User login
Ceftolozane-tazobactam found effective in critically ill patients with Pseudomonas aeruginosa infections
, according to the results of a retrospective, observational study conducted in critically ill patients.
The multicenter, observational study assessed 95 patients who received C/T for P. aeruginosa serious infections, according to a report published online in the International Journal of Antimicrobial Agents.
C/T is a novel beta-lactam/ beta-lactamase inhibitor combination active against gram-negative bacteria including P. aeruginosa, “This paper presents the largest real-life experience published on C/T therapy for treating serious P. aeruginosa infections according to researchers Barbara Balandin, MD, of the Hospital Universitario Puerta de Hierro, Majadahonda, Spain, and colleagues.
The main infections treated were nosocomial pneumonia (56.2%), intra-abdominal infection (10.5%), tracheobronchitis (8.4%), and urinary tract infection (6.3%). Most infections were complicated with sepsis (49.5%) or septic shock (45.3%), and bacteremia (10.5%).
A total of 46 episodes were treated with high-dose C/T (3 g every 8 hours), and 38 episodes were treated with standard dosage (1.5 g every 8 hours). Almost half (44.2%) of the patients were treated with C/T monotherapy, and the remaining group received combination therapy with other antibiotics, according to the researchers.
The primary outcome of the study was to assess the efficacy and toxicity of C/T therapy. The secondary outcome was to evaluate the risk factors for all-cause 30-day mortality from the first day of therapy.
Favorable results
Most of the infections (93.7%) were severe and included the presence of sepsis (49.5%) or septic shock (45.3%). Bacteremia was observed in 15 (15.7%) patients. Bacteremia was secondary to nosocomial pneumonia in eight cases, catheter infection in five, urinary tract infection in one, and soft tissue infection in one. According to their susceptibility profiles, 46 (48.4%) of the strains were classified as extensively drug-resistant (XDR) P. aeruginosa and 35 (36.5%) were multidrug-resistant (MDR) P. aeruginosa.
Sixty-eight (71.6%) patients presented a favorable clinical response, which was defined as a resolution of presenting symptoms and signs of the infection by the end of therapy. An unfavorable clinical response was considered as persistence or worsening of the presenting symptoms and signs or death occurring during treatment with no other cause identified. Death associated with infection was defined as persistence of signs and symptoms of P. aeruginosa infection during C/T therapy with no other cause identified.
Microbiological eradication was documented in 42.1% (40/95) of the episodes. However, the global ICU mortality was still high, at 36.5%, with mortality mainly related to the severity of the infection.
Mortality was found to be significantly correlated with the Charlson Comorbidity Index (5.7 vs. 4.3; P = .04) and the need for life-supporting therapies such as vasopressors (66.6% vs. 46.9%; P = .03) and renal replacement therapy (46.6% vs. 18.1%; P = .002). In addition, mortality was significantly associated with a higher sequential organ failure assessment (SOFA) score during C/T therapy (SOFA1, SOFA 3, and SOFA 7; P < .001).
No significant differences in outcomes were correlated with demographic features, type and severity of infection, and dose of C/T. Also, there were no differences seen in outcomes between patients treated with C/T monotherapy and combined therapy (30.9% vs. 30.1%; P = .55).
“The lack of a positive effect from combined therapy suggests that C/T monotherapy may be sufficient for treating P. aeruginosa isolates that are susceptible to that agent,” the researchers suggested. “This study shows that C/T appears to be a suitable, effective, and safe drug for treating severe infections due to P. aeruginosa, highlighting nosocomial pneumonia caused by MDR/XDR P. aeruginosa in ICU patients with multiple comorbidities, such as immunosuppression, and needing life-sustaining therapies,” they concluded.
The authors reported that they had no outside funding source and had no conflicts of interest.
, according to the results of a retrospective, observational study conducted in critically ill patients.
The multicenter, observational study assessed 95 patients who received C/T for P. aeruginosa serious infections, according to a report published online in the International Journal of Antimicrobial Agents.
C/T is a novel beta-lactam/ beta-lactamase inhibitor combination active against gram-negative bacteria including P. aeruginosa, “This paper presents the largest real-life experience published on C/T therapy for treating serious P. aeruginosa infections according to researchers Barbara Balandin, MD, of the Hospital Universitario Puerta de Hierro, Majadahonda, Spain, and colleagues.
The main infections treated were nosocomial pneumonia (56.2%), intra-abdominal infection (10.5%), tracheobronchitis (8.4%), and urinary tract infection (6.3%). Most infections were complicated with sepsis (49.5%) or septic shock (45.3%), and bacteremia (10.5%).
A total of 46 episodes were treated with high-dose C/T (3 g every 8 hours), and 38 episodes were treated with standard dosage (1.5 g every 8 hours). Almost half (44.2%) of the patients were treated with C/T monotherapy, and the remaining group received combination therapy with other antibiotics, according to the researchers.
The primary outcome of the study was to assess the efficacy and toxicity of C/T therapy. The secondary outcome was to evaluate the risk factors for all-cause 30-day mortality from the first day of therapy.
Favorable results
Most of the infections (93.7%) were severe and included the presence of sepsis (49.5%) or septic shock (45.3%). Bacteremia was observed in 15 (15.7%) patients. Bacteremia was secondary to nosocomial pneumonia in eight cases, catheter infection in five, urinary tract infection in one, and soft tissue infection in one. According to their susceptibility profiles, 46 (48.4%) of the strains were classified as extensively drug-resistant (XDR) P. aeruginosa and 35 (36.5%) were multidrug-resistant (MDR) P. aeruginosa.
Sixty-eight (71.6%) patients presented a favorable clinical response, which was defined as a resolution of presenting symptoms and signs of the infection by the end of therapy. An unfavorable clinical response was considered as persistence or worsening of the presenting symptoms and signs or death occurring during treatment with no other cause identified. Death associated with infection was defined as persistence of signs and symptoms of P. aeruginosa infection during C/T therapy with no other cause identified.
Microbiological eradication was documented in 42.1% (40/95) of the episodes. However, the global ICU mortality was still high, at 36.5%, with mortality mainly related to the severity of the infection.
Mortality was found to be significantly correlated with the Charlson Comorbidity Index (5.7 vs. 4.3; P = .04) and the need for life-supporting therapies such as vasopressors (66.6% vs. 46.9%; P = .03) and renal replacement therapy (46.6% vs. 18.1%; P = .002). In addition, mortality was significantly associated with a higher sequential organ failure assessment (SOFA) score during C/T therapy (SOFA1, SOFA 3, and SOFA 7; P < .001).
No significant differences in outcomes were correlated with demographic features, type and severity of infection, and dose of C/T. Also, there were no differences seen in outcomes between patients treated with C/T monotherapy and combined therapy (30.9% vs. 30.1%; P = .55).
“The lack of a positive effect from combined therapy suggests that C/T monotherapy may be sufficient for treating P. aeruginosa isolates that are susceptible to that agent,” the researchers suggested. “This study shows that C/T appears to be a suitable, effective, and safe drug for treating severe infections due to P. aeruginosa, highlighting nosocomial pneumonia caused by MDR/XDR P. aeruginosa in ICU patients with multiple comorbidities, such as immunosuppression, and needing life-sustaining therapies,” they concluded.
The authors reported that they had no outside funding source and had no conflicts of interest.
, according to the results of a retrospective, observational study conducted in critically ill patients.
The multicenter, observational study assessed 95 patients who received C/T for P. aeruginosa serious infections, according to a report published online in the International Journal of Antimicrobial Agents.
C/T is a novel beta-lactam/ beta-lactamase inhibitor combination active against gram-negative bacteria including P. aeruginosa, “This paper presents the largest real-life experience published on C/T therapy for treating serious P. aeruginosa infections according to researchers Barbara Balandin, MD, of the Hospital Universitario Puerta de Hierro, Majadahonda, Spain, and colleagues.
The main infections treated were nosocomial pneumonia (56.2%), intra-abdominal infection (10.5%), tracheobronchitis (8.4%), and urinary tract infection (6.3%). Most infections were complicated with sepsis (49.5%) or septic shock (45.3%), and bacteremia (10.5%).
A total of 46 episodes were treated with high-dose C/T (3 g every 8 hours), and 38 episodes were treated with standard dosage (1.5 g every 8 hours). Almost half (44.2%) of the patients were treated with C/T monotherapy, and the remaining group received combination therapy with other antibiotics, according to the researchers.
The primary outcome of the study was to assess the efficacy and toxicity of C/T therapy. The secondary outcome was to evaluate the risk factors for all-cause 30-day mortality from the first day of therapy.
Favorable results
Most of the infections (93.7%) were severe and included the presence of sepsis (49.5%) or septic shock (45.3%). Bacteremia was observed in 15 (15.7%) patients. Bacteremia was secondary to nosocomial pneumonia in eight cases, catheter infection in five, urinary tract infection in one, and soft tissue infection in one. According to their susceptibility profiles, 46 (48.4%) of the strains were classified as extensively drug-resistant (XDR) P. aeruginosa and 35 (36.5%) were multidrug-resistant (MDR) P. aeruginosa.
Sixty-eight (71.6%) patients presented a favorable clinical response, which was defined as a resolution of presenting symptoms and signs of the infection by the end of therapy. An unfavorable clinical response was considered as persistence or worsening of the presenting symptoms and signs or death occurring during treatment with no other cause identified. Death associated with infection was defined as persistence of signs and symptoms of P. aeruginosa infection during C/T therapy with no other cause identified.
Microbiological eradication was documented in 42.1% (40/95) of the episodes. However, the global ICU mortality was still high, at 36.5%, with mortality mainly related to the severity of the infection.
Mortality was found to be significantly correlated with the Charlson Comorbidity Index (5.7 vs. 4.3; P = .04) and the need for life-supporting therapies such as vasopressors (66.6% vs. 46.9%; P = .03) and renal replacement therapy (46.6% vs. 18.1%; P = .002). In addition, mortality was significantly associated with a higher sequential organ failure assessment (SOFA) score during C/T therapy (SOFA1, SOFA 3, and SOFA 7; P < .001).
No significant differences in outcomes were correlated with demographic features, type and severity of infection, and dose of C/T. Also, there were no differences seen in outcomes between patients treated with C/T monotherapy and combined therapy (30.9% vs. 30.1%; P = .55).
“The lack of a positive effect from combined therapy suggests that C/T monotherapy may be sufficient for treating P. aeruginosa isolates that are susceptible to that agent,” the researchers suggested. “This study shows that C/T appears to be a suitable, effective, and safe drug for treating severe infections due to P. aeruginosa, highlighting nosocomial pneumonia caused by MDR/XDR P. aeruginosa in ICU patients with multiple comorbidities, such as immunosuppression, and needing life-sustaining therapies,” they concluded.
The authors reported that they had no outside funding source and had no conflicts of interest.
FROM THE INTERNATIONAL JOURNAL OF ANTIMICROBIAL AGENTS
Expert offers tips for sorting out pink lesions on dermoscopy
Even in the most experienced hands, .
“For me, pink lesions are challenging,” Jennifer A. Stein, MD, PhD, said during the virtual Orlando Dermatology Aesthetic and Clinical Conference. “How can dermoscopy help us distinguish between Spitz nevus, melanoma, clear cell acanthoma, psoriasis, basal cell carcinoma, and squamous cell carcinoma?”
Dr. Stein, professor of dermatology at New York University, offered four tips. First, look for the shiny white perpendicular lines, otherwise known as the chrysalis or crystalline pattern. “You can only see this feature when you’re looking with polarized light,” she said. “This is why you want a dermatoscope that has polarized light, and better yet, one that you’re able to turn on and off, the hybrid kind, because then you can convince yourself that you’re looking at this feature, because it blinks on and off.”
The differential diagnosis for white shiny perpendicular lines includes dermatofibroma/scars (which is most common), Spitz and atypical genital nevi, BCC, and melanoma. “Dermatofibromas sometimes have white circles or rings in the center,” Dr. Stein said. “In BCC, the lines aren’t always perpendicular. Sometimes it’s more of a blotch or strands.”
A second tip for managing a pink lesion on dermoscopy is to look for any brown color. “When you see that combo together you have to worry,” she said. “When you see pigment network on dermoscopy, you have to put melanoma in your differential. If you see shiny white lines in something that is melanocytic, there’s a 98% specificity for melanoma.”
A third tip she offered for managing pink lesions is to check the blood vessels for clues. “For years, I was just naming the vessels based on making the diagnosis and then deciding, ‘that’s a basal cell carcinoma; those must be branching vessels,’ ” said Dr. Stein, who manages NYU’s medical dermatology faculty group practice.
However, blood vessel patterns differ. For example, branching or arborizing vessels are suggestive of BCC. “These vessels are very crisp-looking on dermoscopy,” she said. “They’re all in the same plane of focus and they look like they were drawn in with a fine point marker. That’s different from other blood vessel patterns.” She also pointed out that superficial basal cells have short, fine telangiectasias. “When you put on the polarized light, the clue is the white, shiny structures,” she said.
Dotted vessels, meanwhile, appear on dermoscopy as small red dots aligned perpendicular to the skin surface. The differential includes inflammatory lesions like psoriasis, stasis, and trauma; clear cell acanthoma (characterized by a “string of pearls” arrangement), nevi, and melanoma. “I find dermoscopy most useful in diagnosing SCC – especially squamous cell in situ,” she said. “Important clinical clues suggestive of SCC or melanoma include a solitary lesion, it’s new, it’s growing, and it’s not going away with a topical steroid.”
An additional pattern to be aware of are hairpin vessels, which are looped and feature a sharp bend at one end. These are often seen in seborrheic keratoses. “You can’t count on the hairpin vessels alone, because you can see this in anything keratotic, such as in keratoacanthoma (at the periphery with a yellow keratotic center), warts, SCC, BCC, as well as in dermal nevi and Spitz nevi,” said Dr. Stein, who recommended dermoscopedia.org as resource.
Comma vessels, meanwhile, appear in dermal or compound nevi. She described these as “slightly curved vessels that are much less in focus than branched vessels, because they come in and out of the plane of focus,” she said. “If you put your dermatoscope on top of the nevus and wobble it around you can appreciate the curve. If you look at it from the side, it looks like a curve. If you look at it straight on it will look more like a line. If you look at from the end it will look like a dot.”
Another vessel type she discussed are linear irregular and polymorphous vessels, which she described as “any combination of different types of vessels. We get most worried when we see dotted and linear irregular vessels together. In that case, you worry about melanoma. These can also be seen in nevi and other tumors, such as BCC.”
Dr. Stein’s fourth tip of the presentation was a reminder to consider dermoscopy as one piece of the clinical exam. “Always think about the lesion in context of the rest of the clinical picture and history,” she said. “Don’t get discouraged if it’s hard; just keep practicing. Look for any brown and use your clinical clues to put together to make the right decision.”
She disclosed that NYU receives compensation from MoleSafe for her telemedicine dermoscopic diagnoses.
Even in the most experienced hands, .

“For me, pink lesions are challenging,” Jennifer A. Stein, MD, PhD, said during the virtual Orlando Dermatology Aesthetic and Clinical Conference. “How can dermoscopy help us distinguish between Spitz nevus, melanoma, clear cell acanthoma, psoriasis, basal cell carcinoma, and squamous cell carcinoma?”
Dr. Stein, professor of dermatology at New York University, offered four tips. First, look for the shiny white perpendicular lines, otherwise known as the chrysalis or crystalline pattern. “You can only see this feature when you’re looking with polarized light,” she said. “This is why you want a dermatoscope that has polarized light, and better yet, one that you’re able to turn on and off, the hybrid kind, because then you can convince yourself that you’re looking at this feature, because it blinks on and off.”
The differential diagnosis for white shiny perpendicular lines includes dermatofibroma/scars (which is most common), Spitz and atypical genital nevi, BCC, and melanoma. “Dermatofibromas sometimes have white circles or rings in the center,” Dr. Stein said. “In BCC, the lines aren’t always perpendicular. Sometimes it’s more of a blotch or strands.”
A second tip for managing a pink lesion on dermoscopy is to look for any brown color. “When you see that combo together you have to worry,” she said. “When you see pigment network on dermoscopy, you have to put melanoma in your differential. If you see shiny white lines in something that is melanocytic, there’s a 98% specificity for melanoma.”
A third tip she offered for managing pink lesions is to check the blood vessels for clues. “For years, I was just naming the vessels based on making the diagnosis and then deciding, ‘that’s a basal cell carcinoma; those must be branching vessels,’ ” said Dr. Stein, who manages NYU’s medical dermatology faculty group practice.
However, blood vessel patterns differ. For example, branching or arborizing vessels are suggestive of BCC. “These vessels are very crisp-looking on dermoscopy,” she said. “They’re all in the same plane of focus and they look like they were drawn in with a fine point marker. That’s different from other blood vessel patterns.” She also pointed out that superficial basal cells have short, fine telangiectasias. “When you put on the polarized light, the clue is the white, shiny structures,” she said.
Dotted vessels, meanwhile, appear on dermoscopy as small red dots aligned perpendicular to the skin surface. The differential includes inflammatory lesions like psoriasis, stasis, and trauma; clear cell acanthoma (characterized by a “string of pearls” arrangement), nevi, and melanoma. “I find dermoscopy most useful in diagnosing SCC – especially squamous cell in situ,” she said. “Important clinical clues suggestive of SCC or melanoma include a solitary lesion, it’s new, it’s growing, and it’s not going away with a topical steroid.”
An additional pattern to be aware of are hairpin vessels, which are looped and feature a sharp bend at one end. These are often seen in seborrheic keratoses. “You can’t count on the hairpin vessels alone, because you can see this in anything keratotic, such as in keratoacanthoma (at the periphery with a yellow keratotic center), warts, SCC, BCC, as well as in dermal nevi and Spitz nevi,” said Dr. Stein, who recommended dermoscopedia.org as resource.
Comma vessels, meanwhile, appear in dermal or compound nevi. She described these as “slightly curved vessels that are much less in focus than branched vessels, because they come in and out of the plane of focus,” she said. “If you put your dermatoscope on top of the nevus and wobble it around you can appreciate the curve. If you look at it from the side, it looks like a curve. If you look at it straight on it will look more like a line. If you look at from the end it will look like a dot.”
Another vessel type she discussed are linear irregular and polymorphous vessels, which she described as “any combination of different types of vessels. We get most worried when we see dotted and linear irregular vessels together. In that case, you worry about melanoma. These can also be seen in nevi and other tumors, such as BCC.”
Dr. Stein’s fourth tip of the presentation was a reminder to consider dermoscopy as one piece of the clinical exam. “Always think about the lesion in context of the rest of the clinical picture and history,” she said. “Don’t get discouraged if it’s hard; just keep practicing. Look for any brown and use your clinical clues to put together to make the right decision.”
She disclosed that NYU receives compensation from MoleSafe for her telemedicine dermoscopic diagnoses.
Even in the most experienced hands, .

“For me, pink lesions are challenging,” Jennifer A. Stein, MD, PhD, said during the virtual Orlando Dermatology Aesthetic and Clinical Conference. “How can dermoscopy help us distinguish between Spitz nevus, melanoma, clear cell acanthoma, psoriasis, basal cell carcinoma, and squamous cell carcinoma?”
Dr. Stein, professor of dermatology at New York University, offered four tips. First, look for the shiny white perpendicular lines, otherwise known as the chrysalis or crystalline pattern. “You can only see this feature when you’re looking with polarized light,” she said. “This is why you want a dermatoscope that has polarized light, and better yet, one that you’re able to turn on and off, the hybrid kind, because then you can convince yourself that you’re looking at this feature, because it blinks on and off.”
The differential diagnosis for white shiny perpendicular lines includes dermatofibroma/scars (which is most common), Spitz and atypical genital nevi, BCC, and melanoma. “Dermatofibromas sometimes have white circles or rings in the center,” Dr. Stein said. “In BCC, the lines aren’t always perpendicular. Sometimes it’s more of a blotch or strands.”
A second tip for managing a pink lesion on dermoscopy is to look for any brown color. “When you see that combo together you have to worry,” she said. “When you see pigment network on dermoscopy, you have to put melanoma in your differential. If you see shiny white lines in something that is melanocytic, there’s a 98% specificity for melanoma.”
A third tip she offered for managing pink lesions is to check the blood vessels for clues. “For years, I was just naming the vessels based on making the diagnosis and then deciding, ‘that’s a basal cell carcinoma; those must be branching vessels,’ ” said Dr. Stein, who manages NYU’s medical dermatology faculty group practice.
However, blood vessel patterns differ. For example, branching or arborizing vessels are suggestive of BCC. “These vessels are very crisp-looking on dermoscopy,” she said. “They’re all in the same plane of focus and they look like they were drawn in with a fine point marker. That’s different from other blood vessel patterns.” She also pointed out that superficial basal cells have short, fine telangiectasias. “When you put on the polarized light, the clue is the white, shiny structures,” she said.
Dotted vessels, meanwhile, appear on dermoscopy as small red dots aligned perpendicular to the skin surface. The differential includes inflammatory lesions like psoriasis, stasis, and trauma; clear cell acanthoma (characterized by a “string of pearls” arrangement), nevi, and melanoma. “I find dermoscopy most useful in diagnosing SCC – especially squamous cell in situ,” she said. “Important clinical clues suggestive of SCC or melanoma include a solitary lesion, it’s new, it’s growing, and it’s not going away with a topical steroid.”
An additional pattern to be aware of are hairpin vessels, which are looped and feature a sharp bend at one end. These are often seen in seborrheic keratoses. “You can’t count on the hairpin vessels alone, because you can see this in anything keratotic, such as in keratoacanthoma (at the periphery with a yellow keratotic center), warts, SCC, BCC, as well as in dermal nevi and Spitz nevi,” said Dr. Stein, who recommended dermoscopedia.org as resource.
Comma vessels, meanwhile, appear in dermal or compound nevi. She described these as “slightly curved vessels that are much less in focus than branched vessels, because they come in and out of the plane of focus,” she said. “If you put your dermatoscope on top of the nevus and wobble it around you can appreciate the curve. If you look at it from the side, it looks like a curve. If you look at it straight on it will look more like a line. If you look at from the end it will look like a dot.”
Another vessel type she discussed are linear irregular and polymorphous vessels, which she described as “any combination of different types of vessels. We get most worried when we see dotted and linear irregular vessels together. In that case, you worry about melanoma. These can also be seen in nevi and other tumors, such as BCC.”
Dr. Stein’s fourth tip of the presentation was a reminder to consider dermoscopy as one piece of the clinical exam. “Always think about the lesion in context of the rest of the clinical picture and history,” she said. “Don’t get discouraged if it’s hard; just keep practicing. Look for any brown and use your clinical clues to put together to make the right decision.”
She disclosed that NYU receives compensation from MoleSafe for her telemedicine dermoscopic diagnoses.
FROM ODAC 2021
TACTICS: TACE plus sorafenib improves PFS in unresectable HCC
The lack of a statistically significant difference in OS may have been due to the fact that patients randomized to receive TACE alone had more frequent post-trial therapies compared with patients assigned to TACE plus sorafenib, said study investigator Masatoshi Kudo, MD, PhD, of the Kindai University faculty of medicine in Osaka, Japan.
“These subsequent anticancer procedures and active systemic therapies have potentially diluted OS benefit in TACE plus sorafenib by extending post-progression survival and confounding survival analysis, implying the OS endpoint is not feasible anymore for TACE combination trials in the era of multitargeted agents and immune checkpoint inhibitors,” Dr. Kudo said at the 2021 Gastrointestinal Cancers Symposium (abstract 270).
Unresectable HCC
The TACTICS trial was launched in October 2010. Investigators enrolled 156 patients with unresectable HCC, Child-Pugh scores of 7 or less, treatable tumors (10 or fewer nodules of 10 cm or less) and adequate organ function.
Patients were randomized to receive TACE alone or with sorafenib. Sorafenib was delivered at a dose of 400 mg daily starting 2-3 weeks before the first TACE procedure to assess tolerability, followed by 800-mg daily doses. Sorafenib was interrupted for 2 days before and 3 days after each TACE session.
The trial had a gate-keeping design, which specified that OS would be formally analyzed only if PFS results were positive.
As reported in GUT in 2020, the trial met its PFS coprimary endpoint, with a median PFS of 25.2 months for the combination, compared with 13.5 months for TACE alone, at a median follow-up of 122.3 weeks. The hazard ratio (HR) for progression with the combination was 0.59 (P = .006).
Updated results
At the symposium, Dr. Kudo presented updated PFS results. At a median follow-up for all randomized patients of 33.4 months, the median PFS with the combination was 22.8 months, compared with 13.5 months for TACE alone (HR, 0.661; P = .02).
However, OS did not differ significantly between the groups, with a median of 36.2 months for the combination and 30.8 months for TACE alone (HR, 0.861; P = .40)
In a subgroup analysis of OS, there were small trends in favor of the combination compared with TACE alone in most categories, but the benefit of the combination was statistically significant only for the 12 patients with HCC of hepatitis B virus etiology (HR, 0.72; 95% CI, 0.006-0.808).
There were also trends favoring TACE plus sorafenib for PFS in a subgroup analysis, but none of the differences were statistically significant, except for patients who had received one or two TACE treatments prior to study entry (HR, 0.474; 95% CI, 0.276-0.812).
Treatment-emergent adverse events were consistent with those seen in the primary analysis, with no new safety signals seen at the last follow-up, Dr. Kudo said.
A majority of patients in both arms had subsequent anticancer therapy – 76.3% of the TACE-alone arm and 58.8% of the combination arm.
Patients in the TACE-alone arm were more likely than were those in the combination arm to have ablation (22.4% vs. 14.9%) or additional sorafenib (50% vs. 10.6%). Patients in the TACE-alone arm were also more likely to receive hepatic artery infusion chemotherapy a single time (27.6% vs. 19.1%) but less likely to receive it continuously (10.3% vs. 19.1%).
Dr. Kudo noted that in six trials in which TACE was combined with another agent, the correlation coefficient between PFS and OS was low, and the slope of weighted linear regression was more gentle than that seen in trials of other therapies for advanced HCC, “suggesting that long post-progression survivals strongly affected the OS in TACE combination trials.”
The TACTICS study was funded by the Japan Liver Oncology Group. Dr. Kudo disclosed relationships with Bayer, codeveloper of sorafenib, and multiple other companies.
The Gastrointestinal Cancers Symposium is sponsored by the American Gastroenterological Association, the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
The lack of a statistically significant difference in OS may have been due to the fact that patients randomized to receive TACE alone had more frequent post-trial therapies compared with patients assigned to TACE plus sorafenib, said study investigator Masatoshi Kudo, MD, PhD, of the Kindai University faculty of medicine in Osaka, Japan.
“These subsequent anticancer procedures and active systemic therapies have potentially diluted OS benefit in TACE plus sorafenib by extending post-progression survival and confounding survival analysis, implying the OS endpoint is not feasible anymore for TACE combination trials in the era of multitargeted agents and immune checkpoint inhibitors,” Dr. Kudo said at the 2021 Gastrointestinal Cancers Symposium (abstract 270).
Unresectable HCC
The TACTICS trial was launched in October 2010. Investigators enrolled 156 patients with unresectable HCC, Child-Pugh scores of 7 or less, treatable tumors (10 or fewer nodules of 10 cm or less) and adequate organ function.
Patients were randomized to receive TACE alone or with sorafenib. Sorafenib was delivered at a dose of 400 mg daily starting 2-3 weeks before the first TACE procedure to assess tolerability, followed by 800-mg daily doses. Sorafenib was interrupted for 2 days before and 3 days after each TACE session.
The trial had a gate-keeping design, which specified that OS would be formally analyzed only if PFS results were positive.
As reported in GUT in 2020, the trial met its PFS coprimary endpoint, with a median PFS of 25.2 months for the combination, compared with 13.5 months for TACE alone, at a median follow-up of 122.3 weeks. The hazard ratio (HR) for progression with the combination was 0.59 (P = .006).
Updated results
At the symposium, Dr. Kudo presented updated PFS results. At a median follow-up for all randomized patients of 33.4 months, the median PFS with the combination was 22.8 months, compared with 13.5 months for TACE alone (HR, 0.661; P = .02).
However, OS did not differ significantly between the groups, with a median of 36.2 months for the combination and 30.8 months for TACE alone (HR, 0.861; P = .40)
In a subgroup analysis of OS, there were small trends in favor of the combination compared with TACE alone in most categories, but the benefit of the combination was statistically significant only for the 12 patients with HCC of hepatitis B virus etiology (HR, 0.72; 95% CI, 0.006-0.808).
There were also trends favoring TACE plus sorafenib for PFS in a subgroup analysis, but none of the differences were statistically significant, except for patients who had received one or two TACE treatments prior to study entry (HR, 0.474; 95% CI, 0.276-0.812).
Treatment-emergent adverse events were consistent with those seen in the primary analysis, with no new safety signals seen at the last follow-up, Dr. Kudo said.
A majority of patients in both arms had subsequent anticancer therapy – 76.3% of the TACE-alone arm and 58.8% of the combination arm.
Patients in the TACE-alone arm were more likely than were those in the combination arm to have ablation (22.4% vs. 14.9%) or additional sorafenib (50% vs. 10.6%). Patients in the TACE-alone arm were also more likely to receive hepatic artery infusion chemotherapy a single time (27.6% vs. 19.1%) but less likely to receive it continuously (10.3% vs. 19.1%).
Dr. Kudo noted that in six trials in which TACE was combined with another agent, the correlation coefficient between PFS and OS was low, and the slope of weighted linear regression was more gentle than that seen in trials of other therapies for advanced HCC, “suggesting that long post-progression survivals strongly affected the OS in TACE combination trials.”
The TACTICS study was funded by the Japan Liver Oncology Group. Dr. Kudo disclosed relationships with Bayer, codeveloper of sorafenib, and multiple other companies.
The Gastrointestinal Cancers Symposium is sponsored by the American Gastroenterological Association, the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
The lack of a statistically significant difference in OS may have been due to the fact that patients randomized to receive TACE alone had more frequent post-trial therapies compared with patients assigned to TACE plus sorafenib, said study investigator Masatoshi Kudo, MD, PhD, of the Kindai University faculty of medicine in Osaka, Japan.
“These subsequent anticancer procedures and active systemic therapies have potentially diluted OS benefit in TACE plus sorafenib by extending post-progression survival and confounding survival analysis, implying the OS endpoint is not feasible anymore for TACE combination trials in the era of multitargeted agents and immune checkpoint inhibitors,” Dr. Kudo said at the 2021 Gastrointestinal Cancers Symposium (abstract 270).
Unresectable HCC
The TACTICS trial was launched in October 2010. Investigators enrolled 156 patients with unresectable HCC, Child-Pugh scores of 7 or less, treatable tumors (10 or fewer nodules of 10 cm or less) and adequate organ function.
Patients were randomized to receive TACE alone or with sorafenib. Sorafenib was delivered at a dose of 400 mg daily starting 2-3 weeks before the first TACE procedure to assess tolerability, followed by 800-mg daily doses. Sorafenib was interrupted for 2 days before and 3 days after each TACE session.
The trial had a gate-keeping design, which specified that OS would be formally analyzed only if PFS results were positive.
As reported in GUT in 2020, the trial met its PFS coprimary endpoint, with a median PFS of 25.2 months for the combination, compared with 13.5 months for TACE alone, at a median follow-up of 122.3 weeks. The hazard ratio (HR) for progression with the combination was 0.59 (P = .006).
Updated results
At the symposium, Dr. Kudo presented updated PFS results. At a median follow-up for all randomized patients of 33.4 months, the median PFS with the combination was 22.8 months, compared with 13.5 months for TACE alone (HR, 0.661; P = .02).
However, OS did not differ significantly between the groups, with a median of 36.2 months for the combination and 30.8 months for TACE alone (HR, 0.861; P = .40)
In a subgroup analysis of OS, there were small trends in favor of the combination compared with TACE alone in most categories, but the benefit of the combination was statistically significant only for the 12 patients with HCC of hepatitis B virus etiology (HR, 0.72; 95% CI, 0.006-0.808).
There were also trends favoring TACE plus sorafenib for PFS in a subgroup analysis, but none of the differences were statistically significant, except for patients who had received one or two TACE treatments prior to study entry (HR, 0.474; 95% CI, 0.276-0.812).
Treatment-emergent adverse events were consistent with those seen in the primary analysis, with no new safety signals seen at the last follow-up, Dr. Kudo said.
A majority of patients in both arms had subsequent anticancer therapy – 76.3% of the TACE-alone arm and 58.8% of the combination arm.
Patients in the TACE-alone arm were more likely than were those in the combination arm to have ablation (22.4% vs. 14.9%) or additional sorafenib (50% vs. 10.6%). Patients in the TACE-alone arm were also more likely to receive hepatic artery infusion chemotherapy a single time (27.6% vs. 19.1%) but less likely to receive it continuously (10.3% vs. 19.1%).
Dr. Kudo noted that in six trials in which TACE was combined with another agent, the correlation coefficient between PFS and OS was low, and the slope of weighted linear regression was more gentle than that seen in trials of other therapies for advanced HCC, “suggesting that long post-progression survivals strongly affected the OS in TACE combination trials.”
The TACTICS study was funded by the Japan Liver Oncology Group. Dr. Kudo disclosed relationships with Bayer, codeveloper of sorafenib, and multiple other companies.
The Gastrointestinal Cancers Symposium is sponsored by the American Gastroenterological Association, the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
FROM GI CANCERS SYMPOSIUM 2021
High cost of pancreatic enzymes a barrier for patients with cancer
Pancreatic enzyme replacement therapy (PERT) is often an essential component of the treatment regimen for patients with pancreatic cancer, but it can be very pricey.
“Out-of-pocket costs for a 30-day supply of enzymes for Medicare beneficiaries can be as high as $1,000,” commented Arjun Gupta, MD, an oncology fellow at Johns Hopkins Sidney Kimmel Comprehensive Cancer Center, Baltimore.
This can contribute to financial toxicity for patients who already have a high symptom burden and distress. The high cost of this supportive care has been underappreciated, he said.
In addition to its use for patients with pancreatic cancer, PERT is also prescribed to patients with chronic pancreatitis and cystic fibrosis. These enzymes can reduce symptoms of indigestion and improve nutrition for patients with exocrine pancreatic insufficiency, he explained.
“Out-of-pocket costs for two large pancreas enzyme capsules, which are often required for a meal, may be $15. And these need to be taken at every meal and may be more expensive than the meal itself,” he said in an interview.
Dr. Gupta led a new study which showed that, among Medicare beneficiaries, the expected out-of-pocket costs for a 30-day supply of optimally dosed PERT averaged $999 across formulations. Patients’ costs, including deductibles and coinsurance, ranged from $853 to $1,536.
The out-of-pocket costs were lower after patients met the deductible ($673; range, $527-$1,210) and continued to decrease after reaching catastrophic coverage ($135; range, $105-$242).
The findings were presented at the 2021 Gastrointestinal Cancers Symposium.
Dr. Gupta noted that there has been a lot of publicity about very expensive anticancer drugs, but little has been said about the costs of products used in supportive care. “While it’s true that many patients cannot afford the drugs, there are patient-assistance programs where they can often get them free of charge,” he said. “But supportive care agents, such as those for constipation or the enzymes – all of those can nickel and dime you and end up being very costly.”
These agents add substantially to the drug cost burden. “Some patients also need insulin, which is also insanely expensive,” he said.
One of the reasons for the high cost of PERT is that there are very few options, and all the available products are brand-name agents. Dr. Gupta noted that clinicians often underprescribe pancreatic enzymes in clinical practice. “Because of this, we wanted to look at what are the estimated out-of-pocket costs for patients directly when they’re prescribed an optimal regimen of pancreatic enzymes,” he said.
Study details
For their study, Dr. Gupta and colleagues assessed PERT costs using the Medicare Part D formulary and pricing files for the first quarter of 2020. Point-of-sale and out-of-pocket costs for each PERT formulation were calculated among Part D standalone and Medicare Advantage prescription drug plans.
Costs were then assessed using three scenarios: the standard-benefit design, with a $435 deductible and 25% coinsurance after the deductible is met; 25% coinsurance to fill a prescription after the deductible while in the coverage gap until the patient spends $6,350 out of pocket; and 5% coinsurance once catastrophic coverage is reached.
Across 3,974 plans nationwide, four formulations in 17 different doses were covered by Medicare plans during the study period. Doses ranged from 3,000 to 40,000 lipase units, and the per-unit list price ranged from $1.44 to $13.89.
The point-of-sale price for a 30-day supply of optimally dosed PERT ranged from $2,109 to $4,840.
Dr. Gupta noted that a “good-sized meal often requires 80,000 units of lipase, or two of the very largest pills. Of note, these pills need to be taken meal after meal every meal throughout a patient’s life.”
Prescribers and dietitians try to find the least expensive options, including patient-assistance programs, but in the end, they are sometimes forced to underprescribe. “Some patients will go and buy over-the-counter pancreatic enzyme supplements, and it seems like a good way to cut costs,” said Dr. Gupta, “but it is not recommended for people with pancreatic cancer.”
The problem with these formulations is that they are not regulated. “The enzyme content in them is also minuscule, in the range of hundreds of units instead of the 50,000 units needed per meal,” he said. “Patients end up spending much more for ineffective therapies.”
The study received no outside funding. Dr. Gupta disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pancreatic enzyme replacement therapy (PERT) is often an essential component of the treatment regimen for patients with pancreatic cancer, but it can be very pricey.
“Out-of-pocket costs for a 30-day supply of enzymes for Medicare beneficiaries can be as high as $1,000,” commented Arjun Gupta, MD, an oncology fellow at Johns Hopkins Sidney Kimmel Comprehensive Cancer Center, Baltimore.
This can contribute to financial toxicity for patients who already have a high symptom burden and distress. The high cost of this supportive care has been underappreciated, he said.
In addition to its use for patients with pancreatic cancer, PERT is also prescribed to patients with chronic pancreatitis and cystic fibrosis. These enzymes can reduce symptoms of indigestion and improve nutrition for patients with exocrine pancreatic insufficiency, he explained.
“Out-of-pocket costs for two large pancreas enzyme capsules, which are often required for a meal, may be $15. And these need to be taken at every meal and may be more expensive than the meal itself,” he said in an interview.
Dr. Gupta led a new study which showed that, among Medicare beneficiaries, the expected out-of-pocket costs for a 30-day supply of optimally dosed PERT averaged $999 across formulations. Patients’ costs, including deductibles and coinsurance, ranged from $853 to $1,536.
The out-of-pocket costs were lower after patients met the deductible ($673; range, $527-$1,210) and continued to decrease after reaching catastrophic coverage ($135; range, $105-$242).
The findings were presented at the 2021 Gastrointestinal Cancers Symposium.
Dr. Gupta noted that there has been a lot of publicity about very expensive anticancer drugs, but little has been said about the costs of products used in supportive care. “While it’s true that many patients cannot afford the drugs, there are patient-assistance programs where they can often get them free of charge,” he said. “But supportive care agents, such as those for constipation or the enzymes – all of those can nickel and dime you and end up being very costly.”
These agents add substantially to the drug cost burden. “Some patients also need insulin, which is also insanely expensive,” he said.
One of the reasons for the high cost of PERT is that there are very few options, and all the available products are brand-name agents. Dr. Gupta noted that clinicians often underprescribe pancreatic enzymes in clinical practice. “Because of this, we wanted to look at what are the estimated out-of-pocket costs for patients directly when they’re prescribed an optimal regimen of pancreatic enzymes,” he said.
Study details
For their study, Dr. Gupta and colleagues assessed PERT costs using the Medicare Part D formulary and pricing files for the first quarter of 2020. Point-of-sale and out-of-pocket costs for each PERT formulation were calculated among Part D standalone and Medicare Advantage prescription drug plans.
Costs were then assessed using three scenarios: the standard-benefit design, with a $435 deductible and 25% coinsurance after the deductible is met; 25% coinsurance to fill a prescription after the deductible while in the coverage gap until the patient spends $6,350 out of pocket; and 5% coinsurance once catastrophic coverage is reached.
Across 3,974 plans nationwide, four formulations in 17 different doses were covered by Medicare plans during the study period. Doses ranged from 3,000 to 40,000 lipase units, and the per-unit list price ranged from $1.44 to $13.89.
The point-of-sale price for a 30-day supply of optimally dosed PERT ranged from $2,109 to $4,840.
Dr. Gupta noted that a “good-sized meal often requires 80,000 units of lipase, or two of the very largest pills. Of note, these pills need to be taken meal after meal every meal throughout a patient’s life.”
Prescribers and dietitians try to find the least expensive options, including patient-assistance programs, but in the end, they are sometimes forced to underprescribe. “Some patients will go and buy over-the-counter pancreatic enzyme supplements, and it seems like a good way to cut costs,” said Dr. Gupta, “but it is not recommended for people with pancreatic cancer.”
The problem with these formulations is that they are not regulated. “The enzyme content in them is also minuscule, in the range of hundreds of units instead of the 50,000 units needed per meal,” he said. “Patients end up spending much more for ineffective therapies.”
The study received no outside funding. Dr. Gupta disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pancreatic enzyme replacement therapy (PERT) is often an essential component of the treatment regimen for patients with pancreatic cancer, but it can be very pricey.
“Out-of-pocket costs for a 30-day supply of enzymes for Medicare beneficiaries can be as high as $1,000,” commented Arjun Gupta, MD, an oncology fellow at Johns Hopkins Sidney Kimmel Comprehensive Cancer Center, Baltimore.
This can contribute to financial toxicity for patients who already have a high symptom burden and distress. The high cost of this supportive care has been underappreciated, he said.
In addition to its use for patients with pancreatic cancer, PERT is also prescribed to patients with chronic pancreatitis and cystic fibrosis. These enzymes can reduce symptoms of indigestion and improve nutrition for patients with exocrine pancreatic insufficiency, he explained.
“Out-of-pocket costs for two large pancreas enzyme capsules, which are often required for a meal, may be $15. And these need to be taken at every meal and may be more expensive than the meal itself,” he said in an interview.
Dr. Gupta led a new study which showed that, among Medicare beneficiaries, the expected out-of-pocket costs for a 30-day supply of optimally dosed PERT averaged $999 across formulations. Patients’ costs, including deductibles and coinsurance, ranged from $853 to $1,536.
The out-of-pocket costs were lower after patients met the deductible ($673; range, $527-$1,210) and continued to decrease after reaching catastrophic coverage ($135; range, $105-$242).
The findings were presented at the 2021 Gastrointestinal Cancers Symposium.
Dr. Gupta noted that there has been a lot of publicity about very expensive anticancer drugs, but little has been said about the costs of products used in supportive care. “While it’s true that many patients cannot afford the drugs, there are patient-assistance programs where they can often get them free of charge,” he said. “But supportive care agents, such as those for constipation or the enzymes – all of those can nickel and dime you and end up being very costly.”
These agents add substantially to the drug cost burden. “Some patients also need insulin, which is also insanely expensive,” he said.
One of the reasons for the high cost of PERT is that there are very few options, and all the available products are brand-name agents. Dr. Gupta noted that clinicians often underprescribe pancreatic enzymes in clinical practice. “Because of this, we wanted to look at what are the estimated out-of-pocket costs for patients directly when they’re prescribed an optimal regimen of pancreatic enzymes,” he said.
Study details
For their study, Dr. Gupta and colleagues assessed PERT costs using the Medicare Part D formulary and pricing files for the first quarter of 2020. Point-of-sale and out-of-pocket costs for each PERT formulation were calculated among Part D standalone and Medicare Advantage prescription drug plans.
Costs were then assessed using three scenarios: the standard-benefit design, with a $435 deductible and 25% coinsurance after the deductible is met; 25% coinsurance to fill a prescription after the deductible while in the coverage gap until the patient spends $6,350 out of pocket; and 5% coinsurance once catastrophic coverage is reached.
Across 3,974 plans nationwide, four formulations in 17 different doses were covered by Medicare plans during the study period. Doses ranged from 3,000 to 40,000 lipase units, and the per-unit list price ranged from $1.44 to $13.89.
The point-of-sale price for a 30-day supply of optimally dosed PERT ranged from $2,109 to $4,840.
Dr. Gupta noted that a “good-sized meal often requires 80,000 units of lipase, or two of the very largest pills. Of note, these pills need to be taken meal after meal every meal throughout a patient’s life.”
Prescribers and dietitians try to find the least expensive options, including patient-assistance programs, but in the end, they are sometimes forced to underprescribe. “Some patients will go and buy over-the-counter pancreatic enzyme supplements, and it seems like a good way to cut costs,” said Dr. Gupta, “but it is not recommended for people with pancreatic cancer.”
The problem with these formulations is that they are not regulated. “The enzyme content in them is also minuscule, in the range of hundreds of units instead of the 50,000 units needed per meal,” he said. “Patients end up spending much more for ineffective therapies.”
The study received no outside funding. Dr. Gupta disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New technique uses voice to evaluate thyroid nodules
However, it has not yet been tested for cancer detection.
The new approach involves holding a linear ultrasound probe to the throat of the patient, who is then requested to vocalize an “eee” sound at 150 Hz. A loudspeaker plays a 150-Hz sound to guide the patient.
The vocal vibrations generated, called shear waves, are detected by the probe as they pass through the thyroid. Software the researchers developed calculates the velocity of the shear waves, which move faster through stiffer tissue. The software produces a stiffness map that is then superimposed onto a gray-scale (B mode) thyroid image from the ultrasound.
Cancerous tissue is stiffer than healthy tissue and benign nodules, so shear waves pass through it more quickly, the researchers explained. Areas of particular stiffness that are revealed by the test are a concern.
The study was published online Jan. 12 in Applied Physics Letters.
The new approach is a noninvasive method that “would reduce the stress of patients during their medical exams. Having to sing during a medical exam can perhaps help release some of the nervous tension even more,” lead investigator Steve Beuve, PhD, of the Université de Tours (France), said in a press release. The main benefit of this technique is that it is “quick and easy,” he added. Data acquisition takes about a second, and no specialized equipment is required. Imaging can be rendered by any Doppler ultrasound set at an ultrafast frame rate to track the shear waves. The computer program automatically calculates wave velocity through various parts of the thyroid.
The technique, dubbed vocal passive elastography (VPE), has not yet been tested to see how well it distinguishes cancerous from benign thyroid nodules.
“We want to cooperate with physicians to propose protocols to verify [VPE’s] relevance,” Dr. Beuve said.
Because no data are currently available on how accurate VPE is in diagnosing malignant nodules, it is not possible to comment on its potential usefulness, Aya Kamaya, MD, a radiology professor at Stanford (Calif.) University Medical Center, said in an interview.
Ultrasound elastography for diagnosing thyroid disease has been in development for years. More than 100 reports have been published in the medical literature since 2005. Various devices are available commercially, but for now, elastography for thyroid nodules remains investigational, she said.
Another expert who was approached for comment was more critical.
Lisa Orloff, MD, a professor and director of endocrine head and neck surgery and the thyroid tumor program at Stanford University, noted that, in general, “elastography has not gained more traction in thyroid evaluation to date because it does not appear to reduce the need for [fine-needle aspiration] of suspicious nodules based on gray-scale ultrasound alone, without elastography.”
As for the French report, she said that “while the voice might be a convenient shear wave source, I am very skeptical at many levels. I get the impression that [this is] a laboratory-based concept that is fraught with confounding factors in attempting real-world application.
“One concern is that the thyroid gland and nodule stiffness are affected by factors including underlying goiter, autoimmune disease, fluid content of nodules, calcifications, and other variables that can be present in benign or malignant conditions. And there are so many variables that would affect an individual patient’s ability (or not) to phonate at 150 Hz,” Dr. Orloff said.
VPE is an extension of passive elastography, which extracts elasticity data from the natural vibrations caused by the heart, blood pulsatility, and muscle activity, the authors explained. The team turned to vocalization at 150 Hz in part to overcome the physiological background noise in the thyroid from carotid pulsation at about 1-10 Hz.
The group is exploring vocalizations at other frequencies and is working to improve the computer program interface. They are also exploring VPE for other organs, including the brain.
The source of funding for the study and the authors’ relevant financial relationships were not reported.
A version of this article first appeared on Medscape.com.
However, it has not yet been tested for cancer detection.
The new approach involves holding a linear ultrasound probe to the throat of the patient, who is then requested to vocalize an “eee” sound at 150 Hz. A loudspeaker plays a 150-Hz sound to guide the patient.
The vocal vibrations generated, called shear waves, are detected by the probe as they pass through the thyroid. Software the researchers developed calculates the velocity of the shear waves, which move faster through stiffer tissue. The software produces a stiffness map that is then superimposed onto a gray-scale (B mode) thyroid image from the ultrasound.
Cancerous tissue is stiffer than healthy tissue and benign nodules, so shear waves pass through it more quickly, the researchers explained. Areas of particular stiffness that are revealed by the test are a concern.
The study was published online Jan. 12 in Applied Physics Letters.
The new approach is a noninvasive method that “would reduce the stress of patients during their medical exams. Having to sing during a medical exam can perhaps help release some of the nervous tension even more,” lead investigator Steve Beuve, PhD, of the Université de Tours (France), said in a press release. The main benefit of this technique is that it is “quick and easy,” he added. Data acquisition takes about a second, and no specialized equipment is required. Imaging can be rendered by any Doppler ultrasound set at an ultrafast frame rate to track the shear waves. The computer program automatically calculates wave velocity through various parts of the thyroid.
The technique, dubbed vocal passive elastography (VPE), has not yet been tested to see how well it distinguishes cancerous from benign thyroid nodules.
“We want to cooperate with physicians to propose protocols to verify [VPE’s] relevance,” Dr. Beuve said.
Because no data are currently available on how accurate VPE is in diagnosing malignant nodules, it is not possible to comment on its potential usefulness, Aya Kamaya, MD, a radiology professor at Stanford (Calif.) University Medical Center, said in an interview.
Ultrasound elastography for diagnosing thyroid disease has been in development for years. More than 100 reports have been published in the medical literature since 2005. Various devices are available commercially, but for now, elastography for thyroid nodules remains investigational, she said.
Another expert who was approached for comment was more critical.
Lisa Orloff, MD, a professor and director of endocrine head and neck surgery and the thyroid tumor program at Stanford University, noted that, in general, “elastography has not gained more traction in thyroid evaluation to date because it does not appear to reduce the need for [fine-needle aspiration] of suspicious nodules based on gray-scale ultrasound alone, without elastography.”
As for the French report, she said that “while the voice might be a convenient shear wave source, I am very skeptical at many levels. I get the impression that [this is] a laboratory-based concept that is fraught with confounding factors in attempting real-world application.
“One concern is that the thyroid gland and nodule stiffness are affected by factors including underlying goiter, autoimmune disease, fluid content of nodules, calcifications, and other variables that can be present in benign or malignant conditions. And there are so many variables that would affect an individual patient’s ability (or not) to phonate at 150 Hz,” Dr. Orloff said.
VPE is an extension of passive elastography, which extracts elasticity data from the natural vibrations caused by the heart, blood pulsatility, and muscle activity, the authors explained. The team turned to vocalization at 150 Hz in part to overcome the physiological background noise in the thyroid from carotid pulsation at about 1-10 Hz.
The group is exploring vocalizations at other frequencies and is working to improve the computer program interface. They are also exploring VPE for other organs, including the brain.
The source of funding for the study and the authors’ relevant financial relationships were not reported.
A version of this article first appeared on Medscape.com.
However, it has not yet been tested for cancer detection.
The new approach involves holding a linear ultrasound probe to the throat of the patient, who is then requested to vocalize an “eee” sound at 150 Hz. A loudspeaker plays a 150-Hz sound to guide the patient.
The vocal vibrations generated, called shear waves, are detected by the probe as they pass through the thyroid. Software the researchers developed calculates the velocity of the shear waves, which move faster through stiffer tissue. The software produces a stiffness map that is then superimposed onto a gray-scale (B mode) thyroid image from the ultrasound.
Cancerous tissue is stiffer than healthy tissue and benign nodules, so shear waves pass through it more quickly, the researchers explained. Areas of particular stiffness that are revealed by the test are a concern.
The study was published online Jan. 12 in Applied Physics Letters.
The new approach is a noninvasive method that “would reduce the stress of patients during their medical exams. Having to sing during a medical exam can perhaps help release some of the nervous tension even more,” lead investigator Steve Beuve, PhD, of the Université de Tours (France), said in a press release. The main benefit of this technique is that it is “quick and easy,” he added. Data acquisition takes about a second, and no specialized equipment is required. Imaging can be rendered by any Doppler ultrasound set at an ultrafast frame rate to track the shear waves. The computer program automatically calculates wave velocity through various parts of the thyroid.
The technique, dubbed vocal passive elastography (VPE), has not yet been tested to see how well it distinguishes cancerous from benign thyroid nodules.
“We want to cooperate with physicians to propose protocols to verify [VPE’s] relevance,” Dr. Beuve said.
Because no data are currently available on how accurate VPE is in diagnosing malignant nodules, it is not possible to comment on its potential usefulness, Aya Kamaya, MD, a radiology professor at Stanford (Calif.) University Medical Center, said in an interview.
Ultrasound elastography for diagnosing thyroid disease has been in development for years. More than 100 reports have been published in the medical literature since 2005. Various devices are available commercially, but for now, elastography for thyroid nodules remains investigational, she said.
Another expert who was approached for comment was more critical.
Lisa Orloff, MD, a professor and director of endocrine head and neck surgery and the thyroid tumor program at Stanford University, noted that, in general, “elastography has not gained more traction in thyroid evaluation to date because it does not appear to reduce the need for [fine-needle aspiration] of suspicious nodules based on gray-scale ultrasound alone, without elastography.”
As for the French report, she said that “while the voice might be a convenient shear wave source, I am very skeptical at many levels. I get the impression that [this is] a laboratory-based concept that is fraught with confounding factors in attempting real-world application.
“One concern is that the thyroid gland and nodule stiffness are affected by factors including underlying goiter, autoimmune disease, fluid content of nodules, calcifications, and other variables that can be present in benign or malignant conditions. And there are so many variables that would affect an individual patient’s ability (or not) to phonate at 150 Hz,” Dr. Orloff said.
VPE is an extension of passive elastography, which extracts elasticity data from the natural vibrations caused by the heart, blood pulsatility, and muscle activity, the authors explained. The team turned to vocalization at 150 Hz in part to overcome the physiological background noise in the thyroid from carotid pulsation at about 1-10 Hz.
The group is exploring vocalizations at other frequencies and is working to improve the computer program interface. They are also exploring VPE for other organs, including the brain.
The source of funding for the study and the authors’ relevant financial relationships were not reported.
A version of this article first appeared on Medscape.com.
Pelvic pain
A 34-year-old woman with no significant past medical history presented as a new patient to our family medicine clinic with 2 weeks of intermittent lower abdominal and pelvic pain. She was sexually active with 1 partner and denied abnormal vaginal discharge or bleeding. She mentioned she’d had an intrauterine contraceptive device (IUD) placed a few weeks ago. The patient was afebrile, and her pelvic examination was unremarkable.
Physical examination showed mild tenderness to palpation over the lower abdomen without rebound tenderness or guarding. A complete metabolic panel revealed no significant abnormalities, and her human chorionic gonadotropin levels were normal.
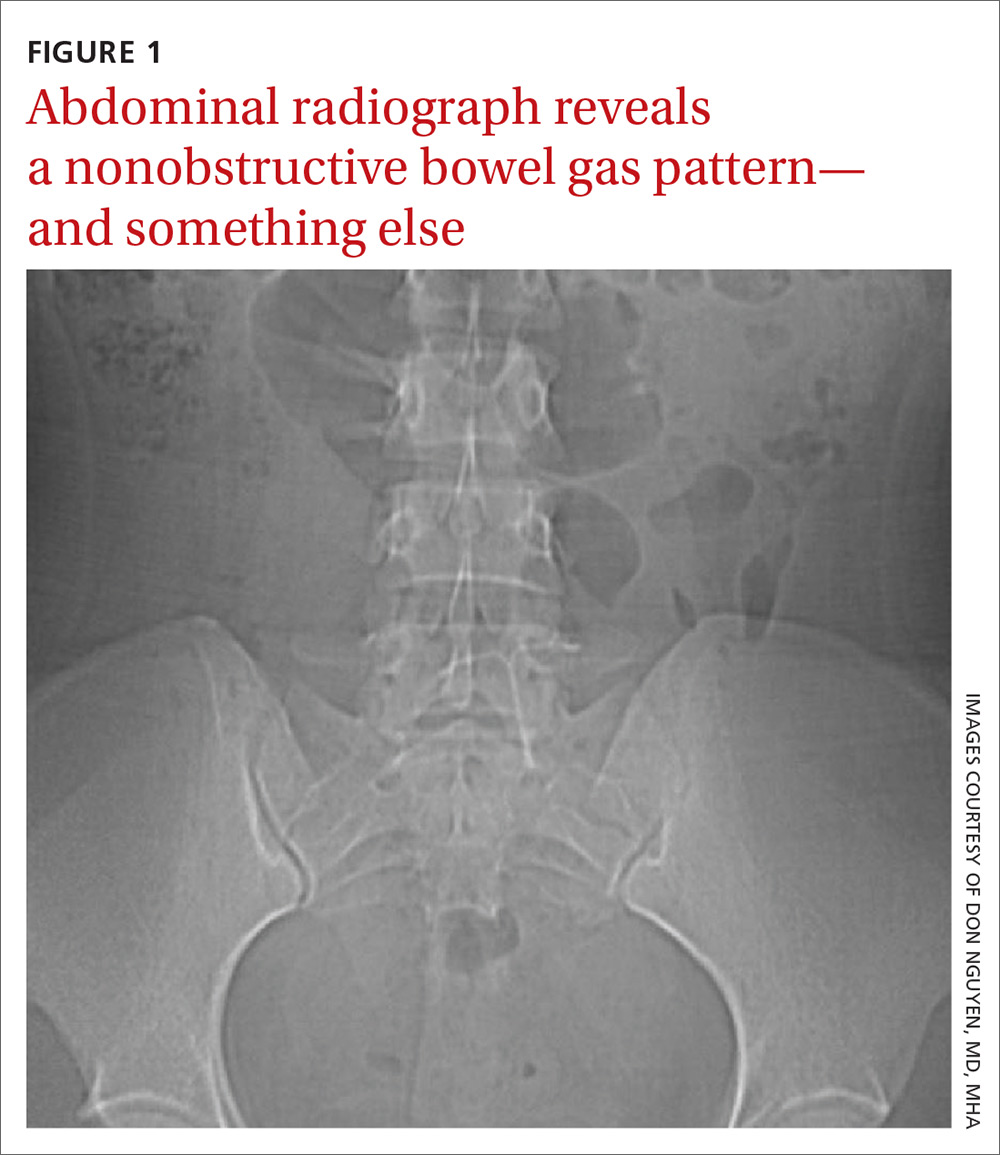
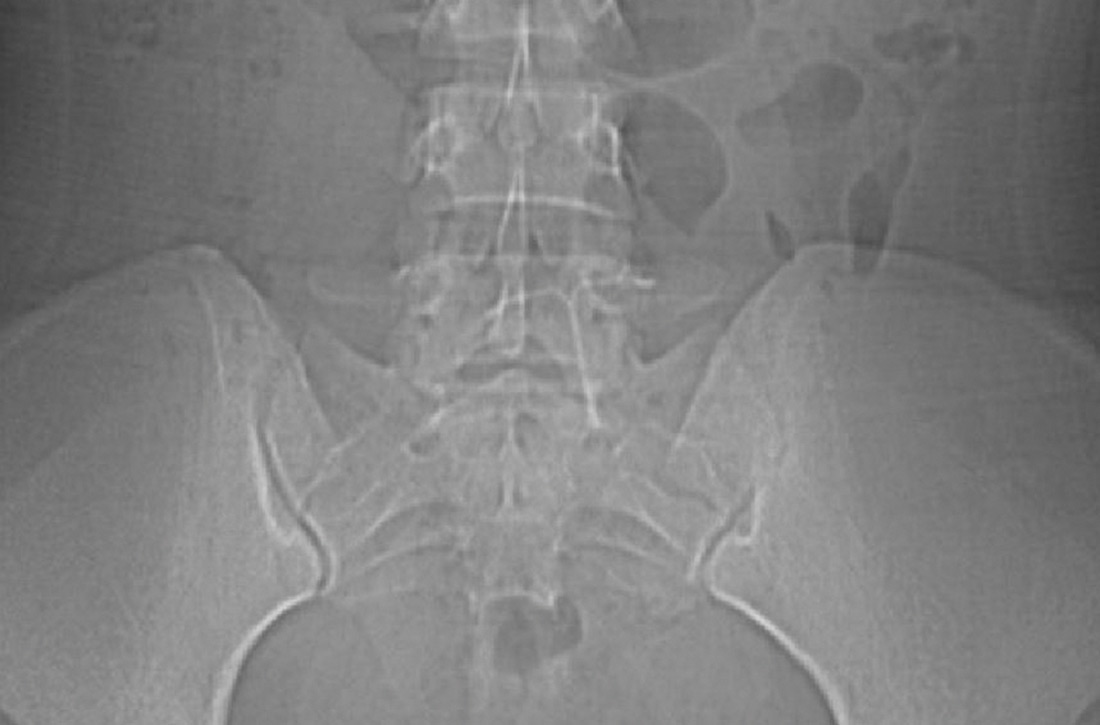
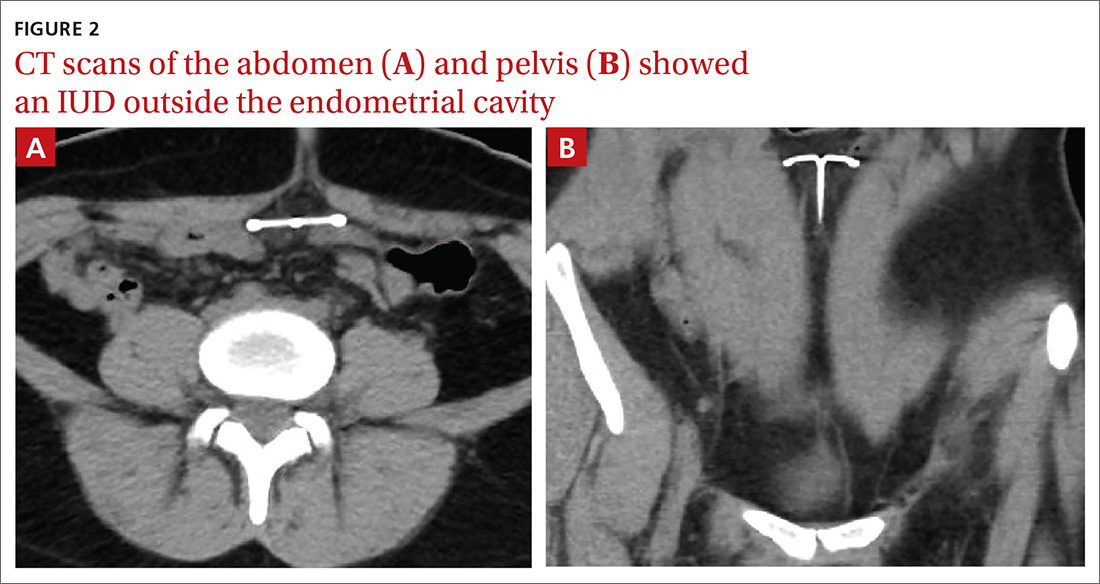
Findings from the physical exam and her clinical history prompted the need for imaging. An abdominal radiograph (FIGURE 1) and noncontrast computed tomography (FIGURES 2A and 2B) were subsequently ordered.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Intra-abdominal IUD migration
The abdominal radiograph revealed a nonobstructive bowel gas pattern with an IUD overlaying the central lower abdomen and pelvis at the L5-S1 level (FIGURE 1). Computed tomography (CT) of her abdomen and pelvis showed that the IUD was outside the endometrial cavity (FIGURES 2A and 2B). There was no evidence of pneumoperitoneum or bowel perforation. Based on the work-up and imaging, the patient’s pain was due to intra-abdominal IUD malpositioning.
Diagnostic criteria for IUD malpositioning include device migration into 1 of several locations, such as the lower uterine segment or cervix. IUD malpositioning can involve the rotation or protrusion of the device into or through the myometrium. On imaging, a well-positioned IUD should have a straight stem contained within the endometrial cavity, with the arms of the IUD extending laterally at the uterine fundus.
For our patient, an abdominal radiograph showed that her IUD was superiorly displaced outside the expected region of the endometrial cavity. CT helped to confirm this.
Complications with IUDs are few
Using an IUD is an increasingly popular method of contraception because it is effective and generally well tolerated, with minimal adverse effects or complications. In a multicenter retrospective chart review of 2138 patients who had IUDs, Aoun et al found that serious complications included pelvic inflammatory disease (2%), IUD expulsion (6%), and pregnancy (1%).1 In a retrospective cohort study examining complications among 90,489 women with IUDs, Berenson et al found ectopic pregnancy and uterine perforation affected < 1%.2
A less serious complication is IUD malpositioning. Although it does seem to occur more often than other, more serious complications, the exact incidence is unknown. In a retrospective case-control study, Braaten et al reported the rate for IUD malpositioning was 10.4% among 182 women.3 Malpositioned IUDs may be more likely to occur in those with suspected adenomyosis.3 In a study by de Kroon et al, the estimated prevalence rate for an abnormal IUD position ranged from 4% to 7.7% among 195 patients.4
Continue to: The clinical presentation of IUD migration
The clinical presentation of IUD migration
Identification of a malpositioned IUD is needed to avoid the possible increased risk for uterine perforation, IUD expulsion, or pregnancy.5
IUDs that have perforated the uterus float freely in the pelvis or abdomen and can result in injury to adjacent structures as well as peritonitis, fistulas, and hemorrhage.5-7 In addition, adhesion formation over the IUD can lead to intestinal obstruction, infertility, and chronic pain.6
Common symptoms of IUD malpositioning include abdominal or pelvic pain and abnormal bleeding, although many patients may be asymptomatic.8 In a retrospective study of 167 patients with IUDs who underwent pelvic ultrasound, 28 patients were found to have an IUD in an abnormal position.8 Rates of bleeding and pain were higher in patients with malpositioned IUDs (35.7% and 39.3%, respectively) than in those with a normally positioned IUD (15.1% and 19.4%, respectively).8
The differential Dx includes endometriosis and fibroids
IUD malpositioning can be distinguished from other diagnoses that cause pelvic pain and have similar presentations—including endometriosis, ectopic pregnancy, and fibroids—through imaging study findings, clinical history, and presentation.
Other conditions that may need to be ruled out include pelvic inflammatory disease, acute appendicitis, and ovarian cysts.9 A thorough history and physical examination can help rule out these conditions by organ system, and laboratory and imaging studies can help to confirm the diagnosis.
Continue to: Which imaging tool to use, and when
Which imaging tool to use, and when
Assessment of intrauterine contraception placement requires evaluation of the uterine cavity; gynecologic examination alone is not sufficient to fully evaluate for IUD position. Certain imaging studies are particularly helpful for revealing possible IUD migration.
Ultrasound—a widely available, radiation-free modality—is the first-line imaging tool for evaluation of an IUD’s position.10 In addition, ultrasound can provide effective evaluation of other pelvic structures, which is helpful in identifying or eliminating other causes of pain or abnormal bleeding.
Conventional radiography. If the IUD is not visualized on ultrasound, the American College of Obstetricians and Gynecologists (ACOG) recommends radiography to determine if the IUD has been expelled or has migrated to an extra-uterine position.6
CT may be best suited for the evaluation of more severe complications of IUD malpositioning, including visceral perforation, abscess formation, or bowel obstruction. CT should be considered if the patient’s clinical presentation is suspicious for a more serious intra-abdominal pathology.
Management depends on the IUD’s position
For patients whose IUD has an uncertain position or nonvisualized intravaginal strings, ACOG’s first-line recommendations include ruling out pregnancy, using an alternative method for contraception, and ordering pelvic ultrasonography.6 ACOG recommendations for the management of IUD malpositioning depend on the device’s location and the patient’s symptomatology.
Continue to: Management of low-lying IUDs
Management of low-lying IUDs is complex. An IUD that is malpositioned in the cervix is considered partially expelled and should be completely removed.6 For asymptomatic patients with an IUD located in the lower uterine segment and above the internal cervical os, there should be strong consideration given to leaving the IUD in place because removal is associated with higher rates of pregnancy given the low rates of initiation of effective contraception following removal.6
IUD malpositioning in the peritoneal cavity requires surgical intervention. Although ACOG’s first-line recommendation is laparoscopic intervention, laparotomy can be considered if laparoscopy does not result in the removal of the IUD or the patient has more severe complications (sepsis or bowel perforation).6 At the time of IUD removal, the clinician should also discuss and/or prescribe interim contraception.
Treatment for our patient included uncomplicated laparoscopic surgical removal of the intra-abdominal IUD. The patient’s symptoms went away following the procedure, and she was subsequently switched to an oral contraceptive.
1. Aoun J, Dines VA, Stovall DW, et al. Effects of age, parity, and device type on complications and discontinuation of intrauterine devices. Obstet Gynecol. 2014;123:585-592.
2. Berenson AB, Tan A, Hirth JM, et al. Complications and continuation of intrauterine device use among commercially insured teenagers. Obstet Gynecol. 2013;121:951-958.
3. Braaten KP, Benson CB, Maurer R, et al. Malpositioned intrauterine contraceptive devices: risk factors, outcomes, and future pregnancies. Obstet Gynecol. 2011;118:1014-1020.
4. de Kroon CD, van Houwelingen JC, Trimbos JB, et al. The value of transvaginal ultrasound to monitor the position of an intrauterine device after insertion. A technology assessment study. Hum Reprod. 2003;18:2323-2327.
5. Thonneau P, Almont T, de La Rochebrochard E, et al. Risk factors for IUD failure: results of a large multicentre case-control study. Hum Reprod. 2006;21:2612-2616.
6. ACOG Committee on Gynecologic Practice. Committee Opinion No 672: clinical challenges of long-acting reversible contraceptive methods. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2016;128:e69-e77.
7. Heinemann K, Reed S, Moehner S, et al. Risk of uterine perforation with levonorgestrel-releasing and copper intrauterine devices in the European Active Surveillance Study on Intrauterine Devices. Contraception. 2015;91:274-279.
8. Benacerraf BR, Shipp TD, Bromley B. Three-dimensional ultrasound detection of abnormally located intrauterine contraceptive devices which are a source of pelvic pain and abnormal bleeding. Ultrasound Obstet Gynecol. 2009;34:110-115.
9. Bhavasr AK, Felner EJ, Shorma T. Common questions about the evaluation of acute pelvic pain. Am Fam Physician. 2016;93:41-48.
10. Peri N, Graham D, Levine D. Imaging of intrauterine contraceptive devices. J Ultrasound Med. 2007;26:1389-1401.
A 34-year-old woman with no significant past medical history presented as a new patient to our family medicine clinic with 2 weeks of intermittent lower abdominal and pelvic pain. She was sexually active with 1 partner and denied abnormal vaginal discharge or bleeding. She mentioned she’d had an intrauterine contraceptive device (IUD) placed a few weeks ago. The patient was afebrile, and her pelvic examination was unremarkable.
Physical examination showed mild tenderness to palpation over the lower abdomen without rebound tenderness or guarding. A complete metabolic panel revealed no significant abnormalities, and her human chorionic gonadotropin levels were normal.

Findings from the physical exam and her clinical history prompted the need for imaging. An abdominal radiograph (FIGURE 1) and noncontrast computed tomography (FIGURES 2A and 2B) were subsequently ordered.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Intra-abdominal IUD migration
The abdominal radiograph revealed a nonobstructive bowel gas pattern with an IUD overlaying the central lower abdomen and pelvis at the L5-S1 level (FIGURE 1). Computed tomography (CT) of her abdomen and pelvis showed that the IUD was outside the endometrial cavity (FIGURES 2A and 2B). There was no evidence of pneumoperitoneum or bowel perforation. Based on the work-up and imaging, the patient’s pain was due to intra-abdominal IUD malpositioning.
Diagnostic criteria for IUD malpositioning include device migration into 1 of several locations, such as the lower uterine segment or cervix. IUD malpositioning can involve the rotation or protrusion of the device into or through the myometrium. On imaging, a well-positioned IUD should have a straight stem contained within the endometrial cavity, with the arms of the IUD extending laterally at the uterine fundus.
For our patient, an abdominal radiograph showed that her IUD was superiorly displaced outside the expected region of the endometrial cavity. CT helped to confirm this.
Complications with IUDs are few
Using an IUD is an increasingly popular method of contraception because it is effective and generally well tolerated, with minimal adverse effects or complications. In a multicenter retrospective chart review of 2138 patients who had IUDs, Aoun et al found that serious complications included pelvic inflammatory disease (2%), IUD expulsion (6%), and pregnancy (1%).1 In a retrospective cohort study examining complications among 90,489 women with IUDs, Berenson et al found ectopic pregnancy and uterine perforation affected < 1%.2
A less serious complication is IUD malpositioning. Although it does seem to occur more often than other, more serious complications, the exact incidence is unknown. In a retrospective case-control study, Braaten et al reported the rate for IUD malpositioning was 10.4% among 182 women.3 Malpositioned IUDs may be more likely to occur in those with suspected adenomyosis.3 In a study by de Kroon et al, the estimated prevalence rate for an abnormal IUD position ranged from 4% to 7.7% among 195 patients.4
Continue to: The clinical presentation of IUD migration
The clinical presentation of IUD migration
Identification of a malpositioned IUD is needed to avoid the possible increased risk for uterine perforation, IUD expulsion, or pregnancy.5
IUDs that have perforated the uterus float freely in the pelvis or abdomen and can result in injury to adjacent structures as well as peritonitis, fistulas, and hemorrhage.5-7 In addition, adhesion formation over the IUD can lead to intestinal obstruction, infertility, and chronic pain.6
Common symptoms of IUD malpositioning include abdominal or pelvic pain and abnormal bleeding, although many patients may be asymptomatic.8 In a retrospective study of 167 patients with IUDs who underwent pelvic ultrasound, 28 patients were found to have an IUD in an abnormal position.8 Rates of bleeding and pain were higher in patients with malpositioned IUDs (35.7% and 39.3%, respectively) than in those with a normally positioned IUD (15.1% and 19.4%, respectively).8
The differential Dx includes endometriosis and fibroids
IUD malpositioning can be distinguished from other diagnoses that cause pelvic pain and have similar presentations—including endometriosis, ectopic pregnancy, and fibroids—through imaging study findings, clinical history, and presentation.
Other conditions that may need to be ruled out include pelvic inflammatory disease, acute appendicitis, and ovarian cysts.9 A thorough history and physical examination can help rule out these conditions by organ system, and laboratory and imaging studies can help to confirm the diagnosis.
Continue to: Which imaging tool to use, and when
Which imaging tool to use, and when
Assessment of intrauterine contraception placement requires evaluation of the uterine cavity; gynecologic examination alone is not sufficient to fully evaluate for IUD position. Certain imaging studies are particularly helpful for revealing possible IUD migration.
Ultrasound—a widely available, radiation-free modality—is the first-line imaging tool for evaluation of an IUD’s position.10 In addition, ultrasound can provide effective evaluation of other pelvic structures, which is helpful in identifying or eliminating other causes of pain or abnormal bleeding.
Conventional radiography. If the IUD is not visualized on ultrasound, the American College of Obstetricians and Gynecologists (ACOG) recommends radiography to determine if the IUD has been expelled or has migrated to an extra-uterine position.6
CT may be best suited for the evaluation of more severe complications of IUD malpositioning, including visceral perforation, abscess formation, or bowel obstruction. CT should be considered if the patient’s clinical presentation is suspicious for a more serious intra-abdominal pathology.
Management depends on the IUD’s position
For patients whose IUD has an uncertain position or nonvisualized intravaginal strings, ACOG’s first-line recommendations include ruling out pregnancy, using an alternative method for contraception, and ordering pelvic ultrasonography.6 ACOG recommendations for the management of IUD malpositioning depend on the device’s location and the patient’s symptomatology.
Continue to: Management of low-lying IUDs
Management of low-lying IUDs is complex. An IUD that is malpositioned in the cervix is considered partially expelled and should be completely removed.6 For asymptomatic patients with an IUD located in the lower uterine segment and above the internal cervical os, there should be strong consideration given to leaving the IUD in place because removal is associated with higher rates of pregnancy given the low rates of initiation of effective contraception following removal.6
IUD malpositioning in the peritoneal cavity requires surgical intervention. Although ACOG’s first-line recommendation is laparoscopic intervention, laparotomy can be considered if laparoscopy does not result in the removal of the IUD or the patient has more severe complications (sepsis or bowel perforation).6 At the time of IUD removal, the clinician should also discuss and/or prescribe interim contraception.
Treatment for our patient included uncomplicated laparoscopic surgical removal of the intra-abdominal IUD. The patient’s symptoms went away following the procedure, and she was subsequently switched to an oral contraceptive.
A 34-year-old woman with no significant past medical history presented as a new patient to our family medicine clinic with 2 weeks of intermittent lower abdominal and pelvic pain. She was sexually active with 1 partner and denied abnormal vaginal discharge or bleeding. She mentioned she’d had an intrauterine contraceptive device (IUD) placed a few weeks ago. The patient was afebrile, and her pelvic examination was unremarkable.
Physical examination showed mild tenderness to palpation over the lower abdomen without rebound tenderness or guarding. A complete metabolic panel revealed no significant abnormalities, and her human chorionic gonadotropin levels were normal.

Findings from the physical exam and her clinical history prompted the need for imaging. An abdominal radiograph (FIGURE 1) and noncontrast computed tomography (FIGURES 2A and 2B) were subsequently ordered.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Intra-abdominal IUD migration
The abdominal radiograph revealed a nonobstructive bowel gas pattern with an IUD overlaying the central lower abdomen and pelvis at the L5-S1 level (FIGURE 1). Computed tomography (CT) of her abdomen and pelvis showed that the IUD was outside the endometrial cavity (FIGURES 2A and 2B). There was no evidence of pneumoperitoneum or bowel perforation. Based on the work-up and imaging, the patient’s pain was due to intra-abdominal IUD malpositioning.
Diagnostic criteria for IUD malpositioning include device migration into 1 of several locations, such as the lower uterine segment or cervix. IUD malpositioning can involve the rotation or protrusion of the device into or through the myometrium. On imaging, a well-positioned IUD should have a straight stem contained within the endometrial cavity, with the arms of the IUD extending laterally at the uterine fundus.
For our patient, an abdominal radiograph showed that her IUD was superiorly displaced outside the expected region of the endometrial cavity. CT helped to confirm this.
Complications with IUDs are few
Using an IUD is an increasingly popular method of contraception because it is effective and generally well tolerated, with minimal adverse effects or complications. In a multicenter retrospective chart review of 2138 patients who had IUDs, Aoun et al found that serious complications included pelvic inflammatory disease (2%), IUD expulsion (6%), and pregnancy (1%).1 In a retrospective cohort study examining complications among 90,489 women with IUDs, Berenson et al found ectopic pregnancy and uterine perforation affected < 1%.2
A less serious complication is IUD malpositioning. Although it does seem to occur more often than other, more serious complications, the exact incidence is unknown. In a retrospective case-control study, Braaten et al reported the rate for IUD malpositioning was 10.4% among 182 women.3 Malpositioned IUDs may be more likely to occur in those with suspected adenomyosis.3 In a study by de Kroon et al, the estimated prevalence rate for an abnormal IUD position ranged from 4% to 7.7% among 195 patients.4
Continue to: The clinical presentation of IUD migration
The clinical presentation of IUD migration
Identification of a malpositioned IUD is needed to avoid the possible increased risk for uterine perforation, IUD expulsion, or pregnancy.5
IUDs that have perforated the uterus float freely in the pelvis or abdomen and can result in injury to adjacent structures as well as peritonitis, fistulas, and hemorrhage.5-7 In addition, adhesion formation over the IUD can lead to intestinal obstruction, infertility, and chronic pain.6
Common symptoms of IUD malpositioning include abdominal or pelvic pain and abnormal bleeding, although many patients may be asymptomatic.8 In a retrospective study of 167 patients with IUDs who underwent pelvic ultrasound, 28 patients were found to have an IUD in an abnormal position.8 Rates of bleeding and pain were higher in patients with malpositioned IUDs (35.7% and 39.3%, respectively) than in those with a normally positioned IUD (15.1% and 19.4%, respectively).8
The differential Dx includes endometriosis and fibroids
IUD malpositioning can be distinguished from other diagnoses that cause pelvic pain and have similar presentations—including endometriosis, ectopic pregnancy, and fibroids—through imaging study findings, clinical history, and presentation.
Other conditions that may need to be ruled out include pelvic inflammatory disease, acute appendicitis, and ovarian cysts.9 A thorough history and physical examination can help rule out these conditions by organ system, and laboratory and imaging studies can help to confirm the diagnosis.
Continue to: Which imaging tool to use, and when
Which imaging tool to use, and when
Assessment of intrauterine contraception placement requires evaluation of the uterine cavity; gynecologic examination alone is not sufficient to fully evaluate for IUD position. Certain imaging studies are particularly helpful for revealing possible IUD migration.
Ultrasound—a widely available, radiation-free modality—is the first-line imaging tool for evaluation of an IUD’s position.10 In addition, ultrasound can provide effective evaluation of other pelvic structures, which is helpful in identifying or eliminating other causes of pain or abnormal bleeding.
Conventional radiography. If the IUD is not visualized on ultrasound, the American College of Obstetricians and Gynecologists (ACOG) recommends radiography to determine if the IUD has been expelled or has migrated to an extra-uterine position.6
CT may be best suited for the evaluation of more severe complications of IUD malpositioning, including visceral perforation, abscess formation, or bowel obstruction. CT should be considered if the patient’s clinical presentation is suspicious for a more serious intra-abdominal pathology.
Management depends on the IUD’s position
For patients whose IUD has an uncertain position or nonvisualized intravaginal strings, ACOG’s first-line recommendations include ruling out pregnancy, using an alternative method for contraception, and ordering pelvic ultrasonography.6 ACOG recommendations for the management of IUD malpositioning depend on the device’s location and the patient’s symptomatology.
Continue to: Management of low-lying IUDs
Management of low-lying IUDs is complex. An IUD that is malpositioned in the cervix is considered partially expelled and should be completely removed.6 For asymptomatic patients with an IUD located in the lower uterine segment and above the internal cervical os, there should be strong consideration given to leaving the IUD in place because removal is associated with higher rates of pregnancy given the low rates of initiation of effective contraception following removal.6
IUD malpositioning in the peritoneal cavity requires surgical intervention. Although ACOG’s first-line recommendation is laparoscopic intervention, laparotomy can be considered if laparoscopy does not result in the removal of the IUD or the patient has more severe complications (sepsis or bowel perforation).6 At the time of IUD removal, the clinician should also discuss and/or prescribe interim contraception.
Treatment for our patient included uncomplicated laparoscopic surgical removal of the intra-abdominal IUD. The patient’s symptoms went away following the procedure, and she was subsequently switched to an oral contraceptive.
1. Aoun J, Dines VA, Stovall DW, et al. Effects of age, parity, and device type on complications and discontinuation of intrauterine devices. Obstet Gynecol. 2014;123:585-592.
2. Berenson AB, Tan A, Hirth JM, et al. Complications and continuation of intrauterine device use among commercially insured teenagers. Obstet Gynecol. 2013;121:951-958.
3. Braaten KP, Benson CB, Maurer R, et al. Malpositioned intrauterine contraceptive devices: risk factors, outcomes, and future pregnancies. Obstet Gynecol. 2011;118:1014-1020.
4. de Kroon CD, van Houwelingen JC, Trimbos JB, et al. The value of transvaginal ultrasound to monitor the position of an intrauterine device after insertion. A technology assessment study. Hum Reprod. 2003;18:2323-2327.
5. Thonneau P, Almont T, de La Rochebrochard E, et al. Risk factors for IUD failure: results of a large multicentre case-control study. Hum Reprod. 2006;21:2612-2616.
6. ACOG Committee on Gynecologic Practice. Committee Opinion No 672: clinical challenges of long-acting reversible contraceptive methods. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2016;128:e69-e77.
7. Heinemann K, Reed S, Moehner S, et al. Risk of uterine perforation with levonorgestrel-releasing and copper intrauterine devices in the European Active Surveillance Study on Intrauterine Devices. Contraception. 2015;91:274-279.
8. Benacerraf BR, Shipp TD, Bromley B. Three-dimensional ultrasound detection of abnormally located intrauterine contraceptive devices which are a source of pelvic pain and abnormal bleeding. Ultrasound Obstet Gynecol. 2009;34:110-115.
9. Bhavasr AK, Felner EJ, Shorma T. Common questions about the evaluation of acute pelvic pain. Am Fam Physician. 2016;93:41-48.
10. Peri N, Graham D, Levine D. Imaging of intrauterine contraceptive devices. J Ultrasound Med. 2007;26:1389-1401.
1. Aoun J, Dines VA, Stovall DW, et al. Effects of age, parity, and device type on complications and discontinuation of intrauterine devices. Obstet Gynecol. 2014;123:585-592.
2. Berenson AB, Tan A, Hirth JM, et al. Complications and continuation of intrauterine device use among commercially insured teenagers. Obstet Gynecol. 2013;121:951-958.
3. Braaten KP, Benson CB, Maurer R, et al. Malpositioned intrauterine contraceptive devices: risk factors, outcomes, and future pregnancies. Obstet Gynecol. 2011;118:1014-1020.
4. de Kroon CD, van Houwelingen JC, Trimbos JB, et al. The value of transvaginal ultrasound to monitor the position of an intrauterine device after insertion. A technology assessment study. Hum Reprod. 2003;18:2323-2327.
5. Thonneau P, Almont T, de La Rochebrochard E, et al. Risk factors for IUD failure: results of a large multicentre case-control study. Hum Reprod. 2006;21:2612-2616.
6. ACOG Committee on Gynecologic Practice. Committee Opinion No 672: clinical challenges of long-acting reversible contraceptive methods. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2016;128:e69-e77.
7. Heinemann K, Reed S, Moehner S, et al. Risk of uterine perforation with levonorgestrel-releasing and copper intrauterine devices in the European Active Surveillance Study on Intrauterine Devices. Contraception. 2015;91:274-279.
8. Benacerraf BR, Shipp TD, Bromley B. Three-dimensional ultrasound detection of abnormally located intrauterine contraceptive devices which are a source of pelvic pain and abnormal bleeding. Ultrasound Obstet Gynecol. 2009;34:110-115.
9. Bhavasr AK, Felner EJ, Shorma T. Common questions about the evaluation of acute pelvic pain. Am Fam Physician. 2016;93:41-48.
10. Peri N, Graham D, Levine D. Imaging of intrauterine contraceptive devices. J Ultrasound Med. 2007;26:1389-1401.
60-year-old man • chronic cough • history of GERD & dyslipidemia • throat tickle • Dx?
THE CASE
A 60-year-old man with a past medical history of gastroesophageal reflux disease (GERD) and dyslipidemia presented to his family physician for evaluation of chronic cough. Five years prior, the patient had developed a high fever and respiratory symptoms, including a cough, and was believed to have had severe otitis media. He was treated with multiple courses of antibiotics and corticosteroids for persistent otitis media. Although the condition eventually resolved, his cough continued.
The persistent cough prompted the patient to consult a succession of specialists. First, he saw a gastroenterologist; following an esophagogastroduodenoscopy, he was prescribed pantoprazole. Despite the proton-pump inhibitor (PPI) therapy, the cough remained. Next, he had multiple visits with an otolaryngologist but that yielded no specific diagnosis for the cough. He also saw an allergist-immunologist, who identified a ragweed allergy, gave him a diagnosis of cough-variant asthma, and prescribed antihistamines and mometasone furoate and formoterol fumarate dihydrate. Neither was helpful.
After 5 years of frustration, the patient complained to his family physician that he still had a cough and “a tickle” in his throat that was worsened by speaking and drinking cold beverages. He denied fever, shortness of breath, nausea, vomiting, or any other associated symptoms.
THE DIAGNOSIS
The failed treatment attempts with antihistamines, corticosteroids, bronchodilators, and PPI therapy excluded multiple etiologies for the cough. The throat discomfort and feeling of a “tickle” prompted us to consider a nerve-related disorder on the differential. The diagnosis of laryngeal sensory neuropathy (LSN) was considered.
DISCUSSION
LSN is a relatively uncommon cause of chronic refractory cough that can also manifest with throat discomfort, dysphagia, and dysphonia.1 It is thought to result from some type of insult to the recurrent laryngeal nerve or superior laryngeal nerve via viral infections, metabolic changes, or mechanical trauma, leading to a change in the firing threshold.2 The hypothesis of nerve damage is supported by the increased incidence of LSN in patients with goiters and those with type 2 diabetes.3,4 When there is a decrease in the laryngeal sensory threshold, dysfunctional laryngeal behavior results, leading to symptoms such as persistent cough and throat clearing.
Diagnosis. LSN is often diagnosed clinically, after GERD, allergies, asthma, angiotensin-converting enzyme inhibitor intake, and psychogenic disorders have been ruled out.1 Our patient had a prior diagnosis or investigation of nearly all of these conditions. Other clues pointing to an LSN diagnosis include a cough lasting 8 weeks or more, recurrent sensory disturbances (such as a tickle) of instantaneous onset before each cough episode, triggers that can include talking or a change in air temperature, daily coughing episodes numbering in the 10s to 100s, and a nonproductive cough.5,6
Beyond clinical clues, laryngeal electromyography, which evaluates the neuromuscular system in the larynx by recording action potentials generated in the laryngeal muscles during contraction, can be used for diagnosis.4 Videostroboscopy, which allows for an enlarged and slow motion view of the vocal cords, can also be used.
Continue to: Treatment
Treatment. To both confirm the diagnosis and treat the patient in a rapid, practical fashion, a trial of a neuromodulating agent such as pregabalin or gabapentin can be employed.6-9 A study identifying 28 LSN patients found symptomatic relief in 68% of patients taking gabapentin 100 to 900 mg/d.2 In another study, 12 LSN patients given pregabalin found relief after a 1-month regimen.1 Another study of 12 patients showed amitriptyline hydrochloride and gabapentin provided a positive response in 2 months, and the addition of reflux precautions and acid-suppression therapy was helpful.9 Finally, a group of 32 patients trialed on 3 different medications (amitriptyline, desipramine, and gabapentin) found similar efficacy among the 3.6
Another option. Aside from medications, botulinum toxin type A has been shown in a case series to directly decrease laryngeal hypertonicity and possibly reduce neurogenic inflammation and neuropeptide-mediated cough.10 Another study found that 18 patients with neurogenic cough who received superior laryngeal nerve blocks had cough severity index scores decrease from an average of 26.8 pretreatment to 14.6 posttreatment (P < .0001).11
Our patient agreed to a trial of gabapentin 300 mg once a day, with titration up to a maximum of 900 mg tid. When the patient returned to the clinic 4 months later, he reported that when he reached 300 mg bid, the cough completely resolved.
THE TAKEAWAY
A persistent cough with minimal identifiable triggers is a huge disruption to a patient’s life; having to visit multiple specialists before receiving a diagnosis compounds that. In our patient’s case, the process took 5 years, which underscores how important it is that LSN be considered in the differential diagnosis. Since this is generally a diagnosis of exclusion, it is important to take a careful history of a patient with a chronic cough. If LSN seems likely, trialing a patient on neuromodulating medication is the next best step, with dose titration if necessary.
Selena R. Pasadyn, 675 West 130th Street, Hinckley, OH, 44233; [email protected]
1. Halum SL, Sycamore DL, McRae BR. A new treatment option for laryngeal sensory neuropathy. Laryngoscope. 2009;119:1844-1847.
2. Lee B, Woo P. Chronic cough as a sign of laryngeal sensory neuropathy: diagnosis and treatment. Ann Otol Rhinol Laryngol. 2005;114:253-257.
3. Hamdan AL, Jabour J, Azar ST. Goiter and laryngeal sensory neuropathy. Int J Otolaryngol. 2013;2013:765265.
4. Hamdan AL, Dowli A, Barazi R, et al. Laryngeal sensory neuropathy in patients with diabetes mellitus. J Laryngol Otol. 2014;128:725-729.
5. Bastian RW, Vaidya AM, Delsupehe KG. Sensory neuropathic cough: a common and treatable cause of chronic cough. Otolaryngol Head Neck Surg. 2006;135:17-21.
6. Bastian ZJ, Bastian RW. The use of neuralgia medications to treat sensory neuropathic cough: our experience in a retrospective cohort of thirty-two patients. PeerJ. 2015;3:e816.
7. Van de Kerkhove C, Goeminne PC, Van Bleyenbergh P, et al. A cohort description and analysis of the effect of gabapentin on idiopathic cough. Cough. 2012;8:9.
8. Mishriki YY. Laryngeal neuropathy as a cause of chronic intractable cough. Am J Med. 2007;120:e5.
9. Norris BK, Schweinfurth JM. Management of recurrent laryngeal sensory neuropathic symptoms. Ann Otol Rhinol Laryngol. 2010;119:188-191.
10. Chu MW, Lieser JD, Sinacori JT. Use of botulinum toxin type a for chronic cough: a neuropathic model. Arch Otolaryngol Head Neck Surg. 2010;136:447.
11. Simpson CB, Tibbetts KM, Loochtan MJ, et al. Treatment of chronic neurogenic cough with in-office superior laryngeal nerve block. Laryngoscope. 2018;128:1898-1903.
THE CASE
A 60-year-old man with a past medical history of gastroesophageal reflux disease (GERD) and dyslipidemia presented to his family physician for evaluation of chronic cough. Five years prior, the patient had developed a high fever and respiratory symptoms, including a cough, and was believed to have had severe otitis media. He was treated with multiple courses of antibiotics and corticosteroids for persistent otitis media. Although the condition eventually resolved, his cough continued.
The persistent cough prompted the patient to consult a succession of specialists. First, he saw a gastroenterologist; following an esophagogastroduodenoscopy, he was prescribed pantoprazole. Despite the proton-pump inhibitor (PPI) therapy, the cough remained. Next, he had multiple visits with an otolaryngologist but that yielded no specific diagnosis for the cough. He also saw an allergist-immunologist, who identified a ragweed allergy, gave him a diagnosis of cough-variant asthma, and prescribed antihistamines and mometasone furoate and formoterol fumarate dihydrate. Neither was helpful.
After 5 years of frustration, the patient complained to his family physician that he still had a cough and “a tickle” in his throat that was worsened by speaking and drinking cold beverages. He denied fever, shortness of breath, nausea, vomiting, or any other associated symptoms.
THE DIAGNOSIS
The failed treatment attempts with antihistamines, corticosteroids, bronchodilators, and PPI therapy excluded multiple etiologies for the cough. The throat discomfort and feeling of a “tickle” prompted us to consider a nerve-related disorder on the differential. The diagnosis of laryngeal sensory neuropathy (LSN) was considered.
DISCUSSION
LSN is a relatively uncommon cause of chronic refractory cough that can also manifest with throat discomfort, dysphagia, and dysphonia.1 It is thought to result from some type of insult to the recurrent laryngeal nerve or superior laryngeal nerve via viral infections, metabolic changes, or mechanical trauma, leading to a change in the firing threshold.2 The hypothesis of nerve damage is supported by the increased incidence of LSN in patients with goiters and those with type 2 diabetes.3,4 When there is a decrease in the laryngeal sensory threshold, dysfunctional laryngeal behavior results, leading to symptoms such as persistent cough and throat clearing.
Diagnosis. LSN is often diagnosed clinically, after GERD, allergies, asthma, angiotensin-converting enzyme inhibitor intake, and psychogenic disorders have been ruled out.1 Our patient had a prior diagnosis or investigation of nearly all of these conditions. Other clues pointing to an LSN diagnosis include a cough lasting 8 weeks or more, recurrent sensory disturbances (such as a tickle) of instantaneous onset before each cough episode, triggers that can include talking or a change in air temperature, daily coughing episodes numbering in the 10s to 100s, and a nonproductive cough.5,6
Beyond clinical clues, laryngeal electromyography, which evaluates the neuromuscular system in the larynx by recording action potentials generated in the laryngeal muscles during contraction, can be used for diagnosis.4 Videostroboscopy, which allows for an enlarged and slow motion view of the vocal cords, can also be used.
Continue to: Treatment
Treatment. To both confirm the diagnosis and treat the patient in a rapid, practical fashion, a trial of a neuromodulating agent such as pregabalin or gabapentin can be employed.6-9 A study identifying 28 LSN patients found symptomatic relief in 68% of patients taking gabapentin 100 to 900 mg/d.2 In another study, 12 LSN patients given pregabalin found relief after a 1-month regimen.1 Another study of 12 patients showed amitriptyline hydrochloride and gabapentin provided a positive response in 2 months, and the addition of reflux precautions and acid-suppression therapy was helpful.9 Finally, a group of 32 patients trialed on 3 different medications (amitriptyline, desipramine, and gabapentin) found similar efficacy among the 3.6
Another option. Aside from medications, botulinum toxin type A has been shown in a case series to directly decrease laryngeal hypertonicity and possibly reduce neurogenic inflammation and neuropeptide-mediated cough.10 Another study found that 18 patients with neurogenic cough who received superior laryngeal nerve blocks had cough severity index scores decrease from an average of 26.8 pretreatment to 14.6 posttreatment (P < .0001).11
Our patient agreed to a trial of gabapentin 300 mg once a day, with titration up to a maximum of 900 mg tid. When the patient returned to the clinic 4 months later, he reported that when he reached 300 mg bid, the cough completely resolved.
THE TAKEAWAY
A persistent cough with minimal identifiable triggers is a huge disruption to a patient’s life; having to visit multiple specialists before receiving a diagnosis compounds that. In our patient’s case, the process took 5 years, which underscores how important it is that LSN be considered in the differential diagnosis. Since this is generally a diagnosis of exclusion, it is important to take a careful history of a patient with a chronic cough. If LSN seems likely, trialing a patient on neuromodulating medication is the next best step, with dose titration if necessary.
Selena R. Pasadyn, 675 West 130th Street, Hinckley, OH, 44233; [email protected]
THE CASE
A 60-year-old man with a past medical history of gastroesophageal reflux disease (GERD) and dyslipidemia presented to his family physician for evaluation of chronic cough. Five years prior, the patient had developed a high fever and respiratory symptoms, including a cough, and was believed to have had severe otitis media. He was treated with multiple courses of antibiotics and corticosteroids for persistent otitis media. Although the condition eventually resolved, his cough continued.
The persistent cough prompted the patient to consult a succession of specialists. First, he saw a gastroenterologist; following an esophagogastroduodenoscopy, he was prescribed pantoprazole. Despite the proton-pump inhibitor (PPI) therapy, the cough remained. Next, he had multiple visits with an otolaryngologist but that yielded no specific diagnosis for the cough. He also saw an allergist-immunologist, who identified a ragweed allergy, gave him a diagnosis of cough-variant asthma, and prescribed antihistamines and mometasone furoate and formoterol fumarate dihydrate. Neither was helpful.
After 5 years of frustration, the patient complained to his family physician that he still had a cough and “a tickle” in his throat that was worsened by speaking and drinking cold beverages. He denied fever, shortness of breath, nausea, vomiting, or any other associated symptoms.
THE DIAGNOSIS
The failed treatment attempts with antihistamines, corticosteroids, bronchodilators, and PPI therapy excluded multiple etiologies for the cough. The throat discomfort and feeling of a “tickle” prompted us to consider a nerve-related disorder on the differential. The diagnosis of laryngeal sensory neuropathy (LSN) was considered.
DISCUSSION
LSN is a relatively uncommon cause of chronic refractory cough that can also manifest with throat discomfort, dysphagia, and dysphonia.1 It is thought to result from some type of insult to the recurrent laryngeal nerve or superior laryngeal nerve via viral infections, metabolic changes, or mechanical trauma, leading to a change in the firing threshold.2 The hypothesis of nerve damage is supported by the increased incidence of LSN in patients with goiters and those with type 2 diabetes.3,4 When there is a decrease in the laryngeal sensory threshold, dysfunctional laryngeal behavior results, leading to symptoms such as persistent cough and throat clearing.
Diagnosis. LSN is often diagnosed clinically, after GERD, allergies, asthma, angiotensin-converting enzyme inhibitor intake, and psychogenic disorders have been ruled out.1 Our patient had a prior diagnosis or investigation of nearly all of these conditions. Other clues pointing to an LSN diagnosis include a cough lasting 8 weeks or more, recurrent sensory disturbances (such as a tickle) of instantaneous onset before each cough episode, triggers that can include talking or a change in air temperature, daily coughing episodes numbering in the 10s to 100s, and a nonproductive cough.5,6
Beyond clinical clues, laryngeal electromyography, which evaluates the neuromuscular system in the larynx by recording action potentials generated in the laryngeal muscles during contraction, can be used for diagnosis.4 Videostroboscopy, which allows for an enlarged and slow motion view of the vocal cords, can also be used.
Continue to: Treatment
Treatment. To both confirm the diagnosis and treat the patient in a rapid, practical fashion, a trial of a neuromodulating agent such as pregabalin or gabapentin can be employed.6-9 A study identifying 28 LSN patients found symptomatic relief in 68% of patients taking gabapentin 100 to 900 mg/d.2 In another study, 12 LSN patients given pregabalin found relief after a 1-month regimen.1 Another study of 12 patients showed amitriptyline hydrochloride and gabapentin provided a positive response in 2 months, and the addition of reflux precautions and acid-suppression therapy was helpful.9 Finally, a group of 32 patients trialed on 3 different medications (amitriptyline, desipramine, and gabapentin) found similar efficacy among the 3.6
Another option. Aside from medications, botulinum toxin type A has been shown in a case series to directly decrease laryngeal hypertonicity and possibly reduce neurogenic inflammation and neuropeptide-mediated cough.10 Another study found that 18 patients with neurogenic cough who received superior laryngeal nerve blocks had cough severity index scores decrease from an average of 26.8 pretreatment to 14.6 posttreatment (P < .0001).11
Our patient agreed to a trial of gabapentin 300 mg once a day, with titration up to a maximum of 900 mg tid. When the patient returned to the clinic 4 months later, he reported that when he reached 300 mg bid, the cough completely resolved.
THE TAKEAWAY
A persistent cough with minimal identifiable triggers is a huge disruption to a patient’s life; having to visit multiple specialists before receiving a diagnosis compounds that. In our patient’s case, the process took 5 years, which underscores how important it is that LSN be considered in the differential diagnosis. Since this is generally a diagnosis of exclusion, it is important to take a careful history of a patient with a chronic cough. If LSN seems likely, trialing a patient on neuromodulating medication is the next best step, with dose titration if necessary.
Selena R. Pasadyn, 675 West 130th Street, Hinckley, OH, 44233; [email protected]
1. Halum SL, Sycamore DL, McRae BR. A new treatment option for laryngeal sensory neuropathy. Laryngoscope. 2009;119:1844-1847.
2. Lee B, Woo P. Chronic cough as a sign of laryngeal sensory neuropathy: diagnosis and treatment. Ann Otol Rhinol Laryngol. 2005;114:253-257.
3. Hamdan AL, Jabour J, Azar ST. Goiter and laryngeal sensory neuropathy. Int J Otolaryngol. 2013;2013:765265.
4. Hamdan AL, Dowli A, Barazi R, et al. Laryngeal sensory neuropathy in patients with diabetes mellitus. J Laryngol Otol. 2014;128:725-729.
5. Bastian RW, Vaidya AM, Delsupehe KG. Sensory neuropathic cough: a common and treatable cause of chronic cough. Otolaryngol Head Neck Surg. 2006;135:17-21.
6. Bastian ZJ, Bastian RW. The use of neuralgia medications to treat sensory neuropathic cough: our experience in a retrospective cohort of thirty-two patients. PeerJ. 2015;3:e816.
7. Van de Kerkhove C, Goeminne PC, Van Bleyenbergh P, et al. A cohort description and analysis of the effect of gabapentin on idiopathic cough. Cough. 2012;8:9.
8. Mishriki YY. Laryngeal neuropathy as a cause of chronic intractable cough. Am J Med. 2007;120:e5.
9. Norris BK, Schweinfurth JM. Management of recurrent laryngeal sensory neuropathic symptoms. Ann Otol Rhinol Laryngol. 2010;119:188-191.
10. Chu MW, Lieser JD, Sinacori JT. Use of botulinum toxin type a for chronic cough: a neuropathic model. Arch Otolaryngol Head Neck Surg. 2010;136:447.
11. Simpson CB, Tibbetts KM, Loochtan MJ, et al. Treatment of chronic neurogenic cough with in-office superior laryngeal nerve block. Laryngoscope. 2018;128:1898-1903.
1. Halum SL, Sycamore DL, McRae BR. A new treatment option for laryngeal sensory neuropathy. Laryngoscope. 2009;119:1844-1847.
2. Lee B, Woo P. Chronic cough as a sign of laryngeal sensory neuropathy: diagnosis and treatment. Ann Otol Rhinol Laryngol. 2005;114:253-257.
3. Hamdan AL, Jabour J, Azar ST. Goiter and laryngeal sensory neuropathy. Int J Otolaryngol. 2013;2013:765265.
4. Hamdan AL, Dowli A, Barazi R, et al. Laryngeal sensory neuropathy in patients with diabetes mellitus. J Laryngol Otol. 2014;128:725-729.
5. Bastian RW, Vaidya AM, Delsupehe KG. Sensory neuropathic cough: a common and treatable cause of chronic cough. Otolaryngol Head Neck Surg. 2006;135:17-21.
6. Bastian ZJ, Bastian RW. The use of neuralgia medications to treat sensory neuropathic cough: our experience in a retrospective cohort of thirty-two patients. PeerJ. 2015;3:e816.
7. Van de Kerkhove C, Goeminne PC, Van Bleyenbergh P, et al. A cohort description and analysis of the effect of gabapentin on idiopathic cough. Cough. 2012;8:9.
8. Mishriki YY. Laryngeal neuropathy as a cause of chronic intractable cough. Am J Med. 2007;120:e5.
9. Norris BK, Schweinfurth JM. Management of recurrent laryngeal sensory neuropathic symptoms. Ann Otol Rhinol Laryngol. 2010;119:188-191.
10. Chu MW, Lieser JD, Sinacori JT. Use of botulinum toxin type a for chronic cough: a neuropathic model. Arch Otolaryngol Head Neck Surg. 2010;136:447.
11. Simpson CB, Tibbetts KM, Loochtan MJ, et al. Treatment of chronic neurogenic cough with in-office superior laryngeal nerve block. Laryngoscope. 2018;128:1898-1903.
Systemic racism: An editor’s note
This month’s editorial was jointly written by the editors of 10 prominent family medicine publications, including JFP, and is being published simultaneously in all 10 publications. In addition to this statement, each editor has developed action steps for their respective journals. At JFP, we plan to take the steps detailed here to help eliminate systemic racism. We will:
- continue to seek Black, Latino, and Native American physicians to serve on the JFP editorial advisory board.
- solicit manuscripts from these underrepresented groups of physicians.
- recruit peer reviewers from underrepresented communities.
- re-evaluate the thoroughness of manuscripts; where there are racial or ethnic differences in presentation of diseases or treatment outcomes, we will ensure that these differences are highlighted.
If you are interested in helping us to achieve these goals, I encourage you to contact me at [email protected].
We must all band together to eliminate disparities and biases in medical education and medical care so that all people receive the same high standard of respect and care that every human being deserves.
This month’s editorial was jointly written by the editors of 10 prominent family medicine publications, including JFP, and is being published simultaneously in all 10 publications. In addition to this statement, each editor has developed action steps for their respective journals. At JFP, we plan to take the steps detailed here to help eliminate systemic racism. We will:
- continue to seek Black, Latino, and Native American physicians to serve on the JFP editorial advisory board.
- solicit manuscripts from these underrepresented groups of physicians.
- recruit peer reviewers from underrepresented communities.
- re-evaluate the thoroughness of manuscripts; where there are racial or ethnic differences in presentation of diseases or treatment outcomes, we will ensure that these differences are highlighted.
If you are interested in helping us to achieve these goals, I encourage you to contact me at [email protected].
We must all band together to eliminate disparities and biases in medical education and medical care so that all people receive the same high standard of respect and care that every human being deserves.
This month’s editorial was jointly written by the editors of 10 prominent family medicine publications, including JFP, and is being published simultaneously in all 10 publications. In addition to this statement, each editor has developed action steps for their respective journals. At JFP, we plan to take the steps detailed here to help eliminate systemic racism. We will:
- continue to seek Black, Latino, and Native American physicians to serve on the JFP editorial advisory board.
- solicit manuscripts from these underrepresented groups of physicians.
- recruit peer reviewers from underrepresented communities.
- re-evaluate the thoroughness of manuscripts; where there are racial or ethnic differences in presentation of diseases or treatment outcomes, we will ensure that these differences are highlighted.
If you are interested in helping us to achieve these goals, I encourage you to contact me at [email protected].
We must all band together to eliminate disparities and biases in medical education and medical care so that all people receive the same high standard of respect and care that every human being deserves.
Dermatology history: University Hospital ‘Saint Louis,’ Paris
The Hospital “Saint Louis” was founded in 1607 by King Henry IV of France to relieve overcrowding of Parisian hospitals during the plague epidemic of 1605-1606. He named it Saint-Louis in memory of his grandfather, King Louis IX.
Today, the Hospital Saint-Louis, a registered historic monument, is used for administrative activities.

Since 1980, a modern building has hosted all the activities of the University Hospital Center, which belongs to the University of Paris 7.

In addition to dermatology, the main departments include hematology and bone marrow transplantation, hemato-oncology, general surgery, endocrinology, gastroenterology, clinical immunology, internal medicine, and nephrology. Saint-Louis Hospital employs 2,500 people, including a medical staff of 1,000. It houses the Institute Inserm U976 – a public research unit that is part of the National Health and Medical Research Institute, which focuses on human immunology, physiopathology and immunotherapy – as well as the René-Touraine Foundation, a private non-profit organization that brings together dermatologists, scientists, pharmaceutical companies, and health authorities to support therapeutic progress in dermatology.
Saint-Louis Hospital is known for its long tradition in hematology; it is the site of the first successful allogeneic bone marrow transplant in 1958, performed by Georges Mathé, MD, Professor Jean Bernard, and one of the recipients of the 1980 Nobel Prize in Medicine, Professor Jean Dausset. The hospital is known for not only its activity in dermatology care and research (such as oncodermatology and inflammatory diseases) but also its long tradition of teaching in dermatology and venereology.
Over the last four centuries, great physicians have practiced their art here and many professors, and clinicians at Saint-Louis Hospital have authored publications and developed manuals of dermatology that have been translated across five continents. Many diseases and semiology signs in dermatology were first described by physicians from this hospital, their names familiar to dermatologists worldwide: Jean-Louis-Marc Alibert, MD; Jean Guillaume Auguste Lugol, MD; Laurent-Théodore Biett, MD; Pierre-Antoine-Ernest Bazin, MD; Pierre Louis Alphée Cazenave, MD; François Henri Hallopeau, MD; Léon Lortat-Jacob, MD; Henri-Alexandre Danlos, MD; Ernest Besnier, MD; Jean Baptiste Emile Vidal, MD; Ferdinand-Jean Darier, MD; Louis Brocq, MD; Bernard Felix Duperrat, MD; Gaston Auguste Milian, MD; Albert Sézary, MD; Achille Civatte, MD; Raymond Sabouraud, MD; Henri Gougerot, MD; Albert Touraine, MD; Arnault Tzanck, MD; and Robert Degos, MD, among others.
The Henri-Feulard library – known as the “Dermatology Wax Museum” – is a fascinating place that houses the world’s largest collection of 4,807 wax casts dedicated to teaching skin diseases and venereal diseases.

The library next to the museum contains numerous outstanding ancient works on dermatology and sexually transmitted diseases, including first issues of dermatology journals from the 19th century and rare dermatology textbooks published in the last 2 centuries.
The recently renovated museum hosts national and international dermatological meetings and is also where the hospital’s dermatology staff meets weekly.
In the dermatology department at Saint-Louis Hospital, patient care is provided in two hospital areas with 18 beds each and a day hospital with 8 beds for patients with inflammatory and dysimmune dermatoses, including a special room with a bathtub for the management of patients with severe genodermatoses. The department is a referral center for genodermatoses and a dedicated center for autoimmune bullous diseases.
Patients with all types of skin tumors, particularly melanomas, carcinomas, sarcomas, and cutaneous lymphomas, are treated at the oncodermatology center, which has a 10-bed day hospital and a very active consultation service. The Saint-Louis Hospital dermatology department is also a National Reference Center for cutaneous lymphomas, providing four Multidisciplinary Consultation Meetings, a national MCM for cutaneous lymphomas, and a multidisciplinary MCM for the diagnosis and treatment of side effects of new targeted therapies and immunotherapies for cancers.
The dermatology polyclinic, an outpatient clinic, provides 54,000 consultations per year. It includes a very active general consultation service, including a wide variety of specialized consultations for atopic dermatitis, psoriasis, hand dermatitis, hidradenitis suppurativa, internal medicine/dermatology, bullous diseases, keloids, angiomas, leprosy, genodermatoses, and medical mycology.
Anonymous, free screening services are available at the Sexually Transmitted Diseases Center through “CeGIDD,” a free center for HIV/AIDS screening and specialized consultations in venereology and mucosal pathologies.
The surgical activity of the department is provided in the Center of Dermo-Surgery. Dedicated medical and paramedical consultations ensure the management of ulcers and therapeutic baths for patients and families with refractory scabies.
The technical platform includes an allergology consultation, a phototherapy center, a Fotofinder diagnosis, a photodynamic therapy unit, and a confocal microscopy unit. The department, completed in September 2019 with a laser center with four devices, also works in close collaboration with the Sabouraud Center, created by Dr. Sabouraud and dedicated to the investigation and treatment of scalp diseases.
We are absolutely aware that working in a hospital so rich in past personalities and discoveries and part of the history of dermatology is not only a huge honor requiring a special commitment to continue the tradition of research and excellence in dermatology initiated hundreds of years ago, but also an important responsibility to focus all our efforts on teaching dermatology to next generations in France and around the world. It is also our responsibility to pursue this historic tradition of excellence by developing dynamic translational research activities that lead to innovations in the field of dermatology.
Professor Bagot is head of the dermatology department of University Hospital Saint-Louis, Paris. Dr. Ionescu is a specialist in dermatology and venereology in the department of dermatology at University Hospital Saint-Louis in Paris and is a member of the Dermatology News editorial board. Write to them at [email protected].
The Hospital “Saint Louis” was founded in 1607 by King Henry IV of France to relieve overcrowding of Parisian hospitals during the plague epidemic of 1605-1606. He named it Saint-Louis in memory of his grandfather, King Louis IX.
Today, the Hospital Saint-Louis, a registered historic monument, is used for administrative activities.

Since 1980, a modern building has hosted all the activities of the University Hospital Center, which belongs to the University of Paris 7.

In addition to dermatology, the main departments include hematology and bone marrow transplantation, hemato-oncology, general surgery, endocrinology, gastroenterology, clinical immunology, internal medicine, and nephrology. Saint-Louis Hospital employs 2,500 people, including a medical staff of 1,000. It houses the Institute Inserm U976 – a public research unit that is part of the National Health and Medical Research Institute, which focuses on human immunology, physiopathology and immunotherapy – as well as the René-Touraine Foundation, a private non-profit organization that brings together dermatologists, scientists, pharmaceutical companies, and health authorities to support therapeutic progress in dermatology.
Saint-Louis Hospital is known for its long tradition in hematology; it is the site of the first successful allogeneic bone marrow transplant in 1958, performed by Georges Mathé, MD, Professor Jean Bernard, and one of the recipients of the 1980 Nobel Prize in Medicine, Professor Jean Dausset. The hospital is known for not only its activity in dermatology care and research (such as oncodermatology and inflammatory diseases) but also its long tradition of teaching in dermatology and venereology.
Over the last four centuries, great physicians have practiced their art here and many professors, and clinicians at Saint-Louis Hospital have authored publications and developed manuals of dermatology that have been translated across five continents. Many diseases and semiology signs in dermatology were first described by physicians from this hospital, their names familiar to dermatologists worldwide: Jean-Louis-Marc Alibert, MD; Jean Guillaume Auguste Lugol, MD; Laurent-Théodore Biett, MD; Pierre-Antoine-Ernest Bazin, MD; Pierre Louis Alphée Cazenave, MD; François Henri Hallopeau, MD; Léon Lortat-Jacob, MD; Henri-Alexandre Danlos, MD; Ernest Besnier, MD; Jean Baptiste Emile Vidal, MD; Ferdinand-Jean Darier, MD; Louis Brocq, MD; Bernard Felix Duperrat, MD; Gaston Auguste Milian, MD; Albert Sézary, MD; Achille Civatte, MD; Raymond Sabouraud, MD; Henri Gougerot, MD; Albert Touraine, MD; Arnault Tzanck, MD; and Robert Degos, MD, among others.
The Henri-Feulard library – known as the “Dermatology Wax Museum” – is a fascinating place that houses the world’s largest collection of 4,807 wax casts dedicated to teaching skin diseases and venereal diseases.

The library next to the museum contains numerous outstanding ancient works on dermatology and sexually transmitted diseases, including first issues of dermatology journals from the 19th century and rare dermatology textbooks published in the last 2 centuries.
The recently renovated museum hosts national and international dermatological meetings and is also where the hospital’s dermatology staff meets weekly.
In the dermatology department at Saint-Louis Hospital, patient care is provided in two hospital areas with 18 beds each and a day hospital with 8 beds for patients with inflammatory and dysimmune dermatoses, including a special room with a bathtub for the management of patients with severe genodermatoses. The department is a referral center for genodermatoses and a dedicated center for autoimmune bullous diseases.
Patients with all types of skin tumors, particularly melanomas, carcinomas, sarcomas, and cutaneous lymphomas, are treated at the oncodermatology center, which has a 10-bed day hospital and a very active consultation service. The Saint-Louis Hospital dermatology department is also a National Reference Center for cutaneous lymphomas, providing four Multidisciplinary Consultation Meetings, a national MCM for cutaneous lymphomas, and a multidisciplinary MCM for the diagnosis and treatment of side effects of new targeted therapies and immunotherapies for cancers.
The dermatology polyclinic, an outpatient clinic, provides 54,000 consultations per year. It includes a very active general consultation service, including a wide variety of specialized consultations for atopic dermatitis, psoriasis, hand dermatitis, hidradenitis suppurativa, internal medicine/dermatology, bullous diseases, keloids, angiomas, leprosy, genodermatoses, and medical mycology.
Anonymous, free screening services are available at the Sexually Transmitted Diseases Center through “CeGIDD,” a free center for HIV/AIDS screening and specialized consultations in venereology and mucosal pathologies.
The surgical activity of the department is provided in the Center of Dermo-Surgery. Dedicated medical and paramedical consultations ensure the management of ulcers and therapeutic baths for patients and families with refractory scabies.
The technical platform includes an allergology consultation, a phototherapy center, a Fotofinder diagnosis, a photodynamic therapy unit, and a confocal microscopy unit. The department, completed in September 2019 with a laser center with four devices, also works in close collaboration with the Sabouraud Center, created by Dr. Sabouraud and dedicated to the investigation and treatment of scalp diseases.
We are absolutely aware that working in a hospital so rich in past personalities and discoveries and part of the history of dermatology is not only a huge honor requiring a special commitment to continue the tradition of research and excellence in dermatology initiated hundreds of years ago, but also an important responsibility to focus all our efforts on teaching dermatology to next generations in France and around the world. It is also our responsibility to pursue this historic tradition of excellence by developing dynamic translational research activities that lead to innovations in the field of dermatology.
Professor Bagot is head of the dermatology department of University Hospital Saint-Louis, Paris. Dr. Ionescu is a specialist in dermatology and venereology in the department of dermatology at University Hospital Saint-Louis in Paris and is a member of the Dermatology News editorial board. Write to them at [email protected].
The Hospital “Saint Louis” was founded in 1607 by King Henry IV of France to relieve overcrowding of Parisian hospitals during the plague epidemic of 1605-1606. He named it Saint-Louis in memory of his grandfather, King Louis IX.
Today, the Hospital Saint-Louis, a registered historic monument, is used for administrative activities.

Since 1980, a modern building has hosted all the activities of the University Hospital Center, which belongs to the University of Paris 7.

In addition to dermatology, the main departments include hematology and bone marrow transplantation, hemato-oncology, general surgery, endocrinology, gastroenterology, clinical immunology, internal medicine, and nephrology. Saint-Louis Hospital employs 2,500 people, including a medical staff of 1,000. It houses the Institute Inserm U976 – a public research unit that is part of the National Health and Medical Research Institute, which focuses on human immunology, physiopathology and immunotherapy – as well as the René-Touraine Foundation, a private non-profit organization that brings together dermatologists, scientists, pharmaceutical companies, and health authorities to support therapeutic progress in dermatology.
Saint-Louis Hospital is known for its long tradition in hematology; it is the site of the first successful allogeneic bone marrow transplant in 1958, performed by Georges Mathé, MD, Professor Jean Bernard, and one of the recipients of the 1980 Nobel Prize in Medicine, Professor Jean Dausset. The hospital is known for not only its activity in dermatology care and research (such as oncodermatology and inflammatory diseases) but also its long tradition of teaching in dermatology and venereology.
Over the last four centuries, great physicians have practiced their art here and many professors, and clinicians at Saint-Louis Hospital have authored publications and developed manuals of dermatology that have been translated across five continents. Many diseases and semiology signs in dermatology were first described by physicians from this hospital, their names familiar to dermatologists worldwide: Jean-Louis-Marc Alibert, MD; Jean Guillaume Auguste Lugol, MD; Laurent-Théodore Biett, MD; Pierre-Antoine-Ernest Bazin, MD; Pierre Louis Alphée Cazenave, MD; François Henri Hallopeau, MD; Léon Lortat-Jacob, MD; Henri-Alexandre Danlos, MD; Ernest Besnier, MD; Jean Baptiste Emile Vidal, MD; Ferdinand-Jean Darier, MD; Louis Brocq, MD; Bernard Felix Duperrat, MD; Gaston Auguste Milian, MD; Albert Sézary, MD; Achille Civatte, MD; Raymond Sabouraud, MD; Henri Gougerot, MD; Albert Touraine, MD; Arnault Tzanck, MD; and Robert Degos, MD, among others.
The Henri-Feulard library – known as the “Dermatology Wax Museum” – is a fascinating place that houses the world’s largest collection of 4,807 wax casts dedicated to teaching skin diseases and venereal diseases.

The library next to the museum contains numerous outstanding ancient works on dermatology and sexually transmitted diseases, including first issues of dermatology journals from the 19th century and rare dermatology textbooks published in the last 2 centuries.
The recently renovated museum hosts national and international dermatological meetings and is also where the hospital’s dermatology staff meets weekly.
In the dermatology department at Saint-Louis Hospital, patient care is provided in two hospital areas with 18 beds each and a day hospital with 8 beds for patients with inflammatory and dysimmune dermatoses, including a special room with a bathtub for the management of patients with severe genodermatoses. The department is a referral center for genodermatoses and a dedicated center for autoimmune bullous diseases.
Patients with all types of skin tumors, particularly melanomas, carcinomas, sarcomas, and cutaneous lymphomas, are treated at the oncodermatology center, which has a 10-bed day hospital and a very active consultation service. The Saint-Louis Hospital dermatology department is also a National Reference Center for cutaneous lymphomas, providing four Multidisciplinary Consultation Meetings, a national MCM for cutaneous lymphomas, and a multidisciplinary MCM for the diagnosis and treatment of side effects of new targeted therapies and immunotherapies for cancers.
The dermatology polyclinic, an outpatient clinic, provides 54,000 consultations per year. It includes a very active general consultation service, including a wide variety of specialized consultations for atopic dermatitis, psoriasis, hand dermatitis, hidradenitis suppurativa, internal medicine/dermatology, bullous diseases, keloids, angiomas, leprosy, genodermatoses, and medical mycology.
Anonymous, free screening services are available at the Sexually Transmitted Diseases Center through “CeGIDD,” a free center for HIV/AIDS screening and specialized consultations in venereology and mucosal pathologies.
The surgical activity of the department is provided in the Center of Dermo-Surgery. Dedicated medical and paramedical consultations ensure the management of ulcers and therapeutic baths for patients and families with refractory scabies.
The technical platform includes an allergology consultation, a phototherapy center, a Fotofinder diagnosis, a photodynamic therapy unit, and a confocal microscopy unit. The department, completed in September 2019 with a laser center with four devices, also works in close collaboration with the Sabouraud Center, created by Dr. Sabouraud and dedicated to the investigation and treatment of scalp diseases.
We are absolutely aware that working in a hospital so rich in past personalities and discoveries and part of the history of dermatology is not only a huge honor requiring a special commitment to continue the tradition of research and excellence in dermatology initiated hundreds of years ago, but also an important responsibility to focus all our efforts on teaching dermatology to next generations in France and around the world. It is also our responsibility to pursue this historic tradition of excellence by developing dynamic translational research activities that lead to innovations in the field of dermatology.
Professor Bagot is head of the dermatology department of University Hospital Saint-Louis, Paris. Dr. Ionescu is a specialist in dermatology and venereology in the department of dermatology at University Hospital Saint-Louis in Paris and is a member of the Dermatology News editorial board. Write to them at [email protected].
Maternal autoimmune disease raises children’s risk of ADHD
Maternal autoimmune diseases significantly increased the risk of ADHD in children, based on data from a large cohort study of more than 800,000 mothers and children and a subsequent meta-analysis.
“There is growing evidence that immune-related cells and proteins play a role in brain development and function and that maternal immune activation, including infection, autoimmune disease, and chronic inflammation during pregnancy, increases the risk of neurodevelopmental disorders among children,” wrote Timothy C. Nielsen, MPH, of the University of Sydney, and colleagues.
Previous research has examined a link between maternal autoimmune disorders and autism spectrum disorders in children, but associations with ADHD have not been well studied, they said.
In a population-based cohort study published in JAMA Pediatrics, the researchers identified 831,718 mothers and their 831,718 singleton infants in Australia. A total of 12,787 infants were born to mothers with an autoimmune diagnosis; 12,610 of them were matched to 50,440 control infants. ADHD was determined based on prescription for a stimulant treatment or a hospital diagnosis; children with a first ADHD event younger than 3 years were excluded.
In the total cohort of 63,050 infants, the presence of any maternal autoimmune disease was associated with a significantly increased risk of ADHD (hazard ratio, 1.30) as was the presence of several specific conditions: type 1 diabetes (HR, 2.23), psoriasis (HR, 1.66), and rheumatic fever or rheumatic carditis (HR, 1.75).
In addition, the researchers conducted a meta-analysis of the current study and four additional studies that yielded similar results. In the meta-analysis, the risk of ADHD was significantly associated with any maternal autoimmune disease in two studies (HR, 1.20); with maternal type 1 diabetes in four studies (HR, 1.53); with maternal hyperthyroidism in three studies (HR 1.15); and with maternal psoriasis in two studies (HR, 1.31).
Type 1 diabetes (T1D) had the highest HR and was the most often studied condition. However, “the observed association may also be related to nonimmune aspects of T1D, such as glycemic control, as nonautoimmune diabetes has been associated with ADHD among children,” the researchers wrote.
The study findings were limited by several factors, including the lack of outpatient and primary care records to identify maternal autoimmune disease, and lack of data on any medication used to managed diseases during pregnancy, as well as a lack of data on children with ADHD who might not have been treated with medication, the researchers noted. In addition, “given differences in study design and definitions, the pooled HRs presented in the meta-analysis need to be treated cautiously.”
However, the results were strengthened by the hybrid study design and large study population, and were generally consistent with previous research supporting an effect of maternal immune function on fetal neurodevelopment, they noted.
“Our study provides justification for future studies that examine the effect of maternal autoimmune diseases, including biomarkers, condition severity, and management in pregnancy and in the periconception period, on neurodevelopmental disorders in children,” they concluded.
Studies need to explore mechanism of action
The current study, with its hybrid design, adds support to the evidence of an association between any maternal autoimmune disease and ADHD in children, as well as an association between the specific conditions of type 1 diabetes, hyperthyroidism, and psoriasis in mothers and ADHD in children, Søren Dalsgaard, MD, of Aarhus (Denmark) University, wrote in an accompanying editorial.
“Importantly, Nielsen et al. emphasized in their article that, for the many different autoimmune diseases, different underlying mechanisms for the associations with disorders of the central nervous system were likely. They mentioned that, for T1D, low glycemic control may play a role, as type 2 diabetes has been associated with ADHD,” said Dr. Dalsgaard.
“Overall, these mechanisms are thought to include shared genetic and environmental risk factors or direct effects of maternal autoantibodies or cytokines crossing the placenta and altering the fetal immune response, which in turns leads to changes in the central nervous system,” Dr. Dalsgaard explained. However, the current study and previous studies have not identified the mechanisms to explain the association between ADHD in children and maternal autoimmune disease.
“To understand more about these associations, future studies should include researchers and data from different scientific disciplines, such as epidemiology, animal modeling, genetics, and neuroimmunology,” he concluded.
Association is not causality
Overall, the study findings add to the evidence of a correlation between autoimmune diseases and neurologic disease, said Herschel Lessin, MD, of Children’s Medical Group, Poughkeepsie, N.Y., in an interview. “Anything that might contribute to behavioral problems is worth investigating.” However, it is important to remember that association is not causation.
“There is some literature and evidence that autoimmune disease is associated with mental health issues, but the mechanisms of action are unknown,” said Dr. Lessin. ADHD is highly heritable, so the association may be caused by a similar genetic predisposition, or it may be something related to autoimmunity that is impacting the fetus by passing through the placenta.
The current study’s strengths include the large size and hybrid design, but limitations such as the identification of ADHD based on medication prescriptions may have led to underreporting, and identifying maternal autoimmune disease via inpatient hospital diagnosis could have selected for more severe disease, he said.
From a clinical standpoint, the study suggests a correlation that should be noted in a family history and potentially used to inform a diagnosis, especially in cases of type 1 diabetes where the association was strongest, Dr. Lessin said. The findings also support the value of further research to look for mechanisms that might explain whether the association between autoimmune disease and ADHD is autoimmune system causality or shared genetic susceptibility.
The study received no outside funding. One coauthor disclosed receiving grants from the National Blood Authority Australia and the Australian National Health and Medical Research Council during the conduct of the study. Dr. Dalsgaard had no financial conflicts to disclose. Dr. Lessin disclosed serving as editor of the ADHD toolkit for the American Academy of Pediatrics and coauthor of the current ADHD clinical guidelines. He also serves in advisory capacity to Cognoa, a company involved in diagnosis of autism, and Corium/KemPharm, companies involved in the development of ADHD treatments.
Maternal autoimmune diseases significantly increased the risk of ADHD in children, based on data from a large cohort study of more than 800,000 mothers and children and a subsequent meta-analysis.
“There is growing evidence that immune-related cells and proteins play a role in brain development and function and that maternal immune activation, including infection, autoimmune disease, and chronic inflammation during pregnancy, increases the risk of neurodevelopmental disorders among children,” wrote Timothy C. Nielsen, MPH, of the University of Sydney, and colleagues.
Previous research has examined a link between maternal autoimmune disorders and autism spectrum disorders in children, but associations with ADHD have not been well studied, they said.
In a population-based cohort study published in JAMA Pediatrics, the researchers identified 831,718 mothers and their 831,718 singleton infants in Australia. A total of 12,787 infants were born to mothers with an autoimmune diagnosis; 12,610 of them were matched to 50,440 control infants. ADHD was determined based on prescription for a stimulant treatment or a hospital diagnosis; children with a first ADHD event younger than 3 years were excluded.
In the total cohort of 63,050 infants, the presence of any maternal autoimmune disease was associated with a significantly increased risk of ADHD (hazard ratio, 1.30) as was the presence of several specific conditions: type 1 diabetes (HR, 2.23), psoriasis (HR, 1.66), and rheumatic fever or rheumatic carditis (HR, 1.75).
In addition, the researchers conducted a meta-analysis of the current study and four additional studies that yielded similar results. In the meta-analysis, the risk of ADHD was significantly associated with any maternal autoimmune disease in two studies (HR, 1.20); with maternal type 1 diabetes in four studies (HR, 1.53); with maternal hyperthyroidism in three studies (HR 1.15); and with maternal psoriasis in two studies (HR, 1.31).
Type 1 diabetes (T1D) had the highest HR and was the most often studied condition. However, “the observed association may also be related to nonimmune aspects of T1D, such as glycemic control, as nonautoimmune diabetes has been associated with ADHD among children,” the researchers wrote.
The study findings were limited by several factors, including the lack of outpatient and primary care records to identify maternal autoimmune disease, and lack of data on any medication used to managed diseases during pregnancy, as well as a lack of data on children with ADHD who might not have been treated with medication, the researchers noted. In addition, “given differences in study design and definitions, the pooled HRs presented in the meta-analysis need to be treated cautiously.”
However, the results were strengthened by the hybrid study design and large study population, and were generally consistent with previous research supporting an effect of maternal immune function on fetal neurodevelopment, they noted.
“Our study provides justification for future studies that examine the effect of maternal autoimmune diseases, including biomarkers, condition severity, and management in pregnancy and in the periconception period, on neurodevelopmental disorders in children,” they concluded.
Studies need to explore mechanism of action
The current study, with its hybrid design, adds support to the evidence of an association between any maternal autoimmune disease and ADHD in children, as well as an association between the specific conditions of type 1 diabetes, hyperthyroidism, and psoriasis in mothers and ADHD in children, Søren Dalsgaard, MD, of Aarhus (Denmark) University, wrote in an accompanying editorial.
“Importantly, Nielsen et al. emphasized in their article that, for the many different autoimmune diseases, different underlying mechanisms for the associations with disorders of the central nervous system were likely. They mentioned that, for T1D, low glycemic control may play a role, as type 2 diabetes has been associated with ADHD,” said Dr. Dalsgaard.
“Overall, these mechanisms are thought to include shared genetic and environmental risk factors or direct effects of maternal autoantibodies or cytokines crossing the placenta and altering the fetal immune response, which in turns leads to changes in the central nervous system,” Dr. Dalsgaard explained. However, the current study and previous studies have not identified the mechanisms to explain the association between ADHD in children and maternal autoimmune disease.
“To understand more about these associations, future studies should include researchers and data from different scientific disciplines, such as epidemiology, animal modeling, genetics, and neuroimmunology,” he concluded.
Association is not causality
Overall, the study findings add to the evidence of a correlation between autoimmune diseases and neurologic disease, said Herschel Lessin, MD, of Children’s Medical Group, Poughkeepsie, N.Y., in an interview. “Anything that might contribute to behavioral problems is worth investigating.” However, it is important to remember that association is not causation.
“There is some literature and evidence that autoimmune disease is associated with mental health issues, but the mechanisms of action are unknown,” said Dr. Lessin. ADHD is highly heritable, so the association may be caused by a similar genetic predisposition, or it may be something related to autoimmunity that is impacting the fetus by passing through the placenta.
The current study’s strengths include the large size and hybrid design, but limitations such as the identification of ADHD based on medication prescriptions may have led to underreporting, and identifying maternal autoimmune disease via inpatient hospital diagnosis could have selected for more severe disease, he said.
From a clinical standpoint, the study suggests a correlation that should be noted in a family history and potentially used to inform a diagnosis, especially in cases of type 1 diabetes where the association was strongest, Dr. Lessin said. The findings also support the value of further research to look for mechanisms that might explain whether the association between autoimmune disease and ADHD is autoimmune system causality or shared genetic susceptibility.
The study received no outside funding. One coauthor disclosed receiving grants from the National Blood Authority Australia and the Australian National Health and Medical Research Council during the conduct of the study. Dr. Dalsgaard had no financial conflicts to disclose. Dr. Lessin disclosed serving as editor of the ADHD toolkit for the American Academy of Pediatrics and coauthor of the current ADHD clinical guidelines. He also serves in advisory capacity to Cognoa, a company involved in diagnosis of autism, and Corium/KemPharm, companies involved in the development of ADHD treatments.
Maternal autoimmune diseases significantly increased the risk of ADHD in children, based on data from a large cohort study of more than 800,000 mothers and children and a subsequent meta-analysis.
“There is growing evidence that immune-related cells and proteins play a role in brain development and function and that maternal immune activation, including infection, autoimmune disease, and chronic inflammation during pregnancy, increases the risk of neurodevelopmental disorders among children,” wrote Timothy C. Nielsen, MPH, of the University of Sydney, and colleagues.
Previous research has examined a link between maternal autoimmune disorders and autism spectrum disorders in children, but associations with ADHD have not been well studied, they said.
In a population-based cohort study published in JAMA Pediatrics, the researchers identified 831,718 mothers and their 831,718 singleton infants in Australia. A total of 12,787 infants were born to mothers with an autoimmune diagnosis; 12,610 of them were matched to 50,440 control infants. ADHD was determined based on prescription for a stimulant treatment or a hospital diagnosis; children with a first ADHD event younger than 3 years were excluded.
In the total cohort of 63,050 infants, the presence of any maternal autoimmune disease was associated with a significantly increased risk of ADHD (hazard ratio, 1.30) as was the presence of several specific conditions: type 1 diabetes (HR, 2.23), psoriasis (HR, 1.66), and rheumatic fever or rheumatic carditis (HR, 1.75).
In addition, the researchers conducted a meta-analysis of the current study and four additional studies that yielded similar results. In the meta-analysis, the risk of ADHD was significantly associated with any maternal autoimmune disease in two studies (HR, 1.20); with maternal type 1 diabetes in four studies (HR, 1.53); with maternal hyperthyroidism in three studies (HR 1.15); and with maternal psoriasis in two studies (HR, 1.31).
Type 1 diabetes (T1D) had the highest HR and was the most often studied condition. However, “the observed association may also be related to nonimmune aspects of T1D, such as glycemic control, as nonautoimmune diabetes has been associated with ADHD among children,” the researchers wrote.
The study findings were limited by several factors, including the lack of outpatient and primary care records to identify maternal autoimmune disease, and lack of data on any medication used to managed diseases during pregnancy, as well as a lack of data on children with ADHD who might not have been treated with medication, the researchers noted. In addition, “given differences in study design and definitions, the pooled HRs presented in the meta-analysis need to be treated cautiously.”
However, the results were strengthened by the hybrid study design and large study population, and were generally consistent with previous research supporting an effect of maternal immune function on fetal neurodevelopment, they noted.
“Our study provides justification for future studies that examine the effect of maternal autoimmune diseases, including biomarkers, condition severity, and management in pregnancy and in the periconception period, on neurodevelopmental disorders in children,” they concluded.
Studies need to explore mechanism of action
The current study, with its hybrid design, adds support to the evidence of an association between any maternal autoimmune disease and ADHD in children, as well as an association between the specific conditions of type 1 diabetes, hyperthyroidism, and psoriasis in mothers and ADHD in children, Søren Dalsgaard, MD, of Aarhus (Denmark) University, wrote in an accompanying editorial.
“Importantly, Nielsen et al. emphasized in their article that, for the many different autoimmune diseases, different underlying mechanisms for the associations with disorders of the central nervous system were likely. They mentioned that, for T1D, low glycemic control may play a role, as type 2 diabetes has been associated with ADHD,” said Dr. Dalsgaard.
“Overall, these mechanisms are thought to include shared genetic and environmental risk factors or direct effects of maternal autoantibodies or cytokines crossing the placenta and altering the fetal immune response, which in turns leads to changes in the central nervous system,” Dr. Dalsgaard explained. However, the current study and previous studies have not identified the mechanisms to explain the association between ADHD in children and maternal autoimmune disease.
“To understand more about these associations, future studies should include researchers and data from different scientific disciplines, such as epidemiology, animal modeling, genetics, and neuroimmunology,” he concluded.
Association is not causality
Overall, the study findings add to the evidence of a correlation between autoimmune diseases and neurologic disease, said Herschel Lessin, MD, of Children’s Medical Group, Poughkeepsie, N.Y., in an interview. “Anything that might contribute to behavioral problems is worth investigating.” However, it is important to remember that association is not causation.
“There is some literature and evidence that autoimmune disease is associated with mental health issues, but the mechanisms of action are unknown,” said Dr. Lessin. ADHD is highly heritable, so the association may be caused by a similar genetic predisposition, or it may be something related to autoimmunity that is impacting the fetus by passing through the placenta.
The current study’s strengths include the large size and hybrid design, but limitations such as the identification of ADHD based on medication prescriptions may have led to underreporting, and identifying maternal autoimmune disease via inpatient hospital diagnosis could have selected for more severe disease, he said.
From a clinical standpoint, the study suggests a correlation that should be noted in a family history and potentially used to inform a diagnosis, especially in cases of type 1 diabetes where the association was strongest, Dr. Lessin said. The findings also support the value of further research to look for mechanisms that might explain whether the association between autoimmune disease and ADHD is autoimmune system causality or shared genetic susceptibility.
The study received no outside funding. One coauthor disclosed receiving grants from the National Blood Authority Australia and the Australian National Health and Medical Research Council during the conduct of the study. Dr. Dalsgaard had no financial conflicts to disclose. Dr. Lessin disclosed serving as editor of the ADHD toolkit for the American Academy of Pediatrics and coauthor of the current ADHD clinical guidelines. He also serves in advisory capacity to Cognoa, a company involved in diagnosis of autism, and Corium/KemPharm, companies involved in the development of ADHD treatments.
FROM JAMA PEDIATRICS