User login
Mindful mentoring
Scenario
A GI faculty member is approached by two medical students who are planning careers in gastroenterology. They are interested in research projects and are very willing to dedicate the necessary time and energy. The faculty member is impressed by their desire and finds themselves recalling their own unsuccessful medical school search for a research mentor. Inspired by their enthusiasm and a desire to “give back,” the faculty member agrees to mentor them and helps them find suitable projects. Primarily because of the students’ hard work and fueled by their desire to produce results that will help their residency applications, the work progresses rapidly. Both students have separate abstracts accepted at a national meeting.
When COVID-19 hits, the faculty member is asked by their department to take on additional administrative and clinical work. They feel they cannot say no. Soon the faculty member finds it difficult to manage these new responsibilities on top of their many research projects, numerous clinical obligations, and additional pressures outside of work. They find they have no time for mentoring or even adequate sleep. Facing burnout, the faculty member is uncertain what to do for these hard-working and very gifted students. How would you recommend they manage their mentoring obligations?
Discussion
Mentorship is a cornerstone of academic medicine. In fact, it has been shown that academic clinicians who serve as mentors publish more papers, get more grants, are promoted faster, and are more likely to stay at their academic institutions with greater career satisfaction.1 However, not every mentor-mentee relationship is mutually beneficial. Usually, it’s the mentees that disproportionately suffer the consequences of a suboptimal relationship.2
Mentorship malpractice occurs when mentors’ behavior crosses a threshold that places the mentees’ success at risk.1,2 While the case above highlights a specific scenario where multiple issues are unfolding, the ability to recognize, address, and most importantly prevent mentorship malpractice ultimately benefits both mentees and mentors.
Understanding the various types of mentorship malpractice is helpful for prevention and course correction. As described by Chopra and colleagues, there are multiple types of passive and active mentorship malpractice.2 The passive forms are characterized by a lack of face-to-face meeting time with mentees and/or a lack of advocacy on the mentees’ behalf. Meanwhile, the active forms occur when the mentor exhibits self-serving behaviors. These can include listing themselves as first author on a mentee’s project or discouraging a mentee from working with other mentors. Mentors must be able to self-check, seek feedback from mentees, and encourage mentees to further their professional networks beyond the boundaries of what the mentor alone can offer. Doing so helps create new opportunities and helps ensure a mutually beneficial relationship.
A great initial step to prevent passive and active mentorship malpractice is to leverage the benefits of team mentorship.2,3 At its core, team mentorship capitalizes on the collective contributions of multiple mentors. Doing so not only provides security during uncertain times, but also allows for a diversity of perspectives, distribution of workload among mentors, and additional support for mentees.3,4 Team mentorship it is particularly important during this current global health crisis, and such an approach from the outset could have significantly improved the scenario above.
For the above scenario, likely a transition in mentorship would be needed. Such transitions, whether short term or long term, require transparency, honesty, and willingness to engage in difficult conversations with mentees. Whether the mentor in the above case engages another faculty to take on the mentees or chooses to find a colleague who will agree to take on other competing demands, it will require time, effort, and energy – all of which are in short supply. When team mentorship is established from the outset, such transitions of mentorship can occur seamlessly and with more ease for all.
Additional considerations for successful mentoring of medical students or early-career physicians include understanding generational differences between the mentor and their mentees. As outlined by Waljee and colleagues, the next generation of trainees and physicians may act in ways that deviate from the norms of academic medicine’s tradition. As a mentor, it is imperative to understand these actions are not intended to disrupt the traditions and norms of health systems.5 For example, the use of technology during rounds can often be misconstrued as disrespectful. However, the underlying intent in many cases is to answer a question or access a helpful reference.
Seeing behavior and actions from the perspective of the mentee is one of the many ways to support and sustain successful mentoring relationships. A mindful approach benefits both mentees and mentors; this includes reflecting on the underlying motives for mentorship and cultivating gratitude for the relationships formed.6 While these steps may seem trivial, gratitude promotes happiness, trust, motivation, and respect. It can be felt by others, including mentees.
As mentors continue to shape the future, they have an ethical obligation to care for themselves, in addition to their mentees. In addition to avoiding mentorship malpractice, engaging in team mentorship, and incorporating mindful mentoring, an emphasis on self-care is critical.7 Taking time to recharge is essential. It allows one to be fully present, while also setting an example for the mentee. Explicitly addressing self-care for both mentor and mentee is a part of mindful mentorship, with benefits for all.6
Three key points:
1. Awareness of mentorship malpractice
2. Importance of team mentorship
3. Benefits of mindful mentorship
Mr. Rodoni is with the University of Michigan Medical School and Stephen M. Ross School of Business, Ann Arbor, Mich. Dr. Fessel is a professor of radiology in the department of radiology at Michigan Medicine, Ann Arbor. They reported having no disclosures relevant to this article.
References:
1. Chopra V et al. JAMA Intern Med. 2018 Feb;178:175-6.
2. Chopra V et al. JAMA. 2016 Apr 12;315:1453-4.
3. Chopra V et al. The Mentoring Guide: Helping Mentors & Mentees Succeed. Ann Arbor: Michigan Publishing, 2019.
4. Rodoni BM et al. Annals of Surgery. 2020 Aug;272(2):e151-2.
5. Waljee JF et al. JAMA. 2018 Apr 17;319(15):1547-8.
6. Chopra V and Saint S. Healthc (Amst). 2020 Mar;8(1):100390.
7. Fessell D et al. “Mentoring During a Crisis.” Harvard Business Review. 2020 Oct 29.
Scenario
A GI faculty member is approached by two medical students who are planning careers in gastroenterology. They are interested in research projects and are very willing to dedicate the necessary time and energy. The faculty member is impressed by their desire and finds themselves recalling their own unsuccessful medical school search for a research mentor. Inspired by their enthusiasm and a desire to “give back,” the faculty member agrees to mentor them and helps them find suitable projects. Primarily because of the students’ hard work and fueled by their desire to produce results that will help their residency applications, the work progresses rapidly. Both students have separate abstracts accepted at a national meeting.
When COVID-19 hits, the faculty member is asked by their department to take on additional administrative and clinical work. They feel they cannot say no. Soon the faculty member finds it difficult to manage these new responsibilities on top of their many research projects, numerous clinical obligations, and additional pressures outside of work. They find they have no time for mentoring or even adequate sleep. Facing burnout, the faculty member is uncertain what to do for these hard-working and very gifted students. How would you recommend they manage their mentoring obligations?
Discussion
Mentorship is a cornerstone of academic medicine. In fact, it has been shown that academic clinicians who serve as mentors publish more papers, get more grants, are promoted faster, and are more likely to stay at their academic institutions with greater career satisfaction.1 However, not every mentor-mentee relationship is mutually beneficial. Usually, it’s the mentees that disproportionately suffer the consequences of a suboptimal relationship.2
Mentorship malpractice occurs when mentors’ behavior crosses a threshold that places the mentees’ success at risk.1,2 While the case above highlights a specific scenario where multiple issues are unfolding, the ability to recognize, address, and most importantly prevent mentorship malpractice ultimately benefits both mentees and mentors.
Understanding the various types of mentorship malpractice is helpful for prevention and course correction. As described by Chopra and colleagues, there are multiple types of passive and active mentorship malpractice.2 The passive forms are characterized by a lack of face-to-face meeting time with mentees and/or a lack of advocacy on the mentees’ behalf. Meanwhile, the active forms occur when the mentor exhibits self-serving behaviors. These can include listing themselves as first author on a mentee’s project or discouraging a mentee from working with other mentors. Mentors must be able to self-check, seek feedback from mentees, and encourage mentees to further their professional networks beyond the boundaries of what the mentor alone can offer. Doing so helps create new opportunities and helps ensure a mutually beneficial relationship.
A great initial step to prevent passive and active mentorship malpractice is to leverage the benefits of team mentorship.2,3 At its core, team mentorship capitalizes on the collective contributions of multiple mentors. Doing so not only provides security during uncertain times, but also allows for a diversity of perspectives, distribution of workload among mentors, and additional support for mentees.3,4 Team mentorship it is particularly important during this current global health crisis, and such an approach from the outset could have significantly improved the scenario above.
For the above scenario, likely a transition in mentorship would be needed. Such transitions, whether short term or long term, require transparency, honesty, and willingness to engage in difficult conversations with mentees. Whether the mentor in the above case engages another faculty to take on the mentees or chooses to find a colleague who will agree to take on other competing demands, it will require time, effort, and energy – all of which are in short supply. When team mentorship is established from the outset, such transitions of mentorship can occur seamlessly and with more ease for all.
Additional considerations for successful mentoring of medical students or early-career physicians include understanding generational differences between the mentor and their mentees. As outlined by Waljee and colleagues, the next generation of trainees and physicians may act in ways that deviate from the norms of academic medicine’s tradition. As a mentor, it is imperative to understand these actions are not intended to disrupt the traditions and norms of health systems.5 For example, the use of technology during rounds can often be misconstrued as disrespectful. However, the underlying intent in many cases is to answer a question or access a helpful reference.
Seeing behavior and actions from the perspective of the mentee is one of the many ways to support and sustain successful mentoring relationships. A mindful approach benefits both mentees and mentors; this includes reflecting on the underlying motives for mentorship and cultivating gratitude for the relationships formed.6 While these steps may seem trivial, gratitude promotes happiness, trust, motivation, and respect. It can be felt by others, including mentees.
As mentors continue to shape the future, they have an ethical obligation to care for themselves, in addition to their mentees. In addition to avoiding mentorship malpractice, engaging in team mentorship, and incorporating mindful mentoring, an emphasis on self-care is critical.7 Taking time to recharge is essential. It allows one to be fully present, while also setting an example for the mentee. Explicitly addressing self-care for both mentor and mentee is a part of mindful mentorship, with benefits for all.6
Three key points:
1. Awareness of mentorship malpractice
2. Importance of team mentorship
3. Benefits of mindful mentorship
Mr. Rodoni is with the University of Michigan Medical School and Stephen M. Ross School of Business, Ann Arbor, Mich. Dr. Fessel is a professor of radiology in the department of radiology at Michigan Medicine, Ann Arbor. They reported having no disclosures relevant to this article.
References:
1. Chopra V et al. JAMA Intern Med. 2018 Feb;178:175-6.
2. Chopra V et al. JAMA. 2016 Apr 12;315:1453-4.
3. Chopra V et al. The Mentoring Guide: Helping Mentors & Mentees Succeed. Ann Arbor: Michigan Publishing, 2019.
4. Rodoni BM et al. Annals of Surgery. 2020 Aug;272(2):e151-2.
5. Waljee JF et al. JAMA. 2018 Apr 17;319(15):1547-8.
6. Chopra V and Saint S. Healthc (Amst). 2020 Mar;8(1):100390.
7. Fessell D et al. “Mentoring During a Crisis.” Harvard Business Review. 2020 Oct 29.
Scenario
A GI faculty member is approached by two medical students who are planning careers in gastroenterology. They are interested in research projects and are very willing to dedicate the necessary time and energy. The faculty member is impressed by their desire and finds themselves recalling their own unsuccessful medical school search for a research mentor. Inspired by their enthusiasm and a desire to “give back,” the faculty member agrees to mentor them and helps them find suitable projects. Primarily because of the students’ hard work and fueled by their desire to produce results that will help their residency applications, the work progresses rapidly. Both students have separate abstracts accepted at a national meeting.
When COVID-19 hits, the faculty member is asked by their department to take on additional administrative and clinical work. They feel they cannot say no. Soon the faculty member finds it difficult to manage these new responsibilities on top of their many research projects, numerous clinical obligations, and additional pressures outside of work. They find they have no time for mentoring or even adequate sleep. Facing burnout, the faculty member is uncertain what to do for these hard-working and very gifted students. How would you recommend they manage their mentoring obligations?
Discussion
Mentorship is a cornerstone of academic medicine. In fact, it has been shown that academic clinicians who serve as mentors publish more papers, get more grants, are promoted faster, and are more likely to stay at their academic institutions with greater career satisfaction.1 However, not every mentor-mentee relationship is mutually beneficial. Usually, it’s the mentees that disproportionately suffer the consequences of a suboptimal relationship.2
Mentorship malpractice occurs when mentors’ behavior crosses a threshold that places the mentees’ success at risk.1,2 While the case above highlights a specific scenario where multiple issues are unfolding, the ability to recognize, address, and most importantly prevent mentorship malpractice ultimately benefits both mentees and mentors.
Understanding the various types of mentorship malpractice is helpful for prevention and course correction. As described by Chopra and colleagues, there are multiple types of passive and active mentorship malpractice.2 The passive forms are characterized by a lack of face-to-face meeting time with mentees and/or a lack of advocacy on the mentees’ behalf. Meanwhile, the active forms occur when the mentor exhibits self-serving behaviors. These can include listing themselves as first author on a mentee’s project or discouraging a mentee from working with other mentors. Mentors must be able to self-check, seek feedback from mentees, and encourage mentees to further their professional networks beyond the boundaries of what the mentor alone can offer. Doing so helps create new opportunities and helps ensure a mutually beneficial relationship.
A great initial step to prevent passive and active mentorship malpractice is to leverage the benefits of team mentorship.2,3 At its core, team mentorship capitalizes on the collective contributions of multiple mentors. Doing so not only provides security during uncertain times, but also allows for a diversity of perspectives, distribution of workload among mentors, and additional support for mentees.3,4 Team mentorship it is particularly important during this current global health crisis, and such an approach from the outset could have significantly improved the scenario above.
For the above scenario, likely a transition in mentorship would be needed. Such transitions, whether short term or long term, require transparency, honesty, and willingness to engage in difficult conversations with mentees. Whether the mentor in the above case engages another faculty to take on the mentees or chooses to find a colleague who will agree to take on other competing demands, it will require time, effort, and energy – all of which are in short supply. When team mentorship is established from the outset, such transitions of mentorship can occur seamlessly and with more ease for all.
Additional considerations for successful mentoring of medical students or early-career physicians include understanding generational differences between the mentor and their mentees. As outlined by Waljee and colleagues, the next generation of trainees and physicians may act in ways that deviate from the norms of academic medicine’s tradition. As a mentor, it is imperative to understand these actions are not intended to disrupt the traditions and norms of health systems.5 For example, the use of technology during rounds can often be misconstrued as disrespectful. However, the underlying intent in many cases is to answer a question or access a helpful reference.
Seeing behavior and actions from the perspective of the mentee is one of the many ways to support and sustain successful mentoring relationships. A mindful approach benefits both mentees and mentors; this includes reflecting on the underlying motives for mentorship and cultivating gratitude for the relationships formed.6 While these steps may seem trivial, gratitude promotes happiness, trust, motivation, and respect. It can be felt by others, including mentees.
As mentors continue to shape the future, they have an ethical obligation to care for themselves, in addition to their mentees. In addition to avoiding mentorship malpractice, engaging in team mentorship, and incorporating mindful mentoring, an emphasis on self-care is critical.7 Taking time to recharge is essential. It allows one to be fully present, while also setting an example for the mentee. Explicitly addressing self-care for both mentor and mentee is a part of mindful mentorship, with benefits for all.6
Three key points:
1. Awareness of mentorship malpractice
2. Importance of team mentorship
3. Benefits of mindful mentorship
Mr. Rodoni is with the University of Michigan Medical School and Stephen M. Ross School of Business, Ann Arbor, Mich. Dr. Fessel is a professor of radiology in the department of radiology at Michigan Medicine, Ann Arbor. They reported having no disclosures relevant to this article.
References:
1. Chopra V et al. JAMA Intern Med. 2018 Feb;178:175-6.
2. Chopra V et al. JAMA. 2016 Apr 12;315:1453-4.
3. Chopra V et al. The Mentoring Guide: Helping Mentors & Mentees Succeed. Ann Arbor: Michigan Publishing, 2019.
4. Rodoni BM et al. Annals of Surgery. 2020 Aug;272(2):e151-2.
5. Waljee JF et al. JAMA. 2018 Apr 17;319(15):1547-8.
6. Chopra V and Saint S. Healthc (Amst). 2020 Mar;8(1):100390.
7. Fessell D et al. “Mentoring During a Crisis.” Harvard Business Review. 2020 Oct 29.
MRI-detected tenosynovitis can predict early RA
Key clinical point: Magnetic resonance imaging (MRI)-detected tenosynovitis is highly predictive of rheumatoid arthritis (RA), irrespective of anti-citrullinated protein antibodies (ACPA) status.
Major finding: The sensitivity of imaging-detected tenosynovitis was high for both, ACPA-positive RA (88%) and ACPA-negative RA (82%). The sensitivity of MRI-detected tenosynovitis for RA was significantly higher than that for psoriatic arthritis (65%; P = .001), peripheral spondylarthritis (53%; P less than .001), reactive arthritis (36%; P less than .001), and self-limiting undifferentiated arthritis (42%; P less than .001).
Study details: The data come from a large cross-sectional MRI study of 1,211 consecutive patients with early arthritis who underwent contrast-enhanced 1.5T MRI of hand and foot at diagnosis.
Disclosures: The study received funding from the Dutch Arthritis Foundation and the European Research Council under the European Union’s Horizon 2020 research and innovation programme. The authors declared no conflicts of interest.
Source: Matthijssen XME et al. Ann Rheum Dis. 2021 Feb 5. doi: 10.1136/annrheumdis-2020-219302.
Key clinical point: Magnetic resonance imaging (MRI)-detected tenosynovitis is highly predictive of rheumatoid arthritis (RA), irrespective of anti-citrullinated protein antibodies (ACPA) status.
Major finding: The sensitivity of imaging-detected tenosynovitis was high for both, ACPA-positive RA (88%) and ACPA-negative RA (82%). The sensitivity of MRI-detected tenosynovitis for RA was significantly higher than that for psoriatic arthritis (65%; P = .001), peripheral spondylarthritis (53%; P less than .001), reactive arthritis (36%; P less than .001), and self-limiting undifferentiated arthritis (42%; P less than .001).
Study details: The data come from a large cross-sectional MRI study of 1,211 consecutive patients with early arthritis who underwent contrast-enhanced 1.5T MRI of hand and foot at diagnosis.
Disclosures: The study received funding from the Dutch Arthritis Foundation and the European Research Council under the European Union’s Horizon 2020 research and innovation programme. The authors declared no conflicts of interest.
Source: Matthijssen XME et al. Ann Rheum Dis. 2021 Feb 5. doi: 10.1136/annrheumdis-2020-219302.
Key clinical point: Magnetic resonance imaging (MRI)-detected tenosynovitis is highly predictive of rheumatoid arthritis (RA), irrespective of anti-citrullinated protein antibodies (ACPA) status.
Major finding: The sensitivity of imaging-detected tenosynovitis was high for both, ACPA-positive RA (88%) and ACPA-negative RA (82%). The sensitivity of MRI-detected tenosynovitis for RA was significantly higher than that for psoriatic arthritis (65%; P = .001), peripheral spondylarthritis (53%; P less than .001), reactive arthritis (36%; P less than .001), and self-limiting undifferentiated arthritis (42%; P less than .001).
Study details: The data come from a large cross-sectional MRI study of 1,211 consecutive patients with early arthritis who underwent contrast-enhanced 1.5T MRI of hand and foot at diagnosis.
Disclosures: The study received funding from the Dutch Arthritis Foundation and the European Research Council under the European Union’s Horizon 2020 research and innovation programme. The authors declared no conflicts of interest.
Source: Matthijssen XME et al. Ann Rheum Dis. 2021 Feb 5. doi: 10.1136/annrheumdis-2020-219302.
Nonsurgical periodontal treatment could improve disease activity in RA patients with periodontitis
Key clinical point: Nonsurgical periodontal treatment (NSPT) was associated with a significant reduction in disease activity score (DAS28), tender joint counts (TJC), swollen joint counts (SJC), visual analogical scale (VAS), and C-reactive protein (CRP) in patients with rheumatoid arthritis (RA) and periodontitis.
Major finding: NSPT significantly reduced DAS28 (P less than .001), TJC (P less than .001), SJC (P = .008), VAS (P = .02), and CRP (P = .01) in patients with RA and periodontitis.
Study details: Data come from a meta-analysis of 9 studies that compared RA-related indicator changes between NSPT and no treatment groups.
Disclosures: The work was supported by the Natural Science Foundation of Tianjin, China, and the Science and Technology Foundation of Tianjin Health Commission, China. The authors declared no conflicts of interest.
Source: Sun J et al. Clin Oral Investig. 2021 Jan 29. doi: 10.1007/s00784-021-03807-w.
Key clinical point: Nonsurgical periodontal treatment (NSPT) was associated with a significant reduction in disease activity score (DAS28), tender joint counts (TJC), swollen joint counts (SJC), visual analogical scale (VAS), and C-reactive protein (CRP) in patients with rheumatoid arthritis (RA) and periodontitis.
Major finding: NSPT significantly reduced DAS28 (P less than .001), TJC (P less than .001), SJC (P = .008), VAS (P = .02), and CRP (P = .01) in patients with RA and periodontitis.
Study details: Data come from a meta-analysis of 9 studies that compared RA-related indicator changes between NSPT and no treatment groups.
Disclosures: The work was supported by the Natural Science Foundation of Tianjin, China, and the Science and Technology Foundation of Tianjin Health Commission, China. The authors declared no conflicts of interest.
Source: Sun J et al. Clin Oral Investig. 2021 Jan 29. doi: 10.1007/s00784-021-03807-w.
Key clinical point: Nonsurgical periodontal treatment (NSPT) was associated with a significant reduction in disease activity score (DAS28), tender joint counts (TJC), swollen joint counts (SJC), visual analogical scale (VAS), and C-reactive protein (CRP) in patients with rheumatoid arthritis (RA) and periodontitis.
Major finding: NSPT significantly reduced DAS28 (P less than .001), TJC (P less than .001), SJC (P = .008), VAS (P = .02), and CRP (P = .01) in patients with RA and periodontitis.
Study details: Data come from a meta-analysis of 9 studies that compared RA-related indicator changes between NSPT and no treatment groups.
Disclosures: The work was supported by the Natural Science Foundation of Tianjin, China, and the Science and Technology Foundation of Tianjin Health Commission, China. The authors declared no conflicts of interest.
Source: Sun J et al. Clin Oral Investig. 2021 Jan 29. doi: 10.1007/s00784-021-03807-w.
Hospital medicine groups are getting larger
What are the implications for your workplace?
Although readers will be forgiven for missing the subtle change, the tables in the 2020 State of Hospital Medicine (SoHM) Report underwent a landmark structural change that echoes the growth of our field. In the latest SoHM Report, the hospital medicine group (HMG) size categories all increased significantly to reflect the fact that hospitalist groups have grown from a median of 9 physician full time equivalents (FTE) in 2016 to a median of 15.2 employed/contracted FTE (excluding FTE provided by locum tenens providers) in 2020.
For many years, the Report considered “large” adult HMGs to be those with 30 or more FTE of physicians, and smaller groups were organized by FTE categories of <5, 5-9, 10-19, and 20-29. Now the SoHM Report describes a large HMG as 50 employed/contracted FTE or greater, a category that represents 12.7% of HMGs serving adults. The other categories expanded to <5, 5-14, 15-29, and 30-49, respectively. Overall, HMGs are growing in size, and the SoHM displays new data slices that help leaders to compare their group to modern peers.
There are some caveats to consider. First, these figures only represent physician FTE, and essentially all these large groups employ NP/PA hospitalists as well. Second, these HMGs typically employ some part-time and contracted PRN physicians in this FTE count. In combination, these two factors mean that large HMGs often employ many more than 50 individual clinicians. In fact, the average number of physicians in this cohort was 72.3 before counting NP/PAs and locums. Third, do not interpret the portion of large groups in the survey (12.7%) as insignificant. Because each one employs so many total hospitalists, large HMGs collectively represent a common work environment for many hospitalists in the US. Lastly, although pediatric HMGs have grown, far fewer (3.1%) have over 50 FTE, so this column focuses on HMGs serving adults.
Why does it matter that groups are growing in size? The SoHM Report offers extensive data to answer this question. Here are a couple of highlights but consider buying the report to dig deeper. First, large groups are far more likely to offer variable scheduling. Although the 7-on, 7-off scheduling pattern is still the norm in all group sizes, large HMGs are most likely to offer something flexible that might enhance career sustainability for hospitalists. Second, large groups are the most likely to employ a few hospitalists with extra training, whether that be geriatrics, palliative care, pediatrics, or a medicine subspecialty. Working in a large group means you can ask for curbside consults from a diverse and well-trained bunch of colleagues. Third, large groups were most likely to employ nocturnists, meaning fewer night shifts are allocated to the hospitalists who want to focus on daytime work. From an individual perspective, there is a lot to like about working in a large HMG.
There are some drawbacks to larger groups, of course. Large groups can be less socially cohesive and the costs of managing 70-100 hospitalists typically grow well past the capacity of a single group leader. My personal belief is that these downsides can be solved through economies of scale and skilled management teams. In addition, a large group can afford to dedicate leadership FTE to niche hospitalist needs, such as career development and coaching, which are difficult to fund in small practices. This also provides more opportunities for staff hospitalists to begin taking on some leadership or administrative duties or branch out into related areas such as quality improvement, case management physician advisor roles, or IT expertise.
Ultimately, large groups typically represent the maturation of an HMG within a large hospital – it signifies that the hospital relies on that group to deliver great patient outcomes in every corner of the hospital. Where you practice remains a personal choice, but the emergence of large groups hints at the clout and sophistication hospitalists can build by banding together. Learn more about the full 2020 SoHM Report at hospitalmedicine.org/sohm.
Dr. White is associate professor of medicine at the University of Washington, Seattle. He is the chair of SHM’s Practice Analysis Committee.
What are the implications for your workplace?
What are the implications for your workplace?
Although readers will be forgiven for missing the subtle change, the tables in the 2020 State of Hospital Medicine (SoHM) Report underwent a landmark structural change that echoes the growth of our field. In the latest SoHM Report, the hospital medicine group (HMG) size categories all increased significantly to reflect the fact that hospitalist groups have grown from a median of 9 physician full time equivalents (FTE) in 2016 to a median of 15.2 employed/contracted FTE (excluding FTE provided by locum tenens providers) in 2020.
For many years, the Report considered “large” adult HMGs to be those with 30 or more FTE of physicians, and smaller groups were organized by FTE categories of <5, 5-9, 10-19, and 20-29. Now the SoHM Report describes a large HMG as 50 employed/contracted FTE or greater, a category that represents 12.7% of HMGs serving adults. The other categories expanded to <5, 5-14, 15-29, and 30-49, respectively. Overall, HMGs are growing in size, and the SoHM displays new data slices that help leaders to compare their group to modern peers.
There are some caveats to consider. First, these figures only represent physician FTE, and essentially all these large groups employ NP/PA hospitalists as well. Second, these HMGs typically employ some part-time and contracted PRN physicians in this FTE count. In combination, these two factors mean that large HMGs often employ many more than 50 individual clinicians. In fact, the average number of physicians in this cohort was 72.3 before counting NP/PAs and locums. Third, do not interpret the portion of large groups in the survey (12.7%) as insignificant. Because each one employs so many total hospitalists, large HMGs collectively represent a common work environment for many hospitalists in the US. Lastly, although pediatric HMGs have grown, far fewer (3.1%) have over 50 FTE, so this column focuses on HMGs serving adults.
Why does it matter that groups are growing in size? The SoHM Report offers extensive data to answer this question. Here are a couple of highlights but consider buying the report to dig deeper. First, large groups are far more likely to offer variable scheduling. Although the 7-on, 7-off scheduling pattern is still the norm in all group sizes, large HMGs are most likely to offer something flexible that might enhance career sustainability for hospitalists. Second, large groups are the most likely to employ a few hospitalists with extra training, whether that be geriatrics, palliative care, pediatrics, or a medicine subspecialty. Working in a large group means you can ask for curbside consults from a diverse and well-trained bunch of colleagues. Third, large groups were most likely to employ nocturnists, meaning fewer night shifts are allocated to the hospitalists who want to focus on daytime work. From an individual perspective, there is a lot to like about working in a large HMG.
There are some drawbacks to larger groups, of course. Large groups can be less socially cohesive and the costs of managing 70-100 hospitalists typically grow well past the capacity of a single group leader. My personal belief is that these downsides can be solved through economies of scale and skilled management teams. In addition, a large group can afford to dedicate leadership FTE to niche hospitalist needs, such as career development and coaching, which are difficult to fund in small practices. This also provides more opportunities for staff hospitalists to begin taking on some leadership or administrative duties or branch out into related areas such as quality improvement, case management physician advisor roles, or IT expertise.
Ultimately, large groups typically represent the maturation of an HMG within a large hospital – it signifies that the hospital relies on that group to deliver great patient outcomes in every corner of the hospital. Where you practice remains a personal choice, but the emergence of large groups hints at the clout and sophistication hospitalists can build by banding together. Learn more about the full 2020 SoHM Report at hospitalmedicine.org/sohm.
Dr. White is associate professor of medicine at the University of Washington, Seattle. He is the chair of SHM’s Practice Analysis Committee.
Although readers will be forgiven for missing the subtle change, the tables in the 2020 State of Hospital Medicine (SoHM) Report underwent a landmark structural change that echoes the growth of our field. In the latest SoHM Report, the hospital medicine group (HMG) size categories all increased significantly to reflect the fact that hospitalist groups have grown from a median of 9 physician full time equivalents (FTE) in 2016 to a median of 15.2 employed/contracted FTE (excluding FTE provided by locum tenens providers) in 2020.
For many years, the Report considered “large” adult HMGs to be those with 30 or more FTE of physicians, and smaller groups were organized by FTE categories of <5, 5-9, 10-19, and 20-29. Now the SoHM Report describes a large HMG as 50 employed/contracted FTE or greater, a category that represents 12.7% of HMGs serving adults. The other categories expanded to <5, 5-14, 15-29, and 30-49, respectively. Overall, HMGs are growing in size, and the SoHM displays new data slices that help leaders to compare their group to modern peers.
There are some caveats to consider. First, these figures only represent physician FTE, and essentially all these large groups employ NP/PA hospitalists as well. Second, these HMGs typically employ some part-time and contracted PRN physicians in this FTE count. In combination, these two factors mean that large HMGs often employ many more than 50 individual clinicians. In fact, the average number of physicians in this cohort was 72.3 before counting NP/PAs and locums. Third, do not interpret the portion of large groups in the survey (12.7%) as insignificant. Because each one employs so many total hospitalists, large HMGs collectively represent a common work environment for many hospitalists in the US. Lastly, although pediatric HMGs have grown, far fewer (3.1%) have over 50 FTE, so this column focuses on HMGs serving adults.
Why does it matter that groups are growing in size? The SoHM Report offers extensive data to answer this question. Here are a couple of highlights but consider buying the report to dig deeper. First, large groups are far more likely to offer variable scheduling. Although the 7-on, 7-off scheduling pattern is still the norm in all group sizes, large HMGs are most likely to offer something flexible that might enhance career sustainability for hospitalists. Second, large groups are the most likely to employ a few hospitalists with extra training, whether that be geriatrics, palliative care, pediatrics, or a medicine subspecialty. Working in a large group means you can ask for curbside consults from a diverse and well-trained bunch of colleagues. Third, large groups were most likely to employ nocturnists, meaning fewer night shifts are allocated to the hospitalists who want to focus on daytime work. From an individual perspective, there is a lot to like about working in a large HMG.
There are some drawbacks to larger groups, of course. Large groups can be less socially cohesive and the costs of managing 70-100 hospitalists typically grow well past the capacity of a single group leader. My personal belief is that these downsides can be solved through economies of scale and skilled management teams. In addition, a large group can afford to dedicate leadership FTE to niche hospitalist needs, such as career development and coaching, which are difficult to fund in small practices. This also provides more opportunities for staff hospitalists to begin taking on some leadership or administrative duties or branch out into related areas such as quality improvement, case management physician advisor roles, or IT expertise.
Ultimately, large groups typically represent the maturation of an HMG within a large hospital – it signifies that the hospital relies on that group to deliver great patient outcomes in every corner of the hospital. Where you practice remains a personal choice, but the emergence of large groups hints at the clout and sophistication hospitalists can build by banding together. Learn more about the full 2020 SoHM Report at hospitalmedicine.org/sohm.
Dr. White is associate professor of medicine at the University of Washington, Seattle. He is the chair of SHM’s Practice Analysis Committee.
MIS-C follow-up proves challenging across pediatric hospitals
The discovery of any novel disease or condition means a steep learning curve as physicians must develop protocols for diagnosis, management, and follow-up on the fly in the midst of admitting and treating patients. Medical society task forces and committees often release interim guidance during the learning process, but each institution ultimately has to determine what works for them based on their resources, clinical experience, and patient population.
But when the novel condition demands the involvement of multiple different specialties, the challenge of management grows even more complex – as does follow-up after patients are discharged. Such has been the story with multisystem inflammatory syndrome in children (MIS-C), a complication of COVID-19 that shares some features with Kawasaki disease.
The similarities to Kawasaki provided physicians a place to start in developing appropriate treatment regimens and involved a similar interdisciplinary team from, at the least, cardiology and rheumatology, plus infectious disease since MIS-C results from COVID-19.
“It literally has it in the name – multisystem essentially hints that there are multiple specialties involved, multiple hands in the pot trying to manage the kids, and so each specialty has their own kind of unique role in the patient’s care even on the outpatient side,” said Samina S. Bhumbra, MD, an infectious disease pediatrician at Riley Hospital for Children and assistant professor of clinical pediatrics at Indiana University in Indianapolis. “This isn’t a disease that falls under one specialty.”
By July, the American College of Rheumatology had issued interim clinical guidance for management that most children’s hospitals have followed or slightly adapted. But ACR guidelines could not address how each institution should handle outpatient follow-up visits, especially since those visits required, again, at least cardiology and rheumatology if not infectious disease or other specialties as well.
“When their kids are admitted to the hospital, to be told at discharge you have to be followed up by all these specialists is a lot to handle,” Dr. Bhumbra said. But just as it’s difficult for parents to deal with the need to see several different doctors after discharge, it can be difficult at some institutions for physicians to design a follow-up schedule that can accommodate families, especially families who live far from the hospital in the first place.
“Some of our follow-up is disjointed because all of our clinics had never been on the same day just because of staff availability,” Dr. Bhumbra said. “But it can be a 2- to 3-hour drive for some of our patients, depending on how far they’re coming.”
Many of them can’t make that drive more than once in the same month, much less the same week.
“If you have multiple visits, it makes it more likely that they’re not showing up,” said Ryan M. Serrano, MD, a pediatric cardiologist at Riley and assistant professor of pediatrics at Indiana University. Riley used telehealth when possible, especially if families could get labs done near home. But pediatric echocardiograms require technicians who have experience with children, so families need to come to the hospital.
Children’s hospitals have therefore had to adapt scheduling strategies or develop pediatric specialty clinics to coordinate across the multiple departments and accommodate a complex follow-up regimen that is still evolving as physicians learn more about MIS-C.
Determining a follow-up regimen
Even before determining how to coordinate appointments, hospitals had to decide what follow-up itself should be.
“How long do we follow these patients and how often do we follow them?” said Melissa S. Oliver, MD, a rheumatologist at Riley and assistant professor of clinical pediatrics at Indiana University.
“We’re seeing that a lot of our patients rapidly respond when they get appropriate therapy, but we don’t know about long-term outcomes yet. We’re all still learning.”
At Children’s Hospital of Philadelphia, infectious disease follows up 4-6 weeks post discharge. The cardiology division came up with a follow-up plan that has evolved over time, said Matthew Elias, MD, an attending cardiologist at CHOP’s Cardiac Center and clinical assistant professor of pediatrics at the University of Pennsylvania, Philadelphia.
Patients get an EKG and echocardiogram at 2 weeks and, if their condition is stable, 6 weeks after discharge. After that, it depends on the patient’s clinical situation. Patients with moderately diminished left ventricular systolic function are recommended to get an MRI scan 3 months after discharge and, if old enough, exercise stress tests. Otherwise, they are seen at 6 months, but that appointment is optional for those whose prior echos have consistently been normal.
Other institutions, including Riley, are following a similar schedule of 2-week, 6-week, and 6-month postdischarge follow-ups, and most plan to do a 1-year follow-up as well, although that 1-year mark hasn’t arrived yet for most. Most do rheumatology labs at the 2-week appointment and use that to determine steroids management and whether labs are needed at the 6-week appointment. If labs have normalized, they aren’t done at 6 months. Small variations in follow-up management exist across institutions, but all are remaining open to changes. Riley, for example, is considering MRI screening for ongoing cardiac inflammation at 6 months to a year for all patients, Dr. Serrano said.
The dedicated clinic model
The two challenges Riley needed to address were the lack of a clear consensus on what MIS-C follow-up should look like and the need for continuity of care, Dr. Serrano said.
Regular discussion in departmental meetings at Riley “progressed from how do we take care of them and what treatments do we give them to how do we follow them and manage them in outpatient,” Dr. Oliver said. In the inpatient setting, they had an interdisciplinary team, but how could they maintain that for outpatients without overwhelming the families?
“I think the main challenge is for the families to identify who is leading the care for them,” said Martha M. Rodriguez, MD, a rheumatologist at Riley and assistant professor of clinical pediatrics at Indiana University. That sometimes led to families picking which follow-up appointments they would attend and which they would skip if they could not make them all – and sometimes they skipped the more important ones. “They would go to the appointment with me and then miss the cardiology appointments and the echocardiogram, which was more important to follow any abnormalities in the heart,” Dr. Rodriguez said.
After trying to coordinate separate follow-up appointments for months, Riley ultimately decided to form a dedicated clinic for MIS-C follow-up – a “one-stop shop” single appointment at each follow-up, Dr. Bhumbra said, that covers labs, EKG, echocardiogram, and any other necessary tests.
“Our goal with the clinic is to make life easier for the families and to be able to coordinate the appointments,” Dr. Rodriguez said. “They will be able to see the three of us, and it would be easier for us to communicate with each other about their plan.”
The clinic began Feb. 11 and occurs twice a month. Though it’s just begun, Dr. Oliver said the first clinic went well, and it’s helping them figure out the role each specialty needs to play in follow-up care.
“For us with rheumatology, after lab values have returned to normal and they’re off steroids, sometimes we think there isn’t much more we can contribute to,” she said. And then there are the patients who didn’t see any rheumatologists while inpatients.
“That’s what we’re trying to figure out as well,” Dr. Oliver said. “Should we be seeing every single kid regardless of whether we were involved in their inpatient [stay] or only seeing the ones we’ve seen?” She expects the coming months will help them work that out.
Texas Children’s Hospital in Houston also uses a dedicated clinic, but they set it up before the first MIS-C patient came through the doors, said Sara Kristen Sexson Tejtel, MD, a pediatric cardiologist at Texas Children’s. The hospital already has other types of multidisciplinary clinics, and they anticipated the challenge of getting families to come to too many appointments in a short period of time.
“Getting someone to come back once is hard enough,” Dr. Sexson Tejtel said. “Getting them to come back twice is impossible.”
Infectious disease is less involved at Texas Children’s, so it’s primarily Dr. Sexson Tejtel and her rheumatologist colleague who see the patients. They hold the clinic once a week, twice if needed.
“It does make the appointment a little longer, but I think the patients appreciate that everything can be addressed with that one visit,” Dr. Sexson Tejtel said. “Being in the hospital as long as some of these kids are is so hard, so making any of that easy as possible is so helpful.” A single appointment also allows the doctors to work together on what labs are needed so that children don’t need multiple labs drawn.
At the appointment, she and the rheumatologist enter the patient’s room and take the patient’s history together.
“It’s nice because it makes the family not to have to repeat things and tell the same story over and over,” she said. “Sometimes I ask questions that then the rheumatologist jumps off of, and then sometimes he’ll ask questions, and I’ll think, ‘Ooh, I’ll ask more questions about that.’ ”
In fact, this team approach at all clinics has made her a more thoughtful, well-rounded physician, she said.
“I have learned so much going to all of my multidisciplinary clinics, and I think I’m able to better care for my patients because I’m not just thinking about it from a cardiac perspective,” she said. “It takes some work, but it’s not hard and I think it is beneficial both for the patient and for the physician. This team approach is definitely where we’re trying to live right now.”
Separate but coordinated appointments
A dedicated clinic isn’t the answer for all institutions, however. At Children’s Hospital of Philadelphia, the size of the networks and all its satellites made a one-stop shop impractical.
“We talked about a consolidated clinic early on, when MIS-C was first emerging and all our groups were collaborating and coming up with our inpatient and outpatient care pathways,” said Sanjeev K. Swami, MD, an infectious disease pediatrician at CHOP and associate professor of clinical pediatrics at the University of Pennsylvania. But timing varies on when each specialist wants to see the families return, and existing clinic schedules and locations varied too much.
So CHOP coordinates appointments individually for each patient, depending on where the patient lives and sometimes stacking them on the same day when possible. Sometimes infectious disease or rheumatology use telehealth, and CHOP, like the other hospitals, prioritizes cardiology, especially for the patients who had cardiac abnormalities in the hospital, Dr. Swami said.
“All three of our groups try to be as flexible as possible. We’ve had a really good collaboration between our groups,” he said, and spreading out follow-up allows specialists to ask about concerns raised at previous appointments, ensuring stronger continuity of care.
“We can make sure things are getting followed up on,” Dr. Swami said. “I think that has been beneficial to make sure things aren’t falling through the cracks.”
CHOP cardiologist Dr. Elias said that ongoing communication, among providers and with families, has been absolutely crucial.
“Everyone’s been talking so frequently about our MIS-C patients while inpatient that by the time they’re an outpatient, it seems to work smoothly, where families are hearing similar items but with a different flair, one from infectious, one from rheumatology, and one from cardiology,” he said.
Children’s Mercy in Kansas City, Mo., also has multiple satellite clinics and follows a model similar to that of CHOP. They discussed having a dedicated multidisciplinary team for each MIS-C patient, but even the logistics of that were difficult, said Emily J. Fox, MD, a rheumatologist and assistant professor of pediatrics at the University of Missouri-Kansas City.
Instead, Children’s Mercy tries to coordinate follow-up appointments to be on the same day and often use telehealth for the rheumatology appointments. Families that live closer to the hospital’s location in Joplin, Mo., go in for their cardiology appointment there, and then Dr. Fox conducts a telehealth appointment with the help of nurses in Joplin.
“We really do try hard, especially since these kids are in the hospital for a long time, to make the coordination as easy as possible,” Dr. Fox said. “This was all was very new, especially in the beginning, but I think at least our group is getting a little bit more comfortable in managing these patients.”
Looking ahead
The biggest question that still looms is what happens to these children, if anything, down the line.
“What was unique about this was this was a new disease we were all learning about together with no baseline,” Dr. Swami said. “None of us had ever seen this condition before.”
So far, the prognosis for the vast majority of children is good. “Most of these kids survive, most of them are doing well, and they almost all recover,” Dr. Serrano said. Labs tend to normalize by 6 weeks post discharge, if not much earlier, and not much cardiac involvement is showing up at later follow-ups. But not even a year has passed, so there’s plenty to learn. “We don’t know if there’s long-term risk. I would not be surprised if 20 years down the road we’re finding out things about this that we had no idea” about, Dr. Serrano said. “Everybody wants answers, and nobody has any, and the answers we have may end up being wrong. That’s how it goes when you’re dealing with something you’ve never seen.”
Research underway will ideally begin providing those answers soon. CHOP is a participating site in an NIH-NHLBI–sponsored study, called COVID MUSIC, that is tracking long-term outcomes for MIS-C at 30 centers across the United States and Canada for 5 years.
“That will really definitely be helpful in answering some of the questions about long-term outcomes,” Dr. Elias said. “We hope this is going to be a transient issue and that patients won’t have any long-term manifestations, but we don’t know that yet.”
Meanwhile, one benefit that has come out of the pandemic is strong collaboration, Dr. Bhumbra said.
“The biggest thing we’re all eagerly waiting and hoping for is standard guidelines on how best to follow-up on these kids, but I know that’s a ways away,” Dr. Bhumbra said. So for now, each institution is doing what it can to develop protocols that they feel best serve the patients’ needs, such as Riley’s new dedicated MIS-C clinic. “It takes a village to take care of these kids, and MIS-C has proven that having a clinic with all three specialties at one clinic is going to be great for the families.”
Dr. Fox serves on a committee for Pfizer unrelated to MIS-C. No other doctors interviewed for this story had relevant conflicts of interest to disclose.
The discovery of any novel disease or condition means a steep learning curve as physicians must develop protocols for diagnosis, management, and follow-up on the fly in the midst of admitting and treating patients. Medical society task forces and committees often release interim guidance during the learning process, but each institution ultimately has to determine what works for them based on their resources, clinical experience, and patient population.
But when the novel condition demands the involvement of multiple different specialties, the challenge of management grows even more complex – as does follow-up after patients are discharged. Such has been the story with multisystem inflammatory syndrome in children (MIS-C), a complication of COVID-19 that shares some features with Kawasaki disease.
The similarities to Kawasaki provided physicians a place to start in developing appropriate treatment regimens and involved a similar interdisciplinary team from, at the least, cardiology and rheumatology, plus infectious disease since MIS-C results from COVID-19.
“It literally has it in the name – multisystem essentially hints that there are multiple specialties involved, multiple hands in the pot trying to manage the kids, and so each specialty has their own kind of unique role in the patient’s care even on the outpatient side,” said Samina S. Bhumbra, MD, an infectious disease pediatrician at Riley Hospital for Children and assistant professor of clinical pediatrics at Indiana University in Indianapolis. “This isn’t a disease that falls under one specialty.”
By July, the American College of Rheumatology had issued interim clinical guidance for management that most children’s hospitals have followed or slightly adapted. But ACR guidelines could not address how each institution should handle outpatient follow-up visits, especially since those visits required, again, at least cardiology and rheumatology if not infectious disease or other specialties as well.
“When their kids are admitted to the hospital, to be told at discharge you have to be followed up by all these specialists is a lot to handle,” Dr. Bhumbra said. But just as it’s difficult for parents to deal with the need to see several different doctors after discharge, it can be difficult at some institutions for physicians to design a follow-up schedule that can accommodate families, especially families who live far from the hospital in the first place.
“Some of our follow-up is disjointed because all of our clinics had never been on the same day just because of staff availability,” Dr. Bhumbra said. “But it can be a 2- to 3-hour drive for some of our patients, depending on how far they’re coming.”
Many of them can’t make that drive more than once in the same month, much less the same week.
“If you have multiple visits, it makes it more likely that they’re not showing up,” said Ryan M. Serrano, MD, a pediatric cardiologist at Riley and assistant professor of pediatrics at Indiana University. Riley used telehealth when possible, especially if families could get labs done near home. But pediatric echocardiograms require technicians who have experience with children, so families need to come to the hospital.
Children’s hospitals have therefore had to adapt scheduling strategies or develop pediatric specialty clinics to coordinate across the multiple departments and accommodate a complex follow-up regimen that is still evolving as physicians learn more about MIS-C.
Determining a follow-up regimen
Even before determining how to coordinate appointments, hospitals had to decide what follow-up itself should be.
“How long do we follow these patients and how often do we follow them?” said Melissa S. Oliver, MD, a rheumatologist at Riley and assistant professor of clinical pediatrics at Indiana University.
“We’re seeing that a lot of our patients rapidly respond when they get appropriate therapy, but we don’t know about long-term outcomes yet. We’re all still learning.”
At Children’s Hospital of Philadelphia, infectious disease follows up 4-6 weeks post discharge. The cardiology division came up with a follow-up plan that has evolved over time, said Matthew Elias, MD, an attending cardiologist at CHOP’s Cardiac Center and clinical assistant professor of pediatrics at the University of Pennsylvania, Philadelphia.
Patients get an EKG and echocardiogram at 2 weeks and, if their condition is stable, 6 weeks after discharge. After that, it depends on the patient’s clinical situation. Patients with moderately diminished left ventricular systolic function are recommended to get an MRI scan 3 months after discharge and, if old enough, exercise stress tests. Otherwise, they are seen at 6 months, but that appointment is optional for those whose prior echos have consistently been normal.
Other institutions, including Riley, are following a similar schedule of 2-week, 6-week, and 6-month postdischarge follow-ups, and most plan to do a 1-year follow-up as well, although that 1-year mark hasn’t arrived yet for most. Most do rheumatology labs at the 2-week appointment and use that to determine steroids management and whether labs are needed at the 6-week appointment. If labs have normalized, they aren’t done at 6 months. Small variations in follow-up management exist across institutions, but all are remaining open to changes. Riley, for example, is considering MRI screening for ongoing cardiac inflammation at 6 months to a year for all patients, Dr. Serrano said.
The dedicated clinic model
The two challenges Riley needed to address were the lack of a clear consensus on what MIS-C follow-up should look like and the need for continuity of care, Dr. Serrano said.
Regular discussion in departmental meetings at Riley “progressed from how do we take care of them and what treatments do we give them to how do we follow them and manage them in outpatient,” Dr. Oliver said. In the inpatient setting, they had an interdisciplinary team, but how could they maintain that for outpatients without overwhelming the families?
“I think the main challenge is for the families to identify who is leading the care for them,” said Martha M. Rodriguez, MD, a rheumatologist at Riley and assistant professor of clinical pediatrics at Indiana University. That sometimes led to families picking which follow-up appointments they would attend and which they would skip if they could not make them all – and sometimes they skipped the more important ones. “They would go to the appointment with me and then miss the cardiology appointments and the echocardiogram, which was more important to follow any abnormalities in the heart,” Dr. Rodriguez said.
After trying to coordinate separate follow-up appointments for months, Riley ultimately decided to form a dedicated clinic for MIS-C follow-up – a “one-stop shop” single appointment at each follow-up, Dr. Bhumbra said, that covers labs, EKG, echocardiogram, and any other necessary tests.
“Our goal with the clinic is to make life easier for the families and to be able to coordinate the appointments,” Dr. Rodriguez said. “They will be able to see the three of us, and it would be easier for us to communicate with each other about their plan.”
The clinic began Feb. 11 and occurs twice a month. Though it’s just begun, Dr. Oliver said the first clinic went well, and it’s helping them figure out the role each specialty needs to play in follow-up care.
“For us with rheumatology, after lab values have returned to normal and they’re off steroids, sometimes we think there isn’t much more we can contribute to,” she said. And then there are the patients who didn’t see any rheumatologists while inpatients.
“That’s what we’re trying to figure out as well,” Dr. Oliver said. “Should we be seeing every single kid regardless of whether we were involved in their inpatient [stay] or only seeing the ones we’ve seen?” She expects the coming months will help them work that out.
Texas Children’s Hospital in Houston also uses a dedicated clinic, but they set it up before the first MIS-C patient came through the doors, said Sara Kristen Sexson Tejtel, MD, a pediatric cardiologist at Texas Children’s. The hospital already has other types of multidisciplinary clinics, and they anticipated the challenge of getting families to come to too many appointments in a short period of time.
“Getting someone to come back once is hard enough,” Dr. Sexson Tejtel said. “Getting them to come back twice is impossible.”
Infectious disease is less involved at Texas Children’s, so it’s primarily Dr. Sexson Tejtel and her rheumatologist colleague who see the patients. They hold the clinic once a week, twice if needed.
“It does make the appointment a little longer, but I think the patients appreciate that everything can be addressed with that one visit,” Dr. Sexson Tejtel said. “Being in the hospital as long as some of these kids are is so hard, so making any of that easy as possible is so helpful.” A single appointment also allows the doctors to work together on what labs are needed so that children don’t need multiple labs drawn.
At the appointment, she and the rheumatologist enter the patient’s room and take the patient’s history together.
“It’s nice because it makes the family not to have to repeat things and tell the same story over and over,” she said. “Sometimes I ask questions that then the rheumatologist jumps off of, and then sometimes he’ll ask questions, and I’ll think, ‘Ooh, I’ll ask more questions about that.’ ”
In fact, this team approach at all clinics has made her a more thoughtful, well-rounded physician, she said.
“I have learned so much going to all of my multidisciplinary clinics, and I think I’m able to better care for my patients because I’m not just thinking about it from a cardiac perspective,” she said. “It takes some work, but it’s not hard and I think it is beneficial both for the patient and for the physician. This team approach is definitely where we’re trying to live right now.”
Separate but coordinated appointments
A dedicated clinic isn’t the answer for all institutions, however. At Children’s Hospital of Philadelphia, the size of the networks and all its satellites made a one-stop shop impractical.
“We talked about a consolidated clinic early on, when MIS-C was first emerging and all our groups were collaborating and coming up with our inpatient and outpatient care pathways,” said Sanjeev K. Swami, MD, an infectious disease pediatrician at CHOP and associate professor of clinical pediatrics at the University of Pennsylvania. But timing varies on when each specialist wants to see the families return, and existing clinic schedules and locations varied too much.
So CHOP coordinates appointments individually for each patient, depending on where the patient lives and sometimes stacking them on the same day when possible. Sometimes infectious disease or rheumatology use telehealth, and CHOP, like the other hospitals, prioritizes cardiology, especially for the patients who had cardiac abnormalities in the hospital, Dr. Swami said.
“All three of our groups try to be as flexible as possible. We’ve had a really good collaboration between our groups,” he said, and spreading out follow-up allows specialists to ask about concerns raised at previous appointments, ensuring stronger continuity of care.
“We can make sure things are getting followed up on,” Dr. Swami said. “I think that has been beneficial to make sure things aren’t falling through the cracks.”
CHOP cardiologist Dr. Elias said that ongoing communication, among providers and with families, has been absolutely crucial.
“Everyone’s been talking so frequently about our MIS-C patients while inpatient that by the time they’re an outpatient, it seems to work smoothly, where families are hearing similar items but with a different flair, one from infectious, one from rheumatology, and one from cardiology,” he said.
Children’s Mercy in Kansas City, Mo., also has multiple satellite clinics and follows a model similar to that of CHOP. They discussed having a dedicated multidisciplinary team for each MIS-C patient, but even the logistics of that were difficult, said Emily J. Fox, MD, a rheumatologist and assistant professor of pediatrics at the University of Missouri-Kansas City.
Instead, Children’s Mercy tries to coordinate follow-up appointments to be on the same day and often use telehealth for the rheumatology appointments. Families that live closer to the hospital’s location in Joplin, Mo., go in for their cardiology appointment there, and then Dr. Fox conducts a telehealth appointment with the help of nurses in Joplin.
“We really do try hard, especially since these kids are in the hospital for a long time, to make the coordination as easy as possible,” Dr. Fox said. “This was all was very new, especially in the beginning, but I think at least our group is getting a little bit more comfortable in managing these patients.”
Looking ahead
The biggest question that still looms is what happens to these children, if anything, down the line.
“What was unique about this was this was a new disease we were all learning about together with no baseline,” Dr. Swami said. “None of us had ever seen this condition before.”
So far, the prognosis for the vast majority of children is good. “Most of these kids survive, most of them are doing well, and they almost all recover,” Dr. Serrano said. Labs tend to normalize by 6 weeks post discharge, if not much earlier, and not much cardiac involvement is showing up at later follow-ups. But not even a year has passed, so there’s plenty to learn. “We don’t know if there’s long-term risk. I would not be surprised if 20 years down the road we’re finding out things about this that we had no idea” about, Dr. Serrano said. “Everybody wants answers, and nobody has any, and the answers we have may end up being wrong. That’s how it goes when you’re dealing with something you’ve never seen.”
Research underway will ideally begin providing those answers soon. CHOP is a participating site in an NIH-NHLBI–sponsored study, called COVID MUSIC, that is tracking long-term outcomes for MIS-C at 30 centers across the United States and Canada for 5 years.
“That will really definitely be helpful in answering some of the questions about long-term outcomes,” Dr. Elias said. “We hope this is going to be a transient issue and that patients won’t have any long-term manifestations, but we don’t know that yet.”
Meanwhile, one benefit that has come out of the pandemic is strong collaboration, Dr. Bhumbra said.
“The biggest thing we’re all eagerly waiting and hoping for is standard guidelines on how best to follow-up on these kids, but I know that’s a ways away,” Dr. Bhumbra said. So for now, each institution is doing what it can to develop protocols that they feel best serve the patients’ needs, such as Riley’s new dedicated MIS-C clinic. “It takes a village to take care of these kids, and MIS-C has proven that having a clinic with all three specialties at one clinic is going to be great for the families.”
Dr. Fox serves on a committee for Pfizer unrelated to MIS-C. No other doctors interviewed for this story had relevant conflicts of interest to disclose.
The discovery of any novel disease or condition means a steep learning curve as physicians must develop protocols for diagnosis, management, and follow-up on the fly in the midst of admitting and treating patients. Medical society task forces and committees often release interim guidance during the learning process, but each institution ultimately has to determine what works for them based on their resources, clinical experience, and patient population.
But when the novel condition demands the involvement of multiple different specialties, the challenge of management grows even more complex – as does follow-up after patients are discharged. Such has been the story with multisystem inflammatory syndrome in children (MIS-C), a complication of COVID-19 that shares some features with Kawasaki disease.
The similarities to Kawasaki provided physicians a place to start in developing appropriate treatment regimens and involved a similar interdisciplinary team from, at the least, cardiology and rheumatology, plus infectious disease since MIS-C results from COVID-19.
“It literally has it in the name – multisystem essentially hints that there are multiple specialties involved, multiple hands in the pot trying to manage the kids, and so each specialty has their own kind of unique role in the patient’s care even on the outpatient side,” said Samina S. Bhumbra, MD, an infectious disease pediatrician at Riley Hospital for Children and assistant professor of clinical pediatrics at Indiana University in Indianapolis. “This isn’t a disease that falls under one specialty.”
By July, the American College of Rheumatology had issued interim clinical guidance for management that most children’s hospitals have followed or slightly adapted. But ACR guidelines could not address how each institution should handle outpatient follow-up visits, especially since those visits required, again, at least cardiology and rheumatology if not infectious disease or other specialties as well.
“When their kids are admitted to the hospital, to be told at discharge you have to be followed up by all these specialists is a lot to handle,” Dr. Bhumbra said. But just as it’s difficult for parents to deal with the need to see several different doctors after discharge, it can be difficult at some institutions for physicians to design a follow-up schedule that can accommodate families, especially families who live far from the hospital in the first place.
“Some of our follow-up is disjointed because all of our clinics had never been on the same day just because of staff availability,” Dr. Bhumbra said. “But it can be a 2- to 3-hour drive for some of our patients, depending on how far they’re coming.”
Many of them can’t make that drive more than once in the same month, much less the same week.
“If you have multiple visits, it makes it more likely that they’re not showing up,” said Ryan M. Serrano, MD, a pediatric cardiologist at Riley and assistant professor of pediatrics at Indiana University. Riley used telehealth when possible, especially if families could get labs done near home. But pediatric echocardiograms require technicians who have experience with children, so families need to come to the hospital.
Children’s hospitals have therefore had to adapt scheduling strategies or develop pediatric specialty clinics to coordinate across the multiple departments and accommodate a complex follow-up regimen that is still evolving as physicians learn more about MIS-C.
Determining a follow-up regimen
Even before determining how to coordinate appointments, hospitals had to decide what follow-up itself should be.
“How long do we follow these patients and how often do we follow them?” said Melissa S. Oliver, MD, a rheumatologist at Riley and assistant professor of clinical pediatrics at Indiana University.
“We’re seeing that a lot of our patients rapidly respond when they get appropriate therapy, but we don’t know about long-term outcomes yet. We’re all still learning.”
At Children’s Hospital of Philadelphia, infectious disease follows up 4-6 weeks post discharge. The cardiology division came up with a follow-up plan that has evolved over time, said Matthew Elias, MD, an attending cardiologist at CHOP’s Cardiac Center and clinical assistant professor of pediatrics at the University of Pennsylvania, Philadelphia.
Patients get an EKG and echocardiogram at 2 weeks and, if their condition is stable, 6 weeks after discharge. After that, it depends on the patient’s clinical situation. Patients with moderately diminished left ventricular systolic function are recommended to get an MRI scan 3 months after discharge and, if old enough, exercise stress tests. Otherwise, they are seen at 6 months, but that appointment is optional for those whose prior echos have consistently been normal.
Other institutions, including Riley, are following a similar schedule of 2-week, 6-week, and 6-month postdischarge follow-ups, and most plan to do a 1-year follow-up as well, although that 1-year mark hasn’t arrived yet for most. Most do rheumatology labs at the 2-week appointment and use that to determine steroids management and whether labs are needed at the 6-week appointment. If labs have normalized, they aren’t done at 6 months. Small variations in follow-up management exist across institutions, but all are remaining open to changes. Riley, for example, is considering MRI screening for ongoing cardiac inflammation at 6 months to a year for all patients, Dr. Serrano said.
The dedicated clinic model
The two challenges Riley needed to address were the lack of a clear consensus on what MIS-C follow-up should look like and the need for continuity of care, Dr. Serrano said.
Regular discussion in departmental meetings at Riley “progressed from how do we take care of them and what treatments do we give them to how do we follow them and manage them in outpatient,” Dr. Oliver said. In the inpatient setting, they had an interdisciplinary team, but how could they maintain that for outpatients without overwhelming the families?
“I think the main challenge is for the families to identify who is leading the care for them,” said Martha M. Rodriguez, MD, a rheumatologist at Riley and assistant professor of clinical pediatrics at Indiana University. That sometimes led to families picking which follow-up appointments they would attend and which they would skip if they could not make them all – and sometimes they skipped the more important ones. “They would go to the appointment with me and then miss the cardiology appointments and the echocardiogram, which was more important to follow any abnormalities in the heart,” Dr. Rodriguez said.
After trying to coordinate separate follow-up appointments for months, Riley ultimately decided to form a dedicated clinic for MIS-C follow-up – a “one-stop shop” single appointment at each follow-up, Dr. Bhumbra said, that covers labs, EKG, echocardiogram, and any other necessary tests.
“Our goal with the clinic is to make life easier for the families and to be able to coordinate the appointments,” Dr. Rodriguez said. “They will be able to see the three of us, and it would be easier for us to communicate with each other about their plan.”
The clinic began Feb. 11 and occurs twice a month. Though it’s just begun, Dr. Oliver said the first clinic went well, and it’s helping them figure out the role each specialty needs to play in follow-up care.
“For us with rheumatology, after lab values have returned to normal and they’re off steroids, sometimes we think there isn’t much more we can contribute to,” she said. And then there are the patients who didn’t see any rheumatologists while inpatients.
“That’s what we’re trying to figure out as well,” Dr. Oliver said. “Should we be seeing every single kid regardless of whether we were involved in their inpatient [stay] or only seeing the ones we’ve seen?” She expects the coming months will help them work that out.
Texas Children’s Hospital in Houston also uses a dedicated clinic, but they set it up before the first MIS-C patient came through the doors, said Sara Kristen Sexson Tejtel, MD, a pediatric cardiologist at Texas Children’s. The hospital already has other types of multidisciplinary clinics, and they anticipated the challenge of getting families to come to too many appointments in a short period of time.
“Getting someone to come back once is hard enough,” Dr. Sexson Tejtel said. “Getting them to come back twice is impossible.”
Infectious disease is less involved at Texas Children’s, so it’s primarily Dr. Sexson Tejtel and her rheumatologist colleague who see the patients. They hold the clinic once a week, twice if needed.
“It does make the appointment a little longer, but I think the patients appreciate that everything can be addressed with that one visit,” Dr. Sexson Tejtel said. “Being in the hospital as long as some of these kids are is so hard, so making any of that easy as possible is so helpful.” A single appointment also allows the doctors to work together on what labs are needed so that children don’t need multiple labs drawn.
At the appointment, she and the rheumatologist enter the patient’s room and take the patient’s history together.
“It’s nice because it makes the family not to have to repeat things and tell the same story over and over,” she said. “Sometimes I ask questions that then the rheumatologist jumps off of, and then sometimes he’ll ask questions, and I’ll think, ‘Ooh, I’ll ask more questions about that.’ ”
In fact, this team approach at all clinics has made her a more thoughtful, well-rounded physician, she said.
“I have learned so much going to all of my multidisciplinary clinics, and I think I’m able to better care for my patients because I’m not just thinking about it from a cardiac perspective,” she said. “It takes some work, but it’s not hard and I think it is beneficial both for the patient and for the physician. This team approach is definitely where we’re trying to live right now.”
Separate but coordinated appointments
A dedicated clinic isn’t the answer for all institutions, however. At Children’s Hospital of Philadelphia, the size of the networks and all its satellites made a one-stop shop impractical.
“We talked about a consolidated clinic early on, when MIS-C was first emerging and all our groups were collaborating and coming up with our inpatient and outpatient care pathways,” said Sanjeev K. Swami, MD, an infectious disease pediatrician at CHOP and associate professor of clinical pediatrics at the University of Pennsylvania. But timing varies on when each specialist wants to see the families return, and existing clinic schedules and locations varied too much.
So CHOP coordinates appointments individually for each patient, depending on where the patient lives and sometimes stacking them on the same day when possible. Sometimes infectious disease or rheumatology use telehealth, and CHOP, like the other hospitals, prioritizes cardiology, especially for the patients who had cardiac abnormalities in the hospital, Dr. Swami said.
“All three of our groups try to be as flexible as possible. We’ve had a really good collaboration between our groups,” he said, and spreading out follow-up allows specialists to ask about concerns raised at previous appointments, ensuring stronger continuity of care.
“We can make sure things are getting followed up on,” Dr. Swami said. “I think that has been beneficial to make sure things aren’t falling through the cracks.”
CHOP cardiologist Dr. Elias said that ongoing communication, among providers and with families, has been absolutely crucial.
“Everyone’s been talking so frequently about our MIS-C patients while inpatient that by the time they’re an outpatient, it seems to work smoothly, where families are hearing similar items but with a different flair, one from infectious, one from rheumatology, and one from cardiology,” he said.
Children’s Mercy in Kansas City, Mo., also has multiple satellite clinics and follows a model similar to that of CHOP. They discussed having a dedicated multidisciplinary team for each MIS-C patient, but even the logistics of that were difficult, said Emily J. Fox, MD, a rheumatologist and assistant professor of pediatrics at the University of Missouri-Kansas City.
Instead, Children’s Mercy tries to coordinate follow-up appointments to be on the same day and often use telehealth for the rheumatology appointments. Families that live closer to the hospital’s location in Joplin, Mo., go in for their cardiology appointment there, and then Dr. Fox conducts a telehealth appointment with the help of nurses in Joplin.
“We really do try hard, especially since these kids are in the hospital for a long time, to make the coordination as easy as possible,” Dr. Fox said. “This was all was very new, especially in the beginning, but I think at least our group is getting a little bit more comfortable in managing these patients.”
Looking ahead
The biggest question that still looms is what happens to these children, if anything, down the line.
“What was unique about this was this was a new disease we were all learning about together with no baseline,” Dr. Swami said. “None of us had ever seen this condition before.”
So far, the prognosis for the vast majority of children is good. “Most of these kids survive, most of them are doing well, and they almost all recover,” Dr. Serrano said. Labs tend to normalize by 6 weeks post discharge, if not much earlier, and not much cardiac involvement is showing up at later follow-ups. But not even a year has passed, so there’s plenty to learn. “We don’t know if there’s long-term risk. I would not be surprised if 20 years down the road we’re finding out things about this that we had no idea” about, Dr. Serrano said. “Everybody wants answers, and nobody has any, and the answers we have may end up being wrong. That’s how it goes when you’re dealing with something you’ve never seen.”
Research underway will ideally begin providing those answers soon. CHOP is a participating site in an NIH-NHLBI–sponsored study, called COVID MUSIC, that is tracking long-term outcomes for MIS-C at 30 centers across the United States and Canada for 5 years.
“That will really definitely be helpful in answering some of the questions about long-term outcomes,” Dr. Elias said. “We hope this is going to be a transient issue and that patients won’t have any long-term manifestations, but we don’t know that yet.”
Meanwhile, one benefit that has come out of the pandemic is strong collaboration, Dr. Bhumbra said.
“The biggest thing we’re all eagerly waiting and hoping for is standard guidelines on how best to follow-up on these kids, but I know that’s a ways away,” Dr. Bhumbra said. So for now, each institution is doing what it can to develop protocols that they feel best serve the patients’ needs, such as Riley’s new dedicated MIS-C clinic. “It takes a village to take care of these kids, and MIS-C has proven that having a clinic with all three specialties at one clinic is going to be great for the families.”
Dr. Fox serves on a committee for Pfizer unrelated to MIS-C. No other doctors interviewed for this story had relevant conflicts of interest to disclose.
Anti-TNF responding RA: Tocilizumab bests rituximab in reducing disease activity
Key clinical point: Tocilizumab is more effective than rituximab for reducing disease activity in patients with rheumatoid arthritis (RA) who had an inadequate response to tumor necrosis factor (TNF) inhibitors stratified for synovial B-cell status.
Major finding: At 16 weeks, the rituximab group vs. the tocilizumab group showed no significant difference in the rate of Clinical Disease Activity Index (CDAI50%; 45% vs. 56%; P = .31). However, the tocilizumab group had a significantly higher response rate compared with the rituximab group for both CDAI50% (63% vs. 36%; P = .035) and CDAImajor treatment response (50% vs. 12%, P = .0012).
Study details: The data come from a 48-week, biopsy-driven, multicenter, open-label, phase 4 trial involving 164 patients randomly assigned to receive either 2 1 gm rituximab infusions at an interval of 2 weeks (n=83) or 8 mg/kg tocilizumab infusions at 4-week intervals (n=81).
Disclosures: The study received funding from the UK National Institute for Health Research. MH Buch, A Nerviani, VC Romão, P Verschueren, B Dasgupta, A Cauli, PC Taylor, CJ Edwards, J Isaacs, E Choy, C Pitzalism, P Sasieni, MJ Lewis, and F Humby reported relationships with various pharmaceutical companies and/or research organizations. C Pitzalis reported a patent relevant to the work. The remaining authors declared no conflicts of interest.
Source: Humby F et al. Lancet. 2021 Jan 23. doi: 10.1016/S0140-6736(20)32341-2.
Key clinical point: Tocilizumab is more effective than rituximab for reducing disease activity in patients with rheumatoid arthritis (RA) who had an inadequate response to tumor necrosis factor (TNF) inhibitors stratified for synovial B-cell status.
Major finding: At 16 weeks, the rituximab group vs. the tocilizumab group showed no significant difference in the rate of Clinical Disease Activity Index (CDAI50%; 45% vs. 56%; P = .31). However, the tocilizumab group had a significantly higher response rate compared with the rituximab group for both CDAI50% (63% vs. 36%; P = .035) and CDAImajor treatment response (50% vs. 12%, P = .0012).
Study details: The data come from a 48-week, biopsy-driven, multicenter, open-label, phase 4 trial involving 164 patients randomly assigned to receive either 2 1 gm rituximab infusions at an interval of 2 weeks (n=83) or 8 mg/kg tocilizumab infusions at 4-week intervals (n=81).
Disclosures: The study received funding from the UK National Institute for Health Research. MH Buch, A Nerviani, VC Romão, P Verschueren, B Dasgupta, A Cauli, PC Taylor, CJ Edwards, J Isaacs, E Choy, C Pitzalism, P Sasieni, MJ Lewis, and F Humby reported relationships with various pharmaceutical companies and/or research organizations. C Pitzalis reported a patent relevant to the work. The remaining authors declared no conflicts of interest.
Source: Humby F et al. Lancet. 2021 Jan 23. doi: 10.1016/S0140-6736(20)32341-2.
Key clinical point: Tocilizumab is more effective than rituximab for reducing disease activity in patients with rheumatoid arthritis (RA) who had an inadequate response to tumor necrosis factor (TNF) inhibitors stratified for synovial B-cell status.
Major finding: At 16 weeks, the rituximab group vs. the tocilizumab group showed no significant difference in the rate of Clinical Disease Activity Index (CDAI50%; 45% vs. 56%; P = .31). However, the tocilizumab group had a significantly higher response rate compared with the rituximab group for both CDAI50% (63% vs. 36%; P = .035) and CDAImajor treatment response (50% vs. 12%, P = .0012).
Study details: The data come from a 48-week, biopsy-driven, multicenter, open-label, phase 4 trial involving 164 patients randomly assigned to receive either 2 1 gm rituximab infusions at an interval of 2 weeks (n=83) or 8 mg/kg tocilizumab infusions at 4-week intervals (n=81).
Disclosures: The study received funding from the UK National Institute for Health Research. MH Buch, A Nerviani, VC Romão, P Verschueren, B Dasgupta, A Cauli, PC Taylor, CJ Edwards, J Isaacs, E Choy, C Pitzalism, P Sasieni, MJ Lewis, and F Humby reported relationships with various pharmaceutical companies and/or research organizations. C Pitzalis reported a patent relevant to the work. The remaining authors declared no conflicts of interest.
Source: Humby F et al. Lancet. 2021 Jan 23. doi: 10.1016/S0140-6736(20)32341-2.
Younger RA patients at greater fracture risk even before age 50 years
Key clinical point: This study found an increased risk of first fracture before age 50 years in people with rheumatoid arthritis (RA) diagnosed before this age.
Major finding: Overall, the rate of first fractures occurring before age 50 was significantly higher in patients diagnosed with RA before age 50 (incidence rate ratio [IRR], 1.24; 95% confidence interval [CI], 1.10-1.40). The IRR of first fracture before age 50 was significantly higher in women (1.29; 95% CI, 1.12-1.49) and not in men diagnosed with RA before age 50 (1.15; 95% CI, 0.92-1.43). IRR of subsequent fractures below age 50 was also higher in both men and women but not significantly so.
Study details: A retrospective observational study of RA cases (n = 36,858) and matched controls (n=110,574) from the U.K. Clinical Practice Research Datalink.
Disclosures: The study was funded by a grant through the Pfizer competitive I-CRP funding program. The authors declared no conflicts of interest.
Source: Erwin J et al. Osteoporos Int. 2021 Feb 11. doi: 10.1007/s00198-021-05862-1.
Key clinical point: This study found an increased risk of first fracture before age 50 years in people with rheumatoid arthritis (RA) diagnosed before this age.
Major finding: Overall, the rate of first fractures occurring before age 50 was significantly higher in patients diagnosed with RA before age 50 (incidence rate ratio [IRR], 1.24; 95% confidence interval [CI], 1.10-1.40). The IRR of first fracture before age 50 was significantly higher in women (1.29; 95% CI, 1.12-1.49) and not in men diagnosed with RA before age 50 (1.15; 95% CI, 0.92-1.43). IRR of subsequent fractures below age 50 was also higher in both men and women but not significantly so.
Study details: A retrospective observational study of RA cases (n = 36,858) and matched controls (n=110,574) from the U.K. Clinical Practice Research Datalink.
Disclosures: The study was funded by a grant through the Pfizer competitive I-CRP funding program. The authors declared no conflicts of interest.
Source: Erwin J et al. Osteoporos Int. 2021 Feb 11. doi: 10.1007/s00198-021-05862-1.
Key clinical point: This study found an increased risk of first fracture before age 50 years in people with rheumatoid arthritis (RA) diagnosed before this age.
Major finding: Overall, the rate of first fractures occurring before age 50 was significantly higher in patients diagnosed with RA before age 50 (incidence rate ratio [IRR], 1.24; 95% confidence interval [CI], 1.10-1.40). The IRR of first fracture before age 50 was significantly higher in women (1.29; 95% CI, 1.12-1.49) and not in men diagnosed with RA before age 50 (1.15; 95% CI, 0.92-1.43). IRR of subsequent fractures below age 50 was also higher in both men and women but not significantly so.
Study details: A retrospective observational study of RA cases (n = 36,858) and matched controls (n=110,574) from the U.K. Clinical Practice Research Datalink.
Disclosures: The study was funded by a grant through the Pfizer competitive I-CRP funding program. The authors declared no conflicts of interest.
Source: Erwin J et al. Osteoporos Int. 2021 Feb 11. doi: 10.1007/s00198-021-05862-1.
Medical professional liability risk and mitigation: An overview for early-career gastroenterologists
Disclaimer: This article is for educational purposes only. All examples are hypothetical and aim to illustrate common clinical scenarios and challenges gastroenterologists may encounter within their scope of practice. The content herein should not be interpreted as legal advice for individual cases nor a substitute for seeking the advice of an attorney.
There are unique potential stressors faced by the gastroenterologist at each career stage, some more so early on. One such stressor, and one particularly important in a procedure-intensive specialty like GI, is medical professional liability (MPL), historically termed “medical malpractice.” Between 2009 and 2018, GI was the second-highest internal medicine subspecialty in both MPL claims made and claims paid,1 yet instruction on MPL risk and mitigation is scarce in fellowship, as is the available GI-related literature on the topic. This scarcity may generate untoward stress and unnecessarily expose gastroenterologists to avoidable MPL pitfalls. Therefore, it is vital for GI trainees, early-career gastroenterologists, and even seasoned gastroenterologists to have a working and updated knowledge of the general principles of MPL and GI-specific considerations. Such understanding can help preserve physician well-being, increase professional satisfaction, strengthen the doctor-patient relationship, and improve health care outcomes.2
To this end, we herein provide a focused review of the following: key MPL concepts, trends in MPL claims, GI-related MPL risk scenarios and considerations, adverse provider defensive mechanisms, documentation tenets, challenges posed by telemedicine, and the concept of “vicarious liability.”
Key MPL concepts
MPL falls under the umbrella of tort law, which itself falls under the umbrella of civil law; that is, civil (as opposed to criminal) justice governs torts – including but not limited to MPL claims – as well as other areas of law concerning noncriminal injury.3 A “tort” is a “civil wrong that unfairly causes another to experience loss or harm resulting in legal liability.”3 MPL claims assert the tort of negligence (similar to the concept of “incompetence”) and endeavor to compensate the harmed patient/individual while simultaneously dissuading suboptimal medical care by the provider in the future.4,5 A successful MPL claim must prove four overlapping elements: that the tortfeasor (here, the gastroenterologist) owed a duty of care to the injured party and breached that duty, which caused damages.6 Given that MPL cases exist within tort law rather than criminal law, the burden of proof for these cases is not “beyond a reasonable doubt”; instead, it’s “to a reasonable medical probability.”7
Trends in MPL claims
According to data compiled by the MPL Association, 278,220 MPL claims were made in the United States from 1985 to 2012.3,8-10 Among these, 1.8% involved gastroenterologists, which puts it at 17th place out of the 20 specialties surveyed.9 While the number of paid claims over this time frame decreased in GI by 34.6% (from 18.5 to 12.1 cases per 1,000 physician-years), there was a concurrent 23.3% increase in average claim compensation; essentially, there were fewer paid GI-related claims but there were higher payouts per paid claim.11,12 From 2009 to 2018, average legal defense costs for paid GI-related claims were $97,392, and average paid amount was $330,876.1
GI-related MPL risk scenarios and considerations
Many MPL claims relate to situations involving medical errors or adverse events (AEs), be they procedural or nonprocedural. However other aspects of GI also carry MPL risk.
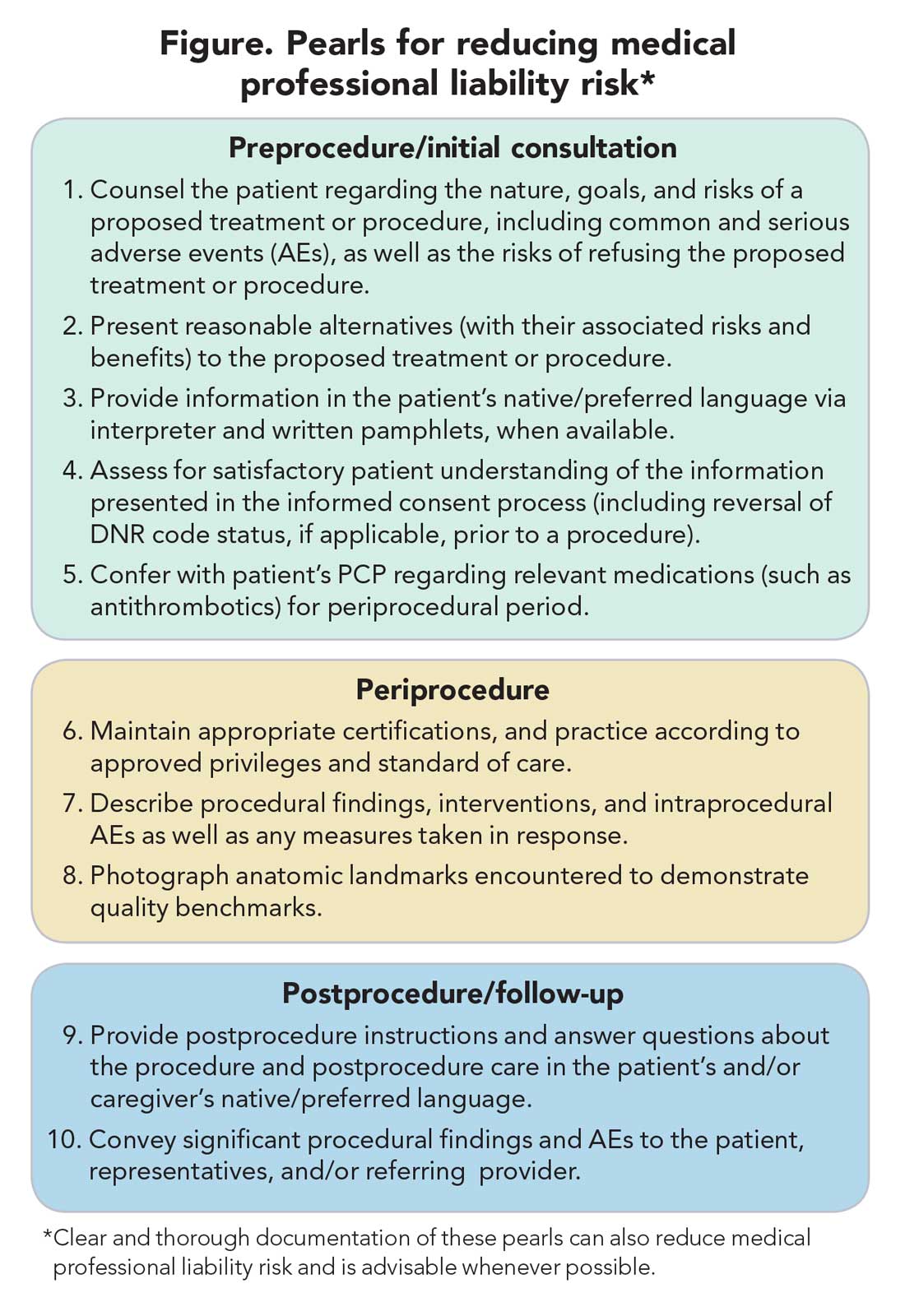
Informed consent
MPL claims may be made not only on the grounds of inadequately informed consent but also inadequately informed refusal.5,13,14 While standards for adequate informed consent vary by state, most states apply the “reasonable patient standard,” i.e., assuming an average patient with enough information to be an active participant in the medical decision-making process. Generally, informed consent should ensure that the patient understands the nature of the procedure/treatment being proposed, there is a discussion of the risks and benefits of undergoing and not undergoing the procedure/treatment, reasonable alternatives are presented, the risks and benefits associated with these alternatives are discussed, and the patient’s comprehension of these things is assessed (Figure).15 Additionally, informed consent should be tailored to each patient and GI procedure/treatment on a case-by-case basis rather than using a one-size-fits-all approach. Moreover, documentation of the patient’s understanding of the (tailored) information provided can concurrently improve quality of the consent and potentially decrease MPL risk (Figure).16
Endoscopic procedures
Procedure-related MPL claims represent approximately 25% of all GI-related claims (8,17). Among these, 52% involve colonoscopy, 16% involve endoscopic retrograde cholangiopancreatography (ERCP), and 11% involve esophagogastroduodenoscopy.8 Albeit generally safe, colonoscopy, as with esophagogastroduodenoscopy, is subject to rare but serious AEs.18,19 Risk of these AEs may be accentuated in certain scenarios (such as severe colonic inflammation or coagulopathy) and, as discussed earlier, may merit tailored informed consent. Regardless of the procedure, in the event of postprocedural development of signs/symptoms (such as tachycardia, fever, chest or abdominal discomfort, or hypotension) indicating a potential AE, stabilizing measures and evaluation (such as blood work and imaging) should be undertaken, and hospital admission (if not already hospitalized) should be considered until discharge is deemed safe.19
ERCP-related MPL claims, for many years, have had the highest average compensation of any GI procedure.11 Though discussion of advanced procedures is beyond the scope of this article, it is worth mentioning the observation that most of such claims involve an allegation that the procedure was not indicated (for example, that it was performed based on inadequate evidence of pancreatobiliary pathology), or was for diagnostic purposes (for example, being done instead of noninvasive imaging) rather than therapeutic.20-23 This emphasizes the importance of appropriate procedure indications.
Percutaneous endoscopic gastrostomy (PEG) placement merits special mention given it can be complicated by ethical challenges (for example, needing a surrogate decision-maker’s consent or representing medical futility) and has a relatively high potential for MPL claims. PEG placement carries a low AE rate (0.1%-1%), but these AEs may result in high morbidity/mortality, in part because of the underlying comorbidities of patients needing PEG placement.24,25 Also, timing of a patient’s demise may coincide with PEG placement, thereby prompting (possibly unfounded) perceptions of causality.24-27 Therefore, such scenarios merit unique additional preprocedure safeguards. For instance, for patients lacking capacity to provide informed consent, especially when family members may differ on whether PEG should be placed, it is advisable to ask the family to select one surrogate decision-maker (if there’s no advance directive) to whom the gastroenterologist should discuss both the risks, benefits, and goals of PEG placement in the context of the patient’s overall clinical trajectory/life expectancy and the need for consent (or refusal) based on what the patient would have wished. In addition, having a medical professional witness this discussion may be useful.27
Antithrombotic agents
Periprocedural management of antithrombotics, including anticoagulants and antiplatelets, can pose challenges for the gastroenterologist. While clinical practice guidelines exist to guide decision-making in this regard, the variables involved may extend beyond the expertise of the gastroenterologist.28 For instance, in addition to the procedural risk for bleeding, the indication for antithrombotic therapy, risk of a thrombotic event, duration of action of the antithrombotic, and available bridging options should all be considered according to recommendations.28,29 While requiring more time on the part of the gastroenterologist, the optimal periprocedural management of antithrombotic agents would usually involve discussion with the provider managing antithrombotic therapy to best conduct a risk-benefit assessment regarding if (and how long) the antithrombotic therapy should be held (Figure). This shared decision-making, which should also include the patient, may help decrease MPL risk and improve outcomes.
Provider defense mechanisms
Physicians may engage in various defensive behaviors in an attempt to mitigate MPL risk; however, these behaviors may, paradoxically, increase risk.30,31
Assurance behaviors
Assurance behaviors refer to the practice of recommending or performing additional services (such as medications, imaging, procedures, and referrals) that are not clearly indicated.2,30,31 Assurance behaviors are driven by fear of MPL risk and/or missing a potential diagnosis. Recent studies have estimated that more than 50% of gastroenterologists worldwide have performed additional invasive procedures without clear indications, and that nearly one-third of endoscopic procedures annually have questionable indications.30,32 While assurance behaviors may seem likely to decrease MPL risk, overall, they may inadvertently increase AE and MPL risk, as well as health care expenditures.3,30,32
Avoidance behaviors
Avoidance behaviors refer to providers avoiding participation in potentially high-risk clinical interventions (for example, the actual procedures), including those for which they are credentialed/certified proficient.30,31 Two clinical scenarios that illustrate this behavior include the following: An advanced endoscopist credentialed to perform ERCP might refer a “high-risk” elderly patient with cholangitis to another provider to perform said ERCP or for percutaneous transhepatic drainage (in the absence of a clear benefit to such), or a gastroenterologist might refer a patient to interventional gastroenterology for resection of a large polyp even though gastroenterologists are usually proficient in this skill and may feel comfortable performing the resection themselves. Avoidance behaviors are driven by a fear of MPL risk and can have several negative consequences.33 For example, patients may not receive indicated interventions. Additionally, patients may have to wait longer for an intervention because they are referred to another provider, which also increases potential for loss to follow-up.2,30,31 This may be viewed as noncompliance with the standard of care, among other hazards, thereby increasing MPL risk.
Documentation tenets
Thorough documentation can decrease MPL risk, especially since it is often used as legal evidence.16 Documenting, for instance, preprocedure discussion of potential risk of AEs (such as bleeding or perforation) or procedural failure (for example, missed lesions)can protect gastroenterologists (Figure).16 While, as discussed previously, these should be covered in the informed consent process (which itself reduces MPL risk), proof of compliance in providing adequate informed consent must come in the form of documentation that indicates that the process took place and specifically what topics were discussed therein. MPL risk may be further decreased by documenting steps taken during a procedure and anatomic landmarks encountered to offer proof of technical competency and compliance with standards of care (Figure).16,34 In this context, it is worth recalling the adage: “If it’s not documented, it did not occur.”
Curbside consults versus consultation
Also germane here is the topic of whether documentation is needed for “curbside consults.” The uncertainty is, in part, semantic; that is, at what point does a “curbside” become a consultation? A curbside is a general question or query (such as anything that could also be answered by searching the Internet or reference materials) in response to which information is provided; once it involves provision of medical advice for a specific patient (for example, when patient identifiers have been shared or their EHR has been accessed), it constitutes a consultation. Based on these definitions, a curbside need not be documented, whereas a consultation – even if seemingly trivial – should be.
Consideration of language and cultural factors
Language barriers should be considered when the gastroenterologist is communicating with the patient, and such efforts, whenever made, should be documented to best protect against MPL.16,35 These considerations arise not only during the consent process but when obtaining a history, providing postprocedure instructions, and during follow-ups. To this end, 24/7 telephone interpreter services may assist the gastroenterologist (when one is communicating with non–English speakers and is not medically certified in the patient’s native/preferred language) and strengthen trust in the provider-patient relationship.36 Additionally, written materials (such as consent forms, procedural information) in patients’ native/preferred languages should be provided, when available, to enhance patient understanding and participation in care (Figure).35
Challenges posed by telemedicine
The COVID-19 pandemic has rapidly led to more virtual encounters. While increased utilization of telemedicine platforms may make health care more accessible, it does not lessen the clinicians’ duty to patients and may actually expose them to greater MPL risk.18,37,38 Therefore, the provider must be cognizant of two key principles to mitigate MPL risk in the context of telemedicine encounters. First, the same standard of care applies to virtual and in-person encounters.18,37,38 Second, patient privacy and HIPAA regulations are not waived during telemedicine encounters, and breaches of such may result in an MPL claim.18,37,38
With regard to the first principle, for patients who have not been physically examined (such as when a telemedicine visit was substituted for an in-person clinic encounter), gastroenterologists should not overlook requesting timely preprocedure anesthesia consultation or obtaining additional laboratory studies as needed to ensure safety and the same standard of care. Moreover, particularly in the context of pandemic-related decreased procedural capacity, triaging procedures can be especially challenging. Standardized institutional criteria which prioritize certain diagnoses/conditions over others, leaving room for justifiable exceptions, are advisable.
Vicarious liability
“Vicarious liability” is defined as that extending to persons who have not committed a wrong but on whose behalf wrongdoers acted.39 Therefore, gastroenterologists may be liable not only for their own actions but also for those of personnel they supervise (such as fellow trainees and non–physician practitioners).39 Vicarious liability aims to ensure that systemic checks and balances are in place so that, if failure occurs, harm can still be mitigated and/or avoided, as illustrated by Reason’s “Swiss Cheese Model.”40
Conclusion
Any gastroenterologist can experience an MPL claim. Such an experience can be especially stressful and confusing to early-career clinicians, especially if they’re unfamiliar with legal proceedings. Although MPL principles are not often taught in medical school or residency, it is important for gastroenterologists to be informed regarding tenets of MPL and cognizant of clinical situations which have relatively higher MPL risk. This can assuage untoward angst regarding MPL and highlight proactive risk-mitigation strategies. In general, gastroenterologist practices that can mitigate MPL risk include effective communication; adequate informed consent/refusal; documentation of preprocedure counseling, periprocedure events, and postprocedure recommendations; and maintenance of proper certification and privileging.
Dr. Azizian and Dr. Dalai are with the University of California, Los Angeles and the department of medicine at Olive View–UCLA Medical Center, Sylmar, Calif. They are co–first authors of this paper. Dr. Dalai is also with the division of gastroenterology at the University of New Mexico, Albuquerque. Dr. Adams is with the Center for Clinical Management Research in Veterans Affairs Ann Arbor Healthcare System, the division of gastroenterology at the University of Michigan Health System, and the Institute for Healthcare Policy and Innovation, all in Ann Arbor, Mich. Dr. Tabibian is with UCLA and the division of gastroenterology at Olive View–UCLA Medical Center. The authors have no conflicts of interest.
References
1. 2020 Data Sharing Project Gastroenterology 2009-2018. Inside Medical Liability: Second Quarter. Accessed 2020 Dec 6.
2. Mello MM et al. Health Aff (Millwood). 2004 Jul-Aug;23(4):42-53.
3. Adams MA et al. JAMA. 2014 Oct;312(13):1348-9.
4. Pegalis SE. American Law of Medical Malpractice 3d, Vol. 2. St. Paul, Minn.: Thomson Reuters, 2005.
5. Feld LD et al. Am J Gastroenterol. 2018 Nov;113(11):1577-9.
6. Sawyer v. Wight, 196 F. Supp. 2d 220, 226 (E.D.N.Y. 2002).
7. Michael A. Sita v. Long Island Jewish-Hillside Medical Center, 22 A.D.3d 743 (N.Y. App. Div. 2005).
8. Conklin LS et al. Clin Gastroenterol Hepatol. 2008 Jun;6(6):677-81.
9. Jena AB et al. N Engl J Med. 2011 Aug 18;365(7):629-36.
10. Kane CK. “Policy Research Perspectives Medical Liability Claim Frequency: A 2007-2008 Snapshot of Physicians.” Chicago: American Medical Association, 2010.
11. Hernandez LV et al. World J Gastrointest Endosc. 2013 Apr 16;5(4):169-73.
12. Schaffer AC et al. JAMA Intern Med. 2017 May 1;177(5):710-8.
13. Natanson v. Kline, 186 Kan. 393, 409, 350 P.2d 1093, 1106, decision clarified on denial of reh’g, 187 Kan. 186, 354 P.2d 670 (1960).
14. Truman v. Thomas, 27 Cal. 3d 285, 292, 611 P.2d 902, 906 (1980).
15. Shah P et al. Informed Consent, in “StatPearls.” Treasure Island, Fla.: StatPearls Publishing, 2020 Jan. Updated 2020 Aug 22.
16. Rex DK. Clin Gastroenterol Hepatol. 2013 Jul;11(7):768-73.
17. Gerstenberger PD, Plumeri PA. Gastrointest Endosc. Mar-Apr 1993;39(2):132-8.
18. Adams MA and Allen JI. Clin Gastroenterol Hepatol. 2019 Nov;17(12):2392-6.e1.
19. Ahlawat R et al. Esophagogastroduodenoscopy, in “StatPearls.” Treasure Island, Fla.: StatPearls Publishing, 2020 Jan. Updated 2020 Dec 9.
20. Cotton PB. Gastrointest Endosc. 2006 Mar;63(3):378-82.
21. Cotton PB. Gastrointest Endosc. 2010 Oct;72(4):904.
22. Adamson TE et al. West J Med. 1989 Mar;150(3):356-60.
23. Trap R et al. Endoscopy. 1999 Feb;31(2):125-30.
24. Funaki B. Semin Intervent Radiol. 2015 Mar;32(1):61-4.
25. Feeding Tube Nursing Home and Hospital Malpractice. Miller & Zois, Attorneys at Law. Accessed 2020 Jun 20.
26. Medical Malpractice Lawsuit Brings $750,000 Settlement: Death of 82-year-old woman from sepsis due to improper placement of feeding tube. Lubin & Meyers PC. Accessed 2020 Jun 20.
27. Brendel RW et al. Med Clin North Am. 2010 Nov;94(6):1229-40, xi-ii.
28. ASGE Standards of Practice Committee; Acosta RD et al. Gastrointest Endosc. 2016 Jan;83(1):3-16.
29. Saleem S and Thomas AL. Cureus. 2018 Jun 25;10(6):e2878.
30. Hiyama T et al. World J Gastroenterol. 2006 Dec 21;12(47):7671-5.
31. Studdert DM et al. JAMA. 2005 Jun 1;293(21):2609-17.
32. Shaheen NJ et al. Gastroenterology. 2018 May;154(7):1993-2003.
33. Oza VM et al. Clin Gastroenterol Hepatol. 2016 Feb;14(2):172-4.
34. Feld AD. Gastrointest Endosc Clin N Am. 2002 Jan;12(1):171-9, viii-ix.
35. Lee JS et al. J Gen Intern Med. 2017 Aug;32(8):863-70.
36. Forrow L and Kontrimas JC. J Gen Intern Med. 2017 Aug;32(8):855-7.
37. Moses RE et al. Am J Gastroenterol. 2014 Aug;109(8):1128-32.
38. Tabibian JH. “The Evolution of Telehealth.” Guidepoint: Legal Solutions Blog. Accessed 2020 Aug 12.
39. Feld AD. Am J Gastroenterol. 2004 Sep;99(9):1641-4.
40. Reason J. BMJ. 2000;320(7237):768‐70.
Disclaimer: This article is for educational purposes only. All examples are hypothetical and aim to illustrate common clinical scenarios and challenges gastroenterologists may encounter within their scope of practice. The content herein should not be interpreted as legal advice for individual cases nor a substitute for seeking the advice of an attorney.
There are unique potential stressors faced by the gastroenterologist at each career stage, some more so early on. One such stressor, and one particularly important in a procedure-intensive specialty like GI, is medical professional liability (MPL), historically termed “medical malpractice.” Between 2009 and 2018, GI was the second-highest internal medicine subspecialty in both MPL claims made and claims paid,1 yet instruction on MPL risk and mitigation is scarce in fellowship, as is the available GI-related literature on the topic. This scarcity may generate untoward stress and unnecessarily expose gastroenterologists to avoidable MPL pitfalls. Therefore, it is vital for GI trainees, early-career gastroenterologists, and even seasoned gastroenterologists to have a working and updated knowledge of the general principles of MPL and GI-specific considerations. Such understanding can help preserve physician well-being, increase professional satisfaction, strengthen the doctor-patient relationship, and improve health care outcomes.2
To this end, we herein provide a focused review of the following: key MPL concepts, trends in MPL claims, GI-related MPL risk scenarios and considerations, adverse provider defensive mechanisms, documentation tenets, challenges posed by telemedicine, and the concept of “vicarious liability.”
Key MPL concepts
MPL falls under the umbrella of tort law, which itself falls under the umbrella of civil law; that is, civil (as opposed to criminal) justice governs torts – including but not limited to MPL claims – as well as other areas of law concerning noncriminal injury.3 A “tort” is a “civil wrong that unfairly causes another to experience loss or harm resulting in legal liability.”3 MPL claims assert the tort of negligence (similar to the concept of “incompetence”) and endeavor to compensate the harmed patient/individual while simultaneously dissuading suboptimal medical care by the provider in the future.4,5 A successful MPL claim must prove four overlapping elements: that the tortfeasor (here, the gastroenterologist) owed a duty of care to the injured party and breached that duty, which caused damages.6 Given that MPL cases exist within tort law rather than criminal law, the burden of proof for these cases is not “beyond a reasonable doubt”; instead, it’s “to a reasonable medical probability.”7
Trends in MPL claims
According to data compiled by the MPL Association, 278,220 MPL claims were made in the United States from 1985 to 2012.3,8-10 Among these, 1.8% involved gastroenterologists, which puts it at 17th place out of the 20 specialties surveyed.9 While the number of paid claims over this time frame decreased in GI by 34.6% (from 18.5 to 12.1 cases per 1,000 physician-years), there was a concurrent 23.3% increase in average claim compensation; essentially, there were fewer paid GI-related claims but there were higher payouts per paid claim.11,12 From 2009 to 2018, average legal defense costs for paid GI-related claims were $97,392, and average paid amount was $330,876.1
GI-related MPL risk scenarios and considerations
Many MPL claims relate to situations involving medical errors or adverse events (AEs), be they procedural or nonprocedural. However other aspects of GI also carry MPL risk.

Informed consent
MPL claims may be made not only on the grounds of inadequately informed consent but also inadequately informed refusal.5,13,14 While standards for adequate informed consent vary by state, most states apply the “reasonable patient standard,” i.e., assuming an average patient with enough information to be an active participant in the medical decision-making process. Generally, informed consent should ensure that the patient understands the nature of the procedure/treatment being proposed, there is a discussion of the risks and benefits of undergoing and not undergoing the procedure/treatment, reasonable alternatives are presented, the risks and benefits associated with these alternatives are discussed, and the patient’s comprehension of these things is assessed (Figure).15 Additionally, informed consent should be tailored to each patient and GI procedure/treatment on a case-by-case basis rather than using a one-size-fits-all approach. Moreover, documentation of the patient’s understanding of the (tailored) information provided can concurrently improve quality of the consent and potentially decrease MPL risk (Figure).16
Endoscopic procedures
Procedure-related MPL claims represent approximately 25% of all GI-related claims (8,17). Among these, 52% involve colonoscopy, 16% involve endoscopic retrograde cholangiopancreatography (ERCP), and 11% involve esophagogastroduodenoscopy.8 Albeit generally safe, colonoscopy, as with esophagogastroduodenoscopy, is subject to rare but serious AEs.18,19 Risk of these AEs may be accentuated in certain scenarios (such as severe colonic inflammation or coagulopathy) and, as discussed earlier, may merit tailored informed consent. Regardless of the procedure, in the event of postprocedural development of signs/symptoms (such as tachycardia, fever, chest or abdominal discomfort, or hypotension) indicating a potential AE, stabilizing measures and evaluation (such as blood work and imaging) should be undertaken, and hospital admission (if not already hospitalized) should be considered until discharge is deemed safe.19
ERCP-related MPL claims, for many years, have had the highest average compensation of any GI procedure.11 Though discussion of advanced procedures is beyond the scope of this article, it is worth mentioning the observation that most of such claims involve an allegation that the procedure was not indicated (for example, that it was performed based on inadequate evidence of pancreatobiliary pathology), or was for diagnostic purposes (for example, being done instead of noninvasive imaging) rather than therapeutic.20-23 This emphasizes the importance of appropriate procedure indications.
Percutaneous endoscopic gastrostomy (PEG) placement merits special mention given it can be complicated by ethical challenges (for example, needing a surrogate decision-maker’s consent or representing medical futility) and has a relatively high potential for MPL claims. PEG placement carries a low AE rate (0.1%-1%), but these AEs may result in high morbidity/mortality, in part because of the underlying comorbidities of patients needing PEG placement.24,25 Also, timing of a patient’s demise may coincide with PEG placement, thereby prompting (possibly unfounded) perceptions of causality.24-27 Therefore, such scenarios merit unique additional preprocedure safeguards. For instance, for patients lacking capacity to provide informed consent, especially when family members may differ on whether PEG should be placed, it is advisable to ask the family to select one surrogate decision-maker (if there’s no advance directive) to whom the gastroenterologist should discuss both the risks, benefits, and goals of PEG placement in the context of the patient’s overall clinical trajectory/life expectancy and the need for consent (or refusal) based on what the patient would have wished. In addition, having a medical professional witness this discussion may be useful.27
Antithrombotic agents
Periprocedural management of antithrombotics, including anticoagulants and antiplatelets, can pose challenges for the gastroenterologist. While clinical practice guidelines exist to guide decision-making in this regard, the variables involved may extend beyond the expertise of the gastroenterologist.28 For instance, in addition to the procedural risk for bleeding, the indication for antithrombotic therapy, risk of a thrombotic event, duration of action of the antithrombotic, and available bridging options should all be considered according to recommendations.28,29 While requiring more time on the part of the gastroenterologist, the optimal periprocedural management of antithrombotic agents would usually involve discussion with the provider managing antithrombotic therapy to best conduct a risk-benefit assessment regarding if (and how long) the antithrombotic therapy should be held (Figure). This shared decision-making, which should also include the patient, may help decrease MPL risk and improve outcomes.
Provider defense mechanisms
Physicians may engage in various defensive behaviors in an attempt to mitigate MPL risk; however, these behaviors may, paradoxically, increase risk.30,31
Assurance behaviors
Assurance behaviors refer to the practice of recommending or performing additional services (such as medications, imaging, procedures, and referrals) that are not clearly indicated.2,30,31 Assurance behaviors are driven by fear of MPL risk and/or missing a potential diagnosis. Recent studies have estimated that more than 50% of gastroenterologists worldwide have performed additional invasive procedures without clear indications, and that nearly one-third of endoscopic procedures annually have questionable indications.30,32 While assurance behaviors may seem likely to decrease MPL risk, overall, they may inadvertently increase AE and MPL risk, as well as health care expenditures.3,30,32
Avoidance behaviors
Avoidance behaviors refer to providers avoiding participation in potentially high-risk clinical interventions (for example, the actual procedures), including those for which they are credentialed/certified proficient.30,31 Two clinical scenarios that illustrate this behavior include the following: An advanced endoscopist credentialed to perform ERCP might refer a “high-risk” elderly patient with cholangitis to another provider to perform said ERCP or for percutaneous transhepatic drainage (in the absence of a clear benefit to such), or a gastroenterologist might refer a patient to interventional gastroenterology for resection of a large polyp even though gastroenterologists are usually proficient in this skill and may feel comfortable performing the resection themselves. Avoidance behaviors are driven by a fear of MPL risk and can have several negative consequences.33 For example, patients may not receive indicated interventions. Additionally, patients may have to wait longer for an intervention because they are referred to another provider, which also increases potential for loss to follow-up.2,30,31 This may be viewed as noncompliance with the standard of care, among other hazards, thereby increasing MPL risk.
Documentation tenets
Thorough documentation can decrease MPL risk, especially since it is often used as legal evidence.16 Documenting, for instance, preprocedure discussion of potential risk of AEs (such as bleeding or perforation) or procedural failure (for example, missed lesions)can protect gastroenterologists (Figure).16 While, as discussed previously, these should be covered in the informed consent process (which itself reduces MPL risk), proof of compliance in providing adequate informed consent must come in the form of documentation that indicates that the process took place and specifically what topics were discussed therein. MPL risk may be further decreased by documenting steps taken during a procedure and anatomic landmarks encountered to offer proof of technical competency and compliance with standards of care (Figure).16,34 In this context, it is worth recalling the adage: “If it’s not documented, it did not occur.”
Curbside consults versus consultation
Also germane here is the topic of whether documentation is needed for “curbside consults.” The uncertainty is, in part, semantic; that is, at what point does a “curbside” become a consultation? A curbside is a general question or query (such as anything that could also be answered by searching the Internet or reference materials) in response to which information is provided; once it involves provision of medical advice for a specific patient (for example, when patient identifiers have been shared or their EHR has been accessed), it constitutes a consultation. Based on these definitions, a curbside need not be documented, whereas a consultation – even if seemingly trivial – should be.
Consideration of language and cultural factors
Language barriers should be considered when the gastroenterologist is communicating with the patient, and such efforts, whenever made, should be documented to best protect against MPL.16,35 These considerations arise not only during the consent process but when obtaining a history, providing postprocedure instructions, and during follow-ups. To this end, 24/7 telephone interpreter services may assist the gastroenterologist (when one is communicating with non–English speakers and is not medically certified in the patient’s native/preferred language) and strengthen trust in the provider-patient relationship.36 Additionally, written materials (such as consent forms, procedural information) in patients’ native/preferred languages should be provided, when available, to enhance patient understanding and participation in care (Figure).35
Challenges posed by telemedicine
The COVID-19 pandemic has rapidly led to more virtual encounters. While increased utilization of telemedicine platforms may make health care more accessible, it does not lessen the clinicians’ duty to patients and may actually expose them to greater MPL risk.18,37,38 Therefore, the provider must be cognizant of two key principles to mitigate MPL risk in the context of telemedicine encounters. First, the same standard of care applies to virtual and in-person encounters.18,37,38 Second, patient privacy and HIPAA regulations are not waived during telemedicine encounters, and breaches of such may result in an MPL claim.18,37,38
With regard to the first principle, for patients who have not been physically examined (such as when a telemedicine visit was substituted for an in-person clinic encounter), gastroenterologists should not overlook requesting timely preprocedure anesthesia consultation or obtaining additional laboratory studies as needed to ensure safety and the same standard of care. Moreover, particularly in the context of pandemic-related decreased procedural capacity, triaging procedures can be especially challenging. Standardized institutional criteria which prioritize certain diagnoses/conditions over others, leaving room for justifiable exceptions, are advisable.
Vicarious liability
“Vicarious liability” is defined as that extending to persons who have not committed a wrong but on whose behalf wrongdoers acted.39 Therefore, gastroenterologists may be liable not only for their own actions but also for those of personnel they supervise (such as fellow trainees and non–physician practitioners).39 Vicarious liability aims to ensure that systemic checks and balances are in place so that, if failure occurs, harm can still be mitigated and/or avoided, as illustrated by Reason’s “Swiss Cheese Model.”40
Conclusion
Any gastroenterologist can experience an MPL claim. Such an experience can be especially stressful and confusing to early-career clinicians, especially if they’re unfamiliar with legal proceedings. Although MPL principles are not often taught in medical school or residency, it is important for gastroenterologists to be informed regarding tenets of MPL and cognizant of clinical situations which have relatively higher MPL risk. This can assuage untoward angst regarding MPL and highlight proactive risk-mitigation strategies. In general, gastroenterologist practices that can mitigate MPL risk include effective communication; adequate informed consent/refusal; documentation of preprocedure counseling, periprocedure events, and postprocedure recommendations; and maintenance of proper certification and privileging.
Dr. Azizian and Dr. Dalai are with the University of California, Los Angeles and the department of medicine at Olive View–UCLA Medical Center, Sylmar, Calif. They are co–first authors of this paper. Dr. Dalai is also with the division of gastroenterology at the University of New Mexico, Albuquerque. Dr. Adams is with the Center for Clinical Management Research in Veterans Affairs Ann Arbor Healthcare System, the division of gastroenterology at the University of Michigan Health System, and the Institute for Healthcare Policy and Innovation, all in Ann Arbor, Mich. Dr. Tabibian is with UCLA and the division of gastroenterology at Olive View–UCLA Medical Center. The authors have no conflicts of interest.
References
1. 2020 Data Sharing Project Gastroenterology 2009-2018. Inside Medical Liability: Second Quarter. Accessed 2020 Dec 6.
2. Mello MM et al. Health Aff (Millwood). 2004 Jul-Aug;23(4):42-53.
3. Adams MA et al. JAMA. 2014 Oct;312(13):1348-9.
4. Pegalis SE. American Law of Medical Malpractice 3d, Vol. 2. St. Paul, Minn.: Thomson Reuters, 2005.
5. Feld LD et al. Am J Gastroenterol. 2018 Nov;113(11):1577-9.
6. Sawyer v. Wight, 196 F. Supp. 2d 220, 226 (E.D.N.Y. 2002).
7. Michael A. Sita v. Long Island Jewish-Hillside Medical Center, 22 A.D.3d 743 (N.Y. App. Div. 2005).
8. Conklin LS et al. Clin Gastroenterol Hepatol. 2008 Jun;6(6):677-81.
9. Jena AB et al. N Engl J Med. 2011 Aug 18;365(7):629-36.
10. Kane CK. “Policy Research Perspectives Medical Liability Claim Frequency: A 2007-2008 Snapshot of Physicians.” Chicago: American Medical Association, 2010.
11. Hernandez LV et al. World J Gastrointest Endosc. 2013 Apr 16;5(4):169-73.
12. Schaffer AC et al. JAMA Intern Med. 2017 May 1;177(5):710-8.
13. Natanson v. Kline, 186 Kan. 393, 409, 350 P.2d 1093, 1106, decision clarified on denial of reh’g, 187 Kan. 186, 354 P.2d 670 (1960).
14. Truman v. Thomas, 27 Cal. 3d 285, 292, 611 P.2d 902, 906 (1980).
15. Shah P et al. Informed Consent, in “StatPearls.” Treasure Island, Fla.: StatPearls Publishing, 2020 Jan. Updated 2020 Aug 22.
16. Rex DK. Clin Gastroenterol Hepatol. 2013 Jul;11(7):768-73.
17. Gerstenberger PD, Plumeri PA. Gastrointest Endosc. Mar-Apr 1993;39(2):132-8.
18. Adams MA and Allen JI. Clin Gastroenterol Hepatol. 2019 Nov;17(12):2392-6.e1.
19. Ahlawat R et al. Esophagogastroduodenoscopy, in “StatPearls.” Treasure Island, Fla.: StatPearls Publishing, 2020 Jan. Updated 2020 Dec 9.
20. Cotton PB. Gastrointest Endosc. 2006 Mar;63(3):378-82.
21. Cotton PB. Gastrointest Endosc. 2010 Oct;72(4):904.
22. Adamson TE et al. West J Med. 1989 Mar;150(3):356-60.
23. Trap R et al. Endoscopy. 1999 Feb;31(2):125-30.
24. Funaki B. Semin Intervent Radiol. 2015 Mar;32(1):61-4.
25. Feeding Tube Nursing Home and Hospital Malpractice. Miller & Zois, Attorneys at Law. Accessed 2020 Jun 20.
26. Medical Malpractice Lawsuit Brings $750,000 Settlement: Death of 82-year-old woman from sepsis due to improper placement of feeding tube. Lubin & Meyers PC. Accessed 2020 Jun 20.
27. Brendel RW et al. Med Clin North Am. 2010 Nov;94(6):1229-40, xi-ii.
28. ASGE Standards of Practice Committee; Acosta RD et al. Gastrointest Endosc. 2016 Jan;83(1):3-16.
29. Saleem S and Thomas AL. Cureus. 2018 Jun 25;10(6):e2878.
30. Hiyama T et al. World J Gastroenterol. 2006 Dec 21;12(47):7671-5.
31. Studdert DM et al. JAMA. 2005 Jun 1;293(21):2609-17.
32. Shaheen NJ et al. Gastroenterology. 2018 May;154(7):1993-2003.
33. Oza VM et al. Clin Gastroenterol Hepatol. 2016 Feb;14(2):172-4.
34. Feld AD. Gastrointest Endosc Clin N Am. 2002 Jan;12(1):171-9, viii-ix.
35. Lee JS et al. J Gen Intern Med. 2017 Aug;32(8):863-70.
36. Forrow L and Kontrimas JC. J Gen Intern Med. 2017 Aug;32(8):855-7.
37. Moses RE et al. Am J Gastroenterol. 2014 Aug;109(8):1128-32.
38. Tabibian JH. “The Evolution of Telehealth.” Guidepoint: Legal Solutions Blog. Accessed 2020 Aug 12.
39. Feld AD. Am J Gastroenterol. 2004 Sep;99(9):1641-4.
40. Reason J. BMJ. 2000;320(7237):768‐70.
Disclaimer: This article is for educational purposes only. All examples are hypothetical and aim to illustrate common clinical scenarios and challenges gastroenterologists may encounter within their scope of practice. The content herein should not be interpreted as legal advice for individual cases nor a substitute for seeking the advice of an attorney.
There are unique potential stressors faced by the gastroenterologist at each career stage, some more so early on. One such stressor, and one particularly important in a procedure-intensive specialty like GI, is medical professional liability (MPL), historically termed “medical malpractice.” Between 2009 and 2018, GI was the second-highest internal medicine subspecialty in both MPL claims made and claims paid,1 yet instruction on MPL risk and mitigation is scarce in fellowship, as is the available GI-related literature on the topic. This scarcity may generate untoward stress and unnecessarily expose gastroenterologists to avoidable MPL pitfalls. Therefore, it is vital for GI trainees, early-career gastroenterologists, and even seasoned gastroenterologists to have a working and updated knowledge of the general principles of MPL and GI-specific considerations. Such understanding can help preserve physician well-being, increase professional satisfaction, strengthen the doctor-patient relationship, and improve health care outcomes.2
To this end, we herein provide a focused review of the following: key MPL concepts, trends in MPL claims, GI-related MPL risk scenarios and considerations, adverse provider defensive mechanisms, documentation tenets, challenges posed by telemedicine, and the concept of “vicarious liability.”
Key MPL concepts
MPL falls under the umbrella of tort law, which itself falls under the umbrella of civil law; that is, civil (as opposed to criminal) justice governs torts – including but not limited to MPL claims – as well as other areas of law concerning noncriminal injury.3 A “tort” is a “civil wrong that unfairly causes another to experience loss or harm resulting in legal liability.”3 MPL claims assert the tort of negligence (similar to the concept of “incompetence”) and endeavor to compensate the harmed patient/individual while simultaneously dissuading suboptimal medical care by the provider in the future.4,5 A successful MPL claim must prove four overlapping elements: that the tortfeasor (here, the gastroenterologist) owed a duty of care to the injured party and breached that duty, which caused damages.6 Given that MPL cases exist within tort law rather than criminal law, the burden of proof for these cases is not “beyond a reasonable doubt”; instead, it’s “to a reasonable medical probability.”7
Trends in MPL claims
According to data compiled by the MPL Association, 278,220 MPL claims were made in the United States from 1985 to 2012.3,8-10 Among these, 1.8% involved gastroenterologists, which puts it at 17th place out of the 20 specialties surveyed.9 While the number of paid claims over this time frame decreased in GI by 34.6% (from 18.5 to 12.1 cases per 1,000 physician-years), there was a concurrent 23.3% increase in average claim compensation; essentially, there were fewer paid GI-related claims but there were higher payouts per paid claim.11,12 From 2009 to 2018, average legal defense costs for paid GI-related claims were $97,392, and average paid amount was $330,876.1
GI-related MPL risk scenarios and considerations
Many MPL claims relate to situations involving medical errors or adverse events (AEs), be they procedural or nonprocedural. However other aspects of GI also carry MPL risk.

Informed consent
MPL claims may be made not only on the grounds of inadequately informed consent but also inadequately informed refusal.5,13,14 While standards for adequate informed consent vary by state, most states apply the “reasonable patient standard,” i.e., assuming an average patient with enough information to be an active participant in the medical decision-making process. Generally, informed consent should ensure that the patient understands the nature of the procedure/treatment being proposed, there is a discussion of the risks and benefits of undergoing and not undergoing the procedure/treatment, reasonable alternatives are presented, the risks and benefits associated with these alternatives are discussed, and the patient’s comprehension of these things is assessed (Figure).15 Additionally, informed consent should be tailored to each patient and GI procedure/treatment on a case-by-case basis rather than using a one-size-fits-all approach. Moreover, documentation of the patient’s understanding of the (tailored) information provided can concurrently improve quality of the consent and potentially decrease MPL risk (Figure).16
Endoscopic procedures
Procedure-related MPL claims represent approximately 25% of all GI-related claims (8,17). Among these, 52% involve colonoscopy, 16% involve endoscopic retrograde cholangiopancreatography (ERCP), and 11% involve esophagogastroduodenoscopy.8 Albeit generally safe, colonoscopy, as with esophagogastroduodenoscopy, is subject to rare but serious AEs.18,19 Risk of these AEs may be accentuated in certain scenarios (such as severe colonic inflammation or coagulopathy) and, as discussed earlier, may merit tailored informed consent. Regardless of the procedure, in the event of postprocedural development of signs/symptoms (such as tachycardia, fever, chest or abdominal discomfort, or hypotension) indicating a potential AE, stabilizing measures and evaluation (such as blood work and imaging) should be undertaken, and hospital admission (if not already hospitalized) should be considered until discharge is deemed safe.19
ERCP-related MPL claims, for many years, have had the highest average compensation of any GI procedure.11 Though discussion of advanced procedures is beyond the scope of this article, it is worth mentioning the observation that most of such claims involve an allegation that the procedure was not indicated (for example, that it was performed based on inadequate evidence of pancreatobiliary pathology), or was for diagnostic purposes (for example, being done instead of noninvasive imaging) rather than therapeutic.20-23 This emphasizes the importance of appropriate procedure indications.
Percutaneous endoscopic gastrostomy (PEG) placement merits special mention given it can be complicated by ethical challenges (for example, needing a surrogate decision-maker’s consent or representing medical futility) and has a relatively high potential for MPL claims. PEG placement carries a low AE rate (0.1%-1%), but these AEs may result in high morbidity/mortality, in part because of the underlying comorbidities of patients needing PEG placement.24,25 Also, timing of a patient’s demise may coincide with PEG placement, thereby prompting (possibly unfounded) perceptions of causality.24-27 Therefore, such scenarios merit unique additional preprocedure safeguards. For instance, for patients lacking capacity to provide informed consent, especially when family members may differ on whether PEG should be placed, it is advisable to ask the family to select one surrogate decision-maker (if there’s no advance directive) to whom the gastroenterologist should discuss both the risks, benefits, and goals of PEG placement in the context of the patient’s overall clinical trajectory/life expectancy and the need for consent (or refusal) based on what the patient would have wished. In addition, having a medical professional witness this discussion may be useful.27
Antithrombotic agents
Periprocedural management of antithrombotics, including anticoagulants and antiplatelets, can pose challenges for the gastroenterologist. While clinical practice guidelines exist to guide decision-making in this regard, the variables involved may extend beyond the expertise of the gastroenterologist.28 For instance, in addition to the procedural risk for bleeding, the indication for antithrombotic therapy, risk of a thrombotic event, duration of action of the antithrombotic, and available bridging options should all be considered according to recommendations.28,29 While requiring more time on the part of the gastroenterologist, the optimal periprocedural management of antithrombotic agents would usually involve discussion with the provider managing antithrombotic therapy to best conduct a risk-benefit assessment regarding if (and how long) the antithrombotic therapy should be held (Figure). This shared decision-making, which should also include the patient, may help decrease MPL risk and improve outcomes.
Provider defense mechanisms
Physicians may engage in various defensive behaviors in an attempt to mitigate MPL risk; however, these behaviors may, paradoxically, increase risk.30,31
Assurance behaviors
Assurance behaviors refer to the practice of recommending or performing additional services (such as medications, imaging, procedures, and referrals) that are not clearly indicated.2,30,31 Assurance behaviors are driven by fear of MPL risk and/or missing a potential diagnosis. Recent studies have estimated that more than 50% of gastroenterologists worldwide have performed additional invasive procedures without clear indications, and that nearly one-third of endoscopic procedures annually have questionable indications.30,32 While assurance behaviors may seem likely to decrease MPL risk, overall, they may inadvertently increase AE and MPL risk, as well as health care expenditures.3,30,32
Avoidance behaviors
Avoidance behaviors refer to providers avoiding participation in potentially high-risk clinical interventions (for example, the actual procedures), including those for which they are credentialed/certified proficient.30,31 Two clinical scenarios that illustrate this behavior include the following: An advanced endoscopist credentialed to perform ERCP might refer a “high-risk” elderly patient with cholangitis to another provider to perform said ERCP or for percutaneous transhepatic drainage (in the absence of a clear benefit to such), or a gastroenterologist might refer a patient to interventional gastroenterology for resection of a large polyp even though gastroenterologists are usually proficient in this skill and may feel comfortable performing the resection themselves. Avoidance behaviors are driven by a fear of MPL risk and can have several negative consequences.33 For example, patients may not receive indicated interventions. Additionally, patients may have to wait longer for an intervention because they are referred to another provider, which also increases potential for loss to follow-up.2,30,31 This may be viewed as noncompliance with the standard of care, among other hazards, thereby increasing MPL risk.
Documentation tenets
Thorough documentation can decrease MPL risk, especially since it is often used as legal evidence.16 Documenting, for instance, preprocedure discussion of potential risk of AEs (such as bleeding or perforation) or procedural failure (for example, missed lesions)can protect gastroenterologists (Figure).16 While, as discussed previously, these should be covered in the informed consent process (which itself reduces MPL risk), proof of compliance in providing adequate informed consent must come in the form of documentation that indicates that the process took place and specifically what topics were discussed therein. MPL risk may be further decreased by documenting steps taken during a procedure and anatomic landmarks encountered to offer proof of technical competency and compliance with standards of care (Figure).16,34 In this context, it is worth recalling the adage: “If it’s not documented, it did not occur.”
Curbside consults versus consultation
Also germane here is the topic of whether documentation is needed for “curbside consults.” The uncertainty is, in part, semantic; that is, at what point does a “curbside” become a consultation? A curbside is a general question or query (such as anything that could also be answered by searching the Internet or reference materials) in response to which information is provided; once it involves provision of medical advice for a specific patient (for example, when patient identifiers have been shared or their EHR has been accessed), it constitutes a consultation. Based on these definitions, a curbside need not be documented, whereas a consultation – even if seemingly trivial – should be.
Consideration of language and cultural factors
Language barriers should be considered when the gastroenterologist is communicating with the patient, and such efforts, whenever made, should be documented to best protect against MPL.16,35 These considerations arise not only during the consent process but when obtaining a history, providing postprocedure instructions, and during follow-ups. To this end, 24/7 telephone interpreter services may assist the gastroenterologist (when one is communicating with non–English speakers and is not medically certified in the patient’s native/preferred language) and strengthen trust in the provider-patient relationship.36 Additionally, written materials (such as consent forms, procedural information) in patients’ native/preferred languages should be provided, when available, to enhance patient understanding and participation in care (Figure).35
Challenges posed by telemedicine
The COVID-19 pandemic has rapidly led to more virtual encounters. While increased utilization of telemedicine platforms may make health care more accessible, it does not lessen the clinicians’ duty to patients and may actually expose them to greater MPL risk.18,37,38 Therefore, the provider must be cognizant of two key principles to mitigate MPL risk in the context of telemedicine encounters. First, the same standard of care applies to virtual and in-person encounters.18,37,38 Second, patient privacy and HIPAA regulations are not waived during telemedicine encounters, and breaches of such may result in an MPL claim.18,37,38
With regard to the first principle, for patients who have not been physically examined (such as when a telemedicine visit was substituted for an in-person clinic encounter), gastroenterologists should not overlook requesting timely preprocedure anesthesia consultation or obtaining additional laboratory studies as needed to ensure safety and the same standard of care. Moreover, particularly in the context of pandemic-related decreased procedural capacity, triaging procedures can be especially challenging. Standardized institutional criteria which prioritize certain diagnoses/conditions over others, leaving room for justifiable exceptions, are advisable.
Vicarious liability
“Vicarious liability” is defined as that extending to persons who have not committed a wrong but on whose behalf wrongdoers acted.39 Therefore, gastroenterologists may be liable not only for their own actions but also for those of personnel they supervise (such as fellow trainees and non–physician practitioners).39 Vicarious liability aims to ensure that systemic checks and balances are in place so that, if failure occurs, harm can still be mitigated and/or avoided, as illustrated by Reason’s “Swiss Cheese Model.”40
Conclusion
Any gastroenterologist can experience an MPL claim. Such an experience can be especially stressful and confusing to early-career clinicians, especially if they’re unfamiliar with legal proceedings. Although MPL principles are not often taught in medical school or residency, it is important for gastroenterologists to be informed regarding tenets of MPL and cognizant of clinical situations which have relatively higher MPL risk. This can assuage untoward angst regarding MPL and highlight proactive risk-mitigation strategies. In general, gastroenterologist practices that can mitigate MPL risk include effective communication; adequate informed consent/refusal; documentation of preprocedure counseling, periprocedure events, and postprocedure recommendations; and maintenance of proper certification and privileging.
Dr. Azizian and Dr. Dalai are with the University of California, Los Angeles and the department of medicine at Olive View–UCLA Medical Center, Sylmar, Calif. They are co–first authors of this paper. Dr. Dalai is also with the division of gastroenterology at the University of New Mexico, Albuquerque. Dr. Adams is with the Center for Clinical Management Research in Veterans Affairs Ann Arbor Healthcare System, the division of gastroenterology at the University of Michigan Health System, and the Institute for Healthcare Policy and Innovation, all in Ann Arbor, Mich. Dr. Tabibian is with UCLA and the division of gastroenterology at Olive View–UCLA Medical Center. The authors have no conflicts of interest.
References
1. 2020 Data Sharing Project Gastroenterology 2009-2018. Inside Medical Liability: Second Quarter. Accessed 2020 Dec 6.
2. Mello MM et al. Health Aff (Millwood). 2004 Jul-Aug;23(4):42-53.
3. Adams MA et al. JAMA. 2014 Oct;312(13):1348-9.
4. Pegalis SE. American Law of Medical Malpractice 3d, Vol. 2. St. Paul, Minn.: Thomson Reuters, 2005.
5. Feld LD et al. Am J Gastroenterol. 2018 Nov;113(11):1577-9.
6. Sawyer v. Wight, 196 F. Supp. 2d 220, 226 (E.D.N.Y. 2002).
7. Michael A. Sita v. Long Island Jewish-Hillside Medical Center, 22 A.D.3d 743 (N.Y. App. Div. 2005).
8. Conklin LS et al. Clin Gastroenterol Hepatol. 2008 Jun;6(6):677-81.
9. Jena AB et al. N Engl J Med. 2011 Aug 18;365(7):629-36.
10. Kane CK. “Policy Research Perspectives Medical Liability Claim Frequency: A 2007-2008 Snapshot of Physicians.” Chicago: American Medical Association, 2010.
11. Hernandez LV et al. World J Gastrointest Endosc. 2013 Apr 16;5(4):169-73.
12. Schaffer AC et al. JAMA Intern Med. 2017 May 1;177(5):710-8.
13. Natanson v. Kline, 186 Kan. 393, 409, 350 P.2d 1093, 1106, decision clarified on denial of reh’g, 187 Kan. 186, 354 P.2d 670 (1960).
14. Truman v. Thomas, 27 Cal. 3d 285, 292, 611 P.2d 902, 906 (1980).
15. Shah P et al. Informed Consent, in “StatPearls.” Treasure Island, Fla.: StatPearls Publishing, 2020 Jan. Updated 2020 Aug 22.
16. Rex DK. Clin Gastroenterol Hepatol. 2013 Jul;11(7):768-73.
17. Gerstenberger PD, Plumeri PA. Gastrointest Endosc. Mar-Apr 1993;39(2):132-8.
18. Adams MA and Allen JI. Clin Gastroenterol Hepatol. 2019 Nov;17(12):2392-6.e1.
19. Ahlawat R et al. Esophagogastroduodenoscopy, in “StatPearls.” Treasure Island, Fla.: StatPearls Publishing, 2020 Jan. Updated 2020 Dec 9.
20. Cotton PB. Gastrointest Endosc. 2006 Mar;63(3):378-82.
21. Cotton PB. Gastrointest Endosc. 2010 Oct;72(4):904.
22. Adamson TE et al. West J Med. 1989 Mar;150(3):356-60.
23. Trap R et al. Endoscopy. 1999 Feb;31(2):125-30.
24. Funaki B. Semin Intervent Radiol. 2015 Mar;32(1):61-4.
25. Feeding Tube Nursing Home and Hospital Malpractice. Miller & Zois, Attorneys at Law. Accessed 2020 Jun 20.
26. Medical Malpractice Lawsuit Brings $750,000 Settlement: Death of 82-year-old woman from sepsis due to improper placement of feeding tube. Lubin & Meyers PC. Accessed 2020 Jun 20.
27. Brendel RW et al. Med Clin North Am. 2010 Nov;94(6):1229-40, xi-ii.
28. ASGE Standards of Practice Committee; Acosta RD et al. Gastrointest Endosc. 2016 Jan;83(1):3-16.
29. Saleem S and Thomas AL. Cureus. 2018 Jun 25;10(6):e2878.
30. Hiyama T et al. World J Gastroenterol. 2006 Dec 21;12(47):7671-5.
31. Studdert DM et al. JAMA. 2005 Jun 1;293(21):2609-17.
32. Shaheen NJ et al. Gastroenterology. 2018 May;154(7):1993-2003.
33. Oza VM et al. Clin Gastroenterol Hepatol. 2016 Feb;14(2):172-4.
34. Feld AD. Gastrointest Endosc Clin N Am. 2002 Jan;12(1):171-9, viii-ix.
35. Lee JS et al. J Gen Intern Med. 2017 Aug;32(8):863-70.
36. Forrow L and Kontrimas JC. J Gen Intern Med. 2017 Aug;32(8):855-7.
37. Moses RE et al. Am J Gastroenterol. 2014 Aug;109(8):1128-32.
38. Tabibian JH. “The Evolution of Telehealth.” Guidepoint: Legal Solutions Blog. Accessed 2020 Aug 12.
39. Feld AD. Am J Gastroenterol. 2004 Sep;99(9):1641-4.
40. Reason J. BMJ. 2000;320(7237):768‐70.
Impact of female hormonal exposure on risk of RA
Key clinical point: Early age at first pregnancy (less than 22 years) and early menopause (45 years and earlier) were associated with a higher risk of rheumatoid arthritis (RA), whereas long-term progestogen use (more than 24 months) before menopause was associated with a reduced risk.
Major finding: The risk of RA was a significantly increased with early age at first pregnancy (less than 22 vs. 27 or more years; hazard ratio [HR], 1.34; Ptrend = .028) and menopause (45 or less vs. 53 or more years; HR, 1.40; Ptrend = .039). Risk of RA was inversely related to exposure to progestogen only in perimenopause (more than 24 vs. 0 months; multi-adjusted HR, 0.77; Ptrend = .004).
Study details: This study included 78,452 women, with 698 incident cases of RA using data from the French E3N-EPIC cohort study.
Disclosures: The study was funded by a research grant from FOREUM Foundation for Research in Rheumatology. The authors declared no conflicts of interest.
Source: Salliot C et al. Rheumatology (Oxford). 2021 Feb 6. doi: 10.1093/rheumatology/keab101.
Key clinical point: Early age at first pregnancy (less than 22 years) and early menopause (45 years and earlier) were associated with a higher risk of rheumatoid arthritis (RA), whereas long-term progestogen use (more than 24 months) before menopause was associated with a reduced risk.
Major finding: The risk of RA was a significantly increased with early age at first pregnancy (less than 22 vs. 27 or more years; hazard ratio [HR], 1.34; Ptrend = .028) and menopause (45 or less vs. 53 or more years; HR, 1.40; Ptrend = .039). Risk of RA was inversely related to exposure to progestogen only in perimenopause (more than 24 vs. 0 months; multi-adjusted HR, 0.77; Ptrend = .004).
Study details: This study included 78,452 women, with 698 incident cases of RA using data from the French E3N-EPIC cohort study.
Disclosures: The study was funded by a research grant from FOREUM Foundation for Research in Rheumatology. The authors declared no conflicts of interest.
Source: Salliot C et al. Rheumatology (Oxford). 2021 Feb 6. doi: 10.1093/rheumatology/keab101.
Key clinical point: Early age at first pregnancy (less than 22 years) and early menopause (45 years and earlier) were associated with a higher risk of rheumatoid arthritis (RA), whereas long-term progestogen use (more than 24 months) before menopause was associated with a reduced risk.
Major finding: The risk of RA was a significantly increased with early age at first pregnancy (less than 22 vs. 27 or more years; hazard ratio [HR], 1.34; Ptrend = .028) and menopause (45 or less vs. 53 or more years; HR, 1.40; Ptrend = .039). Risk of RA was inversely related to exposure to progestogen only in perimenopause (more than 24 vs. 0 months; multi-adjusted HR, 0.77; Ptrend = .004).
Study details: This study included 78,452 women, with 698 incident cases of RA using data from the French E3N-EPIC cohort study.
Disclosures: The study was funded by a research grant from FOREUM Foundation for Research in Rheumatology. The authors declared no conflicts of interest.
Source: Salliot C et al. Rheumatology (Oxford). 2021 Feb 6. doi: 10.1093/rheumatology/keab101.
Modern treatment approach results in LDA and remission in pregnant patients with RA
Key clinical point: A modern treat-to-target (T2T) approach with the use of tumor necrosis factor inhibitors (TNFi) resulted in low disease activity (LDA) and remission in 90% of pregnant patients with rheumatoid arthritis (RA).
Major finding: Among patients treated with T2T, 90.4% and 76.1% of patients were in LDA or remission, respectively, in the third trimester. Mean disease activity over time was lower in patients treated with the T2T approach vs. reference cohort (P less than .001).
Study details: Findings are from an analysis of 309 patients with RA from the PreCARA cohort who wished to conceive or were pregnant. Patients were treated with a T2T approach with the use of TNFi and/or a combination of disease-modifying antirheumatic drugs along with obvious pregnancy restrictions. The reference cohort consisted of pregnant patients with RA treated according to the 2002-2010 standards from the PARA study.
Disclosures: This investigator-initiated study was supported by UCB and Dutch Arthritis Foundation (ReumaNederland). The investigators did not report any competing interests.
Source: Smeele HTW et al. Ann Rheum Dis. 2021 Feb 10. doi: 10.1136/annrheumdis-2020-219547.
Key clinical point: A modern treat-to-target (T2T) approach with the use of tumor necrosis factor inhibitors (TNFi) resulted in low disease activity (LDA) and remission in 90% of pregnant patients with rheumatoid arthritis (RA).
Major finding: Among patients treated with T2T, 90.4% and 76.1% of patients were in LDA or remission, respectively, in the third trimester. Mean disease activity over time was lower in patients treated with the T2T approach vs. reference cohort (P less than .001).
Study details: Findings are from an analysis of 309 patients with RA from the PreCARA cohort who wished to conceive or were pregnant. Patients were treated with a T2T approach with the use of TNFi and/or a combination of disease-modifying antirheumatic drugs along with obvious pregnancy restrictions. The reference cohort consisted of pregnant patients with RA treated according to the 2002-2010 standards from the PARA study.
Disclosures: This investigator-initiated study was supported by UCB and Dutch Arthritis Foundation (ReumaNederland). The investigators did not report any competing interests.
Source: Smeele HTW et al. Ann Rheum Dis. 2021 Feb 10. doi: 10.1136/annrheumdis-2020-219547.
Key clinical point: A modern treat-to-target (T2T) approach with the use of tumor necrosis factor inhibitors (TNFi) resulted in low disease activity (LDA) and remission in 90% of pregnant patients with rheumatoid arthritis (RA).
Major finding: Among patients treated with T2T, 90.4% and 76.1% of patients were in LDA or remission, respectively, in the third trimester. Mean disease activity over time was lower in patients treated with the T2T approach vs. reference cohort (P less than .001).
Study details: Findings are from an analysis of 309 patients with RA from the PreCARA cohort who wished to conceive or were pregnant. Patients were treated with a T2T approach with the use of TNFi and/or a combination of disease-modifying antirheumatic drugs along with obvious pregnancy restrictions. The reference cohort consisted of pregnant patients with RA treated according to the 2002-2010 standards from the PARA study.
Disclosures: This investigator-initiated study was supported by UCB and Dutch Arthritis Foundation (ReumaNederland). The investigators did not report any competing interests.
Source: Smeele HTW et al. Ann Rheum Dis. 2021 Feb 10. doi: 10.1136/annrheumdis-2020-219547.


















