User login
Prospects dim for Medicare drug reimbursement cuts
A proposal to lower Medicare Part B reimbursements for 50 physician-administered drugs and biologics to what drug manufacturers receive for them in other wealthy nations seems unlikely to take effect as planned on January 1, 2021. The proposal has been strongly opposed by the pharmaceutical industry as well as oncologists and other physicians who use the products most often.
At least four lawsuits have been filed in US district courts to block the move, including from Regeneron, manufacturer of the ophthalmic biologic aflibercept, the first agent on the list; the Community Oncology Alliance; the Biotechnology Innovation Organization, California Life Sciences Association, and Biocom; and Pharmaceutical Research and Manufacturers of America, the Association of Community Cancer Centers, the Global Colon Cancer Association, and National Infusion Center Association.
The proposal could hit oncologists/hematologists particularly hard because they are the primary prescribers of about 30 of the 50 agents on the list, including mainstay breast, lung, and prostate cancer treatments and newer immunotherapies. In its filing for injunctive relieve, the Community Oncology Alliance, a trade group for community oncologists, said the proposal exposes “the health and safety of cancer patients and other patients with potentially life-threatening diseases to real danger.”
Hearings are imminent
Hearings on the proposal, which was published by the Centers for Medicare & Medicaid Services (CMS) on November 27 following an executive order September 13, are scheduled for coming days. The first hearing is scheduled for December 18.
Given the looming implementation date, judges are likely to rule quickly on the motions for injunctive relief, said attorney Rachel Sachs, a health law expert and associate professor at the Washington University School of Law, St. Louis, Missouri. The odds are in plaintiffs’ favor based on procedural and Constitutional grounds. “It’s extremely unlikely to survive” the legal onslaught, she told Medscape Medical News.
Among the many issues raised in court filings, the proposal was released as an interim final rule (IFR), meaning it would take effect outside of the usual process of proposed rule, comment period, revision, then implementation. The law allows for bypassing normal rule-making requirements with an IFR, but they are meant for emergency situations — several have been issued in response to the COVID-19 pandemic — and the government must be able to show that delay would be “impracticable, unnecessary, or contrary to the public interest,” Sachs explained.
In contrast, some form of CMS’s new proposal, dubbed the “Most Favored Nation” (MFN) model for drug reimbursements, has been under consideration by the Trump administration since 2018.
“The way the [Trump] administration rolled this rule out at pretty much the last minute opens them up to greater legal challenges than if they pursued more normal regulatory pathways, which they had the opportunity to do. They are attempting to implement this on a time frame that is unprecedented for as large a change as this is,” said Juliette Cubanski, PhD, deputy director of Medicare policy at the Kaiser Family Foundation, San Francisco, California.
Reimbursement less than acquisition costs
In the proposal, CMS sought to offset the higher prices that pharmaceutical companies charge in the United States when compared with other developed nations — the prices are about double on average.
“One of the largest drivers of increasing Medicare spending is the growing prices for physician-administered separately payable Medicare Part B drugs, which have risen an average of 11.5% annually since 2015, with total spending of approximately $30 billion in 2019,” the agency said in a fact sheet. This is due in large part to “lack of competitive market forces on Medicare Part B drug costs,” it added.
The 50 agents covered by the new proposal are the ones Part B spends the most on, almost $3 billion in 2019 for aflibercept alone, followed closely by pembrolizumab, an immunotherapy used in many different cancer types.
A full list of the drugs and biologicals included in the proposal is on page 50 of the IFR.
CMS estimated that its move would cut reimbursements by approximately 65% once fully implemented in 4 years and save $87.8 billion over the 7 years of the proposed model, as well as reduce cost sharing for beneficiaries.
This model puts the onus on providers to negotiate down drug prices with companies to meet proposed reimbursement limits. However, if companies do not lower their prices, acquisition costs could be substantially lower than reimbursements.
Prescribers and their practices would either have to take the financial hit or stop offering the pharmaceuticals, in which case patients would have to do without, try a different option, or seek care elsewhere, including facilities excluded from the proposal: children’s hospitals, critical access hospitals, cancer hospitals, federally qualified health centers, rural health clinics, and extended neoplastic disease care hospitals.
CMS estimates that in the first year of MFN reimbursements, 9% of beneficiaries would forgo MFN therapies, growing to 19% by year 3, figures that were included in cost savings estimates.
The reimbursement cuts are meant to motivate manufactures to lower prices, but “we have not seen this occur with similar efforts in the past, and drug prices have continued to rise,” said American College of Rheumatology President David Karp, MD, PhD, in a press release. Many of the agents on the list are used by rheumatologists.
Under current policy, Medicare Part B prescribers are reimbursed manufactures’ average sales price plus a 6% add-on. Under the new proposal, reimbursements would be pegged to the lowest price charged among nations of the Organization for Economic Co-operation and Development with a gross domestic product per capita of at least 60% of the US price. In addition to the United States, there are 36 other member countries, including Canada, the United Kingdom, Japan, and Germany.
To remove incentives to prescribe more expensive drugs, the 6% add-on would be replaced with a flat add-on payment per dose, pegged at $148.73 for the first quarter of 2021. There is a hardship exemption for providers if the reimbursement cuts are too drastic, but that involves a lot of paperwork.
This article first appeared on Medscape.com.
A proposal to lower Medicare Part B reimbursements for 50 physician-administered drugs and biologics to what drug manufacturers receive for them in other wealthy nations seems unlikely to take effect as planned on January 1, 2021. The proposal has been strongly opposed by the pharmaceutical industry as well as oncologists and other physicians who use the products most often.
At least four lawsuits have been filed in US district courts to block the move, including from Regeneron, manufacturer of the ophthalmic biologic aflibercept, the first agent on the list; the Community Oncology Alliance; the Biotechnology Innovation Organization, California Life Sciences Association, and Biocom; and Pharmaceutical Research and Manufacturers of America, the Association of Community Cancer Centers, the Global Colon Cancer Association, and National Infusion Center Association.
The proposal could hit oncologists/hematologists particularly hard because they are the primary prescribers of about 30 of the 50 agents on the list, including mainstay breast, lung, and prostate cancer treatments and newer immunotherapies. In its filing for injunctive relieve, the Community Oncology Alliance, a trade group for community oncologists, said the proposal exposes “the health and safety of cancer patients and other patients with potentially life-threatening diseases to real danger.”
Hearings are imminent
Hearings on the proposal, which was published by the Centers for Medicare & Medicaid Services (CMS) on November 27 following an executive order September 13, are scheduled for coming days. The first hearing is scheduled for December 18.
Given the looming implementation date, judges are likely to rule quickly on the motions for injunctive relief, said attorney Rachel Sachs, a health law expert and associate professor at the Washington University School of Law, St. Louis, Missouri. The odds are in plaintiffs’ favor based on procedural and Constitutional grounds. “It’s extremely unlikely to survive” the legal onslaught, she told Medscape Medical News.
Among the many issues raised in court filings, the proposal was released as an interim final rule (IFR), meaning it would take effect outside of the usual process of proposed rule, comment period, revision, then implementation. The law allows for bypassing normal rule-making requirements with an IFR, but they are meant for emergency situations — several have been issued in response to the COVID-19 pandemic — and the government must be able to show that delay would be “impracticable, unnecessary, or contrary to the public interest,” Sachs explained.
In contrast, some form of CMS’s new proposal, dubbed the “Most Favored Nation” (MFN) model for drug reimbursements, has been under consideration by the Trump administration since 2018.
“The way the [Trump] administration rolled this rule out at pretty much the last minute opens them up to greater legal challenges than if they pursued more normal regulatory pathways, which they had the opportunity to do. They are attempting to implement this on a time frame that is unprecedented for as large a change as this is,” said Juliette Cubanski, PhD, deputy director of Medicare policy at the Kaiser Family Foundation, San Francisco, California.
Reimbursement less than acquisition costs
In the proposal, CMS sought to offset the higher prices that pharmaceutical companies charge in the United States when compared with other developed nations — the prices are about double on average.
“One of the largest drivers of increasing Medicare spending is the growing prices for physician-administered separately payable Medicare Part B drugs, which have risen an average of 11.5% annually since 2015, with total spending of approximately $30 billion in 2019,” the agency said in a fact sheet. This is due in large part to “lack of competitive market forces on Medicare Part B drug costs,” it added.
The 50 agents covered by the new proposal are the ones Part B spends the most on, almost $3 billion in 2019 for aflibercept alone, followed closely by pembrolizumab, an immunotherapy used in many different cancer types.
A full list of the drugs and biologicals included in the proposal is on page 50 of the IFR.
CMS estimated that its move would cut reimbursements by approximately 65% once fully implemented in 4 years and save $87.8 billion over the 7 years of the proposed model, as well as reduce cost sharing for beneficiaries.
This model puts the onus on providers to negotiate down drug prices with companies to meet proposed reimbursement limits. However, if companies do not lower their prices, acquisition costs could be substantially lower than reimbursements.
Prescribers and their practices would either have to take the financial hit or stop offering the pharmaceuticals, in which case patients would have to do without, try a different option, or seek care elsewhere, including facilities excluded from the proposal: children’s hospitals, critical access hospitals, cancer hospitals, federally qualified health centers, rural health clinics, and extended neoplastic disease care hospitals.
CMS estimates that in the first year of MFN reimbursements, 9% of beneficiaries would forgo MFN therapies, growing to 19% by year 3, figures that were included in cost savings estimates.
The reimbursement cuts are meant to motivate manufactures to lower prices, but “we have not seen this occur with similar efforts in the past, and drug prices have continued to rise,” said American College of Rheumatology President David Karp, MD, PhD, in a press release. Many of the agents on the list are used by rheumatologists.
Under current policy, Medicare Part B prescribers are reimbursed manufactures’ average sales price plus a 6% add-on. Under the new proposal, reimbursements would be pegged to the lowest price charged among nations of the Organization for Economic Co-operation and Development with a gross domestic product per capita of at least 60% of the US price. In addition to the United States, there are 36 other member countries, including Canada, the United Kingdom, Japan, and Germany.
To remove incentives to prescribe more expensive drugs, the 6% add-on would be replaced with a flat add-on payment per dose, pegged at $148.73 for the first quarter of 2021. There is a hardship exemption for providers if the reimbursement cuts are too drastic, but that involves a lot of paperwork.
This article first appeared on Medscape.com.
A proposal to lower Medicare Part B reimbursements for 50 physician-administered drugs and biologics to what drug manufacturers receive for them in other wealthy nations seems unlikely to take effect as planned on January 1, 2021. The proposal has been strongly opposed by the pharmaceutical industry as well as oncologists and other physicians who use the products most often.
At least four lawsuits have been filed in US district courts to block the move, including from Regeneron, manufacturer of the ophthalmic biologic aflibercept, the first agent on the list; the Community Oncology Alliance; the Biotechnology Innovation Organization, California Life Sciences Association, and Biocom; and Pharmaceutical Research and Manufacturers of America, the Association of Community Cancer Centers, the Global Colon Cancer Association, and National Infusion Center Association.
The proposal could hit oncologists/hematologists particularly hard because they are the primary prescribers of about 30 of the 50 agents on the list, including mainstay breast, lung, and prostate cancer treatments and newer immunotherapies. In its filing for injunctive relieve, the Community Oncology Alliance, a trade group for community oncologists, said the proposal exposes “the health and safety of cancer patients and other patients with potentially life-threatening diseases to real danger.”
Hearings are imminent
Hearings on the proposal, which was published by the Centers for Medicare & Medicaid Services (CMS) on November 27 following an executive order September 13, are scheduled for coming days. The first hearing is scheduled for December 18.
Given the looming implementation date, judges are likely to rule quickly on the motions for injunctive relief, said attorney Rachel Sachs, a health law expert and associate professor at the Washington University School of Law, St. Louis, Missouri. The odds are in plaintiffs’ favor based on procedural and Constitutional grounds. “It’s extremely unlikely to survive” the legal onslaught, she told Medscape Medical News.
Among the many issues raised in court filings, the proposal was released as an interim final rule (IFR), meaning it would take effect outside of the usual process of proposed rule, comment period, revision, then implementation. The law allows for bypassing normal rule-making requirements with an IFR, but they are meant for emergency situations — several have been issued in response to the COVID-19 pandemic — and the government must be able to show that delay would be “impracticable, unnecessary, or contrary to the public interest,” Sachs explained.
In contrast, some form of CMS’s new proposal, dubbed the “Most Favored Nation” (MFN) model for drug reimbursements, has been under consideration by the Trump administration since 2018.
“The way the [Trump] administration rolled this rule out at pretty much the last minute opens them up to greater legal challenges than if they pursued more normal regulatory pathways, which they had the opportunity to do. They are attempting to implement this on a time frame that is unprecedented for as large a change as this is,” said Juliette Cubanski, PhD, deputy director of Medicare policy at the Kaiser Family Foundation, San Francisco, California.
Reimbursement less than acquisition costs
In the proposal, CMS sought to offset the higher prices that pharmaceutical companies charge in the United States when compared with other developed nations — the prices are about double on average.
“One of the largest drivers of increasing Medicare spending is the growing prices for physician-administered separately payable Medicare Part B drugs, which have risen an average of 11.5% annually since 2015, with total spending of approximately $30 billion in 2019,” the agency said in a fact sheet. This is due in large part to “lack of competitive market forces on Medicare Part B drug costs,” it added.
The 50 agents covered by the new proposal are the ones Part B spends the most on, almost $3 billion in 2019 for aflibercept alone, followed closely by pembrolizumab, an immunotherapy used in many different cancer types.
A full list of the drugs and biologicals included in the proposal is on page 50 of the IFR.
CMS estimated that its move would cut reimbursements by approximately 65% once fully implemented in 4 years and save $87.8 billion over the 7 years of the proposed model, as well as reduce cost sharing for beneficiaries.
This model puts the onus on providers to negotiate down drug prices with companies to meet proposed reimbursement limits. However, if companies do not lower their prices, acquisition costs could be substantially lower than reimbursements.
Prescribers and their practices would either have to take the financial hit or stop offering the pharmaceuticals, in which case patients would have to do without, try a different option, or seek care elsewhere, including facilities excluded from the proposal: children’s hospitals, critical access hospitals, cancer hospitals, federally qualified health centers, rural health clinics, and extended neoplastic disease care hospitals.
CMS estimates that in the first year of MFN reimbursements, 9% of beneficiaries would forgo MFN therapies, growing to 19% by year 3, figures that were included in cost savings estimates.
The reimbursement cuts are meant to motivate manufactures to lower prices, but “we have not seen this occur with similar efforts in the past, and drug prices have continued to rise,” said American College of Rheumatology President David Karp, MD, PhD, in a press release. Many of the agents on the list are used by rheumatologists.
Under current policy, Medicare Part B prescribers are reimbursed manufactures’ average sales price plus a 6% add-on. Under the new proposal, reimbursements would be pegged to the lowest price charged among nations of the Organization for Economic Co-operation and Development with a gross domestic product per capita of at least 60% of the US price. In addition to the United States, there are 36 other member countries, including Canada, the United Kingdom, Japan, and Germany.
To remove incentives to prescribe more expensive drugs, the 6% add-on would be replaced with a flat add-on payment per dose, pegged at $148.73 for the first quarter of 2021. There is a hardship exemption for providers if the reimbursement cuts are too drastic, but that involves a lot of paperwork.
This article first appeared on Medscape.com.
Ambulatory BP monitoring reliability questioned for HTN diagnosis
Although guidelines generally recommend ambulatory over home blood pressure monitoring for diagnosing hypertension, new research questions home BP monitoring’s role as second fiddle.
One week of home BP monitoring (HBPM) was more reliable than one 24-hour ambulatory BP or nine mercury readings across three office visits among younger, untreated participants in the Improving the Detection of Hypertension study.
The reliability coefficients were 0.938, 0.846, and 0.894 for systolic BP and 0.918, 0.843, and 0.847 for diastolic BP, respectively.
Further, HBPM had the strongest association with left ventricular mass index (LVMI), a predictor of adverse cardiovascular events, according to researchers led by Joseph E. Schwartz, PhD, Stony Brook (N.Y.) University and Columbia University Irving Medical Center, New York.
The association with LVMI also remained after multivariate adjustment and after correcting for regression dilution bias, indicating the results were not a result of differences in the number of readings, they write in the study, published online in the Journal of the American College of Cardiology.
Whenever patients have an elevated blood pressure for the first time or even borderline elevated BP, guidelines recommend clinicians request a 24-hour ambulatory recording or home monitoring, Dr. Schwartz said in an interview. “I think this has the potential, for that purpose, to put ambulatory blood pressure monitoring out of business, even though that’s what I’ve done for 30 years.”
Previous studies have shown that home and ambulatory BP monitoring (ABPM) correlate more strongly with target-organ damage and cardiovascular outcomes than office BP, but head-to-head outcomes trials of the two techniques are lacking. A recent systematic review also found scant evidence supporting one approach over the other for predicting cardiovascular events or mortality.
An accompanying editorial notes that ABPM is largely unavailable to primary care physicians in the United States and poorly reimbursed. “Thus the demonstration that HBPM is more reliable and associates more closely with LVMI than ABPM, if confirmed, would carry the potential to change clinical practice,” wrote Robert M. Carey, MD, University of Virginia Health System in Charlottesville, and Thomas H. Marwick, MBBS, PhD, MPH, Baker Heart and Diabetes Institute, Melbourne.
In a comment, ABPM proponent Raymond R. Townsend, MD, said, “Honestly, it may be that we’ll need to act on this. I’m not quite ready to do that and change my practice patterns but, on the other hand, I can’t sweep this under the rug.”
He noted that it’s ironic the study is coauthored by the late Thomas Pickering, MD, a maven of ABPM who coined the term “white-coat hypertension” and pointed out masked hypertension.
That said, “it raised the bar on ambulatory blood pressure monitoring: Is it really worth our public health dollars? So I think it’s a very good call to arms,” said Dr. Townsend, who directs the hypertension program at the University of Pennsylvania, Philadelphia.
Ambulatory BP monitoring has long been considered the preferred method but, from a cost standpoint, HBPM is more attractive because the devices can be used more than once and track more than one person in a household, he said. The Center for Medicare Management also has a code in the 2020 bundle to reimburse physicians $15 for training patients and has a monthly charge for communicating with those filing regularly. “You’re not going to get rich doing monitoring of home BP, but at least the government is recognizing we are moving more and more to the home base in terms of our managing common conditions like blood pressure.”
One of the attractions of ABPM is the ability to do every half hour to every hour nocturnal pressures, but at least one home monitor, manufactured by Microlife, has added a nocturnal feature, Dr. Townsend noted. “So that’s just one more incoming against the ABPM defenses about why ABPMs are still better.”
The study enrolled a community-based sample of 408 participants who had office BP assessed at three visits (three readings per visit) using a mercury sphygmomanometer, a BpTRU (VSM MedTech) automated oscillometric device, and a home-validated Omron Healthcare oscillometric device.
After 5 minutes of in-office training and receipt of a reference sheet, participants also completed 3 weeks of HBPM with the Omron device as well as two 24-hour ambulatory measurements (Spacelabs Healthcare, Model 90207). Cardiovascular evaluations, including two-dimensional echocardiograms, were performed during the fifth office visit.
The 400 participants who completed all five visits had a mean age of 41 years, mean LVMI of 79.3 g/m2, and mean office systolic BP ranging from 116.0 to 117.2 mm Hg and diastolic BP from 75.6 to 76.5 mm Hg.
Both before and after correction for regression dilution bias, home systolic and diastolic BP were more highly correlated with LVMI than 24-hour ambulatory or office mercury readings. The corrected correlations for systolic BP were 0.501, 0.430, and 0.389, respectively.
After multivariable adjustment including office and 24-hour ambulatory BP, 10 mm Hg higher systolic and diastolic home BP were associated with 5.07 g/m2 (P = .001) and 3.92 g/m2 (P = .07) higher LVMI, respectively. After adjustment for home BP, however, neither systolic or diastolic office BP nor ambulatory BP was associated with LVMI.
Dr. Townsend and editorialists Dr. Carey and Dr. Marwick pointed out the study included a younger population in whom just 30% to 50% would have been classified as having hypertension by the 2017 American College of Cardiology/American Heart Association guidelines, which Dr. Carey helped to pen.
“These people are young and older people have a different kind of blood pressure driven more by the stiffness in their circulation and less by the resistance to blood flow that you find more characteristic in younger people,” Dr. Townsend observed.
“I don’t know that you can extrapolate the findings from this study in healthy, younger untreated people to older, perhaps sicker, and more diabetic people where the real action is and where the endpoints like heart attack, death, and stroke actually occur,” he said.
The results suggest measurement of resting daytime BP may be relatively more important than dynamic daytime and/or nocturnal parameters in predicting subclinical cardiac target organ damage, but this requires further study, Dr. Carey and Dr. Marwick noted.
Commenting further, they wrote that the results suggest “HBPM could be especially important for detecting elevated BP and hypertension early in life, when adults are relatively healthy, but those with hypertension have a high lifetime risk of CVD.”
Dr. Schwartz acknowledged the study didn’t include the typical hypertensive patient but said it goes to the central question of whether the risk associated with blood pressure is because of the heart’s cumulative exposure over its lifetime and, thus, best measured with multiple readings taken under a variety of circumstances or with readings taken only at rest.
“I’ve been posing that question at a conceptual level for 15 years, never in print, and this paper is the first hint, at least with respect to the left ventricular mass index … that getting a better measure of resting blood pressure is more important for controlling risk than the heart’s cumulative exposure to blood pressure, as measured by ambulatory,” he said.
The Improving the Detection of Hypertension study was supported by a grant from the National Heart, Lung, and Blood Institute of the National Institutes of Health. The authors disclosed no relevant financial relationships. Dr. Townsend reported receiving royalties as a writer for UpToDate and serving as an unpaid reviewer for ValidateBP.org. Dr. Carey is principal investigator and project director of a NIH R01 and P01 grant, respectively; vice chair of the 2017 ACC/AHA hypertension guideline writing committee; and chair of the AHA Resistant Hypertension Scientific Statement writing committee. Dr. Marwick disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Although guidelines generally recommend ambulatory over home blood pressure monitoring for diagnosing hypertension, new research questions home BP monitoring’s role as second fiddle.
One week of home BP monitoring (HBPM) was more reliable than one 24-hour ambulatory BP or nine mercury readings across three office visits among younger, untreated participants in the Improving the Detection of Hypertension study.
The reliability coefficients were 0.938, 0.846, and 0.894 for systolic BP and 0.918, 0.843, and 0.847 for diastolic BP, respectively.
Further, HBPM had the strongest association with left ventricular mass index (LVMI), a predictor of adverse cardiovascular events, according to researchers led by Joseph E. Schwartz, PhD, Stony Brook (N.Y.) University and Columbia University Irving Medical Center, New York.
The association with LVMI also remained after multivariate adjustment and after correcting for regression dilution bias, indicating the results were not a result of differences in the number of readings, they write in the study, published online in the Journal of the American College of Cardiology.
Whenever patients have an elevated blood pressure for the first time or even borderline elevated BP, guidelines recommend clinicians request a 24-hour ambulatory recording or home monitoring, Dr. Schwartz said in an interview. “I think this has the potential, for that purpose, to put ambulatory blood pressure monitoring out of business, even though that’s what I’ve done for 30 years.”
Previous studies have shown that home and ambulatory BP monitoring (ABPM) correlate more strongly with target-organ damage and cardiovascular outcomes than office BP, but head-to-head outcomes trials of the two techniques are lacking. A recent systematic review also found scant evidence supporting one approach over the other for predicting cardiovascular events or mortality.
An accompanying editorial notes that ABPM is largely unavailable to primary care physicians in the United States and poorly reimbursed. “Thus the demonstration that HBPM is more reliable and associates more closely with LVMI than ABPM, if confirmed, would carry the potential to change clinical practice,” wrote Robert M. Carey, MD, University of Virginia Health System in Charlottesville, and Thomas H. Marwick, MBBS, PhD, MPH, Baker Heart and Diabetes Institute, Melbourne.
In a comment, ABPM proponent Raymond R. Townsend, MD, said, “Honestly, it may be that we’ll need to act on this. I’m not quite ready to do that and change my practice patterns but, on the other hand, I can’t sweep this under the rug.”
He noted that it’s ironic the study is coauthored by the late Thomas Pickering, MD, a maven of ABPM who coined the term “white-coat hypertension” and pointed out masked hypertension.
That said, “it raised the bar on ambulatory blood pressure monitoring: Is it really worth our public health dollars? So I think it’s a very good call to arms,” said Dr. Townsend, who directs the hypertension program at the University of Pennsylvania, Philadelphia.
Ambulatory BP monitoring has long been considered the preferred method but, from a cost standpoint, HBPM is more attractive because the devices can be used more than once and track more than one person in a household, he said. The Center for Medicare Management also has a code in the 2020 bundle to reimburse physicians $15 for training patients and has a monthly charge for communicating with those filing regularly. “You’re not going to get rich doing monitoring of home BP, but at least the government is recognizing we are moving more and more to the home base in terms of our managing common conditions like blood pressure.”
One of the attractions of ABPM is the ability to do every half hour to every hour nocturnal pressures, but at least one home monitor, manufactured by Microlife, has added a nocturnal feature, Dr. Townsend noted. “So that’s just one more incoming against the ABPM defenses about why ABPMs are still better.”
The study enrolled a community-based sample of 408 participants who had office BP assessed at three visits (three readings per visit) using a mercury sphygmomanometer, a BpTRU (VSM MedTech) automated oscillometric device, and a home-validated Omron Healthcare oscillometric device.
After 5 minutes of in-office training and receipt of a reference sheet, participants also completed 3 weeks of HBPM with the Omron device as well as two 24-hour ambulatory measurements (Spacelabs Healthcare, Model 90207). Cardiovascular evaluations, including two-dimensional echocardiograms, were performed during the fifth office visit.
The 400 participants who completed all five visits had a mean age of 41 years, mean LVMI of 79.3 g/m2, and mean office systolic BP ranging from 116.0 to 117.2 mm Hg and diastolic BP from 75.6 to 76.5 mm Hg.
Both before and after correction for regression dilution bias, home systolic and diastolic BP were more highly correlated with LVMI than 24-hour ambulatory or office mercury readings. The corrected correlations for systolic BP were 0.501, 0.430, and 0.389, respectively.
After multivariable adjustment including office and 24-hour ambulatory BP, 10 mm Hg higher systolic and diastolic home BP were associated with 5.07 g/m2 (P = .001) and 3.92 g/m2 (P = .07) higher LVMI, respectively. After adjustment for home BP, however, neither systolic or diastolic office BP nor ambulatory BP was associated with LVMI.
Dr. Townsend and editorialists Dr. Carey and Dr. Marwick pointed out the study included a younger population in whom just 30% to 50% would have been classified as having hypertension by the 2017 American College of Cardiology/American Heart Association guidelines, which Dr. Carey helped to pen.
“These people are young and older people have a different kind of blood pressure driven more by the stiffness in their circulation and less by the resistance to blood flow that you find more characteristic in younger people,” Dr. Townsend observed.
“I don’t know that you can extrapolate the findings from this study in healthy, younger untreated people to older, perhaps sicker, and more diabetic people where the real action is and where the endpoints like heart attack, death, and stroke actually occur,” he said.
The results suggest measurement of resting daytime BP may be relatively more important than dynamic daytime and/or nocturnal parameters in predicting subclinical cardiac target organ damage, but this requires further study, Dr. Carey and Dr. Marwick noted.
Commenting further, they wrote that the results suggest “HBPM could be especially important for detecting elevated BP and hypertension early in life, when adults are relatively healthy, but those with hypertension have a high lifetime risk of CVD.”
Dr. Schwartz acknowledged the study didn’t include the typical hypertensive patient but said it goes to the central question of whether the risk associated with blood pressure is because of the heart’s cumulative exposure over its lifetime and, thus, best measured with multiple readings taken under a variety of circumstances or with readings taken only at rest.
“I’ve been posing that question at a conceptual level for 15 years, never in print, and this paper is the first hint, at least with respect to the left ventricular mass index … that getting a better measure of resting blood pressure is more important for controlling risk than the heart’s cumulative exposure to blood pressure, as measured by ambulatory,” he said.
The Improving the Detection of Hypertension study was supported by a grant from the National Heart, Lung, and Blood Institute of the National Institutes of Health. The authors disclosed no relevant financial relationships. Dr. Townsend reported receiving royalties as a writer for UpToDate and serving as an unpaid reviewer for ValidateBP.org. Dr. Carey is principal investigator and project director of a NIH R01 and P01 grant, respectively; vice chair of the 2017 ACC/AHA hypertension guideline writing committee; and chair of the AHA Resistant Hypertension Scientific Statement writing committee. Dr. Marwick disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Although guidelines generally recommend ambulatory over home blood pressure monitoring for diagnosing hypertension, new research questions home BP monitoring’s role as second fiddle.
One week of home BP monitoring (HBPM) was more reliable than one 24-hour ambulatory BP or nine mercury readings across three office visits among younger, untreated participants in the Improving the Detection of Hypertension study.
The reliability coefficients were 0.938, 0.846, and 0.894 for systolic BP and 0.918, 0.843, and 0.847 for diastolic BP, respectively.
Further, HBPM had the strongest association with left ventricular mass index (LVMI), a predictor of adverse cardiovascular events, according to researchers led by Joseph E. Schwartz, PhD, Stony Brook (N.Y.) University and Columbia University Irving Medical Center, New York.
The association with LVMI also remained after multivariate adjustment and after correcting for regression dilution bias, indicating the results were not a result of differences in the number of readings, they write in the study, published online in the Journal of the American College of Cardiology.
Whenever patients have an elevated blood pressure for the first time or even borderline elevated BP, guidelines recommend clinicians request a 24-hour ambulatory recording or home monitoring, Dr. Schwartz said in an interview. “I think this has the potential, for that purpose, to put ambulatory blood pressure monitoring out of business, even though that’s what I’ve done for 30 years.”
Previous studies have shown that home and ambulatory BP monitoring (ABPM) correlate more strongly with target-organ damage and cardiovascular outcomes than office BP, but head-to-head outcomes trials of the two techniques are lacking. A recent systematic review also found scant evidence supporting one approach over the other for predicting cardiovascular events or mortality.
An accompanying editorial notes that ABPM is largely unavailable to primary care physicians in the United States and poorly reimbursed. “Thus the demonstration that HBPM is more reliable and associates more closely with LVMI than ABPM, if confirmed, would carry the potential to change clinical practice,” wrote Robert M. Carey, MD, University of Virginia Health System in Charlottesville, and Thomas H. Marwick, MBBS, PhD, MPH, Baker Heart and Diabetes Institute, Melbourne.
In a comment, ABPM proponent Raymond R. Townsend, MD, said, “Honestly, it may be that we’ll need to act on this. I’m not quite ready to do that and change my practice patterns but, on the other hand, I can’t sweep this under the rug.”
He noted that it’s ironic the study is coauthored by the late Thomas Pickering, MD, a maven of ABPM who coined the term “white-coat hypertension” and pointed out masked hypertension.
That said, “it raised the bar on ambulatory blood pressure monitoring: Is it really worth our public health dollars? So I think it’s a very good call to arms,” said Dr. Townsend, who directs the hypertension program at the University of Pennsylvania, Philadelphia.
Ambulatory BP monitoring has long been considered the preferred method but, from a cost standpoint, HBPM is more attractive because the devices can be used more than once and track more than one person in a household, he said. The Center for Medicare Management also has a code in the 2020 bundle to reimburse physicians $15 for training patients and has a monthly charge for communicating with those filing regularly. “You’re not going to get rich doing monitoring of home BP, but at least the government is recognizing we are moving more and more to the home base in terms of our managing common conditions like blood pressure.”
One of the attractions of ABPM is the ability to do every half hour to every hour nocturnal pressures, but at least one home monitor, manufactured by Microlife, has added a nocturnal feature, Dr. Townsend noted. “So that’s just one more incoming against the ABPM defenses about why ABPMs are still better.”
The study enrolled a community-based sample of 408 participants who had office BP assessed at three visits (three readings per visit) using a mercury sphygmomanometer, a BpTRU (VSM MedTech) automated oscillometric device, and a home-validated Omron Healthcare oscillometric device.
After 5 minutes of in-office training and receipt of a reference sheet, participants also completed 3 weeks of HBPM with the Omron device as well as two 24-hour ambulatory measurements (Spacelabs Healthcare, Model 90207). Cardiovascular evaluations, including two-dimensional echocardiograms, were performed during the fifth office visit.
The 400 participants who completed all five visits had a mean age of 41 years, mean LVMI of 79.3 g/m2, and mean office systolic BP ranging from 116.0 to 117.2 mm Hg and diastolic BP from 75.6 to 76.5 mm Hg.
Both before and after correction for regression dilution bias, home systolic and diastolic BP were more highly correlated with LVMI than 24-hour ambulatory or office mercury readings. The corrected correlations for systolic BP were 0.501, 0.430, and 0.389, respectively.
After multivariable adjustment including office and 24-hour ambulatory BP, 10 mm Hg higher systolic and diastolic home BP were associated with 5.07 g/m2 (P = .001) and 3.92 g/m2 (P = .07) higher LVMI, respectively. After adjustment for home BP, however, neither systolic or diastolic office BP nor ambulatory BP was associated with LVMI.
Dr. Townsend and editorialists Dr. Carey and Dr. Marwick pointed out the study included a younger population in whom just 30% to 50% would have been classified as having hypertension by the 2017 American College of Cardiology/American Heart Association guidelines, which Dr. Carey helped to pen.
“These people are young and older people have a different kind of blood pressure driven more by the stiffness in their circulation and less by the resistance to blood flow that you find more characteristic in younger people,” Dr. Townsend observed.
“I don’t know that you can extrapolate the findings from this study in healthy, younger untreated people to older, perhaps sicker, and more diabetic people where the real action is and where the endpoints like heart attack, death, and stroke actually occur,” he said.
The results suggest measurement of resting daytime BP may be relatively more important than dynamic daytime and/or nocturnal parameters in predicting subclinical cardiac target organ damage, but this requires further study, Dr. Carey and Dr. Marwick noted.
Commenting further, they wrote that the results suggest “HBPM could be especially important for detecting elevated BP and hypertension early in life, when adults are relatively healthy, but those with hypertension have a high lifetime risk of CVD.”
Dr. Schwartz acknowledged the study didn’t include the typical hypertensive patient but said it goes to the central question of whether the risk associated with blood pressure is because of the heart’s cumulative exposure over its lifetime and, thus, best measured with multiple readings taken under a variety of circumstances or with readings taken only at rest.
“I’ve been posing that question at a conceptual level for 15 years, never in print, and this paper is the first hint, at least with respect to the left ventricular mass index … that getting a better measure of resting blood pressure is more important for controlling risk than the heart’s cumulative exposure to blood pressure, as measured by ambulatory,” he said.
The Improving the Detection of Hypertension study was supported by a grant from the National Heart, Lung, and Blood Institute of the National Institutes of Health. The authors disclosed no relevant financial relationships. Dr. Townsend reported receiving royalties as a writer for UpToDate and serving as an unpaid reviewer for ValidateBP.org. Dr. Carey is principal investigator and project director of a NIH R01 and P01 grant, respectively; vice chair of the 2017 ACC/AHA hypertension guideline writing committee; and chair of the AHA Resistant Hypertension Scientific Statement writing committee. Dr. Marwick disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
COVID-19 vaccines: Safe for immunocompromised patients?
Coronavirus vaccines have become a reality, as they are now being approved and authorized for use in a growing number of countries including the United States. The U.S. Food and Drug Administration has just issued emergency authorization for the use of the COVID-19 vaccine produced by Pfizer and BioNTech. Close behind is the vaccine developed by Moderna, which has also applied to the FDA for emergency authorization.
The efficacy of a two-dose administration of the vaccine has been pegged at 95.0%, and the FDA has said that the 95% credible interval for the vaccine efficacy was 90.3%-97.6%. But as with many initial clinical trials, whether for drugs or vaccines, not all populations were represented in the trial cohort, including individuals who are immunocompromised. At the current time, it is largely unknown how safe or effective the vaccine may be in this large population, many of whom are at high risk for serious COVID-19 complications.
At a special session held during the recent annual meeting of the American Society of Hematology, Anthony Fauci, MD, the nation’s leading infectious disease expert, said that individuals with compromised immune systems, whether because of chemotherapy or a bone marrow transplant, should plan to be vaccinated when the opportunity arises.
In response to a question from ASH President Stephanie J. Lee, MD, of the Fred Hutchinson Cancer Center, Seattle, Dr. Fauci emphasized that, despite being excluded from clinical trials, this population should get vaccinated. “I think we should recommend that they get vaccinated,” he said. “I mean, it is clear that, if you are on immunosuppressive agents, history tells us that you’re not going to have as robust a response as if you had an intact immune system that was not being compromised. But some degree of immunity is better than no degree of immunity.”
That does seem to be the consensus among experts who spoke in interviews: that as long as these are not live attenuated vaccines, they hold no specific risk to an immunocompromised patient, other than any factors specific to the individual that could be a contraindication.
“Patients, family members, friends, and work contacts should be encouraged to receive the vaccine,” said William Stohl, MD, PhD, chief of the division of rheumatology at the University of Southern California, Los Angeles. “Clinicians should advise patients to obtain the vaccine sooner rather than later.”
Kevin C. Wang, MD, PhD, of the department of dermatology at Stanford (Calif.) University, agreed. “I am 100% with Dr. Fauci. Everyone should get the vaccine, even if it may not be as effective,” he said. “I would treat it exactly like the flu vaccines that we recommend folks get every year.”
Dr. Wang noted that he couldn’t think of any contraindications unless the immunosuppressed patients have a history of severe allergic reactions to prior vaccinations. “But I would even say patients with history of cancer, upon recommendation of their oncologists, are likely to be suitable candidates for the vaccine,” he added. “I would say clinicians should approach counseling the same way they counsel patients for the flu vaccine, and as far as I know, there are no concerns for systemic drugs commonly used in dermatology patients.”
However, guidance has not yet been issued from either the FDA or the Centers for Disease Control and Prevention regarding the use of the vaccine in immunocompromised individuals. Given the lack of data, the FDA has said that “it will be something that providers will need to consider on an individual basis,” and that individuals should consult with physicians to weigh the potential benefits and potential risks.
The CDC’s Advisory Committee on Immunization Practices has said that clinicians need more guidance on whether to use the vaccine in pregnant or breastfeeding women, the immunocompromised, or those who have a history of allergies. The CDC itself has not yet released its formal guidance on vaccine use.
COVID-19 vaccines
Vaccines typically require years of research and testing before reaching the clinic, but this year researchers embarked on a global effort to develop safe and effective coronavirus vaccines in record time. Both the Pfizer/BioNTech and Moderna vaccines have only a few months of phase 3 clinical trial data, so much remains unknown about them, including their duration of effect and any long-term safety signals. In addition to excluding immunocompromised individuals, the clinical trials did not include children or pregnant women, so data are lacking for several population subgroups.
But these will not be the only vaccines available, as the pipeline is already becoming crowded. U.S. clinical trial data from a vaccine jointly being developed by Oxford-AstraZeneca, could potentially be ready, along with a request for FDA emergency use authorization, by late January 2021.
In addition, China and Russia have released vaccines, and there are currently 61 vaccines being investigated in clinical trials and at least 85 preclinical products under active investigation.
The vaccine candidates are using both conventional and novel mechanisms of action to elicit an immune response in patients. Conventional methods include attenuated inactivated (killed) virus and recombinant viral protein vaccines to develop immunity. Novel approaches include replication-deficient, adenovirus vector-based vaccines that contain the viral protein, and mRNA-based vaccines, such as the Pfizer and Moderna vaccines, that encode for a SARS-CoV-2 spike protein.
“The special vaccine concern for immunocompromised individuals is introduction of a live virus,” Dr. Stohl said. “Neither the Moderna nor Pfizer vaccines are live viruses, so there should be no special contraindication for such individuals.”
Live vaccine should be avoided in immunocompromised patients, and currently, live SARS-CoV-2 vaccines are only being developed in India and Turkey.
It is not unusual for vaccine trials to begin with cohorts that exclude participants with various health conditions, including those who are immunocompromised. These groups are generally then evaluated in phase 4 trials, or postmarketing surveillance. While the precise number of immunosuppressed adults in the United States is not known, the numbers are believed to be rising because of increased life expectancy among immunosuppressed adults as a result of advances in treatment and new and wider indications for therapies that can affect the immune system.
According to data from the 2013 National Health Interview Survey, an estimated 2.7% of U.S. adults are immunosuppressed. This population covers a broad array of health conditions and medical specialties; people living with inflammatory or autoimmune conditions, such as inflammatory rheumatic diseases (rheumatoid arthritis, axial spondyloarthritis, lupus); inflammatory bowel disease (Crohn’s disease and ulcerative colitis); psoriasis; multiple sclerosis; organ transplant recipients; patients undergoing chemotherapy; and life-long immunosuppression attributable to HIV infection.
As the vaccines begin to roll out and become available, how should clinicians advise their patients, in the absence of any clinical trial data?
Risk vs. benefit
Gilaad Kaplan, MD, MPH, a gastroenterologist and professor of medicine at the University of Calgary (Alta.), noted that the inflammatory bowel disease (IBD) community has dealt with tremendous anxiety during the pandemic because many are immunocompromised because of the medications they use to treat their disease.
“For example, many patients with IBD are on biologics like anti-TNF [tumor necrosis factor] therapies, which are also used in other immune-mediated inflammatory diseases such as rheumatoid arthritis,” he said. “Understandably, individuals with IBD on immunosuppressive medications are concerned about the risk of severe complications due to COVID-19.”
The entire IBD community, along with the world, celebrated the announcement that multiple vaccines are protective against SARS-CoV-2, he noted. “Vaccines offer the potential to reduce the spread of COVID-19, allowing society to revert back to normalcy,” Dr. Kaplan said. “Moreover, for vulnerable populations, including those who are immunocompromised, vaccines offer the potential to directly protect them from the morbidity and mortality associated with COVID-19.”
That said, even though the news of vaccines are extremely promising, some cautions must be raised regarding their use in immunocompromised populations, such as persons with IBD. “The current trials, to my knowledge, did not include immunocompromised individuals and thus, we can only extrapolate from what we know from other trials of different vaccines,” he explained. “We know from prior vaccines studies that the immune response following vaccination is less robust in those who are immunocompromised as compared to a healthy control population.”
Dr. Kaplan also pointed to recent reports of allergic reactions that have been reported in healthy individuals. “We don’t know whether side effects, like allergic reactions, may be different in unstudied populations,” he said. “Thus, the medical and scientific community should prioritize clinical studies of safety and effectiveness of COVID-19 vaccines in immunocompromised populations.”
So, what does this mean for an individual with an immune-mediated inflammatory disease like Crohn’s disease or ulcerative colitis who is immunocompromised? Dr. Kaplan explained that it is a balance between the potential harm of being infected with COVID-19 and the uncertainty of receiving a vaccine in an understudied population. For those who are highly susceptible to dying from COVID-19, such as an older adult with IBD, or someone who faces high exposure, such as a health care worker, the potential protection of the vaccine greatly outweighs the uncertainty.
“However, for individuals who are at otherwise lower risk – for example, young and able to work from home – then waiting a few extra months for postmarketing surveillance studies in immunocompromised populations may be a reasonable approach, as long as these individuals are taking great care to avoid infection,” he said.
No waiting needed
Joel M. Gelfand, MD, MSCE, professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia, feels that the newly approved vaccine should be safe for most of his patients.
“Patients with psoriatic disease should get the mRNA-based COVID-19 vaccine as soon as possible based on eligibility as determined by the CDC and local public health officials,” he said. “It is not a live vaccine, and therefore patients on biologics or other immune-modulating or immune-suppressing treatment can receive it.”
However, the impact of psoriasis treatment on immune response to the mRNA-based vaccines is not known. Dr. Gelfand noted that, extrapolating from the vaccine literature, there is some evidence that methotrexate reduces response to the influenza vaccine. “However, the clinical significance of this finding is not clear,” he said. “Since the mRNA vaccine needs to be taken twice, a few weeks apart, I do not recommend interrupting or delaying treatment for psoriatic disease while undergoing vaccination for COVID-19.”
Given the reports of allergic reactions, he added that it is advisable for patients with a history of life-threatening allergic reactions such as anaphylaxis or who have been advised to carry an epinephrine autoinjector, to talk with their health care provider to determine if COVID-19 vaccination is medically appropriate.
The National Psoriasis Foundation has issued guidance on COVID-19, explained Steven R. Feldman, MD, PhD, professor of dermatology, pathology, and social sciences & health policy at Wake Forest University, Winston-Salem, N.C., who is also a member of the committee that is working on those guidelines and keeping them up to date. “We are in the process of updating the guidelines with information on COVID vaccines,” he said.
He agreed that there are no contraindications for psoriasis patients to receive the vaccine, regardless of whether they are on immunosuppressive treatment, even though definitive data are lacking. “Fortunately, there’s a lot of good data coming out of Italy that patients with psoriasis on biologics do not appear to be at increased risk of getting COVID or of having worse outcomes from COVID,” he said.
Patients are going to ask about the vaccines, and when counseling them, clinicians should discuss the available data, the residual uncertainty, and patients’ concerns should be considered, Dr. Feldman explained. “There may be some concern that steroids and cyclosporine would reduce the effectiveness of vaccines, but there is no concern that any of the drugs would cause increased risk from nonlive vaccines.”
He added that there is evidence that “patients on biologics who receive nonlive vaccines do develop antibody responses and are immunized.”
Boosting efficacy
Even prior to making their announcement, the American College of Rheumatology had said that they would endorse the vaccine for all patients, explained rheumatologist Brett Smith, DO, from Blount Memorial Physicians Group and East Tennessee Children’s Hospital, Alcoa. “The vaccine is safe for all patients, but the problem may be that it’s not as effective,” he said. “But we don’t know that because it hasn’t been tested.”
With other vaccines, biologic medicines are held for 2 weeks before and afterwards, to get the best response. “But some patients don’t want to stop the medication,” Dr. Smith said. “They are afraid that their symptoms will return.”
As for counseling patients as to whether they should receive this vaccine, he explained that he typically doesn’t try to sway patients one way or another until they are really high risk. “When I counsel, it really depends on the individual situation. And for this vaccine, we have to be open to the fact that many people have already made up their mind.”
There are a lot of questions regarding the vaccine. One is the short time frame of development. “Vaccines typically take 6-10 years to come on the market, and this one is now available after a 3-month study,” Dr. Smith said. “Some have already decided that it’s too new for them.”
The process is also new, and patients need to understand that it doesn’t contain an active virus and “you can’t catch coronavirus from it.”
Dr. Smith also explained that, because the vaccine may be less effective in a person using biologic therapies, there is currently no information available on repeat vaccination. “These are all unanswered questions,” he said. “If the antibodies wane in a short time, can we be revaccinated and in what time frame? We just don’t know that yet.”
Marcelo Bonomi, MD, a medical oncologist from The Ohio State University Comprehensive Cancer Center, Columbus, explained that one way to ensure a more optimal response to the vaccine would be to wait until the patient has finished chemotherapy.* “The vaccine can be offered at that time, and in the meantime, they can take other steps to avoid infection,” he said. “If they are very immunosuppressed, it isn’t worth trying to give the vaccine.”
Cancer patients should be encouraged to stay as healthy as possible, and to wear masks and social distance. “It’s a comprehensive approach. Eat healthy, avoid alcohol and tobacco, and exercise. [These things] will help boost the immune system,” Dr. Bonomi said. “Family members should be encouraged to get vaccinated, which will help them avoid infection and exposing the patient.”
Jim Boonyaratanakornkit, MD, PhD, an infectious disease specialist who cares for cancer patients at the Fred Hutchinson Cancer Research Center, agreed. “Giving a vaccine right after a transplant is a futile endeavor,” he said. “We need to wait 6 months to have an immune response.”
He pointed out there may be a continuing higher number of cases, with high levels peaking in Washington in February and March. “Close friends and family should be vaccinated if possible,” he said, “which will help interrupt transmission.”
The vaccines are using new platforms that are totally different, and there is no clear data as to how long the antibodies will persist. “We know that they last for at least 4 months,” said Dr. Boonyaratanakornkit. “We don’t know what level of antibody will protect them from COVID-19 infection. Current studies are being conducted, but we don’t have that information for anyone yet.”
*Correction, 1/7/21: An earlier version of this article misattributed quotes from Dr. Marcelo Bonomi.
Coronavirus vaccines have become a reality, as they are now being approved and authorized for use in a growing number of countries including the United States. The U.S. Food and Drug Administration has just issued emergency authorization for the use of the COVID-19 vaccine produced by Pfizer and BioNTech. Close behind is the vaccine developed by Moderna, which has also applied to the FDA for emergency authorization.
The efficacy of a two-dose administration of the vaccine has been pegged at 95.0%, and the FDA has said that the 95% credible interval for the vaccine efficacy was 90.3%-97.6%. But as with many initial clinical trials, whether for drugs or vaccines, not all populations were represented in the trial cohort, including individuals who are immunocompromised. At the current time, it is largely unknown how safe or effective the vaccine may be in this large population, many of whom are at high risk for serious COVID-19 complications.
At a special session held during the recent annual meeting of the American Society of Hematology, Anthony Fauci, MD, the nation’s leading infectious disease expert, said that individuals with compromised immune systems, whether because of chemotherapy or a bone marrow transplant, should plan to be vaccinated when the opportunity arises.
In response to a question from ASH President Stephanie J. Lee, MD, of the Fred Hutchinson Cancer Center, Seattle, Dr. Fauci emphasized that, despite being excluded from clinical trials, this population should get vaccinated. “I think we should recommend that they get vaccinated,” he said. “I mean, it is clear that, if you are on immunosuppressive agents, history tells us that you’re not going to have as robust a response as if you had an intact immune system that was not being compromised. But some degree of immunity is better than no degree of immunity.”
That does seem to be the consensus among experts who spoke in interviews: that as long as these are not live attenuated vaccines, they hold no specific risk to an immunocompromised patient, other than any factors specific to the individual that could be a contraindication.
“Patients, family members, friends, and work contacts should be encouraged to receive the vaccine,” said William Stohl, MD, PhD, chief of the division of rheumatology at the University of Southern California, Los Angeles. “Clinicians should advise patients to obtain the vaccine sooner rather than later.”
Kevin C. Wang, MD, PhD, of the department of dermatology at Stanford (Calif.) University, agreed. “I am 100% with Dr. Fauci. Everyone should get the vaccine, even if it may not be as effective,” he said. “I would treat it exactly like the flu vaccines that we recommend folks get every year.”
Dr. Wang noted that he couldn’t think of any contraindications unless the immunosuppressed patients have a history of severe allergic reactions to prior vaccinations. “But I would even say patients with history of cancer, upon recommendation of their oncologists, are likely to be suitable candidates for the vaccine,” he added. “I would say clinicians should approach counseling the same way they counsel patients for the flu vaccine, and as far as I know, there are no concerns for systemic drugs commonly used in dermatology patients.”
However, guidance has not yet been issued from either the FDA or the Centers for Disease Control and Prevention regarding the use of the vaccine in immunocompromised individuals. Given the lack of data, the FDA has said that “it will be something that providers will need to consider on an individual basis,” and that individuals should consult with physicians to weigh the potential benefits and potential risks.
The CDC’s Advisory Committee on Immunization Practices has said that clinicians need more guidance on whether to use the vaccine in pregnant or breastfeeding women, the immunocompromised, or those who have a history of allergies. The CDC itself has not yet released its formal guidance on vaccine use.
COVID-19 vaccines
Vaccines typically require years of research and testing before reaching the clinic, but this year researchers embarked on a global effort to develop safe and effective coronavirus vaccines in record time. Both the Pfizer/BioNTech and Moderna vaccines have only a few months of phase 3 clinical trial data, so much remains unknown about them, including their duration of effect and any long-term safety signals. In addition to excluding immunocompromised individuals, the clinical trials did not include children or pregnant women, so data are lacking for several population subgroups.
But these will not be the only vaccines available, as the pipeline is already becoming crowded. U.S. clinical trial data from a vaccine jointly being developed by Oxford-AstraZeneca, could potentially be ready, along with a request for FDA emergency use authorization, by late January 2021.
In addition, China and Russia have released vaccines, and there are currently 61 vaccines being investigated in clinical trials and at least 85 preclinical products under active investigation.
The vaccine candidates are using both conventional and novel mechanisms of action to elicit an immune response in patients. Conventional methods include attenuated inactivated (killed) virus and recombinant viral protein vaccines to develop immunity. Novel approaches include replication-deficient, adenovirus vector-based vaccines that contain the viral protein, and mRNA-based vaccines, such as the Pfizer and Moderna vaccines, that encode for a SARS-CoV-2 spike protein.
“The special vaccine concern for immunocompromised individuals is introduction of a live virus,” Dr. Stohl said. “Neither the Moderna nor Pfizer vaccines are live viruses, so there should be no special contraindication for such individuals.”
Live vaccine should be avoided in immunocompromised patients, and currently, live SARS-CoV-2 vaccines are only being developed in India and Turkey.
It is not unusual for vaccine trials to begin with cohorts that exclude participants with various health conditions, including those who are immunocompromised. These groups are generally then evaluated in phase 4 trials, or postmarketing surveillance. While the precise number of immunosuppressed adults in the United States is not known, the numbers are believed to be rising because of increased life expectancy among immunosuppressed adults as a result of advances in treatment and new and wider indications for therapies that can affect the immune system.
According to data from the 2013 National Health Interview Survey, an estimated 2.7% of U.S. adults are immunosuppressed. This population covers a broad array of health conditions and medical specialties; people living with inflammatory or autoimmune conditions, such as inflammatory rheumatic diseases (rheumatoid arthritis, axial spondyloarthritis, lupus); inflammatory bowel disease (Crohn’s disease and ulcerative colitis); psoriasis; multiple sclerosis; organ transplant recipients; patients undergoing chemotherapy; and life-long immunosuppression attributable to HIV infection.
As the vaccines begin to roll out and become available, how should clinicians advise their patients, in the absence of any clinical trial data?
Risk vs. benefit
Gilaad Kaplan, MD, MPH, a gastroenterologist and professor of medicine at the University of Calgary (Alta.), noted that the inflammatory bowel disease (IBD) community has dealt with tremendous anxiety during the pandemic because many are immunocompromised because of the medications they use to treat their disease.
“For example, many patients with IBD are on biologics like anti-TNF [tumor necrosis factor] therapies, which are also used in other immune-mediated inflammatory diseases such as rheumatoid arthritis,” he said. “Understandably, individuals with IBD on immunosuppressive medications are concerned about the risk of severe complications due to COVID-19.”
The entire IBD community, along with the world, celebrated the announcement that multiple vaccines are protective against SARS-CoV-2, he noted. “Vaccines offer the potential to reduce the spread of COVID-19, allowing society to revert back to normalcy,” Dr. Kaplan said. “Moreover, for vulnerable populations, including those who are immunocompromised, vaccines offer the potential to directly protect them from the morbidity and mortality associated with COVID-19.”
That said, even though the news of vaccines are extremely promising, some cautions must be raised regarding their use in immunocompromised populations, such as persons with IBD. “The current trials, to my knowledge, did not include immunocompromised individuals and thus, we can only extrapolate from what we know from other trials of different vaccines,” he explained. “We know from prior vaccines studies that the immune response following vaccination is less robust in those who are immunocompromised as compared to a healthy control population.”
Dr. Kaplan also pointed to recent reports of allergic reactions that have been reported in healthy individuals. “We don’t know whether side effects, like allergic reactions, may be different in unstudied populations,” he said. “Thus, the medical and scientific community should prioritize clinical studies of safety and effectiveness of COVID-19 vaccines in immunocompromised populations.”
So, what does this mean for an individual with an immune-mediated inflammatory disease like Crohn’s disease or ulcerative colitis who is immunocompromised? Dr. Kaplan explained that it is a balance between the potential harm of being infected with COVID-19 and the uncertainty of receiving a vaccine in an understudied population. For those who are highly susceptible to dying from COVID-19, such as an older adult with IBD, or someone who faces high exposure, such as a health care worker, the potential protection of the vaccine greatly outweighs the uncertainty.
“However, for individuals who are at otherwise lower risk – for example, young and able to work from home – then waiting a few extra months for postmarketing surveillance studies in immunocompromised populations may be a reasonable approach, as long as these individuals are taking great care to avoid infection,” he said.
No waiting needed
Joel M. Gelfand, MD, MSCE, professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia, feels that the newly approved vaccine should be safe for most of his patients.
“Patients with psoriatic disease should get the mRNA-based COVID-19 vaccine as soon as possible based on eligibility as determined by the CDC and local public health officials,” he said. “It is not a live vaccine, and therefore patients on biologics or other immune-modulating or immune-suppressing treatment can receive it.”
However, the impact of psoriasis treatment on immune response to the mRNA-based vaccines is not known. Dr. Gelfand noted that, extrapolating from the vaccine literature, there is some evidence that methotrexate reduces response to the influenza vaccine. “However, the clinical significance of this finding is not clear,” he said. “Since the mRNA vaccine needs to be taken twice, a few weeks apart, I do not recommend interrupting or delaying treatment for psoriatic disease while undergoing vaccination for COVID-19.”
Given the reports of allergic reactions, he added that it is advisable for patients with a history of life-threatening allergic reactions such as anaphylaxis or who have been advised to carry an epinephrine autoinjector, to talk with their health care provider to determine if COVID-19 vaccination is medically appropriate.
The National Psoriasis Foundation has issued guidance on COVID-19, explained Steven R. Feldman, MD, PhD, professor of dermatology, pathology, and social sciences & health policy at Wake Forest University, Winston-Salem, N.C., who is also a member of the committee that is working on those guidelines and keeping them up to date. “We are in the process of updating the guidelines with information on COVID vaccines,” he said.
He agreed that there are no contraindications for psoriasis patients to receive the vaccine, regardless of whether they are on immunosuppressive treatment, even though definitive data are lacking. “Fortunately, there’s a lot of good data coming out of Italy that patients with psoriasis on biologics do not appear to be at increased risk of getting COVID or of having worse outcomes from COVID,” he said.
Patients are going to ask about the vaccines, and when counseling them, clinicians should discuss the available data, the residual uncertainty, and patients’ concerns should be considered, Dr. Feldman explained. “There may be some concern that steroids and cyclosporine would reduce the effectiveness of vaccines, but there is no concern that any of the drugs would cause increased risk from nonlive vaccines.”
He added that there is evidence that “patients on biologics who receive nonlive vaccines do develop antibody responses and are immunized.”
Boosting efficacy
Even prior to making their announcement, the American College of Rheumatology had said that they would endorse the vaccine for all patients, explained rheumatologist Brett Smith, DO, from Blount Memorial Physicians Group and East Tennessee Children’s Hospital, Alcoa. “The vaccine is safe for all patients, but the problem may be that it’s not as effective,” he said. “But we don’t know that because it hasn’t been tested.”
With other vaccines, biologic medicines are held for 2 weeks before and afterwards, to get the best response. “But some patients don’t want to stop the medication,” Dr. Smith said. “They are afraid that their symptoms will return.”
As for counseling patients as to whether they should receive this vaccine, he explained that he typically doesn’t try to sway patients one way or another until they are really high risk. “When I counsel, it really depends on the individual situation. And for this vaccine, we have to be open to the fact that many people have already made up their mind.”
There are a lot of questions regarding the vaccine. One is the short time frame of development. “Vaccines typically take 6-10 years to come on the market, and this one is now available after a 3-month study,” Dr. Smith said. “Some have already decided that it’s too new for them.”
The process is also new, and patients need to understand that it doesn’t contain an active virus and “you can’t catch coronavirus from it.”
Dr. Smith also explained that, because the vaccine may be less effective in a person using biologic therapies, there is currently no information available on repeat vaccination. “These are all unanswered questions,” he said. “If the antibodies wane in a short time, can we be revaccinated and in what time frame? We just don’t know that yet.”
Marcelo Bonomi, MD, a medical oncologist from The Ohio State University Comprehensive Cancer Center, Columbus, explained that one way to ensure a more optimal response to the vaccine would be to wait until the patient has finished chemotherapy.* “The vaccine can be offered at that time, and in the meantime, they can take other steps to avoid infection,” he said. “If they are very immunosuppressed, it isn’t worth trying to give the vaccine.”
Cancer patients should be encouraged to stay as healthy as possible, and to wear masks and social distance. “It’s a comprehensive approach. Eat healthy, avoid alcohol and tobacco, and exercise. [These things] will help boost the immune system,” Dr. Bonomi said. “Family members should be encouraged to get vaccinated, which will help them avoid infection and exposing the patient.”
Jim Boonyaratanakornkit, MD, PhD, an infectious disease specialist who cares for cancer patients at the Fred Hutchinson Cancer Research Center, agreed. “Giving a vaccine right after a transplant is a futile endeavor,” he said. “We need to wait 6 months to have an immune response.”
He pointed out there may be a continuing higher number of cases, with high levels peaking in Washington in February and March. “Close friends and family should be vaccinated if possible,” he said, “which will help interrupt transmission.”
The vaccines are using new platforms that are totally different, and there is no clear data as to how long the antibodies will persist. “We know that they last for at least 4 months,” said Dr. Boonyaratanakornkit. “We don’t know what level of antibody will protect them from COVID-19 infection. Current studies are being conducted, but we don’t have that information for anyone yet.”
*Correction, 1/7/21: An earlier version of this article misattributed quotes from Dr. Marcelo Bonomi.
Coronavirus vaccines have become a reality, as they are now being approved and authorized for use in a growing number of countries including the United States. The U.S. Food and Drug Administration has just issued emergency authorization for the use of the COVID-19 vaccine produced by Pfizer and BioNTech. Close behind is the vaccine developed by Moderna, which has also applied to the FDA for emergency authorization.
The efficacy of a two-dose administration of the vaccine has been pegged at 95.0%, and the FDA has said that the 95% credible interval for the vaccine efficacy was 90.3%-97.6%. But as with many initial clinical trials, whether for drugs or vaccines, not all populations were represented in the trial cohort, including individuals who are immunocompromised. At the current time, it is largely unknown how safe or effective the vaccine may be in this large population, many of whom are at high risk for serious COVID-19 complications.
At a special session held during the recent annual meeting of the American Society of Hematology, Anthony Fauci, MD, the nation’s leading infectious disease expert, said that individuals with compromised immune systems, whether because of chemotherapy or a bone marrow transplant, should plan to be vaccinated when the opportunity arises.
In response to a question from ASH President Stephanie J. Lee, MD, of the Fred Hutchinson Cancer Center, Seattle, Dr. Fauci emphasized that, despite being excluded from clinical trials, this population should get vaccinated. “I think we should recommend that they get vaccinated,” he said. “I mean, it is clear that, if you are on immunosuppressive agents, history tells us that you’re not going to have as robust a response as if you had an intact immune system that was not being compromised. But some degree of immunity is better than no degree of immunity.”
That does seem to be the consensus among experts who spoke in interviews: that as long as these are not live attenuated vaccines, they hold no specific risk to an immunocompromised patient, other than any factors specific to the individual that could be a contraindication.
“Patients, family members, friends, and work contacts should be encouraged to receive the vaccine,” said William Stohl, MD, PhD, chief of the division of rheumatology at the University of Southern California, Los Angeles. “Clinicians should advise patients to obtain the vaccine sooner rather than later.”
Kevin C. Wang, MD, PhD, of the department of dermatology at Stanford (Calif.) University, agreed. “I am 100% with Dr. Fauci. Everyone should get the vaccine, even if it may not be as effective,” he said. “I would treat it exactly like the flu vaccines that we recommend folks get every year.”
Dr. Wang noted that he couldn’t think of any contraindications unless the immunosuppressed patients have a history of severe allergic reactions to prior vaccinations. “But I would even say patients with history of cancer, upon recommendation of their oncologists, are likely to be suitable candidates for the vaccine,” he added. “I would say clinicians should approach counseling the same way they counsel patients for the flu vaccine, and as far as I know, there are no concerns for systemic drugs commonly used in dermatology patients.”
However, guidance has not yet been issued from either the FDA or the Centers for Disease Control and Prevention regarding the use of the vaccine in immunocompromised individuals. Given the lack of data, the FDA has said that “it will be something that providers will need to consider on an individual basis,” and that individuals should consult with physicians to weigh the potential benefits and potential risks.
The CDC’s Advisory Committee on Immunization Practices has said that clinicians need more guidance on whether to use the vaccine in pregnant or breastfeeding women, the immunocompromised, or those who have a history of allergies. The CDC itself has not yet released its formal guidance on vaccine use.
COVID-19 vaccines
Vaccines typically require years of research and testing before reaching the clinic, but this year researchers embarked on a global effort to develop safe and effective coronavirus vaccines in record time. Both the Pfizer/BioNTech and Moderna vaccines have only a few months of phase 3 clinical trial data, so much remains unknown about them, including their duration of effect and any long-term safety signals. In addition to excluding immunocompromised individuals, the clinical trials did not include children or pregnant women, so data are lacking for several population subgroups.
But these will not be the only vaccines available, as the pipeline is already becoming crowded. U.S. clinical trial data from a vaccine jointly being developed by Oxford-AstraZeneca, could potentially be ready, along with a request for FDA emergency use authorization, by late January 2021.
In addition, China and Russia have released vaccines, and there are currently 61 vaccines being investigated in clinical trials and at least 85 preclinical products under active investigation.
The vaccine candidates are using both conventional and novel mechanisms of action to elicit an immune response in patients. Conventional methods include attenuated inactivated (killed) virus and recombinant viral protein vaccines to develop immunity. Novel approaches include replication-deficient, adenovirus vector-based vaccines that contain the viral protein, and mRNA-based vaccines, such as the Pfizer and Moderna vaccines, that encode for a SARS-CoV-2 spike protein.
“The special vaccine concern for immunocompromised individuals is introduction of a live virus,” Dr. Stohl said. “Neither the Moderna nor Pfizer vaccines are live viruses, so there should be no special contraindication for such individuals.”
Live vaccine should be avoided in immunocompromised patients, and currently, live SARS-CoV-2 vaccines are only being developed in India and Turkey.
It is not unusual for vaccine trials to begin with cohorts that exclude participants with various health conditions, including those who are immunocompromised. These groups are generally then evaluated in phase 4 trials, or postmarketing surveillance. While the precise number of immunosuppressed adults in the United States is not known, the numbers are believed to be rising because of increased life expectancy among immunosuppressed adults as a result of advances in treatment and new and wider indications for therapies that can affect the immune system.
According to data from the 2013 National Health Interview Survey, an estimated 2.7% of U.S. adults are immunosuppressed. This population covers a broad array of health conditions and medical specialties; people living with inflammatory or autoimmune conditions, such as inflammatory rheumatic diseases (rheumatoid arthritis, axial spondyloarthritis, lupus); inflammatory bowel disease (Crohn’s disease and ulcerative colitis); psoriasis; multiple sclerosis; organ transplant recipients; patients undergoing chemotherapy; and life-long immunosuppression attributable to HIV infection.
As the vaccines begin to roll out and become available, how should clinicians advise their patients, in the absence of any clinical trial data?
Risk vs. benefit
Gilaad Kaplan, MD, MPH, a gastroenterologist and professor of medicine at the University of Calgary (Alta.), noted that the inflammatory bowel disease (IBD) community has dealt with tremendous anxiety during the pandemic because many are immunocompromised because of the medications they use to treat their disease.
“For example, many patients with IBD are on biologics like anti-TNF [tumor necrosis factor] therapies, which are also used in other immune-mediated inflammatory diseases such as rheumatoid arthritis,” he said. “Understandably, individuals with IBD on immunosuppressive medications are concerned about the risk of severe complications due to COVID-19.”
The entire IBD community, along with the world, celebrated the announcement that multiple vaccines are protective against SARS-CoV-2, he noted. “Vaccines offer the potential to reduce the spread of COVID-19, allowing society to revert back to normalcy,” Dr. Kaplan said. “Moreover, for vulnerable populations, including those who are immunocompromised, vaccines offer the potential to directly protect them from the morbidity and mortality associated with COVID-19.”
That said, even though the news of vaccines are extremely promising, some cautions must be raised regarding their use in immunocompromised populations, such as persons with IBD. “The current trials, to my knowledge, did not include immunocompromised individuals and thus, we can only extrapolate from what we know from other trials of different vaccines,” he explained. “We know from prior vaccines studies that the immune response following vaccination is less robust in those who are immunocompromised as compared to a healthy control population.”
Dr. Kaplan also pointed to recent reports of allergic reactions that have been reported in healthy individuals. “We don’t know whether side effects, like allergic reactions, may be different in unstudied populations,” he said. “Thus, the medical and scientific community should prioritize clinical studies of safety and effectiveness of COVID-19 vaccines in immunocompromised populations.”
So, what does this mean for an individual with an immune-mediated inflammatory disease like Crohn’s disease or ulcerative colitis who is immunocompromised? Dr. Kaplan explained that it is a balance between the potential harm of being infected with COVID-19 and the uncertainty of receiving a vaccine in an understudied population. For those who are highly susceptible to dying from COVID-19, such as an older adult with IBD, or someone who faces high exposure, such as a health care worker, the potential protection of the vaccine greatly outweighs the uncertainty.
“However, for individuals who are at otherwise lower risk – for example, young and able to work from home – then waiting a few extra months for postmarketing surveillance studies in immunocompromised populations may be a reasonable approach, as long as these individuals are taking great care to avoid infection,” he said.
No waiting needed
Joel M. Gelfand, MD, MSCE, professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia, feels that the newly approved vaccine should be safe for most of his patients.
“Patients with psoriatic disease should get the mRNA-based COVID-19 vaccine as soon as possible based on eligibility as determined by the CDC and local public health officials,” he said. “It is not a live vaccine, and therefore patients on biologics or other immune-modulating or immune-suppressing treatment can receive it.”
However, the impact of psoriasis treatment on immune response to the mRNA-based vaccines is not known. Dr. Gelfand noted that, extrapolating from the vaccine literature, there is some evidence that methotrexate reduces response to the influenza vaccine. “However, the clinical significance of this finding is not clear,” he said. “Since the mRNA vaccine needs to be taken twice, a few weeks apart, I do not recommend interrupting or delaying treatment for psoriatic disease while undergoing vaccination for COVID-19.”
Given the reports of allergic reactions, he added that it is advisable for patients with a history of life-threatening allergic reactions such as anaphylaxis or who have been advised to carry an epinephrine autoinjector, to talk with their health care provider to determine if COVID-19 vaccination is medically appropriate.
The National Psoriasis Foundation has issued guidance on COVID-19, explained Steven R. Feldman, MD, PhD, professor of dermatology, pathology, and social sciences & health policy at Wake Forest University, Winston-Salem, N.C., who is also a member of the committee that is working on those guidelines and keeping them up to date. “We are in the process of updating the guidelines with information on COVID vaccines,” he said.
He agreed that there are no contraindications for psoriasis patients to receive the vaccine, regardless of whether they are on immunosuppressive treatment, even though definitive data are lacking. “Fortunately, there’s a lot of good data coming out of Italy that patients with psoriasis on biologics do not appear to be at increased risk of getting COVID or of having worse outcomes from COVID,” he said.
Patients are going to ask about the vaccines, and when counseling them, clinicians should discuss the available data, the residual uncertainty, and patients’ concerns should be considered, Dr. Feldman explained. “There may be some concern that steroids and cyclosporine would reduce the effectiveness of vaccines, but there is no concern that any of the drugs would cause increased risk from nonlive vaccines.”
He added that there is evidence that “patients on biologics who receive nonlive vaccines do develop antibody responses and are immunized.”
Boosting efficacy
Even prior to making their announcement, the American College of Rheumatology had said that they would endorse the vaccine for all patients, explained rheumatologist Brett Smith, DO, from Blount Memorial Physicians Group and East Tennessee Children’s Hospital, Alcoa. “The vaccine is safe for all patients, but the problem may be that it’s not as effective,” he said. “But we don’t know that because it hasn’t been tested.”
With other vaccines, biologic medicines are held for 2 weeks before and afterwards, to get the best response. “But some patients don’t want to stop the medication,” Dr. Smith said. “They are afraid that their symptoms will return.”
As for counseling patients as to whether they should receive this vaccine, he explained that he typically doesn’t try to sway patients one way or another until they are really high risk. “When I counsel, it really depends on the individual situation. And for this vaccine, we have to be open to the fact that many people have already made up their mind.”
There are a lot of questions regarding the vaccine. One is the short time frame of development. “Vaccines typically take 6-10 years to come on the market, and this one is now available after a 3-month study,” Dr. Smith said. “Some have already decided that it’s too new for them.”
The process is also new, and patients need to understand that it doesn’t contain an active virus and “you can’t catch coronavirus from it.”
Dr. Smith also explained that, because the vaccine may be less effective in a person using biologic therapies, there is currently no information available on repeat vaccination. “These are all unanswered questions,” he said. “If the antibodies wane in a short time, can we be revaccinated and in what time frame? We just don’t know that yet.”
Marcelo Bonomi, MD, a medical oncologist from The Ohio State University Comprehensive Cancer Center, Columbus, explained that one way to ensure a more optimal response to the vaccine would be to wait until the patient has finished chemotherapy.* “The vaccine can be offered at that time, and in the meantime, they can take other steps to avoid infection,” he said. “If they are very immunosuppressed, it isn’t worth trying to give the vaccine.”
Cancer patients should be encouraged to stay as healthy as possible, and to wear masks and social distance. “It’s a comprehensive approach. Eat healthy, avoid alcohol and tobacco, and exercise. [These things] will help boost the immune system,” Dr. Bonomi said. “Family members should be encouraged to get vaccinated, which will help them avoid infection and exposing the patient.”
Jim Boonyaratanakornkit, MD, PhD, an infectious disease specialist who cares for cancer patients at the Fred Hutchinson Cancer Research Center, agreed. “Giving a vaccine right after a transplant is a futile endeavor,” he said. “We need to wait 6 months to have an immune response.”
He pointed out there may be a continuing higher number of cases, with high levels peaking in Washington in February and March. “Close friends and family should be vaccinated if possible,” he said, “which will help interrupt transmission.”
The vaccines are using new platforms that are totally different, and there is no clear data as to how long the antibodies will persist. “We know that they last for at least 4 months,” said Dr. Boonyaratanakornkit. “We don’t know what level of antibody will protect them from COVID-19 infection. Current studies are being conducted, but we don’t have that information for anyone yet.”
*Correction, 1/7/21: An earlier version of this article misattributed quotes from Dr. Marcelo Bonomi.
Parents favored virtual learning over in-person school attendance
Parents of school-aged children were generally more comfortable with full-time virtual learning in schools in the fall of 2020, compared with full-capacity in-person attendance, according to a survey conducted in July.
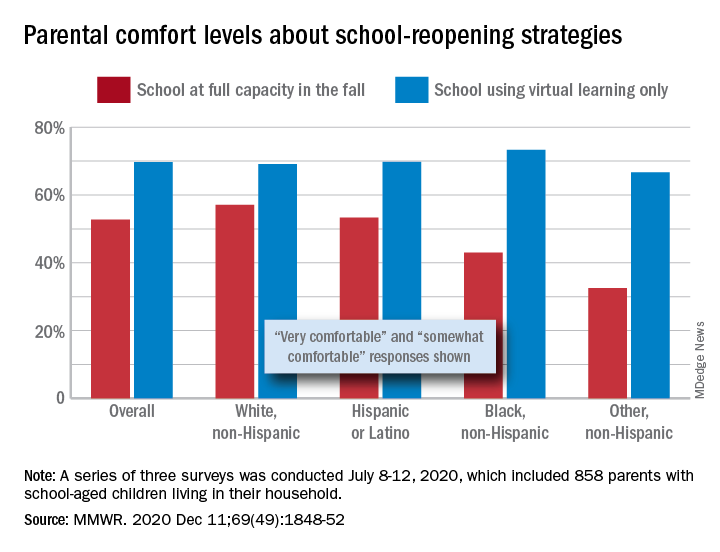
Those of racial/ethnic minorities, however, “were less likely to feel that schools should reopen for all students and were more concerned about” several aspects of in-person instruction than were White parents, Leah K. Gilbert, MD, and associates at the Centers for Disease Control and Prevention’s COVID-19 Response Team said in the Morbidity and Mortality Weekly Report.
A slim majority, just under 53% of the 858 parents surveyed, said that they were very or somewhat comfortable with their children returning to schools that were reopening at full capacity, while almost 70% said they were very/somewhat comfortable with schools going exclusively with virtual learning, the investigators reported.
The question about full-capacity attendance in particular showed considerable variation by race and ethnicity, with 57% of White parents saying they were very/somewhat comfortable, versus 53% of Hispanic or Latino parents, 43% of Black parents, and 32.5% of parents of other races/ethnicities (American Indian/Alaska Native, Asian, or multiracial).
Comfort levels were closer regarding virtual learning: Parents of other races/ethnicities were lowest at 67% and Black parents were highest at 73%. When asked about schools reopening at 50% capacity and 50% virtual learning, Black parents were again lowest at 58% with strong or moderate comfort and White parents were highest at 68%, Dr. Gilbert and associates said.
“Although the majority of parent respondents had concerns about both school reopening for in-person instruction and virtual learning, the perceived risk for SARS-CoV-2 infection and poor health outcomes might account for the differences in parental attitudes and concerns by race and ethnicity,” they wrote.
SOURCE: Gilbert LK et al. MMWR. 2020 Dec 11;69(49):1848-52.
Parents of school-aged children were generally more comfortable with full-time virtual learning in schools in the fall of 2020, compared with full-capacity in-person attendance, according to a survey conducted in July.

Those of racial/ethnic minorities, however, “were less likely to feel that schools should reopen for all students and were more concerned about” several aspects of in-person instruction than were White parents, Leah K. Gilbert, MD, and associates at the Centers for Disease Control and Prevention’s COVID-19 Response Team said in the Morbidity and Mortality Weekly Report.
A slim majority, just under 53% of the 858 parents surveyed, said that they were very or somewhat comfortable with their children returning to schools that were reopening at full capacity, while almost 70% said they were very/somewhat comfortable with schools going exclusively with virtual learning, the investigators reported.
The question about full-capacity attendance in particular showed considerable variation by race and ethnicity, with 57% of White parents saying they were very/somewhat comfortable, versus 53% of Hispanic or Latino parents, 43% of Black parents, and 32.5% of parents of other races/ethnicities (American Indian/Alaska Native, Asian, or multiracial).
Comfort levels were closer regarding virtual learning: Parents of other races/ethnicities were lowest at 67% and Black parents were highest at 73%. When asked about schools reopening at 50% capacity and 50% virtual learning, Black parents were again lowest at 58% with strong or moderate comfort and White parents were highest at 68%, Dr. Gilbert and associates said.
“Although the majority of parent respondents had concerns about both school reopening for in-person instruction and virtual learning, the perceived risk for SARS-CoV-2 infection and poor health outcomes might account for the differences in parental attitudes and concerns by race and ethnicity,” they wrote.
SOURCE: Gilbert LK et al. MMWR. 2020 Dec 11;69(49):1848-52.
Parents of school-aged children were generally more comfortable with full-time virtual learning in schools in the fall of 2020, compared with full-capacity in-person attendance, according to a survey conducted in July.

Those of racial/ethnic minorities, however, “were less likely to feel that schools should reopen for all students and were more concerned about” several aspects of in-person instruction than were White parents, Leah K. Gilbert, MD, and associates at the Centers for Disease Control and Prevention’s COVID-19 Response Team said in the Morbidity and Mortality Weekly Report.
A slim majority, just under 53% of the 858 parents surveyed, said that they were very or somewhat comfortable with their children returning to schools that were reopening at full capacity, while almost 70% said they were very/somewhat comfortable with schools going exclusively with virtual learning, the investigators reported.
The question about full-capacity attendance in particular showed considerable variation by race and ethnicity, with 57% of White parents saying they were very/somewhat comfortable, versus 53% of Hispanic or Latino parents, 43% of Black parents, and 32.5% of parents of other races/ethnicities (American Indian/Alaska Native, Asian, or multiracial).
Comfort levels were closer regarding virtual learning: Parents of other races/ethnicities were lowest at 67% and Black parents were highest at 73%. When asked about schools reopening at 50% capacity and 50% virtual learning, Black parents were again lowest at 58% with strong or moderate comfort and White parents were highest at 68%, Dr. Gilbert and associates said.
“Although the majority of parent respondents had concerns about both school reopening for in-person instruction and virtual learning, the perceived risk for SARS-CoV-2 infection and poor health outcomes might account for the differences in parental attitudes and concerns by race and ethnicity,” they wrote.
SOURCE: Gilbert LK et al. MMWR. 2020 Dec 11;69(49):1848-52.
FROM MMWR
Distinguishing between joy and pleasure during the pandemic
You can now buy vegan eggnog, made from almond milk. The fact that someone created this wasn’t a surprise – plant milks are taking over. That it gave me such pleasure was. It’s rich, and if you love eggnog, like all normal people, it’s amazingly satisfying when mixed in a Nespresso latte swirled creamy white and brown. It seems some things, like Netflix’s The Crown, my Peloton spin classes, long Sunday walks on the beach, and the best mushroom risotto I ever made were still pleasurable this year, despite all. I’d daresay, there was joy even in the time of COVID.
.
Pleasure is pretty constant. It pops up even in the worst times. It seems, there’s plenty to be found even now. Unless, perhaps it’s just me. The label my mother pinned on me as a boy has remained into adulthood: “Easy to please.” There’s hardly a movie I’ve seen that I didn’t like. I’m quite comfortable in the middle seat. I thought the EPIC updates this year were nice. I’ve liked the vast majority of pizzas I’ve ever eaten – even those contaminated with Truffle salt. Easy to please is a gift, not something I’ve acquired through hours of meditation or aesthetic fasts. But surely pleasure isn’t the same as joy. No one has tears of pleasure. (Not to mention, pleasure as a verb has obvious NSFW connotations; not true of joy).
No, joy is waaay bigger. Joy is shared. Joy is to the whole world. Joy is what happens when you have a baby. Pleasure is what happens when you remembered to put a burp cloth in the car. Pleasure is when three patients in a row take merely 5 minutes each. Joy is when an itchy patient is cured.
2020 was one of the most miserable years in the last century. We didn’t expect it, but we ought to have. I mean really, how many plagues have we endured? How many times has inequality led to social unrest? Many times. We, by luck and dint of hard work, have always managed to get through. Although suffering would surely have been greater during those times of sickness and loss, I don’t believe joy would have been less. Indeed, maybe it is those difficulties and that suffering that allows us to feel joy in the first place. It is only once you summit that you experience joy. The run-up is just pain.
It is no coincidence that it is now during this cold, dark, difficult part of the year that we wish joy. We’ve made it. We light the darkness with candles to joyously celebrate Mawlid, Diwali, then Hanukkah and Christmas. Had malls been open now, you’d hear amongst the din of ringing bells Rejoice! Rejoice! O Emmanuel! You’d sing along, “Joy to the world, now we sing, let the Angel voices ring.” Joy: A pleasure so great and so deserved, it is shared by all. It is good news, hope, gratitude.
A joy shared amongst us all is also coming. Through the wrenching pain of watching patients suffocate, fogged shields, and bleached masks, through canceled Thanksgivings, through weekends spent in the OR on the backlog of patients, after months spent sitting in empty clinics, though the long, orange-cone-winding lines of testing, at last, at last a vaccine is here to light the darkness.
Let the sea resound, and everything in it,
Let the rivers clap their hands,
let the mountains sing together for joy.
Joy to the world.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
You can now buy vegan eggnog, made from almond milk. The fact that someone created this wasn’t a surprise – plant milks are taking over. That it gave me such pleasure was. It’s rich, and if you love eggnog, like all normal people, it’s amazingly satisfying when mixed in a Nespresso latte swirled creamy white and brown. It seems some things, like Netflix’s The Crown, my Peloton spin classes, long Sunday walks on the beach, and the best mushroom risotto I ever made were still pleasurable this year, despite all. I’d daresay, there was joy even in the time of COVID.
.
Pleasure is pretty constant. It pops up even in the worst times. It seems, there’s plenty to be found even now. Unless, perhaps it’s just me. The label my mother pinned on me as a boy has remained into adulthood: “Easy to please.” There’s hardly a movie I’ve seen that I didn’t like. I’m quite comfortable in the middle seat. I thought the EPIC updates this year were nice. I’ve liked the vast majority of pizzas I’ve ever eaten – even those contaminated with Truffle salt. Easy to please is a gift, not something I’ve acquired through hours of meditation or aesthetic fasts. But surely pleasure isn’t the same as joy. No one has tears of pleasure. (Not to mention, pleasure as a verb has obvious NSFW connotations; not true of joy).
No, joy is waaay bigger. Joy is shared. Joy is to the whole world. Joy is what happens when you have a baby. Pleasure is what happens when you remembered to put a burp cloth in the car. Pleasure is when three patients in a row take merely 5 minutes each. Joy is when an itchy patient is cured.
2020 was one of the most miserable years in the last century. We didn’t expect it, but we ought to have. I mean really, how many plagues have we endured? How many times has inequality led to social unrest? Many times. We, by luck and dint of hard work, have always managed to get through. Although suffering would surely have been greater during those times of sickness and loss, I don’t believe joy would have been less. Indeed, maybe it is those difficulties and that suffering that allows us to feel joy in the first place. It is only once you summit that you experience joy. The run-up is just pain.
It is no coincidence that it is now during this cold, dark, difficult part of the year that we wish joy. We’ve made it. We light the darkness with candles to joyously celebrate Mawlid, Diwali, then Hanukkah and Christmas. Had malls been open now, you’d hear amongst the din of ringing bells Rejoice! Rejoice! O Emmanuel! You’d sing along, “Joy to the world, now we sing, let the Angel voices ring.” Joy: A pleasure so great and so deserved, it is shared by all. It is good news, hope, gratitude.
A joy shared amongst us all is also coming. Through the wrenching pain of watching patients suffocate, fogged shields, and bleached masks, through canceled Thanksgivings, through weekends spent in the OR on the backlog of patients, after months spent sitting in empty clinics, though the long, orange-cone-winding lines of testing, at last, at last a vaccine is here to light the darkness.
Let the sea resound, and everything in it,
Let the rivers clap their hands,
let the mountains sing together for joy.
Joy to the world.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
You can now buy vegan eggnog, made from almond milk. The fact that someone created this wasn’t a surprise – plant milks are taking over. That it gave me such pleasure was. It’s rich, and if you love eggnog, like all normal people, it’s amazingly satisfying when mixed in a Nespresso latte swirled creamy white and brown. It seems some things, like Netflix’s The Crown, my Peloton spin classes, long Sunday walks on the beach, and the best mushroom risotto I ever made were still pleasurable this year, despite all. I’d daresay, there was joy even in the time of COVID.
.
Pleasure is pretty constant. It pops up even in the worst times. It seems, there’s plenty to be found even now. Unless, perhaps it’s just me. The label my mother pinned on me as a boy has remained into adulthood: “Easy to please.” There’s hardly a movie I’ve seen that I didn’t like. I’m quite comfortable in the middle seat. I thought the EPIC updates this year were nice. I’ve liked the vast majority of pizzas I’ve ever eaten – even those contaminated with Truffle salt. Easy to please is a gift, not something I’ve acquired through hours of meditation or aesthetic fasts. But surely pleasure isn’t the same as joy. No one has tears of pleasure. (Not to mention, pleasure as a verb has obvious NSFW connotations; not true of joy).
No, joy is waaay bigger. Joy is shared. Joy is to the whole world. Joy is what happens when you have a baby. Pleasure is what happens when you remembered to put a burp cloth in the car. Pleasure is when three patients in a row take merely 5 minutes each. Joy is when an itchy patient is cured.
2020 was one of the most miserable years in the last century. We didn’t expect it, but we ought to have. I mean really, how many plagues have we endured? How many times has inequality led to social unrest? Many times. We, by luck and dint of hard work, have always managed to get through. Although suffering would surely have been greater during those times of sickness and loss, I don’t believe joy would have been less. Indeed, maybe it is those difficulties and that suffering that allows us to feel joy in the first place. It is only once you summit that you experience joy. The run-up is just pain.
It is no coincidence that it is now during this cold, dark, difficult part of the year that we wish joy. We’ve made it. We light the darkness with candles to joyously celebrate Mawlid, Diwali, then Hanukkah and Christmas. Had malls been open now, you’d hear amongst the din of ringing bells Rejoice! Rejoice! O Emmanuel! You’d sing along, “Joy to the world, now we sing, let the Angel voices ring.” Joy: A pleasure so great and so deserved, it is shared by all. It is good news, hope, gratitude.
A joy shared amongst us all is also coming. Through the wrenching pain of watching patients suffocate, fogged shields, and bleached masks, through canceled Thanksgivings, through weekends spent in the OR on the backlog of patients, after months spent sitting in empty clinics, though the long, orange-cone-winding lines of testing, at last, at last a vaccine is here to light the darkness.
Let the sea resound, and everything in it,
Let the rivers clap their hands,
let the mountains sing together for joy.
Joy to the world.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
On the horizon: Asciminib, a new drug for treating r/r CML
The investigational drug asciminib (being developed by Novartis) may become the new kid on the block for the treatment of chronic phase chronic myeloid leukemia (CMP-CP) for patients who have relapsed on or are refractory to at least two prior tyrosine kinase inhibitors (TKIs).
New results from the ASCEMBL study (NCT03106779) show that patients who received asciminib, which works differently from currently approved therapies for CML-CP, achieved better responses, compared with bosutinib (Bosulif) as third-line therapy.
“The ASCEMBL study opens a new chapter for CML, proving comparatively superior efficacy and excellent safety for a new class of ABL inhibitors,” coinvestigator Michael J. Mauro, MD, from Memorial Sloan Kettering Cancer Center, New York, said in an interview.
The trial was presented as a late-breaking abstract at the annual meeting of the American Society of Hematology.
Asciminib is a first-of-a-kind STAMP (Specifically Targeting the ABL Myristoyl Pocket) inhibitor that works differently from currently approved TKIs, which are adenosine triphosphate (ATP)-–competitive ABL inhibitors.
Five TKIs have been approved by the Food and Drug Administration to treat CML: imatinib (Gleevec; generics), nilotinib (Tasigna), dasatinib (Sprycel), bosutinib, and ponatinib (Iclusig).
All of them inhibit BCR/ABL tyrosine kinase by binding to the ATP-binding pocket.
Most patients with TKI resistant disease develop mutations in the ATP-binding pocket, explained Michael Jay Styler, MD, associate professor at Fox Chase–Temple University Hospital bone marrow transplant program, Fox Chase Cancer Center, Philadelphia.
By inactivating the protein through binding outside the ATP site, asciminib is a novel BCR-ABL inhibitor and may be a superior alternative to further traditional TKIs. “This agent promises to be an important addition to our treatment armamentarium for CML,” Dr. Styler said in an interview.
Another expert agreed. “Although we have many excellent therapies for CML, having a new medication that targets BCR-ABL in a novel way is still welcome to help us better care for CML patients,” Gabriela S. Hobbs, MD, said in an interview. Dr. Hobbs is the clinical director of leukemia services at Boston’s Mass General Cancer Center.
Patients in this study had previously been receiving at least two different types of TKIs. “The responses looked very encouraging for this group of heavily pretreated patients. Although CML patients do very well on current therapies, those that don’t get a response with TKI remain a difficult clinical challenge,” Dr. Hobbs said.
“This is the first study comparing asciminib to a TKI directly (in this case bosutinib) and it showed safety as well as preliminary evidence of efficacy. I look forward to seeing additional studies with this promising drug and to have a new drug to add to the CML arsenal,” she added.
Identifying patients who will benefit from asciminib
Patients with CML are currently sequenced through more than one second-generation TKI, Dr. Mauro commented. “If imatinib and a second-generation TKI have not served a patient well, only bosutinib has been studied in the third line and comparatively in the ASCEMBL study.” Asciminib was shown to be superior and could offer a clear alternative to ponatinib, which would be the other choice and is typically given even later after sequencing all other available options.
Dr. Hobbs agreed. “This is a challenging group of patients to manage as their options are limited. Ponatinib is often the drug of choice in these scenarios, as well as bone marrow transplant.”
They also agreed that it may be effective (alone or in combination) in treating patients with T315I-mutation CML, which is a particularly challenging disease.
Senior study author Andreas Hochhaus, MD, of the Klinik für Innere Medizin II in Jena, Germany, who presented the data at the meeting, noted new trials to test the efficacy of asciminib alone or in combination in earlier lines of therapy are ongoing and include the investigator-initiated FASCINATION study (first-line asciminib in combination) in Germany (NCT03906292).
ASCEMBL study details
ASCEMBL is a phase 3 study in which patients with CML who had received at least two previous TKIs were randomized to asciminib (n = 157) 40 mg twice daily or bosutinib (n = 76) 500 mg once daily. In a protocol amendment, patients with documented failure on bosutinib were allowed to switch to asciminib.
The main reason for discontinuing the last TKI therapy was lack of efficacy in approximately two-thirds of patients. More patients in the asciminib than the bosutinib group received two prior lines of therapy (52% vs. 40%); the others had received three or more prior lines of therapy.
Median follow-up for the data cutoff was 14.9 months.
Dr. Hochhaus reported that treatment discontinuation was lower in patients receiving asciminib than bosutinib (38% vs. 70%) and was mostly due to lack of efficacy (21% vs. 32%) or adverse events (5% vs. 21%).
The study met its primary endpoint: major molecular response (MMR) was approximately twice as high with asciminib than bosutinib at 24 weeks (25.5% vs. 13.2%; P = .029). Treatment effect for MMR was 12.2%. Median duration of exposure to asciminib was 43.4 weeks for asciminib and 29.2 weeks for bosutinib.
“Consistent treatment effect was seen across all subgroups of patients, and MMR rates were consistently high for patients on asciminib across all prior lines of therapy,” Dr. Hochhaus reported.
The probability of achieving MMR at 24 weeks was higher for patients receiving asciminib (25% vs. 11.9%) and started at week 12, he noted. Complete cytogenetic response was also higher for patients receiving asciminib (40.8% vs. 24.2%).
The occurrence of grade 3 or higher adverse events was lower with asciminib than bosutinib (51% vs. 61%). Thrombocytopenia and neutropenia were more common with asciminib and gastrointestinal events were more common with bosutinib. Arterial occlusion events were reported in five patients receiving asciminib and one patient receiving bosutinib. Most of these patients had prior exposure to imatinib, nilotinib, and/or dasatinib.
Dr. Mauro, a coinvestigator of the phase 3 study, also treated patients with the drug in the phase 1 study. “I feel asciminib has proven to be very well tolerated, with rare to absent cases of intolerance,” he said. Cardiovascular and cardiopulmonary adverse events are exceedingly rare as well.
Longer follow-up of the ASCEMBL study and continued follow-up of the myriad of groups from the phase 1 trial (T315I-positive patients treated with higher-dose asciminib, combination therapy with imatinib/nilotinib/dasatinib plus asciminib, and others) will be essential to settle any questions regarding selective adverse events of interest such as vascular occlusion, Dr. Mauro noted.
Dr. Hochhaus has reported receiving research funding from Novartis, Incyte, Pfizer, and Bristol-Myers Squibb. Dr. Hobbs has reported serving on advisory boards for Novartis. Dr. Mauro has reported financial relationships with Bristol-Myers Squibb, Novartis, Takeda, Pfizer, and Sun Pharma/SPARC.
A version of this article first appeared on Medscape.com.
The investigational drug asciminib (being developed by Novartis) may become the new kid on the block for the treatment of chronic phase chronic myeloid leukemia (CMP-CP) for patients who have relapsed on or are refractory to at least two prior tyrosine kinase inhibitors (TKIs).
New results from the ASCEMBL study (NCT03106779) show that patients who received asciminib, which works differently from currently approved therapies for CML-CP, achieved better responses, compared with bosutinib (Bosulif) as third-line therapy.
“The ASCEMBL study opens a new chapter for CML, proving comparatively superior efficacy and excellent safety for a new class of ABL inhibitors,” coinvestigator Michael J. Mauro, MD, from Memorial Sloan Kettering Cancer Center, New York, said in an interview.
The trial was presented as a late-breaking abstract at the annual meeting of the American Society of Hematology.
Asciminib is a first-of-a-kind STAMP (Specifically Targeting the ABL Myristoyl Pocket) inhibitor that works differently from currently approved TKIs, which are adenosine triphosphate (ATP)-–competitive ABL inhibitors.
Five TKIs have been approved by the Food and Drug Administration to treat CML: imatinib (Gleevec; generics), nilotinib (Tasigna), dasatinib (Sprycel), bosutinib, and ponatinib (Iclusig).
All of them inhibit BCR/ABL tyrosine kinase by binding to the ATP-binding pocket.
Most patients with TKI resistant disease develop mutations in the ATP-binding pocket, explained Michael Jay Styler, MD, associate professor at Fox Chase–Temple University Hospital bone marrow transplant program, Fox Chase Cancer Center, Philadelphia.
By inactivating the protein through binding outside the ATP site, asciminib is a novel BCR-ABL inhibitor and may be a superior alternative to further traditional TKIs. “This agent promises to be an important addition to our treatment armamentarium for CML,” Dr. Styler said in an interview.
Another expert agreed. “Although we have many excellent therapies for CML, having a new medication that targets BCR-ABL in a novel way is still welcome to help us better care for CML patients,” Gabriela S. Hobbs, MD, said in an interview. Dr. Hobbs is the clinical director of leukemia services at Boston’s Mass General Cancer Center.
Patients in this study had previously been receiving at least two different types of TKIs. “The responses looked very encouraging for this group of heavily pretreated patients. Although CML patients do very well on current therapies, those that don’t get a response with TKI remain a difficult clinical challenge,” Dr. Hobbs said.
“This is the first study comparing asciminib to a TKI directly (in this case bosutinib) and it showed safety as well as preliminary evidence of efficacy. I look forward to seeing additional studies with this promising drug and to have a new drug to add to the CML arsenal,” she added.
Identifying patients who will benefit from asciminib
Patients with CML are currently sequenced through more than one second-generation TKI, Dr. Mauro commented. “If imatinib and a second-generation TKI have not served a patient well, only bosutinib has been studied in the third line and comparatively in the ASCEMBL study.” Asciminib was shown to be superior and could offer a clear alternative to ponatinib, which would be the other choice and is typically given even later after sequencing all other available options.
Dr. Hobbs agreed. “This is a challenging group of patients to manage as their options are limited. Ponatinib is often the drug of choice in these scenarios, as well as bone marrow transplant.”
They also agreed that it may be effective (alone or in combination) in treating patients with T315I-mutation CML, which is a particularly challenging disease.
Senior study author Andreas Hochhaus, MD, of the Klinik für Innere Medizin II in Jena, Germany, who presented the data at the meeting, noted new trials to test the efficacy of asciminib alone or in combination in earlier lines of therapy are ongoing and include the investigator-initiated FASCINATION study (first-line asciminib in combination) in Germany (NCT03906292).
ASCEMBL study details
ASCEMBL is a phase 3 study in which patients with CML who had received at least two previous TKIs were randomized to asciminib (n = 157) 40 mg twice daily or bosutinib (n = 76) 500 mg once daily. In a protocol amendment, patients with documented failure on bosutinib were allowed to switch to asciminib.
The main reason for discontinuing the last TKI therapy was lack of efficacy in approximately two-thirds of patients. More patients in the asciminib than the bosutinib group received two prior lines of therapy (52% vs. 40%); the others had received three or more prior lines of therapy.
Median follow-up for the data cutoff was 14.9 months.
Dr. Hochhaus reported that treatment discontinuation was lower in patients receiving asciminib than bosutinib (38% vs. 70%) and was mostly due to lack of efficacy (21% vs. 32%) or adverse events (5% vs. 21%).
The study met its primary endpoint: major molecular response (MMR) was approximately twice as high with asciminib than bosutinib at 24 weeks (25.5% vs. 13.2%; P = .029). Treatment effect for MMR was 12.2%. Median duration of exposure to asciminib was 43.4 weeks for asciminib and 29.2 weeks for bosutinib.
“Consistent treatment effect was seen across all subgroups of patients, and MMR rates were consistently high for patients on asciminib across all prior lines of therapy,” Dr. Hochhaus reported.
The probability of achieving MMR at 24 weeks was higher for patients receiving asciminib (25% vs. 11.9%) and started at week 12, he noted. Complete cytogenetic response was also higher for patients receiving asciminib (40.8% vs. 24.2%).
The occurrence of grade 3 or higher adverse events was lower with asciminib than bosutinib (51% vs. 61%). Thrombocytopenia and neutropenia were more common with asciminib and gastrointestinal events were more common with bosutinib. Arterial occlusion events were reported in five patients receiving asciminib and one patient receiving bosutinib. Most of these patients had prior exposure to imatinib, nilotinib, and/or dasatinib.
Dr. Mauro, a coinvestigator of the phase 3 study, also treated patients with the drug in the phase 1 study. “I feel asciminib has proven to be very well tolerated, with rare to absent cases of intolerance,” he said. Cardiovascular and cardiopulmonary adverse events are exceedingly rare as well.
Longer follow-up of the ASCEMBL study and continued follow-up of the myriad of groups from the phase 1 trial (T315I-positive patients treated with higher-dose asciminib, combination therapy with imatinib/nilotinib/dasatinib plus asciminib, and others) will be essential to settle any questions regarding selective adverse events of interest such as vascular occlusion, Dr. Mauro noted.
Dr. Hochhaus has reported receiving research funding from Novartis, Incyte, Pfizer, and Bristol-Myers Squibb. Dr. Hobbs has reported serving on advisory boards for Novartis. Dr. Mauro has reported financial relationships with Bristol-Myers Squibb, Novartis, Takeda, Pfizer, and Sun Pharma/SPARC.
A version of this article first appeared on Medscape.com.
The investigational drug asciminib (being developed by Novartis) may become the new kid on the block for the treatment of chronic phase chronic myeloid leukemia (CMP-CP) for patients who have relapsed on or are refractory to at least two prior tyrosine kinase inhibitors (TKIs).
New results from the ASCEMBL study (NCT03106779) show that patients who received asciminib, which works differently from currently approved therapies for CML-CP, achieved better responses, compared with bosutinib (Bosulif) as third-line therapy.
“The ASCEMBL study opens a new chapter for CML, proving comparatively superior efficacy and excellent safety for a new class of ABL inhibitors,” coinvestigator Michael J. Mauro, MD, from Memorial Sloan Kettering Cancer Center, New York, said in an interview.
The trial was presented as a late-breaking abstract at the annual meeting of the American Society of Hematology.
Asciminib is a first-of-a-kind STAMP (Specifically Targeting the ABL Myristoyl Pocket) inhibitor that works differently from currently approved TKIs, which are adenosine triphosphate (ATP)-–competitive ABL inhibitors.
Five TKIs have been approved by the Food and Drug Administration to treat CML: imatinib (Gleevec; generics), nilotinib (Tasigna), dasatinib (Sprycel), bosutinib, and ponatinib (Iclusig).
All of them inhibit BCR/ABL tyrosine kinase by binding to the ATP-binding pocket.
Most patients with TKI resistant disease develop mutations in the ATP-binding pocket, explained Michael Jay Styler, MD, associate professor at Fox Chase–Temple University Hospital bone marrow transplant program, Fox Chase Cancer Center, Philadelphia.
By inactivating the protein through binding outside the ATP site, asciminib is a novel BCR-ABL inhibitor and may be a superior alternative to further traditional TKIs. “This agent promises to be an important addition to our treatment armamentarium for CML,” Dr. Styler said in an interview.
Another expert agreed. “Although we have many excellent therapies for CML, having a new medication that targets BCR-ABL in a novel way is still welcome to help us better care for CML patients,” Gabriela S. Hobbs, MD, said in an interview. Dr. Hobbs is the clinical director of leukemia services at Boston’s Mass General Cancer Center.
Patients in this study had previously been receiving at least two different types of TKIs. “The responses looked very encouraging for this group of heavily pretreated patients. Although CML patients do very well on current therapies, those that don’t get a response with TKI remain a difficult clinical challenge,” Dr. Hobbs said.
“This is the first study comparing asciminib to a TKI directly (in this case bosutinib) and it showed safety as well as preliminary evidence of efficacy. I look forward to seeing additional studies with this promising drug and to have a new drug to add to the CML arsenal,” she added.
Identifying patients who will benefit from asciminib
Patients with CML are currently sequenced through more than one second-generation TKI, Dr. Mauro commented. “If imatinib and a second-generation TKI have not served a patient well, only bosutinib has been studied in the third line and comparatively in the ASCEMBL study.” Asciminib was shown to be superior and could offer a clear alternative to ponatinib, which would be the other choice and is typically given even later after sequencing all other available options.
Dr. Hobbs agreed. “This is a challenging group of patients to manage as their options are limited. Ponatinib is often the drug of choice in these scenarios, as well as bone marrow transplant.”
They also agreed that it may be effective (alone or in combination) in treating patients with T315I-mutation CML, which is a particularly challenging disease.
Senior study author Andreas Hochhaus, MD, of the Klinik für Innere Medizin II in Jena, Germany, who presented the data at the meeting, noted new trials to test the efficacy of asciminib alone or in combination in earlier lines of therapy are ongoing and include the investigator-initiated FASCINATION study (first-line asciminib in combination) in Germany (NCT03906292).
ASCEMBL study details
ASCEMBL is a phase 3 study in which patients with CML who had received at least two previous TKIs were randomized to asciminib (n = 157) 40 mg twice daily or bosutinib (n = 76) 500 mg once daily. In a protocol amendment, patients with documented failure on bosutinib were allowed to switch to asciminib.
The main reason for discontinuing the last TKI therapy was lack of efficacy in approximately two-thirds of patients. More patients in the asciminib than the bosutinib group received two prior lines of therapy (52% vs. 40%); the others had received three or more prior lines of therapy.
Median follow-up for the data cutoff was 14.9 months.
Dr. Hochhaus reported that treatment discontinuation was lower in patients receiving asciminib than bosutinib (38% vs. 70%) and was mostly due to lack of efficacy (21% vs. 32%) or adverse events (5% vs. 21%).
The study met its primary endpoint: major molecular response (MMR) was approximately twice as high with asciminib than bosutinib at 24 weeks (25.5% vs. 13.2%; P = .029). Treatment effect for MMR was 12.2%. Median duration of exposure to asciminib was 43.4 weeks for asciminib and 29.2 weeks for bosutinib.
“Consistent treatment effect was seen across all subgroups of patients, and MMR rates were consistently high for patients on asciminib across all prior lines of therapy,” Dr. Hochhaus reported.
The probability of achieving MMR at 24 weeks was higher for patients receiving asciminib (25% vs. 11.9%) and started at week 12, he noted. Complete cytogenetic response was also higher for patients receiving asciminib (40.8% vs. 24.2%).
The occurrence of grade 3 or higher adverse events was lower with asciminib than bosutinib (51% vs. 61%). Thrombocytopenia and neutropenia were more common with asciminib and gastrointestinal events were more common with bosutinib. Arterial occlusion events were reported in five patients receiving asciminib and one patient receiving bosutinib. Most of these patients had prior exposure to imatinib, nilotinib, and/or dasatinib.
Dr. Mauro, a coinvestigator of the phase 3 study, also treated patients with the drug in the phase 1 study. “I feel asciminib has proven to be very well tolerated, with rare to absent cases of intolerance,” he said. Cardiovascular and cardiopulmonary adverse events are exceedingly rare as well.
Longer follow-up of the ASCEMBL study and continued follow-up of the myriad of groups from the phase 1 trial (T315I-positive patients treated with higher-dose asciminib, combination therapy with imatinib/nilotinib/dasatinib plus asciminib, and others) will be essential to settle any questions regarding selective adverse events of interest such as vascular occlusion, Dr. Mauro noted.
Dr. Hochhaus has reported receiving research funding from Novartis, Incyte, Pfizer, and Bristol-Myers Squibb. Dr. Hobbs has reported serving on advisory boards for Novartis. Dr. Mauro has reported financial relationships with Bristol-Myers Squibb, Novartis, Takeda, Pfizer, and Sun Pharma/SPARC.
A version of this article first appeared on Medscape.com.
Preadolescent acne: Management from birth requires increasing vigilance
No treatment may be necessary for acne in the first few months of life, but the condition can leave scars in children as young as ages 3-6 months, said Andrea L. Zaenglein, MD, professor of dermatology and pediatric dermatology, Penn State University, Hershey, Penn., said in a presentation at MedscapeLive’s virtual Women’s & Pediatric Dermatology Seminar.
Neonatal acne occurs in more than 20% of newborns aged 2 weeks to 3 months. “Typically we don’t need to treat it. But if you do, you could use a topical antifungal like clotrimazole cream twice a day,” but in most babies, “this will just improve over time and resolve without any scarring or sequelae,” she said.
Infantile acne begins about 3-6 months of age typically, or a little bit older, and lasts up to 2 years of age, Dr. Zaenglein said. “You will see comedones in infantile acne, so this is actually a true form of acne. It’s due to increased adrenal production of androgens.”
She added: “The scarring can be permanent. It’s important that you recognize infantile acne and treat it, even though it seems pretty mild.”
For infantile acne, she recommends performing a full-skin exam to rule out hyperandrogenic disorders such as Cushing syndrome, congenital adrenal hyperplasia, premature adrenarche, a gonadal/adrenal tumor and precocious puberty.
Treatments are similar to those in teenagers, she said, “but make sure you use baby-friendly formulations,” with lower concentrations of active ingredients – and avoid tetracyclines and benzoyl peroxide (BPO) washes. BPO can be used in leave-on formulations/creams at lower strengths (2.5%-5%).
One possible combination option is tretinoin 0.025% cream or adapalene 0.1% gel plus BPO 2.5% cream or clindamycin/BPO gel. Another combination is adapalene/BPO 2.5% gel.
Erythromycin can be appropriate at 30-50 mg/kg per day divided in two or three doses a day, but beware of possible gastrointestinal upset. Azithromycin at 5 mg/kg per day is another option.
“Rarely do we have to go to isotretinoin,” Dr. Zaenglein said. “I think in all my years, I’ve only treated one baby with isotretinoin for infantile acne. But severe forms can occur.”
Midchildhood and preadolescent acne conditions occur in children starting at ages 1 up to 10 years, Dr. Zaenglein said. In this population, she also recommends ruling out hyperandrogenism by looking for secondary sexual characteristics with full-body skin exams. “The workup can be broad and includes looking at adrenal androgens and total and free testosterone, as well as looking at growth charts and bone age. Typically, you’ll refer these kids to pediatric endocrinology.”
Keep in mind, she said, that early adrenarche starts at ages 6-7 years in girls and 7-8 years in boys. “That’s when we expect to start seeing that very early acne. You can see it even earlier in patients with elevated BMI, and it’s more common in Hispanic and Black children as well.”
She added that it’s important to remember that early adrenarche is a risk factor for polycystic ovarian syndrome (PCOS). “So ask patients about their family history and look for other signs of PCOS as they move further into adolescence.”
Milder cases of acne in this age group can be treated with “salicylic acid wipes and things that are kind of a rite of passage. But if they have any more severe acne, you’re going to want to treat it more or less like you do adolescent acne.”
MedscapeLive and this news organization are owned by the same parent company. Dr. Zaenglein disclosed receiving consulting fees from Cassiopea, Dermata, and Regeneron and fees for contracted research support from Incyte.
No treatment may be necessary for acne in the first few months of life, but the condition can leave scars in children as young as ages 3-6 months, said Andrea L. Zaenglein, MD, professor of dermatology and pediatric dermatology, Penn State University, Hershey, Penn., said in a presentation at MedscapeLive’s virtual Women’s & Pediatric Dermatology Seminar.
Neonatal acne occurs in more than 20% of newborns aged 2 weeks to 3 months. “Typically we don’t need to treat it. But if you do, you could use a topical antifungal like clotrimazole cream twice a day,” but in most babies, “this will just improve over time and resolve without any scarring or sequelae,” she said.
Infantile acne begins about 3-6 months of age typically, or a little bit older, and lasts up to 2 years of age, Dr. Zaenglein said. “You will see comedones in infantile acne, so this is actually a true form of acne. It’s due to increased adrenal production of androgens.”
She added: “The scarring can be permanent. It’s important that you recognize infantile acne and treat it, even though it seems pretty mild.”
For infantile acne, she recommends performing a full-skin exam to rule out hyperandrogenic disorders such as Cushing syndrome, congenital adrenal hyperplasia, premature adrenarche, a gonadal/adrenal tumor and precocious puberty.
Treatments are similar to those in teenagers, she said, “but make sure you use baby-friendly formulations,” with lower concentrations of active ingredients – and avoid tetracyclines and benzoyl peroxide (BPO) washes. BPO can be used in leave-on formulations/creams at lower strengths (2.5%-5%).
One possible combination option is tretinoin 0.025% cream or adapalene 0.1% gel plus BPO 2.5% cream or clindamycin/BPO gel. Another combination is adapalene/BPO 2.5% gel.
Erythromycin can be appropriate at 30-50 mg/kg per day divided in two or three doses a day, but beware of possible gastrointestinal upset. Azithromycin at 5 mg/kg per day is another option.
“Rarely do we have to go to isotretinoin,” Dr. Zaenglein said. “I think in all my years, I’ve only treated one baby with isotretinoin for infantile acne. But severe forms can occur.”
Midchildhood and preadolescent acne conditions occur in children starting at ages 1 up to 10 years, Dr. Zaenglein said. In this population, she also recommends ruling out hyperandrogenism by looking for secondary sexual characteristics with full-body skin exams. “The workup can be broad and includes looking at adrenal androgens and total and free testosterone, as well as looking at growth charts and bone age. Typically, you’ll refer these kids to pediatric endocrinology.”
Keep in mind, she said, that early adrenarche starts at ages 6-7 years in girls and 7-8 years in boys. “That’s when we expect to start seeing that very early acne. You can see it even earlier in patients with elevated BMI, and it’s more common in Hispanic and Black children as well.”
She added that it’s important to remember that early adrenarche is a risk factor for polycystic ovarian syndrome (PCOS). “So ask patients about their family history and look for other signs of PCOS as they move further into adolescence.”
Milder cases of acne in this age group can be treated with “salicylic acid wipes and things that are kind of a rite of passage. But if they have any more severe acne, you’re going to want to treat it more or less like you do adolescent acne.”
MedscapeLive and this news organization are owned by the same parent company. Dr. Zaenglein disclosed receiving consulting fees from Cassiopea, Dermata, and Regeneron and fees for contracted research support from Incyte.
No treatment may be necessary for acne in the first few months of life, but the condition can leave scars in children as young as ages 3-6 months, said Andrea L. Zaenglein, MD, professor of dermatology and pediatric dermatology, Penn State University, Hershey, Penn., said in a presentation at MedscapeLive’s virtual Women’s & Pediatric Dermatology Seminar.
Neonatal acne occurs in more than 20% of newborns aged 2 weeks to 3 months. “Typically we don’t need to treat it. But if you do, you could use a topical antifungal like clotrimazole cream twice a day,” but in most babies, “this will just improve over time and resolve without any scarring or sequelae,” she said.
Infantile acne begins about 3-6 months of age typically, or a little bit older, and lasts up to 2 years of age, Dr. Zaenglein said. “You will see comedones in infantile acne, so this is actually a true form of acne. It’s due to increased adrenal production of androgens.”
She added: “The scarring can be permanent. It’s important that you recognize infantile acne and treat it, even though it seems pretty mild.”
For infantile acne, she recommends performing a full-skin exam to rule out hyperandrogenic disorders such as Cushing syndrome, congenital adrenal hyperplasia, premature adrenarche, a gonadal/adrenal tumor and precocious puberty.
Treatments are similar to those in teenagers, she said, “but make sure you use baby-friendly formulations,” with lower concentrations of active ingredients – and avoid tetracyclines and benzoyl peroxide (BPO) washes. BPO can be used in leave-on formulations/creams at lower strengths (2.5%-5%).
One possible combination option is tretinoin 0.025% cream or adapalene 0.1% gel plus BPO 2.5% cream or clindamycin/BPO gel. Another combination is adapalene/BPO 2.5% gel.
Erythromycin can be appropriate at 30-50 mg/kg per day divided in two or three doses a day, but beware of possible gastrointestinal upset. Azithromycin at 5 mg/kg per day is another option.
“Rarely do we have to go to isotretinoin,” Dr. Zaenglein said. “I think in all my years, I’ve only treated one baby with isotretinoin for infantile acne. But severe forms can occur.”
Midchildhood and preadolescent acne conditions occur in children starting at ages 1 up to 10 years, Dr. Zaenglein said. In this population, she also recommends ruling out hyperandrogenism by looking for secondary sexual characteristics with full-body skin exams. “The workup can be broad and includes looking at adrenal androgens and total and free testosterone, as well as looking at growth charts and bone age. Typically, you’ll refer these kids to pediatric endocrinology.”
Keep in mind, she said, that early adrenarche starts at ages 6-7 years in girls and 7-8 years in boys. “That’s when we expect to start seeing that very early acne. You can see it even earlier in patients with elevated BMI, and it’s more common in Hispanic and Black children as well.”
She added that it’s important to remember that early adrenarche is a risk factor for polycystic ovarian syndrome (PCOS). “So ask patients about their family history and look for other signs of PCOS as they move further into adolescence.”
Milder cases of acne in this age group can be treated with “salicylic acid wipes and things that are kind of a rite of passage. But if they have any more severe acne, you’re going to want to treat it more or less like you do adolescent acne.”
MedscapeLive and this news organization are owned by the same parent company. Dr. Zaenglein disclosed receiving consulting fees from Cassiopea, Dermata, and Regeneron and fees for contracted research support from Incyte.
FROM MEDSCAPELIVE WOMEN’S & PEDIATRIC DERMATOLOGY SEMINAR
Pembro benefits in mTNBC regardless of chemo type
Adding pembrolizumab to chemotherapy substantially increases progression-free survival (PFS) in treatment-naive advanced or metastatic triple-negative breast cancer (TNBC) regardless of chemotherapy type, suggests an analysis of the clinical trial KEYNOTE-355.
There was also a trend for improved outcomes with increasing programmed death–ligand 1 (PD-L1) expression in the tumor, as measured by combined positive score (CPS).
“These data further support a role for the addition of pembro to standard chemo for the first-line treatment of metastatic TNBC,” said study presenter Hope S. Rugo, MD, from the Helen Diller Family Comprehensive Cancer Center, University of California, San Francisco.
The research was presented at the 2020 San Antonio Breast Cancer Symposium on Dec. 10.
Last month, pembrolizumab was granted accelerated approval by the Food and Drug Administration in combination with chemotherapy for the treatment of patients with locally recurrent unresectable or metastatic TNBC whose tumors express PD-L1 (CPS ≥10).
The approval was based on data from KEYNOTE-355, which involved almost 850 women with previously untreated locally recurrent inoperable or metastatic TNBC randomized 2:1 to pembrolizumab plus investigator’s choice of chemotherapy who were followed for 2 years.
For the current analysis, patients were stratified by PD-L1 CPS in the tumor, including over 320 patients with CPS ≥10, and by accompanying chemotherapy regimen.
In the overall intention-to-treat (ITT) population (n = 847), median PFS was longer with pembrolizumab plus chemotherapy versus placebo plus chemotherapy, at 9.7 months versus 5.6 months (hazard ratio, 0.82).
PFS improved step-wise with increased PD-L1 expression. In patients with PD-L1 CPS ≥1, the HR was 0.74, and in those with PD-L1 CPS ≥10, it was 0.65.
A similar incremental improvement by PD-L1 expression was seen in the overall response rate, with the rate topping out at 53.2% in the pembrolizumab plus chemotherapy arm, among the PD-L1 CPS ≥10 group.
Duration of response told a similar story, with the pembro-chemo combination providing superior results and the treatment effect increasing with PD-L1 enrichment.
Study discussant Sylvia Adams, MD, New York University Perlmutter Cancer Center, New York, said the “consistency of treatment effect” with different chemotherapy backbones seen in the study is “very important, as it is currently unknown what the optimal backbone is.”
She also noted that the chemotherapy analysis presented by Dr. Rugo was “exploratory” and “not powered to show the winner of the chemotherapy backbone.”
Nevertheless, in the postpresentation debate, Ian Krop, MD, PhD, Dana-Farber Cancer Institute, Boston, said that there are “several questions over the chemotherapy partner,” including whether there were differences in the populations who received each type of regimen.
Dr. Rugo replied that “because the trial wasn’t powered to look at the separate chemotherapy groups with any statistical significance ... it’s really impossible to draw any specific conclusions because it’s the overall population that’s evaluated.”
Asked about when overall survival results will be presented, Dr. Rugo said that “everybody is very interested” in that, “and we expect these results to be available next year.”
Study details
For KEYNOTE-355, researchers recruited women with previously untreated metastatic TNBC who had completed treatment with curative intent ≥6 months prior to their first disease recurrence.
They were randomized 2:1 to pembrolizumab or placebo plus investigator’s choice of chemotherapy from nab-paclitaxel, paclitaxel, or gemcitabine/carboplatin for up to 35 administrations of pembrolizumab or placebo or until progression, intolerable toxicity, or cessation of treatment.
Crossover was not allowed. Patients were stratified by type of chemotherapy, PD-L1 expression in the tumor, and prior treatment in the neoadjuvant or adjuvant setting with the same class of chemotherapy.
Response was assessed with imaging every 8 weeks until week 24, then every 9 weeks during the first year of follow-up, and then every 12 weeks.
Of 847 randomized patients, 566 received pembrolizumab plus chemotherapy and 281 were assigned to the placebo group. The median age was 53 years in both groups.
The majority (75.1%) of patients in both groups were PD-L1 positive with a centrally assessed CPS ≥1, while 38.9% of patients in the pembrolizumab arm and 36.7% of those given placebo had a CPS ≥10.
After a median follow-up of 25.9 months, 16 patients given pembrolizumab had completed the study and 33 were still ongoing.
This compares with five patients having completed the placebo arm, and 12 still ongoing, after a median follow-up of 26.3 months.
The overall response rate was higher with pembrolizumab plus chemotherapy in the ITT population, at 41.0% versus 35.9%, rising to 45.2% versus 37.9% in patients with PD-L1 CPS ≥1 and 53.2% versus 39.8% in the PD-L1 CPS ≥10 group.
Again, when the groups were stratified by on-study chemotherapy, the overall response rate was higher with pembrolizumab versus placebo regardless of the chemotherapy partner.
Finally, the duration of response with pembrolizumab plus chemotherapy was longer than that seen with placebo, at a median of 10.1 months versus 6.4 months in the ITT population.
In the PD-L1 CPS ≥1 group, the duration of response was 10.1 months versus 6.5 months, rising to 19.3 months versus 7.3 months in the PD-L1 CPS ≥10 group.
Dr. Adams nevertheless said that PD-L1 remains an “imperfect” biomarker in metastatic TNBC, although it is “the best to date.” Furthermore, the IMpassion130 trial, featuring atezolizumab, showed that there is “very poor” analytic and clinical concordance between assays, which “complicates clinical decision-making.”
This study was sponsored by Merck. Dr. Rugo, Dr. Adams, and Dr. Krop have disclosed financial ties to multiple pharmaceutical companies, including Merck.
A version of this article first appeared on Medscape.com.
Adding pembrolizumab to chemotherapy substantially increases progression-free survival (PFS) in treatment-naive advanced or metastatic triple-negative breast cancer (TNBC) regardless of chemotherapy type, suggests an analysis of the clinical trial KEYNOTE-355.
There was also a trend for improved outcomes with increasing programmed death–ligand 1 (PD-L1) expression in the tumor, as measured by combined positive score (CPS).
“These data further support a role for the addition of pembro to standard chemo for the first-line treatment of metastatic TNBC,” said study presenter Hope S. Rugo, MD, from the Helen Diller Family Comprehensive Cancer Center, University of California, San Francisco.
The research was presented at the 2020 San Antonio Breast Cancer Symposium on Dec. 10.
Last month, pembrolizumab was granted accelerated approval by the Food and Drug Administration in combination with chemotherapy for the treatment of patients with locally recurrent unresectable or metastatic TNBC whose tumors express PD-L1 (CPS ≥10).
The approval was based on data from KEYNOTE-355, which involved almost 850 women with previously untreated locally recurrent inoperable or metastatic TNBC randomized 2:1 to pembrolizumab plus investigator’s choice of chemotherapy who were followed for 2 years.
For the current analysis, patients were stratified by PD-L1 CPS in the tumor, including over 320 patients with CPS ≥10, and by accompanying chemotherapy regimen.
In the overall intention-to-treat (ITT) population (n = 847), median PFS was longer with pembrolizumab plus chemotherapy versus placebo plus chemotherapy, at 9.7 months versus 5.6 months (hazard ratio, 0.82).
PFS improved step-wise with increased PD-L1 expression. In patients with PD-L1 CPS ≥1, the HR was 0.74, and in those with PD-L1 CPS ≥10, it was 0.65.
A similar incremental improvement by PD-L1 expression was seen in the overall response rate, with the rate topping out at 53.2% in the pembrolizumab plus chemotherapy arm, among the PD-L1 CPS ≥10 group.
Duration of response told a similar story, with the pembro-chemo combination providing superior results and the treatment effect increasing with PD-L1 enrichment.
Study discussant Sylvia Adams, MD, New York University Perlmutter Cancer Center, New York, said the “consistency of treatment effect” with different chemotherapy backbones seen in the study is “very important, as it is currently unknown what the optimal backbone is.”
She also noted that the chemotherapy analysis presented by Dr. Rugo was “exploratory” and “not powered to show the winner of the chemotherapy backbone.”
Nevertheless, in the postpresentation debate, Ian Krop, MD, PhD, Dana-Farber Cancer Institute, Boston, said that there are “several questions over the chemotherapy partner,” including whether there were differences in the populations who received each type of regimen.
Dr. Rugo replied that “because the trial wasn’t powered to look at the separate chemotherapy groups with any statistical significance ... it’s really impossible to draw any specific conclusions because it’s the overall population that’s evaluated.”
Asked about when overall survival results will be presented, Dr. Rugo said that “everybody is very interested” in that, “and we expect these results to be available next year.”
Study details
For KEYNOTE-355, researchers recruited women with previously untreated metastatic TNBC who had completed treatment with curative intent ≥6 months prior to their first disease recurrence.
They were randomized 2:1 to pembrolizumab or placebo plus investigator’s choice of chemotherapy from nab-paclitaxel, paclitaxel, or gemcitabine/carboplatin for up to 35 administrations of pembrolizumab or placebo or until progression, intolerable toxicity, or cessation of treatment.
Crossover was not allowed. Patients were stratified by type of chemotherapy, PD-L1 expression in the tumor, and prior treatment in the neoadjuvant or adjuvant setting with the same class of chemotherapy.
Response was assessed with imaging every 8 weeks until week 24, then every 9 weeks during the first year of follow-up, and then every 12 weeks.
Of 847 randomized patients, 566 received pembrolizumab plus chemotherapy and 281 were assigned to the placebo group. The median age was 53 years in both groups.
The majority (75.1%) of patients in both groups were PD-L1 positive with a centrally assessed CPS ≥1, while 38.9% of patients in the pembrolizumab arm and 36.7% of those given placebo had a CPS ≥10.
After a median follow-up of 25.9 months, 16 patients given pembrolizumab had completed the study and 33 were still ongoing.
This compares with five patients having completed the placebo arm, and 12 still ongoing, after a median follow-up of 26.3 months.
The overall response rate was higher with pembrolizumab plus chemotherapy in the ITT population, at 41.0% versus 35.9%, rising to 45.2% versus 37.9% in patients with PD-L1 CPS ≥1 and 53.2% versus 39.8% in the PD-L1 CPS ≥10 group.
Again, when the groups were stratified by on-study chemotherapy, the overall response rate was higher with pembrolizumab versus placebo regardless of the chemotherapy partner.
Finally, the duration of response with pembrolizumab plus chemotherapy was longer than that seen with placebo, at a median of 10.1 months versus 6.4 months in the ITT population.
In the PD-L1 CPS ≥1 group, the duration of response was 10.1 months versus 6.5 months, rising to 19.3 months versus 7.3 months in the PD-L1 CPS ≥10 group.
Dr. Adams nevertheless said that PD-L1 remains an “imperfect” biomarker in metastatic TNBC, although it is “the best to date.” Furthermore, the IMpassion130 trial, featuring atezolizumab, showed that there is “very poor” analytic and clinical concordance between assays, which “complicates clinical decision-making.”
This study was sponsored by Merck. Dr. Rugo, Dr. Adams, and Dr. Krop have disclosed financial ties to multiple pharmaceutical companies, including Merck.
A version of this article first appeared on Medscape.com.
Adding pembrolizumab to chemotherapy substantially increases progression-free survival (PFS) in treatment-naive advanced or metastatic triple-negative breast cancer (TNBC) regardless of chemotherapy type, suggests an analysis of the clinical trial KEYNOTE-355.
There was also a trend for improved outcomes with increasing programmed death–ligand 1 (PD-L1) expression in the tumor, as measured by combined positive score (CPS).
“These data further support a role for the addition of pembro to standard chemo for the first-line treatment of metastatic TNBC,” said study presenter Hope S. Rugo, MD, from the Helen Diller Family Comprehensive Cancer Center, University of California, San Francisco.
The research was presented at the 2020 San Antonio Breast Cancer Symposium on Dec. 10.
Last month, pembrolizumab was granted accelerated approval by the Food and Drug Administration in combination with chemotherapy for the treatment of patients with locally recurrent unresectable or metastatic TNBC whose tumors express PD-L1 (CPS ≥10).
The approval was based on data from KEYNOTE-355, which involved almost 850 women with previously untreated locally recurrent inoperable or metastatic TNBC randomized 2:1 to pembrolizumab plus investigator’s choice of chemotherapy who were followed for 2 years.
For the current analysis, patients were stratified by PD-L1 CPS in the tumor, including over 320 patients with CPS ≥10, and by accompanying chemotherapy regimen.
In the overall intention-to-treat (ITT) population (n = 847), median PFS was longer with pembrolizumab plus chemotherapy versus placebo plus chemotherapy, at 9.7 months versus 5.6 months (hazard ratio, 0.82).
PFS improved step-wise with increased PD-L1 expression. In patients with PD-L1 CPS ≥1, the HR was 0.74, and in those with PD-L1 CPS ≥10, it was 0.65.
A similar incremental improvement by PD-L1 expression was seen in the overall response rate, with the rate topping out at 53.2% in the pembrolizumab plus chemotherapy arm, among the PD-L1 CPS ≥10 group.
Duration of response told a similar story, with the pembro-chemo combination providing superior results and the treatment effect increasing with PD-L1 enrichment.
Study discussant Sylvia Adams, MD, New York University Perlmutter Cancer Center, New York, said the “consistency of treatment effect” with different chemotherapy backbones seen in the study is “very important, as it is currently unknown what the optimal backbone is.”
She also noted that the chemotherapy analysis presented by Dr. Rugo was “exploratory” and “not powered to show the winner of the chemotherapy backbone.”
Nevertheless, in the postpresentation debate, Ian Krop, MD, PhD, Dana-Farber Cancer Institute, Boston, said that there are “several questions over the chemotherapy partner,” including whether there were differences in the populations who received each type of regimen.
Dr. Rugo replied that “because the trial wasn’t powered to look at the separate chemotherapy groups with any statistical significance ... it’s really impossible to draw any specific conclusions because it’s the overall population that’s evaluated.”
Asked about when overall survival results will be presented, Dr. Rugo said that “everybody is very interested” in that, “and we expect these results to be available next year.”
Study details
For KEYNOTE-355, researchers recruited women with previously untreated metastatic TNBC who had completed treatment with curative intent ≥6 months prior to their first disease recurrence.
They were randomized 2:1 to pembrolizumab or placebo plus investigator’s choice of chemotherapy from nab-paclitaxel, paclitaxel, or gemcitabine/carboplatin for up to 35 administrations of pembrolizumab or placebo or until progression, intolerable toxicity, or cessation of treatment.
Crossover was not allowed. Patients were stratified by type of chemotherapy, PD-L1 expression in the tumor, and prior treatment in the neoadjuvant or adjuvant setting with the same class of chemotherapy.
Response was assessed with imaging every 8 weeks until week 24, then every 9 weeks during the first year of follow-up, and then every 12 weeks.
Of 847 randomized patients, 566 received pembrolizumab plus chemotherapy and 281 were assigned to the placebo group. The median age was 53 years in both groups.
The majority (75.1%) of patients in both groups were PD-L1 positive with a centrally assessed CPS ≥1, while 38.9% of patients in the pembrolizumab arm and 36.7% of those given placebo had a CPS ≥10.
After a median follow-up of 25.9 months, 16 patients given pembrolizumab had completed the study and 33 were still ongoing.
This compares with five patients having completed the placebo arm, and 12 still ongoing, after a median follow-up of 26.3 months.
The overall response rate was higher with pembrolizumab plus chemotherapy in the ITT population, at 41.0% versus 35.9%, rising to 45.2% versus 37.9% in patients with PD-L1 CPS ≥1 and 53.2% versus 39.8% in the PD-L1 CPS ≥10 group.
Again, when the groups were stratified by on-study chemotherapy, the overall response rate was higher with pembrolizumab versus placebo regardless of the chemotherapy partner.
Finally, the duration of response with pembrolizumab plus chemotherapy was longer than that seen with placebo, at a median of 10.1 months versus 6.4 months in the ITT population.
In the PD-L1 CPS ≥1 group, the duration of response was 10.1 months versus 6.5 months, rising to 19.3 months versus 7.3 months in the PD-L1 CPS ≥10 group.
Dr. Adams nevertheless said that PD-L1 remains an “imperfect” biomarker in metastatic TNBC, although it is “the best to date.” Furthermore, the IMpassion130 trial, featuring atezolizumab, showed that there is “very poor” analytic and clinical concordance between assays, which “complicates clinical decision-making.”
This study was sponsored by Merck. Dr. Rugo, Dr. Adams, and Dr. Krop have disclosed financial ties to multiple pharmaceutical companies, including Merck.
A version of this article first appeared on Medscape.com.
Etanercept may not help some with suspected nonradiographic axial spondyloarthritis
Treatment with etanercept does not appear to achieve significant clinical improvement in patients suspected of having nonradiographic axial spondyloarthritis (nr-axSpA) but without positive MRI signs of sacroiliitis and/or elevated C-reactive protein (CRP) levels, research suggests.
A paper published in Arthritis & Rheumatology presents the outcomes of a randomized, double-blind, placebo-controlled trial of a 16-week course of etanercept at 25 mg twice weekly in 80 tumor necrosis factor inhibitor (TNFi)–naive patients with suspected nr-axSpA. Patients all had chronic inflammatory back pain, at least two spondyloarthritis features – such as HLA-B27 positivity, asymmetrical arthritis, or family history of ankylosing spondylitis – as well as high disease activity and insufficient response to at least two NSAIDs. These patients meet the “clinical arm” of Assessment of SpondyloArthritis international Society (ASAS) criteria for classifying axSpA at an early stage of disease but not “imaging-arm” requirements for the presence of active inflammatory lesions of the sacroiliac joints (SIJ) on MRI and one additional SpA feature.
Whether these imaging criteria and objective evidence of elevated inflammation are necessary to fulfill when considering TNFi treatment for patients with suspected nr-axSpA in daily practice is an important question to address, the authors pointed out, because “in many studies, the presence of a positive MRI-SIJ is one of the prerequisites to start a TNF inhibitor treatment in patients with nr-axSpA.” In addition, starting a TNFi is dependent on failure of at least two NSAIDs and an elevated CRP level when the MRI is negative, which is problematic since in some studies raised CRP levels were found in only 30% of the nr-axSpA patients and 59%-64% of nr-axSpA patients with high disease activity do not have active inflammatory SIJ lesions on MRI. On top of these concerns is the fact that many people who do not have axSpA show false-positive results of bone marrow edema on MRI of the SIJ, such as postpartum women, recreational runners, professional athletes, and army recruits undergoing physical training, they added.
In the current study at the end of the 16-week course of treatment, researchers found no statistically significant difference between the treatment and placebo group in the number of patients who achieved a 20% improvement in ASAS response criteria (16.7% vs. 11.1%; P = .5), nor in those who had at least 40% improvement (8.3% in both groups). This was regardless of sex, age, NSAID or disease-modifying antirheumatic drug use, HLA-B27 status, or other clinical factors.
Similarly, there was no statistically significant difference between the two groups in the number of patients who met response criteria for the Ankylosing Spondylitis Disease Activity Score based on CRP for either clinical improvement or major improvement.
Participants underwent MRI at baseline and at 16 and 24 weeks, which revealed similar numbers of active inflammatory SIJ lesions in each group. The two groups also had similar Spondyloarthritis Research Consortium of Canada scores at baseline and 16 weeks, but a slightly – yet statistically significant – higher score in the etanercept group at 24 weeks.
However, during the first 16 weeks of the study, patients in the etanercept group showed greater improvements in pain and erythrocyte sedimentation rate (ESR), compared with those in the placebo group.
After the 16-week treatment course, participants were followed for another 8 weeks. During this time, participants in the etanercept group showed a worsening in their mean Bath Ankylosing Spondylitis Metrology Index score, CRP level, and ESR, compared with the placebo group.
While the number of participants who experienced an adverse event by 16 weeks was similar in both groups, more patients in the etanercept group experienced an adverse effect likely related to the study drug.
Study results in the context of previous findings
Commenting on their findings, first author Tamara Rusman, of the Amsterdam University Medical Center, and coauthors wrote that the results suggested early treatment with etanercept in patients without a positive MRI and raised CRP levels was not effective.
However, they acknowledged that two previous placebo-controlled studies had specifically included patients with nr-axSpA and found a significantly better treatment response to TNF inhibitors than to placebo. One of these studies included a significant number of patients with MRI-detected active inflammatory SIJ lesions at baseline, which is a known predictor of treatment response.
“The relatively low number of patients with either a positive MRI-SIJ (23%) and/or elevated CRP level (13%) at baseline in our study could be an explanation for the absence of a treatment effect in favor of etanercept,” they wrote.
They also raised the possibility that their choice of study population didn’t truly capture patients with nr-axSpA, and that it was not powered to compare patients with or without a positive MRI or raised CRP level at baseline.
“It would be interesting to know whether our study results will be replicated by others in comparable study populations with equal numbers of patients with and without a positive MRI-SIJ and raised CRP in the future,” they wrote.
The study was supported by an unrestricted financial grant from Pfizer and ReumaNederland. No conflicts of interest were declared.
SOURCE: Rusman T et al. Arthritis Rheumatol. 2020 Dec 5. doi: 10.1002/art.41607.
Treatment with etanercept does not appear to achieve significant clinical improvement in patients suspected of having nonradiographic axial spondyloarthritis (nr-axSpA) but without positive MRI signs of sacroiliitis and/or elevated C-reactive protein (CRP) levels, research suggests.
A paper published in Arthritis & Rheumatology presents the outcomes of a randomized, double-blind, placebo-controlled trial of a 16-week course of etanercept at 25 mg twice weekly in 80 tumor necrosis factor inhibitor (TNFi)–naive patients with suspected nr-axSpA. Patients all had chronic inflammatory back pain, at least two spondyloarthritis features – such as HLA-B27 positivity, asymmetrical arthritis, or family history of ankylosing spondylitis – as well as high disease activity and insufficient response to at least two NSAIDs. These patients meet the “clinical arm” of Assessment of SpondyloArthritis international Society (ASAS) criteria for classifying axSpA at an early stage of disease but not “imaging-arm” requirements for the presence of active inflammatory lesions of the sacroiliac joints (SIJ) on MRI and one additional SpA feature.
Whether these imaging criteria and objective evidence of elevated inflammation are necessary to fulfill when considering TNFi treatment for patients with suspected nr-axSpA in daily practice is an important question to address, the authors pointed out, because “in many studies, the presence of a positive MRI-SIJ is one of the prerequisites to start a TNF inhibitor treatment in patients with nr-axSpA.” In addition, starting a TNFi is dependent on failure of at least two NSAIDs and an elevated CRP level when the MRI is negative, which is problematic since in some studies raised CRP levels were found in only 30% of the nr-axSpA patients and 59%-64% of nr-axSpA patients with high disease activity do not have active inflammatory SIJ lesions on MRI. On top of these concerns is the fact that many people who do not have axSpA show false-positive results of bone marrow edema on MRI of the SIJ, such as postpartum women, recreational runners, professional athletes, and army recruits undergoing physical training, they added.
In the current study at the end of the 16-week course of treatment, researchers found no statistically significant difference between the treatment and placebo group in the number of patients who achieved a 20% improvement in ASAS response criteria (16.7% vs. 11.1%; P = .5), nor in those who had at least 40% improvement (8.3% in both groups). This was regardless of sex, age, NSAID or disease-modifying antirheumatic drug use, HLA-B27 status, or other clinical factors.
Similarly, there was no statistically significant difference between the two groups in the number of patients who met response criteria for the Ankylosing Spondylitis Disease Activity Score based on CRP for either clinical improvement or major improvement.
Participants underwent MRI at baseline and at 16 and 24 weeks, which revealed similar numbers of active inflammatory SIJ lesions in each group. The two groups also had similar Spondyloarthritis Research Consortium of Canada scores at baseline and 16 weeks, but a slightly – yet statistically significant – higher score in the etanercept group at 24 weeks.
However, during the first 16 weeks of the study, patients in the etanercept group showed greater improvements in pain and erythrocyte sedimentation rate (ESR), compared with those in the placebo group.
After the 16-week treatment course, participants were followed for another 8 weeks. During this time, participants in the etanercept group showed a worsening in their mean Bath Ankylosing Spondylitis Metrology Index score, CRP level, and ESR, compared with the placebo group.
While the number of participants who experienced an adverse event by 16 weeks was similar in both groups, more patients in the etanercept group experienced an adverse effect likely related to the study drug.
Study results in the context of previous findings
Commenting on their findings, first author Tamara Rusman, of the Amsterdam University Medical Center, and coauthors wrote that the results suggested early treatment with etanercept in patients without a positive MRI and raised CRP levels was not effective.
However, they acknowledged that two previous placebo-controlled studies had specifically included patients with nr-axSpA and found a significantly better treatment response to TNF inhibitors than to placebo. One of these studies included a significant number of patients with MRI-detected active inflammatory SIJ lesions at baseline, which is a known predictor of treatment response.
“The relatively low number of patients with either a positive MRI-SIJ (23%) and/or elevated CRP level (13%) at baseline in our study could be an explanation for the absence of a treatment effect in favor of etanercept,” they wrote.
They also raised the possibility that their choice of study population didn’t truly capture patients with nr-axSpA, and that it was not powered to compare patients with or without a positive MRI or raised CRP level at baseline.
“It would be interesting to know whether our study results will be replicated by others in comparable study populations with equal numbers of patients with and without a positive MRI-SIJ and raised CRP in the future,” they wrote.
The study was supported by an unrestricted financial grant from Pfizer and ReumaNederland. No conflicts of interest were declared.
SOURCE: Rusman T et al. Arthritis Rheumatol. 2020 Dec 5. doi: 10.1002/art.41607.
Treatment with etanercept does not appear to achieve significant clinical improvement in patients suspected of having nonradiographic axial spondyloarthritis (nr-axSpA) but without positive MRI signs of sacroiliitis and/or elevated C-reactive protein (CRP) levels, research suggests.
A paper published in Arthritis & Rheumatology presents the outcomes of a randomized, double-blind, placebo-controlled trial of a 16-week course of etanercept at 25 mg twice weekly in 80 tumor necrosis factor inhibitor (TNFi)–naive patients with suspected nr-axSpA. Patients all had chronic inflammatory back pain, at least two spondyloarthritis features – such as HLA-B27 positivity, asymmetrical arthritis, or family history of ankylosing spondylitis – as well as high disease activity and insufficient response to at least two NSAIDs. These patients meet the “clinical arm” of Assessment of SpondyloArthritis international Society (ASAS) criteria for classifying axSpA at an early stage of disease but not “imaging-arm” requirements for the presence of active inflammatory lesions of the sacroiliac joints (SIJ) on MRI and one additional SpA feature.
Whether these imaging criteria and objective evidence of elevated inflammation are necessary to fulfill when considering TNFi treatment for patients with suspected nr-axSpA in daily practice is an important question to address, the authors pointed out, because “in many studies, the presence of a positive MRI-SIJ is one of the prerequisites to start a TNF inhibitor treatment in patients with nr-axSpA.” In addition, starting a TNFi is dependent on failure of at least two NSAIDs and an elevated CRP level when the MRI is negative, which is problematic since in some studies raised CRP levels were found in only 30% of the nr-axSpA patients and 59%-64% of nr-axSpA patients with high disease activity do not have active inflammatory SIJ lesions on MRI. On top of these concerns is the fact that many people who do not have axSpA show false-positive results of bone marrow edema on MRI of the SIJ, such as postpartum women, recreational runners, professional athletes, and army recruits undergoing physical training, they added.
In the current study at the end of the 16-week course of treatment, researchers found no statistically significant difference between the treatment and placebo group in the number of patients who achieved a 20% improvement in ASAS response criteria (16.7% vs. 11.1%; P = .5), nor in those who had at least 40% improvement (8.3% in both groups). This was regardless of sex, age, NSAID or disease-modifying antirheumatic drug use, HLA-B27 status, or other clinical factors.
Similarly, there was no statistically significant difference between the two groups in the number of patients who met response criteria for the Ankylosing Spondylitis Disease Activity Score based on CRP for either clinical improvement or major improvement.
Participants underwent MRI at baseline and at 16 and 24 weeks, which revealed similar numbers of active inflammatory SIJ lesions in each group. The two groups also had similar Spondyloarthritis Research Consortium of Canada scores at baseline and 16 weeks, but a slightly – yet statistically significant – higher score in the etanercept group at 24 weeks.
However, during the first 16 weeks of the study, patients in the etanercept group showed greater improvements in pain and erythrocyte sedimentation rate (ESR), compared with those in the placebo group.
After the 16-week treatment course, participants were followed for another 8 weeks. During this time, participants in the etanercept group showed a worsening in their mean Bath Ankylosing Spondylitis Metrology Index score, CRP level, and ESR, compared with the placebo group.
While the number of participants who experienced an adverse event by 16 weeks was similar in both groups, more patients in the etanercept group experienced an adverse effect likely related to the study drug.
Study results in the context of previous findings
Commenting on their findings, first author Tamara Rusman, of the Amsterdam University Medical Center, and coauthors wrote that the results suggested early treatment with etanercept in patients without a positive MRI and raised CRP levels was not effective.
However, they acknowledged that two previous placebo-controlled studies had specifically included patients with nr-axSpA and found a significantly better treatment response to TNF inhibitors than to placebo. One of these studies included a significant number of patients with MRI-detected active inflammatory SIJ lesions at baseline, which is a known predictor of treatment response.
“The relatively low number of patients with either a positive MRI-SIJ (23%) and/or elevated CRP level (13%) at baseline in our study could be an explanation for the absence of a treatment effect in favor of etanercept,” they wrote.
They also raised the possibility that their choice of study population didn’t truly capture patients with nr-axSpA, and that it was not powered to compare patients with or without a positive MRI or raised CRP level at baseline.
“It would be interesting to know whether our study results will be replicated by others in comparable study populations with equal numbers of patients with and without a positive MRI-SIJ and raised CRP in the future,” they wrote.
The study was supported by an unrestricted financial grant from Pfizer and ReumaNederland. No conflicts of interest were declared.
SOURCE: Rusman T et al. Arthritis Rheumatol. 2020 Dec 5. doi: 10.1002/art.41607.
FROM ARTHRITIS & RHEUMATOLOGY
Four-item prognostic index predicts survival in adult Burkitt lymphoma
A newly devised, validated prognostic tool – the Burkitt Lymphoma International Prognostic Index – can consistently identify low-risk patients who might benefit from treatment de-escalation, and high-risk patients who are unlikely to be cured with current therapies and may require novel approaches, investigators said.
In a cohort of patients treated at international sites, patients with a low-risk score on the BL-IPI had a 3-year progression-free survival (PFS) rate of 96%, and 3-year overall survival rate (OS) of 99%. In contrast, the 3-year PFS rate for patients in the high-risk category was 63%, and the 3-year OS rate was 64%, reported Adam J Olszewski, MD, from the Lifespan Cancer Institute at Rhode Island Hospital and The Miriam Hospital, both in Providence.
“The Burkitt Lymphoma International Prognostic Index – or the ‘BLI-PI’ [‘blippy’] as it was inevitably called – is a novel prognostic index that is specific to Burkitt lymphoma. It has been validated with sufficient calibration and discrimination in external data sets to allow for simple stratification and comparison of risk distribution in geographically diverse cohorts,” he said in an oral abstract presented virtually during the annual meeting of the American Society of Hematology.
Inconsistent criteria
There is a need for a Burkitt-specific index, he said, because of significant differences in age, stage at presentation, and abnormal lactate dehydrogenase (LDH) levels between patients with Burkitt and those with diffuse large B-cell lymphoma (DLBCL), and because historical definitions of “low-risk” Burkitt lymphoma have been inconsistent, with less than 10% of patients falling into this group, leaving the remainder in a undifferentiated “high-risk” category.
“Burkitt lymphoma is considered highly curable, but current therapy requires administration of dose-intense chemoimmunotherapy for which there are many chemotherapy backbone regimens developed across the world, and used mostly locally. These are often studied in phase 2 studies with limited sample sizes, which makes it difficult to compare populations across trials,” Dr. Olszewski said.
A validated prognostic index can help clinicians and researchers compare cohorts and can be used to help design future trials, he added.
To devise the BL-IPI, the investigators first selected a retrospective cohort of 570 adults with Burkitt lymphoma treated at 30 U.S. centers for whom data on outcomes were available.
They determined the best prognostic cutoffs for age, LDH, hemoglobin and albumin levels, and identified independent risk factors using stepwise selection in Cox regression and lasso regression analysis, a machine learning approach. The variables included age; sex; HIV-positivity status; loss of MYC rearrangement; performance status; stage; nodal involvement; marrow involvement; central nervous system involvement; and LDH, hemoglobin, and albumin levels.
For validation, they pooled data from European, Canadian, Australian, and U.K. studies to identify 457 patients for whom retrospective treatment and outcomes data were available.
The derivation and validation cohorts were similar in most respects, expect for a higher proportion of patients with Eastern Cooperative Oncology Group performance status scores of 2 or higher in the validation cohort (22% vs. 35%), and a higher proportion of patients with CNS involvement in the U.S.-based derivation cohort (19% vs. 10%, respectively).
Therapy also differed markedly between the U.S. and international cohorts, with about 30% each of U.S. patients receiving either the CODOX-M/IVAC (cyclophosphamide, vincristine, doxorubicin, high-dose methotrexate/ifosfamide, etoposide, and high-dose cytarabine) regimen, DA-EPOCH-R (dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab) regimen, or hCVAD/MA (fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with high-dose methotrexate and cytarabine) regimen, and the remaining 10% receiving other, unspecified therapy.
In contrast, 65% of the patients in the international (validation) cohort received CODOX-M/IVAC, 10% and 9%, respectively, received DA-EPOCH-R and hCVAD/MA, and 16% receiving other regimens.
Rituximab was administered to 91% of U.S. and 95% of international patients.
Higher survival rates outside US
Both PFS and OS were higher in the international versus U.S. cohort. At a median follow-up of 45 months, the PFS rate in the United States was 65%, and the OS rate was 70%.
In the international cohort, after a median follow-up of 52 months, the PFS rate was 75%, and the OS rate was 76%, the investigators found.
Reasons for the differences may be because of differences in treatment regimens, socioeconomic and racial disparities in the United States versus other countries, or to decentralized Burkitt lymphoma therapy in the United States, Dr. Olszewski said.
In univariate analysis, factors significantly predictive of worse PFS included age 40 years or older, ECOG performance status 2 or greater, stage 3 or 4 disease, marrow involvement, CNS involvement, LDH more than three times the upper limit of normal, and hemoglobin <11.5 g/dL (P < .001 for all preceding), as well as albumin <3.5 g/dL (P = .001).
“However, the multivariable analysis was more complicated, because many of these factors were overlapping, and most patients with high LDH also had advanced disease, and this group also encompassed patients who had bone marrow and CNS involvement,” he said.
Using the two types of regression analysis mentioned before, investigators identified ECOG performance status 2 or greater (P = .001), age 40 and older (P = .005), LDH greater than three times the upper limit of normal (P < .001) and CNS involvement (P = .002) as significant predictors for worse outcomes in multivariable analysis, and were included in the final model.
“We initially had five groups according to the number of these factors, but we observed that the survival curves for patients with two, three, or four factors were overlapping, and not significantly different, so ultimately we had three risk groups. In the derivation (U.S.) cohort, patients in the low-risk group, with no risk factors, a 3-year PFS of 92%, compared with 72% for patients with one risk factor (intermediate risk), and 53% for patients with two to four risk factors (high risk).
Respective hazard ratios for worse PFS in the low-, intermediate-, and high-risk groups were 1 (reference), 4.15 (95% confidence interval, 1.99-8.68), and 8.83 (95% CI, 4.32-18.03).
Respective HR for worse OS was 1, 7.06 (95% CI, 2.55-19.53), and 15.12 (95% CI, 5.58-40.99).
There were no significant differences in either PFS or OS when either LDH or stage was added into the model.
The BL-IPI was prognostic for PFS and OS in all subgroups, including HIV-positive or -negative patients, those with MYC rearrangements, stage 1 or 2 versus stage 3 or 4, or those treated with rituximab versus those who were not.
As noted before, 3-year PFS rates in the validation cohort for low, intermediate, high-risk groups were 96%, 82%, and 63% respectively, and 3-year OS rates were 99%, 85%, and 64%.
Why the CNS discrepancy?
In the question and answer session following the presentation, comoderator Christopher J. Melani, MD, from the Lymphoid Malignancies Branch at the National Cancer Institute in Bethesda, Md., said that “it was interesting to see the difference between CNS involvement in both the U.S. and the international cohort,” and asked whether Dr. Olszweski could elaborate on whether baseline CNS involvement was assessed by contrast-enhanced MRI of flow cytometry studies of cerebrospinal fluid.
“Could some of these differences between the U.S. and the international cohort be from the baseline assessment differing between the two?” he asked.
Dr. Olszewski replied that the retrospective nature of the data precluded capturing those data, but added that “I do suspect there may be some differences in the way that central nervous system is staged in different countries. In the United States the use of flow cytometry is more commonly employed, but we don’t know how it is used internationally. We do not know how often this is staged radiographically.”
Asked by others who viewed the presentation whether extranodal disease or peripheral blood involvement were prognostic in the final model, Dr. Olszewski replied that “one has to understand that, when one constructs a prognostic index, there is a balance between trying to input as much information as possible and to create something that is useful, clinically meaningful, and accurate.”
He said that, despite trying different models with different factors, “we couldn’t get the discrimination to be much better than the basic model that we ultimately created, so we favored using a more parsimonious model.”
No study funding source was reported. Dr. Olszewski reported research funding from Spectrum Pharmaceuticals, Genentech, TG Therapeutics, and Adaptive Biotechnologies. Dr. Melani reported having no relevant conflicts of interest.
SOURCE: Olszewski AJ et al. ASH 2020, Abstract 705.
A newly devised, validated prognostic tool – the Burkitt Lymphoma International Prognostic Index – can consistently identify low-risk patients who might benefit from treatment de-escalation, and high-risk patients who are unlikely to be cured with current therapies and may require novel approaches, investigators said.
In a cohort of patients treated at international sites, patients with a low-risk score on the BL-IPI had a 3-year progression-free survival (PFS) rate of 96%, and 3-year overall survival rate (OS) of 99%. In contrast, the 3-year PFS rate for patients in the high-risk category was 63%, and the 3-year OS rate was 64%, reported Adam J Olszewski, MD, from the Lifespan Cancer Institute at Rhode Island Hospital and The Miriam Hospital, both in Providence.
“The Burkitt Lymphoma International Prognostic Index – or the ‘BLI-PI’ [‘blippy’] as it was inevitably called – is a novel prognostic index that is specific to Burkitt lymphoma. It has been validated with sufficient calibration and discrimination in external data sets to allow for simple stratification and comparison of risk distribution in geographically diverse cohorts,” he said in an oral abstract presented virtually during the annual meeting of the American Society of Hematology.
Inconsistent criteria
There is a need for a Burkitt-specific index, he said, because of significant differences in age, stage at presentation, and abnormal lactate dehydrogenase (LDH) levels between patients with Burkitt and those with diffuse large B-cell lymphoma (DLBCL), and because historical definitions of “low-risk” Burkitt lymphoma have been inconsistent, with less than 10% of patients falling into this group, leaving the remainder in a undifferentiated “high-risk” category.
“Burkitt lymphoma is considered highly curable, but current therapy requires administration of dose-intense chemoimmunotherapy for which there are many chemotherapy backbone regimens developed across the world, and used mostly locally. These are often studied in phase 2 studies with limited sample sizes, which makes it difficult to compare populations across trials,” Dr. Olszewski said.
A validated prognostic index can help clinicians and researchers compare cohorts and can be used to help design future trials, he added.
To devise the BL-IPI, the investigators first selected a retrospective cohort of 570 adults with Burkitt lymphoma treated at 30 U.S. centers for whom data on outcomes were available.
They determined the best prognostic cutoffs for age, LDH, hemoglobin and albumin levels, and identified independent risk factors using stepwise selection in Cox regression and lasso regression analysis, a machine learning approach. The variables included age; sex; HIV-positivity status; loss of MYC rearrangement; performance status; stage; nodal involvement; marrow involvement; central nervous system involvement; and LDH, hemoglobin, and albumin levels.
For validation, they pooled data from European, Canadian, Australian, and U.K. studies to identify 457 patients for whom retrospective treatment and outcomes data were available.
The derivation and validation cohorts were similar in most respects, expect for a higher proportion of patients with Eastern Cooperative Oncology Group performance status scores of 2 or higher in the validation cohort (22% vs. 35%), and a higher proportion of patients with CNS involvement in the U.S.-based derivation cohort (19% vs. 10%, respectively).
Therapy also differed markedly between the U.S. and international cohorts, with about 30% each of U.S. patients receiving either the CODOX-M/IVAC (cyclophosphamide, vincristine, doxorubicin, high-dose methotrexate/ifosfamide, etoposide, and high-dose cytarabine) regimen, DA-EPOCH-R (dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab) regimen, or hCVAD/MA (fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with high-dose methotrexate and cytarabine) regimen, and the remaining 10% receiving other, unspecified therapy.
In contrast, 65% of the patients in the international (validation) cohort received CODOX-M/IVAC, 10% and 9%, respectively, received DA-EPOCH-R and hCVAD/MA, and 16% receiving other regimens.
Rituximab was administered to 91% of U.S. and 95% of international patients.
Higher survival rates outside US
Both PFS and OS were higher in the international versus U.S. cohort. At a median follow-up of 45 months, the PFS rate in the United States was 65%, and the OS rate was 70%.
In the international cohort, after a median follow-up of 52 months, the PFS rate was 75%, and the OS rate was 76%, the investigators found.
Reasons for the differences may be because of differences in treatment regimens, socioeconomic and racial disparities in the United States versus other countries, or to decentralized Burkitt lymphoma therapy in the United States, Dr. Olszewski said.
In univariate analysis, factors significantly predictive of worse PFS included age 40 years or older, ECOG performance status 2 or greater, stage 3 or 4 disease, marrow involvement, CNS involvement, LDH more than three times the upper limit of normal, and hemoglobin <11.5 g/dL (P < .001 for all preceding), as well as albumin <3.5 g/dL (P = .001).
“However, the multivariable analysis was more complicated, because many of these factors were overlapping, and most patients with high LDH also had advanced disease, and this group also encompassed patients who had bone marrow and CNS involvement,” he said.
Using the two types of regression analysis mentioned before, investigators identified ECOG performance status 2 or greater (P = .001), age 40 and older (P = .005), LDH greater than three times the upper limit of normal (P < .001) and CNS involvement (P = .002) as significant predictors for worse outcomes in multivariable analysis, and were included in the final model.
“We initially had five groups according to the number of these factors, but we observed that the survival curves for patients with two, three, or four factors were overlapping, and not significantly different, so ultimately we had three risk groups. In the derivation (U.S.) cohort, patients in the low-risk group, with no risk factors, a 3-year PFS of 92%, compared with 72% for patients with one risk factor (intermediate risk), and 53% for patients with two to four risk factors (high risk).
Respective hazard ratios for worse PFS in the low-, intermediate-, and high-risk groups were 1 (reference), 4.15 (95% confidence interval, 1.99-8.68), and 8.83 (95% CI, 4.32-18.03).
Respective HR for worse OS was 1, 7.06 (95% CI, 2.55-19.53), and 15.12 (95% CI, 5.58-40.99).
There were no significant differences in either PFS or OS when either LDH or stage was added into the model.
The BL-IPI was prognostic for PFS and OS in all subgroups, including HIV-positive or -negative patients, those with MYC rearrangements, stage 1 or 2 versus stage 3 or 4, or those treated with rituximab versus those who were not.
As noted before, 3-year PFS rates in the validation cohort for low, intermediate, high-risk groups were 96%, 82%, and 63% respectively, and 3-year OS rates were 99%, 85%, and 64%.
Why the CNS discrepancy?
In the question and answer session following the presentation, comoderator Christopher J. Melani, MD, from the Lymphoid Malignancies Branch at the National Cancer Institute in Bethesda, Md., said that “it was interesting to see the difference between CNS involvement in both the U.S. and the international cohort,” and asked whether Dr. Olszweski could elaborate on whether baseline CNS involvement was assessed by contrast-enhanced MRI of flow cytometry studies of cerebrospinal fluid.
“Could some of these differences between the U.S. and the international cohort be from the baseline assessment differing between the two?” he asked.
Dr. Olszewski replied that the retrospective nature of the data precluded capturing those data, but added that “I do suspect there may be some differences in the way that central nervous system is staged in different countries. In the United States the use of flow cytometry is more commonly employed, but we don’t know how it is used internationally. We do not know how often this is staged radiographically.”
Asked by others who viewed the presentation whether extranodal disease or peripheral blood involvement were prognostic in the final model, Dr. Olszewski replied that “one has to understand that, when one constructs a prognostic index, there is a balance between trying to input as much information as possible and to create something that is useful, clinically meaningful, and accurate.”
He said that, despite trying different models with different factors, “we couldn’t get the discrimination to be much better than the basic model that we ultimately created, so we favored using a more parsimonious model.”
No study funding source was reported. Dr. Olszewski reported research funding from Spectrum Pharmaceuticals, Genentech, TG Therapeutics, and Adaptive Biotechnologies. Dr. Melani reported having no relevant conflicts of interest.
SOURCE: Olszewski AJ et al. ASH 2020, Abstract 705.
A newly devised, validated prognostic tool – the Burkitt Lymphoma International Prognostic Index – can consistently identify low-risk patients who might benefit from treatment de-escalation, and high-risk patients who are unlikely to be cured with current therapies and may require novel approaches, investigators said.
In a cohort of patients treated at international sites, patients with a low-risk score on the BL-IPI had a 3-year progression-free survival (PFS) rate of 96%, and 3-year overall survival rate (OS) of 99%. In contrast, the 3-year PFS rate for patients in the high-risk category was 63%, and the 3-year OS rate was 64%, reported Adam J Olszewski, MD, from the Lifespan Cancer Institute at Rhode Island Hospital and The Miriam Hospital, both in Providence.
“The Burkitt Lymphoma International Prognostic Index – or the ‘BLI-PI’ [‘blippy’] as it was inevitably called – is a novel prognostic index that is specific to Burkitt lymphoma. It has been validated with sufficient calibration and discrimination in external data sets to allow for simple stratification and comparison of risk distribution in geographically diverse cohorts,” he said in an oral abstract presented virtually during the annual meeting of the American Society of Hematology.
Inconsistent criteria
There is a need for a Burkitt-specific index, he said, because of significant differences in age, stage at presentation, and abnormal lactate dehydrogenase (LDH) levels between patients with Burkitt and those with diffuse large B-cell lymphoma (DLBCL), and because historical definitions of “low-risk” Burkitt lymphoma have been inconsistent, with less than 10% of patients falling into this group, leaving the remainder in a undifferentiated “high-risk” category.
“Burkitt lymphoma is considered highly curable, but current therapy requires administration of dose-intense chemoimmunotherapy for which there are many chemotherapy backbone regimens developed across the world, and used mostly locally. These are often studied in phase 2 studies with limited sample sizes, which makes it difficult to compare populations across trials,” Dr. Olszewski said.
A validated prognostic index can help clinicians and researchers compare cohorts and can be used to help design future trials, he added.
To devise the BL-IPI, the investigators first selected a retrospective cohort of 570 adults with Burkitt lymphoma treated at 30 U.S. centers for whom data on outcomes were available.
They determined the best prognostic cutoffs for age, LDH, hemoglobin and albumin levels, and identified independent risk factors using stepwise selection in Cox regression and lasso regression analysis, a machine learning approach. The variables included age; sex; HIV-positivity status; loss of MYC rearrangement; performance status; stage; nodal involvement; marrow involvement; central nervous system involvement; and LDH, hemoglobin, and albumin levels.
For validation, they pooled data from European, Canadian, Australian, and U.K. studies to identify 457 patients for whom retrospective treatment and outcomes data were available.
The derivation and validation cohorts were similar in most respects, expect for a higher proportion of patients with Eastern Cooperative Oncology Group performance status scores of 2 or higher in the validation cohort (22% vs. 35%), and a higher proportion of patients with CNS involvement in the U.S.-based derivation cohort (19% vs. 10%, respectively).
Therapy also differed markedly between the U.S. and international cohorts, with about 30% each of U.S. patients receiving either the CODOX-M/IVAC (cyclophosphamide, vincristine, doxorubicin, high-dose methotrexate/ifosfamide, etoposide, and high-dose cytarabine) regimen, DA-EPOCH-R (dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab) regimen, or hCVAD/MA (fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with high-dose methotrexate and cytarabine) regimen, and the remaining 10% receiving other, unspecified therapy.
In contrast, 65% of the patients in the international (validation) cohort received CODOX-M/IVAC, 10% and 9%, respectively, received DA-EPOCH-R and hCVAD/MA, and 16% receiving other regimens.
Rituximab was administered to 91% of U.S. and 95% of international patients.
Higher survival rates outside US
Both PFS and OS were higher in the international versus U.S. cohort. At a median follow-up of 45 months, the PFS rate in the United States was 65%, and the OS rate was 70%.
In the international cohort, after a median follow-up of 52 months, the PFS rate was 75%, and the OS rate was 76%, the investigators found.
Reasons for the differences may be because of differences in treatment regimens, socioeconomic and racial disparities in the United States versus other countries, or to decentralized Burkitt lymphoma therapy in the United States, Dr. Olszewski said.
In univariate analysis, factors significantly predictive of worse PFS included age 40 years or older, ECOG performance status 2 or greater, stage 3 or 4 disease, marrow involvement, CNS involvement, LDH more than three times the upper limit of normal, and hemoglobin <11.5 g/dL (P < .001 for all preceding), as well as albumin <3.5 g/dL (P = .001).
“However, the multivariable analysis was more complicated, because many of these factors were overlapping, and most patients with high LDH also had advanced disease, and this group also encompassed patients who had bone marrow and CNS involvement,” he said.
Using the two types of regression analysis mentioned before, investigators identified ECOG performance status 2 or greater (P = .001), age 40 and older (P = .005), LDH greater than three times the upper limit of normal (P < .001) and CNS involvement (P = .002) as significant predictors for worse outcomes in multivariable analysis, and were included in the final model.
“We initially had five groups according to the number of these factors, but we observed that the survival curves for patients with two, three, or four factors were overlapping, and not significantly different, so ultimately we had three risk groups. In the derivation (U.S.) cohort, patients in the low-risk group, with no risk factors, a 3-year PFS of 92%, compared with 72% for patients with one risk factor (intermediate risk), and 53% for patients with two to four risk factors (high risk).
Respective hazard ratios for worse PFS in the low-, intermediate-, and high-risk groups were 1 (reference), 4.15 (95% confidence interval, 1.99-8.68), and 8.83 (95% CI, 4.32-18.03).
Respective HR for worse OS was 1, 7.06 (95% CI, 2.55-19.53), and 15.12 (95% CI, 5.58-40.99).
There were no significant differences in either PFS or OS when either LDH or stage was added into the model.
The BL-IPI was prognostic for PFS and OS in all subgroups, including HIV-positive or -negative patients, those with MYC rearrangements, stage 1 or 2 versus stage 3 or 4, or those treated with rituximab versus those who were not.
As noted before, 3-year PFS rates in the validation cohort for low, intermediate, high-risk groups were 96%, 82%, and 63% respectively, and 3-year OS rates were 99%, 85%, and 64%.
Why the CNS discrepancy?
In the question and answer session following the presentation, comoderator Christopher J. Melani, MD, from the Lymphoid Malignancies Branch at the National Cancer Institute in Bethesda, Md., said that “it was interesting to see the difference between CNS involvement in both the U.S. and the international cohort,” and asked whether Dr. Olszweski could elaborate on whether baseline CNS involvement was assessed by contrast-enhanced MRI of flow cytometry studies of cerebrospinal fluid.
“Could some of these differences between the U.S. and the international cohort be from the baseline assessment differing between the two?” he asked.
Dr. Olszewski replied that the retrospective nature of the data precluded capturing those data, but added that “I do suspect there may be some differences in the way that central nervous system is staged in different countries. In the United States the use of flow cytometry is more commonly employed, but we don’t know how it is used internationally. We do not know how often this is staged radiographically.”
Asked by others who viewed the presentation whether extranodal disease or peripheral blood involvement were prognostic in the final model, Dr. Olszewski replied that “one has to understand that, when one constructs a prognostic index, there is a balance between trying to input as much information as possible and to create something that is useful, clinically meaningful, and accurate.”
He said that, despite trying different models with different factors, “we couldn’t get the discrimination to be much better than the basic model that we ultimately created, so we favored using a more parsimonious model.”
No study funding source was reported. Dr. Olszewski reported research funding from Spectrum Pharmaceuticals, Genentech, TG Therapeutics, and Adaptive Biotechnologies. Dr. Melani reported having no relevant conflicts of interest.
SOURCE: Olszewski AJ et al. ASH 2020, Abstract 705.
FROM ASH 2020