User login
First mammography guidelines for older breast cancer survivors
For women who have a life expectancy of 5-10 years, the guidelines recommend that consideration be given to discontinuing mammography.
Overall, the guidelines encourage shared decision-making that is individualized for each woman after weighing the benefits and harms associated with surveillance mammography and patient preferences.
The panel also recommended that patients with clinical findings and symptoms receive ongoing clinical breast examinations and diagnostic mammography and that patients be reassured that these practices will continue.
Guidelines on breast cancer screening for healthy women already “acknowledge the limitations of mammograms and the need to consider one’s health status and preferences when making decisions on how and when to stop routine mammograms,” said the article’s first author, Rachel A. Freedman, MD, MPH, of the Dana-Farber Cancer Institute, Boston.
However, “we don’t have this kind of consensus for women with a history of breast cancer,” she continued. “Current follow-up care guidelines simply state that women with a history of breast cancer with intact breasts should have annual mammography without any guidance.
“In practice, the use of mammograms is highly variable, with less than 50% of breast cancer survivors who have limited life expectancy having annual mammograms, according to survey data we have from prior work,” Dr. Freedman said in an interview.
The guidelines were published online Jan. 28 in JAMA Oncology.
Clinicians discuss how to have these discussions
As part of the process of developing these expert consensus guidelines, the researchers held several clinical focus groups that involved primary care physicians from Brigham and Women’s Hospital and oncology clinicians (including breast surgeons and medical oncologists) from the Dana-Farber Cancer Institute.
All clinicians felt that having expert guidelines and talking points to guide discussions would be helpful, the researchers report.
“However, some oncology clinicians felt that 75 years is often ‘too young’ to stop surveillance mammography and that 80 years may be a more comfortable age to stop routine testing,” they write. “Most clinicians felt that estimations of life expectancy, more than age, should inform the timing of this discussion.”
In contrast to primary and geriatric care clinicians, oncology clinicians reported discomfort with such discussions. They appreciated having the information but “felt it was easier to communicate findings indirectly, without specifically revealing life expectancy to patients. One oncology clinician, however, felt it would be ‘sneaky’ to calculate life expectancy without communicating this to patients, supporting more open discussions,” the authors report.
“All clinicians acknowledged that framing the conversation around patients’ low risk for in-breast cancer events and how mammography will not benefit them was more appealing than discussing life expectancy,” the researchers continue. Their literature review found that the risk of these individuals developing second breast cancers was similar to that of a healthy woman developing a first breast cancer, leading one clinician to comment: “If their risk is really equivalent to the general population – that is very powerful.”
“Some clinicians reported that they ‘focus on the risks’ or frame such discussions by asking: ‘If you were to find something on [a] mammogram, would you do anything about it?’ If a patient answered no, clinicians felt this was a signal to stop mammography,” they noted.
Literature review finds very low risk
Dr. Freedman and colleagues conducted a literature review of the risk for ipsilateral and contralateral breast cancer events among survivors and of the harms and benefits associated with mammography. Following the literature review, a multidisciplinary expert panel, which included patients and patient advocates, was convened to develop consensus guidelines.
The literature review confirmed that there was a low risk for in-breast cancer events in this population and that the risk was particularly low among patients who undergo treatment with endocrine therapy. Among those who did not receive systemic therapy for ERBB2-positive or triple-negative cancers, the rates of ipsilateral recurrence were estimated to be higher.
On the basis of the literature review, the estimated 10-year risk for in-breast cancer events ranged from 1% to 15% for ipsilateral breast cancers and from 1% to 5% for contralateral cancers. Among women in the same age group who did not have a history of breast cancer, the 5-year risk of developing the disease (average risk) was 2.2%.
The authors note that these findings mirror their estimates for new breast cancers among survivors who had low-risk disease. The findings are also similar to those cited in a large-scale mammography study, in which breast cancer survivors aged 70-80 years had a 1.1% annual risk for in-breast cancers. The risk was 0.7%-0.9% for similarly aged patients who did not have a history of breast cancer.
The benefits associated with mammography for older women are not well defined, but the literature suggests that mammography offers little to modest clinical benefit for patients in this age group. The limited benefits are likely because of the more than 10-year time lag that is needed to detect the small improvements in breast cancer mortality; slow-growing tumors generally do not affect the life expectancy of older women, they point out.
“Through our expert consensus process and after iterative feedback from clinicians, we created guidelines to support patients and clinicians in making individualized decisions on how and when to stop mammography,” said Dr. Freedman. “These guidelines are based on the risk of a breast cancer returning in the breast, one’s underlying health, and one’s preferences.”
The guidelines are also intended to provide information to patients on the benefits and harms of mammography in this setting, in addition to “how much we anticipate a mammogram may or may not continue to help a woman over time,” she said.
A companion guide for patients on these guidelines will be published in the coming months.
Dr. Freedman has received institutional clinical trial funding from Eisai and Puma Biotechnology outside the submitted work.
A version of this article first appeared on Medscape.com.
For women who have a life expectancy of 5-10 years, the guidelines recommend that consideration be given to discontinuing mammography.
Overall, the guidelines encourage shared decision-making that is individualized for each woman after weighing the benefits and harms associated with surveillance mammography and patient preferences.
The panel also recommended that patients with clinical findings and symptoms receive ongoing clinical breast examinations and diagnostic mammography and that patients be reassured that these practices will continue.
Guidelines on breast cancer screening for healthy women already “acknowledge the limitations of mammograms and the need to consider one’s health status and preferences when making decisions on how and when to stop routine mammograms,” said the article’s first author, Rachel A. Freedman, MD, MPH, of the Dana-Farber Cancer Institute, Boston.
However, “we don’t have this kind of consensus for women with a history of breast cancer,” she continued. “Current follow-up care guidelines simply state that women with a history of breast cancer with intact breasts should have annual mammography without any guidance.
“In practice, the use of mammograms is highly variable, with less than 50% of breast cancer survivors who have limited life expectancy having annual mammograms, according to survey data we have from prior work,” Dr. Freedman said in an interview.
The guidelines were published online Jan. 28 in JAMA Oncology.
Clinicians discuss how to have these discussions
As part of the process of developing these expert consensus guidelines, the researchers held several clinical focus groups that involved primary care physicians from Brigham and Women’s Hospital and oncology clinicians (including breast surgeons and medical oncologists) from the Dana-Farber Cancer Institute.
All clinicians felt that having expert guidelines and talking points to guide discussions would be helpful, the researchers report.
“However, some oncology clinicians felt that 75 years is often ‘too young’ to stop surveillance mammography and that 80 years may be a more comfortable age to stop routine testing,” they write. “Most clinicians felt that estimations of life expectancy, more than age, should inform the timing of this discussion.”
In contrast to primary and geriatric care clinicians, oncology clinicians reported discomfort with such discussions. They appreciated having the information but “felt it was easier to communicate findings indirectly, without specifically revealing life expectancy to patients. One oncology clinician, however, felt it would be ‘sneaky’ to calculate life expectancy without communicating this to patients, supporting more open discussions,” the authors report.
“All clinicians acknowledged that framing the conversation around patients’ low risk for in-breast cancer events and how mammography will not benefit them was more appealing than discussing life expectancy,” the researchers continue. Their literature review found that the risk of these individuals developing second breast cancers was similar to that of a healthy woman developing a first breast cancer, leading one clinician to comment: “If their risk is really equivalent to the general population – that is very powerful.”
“Some clinicians reported that they ‘focus on the risks’ or frame such discussions by asking: ‘If you were to find something on [a] mammogram, would you do anything about it?’ If a patient answered no, clinicians felt this was a signal to stop mammography,” they noted.
Literature review finds very low risk
Dr. Freedman and colleagues conducted a literature review of the risk for ipsilateral and contralateral breast cancer events among survivors and of the harms and benefits associated with mammography. Following the literature review, a multidisciplinary expert panel, which included patients and patient advocates, was convened to develop consensus guidelines.
The literature review confirmed that there was a low risk for in-breast cancer events in this population and that the risk was particularly low among patients who undergo treatment with endocrine therapy. Among those who did not receive systemic therapy for ERBB2-positive or triple-negative cancers, the rates of ipsilateral recurrence were estimated to be higher.
On the basis of the literature review, the estimated 10-year risk for in-breast cancer events ranged from 1% to 15% for ipsilateral breast cancers and from 1% to 5% for contralateral cancers. Among women in the same age group who did not have a history of breast cancer, the 5-year risk of developing the disease (average risk) was 2.2%.
The authors note that these findings mirror their estimates for new breast cancers among survivors who had low-risk disease. The findings are also similar to those cited in a large-scale mammography study, in which breast cancer survivors aged 70-80 years had a 1.1% annual risk for in-breast cancers. The risk was 0.7%-0.9% for similarly aged patients who did not have a history of breast cancer.
The benefits associated with mammography for older women are not well defined, but the literature suggests that mammography offers little to modest clinical benefit for patients in this age group. The limited benefits are likely because of the more than 10-year time lag that is needed to detect the small improvements in breast cancer mortality; slow-growing tumors generally do not affect the life expectancy of older women, they point out.
“Through our expert consensus process and after iterative feedback from clinicians, we created guidelines to support patients and clinicians in making individualized decisions on how and when to stop mammography,” said Dr. Freedman. “These guidelines are based on the risk of a breast cancer returning in the breast, one’s underlying health, and one’s preferences.”
The guidelines are also intended to provide information to patients on the benefits and harms of mammography in this setting, in addition to “how much we anticipate a mammogram may or may not continue to help a woman over time,” she said.
A companion guide for patients on these guidelines will be published in the coming months.
Dr. Freedman has received institutional clinical trial funding from Eisai and Puma Biotechnology outside the submitted work.
A version of this article first appeared on Medscape.com.
For women who have a life expectancy of 5-10 years, the guidelines recommend that consideration be given to discontinuing mammography.
Overall, the guidelines encourage shared decision-making that is individualized for each woman after weighing the benefits and harms associated with surveillance mammography and patient preferences.
The panel also recommended that patients with clinical findings and symptoms receive ongoing clinical breast examinations and diagnostic mammography and that patients be reassured that these practices will continue.
Guidelines on breast cancer screening for healthy women already “acknowledge the limitations of mammograms and the need to consider one’s health status and preferences when making decisions on how and when to stop routine mammograms,” said the article’s first author, Rachel A. Freedman, MD, MPH, of the Dana-Farber Cancer Institute, Boston.
However, “we don’t have this kind of consensus for women with a history of breast cancer,” she continued. “Current follow-up care guidelines simply state that women with a history of breast cancer with intact breasts should have annual mammography without any guidance.
“In practice, the use of mammograms is highly variable, with less than 50% of breast cancer survivors who have limited life expectancy having annual mammograms, according to survey data we have from prior work,” Dr. Freedman said in an interview.
The guidelines were published online Jan. 28 in JAMA Oncology.
Clinicians discuss how to have these discussions
As part of the process of developing these expert consensus guidelines, the researchers held several clinical focus groups that involved primary care physicians from Brigham and Women’s Hospital and oncology clinicians (including breast surgeons and medical oncologists) from the Dana-Farber Cancer Institute.
All clinicians felt that having expert guidelines and talking points to guide discussions would be helpful, the researchers report.
“However, some oncology clinicians felt that 75 years is often ‘too young’ to stop surveillance mammography and that 80 years may be a more comfortable age to stop routine testing,” they write. “Most clinicians felt that estimations of life expectancy, more than age, should inform the timing of this discussion.”
In contrast to primary and geriatric care clinicians, oncology clinicians reported discomfort with such discussions. They appreciated having the information but “felt it was easier to communicate findings indirectly, without specifically revealing life expectancy to patients. One oncology clinician, however, felt it would be ‘sneaky’ to calculate life expectancy without communicating this to patients, supporting more open discussions,” the authors report.
“All clinicians acknowledged that framing the conversation around patients’ low risk for in-breast cancer events and how mammography will not benefit them was more appealing than discussing life expectancy,” the researchers continue. Their literature review found that the risk of these individuals developing second breast cancers was similar to that of a healthy woman developing a first breast cancer, leading one clinician to comment: “If their risk is really equivalent to the general population – that is very powerful.”
“Some clinicians reported that they ‘focus on the risks’ or frame such discussions by asking: ‘If you were to find something on [a] mammogram, would you do anything about it?’ If a patient answered no, clinicians felt this was a signal to stop mammography,” they noted.
Literature review finds very low risk
Dr. Freedman and colleagues conducted a literature review of the risk for ipsilateral and contralateral breast cancer events among survivors and of the harms and benefits associated with mammography. Following the literature review, a multidisciplinary expert panel, which included patients and patient advocates, was convened to develop consensus guidelines.
The literature review confirmed that there was a low risk for in-breast cancer events in this population and that the risk was particularly low among patients who undergo treatment with endocrine therapy. Among those who did not receive systemic therapy for ERBB2-positive or triple-negative cancers, the rates of ipsilateral recurrence were estimated to be higher.
On the basis of the literature review, the estimated 10-year risk for in-breast cancer events ranged from 1% to 15% for ipsilateral breast cancers and from 1% to 5% for contralateral cancers. Among women in the same age group who did not have a history of breast cancer, the 5-year risk of developing the disease (average risk) was 2.2%.
The authors note that these findings mirror their estimates for new breast cancers among survivors who had low-risk disease. The findings are also similar to those cited in a large-scale mammography study, in which breast cancer survivors aged 70-80 years had a 1.1% annual risk for in-breast cancers. The risk was 0.7%-0.9% for similarly aged patients who did not have a history of breast cancer.
The benefits associated with mammography for older women are not well defined, but the literature suggests that mammography offers little to modest clinical benefit for patients in this age group. The limited benefits are likely because of the more than 10-year time lag that is needed to detect the small improvements in breast cancer mortality; slow-growing tumors generally do not affect the life expectancy of older women, they point out.
“Through our expert consensus process and after iterative feedback from clinicians, we created guidelines to support patients and clinicians in making individualized decisions on how and when to stop mammography,” said Dr. Freedman. “These guidelines are based on the risk of a breast cancer returning in the breast, one’s underlying health, and one’s preferences.”
The guidelines are also intended to provide information to patients on the benefits and harms of mammography in this setting, in addition to “how much we anticipate a mammogram may or may not continue to help a woman over time,” she said.
A companion guide for patients on these guidelines will be published in the coming months.
Dr. Freedman has received institutional clinical trial funding from Eisai and Puma Biotechnology outside the submitted work.
A version of this article first appeared on Medscape.com.
Global thyroid cancer overdiagnosis in children and adolescents
Global patterns of the incidence of thyroid cancer in children and adolescents closely correspond to the increases seen in recent decades in adults. The patterns point to the same culprit in both groups – overdiagnosis. The finding underscores recommendations to limit screening.
“Our findings suggest that recommendations against screening for thyroid cancer in the asymptomatic adult population who are free from risk factors should be extended to explicitly recommend against screening for thyroid cancer in similar populations of children and adolescents,” say the authors, led by Salvatore Vaccarella, PhD, of the International Agency for Research on Cancer, in Lyon, France.
The study was published online Jan. 19 in The Lancet Diabetes and Endocrinology.
In an accompanying comment, Livia Lamartina and colleagues from the department of nuclear medicine and endocrine oncology, Institut Gustave Roussy and the University Paris-Saclay, Villejuif, France, emphasize that unnecessary screening of thyroid cancer in children can have substantial implications.
“Overdiagnosis might transform a child into a thyroid cancer patient for the rest of their life, and overtreatment might induce complications and possibly lead to the requirement of lifelong thyroid hormone treatment,” they write.
“Therefore, screening with ultrasonography should not be recommended in asymptomatic children and adolescents,” they conclude.
Study findings
For the study, Dr. Vaccarella and colleagues evaluated the incidence of thyroid cancer in 49 countries and territories and mortality in 27 countries, using the most up-to-date data from the International Incidence of Childhood Cancer Volume 3 study, the Cancer in Five Continents database, and the World Health Organization mortality database.
Although there was considerable variability between countries, the incidence of thyroid cancer in children and adolescents aged 0-19 years increased rapidly between 1998 and 2002 and again between 2008 and 2012 in nearly all countries.
Country-specific incidence rates strongly correlated with rates in adults (r > 0.8), including the temporal aspects of the incidence rates (r > .0.6).
Of the 8049 thyroid cancers that were detected, 6935 (86.2%) were papillary carcinomas, 682 (8.5%) were follicular carcinomas, and 307 (3.8%) were medullary carcinomas, as determined on the basis of the WHO classification of thyroid carcinomas. Sixty-four tumors (0.8%) were of unspecified subtype. As is commonly observed in adults, rates were higher in girls than in boys and increased with older age for both sexes.
The strong correlation between children and adults in the timing of the increases in incidence was especially notable in countries where overdiagnosis has been identified as having a major role in the increasing thyroid cancer rates. Those countries are South Korea, the United States, Italy, France, and Australia, where 60%-90% of thyroid cancer diagnoses are attributable to overdiagnosis. Overall, the incidence of thyroid cancer was less than 1.5 per one million person-years in children younger than 10 years. There were small variations by country and sex.
Thyroid cancer mortalities remain low
Overall, the rate of thyroid cancer mortality among those younger than 20 years in each country was less than 0.1 per 10 million person-years, “corresponding to less than 10 deaths per year in all of the included countries collectively,” note Dr. Vaccarella and colleagues.
“The epidemiological pattern seen in children and adolescents mirrored that seen in adults. These findings suggest that, in affected countries and territories, there might be overdiagnosis in children and adolescents, as has been observed in adults,” they write.
The incidence of thyroid cancer in children and adolescents between 2008 and 2012 ranged from 0.4 per one million person-years in Uganda and Kenya and 13.4 per 1 million person-years in Belarus, where the increase is believed to be related to the Chernobyl nuclear power plant accident and to increased screening in the years following the accident.
Subclinical discoveries may lead to unnecessary measures
Thyroid cancer was once a rare condition. Rates began to increase steadily in the 1990s, corresponding with rapid advances in noninvasive diagnostic imaging. Currently, thyroid cancer is the fifth most diagnosed cancer worldwide in adult women and the third most common in women aged 50 years and younger.
Diagnostic measures ranging from ultrasound and MRI to fine-needle aspiration biopsy have played a large role in the increase in diagnoses. The diagnostic techniques are revealing subclinical cancers in thyroid glands that previously went undetected and that usually do not cause harm over a person’s lifetime. According to Dr. Vaccarella and colleagues, such discoveries can open the door to a wide range of unnecessary measures.
The possible consequences of overdiagnosis include unnecessary treatments, the need to undergo lifelong medical care, and potential adverse effects, which could negatively affect quality of life.
Recent research from the International Agency for Research on Cancer has indicated that there has been an “epidemic of overdiagnosis” of thyroid cancer. The pattern has even reached less affluent regions as diagnostic technologies have become widely available.
“What is surprising is the magnitude of this,” Dr. Vaccarella said in an interview.
“Without overdiagnosis, thyroid cancer would probably still be a relatively rare cancer,” he said.
The study authors have disclosed no relevant financial relationships. Dr. Lamartina has received personal, advisory board, and clinical trial principal investigator fees from Bayer, personal fees from Eisai, and clinical trial principal investigator fees from AstraZeneca. The other editorialists’ financial relationships are listed in the original article.
A version of this article first appeared on Medscape.com.
Global patterns of the incidence of thyroid cancer in children and adolescents closely correspond to the increases seen in recent decades in adults. The patterns point to the same culprit in both groups – overdiagnosis. The finding underscores recommendations to limit screening.
“Our findings suggest that recommendations against screening for thyroid cancer in the asymptomatic adult population who are free from risk factors should be extended to explicitly recommend against screening for thyroid cancer in similar populations of children and adolescents,” say the authors, led by Salvatore Vaccarella, PhD, of the International Agency for Research on Cancer, in Lyon, France.
The study was published online Jan. 19 in The Lancet Diabetes and Endocrinology.
In an accompanying comment, Livia Lamartina and colleagues from the department of nuclear medicine and endocrine oncology, Institut Gustave Roussy and the University Paris-Saclay, Villejuif, France, emphasize that unnecessary screening of thyroid cancer in children can have substantial implications.
“Overdiagnosis might transform a child into a thyroid cancer patient for the rest of their life, and overtreatment might induce complications and possibly lead to the requirement of lifelong thyroid hormone treatment,” they write.
“Therefore, screening with ultrasonography should not be recommended in asymptomatic children and adolescents,” they conclude.
Study findings
For the study, Dr. Vaccarella and colleagues evaluated the incidence of thyroid cancer in 49 countries and territories and mortality in 27 countries, using the most up-to-date data from the International Incidence of Childhood Cancer Volume 3 study, the Cancer in Five Continents database, and the World Health Organization mortality database.
Although there was considerable variability between countries, the incidence of thyroid cancer in children and adolescents aged 0-19 years increased rapidly between 1998 and 2002 and again between 2008 and 2012 in nearly all countries.
Country-specific incidence rates strongly correlated with rates in adults (r > 0.8), including the temporal aspects of the incidence rates (r > .0.6).
Of the 8049 thyroid cancers that were detected, 6935 (86.2%) were papillary carcinomas, 682 (8.5%) were follicular carcinomas, and 307 (3.8%) were medullary carcinomas, as determined on the basis of the WHO classification of thyroid carcinomas. Sixty-four tumors (0.8%) were of unspecified subtype. As is commonly observed in adults, rates were higher in girls than in boys and increased with older age for both sexes.
The strong correlation between children and adults in the timing of the increases in incidence was especially notable in countries where overdiagnosis has been identified as having a major role in the increasing thyroid cancer rates. Those countries are South Korea, the United States, Italy, France, and Australia, where 60%-90% of thyroid cancer diagnoses are attributable to overdiagnosis. Overall, the incidence of thyroid cancer was less than 1.5 per one million person-years in children younger than 10 years. There were small variations by country and sex.
Thyroid cancer mortalities remain low
Overall, the rate of thyroid cancer mortality among those younger than 20 years in each country was less than 0.1 per 10 million person-years, “corresponding to less than 10 deaths per year in all of the included countries collectively,” note Dr. Vaccarella and colleagues.
“The epidemiological pattern seen in children and adolescents mirrored that seen in adults. These findings suggest that, in affected countries and territories, there might be overdiagnosis in children and adolescents, as has been observed in adults,” they write.
The incidence of thyroid cancer in children and adolescents between 2008 and 2012 ranged from 0.4 per one million person-years in Uganda and Kenya and 13.4 per 1 million person-years in Belarus, where the increase is believed to be related to the Chernobyl nuclear power plant accident and to increased screening in the years following the accident.
Subclinical discoveries may lead to unnecessary measures
Thyroid cancer was once a rare condition. Rates began to increase steadily in the 1990s, corresponding with rapid advances in noninvasive diagnostic imaging. Currently, thyroid cancer is the fifth most diagnosed cancer worldwide in adult women and the third most common in women aged 50 years and younger.
Diagnostic measures ranging from ultrasound and MRI to fine-needle aspiration biopsy have played a large role in the increase in diagnoses. The diagnostic techniques are revealing subclinical cancers in thyroid glands that previously went undetected and that usually do not cause harm over a person’s lifetime. According to Dr. Vaccarella and colleagues, such discoveries can open the door to a wide range of unnecessary measures.
The possible consequences of overdiagnosis include unnecessary treatments, the need to undergo lifelong medical care, and potential adverse effects, which could negatively affect quality of life.
Recent research from the International Agency for Research on Cancer has indicated that there has been an “epidemic of overdiagnosis” of thyroid cancer. The pattern has even reached less affluent regions as diagnostic technologies have become widely available.
“What is surprising is the magnitude of this,” Dr. Vaccarella said in an interview.
“Without overdiagnosis, thyroid cancer would probably still be a relatively rare cancer,” he said.
The study authors have disclosed no relevant financial relationships. Dr. Lamartina has received personal, advisory board, and clinical trial principal investigator fees from Bayer, personal fees from Eisai, and clinical trial principal investigator fees from AstraZeneca. The other editorialists’ financial relationships are listed in the original article.
A version of this article first appeared on Medscape.com.
Global patterns of the incidence of thyroid cancer in children and adolescents closely correspond to the increases seen in recent decades in adults. The patterns point to the same culprit in both groups – overdiagnosis. The finding underscores recommendations to limit screening.
“Our findings suggest that recommendations against screening for thyroid cancer in the asymptomatic adult population who are free from risk factors should be extended to explicitly recommend against screening for thyroid cancer in similar populations of children and adolescents,” say the authors, led by Salvatore Vaccarella, PhD, of the International Agency for Research on Cancer, in Lyon, France.
The study was published online Jan. 19 in The Lancet Diabetes and Endocrinology.
In an accompanying comment, Livia Lamartina and colleagues from the department of nuclear medicine and endocrine oncology, Institut Gustave Roussy and the University Paris-Saclay, Villejuif, France, emphasize that unnecessary screening of thyroid cancer in children can have substantial implications.
“Overdiagnosis might transform a child into a thyroid cancer patient for the rest of their life, and overtreatment might induce complications and possibly lead to the requirement of lifelong thyroid hormone treatment,” they write.
“Therefore, screening with ultrasonography should not be recommended in asymptomatic children and adolescents,” they conclude.
Study findings
For the study, Dr. Vaccarella and colleagues evaluated the incidence of thyroid cancer in 49 countries and territories and mortality in 27 countries, using the most up-to-date data from the International Incidence of Childhood Cancer Volume 3 study, the Cancer in Five Continents database, and the World Health Organization mortality database.
Although there was considerable variability between countries, the incidence of thyroid cancer in children and adolescents aged 0-19 years increased rapidly between 1998 and 2002 and again between 2008 and 2012 in nearly all countries.
Country-specific incidence rates strongly correlated with rates in adults (r > 0.8), including the temporal aspects of the incidence rates (r > .0.6).
Of the 8049 thyroid cancers that were detected, 6935 (86.2%) were papillary carcinomas, 682 (8.5%) were follicular carcinomas, and 307 (3.8%) were medullary carcinomas, as determined on the basis of the WHO classification of thyroid carcinomas. Sixty-four tumors (0.8%) were of unspecified subtype. As is commonly observed in adults, rates were higher in girls than in boys and increased with older age for both sexes.
The strong correlation between children and adults in the timing of the increases in incidence was especially notable in countries where overdiagnosis has been identified as having a major role in the increasing thyroid cancer rates. Those countries are South Korea, the United States, Italy, France, and Australia, where 60%-90% of thyroid cancer diagnoses are attributable to overdiagnosis. Overall, the incidence of thyroid cancer was less than 1.5 per one million person-years in children younger than 10 years. There were small variations by country and sex.
Thyroid cancer mortalities remain low
Overall, the rate of thyroid cancer mortality among those younger than 20 years in each country was less than 0.1 per 10 million person-years, “corresponding to less than 10 deaths per year in all of the included countries collectively,” note Dr. Vaccarella and colleagues.
“The epidemiological pattern seen in children and adolescents mirrored that seen in adults. These findings suggest that, in affected countries and territories, there might be overdiagnosis in children and adolescents, as has been observed in adults,” they write.
The incidence of thyroid cancer in children and adolescents between 2008 and 2012 ranged from 0.4 per one million person-years in Uganda and Kenya and 13.4 per 1 million person-years in Belarus, where the increase is believed to be related to the Chernobyl nuclear power plant accident and to increased screening in the years following the accident.
Subclinical discoveries may lead to unnecessary measures
Thyroid cancer was once a rare condition. Rates began to increase steadily in the 1990s, corresponding with rapid advances in noninvasive diagnostic imaging. Currently, thyroid cancer is the fifth most diagnosed cancer worldwide in adult women and the third most common in women aged 50 years and younger.
Diagnostic measures ranging from ultrasound and MRI to fine-needle aspiration biopsy have played a large role in the increase in diagnoses. The diagnostic techniques are revealing subclinical cancers in thyroid glands that previously went undetected and that usually do not cause harm over a person’s lifetime. According to Dr. Vaccarella and colleagues, such discoveries can open the door to a wide range of unnecessary measures.
The possible consequences of overdiagnosis include unnecessary treatments, the need to undergo lifelong medical care, and potential adverse effects, which could negatively affect quality of life.
Recent research from the International Agency for Research on Cancer has indicated that there has been an “epidemic of overdiagnosis” of thyroid cancer. The pattern has even reached less affluent regions as diagnostic technologies have become widely available.
“What is surprising is the magnitude of this,” Dr. Vaccarella said in an interview.
“Without overdiagnosis, thyroid cancer would probably still be a relatively rare cancer,” he said.
The study authors have disclosed no relevant financial relationships. Dr. Lamartina has received personal, advisory board, and clinical trial principal investigator fees from Bayer, personal fees from Eisai, and clinical trial principal investigator fees from AstraZeneca. The other editorialists’ financial relationships are listed in the original article.
A version of this article first appeared on Medscape.com.
Large study weighs in on ‘fat but fit’ paradox
Physical activity mitigated the impact of high body mass index (BMI) on cardiovascular risk factors, but not overall cardiovascular disease risk, according to an observational study of half a million individuals.
Despite the historically high rates of overweight and obesity worldwide, some evidence suggests that cardiorespiratory fitness could reduce the effects of excess weight on cardiovascular disease risk, wrote Pedro L. Valenzuela, PhD, of the University of Alcalá, Madrid, and colleagues.
“To clarify the existence of the ‘fat-but-fit’ [or ‘elevated BMI but active’] paradox, in this observational study, we assessed the joint association between different BMI categories and physical activity levels, respectively, and the prevalence of major CVD risk factors,” they said.
In a population-based cohort study published in the European Journal of Preventive Cardiology, the researchers identified 527,662 adults aged 18-64 years who were insured by an occupational risk–prevention company and underwent annual medical exams as part of their coverage. The average age of the participants was 42 years, 32% were women, and the average BMI was 26.2 kg/m2.
The participants were categorized as normal weight (42%), overweight (41%), and obese (18%), and their activity levels were categorized as inactive (64%), insufficiently active (12%), and regularly active (24%). In addition, 30% had hypercholesterolemia, 15% had hypertension, and 3% had diabetes.
Overall, compared with inactivity, insufficient activity or regular activity reduced CVD risk factors within each BMI category, and subgroups. “However, regular/insufficient PA did not compensate for the negative effects of overweight/obesity, as individuals with overweight/obesity were at greater CVD risk than their peers with normal weight, irrespective of PA levels,” the researchers said. Compared with active normal-weight men, the odds ratios for hypertension in active overweight men and active obese men were 1.98 and 4.93, respectively; the odds ratios for hypercholesterolemia were 1.61 and 2.03, respectively, and the odds ratios for diabetes were 1.33 and 3.62, respectively (P < .001 for all). Trends were similar for women.
The study results were limited by the cross-sectional design; inability to control for participants’ diet, and the reliance of self-reports of leisure-time physical activity. However, the findings were strengthened by the large sample size and “refute the notion that a physically active lifestyle can completely negate the deleterious effects of overweight/obesity,” the researchers said.
Although increasing physical activity should remain a priority for health policies, “weight loss per se should remain a primary target for health policies aimed at reducing CVD risk in people with overweight/obesity,” they concluded.
Interpret findings with caution
“With the ever-increasing public health problem of overweight and obesity, it is useful to assess any measure or measures that can have a favorable or adverse effect on cardiometabolic risk factors and the risk of CVD” Prakash Deedwania, MD, of the University of California, San Francisco, said in an interview.
Dr. Deedwania said he was not entirely surprised by the study findings. “The investigators have correlated only the self-reported level of physical activity (which is not always reliable) to the presence of three cardiac risk factors: hypertension, hypercholesterolemia, and diabetes.”
The study “is not comparable to prior reports that had shown a favorable impact of carefully assessed cardiorespiratory fitness with the risk of CVD,” Dr. Deedwania noted. “However, this is one of the largest population-wide surveillance studies of more than a half million active workers across Spain, and it does show that, despite self-reported physical activity, overweight and obesity are associated with higher risks of hypertension, diabetes, and hypercholesterolemia,” he explained.
“The main message of these findings is that, although physical activity does have a dose-dependent favorable impact on CV risk, the main public health intervention to reduce the risk of CV risk should focus on weight loss in overweight and obese individuals,” Dr. Deedwania emphasized.
“Future studies should focus on comparing various levels of daily activities and routine exercise such as walking, bicycling, etc., with the beneficial impact on cardiometabolic risk factors in overweight and obese individuals,” he said.
Dr. Valenzuela disclosed support from the University of Alcalá. Research by corresponding author Dr. Lucia was funded by grants from Spanish Ministry of Science and Innovation and Fondos FEDER. Dr. Deedwania had no financial conflicts to disclose.
Physical activity mitigated the impact of high body mass index (BMI) on cardiovascular risk factors, but not overall cardiovascular disease risk, according to an observational study of half a million individuals.
Despite the historically high rates of overweight and obesity worldwide, some evidence suggests that cardiorespiratory fitness could reduce the effects of excess weight on cardiovascular disease risk, wrote Pedro L. Valenzuela, PhD, of the University of Alcalá, Madrid, and colleagues.
“To clarify the existence of the ‘fat-but-fit’ [or ‘elevated BMI but active’] paradox, in this observational study, we assessed the joint association between different BMI categories and physical activity levels, respectively, and the prevalence of major CVD risk factors,” they said.
In a population-based cohort study published in the European Journal of Preventive Cardiology, the researchers identified 527,662 adults aged 18-64 years who were insured by an occupational risk–prevention company and underwent annual medical exams as part of their coverage. The average age of the participants was 42 years, 32% were women, and the average BMI was 26.2 kg/m2.
The participants were categorized as normal weight (42%), overweight (41%), and obese (18%), and their activity levels were categorized as inactive (64%), insufficiently active (12%), and regularly active (24%). In addition, 30% had hypercholesterolemia, 15% had hypertension, and 3% had diabetes.
Overall, compared with inactivity, insufficient activity or regular activity reduced CVD risk factors within each BMI category, and subgroups. “However, regular/insufficient PA did not compensate for the negative effects of overweight/obesity, as individuals with overweight/obesity were at greater CVD risk than their peers with normal weight, irrespective of PA levels,” the researchers said. Compared with active normal-weight men, the odds ratios for hypertension in active overweight men and active obese men were 1.98 and 4.93, respectively; the odds ratios for hypercholesterolemia were 1.61 and 2.03, respectively, and the odds ratios for diabetes were 1.33 and 3.62, respectively (P < .001 for all). Trends were similar for women.
The study results were limited by the cross-sectional design; inability to control for participants’ diet, and the reliance of self-reports of leisure-time physical activity. However, the findings were strengthened by the large sample size and “refute the notion that a physically active lifestyle can completely negate the deleterious effects of overweight/obesity,” the researchers said.
Although increasing physical activity should remain a priority for health policies, “weight loss per se should remain a primary target for health policies aimed at reducing CVD risk in people with overweight/obesity,” they concluded.
Interpret findings with caution
“With the ever-increasing public health problem of overweight and obesity, it is useful to assess any measure or measures that can have a favorable or adverse effect on cardiometabolic risk factors and the risk of CVD” Prakash Deedwania, MD, of the University of California, San Francisco, said in an interview.
Dr. Deedwania said he was not entirely surprised by the study findings. “The investigators have correlated only the self-reported level of physical activity (which is not always reliable) to the presence of three cardiac risk factors: hypertension, hypercholesterolemia, and diabetes.”
The study “is not comparable to prior reports that had shown a favorable impact of carefully assessed cardiorespiratory fitness with the risk of CVD,” Dr. Deedwania noted. “However, this is one of the largest population-wide surveillance studies of more than a half million active workers across Spain, and it does show that, despite self-reported physical activity, overweight and obesity are associated with higher risks of hypertension, diabetes, and hypercholesterolemia,” he explained.
“The main message of these findings is that, although physical activity does have a dose-dependent favorable impact on CV risk, the main public health intervention to reduce the risk of CV risk should focus on weight loss in overweight and obese individuals,” Dr. Deedwania emphasized.
“Future studies should focus on comparing various levels of daily activities and routine exercise such as walking, bicycling, etc., with the beneficial impact on cardiometabolic risk factors in overweight and obese individuals,” he said.
Dr. Valenzuela disclosed support from the University of Alcalá. Research by corresponding author Dr. Lucia was funded by grants from Spanish Ministry of Science and Innovation and Fondos FEDER. Dr. Deedwania had no financial conflicts to disclose.
Physical activity mitigated the impact of high body mass index (BMI) on cardiovascular risk factors, but not overall cardiovascular disease risk, according to an observational study of half a million individuals.
Despite the historically high rates of overweight and obesity worldwide, some evidence suggests that cardiorespiratory fitness could reduce the effects of excess weight on cardiovascular disease risk, wrote Pedro L. Valenzuela, PhD, of the University of Alcalá, Madrid, and colleagues.
“To clarify the existence of the ‘fat-but-fit’ [or ‘elevated BMI but active’] paradox, in this observational study, we assessed the joint association between different BMI categories and physical activity levels, respectively, and the prevalence of major CVD risk factors,” they said.
In a population-based cohort study published in the European Journal of Preventive Cardiology, the researchers identified 527,662 adults aged 18-64 years who were insured by an occupational risk–prevention company and underwent annual medical exams as part of their coverage. The average age of the participants was 42 years, 32% were women, and the average BMI was 26.2 kg/m2.
The participants were categorized as normal weight (42%), overweight (41%), and obese (18%), and their activity levels were categorized as inactive (64%), insufficiently active (12%), and regularly active (24%). In addition, 30% had hypercholesterolemia, 15% had hypertension, and 3% had diabetes.
Overall, compared with inactivity, insufficient activity or regular activity reduced CVD risk factors within each BMI category, and subgroups. “However, regular/insufficient PA did not compensate for the negative effects of overweight/obesity, as individuals with overweight/obesity were at greater CVD risk than their peers with normal weight, irrespective of PA levels,” the researchers said. Compared with active normal-weight men, the odds ratios for hypertension in active overweight men and active obese men were 1.98 and 4.93, respectively; the odds ratios for hypercholesterolemia were 1.61 and 2.03, respectively, and the odds ratios for diabetes were 1.33 and 3.62, respectively (P < .001 for all). Trends were similar for women.
The study results were limited by the cross-sectional design; inability to control for participants’ diet, and the reliance of self-reports of leisure-time physical activity. However, the findings were strengthened by the large sample size and “refute the notion that a physically active lifestyle can completely negate the deleterious effects of overweight/obesity,” the researchers said.
Although increasing physical activity should remain a priority for health policies, “weight loss per se should remain a primary target for health policies aimed at reducing CVD risk in people with overweight/obesity,” they concluded.
Interpret findings with caution
“With the ever-increasing public health problem of overweight and obesity, it is useful to assess any measure or measures that can have a favorable or adverse effect on cardiometabolic risk factors and the risk of CVD” Prakash Deedwania, MD, of the University of California, San Francisco, said in an interview.
Dr. Deedwania said he was not entirely surprised by the study findings. “The investigators have correlated only the self-reported level of physical activity (which is not always reliable) to the presence of three cardiac risk factors: hypertension, hypercholesterolemia, and diabetes.”
The study “is not comparable to prior reports that had shown a favorable impact of carefully assessed cardiorespiratory fitness with the risk of CVD,” Dr. Deedwania noted. “However, this is one of the largest population-wide surveillance studies of more than a half million active workers across Spain, and it does show that, despite self-reported physical activity, overweight and obesity are associated with higher risks of hypertension, diabetes, and hypercholesterolemia,” he explained.
“The main message of these findings is that, although physical activity does have a dose-dependent favorable impact on CV risk, the main public health intervention to reduce the risk of CV risk should focus on weight loss in overweight and obese individuals,” Dr. Deedwania emphasized.
“Future studies should focus on comparing various levels of daily activities and routine exercise such as walking, bicycling, etc., with the beneficial impact on cardiometabolic risk factors in overweight and obese individuals,” he said.
Dr. Valenzuela disclosed support from the University of Alcalá. Research by corresponding author Dr. Lucia was funded by grants from Spanish Ministry of Science and Innovation and Fondos FEDER. Dr. Deedwania had no financial conflicts to disclose.
FROM THE EUROPEAN JOURNAL OF PREVENTIVE CARDIOLOGY
Meta-analysis finds much less lupus than expected
The prevalence of systemic lupus erythematosus (SLE) appears to be much lower than previously believed and may pose “a potential risk to research funding for the disease,” according to results of a meta-analysis involving a network of population-based registries.
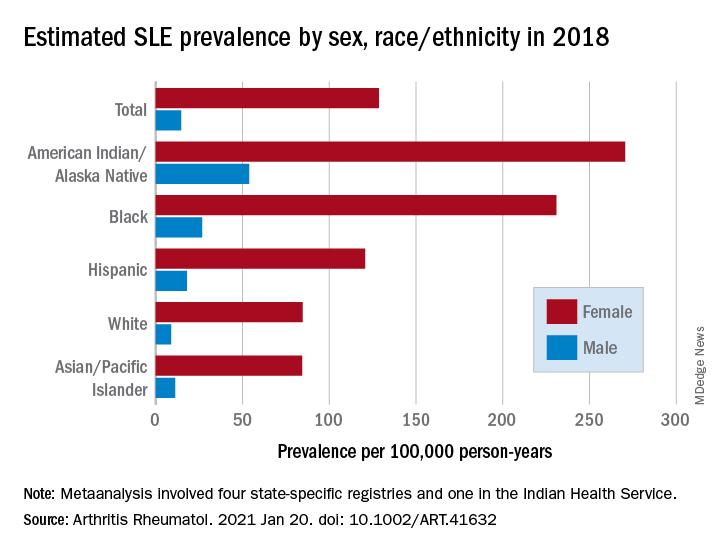
“When we started this study, a widely cited lupus statistic was that approximately 1.5 million Americans were affected. Our meta-analysis found the actual prevalence to be slightly more than 200,000: a number that approaches the [Food and Drug Administration’s] definition of a rare disease,” Emily Somers, PhD, ScM, senior author and associate professor of rheumatology and environmental health sciences at the University of Michigan, Ann Arbor, said in a written statement.
Their estimates, published online in Arthritis & Rheumatology, put the overall SLE prevalence in the United States at 72.8 per 100,000 person-years in 2018, with nearly nine times more females affected (128.7 cases per 100,000) than males (14.6 per 100,000). Race and ethnicity also play a role, as prevalence was highest among American Indian/Alaska Native and Black females, with Hispanic females lower but still higher than White and Asian/Pacific Islander females, Peter M. Izmirly, MD, MSc, of New York University, the lead author, and associates said.
SLE prevalence was distributed similarly in men, although there was a greater relative margin between American Indians/Alaska Natives (53.8 cases per 100,000 person-years) and Blacks (26.7 per 100,000), and Asians/Pacific Islanders were higher than Whites (11.2 vs. 8.9), the investigators reported.
The meta-analysis leveraged data from the Centers for Disease Control and Prevention’s national lupus registries, which include four state-specific SLE registries and a fifth in the Indian Health Service. All cases of SLE occurred in 2002-2009, and the data were age adjusted to the 2000 U.S. population and separately extrapolated to the 2018 U.S. Census population, they explained.
The analysis was funded by cooperative agreements between the New York City Department of Health and Mental Hygiene and New York University, and the CDC and National Institute of Health.
The prevalence of systemic lupus erythematosus (SLE) appears to be much lower than previously believed and may pose “a potential risk to research funding for the disease,” according to results of a meta-analysis involving a network of population-based registries.

“When we started this study, a widely cited lupus statistic was that approximately 1.5 million Americans were affected. Our meta-analysis found the actual prevalence to be slightly more than 200,000: a number that approaches the [Food and Drug Administration’s] definition of a rare disease,” Emily Somers, PhD, ScM, senior author and associate professor of rheumatology and environmental health sciences at the University of Michigan, Ann Arbor, said in a written statement.
Their estimates, published online in Arthritis & Rheumatology, put the overall SLE prevalence in the United States at 72.8 per 100,000 person-years in 2018, with nearly nine times more females affected (128.7 cases per 100,000) than males (14.6 per 100,000). Race and ethnicity also play a role, as prevalence was highest among American Indian/Alaska Native and Black females, with Hispanic females lower but still higher than White and Asian/Pacific Islander females, Peter M. Izmirly, MD, MSc, of New York University, the lead author, and associates said.
SLE prevalence was distributed similarly in men, although there was a greater relative margin between American Indians/Alaska Natives (53.8 cases per 100,000 person-years) and Blacks (26.7 per 100,000), and Asians/Pacific Islanders were higher than Whites (11.2 vs. 8.9), the investigators reported.
The meta-analysis leveraged data from the Centers for Disease Control and Prevention’s national lupus registries, which include four state-specific SLE registries and a fifth in the Indian Health Service. All cases of SLE occurred in 2002-2009, and the data were age adjusted to the 2000 U.S. population and separately extrapolated to the 2018 U.S. Census population, they explained.
The analysis was funded by cooperative agreements between the New York City Department of Health and Mental Hygiene and New York University, and the CDC and National Institute of Health.
The prevalence of systemic lupus erythematosus (SLE) appears to be much lower than previously believed and may pose “a potential risk to research funding for the disease,” according to results of a meta-analysis involving a network of population-based registries.

“When we started this study, a widely cited lupus statistic was that approximately 1.5 million Americans were affected. Our meta-analysis found the actual prevalence to be slightly more than 200,000: a number that approaches the [Food and Drug Administration’s] definition of a rare disease,” Emily Somers, PhD, ScM, senior author and associate professor of rheumatology and environmental health sciences at the University of Michigan, Ann Arbor, said in a written statement.
Their estimates, published online in Arthritis & Rheumatology, put the overall SLE prevalence in the United States at 72.8 per 100,000 person-years in 2018, with nearly nine times more females affected (128.7 cases per 100,000) than males (14.6 per 100,000). Race and ethnicity also play a role, as prevalence was highest among American Indian/Alaska Native and Black females, with Hispanic females lower but still higher than White and Asian/Pacific Islander females, Peter M. Izmirly, MD, MSc, of New York University, the lead author, and associates said.
SLE prevalence was distributed similarly in men, although there was a greater relative margin between American Indians/Alaska Natives (53.8 cases per 100,000 person-years) and Blacks (26.7 per 100,000), and Asians/Pacific Islanders were higher than Whites (11.2 vs. 8.9), the investigators reported.
The meta-analysis leveraged data from the Centers for Disease Control and Prevention’s national lupus registries, which include four state-specific SLE registries and a fifth in the Indian Health Service. All cases of SLE occurred in 2002-2009, and the data were age adjusted to the 2000 U.S. population and separately extrapolated to the 2018 U.S. Census population, they explained.
The analysis was funded by cooperative agreements between the New York City Department of Health and Mental Hygiene and New York University, and the CDC and National Institute of Health.
FROM ARTHRITIS & RHEUMATOLOGY
Defining wellness in IBD
Physicians treating patients with IBD typically focus on disease and symptom management along with quality of life measures, but the latter are not the final word on patient well-being. Social well-being is another outcome that can more accurately portray a patient’s satisfaction with their treatment.
That was the message delivered by Laurie Keefer, PhD, at a session on diet, stress, health literacy, and disparities in IBD treatment at the annual congress of the Crohn’s & Colitis Foundation and the American Gastroenterological Association. “When we talk about disease management, we’re talking about these outcomes of mucosal healing, remission, and lack of hospitalizations, but we don’t always talk about wellness,” said Dr. Keefer, director of psychobehavioral research in the department of gastroenterology at Icahn School of Medicine at Mount Sinai, New York.
Dr. Keefer advocated for incorporating measures that focus on the patient’s ability to feel fulfilled, pursue happiness, and contribute to the community. “Wellness is defined as a state of complete physical, mental, and social well-being. It’s a holistic definition, not merely the absence of those things,” she said during her talk.
Social determinants of health, such as income, inequality, health literacy, numeracy, financial stress, social connections, community, place of resonance, and housing coresidents, play important roles.
“Subjective well-being is a state in which an individual feels they are able to do work productively and creatively, have relationships, and contribute to their community. We want them to thrive. We want them to live well. We want them to reach their potential. There’s no reason you cannot reach your potential even though you’re living with IBD,” said Dr. Keefer.
Subjective well-being doesn’t replace quality of life assessment. “Absolutely, quality of life is an important metric, [but I want to] make a plug that maybe we should start to add subjective well-being into these outcome measures,” said Dr. Keefer.
The approach does away with specific measures of health, employment, financial security, or even living situation. “It takes away all of those things we just assume are part of being well. It measures it differently. It measures what makes us happy, divided by the degree of happiness we obtain,” said Dr. Keefer. She presented examples from a study her group is conducting that showed patients’ responses to what made them want to be well. “Some people want to be well to take care of their children or families or a parent, some people want to be well so they can go adventure skydiving, other people just want to be able to exercise and take care of their health. That’s what the target needs to be for wellness. In that sense, wellness is an achievement of best health possible in all domains, not just one. It’s a lifelong pursuit. It forces us to ask not just ‘Are my patient’s symptoms gone? Are they in clinical remission? Are they in histological remission? Are they in deep remission?’ but ‘Is my patient thriving? Are they meeting their potential? Are they getting what they want out of treatment? Are they happy?’ ”
Quality of life measures can provide some insight, but they are limited because they are anchored in physical symptoms, and they focus on a narrow, recent window, usually the past week. “You can imagine that as symptoms improve, those metrics kind of improve, and it looks like quality of life is great. But that’s not always the case, and we’re really missing an opportunity to go deeper. It’s also less sensitive when somebody is in remission, so it’s also very difficult to continue that proactive [approach] of thriving and living well when you’re already coming up positive on quality of life indices,” said Dr. Keefer.
Subjective well-being measures ignore physical symptoms, and focus instead on questions like the patient’s ability to work, socialize, and maintain relationships with family, and whether the patient feels able to contribute meaningfully to society. The measure is insensitive to factors such as inflammation, trauma, or changes to medication. As a result, measures can be used much less frequently – every 6 months, or even once a year.
Subjective well-being can also rely on the patient to define well-being, and that makes it more culturally sensitive. “It can allow for people to be well in whatever way they think they want to be well,” said Dr. Keefer.
There are various resources for measuring subjective well-being. The Organization for Economic Cooperation and Development has guidelines for measuring subjective well-being. The National Institutes of Health PROMIS includes useful measures of psychological well-being, positive affect, and general life satisfaction; they are available for free and include 6-8 items. Other useful measures include the Satisfaction with Life scale, the Positive and Negative Affect scale, and the Harmony in Life scale. “All of those have been well validated and used internationally as measures of well-being,” said Dr. Keefer.
Physicians can also address patients directly, asking them about how satisfied they are with their life. “You’re opening up that discussion to ask them not just, ‘How is your IBD and how is your IBD affecting your work?’ but ‘How is your life going?’ You’re proactively trying to help your patients thrive,” said Dr. Keefer.
Session moderators praised Dr. Keefer’s presentation as an appropriate wrap-up to talks that looked at stress, diet, economic disparities, health literacy, and numeracy.
“We capped it all with a discussion around what is well-being. We often talk about biologics or medicines or surgery when it comes to Crohn’s disease and ulcerative colitis, but what about holistic wellness? It’s all of this. It’s the medication piece, but it’s all of these other pillars involved in the process as well. I think looking at this from many different angles is very important so that patients can achieve the best quality of life possible,” said comoderator Tina Aswani Omprakash, a patient advocate who is pursuing a master’s degree in public health at Mount Sinai’s Icahn School of Medicine.
The other comoderator, Kelly Issokson, MS, RD, CNSC, agreed. “You can’t adequately treat patients with diet alone or stress management alone. You really need a holistic approach for best outcomes,” said Ms. Issokson, clinical nutritional coordinator at the digestive disease clinic at Cedars-Sinai Medical Center in Los Angeles.
Dr. Keefer has received research funding from AbbVie and is a cofounder and equity holder in Trellus Health. Ms. Aswani Omprakash has consulted for Genentech, AbbVie, Janssen, and Arena Pharmaceuticals. Ms. Issokson has no relevant financial disclosures.
Physicians treating patients with IBD typically focus on disease and symptom management along with quality of life measures, but the latter are not the final word on patient well-being. Social well-being is another outcome that can more accurately portray a patient’s satisfaction with their treatment.
That was the message delivered by Laurie Keefer, PhD, at a session on diet, stress, health literacy, and disparities in IBD treatment at the annual congress of the Crohn’s & Colitis Foundation and the American Gastroenterological Association. “When we talk about disease management, we’re talking about these outcomes of mucosal healing, remission, and lack of hospitalizations, but we don’t always talk about wellness,” said Dr. Keefer, director of psychobehavioral research in the department of gastroenterology at Icahn School of Medicine at Mount Sinai, New York.
Dr. Keefer advocated for incorporating measures that focus on the patient’s ability to feel fulfilled, pursue happiness, and contribute to the community. “Wellness is defined as a state of complete physical, mental, and social well-being. It’s a holistic definition, not merely the absence of those things,” she said during her talk.
Social determinants of health, such as income, inequality, health literacy, numeracy, financial stress, social connections, community, place of resonance, and housing coresidents, play important roles.
“Subjective well-being is a state in which an individual feels they are able to do work productively and creatively, have relationships, and contribute to their community. We want them to thrive. We want them to live well. We want them to reach their potential. There’s no reason you cannot reach your potential even though you’re living with IBD,” said Dr. Keefer.
Subjective well-being doesn’t replace quality of life assessment. “Absolutely, quality of life is an important metric, [but I want to] make a plug that maybe we should start to add subjective well-being into these outcome measures,” said Dr. Keefer.
The approach does away with specific measures of health, employment, financial security, or even living situation. “It takes away all of those things we just assume are part of being well. It measures it differently. It measures what makes us happy, divided by the degree of happiness we obtain,” said Dr. Keefer. She presented examples from a study her group is conducting that showed patients’ responses to what made them want to be well. “Some people want to be well to take care of their children or families or a parent, some people want to be well so they can go adventure skydiving, other people just want to be able to exercise and take care of their health. That’s what the target needs to be for wellness. In that sense, wellness is an achievement of best health possible in all domains, not just one. It’s a lifelong pursuit. It forces us to ask not just ‘Are my patient’s symptoms gone? Are they in clinical remission? Are they in histological remission? Are they in deep remission?’ but ‘Is my patient thriving? Are they meeting their potential? Are they getting what they want out of treatment? Are they happy?’ ”
Quality of life measures can provide some insight, but they are limited because they are anchored in physical symptoms, and they focus on a narrow, recent window, usually the past week. “You can imagine that as symptoms improve, those metrics kind of improve, and it looks like quality of life is great. But that’s not always the case, and we’re really missing an opportunity to go deeper. It’s also less sensitive when somebody is in remission, so it’s also very difficult to continue that proactive [approach] of thriving and living well when you’re already coming up positive on quality of life indices,” said Dr. Keefer.
Subjective well-being measures ignore physical symptoms, and focus instead on questions like the patient’s ability to work, socialize, and maintain relationships with family, and whether the patient feels able to contribute meaningfully to society. The measure is insensitive to factors such as inflammation, trauma, or changes to medication. As a result, measures can be used much less frequently – every 6 months, or even once a year.
Subjective well-being can also rely on the patient to define well-being, and that makes it more culturally sensitive. “It can allow for people to be well in whatever way they think they want to be well,” said Dr. Keefer.
There are various resources for measuring subjective well-being. The Organization for Economic Cooperation and Development has guidelines for measuring subjective well-being. The National Institutes of Health PROMIS includes useful measures of psychological well-being, positive affect, and general life satisfaction; they are available for free and include 6-8 items. Other useful measures include the Satisfaction with Life scale, the Positive and Negative Affect scale, and the Harmony in Life scale. “All of those have been well validated and used internationally as measures of well-being,” said Dr. Keefer.
Physicians can also address patients directly, asking them about how satisfied they are with their life. “You’re opening up that discussion to ask them not just, ‘How is your IBD and how is your IBD affecting your work?’ but ‘How is your life going?’ You’re proactively trying to help your patients thrive,” said Dr. Keefer.
Session moderators praised Dr. Keefer’s presentation as an appropriate wrap-up to talks that looked at stress, diet, economic disparities, health literacy, and numeracy.
“We capped it all with a discussion around what is well-being. We often talk about biologics or medicines or surgery when it comes to Crohn’s disease and ulcerative colitis, but what about holistic wellness? It’s all of this. It’s the medication piece, but it’s all of these other pillars involved in the process as well. I think looking at this from many different angles is very important so that patients can achieve the best quality of life possible,” said comoderator Tina Aswani Omprakash, a patient advocate who is pursuing a master’s degree in public health at Mount Sinai’s Icahn School of Medicine.
The other comoderator, Kelly Issokson, MS, RD, CNSC, agreed. “You can’t adequately treat patients with diet alone or stress management alone. You really need a holistic approach for best outcomes,” said Ms. Issokson, clinical nutritional coordinator at the digestive disease clinic at Cedars-Sinai Medical Center in Los Angeles.
Dr. Keefer has received research funding from AbbVie and is a cofounder and equity holder in Trellus Health. Ms. Aswani Omprakash has consulted for Genentech, AbbVie, Janssen, and Arena Pharmaceuticals. Ms. Issokson has no relevant financial disclosures.
Physicians treating patients with IBD typically focus on disease and symptom management along with quality of life measures, but the latter are not the final word on patient well-being. Social well-being is another outcome that can more accurately portray a patient’s satisfaction with their treatment.
That was the message delivered by Laurie Keefer, PhD, at a session on diet, stress, health literacy, and disparities in IBD treatment at the annual congress of the Crohn’s & Colitis Foundation and the American Gastroenterological Association. “When we talk about disease management, we’re talking about these outcomes of mucosal healing, remission, and lack of hospitalizations, but we don’t always talk about wellness,” said Dr. Keefer, director of psychobehavioral research in the department of gastroenterology at Icahn School of Medicine at Mount Sinai, New York.
Dr. Keefer advocated for incorporating measures that focus on the patient’s ability to feel fulfilled, pursue happiness, and contribute to the community. “Wellness is defined as a state of complete physical, mental, and social well-being. It’s a holistic definition, not merely the absence of those things,” she said during her talk.
Social determinants of health, such as income, inequality, health literacy, numeracy, financial stress, social connections, community, place of resonance, and housing coresidents, play important roles.
“Subjective well-being is a state in which an individual feels they are able to do work productively and creatively, have relationships, and contribute to their community. We want them to thrive. We want them to live well. We want them to reach their potential. There’s no reason you cannot reach your potential even though you’re living with IBD,” said Dr. Keefer.
Subjective well-being doesn’t replace quality of life assessment. “Absolutely, quality of life is an important metric, [but I want to] make a plug that maybe we should start to add subjective well-being into these outcome measures,” said Dr. Keefer.
The approach does away with specific measures of health, employment, financial security, or even living situation. “It takes away all of those things we just assume are part of being well. It measures it differently. It measures what makes us happy, divided by the degree of happiness we obtain,” said Dr. Keefer. She presented examples from a study her group is conducting that showed patients’ responses to what made them want to be well. “Some people want to be well to take care of their children or families or a parent, some people want to be well so they can go adventure skydiving, other people just want to be able to exercise and take care of their health. That’s what the target needs to be for wellness. In that sense, wellness is an achievement of best health possible in all domains, not just one. It’s a lifelong pursuit. It forces us to ask not just ‘Are my patient’s symptoms gone? Are they in clinical remission? Are they in histological remission? Are they in deep remission?’ but ‘Is my patient thriving? Are they meeting their potential? Are they getting what they want out of treatment? Are they happy?’ ”
Quality of life measures can provide some insight, but they are limited because they are anchored in physical symptoms, and they focus on a narrow, recent window, usually the past week. “You can imagine that as symptoms improve, those metrics kind of improve, and it looks like quality of life is great. But that’s not always the case, and we’re really missing an opportunity to go deeper. It’s also less sensitive when somebody is in remission, so it’s also very difficult to continue that proactive [approach] of thriving and living well when you’re already coming up positive on quality of life indices,” said Dr. Keefer.
Subjective well-being measures ignore physical symptoms, and focus instead on questions like the patient’s ability to work, socialize, and maintain relationships with family, and whether the patient feels able to contribute meaningfully to society. The measure is insensitive to factors such as inflammation, trauma, or changes to medication. As a result, measures can be used much less frequently – every 6 months, or even once a year.
Subjective well-being can also rely on the patient to define well-being, and that makes it more culturally sensitive. “It can allow for people to be well in whatever way they think they want to be well,” said Dr. Keefer.
There are various resources for measuring subjective well-being. The Organization for Economic Cooperation and Development has guidelines for measuring subjective well-being. The National Institutes of Health PROMIS includes useful measures of psychological well-being, positive affect, and general life satisfaction; they are available for free and include 6-8 items. Other useful measures include the Satisfaction with Life scale, the Positive and Negative Affect scale, and the Harmony in Life scale. “All of those have been well validated and used internationally as measures of well-being,” said Dr. Keefer.
Physicians can also address patients directly, asking them about how satisfied they are with their life. “You’re opening up that discussion to ask them not just, ‘How is your IBD and how is your IBD affecting your work?’ but ‘How is your life going?’ You’re proactively trying to help your patients thrive,” said Dr. Keefer.
Session moderators praised Dr. Keefer’s presentation as an appropriate wrap-up to talks that looked at stress, diet, economic disparities, health literacy, and numeracy.
“We capped it all with a discussion around what is well-being. We often talk about biologics or medicines or surgery when it comes to Crohn’s disease and ulcerative colitis, but what about holistic wellness? It’s all of this. It’s the medication piece, but it’s all of these other pillars involved in the process as well. I think looking at this from many different angles is very important so that patients can achieve the best quality of life possible,” said comoderator Tina Aswani Omprakash, a patient advocate who is pursuing a master’s degree in public health at Mount Sinai’s Icahn School of Medicine.
The other comoderator, Kelly Issokson, MS, RD, CNSC, agreed. “You can’t adequately treat patients with diet alone or stress management alone. You really need a holistic approach for best outcomes,” said Ms. Issokson, clinical nutritional coordinator at the digestive disease clinic at Cedars-Sinai Medical Center in Los Angeles.
Dr. Keefer has received research funding from AbbVie and is a cofounder and equity holder in Trellus Health. Ms. Aswani Omprakash has consulted for Genentech, AbbVie, Janssen, and Arena Pharmaceuticals. Ms. Issokson has no relevant financial disclosures.
FROM THE CROHN’S & COLITIS CONGRESS
Incidence of autoimmune hepatitis may be rising
The incidence of autoimmune hepatitis (AIH) may be rising, according to a prospective population-based study conducted in New Zealand.
From 2008 to 2016, the rising incidence of AIH led to a 40% increase in point prevalence, reported lead author Mehul Lamba, MD, of Christchurch (New Zealand) Hospital and colleagues.
The present study, which also assessed rates of primary biliary cholangitis (PBC) and primary sclerosing cholangitis (PSC), adds data to an area of inquiry historically characterized by limited and inconsistent results, the investigators wrote in Clinical Gastroenterology and Hepatology. They suggested that mixed findings from previous studies may be because of differences in population and environmental factors, but also varying diagnostic criteria.
“The epidemiological trends of these autoimmune liver diseases therefore remain incompletely understood,” wrote Dr. Lamba and colleagues.
Their study evaluated trends in autoimmune liver diseases over a 9-year time frame in Canterbury, New Zealand. According to the investigators, this region is well suited to an epidemiological investigation because it is a clearly defined geographic area with approximately 600,000 people, most of whom rely on one tertiary care center: Christchurch Hospital. The bulk of the data therefore came from this center, while a minority of cases were gathered from local private gastroenterology practices, “making complete case ascertainment possible.”
Incidence of AIH, PBC, and PSC was assessed at three time points: 2008-2010, 2011-2013, and 2014-2016. AIH had the highest overall incidence, at 1.93 cases per 100,000 people, followed by PSC (0.92) and PBC (0.51).
While the rates of PBC and PSC did not change significantly over time, the incidence of AIH rose from 1.37 cases per 100,000 people in the period from 2008-2010 to 2.39 per 100,000 in 2014-2016 (P = .04), which computes to an incidence rate ratio of 1.69 (95% confidence interval, 1.02-2.84). Point prevalence was also significantly higher in 2016, compared with 2008, at 27.5 per 100,000 versus 19.7 per 100,000 (P < .01). The investigators described a bimodal age of presentation, with the first peak among patients younger than 20 years, and a second, larger peak among individuals aged 50-69 years.
According to the investigators, these findings “are concordant with the results observed in the European cohort,” citing a Danish study spanning 1994-2012 and a Dutch study spanning 2000-2010. They noted that the Danish study also reported a bimodal distribution of age incidence, as did a Swedish study, and another study from New Zealand. The stable levels of PBC and PSC align with two recent retrospective studies conducted in the United States and, they added.
“We believe that the observed differential trends in the incidence of these autoimmune liver diseases truly reflects their contemporary epidemiology,” the investigators wrote. They went on to suggest that the findings did not stem from an increase in diagnostic scrutiny because the study period did not include any significant changes in gastroenterology service, coding, or diagnostic criteria in the region studied.
“The increased incidence of AIH parallels rising incidence and prevalence of other autoimmune disorders such as [inflammatory bowel disease], type 1 diabetes, and multiple sclerosis in New Zealand, and it is unclear whether these autoimmune conditions share a common local environmental trigger,” they wrote. “Environmental factors likely play a central role augmenting phenotypic expression in genetically predisposed individuals.”
While Dr. Lamba and colleagues proposed several possible factors, such as increased exposure to pharmaceuticals, definitive factors remain elusive, which the authors cited as one limitation of their study. Another limitation they cited is the possibility that other etiologies were mistakenly classified as “probable” AIH; however, the chances of that are small, and the proportion of probable versus definitive AIH noted in this study do reflect those seen in other epidemiological studies.
“The reason for observed differential change in incidence of these autoimmune liver diseases is unclear,” they wrote, “and future collaborative prospective epidemiological study would be required to assess this further.”
The investigators reported no conflicts of interest.
The incidence of autoimmune hepatitis (AIH) may be rising, according to a prospective population-based study conducted in New Zealand.
From 2008 to 2016, the rising incidence of AIH led to a 40% increase in point prevalence, reported lead author Mehul Lamba, MD, of Christchurch (New Zealand) Hospital and colleagues.
The present study, which also assessed rates of primary biliary cholangitis (PBC) and primary sclerosing cholangitis (PSC), adds data to an area of inquiry historically characterized by limited and inconsistent results, the investigators wrote in Clinical Gastroenterology and Hepatology. They suggested that mixed findings from previous studies may be because of differences in population and environmental factors, but also varying diagnostic criteria.
“The epidemiological trends of these autoimmune liver diseases therefore remain incompletely understood,” wrote Dr. Lamba and colleagues.
Their study evaluated trends in autoimmune liver diseases over a 9-year time frame in Canterbury, New Zealand. According to the investigators, this region is well suited to an epidemiological investigation because it is a clearly defined geographic area with approximately 600,000 people, most of whom rely on one tertiary care center: Christchurch Hospital. The bulk of the data therefore came from this center, while a minority of cases were gathered from local private gastroenterology practices, “making complete case ascertainment possible.”
Incidence of AIH, PBC, and PSC was assessed at three time points: 2008-2010, 2011-2013, and 2014-2016. AIH had the highest overall incidence, at 1.93 cases per 100,000 people, followed by PSC (0.92) and PBC (0.51).
While the rates of PBC and PSC did not change significantly over time, the incidence of AIH rose from 1.37 cases per 100,000 people in the period from 2008-2010 to 2.39 per 100,000 in 2014-2016 (P = .04), which computes to an incidence rate ratio of 1.69 (95% confidence interval, 1.02-2.84). Point prevalence was also significantly higher in 2016, compared with 2008, at 27.5 per 100,000 versus 19.7 per 100,000 (P < .01). The investigators described a bimodal age of presentation, with the first peak among patients younger than 20 years, and a second, larger peak among individuals aged 50-69 years.
According to the investigators, these findings “are concordant with the results observed in the European cohort,” citing a Danish study spanning 1994-2012 and a Dutch study spanning 2000-2010. They noted that the Danish study also reported a bimodal distribution of age incidence, as did a Swedish study, and another study from New Zealand. The stable levels of PBC and PSC align with two recent retrospective studies conducted in the United States and, they added.
“We believe that the observed differential trends in the incidence of these autoimmune liver diseases truly reflects their contemporary epidemiology,” the investigators wrote. They went on to suggest that the findings did not stem from an increase in diagnostic scrutiny because the study period did not include any significant changes in gastroenterology service, coding, or diagnostic criteria in the region studied.
“The increased incidence of AIH parallels rising incidence and prevalence of other autoimmune disorders such as [inflammatory bowel disease], type 1 diabetes, and multiple sclerosis in New Zealand, and it is unclear whether these autoimmune conditions share a common local environmental trigger,” they wrote. “Environmental factors likely play a central role augmenting phenotypic expression in genetically predisposed individuals.”
While Dr. Lamba and colleagues proposed several possible factors, such as increased exposure to pharmaceuticals, definitive factors remain elusive, which the authors cited as one limitation of their study. Another limitation they cited is the possibility that other etiologies were mistakenly classified as “probable” AIH; however, the chances of that are small, and the proportion of probable versus definitive AIH noted in this study do reflect those seen in other epidemiological studies.
“The reason for observed differential change in incidence of these autoimmune liver diseases is unclear,” they wrote, “and future collaborative prospective epidemiological study would be required to assess this further.”
The investigators reported no conflicts of interest.
The incidence of autoimmune hepatitis (AIH) may be rising, according to a prospective population-based study conducted in New Zealand.
From 2008 to 2016, the rising incidence of AIH led to a 40% increase in point prevalence, reported lead author Mehul Lamba, MD, of Christchurch (New Zealand) Hospital and colleagues.
The present study, which also assessed rates of primary biliary cholangitis (PBC) and primary sclerosing cholangitis (PSC), adds data to an area of inquiry historically characterized by limited and inconsistent results, the investigators wrote in Clinical Gastroenterology and Hepatology. They suggested that mixed findings from previous studies may be because of differences in population and environmental factors, but also varying diagnostic criteria.
“The epidemiological trends of these autoimmune liver diseases therefore remain incompletely understood,” wrote Dr. Lamba and colleagues.
Their study evaluated trends in autoimmune liver diseases over a 9-year time frame in Canterbury, New Zealand. According to the investigators, this region is well suited to an epidemiological investigation because it is a clearly defined geographic area with approximately 600,000 people, most of whom rely on one tertiary care center: Christchurch Hospital. The bulk of the data therefore came from this center, while a minority of cases were gathered from local private gastroenterology practices, “making complete case ascertainment possible.”
Incidence of AIH, PBC, and PSC was assessed at three time points: 2008-2010, 2011-2013, and 2014-2016. AIH had the highest overall incidence, at 1.93 cases per 100,000 people, followed by PSC (0.92) and PBC (0.51).
While the rates of PBC and PSC did not change significantly over time, the incidence of AIH rose from 1.37 cases per 100,000 people in the period from 2008-2010 to 2.39 per 100,000 in 2014-2016 (P = .04), which computes to an incidence rate ratio of 1.69 (95% confidence interval, 1.02-2.84). Point prevalence was also significantly higher in 2016, compared with 2008, at 27.5 per 100,000 versus 19.7 per 100,000 (P < .01). The investigators described a bimodal age of presentation, with the first peak among patients younger than 20 years, and a second, larger peak among individuals aged 50-69 years.
According to the investigators, these findings “are concordant with the results observed in the European cohort,” citing a Danish study spanning 1994-2012 and a Dutch study spanning 2000-2010. They noted that the Danish study also reported a bimodal distribution of age incidence, as did a Swedish study, and another study from New Zealand. The stable levels of PBC and PSC align with two recent retrospective studies conducted in the United States and, they added.
“We believe that the observed differential trends in the incidence of these autoimmune liver diseases truly reflects their contemporary epidemiology,” the investigators wrote. They went on to suggest that the findings did not stem from an increase in diagnostic scrutiny because the study period did not include any significant changes in gastroenterology service, coding, or diagnostic criteria in the region studied.
“The increased incidence of AIH parallels rising incidence and prevalence of other autoimmune disorders such as [inflammatory bowel disease], type 1 diabetes, and multiple sclerosis in New Zealand, and it is unclear whether these autoimmune conditions share a common local environmental trigger,” they wrote. “Environmental factors likely play a central role augmenting phenotypic expression in genetically predisposed individuals.”
While Dr. Lamba and colleagues proposed several possible factors, such as increased exposure to pharmaceuticals, definitive factors remain elusive, which the authors cited as one limitation of their study. Another limitation they cited is the possibility that other etiologies were mistakenly classified as “probable” AIH; however, the chances of that are small, and the proportion of probable versus definitive AIH noted in this study do reflect those seen in other epidemiological studies.
“The reason for observed differential change in incidence of these autoimmune liver diseases is unclear,” they wrote, “and future collaborative prospective epidemiological study would be required to assess this further.”
The investigators reported no conflicts of interest.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Antipsychotic administration fails to treat delirium in hospitalized adults
Background: Delirium is a common disorder in hospitalized adults and is associated with poor outcomes. Antipsychotics are used clinically to treat delirium, but benefits and harms remain unclear.
Study design: A systematic review evaluating treatment of delirium in 16 randomized, controlled trials (RCTs) of antipsychotics vs. placebo or other antipsychotics, as well as 10 prospective observational studies reporting harm.
Setting: Data obtained from PubMed, Embase, CENTRAL, CINAHL, and PsycINFO from inception to July 2019 without language restrictions.
Synopsis: For 5,607 adult inpatients, treatment of delirium with haloperidol showed no difference in sedation status, duration of delirium, hospital length of stay, or mortality when compared with second-generation antipsychotics or placebo (low and moderate strength of evidence). Regarding second-generation antipsychotics versus haloperidol, no difference was found in delirium severity and cognitive function (low strength of evidence). Direct comparisons between second-generation antipsychotics showed no difference in mortality.
Limitations include heterogeneous use of agents, routes, dose, and measurement tools, which limits generalization of evidence. Multiple RCTs excluded patients with underlying cardiac and neurologic conditions that likely led to underrepresentation of harm in routine use. Insufficient evidence still exists for multiple clinically relevant outcomes including long-term cognitive function.
Bottom line: Evidence from several studies does not support the use of haloperidol or newer antipsychotics to treat delirium.
Citation: Nikooie R et al. Antipsychotics for delirium treatment in adults: A systematic review. Ann Intern Med. 2019 Oct 1;171(7):485-95.
Dr. Berry is assistant professor of medicine, hospital medicine, at the Rocky Mountain Veterans Affairs Regional Medical Center, Aurora, Colo.
Background: Delirium is a common disorder in hospitalized adults and is associated with poor outcomes. Antipsychotics are used clinically to treat delirium, but benefits and harms remain unclear.
Study design: A systematic review evaluating treatment of delirium in 16 randomized, controlled trials (RCTs) of antipsychotics vs. placebo or other antipsychotics, as well as 10 prospective observational studies reporting harm.
Setting: Data obtained from PubMed, Embase, CENTRAL, CINAHL, and PsycINFO from inception to July 2019 without language restrictions.
Synopsis: For 5,607 adult inpatients, treatment of delirium with haloperidol showed no difference in sedation status, duration of delirium, hospital length of stay, or mortality when compared with second-generation antipsychotics or placebo (low and moderate strength of evidence). Regarding second-generation antipsychotics versus haloperidol, no difference was found in delirium severity and cognitive function (low strength of evidence). Direct comparisons between second-generation antipsychotics showed no difference in mortality.
Limitations include heterogeneous use of agents, routes, dose, and measurement tools, which limits generalization of evidence. Multiple RCTs excluded patients with underlying cardiac and neurologic conditions that likely led to underrepresentation of harm in routine use. Insufficient evidence still exists for multiple clinically relevant outcomes including long-term cognitive function.
Bottom line: Evidence from several studies does not support the use of haloperidol or newer antipsychotics to treat delirium.
Citation: Nikooie R et al. Antipsychotics for delirium treatment in adults: A systematic review. Ann Intern Med. 2019 Oct 1;171(7):485-95.
Dr. Berry is assistant professor of medicine, hospital medicine, at the Rocky Mountain Veterans Affairs Regional Medical Center, Aurora, Colo.
Background: Delirium is a common disorder in hospitalized adults and is associated with poor outcomes. Antipsychotics are used clinically to treat delirium, but benefits and harms remain unclear.
Study design: A systematic review evaluating treatment of delirium in 16 randomized, controlled trials (RCTs) of antipsychotics vs. placebo or other antipsychotics, as well as 10 prospective observational studies reporting harm.
Setting: Data obtained from PubMed, Embase, CENTRAL, CINAHL, and PsycINFO from inception to July 2019 without language restrictions.
Synopsis: For 5,607 adult inpatients, treatment of delirium with haloperidol showed no difference in sedation status, duration of delirium, hospital length of stay, or mortality when compared with second-generation antipsychotics or placebo (low and moderate strength of evidence). Regarding second-generation antipsychotics versus haloperidol, no difference was found in delirium severity and cognitive function (low strength of evidence). Direct comparisons between second-generation antipsychotics showed no difference in mortality.
Limitations include heterogeneous use of agents, routes, dose, and measurement tools, which limits generalization of evidence. Multiple RCTs excluded patients with underlying cardiac and neurologic conditions that likely led to underrepresentation of harm in routine use. Insufficient evidence still exists for multiple clinically relevant outcomes including long-term cognitive function.
Bottom line: Evidence from several studies does not support the use of haloperidol or newer antipsychotics to treat delirium.
Citation: Nikooie R et al. Antipsychotics for delirium treatment in adults: A systematic review. Ann Intern Med. 2019 Oct 1;171(7):485-95.
Dr. Berry is assistant professor of medicine, hospital medicine, at the Rocky Mountain Veterans Affairs Regional Medical Center, Aurora, Colo.
DDSEP® 9 Quick Quiz
Q2. Correct answer: E
Rationale
This patient has nausea and vomiting of pregnancy (NVP), and has tried conservative management. Doxylamine and vitamin B6 have been found to be safe and effective for NVP and are considered first-line therapy. Further testing with gastric-emptying study is not necessary because NVP has a high prevalence at weeks 4-6 of gestation and peaks at week 9-16. A nuclear test such as gastric emptying is not appropriate during pregnancy, though decreased gastric emptying due to estrogen and progesterone is thought to be related to NVP. Upper endoscopy would be considered if the nausea and vomiting is refractory. Ondansetron can be considered, but there have been some questions raised regarding safety and it is not considered first line. Meals high in protein have been found to decrease nausea more that carbohydrate-rich meals.
Reference
ACOG Committee on Practice Bulletins-Obstetrics. Obstet Gynecol. 2018 Jan;131(1):e15-e30.
Q2. Correct answer: E
Rationale
This patient has nausea and vomiting of pregnancy (NVP), and has tried conservative management. Doxylamine and vitamin B6 have been found to be safe and effective for NVP and are considered first-line therapy. Further testing with gastric-emptying study is not necessary because NVP has a high prevalence at weeks 4-6 of gestation and peaks at week 9-16. A nuclear test such as gastric emptying is not appropriate during pregnancy, though decreased gastric emptying due to estrogen and progesterone is thought to be related to NVP. Upper endoscopy would be considered if the nausea and vomiting is refractory. Ondansetron can be considered, but there have been some questions raised regarding safety and it is not considered first line. Meals high in protein have been found to decrease nausea more that carbohydrate-rich meals.
Reference
ACOG Committee on Practice Bulletins-Obstetrics. Obstet Gynecol. 2018 Jan;131(1):e15-e30.
Q2. Correct answer: E
Rationale
This patient has nausea and vomiting of pregnancy (NVP), and has tried conservative management. Doxylamine and vitamin B6 have been found to be safe and effective for NVP and are considered first-line therapy. Further testing with gastric-emptying study is not necessary because NVP has a high prevalence at weeks 4-6 of gestation and peaks at week 9-16. A nuclear test such as gastric emptying is not appropriate during pregnancy, though decreased gastric emptying due to estrogen and progesterone is thought to be related to NVP. Upper endoscopy would be considered if the nausea and vomiting is refractory. Ondansetron can be considered, but there have been some questions raised regarding safety and it is not considered first line. Meals high in protein have been found to decrease nausea more that carbohydrate-rich meals.
Reference
ACOG Committee on Practice Bulletins-Obstetrics. Obstet Gynecol. 2018 Jan;131(1):e15-e30.
Q2. A 26-year-old female who is 7 weeks pregnant presents with nausea and vomiting. She describes nausea that lasts most of the day with vomiting. She has tried rest and hydration, ginger supplementation, and a wrist band she purchased over the counter. However, she comes to clinic to request further management.
DDSEP® 9 Quick Quiz
Q1. Correct answer: E
Rationale
Transient lower esophageal sphincter relaxation (TLESR) is a physiologic phenomenon that allows venting of swallowed air from the stomach in response to distension of the proximal stomach. Patients with GERD typically reflux gastric content through a compliant esophagogastric junction into the esophagus during a TLESR; the frequency of TLESRs may also be higher in patients with GERD. TLESRs are suppressed during deep sleep, and are less frequent when LES relaxation is abnormal (e.g., esophageal outflow obstruction). Baclofen, a GABAB receptor agonist, can reduce TLESR frequency, and can reduce reflux episodes in patients with reflux. Obese patients and those with obstructive sleep apnea can have increased frequency of TLESRs. The frequency of TLESR is not related to degree of gastric acid secretion in the stomach.
References
Kuribayashi S et al. Neurogastroenterol Motil. 2010 Jun;22(6):611-e172.
Hershcovici T et al. Neurogastroenterol Motil. 2011 Sep;23(9):819-30.
Q1. Correct answer: E
Rationale
Transient lower esophageal sphincter relaxation (TLESR) is a physiologic phenomenon that allows venting of swallowed air from the stomach in response to distension of the proximal stomach. Patients with GERD typically reflux gastric content through a compliant esophagogastric junction into the esophagus during a TLESR; the frequency of TLESRs may also be higher in patients with GERD. TLESRs are suppressed during deep sleep, and are less frequent when LES relaxation is abnormal (e.g., esophageal outflow obstruction). Baclofen, a GABAB receptor agonist, can reduce TLESR frequency, and can reduce reflux episodes in patients with reflux. Obese patients and those with obstructive sleep apnea can have increased frequency of TLESRs. The frequency of TLESR is not related to degree of gastric acid secretion in the stomach.
References
Kuribayashi S et al. Neurogastroenterol Motil. 2010 Jun;22(6):611-e172.
Hershcovici T et al. Neurogastroenterol Motil. 2011 Sep;23(9):819-30.
Q1. Correct answer: E
Rationale
Transient lower esophageal sphincter relaxation (TLESR) is a physiologic phenomenon that allows venting of swallowed air from the stomach in response to distension of the proximal stomach. Patients with GERD typically reflux gastric content through a compliant esophagogastric junction into the esophagus during a TLESR; the frequency of TLESRs may also be higher in patients with GERD. TLESRs are suppressed during deep sleep, and are less frequent when LES relaxation is abnormal (e.g., esophageal outflow obstruction). Baclofen, a GABAB receptor agonist, can reduce TLESR frequency, and can reduce reflux episodes in patients with reflux. Obese patients and those with obstructive sleep apnea can have increased frequency of TLESRs. The frequency of TLESR is not related to degree of gastric acid secretion in the stomach.
References
Kuribayashi S et al. Neurogastroenterol Motil. 2010 Jun;22(6):611-e172.
Hershcovici T et al. Neurogastroenterol Motil. 2011 Sep;23(9):819-30.
Question 1:
COVID-19 may alter gut microbiota
COVID-19 infection altered the gut microbiota of adult patients and caused depletion of several types of bacteria with known immunomodulatory properties, based on data from a cohort study of 100 patients with confirmed COVID-19 infections from two hospitals.
“As the GI tract is the largest immunological organ in the body and its resident microbiota are known to modulate host immune responses, we hypothesized that the gut microbiota is associated with host inflammatory immune responses in COVID19,” wrote Yun Kit Yeoh, PhD, of the Chinese University of Hong Kong, and colleagues.
In a study published in Gut, the researchers investigated patient microbiota by collecting blood, stool, and patient records between February and May 2020 from 100 confirmed SARS-CoV-2–infected patients in Hong Kong during hospitalization, as well as follow-up stool samples from 27 patients up to 30 days after they cleared the COVID-19 virus; these observations were compared with 78 non–COVID-19 controls.
Overall, 274 stool samples were sequenced. Samples collected from patients during hospitalization for COVID-19 were compared with non–COVID-19 controls. The presence of phylum Bacteroidetes was significantly higher in COVID-19 patients compared with controls (23.9% vs. 12.8%; P < .001), as were Actinobacteria (26.1% vs. 19.0%; P < .001).
After controlling for antibiotics, the investigators found that “differences between cohorts were primarily linked to enrichment of taxa such as Parabacteroides, Sutterella wadsworthensis, and Bacteroides caccae and depletion of Adlercreutzia equolifaciens, Dorea formicigenerans, and Clostridium leptum in COVID-19 relative to non-COVID-19” (P < .05). In addition, Faecalibacterium prausnitzii and Bifidobacterium bifidum were negatively correlated with COVID-19 severity after investigators controlled for patient age and antibiotic use (P < .05).
The researchers also examined bacteria in COVID-19 patients and controls in the context of cytokines and other inflammatory markers. “We hypothesized that these compositional changes play a role in exacerbating disease by contributing to dysregulation of the immune response,” they said.
In fact, species depleted in COVID-19 patients including included B. adolescentis, E. rectale, and F. prausnitzii were negatively correlated with inflammatory markers including CXCL10, IL-10, TNF-alpha, and CCL2.
In addition, 42 stool samples from 27 patients showed significantly distinct gut microbiota from controls up to 30 days (median, 6 days) after virus clearance, regardless of antibiotics use (P < .05), the researchers said.
Long-term data needed
The study findings were limited by several factors, including the potential confounding of microbial signatures associated with COVID-19 because of heterogeneous patient management in the clinical setting and the potential that gut microbiota reflects a patient’s health with no impact on disease severity, as well as lack of data on the role of antibiotics for severe and critical patients, the researchers noted. In addition, “gut microbiota composition is highly heterogeneous across human populations and changes in compositions reported here may not necessarily be reflected in patients with COVID-19 from other biogeographies,” they wrote.
The “longer follow-up of patients with COVID-19 (e.g., 3 months to 1 year after clearing the virus) is needed to address questions related to the duration of gut microbiota dysbiosis post recovery, link between microbiota dysbiosis and long-term persistent symptoms, and whether the dysbiosis or enrichment/depletion of specific gut microorganisms predisposes recovered individuals to future health problems,” they wrote.
However, the results suggest a likely role for gut microorganisms in host inflammatory responses to COVID-19 infection, and “underscore an urgent need to understand the specific roles of gut microorganisms in human immune function and systemic inflammation,” they concluded.
More than infectious
“A growing body of evidence suggests that severity of illness from COVID-19 is largely determined by the patient’s aberrant immune response to the virus,” Jatin Roper, MD, of Duke University, Durham, N.C., said in an interview. “Therefore, a critical question is: What patient factors determine this immune response? The gut microbiota closely interact with the host immune system and are altered in many immunological diseases,” he said. “Furthermore, the SARS-CoV-2 virus infects enterocytes in the intestine and causes symptomatic gastrointestinal disease in a subset of patients. Therefore, understanding a possible association between gut microbiota and COVID-19 may reveal microbial species involved in disease pathogenesis,” he emphasized.
In the current study, “I was surprised to find that COVID-19 infection is associated with depletion of immunomodulatory gut bacteria,” said Dr. Roper. “An open question is whether these changes are caused by the SARS-CoV-2 virus and then result in altered immune response. Alternatively, the changes in gut microbiota may be a result of the immune response or other changes associated with the disease,” he said.
“COVID-19 is an immunological disease, not just an infectious disease,” explained Dr. Roper. “The gut microbiota may play an important role in the pathogenesis of the disease. Thus, specific gut microbes could one day be analyzed to risk stratify patients, or even modified to treat the disease,” he noted.
Beyond COVID-19
“Given the impact of the gut microbiota on health and disease, as well as the impact of diseases on the microbiota, I am not at all surprised to find that there were significant changes in the microbiota of COVID-19 patients and that these changes are associated with inflammatory cytokines, chemokines, and blood markers of tissue damage,” said Anthony Sung, MD, also of Duke University.
According to Dr. Sung, researchers have already been investigating possible connections between gut microbiota and other conditions such as Alzheimer’s disease, and it’s been hypothesized that these connections are mediated by interactions between the gut microbiota and the immune system.
“While this is an important paper in our understanding of COVID-19, and highlights the microbiome as a potential therapeutic target, we need to conduct clinical trials of microbiota-based interventions before we can fully realize the clinical implications of these findings,” he said.
The study was supported by the Health and Medical Research Fund, the Food and Health Bureau, The Government of the Hong Kong Special Administrative Region, and donations from Hui Hoy & Chow Sin Lan Charity Fund Limited, Pine and Crane Company Limited, Mr. Hui Ming, and The D.H. Chen Foundation. The researchers had no financial conflicts to disclose. Dr. Roper and Dr. Sung had no financial conflicts to disclose.
COVID-19 infection altered the gut microbiota of adult patients and caused depletion of several types of bacteria with known immunomodulatory properties, based on data from a cohort study of 100 patients with confirmed COVID-19 infections from two hospitals.
“As the GI tract is the largest immunological organ in the body and its resident microbiota are known to modulate host immune responses, we hypothesized that the gut microbiota is associated with host inflammatory immune responses in COVID19,” wrote Yun Kit Yeoh, PhD, of the Chinese University of Hong Kong, and colleagues.
In a study published in Gut, the researchers investigated patient microbiota by collecting blood, stool, and patient records between February and May 2020 from 100 confirmed SARS-CoV-2–infected patients in Hong Kong during hospitalization, as well as follow-up stool samples from 27 patients up to 30 days after they cleared the COVID-19 virus; these observations were compared with 78 non–COVID-19 controls.
Overall, 274 stool samples were sequenced. Samples collected from patients during hospitalization for COVID-19 were compared with non–COVID-19 controls. The presence of phylum Bacteroidetes was significantly higher in COVID-19 patients compared with controls (23.9% vs. 12.8%; P < .001), as were Actinobacteria (26.1% vs. 19.0%; P < .001).
After controlling for antibiotics, the investigators found that “differences between cohorts were primarily linked to enrichment of taxa such as Parabacteroides, Sutterella wadsworthensis, and Bacteroides caccae and depletion of Adlercreutzia equolifaciens, Dorea formicigenerans, and Clostridium leptum in COVID-19 relative to non-COVID-19” (P < .05). In addition, Faecalibacterium prausnitzii and Bifidobacterium bifidum were negatively correlated with COVID-19 severity after investigators controlled for patient age and antibiotic use (P < .05).
The researchers also examined bacteria in COVID-19 patients and controls in the context of cytokines and other inflammatory markers. “We hypothesized that these compositional changes play a role in exacerbating disease by contributing to dysregulation of the immune response,” they said.
In fact, species depleted in COVID-19 patients including included B. adolescentis, E. rectale, and F. prausnitzii were negatively correlated with inflammatory markers including CXCL10, IL-10, TNF-alpha, and CCL2.
In addition, 42 stool samples from 27 patients showed significantly distinct gut microbiota from controls up to 30 days (median, 6 days) after virus clearance, regardless of antibiotics use (P < .05), the researchers said.
Long-term data needed
The study findings were limited by several factors, including the potential confounding of microbial signatures associated with COVID-19 because of heterogeneous patient management in the clinical setting and the potential that gut microbiota reflects a patient’s health with no impact on disease severity, as well as lack of data on the role of antibiotics for severe and critical patients, the researchers noted. In addition, “gut microbiota composition is highly heterogeneous across human populations and changes in compositions reported here may not necessarily be reflected in patients with COVID-19 from other biogeographies,” they wrote.
The “longer follow-up of patients with COVID-19 (e.g., 3 months to 1 year after clearing the virus) is needed to address questions related to the duration of gut microbiota dysbiosis post recovery, link between microbiota dysbiosis and long-term persistent symptoms, and whether the dysbiosis or enrichment/depletion of specific gut microorganisms predisposes recovered individuals to future health problems,” they wrote.
However, the results suggest a likely role for gut microorganisms in host inflammatory responses to COVID-19 infection, and “underscore an urgent need to understand the specific roles of gut microorganisms in human immune function and systemic inflammation,” they concluded.
More than infectious
“A growing body of evidence suggests that severity of illness from COVID-19 is largely determined by the patient’s aberrant immune response to the virus,” Jatin Roper, MD, of Duke University, Durham, N.C., said in an interview. “Therefore, a critical question is: What patient factors determine this immune response? The gut microbiota closely interact with the host immune system and are altered in many immunological diseases,” he said. “Furthermore, the SARS-CoV-2 virus infects enterocytes in the intestine and causes symptomatic gastrointestinal disease in a subset of patients. Therefore, understanding a possible association between gut microbiota and COVID-19 may reveal microbial species involved in disease pathogenesis,” he emphasized.
In the current study, “I was surprised to find that COVID-19 infection is associated with depletion of immunomodulatory gut bacteria,” said Dr. Roper. “An open question is whether these changes are caused by the SARS-CoV-2 virus and then result in altered immune response. Alternatively, the changes in gut microbiota may be a result of the immune response or other changes associated with the disease,” he said.
“COVID-19 is an immunological disease, not just an infectious disease,” explained Dr. Roper. “The gut microbiota may play an important role in the pathogenesis of the disease. Thus, specific gut microbes could one day be analyzed to risk stratify patients, or even modified to treat the disease,” he noted.
Beyond COVID-19
“Given the impact of the gut microbiota on health and disease, as well as the impact of diseases on the microbiota, I am not at all surprised to find that there were significant changes in the microbiota of COVID-19 patients and that these changes are associated with inflammatory cytokines, chemokines, and blood markers of tissue damage,” said Anthony Sung, MD, also of Duke University.
According to Dr. Sung, researchers have already been investigating possible connections between gut microbiota and other conditions such as Alzheimer’s disease, and it’s been hypothesized that these connections are mediated by interactions between the gut microbiota and the immune system.
“While this is an important paper in our understanding of COVID-19, and highlights the microbiome as a potential therapeutic target, we need to conduct clinical trials of microbiota-based interventions before we can fully realize the clinical implications of these findings,” he said.
The study was supported by the Health and Medical Research Fund, the Food and Health Bureau, The Government of the Hong Kong Special Administrative Region, and donations from Hui Hoy & Chow Sin Lan Charity Fund Limited, Pine and Crane Company Limited, Mr. Hui Ming, and The D.H. Chen Foundation. The researchers had no financial conflicts to disclose. Dr. Roper and Dr. Sung had no financial conflicts to disclose.
COVID-19 infection altered the gut microbiota of adult patients and caused depletion of several types of bacteria with known immunomodulatory properties, based on data from a cohort study of 100 patients with confirmed COVID-19 infections from two hospitals.
“As the GI tract is the largest immunological organ in the body and its resident microbiota are known to modulate host immune responses, we hypothesized that the gut microbiota is associated with host inflammatory immune responses in COVID19,” wrote Yun Kit Yeoh, PhD, of the Chinese University of Hong Kong, and colleagues.
In a study published in Gut, the researchers investigated patient microbiota by collecting blood, stool, and patient records between February and May 2020 from 100 confirmed SARS-CoV-2–infected patients in Hong Kong during hospitalization, as well as follow-up stool samples from 27 patients up to 30 days after they cleared the COVID-19 virus; these observations were compared with 78 non–COVID-19 controls.
Overall, 274 stool samples were sequenced. Samples collected from patients during hospitalization for COVID-19 were compared with non–COVID-19 controls. The presence of phylum Bacteroidetes was significantly higher in COVID-19 patients compared with controls (23.9% vs. 12.8%; P < .001), as were Actinobacteria (26.1% vs. 19.0%; P < .001).
After controlling for antibiotics, the investigators found that “differences between cohorts were primarily linked to enrichment of taxa such as Parabacteroides, Sutterella wadsworthensis, and Bacteroides caccae and depletion of Adlercreutzia equolifaciens, Dorea formicigenerans, and Clostridium leptum in COVID-19 relative to non-COVID-19” (P < .05). In addition, Faecalibacterium prausnitzii and Bifidobacterium bifidum were negatively correlated with COVID-19 severity after investigators controlled for patient age and antibiotic use (P < .05).
The researchers also examined bacteria in COVID-19 patients and controls in the context of cytokines and other inflammatory markers. “We hypothesized that these compositional changes play a role in exacerbating disease by contributing to dysregulation of the immune response,” they said.
In fact, species depleted in COVID-19 patients including included B. adolescentis, E. rectale, and F. prausnitzii were negatively correlated with inflammatory markers including CXCL10, IL-10, TNF-alpha, and CCL2.
In addition, 42 stool samples from 27 patients showed significantly distinct gut microbiota from controls up to 30 days (median, 6 days) after virus clearance, regardless of antibiotics use (P < .05), the researchers said.
Long-term data needed
The study findings were limited by several factors, including the potential confounding of microbial signatures associated with COVID-19 because of heterogeneous patient management in the clinical setting and the potential that gut microbiota reflects a patient’s health with no impact on disease severity, as well as lack of data on the role of antibiotics for severe and critical patients, the researchers noted. In addition, “gut microbiota composition is highly heterogeneous across human populations and changes in compositions reported here may not necessarily be reflected in patients with COVID-19 from other biogeographies,” they wrote.
The “longer follow-up of patients with COVID-19 (e.g., 3 months to 1 year after clearing the virus) is needed to address questions related to the duration of gut microbiota dysbiosis post recovery, link between microbiota dysbiosis and long-term persistent symptoms, and whether the dysbiosis or enrichment/depletion of specific gut microorganisms predisposes recovered individuals to future health problems,” they wrote.
However, the results suggest a likely role for gut microorganisms in host inflammatory responses to COVID-19 infection, and “underscore an urgent need to understand the specific roles of gut microorganisms in human immune function and systemic inflammation,” they concluded.
More than infectious
“A growing body of evidence suggests that severity of illness from COVID-19 is largely determined by the patient’s aberrant immune response to the virus,” Jatin Roper, MD, of Duke University, Durham, N.C., said in an interview. “Therefore, a critical question is: What patient factors determine this immune response? The gut microbiota closely interact with the host immune system and are altered in many immunological diseases,” he said. “Furthermore, the SARS-CoV-2 virus infects enterocytes in the intestine and causes symptomatic gastrointestinal disease in a subset of patients. Therefore, understanding a possible association between gut microbiota and COVID-19 may reveal microbial species involved in disease pathogenesis,” he emphasized.
In the current study, “I was surprised to find that COVID-19 infection is associated with depletion of immunomodulatory gut bacteria,” said Dr. Roper. “An open question is whether these changes are caused by the SARS-CoV-2 virus and then result in altered immune response. Alternatively, the changes in gut microbiota may be a result of the immune response or other changes associated with the disease,” he said.
“COVID-19 is an immunological disease, not just an infectious disease,” explained Dr. Roper. “The gut microbiota may play an important role in the pathogenesis of the disease. Thus, specific gut microbes could one day be analyzed to risk stratify patients, or even modified to treat the disease,” he noted.
Beyond COVID-19
“Given the impact of the gut microbiota on health and disease, as well as the impact of diseases on the microbiota, I am not at all surprised to find that there were significant changes in the microbiota of COVID-19 patients and that these changes are associated with inflammatory cytokines, chemokines, and blood markers of tissue damage,” said Anthony Sung, MD, also of Duke University.
According to Dr. Sung, researchers have already been investigating possible connections between gut microbiota and other conditions such as Alzheimer’s disease, and it’s been hypothesized that these connections are mediated by interactions between the gut microbiota and the immune system.
“While this is an important paper in our understanding of COVID-19, and highlights the microbiome as a potential therapeutic target, we need to conduct clinical trials of microbiota-based interventions before we can fully realize the clinical implications of these findings,” he said.
The study was supported by the Health and Medical Research Fund, the Food and Health Bureau, The Government of the Hong Kong Special Administrative Region, and donations from Hui Hoy & Chow Sin Lan Charity Fund Limited, Pine and Crane Company Limited, Mr. Hui Ming, and The D.H. Chen Foundation. The researchers had no financial conflicts to disclose. Dr. Roper and Dr. Sung had no financial conflicts to disclose.
FROM GUT







