User login
Cardiologist forks out $2M to resolve unnecessary testing claims
Michigan cardiologist Dinesh M. Shah, MD, has paid the United States $2 million to resolve claims he violated the False Claims Act by knowingly billing federal health care programs for diagnostic tests that were unnecessary or not performed, the Department of Justice announced.
The settlement resolves allegations that, from 2006 to 2017, Dr. Shah and his practice, Michigan Physicians Group (MPG), of which he is sole owner, billed Medicare, Medicaid, and TRICARE for unnecessary diagnostic tests, including ankle brachial index and toe brachial index tests that were routinely performed on patients without first being ordered by a physician and without regard to medical necessity.
The prosecutors also alleged that Dr. Shah was routinely ordering, and MPG was providing, unnecessary nuclear stress tests to some patients.
“Subjecting patients to unnecessary testing in order to fill one’s pockets with taxpayer funds will not be tolerated. Such practices are particularly concerning because overuse of some tests can be harmful to patients,” acting U.S. Attorney Saima Mohsin said in the news release. “With these lawsuits and the accompanying resolution, Dr. Shah and Michigan Physicians Group are being held to account for these exploitative and improper past practices.”
In addition to the settlement, Dr. Shah and MPG entered into an Integrity Agreement with the Office of Inspector General for the Department of Health & Human Services, which will provide oversight of Dr. Shah and MPG’s billing practices for a 3-year period.
There was “no determination of liability” with the settlement, according to the Department of Justice. Dr. Shah’s case was sparked by two whistleblower lawsuits filed by Arlene Klinke and Khrystyna Malva, both former MPG employees.
The settlement comes after a years-long investigation by the HHS acting on behalf of TRICARE, a health care program for active and retired military members. Allegations that William Beaumont Hospital in Royal Oak, Mich., paid eight physicians excessive compensation to increase patient referrals led to an $84.5 million settlement in 2018.
Dr. Shah was one of three private practice cardiologists who denied involvement in the scheme but were named in the settlement, according to Crain’s Detroit Business.
A version of this article first appeared on Medscape.com.
Michigan cardiologist Dinesh M. Shah, MD, has paid the United States $2 million to resolve claims he violated the False Claims Act by knowingly billing federal health care programs for diagnostic tests that were unnecessary or not performed, the Department of Justice announced.
The settlement resolves allegations that, from 2006 to 2017, Dr. Shah and his practice, Michigan Physicians Group (MPG), of which he is sole owner, billed Medicare, Medicaid, and TRICARE for unnecessary diagnostic tests, including ankle brachial index and toe brachial index tests that were routinely performed on patients without first being ordered by a physician and without regard to medical necessity.
The prosecutors also alleged that Dr. Shah was routinely ordering, and MPG was providing, unnecessary nuclear stress tests to some patients.
“Subjecting patients to unnecessary testing in order to fill one’s pockets with taxpayer funds will not be tolerated. Such practices are particularly concerning because overuse of some tests can be harmful to patients,” acting U.S. Attorney Saima Mohsin said in the news release. “With these lawsuits and the accompanying resolution, Dr. Shah and Michigan Physicians Group are being held to account for these exploitative and improper past practices.”
In addition to the settlement, Dr. Shah and MPG entered into an Integrity Agreement with the Office of Inspector General for the Department of Health & Human Services, which will provide oversight of Dr. Shah and MPG’s billing practices for a 3-year period.
There was “no determination of liability” with the settlement, according to the Department of Justice. Dr. Shah’s case was sparked by two whistleblower lawsuits filed by Arlene Klinke and Khrystyna Malva, both former MPG employees.
The settlement comes after a years-long investigation by the HHS acting on behalf of TRICARE, a health care program for active and retired military members. Allegations that William Beaumont Hospital in Royal Oak, Mich., paid eight physicians excessive compensation to increase patient referrals led to an $84.5 million settlement in 2018.
Dr. Shah was one of three private practice cardiologists who denied involvement in the scheme but were named in the settlement, according to Crain’s Detroit Business.
A version of this article first appeared on Medscape.com.
Michigan cardiologist Dinesh M. Shah, MD, has paid the United States $2 million to resolve claims he violated the False Claims Act by knowingly billing federal health care programs for diagnostic tests that were unnecessary or not performed, the Department of Justice announced.
The settlement resolves allegations that, from 2006 to 2017, Dr. Shah and his practice, Michigan Physicians Group (MPG), of which he is sole owner, billed Medicare, Medicaid, and TRICARE for unnecessary diagnostic tests, including ankle brachial index and toe brachial index tests that were routinely performed on patients without first being ordered by a physician and without regard to medical necessity.
The prosecutors also alleged that Dr. Shah was routinely ordering, and MPG was providing, unnecessary nuclear stress tests to some patients.
“Subjecting patients to unnecessary testing in order to fill one’s pockets with taxpayer funds will not be tolerated. Such practices are particularly concerning because overuse of some tests can be harmful to patients,” acting U.S. Attorney Saima Mohsin said in the news release. “With these lawsuits and the accompanying resolution, Dr. Shah and Michigan Physicians Group are being held to account for these exploitative and improper past practices.”
In addition to the settlement, Dr. Shah and MPG entered into an Integrity Agreement with the Office of Inspector General for the Department of Health & Human Services, which will provide oversight of Dr. Shah and MPG’s billing practices for a 3-year period.
There was “no determination of liability” with the settlement, according to the Department of Justice. Dr. Shah’s case was sparked by two whistleblower lawsuits filed by Arlene Klinke and Khrystyna Malva, both former MPG employees.
The settlement comes after a years-long investigation by the HHS acting on behalf of TRICARE, a health care program for active and retired military members. Allegations that William Beaumont Hospital in Royal Oak, Mich., paid eight physicians excessive compensation to increase patient referrals led to an $84.5 million settlement in 2018.
Dr. Shah was one of three private practice cardiologists who denied involvement in the scheme but were named in the settlement, according to Crain’s Detroit Business.
A version of this article first appeared on Medscape.com.
Can benefits of SBRT outweigh risks in ultra-central lung tumors?
Of the 72 patients studied, 15 (21%) experienced grade 3 or higher toxicity and 10 (14%) died of bronchopulmonary hemorrhage.
This doesn’t completely write off the use of SBRT for ultra-central lung tumors, according to Joyce Lodeweges, MD, of University Medical Center (UMC) Utrecht in the Netherlands.
“We have to inform the patient very well that there are some high risks to this treatment,” she said at the European Lung Cancer Virtual Congress 2021 (Abstract 61M0).
Dr. Lodeweges noted that keeping the biologically effective dose of radiation to the main bronchus below a certain threshold (< 90 Gy) could reduce the risk of toxicity significantly, making SBRT a viable option for some patients. In addition, MRI-guided daily adaptation of the radiation treatment to organs at risk may make the treatment safer.
Varying definitions, regimens spur debate
SBRT is standard care in peripherally located, stage I non–small cell lung cancer that is inoperable or if the patient refuses surgery, noted study discussant Yolande Lievens, MD, PhD, of Ghent University Hospital in Belgium.
“[SBRT] has good local control rates with low toxicity even in patients with COPD or being elderly,” Dr. Lievens said.
“In more moderately central tumors, there is quite some evidence that risk-adapted fractionation schemes can be delivered in a safe way and lead to high local control rates,” she added. “For ultra-central legions, there’s still not a recommendation to treat with SBRT because we see a lot of increased toxicity.”
“For ultra-central tumors, SBRT is still under debate,” agreed Dr. Lodeweges. “This is because of the varying definitions in the literature and the varying fractionation schemes used.”
How the location of tumors is defined is important. Central tumors are those that are at least 2 cm away from the main bronchial tree, whereas ultra-central tumors are those that butt onto it or overlap it.
In Dr. Lodeweges’s study, ultra-central tumors were defined as those with a planning target volume (PTV) abutting or overlapping the main bronchi, trachea, and/or esophagus.
Study details
Between 2012 and 2020, there were 72 patients with ultra-central lung tumors treated at UMC Utrecht. Most patients (78%) had a PTV covering the main bronchus, with 21% each having PTVs overlapping the trachea or esophagus.
Patients received a protracted SBRT regimen of 60 Gy given in 12 fractions. The median follow-up was 19 months.
The local failure-free survival rate was 98% at 1 year and 85% at 2 years. Overall survival rates were 77% and 52%, respectively.
Receiving a biologically effective dose of more than 90 Gy to the main bronchus increased the risk of grade 3 or higher toxicity. On the other hand, patient age and tumor histology did not affect the risk of adverse events.
The use of antithrombotic therapy didn’t have any bearing on toxicity either, but it’s a possible risk factor to consider, Dr. Lodeweges said. Peri- or endobronchial tumor location is another consideration.
Findings in context
How do the results of the current study sit with other studies of SBRT in non–small cell lung cancer? Dr. Lievens pointed out that overall survival at 2 years was lower in the current trial (52%) than in patients with central tumors treated in the RTOG 0813 trial (68%-73%) or those with peripheral tumors in the CHISEL trial (77%).
There were, of course, different fractions and doses of radiotherapy used in these trials, with lower doses and more fractions in the UMC Utrecht study, and there was higher toxicity when ultra-central lesions were treated.
“Optimized radiotherapy dose fractionation regimens are investigated quite intensively to improve the clinical benefit. This is an important area of research,” Dr. Lievens said.
The high local control rates but serious risk of bronchopulmonary hemorrhage seen in the current study “calls for further investigation of dose/volume parameters in the context of the location of the tumor but also in the context of other treatment modalities,” she added. “Advanced technologies in radiotherapy, which allow better imaging and daily adaptation, such as the MR-Linac, can optimize clinical benefits.”
The study was supported by UMC Utrecht and received no commercial funding. Dr. Lodeweges and Dr. Lievens had no relevant conflicts of interest.
Of the 72 patients studied, 15 (21%) experienced grade 3 or higher toxicity and 10 (14%) died of bronchopulmonary hemorrhage.
This doesn’t completely write off the use of SBRT for ultra-central lung tumors, according to Joyce Lodeweges, MD, of University Medical Center (UMC) Utrecht in the Netherlands.
“We have to inform the patient very well that there are some high risks to this treatment,” she said at the European Lung Cancer Virtual Congress 2021 (Abstract 61M0).
Dr. Lodeweges noted that keeping the biologically effective dose of radiation to the main bronchus below a certain threshold (< 90 Gy) could reduce the risk of toxicity significantly, making SBRT a viable option for some patients. In addition, MRI-guided daily adaptation of the radiation treatment to organs at risk may make the treatment safer.
Varying definitions, regimens spur debate
SBRT is standard care in peripherally located, stage I non–small cell lung cancer that is inoperable or if the patient refuses surgery, noted study discussant Yolande Lievens, MD, PhD, of Ghent University Hospital in Belgium.
“[SBRT] has good local control rates with low toxicity even in patients with COPD or being elderly,” Dr. Lievens said.
“In more moderately central tumors, there is quite some evidence that risk-adapted fractionation schemes can be delivered in a safe way and lead to high local control rates,” she added. “For ultra-central legions, there’s still not a recommendation to treat with SBRT because we see a lot of increased toxicity.”
“For ultra-central tumors, SBRT is still under debate,” agreed Dr. Lodeweges. “This is because of the varying definitions in the literature and the varying fractionation schemes used.”
How the location of tumors is defined is important. Central tumors are those that are at least 2 cm away from the main bronchial tree, whereas ultra-central tumors are those that butt onto it or overlap it.
In Dr. Lodeweges’s study, ultra-central tumors were defined as those with a planning target volume (PTV) abutting or overlapping the main bronchi, trachea, and/or esophagus.
Study details
Between 2012 and 2020, there were 72 patients with ultra-central lung tumors treated at UMC Utrecht. Most patients (78%) had a PTV covering the main bronchus, with 21% each having PTVs overlapping the trachea or esophagus.
Patients received a protracted SBRT regimen of 60 Gy given in 12 fractions. The median follow-up was 19 months.
The local failure-free survival rate was 98% at 1 year and 85% at 2 years. Overall survival rates were 77% and 52%, respectively.
Receiving a biologically effective dose of more than 90 Gy to the main bronchus increased the risk of grade 3 or higher toxicity. On the other hand, patient age and tumor histology did not affect the risk of adverse events.
The use of antithrombotic therapy didn’t have any bearing on toxicity either, but it’s a possible risk factor to consider, Dr. Lodeweges said. Peri- or endobronchial tumor location is another consideration.
Findings in context
How do the results of the current study sit with other studies of SBRT in non–small cell lung cancer? Dr. Lievens pointed out that overall survival at 2 years was lower in the current trial (52%) than in patients with central tumors treated in the RTOG 0813 trial (68%-73%) or those with peripheral tumors in the CHISEL trial (77%).
There were, of course, different fractions and doses of radiotherapy used in these trials, with lower doses and more fractions in the UMC Utrecht study, and there was higher toxicity when ultra-central lesions were treated.
“Optimized radiotherapy dose fractionation regimens are investigated quite intensively to improve the clinical benefit. This is an important area of research,” Dr. Lievens said.
The high local control rates but serious risk of bronchopulmonary hemorrhage seen in the current study “calls for further investigation of dose/volume parameters in the context of the location of the tumor but also in the context of other treatment modalities,” she added. “Advanced technologies in radiotherapy, which allow better imaging and daily adaptation, such as the MR-Linac, can optimize clinical benefits.”
The study was supported by UMC Utrecht and received no commercial funding. Dr. Lodeweges and Dr. Lievens had no relevant conflicts of interest.
Of the 72 patients studied, 15 (21%) experienced grade 3 or higher toxicity and 10 (14%) died of bronchopulmonary hemorrhage.
This doesn’t completely write off the use of SBRT for ultra-central lung tumors, according to Joyce Lodeweges, MD, of University Medical Center (UMC) Utrecht in the Netherlands.
“We have to inform the patient very well that there are some high risks to this treatment,” she said at the European Lung Cancer Virtual Congress 2021 (Abstract 61M0).
Dr. Lodeweges noted that keeping the biologically effective dose of radiation to the main bronchus below a certain threshold (< 90 Gy) could reduce the risk of toxicity significantly, making SBRT a viable option for some patients. In addition, MRI-guided daily adaptation of the radiation treatment to organs at risk may make the treatment safer.
Varying definitions, regimens spur debate
SBRT is standard care in peripherally located, stage I non–small cell lung cancer that is inoperable or if the patient refuses surgery, noted study discussant Yolande Lievens, MD, PhD, of Ghent University Hospital in Belgium.
“[SBRT] has good local control rates with low toxicity even in patients with COPD or being elderly,” Dr. Lievens said.
“In more moderately central tumors, there is quite some evidence that risk-adapted fractionation schemes can be delivered in a safe way and lead to high local control rates,” she added. “For ultra-central legions, there’s still not a recommendation to treat with SBRT because we see a lot of increased toxicity.”
“For ultra-central tumors, SBRT is still under debate,” agreed Dr. Lodeweges. “This is because of the varying definitions in the literature and the varying fractionation schemes used.”
How the location of tumors is defined is important. Central tumors are those that are at least 2 cm away from the main bronchial tree, whereas ultra-central tumors are those that butt onto it or overlap it.
In Dr. Lodeweges’s study, ultra-central tumors were defined as those with a planning target volume (PTV) abutting or overlapping the main bronchi, trachea, and/or esophagus.
Study details
Between 2012 and 2020, there were 72 patients with ultra-central lung tumors treated at UMC Utrecht. Most patients (78%) had a PTV covering the main bronchus, with 21% each having PTVs overlapping the trachea or esophagus.
Patients received a protracted SBRT regimen of 60 Gy given in 12 fractions. The median follow-up was 19 months.
The local failure-free survival rate was 98% at 1 year and 85% at 2 years. Overall survival rates were 77% and 52%, respectively.
Receiving a biologically effective dose of more than 90 Gy to the main bronchus increased the risk of grade 3 or higher toxicity. On the other hand, patient age and tumor histology did not affect the risk of adverse events.
The use of antithrombotic therapy didn’t have any bearing on toxicity either, but it’s a possible risk factor to consider, Dr. Lodeweges said. Peri- or endobronchial tumor location is another consideration.
Findings in context
How do the results of the current study sit with other studies of SBRT in non–small cell lung cancer? Dr. Lievens pointed out that overall survival at 2 years was lower in the current trial (52%) than in patients with central tumors treated in the RTOG 0813 trial (68%-73%) or those with peripheral tumors in the CHISEL trial (77%).
There were, of course, different fractions and doses of radiotherapy used in these trials, with lower doses and more fractions in the UMC Utrecht study, and there was higher toxicity when ultra-central lesions were treated.
“Optimized radiotherapy dose fractionation regimens are investigated quite intensively to improve the clinical benefit. This is an important area of research,” Dr. Lievens said.
The high local control rates but serious risk of bronchopulmonary hemorrhage seen in the current study “calls for further investigation of dose/volume parameters in the context of the location of the tumor but also in the context of other treatment modalities,” she added. “Advanced technologies in radiotherapy, which allow better imaging and daily adaptation, such as the MR-Linac, can optimize clinical benefits.”
The study was supported by UMC Utrecht and received no commercial funding. Dr. Lodeweges and Dr. Lievens had no relevant conflicts of interest.
FROM ELCC 2021
COVID-19 ‘long-haul’ symptoms overlap with ME/CFS
People experiencing long-term symptoms following acute COVID-19 infection are increasingly meeting criteria for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), a phenomenon that highlights the need for unified research and clinical approaches, speakers said at a press briefing March 25 held by the advocacy group MEAction.
“Post-COVID lingering illness was predictable. Similar lingering fatigue syndromes have been reported in the scientific literature for nearly 100 years, following a variety of well-documented infections with viruses, bacteria, fungi, and even protozoa,” said Anthony Komaroff, MD, professor of medicine at Harvard Medical School, Boston.
Core criteria for ME/CFS established by the Institute of Medicine in 2015 include substantial decrement in functioning for at least 6 months, postexertional malaise (PEM), or a worsening of symptoms following even minor exertion (often described as “crashes”), unrefreshing sleep, and cognitive impairment and/or orthostatic intolerance.
Patients with ME/CFS also commonly experience painful headaches, muscle or joint aches, and allergies/other sensitivities. Although many patients can trace their symptoms to an initiating infection, “the cause is often unclear because the diagnosis is often delayed for months or years after symptom onset,” said Lucinda Bateman, MD, founder of the Bateman Horne Center, Salt Lake City, who leads a clinician coalition that aims to improve ME/CFS management.
In an international survey of 3762 COVID-19 “long-haulers” published in a preprint in December of 2020, the most frequent symptoms reported at least 6 months after illness onset were fatigue in 78%, PEM in 72%, and cognitive dysfunction (“brain fog”) in 55%. At the time of the survey, 45% reported requiring reduced work schedules because of their illness, and 22% reported being unable to work at all.
Dr. Bateman said those findings align with her experience so far with 12 COVID-19 “long haulers” who self-referred to her ME/CFS and fibromyalgia specialty clinic. Nine of the 12 met criteria for postural orthostatic tachycardia syndrome (POTS) based on the 10-minute NASA Lean Test, she said, and half also met the 2016 American College of Rheumatology criteria for fibromyalgia.
“Some were severely impaired. We suspect a small fiber polyneuropathy in about half, and mast cell activation syndrome in more than half. We look forward to doing more testing,” Dr. Bateman said.
To be sure, Dr. Komaroff noted, there are some differences. “Long COVID” patients will often experience breathlessness and ongoing anosmia (loss of taste and smell), which aren’t typical of ME/CFS.
But, he said, “many of the symptoms are quite similar ... My guess is that ME/CFS is an illness with a final common pathway that can be triggered by different things,” said Dr. Komaroff, a senior physician at Brigham and Women’s Hospital in Boston, and editor-in-chief of the Harvard Health Letter.
Based on previous data about CFS suggesting a 10% rate of symptoms persisting at least a year following a variety of infectious agents and the predicted 200 million COVID-19 cases globally by the end of 2021, Dr. Komaroff estimated that about 20 million cases of “long COVID” would be expected in the next year.
‘A huge investment’
On the research side, the National Institutes of Health recently appropriated $1.15 billion dollars over the next 4 years to investigate “the heterogeneity in the recovery process after COVID and to develop treatments for those suffering from [postacute COVID-19 syndrome]” according to a Feb. 5, 2021, blog from the National Institute of Neurological Disorders and Stroke (NINDS).
That same day, another NINDS blog announced “new resources for large-scale ME/CFS research” and emphasized the tie-in with long–COVID-19 syndrome.
“That’s a huge investment. In my opinion, there will be several lingering illnesses following COVID,” Dr. Komaroff said, adding, “It’s my bet that long COVID will prove to be caused by certain kinds of abnormalities in the brain, some of the same abnormalities already identified in ME/CFS. Research will determine whether that’s right or wrong.”
In 2017, NINDS had announced a large increase in funding for ME/CFS research, including the creation of four dedicated research centers. In April 2019, NINDS held a 2-day conference highlighting that ongoing work, as reported by Medscape Medical News.
During the briefing, NINDS clinical director Avindra Nath, MD, described a comprehensive ongoing ME/CFS intramural study he’s been leading since 2016.
He’s now also overseeing two long–COVID-19 studies, one of which has a protocol similar to that of the ME/CFS study and will include individuals who are still experiencing long-term symptoms following confirmed cases of COVID-19. The aim is to screen about 1,300 patients. Several task forces are now examining all of these data together.
“Each aspect is now being analyzed … What we learn from one applies to the other,” Dr. Nath said.
Advice for clinicians
In interviews, Dr. Bateman and Dr. Nath offered clinical advice for managing patients who meet ME/CFS criteria, whether they had confirmed or suspected COVID-19, a different infection, or unknown trigger(s).
Dr. Bateman advised that clinicians assess patients for each of the symptoms individually. “Besides exercise intolerance and PEM, the most commonly missed is orthostatic intolerance. It really doesn’t matter what the cause is, it’s amenable to supportive treatment. It’s one aspect of the illness that contributes to severely impaired function. My plea to all physicians would be for sure to assess for [orthostatic intolerance], and gain an understanding about activity management and avoiding PEM symptoms.”
Dr. Nath noted that an often-challenging situation is when tests for the infectious agent and other blood work come back negative, yet the patient still reports multiple debilitating symptoms. This has been a particular issue with long COVID-19, since many patients became ill early in the pandemic before the polymerase chain reaction (PCR) tests for SARS-CoV-2 were widely available.
“The physician can only order tests that are available at their labs. I think what the physician should do is handle symptoms symptomatically but also refer patients to specialists who are taking care of these patients or to research studies,” he said.
Dr. Bateman added, “Whether they had a documented COVID infection – we just have to let go of that in 2020. Way too many people didn’t have access to a test or the timing wasn’t amenable. If people meet criteria for ME/CFS, it’s irrelevant … It’s mainly a clinical diagnosis. It’s not reliant on identifying the infectious trigger.”
Dr. Komaroff, who began caring for then-termed “chronic fatigue syndrome” patients and researching the condition more than 30 years ago, said that “every cloud has its silver lining. The increased focus on postinfectious fatigue syndrome is a silver lining in my mind around the terrible dark cloud that is the pandemic of COVID.”
Dr. Komaroff has received personal fees from Serimmune Inc., Ono Pharma, and Deallus, and grants from the NIH. Dr. Bateman is employed by the Bateman Horne Center, which receives grants from the NIH, and fees from Exagen Inc., and Teva Pharmaceutical. Dr. Nath is an NIH employee.
A version of this article first appeared on Medscape.com.
People experiencing long-term symptoms following acute COVID-19 infection are increasingly meeting criteria for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), a phenomenon that highlights the need for unified research and clinical approaches, speakers said at a press briefing March 25 held by the advocacy group MEAction.
“Post-COVID lingering illness was predictable. Similar lingering fatigue syndromes have been reported in the scientific literature for nearly 100 years, following a variety of well-documented infections with viruses, bacteria, fungi, and even protozoa,” said Anthony Komaroff, MD, professor of medicine at Harvard Medical School, Boston.
Core criteria for ME/CFS established by the Institute of Medicine in 2015 include substantial decrement in functioning for at least 6 months, postexertional malaise (PEM), or a worsening of symptoms following even minor exertion (often described as “crashes”), unrefreshing sleep, and cognitive impairment and/or orthostatic intolerance.
Patients with ME/CFS also commonly experience painful headaches, muscle or joint aches, and allergies/other sensitivities. Although many patients can trace their symptoms to an initiating infection, “the cause is often unclear because the diagnosis is often delayed for months or years after symptom onset,” said Lucinda Bateman, MD, founder of the Bateman Horne Center, Salt Lake City, who leads a clinician coalition that aims to improve ME/CFS management.
In an international survey of 3762 COVID-19 “long-haulers” published in a preprint in December of 2020, the most frequent symptoms reported at least 6 months after illness onset were fatigue in 78%, PEM in 72%, and cognitive dysfunction (“brain fog”) in 55%. At the time of the survey, 45% reported requiring reduced work schedules because of their illness, and 22% reported being unable to work at all.
Dr. Bateman said those findings align with her experience so far with 12 COVID-19 “long haulers” who self-referred to her ME/CFS and fibromyalgia specialty clinic. Nine of the 12 met criteria for postural orthostatic tachycardia syndrome (POTS) based on the 10-minute NASA Lean Test, she said, and half also met the 2016 American College of Rheumatology criteria for fibromyalgia.
“Some were severely impaired. We suspect a small fiber polyneuropathy in about half, and mast cell activation syndrome in more than half. We look forward to doing more testing,” Dr. Bateman said.
To be sure, Dr. Komaroff noted, there are some differences. “Long COVID” patients will often experience breathlessness and ongoing anosmia (loss of taste and smell), which aren’t typical of ME/CFS.
But, he said, “many of the symptoms are quite similar ... My guess is that ME/CFS is an illness with a final common pathway that can be triggered by different things,” said Dr. Komaroff, a senior physician at Brigham and Women’s Hospital in Boston, and editor-in-chief of the Harvard Health Letter.
Based on previous data about CFS suggesting a 10% rate of symptoms persisting at least a year following a variety of infectious agents and the predicted 200 million COVID-19 cases globally by the end of 2021, Dr. Komaroff estimated that about 20 million cases of “long COVID” would be expected in the next year.
‘A huge investment’
On the research side, the National Institutes of Health recently appropriated $1.15 billion dollars over the next 4 years to investigate “the heterogeneity in the recovery process after COVID and to develop treatments for those suffering from [postacute COVID-19 syndrome]” according to a Feb. 5, 2021, blog from the National Institute of Neurological Disorders and Stroke (NINDS).
That same day, another NINDS blog announced “new resources for large-scale ME/CFS research” and emphasized the tie-in with long–COVID-19 syndrome.
“That’s a huge investment. In my opinion, there will be several lingering illnesses following COVID,” Dr. Komaroff said, adding, “It’s my bet that long COVID will prove to be caused by certain kinds of abnormalities in the brain, some of the same abnormalities already identified in ME/CFS. Research will determine whether that’s right or wrong.”
In 2017, NINDS had announced a large increase in funding for ME/CFS research, including the creation of four dedicated research centers. In April 2019, NINDS held a 2-day conference highlighting that ongoing work, as reported by Medscape Medical News.
During the briefing, NINDS clinical director Avindra Nath, MD, described a comprehensive ongoing ME/CFS intramural study he’s been leading since 2016.
He’s now also overseeing two long–COVID-19 studies, one of which has a protocol similar to that of the ME/CFS study and will include individuals who are still experiencing long-term symptoms following confirmed cases of COVID-19. The aim is to screen about 1,300 patients. Several task forces are now examining all of these data together.
“Each aspect is now being analyzed … What we learn from one applies to the other,” Dr. Nath said.
Advice for clinicians
In interviews, Dr. Bateman and Dr. Nath offered clinical advice for managing patients who meet ME/CFS criteria, whether they had confirmed or suspected COVID-19, a different infection, or unknown trigger(s).
Dr. Bateman advised that clinicians assess patients for each of the symptoms individually. “Besides exercise intolerance and PEM, the most commonly missed is orthostatic intolerance. It really doesn’t matter what the cause is, it’s amenable to supportive treatment. It’s one aspect of the illness that contributes to severely impaired function. My plea to all physicians would be for sure to assess for [orthostatic intolerance], and gain an understanding about activity management and avoiding PEM symptoms.”
Dr. Nath noted that an often-challenging situation is when tests for the infectious agent and other blood work come back negative, yet the patient still reports multiple debilitating symptoms. This has been a particular issue with long COVID-19, since many patients became ill early in the pandemic before the polymerase chain reaction (PCR) tests for SARS-CoV-2 were widely available.
“The physician can only order tests that are available at their labs. I think what the physician should do is handle symptoms symptomatically but also refer patients to specialists who are taking care of these patients or to research studies,” he said.
Dr. Bateman added, “Whether they had a documented COVID infection – we just have to let go of that in 2020. Way too many people didn’t have access to a test or the timing wasn’t amenable. If people meet criteria for ME/CFS, it’s irrelevant … It’s mainly a clinical diagnosis. It’s not reliant on identifying the infectious trigger.”
Dr. Komaroff, who began caring for then-termed “chronic fatigue syndrome” patients and researching the condition more than 30 years ago, said that “every cloud has its silver lining. The increased focus on postinfectious fatigue syndrome is a silver lining in my mind around the terrible dark cloud that is the pandemic of COVID.”
Dr. Komaroff has received personal fees from Serimmune Inc., Ono Pharma, and Deallus, and grants from the NIH. Dr. Bateman is employed by the Bateman Horne Center, which receives grants from the NIH, and fees from Exagen Inc., and Teva Pharmaceutical. Dr. Nath is an NIH employee.
A version of this article first appeared on Medscape.com.
People experiencing long-term symptoms following acute COVID-19 infection are increasingly meeting criteria for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), a phenomenon that highlights the need for unified research and clinical approaches, speakers said at a press briefing March 25 held by the advocacy group MEAction.
“Post-COVID lingering illness was predictable. Similar lingering fatigue syndromes have been reported in the scientific literature for nearly 100 years, following a variety of well-documented infections with viruses, bacteria, fungi, and even protozoa,” said Anthony Komaroff, MD, professor of medicine at Harvard Medical School, Boston.
Core criteria for ME/CFS established by the Institute of Medicine in 2015 include substantial decrement in functioning for at least 6 months, postexertional malaise (PEM), or a worsening of symptoms following even minor exertion (often described as “crashes”), unrefreshing sleep, and cognitive impairment and/or orthostatic intolerance.
Patients with ME/CFS also commonly experience painful headaches, muscle or joint aches, and allergies/other sensitivities. Although many patients can trace their symptoms to an initiating infection, “the cause is often unclear because the diagnosis is often delayed for months or years after symptom onset,” said Lucinda Bateman, MD, founder of the Bateman Horne Center, Salt Lake City, who leads a clinician coalition that aims to improve ME/CFS management.
In an international survey of 3762 COVID-19 “long-haulers” published in a preprint in December of 2020, the most frequent symptoms reported at least 6 months after illness onset were fatigue in 78%, PEM in 72%, and cognitive dysfunction (“brain fog”) in 55%. At the time of the survey, 45% reported requiring reduced work schedules because of their illness, and 22% reported being unable to work at all.
Dr. Bateman said those findings align with her experience so far with 12 COVID-19 “long haulers” who self-referred to her ME/CFS and fibromyalgia specialty clinic. Nine of the 12 met criteria for postural orthostatic tachycardia syndrome (POTS) based on the 10-minute NASA Lean Test, she said, and half also met the 2016 American College of Rheumatology criteria for fibromyalgia.
“Some were severely impaired. We suspect a small fiber polyneuropathy in about half, and mast cell activation syndrome in more than half. We look forward to doing more testing,” Dr. Bateman said.
To be sure, Dr. Komaroff noted, there are some differences. “Long COVID” patients will often experience breathlessness and ongoing anosmia (loss of taste and smell), which aren’t typical of ME/CFS.
But, he said, “many of the symptoms are quite similar ... My guess is that ME/CFS is an illness with a final common pathway that can be triggered by different things,” said Dr. Komaroff, a senior physician at Brigham and Women’s Hospital in Boston, and editor-in-chief of the Harvard Health Letter.
Based on previous data about CFS suggesting a 10% rate of symptoms persisting at least a year following a variety of infectious agents and the predicted 200 million COVID-19 cases globally by the end of 2021, Dr. Komaroff estimated that about 20 million cases of “long COVID” would be expected in the next year.
‘A huge investment’
On the research side, the National Institutes of Health recently appropriated $1.15 billion dollars over the next 4 years to investigate “the heterogeneity in the recovery process after COVID and to develop treatments for those suffering from [postacute COVID-19 syndrome]” according to a Feb. 5, 2021, blog from the National Institute of Neurological Disorders and Stroke (NINDS).
That same day, another NINDS blog announced “new resources for large-scale ME/CFS research” and emphasized the tie-in with long–COVID-19 syndrome.
“That’s a huge investment. In my opinion, there will be several lingering illnesses following COVID,” Dr. Komaroff said, adding, “It’s my bet that long COVID will prove to be caused by certain kinds of abnormalities in the brain, some of the same abnormalities already identified in ME/CFS. Research will determine whether that’s right or wrong.”
In 2017, NINDS had announced a large increase in funding for ME/CFS research, including the creation of four dedicated research centers. In April 2019, NINDS held a 2-day conference highlighting that ongoing work, as reported by Medscape Medical News.
During the briefing, NINDS clinical director Avindra Nath, MD, described a comprehensive ongoing ME/CFS intramural study he’s been leading since 2016.
He’s now also overseeing two long–COVID-19 studies, one of which has a protocol similar to that of the ME/CFS study and will include individuals who are still experiencing long-term symptoms following confirmed cases of COVID-19. The aim is to screen about 1,300 patients. Several task forces are now examining all of these data together.
“Each aspect is now being analyzed … What we learn from one applies to the other,” Dr. Nath said.
Advice for clinicians
In interviews, Dr. Bateman and Dr. Nath offered clinical advice for managing patients who meet ME/CFS criteria, whether they had confirmed or suspected COVID-19, a different infection, or unknown trigger(s).
Dr. Bateman advised that clinicians assess patients for each of the symptoms individually. “Besides exercise intolerance and PEM, the most commonly missed is orthostatic intolerance. It really doesn’t matter what the cause is, it’s amenable to supportive treatment. It’s one aspect of the illness that contributes to severely impaired function. My plea to all physicians would be for sure to assess for [orthostatic intolerance], and gain an understanding about activity management and avoiding PEM symptoms.”
Dr. Nath noted that an often-challenging situation is when tests for the infectious agent and other blood work come back negative, yet the patient still reports multiple debilitating symptoms. This has been a particular issue with long COVID-19, since many patients became ill early in the pandemic before the polymerase chain reaction (PCR) tests for SARS-CoV-2 were widely available.
“The physician can only order tests that are available at their labs. I think what the physician should do is handle symptoms symptomatically but also refer patients to specialists who are taking care of these patients or to research studies,” he said.
Dr. Bateman added, “Whether they had a documented COVID infection – we just have to let go of that in 2020. Way too many people didn’t have access to a test or the timing wasn’t amenable. If people meet criteria for ME/CFS, it’s irrelevant … It’s mainly a clinical diagnosis. It’s not reliant on identifying the infectious trigger.”
Dr. Komaroff, who began caring for then-termed “chronic fatigue syndrome” patients and researching the condition more than 30 years ago, said that “every cloud has its silver lining. The increased focus on postinfectious fatigue syndrome is a silver lining in my mind around the terrible dark cloud that is the pandemic of COVID.”
Dr. Komaroff has received personal fees from Serimmune Inc., Ono Pharma, and Deallus, and grants from the NIH. Dr. Bateman is employed by the Bateman Horne Center, which receives grants from the NIH, and fees from Exagen Inc., and Teva Pharmaceutical. Dr. Nath is an NIH employee.
A version of this article first appeared on Medscape.com.
FDA okays transcatheter pulmonary valve for congenital heart disease
The Food and Drug Administration has approved Medtronic’s Harmony Transcatheter Pulmonary Valve (TPV) System to treat severe pulmonary regurgitation in pediatric and adult patients who have a native or surgically repaired right ventricular outflow tract (RVOT).
The Harmony TPV is the first nonsurgical heart valve to treat severe pulmonary valve regurgitation, which is common in patients with congenital heart disease, the agency said in a news release. Its use can delay the time before a patient needs open-heart surgery and potentially reduce the number of these surgeries required over a lifetime.
“The Harmony TPV provides a new treatment option for adult and pediatric patients with certain types of congenital heart disease,” Bram Zuckerman, MD, director of the Office of Cardiovascular Devices in the FDA’s Center for Devices and Radiological Health, said in the statement.
“It offers a less-invasive treatment alternative to open-heart surgery to patients with a leaky native or surgically repaired RVOT and may help patients improve their quality of life and return to their normal activities more quickly, thus fulfilling an unmet clinical need of many patients with congenital heart disease,” he said.
The Harmony valve, which was granted breakthrough device designation, is a 22-mm or 25-mm porcine pericardium valve, sewn to a nitinol frame. It is implanted with a 25-French delivery system using a coil-loading catheter.
The FDA approval was based on the 70-patient prospective, nonrandomized, multicenter Harmony TPV Clinical study, in which 100% of patients achieved the primary safety endpoint of no procedure or device-related deaths 30 days after implantation.
Among 65 patients with evaluable echocardiographic data, 89.2% met the primary effectiveness endpoint of no additional surgical or interventional device-related procedures and acceptable heart blood flow at 6 months.
Adverse events included irregular or abnormal heart rhythms in 23.9% of patients, including 14.1% ventricular tachycardia; leakage around the valve in 8.5%, including 1.4% major leakage; minor bleeding in 7.0%, narrowing of the pulmonary valve in 4.2%, and movement of the implant in 4.2%.
Follow-up was scheduled annually through 5 years and has been extended to 10 years as part of the postapproval study, the FDA noted.
The Harmony TPV device is contraindicated for patients with an infection in the heart or elsewhere, for patients who cannot tolerate blood-thinning medicines, and for those with a sensitivity to nitinol (titanium or nickel).
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved Medtronic’s Harmony Transcatheter Pulmonary Valve (TPV) System to treat severe pulmonary regurgitation in pediatric and adult patients who have a native or surgically repaired right ventricular outflow tract (RVOT).
The Harmony TPV is the first nonsurgical heart valve to treat severe pulmonary valve regurgitation, which is common in patients with congenital heart disease, the agency said in a news release. Its use can delay the time before a patient needs open-heart surgery and potentially reduce the number of these surgeries required over a lifetime.
“The Harmony TPV provides a new treatment option for adult and pediatric patients with certain types of congenital heart disease,” Bram Zuckerman, MD, director of the Office of Cardiovascular Devices in the FDA’s Center for Devices and Radiological Health, said in the statement.
“It offers a less-invasive treatment alternative to open-heart surgery to patients with a leaky native or surgically repaired RVOT and may help patients improve their quality of life and return to their normal activities more quickly, thus fulfilling an unmet clinical need of many patients with congenital heart disease,” he said.
The Harmony valve, which was granted breakthrough device designation, is a 22-mm or 25-mm porcine pericardium valve, sewn to a nitinol frame. It is implanted with a 25-French delivery system using a coil-loading catheter.
The FDA approval was based on the 70-patient prospective, nonrandomized, multicenter Harmony TPV Clinical study, in which 100% of patients achieved the primary safety endpoint of no procedure or device-related deaths 30 days after implantation.
Among 65 patients with evaluable echocardiographic data, 89.2% met the primary effectiveness endpoint of no additional surgical or interventional device-related procedures and acceptable heart blood flow at 6 months.
Adverse events included irregular or abnormal heart rhythms in 23.9% of patients, including 14.1% ventricular tachycardia; leakage around the valve in 8.5%, including 1.4% major leakage; minor bleeding in 7.0%, narrowing of the pulmonary valve in 4.2%, and movement of the implant in 4.2%.
Follow-up was scheduled annually through 5 years and has been extended to 10 years as part of the postapproval study, the FDA noted.
The Harmony TPV device is contraindicated for patients with an infection in the heart or elsewhere, for patients who cannot tolerate blood-thinning medicines, and for those with a sensitivity to nitinol (titanium or nickel).
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved Medtronic’s Harmony Transcatheter Pulmonary Valve (TPV) System to treat severe pulmonary regurgitation in pediatric and adult patients who have a native or surgically repaired right ventricular outflow tract (RVOT).
The Harmony TPV is the first nonsurgical heart valve to treat severe pulmonary valve regurgitation, which is common in patients with congenital heart disease, the agency said in a news release. Its use can delay the time before a patient needs open-heart surgery and potentially reduce the number of these surgeries required over a lifetime.
“The Harmony TPV provides a new treatment option for adult and pediatric patients with certain types of congenital heart disease,” Bram Zuckerman, MD, director of the Office of Cardiovascular Devices in the FDA’s Center for Devices and Radiological Health, said in the statement.
“It offers a less-invasive treatment alternative to open-heart surgery to patients with a leaky native or surgically repaired RVOT and may help patients improve their quality of life and return to their normal activities more quickly, thus fulfilling an unmet clinical need of many patients with congenital heart disease,” he said.
The Harmony valve, which was granted breakthrough device designation, is a 22-mm or 25-mm porcine pericardium valve, sewn to a nitinol frame. It is implanted with a 25-French delivery system using a coil-loading catheter.
The FDA approval was based on the 70-patient prospective, nonrandomized, multicenter Harmony TPV Clinical study, in which 100% of patients achieved the primary safety endpoint of no procedure or device-related deaths 30 days after implantation.
Among 65 patients with evaluable echocardiographic data, 89.2% met the primary effectiveness endpoint of no additional surgical or interventional device-related procedures and acceptable heart blood flow at 6 months.
Adverse events included irregular or abnormal heart rhythms in 23.9% of patients, including 14.1% ventricular tachycardia; leakage around the valve in 8.5%, including 1.4% major leakage; minor bleeding in 7.0%, narrowing of the pulmonary valve in 4.2%, and movement of the implant in 4.2%.
Follow-up was scheduled annually through 5 years and has been extended to 10 years as part of the postapproval study, the FDA noted.
The Harmony TPV device is contraindicated for patients with an infection in the heart or elsewhere, for patients who cannot tolerate blood-thinning medicines, and for those with a sensitivity to nitinol (titanium or nickel).
A version of this article first appeared on Medscape.com.
Step therapy: Inside the fight against insurance companies and fail-first medicine
Every day Melissa Fulton, RN, MSN, FNP, APRN-C, shows up to work, she’s ready for another fight. An advanced practice nurse who specializes in multiple sclerosis care, Ms. Fulton said she typically spends more than a third of her time battling it out with insurance companies over drugs she knows her patients need but that insurers don’t want to cover. Instead, they want the patient to first receive less expensive and often less efficacious drugs, even if that goes against recommendations and, in some cases, against the patient’s medical history.
The maddening protocol – familiar to health care providers everywhere – is known as “step therapy.” It forces patients to try alternative medications – medications that often fail – before receiving the one initially prescribed. The process can take weeks or months, which is time that some patients don’t have. Step therapy was sold as a way to lower costs. However, beyond the ethically problematic notion of forcing sick patients to receiver cheaper alternatives that are ineffective, research has also shown it may actually be more costly in the long run.
Ms. Fulton, who works at Saunders Medical Center in Wahoo, Neb., is a veteran in the war against step therapy. She is used to pushing her appeals up the insurance company chain of command, past nonmedical reviewers, until her patient’s case finally lands on the desk of someone with a neurology background. She said that can take three or four appeals – a judge might even get involved – and the patient could still lose. “This happens constantly,” she said, “but we fight like hell.”
Fortunately, life may soon get a little easier for Ms. Fulton. In late March, a bill to restrict step therapy made it through the Nebraska state legislature and is on its way to the governor’s desk. The Step Therapy Reform Act doesn’t outright ban the practice; however, it will put guardrails in place. It requires that insurers respond to appeals within certain time frames, and it creates key exemptions.
When the governor signs off, Nebraska will join more than two dozen other states that already have step therapy restrictions on the books, according to Hannah Lynch, MPS, associate director of federal government relations and health policy at the National Psoriasis Foundation, a leading advocate to reform and protect against the insurance practice. “There’s a lot of frustration out there,” Ms. Lynch said. “It really hinders providers’ ability to make decisions they think will have the best outcomes.”
Driven by coalitions of doctors, nurses, and patients, laws reining in step therapy have been adopted at a relatively quick clip, mostly within the past 5 years. Recent additions include South Dakota and North Carolina, which adopted step therapy laws in 2020, and Arkansas, which passed a law earlier this year.
Ms. Lynch attributed growing support to rising out-of-pocket drug costs and the introduction of biologic drugs, which are often more effective but also more expensive. Like Nebraska’s law, most step therapy reform legislation carves out exemptions and requires timely appeals processes; however, many of the laws still have significant gaps, such as not including certain types of insurance plans.
Ideally, Ms. Lynch said, the protections would apply to all types of health plans that are regulated at the state level, such as Medicaid, state employee health plans, and coverage sold through state insurance exchanges. Closing loopholes in the laws is a top priority for advocates, she added, pointing to work currently underway in Arkansas to extend its new protections to Medicaid expansion patients.
“With so many outside stakeholders, you have to compromise – it’s a give and take,” Ms. Lynch said. Still, when it comes to fighting step therapy, she says, “Any protection on the books is always our first goal when we go into a state.”
Putting patients first
Lisa Arkin, MD, a pediatric dermatologist at the University of Wisconsin–Madison, said she finds herself “swimming upstream every day in the fight with insurance.” Her patients are typically on their second or third stop and have more complex disorders. Dr. Arkin said that the problem with step therapy is that it tries to squeeze all patients into the same box, even if the circumstances don’t fit.
Her state passed restrictions on step therapy in 2019, but the measures only went into effect last year. Under the Wisconsin law, patients can be granted an exemption if an alternative treatment is contraindicated, likely to cause harm, or expected to be ineffective. Patients can also be exempt if their current treatment is working.
Dr. Arkin, an outspoken advocate for curbing step therapy, says the Wisconsin law is “very strong.” However, because it only applies to certain health plans – state employee health plans and those purchased in the state’s health insurance exchange – fewer than half the state’s patients benefit from its protections. She notes that some of the most severe presentations she treats occur in patients who rely on Medicaid coverage and already face barriers to care.
“I’m a doctor who puts up a fuss [with insurers], but that’s not fair – we shouldn’t have to do that,” Dr. Arkin said. “To me, it’s really critical to make this an even playing field so this law affords protection to everyone I see in the clinic.”
Major medical associations caution against step therapy as well. The American Society of Clinical Oncology and the American Medical Association have called out the risks to patient safety and health. In fact, in 2019, after the Centers for Medicare & Medicaid Services gave new authority to Medicare Advantage plans to start using step therapy, dozens of national medical groups called out the agency for allowing a practice that could potentially hurt patients and undercut the physician-patient decision-making process.
Last year, in a new position paper from the American College of Physicians, authors laid out recommendations for combating step therapy’s side effects. These recommendations included making related data transparent to the public and minimizing the policy’s disruptions to care. Jacqueline W. Fincher, MD, MACP, a member of the committee that issued the position paper and who is a primary care physician in Georgia, said such insurance practices need to be designed with “strong input from frontline physicians, not clipboard physicians.
“What we want from insurers is understanding, transparency, and the least burdensome protocol to provide patients the care they need at a cost-effective price they can afford,” said Dr. Fincher, who is also the current president of the ACP. “The focus needs to be on what’s in the patient’s best interest.”
Every year a new fight
“We all dread January,” said Dr. Fincher. That is the worst month, she added, because new health benefits go into effect, which means patients who are responding well to certain treatments may suddenly face new restrictions.
Another aggravating aspect of step therapy? It is often difficult – if not impossible – to access information on specific step therapy protocols in a patient’s health plan in real time in the exam room, where treatment conversations actually take place. In a more patient-centered world, Dr. Fincher said, she would be able to use the electronic health record system to quickly identify whether a patient’s plan covers a particular treatment and, if not, what the alternatives are.
Georgia’s new step therapy law went into effect last year. Like laws in other states, it spells out step therapy exemptions and sets time frames in which insurers must respond to exceptions and appeals. Dr. Fincher, who spoke in favor of the new law, said she’s “happy for any step forward.” Still, the growing burden of prior authorization rules are an utter “time sink” for her and her staff.
“I have to justify my decisions to nondoctors before I even get to a doctor, and that’s really frustrating,” she said. “We’re talking about people here, not widgets.”
Advocates in Nevada are hoping this is the year a step therapy bill will make it into law in their state. As of March, one had yet to be introduced in the state legislature. Tom McCoy, director of state government affairs at the Nevada Chronic Care Collaborative, said existing Nevada law already prohibits nonmedical drug switching during a policy year; however, insurers can still make changes the following year.
A bill to rein in step therapy was proposed previously, Mr. McCoy said, but it never got off the ground. The collaborative, as well as about two dozen organizations representing Nevada providers and patients, are now calling on state lawmakers to make the issue a priority in the current session.
“The health plans have a lot of power – a lot,” Mr. McCoy said. “We’re hoping to get a [legislative] sponsor in 2021 ... but it’s also been a really hard year to connect legislators with patients and doctors, and being able to hear their stories really does make a difference.”
In Nebraska, Marcus Snow, MD, a rheumatologist at Nebraska Medicine, in Omaha, said that the state’s new step therapy law will be a “great first step in helping to provide some guardrails” around the practice. He noted that turnaround requirements for insurer responses are “sorely needed.” However, he said that, because the bill doesn’t apply to all health plans, many Nebraskans still won’t benefit.
Dealing with step therapy is a daily “headache” for Dr. Snow, who says navigating the bureaucracy of prior authorization seems to be getting worse every year. Like his peers around the country, he spends an inordinate amount of time pushing appeals up the insurance company ranks to get access to treatments he believes will be most effective. But Snow says that, more than just being a mountain of tiresome red tape, these practices also intrude on the patient-provider relationship, casting an unsettling sense of uncertainty that the ultimate decision about the best course of action isn’t up to the doctor and patient at all.
“In the end, the insurance company is the judge and jury of my prescription,” Dr. Snow said. “They’d argue I can still prescribe it, but if it costs $70,000 a year – I don’t know who can afford that.”
Ms. Lynch, at the National Psoriasis Foundation, said their step therapy advocacy will continue to take a two-pronged approach. They will push for new and expanded protections at both state and federal levels. Protections are needed at both levels to make sure that all health plans regulated by all entities are covered. In the U.S. Senate and the House, step therapy bills were reintroduced this year. They would apply to health plans subject to the federal Employee Retirement Income Security Act, which governs employer-sponsored health coverage, and could close a big gap in existing protections. Oregon, New Jersey, and Arizona are at the top of the foundation’s advocacy list this year, according to Ms. Lynch.
“Folks are really starting to pay more attention to this issue,” she said. “And hearing those real-world stories and frustrations is definitely one of the most effective tools we have.”
A version of this article first appeared on Medscape.com.
Every day Melissa Fulton, RN, MSN, FNP, APRN-C, shows up to work, she’s ready for another fight. An advanced practice nurse who specializes in multiple sclerosis care, Ms. Fulton said she typically spends more than a third of her time battling it out with insurance companies over drugs she knows her patients need but that insurers don’t want to cover. Instead, they want the patient to first receive less expensive and often less efficacious drugs, even if that goes against recommendations and, in some cases, against the patient’s medical history.
The maddening protocol – familiar to health care providers everywhere – is known as “step therapy.” It forces patients to try alternative medications – medications that often fail – before receiving the one initially prescribed. The process can take weeks or months, which is time that some patients don’t have. Step therapy was sold as a way to lower costs. However, beyond the ethically problematic notion of forcing sick patients to receiver cheaper alternatives that are ineffective, research has also shown it may actually be more costly in the long run.
Ms. Fulton, who works at Saunders Medical Center in Wahoo, Neb., is a veteran in the war against step therapy. She is used to pushing her appeals up the insurance company chain of command, past nonmedical reviewers, until her patient’s case finally lands on the desk of someone with a neurology background. She said that can take three or four appeals – a judge might even get involved – and the patient could still lose. “This happens constantly,” she said, “but we fight like hell.”
Fortunately, life may soon get a little easier for Ms. Fulton. In late March, a bill to restrict step therapy made it through the Nebraska state legislature and is on its way to the governor’s desk. The Step Therapy Reform Act doesn’t outright ban the practice; however, it will put guardrails in place. It requires that insurers respond to appeals within certain time frames, and it creates key exemptions.
When the governor signs off, Nebraska will join more than two dozen other states that already have step therapy restrictions on the books, according to Hannah Lynch, MPS, associate director of federal government relations and health policy at the National Psoriasis Foundation, a leading advocate to reform and protect against the insurance practice. “There’s a lot of frustration out there,” Ms. Lynch said. “It really hinders providers’ ability to make decisions they think will have the best outcomes.”
Driven by coalitions of doctors, nurses, and patients, laws reining in step therapy have been adopted at a relatively quick clip, mostly within the past 5 years. Recent additions include South Dakota and North Carolina, which adopted step therapy laws in 2020, and Arkansas, which passed a law earlier this year.
Ms. Lynch attributed growing support to rising out-of-pocket drug costs and the introduction of biologic drugs, which are often more effective but also more expensive. Like Nebraska’s law, most step therapy reform legislation carves out exemptions and requires timely appeals processes; however, many of the laws still have significant gaps, such as not including certain types of insurance plans.
Ideally, Ms. Lynch said, the protections would apply to all types of health plans that are regulated at the state level, such as Medicaid, state employee health plans, and coverage sold through state insurance exchanges. Closing loopholes in the laws is a top priority for advocates, she added, pointing to work currently underway in Arkansas to extend its new protections to Medicaid expansion patients.
“With so many outside stakeholders, you have to compromise – it’s a give and take,” Ms. Lynch said. Still, when it comes to fighting step therapy, she says, “Any protection on the books is always our first goal when we go into a state.”
Putting patients first
Lisa Arkin, MD, a pediatric dermatologist at the University of Wisconsin–Madison, said she finds herself “swimming upstream every day in the fight with insurance.” Her patients are typically on their second or third stop and have more complex disorders. Dr. Arkin said that the problem with step therapy is that it tries to squeeze all patients into the same box, even if the circumstances don’t fit.
Her state passed restrictions on step therapy in 2019, but the measures only went into effect last year. Under the Wisconsin law, patients can be granted an exemption if an alternative treatment is contraindicated, likely to cause harm, or expected to be ineffective. Patients can also be exempt if their current treatment is working.
Dr. Arkin, an outspoken advocate for curbing step therapy, says the Wisconsin law is “very strong.” However, because it only applies to certain health plans – state employee health plans and those purchased in the state’s health insurance exchange – fewer than half the state’s patients benefit from its protections. She notes that some of the most severe presentations she treats occur in patients who rely on Medicaid coverage and already face barriers to care.
“I’m a doctor who puts up a fuss [with insurers], but that’s not fair – we shouldn’t have to do that,” Dr. Arkin said. “To me, it’s really critical to make this an even playing field so this law affords protection to everyone I see in the clinic.”
Major medical associations caution against step therapy as well. The American Society of Clinical Oncology and the American Medical Association have called out the risks to patient safety and health. In fact, in 2019, after the Centers for Medicare & Medicaid Services gave new authority to Medicare Advantage plans to start using step therapy, dozens of national medical groups called out the agency for allowing a practice that could potentially hurt patients and undercut the physician-patient decision-making process.
Last year, in a new position paper from the American College of Physicians, authors laid out recommendations for combating step therapy’s side effects. These recommendations included making related data transparent to the public and minimizing the policy’s disruptions to care. Jacqueline W. Fincher, MD, MACP, a member of the committee that issued the position paper and who is a primary care physician in Georgia, said such insurance practices need to be designed with “strong input from frontline physicians, not clipboard physicians.
“What we want from insurers is understanding, transparency, and the least burdensome protocol to provide patients the care they need at a cost-effective price they can afford,” said Dr. Fincher, who is also the current president of the ACP. “The focus needs to be on what’s in the patient’s best interest.”
Every year a new fight
“We all dread January,” said Dr. Fincher. That is the worst month, she added, because new health benefits go into effect, which means patients who are responding well to certain treatments may suddenly face new restrictions.
Another aggravating aspect of step therapy? It is often difficult – if not impossible – to access information on specific step therapy protocols in a patient’s health plan in real time in the exam room, where treatment conversations actually take place. In a more patient-centered world, Dr. Fincher said, she would be able to use the electronic health record system to quickly identify whether a patient’s plan covers a particular treatment and, if not, what the alternatives are.
Georgia’s new step therapy law went into effect last year. Like laws in other states, it spells out step therapy exemptions and sets time frames in which insurers must respond to exceptions and appeals. Dr. Fincher, who spoke in favor of the new law, said she’s “happy for any step forward.” Still, the growing burden of prior authorization rules are an utter “time sink” for her and her staff.
“I have to justify my decisions to nondoctors before I even get to a doctor, and that’s really frustrating,” she said. “We’re talking about people here, not widgets.”
Advocates in Nevada are hoping this is the year a step therapy bill will make it into law in their state. As of March, one had yet to be introduced in the state legislature. Tom McCoy, director of state government affairs at the Nevada Chronic Care Collaborative, said existing Nevada law already prohibits nonmedical drug switching during a policy year; however, insurers can still make changes the following year.
A bill to rein in step therapy was proposed previously, Mr. McCoy said, but it never got off the ground. The collaborative, as well as about two dozen organizations representing Nevada providers and patients, are now calling on state lawmakers to make the issue a priority in the current session.
“The health plans have a lot of power – a lot,” Mr. McCoy said. “We’re hoping to get a [legislative] sponsor in 2021 ... but it’s also been a really hard year to connect legislators with patients and doctors, and being able to hear their stories really does make a difference.”
In Nebraska, Marcus Snow, MD, a rheumatologist at Nebraska Medicine, in Omaha, said that the state’s new step therapy law will be a “great first step in helping to provide some guardrails” around the practice. He noted that turnaround requirements for insurer responses are “sorely needed.” However, he said that, because the bill doesn’t apply to all health plans, many Nebraskans still won’t benefit.
Dealing with step therapy is a daily “headache” for Dr. Snow, who says navigating the bureaucracy of prior authorization seems to be getting worse every year. Like his peers around the country, he spends an inordinate amount of time pushing appeals up the insurance company ranks to get access to treatments he believes will be most effective. But Snow says that, more than just being a mountain of tiresome red tape, these practices also intrude on the patient-provider relationship, casting an unsettling sense of uncertainty that the ultimate decision about the best course of action isn’t up to the doctor and patient at all.
“In the end, the insurance company is the judge and jury of my prescription,” Dr. Snow said. “They’d argue I can still prescribe it, but if it costs $70,000 a year – I don’t know who can afford that.”
Ms. Lynch, at the National Psoriasis Foundation, said their step therapy advocacy will continue to take a two-pronged approach. They will push for new and expanded protections at both state and federal levels. Protections are needed at both levels to make sure that all health plans regulated by all entities are covered. In the U.S. Senate and the House, step therapy bills were reintroduced this year. They would apply to health plans subject to the federal Employee Retirement Income Security Act, which governs employer-sponsored health coverage, and could close a big gap in existing protections. Oregon, New Jersey, and Arizona are at the top of the foundation’s advocacy list this year, according to Ms. Lynch.
“Folks are really starting to pay more attention to this issue,” she said. “And hearing those real-world stories and frustrations is definitely one of the most effective tools we have.”
A version of this article first appeared on Medscape.com.
Every day Melissa Fulton, RN, MSN, FNP, APRN-C, shows up to work, she’s ready for another fight. An advanced practice nurse who specializes in multiple sclerosis care, Ms. Fulton said she typically spends more than a third of her time battling it out with insurance companies over drugs she knows her patients need but that insurers don’t want to cover. Instead, they want the patient to first receive less expensive and often less efficacious drugs, even if that goes against recommendations and, in some cases, against the patient’s medical history.
The maddening protocol – familiar to health care providers everywhere – is known as “step therapy.” It forces patients to try alternative medications – medications that often fail – before receiving the one initially prescribed. The process can take weeks or months, which is time that some patients don’t have. Step therapy was sold as a way to lower costs. However, beyond the ethically problematic notion of forcing sick patients to receiver cheaper alternatives that are ineffective, research has also shown it may actually be more costly in the long run.
Ms. Fulton, who works at Saunders Medical Center in Wahoo, Neb., is a veteran in the war against step therapy. She is used to pushing her appeals up the insurance company chain of command, past nonmedical reviewers, until her patient’s case finally lands on the desk of someone with a neurology background. She said that can take three or four appeals – a judge might even get involved – and the patient could still lose. “This happens constantly,” she said, “but we fight like hell.”
Fortunately, life may soon get a little easier for Ms. Fulton. In late March, a bill to restrict step therapy made it through the Nebraska state legislature and is on its way to the governor’s desk. The Step Therapy Reform Act doesn’t outright ban the practice; however, it will put guardrails in place. It requires that insurers respond to appeals within certain time frames, and it creates key exemptions.
When the governor signs off, Nebraska will join more than two dozen other states that already have step therapy restrictions on the books, according to Hannah Lynch, MPS, associate director of federal government relations and health policy at the National Psoriasis Foundation, a leading advocate to reform and protect against the insurance practice. “There’s a lot of frustration out there,” Ms. Lynch said. “It really hinders providers’ ability to make decisions they think will have the best outcomes.”
Driven by coalitions of doctors, nurses, and patients, laws reining in step therapy have been adopted at a relatively quick clip, mostly within the past 5 years. Recent additions include South Dakota and North Carolina, which adopted step therapy laws in 2020, and Arkansas, which passed a law earlier this year.
Ms. Lynch attributed growing support to rising out-of-pocket drug costs and the introduction of biologic drugs, which are often more effective but also more expensive. Like Nebraska’s law, most step therapy reform legislation carves out exemptions and requires timely appeals processes; however, many of the laws still have significant gaps, such as not including certain types of insurance plans.
Ideally, Ms. Lynch said, the protections would apply to all types of health plans that are regulated at the state level, such as Medicaid, state employee health plans, and coverage sold through state insurance exchanges. Closing loopholes in the laws is a top priority for advocates, she added, pointing to work currently underway in Arkansas to extend its new protections to Medicaid expansion patients.
“With so many outside stakeholders, you have to compromise – it’s a give and take,” Ms. Lynch said. Still, when it comes to fighting step therapy, she says, “Any protection on the books is always our first goal when we go into a state.”
Putting patients first
Lisa Arkin, MD, a pediatric dermatologist at the University of Wisconsin–Madison, said she finds herself “swimming upstream every day in the fight with insurance.” Her patients are typically on their second or third stop and have more complex disorders. Dr. Arkin said that the problem with step therapy is that it tries to squeeze all patients into the same box, even if the circumstances don’t fit.
Her state passed restrictions on step therapy in 2019, but the measures only went into effect last year. Under the Wisconsin law, patients can be granted an exemption if an alternative treatment is contraindicated, likely to cause harm, or expected to be ineffective. Patients can also be exempt if their current treatment is working.
Dr. Arkin, an outspoken advocate for curbing step therapy, says the Wisconsin law is “very strong.” However, because it only applies to certain health plans – state employee health plans and those purchased in the state’s health insurance exchange – fewer than half the state’s patients benefit from its protections. She notes that some of the most severe presentations she treats occur in patients who rely on Medicaid coverage and already face barriers to care.
“I’m a doctor who puts up a fuss [with insurers], but that’s not fair – we shouldn’t have to do that,” Dr. Arkin said. “To me, it’s really critical to make this an even playing field so this law affords protection to everyone I see in the clinic.”
Major medical associations caution against step therapy as well. The American Society of Clinical Oncology and the American Medical Association have called out the risks to patient safety and health. In fact, in 2019, after the Centers for Medicare & Medicaid Services gave new authority to Medicare Advantage plans to start using step therapy, dozens of national medical groups called out the agency for allowing a practice that could potentially hurt patients and undercut the physician-patient decision-making process.
Last year, in a new position paper from the American College of Physicians, authors laid out recommendations for combating step therapy’s side effects. These recommendations included making related data transparent to the public and minimizing the policy’s disruptions to care. Jacqueline W. Fincher, MD, MACP, a member of the committee that issued the position paper and who is a primary care physician in Georgia, said such insurance practices need to be designed with “strong input from frontline physicians, not clipboard physicians.
“What we want from insurers is understanding, transparency, and the least burdensome protocol to provide patients the care they need at a cost-effective price they can afford,” said Dr. Fincher, who is also the current president of the ACP. “The focus needs to be on what’s in the patient’s best interest.”
Every year a new fight
“We all dread January,” said Dr. Fincher. That is the worst month, she added, because new health benefits go into effect, which means patients who are responding well to certain treatments may suddenly face new restrictions.
Another aggravating aspect of step therapy? It is often difficult – if not impossible – to access information on specific step therapy protocols in a patient’s health plan in real time in the exam room, where treatment conversations actually take place. In a more patient-centered world, Dr. Fincher said, she would be able to use the electronic health record system to quickly identify whether a patient’s plan covers a particular treatment and, if not, what the alternatives are.
Georgia’s new step therapy law went into effect last year. Like laws in other states, it spells out step therapy exemptions and sets time frames in which insurers must respond to exceptions and appeals. Dr. Fincher, who spoke in favor of the new law, said she’s “happy for any step forward.” Still, the growing burden of prior authorization rules are an utter “time sink” for her and her staff.
“I have to justify my decisions to nondoctors before I even get to a doctor, and that’s really frustrating,” she said. “We’re talking about people here, not widgets.”
Advocates in Nevada are hoping this is the year a step therapy bill will make it into law in their state. As of March, one had yet to be introduced in the state legislature. Tom McCoy, director of state government affairs at the Nevada Chronic Care Collaborative, said existing Nevada law already prohibits nonmedical drug switching during a policy year; however, insurers can still make changes the following year.
A bill to rein in step therapy was proposed previously, Mr. McCoy said, but it never got off the ground. The collaborative, as well as about two dozen organizations representing Nevada providers and patients, are now calling on state lawmakers to make the issue a priority in the current session.
“The health plans have a lot of power – a lot,” Mr. McCoy said. “We’re hoping to get a [legislative] sponsor in 2021 ... but it’s also been a really hard year to connect legislators with patients and doctors, and being able to hear their stories really does make a difference.”
In Nebraska, Marcus Snow, MD, a rheumatologist at Nebraska Medicine, in Omaha, said that the state’s new step therapy law will be a “great first step in helping to provide some guardrails” around the practice. He noted that turnaround requirements for insurer responses are “sorely needed.” However, he said that, because the bill doesn’t apply to all health plans, many Nebraskans still won’t benefit.
Dealing with step therapy is a daily “headache” for Dr. Snow, who says navigating the bureaucracy of prior authorization seems to be getting worse every year. Like his peers around the country, he spends an inordinate amount of time pushing appeals up the insurance company ranks to get access to treatments he believes will be most effective. But Snow says that, more than just being a mountain of tiresome red tape, these practices also intrude on the patient-provider relationship, casting an unsettling sense of uncertainty that the ultimate decision about the best course of action isn’t up to the doctor and patient at all.
“In the end, the insurance company is the judge and jury of my prescription,” Dr. Snow said. “They’d argue I can still prescribe it, but if it costs $70,000 a year – I don’t know who can afford that.”
Ms. Lynch, at the National Psoriasis Foundation, said their step therapy advocacy will continue to take a two-pronged approach. They will push for new and expanded protections at both state and federal levels. Protections are needed at both levels to make sure that all health plans regulated by all entities are covered. In the U.S. Senate and the House, step therapy bills were reintroduced this year. They would apply to health plans subject to the federal Employee Retirement Income Security Act, which governs employer-sponsored health coverage, and could close a big gap in existing protections. Oregon, New Jersey, and Arizona are at the top of the foundation’s advocacy list this year, according to Ms. Lynch.
“Folks are really starting to pay more attention to this issue,” she said. “And hearing those real-world stories and frustrations is definitely one of the most effective tools we have.”
A version of this article first appeared on Medscape.com.
Encephalopathy common, often lethal in hospitalized patients with COVID-19
, new research shows. Results of a retrospective study show that of almost 4,500 patients with COVID-19, 12% were diagnosed with TME. Of these, 78% developed encephalopathy immediately prior to hospital admission. Septic encephalopathy, hypoxic-ischemic encephalopathy (HIE), and uremia were the most common causes, although multiple causes were present in close to 80% of patients. TME was also associated with a 24% higher risk of in-hospital death.
“We found that close to one in eight patients who were hospitalized with COVID-19 had TME that was not attributed to the effects of sedatives, and that this is incredibly common among these patients who are critically ill” said lead author Jennifer A. Frontera, MD, New York University.
“The general principle of our findings is to be more aggressive in TME; and from a neurologist perspective, the way to do this is to eliminate the effects of sedation, which is a confounder,” she said.
The study was published online March 16 in Neurocritical Care.
Drilling down
“Many neurological complications of COVID-19 are sequelae of severe illness or secondary effects of multisystem organ failure, but our previous work identified TME as the most common neurological complication,” Dr. Frontera said.
Previous research investigating encephalopathy among patients with COVID-19 included patients who may have been sedated or have had a positive Confusion Assessment Method (CAM) result.
“A lot of the delirium literature is effectively heterogeneous because there are a number of patients who are on sedative medication that, if you could turn it off, these patients would return to normal. Some may have underlying neurological issues that can be addressed, but you can›t get to the bottom of this unless you turn off the sedation,” Dr. Frontera noted.
“We wanted to be specific and try to drill down to see what the underlying cause of the encephalopathy was,” she said.
The researchers retrospectively analyzed data on 4,491 patients (≥ 18 years old) with COVID-19 who were admitted to four New York City hospitals between March 1, 2020, and May 20, 2020. Of these, 559 (12%) with TME were compared with 3,932 patients without TME.
The researchers looked at index admissions and included patients who had:
- New changes in mental status or significant worsening of mental status (in patients with baseline abnormal mental status).
- Hyperglycemia or with transient focal neurologic deficits that resolved with glucose correction.
- An adequate washout of sedating medications (when relevant) prior to mental status assessment.
Potential etiologies included electrolyte abnormalities, organ failure, hypertensive encephalopathy, sepsis or active infection, fever, nutritional deficiency, and environmental injury.
Foreign environment
Most (78%) of the 559 patients diagnosed with TME had already developed encephalopathy immediately prior to hospital admission, the authors report. The most common etiologies of TME among hospitalized patients with COVID-19 are listed below.
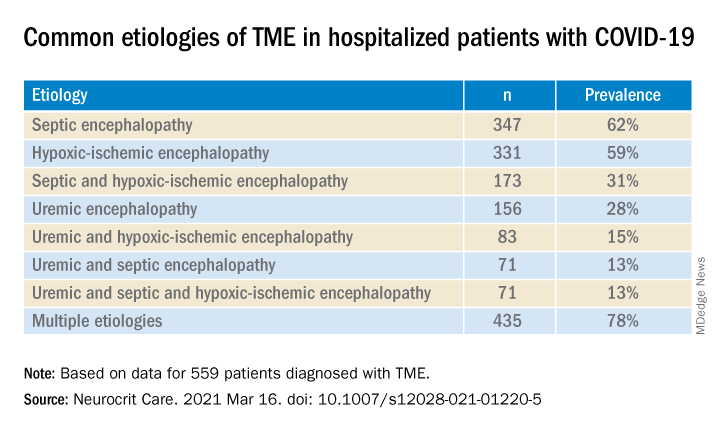
Compared with patients without TME, those with TME – (all Ps < .001):
- Were older (76 vs. 62 years).
- Had higher rates of dementia (27% vs. 3%).
- Had higher rates of psychiatric history (20% vs. 10%).
- Were more often intubated (37% vs. 20%).
- Had a longer length of hospital stay (7.9 vs. 6.0 days).
- Were less often discharged home (25% vs. 66%).
“It’s no surprise that older patients and people with dementia or psychiatric illness are predisposed to becoming encephalopathic,” said Dr. Frontera. “Being in a foreign environment, such as a hospital, or being sleep-deprived in the ICU is likely to make them more confused during their hospital stay.”
Delirium as a symptom
In-hospital mortality or discharge to hospice was considerably higher in the TME versus non-TME patients (44% vs. 18%, respectively).
When the researchers adjusted for confounders (age, sex, race, worse Sequential Organ Failure Assessment score during hospitalization, ventilator status, study week, hospital location, and ICU care level) and excluded patients receiving only comfort care, they found that TME was associated with a 24% increased risk of in-hospital death (30% in patients with TME vs. 16% in those without TME).
The highest mortality risk was associated with hypoxemia, with 42% of patients with HIE dying during hospitalization, compared with 16% of patients without HIE (adjusted hazard ratio 1.56; 95% confidence interval, 1.21-2.00; P = .001).
“Not all patients who are intubated require sedation, but there’s generally a lot of hesitation in reducing or stopping sedation in some patients,” Dr. Frontera observed.
She acknowledged there are “many extremely sick patients whom you can’t ventilate without sedation.”
Nevertheless, “delirium in and of itself does not cause death. It’s a symptom, not a disease, and we have to figure out what causes it. Delirium might not need to be sedated, and it’s more important to see what the causal problem is.”
Independent predictor of death
Commenting on the study, Panayiotis N. Varelas, MD, PhD, vice president of the Neurocritical Care Society, said the study “approached the TME issue better than previously, namely allowing time for sedatives to wear off to have a better sample of patients with this syndrome.”
Dr. Varelas, who is chairman of the department of neurology and professor of neurology at Albany (N.Y.) Medical College, emphasized that TME “is not benign and, in patients with COVID-19, it is an independent predictor of in-hospital mortality.”
“One should take all possible measures … to avoid desaturation and hypotensive episodes and also aggressively treat SAE and uremic encephalopathy in hopes of improving the outcomes,” added Dr. Varelas, who was not involved with the study.
Also commenting on the study, Mitchell Elkind, MD, professor of neurology and epidemiology at Columbia University in New York, who was not associated with the research, said it “nicely distinguishes among the different causes of encephalopathy, including sepsis, hypoxia, and kidney failure … emphasizing just how sick these patients are.”
The study received no direct funding. Individual investigators were supported by grants from the National Institute on Aging and the National Institute of Neurological Disorders and Stroke. The investigators, Dr. Varelas, and Dr. Elkind have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research shows. Results of a retrospective study show that of almost 4,500 patients with COVID-19, 12% were diagnosed with TME. Of these, 78% developed encephalopathy immediately prior to hospital admission. Septic encephalopathy, hypoxic-ischemic encephalopathy (HIE), and uremia were the most common causes, although multiple causes were present in close to 80% of patients. TME was also associated with a 24% higher risk of in-hospital death.
“We found that close to one in eight patients who were hospitalized with COVID-19 had TME that was not attributed to the effects of sedatives, and that this is incredibly common among these patients who are critically ill” said lead author Jennifer A. Frontera, MD, New York University.
“The general principle of our findings is to be more aggressive in TME; and from a neurologist perspective, the way to do this is to eliminate the effects of sedation, which is a confounder,” she said.
The study was published online March 16 in Neurocritical Care.
Drilling down
“Many neurological complications of COVID-19 are sequelae of severe illness or secondary effects of multisystem organ failure, but our previous work identified TME as the most common neurological complication,” Dr. Frontera said.
Previous research investigating encephalopathy among patients with COVID-19 included patients who may have been sedated or have had a positive Confusion Assessment Method (CAM) result.
“A lot of the delirium literature is effectively heterogeneous because there are a number of patients who are on sedative medication that, if you could turn it off, these patients would return to normal. Some may have underlying neurological issues that can be addressed, but you can›t get to the bottom of this unless you turn off the sedation,” Dr. Frontera noted.
“We wanted to be specific and try to drill down to see what the underlying cause of the encephalopathy was,” she said.
The researchers retrospectively analyzed data on 4,491 patients (≥ 18 years old) with COVID-19 who were admitted to four New York City hospitals between March 1, 2020, and May 20, 2020. Of these, 559 (12%) with TME were compared with 3,932 patients without TME.
The researchers looked at index admissions and included patients who had:
- New changes in mental status or significant worsening of mental status (in patients with baseline abnormal mental status).
- Hyperglycemia or with transient focal neurologic deficits that resolved with glucose correction.
- An adequate washout of sedating medications (when relevant) prior to mental status assessment.
Potential etiologies included electrolyte abnormalities, organ failure, hypertensive encephalopathy, sepsis or active infection, fever, nutritional deficiency, and environmental injury.
Foreign environment
Most (78%) of the 559 patients diagnosed with TME had already developed encephalopathy immediately prior to hospital admission, the authors report. The most common etiologies of TME among hospitalized patients with COVID-19 are listed below.

Compared with patients without TME, those with TME – (all Ps < .001):
- Were older (76 vs. 62 years).
- Had higher rates of dementia (27% vs. 3%).
- Had higher rates of psychiatric history (20% vs. 10%).
- Were more often intubated (37% vs. 20%).
- Had a longer length of hospital stay (7.9 vs. 6.0 days).
- Were less often discharged home (25% vs. 66%).
“It’s no surprise that older patients and people with dementia or psychiatric illness are predisposed to becoming encephalopathic,” said Dr. Frontera. “Being in a foreign environment, such as a hospital, or being sleep-deprived in the ICU is likely to make them more confused during their hospital stay.”
Delirium as a symptom
In-hospital mortality or discharge to hospice was considerably higher in the TME versus non-TME patients (44% vs. 18%, respectively).
When the researchers adjusted for confounders (age, sex, race, worse Sequential Organ Failure Assessment score during hospitalization, ventilator status, study week, hospital location, and ICU care level) and excluded patients receiving only comfort care, they found that TME was associated with a 24% increased risk of in-hospital death (30% in patients with TME vs. 16% in those without TME).
The highest mortality risk was associated with hypoxemia, with 42% of patients with HIE dying during hospitalization, compared with 16% of patients without HIE (adjusted hazard ratio 1.56; 95% confidence interval, 1.21-2.00; P = .001).
“Not all patients who are intubated require sedation, but there’s generally a lot of hesitation in reducing or stopping sedation in some patients,” Dr. Frontera observed.
She acknowledged there are “many extremely sick patients whom you can’t ventilate without sedation.”
Nevertheless, “delirium in and of itself does not cause death. It’s a symptom, not a disease, and we have to figure out what causes it. Delirium might not need to be sedated, and it’s more important to see what the causal problem is.”
Independent predictor of death
Commenting on the study, Panayiotis N. Varelas, MD, PhD, vice president of the Neurocritical Care Society, said the study “approached the TME issue better than previously, namely allowing time for sedatives to wear off to have a better sample of patients with this syndrome.”
Dr. Varelas, who is chairman of the department of neurology and professor of neurology at Albany (N.Y.) Medical College, emphasized that TME “is not benign and, in patients with COVID-19, it is an independent predictor of in-hospital mortality.”
“One should take all possible measures … to avoid desaturation and hypotensive episodes and also aggressively treat SAE and uremic encephalopathy in hopes of improving the outcomes,” added Dr. Varelas, who was not involved with the study.
Also commenting on the study, Mitchell Elkind, MD, professor of neurology and epidemiology at Columbia University in New York, who was not associated with the research, said it “nicely distinguishes among the different causes of encephalopathy, including sepsis, hypoxia, and kidney failure … emphasizing just how sick these patients are.”
The study received no direct funding. Individual investigators were supported by grants from the National Institute on Aging and the National Institute of Neurological Disorders and Stroke. The investigators, Dr. Varelas, and Dr. Elkind have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research shows. Results of a retrospective study show that of almost 4,500 patients with COVID-19, 12% were diagnosed with TME. Of these, 78% developed encephalopathy immediately prior to hospital admission. Septic encephalopathy, hypoxic-ischemic encephalopathy (HIE), and uremia were the most common causes, although multiple causes were present in close to 80% of patients. TME was also associated with a 24% higher risk of in-hospital death.
“We found that close to one in eight patients who were hospitalized with COVID-19 had TME that was not attributed to the effects of sedatives, and that this is incredibly common among these patients who are critically ill” said lead author Jennifer A. Frontera, MD, New York University.
“The general principle of our findings is to be more aggressive in TME; and from a neurologist perspective, the way to do this is to eliminate the effects of sedation, which is a confounder,” she said.
The study was published online March 16 in Neurocritical Care.
Drilling down
“Many neurological complications of COVID-19 are sequelae of severe illness or secondary effects of multisystem organ failure, but our previous work identified TME as the most common neurological complication,” Dr. Frontera said.
Previous research investigating encephalopathy among patients with COVID-19 included patients who may have been sedated or have had a positive Confusion Assessment Method (CAM) result.
“A lot of the delirium literature is effectively heterogeneous because there are a number of patients who are on sedative medication that, if you could turn it off, these patients would return to normal. Some may have underlying neurological issues that can be addressed, but you can›t get to the bottom of this unless you turn off the sedation,” Dr. Frontera noted.
“We wanted to be specific and try to drill down to see what the underlying cause of the encephalopathy was,” she said.
The researchers retrospectively analyzed data on 4,491 patients (≥ 18 years old) with COVID-19 who were admitted to four New York City hospitals between March 1, 2020, and May 20, 2020. Of these, 559 (12%) with TME were compared with 3,932 patients without TME.
The researchers looked at index admissions and included patients who had:
- New changes in mental status or significant worsening of mental status (in patients with baseline abnormal mental status).
- Hyperglycemia or with transient focal neurologic deficits that resolved with glucose correction.
- An adequate washout of sedating medications (when relevant) prior to mental status assessment.
Potential etiologies included electrolyte abnormalities, organ failure, hypertensive encephalopathy, sepsis or active infection, fever, nutritional deficiency, and environmental injury.
Foreign environment
Most (78%) of the 559 patients diagnosed with TME had already developed encephalopathy immediately prior to hospital admission, the authors report. The most common etiologies of TME among hospitalized patients with COVID-19 are listed below.

Compared with patients without TME, those with TME – (all Ps < .001):
- Were older (76 vs. 62 years).
- Had higher rates of dementia (27% vs. 3%).
- Had higher rates of psychiatric history (20% vs. 10%).
- Were more often intubated (37% vs. 20%).
- Had a longer length of hospital stay (7.9 vs. 6.0 days).
- Were less often discharged home (25% vs. 66%).
“It’s no surprise that older patients and people with dementia or psychiatric illness are predisposed to becoming encephalopathic,” said Dr. Frontera. “Being in a foreign environment, such as a hospital, or being sleep-deprived in the ICU is likely to make them more confused during their hospital stay.”
Delirium as a symptom
In-hospital mortality or discharge to hospice was considerably higher in the TME versus non-TME patients (44% vs. 18%, respectively).
When the researchers adjusted for confounders (age, sex, race, worse Sequential Organ Failure Assessment score during hospitalization, ventilator status, study week, hospital location, and ICU care level) and excluded patients receiving only comfort care, they found that TME was associated with a 24% increased risk of in-hospital death (30% in patients with TME vs. 16% in those without TME).
The highest mortality risk was associated with hypoxemia, with 42% of patients with HIE dying during hospitalization, compared with 16% of patients without HIE (adjusted hazard ratio 1.56; 95% confidence interval, 1.21-2.00; P = .001).
“Not all patients who are intubated require sedation, but there’s generally a lot of hesitation in reducing or stopping sedation in some patients,” Dr. Frontera observed.
She acknowledged there are “many extremely sick patients whom you can’t ventilate without sedation.”
Nevertheless, “delirium in and of itself does not cause death. It’s a symptom, not a disease, and we have to figure out what causes it. Delirium might not need to be sedated, and it’s more important to see what the causal problem is.”
Independent predictor of death
Commenting on the study, Panayiotis N. Varelas, MD, PhD, vice president of the Neurocritical Care Society, said the study “approached the TME issue better than previously, namely allowing time for sedatives to wear off to have a better sample of patients with this syndrome.”
Dr. Varelas, who is chairman of the department of neurology and professor of neurology at Albany (N.Y.) Medical College, emphasized that TME “is not benign and, in patients with COVID-19, it is an independent predictor of in-hospital mortality.”
“One should take all possible measures … to avoid desaturation and hypotensive episodes and also aggressively treat SAE and uremic encephalopathy in hopes of improving the outcomes,” added Dr. Varelas, who was not involved with the study.
Also commenting on the study, Mitchell Elkind, MD, professor of neurology and epidemiology at Columbia University in New York, who was not associated with the research, said it “nicely distinguishes among the different causes of encephalopathy, including sepsis, hypoxia, and kidney failure … emphasizing just how sick these patients are.”
The study received no direct funding. Individual investigators were supported by grants from the National Institute on Aging and the National Institute of Neurological Disorders and Stroke. The investigators, Dr. Varelas, and Dr. Elkind have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NEUROCRITICAL CARE
Experts highlight recent breakthroughs in psoriatic arthritis
Apremilast (Otezla) monotherapy may be an effective option in oligoarticular psoriatic arthritis, Alexis R. Ogdie, MD, reported at the 2021 Rheumatology Winter Clinical Symposium.
Her analysis of apremilast data from the CORRONA Registry was among several recent highlights in psoriatic arthritis (PsA) cited by speakers at the meeting. Other significant developments included a large pan-Scandinavian study that reassuringly found no increased risk of solid cancers in tumor necrosis factor (TNF) inhibitor–treated patients with PsA, and evidence to suggest a sex difference in the efficacy of both secukinumab (Cosentyx) and adalimumab (Humira), with men responding better than women to two biologics with differing mechanisms of action.
A role for apremilast in oligoarticular disease?
Dr. Ogdie presented an analysis of 150 patients in the U.S. observational CORRONA Registry who initiated monotherapy for oligoarticular PsA and were followed for 6 months. Thirty-four started on apremilast, 15 on methotrexate, and 101 on a biologic. Even though the apremilast group had higher baseline disease activity than did those who started on methotrexate, at 6 months a swollen joint count of 1 or 0 was present in 41% of the apremilast-treated patients, compared with none on methotrexate and 15% on a biologic agent.
A tender joint count of 0-1 was documented at 6 months in 24% of patients on apremilast, 13% with methotrexate, and 21% on a biologic agent. Apremilast’s numeric superiority in outcomes compared to methotrexate in this exploratory study wasn’t subjected to statistical analysis because of the small sample size. However, the ongoing phase 4, double-blind, placebo-controlled, multicenter FOREMOST trial in 330 patients with early oligoarticular PsA should provide more definitive efficacy data, noted Dr. Ogdie, a rheumatologist and epidemiologist at the University of Pennsylvania, Philadelphia.
RWCS program director Arthur Kavanaugh, MD, said, “The most recent EULAR [European Alliance of Associations for Rheumatology] PsA guidelines totally discount apremilast, and I think mostly on the basis of cost, but then they also say that in groups of people it’s not as effective as methotrexate.”
“This study shows to me that, even though it’s a registry, with all the caveats about getting data from registries, apremilast certainly can be an effective drug,” said Dr. Kavanaugh, a rheumatologist and professor of medicine at the University of California, San Diego.
Another valuable piece of information from the CORRONA analysis is that it zeros in on patients with oligoarticular PsA.
“Almost all of our PsA studies are focused on people with polyarticular disease. What about those who have lesser involvement? That, of course, is important in the clinic,” he noted.
Dr. Ogdie concurred.
“If we study only polyarticular disease and we make all of our assumptions based on polyarticular disease, we might be leaving out at least half of the patients with PsA. And those patients may not need a bigger gun. Apremilast and methotrexate are kind of in the same group for that mild oligoarticular disease, and they probably work just fine,” she said.
A final point: “We really don’t have good outcome measures to study oligoarticular disease well. The ACR20 is not good because a 20% improvement in three joints is not readily measurable. That’s why trialists enroll patients with high joint count numbers,” according to the rheumatologist.
No increased risk of solid cancers in PsA patients treated with TNF inhibitors
A new analysis of clinical rheumatology registries in five Nordic countries finally puts to rest any concerns that treatment of PsA with TNF inhibitors is associated with increased risk of solid cancers. The same group previously reported no link between TNF inhibitors and lymphoma in PsA.
The solid cancers study included 9,655 PsA patients who started a first TNF inhibitor during 2001-2017, 14,809 others not treated with biologics, and 31,350 matched general population controls. Linkage to Swedish, Norwegian, Danish, Icelandic, and Finnish national cancer registries showed that the adjusted risk for solid cancer in TNF inhibitor–treated, compared with biologic-naive PsA patients, was 1.0. Similarly, the pooled standardized incidence ratio of solid cancer in TNF inhibitor–treated PsA patients compared to the general population was 1.0. There was no signal of a differential risk for incident cancer for any of the eight malignancies studied: lung, colorectal, breast, prostate, uterine, brain, liver, and pancreatic cancer.
“I like this study a lot because it’s specific to PsA rather than extrapolating from rheumatoid arthritis data, where we have a bunch more information for a much longer period of time, but it’s a different population,” Dr. Kavanaugh said.
Dr. Ogdie said, “I talk to my patients about this particular study or the same group’s earlier lymphoma study all the time.”
“I have to say, these are important data for the dermatology world because there are dermatologists who are still not convinced that TNF inhibitors don’t have an increased risk of malignancy. This kind of information is going to be helpful,” observed Eric M. Ruderman, MD, professor of medicine (rheumatology) at Northwestern University, Chicago.
Greater efficacy for biologics in males than females with PsA?
A secondary analysis of the phase 3b EXCEED trial raised the intriguing possibility that both secukinumab, an interleukin-17A inhibitor, and adalimumab, a TNF inhibitor, have greater efficacy in men than in women with PsA. In this randomized trial of 853 biologic-naive patients with PsA, the ACR20 response rate to secukinumab at week 52 was 61% in females versus 74% in males, with ACR50 rates of 43% in females and 55.3% in males. The ACR20 rate with adalimumab was 51.5% in females and 70.2% in males. Similarly, the corresponding ACR50s were 32.6% and 55.3%, respectively. Minimal disease activity was achieved in 36.2% of women and 51% of men on secukinumab, and in 24.2% of women and 49.8% of men on adalimumab.
“These are randomized patients, so you really shouldn’t see these big differences in minimal disease activity,” Dr. Ogdie noted. “The question is why do men seem to respond better to therapy than women? I don’t think it’s the fibromyalgia-ness. There’s probably some biologic rationale for this that we just don’t understand yet. Maybe hormonal interactions.”
This gender difference in response is an important issue because it can potentially distort outcomes in head-to-head drug trials, Dr. Ruderman added.
“That gender difference is not likely to be huge if you’re looking at a placebo-controlled trial because the difference between the active drug and placebo is going to outweigh it. But when you have two active drugs, if there’s an imbalance in terms of how many men or women there are on each of the two drugs, you may end up with an efficacy difference that’s not real but is based on gender and not response to the drug,” he explained.
Roy M. Fleischmann, MD, a rheumatologist and clinical trialist at the University of Texas, Dallas, rose from the audience to pronounce the EXCEED male-versus-female analysis “very interesting.”
“We should go back and look at other trials and see if that occurred, and if it did, then we have to think about that going forward,” he proposed.
Dr. Ogdie, Dr. Kavanaugh, and Dr. Ruderman reported having financial relationships with numerous pharmaceutical companies.
Apremilast (Otezla) monotherapy may be an effective option in oligoarticular psoriatic arthritis, Alexis R. Ogdie, MD, reported at the 2021 Rheumatology Winter Clinical Symposium.
Her analysis of apremilast data from the CORRONA Registry was among several recent highlights in psoriatic arthritis (PsA) cited by speakers at the meeting. Other significant developments included a large pan-Scandinavian study that reassuringly found no increased risk of solid cancers in tumor necrosis factor (TNF) inhibitor–treated patients with PsA, and evidence to suggest a sex difference in the efficacy of both secukinumab (Cosentyx) and adalimumab (Humira), with men responding better than women to two biologics with differing mechanisms of action.
A role for apremilast in oligoarticular disease?
Dr. Ogdie presented an analysis of 150 patients in the U.S. observational CORRONA Registry who initiated monotherapy for oligoarticular PsA and were followed for 6 months. Thirty-four started on apremilast, 15 on methotrexate, and 101 on a biologic. Even though the apremilast group had higher baseline disease activity than did those who started on methotrexate, at 6 months a swollen joint count of 1 or 0 was present in 41% of the apremilast-treated patients, compared with none on methotrexate and 15% on a biologic agent.
A tender joint count of 0-1 was documented at 6 months in 24% of patients on apremilast, 13% with methotrexate, and 21% on a biologic agent. Apremilast’s numeric superiority in outcomes compared to methotrexate in this exploratory study wasn’t subjected to statistical analysis because of the small sample size. However, the ongoing phase 4, double-blind, placebo-controlled, multicenter FOREMOST trial in 330 patients with early oligoarticular PsA should provide more definitive efficacy data, noted Dr. Ogdie, a rheumatologist and epidemiologist at the University of Pennsylvania, Philadelphia.
RWCS program director Arthur Kavanaugh, MD, said, “The most recent EULAR [European Alliance of Associations for Rheumatology] PsA guidelines totally discount apremilast, and I think mostly on the basis of cost, but then they also say that in groups of people it’s not as effective as methotrexate.”
“This study shows to me that, even though it’s a registry, with all the caveats about getting data from registries, apremilast certainly can be an effective drug,” said Dr. Kavanaugh, a rheumatologist and professor of medicine at the University of California, San Diego.
Another valuable piece of information from the CORRONA analysis is that it zeros in on patients with oligoarticular PsA.
“Almost all of our PsA studies are focused on people with polyarticular disease. What about those who have lesser involvement? That, of course, is important in the clinic,” he noted.
Dr. Ogdie concurred.
“If we study only polyarticular disease and we make all of our assumptions based on polyarticular disease, we might be leaving out at least half of the patients with PsA. And those patients may not need a bigger gun. Apremilast and methotrexate are kind of in the same group for that mild oligoarticular disease, and they probably work just fine,” she said.
A final point: “We really don’t have good outcome measures to study oligoarticular disease well. The ACR20 is not good because a 20% improvement in three joints is not readily measurable. That’s why trialists enroll patients with high joint count numbers,” according to the rheumatologist.
No increased risk of solid cancers in PsA patients treated with TNF inhibitors
A new analysis of clinical rheumatology registries in five Nordic countries finally puts to rest any concerns that treatment of PsA with TNF inhibitors is associated with increased risk of solid cancers. The same group previously reported no link between TNF inhibitors and lymphoma in PsA.
The solid cancers study included 9,655 PsA patients who started a first TNF inhibitor during 2001-2017, 14,809 others not treated with biologics, and 31,350 matched general population controls. Linkage to Swedish, Norwegian, Danish, Icelandic, and Finnish national cancer registries showed that the adjusted risk for solid cancer in TNF inhibitor–treated, compared with biologic-naive PsA patients, was 1.0. Similarly, the pooled standardized incidence ratio of solid cancer in TNF inhibitor–treated PsA patients compared to the general population was 1.0. There was no signal of a differential risk for incident cancer for any of the eight malignancies studied: lung, colorectal, breast, prostate, uterine, brain, liver, and pancreatic cancer.
“I like this study a lot because it’s specific to PsA rather than extrapolating from rheumatoid arthritis data, where we have a bunch more information for a much longer period of time, but it’s a different population,” Dr. Kavanaugh said.
Dr. Ogdie said, “I talk to my patients about this particular study or the same group’s earlier lymphoma study all the time.”
“I have to say, these are important data for the dermatology world because there are dermatologists who are still not convinced that TNF inhibitors don’t have an increased risk of malignancy. This kind of information is going to be helpful,” observed Eric M. Ruderman, MD, professor of medicine (rheumatology) at Northwestern University, Chicago.
Greater efficacy for biologics in males than females with PsA?
A secondary analysis of the phase 3b EXCEED trial raised the intriguing possibility that both secukinumab, an interleukin-17A inhibitor, and adalimumab, a TNF inhibitor, have greater efficacy in men than in women with PsA. In this randomized trial of 853 biologic-naive patients with PsA, the ACR20 response rate to secukinumab at week 52 was 61% in females versus 74% in males, with ACR50 rates of 43% in females and 55.3% in males. The ACR20 rate with adalimumab was 51.5% in females and 70.2% in males. Similarly, the corresponding ACR50s were 32.6% and 55.3%, respectively. Minimal disease activity was achieved in 36.2% of women and 51% of men on secukinumab, and in 24.2% of women and 49.8% of men on adalimumab.
“These are randomized patients, so you really shouldn’t see these big differences in minimal disease activity,” Dr. Ogdie noted. “The question is why do men seem to respond better to therapy than women? I don’t think it’s the fibromyalgia-ness. There’s probably some biologic rationale for this that we just don’t understand yet. Maybe hormonal interactions.”
This gender difference in response is an important issue because it can potentially distort outcomes in head-to-head drug trials, Dr. Ruderman added.
“That gender difference is not likely to be huge if you’re looking at a placebo-controlled trial because the difference between the active drug and placebo is going to outweigh it. But when you have two active drugs, if there’s an imbalance in terms of how many men or women there are on each of the two drugs, you may end up with an efficacy difference that’s not real but is based on gender and not response to the drug,” he explained.
Roy M. Fleischmann, MD, a rheumatologist and clinical trialist at the University of Texas, Dallas, rose from the audience to pronounce the EXCEED male-versus-female analysis “very interesting.”
“We should go back and look at other trials and see if that occurred, and if it did, then we have to think about that going forward,” he proposed.
Dr. Ogdie, Dr. Kavanaugh, and Dr. Ruderman reported having financial relationships with numerous pharmaceutical companies.
Apremilast (Otezla) monotherapy may be an effective option in oligoarticular psoriatic arthritis, Alexis R. Ogdie, MD, reported at the 2021 Rheumatology Winter Clinical Symposium.
Her analysis of apremilast data from the CORRONA Registry was among several recent highlights in psoriatic arthritis (PsA) cited by speakers at the meeting. Other significant developments included a large pan-Scandinavian study that reassuringly found no increased risk of solid cancers in tumor necrosis factor (TNF) inhibitor–treated patients with PsA, and evidence to suggest a sex difference in the efficacy of both secukinumab (Cosentyx) and adalimumab (Humira), with men responding better than women to two biologics with differing mechanisms of action.
A role for apremilast in oligoarticular disease?
Dr. Ogdie presented an analysis of 150 patients in the U.S. observational CORRONA Registry who initiated monotherapy for oligoarticular PsA and were followed for 6 months. Thirty-four started on apremilast, 15 on methotrexate, and 101 on a biologic. Even though the apremilast group had higher baseline disease activity than did those who started on methotrexate, at 6 months a swollen joint count of 1 or 0 was present in 41% of the apremilast-treated patients, compared with none on methotrexate and 15% on a biologic agent.
A tender joint count of 0-1 was documented at 6 months in 24% of patients on apremilast, 13% with methotrexate, and 21% on a biologic agent. Apremilast’s numeric superiority in outcomes compared to methotrexate in this exploratory study wasn’t subjected to statistical analysis because of the small sample size. However, the ongoing phase 4, double-blind, placebo-controlled, multicenter FOREMOST trial in 330 patients with early oligoarticular PsA should provide more definitive efficacy data, noted Dr. Ogdie, a rheumatologist and epidemiologist at the University of Pennsylvania, Philadelphia.
RWCS program director Arthur Kavanaugh, MD, said, “The most recent EULAR [European Alliance of Associations for Rheumatology] PsA guidelines totally discount apremilast, and I think mostly on the basis of cost, but then they also say that in groups of people it’s not as effective as methotrexate.”
“This study shows to me that, even though it’s a registry, with all the caveats about getting data from registries, apremilast certainly can be an effective drug,” said Dr. Kavanaugh, a rheumatologist and professor of medicine at the University of California, San Diego.
Another valuable piece of information from the CORRONA analysis is that it zeros in on patients with oligoarticular PsA.
“Almost all of our PsA studies are focused on people with polyarticular disease. What about those who have lesser involvement? That, of course, is important in the clinic,” he noted.
Dr. Ogdie concurred.
“If we study only polyarticular disease and we make all of our assumptions based on polyarticular disease, we might be leaving out at least half of the patients with PsA. And those patients may not need a bigger gun. Apremilast and methotrexate are kind of in the same group for that mild oligoarticular disease, and they probably work just fine,” she said.
A final point: “We really don’t have good outcome measures to study oligoarticular disease well. The ACR20 is not good because a 20% improvement in three joints is not readily measurable. That’s why trialists enroll patients with high joint count numbers,” according to the rheumatologist.
No increased risk of solid cancers in PsA patients treated with TNF inhibitors
A new analysis of clinical rheumatology registries in five Nordic countries finally puts to rest any concerns that treatment of PsA with TNF inhibitors is associated with increased risk of solid cancers. The same group previously reported no link between TNF inhibitors and lymphoma in PsA.
The solid cancers study included 9,655 PsA patients who started a first TNF inhibitor during 2001-2017, 14,809 others not treated with biologics, and 31,350 matched general population controls. Linkage to Swedish, Norwegian, Danish, Icelandic, and Finnish national cancer registries showed that the adjusted risk for solid cancer in TNF inhibitor–treated, compared with biologic-naive PsA patients, was 1.0. Similarly, the pooled standardized incidence ratio of solid cancer in TNF inhibitor–treated PsA patients compared to the general population was 1.0. There was no signal of a differential risk for incident cancer for any of the eight malignancies studied: lung, colorectal, breast, prostate, uterine, brain, liver, and pancreatic cancer.
“I like this study a lot because it’s specific to PsA rather than extrapolating from rheumatoid arthritis data, where we have a bunch more information for a much longer period of time, but it’s a different population,” Dr. Kavanaugh said.
Dr. Ogdie said, “I talk to my patients about this particular study or the same group’s earlier lymphoma study all the time.”
“I have to say, these are important data for the dermatology world because there are dermatologists who are still not convinced that TNF inhibitors don’t have an increased risk of malignancy. This kind of information is going to be helpful,” observed Eric M. Ruderman, MD, professor of medicine (rheumatology) at Northwestern University, Chicago.
Greater efficacy for biologics in males than females with PsA?
A secondary analysis of the phase 3b EXCEED trial raised the intriguing possibility that both secukinumab, an interleukin-17A inhibitor, and adalimumab, a TNF inhibitor, have greater efficacy in men than in women with PsA. In this randomized trial of 853 biologic-naive patients with PsA, the ACR20 response rate to secukinumab at week 52 was 61% in females versus 74% in males, with ACR50 rates of 43% in females and 55.3% in males. The ACR20 rate with adalimumab was 51.5% in females and 70.2% in males. Similarly, the corresponding ACR50s were 32.6% and 55.3%, respectively. Minimal disease activity was achieved in 36.2% of women and 51% of men on secukinumab, and in 24.2% of women and 49.8% of men on adalimumab.
“These are randomized patients, so you really shouldn’t see these big differences in minimal disease activity,” Dr. Ogdie noted. “The question is why do men seem to respond better to therapy than women? I don’t think it’s the fibromyalgia-ness. There’s probably some biologic rationale for this that we just don’t understand yet. Maybe hormonal interactions.”
This gender difference in response is an important issue because it can potentially distort outcomes in head-to-head drug trials, Dr. Ruderman added.
“That gender difference is not likely to be huge if you’re looking at a placebo-controlled trial because the difference between the active drug and placebo is going to outweigh it. But when you have two active drugs, if there’s an imbalance in terms of how many men or women there are on each of the two drugs, you may end up with an efficacy difference that’s not real but is based on gender and not response to the drug,” he explained.
Roy M. Fleischmann, MD, a rheumatologist and clinical trialist at the University of Texas, Dallas, rose from the audience to pronounce the EXCEED male-versus-female analysis “very interesting.”
“We should go back and look at other trials and see if that occurred, and if it did, then we have to think about that going forward,” he proposed.
Dr. Ogdie, Dr. Kavanaugh, and Dr. Ruderman reported having financial relationships with numerous pharmaceutical companies.
FROM RWCS 2021
In-hospital mobility impairment in older MI patients predicts postdischarge functional decline
Background: The ability to independently perform daily activities is highly valued by patients, yet it is commonly impaired in older adults after hospitalization for MI. Risk of functional decline in this population is not well understood, but may relate to reduced mobility while hospitalized.
Study design: Prospective cohort.
Setting: A total of 94 academic and community hospitals in the United States.
Synopsis: More than 3,000 adults aged 75 years and older who were hospitalized for acute myocardial infarction were enrolled in the prospective cohort SILVER-AMI; 2,587 patients within this cohort were evaluated for in-hospital mobility with the Timed “Up and Go” test. At 6-month follow-up, loss of independent performance of activities of daily living (ADL) and of the ability to walk 0.25 miles were both associated in a dose-dependent manner with in-hospital mobility. Severe in-hospital mobility impairment was associated with ADL decline with an adjusted odds ratio of 5.45 (95% confidence interval, 3.29-9.01).
While in-hospital mobility is predictive of future functional decline in this population, this observational study cannot establish whether attempts to improve mobility in hospitalized patients will prevent future functional decline.
Bottom line: Lower performance on the Timed “Up and Go” test of mobility among older patients hospitalized for MI is associated with functional decline 6 months after hospitalization.
Citation: Hajduk AM et al. Association between mobility measured during hospitalization and functional outcomes in older adults with acute myocardial infarction in the SILVER-AMI study. JAMA Intern Med. 2019 Oct 7. doi: 10.1001/jamainternmed.2019.4114.
Dr. Gerstenberger is a hospitalist and clinical assistant professor of medicine at the University of Utah, Salt Lake City.
Background: The ability to independently perform daily activities is highly valued by patients, yet it is commonly impaired in older adults after hospitalization for MI. Risk of functional decline in this population is not well understood, but may relate to reduced mobility while hospitalized.
Study design: Prospective cohort.
Setting: A total of 94 academic and community hospitals in the United States.
Synopsis: More than 3,000 adults aged 75 years and older who were hospitalized for acute myocardial infarction were enrolled in the prospective cohort SILVER-AMI; 2,587 patients within this cohort were evaluated for in-hospital mobility with the Timed “Up and Go” test. At 6-month follow-up, loss of independent performance of activities of daily living (ADL) and of the ability to walk 0.25 miles were both associated in a dose-dependent manner with in-hospital mobility. Severe in-hospital mobility impairment was associated with ADL decline with an adjusted odds ratio of 5.45 (95% confidence interval, 3.29-9.01).
While in-hospital mobility is predictive of future functional decline in this population, this observational study cannot establish whether attempts to improve mobility in hospitalized patients will prevent future functional decline.
Bottom line: Lower performance on the Timed “Up and Go” test of mobility among older patients hospitalized for MI is associated with functional decline 6 months after hospitalization.
Citation: Hajduk AM et al. Association between mobility measured during hospitalization and functional outcomes in older adults with acute myocardial infarction in the SILVER-AMI study. JAMA Intern Med. 2019 Oct 7. doi: 10.1001/jamainternmed.2019.4114.
Dr. Gerstenberger is a hospitalist and clinical assistant professor of medicine at the University of Utah, Salt Lake City.
Background: The ability to independently perform daily activities is highly valued by patients, yet it is commonly impaired in older adults after hospitalization for MI. Risk of functional decline in this population is not well understood, but may relate to reduced mobility while hospitalized.
Study design: Prospective cohort.
Setting: A total of 94 academic and community hospitals in the United States.
Synopsis: More than 3,000 adults aged 75 years and older who were hospitalized for acute myocardial infarction were enrolled in the prospective cohort SILVER-AMI; 2,587 patients within this cohort were evaluated for in-hospital mobility with the Timed “Up and Go” test. At 6-month follow-up, loss of independent performance of activities of daily living (ADL) and of the ability to walk 0.25 miles were both associated in a dose-dependent manner with in-hospital mobility. Severe in-hospital mobility impairment was associated with ADL decline with an adjusted odds ratio of 5.45 (95% confidence interval, 3.29-9.01).
While in-hospital mobility is predictive of future functional decline in this population, this observational study cannot establish whether attempts to improve mobility in hospitalized patients will prevent future functional decline.
Bottom line: Lower performance on the Timed “Up and Go” test of mobility among older patients hospitalized for MI is associated with functional decline 6 months after hospitalization.
Citation: Hajduk AM et al. Association between mobility measured during hospitalization and functional outcomes in older adults with acute myocardial infarction in the SILVER-AMI study. JAMA Intern Med. 2019 Oct 7. doi: 10.1001/jamainternmed.2019.4114.
Dr. Gerstenberger is a hospitalist and clinical assistant professor of medicine at the University of Utah, Salt Lake City.
Huge, struggling breast cancer screening trial gets lifeline
But mammography trends can’t be ignored.
A controversial, big-budget breast cancer screening trial that has been chronically unable to attract enough female participants since its debut in 2017 got a vote of confidence from a special working group of the National Cancer Institute (NCI) on March 17.
The Tomosynthesis Mammography Imaging Screening Trial (TMIST) should continue, but with modification, the expert group concluded in its report.
The randomized trial, with an estimated cost of $100 million, compares two kinds of mammography screenings for breast cancer in healthy women. One group of patients is screened with digital breast tomosynthesis, also known as 3-D mammography; the other is screened with the older, less expensive 2-D digital technology.
Tomosynthesis is already considered superior in detecting small cancers and reducing callbacks and is increasingly being used in the real world, leading some experts in the field to say that TMIST is critically hampered by women’s and radiologists’ preference for 3-D mammography.
At a meeting of an NCI advisory board in September 2020, there was a suggestion that the federal agency may kill the trial.
But at the latest meeting, the working group proposed that the trial should live on.
One of the main problems with the trial has been recruitment; the recommended changes discussed at the meeting include reducing the number of women needed in the study (from 165,000 to 102,000), which would allow patient “accrual to be completed more quickly,” the working group noted. In addition, the target date for completing patient accrual would be moved to 2023, 3 years after the original completion date of 2020.
The group’s recommendations now go to NCI staff for scientific review. The NCI will then decide about implementing the proposed changes.
The trial, which is the NCI’s largest and most expensive screening study, has never come close to targeted monthly enrollment. It was enrolling fewer than 1,500 patients per month over a 2-year period, instead of the projected 5,500 per month, Philip Castle, PhD, MPH, director of the NCI’s Division of Cancer Prevention, said last year. He called for a review of TMIST’s “feasibility and relevance” in view of the increasing use of tomosynthesis in the United States, as well as other factors.
The new technology has been “rapidly adopted” by facilities in North America, the working group noted. As of December 2020, approximately 74% of breast cancer screening clinics in the United States had at least one tomosynthesis or 3-D system; 42% of the mammography machines were 3-D.
This trend of increasing use of 3-D machines might be too much for TMIST to surmount, said Nancy Davidson, MD, of the Fred Hutchinson Cancer Research Center, in Seattle, who chaired the working group.
“We are worried the challenges [to patient accrual] may persist due to the increasing adoption of three-dimensional breast tomosynthesis in the United States over time,” she said during the working group’s virtual presentation of the report to the NCI’s Clinical Trials and Translational Research Advisory Committee.
Committee member Smita Bhatia, MD, PhD, of the University of Alabama at Birmingham, wondered, “What are the ongoing barriers that [TMIST investigators] are going to face beside recruitment?”
Dr. Davidson answered by speaking, again, about market penetration of tomosynthesis machines and suggested that the recruitment problems and the availability of 3-D mammography are intertwined.
“Is this a technology that has or will arrive, at which point it may not be very easy to put the genie back in the bottle?” she asked.
That question has already been answered – widespread use of tomosynthesis is here to stay, argued Daniel Kopans, MD, of Harvard Medical School, Boston, who invented digital breast tomosynthesis but no longer benefits financially from his invention because the patent has expired.
“The horse is out of the barn,” Dr. Kopans said in an interview. By the time the study results are available, digital mammography will be a tool of the past, he said.
TMIST is a trial “that should never have been started in the first place, and it’s failing,” he said. “I was hoping they [the NCI] would say, ‘Let’s stop this because there’s not enough accrual.’ But it looks like they’re not.”
“TMIST is a waste of money,” said Dr. Kopans, repeating a criticism he has made in the past.
A drop in study power
The new recommendations for TMIST come about 1 year after Medscape Medical News reported that the study was lagging in enrollment of both patients and participating sites/physicians.
Last year, two TMIST study investigators said it had been difficult enlisting sites, in part because many radiologists and facilities – informed by their experience and previous research results – already believe that the 3-D technology is superior.
Currently, most 3-D systems are used in conjunction with 2-D. First, two static images of the breast are taken (2-D), and then the unit moves in an arc, taking multiple images of the breast (3-D). Thus, 3-D is widely described as allowing clinicians to flip through the images like “pages in a book” and as offering a superior read of the breast.
The NCI working group concedes that “there is evidence that screening utilizing tomosynthesis may reduce recall rates and improve cancer detection,” but it says the trial is needed to address “questions that still remain regarding the overall benefit to patients.”
Furthermore, tomosynthesis “may carry higher out-of-pocket costs for women and is more labor intensive and costly for health care systems in that it requires about twice as much reader time for interpretation,” the working group said.
The “main hypothesis of TMIST” is that “tomosynthesis will decrease the cumulative incidence of advanced breast cancers, a surrogate for mortality, compared to standard digital mammography,” posits the group.
Advanced breast cancer is defined in TMIST as invasive breast cancers that meet any of the following criteria:
- Distant metastases
- At least one lymph node macrometastasis
- Tumor size >1 cm and triple-negative or positive for human epidermal growth factor receptor
- Tumor size ≥ 20 mm unless of pure mucinous or other favorable histologies.
In the original study design, the sample size was estimated to be sufficient to provide 90% power to detect a 20% relative reduction in the proportion of advanced cancers in the intervention arm (tomosynthesis, or 3-D) compared to the control arm (digital mammography, or 2-D) 4.5 years from randomization.
Now, with fewer patients and a revised analytic approach, the study’s statistical power will be decreased to 80% from the original 90%.
Also, an advanced cancer is counted “if it occurs at any time while the participant is on study.”
Dr. Kopans says that is a problem.
“That is a huge mistake, since digital breast tomosynthesis cannot impact prevalent cancers. They are already there. This means that their ‘power calculation’ is wrong, and they won’t have the power that they are claiming,” he said.
Dr. Kopans explained that the first screen in TMIST will have “no effect on the number of advanced cancers.” That’s because the cancers will have already grown enough to become advanced, he said.
On the other hand, an initial screening might detect and thus lead to the removal of nonadvanced, smaller cancers, which, had they not been detected and removed, would have grown to become advanced cancers by the next year. Thus, the screenings done after the first year are the ones that potentially prove the effect of the intervention.
However, the working group report says that changes to the study will not affect anything other than a 10% reduction in the study’s power.
The working group is concerned about TMIST going on for years and years. For that reason, they recommended that the NCI establish “strict criteria for termination of the study” if accrual goals are not met. However, those parameters have not been developed, and it was not part of the study group’s mission to establish them.
The working group was sponsored by the NCI. Dr. Kopans reports consulting with DART Imaging in China.
A version of this article first appeared on Medscape.com.
But mammography trends can’t be ignored.
But mammography trends can’t be ignored.
A controversial, big-budget breast cancer screening trial that has been chronically unable to attract enough female participants since its debut in 2017 got a vote of confidence from a special working group of the National Cancer Institute (NCI) on March 17.
The Tomosynthesis Mammography Imaging Screening Trial (TMIST) should continue, but with modification, the expert group concluded in its report.
The randomized trial, with an estimated cost of $100 million, compares two kinds of mammography screenings for breast cancer in healthy women. One group of patients is screened with digital breast tomosynthesis, also known as 3-D mammography; the other is screened with the older, less expensive 2-D digital technology.
Tomosynthesis is already considered superior in detecting small cancers and reducing callbacks and is increasingly being used in the real world, leading some experts in the field to say that TMIST is critically hampered by women’s and radiologists’ preference for 3-D mammography.
At a meeting of an NCI advisory board in September 2020, there was a suggestion that the federal agency may kill the trial.
But at the latest meeting, the working group proposed that the trial should live on.
One of the main problems with the trial has been recruitment; the recommended changes discussed at the meeting include reducing the number of women needed in the study (from 165,000 to 102,000), which would allow patient “accrual to be completed more quickly,” the working group noted. In addition, the target date for completing patient accrual would be moved to 2023, 3 years after the original completion date of 2020.
The group’s recommendations now go to NCI staff for scientific review. The NCI will then decide about implementing the proposed changes.
The trial, which is the NCI’s largest and most expensive screening study, has never come close to targeted monthly enrollment. It was enrolling fewer than 1,500 patients per month over a 2-year period, instead of the projected 5,500 per month, Philip Castle, PhD, MPH, director of the NCI’s Division of Cancer Prevention, said last year. He called for a review of TMIST’s “feasibility and relevance” in view of the increasing use of tomosynthesis in the United States, as well as other factors.
The new technology has been “rapidly adopted” by facilities in North America, the working group noted. As of December 2020, approximately 74% of breast cancer screening clinics in the United States had at least one tomosynthesis or 3-D system; 42% of the mammography machines were 3-D.
This trend of increasing use of 3-D machines might be too much for TMIST to surmount, said Nancy Davidson, MD, of the Fred Hutchinson Cancer Research Center, in Seattle, who chaired the working group.
“We are worried the challenges [to patient accrual] may persist due to the increasing adoption of three-dimensional breast tomosynthesis in the United States over time,” she said during the working group’s virtual presentation of the report to the NCI’s Clinical Trials and Translational Research Advisory Committee.
Committee member Smita Bhatia, MD, PhD, of the University of Alabama at Birmingham, wondered, “What are the ongoing barriers that [TMIST investigators] are going to face beside recruitment?”
Dr. Davidson answered by speaking, again, about market penetration of tomosynthesis machines and suggested that the recruitment problems and the availability of 3-D mammography are intertwined.
“Is this a technology that has or will arrive, at which point it may not be very easy to put the genie back in the bottle?” she asked.
That question has already been answered – widespread use of tomosynthesis is here to stay, argued Daniel Kopans, MD, of Harvard Medical School, Boston, who invented digital breast tomosynthesis but no longer benefits financially from his invention because the patent has expired.
“The horse is out of the barn,” Dr. Kopans said in an interview. By the time the study results are available, digital mammography will be a tool of the past, he said.
TMIST is a trial “that should never have been started in the first place, and it’s failing,” he said. “I was hoping they [the NCI] would say, ‘Let’s stop this because there’s not enough accrual.’ But it looks like they’re not.”
“TMIST is a waste of money,” said Dr. Kopans, repeating a criticism he has made in the past.
A drop in study power
The new recommendations for TMIST come about 1 year after Medscape Medical News reported that the study was lagging in enrollment of both patients and participating sites/physicians.
Last year, two TMIST study investigators said it had been difficult enlisting sites, in part because many radiologists and facilities – informed by their experience and previous research results – already believe that the 3-D technology is superior.
Currently, most 3-D systems are used in conjunction with 2-D. First, two static images of the breast are taken (2-D), and then the unit moves in an arc, taking multiple images of the breast (3-D). Thus, 3-D is widely described as allowing clinicians to flip through the images like “pages in a book” and as offering a superior read of the breast.
The NCI working group concedes that “there is evidence that screening utilizing tomosynthesis may reduce recall rates and improve cancer detection,” but it says the trial is needed to address “questions that still remain regarding the overall benefit to patients.”
Furthermore, tomosynthesis “may carry higher out-of-pocket costs for women and is more labor intensive and costly for health care systems in that it requires about twice as much reader time for interpretation,” the working group said.
The “main hypothesis of TMIST” is that “tomosynthesis will decrease the cumulative incidence of advanced breast cancers, a surrogate for mortality, compared to standard digital mammography,” posits the group.
Advanced breast cancer is defined in TMIST as invasive breast cancers that meet any of the following criteria:
- Distant metastases
- At least one lymph node macrometastasis
- Tumor size >1 cm and triple-negative or positive for human epidermal growth factor receptor
- Tumor size ≥ 20 mm unless of pure mucinous or other favorable histologies.
In the original study design, the sample size was estimated to be sufficient to provide 90% power to detect a 20% relative reduction in the proportion of advanced cancers in the intervention arm (tomosynthesis, or 3-D) compared to the control arm (digital mammography, or 2-D) 4.5 years from randomization.
Now, with fewer patients and a revised analytic approach, the study’s statistical power will be decreased to 80% from the original 90%.
Also, an advanced cancer is counted “if it occurs at any time while the participant is on study.”
Dr. Kopans says that is a problem.
“That is a huge mistake, since digital breast tomosynthesis cannot impact prevalent cancers. They are already there. This means that their ‘power calculation’ is wrong, and they won’t have the power that they are claiming,” he said.
Dr. Kopans explained that the first screen in TMIST will have “no effect on the number of advanced cancers.” That’s because the cancers will have already grown enough to become advanced, he said.
On the other hand, an initial screening might detect and thus lead to the removal of nonadvanced, smaller cancers, which, had they not been detected and removed, would have grown to become advanced cancers by the next year. Thus, the screenings done after the first year are the ones that potentially prove the effect of the intervention.
However, the working group report says that changes to the study will not affect anything other than a 10% reduction in the study’s power.
The working group is concerned about TMIST going on for years and years. For that reason, they recommended that the NCI establish “strict criteria for termination of the study” if accrual goals are not met. However, those parameters have not been developed, and it was not part of the study group’s mission to establish them.
The working group was sponsored by the NCI. Dr. Kopans reports consulting with DART Imaging in China.
A version of this article first appeared on Medscape.com.
A controversial, big-budget breast cancer screening trial that has been chronically unable to attract enough female participants since its debut in 2017 got a vote of confidence from a special working group of the National Cancer Institute (NCI) on March 17.
The Tomosynthesis Mammography Imaging Screening Trial (TMIST) should continue, but with modification, the expert group concluded in its report.
The randomized trial, with an estimated cost of $100 million, compares two kinds of mammography screenings for breast cancer in healthy women. One group of patients is screened with digital breast tomosynthesis, also known as 3-D mammography; the other is screened with the older, less expensive 2-D digital technology.
Tomosynthesis is already considered superior in detecting small cancers and reducing callbacks and is increasingly being used in the real world, leading some experts in the field to say that TMIST is critically hampered by women’s and radiologists’ preference for 3-D mammography.
At a meeting of an NCI advisory board in September 2020, there was a suggestion that the federal agency may kill the trial.
But at the latest meeting, the working group proposed that the trial should live on.
One of the main problems with the trial has been recruitment; the recommended changes discussed at the meeting include reducing the number of women needed in the study (from 165,000 to 102,000), which would allow patient “accrual to be completed more quickly,” the working group noted. In addition, the target date for completing patient accrual would be moved to 2023, 3 years after the original completion date of 2020.
The group’s recommendations now go to NCI staff for scientific review. The NCI will then decide about implementing the proposed changes.
The trial, which is the NCI’s largest and most expensive screening study, has never come close to targeted monthly enrollment. It was enrolling fewer than 1,500 patients per month over a 2-year period, instead of the projected 5,500 per month, Philip Castle, PhD, MPH, director of the NCI’s Division of Cancer Prevention, said last year. He called for a review of TMIST’s “feasibility and relevance” in view of the increasing use of tomosynthesis in the United States, as well as other factors.
The new technology has been “rapidly adopted” by facilities in North America, the working group noted. As of December 2020, approximately 74% of breast cancer screening clinics in the United States had at least one tomosynthesis or 3-D system; 42% of the mammography machines were 3-D.
This trend of increasing use of 3-D machines might be too much for TMIST to surmount, said Nancy Davidson, MD, of the Fred Hutchinson Cancer Research Center, in Seattle, who chaired the working group.
“We are worried the challenges [to patient accrual] may persist due to the increasing adoption of three-dimensional breast tomosynthesis in the United States over time,” she said during the working group’s virtual presentation of the report to the NCI’s Clinical Trials and Translational Research Advisory Committee.
Committee member Smita Bhatia, MD, PhD, of the University of Alabama at Birmingham, wondered, “What are the ongoing barriers that [TMIST investigators] are going to face beside recruitment?”
Dr. Davidson answered by speaking, again, about market penetration of tomosynthesis machines and suggested that the recruitment problems and the availability of 3-D mammography are intertwined.
“Is this a technology that has or will arrive, at which point it may not be very easy to put the genie back in the bottle?” she asked.
That question has already been answered – widespread use of tomosynthesis is here to stay, argued Daniel Kopans, MD, of Harvard Medical School, Boston, who invented digital breast tomosynthesis but no longer benefits financially from his invention because the patent has expired.
“The horse is out of the barn,” Dr. Kopans said in an interview. By the time the study results are available, digital mammography will be a tool of the past, he said.
TMIST is a trial “that should never have been started in the first place, and it’s failing,” he said. “I was hoping they [the NCI] would say, ‘Let’s stop this because there’s not enough accrual.’ But it looks like they’re not.”
“TMIST is a waste of money,” said Dr. Kopans, repeating a criticism he has made in the past.
A drop in study power
The new recommendations for TMIST come about 1 year after Medscape Medical News reported that the study was lagging in enrollment of both patients and participating sites/physicians.
Last year, two TMIST study investigators said it had been difficult enlisting sites, in part because many radiologists and facilities – informed by their experience and previous research results – already believe that the 3-D technology is superior.
Currently, most 3-D systems are used in conjunction with 2-D. First, two static images of the breast are taken (2-D), and then the unit moves in an arc, taking multiple images of the breast (3-D). Thus, 3-D is widely described as allowing clinicians to flip through the images like “pages in a book” and as offering a superior read of the breast.
The NCI working group concedes that “there is evidence that screening utilizing tomosynthesis may reduce recall rates and improve cancer detection,” but it says the trial is needed to address “questions that still remain regarding the overall benefit to patients.”
Furthermore, tomosynthesis “may carry higher out-of-pocket costs for women and is more labor intensive and costly for health care systems in that it requires about twice as much reader time for interpretation,” the working group said.
The “main hypothesis of TMIST” is that “tomosynthesis will decrease the cumulative incidence of advanced breast cancers, a surrogate for mortality, compared to standard digital mammography,” posits the group.
Advanced breast cancer is defined in TMIST as invasive breast cancers that meet any of the following criteria:
- Distant metastases
- At least one lymph node macrometastasis
- Tumor size >1 cm and triple-negative or positive for human epidermal growth factor receptor
- Tumor size ≥ 20 mm unless of pure mucinous or other favorable histologies.
In the original study design, the sample size was estimated to be sufficient to provide 90% power to detect a 20% relative reduction in the proportion of advanced cancers in the intervention arm (tomosynthesis, or 3-D) compared to the control arm (digital mammography, or 2-D) 4.5 years from randomization.
Now, with fewer patients and a revised analytic approach, the study’s statistical power will be decreased to 80% from the original 90%.
Also, an advanced cancer is counted “if it occurs at any time while the participant is on study.”
Dr. Kopans says that is a problem.
“That is a huge mistake, since digital breast tomosynthesis cannot impact prevalent cancers. They are already there. This means that their ‘power calculation’ is wrong, and they won’t have the power that they are claiming,” he said.
Dr. Kopans explained that the first screen in TMIST will have “no effect on the number of advanced cancers.” That’s because the cancers will have already grown enough to become advanced, he said.
On the other hand, an initial screening might detect and thus lead to the removal of nonadvanced, smaller cancers, which, had they not been detected and removed, would have grown to become advanced cancers by the next year. Thus, the screenings done after the first year are the ones that potentially prove the effect of the intervention.
However, the working group report says that changes to the study will not affect anything other than a 10% reduction in the study’s power.
The working group is concerned about TMIST going on for years and years. For that reason, they recommended that the NCI establish “strict criteria for termination of the study” if accrual goals are not met. However, those parameters have not been developed, and it was not part of the study group’s mission to establish them.
The working group was sponsored by the NCI. Dr. Kopans reports consulting with DART Imaging in China.
A version of this article first appeared on Medscape.com.
A Rising Tide: No Hospital Is an Island Unto Itself in the Era of COVID-19
The early phase of the COVID-19 pandemic was an extraordinarily uncertain, yet innovative, time.1 Few data describe site-level effects of the many adaptations made to deal with surging case numbers, but studies of larger hospital referral regions (HRR) provide important clues.
In this issue of the Journal of Hospital Medicine, Janke et al2 describe how availability of hospital resources in a region relate to COVID-19 mortality between March and June 2020.The authors’ findings suggest that, at least for early periods of the pandemic, having more intensive care unit (ICU), hospital bed, or nursing capacity per COVID-19 case was associated with lower mortality, while physician availability was not. Moreover, months later there were no associations between service or physician availability and HRR COVID-19 mortality. The authors observed variations in mortality rates in places commonly thought to have been overwhelmed early in the pandemic (April 2020), as well as in cities (Boston, Philadelphia, Hartford, Detroit, and Camden, New Jersey) that had a less prominent place in the news at that time.
Larger hospitals tend to have the resources necessary to make wholesale changes when preparing for a pandemic wave. Thus, Janke et al’s results may not have fully captured the pandemic’s potential impact in settings with fewer resources or in smaller hospitals, which are currently being overwhelmed.3
The number of cases and hospitalizations in this third wave of COVID-19 continues to rise, and the strain on healthcare resources has been felt across entire regions, making the results of this study even more salient. Hospital outcomes for COVID-19 are sensitive to limitations in physical locations (number of beds, ICU capacity) and nursing capacity. Nurses more often are assigned specifically to a bed or unit, and the number of patients per nurse is limited by state or local statute. Innovations such as COVID-19 field hospitals or redeploying existing beds (eg, converting postanesthesia care units to ICUs) offset physically constrained resources.4 On the other hand, lower acuity in this phase of the pandemic (eg, fewer ICU admissions) and shorter lengths of stay may produce higher turnover, producing more workforce stress, regardless of bed availability.
Early work of our COVID-19 collaborative5 suggests that the focus on localizing patients to geographic units or teams has given way to strategies that utilize more flexible team and bed-finding approaches. Clinical care has evolved to focus on more aggressive discharge strategies, with remote monitoring and hospital-at-home capabilities. Overall, the pandemic is providing fodder for future studies examining interaction between case volumes, physician and nurse availability, and evolution in clinical care practices. Most critically, it provides an opportunity to study health system flexibility and robustness with a lens that incorporates a view of the hospital and its surroundings as tightly related parts of care delivery. Because if there is one thing the pandemic is teaching us, it is that, more than ever, no hospital can be an island unto itself, and each hospital is part of a larger ecosystem where rising tides are felt throughout.
1. Auerbach A, O’Leary KJ, Greysen SR, et al; HOMERuN COVID-19 Collaborative Group. Hospital ward adaptation during the COVID-19 pandemic: a national survey of academic medical centers. J Hosp Med. 2020;15(8):483-488. https://doi.org/10.12788/jhm.3476
2. Janke AT, Mei H, Rothenberg C, Becher RD, Lin Z, Venkatesh AK. Analysis of hospital resource availability and COVID-19 mortality across the United States. J Hosp Med. 2021;16(4):211-214.
3. Achenbach J, Brulliard K, Shammas B, Dupree J. Hospitals in nearly every region report a flood of covid-19 patients. Washington Post. October 26, 2020. Accessed March 4, 2021. https://www.washingtonpost.com/health/covid-hospitals-record-patients/2020/10/26/0bc362cc-17b2-11eb-befb-8864259bd2d8_story.html
4. Chaudhary MJ, Howell E, Ficke JR, et al. Caring for patients at a COVID-19 field hospital. J Hosp Med. 2021;16(2):117-119. https://doi.org/10.12788/jhm.3551
5. Welcome to the COVID-19 response working team knowledge base. HOMERun Hospital Medicine Reengineering Network COVID-19 Collaboration. Accessed March 4, 2021. https://www.hospitalinnovate.org/covid19/
The early phase of the COVID-19 pandemic was an extraordinarily uncertain, yet innovative, time.1 Few data describe site-level effects of the many adaptations made to deal with surging case numbers, but studies of larger hospital referral regions (HRR) provide important clues.
In this issue of the Journal of Hospital Medicine, Janke et al2 describe how availability of hospital resources in a region relate to COVID-19 mortality between March and June 2020.The authors’ findings suggest that, at least for early periods of the pandemic, having more intensive care unit (ICU), hospital bed, or nursing capacity per COVID-19 case was associated with lower mortality, while physician availability was not. Moreover, months later there were no associations between service or physician availability and HRR COVID-19 mortality. The authors observed variations in mortality rates in places commonly thought to have been overwhelmed early in the pandemic (April 2020), as well as in cities (Boston, Philadelphia, Hartford, Detroit, and Camden, New Jersey) that had a less prominent place in the news at that time.
Larger hospitals tend to have the resources necessary to make wholesale changes when preparing for a pandemic wave. Thus, Janke et al’s results may not have fully captured the pandemic’s potential impact in settings with fewer resources or in smaller hospitals, which are currently being overwhelmed.3
The number of cases and hospitalizations in this third wave of COVID-19 continues to rise, and the strain on healthcare resources has been felt across entire regions, making the results of this study even more salient. Hospital outcomes for COVID-19 are sensitive to limitations in physical locations (number of beds, ICU capacity) and nursing capacity. Nurses more often are assigned specifically to a bed or unit, and the number of patients per nurse is limited by state or local statute. Innovations such as COVID-19 field hospitals or redeploying existing beds (eg, converting postanesthesia care units to ICUs) offset physically constrained resources.4 On the other hand, lower acuity in this phase of the pandemic (eg, fewer ICU admissions) and shorter lengths of stay may produce higher turnover, producing more workforce stress, regardless of bed availability.
Early work of our COVID-19 collaborative5 suggests that the focus on localizing patients to geographic units or teams has given way to strategies that utilize more flexible team and bed-finding approaches. Clinical care has evolved to focus on more aggressive discharge strategies, with remote monitoring and hospital-at-home capabilities. Overall, the pandemic is providing fodder for future studies examining interaction between case volumes, physician and nurse availability, and evolution in clinical care practices. Most critically, it provides an opportunity to study health system flexibility and robustness with a lens that incorporates a view of the hospital and its surroundings as tightly related parts of care delivery. Because if there is one thing the pandemic is teaching us, it is that, more than ever, no hospital can be an island unto itself, and each hospital is part of a larger ecosystem where rising tides are felt throughout.
The early phase of the COVID-19 pandemic was an extraordinarily uncertain, yet innovative, time.1 Few data describe site-level effects of the many adaptations made to deal with surging case numbers, but studies of larger hospital referral regions (HRR) provide important clues.
In this issue of the Journal of Hospital Medicine, Janke et al2 describe how availability of hospital resources in a region relate to COVID-19 mortality between March and June 2020.The authors’ findings suggest that, at least for early periods of the pandemic, having more intensive care unit (ICU), hospital bed, or nursing capacity per COVID-19 case was associated with lower mortality, while physician availability was not. Moreover, months later there were no associations between service or physician availability and HRR COVID-19 mortality. The authors observed variations in mortality rates in places commonly thought to have been overwhelmed early in the pandemic (April 2020), as well as in cities (Boston, Philadelphia, Hartford, Detroit, and Camden, New Jersey) that had a less prominent place in the news at that time.
Larger hospitals tend to have the resources necessary to make wholesale changes when preparing for a pandemic wave. Thus, Janke et al’s results may not have fully captured the pandemic’s potential impact in settings with fewer resources or in smaller hospitals, which are currently being overwhelmed.3
The number of cases and hospitalizations in this third wave of COVID-19 continues to rise, and the strain on healthcare resources has been felt across entire regions, making the results of this study even more salient. Hospital outcomes for COVID-19 are sensitive to limitations in physical locations (number of beds, ICU capacity) and nursing capacity. Nurses more often are assigned specifically to a bed or unit, and the number of patients per nurse is limited by state or local statute. Innovations such as COVID-19 field hospitals or redeploying existing beds (eg, converting postanesthesia care units to ICUs) offset physically constrained resources.4 On the other hand, lower acuity in this phase of the pandemic (eg, fewer ICU admissions) and shorter lengths of stay may produce higher turnover, producing more workforce stress, regardless of bed availability.
Early work of our COVID-19 collaborative5 suggests that the focus on localizing patients to geographic units or teams has given way to strategies that utilize more flexible team and bed-finding approaches. Clinical care has evolved to focus on more aggressive discharge strategies, with remote monitoring and hospital-at-home capabilities. Overall, the pandemic is providing fodder for future studies examining interaction between case volumes, physician and nurse availability, and evolution in clinical care practices. Most critically, it provides an opportunity to study health system flexibility and robustness with a lens that incorporates a view of the hospital and its surroundings as tightly related parts of care delivery. Because if there is one thing the pandemic is teaching us, it is that, more than ever, no hospital can be an island unto itself, and each hospital is part of a larger ecosystem where rising tides are felt throughout.
1. Auerbach A, O’Leary KJ, Greysen SR, et al; HOMERuN COVID-19 Collaborative Group. Hospital ward adaptation during the COVID-19 pandemic: a national survey of academic medical centers. J Hosp Med. 2020;15(8):483-488. https://doi.org/10.12788/jhm.3476
2. Janke AT, Mei H, Rothenberg C, Becher RD, Lin Z, Venkatesh AK. Analysis of hospital resource availability and COVID-19 mortality across the United States. J Hosp Med. 2021;16(4):211-214.
3. Achenbach J, Brulliard K, Shammas B, Dupree J. Hospitals in nearly every region report a flood of covid-19 patients. Washington Post. October 26, 2020. Accessed March 4, 2021. https://www.washingtonpost.com/health/covid-hospitals-record-patients/2020/10/26/0bc362cc-17b2-11eb-befb-8864259bd2d8_story.html
4. Chaudhary MJ, Howell E, Ficke JR, et al. Caring for patients at a COVID-19 field hospital. J Hosp Med. 2021;16(2):117-119. https://doi.org/10.12788/jhm.3551
5. Welcome to the COVID-19 response working team knowledge base. HOMERun Hospital Medicine Reengineering Network COVID-19 Collaboration. Accessed March 4, 2021. https://www.hospitalinnovate.org/covid19/
1. Auerbach A, O’Leary KJ, Greysen SR, et al; HOMERuN COVID-19 Collaborative Group. Hospital ward adaptation during the COVID-19 pandemic: a national survey of academic medical centers. J Hosp Med. 2020;15(8):483-488. https://doi.org/10.12788/jhm.3476
2. Janke AT, Mei H, Rothenberg C, Becher RD, Lin Z, Venkatesh AK. Analysis of hospital resource availability and COVID-19 mortality across the United States. J Hosp Med. 2021;16(4):211-214.
3. Achenbach J, Brulliard K, Shammas B, Dupree J. Hospitals in nearly every region report a flood of covid-19 patients. Washington Post. October 26, 2020. Accessed March 4, 2021. https://www.washingtonpost.com/health/covid-hospitals-record-patients/2020/10/26/0bc362cc-17b2-11eb-befb-8864259bd2d8_story.html
4. Chaudhary MJ, Howell E, Ficke JR, et al. Caring for patients at a COVID-19 field hospital. J Hosp Med. 2021;16(2):117-119. https://doi.org/10.12788/jhm.3551
5. Welcome to the COVID-19 response working team knowledge base. HOMERun Hospital Medicine Reengineering Network COVID-19 Collaboration. Accessed March 4, 2021. https://www.hospitalinnovate.org/covid19/
© 2021 Society of Hospital Medicine

