User login
Vitiligo patients share their experiences, frustrations with treatment options with FDA
Patients with vitiligo have faced significant impacts psychosocially and in many cases, profound losses of identity – and they’ve had only minimal success with treatment, according to participants who spoke at and provided input at a public meeting on patient-focused drug development for the disease.
The virtual meeting, held in March, was part of the Food and Drug Administration’s Patient-Focused Drug Development (PFDD) initiative, which began in 2012 and aims to provide a systematic way for patients’ experiences, needs and priorities to be “captured and meaningfully incorporated” into drug development and evaluation.
Seemal Desai, MD, of the department of dermatology at the University of Texas, Dallas, who attended the meeting as an observer, said in a later interview that while “all skin diseases have a psychosocial component … vitiligo is a really unique one, because it really relates to the patient’s own identity.
“What I heard loud and clear from the FDA [leaders who ran and attended the meeting] is recognition that patients are suffering. They needed to hear about the emotional devastation of the disease and how it is a medical condition,” Dr. Desai said.
The meeting was the “first-ever vitiligo meeting at the FDA” and was a “historic moment for the vitiligo community,” he added.
The pigmentation disorder affects 1% of the world’s population. Nearly 50% have an onset before age 20, and onset before age 12 is common, Brenda Carr, MD, medical officer with the FDA’s Division of Dermatology and Dentistry in the Center for Drug Evaluation and Research, said in an introductory overview.
The only FDA-approved treatment for vitiligo is monobenzone cream, but this is indicated for final depigmentation in extensive vitiligo and is no longer marketed. Treatment options include corticosteroids, calcineurin inhibitors, vitamin D analogues, phototherapy, surgical treatments (tissue grafts and cellular grafts), and camouflage (make-up, tattoos, self-tanning products), Dr. Carr said.
Patients participated in one of two panels – one about the health effects and daily impacts of vitiligo and the other about treatments – or submitted input electronically. All patients were invited to answer poll questions and open-ended queries, including questions about how they would assess new treatments.
Several panel members who are Black shared series of photos that showed the evolution of defined white patches into widespread, generalized depigmentation. One man with skin of color who lives in the Netherlands said he has had vitiligo since the age of 12, but that when he became older, over a 4-year period, he was “transformed from a man of Indonesian roots to a totally white man.”
Experiencing only minimal benefit from treatment and the short-term effectiveness of treatments were the top two answers to a poll question asking participants about the most burdensome impacts of the medical products and interventions they have used. Difficulty in accessing treatment, concern about serious risks of treatment, and uncertainty about long-term effects of treatment were other frequently chosen answers.
Patients described the onerous nature of phototherapy (treatments repeated several times a week over long periods) and other treatments, and several described feeling that some physicians did not take the condition seriously or fully know of treatment options.
In her closing remarks, Kendall Marcus, MD, director of the Division of Dermatology and Dentistry at the FDA, acknowledged the input. “Some of you have had difficulty having your disease taken seriously by physicians who view it as a cosmetic condition and are reluctant to treat because they believe your expectations will not be met, that it will be an exercise in frustration,” she said.
Regarding the impacts of treatments that have been utilized, “some of the treatments make it impossible to do other activities such as work or care for yourself in other ways,” Dr. Marcus said. “Certainly that’s not the kind of treatment … that anybody wants to have.”
Dr. Desai, who utilizes an array of oral and topical treatments and phototherapies in his practice, said he was surprised and disheartened to hear the level of concern about side effects of treatment. Most of those who expressed concerns alluded to phototherapy. “I think light treatments are very safe and effective,” he said in the interview. “I might equate [such concerns] to the older PUVA [psoralen plus UVA ultraviolet light] therapy, but not so much the newer therapies.”
The FDA participants probed patients for their perspective on a meaningful level of repigmentation and an acceptable level of risk for any new hypothetical treatment. Specifically, they asked whether patients would use a new topical cream approved for vitiligo if the cream needed to be applied once a day, would have up to 50% efficacy in some people, and would have common side effects of redness and irritation at the application site, mild acne, and burning, as well as several rarer but more serious side effects.
Only 36% answered yes; 24% said no, and 40% answered maybe. Some patients said during the meeting that they had accepted their condition and were not pursuing any treatment. Others said they were very interested in treatment but only if the level of repigmentation were significantly higher than 50%. Some described their fear that positive treatment effects would be short term only.
Meri Izrail Kohen, who lives in France and has lost half of her skin’s pigmentation, said that treatment efficacy is “not only about how much recovery of pigment it allows, but how long the recovery will last.” Some treatments will work for some patients, she said, “but even in these cases when we stop the treatment, it will come back somehow.”
Lee Thomas, a TV anchor in Detroit, and a reporter and author of the book “Turning White,” described how he tried “every treatment he could afford” but stopped trying 10 years ago. A treatment in Germany “gave me 80% of my pigment back, but it has gone again,” he said. “I would love to have my face back again. I was born a Black child, and I’d like to die a Black man.”
Patients also spoke of their skin burning easily outdoors; skin sensitivity, itchiness, and burning with the spread of disease; treatment expenses and not being able to afford treatment; and worsening of their vitiligo with the stress of the pandemic. Parents expressed having fear that their children would develop vitiligo and experience bullying, isolation, or other emotional or psychosocial impacts that they had experienced; one described having an almost-paralyzing anxiety when he saw patchy white spots on his 20-month-old daughter (it was not diagnosed as vitiligo).
Calls for further advancement with home phototherapy – which Dr. Desai said is a growing market but not yet adequately covered by insurance plans – were also made, as were pleas for research on the root causes of the disease.
Patients clearly indicated “that they need more efficacious treatments, and more comprehensive treatments,” said Dr. Desai, who chairs the advisory committee of the Global Vitiligo Foundation. “It’s disappointing to me that patients come in with a not fully optimistic viewpoint, with a lot of anxiety and angst that treatments are not going to work. … But the Agency needs to hear that. This means that there haven’t been good treatments and we need more.”
The FDA will accept public comments until May 10, 2021, at which time comments will be compiled into a summary report. FDA officials assured patients that the report would be visible and circulated not only within the FDA but among drug companies, researchers, and other product developers.
Patients with vitiligo have faced significant impacts psychosocially and in many cases, profound losses of identity – and they’ve had only minimal success with treatment, according to participants who spoke at and provided input at a public meeting on patient-focused drug development for the disease.
The virtual meeting, held in March, was part of the Food and Drug Administration’s Patient-Focused Drug Development (PFDD) initiative, which began in 2012 and aims to provide a systematic way for patients’ experiences, needs and priorities to be “captured and meaningfully incorporated” into drug development and evaluation.
Seemal Desai, MD, of the department of dermatology at the University of Texas, Dallas, who attended the meeting as an observer, said in a later interview that while “all skin diseases have a psychosocial component … vitiligo is a really unique one, because it really relates to the patient’s own identity.
“What I heard loud and clear from the FDA [leaders who ran and attended the meeting] is recognition that patients are suffering. They needed to hear about the emotional devastation of the disease and how it is a medical condition,” Dr. Desai said.
The meeting was the “first-ever vitiligo meeting at the FDA” and was a “historic moment for the vitiligo community,” he added.
The pigmentation disorder affects 1% of the world’s population. Nearly 50% have an onset before age 20, and onset before age 12 is common, Brenda Carr, MD, medical officer with the FDA’s Division of Dermatology and Dentistry in the Center for Drug Evaluation and Research, said in an introductory overview.
The only FDA-approved treatment for vitiligo is monobenzone cream, but this is indicated for final depigmentation in extensive vitiligo and is no longer marketed. Treatment options include corticosteroids, calcineurin inhibitors, vitamin D analogues, phototherapy, surgical treatments (tissue grafts and cellular grafts), and camouflage (make-up, tattoos, self-tanning products), Dr. Carr said.
Patients participated in one of two panels – one about the health effects and daily impacts of vitiligo and the other about treatments – or submitted input electronically. All patients were invited to answer poll questions and open-ended queries, including questions about how they would assess new treatments.
Several panel members who are Black shared series of photos that showed the evolution of defined white patches into widespread, generalized depigmentation. One man with skin of color who lives in the Netherlands said he has had vitiligo since the age of 12, but that when he became older, over a 4-year period, he was “transformed from a man of Indonesian roots to a totally white man.”
Experiencing only minimal benefit from treatment and the short-term effectiveness of treatments were the top two answers to a poll question asking participants about the most burdensome impacts of the medical products and interventions they have used. Difficulty in accessing treatment, concern about serious risks of treatment, and uncertainty about long-term effects of treatment were other frequently chosen answers.
Patients described the onerous nature of phototherapy (treatments repeated several times a week over long periods) and other treatments, and several described feeling that some physicians did not take the condition seriously or fully know of treatment options.
In her closing remarks, Kendall Marcus, MD, director of the Division of Dermatology and Dentistry at the FDA, acknowledged the input. “Some of you have had difficulty having your disease taken seriously by physicians who view it as a cosmetic condition and are reluctant to treat because they believe your expectations will not be met, that it will be an exercise in frustration,” she said.
Regarding the impacts of treatments that have been utilized, “some of the treatments make it impossible to do other activities such as work or care for yourself in other ways,” Dr. Marcus said. “Certainly that’s not the kind of treatment … that anybody wants to have.”
Dr. Desai, who utilizes an array of oral and topical treatments and phototherapies in his practice, said he was surprised and disheartened to hear the level of concern about side effects of treatment. Most of those who expressed concerns alluded to phototherapy. “I think light treatments are very safe and effective,” he said in the interview. “I might equate [such concerns] to the older PUVA [psoralen plus UVA ultraviolet light] therapy, but not so much the newer therapies.”
The FDA participants probed patients for their perspective on a meaningful level of repigmentation and an acceptable level of risk for any new hypothetical treatment. Specifically, they asked whether patients would use a new topical cream approved for vitiligo if the cream needed to be applied once a day, would have up to 50% efficacy in some people, and would have common side effects of redness and irritation at the application site, mild acne, and burning, as well as several rarer but more serious side effects.
Only 36% answered yes; 24% said no, and 40% answered maybe. Some patients said during the meeting that they had accepted their condition and were not pursuing any treatment. Others said they were very interested in treatment but only if the level of repigmentation were significantly higher than 50%. Some described their fear that positive treatment effects would be short term only.
Meri Izrail Kohen, who lives in France and has lost half of her skin’s pigmentation, said that treatment efficacy is “not only about how much recovery of pigment it allows, but how long the recovery will last.” Some treatments will work for some patients, she said, “but even in these cases when we stop the treatment, it will come back somehow.”
Lee Thomas, a TV anchor in Detroit, and a reporter and author of the book “Turning White,” described how he tried “every treatment he could afford” but stopped trying 10 years ago. A treatment in Germany “gave me 80% of my pigment back, but it has gone again,” he said. “I would love to have my face back again. I was born a Black child, and I’d like to die a Black man.”
Patients also spoke of their skin burning easily outdoors; skin sensitivity, itchiness, and burning with the spread of disease; treatment expenses and not being able to afford treatment; and worsening of their vitiligo with the stress of the pandemic. Parents expressed having fear that their children would develop vitiligo and experience bullying, isolation, or other emotional or psychosocial impacts that they had experienced; one described having an almost-paralyzing anxiety when he saw patchy white spots on his 20-month-old daughter (it was not diagnosed as vitiligo).
Calls for further advancement with home phototherapy – which Dr. Desai said is a growing market but not yet adequately covered by insurance plans – were also made, as were pleas for research on the root causes of the disease.
Patients clearly indicated “that they need more efficacious treatments, and more comprehensive treatments,” said Dr. Desai, who chairs the advisory committee of the Global Vitiligo Foundation. “It’s disappointing to me that patients come in with a not fully optimistic viewpoint, with a lot of anxiety and angst that treatments are not going to work. … But the Agency needs to hear that. This means that there haven’t been good treatments and we need more.”
The FDA will accept public comments until May 10, 2021, at which time comments will be compiled into a summary report. FDA officials assured patients that the report would be visible and circulated not only within the FDA but among drug companies, researchers, and other product developers.
Patients with vitiligo have faced significant impacts psychosocially and in many cases, profound losses of identity – and they’ve had only minimal success with treatment, according to participants who spoke at and provided input at a public meeting on patient-focused drug development for the disease.
The virtual meeting, held in March, was part of the Food and Drug Administration’s Patient-Focused Drug Development (PFDD) initiative, which began in 2012 and aims to provide a systematic way for patients’ experiences, needs and priorities to be “captured and meaningfully incorporated” into drug development and evaluation.
Seemal Desai, MD, of the department of dermatology at the University of Texas, Dallas, who attended the meeting as an observer, said in a later interview that while “all skin diseases have a psychosocial component … vitiligo is a really unique one, because it really relates to the patient’s own identity.
“What I heard loud and clear from the FDA [leaders who ran and attended the meeting] is recognition that patients are suffering. They needed to hear about the emotional devastation of the disease and how it is a medical condition,” Dr. Desai said.
The meeting was the “first-ever vitiligo meeting at the FDA” and was a “historic moment for the vitiligo community,” he added.
The pigmentation disorder affects 1% of the world’s population. Nearly 50% have an onset before age 20, and onset before age 12 is common, Brenda Carr, MD, medical officer with the FDA’s Division of Dermatology and Dentistry in the Center for Drug Evaluation and Research, said in an introductory overview.
The only FDA-approved treatment for vitiligo is monobenzone cream, but this is indicated for final depigmentation in extensive vitiligo and is no longer marketed. Treatment options include corticosteroids, calcineurin inhibitors, vitamin D analogues, phototherapy, surgical treatments (tissue grafts and cellular grafts), and camouflage (make-up, tattoos, self-tanning products), Dr. Carr said.
Patients participated in one of two panels – one about the health effects and daily impacts of vitiligo and the other about treatments – or submitted input electronically. All patients were invited to answer poll questions and open-ended queries, including questions about how they would assess new treatments.
Several panel members who are Black shared series of photos that showed the evolution of defined white patches into widespread, generalized depigmentation. One man with skin of color who lives in the Netherlands said he has had vitiligo since the age of 12, but that when he became older, over a 4-year period, he was “transformed from a man of Indonesian roots to a totally white man.”
Experiencing only minimal benefit from treatment and the short-term effectiveness of treatments were the top two answers to a poll question asking participants about the most burdensome impacts of the medical products and interventions they have used. Difficulty in accessing treatment, concern about serious risks of treatment, and uncertainty about long-term effects of treatment were other frequently chosen answers.
Patients described the onerous nature of phototherapy (treatments repeated several times a week over long periods) and other treatments, and several described feeling that some physicians did not take the condition seriously or fully know of treatment options.
In her closing remarks, Kendall Marcus, MD, director of the Division of Dermatology and Dentistry at the FDA, acknowledged the input. “Some of you have had difficulty having your disease taken seriously by physicians who view it as a cosmetic condition and are reluctant to treat because they believe your expectations will not be met, that it will be an exercise in frustration,” she said.
Regarding the impacts of treatments that have been utilized, “some of the treatments make it impossible to do other activities such as work or care for yourself in other ways,” Dr. Marcus said. “Certainly that’s not the kind of treatment … that anybody wants to have.”
Dr. Desai, who utilizes an array of oral and topical treatments and phototherapies in his practice, said he was surprised and disheartened to hear the level of concern about side effects of treatment. Most of those who expressed concerns alluded to phototherapy. “I think light treatments are very safe and effective,” he said in the interview. “I might equate [such concerns] to the older PUVA [psoralen plus UVA ultraviolet light] therapy, but not so much the newer therapies.”
The FDA participants probed patients for their perspective on a meaningful level of repigmentation and an acceptable level of risk for any new hypothetical treatment. Specifically, they asked whether patients would use a new topical cream approved for vitiligo if the cream needed to be applied once a day, would have up to 50% efficacy in some people, and would have common side effects of redness and irritation at the application site, mild acne, and burning, as well as several rarer but more serious side effects.
Only 36% answered yes; 24% said no, and 40% answered maybe. Some patients said during the meeting that they had accepted their condition and were not pursuing any treatment. Others said they were very interested in treatment but only if the level of repigmentation were significantly higher than 50%. Some described their fear that positive treatment effects would be short term only.
Meri Izrail Kohen, who lives in France and has lost half of her skin’s pigmentation, said that treatment efficacy is “not only about how much recovery of pigment it allows, but how long the recovery will last.” Some treatments will work for some patients, she said, “but even in these cases when we stop the treatment, it will come back somehow.”
Lee Thomas, a TV anchor in Detroit, and a reporter and author of the book “Turning White,” described how he tried “every treatment he could afford” but stopped trying 10 years ago. A treatment in Germany “gave me 80% of my pigment back, but it has gone again,” he said. “I would love to have my face back again. I was born a Black child, and I’d like to die a Black man.”
Patients also spoke of their skin burning easily outdoors; skin sensitivity, itchiness, and burning with the spread of disease; treatment expenses and not being able to afford treatment; and worsening of their vitiligo with the stress of the pandemic. Parents expressed having fear that their children would develop vitiligo and experience bullying, isolation, or other emotional or psychosocial impacts that they had experienced; one described having an almost-paralyzing anxiety when he saw patchy white spots on his 20-month-old daughter (it was not diagnosed as vitiligo).
Calls for further advancement with home phototherapy – which Dr. Desai said is a growing market but not yet adequately covered by insurance plans – were also made, as were pleas for research on the root causes of the disease.
Patients clearly indicated “that they need more efficacious treatments, and more comprehensive treatments,” said Dr. Desai, who chairs the advisory committee of the Global Vitiligo Foundation. “It’s disappointing to me that patients come in with a not fully optimistic viewpoint, with a lot of anxiety and angst that treatments are not going to work. … But the Agency needs to hear that. This means that there haven’t been good treatments and we need more.”
The FDA will accept public comments until May 10, 2021, at which time comments will be compiled into a summary report. FDA officials assured patients that the report would be visible and circulated not only within the FDA but among drug companies, researchers, and other product developers.
FROM AN FDA PATIENT-FOCUSED DRUG DEVELOPMENT MEETING
Incontinentia Pigmenti: Initial Presentation of Encephalopathy and Seizures
To the Editor:
A 7-day-old full-term infant presented to the neonatal intensive care unit with poor feeding and altered consciousness. She was born at 39 weeks and 3 days to a gravida 1 mother with a pregnancy history complicated by maternal chorioamnionitis and gestational diabetes. During labor, nonreassuring fetal heart tones and arrest of labor prompted an uncomplicated cesarean delivery with normal Apgar scores at birth. The infant’s family history revealed only beta thalassemia minor in her father. At 5 to 7 days of life, the mother noted difficulty with feeding and poor latch along with lethargy and depressed consciousness in the infant.
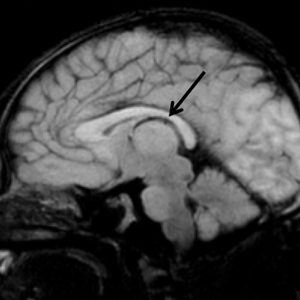
Upon arrival to the neonatal intensive care unit, the infant was noted to have rhythmic lip-smacking behavior, intermittent nystagmus, mild hypotonia, and clonic movements of the left upper extremity. An electroencephalogram was markedly abnormal, capturing multiple seizures in the bilateral cortical hemispheres. She was loaded with phenobarbital with no further seizure activity. Brain magnetic resonance imaging revealed innumerable punctate foci of restricted diffusion with corresponding punctate hemorrhage within the frontal and parietal white matter, as well as cortical diffusion restriction within the occipital lobe, inferior temporal lobe, bilateral thalami, and corpus callosum (Figure 1). An exhaustive infectious workup also was completed and was unremarkable, though she was treated with broad-spectrum antimicrobials, including intravenous acyclovir.
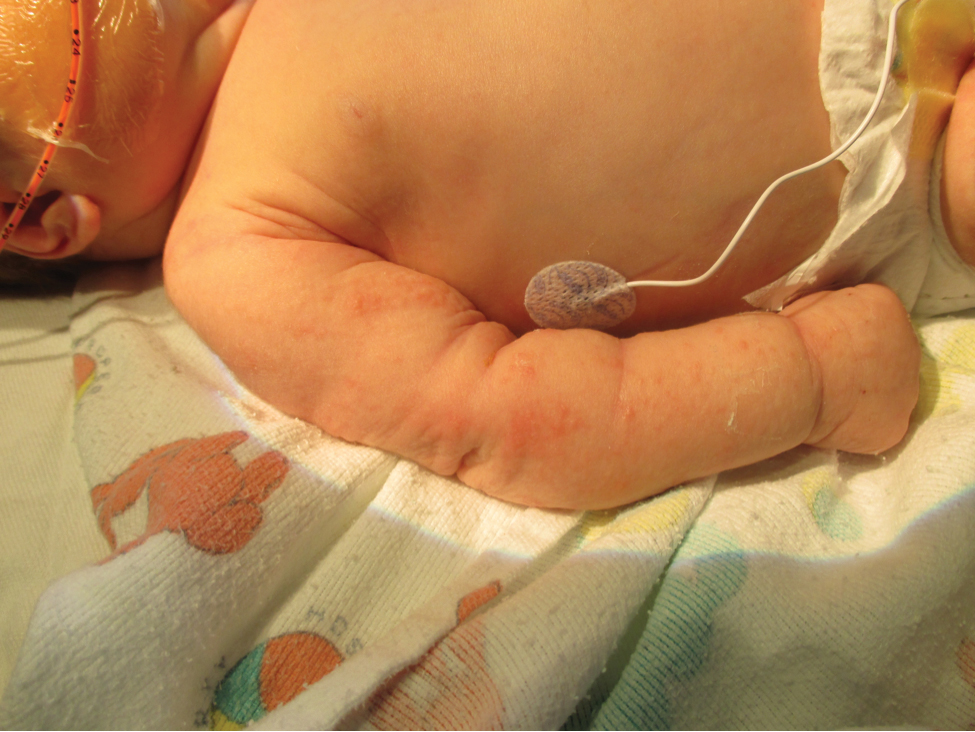
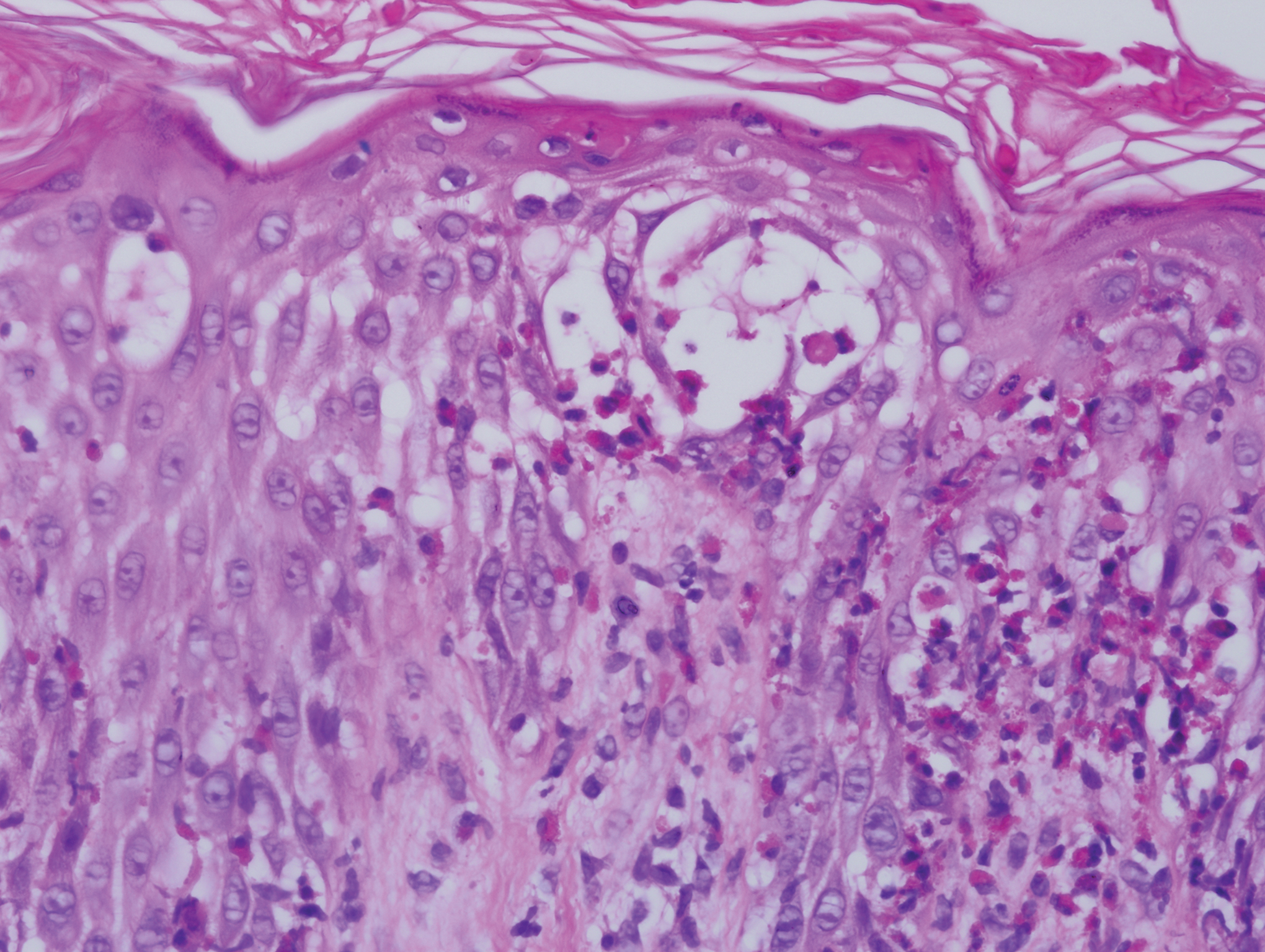
Five days after being hospitalized (day 10 of life), a vesicular rash was noted on the arms and legs (Figure 2). Discussion with the patient’s mother revealed that the first signs of unusual skin lesions occurred as early as several days prior. There were no oral mucosal lesions or gross ocular abnormalities. No nail changes were appreciated. A bedside Tzanck preparation was negative for viral cytopathic changes. A skin biopsy was performed that demonstrated eosinophilic spongiosis with necrotic keratinocytes, typical of the vesicular stage of incontinentia pigmenti (IP)(Figure 3). An ophthalmology examination showed an arteriovenous malformation of the right eye with subtle neovascularization at the infratemporal periphery, consistent with known ocular manifestations of IP. The infant’s mother reported no history of notable dental abnormalities, hair loss, skin rashes, or nail changes. Genetic testing demonstrated the common IKBKG (inhibitor of κ light polypeptide gene enhancer in B cells, kinase gamma [formerly known as NEMO]) gene deletion on the X chromosome, consistent with IP.
She successfully underwent retinal laser ablative therapy for the ocular manifestations without further evidence of neovascularization. She developed a mild cataract that was not visually significant and required no intervention. Her brain abnormalities were thought to represent foci of necrosis with superimposed hemorrhagic transformation due to spontaneous degeneration of brain cells in which the mutated X chromosome was activated. No further treatment was indicated beyond suppression of the consequent seizures. There was no notable cortical edema or other medical indication for systemic glucocorticoid therapy. Phenobarbital was continued without further seizure events.
Several months after the initial presentation, a follow-up electroencephalogram was normal. Phenobarbital was slowly weaned and finally discontinued approximately 6 months after the initial event with no other reported seizures. She currently is achieving normal developmental milestones with the exception of slight motor delay and expected residual hypotonia.
Incontinentia pigmenti, also known as Bloch-Sulzberger syndrome, is a rare multisystem neuroectodermal disorder, primarily affecting the skin, central nervous system (CNS), and retinas. The disorder can be inherited in an X-linked dominant fashion and appears almost exclusively in women with typical in utero lethality seen in males. Most affected individuals have a sporadic, or de novo, mutation, which was likely the case in our patient given that her mother demonstrated no signs or symptoms.1 The pathogenesis of disease is a defect at chromosome Xq28 that is a region encoding the nuclear factor–κB essential modulator, IKBKG. Absence or mutation of IKBKG in IP results in failure to activate nuclear factor–κB and leaves cells vulnerable to cytokine-mediated apoptosis, especially after exposure to tumor necrosis factor α.2
Clinical manifestations of IP are present at or soon after birth. The cutaneous findings of this disorder are classically described as a step-wise progression through 4 distinct stages: (1) a linear and/or whorled vesicular eruption predominantly on the extremities at birth or within the first few weeks of life; (2) thickened linear or whorled verrucous plaques; (3) hyperpigmented streaks and whorls that may or may not correspond with prior affected areas that may resolve by adolescence; and (4) hypopigmented, possibly atrophic plaques on the extremities that may persist lifelong. Importantly, not every patient will experience each of these stages. Overlap can occur, and the time course of each stage is highly variable. Other ectodermal manifestations include dental abnormalities such as small, misshaped, or missing teeth; alopecia; and nail abnormalities. Ocular abnormalities associated with IP primarily occur in the retina, including vascular occlusion, neovascularization, hemorrhages, foveal abnormalities, as well as exudative and tractional detachments.3,4
It is crucial to recognize CNS anomalies in association with the cutaneous findings of IP, as CNS pathology can be severe with profound developmental implications. Central nervous system findings have been noted to correlate with the appearance of the vesicular stage of IP. A high index of suspicion is needed, as the disease can demonstrate progression within a short time.5-8 The most frequent anomalies include seizures, motor impairment, intellectual disability, and microcephaly.9,10 Some of the most commonly identified CNS lesions on imaging include necrosis or brain infarcts, atrophy, and lesions of the corpus callosum.7
The pathogenesis of observed CNS changes in IP is not well understood. There have been numerous proposals of a vascular mechanism, and a microangiopathic process appears to be most plausible. Mutations in IKBKG may result in interruption of signaling via vascular endothelial growth factor receptor 3 with a consequent impact on angiogenesis, supporting a vascular mechanism. Additionally, mutations in IKBKG lead to activation of eotaxin, an eosinophil-selective chemokine.9 Eotaxin activation results in eosinophilic degranulation that mediates the classic eosinophilic infiltrate seen in the classic skin histology of IP. Additionally, it has been shown that eotaxin is strongly expressed by endothelial cells in IP, and more abundant eosinophil degranulation may play a role in mediating vaso-occlusion.7 Other studies have found that the highest expression level of the IKBKG gene is in the CNS, potentially explaining the extensive imaging findings of hemorrhage and diffusion restriction in our patient. These features likely are attributable to apoptosis of cells possessing the mutated IKBKG gene.9-11
- Ehrenreich M, Tarlow MM, Godlewska-Janusz E, et al. Incontinentia pigmenti (Bloch-Sulzberger syndrome): a systemic disorder. Cutis. 2007;79:355-362.
- Smahi A, Courtois G, Rabia SH, et al. The NF-kappaB signaling pathway in human diseases: from incontinentia pigmenti to ectodermal dysplasias and immune-deficiency syndromes. Hum Mol Genet. 2002;11:2371-2375.
- O’Doherty M, McCreery K, Green AJ, et al. Incontinentia pigmenti—ophthalmological observation of a series of cases and review of the literature. Br J Ophthalmol. 2011;95:11-16.
- Swinney CC, Han DP, Karth PA. Incontinentia pigmenti: a comprehensive review and update. Ophthalmic Surg Lasers Imaging Retina. 2015;46:650-657.
- Hennel SJ, Ekert PG, Volpe JJ, et al. Insights into the pathogenesis of cerebral lesions in incontinentia pigmenti. Pediatr Neurol. 2003;29:148-150.
- Maingay-de Groof F, Lequin MH, Roofthooft DW, et al. Extensive cerebral infarction in the newborn due to incontinentia pigmenti. Eur J Paediatr Neurol. 2008;12:284-289.
- Minic´ S, Trpinac D, Obradovic´ M. Systematic review of central nervous system anomalies in incontinentia pigmenti. Orphanet J Rare Dis. 2013;8:25-35.
- Wolf NI, Kramer N, Harting I, et al. Diffuse cortical necrosis in a neonate with incontinentia pigmenti and an encephalitis-like presentation. AJNR Am J Neuroradiol. 2005;26:1580-1582.
- Phan TA, Wargon O, Turner AM. Incontinentia pigmenti case series: clinical spectrum of incontinentia pigmenti in 53 female patients and their relatives. Clin Exp Dermatol. 2005;30:474-480.
- Volpe J. Neurobiology of periventricular leukomalacia in the premature infant. Pediatr Res. 2001;50:553-562.
- Pascual-Castroviejo I, Pascual-Pascual SI, Velazquez-Fragua R, et al. Incontinentia pigmenti: clinical and neuroimaging findings in a series of 12 patients. Neurologia. 2006;21:239-248.
To the Editor:
A 7-day-old full-term infant presented to the neonatal intensive care unit with poor feeding and altered consciousness. She was born at 39 weeks and 3 days to a gravida 1 mother with a pregnancy history complicated by maternal chorioamnionitis and gestational diabetes. During labor, nonreassuring fetal heart tones and arrest of labor prompted an uncomplicated cesarean delivery with normal Apgar scores at birth. The infant’s family history revealed only beta thalassemia minor in her father. At 5 to 7 days of life, the mother noted difficulty with feeding and poor latch along with lethargy and depressed consciousness in the infant.
Upon arrival to the neonatal intensive care unit, the infant was noted to have rhythmic lip-smacking behavior, intermittent nystagmus, mild hypotonia, and clonic movements of the left upper extremity. An electroencephalogram was markedly abnormal, capturing multiple seizures in the bilateral cortical hemispheres. She was loaded with phenobarbital with no further seizure activity. Brain magnetic resonance imaging revealed innumerable punctate foci of restricted diffusion with corresponding punctate hemorrhage within the frontal and parietal white matter, as well as cortical diffusion restriction within the occipital lobe, inferior temporal lobe, bilateral thalami, and corpus callosum (Figure 1). An exhaustive infectious workup also was completed and was unremarkable, though she was treated with broad-spectrum antimicrobials, including intravenous acyclovir.

Five days after being hospitalized (day 10 of life), a vesicular rash was noted on the arms and legs (Figure 2). Discussion with the patient’s mother revealed that the first signs of unusual skin lesions occurred as early as several days prior. There were no oral mucosal lesions or gross ocular abnormalities. No nail changes were appreciated. A bedside Tzanck preparation was negative for viral cytopathic changes. A skin biopsy was performed that demonstrated eosinophilic spongiosis with necrotic keratinocytes, typical of the vesicular stage of incontinentia pigmenti (IP)(Figure 3). An ophthalmology examination showed an arteriovenous malformation of the right eye with subtle neovascularization at the infratemporal periphery, consistent with known ocular manifestations of IP. The infant’s mother reported no history of notable dental abnormalities, hair loss, skin rashes, or nail changes. Genetic testing demonstrated the common IKBKG (inhibitor of κ light polypeptide gene enhancer in B cells, kinase gamma [formerly known as NEMO]) gene deletion on the X chromosome, consistent with IP.


She successfully underwent retinal laser ablative therapy for the ocular manifestations without further evidence of neovascularization. She developed a mild cataract that was not visually significant and required no intervention. Her brain abnormalities were thought to represent foci of necrosis with superimposed hemorrhagic transformation due to spontaneous degeneration of brain cells in which the mutated X chromosome was activated. No further treatment was indicated beyond suppression of the consequent seizures. There was no notable cortical edema or other medical indication for systemic glucocorticoid therapy. Phenobarbital was continued without further seizure events.
Several months after the initial presentation, a follow-up electroencephalogram was normal. Phenobarbital was slowly weaned and finally discontinued approximately 6 months after the initial event with no other reported seizures. She currently is achieving normal developmental milestones with the exception of slight motor delay and expected residual hypotonia.
Incontinentia pigmenti, also known as Bloch-Sulzberger syndrome, is a rare multisystem neuroectodermal disorder, primarily affecting the skin, central nervous system (CNS), and retinas. The disorder can be inherited in an X-linked dominant fashion and appears almost exclusively in women with typical in utero lethality seen in males. Most affected individuals have a sporadic, or de novo, mutation, which was likely the case in our patient given that her mother demonstrated no signs or symptoms.1 The pathogenesis of disease is a defect at chromosome Xq28 that is a region encoding the nuclear factor–κB essential modulator, IKBKG. Absence or mutation of IKBKG in IP results in failure to activate nuclear factor–κB and leaves cells vulnerable to cytokine-mediated apoptosis, especially after exposure to tumor necrosis factor α.2
Clinical manifestations of IP are present at or soon after birth. The cutaneous findings of this disorder are classically described as a step-wise progression through 4 distinct stages: (1) a linear and/or whorled vesicular eruption predominantly on the extremities at birth or within the first few weeks of life; (2) thickened linear or whorled verrucous plaques; (3) hyperpigmented streaks and whorls that may or may not correspond with prior affected areas that may resolve by adolescence; and (4) hypopigmented, possibly atrophic plaques on the extremities that may persist lifelong. Importantly, not every patient will experience each of these stages. Overlap can occur, and the time course of each stage is highly variable. Other ectodermal manifestations include dental abnormalities such as small, misshaped, or missing teeth; alopecia; and nail abnormalities. Ocular abnormalities associated with IP primarily occur in the retina, including vascular occlusion, neovascularization, hemorrhages, foveal abnormalities, as well as exudative and tractional detachments.3,4
It is crucial to recognize CNS anomalies in association with the cutaneous findings of IP, as CNS pathology can be severe with profound developmental implications. Central nervous system findings have been noted to correlate with the appearance of the vesicular stage of IP. A high index of suspicion is needed, as the disease can demonstrate progression within a short time.5-8 The most frequent anomalies include seizures, motor impairment, intellectual disability, and microcephaly.9,10 Some of the most commonly identified CNS lesions on imaging include necrosis or brain infarcts, atrophy, and lesions of the corpus callosum.7
The pathogenesis of observed CNS changes in IP is not well understood. There have been numerous proposals of a vascular mechanism, and a microangiopathic process appears to be most plausible. Mutations in IKBKG may result in interruption of signaling via vascular endothelial growth factor receptor 3 with a consequent impact on angiogenesis, supporting a vascular mechanism. Additionally, mutations in IKBKG lead to activation of eotaxin, an eosinophil-selective chemokine.9 Eotaxin activation results in eosinophilic degranulation that mediates the classic eosinophilic infiltrate seen in the classic skin histology of IP. Additionally, it has been shown that eotaxin is strongly expressed by endothelial cells in IP, and more abundant eosinophil degranulation may play a role in mediating vaso-occlusion.7 Other studies have found that the highest expression level of the IKBKG gene is in the CNS, potentially explaining the extensive imaging findings of hemorrhage and diffusion restriction in our patient. These features likely are attributable to apoptosis of cells possessing the mutated IKBKG gene.9-11
To the Editor:
A 7-day-old full-term infant presented to the neonatal intensive care unit with poor feeding and altered consciousness. She was born at 39 weeks and 3 days to a gravida 1 mother with a pregnancy history complicated by maternal chorioamnionitis and gestational diabetes. During labor, nonreassuring fetal heart tones and arrest of labor prompted an uncomplicated cesarean delivery with normal Apgar scores at birth. The infant’s family history revealed only beta thalassemia minor in her father. At 5 to 7 days of life, the mother noted difficulty with feeding and poor latch along with lethargy and depressed consciousness in the infant.
Upon arrival to the neonatal intensive care unit, the infant was noted to have rhythmic lip-smacking behavior, intermittent nystagmus, mild hypotonia, and clonic movements of the left upper extremity. An electroencephalogram was markedly abnormal, capturing multiple seizures in the bilateral cortical hemispheres. She was loaded with phenobarbital with no further seizure activity. Brain magnetic resonance imaging revealed innumerable punctate foci of restricted diffusion with corresponding punctate hemorrhage within the frontal and parietal white matter, as well as cortical diffusion restriction within the occipital lobe, inferior temporal lobe, bilateral thalami, and corpus callosum (Figure 1). An exhaustive infectious workup also was completed and was unremarkable, though she was treated with broad-spectrum antimicrobials, including intravenous acyclovir.

Five days after being hospitalized (day 10 of life), a vesicular rash was noted on the arms and legs (Figure 2). Discussion with the patient’s mother revealed that the first signs of unusual skin lesions occurred as early as several days prior. There were no oral mucosal lesions or gross ocular abnormalities. No nail changes were appreciated. A bedside Tzanck preparation was negative for viral cytopathic changes. A skin biopsy was performed that demonstrated eosinophilic spongiosis with necrotic keratinocytes, typical of the vesicular stage of incontinentia pigmenti (IP)(Figure 3). An ophthalmology examination showed an arteriovenous malformation of the right eye with subtle neovascularization at the infratemporal periphery, consistent with known ocular manifestations of IP. The infant’s mother reported no history of notable dental abnormalities, hair loss, skin rashes, or nail changes. Genetic testing demonstrated the common IKBKG (inhibitor of κ light polypeptide gene enhancer in B cells, kinase gamma [formerly known as NEMO]) gene deletion on the X chromosome, consistent with IP.


She successfully underwent retinal laser ablative therapy for the ocular manifestations without further evidence of neovascularization. She developed a mild cataract that was not visually significant and required no intervention. Her brain abnormalities were thought to represent foci of necrosis with superimposed hemorrhagic transformation due to spontaneous degeneration of brain cells in which the mutated X chromosome was activated. No further treatment was indicated beyond suppression of the consequent seizures. There was no notable cortical edema or other medical indication for systemic glucocorticoid therapy. Phenobarbital was continued without further seizure events.
Several months after the initial presentation, a follow-up electroencephalogram was normal. Phenobarbital was slowly weaned and finally discontinued approximately 6 months after the initial event with no other reported seizures. She currently is achieving normal developmental milestones with the exception of slight motor delay and expected residual hypotonia.
Incontinentia pigmenti, also known as Bloch-Sulzberger syndrome, is a rare multisystem neuroectodermal disorder, primarily affecting the skin, central nervous system (CNS), and retinas. The disorder can be inherited in an X-linked dominant fashion and appears almost exclusively in women with typical in utero lethality seen in males. Most affected individuals have a sporadic, or de novo, mutation, which was likely the case in our patient given that her mother demonstrated no signs or symptoms.1 The pathogenesis of disease is a defect at chromosome Xq28 that is a region encoding the nuclear factor–κB essential modulator, IKBKG. Absence or mutation of IKBKG in IP results in failure to activate nuclear factor–κB and leaves cells vulnerable to cytokine-mediated apoptosis, especially after exposure to tumor necrosis factor α.2
Clinical manifestations of IP are present at or soon after birth. The cutaneous findings of this disorder are classically described as a step-wise progression through 4 distinct stages: (1) a linear and/or whorled vesicular eruption predominantly on the extremities at birth or within the first few weeks of life; (2) thickened linear or whorled verrucous plaques; (3) hyperpigmented streaks and whorls that may or may not correspond with prior affected areas that may resolve by adolescence; and (4) hypopigmented, possibly atrophic plaques on the extremities that may persist lifelong. Importantly, not every patient will experience each of these stages. Overlap can occur, and the time course of each stage is highly variable. Other ectodermal manifestations include dental abnormalities such as small, misshaped, or missing teeth; alopecia; and nail abnormalities. Ocular abnormalities associated with IP primarily occur in the retina, including vascular occlusion, neovascularization, hemorrhages, foveal abnormalities, as well as exudative and tractional detachments.3,4
It is crucial to recognize CNS anomalies in association with the cutaneous findings of IP, as CNS pathology can be severe with profound developmental implications. Central nervous system findings have been noted to correlate with the appearance of the vesicular stage of IP. A high index of suspicion is needed, as the disease can demonstrate progression within a short time.5-8 The most frequent anomalies include seizures, motor impairment, intellectual disability, and microcephaly.9,10 Some of the most commonly identified CNS lesions on imaging include necrosis or brain infarcts, atrophy, and lesions of the corpus callosum.7
The pathogenesis of observed CNS changes in IP is not well understood. There have been numerous proposals of a vascular mechanism, and a microangiopathic process appears to be most plausible. Mutations in IKBKG may result in interruption of signaling via vascular endothelial growth factor receptor 3 with a consequent impact on angiogenesis, supporting a vascular mechanism. Additionally, mutations in IKBKG lead to activation of eotaxin, an eosinophil-selective chemokine.9 Eotaxin activation results in eosinophilic degranulation that mediates the classic eosinophilic infiltrate seen in the classic skin histology of IP. Additionally, it has been shown that eotaxin is strongly expressed by endothelial cells in IP, and more abundant eosinophil degranulation may play a role in mediating vaso-occlusion.7 Other studies have found that the highest expression level of the IKBKG gene is in the CNS, potentially explaining the extensive imaging findings of hemorrhage and diffusion restriction in our patient. These features likely are attributable to apoptosis of cells possessing the mutated IKBKG gene.9-11
- Ehrenreich M, Tarlow MM, Godlewska-Janusz E, et al. Incontinentia pigmenti (Bloch-Sulzberger syndrome): a systemic disorder. Cutis. 2007;79:355-362.
- Smahi A, Courtois G, Rabia SH, et al. The NF-kappaB signaling pathway in human diseases: from incontinentia pigmenti to ectodermal dysplasias and immune-deficiency syndromes. Hum Mol Genet. 2002;11:2371-2375.
- O’Doherty M, McCreery K, Green AJ, et al. Incontinentia pigmenti—ophthalmological observation of a series of cases and review of the literature. Br J Ophthalmol. 2011;95:11-16.
- Swinney CC, Han DP, Karth PA. Incontinentia pigmenti: a comprehensive review and update. Ophthalmic Surg Lasers Imaging Retina. 2015;46:650-657.
- Hennel SJ, Ekert PG, Volpe JJ, et al. Insights into the pathogenesis of cerebral lesions in incontinentia pigmenti. Pediatr Neurol. 2003;29:148-150.
- Maingay-de Groof F, Lequin MH, Roofthooft DW, et al. Extensive cerebral infarction in the newborn due to incontinentia pigmenti. Eur J Paediatr Neurol. 2008;12:284-289.
- Minic´ S, Trpinac D, Obradovic´ M. Systematic review of central nervous system anomalies in incontinentia pigmenti. Orphanet J Rare Dis. 2013;8:25-35.
- Wolf NI, Kramer N, Harting I, et al. Diffuse cortical necrosis in a neonate with incontinentia pigmenti and an encephalitis-like presentation. AJNR Am J Neuroradiol. 2005;26:1580-1582.
- Phan TA, Wargon O, Turner AM. Incontinentia pigmenti case series: clinical spectrum of incontinentia pigmenti in 53 female patients and their relatives. Clin Exp Dermatol. 2005;30:474-480.
- Volpe J. Neurobiology of periventricular leukomalacia in the premature infant. Pediatr Res. 2001;50:553-562.
- Pascual-Castroviejo I, Pascual-Pascual SI, Velazquez-Fragua R, et al. Incontinentia pigmenti: clinical and neuroimaging findings in a series of 12 patients. Neurologia. 2006;21:239-248.
- Ehrenreich M, Tarlow MM, Godlewska-Janusz E, et al. Incontinentia pigmenti (Bloch-Sulzberger syndrome): a systemic disorder. Cutis. 2007;79:355-362.
- Smahi A, Courtois G, Rabia SH, et al. The NF-kappaB signaling pathway in human diseases: from incontinentia pigmenti to ectodermal dysplasias and immune-deficiency syndromes. Hum Mol Genet. 2002;11:2371-2375.
- O’Doherty M, McCreery K, Green AJ, et al. Incontinentia pigmenti—ophthalmological observation of a series of cases and review of the literature. Br J Ophthalmol. 2011;95:11-16.
- Swinney CC, Han DP, Karth PA. Incontinentia pigmenti: a comprehensive review and update. Ophthalmic Surg Lasers Imaging Retina. 2015;46:650-657.
- Hennel SJ, Ekert PG, Volpe JJ, et al. Insights into the pathogenesis of cerebral lesions in incontinentia pigmenti. Pediatr Neurol. 2003;29:148-150.
- Maingay-de Groof F, Lequin MH, Roofthooft DW, et al. Extensive cerebral infarction in the newborn due to incontinentia pigmenti. Eur J Paediatr Neurol. 2008;12:284-289.
- Minic´ S, Trpinac D, Obradovic´ M. Systematic review of central nervous system anomalies in incontinentia pigmenti. Orphanet J Rare Dis. 2013;8:25-35.
- Wolf NI, Kramer N, Harting I, et al. Diffuse cortical necrosis in a neonate with incontinentia pigmenti and an encephalitis-like presentation. AJNR Am J Neuroradiol. 2005;26:1580-1582.
- Phan TA, Wargon O, Turner AM. Incontinentia pigmenti case series: clinical spectrum of incontinentia pigmenti in 53 female patients and their relatives. Clin Exp Dermatol. 2005;30:474-480.
- Volpe J. Neurobiology of periventricular leukomalacia in the premature infant. Pediatr Res. 2001;50:553-562.
- Pascual-Castroviejo I, Pascual-Pascual SI, Velazquez-Fragua R, et al. Incontinentia pigmenti: clinical and neuroimaging findings in a series of 12 patients. Neurologia. 2006;21:239-248.
Practice Points
- Central nervous system involvement in incontinentia pigmenti (IP) may be profound and can present prior to the classic cutaneous findings.
- A high index of suspicion for IP should be maintained in neonatal vesicular eruptions of unclear etiology, especially in the setting of unexplained seizures and/or abnormal brain imaging.
Delirium risk factors identified in ICU cancer patients
Hematology-oncology patients who receive treatment in the intensive care unit often develop delirium, and according to new findings, mechanical ventilation, high-dose corticosteroid use, and brain metastases were identified as independent risk factors.
Roughly half of all hematology-oncology patients who were admitted to the ICU experienced delirium, explained lead author Rachel Klosko, PharmD, PGY-2 cardiology pharmacy resident at the Ohio State University, Columbus.
“Delirium was associated with increased mortality, an increase in hospital length of stay, and increased length of stay in the ICU,” she said.
Dr. Klosko presented the study results at the at the Critical Care Congress sponsored by the Society of Critical Care Medicine (SCCM), which was held virtually this year.
Delirium is an acute and fluctuating disturbance of consciousness and cognition and fluctuates in severity. Critically ill patients are subject to numerous risk factors for delirium. “It can occur in independently of any known neurological disorder,” said Dr. Klosko, adding that its occurrence has been associated with poorer outcomes in ICU patients.
In this study, Dr. Klosko and colleagues sought to determine the incidence of delirium in cancer patients who were admitted to the ICU, as well as identify the associated risk factors and recognize potential consequences of the development of delirium in this patient population.
They conducted a single center, retrospective, cohort study that evaluated patients between the ages of 18 and 89 years who were admitted to the hematology-oncology medical or surgical ICU between July 1, 2018, and June 30, 2019.
The study’s primary endpoint was the incidence of delirium within 7 days of ICU admission, defined as two positive Confusion Assessment Method for the ICU (CAM-ICU) assessments within 24 hours. Patients identified with delirium were compared to those without it, for the evaluation of secondary endpoints that included hospital mortality and ICU and hospital length of stay. The researchers also sought to identify independent risk factors for delirium in this population.
A total of 244 patients were included in the final analysis. Of this group, 125 (51.2%) experienced delirium during their stay in the ICU, and 119 (48.8%) did not.
Mortality in the delirium group was significantly higher at 32.8% vs. 15.1% (P = .001). In addition, the delirium group was associated with significantly higher ICU length of stay (6 days vs. 3 days, P < .001) and hospital length of stay (21 days vs. 12 days, P < .001).
“When comparing the baseline characteristics between the two groups, the delirium group had a longer hospital length prior to ICU admission, a higher SOFA score, a higher rate of brain metastases, a higher rate of shock, and higher receipt of high-dose steroids, benzodiazepines, and immunotherapy,” said Dr. Klosko.
After multivariable regression, four variables were included in the final model. Among patients with delirium, the SOFA score increased by 25% (odds ratio[OR] 1.25, P < .001), while the odds of delirium were almost four times higher among those treated with high-dose corticosteroids (OR 3.79, P = .004). Delirium was also eight times higher (OR 8.48, P < .001) among those who received mechanical ventilation and five times higher in (OR 5.38, P = .015) in patients with brain metastases.
Dr. Klosko noted that the main limitations for this study were that it was a single center retrospective analysis, and that patients were reviewed within the first 7 days of ICU admission. “This potentially missed patients who developed delirium outside of this time frame,” she said. In addition, “too few patients received high-dose benzodiazepines,” and “none of the patients received continuous neuromuscular blockade.”
However, in “contrast to these limitations, this is the largest study to date that has analyzed delirium in this population,” Dr. Klosko said.
Commenting on the study, Brenda Pun, DNP, RN, director of data quality at the Vanderbilt Critical Illness, Brain Dysfunction, and Survivorship Center, Nashville, Tenn., pointed out that the goal of this study was to describe delirium in this specific population. “But I will take a step backward and say that they are just confirming that these patients look like other ICU patients in many regards,” she said.
She explained that the sicker patients are, the higher the rates of delirium. “We have implemented strategies to lower these rates, and they have improved,” Dr. Pun said. “Ten years ago, I would say that 80% of patients who were on a ventilator would have delirium but now the rates are around 50% and that’s what we are typically seeing now.”
Dr. Pun emphasized that this study shows that delirium is like the “canary in the coal mine” or a red flag. “It’s a sign that something is wrong and that we need to pay attention, because the patient’s outcome may be worse,” she said. “So this is saying that we need to see if there is something that can be changed or modified to decrease the incidence of delirium—these are important questions.”
There was no outside sponsor. The authors had no disclosures. Dr. Pun has no disclosures.
Hematology-oncology patients who receive treatment in the intensive care unit often develop delirium, and according to new findings, mechanical ventilation, high-dose corticosteroid use, and brain metastases were identified as independent risk factors.
Roughly half of all hematology-oncology patients who were admitted to the ICU experienced delirium, explained lead author Rachel Klosko, PharmD, PGY-2 cardiology pharmacy resident at the Ohio State University, Columbus.
“Delirium was associated with increased mortality, an increase in hospital length of stay, and increased length of stay in the ICU,” she said.
Dr. Klosko presented the study results at the at the Critical Care Congress sponsored by the Society of Critical Care Medicine (SCCM), which was held virtually this year.
Delirium is an acute and fluctuating disturbance of consciousness and cognition and fluctuates in severity. Critically ill patients are subject to numerous risk factors for delirium. “It can occur in independently of any known neurological disorder,” said Dr. Klosko, adding that its occurrence has been associated with poorer outcomes in ICU patients.
In this study, Dr. Klosko and colleagues sought to determine the incidence of delirium in cancer patients who were admitted to the ICU, as well as identify the associated risk factors and recognize potential consequences of the development of delirium in this patient population.
They conducted a single center, retrospective, cohort study that evaluated patients between the ages of 18 and 89 years who were admitted to the hematology-oncology medical or surgical ICU between July 1, 2018, and June 30, 2019.
The study’s primary endpoint was the incidence of delirium within 7 days of ICU admission, defined as two positive Confusion Assessment Method for the ICU (CAM-ICU) assessments within 24 hours. Patients identified with delirium were compared to those without it, for the evaluation of secondary endpoints that included hospital mortality and ICU and hospital length of stay. The researchers also sought to identify independent risk factors for delirium in this population.
A total of 244 patients were included in the final analysis. Of this group, 125 (51.2%) experienced delirium during their stay in the ICU, and 119 (48.8%) did not.
Mortality in the delirium group was significantly higher at 32.8% vs. 15.1% (P = .001). In addition, the delirium group was associated with significantly higher ICU length of stay (6 days vs. 3 days, P < .001) and hospital length of stay (21 days vs. 12 days, P < .001).
“When comparing the baseline characteristics between the two groups, the delirium group had a longer hospital length prior to ICU admission, a higher SOFA score, a higher rate of brain metastases, a higher rate of shock, and higher receipt of high-dose steroids, benzodiazepines, and immunotherapy,” said Dr. Klosko.
After multivariable regression, four variables were included in the final model. Among patients with delirium, the SOFA score increased by 25% (odds ratio[OR] 1.25, P < .001), while the odds of delirium were almost four times higher among those treated with high-dose corticosteroids (OR 3.79, P = .004). Delirium was also eight times higher (OR 8.48, P < .001) among those who received mechanical ventilation and five times higher in (OR 5.38, P = .015) in patients with brain metastases.
Dr. Klosko noted that the main limitations for this study were that it was a single center retrospective analysis, and that patients were reviewed within the first 7 days of ICU admission. “This potentially missed patients who developed delirium outside of this time frame,” she said. In addition, “too few patients received high-dose benzodiazepines,” and “none of the patients received continuous neuromuscular blockade.”
However, in “contrast to these limitations, this is the largest study to date that has analyzed delirium in this population,” Dr. Klosko said.
Commenting on the study, Brenda Pun, DNP, RN, director of data quality at the Vanderbilt Critical Illness, Brain Dysfunction, and Survivorship Center, Nashville, Tenn., pointed out that the goal of this study was to describe delirium in this specific population. “But I will take a step backward and say that they are just confirming that these patients look like other ICU patients in many regards,” she said.
She explained that the sicker patients are, the higher the rates of delirium. “We have implemented strategies to lower these rates, and they have improved,” Dr. Pun said. “Ten years ago, I would say that 80% of patients who were on a ventilator would have delirium but now the rates are around 50% and that’s what we are typically seeing now.”
Dr. Pun emphasized that this study shows that delirium is like the “canary in the coal mine” or a red flag. “It’s a sign that something is wrong and that we need to pay attention, because the patient’s outcome may be worse,” she said. “So this is saying that we need to see if there is something that can be changed or modified to decrease the incidence of delirium—these are important questions.”
There was no outside sponsor. The authors had no disclosures. Dr. Pun has no disclosures.
Hematology-oncology patients who receive treatment in the intensive care unit often develop delirium, and according to new findings, mechanical ventilation, high-dose corticosteroid use, and brain metastases were identified as independent risk factors.
Roughly half of all hematology-oncology patients who were admitted to the ICU experienced delirium, explained lead author Rachel Klosko, PharmD, PGY-2 cardiology pharmacy resident at the Ohio State University, Columbus.
“Delirium was associated with increased mortality, an increase in hospital length of stay, and increased length of stay in the ICU,” she said.
Dr. Klosko presented the study results at the at the Critical Care Congress sponsored by the Society of Critical Care Medicine (SCCM), which was held virtually this year.
Delirium is an acute and fluctuating disturbance of consciousness and cognition and fluctuates in severity. Critically ill patients are subject to numerous risk factors for delirium. “It can occur in independently of any known neurological disorder,” said Dr. Klosko, adding that its occurrence has been associated with poorer outcomes in ICU patients.
In this study, Dr. Klosko and colleagues sought to determine the incidence of delirium in cancer patients who were admitted to the ICU, as well as identify the associated risk factors and recognize potential consequences of the development of delirium in this patient population.
They conducted a single center, retrospective, cohort study that evaluated patients between the ages of 18 and 89 years who were admitted to the hematology-oncology medical or surgical ICU between July 1, 2018, and June 30, 2019.
The study’s primary endpoint was the incidence of delirium within 7 days of ICU admission, defined as two positive Confusion Assessment Method for the ICU (CAM-ICU) assessments within 24 hours. Patients identified with delirium were compared to those without it, for the evaluation of secondary endpoints that included hospital mortality and ICU and hospital length of stay. The researchers also sought to identify independent risk factors for delirium in this population.
A total of 244 patients were included in the final analysis. Of this group, 125 (51.2%) experienced delirium during their stay in the ICU, and 119 (48.8%) did not.
Mortality in the delirium group was significantly higher at 32.8% vs. 15.1% (P = .001). In addition, the delirium group was associated with significantly higher ICU length of stay (6 days vs. 3 days, P < .001) and hospital length of stay (21 days vs. 12 days, P < .001).
“When comparing the baseline characteristics between the two groups, the delirium group had a longer hospital length prior to ICU admission, a higher SOFA score, a higher rate of brain metastases, a higher rate of shock, and higher receipt of high-dose steroids, benzodiazepines, and immunotherapy,” said Dr. Klosko.
After multivariable regression, four variables were included in the final model. Among patients with delirium, the SOFA score increased by 25% (odds ratio[OR] 1.25, P < .001), while the odds of delirium were almost four times higher among those treated with high-dose corticosteroids (OR 3.79, P = .004). Delirium was also eight times higher (OR 8.48, P < .001) among those who received mechanical ventilation and five times higher in (OR 5.38, P = .015) in patients with brain metastases.
Dr. Klosko noted that the main limitations for this study were that it was a single center retrospective analysis, and that patients were reviewed within the first 7 days of ICU admission. “This potentially missed patients who developed delirium outside of this time frame,” she said. In addition, “too few patients received high-dose benzodiazepines,” and “none of the patients received continuous neuromuscular blockade.”
However, in “contrast to these limitations, this is the largest study to date that has analyzed delirium in this population,” Dr. Klosko said.
Commenting on the study, Brenda Pun, DNP, RN, director of data quality at the Vanderbilt Critical Illness, Brain Dysfunction, and Survivorship Center, Nashville, Tenn., pointed out that the goal of this study was to describe delirium in this specific population. “But I will take a step backward and say that they are just confirming that these patients look like other ICU patients in many regards,” she said.
She explained that the sicker patients are, the higher the rates of delirium. “We have implemented strategies to lower these rates, and they have improved,” Dr. Pun said. “Ten years ago, I would say that 80% of patients who were on a ventilator would have delirium but now the rates are around 50% and that’s what we are typically seeing now.”
Dr. Pun emphasized that this study shows that delirium is like the “canary in the coal mine” or a red flag. “It’s a sign that something is wrong and that we need to pay attention, because the patient’s outcome may be worse,” she said. “So this is saying that we need to see if there is something that can be changed or modified to decrease the incidence of delirium—these are important questions.”
There was no outside sponsor. The authors had no disclosures. Dr. Pun has no disclosures.
FROM CCC50
PCI and CABG for left main disease have equal outcomes at 5 years
Background: While PCI with drug-eluting stents has become more accepted as treatment for some patients with left main disease, long-term outcomes from randomized control trials comparing PCI with CABG have yet to be clearly established.
Study design: International, open-label, multicenter, randomized trial.
Setting: A total of 126 sites in 17 countries.
Synopsis: Patients with low or intermediate anatomical complexity with 70% visual stenosis of the left main coronary artery or 50%-70% stenosis by noninvasive testing were randomized to either PCI (948) or CABG (957). Dual-antiplatelet therapy was given to PCI patients and aspirin to CABG patients. At 5 years there was no significant difference in the rate of the composite of death, stroke, or myocardial infarction (22.0% with PCI vs. 19.2% with CABG; difference, 2.8 percentage points; 95% CI, –0.9 to 6.5; P = .13). This was consistent across subgroups.
There were numerical differences in nonpowered secondary outcomes that may represent effects but should be interpreted cautiously: ischemia-driven revascularization (16.9% with PCI vs. 10% with CABG), transient ischemic attack plus stroke (3.3% with PCI vs. 5.2% with CABG), and death from any cause (3% with PCI vs. 9.9% with CABG). There was no significant difference in cardiovascular events, MI, or stroke.
One interesting limitation was that patients who had PCI were more commonly on dual-antiplatelet therapy and angiotensin converting–enzyme inhibitors, whereas CABG patients were more often on beta-blockers, diuretics, anticoagulants, and antiarrhythmics.
Bottom line: PCI and CABG treatments for left main disease have no significant difference in the composite outcome of death, stroke, or MI at 5 years.
Citation: Stone GW et al. Five-year outcomes after PCI or CABG for left main coronary disease. N Engl J Med. 2019;381:1820-30.
Dr. Horton is a hospitalist and clinical instructor of medicine at the University of Utah, Salt Lake City.
Background: While PCI with drug-eluting stents has become more accepted as treatment for some patients with left main disease, long-term outcomes from randomized control trials comparing PCI with CABG have yet to be clearly established.
Study design: International, open-label, multicenter, randomized trial.
Setting: A total of 126 sites in 17 countries.
Synopsis: Patients with low or intermediate anatomical complexity with 70% visual stenosis of the left main coronary artery or 50%-70% stenosis by noninvasive testing were randomized to either PCI (948) or CABG (957). Dual-antiplatelet therapy was given to PCI patients and aspirin to CABG patients. At 5 years there was no significant difference in the rate of the composite of death, stroke, or myocardial infarction (22.0% with PCI vs. 19.2% with CABG; difference, 2.8 percentage points; 95% CI, –0.9 to 6.5; P = .13). This was consistent across subgroups.
There were numerical differences in nonpowered secondary outcomes that may represent effects but should be interpreted cautiously: ischemia-driven revascularization (16.9% with PCI vs. 10% with CABG), transient ischemic attack plus stroke (3.3% with PCI vs. 5.2% with CABG), and death from any cause (3% with PCI vs. 9.9% with CABG). There was no significant difference in cardiovascular events, MI, or stroke.
One interesting limitation was that patients who had PCI were more commonly on dual-antiplatelet therapy and angiotensin converting–enzyme inhibitors, whereas CABG patients were more often on beta-blockers, diuretics, anticoagulants, and antiarrhythmics.
Bottom line: PCI and CABG treatments for left main disease have no significant difference in the composite outcome of death, stroke, or MI at 5 years.
Citation: Stone GW et al. Five-year outcomes after PCI or CABG for left main coronary disease. N Engl J Med. 2019;381:1820-30.
Dr. Horton is a hospitalist and clinical instructor of medicine at the University of Utah, Salt Lake City.
Background: While PCI with drug-eluting stents has become more accepted as treatment for some patients with left main disease, long-term outcomes from randomized control trials comparing PCI with CABG have yet to be clearly established.
Study design: International, open-label, multicenter, randomized trial.
Setting: A total of 126 sites in 17 countries.
Synopsis: Patients with low or intermediate anatomical complexity with 70% visual stenosis of the left main coronary artery or 50%-70% stenosis by noninvasive testing were randomized to either PCI (948) or CABG (957). Dual-antiplatelet therapy was given to PCI patients and aspirin to CABG patients. At 5 years there was no significant difference in the rate of the composite of death, stroke, or myocardial infarction (22.0% with PCI vs. 19.2% with CABG; difference, 2.8 percentage points; 95% CI, –0.9 to 6.5; P = .13). This was consistent across subgroups.
There were numerical differences in nonpowered secondary outcomes that may represent effects but should be interpreted cautiously: ischemia-driven revascularization (16.9% with PCI vs. 10% with CABG), transient ischemic attack plus stroke (3.3% with PCI vs. 5.2% with CABG), and death from any cause (3% with PCI vs. 9.9% with CABG). There was no significant difference in cardiovascular events, MI, or stroke.
One interesting limitation was that patients who had PCI were more commonly on dual-antiplatelet therapy and angiotensin converting–enzyme inhibitors, whereas CABG patients were more often on beta-blockers, diuretics, anticoagulants, and antiarrhythmics.
Bottom line: PCI and CABG treatments for left main disease have no significant difference in the composite outcome of death, stroke, or MI at 5 years.
Citation: Stone GW et al. Five-year outcomes after PCI or CABG for left main coronary disease. N Engl J Med. 2019;381:1820-30.
Dr. Horton is a hospitalist and clinical instructor of medicine at the University of Utah, Salt Lake City.
Question 2
Correct answer: B. Prednisone
Rationale
This is a case of Henoch-Schönlein purpura, which is a self-limited, systemic, nongranulomatous, autoimmune complex, small-vessel vasculitis with multiorgan involvement. It is characterized by a classic tetrad of nonthrombocytopenic palpable purpura, arthritis or arthralgias, gastrointestinal involvement, and renal involvement. GI involvement may mimic Crohn's disease, although the biopsies are usually diagnostic. Most cases are self-limiting, but oral steroids are indicated in patients with severe colicky abdominal pain; usually they're started as prednisone or methylprednisolone at 1-2 mg/kg per day for 1-2 weeks and then tapering to a stop in the next 1-2 weeks. Steroids may prevent major complications such as gastrointestinal bleeding or intussusception. Immunosuppressive drugs (cyclophosphamide, azathioprine, cyclosporine A, and mycophenolate mofetil) in combination with high-dose IV pulse steroids are recommended if there is no benefit from steroids alone.
Reference
Sohagia AB et al. Gastroenterol Res Pract. 2010. doi: 10.1155/2010/59764.
Correct answer: B. Prednisone
Rationale
This is a case of Henoch-Schönlein purpura, which is a self-limited, systemic, nongranulomatous, autoimmune complex, small-vessel vasculitis with multiorgan involvement. It is characterized by a classic tetrad of nonthrombocytopenic palpable purpura, arthritis or arthralgias, gastrointestinal involvement, and renal involvement. GI involvement may mimic Crohn's disease, although the biopsies are usually diagnostic. Most cases are self-limiting, but oral steroids are indicated in patients with severe colicky abdominal pain; usually they're started as prednisone or methylprednisolone at 1-2 mg/kg per day for 1-2 weeks and then tapering to a stop in the next 1-2 weeks. Steroids may prevent major complications such as gastrointestinal bleeding or intussusception. Immunosuppressive drugs (cyclophosphamide, azathioprine, cyclosporine A, and mycophenolate mofetil) in combination with high-dose IV pulse steroids are recommended if there is no benefit from steroids alone.
Reference
Sohagia AB et al. Gastroenterol Res Pract. 2010. doi: 10.1155/2010/59764.
Correct answer: B. Prednisone
Rationale
This is a case of Henoch-Schönlein purpura, which is a self-limited, systemic, nongranulomatous, autoimmune complex, small-vessel vasculitis with multiorgan involvement. It is characterized by a classic tetrad of nonthrombocytopenic palpable purpura, arthritis or arthralgias, gastrointestinal involvement, and renal involvement. GI involvement may mimic Crohn's disease, although the biopsies are usually diagnostic. Most cases are self-limiting, but oral steroids are indicated in patients with severe colicky abdominal pain; usually they're started as prednisone or methylprednisolone at 1-2 mg/kg per day for 1-2 weeks and then tapering to a stop in the next 1-2 weeks. Steroids may prevent major complications such as gastrointestinal bleeding or intussusception. Immunosuppressive drugs (cyclophosphamide, azathioprine, cyclosporine A, and mycophenolate mofetil) in combination with high-dose IV pulse steroids are recommended if there is no benefit from steroids alone.
Reference
Sohagia AB et al. Gastroenterol Res Pract. 2010. doi: 10.1155/2010/59764.
Q2. A 26-year-old White male presented with fever and sore throat for 5 days along with erythematous, nonpruritic rash involving the extremities and arthralgias. He subsequently developed right lower-quadrant pain, aggravated with meals, and associated with watery diarrhea. Labs show showed white blood cell count of 14,900/microL and a C-reactive protein level of 12.6 mg/dL. A magnetic resonance enterography showed 20 cm of thickened ileum. An upper endoscopy showed multiple erosions in the duodenum and antrum while a colonoscopy showed erythema and inflammation in the terminal ileum and cecum. Biopsies from both areas demonstrated evidence of leukocytoclastic vasculitis. Skin biopsy also showed leukocytoclastic vasculitis.
Question 1
Correct answer: D. Antienterocyte antibodies
Rationale
Autoimmune enteropathy (AIE) is characterized by a severe malabsorption and secretory diarrhea, and is differentiated from celiac disease on small-bowel biopsy by the decreased numbers or absence of surface intraepithelial lymphocytes, apoptotic bodies present in the intestinal crypts, and absent goblet and Paneth cells. Patients with AIE may also carry other autoimmune conditions such as rheumatoid arthritis and multiple sclerosis. A group at the Mayo Clinic has published a set of diagnostic criteria based on their case series of adult AIE that requires ruling out other causes of chronic diarrhea in adults, specific histology supportive of AIE, and presence of malabsorption and ruling out other causes of villous atrophy. The presence of antienterocyte or antigoblet cell antibodies are supportive of a diagnosis of AIE, but their absence does not exclude the diagnosis.
References
Akram S et al. Clin Gastroenterol Hepatol. 2007;5(11):1282-90.
Montalto M et al. Scan J Gastroenterol. 2009;44(9):1029-36.
Correct answer: D. Antienterocyte antibodies
Rationale
Autoimmune enteropathy (AIE) is characterized by a severe malabsorption and secretory diarrhea, and is differentiated from celiac disease on small-bowel biopsy by the decreased numbers or absence of surface intraepithelial lymphocytes, apoptotic bodies present in the intestinal crypts, and absent goblet and Paneth cells. Patients with AIE may also carry other autoimmune conditions such as rheumatoid arthritis and multiple sclerosis. A group at the Mayo Clinic has published a set of diagnostic criteria based on their case series of adult AIE that requires ruling out other causes of chronic diarrhea in adults, specific histology supportive of AIE, and presence of malabsorption and ruling out other causes of villous atrophy. The presence of antienterocyte or antigoblet cell antibodies are supportive of a diagnosis of AIE, but their absence does not exclude the diagnosis.
References
Akram S et al. Clin Gastroenterol Hepatol. 2007;5(11):1282-90.
Montalto M et al. Scan J Gastroenterol. 2009;44(9):1029-36.
Correct answer: D. Antienterocyte antibodies
Rationale
Autoimmune enteropathy (AIE) is characterized by a severe malabsorption and secretory diarrhea, and is differentiated from celiac disease on small-bowel biopsy by the decreased numbers or absence of surface intraepithelial lymphocytes, apoptotic bodies present in the intestinal crypts, and absent goblet and Paneth cells. Patients with AIE may also carry other autoimmune conditions such as rheumatoid arthritis and multiple sclerosis. A group at the Mayo Clinic has published a set of diagnostic criteria based on their case series of adult AIE that requires ruling out other causes of chronic diarrhea in adults, specific histology supportive of AIE, and presence of malabsorption and ruling out other causes of villous atrophy. The presence of antienterocyte or antigoblet cell antibodies are supportive of a diagnosis of AIE, but their absence does not exclude the diagnosis.
References
Akram S et al. Clin Gastroenterol Hepatol. 2007;5(11):1282-90.
Montalto M et al. Scan J Gastroenterol. 2009;44(9):1029-36.
Q1. A 55-year-old woman presents with a one-year history of large volume foul-smelling stools that float in water associated with 40-pound weight loss. Laboratory evaluation reveals low vitamin A and D levels. An upper endoscopy with duodenal biopsies reveals complete villous blunting with decreased goblet and Paneth cells, absence of surface intraepithelial lymphocytes, and increased crypt apoptosis. She denies nonsteroidal anti-inflammatory drug use, celiac serologies were not elevated, and a glucose hydrogen breath test was negative. She also has coexisting rheumatoid arthritis and multiple sclerosis.
Treatment paradigm for chronic HBV in flux
These days deciding when to stop targeted treatment for chronic hepatitis B is a bigger challenge than knowing when to start, Norah A. Terrault, MD, MPH, observed at the Gastroenterology Updates, IBD, Liver Disease Conference.
That’s because the treatment paradigm is in flux. The strategy is shifting from achieving hepatitis B virus (HBV) DNA suppression through indefinite use of nucleoside analogues to striving for functional cure, which means eliminating hepatitis B surface antigen (HBsAg) and sustained inactive chronic hepatitis B off therapy. It’s a goal that recognizes that, while suppression is worthwhile because it reduces a patient’s risk of hepatocellular carcinoma, HBsAg clearance is better because it’s associated with an even lower risk of the malignancy, explained Dr. Terrault, professor of medicine and chief of gastroenterology and liver diseases at the University of Southern California, Los Angeles.
The current strategy in patients who are hepatitis B e antigen (HBeAg) positive at the outset is to treat with a nucleoside analogue until seroconversion, followed by a further year or more of consolidation therapy then treatment withdrawal. It’s a rational approach whose primary benefit is it allows identification of the roughly 50% of patients who can remain off treatment with inactive chronic hepatitis B. The other 50% – those who experience clinical relapse – will need retreatment.
Factors predictive of increased likelihood of a sustained off-treatment response include age younger than 40 years at the time of seroconversion, more than 1 year of consolidation therapy, and undetectable HBV DNA at cessation of treatment.
“In my own practice now, I actually extend the consolidation period for 2 years before I consider stopping, and I really favor doing a trial of stopping treatment in those who are younger,” Dr. Terrault said.
The biggest change in thinking involves the duration of therapy in patients who are HBeAg negative. The strategy has been to treat indefinitely unless there is a compelling reason to stop, such as toxicity, cost, or patient preference. However, it has now been demonstrated in at least nine published studies that withdrawal of therapy has a favorable immunologic effect in noncirrhotic patients with HBeAg-negative chronic hepatitis B who have been HBV DNA negative on nucleoside analogues for at least 3 years. This trial off therapy can bring major benefits because roughly 50% of patients will have sustained inactive chronic hepatitis B off-treatment and 20% of patients will become HbsAg negative with functional cure at 3-5 years of follow-up.
“This is what’s impressive: that 20% of patients have lost surface antigen, because if you continue HbeAg-negative patients on nucleoside analogue therapy, essentially none of them lose surface antigen. This is an impressive number, and you’re also able to identify about 50% of patients who didn’t need to be on treatment because they now have immune control and can remain inactive carriers off treatment,” the gastroenterologist commented.
Treatment withdrawal in HBeAg-negative patients usually is followed by disease flares 8-12 weeks later because of host immune clearance, and therein lies a problem.
“The challenge with the withdrawal strategy is these flares that appear to be necessary and important, can be good or bad, and we’re really not very good at predicting what the flare is going to look like and how severe it’s going to be,” according to Dr. Terrault, first author of the current American Association for the Study of Liver Diseases guidance on prevention, diagnosis, and treatment of chronic hepatitis B.
The good flares are accompanied by a reductions in HBV DNA and viral proteins, loss of HbsAg, and preserved liver function. The bad flares entail excessive host immune clearance leading to liver dysfunction or failure, with no reduction in viral proteins. The search is on for predictors of response to treatment withdrawal in HbeAg-negative patients. Potential differences in outcomes with the three available nucleoside analogues are being looked at, as are duration of viral suppression on treatment and differences in patient characteristics. A low quantitative HbsAg level at the time of drug withdrawal may also be important as a predictor of a higher likelihood of HBsAg loss over time off treatment.
“The studies that have been done are basically withdrawing everyone and then seeing what happens. I think we want to have a more refined approach,” she said.
This is an unfolding story. The encouraging news is that the drug development pipeline is rich with agents with a variety of mechanisms aimed at achieving HbsAg loss with finite therapy. Some of the studies are now in phase 2 and 3.
“We should be extremely excited,” Dr. Terrault said. “I think in the future we’re very likely to have curative therapies in a much greater proportion of our patients.”
When to start nucleoside analogues
Three antiviral oral nucleoside analogues are available as preferred therapies for chronic HBV: entecavir (Baraclude), tenofovir alafenamide (Vemlidy), and tenofovir disoproxil (Viread). All three provide high antiviral efficacy and low risk for resistance. The treatment goal is to prevent disease progression and HBV complications, including hepatocellular carcinoma, in individuals with active chronic hepatitis B.
The major liver disease medical societies differ only slightly on the criteria for starting treatment. Broadly, they recommend starting therapy in all patients with cirrhosis, as well as in patients without cirrhosis who have both a serum ALT level more than twice the upper limit of normal and elevated HBV DNA levels. The treatment threshold for HBV DNA levels is higher in patients who are HBeAg positive than it is for patients who are HBeAg negative; for example, the American Association for the Study of Liver Diseases recommends that an HbeAg-positive patient should have a HBV DNA titer greater than 20,000 IU/mL, which is a level 10 times higher than the group’s treatment threshold in HBeAg-negative patients. However, these thresholds are intended as guidance, not absolute rules, Dr. Terrault emphasized. Nearly 40% of patients don’t meet the dual ALT and HBV DNA thresholds, and serial monitoring of such patients for 6-12 months is recommended because they may be in transition.
The choice of nucleoside analogue is largely based on comorbidities. Any of the three preferred antivirals can be used when there are none. Tenofovir disoproxil is preferred in pregnancy because of its safety profile in that setting. In patients who are aged over 60 years or have bone disease or renal impairment, tenofovir alafenamide and entecavir are preferred. Entecavir should be avoided in favor of either form of tenofovir in patients who are HIV positive or have prior exposure to lamivudine.
Regarding treatment with these drugs, the recommendations target those whose liver disease is being driven by active HBV rather than fatty liver disease or some other cause. That’s the reason for the reserving treatment for patients with both high HBV DNA and high serum ALT.
“There’s definitely a camp that feels these are safe drugs, easy to use, and we should treat more people. I have to say I’m not hanging out in that camp. I still feel we should do targeted treatment, especially since there are many new drugs coming where we’re going to be able to offer cure to more people. So I feel like putting everybody on suppressive therapy isn’t the answer,” she said.
Dr. Terrault receives research grants from and/or serves as a consultant to numerous pharmaceutical companies.
These days deciding when to stop targeted treatment for chronic hepatitis B is a bigger challenge than knowing when to start, Norah A. Terrault, MD, MPH, observed at the Gastroenterology Updates, IBD, Liver Disease Conference.
That’s because the treatment paradigm is in flux. The strategy is shifting from achieving hepatitis B virus (HBV) DNA suppression through indefinite use of nucleoside analogues to striving for functional cure, which means eliminating hepatitis B surface antigen (HBsAg) and sustained inactive chronic hepatitis B off therapy. It’s a goal that recognizes that, while suppression is worthwhile because it reduces a patient’s risk of hepatocellular carcinoma, HBsAg clearance is better because it’s associated with an even lower risk of the malignancy, explained Dr. Terrault, professor of medicine and chief of gastroenterology and liver diseases at the University of Southern California, Los Angeles.
The current strategy in patients who are hepatitis B e antigen (HBeAg) positive at the outset is to treat with a nucleoside analogue until seroconversion, followed by a further year or more of consolidation therapy then treatment withdrawal. It’s a rational approach whose primary benefit is it allows identification of the roughly 50% of patients who can remain off treatment with inactive chronic hepatitis B. The other 50% – those who experience clinical relapse – will need retreatment.
Factors predictive of increased likelihood of a sustained off-treatment response include age younger than 40 years at the time of seroconversion, more than 1 year of consolidation therapy, and undetectable HBV DNA at cessation of treatment.
“In my own practice now, I actually extend the consolidation period for 2 years before I consider stopping, and I really favor doing a trial of stopping treatment in those who are younger,” Dr. Terrault said.
The biggest change in thinking involves the duration of therapy in patients who are HBeAg negative. The strategy has been to treat indefinitely unless there is a compelling reason to stop, such as toxicity, cost, or patient preference. However, it has now been demonstrated in at least nine published studies that withdrawal of therapy has a favorable immunologic effect in noncirrhotic patients with HBeAg-negative chronic hepatitis B who have been HBV DNA negative on nucleoside analogues for at least 3 years. This trial off therapy can bring major benefits because roughly 50% of patients will have sustained inactive chronic hepatitis B off-treatment and 20% of patients will become HbsAg negative with functional cure at 3-5 years of follow-up.
“This is what’s impressive: that 20% of patients have lost surface antigen, because if you continue HbeAg-negative patients on nucleoside analogue therapy, essentially none of them lose surface antigen. This is an impressive number, and you’re also able to identify about 50% of patients who didn’t need to be on treatment because they now have immune control and can remain inactive carriers off treatment,” the gastroenterologist commented.
Treatment withdrawal in HBeAg-negative patients usually is followed by disease flares 8-12 weeks later because of host immune clearance, and therein lies a problem.
“The challenge with the withdrawal strategy is these flares that appear to be necessary and important, can be good or bad, and we’re really not very good at predicting what the flare is going to look like and how severe it’s going to be,” according to Dr. Terrault, first author of the current American Association for the Study of Liver Diseases guidance on prevention, diagnosis, and treatment of chronic hepatitis B.
The good flares are accompanied by a reductions in HBV DNA and viral proteins, loss of HbsAg, and preserved liver function. The bad flares entail excessive host immune clearance leading to liver dysfunction or failure, with no reduction in viral proteins. The search is on for predictors of response to treatment withdrawal in HbeAg-negative patients. Potential differences in outcomes with the three available nucleoside analogues are being looked at, as are duration of viral suppression on treatment and differences in patient characteristics. A low quantitative HbsAg level at the time of drug withdrawal may also be important as a predictor of a higher likelihood of HBsAg loss over time off treatment.
“The studies that have been done are basically withdrawing everyone and then seeing what happens. I think we want to have a more refined approach,” she said.
This is an unfolding story. The encouraging news is that the drug development pipeline is rich with agents with a variety of mechanisms aimed at achieving HbsAg loss with finite therapy. Some of the studies are now in phase 2 and 3.
“We should be extremely excited,” Dr. Terrault said. “I think in the future we’re very likely to have curative therapies in a much greater proportion of our patients.”
When to start nucleoside analogues
Three antiviral oral nucleoside analogues are available as preferred therapies for chronic HBV: entecavir (Baraclude), tenofovir alafenamide (Vemlidy), and tenofovir disoproxil (Viread). All three provide high antiviral efficacy and low risk for resistance. The treatment goal is to prevent disease progression and HBV complications, including hepatocellular carcinoma, in individuals with active chronic hepatitis B.
The major liver disease medical societies differ only slightly on the criteria for starting treatment. Broadly, they recommend starting therapy in all patients with cirrhosis, as well as in patients without cirrhosis who have both a serum ALT level more than twice the upper limit of normal and elevated HBV DNA levels. The treatment threshold for HBV DNA levels is higher in patients who are HBeAg positive than it is for patients who are HBeAg negative; for example, the American Association for the Study of Liver Diseases recommends that an HbeAg-positive patient should have a HBV DNA titer greater than 20,000 IU/mL, which is a level 10 times higher than the group’s treatment threshold in HBeAg-negative patients. However, these thresholds are intended as guidance, not absolute rules, Dr. Terrault emphasized. Nearly 40% of patients don’t meet the dual ALT and HBV DNA thresholds, and serial monitoring of such patients for 6-12 months is recommended because they may be in transition.
The choice of nucleoside analogue is largely based on comorbidities. Any of the three preferred antivirals can be used when there are none. Tenofovir disoproxil is preferred in pregnancy because of its safety profile in that setting. In patients who are aged over 60 years or have bone disease or renal impairment, tenofovir alafenamide and entecavir are preferred. Entecavir should be avoided in favor of either form of tenofovir in patients who are HIV positive or have prior exposure to lamivudine.
Regarding treatment with these drugs, the recommendations target those whose liver disease is being driven by active HBV rather than fatty liver disease or some other cause. That’s the reason for the reserving treatment for patients with both high HBV DNA and high serum ALT.
“There’s definitely a camp that feels these are safe drugs, easy to use, and we should treat more people. I have to say I’m not hanging out in that camp. I still feel we should do targeted treatment, especially since there are many new drugs coming where we’re going to be able to offer cure to more people. So I feel like putting everybody on suppressive therapy isn’t the answer,” she said.
Dr. Terrault receives research grants from and/or serves as a consultant to numerous pharmaceutical companies.
These days deciding when to stop targeted treatment for chronic hepatitis B is a bigger challenge than knowing when to start, Norah A. Terrault, MD, MPH, observed at the Gastroenterology Updates, IBD, Liver Disease Conference.
That’s because the treatment paradigm is in flux. The strategy is shifting from achieving hepatitis B virus (HBV) DNA suppression through indefinite use of nucleoside analogues to striving for functional cure, which means eliminating hepatitis B surface antigen (HBsAg) and sustained inactive chronic hepatitis B off therapy. It’s a goal that recognizes that, while suppression is worthwhile because it reduces a patient’s risk of hepatocellular carcinoma, HBsAg clearance is better because it’s associated with an even lower risk of the malignancy, explained Dr. Terrault, professor of medicine and chief of gastroenterology and liver diseases at the University of Southern California, Los Angeles.
The current strategy in patients who are hepatitis B e antigen (HBeAg) positive at the outset is to treat with a nucleoside analogue until seroconversion, followed by a further year or more of consolidation therapy then treatment withdrawal. It’s a rational approach whose primary benefit is it allows identification of the roughly 50% of patients who can remain off treatment with inactive chronic hepatitis B. The other 50% – those who experience clinical relapse – will need retreatment.
Factors predictive of increased likelihood of a sustained off-treatment response include age younger than 40 years at the time of seroconversion, more than 1 year of consolidation therapy, and undetectable HBV DNA at cessation of treatment.
“In my own practice now, I actually extend the consolidation period for 2 years before I consider stopping, and I really favor doing a trial of stopping treatment in those who are younger,” Dr. Terrault said.
The biggest change in thinking involves the duration of therapy in patients who are HBeAg negative. The strategy has been to treat indefinitely unless there is a compelling reason to stop, such as toxicity, cost, or patient preference. However, it has now been demonstrated in at least nine published studies that withdrawal of therapy has a favorable immunologic effect in noncirrhotic patients with HBeAg-negative chronic hepatitis B who have been HBV DNA negative on nucleoside analogues for at least 3 years. This trial off therapy can bring major benefits because roughly 50% of patients will have sustained inactive chronic hepatitis B off-treatment and 20% of patients will become HbsAg negative with functional cure at 3-5 years of follow-up.
“This is what’s impressive: that 20% of patients have lost surface antigen, because if you continue HbeAg-negative patients on nucleoside analogue therapy, essentially none of them lose surface antigen. This is an impressive number, and you’re also able to identify about 50% of patients who didn’t need to be on treatment because they now have immune control and can remain inactive carriers off treatment,” the gastroenterologist commented.
Treatment withdrawal in HBeAg-negative patients usually is followed by disease flares 8-12 weeks later because of host immune clearance, and therein lies a problem.
“The challenge with the withdrawal strategy is these flares that appear to be necessary and important, can be good or bad, and we’re really not very good at predicting what the flare is going to look like and how severe it’s going to be,” according to Dr. Terrault, first author of the current American Association for the Study of Liver Diseases guidance on prevention, diagnosis, and treatment of chronic hepatitis B.
The good flares are accompanied by a reductions in HBV DNA and viral proteins, loss of HbsAg, and preserved liver function. The bad flares entail excessive host immune clearance leading to liver dysfunction or failure, with no reduction in viral proteins. The search is on for predictors of response to treatment withdrawal in HbeAg-negative patients. Potential differences in outcomes with the three available nucleoside analogues are being looked at, as are duration of viral suppression on treatment and differences in patient characteristics. A low quantitative HbsAg level at the time of drug withdrawal may also be important as a predictor of a higher likelihood of HBsAg loss over time off treatment.
“The studies that have been done are basically withdrawing everyone and then seeing what happens. I think we want to have a more refined approach,” she said.
This is an unfolding story. The encouraging news is that the drug development pipeline is rich with agents with a variety of mechanisms aimed at achieving HbsAg loss with finite therapy. Some of the studies are now in phase 2 and 3.
“We should be extremely excited,” Dr. Terrault said. “I think in the future we’re very likely to have curative therapies in a much greater proportion of our patients.”
When to start nucleoside analogues
Three antiviral oral nucleoside analogues are available as preferred therapies for chronic HBV: entecavir (Baraclude), tenofovir alafenamide (Vemlidy), and tenofovir disoproxil (Viread). All three provide high antiviral efficacy and low risk for resistance. The treatment goal is to prevent disease progression and HBV complications, including hepatocellular carcinoma, in individuals with active chronic hepatitis B.
The major liver disease medical societies differ only slightly on the criteria for starting treatment. Broadly, they recommend starting therapy in all patients with cirrhosis, as well as in patients without cirrhosis who have both a serum ALT level more than twice the upper limit of normal and elevated HBV DNA levels. The treatment threshold for HBV DNA levels is higher in patients who are HBeAg positive than it is for patients who are HBeAg negative; for example, the American Association for the Study of Liver Diseases recommends that an HbeAg-positive patient should have a HBV DNA titer greater than 20,000 IU/mL, which is a level 10 times higher than the group’s treatment threshold in HBeAg-negative patients. However, these thresholds are intended as guidance, not absolute rules, Dr. Terrault emphasized. Nearly 40% of patients don’t meet the dual ALT and HBV DNA thresholds, and serial monitoring of such patients for 6-12 months is recommended because they may be in transition.
The choice of nucleoside analogue is largely based on comorbidities. Any of the three preferred antivirals can be used when there are none. Tenofovir disoproxil is preferred in pregnancy because of its safety profile in that setting. In patients who are aged over 60 years or have bone disease or renal impairment, tenofovir alafenamide and entecavir are preferred. Entecavir should be avoided in favor of either form of tenofovir in patients who are HIV positive or have prior exposure to lamivudine.
Regarding treatment with these drugs, the recommendations target those whose liver disease is being driven by active HBV rather than fatty liver disease or some other cause. That’s the reason for the reserving treatment for patients with both high HBV DNA and high serum ALT.
“There’s definitely a camp that feels these are safe drugs, easy to use, and we should treat more people. I have to say I’m not hanging out in that camp. I still feel we should do targeted treatment, especially since there are many new drugs coming where we’re going to be able to offer cure to more people. So I feel like putting everybody on suppressive therapy isn’t the answer,” she said.
Dr. Terrault receives research grants from and/or serves as a consultant to numerous pharmaceutical companies.
FROM GUILD 2021
Vaccine mismatch: What to do after dose 1 when plans change
Ideally, Americans receiving their Pfizer/BioNTech or Moderna COVID-19 vaccines will get both doses from the same manufacturer, said Gregory Poland, MD, a vaccinologist at the Mayo Clinic in Rochester, Minn.
After all, that’s how they were tested for efficacy and safety, and it was results from those studies that led to emergency use authorization (EUA) being granted by the Food and Drug Administration.
But states and countries have struggled to keep up with the demand for vaccine, and more flexible vaccination schedules could help.
So researchers are exploring whether it is safe and effective to get the first and second doses from different manufacturers. And they are even wondering whether mixing doses from different manufacturers could increase effectiveness, particularly in light of emerging variants.
It’s called the “interchangeability issue,” said Dr. Poland, who has gotten a steady stream of questions about it.
For example, a patient recently asked about options for his father, who had gotten his first dose of the AstraZeneca vaccine in Ecuador, but had since moved to the United States, where that product has not been approved for use.
Dr. Poland said in an interview that he prefaces each answer with: “I’ve got no science for what I’m about to tell you.”
In this particular case, he recommended that the man’s father talk with his doctor about his level of COVID-19 risk and consider whether he should gamble on the AstraZeneca vaccine getting approved in the United States soon, or whether he should ask for a second dose from one of the three vaccines currently approved.
On March 22, 2021, AstraZeneca released positive results from its phase 3 trial, which will likely speed its path toward use in the United States.
Although clinical trials have started to test combinations and boosters, there’s currently no definitive evidence from human trials on mixing COVID vaccines, Dr. Poland pointed out.
But a study of a mixed-vaccine regimen is currently underway in the United Kingdom.
Participants in that 13-month trial will be given the Oxford/AstraZeneca and Pfizer/BioNTech vaccines in different combinations and at different intervals. The first results from that trial are expected this summer.
And interim results from a trial combining Russia’s Sputnik V and the AstraZeneca vaccines are expected in 2 months, according to a Reuters report.
Mix only in ‘exceptional situations’
The Centers for Disease Control and Prevention has been hesitant to open the door to mixing Pfizer and Moderna vaccinations, noting that the two “are not interchangeable.” But CDC guidance has changed slightly. Now, instead of saying the two vaccines should not be mixed, CDC guidance says they can be mixed in “exceptional situations,” and that the second dose can be administered up to 6 weeks after the first dose.
It is reasonable to assume that mixing COVID-19 vaccines that use the same platform – such as the mRNA platform used by both the Pfizer and Moderna vaccines – will be acceptable, Dr. Poland said, although human trials have not proven that.
However, it is unclear whether vaccines that use different platforms can be mixed. Can the first dose of an mRNA vaccine be followed by an adenovirus-based vaccine, like the Johnson & Johnson product or Novavax, if that vaccine is granted an EUA?
Ross Kedl, PhD, a vaccine researcher and professor of immunology at the University of Colorado at Denver, Aurora, said matching vaccine platforms might not be the preferred vaccination strategy.
He disagreed that there’s a lack of science surrounding the issue, and said all signs point to mixing as not only a good option, but probably a better one.
Researcher says science backs mixing
A mix of two different vaccine platforms likely enhances immunity, Dr. Kedl said. The heterologous prime-boost strategy has been used in animal studies for decades, “and it is well known that this promotes a much better immune response than when immunizing with the same vaccine twice.
“If you think about it in a Venn diagram sort of way, it makes sense,” he said in an interview. “Each vaccine has a number of components in it that influence immunity in various ways, but between the two of them, they only have one component that is similar. In the case of the coronavirus vaccines, the one thing both have in common is the spike protein from SARS-CoV-2. In essence, this gives you two shots at generating immunity against the one thing in each vaccine you care most about, but only one shot for the other vaccine components in each platform, resulting in an amplified response against the common target.”
In fact, the heterologous prime-boost vaccination strategy has proven to be effective in humans in early studies.
For example, an Ebola regimen that consisted of an adenovirus vector, similar to the AstraZeneca COVID vaccine, and a modified vaccinia virus vector showed promise in a phase 1 study. And an HIV regimen that consisted of the combination of a DNA vaccine, similar to the Pfizer and Moderna mRNA vaccines, and another viral vector showed encouraging results in a proof-of-concept study.
In both these cases, the heterologous prime-boost strategy was far better than single-vaccine prime-boost regimens, Dr. Kedl pointed out. And neither study reported any safety issues with the combinations.
For now, it’s best to stick with the same manufacturer for both shots, as the CDC guidance suggests, he said, agreeing with Dr. Poland.
But “I would be very surprised if we didn’t move to a mixing of vaccine platforms for the population,” Dr. Kedl said.
A version of this article first appeared on Medscape.com.
Ideally, Americans receiving their Pfizer/BioNTech or Moderna COVID-19 vaccines will get both doses from the same manufacturer, said Gregory Poland, MD, a vaccinologist at the Mayo Clinic in Rochester, Minn.
After all, that’s how they were tested for efficacy and safety, and it was results from those studies that led to emergency use authorization (EUA) being granted by the Food and Drug Administration.
But states and countries have struggled to keep up with the demand for vaccine, and more flexible vaccination schedules could help.
So researchers are exploring whether it is safe and effective to get the first and second doses from different manufacturers. And they are even wondering whether mixing doses from different manufacturers could increase effectiveness, particularly in light of emerging variants.
It’s called the “interchangeability issue,” said Dr. Poland, who has gotten a steady stream of questions about it.
For example, a patient recently asked about options for his father, who had gotten his first dose of the AstraZeneca vaccine in Ecuador, but had since moved to the United States, where that product has not been approved for use.
Dr. Poland said in an interview that he prefaces each answer with: “I’ve got no science for what I’m about to tell you.”
In this particular case, he recommended that the man’s father talk with his doctor about his level of COVID-19 risk and consider whether he should gamble on the AstraZeneca vaccine getting approved in the United States soon, or whether he should ask for a second dose from one of the three vaccines currently approved.
On March 22, 2021, AstraZeneca released positive results from its phase 3 trial, which will likely speed its path toward use in the United States.
Although clinical trials have started to test combinations and boosters, there’s currently no definitive evidence from human trials on mixing COVID vaccines, Dr. Poland pointed out.
But a study of a mixed-vaccine regimen is currently underway in the United Kingdom.
Participants in that 13-month trial will be given the Oxford/AstraZeneca and Pfizer/BioNTech vaccines in different combinations and at different intervals. The first results from that trial are expected this summer.
And interim results from a trial combining Russia’s Sputnik V and the AstraZeneca vaccines are expected in 2 months, according to a Reuters report.
Mix only in ‘exceptional situations’
The Centers for Disease Control and Prevention has been hesitant to open the door to mixing Pfizer and Moderna vaccinations, noting that the two “are not interchangeable.” But CDC guidance has changed slightly. Now, instead of saying the two vaccines should not be mixed, CDC guidance says they can be mixed in “exceptional situations,” and that the second dose can be administered up to 6 weeks after the first dose.
It is reasonable to assume that mixing COVID-19 vaccines that use the same platform – such as the mRNA platform used by both the Pfizer and Moderna vaccines – will be acceptable, Dr. Poland said, although human trials have not proven that.
However, it is unclear whether vaccines that use different platforms can be mixed. Can the first dose of an mRNA vaccine be followed by an adenovirus-based vaccine, like the Johnson & Johnson product or Novavax, if that vaccine is granted an EUA?
Ross Kedl, PhD, a vaccine researcher and professor of immunology at the University of Colorado at Denver, Aurora, said matching vaccine platforms might not be the preferred vaccination strategy.
He disagreed that there’s a lack of science surrounding the issue, and said all signs point to mixing as not only a good option, but probably a better one.
Researcher says science backs mixing
A mix of two different vaccine platforms likely enhances immunity, Dr. Kedl said. The heterologous prime-boost strategy has been used in animal studies for decades, “and it is well known that this promotes a much better immune response than when immunizing with the same vaccine twice.
“If you think about it in a Venn diagram sort of way, it makes sense,” he said in an interview. “Each vaccine has a number of components in it that influence immunity in various ways, but between the two of them, they only have one component that is similar. In the case of the coronavirus vaccines, the one thing both have in common is the spike protein from SARS-CoV-2. In essence, this gives you two shots at generating immunity against the one thing in each vaccine you care most about, but only one shot for the other vaccine components in each platform, resulting in an amplified response against the common target.”
In fact, the heterologous prime-boost vaccination strategy has proven to be effective in humans in early studies.
For example, an Ebola regimen that consisted of an adenovirus vector, similar to the AstraZeneca COVID vaccine, and a modified vaccinia virus vector showed promise in a phase 1 study. And an HIV regimen that consisted of the combination of a DNA vaccine, similar to the Pfizer and Moderna mRNA vaccines, and another viral vector showed encouraging results in a proof-of-concept study.
In both these cases, the heterologous prime-boost strategy was far better than single-vaccine prime-boost regimens, Dr. Kedl pointed out. And neither study reported any safety issues with the combinations.
For now, it’s best to stick with the same manufacturer for both shots, as the CDC guidance suggests, he said, agreeing with Dr. Poland.
But “I would be very surprised if we didn’t move to a mixing of vaccine platforms for the population,” Dr. Kedl said.
A version of this article first appeared on Medscape.com.
Ideally, Americans receiving their Pfizer/BioNTech or Moderna COVID-19 vaccines will get both doses from the same manufacturer, said Gregory Poland, MD, a vaccinologist at the Mayo Clinic in Rochester, Minn.
After all, that’s how they were tested for efficacy and safety, and it was results from those studies that led to emergency use authorization (EUA) being granted by the Food and Drug Administration.
But states and countries have struggled to keep up with the demand for vaccine, and more flexible vaccination schedules could help.
So researchers are exploring whether it is safe and effective to get the first and second doses from different manufacturers. And they are even wondering whether mixing doses from different manufacturers could increase effectiveness, particularly in light of emerging variants.
It’s called the “interchangeability issue,” said Dr. Poland, who has gotten a steady stream of questions about it.
For example, a patient recently asked about options for his father, who had gotten his first dose of the AstraZeneca vaccine in Ecuador, but had since moved to the United States, where that product has not been approved for use.
Dr. Poland said in an interview that he prefaces each answer with: “I’ve got no science for what I’m about to tell you.”
In this particular case, he recommended that the man’s father talk with his doctor about his level of COVID-19 risk and consider whether he should gamble on the AstraZeneca vaccine getting approved in the United States soon, or whether he should ask for a second dose from one of the three vaccines currently approved.
On March 22, 2021, AstraZeneca released positive results from its phase 3 trial, which will likely speed its path toward use in the United States.
Although clinical trials have started to test combinations and boosters, there’s currently no definitive evidence from human trials on mixing COVID vaccines, Dr. Poland pointed out.
But a study of a mixed-vaccine regimen is currently underway in the United Kingdom.
Participants in that 13-month trial will be given the Oxford/AstraZeneca and Pfizer/BioNTech vaccines in different combinations and at different intervals. The first results from that trial are expected this summer.
And interim results from a trial combining Russia’s Sputnik V and the AstraZeneca vaccines are expected in 2 months, according to a Reuters report.
Mix only in ‘exceptional situations’
The Centers for Disease Control and Prevention has been hesitant to open the door to mixing Pfizer and Moderna vaccinations, noting that the two “are not interchangeable.” But CDC guidance has changed slightly. Now, instead of saying the two vaccines should not be mixed, CDC guidance says they can be mixed in “exceptional situations,” and that the second dose can be administered up to 6 weeks after the first dose.
It is reasonable to assume that mixing COVID-19 vaccines that use the same platform – such as the mRNA platform used by both the Pfizer and Moderna vaccines – will be acceptable, Dr. Poland said, although human trials have not proven that.
However, it is unclear whether vaccines that use different platforms can be mixed. Can the first dose of an mRNA vaccine be followed by an adenovirus-based vaccine, like the Johnson & Johnson product or Novavax, if that vaccine is granted an EUA?
Ross Kedl, PhD, a vaccine researcher and professor of immunology at the University of Colorado at Denver, Aurora, said matching vaccine platforms might not be the preferred vaccination strategy.
He disagreed that there’s a lack of science surrounding the issue, and said all signs point to mixing as not only a good option, but probably a better one.
Researcher says science backs mixing
A mix of two different vaccine platforms likely enhances immunity, Dr. Kedl said. The heterologous prime-boost strategy has been used in animal studies for decades, “and it is well known that this promotes a much better immune response than when immunizing with the same vaccine twice.
“If you think about it in a Venn diagram sort of way, it makes sense,” he said in an interview. “Each vaccine has a number of components in it that influence immunity in various ways, but between the two of them, they only have one component that is similar. In the case of the coronavirus vaccines, the one thing both have in common is the spike protein from SARS-CoV-2. In essence, this gives you two shots at generating immunity against the one thing in each vaccine you care most about, but only one shot for the other vaccine components in each platform, resulting in an amplified response against the common target.”
In fact, the heterologous prime-boost vaccination strategy has proven to be effective in humans in early studies.
For example, an Ebola regimen that consisted of an adenovirus vector, similar to the AstraZeneca COVID vaccine, and a modified vaccinia virus vector showed promise in a phase 1 study. And an HIV regimen that consisted of the combination of a DNA vaccine, similar to the Pfizer and Moderna mRNA vaccines, and another viral vector showed encouraging results in a proof-of-concept study.
In both these cases, the heterologous prime-boost strategy was far better than single-vaccine prime-boost regimens, Dr. Kedl pointed out. And neither study reported any safety issues with the combinations.
For now, it’s best to stick with the same manufacturer for both shots, as the CDC guidance suggests, he said, agreeing with Dr. Poland.
But “I would be very surprised if we didn’t move to a mixing of vaccine platforms for the population,” Dr. Kedl said.
A version of this article first appeared on Medscape.com.
Adding oxaliplatin improves OS, DFS in MSI colon cancer
Adding oxaliplatin to fluoropyrimidine improves survival outcomes in patients with stage 3 N1 colon cancer whose tumors show microsatellite instability (MSI), according to a pooled analysis of individual patient data from 12 adjuvant trials.
The findings suggest the treatment combination should be the standard of care adjuvant treatment in this setting, and that N stage “should at least be a stratification parameter” in future trials in the MSI population, the investigators said.
Overall survival (OS) in 185 patients with MSI, which is associated with a deficient DNA mismatch repair system (dMMR), and 1,440 patients with microsatellite stability (MSS), which is associated with a proficient mismatch repair (pMMR), was better with the combination therapy versus fluoropyrimidine alone (adjusted hazard ratios, 0.52 and 0.89 for the groups, respectively), Romain Cohen, MD, PhD, of Saint-Antoine Hospital, medical oncology department, Sorbonne Universite, both in Paris, and colleagues reported online in the Journal of Clinical Oncology.
Encouraging results
Disease-free survival (DFS) was similarly improved with the combination therapy in the MSI and MSS groups (aHRs, 0.47 and 0.82, respectively).
Further, in 461 MSI/dMMR and 3,789 MSS/pMMR patients treated with the combination therapy, MSI status had different prognostic effects depending on N stage category.
“Compared with MSS/pMMR, MSI/dMMR was associated with better OS in the N1 population (HR, 0.66) but a similar OS in the N2 population (HR, 1.13),” they wrote.
Multivariate analysis showed that prognosticators for OS were N stage (HR for OS in N2 vs. N1, 3.10), T stage (HR for OS with T4 vs. T1-3, 2.39), and sex (HR for OS in men vs. women, HR, 1.71).
The 3-year DFS estimates for N2 vs. N1 disease in the MSI/dMMR group were 65% versus 87%, for T4 versus T1-3 they were 60.4% versus 82.1%, and for high- versus low-risk MSI/dMMR colorectal cancer patients they were 64.5% versus 90.1%.
“MSI/dMMR has become a major theranostic biomarker, harboring a high discrimination capacity for the efficacy of immune checkpoint inhibitors among patients with colorectal cancer,” the authors noted. “Given their impressive activity in the metastatic setting, this justifies evaluating these antibodies in the adjuvant setting.”
To date, the efficacy of the adding oxaliplatin to fluoropyrimidine inpatients with stage 3 colon cancer and MSI has not been clearly demonstrated, they added.
Therefore, they analyzed individual patient data from 12 randomized, controlled, phase 3 trials in the ACCENT database and adjusted for demographic and clinicopathologic factors.
“Here, in the T4 and/or N2 MSI/dMMR groups, we were able to identify populations of patients with MSI/dMMR [stage 3 colorectal cancer] with a high risk of disease recurrence. Indeed, the estimated 3-year DFS rates of patients with MSI/dMMR T4 stage III and N2 patients were, respectively, 60.4% and 64.9%, the latter experiencing poorer survival than the MSS/pMMR N2 population,” they wrote, concluding that, “with one-third of T4 and/or N2 high-risk ... patients experiencing disease recurrence or death within 2 years after curative tumor resection, therapeutic innovations should be sought for this patient population.”
This study was supported by the National Cancer Institute, the ARCAD foundation, and research grants from Nuovo-Soldati Foundation, ARC Foundation for Cancer Research, and Servier Institute. Dr. Cohen reported research funding and/or honoraria from Servier and MSD Oncology.
Adding oxaliplatin to fluoropyrimidine improves survival outcomes in patients with stage 3 N1 colon cancer whose tumors show microsatellite instability (MSI), according to a pooled analysis of individual patient data from 12 adjuvant trials.
The findings suggest the treatment combination should be the standard of care adjuvant treatment in this setting, and that N stage “should at least be a stratification parameter” in future trials in the MSI population, the investigators said.
Overall survival (OS) in 185 patients with MSI, which is associated with a deficient DNA mismatch repair system (dMMR), and 1,440 patients with microsatellite stability (MSS), which is associated with a proficient mismatch repair (pMMR), was better with the combination therapy versus fluoropyrimidine alone (adjusted hazard ratios, 0.52 and 0.89 for the groups, respectively), Romain Cohen, MD, PhD, of Saint-Antoine Hospital, medical oncology department, Sorbonne Universite, both in Paris, and colleagues reported online in the Journal of Clinical Oncology.
Encouraging results
Disease-free survival (DFS) was similarly improved with the combination therapy in the MSI and MSS groups (aHRs, 0.47 and 0.82, respectively).
Further, in 461 MSI/dMMR and 3,789 MSS/pMMR patients treated with the combination therapy, MSI status had different prognostic effects depending on N stage category.
“Compared with MSS/pMMR, MSI/dMMR was associated with better OS in the N1 population (HR, 0.66) but a similar OS in the N2 population (HR, 1.13),” they wrote.
Multivariate analysis showed that prognosticators for OS were N stage (HR for OS in N2 vs. N1, 3.10), T stage (HR for OS with T4 vs. T1-3, 2.39), and sex (HR for OS in men vs. women, HR, 1.71).
The 3-year DFS estimates for N2 vs. N1 disease in the MSI/dMMR group were 65% versus 87%, for T4 versus T1-3 they were 60.4% versus 82.1%, and for high- versus low-risk MSI/dMMR colorectal cancer patients they were 64.5% versus 90.1%.
“MSI/dMMR has become a major theranostic biomarker, harboring a high discrimination capacity for the efficacy of immune checkpoint inhibitors among patients with colorectal cancer,” the authors noted. “Given their impressive activity in the metastatic setting, this justifies evaluating these antibodies in the adjuvant setting.”
To date, the efficacy of the adding oxaliplatin to fluoropyrimidine inpatients with stage 3 colon cancer and MSI has not been clearly demonstrated, they added.
Therefore, they analyzed individual patient data from 12 randomized, controlled, phase 3 trials in the ACCENT database and adjusted for demographic and clinicopathologic factors.
“Here, in the T4 and/or N2 MSI/dMMR groups, we were able to identify populations of patients with MSI/dMMR [stage 3 colorectal cancer] with a high risk of disease recurrence. Indeed, the estimated 3-year DFS rates of patients with MSI/dMMR T4 stage III and N2 patients were, respectively, 60.4% and 64.9%, the latter experiencing poorer survival than the MSS/pMMR N2 population,” they wrote, concluding that, “with one-third of T4 and/or N2 high-risk ... patients experiencing disease recurrence or death within 2 years after curative tumor resection, therapeutic innovations should be sought for this patient population.”
This study was supported by the National Cancer Institute, the ARCAD foundation, and research grants from Nuovo-Soldati Foundation, ARC Foundation for Cancer Research, and Servier Institute. Dr. Cohen reported research funding and/or honoraria from Servier and MSD Oncology.
Adding oxaliplatin to fluoropyrimidine improves survival outcomes in patients with stage 3 N1 colon cancer whose tumors show microsatellite instability (MSI), according to a pooled analysis of individual patient data from 12 adjuvant trials.
The findings suggest the treatment combination should be the standard of care adjuvant treatment in this setting, and that N stage “should at least be a stratification parameter” in future trials in the MSI population, the investigators said.
Overall survival (OS) in 185 patients with MSI, which is associated with a deficient DNA mismatch repair system (dMMR), and 1,440 patients with microsatellite stability (MSS), which is associated with a proficient mismatch repair (pMMR), was better with the combination therapy versus fluoropyrimidine alone (adjusted hazard ratios, 0.52 and 0.89 for the groups, respectively), Romain Cohen, MD, PhD, of Saint-Antoine Hospital, medical oncology department, Sorbonne Universite, both in Paris, and colleagues reported online in the Journal of Clinical Oncology.
Encouraging results
Disease-free survival (DFS) was similarly improved with the combination therapy in the MSI and MSS groups (aHRs, 0.47 and 0.82, respectively).
Further, in 461 MSI/dMMR and 3,789 MSS/pMMR patients treated with the combination therapy, MSI status had different prognostic effects depending on N stage category.
“Compared with MSS/pMMR, MSI/dMMR was associated with better OS in the N1 population (HR, 0.66) but a similar OS in the N2 population (HR, 1.13),” they wrote.
Multivariate analysis showed that prognosticators for OS were N stage (HR for OS in N2 vs. N1, 3.10), T stage (HR for OS with T4 vs. T1-3, 2.39), and sex (HR for OS in men vs. women, HR, 1.71).
The 3-year DFS estimates for N2 vs. N1 disease in the MSI/dMMR group were 65% versus 87%, for T4 versus T1-3 they were 60.4% versus 82.1%, and for high- versus low-risk MSI/dMMR colorectal cancer patients they were 64.5% versus 90.1%.
“MSI/dMMR has become a major theranostic biomarker, harboring a high discrimination capacity for the efficacy of immune checkpoint inhibitors among patients with colorectal cancer,” the authors noted. “Given their impressive activity in the metastatic setting, this justifies evaluating these antibodies in the adjuvant setting.”
To date, the efficacy of the adding oxaliplatin to fluoropyrimidine inpatients with stage 3 colon cancer and MSI has not been clearly demonstrated, they added.
Therefore, they analyzed individual patient data from 12 randomized, controlled, phase 3 trials in the ACCENT database and adjusted for demographic and clinicopathologic factors.
“Here, in the T4 and/or N2 MSI/dMMR groups, we were able to identify populations of patients with MSI/dMMR [stage 3 colorectal cancer] with a high risk of disease recurrence. Indeed, the estimated 3-year DFS rates of patients with MSI/dMMR T4 stage III and N2 patients were, respectively, 60.4% and 64.9%, the latter experiencing poorer survival than the MSS/pMMR N2 population,” they wrote, concluding that, “with one-third of T4 and/or N2 high-risk ... patients experiencing disease recurrence or death within 2 years after curative tumor resection, therapeutic innovations should be sought for this patient population.”
This study was supported by the National Cancer Institute, the ARCAD foundation, and research grants from Nuovo-Soldati Foundation, ARC Foundation for Cancer Research, and Servier Institute. Dr. Cohen reported research funding and/or honoraria from Servier and MSD Oncology.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Upfront asymptomatic primary tumor resection: No benefit in advanced CRC
Upfront resection of an asymptomatic primary tumor does not improve survival over chemotherapy alone in stage IV colorectal cancer with unresectable synchronous metastases, according to a randomized trial in Japan with 165 patients.
Median overall survival was slightly more than 2 years with or without primary resection, plus upfront surgery delayed systemic treatment and had a 4% risk of fatal complications. The trial was terminated early because of futility.
“PTR [primary tumor resection] should no longer be considered a standard of care for patients with CRC with asymptomatic primary tumors and synchronous unresectable metastases,” said investigators led by Yukihide Kanemitsu, MD, a colorectal surgeon at National Cancer Center Hospital in Tokyo.
“This paper is important for establishing solid evidence-based decisions. Why perform an invasive procedure on a patient that introduces additional risks if it won’t change their disease course?” said colorectal surgeon Deborah Keller, MD, clinical assistant professor of surgery at the University of California at Davis Medical Center, when asked for comment.
She explained that, in general, when the primary tumor is not obstructing, the standard of care is upfront chemotherapy, as supported by National Comprehensive Cancer Network guidelines.
“However, there was a change in practice over the last few years” after several retrospective studies reported better overall survival with surgery before chemotherapy, but “the studies were not the highest quality of evidence,” she said.
To bring better data to bear, the Japanese team randomized 84 patients to chemotherapy alone and 81 to PTR followed by chemotherapy. Primary tumors were asymptomatic, and patients had no more than three unresectable metastases in the liver, lungs, distant lymph nodes, or peritoneum. Chemotherapy was either mFOLFOX6 plus bevacizumab or CapeOX plus bevacizumab at investigator discretion.
The trial was halted at interim analysis in September 2019. With a median follow-up of 22 months, median overall survival – the primary endpoint – was 25.9 months in the surgery-first arm and 26.7 months in the chemotherapy-alone group (P = .69). Subgroup analysis found no populations that benefited from PTR. Median progression free survival was 10.4 months with PTR first and 12.1 months with chemotherapy alone (P = .48)
Twenty-seven patients in the PTR arm had grade 3 or worse surgery-related adverse events, including anastomotic leakage in 3 and increased aspartate aminotransferase in 13. Three patients (4%) died within 30 days of surgery. Chemotherapy-related grade 3 or worse adverse events were more frequent and severe in the PTR arm (48% PTR versus 34% chemotherapy alone).
“For those who had been performing resections, this could push the paradigm back to chemotherapy alone,” Dr. Keller said.
Eleven patients (13%) in the chemotherapy-alone arm required surgery for intestinal obstruction or other primary tumor symptoms that developed after randomization, which means that 87% avoided surgery entirely, the investigators noted.
In the PTR group, surgery was performed within 21 days of enrollment, and chemotherapy started a median of 34 days after PTR. Chemotherapy was started within 14 days of enrollment in the chemotherapy-alone arm.
The groups were well balanced, including colon and rectosigmoid tumor locations in 93% of both arms and liver metastases in 73% of both. A bit over half the subjects were men and the median age was 65 years.
The team noted that early termination meant that the planned sample size was not achieved, which in turn limited the statistical power of the conclusions. “It is hoped that the comprehensive results of [ongoing trials] will clearly demonstrate the role of PTR for these patients,” they said.
The study was conducted by the Japan Clinical Oncology Group at 38 cancer centers in Japan. The work was funded by the Ministry of Health of Japan. The investigators had numerous industry ties, including Dr. Kanemitsu, who reported honoraria from Chugai Pharma, Ethicon, Covidien, and Intuitive Surgical, and being a Covidien adviser.
Upfront resection of an asymptomatic primary tumor does not improve survival over chemotherapy alone in stage IV colorectal cancer with unresectable synchronous metastases, according to a randomized trial in Japan with 165 patients.
Median overall survival was slightly more than 2 years with or without primary resection, plus upfront surgery delayed systemic treatment and had a 4% risk of fatal complications. The trial was terminated early because of futility.
“PTR [primary tumor resection] should no longer be considered a standard of care for patients with CRC with asymptomatic primary tumors and synchronous unresectable metastases,” said investigators led by Yukihide Kanemitsu, MD, a colorectal surgeon at National Cancer Center Hospital in Tokyo.
“This paper is important for establishing solid evidence-based decisions. Why perform an invasive procedure on a patient that introduces additional risks if it won’t change their disease course?” said colorectal surgeon Deborah Keller, MD, clinical assistant professor of surgery at the University of California at Davis Medical Center, when asked for comment.
She explained that, in general, when the primary tumor is not obstructing, the standard of care is upfront chemotherapy, as supported by National Comprehensive Cancer Network guidelines.
“However, there was a change in practice over the last few years” after several retrospective studies reported better overall survival with surgery before chemotherapy, but “the studies were not the highest quality of evidence,” she said.
To bring better data to bear, the Japanese team randomized 84 patients to chemotherapy alone and 81 to PTR followed by chemotherapy. Primary tumors were asymptomatic, and patients had no more than three unresectable metastases in the liver, lungs, distant lymph nodes, or peritoneum. Chemotherapy was either mFOLFOX6 plus bevacizumab or CapeOX plus bevacizumab at investigator discretion.
The trial was halted at interim analysis in September 2019. With a median follow-up of 22 months, median overall survival – the primary endpoint – was 25.9 months in the surgery-first arm and 26.7 months in the chemotherapy-alone group (P = .69). Subgroup analysis found no populations that benefited from PTR. Median progression free survival was 10.4 months with PTR first and 12.1 months with chemotherapy alone (P = .48)
Twenty-seven patients in the PTR arm had grade 3 or worse surgery-related adverse events, including anastomotic leakage in 3 and increased aspartate aminotransferase in 13. Three patients (4%) died within 30 days of surgery. Chemotherapy-related grade 3 or worse adverse events were more frequent and severe in the PTR arm (48% PTR versus 34% chemotherapy alone).
“For those who had been performing resections, this could push the paradigm back to chemotherapy alone,” Dr. Keller said.
Eleven patients (13%) in the chemotherapy-alone arm required surgery for intestinal obstruction or other primary tumor symptoms that developed after randomization, which means that 87% avoided surgery entirely, the investigators noted.
In the PTR group, surgery was performed within 21 days of enrollment, and chemotherapy started a median of 34 days after PTR. Chemotherapy was started within 14 days of enrollment in the chemotherapy-alone arm.
The groups were well balanced, including colon and rectosigmoid tumor locations in 93% of both arms and liver metastases in 73% of both. A bit over half the subjects were men and the median age was 65 years.
The team noted that early termination meant that the planned sample size was not achieved, which in turn limited the statistical power of the conclusions. “It is hoped that the comprehensive results of [ongoing trials] will clearly demonstrate the role of PTR for these patients,” they said.
The study was conducted by the Japan Clinical Oncology Group at 38 cancer centers in Japan. The work was funded by the Ministry of Health of Japan. The investigators had numerous industry ties, including Dr. Kanemitsu, who reported honoraria from Chugai Pharma, Ethicon, Covidien, and Intuitive Surgical, and being a Covidien adviser.
Upfront resection of an asymptomatic primary tumor does not improve survival over chemotherapy alone in stage IV colorectal cancer with unresectable synchronous metastases, according to a randomized trial in Japan with 165 patients.
Median overall survival was slightly more than 2 years with or without primary resection, plus upfront surgery delayed systemic treatment and had a 4% risk of fatal complications. The trial was terminated early because of futility.
“PTR [primary tumor resection] should no longer be considered a standard of care for patients with CRC with asymptomatic primary tumors and synchronous unresectable metastases,” said investigators led by Yukihide Kanemitsu, MD, a colorectal surgeon at National Cancer Center Hospital in Tokyo.
“This paper is important for establishing solid evidence-based decisions. Why perform an invasive procedure on a patient that introduces additional risks if it won’t change their disease course?” said colorectal surgeon Deborah Keller, MD, clinical assistant professor of surgery at the University of California at Davis Medical Center, when asked for comment.
She explained that, in general, when the primary tumor is not obstructing, the standard of care is upfront chemotherapy, as supported by National Comprehensive Cancer Network guidelines.
“However, there was a change in practice over the last few years” after several retrospective studies reported better overall survival with surgery before chemotherapy, but “the studies were not the highest quality of evidence,” she said.
To bring better data to bear, the Japanese team randomized 84 patients to chemotherapy alone and 81 to PTR followed by chemotherapy. Primary tumors were asymptomatic, and patients had no more than three unresectable metastases in the liver, lungs, distant lymph nodes, or peritoneum. Chemotherapy was either mFOLFOX6 plus bevacizumab or CapeOX plus bevacizumab at investigator discretion.
The trial was halted at interim analysis in September 2019. With a median follow-up of 22 months, median overall survival – the primary endpoint – was 25.9 months in the surgery-first arm and 26.7 months in the chemotherapy-alone group (P = .69). Subgroup analysis found no populations that benefited from PTR. Median progression free survival was 10.4 months with PTR first and 12.1 months with chemotherapy alone (P = .48)
Twenty-seven patients in the PTR arm had grade 3 or worse surgery-related adverse events, including anastomotic leakage in 3 and increased aspartate aminotransferase in 13. Three patients (4%) died within 30 days of surgery. Chemotherapy-related grade 3 or worse adverse events were more frequent and severe in the PTR arm (48% PTR versus 34% chemotherapy alone).
“For those who had been performing resections, this could push the paradigm back to chemotherapy alone,” Dr. Keller said.
Eleven patients (13%) in the chemotherapy-alone arm required surgery for intestinal obstruction or other primary tumor symptoms that developed after randomization, which means that 87% avoided surgery entirely, the investigators noted.
In the PTR group, surgery was performed within 21 days of enrollment, and chemotherapy started a median of 34 days after PTR. Chemotherapy was started within 14 days of enrollment in the chemotherapy-alone arm.
The groups were well balanced, including colon and rectosigmoid tumor locations in 93% of both arms and liver metastases in 73% of both. A bit over half the subjects were men and the median age was 65 years.
The team noted that early termination meant that the planned sample size was not achieved, which in turn limited the statistical power of the conclusions. “It is hoped that the comprehensive results of [ongoing trials] will clearly demonstrate the role of PTR for these patients,” they said.
The study was conducted by the Japan Clinical Oncology Group at 38 cancer centers in Japan. The work was funded by the Ministry of Health of Japan. The investigators had numerous industry ties, including Dr. Kanemitsu, who reported honoraria from Chugai Pharma, Ethicon, Covidien, and Intuitive Surgical, and being a Covidien adviser.
FROM THE JOURNAL OF CLINICAL ONCOLOGY