatin, and rosuvastatin for safety and efficacy (quantity and quality of LDL): the PATROL trial. Circ J. 2011;75(6):1493-1505. doi:10.1253/circj.cj-10-1281
5. Wald JJ. The effects of toxins on muscle. Neurol Clin. 2000;18(3):695-718. doi:10.1016/s0733-8619(05)70219-x
6. Hoffman KB, Kraus C, Dimbil M, Golomb BA. A survey of the FDA’s AERS database regarding muscle and tendon adverse events linked to the statin drug class. PLoS One. 2012;7(8):e42866. doi:10.1371/journal.pone.0042866
7. Sakaeda T, Kadoyama K, Okuno Y. Statin-associated muscular and renal adverse events: data mining of the public version of the FDA adverse event reporting system. PLoS One. 2011;6(12):e28124. doi:10.1371/journal.pone.0028124
8. Tiwari A. An overview of statin-associated proteinuria. Drug Discov Today. 2006;11(9-10):458-464. doi:10.1016/j.drudis.2006.03.017
9. Verhulst A, Sayer R, De Broe ME, D’Haese PC, Brown CD. Human proximal tubular epithelium actively secretes but does not retain rosuvastatin. Mol Pharmacol. 2008;74(4):1084-1091. doi:10.1124/mol.108.047647
10. Jones PH, Davidson MH, Stein EA, et al. Comparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses (STELLAR* Trial). Am J Cardiol. 2003;92(2):152-160. doi:10.1016/s0002-9149(03)00530-7
11. Wanner C, Krane V, März W, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis [published correction appears in N Engl J Med. 2005 Oct 13;353(15):1640]. N Engl J Med. 2005;353(3):238-248. doi:10.1056/NEJMoa043545
12. Fellström BC, Jardine AG, Schmieder RE, et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis [published correction appears in N Engl J Med. 2010 Apr 15;362(15):1450]. N Engl J Med. 2009;360(14):1395-1407. doi:10.1056/NEJMoa0810177
13. Holdaas H, Holme I, Schmieder RE, et al. Rosuvastatin in diabetic hemodialysis patients. J Am Soc Nephrol. 2011;22(7):1335-1341. doi:10.1681/ASN.2010090987
14. Naci H, Brugts JJ, Fleurence R, Tsoi B, Toor H, Ades AE. Comparative benefits of statins in the primary and secondary prevention of major coronary events and all-cause mortality: a network meta-analysis of placebo-controlled and active-comparator trials. Eur J Prev Cardiol. 2013;20(4):641-657. doi:10.1177/2047487313480435
15. Alsheikh-Ali AA, Ambrose MS, Kuvin JT, Karas RH. The safety of rosuvastatin as used in common clinical practice: a postmarketing analysis. Circulation. 2005;111(23):3051-3057. doi:10.1161/CIRCULATIONAHA.105.555482
16. Ward NC, Watts GF, Eckel RH. Statin toxicity. Circ Res. 2019;124(2):328-350. doi:10.1161/CIRCRESAHA.118.312782
17. Selva-O’Callaghan A, Alvarado-Cardenas M, Pinal-Fernández I, et al. Statin-induced myalgia and myositis: an update on pathogenesis and clinical recommendations. Expert Rev Clin Immunol. 2018;14(3):215-224. doi:10.1080/1744666X.2018.1440206
18. Koslik HJ, Meskimen AH, Golomb BA. Physicians’ Experiences as patients with statin side effects: a case series. Drug Saf Case Rep. 2017;4(1):3. doi:10.1007/s40800-017-0045-0
Pfizer seeks EUA expansion for COVID-19 booster
Pfizer and its European partner BioNTech on Nov. 9 asked the U.S. government to expand emergency use authorization (EUA) to allow everybody over 18 to receive their COVID-19 booster shots.
If the request is approved, He announced the goal last August but backed off after some scientists said younger people may not need boosters, especially with large parts of the world unvaccinated.
Pfizer is submitting a study of booster effects on 10,000 people to make its case, according to The Associated Press.
This would be Pfizer’s second attempt. In September, a Food and Drug Administration advisory panel turned down Pfizer’s idea of booster shots for everybody over 18.
However, the committee recommended Pfizer booster shots for people 65 and over, essential workers, and people with underlying health conditions.
The FDA and the Centers for Disease Control and Prevention authorized the Pfizer booster for those other groups and later authorization was granted for the same groups with Moderna and Johnson & Johnson boosters. People who got the two-shot Pfizer or Moderna vaccines should get a booster 6 months after the second dose and people who got the one-dose J&J vaccine should get a booster 2 months later.
The pro-booster argument has strengthened because new data have come in from Israel that confirm boosters provide protection as vaccine effectiveness wanes over time, The Washington Post reported. Also, health officials are worried about a post-holiday surge and because COVID-19 case counts and deaths are not dropping in every part of the country, though they are declining overall, according to the The Post report.
The regulatory path for a booster-for-all application is unclear. The Post, citing two unnamed officials, said the FDA probably won’t send the Pfizer application to the FDA advisory committee this time because the committee has already had extensive discussions about boosters. If the FDA gives the green light, CDC Director Rochelle Walensky, MD, would have to make updated recommendations on boosters, The Post article noted.
A version of this article first appeared on WebMD.com.
Pfizer and its European partner BioNTech on Nov. 9 asked the U.S. government to expand emergency use authorization (EUA) to allow everybody over 18 to receive their COVID-19 booster shots.
If the request is approved, He announced the goal last August but backed off after some scientists said younger people may not need boosters, especially with large parts of the world unvaccinated.
Pfizer is submitting a study of booster effects on 10,000 people to make its case, according to The Associated Press.
This would be Pfizer’s second attempt. In September, a Food and Drug Administration advisory panel turned down Pfizer’s idea of booster shots for everybody over 18.
However, the committee recommended Pfizer booster shots for people 65 and over, essential workers, and people with underlying health conditions.
The FDA and the Centers for Disease Control and Prevention authorized the Pfizer booster for those other groups and later authorization was granted for the same groups with Moderna and Johnson & Johnson boosters. People who got the two-shot Pfizer or Moderna vaccines should get a booster 6 months after the second dose and people who got the one-dose J&J vaccine should get a booster 2 months later.
The pro-booster argument has strengthened because new data have come in from Israel that confirm boosters provide protection as vaccine effectiveness wanes over time, The Washington Post reported. Also, health officials are worried about a post-holiday surge and because COVID-19 case counts and deaths are not dropping in every part of the country, though they are declining overall, according to the The Post report.
The regulatory path for a booster-for-all application is unclear. The Post, citing two unnamed officials, said the FDA probably won’t send the Pfizer application to the FDA advisory committee this time because the committee has already had extensive discussions about boosters. If the FDA gives the green light, CDC Director Rochelle Walensky, MD, would have to make updated recommendations on boosters, The Post article noted.
A version of this article first appeared on WebMD.com.
Pfizer and its European partner BioNTech on Nov. 9 asked the U.S. government to expand emergency use authorization (EUA) to allow everybody over 18 to receive their COVID-19 booster shots.
If the request is approved, He announced the goal last August but backed off after some scientists said younger people may not need boosters, especially with large parts of the world unvaccinated.
Pfizer is submitting a study of booster effects on 10,000 people to make its case, according to The Associated Press.
This would be Pfizer’s second attempt. In September, a Food and Drug Administration advisory panel turned down Pfizer’s idea of booster shots for everybody over 18.
However, the committee recommended Pfizer booster shots for people 65 and over, essential workers, and people with underlying health conditions.
The FDA and the Centers for Disease Control and Prevention authorized the Pfizer booster for those other groups and later authorization was granted for the same groups with Moderna and Johnson & Johnson boosters. People who got the two-shot Pfizer or Moderna vaccines should get a booster 6 months after the second dose and people who got the one-dose J&J vaccine should get a booster 2 months later.
The pro-booster argument has strengthened because new data have come in from Israel that confirm boosters provide protection as vaccine effectiveness wanes over time, The Washington Post reported. Also, health officials are worried about a post-holiday surge and because COVID-19 case counts and deaths are not dropping in every part of the country, though they are declining overall, according to the The Post report.
The regulatory path for a booster-for-all application is unclear. The Post, citing two unnamed officials, said the FDA probably won’t send the Pfizer application to the FDA advisory committee this time because the committee has already had extensive discussions about boosters. If the FDA gives the green light, CDC Director Rochelle Walensky, MD, would have to make updated recommendations on boosters, The Post article noted.
A version of this article first appeared on WebMD.com.
Gastric cancer prevalent in hereditary breast cancer patients
a prospective cohort study has shown.
“In short, what we are putting forward with these data is that pathogenic/likely pathogenic (P/LP) variants in the CDH1 gene confer a very high risk, at the very least, of occult early-stage gastric cancer in patients with HLBC,” said Jeremy Davis, MD, of the surgical oncology program, Center for Cancer Research at the National Cancer Institute.
“So patients that are referred to as ‘HLBC’ due to a CDH1 variant should at least undergo annual endoscopic surveillance but the real questions is whether or not they should also consider prophylactic total gastrectomy – as many patients in our study did,” he said.
The study, which was published online Oct. 13, 2021, in JAMA Surgery, included a cohort of 151 families totaling 283 patients with a CDH1 pathogenic or likely pathogenic (P/LP) variant. Analyses were conducted on three patient groups, which included those with HLBC and a family history of breast cancer but no gastric cancer, those with hereditary diffuse gastric cancer (HDGC) but no history of breast cancer, and those with a family history of both gastric and breast cancer in the mixed group. Of these, 15.5% had a history of HLBC, 16.2% had a history of HDGC, and 52.6% made up the mixed group.
“We examined the HLBC group with specific attention to CDH1 genotype and prevalence of occult gastric cancer,” the authors explained. The group consisted of 31 families with 19 CDH1 variants, 10 of which were also present in the HDGC and mixed groups.
Among this group of patients, almost 73% underwent one or more surveillance endoscopies and on endoscopy, occult signet ring cell carcinoma was detected in over one-third of patients.
The median age at the time of endoscopic carcinoma detection was only 33 years.
“Nearly all of the patients with HLBC (93.8%) ... who elected for risk-reducing total gastrectomy owing to their underlying CDH1 P/LP variant harbored occult signet ring cell gastric adenocarcinoma on final pathology,” investigators observed.
The median age at the time patients elected to undergo total gastrectomy was 50 years.
The prevalence of occult gastric cancer among asymptomatic patients in the HDGC group was similarly high, affecting almost 95% of this group of patients.
Some 18 out of 19 CDH1 P/LP variants were responsible for this high prevalence of occult gastric cancer, as the investigators pointed out.
“Hereditary cancer risk is informed by the presence of a germline gene variant more so than by family history of cancer,” the authors stressed. “[And we found that] germline CDH1 P/LP variants appear to have a highly penetrant gastric phenotype irrespective of family history.”
Given this finding, the authors stressed that it is “paramount” patients previously assigned a diagnosis of HLBC not be excluded from undergoing gastric cancer risk assessment and counseling.
Furthermore, “the mere presence of a germline CDH1 P/LP variant, regardless of family history, may be reason enough to consider prophylactic total gastrectomy,” the authors wrote.
Limitations of the study included the fact that the disease phenotype was established from family pedigrees which has the potential for recall bias by family members.
The study was supported in part by the Intramural Research Program of the National Cancer Institute. None of the authors had conflicts of interest to disclose.
a prospective cohort study has shown.
“In short, what we are putting forward with these data is that pathogenic/likely pathogenic (P/LP) variants in the CDH1 gene confer a very high risk, at the very least, of occult early-stage gastric cancer in patients with HLBC,” said Jeremy Davis, MD, of the surgical oncology program, Center for Cancer Research at the National Cancer Institute.
“So patients that are referred to as ‘HLBC’ due to a CDH1 variant should at least undergo annual endoscopic surveillance but the real questions is whether or not they should also consider prophylactic total gastrectomy – as many patients in our study did,” he said.
The study, which was published online Oct. 13, 2021, in JAMA Surgery, included a cohort of 151 families totaling 283 patients with a CDH1 pathogenic or likely pathogenic (P/LP) variant. Analyses were conducted on three patient groups, which included those with HLBC and a family history of breast cancer but no gastric cancer, those with hereditary diffuse gastric cancer (HDGC) but no history of breast cancer, and those with a family history of both gastric and breast cancer in the mixed group. Of these, 15.5% had a history of HLBC, 16.2% had a history of HDGC, and 52.6% made up the mixed group.
“We examined the HLBC group with specific attention to CDH1 genotype and prevalence of occult gastric cancer,” the authors explained. The group consisted of 31 families with 19 CDH1 variants, 10 of which were also present in the HDGC and mixed groups.
Among this group of patients, almost 73% underwent one or more surveillance endoscopies and on endoscopy, occult signet ring cell carcinoma was detected in over one-third of patients.
The median age at the time of endoscopic carcinoma detection was only 33 years.
“Nearly all of the patients with HLBC (93.8%) ... who elected for risk-reducing total gastrectomy owing to their underlying CDH1 P/LP variant harbored occult signet ring cell gastric adenocarcinoma on final pathology,” investigators observed.
The median age at the time patients elected to undergo total gastrectomy was 50 years.
The prevalence of occult gastric cancer among asymptomatic patients in the HDGC group was similarly high, affecting almost 95% of this group of patients.
Some 18 out of 19 CDH1 P/LP variants were responsible for this high prevalence of occult gastric cancer, as the investigators pointed out.
“Hereditary cancer risk is informed by the presence of a germline gene variant more so than by family history of cancer,” the authors stressed. “[And we found that] germline CDH1 P/LP variants appear to have a highly penetrant gastric phenotype irrespective of family history.”
Given this finding, the authors stressed that it is “paramount” patients previously assigned a diagnosis of HLBC not be excluded from undergoing gastric cancer risk assessment and counseling.
Furthermore, “the mere presence of a germline CDH1 P/LP variant, regardless of family history, may be reason enough to consider prophylactic total gastrectomy,” the authors wrote.
Limitations of the study included the fact that the disease phenotype was established from family pedigrees which has the potential for recall bias by family members.
The study was supported in part by the Intramural Research Program of the National Cancer Institute. None of the authors had conflicts of interest to disclose.
a prospective cohort study has shown.
“In short, what we are putting forward with these data is that pathogenic/likely pathogenic (P/LP) variants in the CDH1 gene confer a very high risk, at the very least, of occult early-stage gastric cancer in patients with HLBC,” said Jeremy Davis, MD, of the surgical oncology program, Center for Cancer Research at the National Cancer Institute.
“So patients that are referred to as ‘HLBC’ due to a CDH1 variant should at least undergo annual endoscopic surveillance but the real questions is whether or not they should also consider prophylactic total gastrectomy – as many patients in our study did,” he said.
The study, which was published online Oct. 13, 2021, in JAMA Surgery, included a cohort of 151 families totaling 283 patients with a CDH1 pathogenic or likely pathogenic (P/LP) variant. Analyses were conducted on three patient groups, which included those with HLBC and a family history of breast cancer but no gastric cancer, those with hereditary diffuse gastric cancer (HDGC) but no history of breast cancer, and those with a family history of both gastric and breast cancer in the mixed group. Of these, 15.5% had a history of HLBC, 16.2% had a history of HDGC, and 52.6% made up the mixed group.
“We examined the HLBC group with specific attention to CDH1 genotype and prevalence of occult gastric cancer,” the authors explained. The group consisted of 31 families with 19 CDH1 variants, 10 of which were also present in the HDGC and mixed groups.
Among this group of patients, almost 73% underwent one or more surveillance endoscopies and on endoscopy, occult signet ring cell carcinoma was detected in over one-third of patients.
The median age at the time of endoscopic carcinoma detection was only 33 years.
“Nearly all of the patients with HLBC (93.8%) ... who elected for risk-reducing total gastrectomy owing to their underlying CDH1 P/LP variant harbored occult signet ring cell gastric adenocarcinoma on final pathology,” investigators observed.
The median age at the time patients elected to undergo total gastrectomy was 50 years.
The prevalence of occult gastric cancer among asymptomatic patients in the HDGC group was similarly high, affecting almost 95% of this group of patients.
Some 18 out of 19 CDH1 P/LP variants were responsible for this high prevalence of occult gastric cancer, as the investigators pointed out.
“Hereditary cancer risk is informed by the presence of a germline gene variant more so than by family history of cancer,” the authors stressed. “[And we found that] germline CDH1 P/LP variants appear to have a highly penetrant gastric phenotype irrespective of family history.”
Given this finding, the authors stressed that it is “paramount” patients previously assigned a diagnosis of HLBC not be excluded from undergoing gastric cancer risk assessment and counseling.
Furthermore, “the mere presence of a germline CDH1 P/LP variant, regardless of family history, may be reason enough to consider prophylactic total gastrectomy,” the authors wrote.
Limitations of the study included the fact that the disease phenotype was established from family pedigrees which has the potential for recall bias by family members.
The study was supported in part by the Intramural Research Program of the National Cancer Institute. None of the authors had conflicts of interest to disclose.
FROM JAMA SURGERY
Chemotherapy with FOLFOX superior to TACE in liver cancer
Hepatic arterial infusion chemotherapy (HAIC) with FOLFOX led to superior patient outcomes and less toxicity than standard of care transarterial chemoembolization (TACE) for patients with large, unresectable hepatocellular carcinoma (HCC), a randomized, phase 3 trial has shown.
In a group of 315 patients with unresectable tumors at least 7 cm in diameter and no macrovascular invasion or extrahepatic spread, overall survival (OS) was 42% longer at 23.1 months for patients treated with FOLFOX-HAIC, compared with 16.1 months for TACE, at a hazard ratio of 0.58 (P < .001), Ming Shi, MD, Sun Yat-sen University, Guangzhou, China, and colleagues reported recently in the Journal of Clinical Oncology.
Similarly, median progression-free survival (PFS) was also longer for FOLFOX-HAIC patients at 9.6 months, compared with 5.4 months for TACE patients, as was median symptomatic PFS at 17.9 months, compared with 10.4 months, respectively (P < .001).
The frequency of grade 3-4 elevated liver ALT at 8% versus 19% (P = .005), elevated AST at 18% versus 28%, and hyperbilirubinemia at 1% versus 6% (P = .01) were all significantly higher in the TACE group than in the FOLFOX-HAIC group as was the overall incidence of serious adverse events at 30% versus 19%, respectively (P = .03).
The frequency of treatment delay and discontinuation of TACE because of adverse events were also higher than that in the FOLFOX-HAIC group.
“Transarterial chemoembolization (TACE) is the current standard of care for intermediate-stage hepatocellular carcinoma ... but the efficacy of TACE is largely dependent upon tumor size ... and for patients with particularly large tumors (>7 cm), the OS after TACE is only 11.2-13.2 months,” Dr. Shi and colleagues explained.
“Although the need for technical expertise may limit its generalizability, hepatic arterial infusion chemotherapy represents an appropriate frontline locoregional intervention in select patients with large unresectable hepatocellular carcinoma,” they observed.
TACE vs. HAIC
During TACE, a catheter was inserted into the celiac trunk or superior mesenteric artery for arteriography, after which a microcatheter was selectively placed into the feeding arteries of the tumors.
“Chemolipiodolization was performed using 50 mg of epirubicin and 50 mg of lobaplatin mixed with lipiodol,” the investigators noted, and subsequent embolization was done with the injection of polyvinyl alcohol particles.
TACE was repeated every 6 weeks.
HAIC in turn was divided into 3-week cycles during which a microcatheter was advanced into the hepatic artery on day 1 of each treatment cycle and FOLFOX was infused via the hepatic artery. FOLFOX consisted of oxaliplatin, 130 mg/m2; leucovorin, 400 mg/m2; and fluorouracil, 400 mg/m2, all given on day 1.
Subgroup analyses showed that HAIC provided a clinical benefit for PFS in most subgroups except females; those with a Child-Pug score of 6, and those who were negative for HBV.
The main drawback to the FOLFOX-HAIC regimen appears to be abdominal pain which some patients experienced when oxaliplatin was injected but which subsequently resolved when the injection was stopped.
As the authors pointed out, the overall response rate at 46% among patients treated with FOLFOX-HAIC was more than twice that with TACE at 18%.
“One possible reason is that FOLFOX-HAIC can provide stable local high concentrations of the chemotherapy agents in the tumor for more than 24 hours,” they speculated, “whereas most chemotherapeutic agents delivered through TACE ... will be released into the systemic circulation within less than 1 hour.”
Recently, drugs such as atezolizumab (Tecentriq, Roche) plus bevacizumab (Avastin, Genentech) have been shown to lead to high response rates in intermediate-stage HCC, as shown by the IMbrave 150 study.
“Therefore, in some latest practice guidelines and expert consensus statements, a switch of first-line treatment modality from TACE to systemic treatment is proposed for these patients,” the researchers noted.
Limitations of the study include its open-label design and the fact that more patients in the FOLFOX-HAIC group underwent hepatic resection whereas more patients in the TACE group crossed over to the other treatment arm.
Dr. Shi declared no conflicts of interest.
Hepatic arterial infusion chemotherapy (HAIC) with FOLFOX led to superior patient outcomes and less toxicity than standard of care transarterial chemoembolization (TACE) for patients with large, unresectable hepatocellular carcinoma (HCC), a randomized, phase 3 trial has shown.
In a group of 315 patients with unresectable tumors at least 7 cm in diameter and no macrovascular invasion or extrahepatic spread, overall survival (OS) was 42% longer at 23.1 months for patients treated with FOLFOX-HAIC, compared with 16.1 months for TACE, at a hazard ratio of 0.58 (P < .001), Ming Shi, MD, Sun Yat-sen University, Guangzhou, China, and colleagues reported recently in the Journal of Clinical Oncology.
Similarly, median progression-free survival (PFS) was also longer for FOLFOX-HAIC patients at 9.6 months, compared with 5.4 months for TACE patients, as was median symptomatic PFS at 17.9 months, compared with 10.4 months, respectively (P < .001).
The frequency of grade 3-4 elevated liver ALT at 8% versus 19% (P = .005), elevated AST at 18% versus 28%, and hyperbilirubinemia at 1% versus 6% (P = .01) were all significantly higher in the TACE group than in the FOLFOX-HAIC group as was the overall incidence of serious adverse events at 30% versus 19%, respectively (P = .03).
The frequency of treatment delay and discontinuation of TACE because of adverse events were also higher than that in the FOLFOX-HAIC group.
“Transarterial chemoembolization (TACE) is the current standard of care for intermediate-stage hepatocellular carcinoma ... but the efficacy of TACE is largely dependent upon tumor size ... and for patients with particularly large tumors (>7 cm), the OS after TACE is only 11.2-13.2 months,” Dr. Shi and colleagues explained.
“Although the need for technical expertise may limit its generalizability, hepatic arterial infusion chemotherapy represents an appropriate frontline locoregional intervention in select patients with large unresectable hepatocellular carcinoma,” they observed.
TACE vs. HAIC
During TACE, a catheter was inserted into the celiac trunk or superior mesenteric artery for arteriography, after which a microcatheter was selectively placed into the feeding arteries of the tumors.
“Chemolipiodolization was performed using 50 mg of epirubicin and 50 mg of lobaplatin mixed with lipiodol,” the investigators noted, and subsequent embolization was done with the injection of polyvinyl alcohol particles.
TACE was repeated every 6 weeks.
HAIC in turn was divided into 3-week cycles during which a microcatheter was advanced into the hepatic artery on day 1 of each treatment cycle and FOLFOX was infused via the hepatic artery. FOLFOX consisted of oxaliplatin, 130 mg/m2; leucovorin, 400 mg/m2; and fluorouracil, 400 mg/m2, all given on day 1.
Subgroup analyses showed that HAIC provided a clinical benefit for PFS in most subgroups except females; those with a Child-Pug score of 6, and those who were negative for HBV.
The main drawback to the FOLFOX-HAIC regimen appears to be abdominal pain which some patients experienced when oxaliplatin was injected but which subsequently resolved when the injection was stopped.
As the authors pointed out, the overall response rate at 46% among patients treated with FOLFOX-HAIC was more than twice that with TACE at 18%.
“One possible reason is that FOLFOX-HAIC can provide stable local high concentrations of the chemotherapy agents in the tumor for more than 24 hours,” they speculated, “whereas most chemotherapeutic agents delivered through TACE ... will be released into the systemic circulation within less than 1 hour.”
Recently, drugs such as atezolizumab (Tecentriq, Roche) plus bevacizumab (Avastin, Genentech) have been shown to lead to high response rates in intermediate-stage HCC, as shown by the IMbrave 150 study.
“Therefore, in some latest practice guidelines and expert consensus statements, a switch of first-line treatment modality from TACE to systemic treatment is proposed for these patients,” the researchers noted.
Limitations of the study include its open-label design and the fact that more patients in the FOLFOX-HAIC group underwent hepatic resection whereas more patients in the TACE group crossed over to the other treatment arm.
Dr. Shi declared no conflicts of interest.
Hepatic arterial infusion chemotherapy (HAIC) with FOLFOX led to superior patient outcomes and less toxicity than standard of care transarterial chemoembolization (TACE) for patients with large, unresectable hepatocellular carcinoma (HCC), a randomized, phase 3 trial has shown.
In a group of 315 patients with unresectable tumors at least 7 cm in diameter and no macrovascular invasion or extrahepatic spread, overall survival (OS) was 42% longer at 23.1 months for patients treated with FOLFOX-HAIC, compared with 16.1 months for TACE, at a hazard ratio of 0.58 (P < .001), Ming Shi, MD, Sun Yat-sen University, Guangzhou, China, and colleagues reported recently in the Journal of Clinical Oncology.
Similarly, median progression-free survival (PFS) was also longer for FOLFOX-HAIC patients at 9.6 months, compared with 5.4 months for TACE patients, as was median symptomatic PFS at 17.9 months, compared with 10.4 months, respectively (P < .001).
The frequency of grade 3-4 elevated liver ALT at 8% versus 19% (P = .005), elevated AST at 18% versus 28%, and hyperbilirubinemia at 1% versus 6% (P = .01) were all significantly higher in the TACE group than in the FOLFOX-HAIC group as was the overall incidence of serious adverse events at 30% versus 19%, respectively (P = .03).
The frequency of treatment delay and discontinuation of TACE because of adverse events were also higher than that in the FOLFOX-HAIC group.
“Transarterial chemoembolization (TACE) is the current standard of care for intermediate-stage hepatocellular carcinoma ... but the efficacy of TACE is largely dependent upon tumor size ... and for patients with particularly large tumors (>7 cm), the OS after TACE is only 11.2-13.2 months,” Dr. Shi and colleagues explained.
“Although the need for technical expertise may limit its generalizability, hepatic arterial infusion chemotherapy represents an appropriate frontline locoregional intervention in select patients with large unresectable hepatocellular carcinoma,” they observed.
TACE vs. HAIC
During TACE, a catheter was inserted into the celiac trunk or superior mesenteric artery for arteriography, after which a microcatheter was selectively placed into the feeding arteries of the tumors.
“Chemolipiodolization was performed using 50 mg of epirubicin and 50 mg of lobaplatin mixed with lipiodol,” the investigators noted, and subsequent embolization was done with the injection of polyvinyl alcohol particles.
TACE was repeated every 6 weeks.
HAIC in turn was divided into 3-week cycles during which a microcatheter was advanced into the hepatic artery on day 1 of each treatment cycle and FOLFOX was infused via the hepatic artery. FOLFOX consisted of oxaliplatin, 130 mg/m2; leucovorin, 400 mg/m2; and fluorouracil, 400 mg/m2, all given on day 1.
Subgroup analyses showed that HAIC provided a clinical benefit for PFS in most subgroups except females; those with a Child-Pug score of 6, and those who were negative for HBV.
The main drawback to the FOLFOX-HAIC regimen appears to be abdominal pain which some patients experienced when oxaliplatin was injected but which subsequently resolved when the injection was stopped.
As the authors pointed out, the overall response rate at 46% among patients treated with FOLFOX-HAIC was more than twice that with TACE at 18%.
“One possible reason is that FOLFOX-HAIC can provide stable local high concentrations of the chemotherapy agents in the tumor for more than 24 hours,” they speculated, “whereas most chemotherapeutic agents delivered through TACE ... will be released into the systemic circulation within less than 1 hour.”
Recently, drugs such as atezolizumab (Tecentriq, Roche) plus bevacizumab (Avastin, Genentech) have been shown to lead to high response rates in intermediate-stage HCC, as shown by the IMbrave 150 study.
“Therefore, in some latest practice guidelines and expert consensus statements, a switch of first-line treatment modality from TACE to systemic treatment is proposed for these patients,” the researchers noted.
Limitations of the study include its open-label design and the fact that more patients in the FOLFOX-HAIC group underwent hepatic resection whereas more patients in the TACE group crossed over to the other treatment arm.
Dr. Shi declared no conflicts of interest.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Success in closing racial survival gap in lung and breast cancer
When barriers to completing radiation therapy were identified and addressed in a cohort of patients with early-stage lung and breast cancer, 5-year survival rates improved for all patients and closed the racial disparity gap, researchers reported at the annual meeting of the American Society for Radiation Oncology (ASTRO).
The findings come from the ACCURE clinical trial. This is the first prospective study designed to erase gaps in cancer treatment completion and survival among Black and White patient populations, explained lead author Matthew A. Manning, MD, a radiation oncologist and chief of oncology at Cone Health in Greensboro, N.C.
“Thousands of studies have looked at racial disparities in health care, but until recently, very few studies have implemented interventions to eliminate those disparities,” he said.
“This study shows that the implementation of ‘systems-change’ can eliminate racial disparities in cancer survival while improving survival for all,” he added.
“These results add to a growing body of evidence that health care disparities in cancer outcomes are eliminated or minimized by providing supportive, timely, and guideline-directed care,” said Lannis Hall, MD, MPH, director of radiation oncology, Siteman Cancer Center, and associate professor of radiation oncology at Washington University School of Medicine, St. Louis, who was approached for comment
“This research supports that access to care and timely treatment completion is critical to eliminating health care disparities,” she told this news organization. The system-based intervention in this trial was designed to reduce treatment delays and provide a supportive matrix for patients confronting real-world difficulties like transportation issues, childcare complications, and work absence, she explained.
Eliminating racial disparities
Previous findings from the ACCURE trial showed that it eliminated Black-White disparities in treatment completion rates, which was the study’s primary endpoint (Cancer Med. 2019;8:1095-1102). “It also improved treatment for all patients,” said Dr. Manning. “The current study is a follow-up on the survival of eligible patients treated during the ACCURE enrollment as compared to historical data.”
ACCURE was a multi-institutional trial designed to test a community-created intervention to reduce racial disparities. The intervention involved multiple changes to the way patients were supported while receiving cancer treatment and had four components:
- an electronic health record with automatic alerts to flag missed appointments or unmet milestones in expected care
- a nurse navigator trained in race-specific barriers to help patients overcome obstacles to care when alerts are flagged
- a physician champion to engage health care teams with race-related feedback on treatment completion
- regular health equity education training sessions for staff
The cohort was comprised of 1,413 patients with lung and breast cancer (stage 0-II) who were diagnosed from 2013-2015, and survival was compared to historical cases – 2,016 patients who had been treated from 2007-2011.
The results showed a significant improvement in survival for both Black and White patients with breast and lung cancer over time, and the racial gap in survival was reduced.
The 5-year survival rate for breast cancer increased from 91% for White patients and 89% in Black patients in historical cases, to 94% for both during the study period.
For patients with lung cancer, the 5-year survival rate improved from 43% in White patients and 37% in Black patients to 56% and 54%, respectively.
A subgroup analysis showed that patients with lung cancer who underwent surgery had 5-year survival rates of 78.5% for White and 70.1% for Black patients, whereas for those who underwent stereotactic body radiation therapy (SBRT) the rates were 41.9% and 50% respectively.
“We’ve shown it’s possible to eliminate disparities in cancer treatment completion and that this change has the potential to close cancer survival gaps downstream,” said Dr. Manning. “But we think the application can be much broader.”
The ACCURE study was sponsored by the National Institutes of Health. Dr. Manning and Dr. Hall have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
When barriers to completing radiation therapy were identified and addressed in a cohort of patients with early-stage lung and breast cancer, 5-year survival rates improved for all patients and closed the racial disparity gap, researchers reported at the annual meeting of the American Society for Radiation Oncology (ASTRO).
The findings come from the ACCURE clinical trial. This is the first prospective study designed to erase gaps in cancer treatment completion and survival among Black and White patient populations, explained lead author Matthew A. Manning, MD, a radiation oncologist and chief of oncology at Cone Health in Greensboro, N.C.
“Thousands of studies have looked at racial disparities in health care, but until recently, very few studies have implemented interventions to eliminate those disparities,” he said.
“This study shows that the implementation of ‘systems-change’ can eliminate racial disparities in cancer survival while improving survival for all,” he added.
“These results add to a growing body of evidence that health care disparities in cancer outcomes are eliminated or minimized by providing supportive, timely, and guideline-directed care,” said Lannis Hall, MD, MPH, director of radiation oncology, Siteman Cancer Center, and associate professor of radiation oncology at Washington University School of Medicine, St. Louis, who was approached for comment
“This research supports that access to care and timely treatment completion is critical to eliminating health care disparities,” she told this news organization. The system-based intervention in this trial was designed to reduce treatment delays and provide a supportive matrix for patients confronting real-world difficulties like transportation issues, childcare complications, and work absence, she explained.
Eliminating racial disparities
Previous findings from the ACCURE trial showed that it eliminated Black-White disparities in treatment completion rates, which was the study’s primary endpoint (Cancer Med. 2019;8:1095-1102). “It also improved treatment for all patients,” said Dr. Manning. “The current study is a follow-up on the survival of eligible patients treated during the ACCURE enrollment as compared to historical data.”
ACCURE was a multi-institutional trial designed to test a community-created intervention to reduce racial disparities. The intervention involved multiple changes to the way patients were supported while receiving cancer treatment and had four components:
- an electronic health record with automatic alerts to flag missed appointments or unmet milestones in expected care
- a nurse navigator trained in race-specific barriers to help patients overcome obstacles to care when alerts are flagged
- a physician champion to engage health care teams with race-related feedback on treatment completion
- regular health equity education training sessions for staff
The cohort was comprised of 1,413 patients with lung and breast cancer (stage 0-II) who were diagnosed from 2013-2015, and survival was compared to historical cases – 2,016 patients who had been treated from 2007-2011.
The results showed a significant improvement in survival for both Black and White patients with breast and lung cancer over time, and the racial gap in survival was reduced.
The 5-year survival rate for breast cancer increased from 91% for White patients and 89% in Black patients in historical cases, to 94% for both during the study period.
For patients with lung cancer, the 5-year survival rate improved from 43% in White patients and 37% in Black patients to 56% and 54%, respectively.
A subgroup analysis showed that patients with lung cancer who underwent surgery had 5-year survival rates of 78.5% for White and 70.1% for Black patients, whereas for those who underwent stereotactic body radiation therapy (SBRT) the rates were 41.9% and 50% respectively.
“We’ve shown it’s possible to eliminate disparities in cancer treatment completion and that this change has the potential to close cancer survival gaps downstream,” said Dr. Manning. “But we think the application can be much broader.”
The ACCURE study was sponsored by the National Institutes of Health. Dr. Manning and Dr. Hall have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
When barriers to completing radiation therapy were identified and addressed in a cohort of patients with early-stage lung and breast cancer, 5-year survival rates improved for all patients and closed the racial disparity gap, researchers reported at the annual meeting of the American Society for Radiation Oncology (ASTRO).
The findings come from the ACCURE clinical trial. This is the first prospective study designed to erase gaps in cancer treatment completion and survival among Black and White patient populations, explained lead author Matthew A. Manning, MD, a radiation oncologist and chief of oncology at Cone Health in Greensboro, N.C.
“Thousands of studies have looked at racial disparities in health care, but until recently, very few studies have implemented interventions to eliminate those disparities,” he said.
“This study shows that the implementation of ‘systems-change’ can eliminate racial disparities in cancer survival while improving survival for all,” he added.
“These results add to a growing body of evidence that health care disparities in cancer outcomes are eliminated or minimized by providing supportive, timely, and guideline-directed care,” said Lannis Hall, MD, MPH, director of radiation oncology, Siteman Cancer Center, and associate professor of radiation oncology at Washington University School of Medicine, St. Louis, who was approached for comment
“This research supports that access to care and timely treatment completion is critical to eliminating health care disparities,” she told this news organization. The system-based intervention in this trial was designed to reduce treatment delays and provide a supportive matrix for patients confronting real-world difficulties like transportation issues, childcare complications, and work absence, she explained.
Eliminating racial disparities
Previous findings from the ACCURE trial showed that it eliminated Black-White disparities in treatment completion rates, which was the study’s primary endpoint (Cancer Med. 2019;8:1095-1102). “It also improved treatment for all patients,” said Dr. Manning. “The current study is a follow-up on the survival of eligible patients treated during the ACCURE enrollment as compared to historical data.”
ACCURE was a multi-institutional trial designed to test a community-created intervention to reduce racial disparities. The intervention involved multiple changes to the way patients were supported while receiving cancer treatment and had four components:
- an electronic health record with automatic alerts to flag missed appointments or unmet milestones in expected care
- a nurse navigator trained in race-specific barriers to help patients overcome obstacles to care when alerts are flagged
- a physician champion to engage health care teams with race-related feedback on treatment completion
- regular health equity education training sessions for staff
The cohort was comprised of 1,413 patients with lung and breast cancer (stage 0-II) who were diagnosed from 2013-2015, and survival was compared to historical cases – 2,016 patients who had been treated from 2007-2011.
The results showed a significant improvement in survival for both Black and White patients with breast and lung cancer over time, and the racial gap in survival was reduced.
The 5-year survival rate for breast cancer increased from 91% for White patients and 89% in Black patients in historical cases, to 94% for both during the study period.
For patients with lung cancer, the 5-year survival rate improved from 43% in White patients and 37% in Black patients to 56% and 54%, respectively.
A subgroup analysis showed that patients with lung cancer who underwent surgery had 5-year survival rates of 78.5% for White and 70.1% for Black patients, whereas for those who underwent stereotactic body radiation therapy (SBRT) the rates were 41.9% and 50% respectively.
“We’ve shown it’s possible to eliminate disparities in cancer treatment completion and that this change has the potential to close cancer survival gaps downstream,” said Dr. Manning. “But we think the application can be much broader.”
The ACCURE study was sponsored by the National Institutes of Health. Dr. Manning and Dr. Hall have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ASTRO 2021