User login
More U.S. adults vaccinated, but partisan divide remains: Survey
The number of U.S. adults who are at least partially vaccinated rose five percentage points to 72% in August, a slightly faster increase than in previous months, according to the latest monthly COVID-19 Vaccine Monitor report of the Kaiser Family Foundation (KFF).
The largest increases in vaccine uptake between July and September were among Hispanic adults and people aged 18-29 years. Roughly equal shares of adults now report being vaccinated across racial and ethnic groups: 71% of White adults, 70% of Black adults, and 73% of Hispanic adults.
Overall, the big takeaway of the latest Kaiser COVID-19 survey is that the partisan divide on all aspects of the pandemic, from vaccination status to attitudes toward vaccination and mask mandates, remains as wide as ever.
The only thing that Republicans, Democrats, and Independents mostly agree on is that COVID-19 will probably become an endemic disease like influenza. Seventy-nine percent of respondents agreed with that statement; 14% predicted that COVID-19 would probably be largely eliminated in future years, like polio.
Delta motivated many
The most important factor that recently led people toward being vaccinated against COVID-19 was the surge in cases, hospitalizations, and deaths due to the Delta variant, KFF reports.
Full approval of the Pfizer vaccine by the Food and Drug Administration and the increasing prevalence of vaccine mandates played secondary roles in the vaccination uptick.
Specifically, 10% of the recently vaccinated said the main reason they got shots was the increase in COVID-19 cases due to the Delta variant. Concern about reports of local hospitals and intensive care units filling up with COVID-19 patients was the main motivator for 12% of those who just got shots. Fourteen percent of the recently vaccinated got inoculated mainly because someone they knew had become seriously ill or had died from COVID-19.
The role of the Delta threat is also evident with regard to where those who were recently vaccinated live. Twenty-four percent of those who received their first dose of vaccine after June 1, 2021, reside in counties with a high COVID-19 case rate; 15% of them live in counties with a relatively low case rate.
Despite the recent surge in vaccinations, however, 7% of adults are still taking a wait-and-see approach; 4% said they’d get a shot only if required; and 12% said they definitely wouldn’t get vaccinated. The latter figure has barely budged since January of this year.
Ninety percent of Democrats said they had received at least one dose of the COVID-19 vaccine, vs. 68% of Independents, and 58% of Republicans.
Wealthier, better educated, urban, and older people were more likely to be vaccinated, with one exception: Sixty-eight percent of those aged 18-29 were vaccinated, vs. 66% of those aged 30-49. The group least likely to be vaccinated were uninsured people younger than 65, suggesting that some of them were unaware that the shot is free.
Partisan affiliation
Attitudes toward vaccine booster shots – which are now recommended for people older than 65, the immunocompromised, and certain frontline workers – largely fell along party lines and/or reflected whether respondents had been vaccinated.
Discussion of the boosters, KFF said, “appears to be a net positive for people who are already vaccinated, but a net negative for the unvaccinated. While a larger share of vaccinated adults say the information they have seen about boosters has been helpful (54%) than find it confusing (35%), among the unvaccinated almost twice as many find the information confusing as find it helpful (45% vs. 24%).”
Among fully vaccinated adults, 68% of Democrats say they’d definitely get a booster, and 20% say they probably would. Among Republicans, those percentages are 36% and 33%, respectively. Independents fall in between the other groups.
Although 82% of Democrats say the boosters show that scientists are continuing to make vaccines more effective, 52% of Republicans say that it shows that the vaccines are not working as well as promised.
Similarly, partisan attitudes emerged in questions about breakthrough infections. The fact that COVID-19 cases are fairly mild when they occur among persons who have been vaccinated indicates that the vaccines are working, said 87% of Democrats, but only 55% of Republicans agreed with that assessment.
In contrast, just 10% of Democrats but 39% of Republicans said that breakthrough infections mean the vaccines are not working.
Vaccine requirements
The public is more evenly divided on vaccine requirements. About 6 in 10 respondents said that vaccines should be required for health care workers (62%) and schoolteachers (58%). Slim majorities supported mandates for federal government employees (55%), college students (55%), and state and local government employees (54%).
On the question of whether employers in general should require their workers to be vaccinated, 48% of respondents said they should, and 50% said they should not.
Similarly, 52% said that all schools should mandate vaccines for eligible students; 46% didn’t approve of such requirements.
Three in four Democrats supported employer vaccine mandates, compared to 45% of Independents and just 20% of Republicans. Large partisan gaps were also seen for government, school, and health care vaccine mandates.
In contrast, 78% of the public favored requiring large employers to give their workers paid time off to get vaccinated and to recover from any side effects.
Twenty percent of workers under mandates
One in five workers said their employers require COVID-19 vaccination. Twenty-eight percent of employed people want their employers to require vaccination, and 50% don’t.
Again, the responses broke down along party lines, with 52% of Democrats, 21% of Independents, and 10% of Republicans favoring a vaccine mandate by their employers.
Most unvaccinated people didn’t support an employer mandate. A third of unvaccinated workers said they’d be likely to get vaccinated if their companies required it, but most of them said they would choose weekly testing if offered the option.
Being unable to use gyms, restaurants, or indoor entertainment venues that require vaccination was cited by 13% of the recently vaccinated as the main reason why they got shots.
The public was evenly divided on whether states or local governments should require such businesses to mandate that staff and customers show proof of vaccination. Although the views of the public were tied on this issue overall, 79% of Democrats, 43% of Independents, and 21% of Republicans supported having these kinds of businesses require proof of vaccination.
On school mask mandates, 56% of the respondents supported requiring all students and staff to wear masks. Favoring this kind of mandate were 83% of Democrats, 53% of Independents, and 29% of Republicans.
Partisanship also defined how Democrats and Republicans viewed the current high number of COVID-19 cases. Most Democrats blamed people who don’t wear masks and those who don’t get vaccinated, whereas Republicans were more likely to blame immigrants and tourists bringing COVID-19 into the United States.
A version of this article first appeared on Medscape.com.
The number of U.S. adults who are at least partially vaccinated rose five percentage points to 72% in August, a slightly faster increase than in previous months, according to the latest monthly COVID-19 Vaccine Monitor report of the Kaiser Family Foundation (KFF).
The largest increases in vaccine uptake between July and September were among Hispanic adults and people aged 18-29 years. Roughly equal shares of adults now report being vaccinated across racial and ethnic groups: 71% of White adults, 70% of Black adults, and 73% of Hispanic adults.
Overall, the big takeaway of the latest Kaiser COVID-19 survey is that the partisan divide on all aspects of the pandemic, from vaccination status to attitudes toward vaccination and mask mandates, remains as wide as ever.
The only thing that Republicans, Democrats, and Independents mostly agree on is that COVID-19 will probably become an endemic disease like influenza. Seventy-nine percent of respondents agreed with that statement; 14% predicted that COVID-19 would probably be largely eliminated in future years, like polio.
Delta motivated many
The most important factor that recently led people toward being vaccinated against COVID-19 was the surge in cases, hospitalizations, and deaths due to the Delta variant, KFF reports.
Full approval of the Pfizer vaccine by the Food and Drug Administration and the increasing prevalence of vaccine mandates played secondary roles in the vaccination uptick.
Specifically, 10% of the recently vaccinated said the main reason they got shots was the increase in COVID-19 cases due to the Delta variant. Concern about reports of local hospitals and intensive care units filling up with COVID-19 patients was the main motivator for 12% of those who just got shots. Fourteen percent of the recently vaccinated got inoculated mainly because someone they knew had become seriously ill or had died from COVID-19.
The role of the Delta threat is also evident with regard to where those who were recently vaccinated live. Twenty-four percent of those who received their first dose of vaccine after June 1, 2021, reside in counties with a high COVID-19 case rate; 15% of them live in counties with a relatively low case rate.
Despite the recent surge in vaccinations, however, 7% of adults are still taking a wait-and-see approach; 4% said they’d get a shot only if required; and 12% said they definitely wouldn’t get vaccinated. The latter figure has barely budged since January of this year.
Ninety percent of Democrats said they had received at least one dose of the COVID-19 vaccine, vs. 68% of Independents, and 58% of Republicans.
Wealthier, better educated, urban, and older people were more likely to be vaccinated, with one exception: Sixty-eight percent of those aged 18-29 were vaccinated, vs. 66% of those aged 30-49. The group least likely to be vaccinated were uninsured people younger than 65, suggesting that some of them were unaware that the shot is free.
Partisan affiliation
Attitudes toward vaccine booster shots – which are now recommended for people older than 65, the immunocompromised, and certain frontline workers – largely fell along party lines and/or reflected whether respondents had been vaccinated.
Discussion of the boosters, KFF said, “appears to be a net positive for people who are already vaccinated, but a net negative for the unvaccinated. While a larger share of vaccinated adults say the information they have seen about boosters has been helpful (54%) than find it confusing (35%), among the unvaccinated almost twice as many find the information confusing as find it helpful (45% vs. 24%).”
Among fully vaccinated adults, 68% of Democrats say they’d definitely get a booster, and 20% say they probably would. Among Republicans, those percentages are 36% and 33%, respectively. Independents fall in between the other groups.
Although 82% of Democrats say the boosters show that scientists are continuing to make vaccines more effective, 52% of Republicans say that it shows that the vaccines are not working as well as promised.
Similarly, partisan attitudes emerged in questions about breakthrough infections. The fact that COVID-19 cases are fairly mild when they occur among persons who have been vaccinated indicates that the vaccines are working, said 87% of Democrats, but only 55% of Republicans agreed with that assessment.
In contrast, just 10% of Democrats but 39% of Republicans said that breakthrough infections mean the vaccines are not working.
Vaccine requirements
The public is more evenly divided on vaccine requirements. About 6 in 10 respondents said that vaccines should be required for health care workers (62%) and schoolteachers (58%). Slim majorities supported mandates for federal government employees (55%), college students (55%), and state and local government employees (54%).
On the question of whether employers in general should require their workers to be vaccinated, 48% of respondents said they should, and 50% said they should not.
Similarly, 52% said that all schools should mandate vaccines for eligible students; 46% didn’t approve of such requirements.
Three in four Democrats supported employer vaccine mandates, compared to 45% of Independents and just 20% of Republicans. Large partisan gaps were also seen for government, school, and health care vaccine mandates.
In contrast, 78% of the public favored requiring large employers to give their workers paid time off to get vaccinated and to recover from any side effects.
Twenty percent of workers under mandates
One in five workers said their employers require COVID-19 vaccination. Twenty-eight percent of employed people want their employers to require vaccination, and 50% don’t.
Again, the responses broke down along party lines, with 52% of Democrats, 21% of Independents, and 10% of Republicans favoring a vaccine mandate by their employers.
Most unvaccinated people didn’t support an employer mandate. A third of unvaccinated workers said they’d be likely to get vaccinated if their companies required it, but most of them said they would choose weekly testing if offered the option.
Being unable to use gyms, restaurants, or indoor entertainment venues that require vaccination was cited by 13% of the recently vaccinated as the main reason why they got shots.
The public was evenly divided on whether states or local governments should require such businesses to mandate that staff and customers show proof of vaccination. Although the views of the public were tied on this issue overall, 79% of Democrats, 43% of Independents, and 21% of Republicans supported having these kinds of businesses require proof of vaccination.
On school mask mandates, 56% of the respondents supported requiring all students and staff to wear masks. Favoring this kind of mandate were 83% of Democrats, 53% of Independents, and 29% of Republicans.
Partisanship also defined how Democrats and Republicans viewed the current high number of COVID-19 cases. Most Democrats blamed people who don’t wear masks and those who don’t get vaccinated, whereas Republicans were more likely to blame immigrants and tourists bringing COVID-19 into the United States.
A version of this article first appeared on Medscape.com.
The number of U.S. adults who are at least partially vaccinated rose five percentage points to 72% in August, a slightly faster increase than in previous months, according to the latest monthly COVID-19 Vaccine Monitor report of the Kaiser Family Foundation (KFF).
The largest increases in vaccine uptake between July and September were among Hispanic adults and people aged 18-29 years. Roughly equal shares of adults now report being vaccinated across racial and ethnic groups: 71% of White adults, 70% of Black adults, and 73% of Hispanic adults.
Overall, the big takeaway of the latest Kaiser COVID-19 survey is that the partisan divide on all aspects of the pandemic, from vaccination status to attitudes toward vaccination and mask mandates, remains as wide as ever.
The only thing that Republicans, Democrats, and Independents mostly agree on is that COVID-19 will probably become an endemic disease like influenza. Seventy-nine percent of respondents agreed with that statement; 14% predicted that COVID-19 would probably be largely eliminated in future years, like polio.
Delta motivated many
The most important factor that recently led people toward being vaccinated against COVID-19 was the surge in cases, hospitalizations, and deaths due to the Delta variant, KFF reports.
Full approval of the Pfizer vaccine by the Food and Drug Administration and the increasing prevalence of vaccine mandates played secondary roles in the vaccination uptick.
Specifically, 10% of the recently vaccinated said the main reason they got shots was the increase in COVID-19 cases due to the Delta variant. Concern about reports of local hospitals and intensive care units filling up with COVID-19 patients was the main motivator for 12% of those who just got shots. Fourteen percent of the recently vaccinated got inoculated mainly because someone they knew had become seriously ill or had died from COVID-19.
The role of the Delta threat is also evident with regard to where those who were recently vaccinated live. Twenty-four percent of those who received their first dose of vaccine after June 1, 2021, reside in counties with a high COVID-19 case rate; 15% of them live in counties with a relatively low case rate.
Despite the recent surge in vaccinations, however, 7% of adults are still taking a wait-and-see approach; 4% said they’d get a shot only if required; and 12% said they definitely wouldn’t get vaccinated. The latter figure has barely budged since January of this year.
Ninety percent of Democrats said they had received at least one dose of the COVID-19 vaccine, vs. 68% of Independents, and 58% of Republicans.
Wealthier, better educated, urban, and older people were more likely to be vaccinated, with one exception: Sixty-eight percent of those aged 18-29 were vaccinated, vs. 66% of those aged 30-49. The group least likely to be vaccinated were uninsured people younger than 65, suggesting that some of them were unaware that the shot is free.
Partisan affiliation
Attitudes toward vaccine booster shots – which are now recommended for people older than 65, the immunocompromised, and certain frontline workers – largely fell along party lines and/or reflected whether respondents had been vaccinated.
Discussion of the boosters, KFF said, “appears to be a net positive for people who are already vaccinated, but a net negative for the unvaccinated. While a larger share of vaccinated adults say the information they have seen about boosters has been helpful (54%) than find it confusing (35%), among the unvaccinated almost twice as many find the information confusing as find it helpful (45% vs. 24%).”
Among fully vaccinated adults, 68% of Democrats say they’d definitely get a booster, and 20% say they probably would. Among Republicans, those percentages are 36% and 33%, respectively. Independents fall in between the other groups.
Although 82% of Democrats say the boosters show that scientists are continuing to make vaccines more effective, 52% of Republicans say that it shows that the vaccines are not working as well as promised.
Similarly, partisan attitudes emerged in questions about breakthrough infections. The fact that COVID-19 cases are fairly mild when they occur among persons who have been vaccinated indicates that the vaccines are working, said 87% of Democrats, but only 55% of Republicans agreed with that assessment.
In contrast, just 10% of Democrats but 39% of Republicans said that breakthrough infections mean the vaccines are not working.
Vaccine requirements
The public is more evenly divided on vaccine requirements. About 6 in 10 respondents said that vaccines should be required for health care workers (62%) and schoolteachers (58%). Slim majorities supported mandates for federal government employees (55%), college students (55%), and state and local government employees (54%).
On the question of whether employers in general should require their workers to be vaccinated, 48% of respondents said they should, and 50% said they should not.
Similarly, 52% said that all schools should mandate vaccines for eligible students; 46% didn’t approve of such requirements.
Three in four Democrats supported employer vaccine mandates, compared to 45% of Independents and just 20% of Republicans. Large partisan gaps were also seen for government, school, and health care vaccine mandates.
In contrast, 78% of the public favored requiring large employers to give their workers paid time off to get vaccinated and to recover from any side effects.
Twenty percent of workers under mandates
One in five workers said their employers require COVID-19 vaccination. Twenty-eight percent of employed people want their employers to require vaccination, and 50% don’t.
Again, the responses broke down along party lines, with 52% of Democrats, 21% of Independents, and 10% of Republicans favoring a vaccine mandate by their employers.
Most unvaccinated people didn’t support an employer mandate. A third of unvaccinated workers said they’d be likely to get vaccinated if their companies required it, but most of them said they would choose weekly testing if offered the option.
Being unable to use gyms, restaurants, or indoor entertainment venues that require vaccination was cited by 13% of the recently vaccinated as the main reason why they got shots.
The public was evenly divided on whether states or local governments should require such businesses to mandate that staff and customers show proof of vaccination. Although the views of the public were tied on this issue overall, 79% of Democrats, 43% of Independents, and 21% of Republicans supported having these kinds of businesses require proof of vaccination.
On school mask mandates, 56% of the respondents supported requiring all students and staff to wear masks. Favoring this kind of mandate were 83% of Democrats, 53% of Independents, and 29% of Republicans.
Partisanship also defined how Democrats and Republicans viewed the current high number of COVID-19 cases. Most Democrats blamed people who don’t wear masks and those who don’t get vaccinated, whereas Republicans were more likely to blame immigrants and tourists bringing COVID-19 into the United States.
A version of this article first appeared on Medscape.com.
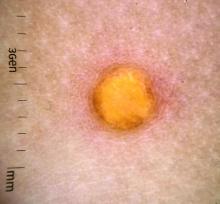
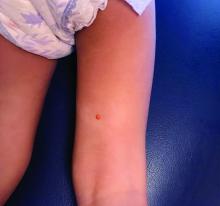
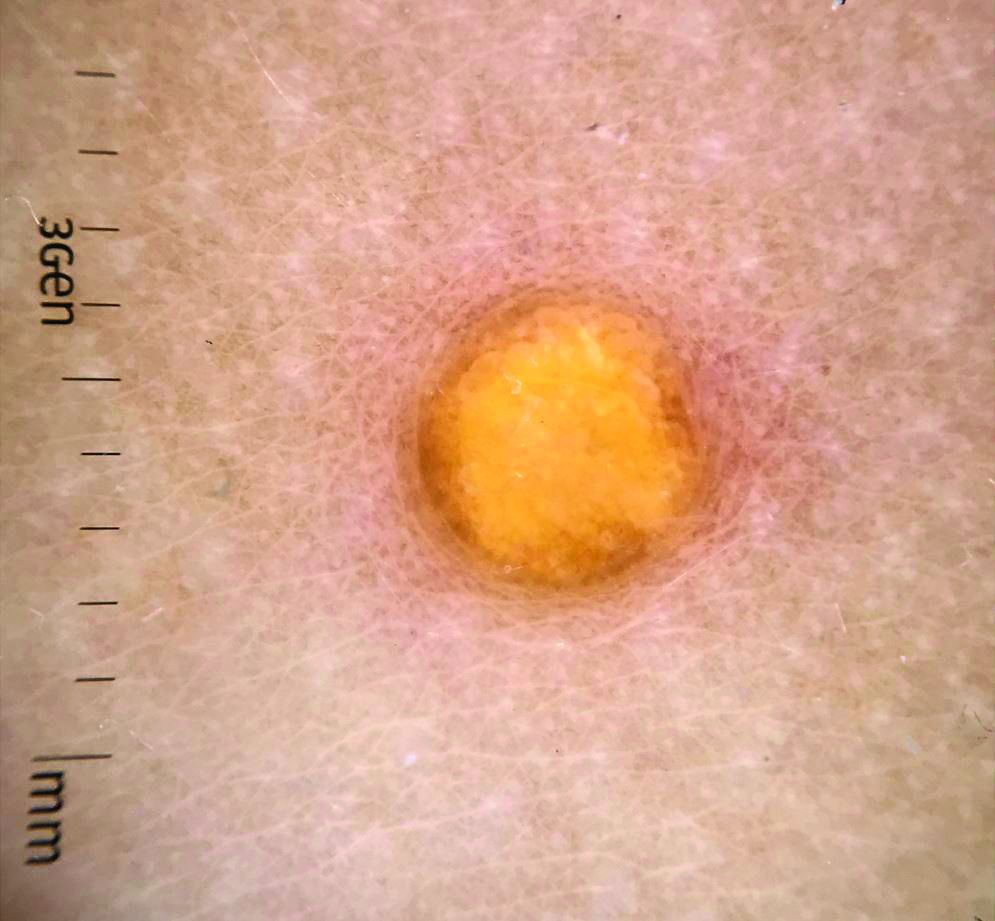
A female toddler presents with an itchy yellow nodule
Juvenile xanthogranuloma (JXG) is a benign disorder presenting as firm, yellow-red skin papules or nodules, usually in infancy or early childhood. It derives its name based on its yellowish color and the histologic finding of lipid-filled histiocytes. In fact, it is a form of non-Langerhans’ cell histiocytosis. It most commonly presents on the head, neck, and trunk, but can arise anywhere on the body as demonstrated by this case. While often pink to reddish early on, the characteristic yellow or orange, brown appearance over time is common, occasionally with overlying telangiectasia, and ranging in size from 1 mm to 2 cm. While typically asymptomatic, it is possible for lesions to itch. JXG is usually self-limiting, and spontaneously resolves over several years. On dermoscopy (with polarized light), it has a characteristic “setting sun” appearance because of its central yellow area surrounded by a reddish periphery.
JXGs have been associated with neurofibromatosis-1 and a “triple association” of NF-1, JXG, and juvenile myelomonocytic leukemia (JMML) has been debated. Many cases are diagnosed on clinical grounds without histologic confirmation, so while the absolute incidence is unknown, they are not uncommon.
What is on the differential?
Spitz nevus is a melanocytic lesion which typically presents as a sharply circumscribed, dome-shaped, pink-red or brown papule or nodule, and is composed of large epithelioid and/or spindled cells. These nevi can present with a spectrum of morphology and biologic activity; commonly with benign melanocytic proliferations and a symmetric appearance or, rarely, with atypical tumors or lesions, characterized as Spitzoid melanomas. The yellowish color of JXG is distinct from the appearance of Spitz tumors.
Molluscum contagiosum is a common pox viral infection seen in children that presents with round, flat-topped firm papules on the skin and distinctive whitish centers with or without umbilication. Like JXG, molluscum contagiosum papules may grow over time and cause pruritus. However, this diagnosis is less likely given the absence of other lesions on the skin, lack of known contacts with similar lesions, and yellowish color without a more typical appearance of molluscum.
Dermatofibromas occur in people of all ages, although more commonly between the ages of 20 and 40 and in those with a history of trauma at the lesion. Like JXGs, dermatofibromas tend to be firm, solitary papules or nodules. They usually are hyperpigmented, and classically “dimple when pinched” as they are fixed to the subcutaneous tissue. However, this patient’s age, lack of trauma, and the lesion morphology are not consistent with dermatofibromas.
Like XJGs, mastocytomas commonly present in the first 2 years of life with maculopapular or nodular lesions that itch. However, the history of new-onset itch in recent months as the lesion grew larger and the yellow color on dermoscopy are more consistent with JXG.
Eruptive xanthomas typically appear suddenly as multiple erythematous yellow, dome-shaped papules on the extensor surfaces of the extremities, buttocks, and hands. They are usually present with hypertriglyceridemia and are very rare in young children. The presence of a solitary lesion in a 6-month-old patient without a history of lipid abnormalities favors the diagnosis of XJG.
Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Ms. Kleinman is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology, University of California, San Diego, and Rady Children’s Hospital. Dr. Eichenfield and Ms. Kleinman have no relevant financial disclosures.
References
Hernandez-Martin A et al. J Am Acad Dermatol. 1997 Mar;36(3 Pt 1):355-67.
Prendiville J. Lumps, bumps and hamartomas in “Neonatal and Infant Dermatology,” 3rd ed. (Philadelphia: Elsevier, 2015).
Püttgen KB. Juvenile xanthogranuloma. UpToDate, 2021.
Schaffer JV. Am J Clin Dermatol. 2021 Mar;22(2):205-20.
Juvenile xanthogranuloma (JXG) is a benign disorder presenting as firm, yellow-red skin papules or nodules, usually in infancy or early childhood. It derives its name based on its yellowish color and the histologic finding of lipid-filled histiocytes. In fact, it is a form of non-Langerhans’ cell histiocytosis. It most commonly presents on the head, neck, and trunk, but can arise anywhere on the body as demonstrated by this case. While often pink to reddish early on, the characteristic yellow or orange, brown appearance over time is common, occasionally with overlying telangiectasia, and ranging in size from 1 mm to 2 cm. While typically asymptomatic, it is possible for lesions to itch. JXG is usually self-limiting, and spontaneously resolves over several years. On dermoscopy (with polarized light), it has a characteristic “setting sun” appearance because of its central yellow area surrounded by a reddish periphery.
JXGs have been associated with neurofibromatosis-1 and a “triple association” of NF-1, JXG, and juvenile myelomonocytic leukemia (JMML) has been debated. Many cases are diagnosed on clinical grounds without histologic confirmation, so while the absolute incidence is unknown, they are not uncommon.
What is on the differential?
Spitz nevus is a melanocytic lesion which typically presents as a sharply circumscribed, dome-shaped, pink-red or brown papule or nodule, and is composed of large epithelioid and/or spindled cells. These nevi can present with a spectrum of morphology and biologic activity; commonly with benign melanocytic proliferations and a symmetric appearance or, rarely, with atypical tumors or lesions, characterized as Spitzoid melanomas. The yellowish color of JXG is distinct from the appearance of Spitz tumors.
Molluscum contagiosum is a common pox viral infection seen in children that presents with round, flat-topped firm papules on the skin and distinctive whitish centers with or without umbilication. Like JXG, molluscum contagiosum papules may grow over time and cause pruritus. However, this diagnosis is less likely given the absence of other lesions on the skin, lack of known contacts with similar lesions, and yellowish color without a more typical appearance of molluscum.
Dermatofibromas occur in people of all ages, although more commonly between the ages of 20 and 40 and in those with a history of trauma at the lesion. Like JXGs, dermatofibromas tend to be firm, solitary papules or nodules. They usually are hyperpigmented, and classically “dimple when pinched” as they are fixed to the subcutaneous tissue. However, this patient’s age, lack of trauma, and the lesion morphology are not consistent with dermatofibromas.
Like XJGs, mastocytomas commonly present in the first 2 years of life with maculopapular or nodular lesions that itch. However, the history of new-onset itch in recent months as the lesion grew larger and the yellow color on dermoscopy are more consistent with JXG.
Eruptive xanthomas typically appear suddenly as multiple erythematous yellow, dome-shaped papules on the extensor surfaces of the extremities, buttocks, and hands. They are usually present with hypertriglyceridemia and are very rare in young children. The presence of a solitary lesion in a 6-month-old patient without a history of lipid abnormalities favors the diagnosis of XJG.
Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Ms. Kleinman is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology, University of California, San Diego, and Rady Children’s Hospital. Dr. Eichenfield and Ms. Kleinman have no relevant financial disclosures.
References
Hernandez-Martin A et al. J Am Acad Dermatol. 1997 Mar;36(3 Pt 1):355-67.
Prendiville J. Lumps, bumps and hamartomas in “Neonatal and Infant Dermatology,” 3rd ed. (Philadelphia: Elsevier, 2015).
Püttgen KB. Juvenile xanthogranuloma. UpToDate, 2021.
Schaffer JV. Am J Clin Dermatol. 2021 Mar;22(2):205-20.
Juvenile xanthogranuloma (JXG) is a benign disorder presenting as firm, yellow-red skin papules or nodules, usually in infancy or early childhood. It derives its name based on its yellowish color and the histologic finding of lipid-filled histiocytes. In fact, it is a form of non-Langerhans’ cell histiocytosis. It most commonly presents on the head, neck, and trunk, but can arise anywhere on the body as demonstrated by this case. While often pink to reddish early on, the characteristic yellow or orange, brown appearance over time is common, occasionally with overlying telangiectasia, and ranging in size from 1 mm to 2 cm. While typically asymptomatic, it is possible for lesions to itch. JXG is usually self-limiting, and spontaneously resolves over several years. On dermoscopy (with polarized light), it has a characteristic “setting sun” appearance because of its central yellow area surrounded by a reddish periphery.
JXGs have been associated with neurofibromatosis-1 and a “triple association” of NF-1, JXG, and juvenile myelomonocytic leukemia (JMML) has been debated. Many cases are diagnosed on clinical grounds without histologic confirmation, so while the absolute incidence is unknown, they are not uncommon.
What is on the differential?
Spitz nevus is a melanocytic lesion which typically presents as a sharply circumscribed, dome-shaped, pink-red or brown papule or nodule, and is composed of large epithelioid and/or spindled cells. These nevi can present with a spectrum of morphology and biologic activity; commonly with benign melanocytic proliferations and a symmetric appearance or, rarely, with atypical tumors or lesions, characterized as Spitzoid melanomas. The yellowish color of JXG is distinct from the appearance of Spitz tumors.
Molluscum contagiosum is a common pox viral infection seen in children that presents with round, flat-topped firm papules on the skin and distinctive whitish centers with or without umbilication. Like JXG, molluscum contagiosum papules may grow over time and cause pruritus. However, this diagnosis is less likely given the absence of other lesions on the skin, lack of known contacts with similar lesions, and yellowish color without a more typical appearance of molluscum.
Dermatofibromas occur in people of all ages, although more commonly between the ages of 20 and 40 and in those with a history of trauma at the lesion. Like JXGs, dermatofibromas tend to be firm, solitary papules or nodules. They usually are hyperpigmented, and classically “dimple when pinched” as they are fixed to the subcutaneous tissue. However, this patient’s age, lack of trauma, and the lesion morphology are not consistent with dermatofibromas.
Like XJGs, mastocytomas commonly present in the first 2 years of life with maculopapular or nodular lesions that itch. However, the history of new-onset itch in recent months as the lesion grew larger and the yellow color on dermoscopy are more consistent with JXG.
Eruptive xanthomas typically appear suddenly as multiple erythematous yellow, dome-shaped papules on the extensor surfaces of the extremities, buttocks, and hands. They are usually present with hypertriglyceridemia and are very rare in young children. The presence of a solitary lesion in a 6-month-old patient without a history of lipid abnormalities favors the diagnosis of XJG.
Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Ms. Kleinman is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology, University of California, San Diego, and Rady Children’s Hospital. Dr. Eichenfield and Ms. Kleinman have no relevant financial disclosures.
References
Hernandez-Martin A et al. J Am Acad Dermatol. 1997 Mar;36(3 Pt 1):355-67.
Prendiville J. Lumps, bumps and hamartomas in “Neonatal and Infant Dermatology,” 3rd ed. (Philadelphia: Elsevier, 2015).
Püttgen KB. Juvenile xanthogranuloma. UpToDate, 2021.
Schaffer JV. Am J Clin Dermatol. 2021 Mar;22(2):205-20.
VA Turns to Telehealth to Address Delays in Genetic Counseling
The U.S. Department of Veteran Affairs (VA) has been unable to provide genetic counseling to veterans at the same level as the civilian community, and other gaps exist, a genetic counselor told oncologist and hematologist colleagues. The good news is that telemedicine is turning out to be a valuable and proven way to reach veterans who need this kind of care, she said, although certain patients are being left behind.
“To me, telehealth is no doubt the way to go. But it is really important that we continue to look into these disparities, what's causing them, and how we can find a path forward,” said
Deborah Hartzfeld, MS, CGC, of the Genomic Medicine Service based in Salt Lake City, Utah. She spoke in a presentation at the 2021 annual meeting of the Association of VA Hematology/Oncology (AVAHO) that was held virtually and in person in Denver, Colorado, from September 24 to September 26, 2021.
As Harzfeld explained, the genetic counselor workforce is expanding along with the number of indications for genetic testing, especially in cancer, “where the need for germline genetic testing for inherited cancer genes becomes broader every year.”
Genetic counselors are a homogenous group, she said, as revealed by a 2021 survey of most of the nation’s 5,629 certified generic counselors. The North American survey, by the National Society of Genetic Counselors, found that 94% of respondents identified themselves as female, and 90% were white/non-Hispanic.
The survey report also noted that “the genetic counseling profession has grown by over 100% in the last 10 years and is expected to grow another 100% over the next 10 years. By 2025 there should be nearly 7,500 certified genetic counselors, and by 2030 there are likely to be over 10,000.”
Genetic counseling within the VA has also grown rapidly. In 2010, Harzfeld said, about 737 veterans were referred for the service. In 2020, the number was about 10,000, with about half referred for personal or family history of cancer.
The VA has 18 genetic counselors, not all of whom are actively seeing patients or working full time, she said. “Per the National Society of Genetic Counselors, there's one clinical genetic counselor per 100,000 people in the general population,” she said. “It's one for about 474,000 in the VA.”
Wait times for genetic counseling within the VA exceed Mission Act standards outside of urgent referrals in matters such as surgical or medical management, she said. “We usually see those patients within a week, but other folks have to wait or are referred into the community. It remains unclear how many of our patients could access care easily in the community or what the wait times at any individual VA will be.”
Fortunately, she said, telemedicine has increased access to genetic counseling within the general population and the VA, Harzfeld said. “A recent systematic evidence review found providing genetic counseling via video or telephone is comparable to in-person care, it increases access and it's likely feasible and acceptable to major stakeholders. It's worth noting that the data in this evidence review was collected prior to COVID-19 when fewer programs were using telehealth.”
Genetic counseling works especially well via telehealth because counselors don’t perform physical examinations, she said. “Prior to COVID, service probably saw maybe 4 VVC [VA Video Connect] appointments per month for genetic counseling. Now, VVC makes up about 70% of our new patient encounters. About 25% are telephone and about 5% are clinical video telehealth where the veteran goes into their clinic to be seated in front of the machine.”
Research has suggested that non-White patients are 40 to 50% less likely to be referred to telehealth for genetic counseling vs. in-person encounters, she said, although women in general (including black women) are more likely to be referred.
Harzfeld highlighted several challenges facing genetic counseling in the VA. She notes that contracted laboratories aren’t “really set up to be experts in germline genetic testing, so they’re not as nimble, and their test catalogs are not most likely going to be as comprehensive enough for what is needed.” Also, she said, “test ordering can be quite burdensome.”
“We need to continue working with various partners to increase access and the ease of ordering genetic testing,” she said.
Hartzfeld reports no disclosures.
The U.S. Department of Veteran Affairs (VA) has been unable to provide genetic counseling to veterans at the same level as the civilian community, and other gaps exist, a genetic counselor told oncologist and hematologist colleagues. The good news is that telemedicine is turning out to be a valuable and proven way to reach veterans who need this kind of care, she said, although certain patients are being left behind.
“To me, telehealth is no doubt the way to go. But it is really important that we continue to look into these disparities, what's causing them, and how we can find a path forward,” said
Deborah Hartzfeld, MS, CGC, of the Genomic Medicine Service based in Salt Lake City, Utah. She spoke in a presentation at the 2021 annual meeting of the Association of VA Hematology/Oncology (AVAHO) that was held virtually and in person in Denver, Colorado, from September 24 to September 26, 2021.
As Harzfeld explained, the genetic counselor workforce is expanding along with the number of indications for genetic testing, especially in cancer, “where the need for germline genetic testing for inherited cancer genes becomes broader every year.”
Genetic counselors are a homogenous group, she said, as revealed by a 2021 survey of most of the nation’s 5,629 certified generic counselors. The North American survey, by the National Society of Genetic Counselors, found that 94% of respondents identified themselves as female, and 90% were white/non-Hispanic.
The survey report also noted that “the genetic counseling profession has grown by over 100% in the last 10 years and is expected to grow another 100% over the next 10 years. By 2025 there should be nearly 7,500 certified genetic counselors, and by 2030 there are likely to be over 10,000.”
Genetic counseling within the VA has also grown rapidly. In 2010, Harzfeld said, about 737 veterans were referred for the service. In 2020, the number was about 10,000, with about half referred for personal or family history of cancer.
The VA has 18 genetic counselors, not all of whom are actively seeing patients or working full time, she said. “Per the National Society of Genetic Counselors, there's one clinical genetic counselor per 100,000 people in the general population,” she said. “It's one for about 474,000 in the VA.”
Wait times for genetic counseling within the VA exceed Mission Act standards outside of urgent referrals in matters such as surgical or medical management, she said. “We usually see those patients within a week, but other folks have to wait or are referred into the community. It remains unclear how many of our patients could access care easily in the community or what the wait times at any individual VA will be.”
Fortunately, she said, telemedicine has increased access to genetic counseling within the general population and the VA, Harzfeld said. “A recent systematic evidence review found providing genetic counseling via video or telephone is comparable to in-person care, it increases access and it's likely feasible and acceptable to major stakeholders. It's worth noting that the data in this evidence review was collected prior to COVID-19 when fewer programs were using telehealth.”
Genetic counseling works especially well via telehealth because counselors don’t perform physical examinations, she said. “Prior to COVID, service probably saw maybe 4 VVC [VA Video Connect] appointments per month for genetic counseling. Now, VVC makes up about 70% of our new patient encounters. About 25% are telephone and about 5% are clinical video telehealth where the veteran goes into their clinic to be seated in front of the machine.”
Research has suggested that non-White patients are 40 to 50% less likely to be referred to telehealth for genetic counseling vs. in-person encounters, she said, although women in general (including black women) are more likely to be referred.
Harzfeld highlighted several challenges facing genetic counseling in the VA. She notes that contracted laboratories aren’t “really set up to be experts in germline genetic testing, so they’re not as nimble, and their test catalogs are not most likely going to be as comprehensive enough for what is needed.” Also, she said, “test ordering can be quite burdensome.”
“We need to continue working with various partners to increase access and the ease of ordering genetic testing,” she said.
Hartzfeld reports no disclosures.
The U.S. Department of Veteran Affairs (VA) has been unable to provide genetic counseling to veterans at the same level as the civilian community, and other gaps exist, a genetic counselor told oncologist and hematologist colleagues. The good news is that telemedicine is turning out to be a valuable and proven way to reach veterans who need this kind of care, she said, although certain patients are being left behind.
“To me, telehealth is no doubt the way to go. But it is really important that we continue to look into these disparities, what's causing them, and how we can find a path forward,” said
Deborah Hartzfeld, MS, CGC, of the Genomic Medicine Service based in Salt Lake City, Utah. She spoke in a presentation at the 2021 annual meeting of the Association of VA Hematology/Oncology (AVAHO) that was held virtually and in person in Denver, Colorado, from September 24 to September 26, 2021.
As Harzfeld explained, the genetic counselor workforce is expanding along with the number of indications for genetic testing, especially in cancer, “where the need for germline genetic testing for inherited cancer genes becomes broader every year.”
Genetic counselors are a homogenous group, she said, as revealed by a 2021 survey of most of the nation’s 5,629 certified generic counselors. The North American survey, by the National Society of Genetic Counselors, found that 94% of respondents identified themselves as female, and 90% were white/non-Hispanic.
The survey report also noted that “the genetic counseling profession has grown by over 100% in the last 10 years and is expected to grow another 100% over the next 10 years. By 2025 there should be nearly 7,500 certified genetic counselors, and by 2030 there are likely to be over 10,000.”
Genetic counseling within the VA has also grown rapidly. In 2010, Harzfeld said, about 737 veterans were referred for the service. In 2020, the number was about 10,000, with about half referred for personal or family history of cancer.
The VA has 18 genetic counselors, not all of whom are actively seeing patients or working full time, she said. “Per the National Society of Genetic Counselors, there's one clinical genetic counselor per 100,000 people in the general population,” she said. “It's one for about 474,000 in the VA.”
Wait times for genetic counseling within the VA exceed Mission Act standards outside of urgent referrals in matters such as surgical or medical management, she said. “We usually see those patients within a week, but other folks have to wait or are referred into the community. It remains unclear how many of our patients could access care easily in the community or what the wait times at any individual VA will be.”
Fortunately, she said, telemedicine has increased access to genetic counseling within the general population and the VA, Harzfeld said. “A recent systematic evidence review found providing genetic counseling via video or telephone is comparable to in-person care, it increases access and it's likely feasible and acceptable to major stakeholders. It's worth noting that the data in this evidence review was collected prior to COVID-19 when fewer programs were using telehealth.”
Genetic counseling works especially well via telehealth because counselors don’t perform physical examinations, she said. “Prior to COVID, service probably saw maybe 4 VVC [VA Video Connect] appointments per month for genetic counseling. Now, VVC makes up about 70% of our new patient encounters. About 25% are telephone and about 5% are clinical video telehealth where the veteran goes into their clinic to be seated in front of the machine.”
Research has suggested that non-White patients are 40 to 50% less likely to be referred to telehealth for genetic counseling vs. in-person encounters, she said, although women in general (including black women) are more likely to be referred.
Harzfeld highlighted several challenges facing genetic counseling in the VA. She notes that contracted laboratories aren’t “really set up to be experts in germline genetic testing, so they’re not as nimble, and their test catalogs are not most likely going to be as comprehensive enough for what is needed.” Also, she said, “test ordering can be quite burdensome.”
“We need to continue working with various partners to increase access and the ease of ordering genetic testing,” she said.
Hartzfeld reports no disclosures.
Drug cocktail significantly reduced severe COVID, death in outpatients
A monoclonal antibody combination of casirivimab and imdevimab (REGEN-COV) significantly reduced the risk of COVID-19–related hospitalizations and death from any cause in the phase 3 portion of an adaptive trial of outpatients.
Researchers, led by David Weinreich, MD, MBA, executive vice president of the drug cocktail’s manufacturer Regeneron, found in the randomized trial that the combination also resolved symptoms and reduced the SARS-CoV-2 viral load more quickly, compared with placebo.
Findings were published in the New England Journal of Medicine.
COVID-related hospitalization or death from any cause occurred in 18 of 1,355 patients (1.3%) in the group getting 2,400 mg infusions of the study drug, compared with 62 (4.6%) of 1,341 in the matching placebo group, indicating a relative risk reduction of 71.3%; P < .001.
Sunil Joshi, MD, president of the Duval County Medical Society Foundation and an immunologist in Jacksonville, Fla., said in an interview that these findings confirm benefits of REGEN-COV and are very good news for a patient group that includes those age 65 and older with high blood pressure, diabetes, or obesity; and for people not vaccinated, who are all at high risk of hospitalization or death if they get COVID-19.
“Vaccines are critically important,” he said, “but if you were to be infected and know that there’s a way to keep yourself out of the hospital, this is very good news.”
Researchers seek lowest doses
This trial found that the effect was similar when researchers cut the doses in half. These outcomes occurred in 7 of 736 (1%) of patients given 1,200 mg of REGEN-COV and in 24 (3.2%) of 748 in the matching placebo group (relative risk reduction, 70.4%; P = .002).
Symptoms were resolved on average 4 days earlier with each REGEN-COV dose than with placebo (10 days vs. 14 days; P < .001 for both comparisons).
Dr. Weinreich said in an interview that trials will continue to find the lowest effective doses that can stand up to all evolving variants.
“This is one of those settings where you don’t want to underdose. You’ve got one shot at this,” he said. “We’d love to do lower doses. It would be more convenient and we could treat more patients, but if it generates more clinical failures or doesn’t work with certain variants, then you’ve done a huge disservice to the world.”
Also new in this study is that researchers tested not only seronegative patients, but patients at high risk regardless of blood antibody status, he said.
“It’s the first suggestion of data that if you’re breaking through a vaccine and you’re at high risk, the use of the cocktail is something to strongly consider because treatment early is better than treatment later,” Dr. Weinreich said.
In addition to efficacy, the phase 3 trial demonstrated the cocktail had a good safety profile. Serious adverse events occurred more often in the placebo group (4%) than in the 1,200-mg group (1.1%) and the 2,400-mg group (1.3%). Infusion reactions (grade 2 or higher) occurred in less than 0.3% of patients in all groups.
William Fales, MD, state medical director for the Michigan Department of Health and Human Services, said the results confirm the promise of REGEN-COV for reducing hospitalizations and death in a peer-reviewed publication.
COVID-19 a moving target
However, Dr. Fales noted that COVID-19 is a moving target with emerging variants. The criteria for populations at high risk have also broadened since the start of the study, he said.
“A great example is pregnancy is now included as high risk, and that would have likely been a specific contraindication of patients in this clinical trial,” he said.
Dr. Fales said Michigan has been using both REGEN-COV and the Eli Lilly combination of bamlanivimab and etesevimab, which also has an emergency use authorization (EUA) from the Food and Drug Administration, with positive results.
REGEN-COV has an EUA to treat people who are at high risk of serious consequences from COVID-19, including those who are already infected (nonhospitalized) or those in certain postexposure prophylaxis settings.
“We’re seeing very low hospitalization rates and few deaths in a state that is predominately Delta,” Dr. Fales said. “So, this makes us feel that we’re doing the right thing and supports the current efforts around the country to make monoclonal antibody therapy available to high-risk patients.”
Dr. Joshi noted that trial results have been emerging from other monoclonal antibody cocktails with different COVID-19 patient groups.
However, he said in an interview, “how much more effective they would be than this is something we’d have to look at, as 71% effectiveness in keeping people out of the hospital is pretty good for any treatment.”
“These are great numbers, but vaccination itself keeps you from getting the disease in the first place and not just for a short time period. This treatment is just that – a treatment. It gets you through that episode but it doesn’t mean you won’t get sick again. You don’t develop an immune response as you do with the vaccine,” he said.
Dr. Weinreich agreed: “This is not a substitute for a vaccine except for the small group who get the vaccine and their bodies can’t respond to it because they’re significantly immunocompromised.”
The results from this paper “are one piece of a large, multistudy, phase 3 program that basically spans from prophylaxis all the way to hospitalization and pretty much the gamut – all of them – have worked. All of these studies have shown dramatic improvement in whatever the definitive regulatory endpoint is,” Dr. Weinreich said.
He said discussions are ongoing for full regulatory approval in the United States and for expanding the EUA for other populations, including pre-exposure prophylaxis, “which the [United Kingdom’s] authority has already granted us but the FDA has not.”
The study is funded by Regeneron and the Department of Health & Human Services. Dr. Weinreich is a vice president of Regeneron. Dr. Joshi reported no relevant financial relationships. Dr. Fales holds stock in Eli Lilly.
A version of this article first appeared on Medscape.com.
A monoclonal antibody combination of casirivimab and imdevimab (REGEN-COV) significantly reduced the risk of COVID-19–related hospitalizations and death from any cause in the phase 3 portion of an adaptive trial of outpatients.
Researchers, led by David Weinreich, MD, MBA, executive vice president of the drug cocktail’s manufacturer Regeneron, found in the randomized trial that the combination also resolved symptoms and reduced the SARS-CoV-2 viral load more quickly, compared with placebo.
Findings were published in the New England Journal of Medicine.
COVID-related hospitalization or death from any cause occurred in 18 of 1,355 patients (1.3%) in the group getting 2,400 mg infusions of the study drug, compared with 62 (4.6%) of 1,341 in the matching placebo group, indicating a relative risk reduction of 71.3%; P < .001.
Sunil Joshi, MD, president of the Duval County Medical Society Foundation and an immunologist in Jacksonville, Fla., said in an interview that these findings confirm benefits of REGEN-COV and are very good news for a patient group that includes those age 65 and older with high blood pressure, diabetes, or obesity; and for people not vaccinated, who are all at high risk of hospitalization or death if they get COVID-19.
“Vaccines are critically important,” he said, “but if you were to be infected and know that there’s a way to keep yourself out of the hospital, this is very good news.”
Researchers seek lowest doses
This trial found that the effect was similar when researchers cut the doses in half. These outcomes occurred in 7 of 736 (1%) of patients given 1,200 mg of REGEN-COV and in 24 (3.2%) of 748 in the matching placebo group (relative risk reduction, 70.4%; P = .002).
Symptoms were resolved on average 4 days earlier with each REGEN-COV dose than with placebo (10 days vs. 14 days; P < .001 for both comparisons).
Dr. Weinreich said in an interview that trials will continue to find the lowest effective doses that can stand up to all evolving variants.
“This is one of those settings where you don’t want to underdose. You’ve got one shot at this,” he said. “We’d love to do lower doses. It would be more convenient and we could treat more patients, but if it generates more clinical failures or doesn’t work with certain variants, then you’ve done a huge disservice to the world.”
Also new in this study is that researchers tested not only seronegative patients, but patients at high risk regardless of blood antibody status, he said.
“It’s the first suggestion of data that if you’re breaking through a vaccine and you’re at high risk, the use of the cocktail is something to strongly consider because treatment early is better than treatment later,” Dr. Weinreich said.
In addition to efficacy, the phase 3 trial demonstrated the cocktail had a good safety profile. Serious adverse events occurred more often in the placebo group (4%) than in the 1,200-mg group (1.1%) and the 2,400-mg group (1.3%). Infusion reactions (grade 2 or higher) occurred in less than 0.3% of patients in all groups.
William Fales, MD, state medical director for the Michigan Department of Health and Human Services, said the results confirm the promise of REGEN-COV for reducing hospitalizations and death in a peer-reviewed publication.
COVID-19 a moving target
However, Dr. Fales noted that COVID-19 is a moving target with emerging variants. The criteria for populations at high risk have also broadened since the start of the study, he said.
“A great example is pregnancy is now included as high risk, and that would have likely been a specific contraindication of patients in this clinical trial,” he said.
Dr. Fales said Michigan has been using both REGEN-COV and the Eli Lilly combination of bamlanivimab and etesevimab, which also has an emergency use authorization (EUA) from the Food and Drug Administration, with positive results.
REGEN-COV has an EUA to treat people who are at high risk of serious consequences from COVID-19, including those who are already infected (nonhospitalized) or those in certain postexposure prophylaxis settings.
“We’re seeing very low hospitalization rates and few deaths in a state that is predominately Delta,” Dr. Fales said. “So, this makes us feel that we’re doing the right thing and supports the current efforts around the country to make monoclonal antibody therapy available to high-risk patients.”
Dr. Joshi noted that trial results have been emerging from other monoclonal antibody cocktails with different COVID-19 patient groups.
However, he said in an interview, “how much more effective they would be than this is something we’d have to look at, as 71% effectiveness in keeping people out of the hospital is pretty good for any treatment.”
“These are great numbers, but vaccination itself keeps you from getting the disease in the first place and not just for a short time period. This treatment is just that – a treatment. It gets you through that episode but it doesn’t mean you won’t get sick again. You don’t develop an immune response as you do with the vaccine,” he said.
Dr. Weinreich agreed: “This is not a substitute for a vaccine except for the small group who get the vaccine and their bodies can’t respond to it because they’re significantly immunocompromised.”
The results from this paper “are one piece of a large, multistudy, phase 3 program that basically spans from prophylaxis all the way to hospitalization and pretty much the gamut – all of them – have worked. All of these studies have shown dramatic improvement in whatever the definitive regulatory endpoint is,” Dr. Weinreich said.
He said discussions are ongoing for full regulatory approval in the United States and for expanding the EUA for other populations, including pre-exposure prophylaxis, “which the [United Kingdom’s] authority has already granted us but the FDA has not.”
The study is funded by Regeneron and the Department of Health & Human Services. Dr. Weinreich is a vice president of Regeneron. Dr. Joshi reported no relevant financial relationships. Dr. Fales holds stock in Eli Lilly.
A version of this article first appeared on Medscape.com.
A monoclonal antibody combination of casirivimab and imdevimab (REGEN-COV) significantly reduced the risk of COVID-19–related hospitalizations and death from any cause in the phase 3 portion of an adaptive trial of outpatients.
Researchers, led by David Weinreich, MD, MBA, executive vice president of the drug cocktail’s manufacturer Regeneron, found in the randomized trial that the combination also resolved symptoms and reduced the SARS-CoV-2 viral load more quickly, compared with placebo.
Findings were published in the New England Journal of Medicine.
COVID-related hospitalization or death from any cause occurred in 18 of 1,355 patients (1.3%) in the group getting 2,400 mg infusions of the study drug, compared with 62 (4.6%) of 1,341 in the matching placebo group, indicating a relative risk reduction of 71.3%; P < .001.
Sunil Joshi, MD, president of the Duval County Medical Society Foundation and an immunologist in Jacksonville, Fla., said in an interview that these findings confirm benefits of REGEN-COV and are very good news for a patient group that includes those age 65 and older with high blood pressure, diabetes, or obesity; and for people not vaccinated, who are all at high risk of hospitalization or death if they get COVID-19.
“Vaccines are critically important,” he said, “but if you were to be infected and know that there’s a way to keep yourself out of the hospital, this is very good news.”
Researchers seek lowest doses
This trial found that the effect was similar when researchers cut the doses in half. These outcomes occurred in 7 of 736 (1%) of patients given 1,200 mg of REGEN-COV and in 24 (3.2%) of 748 in the matching placebo group (relative risk reduction, 70.4%; P = .002).
Symptoms were resolved on average 4 days earlier with each REGEN-COV dose than with placebo (10 days vs. 14 days; P < .001 for both comparisons).
Dr. Weinreich said in an interview that trials will continue to find the lowest effective doses that can stand up to all evolving variants.
“This is one of those settings where you don’t want to underdose. You’ve got one shot at this,” he said. “We’d love to do lower doses. It would be more convenient and we could treat more patients, but if it generates more clinical failures or doesn’t work with certain variants, then you’ve done a huge disservice to the world.”
Also new in this study is that researchers tested not only seronegative patients, but patients at high risk regardless of blood antibody status, he said.
“It’s the first suggestion of data that if you’re breaking through a vaccine and you’re at high risk, the use of the cocktail is something to strongly consider because treatment early is better than treatment later,” Dr. Weinreich said.
In addition to efficacy, the phase 3 trial demonstrated the cocktail had a good safety profile. Serious adverse events occurred more often in the placebo group (4%) than in the 1,200-mg group (1.1%) and the 2,400-mg group (1.3%). Infusion reactions (grade 2 or higher) occurred in less than 0.3% of patients in all groups.
William Fales, MD, state medical director for the Michigan Department of Health and Human Services, said the results confirm the promise of REGEN-COV for reducing hospitalizations and death in a peer-reviewed publication.
COVID-19 a moving target
However, Dr. Fales noted that COVID-19 is a moving target with emerging variants. The criteria for populations at high risk have also broadened since the start of the study, he said.
“A great example is pregnancy is now included as high risk, and that would have likely been a specific contraindication of patients in this clinical trial,” he said.
Dr. Fales said Michigan has been using both REGEN-COV and the Eli Lilly combination of bamlanivimab and etesevimab, which also has an emergency use authorization (EUA) from the Food and Drug Administration, with positive results.
REGEN-COV has an EUA to treat people who are at high risk of serious consequences from COVID-19, including those who are already infected (nonhospitalized) or those in certain postexposure prophylaxis settings.
“We’re seeing very low hospitalization rates and few deaths in a state that is predominately Delta,” Dr. Fales said. “So, this makes us feel that we’re doing the right thing and supports the current efforts around the country to make monoclonal antibody therapy available to high-risk patients.”
Dr. Joshi noted that trial results have been emerging from other monoclonal antibody cocktails with different COVID-19 patient groups.
However, he said in an interview, “how much more effective they would be than this is something we’d have to look at, as 71% effectiveness in keeping people out of the hospital is pretty good for any treatment.”
“These are great numbers, but vaccination itself keeps you from getting the disease in the first place and not just for a short time period. This treatment is just that – a treatment. It gets you through that episode but it doesn’t mean you won’t get sick again. You don’t develop an immune response as you do with the vaccine,” he said.
Dr. Weinreich agreed: “This is not a substitute for a vaccine except for the small group who get the vaccine and their bodies can’t respond to it because they’re significantly immunocompromised.”
The results from this paper “are one piece of a large, multistudy, phase 3 program that basically spans from prophylaxis all the way to hospitalization and pretty much the gamut – all of them – have worked. All of these studies have shown dramatic improvement in whatever the definitive regulatory endpoint is,” Dr. Weinreich said.
He said discussions are ongoing for full regulatory approval in the United States and for expanding the EUA for other populations, including pre-exposure prophylaxis, “which the [United Kingdom’s] authority has already granted us but the FDA has not.”
The study is funded by Regeneron and the Department of Health & Human Services. Dr. Weinreich is a vice president of Regeneron. Dr. Joshi reported no relevant financial relationships. Dr. Fales holds stock in Eli Lilly.
A version of this article first appeared on Medscape.com.
Linked-color imaging outperforms other modalities at adenoma detection
Linked-color imaging (LCI) significantly increases the detection of adenomas in screening colonoscopies compared to white-light imaging (WLI) and blue-laser imaging (BLI)–bright, according to data from 205 adults who underwent screening colonoscopies.
LCI is a relatively new image-enhancement method designed to better identify adenomatous lesions by increasing the contrast of the mucosal surface, wrote Carlos E.O. dos Santos, MD, of Pontifícia Universidade Católica do Rio Grande do Sul in Porto Alegre, Brazil, and colleagues. Their report is in the Journal of Clinical Gastroenterology. With LCI, the lesions are more vascularized, and thus become reddish due to color contrast of hemoglobin present in capillary vessels, whereas the surrounding mucosa becomes whitish. Until this new study, the potential of LCI to detect adenomas compared with other imaging had not been evaluated.
The researchers randomized 205 patients with a total of 296 colorectal lesions to WLI, BLI-bright, or LCI; 70 patients were examined by WLI, 66 by BLI-bright, and 69 by LCI. The average age of the patients was 59 years, and 52% were women. The primary outcome measures were adenoma detection rate (ADR), mean number of adenomas per patient, and withdrawal time.
A total of 251 adenomas were detected, with an overall ADR of 62%. The total number of adenomas detected by each method were 112 by LCI, 71 by WLI, and 68 by BLI-bright.
The ADR was significantly higher for patients in the LCI group compared with those in the WLI group (71% vs. 52.9%, P = .04). ADR for LCI was greater than the ADR for BLI-bright, but the difference was not significant (71% vs. 62.1%, P = .28). No significant differences in ADR were noted between the WLI and BLI-bright groups.
The mean number of adenomas identified per patient was 1.17 overall, but significantly higher in the LCI group compared to the WLI and BLI-bright groups (1.62, 1.01, and 1.03, respectively, P = .02). Mean withdrawal times were not significantly different among the three groups and ranged from approximately 10 to 11 minutes. An analysis of secondary outcomes showed no differences among the groups in terms of size and morphology of the adenomas, or in the detection of sessile serrated adenomas or polyps.
The researchers noted that the study findings were limited by several factors including the use of data from a single center with a high level of experience in image-enhanced endoscopy and by the relatively small sample size.
Nevertheless, concluded the researchers, “It is evident that better visibility of the mucosa is a key factor for the detection of neoplastic lesions,” and the results support the potential of LCI given the demonstrated superiority of LCI over WLI for colorectal adenoma detection and the mean number of adenomas detected per patient.
The researchers said that further single and multicenter randomized studies are needed to validate the results and to confirm whether one image-enhancement system is superior to the other for increasing the ADR.
Door is open for better detection tools
In an interview, Atsushi Sakuraba, MD, of the University of Chicago, who was not involved with the study, said that colonoscopy is considered the best method for colorectal cancer screening and prevention, but is associated with a certain risk of missing adenomas, so new methods and technologies to improve detection rate are needed. “Linked-color imaging provides an increased contrast of the mucosal surface and enhances the findings of adenomatous lesions in comparison to white-light endoscopy and has been shown to be effective in detecting adenomas, so the findings of the present study are not surprising,” said Dr. Sakuraba.
LCI provides clearer and brighter images by enhancing the differences in color contrast, and therefore does not cause the impaired visibility that can occur with narrow band imaging or BLI images, Dr. Sakuraba said. However, he noted, not all endoscopy centers carry the scopes equipped with LCI, which is a barrier to widespread use.
Dr. Sakuraba said that multicenter studies need to be undertaken to confirm the generalizability of the results of the present study.
“There is now convincing evidence that increasing adenoma detection rate is associated with fewer missed cancers and lower mortality from colorectal cancer,” said Ziad F. Gellad, MD, of Duke University, Durham, N.C., who was also not involved with the study. “As such, utilizing tools that enhance ADR may improve our ability to prevent colorectal cancer. ... Understanding the relative benefits and drawbacks of available tools and technologies in the market can help practicing gastroenterologists decide where to invest their time and resources to improve care.”
Dr. Gellad said he was not surprised by the enhanced detection using LCI, as the study is not the first to evaluate this technology. “However, I was surprised by how high the ADR was in the screening population (62%),” said Dr. Gellad, observing that this exceeds benchmarks set by the society. “We don’t have a full understanding of the demographic characteristics of this screening population. ... Nonetheless, I think this paper adds to accumulating data that current benchmarks may be too low.”
Dr. Gellad said he didn’t think the findings of the study are strong enough to change practice, but the results are a “valuable contribution to the literature and will empower future larger studies as well as meta-analyses.” He called for larger studies in nonspecialized centers to relate the findings from this small study to general practice.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Sakuraba disclosed collaborative research relationships with Fuji, the manufacturer of the imaging equipment used in the study. Dr. Gellad had no financial conflicts to disclose but serves on the editorial board of GI & Hepatology News.
Linked-color imaging (LCI) significantly increases the detection of adenomas in screening colonoscopies compared to white-light imaging (WLI) and blue-laser imaging (BLI)–bright, according to data from 205 adults who underwent screening colonoscopies.
LCI is a relatively new image-enhancement method designed to better identify adenomatous lesions by increasing the contrast of the mucosal surface, wrote Carlos E.O. dos Santos, MD, of Pontifícia Universidade Católica do Rio Grande do Sul in Porto Alegre, Brazil, and colleagues. Their report is in the Journal of Clinical Gastroenterology. With LCI, the lesions are more vascularized, and thus become reddish due to color contrast of hemoglobin present in capillary vessels, whereas the surrounding mucosa becomes whitish. Until this new study, the potential of LCI to detect adenomas compared with other imaging had not been evaluated.
The researchers randomized 205 patients with a total of 296 colorectal lesions to WLI, BLI-bright, or LCI; 70 patients were examined by WLI, 66 by BLI-bright, and 69 by LCI. The average age of the patients was 59 years, and 52% were women. The primary outcome measures were adenoma detection rate (ADR), mean number of adenomas per patient, and withdrawal time.
A total of 251 adenomas were detected, with an overall ADR of 62%. The total number of adenomas detected by each method were 112 by LCI, 71 by WLI, and 68 by BLI-bright.
The ADR was significantly higher for patients in the LCI group compared with those in the WLI group (71% vs. 52.9%, P = .04). ADR for LCI was greater than the ADR for BLI-bright, but the difference was not significant (71% vs. 62.1%, P = .28). No significant differences in ADR were noted between the WLI and BLI-bright groups.
The mean number of adenomas identified per patient was 1.17 overall, but significantly higher in the LCI group compared to the WLI and BLI-bright groups (1.62, 1.01, and 1.03, respectively, P = .02). Mean withdrawal times were not significantly different among the three groups and ranged from approximately 10 to 11 minutes. An analysis of secondary outcomes showed no differences among the groups in terms of size and morphology of the adenomas, or in the detection of sessile serrated adenomas or polyps.
The researchers noted that the study findings were limited by several factors including the use of data from a single center with a high level of experience in image-enhanced endoscopy and by the relatively small sample size.
Nevertheless, concluded the researchers, “It is evident that better visibility of the mucosa is a key factor for the detection of neoplastic lesions,” and the results support the potential of LCI given the demonstrated superiority of LCI over WLI for colorectal adenoma detection and the mean number of adenomas detected per patient.
The researchers said that further single and multicenter randomized studies are needed to validate the results and to confirm whether one image-enhancement system is superior to the other for increasing the ADR.
Door is open for better detection tools
In an interview, Atsushi Sakuraba, MD, of the University of Chicago, who was not involved with the study, said that colonoscopy is considered the best method for colorectal cancer screening and prevention, but is associated with a certain risk of missing adenomas, so new methods and technologies to improve detection rate are needed. “Linked-color imaging provides an increased contrast of the mucosal surface and enhances the findings of adenomatous lesions in comparison to white-light endoscopy and has been shown to be effective in detecting adenomas, so the findings of the present study are not surprising,” said Dr. Sakuraba.
LCI provides clearer and brighter images by enhancing the differences in color contrast, and therefore does not cause the impaired visibility that can occur with narrow band imaging or BLI images, Dr. Sakuraba said. However, he noted, not all endoscopy centers carry the scopes equipped with LCI, which is a barrier to widespread use.
Dr. Sakuraba said that multicenter studies need to be undertaken to confirm the generalizability of the results of the present study.
“There is now convincing evidence that increasing adenoma detection rate is associated with fewer missed cancers and lower mortality from colorectal cancer,” said Ziad F. Gellad, MD, of Duke University, Durham, N.C., who was also not involved with the study. “As such, utilizing tools that enhance ADR may improve our ability to prevent colorectal cancer. ... Understanding the relative benefits and drawbacks of available tools and technologies in the market can help practicing gastroenterologists decide where to invest their time and resources to improve care.”
Dr. Gellad said he was not surprised by the enhanced detection using LCI, as the study is not the first to evaluate this technology. “However, I was surprised by how high the ADR was in the screening population (62%),” said Dr. Gellad, observing that this exceeds benchmarks set by the society. “We don’t have a full understanding of the demographic characteristics of this screening population. ... Nonetheless, I think this paper adds to accumulating data that current benchmarks may be too low.”
Dr. Gellad said he didn’t think the findings of the study are strong enough to change practice, but the results are a “valuable contribution to the literature and will empower future larger studies as well as meta-analyses.” He called for larger studies in nonspecialized centers to relate the findings from this small study to general practice.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Sakuraba disclosed collaborative research relationships with Fuji, the manufacturer of the imaging equipment used in the study. Dr. Gellad had no financial conflicts to disclose but serves on the editorial board of GI & Hepatology News.
Linked-color imaging (LCI) significantly increases the detection of adenomas in screening colonoscopies compared to white-light imaging (WLI) and blue-laser imaging (BLI)–bright, according to data from 205 adults who underwent screening colonoscopies.
LCI is a relatively new image-enhancement method designed to better identify adenomatous lesions by increasing the contrast of the mucosal surface, wrote Carlos E.O. dos Santos, MD, of Pontifícia Universidade Católica do Rio Grande do Sul in Porto Alegre, Brazil, and colleagues. Their report is in the Journal of Clinical Gastroenterology. With LCI, the lesions are more vascularized, and thus become reddish due to color contrast of hemoglobin present in capillary vessels, whereas the surrounding mucosa becomes whitish. Until this new study, the potential of LCI to detect adenomas compared with other imaging had not been evaluated.
The researchers randomized 205 patients with a total of 296 colorectal lesions to WLI, BLI-bright, or LCI; 70 patients were examined by WLI, 66 by BLI-bright, and 69 by LCI. The average age of the patients was 59 years, and 52% were women. The primary outcome measures were adenoma detection rate (ADR), mean number of adenomas per patient, and withdrawal time.
A total of 251 adenomas were detected, with an overall ADR of 62%. The total number of adenomas detected by each method were 112 by LCI, 71 by WLI, and 68 by BLI-bright.
The ADR was significantly higher for patients in the LCI group compared with those in the WLI group (71% vs. 52.9%, P = .04). ADR for LCI was greater than the ADR for BLI-bright, but the difference was not significant (71% vs. 62.1%, P = .28). No significant differences in ADR were noted between the WLI and BLI-bright groups.
The mean number of adenomas identified per patient was 1.17 overall, but significantly higher in the LCI group compared to the WLI and BLI-bright groups (1.62, 1.01, and 1.03, respectively, P = .02). Mean withdrawal times were not significantly different among the three groups and ranged from approximately 10 to 11 minutes. An analysis of secondary outcomes showed no differences among the groups in terms of size and morphology of the adenomas, or in the detection of sessile serrated adenomas or polyps.
The researchers noted that the study findings were limited by several factors including the use of data from a single center with a high level of experience in image-enhanced endoscopy and by the relatively small sample size.
Nevertheless, concluded the researchers, “It is evident that better visibility of the mucosa is a key factor for the detection of neoplastic lesions,” and the results support the potential of LCI given the demonstrated superiority of LCI over WLI for colorectal adenoma detection and the mean number of adenomas detected per patient.
The researchers said that further single and multicenter randomized studies are needed to validate the results and to confirm whether one image-enhancement system is superior to the other for increasing the ADR.
Door is open for better detection tools
In an interview, Atsushi Sakuraba, MD, of the University of Chicago, who was not involved with the study, said that colonoscopy is considered the best method for colorectal cancer screening and prevention, but is associated with a certain risk of missing adenomas, so new methods and technologies to improve detection rate are needed. “Linked-color imaging provides an increased contrast of the mucosal surface and enhances the findings of adenomatous lesions in comparison to white-light endoscopy and has been shown to be effective in detecting adenomas, so the findings of the present study are not surprising,” said Dr. Sakuraba.
LCI provides clearer and brighter images by enhancing the differences in color contrast, and therefore does not cause the impaired visibility that can occur with narrow band imaging or BLI images, Dr. Sakuraba said. However, he noted, not all endoscopy centers carry the scopes equipped with LCI, which is a barrier to widespread use.
Dr. Sakuraba said that multicenter studies need to be undertaken to confirm the generalizability of the results of the present study.
“There is now convincing evidence that increasing adenoma detection rate is associated with fewer missed cancers and lower mortality from colorectal cancer,” said Ziad F. Gellad, MD, of Duke University, Durham, N.C., who was also not involved with the study. “As such, utilizing tools that enhance ADR may improve our ability to prevent colorectal cancer. ... Understanding the relative benefits and drawbacks of available tools and technologies in the market can help practicing gastroenterologists decide where to invest their time and resources to improve care.”
Dr. Gellad said he was not surprised by the enhanced detection using LCI, as the study is not the first to evaluate this technology. “However, I was surprised by how high the ADR was in the screening population (62%),” said Dr. Gellad, observing that this exceeds benchmarks set by the society. “We don’t have a full understanding of the demographic characteristics of this screening population. ... Nonetheless, I think this paper adds to accumulating data that current benchmarks may be too low.”
Dr. Gellad said he didn’t think the findings of the study are strong enough to change practice, but the results are a “valuable contribution to the literature and will empower future larger studies as well as meta-analyses.” He called for larger studies in nonspecialized centers to relate the findings from this small study to general practice.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Sakuraba disclosed collaborative research relationships with Fuji, the manufacturer of the imaging equipment used in the study. Dr. Gellad had no financial conflicts to disclose but serves on the editorial board of GI & Hepatology News.
FROM THE JOURNAL OF CLINICAL GASTROENTEROLOGY
Flu shot highly recommended this year
With the Delta variant of COVID-19 still raging in the United States and ICUs in parts of the country filled with patients with the coronavirus, experts are voicing concern about the added risk of a difficult flu season.
Two mathematical models are predicting a big rebound in the number and severity of flu cases in the 2021-22 season after 2020-2021’s flu season failed to show up when public health measures brought in to control COVID-19 seemed to have the added benefit of stopping the flu.
But both analyses, posted to the medRxiv preprint server and not yet peer reviewed by other experts, have come to the same conclusion: The flu could make a comeback this year.
In the worst-case scenario, the United States could see an extra 300,000-400,000 hospitalizations from the flu – almost double the usual number – according to senior study author Mark Roberts, MD, director of the Public Health Dynamics Laboratory at the University of Pittsburgh. These numbers could be a disaster in areas where hospitals are already filled with COVID-19 patients.
Waning natural immunity in the public because of 2020-2021’s missing flu season could make people, especially young children, more likely to get the virus.
“Usually, a combination of natural immunity and vaccination helps tamp down seasonal influenza,” said Dr. Roberts. “If we don’t have the first part, we’ll have to rely more on the vaccine.”
In a typical year, about half of Americans get the flu shot. The new mathematical models predict that the vaccination rate would need to rise to about 75% to avoid the extra hospitalizations. But even a 10% increase in vaccination rates could reduce hospitalizations by 6%-46%, depending on what strains are dominant.
Usually, the Southern Hemisphere flu season, from February to August, helps show what the Northern Hemisphere can expect over the coming winter. But with strict COVID-19 measures and limits on international travel still in place in countries like Australia and New Zealand and much of South America, it has been another record-low year for flu infections, said Ian Barr, PhD, deputy director of the World Health Organization’s Collaborating Center for Reference and Research on Influenza in Melbourne.
Australia detected only around 500 cases in 2021, compared with about 300,000 in a normal year, and recorded no hospitalizations or deaths from the flu. New Zealand recorded just two cases.
“I’ve never seen anything like this,” Dr. Barr said.
In Australia, the mild flu season led to fewer people getting their flu shot than usual. The rate fell from around 50% to just 33%, said Dr. Barr. “If that happens in the U.S., the population will be even more vulnerable because there has been almost no flu for more than 12 months,” he said.
Both Dr. Roberts and Dr. Barr say it is vital that as many people as possible get vaccinated during the upcoming flu season, especially children who will have almost no natural immunity to the virus.
“The vaccine is our best weapon against the flu, especially for the most at-risk groups,” said Dr. Barr.
Other parts of the world had mixed results. India saw a high number of flu cases, while neighboring Sri Lanka had very few. West Africa also saw quite a high level of circulating virus. Overall, the flu was detected in 45 countries during the Southern Hemisphere season, less than half of what might be expected in a normal year, said Dr. Barr.
Despite the overall low numbers, the WHO saw enough in the data to make two changes to 2022’s Southern Hemisphere vaccine formulation at its meeting on Sept. 24, after changing just one of the strains for the Northern Hemisphere vaccine at its meeting in February.
The CDC recommends that everyone 6 months or older get the flu shot, with few exceptions.
A version of this article first appeared on WebMD.com.
With the Delta variant of COVID-19 still raging in the United States and ICUs in parts of the country filled with patients with the coronavirus, experts are voicing concern about the added risk of a difficult flu season.
Two mathematical models are predicting a big rebound in the number and severity of flu cases in the 2021-22 season after 2020-2021’s flu season failed to show up when public health measures brought in to control COVID-19 seemed to have the added benefit of stopping the flu.
But both analyses, posted to the medRxiv preprint server and not yet peer reviewed by other experts, have come to the same conclusion: The flu could make a comeback this year.
In the worst-case scenario, the United States could see an extra 300,000-400,000 hospitalizations from the flu – almost double the usual number – according to senior study author Mark Roberts, MD, director of the Public Health Dynamics Laboratory at the University of Pittsburgh. These numbers could be a disaster in areas where hospitals are already filled with COVID-19 patients.
Waning natural immunity in the public because of 2020-2021’s missing flu season could make people, especially young children, more likely to get the virus.
“Usually, a combination of natural immunity and vaccination helps tamp down seasonal influenza,” said Dr. Roberts. “If we don’t have the first part, we’ll have to rely more on the vaccine.”
In a typical year, about half of Americans get the flu shot. The new mathematical models predict that the vaccination rate would need to rise to about 75% to avoid the extra hospitalizations. But even a 10% increase in vaccination rates could reduce hospitalizations by 6%-46%, depending on what strains are dominant.
Usually, the Southern Hemisphere flu season, from February to August, helps show what the Northern Hemisphere can expect over the coming winter. But with strict COVID-19 measures and limits on international travel still in place in countries like Australia and New Zealand and much of South America, it has been another record-low year for flu infections, said Ian Barr, PhD, deputy director of the World Health Organization’s Collaborating Center for Reference and Research on Influenza in Melbourne.
Australia detected only around 500 cases in 2021, compared with about 300,000 in a normal year, and recorded no hospitalizations or deaths from the flu. New Zealand recorded just two cases.
“I’ve never seen anything like this,” Dr. Barr said.
In Australia, the mild flu season led to fewer people getting their flu shot than usual. The rate fell from around 50% to just 33%, said Dr. Barr. “If that happens in the U.S., the population will be even more vulnerable because there has been almost no flu for more than 12 months,” he said.
Both Dr. Roberts and Dr. Barr say it is vital that as many people as possible get vaccinated during the upcoming flu season, especially children who will have almost no natural immunity to the virus.
“The vaccine is our best weapon against the flu, especially for the most at-risk groups,” said Dr. Barr.
Other parts of the world had mixed results. India saw a high number of flu cases, while neighboring Sri Lanka had very few. West Africa also saw quite a high level of circulating virus. Overall, the flu was detected in 45 countries during the Southern Hemisphere season, less than half of what might be expected in a normal year, said Dr. Barr.
Despite the overall low numbers, the WHO saw enough in the data to make two changes to 2022’s Southern Hemisphere vaccine formulation at its meeting on Sept. 24, after changing just one of the strains for the Northern Hemisphere vaccine at its meeting in February.
The CDC recommends that everyone 6 months or older get the flu shot, with few exceptions.
A version of this article first appeared on WebMD.com.
With the Delta variant of COVID-19 still raging in the United States and ICUs in parts of the country filled with patients with the coronavirus, experts are voicing concern about the added risk of a difficult flu season.
Two mathematical models are predicting a big rebound in the number and severity of flu cases in the 2021-22 season after 2020-2021’s flu season failed to show up when public health measures brought in to control COVID-19 seemed to have the added benefit of stopping the flu.
But both analyses, posted to the medRxiv preprint server and not yet peer reviewed by other experts, have come to the same conclusion: The flu could make a comeback this year.
In the worst-case scenario, the United States could see an extra 300,000-400,000 hospitalizations from the flu – almost double the usual number – according to senior study author Mark Roberts, MD, director of the Public Health Dynamics Laboratory at the University of Pittsburgh. These numbers could be a disaster in areas where hospitals are already filled with COVID-19 patients.
Waning natural immunity in the public because of 2020-2021’s missing flu season could make people, especially young children, more likely to get the virus.
“Usually, a combination of natural immunity and vaccination helps tamp down seasonal influenza,” said Dr. Roberts. “If we don’t have the first part, we’ll have to rely more on the vaccine.”
In a typical year, about half of Americans get the flu shot. The new mathematical models predict that the vaccination rate would need to rise to about 75% to avoid the extra hospitalizations. But even a 10% increase in vaccination rates could reduce hospitalizations by 6%-46%, depending on what strains are dominant.
Usually, the Southern Hemisphere flu season, from February to August, helps show what the Northern Hemisphere can expect over the coming winter. But with strict COVID-19 measures and limits on international travel still in place in countries like Australia and New Zealand and much of South America, it has been another record-low year for flu infections, said Ian Barr, PhD, deputy director of the World Health Organization’s Collaborating Center for Reference and Research on Influenza in Melbourne.
Australia detected only around 500 cases in 2021, compared with about 300,000 in a normal year, and recorded no hospitalizations or deaths from the flu. New Zealand recorded just two cases.
“I’ve never seen anything like this,” Dr. Barr said.
In Australia, the mild flu season led to fewer people getting their flu shot than usual. The rate fell from around 50% to just 33%, said Dr. Barr. “If that happens in the U.S., the population will be even more vulnerable because there has been almost no flu for more than 12 months,” he said.
Both Dr. Roberts and Dr. Barr say it is vital that as many people as possible get vaccinated during the upcoming flu season, especially children who will have almost no natural immunity to the virus.
“The vaccine is our best weapon against the flu, especially for the most at-risk groups,” said Dr. Barr.
Other parts of the world had mixed results. India saw a high number of flu cases, while neighboring Sri Lanka had very few. West Africa also saw quite a high level of circulating virus. Overall, the flu was detected in 45 countries during the Southern Hemisphere season, less than half of what might be expected in a normal year, said Dr. Barr.
Despite the overall low numbers, the WHO saw enough in the data to make two changes to 2022’s Southern Hemisphere vaccine formulation at its meeting on Sept. 24, after changing just one of the strains for the Northern Hemisphere vaccine at its meeting in February.
The CDC recommends that everyone 6 months or older get the flu shot, with few exceptions.
A version of this article first appeared on WebMD.com.
NIAMS director reflects on her mentors, spotlights research projects underway
After many years at the University of California, San Francisco, Lindsey A. Criswell, MD, MPH, DSc, began a new chapter in February 2021 as the director of the National Institute of Arthritis and Musculoskeletal and Skin Disease, part of the National Institutes of Health. NIH Director Francis S. Collins, MD, PhD, selected her for the post.
“Dr. Criswell has rich experience as a clinician, researcher, and administrator,” Dr. Collins said in a prepared statement. “Her ability to oversee the research program of one of the country’s top research-intensive medical schools, and her expertise in autoimmune diseases, including rheumatoid arthritis and lupus, make her well positioned to direct NIAMS.” Dr. Criswell, a rheumatologist, was named a full professor of medicine at UCSF in 2007 and had served as vice chancellor of research at the university since 2017. She has authored more than 250 peer-reviewed scientific papers, and her efforts have contributed to the identification of more than 30 genes linked to autoimmune disorders. In her first media interview, Dr. Criswell opens up about her mentors, operational challenges posed by the COVID-19 pandemic, and highlights many NIAMS research projects underway.
Who inspired you most early in your career as a physician scientist? I have had great opportunities to work with fabulous mentors. Wallace (Wally) Epstein, MD, was my mentor when I was a rheumatology fellow and junior faculty member at UCSF. He was broadly admired for the breadth of his experience as a clinician and a researcher, and he was noteworthy at that time for his strong support for women and students of color. One of the many things I appreciated about him was his diverse range of interests outside of work, which included cello playing and woodworking.
Another mentor was Ephraim (Eph) Engleman, MD, the first academic rheumatologist in California. Eph continued to see patients beyond the age of 100. Perhaps his most important contributions were his efforts towards advocacy for funding for research and education in rheumatology. A prodigy violinist, he too had a broad range of personal interests.
What research into the genetics and epidemiology of human autoimmune disease that you have been a part of has most surprised you, in term of its ultimate clinical impact? Some of my most rewarding and impactful work has focused on the shared genetic basis of autoimmune diseases. We’ve identified dozens of genes that contribute to the risk and outcome of rheumatoid arthritis, lupus, and other autoimmune disorders. These discoveries regarding shared genes and pathways among such a diverse set of conditions have helped to inform optimal therapeutic target and treatment strategies across multiple diseases. For example, exploration of RA genes and pathways has revealed that approved agents for other conditions, such as cancer, may be appropriately repurposed for the treatment of RA. These are critical observations that have the potential to dramatically accelerate progress in developing new therapies for autoimmune diseases, such as RA.
Did you have much interaction with Stephen I. Katz, MD, PhD, your longtime predecessor who passed away unexpectedly in 2018? If so, what do you remember most about him? I regret that I had very little interaction with Steve, but I am well aware of the impact he had on NIAMS, NIH, and the research enterprise overall. He inspired so many people in a personal way, and I am energized by the legacy that he left behind.
What are your goals for the early part of your tenure as the new director of NIAMS? An important goal is getting to know the NIAMS community and expanding my knowledge of the Institute’s musculoskeletal and skin portfolios. I am also conducting outreach to Institute/Center directors and other NIH leadership to increase opportunities for input and advice. In doing this, I am identifying shared research interests, best practices, and potential partners for possible future collaborations. Another important goal is to increase NIAMS’ visibility within and beyond NIH. Ultimately, I want to contribute to the great work of the Institute and improve the lives of people with rheumatic, musculoskeletal, and skin diseases.
How would you characterize your management style? I like to lead with a flat hierarchy and work collectively to address opportunities and challenges. I value team building and tend to tap a variety of perspectives and expertise at all levels to achieve consensus, where possible.
The Accelerating Medicines Partnership (AMP) program was launched in 2014, with projects in three disease areas including the autoimmune disorders RA and lupus. What are some recent highlights from this program with respect to RA and lupus? AMP RA/SLE was dedicated to identifying promising therapeutic targets for RA and systemic lupus erythematosus. AMP-funded researchers have applied cutting-edge technologies to study cells from the synovial tissues of the joints of people with RA, and from the kidneys of people with lupus nephritis. In 2014, studying tissues in patients where the disease is active was a novel approach, since most research was conducted in mouse models or human blood samples.
The AMP RA/SLE Network developed a rich dataset that is available to the research community. Investigators are now using the data to facilitate RA and lupus research. For example, using AMP data, NIAMS-supported researchers identified potential biomarkers that could help predict an imminent RA flare. Work from another NIAMS-supported group suggests that targeting the regulatory transcription factor HIF-1, which drives inflammation and tissue damage, might be an effective approach for treating renal injury in lupus.
The data generated are accessible to the scientific community through two NIH websites: the database of Genotypes and Phenotypes (dbGaP) and the Immunology Database and Analysis Portal (IMMPORT).
Given the success of AMP RA/SLE, NIH plans to launch an “AMP 2.0” later in 2021. The AMP Autoimmune and Immune-Mediated Diseases (AMP AIM) program will provide an opportunity to leverage the accomplishments of AMP RA/SLE to new conditions, including psoriatic spectrum diseases and Sjögren’s syndrome.
What are some recent highlights from NIAMS-supported research in skin diseases? NIAMS-supported investigators continue to make significant strides in our understanding of skin biology and disease. For example, researchers recently demonstrated that imiquimod, a drug used to treat precancerous skin lesions, can help mouse ear wounds heal without scarring.
Another team addressed the safety and potential benefit of Staphylococcus hominis A9, a bacterium isolated from healthy human skin, as a topical therapy for atopic dermatitis.
Moving forward, AMP AIM will refine and extend the single-cell analysis of tissues to additional diseases, including psoriasis, setting the stage for the discovery of new therapeutic targets for the disease.
How has the COVID-19 pandemic changed the landscape of research, at least for the short term? This is a once-in-a-century pandemic that none of us were fully prepared for. We understand that it has been particularly challenging for women scientists, scientists with young children, and trainees and junior faculty who are at critically important and vulnerable stages of their careers. There isn’t a lab or clinical setting that hasn’t been negatively impacted in some way.
During the pandemic, the NIH instituted administrative flexibilities to support the grantee community, including extensions in time. In addition, the agency has issued several funding opportunities specific to COVID-19, some of which involve NIAMS participation.
What is NIAMS doing to help early/young investigators as well as female investigators and those from minority groups? Structural racism in biomedical research is a heightened concern. Earlier this year, Dr. Collins established the UNITE initiative to address structural racism and promote racial equity and inclusion at the NIH and within the larger biomedical community that we support. NIAMS is fully committed to this effort. One example is the Diversity Supplement Program, which is designed to attract and encourage eligible individuals from underrepresented populations to research careers.
Early-stage investigators are another top priority. In a tribute to the beloved former NIAMS director, NIH recently established the Stephen I. Katz Early Stage Investigator Research Grant Program. The R01 award provides support for a project unrelated to an early investigator’s area of postdoctoral study. (No preliminary data are allowed.) This award mechanism is a unique opportunity for early-stage investigators to take their research in a completely new direction.
Managing work and family life is an important concern, particularly for female investigators. Many NIH grant awards allow for reimbursement of actual, allowable costs incurred for childcare and parental leave. The NIH is exploring initiatives to promote research continuity and retention of eligible investigators facing major life events, such as pregnancy, childbirth, and adoption, at vulnerable career stages.
Who inspires you most in your work today? I am inspired by the ongoing struggles of our patients, junior investigators, and by the committed staff members on my team.
After many years at the University of California, San Francisco, Lindsey A. Criswell, MD, MPH, DSc, began a new chapter in February 2021 as the director of the National Institute of Arthritis and Musculoskeletal and Skin Disease, part of the National Institutes of Health. NIH Director Francis S. Collins, MD, PhD, selected her for the post.
“Dr. Criswell has rich experience as a clinician, researcher, and administrator,” Dr. Collins said in a prepared statement. “Her ability to oversee the research program of one of the country’s top research-intensive medical schools, and her expertise in autoimmune diseases, including rheumatoid arthritis and lupus, make her well positioned to direct NIAMS.” Dr. Criswell, a rheumatologist, was named a full professor of medicine at UCSF in 2007 and had served as vice chancellor of research at the university since 2017. She has authored more than 250 peer-reviewed scientific papers, and her efforts have contributed to the identification of more than 30 genes linked to autoimmune disorders. In her first media interview, Dr. Criswell opens up about her mentors, operational challenges posed by the COVID-19 pandemic, and highlights many NIAMS research projects underway.
Who inspired you most early in your career as a physician scientist? I have had great opportunities to work with fabulous mentors. Wallace (Wally) Epstein, MD, was my mentor when I was a rheumatology fellow and junior faculty member at UCSF. He was broadly admired for the breadth of his experience as a clinician and a researcher, and he was noteworthy at that time for his strong support for women and students of color. One of the many things I appreciated about him was his diverse range of interests outside of work, which included cello playing and woodworking.
Another mentor was Ephraim (Eph) Engleman, MD, the first academic rheumatologist in California. Eph continued to see patients beyond the age of 100. Perhaps his most important contributions were his efforts towards advocacy for funding for research and education in rheumatology. A prodigy violinist, he too had a broad range of personal interests.
What research into the genetics and epidemiology of human autoimmune disease that you have been a part of has most surprised you, in term of its ultimate clinical impact? Some of my most rewarding and impactful work has focused on the shared genetic basis of autoimmune diseases. We’ve identified dozens of genes that contribute to the risk and outcome of rheumatoid arthritis, lupus, and other autoimmune disorders. These discoveries regarding shared genes and pathways among such a diverse set of conditions have helped to inform optimal therapeutic target and treatment strategies across multiple diseases. For example, exploration of RA genes and pathways has revealed that approved agents for other conditions, such as cancer, may be appropriately repurposed for the treatment of RA. These are critical observations that have the potential to dramatically accelerate progress in developing new therapies for autoimmune diseases, such as RA.
Did you have much interaction with Stephen I. Katz, MD, PhD, your longtime predecessor who passed away unexpectedly in 2018? If so, what do you remember most about him? I regret that I had very little interaction with Steve, but I am well aware of the impact he had on NIAMS, NIH, and the research enterprise overall. He inspired so many people in a personal way, and I am energized by the legacy that he left behind.
What are your goals for the early part of your tenure as the new director of NIAMS? An important goal is getting to know the NIAMS community and expanding my knowledge of the Institute’s musculoskeletal and skin portfolios. I am also conducting outreach to Institute/Center directors and other NIH leadership to increase opportunities for input and advice. In doing this, I am identifying shared research interests, best practices, and potential partners for possible future collaborations. Another important goal is to increase NIAMS’ visibility within and beyond NIH. Ultimately, I want to contribute to the great work of the Institute and improve the lives of people with rheumatic, musculoskeletal, and skin diseases.
How would you characterize your management style? I like to lead with a flat hierarchy and work collectively to address opportunities and challenges. I value team building and tend to tap a variety of perspectives and expertise at all levels to achieve consensus, where possible.
The Accelerating Medicines Partnership (AMP) program was launched in 2014, with projects in three disease areas including the autoimmune disorders RA and lupus. What are some recent highlights from this program with respect to RA and lupus? AMP RA/SLE was dedicated to identifying promising therapeutic targets for RA and systemic lupus erythematosus. AMP-funded researchers have applied cutting-edge technologies to study cells from the synovial tissues of the joints of people with RA, and from the kidneys of people with lupus nephritis. In 2014, studying tissues in patients where the disease is active was a novel approach, since most research was conducted in mouse models or human blood samples.
The AMP RA/SLE Network developed a rich dataset that is available to the research community. Investigators are now using the data to facilitate RA and lupus research. For example, using AMP data, NIAMS-supported researchers identified potential biomarkers that could help predict an imminent RA flare. Work from another NIAMS-supported group suggests that targeting the regulatory transcription factor HIF-1, which drives inflammation and tissue damage, might be an effective approach for treating renal injury in lupus.
The data generated are accessible to the scientific community through two NIH websites: the database of Genotypes and Phenotypes (dbGaP) and the Immunology Database and Analysis Portal (IMMPORT).
Given the success of AMP RA/SLE, NIH plans to launch an “AMP 2.0” later in 2021. The AMP Autoimmune and Immune-Mediated Diseases (AMP AIM) program will provide an opportunity to leverage the accomplishments of AMP RA/SLE to new conditions, including psoriatic spectrum diseases and Sjögren’s syndrome.
What are some recent highlights from NIAMS-supported research in skin diseases? NIAMS-supported investigators continue to make significant strides in our understanding of skin biology and disease. For example, researchers recently demonstrated that imiquimod, a drug used to treat precancerous skin lesions, can help mouse ear wounds heal without scarring.
Another team addressed the safety and potential benefit of Staphylococcus hominis A9, a bacterium isolated from healthy human skin, as a topical therapy for atopic dermatitis.
Moving forward, AMP AIM will refine and extend the single-cell analysis of tissues to additional diseases, including psoriasis, setting the stage for the discovery of new therapeutic targets for the disease.
How has the COVID-19 pandemic changed the landscape of research, at least for the short term? This is a once-in-a-century pandemic that none of us were fully prepared for. We understand that it has been particularly challenging for women scientists, scientists with young children, and trainees and junior faculty who are at critically important and vulnerable stages of their careers. There isn’t a lab or clinical setting that hasn’t been negatively impacted in some way.
During the pandemic, the NIH instituted administrative flexibilities to support the grantee community, including extensions in time. In addition, the agency has issued several funding opportunities specific to COVID-19, some of which involve NIAMS participation.
What is NIAMS doing to help early/young investigators as well as female investigators and those from minority groups? Structural racism in biomedical research is a heightened concern. Earlier this year, Dr. Collins established the UNITE initiative to address structural racism and promote racial equity and inclusion at the NIH and within the larger biomedical community that we support. NIAMS is fully committed to this effort. One example is the Diversity Supplement Program, which is designed to attract and encourage eligible individuals from underrepresented populations to research careers.
Early-stage investigators are another top priority. In a tribute to the beloved former NIAMS director, NIH recently established the Stephen I. Katz Early Stage Investigator Research Grant Program. The R01 award provides support for a project unrelated to an early investigator’s area of postdoctoral study. (No preliminary data are allowed.) This award mechanism is a unique opportunity for early-stage investigators to take their research in a completely new direction.
Managing work and family life is an important concern, particularly for female investigators. Many NIH grant awards allow for reimbursement of actual, allowable costs incurred for childcare and parental leave. The NIH is exploring initiatives to promote research continuity and retention of eligible investigators facing major life events, such as pregnancy, childbirth, and adoption, at vulnerable career stages.
Who inspires you most in your work today? I am inspired by the ongoing struggles of our patients, junior investigators, and by the committed staff members on my team.
After many years at the University of California, San Francisco, Lindsey A. Criswell, MD, MPH, DSc, began a new chapter in February 2021 as the director of the National Institute of Arthritis and Musculoskeletal and Skin Disease, part of the National Institutes of Health. NIH Director Francis S. Collins, MD, PhD, selected her for the post.
“Dr. Criswell has rich experience as a clinician, researcher, and administrator,” Dr. Collins said in a prepared statement. “Her ability to oversee the research program of one of the country’s top research-intensive medical schools, and her expertise in autoimmune diseases, including rheumatoid arthritis and lupus, make her well positioned to direct NIAMS.” Dr. Criswell, a rheumatologist, was named a full professor of medicine at UCSF in 2007 and had served as vice chancellor of research at the university since 2017. She has authored more than 250 peer-reviewed scientific papers, and her efforts have contributed to the identification of more than 30 genes linked to autoimmune disorders. In her first media interview, Dr. Criswell opens up about her mentors, operational challenges posed by the COVID-19 pandemic, and highlights many NIAMS research projects underway.
Who inspired you most early in your career as a physician scientist? I have had great opportunities to work with fabulous mentors. Wallace (Wally) Epstein, MD, was my mentor when I was a rheumatology fellow and junior faculty member at UCSF. He was broadly admired for the breadth of his experience as a clinician and a researcher, and he was noteworthy at that time for his strong support for women and students of color. One of the many things I appreciated about him was his diverse range of interests outside of work, which included cello playing and woodworking.
Another mentor was Ephraim (Eph) Engleman, MD, the first academic rheumatologist in California. Eph continued to see patients beyond the age of 100. Perhaps his most important contributions were his efforts towards advocacy for funding for research and education in rheumatology. A prodigy violinist, he too had a broad range of personal interests.
What research into the genetics and epidemiology of human autoimmune disease that you have been a part of has most surprised you, in term of its ultimate clinical impact? Some of my most rewarding and impactful work has focused on the shared genetic basis of autoimmune diseases. We’ve identified dozens of genes that contribute to the risk and outcome of rheumatoid arthritis, lupus, and other autoimmune disorders. These discoveries regarding shared genes and pathways among such a diverse set of conditions have helped to inform optimal therapeutic target and treatment strategies across multiple diseases. For example, exploration of RA genes and pathways has revealed that approved agents for other conditions, such as cancer, may be appropriately repurposed for the treatment of RA. These are critical observations that have the potential to dramatically accelerate progress in developing new therapies for autoimmune diseases, such as RA.
Did you have much interaction with Stephen I. Katz, MD, PhD, your longtime predecessor who passed away unexpectedly in 2018? If so, what do you remember most about him? I regret that I had very little interaction with Steve, but I am well aware of the impact he had on NIAMS, NIH, and the research enterprise overall. He inspired so many people in a personal way, and I am energized by the legacy that he left behind.
What are your goals for the early part of your tenure as the new director of NIAMS? An important goal is getting to know the NIAMS community and expanding my knowledge of the Institute’s musculoskeletal and skin portfolios. I am also conducting outreach to Institute/Center directors and other NIH leadership to increase opportunities for input and advice. In doing this, I am identifying shared research interests, best practices, and potential partners for possible future collaborations. Another important goal is to increase NIAMS’ visibility within and beyond NIH. Ultimately, I want to contribute to the great work of the Institute and improve the lives of people with rheumatic, musculoskeletal, and skin diseases.
How would you characterize your management style? I like to lead with a flat hierarchy and work collectively to address opportunities and challenges. I value team building and tend to tap a variety of perspectives and expertise at all levels to achieve consensus, where possible.
The Accelerating Medicines Partnership (AMP) program was launched in 2014, with projects in three disease areas including the autoimmune disorders RA and lupus. What are some recent highlights from this program with respect to RA and lupus? AMP RA/SLE was dedicated to identifying promising therapeutic targets for RA and systemic lupus erythematosus. AMP-funded researchers have applied cutting-edge technologies to study cells from the synovial tissues of the joints of people with RA, and from the kidneys of people with lupus nephritis. In 2014, studying tissues in patients where the disease is active was a novel approach, since most research was conducted in mouse models or human blood samples.
The AMP RA/SLE Network developed a rich dataset that is available to the research community. Investigators are now using the data to facilitate RA and lupus research. For example, using AMP data, NIAMS-supported researchers identified potential biomarkers that could help predict an imminent RA flare. Work from another NIAMS-supported group suggests that targeting the regulatory transcription factor HIF-1, which drives inflammation and tissue damage, might be an effective approach for treating renal injury in lupus.
The data generated are accessible to the scientific community through two NIH websites: the database of Genotypes and Phenotypes (dbGaP) and the Immunology Database and Analysis Portal (IMMPORT).
Given the success of AMP RA/SLE, NIH plans to launch an “AMP 2.0” later in 2021. The AMP Autoimmune and Immune-Mediated Diseases (AMP AIM) program will provide an opportunity to leverage the accomplishments of AMP RA/SLE to new conditions, including psoriatic spectrum diseases and Sjögren’s syndrome.
What are some recent highlights from NIAMS-supported research in skin diseases? NIAMS-supported investigators continue to make significant strides in our understanding of skin biology and disease. For example, researchers recently demonstrated that imiquimod, a drug used to treat precancerous skin lesions, can help mouse ear wounds heal without scarring.
Another team addressed the safety and potential benefit of Staphylococcus hominis A9, a bacterium isolated from healthy human skin, as a topical therapy for atopic dermatitis.
Moving forward, AMP AIM will refine and extend the single-cell analysis of tissues to additional diseases, including psoriasis, setting the stage for the discovery of new therapeutic targets for the disease.
How has the COVID-19 pandemic changed the landscape of research, at least for the short term? This is a once-in-a-century pandemic that none of us were fully prepared for. We understand that it has been particularly challenging for women scientists, scientists with young children, and trainees and junior faculty who are at critically important and vulnerable stages of their careers. There isn’t a lab or clinical setting that hasn’t been negatively impacted in some way.
During the pandemic, the NIH instituted administrative flexibilities to support the grantee community, including extensions in time. In addition, the agency has issued several funding opportunities specific to COVID-19, some of which involve NIAMS participation.
What is NIAMS doing to help early/young investigators as well as female investigators and those from minority groups? Structural racism in biomedical research is a heightened concern. Earlier this year, Dr. Collins established the UNITE initiative to address structural racism and promote racial equity and inclusion at the NIH and within the larger biomedical community that we support. NIAMS is fully committed to this effort. One example is the Diversity Supplement Program, which is designed to attract and encourage eligible individuals from underrepresented populations to research careers.
Early-stage investigators are another top priority. In a tribute to the beloved former NIAMS director, NIH recently established the Stephen I. Katz Early Stage Investigator Research Grant Program. The R01 award provides support for a project unrelated to an early investigator’s area of postdoctoral study. (No preliminary data are allowed.) This award mechanism is a unique opportunity for early-stage investigators to take their research in a completely new direction.
Managing work and family life is an important concern, particularly for female investigators. Many NIH grant awards allow for reimbursement of actual, allowable costs incurred for childcare and parental leave. The NIH is exploring initiatives to promote research continuity and retention of eligible investigators facing major life events, such as pregnancy, childbirth, and adoption, at vulnerable career stages.
Who inspires you most in your work today? I am inspired by the ongoing struggles of our patients, junior investigators, and by the committed staff members on my team.
Management of pediatric food allergies evolving
The treatment of atopic dermatitis (AD) is undergoing a revolution thanks to biologics. Now, an allergist and a dietitian told pediatric dermatologists that the treatment of a related condition – food allergy – is also undergoing a dramatic transformation as the management approach evolves away from blanket avoidance of allergens.
“Over the past 15 years, we’ve seen a shift from a very passive approach where generally we just advised patients to avoid the things they’re allergic to,” said U.K. pediatric allergist Adam Fox, MBBS, MD, in a presentation at The World Congress of Pediatric Dermatology (WCPD) 2021 Annual Meeting. “Now, we have a much better understanding of how allergy develops and strategies to minimize the risk of allergy happening in the first place,” he said.
According to Carina Venter, PhD, RD, associate professor of pediatrics-allergy/immunology at the University of Colorado, Denver, who also spoke at the conference, an estimated 20% to 30% of patients with AD also have food allergies, and up to 90% of infants with cow’s milk allergy develop skin symptoms.
It may not be necessary for a breastfeeding mother to avoid food allergens if a child is allergic, said Dr. Fox, of Guy’s and St. Thomas’ NHS Foundation Trust, London. “A lot of parents will automatically assume that if their child has an egg or milk allergy, then it’s a good idea to completely eliminate that from their diet if they’re breastfeeding,” but it is “surprisingly uncommon” that this approach makes a difference, he said. “Less goes through the breast milk than people imagine,” he said.
He noted that eliminating foods from the breastfeeding mother’s diet may have negative consequences. “There’s always that risk that if you make life harder for the breastfeeding mom because they’re going to have to avoid all sorts of foods, they’ll be more likely to discontinue breastfeeding. You really need a compelling reason to stop the food.”
As for children themselves, Dr. Fox suggested that there’s often no connection between AD and food allergies. “What will commonly happen when you see and diagnose these kids is that their eczema has been quite significantly undertreated,” he said. “Once you just get them on the right [regimen], they don’t need to be cutting the food out of their diet. It’s just making their life unnecessarily harder.”
Dr. Venter said there may be little choice but to avoid a trigger food if a child develops AD with exposure. However, she noted, it’s important to understand that avoidance of certain foods could make the allergy – and AD – worse. “If you have a child or an adult with atopic dermatitis that’s not controlled by an optimal topical treatment, and you do consider avoidance, we need to be aware that development of more severe IgA-mediated symptoms can happen in a short period of time,” she said.
In a slide that Dr. Venter presented, the dilemma for physicians was expressed this way: “The potential benefit of food avoidance as a management strategy for some patients with AD must now be weighed against the strong evidence that unnecessarily avoiding a food in kids with AD increases the risk of developing anaphylaxis to that food.”
What should pediatric dermatologists do to balance the risks of allergen exposure to the risks that children will develop permanent allergies? Dr. Venter pointed to guidelines about AD that were developed by the U.K.’s National Institute for Health and Care Excellence. She also highlighted the International Milk Allergy in Primary Care recommendations.
She suggested considering creative ways to bypass complete avoidance and boost a child’s tolerance of allergens if possible. “If we’re going to keep a child with eczema on a mold-free diet for a longer period of time, is there perhaps a role for regularly introducing small amounts of yogurt or even small amounts of milk in the child’s diet to at least keep immune tolerance without necessarily aggravating eczema symptoms?”
Dr. Fox has consulted for DBV and Aimmune through his employer, NHS Trust. He serves as president of the British Society for Allergy and Clinical Immunology and as chair of the Allergy UK Health Advisory Board, both of which receive funding from drug companies. Dr. Venter has received support for allergy-related research from the National Peanut Board.
A version of this article first appeared on Medscape.com.
The treatment of atopic dermatitis (AD) is undergoing a revolution thanks to biologics. Now, an allergist and a dietitian told pediatric dermatologists that the treatment of a related condition – food allergy – is also undergoing a dramatic transformation as the management approach evolves away from blanket avoidance of allergens.
“Over the past 15 years, we’ve seen a shift from a very passive approach where generally we just advised patients to avoid the things they’re allergic to,” said U.K. pediatric allergist Adam Fox, MBBS, MD, in a presentation at The World Congress of Pediatric Dermatology (WCPD) 2021 Annual Meeting. “Now, we have a much better understanding of how allergy develops and strategies to minimize the risk of allergy happening in the first place,” he said.
According to Carina Venter, PhD, RD, associate professor of pediatrics-allergy/immunology at the University of Colorado, Denver, who also spoke at the conference, an estimated 20% to 30% of patients with AD also have food allergies, and up to 90% of infants with cow’s milk allergy develop skin symptoms.
It may not be necessary for a breastfeeding mother to avoid food allergens if a child is allergic, said Dr. Fox, of Guy’s and St. Thomas’ NHS Foundation Trust, London. “A lot of parents will automatically assume that if their child has an egg or milk allergy, then it’s a good idea to completely eliminate that from their diet if they’re breastfeeding,” but it is “surprisingly uncommon” that this approach makes a difference, he said. “Less goes through the breast milk than people imagine,” he said.
He noted that eliminating foods from the breastfeeding mother’s diet may have negative consequences. “There’s always that risk that if you make life harder for the breastfeeding mom because they’re going to have to avoid all sorts of foods, they’ll be more likely to discontinue breastfeeding. You really need a compelling reason to stop the food.”
As for children themselves, Dr. Fox suggested that there’s often no connection between AD and food allergies. “What will commonly happen when you see and diagnose these kids is that their eczema has been quite significantly undertreated,” he said. “Once you just get them on the right [regimen], they don’t need to be cutting the food out of their diet. It’s just making their life unnecessarily harder.”
Dr. Venter said there may be little choice but to avoid a trigger food if a child develops AD with exposure. However, she noted, it’s important to understand that avoidance of certain foods could make the allergy – and AD – worse. “If you have a child or an adult with atopic dermatitis that’s not controlled by an optimal topical treatment, and you do consider avoidance, we need to be aware that development of more severe IgA-mediated symptoms can happen in a short period of time,” she said.
In a slide that Dr. Venter presented, the dilemma for physicians was expressed this way: “The potential benefit of food avoidance as a management strategy for some patients with AD must now be weighed against the strong evidence that unnecessarily avoiding a food in kids with AD increases the risk of developing anaphylaxis to that food.”
What should pediatric dermatologists do to balance the risks of allergen exposure to the risks that children will develop permanent allergies? Dr. Venter pointed to guidelines about AD that were developed by the U.K.’s National Institute for Health and Care Excellence. She also highlighted the International Milk Allergy in Primary Care recommendations.
She suggested considering creative ways to bypass complete avoidance and boost a child’s tolerance of allergens if possible. “If we’re going to keep a child with eczema on a mold-free diet for a longer period of time, is there perhaps a role for regularly introducing small amounts of yogurt or even small amounts of milk in the child’s diet to at least keep immune tolerance without necessarily aggravating eczema symptoms?”
Dr. Fox has consulted for DBV and Aimmune through his employer, NHS Trust. He serves as president of the British Society for Allergy and Clinical Immunology and as chair of the Allergy UK Health Advisory Board, both of which receive funding from drug companies. Dr. Venter has received support for allergy-related research from the National Peanut Board.
A version of this article first appeared on Medscape.com.
The treatment of atopic dermatitis (AD) is undergoing a revolution thanks to biologics. Now, an allergist and a dietitian told pediatric dermatologists that the treatment of a related condition – food allergy – is also undergoing a dramatic transformation as the management approach evolves away from blanket avoidance of allergens.
“Over the past 15 years, we’ve seen a shift from a very passive approach where generally we just advised patients to avoid the things they’re allergic to,” said U.K. pediatric allergist Adam Fox, MBBS, MD, in a presentation at The World Congress of Pediatric Dermatology (WCPD) 2021 Annual Meeting. “Now, we have a much better understanding of how allergy develops and strategies to minimize the risk of allergy happening in the first place,” he said.
According to Carina Venter, PhD, RD, associate professor of pediatrics-allergy/immunology at the University of Colorado, Denver, who also spoke at the conference, an estimated 20% to 30% of patients with AD also have food allergies, and up to 90% of infants with cow’s milk allergy develop skin symptoms.
It may not be necessary for a breastfeeding mother to avoid food allergens if a child is allergic, said Dr. Fox, of Guy’s and St. Thomas’ NHS Foundation Trust, London. “A lot of parents will automatically assume that if their child has an egg or milk allergy, then it’s a good idea to completely eliminate that from their diet if they’re breastfeeding,” but it is “surprisingly uncommon” that this approach makes a difference, he said. “Less goes through the breast milk than people imagine,” he said.
He noted that eliminating foods from the breastfeeding mother’s diet may have negative consequences. “There’s always that risk that if you make life harder for the breastfeeding mom because they’re going to have to avoid all sorts of foods, they’ll be more likely to discontinue breastfeeding. You really need a compelling reason to stop the food.”
As for children themselves, Dr. Fox suggested that there’s often no connection between AD and food allergies. “What will commonly happen when you see and diagnose these kids is that their eczema has been quite significantly undertreated,” he said. “Once you just get them on the right [regimen], they don’t need to be cutting the food out of their diet. It’s just making their life unnecessarily harder.”
Dr. Venter said there may be little choice but to avoid a trigger food if a child develops AD with exposure. However, she noted, it’s important to understand that avoidance of certain foods could make the allergy – and AD – worse. “If you have a child or an adult with atopic dermatitis that’s not controlled by an optimal topical treatment, and you do consider avoidance, we need to be aware that development of more severe IgA-mediated symptoms can happen in a short period of time,” she said.
In a slide that Dr. Venter presented, the dilemma for physicians was expressed this way: “The potential benefit of food avoidance as a management strategy for some patients with AD must now be weighed against the strong evidence that unnecessarily avoiding a food in kids with AD increases the risk of developing anaphylaxis to that food.”
What should pediatric dermatologists do to balance the risks of allergen exposure to the risks that children will develop permanent allergies? Dr. Venter pointed to guidelines about AD that were developed by the U.K.’s National Institute for Health and Care Excellence. She also highlighted the International Milk Allergy in Primary Care recommendations.
She suggested considering creative ways to bypass complete avoidance and boost a child’s tolerance of allergens if possible. “If we’re going to keep a child with eczema on a mold-free diet for a longer period of time, is there perhaps a role for regularly introducing small amounts of yogurt or even small amounts of milk in the child’s diet to at least keep immune tolerance without necessarily aggravating eczema symptoms?”
Dr. Fox has consulted for DBV and Aimmune through his employer, NHS Trust. He serves as president of the British Society for Allergy and Clinical Immunology and as chair of the Allergy UK Health Advisory Board, both of which receive funding from drug companies. Dr. Venter has received support for allergy-related research from the National Peanut Board.
A version of this article first appeared on Medscape.com.
Early interventions for psychosis
Neuroscience research over the past half century has failed to significantly advance the treatment of severe mental illness.1,2 Hence, evidence that a longer duration of untreated psychosis (DUP) aggravates—and early intervention with medication and social supports ameliorates—the long-term adverse consequences of psychotic disorders generated a great deal of interest.3,4 This knowledge led to the development of diverse early intervention services worldwide aimed at this putative “critical window.” It raised the possibility that appropriate interventions could prevent the long-term disability that makes chronic psychosis one of the most debilitating disorders.5,6 However, even beyond the varied cultural and economic confounds, it is difficult to assess, compare, and optimize program effectiveness.7 Obstacles include paucity of sufficiently powered, well-designed randomized controlled trials (RCTs), the absence of diagnostic biomarkers or other prognostic indicators to better account for the inherent heterogeneity in the population and associated outcomes, and the absence of modifiable risk factors that can guide interventions and provide intermediate outcomes.4,8-10
To better appreciate these issues, it is important to distinguish whether a program is designed to prevent psychosis, or to mitigate the effects of psychosis. Two models include the:
- Prevention model, which focuses on young individuals who are not yet overtly psychotic but at high risk
- First-episode recovery model, which focuses on those who have experienced a first episode of psychosis (FEP) but have not yet developed a chronic disorder.
Both models share long-term goals and are hampered by many of the same issues summarized above. They both deviate markedly from the standard medical model by including psychosocial services designed to promote restoration of a self-defined trajectory to greater independence.11-14 The 2 differ, however, in the challenges they must overcome to produce their sample populations and establish effective interventions.10,15,16
In this article, we provide a succinct overview of these issues and a set of recommendations based on a “strength-based” approach. This approach focuses on finding common ground between patients, their support system, and the treatment team in the service of empowering patients to resume responsibility for transition to adulthood.
The prevention model
While most prevention initiatives in medicine rely on the growing ability to target specific pathophysiologic pathways,3 preventing psychosis relies on clinical evidence showing that DUP and early interventions predict a better course of severe mental illness.17 In contrast, initiatives such as normalizing neonatal neuronal pathways are more consistent with the strategy utilized in other fields but have yet to yield a pathophysiologic target for psychosis.3,18
Initial efforts to identify ‘at-risk’ individuals
The prevention model of psychosis is based on the ability to identify young individuals at high risk for developing a psychotic disorder (Figure). The first screening measures were focused on prodromal psychosis (eg, significant loss of function, family history, and “intermittent” and “attenuated” psychotic symptoms). When applied to referred (ie, pre-screened) samples, 30% to 40% of this group who met criteria transitioned to psychosis over the next 1 to 3 years despite antidepressant and psychosocial interventions.19 Comprising 8 academic medical centers, the North American Prodrome Longitudinal Study (NAPLS) produced similar results using the Structured Interview for Prodromal Syndromes (SIPS).17 Thus, 30% to 50% of pre-screened individuals referred by school counselors and mental health professionals met SIPS criteria, and 35% of these individuals transitioned to psychosis over 30 months. The validity of this measure was further supported by the fact that higher baseline levels of unusual thought content, suspicion/paranoia, social impairment, and substance abuse successfully distinguished approximately 80% of those who transitioned to psychosis. The results of this first generation of screening studies were exciting because they seemed to demonstrate that highly concentrated samples of young persons at high risk of developing psychosis could be identified, and that fine-tuning the screening criteria could produce even more enriched samples (ie, positive predictive power).

Initial interventions produced promising results
The development of effective screening measures led to reports of effective treatment interventions. These were largely applied in a clinical staging model that restricted antipsychotic medications to those who failed to improve after receiving potentially “less toxic” interventions (eg, omega-3 polyunsaturated fatty acids and other antioxidants; psychotherapy; cognitive-behavioral therapy [CBT]; family therapy).5 While study designs were typically quasi-experimental, the interventions appeared to dramatically diminish the transition to psychosis (ie, approximately 50%).
Continue to: The first generation...
The first generation of RCTs appeared to confirm these results, although sample sizes were small, and most study designs assessed only a single intervention. Initial meta-analyses of these data reported that both CBT and antipsychotics appeared to prevent approximately one-half of individuals from becoming psychotic at 12 months, and more than one-third at 2 to 4 years, compared with treatment as usual.20
While some researchers challenged the validity of these findings,21-23 the results generated tremendous international enthusiasm and calls for widespread implementation.6 The number of early intervention services (EIS) centers increased dramatically worldwide, and in 2014 the National Institute for Health and Care Excellence released standards for interventions to prevent transition to psychosis.24 These included close monitoring, CBT and family interventions, and avoiding antipsychotics when possible.24
Focusing on sensitivity over specificity
The first generation of studies generated by the prevention model relied on outreach programs or referrals, which produced small samples of carefully selected, pre-screened individuals (Figure, Pre-screened) who were then screened again to establish the high-risk sample.25 While approximately 33% of these individuals became psychotic, the screening process required a very efficient means of eliminating those not at high-risk (given the ultimate target population represented only approximately .5% of young people) (Figure). The pre-screening and screening processes in these first-generation studies were labor-intensive but could only identify approximately 5% of those individuals destined to become psychotic over the next 2 or 3 years. Thus, alternative methods to enhance sensitivity were needed to extend programming to the general population.
Second-generation pre-screening (Figure; Step 1). New pre-screening methods were identified that captured more individuals destined to become psychotic. For example, approximately 90% of this population were registered in health care organizations (eg, health maintenance organizations) and received a psychiatric diagnosis in the year prior to the onset of psychosis (true positives).8 These samples, however, contained a much higher percentage of persons not destined to become psychotic, and somehow the issue of specificity (decreasing false positives) was minimized.8,9 For example, pre-screened samples contained 20 to 50 individuals not destined to become psychotic for each one who did.26 Since screening measures could only eliminate approximately 20% of this group (Figure, Step 2, page 25), second-generation transition rates fell from 30% to 40% to 2% to 10%.27,28
Other pre-screening approaches were introduced, but they also focused on capturing more of those destined to become psychotic (sensitivity) than eliminating those who would not (specificity). For instance, Australia opened more than 100 “Headspace” community centers nationwide designed to promote engagement and self-esteem in youth experiencing anxiety; depression; stress; relationship, work, or school problems; or bullying.13 Most services were free and included mental health staff who screened for psychosis and provided a wide range of services in a destigmatized setting. These methods identified at least an additional 5% to 7% of individuals destined to become psychotic, but to our knowledge, no data have been published on whether they helped eliminate those who did not.
Continue to: Second-generation screening
Second-generation screening (Figure, Step 2). A second screening aims to retain those pre-screened individuals who will become psychotic (ie, minimizing false negatives) while further minimizing those who do not (ie, minimizing false positives). The addition of cognitive, neural (eg, structural MRI; neurophysiologic), and biochemical (eg, inflammatory immune and stress) markers to the risk calculators have produced a sensitivity close to 100%.8,9 Unfortunately, these studies downplayed specificity, which remained approximately 20%.8,9 Specificity is critical not just because of concerns about stigma (ie, labeling people as pre-psychotic when they are not) but also because of the adverse effects of antipsychotic medications and the effects on future program development (interventions are costly and labor-intensive). Also, diluting the pool with individuals not at risk makes it nearly impossible to identify effective interventions (ie, power).27,28
While some studies focused on increasing specificity (to approximately 75%), this leads to an unacceptable loss of sensitivity (from 90% to 60%),29 with 40% of pre-screened individuals who would become psychotic being eliminated from the study population. The addition of other biological markers (eg, salivary cortisol)30 and use of learning health systems may be able to enhance these numbers (initial reports of specificity = 87% and sensitivity = 85%).8,9 This is accomplished by integrating artificial and human intelligence measures of clinical (symptom and neurocognitive measures) and biological (eg, polygenetic risk scores; gray matter volume) variables.31 However, even if these results are replicated, more effective pre-screening measures will be required.
Identifying a suitable sample population for prevention program studies is clearly more complicated than for FEP studies, where one can usually identify many of those in the at-risk population by their first hospitalization for psychotic symptoms. The issues of false positives (eg, substance-induced psychosis) and negatives (eg, slow deterioration, prominent negative symptoms) are important concerns, but proportionately far less significant.
Prevention and FEP interventions
Once a study sample is constituted, 1 to 3 years of treatment interventions are initiated. Interventions for prevention programs typically include CBT directed at attenuated psychosis (eg, reframing or de-catastrophizing unusual thoughts and minimizing distress associated with unusual perceptions); case management to facilitate personal, educational, and vocational goals; and family therapy in single or multi-group formats to educate one’s support system about the risk state and to minimize adverse familial responses.14 Many programs also include supported education or employment services to promote reintegration in age-appropriate activities; group therapy focused on substance abuse and social skills training; cognitive remediation to ameliorate the cognitive dysfunction; and an array of pharmacologic interventions designed to delay or prevent transition to psychosis or to alleviate symptoms. While most interventions are similar, FEP programs have recently included peer support staff. This appears to instill hope in newly diagnosed patients, provide role models, and provide peer supporters an opportunity to use their experiences to help others and earn income.32
The breadth and depth of these services are critical because retention in the program is highly dependent on participant engagement, which in turn is highly dependent on whether the program can help individuals get what they want (eg, friends, employment, education, more autonomy, physical health). The setting and atmosphere of the treatment program and the willingness/ability of staff to meet participants in the community are also important elements.11,12 In this context, the Headspace community centers are having an impact far beyond Australia and may prove to be a particularly good model.13
Continue to: Assessing prevention and FEP interventions
Assessing prevention and FEP interventions
The second generation of studies of prevention programs has not confirmed, let alone extended, the earlier findings and meta-analyses. A 2020 report concluded CBT was still the most promising intervention; it was more effective than control treatments at 12 and 18 months, although not at 6, 24, or 48 months.33 This review included controlled, open-label, and naturalistic studies that assessed family therapy; omega-3 polyunsaturated fatty acids; integrated psychological therapy (a package of interventions that included family education, CBT, social skills training, and cognitive remediation); N-methyl-
While these disappointing findings are at least partly attributable to the methodological challenges described above and in the Figure, other factors may hinder establishing effective interventions. In contrast to FEP studies, those focused on prevention had a very ambitious agenda (eliminating psychosis) and tended to downplay more modest intermediate outcomes. These studies also tended to assess new ideas with small samples rather than pursue promising findings with larger multi-site studies focused on a group of interventions. The authors of a Cochrane review observed “There is the impression that in this whole area there is a triumph of hope over adversity. There is the repeated hope invested in another—often unique—study question and then a study of fewer than 100 participants are completed. This results in the set of comparisons reported here, all 9 of which are too underpowered to really highlight clear differences.”34 To use a baseball analogy, it seems that investigators are “swinging for the fence” when a few singles are what’s really needed.
From the outset, the goals of FEP studies were more modest, largely ignoring the task of developing consensus definitions of recovery that require following patients for up to 5 to 10 years. Instead, they use intermediate endpoints based on adapting treatments that already appeared effective in patients with chronic mental disorders.35 As a consequence, researchers examining FEP demonstrated clear, albeit limited, salutary effects using large multi-site trials and previously established outcome measures.3,10,36 For instance, the Recovery After an Initial Schizophrenia Episode-Early Treatment Program (RAISE-ETP) study was a 2-year, multi-site RCT (N = 404) funded by the National Institute of Mental Health (NIMH). The investigators reported improved indices of social function (eg, quality of life; education and work participation) and total ratings of psychopathology and depression compared with treatment as usual. Furthermore, they established that DUP predicted treatment response.35 The latter finding was underscored by improvement being limited to the 50% with <74 weeks DUP. Annual costs of the program per 1 standard deviation improvement in quality of life were approximately $1,000 for patients with <74 weeks DUP and $40,000 for those with >74 weeks DUP. Concurrent meta-analyses confirmed and extended these findings,16 showing higher remission rates; diminished relapses and hospital admissions; greater engagement in programming; greater involvement in work and school; improved quality of life; and other steps toward recovery. These studies were also able to establish a clear benefit of antipsychotic medications, particularly a high acceptance of long-acting injectable antipsychotic formulations, which promoted adherence and decreased some adverse events37; and early use of clozapine therapy, which improved remission rates and longer-term outcomes.38 Other findings underscored the need to anticipate and address new problems associated with effective antipsychotic therapy (eg, antipsychotic response correlates with weight gain, a particularly intolerable adverse event for this age group).39 Providing pre-emptive strategies such as exercise groups and nutritional education may be necessary to maintain adherence.
Limitations of FEP studies
The effect sizes in these FEP studies were small to medium on outcome measures tracking recovery and associated indicators (eg, global functioning, school/work participation, treatment engagement); the number needed to treat for each of these was >10. There is no clear evidence that recovery programs such as RAISE-ETP actually reduce longer-term disability. Most studies showed disability payments increased while clinical benefits tended to fade over time. In addition, by grouping interventions together, the studies made it difficult to identify effective vs ineffective treatments, let alone determine how best to personalize therapy for participants in future studies.
The next generation of FEP studies
While limited in scope, the results of the recent FEP studies justify a next generation of recovery interventions designed to address these shortcomings and optimize program outcomes.39 Most previous FEP studies were conducted in community mental health center settings, thus eliminating the need to transition services developed in academia into the “real world.” The next generation of NIMH studies will be primarily conducted in analogous settings under the Early Psychosis Intervention Network (EPINET).40 EPINET’s study design echoes that responsible for the stepwise successes in the late 20th century that produced cures for the deadliest childhood cancer, acute lymphoblastic leukemia (ALL). This disease was successfully treated by modifying diverse evidence-based practices without relying on pharmacologic or other major treatment breakthroughs. Despite this, the effort yielded successful personalized interventions that were not obtainable for other severe childhood conditions.40 EPINET hopes to automate much of these stepwise advances with a learning health system. This program relies on data routinely collected in clinical practice to drive the process of scientific discovery. Specifically, it determines the relationships between clinical features, biologic measures, treatment characteristics, and symptomatic and functional outcomes. EPINET aims to accelerate our understanding of biomarkers of psychosis risk and onset, as well as factors associated with recovery and cure. Dashboard displays of outcomes will allow for real-time comparisons within and across early intervention clinics. This in turn identifies performance gaps and drives continuous quality improvement.
Continue to: Barriers to optimizing program efficacy for both models
Barriers to optimizing program efficacy for both models
Unfortunately, there are stark differences between ALL and severe mental disorders that potentially jeopardize the achievement of these aims, despite the advances in data analytic abilities that drive the learning health system. Specifically, the heterogeneity of psychotic illnesses and the absence of reliable prognostic and modifiable risk markers (responsible for failed efforts to enhance treatment of serious mental illness over the last half century1,2,41) are unlikely to be resolved by a learning health system. These measures are vital to determine whether specific interventions are effective, particularly given the absence of a randomized control group in the EPINET/learning health system design. Fortunately, however, the National Institutes for Health has recently initiated the Accelerating Medicines Partnership–Schizophrenia (AMP-SCZ). This approach seeks “promising biological markers that can help identify those at risk of developing schizophrenia as early as possible, track the progression of symptoms and other outcomes and ultimately define targets for treatment development.”42 The Box1,4,9,10,36,41,43-45 describes some of the challenges involved in identifying biomarkers of severe mental illness.
Box
Biomarkers and modifiable risk factors4,9,10,41,43 are at the core of personalized medicine and its ultimate objective (ie, theragnostics). This is the ability to identify the correct intervention for a disorder based on a biomarker of the illness.10,36 The inability to identify biomarkers of severe mental illness is multifactorial but in part may be attributable to “looking in all the wrong places.”41 By focusing on neural processes that generate psychiatric symptomatology, investigators are assuming they can bridge the “mind gap”1 and specifically distinguish between pathological, compensatory, or collateral measures of poorly characterized limbic neural functions.41
It may be more productive to identify a pathological process within the limbic system that produces a medical condition as well as the mental disorder. If one can isolate the pathologic limbic circuit activity responsible for a medical condition, one may be able to reproduce this in animal models and determine whether analogous processes contribute to the core features of the mental illness. Characterization of the aberrant neural circuit in animal models also could yield targets for future therapies. For example, episodic water intoxication in a discrete subset of patients with schizophrenia44 appears to arise from a stress diathesis produced by anterior hippocampal pathology that disrupts regulation of antidiuretic hormone, oxytocin, and hypothalamic-pituitary-adrenal axis secretion. These patients also exhibit psychogenic polydipsia that may be a consequence of the same hippocampal pathology that disrupts ventral striatal and lateral hypothalamic circuits. These circuits, in turn, also modulate motivated behaviors and cognitive processes likely relevant to psychosis.45
A strength-based approach
The absence of sufficiently powered RCTs for prevention studies and the reliance on intermediate outcomes for FEP studies leaves unanswered whether such programs can effectively prevent chronic psychosis at a cost society is willing to pay. Still, substantial evidence indicates that outreach, long-acting injectable antipsychotics, early consideration of clozapine, family therapy, CBT for psychosis/attenuated psychosis, and services focused on competitive employment can preserve social and occupational functioning.16,34 Until these broader questions are more definitively addressed, it seems reasonable to apply what we have learned (Table11,12,35,37-39,46).
Simply avoiding the most divisive aspects of the medical model that inadvertently promote stigma and undercut self-confidence may help maintain patients’ willingness to learn how best to apply their strengths and manage their limitations.11 The progression to enduring psychotic features (eg, fixed delusions) may reflect ongoing social isolation and alienation. A strength-based approach seeks first to establish common goals (eg, school, work, friends, family support, housing, leaving home) and then works to empower the patient to successfully reach those goals.35 This typically involves giving them the opportunity to fail, avoiding criticism when they do, and focusing on these experiences as learning opportunities from which success can ultimately result.
It is difficult to offer all these services in a typical private practice setting. Instead, it may make more sense to use one of the hundreds of early intervention services programs in the United States.46 If a psychiatric clinician is dedicated to working with this population, it may also be possible to establish ongoing relationships with primary care physicians, family and CBT therapists, family support services (eg, National Alliance on Mental Illness), caseworkers and employment counselors. In essence, a psychiatrist may be able re-create a multidisciplinary effort by taking advantage of the expertise of these various professionals. The challenge is to create a consistent message for patients and families in the absence of regular meetings with the clinical team, although the recent reliance on and improved sophistication of virtual meetings may help. Psychiatrists often play a critical role even when the patient is not prescribed medication, partly because they are most comfortable handling the risks and may have the most comprehensive understanding of the issues at play. When medications are appropriate and patients with FEP are willing to take them, early consideration of long-acting injectable antipsychotics and clozapine may provide better stabilization and diminish the risk of earlier and more frequent relapses.
Bottom Line
Early interventions for psychosis include the prevention model and the first-episode recovery model. It is difficult to assess, compare, and optimize the effectiveness of such programs. Current evidence supports a ‘strength-based’ approach focused on finding common ground between patients, their support system, and the treatment team.
Related Resources
- Early Assessment and Support Alliance. National Early Psychosis Directory. https://easacommunity.org/nationaldirectory.php
- Kane JM, Robinson DG, Schooler NR, et al. Comprehensive versus usual community care for first-episode psychosis: 2-year outcomes from the NIMH RAISE Early Treatment Program. Am J Psychiatry. 2016 ;173(4):362-372
Drug Brand Name
Clozapine • Clozaril
1. Hyman SE. Revolution stalled. Sci Transl Med. 2012;4(155):155cm11. doi: 10.1126/scitranslmed.3003142
2. Harrington A. Mind fixers: psychiatry’s troubled search for the biology of mental illness. W.W. Norton & Company; 2019.
3. Millan MJ, Andrieux A, Bartzokis G, et al. Altering the course of schizophrenia: progress and perspectives. Nat Rev Drug Discov. 2016;15(7):485-515.
4. Lieberman JA, Small SA, Girgis RR. Early detection and preventive intervention in schizophrenia: from fantasy to reality. Am J Psychiatry. 2019;176(10):794-810.
5. McGorry PD, Nelson B, Nordentoft M, et al. Intervention in individuals at ultra-high risk for psychosis: a review and future directions. J Clin Psychiatry. 2009;70(9):1206-1212.
6. Csillag C, Nordentoft M, Mizuno M, et al. Early intervention in psychosis: From clinical intervention to health system implementation. Early Interv Psychiatry. 2018;12(4):757-764.
7. McGorry PD, Ratheesh A, O’Donoghue B. Early intervention—an implementation challenge for 21st century mental health care. JAMA Psychiatry. 2018;75(6):545-546.
8. Rosenheck R. Toward dissemination of secondary prevention for psychosis. Am J Psychiatry. 2018;175(5):393-394.
9. Fusar-Poli P, Salazar de Pablo G, Correll CU, et al. Prevention of psychosis: advances in detection, prognosis, and intervention. JAMA Psychiatry. 2020;77(7):755-765.
10. Oliver D, Reilly TJ, Baccaredda Boy O, et al. What causes the onset of psychosis in individuals at clinical high risk? A meta-analysis of risk and protective factors. Schizophr Bull. 2020;46(1):110-120.
11. Tindall R, Simmons M, Allott K, et al. Disengagement processes within an early intervention service for first-episode psychosis: a longitudinal, qualitative, multi-perspective study. Front Psychiatry. 2020;11:565-565.
12. Dixon LB, Holoshitz Y, Nossel I. Treatment engagement of individuals experiencing mental illness: review and update. World Psychiatry. 2016;15(1):13-20.
13. Rickwood D, Paraskakis M, Quin D, et al. Australia’s innovation in youth mental health care: The headspace centre model. Early Interv Psychiatry. 2019;13(1):159-166.
14. Woodberry KA, Shapiro DI, Bryant C, et al. Progress and future directions in research on the psychosis prodrome: a review for clinicians. Harv Rev Psychiatry. 2016;24(2):87-103.
15. Gupta T, Mittal VA. Advances in clinical staging, early intervention, and the prevention of psychosis. F1000Res. 2019;8:F1000 Faculty Rev-2027. doi: 10.12688/f1000research.20346.1
16. Correll CU, Galling B, Pawar A, et al. Comparison of early intervention services vs treatment as usual for early-phase psychosis: a systematic review, meta-analysis, and meta-regression. JAMA Psychiatry. 2018;75(6):555-565.
17. Cannon TD, Cadenhead K, Cornblatt B, et al. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry. 2008;65(1):28-37.
18. Sommer IE, Bearden CE, van Dellen E, et al. Early interventions in risk groups for schizophrenia: what are we waiting for? NPJ Schizophr. 2016;2(1):16003-16003.
19. McGorry PD, Nelson B. Clinical high risk for psychosis—not seeing the trees for the wood. JAMA Psychiatry. 2020;77(7):559-560.
20. van der Gaag M, Smit F, Bechdolf A, et al. Preventing a first episode of psychosis: meta-analysis of randomized controlled prevention trials of 12 month and longer-term follow-ups. Schizophr Res. 2013;149(1):56-62.
21. Marshall M, Rathbone J. Early intervention for psychosis. Cochrane Database Syst Rev. 2011;(6):CD004718. doi: 10.1002/14651858.CD004718.pub3
22. Heinssen RK, Insel TR. Preventing the onset of psychosis: not quite there yet. Schizophr Bull. 2015;41(1):28-29.
23. Amos AJ. Evidence that treatment prevents transition to psychosis in ultra-high-risk patients remains questionable. Schizophr Res. 2014;153(1):240.
24. National Institute for Health and Care Excellence. Psychosis and schizophrenia in adults: prevention and management. Clinical guideline [CG178]. 1.3.7 How to deliver psychological interventions. Published February 12, 2014. Updated March 1, 2014. Accessed August 30, 2021. https://www.nice.org.uk/guidance/cg178/chapter/recommendations#how-to-deliver-psychological-interventions
25. Fusar-Poli P, Werbeloff N, Rutigliano G, et al. Transdiagnostic risk calculator for the automatic detection of individuals at risk and the prediction of psychosis: second replication in an independent National Health Service Trust. Schizophr Bull. 2019;45(3):562-570.
26. Fusar-Poli P, Oliver D, Spada G, et al. The case for improved transdiagnostic detection of first-episode psychosis: electronic health record cohort study. Schizophr Res. 2021;228:547-554.
27. Fusar-Poli P. Negative psychosis prevention trials. JAMA Psychiatry. 2017;74(6):651.
28. Cuijpers P, Smit F, Furukawa TA. Most at‐risk individuals will not develop a mental disorder: the limited predictive strength of risk factors. World Psychiatry. 2021;20(2):224-225.
29. Carrión RE, Cornblatt BA, Burton CZ, et al. Personalized prediction of psychosis: external validation of the NAPLS-2 psychosis risk calculator with the EDIPPP Project. Am J Psychiatry. 2016;173(10):989-996.
30. Worthington MA, Walker EF, Addington J, et al. Incorporating cortisol into the NAPLS2 individualized risk calculator for prediction of psychosis. Schizophr Res. 2021;227:95-100.
31. Koutsouleris N, Dwyer DB, Degenhardt F, et al. Multimodal machine learning workflows for prediction of psychosis in patients with clinical high-risk syndromes and recent-onset depression. JAMA Psychiatry. 2021;78(2):195-209.
32. Simmons MB, Grace D, Fava NJ, et al. The experiences of youth mental health peer workers over time: a qualitative study with longitudinal analysis. Community Ment Health J. 2020;56(5):906-914.
33. Devoe DJ, Farris MS, Townes P, et al. Interventions and transition in youth at risk of psychosis: a systematic review and meta-analyses. J Clin Psychiatry. 2020;81(3):17r12053. doi: 10.4088/JCP.17r12053
34. Bosnjak Kuharic D, Kekin I, Hew J, et al. Interventions for prodromal stage of psychosis. Cochrane Database Syst Rev. 2019;2019(11):CD012236
35. Dixon LB, Goldman HH, Srihari VH, et al. Transforming the treatment of schizophrenia in the United States: The RAISE Initiative. Annu Rev Clin Psychol. 2018;14:237-258.
36. Friedman-Yakoobian MS, Parrish EM, Eack SM, et al. Neurocognitive and social cognitive training for youth at clinical high risk (CHR) for psychosis: a randomized controlled feasibility trial. Schizophr Res. 2020;S0920-9964(20)30461-8. doi: 10.1016/j.schres.2020.09.005
37. Kane JM, Schooler NR, Marcy P, et al. Effect of long-acting injectable antipsychotics vs usual care on time to first hospitalization in early-phase schizophrenia: a randomized clinical trial. JAMA Psychiatry. 2020;77(12):1217-1224.
38. Morrison AP, Pyle M, Maughan D, et al. Antipsychotic medication versus psychological intervention versus a combination of both in adolescents with first-episode psychosis (MAPS): a multicentre, three-arm, randomised controlled pilot and feasibility study. Lancet Psychiatry. 2020;7(9):788-800.
39. Chen YQ, Li XR, Zhang L, et al. Therapeutic response is associated with antipsychotic-induced weight gain in drug-naive first-episode patients with schizophrenia: an 8-week prospective study. J Clin Psychiatry. 2021;82(3):20m13469. doi: 10.4088/JCP.20m13469
40. Insel TR. RAISE-ing our expectations for first-episode psychosis. Am J Psychiatry. 2016;173(4):311-312.
41. Tandon R, Goldman M. Overview of neurobiology. In: Janicak PG, Marder SR, Tandon R, et al, eds. Schizophrenia: recent advances in diagnosis and treatment. Springer; 2014:27-33.
42. National Institutes of Health. Accelerating Medicines Partnership. Schizophrenia. Accessed August 30, 2021. https://www.nih.gov/research-training/accelerating-medicines-partnership-amp/schizophrenia
43. Guloksuz S, van Os J. The slow death of the concept of schizophrenia and the painful birth of the psychosis spectrum. Psychol Med. 2018;48(2):229-244.
44. Christ-Crain M, Bichet DG, Fenske WK, et al. Diabetes insipidus. Nat Rev Dis Primers. 2019;5(1):54.
45. Ahmadi L, Goldman MB. Primary polydipsia: update. Best Pract Res Clin Endocrinol Metab. 2020;34(5):101469. doi: 10.1016/j.beem.2020.101469
46. Early Assessment and Support Alliance. National Early Psychosis Directory. Accessed August 30, 2021. https://easacommunity.org/national-directory.php
Neuroscience research over the past half century has failed to significantly advance the treatment of severe mental illness.1,2 Hence, evidence that a longer duration of untreated psychosis (DUP) aggravates—and early intervention with medication and social supports ameliorates—the long-term adverse consequences of psychotic disorders generated a great deal of interest.3,4 This knowledge led to the development of diverse early intervention services worldwide aimed at this putative “critical window.” It raised the possibility that appropriate interventions could prevent the long-term disability that makes chronic psychosis one of the most debilitating disorders.5,6 However, even beyond the varied cultural and economic confounds, it is difficult to assess, compare, and optimize program effectiveness.7 Obstacles include paucity of sufficiently powered, well-designed randomized controlled trials (RCTs), the absence of diagnostic biomarkers or other prognostic indicators to better account for the inherent heterogeneity in the population and associated outcomes, and the absence of modifiable risk factors that can guide interventions and provide intermediate outcomes.4,8-10
To better appreciate these issues, it is important to distinguish whether a program is designed to prevent psychosis, or to mitigate the effects of psychosis. Two models include the:
- Prevention model, which focuses on young individuals who are not yet overtly psychotic but at high risk
- First-episode recovery model, which focuses on those who have experienced a first episode of psychosis (FEP) but have not yet developed a chronic disorder.
Both models share long-term goals and are hampered by many of the same issues summarized above. They both deviate markedly from the standard medical model by including psychosocial services designed to promote restoration of a self-defined trajectory to greater independence.11-14 The 2 differ, however, in the challenges they must overcome to produce their sample populations and establish effective interventions.10,15,16
In this article, we provide a succinct overview of these issues and a set of recommendations based on a “strength-based” approach. This approach focuses on finding common ground between patients, their support system, and the treatment team in the service of empowering patients to resume responsibility for transition to adulthood.
The prevention model
While most prevention initiatives in medicine rely on the growing ability to target specific pathophysiologic pathways,3 preventing psychosis relies on clinical evidence showing that DUP and early interventions predict a better course of severe mental illness.17 In contrast, initiatives such as normalizing neonatal neuronal pathways are more consistent with the strategy utilized in other fields but have yet to yield a pathophysiologic target for psychosis.3,18
Initial efforts to identify ‘at-risk’ individuals
The prevention model of psychosis is based on the ability to identify young individuals at high risk for developing a psychotic disorder (Figure). The first screening measures were focused on prodromal psychosis (eg, significant loss of function, family history, and “intermittent” and “attenuated” psychotic symptoms). When applied to referred (ie, pre-screened) samples, 30% to 40% of this group who met criteria transitioned to psychosis over the next 1 to 3 years despite antidepressant and psychosocial interventions.19 Comprising 8 academic medical centers, the North American Prodrome Longitudinal Study (NAPLS) produced similar results using the Structured Interview for Prodromal Syndromes (SIPS).17 Thus, 30% to 50% of pre-screened individuals referred by school counselors and mental health professionals met SIPS criteria, and 35% of these individuals transitioned to psychosis over 30 months. The validity of this measure was further supported by the fact that higher baseline levels of unusual thought content, suspicion/paranoia, social impairment, and substance abuse successfully distinguished approximately 80% of those who transitioned to psychosis. The results of this first generation of screening studies were exciting because they seemed to demonstrate that highly concentrated samples of young persons at high risk of developing psychosis could be identified, and that fine-tuning the screening criteria could produce even more enriched samples (ie, positive predictive power).

Initial interventions produced promising results
The development of effective screening measures led to reports of effective treatment interventions. These were largely applied in a clinical staging model that restricted antipsychotic medications to those who failed to improve after receiving potentially “less toxic” interventions (eg, omega-3 polyunsaturated fatty acids and other antioxidants; psychotherapy; cognitive-behavioral therapy [CBT]; family therapy).5 While study designs were typically quasi-experimental, the interventions appeared to dramatically diminish the transition to psychosis (ie, approximately 50%).
Continue to: The first generation...
The first generation of RCTs appeared to confirm these results, although sample sizes were small, and most study designs assessed only a single intervention. Initial meta-analyses of these data reported that both CBT and antipsychotics appeared to prevent approximately one-half of individuals from becoming psychotic at 12 months, and more than one-third at 2 to 4 years, compared with treatment as usual.20
While some researchers challenged the validity of these findings,21-23 the results generated tremendous international enthusiasm and calls for widespread implementation.6 The number of early intervention services (EIS) centers increased dramatically worldwide, and in 2014 the National Institute for Health and Care Excellence released standards for interventions to prevent transition to psychosis.24 These included close monitoring, CBT and family interventions, and avoiding antipsychotics when possible.24
Focusing on sensitivity over specificity
The first generation of studies generated by the prevention model relied on outreach programs or referrals, which produced small samples of carefully selected, pre-screened individuals (Figure, Pre-screened) who were then screened again to establish the high-risk sample.25 While approximately 33% of these individuals became psychotic, the screening process required a very efficient means of eliminating those not at high-risk (given the ultimate target population represented only approximately .5% of young people) (Figure). The pre-screening and screening processes in these first-generation studies were labor-intensive but could only identify approximately 5% of those individuals destined to become psychotic over the next 2 or 3 years. Thus, alternative methods to enhance sensitivity were needed to extend programming to the general population.
Second-generation pre-screening (Figure; Step 1). New pre-screening methods were identified that captured more individuals destined to become psychotic. For example, approximately 90% of this population were registered in health care organizations (eg, health maintenance organizations) and received a psychiatric diagnosis in the year prior to the onset of psychosis (true positives).8 These samples, however, contained a much higher percentage of persons not destined to become psychotic, and somehow the issue of specificity (decreasing false positives) was minimized.8,9 For example, pre-screened samples contained 20 to 50 individuals not destined to become psychotic for each one who did.26 Since screening measures could only eliminate approximately 20% of this group (Figure, Step 2, page 25), second-generation transition rates fell from 30% to 40% to 2% to 10%.27,28
Other pre-screening approaches were introduced, but they also focused on capturing more of those destined to become psychotic (sensitivity) than eliminating those who would not (specificity). For instance, Australia opened more than 100 “Headspace” community centers nationwide designed to promote engagement and self-esteem in youth experiencing anxiety; depression; stress; relationship, work, or school problems; or bullying.13 Most services were free and included mental health staff who screened for psychosis and provided a wide range of services in a destigmatized setting. These methods identified at least an additional 5% to 7% of individuals destined to become psychotic, but to our knowledge, no data have been published on whether they helped eliminate those who did not.
Continue to: Second-generation screening
Second-generation screening (Figure, Step 2). A second screening aims to retain those pre-screened individuals who will become psychotic (ie, minimizing false negatives) while further minimizing those who do not (ie, minimizing false positives). The addition of cognitive, neural (eg, structural MRI; neurophysiologic), and biochemical (eg, inflammatory immune and stress) markers to the risk calculators have produced a sensitivity close to 100%.8,9 Unfortunately, these studies downplayed specificity, which remained approximately 20%.8,9 Specificity is critical not just because of concerns about stigma (ie, labeling people as pre-psychotic when they are not) but also because of the adverse effects of antipsychotic medications and the effects on future program development (interventions are costly and labor-intensive). Also, diluting the pool with individuals not at risk makes it nearly impossible to identify effective interventions (ie, power).27,28
While some studies focused on increasing specificity (to approximately 75%), this leads to an unacceptable loss of sensitivity (from 90% to 60%),29 with 40% of pre-screened individuals who would become psychotic being eliminated from the study population. The addition of other biological markers (eg, salivary cortisol)30 and use of learning health systems may be able to enhance these numbers (initial reports of specificity = 87% and sensitivity = 85%).8,9 This is accomplished by integrating artificial and human intelligence measures of clinical (symptom and neurocognitive measures) and biological (eg, polygenetic risk scores; gray matter volume) variables.31 However, even if these results are replicated, more effective pre-screening measures will be required.
Identifying a suitable sample population for prevention program studies is clearly more complicated than for FEP studies, where one can usually identify many of those in the at-risk population by their first hospitalization for psychotic symptoms. The issues of false positives (eg, substance-induced psychosis) and negatives (eg, slow deterioration, prominent negative symptoms) are important concerns, but proportionately far less significant.
Prevention and FEP interventions
Once a study sample is constituted, 1 to 3 years of treatment interventions are initiated. Interventions for prevention programs typically include CBT directed at attenuated psychosis (eg, reframing or de-catastrophizing unusual thoughts and minimizing distress associated with unusual perceptions); case management to facilitate personal, educational, and vocational goals; and family therapy in single or multi-group formats to educate one’s support system about the risk state and to minimize adverse familial responses.14 Many programs also include supported education or employment services to promote reintegration in age-appropriate activities; group therapy focused on substance abuse and social skills training; cognitive remediation to ameliorate the cognitive dysfunction; and an array of pharmacologic interventions designed to delay or prevent transition to psychosis or to alleviate symptoms. While most interventions are similar, FEP programs have recently included peer support staff. This appears to instill hope in newly diagnosed patients, provide role models, and provide peer supporters an opportunity to use their experiences to help others and earn income.32
The breadth and depth of these services are critical because retention in the program is highly dependent on participant engagement, which in turn is highly dependent on whether the program can help individuals get what they want (eg, friends, employment, education, more autonomy, physical health). The setting and atmosphere of the treatment program and the willingness/ability of staff to meet participants in the community are also important elements.11,12 In this context, the Headspace community centers are having an impact far beyond Australia and may prove to be a particularly good model.13
Continue to: Assessing prevention and FEP interventions
Assessing prevention and FEP interventions
The second generation of studies of prevention programs has not confirmed, let alone extended, the earlier findings and meta-analyses. A 2020 report concluded CBT was still the most promising intervention; it was more effective than control treatments at 12 and 18 months, although not at 6, 24, or 48 months.33 This review included controlled, open-label, and naturalistic studies that assessed family therapy; omega-3 polyunsaturated fatty acids; integrated psychological therapy (a package of interventions that included family education, CBT, social skills training, and cognitive remediation); N-methyl-
While these disappointing findings are at least partly attributable to the methodological challenges described above and in the Figure, other factors may hinder establishing effective interventions. In contrast to FEP studies, those focused on prevention had a very ambitious agenda (eliminating psychosis) and tended to downplay more modest intermediate outcomes. These studies also tended to assess new ideas with small samples rather than pursue promising findings with larger multi-site studies focused on a group of interventions. The authors of a Cochrane review observed “There is the impression that in this whole area there is a triumph of hope over adversity. There is the repeated hope invested in another—often unique—study question and then a study of fewer than 100 participants are completed. This results in the set of comparisons reported here, all 9 of which are too underpowered to really highlight clear differences.”34 To use a baseball analogy, it seems that investigators are “swinging for the fence” when a few singles are what’s really needed.
From the outset, the goals of FEP studies were more modest, largely ignoring the task of developing consensus definitions of recovery that require following patients for up to 5 to 10 years. Instead, they use intermediate endpoints based on adapting treatments that already appeared effective in patients with chronic mental disorders.35 As a consequence, researchers examining FEP demonstrated clear, albeit limited, salutary effects using large multi-site trials and previously established outcome measures.3,10,36 For instance, the Recovery After an Initial Schizophrenia Episode-Early Treatment Program (RAISE-ETP) study was a 2-year, multi-site RCT (N = 404) funded by the National Institute of Mental Health (NIMH). The investigators reported improved indices of social function (eg, quality of life; education and work participation) and total ratings of psychopathology and depression compared with treatment as usual. Furthermore, they established that DUP predicted treatment response.35 The latter finding was underscored by improvement being limited to the 50% with <74 weeks DUP. Annual costs of the program per 1 standard deviation improvement in quality of life were approximately $1,000 for patients with <74 weeks DUP and $40,000 for those with >74 weeks DUP. Concurrent meta-analyses confirmed and extended these findings,16 showing higher remission rates; diminished relapses and hospital admissions; greater engagement in programming; greater involvement in work and school; improved quality of life; and other steps toward recovery. These studies were also able to establish a clear benefit of antipsychotic medications, particularly a high acceptance of long-acting injectable antipsychotic formulations, which promoted adherence and decreased some adverse events37; and early use of clozapine therapy, which improved remission rates and longer-term outcomes.38 Other findings underscored the need to anticipate and address new problems associated with effective antipsychotic therapy (eg, antipsychotic response correlates with weight gain, a particularly intolerable adverse event for this age group).39 Providing pre-emptive strategies such as exercise groups and nutritional education may be necessary to maintain adherence.
Limitations of FEP studies
The effect sizes in these FEP studies were small to medium on outcome measures tracking recovery and associated indicators (eg, global functioning, school/work participation, treatment engagement); the number needed to treat for each of these was >10. There is no clear evidence that recovery programs such as RAISE-ETP actually reduce longer-term disability. Most studies showed disability payments increased while clinical benefits tended to fade over time. In addition, by grouping interventions together, the studies made it difficult to identify effective vs ineffective treatments, let alone determine how best to personalize therapy for participants in future studies.
The next generation of FEP studies
While limited in scope, the results of the recent FEP studies justify a next generation of recovery interventions designed to address these shortcomings and optimize program outcomes.39 Most previous FEP studies were conducted in community mental health center settings, thus eliminating the need to transition services developed in academia into the “real world.” The next generation of NIMH studies will be primarily conducted in analogous settings under the Early Psychosis Intervention Network (EPINET).40 EPINET’s study design echoes that responsible for the stepwise successes in the late 20th century that produced cures for the deadliest childhood cancer, acute lymphoblastic leukemia (ALL). This disease was successfully treated by modifying diverse evidence-based practices without relying on pharmacologic or other major treatment breakthroughs. Despite this, the effort yielded successful personalized interventions that were not obtainable for other severe childhood conditions.40 EPINET hopes to automate much of these stepwise advances with a learning health system. This program relies on data routinely collected in clinical practice to drive the process of scientific discovery. Specifically, it determines the relationships between clinical features, biologic measures, treatment characteristics, and symptomatic and functional outcomes. EPINET aims to accelerate our understanding of biomarkers of psychosis risk and onset, as well as factors associated with recovery and cure. Dashboard displays of outcomes will allow for real-time comparisons within and across early intervention clinics. This in turn identifies performance gaps and drives continuous quality improvement.
Continue to: Barriers to optimizing program efficacy for both models
Barriers to optimizing program efficacy for both models
Unfortunately, there are stark differences between ALL and severe mental disorders that potentially jeopardize the achievement of these aims, despite the advances in data analytic abilities that drive the learning health system. Specifically, the heterogeneity of psychotic illnesses and the absence of reliable prognostic and modifiable risk markers (responsible for failed efforts to enhance treatment of serious mental illness over the last half century1,2,41) are unlikely to be resolved by a learning health system. These measures are vital to determine whether specific interventions are effective, particularly given the absence of a randomized control group in the EPINET/learning health system design. Fortunately, however, the National Institutes for Health has recently initiated the Accelerating Medicines Partnership–Schizophrenia (AMP-SCZ). This approach seeks “promising biological markers that can help identify those at risk of developing schizophrenia as early as possible, track the progression of symptoms and other outcomes and ultimately define targets for treatment development.”42 The Box1,4,9,10,36,41,43-45 describes some of the challenges involved in identifying biomarkers of severe mental illness.
Box
Biomarkers and modifiable risk factors4,9,10,41,43 are at the core of personalized medicine and its ultimate objective (ie, theragnostics). This is the ability to identify the correct intervention for a disorder based on a biomarker of the illness.10,36 The inability to identify biomarkers of severe mental illness is multifactorial but in part may be attributable to “looking in all the wrong places.”41 By focusing on neural processes that generate psychiatric symptomatology, investigators are assuming they can bridge the “mind gap”1 and specifically distinguish between pathological, compensatory, or collateral measures of poorly characterized limbic neural functions.41
It may be more productive to identify a pathological process within the limbic system that produces a medical condition as well as the mental disorder. If one can isolate the pathologic limbic circuit activity responsible for a medical condition, one may be able to reproduce this in animal models and determine whether analogous processes contribute to the core features of the mental illness. Characterization of the aberrant neural circuit in animal models also could yield targets for future therapies. For example, episodic water intoxication in a discrete subset of patients with schizophrenia44 appears to arise from a stress diathesis produced by anterior hippocampal pathology that disrupts regulation of antidiuretic hormone, oxytocin, and hypothalamic-pituitary-adrenal axis secretion. These patients also exhibit psychogenic polydipsia that may be a consequence of the same hippocampal pathology that disrupts ventral striatal and lateral hypothalamic circuits. These circuits, in turn, also modulate motivated behaviors and cognitive processes likely relevant to psychosis.45
A strength-based approach
The absence of sufficiently powered RCTs for prevention studies and the reliance on intermediate outcomes for FEP studies leaves unanswered whether such programs can effectively prevent chronic psychosis at a cost society is willing to pay. Still, substantial evidence indicates that outreach, long-acting injectable antipsychotics, early consideration of clozapine, family therapy, CBT for psychosis/attenuated psychosis, and services focused on competitive employment can preserve social and occupational functioning.16,34 Until these broader questions are more definitively addressed, it seems reasonable to apply what we have learned (Table11,12,35,37-39,46).

Simply avoiding the most divisive aspects of the medical model that inadvertently promote stigma and undercut self-confidence may help maintain patients’ willingness to learn how best to apply their strengths and manage their limitations.11 The progression to enduring psychotic features (eg, fixed delusions) may reflect ongoing social isolation and alienation. A strength-based approach seeks first to establish common goals (eg, school, work, friends, family support, housing, leaving home) and then works to empower the patient to successfully reach those goals.35 This typically involves giving them the opportunity to fail, avoiding criticism when they do, and focusing on these experiences as learning opportunities from which success can ultimately result.
It is difficult to offer all these services in a typical private practice setting. Instead, it may make more sense to use one of the hundreds of early intervention services programs in the United States.46 If a psychiatric clinician is dedicated to working with this population, it may also be possible to establish ongoing relationships with primary care physicians, family and CBT therapists, family support services (eg, National Alliance on Mental Illness), caseworkers and employment counselors. In essence, a psychiatrist may be able re-create a multidisciplinary effort by taking advantage of the expertise of these various professionals. The challenge is to create a consistent message for patients and families in the absence of regular meetings with the clinical team, although the recent reliance on and improved sophistication of virtual meetings may help. Psychiatrists often play a critical role even when the patient is not prescribed medication, partly because they are most comfortable handling the risks and may have the most comprehensive understanding of the issues at play. When medications are appropriate and patients with FEP are willing to take them, early consideration of long-acting injectable antipsychotics and clozapine may provide better stabilization and diminish the risk of earlier and more frequent relapses.
Bottom Line
Early interventions for psychosis include the prevention model and the first-episode recovery model. It is difficult to assess, compare, and optimize the effectiveness of such programs. Current evidence supports a ‘strength-based’ approach focused on finding common ground between patients, their support system, and the treatment team.
Related Resources
- Early Assessment and Support Alliance. National Early Psychosis Directory. https://easacommunity.org/nationaldirectory.php
- Kane JM, Robinson DG, Schooler NR, et al. Comprehensive versus usual community care for first-episode psychosis: 2-year outcomes from the NIMH RAISE Early Treatment Program. Am J Psychiatry. 2016 ;173(4):362-372
Drug Brand Name
Clozapine • Clozaril
Neuroscience research over the past half century has failed to significantly advance the treatment of severe mental illness.1,2 Hence, evidence that a longer duration of untreated psychosis (DUP) aggravates—and early intervention with medication and social supports ameliorates—the long-term adverse consequences of psychotic disorders generated a great deal of interest.3,4 This knowledge led to the development of diverse early intervention services worldwide aimed at this putative “critical window.” It raised the possibility that appropriate interventions could prevent the long-term disability that makes chronic psychosis one of the most debilitating disorders.5,6 However, even beyond the varied cultural and economic confounds, it is difficult to assess, compare, and optimize program effectiveness.7 Obstacles include paucity of sufficiently powered, well-designed randomized controlled trials (RCTs), the absence of diagnostic biomarkers or other prognostic indicators to better account for the inherent heterogeneity in the population and associated outcomes, and the absence of modifiable risk factors that can guide interventions and provide intermediate outcomes.4,8-10
To better appreciate these issues, it is important to distinguish whether a program is designed to prevent psychosis, or to mitigate the effects of psychosis. Two models include the:
- Prevention model, which focuses on young individuals who are not yet overtly psychotic but at high risk
- First-episode recovery model, which focuses on those who have experienced a first episode of psychosis (FEP) but have not yet developed a chronic disorder.
Both models share long-term goals and are hampered by many of the same issues summarized above. They both deviate markedly from the standard medical model by including psychosocial services designed to promote restoration of a self-defined trajectory to greater independence.11-14 The 2 differ, however, in the challenges they must overcome to produce their sample populations and establish effective interventions.10,15,16
In this article, we provide a succinct overview of these issues and a set of recommendations based on a “strength-based” approach. This approach focuses on finding common ground between patients, their support system, and the treatment team in the service of empowering patients to resume responsibility for transition to adulthood.
The prevention model
While most prevention initiatives in medicine rely on the growing ability to target specific pathophysiologic pathways,3 preventing psychosis relies on clinical evidence showing that DUP and early interventions predict a better course of severe mental illness.17 In contrast, initiatives such as normalizing neonatal neuronal pathways are more consistent with the strategy utilized in other fields but have yet to yield a pathophysiologic target for psychosis.3,18
Initial efforts to identify ‘at-risk’ individuals
The prevention model of psychosis is based on the ability to identify young individuals at high risk for developing a psychotic disorder (Figure). The first screening measures were focused on prodromal psychosis (eg, significant loss of function, family history, and “intermittent” and “attenuated” psychotic symptoms). When applied to referred (ie, pre-screened) samples, 30% to 40% of this group who met criteria transitioned to psychosis over the next 1 to 3 years despite antidepressant and psychosocial interventions.19 Comprising 8 academic medical centers, the North American Prodrome Longitudinal Study (NAPLS) produced similar results using the Structured Interview for Prodromal Syndromes (SIPS).17 Thus, 30% to 50% of pre-screened individuals referred by school counselors and mental health professionals met SIPS criteria, and 35% of these individuals transitioned to psychosis over 30 months. The validity of this measure was further supported by the fact that higher baseline levels of unusual thought content, suspicion/paranoia, social impairment, and substance abuse successfully distinguished approximately 80% of those who transitioned to psychosis. The results of this first generation of screening studies were exciting because they seemed to demonstrate that highly concentrated samples of young persons at high risk of developing psychosis could be identified, and that fine-tuning the screening criteria could produce even more enriched samples (ie, positive predictive power).

Initial interventions produced promising results
The development of effective screening measures led to reports of effective treatment interventions. These were largely applied in a clinical staging model that restricted antipsychotic medications to those who failed to improve after receiving potentially “less toxic” interventions (eg, omega-3 polyunsaturated fatty acids and other antioxidants; psychotherapy; cognitive-behavioral therapy [CBT]; family therapy).5 While study designs were typically quasi-experimental, the interventions appeared to dramatically diminish the transition to psychosis (ie, approximately 50%).
Continue to: The first generation...
The first generation of RCTs appeared to confirm these results, although sample sizes were small, and most study designs assessed only a single intervention. Initial meta-analyses of these data reported that both CBT and antipsychotics appeared to prevent approximately one-half of individuals from becoming psychotic at 12 months, and more than one-third at 2 to 4 years, compared with treatment as usual.20
While some researchers challenged the validity of these findings,21-23 the results generated tremendous international enthusiasm and calls for widespread implementation.6 The number of early intervention services (EIS) centers increased dramatically worldwide, and in 2014 the National Institute for Health and Care Excellence released standards for interventions to prevent transition to psychosis.24 These included close monitoring, CBT and family interventions, and avoiding antipsychotics when possible.24
Focusing on sensitivity over specificity
The first generation of studies generated by the prevention model relied on outreach programs or referrals, which produced small samples of carefully selected, pre-screened individuals (Figure, Pre-screened) who were then screened again to establish the high-risk sample.25 While approximately 33% of these individuals became psychotic, the screening process required a very efficient means of eliminating those not at high-risk (given the ultimate target population represented only approximately .5% of young people) (Figure). The pre-screening and screening processes in these first-generation studies were labor-intensive but could only identify approximately 5% of those individuals destined to become psychotic over the next 2 or 3 years. Thus, alternative methods to enhance sensitivity were needed to extend programming to the general population.
Second-generation pre-screening (Figure; Step 1). New pre-screening methods were identified that captured more individuals destined to become psychotic. For example, approximately 90% of this population were registered in health care organizations (eg, health maintenance organizations) and received a psychiatric diagnosis in the year prior to the onset of psychosis (true positives).8 These samples, however, contained a much higher percentage of persons not destined to become psychotic, and somehow the issue of specificity (decreasing false positives) was minimized.8,9 For example, pre-screened samples contained 20 to 50 individuals not destined to become psychotic for each one who did.26 Since screening measures could only eliminate approximately 20% of this group (Figure, Step 2, page 25), second-generation transition rates fell from 30% to 40% to 2% to 10%.27,28
Other pre-screening approaches were introduced, but they also focused on capturing more of those destined to become psychotic (sensitivity) than eliminating those who would not (specificity). For instance, Australia opened more than 100 “Headspace” community centers nationwide designed to promote engagement and self-esteem in youth experiencing anxiety; depression; stress; relationship, work, or school problems; or bullying.13 Most services were free and included mental health staff who screened for psychosis and provided a wide range of services in a destigmatized setting. These methods identified at least an additional 5% to 7% of individuals destined to become psychotic, but to our knowledge, no data have been published on whether they helped eliminate those who did not.
Continue to: Second-generation screening
Second-generation screening (Figure, Step 2). A second screening aims to retain those pre-screened individuals who will become psychotic (ie, minimizing false negatives) while further minimizing those who do not (ie, minimizing false positives). The addition of cognitive, neural (eg, structural MRI; neurophysiologic), and biochemical (eg, inflammatory immune and stress) markers to the risk calculators have produced a sensitivity close to 100%.8,9 Unfortunately, these studies downplayed specificity, which remained approximately 20%.8,9 Specificity is critical not just because of concerns about stigma (ie, labeling people as pre-psychotic when they are not) but also because of the adverse effects of antipsychotic medications and the effects on future program development (interventions are costly and labor-intensive). Also, diluting the pool with individuals not at risk makes it nearly impossible to identify effective interventions (ie, power).27,28
While some studies focused on increasing specificity (to approximately 75%), this leads to an unacceptable loss of sensitivity (from 90% to 60%),29 with 40% of pre-screened individuals who would become psychotic being eliminated from the study population. The addition of other biological markers (eg, salivary cortisol)30 and use of learning health systems may be able to enhance these numbers (initial reports of specificity = 87% and sensitivity = 85%).8,9 This is accomplished by integrating artificial and human intelligence measures of clinical (symptom and neurocognitive measures) and biological (eg, polygenetic risk scores; gray matter volume) variables.31 However, even if these results are replicated, more effective pre-screening measures will be required.
Identifying a suitable sample population for prevention program studies is clearly more complicated than for FEP studies, where one can usually identify many of those in the at-risk population by their first hospitalization for psychotic symptoms. The issues of false positives (eg, substance-induced psychosis) and negatives (eg, slow deterioration, prominent negative symptoms) are important concerns, but proportionately far less significant.
Prevention and FEP interventions
Once a study sample is constituted, 1 to 3 years of treatment interventions are initiated. Interventions for prevention programs typically include CBT directed at attenuated psychosis (eg, reframing or de-catastrophizing unusual thoughts and minimizing distress associated with unusual perceptions); case management to facilitate personal, educational, and vocational goals; and family therapy in single or multi-group formats to educate one’s support system about the risk state and to minimize adverse familial responses.14 Many programs also include supported education or employment services to promote reintegration in age-appropriate activities; group therapy focused on substance abuse and social skills training; cognitive remediation to ameliorate the cognitive dysfunction; and an array of pharmacologic interventions designed to delay or prevent transition to psychosis or to alleviate symptoms. While most interventions are similar, FEP programs have recently included peer support staff. This appears to instill hope in newly diagnosed patients, provide role models, and provide peer supporters an opportunity to use their experiences to help others and earn income.32
The breadth and depth of these services are critical because retention in the program is highly dependent on participant engagement, which in turn is highly dependent on whether the program can help individuals get what they want (eg, friends, employment, education, more autonomy, physical health). The setting and atmosphere of the treatment program and the willingness/ability of staff to meet participants in the community are also important elements.11,12 In this context, the Headspace community centers are having an impact far beyond Australia and may prove to be a particularly good model.13
Continue to: Assessing prevention and FEP interventions
Assessing prevention and FEP interventions
The second generation of studies of prevention programs has not confirmed, let alone extended, the earlier findings and meta-analyses. A 2020 report concluded CBT was still the most promising intervention; it was more effective than control treatments at 12 and 18 months, although not at 6, 24, or 48 months.33 This review included controlled, open-label, and naturalistic studies that assessed family therapy; omega-3 polyunsaturated fatty acids; integrated psychological therapy (a package of interventions that included family education, CBT, social skills training, and cognitive remediation); N-methyl-
While these disappointing findings are at least partly attributable to the methodological challenges described above and in the Figure, other factors may hinder establishing effective interventions. In contrast to FEP studies, those focused on prevention had a very ambitious agenda (eliminating psychosis) and tended to downplay more modest intermediate outcomes. These studies also tended to assess new ideas with small samples rather than pursue promising findings with larger multi-site studies focused on a group of interventions. The authors of a Cochrane review observed “There is the impression that in this whole area there is a triumph of hope over adversity. There is the repeated hope invested in another—often unique—study question and then a study of fewer than 100 participants are completed. This results in the set of comparisons reported here, all 9 of which are too underpowered to really highlight clear differences.”34 To use a baseball analogy, it seems that investigators are “swinging for the fence” when a few singles are what’s really needed.
From the outset, the goals of FEP studies were more modest, largely ignoring the task of developing consensus definitions of recovery that require following patients for up to 5 to 10 years. Instead, they use intermediate endpoints based on adapting treatments that already appeared effective in patients with chronic mental disorders.35 As a consequence, researchers examining FEP demonstrated clear, albeit limited, salutary effects using large multi-site trials and previously established outcome measures.3,10,36 For instance, the Recovery After an Initial Schizophrenia Episode-Early Treatment Program (RAISE-ETP) study was a 2-year, multi-site RCT (N = 404) funded by the National Institute of Mental Health (NIMH). The investigators reported improved indices of social function (eg, quality of life; education and work participation) and total ratings of psychopathology and depression compared with treatment as usual. Furthermore, they established that DUP predicted treatment response.35 The latter finding was underscored by improvement being limited to the 50% with <74 weeks DUP. Annual costs of the program per 1 standard deviation improvement in quality of life were approximately $1,000 for patients with <74 weeks DUP and $40,000 for those with >74 weeks DUP. Concurrent meta-analyses confirmed and extended these findings,16 showing higher remission rates; diminished relapses and hospital admissions; greater engagement in programming; greater involvement in work and school; improved quality of life; and other steps toward recovery. These studies were also able to establish a clear benefit of antipsychotic medications, particularly a high acceptance of long-acting injectable antipsychotic formulations, which promoted adherence and decreased some adverse events37; and early use of clozapine therapy, which improved remission rates and longer-term outcomes.38 Other findings underscored the need to anticipate and address new problems associated with effective antipsychotic therapy (eg, antipsychotic response correlates with weight gain, a particularly intolerable adverse event for this age group).39 Providing pre-emptive strategies such as exercise groups and nutritional education may be necessary to maintain adherence.
Limitations of FEP studies
The effect sizes in these FEP studies were small to medium on outcome measures tracking recovery and associated indicators (eg, global functioning, school/work participation, treatment engagement); the number needed to treat for each of these was >10. There is no clear evidence that recovery programs such as RAISE-ETP actually reduce longer-term disability. Most studies showed disability payments increased while clinical benefits tended to fade over time. In addition, by grouping interventions together, the studies made it difficult to identify effective vs ineffective treatments, let alone determine how best to personalize therapy for participants in future studies.
The next generation of FEP studies
While limited in scope, the results of the recent FEP studies justify a next generation of recovery interventions designed to address these shortcomings and optimize program outcomes.39 Most previous FEP studies were conducted in community mental health center settings, thus eliminating the need to transition services developed in academia into the “real world.” The next generation of NIMH studies will be primarily conducted in analogous settings under the Early Psychosis Intervention Network (EPINET).40 EPINET’s study design echoes that responsible for the stepwise successes in the late 20th century that produced cures for the deadliest childhood cancer, acute lymphoblastic leukemia (ALL). This disease was successfully treated by modifying diverse evidence-based practices without relying on pharmacologic or other major treatment breakthroughs. Despite this, the effort yielded successful personalized interventions that were not obtainable for other severe childhood conditions.40 EPINET hopes to automate much of these stepwise advances with a learning health system. This program relies on data routinely collected in clinical practice to drive the process of scientific discovery. Specifically, it determines the relationships between clinical features, biologic measures, treatment characteristics, and symptomatic and functional outcomes. EPINET aims to accelerate our understanding of biomarkers of psychosis risk and onset, as well as factors associated with recovery and cure. Dashboard displays of outcomes will allow for real-time comparisons within and across early intervention clinics. This in turn identifies performance gaps and drives continuous quality improvement.
Continue to: Barriers to optimizing program efficacy for both models
Barriers to optimizing program efficacy for both models
Unfortunately, there are stark differences between ALL and severe mental disorders that potentially jeopardize the achievement of these aims, despite the advances in data analytic abilities that drive the learning health system. Specifically, the heterogeneity of psychotic illnesses and the absence of reliable prognostic and modifiable risk markers (responsible for failed efforts to enhance treatment of serious mental illness over the last half century1,2,41) are unlikely to be resolved by a learning health system. These measures are vital to determine whether specific interventions are effective, particularly given the absence of a randomized control group in the EPINET/learning health system design. Fortunately, however, the National Institutes for Health has recently initiated the Accelerating Medicines Partnership–Schizophrenia (AMP-SCZ). This approach seeks “promising biological markers that can help identify those at risk of developing schizophrenia as early as possible, track the progression of symptoms and other outcomes and ultimately define targets for treatment development.”42 The Box1,4,9,10,36,41,43-45 describes some of the challenges involved in identifying biomarkers of severe mental illness.
Box
Biomarkers and modifiable risk factors4,9,10,41,43 are at the core of personalized medicine and its ultimate objective (ie, theragnostics). This is the ability to identify the correct intervention for a disorder based on a biomarker of the illness.10,36 The inability to identify biomarkers of severe mental illness is multifactorial but in part may be attributable to “looking in all the wrong places.”41 By focusing on neural processes that generate psychiatric symptomatology, investigators are assuming they can bridge the “mind gap”1 and specifically distinguish between pathological, compensatory, or collateral measures of poorly characterized limbic neural functions.41
It may be more productive to identify a pathological process within the limbic system that produces a medical condition as well as the mental disorder. If one can isolate the pathologic limbic circuit activity responsible for a medical condition, one may be able to reproduce this in animal models and determine whether analogous processes contribute to the core features of the mental illness. Characterization of the aberrant neural circuit in animal models also could yield targets for future therapies. For example, episodic water intoxication in a discrete subset of patients with schizophrenia44 appears to arise from a stress diathesis produced by anterior hippocampal pathology that disrupts regulation of antidiuretic hormone, oxytocin, and hypothalamic-pituitary-adrenal axis secretion. These patients also exhibit psychogenic polydipsia that may be a consequence of the same hippocampal pathology that disrupts ventral striatal and lateral hypothalamic circuits. These circuits, in turn, also modulate motivated behaviors and cognitive processes likely relevant to psychosis.45
A strength-based approach
The absence of sufficiently powered RCTs for prevention studies and the reliance on intermediate outcomes for FEP studies leaves unanswered whether such programs can effectively prevent chronic psychosis at a cost society is willing to pay. Still, substantial evidence indicates that outreach, long-acting injectable antipsychotics, early consideration of clozapine, family therapy, CBT for psychosis/attenuated psychosis, and services focused on competitive employment can preserve social and occupational functioning.16,34 Until these broader questions are more definitively addressed, it seems reasonable to apply what we have learned (Table11,12,35,37-39,46).

Simply avoiding the most divisive aspects of the medical model that inadvertently promote stigma and undercut self-confidence may help maintain patients’ willingness to learn how best to apply their strengths and manage their limitations.11 The progression to enduring psychotic features (eg, fixed delusions) may reflect ongoing social isolation and alienation. A strength-based approach seeks first to establish common goals (eg, school, work, friends, family support, housing, leaving home) and then works to empower the patient to successfully reach those goals.35 This typically involves giving them the opportunity to fail, avoiding criticism when they do, and focusing on these experiences as learning opportunities from which success can ultimately result.
It is difficult to offer all these services in a typical private practice setting. Instead, it may make more sense to use one of the hundreds of early intervention services programs in the United States.46 If a psychiatric clinician is dedicated to working with this population, it may also be possible to establish ongoing relationships with primary care physicians, family and CBT therapists, family support services (eg, National Alliance on Mental Illness), caseworkers and employment counselors. In essence, a psychiatrist may be able re-create a multidisciplinary effort by taking advantage of the expertise of these various professionals. The challenge is to create a consistent message for patients and families in the absence of regular meetings with the clinical team, although the recent reliance on and improved sophistication of virtual meetings may help. Psychiatrists often play a critical role even when the patient is not prescribed medication, partly because they are most comfortable handling the risks and may have the most comprehensive understanding of the issues at play. When medications are appropriate and patients with FEP are willing to take them, early consideration of long-acting injectable antipsychotics and clozapine may provide better stabilization and diminish the risk of earlier and more frequent relapses.
Bottom Line
Early interventions for psychosis include the prevention model and the first-episode recovery model. It is difficult to assess, compare, and optimize the effectiveness of such programs. Current evidence supports a ‘strength-based’ approach focused on finding common ground between patients, their support system, and the treatment team.
Related Resources
- Early Assessment and Support Alliance. National Early Psychosis Directory. https://easacommunity.org/nationaldirectory.php
- Kane JM, Robinson DG, Schooler NR, et al. Comprehensive versus usual community care for first-episode psychosis: 2-year outcomes from the NIMH RAISE Early Treatment Program. Am J Psychiatry. 2016 ;173(4):362-372
Drug Brand Name
Clozapine • Clozaril
1. Hyman SE. Revolution stalled. Sci Transl Med. 2012;4(155):155cm11. doi: 10.1126/scitranslmed.3003142
2. Harrington A. Mind fixers: psychiatry’s troubled search for the biology of mental illness. W.W. Norton & Company; 2019.
3. Millan MJ, Andrieux A, Bartzokis G, et al. Altering the course of schizophrenia: progress and perspectives. Nat Rev Drug Discov. 2016;15(7):485-515.
4. Lieberman JA, Small SA, Girgis RR. Early detection and preventive intervention in schizophrenia: from fantasy to reality. Am J Psychiatry. 2019;176(10):794-810.
5. McGorry PD, Nelson B, Nordentoft M, et al. Intervention in individuals at ultra-high risk for psychosis: a review and future directions. J Clin Psychiatry. 2009;70(9):1206-1212.
6. Csillag C, Nordentoft M, Mizuno M, et al. Early intervention in psychosis: From clinical intervention to health system implementation. Early Interv Psychiatry. 2018;12(4):757-764.
7. McGorry PD, Ratheesh A, O’Donoghue B. Early intervention—an implementation challenge for 21st century mental health care. JAMA Psychiatry. 2018;75(6):545-546.
8. Rosenheck R. Toward dissemination of secondary prevention for psychosis. Am J Psychiatry. 2018;175(5):393-394.
9. Fusar-Poli P, Salazar de Pablo G, Correll CU, et al. Prevention of psychosis: advances in detection, prognosis, and intervention. JAMA Psychiatry. 2020;77(7):755-765.
10. Oliver D, Reilly TJ, Baccaredda Boy O, et al. What causes the onset of psychosis in individuals at clinical high risk? A meta-analysis of risk and protective factors. Schizophr Bull. 2020;46(1):110-120.
11. Tindall R, Simmons M, Allott K, et al. Disengagement processes within an early intervention service for first-episode psychosis: a longitudinal, qualitative, multi-perspective study. Front Psychiatry. 2020;11:565-565.
12. Dixon LB, Holoshitz Y, Nossel I. Treatment engagement of individuals experiencing mental illness: review and update. World Psychiatry. 2016;15(1):13-20.
13. Rickwood D, Paraskakis M, Quin D, et al. Australia’s innovation in youth mental health care: The headspace centre model. Early Interv Psychiatry. 2019;13(1):159-166.
14. Woodberry KA, Shapiro DI, Bryant C, et al. Progress and future directions in research on the psychosis prodrome: a review for clinicians. Harv Rev Psychiatry. 2016;24(2):87-103.
15. Gupta T, Mittal VA. Advances in clinical staging, early intervention, and the prevention of psychosis. F1000Res. 2019;8:F1000 Faculty Rev-2027. doi: 10.12688/f1000research.20346.1
16. Correll CU, Galling B, Pawar A, et al. Comparison of early intervention services vs treatment as usual for early-phase psychosis: a systematic review, meta-analysis, and meta-regression. JAMA Psychiatry. 2018;75(6):555-565.
17. Cannon TD, Cadenhead K, Cornblatt B, et al. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry. 2008;65(1):28-37.
18. Sommer IE, Bearden CE, van Dellen E, et al. Early interventions in risk groups for schizophrenia: what are we waiting for? NPJ Schizophr. 2016;2(1):16003-16003.
19. McGorry PD, Nelson B. Clinical high risk for psychosis—not seeing the trees for the wood. JAMA Psychiatry. 2020;77(7):559-560.
20. van der Gaag M, Smit F, Bechdolf A, et al. Preventing a first episode of psychosis: meta-analysis of randomized controlled prevention trials of 12 month and longer-term follow-ups. Schizophr Res. 2013;149(1):56-62.
21. Marshall M, Rathbone J. Early intervention for psychosis. Cochrane Database Syst Rev. 2011;(6):CD004718. doi: 10.1002/14651858.CD004718.pub3
22. Heinssen RK, Insel TR. Preventing the onset of psychosis: not quite there yet. Schizophr Bull. 2015;41(1):28-29.
23. Amos AJ. Evidence that treatment prevents transition to psychosis in ultra-high-risk patients remains questionable. Schizophr Res. 2014;153(1):240.
24. National Institute for Health and Care Excellence. Psychosis and schizophrenia in adults: prevention and management. Clinical guideline [CG178]. 1.3.7 How to deliver psychological interventions. Published February 12, 2014. Updated March 1, 2014. Accessed August 30, 2021. https://www.nice.org.uk/guidance/cg178/chapter/recommendations#how-to-deliver-psychological-interventions
25. Fusar-Poli P, Werbeloff N, Rutigliano G, et al. Transdiagnostic risk calculator for the automatic detection of individuals at risk and the prediction of psychosis: second replication in an independent National Health Service Trust. Schizophr Bull. 2019;45(3):562-570.
26. Fusar-Poli P, Oliver D, Spada G, et al. The case for improved transdiagnostic detection of first-episode psychosis: electronic health record cohort study. Schizophr Res. 2021;228:547-554.
27. Fusar-Poli P. Negative psychosis prevention trials. JAMA Psychiatry. 2017;74(6):651.
28. Cuijpers P, Smit F, Furukawa TA. Most at‐risk individuals will not develop a mental disorder: the limited predictive strength of risk factors. World Psychiatry. 2021;20(2):224-225.
29. Carrión RE, Cornblatt BA, Burton CZ, et al. Personalized prediction of psychosis: external validation of the NAPLS-2 psychosis risk calculator with the EDIPPP Project. Am J Psychiatry. 2016;173(10):989-996.
30. Worthington MA, Walker EF, Addington J, et al. Incorporating cortisol into the NAPLS2 individualized risk calculator for prediction of psychosis. Schizophr Res. 2021;227:95-100.
31. Koutsouleris N, Dwyer DB, Degenhardt F, et al. Multimodal machine learning workflows for prediction of psychosis in patients with clinical high-risk syndromes and recent-onset depression. JAMA Psychiatry. 2021;78(2):195-209.
32. Simmons MB, Grace D, Fava NJ, et al. The experiences of youth mental health peer workers over time: a qualitative study with longitudinal analysis. Community Ment Health J. 2020;56(5):906-914.
33. Devoe DJ, Farris MS, Townes P, et al. Interventions and transition in youth at risk of psychosis: a systematic review and meta-analyses. J Clin Psychiatry. 2020;81(3):17r12053. doi: 10.4088/JCP.17r12053
34. Bosnjak Kuharic D, Kekin I, Hew J, et al. Interventions for prodromal stage of psychosis. Cochrane Database Syst Rev. 2019;2019(11):CD012236
35. Dixon LB, Goldman HH, Srihari VH, et al. Transforming the treatment of schizophrenia in the United States: The RAISE Initiative. Annu Rev Clin Psychol. 2018;14:237-258.
36. Friedman-Yakoobian MS, Parrish EM, Eack SM, et al. Neurocognitive and social cognitive training for youth at clinical high risk (CHR) for psychosis: a randomized controlled feasibility trial. Schizophr Res. 2020;S0920-9964(20)30461-8. doi: 10.1016/j.schres.2020.09.005
37. Kane JM, Schooler NR, Marcy P, et al. Effect of long-acting injectable antipsychotics vs usual care on time to first hospitalization in early-phase schizophrenia: a randomized clinical trial. JAMA Psychiatry. 2020;77(12):1217-1224.
38. Morrison AP, Pyle M, Maughan D, et al. Antipsychotic medication versus psychological intervention versus a combination of both in adolescents with first-episode psychosis (MAPS): a multicentre, three-arm, randomised controlled pilot and feasibility study. Lancet Psychiatry. 2020;7(9):788-800.
39. Chen YQ, Li XR, Zhang L, et al. Therapeutic response is associated with antipsychotic-induced weight gain in drug-naive first-episode patients with schizophrenia: an 8-week prospective study. J Clin Psychiatry. 2021;82(3):20m13469. doi: 10.4088/JCP.20m13469
40. Insel TR. RAISE-ing our expectations for first-episode psychosis. Am J Psychiatry. 2016;173(4):311-312.
41. Tandon R, Goldman M. Overview of neurobiology. In: Janicak PG, Marder SR, Tandon R, et al, eds. Schizophrenia: recent advances in diagnosis and treatment. Springer; 2014:27-33.
42. National Institutes of Health. Accelerating Medicines Partnership. Schizophrenia. Accessed August 30, 2021. https://www.nih.gov/research-training/accelerating-medicines-partnership-amp/schizophrenia
43. Guloksuz S, van Os J. The slow death of the concept of schizophrenia and the painful birth of the psychosis spectrum. Psychol Med. 2018;48(2):229-244.
44. Christ-Crain M, Bichet DG, Fenske WK, et al. Diabetes insipidus. Nat Rev Dis Primers. 2019;5(1):54.
45. Ahmadi L, Goldman MB. Primary polydipsia: update. Best Pract Res Clin Endocrinol Metab. 2020;34(5):101469. doi: 10.1016/j.beem.2020.101469
46. Early Assessment and Support Alliance. National Early Psychosis Directory. Accessed August 30, 2021. https://easacommunity.org/national-directory.php
1. Hyman SE. Revolution stalled. Sci Transl Med. 2012;4(155):155cm11. doi: 10.1126/scitranslmed.3003142
2. Harrington A. Mind fixers: psychiatry’s troubled search for the biology of mental illness. W.W. Norton & Company; 2019.
3. Millan MJ, Andrieux A, Bartzokis G, et al. Altering the course of schizophrenia: progress and perspectives. Nat Rev Drug Discov. 2016;15(7):485-515.
4. Lieberman JA, Small SA, Girgis RR. Early detection and preventive intervention in schizophrenia: from fantasy to reality. Am J Psychiatry. 2019;176(10):794-810.
5. McGorry PD, Nelson B, Nordentoft M, et al. Intervention in individuals at ultra-high risk for psychosis: a review and future directions. J Clin Psychiatry. 2009;70(9):1206-1212.
6. Csillag C, Nordentoft M, Mizuno M, et al. Early intervention in psychosis: From clinical intervention to health system implementation. Early Interv Psychiatry. 2018;12(4):757-764.
7. McGorry PD, Ratheesh A, O’Donoghue B. Early intervention—an implementation challenge for 21st century mental health care. JAMA Psychiatry. 2018;75(6):545-546.
8. Rosenheck R. Toward dissemination of secondary prevention for psychosis. Am J Psychiatry. 2018;175(5):393-394.
9. Fusar-Poli P, Salazar de Pablo G, Correll CU, et al. Prevention of psychosis: advances in detection, prognosis, and intervention. JAMA Psychiatry. 2020;77(7):755-765.
10. Oliver D, Reilly TJ, Baccaredda Boy O, et al. What causes the onset of psychosis in individuals at clinical high risk? A meta-analysis of risk and protective factors. Schizophr Bull. 2020;46(1):110-120.
11. Tindall R, Simmons M, Allott K, et al. Disengagement processes within an early intervention service for first-episode psychosis: a longitudinal, qualitative, multi-perspective study. Front Psychiatry. 2020;11:565-565.
12. Dixon LB, Holoshitz Y, Nossel I. Treatment engagement of individuals experiencing mental illness: review and update. World Psychiatry. 2016;15(1):13-20.
13. Rickwood D, Paraskakis M, Quin D, et al. Australia’s innovation in youth mental health care: The headspace centre model. Early Interv Psychiatry. 2019;13(1):159-166.
14. Woodberry KA, Shapiro DI, Bryant C, et al. Progress and future directions in research on the psychosis prodrome: a review for clinicians. Harv Rev Psychiatry. 2016;24(2):87-103.
15. Gupta T, Mittal VA. Advances in clinical staging, early intervention, and the prevention of psychosis. F1000Res. 2019;8:F1000 Faculty Rev-2027. doi: 10.12688/f1000research.20346.1
16. Correll CU, Galling B, Pawar A, et al. Comparison of early intervention services vs treatment as usual for early-phase psychosis: a systematic review, meta-analysis, and meta-regression. JAMA Psychiatry. 2018;75(6):555-565.
17. Cannon TD, Cadenhead K, Cornblatt B, et al. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry. 2008;65(1):28-37.
18. Sommer IE, Bearden CE, van Dellen E, et al. Early interventions in risk groups for schizophrenia: what are we waiting for? NPJ Schizophr. 2016;2(1):16003-16003.
19. McGorry PD, Nelson B. Clinical high risk for psychosis—not seeing the trees for the wood. JAMA Psychiatry. 2020;77(7):559-560.
20. van der Gaag M, Smit F, Bechdolf A, et al. Preventing a first episode of psychosis: meta-analysis of randomized controlled prevention trials of 12 month and longer-term follow-ups. Schizophr Res. 2013;149(1):56-62.
21. Marshall M, Rathbone J. Early intervention for psychosis. Cochrane Database Syst Rev. 2011;(6):CD004718. doi: 10.1002/14651858.CD004718.pub3
22. Heinssen RK, Insel TR. Preventing the onset of psychosis: not quite there yet. Schizophr Bull. 2015;41(1):28-29.
23. Amos AJ. Evidence that treatment prevents transition to psychosis in ultra-high-risk patients remains questionable. Schizophr Res. 2014;153(1):240.
24. National Institute for Health and Care Excellence. Psychosis and schizophrenia in adults: prevention and management. Clinical guideline [CG178]. 1.3.7 How to deliver psychological interventions. Published February 12, 2014. Updated March 1, 2014. Accessed August 30, 2021. https://www.nice.org.uk/guidance/cg178/chapter/recommendations#how-to-deliver-psychological-interventions
25. Fusar-Poli P, Werbeloff N, Rutigliano G, et al. Transdiagnostic risk calculator for the automatic detection of individuals at risk and the prediction of psychosis: second replication in an independent National Health Service Trust. Schizophr Bull. 2019;45(3):562-570.
26. Fusar-Poli P, Oliver D, Spada G, et al. The case for improved transdiagnostic detection of first-episode psychosis: electronic health record cohort study. Schizophr Res. 2021;228:547-554.
27. Fusar-Poli P. Negative psychosis prevention trials. JAMA Psychiatry. 2017;74(6):651.
28. Cuijpers P, Smit F, Furukawa TA. Most at‐risk individuals will not develop a mental disorder: the limited predictive strength of risk factors. World Psychiatry. 2021;20(2):224-225.
29. Carrión RE, Cornblatt BA, Burton CZ, et al. Personalized prediction of psychosis: external validation of the NAPLS-2 psychosis risk calculator with the EDIPPP Project. Am J Psychiatry. 2016;173(10):989-996.
30. Worthington MA, Walker EF, Addington J, et al. Incorporating cortisol into the NAPLS2 individualized risk calculator for prediction of psychosis. Schizophr Res. 2021;227:95-100.
31. Koutsouleris N, Dwyer DB, Degenhardt F, et al. Multimodal machine learning workflows for prediction of psychosis in patients with clinical high-risk syndromes and recent-onset depression. JAMA Psychiatry. 2021;78(2):195-209.
32. Simmons MB, Grace D, Fava NJ, et al. The experiences of youth mental health peer workers over time: a qualitative study with longitudinal analysis. Community Ment Health J. 2020;56(5):906-914.
33. Devoe DJ, Farris MS, Townes P, et al. Interventions and transition in youth at risk of psychosis: a systematic review and meta-analyses. J Clin Psychiatry. 2020;81(3):17r12053. doi: 10.4088/JCP.17r12053
34. Bosnjak Kuharic D, Kekin I, Hew J, et al. Interventions for prodromal stage of psychosis. Cochrane Database Syst Rev. 2019;2019(11):CD012236
35. Dixon LB, Goldman HH, Srihari VH, et al. Transforming the treatment of schizophrenia in the United States: The RAISE Initiative. Annu Rev Clin Psychol. 2018;14:237-258.
36. Friedman-Yakoobian MS, Parrish EM, Eack SM, et al. Neurocognitive and social cognitive training for youth at clinical high risk (CHR) for psychosis: a randomized controlled feasibility trial. Schizophr Res. 2020;S0920-9964(20)30461-8. doi: 10.1016/j.schres.2020.09.005
37. Kane JM, Schooler NR, Marcy P, et al. Effect of long-acting injectable antipsychotics vs usual care on time to first hospitalization in early-phase schizophrenia: a randomized clinical trial. JAMA Psychiatry. 2020;77(12):1217-1224.
38. Morrison AP, Pyle M, Maughan D, et al. Antipsychotic medication versus psychological intervention versus a combination of both in adolescents with first-episode psychosis (MAPS): a multicentre, three-arm, randomised controlled pilot and feasibility study. Lancet Psychiatry. 2020;7(9):788-800.
39. Chen YQ, Li XR, Zhang L, et al. Therapeutic response is associated with antipsychotic-induced weight gain in drug-naive first-episode patients with schizophrenia: an 8-week prospective study. J Clin Psychiatry. 2021;82(3):20m13469. doi: 10.4088/JCP.20m13469
40. Insel TR. RAISE-ing our expectations for first-episode psychosis. Am J Psychiatry. 2016;173(4):311-312.
41. Tandon R, Goldman M. Overview of neurobiology. In: Janicak PG, Marder SR, Tandon R, et al, eds. Schizophrenia: recent advances in diagnosis and treatment. Springer; 2014:27-33.
42. National Institutes of Health. Accelerating Medicines Partnership. Schizophrenia. Accessed August 30, 2021. https://www.nih.gov/research-training/accelerating-medicines-partnership-amp/schizophrenia
43. Guloksuz S, van Os J. The slow death of the concept of schizophrenia and the painful birth of the psychosis spectrum. Psychol Med. 2018;48(2):229-244.
44. Christ-Crain M, Bichet DG, Fenske WK, et al. Diabetes insipidus. Nat Rev Dis Primers. 2019;5(1):54.
45. Ahmadi L, Goldman MB. Primary polydipsia: update. Best Pract Res Clin Endocrinol Metab. 2020;34(5):101469. doi: 10.1016/j.beem.2020.101469
46. Early Assessment and Support Alliance. National Early Psychosis Directory. Accessed August 30, 2021. https://easacommunity.org/national-directory.php
Nontraditional therapies for treatment-resistant depression: Part 2
When patients with major depressive disorder (MDD) do not achieve optimal outcomes after FDA-approved first-line treatments and standard adjunctive strategies, clinicians look for additional approaches to alleviate their patients’ symptoms. Recent research suggests that several “nontraditional” treatments used primarily as adjuncts to standard antidepressants have promise for treatment-resistant depression.
In Part 1 of this article (
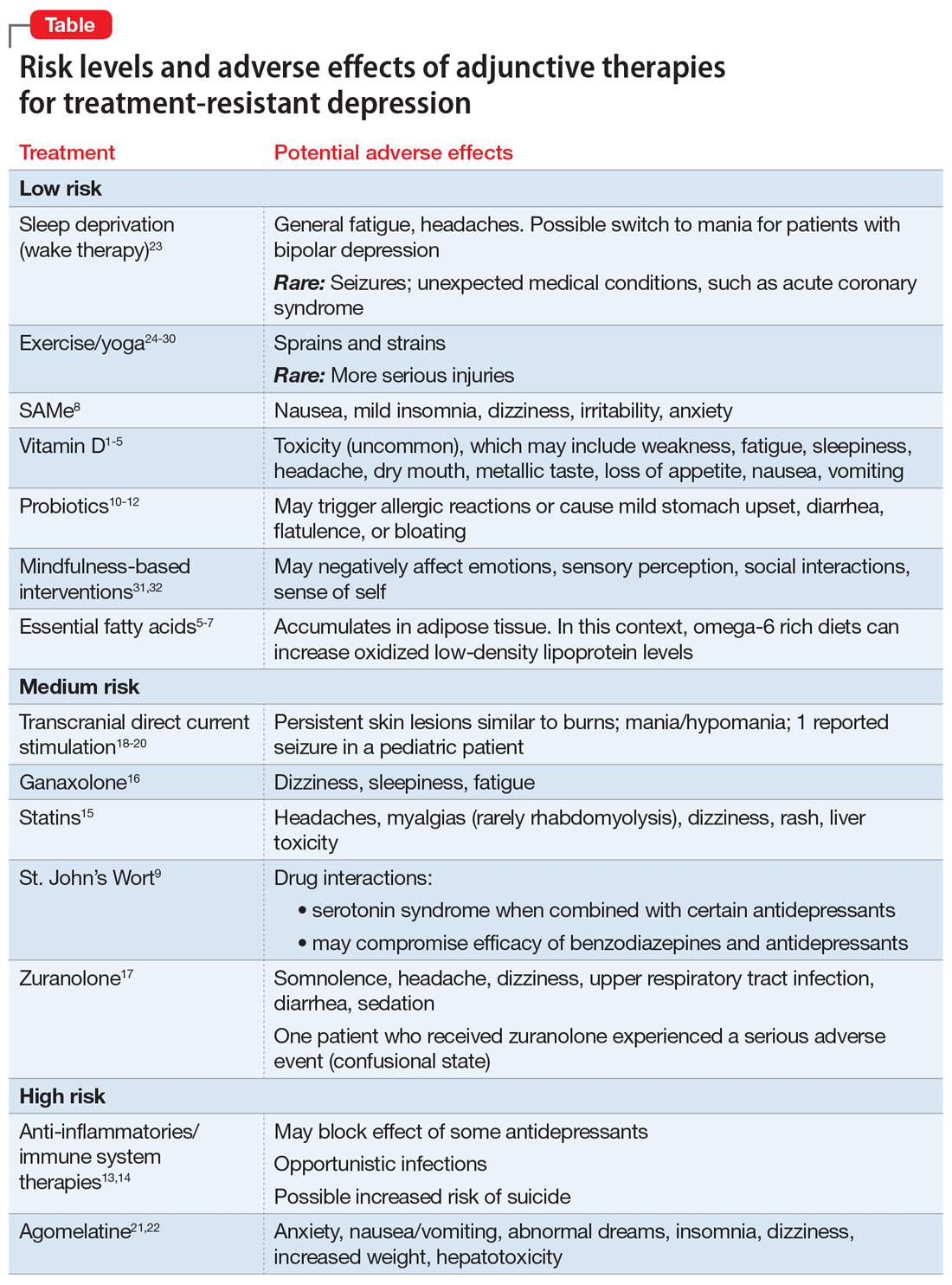
Herbal/nutraceutical agents
This category encompasses a variety of commonly available “natural” options patients often ask about and at times self-prescribe. Examples evaluated in clinical trials include:
- vitamin D
- essential fatty acids (omega-3, omega-6)
- S-adenosyl-L-methionine (SAMe)
- hypericum perforatum (St. John’s Wort)
- probiotics.
Vitamin D deficiency has been linked to depression, possibly by lowering serotonin, norepinephrine, and dopamine concentrations.1-3
A meta-analysis of 3 prospective, observational studies (N = 8,815) found an elevated risk of affective disorders in patients with low vitamin D levels.4 In addition, a systematic review and meta-analysis supported a potential role for vitamin D supplementation for patients with treatment-resistant depresssion.5
Toxicity can occur at levels >100 ng/mL, and resulting adverse effects may include weakness, fatigue, sleepiness, headache, loss of appetite, dry mouth, metallic taste, nausea, and vomiting. This vitamin can be considered as an adjunct to standard antidepressants, particularly in patients with treatment-resistant depression who have low vitamin D levels, but regular monitoring is necessary to avoid toxicity.
Essential fatty acids. Protein receptors embedded in lipid membranes and their binding affinities are influenced by omega-3 and omega-6 polyunsaturated fatty acids. Thus, essential fatty acids may benefit depression by maintaining membrane integrity and fluidity, as well as via their anti-inflammatory activity.
Continue to: Although results from...
Although results from controlled trials are mixed, a systematic review and meta-analysis of adjunctive nutraceuticals supported a potential role for essential fatty acids, primarily eicosapentaenoic acid (EPA), by itself or in combination with docosahexaenoic acid (DHA), with total EPA >60%.5 A second meta-analysis of 26 studies (N = 2,160) that considered only essential fatty acids concluded that EPA ≥60% at ≤1 g/d could benefit depression.6 Furthermore, omega-3 fatty acids may be helpful as an add-on agent for postpartum depression.7
Be aware that a diet rich in omega-6 greatly increases oxidized low-density lipoprotein levels in adipose tissue, potentially posing a cardiac risk factor. Clinicians need to be aware that self-prescribed use of essential fatty acids is common, and to ask about and monitor their patients’ use of these agents.
S-adenosyl-L-methionine (SAMe) is an intracellular amino acid and methyl donor. Among other actions, it is involved in the biosynthesis of hormones and neurotransmitters. There is promising but limited preliminary evidence of its efficacy and safety as a monotherapy or for antidepressant augmentation.
- Five out of 6 earlier controlled studies reported SAMe IV (200 to 400 mg/d) or IM (45 to 50 mg/d) was more effective than placebo
- When the above studies were added to 14 subsequent studies for a meta-analysis, 12 of 19 RCTs reported that parenteral or oral SAMe was significantly more effective than placebo for depression (P < .05).
Overall, the safety and tolerability of SAMe are good. Common adverse effects include nausea, mild insomnia, dizziness, irritability, and anxiety. This is another compound widely available without a prescription and at times self-prescribed. It carries an acceptable risk/benefit balance, with decades of experience.
Hypericum perforatum (St. John’s Wort) is widely prescribed for depression in China and Europe, typically in doses ranging from 500 to 900 mg/d. Its mechanism of action in depression may relate to inhibition of serotonin, dopamine, and norepinephrine uptake from the synaptic cleft of these interconnecting neurotransmitter systems.
Continue to: A meta-analysis of 7 clinical trials...
A meta-analysis of 7 clinical trials (N = 3,808) comparing St. John’s Wort with various selective serotonin reuptake inhibitors (SSRIs) reported comparable rates of response (pooled relative risk .983, 95% CI .924 to 1.042; P < .001) and remission (pooled relative risk 1.013, 95% CI .892 to 1.134; P < .001).9 Further, there were significantly lower discontinuation/dropout rates (pooled odds ratio .587, 95% CI .478 to 0.697; P < .001) for St. John’s Wort compared with the SSRIs.
Existing evidence on the long-term efficacy and safety is limited (studies ranged from 4 to 12 weeks), as is evidence for patients with more severe depression or high suicidality.
Serious drug interactions include the potential for serotonin syndrome when St. John’s Wort is combined with certain antidepressants, compromised efficacy of benzodiazepines and standard antidepressants, and severe skin reactions to sun exposure. In addition, St. John’s Wort may not be safe to use during pregnancy or while breastfeeding. Because potential drug interactions can be serious and individuals often self-prescribe this agent, it is important to ask patients about their use of St. John’s Wort, and to be vigilant for such potential adverse interactions.
Probiotics. These agents produce neuroactive substances that act on the brain/gut axis. Preliminary evidence suggests that these “psychobiotics” confer mental health benefits.10-12 Relative to other approaches, their low-risk profile make them an attractive option for some patients.
Anti-inflammatory/immune system therapies
Inflammation is linked to various medical and brain disorders. For example, patients with depression often demonstrate increased levels of peripheral blood inflammatory biomarkers (such as C-reactive protein and interleukin-6 and -17) that are known to alter norepinephrine, neuroendocrine (eg, the hypothalamic-pituitary-adrenal axis), and microglia function in addition to neuroplasticity. Thus, targeting inflammation may facilitate the development of novel antidepressants. In addition, these agents may benefit depression associated with comorbid autoimmune disorders, such as psoriasis or rheumatoid arthritis. A systematic review and meta-analysis of 36 RCTs (N = 10,000) found 5 out of 6 anti-inflammatory agents improved depression.13,14 In general, reported disadvantages of anti-inflammatories/immunosuppressants include the potential to block the antidepressant effect of some agents, the risk of opportunistic infections, and an increased risk of suicide.
Continue to: Statins
Statins
In a meta-analysis of 3 randomized, double-blind trials, 3 statins (lovastatin, atorvastatin, and simvastatin) significantly improved depression scores when used as an adjunctive therapy to fluoxetine and citalopram, compared with adjunctive placebo (N = 165, P < .001).15
Specific adverse effects of statins include headaches, muscle pain (rarely rhabdomyolysis), dizziness, rash, and liver damage. Statins also have the potential for adverse interactions with other medications. Given the limited efficacy literature on statins for depression and the potential for serious adverse effects, these agents probably should be limited to patients with treatment-resistant depression for whom a statin is indicated for a comorbid medical disorder, such as hypercholesteremia.
Neurosteroids
Brexanolone is FDA-approved for the treatment of postpartum depression. It is an IV formulation of the neuroactive steroid hormone allopregnanolone (a metabolite of progesterone), which acts as a positive allosteric modulator of the GABA-A receptor. Unfortunately, the infusion needs to occur over a 60-hour period.
Ganaxolone is an oral analog formulation of allopregnanolone. In an uncontrolled, open-label pilot study, this medication was administered for 8 weeks as an adjunct to an adequately dosed antidepressant to 10 postmenopausal women with persistent MDD.16 Of the 9 women who completed the study, 4 (44%) improved significantly (P < .019) and the benefit was sustained for 2 additional weeks.16 Adverse effects of ganaxolone included dizziness in 60% of participants, and sleepiness and fatigue in all of them with twice-daily dosing. If the FDA approves ganaxolone, it would become an easier-to-administer option to brexanolone.
Zuranolone is an investigational agent being studied as a treatment for postpartum depression. In a double-blind RCT that evaluated 151 women with postpartum depression, those who took oral zuranolone, 30 mg daily at bedtime for 2 weeks, experienced significant reductions in Hamilton Depression Rating Scale-17 (HDRS-17) scores compared with placebo (P < .003).17 Improvement in core depression symptom ratings was seen as early as Day 3 and persisted through Day 45.
Continue to: The most common...
The most common (≥5%) treatment-emergent adverse effects were somnolence (15%), headache (9%), dizziness (8%), upper respiratory tract infection (8%), diarrhea (6%), and sedation (5%). Two patients experienced a serious adverse event: one who received zuranolone (confusional state) and one who received placebo (pancreatitis). One patient discontinued zuranolone due to adverse effects vs no discontinuations among those who received placebo. The risk of taking zuranolone while breastfeeding is not known.
Device-based strategies
In addition to FDA-cleared approaches (eg, electroconvulsive therapy [ECT], vagus nerve stimulation [VNS], transcranial magnetic stimulation [TMS]), other devices have also demonstrated promising results.
Transcranial direct current stimulation (tDCS) involves delivering weak electrical current to the cerebral cortex through small scalp electrodes to produce the following effects:
- anodal tDCS enhances cortical excitability
- cathodal tDCS reduces cortical excitability.
A typical protocol consists of delivering 1 to 2 mA over 20 minutes with scalp electrodes placed in different configurations based on the targeted symptom(s).
While tDCS has been evaluated as a treatment for various neuropsychiatric disorders, including bipolar depression, Parkinson’s disease, and schizophrenia, most trials have looked at its use for treating depression. Results have been promising but mixed. For example, 1 meta-analysis of 6 RCTs (comprising 96 active and 80 sham tDCS courses) reported that active tDCS was superior to a sham procedure (Hedges’ g = 0.743) for symptoms of depression.18 By contrast, another meta-analysis of 6 RCTs (N = 200) did not find a significant difference between active and sham tDCS for response and remission rates.19 More recently, a group of experts created an evidence-based guideline using a systematic review of the controlled trial literature. These authors concluded there is “probable efficacy for anodal tDCS of the left dorsolateral prefrontal cortex (DLPFC) (with right orbitofrontal cathode) in major depressive episodes without drug resistance but probable inefficacy for drug-resistant major depressive episodes.”20
Continue to: Adverse effects of tDCS...
Adverse effects of tDCS are typically mild but may include persistent skin lesions similar to burns; mania or hypomania; and one reported seizure in a pediatric patient.
Because various over-the-counter direct current stimulation devices are available for purchase at modest cost, clinicians should ask patients if they have been self-administering this treatment.
Chronotherapy strategies
Agomelatine combines serotonergic (5-HT2B and 5-HT2C antagonist) and melatonergic (MT1-MT2 agonist in the suprachiasmatic nucleus) actions that contribute to stabilization of circadian rhythms and subsequent improvement in sleep patterns. Agomelatine (n = 1,274) significantly lowered depression symptoms compared with placebo (n = 689) (standardized mean difference −0.26; P < 3.48×10-11), but the clinical relevance was questionable.21 A recent review of the literature and expert opinion suggest this agent may also have efficacy for anhedonia; however, in placebo-controlled, relapse prevention studies, its long-term efficacy was not consistent.22
Common adverse effects include anxiety; nausea, vomiting, and stomach pain; abnormal dreams and insomnia; dizziness; drowsiness and fatigue; and weight gain. Some reviewers have expressed concerns about agomelatine’s potential for hepatotoxicity and the need for repeated clinical laboratory tests. Although agomelatine is approved outside of the United States, limited efficacy data and the potential for serious adverse effects have precluded FDA approval of this agent.
Sleep deprivation as a treatment technique for depression has been developed over the past 50 years. With total sleep deprivation (TSD) over 1 cycle, patients stay awake for approximately 36 hours, from daytime until the next day’s evening. While 1 to 6 cycles can produce acute antidepressant effects, prompt relapse after sleep recovery is common.
Continue to: In a systematic review...
In a systematic review and meta-analysis of 7 studies that included a total of 311 patients with bipolar depression23:
- TSD plus medications resulted in a significant decrease in depressive symptoms at 1 week compared with medications alone
- higher response rates were maintained after 3 months with lithium.
Adverse effects commonly include general fatigue and headaches; possible switch into mania with bipolar depression; and rarely, seizures or other unexpected medical conditions (eg, acute coronary syndrome). Presently, this approach is limited to research laboratories with the appropriate sophistication to safely conduct such trials.
Other nontraditional strategies
Cardiovascular exercise, resistance training, mindfulness, and yoga have been shown to decrease severe depressive symptoms when used as adjuncts for patients with treatment-resistant depression, or as monotherapy to treat patients with milder depression.
Exercise. The significant benefits of exercise in various forms as treatment for mild to moderate depression are well described in the literature, but it is less clear if it is effective for treatment-resistant depression. A 2013 Cochrane report24 (39 studies with 2,326 participants total) and 2 meta-analyses undertaken in 2015 (Kvam et al25 included 23 studies with 977 participants, and Schuh et al26 included 25 trials with 1,487 participants) reported that various types of exercise ameliorate depression of differing subtypes and severity, with effect sizes ranging from small to large. Schuh et al26 found that publication bias underestimated effect size. Also, not surprisingly, separate analysis of only higher-quality trials decreased effect size.24-26 A meta-analysis that included tai chi and yoga in addition to aerobic exercise and strength training (25 trials with 2,083 participants) found low to moderate benefit for exercise and yoga.27 Finally, a meta-analysis by Cramer et al28 that included 12 RCTs (N = 619) supported the use of yoga plus controlled breathing techniques as an ancillary treatment for depression.
Two small exercise trials specifically evaluated patients with treatment-resistant depression.29,30 Mota-Pereira et al29 compared 22 participants who walked for 30 to 45 minutes, 5 days a week for 12 weeks in addition to pharmacotherapy with 11 patients who received pharmacotherapy only. Exercise improved all outcomes, including HDRS score (both compared to baseline and to the control group). Moreover, 26% of the exercise group went into remission. Pilu et al30 evaluated strength training as an adjunctive treatment. Participants received 1 hour of strength training twice weekly for 8 months (n = 10), or pharmacotherapy only (n = 20). The adjunct strength training group had a statistically significant (P < .0001) improvement in HDRS scores at the end of the 8 months, whereas the control group did not (P < .28).
Continue to: Adverse effects...
Adverse effects of exercise are typically limited to sprains or strains; rarely, participants experience serious injuries.
Mindfulness-based interventions involve purposely paying attention in the present moment to enhance self-understanding and decrease anxiety about the future and regrets about the past, both of which complicate depression. A meta-analysis of 12 RCTs (N = 578) found this approach significantly reduced depression severity when used as an adjunctive therapy.31 There may be risks if mindfulness-based interventions are practiced incorrectly. For example, some reports have linked mindfulness-based interventions to psychotic episodes, meditation addiction, and antisocial or asocial behavior.32
Bottom Line
Nonpharmacologic options for patients with treatment-resistant depression include herbal/nutraceuticals, anti-inflammatory/immune system therapies, and devices. While research suggests some of these approaches are promising, clinicians need to carefully consider potential adverse effects, some of which may be serious.
Related Resources
- Kaur M, Sanches M. Experimental therapeutics in treatmentresistant major depressive disorder. J Exp Pharmacol. 2021;13:181-196.
- Janicak PG. What’s new in transcranial magnetic stimulation. Current Psychiatry. 2019;18(3):10-16.
Drug Brand Names
Atorvastatin • Lipitor
Brexanolone • Zulresso
Citalopram • Celexa
Fluoxetine • Prozac
Lithium • Eskalith, Lithobid
Lovastatin • Altoprev, Mevacor
Minocycline • Dynacin, Minocin
Simvastatin • Flolipid, Zocor
1. Pittampalli S, Mekala HM, Upadhyayula, S, et al. Does vitamin D deficiency cause depression? Prim Care Companion CNS Disord. 2018;20(5):17l02263.
2. Parker GB, Brotchie H, Graham RK. Vitamin D and depression. J Affect Disord. 2017;208:56-61.
3. Berridge MJ. Vitamin D and depression: cellular and regulatory mechanisms. Pharmacol Rev. 2017;69(2):80-92.
4. Anglin RE, Samaan Z, Walter SD, et al. Vitamin D deficiency and depression in adults: systematic review and meta-analysis. Br J Psychiatry. 2013;202:100-107.
5. Sarris J, Murphy J, Mischoulon D, et al. Adjunctive nutraceuticals for depression: a systematic review and meta-analyses. Am J Psychiatry 2016;173(6);575-587.
6. Liao Y, Xie B, Zhang H, et al. Efficacy of omega-3 PUFAs in depression: a meta-analysis. Transl Psychiatry. 2019;9(1):190.
7. Mocking RJT, Steijn K, Roos C, et al. Omega-3 fatty acid supplementation for perinatal depression: a meta-analysis. J Clin Psychiatry. 2020;81(5):19r13106.
8. Sharma A, Gerbarg P, Bottiglieri T, et al; Work Group of the American Psychiatric Association Council on Research. S-Adenosylmethionine (SAMe) for neuropsychiatric disorders: a clinician-oriented review of research. J Clin Psychiatry. 2017;78(6):e656-e667.
9. Ng QX, Venkatanarayanan N, Ho CY. Clinical use of hypericum perforatum (St John’s wort) in depression: a meta-analysis. J Affect Disord 2017;210:211-221.
10. Huang R, Wang K, Hu J. Effect of probiotics on depression: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2016;8(8):483.
11. Liu RT, Walsh RFL, Sheehan AE. Prebiotics and probiotics for depression and anxiety: a systematic review and meta-analysis of controlled clinical trials. Neurosci Biobehav Rev. 2019;102:13-23.
12. Wallace CJK, Milev RV. The efficacy, safety, and tolerability of probiotics on depression: clinical results from an open-label pilot study. Front Psychiatry. 2021;12(132):618279.
13. Köhler-Forsberg O, N Lyndholm C, Hjorthøj C, et al. Efficacy of anti-inflammatory treatment on major depressive disorder or depressive symptoms: meta-analysis of clinical trials. Acta Psychiatr Scand. 2019;139(5):404-419.
14. Jha MK. Anti-inflammatory treatments for major depressive disorder: what’s on the horizon? J Clin Psychiatry. 2019;80(6)18ac12630.
15. Salagre E, Fernandes BS, Dodd S, et al. Statins for the treatment of depression: a meta-analysis of randomized, double-blind, placebo-controlled trials. J Affect Disord. 2016;200:235-242.
16. Dichtel LE, Nyer M, Dording C, et al. Effects of open-label, adjunctive ganaxolone on persistent depression despite adequate antidepressant treatment in postmenopausal women: a pilot study. J Clin Psychiatry. 2020;81(4):19m12887.
17. Deligiannidis KM, Meltzer-Brody S, Gunduz-Bruce H, et al. Effect of zuranolone vs placebo in postpartum depression: a randomized clinical trial. JAMA Psychiatry. 2021;78(9):951-959.
18. Kalu UG, Sexton CE, Loo CK, et al. Transcranial direct current stimulation in the treatment of major depression: a meta-analysis. Psychol Med. 2012;42(9):1791-800.
19. Berlim MT, Van den Eynde F, Daskalakis ZJ. Clinical utility of transcranial direct current stimulation (tDCS) for treating major depression: a systematic review and meta-analysis of randomized, double-blind and sham-controlled trials. J Psychiatr Res. 2013;47(1):1-7.
20. Lefaucheur JP, Antal A, Ayache SS, et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin Neurophysiol. 2017;128(1):56-92.
21. Singh SP, Singh V, Kar N. Efficacy of agomelatine in major depressive disorder: meta-analysis and appraisal. Int J Neuropsychopharmacol. 2012;15(3):417-428.
22. Norman TR, Olver JS. Agomelatine for depression: expanding the horizons? Expert Opin Pharmacother. 2019;20(6):647-656.
23. Ramirez-Mahaluf JP, Rozas-Serri E, Ivanovic-Zuvic F, et al. Effectiveness of sleep deprivation in treating acute bipolar depression as augmentation strategy: a systematic review and meta-analysis. Front Psychiatry. 2020;11:70.
24. Cooney GM, Dwan K, Greig CA, et al. Exercise for depression. Cochrane Database Syst Rev. 2013;(9):CD004366.
25. Kvam S, Kleppe CL, Nordhus IH, et al. Exercise as a treatment for depression: a meta-analysis. J Affect Disord. 2016;202:67-86.
26. Schuch FB, Vancampfort D, Richards J, et al. Exercise as a treatment for depression: a meta-analysis adjusting for publication bias. J Psychiatr Res. 2016;77:42-51.
27. Seshadri A, Adaji A, Orth SS, et al. Exercise, yoga, and tai chi for treatment of major depressive disorder in outpatient settings: a systematic review and meta-analysis. Prim Care Companion CNS Disord. 2020;23(1):20r02722.
28. Cramer H, Lauche R, Langhorst J, et al. Yoga for depression: a systematic review and meta-analysis. Depress Anxiety. 2013;30(11):1068-1083.
29. Mota-Pereira J, Silverio J, Carvalho S, et al. Moderate exercise improves depression parameters in treatment-resistant patients with major depressive disorder. J Psychiatr Res. 2011;45(8):1005-1011.
30. Pilu A, Sorba M, Hardoy MC, et al. Efficacy of physical activity in the adjunctive treatment of major depressive disorders: preliminary results. Clin Pract Epidemiol Ment Health. 2007;3:8.
31. Strauss C, Cavanagh K, Oliver A, et al. Mindfulness-based interventions for people diagnosed with a current episode of an anxiety or depressive disorder: a meta-analysis of randomised controlled trials. PLoS One. 2014;9(4):e96110.
32. Shonin E, Van Gordon W, Griffiths MD. Are there risks associated with using mindfulness for the treatment of psychopathology? Clinical Practice. 2014;11(4):389-392.
When patients with major depressive disorder (MDD) do not achieve optimal outcomes after FDA-approved first-line treatments and standard adjunctive strategies, clinicians look for additional approaches to alleviate their patients’ symptoms. Recent research suggests that several “nontraditional” treatments used primarily as adjuncts to standard antidepressants have promise for treatment-resistant depression.
In Part 1 of this article (

Herbal/nutraceutical agents
This category encompasses a variety of commonly available “natural” options patients often ask about and at times self-prescribe. Examples evaluated in clinical trials include:
- vitamin D
- essential fatty acids (omega-3, omega-6)
- S-adenosyl-L-methionine (SAMe)
- hypericum perforatum (St. John’s Wort)
- probiotics.
Vitamin D deficiency has been linked to depression, possibly by lowering serotonin, norepinephrine, and dopamine concentrations.1-3
A meta-analysis of 3 prospective, observational studies (N = 8,815) found an elevated risk of affective disorders in patients with low vitamin D levels.4 In addition, a systematic review and meta-analysis supported a potential role for vitamin D supplementation for patients with treatment-resistant depresssion.5
Toxicity can occur at levels >100 ng/mL, and resulting adverse effects may include weakness, fatigue, sleepiness, headache, loss of appetite, dry mouth, metallic taste, nausea, and vomiting. This vitamin can be considered as an adjunct to standard antidepressants, particularly in patients with treatment-resistant depression who have low vitamin D levels, but regular monitoring is necessary to avoid toxicity.
Essential fatty acids. Protein receptors embedded in lipid membranes and their binding affinities are influenced by omega-3 and omega-6 polyunsaturated fatty acids. Thus, essential fatty acids may benefit depression by maintaining membrane integrity and fluidity, as well as via their anti-inflammatory activity.
Continue to: Although results from...
Although results from controlled trials are mixed, a systematic review and meta-analysis of adjunctive nutraceuticals supported a potential role for essential fatty acids, primarily eicosapentaenoic acid (EPA), by itself or in combination with docosahexaenoic acid (DHA), with total EPA >60%.5 A second meta-analysis of 26 studies (N = 2,160) that considered only essential fatty acids concluded that EPA ≥60% at ≤1 g/d could benefit depression.6 Furthermore, omega-3 fatty acids may be helpful as an add-on agent for postpartum depression.7
Be aware that a diet rich in omega-6 greatly increases oxidized low-density lipoprotein levels in adipose tissue, potentially posing a cardiac risk factor. Clinicians need to be aware that self-prescribed use of essential fatty acids is common, and to ask about and monitor their patients’ use of these agents.
S-adenosyl-L-methionine (SAMe) is an intracellular amino acid and methyl donor. Among other actions, it is involved in the biosynthesis of hormones and neurotransmitters. There is promising but limited preliminary evidence of its efficacy and safety as a monotherapy or for antidepressant augmentation.
- Five out of 6 earlier controlled studies reported SAMe IV (200 to 400 mg/d) or IM (45 to 50 mg/d) was more effective than placebo
- When the above studies were added to 14 subsequent studies for a meta-analysis, 12 of 19 RCTs reported that parenteral or oral SAMe was significantly more effective than placebo for depression (P < .05).
Overall, the safety and tolerability of SAMe are good. Common adverse effects include nausea, mild insomnia, dizziness, irritability, and anxiety. This is another compound widely available without a prescription and at times self-prescribed. It carries an acceptable risk/benefit balance, with decades of experience.
Hypericum perforatum (St. John’s Wort) is widely prescribed for depression in China and Europe, typically in doses ranging from 500 to 900 mg/d. Its mechanism of action in depression may relate to inhibition of serotonin, dopamine, and norepinephrine uptake from the synaptic cleft of these interconnecting neurotransmitter systems.
Continue to: A meta-analysis of 7 clinical trials...
A meta-analysis of 7 clinical trials (N = 3,808) comparing St. John’s Wort with various selective serotonin reuptake inhibitors (SSRIs) reported comparable rates of response (pooled relative risk .983, 95% CI .924 to 1.042; P < .001) and remission (pooled relative risk 1.013, 95% CI .892 to 1.134; P < .001).9 Further, there were significantly lower discontinuation/dropout rates (pooled odds ratio .587, 95% CI .478 to 0.697; P < .001) for St. John’s Wort compared with the SSRIs.
Existing evidence on the long-term efficacy and safety is limited (studies ranged from 4 to 12 weeks), as is evidence for patients with more severe depression or high suicidality.
Serious drug interactions include the potential for serotonin syndrome when St. John’s Wort is combined with certain antidepressants, compromised efficacy of benzodiazepines and standard antidepressants, and severe skin reactions to sun exposure. In addition, St. John’s Wort may not be safe to use during pregnancy or while breastfeeding. Because potential drug interactions can be serious and individuals often self-prescribe this agent, it is important to ask patients about their use of St. John’s Wort, and to be vigilant for such potential adverse interactions.
Probiotics. These agents produce neuroactive substances that act on the brain/gut axis. Preliminary evidence suggests that these “psychobiotics” confer mental health benefits.10-12 Relative to other approaches, their low-risk profile make them an attractive option for some patients.
Anti-inflammatory/immune system therapies
Inflammation is linked to various medical and brain disorders. For example, patients with depression often demonstrate increased levels of peripheral blood inflammatory biomarkers (such as C-reactive protein and interleukin-6 and -17) that are known to alter norepinephrine, neuroendocrine (eg, the hypothalamic-pituitary-adrenal axis), and microglia function in addition to neuroplasticity. Thus, targeting inflammation may facilitate the development of novel antidepressants. In addition, these agents may benefit depression associated with comorbid autoimmune disorders, such as psoriasis or rheumatoid arthritis. A systematic review and meta-analysis of 36 RCTs (N = 10,000) found 5 out of 6 anti-inflammatory agents improved depression.13,14 In general, reported disadvantages of anti-inflammatories/immunosuppressants include the potential to block the antidepressant effect of some agents, the risk of opportunistic infections, and an increased risk of suicide.
Continue to: Statins
Statins
In a meta-analysis of 3 randomized, double-blind trials, 3 statins (lovastatin, atorvastatin, and simvastatin) significantly improved depression scores when used as an adjunctive therapy to fluoxetine and citalopram, compared with adjunctive placebo (N = 165, P < .001).15
Specific adverse effects of statins include headaches, muscle pain (rarely rhabdomyolysis), dizziness, rash, and liver damage. Statins also have the potential for adverse interactions with other medications. Given the limited efficacy literature on statins for depression and the potential for serious adverse effects, these agents probably should be limited to patients with treatment-resistant depression for whom a statin is indicated for a comorbid medical disorder, such as hypercholesteremia.
Neurosteroids
Brexanolone is FDA-approved for the treatment of postpartum depression. It is an IV formulation of the neuroactive steroid hormone allopregnanolone (a metabolite of progesterone), which acts as a positive allosteric modulator of the GABA-A receptor. Unfortunately, the infusion needs to occur over a 60-hour period.
Ganaxolone is an oral analog formulation of allopregnanolone. In an uncontrolled, open-label pilot study, this medication was administered for 8 weeks as an adjunct to an adequately dosed antidepressant to 10 postmenopausal women with persistent MDD.16 Of the 9 women who completed the study, 4 (44%) improved significantly (P < .019) and the benefit was sustained for 2 additional weeks.16 Adverse effects of ganaxolone included dizziness in 60% of participants, and sleepiness and fatigue in all of them with twice-daily dosing. If the FDA approves ganaxolone, it would become an easier-to-administer option to brexanolone.
Zuranolone is an investigational agent being studied as a treatment for postpartum depression. In a double-blind RCT that evaluated 151 women with postpartum depression, those who took oral zuranolone, 30 mg daily at bedtime for 2 weeks, experienced significant reductions in Hamilton Depression Rating Scale-17 (HDRS-17) scores compared with placebo (P < .003).17 Improvement in core depression symptom ratings was seen as early as Day 3 and persisted through Day 45.
Continue to: The most common...
The most common (≥5%) treatment-emergent adverse effects were somnolence (15%), headache (9%), dizziness (8%), upper respiratory tract infection (8%), diarrhea (6%), and sedation (5%). Two patients experienced a serious adverse event: one who received zuranolone (confusional state) and one who received placebo (pancreatitis). One patient discontinued zuranolone due to adverse effects vs no discontinuations among those who received placebo. The risk of taking zuranolone while breastfeeding is not known.
Device-based strategies
In addition to FDA-cleared approaches (eg, electroconvulsive therapy [ECT], vagus nerve stimulation [VNS], transcranial magnetic stimulation [TMS]), other devices have also demonstrated promising results.
Transcranial direct current stimulation (tDCS) involves delivering weak electrical current to the cerebral cortex through small scalp electrodes to produce the following effects:
- anodal tDCS enhances cortical excitability
- cathodal tDCS reduces cortical excitability.
A typical protocol consists of delivering 1 to 2 mA over 20 minutes with scalp electrodes placed in different configurations based on the targeted symptom(s).
While tDCS has been evaluated as a treatment for various neuropsychiatric disorders, including bipolar depression, Parkinson’s disease, and schizophrenia, most trials have looked at its use for treating depression. Results have been promising but mixed. For example, 1 meta-analysis of 6 RCTs (comprising 96 active and 80 sham tDCS courses) reported that active tDCS was superior to a sham procedure (Hedges’ g = 0.743) for symptoms of depression.18 By contrast, another meta-analysis of 6 RCTs (N = 200) did not find a significant difference between active and sham tDCS for response and remission rates.19 More recently, a group of experts created an evidence-based guideline using a systematic review of the controlled trial literature. These authors concluded there is “probable efficacy for anodal tDCS of the left dorsolateral prefrontal cortex (DLPFC) (with right orbitofrontal cathode) in major depressive episodes without drug resistance but probable inefficacy for drug-resistant major depressive episodes.”20
Continue to: Adverse effects of tDCS...
Adverse effects of tDCS are typically mild but may include persistent skin lesions similar to burns; mania or hypomania; and one reported seizure in a pediatric patient.
Because various over-the-counter direct current stimulation devices are available for purchase at modest cost, clinicians should ask patients if they have been self-administering this treatment.
Chronotherapy strategies
Agomelatine combines serotonergic (5-HT2B and 5-HT2C antagonist) and melatonergic (MT1-MT2 agonist in the suprachiasmatic nucleus) actions that contribute to stabilization of circadian rhythms and subsequent improvement in sleep patterns. Agomelatine (n = 1,274) significantly lowered depression symptoms compared with placebo (n = 689) (standardized mean difference −0.26; P < 3.48×10-11), but the clinical relevance was questionable.21 A recent review of the literature and expert opinion suggest this agent may also have efficacy for anhedonia; however, in placebo-controlled, relapse prevention studies, its long-term efficacy was not consistent.22
Common adverse effects include anxiety; nausea, vomiting, and stomach pain; abnormal dreams and insomnia; dizziness; drowsiness and fatigue; and weight gain. Some reviewers have expressed concerns about agomelatine’s potential for hepatotoxicity and the need for repeated clinical laboratory tests. Although agomelatine is approved outside of the United States, limited efficacy data and the potential for serious adverse effects have precluded FDA approval of this agent.
Sleep deprivation as a treatment technique for depression has been developed over the past 50 years. With total sleep deprivation (TSD) over 1 cycle, patients stay awake for approximately 36 hours, from daytime until the next day’s evening. While 1 to 6 cycles can produce acute antidepressant effects, prompt relapse after sleep recovery is common.
Continue to: In a systematic review...
In a systematic review and meta-analysis of 7 studies that included a total of 311 patients with bipolar depression23:
- TSD plus medications resulted in a significant decrease in depressive symptoms at 1 week compared with medications alone
- higher response rates were maintained after 3 months with lithium.
Adverse effects commonly include general fatigue and headaches; possible switch into mania with bipolar depression; and rarely, seizures or other unexpected medical conditions (eg, acute coronary syndrome). Presently, this approach is limited to research laboratories with the appropriate sophistication to safely conduct such trials.
Other nontraditional strategies
Cardiovascular exercise, resistance training, mindfulness, and yoga have been shown to decrease severe depressive symptoms when used as adjuncts for patients with treatment-resistant depression, or as monotherapy to treat patients with milder depression.
Exercise. The significant benefits of exercise in various forms as treatment for mild to moderate depression are well described in the literature, but it is less clear if it is effective for treatment-resistant depression. A 2013 Cochrane report24 (39 studies with 2,326 participants total) and 2 meta-analyses undertaken in 2015 (Kvam et al25 included 23 studies with 977 participants, and Schuh et al26 included 25 trials with 1,487 participants) reported that various types of exercise ameliorate depression of differing subtypes and severity, with effect sizes ranging from small to large. Schuh et al26 found that publication bias underestimated effect size. Also, not surprisingly, separate analysis of only higher-quality trials decreased effect size.24-26 A meta-analysis that included tai chi and yoga in addition to aerobic exercise and strength training (25 trials with 2,083 participants) found low to moderate benefit for exercise and yoga.27 Finally, a meta-analysis by Cramer et al28 that included 12 RCTs (N = 619) supported the use of yoga plus controlled breathing techniques as an ancillary treatment for depression.
Two small exercise trials specifically evaluated patients with treatment-resistant depression.29,30 Mota-Pereira et al29 compared 22 participants who walked for 30 to 45 minutes, 5 days a week for 12 weeks in addition to pharmacotherapy with 11 patients who received pharmacotherapy only. Exercise improved all outcomes, including HDRS score (both compared to baseline and to the control group). Moreover, 26% of the exercise group went into remission. Pilu et al30 evaluated strength training as an adjunctive treatment. Participants received 1 hour of strength training twice weekly for 8 months (n = 10), or pharmacotherapy only (n = 20). The adjunct strength training group had a statistically significant (P < .0001) improvement in HDRS scores at the end of the 8 months, whereas the control group did not (P < .28).
Continue to: Adverse effects...
Adverse effects of exercise are typically limited to sprains or strains; rarely, participants experience serious injuries.
Mindfulness-based interventions involve purposely paying attention in the present moment to enhance self-understanding and decrease anxiety about the future and regrets about the past, both of which complicate depression. A meta-analysis of 12 RCTs (N = 578) found this approach significantly reduced depression severity when used as an adjunctive therapy.31 There may be risks if mindfulness-based interventions are practiced incorrectly. For example, some reports have linked mindfulness-based interventions to psychotic episodes, meditation addiction, and antisocial or asocial behavior.32
Bottom Line
Nonpharmacologic options for patients with treatment-resistant depression include herbal/nutraceuticals, anti-inflammatory/immune system therapies, and devices. While research suggests some of these approaches are promising, clinicians need to carefully consider potential adverse effects, some of which may be serious.
Related Resources
- Kaur M, Sanches M. Experimental therapeutics in treatmentresistant major depressive disorder. J Exp Pharmacol. 2021;13:181-196.
- Janicak PG. What’s new in transcranial magnetic stimulation. Current Psychiatry. 2019;18(3):10-16.
Drug Brand Names
Atorvastatin • Lipitor
Brexanolone • Zulresso
Citalopram • Celexa
Fluoxetine • Prozac
Lithium • Eskalith, Lithobid
Lovastatin • Altoprev, Mevacor
Minocycline • Dynacin, Minocin
Simvastatin • Flolipid, Zocor
When patients with major depressive disorder (MDD) do not achieve optimal outcomes after FDA-approved first-line treatments and standard adjunctive strategies, clinicians look for additional approaches to alleviate their patients’ symptoms. Recent research suggests that several “nontraditional” treatments used primarily as adjuncts to standard antidepressants have promise for treatment-resistant depression.
In Part 1 of this article (

Herbal/nutraceutical agents
This category encompasses a variety of commonly available “natural” options patients often ask about and at times self-prescribe. Examples evaluated in clinical trials include:
- vitamin D
- essential fatty acids (omega-3, omega-6)
- S-adenosyl-L-methionine (SAMe)
- hypericum perforatum (St. John’s Wort)
- probiotics.
Vitamin D deficiency has been linked to depression, possibly by lowering serotonin, norepinephrine, and dopamine concentrations.1-3
A meta-analysis of 3 prospective, observational studies (N = 8,815) found an elevated risk of affective disorders in patients with low vitamin D levels.4 In addition, a systematic review and meta-analysis supported a potential role for vitamin D supplementation for patients with treatment-resistant depresssion.5
Toxicity can occur at levels >100 ng/mL, and resulting adverse effects may include weakness, fatigue, sleepiness, headache, loss of appetite, dry mouth, metallic taste, nausea, and vomiting. This vitamin can be considered as an adjunct to standard antidepressants, particularly in patients with treatment-resistant depression who have low vitamin D levels, but regular monitoring is necessary to avoid toxicity.
Essential fatty acids. Protein receptors embedded in lipid membranes and their binding affinities are influenced by omega-3 and omega-6 polyunsaturated fatty acids. Thus, essential fatty acids may benefit depression by maintaining membrane integrity and fluidity, as well as via their anti-inflammatory activity.
Continue to: Although results from...
Although results from controlled trials are mixed, a systematic review and meta-analysis of adjunctive nutraceuticals supported a potential role for essential fatty acids, primarily eicosapentaenoic acid (EPA), by itself or in combination with docosahexaenoic acid (DHA), with total EPA >60%.5 A second meta-analysis of 26 studies (N = 2,160) that considered only essential fatty acids concluded that EPA ≥60% at ≤1 g/d could benefit depression.6 Furthermore, omega-3 fatty acids may be helpful as an add-on agent for postpartum depression.7
Be aware that a diet rich in omega-6 greatly increases oxidized low-density lipoprotein levels in adipose tissue, potentially posing a cardiac risk factor. Clinicians need to be aware that self-prescribed use of essential fatty acids is common, and to ask about and monitor their patients’ use of these agents.
S-adenosyl-L-methionine (SAMe) is an intracellular amino acid and methyl donor. Among other actions, it is involved in the biosynthesis of hormones and neurotransmitters. There is promising but limited preliminary evidence of its efficacy and safety as a monotherapy or for antidepressant augmentation.
- Five out of 6 earlier controlled studies reported SAMe IV (200 to 400 mg/d) or IM (45 to 50 mg/d) was more effective than placebo
- When the above studies were added to 14 subsequent studies for a meta-analysis, 12 of 19 RCTs reported that parenteral or oral SAMe was significantly more effective than placebo for depression (P < .05).
Overall, the safety and tolerability of SAMe are good. Common adverse effects include nausea, mild insomnia, dizziness, irritability, and anxiety. This is another compound widely available without a prescription and at times self-prescribed. It carries an acceptable risk/benefit balance, with decades of experience.
Hypericum perforatum (St. John’s Wort) is widely prescribed for depression in China and Europe, typically in doses ranging from 500 to 900 mg/d. Its mechanism of action in depression may relate to inhibition of serotonin, dopamine, and norepinephrine uptake from the synaptic cleft of these interconnecting neurotransmitter systems.
Continue to: A meta-analysis of 7 clinical trials...
A meta-analysis of 7 clinical trials (N = 3,808) comparing St. John’s Wort with various selective serotonin reuptake inhibitors (SSRIs) reported comparable rates of response (pooled relative risk .983, 95% CI .924 to 1.042; P < .001) and remission (pooled relative risk 1.013, 95% CI .892 to 1.134; P < .001).9 Further, there were significantly lower discontinuation/dropout rates (pooled odds ratio .587, 95% CI .478 to 0.697; P < .001) for St. John’s Wort compared with the SSRIs.
Existing evidence on the long-term efficacy and safety is limited (studies ranged from 4 to 12 weeks), as is evidence for patients with more severe depression or high suicidality.
Serious drug interactions include the potential for serotonin syndrome when St. John’s Wort is combined with certain antidepressants, compromised efficacy of benzodiazepines and standard antidepressants, and severe skin reactions to sun exposure. In addition, St. John’s Wort may not be safe to use during pregnancy or while breastfeeding. Because potential drug interactions can be serious and individuals often self-prescribe this agent, it is important to ask patients about their use of St. John’s Wort, and to be vigilant for such potential adverse interactions.
Probiotics. These agents produce neuroactive substances that act on the brain/gut axis. Preliminary evidence suggests that these “psychobiotics” confer mental health benefits.10-12 Relative to other approaches, their low-risk profile make them an attractive option for some patients.
Anti-inflammatory/immune system therapies
Inflammation is linked to various medical and brain disorders. For example, patients with depression often demonstrate increased levels of peripheral blood inflammatory biomarkers (such as C-reactive protein and interleukin-6 and -17) that are known to alter norepinephrine, neuroendocrine (eg, the hypothalamic-pituitary-adrenal axis), and microglia function in addition to neuroplasticity. Thus, targeting inflammation may facilitate the development of novel antidepressants. In addition, these agents may benefit depression associated with comorbid autoimmune disorders, such as psoriasis or rheumatoid arthritis. A systematic review and meta-analysis of 36 RCTs (N = 10,000) found 5 out of 6 anti-inflammatory agents improved depression.13,14 In general, reported disadvantages of anti-inflammatories/immunosuppressants include the potential to block the antidepressant effect of some agents, the risk of opportunistic infections, and an increased risk of suicide.
Continue to: Statins
Statins
In a meta-analysis of 3 randomized, double-blind trials, 3 statins (lovastatin, atorvastatin, and simvastatin) significantly improved depression scores when used as an adjunctive therapy to fluoxetine and citalopram, compared with adjunctive placebo (N = 165, P < .001).15
Specific adverse effects of statins include headaches, muscle pain (rarely rhabdomyolysis), dizziness, rash, and liver damage. Statins also have the potential for adverse interactions with other medications. Given the limited efficacy literature on statins for depression and the potential for serious adverse effects, these agents probably should be limited to patients with treatment-resistant depression for whom a statin is indicated for a comorbid medical disorder, such as hypercholesteremia.
Neurosteroids
Brexanolone is FDA-approved for the treatment of postpartum depression. It is an IV formulation of the neuroactive steroid hormone allopregnanolone (a metabolite of progesterone), which acts as a positive allosteric modulator of the GABA-A receptor. Unfortunately, the infusion needs to occur over a 60-hour period.
Ganaxolone is an oral analog formulation of allopregnanolone. In an uncontrolled, open-label pilot study, this medication was administered for 8 weeks as an adjunct to an adequately dosed antidepressant to 10 postmenopausal women with persistent MDD.16 Of the 9 women who completed the study, 4 (44%) improved significantly (P < .019) and the benefit was sustained for 2 additional weeks.16 Adverse effects of ganaxolone included dizziness in 60% of participants, and sleepiness and fatigue in all of them with twice-daily dosing. If the FDA approves ganaxolone, it would become an easier-to-administer option to brexanolone.
Zuranolone is an investigational agent being studied as a treatment for postpartum depression. In a double-blind RCT that evaluated 151 women with postpartum depression, those who took oral zuranolone, 30 mg daily at bedtime for 2 weeks, experienced significant reductions in Hamilton Depression Rating Scale-17 (HDRS-17) scores compared with placebo (P < .003).17 Improvement in core depression symptom ratings was seen as early as Day 3 and persisted through Day 45.
Continue to: The most common...
The most common (≥5%) treatment-emergent adverse effects were somnolence (15%), headache (9%), dizziness (8%), upper respiratory tract infection (8%), diarrhea (6%), and sedation (5%). Two patients experienced a serious adverse event: one who received zuranolone (confusional state) and one who received placebo (pancreatitis). One patient discontinued zuranolone due to adverse effects vs no discontinuations among those who received placebo. The risk of taking zuranolone while breastfeeding is not known.
Device-based strategies
In addition to FDA-cleared approaches (eg, electroconvulsive therapy [ECT], vagus nerve stimulation [VNS], transcranial magnetic stimulation [TMS]), other devices have also demonstrated promising results.
Transcranial direct current stimulation (tDCS) involves delivering weak electrical current to the cerebral cortex through small scalp electrodes to produce the following effects:
- anodal tDCS enhances cortical excitability
- cathodal tDCS reduces cortical excitability.
A typical protocol consists of delivering 1 to 2 mA over 20 minutes with scalp electrodes placed in different configurations based on the targeted symptom(s).
While tDCS has been evaluated as a treatment for various neuropsychiatric disorders, including bipolar depression, Parkinson’s disease, and schizophrenia, most trials have looked at its use for treating depression. Results have been promising but mixed. For example, 1 meta-analysis of 6 RCTs (comprising 96 active and 80 sham tDCS courses) reported that active tDCS was superior to a sham procedure (Hedges’ g = 0.743) for symptoms of depression.18 By contrast, another meta-analysis of 6 RCTs (N = 200) did not find a significant difference between active and sham tDCS for response and remission rates.19 More recently, a group of experts created an evidence-based guideline using a systematic review of the controlled trial literature. These authors concluded there is “probable efficacy for anodal tDCS of the left dorsolateral prefrontal cortex (DLPFC) (with right orbitofrontal cathode) in major depressive episodes without drug resistance but probable inefficacy for drug-resistant major depressive episodes.”20
Continue to: Adverse effects of tDCS...
Adverse effects of tDCS are typically mild but may include persistent skin lesions similar to burns; mania or hypomania; and one reported seizure in a pediatric patient.
Because various over-the-counter direct current stimulation devices are available for purchase at modest cost, clinicians should ask patients if they have been self-administering this treatment.
Chronotherapy strategies
Agomelatine combines serotonergic (5-HT2B and 5-HT2C antagonist) and melatonergic (MT1-MT2 agonist in the suprachiasmatic nucleus) actions that contribute to stabilization of circadian rhythms and subsequent improvement in sleep patterns. Agomelatine (n = 1,274) significantly lowered depression symptoms compared with placebo (n = 689) (standardized mean difference −0.26; P < 3.48×10-11), but the clinical relevance was questionable.21 A recent review of the literature and expert opinion suggest this agent may also have efficacy for anhedonia; however, in placebo-controlled, relapse prevention studies, its long-term efficacy was not consistent.22
Common adverse effects include anxiety; nausea, vomiting, and stomach pain; abnormal dreams and insomnia; dizziness; drowsiness and fatigue; and weight gain. Some reviewers have expressed concerns about agomelatine’s potential for hepatotoxicity and the need for repeated clinical laboratory tests. Although agomelatine is approved outside of the United States, limited efficacy data and the potential for serious adverse effects have precluded FDA approval of this agent.
Sleep deprivation as a treatment technique for depression has been developed over the past 50 years. With total sleep deprivation (TSD) over 1 cycle, patients stay awake for approximately 36 hours, from daytime until the next day’s evening. While 1 to 6 cycles can produce acute antidepressant effects, prompt relapse after sleep recovery is common.
Continue to: In a systematic review...
In a systematic review and meta-analysis of 7 studies that included a total of 311 patients with bipolar depression23:
- TSD plus medications resulted in a significant decrease in depressive symptoms at 1 week compared with medications alone
- higher response rates were maintained after 3 months with lithium.
Adverse effects commonly include general fatigue and headaches; possible switch into mania with bipolar depression; and rarely, seizures or other unexpected medical conditions (eg, acute coronary syndrome). Presently, this approach is limited to research laboratories with the appropriate sophistication to safely conduct such trials.
Other nontraditional strategies
Cardiovascular exercise, resistance training, mindfulness, and yoga have been shown to decrease severe depressive symptoms when used as adjuncts for patients with treatment-resistant depression, or as monotherapy to treat patients with milder depression.
Exercise. The significant benefits of exercise in various forms as treatment for mild to moderate depression are well described in the literature, but it is less clear if it is effective for treatment-resistant depression. A 2013 Cochrane report24 (39 studies with 2,326 participants total) and 2 meta-analyses undertaken in 2015 (Kvam et al25 included 23 studies with 977 participants, and Schuh et al26 included 25 trials with 1,487 participants) reported that various types of exercise ameliorate depression of differing subtypes and severity, with effect sizes ranging from small to large. Schuh et al26 found that publication bias underestimated effect size. Also, not surprisingly, separate analysis of only higher-quality trials decreased effect size.24-26 A meta-analysis that included tai chi and yoga in addition to aerobic exercise and strength training (25 trials with 2,083 participants) found low to moderate benefit for exercise and yoga.27 Finally, a meta-analysis by Cramer et al28 that included 12 RCTs (N = 619) supported the use of yoga plus controlled breathing techniques as an ancillary treatment for depression.
Two small exercise trials specifically evaluated patients with treatment-resistant depression.29,30 Mota-Pereira et al29 compared 22 participants who walked for 30 to 45 minutes, 5 days a week for 12 weeks in addition to pharmacotherapy with 11 patients who received pharmacotherapy only. Exercise improved all outcomes, including HDRS score (both compared to baseline and to the control group). Moreover, 26% of the exercise group went into remission. Pilu et al30 evaluated strength training as an adjunctive treatment. Participants received 1 hour of strength training twice weekly for 8 months (n = 10), or pharmacotherapy only (n = 20). The adjunct strength training group had a statistically significant (P < .0001) improvement in HDRS scores at the end of the 8 months, whereas the control group did not (P < .28).
Continue to: Adverse effects...
Adverse effects of exercise are typically limited to sprains or strains; rarely, participants experience serious injuries.
Mindfulness-based interventions involve purposely paying attention in the present moment to enhance self-understanding and decrease anxiety about the future and regrets about the past, both of which complicate depression. A meta-analysis of 12 RCTs (N = 578) found this approach significantly reduced depression severity when used as an adjunctive therapy.31 There may be risks if mindfulness-based interventions are practiced incorrectly. For example, some reports have linked mindfulness-based interventions to psychotic episodes, meditation addiction, and antisocial or asocial behavior.32
Bottom Line
Nonpharmacologic options for patients with treatment-resistant depression include herbal/nutraceuticals, anti-inflammatory/immune system therapies, and devices. While research suggests some of these approaches are promising, clinicians need to carefully consider potential adverse effects, some of which may be serious.
Related Resources
- Kaur M, Sanches M. Experimental therapeutics in treatmentresistant major depressive disorder. J Exp Pharmacol. 2021;13:181-196.
- Janicak PG. What’s new in transcranial magnetic stimulation. Current Psychiatry. 2019;18(3):10-16.
Drug Brand Names
Atorvastatin • Lipitor
Brexanolone • Zulresso
Citalopram • Celexa
Fluoxetine • Prozac
Lithium • Eskalith, Lithobid
Lovastatin • Altoprev, Mevacor
Minocycline • Dynacin, Minocin
Simvastatin • Flolipid, Zocor
1. Pittampalli S, Mekala HM, Upadhyayula, S, et al. Does vitamin D deficiency cause depression? Prim Care Companion CNS Disord. 2018;20(5):17l02263.
2. Parker GB, Brotchie H, Graham RK. Vitamin D and depression. J Affect Disord. 2017;208:56-61.
3. Berridge MJ. Vitamin D and depression: cellular and regulatory mechanisms. Pharmacol Rev. 2017;69(2):80-92.
4. Anglin RE, Samaan Z, Walter SD, et al. Vitamin D deficiency and depression in adults: systematic review and meta-analysis. Br J Psychiatry. 2013;202:100-107.
5. Sarris J, Murphy J, Mischoulon D, et al. Adjunctive nutraceuticals for depression: a systematic review and meta-analyses. Am J Psychiatry 2016;173(6);575-587.
6. Liao Y, Xie B, Zhang H, et al. Efficacy of omega-3 PUFAs in depression: a meta-analysis. Transl Psychiatry. 2019;9(1):190.
7. Mocking RJT, Steijn K, Roos C, et al. Omega-3 fatty acid supplementation for perinatal depression: a meta-analysis. J Clin Psychiatry. 2020;81(5):19r13106.
8. Sharma A, Gerbarg P, Bottiglieri T, et al; Work Group of the American Psychiatric Association Council on Research. S-Adenosylmethionine (SAMe) for neuropsychiatric disorders: a clinician-oriented review of research. J Clin Psychiatry. 2017;78(6):e656-e667.
9. Ng QX, Venkatanarayanan N, Ho CY. Clinical use of hypericum perforatum (St John’s wort) in depression: a meta-analysis. J Affect Disord 2017;210:211-221.
10. Huang R, Wang K, Hu J. Effect of probiotics on depression: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2016;8(8):483.
11. Liu RT, Walsh RFL, Sheehan AE. Prebiotics and probiotics for depression and anxiety: a systematic review and meta-analysis of controlled clinical trials. Neurosci Biobehav Rev. 2019;102:13-23.
12. Wallace CJK, Milev RV. The efficacy, safety, and tolerability of probiotics on depression: clinical results from an open-label pilot study. Front Psychiatry. 2021;12(132):618279.
13. Köhler-Forsberg O, N Lyndholm C, Hjorthøj C, et al. Efficacy of anti-inflammatory treatment on major depressive disorder or depressive symptoms: meta-analysis of clinical trials. Acta Psychiatr Scand. 2019;139(5):404-419.
14. Jha MK. Anti-inflammatory treatments for major depressive disorder: what’s on the horizon? J Clin Psychiatry. 2019;80(6)18ac12630.
15. Salagre E, Fernandes BS, Dodd S, et al. Statins for the treatment of depression: a meta-analysis of randomized, double-blind, placebo-controlled trials. J Affect Disord. 2016;200:235-242.
16. Dichtel LE, Nyer M, Dording C, et al. Effects of open-label, adjunctive ganaxolone on persistent depression despite adequate antidepressant treatment in postmenopausal women: a pilot study. J Clin Psychiatry. 2020;81(4):19m12887.
17. Deligiannidis KM, Meltzer-Brody S, Gunduz-Bruce H, et al. Effect of zuranolone vs placebo in postpartum depression: a randomized clinical trial. JAMA Psychiatry. 2021;78(9):951-959.
18. Kalu UG, Sexton CE, Loo CK, et al. Transcranial direct current stimulation in the treatment of major depression: a meta-analysis. Psychol Med. 2012;42(9):1791-800.
19. Berlim MT, Van den Eynde F, Daskalakis ZJ. Clinical utility of transcranial direct current stimulation (tDCS) for treating major depression: a systematic review and meta-analysis of randomized, double-blind and sham-controlled trials. J Psychiatr Res. 2013;47(1):1-7.
20. Lefaucheur JP, Antal A, Ayache SS, et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin Neurophysiol. 2017;128(1):56-92.
21. Singh SP, Singh V, Kar N. Efficacy of agomelatine in major depressive disorder: meta-analysis and appraisal. Int J Neuropsychopharmacol. 2012;15(3):417-428.
22. Norman TR, Olver JS. Agomelatine for depression: expanding the horizons? Expert Opin Pharmacother. 2019;20(6):647-656.
23. Ramirez-Mahaluf JP, Rozas-Serri E, Ivanovic-Zuvic F, et al. Effectiveness of sleep deprivation in treating acute bipolar depression as augmentation strategy: a systematic review and meta-analysis. Front Psychiatry. 2020;11:70.
24. Cooney GM, Dwan K, Greig CA, et al. Exercise for depression. Cochrane Database Syst Rev. 2013;(9):CD004366.
25. Kvam S, Kleppe CL, Nordhus IH, et al. Exercise as a treatment for depression: a meta-analysis. J Affect Disord. 2016;202:67-86.
26. Schuch FB, Vancampfort D, Richards J, et al. Exercise as a treatment for depression: a meta-analysis adjusting for publication bias. J Psychiatr Res. 2016;77:42-51.
27. Seshadri A, Adaji A, Orth SS, et al. Exercise, yoga, and tai chi for treatment of major depressive disorder in outpatient settings: a systematic review and meta-analysis. Prim Care Companion CNS Disord. 2020;23(1):20r02722.
28. Cramer H, Lauche R, Langhorst J, et al. Yoga for depression: a systematic review and meta-analysis. Depress Anxiety. 2013;30(11):1068-1083.
29. Mota-Pereira J, Silverio J, Carvalho S, et al. Moderate exercise improves depression parameters in treatment-resistant patients with major depressive disorder. J Psychiatr Res. 2011;45(8):1005-1011.
30. Pilu A, Sorba M, Hardoy MC, et al. Efficacy of physical activity in the adjunctive treatment of major depressive disorders: preliminary results. Clin Pract Epidemiol Ment Health. 2007;3:8.
31. Strauss C, Cavanagh K, Oliver A, et al. Mindfulness-based interventions for people diagnosed with a current episode of an anxiety or depressive disorder: a meta-analysis of randomised controlled trials. PLoS One. 2014;9(4):e96110.
32. Shonin E, Van Gordon W, Griffiths MD. Are there risks associated with using mindfulness for the treatment of psychopathology? Clinical Practice. 2014;11(4):389-392.
1. Pittampalli S, Mekala HM, Upadhyayula, S, et al. Does vitamin D deficiency cause depression? Prim Care Companion CNS Disord. 2018;20(5):17l02263.
2. Parker GB, Brotchie H, Graham RK. Vitamin D and depression. J Affect Disord. 2017;208:56-61.
3. Berridge MJ. Vitamin D and depression: cellular and regulatory mechanisms. Pharmacol Rev. 2017;69(2):80-92.
4. Anglin RE, Samaan Z, Walter SD, et al. Vitamin D deficiency and depression in adults: systematic review and meta-analysis. Br J Psychiatry. 2013;202:100-107.
5. Sarris J, Murphy J, Mischoulon D, et al. Adjunctive nutraceuticals for depression: a systematic review and meta-analyses. Am J Psychiatry 2016;173(6);575-587.
6. Liao Y, Xie B, Zhang H, et al. Efficacy of omega-3 PUFAs in depression: a meta-analysis. Transl Psychiatry. 2019;9(1):190.
7. Mocking RJT, Steijn K, Roos C, et al. Omega-3 fatty acid supplementation for perinatal depression: a meta-analysis. J Clin Psychiatry. 2020;81(5):19r13106.
8. Sharma A, Gerbarg P, Bottiglieri T, et al; Work Group of the American Psychiatric Association Council on Research. S-Adenosylmethionine (SAMe) for neuropsychiatric disorders: a clinician-oriented review of research. J Clin Psychiatry. 2017;78(6):e656-e667.
9. Ng QX, Venkatanarayanan N, Ho CY. Clinical use of hypericum perforatum (St John’s wort) in depression: a meta-analysis. J Affect Disord 2017;210:211-221.
10. Huang R, Wang K, Hu J. Effect of probiotics on depression: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2016;8(8):483.
11. Liu RT, Walsh RFL, Sheehan AE. Prebiotics and probiotics for depression and anxiety: a systematic review and meta-analysis of controlled clinical trials. Neurosci Biobehav Rev. 2019;102:13-23.
12. Wallace CJK, Milev RV. The efficacy, safety, and tolerability of probiotics on depression: clinical results from an open-label pilot study. Front Psychiatry. 2021;12(132):618279.
13. Köhler-Forsberg O, N Lyndholm C, Hjorthøj C, et al. Efficacy of anti-inflammatory treatment on major depressive disorder or depressive symptoms: meta-analysis of clinical trials. Acta Psychiatr Scand. 2019;139(5):404-419.
14. Jha MK. Anti-inflammatory treatments for major depressive disorder: what’s on the horizon? J Clin Psychiatry. 2019;80(6)18ac12630.
15. Salagre E, Fernandes BS, Dodd S, et al. Statins for the treatment of depression: a meta-analysis of randomized, double-blind, placebo-controlled trials. J Affect Disord. 2016;200:235-242.
16. Dichtel LE, Nyer M, Dording C, et al. Effects of open-label, adjunctive ganaxolone on persistent depression despite adequate antidepressant treatment in postmenopausal women: a pilot study. J Clin Psychiatry. 2020;81(4):19m12887.
17. Deligiannidis KM, Meltzer-Brody S, Gunduz-Bruce H, et al. Effect of zuranolone vs placebo in postpartum depression: a randomized clinical trial. JAMA Psychiatry. 2021;78(9):951-959.
18. Kalu UG, Sexton CE, Loo CK, et al. Transcranial direct current stimulation in the treatment of major depression: a meta-analysis. Psychol Med. 2012;42(9):1791-800.
19. Berlim MT, Van den Eynde F, Daskalakis ZJ. Clinical utility of transcranial direct current stimulation (tDCS) for treating major depression: a systematic review and meta-analysis of randomized, double-blind and sham-controlled trials. J Psychiatr Res. 2013;47(1):1-7.
20. Lefaucheur JP, Antal A, Ayache SS, et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin Neurophysiol. 2017;128(1):56-92.
21. Singh SP, Singh V, Kar N. Efficacy of agomelatine in major depressive disorder: meta-analysis and appraisal. Int J Neuropsychopharmacol. 2012;15(3):417-428.
22. Norman TR, Olver JS. Agomelatine for depression: expanding the horizons? Expert Opin Pharmacother. 2019;20(6):647-656.
23. Ramirez-Mahaluf JP, Rozas-Serri E, Ivanovic-Zuvic F, et al. Effectiveness of sleep deprivation in treating acute bipolar depression as augmentation strategy: a systematic review and meta-analysis. Front Psychiatry. 2020;11:70.
24. Cooney GM, Dwan K, Greig CA, et al. Exercise for depression. Cochrane Database Syst Rev. 2013;(9):CD004366.
25. Kvam S, Kleppe CL, Nordhus IH, et al. Exercise as a treatment for depression: a meta-analysis. J Affect Disord. 2016;202:67-86.
26. Schuch FB, Vancampfort D, Richards J, et al. Exercise as a treatment for depression: a meta-analysis adjusting for publication bias. J Psychiatr Res. 2016;77:42-51.
27. Seshadri A, Adaji A, Orth SS, et al. Exercise, yoga, and tai chi for treatment of major depressive disorder in outpatient settings: a systematic review and meta-analysis. Prim Care Companion CNS Disord. 2020;23(1):20r02722.
28. Cramer H, Lauche R, Langhorst J, et al. Yoga for depression: a systematic review and meta-analysis. Depress Anxiety. 2013;30(11):1068-1083.
29. Mota-Pereira J, Silverio J, Carvalho S, et al. Moderate exercise improves depression parameters in treatment-resistant patients with major depressive disorder. J Psychiatr Res. 2011;45(8):1005-1011.
30. Pilu A, Sorba M, Hardoy MC, et al. Efficacy of physical activity in the adjunctive treatment of major depressive disorders: preliminary results. Clin Pract Epidemiol Ment Health. 2007;3:8.
31. Strauss C, Cavanagh K, Oliver A, et al. Mindfulness-based interventions for people diagnosed with a current episode of an anxiety or depressive disorder: a meta-analysis of randomised controlled trials. PLoS One. 2014;9(4):e96110.
32. Shonin E, Van Gordon W, Griffiths MD. Are there risks associated with using mindfulness for the treatment of psychopathology? Clinical Practice. 2014;11(4):389-392.