User login
Tolerance in medicine
There is a narrative being pushed now about health care professionals being “frustrated” and “tired” in the midst of this current delta COVID wave. This stems from the idea that this current wave was potentially preventable if everyone received the COVID vaccines when they were made available.
I certainly understand this frustration and am tired of dealing with COVID restrictions and wearing masks. Above all I’m tired of talking about it. But frustration and fatigue are nothing new for those in the health care profession. Part of our training is that we should care for everyone, no matter what. Compassion for the ill should not be restricted to patients with a certain financial status, immigration status, race, gender, sexual orientation, or education level. Socially and politically, we are having a reckoning with how we treat people and how we need to do better to create a more just society. A key virtue in all of this is tolerance.
If we are going to have a free society, tolerance is essential. This is because in a free society people are going to, well, be free. In medicine we tolerate people who are morbidly obese, drink alcohol excessively, smoke, refuse to take their medications, won’t exercise, won’t sleep, and do drugs. The overwhelming majority of these people know that what they are doing is bad for their health. Not only do we tolerate them, we are taught to treat them indiscriminately. When someone who is morbidly obese has a heart attack, we treat them, give them medicine, and tell them the importance of losing weight. We do not tell them, “you shouldn’t have eaten so much and gotten so fat,” or “don’t you wish you didn’t get so fat?”
What I am trying to circle back to here is that if you could force people into doing everything they could for their health and eliminate all “preventable” diseases, then the need for health care in this country – including doctors, nurses, hospitals, and pharmaceuticals, just to name a few – would be cut dramatically. While the frustration for the continued COVID surges is understandable, I urge people to remember that in the business of health care we deal with preventable diseases all the time, every day. We are taught to show compassion for everyone, and for good reason. We have no idea what many people’s backstories are, we just know that they are sick and need help.
I urge everyone to put the unvaccinated under the same umbrella you put other people with preventable diseases, which, sadly, is a lot of patients. Continue to educate those about the vaccine as you should about every other aspect of their health. Education is part of our job as health care professionals but judgment is not.
Dr. Matuszak works for Sound Physicians and is a nocturnist at a hospital in the San Francisco Bay Area.
There is a narrative being pushed now about health care professionals being “frustrated” and “tired” in the midst of this current delta COVID wave. This stems from the idea that this current wave was potentially preventable if everyone received the COVID vaccines when they were made available.
I certainly understand this frustration and am tired of dealing with COVID restrictions and wearing masks. Above all I’m tired of talking about it. But frustration and fatigue are nothing new for those in the health care profession. Part of our training is that we should care for everyone, no matter what. Compassion for the ill should not be restricted to patients with a certain financial status, immigration status, race, gender, sexual orientation, or education level. Socially and politically, we are having a reckoning with how we treat people and how we need to do better to create a more just society. A key virtue in all of this is tolerance.
If we are going to have a free society, tolerance is essential. This is because in a free society people are going to, well, be free. In medicine we tolerate people who are morbidly obese, drink alcohol excessively, smoke, refuse to take their medications, won’t exercise, won’t sleep, and do drugs. The overwhelming majority of these people know that what they are doing is bad for their health. Not only do we tolerate them, we are taught to treat them indiscriminately. When someone who is morbidly obese has a heart attack, we treat them, give them medicine, and tell them the importance of losing weight. We do not tell them, “you shouldn’t have eaten so much and gotten so fat,” or “don’t you wish you didn’t get so fat?”
What I am trying to circle back to here is that if you could force people into doing everything they could for their health and eliminate all “preventable” diseases, then the need for health care in this country – including doctors, nurses, hospitals, and pharmaceuticals, just to name a few – would be cut dramatically. While the frustration for the continued COVID surges is understandable, I urge people to remember that in the business of health care we deal with preventable diseases all the time, every day. We are taught to show compassion for everyone, and for good reason. We have no idea what many people’s backstories are, we just know that they are sick and need help.
I urge everyone to put the unvaccinated under the same umbrella you put other people with preventable diseases, which, sadly, is a lot of patients. Continue to educate those about the vaccine as you should about every other aspect of their health. Education is part of our job as health care professionals but judgment is not.
Dr. Matuszak works for Sound Physicians and is a nocturnist at a hospital in the San Francisco Bay Area.
There is a narrative being pushed now about health care professionals being “frustrated” and “tired” in the midst of this current delta COVID wave. This stems from the idea that this current wave was potentially preventable if everyone received the COVID vaccines when they were made available.
I certainly understand this frustration and am tired of dealing with COVID restrictions and wearing masks. Above all I’m tired of talking about it. But frustration and fatigue are nothing new for those in the health care profession. Part of our training is that we should care for everyone, no matter what. Compassion for the ill should not be restricted to patients with a certain financial status, immigration status, race, gender, sexual orientation, or education level. Socially and politically, we are having a reckoning with how we treat people and how we need to do better to create a more just society. A key virtue in all of this is tolerance.
If we are going to have a free society, tolerance is essential. This is because in a free society people are going to, well, be free. In medicine we tolerate people who are morbidly obese, drink alcohol excessively, smoke, refuse to take their medications, won’t exercise, won’t sleep, and do drugs. The overwhelming majority of these people know that what they are doing is bad for their health. Not only do we tolerate them, we are taught to treat them indiscriminately. When someone who is morbidly obese has a heart attack, we treat them, give them medicine, and tell them the importance of losing weight. We do not tell them, “you shouldn’t have eaten so much and gotten so fat,” or “don’t you wish you didn’t get so fat?”
What I am trying to circle back to here is that if you could force people into doing everything they could for their health and eliminate all “preventable” diseases, then the need for health care in this country – including doctors, nurses, hospitals, and pharmaceuticals, just to name a few – would be cut dramatically. While the frustration for the continued COVID surges is understandable, I urge people to remember that in the business of health care we deal with preventable diseases all the time, every day. We are taught to show compassion for everyone, and for good reason. We have no idea what many people’s backstories are, we just know that they are sick and need help.
I urge everyone to put the unvaccinated under the same umbrella you put other people with preventable diseases, which, sadly, is a lot of patients. Continue to educate those about the vaccine as you should about every other aspect of their health. Education is part of our job as health care professionals but judgment is not.
Dr. Matuszak works for Sound Physicians and is a nocturnist at a hospital in the San Francisco Bay Area.
Clinical Edge Journal Scan Commentary: COVID-19 October 2021
Marconi et al report out the results of the COV-Barrier study, a phase 3 randomized placebo controlled (RCT) trial with baricitinib, a JAK-STAT pathway inhibitor generally used to suppress proinflammatory cytokine production and systemic inflammation in rheumatoid arthritis, in hospitalized COVID-19 patients. The 1525 participants equally divided between placebo and baricitinib in an intention to treat analysis, were recruited from 12 countries, and most were on systemic corticosteroids (79.3%) and about one fifth received the antiviral, remdesivir. The study did not show a difference in the primary outcome (a composite of progression to more severe disease including need for high flow oxygen, non-invasive or invasive mechanical ventilation or death by 28 days), but did note a 5% absolute reduction in mortality at 28 days. Based on this results, Baricitinib may have a role similar role in the care of COVID-19 patients as tocilizumab (an IL-6 inhibitor) when given in combination with steroids, but may be used in the care of patients earlier in their disease including those on high flow or noninvasive oxygen as well as those requiring invasive mechanical ventilation, compared to tocilizumab, which is currently preferred by many clinicians in those on more intensive respiratory support and within 24-48 hours of ICU admission. Like other immunomodulators, there is a concern for increased secondary infections with the use of this therapy, but JAK inhibitors also carry a risk for increase in venous thromboembolic events. Interestingly, in this trial, there was no difference in the incidence of either of these adverse effects between the placebo and treatment groups.
The results of a phase 3 RCT with Canakinumab, an anti-interleukin-1B monoclonal antibody, on the other hand, did not show a benefit in primary outcome of survival without need for mechanical ventilation day 3 to day 29, or recovery time, in hospitalized patients who had elevated C-reactive protein and ferritin but were not yet intubated. Canakinumab was evaluated because IL-1B has been identified as one of the signature elevated cytokines in a maladaptive immune response to SARS-CoV-2 infection and because had shown some promise in small and retrospective studies.
Lastly, on the prevention side, a study of Regeneron’s anti-SARS-CoV-2 monoclonal antibody cocktail (casirivimab and imdevimab) was evaluated in a double blind placebo controlled RCT in prevention of development of symptomatic SARS-CoV-2 infection within 28 days among household contacts who did not have prior immunity. In the study, 1.5% of those who received the monoclonal therapy subcutaneously versus 7.8% of those who received placebo met the primary endpoint. Additionally, those who received the therapy and were symptomatic, resolved their symptoms two weeks earlier. The real-world utility of the results, however, maybe limited to specific groups, given that need for early identification of contacts, the need to access medical facility for administration, as well as the cost of the drug. There may be a role in high risk and immunocompromised contacts who do not develop adequate immune response to vaccination.
Marconi et al report out the results of the COV-Barrier study, a phase 3 randomized placebo controlled (RCT) trial with baricitinib, a JAK-STAT pathway inhibitor generally used to suppress proinflammatory cytokine production and systemic inflammation in rheumatoid arthritis, in hospitalized COVID-19 patients. The 1525 participants equally divided between placebo and baricitinib in an intention to treat analysis, were recruited from 12 countries, and most were on systemic corticosteroids (79.3%) and about one fifth received the antiviral, remdesivir. The study did not show a difference in the primary outcome (a composite of progression to more severe disease including need for high flow oxygen, non-invasive or invasive mechanical ventilation or death by 28 days), but did note a 5% absolute reduction in mortality at 28 days. Based on this results, Baricitinib may have a role similar role in the care of COVID-19 patients as tocilizumab (an IL-6 inhibitor) when given in combination with steroids, but may be used in the care of patients earlier in their disease including those on high flow or noninvasive oxygen as well as those requiring invasive mechanical ventilation, compared to tocilizumab, which is currently preferred by many clinicians in those on more intensive respiratory support and within 24-48 hours of ICU admission. Like other immunomodulators, there is a concern for increased secondary infections with the use of this therapy, but JAK inhibitors also carry a risk for increase in venous thromboembolic events. Interestingly, in this trial, there was no difference in the incidence of either of these adverse effects between the placebo and treatment groups.
The results of a phase 3 RCT with Canakinumab, an anti-interleukin-1B monoclonal antibody, on the other hand, did not show a benefit in primary outcome of survival without need for mechanical ventilation day 3 to day 29, or recovery time, in hospitalized patients who had elevated C-reactive protein and ferritin but were not yet intubated. Canakinumab was evaluated because IL-1B has been identified as one of the signature elevated cytokines in a maladaptive immune response to SARS-CoV-2 infection and because had shown some promise in small and retrospective studies.
Lastly, on the prevention side, a study of Regeneron’s anti-SARS-CoV-2 monoclonal antibody cocktail (casirivimab and imdevimab) was evaluated in a double blind placebo controlled RCT in prevention of development of symptomatic SARS-CoV-2 infection within 28 days among household contacts who did not have prior immunity. In the study, 1.5% of those who received the monoclonal therapy subcutaneously versus 7.8% of those who received placebo met the primary endpoint. Additionally, those who received the therapy and were symptomatic, resolved their symptoms two weeks earlier. The real-world utility of the results, however, maybe limited to specific groups, given that need for early identification of contacts, the need to access medical facility for administration, as well as the cost of the drug. There may be a role in high risk and immunocompromised contacts who do not develop adequate immune response to vaccination.
Marconi et al report out the results of the COV-Barrier study, a phase 3 randomized placebo controlled (RCT) trial with baricitinib, a JAK-STAT pathway inhibitor generally used to suppress proinflammatory cytokine production and systemic inflammation in rheumatoid arthritis, in hospitalized COVID-19 patients. The 1525 participants equally divided between placebo and baricitinib in an intention to treat analysis, were recruited from 12 countries, and most were on systemic corticosteroids (79.3%) and about one fifth received the antiviral, remdesivir. The study did not show a difference in the primary outcome (a composite of progression to more severe disease including need for high flow oxygen, non-invasive or invasive mechanical ventilation or death by 28 days), but did note a 5% absolute reduction in mortality at 28 days. Based on this results, Baricitinib may have a role similar role in the care of COVID-19 patients as tocilizumab (an IL-6 inhibitor) when given in combination with steroids, but may be used in the care of patients earlier in their disease including those on high flow or noninvasive oxygen as well as those requiring invasive mechanical ventilation, compared to tocilizumab, which is currently preferred by many clinicians in those on more intensive respiratory support and within 24-48 hours of ICU admission. Like other immunomodulators, there is a concern for increased secondary infections with the use of this therapy, but JAK inhibitors also carry a risk for increase in venous thromboembolic events. Interestingly, in this trial, there was no difference in the incidence of either of these adverse effects between the placebo and treatment groups.
The results of a phase 3 RCT with Canakinumab, an anti-interleukin-1B monoclonal antibody, on the other hand, did not show a benefit in primary outcome of survival without need for mechanical ventilation day 3 to day 29, or recovery time, in hospitalized patients who had elevated C-reactive protein and ferritin but were not yet intubated. Canakinumab was evaluated because IL-1B has been identified as one of the signature elevated cytokines in a maladaptive immune response to SARS-CoV-2 infection and because had shown some promise in small and retrospective studies.
Lastly, on the prevention side, a study of Regeneron’s anti-SARS-CoV-2 monoclonal antibody cocktail (casirivimab and imdevimab) was evaluated in a double blind placebo controlled RCT in prevention of development of symptomatic SARS-CoV-2 infection within 28 days among household contacts who did not have prior immunity. In the study, 1.5% of those who received the monoclonal therapy subcutaneously versus 7.8% of those who received placebo met the primary endpoint. Additionally, those who received the therapy and were symptomatic, resolved their symptoms two weeks earlier. The real-world utility of the results, however, maybe limited to specific groups, given that need for early identification of contacts, the need to access medical facility for administration, as well as the cost of the drug. There may be a role in high risk and immunocompromised contacts who do not develop adequate immune response to vaccination.
More than half of U.S. children under 6 years show detectable blood lead levels
Lead poisoning remains a significant threat to the health of young children in the United States, based on data from blood tests of more than 1 million children.
Any level of lead is potentially harmful, although blood lead levels have decreased over the past several decades in part because of the elimination of lead from many consumer products, as well as from gas, paint, and plumbing fixtures, wrote Marissa Hauptman, MD, of Boston Children’s Hospital and colleagues.
However, “numerous environmental sources of legacy lead still exist,” and children living in poverty and in older housing in particular remain at increased risk for lead exposure, they noted.
In a study published in JAMA Pediatrics, the researchers analyzed deidentified results from blood lead tests performed at a single clinical laboratory for 1,141,441 children younger than 6 years between Oct. 1, 2018, and Feb. 29, 2020. The mean age of the children was 2.3 years; approximately half were boys.
Overall, 50.5% of the children tested (576,092 children) had detectable blood lead levels (BLLs), defined as 1.0 mcg/dL or higher, and 1.9% (21,172 children) had elevated BLLs, defined as 5.0 mcg/dL or higher.
In multivariate analysis, both detectable BLLs and elevated BLLs were significantly more common among children with public insurance (adjusted odds ratios, 2.01 and 1.08, respectively).
Children in the highest vs. lowest quintile of pre-1950s housing had significantly greater odds of both detectable and elevated BLLs (aOR, 1.65 and aOR, 3.06); those in the highest vs. lowest quintiles of poverty showed similarly increased risk of detectable and elevated BLLs (aOR, 1.89 and aOR, 1.99, respectively; P < .001 for all).
When the data were broken out by ZIP code, children in predominantly Black non-Hispanic and non-Latino neighborhoods were more likely than those living in other ZIP codes to have detectable BLLs (aOR, 1.13), but less likely to have elevated BLLs (aOR, 0.83). States with the highest overall proportions of children with detectable BLLs were Nebraska (83%), Missouri (82%), and Michigan (78%).
The study findings were limited by several factors, especially the potential for selection bias because of the use of a single reference laboratory (Quest Diagnostics), that does not perform all lead testing in the United States, the researchers noted. Other limitations included variability in testing at the state level, and the use of ZIP code–level data to estimate race, ethnicity, housing, and poverty, they said.
However, the results suggest that lead exposure remains a problem in young children, with significant disparities at the individual and community level, and national efforts must focus on further reductions of lead exposure in areas of highest risk, they concluded.
Step up lead elimination efforts
“The removal of lead from gasoline and new paint produced a precipitous decrease in blood lead levels from a population mean of 17 mcg/dL (all ages) in 1976 to 4 mcg/dL in the early 1990s to less than 2 mcg/dL today,” wrote Philip J. Landrigan, MD, of Boston College and David Bellinger, PhD, of Harvard University, Boston, in an accompanying editorial. However, “The findings from this study underscore the urgent need to eliminate all sources of lead exposure from U.S. children’s environments,” and highlight the persistent disparities in children’s lead exposure, they said.
The authors emphasized the need to remove existing lead paint from U.S. homes, as not only the paint itself, but the dust that enters the environment as the pain wears over time, continue to account for most detectable and elevated BLLs in children. A comprehensive lead paint removal effort would be an investment that would protect children now and would protect future generations, they emphasized. They proposed “creating a lead paint removal workforce through federally supported partnerships between city governments and major unions,” that would not only protect children from disease and disability, but could potentially provide jobs and vocational programs that would have a significant impact on communities.
Elevated lead levels may be underreported
In fact, the situation of children’s lead exposure in the United States may be more severe than indicated by the study findings, given the variation in testing at the state and local levels, said Karalyn Kinsella, MD, a pediatrician in private practice in Cheshire, Conn.
“There are no available lead test kits in our offices, so I do worry that many elevated lead levels will be missed,” she said.
“The recent case of elevated lead levels in drinking water in Flint, Michigan, was largely detected through pediatric clinic screening and showed that elevated lead levels may remain a major issue in some communities,” said Tim Joos, MD, a clinician in combined internal medicine/pediatrics in Seattle, Wash., in an interview.
“It is important to highlight to what extent baseline and point-source lead contamination still exists, monitor progress towards lowering levels, and identify communities at high risk,” Dr. Joos emphasized. “The exact prevalence of elevated lead levels among the general pediatric populations is hard to estimate from this study because of the methodology, which looked at demographic characteristics of the subset of the pediatric population that had venous samples sent to Quest Lab,” he noted.
“As the authors pointed out, it is hard to know what biases went into deciding whether to screen or not, and whether these were confirmatory tests for elevated point of care testing done earlier in the clinic,” said Dr. Joos. “Nonetheless, it does point to the role of poverty and pre-1950s housing in elevated blood lead levels,” he added. “The study also highlights that, as the CDC considers lowering the level for what is considered an ‘elevated blood lead level’ from 5.0 to perhaps 3.5 mcg/dL, we still have a lot more work to do,” he said.
The study was funded by Quest Diagnostics and the company provided salaries to several coauthors during the study. Dr. Hauptmann disclosed support from the National Institutes of Health/National Institute of Environmental Health Sciences during the current study and support from the Agency for Toxic Substances and Disease Registry and the U.S. Environmental Protection Agency unrelated to the current study. Dr. Landrigan had no financial conflicts to disclose. Dr. Bellinger disclosed fees from attorneys for testimony in cases unrelated to the editorial. Dr. Kinsella had no financial conflicts to disclose, but serves on the Editorial Advisory Board of Pediatric News. Dr. Joos had no financial conflicts to disclose, but serves on the Pediatric News Editorial Advisory Board.
Lead poisoning remains a significant threat to the health of young children in the United States, based on data from blood tests of more than 1 million children.
Any level of lead is potentially harmful, although blood lead levels have decreased over the past several decades in part because of the elimination of lead from many consumer products, as well as from gas, paint, and plumbing fixtures, wrote Marissa Hauptman, MD, of Boston Children’s Hospital and colleagues.
However, “numerous environmental sources of legacy lead still exist,” and children living in poverty and in older housing in particular remain at increased risk for lead exposure, they noted.
In a study published in JAMA Pediatrics, the researchers analyzed deidentified results from blood lead tests performed at a single clinical laboratory for 1,141,441 children younger than 6 years between Oct. 1, 2018, and Feb. 29, 2020. The mean age of the children was 2.3 years; approximately half were boys.
Overall, 50.5% of the children tested (576,092 children) had detectable blood lead levels (BLLs), defined as 1.0 mcg/dL or higher, and 1.9% (21,172 children) had elevated BLLs, defined as 5.0 mcg/dL or higher.
In multivariate analysis, both detectable BLLs and elevated BLLs were significantly more common among children with public insurance (adjusted odds ratios, 2.01 and 1.08, respectively).
Children in the highest vs. lowest quintile of pre-1950s housing had significantly greater odds of both detectable and elevated BLLs (aOR, 1.65 and aOR, 3.06); those in the highest vs. lowest quintiles of poverty showed similarly increased risk of detectable and elevated BLLs (aOR, 1.89 and aOR, 1.99, respectively; P < .001 for all).
When the data were broken out by ZIP code, children in predominantly Black non-Hispanic and non-Latino neighborhoods were more likely than those living in other ZIP codes to have detectable BLLs (aOR, 1.13), but less likely to have elevated BLLs (aOR, 0.83). States with the highest overall proportions of children with detectable BLLs were Nebraska (83%), Missouri (82%), and Michigan (78%).
The study findings were limited by several factors, especially the potential for selection bias because of the use of a single reference laboratory (Quest Diagnostics), that does not perform all lead testing in the United States, the researchers noted. Other limitations included variability in testing at the state level, and the use of ZIP code–level data to estimate race, ethnicity, housing, and poverty, they said.
However, the results suggest that lead exposure remains a problem in young children, with significant disparities at the individual and community level, and national efforts must focus on further reductions of lead exposure in areas of highest risk, they concluded.
Step up lead elimination efforts
“The removal of lead from gasoline and new paint produced a precipitous decrease in blood lead levels from a population mean of 17 mcg/dL (all ages) in 1976 to 4 mcg/dL in the early 1990s to less than 2 mcg/dL today,” wrote Philip J. Landrigan, MD, of Boston College and David Bellinger, PhD, of Harvard University, Boston, in an accompanying editorial. However, “The findings from this study underscore the urgent need to eliminate all sources of lead exposure from U.S. children’s environments,” and highlight the persistent disparities in children’s lead exposure, they said.
The authors emphasized the need to remove existing lead paint from U.S. homes, as not only the paint itself, but the dust that enters the environment as the pain wears over time, continue to account for most detectable and elevated BLLs in children. A comprehensive lead paint removal effort would be an investment that would protect children now and would protect future generations, they emphasized. They proposed “creating a lead paint removal workforce through federally supported partnerships between city governments and major unions,” that would not only protect children from disease and disability, but could potentially provide jobs and vocational programs that would have a significant impact on communities.
Elevated lead levels may be underreported
In fact, the situation of children’s lead exposure in the United States may be more severe than indicated by the study findings, given the variation in testing at the state and local levels, said Karalyn Kinsella, MD, a pediatrician in private practice in Cheshire, Conn.
“There are no available lead test kits in our offices, so I do worry that many elevated lead levels will be missed,” she said.
“The recent case of elevated lead levels in drinking water in Flint, Michigan, was largely detected through pediatric clinic screening and showed that elevated lead levels may remain a major issue in some communities,” said Tim Joos, MD, a clinician in combined internal medicine/pediatrics in Seattle, Wash., in an interview.
“It is important to highlight to what extent baseline and point-source lead contamination still exists, monitor progress towards lowering levels, and identify communities at high risk,” Dr. Joos emphasized. “The exact prevalence of elevated lead levels among the general pediatric populations is hard to estimate from this study because of the methodology, which looked at demographic characteristics of the subset of the pediatric population that had venous samples sent to Quest Lab,” he noted.
“As the authors pointed out, it is hard to know what biases went into deciding whether to screen or not, and whether these were confirmatory tests for elevated point of care testing done earlier in the clinic,” said Dr. Joos. “Nonetheless, it does point to the role of poverty and pre-1950s housing in elevated blood lead levels,” he added. “The study also highlights that, as the CDC considers lowering the level for what is considered an ‘elevated blood lead level’ from 5.0 to perhaps 3.5 mcg/dL, we still have a lot more work to do,” he said.
The study was funded by Quest Diagnostics and the company provided salaries to several coauthors during the study. Dr. Hauptmann disclosed support from the National Institutes of Health/National Institute of Environmental Health Sciences during the current study and support from the Agency for Toxic Substances and Disease Registry and the U.S. Environmental Protection Agency unrelated to the current study. Dr. Landrigan had no financial conflicts to disclose. Dr. Bellinger disclosed fees from attorneys for testimony in cases unrelated to the editorial. Dr. Kinsella had no financial conflicts to disclose, but serves on the Editorial Advisory Board of Pediatric News. Dr. Joos had no financial conflicts to disclose, but serves on the Pediatric News Editorial Advisory Board.
Lead poisoning remains a significant threat to the health of young children in the United States, based on data from blood tests of more than 1 million children.
Any level of lead is potentially harmful, although blood lead levels have decreased over the past several decades in part because of the elimination of lead from many consumer products, as well as from gas, paint, and plumbing fixtures, wrote Marissa Hauptman, MD, of Boston Children’s Hospital and colleagues.
However, “numerous environmental sources of legacy lead still exist,” and children living in poverty and in older housing in particular remain at increased risk for lead exposure, they noted.
In a study published in JAMA Pediatrics, the researchers analyzed deidentified results from blood lead tests performed at a single clinical laboratory for 1,141,441 children younger than 6 years between Oct. 1, 2018, and Feb. 29, 2020. The mean age of the children was 2.3 years; approximately half were boys.
Overall, 50.5% of the children tested (576,092 children) had detectable blood lead levels (BLLs), defined as 1.0 mcg/dL or higher, and 1.9% (21,172 children) had elevated BLLs, defined as 5.0 mcg/dL or higher.
In multivariate analysis, both detectable BLLs and elevated BLLs were significantly more common among children with public insurance (adjusted odds ratios, 2.01 and 1.08, respectively).
Children in the highest vs. lowest quintile of pre-1950s housing had significantly greater odds of both detectable and elevated BLLs (aOR, 1.65 and aOR, 3.06); those in the highest vs. lowest quintiles of poverty showed similarly increased risk of detectable and elevated BLLs (aOR, 1.89 and aOR, 1.99, respectively; P < .001 for all).
When the data were broken out by ZIP code, children in predominantly Black non-Hispanic and non-Latino neighborhoods were more likely than those living in other ZIP codes to have detectable BLLs (aOR, 1.13), but less likely to have elevated BLLs (aOR, 0.83). States with the highest overall proportions of children with detectable BLLs were Nebraska (83%), Missouri (82%), and Michigan (78%).
The study findings were limited by several factors, especially the potential for selection bias because of the use of a single reference laboratory (Quest Diagnostics), that does not perform all lead testing in the United States, the researchers noted. Other limitations included variability in testing at the state level, and the use of ZIP code–level data to estimate race, ethnicity, housing, and poverty, they said.
However, the results suggest that lead exposure remains a problem in young children, with significant disparities at the individual and community level, and national efforts must focus on further reductions of lead exposure in areas of highest risk, they concluded.
Step up lead elimination efforts
“The removal of lead from gasoline and new paint produced a precipitous decrease in blood lead levels from a population mean of 17 mcg/dL (all ages) in 1976 to 4 mcg/dL in the early 1990s to less than 2 mcg/dL today,” wrote Philip J. Landrigan, MD, of Boston College and David Bellinger, PhD, of Harvard University, Boston, in an accompanying editorial. However, “The findings from this study underscore the urgent need to eliminate all sources of lead exposure from U.S. children’s environments,” and highlight the persistent disparities in children’s lead exposure, they said.
The authors emphasized the need to remove existing lead paint from U.S. homes, as not only the paint itself, but the dust that enters the environment as the pain wears over time, continue to account for most detectable and elevated BLLs in children. A comprehensive lead paint removal effort would be an investment that would protect children now and would protect future generations, they emphasized. They proposed “creating a lead paint removal workforce through federally supported partnerships between city governments and major unions,” that would not only protect children from disease and disability, but could potentially provide jobs and vocational programs that would have a significant impact on communities.
Elevated lead levels may be underreported
In fact, the situation of children’s lead exposure in the United States may be more severe than indicated by the study findings, given the variation in testing at the state and local levels, said Karalyn Kinsella, MD, a pediatrician in private practice in Cheshire, Conn.
“There are no available lead test kits in our offices, so I do worry that many elevated lead levels will be missed,” she said.
“The recent case of elevated lead levels in drinking water in Flint, Michigan, was largely detected through pediatric clinic screening and showed that elevated lead levels may remain a major issue in some communities,” said Tim Joos, MD, a clinician in combined internal medicine/pediatrics in Seattle, Wash., in an interview.
“It is important to highlight to what extent baseline and point-source lead contamination still exists, monitor progress towards lowering levels, and identify communities at high risk,” Dr. Joos emphasized. “The exact prevalence of elevated lead levels among the general pediatric populations is hard to estimate from this study because of the methodology, which looked at demographic characteristics of the subset of the pediatric population that had venous samples sent to Quest Lab,” he noted.
“As the authors pointed out, it is hard to know what biases went into deciding whether to screen or not, and whether these were confirmatory tests for elevated point of care testing done earlier in the clinic,” said Dr. Joos. “Nonetheless, it does point to the role of poverty and pre-1950s housing in elevated blood lead levels,” he added. “The study also highlights that, as the CDC considers lowering the level for what is considered an ‘elevated blood lead level’ from 5.0 to perhaps 3.5 mcg/dL, we still have a lot more work to do,” he said.
The study was funded by Quest Diagnostics and the company provided salaries to several coauthors during the study. Dr. Hauptmann disclosed support from the National Institutes of Health/National Institute of Environmental Health Sciences during the current study and support from the Agency for Toxic Substances and Disease Registry and the U.S. Environmental Protection Agency unrelated to the current study. Dr. Landrigan had no financial conflicts to disclose. Dr. Bellinger disclosed fees from attorneys for testimony in cases unrelated to the editorial. Dr. Kinsella had no financial conflicts to disclose, but serves on the Editorial Advisory Board of Pediatric News. Dr. Joos had no financial conflicts to disclose, but serves on the Pediatric News Editorial Advisory Board.
FROM JAMA PEDIATRICS
Rapid response needed for rare filler injection complications
if not promptly addressed, according to an expert explaining the signs of an impending disaster at the Skin of Color Update 2021.
The most serious of the adverse events stem from vascular compromise, which is often signaled immediately by sharp pain and blanching of the skin, according to Hassan Galadari, MD, assistant professor of dermatology at the United Arab Emirates University, Dubai.
“Swift and aggressive treatment is required to avoid irreversible changes,” said Dr. Galadari, warning that blindness and vision impairment can be permanent, and that other events associated with vascular compromise include stroke and other types of embolism, as well as tissue necrosis.
To be swift, Dr. Galadari advised an immediate halt of injections and then a series of steps to abort the vascular insult. The goal is to encourage blood flow to prevent clotting and dissipate the filler.
“Massage the area like crazy. Keep on massaging. The more you massage the better. You are recruiting blood into that area so it remains viable,” Dr. Galadari said.
Hyaluronidase injections helpful
Warm compresses should also be applied for periods ranging from 5 minutes up to an hour, he added. In patients treated with hyaluronic acid, he also commonly introduces hyaluronidase injections of 200-500 IU diluted in lidocaine or saline. The injections are placed 2-3 cm apart and repeated every hour until signs and symptoms improve.
“Flush all of the filler out,” he said, emphasizing the urgency for reversing risk of vascular adverse events.
To sustain blood flow and avoid clots, he also recommends initiating aspirin with maintenance doses sustained over several days. Sildenafil to further improve conditions of blood perfusion can be “considered.”
The risks of vascular compromise, like other complications from filler injections, are low, but they are not zero, and the opportunity to prevent irreversible changes depends on acting quickly, according to Dr. Galadari.
“To prevent embolism, recognize the danger zones,” he advised, identifying the glabella region as the site of highest risk. The risk of vascular compromise from injections into the nasal region is lower but higher than injections of the nasolabial fold and forehead, which are associated with a relatively low risk.
Slow injections reduce risks
Some basic strategies he recommended for preventing vascular compromise included slow injections while keeping pressure low and using small volumes of filler per shot. Fractionated treatment and microdroplet techniques can be appropriate depending on the site of injection.
“Delivery of the filler by cannulas rather than by needles is preferable,” according to Dr. Galadari, who noted that a task force from the American Society for Dermatologic Surgery recently endorsed this approach as part of other recommendations to avoid complications of injectable fillers.
The Food and Drug Administration’s Manufacturer and User Facility Device Experience (MAUDE) database suggests that adverse events of any substantial severity from filler injections, not just those involving vascular compromise, occur at a rate of 1 per 3,600 cases. However, MAUDE is a passive surveillance system dependent on reports provided by clinicians and others, so this event rate might be an underrepresentation.
In the MAUDE database, complication rates are listed for each of the available filler products and show a variation in rates not just overall but also for each of the major types of complications, which include skin-specific complications such as nodules, discoloration, and inflammation, as well as neurologic adverse events, infection, and vascular compromise, Dr. Galadari reported.
Filler products are not interchangeable
Again, because of passive data collection, it is not clear whether the differences between products is a true representation of relative risk. Nevertheless, Dr. Galadari cautioned that these products are not necessarily interchangeable, advising clinicians to avoid products without an established safety track record.
There are a wide variety of fillers, including biostimulatory products, such as poly-L-lactic acid and calcium hydroxyapatite, and permanent fillers, such as silicone, in addition to collagen and hyaluronic acid, which function as temporary fillers, according to Dr. Galadari. He emphasized that the specific risks of each filler vary, but clinicians should always respond quickly whenever there is an adverse reaction or evidence of vascular compromise.
In flushing out filler, Cheryl M. Burgess, MD, of the Center for Dermatology and Dermatologic Surgery, Washington, who spoke at the meeting, also emphasized a prompt response. She too employs hyaluronidase injections to break down excess hyaluronic acid in the event of complications related to this filler.
Importantly, Dr. Burgess pointed out that hyaluronic acid can be considered safe for darker skin types, including Fitzpatrick skin types IV, V, and VI, but she added that speed of injection might be a particularly important variable for cosmetic procedures in skin of color.
“There is less postinflammatory hyperpigmentation with slower injection times and more with serial or multiple puncture injection technique,” she cautioned.
She further concurred with the value of cannulas over needles in most instances for facial contouring applications with filler, but she encouraged clinicians not to be overly ambitious and to move gradually toward goals.
“The desired outcome may require multiple sessions with conservative measures,” she said, indicating that conservative measures also represent a strategy to avoid adverse events.
if not promptly addressed, according to an expert explaining the signs of an impending disaster at the Skin of Color Update 2021.
The most serious of the adverse events stem from vascular compromise, which is often signaled immediately by sharp pain and blanching of the skin, according to Hassan Galadari, MD, assistant professor of dermatology at the United Arab Emirates University, Dubai.
“Swift and aggressive treatment is required to avoid irreversible changes,” said Dr. Galadari, warning that blindness and vision impairment can be permanent, and that other events associated with vascular compromise include stroke and other types of embolism, as well as tissue necrosis.
To be swift, Dr. Galadari advised an immediate halt of injections and then a series of steps to abort the vascular insult. The goal is to encourage blood flow to prevent clotting and dissipate the filler.
“Massage the area like crazy. Keep on massaging. The more you massage the better. You are recruiting blood into that area so it remains viable,” Dr. Galadari said.
Hyaluronidase injections helpful
Warm compresses should also be applied for periods ranging from 5 minutes up to an hour, he added. In patients treated with hyaluronic acid, he also commonly introduces hyaluronidase injections of 200-500 IU diluted in lidocaine or saline. The injections are placed 2-3 cm apart and repeated every hour until signs and symptoms improve.
“Flush all of the filler out,” he said, emphasizing the urgency for reversing risk of vascular adverse events.
To sustain blood flow and avoid clots, he also recommends initiating aspirin with maintenance doses sustained over several days. Sildenafil to further improve conditions of blood perfusion can be “considered.”
The risks of vascular compromise, like other complications from filler injections, are low, but they are not zero, and the opportunity to prevent irreversible changes depends on acting quickly, according to Dr. Galadari.
“To prevent embolism, recognize the danger zones,” he advised, identifying the glabella region as the site of highest risk. The risk of vascular compromise from injections into the nasal region is lower but higher than injections of the nasolabial fold and forehead, which are associated with a relatively low risk.
Slow injections reduce risks
Some basic strategies he recommended for preventing vascular compromise included slow injections while keeping pressure low and using small volumes of filler per shot. Fractionated treatment and microdroplet techniques can be appropriate depending on the site of injection.
“Delivery of the filler by cannulas rather than by needles is preferable,” according to Dr. Galadari, who noted that a task force from the American Society for Dermatologic Surgery recently endorsed this approach as part of other recommendations to avoid complications of injectable fillers.
The Food and Drug Administration’s Manufacturer and User Facility Device Experience (MAUDE) database suggests that adverse events of any substantial severity from filler injections, not just those involving vascular compromise, occur at a rate of 1 per 3,600 cases. However, MAUDE is a passive surveillance system dependent on reports provided by clinicians and others, so this event rate might be an underrepresentation.
In the MAUDE database, complication rates are listed for each of the available filler products and show a variation in rates not just overall but also for each of the major types of complications, which include skin-specific complications such as nodules, discoloration, and inflammation, as well as neurologic adverse events, infection, and vascular compromise, Dr. Galadari reported.
Filler products are not interchangeable
Again, because of passive data collection, it is not clear whether the differences between products is a true representation of relative risk. Nevertheless, Dr. Galadari cautioned that these products are not necessarily interchangeable, advising clinicians to avoid products without an established safety track record.
There are a wide variety of fillers, including biostimulatory products, such as poly-L-lactic acid and calcium hydroxyapatite, and permanent fillers, such as silicone, in addition to collagen and hyaluronic acid, which function as temporary fillers, according to Dr. Galadari. He emphasized that the specific risks of each filler vary, but clinicians should always respond quickly whenever there is an adverse reaction or evidence of vascular compromise.
In flushing out filler, Cheryl M. Burgess, MD, of the Center for Dermatology and Dermatologic Surgery, Washington, who spoke at the meeting, also emphasized a prompt response. She too employs hyaluronidase injections to break down excess hyaluronic acid in the event of complications related to this filler.
Importantly, Dr. Burgess pointed out that hyaluronic acid can be considered safe for darker skin types, including Fitzpatrick skin types IV, V, and VI, but she added that speed of injection might be a particularly important variable for cosmetic procedures in skin of color.
“There is less postinflammatory hyperpigmentation with slower injection times and more with serial or multiple puncture injection technique,” she cautioned.
She further concurred with the value of cannulas over needles in most instances for facial contouring applications with filler, but she encouraged clinicians not to be overly ambitious and to move gradually toward goals.
“The desired outcome may require multiple sessions with conservative measures,” she said, indicating that conservative measures also represent a strategy to avoid adverse events.
if not promptly addressed, according to an expert explaining the signs of an impending disaster at the Skin of Color Update 2021.
The most serious of the adverse events stem from vascular compromise, which is often signaled immediately by sharp pain and blanching of the skin, according to Hassan Galadari, MD, assistant professor of dermatology at the United Arab Emirates University, Dubai.
“Swift and aggressive treatment is required to avoid irreversible changes,” said Dr. Galadari, warning that blindness and vision impairment can be permanent, and that other events associated with vascular compromise include stroke and other types of embolism, as well as tissue necrosis.
To be swift, Dr. Galadari advised an immediate halt of injections and then a series of steps to abort the vascular insult. The goal is to encourage blood flow to prevent clotting and dissipate the filler.
“Massage the area like crazy. Keep on massaging. The more you massage the better. You are recruiting blood into that area so it remains viable,” Dr. Galadari said.
Hyaluronidase injections helpful
Warm compresses should also be applied for periods ranging from 5 minutes up to an hour, he added. In patients treated with hyaluronic acid, he also commonly introduces hyaluronidase injections of 200-500 IU diluted in lidocaine or saline. The injections are placed 2-3 cm apart and repeated every hour until signs and symptoms improve.
“Flush all of the filler out,” he said, emphasizing the urgency for reversing risk of vascular adverse events.
To sustain blood flow and avoid clots, he also recommends initiating aspirin with maintenance doses sustained over several days. Sildenafil to further improve conditions of blood perfusion can be “considered.”
The risks of vascular compromise, like other complications from filler injections, are low, but they are not zero, and the opportunity to prevent irreversible changes depends on acting quickly, according to Dr. Galadari.
“To prevent embolism, recognize the danger zones,” he advised, identifying the glabella region as the site of highest risk. The risk of vascular compromise from injections into the nasal region is lower but higher than injections of the nasolabial fold and forehead, which are associated with a relatively low risk.
Slow injections reduce risks
Some basic strategies he recommended for preventing vascular compromise included slow injections while keeping pressure low and using small volumes of filler per shot. Fractionated treatment and microdroplet techniques can be appropriate depending on the site of injection.
“Delivery of the filler by cannulas rather than by needles is preferable,” according to Dr. Galadari, who noted that a task force from the American Society for Dermatologic Surgery recently endorsed this approach as part of other recommendations to avoid complications of injectable fillers.
The Food and Drug Administration’s Manufacturer and User Facility Device Experience (MAUDE) database suggests that adverse events of any substantial severity from filler injections, not just those involving vascular compromise, occur at a rate of 1 per 3,600 cases. However, MAUDE is a passive surveillance system dependent on reports provided by clinicians and others, so this event rate might be an underrepresentation.
In the MAUDE database, complication rates are listed for each of the available filler products and show a variation in rates not just overall but also for each of the major types of complications, which include skin-specific complications such as nodules, discoloration, and inflammation, as well as neurologic adverse events, infection, and vascular compromise, Dr. Galadari reported.
Filler products are not interchangeable
Again, because of passive data collection, it is not clear whether the differences between products is a true representation of relative risk. Nevertheless, Dr. Galadari cautioned that these products are not necessarily interchangeable, advising clinicians to avoid products without an established safety track record.
There are a wide variety of fillers, including biostimulatory products, such as poly-L-lactic acid and calcium hydroxyapatite, and permanent fillers, such as silicone, in addition to collagen and hyaluronic acid, which function as temporary fillers, according to Dr. Galadari. He emphasized that the specific risks of each filler vary, but clinicians should always respond quickly whenever there is an adverse reaction or evidence of vascular compromise.
In flushing out filler, Cheryl M. Burgess, MD, of the Center for Dermatology and Dermatologic Surgery, Washington, who spoke at the meeting, also emphasized a prompt response. She too employs hyaluronidase injections to break down excess hyaluronic acid in the event of complications related to this filler.
Importantly, Dr. Burgess pointed out that hyaluronic acid can be considered safe for darker skin types, including Fitzpatrick skin types IV, V, and VI, but she added that speed of injection might be a particularly important variable for cosmetic procedures in skin of color.
“There is less postinflammatory hyperpigmentation with slower injection times and more with serial or multiple puncture injection technique,” she cautioned.
She further concurred with the value of cannulas over needles in most instances for facial contouring applications with filler, but she encouraged clinicians not to be overly ambitious and to move gradually toward goals.
“The desired outcome may require multiple sessions with conservative measures,” she said, indicating that conservative measures also represent a strategy to avoid adverse events.
FROM SOC 2021
Opioid prescribing mapped: Alabama highest, New York lowest
Medicare beneficiaries in Alabama were more likely to get a prescription for an opioid than in any other state in 2019, based on newly released data.
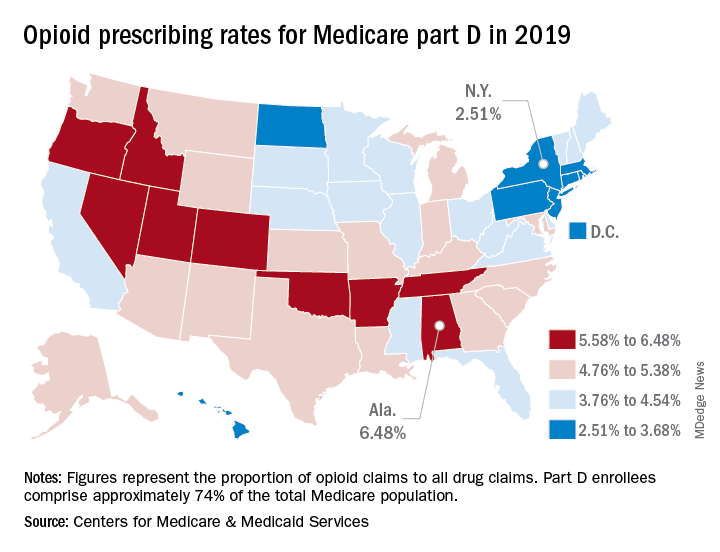
That year, opioids represented 6.48% of all drug claims for part D enrollees in the state, just ahead of Utah at 6.41%. Idaho, at 6.07%, was the only other state with an opioid prescribing rate over 6%, while Oklahoma came in at an even 6.0%, according to the latest update of the Centers for Medicare & Medicaid Services’ dataset.
The lowest rate in 2019 belonged to New York, where 2.51% of drug claims, including original prescriptions and refills, involved an opioid. Rhode Island was next at 2.87%, followed by New Jersey (3.23%), Massachusetts (3.26%), and North Dakota (3.39%),
Altogether, Medicare part D processed 1.5 billion drug claims in 2019, of which 66.1 million, or 4.41%, involved opioids. Both of the opioid numbers were down from 2018, when opioids represented 4.68% (70.2 million) of the 1.5 billion total claims, and from 2014, when opioids were involved in 5.73% (81,026,831) of the 1.41 billion drug claims, the CMS data show. That works out to 5.77% fewer opioids in 2019, compared with 2014, despite the increase in total volume.
from 2014 to 2019, with Hawaii showing the smallest decline as it slipped 0.41 percentage points from 3.9% to 3.49%, according to the CMS.
In 2019, part D beneficiaries in Vermont were the most likely to receive a long-acting opioid, which accounted for 20.14% of all opioid prescriptions in the state, while Kentucky had the lowest share of prescriptions written for long-acting forms at 6.41%. The national average was 11.02%, dropping from 11.79% in 2018 and 12.75% in 2014, the CMS reported.
Medicare beneficiaries in Alabama were more likely to get a prescription for an opioid than in any other state in 2019, based on newly released data.

That year, opioids represented 6.48% of all drug claims for part D enrollees in the state, just ahead of Utah at 6.41%. Idaho, at 6.07%, was the only other state with an opioid prescribing rate over 6%, while Oklahoma came in at an even 6.0%, according to the latest update of the Centers for Medicare & Medicaid Services’ dataset.
The lowest rate in 2019 belonged to New York, where 2.51% of drug claims, including original prescriptions and refills, involved an opioid. Rhode Island was next at 2.87%, followed by New Jersey (3.23%), Massachusetts (3.26%), and North Dakota (3.39%),
Altogether, Medicare part D processed 1.5 billion drug claims in 2019, of which 66.1 million, or 4.41%, involved opioids. Both of the opioid numbers were down from 2018, when opioids represented 4.68% (70.2 million) of the 1.5 billion total claims, and from 2014, when opioids were involved in 5.73% (81,026,831) of the 1.41 billion drug claims, the CMS data show. That works out to 5.77% fewer opioids in 2019, compared with 2014, despite the increase in total volume.
from 2014 to 2019, with Hawaii showing the smallest decline as it slipped 0.41 percentage points from 3.9% to 3.49%, according to the CMS.
In 2019, part D beneficiaries in Vermont were the most likely to receive a long-acting opioid, which accounted for 20.14% of all opioid prescriptions in the state, while Kentucky had the lowest share of prescriptions written for long-acting forms at 6.41%. The national average was 11.02%, dropping from 11.79% in 2018 and 12.75% in 2014, the CMS reported.
Medicare beneficiaries in Alabama were more likely to get a prescription for an opioid than in any other state in 2019, based on newly released data.

That year, opioids represented 6.48% of all drug claims for part D enrollees in the state, just ahead of Utah at 6.41%. Idaho, at 6.07%, was the only other state with an opioid prescribing rate over 6%, while Oklahoma came in at an even 6.0%, according to the latest update of the Centers for Medicare & Medicaid Services’ dataset.
The lowest rate in 2019 belonged to New York, where 2.51% of drug claims, including original prescriptions and refills, involved an opioid. Rhode Island was next at 2.87%, followed by New Jersey (3.23%), Massachusetts (3.26%), and North Dakota (3.39%),
Altogether, Medicare part D processed 1.5 billion drug claims in 2019, of which 66.1 million, or 4.41%, involved opioids. Both of the opioid numbers were down from 2018, when opioids represented 4.68% (70.2 million) of the 1.5 billion total claims, and from 2014, when opioids were involved in 5.73% (81,026,831) of the 1.41 billion drug claims, the CMS data show. That works out to 5.77% fewer opioids in 2019, compared with 2014, despite the increase in total volume.
from 2014 to 2019, with Hawaii showing the smallest decline as it slipped 0.41 percentage points from 3.9% to 3.49%, according to the CMS.
In 2019, part D beneficiaries in Vermont were the most likely to receive a long-acting opioid, which accounted for 20.14% of all opioid prescriptions in the state, while Kentucky had the lowest share of prescriptions written for long-acting forms at 6.41%. The national average was 11.02%, dropping from 11.79% in 2018 and 12.75% in 2014, the CMS reported.
Scientists who unlocked secrets of pain sensation win nobel prize
for their discoveries of receptors for temperature and touch.
Their discoveries paved the way for new treatments for a wide range of disease conditions, including chronic pain.
“Our ability to sense heat, cold, and touch is essential for survival and underpins our interaction with the world around us,” the Nobel committee, in Stockholm, said in a news release.
“In our daily lives we take these sensations for granted, but how are nerve impulses initiated so that temperature and pressure can be perceived? This question has been solved by this year’s Nobel Prize laureates,” the committee added.
Science heats up
Dr. Julius and his collaborators used capsaicin, a pungent compound found in chili peppers that produces a burning sensation, to identify TRPV1, an ion channel activated by painful heat.
“The discovery of TRPV1 was a major breakthrough leading the way to the unravelling of additional temperature-sensing receptors,” the committee said.
Both Dr. Julius and Dr. Patapoutian used menthol to identify another receptor called TRPM8 that is activated by cold. Additional ion channels related to TRPV1 and TRPM8 were identified and found to be activated by a range of different temperatures.
The discoveries fueled other scientists to investigate the roles of these channels in thermal sensation.
“Julius’ discovery of TRPV1 was the breakthrough that allowed us to understand how differences in temperature can induce electrical signals in the nervous system,” the committee noted.
Science under pressure
As the mechanisms for temperature sensation began to unravel, Dr. Patapoutian and his collaborators used cultured pressure-sensitive cells to identify an ion channel activated by mechanical stimuli in the skin and internal organs. It was given the name Piezo1, after the Greek word for pressure.
Through its similarity to Piezo1, a second gene was discovered and named Piezo2. Sensory neurons were found to express high levels of Piezo2 and further studies firmly established that Piezo1 and Piezo2 are ion channels that are directly activated by the exertion of pressure on cell membranes.
“The groundbreaking discoveries of the TRPV1, TRPM8, and Piezo channels by this year’s Nobel Prize laureates have allowed us to understand how heat, cold, and mechanical force can initiate the nerve impulses that allow us to perceive and adapt to the world around us,” the Nobel committee said.
Dr. Julius and Dr. Patapoutian will receive a gold medal and share the $1.14 million prize money.
A version of this article first appeared on Medscape.com.
for their discoveries of receptors for temperature and touch.
Their discoveries paved the way for new treatments for a wide range of disease conditions, including chronic pain.
“Our ability to sense heat, cold, and touch is essential for survival and underpins our interaction with the world around us,” the Nobel committee, in Stockholm, said in a news release.
“In our daily lives we take these sensations for granted, but how are nerve impulses initiated so that temperature and pressure can be perceived? This question has been solved by this year’s Nobel Prize laureates,” the committee added.
Science heats up
Dr. Julius and his collaborators used capsaicin, a pungent compound found in chili peppers that produces a burning sensation, to identify TRPV1, an ion channel activated by painful heat.
“The discovery of TRPV1 was a major breakthrough leading the way to the unravelling of additional temperature-sensing receptors,” the committee said.
Both Dr. Julius and Dr. Patapoutian used menthol to identify another receptor called TRPM8 that is activated by cold. Additional ion channels related to TRPV1 and TRPM8 were identified and found to be activated by a range of different temperatures.
The discoveries fueled other scientists to investigate the roles of these channels in thermal sensation.
“Julius’ discovery of TRPV1 was the breakthrough that allowed us to understand how differences in temperature can induce electrical signals in the nervous system,” the committee noted.
Science under pressure
As the mechanisms for temperature sensation began to unravel, Dr. Patapoutian and his collaborators used cultured pressure-sensitive cells to identify an ion channel activated by mechanical stimuli in the skin and internal organs. It was given the name Piezo1, after the Greek word for pressure.
Through its similarity to Piezo1, a second gene was discovered and named Piezo2. Sensory neurons were found to express high levels of Piezo2 and further studies firmly established that Piezo1 and Piezo2 are ion channels that are directly activated by the exertion of pressure on cell membranes.
“The groundbreaking discoveries of the TRPV1, TRPM8, and Piezo channels by this year’s Nobel Prize laureates have allowed us to understand how heat, cold, and mechanical force can initiate the nerve impulses that allow us to perceive and adapt to the world around us,” the Nobel committee said.
Dr. Julius and Dr. Patapoutian will receive a gold medal and share the $1.14 million prize money.
A version of this article first appeared on Medscape.com.
for their discoveries of receptors for temperature and touch.
Their discoveries paved the way for new treatments for a wide range of disease conditions, including chronic pain.
“Our ability to sense heat, cold, and touch is essential for survival and underpins our interaction with the world around us,” the Nobel committee, in Stockholm, said in a news release.
“In our daily lives we take these sensations for granted, but how are nerve impulses initiated so that temperature and pressure can be perceived? This question has been solved by this year’s Nobel Prize laureates,” the committee added.
Science heats up
Dr. Julius and his collaborators used capsaicin, a pungent compound found in chili peppers that produces a burning sensation, to identify TRPV1, an ion channel activated by painful heat.
“The discovery of TRPV1 was a major breakthrough leading the way to the unravelling of additional temperature-sensing receptors,” the committee said.
Both Dr. Julius and Dr. Patapoutian used menthol to identify another receptor called TRPM8 that is activated by cold. Additional ion channels related to TRPV1 and TRPM8 were identified and found to be activated by a range of different temperatures.
The discoveries fueled other scientists to investigate the roles of these channels in thermal sensation.
“Julius’ discovery of TRPV1 was the breakthrough that allowed us to understand how differences in temperature can induce electrical signals in the nervous system,” the committee noted.
Science under pressure
As the mechanisms for temperature sensation began to unravel, Dr. Patapoutian and his collaborators used cultured pressure-sensitive cells to identify an ion channel activated by mechanical stimuli in the skin and internal organs. It was given the name Piezo1, after the Greek word for pressure.
Through its similarity to Piezo1, a second gene was discovered and named Piezo2. Sensory neurons were found to express high levels of Piezo2 and further studies firmly established that Piezo1 and Piezo2 are ion channels that are directly activated by the exertion of pressure on cell membranes.
“The groundbreaking discoveries of the TRPV1, TRPM8, and Piezo channels by this year’s Nobel Prize laureates have allowed us to understand how heat, cold, and mechanical force can initiate the nerve impulses that allow us to perceive and adapt to the world around us,” the Nobel committee said.
Dr. Julius and Dr. Patapoutian will receive a gold medal and share the $1.14 million prize money.
A version of this article first appeared on Medscape.com.
There’s no place like home to diagnose hypertension
Adults who need to track their blood pressure to find out if they have hypertension prefer to do it at home rather than at a clinic or kiosk or with 24-hour ambulatory BP monitoring (ABPM), according to a new study.
“From a patient-centered perspective, home BP monitoring is the most acceptable method for diagnosing hypertension, although participants were willing to complete ABPM and appreciated its accuracy,” said Beverly Green, MD, MPH, of Kaiser Permanente Washington, Seattle.
Dr. Green presented the study Sept. 29 during the virtual American Heart Association Hypertension Scientific Sessions 2021.
“Health care professionals should work toward relying less on in-clinic visits to diagnose hypertension and supporting their patients in taking their blood pressure measurements at home,” Dr. Green said in an AHA news release.
“Home blood pressure monitoring is empowering and improves our ability to identify and treat hypertension, and to prevent strokes, heart attacks, heart failure, and cardiovascular death,” she added.
Convenience is key
The BP-CHECK study was a three-group, randomized, controlled diagnostic study that tested the accuracy and acceptability of office, home, and kiosk BP monitoring against the gold-standard – ABPM – for diagnosing hypertension. Dr. Green presented the results on patient adherence and acceptability of these methods.
Those assigned to clinic measurements were asked to return to the clinic for at least one additional BP check, as is routine in diagnosing hypertension in clinical practice.
Those in the home group were given and trained to use a Bluetooth/web-enabled home BP monitor and were asked to take their BP twice a day (morning and evening, with two measurements each time) for 5 days.
Those in the kiosk group were trained to use a BP kiosk with a smart card and were asked to return to the kiosk (or a nearby pharmacy with the same kiosk) on 3 separate days and measure their BP three times at each visit.
All participants were asked to complete their group-assigned diagnostic regimens in 3 weeks and then to complete 24-hour ABPM.
The trial enrolled 510 adults who presented to Kaiser Permanente Washington primary care clinics with elevated BP (mean, 150/88 mm Hg) but who had not yet been diagnosed with hypertension. Their mean age was 59 years, 80% of the study participants were White, and 51% were male.
Adherence to the monitoring regimen was highest in the home BP group (90.6%), followed by the clinic group (87.2%), and lowest in the kiosk group (67.9%). Adherence to ABPM among all participants was 91.6%.
Overall, acceptability was highest for the home BP group, followed by the clinic and kiosk groups; 24-hour ABPM monitoring was the least acceptable option.
Home was the “overwhelming” stated preference when asked before randomization and after, Dr. Green said.
The findings come as no surprise to Willie Lawrence Jr., MD, head of the AHA National Hypertension Control Initiative oversight committee. “Patients will do what’s most convenient for them,” he told this news organization.
“We know from other studies that really all you need to do is measure the blood pressure twice a day for 3 days. That will give you a good idea what that patient’s blood pressure is as it relates to future cardiac events,” said Dr. Lawrence, who wasn’t involved in the study.
“We should really begin to focus more on these home, self-measured blood pressures using validated devices, and that’s important because a lot of the devices out there aren’t validated,” he explained.
“Patients with hypertension should have a blood pressure monitor at home that is validated and should be instructed in how to use it properly,” Dr. Lawrence concluded.
Funding for the study was provided by the Patient-Centered Outcomes Research Institute. Dr. Green and Dr. Lawrence have no relevant disclosures.
A version of this article first appeared on Medscape.com.
Adults who need to track their blood pressure to find out if they have hypertension prefer to do it at home rather than at a clinic or kiosk or with 24-hour ambulatory BP monitoring (ABPM), according to a new study.
“From a patient-centered perspective, home BP monitoring is the most acceptable method for diagnosing hypertension, although participants were willing to complete ABPM and appreciated its accuracy,” said Beverly Green, MD, MPH, of Kaiser Permanente Washington, Seattle.
Dr. Green presented the study Sept. 29 during the virtual American Heart Association Hypertension Scientific Sessions 2021.
“Health care professionals should work toward relying less on in-clinic visits to diagnose hypertension and supporting their patients in taking their blood pressure measurements at home,” Dr. Green said in an AHA news release.
“Home blood pressure monitoring is empowering and improves our ability to identify and treat hypertension, and to prevent strokes, heart attacks, heart failure, and cardiovascular death,” she added.
Convenience is key
The BP-CHECK study was a three-group, randomized, controlled diagnostic study that tested the accuracy and acceptability of office, home, and kiosk BP monitoring against the gold-standard – ABPM – for diagnosing hypertension. Dr. Green presented the results on patient adherence and acceptability of these methods.
Those assigned to clinic measurements were asked to return to the clinic for at least one additional BP check, as is routine in diagnosing hypertension in clinical practice.
Those in the home group were given and trained to use a Bluetooth/web-enabled home BP monitor and were asked to take their BP twice a day (morning and evening, with two measurements each time) for 5 days.
Those in the kiosk group were trained to use a BP kiosk with a smart card and were asked to return to the kiosk (or a nearby pharmacy with the same kiosk) on 3 separate days and measure their BP three times at each visit.
All participants were asked to complete their group-assigned diagnostic regimens in 3 weeks and then to complete 24-hour ABPM.
The trial enrolled 510 adults who presented to Kaiser Permanente Washington primary care clinics with elevated BP (mean, 150/88 mm Hg) but who had not yet been diagnosed with hypertension. Their mean age was 59 years, 80% of the study participants were White, and 51% were male.
Adherence to the monitoring regimen was highest in the home BP group (90.6%), followed by the clinic group (87.2%), and lowest in the kiosk group (67.9%). Adherence to ABPM among all participants was 91.6%.
Overall, acceptability was highest for the home BP group, followed by the clinic and kiosk groups; 24-hour ABPM monitoring was the least acceptable option.
Home was the “overwhelming” stated preference when asked before randomization and after, Dr. Green said.
The findings come as no surprise to Willie Lawrence Jr., MD, head of the AHA National Hypertension Control Initiative oversight committee. “Patients will do what’s most convenient for them,” he told this news organization.
“We know from other studies that really all you need to do is measure the blood pressure twice a day for 3 days. That will give you a good idea what that patient’s blood pressure is as it relates to future cardiac events,” said Dr. Lawrence, who wasn’t involved in the study.
“We should really begin to focus more on these home, self-measured blood pressures using validated devices, and that’s important because a lot of the devices out there aren’t validated,” he explained.
“Patients with hypertension should have a blood pressure monitor at home that is validated and should be instructed in how to use it properly,” Dr. Lawrence concluded.
Funding for the study was provided by the Patient-Centered Outcomes Research Institute. Dr. Green and Dr. Lawrence have no relevant disclosures.
A version of this article first appeared on Medscape.com.
Adults who need to track their blood pressure to find out if they have hypertension prefer to do it at home rather than at a clinic or kiosk or with 24-hour ambulatory BP monitoring (ABPM), according to a new study.
“From a patient-centered perspective, home BP monitoring is the most acceptable method for diagnosing hypertension, although participants were willing to complete ABPM and appreciated its accuracy,” said Beverly Green, MD, MPH, of Kaiser Permanente Washington, Seattle.
Dr. Green presented the study Sept. 29 during the virtual American Heart Association Hypertension Scientific Sessions 2021.
“Health care professionals should work toward relying less on in-clinic visits to diagnose hypertension and supporting their patients in taking their blood pressure measurements at home,” Dr. Green said in an AHA news release.
“Home blood pressure monitoring is empowering and improves our ability to identify and treat hypertension, and to prevent strokes, heart attacks, heart failure, and cardiovascular death,” she added.
Convenience is key
The BP-CHECK study was a three-group, randomized, controlled diagnostic study that tested the accuracy and acceptability of office, home, and kiosk BP monitoring against the gold-standard – ABPM – for diagnosing hypertension. Dr. Green presented the results on patient adherence and acceptability of these methods.
Those assigned to clinic measurements were asked to return to the clinic for at least one additional BP check, as is routine in diagnosing hypertension in clinical practice.
Those in the home group were given and trained to use a Bluetooth/web-enabled home BP monitor and were asked to take their BP twice a day (morning and evening, with two measurements each time) for 5 days.
Those in the kiosk group were trained to use a BP kiosk with a smart card and were asked to return to the kiosk (or a nearby pharmacy with the same kiosk) on 3 separate days and measure their BP three times at each visit.
All participants were asked to complete their group-assigned diagnostic regimens in 3 weeks and then to complete 24-hour ABPM.
The trial enrolled 510 adults who presented to Kaiser Permanente Washington primary care clinics with elevated BP (mean, 150/88 mm Hg) but who had not yet been diagnosed with hypertension. Their mean age was 59 years, 80% of the study participants were White, and 51% were male.
Adherence to the monitoring regimen was highest in the home BP group (90.6%), followed by the clinic group (87.2%), and lowest in the kiosk group (67.9%). Adherence to ABPM among all participants was 91.6%.
Overall, acceptability was highest for the home BP group, followed by the clinic and kiosk groups; 24-hour ABPM monitoring was the least acceptable option.
Home was the “overwhelming” stated preference when asked before randomization and after, Dr. Green said.
The findings come as no surprise to Willie Lawrence Jr., MD, head of the AHA National Hypertension Control Initiative oversight committee. “Patients will do what’s most convenient for them,” he told this news organization.
“We know from other studies that really all you need to do is measure the blood pressure twice a day for 3 days. That will give you a good idea what that patient’s blood pressure is as it relates to future cardiac events,” said Dr. Lawrence, who wasn’t involved in the study.
“We should really begin to focus more on these home, self-measured blood pressures using validated devices, and that’s important because a lot of the devices out there aren’t validated,” he explained.
“Patients with hypertension should have a blood pressure monitor at home that is validated and should be instructed in how to use it properly,” Dr. Lawrence concluded.
Funding for the study was provided by the Patient-Centered Outcomes Research Institute. Dr. Green and Dr. Lawrence have no relevant disclosures.
A version of this article first appeared on Medscape.com.
TriMaster study shows precision medicine in diabetes is possible
A uniquely-designed three-drug study has demonstrated that individual clinical characteristics, including patient preference, can be used to guide medication choice in type 2 diabetes.
Results from the TriMaster trial using sitagliptin, pioglitazone, and canagliflozin as second- or third-line therapy in a total of 525 patients with type 2 diabetes were presented September 29 at the virtual European Association for the Study of Diabetes (EASD) 2021 Annual Meeting.
TriMaster is a phase 4, multicenter, randomized, double-blind, 12-month crossover trial examining the effects of all three drugs in subgroups of patients with type 2 diabetes who hadn’t achieved target glucose levels with metformin alone or combined with a sulfonylurea.
While all three drugs lowered glucose similarly overall, pioglitazone did so more effectively among patients with a body mass index (BMI) above 30 kg/m2, while sitagliptin worked better in those with a BMI less than 30 kg/m2. However, pioglitazone resulted in more weight gain.
In a second comparison, canagliflozin (a sodium-glucose cotransporter 2 [SGLT2] inhibitor) was more effective than sitagliptin (a dipeptidyl peptidase-4 [DPP-4] inhibitor) in lowering glucose among patients with an estimated glomerular filtration rate (eGFR) above 90 mL/min/1.73m2, while sitagliptin actually lowered glucose better among individuals with an eGFR 60-90 mL/min/1.73m2 than canagliflozin.
And when participants were asked which drug they preferred, the results were split nearly evenly among the three, correlating with how well the drug worked and the side effect profile for each individual.
“We proved a precision approach worked using predefined clinical criteria to define groups of patients where one drug is better than another. This is the first-ever proof of a precision medicine approach in type 2 diabetes,” chief investigator Andrew Hattersley, DM, professor of molecular medicine at the University of Exeter, U.K., told this news organization.
But, he stressed, “These results do not mean all patients with BMI above 30 should have pioglitazone or that all patients with an eGFR 60-90 should have a DPP-4 inhibitor.”
“Drug choice will need to consider other priorities than glycemia ... Patients with heart failure, cardiovascular disease, and chronic kidney disease should be prescribed SGLT2 inhibitors,” he noted. And “some patients will need to avoid specific drugs due to likely side effects.”
‘Modern era’ study used older drugs
Independent commentator Caroline M. Kistorp, MD, PhD, professor of endocrinology at University Hospital Copenhagen, congratulated the investigators for “moving precision medicine from the retrospective analysis of existing data into the modern era of evidence-based medicine with this randomized clinical trial in patients with type 2 diabetes ... Starting this trial back in 2015 was really ahead of their time.”
However, she questioned the use of a thiazolidinedione (TZD), pioglitazone, in the trial, as they are no longer used in many parts of the world in favor of more “modern” glucose-lowering drugs.
“I’m thinking of GLP-1 receptor agonists, especially if you want to treat type 2 diabetes patients who are obese with a BMI over 30 ... I acknowledge that there is a cost issue, but I still think we should try to give our patients the best treatments, so that’s why I’m not sure how much the [TZDs] will be used in the future, even with this trial,” she said.
Dr. Kistorp also noted the trial didn’t include cardiovascular disease outcomes, for which most SGLT2 inhibitors have shown benefit.
“We have to discuss and consider whether A1c is the most important parameter for these patients ... especially looking at their cardiovascular outcomes.”
Mr. Hattersley responded that the study was designed in 2015, prior to the landmark EMPA-REG OUTCOME trial that began the shift toward use of SGLT2 inhibitors for cardiovascular and kidney disease reduction in addition to glycemic control in the clinical management of type 2 diabetes.
“We will report the cardiovascular profiles, but it wasn’t a specific thing because at that time the evidence didn’t exist, so it wasn’t in our protocol,” he explained.
Regarding pioglitazone, he acknowledged that although it may be an alternative to insulin for some patients, “I think for most people you won’t be considering it in clinical practice,” but because it has a very different mechanism from the other two study drugs, “it did give the greater chance of differential effects ... Partly, what we’re really trying to do is test the question of whether precision medicine exists and can we do it.”
Unique study design had each patient act as their own control
Trial statistician Beverley Shields, PhD, of the University of Exeter, U.K., reported the results. The 525 participants with type 2 diabetes were aged 30-79 years and had A1c levels above 58 mmol/mol (7.5%) but not greater than 110 mmol/mol (12.2%) with metformin alone or combined with a sulfonylurea. Just over half (58%) had a BMI above 30 kg/m2 and 52% had an eGFR greater than 90 mL/min/1.73m2.
Each participant received each of the three medications as second- or third-line oral therapy in random order – in one of six possible sequences – for 16 weeks each, with no washout period in between (to prevent dropouts due to hyperglycemia). Thus, each participant acted as their own control.
A total of 458 participants completed all three study periods.
The drugs work differently in different patient groups
Without stratification by patient type, there was no overall difference in A1c reduction between the three therapies, with all achieving about 59-60 mmol/mol (7.5-7.6%) from a baseline average of 69 mmol/mol (8.9%).
But when stratified by BMI, A1c was 1.48 mmol/mol higher with pioglitazone versus sitagliptin in the group with BMI less than 30 kg/m2 and 1.44 mmol/mol lower with pioglitazone versus sitagliptin in the group with BMI greater than 30 kg/m2, giving a significant overall difference of 2.92 mmol/mol (P = .003).
By eGFR stratification, A1c was 1.74 mmol/mol lower with sitagliptin than canagliflozin in the 60-90 mL/min/1.73m2 group and 1.08 mmol/mol higher in the greater than 90 mL/min/1.73m2 group, giving a significant difference of 2.83 mmol/mol (P = .002).
“So, if we were to treat the patients with the drug that is optimal for their strata ... this would lead to a benefit of about 3 mmol/mol compared to if those patients were treated with the other drug,” Dr. Shields said.
By BMI, there were no significant differences by drug or strata for tolerability, defined as staying on drug for at least 12 weeks (P = .2), nor in the percentage of patients reporting at least one hypoglycemic episode (P = .6).
However, pioglitazone was associated with higher weight gain in both BMI groups, resulting in a 0.93 kg difference overall (P < .001), although it was higher in the higher BMI group (1.9 vs. 0.97 kg).
Similarly, by eGFR there were no differences in tolerability or hypoglycemic episodes between sitagliptin and canagliflozin (P = .09 and P = .6, respectively). And here, there were no differences in weight (P = .6).
Patients compared their own experiences with each drug
Patients were asked about their drug preferences after being reminded about their own changes in A1c and weight with each one. The result was a split: 25.8% picked pioglitazone, 34.8% sitagliptin, and 38.7% canagliflozin.
Looking at study outcomes by therapy, pioglitazone had the lowest rate of nontolerability but the highest weight gain, sitagliptin had the highest nontolerability but the lowest number of side effects, while canagliflozin had the highest number of reported side effects but the lowest weight gain.
Patients’ preferred drugs were associated with the lowest A1c and the fewest side effects for each group. Interestingly, pioglitazone was associated with the highest weight on therapy regardless of preference, so that even those who preferred pioglitazone had a higher weight than they did with the other two drugs.
In response to an audience question about durability of the results given the relatively short trial periods, Mr. Hattersley said: “We’re following up these patients who have chosen their drug, and on the whole, their primary care doctor agreed with them. So we’re following that up as a prospective cohort. We’re looking at tolerance and response and also to see if they’re still happy with that drug. That will be a future analysis.”
The TriMASTER data will be submitted for publication soon.
TriMASTER was funded by the UK Medical Research Council. Mr. Hattersley and Dr. Shields have reported no relevant financial relationships. Dr. Kistorp has reported receiving honoraria from and/or is on advisory boards for AstraZeneca, Novo Nordisk, Boehringer Ingelheim, MSD, Otsuka Pharma, and Chiesi.
A version of this article first appeared on Medscape.com.
A uniquely-designed three-drug study has demonstrated that individual clinical characteristics, including patient preference, can be used to guide medication choice in type 2 diabetes.
Results from the TriMaster trial using sitagliptin, pioglitazone, and canagliflozin as second- or third-line therapy in a total of 525 patients with type 2 diabetes were presented September 29 at the virtual European Association for the Study of Diabetes (EASD) 2021 Annual Meeting.
TriMaster is a phase 4, multicenter, randomized, double-blind, 12-month crossover trial examining the effects of all three drugs in subgroups of patients with type 2 diabetes who hadn’t achieved target glucose levels with metformin alone or combined with a sulfonylurea.
While all three drugs lowered glucose similarly overall, pioglitazone did so more effectively among patients with a body mass index (BMI) above 30 kg/m2, while sitagliptin worked better in those with a BMI less than 30 kg/m2. However, pioglitazone resulted in more weight gain.
In a second comparison, canagliflozin (a sodium-glucose cotransporter 2 [SGLT2] inhibitor) was more effective than sitagliptin (a dipeptidyl peptidase-4 [DPP-4] inhibitor) in lowering glucose among patients with an estimated glomerular filtration rate (eGFR) above 90 mL/min/1.73m2, while sitagliptin actually lowered glucose better among individuals with an eGFR 60-90 mL/min/1.73m2 than canagliflozin.
And when participants were asked which drug they preferred, the results were split nearly evenly among the three, correlating with how well the drug worked and the side effect profile for each individual.
“We proved a precision approach worked using predefined clinical criteria to define groups of patients where one drug is better than another. This is the first-ever proof of a precision medicine approach in type 2 diabetes,” chief investigator Andrew Hattersley, DM, professor of molecular medicine at the University of Exeter, U.K., told this news organization.
But, he stressed, “These results do not mean all patients with BMI above 30 should have pioglitazone or that all patients with an eGFR 60-90 should have a DPP-4 inhibitor.”
“Drug choice will need to consider other priorities than glycemia ... Patients with heart failure, cardiovascular disease, and chronic kidney disease should be prescribed SGLT2 inhibitors,” he noted. And “some patients will need to avoid specific drugs due to likely side effects.”
‘Modern era’ study used older drugs
Independent commentator Caroline M. Kistorp, MD, PhD, professor of endocrinology at University Hospital Copenhagen, congratulated the investigators for “moving precision medicine from the retrospective analysis of existing data into the modern era of evidence-based medicine with this randomized clinical trial in patients with type 2 diabetes ... Starting this trial back in 2015 was really ahead of their time.”
However, she questioned the use of a thiazolidinedione (TZD), pioglitazone, in the trial, as they are no longer used in many parts of the world in favor of more “modern” glucose-lowering drugs.
“I’m thinking of GLP-1 receptor agonists, especially if you want to treat type 2 diabetes patients who are obese with a BMI over 30 ... I acknowledge that there is a cost issue, but I still think we should try to give our patients the best treatments, so that’s why I’m not sure how much the [TZDs] will be used in the future, even with this trial,” she said.
Dr. Kistorp also noted the trial didn’t include cardiovascular disease outcomes, for which most SGLT2 inhibitors have shown benefit.
“We have to discuss and consider whether A1c is the most important parameter for these patients ... especially looking at their cardiovascular outcomes.”
Mr. Hattersley responded that the study was designed in 2015, prior to the landmark EMPA-REG OUTCOME trial that began the shift toward use of SGLT2 inhibitors for cardiovascular and kidney disease reduction in addition to glycemic control in the clinical management of type 2 diabetes.
“We will report the cardiovascular profiles, but it wasn’t a specific thing because at that time the evidence didn’t exist, so it wasn’t in our protocol,” he explained.
Regarding pioglitazone, he acknowledged that although it may be an alternative to insulin for some patients, “I think for most people you won’t be considering it in clinical practice,” but because it has a very different mechanism from the other two study drugs, “it did give the greater chance of differential effects ... Partly, what we’re really trying to do is test the question of whether precision medicine exists and can we do it.”
Unique study design had each patient act as their own control
Trial statistician Beverley Shields, PhD, of the University of Exeter, U.K., reported the results. The 525 participants with type 2 diabetes were aged 30-79 years and had A1c levels above 58 mmol/mol (7.5%) but not greater than 110 mmol/mol (12.2%) with metformin alone or combined with a sulfonylurea. Just over half (58%) had a BMI above 30 kg/m2 and 52% had an eGFR greater than 90 mL/min/1.73m2.
Each participant received each of the three medications as second- or third-line oral therapy in random order – in one of six possible sequences – for 16 weeks each, with no washout period in between (to prevent dropouts due to hyperglycemia). Thus, each participant acted as their own control.
A total of 458 participants completed all three study periods.
The drugs work differently in different patient groups
Without stratification by patient type, there was no overall difference in A1c reduction between the three therapies, with all achieving about 59-60 mmol/mol (7.5-7.6%) from a baseline average of 69 mmol/mol (8.9%).
But when stratified by BMI, A1c was 1.48 mmol/mol higher with pioglitazone versus sitagliptin in the group with BMI less than 30 kg/m2 and 1.44 mmol/mol lower with pioglitazone versus sitagliptin in the group with BMI greater than 30 kg/m2, giving a significant overall difference of 2.92 mmol/mol (P = .003).
By eGFR stratification, A1c was 1.74 mmol/mol lower with sitagliptin than canagliflozin in the 60-90 mL/min/1.73m2 group and 1.08 mmol/mol higher in the greater than 90 mL/min/1.73m2 group, giving a significant difference of 2.83 mmol/mol (P = .002).
“So, if we were to treat the patients with the drug that is optimal for their strata ... this would lead to a benefit of about 3 mmol/mol compared to if those patients were treated with the other drug,” Dr. Shields said.
By BMI, there were no significant differences by drug or strata for tolerability, defined as staying on drug for at least 12 weeks (P = .2), nor in the percentage of patients reporting at least one hypoglycemic episode (P = .6).
However, pioglitazone was associated with higher weight gain in both BMI groups, resulting in a 0.93 kg difference overall (P < .001), although it was higher in the higher BMI group (1.9 vs. 0.97 kg).
Similarly, by eGFR there were no differences in tolerability or hypoglycemic episodes between sitagliptin and canagliflozin (P = .09 and P = .6, respectively). And here, there were no differences in weight (P = .6).
Patients compared their own experiences with each drug
Patients were asked about their drug preferences after being reminded about their own changes in A1c and weight with each one. The result was a split: 25.8% picked pioglitazone, 34.8% sitagliptin, and 38.7% canagliflozin.
Looking at study outcomes by therapy, pioglitazone had the lowest rate of nontolerability but the highest weight gain, sitagliptin had the highest nontolerability but the lowest number of side effects, while canagliflozin had the highest number of reported side effects but the lowest weight gain.
Patients’ preferred drugs were associated with the lowest A1c and the fewest side effects for each group. Interestingly, pioglitazone was associated with the highest weight on therapy regardless of preference, so that even those who preferred pioglitazone had a higher weight than they did with the other two drugs.
In response to an audience question about durability of the results given the relatively short trial periods, Mr. Hattersley said: “We’re following up these patients who have chosen their drug, and on the whole, their primary care doctor agreed with them. So we’re following that up as a prospective cohort. We’re looking at tolerance and response and also to see if they’re still happy with that drug. That will be a future analysis.”
The TriMASTER data will be submitted for publication soon.
TriMASTER was funded by the UK Medical Research Council. Mr. Hattersley and Dr. Shields have reported no relevant financial relationships. Dr. Kistorp has reported receiving honoraria from and/or is on advisory boards for AstraZeneca, Novo Nordisk, Boehringer Ingelheim, MSD, Otsuka Pharma, and Chiesi.
A version of this article first appeared on Medscape.com.
A uniquely-designed three-drug study has demonstrated that individual clinical characteristics, including patient preference, can be used to guide medication choice in type 2 diabetes.
Results from the TriMaster trial using sitagliptin, pioglitazone, and canagliflozin as second- or third-line therapy in a total of 525 patients with type 2 diabetes were presented September 29 at the virtual European Association for the Study of Diabetes (EASD) 2021 Annual Meeting.
TriMaster is a phase 4, multicenter, randomized, double-blind, 12-month crossover trial examining the effects of all three drugs in subgroups of patients with type 2 diabetes who hadn’t achieved target glucose levels with metformin alone or combined with a sulfonylurea.
While all three drugs lowered glucose similarly overall, pioglitazone did so more effectively among patients with a body mass index (BMI) above 30 kg/m2, while sitagliptin worked better in those with a BMI less than 30 kg/m2. However, pioglitazone resulted in more weight gain.
In a second comparison, canagliflozin (a sodium-glucose cotransporter 2 [SGLT2] inhibitor) was more effective than sitagliptin (a dipeptidyl peptidase-4 [DPP-4] inhibitor) in lowering glucose among patients with an estimated glomerular filtration rate (eGFR) above 90 mL/min/1.73m2, while sitagliptin actually lowered glucose better among individuals with an eGFR 60-90 mL/min/1.73m2 than canagliflozin.
And when participants were asked which drug they preferred, the results were split nearly evenly among the three, correlating with how well the drug worked and the side effect profile for each individual.
“We proved a precision approach worked using predefined clinical criteria to define groups of patients where one drug is better than another. This is the first-ever proof of a precision medicine approach in type 2 diabetes,” chief investigator Andrew Hattersley, DM, professor of molecular medicine at the University of Exeter, U.K., told this news organization.
But, he stressed, “These results do not mean all patients with BMI above 30 should have pioglitazone or that all patients with an eGFR 60-90 should have a DPP-4 inhibitor.”
“Drug choice will need to consider other priorities than glycemia ... Patients with heart failure, cardiovascular disease, and chronic kidney disease should be prescribed SGLT2 inhibitors,” he noted. And “some patients will need to avoid specific drugs due to likely side effects.”
‘Modern era’ study used older drugs
Independent commentator Caroline M. Kistorp, MD, PhD, professor of endocrinology at University Hospital Copenhagen, congratulated the investigators for “moving precision medicine from the retrospective analysis of existing data into the modern era of evidence-based medicine with this randomized clinical trial in patients with type 2 diabetes ... Starting this trial back in 2015 was really ahead of their time.”
However, she questioned the use of a thiazolidinedione (TZD), pioglitazone, in the trial, as they are no longer used in many parts of the world in favor of more “modern” glucose-lowering drugs.
“I’m thinking of GLP-1 receptor agonists, especially if you want to treat type 2 diabetes patients who are obese with a BMI over 30 ... I acknowledge that there is a cost issue, but I still think we should try to give our patients the best treatments, so that’s why I’m not sure how much the [TZDs] will be used in the future, even with this trial,” she said.
Dr. Kistorp also noted the trial didn’t include cardiovascular disease outcomes, for which most SGLT2 inhibitors have shown benefit.
“We have to discuss and consider whether A1c is the most important parameter for these patients ... especially looking at their cardiovascular outcomes.”
Mr. Hattersley responded that the study was designed in 2015, prior to the landmark EMPA-REG OUTCOME trial that began the shift toward use of SGLT2 inhibitors for cardiovascular and kidney disease reduction in addition to glycemic control in the clinical management of type 2 diabetes.
“We will report the cardiovascular profiles, but it wasn’t a specific thing because at that time the evidence didn’t exist, so it wasn’t in our protocol,” he explained.
Regarding pioglitazone, he acknowledged that although it may be an alternative to insulin for some patients, “I think for most people you won’t be considering it in clinical practice,” but because it has a very different mechanism from the other two study drugs, “it did give the greater chance of differential effects ... Partly, what we’re really trying to do is test the question of whether precision medicine exists and can we do it.”
Unique study design had each patient act as their own control
Trial statistician Beverley Shields, PhD, of the University of Exeter, U.K., reported the results. The 525 participants with type 2 diabetes were aged 30-79 years and had A1c levels above 58 mmol/mol (7.5%) but not greater than 110 mmol/mol (12.2%) with metformin alone or combined with a sulfonylurea. Just over half (58%) had a BMI above 30 kg/m2 and 52% had an eGFR greater than 90 mL/min/1.73m2.
Each participant received each of the three medications as second- or third-line oral therapy in random order – in one of six possible sequences – for 16 weeks each, with no washout period in between (to prevent dropouts due to hyperglycemia). Thus, each participant acted as their own control.
A total of 458 participants completed all three study periods.
The drugs work differently in different patient groups
Without stratification by patient type, there was no overall difference in A1c reduction between the three therapies, with all achieving about 59-60 mmol/mol (7.5-7.6%) from a baseline average of 69 mmol/mol (8.9%).
But when stratified by BMI, A1c was 1.48 mmol/mol higher with pioglitazone versus sitagliptin in the group with BMI less than 30 kg/m2 and 1.44 mmol/mol lower with pioglitazone versus sitagliptin in the group with BMI greater than 30 kg/m2, giving a significant overall difference of 2.92 mmol/mol (P = .003).
By eGFR stratification, A1c was 1.74 mmol/mol lower with sitagliptin than canagliflozin in the 60-90 mL/min/1.73m2 group and 1.08 mmol/mol higher in the greater than 90 mL/min/1.73m2 group, giving a significant difference of 2.83 mmol/mol (P = .002).
“So, if we were to treat the patients with the drug that is optimal for their strata ... this would lead to a benefit of about 3 mmol/mol compared to if those patients were treated with the other drug,” Dr. Shields said.
By BMI, there were no significant differences by drug or strata for tolerability, defined as staying on drug for at least 12 weeks (P = .2), nor in the percentage of patients reporting at least one hypoglycemic episode (P = .6).
However, pioglitazone was associated with higher weight gain in both BMI groups, resulting in a 0.93 kg difference overall (P < .001), although it was higher in the higher BMI group (1.9 vs. 0.97 kg).
Similarly, by eGFR there were no differences in tolerability or hypoglycemic episodes between sitagliptin and canagliflozin (P = .09 and P = .6, respectively). And here, there were no differences in weight (P = .6).
Patients compared their own experiences with each drug
Patients were asked about their drug preferences after being reminded about their own changes in A1c and weight with each one. The result was a split: 25.8% picked pioglitazone, 34.8% sitagliptin, and 38.7% canagliflozin.
Looking at study outcomes by therapy, pioglitazone had the lowest rate of nontolerability but the highest weight gain, sitagliptin had the highest nontolerability but the lowest number of side effects, while canagliflozin had the highest number of reported side effects but the lowest weight gain.
Patients’ preferred drugs were associated with the lowest A1c and the fewest side effects for each group. Interestingly, pioglitazone was associated with the highest weight on therapy regardless of preference, so that even those who preferred pioglitazone had a higher weight than they did with the other two drugs.
In response to an audience question about durability of the results given the relatively short trial periods, Mr. Hattersley said: “We’re following up these patients who have chosen their drug, and on the whole, their primary care doctor agreed with them. So we’re following that up as a prospective cohort. We’re looking at tolerance and response and also to see if they’re still happy with that drug. That will be a future analysis.”
The TriMASTER data will be submitted for publication soon.
TriMASTER was funded by the UK Medical Research Council. Mr. Hattersley and Dr. Shields have reported no relevant financial relationships. Dr. Kistorp has reported receiving honoraria from and/or is on advisory boards for AstraZeneca, Novo Nordisk, Boehringer Ingelheim, MSD, Otsuka Pharma, and Chiesi.
A version of this article first appeared on Medscape.com.
Nonsteroidal topical found effective for psoriasis in 52-week study
Treatment with presented as a late-breaker at the virtual annual congress of the European Academy of Dermatology and Venereology.
The drug has several unique features with meaningful clinical differences from other topical psoriasis therapies, according to Linda Stein Gold, MD, director of dermatology clinical research, Henry Ford Health System, Detroit.
“The currently available nonsteroidal topical therapies are typically associated with significant irritation. We did not see that with tapinarof,” said Dr. Stein Gold. This is one of several reasons she believes this drug will be a valuable addition if it receives regulatory approval.
Tapinarof is a small-molecule aryl hydrocarbon receptor (AhR) modulating agent. AhR is widely expressed in immune cells, including macrophages, mast cells, and antigen-presenting cells. It is believed that modulation of AhR signaling by tapinarof reverses immune dysregulation that is involved in the formation of psoriatic lesions.
The newly presented PSOARING 3 data with tapinarof 1% build on the data from the 12-week PSOARING 1 and PSOARING 2 trials, which were released in August 2020 but have yet to be published.
The primary endpoint in both of the 12-week trials, each of which enrolled about 500 patients with plaque psoriasis, was a Physician Global Assessment (PGA) score of 0 (clear) or 1 (almost clear). Relative to a placebo response rate of about 6% in both trials, the proportion of patients who achieved scores of 0/1 with tapinarof 1% was 35.4% and 40.2% in the PSOARING 1 and PSOARING 2 trials, respectively (P < .0001 vs. placebo in both studies).
For the key secondary endpoint of at least 75% improvement in the Psoriasis Area and Severity Index (PASI 75), the relative advantage for tapinarof over placebo was similar. The results were highly statistically significant (P < .0001) in both of the 12-week trials.
More than 90% of the patients who participated in PSOARING 1 and PSOARING 2 and were eligible for the open-label PSOARING 3 extension trial, according to Dr. Stein Gold.
For the 79 patients with a score of 0 at the time of enrollment, tapinarof 1% was reapplied only if the PGA score reached at least 2 during the course of the study. For the 680 patients who entered with a PGA score of at least 1, once-daily applications of tapinarof 1% cream were maintained until a PGA score of 0 was achieved.
In the outcome analysis, response was defined as the proportion of patients with an initial PGA score of at least2 who achieved PGA 0. A remittive effect was defined as duration of a PGA score of 0 or 1 while off therapy after achieving a PGA score of 0. Durability of response was defined as the proportion of patients who achieved a PGA sore of 0 or 1 at least once during the study while on therapy. This last outcome provided a test of tachyphylaxis.
“Overall, 40.9% of patients achieved complete disease clearance at least once during the trial, and 58.2% who entered the study with a PGA score of 2 or higher achieved a PGA score of 0 or 1,” Dr. Stein Gold reported.
For the 79 patients who entered PSOARING 3 with a PGA score of 0 and were off treatment, the median duration of a remittive effect was 115 days. For the patients who entered the trial with a higher PGA score but who achieved a score of 0 during the study (312 patients), the mean remittive effect after discontinuing therapy was 130 days.
There was no evidence of tachyphylaxis. Rather, “there was no loss of effect despite intermittent therapy observed over the course of the trial,” Dr. Stein Gold reported.
The most common treatment-emergent adverse events in PSOARING 3, as in the previous PSOARING studies, were folliculitis, which was observed in 24.0% of patients; contact dermatitis, which occurred in 5.9% of patients; and headache, which was reported in 2%. Rates of study drug discontinuations for folliculitis and contact dermatitis were 1.2% and 1.4%, respectively. Headache did not lead to any study discontinuations.
Calling tapinarof a “first-in-class nonsteroidal,” Dr. Stein Gold suggested that this is likely to be a useful adjunctive therapy for psoriasis control. It avoids the adverse events associated with long-term topical steroid use, and its tolerability might be particularly attractive for use in sensitive areas.
“This is likely to be very useful in patients who are looking for a topical therapy for skin folds or the face, where there is a need for well-tolerated topical treatments,” Dr. Stein Gold said.
There are a lot of reasons to be positive about a new, well-tolerated topical agent for psoriasis, particularly as an alternative to topical steroids, agreed Adam Friedman, MD, director of translational research and professor and chair of the department of dermatology at George Washington University, Washington. He considers the data with tapinarof promising in general, but he also likes any new, effective topical psoriasis therapy.
“Patients and physicians are always hungry for new options, especially psoriasis patients, given many have ‘been there and done that’ with topical steroids,” Dr. Friedman said.
“Topical steroids are not irritating, but long-term use beyond recommended dosing can lead to skin thinning, lightening, tachyphylaxis, and, if really abused, HPA [hypothalamic-pituitary-adrenal] axis suppression and adrenal insufficiency,” he observed.
A topical therapy with a durable effect is particularly intriguing.
“The other issue with topical steroids is that psoriatic plaques return rather easily after stopping. The data I have seen with tapinarof show more sustainability after cessation, owing to its mechanism of action,” Dr. Friedman said. Rather than its potential for application to sensitive areas, such as the face, the durability “to me is more interesting.”
He suspects that, owing to “the incurable steroid phobia that haunts many of our patients,” an effective nonsteroidal topical option is also likely to lead to better compliance with topical treatment over time.
“A well-tolerated nonsteroidal topical drug will probably find an important place in the future management of chronic inflammatory diseases,” Marius-Anton Ionescu, MD, PhD, a dermatologist at the Hôpital Saint Louis, Paris, said in an interview. He referred to the positive effects of treatment with tapinarof in clinical trials in adults with atopic dermatitis, in addition to psoriasis.
Tapinarof 1% is also being investigated in a phase 3 study involving patients with moderate to severe atopic dermatitis. In that study, patients are as young as age 2 years. The drug is under review at the Food and Drug Administration for the plaque psoriasis indication in adults.
Dr. Stein Gold has financial relationships with Arcutis, Amgen, Bristol-Myers Squibb, Eli Lilly, Leo Pharma Ortho Dermatologic, UCB, and Dermavant Sciences, which is developing tapinarof and is provided funding for the PSOARING 3 trial. Dr. Friedman reported financial relationships with Amgen, Biogen, Encore, Galderma, GlaxoSmithKline, IntraDerm, Johnson & Johnson, Nerium, Novartis, Oculus, Onset, Pfizer, Sanova, and Valeant Pharmaceuticals. Dr. Ionescu has been a speaker or investigator (honoraria) for Celgene, Novartis, Lilly, and Uriage Cosmetics.
A version of this article first appeared on Medscape.com.
Treatment with presented as a late-breaker at the virtual annual congress of the European Academy of Dermatology and Venereology.
The drug has several unique features with meaningful clinical differences from other topical psoriasis therapies, according to Linda Stein Gold, MD, director of dermatology clinical research, Henry Ford Health System, Detroit.
“The currently available nonsteroidal topical therapies are typically associated with significant irritation. We did not see that with tapinarof,” said Dr. Stein Gold. This is one of several reasons she believes this drug will be a valuable addition if it receives regulatory approval.
Tapinarof is a small-molecule aryl hydrocarbon receptor (AhR) modulating agent. AhR is widely expressed in immune cells, including macrophages, mast cells, and antigen-presenting cells. It is believed that modulation of AhR signaling by tapinarof reverses immune dysregulation that is involved in the formation of psoriatic lesions.
The newly presented PSOARING 3 data with tapinarof 1% build on the data from the 12-week PSOARING 1 and PSOARING 2 trials, which were released in August 2020 but have yet to be published.
The primary endpoint in both of the 12-week trials, each of which enrolled about 500 patients with plaque psoriasis, was a Physician Global Assessment (PGA) score of 0 (clear) or 1 (almost clear). Relative to a placebo response rate of about 6% in both trials, the proportion of patients who achieved scores of 0/1 with tapinarof 1% was 35.4% and 40.2% in the PSOARING 1 and PSOARING 2 trials, respectively (P < .0001 vs. placebo in both studies).
For the key secondary endpoint of at least 75% improvement in the Psoriasis Area and Severity Index (PASI 75), the relative advantage for tapinarof over placebo was similar. The results were highly statistically significant (P < .0001) in both of the 12-week trials.
More than 90% of the patients who participated in PSOARING 1 and PSOARING 2 and were eligible for the open-label PSOARING 3 extension trial, according to Dr. Stein Gold.
For the 79 patients with a score of 0 at the time of enrollment, tapinarof 1% was reapplied only if the PGA score reached at least 2 during the course of the study. For the 680 patients who entered with a PGA score of at least 1, once-daily applications of tapinarof 1% cream were maintained until a PGA score of 0 was achieved.
In the outcome analysis, response was defined as the proportion of patients with an initial PGA score of at least2 who achieved PGA 0. A remittive effect was defined as duration of a PGA score of 0 or 1 while off therapy after achieving a PGA score of 0. Durability of response was defined as the proportion of patients who achieved a PGA sore of 0 or 1 at least once during the study while on therapy. This last outcome provided a test of tachyphylaxis.
“Overall, 40.9% of patients achieved complete disease clearance at least once during the trial, and 58.2% who entered the study with a PGA score of 2 or higher achieved a PGA score of 0 or 1,” Dr. Stein Gold reported.
For the 79 patients who entered PSOARING 3 with a PGA score of 0 and were off treatment, the median duration of a remittive effect was 115 days. For the patients who entered the trial with a higher PGA score but who achieved a score of 0 during the study (312 patients), the mean remittive effect after discontinuing therapy was 130 days.
There was no evidence of tachyphylaxis. Rather, “there was no loss of effect despite intermittent therapy observed over the course of the trial,” Dr. Stein Gold reported.
The most common treatment-emergent adverse events in PSOARING 3, as in the previous PSOARING studies, were folliculitis, which was observed in 24.0% of patients; contact dermatitis, which occurred in 5.9% of patients; and headache, which was reported in 2%. Rates of study drug discontinuations for folliculitis and contact dermatitis were 1.2% and 1.4%, respectively. Headache did not lead to any study discontinuations.
Calling tapinarof a “first-in-class nonsteroidal,” Dr. Stein Gold suggested that this is likely to be a useful adjunctive therapy for psoriasis control. It avoids the adverse events associated with long-term topical steroid use, and its tolerability might be particularly attractive for use in sensitive areas.
“This is likely to be very useful in patients who are looking for a topical therapy for skin folds or the face, where there is a need for well-tolerated topical treatments,” Dr. Stein Gold said.
There are a lot of reasons to be positive about a new, well-tolerated topical agent for psoriasis, particularly as an alternative to topical steroids, agreed Adam Friedman, MD, director of translational research and professor and chair of the department of dermatology at George Washington University, Washington. He considers the data with tapinarof promising in general, but he also likes any new, effective topical psoriasis therapy.
“Patients and physicians are always hungry for new options, especially psoriasis patients, given many have ‘been there and done that’ with topical steroids,” Dr. Friedman said.
“Topical steroids are not irritating, but long-term use beyond recommended dosing can lead to skin thinning, lightening, tachyphylaxis, and, if really abused, HPA [hypothalamic-pituitary-adrenal] axis suppression and adrenal insufficiency,” he observed.
A topical therapy with a durable effect is particularly intriguing.
“The other issue with topical steroids is that psoriatic plaques return rather easily after stopping. The data I have seen with tapinarof show more sustainability after cessation, owing to its mechanism of action,” Dr. Friedman said. Rather than its potential for application to sensitive areas, such as the face, the durability “to me is more interesting.”
He suspects that, owing to “the incurable steroid phobia that haunts many of our patients,” an effective nonsteroidal topical option is also likely to lead to better compliance with topical treatment over time.
“A well-tolerated nonsteroidal topical drug will probably find an important place in the future management of chronic inflammatory diseases,” Marius-Anton Ionescu, MD, PhD, a dermatologist at the Hôpital Saint Louis, Paris, said in an interview. He referred to the positive effects of treatment with tapinarof in clinical trials in adults with atopic dermatitis, in addition to psoriasis.
Tapinarof 1% is also being investigated in a phase 3 study involving patients with moderate to severe atopic dermatitis. In that study, patients are as young as age 2 years. The drug is under review at the Food and Drug Administration for the plaque psoriasis indication in adults.
Dr. Stein Gold has financial relationships with Arcutis, Amgen, Bristol-Myers Squibb, Eli Lilly, Leo Pharma Ortho Dermatologic, UCB, and Dermavant Sciences, which is developing tapinarof and is provided funding for the PSOARING 3 trial. Dr. Friedman reported financial relationships with Amgen, Biogen, Encore, Galderma, GlaxoSmithKline, IntraDerm, Johnson & Johnson, Nerium, Novartis, Oculus, Onset, Pfizer, Sanova, and Valeant Pharmaceuticals. Dr. Ionescu has been a speaker or investigator (honoraria) for Celgene, Novartis, Lilly, and Uriage Cosmetics.
A version of this article first appeared on Medscape.com.
Treatment with presented as a late-breaker at the virtual annual congress of the European Academy of Dermatology and Venereology.
The drug has several unique features with meaningful clinical differences from other topical psoriasis therapies, according to Linda Stein Gold, MD, director of dermatology clinical research, Henry Ford Health System, Detroit.
“The currently available nonsteroidal topical therapies are typically associated with significant irritation. We did not see that with tapinarof,” said Dr. Stein Gold. This is one of several reasons she believes this drug will be a valuable addition if it receives regulatory approval.
Tapinarof is a small-molecule aryl hydrocarbon receptor (AhR) modulating agent. AhR is widely expressed in immune cells, including macrophages, mast cells, and antigen-presenting cells. It is believed that modulation of AhR signaling by tapinarof reverses immune dysregulation that is involved in the formation of psoriatic lesions.
The newly presented PSOARING 3 data with tapinarof 1% build on the data from the 12-week PSOARING 1 and PSOARING 2 trials, which were released in August 2020 but have yet to be published.
The primary endpoint in both of the 12-week trials, each of which enrolled about 500 patients with plaque psoriasis, was a Physician Global Assessment (PGA) score of 0 (clear) or 1 (almost clear). Relative to a placebo response rate of about 6% in both trials, the proportion of patients who achieved scores of 0/1 with tapinarof 1% was 35.4% and 40.2% in the PSOARING 1 and PSOARING 2 trials, respectively (P < .0001 vs. placebo in both studies).
For the key secondary endpoint of at least 75% improvement in the Psoriasis Area and Severity Index (PASI 75), the relative advantage for tapinarof over placebo was similar. The results were highly statistically significant (P < .0001) in both of the 12-week trials.
More than 90% of the patients who participated in PSOARING 1 and PSOARING 2 and were eligible for the open-label PSOARING 3 extension trial, according to Dr. Stein Gold.
For the 79 patients with a score of 0 at the time of enrollment, tapinarof 1% was reapplied only if the PGA score reached at least 2 during the course of the study. For the 680 patients who entered with a PGA score of at least 1, once-daily applications of tapinarof 1% cream were maintained until a PGA score of 0 was achieved.
In the outcome analysis, response was defined as the proportion of patients with an initial PGA score of at least2 who achieved PGA 0. A remittive effect was defined as duration of a PGA score of 0 or 1 while off therapy after achieving a PGA score of 0. Durability of response was defined as the proportion of patients who achieved a PGA sore of 0 or 1 at least once during the study while on therapy. This last outcome provided a test of tachyphylaxis.
“Overall, 40.9% of patients achieved complete disease clearance at least once during the trial, and 58.2% who entered the study with a PGA score of 2 or higher achieved a PGA score of 0 or 1,” Dr. Stein Gold reported.
For the 79 patients who entered PSOARING 3 with a PGA score of 0 and were off treatment, the median duration of a remittive effect was 115 days. For the patients who entered the trial with a higher PGA score but who achieved a score of 0 during the study (312 patients), the mean remittive effect after discontinuing therapy was 130 days.
There was no evidence of tachyphylaxis. Rather, “there was no loss of effect despite intermittent therapy observed over the course of the trial,” Dr. Stein Gold reported.
The most common treatment-emergent adverse events in PSOARING 3, as in the previous PSOARING studies, were folliculitis, which was observed in 24.0% of patients; contact dermatitis, which occurred in 5.9% of patients; and headache, which was reported in 2%. Rates of study drug discontinuations for folliculitis and contact dermatitis were 1.2% and 1.4%, respectively. Headache did not lead to any study discontinuations.
Calling tapinarof a “first-in-class nonsteroidal,” Dr. Stein Gold suggested that this is likely to be a useful adjunctive therapy for psoriasis control. It avoids the adverse events associated with long-term topical steroid use, and its tolerability might be particularly attractive for use in sensitive areas.
“This is likely to be very useful in patients who are looking for a topical therapy for skin folds or the face, where there is a need for well-tolerated topical treatments,” Dr. Stein Gold said.
There are a lot of reasons to be positive about a new, well-tolerated topical agent for psoriasis, particularly as an alternative to topical steroids, agreed Adam Friedman, MD, director of translational research and professor and chair of the department of dermatology at George Washington University, Washington. He considers the data with tapinarof promising in general, but he also likes any new, effective topical psoriasis therapy.
“Patients and physicians are always hungry for new options, especially psoriasis patients, given many have ‘been there and done that’ with topical steroids,” Dr. Friedman said.
“Topical steroids are not irritating, but long-term use beyond recommended dosing can lead to skin thinning, lightening, tachyphylaxis, and, if really abused, HPA [hypothalamic-pituitary-adrenal] axis suppression and adrenal insufficiency,” he observed.
A topical therapy with a durable effect is particularly intriguing.
“The other issue with topical steroids is that psoriatic plaques return rather easily after stopping. The data I have seen with tapinarof show more sustainability after cessation, owing to its mechanism of action,” Dr. Friedman said. Rather than its potential for application to sensitive areas, such as the face, the durability “to me is more interesting.”
He suspects that, owing to “the incurable steroid phobia that haunts many of our patients,” an effective nonsteroidal topical option is also likely to lead to better compliance with topical treatment over time.
“A well-tolerated nonsteroidal topical drug will probably find an important place in the future management of chronic inflammatory diseases,” Marius-Anton Ionescu, MD, PhD, a dermatologist at the Hôpital Saint Louis, Paris, said in an interview. He referred to the positive effects of treatment with tapinarof in clinical trials in adults with atopic dermatitis, in addition to psoriasis.
Tapinarof 1% is also being investigated in a phase 3 study involving patients with moderate to severe atopic dermatitis. In that study, patients are as young as age 2 years. The drug is under review at the Food and Drug Administration for the plaque psoriasis indication in adults.
Dr. Stein Gold has financial relationships with Arcutis, Amgen, Bristol-Myers Squibb, Eli Lilly, Leo Pharma Ortho Dermatologic, UCB, and Dermavant Sciences, which is developing tapinarof and is provided funding for the PSOARING 3 trial. Dr. Friedman reported financial relationships with Amgen, Biogen, Encore, Galderma, GlaxoSmithKline, IntraDerm, Johnson & Johnson, Nerium, Novartis, Oculus, Onset, Pfizer, Sanova, and Valeant Pharmaceuticals. Dr. Ionescu has been a speaker or investigator (honoraria) for Celgene, Novartis, Lilly, and Uriage Cosmetics.
A version of this article first appeared on Medscape.com.
Antibody cocktail reduces chance of developing COVID
A one-time dose of two long-acting monoclonal antibodies reduced the risk of developing symptomatic COVID by 77% in comparison with placebo (P < .001) in a randomized, double-blind, placebo-controlled, phase 3 trial in adults, according to researchers who presented results at IDWeek 2021, an annual scientific meeting on infectious diseases.
The mix of tixagevimab and cilgavimab (AZD7442, Astra Zeneca) in a 300-mg dose is delivered in two intramuscular injections.
“This is the first long-acting combination of monoclonal antibodies that represents a potential new option to augment COVID-19 prevention,” said lead author Myron J. Levin, MD, a professor and pediatric infectious disease specialist at the University of Colorado at Denver, Aurora, who presented the findings of the PROVENT trial.
Both antibodies were taken from B cells donated by patients who had been infected with SARS-CoV-2, and they work synergistically, Dr. Levin said.
“The combination of them is better than adding results of each individually,” he said. “In vitro experiments have already shown that variants of interest and concern, including the Delta variant, are successfully neutralized by this cocktail.”
The trial was conducted in 87 sites in the United States, the United Kingdom, Spain, France, and Belgium. Participants included 5,197 unvaccinated adults who had never been infected with SARS-CoV-2 and either were at higher risk for inadequate response to COVID-19 vaccines because they were immunocompromised or were at high risk for exposure.
“Efficacy was observed through at least 3 months,” Dr. Levin said. “Preliminary pharmacokinetic modeling predicts potential protection for up to 12 months.”
Raymund Razonable, MD, an infectious disease expert with the Mayo Clinic in Rochester, Minn., who was not involved with the trial, told this news organization he was particularly interested in this combination because the developers made use of novel technology that extends the half-life of the antibodies and because of the large number of participants in the study.
Modeling that shows protection could last up to a year is novel and important, he said.
“People won’t need frequent injections,” Dr. Razonable said. With postexposure prophylaxis monoclonal cocktails, people may be given a dose a month, he noted.
Dr. Razonable said, “This is something intended to prevent COVID in people who are unvaccinated. The downside to that is we want people to get vaccinated. The best strategy so far is really vaccination.”
He said AZD7442 could potentially help fill the void for patients who are not able to respond to the COVID vaccines, including some who are immunocompromised or are undergoing chemotherapy.
Dr. Razonable said that, although the 77% reduction for developing symptomatic COVID-19 (95% confidence interval vs. placebo, 46.0-90.0; P < .001) is impressive, it is a reduction in relative risk. Still unknown is how much an individual’s absolute risk is reduced.
He also said it would be helpful to know how many people in the study population were immunocompromised, “because I think that’s where this product will be useful for prevention.”
The primary study endpoints were the first case of SARS-CoV-2 RT-PCR-positive symptomatic illness post dose and prior to day 183 (efficacy) as well as the safety of the product.
The cocktail appeared to be well tolerated. Adverse events occurred in 35% of participants administered AZD7442 and in 34% of the placebo group. Injection-site reactions occurred in 2.4% of the AZD7442 group and in 2.1% of the placebo group. There was one case of severe or critical COVID-19; two COVID-19–related deaths occurred in the placebo group.
AZD7442 is being developed with the help of funding from the U.S. government. Dr. Levin has received support from GlaxoSmithKline companies. Many of the coauthors are employed by AstraZeneca and hold stock in the company. Dr. Razonable has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A one-time dose of two long-acting monoclonal antibodies reduced the risk of developing symptomatic COVID by 77% in comparison with placebo (P < .001) in a randomized, double-blind, placebo-controlled, phase 3 trial in adults, according to researchers who presented results at IDWeek 2021, an annual scientific meeting on infectious diseases.
The mix of tixagevimab and cilgavimab (AZD7442, Astra Zeneca) in a 300-mg dose is delivered in two intramuscular injections.
“This is the first long-acting combination of monoclonal antibodies that represents a potential new option to augment COVID-19 prevention,” said lead author Myron J. Levin, MD, a professor and pediatric infectious disease specialist at the University of Colorado at Denver, Aurora, who presented the findings of the PROVENT trial.
Both antibodies were taken from B cells donated by patients who had been infected with SARS-CoV-2, and they work synergistically, Dr. Levin said.
“The combination of them is better than adding results of each individually,” he said. “In vitro experiments have already shown that variants of interest and concern, including the Delta variant, are successfully neutralized by this cocktail.”
The trial was conducted in 87 sites in the United States, the United Kingdom, Spain, France, and Belgium. Participants included 5,197 unvaccinated adults who had never been infected with SARS-CoV-2 and either were at higher risk for inadequate response to COVID-19 vaccines because they were immunocompromised or were at high risk for exposure.
“Efficacy was observed through at least 3 months,” Dr. Levin said. “Preliminary pharmacokinetic modeling predicts potential protection for up to 12 months.”
Raymund Razonable, MD, an infectious disease expert with the Mayo Clinic in Rochester, Minn., who was not involved with the trial, told this news organization he was particularly interested in this combination because the developers made use of novel technology that extends the half-life of the antibodies and because of the large number of participants in the study.
Modeling that shows protection could last up to a year is novel and important, he said.
“People won’t need frequent injections,” Dr. Razonable said. With postexposure prophylaxis monoclonal cocktails, people may be given a dose a month, he noted.
Dr. Razonable said, “This is something intended to prevent COVID in people who are unvaccinated. The downside to that is we want people to get vaccinated. The best strategy so far is really vaccination.”
He said AZD7442 could potentially help fill the void for patients who are not able to respond to the COVID vaccines, including some who are immunocompromised or are undergoing chemotherapy.
Dr. Razonable said that, although the 77% reduction for developing symptomatic COVID-19 (95% confidence interval vs. placebo, 46.0-90.0; P < .001) is impressive, it is a reduction in relative risk. Still unknown is how much an individual’s absolute risk is reduced.
He also said it would be helpful to know how many people in the study population were immunocompromised, “because I think that’s where this product will be useful for prevention.”
The primary study endpoints were the first case of SARS-CoV-2 RT-PCR-positive symptomatic illness post dose and prior to day 183 (efficacy) as well as the safety of the product.
The cocktail appeared to be well tolerated. Adverse events occurred in 35% of participants administered AZD7442 and in 34% of the placebo group. Injection-site reactions occurred in 2.4% of the AZD7442 group and in 2.1% of the placebo group. There was one case of severe or critical COVID-19; two COVID-19–related deaths occurred in the placebo group.
AZD7442 is being developed with the help of funding from the U.S. government. Dr. Levin has received support from GlaxoSmithKline companies. Many of the coauthors are employed by AstraZeneca and hold stock in the company. Dr. Razonable has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A one-time dose of two long-acting monoclonal antibodies reduced the risk of developing symptomatic COVID by 77% in comparison with placebo (P < .001) in a randomized, double-blind, placebo-controlled, phase 3 trial in adults, according to researchers who presented results at IDWeek 2021, an annual scientific meeting on infectious diseases.
The mix of tixagevimab and cilgavimab (AZD7442, Astra Zeneca) in a 300-mg dose is delivered in two intramuscular injections.
“This is the first long-acting combination of monoclonal antibodies that represents a potential new option to augment COVID-19 prevention,” said lead author Myron J. Levin, MD, a professor and pediatric infectious disease specialist at the University of Colorado at Denver, Aurora, who presented the findings of the PROVENT trial.
Both antibodies were taken from B cells donated by patients who had been infected with SARS-CoV-2, and they work synergistically, Dr. Levin said.
“The combination of them is better than adding results of each individually,” he said. “In vitro experiments have already shown that variants of interest and concern, including the Delta variant, are successfully neutralized by this cocktail.”
The trial was conducted in 87 sites in the United States, the United Kingdom, Spain, France, and Belgium. Participants included 5,197 unvaccinated adults who had never been infected with SARS-CoV-2 and either were at higher risk for inadequate response to COVID-19 vaccines because they were immunocompromised or were at high risk for exposure.
“Efficacy was observed through at least 3 months,” Dr. Levin said. “Preliminary pharmacokinetic modeling predicts potential protection for up to 12 months.”
Raymund Razonable, MD, an infectious disease expert with the Mayo Clinic in Rochester, Minn., who was not involved with the trial, told this news organization he was particularly interested in this combination because the developers made use of novel technology that extends the half-life of the antibodies and because of the large number of participants in the study.
Modeling that shows protection could last up to a year is novel and important, he said.
“People won’t need frequent injections,” Dr. Razonable said. With postexposure prophylaxis monoclonal cocktails, people may be given a dose a month, he noted.
Dr. Razonable said, “This is something intended to prevent COVID in people who are unvaccinated. The downside to that is we want people to get vaccinated. The best strategy so far is really vaccination.”
He said AZD7442 could potentially help fill the void for patients who are not able to respond to the COVID vaccines, including some who are immunocompromised or are undergoing chemotherapy.
Dr. Razonable said that, although the 77% reduction for developing symptomatic COVID-19 (95% confidence interval vs. placebo, 46.0-90.0; P < .001) is impressive, it is a reduction in relative risk. Still unknown is how much an individual’s absolute risk is reduced.
He also said it would be helpful to know how many people in the study population were immunocompromised, “because I think that’s where this product will be useful for prevention.”
The primary study endpoints were the first case of SARS-CoV-2 RT-PCR-positive symptomatic illness post dose and prior to day 183 (efficacy) as well as the safety of the product.
The cocktail appeared to be well tolerated. Adverse events occurred in 35% of participants administered AZD7442 and in 34% of the placebo group. Injection-site reactions occurred in 2.4% of the AZD7442 group and in 2.1% of the placebo group. There was one case of severe or critical COVID-19; two COVID-19–related deaths occurred in the placebo group.
AZD7442 is being developed with the help of funding from the U.S. government. Dr. Levin has received support from GlaxoSmithKline companies. Many of the coauthors are employed by AstraZeneca and hold stock in the company. Dr. Razonable has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.





