User login
Youth e-cigarette use: Assessing for, and halting, the hidden habit
THE CASE
Joe, an 18-year-old, has been your patient for many years and has an uncomplicated medical history. He presents for his preparticipation sports examination for the upcoming high school baseball season. Joe’s mother, who arrives at the office with him, tells you she’s worried because she found an e-cigarette in his backpack last week. Joe says that many of the kids at his school vape and he tried it a while back and now vapes “a lot.”
After talking further with Joe, you realize that he is vaping every day, using a 5% nicotine pod. Based on previous consults with the behavioral health counselor in your clinic, you know that this level of vaping is about the same as smoking 1 pack of cigarettes per day in terms of nicotine exposure. Joe states that he often vapes in the bathroom at school because he cannot concentrate in class if he doesn’t vape. He also reports that he had previously used 1 pod per week but had recently started vaping more to help with his cravings.
You assess his withdrawal symptoms and learn that he feels on edge when he is not able to vape and that he vapes heavily before going into school because he knows he will not be able to vape again until his third passing period.
●
Electronic cigarettes (e-cigarettes; also called “vapes”) are electronic nicotine delivery systems that heat and aerosolize e-liquid or “e-juice” that is inhaled by the user. The e-liquid is made up primarily of propylene glycol, vegetable glycerin, and flavorings, and often includes nicotine. Nicotine levels in e-cigarettes can range from 0 mg/mL to 60 mg/mL (regular cigarettes contain ~12 mg of nicotine). The nicotine level of the pod available from e-cigarette company JUUL (50 mg/mL e-liquid) is equivalent to about 1 pack of cigarettes.1 E-cigarette devices are relatively affordable; popular brands cost $10 to $20, while the replacement pods or e-liquid are typically about $4 each.
The e-cigarette market is quickly evolving and diversifying. Originally, e-cigarettes looked similar to cigarettes (cig-a-likes) but did not efficiently deliver nicotine to the user.2 E-cigarettes have evolved and some now deliver cigarette-like levels of nicotine to the user.3,4 Youth and young adults primarily use pod-mod e-cigarettes, which have a sleek design and produce less vapor than older e-cigarettes, making them easier to conceal. They can look like a USB flash-drive or have a teardrop shape. Pod-mod e-cigarettes dominate the current market, led by companies such as JUUL, NJOY, and Vuse.5
E-cigarette use is proliferating in the United States, particularly among young people and facilitated by the introduction of pod-based e-cigarettes in appealing flavors.6,7 While rates of current e-cigarette use by US adults is around 5.5%,8 recent data show that 32.7% of US high school students say they’ve used an e-cigarette in the past 30 days.9
Continue to: A double-edged sword
A double-edged sword. E-cigarettes are less harmful than traditional cigarettes in the short term and likely benefit adult smokers who completely substitute e-cigarettes for their tobacco cigarettes.10 In randomized trials of adult smokers, e-cigarette use resulted in moderate combustible-cigarette cessation rates that rival or exceed rates achieved with traditional nicotine replacement therapy (NRT).11-13 However, most e-cigarettes contain addictive nicotine, can facilitate transitions to more harmful forms of tobacco use,10,14,15 and have unknown long-term health effects. Therefore, youth, young adults, and those who are otherwise tobacco naïve should not initiate e-cigarette use.
Moreover, cases of e-cigarette or vaping product use–associated lung injury (EVALI)—a disease linked to vaping that causes cough, fever, shortness of breath, and death—were first identified in August 2019 and peaked in September 2019 before new cases decreased dramatically through January 2020.16 Since the initial cases of EVALI arose, product testing has shown that tetrahydrocannabinol (THC) and vitamin E acetate are the main ingredients linked to EVALI cases.17 For this reason, the Centers for Disease Control and Prevention and others strongly recommend against use of THC-containing e-cigarettes.18
Given the high rates of e-cigarette use among youth and young adults and its potential health harms, it is critical to inquire about e-cigarette use at primary care visits, and, as appropriate, to assess frequency and quantity of use. Patients who require intervention will be more likely to succeed in quitting if they are connected with behavioral health counseling and prescribed medication. This article offers evidence-based guidance to assess and advise teens and young adults regarding the potential health impact of e-cigarettes.
A NEW ICD-10-CM CODE AND A BRIEF ASSESSMENT TOOL
According to the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5)19 and the International Classification of Diseases, 10th Revision (ICD-10-CM),20 a tobacco use disorder is a problematic pattern of use leading to clinically significant impairment or distress. Associated features and behavioral markers of frequency and quantity include use within 30 minutes of waking, daily use, and increasing use. However, with youth, consider intervention for use of any nicotine or tobacco product, including e-cigarettes, regardless of whether it meets the threshold for diagnosis.21
The new code.
Continue to: As with other tobacco use...
As with other tobacco use, assess e-cigarette use patterns by asking questions about the frequency, duration, and quantity of use. Additionally, determine the level of nicotine in the e-liquid (discussed earlier) and evaluate whether the individual displays signs of physiologic dependence (eg, failed attempts to reduce or quit e-cigarette use, increased use, nicotine withdrawal symptoms).
A useful assessment tool. While e-cigarette use is not often included on current substance use screening measures, the above questions can be added to the end of measures such as the CRAFFT (Car-Relax-Alone-Forget-Family and Friends-Trouble) test.22 Additionally, if an adolescent reports vaping, the American Academy of Pediatrics (AAP) recommends using a brief screening tool such as the Hooked on Nicotine Checklist (HONC) to establish his or her level of dependence (TABLE 1).23
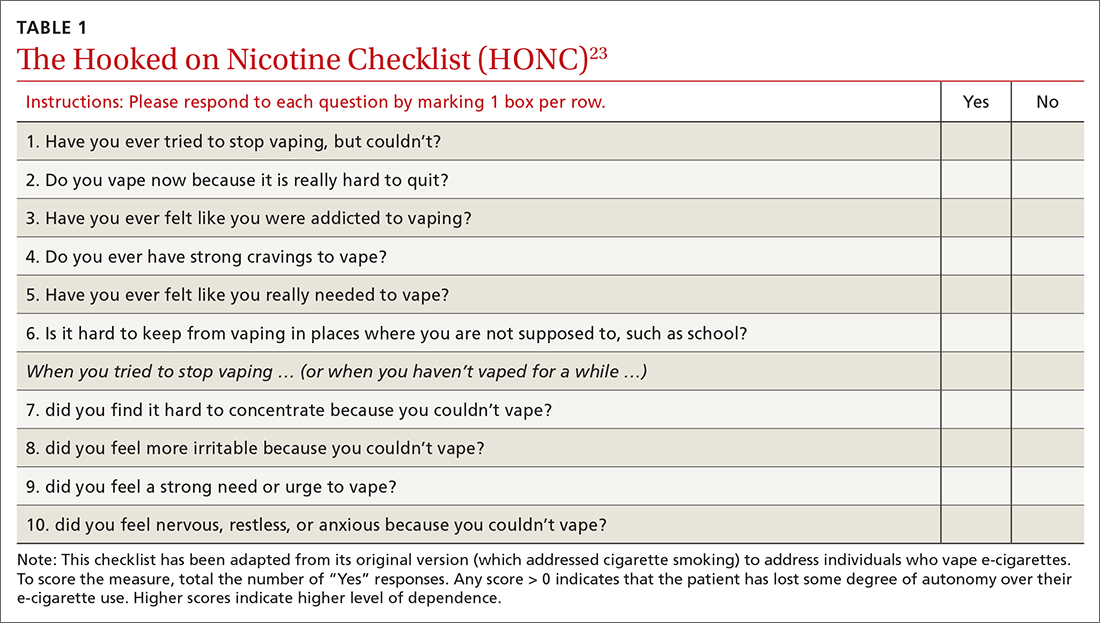
The HONC is ideal for a primary care setting because it is brief and has a high level of sensitivity, minimizing false-negative reports24; a patient’s acknowledgement of any item indicates a loss of autonomy over nicotine. Establishing the level of nicotine dependence is particularly pertinent when making decisions regarding the course of treatment and whether to prescribe NRT (eg, nicotine patch, gum, lozenge). Alternatively, you can quickly assess level of dependence by determining the time to first e-cigarette use in the morning. Tobacco guidelines suggest that if time to first use is > 30 minutes, the individual is “moderately dependent”; if time to first use is < 30 minutes after waking, the individual is “severely dependent.”25
COMBINATION TREATMENT IS MOST SUCCESSFUL
Studies have shown that the most effective treatment for tobacco cessation is pairing behavioral treatment with combination NRT (eg, nicotine gum + patch).25,26 The literature on e-cigarette cessation remains in its infancy, but techniques from traditional smoking cessation can be applied because the behaviors differ only in their mode of nicotine delivery.
Behavioral treatment. There are several options for behavioral treatment for tobacco cessation—and thus, e-cigarette cessation. The first step will depend on the patient’s level of motivation. If the patient is not yet ready to quit, consider using brief motivational interviewing. Once the patient is willing to engage in treatment, options include setting a mutually agreed upon quit date or planning for a reduction in the frequency and duration of vaping.
Continue to: Referrals to the Quitline...
Referrals to the Quitline (800-QUIT-NOW) have long been standard practice and can be used to extend primary care treatment.25 Studies show that it is more effective to connect patients directly to the Quitline at their primary care appointment27 than asking them to call after the visit.28,29 We suggest providing direct assistance in the office to patients as they initiate treatment with the Quitline.
Finally, if the level of dependence is severe or the patient is not motivated to quit, connect them with a behavioral health provider in your clinic or with an outside therapist skilled in cognitive behavioral techniques related to tobacco cessation. Discuss with the patient that quitting nicotine use is difficult for many people and that the best option for success is the combination of counseling and medication.25
Nicotine replacement therapy for e-cigarette use. While over-the-counter NRT (nicotine gum, patches, lozenges) is approved by the US Food and Drug Administration only for sale to adults ≥ 18 years, the AAP issued guidance on prescribing NRT for those < 18 years who use e-cigarettes.30 While the AAP does not suggest a lower age limit for prescribing NRT, national data show that < 6% of middle schoolers report e-cigarette use and that e-cigarette use does not become common (~20% current use) until high school.31 It is therefore unlikely that a child < 14 years would require pharmacotherapy. On their fact sheet, the AAP includes the following guidance:
“Patients who are motivated to quit should use as much safe, FDA-approved NRT as needed to avoid smoking or vaping. When assessing a patient’s current level of nicotine use, it may be helpful to understand that using one JUUL pod per day is equivalent to one pack of cigarettes per day …. Pediatricians and other healthcare providers should work with each patient to determine a starting dosage of NRT that is most likely to help them quit successfully. Dosing is based on the patient’s level of nicotine dependence, which can be measured using a screening tool” (TABLE 123).32
The AAP NRT dosing guidelines can be found at downloads.aap.org/RCE/NRT_and_Adolescents_Pediatrician_Guidance_factsheet.pdf.32 Of note, the dosing guidelines for adolescents are the same as those for adults and are based on level of use and dependence. Moreover, the clinician and patient should work together to choose the initial dose and the plan for weaning NRT over time.
Continue to: THE CASE
Based on your conversation with Joe, you administer the HONC screening tool. He scores 9 out of 10, indicating significant loss of autonomy over nicotine. You consult with a behavioral health counselor, who believes that Joe would benefit from counseling and NRT. You discuss this treatment plan with Joe, who says he is ready to quit because he does not like feeling as if he depends on vaping. Your shared decision is to start the 21-mg patch and 4-mg gum with plans to step down from there.
Joe agrees to set a quit date in the following week. The behavioral health counselor then meets with Joe and they develop a quit plan, which is shared with you so you can follow up at the next visit. Joe also agrees to talk with his parents, who are unaware of his level of use and dependence. Everyone agrees on the quit plan, and a follow-up visit is scheduled.
At the follow-up visit 1 month later, Joe and his parents report that he has quit vaping but is still using the patch and gum. You instruct Joe to reduce his NRT use to the 14-mg patch and 2-mg gum and to stop using them over the next 2 to 3 weeks. Everyone is in agreement with the treatment plan. You also re-administer the HONC screening tool and see that Joe’s score has reduced by 7 points to just 2 out of 10. You recommend that Joe continue to see the behavioral health counselor and follow up as needed. (A noted benefit of having a behavioral health counselor in your clinic is the opportunity for informal briefings on patient progress.33,34)
Following each visit with Joe, you make sure to complete documentation on (1) tobacco/e-cigarette use assessment, (2) diagnoses, (3) discussion of benefits of quitting,(4) assessment of readiness to quit, (5) creation and support of a quit plan, and (6) connection with a behavioral health counselor and planned follow-up. (See TABLE 235 for details onbilling codes.)

CORRESPONDENCE
Eleanor L. S. Leavens, PhD, 3901 Rainbow Boulevard, Mail Stop 1008, Kansas City, KS 66160; [email protected]
1. Prochaska JJ, Vogel EA, Benowitz N. Nicotine delivery and cigarette equivalents from vaping a JUULpod. Tob Control. Published online March 24, 2021. doi: 10.1136/tobaccocontrol- 2020-056367
2. Rüther T, Hagedorn D, Schiela K, et al. Nicotine delivery efficiency of first-and second-generation e-cigarettes and its impact on relief of craving during the acute phase of use. Int J Hyg Environ Health. 2018;221:191-198. doi: 10.1016/j.ijheh.2017.10.012
3. Hajek P, Pittaccio K, Pesola F, et al. Nicotine delivery and users’ reactions to Juul compared with cigarettes and other e‐cigarette products. Addiction. 2020;115:1141-1148. doi: 10.1111/add.14936
4. Wagener TL, Floyd EL, Stepanov I, et al. Have combustible cigarettes met their match? The nicotine delivery profiles and harmful constituent exposures of second-generation and third-generation electronic cigarette users. Tob control. 2017;26:e23-e28. doi: 10.1136/tobaccocontrol-2016-053041
5. Herzog B, Kanada P. Nielsen: Tobacco all channel data thru 8/11 - cig vol decelerates. Published August 21, 2018. Accessed August 19, 2021. https://athra.org.au/wp-content/uploads/2018/09/Wells-Fargo-Nielsen-Tobacco-All-Channel-Report-Period-Ending-8.11.18.pdf
6. Harrell MB, Weaver SR, Loukas A, et al. Flavored e-cigarette use: characterizing youth, young adult, and adult users. Prev Med Rep. 2017;5:33-40. doi: 10.1016/j.pmedr.2016.11.001
7. Morean ME, Butler ER, Bold KW, et al. Preferring more e-cigarette flavors is associated with e-cigarette use frequency among adolescents but not adults. PloS One. 2018;13:e0189015. doi: 10.1371/journal.pone.0189015
8. Obisesan OH, Osei AD, Iftekhar Uddin SM, et al. Trends in e-cigarette use in adults in the United States, 2016-2018. JAMA Intern Med. 2020;180:1394-1398. doi: 10.1001/jamainternmed.2020.2817
9. Creamer MR, Wang TW, Babb S, et al. Tobacco product use and cessation indicators among adults—United States, 2018. MMWR Morb Mortal Wkly Rep. 2019;68:1013-1019. doi: 10.15585/mmwr.mm6845a2
10. NASEM. Public health consequences of e-cigarettes. National Academies Press; 2018. Accessed August 19, 2021. www.ncbi.nlm.nih.gov/books/NBK507171/
11. Hajek P, Phillips-Waller A, Przulj D, et al. A randomized trial of e-cigarettes versus nicotine-replacement therapy. N Engl J Med. 2019;380:629-637. doi: 10.1056/NEJMoa1808779
12. Pulvers K, Nollen NL, Rice M, et al. Effect of pod e-cigarettes vs cigarettes on carcinogen exposure among African American and Latinx smokers: a randomized clinical trial. JAMA Netw Open. 2020;3:e2026324. doi: 10.1001/jamanetworkopen.2020.26324
13. Wang RJ, Bhadriraju S, Glantz SA. E-cigarette use and adult cigarette smoking cessation: a meta-analysis. Am J Public Health. 2021;111:230-246. doi: 10.2105/AJPH.2020.305999
14. Barrington-Trimis JL, Urman R, Berhane K, et al. E-cigarettes and future cigarette use. Pediatrics. 2016;138:e20160379. doi: 10.1542/peds.2016-0379
15. Soneji S, Barrington-Trimis JL, Wills TA, et al. Association between initial use of e-cigarettes and subsequent cigarette smoking among adolescents and young adults: a systematic review and meta-analysis. JAMA Pediatr. 2017;171:788-797. doi: 10.1001/jamapediatrics.2017.1488
16. Krishnasamy VP, Hallowell BD, Ko JY, et al. Update: characteristics of a nationwide outbreak of e-cigarette, or vaping, product use–associated lung injury—United States, August 2019–January 2020. MMWR Morb Mortal Wkly Rep. 2020;69:90-94. doi: 10.15585/mmwr.mm6903e2
17. Blount BC, Karwowski MP, Shields PG, et al. Vitamin E acetate in bronchoalveolar-lavage fluid associated with EVALI. N Engl J Med. 2020;382:697-705. doi: 10.1056/NEJMoa1916433
18. CDC. Outbreak of lung injury associated with use of e-cigarette, or vaping, products. Updated February 25, 2020. Accessed August 19, 2021. www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html
19. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edition (DSM-5). American Psychiatric Publishing; 2013.
20. CDC. International Classification of Diseases, 10th Revision. Updated July 30, 2021. Accessed August 31, 2021. www.cdc.gov/nchs/icd/icd10cm.htm
21. CDC. Surgeon General’s advisory on e-cigarette use among youth. Reviewed April 9, 2019. Accessed August 19, 2021. www.cdc.gov/tobacco/basic_information/e-cigarettes/surgeon-general-advisory/index.html
22. Knight JR, Sherritt L, Shrier LA, et al. Validity of the CRAFFT substance abuse screening test among adolescent clinic patients. Arch Pediatr Adolesc Med. 2002;156:607-614. doi: 10.1001/archpedi.156.6.607
23. DiFranza JR, Savageau JA, Fletcher K, et al. Measuring the loss of autonomy over nicotine use in adolescents: the DANDY (Development and Assessment of Nicotine Dependence in Youths) study. Arch Pediatr Adolesc Med. 2002;156:397-403. doi: 10.1001/archpedi.156.4.397
24. Wellman RJ, Savageau JA, Godiwala S, et al. A comparison of the Hooked on Nicotine Checklist and the Fagerström Test for Nicotine Dependence in adult smokers. Nicotine Tob Res. 2006;8:575-580. doi: 10.1080/14622200600789965
25. Fiore MC, Jaén CR, Baker TB, et al. Treating tobacco use and dependence: 2008 update. Published May 2008. Accessed August 19, 2021. www.aafp.org/dam/AAFP/documents/patient_care/clinical_recommendations/TreatingTobaccoUseandDependence-2008Update.pdf
26. Shah SD, Wilken LA, Winkler SR, et al. Systematic review and meta-analysis of combination therapy for smoking cessation. J Am Pharm Assoc. 2008;48:659-665. doi: 10.1331/JAPhA.2008.07063
27. Vidrine JI, Shete S, Cao Y, et al. Ask-Advise-Connect: a new approach to smoking treatment delivery in health care settings. JAMA Intern Med. 2013;173:458-464. doi: 10.1001/jamainternmed.2013.3751
28. Bentz CJ, Bayley KB, Bonin KE, et al. The feasibility of connecting physician offices to a state-level tobacco quit line. Am J Prev Med. 2006;30:31-37. doi: 10.1016/j.amepre.2005.08.043
29. Borland R, Segan CJ. The potential of quitlines to increase smoking cessation. Drug Alcohol Rev. 2006;25:73-78. doi: 10.1080/09595230500459537
30. Farber HJ, Walley SC, Groner JA, et al. Clinical practice policy to protect children from tobacco, nicotine, and tobacco smoke. Pediatrics. 2015;136:1008-1017. doi: 10.1542/peds.2015-3108
31. Gentzke AS, Wang TW, Jamal A, et al. Tobacco product use among middle and high school students—United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1881-1888. doi: 10.15585/mmwr.mm6950a1
32. AAP. Nicotine replacement therapy and adolescent patients: information for pediatricians. Updated November 2019. Accessed August 19, 2021. https://downloads.aap.org/RCE/NRT_and_Adolescents_Pediatrician_Guidance_factsheet.pdf
33. Blasi PR, Cromp D, McDonald S, et al. Approaches to behavioral health integration at high performing primary care practices. J Am Board Fam Med. 2018;31:691-701. doi: 10.3122/jabfm.2018.05.170468
34. Jacobs C, Brieler JA, Salas J, et al. Integrated behavioral health care in family medicine residencies a CERA survey. Fam Med. 2018;50:380-384. doi: 10.22454/FamMed.2018.639260
35. Oliverez M. Quick guide: billing for smoking cessation services. Capture Billing. Accessed August 26, 2021. https://capturebilling.com/how-bill-smoking-cessation-counseling-99406-99407/
THE CASE
Joe, an 18-year-old, has been your patient for many years and has an uncomplicated medical history. He presents for his preparticipation sports examination for the upcoming high school baseball season. Joe’s mother, who arrives at the office with him, tells you she’s worried because she found an e-cigarette in his backpack last week. Joe says that many of the kids at his school vape and he tried it a while back and now vapes “a lot.”
After talking further with Joe, you realize that he is vaping every day, using a 5% nicotine pod. Based on previous consults with the behavioral health counselor in your clinic, you know that this level of vaping is about the same as smoking 1 pack of cigarettes per day in terms of nicotine exposure. Joe states that he often vapes in the bathroom at school because he cannot concentrate in class if he doesn’t vape. He also reports that he had previously used 1 pod per week but had recently started vaping more to help with his cravings.
You assess his withdrawal symptoms and learn that he feels on edge when he is not able to vape and that he vapes heavily before going into school because he knows he will not be able to vape again until his third passing period.
●
Electronic cigarettes (e-cigarettes; also called “vapes”) are electronic nicotine delivery systems that heat and aerosolize e-liquid or “e-juice” that is inhaled by the user. The e-liquid is made up primarily of propylene glycol, vegetable glycerin, and flavorings, and often includes nicotine. Nicotine levels in e-cigarettes can range from 0 mg/mL to 60 mg/mL (regular cigarettes contain ~12 mg of nicotine). The nicotine level of the pod available from e-cigarette company JUUL (50 mg/mL e-liquid) is equivalent to about 1 pack of cigarettes.1 E-cigarette devices are relatively affordable; popular brands cost $10 to $20, while the replacement pods or e-liquid are typically about $4 each.
The e-cigarette market is quickly evolving and diversifying. Originally, e-cigarettes looked similar to cigarettes (cig-a-likes) but did not efficiently deliver nicotine to the user.2 E-cigarettes have evolved and some now deliver cigarette-like levels of nicotine to the user.3,4 Youth and young adults primarily use pod-mod e-cigarettes, which have a sleek design and produce less vapor than older e-cigarettes, making them easier to conceal. They can look like a USB flash-drive or have a teardrop shape. Pod-mod e-cigarettes dominate the current market, led by companies such as JUUL, NJOY, and Vuse.5
E-cigarette use is proliferating in the United States, particularly among young people and facilitated by the introduction of pod-based e-cigarettes in appealing flavors.6,7 While rates of current e-cigarette use by US adults is around 5.5%,8 recent data show that 32.7% of US high school students say they’ve used an e-cigarette in the past 30 days.9
Continue to: A double-edged sword
A double-edged sword. E-cigarettes are less harmful than traditional cigarettes in the short term and likely benefit adult smokers who completely substitute e-cigarettes for their tobacco cigarettes.10 In randomized trials of adult smokers, e-cigarette use resulted in moderate combustible-cigarette cessation rates that rival or exceed rates achieved with traditional nicotine replacement therapy (NRT).11-13 However, most e-cigarettes contain addictive nicotine, can facilitate transitions to more harmful forms of tobacco use,10,14,15 and have unknown long-term health effects. Therefore, youth, young adults, and those who are otherwise tobacco naïve should not initiate e-cigarette use.
Moreover, cases of e-cigarette or vaping product use–associated lung injury (EVALI)—a disease linked to vaping that causes cough, fever, shortness of breath, and death—were first identified in August 2019 and peaked in September 2019 before new cases decreased dramatically through January 2020.16 Since the initial cases of EVALI arose, product testing has shown that tetrahydrocannabinol (THC) and vitamin E acetate are the main ingredients linked to EVALI cases.17 For this reason, the Centers for Disease Control and Prevention and others strongly recommend against use of THC-containing e-cigarettes.18
Given the high rates of e-cigarette use among youth and young adults and its potential health harms, it is critical to inquire about e-cigarette use at primary care visits, and, as appropriate, to assess frequency and quantity of use. Patients who require intervention will be more likely to succeed in quitting if they are connected with behavioral health counseling and prescribed medication. This article offers evidence-based guidance to assess and advise teens and young adults regarding the potential health impact of e-cigarettes.
A NEW ICD-10-CM CODE AND A BRIEF ASSESSMENT TOOL
According to the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5)19 and the International Classification of Diseases, 10th Revision (ICD-10-CM),20 a tobacco use disorder is a problematic pattern of use leading to clinically significant impairment or distress. Associated features and behavioral markers of frequency and quantity include use within 30 minutes of waking, daily use, and increasing use. However, with youth, consider intervention for use of any nicotine or tobacco product, including e-cigarettes, regardless of whether it meets the threshold for diagnosis.21
The new code.
Continue to: As with other tobacco use...
As with other tobacco use, assess e-cigarette use patterns by asking questions about the frequency, duration, and quantity of use. Additionally, determine the level of nicotine in the e-liquid (discussed earlier) and evaluate whether the individual displays signs of physiologic dependence (eg, failed attempts to reduce or quit e-cigarette use, increased use, nicotine withdrawal symptoms).
A useful assessment tool. While e-cigarette use is not often included on current substance use screening measures, the above questions can be added to the end of measures such as the CRAFFT (Car-Relax-Alone-Forget-Family and Friends-Trouble) test.22 Additionally, if an adolescent reports vaping, the American Academy of Pediatrics (AAP) recommends using a brief screening tool such as the Hooked on Nicotine Checklist (HONC) to establish his or her level of dependence (TABLE 1).23

The HONC is ideal for a primary care setting because it is brief and has a high level of sensitivity, minimizing false-negative reports24; a patient’s acknowledgement of any item indicates a loss of autonomy over nicotine. Establishing the level of nicotine dependence is particularly pertinent when making decisions regarding the course of treatment and whether to prescribe NRT (eg, nicotine patch, gum, lozenge). Alternatively, you can quickly assess level of dependence by determining the time to first e-cigarette use in the morning. Tobacco guidelines suggest that if time to first use is > 30 minutes, the individual is “moderately dependent”; if time to first use is < 30 minutes after waking, the individual is “severely dependent.”25
COMBINATION TREATMENT IS MOST SUCCESSFUL
Studies have shown that the most effective treatment for tobacco cessation is pairing behavioral treatment with combination NRT (eg, nicotine gum + patch).25,26 The literature on e-cigarette cessation remains in its infancy, but techniques from traditional smoking cessation can be applied because the behaviors differ only in their mode of nicotine delivery.
Behavioral treatment. There are several options for behavioral treatment for tobacco cessation—and thus, e-cigarette cessation. The first step will depend on the patient’s level of motivation. If the patient is not yet ready to quit, consider using brief motivational interviewing. Once the patient is willing to engage in treatment, options include setting a mutually agreed upon quit date or planning for a reduction in the frequency and duration of vaping.
Continue to: Referrals to the Quitline...
Referrals to the Quitline (800-QUIT-NOW) have long been standard practice and can be used to extend primary care treatment.25 Studies show that it is more effective to connect patients directly to the Quitline at their primary care appointment27 than asking them to call after the visit.28,29 We suggest providing direct assistance in the office to patients as they initiate treatment with the Quitline.
Finally, if the level of dependence is severe or the patient is not motivated to quit, connect them with a behavioral health provider in your clinic or with an outside therapist skilled in cognitive behavioral techniques related to tobacco cessation. Discuss with the patient that quitting nicotine use is difficult for many people and that the best option for success is the combination of counseling and medication.25
Nicotine replacement therapy for e-cigarette use. While over-the-counter NRT (nicotine gum, patches, lozenges) is approved by the US Food and Drug Administration only for sale to adults ≥ 18 years, the AAP issued guidance on prescribing NRT for those < 18 years who use e-cigarettes.30 While the AAP does not suggest a lower age limit for prescribing NRT, national data show that < 6% of middle schoolers report e-cigarette use and that e-cigarette use does not become common (~20% current use) until high school.31 It is therefore unlikely that a child < 14 years would require pharmacotherapy. On their fact sheet, the AAP includes the following guidance:
“Patients who are motivated to quit should use as much safe, FDA-approved NRT as needed to avoid smoking or vaping. When assessing a patient’s current level of nicotine use, it may be helpful to understand that using one JUUL pod per day is equivalent to one pack of cigarettes per day …. Pediatricians and other healthcare providers should work with each patient to determine a starting dosage of NRT that is most likely to help them quit successfully. Dosing is based on the patient’s level of nicotine dependence, which can be measured using a screening tool” (TABLE 123).32
The AAP NRT dosing guidelines can be found at downloads.aap.org/RCE/NRT_and_Adolescents_Pediatrician_Guidance_factsheet.pdf.32 Of note, the dosing guidelines for adolescents are the same as those for adults and are based on level of use and dependence. Moreover, the clinician and patient should work together to choose the initial dose and the plan for weaning NRT over time.
Continue to: THE CASE
Based on your conversation with Joe, you administer the HONC screening tool. He scores 9 out of 10, indicating significant loss of autonomy over nicotine. You consult with a behavioral health counselor, who believes that Joe would benefit from counseling and NRT. You discuss this treatment plan with Joe, who says he is ready to quit because he does not like feeling as if he depends on vaping. Your shared decision is to start the 21-mg patch and 4-mg gum with plans to step down from there.
Joe agrees to set a quit date in the following week. The behavioral health counselor then meets with Joe and they develop a quit plan, which is shared with you so you can follow up at the next visit. Joe also agrees to talk with his parents, who are unaware of his level of use and dependence. Everyone agrees on the quit plan, and a follow-up visit is scheduled.
At the follow-up visit 1 month later, Joe and his parents report that he has quit vaping but is still using the patch and gum. You instruct Joe to reduce his NRT use to the 14-mg patch and 2-mg gum and to stop using them over the next 2 to 3 weeks. Everyone is in agreement with the treatment plan. You also re-administer the HONC screening tool and see that Joe’s score has reduced by 7 points to just 2 out of 10. You recommend that Joe continue to see the behavioral health counselor and follow up as needed. (A noted benefit of having a behavioral health counselor in your clinic is the opportunity for informal briefings on patient progress.33,34)
Following each visit with Joe, you make sure to complete documentation on (1) tobacco/e-cigarette use assessment, (2) diagnoses, (3) discussion of benefits of quitting,(4) assessment of readiness to quit, (5) creation and support of a quit plan, and (6) connection with a behavioral health counselor and planned follow-up. (See TABLE 235 for details onbilling codes.)

CORRESPONDENCE
Eleanor L. S. Leavens, PhD, 3901 Rainbow Boulevard, Mail Stop 1008, Kansas City, KS 66160; [email protected]
THE CASE
Joe, an 18-year-old, has been your patient for many years and has an uncomplicated medical history. He presents for his preparticipation sports examination for the upcoming high school baseball season. Joe’s mother, who arrives at the office with him, tells you she’s worried because she found an e-cigarette in his backpack last week. Joe says that many of the kids at his school vape and he tried it a while back and now vapes “a lot.”
After talking further with Joe, you realize that he is vaping every day, using a 5% nicotine pod. Based on previous consults with the behavioral health counselor in your clinic, you know that this level of vaping is about the same as smoking 1 pack of cigarettes per day in terms of nicotine exposure. Joe states that he often vapes in the bathroom at school because he cannot concentrate in class if he doesn’t vape. He also reports that he had previously used 1 pod per week but had recently started vaping more to help with his cravings.
You assess his withdrawal symptoms and learn that he feels on edge when he is not able to vape and that he vapes heavily before going into school because he knows he will not be able to vape again until his third passing period.
●
Electronic cigarettes (e-cigarettes; also called “vapes”) are electronic nicotine delivery systems that heat and aerosolize e-liquid or “e-juice” that is inhaled by the user. The e-liquid is made up primarily of propylene glycol, vegetable glycerin, and flavorings, and often includes nicotine. Nicotine levels in e-cigarettes can range from 0 mg/mL to 60 mg/mL (regular cigarettes contain ~12 mg of nicotine). The nicotine level of the pod available from e-cigarette company JUUL (50 mg/mL e-liquid) is equivalent to about 1 pack of cigarettes.1 E-cigarette devices are relatively affordable; popular brands cost $10 to $20, while the replacement pods or e-liquid are typically about $4 each.
The e-cigarette market is quickly evolving and diversifying. Originally, e-cigarettes looked similar to cigarettes (cig-a-likes) but did not efficiently deliver nicotine to the user.2 E-cigarettes have evolved and some now deliver cigarette-like levels of nicotine to the user.3,4 Youth and young adults primarily use pod-mod e-cigarettes, which have a sleek design and produce less vapor than older e-cigarettes, making them easier to conceal. They can look like a USB flash-drive or have a teardrop shape. Pod-mod e-cigarettes dominate the current market, led by companies such as JUUL, NJOY, and Vuse.5
E-cigarette use is proliferating in the United States, particularly among young people and facilitated by the introduction of pod-based e-cigarettes in appealing flavors.6,7 While rates of current e-cigarette use by US adults is around 5.5%,8 recent data show that 32.7% of US high school students say they’ve used an e-cigarette in the past 30 days.9
Continue to: A double-edged sword
A double-edged sword. E-cigarettes are less harmful than traditional cigarettes in the short term and likely benefit adult smokers who completely substitute e-cigarettes for their tobacco cigarettes.10 In randomized trials of adult smokers, e-cigarette use resulted in moderate combustible-cigarette cessation rates that rival or exceed rates achieved with traditional nicotine replacement therapy (NRT).11-13 However, most e-cigarettes contain addictive nicotine, can facilitate transitions to more harmful forms of tobacco use,10,14,15 and have unknown long-term health effects. Therefore, youth, young adults, and those who are otherwise tobacco naïve should not initiate e-cigarette use.
Moreover, cases of e-cigarette or vaping product use–associated lung injury (EVALI)—a disease linked to vaping that causes cough, fever, shortness of breath, and death—were first identified in August 2019 and peaked in September 2019 before new cases decreased dramatically through January 2020.16 Since the initial cases of EVALI arose, product testing has shown that tetrahydrocannabinol (THC) and vitamin E acetate are the main ingredients linked to EVALI cases.17 For this reason, the Centers for Disease Control and Prevention and others strongly recommend against use of THC-containing e-cigarettes.18
Given the high rates of e-cigarette use among youth and young adults and its potential health harms, it is critical to inquire about e-cigarette use at primary care visits, and, as appropriate, to assess frequency and quantity of use. Patients who require intervention will be more likely to succeed in quitting if they are connected with behavioral health counseling and prescribed medication. This article offers evidence-based guidance to assess and advise teens and young adults regarding the potential health impact of e-cigarettes.
A NEW ICD-10-CM CODE AND A BRIEF ASSESSMENT TOOL
According to the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5)19 and the International Classification of Diseases, 10th Revision (ICD-10-CM),20 a tobacco use disorder is a problematic pattern of use leading to clinically significant impairment or distress. Associated features and behavioral markers of frequency and quantity include use within 30 minutes of waking, daily use, and increasing use. However, with youth, consider intervention for use of any nicotine or tobacco product, including e-cigarettes, regardless of whether it meets the threshold for diagnosis.21
The new code.
Continue to: As with other tobacco use...
As with other tobacco use, assess e-cigarette use patterns by asking questions about the frequency, duration, and quantity of use. Additionally, determine the level of nicotine in the e-liquid (discussed earlier) and evaluate whether the individual displays signs of physiologic dependence (eg, failed attempts to reduce or quit e-cigarette use, increased use, nicotine withdrawal symptoms).
A useful assessment tool. While e-cigarette use is not often included on current substance use screening measures, the above questions can be added to the end of measures such as the CRAFFT (Car-Relax-Alone-Forget-Family and Friends-Trouble) test.22 Additionally, if an adolescent reports vaping, the American Academy of Pediatrics (AAP) recommends using a brief screening tool such as the Hooked on Nicotine Checklist (HONC) to establish his or her level of dependence (TABLE 1).23

The HONC is ideal for a primary care setting because it is brief and has a high level of sensitivity, minimizing false-negative reports24; a patient’s acknowledgement of any item indicates a loss of autonomy over nicotine. Establishing the level of nicotine dependence is particularly pertinent when making decisions regarding the course of treatment and whether to prescribe NRT (eg, nicotine patch, gum, lozenge). Alternatively, you can quickly assess level of dependence by determining the time to first e-cigarette use in the morning. Tobacco guidelines suggest that if time to first use is > 30 minutes, the individual is “moderately dependent”; if time to first use is < 30 minutes after waking, the individual is “severely dependent.”25
COMBINATION TREATMENT IS MOST SUCCESSFUL
Studies have shown that the most effective treatment for tobacco cessation is pairing behavioral treatment with combination NRT (eg, nicotine gum + patch).25,26 The literature on e-cigarette cessation remains in its infancy, but techniques from traditional smoking cessation can be applied because the behaviors differ only in their mode of nicotine delivery.
Behavioral treatment. There are several options for behavioral treatment for tobacco cessation—and thus, e-cigarette cessation. The first step will depend on the patient’s level of motivation. If the patient is not yet ready to quit, consider using brief motivational interviewing. Once the patient is willing to engage in treatment, options include setting a mutually agreed upon quit date or planning for a reduction in the frequency and duration of vaping.
Continue to: Referrals to the Quitline...
Referrals to the Quitline (800-QUIT-NOW) have long been standard practice and can be used to extend primary care treatment.25 Studies show that it is more effective to connect patients directly to the Quitline at their primary care appointment27 than asking them to call after the visit.28,29 We suggest providing direct assistance in the office to patients as they initiate treatment with the Quitline.
Finally, if the level of dependence is severe or the patient is not motivated to quit, connect them with a behavioral health provider in your clinic or with an outside therapist skilled in cognitive behavioral techniques related to tobacco cessation. Discuss with the patient that quitting nicotine use is difficult for many people and that the best option for success is the combination of counseling and medication.25
Nicotine replacement therapy for e-cigarette use. While over-the-counter NRT (nicotine gum, patches, lozenges) is approved by the US Food and Drug Administration only for sale to adults ≥ 18 years, the AAP issued guidance on prescribing NRT for those < 18 years who use e-cigarettes.30 While the AAP does not suggest a lower age limit for prescribing NRT, national data show that < 6% of middle schoolers report e-cigarette use and that e-cigarette use does not become common (~20% current use) until high school.31 It is therefore unlikely that a child < 14 years would require pharmacotherapy. On their fact sheet, the AAP includes the following guidance:
“Patients who are motivated to quit should use as much safe, FDA-approved NRT as needed to avoid smoking or vaping. When assessing a patient’s current level of nicotine use, it may be helpful to understand that using one JUUL pod per day is equivalent to one pack of cigarettes per day …. Pediatricians and other healthcare providers should work with each patient to determine a starting dosage of NRT that is most likely to help them quit successfully. Dosing is based on the patient’s level of nicotine dependence, which can be measured using a screening tool” (TABLE 123).32
The AAP NRT dosing guidelines can be found at downloads.aap.org/RCE/NRT_and_Adolescents_Pediatrician_Guidance_factsheet.pdf.32 Of note, the dosing guidelines for adolescents are the same as those for adults and are based on level of use and dependence. Moreover, the clinician and patient should work together to choose the initial dose and the plan for weaning NRT over time.
Continue to: THE CASE
Based on your conversation with Joe, you administer the HONC screening tool. He scores 9 out of 10, indicating significant loss of autonomy over nicotine. You consult with a behavioral health counselor, who believes that Joe would benefit from counseling and NRT. You discuss this treatment plan with Joe, who says he is ready to quit because he does not like feeling as if he depends on vaping. Your shared decision is to start the 21-mg patch and 4-mg gum with plans to step down from there.
Joe agrees to set a quit date in the following week. The behavioral health counselor then meets with Joe and they develop a quit plan, which is shared with you so you can follow up at the next visit. Joe also agrees to talk with his parents, who are unaware of his level of use and dependence. Everyone agrees on the quit plan, and a follow-up visit is scheduled.
At the follow-up visit 1 month later, Joe and his parents report that he has quit vaping but is still using the patch and gum. You instruct Joe to reduce his NRT use to the 14-mg patch and 2-mg gum and to stop using them over the next 2 to 3 weeks. Everyone is in agreement with the treatment plan. You also re-administer the HONC screening tool and see that Joe’s score has reduced by 7 points to just 2 out of 10. You recommend that Joe continue to see the behavioral health counselor and follow up as needed. (A noted benefit of having a behavioral health counselor in your clinic is the opportunity for informal briefings on patient progress.33,34)
Following each visit with Joe, you make sure to complete documentation on (1) tobacco/e-cigarette use assessment, (2) diagnoses, (3) discussion of benefits of quitting,(4) assessment of readiness to quit, (5) creation and support of a quit plan, and (6) connection with a behavioral health counselor and planned follow-up. (See TABLE 235 for details onbilling codes.)

CORRESPONDENCE
Eleanor L. S. Leavens, PhD, 3901 Rainbow Boulevard, Mail Stop 1008, Kansas City, KS 66160; [email protected]
1. Prochaska JJ, Vogel EA, Benowitz N. Nicotine delivery and cigarette equivalents from vaping a JUULpod. Tob Control. Published online March 24, 2021. doi: 10.1136/tobaccocontrol- 2020-056367
2. Rüther T, Hagedorn D, Schiela K, et al. Nicotine delivery efficiency of first-and second-generation e-cigarettes and its impact on relief of craving during the acute phase of use. Int J Hyg Environ Health. 2018;221:191-198. doi: 10.1016/j.ijheh.2017.10.012
3. Hajek P, Pittaccio K, Pesola F, et al. Nicotine delivery and users’ reactions to Juul compared with cigarettes and other e‐cigarette products. Addiction. 2020;115:1141-1148. doi: 10.1111/add.14936
4. Wagener TL, Floyd EL, Stepanov I, et al. Have combustible cigarettes met their match? The nicotine delivery profiles and harmful constituent exposures of second-generation and third-generation electronic cigarette users. Tob control. 2017;26:e23-e28. doi: 10.1136/tobaccocontrol-2016-053041
5. Herzog B, Kanada P. Nielsen: Tobacco all channel data thru 8/11 - cig vol decelerates. Published August 21, 2018. Accessed August 19, 2021. https://athra.org.au/wp-content/uploads/2018/09/Wells-Fargo-Nielsen-Tobacco-All-Channel-Report-Period-Ending-8.11.18.pdf
6. Harrell MB, Weaver SR, Loukas A, et al. Flavored e-cigarette use: characterizing youth, young adult, and adult users. Prev Med Rep. 2017;5:33-40. doi: 10.1016/j.pmedr.2016.11.001
7. Morean ME, Butler ER, Bold KW, et al. Preferring more e-cigarette flavors is associated with e-cigarette use frequency among adolescents but not adults. PloS One. 2018;13:e0189015. doi: 10.1371/journal.pone.0189015
8. Obisesan OH, Osei AD, Iftekhar Uddin SM, et al. Trends in e-cigarette use in adults in the United States, 2016-2018. JAMA Intern Med. 2020;180:1394-1398. doi: 10.1001/jamainternmed.2020.2817
9. Creamer MR, Wang TW, Babb S, et al. Tobacco product use and cessation indicators among adults—United States, 2018. MMWR Morb Mortal Wkly Rep. 2019;68:1013-1019. doi: 10.15585/mmwr.mm6845a2
10. NASEM. Public health consequences of e-cigarettes. National Academies Press; 2018. Accessed August 19, 2021. www.ncbi.nlm.nih.gov/books/NBK507171/
11. Hajek P, Phillips-Waller A, Przulj D, et al. A randomized trial of e-cigarettes versus nicotine-replacement therapy. N Engl J Med. 2019;380:629-637. doi: 10.1056/NEJMoa1808779
12. Pulvers K, Nollen NL, Rice M, et al. Effect of pod e-cigarettes vs cigarettes on carcinogen exposure among African American and Latinx smokers: a randomized clinical trial. JAMA Netw Open. 2020;3:e2026324. doi: 10.1001/jamanetworkopen.2020.26324
13. Wang RJ, Bhadriraju S, Glantz SA. E-cigarette use and adult cigarette smoking cessation: a meta-analysis. Am J Public Health. 2021;111:230-246. doi: 10.2105/AJPH.2020.305999
14. Barrington-Trimis JL, Urman R, Berhane K, et al. E-cigarettes and future cigarette use. Pediatrics. 2016;138:e20160379. doi: 10.1542/peds.2016-0379
15. Soneji S, Barrington-Trimis JL, Wills TA, et al. Association between initial use of e-cigarettes and subsequent cigarette smoking among adolescents and young adults: a systematic review and meta-analysis. JAMA Pediatr. 2017;171:788-797. doi: 10.1001/jamapediatrics.2017.1488
16. Krishnasamy VP, Hallowell BD, Ko JY, et al. Update: characteristics of a nationwide outbreak of e-cigarette, or vaping, product use–associated lung injury—United States, August 2019–January 2020. MMWR Morb Mortal Wkly Rep. 2020;69:90-94. doi: 10.15585/mmwr.mm6903e2
17. Blount BC, Karwowski MP, Shields PG, et al. Vitamin E acetate in bronchoalveolar-lavage fluid associated with EVALI. N Engl J Med. 2020;382:697-705. doi: 10.1056/NEJMoa1916433
18. CDC. Outbreak of lung injury associated with use of e-cigarette, or vaping, products. Updated February 25, 2020. Accessed August 19, 2021. www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html
19. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edition (DSM-5). American Psychiatric Publishing; 2013.
20. CDC. International Classification of Diseases, 10th Revision. Updated July 30, 2021. Accessed August 31, 2021. www.cdc.gov/nchs/icd/icd10cm.htm
21. CDC. Surgeon General’s advisory on e-cigarette use among youth. Reviewed April 9, 2019. Accessed August 19, 2021. www.cdc.gov/tobacco/basic_information/e-cigarettes/surgeon-general-advisory/index.html
22. Knight JR, Sherritt L, Shrier LA, et al. Validity of the CRAFFT substance abuse screening test among adolescent clinic patients. Arch Pediatr Adolesc Med. 2002;156:607-614. doi: 10.1001/archpedi.156.6.607
23. DiFranza JR, Savageau JA, Fletcher K, et al. Measuring the loss of autonomy over nicotine use in adolescents: the DANDY (Development and Assessment of Nicotine Dependence in Youths) study. Arch Pediatr Adolesc Med. 2002;156:397-403. doi: 10.1001/archpedi.156.4.397
24. Wellman RJ, Savageau JA, Godiwala S, et al. A comparison of the Hooked on Nicotine Checklist and the Fagerström Test for Nicotine Dependence in adult smokers. Nicotine Tob Res. 2006;8:575-580. doi: 10.1080/14622200600789965
25. Fiore MC, Jaén CR, Baker TB, et al. Treating tobacco use and dependence: 2008 update. Published May 2008. Accessed August 19, 2021. www.aafp.org/dam/AAFP/documents/patient_care/clinical_recommendations/TreatingTobaccoUseandDependence-2008Update.pdf
26. Shah SD, Wilken LA, Winkler SR, et al. Systematic review and meta-analysis of combination therapy for smoking cessation. J Am Pharm Assoc. 2008;48:659-665. doi: 10.1331/JAPhA.2008.07063
27. Vidrine JI, Shete S, Cao Y, et al. Ask-Advise-Connect: a new approach to smoking treatment delivery in health care settings. JAMA Intern Med. 2013;173:458-464. doi: 10.1001/jamainternmed.2013.3751
28. Bentz CJ, Bayley KB, Bonin KE, et al. The feasibility of connecting physician offices to a state-level tobacco quit line. Am J Prev Med. 2006;30:31-37. doi: 10.1016/j.amepre.2005.08.043
29. Borland R, Segan CJ. The potential of quitlines to increase smoking cessation. Drug Alcohol Rev. 2006;25:73-78. doi: 10.1080/09595230500459537
30. Farber HJ, Walley SC, Groner JA, et al. Clinical practice policy to protect children from tobacco, nicotine, and tobacco smoke. Pediatrics. 2015;136:1008-1017. doi: 10.1542/peds.2015-3108
31. Gentzke AS, Wang TW, Jamal A, et al. Tobacco product use among middle and high school students—United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1881-1888. doi: 10.15585/mmwr.mm6950a1
32. AAP. Nicotine replacement therapy and adolescent patients: information for pediatricians. Updated November 2019. Accessed August 19, 2021. https://downloads.aap.org/RCE/NRT_and_Adolescents_Pediatrician_Guidance_factsheet.pdf
33. Blasi PR, Cromp D, McDonald S, et al. Approaches to behavioral health integration at high performing primary care practices. J Am Board Fam Med. 2018;31:691-701. doi: 10.3122/jabfm.2018.05.170468
34. Jacobs C, Brieler JA, Salas J, et al. Integrated behavioral health care in family medicine residencies a CERA survey. Fam Med. 2018;50:380-384. doi: 10.22454/FamMed.2018.639260
35. Oliverez M. Quick guide: billing for smoking cessation services. Capture Billing. Accessed August 26, 2021. https://capturebilling.com/how-bill-smoking-cessation-counseling-99406-99407/
1. Prochaska JJ, Vogel EA, Benowitz N. Nicotine delivery and cigarette equivalents from vaping a JUULpod. Tob Control. Published online March 24, 2021. doi: 10.1136/tobaccocontrol- 2020-056367
2. Rüther T, Hagedorn D, Schiela K, et al. Nicotine delivery efficiency of first-and second-generation e-cigarettes and its impact on relief of craving during the acute phase of use. Int J Hyg Environ Health. 2018;221:191-198. doi: 10.1016/j.ijheh.2017.10.012
3. Hajek P, Pittaccio K, Pesola F, et al. Nicotine delivery and users’ reactions to Juul compared with cigarettes and other e‐cigarette products. Addiction. 2020;115:1141-1148. doi: 10.1111/add.14936
4. Wagener TL, Floyd EL, Stepanov I, et al. Have combustible cigarettes met their match? The nicotine delivery profiles and harmful constituent exposures of second-generation and third-generation electronic cigarette users. Tob control. 2017;26:e23-e28. doi: 10.1136/tobaccocontrol-2016-053041
5. Herzog B, Kanada P. Nielsen: Tobacco all channel data thru 8/11 - cig vol decelerates. Published August 21, 2018. Accessed August 19, 2021. https://athra.org.au/wp-content/uploads/2018/09/Wells-Fargo-Nielsen-Tobacco-All-Channel-Report-Period-Ending-8.11.18.pdf
6. Harrell MB, Weaver SR, Loukas A, et al. Flavored e-cigarette use: characterizing youth, young adult, and adult users. Prev Med Rep. 2017;5:33-40. doi: 10.1016/j.pmedr.2016.11.001
7. Morean ME, Butler ER, Bold KW, et al. Preferring more e-cigarette flavors is associated with e-cigarette use frequency among adolescents but not adults. PloS One. 2018;13:e0189015. doi: 10.1371/journal.pone.0189015
8. Obisesan OH, Osei AD, Iftekhar Uddin SM, et al. Trends in e-cigarette use in adults in the United States, 2016-2018. JAMA Intern Med. 2020;180:1394-1398. doi: 10.1001/jamainternmed.2020.2817
9. Creamer MR, Wang TW, Babb S, et al. Tobacco product use and cessation indicators among adults—United States, 2018. MMWR Morb Mortal Wkly Rep. 2019;68:1013-1019. doi: 10.15585/mmwr.mm6845a2
10. NASEM. Public health consequences of e-cigarettes. National Academies Press; 2018. Accessed August 19, 2021. www.ncbi.nlm.nih.gov/books/NBK507171/
11. Hajek P, Phillips-Waller A, Przulj D, et al. A randomized trial of e-cigarettes versus nicotine-replacement therapy. N Engl J Med. 2019;380:629-637. doi: 10.1056/NEJMoa1808779
12. Pulvers K, Nollen NL, Rice M, et al. Effect of pod e-cigarettes vs cigarettes on carcinogen exposure among African American and Latinx smokers: a randomized clinical trial. JAMA Netw Open. 2020;3:e2026324. doi: 10.1001/jamanetworkopen.2020.26324
13. Wang RJ, Bhadriraju S, Glantz SA. E-cigarette use and adult cigarette smoking cessation: a meta-analysis. Am J Public Health. 2021;111:230-246. doi: 10.2105/AJPH.2020.305999
14. Barrington-Trimis JL, Urman R, Berhane K, et al. E-cigarettes and future cigarette use. Pediatrics. 2016;138:e20160379. doi: 10.1542/peds.2016-0379
15. Soneji S, Barrington-Trimis JL, Wills TA, et al. Association between initial use of e-cigarettes and subsequent cigarette smoking among adolescents and young adults: a systematic review and meta-analysis. JAMA Pediatr. 2017;171:788-797. doi: 10.1001/jamapediatrics.2017.1488
16. Krishnasamy VP, Hallowell BD, Ko JY, et al. Update: characteristics of a nationwide outbreak of e-cigarette, or vaping, product use–associated lung injury—United States, August 2019–January 2020. MMWR Morb Mortal Wkly Rep. 2020;69:90-94. doi: 10.15585/mmwr.mm6903e2
17. Blount BC, Karwowski MP, Shields PG, et al. Vitamin E acetate in bronchoalveolar-lavage fluid associated with EVALI. N Engl J Med. 2020;382:697-705. doi: 10.1056/NEJMoa1916433
18. CDC. Outbreak of lung injury associated with use of e-cigarette, or vaping, products. Updated February 25, 2020. Accessed August 19, 2021. www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html
19. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edition (DSM-5). American Psychiatric Publishing; 2013.
20. CDC. International Classification of Diseases, 10th Revision. Updated July 30, 2021. Accessed August 31, 2021. www.cdc.gov/nchs/icd/icd10cm.htm
21. CDC. Surgeon General’s advisory on e-cigarette use among youth. Reviewed April 9, 2019. Accessed August 19, 2021. www.cdc.gov/tobacco/basic_information/e-cigarettes/surgeon-general-advisory/index.html
22. Knight JR, Sherritt L, Shrier LA, et al. Validity of the CRAFFT substance abuse screening test among adolescent clinic patients. Arch Pediatr Adolesc Med. 2002;156:607-614. doi: 10.1001/archpedi.156.6.607
23. DiFranza JR, Savageau JA, Fletcher K, et al. Measuring the loss of autonomy over nicotine use in adolescents: the DANDY (Development and Assessment of Nicotine Dependence in Youths) study. Arch Pediatr Adolesc Med. 2002;156:397-403. doi: 10.1001/archpedi.156.4.397
24. Wellman RJ, Savageau JA, Godiwala S, et al. A comparison of the Hooked on Nicotine Checklist and the Fagerström Test for Nicotine Dependence in adult smokers. Nicotine Tob Res. 2006;8:575-580. doi: 10.1080/14622200600789965
25. Fiore MC, Jaén CR, Baker TB, et al. Treating tobacco use and dependence: 2008 update. Published May 2008. Accessed August 19, 2021. www.aafp.org/dam/AAFP/documents/patient_care/clinical_recommendations/TreatingTobaccoUseandDependence-2008Update.pdf
26. Shah SD, Wilken LA, Winkler SR, et al. Systematic review and meta-analysis of combination therapy for smoking cessation. J Am Pharm Assoc. 2008;48:659-665. doi: 10.1331/JAPhA.2008.07063
27. Vidrine JI, Shete S, Cao Y, et al. Ask-Advise-Connect: a new approach to smoking treatment delivery in health care settings. JAMA Intern Med. 2013;173:458-464. doi: 10.1001/jamainternmed.2013.3751
28. Bentz CJ, Bayley KB, Bonin KE, et al. The feasibility of connecting physician offices to a state-level tobacco quit line. Am J Prev Med. 2006;30:31-37. doi: 10.1016/j.amepre.2005.08.043
29. Borland R, Segan CJ. The potential of quitlines to increase smoking cessation. Drug Alcohol Rev. 2006;25:73-78. doi: 10.1080/09595230500459537
30. Farber HJ, Walley SC, Groner JA, et al. Clinical practice policy to protect children from tobacco, nicotine, and tobacco smoke. Pediatrics. 2015;136:1008-1017. doi: 10.1542/peds.2015-3108
31. Gentzke AS, Wang TW, Jamal A, et al. Tobacco product use among middle and high school students—United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1881-1888. doi: 10.15585/mmwr.mm6950a1
32. AAP. Nicotine replacement therapy and adolescent patients: information for pediatricians. Updated November 2019. Accessed August 19, 2021. https://downloads.aap.org/RCE/NRT_and_Adolescents_Pediatrician_Guidance_factsheet.pdf
33. Blasi PR, Cromp D, McDonald S, et al. Approaches to behavioral health integration at high performing primary care practices. J Am Board Fam Med. 2018;31:691-701. doi: 10.3122/jabfm.2018.05.170468
34. Jacobs C, Brieler JA, Salas J, et al. Integrated behavioral health care in family medicine residencies a CERA survey. Fam Med. 2018;50:380-384. doi: 10.22454/FamMed.2018.639260
35. Oliverez M. Quick guide: billing for smoking cessation services. Capture Billing. Accessed August 26, 2021. https://capturebilling.com/how-bill-smoking-cessation-counseling-99406-99407/
Nivo/ipi combo now ‘standard of care’ in mesothelioma
After 3 years, 23% of patients who received combination immunotherapy were still alive, in comparison with 15% of patients in the chemotherapy arm.
Combination immunotherapy continued to provide a “durable and long-term benefit” compared with chemotherapy, commented Solange Peters, MD, from the Oncology Department, Center Hospitalier Universitaire Vaudois, Lausanne, Switzerland.
The new data from the additional 12 months of follow-up “confirm nivolumab plus ipilimumab as a standard of care for unresectable MPM, regardless of histology,” she commented.
She presented the update on September 17 at the annual meeting of the European Society of Medical Oncology (ESMO). She is the current president of the organization.
Previously, 2-year data from this study showed that the combination yielded a median overall survival of 18.1 months, compared to 14.1 months with standard-of-care chemotherapy.
As reported by this news organization, this translated into a 26% improvement in overall survival; 41% of patients in the immunotherapy arm were still alive at 2 years, versus 27% in the chemotherapy group.
On the basis of these data, the combination was subsequently approved in the United States, the European Union, and elsewhere for the first-line treatment of adults with unresectable MPM.
The new data come from a 3-year update, as well as an exploratory biomarker analysis. The new data show significantly improved overall survival with the combination immunotherapy. Among those who responded to immunotherapy, response was ongoing for 28% of patients at 3 years.
Benefit was seen even for patients who discontinued the treatment because of treatment-related adverse events, indicating that discontinuance does not appear to have a negative impact on the long-term benefits, Dr. Peters commented.
In addition, the new analysis suggested that patients with a high score on a four-gene inflammatory signature did particularly well with nivolumab plus ipilimumab, whereas chemotherapy patients did worse if they had nonepithelioid disease, a finding not seen with immunotherapy.
The discussant for this abstract, Pilar Garrido, MD, PhD, associate professor of medicine at the Universidad de Alcalá, Madrid, said that despite the impressive findings, there is a “critical need” to establish predictive biomarkers in MPM.
This is particularly pressing in cases involving early progression, inasmuch as median progression-free survival (PFS) in CheckMate 743 was similar overall, and chemotherapy performed better than immunotherapy in the first 8 months.
There is also a need to be able to identify patients who will have an ongoing response at 3 years, as well as to clarify the impact of toxicity, given that the median duration of response was 20 months following discontinuation of treatment after just 4 months.
Dr. Garrido cautioned that the exploratory analyses were of “limited value,” because RNA data for the gene signature analysis were available for only 54% of patients, and the study was not powered to detect differences on the basis of programmed cell death–ligand-1 (PD-L1) expression.
Summarizing, Dr. Garrido said that although the current results showed that combination immunotherapy “continued to provide” a survival benefit in “a subgroup of patients,” the “better characterization of predictive biomarkers” will be “crucial” to improving these results.
Study details
Dr. Peters reminded the audience that the CheckMate 743 trial involved patients with unresectable MPM who had not previously received any systemic therapy and who had a good performance status.
A total of 605 patients were enrolled. They were randomly assigned in a 1:1 ratio to receive either nivolumab plus ipilimumab for up to 2 years or six cycles of pemetrexed plus cisplatin or carboplatin.
The median age of the patients was 69 years, and 77% were men. The baseline characteristics were well balanced between the two treatment groups; 75% to 76% had epithelioid disease, and for 74% to 80% of patients, baseline PD-L1 expression was greater than or equal to 1%.
Subgroup analysis indicated that combination immunotherapy was beneficial regardless of patient age, sex, performance status, and smoking status.
However, the new analysis suggested that the improvement in overall survival depended on PD-L1 expression, at a hazard ratio for combination immunotherapy versus chemotherapy of 0.71 in patients with expression of greater than or equal to 1%, compared with 0.99 for patients with expression of less than 1%.
Dr. Peters explained that the performance of nivolumab plus ipilimumab was identical in both PD-L1 expression groups, but it was the chemotherapy arm that performed markedly better for patients with expression of less than 1%.
An inverse finding was observed when patients were stratified by tumor histology.
In those with epithelioid disease, the median overall survival with combination immunotherapy was 18.2 months, versus 16.7 with chemotherapy, at a hazard ratio of 0.85.
At 36 months, 24% of immunotherapy patients were still alive, as were 19% of those given standard-of-care chemotherapy.
Among patients with nonepithelioid disease, however, median overall survival was 18.1 months with nivolumab plus ipilimumab, versus just 8.8 months with chemotherapy, at a hazard ratio of 0.48. At 3 years, 22% of patients who received combination immunotherapy were still alive, compared with 4% of those who received chemotherapy.
Other results showed that PFS was only slightly longer with combination immunotherapy, at 6.8 months versus 7.2 months, for a hazard ratio of 0.92.
Yet at 36 months, 14% of patients who received nivolumab plus ipilimumab had not experienced disease progression, versus just 1% of those in the chemotherapy arm.
This difference was even more pronounced when the researchers assessed objective response rates: 28% of patients who received combination immunotherapy were still responding at 36 months, versus 0% among patients given chemotherapy.
This translated into a median duration of response of 11.6 months for nivolumab plus ipilimumab, versus 6.7 months with chemotherapy.
The safety assessment showed that rates of treatment-related adverse events of any grade and of grade 3-4 were similar between the combination immunotherapy and chemotherapy arms.
However, rates of treatment-related adverse events that led to discontinuation of all components of the regimen were higher with immunotherapy, at 17% for events of any grade and 13% for events of grade 3-4, compared with 8% and 5%, respectively, with chemotherapy.
Serious treatment-related adverse events were more common with nivolumab plus ipilimumab. Events of grade 3-4 occurred in 13% of patients with nivolumab plus ipilimumab, versus 5% with chemotherapy.
Dr. Peters showed that this did not severely affect overall survival, however. Among patients who discontinued combination immunotherapy, the median duration of response was 20.0 months.
Median overall survival in these patients was 25.4 months, and the 3-year overall survival rate was 37%.
The study was funded by Bristol-Myers Squibb. Dr. Peters and Dr. Garrido reported relationships with numerous sources in industry.
A version of this article first appeared on Medscape.com.
After 3 years, 23% of patients who received combination immunotherapy were still alive, in comparison with 15% of patients in the chemotherapy arm.
Combination immunotherapy continued to provide a “durable and long-term benefit” compared with chemotherapy, commented Solange Peters, MD, from the Oncology Department, Center Hospitalier Universitaire Vaudois, Lausanne, Switzerland.
The new data from the additional 12 months of follow-up “confirm nivolumab plus ipilimumab as a standard of care for unresectable MPM, regardless of histology,” she commented.
She presented the update on September 17 at the annual meeting of the European Society of Medical Oncology (ESMO). She is the current president of the organization.
Previously, 2-year data from this study showed that the combination yielded a median overall survival of 18.1 months, compared to 14.1 months with standard-of-care chemotherapy.
As reported by this news organization, this translated into a 26% improvement in overall survival; 41% of patients in the immunotherapy arm were still alive at 2 years, versus 27% in the chemotherapy group.
On the basis of these data, the combination was subsequently approved in the United States, the European Union, and elsewhere for the first-line treatment of adults with unresectable MPM.
The new data come from a 3-year update, as well as an exploratory biomarker analysis. The new data show significantly improved overall survival with the combination immunotherapy. Among those who responded to immunotherapy, response was ongoing for 28% of patients at 3 years.
Benefit was seen even for patients who discontinued the treatment because of treatment-related adverse events, indicating that discontinuance does not appear to have a negative impact on the long-term benefits, Dr. Peters commented.
In addition, the new analysis suggested that patients with a high score on a four-gene inflammatory signature did particularly well with nivolumab plus ipilimumab, whereas chemotherapy patients did worse if they had nonepithelioid disease, a finding not seen with immunotherapy.
The discussant for this abstract, Pilar Garrido, MD, PhD, associate professor of medicine at the Universidad de Alcalá, Madrid, said that despite the impressive findings, there is a “critical need” to establish predictive biomarkers in MPM.
This is particularly pressing in cases involving early progression, inasmuch as median progression-free survival (PFS) in CheckMate 743 was similar overall, and chemotherapy performed better than immunotherapy in the first 8 months.
There is also a need to be able to identify patients who will have an ongoing response at 3 years, as well as to clarify the impact of toxicity, given that the median duration of response was 20 months following discontinuation of treatment after just 4 months.
Dr. Garrido cautioned that the exploratory analyses were of “limited value,” because RNA data for the gene signature analysis were available for only 54% of patients, and the study was not powered to detect differences on the basis of programmed cell death–ligand-1 (PD-L1) expression.
Summarizing, Dr. Garrido said that although the current results showed that combination immunotherapy “continued to provide” a survival benefit in “a subgroup of patients,” the “better characterization of predictive biomarkers” will be “crucial” to improving these results.
Study details
Dr. Peters reminded the audience that the CheckMate 743 trial involved patients with unresectable MPM who had not previously received any systemic therapy and who had a good performance status.
A total of 605 patients were enrolled. They were randomly assigned in a 1:1 ratio to receive either nivolumab plus ipilimumab for up to 2 years or six cycles of pemetrexed plus cisplatin or carboplatin.
The median age of the patients was 69 years, and 77% were men. The baseline characteristics were well balanced between the two treatment groups; 75% to 76% had epithelioid disease, and for 74% to 80% of patients, baseline PD-L1 expression was greater than or equal to 1%.
Subgroup analysis indicated that combination immunotherapy was beneficial regardless of patient age, sex, performance status, and smoking status.
However, the new analysis suggested that the improvement in overall survival depended on PD-L1 expression, at a hazard ratio for combination immunotherapy versus chemotherapy of 0.71 in patients with expression of greater than or equal to 1%, compared with 0.99 for patients with expression of less than 1%.
Dr. Peters explained that the performance of nivolumab plus ipilimumab was identical in both PD-L1 expression groups, but it was the chemotherapy arm that performed markedly better for patients with expression of less than 1%.
An inverse finding was observed when patients were stratified by tumor histology.
In those with epithelioid disease, the median overall survival with combination immunotherapy was 18.2 months, versus 16.7 with chemotherapy, at a hazard ratio of 0.85.
At 36 months, 24% of immunotherapy patients were still alive, as were 19% of those given standard-of-care chemotherapy.
Among patients with nonepithelioid disease, however, median overall survival was 18.1 months with nivolumab plus ipilimumab, versus just 8.8 months with chemotherapy, at a hazard ratio of 0.48. At 3 years, 22% of patients who received combination immunotherapy were still alive, compared with 4% of those who received chemotherapy.
Other results showed that PFS was only slightly longer with combination immunotherapy, at 6.8 months versus 7.2 months, for a hazard ratio of 0.92.
Yet at 36 months, 14% of patients who received nivolumab plus ipilimumab had not experienced disease progression, versus just 1% of those in the chemotherapy arm.
This difference was even more pronounced when the researchers assessed objective response rates: 28% of patients who received combination immunotherapy were still responding at 36 months, versus 0% among patients given chemotherapy.
This translated into a median duration of response of 11.6 months for nivolumab plus ipilimumab, versus 6.7 months with chemotherapy.
The safety assessment showed that rates of treatment-related adverse events of any grade and of grade 3-4 were similar between the combination immunotherapy and chemotherapy arms.
However, rates of treatment-related adverse events that led to discontinuation of all components of the regimen were higher with immunotherapy, at 17% for events of any grade and 13% for events of grade 3-4, compared with 8% and 5%, respectively, with chemotherapy.
Serious treatment-related adverse events were more common with nivolumab plus ipilimumab. Events of grade 3-4 occurred in 13% of patients with nivolumab plus ipilimumab, versus 5% with chemotherapy.
Dr. Peters showed that this did not severely affect overall survival, however. Among patients who discontinued combination immunotherapy, the median duration of response was 20.0 months.
Median overall survival in these patients was 25.4 months, and the 3-year overall survival rate was 37%.
The study was funded by Bristol-Myers Squibb. Dr. Peters and Dr. Garrido reported relationships with numerous sources in industry.
A version of this article first appeared on Medscape.com.
After 3 years, 23% of patients who received combination immunotherapy were still alive, in comparison with 15% of patients in the chemotherapy arm.
Combination immunotherapy continued to provide a “durable and long-term benefit” compared with chemotherapy, commented Solange Peters, MD, from the Oncology Department, Center Hospitalier Universitaire Vaudois, Lausanne, Switzerland.
The new data from the additional 12 months of follow-up “confirm nivolumab plus ipilimumab as a standard of care for unresectable MPM, regardless of histology,” she commented.
She presented the update on September 17 at the annual meeting of the European Society of Medical Oncology (ESMO). She is the current president of the organization.
Previously, 2-year data from this study showed that the combination yielded a median overall survival of 18.1 months, compared to 14.1 months with standard-of-care chemotherapy.
As reported by this news organization, this translated into a 26% improvement in overall survival; 41% of patients in the immunotherapy arm were still alive at 2 years, versus 27% in the chemotherapy group.
On the basis of these data, the combination was subsequently approved in the United States, the European Union, and elsewhere for the first-line treatment of adults with unresectable MPM.
The new data come from a 3-year update, as well as an exploratory biomarker analysis. The new data show significantly improved overall survival with the combination immunotherapy. Among those who responded to immunotherapy, response was ongoing for 28% of patients at 3 years.
Benefit was seen even for patients who discontinued the treatment because of treatment-related adverse events, indicating that discontinuance does not appear to have a negative impact on the long-term benefits, Dr. Peters commented.
In addition, the new analysis suggested that patients with a high score on a four-gene inflammatory signature did particularly well with nivolumab plus ipilimumab, whereas chemotherapy patients did worse if they had nonepithelioid disease, a finding not seen with immunotherapy.
The discussant for this abstract, Pilar Garrido, MD, PhD, associate professor of medicine at the Universidad de Alcalá, Madrid, said that despite the impressive findings, there is a “critical need” to establish predictive biomarkers in MPM.
This is particularly pressing in cases involving early progression, inasmuch as median progression-free survival (PFS) in CheckMate 743 was similar overall, and chemotherapy performed better than immunotherapy in the first 8 months.
There is also a need to be able to identify patients who will have an ongoing response at 3 years, as well as to clarify the impact of toxicity, given that the median duration of response was 20 months following discontinuation of treatment after just 4 months.
Dr. Garrido cautioned that the exploratory analyses were of “limited value,” because RNA data for the gene signature analysis were available for only 54% of patients, and the study was not powered to detect differences on the basis of programmed cell death–ligand-1 (PD-L1) expression.
Summarizing, Dr. Garrido said that although the current results showed that combination immunotherapy “continued to provide” a survival benefit in “a subgroup of patients,” the “better characterization of predictive biomarkers” will be “crucial” to improving these results.
Study details
Dr. Peters reminded the audience that the CheckMate 743 trial involved patients with unresectable MPM who had not previously received any systemic therapy and who had a good performance status.
A total of 605 patients were enrolled. They were randomly assigned in a 1:1 ratio to receive either nivolumab plus ipilimumab for up to 2 years or six cycles of pemetrexed plus cisplatin or carboplatin.
The median age of the patients was 69 years, and 77% were men. The baseline characteristics were well balanced between the two treatment groups; 75% to 76% had epithelioid disease, and for 74% to 80% of patients, baseline PD-L1 expression was greater than or equal to 1%.
Subgroup analysis indicated that combination immunotherapy was beneficial regardless of patient age, sex, performance status, and smoking status.
However, the new analysis suggested that the improvement in overall survival depended on PD-L1 expression, at a hazard ratio for combination immunotherapy versus chemotherapy of 0.71 in patients with expression of greater than or equal to 1%, compared with 0.99 for patients with expression of less than 1%.
Dr. Peters explained that the performance of nivolumab plus ipilimumab was identical in both PD-L1 expression groups, but it was the chemotherapy arm that performed markedly better for patients with expression of less than 1%.
An inverse finding was observed when patients were stratified by tumor histology.
In those with epithelioid disease, the median overall survival with combination immunotherapy was 18.2 months, versus 16.7 with chemotherapy, at a hazard ratio of 0.85.
At 36 months, 24% of immunotherapy patients were still alive, as were 19% of those given standard-of-care chemotherapy.
Among patients with nonepithelioid disease, however, median overall survival was 18.1 months with nivolumab plus ipilimumab, versus just 8.8 months with chemotherapy, at a hazard ratio of 0.48. At 3 years, 22% of patients who received combination immunotherapy were still alive, compared with 4% of those who received chemotherapy.
Other results showed that PFS was only slightly longer with combination immunotherapy, at 6.8 months versus 7.2 months, for a hazard ratio of 0.92.
Yet at 36 months, 14% of patients who received nivolumab plus ipilimumab had not experienced disease progression, versus just 1% of those in the chemotherapy arm.
This difference was even more pronounced when the researchers assessed objective response rates: 28% of patients who received combination immunotherapy were still responding at 36 months, versus 0% among patients given chemotherapy.
This translated into a median duration of response of 11.6 months for nivolumab plus ipilimumab, versus 6.7 months with chemotherapy.
The safety assessment showed that rates of treatment-related adverse events of any grade and of grade 3-4 were similar between the combination immunotherapy and chemotherapy arms.
However, rates of treatment-related adverse events that led to discontinuation of all components of the regimen were higher with immunotherapy, at 17% for events of any grade and 13% for events of grade 3-4, compared with 8% and 5%, respectively, with chemotherapy.
Serious treatment-related adverse events were more common with nivolumab plus ipilimumab. Events of grade 3-4 occurred in 13% of patients with nivolumab plus ipilimumab, versus 5% with chemotherapy.
Dr. Peters showed that this did not severely affect overall survival, however. Among patients who discontinued combination immunotherapy, the median duration of response was 20.0 months.
Median overall survival in these patients was 25.4 months, and the 3-year overall survival rate was 37%.
The study was funded by Bristol-Myers Squibb. Dr. Peters and Dr. Garrido reported relationships with numerous sources in industry.
A version of this article first appeared on Medscape.com.
RA treatment responders show unique differences in gut microbiome
The gut microbiome, previously shown to have an association with rheumatoid arthritis, may also provide signals of a patient’s disease prognosis, researchers at the Mayo Clinic have reported.
“We found that the gut microbiome is linked to whether patients with RA improve in their clinical symptoms or not,” cosenior author Jaeyun Sung, PhD, said in an interview. “We found features of the gut microbiome that linked to improvement, and we also put those features in a machine-learning model that can actually predict improvement at a follow-up visit.” Dr. Sung is a computational biologist with Mayo Clinic’s Center for Individualized Medicine in Rochester, Minn.
The retrospective, observational cohort study included 32 patients diagnosed with RA between 1988 and 2014. The researchers performed meta-genome shotgun sequencing on 64 stool samples kept in a biobank and collected at two separate visits 6-12 months apart. Dr. Sung and colleagues observed significantly different microbiome traits between patients who eventually showed minimally clinically important improvement and those who didn’t.
The study also provided a proof of concept for using machine-learning technology to analyze the gut microbiome to predict the course of the disease, Dr. Sung said.
Cosenior author John M. Davis III, MD, a clinical rheumatologist and rheumatology research chair of the Mayo Clinic, noted that their own previous study had confirmed dysbiosis in people with RA when compared with controls. “We had some preliminary insight that it may be linked to some extent to the disease state and maybe treatments,” Dr. Davis said. “So that led us to hypothesize that there may be an association between the gut microbiome and response to treatment or disease activity over time.”
The study found that age was the dominant factor in determining variations in the gut microbiome composition, but the next prevailing factor was minimum clinically important improvement status, which 12 of the 32 study participants achieved at their follow-up visits. At baseline, all patients were on some type of treatment – either biologic or conventional disease-modifying antirheumatic drugs (DMARDs, 46.9% and 87.5%, respectively), or prednisone (46.9%).
Gut microbiome composition
The patients who achieved minimum clinically important improvement had an average decline in Clinical Disease Activity Index of 16.7 units (standard deviation, 12.8) versus a gain of 5.7 (SD, 8.9) in the remaining patients. The study found higher species-level alpha diversity and richness and higher beta diversity in the group that achieved minimum clinically important improvement, compared with those who did not.
They identified six microbial taxa as higher in abundance in the improved patients: Negativicutes (class); Selenomonadales (order); Prevotellaceae (family); Coprococcus (genus); Bacteroides sp. 3_1_19 (species); and Bilophila sp. 4_1_30 (species). In the patients who showed no improvement, Eubacterium sp. 3_1_31 (species) was found to be higher (P < .05). They also found 15 metabolic pathways that were differently abundant between the two groups at baseline.
Two things make this study different from other studies of the gut microbiome in RA, Dr. Sung said: It didn’t have a control group, only RA patients, and it didn’t evaluate a specific drug in RA patients.
“We’re thinking beyond just drug or treatment, independent of prior treatment, independent of prior clinical measurements, independent of age, sex, and other factors, can we predict RA response just using the gut microbiome alone?” Dr. Sung said. “Is there an association between clinical improvement and the gut microbiome?”
The study also showed that the microbiome may be a modifiable target for RA, Dr. Davis said.
“This research is attractive because it may complement medical treatment for RA if we can identify dietary modifications,” he said. “Still, there’s the question if probiotics or prebiotics can influence the gut. Can we modify the gut microbiome to further ameliorate the disease state? That’s something I think is an open question that’s specifically called out in our paper.”
This study included patients with long-term disease, but the group’s ongoing research is focusing on patients with earlier-stage RA, Dr. Davis said. “The next steps have to be in validating [the findings] in additional and external populations and looking at patients with very early disease where a lot of the decision-making is very active and happening in real time.”
James T. Rosenbaum, MD, an ophthalmologist and rheumatologist at Oregon Health & Science University, Portland, acknowledged that this is the first study in RA to find an effect on the gut microbiome using the minimum clinically important improvement endpoint.
“It also raises a ‘chicken-egg’ dilemma,” Dr. Rosenbaum said in an interview. “Did the patients improve and then their microbiome changed, or was the microbiome the first change that led to the clinical improvement? If the latter is correct, we potentially could alter the microbiome, for example, by diet, to treat rheumatic disease.”
He noted that studies with fecal transplants for ulcerative colitis support the therapeutic potential of microbiome modification. “But,” he added, “we are still a long way from putting this in practice.”
“The outcome is promising,” Claudia Mauri, PhD, a professor of immunology at University College London, said of the study. “Obviously, if this can be repeated in a very large cohort of patients, it would give us the possibility to be able to, based on the composition of the microbiota, to predict who is going to respond to treatment or not.”
She noted that, while RA has a broader array of available treatments than other autoimmune diseases, some RA patients don’t respond their first biologic treatment. “If from the outset we would be able to see who may not respond based on the microbiota, we may prepare physicians to better target these patients by, for example, offering them an alternative second biologic agent,” Dr. Mauri said.
Dr. Davis reported receiving research grants from Pfizer. Dr. Sung and other study coauthors have no financial relationships to disclose. Dr. Rosenbaum reported that the National Institutes of Health supports his research. Dr. Mauri has no financial relationships to disclose.
The gut microbiome, previously shown to have an association with rheumatoid arthritis, may also provide signals of a patient’s disease prognosis, researchers at the Mayo Clinic have reported.
“We found that the gut microbiome is linked to whether patients with RA improve in their clinical symptoms or not,” cosenior author Jaeyun Sung, PhD, said in an interview. “We found features of the gut microbiome that linked to improvement, and we also put those features in a machine-learning model that can actually predict improvement at a follow-up visit.” Dr. Sung is a computational biologist with Mayo Clinic’s Center for Individualized Medicine in Rochester, Minn.
The retrospective, observational cohort study included 32 patients diagnosed with RA between 1988 and 2014. The researchers performed meta-genome shotgun sequencing on 64 stool samples kept in a biobank and collected at two separate visits 6-12 months apart. Dr. Sung and colleagues observed significantly different microbiome traits between patients who eventually showed minimally clinically important improvement and those who didn’t.
The study also provided a proof of concept for using machine-learning technology to analyze the gut microbiome to predict the course of the disease, Dr. Sung said.
Cosenior author John M. Davis III, MD, a clinical rheumatologist and rheumatology research chair of the Mayo Clinic, noted that their own previous study had confirmed dysbiosis in people with RA when compared with controls. “We had some preliminary insight that it may be linked to some extent to the disease state and maybe treatments,” Dr. Davis said. “So that led us to hypothesize that there may be an association between the gut microbiome and response to treatment or disease activity over time.”
The study found that age was the dominant factor in determining variations in the gut microbiome composition, but the next prevailing factor was minimum clinically important improvement status, which 12 of the 32 study participants achieved at their follow-up visits. At baseline, all patients were on some type of treatment – either biologic or conventional disease-modifying antirheumatic drugs (DMARDs, 46.9% and 87.5%, respectively), or prednisone (46.9%).
Gut microbiome composition
The patients who achieved minimum clinically important improvement had an average decline in Clinical Disease Activity Index of 16.7 units (standard deviation, 12.8) versus a gain of 5.7 (SD, 8.9) in the remaining patients. The study found higher species-level alpha diversity and richness and higher beta diversity in the group that achieved minimum clinically important improvement, compared with those who did not.
They identified six microbial taxa as higher in abundance in the improved patients: Negativicutes (class); Selenomonadales (order); Prevotellaceae (family); Coprococcus (genus); Bacteroides sp. 3_1_19 (species); and Bilophila sp. 4_1_30 (species). In the patients who showed no improvement, Eubacterium sp. 3_1_31 (species) was found to be higher (P < .05). They also found 15 metabolic pathways that were differently abundant between the two groups at baseline.
Two things make this study different from other studies of the gut microbiome in RA, Dr. Sung said: It didn’t have a control group, only RA patients, and it didn’t evaluate a specific drug in RA patients.
“We’re thinking beyond just drug or treatment, independent of prior treatment, independent of prior clinical measurements, independent of age, sex, and other factors, can we predict RA response just using the gut microbiome alone?” Dr. Sung said. “Is there an association between clinical improvement and the gut microbiome?”
The study also showed that the microbiome may be a modifiable target for RA, Dr. Davis said.
“This research is attractive because it may complement medical treatment for RA if we can identify dietary modifications,” he said. “Still, there’s the question if probiotics or prebiotics can influence the gut. Can we modify the gut microbiome to further ameliorate the disease state? That’s something I think is an open question that’s specifically called out in our paper.”
This study included patients with long-term disease, but the group’s ongoing research is focusing on patients with earlier-stage RA, Dr. Davis said. “The next steps have to be in validating [the findings] in additional and external populations and looking at patients with very early disease where a lot of the decision-making is very active and happening in real time.”
James T. Rosenbaum, MD, an ophthalmologist and rheumatologist at Oregon Health & Science University, Portland, acknowledged that this is the first study in RA to find an effect on the gut microbiome using the minimum clinically important improvement endpoint.
“It also raises a ‘chicken-egg’ dilemma,” Dr. Rosenbaum said in an interview. “Did the patients improve and then their microbiome changed, or was the microbiome the first change that led to the clinical improvement? If the latter is correct, we potentially could alter the microbiome, for example, by diet, to treat rheumatic disease.”
He noted that studies with fecal transplants for ulcerative colitis support the therapeutic potential of microbiome modification. “But,” he added, “we are still a long way from putting this in practice.”
“The outcome is promising,” Claudia Mauri, PhD, a professor of immunology at University College London, said of the study. “Obviously, if this can be repeated in a very large cohort of patients, it would give us the possibility to be able to, based on the composition of the microbiota, to predict who is going to respond to treatment or not.”
She noted that, while RA has a broader array of available treatments than other autoimmune diseases, some RA patients don’t respond their first biologic treatment. “If from the outset we would be able to see who may not respond based on the microbiota, we may prepare physicians to better target these patients by, for example, offering them an alternative second biologic agent,” Dr. Mauri said.
Dr. Davis reported receiving research grants from Pfizer. Dr. Sung and other study coauthors have no financial relationships to disclose. Dr. Rosenbaum reported that the National Institutes of Health supports his research. Dr. Mauri has no financial relationships to disclose.
The gut microbiome, previously shown to have an association with rheumatoid arthritis, may also provide signals of a patient’s disease prognosis, researchers at the Mayo Clinic have reported.
“We found that the gut microbiome is linked to whether patients with RA improve in their clinical symptoms or not,” cosenior author Jaeyun Sung, PhD, said in an interview. “We found features of the gut microbiome that linked to improvement, and we also put those features in a machine-learning model that can actually predict improvement at a follow-up visit.” Dr. Sung is a computational biologist with Mayo Clinic’s Center for Individualized Medicine in Rochester, Minn.
The retrospective, observational cohort study included 32 patients diagnosed with RA between 1988 and 2014. The researchers performed meta-genome shotgun sequencing on 64 stool samples kept in a biobank and collected at two separate visits 6-12 months apart. Dr. Sung and colleagues observed significantly different microbiome traits between patients who eventually showed minimally clinically important improvement and those who didn’t.
The study also provided a proof of concept for using machine-learning technology to analyze the gut microbiome to predict the course of the disease, Dr. Sung said.
Cosenior author John M. Davis III, MD, a clinical rheumatologist and rheumatology research chair of the Mayo Clinic, noted that their own previous study had confirmed dysbiosis in people with RA when compared with controls. “We had some preliminary insight that it may be linked to some extent to the disease state and maybe treatments,” Dr. Davis said. “So that led us to hypothesize that there may be an association between the gut microbiome and response to treatment or disease activity over time.”
The study found that age was the dominant factor in determining variations in the gut microbiome composition, but the next prevailing factor was minimum clinically important improvement status, which 12 of the 32 study participants achieved at their follow-up visits. At baseline, all patients were on some type of treatment – either biologic or conventional disease-modifying antirheumatic drugs (DMARDs, 46.9% and 87.5%, respectively), or prednisone (46.9%).
Gut microbiome composition
The patients who achieved minimum clinically important improvement had an average decline in Clinical Disease Activity Index of 16.7 units (standard deviation, 12.8) versus a gain of 5.7 (SD, 8.9) in the remaining patients. The study found higher species-level alpha diversity and richness and higher beta diversity in the group that achieved minimum clinically important improvement, compared with those who did not.
They identified six microbial taxa as higher in abundance in the improved patients: Negativicutes (class); Selenomonadales (order); Prevotellaceae (family); Coprococcus (genus); Bacteroides sp. 3_1_19 (species); and Bilophila sp. 4_1_30 (species). In the patients who showed no improvement, Eubacterium sp. 3_1_31 (species) was found to be higher (P < .05). They also found 15 metabolic pathways that were differently abundant between the two groups at baseline.
Two things make this study different from other studies of the gut microbiome in RA, Dr. Sung said: It didn’t have a control group, only RA patients, and it didn’t evaluate a specific drug in RA patients.
“We’re thinking beyond just drug or treatment, independent of prior treatment, independent of prior clinical measurements, independent of age, sex, and other factors, can we predict RA response just using the gut microbiome alone?” Dr. Sung said. “Is there an association between clinical improvement and the gut microbiome?”
The study also showed that the microbiome may be a modifiable target for RA, Dr. Davis said.
“This research is attractive because it may complement medical treatment for RA if we can identify dietary modifications,” he said. “Still, there’s the question if probiotics or prebiotics can influence the gut. Can we modify the gut microbiome to further ameliorate the disease state? That’s something I think is an open question that’s specifically called out in our paper.”
This study included patients with long-term disease, but the group’s ongoing research is focusing on patients with earlier-stage RA, Dr. Davis said. “The next steps have to be in validating [the findings] in additional and external populations and looking at patients with very early disease where a lot of the decision-making is very active and happening in real time.”
James T. Rosenbaum, MD, an ophthalmologist and rheumatologist at Oregon Health & Science University, Portland, acknowledged that this is the first study in RA to find an effect on the gut microbiome using the minimum clinically important improvement endpoint.
“It also raises a ‘chicken-egg’ dilemma,” Dr. Rosenbaum said in an interview. “Did the patients improve and then their microbiome changed, or was the microbiome the first change that led to the clinical improvement? If the latter is correct, we potentially could alter the microbiome, for example, by diet, to treat rheumatic disease.”
He noted that studies with fecal transplants for ulcerative colitis support the therapeutic potential of microbiome modification. “But,” he added, “we are still a long way from putting this in practice.”
“The outcome is promising,” Claudia Mauri, PhD, a professor of immunology at University College London, said of the study. “Obviously, if this can be repeated in a very large cohort of patients, it would give us the possibility to be able to, based on the composition of the microbiota, to predict who is going to respond to treatment or not.”
She noted that, while RA has a broader array of available treatments than other autoimmune diseases, some RA patients don’t respond their first biologic treatment. “If from the outset we would be able to see who may not respond based on the microbiota, we may prepare physicians to better target these patients by, for example, offering them an alternative second biologic agent,” Dr. Mauri said.
Dr. Davis reported receiving research grants from Pfizer. Dr. Sung and other study coauthors have no financial relationships to disclose. Dr. Rosenbaum reported that the National Institutes of Health supports his research. Dr. Mauri has no financial relationships to disclose.
FROM GENOME MEDICINE
Recognizing and treating trigger finger
CASE
A 55-year-old right-hand-dominant woman presented to the clinic with a chief complaint of right ring finger pain and stiffness. There was no history of trauma or prior surgery. She had no tingling or numbness. She had a history of type 2 diabetes that was well controlled. She worked as a clerk for a government office for many years, and her painful, limited finger motion interfered with keyboarding and picking up items. Physical examination revealed tenderness to palpation over the palmar aspect of the metacarpophalangeal joint (MCPJ) of the ring finger with no other joint tenderness or swelling. When she made a fist, her ring finger MCPJ, proximal interphalangeal joint (PIPJ), and distal interphalangeal joint (DIPJ) locked in a flexed position that required manipulation to extend the finger. A firm mass was palpated in the palm with finger flexion that moved into the finger with extension.
Stenosing tenosynovitis, also known as trigger finger (TF), is an inflammatory condition that causes pain in the distal palm and proximal digit with associated limited motion. The most commonly affected digits are the middle and ring fingers of the dominant hand.1 The disorder is particularly noticeable when it inhibits day-to-day functioning.
TF affects 2% to 3% of the general population and up to 20% of patients with diabetes.2,3 Patient age and duration of diabetes are commonly cited as contributing factors, although the effect of well-controlled blood glucose and A1C on the frequency and cure rate of TF has not been established.3,4 TF is most commonly seen in individuals ages 40 to 60 years, with a 6 times’ greater frequency in females than males.5
In the United States, there are an estimated 200,000 cases of TF each year, with initial presentation typically being to a primary care physician.6 For this reason, it is essential for primary care physicians to recognize this common pathology and treat symptoms early to prevent progression and the need for surgical intervention.
An impaired gliding motion of the flexor tendons
In each finger, a tendon sheath, consisting of 5 annular pulleys and 3 cruciate pulleys, forms a tunnel around the flexor digitorum profundus (FDP) and flexor digitorum superficialis (FDS). The tendon sheath allows for maximum force by eliminating bowstringing of the tendons when the digit is flexed. Deep to the tendons and surrounding the tendons is a synovial membrane that provides nutrition and reduces friction between the tendons and the tendon sheath.7
The FDP is longer and assists in flexion of the MCPJ and the PIPJ. It is the sole flexor of the DIPJ.
In the thumb, the flexor pollicis longus (FPL) is the only flexor within its tendon sheath. The FPL assists in flexion of the MCPJ and flexes the thumb interphalangeal joint (IPJ). The intrinsic muscles (lumbricals and interossei) do not extend into the tendon sheath and do not contribute to TF.
Continue to: TF occurs when
TF occurs when the tendon sheath, most commonly at the first annular pulley (A1), or the flexor tendons thicken due to fibrocartilaginous metaplasia. This results in impaired gliding motion of the flexor tendons.8 The stenosed A1 pulley can lead to pinching of the flexor tendons and cause the formation of a nodule on the FDS tendon at its bifurcation.9 The nodule of the FDS bifurcation moves proximal to the A1 pulley when the finger is flexed. Upon extension, the tendon nodule may get caught on the A1 pulley. This prevents smooth extension and is the source of pain and triggering (FIGURE 1). In a similar manner, thumb triggering is the result of a stenosed A1 pulley creating a nodule on the FPL tendon, which prevents smooth gliding of the FPL.
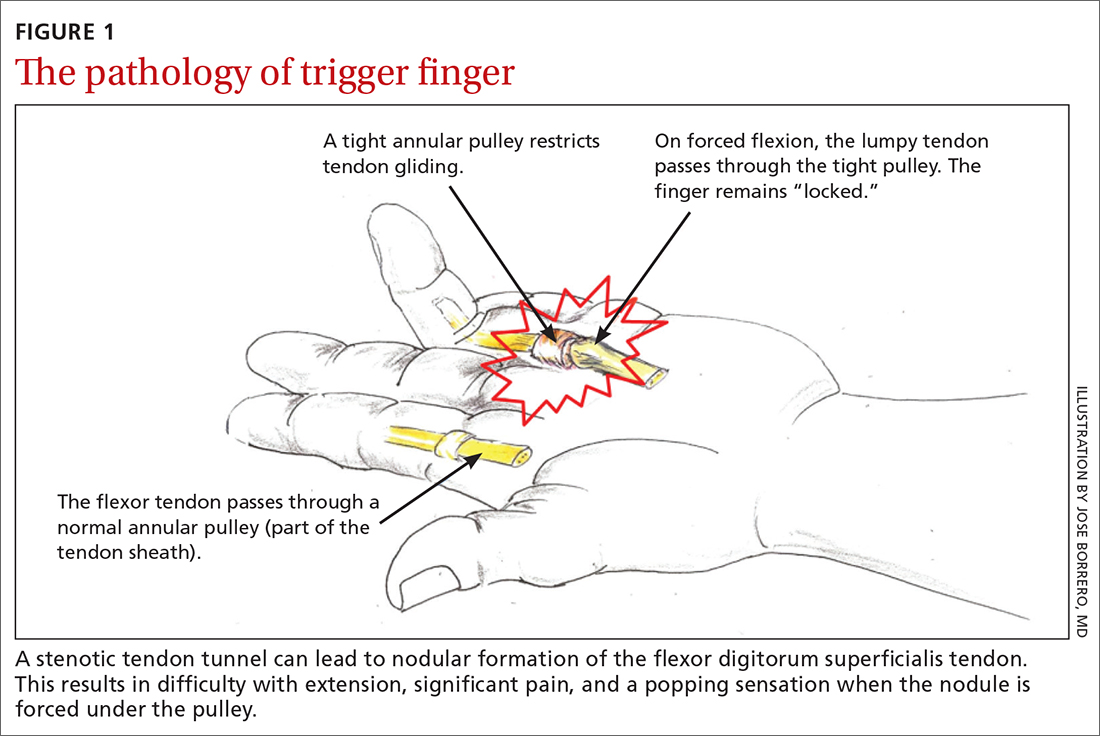
What you’ll see
TF is characterized by locking, popping, or clicking at the base of the finger or thumb.7,10 A small nodule may be palpated on the palmar aspect of the MCPJ when the finger is flexed. This nodule will then move distally when the finger is extended. Patients will present with the affected digit in a flexed position and will have difficulty extending the digit. In some cases, the patient may have to use the other hand to straighten the affected digit. In more severe cases, the digit may be fixed in a position of flexion or extension. The severity of triggering is commonly graded by the Green’s classification system (see TABLE11).
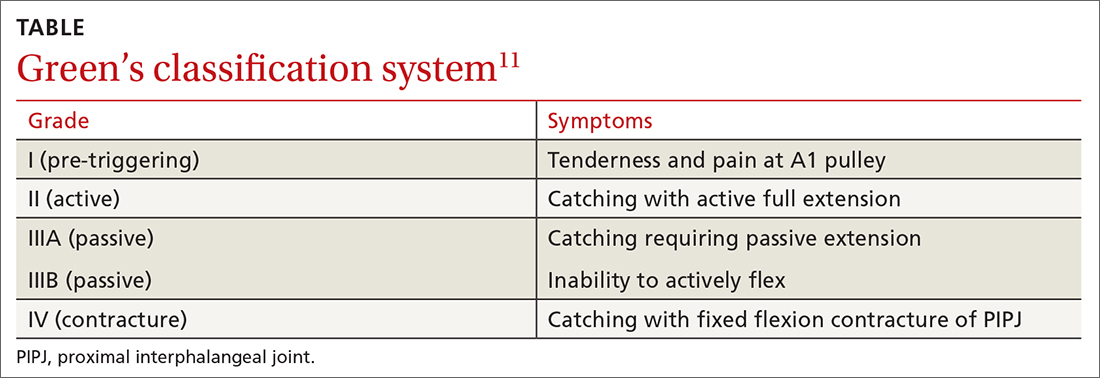
Is it Dupuytren contracture, trigger finger, or something else?
The differential diagnosis for TF includes Dupuytren contracture, MCPJ sprain, calcific peritendinitis, flexor tenosynovitis, diabetic cheiroarthropathy (DCA), rheumatoid arthritis (RA), osteoarthritis (OA), and crystalline arthropathy (gout).5
Dupuytren contracture is usually nonpainful and manifests with a palpable cord in the palm and a fixed flexion contracture that has progressed over time, with no history of catching.
MCPJ sprain is diagnosed with tenderness of the MCPJ and a history of trauma.
Continue to: Calcific peritendinitis
Calcific peritendinitis is characterized by pain, tenderness, and edema near a joint with calcified deposits seen on radiographs.
Flexor tenosynovitis manifests with fusiform swelling of the digit, tenderness over the flexor tendon sheath, and pain with passive extension of the digit; it is more commonly associated with RA.
DCA, RA, OA, and gout usually affect more than 1 digit. DCA is associated with both type 1 and type 2 diabetes and is characterized by thickened, waxy skin and painless, limited extension of the digits. RA and OA are diagnosed by medical history, lab work, and radiographs. Gout is diagnosed with lab work and aspiration of joint fluid.
A thorough history, physical exam, and review of radiographs must be performed to rule out these other disorders. Once the diagnosis of TF is made, available treatment options should be pursued.
Treatment: A conservative or surgical approach?
Current treatment options include both nonsurgical (conservative) and surgical interventions. Nonsurgical interventions include activity modification, splinting, and corticosteroid injections. While nonsteroidal anti-inflammatory drugs are commonly recommended to resolve the local inflammation secondary to triggering, there is no scientific evidence to support their use at this time.7 Surgical interventions, utilized in more severe cases or after conservative treatment has failed, include percutaneous and open release of the tendon sheath.2,7
Continue to: Conservative treatments
Conservative treatments
Splinting is only an option for digits that retain flexibility (Green’s classification grades I, II, and III). The goal of splinting is to keep the affected digit in extension to avoid repeated friction between the tendon and the tendon sheath.12 This ideally allows any cartilaginous metaplasia or inflammation to resolve, subsequently alleviating symptoms. The recommended length of treatment with splinting ranges from 3 to 12 weeks, with an average of 6 weeks.1
Multiple studies have shown long-term alleviation of symptoms with the use of orthotic devices. A retrospective analysis found that 87% of patients who wore their PIPJ orthotic device both day and night for a minimum of 6 weeks required no further treatment at 1-year follow-up.13 In contrast, MCPJ splinting only at night has been shown to resolve symptoms in just 55% of patients after 6 weeks.14 From a practical standpoint, however, patients are more likely to be compliant with night-only splinting, making it a reasonable option. Splinting does remain efficacious for patients even after 6 months of symptomatology.15
Day and night splinting for approximately 8 weeks using a PIPJ orthotic could be considered as an effective first-line intervention.16 Notably, PIPJ splinting is more functional, as it allows motion of the MCPJ and DIPJ.
An adjunct treatment to splinting is tendon-gliding exercises, including passive IPJ flexion, full finger flexion and extension, and hooking.13 Patients may remove the orthotic device to perform these exercises 3 times a day for 5 repetitions, as well as for activities that are not conducive to splinting.13
Corticosteroid injections. Injections of a corticosteroid and 1% lidocaine in a 1:1 mixture for a total volume of 1 cc can be inserted into the tendon sheath, A1 pulley, or adjacent tissue.17 Steroid injections help to decrease inflammation and pain in the affected area, giving symptom relief lasting a few months in as many as 57% to 87% of patients.18
Continue to: While the location of the injection...
While the location of the injection has been debated, recent literature suggests that symptoms can be effectively alleviated regardless of the specific anatomic injection site, such as intra-sheath or extra-sheath (FIGURE 2).19 This allows flexibility for the clinician, as the injection does not have to be placed within the tendon sheath. Corticosteroids should not be injected into the tendon itself, and the needle tip should be slightly withdrawn if there is resistance while injecting. Patients who are averse to injections have been shown to benefit from needle-free jet lidocaine (J-tip) administration prior to the actual steroid injection.20
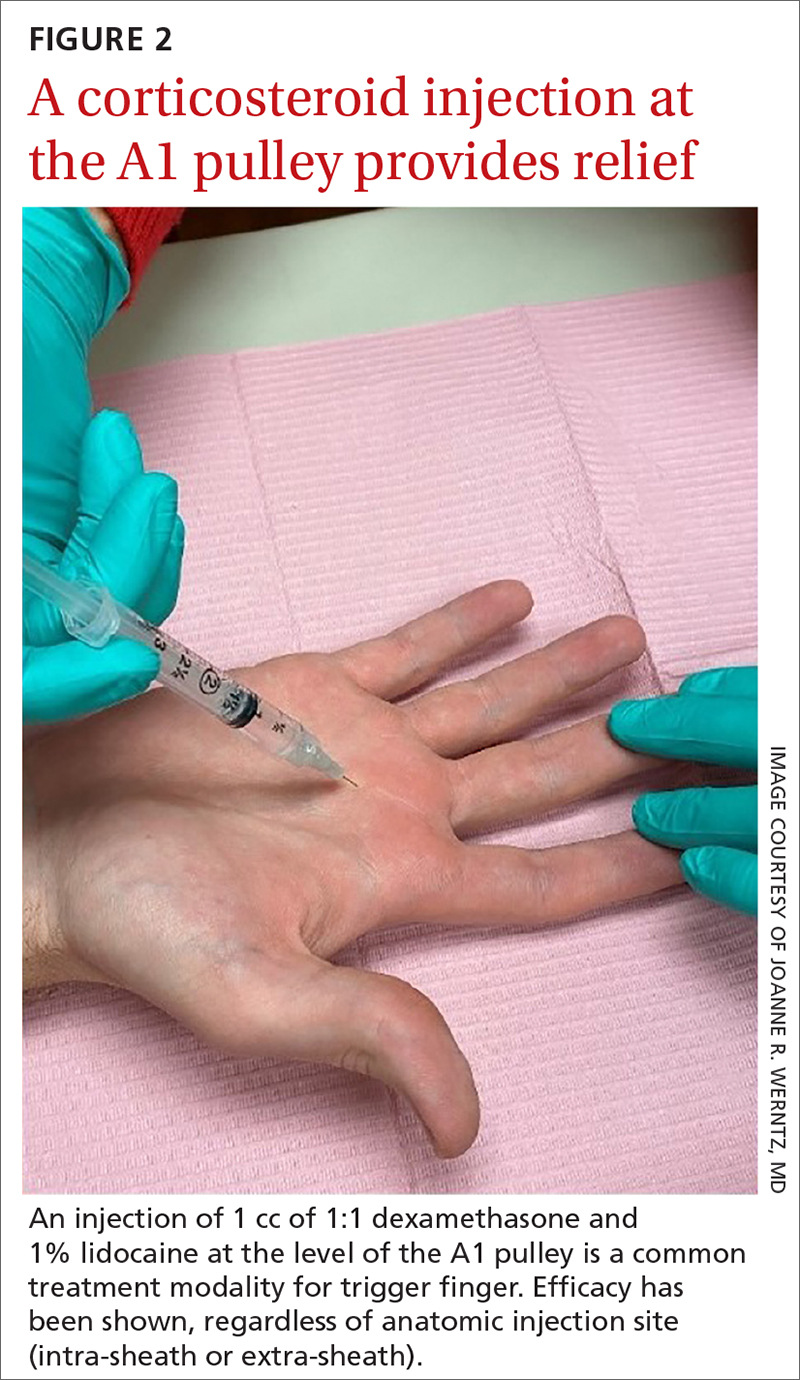
A randomized controlled trial comparing dexamethasone to triamcinolone injections found no difference in outcome at the 3-month follow-up (n = 84).17 This may suggest that the choice of corticosteroid is at the clinician’s discretion. In terms of long-term efficacy of steroid injections, it has been shown that 70% of trigger digits had complete resolution of symptoms at a mean follow-up of 8 years after just 1 injection (n = 43).21
Some patients, though, may require additional corticosteroid injections to maintain symptom control. If multiple injections are performed, they should not be given in intervals shorter than 4 months between treatments.5 Furthermore, steroids can be administered safely up to 3 times in the same digit before surgery is recommended.22
A patient’s options should be reconsidered if efficacy is not demonstrated with prior injections. Notably, a lower success rate has been shown in patients with type 2 diabetes (66%) compared to those without diabetes (90%).4,23 This difference in success rates is not well understood, as there is no causal relationship between well-controlled diabetes and TF.4 Complications of corticosteroid injections include local pain, fat atrophy, and hypopigmentation at the site of the injection, as well as short-term elevations in blood glucose levels in patients with diabetes.5,24
Surgical correction (to be discussed) remains superior to steroid injections in terms of cure rate and resolution of symptoms. A randomized controlled trial (n = 165) found that an injection-only group reported 86% and 49% success at 3-month and 12-month follow-up, respectively, compared to 99% success at both 3- and 12-month follow-up for the surgical group. Further, at 12-month follow-up, the median pain scores were significantly higher in the injection group (3; range, 1-9) than in the surgery group (1; range, 1-7).25 If conservative treatment modalities lead to unresolved symptoms or recurrence, referral to a hand specialist for surgery is recommended.
Continue to: Surgical treatments in an office setting
Surgical treatments in an office setting
Procedures for TF can be safely performed under conscious sedation or local anesthesia, with or without a tourniquet.26 Wide-awake procedures with local anesthesia and no tourniquet (WALANT) can be performed in an office-based procedure room rather than the operating room. This increases efficiency for the surgeon, reduces the amount of preparation and recovery time for the patient, and helps to keep costs down.
Percutaneous release involves the insertion of a 16-gauge hypodermic needle into the affected A1 pulley. The needle is used to fray and disrupt the pulley by moving the needle tip over the fibrotic A1 pulley.
However, it is not without possible complications.27 Inadvertent A2 pulley damage is particularly troublesome, as it leads to “bowstringing” or protrusion of the flexor tendon into the palm upon flexion. This can cause pain and failure to fully extend or flex the finger.10 Because the anatomy is not well visualized during the percutaneous approach, incomplete release, neurovascular injury, and iatrogenic injury to the A2 pulley or deep tendon may occur.28 Ultrasound-guided percutaneous release techniques have shown effective clinical outcomes with minimal complications compared to nonguided percutaneous release techniques.29,30
Open release is the gold standard surgical treatment for trigger finger (FIGURE 3). A small incision (1-2 cm) is made directly over or proximal to the A1 pulley in the distal palmar crease at the base of the affected digit. After blunt dissection through the subcutaneous tissue, the A1 pulley is sharply incised. An open approach has the clear benefit of avoiding the digital neurovascular bundles, as well as visualizing the resolution of triggering upon flexion and extension prior to closure. The WALANT procedure has the advantage of allowing the awake patient to actively flex and extend the digit to determine if the A1 release has been successful prior to closure of the incision.
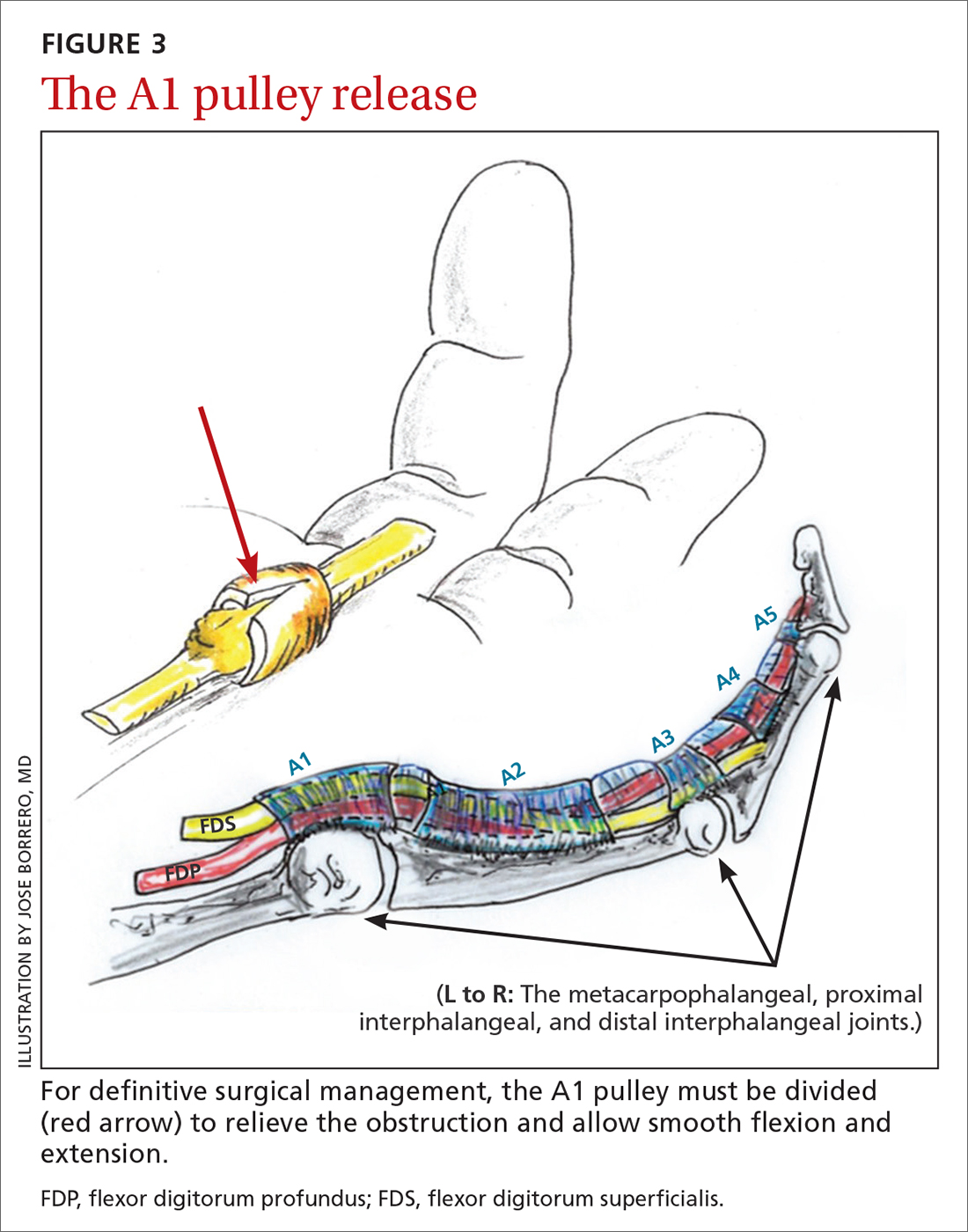
Outcomes and complications of surgery. A recent systematic review and meta-analysis has shown percutaneous techniques to be successful in 94% of cases.27 The success rate of open surgery has been reported at 99% to 100% at varying follow-up intervals up to 1 year.25,30,31 The complication rate for percutaneous release (guided and nonguided) was calculated at 2.2% (n = 2114).27 In another study, the overall complication rate of open releases was calculated at 1% (n = 999).32 When comparing percutaneous release (guided and nonguided) and open release, a meta-analysis found no significant difference in complication rate (RR = 0.84) or failure rate (RR = 0.94).32
Continue to: Several risk factors...
Several risk factors have been associated with postoperative surgical infection, including recent steroid injection (< 80 d), smoking status, increasing age, and pre-operative use of lidocaine with epinephrine.33 Open release has been shown to be an effective and safe treatment modality for patients with and without diabetes alike.34 Overall, definitive surgical correction has been demonstrated to be superior to conservative measures due to a significantly lower rate of recurrence.35
CASE
Given the patient’s presentation with triggering of the digit, tenderness over the A1 pulley, and lack of trauma history, we diagnosed trigger finger in this patient. Potential treatments included splinting, corticosteroid injections, and surgery. After discussion of the risks and benefits of each treatment option, the patient elected to undergo a corticosteroid injection. She was also given a neoprene finger sleeve to wear every night, and in the daytime when possible.
At 12-week follow-up, she noted early improvement in her triggering, which had since recurred. Due to her history of diabetes, the patient was then referred for surgery. She had an open release under local anesthesia. The surgery was uncomplicated, and the abnormality was corrected. At the patient’s 1-year postoperative follow-up visit, there was no evidence of recurrence, and she had regained full active and passive range of motion of her finger.
Acknowledgements
The authors wish to thank Jose Borrero, MD, for contributing his time and creative talents to produce the illustrations in this article.
CORRESPONDENCE
Evan P. Johnson, MD; 506 South Greer Street, Memphis, TN 38111; [email protected]
1. Lunsford D, Valdes K, Hengy S. Conservative management of trigger finger: a systematic review. J Hand Ther. 2019;32:212-221. doi: 10.1016/j.jht.2017.10.016
2. Makkouk AH, Oetgen ME, Swigart CR, et al. Trigger finger: etiology, evaluation, and treatment. Curr Rev Musculoskelet Med. 2008;1:92-96. doi: 10.1007/s12178-007-9012-1
3. Fitzgibbons PG, Weiss AP. Hand manifestations of diabetes mellitus. J Hand Surg Am. 2008;33:771-775. doi: 10.1016/j.jhsa.2008.01.038
4. Junot HSN, Anderson Hertz AFL, Gustavo Vasconcelos GR, et al. Epidemiology of trigger finger: metabolic syndrome as a new perspective of associated disease. Hand (N Y). 2019:1558944719867135. doi: 10.1177/1558944719867135.
5. Matthews A, Smith K, Read L, et al. Trigger finger: an overview of the treatment options. JAAPA. 2019;32:17-21. doi: 10.1097/01.Jaa.0000550281.42592.97
6. Pencle FJ, Waheed A, Molnar JA. Trigger thumb. StatPearls [Internet]. StatPearls Publishing; 2020. www.ncbi.nlm.nih.gov/books/NBK441854/
7. Giugale JM, Fowler JR. Trigger finger: adult and pediatric treatment strategies. Orthop Clin North Am. 2015;46:561-569. doi: 10.1016/j.ocl.2015.06.014
8. Bianchi S, Gitto S, Draghi F. Ultrasound features of trigger finger: review of the literature. J Ultrasound Med. 2019;38:3141-3154. doi: 10.1002/jum.15025
9. Chuang XL, Ooi CC, Chin ST, et al. What triggers in trigger finger? The flexor tendons at the flexor digitorum superficialis bifurcation. J Plast Reconstr Aesthet Surg. 2017;70:1411-1419. doi: 10.1016/j.bjps.2017.05.037
10. Ryzewicz M, Wolf JM. Trigger digits: principles, management, and complications. J Hand Surg Am. 2006;31:135-146. doi: 10.1016/j.jhsa.2005.10.013
11. Chapter 56: Tendinoapthy. In: Wolfe SW, Peterson WC, Kozin SH, Cohen MS. Green’s Operative Hand Surgery. Vol 2. 7th ed. Elsevier; 2017: 1904-1925.
12. Tarbhai K, Hannah S, von Schroeder HP. Trigger finger treatment: a comparison of 2 splint designs. J Hand Surg Am. 2012;37:243-249, 249.e241. doi: 10.1016/j.jhsa.2011.10.038
13. Valdes K. A retrospective review to determine the long-term efficacy of orthotic devices for trigger finger. J Hand Ther. 2012;25:89-95. doi: 10.1016/j.jht.2011.09.005
14. Drijkoningen T, van Berckel M, Becker SJE, et al. Night splinting for idiopathic trigger digits. Hand (N Y). 2018;13:558-562. doi: 10.1177/1558944717725374
15. Colbourn J, Heath N, Manary S, et al. Effectiveness of splinting for the treatment of trigger finger. J Hand Ther. 2008;21:336-343. doi: 10.1197/j.jht.2008.05.001
16. Teo SH, Ng DCL, Wong YKY. Effectiveness of proximal interphalangeal joint-blocking orthosis vs metacarpophalangeal joint-blocking orthosis in trigger digit management: A randomized clinical trial. J Hand Ther. 2018;32:444-451. doi: 10.1016/j.jht.2018.02.007
17. Ring D, Lozano-Calderon S, Shin R, et al. A prospective randomized controlled trial of injection of dexamethasone versus triamcinolone for idiopathic trigger finger. J Hand Surg Am. 2008;33:516-522; discussion 523-514. doi: 10.1016/j.jhsa.2008.01.001
18. Fleisch SB, Spindler KP, Lee DH. Corticosteroid injections in the treatment of trigger finger: A level I and II systematic review. J Am Acad Orthop Surg. 2007;15:166-171. doi: 10.5435/00124635-200703000-00006
19. Shinomiya R, Sunagawa T, Nakashima Y, et al. Impact of corticosteroid injection site on the treatment success rate of trigger finger: a prospective study comparing ultrasound-guided true intra-sheath and true extra-sheath injections. Ultrasound Med Biol. 2016;42:2203-2208. doi: 10.1016/j.ultrasmedbio.2016.05.015
20. Earp BE, Stanbury SJ, Mora AN, et al. Needle-free jet lidocaine administration for preinjection anesthesia in trigger finger injection: a randomized controlled trial. J Hand Surg Am. 2017;42:618-622. doi: 10.1016/j.jhsa.2017.05.001
21. Castellanos J, Munoz-Mahamud E, Dominguez E, et al. Long-term effectiveness of corticosteroid injections for trigger finger and thumb. J Hand Surg Am. 2015;40:121-126. doi: 10.1016/j.jhsa.2014.09.006
22. Dala-Ali BM, Nakhdjevani A, Lloyd MA, et al. The efficacy of steroid injection in the treatment of trigger finger. Clin Orthop Surg. 2012;4:263-268. doi: 10.4055/cios.2012.4.4.263
23. Griggs SM, Weiss AP, Lane LB, et al. Treatment of trigger finger in patients with diabetes mellitus. J Hand Surg Am. 1995;20:787-789. doi: 10.1016/s0363-5023(05)80432-0
24. Stepan JG, London DA, Boyer MI, et al. Blood glucose levels in diabetic patients following corticosteroid injections into the hand and wrist. J Hand Surg Am. 2014;39:706-712. doi: 10.1016/j.jhsa.2014.01.014
25. Hansen RL, Sondergaard M, Lange J. Open surgery versus ultrasound-guided corticosteroid injection for trigger finger: a randomized controlled trial with 1-year follow-up. J Hand Surg Am. 2017;42:359-366. doi: 10.1016/j.jhsa.2017.02.011
26. Mohd Rashid MZ, Sapuan J, Abdullah S. A randomized controlled trial of trigger finger release under digital anesthesia with (WALANT) and without adrenaline. J Orthop Surg (Hong Kong). 2019;27:2309499019833002. doi: 10.1177/2309499019833002
27. Zhao J-G, Kan S-L, Zhao L, et al. Percutaneous first annular pulley release for trigger digits: a systematic review and meta-analysis of current evidence. J Hand Surg Am. 2014;39:2192-2202. doi: 10.1016/j.jhsa.2014.07.044
28. Guler F, Kose O, Ercan EC, et al. Open versus percutaneous release for the treatment of trigger thumb. Orthopedics. 2013;36:e1290-1294. doi: 10.3928/01477447-20130920-22
29. Wu KC, Chern TC, Jou IM. Ultrasound-assisted percutaneous trigger finger release: it is safe [letter]. Hand (N Y). 2009;4:339. doi: 10.1007/s11552-009-9179-6
30. Nikolaou VS, Malahias M-A, Kaseta M-K, et al. Comparative clinical study of ultrasound-guided A1 pulley release vs open surgical intervention in the treatment of trigger finger. World J Orthop. 2017;8:163-169. doi: 10.5312/wjo.v8.i2.163
31. Lim M-H, Lim K-K, Rasheed MZ, et al. Outcome of open trigger digit release. J Hand Surg Eur Vol. 2007;32:457-459. doi: 10.1016/j.Jhsb.2007.02.016
32. Wang J, Zhao J-G, Liang C-C. Percutaneous release, open surgery, or corticosteroid injection, which is the best treatment method for trigger digits? Clin Orthop Relat Res. 2013;471:1879-1886. doi: 10.1007/s11999-012-2716-6
33. Ng WKY, Olmscheid N, Worhacz K, et al. Steroid injection and open trigger finger release outcomes: a retrospective review of 999 digits. Hand (N Y). 2018;15:399-406. doi: 10.1177/1558944718796559
34. Ho SWL, Chia CY, Rajaratnam V. Characteristics and clinical outcomes of open surgery for trigger digits in diabetes. J Hand Microsurg. 2019;11:80-83. doi: 10.1055/s-0038-1670927
35. Sato ES, dos Santos JB, Belloti JC, et al. Percutaneous release of trigger fingers. Hand Clin. 2014;30:39-45. doi: 10.1016/j.hcl.2013.08.017
CASE
A 55-year-old right-hand-dominant woman presented to the clinic with a chief complaint of right ring finger pain and stiffness. There was no history of trauma or prior surgery. She had no tingling or numbness. She had a history of type 2 diabetes that was well controlled. She worked as a clerk for a government office for many years, and her painful, limited finger motion interfered with keyboarding and picking up items. Physical examination revealed tenderness to palpation over the palmar aspect of the metacarpophalangeal joint (MCPJ) of the ring finger with no other joint tenderness or swelling. When she made a fist, her ring finger MCPJ, proximal interphalangeal joint (PIPJ), and distal interphalangeal joint (DIPJ) locked in a flexed position that required manipulation to extend the finger. A firm mass was palpated in the palm with finger flexion that moved into the finger with extension.
Stenosing tenosynovitis, also known as trigger finger (TF), is an inflammatory condition that causes pain in the distal palm and proximal digit with associated limited motion. The most commonly affected digits are the middle and ring fingers of the dominant hand.1 The disorder is particularly noticeable when it inhibits day-to-day functioning.
TF affects 2% to 3% of the general population and up to 20% of patients with diabetes.2,3 Patient age and duration of diabetes are commonly cited as contributing factors, although the effect of well-controlled blood glucose and A1C on the frequency and cure rate of TF has not been established.3,4 TF is most commonly seen in individuals ages 40 to 60 years, with a 6 times’ greater frequency in females than males.5
In the United States, there are an estimated 200,000 cases of TF each year, with initial presentation typically being to a primary care physician.6 For this reason, it is essential for primary care physicians to recognize this common pathology and treat symptoms early to prevent progression and the need for surgical intervention.
An impaired gliding motion of the flexor tendons
In each finger, a tendon sheath, consisting of 5 annular pulleys and 3 cruciate pulleys, forms a tunnel around the flexor digitorum profundus (FDP) and flexor digitorum superficialis (FDS). The tendon sheath allows for maximum force by eliminating bowstringing of the tendons when the digit is flexed. Deep to the tendons and surrounding the tendons is a synovial membrane that provides nutrition and reduces friction between the tendons and the tendon sheath.7
The FDP is longer and assists in flexion of the MCPJ and the PIPJ. It is the sole flexor of the DIPJ.
In the thumb, the flexor pollicis longus (FPL) is the only flexor within its tendon sheath. The FPL assists in flexion of the MCPJ and flexes the thumb interphalangeal joint (IPJ). The intrinsic muscles (lumbricals and interossei) do not extend into the tendon sheath and do not contribute to TF.
Continue to: TF occurs when
TF occurs when the tendon sheath, most commonly at the first annular pulley (A1), or the flexor tendons thicken due to fibrocartilaginous metaplasia. This results in impaired gliding motion of the flexor tendons.8 The stenosed A1 pulley can lead to pinching of the flexor tendons and cause the formation of a nodule on the FDS tendon at its bifurcation.9 The nodule of the FDS bifurcation moves proximal to the A1 pulley when the finger is flexed. Upon extension, the tendon nodule may get caught on the A1 pulley. This prevents smooth extension and is the source of pain and triggering (FIGURE 1). In a similar manner, thumb triggering is the result of a stenosed A1 pulley creating a nodule on the FPL tendon, which prevents smooth gliding of the FPL.

What you’ll see
TF is characterized by locking, popping, or clicking at the base of the finger or thumb.7,10 A small nodule may be palpated on the palmar aspect of the MCPJ when the finger is flexed. This nodule will then move distally when the finger is extended. Patients will present with the affected digit in a flexed position and will have difficulty extending the digit. In some cases, the patient may have to use the other hand to straighten the affected digit. In more severe cases, the digit may be fixed in a position of flexion or extension. The severity of triggering is commonly graded by the Green’s classification system (see TABLE11).

Is it Dupuytren contracture, trigger finger, or something else?
The differential diagnosis for TF includes Dupuytren contracture, MCPJ sprain, calcific peritendinitis, flexor tenosynovitis, diabetic cheiroarthropathy (DCA), rheumatoid arthritis (RA), osteoarthritis (OA), and crystalline arthropathy (gout).5
Dupuytren contracture is usually nonpainful and manifests with a palpable cord in the palm and a fixed flexion contracture that has progressed over time, with no history of catching.
MCPJ sprain is diagnosed with tenderness of the MCPJ and a history of trauma.
Continue to: Calcific peritendinitis
Calcific peritendinitis is characterized by pain, tenderness, and edema near a joint with calcified deposits seen on radiographs.
Flexor tenosynovitis manifests with fusiform swelling of the digit, tenderness over the flexor tendon sheath, and pain with passive extension of the digit; it is more commonly associated with RA.
DCA, RA, OA, and gout usually affect more than 1 digit. DCA is associated with both type 1 and type 2 diabetes and is characterized by thickened, waxy skin and painless, limited extension of the digits. RA and OA are diagnosed by medical history, lab work, and radiographs. Gout is diagnosed with lab work and aspiration of joint fluid.
A thorough history, physical exam, and review of radiographs must be performed to rule out these other disorders. Once the diagnosis of TF is made, available treatment options should be pursued.
Treatment: A conservative or surgical approach?
Current treatment options include both nonsurgical (conservative) and surgical interventions. Nonsurgical interventions include activity modification, splinting, and corticosteroid injections. While nonsteroidal anti-inflammatory drugs are commonly recommended to resolve the local inflammation secondary to triggering, there is no scientific evidence to support their use at this time.7 Surgical interventions, utilized in more severe cases or after conservative treatment has failed, include percutaneous and open release of the tendon sheath.2,7
Continue to: Conservative treatments
Conservative treatments
Splinting is only an option for digits that retain flexibility (Green’s classification grades I, II, and III). The goal of splinting is to keep the affected digit in extension to avoid repeated friction between the tendon and the tendon sheath.12 This ideally allows any cartilaginous metaplasia or inflammation to resolve, subsequently alleviating symptoms. The recommended length of treatment with splinting ranges from 3 to 12 weeks, with an average of 6 weeks.1
Multiple studies have shown long-term alleviation of symptoms with the use of orthotic devices. A retrospective analysis found that 87% of patients who wore their PIPJ orthotic device both day and night for a minimum of 6 weeks required no further treatment at 1-year follow-up.13 In contrast, MCPJ splinting only at night has been shown to resolve symptoms in just 55% of patients after 6 weeks.14 From a practical standpoint, however, patients are more likely to be compliant with night-only splinting, making it a reasonable option. Splinting does remain efficacious for patients even after 6 months of symptomatology.15
Day and night splinting for approximately 8 weeks using a PIPJ orthotic could be considered as an effective first-line intervention.16 Notably, PIPJ splinting is more functional, as it allows motion of the MCPJ and DIPJ.
An adjunct treatment to splinting is tendon-gliding exercises, including passive IPJ flexion, full finger flexion and extension, and hooking.13 Patients may remove the orthotic device to perform these exercises 3 times a day for 5 repetitions, as well as for activities that are not conducive to splinting.13
Corticosteroid injections. Injections of a corticosteroid and 1% lidocaine in a 1:1 mixture for a total volume of 1 cc can be inserted into the tendon sheath, A1 pulley, or adjacent tissue.17 Steroid injections help to decrease inflammation and pain in the affected area, giving symptom relief lasting a few months in as many as 57% to 87% of patients.18
Continue to: While the location of the injection...
While the location of the injection has been debated, recent literature suggests that symptoms can be effectively alleviated regardless of the specific anatomic injection site, such as intra-sheath or extra-sheath (FIGURE 2).19 This allows flexibility for the clinician, as the injection does not have to be placed within the tendon sheath. Corticosteroids should not be injected into the tendon itself, and the needle tip should be slightly withdrawn if there is resistance while injecting. Patients who are averse to injections have been shown to benefit from needle-free jet lidocaine (J-tip) administration prior to the actual steroid injection.20

A randomized controlled trial comparing dexamethasone to triamcinolone injections found no difference in outcome at the 3-month follow-up (n = 84).17 This may suggest that the choice of corticosteroid is at the clinician’s discretion. In terms of long-term efficacy of steroid injections, it has been shown that 70% of trigger digits had complete resolution of symptoms at a mean follow-up of 8 years after just 1 injection (n = 43).21
Some patients, though, may require additional corticosteroid injections to maintain symptom control. If multiple injections are performed, they should not be given in intervals shorter than 4 months between treatments.5 Furthermore, steroids can be administered safely up to 3 times in the same digit before surgery is recommended.22
A patient’s options should be reconsidered if efficacy is not demonstrated with prior injections. Notably, a lower success rate has been shown in patients with type 2 diabetes (66%) compared to those without diabetes (90%).4,23 This difference in success rates is not well understood, as there is no causal relationship between well-controlled diabetes and TF.4 Complications of corticosteroid injections include local pain, fat atrophy, and hypopigmentation at the site of the injection, as well as short-term elevations in blood glucose levels in patients with diabetes.5,24
Surgical correction (to be discussed) remains superior to steroid injections in terms of cure rate and resolution of symptoms. A randomized controlled trial (n = 165) found that an injection-only group reported 86% and 49% success at 3-month and 12-month follow-up, respectively, compared to 99% success at both 3- and 12-month follow-up for the surgical group. Further, at 12-month follow-up, the median pain scores were significantly higher in the injection group (3; range, 1-9) than in the surgery group (1; range, 1-7).25 If conservative treatment modalities lead to unresolved symptoms or recurrence, referral to a hand specialist for surgery is recommended.
Continue to: Surgical treatments in an office setting
Surgical treatments in an office setting
Procedures for TF can be safely performed under conscious sedation or local anesthesia, with or without a tourniquet.26 Wide-awake procedures with local anesthesia and no tourniquet (WALANT) can be performed in an office-based procedure room rather than the operating room. This increases efficiency for the surgeon, reduces the amount of preparation and recovery time for the patient, and helps to keep costs down.
Percutaneous release involves the insertion of a 16-gauge hypodermic needle into the affected A1 pulley. The needle is used to fray and disrupt the pulley by moving the needle tip over the fibrotic A1 pulley.
However, it is not without possible complications.27 Inadvertent A2 pulley damage is particularly troublesome, as it leads to “bowstringing” or protrusion of the flexor tendon into the palm upon flexion. This can cause pain and failure to fully extend or flex the finger.10 Because the anatomy is not well visualized during the percutaneous approach, incomplete release, neurovascular injury, and iatrogenic injury to the A2 pulley or deep tendon may occur.28 Ultrasound-guided percutaneous release techniques have shown effective clinical outcomes with minimal complications compared to nonguided percutaneous release techniques.29,30
Open release is the gold standard surgical treatment for trigger finger (FIGURE 3). A small incision (1-2 cm) is made directly over or proximal to the A1 pulley in the distal palmar crease at the base of the affected digit. After blunt dissection through the subcutaneous tissue, the A1 pulley is sharply incised. An open approach has the clear benefit of avoiding the digital neurovascular bundles, as well as visualizing the resolution of triggering upon flexion and extension prior to closure. The WALANT procedure has the advantage of allowing the awake patient to actively flex and extend the digit to determine if the A1 release has been successful prior to closure of the incision.

Outcomes and complications of surgery. A recent systematic review and meta-analysis has shown percutaneous techniques to be successful in 94% of cases.27 The success rate of open surgery has been reported at 99% to 100% at varying follow-up intervals up to 1 year.25,30,31 The complication rate for percutaneous release (guided and nonguided) was calculated at 2.2% (n = 2114).27 In another study, the overall complication rate of open releases was calculated at 1% (n = 999).32 When comparing percutaneous release (guided and nonguided) and open release, a meta-analysis found no significant difference in complication rate (RR = 0.84) or failure rate (RR = 0.94).32
Continue to: Several risk factors...
Several risk factors have been associated with postoperative surgical infection, including recent steroid injection (< 80 d), smoking status, increasing age, and pre-operative use of lidocaine with epinephrine.33 Open release has been shown to be an effective and safe treatment modality for patients with and without diabetes alike.34 Overall, definitive surgical correction has been demonstrated to be superior to conservative measures due to a significantly lower rate of recurrence.35
CASE
Given the patient’s presentation with triggering of the digit, tenderness over the A1 pulley, and lack of trauma history, we diagnosed trigger finger in this patient. Potential treatments included splinting, corticosteroid injections, and surgery. After discussion of the risks and benefits of each treatment option, the patient elected to undergo a corticosteroid injection. She was also given a neoprene finger sleeve to wear every night, and in the daytime when possible.
At 12-week follow-up, she noted early improvement in her triggering, which had since recurred. Due to her history of diabetes, the patient was then referred for surgery. She had an open release under local anesthesia. The surgery was uncomplicated, and the abnormality was corrected. At the patient’s 1-year postoperative follow-up visit, there was no evidence of recurrence, and she had regained full active and passive range of motion of her finger.
Acknowledgements
The authors wish to thank Jose Borrero, MD, for contributing his time and creative talents to produce the illustrations in this article.
CORRESPONDENCE
Evan P. Johnson, MD; 506 South Greer Street, Memphis, TN 38111; [email protected]
CASE
A 55-year-old right-hand-dominant woman presented to the clinic with a chief complaint of right ring finger pain and stiffness. There was no history of trauma or prior surgery. She had no tingling or numbness. She had a history of type 2 diabetes that was well controlled. She worked as a clerk for a government office for many years, and her painful, limited finger motion interfered with keyboarding and picking up items. Physical examination revealed tenderness to palpation over the palmar aspect of the metacarpophalangeal joint (MCPJ) of the ring finger with no other joint tenderness or swelling. When she made a fist, her ring finger MCPJ, proximal interphalangeal joint (PIPJ), and distal interphalangeal joint (DIPJ) locked in a flexed position that required manipulation to extend the finger. A firm mass was palpated in the palm with finger flexion that moved into the finger with extension.
Stenosing tenosynovitis, also known as trigger finger (TF), is an inflammatory condition that causes pain in the distal palm and proximal digit with associated limited motion. The most commonly affected digits are the middle and ring fingers of the dominant hand.1 The disorder is particularly noticeable when it inhibits day-to-day functioning.
TF affects 2% to 3% of the general population and up to 20% of patients with diabetes.2,3 Patient age and duration of diabetes are commonly cited as contributing factors, although the effect of well-controlled blood glucose and A1C on the frequency and cure rate of TF has not been established.3,4 TF is most commonly seen in individuals ages 40 to 60 years, with a 6 times’ greater frequency in females than males.5
In the United States, there are an estimated 200,000 cases of TF each year, with initial presentation typically being to a primary care physician.6 For this reason, it is essential for primary care physicians to recognize this common pathology and treat symptoms early to prevent progression and the need for surgical intervention.
An impaired gliding motion of the flexor tendons
In each finger, a tendon sheath, consisting of 5 annular pulleys and 3 cruciate pulleys, forms a tunnel around the flexor digitorum profundus (FDP) and flexor digitorum superficialis (FDS). The tendon sheath allows for maximum force by eliminating bowstringing of the tendons when the digit is flexed. Deep to the tendons and surrounding the tendons is a synovial membrane that provides nutrition and reduces friction between the tendons and the tendon sheath.7
The FDP is longer and assists in flexion of the MCPJ and the PIPJ. It is the sole flexor of the DIPJ.
In the thumb, the flexor pollicis longus (FPL) is the only flexor within its tendon sheath. The FPL assists in flexion of the MCPJ and flexes the thumb interphalangeal joint (IPJ). The intrinsic muscles (lumbricals and interossei) do not extend into the tendon sheath and do not contribute to TF.
Continue to: TF occurs when
TF occurs when the tendon sheath, most commonly at the first annular pulley (A1), or the flexor tendons thicken due to fibrocartilaginous metaplasia. This results in impaired gliding motion of the flexor tendons.8 The stenosed A1 pulley can lead to pinching of the flexor tendons and cause the formation of a nodule on the FDS tendon at its bifurcation.9 The nodule of the FDS bifurcation moves proximal to the A1 pulley when the finger is flexed. Upon extension, the tendon nodule may get caught on the A1 pulley. This prevents smooth extension and is the source of pain and triggering (FIGURE 1). In a similar manner, thumb triggering is the result of a stenosed A1 pulley creating a nodule on the FPL tendon, which prevents smooth gliding of the FPL.

What you’ll see
TF is characterized by locking, popping, or clicking at the base of the finger or thumb.7,10 A small nodule may be palpated on the palmar aspect of the MCPJ when the finger is flexed. This nodule will then move distally when the finger is extended. Patients will present with the affected digit in a flexed position and will have difficulty extending the digit. In some cases, the patient may have to use the other hand to straighten the affected digit. In more severe cases, the digit may be fixed in a position of flexion or extension. The severity of triggering is commonly graded by the Green’s classification system (see TABLE11).

Is it Dupuytren contracture, trigger finger, or something else?
The differential diagnosis for TF includes Dupuytren contracture, MCPJ sprain, calcific peritendinitis, flexor tenosynovitis, diabetic cheiroarthropathy (DCA), rheumatoid arthritis (RA), osteoarthritis (OA), and crystalline arthropathy (gout).5
Dupuytren contracture is usually nonpainful and manifests with a palpable cord in the palm and a fixed flexion contracture that has progressed over time, with no history of catching.
MCPJ sprain is diagnosed with tenderness of the MCPJ and a history of trauma.
Continue to: Calcific peritendinitis
Calcific peritendinitis is characterized by pain, tenderness, and edema near a joint with calcified deposits seen on radiographs.
Flexor tenosynovitis manifests with fusiform swelling of the digit, tenderness over the flexor tendon sheath, and pain with passive extension of the digit; it is more commonly associated with RA.
DCA, RA, OA, and gout usually affect more than 1 digit. DCA is associated with both type 1 and type 2 diabetes and is characterized by thickened, waxy skin and painless, limited extension of the digits. RA and OA are diagnosed by medical history, lab work, and radiographs. Gout is diagnosed with lab work and aspiration of joint fluid.
A thorough history, physical exam, and review of radiographs must be performed to rule out these other disorders. Once the diagnosis of TF is made, available treatment options should be pursued.
Treatment: A conservative or surgical approach?
Current treatment options include both nonsurgical (conservative) and surgical interventions. Nonsurgical interventions include activity modification, splinting, and corticosteroid injections. While nonsteroidal anti-inflammatory drugs are commonly recommended to resolve the local inflammation secondary to triggering, there is no scientific evidence to support their use at this time.7 Surgical interventions, utilized in more severe cases or after conservative treatment has failed, include percutaneous and open release of the tendon sheath.2,7
Continue to: Conservative treatments
Conservative treatments
Splinting is only an option for digits that retain flexibility (Green’s classification grades I, II, and III). The goal of splinting is to keep the affected digit in extension to avoid repeated friction between the tendon and the tendon sheath.12 This ideally allows any cartilaginous metaplasia or inflammation to resolve, subsequently alleviating symptoms. The recommended length of treatment with splinting ranges from 3 to 12 weeks, with an average of 6 weeks.1
Multiple studies have shown long-term alleviation of symptoms with the use of orthotic devices. A retrospective analysis found that 87% of patients who wore their PIPJ orthotic device both day and night for a minimum of 6 weeks required no further treatment at 1-year follow-up.13 In contrast, MCPJ splinting only at night has been shown to resolve symptoms in just 55% of patients after 6 weeks.14 From a practical standpoint, however, patients are more likely to be compliant with night-only splinting, making it a reasonable option. Splinting does remain efficacious for patients even after 6 months of symptomatology.15
Day and night splinting for approximately 8 weeks using a PIPJ orthotic could be considered as an effective first-line intervention.16 Notably, PIPJ splinting is more functional, as it allows motion of the MCPJ and DIPJ.
An adjunct treatment to splinting is tendon-gliding exercises, including passive IPJ flexion, full finger flexion and extension, and hooking.13 Patients may remove the orthotic device to perform these exercises 3 times a day for 5 repetitions, as well as for activities that are not conducive to splinting.13
Corticosteroid injections. Injections of a corticosteroid and 1% lidocaine in a 1:1 mixture for a total volume of 1 cc can be inserted into the tendon sheath, A1 pulley, or adjacent tissue.17 Steroid injections help to decrease inflammation and pain in the affected area, giving symptom relief lasting a few months in as many as 57% to 87% of patients.18
Continue to: While the location of the injection...
While the location of the injection has been debated, recent literature suggests that symptoms can be effectively alleviated regardless of the specific anatomic injection site, such as intra-sheath or extra-sheath (FIGURE 2).19 This allows flexibility for the clinician, as the injection does not have to be placed within the tendon sheath. Corticosteroids should not be injected into the tendon itself, and the needle tip should be slightly withdrawn if there is resistance while injecting. Patients who are averse to injections have been shown to benefit from needle-free jet lidocaine (J-tip) administration prior to the actual steroid injection.20

A randomized controlled trial comparing dexamethasone to triamcinolone injections found no difference in outcome at the 3-month follow-up (n = 84).17 This may suggest that the choice of corticosteroid is at the clinician’s discretion. In terms of long-term efficacy of steroid injections, it has been shown that 70% of trigger digits had complete resolution of symptoms at a mean follow-up of 8 years after just 1 injection (n = 43).21
Some patients, though, may require additional corticosteroid injections to maintain symptom control. If multiple injections are performed, they should not be given in intervals shorter than 4 months between treatments.5 Furthermore, steroids can be administered safely up to 3 times in the same digit before surgery is recommended.22
A patient’s options should be reconsidered if efficacy is not demonstrated with prior injections. Notably, a lower success rate has been shown in patients with type 2 diabetes (66%) compared to those without diabetes (90%).4,23 This difference in success rates is not well understood, as there is no causal relationship between well-controlled diabetes and TF.4 Complications of corticosteroid injections include local pain, fat atrophy, and hypopigmentation at the site of the injection, as well as short-term elevations in blood glucose levels in patients with diabetes.5,24
Surgical correction (to be discussed) remains superior to steroid injections in terms of cure rate and resolution of symptoms. A randomized controlled trial (n = 165) found that an injection-only group reported 86% and 49% success at 3-month and 12-month follow-up, respectively, compared to 99% success at both 3- and 12-month follow-up for the surgical group. Further, at 12-month follow-up, the median pain scores were significantly higher in the injection group (3; range, 1-9) than in the surgery group (1; range, 1-7).25 If conservative treatment modalities lead to unresolved symptoms or recurrence, referral to a hand specialist for surgery is recommended.
Continue to: Surgical treatments in an office setting
Surgical treatments in an office setting
Procedures for TF can be safely performed under conscious sedation or local anesthesia, with or without a tourniquet.26 Wide-awake procedures with local anesthesia and no tourniquet (WALANT) can be performed in an office-based procedure room rather than the operating room. This increases efficiency for the surgeon, reduces the amount of preparation and recovery time for the patient, and helps to keep costs down.
Percutaneous release involves the insertion of a 16-gauge hypodermic needle into the affected A1 pulley. The needle is used to fray and disrupt the pulley by moving the needle tip over the fibrotic A1 pulley.
However, it is not without possible complications.27 Inadvertent A2 pulley damage is particularly troublesome, as it leads to “bowstringing” or protrusion of the flexor tendon into the palm upon flexion. This can cause pain and failure to fully extend or flex the finger.10 Because the anatomy is not well visualized during the percutaneous approach, incomplete release, neurovascular injury, and iatrogenic injury to the A2 pulley or deep tendon may occur.28 Ultrasound-guided percutaneous release techniques have shown effective clinical outcomes with minimal complications compared to nonguided percutaneous release techniques.29,30
Open release is the gold standard surgical treatment for trigger finger (FIGURE 3). A small incision (1-2 cm) is made directly over or proximal to the A1 pulley in the distal palmar crease at the base of the affected digit. After blunt dissection through the subcutaneous tissue, the A1 pulley is sharply incised. An open approach has the clear benefit of avoiding the digital neurovascular bundles, as well as visualizing the resolution of triggering upon flexion and extension prior to closure. The WALANT procedure has the advantage of allowing the awake patient to actively flex and extend the digit to determine if the A1 release has been successful prior to closure of the incision.

Outcomes and complications of surgery. A recent systematic review and meta-analysis has shown percutaneous techniques to be successful in 94% of cases.27 The success rate of open surgery has been reported at 99% to 100% at varying follow-up intervals up to 1 year.25,30,31 The complication rate for percutaneous release (guided and nonguided) was calculated at 2.2% (n = 2114).27 In another study, the overall complication rate of open releases was calculated at 1% (n = 999).32 When comparing percutaneous release (guided and nonguided) and open release, a meta-analysis found no significant difference in complication rate (RR = 0.84) or failure rate (RR = 0.94).32
Continue to: Several risk factors...
Several risk factors have been associated with postoperative surgical infection, including recent steroid injection (< 80 d), smoking status, increasing age, and pre-operative use of lidocaine with epinephrine.33 Open release has been shown to be an effective and safe treatment modality for patients with and without diabetes alike.34 Overall, definitive surgical correction has been demonstrated to be superior to conservative measures due to a significantly lower rate of recurrence.35
CASE
Given the patient’s presentation with triggering of the digit, tenderness over the A1 pulley, and lack of trauma history, we diagnosed trigger finger in this patient. Potential treatments included splinting, corticosteroid injections, and surgery. After discussion of the risks and benefits of each treatment option, the patient elected to undergo a corticosteroid injection. She was also given a neoprene finger sleeve to wear every night, and in the daytime when possible.
At 12-week follow-up, she noted early improvement in her triggering, which had since recurred. Due to her history of diabetes, the patient was then referred for surgery. She had an open release under local anesthesia. The surgery was uncomplicated, and the abnormality was corrected. At the patient’s 1-year postoperative follow-up visit, there was no evidence of recurrence, and she had regained full active and passive range of motion of her finger.
Acknowledgements
The authors wish to thank Jose Borrero, MD, for contributing his time and creative talents to produce the illustrations in this article.
CORRESPONDENCE
Evan P. Johnson, MD; 506 South Greer Street, Memphis, TN 38111; [email protected]
1. Lunsford D, Valdes K, Hengy S. Conservative management of trigger finger: a systematic review. J Hand Ther. 2019;32:212-221. doi: 10.1016/j.jht.2017.10.016
2. Makkouk AH, Oetgen ME, Swigart CR, et al. Trigger finger: etiology, evaluation, and treatment. Curr Rev Musculoskelet Med. 2008;1:92-96. doi: 10.1007/s12178-007-9012-1
3. Fitzgibbons PG, Weiss AP. Hand manifestations of diabetes mellitus. J Hand Surg Am. 2008;33:771-775. doi: 10.1016/j.jhsa.2008.01.038
4. Junot HSN, Anderson Hertz AFL, Gustavo Vasconcelos GR, et al. Epidemiology of trigger finger: metabolic syndrome as a new perspective of associated disease. Hand (N Y). 2019:1558944719867135. doi: 10.1177/1558944719867135.
5. Matthews A, Smith K, Read L, et al. Trigger finger: an overview of the treatment options. JAAPA. 2019;32:17-21. doi: 10.1097/01.Jaa.0000550281.42592.97
6. Pencle FJ, Waheed A, Molnar JA. Trigger thumb. StatPearls [Internet]. StatPearls Publishing; 2020. www.ncbi.nlm.nih.gov/books/NBK441854/
7. Giugale JM, Fowler JR. Trigger finger: adult and pediatric treatment strategies. Orthop Clin North Am. 2015;46:561-569. doi: 10.1016/j.ocl.2015.06.014
8. Bianchi S, Gitto S, Draghi F. Ultrasound features of trigger finger: review of the literature. J Ultrasound Med. 2019;38:3141-3154. doi: 10.1002/jum.15025
9. Chuang XL, Ooi CC, Chin ST, et al. What triggers in trigger finger? The flexor tendons at the flexor digitorum superficialis bifurcation. J Plast Reconstr Aesthet Surg. 2017;70:1411-1419. doi: 10.1016/j.bjps.2017.05.037
10. Ryzewicz M, Wolf JM. Trigger digits: principles, management, and complications. J Hand Surg Am. 2006;31:135-146. doi: 10.1016/j.jhsa.2005.10.013
11. Chapter 56: Tendinoapthy. In: Wolfe SW, Peterson WC, Kozin SH, Cohen MS. Green’s Operative Hand Surgery. Vol 2. 7th ed. Elsevier; 2017: 1904-1925.
12. Tarbhai K, Hannah S, von Schroeder HP. Trigger finger treatment: a comparison of 2 splint designs. J Hand Surg Am. 2012;37:243-249, 249.e241. doi: 10.1016/j.jhsa.2011.10.038
13. Valdes K. A retrospective review to determine the long-term efficacy of orthotic devices for trigger finger. J Hand Ther. 2012;25:89-95. doi: 10.1016/j.jht.2011.09.005
14. Drijkoningen T, van Berckel M, Becker SJE, et al. Night splinting for idiopathic trigger digits. Hand (N Y). 2018;13:558-562. doi: 10.1177/1558944717725374
15. Colbourn J, Heath N, Manary S, et al. Effectiveness of splinting for the treatment of trigger finger. J Hand Ther. 2008;21:336-343. doi: 10.1197/j.jht.2008.05.001
16. Teo SH, Ng DCL, Wong YKY. Effectiveness of proximal interphalangeal joint-blocking orthosis vs metacarpophalangeal joint-blocking orthosis in trigger digit management: A randomized clinical trial. J Hand Ther. 2018;32:444-451. doi: 10.1016/j.jht.2018.02.007
17. Ring D, Lozano-Calderon S, Shin R, et al. A prospective randomized controlled trial of injection of dexamethasone versus triamcinolone for idiopathic trigger finger. J Hand Surg Am. 2008;33:516-522; discussion 523-514. doi: 10.1016/j.jhsa.2008.01.001
18. Fleisch SB, Spindler KP, Lee DH. Corticosteroid injections in the treatment of trigger finger: A level I and II systematic review. J Am Acad Orthop Surg. 2007;15:166-171. doi: 10.5435/00124635-200703000-00006
19. Shinomiya R, Sunagawa T, Nakashima Y, et al. Impact of corticosteroid injection site on the treatment success rate of trigger finger: a prospective study comparing ultrasound-guided true intra-sheath and true extra-sheath injections. Ultrasound Med Biol. 2016;42:2203-2208. doi: 10.1016/j.ultrasmedbio.2016.05.015
20. Earp BE, Stanbury SJ, Mora AN, et al. Needle-free jet lidocaine administration for preinjection anesthesia in trigger finger injection: a randomized controlled trial. J Hand Surg Am. 2017;42:618-622. doi: 10.1016/j.jhsa.2017.05.001
21. Castellanos J, Munoz-Mahamud E, Dominguez E, et al. Long-term effectiveness of corticosteroid injections for trigger finger and thumb. J Hand Surg Am. 2015;40:121-126. doi: 10.1016/j.jhsa.2014.09.006
22. Dala-Ali BM, Nakhdjevani A, Lloyd MA, et al. The efficacy of steroid injection in the treatment of trigger finger. Clin Orthop Surg. 2012;4:263-268. doi: 10.4055/cios.2012.4.4.263
23. Griggs SM, Weiss AP, Lane LB, et al. Treatment of trigger finger in patients with diabetes mellitus. J Hand Surg Am. 1995;20:787-789. doi: 10.1016/s0363-5023(05)80432-0
24. Stepan JG, London DA, Boyer MI, et al. Blood glucose levels in diabetic patients following corticosteroid injections into the hand and wrist. J Hand Surg Am. 2014;39:706-712. doi: 10.1016/j.jhsa.2014.01.014
25. Hansen RL, Sondergaard M, Lange J. Open surgery versus ultrasound-guided corticosteroid injection for trigger finger: a randomized controlled trial with 1-year follow-up. J Hand Surg Am. 2017;42:359-366. doi: 10.1016/j.jhsa.2017.02.011
26. Mohd Rashid MZ, Sapuan J, Abdullah S. A randomized controlled trial of trigger finger release under digital anesthesia with (WALANT) and without adrenaline. J Orthop Surg (Hong Kong). 2019;27:2309499019833002. doi: 10.1177/2309499019833002
27. Zhao J-G, Kan S-L, Zhao L, et al. Percutaneous first annular pulley release for trigger digits: a systematic review and meta-analysis of current evidence. J Hand Surg Am. 2014;39:2192-2202. doi: 10.1016/j.jhsa.2014.07.044
28. Guler F, Kose O, Ercan EC, et al. Open versus percutaneous release for the treatment of trigger thumb. Orthopedics. 2013;36:e1290-1294. doi: 10.3928/01477447-20130920-22
29. Wu KC, Chern TC, Jou IM. Ultrasound-assisted percutaneous trigger finger release: it is safe [letter]. Hand (N Y). 2009;4:339. doi: 10.1007/s11552-009-9179-6
30. Nikolaou VS, Malahias M-A, Kaseta M-K, et al. Comparative clinical study of ultrasound-guided A1 pulley release vs open surgical intervention in the treatment of trigger finger. World J Orthop. 2017;8:163-169. doi: 10.5312/wjo.v8.i2.163
31. Lim M-H, Lim K-K, Rasheed MZ, et al. Outcome of open trigger digit release. J Hand Surg Eur Vol. 2007;32:457-459. doi: 10.1016/j.Jhsb.2007.02.016
32. Wang J, Zhao J-G, Liang C-C. Percutaneous release, open surgery, or corticosteroid injection, which is the best treatment method for trigger digits? Clin Orthop Relat Res. 2013;471:1879-1886. doi: 10.1007/s11999-012-2716-6
33. Ng WKY, Olmscheid N, Worhacz K, et al. Steroid injection and open trigger finger release outcomes: a retrospective review of 999 digits. Hand (N Y). 2018;15:399-406. doi: 10.1177/1558944718796559
34. Ho SWL, Chia CY, Rajaratnam V. Characteristics and clinical outcomes of open surgery for trigger digits in diabetes. J Hand Microsurg. 2019;11:80-83. doi: 10.1055/s-0038-1670927
35. Sato ES, dos Santos JB, Belloti JC, et al. Percutaneous release of trigger fingers. Hand Clin. 2014;30:39-45. doi: 10.1016/j.hcl.2013.08.017
1. Lunsford D, Valdes K, Hengy S. Conservative management of trigger finger: a systematic review. J Hand Ther. 2019;32:212-221. doi: 10.1016/j.jht.2017.10.016
2. Makkouk AH, Oetgen ME, Swigart CR, et al. Trigger finger: etiology, evaluation, and treatment. Curr Rev Musculoskelet Med. 2008;1:92-96. doi: 10.1007/s12178-007-9012-1
3. Fitzgibbons PG, Weiss AP. Hand manifestations of diabetes mellitus. J Hand Surg Am. 2008;33:771-775. doi: 10.1016/j.jhsa.2008.01.038
4. Junot HSN, Anderson Hertz AFL, Gustavo Vasconcelos GR, et al. Epidemiology of trigger finger: metabolic syndrome as a new perspective of associated disease. Hand (N Y). 2019:1558944719867135. doi: 10.1177/1558944719867135.
5. Matthews A, Smith K, Read L, et al. Trigger finger: an overview of the treatment options. JAAPA. 2019;32:17-21. doi: 10.1097/01.Jaa.0000550281.42592.97
6. Pencle FJ, Waheed A, Molnar JA. Trigger thumb. StatPearls [Internet]. StatPearls Publishing; 2020. www.ncbi.nlm.nih.gov/books/NBK441854/
7. Giugale JM, Fowler JR. Trigger finger: adult and pediatric treatment strategies. Orthop Clin North Am. 2015;46:561-569. doi: 10.1016/j.ocl.2015.06.014
8. Bianchi S, Gitto S, Draghi F. Ultrasound features of trigger finger: review of the literature. J Ultrasound Med. 2019;38:3141-3154. doi: 10.1002/jum.15025
9. Chuang XL, Ooi CC, Chin ST, et al. What triggers in trigger finger? The flexor tendons at the flexor digitorum superficialis bifurcation. J Plast Reconstr Aesthet Surg. 2017;70:1411-1419. doi: 10.1016/j.bjps.2017.05.037
10. Ryzewicz M, Wolf JM. Trigger digits: principles, management, and complications. J Hand Surg Am. 2006;31:135-146. doi: 10.1016/j.jhsa.2005.10.013
11. Chapter 56: Tendinoapthy. In: Wolfe SW, Peterson WC, Kozin SH, Cohen MS. Green’s Operative Hand Surgery. Vol 2. 7th ed. Elsevier; 2017: 1904-1925.
12. Tarbhai K, Hannah S, von Schroeder HP. Trigger finger treatment: a comparison of 2 splint designs. J Hand Surg Am. 2012;37:243-249, 249.e241. doi: 10.1016/j.jhsa.2011.10.038
13. Valdes K. A retrospective review to determine the long-term efficacy of orthotic devices for trigger finger. J Hand Ther. 2012;25:89-95. doi: 10.1016/j.jht.2011.09.005
14. Drijkoningen T, van Berckel M, Becker SJE, et al. Night splinting for idiopathic trigger digits. Hand (N Y). 2018;13:558-562. doi: 10.1177/1558944717725374
15. Colbourn J, Heath N, Manary S, et al. Effectiveness of splinting for the treatment of trigger finger. J Hand Ther. 2008;21:336-343. doi: 10.1197/j.jht.2008.05.001
16. Teo SH, Ng DCL, Wong YKY. Effectiveness of proximal interphalangeal joint-blocking orthosis vs metacarpophalangeal joint-blocking orthosis in trigger digit management: A randomized clinical trial. J Hand Ther. 2018;32:444-451. doi: 10.1016/j.jht.2018.02.007
17. Ring D, Lozano-Calderon S, Shin R, et al. A prospective randomized controlled trial of injection of dexamethasone versus triamcinolone for idiopathic trigger finger. J Hand Surg Am. 2008;33:516-522; discussion 523-514. doi: 10.1016/j.jhsa.2008.01.001
18. Fleisch SB, Spindler KP, Lee DH. Corticosteroid injections in the treatment of trigger finger: A level I and II systematic review. J Am Acad Orthop Surg. 2007;15:166-171. doi: 10.5435/00124635-200703000-00006
19. Shinomiya R, Sunagawa T, Nakashima Y, et al. Impact of corticosteroid injection site on the treatment success rate of trigger finger: a prospective study comparing ultrasound-guided true intra-sheath and true extra-sheath injections. Ultrasound Med Biol. 2016;42:2203-2208. doi: 10.1016/j.ultrasmedbio.2016.05.015
20. Earp BE, Stanbury SJ, Mora AN, et al. Needle-free jet lidocaine administration for preinjection anesthesia in trigger finger injection: a randomized controlled trial. J Hand Surg Am. 2017;42:618-622. doi: 10.1016/j.jhsa.2017.05.001
21. Castellanos J, Munoz-Mahamud E, Dominguez E, et al. Long-term effectiveness of corticosteroid injections for trigger finger and thumb. J Hand Surg Am. 2015;40:121-126. doi: 10.1016/j.jhsa.2014.09.006
22. Dala-Ali BM, Nakhdjevani A, Lloyd MA, et al. The efficacy of steroid injection in the treatment of trigger finger. Clin Orthop Surg. 2012;4:263-268. doi: 10.4055/cios.2012.4.4.263
23. Griggs SM, Weiss AP, Lane LB, et al. Treatment of trigger finger in patients with diabetes mellitus. J Hand Surg Am. 1995;20:787-789. doi: 10.1016/s0363-5023(05)80432-0
24. Stepan JG, London DA, Boyer MI, et al. Blood glucose levels in diabetic patients following corticosteroid injections into the hand and wrist. J Hand Surg Am. 2014;39:706-712. doi: 10.1016/j.jhsa.2014.01.014
25. Hansen RL, Sondergaard M, Lange J. Open surgery versus ultrasound-guided corticosteroid injection for trigger finger: a randomized controlled trial with 1-year follow-up. J Hand Surg Am. 2017;42:359-366. doi: 10.1016/j.jhsa.2017.02.011
26. Mohd Rashid MZ, Sapuan J, Abdullah S. A randomized controlled trial of trigger finger release under digital anesthesia with (WALANT) and without adrenaline. J Orthop Surg (Hong Kong). 2019;27:2309499019833002. doi: 10.1177/2309499019833002
27. Zhao J-G, Kan S-L, Zhao L, et al. Percutaneous first annular pulley release for trigger digits: a systematic review and meta-analysis of current evidence. J Hand Surg Am. 2014;39:2192-2202. doi: 10.1016/j.jhsa.2014.07.044
28. Guler F, Kose O, Ercan EC, et al. Open versus percutaneous release for the treatment of trigger thumb. Orthopedics. 2013;36:e1290-1294. doi: 10.3928/01477447-20130920-22
29. Wu KC, Chern TC, Jou IM. Ultrasound-assisted percutaneous trigger finger release: it is safe [letter]. Hand (N Y). 2009;4:339. doi: 10.1007/s11552-009-9179-6
30. Nikolaou VS, Malahias M-A, Kaseta M-K, et al. Comparative clinical study of ultrasound-guided A1 pulley release vs open surgical intervention in the treatment of trigger finger. World J Orthop. 2017;8:163-169. doi: 10.5312/wjo.v8.i2.163
31. Lim M-H, Lim K-K, Rasheed MZ, et al. Outcome of open trigger digit release. J Hand Surg Eur Vol. 2007;32:457-459. doi: 10.1016/j.Jhsb.2007.02.016
32. Wang J, Zhao J-G, Liang C-C. Percutaneous release, open surgery, or corticosteroid injection, which is the best treatment method for trigger digits? Clin Orthop Relat Res. 2013;471:1879-1886. doi: 10.1007/s11999-012-2716-6
33. Ng WKY, Olmscheid N, Worhacz K, et al. Steroid injection and open trigger finger release outcomes: a retrospective review of 999 digits. Hand (N Y). 2018;15:399-406. doi: 10.1177/1558944718796559
34. Ho SWL, Chia CY, Rajaratnam V. Characteristics and clinical outcomes of open surgery for trigger digits in diabetes. J Hand Microsurg. 2019;11:80-83. doi: 10.1055/s-0038-1670927
35. Sato ES, dos Santos JB, Belloti JC, et al. Percutaneous release of trigger fingers. Hand Clin. 2014;30:39-45. doi: 10.1016/j.hcl.2013.08.017
PRACTICE RECOMMENDATIONS
› Recommend splinting as a first-line conservative treatment for trigger finger if there is not a fixed contracture. B
› Prescribe corticosteroids, which may completely resolve trigger finger in the majority of patients without diabetes. A
› Refer patients for surgical release if they do not respond to conservative management. The surgical success rate is as high as 99%. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Do carotid artery calcifications seen on radiographs predict stenosis in asymptomatic adults?
EVIDENCE SUMMARY
Mixed results, quality issues do not support screening asymptomatic patients
A meta-analysis (12 observational studies; n = 1002) compared the diagnostic accuracy of
In a retrospective cohort study (n = 778) from the United States, researchers identified carotid artery calcifications on routine dental radiographs in patients ≥ 55 years old and prospectively performed duplex ultrasound (DUS) to assess for significant carotid stenosis (≥ 50%).2 Twenty-seven patients (3.5%) had carotid artery calcifications on radiographs, and 20 of those patients underwent DUS of bilateral carotid arteries (40 sides). Of 26 sides with calcifications on radiograph, 13 (50%) had stenosis confirmed with DUS. Of the 14 sides without calcification on radiograph, 3 (21%) had stenosis on DUS. The positive predictive value for calcification on PR predicting significant carotid stenosis was between 40% and 80%.
In a cross-sectional study from Sweden, investigators sought surgical candidates for asymptomatic carotid endarterectomy and performed PRs of 1182 patients.3 Calcifications were found in 176 people; 117 of them were eligible for asymptomatic carotid endarterectomy (ages 18-74; no cancer or other serious comorbidity; and no prior stroke or transient ischemic attack) and underwent ultrasound to assess for significant carotid stenosis (≥ 50%). Of the 117 participants who underwent ultrasound, 8 (6.8%; 95% CI, 2.2%-11.5%), all men, were found to have significant carotid stenosis. Compared to a sex- and age-matched reference group (n = 119) with no calcifications on PR, the prevalence of carotid stenosis was significantly higher in men (12.5%; 95% CI, 4.2%-20.8%) and in patients who were current smokers (19%; 95% CI, 0.7%-37.4%), were taking cholesterol medications (13.1%; 95% CI 4.4%-21.8%), and had a cardiovascular event history (15.9%; 95% CI, 7%-27.2%).
Recommendations from others
The US Preventive Services Task Force (USPSTF) and the American Academy of Family Physicians do not mention carotid screening with radiographs but recommend against
Editor’s takeaway
If you see calcification of the carotid artery on an x-ray of an asymptomatic patient, ignore it. The positive and negative predictive values for carotid stenosis are poor, and you should not pursue further testing.
1. Schroder AGD, de Araujo CM, Guariza-Filho O, et al. Diagnostic accuracy of panoramic radiography in the detection of calcified carotid artery atheroma: a meta-analysis. Clin Oral Investig. 2019;23:2021-2040. https://doi.org/10.1007/s00784-019-02880-6
2. Almog DM, Horev T, Illig KA, et al. Correlating carotid artery stenosis detected by panoramic radiography with clinically relevant carotid artery stenosis determined by duplex ultrasound. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:768-773. doi: 10.1067/moe.2002.128965
3. Johansson EP, Ahlqvist J, Garoff M, et al. Ultrasound screening for asymptomatic carotid stenosis in subjects with calcifications in the area of the carotid arteries on panoramic radiographs: a cross-sectional study. BMC Cardiovasc Disord. 2011;11:44. doi: 10.1186/1471-2261-11-44
4. USPSTF. Carotid artery stenosis: screening. Updated February 2, 2021. Accessed September 1, 2021. www.uspreventiveservicestaskforce.org/uspstf/recommendation/carotid-artery-stenosis-screening
5. American Academy of Family Physicians. Don’t screen for carotid artery stenosis (CAS) in asymptomatic adult patients. Choosing Wisely website. Published February 21, 2013. Accessed August 29, 2020. www.choosingwisely.org/clinician-lists/american-academy-family-physicians-carotid-artery-stenosis/
EVIDENCE SUMMARY
Mixed results, quality issues do not support screening asymptomatic patients
A meta-analysis (12 observational studies; n = 1002) compared the diagnostic accuracy of
In a retrospective cohort study (n = 778) from the United States, researchers identified carotid artery calcifications on routine dental radiographs in patients ≥ 55 years old and prospectively performed duplex ultrasound (DUS) to assess for significant carotid stenosis (≥ 50%).2 Twenty-seven patients (3.5%) had carotid artery calcifications on radiographs, and 20 of those patients underwent DUS of bilateral carotid arteries (40 sides). Of 26 sides with calcifications on radiograph, 13 (50%) had stenosis confirmed with DUS. Of the 14 sides without calcification on radiograph, 3 (21%) had stenosis on DUS. The positive predictive value for calcification on PR predicting significant carotid stenosis was between 40% and 80%.
In a cross-sectional study from Sweden, investigators sought surgical candidates for asymptomatic carotid endarterectomy and performed PRs of 1182 patients.3 Calcifications were found in 176 people; 117 of them were eligible for asymptomatic carotid endarterectomy (ages 18-74; no cancer or other serious comorbidity; and no prior stroke or transient ischemic attack) and underwent ultrasound to assess for significant carotid stenosis (≥ 50%). Of the 117 participants who underwent ultrasound, 8 (6.8%; 95% CI, 2.2%-11.5%), all men, were found to have significant carotid stenosis. Compared to a sex- and age-matched reference group (n = 119) with no calcifications on PR, the prevalence of carotid stenosis was significantly higher in men (12.5%; 95% CI, 4.2%-20.8%) and in patients who were current smokers (19%; 95% CI, 0.7%-37.4%), were taking cholesterol medications (13.1%; 95% CI 4.4%-21.8%), and had a cardiovascular event history (15.9%; 95% CI, 7%-27.2%).
Recommendations from others
The US Preventive Services Task Force (USPSTF) and the American Academy of Family Physicians do not mention carotid screening with radiographs but recommend against
Editor’s takeaway
If you see calcification of the carotid artery on an x-ray of an asymptomatic patient, ignore it. The positive and negative predictive values for carotid stenosis are poor, and you should not pursue further testing.
EVIDENCE SUMMARY
Mixed results, quality issues do not support screening asymptomatic patients
A meta-analysis (12 observational studies; n = 1002) compared the diagnostic accuracy of
In a retrospective cohort study (n = 778) from the United States, researchers identified carotid artery calcifications on routine dental radiographs in patients ≥ 55 years old and prospectively performed duplex ultrasound (DUS) to assess for significant carotid stenosis (≥ 50%).2 Twenty-seven patients (3.5%) had carotid artery calcifications on radiographs, and 20 of those patients underwent DUS of bilateral carotid arteries (40 sides). Of 26 sides with calcifications on radiograph, 13 (50%) had stenosis confirmed with DUS. Of the 14 sides without calcification on radiograph, 3 (21%) had stenosis on DUS. The positive predictive value for calcification on PR predicting significant carotid stenosis was between 40% and 80%.
In a cross-sectional study from Sweden, investigators sought surgical candidates for asymptomatic carotid endarterectomy and performed PRs of 1182 patients.3 Calcifications were found in 176 people; 117 of them were eligible for asymptomatic carotid endarterectomy (ages 18-74; no cancer or other serious comorbidity; and no prior stroke or transient ischemic attack) and underwent ultrasound to assess for significant carotid stenosis (≥ 50%). Of the 117 participants who underwent ultrasound, 8 (6.8%; 95% CI, 2.2%-11.5%), all men, were found to have significant carotid stenosis. Compared to a sex- and age-matched reference group (n = 119) with no calcifications on PR, the prevalence of carotid stenosis was significantly higher in men (12.5%; 95% CI, 4.2%-20.8%) and in patients who were current smokers (19%; 95% CI, 0.7%-37.4%), were taking cholesterol medications (13.1%; 95% CI 4.4%-21.8%), and had a cardiovascular event history (15.9%; 95% CI, 7%-27.2%).
Recommendations from others
The US Preventive Services Task Force (USPSTF) and the American Academy of Family Physicians do not mention carotid screening with radiographs but recommend against
Editor’s takeaway
If you see calcification of the carotid artery on an x-ray of an asymptomatic patient, ignore it. The positive and negative predictive values for carotid stenosis are poor, and you should not pursue further testing.
1. Schroder AGD, de Araujo CM, Guariza-Filho O, et al. Diagnostic accuracy of panoramic radiography in the detection of calcified carotid artery atheroma: a meta-analysis. Clin Oral Investig. 2019;23:2021-2040. https://doi.org/10.1007/s00784-019-02880-6
2. Almog DM, Horev T, Illig KA, et al. Correlating carotid artery stenosis detected by panoramic radiography with clinically relevant carotid artery stenosis determined by duplex ultrasound. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:768-773. doi: 10.1067/moe.2002.128965
3. Johansson EP, Ahlqvist J, Garoff M, et al. Ultrasound screening for asymptomatic carotid stenosis in subjects with calcifications in the area of the carotid arteries on panoramic radiographs: a cross-sectional study. BMC Cardiovasc Disord. 2011;11:44. doi: 10.1186/1471-2261-11-44
4. USPSTF. Carotid artery stenosis: screening. Updated February 2, 2021. Accessed September 1, 2021. www.uspreventiveservicestaskforce.org/uspstf/recommendation/carotid-artery-stenosis-screening
5. American Academy of Family Physicians. Don’t screen for carotid artery stenosis (CAS) in asymptomatic adult patients. Choosing Wisely website. Published February 21, 2013. Accessed August 29, 2020. www.choosingwisely.org/clinician-lists/american-academy-family-physicians-carotid-artery-stenosis/
1. Schroder AGD, de Araujo CM, Guariza-Filho O, et al. Diagnostic accuracy of panoramic radiography in the detection of calcified carotid artery atheroma: a meta-analysis. Clin Oral Investig. 2019;23:2021-2040. https://doi.org/10.1007/s00784-019-02880-6
2. Almog DM, Horev T, Illig KA, et al. Correlating carotid artery stenosis detected by panoramic radiography with clinically relevant carotid artery stenosis determined by duplex ultrasound. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:768-773. doi: 10.1067/moe.2002.128965
3. Johansson EP, Ahlqvist J, Garoff M, et al. Ultrasound screening for asymptomatic carotid stenosis in subjects with calcifications in the area of the carotid arteries on panoramic radiographs: a cross-sectional study. BMC Cardiovasc Disord. 2011;11:44. doi: 10.1186/1471-2261-11-44
4. USPSTF. Carotid artery stenosis: screening. Updated February 2, 2021. Accessed September 1, 2021. www.uspreventiveservicestaskforce.org/uspstf/recommendation/carotid-artery-stenosis-screening
5. American Academy of Family Physicians. Don’t screen for carotid artery stenosis (CAS) in asymptomatic adult patients. Choosing Wisely website. Published February 21, 2013. Accessed August 29, 2020. www.choosingwisely.org/clinician-lists/american-academy-family-physicians-carotid-artery-stenosis/
EVIDENCE-BASED ANSWER:
Not very well. In asymptomatic patients, carotid artery calcification seen on radiograph has a positive predictive value of 70% and a negative predictive value of 75% for carotid artery stenosis (strength of recommendation [SOR]: B, systematic review of observational studies with heterogeneous results and a retrospective cohort study). Carotid calcifications on radiographs may be more predictive of carotid stenosis in people with atherosclerotic risk factors (SOR: C, cross-sectional study). Harms outweigh benefits in screening for carotid artery stenosis in asymptomatic adults (SOR: B, multiple cohort studies); therefore, incidental radiographic carotid artery calcifications in asymptomatic patients should not prompt further testing.
FDA approves topical ruxolitinib for atopic dermatitis, first JAK inhibitor for this indication in the U.S.
The , making it the first topical JAK inhibitor approved for AD – and the first JAK inhibitor approved for this indication – in the United States.
The approval is limited to patients whose AD is not adequately controlled with topical prescription therapies, or when those therapies are not advisable.
“Approval of topical ruxolitinib fills a major gap in the treatment of atopic dermatitis: a safe, effective, and tolerable non-steroidal topical therapy,” Eric L. Simpson, MD, professor of dermatology and director of the Oregon Health & Science University Dermatology Clinical Research Center, Portland, told this news organization. “This approval will allow for long-term treatment without the concern of steroid side effects. From earlier studies, ruxolitinib cream appears to be as effective as a medium-potency topical steroid. These efficacy levels and low incidence of burning will be a welcome addition to our current nonsteroidal therapies.”
The drug’s approval was based on results from two phase 3, randomized studies of identical design involving 1,249 patients aged 12 years and older with AD: TRuE-AD1 and TRuE-AD2. In these studies, ruxolitinib cream demonstrated anti-inflammatory activity, with rapid and sustained antipruritic action, compared with vehicle. In the trials, patients with an Investigator’s Global Assessment (IGA) score of 2 or 3 and 3%-20% of affected body surface area (BSA) were randomized (2:2:1) to twice-daily 0.75% ruxolitinib cream, 1.5% ruxolitinib cream, or vehicle cream for 8 continuous weeks. The 1.5% concentration was approved by the FDA.
A study first published in May of 2021 found that significantly more patients in TRuE-AD1 and TRuE-AD2 achieved IGA treatment success with 0.75% (50% vs. 39%, respectively) and 1.5% ruxolitinib cream (53.8% vs. 51.3%), compared with vehicle (15.1% vs. 7.6%; P < .0001) at week 8. In addition, significant reductions in itch, compared with vehicle, were reported within 12 hours of first applying 1.5% ruxolitinib cream (P < .05).
More key findings from TRuE-AD1 and TRuE-AD2 are scheduled to be presented during the upcoming European Academy of Dermatology and Venereology meeting Sept. 29-Oct. 2, but during the Revolutionizing Atopic Dermatitis Symposium on June 13, Kim Papp, MD, PhD, presented long-term safety data of ruxolitinib cream in patients who were followed for an additional 44 weeks. Dr. Papp, a dermatologist and founder of Probity Medical Research, Waterloo, Ont., reported that 543 patients from TRuE-AD1 and 530 from TRuE-AD2 entered the long-term analysis and that about 78% of these patients completed the study. From weeks 12 to 52, the proportion of patients with an IGA score of 0 or 1 with 0.75% and 1.5% ruxolitinib cream ranged from 62%-77% and 67%-77%, respectively, in TRuE-AD1, to 60%-77% and 72%-80% in TRuE-AD2.
The measured mean total affected BSA was less than 3% throughout the follow-up period in the 1.5% ruxolitinib cream arm in TRuE-AD1 and TRuE-AD2 and was less than 3% in the 0.75% ruxolitinib cream arm during most of the study period.
In a pooled safety analysis, treatment-emergent adverse events (TEAEs) were reported in 60% and 54% of patients who applied 0.75% and 1.5% ruxolitinib cream, respectively, over 44 weeks. The frequency of application-site reactions remained low. Specifically, treatment-related adverse events were reported in 5% of patients who applied 0.75% ruxolitinib cream and in 3% of patients who applied 1.5% ruxolitinib cream; none were serious. TEAEs led to discontinuation in 2% of patients in the 0.75% ruxolitinib cream group, and no patients in the 1.5% ruxolitinib cream group.
Dr. Papp and his colleagues observed that the most common treatment adverse events were upper respiratory tract infections and nasopharyngitis. According to Incyte’s press release, the most common treatment-emergent adverse reactions in patients treated with ruxolitinib during clinical trials were nasopharyngitis, diarrhea, bronchitis, ear infection, eosinophil count increases, urticaria, folliculitis, tonsillitis, and rhinorrhea. The labeling includes boxed warnings for serious infections, mortality, malignancy, major adverse cardiovascular events, and thrombosis, seen with oral JAK inhibitors for inflammatory conditions.
Incyte will market ruxolitinib under the trade name Opzelura.
Dr. Simpson disclosed that he is a consultant to and/or an investigator for several pharmaceutical companies, including Incyte, Regeneron/Sanofi, Eli Lilly and Company, AbbVie, and Pfizer.
Dr. Papp disclosed that he has received honoraria or clinical research grants as a consultant, speaker, scientific officer, advisory board member, and/or steering committee member for several pharmaceutical companies, including Incyte.
Commentary by Robert Sidbury, MD, MPH
Another nonsteroidal topical medication approved for atopic dermatitis (AD)? Thank goodness. Topical ruxolitinib 1.5% cream twice daily for mild to moderate AD demonstrated excellent efficacy vs. placebo in duplicative trials (53.8/51.3% vs. 15.1%/7.6%; P < .001), with a reassuring safety profile. Application site reactions were uncommon, though past experience with other new nonsteroidal agents suggests judgment be reserved on that score. More compelling was the fact that no patients discontinued therapy in the 1.5% arm, and adverse events were mild and self-limited such as nasopharyngitis and diarrhea. This stands in contradistinction to the boxed warning attached to JAK inhibitors (topical and systemic) against a daunting list of destructive possibilities: malignancy, infection, cardiovascular disease, and blood clots. None of these things was seen in these topical ruxolitinib trials.
Dr. Sidbury is chief of dermatology at Seattle Children's Hospital and professor, department of pediatrics, University of Washington, Seattle. He is a site principal investigator for dupilumab trials, for which the hospital has a contract with Regeneron.
This article was updated 6/16/22.
The , making it the first topical JAK inhibitor approved for AD – and the first JAK inhibitor approved for this indication – in the United States.
The approval is limited to patients whose AD is not adequately controlled with topical prescription therapies, or when those therapies are not advisable.
“Approval of topical ruxolitinib fills a major gap in the treatment of atopic dermatitis: a safe, effective, and tolerable non-steroidal topical therapy,” Eric L. Simpson, MD, professor of dermatology and director of the Oregon Health & Science University Dermatology Clinical Research Center, Portland, told this news organization. “This approval will allow for long-term treatment without the concern of steroid side effects. From earlier studies, ruxolitinib cream appears to be as effective as a medium-potency topical steroid. These efficacy levels and low incidence of burning will be a welcome addition to our current nonsteroidal therapies.”
The drug’s approval was based on results from two phase 3, randomized studies of identical design involving 1,249 patients aged 12 years and older with AD: TRuE-AD1 and TRuE-AD2. In these studies, ruxolitinib cream demonstrated anti-inflammatory activity, with rapid and sustained antipruritic action, compared with vehicle. In the trials, patients with an Investigator’s Global Assessment (IGA) score of 2 or 3 and 3%-20% of affected body surface area (BSA) were randomized (2:2:1) to twice-daily 0.75% ruxolitinib cream, 1.5% ruxolitinib cream, or vehicle cream for 8 continuous weeks. The 1.5% concentration was approved by the FDA.
A study first published in May of 2021 found that significantly more patients in TRuE-AD1 and TRuE-AD2 achieved IGA treatment success with 0.75% (50% vs. 39%, respectively) and 1.5% ruxolitinib cream (53.8% vs. 51.3%), compared with vehicle (15.1% vs. 7.6%; P < .0001) at week 8. In addition, significant reductions in itch, compared with vehicle, were reported within 12 hours of first applying 1.5% ruxolitinib cream (P < .05).
More key findings from TRuE-AD1 and TRuE-AD2 are scheduled to be presented during the upcoming European Academy of Dermatology and Venereology meeting Sept. 29-Oct. 2, but during the Revolutionizing Atopic Dermatitis Symposium on June 13, Kim Papp, MD, PhD, presented long-term safety data of ruxolitinib cream in patients who were followed for an additional 44 weeks. Dr. Papp, a dermatologist and founder of Probity Medical Research, Waterloo, Ont., reported that 543 patients from TRuE-AD1 and 530 from TRuE-AD2 entered the long-term analysis and that about 78% of these patients completed the study. From weeks 12 to 52, the proportion of patients with an IGA score of 0 or 1 with 0.75% and 1.5% ruxolitinib cream ranged from 62%-77% and 67%-77%, respectively, in TRuE-AD1, to 60%-77% and 72%-80% in TRuE-AD2.
The measured mean total affected BSA was less than 3% throughout the follow-up period in the 1.5% ruxolitinib cream arm in TRuE-AD1 and TRuE-AD2 and was less than 3% in the 0.75% ruxolitinib cream arm during most of the study period.
In a pooled safety analysis, treatment-emergent adverse events (TEAEs) were reported in 60% and 54% of patients who applied 0.75% and 1.5% ruxolitinib cream, respectively, over 44 weeks. The frequency of application-site reactions remained low. Specifically, treatment-related adverse events were reported in 5% of patients who applied 0.75% ruxolitinib cream and in 3% of patients who applied 1.5% ruxolitinib cream; none were serious. TEAEs led to discontinuation in 2% of patients in the 0.75% ruxolitinib cream group, and no patients in the 1.5% ruxolitinib cream group.
Dr. Papp and his colleagues observed that the most common treatment adverse events were upper respiratory tract infections and nasopharyngitis. According to Incyte’s press release, the most common treatment-emergent adverse reactions in patients treated with ruxolitinib during clinical trials were nasopharyngitis, diarrhea, bronchitis, ear infection, eosinophil count increases, urticaria, folliculitis, tonsillitis, and rhinorrhea. The labeling includes boxed warnings for serious infections, mortality, malignancy, major adverse cardiovascular events, and thrombosis, seen with oral JAK inhibitors for inflammatory conditions.
Incyte will market ruxolitinib under the trade name Opzelura.
Dr. Simpson disclosed that he is a consultant to and/or an investigator for several pharmaceutical companies, including Incyte, Regeneron/Sanofi, Eli Lilly and Company, AbbVie, and Pfizer.
Dr. Papp disclosed that he has received honoraria or clinical research grants as a consultant, speaker, scientific officer, advisory board member, and/or steering committee member for several pharmaceutical companies, including Incyte.
Commentary by Robert Sidbury, MD, MPH
Another nonsteroidal topical medication approved for atopic dermatitis (AD)? Thank goodness. Topical ruxolitinib 1.5% cream twice daily for mild to moderate AD demonstrated excellent efficacy vs. placebo in duplicative trials (53.8/51.3% vs. 15.1%/7.6%; P < .001), with a reassuring safety profile. Application site reactions were uncommon, though past experience with other new nonsteroidal agents suggests judgment be reserved on that score. More compelling was the fact that no patients discontinued therapy in the 1.5% arm, and adverse events were mild and self-limited such as nasopharyngitis and diarrhea. This stands in contradistinction to the boxed warning attached to JAK inhibitors (topical and systemic) against a daunting list of destructive possibilities: malignancy, infection, cardiovascular disease, and blood clots. None of these things was seen in these topical ruxolitinib trials.
Dr. Sidbury is chief of dermatology at Seattle Children's Hospital and professor, department of pediatrics, University of Washington, Seattle. He is a site principal investigator for dupilumab trials, for which the hospital has a contract with Regeneron.
This article was updated 6/16/22.
The , making it the first topical JAK inhibitor approved for AD – and the first JAK inhibitor approved for this indication – in the United States.
The approval is limited to patients whose AD is not adequately controlled with topical prescription therapies, or when those therapies are not advisable.
“Approval of topical ruxolitinib fills a major gap in the treatment of atopic dermatitis: a safe, effective, and tolerable non-steroidal topical therapy,” Eric L. Simpson, MD, professor of dermatology and director of the Oregon Health & Science University Dermatology Clinical Research Center, Portland, told this news organization. “This approval will allow for long-term treatment without the concern of steroid side effects. From earlier studies, ruxolitinib cream appears to be as effective as a medium-potency topical steroid. These efficacy levels and low incidence of burning will be a welcome addition to our current nonsteroidal therapies.”
The drug’s approval was based on results from two phase 3, randomized studies of identical design involving 1,249 patients aged 12 years and older with AD: TRuE-AD1 and TRuE-AD2. In these studies, ruxolitinib cream demonstrated anti-inflammatory activity, with rapid and sustained antipruritic action, compared with vehicle. In the trials, patients with an Investigator’s Global Assessment (IGA) score of 2 or 3 and 3%-20% of affected body surface area (BSA) were randomized (2:2:1) to twice-daily 0.75% ruxolitinib cream, 1.5% ruxolitinib cream, or vehicle cream for 8 continuous weeks. The 1.5% concentration was approved by the FDA.
A study first published in May of 2021 found that significantly more patients in TRuE-AD1 and TRuE-AD2 achieved IGA treatment success with 0.75% (50% vs. 39%, respectively) and 1.5% ruxolitinib cream (53.8% vs. 51.3%), compared with vehicle (15.1% vs. 7.6%; P < .0001) at week 8. In addition, significant reductions in itch, compared with vehicle, were reported within 12 hours of first applying 1.5% ruxolitinib cream (P < .05).
More key findings from TRuE-AD1 and TRuE-AD2 are scheduled to be presented during the upcoming European Academy of Dermatology and Venereology meeting Sept. 29-Oct. 2, but during the Revolutionizing Atopic Dermatitis Symposium on June 13, Kim Papp, MD, PhD, presented long-term safety data of ruxolitinib cream in patients who were followed for an additional 44 weeks. Dr. Papp, a dermatologist and founder of Probity Medical Research, Waterloo, Ont., reported that 543 patients from TRuE-AD1 and 530 from TRuE-AD2 entered the long-term analysis and that about 78% of these patients completed the study. From weeks 12 to 52, the proportion of patients with an IGA score of 0 or 1 with 0.75% and 1.5% ruxolitinib cream ranged from 62%-77% and 67%-77%, respectively, in TRuE-AD1, to 60%-77% and 72%-80% in TRuE-AD2.
The measured mean total affected BSA was less than 3% throughout the follow-up period in the 1.5% ruxolitinib cream arm in TRuE-AD1 and TRuE-AD2 and was less than 3% in the 0.75% ruxolitinib cream arm during most of the study period.
In a pooled safety analysis, treatment-emergent adverse events (TEAEs) were reported in 60% and 54% of patients who applied 0.75% and 1.5% ruxolitinib cream, respectively, over 44 weeks. The frequency of application-site reactions remained low. Specifically, treatment-related adverse events were reported in 5% of patients who applied 0.75% ruxolitinib cream and in 3% of patients who applied 1.5% ruxolitinib cream; none were serious. TEAEs led to discontinuation in 2% of patients in the 0.75% ruxolitinib cream group, and no patients in the 1.5% ruxolitinib cream group.
Dr. Papp and his colleagues observed that the most common treatment adverse events were upper respiratory tract infections and nasopharyngitis. According to Incyte’s press release, the most common treatment-emergent adverse reactions in patients treated with ruxolitinib during clinical trials were nasopharyngitis, diarrhea, bronchitis, ear infection, eosinophil count increases, urticaria, folliculitis, tonsillitis, and rhinorrhea. The labeling includes boxed warnings for serious infections, mortality, malignancy, major adverse cardiovascular events, and thrombosis, seen with oral JAK inhibitors for inflammatory conditions.
Incyte will market ruxolitinib under the trade name Opzelura.
Dr. Simpson disclosed that he is a consultant to and/or an investigator for several pharmaceutical companies, including Incyte, Regeneron/Sanofi, Eli Lilly and Company, AbbVie, and Pfizer.
Dr. Papp disclosed that he has received honoraria or clinical research grants as a consultant, speaker, scientific officer, advisory board member, and/or steering committee member for several pharmaceutical companies, including Incyte.
Commentary by Robert Sidbury, MD, MPH
Another nonsteroidal topical medication approved for atopic dermatitis (AD)? Thank goodness. Topical ruxolitinib 1.5% cream twice daily for mild to moderate AD demonstrated excellent efficacy vs. placebo in duplicative trials (53.8/51.3% vs. 15.1%/7.6%; P < .001), with a reassuring safety profile. Application site reactions were uncommon, though past experience with other new nonsteroidal agents suggests judgment be reserved on that score. More compelling was the fact that no patients discontinued therapy in the 1.5% arm, and adverse events were mild and self-limited such as nasopharyngitis and diarrhea. This stands in contradistinction to the boxed warning attached to JAK inhibitors (topical and systemic) against a daunting list of destructive possibilities: malignancy, infection, cardiovascular disease, and blood clots. None of these things was seen in these topical ruxolitinib trials.
Dr. Sidbury is chief of dermatology at Seattle Children's Hospital and professor, department of pediatrics, University of Washington, Seattle. He is a site principal investigator for dupilumab trials, for which the hospital has a contract with Regeneron.
This article was updated 6/16/22.
Supreme Court sets date for case that challenges Roe v. Wade
The Supreme Court will hear arguments in a major Mississippi abortion case on Dec. 1, which could challenge the landmark Roe v. Wade decision that guarantees a woman’s right to an abortion.
On Sept. 20, the court issued its calendar for arguments that will be heard in late November and early December, The Associated Press reported.
The Mississippi case, Dobbs v. Jackson Women’s Health Organization, is seeking to overturn Roe v. Wade by asking the Supreme Court to uphold a ban on most abortions after the 15th week of pregnancy. The state also said the court should overrule the 1992 decision in Planned Parenthood v. Casey that prevents states from banning abortion before viability, which is around 24 weeks of pregnancy.
Earlier in September, the Supreme Court allowed a Texas law to take effect that bans abortions after cardiac activity can be detected, which is around 6 weeks of pregnancy and often before many women know they’re pregnant. The court, which was split 5-4, didn’t rule on the constitutional nature of the law, instead declining to block its enforcement.
Hundreds of legal briefs have been filed on both sides of the case, the AP reported. On Sept. 20, more than 500 women athletes, including members of the Women’s National Basketball Players Association, the National Women’s Soccer League Players Association, and Olympic medalists, filed a brief that said an abortion ban would be devastating for female athletes.
The Mississippi law was enacted in 2018 but was blocked after a challenge at the federal court level. The state’s only abortion clinic, Jackson Women’s Health Organization, remains open and offers abortions up to 16 weeks of pregnancy, the AP reported. About 100 abortions a year are completed after 15 weeks, the organization said.
More than 90% of abortions in the United States occur in the first 13 weeks of pregnancy, the AP said.
The Supreme Court justices will return to the courtroom in October to hear arguments now that all of them have been vaccinated, the AP reported. The justices had been hearing cases by phone during the pandemic.
The public won’t be able to attend sessions, but the court will allow live audio of the session.
A version of this article first appeared on WebMD.com.
The Supreme Court will hear arguments in a major Mississippi abortion case on Dec. 1, which could challenge the landmark Roe v. Wade decision that guarantees a woman’s right to an abortion.
On Sept. 20, the court issued its calendar for arguments that will be heard in late November and early December, The Associated Press reported.
The Mississippi case, Dobbs v. Jackson Women’s Health Organization, is seeking to overturn Roe v. Wade by asking the Supreme Court to uphold a ban on most abortions after the 15th week of pregnancy. The state also said the court should overrule the 1992 decision in Planned Parenthood v. Casey that prevents states from banning abortion before viability, which is around 24 weeks of pregnancy.
Earlier in September, the Supreme Court allowed a Texas law to take effect that bans abortions after cardiac activity can be detected, which is around 6 weeks of pregnancy and often before many women know they’re pregnant. The court, which was split 5-4, didn’t rule on the constitutional nature of the law, instead declining to block its enforcement.
Hundreds of legal briefs have been filed on both sides of the case, the AP reported. On Sept. 20, more than 500 women athletes, including members of the Women’s National Basketball Players Association, the National Women’s Soccer League Players Association, and Olympic medalists, filed a brief that said an abortion ban would be devastating for female athletes.
The Mississippi law was enacted in 2018 but was blocked after a challenge at the federal court level. The state’s only abortion clinic, Jackson Women’s Health Organization, remains open and offers abortions up to 16 weeks of pregnancy, the AP reported. About 100 abortions a year are completed after 15 weeks, the organization said.
More than 90% of abortions in the United States occur in the first 13 weeks of pregnancy, the AP said.
The Supreme Court justices will return to the courtroom in October to hear arguments now that all of them have been vaccinated, the AP reported. The justices had been hearing cases by phone during the pandemic.
The public won’t be able to attend sessions, but the court will allow live audio of the session.
A version of this article first appeared on WebMD.com.
The Supreme Court will hear arguments in a major Mississippi abortion case on Dec. 1, which could challenge the landmark Roe v. Wade decision that guarantees a woman’s right to an abortion.
On Sept. 20, the court issued its calendar for arguments that will be heard in late November and early December, The Associated Press reported.
The Mississippi case, Dobbs v. Jackson Women’s Health Organization, is seeking to overturn Roe v. Wade by asking the Supreme Court to uphold a ban on most abortions after the 15th week of pregnancy. The state also said the court should overrule the 1992 decision in Planned Parenthood v. Casey that prevents states from banning abortion before viability, which is around 24 weeks of pregnancy.
Earlier in September, the Supreme Court allowed a Texas law to take effect that bans abortions after cardiac activity can be detected, which is around 6 weeks of pregnancy and often before many women know they’re pregnant. The court, which was split 5-4, didn’t rule on the constitutional nature of the law, instead declining to block its enforcement.
Hundreds of legal briefs have been filed on both sides of the case, the AP reported. On Sept. 20, more than 500 women athletes, including members of the Women’s National Basketball Players Association, the National Women’s Soccer League Players Association, and Olympic medalists, filed a brief that said an abortion ban would be devastating for female athletes.
The Mississippi law was enacted in 2018 but was blocked after a challenge at the federal court level. The state’s only abortion clinic, Jackson Women’s Health Organization, remains open and offers abortions up to 16 weeks of pregnancy, the AP reported. About 100 abortions a year are completed after 15 weeks, the organization said.
More than 90% of abortions in the United States occur in the first 13 weeks of pregnancy, the AP said.
The Supreme Court justices will return to the courtroom in October to hear arguments now that all of them have been vaccinated, the AP reported. The justices had been hearing cases by phone during the pandemic.
The public won’t be able to attend sessions, but the court will allow live audio of the session.
A version of this article first appeared on WebMD.com.
Most community-based oncologists skip biomarker testing
A recent survey shows that fewer than half of community oncologists use biomarker testing to guide patient discussions about treatment, which compares with 73% of academic clinicians.
The findings, reported at the 2020 World Conference on Lung Cancer, which was rescheduled for January 2021, highlight the potential for unequal application of the latest advances in cancer genomics and targeted therapies throughout the health care system, which could worsen existing disparities in underserved populations, according to Leigh Boehmer, PharmD, medical director for the Association of Community Cancer Centers, Rockville, Md.
The survey – a mixed-methods approach for assessing practice patterns, attitudes, barriers, and resource needs related to biomarker testing among clinicians – was developed by the ACCC in partnership with the LUNGevity Foundation and administered to clinicians caring for patients with non–small cell lung cancer who are uninsured or covered by Medicaid.
Of 99 respondents, more than 85% were physicians and 68% worked in a community setting. Only 40% indicated they were very familiar or extremely familiar with 2018 Molecular Testing Guidelines for Lung Cancer from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology.
Clinicians were most confident about selecting appropriate tests to use, interpreting test results, and prognosticating based on test results, with 77%, 74%, and 74%, respectively, saying they are very confident or extremely confident in those areas. They were less confident about determining when to order testing and in coordinating care across the multidisciplinary team, with 59% and 64%, respectively, saying they were very confident or extremely confident in those areas, Dr. Boehmer reported at the conference.
The shortcomings with respect to communication across teams were echoed in two focus groups convened to further validate the survey results, he noted.
As for the reasons why clinicians ordered biomarker testing, 88% and 82% of community and academic clinicians, respectively, said they did so to help make targeted treatment decisions.
“Only 48% of community clinicians indicated that they use biomarker testing to guide patient discussions, compared to 73% of academic clinicians,” he said. “That finding was considered statistically significant.”
With respect to decision-making about biomarker testing, 41% said they prefer to share the responsibility with patients, whereas 52% said they prefer to make the final decision.
“Shedding further light on this situation, focus group participants expressed that patients lacked comprehension and interest about what testing entails and what testing means for their treatment options,” Dr. Boehmer noted.
In order to make more informed decisions about biomarker testing, respondents said they need more information on financial resources for patient assistance (26%) and education around both published guidelines and practical implications of the clinical data (21%).
When asked about patients’ information needs, 23% said their patients need psychosocial support, 22% said they need financial assistance, and 9% said their patients have no additional resource needs.
However, only 27% said they provide patients with resources related to psychosocial support services, and only 44% share financial assistance information, he said.
Further, the fact that 9% said their patients need no additional resources represents “a disconnect” from the findings of the survey and focus groups, he added.
“We believe that this study identifies key areas of ongoing clinician need related to biomarker testing, including things like increased guideline familiarity, practical applications of guideline-concordant testing, and … how to optimally coordinate multidisciplinary care delivery,” Dr. Boehmer said. “Professional organizations … in partnership with patient advocacy organizations or groups should focus on developing those patient education materials … and tools for improving patient-clinician discussions about biomarker testing.”
The ACCC will be working with the LUNGevity Foundation and the Center for Business Models in Healthcare to develop an intervention to ensure that such discussions are “easily integrated into the care process for every patient,” he noted.
Such efforts are important for ensuring that clinicians are informed about the value of biomarker testing and about guidelines for testing so that patients receive the best possible care, said invited discussant Joshua Sabari, MD, of New York University Langone Health’s Perlmutter Cancer Center.
“I know that, in clinic, when meeting a new patient with non–small cell lung cancer, it’s critical to understand the driver alteration, not only for prognosis, but also for goals-of-care discussion, as well as potential treatment option,” Dr. Sabari said.
Dr. Boehmer reported consulting for Pfizer. Dr. Sabari reported consulting and advisory board membership for multiple pharmaceutical companies.
A recent survey shows that fewer than half of community oncologists use biomarker testing to guide patient discussions about treatment, which compares with 73% of academic clinicians.
The findings, reported at the 2020 World Conference on Lung Cancer, which was rescheduled for January 2021, highlight the potential for unequal application of the latest advances in cancer genomics and targeted therapies throughout the health care system, which could worsen existing disparities in underserved populations, according to Leigh Boehmer, PharmD, medical director for the Association of Community Cancer Centers, Rockville, Md.
The survey – a mixed-methods approach for assessing practice patterns, attitudes, barriers, and resource needs related to biomarker testing among clinicians – was developed by the ACCC in partnership with the LUNGevity Foundation and administered to clinicians caring for patients with non–small cell lung cancer who are uninsured or covered by Medicaid.
Of 99 respondents, more than 85% were physicians and 68% worked in a community setting. Only 40% indicated they were very familiar or extremely familiar with 2018 Molecular Testing Guidelines for Lung Cancer from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology.
Clinicians were most confident about selecting appropriate tests to use, interpreting test results, and prognosticating based on test results, with 77%, 74%, and 74%, respectively, saying they are very confident or extremely confident in those areas. They were less confident about determining when to order testing and in coordinating care across the multidisciplinary team, with 59% and 64%, respectively, saying they were very confident or extremely confident in those areas, Dr. Boehmer reported at the conference.
The shortcomings with respect to communication across teams were echoed in two focus groups convened to further validate the survey results, he noted.
As for the reasons why clinicians ordered biomarker testing, 88% and 82% of community and academic clinicians, respectively, said they did so to help make targeted treatment decisions.
“Only 48% of community clinicians indicated that they use biomarker testing to guide patient discussions, compared to 73% of academic clinicians,” he said. “That finding was considered statistically significant.”
With respect to decision-making about biomarker testing, 41% said they prefer to share the responsibility with patients, whereas 52% said they prefer to make the final decision.
“Shedding further light on this situation, focus group participants expressed that patients lacked comprehension and interest about what testing entails and what testing means for their treatment options,” Dr. Boehmer noted.
In order to make more informed decisions about biomarker testing, respondents said they need more information on financial resources for patient assistance (26%) and education around both published guidelines and practical implications of the clinical data (21%).
When asked about patients’ information needs, 23% said their patients need psychosocial support, 22% said they need financial assistance, and 9% said their patients have no additional resource needs.
However, only 27% said they provide patients with resources related to psychosocial support services, and only 44% share financial assistance information, he said.
Further, the fact that 9% said their patients need no additional resources represents “a disconnect” from the findings of the survey and focus groups, he added.
“We believe that this study identifies key areas of ongoing clinician need related to biomarker testing, including things like increased guideline familiarity, practical applications of guideline-concordant testing, and … how to optimally coordinate multidisciplinary care delivery,” Dr. Boehmer said. “Professional organizations … in partnership with patient advocacy organizations or groups should focus on developing those patient education materials … and tools for improving patient-clinician discussions about biomarker testing.”
The ACCC will be working with the LUNGevity Foundation and the Center for Business Models in Healthcare to develop an intervention to ensure that such discussions are “easily integrated into the care process for every patient,” he noted.
Such efforts are important for ensuring that clinicians are informed about the value of biomarker testing and about guidelines for testing so that patients receive the best possible care, said invited discussant Joshua Sabari, MD, of New York University Langone Health’s Perlmutter Cancer Center.
“I know that, in clinic, when meeting a new patient with non–small cell lung cancer, it’s critical to understand the driver alteration, not only for prognosis, but also for goals-of-care discussion, as well as potential treatment option,” Dr. Sabari said.
Dr. Boehmer reported consulting for Pfizer. Dr. Sabari reported consulting and advisory board membership for multiple pharmaceutical companies.
A recent survey shows that fewer than half of community oncologists use biomarker testing to guide patient discussions about treatment, which compares with 73% of academic clinicians.
The findings, reported at the 2020 World Conference on Lung Cancer, which was rescheduled for January 2021, highlight the potential for unequal application of the latest advances in cancer genomics and targeted therapies throughout the health care system, which could worsen existing disparities in underserved populations, according to Leigh Boehmer, PharmD, medical director for the Association of Community Cancer Centers, Rockville, Md.
The survey – a mixed-methods approach for assessing practice patterns, attitudes, barriers, and resource needs related to biomarker testing among clinicians – was developed by the ACCC in partnership with the LUNGevity Foundation and administered to clinicians caring for patients with non–small cell lung cancer who are uninsured or covered by Medicaid.
Of 99 respondents, more than 85% were physicians and 68% worked in a community setting. Only 40% indicated they were very familiar or extremely familiar with 2018 Molecular Testing Guidelines for Lung Cancer from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology.
Clinicians were most confident about selecting appropriate tests to use, interpreting test results, and prognosticating based on test results, with 77%, 74%, and 74%, respectively, saying they are very confident or extremely confident in those areas. They were less confident about determining when to order testing and in coordinating care across the multidisciplinary team, with 59% and 64%, respectively, saying they were very confident or extremely confident in those areas, Dr. Boehmer reported at the conference.
The shortcomings with respect to communication across teams were echoed in two focus groups convened to further validate the survey results, he noted.
As for the reasons why clinicians ordered biomarker testing, 88% and 82% of community and academic clinicians, respectively, said they did so to help make targeted treatment decisions.
“Only 48% of community clinicians indicated that they use biomarker testing to guide patient discussions, compared to 73% of academic clinicians,” he said. “That finding was considered statistically significant.”
With respect to decision-making about biomarker testing, 41% said they prefer to share the responsibility with patients, whereas 52% said they prefer to make the final decision.
“Shedding further light on this situation, focus group participants expressed that patients lacked comprehension and interest about what testing entails and what testing means for their treatment options,” Dr. Boehmer noted.
In order to make more informed decisions about biomarker testing, respondents said they need more information on financial resources for patient assistance (26%) and education around both published guidelines and practical implications of the clinical data (21%).
When asked about patients’ information needs, 23% said their patients need psychosocial support, 22% said they need financial assistance, and 9% said their patients have no additional resource needs.
However, only 27% said they provide patients with resources related to psychosocial support services, and only 44% share financial assistance information, he said.
Further, the fact that 9% said their patients need no additional resources represents “a disconnect” from the findings of the survey and focus groups, he added.
“We believe that this study identifies key areas of ongoing clinician need related to biomarker testing, including things like increased guideline familiarity, practical applications of guideline-concordant testing, and … how to optimally coordinate multidisciplinary care delivery,” Dr. Boehmer said. “Professional organizations … in partnership with patient advocacy organizations or groups should focus on developing those patient education materials … and tools for improving patient-clinician discussions about biomarker testing.”
The ACCC will be working with the LUNGevity Foundation and the Center for Business Models in Healthcare to develop an intervention to ensure that such discussions are “easily integrated into the care process for every patient,” he noted.
Such efforts are important for ensuring that clinicians are informed about the value of biomarker testing and about guidelines for testing so that patients receive the best possible care, said invited discussant Joshua Sabari, MD, of New York University Langone Health’s Perlmutter Cancer Center.
“I know that, in clinic, when meeting a new patient with non–small cell lung cancer, it’s critical to understand the driver alteration, not only for prognosis, but also for goals-of-care discussion, as well as potential treatment option,” Dr. Sabari said.
Dr. Boehmer reported consulting for Pfizer. Dr. Sabari reported consulting and advisory board membership for multiple pharmaceutical companies.
REPORTING FROM WCLC 2020
Nurses ‘at the breaking point,’ consider quitting due to COVID issues: Survey
In the best of times, critical care nurses have one of the most difficult and stressful jobs in health care. The COVID-19 pandemic has made that immeasurably worse. As hospitals have been flooded with critically ill patients, nurses have been overwhelmed.
“What we’re hearing from our nurses is really shocking,” Amanda Bettencourt, PhD, APRN, CCRN-K, president-elect of the American Association of Critical-Care Nurses (AACN), told this news organization. “They’re saying they’re at the breaking point.”
Between August 26 and August 30, the AACN surveyed more than 6,000 critical care nurses, zeroing in on four key questions regarding the pandemic and its impact on nursing. The results were alarming – not only with regard to individual nurses but also for the nursing profession and the future of health care. A full 66% of those surveyed said their experiences during the pandemic have caused them to consider leaving nursing. The respondents’ take on their colleagues was even more concerning. Ninety-two percent agreed with the following two statements: “I believe the pandemic has depleted nurses at my hospital. Their careers will be shorter than they intended.”
“This puts the entire health care system at risk,” says Dr. Bettencourt, who is assistant professor in the department of family and community health at the University of Pennsylvania School of Nursing, Philadelphia. Intensive care unit (ICU) nurses are highly trained and are skilled in caring for critically ill patients with complex medical needs. “It’s not easy to replace a critical care nurse when one leaves,” she says.
And when nurses leave, patients suffer, says Beth Wathen, MSN, RN, CCRN-K, president of the ACCN and frontline nurse at Children’s Hospital Colorado, Aurora. “Hospitals can have all the beds and all the rooms and all the equipment they want, but without nurses and others at the front lines to provide that essential care, none of it really matters, whether we’re talking about caring for COVID patients or caring for patients with other health ailments.”
Heartbreak of the unvaccinated
The problem is not just overwork because of the flood of COVID-19 patients. The emotional strain is enormous as well. “What’s demoralizing for us is not that patients are sick and that it’s physically exhausting to take care of sick patients. We’re used to that,” says Dr. Bettencourt.
But few nurses have experienced the sheer magnitude of patients caused by this pandemic. “The past 18 months have been grueling,” says Ms. Wathen. “The burden on frontline caregivers and our nurses at the front line has been immense.”
The situation is made worse by how unnecessary much of the suffering is at this point. Seventy-six percent of the survey’s respondents agreed with the following statement: “People who hold out on getting vaccinated undermine nurses’ physical and mental well-being.” That comment doesn’t convey the nature or extent of the effect on caregivers’ well-being. “That 9 out of 10 of the people we’re seeing in ICU right now are unvaccinated just adds to the sense of heartbreak and frustration,” says Ms. Wathen. “These deaths don’t have to be happening right now. And that’s hard to bear witness to.”
The politicization of public health has also taken a toll. “That’s been the hard part of this entire pandemic,” says Ms. Wathen. “This really isn’t at all about politics. This is about your health; this is about my health. This is about our collective health as a community and as a country.”
Like the rest of the world, nurses are also concerned about their own loved ones. The survey statement, “I fear taking care of patients with COVID puts my family’s health at risk,” garnered 67% agreement. Ms. Wathen points out that nurses take the appropriate precautions but still worry about taking infection home to their families. “This disease is a tricky one,” she says. She points out that until this pandemic is over, in addition to being vaccinated, nurses and the public still need to be vigilant about wearing masks, social distancing, and taking other precautions to ensure the safety of us all. “Our individual decisions don’t just affect ourselves. They affect our family, the people in our circle, and the people in our community,” she says.
Avoiding a professional exodus
It’s too early yet to have reliable national data on how many nurses have already left their jobs because of COVID-19, but it is clear that there are too few nurses of all kinds. Earlier this month, the American Nurses Association sent a letter to the U.S. Secretary of Health and Human Services urging the agency to declare the nursing shortage a crisis and to take immediate steps to find solutions.
The nursing shortage predates the pandemic, and COVID-19 has brought a simmering problem to a boil. Nurses are calling on the public and the health care system for help. From inside the industry, the needs are pretty much what they were before the pandemic. Dr. Bettencourt and Ms. Wathen point to the need for supportive leadership, healthy work environments, sufficient staffing to meet patients’ needs, and a voice in decisions, such as decisions about staffing, that affect nurses and their patients. Nurses want to be heard and appreciated. “It’s not that these are new things,” says Dr. Bettencourt. “We just need them even more now because we’re stressed even more than we were before.”
Critical care nurses have a different request of the public. They’re asking – pleading, actually – with the public to get vaccinated, wear masks in public, practice social distancing, and bring this pandemic to an end.
“COVID kills, and it’s a really difficult, tragic, and lonely death,” says Ms. Wathen. “We’ve witnessed hundreds of thousands of those deaths. But now we have a way to stop it. If many more people get vaccinated, we can stop this pandemic. And hopefully that will stop this current trend of nurses leaving.”
A version of this article first appeared on Medscape.com.
In the best of times, critical care nurses have one of the most difficult and stressful jobs in health care. The COVID-19 pandemic has made that immeasurably worse. As hospitals have been flooded with critically ill patients, nurses have been overwhelmed.
“What we’re hearing from our nurses is really shocking,” Amanda Bettencourt, PhD, APRN, CCRN-K, president-elect of the American Association of Critical-Care Nurses (AACN), told this news organization. “They’re saying they’re at the breaking point.”
Between August 26 and August 30, the AACN surveyed more than 6,000 critical care nurses, zeroing in on four key questions regarding the pandemic and its impact on nursing. The results were alarming – not only with regard to individual nurses but also for the nursing profession and the future of health care. A full 66% of those surveyed said their experiences during the pandemic have caused them to consider leaving nursing. The respondents’ take on their colleagues was even more concerning. Ninety-two percent agreed with the following two statements: “I believe the pandemic has depleted nurses at my hospital. Their careers will be shorter than they intended.”
“This puts the entire health care system at risk,” says Dr. Bettencourt, who is assistant professor in the department of family and community health at the University of Pennsylvania School of Nursing, Philadelphia. Intensive care unit (ICU) nurses are highly trained and are skilled in caring for critically ill patients with complex medical needs. “It’s not easy to replace a critical care nurse when one leaves,” she says.
And when nurses leave, patients suffer, says Beth Wathen, MSN, RN, CCRN-K, president of the ACCN and frontline nurse at Children’s Hospital Colorado, Aurora. “Hospitals can have all the beds and all the rooms and all the equipment they want, but without nurses and others at the front lines to provide that essential care, none of it really matters, whether we’re talking about caring for COVID patients or caring for patients with other health ailments.”
Heartbreak of the unvaccinated
The problem is not just overwork because of the flood of COVID-19 patients. The emotional strain is enormous as well. “What’s demoralizing for us is not that patients are sick and that it’s physically exhausting to take care of sick patients. We’re used to that,” says Dr. Bettencourt.
But few nurses have experienced the sheer magnitude of patients caused by this pandemic. “The past 18 months have been grueling,” says Ms. Wathen. “The burden on frontline caregivers and our nurses at the front line has been immense.”
The situation is made worse by how unnecessary much of the suffering is at this point. Seventy-six percent of the survey’s respondents agreed with the following statement: “People who hold out on getting vaccinated undermine nurses’ physical and mental well-being.” That comment doesn’t convey the nature or extent of the effect on caregivers’ well-being. “That 9 out of 10 of the people we’re seeing in ICU right now are unvaccinated just adds to the sense of heartbreak and frustration,” says Ms. Wathen. “These deaths don’t have to be happening right now. And that’s hard to bear witness to.”
The politicization of public health has also taken a toll. “That’s been the hard part of this entire pandemic,” says Ms. Wathen. “This really isn’t at all about politics. This is about your health; this is about my health. This is about our collective health as a community and as a country.”
Like the rest of the world, nurses are also concerned about their own loved ones. The survey statement, “I fear taking care of patients with COVID puts my family’s health at risk,” garnered 67% agreement. Ms. Wathen points out that nurses take the appropriate precautions but still worry about taking infection home to their families. “This disease is a tricky one,” she says. She points out that until this pandemic is over, in addition to being vaccinated, nurses and the public still need to be vigilant about wearing masks, social distancing, and taking other precautions to ensure the safety of us all. “Our individual decisions don’t just affect ourselves. They affect our family, the people in our circle, and the people in our community,” she says.
Avoiding a professional exodus
It’s too early yet to have reliable national data on how many nurses have already left their jobs because of COVID-19, but it is clear that there are too few nurses of all kinds. Earlier this month, the American Nurses Association sent a letter to the U.S. Secretary of Health and Human Services urging the agency to declare the nursing shortage a crisis and to take immediate steps to find solutions.
The nursing shortage predates the pandemic, and COVID-19 has brought a simmering problem to a boil. Nurses are calling on the public and the health care system for help. From inside the industry, the needs are pretty much what they were before the pandemic. Dr. Bettencourt and Ms. Wathen point to the need for supportive leadership, healthy work environments, sufficient staffing to meet patients’ needs, and a voice in decisions, such as decisions about staffing, that affect nurses and their patients. Nurses want to be heard and appreciated. “It’s not that these are new things,” says Dr. Bettencourt. “We just need them even more now because we’re stressed even more than we were before.”
Critical care nurses have a different request of the public. They’re asking – pleading, actually – with the public to get vaccinated, wear masks in public, practice social distancing, and bring this pandemic to an end.
“COVID kills, and it’s a really difficult, tragic, and lonely death,” says Ms. Wathen. “We’ve witnessed hundreds of thousands of those deaths. But now we have a way to stop it. If many more people get vaccinated, we can stop this pandemic. And hopefully that will stop this current trend of nurses leaving.”
A version of this article first appeared on Medscape.com.
In the best of times, critical care nurses have one of the most difficult and stressful jobs in health care. The COVID-19 pandemic has made that immeasurably worse. As hospitals have been flooded with critically ill patients, nurses have been overwhelmed.
“What we’re hearing from our nurses is really shocking,” Amanda Bettencourt, PhD, APRN, CCRN-K, president-elect of the American Association of Critical-Care Nurses (AACN), told this news organization. “They’re saying they’re at the breaking point.”
Between August 26 and August 30, the AACN surveyed more than 6,000 critical care nurses, zeroing in on four key questions regarding the pandemic and its impact on nursing. The results were alarming – not only with regard to individual nurses but also for the nursing profession and the future of health care. A full 66% of those surveyed said their experiences during the pandemic have caused them to consider leaving nursing. The respondents’ take on their colleagues was even more concerning. Ninety-two percent agreed with the following two statements: “I believe the pandemic has depleted nurses at my hospital. Their careers will be shorter than they intended.”
“This puts the entire health care system at risk,” says Dr. Bettencourt, who is assistant professor in the department of family and community health at the University of Pennsylvania School of Nursing, Philadelphia. Intensive care unit (ICU) nurses are highly trained and are skilled in caring for critically ill patients with complex medical needs. “It’s not easy to replace a critical care nurse when one leaves,” she says.
And when nurses leave, patients suffer, says Beth Wathen, MSN, RN, CCRN-K, president of the ACCN and frontline nurse at Children’s Hospital Colorado, Aurora. “Hospitals can have all the beds and all the rooms and all the equipment they want, but without nurses and others at the front lines to provide that essential care, none of it really matters, whether we’re talking about caring for COVID patients or caring for patients with other health ailments.”
Heartbreak of the unvaccinated
The problem is not just overwork because of the flood of COVID-19 patients. The emotional strain is enormous as well. “What’s demoralizing for us is not that patients are sick and that it’s physically exhausting to take care of sick patients. We’re used to that,” says Dr. Bettencourt.
But few nurses have experienced the sheer magnitude of patients caused by this pandemic. “The past 18 months have been grueling,” says Ms. Wathen. “The burden on frontline caregivers and our nurses at the front line has been immense.”
The situation is made worse by how unnecessary much of the suffering is at this point. Seventy-six percent of the survey’s respondents agreed with the following statement: “People who hold out on getting vaccinated undermine nurses’ physical and mental well-being.” That comment doesn’t convey the nature or extent of the effect on caregivers’ well-being. “That 9 out of 10 of the people we’re seeing in ICU right now are unvaccinated just adds to the sense of heartbreak and frustration,” says Ms. Wathen. “These deaths don’t have to be happening right now. And that’s hard to bear witness to.”
The politicization of public health has also taken a toll. “That’s been the hard part of this entire pandemic,” says Ms. Wathen. “This really isn’t at all about politics. This is about your health; this is about my health. This is about our collective health as a community and as a country.”
Like the rest of the world, nurses are also concerned about their own loved ones. The survey statement, “I fear taking care of patients with COVID puts my family’s health at risk,” garnered 67% agreement. Ms. Wathen points out that nurses take the appropriate precautions but still worry about taking infection home to their families. “This disease is a tricky one,” she says. She points out that until this pandemic is over, in addition to being vaccinated, nurses and the public still need to be vigilant about wearing masks, social distancing, and taking other precautions to ensure the safety of us all. “Our individual decisions don’t just affect ourselves. They affect our family, the people in our circle, and the people in our community,” she says.
Avoiding a professional exodus
It’s too early yet to have reliable national data on how many nurses have already left their jobs because of COVID-19, but it is clear that there are too few nurses of all kinds. Earlier this month, the American Nurses Association sent a letter to the U.S. Secretary of Health and Human Services urging the agency to declare the nursing shortage a crisis and to take immediate steps to find solutions.
The nursing shortage predates the pandemic, and COVID-19 has brought a simmering problem to a boil. Nurses are calling on the public and the health care system for help. From inside the industry, the needs are pretty much what they were before the pandemic. Dr. Bettencourt and Ms. Wathen point to the need for supportive leadership, healthy work environments, sufficient staffing to meet patients’ needs, and a voice in decisions, such as decisions about staffing, that affect nurses and their patients. Nurses want to be heard and appreciated. “It’s not that these are new things,” says Dr. Bettencourt. “We just need them even more now because we’re stressed even more than we were before.”
Critical care nurses have a different request of the public. They’re asking – pleading, actually – with the public to get vaccinated, wear masks in public, practice social distancing, and bring this pandemic to an end.
“COVID kills, and it’s a really difficult, tragic, and lonely death,” says Ms. Wathen. “We’ve witnessed hundreds of thousands of those deaths. But now we have a way to stop it. If many more people get vaccinated, we can stop this pandemic. And hopefully that will stop this current trend of nurses leaving.”
A version of this article first appeared on Medscape.com.
Decline in child COVID may signal end of latest surge
A second consecutive week of falling COVID-19 cases in children, along with continued declines in new admissions, may indicate that the latest surge has peaked.
Children made up over 25% of all new cases each week over that 3-week period covering the end of August and the first half of September, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
New hospitalizations in children aged 0-17 years peaked on Sept. 4 – when the rate reached 0.51 per 100,000 population – and were down to 0.47 as of Sept. 11, the latest date for which data should be considered reliable, the Centers for Disease Control and Prevention said.
The CDC’s data largely agree with the AAP/CHA report, showing that cases peaked during the week of Aug. 22-28. Cases per 100,000 for children that week looked like this: 154.7 (age 0-4 years), 276.6 (5-11 years), 320.0 (12-15), and 334.1 (16-17). The highest rates that week among adults were 288.6 per 100,000 in 30- to 39-year-olds and 286.5 for those aged 18-29, the CDC said on its COVID Data Tracker.
By the week of Sept. 5-11 – reporting delays can affect more recent data – the rates in children were down more than 20% in each of the four age groups, according to the CDC.
Vaccinations among children, unfortunately, continue to decline. Vaccine initiations for 12- to 15-year-olds slipped from 199,000 (Sept. 7-13) to 179,000 during the week of Sept. 14-20, while the 16- to 17-year-olds went from almost 83,000 down to 75,000. Initiations have dropped for 6 straight weeks in both age groups, based on the CDC data.
Despite those declines, however, the 16- and 17-year-olds just passed a couple of vaccination milestones. More than 60% – 60.9%, to be exact – have now received at least one dose of COVID vaccine, and 50.3% can be considered fully vaccinated. For those aged 12-15, the corresponding figures are 53.1% and 42.0%, the CDC reported.
When children under age 12 years are included – through clinical trial involvement or incorrect birth dates – the CDC data put the total count of Americans under age 18 who have received at least one dose of vaccine at almost 12.8 million, with vaccination complete in 10.3 million.
Total cases, as calculated by the APA and CHA, are now over 5.5 million, although that figure includes cases in individuals as old as 20 years, since many states differ from the CDC on the age range for a child. The CDC’s COVID Data Tracker put the total for children aged 0-17 at nearly 4.6 million.
The total number of COVID-related deaths in children is 480 as of Sept. 16, the AAP and CHA said, based on data from 45 states, New York, City, Puerto Rico, and Guam, but the CDC provides a higher number, 548, since the pandemic began. Children aged 0-4 years represent the largest share (32.3%) of those 548 deaths, followed by the 12- to 15-year-olds (26.5%), based on the CDC data.
A second consecutive week of falling COVID-19 cases in children, along with continued declines in new admissions, may indicate that the latest surge has peaked.
Children made up over 25% of all new cases each week over that 3-week period covering the end of August and the first half of September, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
New hospitalizations in children aged 0-17 years peaked on Sept. 4 – when the rate reached 0.51 per 100,000 population – and were down to 0.47 as of Sept. 11, the latest date for which data should be considered reliable, the Centers for Disease Control and Prevention said.
The CDC’s data largely agree with the AAP/CHA report, showing that cases peaked during the week of Aug. 22-28. Cases per 100,000 for children that week looked like this: 154.7 (age 0-4 years), 276.6 (5-11 years), 320.0 (12-15), and 334.1 (16-17). The highest rates that week among adults were 288.6 per 100,000 in 30- to 39-year-olds and 286.5 for those aged 18-29, the CDC said on its COVID Data Tracker.
By the week of Sept. 5-11 – reporting delays can affect more recent data – the rates in children were down more than 20% in each of the four age groups, according to the CDC.
Vaccinations among children, unfortunately, continue to decline. Vaccine initiations for 12- to 15-year-olds slipped from 199,000 (Sept. 7-13) to 179,000 during the week of Sept. 14-20, while the 16- to 17-year-olds went from almost 83,000 down to 75,000. Initiations have dropped for 6 straight weeks in both age groups, based on the CDC data.
Despite those declines, however, the 16- and 17-year-olds just passed a couple of vaccination milestones. More than 60% – 60.9%, to be exact – have now received at least one dose of COVID vaccine, and 50.3% can be considered fully vaccinated. For those aged 12-15, the corresponding figures are 53.1% and 42.0%, the CDC reported.
When children under age 12 years are included – through clinical trial involvement or incorrect birth dates – the CDC data put the total count of Americans under age 18 who have received at least one dose of vaccine at almost 12.8 million, with vaccination complete in 10.3 million.
Total cases, as calculated by the APA and CHA, are now over 5.5 million, although that figure includes cases in individuals as old as 20 years, since many states differ from the CDC on the age range for a child. The CDC’s COVID Data Tracker put the total for children aged 0-17 at nearly 4.6 million.
The total number of COVID-related deaths in children is 480 as of Sept. 16, the AAP and CHA said, based on data from 45 states, New York, City, Puerto Rico, and Guam, but the CDC provides a higher number, 548, since the pandemic began. Children aged 0-4 years represent the largest share (32.3%) of those 548 deaths, followed by the 12- to 15-year-olds (26.5%), based on the CDC data.
A second consecutive week of falling COVID-19 cases in children, along with continued declines in new admissions, may indicate that the latest surge has peaked.
Children made up over 25% of all new cases each week over that 3-week period covering the end of August and the first half of September, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
New hospitalizations in children aged 0-17 years peaked on Sept. 4 – when the rate reached 0.51 per 100,000 population – and were down to 0.47 as of Sept. 11, the latest date for which data should be considered reliable, the Centers for Disease Control and Prevention said.
The CDC’s data largely agree with the AAP/CHA report, showing that cases peaked during the week of Aug. 22-28. Cases per 100,000 for children that week looked like this: 154.7 (age 0-4 years), 276.6 (5-11 years), 320.0 (12-15), and 334.1 (16-17). The highest rates that week among adults were 288.6 per 100,000 in 30- to 39-year-olds and 286.5 for those aged 18-29, the CDC said on its COVID Data Tracker.
By the week of Sept. 5-11 – reporting delays can affect more recent data – the rates in children were down more than 20% in each of the four age groups, according to the CDC.
Vaccinations among children, unfortunately, continue to decline. Vaccine initiations for 12- to 15-year-olds slipped from 199,000 (Sept. 7-13) to 179,000 during the week of Sept. 14-20, while the 16- to 17-year-olds went from almost 83,000 down to 75,000. Initiations have dropped for 6 straight weeks in both age groups, based on the CDC data.
Despite those declines, however, the 16- and 17-year-olds just passed a couple of vaccination milestones. More than 60% – 60.9%, to be exact – have now received at least one dose of COVID vaccine, and 50.3% can be considered fully vaccinated. For those aged 12-15, the corresponding figures are 53.1% and 42.0%, the CDC reported.
When children under age 12 years are included – through clinical trial involvement or incorrect birth dates – the CDC data put the total count of Americans under age 18 who have received at least one dose of vaccine at almost 12.8 million, with vaccination complete in 10.3 million.
Total cases, as calculated by the APA and CHA, are now over 5.5 million, although that figure includes cases in individuals as old as 20 years, since many states differ from the CDC on the age range for a child. The CDC’s COVID Data Tracker put the total for children aged 0-17 at nearly 4.6 million.
The total number of COVID-related deaths in children is 480 as of Sept. 16, the AAP and CHA said, based on data from 45 states, New York, City, Puerto Rico, and Guam, but the CDC provides a higher number, 548, since the pandemic began. Children aged 0-4 years represent the largest share (32.3%) of those 548 deaths, followed by the 12- to 15-year-olds (26.5%), based on the CDC data.