User login
Optimal Treatment for HR+/HER2- Breast Cancer Patients With Visceral Metastasis
In the past, chemotherapy was the go-to treatment for patients with HR+/HER2- breast cancer with visceral metastases, which most commonly involve lung and liver. Dr Adam M. Brufsky, of the University of Pittsburgh School of Medicine, reports that the advent of CDK4/6 inhibitors offers an opportunity for several different courses of action appropriate to subgroups within this population of patients.
Three CDK4/6 inhibitors are FDA approved for clinical practice. Dr Brufsky summarizes results from three studies of CDK4/6 inhibitors — abemaciclib, ribociclib, and palbociclib — where these agents were used as second-line therapy after progression on a nonsteroidal aromatase inhibitor. In combination with fulvestrant, all three offered significant benefits in progression-free survival (PFS) and two in overall survival (OS). Among those who derived survival benefit were patients who had visceral metatases, who constituted 56%, 27% and 60% of the respective studies.
For patients who show progression on CDK4/6 inhibitors, additional treatment can be determined by genomic sequencing. Combination therapy with alpelisib and fulvestrant has been shown to lengthen PFS in patients whose tumors have PIK3 mutations. Patients without PIK3 mutations can be treated with fulvestrant or a combination of fulvestrant and everolimus.
--
Adam M. Brufsky, MD, PhD, Professor, Department of Medicine, Division of Hematology-Oncology, University of Pittsburgh School of Medicine; Associate Chief, Department of Medicine, Division of Hematology-Oncology, UPMC-Hillman Cancer Center, Pittsburgh, Pennsylvania.
Adam M. Brufsky, MD, PhD, has disclosed the following relevant financial relationships:
Received income in an amount equal to or greater than $250 from: Seattle Genetics; Roche; AstraZeneca; Puma; Daiichi Sankyo.
In the past, chemotherapy was the go-to treatment for patients with HR+/HER2- breast cancer with visceral metastases, which most commonly involve lung and liver. Dr Adam M. Brufsky, of the University of Pittsburgh School of Medicine, reports that the advent of CDK4/6 inhibitors offers an opportunity for several different courses of action appropriate to subgroups within this population of patients.
Three CDK4/6 inhibitors are FDA approved for clinical practice. Dr Brufsky summarizes results from three studies of CDK4/6 inhibitors — abemaciclib, ribociclib, and palbociclib — where these agents were used as second-line therapy after progression on a nonsteroidal aromatase inhibitor. In combination with fulvestrant, all three offered significant benefits in progression-free survival (PFS) and two in overall survival (OS). Among those who derived survival benefit were patients who had visceral metatases, who constituted 56%, 27% and 60% of the respective studies.
For patients who show progression on CDK4/6 inhibitors, additional treatment can be determined by genomic sequencing. Combination therapy with alpelisib and fulvestrant has been shown to lengthen PFS in patients whose tumors have PIK3 mutations. Patients without PIK3 mutations can be treated with fulvestrant or a combination of fulvestrant and everolimus.
--
Adam M. Brufsky, MD, PhD, Professor, Department of Medicine, Division of Hematology-Oncology, University of Pittsburgh School of Medicine; Associate Chief, Department of Medicine, Division of Hematology-Oncology, UPMC-Hillman Cancer Center, Pittsburgh, Pennsylvania.
Adam M. Brufsky, MD, PhD, has disclosed the following relevant financial relationships:
Received income in an amount equal to or greater than $250 from: Seattle Genetics; Roche; AstraZeneca; Puma; Daiichi Sankyo.
In the past, chemotherapy was the go-to treatment for patients with HR+/HER2- breast cancer with visceral metastases, which most commonly involve lung and liver. Dr Adam M. Brufsky, of the University of Pittsburgh School of Medicine, reports that the advent of CDK4/6 inhibitors offers an opportunity for several different courses of action appropriate to subgroups within this population of patients.
Three CDK4/6 inhibitors are FDA approved for clinical practice. Dr Brufsky summarizes results from three studies of CDK4/6 inhibitors — abemaciclib, ribociclib, and palbociclib — where these agents were used as second-line therapy after progression on a nonsteroidal aromatase inhibitor. In combination with fulvestrant, all three offered significant benefits in progression-free survival (PFS) and two in overall survival (OS). Among those who derived survival benefit were patients who had visceral metatases, who constituted 56%, 27% and 60% of the respective studies.
For patients who show progression on CDK4/6 inhibitors, additional treatment can be determined by genomic sequencing. Combination therapy with alpelisib and fulvestrant has been shown to lengthen PFS in patients whose tumors have PIK3 mutations. Patients without PIK3 mutations can be treated with fulvestrant or a combination of fulvestrant and everolimus.
--
Adam M. Brufsky, MD, PhD, Professor, Department of Medicine, Division of Hematology-Oncology, University of Pittsburgh School of Medicine; Associate Chief, Department of Medicine, Division of Hematology-Oncology, UPMC-Hillman Cancer Center, Pittsburgh, Pennsylvania.
Adam M. Brufsky, MD, PhD, has disclosed the following relevant financial relationships:
Received income in an amount equal to or greater than $250 from: Seattle Genetics; Roche; AstraZeneca; Puma; Daiichi Sankyo.

Storing patients’ credit card information: Keep it safe
Credit cards have made it easier for psychiatrists who work in outpatient settings to collect payment for their services. Accepting credit cards saves time in sessions for clinical matters, leads to higher rates of collecting payments for patients who do not show up for appointments, and avoids having to manage bounced checks and collection agencies.1 No federal or state laws prohibit businesses from storing consumers’ credit card information. However, psychiatric practices are legally obligated to have safeguards in place to protect sensitive information and limit liability exposures.2 There are several steps to take when storing your patients’ credit card information.
Establish a payment policy. Create a policy that outlines your practice’s credit card procedures, including when credit cards will be charged and under what circumstances, how patients will be notified, and how credit card information will be stored.2 Give your patients a copy of this policy and review it with them at their first appointment and any time you change this policy.2 Get consent from your patients before using and storing their credit card information.2
Use secure methods to store this information. Most medical practices photocopy/write down their patients’ credit card information and store it in the patient’s electronic/paper medical record, or they use an online service to store it electronically.2 Online services usually provide a higher level of protection than the patient’s medical record.2 Ensure that electronic data that includes credit card numbers is robustly encrypted, or that paper records are locked in a secure place, such as in a safe or file drawer that requires a key/combination lock.3 Payment Card Industry (PCI) regulations prohibit storing a credit card’s security code (a three- or four-digit number on the front or back of the card).3 This code is used to allow merchants to verify whether a customer authorizing a transaction over the phone or via the internet physically possesses the card.3 PCI regulations also prohibit storing data contained in the card’s magnetic strip.3 This data contains information about the account that is not displayed on the card, assists with authorizing transactions, and ensures that credit cards cannot be easily counterfeited.3
Understand all federal and state laws and regulations. If your practice collects patient billing information, you are considered a “merchant” and are subject to federal and state laws and regulations that protect consumer credit card information.2 These laws and regulations include (but are not limited to)2:
- Health Insurance Portability and Accountability Act (HIPAA) and similar state privacy laws
- Federal Trade Commission Act (FTCA) and similar state business laws
- Payment Card Industry Data Security Standard (PCI DSS), which was not devised by federal or state government.
HIPAA and state privacy laws require psychiatrists to implement “reasonable” security measures to protect payment information, regardless of how that information is stored.2,4 Because HIPAA does not define “reasonable,” psychiatrists have latitude in determining which security measures to implement.2,4 Locking the information in a file cabinet and locking the room where the file cabinet is kept (for paper storage) or using HIPAA-compliant encrypted storage programs (for electronic storage) are examples of “reasonable” security measures.2
FTCA requires businesses to use “appropriate” and “reasonable” security measures to protect credit card information.2,5 Because FTCA does not specify these terms,2,5 psychiatrists have leeway in determining which security measures to implement. Federal law requires all businesses to delete a card’s expiration date and shorten the account information to include no more than the last 5 digits of the card number that is printed on all sales receipts.6 FTCA also requires businesses to get prior authorization from individuals before charging their credit card.2 For example, if a patient previously used a credit card to pay for a session, the psychiatrist cannot later use the credit card to charge for a missed appointment without notifying the patient and getting their authorization.2
PCI DSS applies to entities that store, process, and/or transmit cardholder data.7 Any business that accepts credit card payments must comply with PCI DSS, which includes 12 requirements.7 Examples of these requirements include using firewalls to protect cardholder data and restricting access to cardholder data to a “need-to-know” basis. Businesses that do not comply with PCI DSS can be subjected to fines and/or have their contracts terminated by the credit card companies.2 Fines can range from $5,000 to $100,000 per month for data breaches where you are found negligible.1
1. Braslow K. Benefits and costs of accepting credit cards in your practice. Current Psychiatry. 2017;16(5):17,29.
2. Wertheimer M. Keeping patient credit card and payment information on file. Psychiatric News. 2019;54(11):8.
3. Hephner L. 5 tips for proper handling of credit card information. Accessed April 22, 2020. https://paysimple.com/blog/5-tips-for-proper-handling-of-customer-credit-card-account-information/
4. Health Insurance Portability and Accountability Act of 1996. Public Law No. 104–191, 110 Stat. 1936 (1996).
5. Federal Trade Commission Act of 1914. 15 U.S.C. §§ 41-58, as amended (1914).
6. Federal Trade Commission. Slip showing? Federal law requires all businesses to truncate credit card information on receipts. Accessed April 22, 2020. https://www.ftc.gov/tips-advice/business-center/guidance/slip-showing-federal-law-requires-all-businesses-truncate
7. PCI Security Standards Council. Accessed April 22, 2020. https://www.pcisecuritystandards.org/
Credit cards have made it easier for psychiatrists who work in outpatient settings to collect payment for their services. Accepting credit cards saves time in sessions for clinical matters, leads to higher rates of collecting payments for patients who do not show up for appointments, and avoids having to manage bounced checks and collection agencies.1 No federal or state laws prohibit businesses from storing consumers’ credit card information. However, psychiatric practices are legally obligated to have safeguards in place to protect sensitive information and limit liability exposures.2 There are several steps to take when storing your patients’ credit card information.
Establish a payment policy. Create a policy that outlines your practice’s credit card procedures, including when credit cards will be charged and under what circumstances, how patients will be notified, and how credit card information will be stored.2 Give your patients a copy of this policy and review it with them at their first appointment and any time you change this policy.2 Get consent from your patients before using and storing their credit card information.2
Use secure methods to store this information. Most medical practices photocopy/write down their patients’ credit card information and store it in the patient’s electronic/paper medical record, or they use an online service to store it electronically.2 Online services usually provide a higher level of protection than the patient’s medical record.2 Ensure that electronic data that includes credit card numbers is robustly encrypted, or that paper records are locked in a secure place, such as in a safe or file drawer that requires a key/combination lock.3 Payment Card Industry (PCI) regulations prohibit storing a credit card’s security code (a three- or four-digit number on the front or back of the card).3 This code is used to allow merchants to verify whether a customer authorizing a transaction over the phone or via the internet physically possesses the card.3 PCI regulations also prohibit storing data contained in the card’s magnetic strip.3 This data contains information about the account that is not displayed on the card, assists with authorizing transactions, and ensures that credit cards cannot be easily counterfeited.3
Understand all federal and state laws and regulations. If your practice collects patient billing information, you are considered a “merchant” and are subject to federal and state laws and regulations that protect consumer credit card information.2 These laws and regulations include (but are not limited to)2:
- Health Insurance Portability and Accountability Act (HIPAA) and similar state privacy laws
- Federal Trade Commission Act (FTCA) and similar state business laws
- Payment Card Industry Data Security Standard (PCI DSS), which was not devised by federal or state government.
HIPAA and state privacy laws require psychiatrists to implement “reasonable” security measures to protect payment information, regardless of how that information is stored.2,4 Because HIPAA does not define “reasonable,” psychiatrists have latitude in determining which security measures to implement.2,4 Locking the information in a file cabinet and locking the room where the file cabinet is kept (for paper storage) or using HIPAA-compliant encrypted storage programs (for electronic storage) are examples of “reasonable” security measures.2
FTCA requires businesses to use “appropriate” and “reasonable” security measures to protect credit card information.2,5 Because FTCA does not specify these terms,2,5 psychiatrists have leeway in determining which security measures to implement. Federal law requires all businesses to delete a card’s expiration date and shorten the account information to include no more than the last 5 digits of the card number that is printed on all sales receipts.6 FTCA also requires businesses to get prior authorization from individuals before charging their credit card.2 For example, if a patient previously used a credit card to pay for a session, the psychiatrist cannot later use the credit card to charge for a missed appointment without notifying the patient and getting their authorization.2
PCI DSS applies to entities that store, process, and/or transmit cardholder data.7 Any business that accepts credit card payments must comply with PCI DSS, which includes 12 requirements.7 Examples of these requirements include using firewalls to protect cardholder data and restricting access to cardholder data to a “need-to-know” basis. Businesses that do not comply with PCI DSS can be subjected to fines and/or have their contracts terminated by the credit card companies.2 Fines can range from $5,000 to $100,000 per month for data breaches where you are found negligible.1
Credit cards have made it easier for psychiatrists who work in outpatient settings to collect payment for their services. Accepting credit cards saves time in sessions for clinical matters, leads to higher rates of collecting payments for patients who do not show up for appointments, and avoids having to manage bounced checks and collection agencies.1 No federal or state laws prohibit businesses from storing consumers’ credit card information. However, psychiatric practices are legally obligated to have safeguards in place to protect sensitive information and limit liability exposures.2 There are several steps to take when storing your patients’ credit card information.
Establish a payment policy. Create a policy that outlines your practice’s credit card procedures, including when credit cards will be charged and under what circumstances, how patients will be notified, and how credit card information will be stored.2 Give your patients a copy of this policy and review it with them at their first appointment and any time you change this policy.2 Get consent from your patients before using and storing their credit card information.2
Use secure methods to store this information. Most medical practices photocopy/write down their patients’ credit card information and store it in the patient’s electronic/paper medical record, or they use an online service to store it electronically.2 Online services usually provide a higher level of protection than the patient’s medical record.2 Ensure that electronic data that includes credit card numbers is robustly encrypted, or that paper records are locked in a secure place, such as in a safe or file drawer that requires a key/combination lock.3 Payment Card Industry (PCI) regulations prohibit storing a credit card’s security code (a three- or four-digit number on the front or back of the card).3 This code is used to allow merchants to verify whether a customer authorizing a transaction over the phone or via the internet physically possesses the card.3 PCI regulations also prohibit storing data contained in the card’s magnetic strip.3 This data contains information about the account that is not displayed on the card, assists with authorizing transactions, and ensures that credit cards cannot be easily counterfeited.3
Understand all federal and state laws and regulations. If your practice collects patient billing information, you are considered a “merchant” and are subject to federal and state laws and regulations that protect consumer credit card information.2 These laws and regulations include (but are not limited to)2:
- Health Insurance Portability and Accountability Act (HIPAA) and similar state privacy laws
- Federal Trade Commission Act (FTCA) and similar state business laws
- Payment Card Industry Data Security Standard (PCI DSS), which was not devised by federal or state government.
HIPAA and state privacy laws require psychiatrists to implement “reasonable” security measures to protect payment information, regardless of how that information is stored.2,4 Because HIPAA does not define “reasonable,” psychiatrists have latitude in determining which security measures to implement.2,4 Locking the information in a file cabinet and locking the room where the file cabinet is kept (for paper storage) or using HIPAA-compliant encrypted storage programs (for electronic storage) are examples of “reasonable” security measures.2
FTCA requires businesses to use “appropriate” and “reasonable” security measures to protect credit card information.2,5 Because FTCA does not specify these terms,2,5 psychiatrists have leeway in determining which security measures to implement. Federal law requires all businesses to delete a card’s expiration date and shorten the account information to include no more than the last 5 digits of the card number that is printed on all sales receipts.6 FTCA also requires businesses to get prior authorization from individuals before charging their credit card.2 For example, if a patient previously used a credit card to pay for a session, the psychiatrist cannot later use the credit card to charge for a missed appointment without notifying the patient and getting their authorization.2
PCI DSS applies to entities that store, process, and/or transmit cardholder data.7 Any business that accepts credit card payments must comply with PCI DSS, which includes 12 requirements.7 Examples of these requirements include using firewalls to protect cardholder data and restricting access to cardholder data to a “need-to-know” basis. Businesses that do not comply with PCI DSS can be subjected to fines and/or have their contracts terminated by the credit card companies.2 Fines can range from $5,000 to $100,000 per month for data breaches where you are found negligible.1
1. Braslow K. Benefits and costs of accepting credit cards in your practice. Current Psychiatry. 2017;16(5):17,29.
2. Wertheimer M. Keeping patient credit card and payment information on file. Psychiatric News. 2019;54(11):8.
3. Hephner L. 5 tips for proper handling of credit card information. Accessed April 22, 2020. https://paysimple.com/blog/5-tips-for-proper-handling-of-customer-credit-card-account-information/
4. Health Insurance Portability and Accountability Act of 1996. Public Law No. 104–191, 110 Stat. 1936 (1996).
5. Federal Trade Commission Act of 1914. 15 U.S.C. §§ 41-58, as amended (1914).
6. Federal Trade Commission. Slip showing? Federal law requires all businesses to truncate credit card information on receipts. Accessed April 22, 2020. https://www.ftc.gov/tips-advice/business-center/guidance/slip-showing-federal-law-requires-all-businesses-truncate
7. PCI Security Standards Council. Accessed April 22, 2020. https://www.pcisecuritystandards.org/
1. Braslow K. Benefits and costs of accepting credit cards in your practice. Current Psychiatry. 2017;16(5):17,29.
2. Wertheimer M. Keeping patient credit card and payment information on file. Psychiatric News. 2019;54(11):8.
3. Hephner L. 5 tips for proper handling of credit card information. Accessed April 22, 2020. https://paysimple.com/blog/5-tips-for-proper-handling-of-customer-credit-card-account-information/
4. Health Insurance Portability and Accountability Act of 1996. Public Law No. 104–191, 110 Stat. 1936 (1996).
5. Federal Trade Commission Act of 1914. 15 U.S.C. §§ 41-58, as amended (1914).
6. Federal Trade Commission. Slip showing? Federal law requires all businesses to truncate credit card information on receipts. Accessed April 22, 2020. https://www.ftc.gov/tips-advice/business-center/guidance/slip-showing-federal-law-requires-all-businesses-truncate
7. PCI Security Standards Council. Accessed April 22, 2020. https://www.pcisecuritystandards.org/
Harassment of health care workers: A survey
During the course of my residency training, I have experienced and witnessed patients and visitors harassing health care workers (HCWs) by cursing or directing racial slurs at them, making sexist comments, or threatening their lives. What should be the correct response to this harassment? To say nothing may avoid conflict, but the silence perpetuates such abuse. To speak up may provoke aggression or even a physical assault. Further, does our response change if it is not the patient but someone who is accompanying them who exhibits this behavior?
I conducted a survey of psychiatry HCWs at our institution to evaluate the prevalence of and factors associated with such harassment.
An all-too-common problem
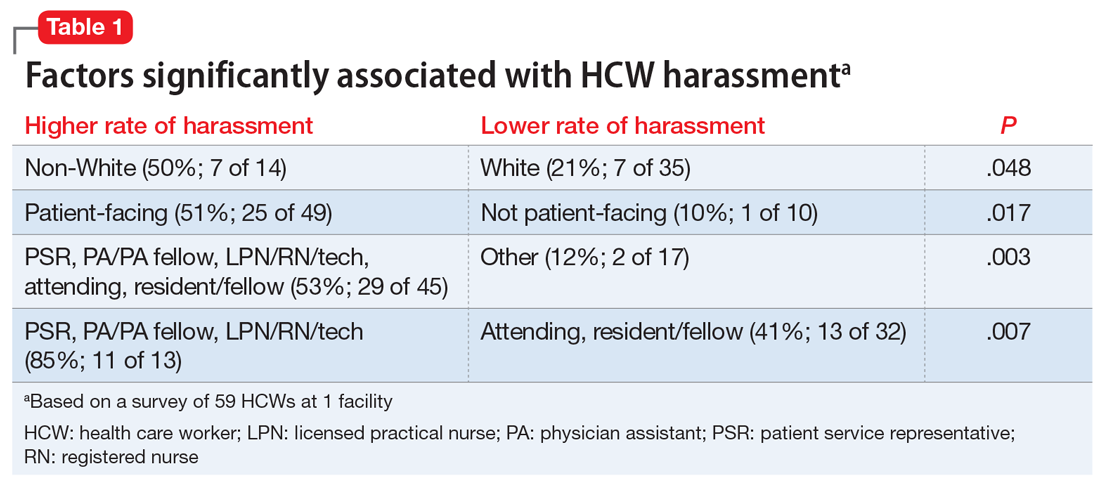
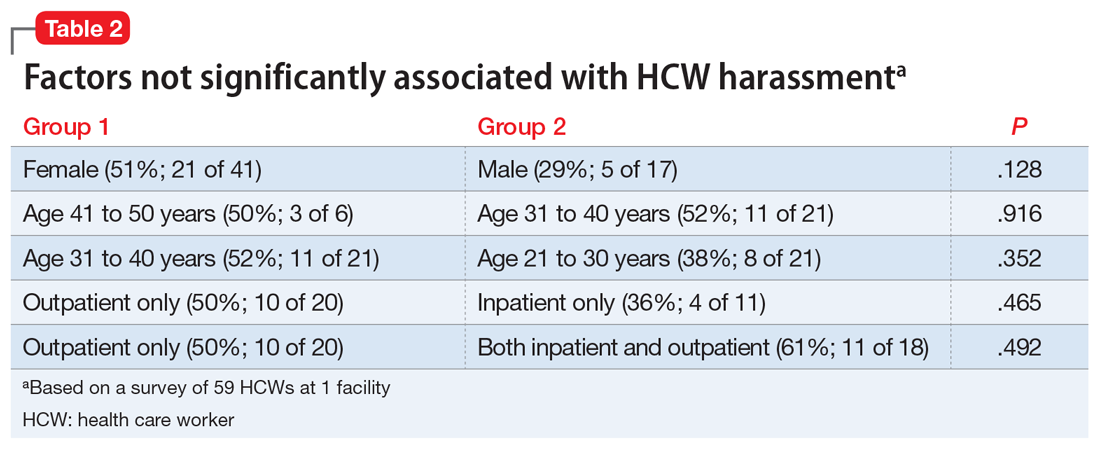
In a December 2020 internal survey at the University of Missouri Department of Psychiatry, 59 of 158 HCWs responded, and 26 (44%) reported experiencing or witnessing on-the-job harassment or abuse. Factors that were statistically significantly associated with experiencing or witnessing on-the-job harassment or abuse included being non-White, working in a patient-facing position, and being a nonphysician patient-facing HCW (Table 1). Factors that were not significantly associated with experiencing or witnessing on-the-job harassment or abuse included clinical setting, HCW age, and HCW gender (Table 2).
In addition to comments from patients and visitors, respondents stated that the harassment or abuse also included:
- physically threatening behavior and assault
- reporting a HCW for HIPAA (Health Insurance Portability and Accountability Act) violations after the HCW declined to provide an early refill of a controlled substance
- being accused of being a bad person for declining to prescribe a specific medication
- insults about not being intelligent enough to be on the treatment team
- comments from colleagues.
At the most basic level of response, the emergency department (ED) remains under the Emergency Medical Treatment and Labor Act (EMTALA) obligation to see, screen, and stabilize any patient, and if psychiatry is consulted in the ED, we should similarly provide this standard of care. Beyond this, we can create behavioral plans for when a relevant diagnosis exists or does not exist, and patients and/or visitors can be terminated from their stay at the location/service/health care system. Whether or not a patient is receiving psychiatric care and/or treatment is irrelevant to the responses to harassment we might consider.
During the incident itself, we are empowered to remove ourselves from the patient encounter. Historically, HCWs have had strong opinions on the next steps, either deciding, “Yes, I am a professional and I will not be bullied,” or “No, I am a professional and I don’t need to deal with this.” Just as we prioritize our patients’ dignities, we should also respect our own and our colleagues’ dignities.
How harassment is handled at our facility
HCWs are commonly unsure whether to “call out” abusive comments during the encounter itself or afterwards. In our hospital, HCWs are encouraged to independently choose to immediately respond, immediately report to a supervisor or hospital security, or defer and report to leadership afterwards via the Patient Safety Network (PSN). The PSN is our hospital’s reporting system for medical errors, near misses, and abuse, neglect, and workplace violence. Relevant examples of abuse, neglect, and workplace violence include:
- Threats. Expression of intent to cause harm, including verbal or written threats and threatening body language
- Physical assault. Attacks ranging from slapping and beating to rape, the use of weapons, or homicide
- Sexual assault. Any type of sexual contact or behavior that occurs without the explicit consent of the recipient, such as forced sexual intercourse, forcible sodomy, child molestation, incest, fondling, and attempted rape.
Continue to: Once complete...
Once complete, the PSN report is sent to Risk Management and other relevant groups, such as a 5-person team of security investigators, who are trained in trauma-informed interviewing and re-directive techniques. This team can immediately speak to the patient face-to-face in the inpatient setting or follow-up via phone in the outpatient setting.
The PSN report may result in the creation of a behavior plan for the patient that outlines the behaviors of concern, staff interventions, and consequences for persistent violations. The behavior plan is saved in the patient’s medical chart, and an alert pops up every time the chart is opened. The behavior plan is reviewed once annually for revision or deletion, as appropriate.
Lessons from our facility’s policy
In our health care system, our primary response to HCW harassment is to create a patient behavior plan that lays out specific expectations, care parameters, and consequences (up to terminating a patient from the entire health care system, except for EMTALA-level care). Clinicians are encouraged to report harassment to hospital administration, and a team of security investigators discusses expectations with the patient and/or visitors to prevent further abuse. We believe that describing our policies may be helpful to other health care systems and HCWs who confront this widespread issue.
During the course of my residency training, I have experienced and witnessed patients and visitors harassing health care workers (HCWs) by cursing or directing racial slurs at them, making sexist comments, or threatening their lives. What should be the correct response to this harassment? To say nothing may avoid conflict, but the silence perpetuates such abuse. To speak up may provoke aggression or even a physical assault. Further, does our response change if it is not the patient but someone who is accompanying them who exhibits this behavior?

I conducted a survey of psychiatry HCWs at our institution to evaluate the prevalence of and factors associated with such harassment.
An all-too-common problem
In a December 2020 internal survey at the University of Missouri Department of Psychiatry, 59 of 158 HCWs responded, and 26 (44%) reported experiencing or witnessing on-the-job harassment or abuse. Factors that were statistically significantly associated with experiencing or witnessing on-the-job harassment or abuse included being non-White, working in a patient-facing position, and being a nonphysician patient-facing HCW (Table 1). Factors that were not significantly associated with experiencing or witnessing on-the-job harassment or abuse included clinical setting, HCW age, and HCW gender (Table 2).

In addition to comments from patients and visitors, respondents stated that the harassment or abuse also included:
- physically threatening behavior and assault
- reporting a HCW for HIPAA (Health Insurance Portability and Accountability Act) violations after the HCW declined to provide an early refill of a controlled substance
- being accused of being a bad person for declining to prescribe a specific medication
- insults about not being intelligent enough to be on the treatment team
- comments from colleagues.
At the most basic level of response, the emergency department (ED) remains under the Emergency Medical Treatment and Labor Act (EMTALA) obligation to see, screen, and stabilize any patient, and if psychiatry is consulted in the ED, we should similarly provide this standard of care. Beyond this, we can create behavioral plans for when a relevant diagnosis exists or does not exist, and patients and/or visitors can be terminated from their stay at the location/service/health care system. Whether or not a patient is receiving psychiatric care and/or treatment is irrelevant to the responses to harassment we might consider.
During the incident itself, we are empowered to remove ourselves from the patient encounter. Historically, HCWs have had strong opinions on the next steps, either deciding, “Yes, I am a professional and I will not be bullied,” or “No, I am a professional and I don’t need to deal with this.” Just as we prioritize our patients’ dignities, we should also respect our own and our colleagues’ dignities.
How harassment is handled at our facility
HCWs are commonly unsure whether to “call out” abusive comments during the encounter itself or afterwards. In our hospital, HCWs are encouraged to independently choose to immediately respond, immediately report to a supervisor or hospital security, or defer and report to leadership afterwards via the Patient Safety Network (PSN). The PSN is our hospital’s reporting system for medical errors, near misses, and abuse, neglect, and workplace violence. Relevant examples of abuse, neglect, and workplace violence include:
- Threats. Expression of intent to cause harm, including verbal or written threats and threatening body language
- Physical assault. Attacks ranging from slapping and beating to rape, the use of weapons, or homicide
- Sexual assault. Any type of sexual contact or behavior that occurs without the explicit consent of the recipient, such as forced sexual intercourse, forcible sodomy, child molestation, incest, fondling, and attempted rape.
Continue to: Once complete...
Once complete, the PSN report is sent to Risk Management and other relevant groups, such as a 5-person team of security investigators, who are trained in trauma-informed interviewing and re-directive techniques. This team can immediately speak to the patient face-to-face in the inpatient setting or follow-up via phone in the outpatient setting.
The PSN report may result in the creation of a behavior plan for the patient that outlines the behaviors of concern, staff interventions, and consequences for persistent violations. The behavior plan is saved in the patient’s medical chart, and an alert pops up every time the chart is opened. The behavior plan is reviewed once annually for revision or deletion, as appropriate.
Lessons from our facility’s policy
In our health care system, our primary response to HCW harassment is to create a patient behavior plan that lays out specific expectations, care parameters, and consequences (up to terminating a patient from the entire health care system, except for EMTALA-level care). Clinicians are encouraged to report harassment to hospital administration, and a team of security investigators discusses expectations with the patient and/or visitors to prevent further abuse. We believe that describing our policies may be helpful to other health care systems and HCWs who confront this widespread issue.
During the course of my residency training, I have experienced and witnessed patients and visitors harassing health care workers (HCWs) by cursing or directing racial slurs at them, making sexist comments, or threatening their lives. What should be the correct response to this harassment? To say nothing may avoid conflict, but the silence perpetuates such abuse. To speak up may provoke aggression or even a physical assault. Further, does our response change if it is not the patient but someone who is accompanying them who exhibits this behavior?

I conducted a survey of psychiatry HCWs at our institution to evaluate the prevalence of and factors associated with such harassment.
An all-too-common problem
In a December 2020 internal survey at the University of Missouri Department of Psychiatry, 59 of 158 HCWs responded, and 26 (44%) reported experiencing or witnessing on-the-job harassment or abuse. Factors that were statistically significantly associated with experiencing or witnessing on-the-job harassment or abuse included being non-White, working in a patient-facing position, and being a nonphysician patient-facing HCW (Table 1). Factors that were not significantly associated with experiencing or witnessing on-the-job harassment or abuse included clinical setting, HCW age, and HCW gender (Table 2).

In addition to comments from patients and visitors, respondents stated that the harassment or abuse also included:
- physically threatening behavior and assault
- reporting a HCW for HIPAA (Health Insurance Portability and Accountability Act) violations after the HCW declined to provide an early refill of a controlled substance
- being accused of being a bad person for declining to prescribe a specific medication
- insults about not being intelligent enough to be on the treatment team
- comments from colleagues.
At the most basic level of response, the emergency department (ED) remains under the Emergency Medical Treatment and Labor Act (EMTALA) obligation to see, screen, and stabilize any patient, and if psychiatry is consulted in the ED, we should similarly provide this standard of care. Beyond this, we can create behavioral plans for when a relevant diagnosis exists or does not exist, and patients and/or visitors can be terminated from their stay at the location/service/health care system. Whether or not a patient is receiving psychiatric care and/or treatment is irrelevant to the responses to harassment we might consider.
During the incident itself, we are empowered to remove ourselves from the patient encounter. Historically, HCWs have had strong opinions on the next steps, either deciding, “Yes, I am a professional and I will not be bullied,” or “No, I am a professional and I don’t need to deal with this.” Just as we prioritize our patients’ dignities, we should also respect our own and our colleagues’ dignities.
How harassment is handled at our facility
HCWs are commonly unsure whether to “call out” abusive comments during the encounter itself or afterwards. In our hospital, HCWs are encouraged to independently choose to immediately respond, immediately report to a supervisor or hospital security, or defer and report to leadership afterwards via the Patient Safety Network (PSN). The PSN is our hospital’s reporting system for medical errors, near misses, and abuse, neglect, and workplace violence. Relevant examples of abuse, neglect, and workplace violence include:
- Threats. Expression of intent to cause harm, including verbal or written threats and threatening body language
- Physical assault. Attacks ranging from slapping and beating to rape, the use of weapons, or homicide
- Sexual assault. Any type of sexual contact or behavior that occurs without the explicit consent of the recipient, such as forced sexual intercourse, forcible sodomy, child molestation, incest, fondling, and attempted rape.
Continue to: Once complete...
Once complete, the PSN report is sent to Risk Management and other relevant groups, such as a 5-person team of security investigators, who are trained in trauma-informed interviewing and re-directive techniques. This team can immediately speak to the patient face-to-face in the inpatient setting or follow-up via phone in the outpatient setting.
The PSN report may result in the creation of a behavior plan for the patient that outlines the behaviors of concern, staff interventions, and consequences for persistent violations. The behavior plan is saved in the patient’s medical chart, and an alert pops up every time the chart is opened. The behavior plan is reviewed once annually for revision or deletion, as appropriate.
Lessons from our facility’s policy
In our health care system, our primary response to HCW harassment is to create a patient behavior plan that lays out specific expectations, care parameters, and consequences (up to terminating a patient from the entire health care system, except for EMTALA-level care). Clinicians are encouraged to report harassment to hospital administration, and a team of security investigators discusses expectations with the patient and/or visitors to prevent further abuse. We believe that describing our policies may be helpful to other health care systems and HCWs who confront this widespread issue.
Garbage out: How much trash does a Mohs surgery practice produce?
left behind after surgical procedures. Their findings: Just two physicians – a surgeon and a surgical fellow – manage to produce nearly a ton of noncontaminated surgical waste annually even though they only see patients twice a week.
“While our emissions as Mohs surgeons are relatively small compared to other types of surgeries, we still emit a notable amount of greenhouse gases compared to nonmedical fields. Mohs surgeons tend to produce the most noncontaminated waste versus other categories, and that’s the category that could be most recyclable,” said Mohs surgeon Simon S. Yoo, MD, of Northwestern University, Chicago, who presented the results at the annual meeting of the American College of Mohs Surgery.
Dr. Yoo, who spoke in an interview, said the coronavirus pandemic spurred the waste analysis. “In the past year, there seemed to be many questions as to the environmental causes and impacts of the pandemic,” he said. “We decided to investigate the environmental impact of Mohs surgery.”
He and surgical fellow Alvin Li, MD, analyzed all waste produced by their clinic over a 3-week period when 106 procedures were performed. They discovered that the surgeries produced 25.8 kg of biohazardous waste (29%), 2.2 kg of packaging waste (3%), 56.4 kg of noncontaminated waste (63%), and 7.5 kg of sharps waste (8%).
“The majority of the waste we produced was noncontaminated and possibly recyclable,” Dr. Yoo said. “However, most of this waste and its packaging did not have clear recycling instructions and presented a significant barrier to recycling by our staff.”
The study authors extrapolated the waste amount to annual totals of 413.5 kg of biohazardous waste, 34.9 kg of packaging waste, 902.3 kg of noncontaminated waste, and 119.9 kg of sharps waste. That adds up to 1,471 kg. The total of noncontaminated waste is the equivalent of nearly 2,000 pounds – a ton.
Dr. Yoo and Dr. Li estimate that the waste produced annual emissions equal to 6.5 metric tons of carbon dioxide equivalent. They estimate that the amount of emissions produced by Mohs surgeons nationally each year is 7,592 metric tons of carbon dioxide equivalent, equal to emissions produced by 19 million miles of passenger automobile travel.
Still, Dr. Yoo said, Mohs surgeries appear to produce fewer emissions than some other operations. “We estimate that an individual Mohs procedure generates around 10 kg of carbon dioxide equivalent whereas a single hysterectomy generates about 380 kg; much of this is due to the use of volatile anesthetics.”
Environmental protection advocate Mary Maloney, MD, professor of medicine and director of dermatologic surgery at the University of Massachusetts, Worcester, urged colleagues to launch a similar waste-weighing project in their own clinics. “I challenge dermatologists to take a bag of your daily plastic waste and weigh it,” she said. “We’ll all be astounded by how much we throw away each day. Until you do that experiment yourself, you’ll have a hard time getting your arms around how much plastic we’re using.”
Dr. Maloney, a member of the American Academy of Dermatology Expert Resource Group for Climate Change and Environmental Issues, urged colleagues to consider strategies to reduce plastic use specifically. “Look at everything you use and see if there’s a nonplastic equivalent,” she said. Even reducing the use of plastic writing pens can make a difference, she said, as can cutting back on syringes and revising procedures so gloves don’t have to be changed as often.
No study funding was reported. Dr. Yoo and Dr. Maloney report no disclosures.
left behind after surgical procedures. Their findings: Just two physicians – a surgeon and a surgical fellow – manage to produce nearly a ton of noncontaminated surgical waste annually even though they only see patients twice a week.
“While our emissions as Mohs surgeons are relatively small compared to other types of surgeries, we still emit a notable amount of greenhouse gases compared to nonmedical fields. Mohs surgeons tend to produce the most noncontaminated waste versus other categories, and that’s the category that could be most recyclable,” said Mohs surgeon Simon S. Yoo, MD, of Northwestern University, Chicago, who presented the results at the annual meeting of the American College of Mohs Surgery.
Dr. Yoo, who spoke in an interview, said the coronavirus pandemic spurred the waste analysis. “In the past year, there seemed to be many questions as to the environmental causes and impacts of the pandemic,” he said. “We decided to investigate the environmental impact of Mohs surgery.”
He and surgical fellow Alvin Li, MD, analyzed all waste produced by their clinic over a 3-week period when 106 procedures were performed. They discovered that the surgeries produced 25.8 kg of biohazardous waste (29%), 2.2 kg of packaging waste (3%), 56.4 kg of noncontaminated waste (63%), and 7.5 kg of sharps waste (8%).
“The majority of the waste we produced was noncontaminated and possibly recyclable,” Dr. Yoo said. “However, most of this waste and its packaging did not have clear recycling instructions and presented a significant barrier to recycling by our staff.”
The study authors extrapolated the waste amount to annual totals of 413.5 kg of biohazardous waste, 34.9 kg of packaging waste, 902.3 kg of noncontaminated waste, and 119.9 kg of sharps waste. That adds up to 1,471 kg. The total of noncontaminated waste is the equivalent of nearly 2,000 pounds – a ton.
Dr. Yoo and Dr. Li estimate that the waste produced annual emissions equal to 6.5 metric tons of carbon dioxide equivalent. They estimate that the amount of emissions produced by Mohs surgeons nationally each year is 7,592 metric tons of carbon dioxide equivalent, equal to emissions produced by 19 million miles of passenger automobile travel.
Still, Dr. Yoo said, Mohs surgeries appear to produce fewer emissions than some other operations. “We estimate that an individual Mohs procedure generates around 10 kg of carbon dioxide equivalent whereas a single hysterectomy generates about 380 kg; much of this is due to the use of volatile anesthetics.”
Environmental protection advocate Mary Maloney, MD, professor of medicine and director of dermatologic surgery at the University of Massachusetts, Worcester, urged colleagues to launch a similar waste-weighing project in their own clinics. “I challenge dermatologists to take a bag of your daily plastic waste and weigh it,” she said. “We’ll all be astounded by how much we throw away each day. Until you do that experiment yourself, you’ll have a hard time getting your arms around how much plastic we’re using.”
Dr. Maloney, a member of the American Academy of Dermatology Expert Resource Group for Climate Change and Environmental Issues, urged colleagues to consider strategies to reduce plastic use specifically. “Look at everything you use and see if there’s a nonplastic equivalent,” she said. Even reducing the use of plastic writing pens can make a difference, she said, as can cutting back on syringes and revising procedures so gloves don’t have to be changed as often.
No study funding was reported. Dr. Yoo and Dr. Maloney report no disclosures.
left behind after surgical procedures. Their findings: Just two physicians – a surgeon and a surgical fellow – manage to produce nearly a ton of noncontaminated surgical waste annually even though they only see patients twice a week.
“While our emissions as Mohs surgeons are relatively small compared to other types of surgeries, we still emit a notable amount of greenhouse gases compared to nonmedical fields. Mohs surgeons tend to produce the most noncontaminated waste versus other categories, and that’s the category that could be most recyclable,” said Mohs surgeon Simon S. Yoo, MD, of Northwestern University, Chicago, who presented the results at the annual meeting of the American College of Mohs Surgery.
Dr. Yoo, who spoke in an interview, said the coronavirus pandemic spurred the waste analysis. “In the past year, there seemed to be many questions as to the environmental causes and impacts of the pandemic,” he said. “We decided to investigate the environmental impact of Mohs surgery.”
He and surgical fellow Alvin Li, MD, analyzed all waste produced by their clinic over a 3-week period when 106 procedures were performed. They discovered that the surgeries produced 25.8 kg of biohazardous waste (29%), 2.2 kg of packaging waste (3%), 56.4 kg of noncontaminated waste (63%), and 7.5 kg of sharps waste (8%).
“The majority of the waste we produced was noncontaminated and possibly recyclable,” Dr. Yoo said. “However, most of this waste and its packaging did not have clear recycling instructions and presented a significant barrier to recycling by our staff.”
The study authors extrapolated the waste amount to annual totals of 413.5 kg of biohazardous waste, 34.9 kg of packaging waste, 902.3 kg of noncontaminated waste, and 119.9 kg of sharps waste. That adds up to 1,471 kg. The total of noncontaminated waste is the equivalent of nearly 2,000 pounds – a ton.
Dr. Yoo and Dr. Li estimate that the waste produced annual emissions equal to 6.5 metric tons of carbon dioxide equivalent. They estimate that the amount of emissions produced by Mohs surgeons nationally each year is 7,592 metric tons of carbon dioxide equivalent, equal to emissions produced by 19 million miles of passenger automobile travel.
Still, Dr. Yoo said, Mohs surgeries appear to produce fewer emissions than some other operations. “We estimate that an individual Mohs procedure generates around 10 kg of carbon dioxide equivalent whereas a single hysterectomy generates about 380 kg; much of this is due to the use of volatile anesthetics.”
Environmental protection advocate Mary Maloney, MD, professor of medicine and director of dermatologic surgery at the University of Massachusetts, Worcester, urged colleagues to launch a similar waste-weighing project in their own clinics. “I challenge dermatologists to take a bag of your daily plastic waste and weigh it,” she said. “We’ll all be astounded by how much we throw away each day. Until you do that experiment yourself, you’ll have a hard time getting your arms around how much plastic we’re using.”
Dr. Maloney, a member of the American Academy of Dermatology Expert Resource Group for Climate Change and Environmental Issues, urged colleagues to consider strategies to reduce plastic use specifically. “Look at everything you use and see if there’s a nonplastic equivalent,” she said. Even reducing the use of plastic writing pens can make a difference, she said, as can cutting back on syringes and revising procedures so gloves don’t have to be changed as often.
No study funding was reported. Dr. Yoo and Dr. Maloney report no disclosures.
FROM THE ACMS ANNUAL MEETING
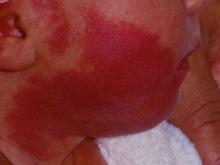
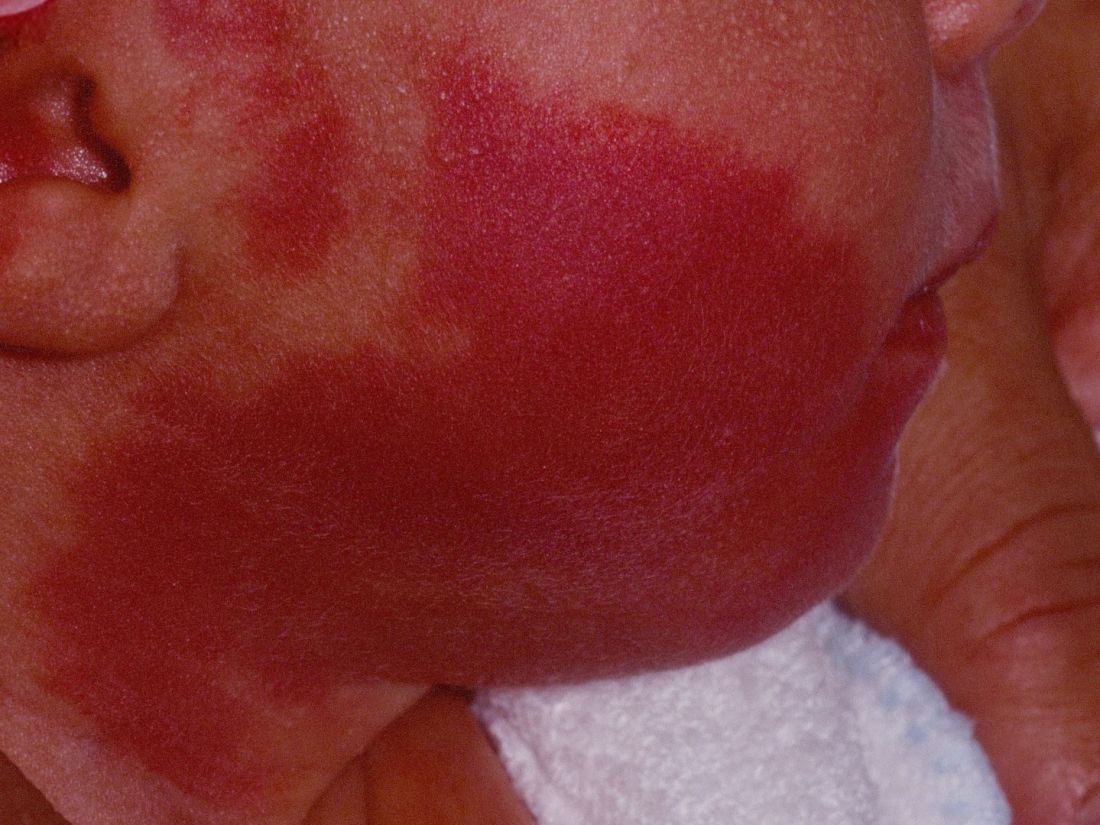
How early can laser treatment for port wine stains in infants be initiated?
without any complications, results from a single-center study showed.
“The current modality of choice for the treatment of port wine birthmarks is pulsed dye laser,” Chelsea Grimes Fidai, MD, said during the annual conference of the American Society for Laser Medicine and Surgery. “When performed by a highly trained expert at efficient frequencies, PDL is a safe, effective treatment that is successful in the majority of patients. We know that earlier treatment yields maximal clearance. However, just how early can you initiate treatment?”
To find out, Dr. Fidai, Roy G. Geronemus, MD, and colleagues at the Laser and Skin Surgery Center of New York, conducted a retrospective chart review of 39 infants with port wine birthmarks who were treated with a 595-nm PDL between 2015 and 2020 at the center. Of the 39 infants, the average age at first treatment was 18 days, with a range from 5 to 29 days. The youngest patient was born prematurely at 35 weeks’ gestation and presented for his first treatment even before his expected due date. Most (74%) had facial lesions with the remaining distributed on the trunk or extremities. The average number of treatments was 15 over the course of 15 months.
The initial settings chosen for facial lesions were a 10-mm spot size, a fluence of 8.0 J/cm2, and a 1.5-millisecond pulse duration. For body lesions, the typical initial settings were a 12-mm spot size, a fluence of 6.7 J/cm2, and 1.5-millisecond pulse duration. Corneal eye shields were placed for all cases with port wine birthmarks approaching the eyelid. “We do recommend a treatment interval of every 2-3 weeks, with longer intervals for patients of darker skin type until the child is 2 years old, at which time the interval is increased to every 3-6 months,” said Dr. Fidai.
Patients in the study experienced the expected short-term side effects of erythema, edema, purpura, and mild transient postinflammatory hyperpigmentation, but there were no cases of atrophy, scarring, infection, or permanent pigmentary change.
“Families seeking early treatment of port wine birthmarks can be reassured that it can be safely initiated within the first few days after birth,” Dr. Fidai concluded. “This procedure can be quickly and confidently performed as an in-office procedure without any complications. The early intervention allows for treatment without general anesthesia and it maximizes the chance of significant clearance as early in life as possible.”
During a question-and-answer session, the abstract section chair, Albert Wolkerstorfer, MD, PhD, expressed concern about the effect of PDL on developing infants. “We do repeated treatments at this young age without any type of anesthesia,” said Dr. Wolkerstorfer, a dermatologist at the Netherlands Institute for Pigment Disorders, department of dermatology, University of Amsterdam.
“Will that influence the development of the child, especially when I hear there might be 15 or 20 treatments done within the first year of life? I think this is a problem where we need to ask the experts in the field of pain management in children, like pediatric anesthesiologists, to find the right way, because I think that the results that you showed are fantastic. I don’t think we can achieve that at a later age, although there’s no direct comparison at this moment.”
Dr. Fidai said that she understood the concern, but pointed to a 2020 article by Dr. Geronemus and colleagues that assessed treatment tolerance and parental perspective of outpatient PDL treatment for port-wine birthmarks without general anesthesia in infants and toddlers. “The kids recover pretty quickly after the treatment,” she said. “There has never been any longstanding issue from the parents’ perspective.”
Dr. Fidai reported having no financial disclosures. Dr. Geronemus disclosed having financial conflicts with numerous device and pharmaceutical companies. Dr. Wolkerstorfer disclosed that he has received consulting fees from Lumenis and InCyte and equipment from Humeca and PerfAction Technologies. He has also received grant funding from Novartis and InCyte and he is a member of InCyte’s advisory board.
without any complications, results from a single-center study showed.
“The current modality of choice for the treatment of port wine birthmarks is pulsed dye laser,” Chelsea Grimes Fidai, MD, said during the annual conference of the American Society for Laser Medicine and Surgery. “When performed by a highly trained expert at efficient frequencies, PDL is a safe, effective treatment that is successful in the majority of patients. We know that earlier treatment yields maximal clearance. However, just how early can you initiate treatment?”
To find out, Dr. Fidai, Roy G. Geronemus, MD, and colleagues at the Laser and Skin Surgery Center of New York, conducted a retrospective chart review of 39 infants with port wine birthmarks who were treated with a 595-nm PDL between 2015 and 2020 at the center. Of the 39 infants, the average age at first treatment was 18 days, with a range from 5 to 29 days. The youngest patient was born prematurely at 35 weeks’ gestation and presented for his first treatment even before his expected due date. Most (74%) had facial lesions with the remaining distributed on the trunk or extremities. The average number of treatments was 15 over the course of 15 months.
The initial settings chosen for facial lesions were a 10-mm spot size, a fluence of 8.0 J/cm2, and a 1.5-millisecond pulse duration. For body lesions, the typical initial settings were a 12-mm spot size, a fluence of 6.7 J/cm2, and 1.5-millisecond pulse duration. Corneal eye shields were placed for all cases with port wine birthmarks approaching the eyelid. “We do recommend a treatment interval of every 2-3 weeks, with longer intervals for patients of darker skin type until the child is 2 years old, at which time the interval is increased to every 3-6 months,” said Dr. Fidai.
Patients in the study experienced the expected short-term side effects of erythema, edema, purpura, and mild transient postinflammatory hyperpigmentation, but there were no cases of atrophy, scarring, infection, or permanent pigmentary change.
“Families seeking early treatment of port wine birthmarks can be reassured that it can be safely initiated within the first few days after birth,” Dr. Fidai concluded. “This procedure can be quickly and confidently performed as an in-office procedure without any complications. The early intervention allows for treatment without general anesthesia and it maximizes the chance of significant clearance as early in life as possible.”
During a question-and-answer session, the abstract section chair, Albert Wolkerstorfer, MD, PhD, expressed concern about the effect of PDL on developing infants. “We do repeated treatments at this young age without any type of anesthesia,” said Dr. Wolkerstorfer, a dermatologist at the Netherlands Institute for Pigment Disorders, department of dermatology, University of Amsterdam.
“Will that influence the development of the child, especially when I hear there might be 15 or 20 treatments done within the first year of life? I think this is a problem where we need to ask the experts in the field of pain management in children, like pediatric anesthesiologists, to find the right way, because I think that the results that you showed are fantastic. I don’t think we can achieve that at a later age, although there’s no direct comparison at this moment.”
Dr. Fidai said that she understood the concern, but pointed to a 2020 article by Dr. Geronemus and colleagues that assessed treatment tolerance and parental perspective of outpatient PDL treatment for port-wine birthmarks without general anesthesia in infants and toddlers. “The kids recover pretty quickly after the treatment,” she said. “There has never been any longstanding issue from the parents’ perspective.”
Dr. Fidai reported having no financial disclosures. Dr. Geronemus disclosed having financial conflicts with numerous device and pharmaceutical companies. Dr. Wolkerstorfer disclosed that he has received consulting fees from Lumenis and InCyte and equipment from Humeca and PerfAction Technologies. He has also received grant funding from Novartis and InCyte and he is a member of InCyte’s advisory board.
without any complications, results from a single-center study showed.
“The current modality of choice for the treatment of port wine birthmarks is pulsed dye laser,” Chelsea Grimes Fidai, MD, said during the annual conference of the American Society for Laser Medicine and Surgery. “When performed by a highly trained expert at efficient frequencies, PDL is a safe, effective treatment that is successful in the majority of patients. We know that earlier treatment yields maximal clearance. However, just how early can you initiate treatment?”
To find out, Dr. Fidai, Roy G. Geronemus, MD, and colleagues at the Laser and Skin Surgery Center of New York, conducted a retrospective chart review of 39 infants with port wine birthmarks who were treated with a 595-nm PDL between 2015 and 2020 at the center. Of the 39 infants, the average age at first treatment was 18 days, with a range from 5 to 29 days. The youngest patient was born prematurely at 35 weeks’ gestation and presented for his first treatment even before his expected due date. Most (74%) had facial lesions with the remaining distributed on the trunk or extremities. The average number of treatments was 15 over the course of 15 months.
The initial settings chosen for facial lesions were a 10-mm spot size, a fluence of 8.0 J/cm2, and a 1.5-millisecond pulse duration. For body lesions, the typical initial settings were a 12-mm spot size, a fluence of 6.7 J/cm2, and 1.5-millisecond pulse duration. Corneal eye shields were placed for all cases with port wine birthmarks approaching the eyelid. “We do recommend a treatment interval of every 2-3 weeks, with longer intervals for patients of darker skin type until the child is 2 years old, at which time the interval is increased to every 3-6 months,” said Dr. Fidai.
Patients in the study experienced the expected short-term side effects of erythema, edema, purpura, and mild transient postinflammatory hyperpigmentation, but there were no cases of atrophy, scarring, infection, or permanent pigmentary change.
“Families seeking early treatment of port wine birthmarks can be reassured that it can be safely initiated within the first few days after birth,” Dr. Fidai concluded. “This procedure can be quickly and confidently performed as an in-office procedure without any complications. The early intervention allows for treatment without general anesthesia and it maximizes the chance of significant clearance as early in life as possible.”
During a question-and-answer session, the abstract section chair, Albert Wolkerstorfer, MD, PhD, expressed concern about the effect of PDL on developing infants. “We do repeated treatments at this young age without any type of anesthesia,” said Dr. Wolkerstorfer, a dermatologist at the Netherlands Institute for Pigment Disorders, department of dermatology, University of Amsterdam.
“Will that influence the development of the child, especially when I hear there might be 15 or 20 treatments done within the first year of life? I think this is a problem where we need to ask the experts in the field of pain management in children, like pediatric anesthesiologists, to find the right way, because I think that the results that you showed are fantastic. I don’t think we can achieve that at a later age, although there’s no direct comparison at this moment.”
Dr. Fidai said that she understood the concern, but pointed to a 2020 article by Dr. Geronemus and colleagues that assessed treatment tolerance and parental perspective of outpatient PDL treatment for port-wine birthmarks without general anesthesia in infants and toddlers. “The kids recover pretty quickly after the treatment,” she said. “There has never been any longstanding issue from the parents’ perspective.”
Dr. Fidai reported having no financial disclosures. Dr. Geronemus disclosed having financial conflicts with numerous device and pharmaceutical companies. Dr. Wolkerstorfer disclosed that he has received consulting fees from Lumenis and InCyte and equipment from Humeca and PerfAction Technologies. He has also received grant funding from Novartis and InCyte and he is a member of InCyte’s advisory board.
FROM ASLMS 2021
Private practice: The basics for psychiatry trainees
Many psychiatry trainees consider private practice as a career option or form of supplemental income. In my experience, however, residency training may provide limited introduction to the general steps involved in starting a practice. In this article, I briefly summarize what I learned while exploring the private practice option as a psychiatry resident.
A good specialty for private practice
Trainees in the earlier stages of their education should be aware that the first step toward private practice may actually occur during medical school, when they are considering which specialty to pursue. If a student is particularly interested in solo private practice, they may want to select a specialty with the potential for less overhead in an independent setting. Psychiatry typically has lower overhead costs than some other specialties. This gap widens even further with the increased popularity and acceptance of telepsychiatry.
Budgeting and finance
Once you decide to pursue private practice, you will want to consider whether you prefer solo practice or group practice, and part-time or full-time. If working for yourself, you will need to understand business planning and budgeting, including how to project revenue and expenses. When first starting in solo practice—especially if you are not taking over a previously established practice—it is useful to have secondary sources of income. This can be a part-time clinical position, working with on-demand health care companies, contracting, consulting, etc. Many new physicians begin with a full-time position and decide to initiate their private practice on a part-time basis. This approach provides a level of financial security that you otherwise would not have. However, a full-time position requires full-time energy, hours, and attention, and it can be challenging to balance full-time and part-time work. Whichever approach you decide to take, it can be most helpful to simply keep an open mind and always consider looking further into any new opportunity that interests you.
Insurance and licensing
You don’t have to wait to establish your own practice to purchase malpractice insurance. Shop around for the best rates and the coverage that most comprehensively fits your needs. If your training program allows “moonlighting,” you might need your own insurance to work at sites other than your training hospital. Many residents begin to apply for independent state licensure at the same time they begin pursuing moonlighting opportunities. It may be helpful not to wait until the last minute to do this, because the process has quite a few steps and can take a while. If your state requires letters of reference, think about which of your supervisors you can ask for one. If you plan to work in a state other than that of your training location, it may be helpful to simultaneously apply for your medical license in that state, because you will already be going through the process. Certain states offer reciprocity regarding medical licenses. The Interstate Medical Licensure Compact offers an expedited pathway to licensure for qualified physicians who want to practice in multiple states.1
Marketing your practice
Potential sources for building a panel of patients include referral networks, insurance panels, professional organizations, social media, networking, directories, and word of mouth. If you plan to accept health insurance, the directories provided by insurance panels will allow potential patients to find you when searching for practitioners who accept their plan. Professional organizations offer similar directories, and some private companies also provide directories, either for free or for a fee.
Use technology to your advantage
The exciting thing about starting a private practice today is that the technology available to support a small practice has drastically improved. Many software applications can help with scheduling and billing, which minimizes the need for office staff and enables you to be more productive. These programs typically are available via an online subscription that gives you access to an electronic medical record and other features for a monthly fee. Many of these programs provide add-ons such as a website for your practice and integrated telehealth services. While these programs typically perform many of the same functions, each has a different setup and varying workflows. An online search can facilitate a side-by-side comparison of the software programs that most closely meet your needs.
Seek out mentors and consultants
Finally, try to find a private practice mentor, and reach out to as many people as possible who have worked in any type of private practice setting. A mentor can alert you to factors you might not otherwise have considered. It also may be helpful to establish some form of supervision; such opportunities can be found through professional societies and other groups for private practice clinicians. In these groups, you also can ask other clinicians to recommend private practice and practice management consultants.
Stepping into the unknown can be an intimidating experience; however, you will not know what you are capable of until you try. Fortunately, psychiatry offers the flexibility to create a hybrid career that allows you to follow your passion and maintain your level of comfort. The American Psychiatric Association offers members additional information in the practice management resources section of its website.2
1. Interstate Medical Licensure Compact. Information for physicians. 2020. Accessed March 8, 2021. https://www.imlcc.org/information-for-physicians
2. American Psychiatric Association. Online practice handbook. 2021. Accessed March 21, 2021. https://www.psychiatry.org/psychiatrists/practice/practice-management/starting-a-practice/online-practice-handbook
Many psychiatry trainees consider private practice as a career option or form of supplemental income. In my experience, however, residency training may provide limited introduction to the general steps involved in starting a practice. In this article, I briefly summarize what I learned while exploring the private practice option as a psychiatry resident.
A good specialty for private practice
Trainees in the earlier stages of their education should be aware that the first step toward private practice may actually occur during medical school, when they are considering which specialty to pursue. If a student is particularly interested in solo private practice, they may want to select a specialty with the potential for less overhead in an independent setting. Psychiatry typically has lower overhead costs than some other specialties. This gap widens even further with the increased popularity and acceptance of telepsychiatry.
Budgeting and finance
Once you decide to pursue private practice, you will want to consider whether you prefer solo practice or group practice, and part-time or full-time. If working for yourself, you will need to understand business planning and budgeting, including how to project revenue and expenses. When first starting in solo practice—especially if you are not taking over a previously established practice—it is useful to have secondary sources of income. This can be a part-time clinical position, working with on-demand health care companies, contracting, consulting, etc. Many new physicians begin with a full-time position and decide to initiate their private practice on a part-time basis. This approach provides a level of financial security that you otherwise would not have. However, a full-time position requires full-time energy, hours, and attention, and it can be challenging to balance full-time and part-time work. Whichever approach you decide to take, it can be most helpful to simply keep an open mind and always consider looking further into any new opportunity that interests you.
Insurance and licensing
You don’t have to wait to establish your own practice to purchase malpractice insurance. Shop around for the best rates and the coverage that most comprehensively fits your needs. If your training program allows “moonlighting,” you might need your own insurance to work at sites other than your training hospital. Many residents begin to apply for independent state licensure at the same time they begin pursuing moonlighting opportunities. It may be helpful not to wait until the last minute to do this, because the process has quite a few steps and can take a while. If your state requires letters of reference, think about which of your supervisors you can ask for one. If you plan to work in a state other than that of your training location, it may be helpful to simultaneously apply for your medical license in that state, because you will already be going through the process. Certain states offer reciprocity regarding medical licenses. The Interstate Medical Licensure Compact offers an expedited pathway to licensure for qualified physicians who want to practice in multiple states.1
Marketing your practice
Potential sources for building a panel of patients include referral networks, insurance panels, professional organizations, social media, networking, directories, and word of mouth. If you plan to accept health insurance, the directories provided by insurance panels will allow potential patients to find you when searching for practitioners who accept their plan. Professional organizations offer similar directories, and some private companies also provide directories, either for free or for a fee.
Use technology to your advantage
The exciting thing about starting a private practice today is that the technology available to support a small practice has drastically improved. Many software applications can help with scheduling and billing, which minimizes the need for office staff and enables you to be more productive. These programs typically are available via an online subscription that gives you access to an electronic medical record and other features for a monthly fee. Many of these programs provide add-ons such as a website for your practice and integrated telehealth services. While these programs typically perform many of the same functions, each has a different setup and varying workflows. An online search can facilitate a side-by-side comparison of the software programs that most closely meet your needs.
Seek out mentors and consultants
Finally, try to find a private practice mentor, and reach out to as many people as possible who have worked in any type of private practice setting. A mentor can alert you to factors you might not otherwise have considered. It also may be helpful to establish some form of supervision; such opportunities can be found through professional societies and other groups for private practice clinicians. In these groups, you also can ask other clinicians to recommend private practice and practice management consultants.
Stepping into the unknown can be an intimidating experience; however, you will not know what you are capable of until you try. Fortunately, psychiatry offers the flexibility to create a hybrid career that allows you to follow your passion and maintain your level of comfort. The American Psychiatric Association offers members additional information in the practice management resources section of its website.2
Many psychiatry trainees consider private practice as a career option or form of supplemental income. In my experience, however, residency training may provide limited introduction to the general steps involved in starting a practice. In this article, I briefly summarize what I learned while exploring the private practice option as a psychiatry resident.
A good specialty for private practice
Trainees in the earlier stages of their education should be aware that the first step toward private practice may actually occur during medical school, when they are considering which specialty to pursue. If a student is particularly interested in solo private practice, they may want to select a specialty with the potential for less overhead in an independent setting. Psychiatry typically has lower overhead costs than some other specialties. This gap widens even further with the increased popularity and acceptance of telepsychiatry.
Budgeting and finance
Once you decide to pursue private practice, you will want to consider whether you prefer solo practice or group practice, and part-time or full-time. If working for yourself, you will need to understand business planning and budgeting, including how to project revenue and expenses. When first starting in solo practice—especially if you are not taking over a previously established practice—it is useful to have secondary sources of income. This can be a part-time clinical position, working with on-demand health care companies, contracting, consulting, etc. Many new physicians begin with a full-time position and decide to initiate their private practice on a part-time basis. This approach provides a level of financial security that you otherwise would not have. However, a full-time position requires full-time energy, hours, and attention, and it can be challenging to balance full-time and part-time work. Whichever approach you decide to take, it can be most helpful to simply keep an open mind and always consider looking further into any new opportunity that interests you.
Insurance and licensing
You don’t have to wait to establish your own practice to purchase malpractice insurance. Shop around for the best rates and the coverage that most comprehensively fits your needs. If your training program allows “moonlighting,” you might need your own insurance to work at sites other than your training hospital. Many residents begin to apply for independent state licensure at the same time they begin pursuing moonlighting opportunities. It may be helpful not to wait until the last minute to do this, because the process has quite a few steps and can take a while. If your state requires letters of reference, think about which of your supervisors you can ask for one. If you plan to work in a state other than that of your training location, it may be helpful to simultaneously apply for your medical license in that state, because you will already be going through the process. Certain states offer reciprocity regarding medical licenses. The Interstate Medical Licensure Compact offers an expedited pathway to licensure for qualified physicians who want to practice in multiple states.1
Marketing your practice
Potential sources for building a panel of patients include referral networks, insurance panels, professional organizations, social media, networking, directories, and word of mouth. If you plan to accept health insurance, the directories provided by insurance panels will allow potential patients to find you when searching for practitioners who accept their plan. Professional organizations offer similar directories, and some private companies also provide directories, either for free or for a fee.
Use technology to your advantage
The exciting thing about starting a private practice today is that the technology available to support a small practice has drastically improved. Many software applications can help with scheduling and billing, which minimizes the need for office staff and enables you to be more productive. These programs typically are available via an online subscription that gives you access to an electronic medical record and other features for a monthly fee. Many of these programs provide add-ons such as a website for your practice and integrated telehealth services. While these programs typically perform many of the same functions, each has a different setup and varying workflows. An online search can facilitate a side-by-side comparison of the software programs that most closely meet your needs.
Seek out mentors and consultants
Finally, try to find a private practice mentor, and reach out to as many people as possible who have worked in any type of private practice setting. A mentor can alert you to factors you might not otherwise have considered. It also may be helpful to establish some form of supervision; such opportunities can be found through professional societies and other groups for private practice clinicians. In these groups, you also can ask other clinicians to recommend private practice and practice management consultants.
Stepping into the unknown can be an intimidating experience; however, you will not know what you are capable of until you try. Fortunately, psychiatry offers the flexibility to create a hybrid career that allows you to follow your passion and maintain your level of comfort. The American Psychiatric Association offers members additional information in the practice management resources section of its website.2
1. Interstate Medical Licensure Compact. Information for physicians. 2020. Accessed March 8, 2021. https://www.imlcc.org/information-for-physicians
2. American Psychiatric Association. Online practice handbook. 2021. Accessed March 21, 2021. https://www.psychiatry.org/psychiatrists/practice/practice-management/starting-a-practice/online-practice-handbook
1. Interstate Medical Licensure Compact. Information for physicians. 2020. Accessed March 8, 2021. https://www.imlcc.org/information-for-physicians
2. American Psychiatric Association. Online practice handbook. 2021. Accessed March 21, 2021. https://www.psychiatry.org/psychiatrists/practice/practice-management/starting-a-practice/online-practice-handbook
Efficacy and safety of high-dose antipsychotic therapy
Mr. K, age 21, is admitted to the psychiatry unit with agitation, disorganized behavior, and paranoia. Upon presentation, he has no known medical history or current medications. He is diagnosed with schizophrenia and subsequently tolerates but does not respond to adequate durations of treatment with fluphenazine, 20 mg/d; aripiprazole, 30 mg/d; and risperidone, 6 mg/d. Medication adherence is verified, but Mr. K is reluctant to try a fourth antipsychotic. The treatment team suspects that Mr. K may be a cytochrome P450 (CYP) 2D6 ultra-rapid metabolizer, so they obtain a serum risperidone level. The serum risperidone concentration is subtherapeutic (10 ng/mL). What should be considered next?
Several factors must be considered when a patient with psychosis does not experience significant symptomatic improvement with an adequate antipsychotic trial. This article focuses on high-dose second-generation antipsychotic (SGA) therapy in adults with psychosis. “High-dose” antipsychotic therapy is dosing that exceeds the standard maximum dosage for a given antipsychotic. Existing evidence on the use of high-dose SGAs consists of open-label studies and case reports, as well as a handful of randomized controlled trials (RCTs) with small sample sizes and high dropout rates. In some studies, the use of concomitant interventions (eg, duplicate antipsychotic therapy) limit the interpretation of data. High-dose first-generation antipsychotic therapy is discouraged because of a heightened risk of extrapyramidal symptoms (EPS).
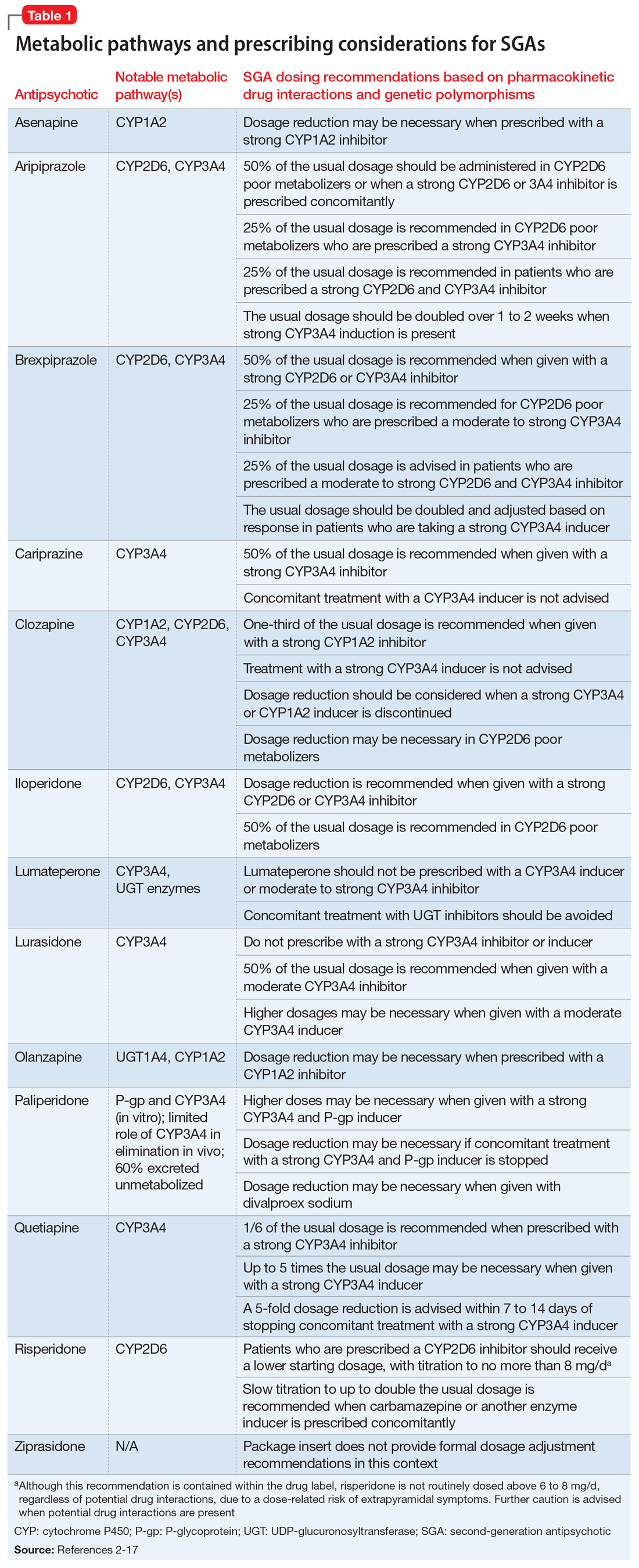
Steps to take before increasing the dose
When considering prescribing high-dose antipsychotic therapy, first confirm that the patient has been adherent to the current medication regimen. Also, screen for factors that might impair drug absorption, such as bariatric surgery or noncompliance with administration precautions.1 For example, administration of lurasidone with less than 350 calories may considerably decrease absorption.2 Dosage requirements may vary based on ethnicity, gender, CYP polymorphisms, and pharmacokinetic drug interactions (Table 12-17).1,18,19 Causes of inadequate efficacy should be addressed before considering the use of high-dose antipsychotic therapy.1 Under certain circumstances, serum drug concentrations may be used to guide antipsychotic dosing (Table 22-17). Inadequate response despite a therapeutic serum concentration may indicate pharmacodynamic failure.1 Inadequate response in the context of subtherapeutic serum concentrations, good medication adherence, and compliance to administration precautions may be indicative of a genetic polymorphism or drug interaction.1 Changes in antipsychotic dosing or selection may be warranted, depending on associated risks and benefits.
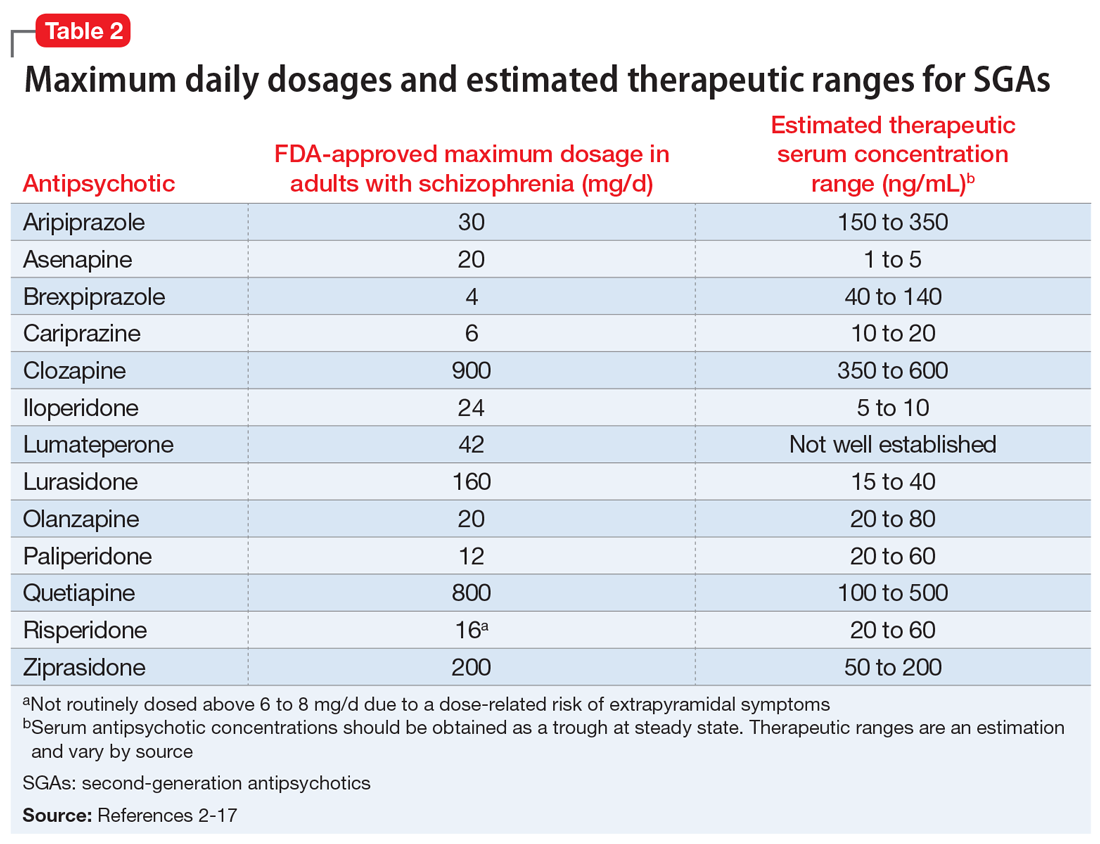
SGAs and high-dose administration
The SGA with the greatest evidence for high-dose administration is olanzapine, which is similar in structure and receptor pharmacology to clozapine.20,21 The use of high-dose olanzapine is controversial. High-dose olanzapine has been compared to clozapine in patients with treatment-resistant schizophrenia (TRS) and schizoaffective disorder. Meltzer et al22 reported similar efficacy with clozapine, 300 to 900 mg/d, and olanzapine, 25 to 45 mg/d. In this study, high-dose olanzapine caused more weight gain when compared to clozapine. Olanzapine dosages of up to 100 mg/d have been prescribed for TRS; however, this is not common practice.23 A study comparing 10, 20, and 40 mg/d in patients with non-TRS or schizoaffective disorder showed no advantage with higher dosages.24
There is limited data on high-dose treatment with other SGAs.17 Orthostasis may limit iloperidone’s safety at high doses, and single doses of asenapine should not exceed 10 mg.25 Limited sublingual surface area and saliva saturation result in decreased bioavailability with higher asenapine doses.25,26 In a small RCT of patients with stable schizophrenia or schizoaffective disorder, aripiprazole was relatively well-tolerated up to 75 mg/d, whereas akathisia and tachycardia occurred with 90 mg/d.27 Case reports have documented successful treatment with aripiprazole, 60 to 75 mg/d; however, dizziness and worsening psychosis, agitation, and confusion have been observed.28-31
There is a paucity of data on high-dose risperidone and paliperidone, possibly due to their potent dopamine-2 (D2) receptor antagonism and dose-related risk of EPS.1 At risperidone dosages >6 mg/d, the balance between D2 and serotonin-2A (5-HT2A) receptor potency is lost, which increases the potential for EPS.32 In one RCT, long-acting injectable (LAI) risperidone, up to 100 mg biweekly, was well-tolerated but no more effective for TRS than 50 mg biweekly.33 A case report suggested improvement of TRS in a patient administered risperidone LAI, 75 mg vs 37.5 mg biweekly, but it is unclear if a 50-mg dosage was tried.34 Another case report documented improvement in schizophrenia symptoms with risperidone LAI, 125 mg biweekly; however, anticholinergic therapy was required for EPS.35
Dose-dependent adverse effects, including EPS, sedation, anticholinergic effects, orthostasis, hyperprolactinemia, and QTc prolongation, may limit the safety of high-dose antipsychotic therapy.1,20,36 Two studies showed no correlation between QTc prolongation and ziprasidone dosages of up to 320 mg/d for psychosis.37,38 QTc prolongation was more likely at higher ziprasidone concentrations.37 Higher concentrations, but not higher dosages, also trended toward improvement in positive symptoms, and concentrations >100 ng/mL were associated with more negative symptoms.37 A case report described improvement in positive symptoms of schizoaffective disorder with ziprasidone, 320 mg/d, but activation, hostility, and depression worsened.39
Continue to: Compared with other antipsychotics...
Compared with other antipsychotics, high-dose clozapine and quetiapine may be less likely to cause EPS due to lower D2 receptor occupancies.40 Nevertheless, increased activity at other postsynaptic receptors may lead to constipation, metabolic effects, and sedation.1,41,42 Case reports suggest efficacy with quetiapine, 1,200 to 2,400 mg/d, vs lower dosages for patients with TRS.43,44 However, RCTs of quetiapine, 600 and 800 mg/d vs 1,200 mg/d, have not demonstrated an efficacy advantage with high-dose treatment in patients with schizophrenia or schizoaffective disorder.41,45 High-dose quetiapine has also resulted in photopsia, cardiotoxicity, orthostasis, dysphagia, and sedation.43,46,47
Proceed with caution
In light of safety concerns and a lack of high-quality evidence for high-dose antipsychotic therapy, alternative solutions for inadequate response to treatment should be considered. Underlying causes of poor response should be addressed, and alternative antipsychotics should be utilized, when appropriate. A clozapine trial remains first-line for TRS. Olanzapine may be the best-supported high-dose antipsychotic alternative when clozapine is not an option. High antipsychotic dosages are not well-studied in patients with genetic polymorphisms or unavoidable drug interactions. Serum antipsychotic concentrations may facilitate dosing in these patients.
If high-dose antipsychotic therapy is deemed necessary, its ongoing appropriateness should be continually re-evaluated. Higher antipsychotic dosages and D2 receptor occupancies may be required to manage acute psychosis, but efficacy may be maintained and adverse effects limited with the use of lower dosages during maintenance treatment.48,49 Long-term treatment with high-dose antipsychotic therapy should be avoided, when possible. If high-dose antipsychotic therapy is prescribed, the rationale should be well-documented. Dosage adjustments should not be made until steady state is reached on a given dosage. Electrocardiograms should be obtained at baseline, steady state, and routinely thereafter.3,20 Tolerability should be assessed regularly, and screening for drug interactions should be conducted when new medications are initiated.
Case CONTINUED
Because Mr. K’s serum risperidone level is subtherapeutic (10 ng/mL), his risperidone dosage is cautiously titrated to 10 mg/d, divided (serum concentration: 22 ng/mL). Mr. K develops mild orthostasis but denies other adverse effects. His psychotic symptoms resolve, and he is discharged with education on nonpharmacologic management of orthostasis. The rationale for high-dose risperidone is relayed to his outpatient psychiatrist, as well as a recommendation to monitor Mr. K closely for continued efficacy and tolerability.
Related Resource
- Barnes TRE, Drake R, Paton C, et al. Evidence-based guidelines for the pharmacological treatment of schizophrenia: updated recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2020;34(1):3-78.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Brexpiprazole • Rexulti
Cariprazine • Vraylar
Clozapine • Clozaril
Iloperidone • Fanapt
Lumateperone • Caplyta
Lurasidone • Latuda
Olanzapine • Zyprexa
Paliperidone • Invega
Quetiapine • Seroquel
Risperidone • Risperdal
Risperidone long-acting injection • Risperdal Consta
Ziprasidone • Geodon
1. Morrissette DA, Stahl SM. Treating the violence patient with psychosis or impulsivity utilizing antipsychotic polypharmacy and high-dose monotherapy. CNS Spectrums. 2014;19(5):439-448.
2. Latuda [package insert]. Marlborough, MA: Sunovion Pharmaceuticals Inc.; 2019.
3. Taylor D, Paton C, Kapur S. The Maudsley prescribing guidelines in psychiatry. 12th ed. Wiley Blackwell; 2015.
4. Vyas P, Hwang BJ, Brasic JR. An evaluation of lumateperone tosylate for the treatment of schizophrenia. Expert Opin Pharmacother. 2020;21(2):139-145.
5. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-02):9-62.
6. Saphris [package insert]. Irvine, CA: Allergan USA, Inc; 2017.
7. Abilify [package insert]. Tokyo, Japan: Otsuka America Pharmaceutical, Inc.; 2014.
8. Rexulti [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc.; 2020.
9. Vraylar [package insert]. Madison, NJ: Allergan USA, Inc.; 2019.
10. Clozaril [package insert]. Rosemont, PA: Novartis Pharmaceuticals Corporation; 2017.
11. Fanapt [package insert]. Washington, DC: Vanda Pharmaceuticals Inc.; 2016.
12. Caplyta [package insert]. New York, NY: Intra-Cellular Therapies, Inc.; 2019.
13. Zyprexa [package insert]. Indianapolis, IN: Lilly USA, LLC.; 2020.
14. Invega [package insert]. Titusville, NJ: Janssen Pharmaceutical Companies; 2019.
15. Seroquel [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2016.
16. Risperdal [package insert]. Titusville, NJ: Janssen Pharmaceutical Companies; 2020.
17. Geodon [package insert]. New York, NY: Pfizer Inc.; 2020.
18. Chaudhry IB, Neelam K, Duddu V, et al. Ethnicity and psychopharmacology. J Psychopharmacol. 2008;22(6):673-680.
19. Seeman MV. Men and women respond differently to antipsychotic drugs. Neuropharmacology. 2020;163:107631. doi: 10.1016/j.neuropharm.2019.05.008
20. Barnes TRE, Drake R, Paton C, et al. Evidence-based guidelines for the pharmacological treatment of schizophrenia: updated recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2020;34(1):3-78.
21. Citrome L, McEvoy JP, Todtenkopf MS, et al. A commentary on the efficacy of olanzapine for the treatment of schizophrenia: the past, present, and future. Neuropsych Dis Treat. 2019;15:2559-2569.
22. Meltzer HY, Bobo WV, Ajanta R, et al. A randomized, double-blind comparison of clozapine and high-dose olanzapine in treatment-resistant patients with schizophrenia. J Clin Psychiatry. 2008;69(2):274-285.
23. Batail JM, Langree B, Robert G, et al. Use of very-high-dose olanzapine in treatment-resistant schizophrenia. Schizophr Res. 2014;159(2-3):411-414.
24. Kinon BJ, Volavka J, Stauffer V, et al. Standard and higher dose of olanzapine in patients with schizophrenia or schizoaffective disorder. J Clin Psychopharmacol. 2008;28(4):392-400.
25. Stahl SM. Stahl’s essential psychopharmacology prescriber’s guide. 6th ed. Cambridge University Press; 2017.
26. Bartlett JA, van der Voort Maarschalk K. Understanding the oral mucosal absorption and resulting clinical pharmacokinetics of asenapine. AAPS PharmSciTech. 2012;13(4):1110-1115.
27. Auby P, Saha A, Ali M, et al. Safety and tolerability of aripiprazole at doses higher than 30 mg. Eur Neuropsychopharm. 2002;12(3):288.
28. Chavez B, Poveda R. Efficacy with high-dose aripiprazole after olanzapine-related metabolic disturbances. Ann Pharmacother. 2006;40(12):2265-2268.
29. Duggal HS, Mendhekar DN. High-dose aripiprazole in treatment-resistant schizophrenia. J Clin Psychiatry. 2006;67(4):674-675.
30. Thone J. Worsened agitation and confusion in schizophrenia subsequent to high-dose aripiprazole. J Neuropsychiatry Clin Neurosci. 2007;19(4):481-482.
31. Saatcioglu O, Gumus S, Kamberyan K, et al. Efficacy of high-dose aripiprazole for treatment-resistant schizoaffective disorder: a case report. Psychopharmacol Bull. 2010;43(4):70-72.
32. Thomson SR, Chogtu B, Bhattacharjee D, et al. Extrapyramidal symptoms probably related to risperidone treatment: a case series. Ann Neurosci. 2017;24(3):155-163.
33. Meltzer HY, Lindenmayer JP, Kwentus J, et al. A six month randomized controlled trial of long acting injectable risperidone 50 and 100 mg in treatment resistant schizophrenia. Schizophr Res. 2014;154(1-3):14-22.
34. Hou Y, Lai C. The response of psychotic symptoms in a patient with resistant schizophrenia under treatment of high-dose risperidone long-acting injection. J Neuropsychiatry Clin Neurosci. 2014;26(3):E16-E17. doi: 10.1176/appi.neuropsych.13070150
35. Albrecht A, Morena PG, Baumann P, et al. High dose of depot risperidone in a nonresponder schizophrenic patient. J Clin Psychopharmacol. 2004;24(6):673-674.
36. Mace S, Taylor D. Reducing the rates of prescribing high-dose antipsychotics and polypharmacy on psychiatric inpatient and intensive care units: results of a 6-year quality improvement programme. Ther Adv Psychopharmacol. 2015;5(1):4-12.
37. Goff DC, McEvoy JP, Citrome L, et al. High-dose oral ziprasidone versus conventional dosing in schizophrenia patients with residual symptoms. J Clin Psychopharmacol. 2013;33:485-490.
38. Levy WO, Robichaux-Keene NR, Nunez C. No significant QTc interval changes with high-dose ziprasidone: a case series. J Psychiatr Pract. 2004;10(4):227-232.
39. Kaushik S, Maccabee N, Kaushik S, et al. Activation induced by high-dose ziprasidone: a case report. J Clin Psychiatry. 2009;70(9):1326-1327.
40. Seeman P. Targeting the dopamine D2 receptor in schizophrenia. Expert Opin Ther Targets. 2006;10(4):515-531.
41. Honer WG, MacEwan W, Gendron A, et al. A randomized, double-blind, placebo-controlled study of safety and tolerability of high-dose quetiapine in patients with persistent symptoms of schizophrenia or schizoaffective disorder. J Clin Psychiatry. 2012;73(1):13-20.
42. Sokolski KN, Brown BJ, Meldon M. Urinary retention following repeated high-dose quetiapine. Ann Pharmacother. 2004;38(5):899-890.
43. Chandrappa P, Ho L. Case reports of patients with treatment-resistant schizophrenia and related psychotic disorders intolerant to clozapine responding to high doses of quetiapine. Ther Adv Psychopharmacol. 2012;2(5):207-209.
44. Pierre JM, Wirshing DA, Wirshing WC, et al. High-dose quetiapine in treatment refractory schizophrenia. Schizophr Res. 2005;73:373-375.
45. Lindenmyer JP, Citrome L, Khan A, et al. A randomized, double-blind parallel-group, fixed-dose, clinical trial of quetiapine at 600 vs. 1200 mg/d for patients with treatment-resistant schizophrenia or schizoaffective disorder. J Clin Psychopharmacol. 2011;31(2):160-168.
46. Hazra M, Culo S, Mamo D. High-dose quetiapine and photopsia. J Clin Psychopharmacol. 2006;26(5):546-547.
47. Smolders DME, Smolders WAP. Case report and review of the literature: cardiomyopathy in a young woman on high-dose quetiapine. Cardiovasc Toxicol. 2017;17(4):478-481.
48. Takeuchi H, Suzuki T, Bies RR, et al. Dose reduction of risperidone and olanzapine and estimated D2 receptor occupancy in stable patients with schizophrenia: findings from an open-label, randomized, controlled study. J Clin Psychiatry. 2014;75(11):1209-1214.
49. Kumar V, Rao NP, Narasimha V, et al. Antipsychotic dose in maintenance treatment of schizophrenia: a retrospective study. Psychiatry Res. 2016;245:311-316.
Mr. K, age 21, is admitted to the psychiatry unit with agitation, disorganized behavior, and paranoia. Upon presentation, he has no known medical history or current medications. He is diagnosed with schizophrenia and subsequently tolerates but does not respond to adequate durations of treatment with fluphenazine, 20 mg/d; aripiprazole, 30 mg/d; and risperidone, 6 mg/d. Medication adherence is verified, but Mr. K is reluctant to try a fourth antipsychotic. The treatment team suspects that Mr. K may be a cytochrome P450 (CYP) 2D6 ultra-rapid metabolizer, so they obtain a serum risperidone level. The serum risperidone concentration is subtherapeutic (10 ng/mL). What should be considered next?
Several factors must be considered when a patient with psychosis does not experience significant symptomatic improvement with an adequate antipsychotic trial. This article focuses on high-dose second-generation antipsychotic (SGA) therapy in adults with psychosis. “High-dose” antipsychotic therapy is dosing that exceeds the standard maximum dosage for a given antipsychotic. Existing evidence on the use of high-dose SGAs consists of open-label studies and case reports, as well as a handful of randomized controlled trials (RCTs) with small sample sizes and high dropout rates. In some studies, the use of concomitant interventions (eg, duplicate antipsychotic therapy) limit the interpretation of data. High-dose first-generation antipsychotic therapy is discouraged because of a heightened risk of extrapyramidal symptoms (EPS).

Steps to take before increasing the dose
When considering prescribing high-dose antipsychotic therapy, first confirm that the patient has been adherent to the current medication regimen. Also, screen for factors that might impair drug absorption, such as bariatric surgery or noncompliance with administration precautions.1 For example, administration of lurasidone with less than 350 calories may considerably decrease absorption.2 Dosage requirements may vary based on ethnicity, gender, CYP polymorphisms, and pharmacokinetic drug interactions (Table 12-17).1,18,19 Causes of inadequate efficacy should be addressed before considering the use of high-dose antipsychotic therapy.1 Under certain circumstances, serum drug concentrations may be used to guide antipsychotic dosing (Table 22-17). Inadequate response despite a therapeutic serum concentration may indicate pharmacodynamic failure.1 Inadequate response in the context of subtherapeutic serum concentrations, good medication adherence, and compliance to administration precautions may be indicative of a genetic polymorphism or drug interaction.1 Changes in antipsychotic dosing or selection may be warranted, depending on associated risks and benefits.

SGAs and high-dose administration
The SGA with the greatest evidence for high-dose administration is olanzapine, which is similar in structure and receptor pharmacology to clozapine.20,21 The use of high-dose olanzapine is controversial. High-dose olanzapine has been compared to clozapine in patients with treatment-resistant schizophrenia (TRS) and schizoaffective disorder. Meltzer et al22 reported similar efficacy with clozapine, 300 to 900 mg/d, and olanzapine, 25 to 45 mg/d. In this study, high-dose olanzapine caused more weight gain when compared to clozapine. Olanzapine dosages of up to 100 mg/d have been prescribed for TRS; however, this is not common practice.23 A study comparing 10, 20, and 40 mg/d in patients with non-TRS or schizoaffective disorder showed no advantage with higher dosages.24
There is limited data on high-dose treatment with other SGAs.17 Orthostasis may limit iloperidone’s safety at high doses, and single doses of asenapine should not exceed 10 mg.25 Limited sublingual surface area and saliva saturation result in decreased bioavailability with higher asenapine doses.25,26 In a small RCT of patients with stable schizophrenia or schizoaffective disorder, aripiprazole was relatively well-tolerated up to 75 mg/d, whereas akathisia and tachycardia occurred with 90 mg/d.27 Case reports have documented successful treatment with aripiprazole, 60 to 75 mg/d; however, dizziness and worsening psychosis, agitation, and confusion have been observed.28-31
There is a paucity of data on high-dose risperidone and paliperidone, possibly due to their potent dopamine-2 (D2) receptor antagonism and dose-related risk of EPS.1 At risperidone dosages >6 mg/d, the balance between D2 and serotonin-2A (5-HT2A) receptor potency is lost, which increases the potential for EPS.32 In one RCT, long-acting injectable (LAI) risperidone, up to 100 mg biweekly, was well-tolerated but no more effective for TRS than 50 mg biweekly.33 A case report suggested improvement of TRS in a patient administered risperidone LAI, 75 mg vs 37.5 mg biweekly, but it is unclear if a 50-mg dosage was tried.34 Another case report documented improvement in schizophrenia symptoms with risperidone LAI, 125 mg biweekly; however, anticholinergic therapy was required for EPS.35
Dose-dependent adverse effects, including EPS, sedation, anticholinergic effects, orthostasis, hyperprolactinemia, and QTc prolongation, may limit the safety of high-dose antipsychotic therapy.1,20,36 Two studies showed no correlation between QTc prolongation and ziprasidone dosages of up to 320 mg/d for psychosis.37,38 QTc prolongation was more likely at higher ziprasidone concentrations.37 Higher concentrations, but not higher dosages, also trended toward improvement in positive symptoms, and concentrations >100 ng/mL were associated with more negative symptoms.37 A case report described improvement in positive symptoms of schizoaffective disorder with ziprasidone, 320 mg/d, but activation, hostility, and depression worsened.39
Continue to: Compared with other antipsychotics...
Compared with other antipsychotics, high-dose clozapine and quetiapine may be less likely to cause EPS due to lower D2 receptor occupancies.40 Nevertheless, increased activity at other postsynaptic receptors may lead to constipation, metabolic effects, and sedation.1,41,42 Case reports suggest efficacy with quetiapine, 1,200 to 2,400 mg/d, vs lower dosages for patients with TRS.43,44 However, RCTs of quetiapine, 600 and 800 mg/d vs 1,200 mg/d, have not demonstrated an efficacy advantage with high-dose treatment in patients with schizophrenia or schizoaffective disorder.41,45 High-dose quetiapine has also resulted in photopsia, cardiotoxicity, orthostasis, dysphagia, and sedation.43,46,47
Proceed with caution
In light of safety concerns and a lack of high-quality evidence for high-dose antipsychotic therapy, alternative solutions for inadequate response to treatment should be considered. Underlying causes of poor response should be addressed, and alternative antipsychotics should be utilized, when appropriate. A clozapine trial remains first-line for TRS. Olanzapine may be the best-supported high-dose antipsychotic alternative when clozapine is not an option. High antipsychotic dosages are not well-studied in patients with genetic polymorphisms or unavoidable drug interactions. Serum antipsychotic concentrations may facilitate dosing in these patients.
If high-dose antipsychotic therapy is deemed necessary, its ongoing appropriateness should be continually re-evaluated. Higher antipsychotic dosages and D2 receptor occupancies may be required to manage acute psychosis, but efficacy may be maintained and adverse effects limited with the use of lower dosages during maintenance treatment.48,49 Long-term treatment with high-dose antipsychotic therapy should be avoided, when possible. If high-dose antipsychotic therapy is prescribed, the rationale should be well-documented. Dosage adjustments should not be made until steady state is reached on a given dosage. Electrocardiograms should be obtained at baseline, steady state, and routinely thereafter.3,20 Tolerability should be assessed regularly, and screening for drug interactions should be conducted when new medications are initiated.
Case CONTINUED
Because Mr. K’s serum risperidone level is subtherapeutic (10 ng/mL), his risperidone dosage is cautiously titrated to 10 mg/d, divided (serum concentration: 22 ng/mL). Mr. K develops mild orthostasis but denies other adverse effects. His psychotic symptoms resolve, and he is discharged with education on nonpharmacologic management of orthostasis. The rationale for high-dose risperidone is relayed to his outpatient psychiatrist, as well as a recommendation to monitor Mr. K closely for continued efficacy and tolerability.
Related Resource
- Barnes TRE, Drake R, Paton C, et al. Evidence-based guidelines for the pharmacological treatment of schizophrenia: updated recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2020;34(1):3-78.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Brexpiprazole • Rexulti
Cariprazine • Vraylar
Clozapine • Clozaril
Iloperidone • Fanapt
Lumateperone • Caplyta
Lurasidone • Latuda
Olanzapine • Zyprexa
Paliperidone • Invega
Quetiapine • Seroquel
Risperidone • Risperdal
Risperidone long-acting injection • Risperdal Consta
Ziprasidone • Geodon
Mr. K, age 21, is admitted to the psychiatry unit with agitation, disorganized behavior, and paranoia. Upon presentation, he has no known medical history or current medications. He is diagnosed with schizophrenia and subsequently tolerates but does not respond to adequate durations of treatment with fluphenazine, 20 mg/d; aripiprazole, 30 mg/d; and risperidone, 6 mg/d. Medication adherence is verified, but Mr. K is reluctant to try a fourth antipsychotic. The treatment team suspects that Mr. K may be a cytochrome P450 (CYP) 2D6 ultra-rapid metabolizer, so they obtain a serum risperidone level. The serum risperidone concentration is subtherapeutic (10 ng/mL). What should be considered next?
Several factors must be considered when a patient with psychosis does not experience significant symptomatic improvement with an adequate antipsychotic trial. This article focuses on high-dose second-generation antipsychotic (SGA) therapy in adults with psychosis. “High-dose” antipsychotic therapy is dosing that exceeds the standard maximum dosage for a given antipsychotic. Existing evidence on the use of high-dose SGAs consists of open-label studies and case reports, as well as a handful of randomized controlled trials (RCTs) with small sample sizes and high dropout rates. In some studies, the use of concomitant interventions (eg, duplicate antipsychotic therapy) limit the interpretation of data. High-dose first-generation antipsychotic therapy is discouraged because of a heightened risk of extrapyramidal symptoms (EPS).

Steps to take before increasing the dose
When considering prescribing high-dose antipsychotic therapy, first confirm that the patient has been adherent to the current medication regimen. Also, screen for factors that might impair drug absorption, such as bariatric surgery or noncompliance with administration precautions.1 For example, administration of lurasidone with less than 350 calories may considerably decrease absorption.2 Dosage requirements may vary based on ethnicity, gender, CYP polymorphisms, and pharmacokinetic drug interactions (Table 12-17).1,18,19 Causes of inadequate efficacy should be addressed before considering the use of high-dose antipsychotic therapy.1 Under certain circumstances, serum drug concentrations may be used to guide antipsychotic dosing (Table 22-17). Inadequate response despite a therapeutic serum concentration may indicate pharmacodynamic failure.1 Inadequate response in the context of subtherapeutic serum concentrations, good medication adherence, and compliance to administration precautions may be indicative of a genetic polymorphism or drug interaction.1 Changes in antipsychotic dosing or selection may be warranted, depending on associated risks and benefits.

SGAs and high-dose administration
The SGA with the greatest evidence for high-dose administration is olanzapine, which is similar in structure and receptor pharmacology to clozapine.20,21 The use of high-dose olanzapine is controversial. High-dose olanzapine has been compared to clozapine in patients with treatment-resistant schizophrenia (TRS) and schizoaffective disorder. Meltzer et al22 reported similar efficacy with clozapine, 300 to 900 mg/d, and olanzapine, 25 to 45 mg/d. In this study, high-dose olanzapine caused more weight gain when compared to clozapine. Olanzapine dosages of up to 100 mg/d have been prescribed for TRS; however, this is not common practice.23 A study comparing 10, 20, and 40 mg/d in patients with non-TRS or schizoaffective disorder showed no advantage with higher dosages.24
There is limited data on high-dose treatment with other SGAs.17 Orthostasis may limit iloperidone’s safety at high doses, and single doses of asenapine should not exceed 10 mg.25 Limited sublingual surface area and saliva saturation result in decreased bioavailability with higher asenapine doses.25,26 In a small RCT of patients with stable schizophrenia or schizoaffective disorder, aripiprazole was relatively well-tolerated up to 75 mg/d, whereas akathisia and tachycardia occurred with 90 mg/d.27 Case reports have documented successful treatment with aripiprazole, 60 to 75 mg/d; however, dizziness and worsening psychosis, agitation, and confusion have been observed.28-31
There is a paucity of data on high-dose risperidone and paliperidone, possibly due to their potent dopamine-2 (D2) receptor antagonism and dose-related risk of EPS.1 At risperidone dosages >6 mg/d, the balance between D2 and serotonin-2A (5-HT2A) receptor potency is lost, which increases the potential for EPS.32 In one RCT, long-acting injectable (LAI) risperidone, up to 100 mg biweekly, was well-tolerated but no more effective for TRS than 50 mg biweekly.33 A case report suggested improvement of TRS in a patient administered risperidone LAI, 75 mg vs 37.5 mg biweekly, but it is unclear if a 50-mg dosage was tried.34 Another case report documented improvement in schizophrenia symptoms with risperidone LAI, 125 mg biweekly; however, anticholinergic therapy was required for EPS.35
Dose-dependent adverse effects, including EPS, sedation, anticholinergic effects, orthostasis, hyperprolactinemia, and QTc prolongation, may limit the safety of high-dose antipsychotic therapy.1,20,36 Two studies showed no correlation between QTc prolongation and ziprasidone dosages of up to 320 mg/d for psychosis.37,38 QTc prolongation was more likely at higher ziprasidone concentrations.37 Higher concentrations, but not higher dosages, also trended toward improvement in positive symptoms, and concentrations >100 ng/mL were associated with more negative symptoms.37 A case report described improvement in positive symptoms of schizoaffective disorder with ziprasidone, 320 mg/d, but activation, hostility, and depression worsened.39
Continue to: Compared with other antipsychotics...
Compared with other antipsychotics, high-dose clozapine and quetiapine may be less likely to cause EPS due to lower D2 receptor occupancies.40 Nevertheless, increased activity at other postsynaptic receptors may lead to constipation, metabolic effects, and sedation.1,41,42 Case reports suggest efficacy with quetiapine, 1,200 to 2,400 mg/d, vs lower dosages for patients with TRS.43,44 However, RCTs of quetiapine, 600 and 800 mg/d vs 1,200 mg/d, have not demonstrated an efficacy advantage with high-dose treatment in patients with schizophrenia or schizoaffective disorder.41,45 High-dose quetiapine has also resulted in photopsia, cardiotoxicity, orthostasis, dysphagia, and sedation.43,46,47
Proceed with caution
In light of safety concerns and a lack of high-quality evidence for high-dose antipsychotic therapy, alternative solutions for inadequate response to treatment should be considered. Underlying causes of poor response should be addressed, and alternative antipsychotics should be utilized, when appropriate. A clozapine trial remains first-line for TRS. Olanzapine may be the best-supported high-dose antipsychotic alternative when clozapine is not an option. High antipsychotic dosages are not well-studied in patients with genetic polymorphisms or unavoidable drug interactions. Serum antipsychotic concentrations may facilitate dosing in these patients.
If high-dose antipsychotic therapy is deemed necessary, its ongoing appropriateness should be continually re-evaluated. Higher antipsychotic dosages and D2 receptor occupancies may be required to manage acute psychosis, but efficacy may be maintained and adverse effects limited with the use of lower dosages during maintenance treatment.48,49 Long-term treatment with high-dose antipsychotic therapy should be avoided, when possible. If high-dose antipsychotic therapy is prescribed, the rationale should be well-documented. Dosage adjustments should not be made until steady state is reached on a given dosage. Electrocardiograms should be obtained at baseline, steady state, and routinely thereafter.3,20 Tolerability should be assessed regularly, and screening for drug interactions should be conducted when new medications are initiated.
Case CONTINUED
Because Mr. K’s serum risperidone level is subtherapeutic (10 ng/mL), his risperidone dosage is cautiously titrated to 10 mg/d, divided (serum concentration: 22 ng/mL). Mr. K develops mild orthostasis but denies other adverse effects. His psychotic symptoms resolve, and he is discharged with education on nonpharmacologic management of orthostasis. The rationale for high-dose risperidone is relayed to his outpatient psychiatrist, as well as a recommendation to monitor Mr. K closely for continued efficacy and tolerability.
Related Resource
- Barnes TRE, Drake R, Paton C, et al. Evidence-based guidelines for the pharmacological treatment of schizophrenia: updated recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2020;34(1):3-78.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Brexpiprazole • Rexulti
Cariprazine • Vraylar
Clozapine • Clozaril
Iloperidone • Fanapt
Lumateperone • Caplyta
Lurasidone • Latuda
Olanzapine • Zyprexa
Paliperidone • Invega
Quetiapine • Seroquel
Risperidone • Risperdal
Risperidone long-acting injection • Risperdal Consta
Ziprasidone • Geodon
1. Morrissette DA, Stahl SM. Treating the violence patient with psychosis or impulsivity utilizing antipsychotic polypharmacy and high-dose monotherapy. CNS Spectrums. 2014;19(5):439-448.
2. Latuda [package insert]. Marlborough, MA: Sunovion Pharmaceuticals Inc.; 2019.
3. Taylor D, Paton C, Kapur S. The Maudsley prescribing guidelines in psychiatry. 12th ed. Wiley Blackwell; 2015.
4. Vyas P, Hwang BJ, Brasic JR. An evaluation of lumateperone tosylate for the treatment of schizophrenia. Expert Opin Pharmacother. 2020;21(2):139-145.
5. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-02):9-62.
6. Saphris [package insert]. Irvine, CA: Allergan USA, Inc; 2017.
7. Abilify [package insert]. Tokyo, Japan: Otsuka America Pharmaceutical, Inc.; 2014.
8. Rexulti [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc.; 2020.
9. Vraylar [package insert]. Madison, NJ: Allergan USA, Inc.; 2019.
10. Clozaril [package insert]. Rosemont, PA: Novartis Pharmaceuticals Corporation; 2017.
11. Fanapt [package insert]. Washington, DC: Vanda Pharmaceuticals Inc.; 2016.
12. Caplyta [package insert]. New York, NY: Intra-Cellular Therapies, Inc.; 2019.
13. Zyprexa [package insert]. Indianapolis, IN: Lilly USA, LLC.; 2020.
14. Invega [package insert]. Titusville, NJ: Janssen Pharmaceutical Companies; 2019.
15. Seroquel [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2016.
16. Risperdal [package insert]. Titusville, NJ: Janssen Pharmaceutical Companies; 2020.
17. Geodon [package insert]. New York, NY: Pfizer Inc.; 2020.
18. Chaudhry IB, Neelam K, Duddu V, et al. Ethnicity and psychopharmacology. J Psychopharmacol. 2008;22(6):673-680.
19. Seeman MV. Men and women respond differently to antipsychotic drugs. Neuropharmacology. 2020;163:107631. doi: 10.1016/j.neuropharm.2019.05.008
20. Barnes TRE, Drake R, Paton C, et al. Evidence-based guidelines for the pharmacological treatment of schizophrenia: updated recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2020;34(1):3-78.
21. Citrome L, McEvoy JP, Todtenkopf MS, et al. A commentary on the efficacy of olanzapine for the treatment of schizophrenia: the past, present, and future. Neuropsych Dis Treat. 2019;15:2559-2569.
22. Meltzer HY, Bobo WV, Ajanta R, et al. A randomized, double-blind comparison of clozapine and high-dose olanzapine in treatment-resistant patients with schizophrenia. J Clin Psychiatry. 2008;69(2):274-285.
23. Batail JM, Langree B, Robert G, et al. Use of very-high-dose olanzapine in treatment-resistant schizophrenia. Schizophr Res. 2014;159(2-3):411-414.
24. Kinon BJ, Volavka J, Stauffer V, et al. Standard and higher dose of olanzapine in patients with schizophrenia or schizoaffective disorder. J Clin Psychopharmacol. 2008;28(4):392-400.
25. Stahl SM. Stahl’s essential psychopharmacology prescriber’s guide. 6th ed. Cambridge University Press; 2017.
26. Bartlett JA, van der Voort Maarschalk K. Understanding the oral mucosal absorption and resulting clinical pharmacokinetics of asenapine. AAPS PharmSciTech. 2012;13(4):1110-1115.
27. Auby P, Saha A, Ali M, et al. Safety and tolerability of aripiprazole at doses higher than 30 mg. Eur Neuropsychopharm. 2002;12(3):288.
28. Chavez B, Poveda R. Efficacy with high-dose aripiprazole after olanzapine-related metabolic disturbances. Ann Pharmacother. 2006;40(12):2265-2268.
29. Duggal HS, Mendhekar DN. High-dose aripiprazole in treatment-resistant schizophrenia. J Clin Psychiatry. 2006;67(4):674-675.
30. Thone J. Worsened agitation and confusion in schizophrenia subsequent to high-dose aripiprazole. J Neuropsychiatry Clin Neurosci. 2007;19(4):481-482.
31. Saatcioglu O, Gumus S, Kamberyan K, et al. Efficacy of high-dose aripiprazole for treatment-resistant schizoaffective disorder: a case report. Psychopharmacol Bull. 2010;43(4):70-72.
32. Thomson SR, Chogtu B, Bhattacharjee D, et al. Extrapyramidal symptoms probably related to risperidone treatment: a case series. Ann Neurosci. 2017;24(3):155-163.
33. Meltzer HY, Lindenmayer JP, Kwentus J, et al. A six month randomized controlled trial of long acting injectable risperidone 50 and 100 mg in treatment resistant schizophrenia. Schizophr Res. 2014;154(1-3):14-22.
34. Hou Y, Lai C. The response of psychotic symptoms in a patient with resistant schizophrenia under treatment of high-dose risperidone long-acting injection. J Neuropsychiatry Clin Neurosci. 2014;26(3):E16-E17. doi: 10.1176/appi.neuropsych.13070150
35. Albrecht A, Morena PG, Baumann P, et al. High dose of depot risperidone in a nonresponder schizophrenic patient. J Clin Psychopharmacol. 2004;24(6):673-674.
36. Mace S, Taylor D. Reducing the rates of prescribing high-dose antipsychotics and polypharmacy on psychiatric inpatient and intensive care units: results of a 6-year quality improvement programme. Ther Adv Psychopharmacol. 2015;5(1):4-12.
37. Goff DC, McEvoy JP, Citrome L, et al. High-dose oral ziprasidone versus conventional dosing in schizophrenia patients with residual symptoms. J Clin Psychopharmacol. 2013;33:485-490.
38. Levy WO, Robichaux-Keene NR, Nunez C. No significant QTc interval changes with high-dose ziprasidone: a case series. J Psychiatr Pract. 2004;10(4):227-232.
39. Kaushik S, Maccabee N, Kaushik S, et al. Activation induced by high-dose ziprasidone: a case report. J Clin Psychiatry. 2009;70(9):1326-1327.
40. Seeman P. Targeting the dopamine D2 receptor in schizophrenia. Expert Opin Ther Targets. 2006;10(4):515-531.
41. Honer WG, MacEwan W, Gendron A, et al. A randomized, double-blind, placebo-controlled study of safety and tolerability of high-dose quetiapine in patients with persistent symptoms of schizophrenia or schizoaffective disorder. J Clin Psychiatry. 2012;73(1):13-20.
42. Sokolski KN, Brown BJ, Meldon M. Urinary retention following repeated high-dose quetiapine. Ann Pharmacother. 2004;38(5):899-890.
43. Chandrappa P, Ho L. Case reports of patients with treatment-resistant schizophrenia and related psychotic disorders intolerant to clozapine responding to high doses of quetiapine. Ther Adv Psychopharmacol. 2012;2(5):207-209.
44. Pierre JM, Wirshing DA, Wirshing WC, et al. High-dose quetiapine in treatment refractory schizophrenia. Schizophr Res. 2005;73:373-375.
45. Lindenmyer JP, Citrome L, Khan A, et al. A randomized, double-blind parallel-group, fixed-dose, clinical trial of quetiapine at 600 vs. 1200 mg/d for patients with treatment-resistant schizophrenia or schizoaffective disorder. J Clin Psychopharmacol. 2011;31(2):160-168.
46. Hazra M, Culo S, Mamo D. High-dose quetiapine and photopsia. J Clin Psychopharmacol. 2006;26(5):546-547.
47. Smolders DME, Smolders WAP. Case report and review of the literature: cardiomyopathy in a young woman on high-dose quetiapine. Cardiovasc Toxicol. 2017;17(4):478-481.
48. Takeuchi H, Suzuki T, Bies RR, et al. Dose reduction of risperidone and olanzapine and estimated D2 receptor occupancy in stable patients with schizophrenia: findings from an open-label, randomized, controlled study. J Clin Psychiatry. 2014;75(11):1209-1214.
49. Kumar V, Rao NP, Narasimha V, et al. Antipsychotic dose in maintenance treatment of schizophrenia: a retrospective study. Psychiatry Res. 2016;245:311-316.
1. Morrissette DA, Stahl SM. Treating the violence patient with psychosis or impulsivity utilizing antipsychotic polypharmacy and high-dose monotherapy. CNS Spectrums. 2014;19(5):439-448.
2. Latuda [package insert]. Marlborough, MA: Sunovion Pharmaceuticals Inc.; 2019.
3. Taylor D, Paton C, Kapur S. The Maudsley prescribing guidelines in psychiatry. 12th ed. Wiley Blackwell; 2015.
4. Vyas P, Hwang BJ, Brasic JR. An evaluation of lumateperone tosylate for the treatment of schizophrenia. Expert Opin Pharmacother. 2020;21(2):139-145.
5. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-02):9-62.
6. Saphris [package insert]. Irvine, CA: Allergan USA, Inc; 2017.
7. Abilify [package insert]. Tokyo, Japan: Otsuka America Pharmaceutical, Inc.; 2014.
8. Rexulti [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc.; 2020.
9. Vraylar [package insert]. Madison, NJ: Allergan USA, Inc.; 2019.
10. Clozaril [package insert]. Rosemont, PA: Novartis Pharmaceuticals Corporation; 2017.
11. Fanapt [package insert]. Washington, DC: Vanda Pharmaceuticals Inc.; 2016.
12. Caplyta [package insert]. New York, NY: Intra-Cellular Therapies, Inc.; 2019.
13. Zyprexa [package insert]. Indianapolis, IN: Lilly USA, LLC.; 2020.
14. Invega [package insert]. Titusville, NJ: Janssen Pharmaceutical Companies; 2019.
15. Seroquel [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2016.
16. Risperdal [package insert]. Titusville, NJ: Janssen Pharmaceutical Companies; 2020.
17. Geodon [package insert]. New York, NY: Pfizer Inc.; 2020.
18. Chaudhry IB, Neelam K, Duddu V, et al. Ethnicity and psychopharmacology. J Psychopharmacol. 2008;22(6):673-680.
19. Seeman MV. Men and women respond differently to antipsychotic drugs. Neuropharmacology. 2020;163:107631. doi: 10.1016/j.neuropharm.2019.05.008
20. Barnes TRE, Drake R, Paton C, et al. Evidence-based guidelines for the pharmacological treatment of schizophrenia: updated recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2020;34(1):3-78.
21. Citrome L, McEvoy JP, Todtenkopf MS, et al. A commentary on the efficacy of olanzapine for the treatment of schizophrenia: the past, present, and future. Neuropsych Dis Treat. 2019;15:2559-2569.
22. Meltzer HY, Bobo WV, Ajanta R, et al. A randomized, double-blind comparison of clozapine and high-dose olanzapine in treatment-resistant patients with schizophrenia. J Clin Psychiatry. 2008;69(2):274-285.
23. Batail JM, Langree B, Robert G, et al. Use of very-high-dose olanzapine in treatment-resistant schizophrenia. Schizophr Res. 2014;159(2-3):411-414.
24. Kinon BJ, Volavka J, Stauffer V, et al. Standard and higher dose of olanzapine in patients with schizophrenia or schizoaffective disorder. J Clin Psychopharmacol. 2008;28(4):392-400.
25. Stahl SM. Stahl’s essential psychopharmacology prescriber’s guide. 6th ed. Cambridge University Press; 2017.
26. Bartlett JA, van der Voort Maarschalk K. Understanding the oral mucosal absorption and resulting clinical pharmacokinetics of asenapine. AAPS PharmSciTech. 2012;13(4):1110-1115.
27. Auby P, Saha A, Ali M, et al. Safety and tolerability of aripiprazole at doses higher than 30 mg. Eur Neuropsychopharm. 2002;12(3):288.
28. Chavez B, Poveda R. Efficacy with high-dose aripiprazole after olanzapine-related metabolic disturbances. Ann Pharmacother. 2006;40(12):2265-2268.
29. Duggal HS, Mendhekar DN. High-dose aripiprazole in treatment-resistant schizophrenia. J Clin Psychiatry. 2006;67(4):674-675.
30. Thone J. Worsened agitation and confusion in schizophrenia subsequent to high-dose aripiprazole. J Neuropsychiatry Clin Neurosci. 2007;19(4):481-482.
31. Saatcioglu O, Gumus S, Kamberyan K, et al. Efficacy of high-dose aripiprazole for treatment-resistant schizoaffective disorder: a case report. Psychopharmacol Bull. 2010;43(4):70-72.
32. Thomson SR, Chogtu B, Bhattacharjee D, et al. Extrapyramidal symptoms probably related to risperidone treatment: a case series. Ann Neurosci. 2017;24(3):155-163.
33. Meltzer HY, Lindenmayer JP, Kwentus J, et al. A six month randomized controlled trial of long acting injectable risperidone 50 and 100 mg in treatment resistant schizophrenia. Schizophr Res. 2014;154(1-3):14-22.
34. Hou Y, Lai C. The response of psychotic symptoms in a patient with resistant schizophrenia under treatment of high-dose risperidone long-acting injection. J Neuropsychiatry Clin Neurosci. 2014;26(3):E16-E17. doi: 10.1176/appi.neuropsych.13070150
35. Albrecht A, Morena PG, Baumann P, et al. High dose of depot risperidone in a nonresponder schizophrenic patient. J Clin Psychopharmacol. 2004;24(6):673-674.
36. Mace S, Taylor D. Reducing the rates of prescribing high-dose antipsychotics and polypharmacy on psychiatric inpatient and intensive care units: results of a 6-year quality improvement programme. Ther Adv Psychopharmacol. 2015;5(1):4-12.
37. Goff DC, McEvoy JP, Citrome L, et al. High-dose oral ziprasidone versus conventional dosing in schizophrenia patients with residual symptoms. J Clin Psychopharmacol. 2013;33:485-490.
38. Levy WO, Robichaux-Keene NR, Nunez C. No significant QTc interval changes with high-dose ziprasidone: a case series. J Psychiatr Pract. 2004;10(4):227-232.
39. Kaushik S, Maccabee N, Kaushik S, et al. Activation induced by high-dose ziprasidone: a case report. J Clin Psychiatry. 2009;70(9):1326-1327.
40. Seeman P. Targeting the dopamine D2 receptor in schizophrenia. Expert Opin Ther Targets. 2006;10(4):515-531.
41. Honer WG, MacEwan W, Gendron A, et al. A randomized, double-blind, placebo-controlled study of safety and tolerability of high-dose quetiapine in patients with persistent symptoms of schizophrenia or schizoaffective disorder. J Clin Psychiatry. 2012;73(1):13-20.
42. Sokolski KN, Brown BJ, Meldon M. Urinary retention following repeated high-dose quetiapine. Ann Pharmacother. 2004;38(5):899-890.
43. Chandrappa P, Ho L. Case reports of patients with treatment-resistant schizophrenia and related psychotic disorders intolerant to clozapine responding to high doses of quetiapine. Ther Adv Psychopharmacol. 2012;2(5):207-209.
44. Pierre JM, Wirshing DA, Wirshing WC, et al. High-dose quetiapine in treatment refractory schizophrenia. Schizophr Res. 2005;73:373-375.
45. Lindenmyer JP, Citrome L, Khan A, et al. A randomized, double-blind parallel-group, fixed-dose, clinical trial of quetiapine at 600 vs. 1200 mg/d for patients with treatment-resistant schizophrenia or schizoaffective disorder. J Clin Psychopharmacol. 2011;31(2):160-168.
46. Hazra M, Culo S, Mamo D. High-dose quetiapine and photopsia. J Clin Psychopharmacol. 2006;26(5):546-547.
47. Smolders DME, Smolders WAP. Case report and review of the literature: cardiomyopathy in a young woman on high-dose quetiapine. Cardiovasc Toxicol. 2017;17(4):478-481.
48. Takeuchi H, Suzuki T, Bies RR, et al. Dose reduction of risperidone and olanzapine and estimated D2 receptor occupancy in stable patients with schizophrenia: findings from an open-label, randomized, controlled study. J Clin Psychiatry. 2014;75(11):1209-1214.
49. Kumar V, Rao NP, Narasimha V, et al. Antipsychotic dose in maintenance treatment of schizophrenia: a retrospective study. Psychiatry Res. 2016;245:311-316.
Vaping and pregnancy: Inhaled toxins among reasons for pause
Researchers are trying to understand how e-cigarette use affects pregnancy and birth outcomes. This question may become more relevant as younger vapers, among whom the devices gained considerable popularity, start having children.
Limited emerging data from animal experiments and human epidemiologic studies suggest that vaping may have negative effects on fertility and pregnancy. “Even if these impacts are less severe than conventional smoking, we really should be thinking about alternate options that may be safer for our patients than inhalation of this aerosol,” said Blair J. Wylie, MD, MPH, a maternal-fetal medicine physician at Beth Israel Deaconess Medical Center in Boston.
Dr. Wylie reviewed what is known about vaping, including chemicals other than nicotine that have been detected in vape aerosols, and pregnancy at the 2021 virtual meeting of the American College of Obstetricians and Gynecologists.
“There’s a lot we don’t know,” she said. “These products were only introduced recently, in 2003. They are marketed aggressively to our youth and have gained tremendous popularity among that population. And it’s only a matter of time, I think, before we see a lot of use in our own patient population.”
In a separate study presented at the ACOG meeting, Nicole Izhakoff, a researcher at Florida International University, Miami, and colleagues evaluated the association between e-cigarette use during pregnancy and unfavorable birth outcomes, such as preterm birth, low birth weight, or extended hospital stay for the newborn.
The investigators used 2016-2017 survey data from the Pregnancy Risk Assessment Monitoring System. In all, 71,940 women completed the survey, including 859 who reported e-cigarette use during pregnancy.
After adjusting for age, race, ethnicity, insurance, maternal education, prenatal care, abuse during pregnancy, and complications during pregnancy, the researchers estimated that the odds of an unfavorable birth outcome were 62% greater among women who used e-cigarettes during pregnancy, compared with those who did not.
The researchers lacked information about simultaneous use of alcohol, traditional tobacco, or other drugs, however.
“Physicians of all subspecialties, especially those of obstetrics-gynecology and pediatrics, need to increase the implementation of screening for past or current e-cigarette use in at-risk patients,” Ms. Izhakoff and coauthors concluded. “Further research regarding the long-term health effects of e-cigarettes is warranted.”
Dr. Wylie coauthored another study related to this topic that was published online May 24, 2021, in the Journal of Maternal-Fetal & Neonatal Medicine.
The researchers examined birth weights of children whose mothers use e-cigarettes alone, those whose mothers used both e-cigarettes and conventional cigarettes, and those whose mothers smoked conventional cigarettes only. Their estimates were imprecise, but signaled that e-cigarette use may reduce birth weight. The use of e-cigarettes alone appeared to have less of an impact on birth weight than the dual use of conventional cigarettes and e-cigarettes did.
Dr. Wylie cautioned that outcomes like birth weight are “pretty crude measures of whether an exposure is okay or not in pregnancy. Many of these toxins that we know that are in the aerosols can cause harm, but they may not be reflected in the absolute value of the birth weight.”
In addition, clinicians should avoid focusing on the wrong question when caring for patients.
“I think the wrong question is: Is vaping safer than smoking?” Dr. Wylie said in an interview. “Metals are going into your lungs. Plastics are going into your lungs. It is hard for me to think that we are going to identify that as our champion smoking cessation strategy in pregnancy.”
Rapidly changing landscape
Answering the question of which is safer is a challenge anyway because researchers likely have incomplete information about who vapes, who smokes, and who does both.
Still, the new research illustrates that “people are starting to think about this and beginning to do some analysis that is really hypothesis generating at this point,” Dr. Wylie said. Such studies may prompt clinicians to ask their patients about e-cigarette use. “Marijuana is sort of a similar thing where patients’ perception of safety, because things are legal, can lead to use during pregnancy without ... letting their care teams know,” she said. “Things are changing so rapidly in terms of what’s available to people to use that we need to stay on top of that as obstetricians and ask the right questions and try to understand what the risks are and potential benefits.”
Dr. Wylie is an obstetric consultant to the New England Pediatric Environmental Health Specialty Unit, which is where she heard pediatricians discussing widespread e-cigarette use among youth. It occurred to her that some of these teens eventually would be seeing obstetricians. She also saw parallels to prior research she conducted that focused on household air pollution or cooking from wood-burning fires in Africa.
“What is frightening, I think, about these electronic cigarettes is that you’re heating this liquid to extraordinarily high temperatures to create the vapor,” and the extreme heat vaporizes plastics and metals as well as nicotine, Dr. Wylie said.
An ACOG committee opinion discusses approaches to smoking and vaping cessation such as counseling, behavioral therapy, and medication.
The publication also lists a host of elements have been isolated from vape aerosol, including “carbonyl compounds (formaldehyde, acetaldehyde, acetone, and acrolein); volatile organic compounds (benzene and toluene); nitrosamines; particulate matter; and heavy metals such as copper, lead, zinc, and tin.”
In addition to the nicotine in e-cigarette liquids, which is harmful in itself, there is “all of this other company that it keeps,” including solvent byproducts, known carcinogens, and lung irritants, Dr. Wylie said. Fine particulate matter “can land in the small airways and cause inflammation, even translocate into the systemic circulation and cause systemic inflammation.”
The use of flavoring “likely alters perceptions of harm” and contributes to the popularity of vaping, Dr. Wylie noted. At the same time, the use of flavoring also has little regulatory oversight. Flavors usually are approved for marketing based on safety for ingestion, but that may not translate into safety for inhalation.
Parsing the health effects
People who vape have increased cough, wheezing, and phlegm production, compared with people who do not vape. Vaping also may worsen underlying lung disease like asthma. Lung function on spirometry decreases after e-cigarette use, studies have shown.
In 2019, researchers described e-cigarette or vaping product use–related acute lung injury (EVALI), which has caused more than 60 deaths in the United States. The condition may be related to vitamin E acetate, a component that had been used in some liquids used by patients with EVALI.
And the nicotine in e-cigarettes can accelerate atherogenesis and affect blood pressure, heart rate, and arterial stiffness.
Initially introduced as a smoking cessation tool, e-cigarettes now often are used on their own or in addition to cigarettes, rather than strictly for smoking cessation.
A Cochrane review suggests that e-cigarettes may be more effective than other approaches to smoking cessation. But “the effect is modest at best,” Dr. Wylie said. Among 100 people attempting to quit cigarette smoking, there might four to six more quitters with the use of e-cigarettes as a smoking cessation intervention, compared with other approaches.
Animal models provide other reasons for caution. One experiment in mice showed that exposure to e-cigarette aerosol impaired implantation and fetal health. The results suggest “that there might be some negative impacts across generations,” Dr. Wylie said.
Another study has suggested the possibility that women who currently use e-cigarettes may have slightly diminished fecundability. The results were not statistically significant, but the study “gives us pause about whether there could be some impact on early pregnancy and fertility,” Dr. Wylie said.
In mouse models, prenatal exposure to e-cigarette aerosol has decreased fetal weight and length, altered neurodevelopment and neuroregulatory gene expression, and increased proinflammatory cytokines. E-cigarette aerosol also has caused birth defects in zebrafish and facial clefting in frogs. Whether and how these data relate to human pregnancy is unclear.
While e-cigarette ads may convey a sense of style and harmlessness, clinicians have reasons to worry about the effects. “We have to be a little bit more cautious when we are talking about this with our patients,” Dr. Wylie said.
Dr. Wylie had no relevant financial disclosures. She is a Society for Maternal-Fetal Medicine board member and receives grant support related to research of household air pollution and pregnancy, prenatal pesticide exposure, preeclampsia in low income settings, and malaria during pregnancy. Ms. Izhakoff and coauthors had no disclosures.
Researchers are trying to understand how e-cigarette use affects pregnancy and birth outcomes. This question may become more relevant as younger vapers, among whom the devices gained considerable popularity, start having children.
Limited emerging data from animal experiments and human epidemiologic studies suggest that vaping may have negative effects on fertility and pregnancy. “Even if these impacts are less severe than conventional smoking, we really should be thinking about alternate options that may be safer for our patients than inhalation of this aerosol,” said Blair J. Wylie, MD, MPH, a maternal-fetal medicine physician at Beth Israel Deaconess Medical Center in Boston.
Dr. Wylie reviewed what is known about vaping, including chemicals other than nicotine that have been detected in vape aerosols, and pregnancy at the 2021 virtual meeting of the American College of Obstetricians and Gynecologists.
“There’s a lot we don’t know,” she said. “These products were only introduced recently, in 2003. They are marketed aggressively to our youth and have gained tremendous popularity among that population. And it’s only a matter of time, I think, before we see a lot of use in our own patient population.”
In a separate study presented at the ACOG meeting, Nicole Izhakoff, a researcher at Florida International University, Miami, and colleagues evaluated the association between e-cigarette use during pregnancy and unfavorable birth outcomes, such as preterm birth, low birth weight, or extended hospital stay for the newborn.
The investigators used 2016-2017 survey data from the Pregnancy Risk Assessment Monitoring System. In all, 71,940 women completed the survey, including 859 who reported e-cigarette use during pregnancy.
After adjusting for age, race, ethnicity, insurance, maternal education, prenatal care, abuse during pregnancy, and complications during pregnancy, the researchers estimated that the odds of an unfavorable birth outcome were 62% greater among women who used e-cigarettes during pregnancy, compared with those who did not.
The researchers lacked information about simultaneous use of alcohol, traditional tobacco, or other drugs, however.
“Physicians of all subspecialties, especially those of obstetrics-gynecology and pediatrics, need to increase the implementation of screening for past or current e-cigarette use in at-risk patients,” Ms. Izhakoff and coauthors concluded. “Further research regarding the long-term health effects of e-cigarettes is warranted.”
Dr. Wylie coauthored another study related to this topic that was published online May 24, 2021, in the Journal of Maternal-Fetal & Neonatal Medicine.
The researchers examined birth weights of children whose mothers use e-cigarettes alone, those whose mothers used both e-cigarettes and conventional cigarettes, and those whose mothers smoked conventional cigarettes only. Their estimates were imprecise, but signaled that e-cigarette use may reduce birth weight. The use of e-cigarettes alone appeared to have less of an impact on birth weight than the dual use of conventional cigarettes and e-cigarettes did.
Dr. Wylie cautioned that outcomes like birth weight are “pretty crude measures of whether an exposure is okay or not in pregnancy. Many of these toxins that we know that are in the aerosols can cause harm, but they may not be reflected in the absolute value of the birth weight.”
In addition, clinicians should avoid focusing on the wrong question when caring for patients.
“I think the wrong question is: Is vaping safer than smoking?” Dr. Wylie said in an interview. “Metals are going into your lungs. Plastics are going into your lungs. It is hard for me to think that we are going to identify that as our champion smoking cessation strategy in pregnancy.”
Rapidly changing landscape
Answering the question of which is safer is a challenge anyway because researchers likely have incomplete information about who vapes, who smokes, and who does both.
Still, the new research illustrates that “people are starting to think about this and beginning to do some analysis that is really hypothesis generating at this point,” Dr. Wylie said. Such studies may prompt clinicians to ask their patients about e-cigarette use. “Marijuana is sort of a similar thing where patients’ perception of safety, because things are legal, can lead to use during pregnancy without ... letting their care teams know,” she said. “Things are changing so rapidly in terms of what’s available to people to use that we need to stay on top of that as obstetricians and ask the right questions and try to understand what the risks are and potential benefits.”
Dr. Wylie is an obstetric consultant to the New England Pediatric Environmental Health Specialty Unit, which is where she heard pediatricians discussing widespread e-cigarette use among youth. It occurred to her that some of these teens eventually would be seeing obstetricians. She also saw parallels to prior research she conducted that focused on household air pollution or cooking from wood-burning fires in Africa.
“What is frightening, I think, about these electronic cigarettes is that you’re heating this liquid to extraordinarily high temperatures to create the vapor,” and the extreme heat vaporizes plastics and metals as well as nicotine, Dr. Wylie said.
An ACOG committee opinion discusses approaches to smoking and vaping cessation such as counseling, behavioral therapy, and medication.
The publication also lists a host of elements have been isolated from vape aerosol, including “carbonyl compounds (formaldehyde, acetaldehyde, acetone, and acrolein); volatile organic compounds (benzene and toluene); nitrosamines; particulate matter; and heavy metals such as copper, lead, zinc, and tin.”
In addition to the nicotine in e-cigarette liquids, which is harmful in itself, there is “all of this other company that it keeps,” including solvent byproducts, known carcinogens, and lung irritants, Dr. Wylie said. Fine particulate matter “can land in the small airways and cause inflammation, even translocate into the systemic circulation and cause systemic inflammation.”
The use of flavoring “likely alters perceptions of harm” and contributes to the popularity of vaping, Dr. Wylie noted. At the same time, the use of flavoring also has little regulatory oversight. Flavors usually are approved for marketing based on safety for ingestion, but that may not translate into safety for inhalation.
Parsing the health effects
People who vape have increased cough, wheezing, and phlegm production, compared with people who do not vape. Vaping also may worsen underlying lung disease like asthma. Lung function on spirometry decreases after e-cigarette use, studies have shown.
In 2019, researchers described e-cigarette or vaping product use–related acute lung injury (EVALI), which has caused more than 60 deaths in the United States. The condition may be related to vitamin E acetate, a component that had been used in some liquids used by patients with EVALI.
And the nicotine in e-cigarettes can accelerate atherogenesis and affect blood pressure, heart rate, and arterial stiffness.
Initially introduced as a smoking cessation tool, e-cigarettes now often are used on their own or in addition to cigarettes, rather than strictly for smoking cessation.
A Cochrane review suggests that e-cigarettes may be more effective than other approaches to smoking cessation. But “the effect is modest at best,” Dr. Wylie said. Among 100 people attempting to quit cigarette smoking, there might four to six more quitters with the use of e-cigarettes as a smoking cessation intervention, compared with other approaches.
Animal models provide other reasons for caution. One experiment in mice showed that exposure to e-cigarette aerosol impaired implantation and fetal health. The results suggest “that there might be some negative impacts across generations,” Dr. Wylie said.
Another study has suggested the possibility that women who currently use e-cigarettes may have slightly diminished fecundability. The results were not statistically significant, but the study “gives us pause about whether there could be some impact on early pregnancy and fertility,” Dr. Wylie said.
In mouse models, prenatal exposure to e-cigarette aerosol has decreased fetal weight and length, altered neurodevelopment and neuroregulatory gene expression, and increased proinflammatory cytokines. E-cigarette aerosol also has caused birth defects in zebrafish and facial clefting in frogs. Whether and how these data relate to human pregnancy is unclear.
While e-cigarette ads may convey a sense of style and harmlessness, clinicians have reasons to worry about the effects. “We have to be a little bit more cautious when we are talking about this with our patients,” Dr. Wylie said.
Dr. Wylie had no relevant financial disclosures. She is a Society for Maternal-Fetal Medicine board member and receives grant support related to research of household air pollution and pregnancy, prenatal pesticide exposure, preeclampsia in low income settings, and malaria during pregnancy. Ms. Izhakoff and coauthors had no disclosures.
Researchers are trying to understand how e-cigarette use affects pregnancy and birth outcomes. This question may become more relevant as younger vapers, among whom the devices gained considerable popularity, start having children.
Limited emerging data from animal experiments and human epidemiologic studies suggest that vaping may have negative effects on fertility and pregnancy. “Even if these impacts are less severe than conventional smoking, we really should be thinking about alternate options that may be safer for our patients than inhalation of this aerosol,” said Blair J. Wylie, MD, MPH, a maternal-fetal medicine physician at Beth Israel Deaconess Medical Center in Boston.
Dr. Wylie reviewed what is known about vaping, including chemicals other than nicotine that have been detected in vape aerosols, and pregnancy at the 2021 virtual meeting of the American College of Obstetricians and Gynecologists.
“There’s a lot we don’t know,” she said. “These products were only introduced recently, in 2003. They are marketed aggressively to our youth and have gained tremendous popularity among that population. And it’s only a matter of time, I think, before we see a lot of use in our own patient population.”
In a separate study presented at the ACOG meeting, Nicole Izhakoff, a researcher at Florida International University, Miami, and colleagues evaluated the association between e-cigarette use during pregnancy and unfavorable birth outcomes, such as preterm birth, low birth weight, or extended hospital stay for the newborn.
The investigators used 2016-2017 survey data from the Pregnancy Risk Assessment Monitoring System. In all, 71,940 women completed the survey, including 859 who reported e-cigarette use during pregnancy.
After adjusting for age, race, ethnicity, insurance, maternal education, prenatal care, abuse during pregnancy, and complications during pregnancy, the researchers estimated that the odds of an unfavorable birth outcome were 62% greater among women who used e-cigarettes during pregnancy, compared with those who did not.
The researchers lacked information about simultaneous use of alcohol, traditional tobacco, or other drugs, however.
“Physicians of all subspecialties, especially those of obstetrics-gynecology and pediatrics, need to increase the implementation of screening for past or current e-cigarette use in at-risk patients,” Ms. Izhakoff and coauthors concluded. “Further research regarding the long-term health effects of e-cigarettes is warranted.”
Dr. Wylie coauthored another study related to this topic that was published online May 24, 2021, in the Journal of Maternal-Fetal & Neonatal Medicine.
The researchers examined birth weights of children whose mothers use e-cigarettes alone, those whose mothers used both e-cigarettes and conventional cigarettes, and those whose mothers smoked conventional cigarettes only. Their estimates were imprecise, but signaled that e-cigarette use may reduce birth weight. The use of e-cigarettes alone appeared to have less of an impact on birth weight than the dual use of conventional cigarettes and e-cigarettes did.
Dr. Wylie cautioned that outcomes like birth weight are “pretty crude measures of whether an exposure is okay or not in pregnancy. Many of these toxins that we know that are in the aerosols can cause harm, but they may not be reflected in the absolute value of the birth weight.”
In addition, clinicians should avoid focusing on the wrong question when caring for patients.
“I think the wrong question is: Is vaping safer than smoking?” Dr. Wylie said in an interview. “Metals are going into your lungs. Plastics are going into your lungs. It is hard for me to think that we are going to identify that as our champion smoking cessation strategy in pregnancy.”
Rapidly changing landscape
Answering the question of which is safer is a challenge anyway because researchers likely have incomplete information about who vapes, who smokes, and who does both.
Still, the new research illustrates that “people are starting to think about this and beginning to do some analysis that is really hypothesis generating at this point,” Dr. Wylie said. Such studies may prompt clinicians to ask their patients about e-cigarette use. “Marijuana is sort of a similar thing where patients’ perception of safety, because things are legal, can lead to use during pregnancy without ... letting their care teams know,” she said. “Things are changing so rapidly in terms of what’s available to people to use that we need to stay on top of that as obstetricians and ask the right questions and try to understand what the risks are and potential benefits.”
Dr. Wylie is an obstetric consultant to the New England Pediatric Environmental Health Specialty Unit, which is where she heard pediatricians discussing widespread e-cigarette use among youth. It occurred to her that some of these teens eventually would be seeing obstetricians. She also saw parallels to prior research she conducted that focused on household air pollution or cooking from wood-burning fires in Africa.
“What is frightening, I think, about these electronic cigarettes is that you’re heating this liquid to extraordinarily high temperatures to create the vapor,” and the extreme heat vaporizes plastics and metals as well as nicotine, Dr. Wylie said.
An ACOG committee opinion discusses approaches to smoking and vaping cessation such as counseling, behavioral therapy, and medication.
The publication also lists a host of elements have been isolated from vape aerosol, including “carbonyl compounds (formaldehyde, acetaldehyde, acetone, and acrolein); volatile organic compounds (benzene and toluene); nitrosamines; particulate matter; and heavy metals such as copper, lead, zinc, and tin.”
In addition to the nicotine in e-cigarette liquids, which is harmful in itself, there is “all of this other company that it keeps,” including solvent byproducts, known carcinogens, and lung irritants, Dr. Wylie said. Fine particulate matter “can land in the small airways and cause inflammation, even translocate into the systemic circulation and cause systemic inflammation.”
The use of flavoring “likely alters perceptions of harm” and contributes to the popularity of vaping, Dr. Wylie noted. At the same time, the use of flavoring also has little regulatory oversight. Flavors usually are approved for marketing based on safety for ingestion, but that may not translate into safety for inhalation.
Parsing the health effects
People who vape have increased cough, wheezing, and phlegm production, compared with people who do not vape. Vaping also may worsen underlying lung disease like asthma. Lung function on spirometry decreases after e-cigarette use, studies have shown.
In 2019, researchers described e-cigarette or vaping product use–related acute lung injury (EVALI), which has caused more than 60 deaths in the United States. The condition may be related to vitamin E acetate, a component that had been used in some liquids used by patients with EVALI.
And the nicotine in e-cigarettes can accelerate atherogenesis and affect blood pressure, heart rate, and arterial stiffness.
Initially introduced as a smoking cessation tool, e-cigarettes now often are used on their own or in addition to cigarettes, rather than strictly for smoking cessation.
A Cochrane review suggests that e-cigarettes may be more effective than other approaches to smoking cessation. But “the effect is modest at best,” Dr. Wylie said. Among 100 people attempting to quit cigarette smoking, there might four to six more quitters with the use of e-cigarettes as a smoking cessation intervention, compared with other approaches.
Animal models provide other reasons for caution. One experiment in mice showed that exposure to e-cigarette aerosol impaired implantation and fetal health. The results suggest “that there might be some negative impacts across generations,” Dr. Wylie said.
Another study has suggested the possibility that women who currently use e-cigarettes may have slightly diminished fecundability. The results were not statistically significant, but the study “gives us pause about whether there could be some impact on early pregnancy and fertility,” Dr. Wylie said.
In mouse models, prenatal exposure to e-cigarette aerosol has decreased fetal weight and length, altered neurodevelopment and neuroregulatory gene expression, and increased proinflammatory cytokines. E-cigarette aerosol also has caused birth defects in zebrafish and facial clefting in frogs. Whether and how these data relate to human pregnancy is unclear.
While e-cigarette ads may convey a sense of style and harmlessness, clinicians have reasons to worry about the effects. “We have to be a little bit more cautious when we are talking about this with our patients,” Dr. Wylie said.
Dr. Wylie had no relevant financial disclosures. She is a Society for Maternal-Fetal Medicine board member and receives grant support related to research of household air pollution and pregnancy, prenatal pesticide exposure, preeclampsia in low income settings, and malaria during pregnancy. Ms. Izhakoff and coauthors had no disclosures.
FROM ACOG 2021
GI symptoms and chronic fatigue may persist months after COVID-19
Gastrointestinal symptoms and chronic fatigue may persist months after the COVID-19 virus infection resolves, results of a recent cohort-controlled study suggest.
About 5 months after SARS-CoV-2 infection, relative risks of loose stools, somatization, and chronic fatigue were increased by approximately two- to three fold, compared to individuals who had not been infected, according to study results presented at the annual Digestive Disease Week® (DDW).
These longer-term consequences of SARS-CoV-2 appeared to be more severe in patients who had experienced diarrhea during the acute infection, according to investigator Daniele Noviello, MD, a second-year resident in gastroenterology and hepatology at the University of Milan.
This is the first cohort-controlled study that specifically investigates gastrointestinal symptoms and somatoform disorders, Dr. Noviello said in a virtual presentation of the results.
“Based on our data, chronic fatigue, gastrointestinal, and somatoform symptoms may have a common postinfectious origin, and they should be investigated in the follow-up of SARS-CoV-2 patients,” he said.
Links between SARS-CoV-2 and gastrointestinal symptoms
Gastrointestinal symptoms are known to be relatively common during acute infection. According to Dr. Noviello, the most frequent gastrointestinal symptom associated with SARS-CoV-2 is diarrhea, occurring in 4% to nearly 40% of patients in case series to date.
However, data on the longer-term gastrointestinal impacts of SARS-CoV-2 remain scarce.
In one noncontrolled cohort study in China, loss of appetite, nausea, acid reflux, and diarrhea were seen in 15%-24% of patients 3 months after the infection, Dr. Noviello said. In another cohort study in China, diarrhea and vomiting were reported in 5% of patients 6 months after infection.
In any case, it is known that viral, bacterial, and protozoal infections of the gastrointestinal tract are a risk factor for development of functional disorders including irritable bowel syndrome (IBS), functional dyspepsia, and chronic fatigue, according to Dr. Noviello.
Accordingly, the results of the present study suggest that SARS-CoV-2 also “may affect the brain-gut axis in the long term,” Dr. Noviello and coauthors wrote in an abstract of the study.
It is plausible that SARS-CoV-2 infection could be a trigger for longer-term gastrointestinal symptoms, especially given the previous evidence linking infections and IBS symptoms, or postinfectious IBS, said Juan Pablo Stefanolo, MD, a physician with the neurogastroenterology and motility section, Hospital de Clínicas José de San Martín, Buenos Aires University.
“If it is demonstrated [that SARS-CoV-2 infection is a trigger], the microbiota-gut-brain axis concept in IBS pathophysiology is reinforced,” Dr. Stefanolo said in an interview.
In the meantime, practitioners may want to take into account COVID-19 infection history in the evaluation of a patient with IBS-like symptoms and, in case of a known positive COVID-19 result in an IBS patient, be aware of the possibility of symptom exacerbation, Dr. Stefanolo said.
Pandemic in Italy: Unique study opportunity
The severe outbreak in the Milan region early in the COVID-19 pandemic provided a “unique opportunity” to assess the long-term impact of infection on gastrointestinal and extraintestinal somatoform symptoms, said Dr. Noviello.
The investigators sent an online questionnaire to patients who had a molecular diagnosis of SARS-CoV-2 infection by nasal swab between February and April of 2020. To form a control group, they also sent questionnaires to hospital employees and health care providers who had tested negative over that same time period.
In all, 378 questionnaires were completed by 177 SARS-CoV-2–positive individuals and 201 controls. The SARS-CoV-2–positive patients were somewhat older (about 44 years vs. 40 years for controls), were less often female (40% vs. 61%), had a lower education level, and smoked less than did controls, according to the investigators.
A mean of 4.8 months had elapsed between the time of SARS-CoV-2 infection and when the questionnaires were compiled, said Dr. Noviello.
In the acute phase, diarrhea was the most common gastrointestinal symptom among virus-positive individuals, occurring in about 50% compared to 20% of controls (P < .001), data show. Other symptoms reported by 40% of SARS-CoV-2–infected individuals included fever, dyspnea, loss of smell or taste, weight loss, myalgia, arthralgia, and asthenia in the acute phase controls in the acute phase, Dr. Noviello said.
Persistent gastrointestinal symptoms after SARS-CoV-2
Persistent symptoms included loose stools, as measured by the Bristol Stool scale, occurring in 17.8% of SARS-CoV-2–positive individuals, but only 9.3% of the SARS-CoV-2–negative controls, according to Dr. Noviello, with an adjusted risk ratio of 1.88 (95% confidence interval, 0.99-3.54).
Chronic fatigue symptoms, as measured by the Structured Assessment of Gastrointestinal Symptoms questionnaire, were reported by about 30% of SARS-CoV-2–positive patients and about 15% of controls, for an adjusted risk ratio of 2.24 (95% CI, 1.48-3.37), according to Dr. Noviello’s presentation.
The mean t-score on the Symptom Checklist–12 for somatoform disorders was higher for the virus-positive patients compared to controls, according to Dr. Noviello. The scores were 54.6 and 50.5, respectively, with an adjusted score difference of 3.6 (95% CI, 1.0-6.2).
The longer-term sequelae of SARS-CoV-2 infection might be more severe in individuals who experienced diarrhea during acute infection, according to Dr. Noviello. In a post hoc analysis, reports of irritable bowel syndrome and loose stools were significantly higher in SARS-CoV-2–infected individuals who had diarrhea in the acute phase compared to those who did not experience diarrhea, he said.
Somatoform disorder scores were significantly higher, and reports of headache, back pain, and chronic fatigue were significantly more common, in individuals who had diarrhea at the time of SARS-CoV-2 infection, he added.
Dr. Noviello and coauthors reported no competing interests related to the study. Dr. Stefanolo had no disclosures to report.
Gastrointestinal symptoms and chronic fatigue may persist months after the COVID-19 virus infection resolves, results of a recent cohort-controlled study suggest.
About 5 months after SARS-CoV-2 infection, relative risks of loose stools, somatization, and chronic fatigue were increased by approximately two- to three fold, compared to individuals who had not been infected, according to study results presented at the annual Digestive Disease Week® (DDW).
These longer-term consequences of SARS-CoV-2 appeared to be more severe in patients who had experienced diarrhea during the acute infection, according to investigator Daniele Noviello, MD, a second-year resident in gastroenterology and hepatology at the University of Milan.
This is the first cohort-controlled study that specifically investigates gastrointestinal symptoms and somatoform disorders, Dr. Noviello said in a virtual presentation of the results.
“Based on our data, chronic fatigue, gastrointestinal, and somatoform symptoms may have a common postinfectious origin, and they should be investigated in the follow-up of SARS-CoV-2 patients,” he said.
Links between SARS-CoV-2 and gastrointestinal symptoms
Gastrointestinal symptoms are known to be relatively common during acute infection. According to Dr. Noviello, the most frequent gastrointestinal symptom associated with SARS-CoV-2 is diarrhea, occurring in 4% to nearly 40% of patients in case series to date.
However, data on the longer-term gastrointestinal impacts of SARS-CoV-2 remain scarce.
In one noncontrolled cohort study in China, loss of appetite, nausea, acid reflux, and diarrhea were seen in 15%-24% of patients 3 months after the infection, Dr. Noviello said. In another cohort study in China, diarrhea and vomiting were reported in 5% of patients 6 months after infection.
In any case, it is known that viral, bacterial, and protozoal infections of the gastrointestinal tract are a risk factor for development of functional disorders including irritable bowel syndrome (IBS), functional dyspepsia, and chronic fatigue, according to Dr. Noviello.
Accordingly, the results of the present study suggest that SARS-CoV-2 also “may affect the brain-gut axis in the long term,” Dr. Noviello and coauthors wrote in an abstract of the study.
It is plausible that SARS-CoV-2 infection could be a trigger for longer-term gastrointestinal symptoms, especially given the previous evidence linking infections and IBS symptoms, or postinfectious IBS, said Juan Pablo Stefanolo, MD, a physician with the neurogastroenterology and motility section, Hospital de Clínicas José de San Martín, Buenos Aires University.
“If it is demonstrated [that SARS-CoV-2 infection is a trigger], the microbiota-gut-brain axis concept in IBS pathophysiology is reinforced,” Dr. Stefanolo said in an interview.
In the meantime, practitioners may want to take into account COVID-19 infection history in the evaluation of a patient with IBS-like symptoms and, in case of a known positive COVID-19 result in an IBS patient, be aware of the possibility of symptom exacerbation, Dr. Stefanolo said.
Pandemic in Italy: Unique study opportunity
The severe outbreak in the Milan region early in the COVID-19 pandemic provided a “unique opportunity” to assess the long-term impact of infection on gastrointestinal and extraintestinal somatoform symptoms, said Dr. Noviello.
The investigators sent an online questionnaire to patients who had a molecular diagnosis of SARS-CoV-2 infection by nasal swab between February and April of 2020. To form a control group, they also sent questionnaires to hospital employees and health care providers who had tested negative over that same time period.
In all, 378 questionnaires were completed by 177 SARS-CoV-2–positive individuals and 201 controls. The SARS-CoV-2–positive patients were somewhat older (about 44 years vs. 40 years for controls), were less often female (40% vs. 61%), had a lower education level, and smoked less than did controls, according to the investigators.
A mean of 4.8 months had elapsed between the time of SARS-CoV-2 infection and when the questionnaires were compiled, said Dr. Noviello.
In the acute phase, diarrhea was the most common gastrointestinal symptom among virus-positive individuals, occurring in about 50% compared to 20% of controls (P < .001), data show. Other symptoms reported by 40% of SARS-CoV-2–infected individuals included fever, dyspnea, loss of smell or taste, weight loss, myalgia, arthralgia, and asthenia in the acute phase controls in the acute phase, Dr. Noviello said.
Persistent gastrointestinal symptoms after SARS-CoV-2
Persistent symptoms included loose stools, as measured by the Bristol Stool scale, occurring in 17.8% of SARS-CoV-2–positive individuals, but only 9.3% of the SARS-CoV-2–negative controls, according to Dr. Noviello, with an adjusted risk ratio of 1.88 (95% confidence interval, 0.99-3.54).
Chronic fatigue symptoms, as measured by the Structured Assessment of Gastrointestinal Symptoms questionnaire, were reported by about 30% of SARS-CoV-2–positive patients and about 15% of controls, for an adjusted risk ratio of 2.24 (95% CI, 1.48-3.37), according to Dr. Noviello’s presentation.
The mean t-score on the Symptom Checklist–12 for somatoform disorders was higher for the virus-positive patients compared to controls, according to Dr. Noviello. The scores were 54.6 and 50.5, respectively, with an adjusted score difference of 3.6 (95% CI, 1.0-6.2).
The longer-term sequelae of SARS-CoV-2 infection might be more severe in individuals who experienced diarrhea during acute infection, according to Dr. Noviello. In a post hoc analysis, reports of irritable bowel syndrome and loose stools were significantly higher in SARS-CoV-2–infected individuals who had diarrhea in the acute phase compared to those who did not experience diarrhea, he said.
Somatoform disorder scores were significantly higher, and reports of headache, back pain, and chronic fatigue were significantly more common, in individuals who had diarrhea at the time of SARS-CoV-2 infection, he added.
Dr. Noviello and coauthors reported no competing interests related to the study. Dr. Stefanolo had no disclosures to report.
Gastrointestinal symptoms and chronic fatigue may persist months after the COVID-19 virus infection resolves, results of a recent cohort-controlled study suggest.
About 5 months after SARS-CoV-2 infection, relative risks of loose stools, somatization, and chronic fatigue were increased by approximately two- to three fold, compared to individuals who had not been infected, according to study results presented at the annual Digestive Disease Week® (DDW).
These longer-term consequences of SARS-CoV-2 appeared to be more severe in patients who had experienced diarrhea during the acute infection, according to investigator Daniele Noviello, MD, a second-year resident in gastroenterology and hepatology at the University of Milan.
This is the first cohort-controlled study that specifically investigates gastrointestinal symptoms and somatoform disorders, Dr. Noviello said in a virtual presentation of the results.
“Based on our data, chronic fatigue, gastrointestinal, and somatoform symptoms may have a common postinfectious origin, and they should be investigated in the follow-up of SARS-CoV-2 patients,” he said.
Links between SARS-CoV-2 and gastrointestinal symptoms
Gastrointestinal symptoms are known to be relatively common during acute infection. According to Dr. Noviello, the most frequent gastrointestinal symptom associated with SARS-CoV-2 is diarrhea, occurring in 4% to nearly 40% of patients in case series to date.
However, data on the longer-term gastrointestinal impacts of SARS-CoV-2 remain scarce.
In one noncontrolled cohort study in China, loss of appetite, nausea, acid reflux, and diarrhea were seen in 15%-24% of patients 3 months after the infection, Dr. Noviello said. In another cohort study in China, diarrhea and vomiting were reported in 5% of patients 6 months after infection.
In any case, it is known that viral, bacterial, and protozoal infections of the gastrointestinal tract are a risk factor for development of functional disorders including irritable bowel syndrome (IBS), functional dyspepsia, and chronic fatigue, according to Dr. Noviello.
Accordingly, the results of the present study suggest that SARS-CoV-2 also “may affect the brain-gut axis in the long term,” Dr. Noviello and coauthors wrote in an abstract of the study.
It is plausible that SARS-CoV-2 infection could be a trigger for longer-term gastrointestinal symptoms, especially given the previous evidence linking infections and IBS symptoms, or postinfectious IBS, said Juan Pablo Stefanolo, MD, a physician with the neurogastroenterology and motility section, Hospital de Clínicas José de San Martín, Buenos Aires University.
“If it is demonstrated [that SARS-CoV-2 infection is a trigger], the microbiota-gut-brain axis concept in IBS pathophysiology is reinforced,” Dr. Stefanolo said in an interview.
In the meantime, practitioners may want to take into account COVID-19 infection history in the evaluation of a patient with IBS-like symptoms and, in case of a known positive COVID-19 result in an IBS patient, be aware of the possibility of symptom exacerbation, Dr. Stefanolo said.
Pandemic in Italy: Unique study opportunity
The severe outbreak in the Milan region early in the COVID-19 pandemic provided a “unique opportunity” to assess the long-term impact of infection on gastrointestinal and extraintestinal somatoform symptoms, said Dr. Noviello.
The investigators sent an online questionnaire to patients who had a molecular diagnosis of SARS-CoV-2 infection by nasal swab between February and April of 2020. To form a control group, they also sent questionnaires to hospital employees and health care providers who had tested negative over that same time period.
In all, 378 questionnaires were completed by 177 SARS-CoV-2–positive individuals and 201 controls. The SARS-CoV-2–positive patients were somewhat older (about 44 years vs. 40 years for controls), were less often female (40% vs. 61%), had a lower education level, and smoked less than did controls, according to the investigators.
A mean of 4.8 months had elapsed between the time of SARS-CoV-2 infection and when the questionnaires were compiled, said Dr. Noviello.
In the acute phase, diarrhea was the most common gastrointestinal symptom among virus-positive individuals, occurring in about 50% compared to 20% of controls (P < .001), data show. Other symptoms reported by 40% of SARS-CoV-2–infected individuals included fever, dyspnea, loss of smell or taste, weight loss, myalgia, arthralgia, and asthenia in the acute phase controls in the acute phase, Dr. Noviello said.
Persistent gastrointestinal symptoms after SARS-CoV-2
Persistent symptoms included loose stools, as measured by the Bristol Stool scale, occurring in 17.8% of SARS-CoV-2–positive individuals, but only 9.3% of the SARS-CoV-2–negative controls, according to Dr. Noviello, with an adjusted risk ratio of 1.88 (95% confidence interval, 0.99-3.54).
Chronic fatigue symptoms, as measured by the Structured Assessment of Gastrointestinal Symptoms questionnaire, were reported by about 30% of SARS-CoV-2–positive patients and about 15% of controls, for an adjusted risk ratio of 2.24 (95% CI, 1.48-3.37), according to Dr. Noviello’s presentation.
The mean t-score on the Symptom Checklist–12 for somatoform disorders was higher for the virus-positive patients compared to controls, according to Dr. Noviello. The scores were 54.6 and 50.5, respectively, with an adjusted score difference of 3.6 (95% CI, 1.0-6.2).
The longer-term sequelae of SARS-CoV-2 infection might be more severe in individuals who experienced diarrhea during acute infection, according to Dr. Noviello. In a post hoc analysis, reports of irritable bowel syndrome and loose stools were significantly higher in SARS-CoV-2–infected individuals who had diarrhea in the acute phase compared to those who did not experience diarrhea, he said.
Somatoform disorder scores were significantly higher, and reports of headache, back pain, and chronic fatigue were significantly more common, in individuals who had diarrhea at the time of SARS-CoV-2 infection, he added.
Dr. Noviello and coauthors reported no competing interests related to the study. Dr. Stefanolo had no disclosures to report.
FROM DDW 2021
Question 2
Q2. Correct answer: D. Glucose hydrogen breath test.
Rationale
The etiology of small intestinal bacterial overgrowth (SIBO) is complex but may include an issue with altered antibacterial defense mechanisms, such as achlorhydria from atrophic gastritis. SIBO can be diagnosed by a glucose hydrogen breath test, and therefore, it is the best next step in the management of this patient's symptoms given the history of atrophic gastritis. Chromogranin A testing and video capsule endoscopy are used in the diagnostic evaluation of suspected carcinoid syndrome and inflammatory bowel disease, respectively, and may be indicated in the subsequent evaluation of this patient's symptoms. In addition, both of these diagnoses are unlikely to cause intermittent diarrhea. Eluxadoline is an agent that combines a mu-opioid receptor agonist and a delta-opioid receptor antagonist and is indicated for the treatment of irritable bowel syndrome - diarrhea predominant (IBS-D). The diagnosis of IBS requires the presence of abdominal pain and is unlikely in an elderly patient with new onset of symptoms; therefore, this is not the diagnosis in this patient's case.
Reference
Jan Bures et al. World J Gastroenterol. 2010 Jun 28;16(24):2978-90.
Q2. Correct answer: D. Glucose hydrogen breath test.
Rationale
The etiology of small intestinal bacterial overgrowth (SIBO) is complex but may include an issue with altered antibacterial defense mechanisms, such as achlorhydria from atrophic gastritis. SIBO can be diagnosed by a glucose hydrogen breath test, and therefore, it is the best next step in the management of this patient's symptoms given the history of atrophic gastritis. Chromogranin A testing and video capsule endoscopy are used in the diagnostic evaluation of suspected carcinoid syndrome and inflammatory bowel disease, respectively, and may be indicated in the subsequent evaluation of this patient's symptoms. In addition, both of these diagnoses are unlikely to cause intermittent diarrhea. Eluxadoline is an agent that combines a mu-opioid receptor agonist and a delta-opioid receptor antagonist and is indicated for the treatment of irritable bowel syndrome - diarrhea predominant (IBS-D). The diagnosis of IBS requires the presence of abdominal pain and is unlikely in an elderly patient with new onset of symptoms; therefore, this is not the diagnosis in this patient's case.
Reference
Jan Bures et al. World J Gastroenterol. 2010 Jun 28;16(24):2978-90.
Q2. Correct answer: D. Glucose hydrogen breath test.
Rationale
The etiology of small intestinal bacterial overgrowth (SIBO) is complex but may include an issue with altered antibacterial defense mechanisms, such as achlorhydria from atrophic gastritis. SIBO can be diagnosed by a glucose hydrogen breath test, and therefore, it is the best next step in the management of this patient's symptoms given the history of atrophic gastritis. Chromogranin A testing and video capsule endoscopy are used in the diagnostic evaluation of suspected carcinoid syndrome and inflammatory bowel disease, respectively, and may be indicated in the subsequent evaluation of this patient's symptoms. In addition, both of these diagnoses are unlikely to cause intermittent diarrhea. Eluxadoline is an agent that combines a mu-opioid receptor agonist and a delta-opioid receptor antagonist and is indicated for the treatment of irritable bowel syndrome - diarrhea predominant (IBS-D). The diagnosis of IBS requires the presence of abdominal pain and is unlikely in an elderly patient with new onset of symptoms; therefore, this is not the diagnosis in this patient's case.
Reference
Jan Bures et al. World J Gastroenterol. 2010 Jun 28;16(24):2978-90.
A 66-year-old woman with a history of atrophic gastritis presents for evaluation of intermittent diarrhea. She denies abdominal pain, weight loss, GI bleeding or a family history of colorectal neoplasia or IBD. Physical exam is normal. Labs including thyroid function testing, celiac screen and CRP are normal. A colonoscopy with random colon biopsies is normal.