User login
Elevated disease activity, cytokine levels linked to diabetes risk in RA
Key clinical point: Higher disease activity and elevated levels of cytokines/chemokines are associated with a greater risk of diabetes mellitus (DM) in patients with rheumatoid arthritis (RA).
Major finding: High Disease activity Score (DAS28)-C reactive protein (CRP) was associated with an increased risk of DM (test for trend: P less than .001). Several cytokines/chemokines analyzed showed independent association with DM risk including interleukin (IL)-1, IL-6, and select macrophage-derived cytokines/chemokines (hazard ratio per standard deviation range, 1.11-1.26). These associations were independent of the DAS28-CRP.
Study details: This longitudinal analysis included 1,866 patients with RA without prevalent DM from Veteran’s Affairs Rheumatoid Arthritis Registry. Thirty cytokines and chemokines were measured in banked serum obtained at the time of enrolment.
Disclosures: No study sponsor was identified. The presenting author has received consulting fees from Bristol-Myers Squibb and Gilead.
Source: Baker JF. Ann Rheum Dis. 2021 Jan 4. doi: 10.1136/annrheumdis-2020-219140
Key clinical point: Higher disease activity and elevated levels of cytokines/chemokines are associated with a greater risk of diabetes mellitus (DM) in patients with rheumatoid arthritis (RA).
Major finding: High Disease activity Score (DAS28)-C reactive protein (CRP) was associated with an increased risk of DM (test for trend: P less than .001). Several cytokines/chemokines analyzed showed independent association with DM risk including interleukin (IL)-1, IL-6, and select macrophage-derived cytokines/chemokines (hazard ratio per standard deviation range, 1.11-1.26). These associations were independent of the DAS28-CRP.
Study details: This longitudinal analysis included 1,866 patients with RA without prevalent DM from Veteran’s Affairs Rheumatoid Arthritis Registry. Thirty cytokines and chemokines were measured in banked serum obtained at the time of enrolment.
Disclosures: No study sponsor was identified. The presenting author has received consulting fees from Bristol-Myers Squibb and Gilead.
Source: Baker JF. Ann Rheum Dis. 2021 Jan 4. doi: 10.1136/annrheumdis-2020-219140
Key clinical point: Higher disease activity and elevated levels of cytokines/chemokines are associated with a greater risk of diabetes mellitus (DM) in patients with rheumatoid arthritis (RA).
Major finding: High Disease activity Score (DAS28)-C reactive protein (CRP) was associated with an increased risk of DM (test for trend: P less than .001). Several cytokines/chemokines analyzed showed independent association with DM risk including interleukin (IL)-1, IL-6, and select macrophage-derived cytokines/chemokines (hazard ratio per standard deviation range, 1.11-1.26). These associations were independent of the DAS28-CRP.
Study details: This longitudinal analysis included 1,866 patients with RA without prevalent DM from Veteran’s Affairs Rheumatoid Arthritis Registry. Thirty cytokines and chemokines were measured in banked serum obtained at the time of enrolment.
Disclosures: No study sponsor was identified. The presenting author has received consulting fees from Bristol-Myers Squibb and Gilead.
Source: Baker JF. Ann Rheum Dis. 2021 Jan 4. doi: 10.1136/annrheumdis-2020-219140
Hydroxychloroquine use not linked to heart failure risk in patients with RA
Key clinical point: Use of hydroxychloroquine (HCQ) is not associated with increased risk of developing heart failure (HF) in patients with rheumatoid arthritis (RA).
Major finding: HCQ cumulative dose was not associated with HF (odds ratio [OR], 0.96 [95% confidence interval (CI), 0.90-1.03] per 100 g). No statistically significant association was found for patients with a cumulative dose of 300 g or more (OR, 0.92; 95% CI, 0.41-2.08). Duration of HCQ use prior to index was not associated with HF (OR, 0.98; 95% CI, 0.91-1.05).
Study details: The data come from a nested case-control study of 143 RA cases diagnosed with HF and 143 non-HF RA controls.
Disclosures: The study was funded by grants from the National Institutes of Health. The authors declared no conflicts of interest.
Source: Sorour AA et al. J Rheumatol. 2021 Jan 15. doi: 10.3899/jrheum.201180
Key clinical point: Use of hydroxychloroquine (HCQ) is not associated with increased risk of developing heart failure (HF) in patients with rheumatoid arthritis (RA).
Major finding: HCQ cumulative dose was not associated with HF (odds ratio [OR], 0.96 [95% confidence interval (CI), 0.90-1.03] per 100 g). No statistically significant association was found for patients with a cumulative dose of 300 g or more (OR, 0.92; 95% CI, 0.41-2.08). Duration of HCQ use prior to index was not associated with HF (OR, 0.98; 95% CI, 0.91-1.05).
Study details: The data come from a nested case-control study of 143 RA cases diagnosed with HF and 143 non-HF RA controls.
Disclosures: The study was funded by grants from the National Institutes of Health. The authors declared no conflicts of interest.
Source: Sorour AA et al. J Rheumatol. 2021 Jan 15. doi: 10.3899/jrheum.201180
Key clinical point: Use of hydroxychloroquine (HCQ) is not associated with increased risk of developing heart failure (HF) in patients with rheumatoid arthritis (RA).
Major finding: HCQ cumulative dose was not associated with HF (odds ratio [OR], 0.96 [95% confidence interval (CI), 0.90-1.03] per 100 g). No statistically significant association was found for patients with a cumulative dose of 300 g or more (OR, 0.92; 95% CI, 0.41-2.08). Duration of HCQ use prior to index was not associated with HF (OR, 0.98; 95% CI, 0.91-1.05).
Study details: The data come from a nested case-control study of 143 RA cases diagnosed with HF and 143 non-HF RA controls.
Disclosures: The study was funded by grants from the National Institutes of Health. The authors declared no conflicts of interest.
Source: Sorour AA et al. J Rheumatol. 2021 Jan 15. doi: 10.3899/jrheum.201180
Gestational diabetes carries CVD risk years later
Women who’ve had gestational diabetes are 40% more likely to develop coronary artery calcification later in life than are women haven’t, and attaining normal glycemic levels doesn’t diminish their midlife risk for atherosclerotic cardiovascular disease.
“The new finding from this study is that women with gestational diabetes had twice the risk of coronary artery calcium, compared to women who never had gestational diabetes, even though both groups attained normal blood sugar levels many years after pregnancy,” lead author Erica P. Gunderson, PhD, MS, MPH, said in an interview about a community-based prospective cohort study of young adults followed for up to 25 years, which was published in Circulation (2021 Feb 1. doi: 10.1161/CIRCULATIONAHA.120.047320).
Previous studies have reported a higher risk of heart disease in women who had gestational diabetes (GD) and later developed type 2 diabetes, but they didn’t elucidate whether that risk carried over in GD patients whose glycemic levels were normal after pregnancy. In 2018, the American College of Cardiology/American Heart Association Cholesterol Clinical Practice Guidelines specified that a history of GD increases women’s risk for coronary artery calcification (CAC).
This study analyzed data of 1,133 women ages 18-30 enrolled in the Coronary Artery Risk Development in Young Adults (CARDIA) study who had no diabetes in the baseline years of 1985-1986 and had given birth at least once in the ensuing 25 years. They had glucose tolerance testing at baseline and up to five times through the study period, along with evaluation for GD status and coronary artery calcification CAC measurements at least once at years 15, 20 and 25 (2001-2011).
CARDIA enrolled 5,155 young Black and White men and women ages 18-30 from four distinct geographic areas: Birmingham, Ala.; Chicago; Minneapolis; and Oakland, Calif. About 52% of the study population was Black.
Of the women who’d given birth, 139 (12%) had GD. Their average age at follow-up was 47.6 years, and 25% of the GD patients (34) had CAC, compared with 15% (149/994) in the non-GD group.
Dr. Gunderson noted that the same relative risk for CAC applied to women who had GD and went on to develop prediabetes or were diagnosed with type 2 diabetes during follow-up.
Risks persist even in normoglycemia
In the GD group, the adjusted hazard ratio for having CAC with normoglycemia was 2.3 (95% confidence interval, 1.34-4.09). The researchers also calculated HRs for prediabetes and incident diabetes: 1.5 (95% CI, 1.06-2.24) in no-GD and 2.1 (95% CI, 1.09-4.17) for GD for prediabetes; and 2.2 (95% CI, 1.3-3.62) and 2.02 (95% CI, 0.98-4.19), respectively, for incident diabetes (P = .003).
“This means the risk of heart disease may be increased substantially in women with a history of gestational diabetes and may not diminish even if their blood-sugar levels remain normal for years later,” said Dr. Gunderson, an epidemiologist and senior research scientist at the Kaiser Permanente Northern California Division of Research in Oakland.
“The clinical implications of our findings are that women with previous GD may benefit from enhanced traditional CVD [cardiovascular disease] risk factor testing – i.e., for hypertension, dyslipidemia, and hyperinsulinemia,” Dr. Gunderson said. “Our findings also suggest that it could be beneficial to incorporate history of GD into risk calculators to improve CVD risk stratification and prevention.”
Strong findings argue for more frequent CVD screening
These study results may be the strongest data to date on the long-term effects of GD, said Prakash Deedwania, MD, professor of cardiology at the University of California, San Francisco. “It’s the strongest in the sense in that it’s sponsored, involved four different communities in different parts of the United States, enrolled individuals when they were young and followed them, and saw very few patients drop out for such a long-term study.” The study reported follow-up data on 72% of patients at 25 years, a rate Dr. Deedwania noted was “excellent.”
“Patients who have had GD should be screened aggressively – for not only diabetes, but other cardiovascular risk factors – early on to minimize the subsequent risk of cardiovascular disease is a very important point of this study,” he added. In the absence of a clinical guideline, Dr. Deedwania suggested women with GD might have screening for CV risk factors every 5-7 years depending on their risk profile, but emphasized that parameter isn’t settled.
Future research should focus on the link between GD and CVD risk, Dr. Gunderson said. “Research is needed to better characterize the severity of GD in relation to CVD outcomes, and to identify critical pregnancy-related periods to modify cardiometabolic risk.” The latter would include life-course studies across the full pregnancy continuum from preconception to lactation. “Interventions for primary prevention of CVD and the importance of modifiable lifestyle behaviors with the highest relevance to reduce both diabetes and CVD risks during the first year post partum merit increased research investigation,” she added.
Future studies might also explore the role of inflammation in the GD-CVD relationship, Dr. Deedwania said. “My hypothesis is, and it’s purely a hypothesis, that perhaps the presence of coronary artery calcification scores score in these individuals who were described as having normal glucose but who could be at risk could very well be related to the beginning of inflammation.”
Dr. Gunderson and Dr. Deedwania have no financial relationships to disclose. The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases and the National Heart, Lung, and Blood Institute.
Women who’ve had gestational diabetes are 40% more likely to develop coronary artery calcification later in life than are women haven’t, and attaining normal glycemic levels doesn’t diminish their midlife risk for atherosclerotic cardiovascular disease.
“The new finding from this study is that women with gestational diabetes had twice the risk of coronary artery calcium, compared to women who never had gestational diabetes, even though both groups attained normal blood sugar levels many years after pregnancy,” lead author Erica P. Gunderson, PhD, MS, MPH, said in an interview about a community-based prospective cohort study of young adults followed for up to 25 years, which was published in Circulation (2021 Feb 1. doi: 10.1161/CIRCULATIONAHA.120.047320).
Previous studies have reported a higher risk of heart disease in women who had gestational diabetes (GD) and later developed type 2 diabetes, but they didn’t elucidate whether that risk carried over in GD patients whose glycemic levels were normal after pregnancy. In 2018, the American College of Cardiology/American Heart Association Cholesterol Clinical Practice Guidelines specified that a history of GD increases women’s risk for coronary artery calcification (CAC).
This study analyzed data of 1,133 women ages 18-30 enrolled in the Coronary Artery Risk Development in Young Adults (CARDIA) study who had no diabetes in the baseline years of 1985-1986 and had given birth at least once in the ensuing 25 years. They had glucose tolerance testing at baseline and up to five times through the study period, along with evaluation for GD status and coronary artery calcification CAC measurements at least once at years 15, 20 and 25 (2001-2011).
CARDIA enrolled 5,155 young Black and White men and women ages 18-30 from four distinct geographic areas: Birmingham, Ala.; Chicago; Minneapolis; and Oakland, Calif. About 52% of the study population was Black.
Of the women who’d given birth, 139 (12%) had GD. Their average age at follow-up was 47.6 years, and 25% of the GD patients (34) had CAC, compared with 15% (149/994) in the non-GD group.
Dr. Gunderson noted that the same relative risk for CAC applied to women who had GD and went on to develop prediabetes or were diagnosed with type 2 diabetes during follow-up.
Risks persist even in normoglycemia
In the GD group, the adjusted hazard ratio for having CAC with normoglycemia was 2.3 (95% confidence interval, 1.34-4.09). The researchers also calculated HRs for prediabetes and incident diabetes: 1.5 (95% CI, 1.06-2.24) in no-GD and 2.1 (95% CI, 1.09-4.17) for GD for prediabetes; and 2.2 (95% CI, 1.3-3.62) and 2.02 (95% CI, 0.98-4.19), respectively, for incident diabetes (P = .003).
“This means the risk of heart disease may be increased substantially in women with a history of gestational diabetes and may not diminish even if their blood-sugar levels remain normal for years later,” said Dr. Gunderson, an epidemiologist and senior research scientist at the Kaiser Permanente Northern California Division of Research in Oakland.
“The clinical implications of our findings are that women with previous GD may benefit from enhanced traditional CVD [cardiovascular disease] risk factor testing – i.e., for hypertension, dyslipidemia, and hyperinsulinemia,” Dr. Gunderson said. “Our findings also suggest that it could be beneficial to incorporate history of GD into risk calculators to improve CVD risk stratification and prevention.”
Strong findings argue for more frequent CVD screening
These study results may be the strongest data to date on the long-term effects of GD, said Prakash Deedwania, MD, professor of cardiology at the University of California, San Francisco. “It’s the strongest in the sense in that it’s sponsored, involved four different communities in different parts of the United States, enrolled individuals when they were young and followed them, and saw very few patients drop out for such a long-term study.” The study reported follow-up data on 72% of patients at 25 years, a rate Dr. Deedwania noted was “excellent.”
“Patients who have had GD should be screened aggressively – for not only diabetes, but other cardiovascular risk factors – early on to minimize the subsequent risk of cardiovascular disease is a very important point of this study,” he added. In the absence of a clinical guideline, Dr. Deedwania suggested women with GD might have screening for CV risk factors every 5-7 years depending on their risk profile, but emphasized that parameter isn’t settled.
Future research should focus on the link between GD and CVD risk, Dr. Gunderson said. “Research is needed to better characterize the severity of GD in relation to CVD outcomes, and to identify critical pregnancy-related periods to modify cardiometabolic risk.” The latter would include life-course studies across the full pregnancy continuum from preconception to lactation. “Interventions for primary prevention of CVD and the importance of modifiable lifestyle behaviors with the highest relevance to reduce both diabetes and CVD risks during the first year post partum merit increased research investigation,” she added.
Future studies might also explore the role of inflammation in the GD-CVD relationship, Dr. Deedwania said. “My hypothesis is, and it’s purely a hypothesis, that perhaps the presence of coronary artery calcification scores score in these individuals who were described as having normal glucose but who could be at risk could very well be related to the beginning of inflammation.”
Dr. Gunderson and Dr. Deedwania have no financial relationships to disclose. The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases and the National Heart, Lung, and Blood Institute.
Women who’ve had gestational diabetes are 40% more likely to develop coronary artery calcification later in life than are women haven’t, and attaining normal glycemic levels doesn’t diminish their midlife risk for atherosclerotic cardiovascular disease.
“The new finding from this study is that women with gestational diabetes had twice the risk of coronary artery calcium, compared to women who never had gestational diabetes, even though both groups attained normal blood sugar levels many years after pregnancy,” lead author Erica P. Gunderson, PhD, MS, MPH, said in an interview about a community-based prospective cohort study of young adults followed for up to 25 years, which was published in Circulation (2021 Feb 1. doi: 10.1161/CIRCULATIONAHA.120.047320).
Previous studies have reported a higher risk of heart disease in women who had gestational diabetes (GD) and later developed type 2 diabetes, but they didn’t elucidate whether that risk carried over in GD patients whose glycemic levels were normal after pregnancy. In 2018, the American College of Cardiology/American Heart Association Cholesterol Clinical Practice Guidelines specified that a history of GD increases women’s risk for coronary artery calcification (CAC).
This study analyzed data of 1,133 women ages 18-30 enrolled in the Coronary Artery Risk Development in Young Adults (CARDIA) study who had no diabetes in the baseline years of 1985-1986 and had given birth at least once in the ensuing 25 years. They had glucose tolerance testing at baseline and up to five times through the study period, along with evaluation for GD status and coronary artery calcification CAC measurements at least once at years 15, 20 and 25 (2001-2011).
CARDIA enrolled 5,155 young Black and White men and women ages 18-30 from four distinct geographic areas: Birmingham, Ala.; Chicago; Minneapolis; and Oakland, Calif. About 52% of the study population was Black.
Of the women who’d given birth, 139 (12%) had GD. Their average age at follow-up was 47.6 years, and 25% of the GD patients (34) had CAC, compared with 15% (149/994) in the non-GD group.
Dr. Gunderson noted that the same relative risk for CAC applied to women who had GD and went on to develop prediabetes or were diagnosed with type 2 diabetes during follow-up.
Risks persist even in normoglycemia
In the GD group, the adjusted hazard ratio for having CAC with normoglycemia was 2.3 (95% confidence interval, 1.34-4.09). The researchers also calculated HRs for prediabetes and incident diabetes: 1.5 (95% CI, 1.06-2.24) in no-GD and 2.1 (95% CI, 1.09-4.17) for GD for prediabetes; and 2.2 (95% CI, 1.3-3.62) and 2.02 (95% CI, 0.98-4.19), respectively, for incident diabetes (P = .003).
“This means the risk of heart disease may be increased substantially in women with a history of gestational diabetes and may not diminish even if their blood-sugar levels remain normal for years later,” said Dr. Gunderson, an epidemiologist and senior research scientist at the Kaiser Permanente Northern California Division of Research in Oakland.
“The clinical implications of our findings are that women with previous GD may benefit from enhanced traditional CVD [cardiovascular disease] risk factor testing – i.e., for hypertension, dyslipidemia, and hyperinsulinemia,” Dr. Gunderson said. “Our findings also suggest that it could be beneficial to incorporate history of GD into risk calculators to improve CVD risk stratification and prevention.”
Strong findings argue for more frequent CVD screening
These study results may be the strongest data to date on the long-term effects of GD, said Prakash Deedwania, MD, professor of cardiology at the University of California, San Francisco. “It’s the strongest in the sense in that it’s sponsored, involved four different communities in different parts of the United States, enrolled individuals when they were young and followed them, and saw very few patients drop out for such a long-term study.” The study reported follow-up data on 72% of patients at 25 years, a rate Dr. Deedwania noted was “excellent.”
“Patients who have had GD should be screened aggressively – for not only diabetes, but other cardiovascular risk factors – early on to minimize the subsequent risk of cardiovascular disease is a very important point of this study,” he added. In the absence of a clinical guideline, Dr. Deedwania suggested women with GD might have screening for CV risk factors every 5-7 years depending on their risk profile, but emphasized that parameter isn’t settled.
Future research should focus on the link between GD and CVD risk, Dr. Gunderson said. “Research is needed to better characterize the severity of GD in relation to CVD outcomes, and to identify critical pregnancy-related periods to modify cardiometabolic risk.” The latter would include life-course studies across the full pregnancy continuum from preconception to lactation. “Interventions for primary prevention of CVD and the importance of modifiable lifestyle behaviors with the highest relevance to reduce both diabetes and CVD risks during the first year post partum merit increased research investigation,” she added.
Future studies might also explore the role of inflammation in the GD-CVD relationship, Dr. Deedwania said. “My hypothesis is, and it’s purely a hypothesis, that perhaps the presence of coronary artery calcification scores score in these individuals who were described as having normal glucose but who could be at risk could very well be related to the beginning of inflammation.”
Dr. Gunderson and Dr. Deedwania have no financial relationships to disclose. The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases and the National Heart, Lung, and Blood Institute.
FROM CIRCULATION
Long-acting injectable antipsychotics during COVID-19
Long-acting injectable antipsychotics (LAIs) are an essential tool in the treatment of patients with psychotic disorders, allowing for periods of stable drug plasma concentration and confirmed adherence.1 The current coronavirus disease 2019 (COVID-19) pandemic presents unique challenges for administering LAIs and requires a thoughtful and prospective approach in order to ensure continuity of psychiatric care while minimizing the risk of infection with COVID-19. Ideally, patients should be seen in person as infrequently as clinically prudent during this public health emergency; however, LAI administration necessitates direct physical contact between patient and clinician.
Patients with serious mental illness (SMI), who comprise the majority of individuals who receive LAIs, are at heightened risk for cardiovascular and pulmonary comorbidities. These factors are the primary reason the life expectancy of a patient with SMI is nearly 30 years shorter than that of the general population.2-5 The risk of health care workers becoming infected or inadvertently spreading COVID-19 is heightened when working with patients in group living environments (ie, a shelter or group home), who have both increased exposure and increased risk of further transmission.6 Additional patient populations, including older adults, immunocompromised individuals, and those with preexisting conditions, are at heightened risk for serious complications if they were to contract COVID-19.7,8
Thus, the questions of whether LAIs should be administered, and how to do so safely (both during the ongoing, acute phase of the pandemic as well as during the subsequent recovery period until the pandemic abates) need to be carefully considered. In this article, we provide concrete advice for clinicians and clinics on these topics, with the goal of maintaining patients’ psychiatric stability while protecting patients, health care workers, and the broader society from COVID-19 infection. Table 1 summarizes the questions regarding LAIs that clinicians need to address during this crisis. While we focus on outpatient care, inpatient teams should keep these considerations in mind if they are starting and discharging a patient on an LAI. More than ever, close collaboration and communication between inpatient and outpatient teams is critical.
Should an LAI be continued?
An important first step to approaching this challenge is to create a spreadsheet for all patients receiving LAIs. Focusing on a population-based approach is helpful to be systematic and ensure that no patients fall through the cracks during this public health emergency.9 Once all patients have been identified, the treatment team should review each patient to determine if continuing to administer the antipsychotic as an LAI formulation is essential, taking into account the patient’s current psychiatric status, historical medication adherence, potential severity and dangerousness of decompensation if nonadherent, and structures to support stability. For example, can a patient move in with family who can monitor medication adherence during the pandemic? Is it possible for the group home to assume medication administration? Additional consideration should be given to the living environment and health-vulnerability of the patient and the individuals living with them.
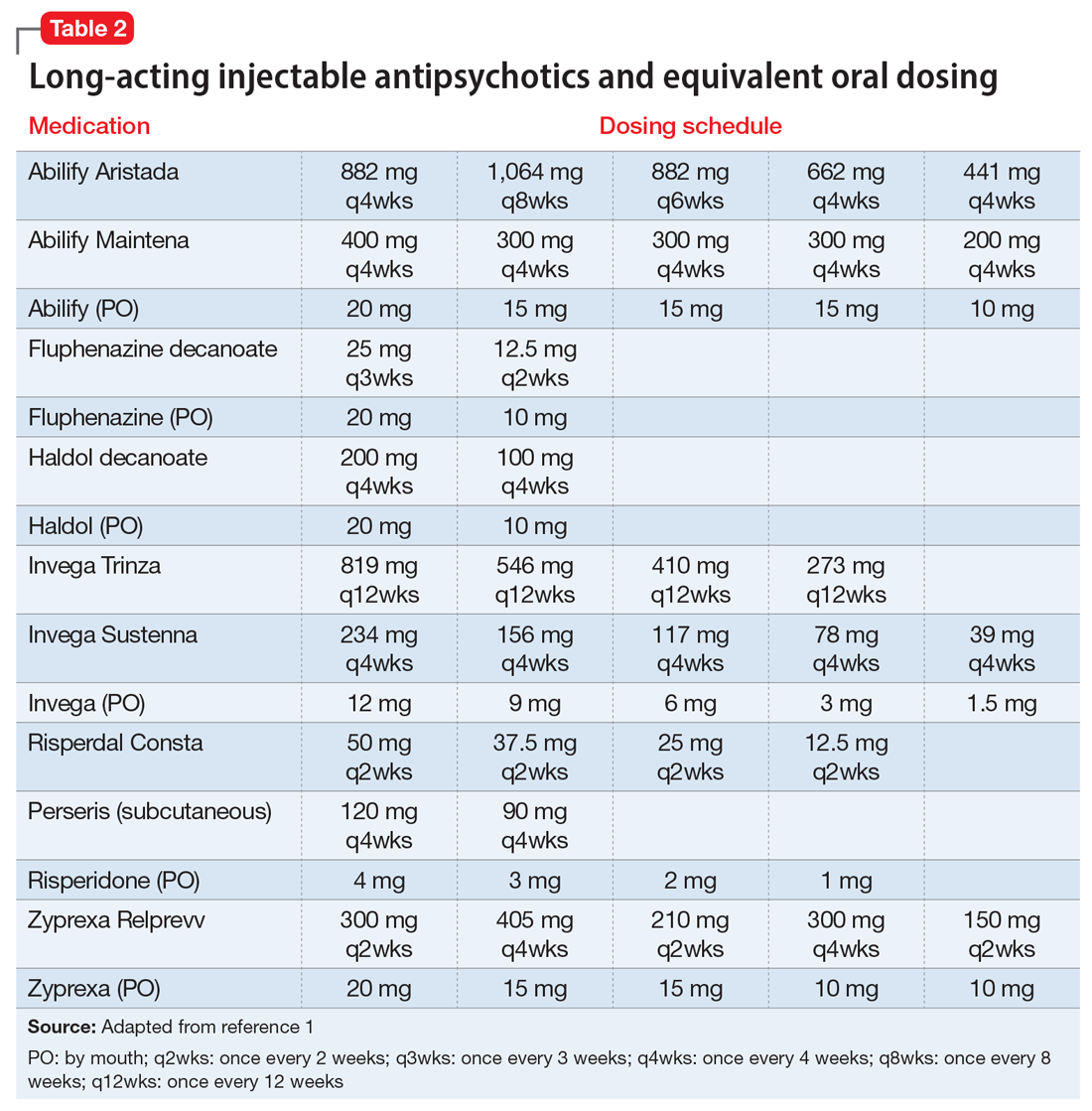
If the risk calculation does not point strongly towards a need for continuing the LAI, it may be prudent to temporarily transition the patient to the corresponding oral antipsychotic preparation. Table 2 lists all LAIs available in the United States and their approximate equivalent oral dosing. It is important to note that such transitions are not without clinical risk, to emphasize to the patient that the transition is intended as a temporary measure, and to discuss a proposed timeline for re-initiating the LAI. Also, emphasize to the patient and family that this transition does not diminish the previous reasoning for needing an LAI, but is a temporary measure taken in light of weighing the risks and benefits during a pandemic.
Which LAI should be administered?
If continuing the LAI is determined to be clinically necessary, consider switching the patient to a longer-acting preparation to maximize intervals between administrations and minimize the potential for infection. From a public health perspective, the longest clinically prudent interval between injections may be the most important consideration, provided the patient can receive a dose necessary to retain stability, and the LAI should be chosen accordingly. Deltoid injections may be able to be administered with reduced contact, or on a “drive-up” basis.10 Consider transitioning a patient who is receiving olanzapine pamoate to an alternate LAI or oral formulation, because the 3-hour observation period that is required after olanzapine pamoate administration is particularly problematic. While it may not be ideal to make medication changes during a pandemic, it is worth carefully weighing the patient’s stability and historical experience with other LAIs to determine if a safer/longer-spaced option is worth trying.11
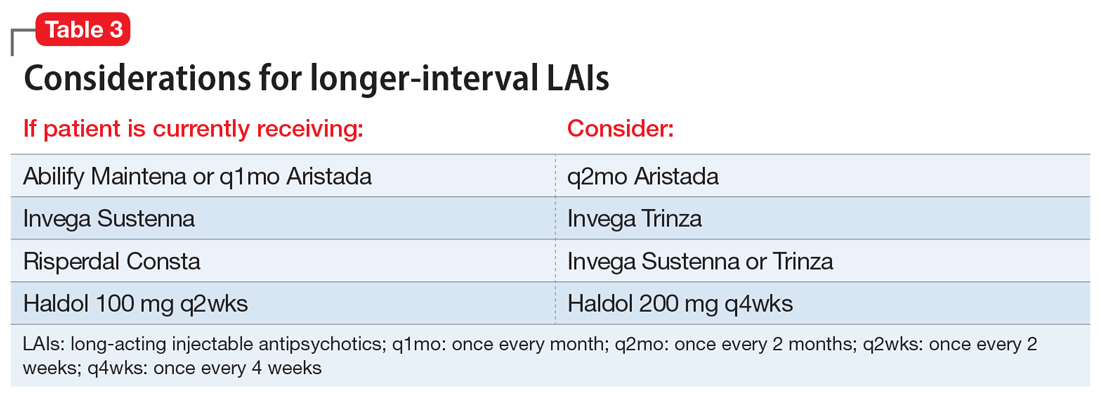
We recommend only switching among similar antipsychotics (ie, risperidone to paliperidone), or between different preparations of the same drug (ie, Abilify Maintena to Aristada), if possible, as these are the lowest risk transitions with regards to relapse. Table 3 provides examples.
Continue to: When should the LAI be administered?
When should the LAI be administered?
The pharmacokinetics of LAIs allow for some flexibility in terms of when an LAI needs to be administered. The package inserts of all second-generation LAIs include missed-dose guidelines. These guidelines provide information on how long one can wait before the next injection is due, and what additional measures must be taken when beyond that date. Delaying an injection may be prudent, and the missed dose guidelines will indicate when one must consider supplementing with oral medications. For patients who are in quarantine, it may be better to delay an injection until the patient ends their quarantine than to deliver the dose during quarantine. Administering an injection earlier also is usually safe; off-cycle visits may help minimize patient contact (ie, if the patient happens to be coming into the vicinity of the clinic, or requires phlebotomy for therapeutic drug monitoring), and assist in planning for possible resurgences. When appropriate, and after considering the risk of worsening adverse effects, administering a higher dose than the usual maintenance dose would provide a buffer if the next injection was to be delayed. Therapeutic drug monitoring can help to optimize dosing and avoid low plasma drug levels, which may be not be sufficient, particularly during this time of stress.12 To provide optimal protection against relapse, consider administering a dose that puts patients at the higher range of plasma drug levels.
Where can the LAI be administered, and who can give it?
For patients who usually travel to a clinic, consider arranging for a more local injection (ie, at the patient’s primary care clinic in their hometown, or at a local mental health center), and explore if the patient may be able to receive their injection in their home through a visiting nurse association (VNA). In many states (approximately 30 currently), clinicians at pharmacies are also able to administer patient injections. Clinics would do well to at least plan for alternate staffing models in the event of staff illness. A pool of individuals should be available to give injections; consider training additional staff members (including MDs who may have never previously administered an LAI but could be quickly instructed to do so) to administer LAIs. Theoretically, during a public health emergency, family members, particularly those who have a background in health care, could be trained to give an injection and provided education on LAI storage and post-injection monitoring. This approach would not be consistent with FDA labeling, however, and should only be considered as a last resort.
What safety measures can be put in place?
Face-to-face time for injection administration should be kept as brief as possible. Before the encounter, obtain the patient’s clinical information, ideally through telehealth or from an acceptable distance. Medication should be drawn ahead of time, and not in an enclosed space with the patient present. Strongly consider abandoning the traditional enclosed room for the injection, and instead use larger spaces, doorways, or outside, if feasible. As previously noted, some clinics and clinicians have used a drive-up approach for LAI administration, particularly for deltoid injections.10 Individuals who administer the injections should wear personal protective equipment, and the clinic should obtain an adequate supply of this equipment well in advance.
Lessons learned at our clinic
In our community mental health center clinic, planning around these questions has allowed us to provide safe and continuous psychiatric care with LAIs during this public health emergency while reducing the risk of infection. We have worked to transfer LAI administration to VNAs and transition patients to longer-lasting formulations or oral medications where appropriate, which has resulted in an approximately 50% decrease in in-person visits. Reducing the number of in-person visits does not need to result in less frequent clinical follow-up. Telepsychiatry visits can make up for lost in-person visits and have generally been well accepted.
As we are preparing for the next phase, routine medical health monitoring (eg, metabolic monitoring, monitoring for tardive dyskinesia) that has not been at the forefront of concerns should be carefully reintroduced. Challenges encountered have included difficulty in having VNA accept patients for short-term LAI visits, changes to where on the body the injection is delivered, and patients with SMI and their families being reluctant to depart from previous routines and administration schedules.
Continue to: There is great value...
There is great value in the collective lessons learned during this public health emergency (eg, the need for a flexible, population health-based approach; acceptability of combination telehealth and in-person visits) that can lead to more person-centered and accessible care for patients with SMI.
Acknowledgments
The authors thank North Suffolk Mental Health Association, the Freedom Trail Clinic, and their patients.
Bottom Line
When caring for a patient with a psychotic illness during the coronavirus disease 2019 (COVID-19) pandemic, evaluate whether it is necessary to continue a longacting injectable antipsychotic (LAI). If yes, reconsider which LAI should be administered, when and where it should be given, and by whom. Implement safety measures to minimize the risk of COVID-19 exposure and transmission.
Related Resources
- American Association of Community Psychiatrists. Clinical Tip Series. Long acting antipsychotic medications. https://drive.google.com/file/d/1unigjmjFJkqZMbaZ_ftdj8oqog49awZs/view?usp=sharing
- SMI Adviser. What are clinical considerations for giving LAIs during the COVID-19 public health emergency? https://smiadviser.org/knowledge_post/what-are-clinical-considerations-for-giving-lais-during-the-covid-19-public-health-emergency
- CDC. Infection control basics. https://www.cdc.gov/infectioncontrol/basics/index.html
Drug Brand Names
Aripiprazole • Abilify
Aripiprazole for extended- release injectable suspension • Abilify Maintena
Aripiprazole lauroxil • Aristada
Haloperidol • Haldol
Haloperidol injection • Haldol decanoate
Olanzapine • Zyprexa
Olanzapine for extended-release injectable suspension • Zyprexa Relprevv
Paliperidone • Invega
Paliperidone palmitate extended-release injectable suspension • Invega Sustenna
Paliperidone palmitate extended-release injectable suspension • Invega Trinza
Risperidone • Risperdal
Risperidone for extended- release injectable suspension • Perseris
Risperidone injection • Risperdal Consta
1. Freudenreich O. Long-acting injectable antipsychotics. In: Freudenreich O. Psychotic disorders: a practical guide. Springer; 2020:249-261.
2. Olfson M, Gerhard T, Huang C, et al. Premature mortality among adults with schizophrenia in the United States. JAMA Psychiatry. 2015;72(12):1172-1181.
3. Reilly S, Olier I, Planner C, et al. Inequalities in physical comorbidity: a longitudinal comparative cohort study of people with severe mental illness in the UK. BMJ Open. 2015;5(12):e009010.
4. Brown S, Inskip H, Barraclough B. Causes of the excess mortality of schizophrenia. Br J Psychiatry. 2000;177:212-217.
5. Goff DC, Cather C, Evins AE, et al. Medical morbidity and mortality in schizophrenia: guidelines for psychiatrists. J Clin Psychiatry. 2005;66(2):183-194.
6. Baggett TP, Keyes H, Sporn N, et al. Prevalence of SARS-CoV-2 infection in residents of a large homeless shelter in Boston. JAMA. 2020;323(21):2191-2192.
7. CDC COVID-19 Response Team. Severe outcomes among patients with coronavirus disease 2019 (COVID-19)—United States, February 12–March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):343-346.
8. Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985
9. Etches V, Frank J, Di Ruggiero E, et al. Measuring population health: a review of indicators. Annu Rev Public Health. 2006;27:29-55.
10. Chepke C. Drive-up pharmacotherapy during the COVID-19 pandemic. Current Psychiatry. 2020;19(5):29-30.
11. Sajatovic M, Ross R, Legacy SN, et al. Initiating/maintaining long-acting injectable antipsychotics in schizophrenia/schizoaffective or bipolar disorder - expert consensus survey part 2. Neuropsychiatr Dis Treat. 2018;14:1475-1492.
12. Schoretsanitis G, Kane JM, Correll CU, et al; American Society of Clinical Psychopharmacology, Pharmakopsychiatrie TTDMTFOTAFNU. Blood levels to optimize antipsychotic treatment in clinical practice: a joint consensus statement of the American Society of Clinical Psychopharmacology and the Therapeutic Drug Monitoring Task Force of the Arbeitsgemeinschaft für Neuropsychopharmakologie und Pharmakopsychiatrie. J Clin Psychiatry. 2020;81(3):19cs13169. doi: 10.4088/JCP.19cs13169
Long-acting injectable antipsychotics (LAIs) are an essential tool in the treatment of patients with psychotic disorders, allowing for periods of stable drug plasma concentration and confirmed adherence.1 The current coronavirus disease 2019 (COVID-19) pandemic presents unique challenges for administering LAIs and requires a thoughtful and prospective approach in order to ensure continuity of psychiatric care while minimizing the risk of infection with COVID-19. Ideally, patients should be seen in person as infrequently as clinically prudent during this public health emergency; however, LAI administration necessitates direct physical contact between patient and clinician.
Patients with serious mental illness (SMI), who comprise the majority of individuals who receive LAIs, are at heightened risk for cardiovascular and pulmonary comorbidities. These factors are the primary reason the life expectancy of a patient with SMI is nearly 30 years shorter than that of the general population.2-5 The risk of health care workers becoming infected or inadvertently spreading COVID-19 is heightened when working with patients in group living environments (ie, a shelter or group home), who have both increased exposure and increased risk of further transmission.6 Additional patient populations, including older adults, immunocompromised individuals, and those with preexisting conditions, are at heightened risk for serious complications if they were to contract COVID-19.7,8
Thus, the questions of whether LAIs should be administered, and how to do so safely (both during the ongoing, acute phase of the pandemic as well as during the subsequent recovery period until the pandemic abates) need to be carefully considered. In this article, we provide concrete advice for clinicians and clinics on these topics, with the goal of maintaining patients’ psychiatric stability while protecting patients, health care workers, and the broader society from COVID-19 infection. Table 1 summarizes the questions regarding LAIs that clinicians need to address during this crisis. While we focus on outpatient care, inpatient teams should keep these considerations in mind if they are starting and discharging a patient on an LAI. More than ever, close collaboration and communication between inpatient and outpatient teams is critical.
Should an LAI be continued?
An important first step to approaching this challenge is to create a spreadsheet for all patients receiving LAIs. Focusing on a population-based approach is helpful to be systematic and ensure that no patients fall through the cracks during this public health emergency.9 Once all patients have been identified, the treatment team should review each patient to determine if continuing to administer the antipsychotic as an LAI formulation is essential, taking into account the patient’s current psychiatric status, historical medication adherence, potential severity and dangerousness of decompensation if nonadherent, and structures to support stability. For example, can a patient move in with family who can monitor medication adherence during the pandemic? Is it possible for the group home to assume medication administration? Additional consideration should be given to the living environment and health-vulnerability of the patient and the individuals living with them.
If the risk calculation does not point strongly towards a need for continuing the LAI, it may be prudent to temporarily transition the patient to the corresponding oral antipsychotic preparation. Table 2 lists all LAIs available in the United States and their approximate equivalent oral dosing. It is important to note that such transitions are not without clinical risk, to emphasize to the patient that the transition is intended as a temporary measure, and to discuss a proposed timeline for re-initiating the LAI. Also, emphasize to the patient and family that this transition does not diminish the previous reasoning for needing an LAI, but is a temporary measure taken in light of weighing the risks and benefits during a pandemic.

Which LAI should be administered?
If continuing the LAI is determined to be clinically necessary, consider switching the patient to a longer-acting preparation to maximize intervals between administrations and minimize the potential for infection. From a public health perspective, the longest clinically prudent interval between injections may be the most important consideration, provided the patient can receive a dose necessary to retain stability, and the LAI should be chosen accordingly. Deltoid injections may be able to be administered with reduced contact, or on a “drive-up” basis.10 Consider transitioning a patient who is receiving olanzapine pamoate to an alternate LAI or oral formulation, because the 3-hour observation period that is required after olanzapine pamoate administration is particularly problematic. While it may not be ideal to make medication changes during a pandemic, it is worth carefully weighing the patient’s stability and historical experience with other LAIs to determine if a safer/longer-spaced option is worth trying.11
We recommend only switching among similar antipsychotics (ie, risperidone to paliperidone), or between different preparations of the same drug (ie, Abilify Maintena to Aristada), if possible, as these are the lowest risk transitions with regards to relapse. Table 3 provides examples.

Continue to: When should the LAI be administered?
When should the LAI be administered?
The pharmacokinetics of LAIs allow for some flexibility in terms of when an LAI needs to be administered. The package inserts of all second-generation LAIs include missed-dose guidelines. These guidelines provide information on how long one can wait before the next injection is due, and what additional measures must be taken when beyond that date. Delaying an injection may be prudent, and the missed dose guidelines will indicate when one must consider supplementing with oral medications. For patients who are in quarantine, it may be better to delay an injection until the patient ends their quarantine than to deliver the dose during quarantine. Administering an injection earlier also is usually safe; off-cycle visits may help minimize patient contact (ie, if the patient happens to be coming into the vicinity of the clinic, or requires phlebotomy for therapeutic drug monitoring), and assist in planning for possible resurgences. When appropriate, and after considering the risk of worsening adverse effects, administering a higher dose than the usual maintenance dose would provide a buffer if the next injection was to be delayed. Therapeutic drug monitoring can help to optimize dosing and avoid low plasma drug levels, which may be not be sufficient, particularly during this time of stress.12 To provide optimal protection against relapse, consider administering a dose that puts patients at the higher range of plasma drug levels.
Where can the LAI be administered, and who can give it?
For patients who usually travel to a clinic, consider arranging for a more local injection (ie, at the patient’s primary care clinic in their hometown, or at a local mental health center), and explore if the patient may be able to receive their injection in their home through a visiting nurse association (VNA). In many states (approximately 30 currently), clinicians at pharmacies are also able to administer patient injections. Clinics would do well to at least plan for alternate staffing models in the event of staff illness. A pool of individuals should be available to give injections; consider training additional staff members (including MDs who may have never previously administered an LAI but could be quickly instructed to do so) to administer LAIs. Theoretically, during a public health emergency, family members, particularly those who have a background in health care, could be trained to give an injection and provided education on LAI storage and post-injection monitoring. This approach would not be consistent with FDA labeling, however, and should only be considered as a last resort.
What safety measures can be put in place?
Face-to-face time for injection administration should be kept as brief as possible. Before the encounter, obtain the patient’s clinical information, ideally through telehealth or from an acceptable distance. Medication should be drawn ahead of time, and not in an enclosed space with the patient present. Strongly consider abandoning the traditional enclosed room for the injection, and instead use larger spaces, doorways, or outside, if feasible. As previously noted, some clinics and clinicians have used a drive-up approach for LAI administration, particularly for deltoid injections.10 Individuals who administer the injections should wear personal protective equipment, and the clinic should obtain an adequate supply of this equipment well in advance.
Lessons learned at our clinic
In our community mental health center clinic, planning around these questions has allowed us to provide safe and continuous psychiatric care with LAIs during this public health emergency while reducing the risk of infection. We have worked to transfer LAI administration to VNAs and transition patients to longer-lasting formulations or oral medications where appropriate, which has resulted in an approximately 50% decrease in in-person visits. Reducing the number of in-person visits does not need to result in less frequent clinical follow-up. Telepsychiatry visits can make up for lost in-person visits and have generally been well accepted.
As we are preparing for the next phase, routine medical health monitoring (eg, metabolic monitoring, monitoring for tardive dyskinesia) that has not been at the forefront of concerns should be carefully reintroduced. Challenges encountered have included difficulty in having VNA accept patients for short-term LAI visits, changes to where on the body the injection is delivered, and patients with SMI and their families being reluctant to depart from previous routines and administration schedules.
Continue to: There is great value...
There is great value in the collective lessons learned during this public health emergency (eg, the need for a flexible, population health-based approach; acceptability of combination telehealth and in-person visits) that can lead to more person-centered and accessible care for patients with SMI.
Acknowledgments
The authors thank North Suffolk Mental Health Association, the Freedom Trail Clinic, and their patients.
Bottom Line
When caring for a patient with a psychotic illness during the coronavirus disease 2019 (COVID-19) pandemic, evaluate whether it is necessary to continue a longacting injectable antipsychotic (LAI). If yes, reconsider which LAI should be administered, when and where it should be given, and by whom. Implement safety measures to minimize the risk of COVID-19 exposure and transmission.
Related Resources
- American Association of Community Psychiatrists. Clinical Tip Series. Long acting antipsychotic medications. https://drive.google.com/file/d/1unigjmjFJkqZMbaZ_ftdj8oqog49awZs/view?usp=sharing
- SMI Adviser. What are clinical considerations for giving LAIs during the COVID-19 public health emergency? https://smiadviser.org/knowledge_post/what-are-clinical-considerations-for-giving-lais-during-the-covid-19-public-health-emergency
- CDC. Infection control basics. https://www.cdc.gov/infectioncontrol/basics/index.html
Drug Brand Names
Aripiprazole • Abilify
Aripiprazole for extended- release injectable suspension • Abilify Maintena
Aripiprazole lauroxil • Aristada
Haloperidol • Haldol
Haloperidol injection • Haldol decanoate
Olanzapine • Zyprexa
Olanzapine for extended-release injectable suspension • Zyprexa Relprevv
Paliperidone • Invega
Paliperidone palmitate extended-release injectable suspension • Invega Sustenna
Paliperidone palmitate extended-release injectable suspension • Invega Trinza
Risperidone • Risperdal
Risperidone for extended- release injectable suspension • Perseris
Risperidone injection • Risperdal Consta
Long-acting injectable antipsychotics (LAIs) are an essential tool in the treatment of patients with psychotic disorders, allowing for periods of stable drug plasma concentration and confirmed adherence.1 The current coronavirus disease 2019 (COVID-19) pandemic presents unique challenges for administering LAIs and requires a thoughtful and prospective approach in order to ensure continuity of psychiatric care while minimizing the risk of infection with COVID-19. Ideally, patients should be seen in person as infrequently as clinically prudent during this public health emergency; however, LAI administration necessitates direct physical contact between patient and clinician.
Patients with serious mental illness (SMI), who comprise the majority of individuals who receive LAIs, are at heightened risk for cardiovascular and pulmonary comorbidities. These factors are the primary reason the life expectancy of a patient with SMI is nearly 30 years shorter than that of the general population.2-5 The risk of health care workers becoming infected or inadvertently spreading COVID-19 is heightened when working with patients in group living environments (ie, a shelter or group home), who have both increased exposure and increased risk of further transmission.6 Additional patient populations, including older adults, immunocompromised individuals, and those with preexisting conditions, are at heightened risk for serious complications if they were to contract COVID-19.7,8
Thus, the questions of whether LAIs should be administered, and how to do so safely (both during the ongoing, acute phase of the pandemic as well as during the subsequent recovery period until the pandemic abates) need to be carefully considered. In this article, we provide concrete advice for clinicians and clinics on these topics, with the goal of maintaining patients’ psychiatric stability while protecting patients, health care workers, and the broader society from COVID-19 infection. Table 1 summarizes the questions regarding LAIs that clinicians need to address during this crisis. While we focus on outpatient care, inpatient teams should keep these considerations in mind if they are starting and discharging a patient on an LAI. More than ever, close collaboration and communication between inpatient and outpatient teams is critical.
Should an LAI be continued?
An important first step to approaching this challenge is to create a spreadsheet for all patients receiving LAIs. Focusing on a population-based approach is helpful to be systematic and ensure that no patients fall through the cracks during this public health emergency.9 Once all patients have been identified, the treatment team should review each patient to determine if continuing to administer the antipsychotic as an LAI formulation is essential, taking into account the patient’s current psychiatric status, historical medication adherence, potential severity and dangerousness of decompensation if nonadherent, and structures to support stability. For example, can a patient move in with family who can monitor medication adherence during the pandemic? Is it possible for the group home to assume medication administration? Additional consideration should be given to the living environment and health-vulnerability of the patient and the individuals living with them.
If the risk calculation does not point strongly towards a need for continuing the LAI, it may be prudent to temporarily transition the patient to the corresponding oral antipsychotic preparation. Table 2 lists all LAIs available in the United States and their approximate equivalent oral dosing. It is important to note that such transitions are not without clinical risk, to emphasize to the patient that the transition is intended as a temporary measure, and to discuss a proposed timeline for re-initiating the LAI. Also, emphasize to the patient and family that this transition does not diminish the previous reasoning for needing an LAI, but is a temporary measure taken in light of weighing the risks and benefits during a pandemic.

Which LAI should be administered?
If continuing the LAI is determined to be clinically necessary, consider switching the patient to a longer-acting preparation to maximize intervals between administrations and minimize the potential for infection. From a public health perspective, the longest clinically prudent interval between injections may be the most important consideration, provided the patient can receive a dose necessary to retain stability, and the LAI should be chosen accordingly. Deltoid injections may be able to be administered with reduced contact, or on a “drive-up” basis.10 Consider transitioning a patient who is receiving olanzapine pamoate to an alternate LAI or oral formulation, because the 3-hour observation period that is required after olanzapine pamoate administration is particularly problematic. While it may not be ideal to make medication changes during a pandemic, it is worth carefully weighing the patient’s stability and historical experience with other LAIs to determine if a safer/longer-spaced option is worth trying.11
We recommend only switching among similar antipsychotics (ie, risperidone to paliperidone), or between different preparations of the same drug (ie, Abilify Maintena to Aristada), if possible, as these are the lowest risk transitions with regards to relapse. Table 3 provides examples.

Continue to: When should the LAI be administered?
When should the LAI be administered?
The pharmacokinetics of LAIs allow for some flexibility in terms of when an LAI needs to be administered. The package inserts of all second-generation LAIs include missed-dose guidelines. These guidelines provide information on how long one can wait before the next injection is due, and what additional measures must be taken when beyond that date. Delaying an injection may be prudent, and the missed dose guidelines will indicate when one must consider supplementing with oral medications. For patients who are in quarantine, it may be better to delay an injection until the patient ends their quarantine than to deliver the dose during quarantine. Administering an injection earlier also is usually safe; off-cycle visits may help minimize patient contact (ie, if the patient happens to be coming into the vicinity of the clinic, or requires phlebotomy for therapeutic drug monitoring), and assist in planning for possible resurgences. When appropriate, and after considering the risk of worsening adverse effects, administering a higher dose than the usual maintenance dose would provide a buffer if the next injection was to be delayed. Therapeutic drug monitoring can help to optimize dosing and avoid low plasma drug levels, which may be not be sufficient, particularly during this time of stress.12 To provide optimal protection against relapse, consider administering a dose that puts patients at the higher range of plasma drug levels.
Where can the LAI be administered, and who can give it?
For patients who usually travel to a clinic, consider arranging for a more local injection (ie, at the patient’s primary care clinic in their hometown, or at a local mental health center), and explore if the patient may be able to receive their injection in their home through a visiting nurse association (VNA). In many states (approximately 30 currently), clinicians at pharmacies are also able to administer patient injections. Clinics would do well to at least plan for alternate staffing models in the event of staff illness. A pool of individuals should be available to give injections; consider training additional staff members (including MDs who may have never previously administered an LAI but could be quickly instructed to do so) to administer LAIs. Theoretically, during a public health emergency, family members, particularly those who have a background in health care, could be trained to give an injection and provided education on LAI storage and post-injection monitoring. This approach would not be consistent with FDA labeling, however, and should only be considered as a last resort.
What safety measures can be put in place?
Face-to-face time for injection administration should be kept as brief as possible. Before the encounter, obtain the patient’s clinical information, ideally through telehealth or from an acceptable distance. Medication should be drawn ahead of time, and not in an enclosed space with the patient present. Strongly consider abandoning the traditional enclosed room for the injection, and instead use larger spaces, doorways, or outside, if feasible. As previously noted, some clinics and clinicians have used a drive-up approach for LAI administration, particularly for deltoid injections.10 Individuals who administer the injections should wear personal protective equipment, and the clinic should obtain an adequate supply of this equipment well in advance.
Lessons learned at our clinic
In our community mental health center clinic, planning around these questions has allowed us to provide safe and continuous psychiatric care with LAIs during this public health emergency while reducing the risk of infection. We have worked to transfer LAI administration to VNAs and transition patients to longer-lasting formulations or oral medications where appropriate, which has resulted in an approximately 50% decrease in in-person visits. Reducing the number of in-person visits does not need to result in less frequent clinical follow-up. Telepsychiatry visits can make up for lost in-person visits and have generally been well accepted.
As we are preparing for the next phase, routine medical health monitoring (eg, metabolic monitoring, monitoring for tardive dyskinesia) that has not been at the forefront of concerns should be carefully reintroduced. Challenges encountered have included difficulty in having VNA accept patients for short-term LAI visits, changes to where on the body the injection is delivered, and patients with SMI and their families being reluctant to depart from previous routines and administration schedules.
Continue to: There is great value...
There is great value in the collective lessons learned during this public health emergency (eg, the need for a flexible, population health-based approach; acceptability of combination telehealth and in-person visits) that can lead to more person-centered and accessible care for patients with SMI.
Acknowledgments
The authors thank North Suffolk Mental Health Association, the Freedom Trail Clinic, and their patients.
Bottom Line
When caring for a patient with a psychotic illness during the coronavirus disease 2019 (COVID-19) pandemic, evaluate whether it is necessary to continue a longacting injectable antipsychotic (LAI). If yes, reconsider which LAI should be administered, when and where it should be given, and by whom. Implement safety measures to minimize the risk of COVID-19 exposure and transmission.
Related Resources
- American Association of Community Psychiatrists. Clinical Tip Series. Long acting antipsychotic medications. https://drive.google.com/file/d/1unigjmjFJkqZMbaZ_ftdj8oqog49awZs/view?usp=sharing
- SMI Adviser. What are clinical considerations for giving LAIs during the COVID-19 public health emergency? https://smiadviser.org/knowledge_post/what-are-clinical-considerations-for-giving-lais-during-the-covid-19-public-health-emergency
- CDC. Infection control basics. https://www.cdc.gov/infectioncontrol/basics/index.html
Drug Brand Names
Aripiprazole • Abilify
Aripiprazole for extended- release injectable suspension • Abilify Maintena
Aripiprazole lauroxil • Aristada
Haloperidol • Haldol
Haloperidol injection • Haldol decanoate
Olanzapine • Zyprexa
Olanzapine for extended-release injectable suspension • Zyprexa Relprevv
Paliperidone • Invega
Paliperidone palmitate extended-release injectable suspension • Invega Sustenna
Paliperidone palmitate extended-release injectable suspension • Invega Trinza
Risperidone • Risperdal
Risperidone for extended- release injectable suspension • Perseris
Risperidone injection • Risperdal Consta
1. Freudenreich O. Long-acting injectable antipsychotics. In: Freudenreich O. Psychotic disorders: a practical guide. Springer; 2020:249-261.
2. Olfson M, Gerhard T, Huang C, et al. Premature mortality among adults with schizophrenia in the United States. JAMA Psychiatry. 2015;72(12):1172-1181.
3. Reilly S, Olier I, Planner C, et al. Inequalities in physical comorbidity: a longitudinal comparative cohort study of people with severe mental illness in the UK. BMJ Open. 2015;5(12):e009010.
4. Brown S, Inskip H, Barraclough B. Causes of the excess mortality of schizophrenia. Br J Psychiatry. 2000;177:212-217.
5. Goff DC, Cather C, Evins AE, et al. Medical morbidity and mortality in schizophrenia: guidelines for psychiatrists. J Clin Psychiatry. 2005;66(2):183-194.
6. Baggett TP, Keyes H, Sporn N, et al. Prevalence of SARS-CoV-2 infection in residents of a large homeless shelter in Boston. JAMA. 2020;323(21):2191-2192.
7. CDC COVID-19 Response Team. Severe outcomes among patients with coronavirus disease 2019 (COVID-19)—United States, February 12–March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):343-346.
8. Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985
9. Etches V, Frank J, Di Ruggiero E, et al. Measuring population health: a review of indicators. Annu Rev Public Health. 2006;27:29-55.
10. Chepke C. Drive-up pharmacotherapy during the COVID-19 pandemic. Current Psychiatry. 2020;19(5):29-30.
11. Sajatovic M, Ross R, Legacy SN, et al. Initiating/maintaining long-acting injectable antipsychotics in schizophrenia/schizoaffective or bipolar disorder - expert consensus survey part 2. Neuropsychiatr Dis Treat. 2018;14:1475-1492.
12. Schoretsanitis G, Kane JM, Correll CU, et al; American Society of Clinical Psychopharmacology, Pharmakopsychiatrie TTDMTFOTAFNU. Blood levels to optimize antipsychotic treatment in clinical practice: a joint consensus statement of the American Society of Clinical Psychopharmacology and the Therapeutic Drug Monitoring Task Force of the Arbeitsgemeinschaft für Neuropsychopharmakologie und Pharmakopsychiatrie. J Clin Psychiatry. 2020;81(3):19cs13169. doi: 10.4088/JCP.19cs13169
1. Freudenreich O. Long-acting injectable antipsychotics. In: Freudenreich O. Psychotic disorders: a practical guide. Springer; 2020:249-261.
2. Olfson M, Gerhard T, Huang C, et al. Premature mortality among adults with schizophrenia in the United States. JAMA Psychiatry. 2015;72(12):1172-1181.
3. Reilly S, Olier I, Planner C, et al. Inequalities in physical comorbidity: a longitudinal comparative cohort study of people with severe mental illness in the UK. BMJ Open. 2015;5(12):e009010.
4. Brown S, Inskip H, Barraclough B. Causes of the excess mortality of schizophrenia. Br J Psychiatry. 2000;177:212-217.
5. Goff DC, Cather C, Evins AE, et al. Medical morbidity and mortality in schizophrenia: guidelines for psychiatrists. J Clin Psychiatry. 2005;66(2):183-194.
6. Baggett TP, Keyes H, Sporn N, et al. Prevalence of SARS-CoV-2 infection in residents of a large homeless shelter in Boston. JAMA. 2020;323(21):2191-2192.
7. CDC COVID-19 Response Team. Severe outcomes among patients with coronavirus disease 2019 (COVID-19)—United States, February 12–March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):343-346.
8. Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985
9. Etches V, Frank J, Di Ruggiero E, et al. Measuring population health: a review of indicators. Annu Rev Public Health. 2006;27:29-55.
10. Chepke C. Drive-up pharmacotherapy during the COVID-19 pandemic. Current Psychiatry. 2020;19(5):29-30.
11. Sajatovic M, Ross R, Legacy SN, et al. Initiating/maintaining long-acting injectable antipsychotics in schizophrenia/schizoaffective or bipolar disorder - expert consensus survey part 2. Neuropsychiatr Dis Treat. 2018;14:1475-1492.
12. Schoretsanitis G, Kane JM, Correll CU, et al; American Society of Clinical Psychopharmacology, Pharmakopsychiatrie TTDMTFOTAFNU. Blood levels to optimize antipsychotic treatment in clinical practice: a joint consensus statement of the American Society of Clinical Psychopharmacology and the Therapeutic Drug Monitoring Task Force of the Arbeitsgemeinschaft für Neuropsychopharmakologie und Pharmakopsychiatrie. J Clin Psychiatry. 2020;81(3):19cs13169. doi: 10.4088/JCP.19cs13169
COVID-19 and the risk of homicide-suicide among older adults
On March 25, 2020, in Cambridge, United Kingdom, a 71-year-old man stabbed his 71-year-old wife before suffocating himself to death. The couple was reportedly anxious about the coronavirus disease 2019 (COVID-19) pandemic lockdown measures and were on the verge of running out of food and medicine.1
One week later, in Chicago, Illinois, a 54-year-old man shot and killed his female partner, age 54, before killing himself. The couple was tested for COVID-19 2 days earlier and the man believed they had contracted the virus; however, the test results for both of them had come back negative.2
Intimate partner homicide-suicide is the most dramatic domestic abuse outcome.3 Homicide-suicide is defined as “homicide committed by a person who subsequently commits suicide within one week of the homicide. In most cases the subsequent suicide occurs within a 24-hour period.”4 Approximately one-quarter of all homicide-suicides are committed by persons age ≥55 years.5,6 We believe that during the COVID-19 pandemic, the risk of homicide-suicide among older adults may be increased due to several factors, including:
- physical distancing and quarantine measures. Protocols established to slow the spread of the virus may be associated with increased rates of depression and anxiety7 and an increased risk of suicide among older adults8
- increased intimate partner violence9
- increased firearm ownership rates in the United States.10
In this article, we review studies that identified risk factors for homicide-suicide among older adults, discuss the impact the COVID-19 pandemic has had on these risks, and describe steps clinicians can take to intervene.
A review of the literature
To better characterize the perpetrators of older adult homicide-suicide, we conducted a literature search of relevant terms. We identified 9 original research publications that examined homicide-suicide in older adults.
Bourget et al11 (2010) reviewed coroners’ charts of individuals killed by an older (age ≥65) spouse or family member from 1992 through 2007 in Quebec, Canada. They identified 19 cases of homicide-suicide, 17 (90%) of which were perpetrated by men. Perpetrators and victims were married (63%), in common-law relationships (16%), or separated/divorced (16%). A history of domestic violence was documented in 4 (21%) cases. The authors found that 13 of 15 perpetrators (87%) had “major depression” and 2 perpetrators had a psychotic disorder. Substance use at the time of the event was confirmed in 6 (32%) cases. Firearms and strangulation were the top methods used to carry out the homicide-suicide.11
Cheung et al12 (2016) conducted a review of coroners’ records of homicide-suicide cases among individuals age ≥65 in New Zealand from 2007 through 2012. In all 4 cases, the perpetrators were men, and their victims were predominantly female, live-in family members. Two cases involved men with a history of domestic violence who were undergoing significant changes in their home and social lives. Both men had a history suggestive of depression and used a firearm to carry out the homicide-suicide.12
Continue to: Cohen et al
Cohen et al13 (1998) conducted a review of coroners’ records from 1988 through 1994 in 2 regions in Florida. They found 48 intimate partner homicide-suicide cases among “old couples” (age ≥55). All were perpetrated by men. The authors identified sociocultural differences in risk factors between the 2 regions. In west-central Florida, perpetrators and victims were predominantly white and in a spousal relationship. Domestic violence was documented in <4% of cases. Approximately 55% of the couples were reported to be ill, and a substantial proportion were documented to be declining in health. One-quarter of the perpetrators and one-third of the victims had “pain and suffering.” More than one-third of perpetrators were reported to have “depression,” 15% were reported to have talked about suicide, and 4% had a history of a suicide attempt. Only 11% of perpetrators were described as abusing substances.
The authors noted several differences in cases in southeastern Florida. Approximately two-thirds of the couples were Hispanic, and 14% had a history of domestic violence. A minority of the couples were in a live-in relationship. Less than 15% of the perpetrators and victims were described as having a decline in health. Additionally, only 19% of perpetrators were reported to have “depression,” and none of the perpetrators had a documented history of attempted suicide or substance abuse. No information was provided regarding the methods used to carry out the homicide-suicide in the southeastern region.13 Financial stress was not a factor in either region.
Malphurs et al14 (2001) used the same database described in the Cohen et al13 study to compare 27 perpetrators of homicide-suicide to 36 age-matched suicide decedents in west central Florida. They found that homicide-suicide perpetrators were significantly less likely to have health problems and were 3 times more likely to be caregivers to their spouses. Approximately 52% of perpetrators had at least 1 documented psychiatric symptom (“depression” and/or substance abuse or other), but only 5% were seeking mental health services at the time of death.14
De Koning and Piette15 (2014) conducted a retrospective medicolegal chart review from 1935 to 2010 to identify homicide-suicide cases in West and East Flanders, Belgium. They found 19 cases of intimate partner homicide-suicide committed by offenders age ≥55 years. Ninety-five percent of the perpetrators were men who killed their female partners. In one-quarter of the cases, either the perpetrator or the victim had a health issue; 21% of the perpetrators were documented as having depression and 27% had alcohol intoxication at the time of death. A motive was documented in 14 out of 19 cases; “mercy killing” was determined as the motive in 6 (43%) cases and “amorous jealousy” in 5 cases (36%).15 Starting in the 1970s, firearms were the most prevalent method used to kill a partner.
Logan et al16 (2019) used data from the National Violent Death Reporting System between 2003 and 2015 to identify characteristics that differentiated male suicide decedents from male perpetrators of intimate partner homicide-suicide. They found that men age 50 to 64 years were 3 times more likely than men age 18 to 34 years to commit intimate partner homicide-suicide, and that men age ≥65 years were approximately 5 times more likely than men age 18 to 34 years to commit intimate partner homicide-suicide. The authors found that approximately 22% of all perpetrators had a documented history of physical domestic violence, and close to 17% had a prior interaction with the criminal justice system. Furthermore, one-third of perpetrators had relationship difficulties and were in the process of a breakup. Health issues were prevalent in 34% of the victims and 26% of the perpetrators. Perpetrator-caregiver burden was reported as a contributing factor for homicide-suicide in 16% of cases. In 27% of cases, multiple health-related contributing factors were mentioned.16
Continue to: Malphurs and Cohen
Malphurs and Cohen5 (2002) reviewed American newspapers from 1997 through 1999 and identified 673 homicide-suicide events, of which 152 (27%) were committed by individuals age ≥55 years. The victims and perpetrators (95% of which were men) were intimate partners in three-quarters of cases. In nearly one-third of cases, caregiving was a contributing factor for the homicide-suicide. A history of or a pending divorce was reported in nearly 14% of cases. Substance use history was rarely recorded. Firearms were used in 88% of the homicide-suicide cases.5
Malphurs and Cohen17 (2005) reviewed coroner records between 1998 and 1999 in Florida and compared 20 cases of intimate partner homicide-suicide involving perpetrators age ≥55 years with matched suicide decedents. They found that 60% of homicide-suicide perpetrators had documented health issues. The authors reported that a “recent change in health status” was more prevalent among perpetrators compared with decedents. Perpetrators were also more likely to be caregivers to their spouses. The authors found that 65% of perpetrators were reported to have a “depressed mood” and 15% of perpetrators had reportedly threatened suicide prior to the incident. However, none of the perpetrators tested positive for antidepressants as documented on post-mortem toxicology reports. Firearms were used in 100% of homicide-suicide cases.17
Salari3 (2007) reviewed multiple American media sources and published police reports between 1999 and 2005 to retrieve data about intimate partner homicide-suicide events in the United States. There were 225 events identified where the perpetrator and/or the victim were age ≥60 years. Ninety-six percent of the perpetrators were men and most homicide-suicide events were committed at the home. A history of domestic violence was reported in 14% of homicide-suicide cases. Thirteen percent of couples were separated or divorced. The perpetrator and/or victim had health issues in 124 (55%) events. Dementia was reported in 7.5% of cases, but overwhelmingly among the victims. Substance abuse was rarely mentioned as a contributing factor. In three-quarters of cases where a motive was described, the perpetrator was “suicidal”; however, a “suicide pact” was mentioned in only 4% of cases. Firearms were used in 87% of cases.3
Focus on common risk factors
The scarcity and heterogeneity of research regarding older adult homicide-suicide were major limitations to our review. Because most of the studies we identified had a small sample size and limited information regarding the mental health of victims and perpetrators, it would be an overreach to claim to have identified a typical profile of an older perpetrator of homicide-suicide. However, the literature has repeatedly identified several common characteristics of such perpetrators. They are significantly more likely to be men who use firearms to murder their intimate partners and then die by suicide in their home (Table3,5,11-17). Health issues afflicting 1 or both individuals in the couple appear to be a contributing factor, particularly when the perpetrator is in a caregiving role. Relational discord, with or without a history of domestic violence, increases the risk of homicide-suicide. Finally, older perpetrators are highly likely to be depressed and have suicidal ideations.
How COVID-19 affects these risks
Although it is too early to determine if there is a causal relationship between the COVID-19 pandemic and an increase in homicide-suicide, the pandemic is likely to promote risk factors for these events, especially among older adults. Confinement measures put into place during the pandemic context have already been shown to increase rates of domestic violence18 and depression and anxiety among older individuals.7 Furthermore,
Continue to: Late-life psychiatric disorders
Late-life psychiatric disorders
Early recognition and effective treatment of late-life psychiatric disorders is crucial. Unfortunately, depression in geriatric patients is often underdiagnosed and undertreated.20 Older adults have more frequent contact with their primary care physicians, and rarely consult mental health professionals.21,22 Several models of integrated depression care within primary care settings have shown the positive impact of this collaborative approach in treating late-life depression and preventing suicide in older individuals.23 Additionally, because alcohol abuse is also a risk factor for domestic violence and breaking the law in this population,24,25 older adults should be screened for alcohol use disorders, and referred to treatment when necessary.
Take steps to keep patients safe
In the context of the COVID-19 pandemic, there are several steps clinicians need to keep in mind when interacting with older patients:
- Screen for depressive symptoms, suicidality, and alcohol and substance use disorders. Individuals who have tested positive for COVID-19 or who have been in contact with a carrier are a particularly vulnerable population.
- Screen for domestic violence and access to weapons at home.4 Any older adult who has a psychiatric disorder and/or suicide ideation should receive immediate intervention through a social worker that includes providing gun-risk education to other family members or contacting law-enforcement officials.26
- Refer patients with a suspected psychiatric disorder to specialized mental health clinicians. Telemental health services can provide rapid access to subspecialists, allowing patients to be treated from their homes.27 These services need to be promoted among older adults during this critical period and reimbursed by public and private insurance systems to ensure accessibility and affordability.28
- Create psychiatric inpatient units specifically designed for suicidal and/or homicidal patients with COVID-19.
Additionally, informing the public about these major health issues is crucial. The media can raise awareness about domestic violence and depression among older adults; however, this should be done responsibly and with accuracy to prevent the spread of misinformation, confusion, fear, and panic.29
Bottom Line
The mental health burden of the coronavirus disease 2019 pandemic has significantly impacted individuals who are older and most vulnerable. Reducing the incidence of homicide-suicide among older adults requires timely screening and interventions by primary care providers, mental health specialists, social workers, media, and governmental agencies.
Related Resources
- Saeed SA, Hebishi K. The psychiatric consequences of COVID-19: 8 studies. Current Psychiatry. 2020;19(11):22-24,28-30,32-35.
- Schwab-Reese LM, Murfree L, Coppola EC, et al. Homicidesuicide across the lifespan: a mixed methods examination of factors contributing to older adult perpetration. Aging Ment Health. 2020;20:1-9.
1. Christodoulou H. LOCKDOWN ‘MURDER-SUICIDE’ OAP, 71, ‘stabbed wife to death then killed himself as he worried about coping with coronavirus lockdown.’ The Sun. Updated April 4, 2020. Accessed December 22, 2020. https://www.thesun.co.uk/news/11327095/coronavirus-lockdown-murder-suicide-cambridge/
2. Farberov S. Illinois man, 54, shoots dead his wife then kills himself in murder-suicide because he feared they had coronavirus - but tests later show the couple were NOT ill. Updated April 6, 2020. Accessed December 22, 2020. https://www.dailymail.co.uk/news/article-8191933/Man-kills-wife-feared-coronavirus.html
3. Salari S. Patterns of intimate partner homicide suicide in later life: strategies for prevention. Clin Interv Aging. 2007;2(3):441-452.
4. Kotzé C, Roos JL. Homicide–suicide: practical implications for risk reduction and support services at primary care level. South African Family Practice. 2019;61(4):165-169.
5. Malphurs JE, Cohen D. A newspaper surveillance study of homicide-suicide in the United States. Am J Forensic Med Pathol. 2002;23(2):142-148.
6. Eliason S. Murder-suicide: a review of the recent literature. J Am Acad Psychiatry Law. 2009;37(3):371-376.
7. Armitage R, Nellums LB. COVID-19 and the consequences of isolating the elderly. Lancet Public Health. 2020;5(5):e256. doi: 10.1016/S2468-2667(20)30061-X
8. Gunnell D, Appleby L, Arensman E, et al. Suicide risk and prevention during the COVID-19 pandemic. Lancet Psychiatry 2020;7(6):468-471.
9. Gosangi B, Park H, Thomas R, et al. Exacerbation of physical intimate partner violence during COVID-19 pandemic. Radiology. 2021;298(1):E38-E45.
10. Mannix R, Lee LK, Fleegler EW. Coronavirus disease 2019 (COVID-19) and firearms in the United States: will an epidemic of suicide follow? Ann Intern Med. 2020;173(3):228-229.
11. Bourget D, Gagne P, Whitehurst L. Domestic homicide and homicide-suicide: the older offender. J Am Acad Psychiatry Law. 2010;38(3):305-311.
12. Cheung G, Hatters Friedman S, Sundram F. Late-life homicide-suicide: a national case series in New Zealand. Psychogeriatrics. 2016;16(1):76-81.
13. Cohen D, Llorente M, Eisdorfer C. Homicide-suicide in older persons. Am J Psychiatry. 1998;155(3):390-396.
14. Malphurs JE, Eisdorfer C, Cohen D. A comparison of antecedents of homicide-suicide and suicide in older married men. Am J Geriatr Psychiatry. 2001;9(1):49-57.
15. De Koning E, Piette MHA. A retrospective study of murder–suicide at the Forensic Institute of Ghent University, Belgium: 1935–2010. Med Sci Law. 2014;54(2):88-98.
16. Logan JE, Ertl A, Bossarte R. Correlates of intimate partner homicide among male suicide decedents with known intimate partner problems. Suicide Life Threat Behav. 2019;49(6):1693-1706.
17. Malphurs JE, Cohen D. A statewide case-control study of spousal homicide-suicide in older persons. Am J Geriatr Psychiatry. 2005;13(3):211-217.
18. Sanford A. ‘Horrifying surge in domestic violence’ against women amid coronavirus-lockdowns, UN chief warns. Euronews. Published June 4, 2020. Accessed December 22, 2020. https://www.euronews.com/2020/04/06/horrifying-surge-in-domestic-violence-against-women-amid-coronavirus-lockdowns-un-chief-w
19. Appel JM. Intimate partner homicide in elderly populations. In: Friedman SH, ed. Family murder: pathologies of love and hate. American Psychiatric Association Publishing; 2019:131-142.
20. Hall CA, Reynolds-III CF. Late-life depression in the primary care setting: challenges, collaborative care, and prevention. Maturitas. 2014;79(2):147-152.
21. Unützer J. Diagnosis and treatment of older adults with depression in primary care. Biological Psychiatry. 2002;52(3):285-292.
22. Byers AL, Arean PA, Yaffe K. Low use of mental health services among older Americans with mood and anxiety disorders. Psychiatr Serv. 2012;63(1):66-72.
23. Bruce ML, Sirey JA. Integrated care for depression in older primary care patients. Can J Psychiatry. 2018;63(7):439-446.
24. Rao R, Roche A. Substance misuse in older people. BMJ. 2017;358:j3885. doi: 10.1136/bmj.j3885
25. Ghossoub E, Khoury R. Prevalence and correlates of criminal behavior among the non-institutionalized elderly: results from the National Survey on Drug Use and Health. J Geriatr Psychiatry Neurol. 2018;31(4):211-222.
26. Slater MAG. Older adults at risk for suicide. In: Berkman B. Handbook of social work in health and aging. Oxford University Press; 2006:149-161.
27. Hollander JE, Carr BG. Virtually perfect? Telemedicine for Covid-19. N Engl J Med. 2020;382(18):1679-1681.
28. Centers for Medicare & Medicaid Services. President Trump expands telehealth benefits for Medicare beneficiaries during COVID-19 outbreak. Published March 17, 2020. Accessed December 23, 2020. https://www.cms.gov/newsroom/press-releases/president-trump-expands-telehealth-benefits-medicare-beneficiaries-during-covid-19-outbreak
29. Mian A, Khan S. Coronavirus: the spread of misinformation. BMC Med. 2020;18(1):89.
On March 25, 2020, in Cambridge, United Kingdom, a 71-year-old man stabbed his 71-year-old wife before suffocating himself to death. The couple was reportedly anxious about the coronavirus disease 2019 (COVID-19) pandemic lockdown measures and were on the verge of running out of food and medicine.1
One week later, in Chicago, Illinois, a 54-year-old man shot and killed his female partner, age 54, before killing himself. The couple was tested for COVID-19 2 days earlier and the man believed they had contracted the virus; however, the test results for both of them had come back negative.2
Intimate partner homicide-suicide is the most dramatic domestic abuse outcome.3 Homicide-suicide is defined as “homicide committed by a person who subsequently commits suicide within one week of the homicide. In most cases the subsequent suicide occurs within a 24-hour period.”4 Approximately one-quarter of all homicide-suicides are committed by persons age ≥55 years.5,6 We believe that during the COVID-19 pandemic, the risk of homicide-suicide among older adults may be increased due to several factors, including:
- physical distancing and quarantine measures. Protocols established to slow the spread of the virus may be associated with increased rates of depression and anxiety7 and an increased risk of suicide among older adults8
- increased intimate partner violence9
- increased firearm ownership rates in the United States.10
In this article, we review studies that identified risk factors for homicide-suicide among older adults, discuss the impact the COVID-19 pandemic has had on these risks, and describe steps clinicians can take to intervene.
A review of the literature
To better characterize the perpetrators of older adult homicide-suicide, we conducted a literature search of relevant terms. We identified 9 original research publications that examined homicide-suicide in older adults.
Bourget et al11 (2010) reviewed coroners’ charts of individuals killed by an older (age ≥65) spouse or family member from 1992 through 2007 in Quebec, Canada. They identified 19 cases of homicide-suicide, 17 (90%) of which were perpetrated by men. Perpetrators and victims were married (63%), in common-law relationships (16%), or separated/divorced (16%). A history of domestic violence was documented in 4 (21%) cases. The authors found that 13 of 15 perpetrators (87%) had “major depression” and 2 perpetrators had a psychotic disorder. Substance use at the time of the event was confirmed in 6 (32%) cases. Firearms and strangulation were the top methods used to carry out the homicide-suicide.11
Cheung et al12 (2016) conducted a review of coroners’ records of homicide-suicide cases among individuals age ≥65 in New Zealand from 2007 through 2012. In all 4 cases, the perpetrators were men, and their victims were predominantly female, live-in family members. Two cases involved men with a history of domestic violence who were undergoing significant changes in their home and social lives. Both men had a history suggestive of depression and used a firearm to carry out the homicide-suicide.12
Continue to: Cohen et al
Cohen et al13 (1998) conducted a review of coroners’ records from 1988 through 1994 in 2 regions in Florida. They found 48 intimate partner homicide-suicide cases among “old couples” (age ≥55). All were perpetrated by men. The authors identified sociocultural differences in risk factors between the 2 regions. In west-central Florida, perpetrators and victims were predominantly white and in a spousal relationship. Domestic violence was documented in <4% of cases. Approximately 55% of the couples were reported to be ill, and a substantial proportion were documented to be declining in health. One-quarter of the perpetrators and one-third of the victims had “pain and suffering.” More than one-third of perpetrators were reported to have “depression,” 15% were reported to have talked about suicide, and 4% had a history of a suicide attempt. Only 11% of perpetrators were described as abusing substances.
The authors noted several differences in cases in southeastern Florida. Approximately two-thirds of the couples were Hispanic, and 14% had a history of domestic violence. A minority of the couples were in a live-in relationship. Less than 15% of the perpetrators and victims were described as having a decline in health. Additionally, only 19% of perpetrators were reported to have “depression,” and none of the perpetrators had a documented history of attempted suicide or substance abuse. No information was provided regarding the methods used to carry out the homicide-suicide in the southeastern region.13 Financial stress was not a factor in either region.
Malphurs et al14 (2001) used the same database described in the Cohen et al13 study to compare 27 perpetrators of homicide-suicide to 36 age-matched suicide decedents in west central Florida. They found that homicide-suicide perpetrators were significantly less likely to have health problems and were 3 times more likely to be caregivers to their spouses. Approximately 52% of perpetrators had at least 1 documented psychiatric symptom (“depression” and/or substance abuse or other), but only 5% were seeking mental health services at the time of death.14
De Koning and Piette15 (2014) conducted a retrospective medicolegal chart review from 1935 to 2010 to identify homicide-suicide cases in West and East Flanders, Belgium. They found 19 cases of intimate partner homicide-suicide committed by offenders age ≥55 years. Ninety-five percent of the perpetrators were men who killed their female partners. In one-quarter of the cases, either the perpetrator or the victim had a health issue; 21% of the perpetrators were documented as having depression and 27% had alcohol intoxication at the time of death. A motive was documented in 14 out of 19 cases; “mercy killing” was determined as the motive in 6 (43%) cases and “amorous jealousy” in 5 cases (36%).15 Starting in the 1970s, firearms were the most prevalent method used to kill a partner.
Logan et al16 (2019) used data from the National Violent Death Reporting System between 2003 and 2015 to identify characteristics that differentiated male suicide decedents from male perpetrators of intimate partner homicide-suicide. They found that men age 50 to 64 years were 3 times more likely than men age 18 to 34 years to commit intimate partner homicide-suicide, and that men age ≥65 years were approximately 5 times more likely than men age 18 to 34 years to commit intimate partner homicide-suicide. The authors found that approximately 22% of all perpetrators had a documented history of physical domestic violence, and close to 17% had a prior interaction with the criminal justice system. Furthermore, one-third of perpetrators had relationship difficulties and were in the process of a breakup. Health issues were prevalent in 34% of the victims and 26% of the perpetrators. Perpetrator-caregiver burden was reported as a contributing factor for homicide-suicide in 16% of cases. In 27% of cases, multiple health-related contributing factors were mentioned.16
Continue to: Malphurs and Cohen
Malphurs and Cohen5 (2002) reviewed American newspapers from 1997 through 1999 and identified 673 homicide-suicide events, of which 152 (27%) were committed by individuals age ≥55 years. The victims and perpetrators (95% of which were men) were intimate partners in three-quarters of cases. In nearly one-third of cases, caregiving was a contributing factor for the homicide-suicide. A history of or a pending divorce was reported in nearly 14% of cases. Substance use history was rarely recorded. Firearms were used in 88% of the homicide-suicide cases.5
Malphurs and Cohen17 (2005) reviewed coroner records between 1998 and 1999 in Florida and compared 20 cases of intimate partner homicide-suicide involving perpetrators age ≥55 years with matched suicide decedents. They found that 60% of homicide-suicide perpetrators had documented health issues. The authors reported that a “recent change in health status” was more prevalent among perpetrators compared with decedents. Perpetrators were also more likely to be caregivers to their spouses. The authors found that 65% of perpetrators were reported to have a “depressed mood” and 15% of perpetrators had reportedly threatened suicide prior to the incident. However, none of the perpetrators tested positive for antidepressants as documented on post-mortem toxicology reports. Firearms were used in 100% of homicide-suicide cases.17
Salari3 (2007) reviewed multiple American media sources and published police reports between 1999 and 2005 to retrieve data about intimate partner homicide-suicide events in the United States. There were 225 events identified where the perpetrator and/or the victim were age ≥60 years. Ninety-six percent of the perpetrators were men and most homicide-suicide events were committed at the home. A history of domestic violence was reported in 14% of homicide-suicide cases. Thirteen percent of couples were separated or divorced. The perpetrator and/or victim had health issues in 124 (55%) events. Dementia was reported in 7.5% of cases, but overwhelmingly among the victims. Substance abuse was rarely mentioned as a contributing factor. In three-quarters of cases where a motive was described, the perpetrator was “suicidal”; however, a “suicide pact” was mentioned in only 4% of cases. Firearms were used in 87% of cases.3
Focus on common risk factors
The scarcity and heterogeneity of research regarding older adult homicide-suicide were major limitations to our review. Because most of the studies we identified had a small sample size and limited information regarding the mental health of victims and perpetrators, it would be an overreach to claim to have identified a typical profile of an older perpetrator of homicide-suicide. However, the literature has repeatedly identified several common characteristics of such perpetrators. They are significantly more likely to be men who use firearms to murder their intimate partners and then die by suicide in their home (Table3,5,11-17). Health issues afflicting 1 or both individuals in the couple appear to be a contributing factor, particularly when the perpetrator is in a caregiving role. Relational discord, with or without a history of domestic violence, increases the risk of homicide-suicide. Finally, older perpetrators are highly likely to be depressed and have suicidal ideations.
How COVID-19 affects these risks
Although it is too early to determine if there is a causal relationship between the COVID-19 pandemic and an increase in homicide-suicide, the pandemic is likely to promote risk factors for these events, especially among older adults. Confinement measures put into place during the pandemic context have already been shown to increase rates of domestic violence18 and depression and anxiety among older individuals.7 Furthermore,
Continue to: Late-life psychiatric disorders
Late-life psychiatric disorders
Early recognition and effective treatment of late-life psychiatric disorders is crucial. Unfortunately, depression in geriatric patients is often underdiagnosed and undertreated.20 Older adults have more frequent contact with their primary care physicians, and rarely consult mental health professionals.21,22 Several models of integrated depression care within primary care settings have shown the positive impact of this collaborative approach in treating late-life depression and preventing suicide in older individuals.23 Additionally, because alcohol abuse is also a risk factor for domestic violence and breaking the law in this population,24,25 older adults should be screened for alcohol use disorders, and referred to treatment when necessary.
Take steps to keep patients safe
In the context of the COVID-19 pandemic, there are several steps clinicians need to keep in mind when interacting with older patients:
- Screen for depressive symptoms, suicidality, and alcohol and substance use disorders. Individuals who have tested positive for COVID-19 or who have been in contact with a carrier are a particularly vulnerable population.
- Screen for domestic violence and access to weapons at home.4 Any older adult who has a psychiatric disorder and/or suicide ideation should receive immediate intervention through a social worker that includes providing gun-risk education to other family members or contacting law-enforcement officials.26
- Refer patients with a suspected psychiatric disorder to specialized mental health clinicians. Telemental health services can provide rapid access to subspecialists, allowing patients to be treated from their homes.27 These services need to be promoted among older adults during this critical period and reimbursed by public and private insurance systems to ensure accessibility and affordability.28
- Create psychiatric inpatient units specifically designed for suicidal and/or homicidal patients with COVID-19.
Additionally, informing the public about these major health issues is crucial. The media can raise awareness about domestic violence and depression among older adults; however, this should be done responsibly and with accuracy to prevent the spread of misinformation, confusion, fear, and panic.29
Bottom Line
The mental health burden of the coronavirus disease 2019 pandemic has significantly impacted individuals who are older and most vulnerable. Reducing the incidence of homicide-suicide among older adults requires timely screening and interventions by primary care providers, mental health specialists, social workers, media, and governmental agencies.
Related Resources
- Saeed SA, Hebishi K. The psychiatric consequences of COVID-19: 8 studies. Current Psychiatry. 2020;19(11):22-24,28-30,32-35.
- Schwab-Reese LM, Murfree L, Coppola EC, et al. Homicidesuicide across the lifespan: a mixed methods examination of factors contributing to older adult perpetration. Aging Ment Health. 2020;20:1-9.
On March 25, 2020, in Cambridge, United Kingdom, a 71-year-old man stabbed his 71-year-old wife before suffocating himself to death. The couple was reportedly anxious about the coronavirus disease 2019 (COVID-19) pandemic lockdown measures and were on the verge of running out of food and medicine.1
One week later, in Chicago, Illinois, a 54-year-old man shot and killed his female partner, age 54, before killing himself. The couple was tested for COVID-19 2 days earlier and the man believed they had contracted the virus; however, the test results for both of them had come back negative.2
Intimate partner homicide-suicide is the most dramatic domestic abuse outcome.3 Homicide-suicide is defined as “homicide committed by a person who subsequently commits suicide within one week of the homicide. In most cases the subsequent suicide occurs within a 24-hour period.”4 Approximately one-quarter of all homicide-suicides are committed by persons age ≥55 years.5,6 We believe that during the COVID-19 pandemic, the risk of homicide-suicide among older adults may be increased due to several factors, including:
- physical distancing and quarantine measures. Protocols established to slow the spread of the virus may be associated with increased rates of depression and anxiety7 and an increased risk of suicide among older adults8
- increased intimate partner violence9
- increased firearm ownership rates in the United States.10
In this article, we review studies that identified risk factors for homicide-suicide among older adults, discuss the impact the COVID-19 pandemic has had on these risks, and describe steps clinicians can take to intervene.
A review of the literature
To better characterize the perpetrators of older adult homicide-suicide, we conducted a literature search of relevant terms. We identified 9 original research publications that examined homicide-suicide in older adults.
Bourget et al11 (2010) reviewed coroners’ charts of individuals killed by an older (age ≥65) spouse or family member from 1992 through 2007 in Quebec, Canada. They identified 19 cases of homicide-suicide, 17 (90%) of which were perpetrated by men. Perpetrators and victims were married (63%), in common-law relationships (16%), or separated/divorced (16%). A history of domestic violence was documented in 4 (21%) cases. The authors found that 13 of 15 perpetrators (87%) had “major depression” and 2 perpetrators had a psychotic disorder. Substance use at the time of the event was confirmed in 6 (32%) cases. Firearms and strangulation were the top methods used to carry out the homicide-suicide.11
Cheung et al12 (2016) conducted a review of coroners’ records of homicide-suicide cases among individuals age ≥65 in New Zealand from 2007 through 2012. In all 4 cases, the perpetrators were men, and their victims were predominantly female, live-in family members. Two cases involved men with a history of domestic violence who were undergoing significant changes in their home and social lives. Both men had a history suggestive of depression and used a firearm to carry out the homicide-suicide.12
Continue to: Cohen et al
Cohen et al13 (1998) conducted a review of coroners’ records from 1988 through 1994 in 2 regions in Florida. They found 48 intimate partner homicide-suicide cases among “old couples” (age ≥55). All were perpetrated by men. The authors identified sociocultural differences in risk factors between the 2 regions. In west-central Florida, perpetrators and victims were predominantly white and in a spousal relationship. Domestic violence was documented in <4% of cases. Approximately 55% of the couples were reported to be ill, and a substantial proportion were documented to be declining in health. One-quarter of the perpetrators and one-third of the victims had “pain and suffering.” More than one-third of perpetrators were reported to have “depression,” 15% were reported to have talked about suicide, and 4% had a history of a suicide attempt. Only 11% of perpetrators were described as abusing substances.
The authors noted several differences in cases in southeastern Florida. Approximately two-thirds of the couples were Hispanic, and 14% had a history of domestic violence. A minority of the couples were in a live-in relationship. Less than 15% of the perpetrators and victims were described as having a decline in health. Additionally, only 19% of perpetrators were reported to have “depression,” and none of the perpetrators had a documented history of attempted suicide or substance abuse. No information was provided regarding the methods used to carry out the homicide-suicide in the southeastern region.13 Financial stress was not a factor in either region.
Malphurs et al14 (2001) used the same database described in the Cohen et al13 study to compare 27 perpetrators of homicide-suicide to 36 age-matched suicide decedents in west central Florida. They found that homicide-suicide perpetrators were significantly less likely to have health problems and were 3 times more likely to be caregivers to their spouses. Approximately 52% of perpetrators had at least 1 documented psychiatric symptom (“depression” and/or substance abuse or other), but only 5% were seeking mental health services at the time of death.14
De Koning and Piette15 (2014) conducted a retrospective medicolegal chart review from 1935 to 2010 to identify homicide-suicide cases in West and East Flanders, Belgium. They found 19 cases of intimate partner homicide-suicide committed by offenders age ≥55 years. Ninety-five percent of the perpetrators were men who killed their female partners. In one-quarter of the cases, either the perpetrator or the victim had a health issue; 21% of the perpetrators were documented as having depression and 27% had alcohol intoxication at the time of death. A motive was documented in 14 out of 19 cases; “mercy killing” was determined as the motive in 6 (43%) cases and “amorous jealousy” in 5 cases (36%).15 Starting in the 1970s, firearms were the most prevalent method used to kill a partner.
Logan et al16 (2019) used data from the National Violent Death Reporting System between 2003 and 2015 to identify characteristics that differentiated male suicide decedents from male perpetrators of intimate partner homicide-suicide. They found that men age 50 to 64 years were 3 times more likely than men age 18 to 34 years to commit intimate partner homicide-suicide, and that men age ≥65 years were approximately 5 times more likely than men age 18 to 34 years to commit intimate partner homicide-suicide. The authors found that approximately 22% of all perpetrators had a documented history of physical domestic violence, and close to 17% had a prior interaction with the criminal justice system. Furthermore, one-third of perpetrators had relationship difficulties and were in the process of a breakup. Health issues were prevalent in 34% of the victims and 26% of the perpetrators. Perpetrator-caregiver burden was reported as a contributing factor for homicide-suicide in 16% of cases. In 27% of cases, multiple health-related contributing factors were mentioned.16
Continue to: Malphurs and Cohen
Malphurs and Cohen5 (2002) reviewed American newspapers from 1997 through 1999 and identified 673 homicide-suicide events, of which 152 (27%) were committed by individuals age ≥55 years. The victims and perpetrators (95% of which were men) were intimate partners in three-quarters of cases. In nearly one-third of cases, caregiving was a contributing factor for the homicide-suicide. A history of or a pending divorce was reported in nearly 14% of cases. Substance use history was rarely recorded. Firearms were used in 88% of the homicide-suicide cases.5
Malphurs and Cohen17 (2005) reviewed coroner records between 1998 and 1999 in Florida and compared 20 cases of intimate partner homicide-suicide involving perpetrators age ≥55 years with matched suicide decedents. They found that 60% of homicide-suicide perpetrators had documented health issues. The authors reported that a “recent change in health status” was more prevalent among perpetrators compared with decedents. Perpetrators were also more likely to be caregivers to their spouses. The authors found that 65% of perpetrators were reported to have a “depressed mood” and 15% of perpetrators had reportedly threatened suicide prior to the incident. However, none of the perpetrators tested positive for antidepressants as documented on post-mortem toxicology reports. Firearms were used in 100% of homicide-suicide cases.17
Salari3 (2007) reviewed multiple American media sources and published police reports between 1999 and 2005 to retrieve data about intimate partner homicide-suicide events in the United States. There were 225 events identified where the perpetrator and/or the victim were age ≥60 years. Ninety-six percent of the perpetrators were men and most homicide-suicide events were committed at the home. A history of domestic violence was reported in 14% of homicide-suicide cases. Thirteen percent of couples were separated or divorced. The perpetrator and/or victim had health issues in 124 (55%) events. Dementia was reported in 7.5% of cases, but overwhelmingly among the victims. Substance abuse was rarely mentioned as a contributing factor. In three-quarters of cases where a motive was described, the perpetrator was “suicidal”; however, a “suicide pact” was mentioned in only 4% of cases. Firearms were used in 87% of cases.3
Focus on common risk factors
The scarcity and heterogeneity of research regarding older adult homicide-suicide were major limitations to our review. Because most of the studies we identified had a small sample size and limited information regarding the mental health of victims and perpetrators, it would be an overreach to claim to have identified a typical profile of an older perpetrator of homicide-suicide. However, the literature has repeatedly identified several common characteristics of such perpetrators. They are significantly more likely to be men who use firearms to murder their intimate partners and then die by suicide in their home (Table3,5,11-17). Health issues afflicting 1 or both individuals in the couple appear to be a contributing factor, particularly when the perpetrator is in a caregiving role. Relational discord, with or without a history of domestic violence, increases the risk of homicide-suicide. Finally, older perpetrators are highly likely to be depressed and have suicidal ideations.
How COVID-19 affects these risks
Although it is too early to determine if there is a causal relationship between the COVID-19 pandemic and an increase in homicide-suicide, the pandemic is likely to promote risk factors for these events, especially among older adults. Confinement measures put into place during the pandemic context have already been shown to increase rates of domestic violence18 and depression and anxiety among older individuals.7 Furthermore,
Continue to: Late-life psychiatric disorders
Late-life psychiatric disorders
Early recognition and effective treatment of late-life psychiatric disorders is crucial. Unfortunately, depression in geriatric patients is often underdiagnosed and undertreated.20 Older adults have more frequent contact with their primary care physicians, and rarely consult mental health professionals.21,22 Several models of integrated depression care within primary care settings have shown the positive impact of this collaborative approach in treating late-life depression and preventing suicide in older individuals.23 Additionally, because alcohol abuse is also a risk factor for domestic violence and breaking the law in this population,24,25 older adults should be screened for alcohol use disorders, and referred to treatment when necessary.
Take steps to keep patients safe
In the context of the COVID-19 pandemic, there are several steps clinicians need to keep in mind when interacting with older patients:
- Screen for depressive symptoms, suicidality, and alcohol and substance use disorders. Individuals who have tested positive for COVID-19 or who have been in contact with a carrier are a particularly vulnerable population.
- Screen for domestic violence and access to weapons at home.4 Any older adult who has a psychiatric disorder and/or suicide ideation should receive immediate intervention through a social worker that includes providing gun-risk education to other family members or contacting law-enforcement officials.26
- Refer patients with a suspected psychiatric disorder to specialized mental health clinicians. Telemental health services can provide rapid access to subspecialists, allowing patients to be treated from their homes.27 These services need to be promoted among older adults during this critical period and reimbursed by public and private insurance systems to ensure accessibility and affordability.28
- Create psychiatric inpatient units specifically designed for suicidal and/or homicidal patients with COVID-19.
Additionally, informing the public about these major health issues is crucial. The media can raise awareness about domestic violence and depression among older adults; however, this should be done responsibly and with accuracy to prevent the spread of misinformation, confusion, fear, and panic.29
Bottom Line
The mental health burden of the coronavirus disease 2019 pandemic has significantly impacted individuals who are older and most vulnerable. Reducing the incidence of homicide-suicide among older adults requires timely screening and interventions by primary care providers, mental health specialists, social workers, media, and governmental agencies.
Related Resources
- Saeed SA, Hebishi K. The psychiatric consequences of COVID-19: 8 studies. Current Psychiatry. 2020;19(11):22-24,28-30,32-35.
- Schwab-Reese LM, Murfree L, Coppola EC, et al. Homicidesuicide across the lifespan: a mixed methods examination of factors contributing to older adult perpetration. Aging Ment Health. 2020;20:1-9.
1. Christodoulou H. LOCKDOWN ‘MURDER-SUICIDE’ OAP, 71, ‘stabbed wife to death then killed himself as he worried about coping with coronavirus lockdown.’ The Sun. Updated April 4, 2020. Accessed December 22, 2020. https://www.thesun.co.uk/news/11327095/coronavirus-lockdown-murder-suicide-cambridge/
2. Farberov S. Illinois man, 54, shoots dead his wife then kills himself in murder-suicide because he feared they had coronavirus - but tests later show the couple were NOT ill. Updated April 6, 2020. Accessed December 22, 2020. https://www.dailymail.co.uk/news/article-8191933/Man-kills-wife-feared-coronavirus.html
3. Salari S. Patterns of intimate partner homicide suicide in later life: strategies for prevention. Clin Interv Aging. 2007;2(3):441-452.
4. Kotzé C, Roos JL. Homicide–suicide: practical implications for risk reduction and support services at primary care level. South African Family Practice. 2019;61(4):165-169.
5. Malphurs JE, Cohen D. A newspaper surveillance study of homicide-suicide in the United States. Am J Forensic Med Pathol. 2002;23(2):142-148.
6. Eliason S. Murder-suicide: a review of the recent literature. J Am Acad Psychiatry Law. 2009;37(3):371-376.
7. Armitage R, Nellums LB. COVID-19 and the consequences of isolating the elderly. Lancet Public Health. 2020;5(5):e256. doi: 10.1016/S2468-2667(20)30061-X
8. Gunnell D, Appleby L, Arensman E, et al. Suicide risk and prevention during the COVID-19 pandemic. Lancet Psychiatry 2020;7(6):468-471.
9. Gosangi B, Park H, Thomas R, et al. Exacerbation of physical intimate partner violence during COVID-19 pandemic. Radiology. 2021;298(1):E38-E45.
10. Mannix R, Lee LK, Fleegler EW. Coronavirus disease 2019 (COVID-19) and firearms in the United States: will an epidemic of suicide follow? Ann Intern Med. 2020;173(3):228-229.
11. Bourget D, Gagne P, Whitehurst L. Domestic homicide and homicide-suicide: the older offender. J Am Acad Psychiatry Law. 2010;38(3):305-311.
12. Cheung G, Hatters Friedman S, Sundram F. Late-life homicide-suicide: a national case series in New Zealand. Psychogeriatrics. 2016;16(1):76-81.
13. Cohen D, Llorente M, Eisdorfer C. Homicide-suicide in older persons. Am J Psychiatry. 1998;155(3):390-396.
14. Malphurs JE, Eisdorfer C, Cohen D. A comparison of antecedents of homicide-suicide and suicide in older married men. Am J Geriatr Psychiatry. 2001;9(1):49-57.
15. De Koning E, Piette MHA. A retrospective study of murder–suicide at the Forensic Institute of Ghent University, Belgium: 1935–2010. Med Sci Law. 2014;54(2):88-98.
16. Logan JE, Ertl A, Bossarte R. Correlates of intimate partner homicide among male suicide decedents with known intimate partner problems. Suicide Life Threat Behav. 2019;49(6):1693-1706.
17. Malphurs JE, Cohen D. A statewide case-control study of spousal homicide-suicide in older persons. Am J Geriatr Psychiatry. 2005;13(3):211-217.
18. Sanford A. ‘Horrifying surge in domestic violence’ against women amid coronavirus-lockdowns, UN chief warns. Euronews. Published June 4, 2020. Accessed December 22, 2020. https://www.euronews.com/2020/04/06/horrifying-surge-in-domestic-violence-against-women-amid-coronavirus-lockdowns-un-chief-w
19. Appel JM. Intimate partner homicide in elderly populations. In: Friedman SH, ed. Family murder: pathologies of love and hate. American Psychiatric Association Publishing; 2019:131-142.
20. Hall CA, Reynolds-III CF. Late-life depression in the primary care setting: challenges, collaborative care, and prevention. Maturitas. 2014;79(2):147-152.
21. Unützer J. Diagnosis and treatment of older adults with depression in primary care. Biological Psychiatry. 2002;52(3):285-292.
22. Byers AL, Arean PA, Yaffe K. Low use of mental health services among older Americans with mood and anxiety disorders. Psychiatr Serv. 2012;63(1):66-72.
23. Bruce ML, Sirey JA. Integrated care for depression in older primary care patients. Can J Psychiatry. 2018;63(7):439-446.
24. Rao R, Roche A. Substance misuse in older people. BMJ. 2017;358:j3885. doi: 10.1136/bmj.j3885
25. Ghossoub E, Khoury R. Prevalence and correlates of criminal behavior among the non-institutionalized elderly: results from the National Survey on Drug Use and Health. J Geriatr Psychiatry Neurol. 2018;31(4):211-222.
26. Slater MAG. Older adults at risk for suicide. In: Berkman B. Handbook of social work in health and aging. Oxford University Press; 2006:149-161.
27. Hollander JE, Carr BG. Virtually perfect? Telemedicine for Covid-19. N Engl J Med. 2020;382(18):1679-1681.
28. Centers for Medicare & Medicaid Services. President Trump expands telehealth benefits for Medicare beneficiaries during COVID-19 outbreak. Published March 17, 2020. Accessed December 23, 2020. https://www.cms.gov/newsroom/press-releases/president-trump-expands-telehealth-benefits-medicare-beneficiaries-during-covid-19-outbreak
29. Mian A, Khan S. Coronavirus: the spread of misinformation. BMC Med. 2020;18(1):89.
1. Christodoulou H. LOCKDOWN ‘MURDER-SUICIDE’ OAP, 71, ‘stabbed wife to death then killed himself as he worried about coping with coronavirus lockdown.’ The Sun. Updated April 4, 2020. Accessed December 22, 2020. https://www.thesun.co.uk/news/11327095/coronavirus-lockdown-murder-suicide-cambridge/
2. Farberov S. Illinois man, 54, shoots dead his wife then kills himself in murder-suicide because he feared they had coronavirus - but tests later show the couple were NOT ill. Updated April 6, 2020. Accessed December 22, 2020. https://www.dailymail.co.uk/news/article-8191933/Man-kills-wife-feared-coronavirus.html
3. Salari S. Patterns of intimate partner homicide suicide in later life: strategies for prevention. Clin Interv Aging. 2007;2(3):441-452.
4. Kotzé C, Roos JL. Homicide–suicide: practical implications for risk reduction and support services at primary care level. South African Family Practice. 2019;61(4):165-169.
5. Malphurs JE, Cohen D. A newspaper surveillance study of homicide-suicide in the United States. Am J Forensic Med Pathol. 2002;23(2):142-148.
6. Eliason S. Murder-suicide: a review of the recent literature. J Am Acad Psychiatry Law. 2009;37(3):371-376.
7. Armitage R, Nellums LB. COVID-19 and the consequences of isolating the elderly. Lancet Public Health. 2020;5(5):e256. doi: 10.1016/S2468-2667(20)30061-X
8. Gunnell D, Appleby L, Arensman E, et al. Suicide risk and prevention during the COVID-19 pandemic. Lancet Psychiatry 2020;7(6):468-471.
9. Gosangi B, Park H, Thomas R, et al. Exacerbation of physical intimate partner violence during COVID-19 pandemic. Radiology. 2021;298(1):E38-E45.
10. Mannix R, Lee LK, Fleegler EW. Coronavirus disease 2019 (COVID-19) and firearms in the United States: will an epidemic of suicide follow? Ann Intern Med. 2020;173(3):228-229.
11. Bourget D, Gagne P, Whitehurst L. Domestic homicide and homicide-suicide: the older offender. J Am Acad Psychiatry Law. 2010;38(3):305-311.
12. Cheung G, Hatters Friedman S, Sundram F. Late-life homicide-suicide: a national case series in New Zealand. Psychogeriatrics. 2016;16(1):76-81.
13. Cohen D, Llorente M, Eisdorfer C. Homicide-suicide in older persons. Am J Psychiatry. 1998;155(3):390-396.
14. Malphurs JE, Eisdorfer C, Cohen D. A comparison of antecedents of homicide-suicide and suicide in older married men. Am J Geriatr Psychiatry. 2001;9(1):49-57.
15. De Koning E, Piette MHA. A retrospective study of murder–suicide at the Forensic Institute of Ghent University, Belgium: 1935–2010. Med Sci Law. 2014;54(2):88-98.
16. Logan JE, Ertl A, Bossarte R. Correlates of intimate partner homicide among male suicide decedents with known intimate partner problems. Suicide Life Threat Behav. 2019;49(6):1693-1706.
17. Malphurs JE, Cohen D. A statewide case-control study of spousal homicide-suicide in older persons. Am J Geriatr Psychiatry. 2005;13(3):211-217.
18. Sanford A. ‘Horrifying surge in domestic violence’ against women amid coronavirus-lockdowns, UN chief warns. Euronews. Published June 4, 2020. Accessed December 22, 2020. https://www.euronews.com/2020/04/06/horrifying-surge-in-domestic-violence-against-women-amid-coronavirus-lockdowns-un-chief-w
19. Appel JM. Intimate partner homicide in elderly populations. In: Friedman SH, ed. Family murder: pathologies of love and hate. American Psychiatric Association Publishing; 2019:131-142.
20. Hall CA, Reynolds-III CF. Late-life depression in the primary care setting: challenges, collaborative care, and prevention. Maturitas. 2014;79(2):147-152.
21. Unützer J. Diagnosis and treatment of older adults with depression in primary care. Biological Psychiatry. 2002;52(3):285-292.
22. Byers AL, Arean PA, Yaffe K. Low use of mental health services among older Americans with mood and anxiety disorders. Psychiatr Serv. 2012;63(1):66-72.
23. Bruce ML, Sirey JA. Integrated care for depression in older primary care patients. Can J Psychiatry. 2018;63(7):439-446.
24. Rao R, Roche A. Substance misuse in older people. BMJ. 2017;358:j3885. doi: 10.1136/bmj.j3885
25. Ghossoub E, Khoury R. Prevalence and correlates of criminal behavior among the non-institutionalized elderly: results from the National Survey on Drug Use and Health. J Geriatr Psychiatry Neurol. 2018;31(4):211-222.
26. Slater MAG. Older adults at risk for suicide. In: Berkman B. Handbook of social work in health and aging. Oxford University Press; 2006:149-161.
27. Hollander JE, Carr BG. Virtually perfect? Telemedicine for Covid-19. N Engl J Med. 2020;382(18):1679-1681.
28. Centers for Medicare & Medicaid Services. President Trump expands telehealth benefits for Medicare beneficiaries during COVID-19 outbreak. Published March 17, 2020. Accessed December 23, 2020. https://www.cms.gov/newsroom/press-releases/president-trump-expands-telehealth-benefits-medicare-beneficiaries-during-covid-19-outbreak
29. Mian A, Khan S. Coronavirus: the spread of misinformation. BMC Med. 2020;18(1):89.
Anticonvulsants for alcohol withdrawal: A review of the evidence
Abrupt cessation or reduction of alcohol consumption may result in alcohol withdrawal syndrome (AWS), which is a medical emergency that can lead to serious complications when unrecognized or treatment is delayed. Symptoms of AWS include tremors, anxiety attacks, cognitive impairment, hallucinations, seizures, delirium tremens (DT), and in severe, untreated cases, death.1 Low to moderate alcohol consumption produces euphoria and excitation via activation of glutamatergic neurotransmission, while higher concentrations produce severe intoxication via GABAergic mechanisms. Acute withdrawal unmasks the hyper-excitatory state of the brain, causing anxiety, agitation, and autonomic activation characteristic of AWS, which typically begins 1 to 3 days after the last drink.2 In the 2012-2013 National Epidemiologic Survey on Alcohol and Related Conditions conducted by the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the 12-month and lifetime prevalences of AWS were 13.9% and 29.1%, respectively.3 Within the general inpatient population, AWS can be present in nearly 30% of patients; if left untreated, AWS has a 15% mortality rate, although when AWS is recognized early and treated, the mortality rate falls dramatically to 2%.4
AWS has most commonly been treated with benzodiazepines.5 However, benzodiazepines have the potential for significant adverse effects when used in older adults and in individuals with complicated medical issues, such as obstructive lung disease and sleep apnea.6 Anticonvulsants have been increasingly used to treat alcohol withdrawal, and their use is supported by several retrospective and prospective studies. In this article, we review the data from randomized control trials (RCTs) on the use of anticonvulsants for the treatment of AWS to see if we can make any recommendations for the use of anticonvulsants for treating AWS.
Our literature search
We searched 5 databases (PubMed, Cochrane, Medline, PsycInfo, and Embase) using the following terms: “alcohol withdrawal syndrome treatment”, “anticonvulsants”, “anti-epileptic”, “gabapentin”, “carbamazepine”, “sodium valproate”, “oxcarbazepine”, “phenytoin”, “levetiracetam”, and “lamotrigine.” We included only double-blind RCTs published between January 1, 1976 and September 30, 2016 in English-language journals or that had an official English translation. There were no restrictions on patient age or location of treatment (inpatient vs outpatient). All RCTs that compared anticonvulsants or a combination of an anticonvulsant and an active pharmacotherapeutic agent with either placebo or gold standard treatment for AWS were included. Database reviews, systematic reviews, and meta-analyses were excluded.
We identified 662 articles that met these criteria. However, most were duplicates, review articles, systematic reviews, meta-analyses, case reports, or open-label or non-randomized trials. Only 16 articles met our inclusion criteria. In the following sections, we discuss these 16 studies by medication type and in chronological order.
Gabapentin
The characteristics of the gabapentin studies included in this review are summarized in Table 1.7-13
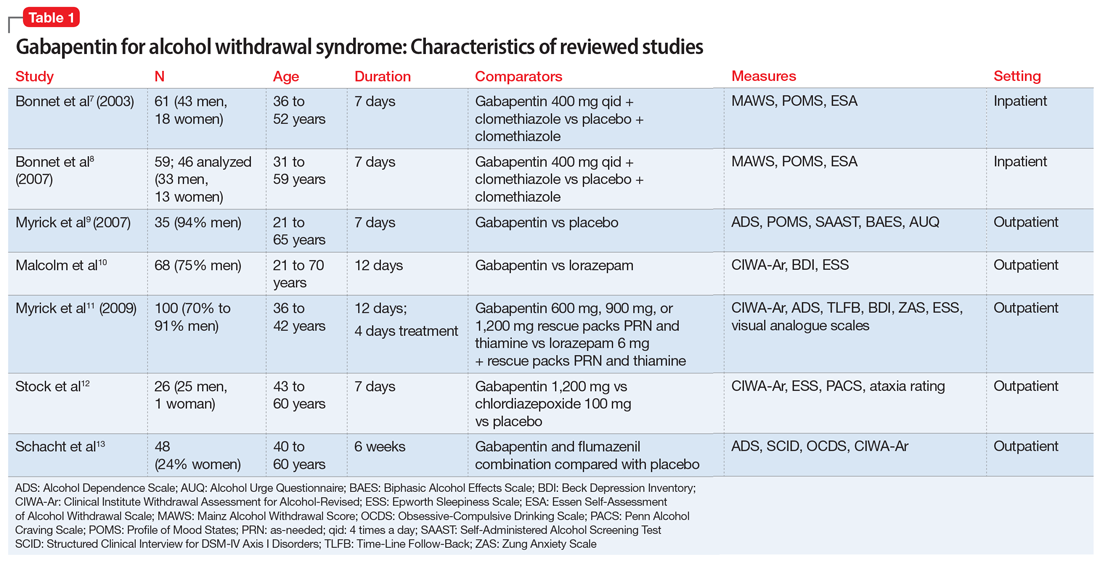
Bonnet et al7 (2003) examined 61 adults who met the clinical criteria for alcohol dependence and displayed moderate or severe AWS according to their Mainz Alcohol Withdrawal Score (MAWS ≥4). They were randomized to receive placebo or gabapentin, 400 mg 4 times a day, along with clomethiazole. The attrition rate was not significantly different between the 2 groups (P = .66). The difference in the number of clomethiazole capsules taken during the first 24 hours between the groups was small and not significant (P = .96). Analysis of MAWS over time revealed no significant main effect for group (P = .26) and a significant effect for the time variable (P < .001). The interaction between group and time was not significant (P =.4). This means that there was a significant decrease in MAWS from baseline over 48 hours, and this decrease in MAWS was considered equal for both study groups. Adverse clinical events were observed in both groups, and there was no significant difference (P = .74) between the groups. Nausea and ataxia, which are specific to gabapentin, were observed more frequently in this group.
Conclusion: The authors concluded that gabapentin, 400 mg 4 times a day, is no better than placebo in reducing the amount of clomethiazole required to treat acute AWS.7
Continue to: Bonnet et al
Bonnet et al8 (2007) also conducted a study examining 59 patients with alcohol dependence who displayed moderate or severe AWS. Participants received placebo or gabapentin, 400 mg, and a rescue medication, clomethiazole, if needed. Subsequently, a capsule of study medication was administered every 6 hours for 2 days and then tapered. During the study, mood was measured by Profile of Mood States (POMS), and subjective complaints of withdrawal were measured using the Essen Self-Assessment of Alcohol Withdrawal Scale (ESA). Of the 59 patients, only 46 were analyzed; 5 patients dropped out, and 8 patients were missing data. Compared with the placebo group, the gabapentin group displayed less dejection, fatigue, and anger, and more vigor. Analysis of variance (ANOVA) measures revealed significant overall changes over time on all 4 scales (all P < .001). A significant (F = 3.62, df 2;43, P = .035) group × time interaction resulted exclusively for vigor. Analysis was repeated using rank-transformed data, resulting in a significant (P = .046) interaction effect. The significant increase in vigor was not apparent after tapering off gabapentin, which suggests gabapentin has a reversible effect on vigor. There was a significant (P < .001) overall decline of subjective withdrawal symptoms complaints, but no group × time interaction (P = .62). Analysis of 11 patients with comorbid mild depression revealed no significant time × group interaction for dejection, fatigue, anger, or subjective withdrawal (all P > .05). However, for vigor, the group × time interaction was significant (P = .022). Throughout the treatment, vigor scores of those mild depressive patients who received gabapentin increased to a level comparable to that of patients without a mood disorder.
Conclusion: The authors authors concluded that gabapentin was markedly more efficacious in improving vigor in the small subgroup of patients with mild depression.8
Myrick et al9 (2007) evaluated the safety and tolerability of gabapentin in patients who abused alcohol, as well as the ability of gabapentin to reduce alcohol craving and consumption. This study included 35 participants randomly assigned to receive gabapentin (n = 17) or placebo (n = 18) for 7 days. All medications were administered in standard gel caps with riboflavin, 25 mg, to assess for compliance via a laboratory-based urinary fluorescence assay. Urine samples were assessed for riboflavin at baseline and Day 6, and a reading of 1,500 ng/mL of riboflavin on Day 6 was interpreted as being compliant. Participants were required to abstain completely from drinking alcohol on Day 6 and the morning of Day 7. At the first session, the following measures were completed: demographic form, alcohol and drug section of the Structured Clinical Interview (SCID), Obsessive-Compulsive Drinking Scale, Self-Administered Alcohol Screening Test (SAAST), and Alcohol Dependence Scale (ADS); there also was collection of a urine sample for detection of abused drugs and a blood sample for liver function and general health screening.
At the second session, patients completed the psychiatric sections of the SCID and the Alcohol Craving Questionnaire, and received a physical exam. To assess the negative clinical effects of gabapentin and alcohol on the CNS, the Epworth Sleepiness Scale (ESS) and POMS were administered at baseline and on Day 6. Also, several other scales were used to identify any impact of gabapentin on acute alcohol effects and craving: the Clinical Institute Withdrawal Assessment of Alcohol, Revised (CIWA-Ar), Biphasic Alcohol Effects Scale (BAES), Subjective High Assessment Scale (SHAS), and Alcohol Urge Questionnaire (AUQ).
Conclusion: Gabapentin was well tolerated, but compared with placebo, gabapentin had no effect on alcohol stimulation (P = .75) or sedation (P = .99) as measured by the BAES. The difference in SHAS scores was also not significant (P = .19). There was also no significant reduction in craving for alcohol as measured on the AUQ scale
Continue to: Malcolm et al
Malcolm et al10 conducted an outpatient treatment study. Patients were men and women age 21 to 70 years from multiple ethnic groups. They were randomized to receive gabapentin or lorazepam; 449 patients were screened and 68 completed the follow-up. Scales used included the CIWA-Ar, Beck Depression Inventory (BDI), and ESS.
Patients receiving lorazepam reported less insomnia and more sleepiness early in treatment than patients receiving gabapentin. However, upon completing treatment and discontinuing medication administration, patients previously treated with lorazepam reported increased insomnia and daytime sleepiness, while patients previously treated with gabapentin continued to report improvements in these self-reported sleep measures. The differences between lorazepam and gabapentin were further evidenced in BDI scores at Day 5, Day 7, and Day 12 in patients who had previously experienced multiple withdrawals. Gabapentin was superior to lorazepam in reducing insomnia as assessed by BDI score, an effect that was sustained throughout the post-treatment week. Participants’ ESS scores indicated less daytime sleepiness in the gabapentin group than in the lorazepam group.
Conclusion: Among patients who abused alcohol and had a history of multiple withdrawals, lorazepam is less effective than gabapentin in reducing insomnia.10 However, this study had several limitations: <25% of individuals who were initially screened were enrolled in the study, and it used subjective tests such as BDI. Objective electrophysiologic measures of sleep and daytime sleepiness would have been very helpful.
Myrick et al11 (2009) also compared gabapentin and lorazepam for treating alcohol withdrawal. One hundred patients were randomized to receive 4 days of fixed-dose taper of gabapentin or lorazepam. Patients could receive 1 of 3 gabapentin dosing regimens (600 mg/d, 900 mg/d, or 1,200 mg/d) for 3 days. Participants who were randomized to receive lorazepam were given 6 mg/d for 3 days and then tapered to 4 mg/d. Also, blinded supplemental medications (rescue packs) were given to each patient on Days 1 to 4 to treat subjective feelings of alcohol withdrawal. All patients also received thiamine for 12 days. Assessment of severity of alcohol withdrawal was measured by the CIWA-Ar. To quantify the severity of alcohol dependence and alcohol use, patients were asked to complete the ADS and Time-Line Follow-Back (TLFB) scales, respectively. Other scales administered included the BDI, Zung Anxiety Scale (ZAS), ESS, and visual analogue scales that assessed craving, ability to perform work, and need for additional medication.
There was a decrease in CIWA-Ar scores over time in all groups. High-dose gabapentin was found to be statistically superior but clinically similar to lorazepam (P = .009). Researchers also found that compared with patients who were treated with lorazepam, patients who were treated with gabapentin experienced reduced craving and anxiety/depressive symptoms, and complained of less subjective discomfort. Compared to patients who were treated with gabapentin, patients who were treated with lorazepam had higher probabilities of drinking on the first day of dose decrease (Day 2) and the second day off medication (Day 6) (P = .0002). During post-treatment, patients who were treated with gabapentin had less probability of drinking during the follow-up post-treatment period (P = .2 for 900 mg/d and P = .3 for 1,200 mg/d) compared with patients who were treated with lorazepam (P = .55).
Continue to: Conclusion
Conclusion: The researchers concluded that gabapentin was well tolerated and effectively diminished the symptoms of alcohol withdrawal, especially at the higher target dose (1,200 mg/d), and that compared with lorazepam, gabapentin decreased the probability of drinking during alcohol withdrawal and in the immediate post-withdrawal week.11
Stock et al12 randomized 26 patients who met criteria for AWS to receive gabapentin or chlordiazepoxide. Gabapentin doses were 1,200 mg/d orally for 3 days, followed by 900 mg/d, 600 mg/d, and 300 mg/d for 1 day each. Chlordiazepoxide doses were 100 mg/d orally for 3 days, followed by 75 mg/d, 50 mg/d, and 25 mg/d for 1 day each. The ESS, Penn Alcohol Craving Scale (PACS), ataxia rating, and CIWA-Ar were administered daily. Thirty-five percent of participants dropped out at the end of the 7-day treatment period. Days 1 to 4 were considered the early treatment period, and Days 5 to 7 were considered the late treatment period. The adjusted mean ESS score did not differ significantly between the randomized groups during the early stage (P = .61) vs the late stage, in which the adjusted mean ESS score was significantly lower with gabapentin compared with chlordiazepoxide (P = .04). The differences in PACS scores between the groups were not statistically significant in either stage (early stage P = .59 vs late stage P = .08), but a trend of lower PACS scores was noted with gabapentin in the later stage. No participant in either group had ataxia during the study. In both groups, CIWA-Ar scores were reduced similarly.
Conclusion: The researchers concluded that gabapentin treatment resulted in a significantly greater reduction in sedation (ESS) and a trend toward reduced alcohol craving (PACS) by the end of treatment compared with chlordiazepoxide treatment.12
Schacht et al13 analyzed functional magnetic resonance imaging data from 48 patients who were alcohol-dependent in a 6-week RCT. Patients were randomized to receive gabapentin up to 1,200 mg/d for 39 days plus flumazenil for 2 days (GP/FMZ group) or an oral placebo and placebo infusions on the same time course. Evaluations included the SCID, ADS, and Obsessive-Compulsive Drinking Scale (OCDS). On Day 1, the CIWA-Ar was administered; it was used to ensure equal distribution of individuals with higher alcohol withdrawal symptoms between medication groups. There were no significant effects of initial alcohol withdrawal symptom status or medication. However, there was a significant interaction between these factors: patients with higher alcohol withdrawal symptoms who received GP/FMZ and those with lower alcohol withdrawal symptoms who received placebo demonstrated greater cue-elicited activation, relative to the other groups, and had less subsequent drinking, which reflected differences in deactivation between alcohol and beverage stimuli, in a cluster that encompassed the dorsal ACC (dACC) (family-wise error-corrected cluster probability of P = .012; 99 voxels; local maxima at [-3, 39, 18] and [6, 33, 9]). In the GP/FMZ group, patients with higher alcohol withdrawal symptoms had significantly greater activation, while in the placebo group, patients with lower alcohol withdrawal symptoms had greater activation.
Conclusion: The researchers concluded that alterations in task-related deactivation of dACC, a component of the default mode network, may predict better alcohol treatment response, while activation of DLPFC, an area associated with selective attention, may predict relapse drinking.13
Continue to: Carbamazepine
Carbamazepine
The characteristics of the carbamazepine studies included in this review are summarized in Table 2.14-19
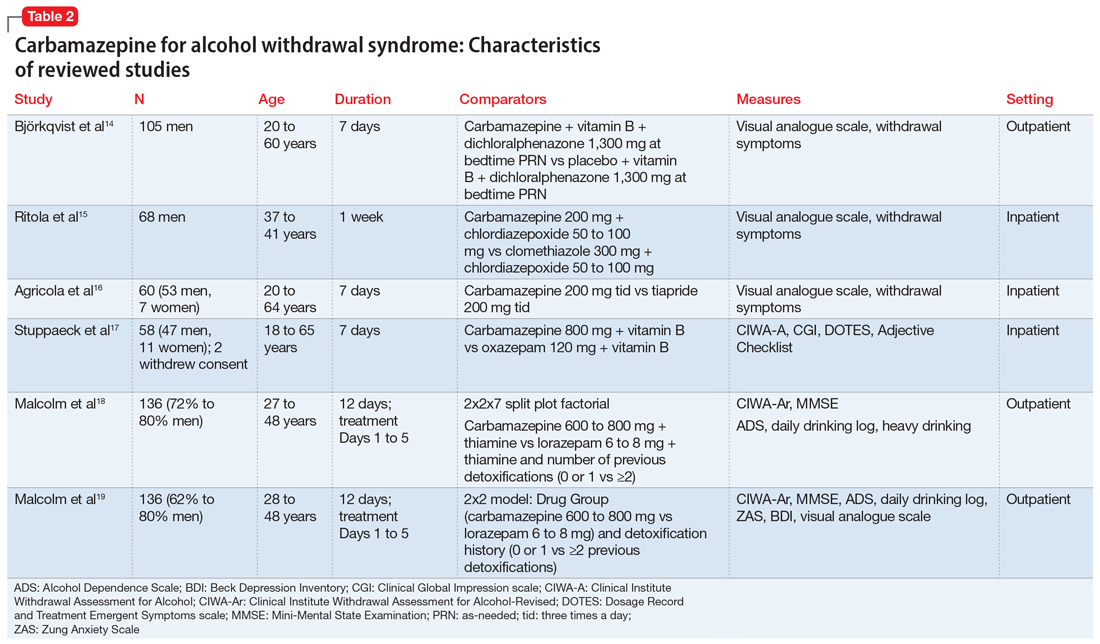
Björkqvist et al14 randomized 105 men with AWS to placebo or carbamazepine. On initial assessment, history, physical examination, relevant labs, and intoxication assessments were recorded. On subsequent visits, nursing staff recorded withdrawal symptoms for patients as 0 to 2 (0 = no specific symptoms, 1 = patient only complained when asked about specific symptoms, 2 = patient complained of withdrawal symptoms without being asked, or if symptoms were severe or obvious to others). Along with the above, vital signs and a visual analogue scale of 0 to 10 (0 = feeling could not be worse, 10 = feeling could not be better) were recorded at each visit. The dose was weight-dependent and administered as follows: on Days 1 and 2, 1+1+2 tablets of carbamazepine, 200 mg, or placebo; Days 3 and 4, 1+1+1 tablets; and Days 5 and 6, 1+0+1 tablets. Every patient received dichloralphenazone as needed. All patients were given vitamin B 3 times a day. Most withdrawal symptoms decreased faster in the carbamazepine group on Day 2 (P = .01) and on Day 4 (P = .1). On the visual analogue scale, scores varied between patients. On Day 1, the mean score was 2.5 times higher in the carbamazepine group compared with the placebo group, and this difference increased to 3 times by Day 7 (P < .01). The patient’s estimated ability to work improved significantly faster in the carbamazepine group than in the placebo group (P < .01).
Conclusion: The authors concluded that compared with placebo, carbamazepine was able to more quickly decrease withdrawal symptoms, especially insomnia and subjective recovery.14
Ritola et al15 randomized 68 hospitalized men with AWS to carbamazepine, 200 mg/d, or clomethiazole, 300 mg/d, for 1 week. The target withdrawal symptoms included gastrointestinal and sleep disturbances; anxiety; aggressiveness; and cardiovascular, depressive, psychotic, and neurologic symptoms. A 4-point rating scale was used for individual symptoms (0 = no symptom, 1 = mild symptom, 2 = moderate symptom, and 3 = severe symptom). On the day of admission (Day 0), all patients were given 50 to 100 mg of chlordiazepoxide IM and 2 tablets and 4 capsules of the trial preparations (either the tablets or capsules were active, and the others were placebos) in the evening. Five patients dropped out of the clomethiazole group and 1 from the carbamazepine group. No significant difference between the 2 treatments were found by the patient, nurse, or physician.
Conclusion: The authors concluded that carbamazepine seemed to be as effective as clomethiazole in the treatment of milder alcohol withdrawal symptoms. Final treatment results were equally good in both groups. Sleep disturbance resolved faster in the carbamazepine group.15
Continue to: Agricola et al
Agricola et al16 compared carbamazepine to tiapride for treatment of acute AWS. In this study, 60 patients were randomized to carbamazepine, 200 mg 3 times a day, or tiapride, 200 mg 3 times a day. All patients were hospitalized with severe AWS preceding DT. The patients were evaluated for withdrawal symptoms (gastrointestinal and cardiovascular symptoms, sleep disturbances, anxiety, aggression, fear, depression, psychotic symptoms, and certain neurologic symptoms). The severity of these symptoms was scored as follows: 0 = no symptoms; 1 = moderate symptoms; and 2 = severe symptoms. At each visit, an overall evaluation of the patient’s clinical condition was made according to a visual analogue scale (100 = worst condition, 0 = best condition). On Day 7, both the doctor and patient evaluated treatment efficacy according to a 4-point scale (1 = no efficacy, 4 = excellent efficacy). There was no significant difference between carbamazepine and tiapride in terms of total symptoms score and visual analogue scale assessment. Carbamazepine was found to have faster relief of symptoms and a significantly greater reduction in symptom score on Day 2 (P < .01). Carbamazepine had a preferential action on fear, nightmares, and hallucinations. The proportion of patients in whom anxiety improved after treatment was 96.2% for carbamazepine and 71.4% for tiapride (P < .05). Aggressiveness and gastrointestinal discomfort resolved faster in the tiapride group. No cases of DT were observed.
Conclusion: The researchers concluded that either carbamazepine or tiapride provides an appropriate alternative in the treatment of inpatients with severe AWS.16
Stuppaeck et al17 compared the efficacy of carbamazepine to oxazepam in 60 inpatients who had symptoms of alcohol withdrawal. Alcohol withdrawal was measured with the CIWA-A, and patients with scores >20 were enrolled in the study. The Clinical Global Impression (CGI) scale and self-rated Adjective Checklist (ACL) were also used. On Days 1 to 3, patients received oxazepam, 120 mg/d, or carbamazepine, 800 mg/d. From Day 4 to 7, doses were decreased to 90 mg/d and 600 mg/d, respectively. After the 7-day trial, all patients were treated with carbamazepine, 200 mg twice a day on Day 8 and 200 mg at night on Day 9. Two patients withdrew consent and 6 dropped out due to adverse effects. During the 7-day trial, when comparing all improvements on CIWA-A, ACL, and CGI scales, carbamazepine was equivalent to oxazepam up to Day 5, and then superior on Days 6 and 7 (P ≤ .05). No decrease in white blood cell count was found in the carbamazepine group.
Conclusion: The authors concluded that carbamazepine is as effective as oxazepam and may be a viable alternative that does not interact with alcohol or cause delirium.17
Malcolm et al18 compared the effects of carbamazepine and lorazepam in patients in an outpatient setting who had single vs multiple previous alcohol withdrawals. The study included 136 patients who satisfied DSM-IV criteria for alcohol dependence and alcohol withdrawal, with a blood alcohol level ≤0.1 g/dL, a Mini-Mental State Examination (MMSE) score ≤26, and a CIWA-Ar score ≤10 on admission. Patients also completed the ADS to quantify the severity of alcohol dependence. Daily drinking was measured by patient report using a daily drinking log and blood alcohol level. Heavy drinking was defined as ≥4 standard drinks per day for women and ≥5 drinks per day for men. On Day 1, patients were randomized to receive carbamazepine, 600 to 800 mg/d,or lorazepam, 6 to 8 mg/d, in divided doses, which was tapered to carbamazepine, 200 mg/d, or lorazepam, 2 mg/d, on Day 5. All patients received thiamine for 12 days. In the immediate post-detoxification period, carbamazepine-treated patients were less likely to relapse, and if they did drink, they drank less than those treated with lorazepam (P = .003). Even in patients who had multiple previous detoxifications, those randomized to carbamazepine drank less than those in lorazepam group (P = .004). Patients in the lorazepam group had significant higher rebound withdrawal symptoms (P = .007).
Continue to: Conclusion
Conclusion: The researchers concluded that carbamazepine and lorazepam were both effective in reducing alcohol withdrawal symptoms. They also concluded that carbamazepine was less likely to cause rebound withdrawal and more likely to reduce post-treatment drinking; among those who did drink, there was less heavy drinking.18
Malcolm et al19 conducted a 5-day double-blind RCT with 136 outpatients who met DSM-IV criteria for alcohol withdrawal. Patients were evaluated by CIWA before getting medications and then daily for 5 days. Patients were randomized to receive carbamazepine, 600 to 800 mg/d on Day 1, 200 mg 3 times a day on Day 2, 200 mg twice a day on Days 3 and 4, and 200 mg once on Day 5. Participants were randomized to receive lorazepam, 6 to 8 mg/d in divided doses on Day 1, 2 mg 3 times a day on Day 2, 2 mg twice a day on Days 3 and 4, and 2 mg once on Day 5. Ability to return to work was self-rated on a 100-mm visual analogue scale, with 0 being “totally unable to return to work’’ and 100 representing “being fully able to return to work.’’ Self-report measures of sleep quality were made using a 100-mm visual analogue scale, with 0 = “the very worst night’s sleep I’ve ever had’’ and 100 = “the very best night’s sleep I’ve ever had.’’ Carbamazepine significantly reduced anxiety (P = .0007). Visual analogue measures of sleep quality indicated a statistically significant main effect of medication on sleep that favored carbamazepine (P = .0186).
Conclusion: The authors concluded that when treating patients with mild to moderate alcohol withdrawal symptoms, carbamazepine was superior to lorazepam in reducing anxiety and improving sleep.19
Sodium valproate
The characteristics of the sodium valproate studies included in this review are summarized in Table 3.20,21
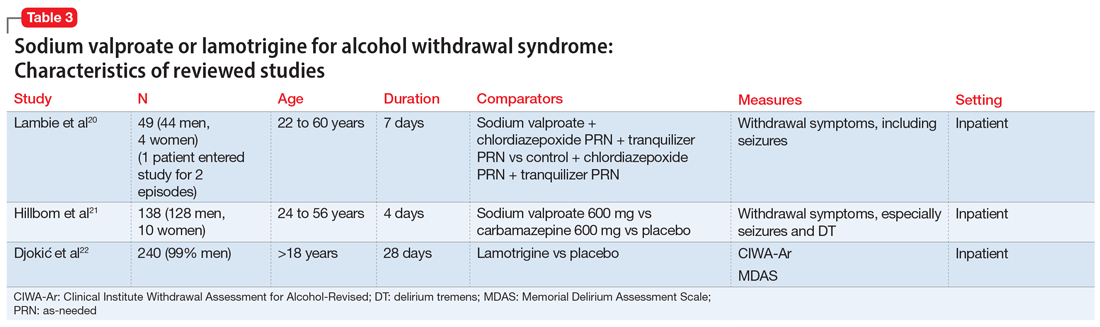
Lambie et al20 evaluated the use of sodium valproate in the treatment of AWS. A total of 49 patients were randomized to a sodium valproate group (n = 22) or a control group (n = 27). All participants were inpatients receiving treatment for alcohol use disorder and substance use disorder for 7 days. Patients in the sodium valproate group received 800 mg every 8 hours for 7 days. Patients were observed daily for occurrence of withdrawal symptoms. Nurses who were blinded to the group assignment graded the degree and severity of symptoms. The trial was initially designed so that chlormethiazole and/or tranquilizers were added to sodium valproate when withdrawal symptoms occurred. However, after treating the first few patients, it became evident that additional medications were not needed. In the treatment group, 13 participants received only sodium valproate, 4 patients needed a tranquilizer, 4 needed chlormethiazole, and 1 needed both. In the control group, 1 received only sodium valproate, 4 received a tranquilizer, 14 received chlormethiazole, and 8 needed both. One patient, who entered the study twice, had a withdrawal seizure when in control group and no seizure on second admission in the sodium valproate group. Physical symptoms disappeared quickly in the sodium valproate group (mean of 2 days vs 2.6 days in the control group). Fourteen patients in the control group received chlormethiazole, compared with only 4 patients in sodium valproate group.
Continue to: Conclusion
Conclusion: The researchers concluded that physical symptoms disappeared quicker in the sodium valproate group than in the control group.20
Hillbom et al21 evaluated the efficacy of sodium valproate vs carbamazepine vs placebo to prevent alcohol withdrawal seizures. A total of 138 participants were studied. Forty-three were assigned to the carbamazepine group, 46 to the sodium valproate group, and 49 to the placebo group. The RCT lasted 4 days. The initial medication doses were 1,200 mg/d. Participants in the carbamazepine group experienced more adverse effects than those in the sodium valproate or placebo groups (P < .001). As a result, approximately one-half of the participants in the carbamazepine group stopped taking the medication. This finding was dependent on the dose of carbamazepine; >800 mg/d resulted in poor tolerance to adverse effects. Seizures occurred among patients in all 3 arms of the study; in the sodium valproate group, 1 participant had a seizure vs 2 participants in the carbamazepine group and 3 in the placebo group. On the other hand, DT occurred only in the sodium valproate and placebo groups.
Conclusion: Researchers concluded that when using sodium valproate or carbamazepine to prevent alcohol withdrawal seizures in an outpatient setting, the adverse effects may outweigh the benefits.21
Lamotrigine
The characteristics of the lamotrigine study included in this review are summarized in Table 3.22
Djokić et al22 evaluated the efficiency of lamotrigine in the treatment of DT. A total of 240 participants who met International Classification of Diseases-10 criteria for DT were randomized to a control group that was treated with anticonvulsants according to an NIAAA protocol (2004), or to an experimental group that was treated with lamotrigine. The CIWA-Ar and the Memorial Delirium Assessment Scale (MDAS) were administered for objective assessment of clinical symptoms, superimposed medical complications, general condition of the patient, adverse effects, and mortality rate. Statistically significant differences between the experimental and control groups were apparent after the third day of therapy, when a drop in the average CIWA-Ar score was observed in the experimental group, while the control group still had high scores (P < .01). After the fifth day of treatment, the differences in scores were more apparent, with the experimental group showing CIWA-Ar scores equal to those of persons with mild/moderate DT, while those in the control group still had high scores. After the tenth day, participants in the experimental group did not have any alcohol withdrawal symptoms, while control group participants were just beginning to get out of life-threatening danger. Death occurred in 4.1% of control group participants and 3.4% of experimental group participants; this difference in mortality rate was not statistically significant.
Continue to: Conclusion
Conclusion: Researchers concluded that lamotrigine is significantly efficacious in the treatment of DT, but does not decrease the mortality rate.22
What to know before you prescribe
AWS is a medical emergency that if left untreated leads to several complications and possibly death. Although benzodiazepines are considered the gold standard for treating AWS, the adverse effects associated with their use advocates for finding alternatives. Anticonvulsants can be an effective alternative for treating AWS. In our literature review, we found 16 double-blind RCTs that used an anticonvulsant medication for the treatment of AWS. Of these, 7 involved gabapentin, 6 involved carbamazepine, 1 involved sodium valproate, 1 involved sodium valproate vs carbamazepine, and 1 involved lamotrigine. Overall, the use of anticonvulsants resulted in significant improvement of mild to moderate symptoms of AWS.
There were more studies of carbamazepine and gabapentin than of other anticonvulsants. All the anticonvulsants offered potential benefits. They decreased the probability of a withdrawal seizure and other complications and effectively reduced alcohol cravings. Anticonvulsants were useful for preventing rebound withdrawal symptoms and reducing post-treatment alcohol consumption, especially in patients who had multiple previous withdrawals. Anticonvulsants were particularly helpful for patients with mood disorders such as depression. In the studies we reviewed, anticonvulsants caused less sedation compared with benzodiazepines, and also decreased the occurrence of relapse.
Dosing recommendations. In the studies included in our review, gabapentin was effective at a dosage of 1,600 mg/d (given as 400 mg 4 times a day). This was tapered as follows: 400 mg 4 times a day on Days 1 to 3, 400 mg 3 times a day on Day 4, 400 mg twice a day on Day 5, and 400 mg once a day on Day 6. Carbamazepine was effective at 600 to 800 mg/d, and was tapered by decreasing by 200 mg as follows: 800 mg/d on Days 1 to 3, 600 mg/d on Day 4, 400 mg on Day 5, and 200 mg/d on Day 6. In the reviewed study, the maximum dose of lamotrigine never exceeded 200 mg/d and was administered for 28 days; the exact dosing and taper plan were not described. The dosing of sodium valproate ranged from 1,200 mg/d to 1600 mg/d for 7 days, followed by decreasing by 200 mg each day. The recommended duration of treatment varied; on average for all anticonvulsants, it was 7 to 12 days, followed by a taper. Carbamazepine was shown to be superior to oxazepam in ameliorating the symptoms of AWS.
Adverse effects. When considering the tolerability, adverse effect profile, duration of action, and effectiveness of the anticonvulsants included in our review, gabapentin appears to be the safest agent to choose. For the other anticonvulsants, the risks might outweigh the benefits. Specifically, in a comparison of sodium valproate and carbamazepine, Hillbom et al21 concluded that in doses >800 mg/d, carbamazepine has potential to cause more adverse effects than benefits. However, Agricola et al16 found that carbamazepine had a preferential action on fear, nightmares, and hallucinations.
Continue to: A few caveats
A few caveats
Our review focused a large collection of data from multiple databases and RCTs only. However, its limitations include:
- there was no measure of heterogeneity
- the studies had short treatment duration
- most studies evaluated predominantly male participants
- some studies were underpowered.
Our review laid a groundwork for future research that includes more well-designed RCTs and/or meta-analyses of recent studies that evaluated the use anticonvulsants for treating AWS.
Bottom Line
Evidence suggests certain anticonvulsants may be an effective alternative to benzodiazepines for the treatment of mild to moderate alcohol withdrawal syndrome. Gabapentin may be the safest anticonvulsant to prescribe. Other anticonvulsants to consider include carbamazepine, sodium valproate, and lamotrigine, but for these agents, the risks might outweigh the benefits.
Related Resources
- Myrick H, Anton RF. Treatment of alcohol withdrawal. Alcohol Health Res World. 1998;22(1):38-43. https://pubs.niaaa.nih.gov/publications/arh22-1/38-43.pdf
- World Health Organization. Management of alcohol withdrawal. Published 2012. https://www.who.int/mental_health/mhgap/evidence/alcohol/q2/en/
Drug Brand Names
Carbamazepine • Tegretol
Gabapentin • Neurontin
Lamotrigine • Lamictal
Levetiracetam • Keppra
Lorazepam • Ativan
Oxcarbazepine • Trileptal
Phenytoin • Dilantin
Sodium valproate • Depakote
Acknowledgments
The authors thank Geetha Manikkara, MD, Madhuri Jakkam Setty, MD, and Elizabeth DeOreo, MD, for their efforts with the systematic review research.
1. Trevisan LA, Boutros N, Petrakis IL, et al. Complications of alcohol withdrawal: pathophysiological insights. Alcohol Health Res World. 1998;22(1):61-66.
2. Borghesani P. Alcohol withdrawal. In: Nordstrom KD, Wilson MP, eds. Quick guide to psychiatric emergencies. Springer: 2018;209-215.
3. Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72(8):757-766.
4. Ungur LA, Neuner B, John S, et al. Prevention and therapy of alcohol withdrawal on intensive care units: systematic review of controlled trials. Alcohol Clin Exp Res. 2013;37(4):675-686.
5. Sachdeva A, Choudhary M, Chandra M. Alcohol withdrawal syndrome: benzodiazepines and beyond. J Clin Diagn Res. 2015;9(9):VE01-VE07.
6. Ashton H. Toxicity and adverse consequences of benzodiazepine use. Psychiatr Ann. 1995;25:158-165.
7. Bonnet U, Banger M, Leweke FM, et al. Treatment of acute alcohol withdrawal with gabapentin: results from a controlled two-center trial. J Clin Psychopharmacol. 2003;23(5):514-519.
8. Bonnet U, Specka M, Leweke FM, et al. Gabapentin’s acute effect on mood profile--a controlled study on patients with alcohol withdrawal. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(2):434-438.
9. Myrick H, Anton R, Voronin K, et al. A double-blind evaluation of gabapentin on alcohol effects and drinking in a clinical laboratory paradigm. Alcohol Clin Exp Res. 2007;31(2):221-227.
10. Malcolm R, Myrick L, Veatch L, et al. Self-reported sleep, sleepiness, and repeated alcohol withdrawals: a randomized, double blind, controlled comparison of lorazepam vs gabapentin. J Clin Sleep Med. 2007;3(1):24-32.
11. Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33(9):1582-1588.
12. Stock CJ, Carpenter L, Ying J, et al. Gabapentin versus chlordiazepoxide for outpatient alcohol detoxification treatment. Ann Pharmacother. 2013;47(7-8):961-969.
13. Schacht JP, Anton RF, Randall PK, et al. Effects of a GABA-ergic medication combination and initial alcohol withdrawal severity on cue-elicited brain activation among treatment-seeking alcoholics. Psychopharmacol. 2013;227(4):627-637.
14. Björkqvist SE, Isohanni M, Mäkelä R, et al. Ambulant treatment of alcohol withdrawal symptoms with carbamazepine: a formal multicenter double blind comparison with placebo. Acta Psychiatr Scand. 1976;53(5):333-342.
15. Ritola E, Malinen L. A double-blind comparison of carbamazepine and clomethiazole in the treatment of alcohol withdrawal syndrome. Acta Psychiatr Scand. 1981;64(3):254-259.
16. Agricola R, Mazzarino M, Urani R, et al. Treatment of acute alcohol withdrawal syndrome with carbamazepine: a double-blind comparison with tiapride. J Int Med Res. 1982;10(3):160-165.
17. Stuppaeck CH, Pycha R, Miller C, et al. Carbamazepine versus oxazepam in the treatment of alcohol withdrawal: a double-blind study. Alcohol Alcohol. 1992;27(2):153-158.
18. Malcolm R, Myrick H, Roberts J, et al. The effects of carbamazepine and lorazepam on single vs multiple previous withdrawals in an outpatient randomized trial. J Gen Intern Med. 2002;17(5):349-355.
19. Malcolm R, Myrick H, Roberts J, et al. The differential effects of medications on mood, sleep disturbance, and work ability in outpatient alcohol detoxification. Am J Addict. 2002;11(2):141-150.
20. Lambie D, Johnson R, Vijayasenan M, et al. Sodium valproate in the treatment of the alcohol withdrawal syndrome. Aust N Z J. 1980;14(3):213-215.
21. Hillbom M, Tokola R, Kuusela V, et al. Prevention of alcohol withdrawal seizures with carbamazepine and valproic acid. Alcohol. 1989;6(3):223-226.
22. Djokic
Abrupt cessation or reduction of alcohol consumption may result in alcohol withdrawal syndrome (AWS), which is a medical emergency that can lead to serious complications when unrecognized or treatment is delayed. Symptoms of AWS include tremors, anxiety attacks, cognitive impairment, hallucinations, seizures, delirium tremens (DT), and in severe, untreated cases, death.1 Low to moderate alcohol consumption produces euphoria and excitation via activation of glutamatergic neurotransmission, while higher concentrations produce severe intoxication via GABAergic mechanisms. Acute withdrawal unmasks the hyper-excitatory state of the brain, causing anxiety, agitation, and autonomic activation characteristic of AWS, which typically begins 1 to 3 days after the last drink.2 In the 2012-2013 National Epidemiologic Survey on Alcohol and Related Conditions conducted by the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the 12-month and lifetime prevalences of AWS were 13.9% and 29.1%, respectively.3 Within the general inpatient population, AWS can be present in nearly 30% of patients; if left untreated, AWS has a 15% mortality rate, although when AWS is recognized early and treated, the mortality rate falls dramatically to 2%.4
AWS has most commonly been treated with benzodiazepines.5 However, benzodiazepines have the potential for significant adverse effects when used in older adults and in individuals with complicated medical issues, such as obstructive lung disease and sleep apnea.6 Anticonvulsants have been increasingly used to treat alcohol withdrawal, and their use is supported by several retrospective and prospective studies. In this article, we review the data from randomized control trials (RCTs) on the use of anticonvulsants for the treatment of AWS to see if we can make any recommendations for the use of anticonvulsants for treating AWS.
Our literature search
We searched 5 databases (PubMed, Cochrane, Medline, PsycInfo, and Embase) using the following terms: “alcohol withdrawal syndrome treatment”, “anticonvulsants”, “anti-epileptic”, “gabapentin”, “carbamazepine”, “sodium valproate”, “oxcarbazepine”, “phenytoin”, “levetiracetam”, and “lamotrigine.” We included only double-blind RCTs published between January 1, 1976 and September 30, 2016 in English-language journals or that had an official English translation. There were no restrictions on patient age or location of treatment (inpatient vs outpatient). All RCTs that compared anticonvulsants or a combination of an anticonvulsant and an active pharmacotherapeutic agent with either placebo or gold standard treatment for AWS were included. Database reviews, systematic reviews, and meta-analyses were excluded.
We identified 662 articles that met these criteria. However, most were duplicates, review articles, systematic reviews, meta-analyses, case reports, or open-label or non-randomized trials. Only 16 articles met our inclusion criteria. In the following sections, we discuss these 16 studies by medication type and in chronological order.
Gabapentin
The characteristics of the gabapentin studies included in this review are summarized in Table 1.7-13

Bonnet et al7 (2003) examined 61 adults who met the clinical criteria for alcohol dependence and displayed moderate or severe AWS according to their Mainz Alcohol Withdrawal Score (MAWS ≥4). They were randomized to receive placebo or gabapentin, 400 mg 4 times a day, along with clomethiazole. The attrition rate was not significantly different between the 2 groups (P = .66). The difference in the number of clomethiazole capsules taken during the first 24 hours between the groups was small and not significant (P = .96). Analysis of MAWS over time revealed no significant main effect for group (P = .26) and a significant effect for the time variable (P < .001). The interaction between group and time was not significant (P =.4). This means that there was a significant decrease in MAWS from baseline over 48 hours, and this decrease in MAWS was considered equal for both study groups. Adverse clinical events were observed in both groups, and there was no significant difference (P = .74) between the groups. Nausea and ataxia, which are specific to gabapentin, were observed more frequently in this group.
Conclusion: The authors concluded that gabapentin, 400 mg 4 times a day, is no better than placebo in reducing the amount of clomethiazole required to treat acute AWS.7
Continue to: Bonnet et al
Bonnet et al8 (2007) also conducted a study examining 59 patients with alcohol dependence who displayed moderate or severe AWS. Participants received placebo or gabapentin, 400 mg, and a rescue medication, clomethiazole, if needed. Subsequently, a capsule of study medication was administered every 6 hours for 2 days and then tapered. During the study, mood was measured by Profile of Mood States (POMS), and subjective complaints of withdrawal were measured using the Essen Self-Assessment of Alcohol Withdrawal Scale (ESA). Of the 59 patients, only 46 were analyzed; 5 patients dropped out, and 8 patients were missing data. Compared with the placebo group, the gabapentin group displayed less dejection, fatigue, and anger, and more vigor. Analysis of variance (ANOVA) measures revealed significant overall changes over time on all 4 scales (all P < .001). A significant (F = 3.62, df 2;43, P = .035) group × time interaction resulted exclusively for vigor. Analysis was repeated using rank-transformed data, resulting in a significant (P = .046) interaction effect. The significant increase in vigor was not apparent after tapering off gabapentin, which suggests gabapentin has a reversible effect on vigor. There was a significant (P < .001) overall decline of subjective withdrawal symptoms complaints, but no group × time interaction (P = .62). Analysis of 11 patients with comorbid mild depression revealed no significant time × group interaction for dejection, fatigue, anger, or subjective withdrawal (all P > .05). However, for vigor, the group × time interaction was significant (P = .022). Throughout the treatment, vigor scores of those mild depressive patients who received gabapentin increased to a level comparable to that of patients without a mood disorder.
Conclusion: The authors authors concluded that gabapentin was markedly more efficacious in improving vigor in the small subgroup of patients with mild depression.8
Myrick et al9 (2007) evaluated the safety and tolerability of gabapentin in patients who abused alcohol, as well as the ability of gabapentin to reduce alcohol craving and consumption. This study included 35 participants randomly assigned to receive gabapentin (n = 17) or placebo (n = 18) for 7 days. All medications were administered in standard gel caps with riboflavin, 25 mg, to assess for compliance via a laboratory-based urinary fluorescence assay. Urine samples were assessed for riboflavin at baseline and Day 6, and a reading of 1,500 ng/mL of riboflavin on Day 6 was interpreted as being compliant. Participants were required to abstain completely from drinking alcohol on Day 6 and the morning of Day 7. At the first session, the following measures were completed: demographic form, alcohol and drug section of the Structured Clinical Interview (SCID), Obsessive-Compulsive Drinking Scale, Self-Administered Alcohol Screening Test (SAAST), and Alcohol Dependence Scale (ADS); there also was collection of a urine sample for detection of abused drugs and a blood sample for liver function and general health screening.
At the second session, patients completed the psychiatric sections of the SCID and the Alcohol Craving Questionnaire, and received a physical exam. To assess the negative clinical effects of gabapentin and alcohol on the CNS, the Epworth Sleepiness Scale (ESS) and POMS were administered at baseline and on Day 6. Also, several other scales were used to identify any impact of gabapentin on acute alcohol effects and craving: the Clinical Institute Withdrawal Assessment of Alcohol, Revised (CIWA-Ar), Biphasic Alcohol Effects Scale (BAES), Subjective High Assessment Scale (SHAS), and Alcohol Urge Questionnaire (AUQ).
Conclusion: Gabapentin was well tolerated, but compared with placebo, gabapentin had no effect on alcohol stimulation (P = .75) or sedation (P = .99) as measured by the BAES. The difference in SHAS scores was also not significant (P = .19). There was also no significant reduction in craving for alcohol as measured on the AUQ scale
Continue to: Malcolm et al
Malcolm et al10 conducted an outpatient treatment study. Patients were men and women age 21 to 70 years from multiple ethnic groups. They were randomized to receive gabapentin or lorazepam; 449 patients were screened and 68 completed the follow-up. Scales used included the CIWA-Ar, Beck Depression Inventory (BDI), and ESS.
Patients receiving lorazepam reported less insomnia and more sleepiness early in treatment than patients receiving gabapentin. However, upon completing treatment and discontinuing medication administration, patients previously treated with lorazepam reported increased insomnia and daytime sleepiness, while patients previously treated with gabapentin continued to report improvements in these self-reported sleep measures. The differences between lorazepam and gabapentin were further evidenced in BDI scores at Day 5, Day 7, and Day 12 in patients who had previously experienced multiple withdrawals. Gabapentin was superior to lorazepam in reducing insomnia as assessed by BDI score, an effect that was sustained throughout the post-treatment week. Participants’ ESS scores indicated less daytime sleepiness in the gabapentin group than in the lorazepam group.
Conclusion: Among patients who abused alcohol and had a history of multiple withdrawals, lorazepam is less effective than gabapentin in reducing insomnia.10 However, this study had several limitations: <25% of individuals who were initially screened were enrolled in the study, and it used subjective tests such as BDI. Objective electrophysiologic measures of sleep and daytime sleepiness would have been very helpful.
Myrick et al11 (2009) also compared gabapentin and lorazepam for treating alcohol withdrawal. One hundred patients were randomized to receive 4 days of fixed-dose taper of gabapentin or lorazepam. Patients could receive 1 of 3 gabapentin dosing regimens (600 mg/d, 900 mg/d, or 1,200 mg/d) for 3 days. Participants who were randomized to receive lorazepam were given 6 mg/d for 3 days and then tapered to 4 mg/d. Also, blinded supplemental medications (rescue packs) were given to each patient on Days 1 to 4 to treat subjective feelings of alcohol withdrawal. All patients also received thiamine for 12 days. Assessment of severity of alcohol withdrawal was measured by the CIWA-Ar. To quantify the severity of alcohol dependence and alcohol use, patients were asked to complete the ADS and Time-Line Follow-Back (TLFB) scales, respectively. Other scales administered included the BDI, Zung Anxiety Scale (ZAS), ESS, and visual analogue scales that assessed craving, ability to perform work, and need for additional medication.
There was a decrease in CIWA-Ar scores over time in all groups. High-dose gabapentin was found to be statistically superior but clinically similar to lorazepam (P = .009). Researchers also found that compared with patients who were treated with lorazepam, patients who were treated with gabapentin experienced reduced craving and anxiety/depressive symptoms, and complained of less subjective discomfort. Compared to patients who were treated with gabapentin, patients who were treated with lorazepam had higher probabilities of drinking on the first day of dose decrease (Day 2) and the second day off medication (Day 6) (P = .0002). During post-treatment, patients who were treated with gabapentin had less probability of drinking during the follow-up post-treatment period (P = .2 for 900 mg/d and P = .3 for 1,200 mg/d) compared with patients who were treated with lorazepam (P = .55).
Continue to: Conclusion
Conclusion: The researchers concluded that gabapentin was well tolerated and effectively diminished the symptoms of alcohol withdrawal, especially at the higher target dose (1,200 mg/d), and that compared with lorazepam, gabapentin decreased the probability of drinking during alcohol withdrawal and in the immediate post-withdrawal week.11
Stock et al12 randomized 26 patients who met criteria for AWS to receive gabapentin or chlordiazepoxide. Gabapentin doses were 1,200 mg/d orally for 3 days, followed by 900 mg/d, 600 mg/d, and 300 mg/d for 1 day each. Chlordiazepoxide doses were 100 mg/d orally for 3 days, followed by 75 mg/d, 50 mg/d, and 25 mg/d for 1 day each. The ESS, Penn Alcohol Craving Scale (PACS), ataxia rating, and CIWA-Ar were administered daily. Thirty-five percent of participants dropped out at the end of the 7-day treatment period. Days 1 to 4 were considered the early treatment period, and Days 5 to 7 were considered the late treatment period. The adjusted mean ESS score did not differ significantly between the randomized groups during the early stage (P = .61) vs the late stage, in which the adjusted mean ESS score was significantly lower with gabapentin compared with chlordiazepoxide (P = .04). The differences in PACS scores between the groups were not statistically significant in either stage (early stage P = .59 vs late stage P = .08), but a trend of lower PACS scores was noted with gabapentin in the later stage. No participant in either group had ataxia during the study. In both groups, CIWA-Ar scores were reduced similarly.
Conclusion: The researchers concluded that gabapentin treatment resulted in a significantly greater reduction in sedation (ESS) and a trend toward reduced alcohol craving (PACS) by the end of treatment compared with chlordiazepoxide treatment.12
Schacht et al13 analyzed functional magnetic resonance imaging data from 48 patients who were alcohol-dependent in a 6-week RCT. Patients were randomized to receive gabapentin up to 1,200 mg/d for 39 days plus flumazenil for 2 days (GP/FMZ group) or an oral placebo and placebo infusions on the same time course. Evaluations included the SCID, ADS, and Obsessive-Compulsive Drinking Scale (OCDS). On Day 1, the CIWA-Ar was administered; it was used to ensure equal distribution of individuals with higher alcohol withdrawal symptoms between medication groups. There were no significant effects of initial alcohol withdrawal symptom status or medication. However, there was a significant interaction between these factors: patients with higher alcohol withdrawal symptoms who received GP/FMZ and those with lower alcohol withdrawal symptoms who received placebo demonstrated greater cue-elicited activation, relative to the other groups, and had less subsequent drinking, which reflected differences in deactivation between alcohol and beverage stimuli, in a cluster that encompassed the dorsal ACC (dACC) (family-wise error-corrected cluster probability of P = .012; 99 voxels; local maxima at [-3, 39, 18] and [6, 33, 9]). In the GP/FMZ group, patients with higher alcohol withdrawal symptoms had significantly greater activation, while in the placebo group, patients with lower alcohol withdrawal symptoms had greater activation.
Conclusion: The researchers concluded that alterations in task-related deactivation of dACC, a component of the default mode network, may predict better alcohol treatment response, while activation of DLPFC, an area associated with selective attention, may predict relapse drinking.13
Continue to: Carbamazepine
Carbamazepine
The characteristics of the carbamazepine studies included in this review are summarized in Table 2.14-19

Björkqvist et al14 randomized 105 men with AWS to placebo or carbamazepine. On initial assessment, history, physical examination, relevant labs, and intoxication assessments were recorded. On subsequent visits, nursing staff recorded withdrawal symptoms for patients as 0 to 2 (0 = no specific symptoms, 1 = patient only complained when asked about specific symptoms, 2 = patient complained of withdrawal symptoms without being asked, or if symptoms were severe or obvious to others). Along with the above, vital signs and a visual analogue scale of 0 to 10 (0 = feeling could not be worse, 10 = feeling could not be better) were recorded at each visit. The dose was weight-dependent and administered as follows: on Days 1 and 2, 1+1+2 tablets of carbamazepine, 200 mg, or placebo; Days 3 and 4, 1+1+1 tablets; and Days 5 and 6, 1+0+1 tablets. Every patient received dichloralphenazone as needed. All patients were given vitamin B 3 times a day. Most withdrawal symptoms decreased faster in the carbamazepine group on Day 2 (P = .01) and on Day 4 (P = .1). On the visual analogue scale, scores varied between patients. On Day 1, the mean score was 2.5 times higher in the carbamazepine group compared with the placebo group, and this difference increased to 3 times by Day 7 (P < .01). The patient’s estimated ability to work improved significantly faster in the carbamazepine group than in the placebo group (P < .01).
Conclusion: The authors concluded that compared with placebo, carbamazepine was able to more quickly decrease withdrawal symptoms, especially insomnia and subjective recovery.14
Ritola et al15 randomized 68 hospitalized men with AWS to carbamazepine, 200 mg/d, or clomethiazole, 300 mg/d, for 1 week. The target withdrawal symptoms included gastrointestinal and sleep disturbances; anxiety; aggressiveness; and cardiovascular, depressive, psychotic, and neurologic symptoms. A 4-point rating scale was used for individual symptoms (0 = no symptom, 1 = mild symptom, 2 = moderate symptom, and 3 = severe symptom). On the day of admission (Day 0), all patients were given 50 to 100 mg of chlordiazepoxide IM and 2 tablets and 4 capsules of the trial preparations (either the tablets or capsules were active, and the others were placebos) in the evening. Five patients dropped out of the clomethiazole group and 1 from the carbamazepine group. No significant difference between the 2 treatments were found by the patient, nurse, or physician.
Conclusion: The authors concluded that carbamazepine seemed to be as effective as clomethiazole in the treatment of milder alcohol withdrawal symptoms. Final treatment results were equally good in both groups. Sleep disturbance resolved faster in the carbamazepine group.15
Continue to: Agricola et al
Agricola et al16 compared carbamazepine to tiapride for treatment of acute AWS. In this study, 60 patients were randomized to carbamazepine, 200 mg 3 times a day, or tiapride, 200 mg 3 times a day. All patients were hospitalized with severe AWS preceding DT. The patients were evaluated for withdrawal symptoms (gastrointestinal and cardiovascular symptoms, sleep disturbances, anxiety, aggression, fear, depression, psychotic symptoms, and certain neurologic symptoms). The severity of these symptoms was scored as follows: 0 = no symptoms; 1 = moderate symptoms; and 2 = severe symptoms. At each visit, an overall evaluation of the patient’s clinical condition was made according to a visual analogue scale (100 = worst condition, 0 = best condition). On Day 7, both the doctor and patient evaluated treatment efficacy according to a 4-point scale (1 = no efficacy, 4 = excellent efficacy). There was no significant difference between carbamazepine and tiapride in terms of total symptoms score and visual analogue scale assessment. Carbamazepine was found to have faster relief of symptoms and a significantly greater reduction in symptom score on Day 2 (P < .01). Carbamazepine had a preferential action on fear, nightmares, and hallucinations. The proportion of patients in whom anxiety improved after treatment was 96.2% for carbamazepine and 71.4% for tiapride (P < .05). Aggressiveness and gastrointestinal discomfort resolved faster in the tiapride group. No cases of DT were observed.
Conclusion: The researchers concluded that either carbamazepine or tiapride provides an appropriate alternative in the treatment of inpatients with severe AWS.16
Stuppaeck et al17 compared the efficacy of carbamazepine to oxazepam in 60 inpatients who had symptoms of alcohol withdrawal. Alcohol withdrawal was measured with the CIWA-A, and patients with scores >20 were enrolled in the study. The Clinical Global Impression (CGI) scale and self-rated Adjective Checklist (ACL) were also used. On Days 1 to 3, patients received oxazepam, 120 mg/d, or carbamazepine, 800 mg/d. From Day 4 to 7, doses were decreased to 90 mg/d and 600 mg/d, respectively. After the 7-day trial, all patients were treated with carbamazepine, 200 mg twice a day on Day 8 and 200 mg at night on Day 9. Two patients withdrew consent and 6 dropped out due to adverse effects. During the 7-day trial, when comparing all improvements on CIWA-A, ACL, and CGI scales, carbamazepine was equivalent to oxazepam up to Day 5, and then superior on Days 6 and 7 (P ≤ .05). No decrease in white blood cell count was found in the carbamazepine group.
Conclusion: The authors concluded that carbamazepine is as effective as oxazepam and may be a viable alternative that does not interact with alcohol or cause delirium.17
Malcolm et al18 compared the effects of carbamazepine and lorazepam in patients in an outpatient setting who had single vs multiple previous alcohol withdrawals. The study included 136 patients who satisfied DSM-IV criteria for alcohol dependence and alcohol withdrawal, with a blood alcohol level ≤0.1 g/dL, a Mini-Mental State Examination (MMSE) score ≤26, and a CIWA-Ar score ≤10 on admission. Patients also completed the ADS to quantify the severity of alcohol dependence. Daily drinking was measured by patient report using a daily drinking log and blood alcohol level. Heavy drinking was defined as ≥4 standard drinks per day for women and ≥5 drinks per day for men. On Day 1, patients were randomized to receive carbamazepine, 600 to 800 mg/d,or lorazepam, 6 to 8 mg/d, in divided doses, which was tapered to carbamazepine, 200 mg/d, or lorazepam, 2 mg/d, on Day 5. All patients received thiamine for 12 days. In the immediate post-detoxification period, carbamazepine-treated patients were less likely to relapse, and if they did drink, they drank less than those treated with lorazepam (P = .003). Even in patients who had multiple previous detoxifications, those randomized to carbamazepine drank less than those in lorazepam group (P = .004). Patients in the lorazepam group had significant higher rebound withdrawal symptoms (P = .007).
Continue to: Conclusion
Conclusion: The researchers concluded that carbamazepine and lorazepam were both effective in reducing alcohol withdrawal symptoms. They also concluded that carbamazepine was less likely to cause rebound withdrawal and more likely to reduce post-treatment drinking; among those who did drink, there was less heavy drinking.18
Malcolm et al19 conducted a 5-day double-blind RCT with 136 outpatients who met DSM-IV criteria for alcohol withdrawal. Patients were evaluated by CIWA before getting medications and then daily for 5 days. Patients were randomized to receive carbamazepine, 600 to 800 mg/d on Day 1, 200 mg 3 times a day on Day 2, 200 mg twice a day on Days 3 and 4, and 200 mg once on Day 5. Participants were randomized to receive lorazepam, 6 to 8 mg/d in divided doses on Day 1, 2 mg 3 times a day on Day 2, 2 mg twice a day on Days 3 and 4, and 2 mg once on Day 5. Ability to return to work was self-rated on a 100-mm visual analogue scale, with 0 being “totally unable to return to work’’ and 100 representing “being fully able to return to work.’’ Self-report measures of sleep quality were made using a 100-mm visual analogue scale, with 0 = “the very worst night’s sleep I’ve ever had’’ and 100 = “the very best night’s sleep I’ve ever had.’’ Carbamazepine significantly reduced anxiety (P = .0007). Visual analogue measures of sleep quality indicated a statistically significant main effect of medication on sleep that favored carbamazepine (P = .0186).
Conclusion: The authors concluded that when treating patients with mild to moderate alcohol withdrawal symptoms, carbamazepine was superior to lorazepam in reducing anxiety and improving sleep.19
Sodium valproate
The characteristics of the sodium valproate studies included in this review are summarized in Table 3.20,21

Lambie et al20 evaluated the use of sodium valproate in the treatment of AWS. A total of 49 patients were randomized to a sodium valproate group (n = 22) or a control group (n = 27). All participants were inpatients receiving treatment for alcohol use disorder and substance use disorder for 7 days. Patients in the sodium valproate group received 800 mg every 8 hours for 7 days. Patients were observed daily for occurrence of withdrawal symptoms. Nurses who were blinded to the group assignment graded the degree and severity of symptoms. The trial was initially designed so that chlormethiazole and/or tranquilizers were added to sodium valproate when withdrawal symptoms occurred. However, after treating the first few patients, it became evident that additional medications were not needed. In the treatment group, 13 participants received only sodium valproate, 4 patients needed a tranquilizer, 4 needed chlormethiazole, and 1 needed both. In the control group, 1 received only sodium valproate, 4 received a tranquilizer, 14 received chlormethiazole, and 8 needed both. One patient, who entered the study twice, had a withdrawal seizure when in control group and no seizure on second admission in the sodium valproate group. Physical symptoms disappeared quickly in the sodium valproate group (mean of 2 days vs 2.6 days in the control group). Fourteen patients in the control group received chlormethiazole, compared with only 4 patients in sodium valproate group.
Continue to: Conclusion
Conclusion: The researchers concluded that physical symptoms disappeared quicker in the sodium valproate group than in the control group.20
Hillbom et al21 evaluated the efficacy of sodium valproate vs carbamazepine vs placebo to prevent alcohol withdrawal seizures. A total of 138 participants were studied. Forty-three were assigned to the carbamazepine group, 46 to the sodium valproate group, and 49 to the placebo group. The RCT lasted 4 days. The initial medication doses were 1,200 mg/d. Participants in the carbamazepine group experienced more adverse effects than those in the sodium valproate or placebo groups (P < .001). As a result, approximately one-half of the participants in the carbamazepine group stopped taking the medication. This finding was dependent on the dose of carbamazepine; >800 mg/d resulted in poor tolerance to adverse effects. Seizures occurred among patients in all 3 arms of the study; in the sodium valproate group, 1 participant had a seizure vs 2 participants in the carbamazepine group and 3 in the placebo group. On the other hand, DT occurred only in the sodium valproate and placebo groups.
Conclusion: Researchers concluded that when using sodium valproate or carbamazepine to prevent alcohol withdrawal seizures in an outpatient setting, the adverse effects may outweigh the benefits.21
Lamotrigine
The characteristics of the lamotrigine study included in this review are summarized in Table 3.22
Djokić et al22 evaluated the efficiency of lamotrigine in the treatment of DT. A total of 240 participants who met International Classification of Diseases-10 criteria for DT were randomized to a control group that was treated with anticonvulsants according to an NIAAA protocol (2004), or to an experimental group that was treated with lamotrigine. The CIWA-Ar and the Memorial Delirium Assessment Scale (MDAS) were administered for objective assessment of clinical symptoms, superimposed medical complications, general condition of the patient, adverse effects, and mortality rate. Statistically significant differences between the experimental and control groups were apparent after the third day of therapy, when a drop in the average CIWA-Ar score was observed in the experimental group, while the control group still had high scores (P < .01). After the fifth day of treatment, the differences in scores were more apparent, with the experimental group showing CIWA-Ar scores equal to those of persons with mild/moderate DT, while those in the control group still had high scores. After the tenth day, participants in the experimental group did not have any alcohol withdrawal symptoms, while control group participants were just beginning to get out of life-threatening danger. Death occurred in 4.1% of control group participants and 3.4% of experimental group participants; this difference in mortality rate was not statistically significant.
Continue to: Conclusion
Conclusion: Researchers concluded that lamotrigine is significantly efficacious in the treatment of DT, but does not decrease the mortality rate.22
What to know before you prescribe
AWS is a medical emergency that if left untreated leads to several complications and possibly death. Although benzodiazepines are considered the gold standard for treating AWS, the adverse effects associated with their use advocates for finding alternatives. Anticonvulsants can be an effective alternative for treating AWS. In our literature review, we found 16 double-blind RCTs that used an anticonvulsant medication for the treatment of AWS. Of these, 7 involved gabapentin, 6 involved carbamazepine, 1 involved sodium valproate, 1 involved sodium valproate vs carbamazepine, and 1 involved lamotrigine. Overall, the use of anticonvulsants resulted in significant improvement of mild to moderate symptoms of AWS.
There were more studies of carbamazepine and gabapentin than of other anticonvulsants. All the anticonvulsants offered potential benefits. They decreased the probability of a withdrawal seizure and other complications and effectively reduced alcohol cravings. Anticonvulsants were useful for preventing rebound withdrawal symptoms and reducing post-treatment alcohol consumption, especially in patients who had multiple previous withdrawals. Anticonvulsants were particularly helpful for patients with mood disorders such as depression. In the studies we reviewed, anticonvulsants caused less sedation compared with benzodiazepines, and also decreased the occurrence of relapse.
Dosing recommendations. In the studies included in our review, gabapentin was effective at a dosage of 1,600 mg/d (given as 400 mg 4 times a day). This was tapered as follows: 400 mg 4 times a day on Days 1 to 3, 400 mg 3 times a day on Day 4, 400 mg twice a day on Day 5, and 400 mg once a day on Day 6. Carbamazepine was effective at 600 to 800 mg/d, and was tapered by decreasing by 200 mg as follows: 800 mg/d on Days 1 to 3, 600 mg/d on Day 4, 400 mg on Day 5, and 200 mg/d on Day 6. In the reviewed study, the maximum dose of lamotrigine never exceeded 200 mg/d and was administered for 28 days; the exact dosing and taper plan were not described. The dosing of sodium valproate ranged from 1,200 mg/d to 1600 mg/d for 7 days, followed by decreasing by 200 mg each day. The recommended duration of treatment varied; on average for all anticonvulsants, it was 7 to 12 days, followed by a taper. Carbamazepine was shown to be superior to oxazepam in ameliorating the symptoms of AWS.
Adverse effects. When considering the tolerability, adverse effect profile, duration of action, and effectiveness of the anticonvulsants included in our review, gabapentin appears to be the safest agent to choose. For the other anticonvulsants, the risks might outweigh the benefits. Specifically, in a comparison of sodium valproate and carbamazepine, Hillbom et al21 concluded that in doses >800 mg/d, carbamazepine has potential to cause more adverse effects than benefits. However, Agricola et al16 found that carbamazepine had a preferential action on fear, nightmares, and hallucinations.
Continue to: A few caveats
A few caveats
Our review focused a large collection of data from multiple databases and RCTs only. However, its limitations include:
- there was no measure of heterogeneity
- the studies had short treatment duration
- most studies evaluated predominantly male participants
- some studies were underpowered.
Our review laid a groundwork for future research that includes more well-designed RCTs and/or meta-analyses of recent studies that evaluated the use anticonvulsants for treating AWS.
Bottom Line
Evidence suggests certain anticonvulsants may be an effective alternative to benzodiazepines for the treatment of mild to moderate alcohol withdrawal syndrome. Gabapentin may be the safest anticonvulsant to prescribe. Other anticonvulsants to consider include carbamazepine, sodium valproate, and lamotrigine, but for these agents, the risks might outweigh the benefits.
Related Resources
- Myrick H, Anton RF. Treatment of alcohol withdrawal. Alcohol Health Res World. 1998;22(1):38-43. https://pubs.niaaa.nih.gov/publications/arh22-1/38-43.pdf
- World Health Organization. Management of alcohol withdrawal. Published 2012. https://www.who.int/mental_health/mhgap/evidence/alcohol/q2/en/
Drug Brand Names
Carbamazepine • Tegretol
Gabapentin • Neurontin
Lamotrigine • Lamictal
Levetiracetam • Keppra
Lorazepam • Ativan
Oxcarbazepine • Trileptal
Phenytoin • Dilantin
Sodium valproate • Depakote
Acknowledgments
The authors thank Geetha Manikkara, MD, Madhuri Jakkam Setty, MD, and Elizabeth DeOreo, MD, for their efforts with the systematic review research.
Abrupt cessation or reduction of alcohol consumption may result in alcohol withdrawal syndrome (AWS), which is a medical emergency that can lead to serious complications when unrecognized or treatment is delayed. Symptoms of AWS include tremors, anxiety attacks, cognitive impairment, hallucinations, seizures, delirium tremens (DT), and in severe, untreated cases, death.1 Low to moderate alcohol consumption produces euphoria and excitation via activation of glutamatergic neurotransmission, while higher concentrations produce severe intoxication via GABAergic mechanisms. Acute withdrawal unmasks the hyper-excitatory state of the brain, causing anxiety, agitation, and autonomic activation characteristic of AWS, which typically begins 1 to 3 days after the last drink.2 In the 2012-2013 National Epidemiologic Survey on Alcohol and Related Conditions conducted by the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the 12-month and lifetime prevalences of AWS were 13.9% and 29.1%, respectively.3 Within the general inpatient population, AWS can be present in nearly 30% of patients; if left untreated, AWS has a 15% mortality rate, although when AWS is recognized early and treated, the mortality rate falls dramatically to 2%.4
AWS has most commonly been treated with benzodiazepines.5 However, benzodiazepines have the potential for significant adverse effects when used in older adults and in individuals with complicated medical issues, such as obstructive lung disease and sleep apnea.6 Anticonvulsants have been increasingly used to treat alcohol withdrawal, and their use is supported by several retrospective and prospective studies. In this article, we review the data from randomized control trials (RCTs) on the use of anticonvulsants for the treatment of AWS to see if we can make any recommendations for the use of anticonvulsants for treating AWS.
Our literature search
We searched 5 databases (PubMed, Cochrane, Medline, PsycInfo, and Embase) using the following terms: “alcohol withdrawal syndrome treatment”, “anticonvulsants”, “anti-epileptic”, “gabapentin”, “carbamazepine”, “sodium valproate”, “oxcarbazepine”, “phenytoin”, “levetiracetam”, and “lamotrigine.” We included only double-blind RCTs published between January 1, 1976 and September 30, 2016 in English-language journals or that had an official English translation. There were no restrictions on patient age or location of treatment (inpatient vs outpatient). All RCTs that compared anticonvulsants or a combination of an anticonvulsant and an active pharmacotherapeutic agent with either placebo or gold standard treatment for AWS were included. Database reviews, systematic reviews, and meta-analyses were excluded.
We identified 662 articles that met these criteria. However, most were duplicates, review articles, systematic reviews, meta-analyses, case reports, or open-label or non-randomized trials. Only 16 articles met our inclusion criteria. In the following sections, we discuss these 16 studies by medication type and in chronological order.
Gabapentin
The characteristics of the gabapentin studies included in this review are summarized in Table 1.7-13

Bonnet et al7 (2003) examined 61 adults who met the clinical criteria for alcohol dependence and displayed moderate or severe AWS according to their Mainz Alcohol Withdrawal Score (MAWS ≥4). They were randomized to receive placebo or gabapentin, 400 mg 4 times a day, along with clomethiazole. The attrition rate was not significantly different between the 2 groups (P = .66). The difference in the number of clomethiazole capsules taken during the first 24 hours between the groups was small and not significant (P = .96). Analysis of MAWS over time revealed no significant main effect for group (P = .26) and a significant effect for the time variable (P < .001). The interaction between group and time was not significant (P =.4). This means that there was a significant decrease in MAWS from baseline over 48 hours, and this decrease in MAWS was considered equal for both study groups. Adverse clinical events were observed in both groups, and there was no significant difference (P = .74) between the groups. Nausea and ataxia, which are specific to gabapentin, were observed more frequently in this group.
Conclusion: The authors concluded that gabapentin, 400 mg 4 times a day, is no better than placebo in reducing the amount of clomethiazole required to treat acute AWS.7
Continue to: Bonnet et al
Bonnet et al8 (2007) also conducted a study examining 59 patients with alcohol dependence who displayed moderate or severe AWS. Participants received placebo or gabapentin, 400 mg, and a rescue medication, clomethiazole, if needed. Subsequently, a capsule of study medication was administered every 6 hours for 2 days and then tapered. During the study, mood was measured by Profile of Mood States (POMS), and subjective complaints of withdrawal were measured using the Essen Self-Assessment of Alcohol Withdrawal Scale (ESA). Of the 59 patients, only 46 were analyzed; 5 patients dropped out, and 8 patients were missing data. Compared with the placebo group, the gabapentin group displayed less dejection, fatigue, and anger, and more vigor. Analysis of variance (ANOVA) measures revealed significant overall changes over time on all 4 scales (all P < .001). A significant (F = 3.62, df 2;43, P = .035) group × time interaction resulted exclusively for vigor. Analysis was repeated using rank-transformed data, resulting in a significant (P = .046) interaction effect. The significant increase in vigor was not apparent after tapering off gabapentin, which suggests gabapentin has a reversible effect on vigor. There was a significant (P < .001) overall decline of subjective withdrawal symptoms complaints, but no group × time interaction (P = .62). Analysis of 11 patients with comorbid mild depression revealed no significant time × group interaction for dejection, fatigue, anger, or subjective withdrawal (all P > .05). However, for vigor, the group × time interaction was significant (P = .022). Throughout the treatment, vigor scores of those mild depressive patients who received gabapentin increased to a level comparable to that of patients without a mood disorder.
Conclusion: The authors authors concluded that gabapentin was markedly more efficacious in improving vigor in the small subgroup of patients with mild depression.8
Myrick et al9 (2007) evaluated the safety and tolerability of gabapentin in patients who abused alcohol, as well as the ability of gabapentin to reduce alcohol craving and consumption. This study included 35 participants randomly assigned to receive gabapentin (n = 17) or placebo (n = 18) for 7 days. All medications were administered in standard gel caps with riboflavin, 25 mg, to assess for compliance via a laboratory-based urinary fluorescence assay. Urine samples were assessed for riboflavin at baseline and Day 6, and a reading of 1,500 ng/mL of riboflavin on Day 6 was interpreted as being compliant. Participants were required to abstain completely from drinking alcohol on Day 6 and the morning of Day 7. At the first session, the following measures were completed: demographic form, alcohol and drug section of the Structured Clinical Interview (SCID), Obsessive-Compulsive Drinking Scale, Self-Administered Alcohol Screening Test (SAAST), and Alcohol Dependence Scale (ADS); there also was collection of a urine sample for detection of abused drugs and a blood sample for liver function and general health screening.
At the second session, patients completed the psychiatric sections of the SCID and the Alcohol Craving Questionnaire, and received a physical exam. To assess the negative clinical effects of gabapentin and alcohol on the CNS, the Epworth Sleepiness Scale (ESS) and POMS were administered at baseline and on Day 6. Also, several other scales were used to identify any impact of gabapentin on acute alcohol effects and craving: the Clinical Institute Withdrawal Assessment of Alcohol, Revised (CIWA-Ar), Biphasic Alcohol Effects Scale (BAES), Subjective High Assessment Scale (SHAS), and Alcohol Urge Questionnaire (AUQ).
Conclusion: Gabapentin was well tolerated, but compared with placebo, gabapentin had no effect on alcohol stimulation (P = .75) or sedation (P = .99) as measured by the BAES. The difference in SHAS scores was also not significant (P = .19). There was also no significant reduction in craving for alcohol as measured on the AUQ scale
Continue to: Malcolm et al
Malcolm et al10 conducted an outpatient treatment study. Patients were men and women age 21 to 70 years from multiple ethnic groups. They were randomized to receive gabapentin or lorazepam; 449 patients were screened and 68 completed the follow-up. Scales used included the CIWA-Ar, Beck Depression Inventory (BDI), and ESS.
Patients receiving lorazepam reported less insomnia and more sleepiness early in treatment than patients receiving gabapentin. However, upon completing treatment and discontinuing medication administration, patients previously treated with lorazepam reported increased insomnia and daytime sleepiness, while patients previously treated with gabapentin continued to report improvements in these self-reported sleep measures. The differences between lorazepam and gabapentin were further evidenced in BDI scores at Day 5, Day 7, and Day 12 in patients who had previously experienced multiple withdrawals. Gabapentin was superior to lorazepam in reducing insomnia as assessed by BDI score, an effect that was sustained throughout the post-treatment week. Participants’ ESS scores indicated less daytime sleepiness in the gabapentin group than in the lorazepam group.
Conclusion: Among patients who abused alcohol and had a history of multiple withdrawals, lorazepam is less effective than gabapentin in reducing insomnia.10 However, this study had several limitations: <25% of individuals who were initially screened were enrolled in the study, and it used subjective tests such as BDI. Objective electrophysiologic measures of sleep and daytime sleepiness would have been very helpful.
Myrick et al11 (2009) also compared gabapentin and lorazepam for treating alcohol withdrawal. One hundred patients were randomized to receive 4 days of fixed-dose taper of gabapentin or lorazepam. Patients could receive 1 of 3 gabapentin dosing regimens (600 mg/d, 900 mg/d, or 1,200 mg/d) for 3 days. Participants who were randomized to receive lorazepam were given 6 mg/d for 3 days and then tapered to 4 mg/d. Also, blinded supplemental medications (rescue packs) were given to each patient on Days 1 to 4 to treat subjective feelings of alcohol withdrawal. All patients also received thiamine for 12 days. Assessment of severity of alcohol withdrawal was measured by the CIWA-Ar. To quantify the severity of alcohol dependence and alcohol use, patients were asked to complete the ADS and Time-Line Follow-Back (TLFB) scales, respectively. Other scales administered included the BDI, Zung Anxiety Scale (ZAS), ESS, and visual analogue scales that assessed craving, ability to perform work, and need for additional medication.
There was a decrease in CIWA-Ar scores over time in all groups. High-dose gabapentin was found to be statistically superior but clinically similar to lorazepam (P = .009). Researchers also found that compared with patients who were treated with lorazepam, patients who were treated with gabapentin experienced reduced craving and anxiety/depressive symptoms, and complained of less subjective discomfort. Compared to patients who were treated with gabapentin, patients who were treated with lorazepam had higher probabilities of drinking on the first day of dose decrease (Day 2) and the second day off medication (Day 6) (P = .0002). During post-treatment, patients who were treated with gabapentin had less probability of drinking during the follow-up post-treatment period (P = .2 for 900 mg/d and P = .3 for 1,200 mg/d) compared with patients who were treated with lorazepam (P = .55).
Continue to: Conclusion
Conclusion: The researchers concluded that gabapentin was well tolerated and effectively diminished the symptoms of alcohol withdrawal, especially at the higher target dose (1,200 mg/d), and that compared with lorazepam, gabapentin decreased the probability of drinking during alcohol withdrawal and in the immediate post-withdrawal week.11
Stock et al12 randomized 26 patients who met criteria for AWS to receive gabapentin or chlordiazepoxide. Gabapentin doses were 1,200 mg/d orally for 3 days, followed by 900 mg/d, 600 mg/d, and 300 mg/d for 1 day each. Chlordiazepoxide doses were 100 mg/d orally for 3 days, followed by 75 mg/d, 50 mg/d, and 25 mg/d for 1 day each. The ESS, Penn Alcohol Craving Scale (PACS), ataxia rating, and CIWA-Ar were administered daily. Thirty-five percent of participants dropped out at the end of the 7-day treatment period. Days 1 to 4 were considered the early treatment period, and Days 5 to 7 were considered the late treatment period. The adjusted mean ESS score did not differ significantly between the randomized groups during the early stage (P = .61) vs the late stage, in which the adjusted mean ESS score was significantly lower with gabapentin compared with chlordiazepoxide (P = .04). The differences in PACS scores between the groups were not statistically significant in either stage (early stage P = .59 vs late stage P = .08), but a trend of lower PACS scores was noted with gabapentin in the later stage. No participant in either group had ataxia during the study. In both groups, CIWA-Ar scores were reduced similarly.
Conclusion: The researchers concluded that gabapentin treatment resulted in a significantly greater reduction in sedation (ESS) and a trend toward reduced alcohol craving (PACS) by the end of treatment compared with chlordiazepoxide treatment.12
Schacht et al13 analyzed functional magnetic resonance imaging data from 48 patients who were alcohol-dependent in a 6-week RCT. Patients were randomized to receive gabapentin up to 1,200 mg/d for 39 days plus flumazenil for 2 days (GP/FMZ group) or an oral placebo and placebo infusions on the same time course. Evaluations included the SCID, ADS, and Obsessive-Compulsive Drinking Scale (OCDS). On Day 1, the CIWA-Ar was administered; it was used to ensure equal distribution of individuals with higher alcohol withdrawal symptoms between medication groups. There were no significant effects of initial alcohol withdrawal symptom status or medication. However, there was a significant interaction between these factors: patients with higher alcohol withdrawal symptoms who received GP/FMZ and those with lower alcohol withdrawal symptoms who received placebo demonstrated greater cue-elicited activation, relative to the other groups, and had less subsequent drinking, which reflected differences in deactivation between alcohol and beverage stimuli, in a cluster that encompassed the dorsal ACC (dACC) (family-wise error-corrected cluster probability of P = .012; 99 voxels; local maxima at [-3, 39, 18] and [6, 33, 9]). In the GP/FMZ group, patients with higher alcohol withdrawal symptoms had significantly greater activation, while in the placebo group, patients with lower alcohol withdrawal symptoms had greater activation.
Conclusion: The researchers concluded that alterations in task-related deactivation of dACC, a component of the default mode network, may predict better alcohol treatment response, while activation of DLPFC, an area associated with selective attention, may predict relapse drinking.13
Continue to: Carbamazepine
Carbamazepine
The characteristics of the carbamazepine studies included in this review are summarized in Table 2.14-19

Björkqvist et al14 randomized 105 men with AWS to placebo or carbamazepine. On initial assessment, history, physical examination, relevant labs, and intoxication assessments were recorded. On subsequent visits, nursing staff recorded withdrawal symptoms for patients as 0 to 2 (0 = no specific symptoms, 1 = patient only complained when asked about specific symptoms, 2 = patient complained of withdrawal symptoms without being asked, or if symptoms were severe or obvious to others). Along with the above, vital signs and a visual analogue scale of 0 to 10 (0 = feeling could not be worse, 10 = feeling could not be better) were recorded at each visit. The dose was weight-dependent and administered as follows: on Days 1 and 2, 1+1+2 tablets of carbamazepine, 200 mg, or placebo; Days 3 and 4, 1+1+1 tablets; and Days 5 and 6, 1+0+1 tablets. Every patient received dichloralphenazone as needed. All patients were given vitamin B 3 times a day. Most withdrawal symptoms decreased faster in the carbamazepine group on Day 2 (P = .01) and on Day 4 (P = .1). On the visual analogue scale, scores varied between patients. On Day 1, the mean score was 2.5 times higher in the carbamazepine group compared with the placebo group, and this difference increased to 3 times by Day 7 (P < .01). The patient’s estimated ability to work improved significantly faster in the carbamazepine group than in the placebo group (P < .01).
Conclusion: The authors concluded that compared with placebo, carbamazepine was able to more quickly decrease withdrawal symptoms, especially insomnia and subjective recovery.14
Ritola et al15 randomized 68 hospitalized men with AWS to carbamazepine, 200 mg/d, or clomethiazole, 300 mg/d, for 1 week. The target withdrawal symptoms included gastrointestinal and sleep disturbances; anxiety; aggressiveness; and cardiovascular, depressive, psychotic, and neurologic symptoms. A 4-point rating scale was used for individual symptoms (0 = no symptom, 1 = mild symptom, 2 = moderate symptom, and 3 = severe symptom). On the day of admission (Day 0), all patients were given 50 to 100 mg of chlordiazepoxide IM and 2 tablets and 4 capsules of the trial preparations (either the tablets or capsules were active, and the others were placebos) in the evening. Five patients dropped out of the clomethiazole group and 1 from the carbamazepine group. No significant difference between the 2 treatments were found by the patient, nurse, or physician.
Conclusion: The authors concluded that carbamazepine seemed to be as effective as clomethiazole in the treatment of milder alcohol withdrawal symptoms. Final treatment results were equally good in both groups. Sleep disturbance resolved faster in the carbamazepine group.15
Continue to: Agricola et al
Agricola et al16 compared carbamazepine to tiapride for treatment of acute AWS. In this study, 60 patients were randomized to carbamazepine, 200 mg 3 times a day, or tiapride, 200 mg 3 times a day. All patients were hospitalized with severe AWS preceding DT. The patients were evaluated for withdrawal symptoms (gastrointestinal and cardiovascular symptoms, sleep disturbances, anxiety, aggression, fear, depression, psychotic symptoms, and certain neurologic symptoms). The severity of these symptoms was scored as follows: 0 = no symptoms; 1 = moderate symptoms; and 2 = severe symptoms. At each visit, an overall evaluation of the patient’s clinical condition was made according to a visual analogue scale (100 = worst condition, 0 = best condition). On Day 7, both the doctor and patient evaluated treatment efficacy according to a 4-point scale (1 = no efficacy, 4 = excellent efficacy). There was no significant difference between carbamazepine and tiapride in terms of total symptoms score and visual analogue scale assessment. Carbamazepine was found to have faster relief of symptoms and a significantly greater reduction in symptom score on Day 2 (P < .01). Carbamazepine had a preferential action on fear, nightmares, and hallucinations. The proportion of patients in whom anxiety improved after treatment was 96.2% for carbamazepine and 71.4% for tiapride (P < .05). Aggressiveness and gastrointestinal discomfort resolved faster in the tiapride group. No cases of DT were observed.
Conclusion: The researchers concluded that either carbamazepine or tiapride provides an appropriate alternative in the treatment of inpatients with severe AWS.16
Stuppaeck et al17 compared the efficacy of carbamazepine to oxazepam in 60 inpatients who had symptoms of alcohol withdrawal. Alcohol withdrawal was measured with the CIWA-A, and patients with scores >20 were enrolled in the study. The Clinical Global Impression (CGI) scale and self-rated Adjective Checklist (ACL) were also used. On Days 1 to 3, patients received oxazepam, 120 mg/d, or carbamazepine, 800 mg/d. From Day 4 to 7, doses were decreased to 90 mg/d and 600 mg/d, respectively. After the 7-day trial, all patients were treated with carbamazepine, 200 mg twice a day on Day 8 and 200 mg at night on Day 9. Two patients withdrew consent and 6 dropped out due to adverse effects. During the 7-day trial, when comparing all improvements on CIWA-A, ACL, and CGI scales, carbamazepine was equivalent to oxazepam up to Day 5, and then superior on Days 6 and 7 (P ≤ .05). No decrease in white blood cell count was found in the carbamazepine group.
Conclusion: The authors concluded that carbamazepine is as effective as oxazepam and may be a viable alternative that does not interact with alcohol or cause delirium.17
Malcolm et al18 compared the effects of carbamazepine and lorazepam in patients in an outpatient setting who had single vs multiple previous alcohol withdrawals. The study included 136 patients who satisfied DSM-IV criteria for alcohol dependence and alcohol withdrawal, with a blood alcohol level ≤0.1 g/dL, a Mini-Mental State Examination (MMSE) score ≤26, and a CIWA-Ar score ≤10 on admission. Patients also completed the ADS to quantify the severity of alcohol dependence. Daily drinking was measured by patient report using a daily drinking log and blood alcohol level. Heavy drinking was defined as ≥4 standard drinks per day for women and ≥5 drinks per day for men. On Day 1, patients were randomized to receive carbamazepine, 600 to 800 mg/d,or lorazepam, 6 to 8 mg/d, in divided doses, which was tapered to carbamazepine, 200 mg/d, or lorazepam, 2 mg/d, on Day 5. All patients received thiamine for 12 days. In the immediate post-detoxification period, carbamazepine-treated patients were less likely to relapse, and if they did drink, they drank less than those treated with lorazepam (P = .003). Even in patients who had multiple previous detoxifications, those randomized to carbamazepine drank less than those in lorazepam group (P = .004). Patients in the lorazepam group had significant higher rebound withdrawal symptoms (P = .007).
Continue to: Conclusion
Conclusion: The researchers concluded that carbamazepine and lorazepam were both effective in reducing alcohol withdrawal symptoms. They also concluded that carbamazepine was less likely to cause rebound withdrawal and more likely to reduce post-treatment drinking; among those who did drink, there was less heavy drinking.18
Malcolm et al19 conducted a 5-day double-blind RCT with 136 outpatients who met DSM-IV criteria for alcohol withdrawal. Patients were evaluated by CIWA before getting medications and then daily for 5 days. Patients were randomized to receive carbamazepine, 600 to 800 mg/d on Day 1, 200 mg 3 times a day on Day 2, 200 mg twice a day on Days 3 and 4, and 200 mg once on Day 5. Participants were randomized to receive lorazepam, 6 to 8 mg/d in divided doses on Day 1, 2 mg 3 times a day on Day 2, 2 mg twice a day on Days 3 and 4, and 2 mg once on Day 5. Ability to return to work was self-rated on a 100-mm visual analogue scale, with 0 being “totally unable to return to work’’ and 100 representing “being fully able to return to work.’’ Self-report measures of sleep quality were made using a 100-mm visual analogue scale, with 0 = “the very worst night’s sleep I’ve ever had’’ and 100 = “the very best night’s sleep I’ve ever had.’’ Carbamazepine significantly reduced anxiety (P = .0007). Visual analogue measures of sleep quality indicated a statistically significant main effect of medication on sleep that favored carbamazepine (P = .0186).
Conclusion: The authors concluded that when treating patients with mild to moderate alcohol withdrawal symptoms, carbamazepine was superior to lorazepam in reducing anxiety and improving sleep.19
Sodium valproate
The characteristics of the sodium valproate studies included in this review are summarized in Table 3.20,21

Lambie et al20 evaluated the use of sodium valproate in the treatment of AWS. A total of 49 patients were randomized to a sodium valproate group (n = 22) or a control group (n = 27). All participants were inpatients receiving treatment for alcohol use disorder and substance use disorder for 7 days. Patients in the sodium valproate group received 800 mg every 8 hours for 7 days. Patients were observed daily for occurrence of withdrawal symptoms. Nurses who were blinded to the group assignment graded the degree and severity of symptoms. The trial was initially designed so that chlormethiazole and/or tranquilizers were added to sodium valproate when withdrawal symptoms occurred. However, after treating the first few patients, it became evident that additional medications were not needed. In the treatment group, 13 participants received only sodium valproate, 4 patients needed a tranquilizer, 4 needed chlormethiazole, and 1 needed both. In the control group, 1 received only sodium valproate, 4 received a tranquilizer, 14 received chlormethiazole, and 8 needed both. One patient, who entered the study twice, had a withdrawal seizure when in control group and no seizure on second admission in the sodium valproate group. Physical symptoms disappeared quickly in the sodium valproate group (mean of 2 days vs 2.6 days in the control group). Fourteen patients in the control group received chlormethiazole, compared with only 4 patients in sodium valproate group.
Continue to: Conclusion
Conclusion: The researchers concluded that physical symptoms disappeared quicker in the sodium valproate group than in the control group.20
Hillbom et al21 evaluated the efficacy of sodium valproate vs carbamazepine vs placebo to prevent alcohol withdrawal seizures. A total of 138 participants were studied. Forty-three were assigned to the carbamazepine group, 46 to the sodium valproate group, and 49 to the placebo group. The RCT lasted 4 days. The initial medication doses were 1,200 mg/d. Participants in the carbamazepine group experienced more adverse effects than those in the sodium valproate or placebo groups (P < .001). As a result, approximately one-half of the participants in the carbamazepine group stopped taking the medication. This finding was dependent on the dose of carbamazepine; >800 mg/d resulted in poor tolerance to adverse effects. Seizures occurred among patients in all 3 arms of the study; in the sodium valproate group, 1 participant had a seizure vs 2 participants in the carbamazepine group and 3 in the placebo group. On the other hand, DT occurred only in the sodium valproate and placebo groups.
Conclusion: Researchers concluded that when using sodium valproate or carbamazepine to prevent alcohol withdrawal seizures in an outpatient setting, the adverse effects may outweigh the benefits.21
Lamotrigine
The characteristics of the lamotrigine study included in this review are summarized in Table 3.22
Djokić et al22 evaluated the efficiency of lamotrigine in the treatment of DT. A total of 240 participants who met International Classification of Diseases-10 criteria for DT were randomized to a control group that was treated with anticonvulsants according to an NIAAA protocol (2004), or to an experimental group that was treated with lamotrigine. The CIWA-Ar and the Memorial Delirium Assessment Scale (MDAS) were administered for objective assessment of clinical symptoms, superimposed medical complications, general condition of the patient, adverse effects, and mortality rate. Statistically significant differences between the experimental and control groups were apparent after the third day of therapy, when a drop in the average CIWA-Ar score was observed in the experimental group, while the control group still had high scores (P < .01). After the fifth day of treatment, the differences in scores were more apparent, with the experimental group showing CIWA-Ar scores equal to those of persons with mild/moderate DT, while those in the control group still had high scores. After the tenth day, participants in the experimental group did not have any alcohol withdrawal symptoms, while control group participants were just beginning to get out of life-threatening danger. Death occurred in 4.1% of control group participants and 3.4% of experimental group participants; this difference in mortality rate was not statistically significant.
Continue to: Conclusion
Conclusion: Researchers concluded that lamotrigine is significantly efficacious in the treatment of DT, but does not decrease the mortality rate.22
What to know before you prescribe
AWS is a medical emergency that if left untreated leads to several complications and possibly death. Although benzodiazepines are considered the gold standard for treating AWS, the adverse effects associated with their use advocates for finding alternatives. Anticonvulsants can be an effective alternative for treating AWS. In our literature review, we found 16 double-blind RCTs that used an anticonvulsant medication for the treatment of AWS. Of these, 7 involved gabapentin, 6 involved carbamazepine, 1 involved sodium valproate, 1 involved sodium valproate vs carbamazepine, and 1 involved lamotrigine. Overall, the use of anticonvulsants resulted in significant improvement of mild to moderate symptoms of AWS.
There were more studies of carbamazepine and gabapentin than of other anticonvulsants. All the anticonvulsants offered potential benefits. They decreased the probability of a withdrawal seizure and other complications and effectively reduced alcohol cravings. Anticonvulsants were useful for preventing rebound withdrawal symptoms and reducing post-treatment alcohol consumption, especially in patients who had multiple previous withdrawals. Anticonvulsants were particularly helpful for patients with mood disorders such as depression. In the studies we reviewed, anticonvulsants caused less sedation compared with benzodiazepines, and also decreased the occurrence of relapse.
Dosing recommendations. In the studies included in our review, gabapentin was effective at a dosage of 1,600 mg/d (given as 400 mg 4 times a day). This was tapered as follows: 400 mg 4 times a day on Days 1 to 3, 400 mg 3 times a day on Day 4, 400 mg twice a day on Day 5, and 400 mg once a day on Day 6. Carbamazepine was effective at 600 to 800 mg/d, and was tapered by decreasing by 200 mg as follows: 800 mg/d on Days 1 to 3, 600 mg/d on Day 4, 400 mg on Day 5, and 200 mg/d on Day 6. In the reviewed study, the maximum dose of lamotrigine never exceeded 200 mg/d and was administered for 28 days; the exact dosing and taper plan were not described. The dosing of sodium valproate ranged from 1,200 mg/d to 1600 mg/d for 7 days, followed by decreasing by 200 mg each day. The recommended duration of treatment varied; on average for all anticonvulsants, it was 7 to 12 days, followed by a taper. Carbamazepine was shown to be superior to oxazepam in ameliorating the symptoms of AWS.
Adverse effects. When considering the tolerability, adverse effect profile, duration of action, and effectiveness of the anticonvulsants included in our review, gabapentin appears to be the safest agent to choose. For the other anticonvulsants, the risks might outweigh the benefits. Specifically, in a comparison of sodium valproate and carbamazepine, Hillbom et al21 concluded that in doses >800 mg/d, carbamazepine has potential to cause more adverse effects than benefits. However, Agricola et al16 found that carbamazepine had a preferential action on fear, nightmares, and hallucinations.
Continue to: A few caveats
A few caveats
Our review focused a large collection of data from multiple databases and RCTs only. However, its limitations include:
- there was no measure of heterogeneity
- the studies had short treatment duration
- most studies evaluated predominantly male participants
- some studies were underpowered.
Our review laid a groundwork for future research that includes more well-designed RCTs and/or meta-analyses of recent studies that evaluated the use anticonvulsants for treating AWS.
Bottom Line
Evidence suggests certain anticonvulsants may be an effective alternative to benzodiazepines for the treatment of mild to moderate alcohol withdrawal syndrome. Gabapentin may be the safest anticonvulsant to prescribe. Other anticonvulsants to consider include carbamazepine, sodium valproate, and lamotrigine, but for these agents, the risks might outweigh the benefits.
Related Resources
- Myrick H, Anton RF. Treatment of alcohol withdrawal. Alcohol Health Res World. 1998;22(1):38-43. https://pubs.niaaa.nih.gov/publications/arh22-1/38-43.pdf
- World Health Organization. Management of alcohol withdrawal. Published 2012. https://www.who.int/mental_health/mhgap/evidence/alcohol/q2/en/
Drug Brand Names
Carbamazepine • Tegretol
Gabapentin • Neurontin
Lamotrigine • Lamictal
Levetiracetam • Keppra
Lorazepam • Ativan
Oxcarbazepine • Trileptal
Phenytoin • Dilantin
Sodium valproate • Depakote
Acknowledgments
The authors thank Geetha Manikkara, MD, Madhuri Jakkam Setty, MD, and Elizabeth DeOreo, MD, for their efforts with the systematic review research.
1. Trevisan LA, Boutros N, Petrakis IL, et al. Complications of alcohol withdrawal: pathophysiological insights. Alcohol Health Res World. 1998;22(1):61-66.
2. Borghesani P. Alcohol withdrawal. In: Nordstrom KD, Wilson MP, eds. Quick guide to psychiatric emergencies. Springer: 2018;209-215.
3. Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72(8):757-766.
4. Ungur LA, Neuner B, John S, et al. Prevention and therapy of alcohol withdrawal on intensive care units: systematic review of controlled trials. Alcohol Clin Exp Res. 2013;37(4):675-686.
5. Sachdeva A, Choudhary M, Chandra M. Alcohol withdrawal syndrome: benzodiazepines and beyond. J Clin Diagn Res. 2015;9(9):VE01-VE07.
6. Ashton H. Toxicity and adverse consequences of benzodiazepine use. Psychiatr Ann. 1995;25:158-165.
7. Bonnet U, Banger M, Leweke FM, et al. Treatment of acute alcohol withdrawal with gabapentin: results from a controlled two-center trial. J Clin Psychopharmacol. 2003;23(5):514-519.
8. Bonnet U, Specka M, Leweke FM, et al. Gabapentin’s acute effect on mood profile--a controlled study on patients with alcohol withdrawal. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(2):434-438.
9. Myrick H, Anton R, Voronin K, et al. A double-blind evaluation of gabapentin on alcohol effects and drinking in a clinical laboratory paradigm. Alcohol Clin Exp Res. 2007;31(2):221-227.
10. Malcolm R, Myrick L, Veatch L, et al. Self-reported sleep, sleepiness, and repeated alcohol withdrawals: a randomized, double blind, controlled comparison of lorazepam vs gabapentin. J Clin Sleep Med. 2007;3(1):24-32.
11. Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33(9):1582-1588.
12. Stock CJ, Carpenter L, Ying J, et al. Gabapentin versus chlordiazepoxide for outpatient alcohol detoxification treatment. Ann Pharmacother. 2013;47(7-8):961-969.
13. Schacht JP, Anton RF, Randall PK, et al. Effects of a GABA-ergic medication combination and initial alcohol withdrawal severity on cue-elicited brain activation among treatment-seeking alcoholics. Psychopharmacol. 2013;227(4):627-637.
14. Björkqvist SE, Isohanni M, Mäkelä R, et al. Ambulant treatment of alcohol withdrawal symptoms with carbamazepine: a formal multicenter double blind comparison with placebo. Acta Psychiatr Scand. 1976;53(5):333-342.
15. Ritola E, Malinen L. A double-blind comparison of carbamazepine and clomethiazole in the treatment of alcohol withdrawal syndrome. Acta Psychiatr Scand. 1981;64(3):254-259.
16. Agricola R, Mazzarino M, Urani R, et al. Treatment of acute alcohol withdrawal syndrome with carbamazepine: a double-blind comparison with tiapride. J Int Med Res. 1982;10(3):160-165.
17. Stuppaeck CH, Pycha R, Miller C, et al. Carbamazepine versus oxazepam in the treatment of alcohol withdrawal: a double-blind study. Alcohol Alcohol. 1992;27(2):153-158.
18. Malcolm R, Myrick H, Roberts J, et al. The effects of carbamazepine and lorazepam on single vs multiple previous withdrawals in an outpatient randomized trial. J Gen Intern Med. 2002;17(5):349-355.
19. Malcolm R, Myrick H, Roberts J, et al. The differential effects of medications on mood, sleep disturbance, and work ability in outpatient alcohol detoxification. Am J Addict. 2002;11(2):141-150.
20. Lambie D, Johnson R, Vijayasenan M, et al. Sodium valproate in the treatment of the alcohol withdrawal syndrome. Aust N Z J. 1980;14(3):213-215.
21. Hillbom M, Tokola R, Kuusela V, et al. Prevention of alcohol withdrawal seizures with carbamazepine and valproic acid. Alcohol. 1989;6(3):223-226.
22. Djokic
1. Trevisan LA, Boutros N, Petrakis IL, et al. Complications of alcohol withdrawal: pathophysiological insights. Alcohol Health Res World. 1998;22(1):61-66.
2. Borghesani P. Alcohol withdrawal. In: Nordstrom KD, Wilson MP, eds. Quick guide to psychiatric emergencies. Springer: 2018;209-215.
3. Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72(8):757-766.
4. Ungur LA, Neuner B, John S, et al. Prevention and therapy of alcohol withdrawal on intensive care units: systematic review of controlled trials. Alcohol Clin Exp Res. 2013;37(4):675-686.
5. Sachdeva A, Choudhary M, Chandra M. Alcohol withdrawal syndrome: benzodiazepines and beyond. J Clin Diagn Res. 2015;9(9):VE01-VE07.
6. Ashton H. Toxicity and adverse consequences of benzodiazepine use. Psychiatr Ann. 1995;25:158-165.
7. Bonnet U, Banger M, Leweke FM, et al. Treatment of acute alcohol withdrawal with gabapentin: results from a controlled two-center trial. J Clin Psychopharmacol. 2003;23(5):514-519.
8. Bonnet U, Specka M, Leweke FM, et al. Gabapentin’s acute effect on mood profile--a controlled study on patients with alcohol withdrawal. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(2):434-438.
9. Myrick H, Anton R, Voronin K, et al. A double-blind evaluation of gabapentin on alcohol effects and drinking in a clinical laboratory paradigm. Alcohol Clin Exp Res. 2007;31(2):221-227.
10. Malcolm R, Myrick L, Veatch L, et al. Self-reported sleep, sleepiness, and repeated alcohol withdrawals: a randomized, double blind, controlled comparison of lorazepam vs gabapentin. J Clin Sleep Med. 2007;3(1):24-32.
11. Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33(9):1582-1588.
12. Stock CJ, Carpenter L, Ying J, et al. Gabapentin versus chlordiazepoxide for outpatient alcohol detoxification treatment. Ann Pharmacother. 2013;47(7-8):961-969.
13. Schacht JP, Anton RF, Randall PK, et al. Effects of a GABA-ergic medication combination and initial alcohol withdrawal severity on cue-elicited brain activation among treatment-seeking alcoholics. Psychopharmacol. 2013;227(4):627-637.
14. Björkqvist SE, Isohanni M, Mäkelä R, et al. Ambulant treatment of alcohol withdrawal symptoms with carbamazepine: a formal multicenter double blind comparison with placebo. Acta Psychiatr Scand. 1976;53(5):333-342.
15. Ritola E, Malinen L. A double-blind comparison of carbamazepine and clomethiazole in the treatment of alcohol withdrawal syndrome. Acta Psychiatr Scand. 1981;64(3):254-259.
16. Agricola R, Mazzarino M, Urani R, et al. Treatment of acute alcohol withdrawal syndrome with carbamazepine: a double-blind comparison with tiapride. J Int Med Res. 1982;10(3):160-165.
17. Stuppaeck CH, Pycha R, Miller C, et al. Carbamazepine versus oxazepam in the treatment of alcohol withdrawal: a double-blind study. Alcohol Alcohol. 1992;27(2):153-158.
18. Malcolm R, Myrick H, Roberts J, et al. The effects of carbamazepine and lorazepam on single vs multiple previous withdrawals in an outpatient randomized trial. J Gen Intern Med. 2002;17(5):349-355.
19. Malcolm R, Myrick H, Roberts J, et al. The differential effects of medications on mood, sleep disturbance, and work ability in outpatient alcohol detoxification. Am J Addict. 2002;11(2):141-150.
20. Lambie D, Johnson R, Vijayasenan M, et al. Sodium valproate in the treatment of the alcohol withdrawal syndrome. Aust N Z J. 1980;14(3):213-215.
21. Hillbom M, Tokola R, Kuusela V, et al. Prevention of alcohol withdrawal seizures with carbamazepine and valproic acid. Alcohol. 1989;6(3):223-226.
22. Djokic
Psychiatric consequences of nitrous oxide abuse
We would like to describe the case of a patient we treated who developed neuropsychiatric symptoms secondary to recreational use of nitrous oxide (N2O).
Mr. N, a 24-year-old military veteran, presented to the emergency department (ED) with symptoms of numbness, tingling of his entire body, and difficulty walking for the past 3 days. His family recently became concerned when they noted changes in his personality and behavior, including increased irritability, verbal aggression, and paranoia. The family reported that before the recent changes, Mr. N had typically been calm and had a pleasant temperament. When Mr. N’s symptoms progressed to difficulty ambulating, his family brought him to the ED for evaluation.
During his interview, Mr. N reported that he started using N2O 2 years ago for recreational purposes because he learned it is legal to purchase and undetectable on a urine drug screen. He said he had been using >100 N2O canisters per day and had spent approximately $15,000 over the past few months. His use had increasingly escalated up to 3 days before his visit to the ED, which was the last day he used N2O.
Mr. N was admitted to the inpatient medical service. Laboratory testing revealed a low-normal vitamin B12 level of 254 pg/mL (normal range: 200 to 900 pg/mL), an elevated methylmalonic acid blood level of 2,491 nmol/L (normal range: 73 to 376 nmol/L), and an elevated homocysteine blood level of 22.4 μmol/L (normal range: 0 to 15 μmol/L). Magnetic resonance imaging studies showed hyperintensity regions on his cervical spine from the C1 to C6 levels. These changes suggested demyelination due to vitamin B12 deficiency from N2O abuse.
Mr. N was started on vitamin B12 injections and physical therapy, which led to the resolution of his concerning neurologic symptoms. A few weeks after admission, he was discharged with outpatient follow-up services. Unfortunately, he was lost to follow-up.
Approximately 1 year later, Mr. N returned to the ED with anxiety and paranoid ideation. Medical workup at the time was normal (including vitamin B12 and methylmalonic acid blood levels). He denied any recent substance use and was admitted voluntarily to the psychiatric unit. He declined the recommended treatment of risperidone. Because he showed no signs or symptoms that warranted involuntary retention, he was discharged. Over the next few months, he had 4 visits to the ED with similar concerns and poor adherence to outpatient treatment.
On Mr. N’s fourth admission, he agreed to a course of long-acting injectable paliperidone and escitalopram to target his psychotic and anxious symptoms. These treatments stabilized him, and he was discharged. Neuropsychological testing later showed impairment across several cognitive domains, including memory, processing speed, attention, and executive functioning.
Continue to: Identifying N2O use
Identifying N2O use. N2O is not detected on routine drug screen panels. Obtaining a careful psychiatric and substance use history, as well as conducting a neurologic assessment, are helpful to identify N2O use. Both acute and chronic inhalation of N2O can result in vitamin B12 deficiency with hematologic (megaloblastic anemia), neurologic (subacute combined degeneration of spinal cord, motor-sensory polyneuropathy), and psychiatric sequelae (memory loss, depression, hypomania, transient psychosis).1 Patients who exhibit these changes warrant workup for vitamin B12 deficiency, which includes testing for B12, homocysteine, and methylmalonic acid blood levels. Magnetic resonance imaging should be considered for patients who exhibit neurologic symptoms.
The means by which N2O causes neuropsychiatric changes have been explored in the literature. There is general consensus that part of N2O’s deleterious effects is due to the inactivation of vitamin B12 by the irreversible oxidation of Cob(I)alamin to Cob(III)alamin.1
Treatment. The recommended treatment is high-dose oral or parenteral vitamin B12.1 Repletion of vitamin B12 is believed to reverse the course of illness. However, our patient’s symptoms of paranoia and delusions persisted despite resolution of his neurologic symptoms after the underlying vitamin B12 deficiency was corrected.
Due to N2O’s wide availability and growing recreational use, it is important for clinicians to ask their patients about their use of this substance. The abuse of N2O remains an important topic that requires further research, particularly in adolescents, who are still undergoing significant brain development.2,3
Daniel Roberts, MD, MSW
PGY-3 Psychiatric Resident
Department of Psychiatry
New York University Grossman School of Medicine
New York, New York
Pantea Farahmand, MA, MD
Assistant Professor
Department of Psychiatry
New York University Grossman School of Medicine
Inpatient Psychiatrist
Veterans Administration New York Harbor Healthcare System
New York, New York
Adam Wolkin, MD
Associate Professor and Vice Chair
New York University Grossman School of Medicine
Associate Chief of Staff for Mental Health
Veterans Administration New York Harbor Healthcare System
New York, New York
Disclosures: The authors report no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
1. Thompson AG, Leite MI, Lunn MP, et al. Whippits, nitrous oxide and the dangers of legal highs. Pract Neurol. 2015;15(3):207-209.
2. Global Drug Survey 2017. Global Drug Survey. Published May 24, 2017. Accessed January 12, 2021. https://www.globaldrugsurvey.com/past-findings/gds2017-launch/results-released/
3. Kaar SJ, Ferris J, Waldron J, et al. Up: the rise of nitrous oxide abuse. An international survey of contemporary nitrous oxide use. J Psychopharmacol. 2016;30(4):395-401.
We would like to describe the case of a patient we treated who developed neuropsychiatric symptoms secondary to recreational use of nitrous oxide (N2O).
Mr. N, a 24-year-old military veteran, presented to the emergency department (ED) with symptoms of numbness, tingling of his entire body, and difficulty walking for the past 3 days. His family recently became concerned when they noted changes in his personality and behavior, including increased irritability, verbal aggression, and paranoia. The family reported that before the recent changes, Mr. N had typically been calm and had a pleasant temperament. When Mr. N’s symptoms progressed to difficulty ambulating, his family brought him to the ED for evaluation.
During his interview, Mr. N reported that he started using N2O 2 years ago for recreational purposes because he learned it is legal to purchase and undetectable on a urine drug screen. He said he had been using >100 N2O canisters per day and had spent approximately $15,000 over the past few months. His use had increasingly escalated up to 3 days before his visit to the ED, which was the last day he used N2O.
Mr. N was admitted to the inpatient medical service. Laboratory testing revealed a low-normal vitamin B12 level of 254 pg/mL (normal range: 200 to 900 pg/mL), an elevated methylmalonic acid blood level of 2,491 nmol/L (normal range: 73 to 376 nmol/L), and an elevated homocysteine blood level of 22.4 μmol/L (normal range: 0 to 15 μmol/L). Magnetic resonance imaging studies showed hyperintensity regions on his cervical spine from the C1 to C6 levels. These changes suggested demyelination due to vitamin B12 deficiency from N2O abuse.
Mr. N was started on vitamin B12 injections and physical therapy, which led to the resolution of his concerning neurologic symptoms. A few weeks after admission, he was discharged with outpatient follow-up services. Unfortunately, he was lost to follow-up.
Approximately 1 year later, Mr. N returned to the ED with anxiety and paranoid ideation. Medical workup at the time was normal (including vitamin B12 and methylmalonic acid blood levels). He denied any recent substance use and was admitted voluntarily to the psychiatric unit. He declined the recommended treatment of risperidone. Because he showed no signs or symptoms that warranted involuntary retention, he was discharged. Over the next few months, he had 4 visits to the ED with similar concerns and poor adherence to outpatient treatment.
On Mr. N’s fourth admission, he agreed to a course of long-acting injectable paliperidone and escitalopram to target his psychotic and anxious symptoms. These treatments stabilized him, and he was discharged. Neuropsychological testing later showed impairment across several cognitive domains, including memory, processing speed, attention, and executive functioning.
Continue to: Identifying N2O use
Identifying N2O use. N2O is not detected on routine drug screen panels. Obtaining a careful psychiatric and substance use history, as well as conducting a neurologic assessment, are helpful to identify N2O use. Both acute and chronic inhalation of N2O can result in vitamin B12 deficiency with hematologic (megaloblastic anemia), neurologic (subacute combined degeneration of spinal cord, motor-sensory polyneuropathy), and psychiatric sequelae (memory loss, depression, hypomania, transient psychosis).1 Patients who exhibit these changes warrant workup for vitamin B12 deficiency, which includes testing for B12, homocysteine, and methylmalonic acid blood levels. Magnetic resonance imaging should be considered for patients who exhibit neurologic symptoms.
The means by which N2O causes neuropsychiatric changes have been explored in the literature. There is general consensus that part of N2O’s deleterious effects is due to the inactivation of vitamin B12 by the irreversible oxidation of Cob(I)alamin to Cob(III)alamin.1
Treatment. The recommended treatment is high-dose oral or parenteral vitamin B12.1 Repletion of vitamin B12 is believed to reverse the course of illness. However, our patient’s symptoms of paranoia and delusions persisted despite resolution of his neurologic symptoms after the underlying vitamin B12 deficiency was corrected.
Due to N2O’s wide availability and growing recreational use, it is important for clinicians to ask their patients about their use of this substance. The abuse of N2O remains an important topic that requires further research, particularly in adolescents, who are still undergoing significant brain development.2,3
Daniel Roberts, MD, MSW
PGY-3 Psychiatric Resident
Department of Psychiatry
New York University Grossman School of Medicine
New York, New York
Pantea Farahmand, MA, MD
Assistant Professor
Department of Psychiatry
New York University Grossman School of Medicine
Inpatient Psychiatrist
Veterans Administration New York Harbor Healthcare System
New York, New York
Adam Wolkin, MD
Associate Professor and Vice Chair
New York University Grossman School of Medicine
Associate Chief of Staff for Mental Health
Veterans Administration New York Harbor Healthcare System
New York, New York
Disclosures: The authors report no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
We would like to describe the case of a patient we treated who developed neuropsychiatric symptoms secondary to recreational use of nitrous oxide (N2O).
Mr. N, a 24-year-old military veteran, presented to the emergency department (ED) with symptoms of numbness, tingling of his entire body, and difficulty walking for the past 3 days. His family recently became concerned when they noted changes in his personality and behavior, including increased irritability, verbal aggression, and paranoia. The family reported that before the recent changes, Mr. N had typically been calm and had a pleasant temperament. When Mr. N’s symptoms progressed to difficulty ambulating, his family brought him to the ED for evaluation.
During his interview, Mr. N reported that he started using N2O 2 years ago for recreational purposes because he learned it is legal to purchase and undetectable on a urine drug screen. He said he had been using >100 N2O canisters per day and had spent approximately $15,000 over the past few months. His use had increasingly escalated up to 3 days before his visit to the ED, which was the last day he used N2O.
Mr. N was admitted to the inpatient medical service. Laboratory testing revealed a low-normal vitamin B12 level of 254 pg/mL (normal range: 200 to 900 pg/mL), an elevated methylmalonic acid blood level of 2,491 nmol/L (normal range: 73 to 376 nmol/L), and an elevated homocysteine blood level of 22.4 μmol/L (normal range: 0 to 15 μmol/L). Magnetic resonance imaging studies showed hyperintensity regions on his cervical spine from the C1 to C6 levels. These changes suggested demyelination due to vitamin B12 deficiency from N2O abuse.
Mr. N was started on vitamin B12 injections and physical therapy, which led to the resolution of his concerning neurologic symptoms. A few weeks after admission, he was discharged with outpatient follow-up services. Unfortunately, he was lost to follow-up.
Approximately 1 year later, Mr. N returned to the ED with anxiety and paranoid ideation. Medical workup at the time was normal (including vitamin B12 and methylmalonic acid blood levels). He denied any recent substance use and was admitted voluntarily to the psychiatric unit. He declined the recommended treatment of risperidone. Because he showed no signs or symptoms that warranted involuntary retention, he was discharged. Over the next few months, he had 4 visits to the ED with similar concerns and poor adherence to outpatient treatment.
On Mr. N’s fourth admission, he agreed to a course of long-acting injectable paliperidone and escitalopram to target his psychotic and anxious symptoms. These treatments stabilized him, and he was discharged. Neuropsychological testing later showed impairment across several cognitive domains, including memory, processing speed, attention, and executive functioning.
Continue to: Identifying N2O use
Identifying N2O use. N2O is not detected on routine drug screen panels. Obtaining a careful psychiatric and substance use history, as well as conducting a neurologic assessment, are helpful to identify N2O use. Both acute and chronic inhalation of N2O can result in vitamin B12 deficiency with hematologic (megaloblastic anemia), neurologic (subacute combined degeneration of spinal cord, motor-sensory polyneuropathy), and psychiatric sequelae (memory loss, depression, hypomania, transient psychosis).1 Patients who exhibit these changes warrant workup for vitamin B12 deficiency, which includes testing for B12, homocysteine, and methylmalonic acid blood levels. Magnetic resonance imaging should be considered for patients who exhibit neurologic symptoms.
The means by which N2O causes neuropsychiatric changes have been explored in the literature. There is general consensus that part of N2O’s deleterious effects is due to the inactivation of vitamin B12 by the irreversible oxidation of Cob(I)alamin to Cob(III)alamin.1
Treatment. The recommended treatment is high-dose oral or parenteral vitamin B12.1 Repletion of vitamin B12 is believed to reverse the course of illness. However, our patient’s symptoms of paranoia and delusions persisted despite resolution of his neurologic symptoms after the underlying vitamin B12 deficiency was corrected.
Due to N2O’s wide availability and growing recreational use, it is important for clinicians to ask their patients about their use of this substance. The abuse of N2O remains an important topic that requires further research, particularly in adolescents, who are still undergoing significant brain development.2,3
Daniel Roberts, MD, MSW
PGY-3 Psychiatric Resident
Department of Psychiatry
New York University Grossman School of Medicine
New York, New York
Pantea Farahmand, MA, MD
Assistant Professor
Department of Psychiatry
New York University Grossman School of Medicine
Inpatient Psychiatrist
Veterans Administration New York Harbor Healthcare System
New York, New York
Adam Wolkin, MD
Associate Professor and Vice Chair
New York University Grossman School of Medicine
Associate Chief of Staff for Mental Health
Veterans Administration New York Harbor Healthcare System
New York, New York
Disclosures: The authors report no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
1. Thompson AG, Leite MI, Lunn MP, et al. Whippits, nitrous oxide and the dangers of legal highs. Pract Neurol. 2015;15(3):207-209.
2. Global Drug Survey 2017. Global Drug Survey. Published May 24, 2017. Accessed January 12, 2021. https://www.globaldrugsurvey.com/past-findings/gds2017-launch/results-released/
3. Kaar SJ, Ferris J, Waldron J, et al. Up: the rise of nitrous oxide abuse. An international survey of contemporary nitrous oxide use. J Psychopharmacol. 2016;30(4):395-401.
1. Thompson AG, Leite MI, Lunn MP, et al. Whippits, nitrous oxide and the dangers of legal highs. Pract Neurol. 2015;15(3):207-209.
2. Global Drug Survey 2017. Global Drug Survey. Published May 24, 2017. Accessed January 12, 2021. https://www.globaldrugsurvey.com/past-findings/gds2017-launch/results-released/
3. Kaar SJ, Ferris J, Waldron J, et al. Up: the rise of nitrous oxide abuse. An international survey of contemporary nitrous oxide use. J Psychopharmacol. 2016;30(4):395-401.
Plagues that will haunt us long after the COVID-19 pandemic is gone
As we struggle to gradually emerge from the horrid coronavirus disease 2019 (COVID-19) pandemic that has disrupted our lives and killed hundreds of thousands of people in the United States, we harbor the hope that life will return to “normal.” But while it will certainly be a great relief to put this deadly virus behind us, many other epidemics will continue to plague our society and taint our culture.
Scientific ingenuity has led to the development of several vaccines in record time (aka “warp speed”) that will help defeat the deadly scourge of COVID-19. The pandemic is likely to peter out 2 years after its onset. We will all be grateful for such a rapid resolution of the worst health crisis the world has faced in a century, which will enable medical, economic, and social recovery. But as we eventually resume our lives and rejoice in resuming the pursuit of happiness, we will quickly realize that all is not well in our society just because the viral pandemic is gone.
Perhaps the ordeal of the COVID-19 pandemic, and the agony that was universally shared, will open our collective eyes to a jarring reality: many other epidemics will continue to permeate society and cause endless grief and suffering to many of our fellow humans. And thanks to our training as psychiatric physicians, we have developed extra “receptors” to the darker side of the human condition. As we help many of our psychiatric patients rendered sicker under the unbearable stress of the pandemic, we must not overlook the plight of so many others who do not show up in our clinics for health care, yet suffer enormously but imperceptibly. And no vaccine can come to the rescue of those who continue to live in quiet desperation.
Long-standing epidemics
It is truly unfortunate that many of the epidemics I am referring to have persisted for so long that they have become “fixtures” of contemporary societies. They have become “endemic epidemics” with no urgency to squelch them, as with the COVID-19 pandemic. The benign neglect that perpetuates these serious epidemics has had a malignant effect of “grudging resignation” that nothing can be done to reverse them. Unlike the viral epidemic that engulfed everyone around the world and triggered a massive and unified push to defeat the virus, these long-standing epidemics continue to afflict subgroups who are left to fend for themselves. These individuals deserve our empathy and warrant our determination to lift them from their miserable existence.
Consider some of the widespread epidemics that preceded the pandemic and will, in all likelihood, persist after the pandemic’s burden is lifted:
- millions of people living in poverty and hunger
- widespread racism
- smoldering social injustice
- appalling human trafficking, especially targeting children and women
- child abuse and neglect that leads to psychosis, depression, and suicide in adulthood
- gun violence, which kills many innocent people
- domestic violence that inflicts both physical and mental harm on families
- suicide, both attempts and completions, which continues to increase annually
- the festering stigma of mental illness that adds insult to injury for psychiatric patients
- alcohol and drug addictions, which destroy lives and corrode the fabric of society
- lack of access to mental health care for millions of people who need it
- lack of parity for psychiatric disorders, which is so unjust for our patients
- venomous political hatred and hyperpartisanship, which permeates our culture and can lead to violence, as we recently witnessed
- physician burnout, due to many causes, even before the stresses of COVID-19
- the ongoing agony of wars and terrorism, including dangerous cyberattacks
- the deleterious effect of social media on everyone, especially children.
Most of these epidemics claim thousands of lives each year, and yet no concerted public health effort is being mounted to counteract them, as we are seeing with the COVID-19 pandemic. Much is being written about each of them, but there has been little tangible action, so they persist. They have become a perpetual underbelly of our society that is essentially ignored or simply given the usual lip service.
It will take a herculean effort by policymakers, the judicial system, the medical establishment, and faith organizations to put an end to these life-threatening epidemics. It may appear too daunting to mount a war on so many fronts, but that should not deter us all from launching a strategic plan to create meaningful tactics and solutions. And just as was done with the COVID-19 pandemic, both mitigation measures as well as effective interventions must be employed in this campaign against the epidemic “hydra.”
Continue to: It is tragic...
It is tragic that so many fellow humans are allowed to suffer or die while the rest of us watch, or worse, turn a blind eye and never get involved. A civilized society must never neglect so many of its suffering citizens. As psychiatrists, we are aware of those human travesties around us, but we are often so overwhelmed with our work and personal responsibilities that few of us are passionately advocating or setting aside some time for those victimized by one or more of these endemic pandemics. And unless we all decide to be actively, meaningfully involved, many lives will continue to be lost every day, but without the daily “casualty count” displayed on television screens, as is the case with COVID-19 causalities.
Regrettably, maybe that old saw is true: out of sight, out of mind.
As we struggle to gradually emerge from the horrid coronavirus disease 2019 (COVID-19) pandemic that has disrupted our lives and killed hundreds of thousands of people in the United States, we harbor the hope that life will return to “normal.” But while it will certainly be a great relief to put this deadly virus behind us, many other epidemics will continue to plague our society and taint our culture.
Scientific ingenuity has led to the development of several vaccines in record time (aka “warp speed”) that will help defeat the deadly scourge of COVID-19. The pandemic is likely to peter out 2 years after its onset. We will all be grateful for such a rapid resolution of the worst health crisis the world has faced in a century, which will enable medical, economic, and social recovery. But as we eventually resume our lives and rejoice in resuming the pursuit of happiness, we will quickly realize that all is not well in our society just because the viral pandemic is gone.
Perhaps the ordeal of the COVID-19 pandemic, and the agony that was universally shared, will open our collective eyes to a jarring reality: many other epidemics will continue to permeate society and cause endless grief and suffering to many of our fellow humans. And thanks to our training as psychiatric physicians, we have developed extra “receptors” to the darker side of the human condition. As we help many of our psychiatric patients rendered sicker under the unbearable stress of the pandemic, we must not overlook the plight of so many others who do not show up in our clinics for health care, yet suffer enormously but imperceptibly. And no vaccine can come to the rescue of those who continue to live in quiet desperation.
Long-standing epidemics
It is truly unfortunate that many of the epidemics I am referring to have persisted for so long that they have become “fixtures” of contemporary societies. They have become “endemic epidemics” with no urgency to squelch them, as with the COVID-19 pandemic. The benign neglect that perpetuates these serious epidemics has had a malignant effect of “grudging resignation” that nothing can be done to reverse them. Unlike the viral epidemic that engulfed everyone around the world and triggered a massive and unified push to defeat the virus, these long-standing epidemics continue to afflict subgroups who are left to fend for themselves. These individuals deserve our empathy and warrant our determination to lift them from their miserable existence.
Consider some of the widespread epidemics that preceded the pandemic and will, in all likelihood, persist after the pandemic’s burden is lifted:
- millions of people living in poverty and hunger
- widespread racism
- smoldering social injustice
- appalling human trafficking, especially targeting children and women
- child abuse and neglect that leads to psychosis, depression, and suicide in adulthood
- gun violence, which kills many innocent people
- domestic violence that inflicts both physical and mental harm on families
- suicide, both attempts and completions, which continues to increase annually
- the festering stigma of mental illness that adds insult to injury for psychiatric patients
- alcohol and drug addictions, which destroy lives and corrode the fabric of society
- lack of access to mental health care for millions of people who need it
- lack of parity for psychiatric disorders, which is so unjust for our patients
- venomous political hatred and hyperpartisanship, which permeates our culture and can lead to violence, as we recently witnessed
- physician burnout, due to many causes, even before the stresses of COVID-19
- the ongoing agony of wars and terrorism, including dangerous cyberattacks
- the deleterious effect of social media on everyone, especially children.
Most of these epidemics claim thousands of lives each year, and yet no concerted public health effort is being mounted to counteract them, as we are seeing with the COVID-19 pandemic. Much is being written about each of them, but there has been little tangible action, so they persist. They have become a perpetual underbelly of our society that is essentially ignored or simply given the usual lip service.
It will take a herculean effort by policymakers, the judicial system, the medical establishment, and faith organizations to put an end to these life-threatening epidemics. It may appear too daunting to mount a war on so many fronts, but that should not deter us all from launching a strategic plan to create meaningful tactics and solutions. And just as was done with the COVID-19 pandemic, both mitigation measures as well as effective interventions must be employed in this campaign against the epidemic “hydra.”
Continue to: It is tragic...
It is tragic that so many fellow humans are allowed to suffer or die while the rest of us watch, or worse, turn a blind eye and never get involved. A civilized society must never neglect so many of its suffering citizens. As psychiatrists, we are aware of those human travesties around us, but we are often so overwhelmed with our work and personal responsibilities that few of us are passionately advocating or setting aside some time for those victimized by one or more of these endemic pandemics. And unless we all decide to be actively, meaningfully involved, many lives will continue to be lost every day, but without the daily “casualty count” displayed on television screens, as is the case with COVID-19 causalities.
Regrettably, maybe that old saw is true: out of sight, out of mind.
As we struggle to gradually emerge from the horrid coronavirus disease 2019 (COVID-19) pandemic that has disrupted our lives and killed hundreds of thousands of people in the United States, we harbor the hope that life will return to “normal.” But while it will certainly be a great relief to put this deadly virus behind us, many other epidemics will continue to plague our society and taint our culture.
Scientific ingenuity has led to the development of several vaccines in record time (aka “warp speed”) that will help defeat the deadly scourge of COVID-19. The pandemic is likely to peter out 2 years after its onset. We will all be grateful for such a rapid resolution of the worst health crisis the world has faced in a century, which will enable medical, economic, and social recovery. But as we eventually resume our lives and rejoice in resuming the pursuit of happiness, we will quickly realize that all is not well in our society just because the viral pandemic is gone.
Perhaps the ordeal of the COVID-19 pandemic, and the agony that was universally shared, will open our collective eyes to a jarring reality: many other epidemics will continue to permeate society and cause endless grief and suffering to many of our fellow humans. And thanks to our training as psychiatric physicians, we have developed extra “receptors” to the darker side of the human condition. As we help many of our psychiatric patients rendered sicker under the unbearable stress of the pandemic, we must not overlook the plight of so many others who do not show up in our clinics for health care, yet suffer enormously but imperceptibly. And no vaccine can come to the rescue of those who continue to live in quiet desperation.
Long-standing epidemics
It is truly unfortunate that many of the epidemics I am referring to have persisted for so long that they have become “fixtures” of contemporary societies. They have become “endemic epidemics” with no urgency to squelch them, as with the COVID-19 pandemic. The benign neglect that perpetuates these serious epidemics has had a malignant effect of “grudging resignation” that nothing can be done to reverse them. Unlike the viral epidemic that engulfed everyone around the world and triggered a massive and unified push to defeat the virus, these long-standing epidemics continue to afflict subgroups who are left to fend for themselves. These individuals deserve our empathy and warrant our determination to lift them from their miserable existence.
Consider some of the widespread epidemics that preceded the pandemic and will, in all likelihood, persist after the pandemic’s burden is lifted:
- millions of people living in poverty and hunger
- widespread racism
- smoldering social injustice
- appalling human trafficking, especially targeting children and women
- child abuse and neglect that leads to psychosis, depression, and suicide in adulthood
- gun violence, which kills many innocent people
- domestic violence that inflicts both physical and mental harm on families
- suicide, both attempts and completions, which continues to increase annually
- the festering stigma of mental illness that adds insult to injury for psychiatric patients
- alcohol and drug addictions, which destroy lives and corrode the fabric of society
- lack of access to mental health care for millions of people who need it
- lack of parity for psychiatric disorders, which is so unjust for our patients
- venomous political hatred and hyperpartisanship, which permeates our culture and can lead to violence, as we recently witnessed
- physician burnout, due to many causes, even before the stresses of COVID-19
- the ongoing agony of wars and terrorism, including dangerous cyberattacks
- the deleterious effect of social media on everyone, especially children.
Most of these epidemics claim thousands of lives each year, and yet no concerted public health effort is being mounted to counteract them, as we are seeing with the COVID-19 pandemic. Much is being written about each of them, but there has been little tangible action, so they persist. They have become a perpetual underbelly of our society that is essentially ignored or simply given the usual lip service.
It will take a herculean effort by policymakers, the judicial system, the medical establishment, and faith organizations to put an end to these life-threatening epidemics. It may appear too daunting to mount a war on so many fronts, but that should not deter us all from launching a strategic plan to create meaningful tactics and solutions. And just as was done with the COVID-19 pandemic, both mitigation measures as well as effective interventions must be employed in this campaign against the epidemic “hydra.”
Continue to: It is tragic...
It is tragic that so many fellow humans are allowed to suffer or die while the rest of us watch, or worse, turn a blind eye and never get involved. A civilized society must never neglect so many of its suffering citizens. As psychiatrists, we are aware of those human travesties around us, but we are often so overwhelmed with our work and personal responsibilities that few of us are passionately advocating or setting aside some time for those victimized by one or more of these endemic pandemics. And unless we all decide to be actively, meaningfully involved, many lives will continue to be lost every day, but without the daily “casualty count” displayed on television screens, as is the case with COVID-19 causalities.
Regrettably, maybe that old saw is true: out of sight, out of mind.
Management of major depressive disorder with psychotic features
Mrs. C, age 56, has a history of major depressive disorder (MDD). She has been stable for 5 years without medication. Six months ago, she presented to you, along with her son, seeking help. She reported that she had been experiencing insomnia, fatigue, and was not engaging in hobbies. Her son told you that his mother had lost weight and had been avoiding family dinners. Mrs. C reported recurrent thoughts of dying and heard voices vividly telling her that she was a burden and that her family would be better off without her. However, there was no imminent danger of self-harm. At that appointment, you initiated
Since that time, Mrs. C has followed up with you monthly with good response to the medications. Currently, she states her depression is much improved, and she denies hearing voices for approximately 5 months.
Based on her presentation and response, what do the data suggest about her length of treatment, and when should you consider tapering the antipsychotic medication?
In DSM-5, MDD with psychotic features is a severe subtype of MDD that is defined as a major depressive episode characterized by delusions and/or hallucinations.1 In the general population, the lifetime prevalence of this disorder varies from 0.35% to 1%, and the rate is higher in older patients.2 Risk factors include female gender, family history, and concomitant bipolar disorder.2
Epidemiologic studies have shown that psychotic features can occur in 15% to 20% of patients with MDD. The psychotic features that occur during these episodes are delusions and hallucinations.1 These features can be either mood-congruent (related to the depressive themes of worthlessness or guilt) or mood-incongruent (ie, unrelated to depressive themes).1
Treatment options: ECT or pharmacotherapy
Guidelines from the American Psychiatric Association3 and the National Institute for Clinical Excellence4 recommend treating depression with psychosis with electroconvulsive therapy (ECT) or with combined antidepressant and antipsychotic medications as first-line options. The Texas Medication Algorithm Project (TMAP) Algorithm for MDD,5 which closely focuses on treatment of MDD with psychotic features, can be used for treatment decisions (see Related Resources).
Electroconvulsive therapy is known to be efficacious in treating patients with MDD with psychotic features and should be considered as a treatment option. However, medication therapy is often chosen as the initial treatment due to the limitations of ECT, including accessibility, cost, and patient preference. However, in certain cases, ECT is the preferred option because it can provide rapid and significant improvement in patients with severe psychosis, suicidality, or risk of imminent harm.
Continue to: Pharmacotherapy
Pharmacotherapy for the treatment of MDD with psychotic features should consist of a combination of an antidepressant and antipsychotic medication. This combination has been shown to be more effective than either agent alone. Some combinations have been studied specifically for MDD with psychosis. The Study of the Pharmacotherapy of Psychotic Depression (STOP-PD), a 12-week, double-blind, randomized controlled trial, found that the combination of sertraline and olanzapine was efficacious and superior to monotherapy with olanzapine in an acute setting.6 In another study, the combination of olanzapine and
How long should treatment last?
The optimal timeline for treating patients with MDD with psychotic features is unknown. According to the TMAP algorithm and expert opinion, the continuation phase of pharmacotherapy should include treatment for at least 4 months with an antipsychotic medication and at least 2 years to lifetime treatment with an antidepressant.5 The STOP-PD II study, which was a continuation of the 12-week STOP-PD study, examined antipsychotic duration to determine the effects of continuing olanzapine once an episode of psychotic depression had responded to olanzapine and sertraline.11 Patients who had achieved remission after receiving olanzapine and sertraline were randomized to continue to receive this combination or to receive sertraline plus placebo for 36 weeks. The primary outcome was relapse, which was broadly defined as 1 of the following11:
- a Structured Clinical Interview for the DSM (SCID)-rated assessment that revealed the patient had enough symptoms to meet criteria for a DSM-IV major depressive episode
- a 17-item HAM-D scoren of ≥18
- SCID-rated psychosis
- other significant clinical worsening, defined as having a suicide plan or attempting suicide, developing SCID-rated symptoms of mania or hypomania, or being hospitalized in a psychiatric unit.
Compared with sertraline plus placebo, continuing sertraline plus olanzapine reduced the risk of relapse over 36 weeks (hazard ratio, 0.25; 95% confidence interval, 0.13 to 0.48; P < .001).11 However, as expected, the incidence of adverse effects such as weight gain and parkinsonism was higher in the olanzapine group. Therefore, it is important to consider the potential long-term adverse effects of continuing antipsychotic medications.
Weighing the evidence
Electroconvulsive therapy is considered a first-line treatment option for MDD with psychotic features; however, because of limitations associated with this approach, antidepressants plus antipsychotics are often utilized as an initial treatment.
Continue to: CASE
CASE CONTINUED
Based on the results of the STOP-PD and STOP-PD II trials, Mrs. C should be continued on sertraline plus olanzapine for at least another 3 to 6 months before an olanzapine taper should be considered. At that time, the risks and benefits of a taper vs continuing therapy should be considered. Given her history of MDD and the severity of this most recent episode, sertraline therapy should be continued for at least 2 years, and possibly indefinitely.
Related Resources
- Texas Medication Algorithm Project. Algorithm for the treatment of major depressive disorder with psychotic features. https://chsciowa.org/sites/chsciowa.org/files/resource/files/9_-_depression_med_algorithm_supplement.pdf
- Dold M, Bartova L, Kautzky A, et al. Psychotic features in patients with major depressive disorder: a report from the European Group for the Study of Resistant Depression. J Clin Psychiatry. 2019;80(1):17m12090. doi: 10.4088/ JCP.17m12090
- Flint AJ, Meyers BS, Rothschild AJ, et al. Effect of continuing olanzapine vs placebo on relapse among patients with psychotic depression in remission: the STOP-PD II randomized clinical trial. JAMA. 2019;322(7): 622-631.
Drug Brand Names
Amitriptyline • Elavil, Endep
Fluoxetine • Prozac
Haloperidol • Haldol
Olanzapine • Zyprexa
Quetiapine • Seroquel
Sertraline • Zoloft
Venlafaxine • Effexor
1. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
2. Jääskeläinen E, Juola T, Korpela H, et al. Epidemiology of psychotic depression - systematic review and meta-analysis. Psychol Med. 2018;48(6):905-918.
3. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder (revision). Am J Psychiatry. 2000;157(4)(suppl):1-45.
4. National Institute for Clinical Excellence. Depression in adults: recognition and management: clinical guideline [CG90]. National Institute for Health and Clinical Excellence. Published October 28, 2009. Accessed January 12, 2021. https://www.nice.org.uk/guidance/cg90
5. Crimson ML, Trivedi M, Pigott TA, et al. The Texas Medication Algorithm Project: report of the Texas Consensus Conference Panel on medication treatment of major depressive disorder. J Clin Psychiatry. 1999;60(3):142-156.
6. Meyers BS, Flint AJ, Rothschild AJ, et al. A double-blind randomized controlled trial of olanzapine plus sertraline vs olanzapine plus placebo for psychotic depression: the study of pharmacotherapy for psychotic depression -- the STOP-PD study. Arch Gen Psychiatry. 2009;66(8):838-847.
7. Rothschild AJ, Williamson DJ, Tohen MF, et al. A double-blind, randomized study of olanzapine and olanzapine/fluoxetine combination for major depression with psychotic features. J Clin Psychopharmacol. 2004;24(4):365-373.
8. Wijkstra J, Burger H, van den Broek WW, et al. Treatment of unipolar psychotic depression: a randomized, doubleblind study comparing imipramine, venlafaxine, and venlafaxine plus quetiapine. Acta Psychiatr Scand. 2010;21(3):190-200.
9. Muller-Siecheneder F, Muller M, Hillert A, et al. Risperidone versus haloperidol and amitriptyline in the treatment of patients with a combined psychotic and depressive syndrome. J Clin Psychopharm. 1998;18(2):111-120.
10. Spiker DG, Weiss JC, Dealy RS, et al. The pharmacological treatment of delusional depression. Am J Psychiatry. 1985;142(4):430-436.
11. Flint AJ, Meyers BS, Rothschild AJ, et al. Effect of continuing olanzapine vs placebo on relapse among patients with psychotic depression in remission: the STOP-PD II randomized clinical trial. JAMA. 2019;322(7):622-631.
Mrs. C, age 56, has a history of major depressive disorder (MDD). She has been stable for 5 years without medication. Six months ago, she presented to you, along with her son, seeking help. She reported that she had been experiencing insomnia, fatigue, and was not engaging in hobbies. Her son told you that his mother had lost weight and had been avoiding family dinners. Mrs. C reported recurrent thoughts of dying and heard voices vividly telling her that she was a burden and that her family would be better off without her. However, there was no imminent danger of self-harm. At that appointment, you initiated
Since that time, Mrs. C has followed up with you monthly with good response to the medications. Currently, she states her depression is much improved, and she denies hearing voices for approximately 5 months.
Based on her presentation and response, what do the data suggest about her length of treatment, and when should you consider tapering the antipsychotic medication?
In DSM-5, MDD with psychotic features is a severe subtype of MDD that is defined as a major depressive episode characterized by delusions and/or hallucinations.1 In the general population, the lifetime prevalence of this disorder varies from 0.35% to 1%, and the rate is higher in older patients.2 Risk factors include female gender, family history, and concomitant bipolar disorder.2
Epidemiologic studies have shown that psychotic features can occur in 15% to 20% of patients with MDD. The psychotic features that occur during these episodes are delusions and hallucinations.1 These features can be either mood-congruent (related to the depressive themes of worthlessness or guilt) or mood-incongruent (ie, unrelated to depressive themes).1
Treatment options: ECT or pharmacotherapy
Guidelines from the American Psychiatric Association3 and the National Institute for Clinical Excellence4 recommend treating depression with psychosis with electroconvulsive therapy (ECT) or with combined antidepressant and antipsychotic medications as first-line options. The Texas Medication Algorithm Project (TMAP) Algorithm for MDD,5 which closely focuses on treatment of MDD with psychotic features, can be used for treatment decisions (see Related Resources).
Electroconvulsive therapy is known to be efficacious in treating patients with MDD with psychotic features and should be considered as a treatment option. However, medication therapy is often chosen as the initial treatment due to the limitations of ECT, including accessibility, cost, and patient preference. However, in certain cases, ECT is the preferred option because it can provide rapid and significant improvement in patients with severe psychosis, suicidality, or risk of imminent harm.
Continue to: Pharmacotherapy
Pharmacotherapy for the treatment of MDD with psychotic features should consist of a combination of an antidepressant and antipsychotic medication. This combination has been shown to be more effective than either agent alone. Some combinations have been studied specifically for MDD with psychosis. The Study of the Pharmacotherapy of Psychotic Depression (STOP-PD), a 12-week, double-blind, randomized controlled trial, found that the combination of sertraline and olanzapine was efficacious and superior to monotherapy with olanzapine in an acute setting.6 In another study, the combination of olanzapine and
How long should treatment last?
The optimal timeline for treating patients with MDD with psychotic features is unknown. According to the TMAP algorithm and expert opinion, the continuation phase of pharmacotherapy should include treatment for at least 4 months with an antipsychotic medication and at least 2 years to lifetime treatment with an antidepressant.5 The STOP-PD II study, which was a continuation of the 12-week STOP-PD study, examined antipsychotic duration to determine the effects of continuing olanzapine once an episode of psychotic depression had responded to olanzapine and sertraline.11 Patients who had achieved remission after receiving olanzapine and sertraline were randomized to continue to receive this combination or to receive sertraline plus placebo for 36 weeks. The primary outcome was relapse, which was broadly defined as 1 of the following11:
- a Structured Clinical Interview for the DSM (SCID)-rated assessment that revealed the patient had enough symptoms to meet criteria for a DSM-IV major depressive episode
- a 17-item HAM-D scoren of ≥18
- SCID-rated psychosis
- other significant clinical worsening, defined as having a suicide plan or attempting suicide, developing SCID-rated symptoms of mania or hypomania, or being hospitalized in a psychiatric unit.
Compared with sertraline plus placebo, continuing sertraline plus olanzapine reduced the risk of relapse over 36 weeks (hazard ratio, 0.25; 95% confidence interval, 0.13 to 0.48; P < .001).11 However, as expected, the incidence of adverse effects such as weight gain and parkinsonism was higher in the olanzapine group. Therefore, it is important to consider the potential long-term adverse effects of continuing antipsychotic medications.
Weighing the evidence
Electroconvulsive therapy is considered a first-line treatment option for MDD with psychotic features; however, because of limitations associated with this approach, antidepressants plus antipsychotics are often utilized as an initial treatment.
Continue to: CASE
CASE CONTINUED
Based on the results of the STOP-PD and STOP-PD II trials, Mrs. C should be continued on sertraline plus olanzapine for at least another 3 to 6 months before an olanzapine taper should be considered. At that time, the risks and benefits of a taper vs continuing therapy should be considered. Given her history of MDD and the severity of this most recent episode, sertraline therapy should be continued for at least 2 years, and possibly indefinitely.
Related Resources
- Texas Medication Algorithm Project. Algorithm for the treatment of major depressive disorder with psychotic features. https://chsciowa.org/sites/chsciowa.org/files/resource/files/9_-_depression_med_algorithm_supplement.pdf
- Dold M, Bartova L, Kautzky A, et al. Psychotic features in patients with major depressive disorder: a report from the European Group for the Study of Resistant Depression. J Clin Psychiatry. 2019;80(1):17m12090. doi: 10.4088/ JCP.17m12090
- Flint AJ, Meyers BS, Rothschild AJ, et al. Effect of continuing olanzapine vs placebo on relapse among patients with psychotic depression in remission: the STOP-PD II randomized clinical trial. JAMA. 2019;322(7): 622-631.
Drug Brand Names
Amitriptyline • Elavil, Endep
Fluoxetine • Prozac
Haloperidol • Haldol
Olanzapine • Zyprexa
Quetiapine • Seroquel
Sertraline • Zoloft
Venlafaxine • Effexor
Mrs. C, age 56, has a history of major depressive disorder (MDD). She has been stable for 5 years without medication. Six months ago, she presented to you, along with her son, seeking help. She reported that she had been experiencing insomnia, fatigue, and was not engaging in hobbies. Her son told you that his mother had lost weight and had been avoiding family dinners. Mrs. C reported recurrent thoughts of dying and heard voices vividly telling her that she was a burden and that her family would be better off without her. However, there was no imminent danger of self-harm. At that appointment, you initiated
Since that time, Mrs. C has followed up with you monthly with good response to the medications. Currently, she states her depression is much improved, and she denies hearing voices for approximately 5 months.
Based on her presentation and response, what do the data suggest about her length of treatment, and when should you consider tapering the antipsychotic medication?
In DSM-5, MDD with psychotic features is a severe subtype of MDD that is defined as a major depressive episode characterized by delusions and/or hallucinations.1 In the general population, the lifetime prevalence of this disorder varies from 0.35% to 1%, and the rate is higher in older patients.2 Risk factors include female gender, family history, and concomitant bipolar disorder.2
Epidemiologic studies have shown that psychotic features can occur in 15% to 20% of patients with MDD. The psychotic features that occur during these episodes are delusions and hallucinations.1 These features can be either mood-congruent (related to the depressive themes of worthlessness or guilt) or mood-incongruent (ie, unrelated to depressive themes).1
Treatment options: ECT or pharmacotherapy
Guidelines from the American Psychiatric Association3 and the National Institute for Clinical Excellence4 recommend treating depression with psychosis with electroconvulsive therapy (ECT) or with combined antidepressant and antipsychotic medications as first-line options. The Texas Medication Algorithm Project (TMAP) Algorithm for MDD,5 which closely focuses on treatment of MDD with psychotic features, can be used for treatment decisions (see Related Resources).
Electroconvulsive therapy is known to be efficacious in treating patients with MDD with psychotic features and should be considered as a treatment option. However, medication therapy is often chosen as the initial treatment due to the limitations of ECT, including accessibility, cost, and patient preference. However, in certain cases, ECT is the preferred option because it can provide rapid and significant improvement in patients with severe psychosis, suicidality, or risk of imminent harm.
Continue to: Pharmacotherapy
Pharmacotherapy for the treatment of MDD with psychotic features should consist of a combination of an antidepressant and antipsychotic medication. This combination has been shown to be more effective than either agent alone. Some combinations have been studied specifically for MDD with psychosis. The Study of the Pharmacotherapy of Psychotic Depression (STOP-PD), a 12-week, double-blind, randomized controlled trial, found that the combination of sertraline and olanzapine was efficacious and superior to monotherapy with olanzapine in an acute setting.6 In another study, the combination of olanzapine and
How long should treatment last?
The optimal timeline for treating patients with MDD with psychotic features is unknown. According to the TMAP algorithm and expert opinion, the continuation phase of pharmacotherapy should include treatment for at least 4 months with an antipsychotic medication and at least 2 years to lifetime treatment with an antidepressant.5 The STOP-PD II study, which was a continuation of the 12-week STOP-PD study, examined antipsychotic duration to determine the effects of continuing olanzapine once an episode of psychotic depression had responded to olanzapine and sertraline.11 Patients who had achieved remission after receiving olanzapine and sertraline were randomized to continue to receive this combination or to receive sertraline plus placebo for 36 weeks. The primary outcome was relapse, which was broadly defined as 1 of the following11:
- a Structured Clinical Interview for the DSM (SCID)-rated assessment that revealed the patient had enough symptoms to meet criteria for a DSM-IV major depressive episode
- a 17-item HAM-D scoren of ≥18
- SCID-rated psychosis
- other significant clinical worsening, defined as having a suicide plan or attempting suicide, developing SCID-rated symptoms of mania or hypomania, or being hospitalized in a psychiatric unit.
Compared with sertraline plus placebo, continuing sertraline plus olanzapine reduced the risk of relapse over 36 weeks (hazard ratio, 0.25; 95% confidence interval, 0.13 to 0.48; P < .001).11 However, as expected, the incidence of adverse effects such as weight gain and parkinsonism was higher in the olanzapine group. Therefore, it is important to consider the potential long-term adverse effects of continuing antipsychotic medications.
Weighing the evidence
Electroconvulsive therapy is considered a first-line treatment option for MDD with psychotic features; however, because of limitations associated with this approach, antidepressants plus antipsychotics are often utilized as an initial treatment.
Continue to: CASE
CASE CONTINUED
Based on the results of the STOP-PD and STOP-PD II trials, Mrs. C should be continued on sertraline plus olanzapine for at least another 3 to 6 months before an olanzapine taper should be considered. At that time, the risks and benefits of a taper vs continuing therapy should be considered. Given her history of MDD and the severity of this most recent episode, sertraline therapy should be continued for at least 2 years, and possibly indefinitely.
Related Resources
- Texas Medication Algorithm Project. Algorithm for the treatment of major depressive disorder with psychotic features. https://chsciowa.org/sites/chsciowa.org/files/resource/files/9_-_depression_med_algorithm_supplement.pdf
- Dold M, Bartova L, Kautzky A, et al. Psychotic features in patients with major depressive disorder: a report from the European Group for the Study of Resistant Depression. J Clin Psychiatry. 2019;80(1):17m12090. doi: 10.4088/ JCP.17m12090
- Flint AJ, Meyers BS, Rothschild AJ, et al. Effect of continuing olanzapine vs placebo on relapse among patients with psychotic depression in remission: the STOP-PD II randomized clinical trial. JAMA. 2019;322(7): 622-631.
Drug Brand Names
Amitriptyline • Elavil, Endep
Fluoxetine • Prozac
Haloperidol • Haldol
Olanzapine • Zyprexa
Quetiapine • Seroquel
Sertraline • Zoloft
Venlafaxine • Effexor
1. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
2. Jääskeläinen E, Juola T, Korpela H, et al. Epidemiology of psychotic depression - systematic review and meta-analysis. Psychol Med. 2018;48(6):905-918.
3. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder (revision). Am J Psychiatry. 2000;157(4)(suppl):1-45.
4. National Institute for Clinical Excellence. Depression in adults: recognition and management: clinical guideline [CG90]. National Institute for Health and Clinical Excellence. Published October 28, 2009. Accessed January 12, 2021. https://www.nice.org.uk/guidance/cg90
5. Crimson ML, Trivedi M, Pigott TA, et al. The Texas Medication Algorithm Project: report of the Texas Consensus Conference Panel on medication treatment of major depressive disorder. J Clin Psychiatry. 1999;60(3):142-156.
6. Meyers BS, Flint AJ, Rothschild AJ, et al. A double-blind randomized controlled trial of olanzapine plus sertraline vs olanzapine plus placebo for psychotic depression: the study of pharmacotherapy for psychotic depression -- the STOP-PD study. Arch Gen Psychiatry. 2009;66(8):838-847.
7. Rothschild AJ, Williamson DJ, Tohen MF, et al. A double-blind, randomized study of olanzapine and olanzapine/fluoxetine combination for major depression with psychotic features. J Clin Psychopharmacol. 2004;24(4):365-373.
8. Wijkstra J, Burger H, van den Broek WW, et al. Treatment of unipolar psychotic depression: a randomized, doubleblind study comparing imipramine, venlafaxine, and venlafaxine plus quetiapine. Acta Psychiatr Scand. 2010;21(3):190-200.
9. Muller-Siecheneder F, Muller M, Hillert A, et al. Risperidone versus haloperidol and amitriptyline in the treatment of patients with a combined psychotic and depressive syndrome. J Clin Psychopharm. 1998;18(2):111-120.
10. Spiker DG, Weiss JC, Dealy RS, et al. The pharmacological treatment of delusional depression. Am J Psychiatry. 1985;142(4):430-436.
11. Flint AJ, Meyers BS, Rothschild AJ, et al. Effect of continuing olanzapine vs placebo on relapse among patients with psychotic depression in remission: the STOP-PD II randomized clinical trial. JAMA. 2019;322(7):622-631.
1. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
2. Jääskeläinen E, Juola T, Korpela H, et al. Epidemiology of psychotic depression - systematic review and meta-analysis. Psychol Med. 2018;48(6):905-918.
3. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder (revision). Am J Psychiatry. 2000;157(4)(suppl):1-45.
4. National Institute for Clinical Excellence. Depression in adults: recognition and management: clinical guideline [CG90]. National Institute for Health and Clinical Excellence. Published October 28, 2009. Accessed January 12, 2021. https://www.nice.org.uk/guidance/cg90
5. Crimson ML, Trivedi M, Pigott TA, et al. The Texas Medication Algorithm Project: report of the Texas Consensus Conference Panel on medication treatment of major depressive disorder. J Clin Psychiatry. 1999;60(3):142-156.
6. Meyers BS, Flint AJ, Rothschild AJ, et al. A double-blind randomized controlled trial of olanzapine plus sertraline vs olanzapine plus placebo for psychotic depression: the study of pharmacotherapy for psychotic depression -- the STOP-PD study. Arch Gen Psychiatry. 2009;66(8):838-847.
7. Rothschild AJ, Williamson DJ, Tohen MF, et al. A double-blind, randomized study of olanzapine and olanzapine/fluoxetine combination for major depression with psychotic features. J Clin Psychopharmacol. 2004;24(4):365-373.
8. Wijkstra J, Burger H, van den Broek WW, et al. Treatment of unipolar psychotic depression: a randomized, doubleblind study comparing imipramine, venlafaxine, and venlafaxine plus quetiapine. Acta Psychiatr Scand. 2010;21(3):190-200.
9. Muller-Siecheneder F, Muller M, Hillert A, et al. Risperidone versus haloperidol and amitriptyline in the treatment of patients with a combined psychotic and depressive syndrome. J Clin Psychopharm. 1998;18(2):111-120.
10. Spiker DG, Weiss JC, Dealy RS, et al. The pharmacological treatment of delusional depression. Am J Psychiatry. 1985;142(4):430-436.
11. Flint AJ, Meyers BS, Rothschild AJ, et al. Effect of continuing olanzapine vs placebo on relapse among patients with psychotic depression in remission: the STOP-PD II randomized clinical trial. JAMA. 2019;322(7):622-631.
Virtual supervision during the COVID-19 pandemic
The coronavirus disease 2019 (COVID-19) pandemic has fundamentally changed our way of life. It has affected everything from how we go to the grocery store, attend school, worship, and spend time with our loved ones. As vaccinations are becoming available, there’s hope for a time when we can all enjoy a mask-free life again. Despite this, many of us are beginning to sense that the precautions and technology employed in response to COVID-19, and some of the lessons learned as a result, are likely to stay in place long after the virus has been controlled.
Working remotely through audio and visual synchronous communication is now becoming the norm throughout the American workplace and educational system. Hospitals and graduate medical education programs are not exempt from this trend. For at least the foreseeable future, gone are the days of “unsocially distanced” bedside rounds in which 5 to 10 residents and medical students gather around with their attending as a case is presented in front of an agreeable patient.
My experience with ‘virtual’ supervision
Telemedicine has played a key role in the practice of health care during this pandemic, but little has been written about “telesupervision” of residents in the hospital setting. An unprecedented virtual approach to supervising emergency medicine residents was trialed at the University of Alabama a few months prior to my experience with it. This was found to be quite effective and well-received by all involved parties.1
I am a PGY-2 psychiatry resident at ChristianaCare, a large multisite hospital system with more than 1,200 beds that serves the health care needs of Delaware and the surrounding areas. I recently had a novel educational experience working on a busy addiction medicine consult service. On the first day of this rotation, I met with my attending, Dr. Terry Horton, to discuss how the month would proceed. Together we developed a strategy for him to supervise me virtually.
Our arrangement was efficient and simple: I began each day by donning my surgical mask and protective eyewear and reviewing patients that had been placed on the consult list. Dr. Horton and I would have a conversation via telephone early in the morning to discuss the tasks that needed to be completed for the day. I would see and evaluate patients in the standard face-to-face way. After developing a treatment strategy, I contacted Dr. Horton on the phone, presented the patient, shared my plan, and gained information from his experienced perspective.
Then we saw the patient “together.” We used an iPad and Microsoft Teams video conferencing software. The information shared was protected using Microsoft Teams accounts, which were secured with profiles created by our institutional accounts. The iPad was placed on a rolling tripod, and the patient was able to converse with Dr. Horton as though he was physically in the room. I was there to facilitate the call, address any technical issues, and conduct any aspects of a physical exam that could only be done in person. After discussing any other changes to the treatment plan, I placed all medication orders, shared relevant details with nursing staff and other clinicians, wrote my progress note, and rolled my “attending on a stick” over to the next patient. Meanwhile, Dr. Horton was free to respond to pages or any other issues while I worked.
This description of my workflow is not very different from life before the virus. Based on informal feedback gathered from patients, the experience was overall positive. A physician is present; patients feel well cared for, and they look forward to visits and a virtual presence. This virtual approach not only spared unnecessary physical contact, reducing the risk of COVID-19 exposure, it also promoted efficiency.
Continue to: Fortunately, our hospital...
Fortunately, our hospital is surrounded by a solid telecommunications infrastructure. This experience would be limited in more remote areas of the country. At times, sound quality was an issue, which can be especially problematic for certain patients.
Certain psychosocial implications of the pandemic, including (but not limited to)social isolation and financial hardship, are often associated with increased substance use, and early data support the hypothesis that substance use has increased during this period.2 Delaware seems to be included in the national trend. As such, our already-busy service is being stretched even further. Dr. Horton receives calls and is providing critical recommendations continuously throughout the day for multiple hospitals as well as for his outpatient practice. He used to spend a great deal of time traveling between different sites. With increasing need for his expertise, this model became increasingly difficult to practice. Our new model of attending supervision is welcomed in some settings because the attending can virtually be in multiple places at the same time.
For me, this experience has been positive. For a physician in training, virtual rounding can provide a critical balance of autonomy and support. I felt free on the rotation to make my own decisions, but I also did not feel like I was left to care for complicated cases on my own. Furthermore, my education did not suffer. In actuality, the experience enabled me to excel in my training. An attending physician was there for the important steps of plan formulation, but solo problem-solving opportunities were more readily available without his physical presence.
Aside from the medical lessons learned, I believe the participation has given me a glimpse of the future of medical training, health care delivery, and life in the increasingly digital post−COVID-19 world.
Hopefully, my experience will be helpful for other hospital systems as they continue to provide high-quality care to patients and education/training to their resident physicians in the face of the pandemic and the changing landscape of health care.
Acknowledgment
The author thanks Mustafa Mufti, MD, ChristianaCare Psychiatry Residency Program Director; Rachel Bronsther, MD, ChristianaCare Psychiatry Residency Associate Program Director; and Terry Horton, MD, ChristianaCare Addiction Medicine, for their assistance with this article.
1. Schrading WA, Pigott D, Thompson L. Virtual remote attending supervision in an academic emergency department during the COVID-19 pandemic. AEM Educ Train. 2020;4(3):266-269.
2. Czeisler MÉ, Lane RI, Petrosky E, et al. Mental health, substance use, and suicidal ideation during the COVID-19 pandemic - United States, June 24-30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(32):1049-1057.
The coronavirus disease 2019 (COVID-19) pandemic has fundamentally changed our way of life. It has affected everything from how we go to the grocery store, attend school, worship, and spend time with our loved ones. As vaccinations are becoming available, there’s hope for a time when we can all enjoy a mask-free life again. Despite this, many of us are beginning to sense that the precautions and technology employed in response to COVID-19, and some of the lessons learned as a result, are likely to stay in place long after the virus has been controlled.
Working remotely through audio and visual synchronous communication is now becoming the norm throughout the American workplace and educational system. Hospitals and graduate medical education programs are not exempt from this trend. For at least the foreseeable future, gone are the days of “unsocially distanced” bedside rounds in which 5 to 10 residents and medical students gather around with their attending as a case is presented in front of an agreeable patient.
My experience with ‘virtual’ supervision
Telemedicine has played a key role in the practice of health care during this pandemic, but little has been written about “telesupervision” of residents in the hospital setting. An unprecedented virtual approach to supervising emergency medicine residents was trialed at the University of Alabama a few months prior to my experience with it. This was found to be quite effective and well-received by all involved parties.1
I am a PGY-2 psychiatry resident at ChristianaCare, a large multisite hospital system with more than 1,200 beds that serves the health care needs of Delaware and the surrounding areas. I recently had a novel educational experience working on a busy addiction medicine consult service. On the first day of this rotation, I met with my attending, Dr. Terry Horton, to discuss how the month would proceed. Together we developed a strategy for him to supervise me virtually.
Our arrangement was efficient and simple: I began each day by donning my surgical mask and protective eyewear and reviewing patients that had been placed on the consult list. Dr. Horton and I would have a conversation via telephone early in the morning to discuss the tasks that needed to be completed for the day. I would see and evaluate patients in the standard face-to-face way. After developing a treatment strategy, I contacted Dr. Horton on the phone, presented the patient, shared my plan, and gained information from his experienced perspective.
Then we saw the patient “together.” We used an iPad and Microsoft Teams video conferencing software. The information shared was protected using Microsoft Teams accounts, which were secured with profiles created by our institutional accounts. The iPad was placed on a rolling tripod, and the patient was able to converse with Dr. Horton as though he was physically in the room. I was there to facilitate the call, address any technical issues, and conduct any aspects of a physical exam that could only be done in person. After discussing any other changes to the treatment plan, I placed all medication orders, shared relevant details with nursing staff and other clinicians, wrote my progress note, and rolled my “attending on a stick” over to the next patient. Meanwhile, Dr. Horton was free to respond to pages or any other issues while I worked.
This description of my workflow is not very different from life before the virus. Based on informal feedback gathered from patients, the experience was overall positive. A physician is present; patients feel well cared for, and they look forward to visits and a virtual presence. This virtual approach not only spared unnecessary physical contact, reducing the risk of COVID-19 exposure, it also promoted efficiency.
Continue to: Fortunately, our hospital...
Fortunately, our hospital is surrounded by a solid telecommunications infrastructure. This experience would be limited in more remote areas of the country. At times, sound quality was an issue, which can be especially problematic for certain patients.
Certain psychosocial implications of the pandemic, including (but not limited to)social isolation and financial hardship, are often associated with increased substance use, and early data support the hypothesis that substance use has increased during this period.2 Delaware seems to be included in the national trend. As such, our already-busy service is being stretched even further. Dr. Horton receives calls and is providing critical recommendations continuously throughout the day for multiple hospitals as well as for his outpatient practice. He used to spend a great deal of time traveling between different sites. With increasing need for his expertise, this model became increasingly difficult to practice. Our new model of attending supervision is welcomed in some settings because the attending can virtually be in multiple places at the same time.
For me, this experience has been positive. For a physician in training, virtual rounding can provide a critical balance of autonomy and support. I felt free on the rotation to make my own decisions, but I also did not feel like I was left to care for complicated cases on my own. Furthermore, my education did not suffer. In actuality, the experience enabled me to excel in my training. An attending physician was there for the important steps of plan formulation, but solo problem-solving opportunities were more readily available without his physical presence.
Aside from the medical lessons learned, I believe the participation has given me a glimpse of the future of medical training, health care delivery, and life in the increasingly digital post−COVID-19 world.
Hopefully, my experience will be helpful for other hospital systems as they continue to provide high-quality care to patients and education/training to their resident physicians in the face of the pandemic and the changing landscape of health care.
Acknowledgment
The author thanks Mustafa Mufti, MD, ChristianaCare Psychiatry Residency Program Director; Rachel Bronsther, MD, ChristianaCare Psychiatry Residency Associate Program Director; and Terry Horton, MD, ChristianaCare Addiction Medicine, for their assistance with this article.
The coronavirus disease 2019 (COVID-19) pandemic has fundamentally changed our way of life. It has affected everything from how we go to the grocery store, attend school, worship, and spend time with our loved ones. As vaccinations are becoming available, there’s hope for a time when we can all enjoy a mask-free life again. Despite this, many of us are beginning to sense that the precautions and technology employed in response to COVID-19, and some of the lessons learned as a result, are likely to stay in place long after the virus has been controlled.
Working remotely through audio and visual synchronous communication is now becoming the norm throughout the American workplace and educational system. Hospitals and graduate medical education programs are not exempt from this trend. For at least the foreseeable future, gone are the days of “unsocially distanced” bedside rounds in which 5 to 10 residents and medical students gather around with their attending as a case is presented in front of an agreeable patient.
My experience with ‘virtual’ supervision
Telemedicine has played a key role in the practice of health care during this pandemic, but little has been written about “telesupervision” of residents in the hospital setting. An unprecedented virtual approach to supervising emergency medicine residents was trialed at the University of Alabama a few months prior to my experience with it. This was found to be quite effective and well-received by all involved parties.1
I am a PGY-2 psychiatry resident at ChristianaCare, a large multisite hospital system with more than 1,200 beds that serves the health care needs of Delaware and the surrounding areas. I recently had a novel educational experience working on a busy addiction medicine consult service. On the first day of this rotation, I met with my attending, Dr. Terry Horton, to discuss how the month would proceed. Together we developed a strategy for him to supervise me virtually.
Our arrangement was efficient and simple: I began each day by donning my surgical mask and protective eyewear and reviewing patients that had been placed on the consult list. Dr. Horton and I would have a conversation via telephone early in the morning to discuss the tasks that needed to be completed for the day. I would see and evaluate patients in the standard face-to-face way. After developing a treatment strategy, I contacted Dr. Horton on the phone, presented the patient, shared my plan, and gained information from his experienced perspective.
Then we saw the patient “together.” We used an iPad and Microsoft Teams video conferencing software. The information shared was protected using Microsoft Teams accounts, which were secured with profiles created by our institutional accounts. The iPad was placed on a rolling tripod, and the patient was able to converse with Dr. Horton as though he was physically in the room. I was there to facilitate the call, address any technical issues, and conduct any aspects of a physical exam that could only be done in person. After discussing any other changes to the treatment plan, I placed all medication orders, shared relevant details with nursing staff and other clinicians, wrote my progress note, and rolled my “attending on a stick” over to the next patient. Meanwhile, Dr. Horton was free to respond to pages or any other issues while I worked.
This description of my workflow is not very different from life before the virus. Based on informal feedback gathered from patients, the experience was overall positive. A physician is present; patients feel well cared for, and they look forward to visits and a virtual presence. This virtual approach not only spared unnecessary physical contact, reducing the risk of COVID-19 exposure, it also promoted efficiency.
Continue to: Fortunately, our hospital...
Fortunately, our hospital is surrounded by a solid telecommunications infrastructure. This experience would be limited in more remote areas of the country. At times, sound quality was an issue, which can be especially problematic for certain patients.
Certain psychosocial implications of the pandemic, including (but not limited to)social isolation and financial hardship, are often associated with increased substance use, and early data support the hypothesis that substance use has increased during this period.2 Delaware seems to be included in the national trend. As such, our already-busy service is being stretched even further. Dr. Horton receives calls and is providing critical recommendations continuously throughout the day for multiple hospitals as well as for his outpatient practice. He used to spend a great deal of time traveling between different sites. With increasing need for his expertise, this model became increasingly difficult to practice. Our new model of attending supervision is welcomed in some settings because the attending can virtually be in multiple places at the same time.
For me, this experience has been positive. For a physician in training, virtual rounding can provide a critical balance of autonomy and support. I felt free on the rotation to make my own decisions, but I also did not feel like I was left to care for complicated cases on my own. Furthermore, my education did not suffer. In actuality, the experience enabled me to excel in my training. An attending physician was there for the important steps of plan formulation, but solo problem-solving opportunities were more readily available without his physical presence.
Aside from the medical lessons learned, I believe the participation has given me a glimpse of the future of medical training, health care delivery, and life in the increasingly digital post−COVID-19 world.
Hopefully, my experience will be helpful for other hospital systems as they continue to provide high-quality care to patients and education/training to their resident physicians in the face of the pandemic and the changing landscape of health care.
Acknowledgment
The author thanks Mustafa Mufti, MD, ChristianaCare Psychiatry Residency Program Director; Rachel Bronsther, MD, ChristianaCare Psychiatry Residency Associate Program Director; and Terry Horton, MD, ChristianaCare Addiction Medicine, for their assistance with this article.
1. Schrading WA, Pigott D, Thompson L. Virtual remote attending supervision in an academic emergency department during the COVID-19 pandemic. AEM Educ Train. 2020;4(3):266-269.
2. Czeisler MÉ, Lane RI, Petrosky E, et al. Mental health, substance use, and suicidal ideation during the COVID-19 pandemic - United States, June 24-30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(32):1049-1057.
1. Schrading WA, Pigott D, Thompson L. Virtual remote attending supervision in an academic emergency department during the COVID-19 pandemic. AEM Educ Train. 2020;4(3):266-269.
2. Czeisler MÉ, Lane RI, Petrosky E, et al. Mental health, substance use, and suicidal ideation during the COVID-19 pandemic - United States, June 24-30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(32):1049-1057.