User login
COVID-19 drives innovation in addiction treatment
With the onset of the COVID-19 pandemic, there has been a rapid uptick in virtual recovery programs and telemedicine counseling sessions for patients with substance use disorders (SUDs). New research shows that these programs are acceptable and effective alternatives to in-person sessions.
Study results from three research teams at the University of South Carolina School of Medicine Greenville (USCSM-G) show that SUD counselors in the state were satisfied with their experience with telehealth and virtual recovery meetings.
In one of the studies, five counselors who utilized a virtual meeting platform after the COVID-19 pandemic made in-person visits unsafe were surveyed. The respondents said they much preferred in-person meetings. However, they could also see that virtual meetings were filling an important need for their patients.
Two other studies echoed the results from the first. Clinicians who were leery of the new technology at first became more enthusiastic after they gained experience using it.
“We have lived in a society where there has been one right way, which has always been in-person meetings for recovery, such as Alcoholics Anonymous. It is a very structured process,” lead author Haley Fulton, a fourth-year medical student at USCSM-G, said in an interview.
“The onset of COVID really upended a lot of things, but ... now there may not be just one right way for recovery. There are alternatives to offer,” Ms. Fulton said.
The findings were presented at the annual meeting of the American Academy of Addiction Psychiatry, which was held online this year because of the pandemic.
Huge need
“Virtual meetings may not be ideal, but some version of recovery is better than none. If we can make these meetings accessible to more people, this could promote recovery from substance use disorder,” Ms. Fulton said.
There is a huge need for counseling, and past research has shown that failure to attend meetings can precipitate relapse in many individuals.
In Ms. Fulton’s study, counselors were asked to describe how they perceived the efficacy of virtual recovery meetings, compared with that of in-person meetings.
The investigators analyzed how often certain words, phrases, or issues came up during seven in-person recovery meetings held before the COVID-19 pandemic as well as observational data from seven virtual recovery-support meetings held during the pandemic.
On the pro side, the respondents cited convenience, comfort at home, and increased accessibility to counseling for patients.
In addition, because there was no need to travel, virtual meetings were cost effective. Such meetings could expand the recovery world, inasmuch as individuals could attend recovery meetings in other parts of the country.
Perceived disadvantages included challenges involving technology, because learning new apps such as Zoom could be a problem for some patients. Distractions at home and lack of privacy were also cited, but for many, the most important drawback to virtual meetings was the lessening of emotional connection with others.
Impact on SUD treatment
In a second study, another team from USCSM-G reported similar findings when it explored the impact of telehealth on counselors as well as on patients who were undergoing SUD treatment during the pandemic.
Led by fourth-year medical students Elizabeth Whiteside and Kyleigh Connolly, the researchers assessed data from a focus group of six behavioral health counselors representing rural and city agencies throughout South Carolina.
Themes that emerged included concerns about mental health – counselors and patients were experiencing increased stress, depression, and anxiety.
“People had to now home school, there were job layoffs, increased responsibilities at home. Also, Narcan [naloxone] distribution was decreased, and this contributed to rising overdose rates,” Ms. Whiteside said in an interview.
The focus group concluded that the advantages of telehealth included greater ability to accept new patients, an increase in scheduling flexibility, and cost-effectiveness because it obviated the need for child care or transportation.
Disadvantages included problems involving privacy, because for many patients who were undergoing SUD recovery, it was impossible to be alone in a room or a designated area of their own.
“Before COVID happened, [health care] barriers included transport to the actual center and finding care for children,” Ms. Connolly said in an interview.
“That’s where telehealth really bridged the gap for these people, and it actually became a lot easier for them to get in contact with their counselors, get into group meetings, and access other services,” she said.
Many of the study participants were not very optimistic about telehealth at first, Ms. Connolly noted. “They felt a little odd going on telehealth at first, but by the end, everybody said that they loved having it.”
“One of the things that came out often was that patients felt they could be more open and honest because they weren’t looking their counselor right in the face. They didn’t feel so horrible sharing,” Ms. Whiteside added.
Some counselors reported that some clients shared more details with them and that there was an ease of connecting. If a patient was a few minutes late to an appointment, telehealth would put in a call to find out where that patient was.
The counselors also had the ability to determine which of their patients would be good candidates for telehealth counseling and which patients would not do well with telehealth and would instead need in-patient care.
“This is something that really helped the experience go better for the counselors. They were able to determine which patient fit the mold for telehealth working for them. Obviously, patients who have more acute periods of mental health problems would do better with in-person care,” Ms. Whiteside said.
Here to stay?
In the third study from USCSM-G, investigators evaluated data from a focus group of four providers of medications for opioid use disorder (MOUD) who practiced in urban and rural areas throughout the state.
The respondents reflected on their experiences in using telemedicine for prescribing MOUD.
As in the previous studies, the providers had positive experiences with telemedicine. It increased patient access, participation, and satisfaction with treatment, and the benefits of telemedicine outweighed its potential limitations.
Still, technology was cited as a barrier to care, especially in rural areas.
“We found that there was a lack of good internet in certain rural parts of South Carolina, and that lack of the proper electronic devices ... could also make it difficult to access telemedicine,” lead author Kellie Shell said in an interview.
As noted in the other studies, the providers expressed a desire that telemedicine incorporate safeguards that would enable clinicians to identify a particular patient’s location in order that authorities could be dispatched if an emergency were to arise.
The clinicians also said that monitoring for diversion and performing pill counts were more difficult to do via telemedicine.
“We definitely have to improve infrastructure, especially in rural areas, so that all people have access to telemedicine,” Ms. Shell said.
“Overall, the providers were won over with telemedicine, and some predicted telehealth and virtual visits were here to stay, even after COVID,” she added.
The three posters provide useful insight into the potential advantages and disadvantages of telehealth in SUD settings, experts said.
Telehealth data ‘very limited’
Commenting on the research, Lewei (Allison) Lin, MD, University of Michigan, Ann Arbor, noted that “there is such limited information” about the use of telehealth for patients with SUD.
“These insights are helpful for us to start understanding the things that need to be considered, including clinician attitudes and perceptions,” said Dr. Lin, who was not involved with the studies.
“It will be key to have data as use of telemedicine increases during COVID-19 to help us see exactly how it should be used and to better understand the actual impacts and whether or not it is increasing accessibility, and for which patients,” she added.
David Kan, MD, chief medical officer at Bright Heart Health, San Ramon, Calif., has had experience with telehealth for SUD and has found that conducting pill counts with his patients has not been a problem.
“The Shell poster covers telemedicine well,” Dr. Kan said in an interview.
However, “I disagree with their point that diversion prevention is harder via telemedicine. In my experience, it is easier, as you can do pill or wrapper counts almost on demand. You can also do daily observed dosing with pill counts if diversion is suspected,” he said.
Dr. Kan also suggested ways to cope with problems involving privacy. “Privacy concerns are always an issue but can be mitigated with headphones and a scan of the room with the telehealth technology if a privacy concern arises.”
He acknowledged that in-person meetings, especially through well-established programs, such as Alcoholics Anonymous (AA), will always be important. But he pointed out that people are finding ways to meet safely and have in-person connections.
“The AA has been providing virtual recovery meetings long before COVID. The common complaint is the loss of fellowship associated with recovery groups. I don’t know of a way to get around this short of vaccines,” Dr. Kan said. However, “people have adapted impressively with masked outdoor meetings and other forms of safe gathering.”
The investigators, Dr. Lin, and Dr. Kan reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
With the onset of the COVID-19 pandemic, there has been a rapid uptick in virtual recovery programs and telemedicine counseling sessions for patients with substance use disorders (SUDs). New research shows that these programs are acceptable and effective alternatives to in-person sessions.
Study results from three research teams at the University of South Carolina School of Medicine Greenville (USCSM-G) show that SUD counselors in the state were satisfied with their experience with telehealth and virtual recovery meetings.
In one of the studies, five counselors who utilized a virtual meeting platform after the COVID-19 pandemic made in-person visits unsafe were surveyed. The respondents said they much preferred in-person meetings. However, they could also see that virtual meetings were filling an important need for their patients.
Two other studies echoed the results from the first. Clinicians who were leery of the new technology at first became more enthusiastic after they gained experience using it.
“We have lived in a society where there has been one right way, which has always been in-person meetings for recovery, such as Alcoholics Anonymous. It is a very structured process,” lead author Haley Fulton, a fourth-year medical student at USCSM-G, said in an interview.
“The onset of COVID really upended a lot of things, but ... now there may not be just one right way for recovery. There are alternatives to offer,” Ms. Fulton said.
The findings were presented at the annual meeting of the American Academy of Addiction Psychiatry, which was held online this year because of the pandemic.
Huge need
“Virtual meetings may not be ideal, but some version of recovery is better than none. If we can make these meetings accessible to more people, this could promote recovery from substance use disorder,” Ms. Fulton said.
There is a huge need for counseling, and past research has shown that failure to attend meetings can precipitate relapse in many individuals.
In Ms. Fulton’s study, counselors were asked to describe how they perceived the efficacy of virtual recovery meetings, compared with that of in-person meetings.
The investigators analyzed how often certain words, phrases, or issues came up during seven in-person recovery meetings held before the COVID-19 pandemic as well as observational data from seven virtual recovery-support meetings held during the pandemic.
On the pro side, the respondents cited convenience, comfort at home, and increased accessibility to counseling for patients.
In addition, because there was no need to travel, virtual meetings were cost effective. Such meetings could expand the recovery world, inasmuch as individuals could attend recovery meetings in other parts of the country.
Perceived disadvantages included challenges involving technology, because learning new apps such as Zoom could be a problem for some patients. Distractions at home and lack of privacy were also cited, but for many, the most important drawback to virtual meetings was the lessening of emotional connection with others.
Impact on SUD treatment
In a second study, another team from USCSM-G reported similar findings when it explored the impact of telehealth on counselors as well as on patients who were undergoing SUD treatment during the pandemic.
Led by fourth-year medical students Elizabeth Whiteside and Kyleigh Connolly, the researchers assessed data from a focus group of six behavioral health counselors representing rural and city agencies throughout South Carolina.
Themes that emerged included concerns about mental health – counselors and patients were experiencing increased stress, depression, and anxiety.
“People had to now home school, there were job layoffs, increased responsibilities at home. Also, Narcan [naloxone] distribution was decreased, and this contributed to rising overdose rates,” Ms. Whiteside said in an interview.
The focus group concluded that the advantages of telehealth included greater ability to accept new patients, an increase in scheduling flexibility, and cost-effectiveness because it obviated the need for child care or transportation.
Disadvantages included problems involving privacy, because for many patients who were undergoing SUD recovery, it was impossible to be alone in a room or a designated area of their own.
“Before COVID happened, [health care] barriers included transport to the actual center and finding care for children,” Ms. Connolly said in an interview.
“That’s where telehealth really bridged the gap for these people, and it actually became a lot easier for them to get in contact with their counselors, get into group meetings, and access other services,” she said.
Many of the study participants were not very optimistic about telehealth at first, Ms. Connolly noted. “They felt a little odd going on telehealth at first, but by the end, everybody said that they loved having it.”
“One of the things that came out often was that patients felt they could be more open and honest because they weren’t looking their counselor right in the face. They didn’t feel so horrible sharing,” Ms. Whiteside added.
Some counselors reported that some clients shared more details with them and that there was an ease of connecting. If a patient was a few minutes late to an appointment, telehealth would put in a call to find out where that patient was.
The counselors also had the ability to determine which of their patients would be good candidates for telehealth counseling and which patients would not do well with telehealth and would instead need in-patient care.
“This is something that really helped the experience go better for the counselors. They were able to determine which patient fit the mold for telehealth working for them. Obviously, patients who have more acute periods of mental health problems would do better with in-person care,” Ms. Whiteside said.
Here to stay?
In the third study from USCSM-G, investigators evaluated data from a focus group of four providers of medications for opioid use disorder (MOUD) who practiced in urban and rural areas throughout the state.
The respondents reflected on their experiences in using telemedicine for prescribing MOUD.
As in the previous studies, the providers had positive experiences with telemedicine. It increased patient access, participation, and satisfaction with treatment, and the benefits of telemedicine outweighed its potential limitations.
Still, technology was cited as a barrier to care, especially in rural areas.
“We found that there was a lack of good internet in certain rural parts of South Carolina, and that lack of the proper electronic devices ... could also make it difficult to access telemedicine,” lead author Kellie Shell said in an interview.
As noted in the other studies, the providers expressed a desire that telemedicine incorporate safeguards that would enable clinicians to identify a particular patient’s location in order that authorities could be dispatched if an emergency were to arise.
The clinicians also said that monitoring for diversion and performing pill counts were more difficult to do via telemedicine.
“We definitely have to improve infrastructure, especially in rural areas, so that all people have access to telemedicine,” Ms. Shell said.
“Overall, the providers were won over with telemedicine, and some predicted telehealth and virtual visits were here to stay, even after COVID,” she added.
The three posters provide useful insight into the potential advantages and disadvantages of telehealth in SUD settings, experts said.
Telehealth data ‘very limited’
Commenting on the research, Lewei (Allison) Lin, MD, University of Michigan, Ann Arbor, noted that “there is such limited information” about the use of telehealth for patients with SUD.
“These insights are helpful for us to start understanding the things that need to be considered, including clinician attitudes and perceptions,” said Dr. Lin, who was not involved with the studies.
“It will be key to have data as use of telemedicine increases during COVID-19 to help us see exactly how it should be used and to better understand the actual impacts and whether or not it is increasing accessibility, and for which patients,” she added.
David Kan, MD, chief medical officer at Bright Heart Health, San Ramon, Calif., has had experience with telehealth for SUD and has found that conducting pill counts with his patients has not been a problem.
“The Shell poster covers telemedicine well,” Dr. Kan said in an interview.
However, “I disagree with their point that diversion prevention is harder via telemedicine. In my experience, it is easier, as you can do pill or wrapper counts almost on demand. You can also do daily observed dosing with pill counts if diversion is suspected,” he said.
Dr. Kan also suggested ways to cope with problems involving privacy. “Privacy concerns are always an issue but can be mitigated with headphones and a scan of the room with the telehealth technology if a privacy concern arises.”
He acknowledged that in-person meetings, especially through well-established programs, such as Alcoholics Anonymous (AA), will always be important. But he pointed out that people are finding ways to meet safely and have in-person connections.
“The AA has been providing virtual recovery meetings long before COVID. The common complaint is the loss of fellowship associated with recovery groups. I don’t know of a way to get around this short of vaccines,” Dr. Kan said. However, “people have adapted impressively with masked outdoor meetings and other forms of safe gathering.”
The investigators, Dr. Lin, and Dr. Kan reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
With the onset of the COVID-19 pandemic, there has been a rapid uptick in virtual recovery programs and telemedicine counseling sessions for patients with substance use disorders (SUDs). New research shows that these programs are acceptable and effective alternatives to in-person sessions.
Study results from three research teams at the University of South Carolina School of Medicine Greenville (USCSM-G) show that SUD counselors in the state were satisfied with their experience with telehealth and virtual recovery meetings.
In one of the studies, five counselors who utilized a virtual meeting platform after the COVID-19 pandemic made in-person visits unsafe were surveyed. The respondents said they much preferred in-person meetings. However, they could also see that virtual meetings were filling an important need for their patients.
Two other studies echoed the results from the first. Clinicians who were leery of the new technology at first became more enthusiastic after they gained experience using it.
“We have lived in a society where there has been one right way, which has always been in-person meetings for recovery, such as Alcoholics Anonymous. It is a very structured process,” lead author Haley Fulton, a fourth-year medical student at USCSM-G, said in an interview.
“The onset of COVID really upended a lot of things, but ... now there may not be just one right way for recovery. There are alternatives to offer,” Ms. Fulton said.
The findings were presented at the annual meeting of the American Academy of Addiction Psychiatry, which was held online this year because of the pandemic.
Huge need
“Virtual meetings may not be ideal, but some version of recovery is better than none. If we can make these meetings accessible to more people, this could promote recovery from substance use disorder,” Ms. Fulton said.
There is a huge need for counseling, and past research has shown that failure to attend meetings can precipitate relapse in many individuals.
In Ms. Fulton’s study, counselors were asked to describe how they perceived the efficacy of virtual recovery meetings, compared with that of in-person meetings.
The investigators analyzed how often certain words, phrases, or issues came up during seven in-person recovery meetings held before the COVID-19 pandemic as well as observational data from seven virtual recovery-support meetings held during the pandemic.
On the pro side, the respondents cited convenience, comfort at home, and increased accessibility to counseling for patients.
In addition, because there was no need to travel, virtual meetings were cost effective. Such meetings could expand the recovery world, inasmuch as individuals could attend recovery meetings in other parts of the country.
Perceived disadvantages included challenges involving technology, because learning new apps such as Zoom could be a problem for some patients. Distractions at home and lack of privacy were also cited, but for many, the most important drawback to virtual meetings was the lessening of emotional connection with others.
Impact on SUD treatment
In a second study, another team from USCSM-G reported similar findings when it explored the impact of telehealth on counselors as well as on patients who were undergoing SUD treatment during the pandemic.
Led by fourth-year medical students Elizabeth Whiteside and Kyleigh Connolly, the researchers assessed data from a focus group of six behavioral health counselors representing rural and city agencies throughout South Carolina.
Themes that emerged included concerns about mental health – counselors and patients were experiencing increased stress, depression, and anxiety.
“People had to now home school, there were job layoffs, increased responsibilities at home. Also, Narcan [naloxone] distribution was decreased, and this contributed to rising overdose rates,” Ms. Whiteside said in an interview.
The focus group concluded that the advantages of telehealth included greater ability to accept new patients, an increase in scheduling flexibility, and cost-effectiveness because it obviated the need for child care or transportation.
Disadvantages included problems involving privacy, because for many patients who were undergoing SUD recovery, it was impossible to be alone in a room or a designated area of their own.
“Before COVID happened, [health care] barriers included transport to the actual center and finding care for children,” Ms. Connolly said in an interview.
“That’s where telehealth really bridged the gap for these people, and it actually became a lot easier for them to get in contact with their counselors, get into group meetings, and access other services,” she said.
Many of the study participants were not very optimistic about telehealth at first, Ms. Connolly noted. “They felt a little odd going on telehealth at first, but by the end, everybody said that they loved having it.”
“One of the things that came out often was that patients felt they could be more open and honest because they weren’t looking their counselor right in the face. They didn’t feel so horrible sharing,” Ms. Whiteside added.
Some counselors reported that some clients shared more details with them and that there was an ease of connecting. If a patient was a few minutes late to an appointment, telehealth would put in a call to find out where that patient was.
The counselors also had the ability to determine which of their patients would be good candidates for telehealth counseling and which patients would not do well with telehealth and would instead need in-patient care.
“This is something that really helped the experience go better for the counselors. They were able to determine which patient fit the mold for telehealth working for them. Obviously, patients who have more acute periods of mental health problems would do better with in-person care,” Ms. Whiteside said.
Here to stay?
In the third study from USCSM-G, investigators evaluated data from a focus group of four providers of medications for opioid use disorder (MOUD) who practiced in urban and rural areas throughout the state.
The respondents reflected on their experiences in using telemedicine for prescribing MOUD.
As in the previous studies, the providers had positive experiences with telemedicine. It increased patient access, participation, and satisfaction with treatment, and the benefits of telemedicine outweighed its potential limitations.
Still, technology was cited as a barrier to care, especially in rural areas.
“We found that there was a lack of good internet in certain rural parts of South Carolina, and that lack of the proper electronic devices ... could also make it difficult to access telemedicine,” lead author Kellie Shell said in an interview.
As noted in the other studies, the providers expressed a desire that telemedicine incorporate safeguards that would enable clinicians to identify a particular patient’s location in order that authorities could be dispatched if an emergency were to arise.
The clinicians also said that monitoring for diversion and performing pill counts were more difficult to do via telemedicine.
“We definitely have to improve infrastructure, especially in rural areas, so that all people have access to telemedicine,” Ms. Shell said.
“Overall, the providers were won over with telemedicine, and some predicted telehealth and virtual visits were here to stay, even after COVID,” she added.
The three posters provide useful insight into the potential advantages and disadvantages of telehealth in SUD settings, experts said.
Telehealth data ‘very limited’
Commenting on the research, Lewei (Allison) Lin, MD, University of Michigan, Ann Arbor, noted that “there is such limited information” about the use of telehealth for patients with SUD.
“These insights are helpful for us to start understanding the things that need to be considered, including clinician attitudes and perceptions,” said Dr. Lin, who was not involved with the studies.
“It will be key to have data as use of telemedicine increases during COVID-19 to help us see exactly how it should be used and to better understand the actual impacts and whether or not it is increasing accessibility, and for which patients,” she added.
David Kan, MD, chief medical officer at Bright Heart Health, San Ramon, Calif., has had experience with telehealth for SUD and has found that conducting pill counts with his patients has not been a problem.
“The Shell poster covers telemedicine well,” Dr. Kan said in an interview.
However, “I disagree with their point that diversion prevention is harder via telemedicine. In my experience, it is easier, as you can do pill or wrapper counts almost on demand. You can also do daily observed dosing with pill counts if diversion is suspected,” he said.
Dr. Kan also suggested ways to cope with problems involving privacy. “Privacy concerns are always an issue but can be mitigated with headphones and a scan of the room with the telehealth technology if a privacy concern arises.”
He acknowledged that in-person meetings, especially through well-established programs, such as Alcoholics Anonymous (AA), will always be important. But he pointed out that people are finding ways to meet safely and have in-person connections.
“The AA has been providing virtual recovery meetings long before COVID. The common complaint is the loss of fellowship associated with recovery groups. I don’t know of a way to get around this short of vaccines,” Dr. Kan said. However, “people have adapted impressively with masked outdoor meetings and other forms of safe gathering.”
The investigators, Dr. Lin, and Dr. Kan reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A girl presents with blotchy, slightly itchy spots on her chest, back
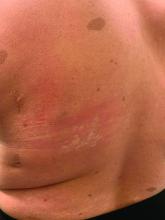
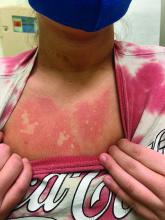
On close evaluation of the picture on her chest, she has pale macules and patches surrounded by erythematous ill-defined patches consistent with nevus anemicus. The findings of the picture raise the suspicion for neurofibromatosis, and it was recommended for her to be evaluated in person.
She comes several days later to the clinic. The caretaker, who is her aunt, reports she does not know much of the girl’s medical history as she recently moved from South America to live with her. The girl is a very nice and pleasant 8-year-old. She reports noticing the spots on her chest for about a year and that they seem to get a little itchier and more noticeable when she is hot or when she is running. She also reports increasing headaches for several months. She is being home schooled, and according to her aunt she is at par with her cousins who are about the same age. There is no history of seizures. She has had back surgery in the past. There is no history of hypertension. There is no family history of any genetic disorder or similar lesions.
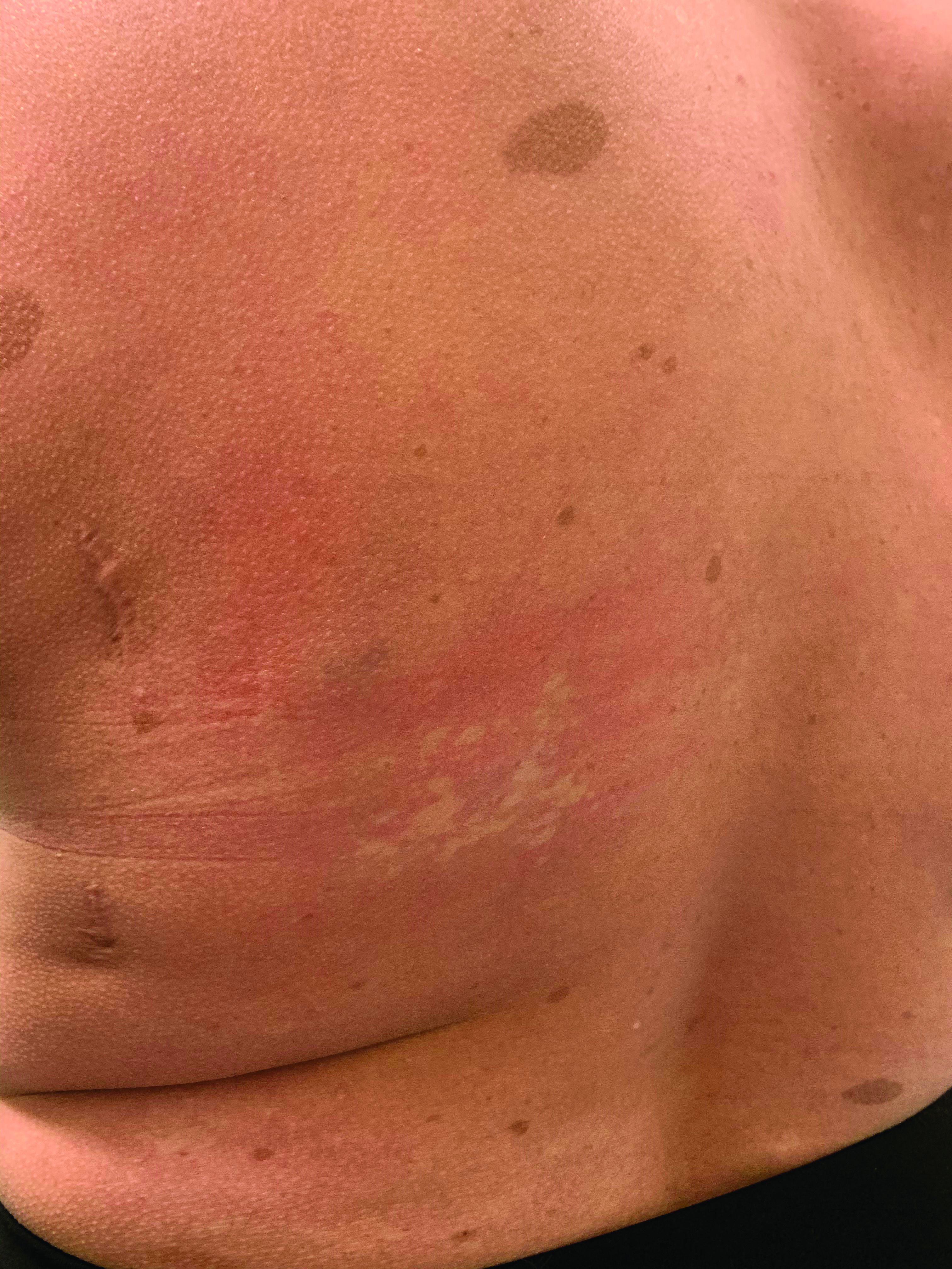
On physical exam, her vital signs are normal, but her head circumference is over the 90th percentile. She is pleasant and interactive. On skin examination, she has slightly noticeable pale macules and patches on the chest and back that become more apparent after rubbing her skin. She has multiple light brown macules and oval patches on the chest, back, and neck. She has no axillary or inguinal freckling. She has scars on the back from her prior surgery.
As she was having worsening headaches, an MRI of the brain was ordered, which showed a left optic glioma. She was then referred to ophthalmology, neurology, and genetics.
Neurofibromatosis type 1 (NF1) is a common genetic autosomal dominant disorder cause by mutations on the NF1 gene on chromosome 17, which encodes for the protein neurofibromin. This protein works in the Ras-mitogen–activated protein kinase pathway as a negative regulator. Based on the National Institute of Health criteria, children need two or more of the following to be diagnosed with NF1: more than six café au lait macules larger than 5 mm in prepubescent children and 2.5 cm after puberty; axillary or inguinal freckling; two or more Lisch nodules; optic gliomas; two or more neurofibromas or one plexiform neurofibroma; or a first degree relative with a diagnosis of NF1. With these criteria, about 70% of the children can be diagnosed before the age of 1 year.1
Nevus anemicus is an uncommon birthmark, sometimes overlooked, that is characterized by pale, hypopigmented, well-defined macules and patches that do not turn red after trauma or changes in temperature. Nevus anemicus is usually localized on the torso but can be seen on the face, neck, and extremities. These lesions are present in 1%-2% of the general population. They are thought to occur because of increased sensitivity of the affected blood vessels to catecholamines, which causes permanent vasoconstriction, which leads to hypopigmentation on the area.2 These lesions are usually present at birth and have been described in patients with tuberous sclerosis, neurofibromatosis, and phakomatosis pigmentovascularis.
Recent studies of patients with neurofibromatosis and other RASopathies have noticed that nevus anemicus is present in about 8.8%-51% of the patients studied with a diagnosis NF1, compared with only 2% of the controls.3,4 The studies failed to report any cases of nevus anemicus in patients with other RASopathies associated with café au lait macules. Bulteel and colleagues recently reported two cases of non-NF1 RASopathies also associated with nevus anemicus in a patient with Legius syndrome and a patient with Noonan syndrome with multiple lentigines.5 The nevus anemicus was reported to occur most commonly on the anterior chest and be multiple, as seen in our patient.
The authors of the published studies advocate for the introduction of nevus anemicus as part of the diagnostic criteria for NF1, especially because it can be an early finding seen in babies, which can aid in early diagnosis of NF1.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. She has no relevant financial disclosures. Email Dr. Matiz at [email protected].
References
1. Pediatrics. 2000 Mar. doi: 10.1542/peds.105.3.608.
2. Nevus Anemicus. StatPearls [Internet] (Treasure Island, Fla.: StatPearls Publishing; 2020 Jan).
3. J Am Acad Dermatol. 2013 Nov. doi: 10.1016/j.jaad.2013.06.039.
4. Pediatr Dermatol. 2015 May-Jun. doi: 10.1111/pde.12525.
5. JAAD Case Rep. 2018 Apr 5. doi: 10.1016/j.jdcr.2017.09.037.
On close evaluation of the picture on her chest, she has pale macules and patches surrounded by erythematous ill-defined patches consistent with nevus anemicus. The findings of the picture raise the suspicion for neurofibromatosis, and it was recommended for her to be evaluated in person.
She comes several days later to the clinic. The caretaker, who is her aunt, reports she does not know much of the girl’s medical history as she recently moved from South America to live with her. The girl is a very nice and pleasant 8-year-old. She reports noticing the spots on her chest for about a year and that they seem to get a little itchier and more noticeable when she is hot or when she is running. She also reports increasing headaches for several months. She is being home schooled, and according to her aunt she is at par with her cousins who are about the same age. There is no history of seizures. She has had back surgery in the past. There is no history of hypertension. There is no family history of any genetic disorder or similar lesions.
On physical exam, her vital signs are normal, but her head circumference is over the 90th percentile. She is pleasant and interactive. On skin examination, she has slightly noticeable pale macules and patches on the chest and back that become more apparent after rubbing her skin. She has multiple light brown macules and oval patches on the chest, back, and neck. She has no axillary or inguinal freckling. She has scars on the back from her prior surgery.
As she was having worsening headaches, an MRI of the brain was ordered, which showed a left optic glioma. She was then referred to ophthalmology, neurology, and genetics.
Neurofibromatosis type 1 (NF1) is a common genetic autosomal dominant disorder cause by mutations on the NF1 gene on chromosome 17, which encodes for the protein neurofibromin. This protein works in the Ras-mitogen–activated protein kinase pathway as a negative regulator. Based on the National Institute of Health criteria, children need two or more of the following to be diagnosed with NF1: more than six café au lait macules larger than 5 mm in prepubescent children and 2.5 cm after puberty; axillary or inguinal freckling; two or more Lisch nodules; optic gliomas; two or more neurofibromas or one plexiform neurofibroma; or a first degree relative with a diagnosis of NF1. With these criteria, about 70% of the children can be diagnosed before the age of 1 year.1
Nevus anemicus is an uncommon birthmark, sometimes overlooked, that is characterized by pale, hypopigmented, well-defined macules and patches that do not turn red after trauma or changes in temperature. Nevus anemicus is usually localized on the torso but can be seen on the face, neck, and extremities. These lesions are present in 1%-2% of the general population. They are thought to occur because of increased sensitivity of the affected blood vessels to catecholamines, which causes permanent vasoconstriction, which leads to hypopigmentation on the area.2 These lesions are usually present at birth and have been described in patients with tuberous sclerosis, neurofibromatosis, and phakomatosis pigmentovascularis.
Recent studies of patients with neurofibromatosis and other RASopathies have noticed that nevus anemicus is present in about 8.8%-51% of the patients studied with a diagnosis NF1, compared with only 2% of the controls.3,4 The studies failed to report any cases of nevus anemicus in patients with other RASopathies associated with café au lait macules. Bulteel and colleagues recently reported two cases of non-NF1 RASopathies also associated with nevus anemicus in a patient with Legius syndrome and a patient with Noonan syndrome with multiple lentigines.5 The nevus anemicus was reported to occur most commonly on the anterior chest and be multiple, as seen in our patient.
The authors of the published studies advocate for the introduction of nevus anemicus as part of the diagnostic criteria for NF1, especially because it can be an early finding seen in babies, which can aid in early diagnosis of NF1.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. She has no relevant financial disclosures. Email Dr. Matiz at [email protected].
References
1. Pediatrics. 2000 Mar. doi: 10.1542/peds.105.3.608.
2. Nevus Anemicus. StatPearls [Internet] (Treasure Island, Fla.: StatPearls Publishing; 2020 Jan).
3. J Am Acad Dermatol. 2013 Nov. doi: 10.1016/j.jaad.2013.06.039.
4. Pediatr Dermatol. 2015 May-Jun. doi: 10.1111/pde.12525.
5. JAAD Case Rep. 2018 Apr 5. doi: 10.1016/j.jdcr.2017.09.037.
On close evaluation of the picture on her chest, she has pale macules and patches surrounded by erythematous ill-defined patches consistent with nevus anemicus. The findings of the picture raise the suspicion for neurofibromatosis, and it was recommended for her to be evaluated in person.
She comes several days later to the clinic. The caretaker, who is her aunt, reports she does not know much of the girl’s medical history as she recently moved from South America to live with her. The girl is a very nice and pleasant 8-year-old. She reports noticing the spots on her chest for about a year and that they seem to get a little itchier and more noticeable when she is hot or when she is running. She also reports increasing headaches for several months. She is being home schooled, and according to her aunt she is at par with her cousins who are about the same age. There is no history of seizures. She has had back surgery in the past. There is no history of hypertension. There is no family history of any genetic disorder or similar lesions.
On physical exam, her vital signs are normal, but her head circumference is over the 90th percentile. She is pleasant and interactive. On skin examination, she has slightly noticeable pale macules and patches on the chest and back that become more apparent after rubbing her skin. She has multiple light brown macules and oval patches on the chest, back, and neck. She has no axillary or inguinal freckling. She has scars on the back from her prior surgery.
As she was having worsening headaches, an MRI of the brain was ordered, which showed a left optic glioma. She was then referred to ophthalmology, neurology, and genetics.
Neurofibromatosis type 1 (NF1) is a common genetic autosomal dominant disorder cause by mutations on the NF1 gene on chromosome 17, which encodes for the protein neurofibromin. This protein works in the Ras-mitogen–activated protein kinase pathway as a negative regulator. Based on the National Institute of Health criteria, children need two or more of the following to be diagnosed with NF1: more than six café au lait macules larger than 5 mm in prepubescent children and 2.5 cm after puberty; axillary or inguinal freckling; two or more Lisch nodules; optic gliomas; two or more neurofibromas or one plexiform neurofibroma; or a first degree relative with a diagnosis of NF1. With these criteria, about 70% of the children can be diagnosed before the age of 1 year.1
Nevus anemicus is an uncommon birthmark, sometimes overlooked, that is characterized by pale, hypopigmented, well-defined macules and patches that do not turn red after trauma or changes in temperature. Nevus anemicus is usually localized on the torso but can be seen on the face, neck, and extremities. These lesions are present in 1%-2% of the general population. They are thought to occur because of increased sensitivity of the affected blood vessels to catecholamines, which causes permanent vasoconstriction, which leads to hypopigmentation on the area.2 These lesions are usually present at birth and have been described in patients with tuberous sclerosis, neurofibromatosis, and phakomatosis pigmentovascularis.
Recent studies of patients with neurofibromatosis and other RASopathies have noticed that nevus anemicus is present in about 8.8%-51% of the patients studied with a diagnosis NF1, compared with only 2% of the controls.3,4 The studies failed to report any cases of nevus anemicus in patients with other RASopathies associated with café au lait macules. Bulteel and colleagues recently reported two cases of non-NF1 RASopathies also associated with nevus anemicus in a patient with Legius syndrome and a patient with Noonan syndrome with multiple lentigines.5 The nevus anemicus was reported to occur most commonly on the anterior chest and be multiple, as seen in our patient.
The authors of the published studies advocate for the introduction of nevus anemicus as part of the diagnostic criteria for NF1, especially because it can be an early finding seen in babies, which can aid in early diagnosis of NF1.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. She has no relevant financial disclosures. Email Dr. Matiz at [email protected].
References
1. Pediatrics. 2000 Mar. doi: 10.1542/peds.105.3.608.
2. Nevus Anemicus. StatPearls [Internet] (Treasure Island, Fla.: StatPearls Publishing; 2020 Jan).
3. J Am Acad Dermatol. 2013 Nov. doi: 10.1016/j.jaad.2013.06.039.
4. Pediatr Dermatol. 2015 May-Jun. doi: 10.1111/pde.12525.
5. JAAD Case Rep. 2018 Apr 5. doi: 10.1016/j.jdcr.2017.09.037.
Study found dual-targeted CAR T highly active against relapsed/refractory multiple myeloma
An investigational chimeric antigen receptor T-cell (CAR T-cell) construct targeting two antigens on multiple myeloma cells showed promise in a first-in-humans trial, investigators said.
Among 16 patients with relapsed/refractory, heavily pretreated multiple myeloma who received the dual-targeting construct GC012F, the overall response rate was 93.8%, and all of six patients who received the cells at the highest of three dose levels had stringent complete responses (sCR) and were negative for minimal residual disease (MRD) at 6 months follow-up, reported Weijun Fu, MD, PhD, from Shanghai (China) Changzheng Hospital in an oral abstract presented during the virtual American Society of Hematology annual meeting.
GC012F is a novel CAR-T cell platform targeting both the B-cell maturation antigen (BCMA), which is universally expressed on malignant plasma cells, and CD19, which is expressed on both multiple myeloma cells and progenitors, Dr. Fu said.
“Targeting CD19 can trigger elimination of malignant cells by CAR T. Our preclinical work demonstrated more effective elimination of multiple myeloma clone-forming cells by BCMA and CD19 dual CAR T, so targeting both BCMA and CD19 antigens could improve efficacy and reduce relapse,” he said.
The construct is created using the FasTCAR platform that, according to manufacturer Gracell Biotechnologies (Shanghai), allows for cell culturing and expansion within 24-36 hours, rather than 2-3 weeks required for other CAR T-cell products.
Investigator-initiated trial
In a phase 1 investigator-initiated trial, 16 patients with a median age of 56 (range 27-71) years were enrolled. The patients all had relapsed or refractory multiple myeloma according to 2016 International Myeloma Working Group criteria, with a life expectancy of at least 3 months and adequate organ function.
The median time since diagnosis was 3 years (range 1-10). All but one of the 16 patients had high-risk disease, 3 had double-hit disease (the presence of two deletions, gain of function, or p53 mutation), and 5 patients had one or more extramedullary plasmacytomas. Four of the patients had received therapy with an anti-CD38 monoclonal antibody.
Following lymphodepletion with fludarabine and cyclophosphamide, the patients received the CAR T cells in a single infusion at dose levels of either 1, 2, or 3 times 105 cells/kg.
As of the cutoff date in July 2020, 15 of the 16 patients had a clinical response, including 9 with a CR or sCR, and 6 with a very good partial response (VGPR). As noted before, all of the six patients treated at the highest dose level had a sCR. At the median follow-up of 7.3 months, the median duration of response had not been reached.
Among all patients evaluable for response at month 1 (14 patients), 11 were MRD negative by flow cytometry. At month 3 all 11 evaluable patients were MRD negative, and all of 10 patients evaluable at 6 months were also MRD negative.
As with other CAR T-cell constructs, all patients developed the cytokine-release syndrome (CRS), with grade 1 or 2 severity in 14 patients, and grade 3 in 2 patients. The median time to onset of CRS was 6 days (range 2-10), and the median duration was 4 days (range 1-8 days).
No cases of immune effector cell–associated neurotoxicity syndrome (ICANS) were observed.
One patient treated at the middle dose level presented with fever and died shortly after day 78 of an unknown cause during the COVID-19 pandemic. Two patients died of extramedullary disease; each had achieved MRD negativity.
Investigators continue to follow the patients and are enrolling new patients in the ongoing study.
‘Interesting approach’
Sandy W. Wong, MD, from the Helen Diller Family Comprehensive Cancer Center at the University of California San Francisco, who was not involved in the study, said in an interview that the dual-targeted approach is interesting, in light of a case report presented at ASH 2020 of a patient with multiple myeloma who had a partial response to CAR T-cell therapy with a different construct and who developed a subsequent biallelic loss of BCMA that resulted in resistance to CAR T-cell therapy.
“This raises the idea that, if we perhaps had a dual-targeted CAR T, perhaps we will prolong progression-free survival, in order to avoid antigen escape. So I do think the concept is very interesting and does deserve further study,” she said.
CD19 is thought to be expressed on myeloma stem cells, “so the question is: Are patients not being cured because there is a reservoir of myeloma cells, and targeting CD19 is thought to get at this putative myeloma stem cell? but that remains to be seen,” she added.
Dr. Wong comoderated the session where Dr. Fu presented the data.
The study was supported by participating medical centers and Gracell Biotechnologies. Dr. Fu and Dr. Wong reported no relevant conflicts of interest to disclose.
SOURCE: Jiang H et al. ASH 2020, Abstract 178.
An investigational chimeric antigen receptor T-cell (CAR T-cell) construct targeting two antigens on multiple myeloma cells showed promise in a first-in-humans trial, investigators said.
Among 16 patients with relapsed/refractory, heavily pretreated multiple myeloma who received the dual-targeting construct GC012F, the overall response rate was 93.8%, and all of six patients who received the cells at the highest of three dose levels had stringent complete responses (sCR) and were negative for minimal residual disease (MRD) at 6 months follow-up, reported Weijun Fu, MD, PhD, from Shanghai (China) Changzheng Hospital in an oral abstract presented during the virtual American Society of Hematology annual meeting.
GC012F is a novel CAR-T cell platform targeting both the B-cell maturation antigen (BCMA), which is universally expressed on malignant plasma cells, and CD19, which is expressed on both multiple myeloma cells and progenitors, Dr. Fu said.
“Targeting CD19 can trigger elimination of malignant cells by CAR T. Our preclinical work demonstrated more effective elimination of multiple myeloma clone-forming cells by BCMA and CD19 dual CAR T, so targeting both BCMA and CD19 antigens could improve efficacy and reduce relapse,” he said.
The construct is created using the FasTCAR platform that, according to manufacturer Gracell Biotechnologies (Shanghai), allows for cell culturing and expansion within 24-36 hours, rather than 2-3 weeks required for other CAR T-cell products.
Investigator-initiated trial
In a phase 1 investigator-initiated trial, 16 patients with a median age of 56 (range 27-71) years were enrolled. The patients all had relapsed or refractory multiple myeloma according to 2016 International Myeloma Working Group criteria, with a life expectancy of at least 3 months and adequate organ function.
The median time since diagnosis was 3 years (range 1-10). All but one of the 16 patients had high-risk disease, 3 had double-hit disease (the presence of two deletions, gain of function, or p53 mutation), and 5 patients had one or more extramedullary plasmacytomas. Four of the patients had received therapy with an anti-CD38 monoclonal antibody.
Following lymphodepletion with fludarabine and cyclophosphamide, the patients received the CAR T cells in a single infusion at dose levels of either 1, 2, or 3 times 105 cells/kg.
As of the cutoff date in July 2020, 15 of the 16 patients had a clinical response, including 9 with a CR or sCR, and 6 with a very good partial response (VGPR). As noted before, all of the six patients treated at the highest dose level had a sCR. At the median follow-up of 7.3 months, the median duration of response had not been reached.
Among all patients evaluable for response at month 1 (14 patients), 11 were MRD negative by flow cytometry. At month 3 all 11 evaluable patients were MRD negative, and all of 10 patients evaluable at 6 months were also MRD negative.
As with other CAR T-cell constructs, all patients developed the cytokine-release syndrome (CRS), with grade 1 or 2 severity in 14 patients, and grade 3 in 2 patients. The median time to onset of CRS was 6 days (range 2-10), and the median duration was 4 days (range 1-8 days).
No cases of immune effector cell–associated neurotoxicity syndrome (ICANS) were observed.
One patient treated at the middle dose level presented with fever and died shortly after day 78 of an unknown cause during the COVID-19 pandemic. Two patients died of extramedullary disease; each had achieved MRD negativity.
Investigators continue to follow the patients and are enrolling new patients in the ongoing study.
‘Interesting approach’
Sandy W. Wong, MD, from the Helen Diller Family Comprehensive Cancer Center at the University of California San Francisco, who was not involved in the study, said in an interview that the dual-targeted approach is interesting, in light of a case report presented at ASH 2020 of a patient with multiple myeloma who had a partial response to CAR T-cell therapy with a different construct and who developed a subsequent biallelic loss of BCMA that resulted in resistance to CAR T-cell therapy.
“This raises the idea that, if we perhaps had a dual-targeted CAR T, perhaps we will prolong progression-free survival, in order to avoid antigen escape. So I do think the concept is very interesting and does deserve further study,” she said.
CD19 is thought to be expressed on myeloma stem cells, “so the question is: Are patients not being cured because there is a reservoir of myeloma cells, and targeting CD19 is thought to get at this putative myeloma stem cell? but that remains to be seen,” she added.
Dr. Wong comoderated the session where Dr. Fu presented the data.
The study was supported by participating medical centers and Gracell Biotechnologies. Dr. Fu and Dr. Wong reported no relevant conflicts of interest to disclose.
SOURCE: Jiang H et al. ASH 2020, Abstract 178.
An investigational chimeric antigen receptor T-cell (CAR T-cell) construct targeting two antigens on multiple myeloma cells showed promise in a first-in-humans trial, investigators said.
Among 16 patients with relapsed/refractory, heavily pretreated multiple myeloma who received the dual-targeting construct GC012F, the overall response rate was 93.8%, and all of six patients who received the cells at the highest of three dose levels had stringent complete responses (sCR) and were negative for minimal residual disease (MRD) at 6 months follow-up, reported Weijun Fu, MD, PhD, from Shanghai (China) Changzheng Hospital in an oral abstract presented during the virtual American Society of Hematology annual meeting.
GC012F is a novel CAR-T cell platform targeting both the B-cell maturation antigen (BCMA), which is universally expressed on malignant plasma cells, and CD19, which is expressed on both multiple myeloma cells and progenitors, Dr. Fu said.
“Targeting CD19 can trigger elimination of malignant cells by CAR T. Our preclinical work demonstrated more effective elimination of multiple myeloma clone-forming cells by BCMA and CD19 dual CAR T, so targeting both BCMA and CD19 antigens could improve efficacy and reduce relapse,” he said.
The construct is created using the FasTCAR platform that, according to manufacturer Gracell Biotechnologies (Shanghai), allows for cell culturing and expansion within 24-36 hours, rather than 2-3 weeks required for other CAR T-cell products.
Investigator-initiated trial
In a phase 1 investigator-initiated trial, 16 patients with a median age of 56 (range 27-71) years were enrolled. The patients all had relapsed or refractory multiple myeloma according to 2016 International Myeloma Working Group criteria, with a life expectancy of at least 3 months and adequate organ function.
The median time since diagnosis was 3 years (range 1-10). All but one of the 16 patients had high-risk disease, 3 had double-hit disease (the presence of two deletions, gain of function, or p53 mutation), and 5 patients had one or more extramedullary plasmacytomas. Four of the patients had received therapy with an anti-CD38 monoclonal antibody.
Following lymphodepletion with fludarabine and cyclophosphamide, the patients received the CAR T cells in a single infusion at dose levels of either 1, 2, or 3 times 105 cells/kg.
As of the cutoff date in July 2020, 15 of the 16 patients had a clinical response, including 9 with a CR or sCR, and 6 with a very good partial response (VGPR). As noted before, all of the six patients treated at the highest dose level had a sCR. At the median follow-up of 7.3 months, the median duration of response had not been reached.
Among all patients evaluable for response at month 1 (14 patients), 11 were MRD negative by flow cytometry. At month 3 all 11 evaluable patients were MRD negative, and all of 10 patients evaluable at 6 months were also MRD negative.
As with other CAR T-cell constructs, all patients developed the cytokine-release syndrome (CRS), with grade 1 or 2 severity in 14 patients, and grade 3 in 2 patients. The median time to onset of CRS was 6 days (range 2-10), and the median duration was 4 days (range 1-8 days).
No cases of immune effector cell–associated neurotoxicity syndrome (ICANS) were observed.
One patient treated at the middle dose level presented with fever and died shortly after day 78 of an unknown cause during the COVID-19 pandemic. Two patients died of extramedullary disease; each had achieved MRD negativity.
Investigators continue to follow the patients and are enrolling new patients in the ongoing study.
‘Interesting approach’
Sandy W. Wong, MD, from the Helen Diller Family Comprehensive Cancer Center at the University of California San Francisco, who was not involved in the study, said in an interview that the dual-targeted approach is interesting, in light of a case report presented at ASH 2020 of a patient with multiple myeloma who had a partial response to CAR T-cell therapy with a different construct and who developed a subsequent biallelic loss of BCMA that resulted in resistance to CAR T-cell therapy.
“This raises the idea that, if we perhaps had a dual-targeted CAR T, perhaps we will prolong progression-free survival, in order to avoid antigen escape. So I do think the concept is very interesting and does deserve further study,” she said.
CD19 is thought to be expressed on myeloma stem cells, “so the question is: Are patients not being cured because there is a reservoir of myeloma cells, and targeting CD19 is thought to get at this putative myeloma stem cell? but that remains to be seen,” she added.
Dr. Wong comoderated the session where Dr. Fu presented the data.
The study was supported by participating medical centers and Gracell Biotechnologies. Dr. Fu and Dr. Wong reported no relevant conflicts of interest to disclose.
SOURCE: Jiang H et al. ASH 2020, Abstract 178.
FROM ASH 2020
Kennedy, NIMH demand urgent action on COVID-19 mental health toll
A public-private partnership, led by mental health advocate Patrick Kennedy and the head of the National Institute of Mental Health, Joshua Gordon, MD, PhD, want urgent action to address the wave of mental illness and suicide caused by COVID-19.
“Our country is in serious denial about the full impact of mental health in this country and certainly as part of this pandemic,” said former congressman Mr. Kennedy, cochair of the Action Alliance’s Mental Health & Suicide Prevention National Response to COVID-19, at a briefing unveiling the group’s new six-priority Action Plan.
“That’s reinforced when all we hear from is Dr. Fauci,” and only about the physical effects of the disease, said Mr. Kennedy, the founder of the Kennedy Forum, a nonprofit dedicated to changing the health system’s approach to mental health and substance use disorders.
“ he said. Mr. Kennedy noted the huge effort to speed therapeutics and vaccines to the American public. “We need to bring that same sense of urgency to these deaths of despair hiding in plain sight.”
Dr. Gordon, NIMH’s director and a cochair of the National Response group, was also at the briefing.
“We know many people report experiencing symptoms of distress, including anxiety, sleep problems, depression, substance use, and suicidal thoughts at rates two to three times higher than we might expect in times before the pandemic. Just as the country has come together to mitigate the physical impacts of pandemic, we also have to identify how to mitigate the mental health impacts,” said Dr. Gordon.
Plan of action
Mr. Kennedy emphasized that it is crucial that federal lawmakers and regulators find a way to increase parity between mental and physical health.
Paramount in that effort would be ensuring stronger enforcement of the Mental Health Parity and Addiction Equity Act, he said.
That 1996 law requires health plans to ensure that benefits for physical and mental health were equivalent, but it has frequently been ignored. In 2019, a U.S. federal court found that one of the nation’s largest behavioral health insurers, United Behavioral Health, had been violating the law. Mr. Kennedy said he expects this decision to continue to have a positive impact on achieving parity.
In November, United was ordered by a federal judge to reprocess 67,000 claims that it illegally denied.
The Alliance’s Action Plan has six priorities:
- Change the national conversation about mental health and suicide.
- Increase access to evidence-based treatments for substance use and mental health disorders in specialty and primary care, and include better reimbursement for services and make permanent reimbursement for telehealth services.
- Increase the use of nonpunitive and supportive crisis intervention services, including keeping people out of the criminal justice system.
- Establish near real-time data collection systems to promptly identify changes in rates of suicide, overdose, and other key events, and of clusters or spikes.
- Ensure the equitable delivery of comprehensive and effective suicide prevention and mental health services for Black Americans, Latin Americans, American Indian/Alaskan Natives, LGBTQ individuals, and others disproportionately impacted by the pandemic.
- Invest in prevention and early intervention approaches that treat the root causes of suicide and mental health problems.
Uptick in distress
Dr. Gordon noted that recent data indicate that, although ED visits for children are still down in 2020, compared with previous years, mental health ED visits are back to prepandemic levels.
A September survey showed an increase in suicidal thoughts and attempts, anxiety, and depression pandemic in youth because of the pandemic. Almost one-quarter of those surveyed said they knew a peer who developed suicidal thoughts since the start of the pandemic and 5% reported making a suicide attempt themselves.
In early December, research reported in JAMA Psychiatry showed the overall rate of overdose-related cardiac arrests in 2020 was about 50% higher than trends in 2018 and 2019, and that all overdose-related incidents were about 17% above baseline in 2020.
COVID-19 also appears to be striking individuals who are living in behavioral health facilities, and some of those facilities are reducing inpatient care and other programs because they don’t have enough personal protective equipment, testing supplies, or staff to cope with the disease.
The facilities are not required to report infections to the federal government. Sen. Elizabeth Warren (D-Mass.), Rep. Carolyn Maloney (D-N.Y.), and Rep. Katie Porter (D-Calif.) issued a report based on their own offices’ survey of 10 large behavioral health program operators.
Eight of those operators – covering 376 facilities and more than 100,000 patients in 40 states and Puerto Rico – provided substantive responses.
More than half had at least one COVID case and 14% had large outbreaks of 10 or more cases. The infection rate for patients was in line with that of the general public.
A version of this article originally appeared on Medscape.com.
A public-private partnership, led by mental health advocate Patrick Kennedy and the head of the National Institute of Mental Health, Joshua Gordon, MD, PhD, want urgent action to address the wave of mental illness and suicide caused by COVID-19.
“Our country is in serious denial about the full impact of mental health in this country and certainly as part of this pandemic,” said former congressman Mr. Kennedy, cochair of the Action Alliance’s Mental Health & Suicide Prevention National Response to COVID-19, at a briefing unveiling the group’s new six-priority Action Plan.
“That’s reinforced when all we hear from is Dr. Fauci,” and only about the physical effects of the disease, said Mr. Kennedy, the founder of the Kennedy Forum, a nonprofit dedicated to changing the health system’s approach to mental health and substance use disorders.
“ he said. Mr. Kennedy noted the huge effort to speed therapeutics and vaccines to the American public. “We need to bring that same sense of urgency to these deaths of despair hiding in plain sight.”
Dr. Gordon, NIMH’s director and a cochair of the National Response group, was also at the briefing.
“We know many people report experiencing symptoms of distress, including anxiety, sleep problems, depression, substance use, and suicidal thoughts at rates two to three times higher than we might expect in times before the pandemic. Just as the country has come together to mitigate the physical impacts of pandemic, we also have to identify how to mitigate the mental health impacts,” said Dr. Gordon.
Plan of action
Mr. Kennedy emphasized that it is crucial that federal lawmakers and regulators find a way to increase parity between mental and physical health.
Paramount in that effort would be ensuring stronger enforcement of the Mental Health Parity and Addiction Equity Act, he said.
That 1996 law requires health plans to ensure that benefits for physical and mental health were equivalent, but it has frequently been ignored. In 2019, a U.S. federal court found that one of the nation’s largest behavioral health insurers, United Behavioral Health, had been violating the law. Mr. Kennedy said he expects this decision to continue to have a positive impact on achieving parity.
In November, United was ordered by a federal judge to reprocess 67,000 claims that it illegally denied.
The Alliance’s Action Plan has six priorities:
- Change the national conversation about mental health and suicide.
- Increase access to evidence-based treatments for substance use and mental health disorders in specialty and primary care, and include better reimbursement for services and make permanent reimbursement for telehealth services.
- Increase the use of nonpunitive and supportive crisis intervention services, including keeping people out of the criminal justice system.
- Establish near real-time data collection systems to promptly identify changes in rates of suicide, overdose, and other key events, and of clusters or spikes.
- Ensure the equitable delivery of comprehensive and effective suicide prevention and mental health services for Black Americans, Latin Americans, American Indian/Alaskan Natives, LGBTQ individuals, and others disproportionately impacted by the pandemic.
- Invest in prevention and early intervention approaches that treat the root causes of suicide and mental health problems.
Uptick in distress
Dr. Gordon noted that recent data indicate that, although ED visits for children are still down in 2020, compared with previous years, mental health ED visits are back to prepandemic levels.
A September survey showed an increase in suicidal thoughts and attempts, anxiety, and depression pandemic in youth because of the pandemic. Almost one-quarter of those surveyed said they knew a peer who developed suicidal thoughts since the start of the pandemic and 5% reported making a suicide attempt themselves.
In early December, research reported in JAMA Psychiatry showed the overall rate of overdose-related cardiac arrests in 2020 was about 50% higher than trends in 2018 and 2019, and that all overdose-related incidents were about 17% above baseline in 2020.
COVID-19 also appears to be striking individuals who are living in behavioral health facilities, and some of those facilities are reducing inpatient care and other programs because they don’t have enough personal protective equipment, testing supplies, or staff to cope with the disease.
The facilities are not required to report infections to the federal government. Sen. Elizabeth Warren (D-Mass.), Rep. Carolyn Maloney (D-N.Y.), and Rep. Katie Porter (D-Calif.) issued a report based on their own offices’ survey of 10 large behavioral health program operators.
Eight of those operators – covering 376 facilities and more than 100,000 patients in 40 states and Puerto Rico – provided substantive responses.
More than half had at least one COVID case and 14% had large outbreaks of 10 or more cases. The infection rate for patients was in line with that of the general public.
A version of this article originally appeared on Medscape.com.
A public-private partnership, led by mental health advocate Patrick Kennedy and the head of the National Institute of Mental Health, Joshua Gordon, MD, PhD, want urgent action to address the wave of mental illness and suicide caused by COVID-19.
“Our country is in serious denial about the full impact of mental health in this country and certainly as part of this pandemic,” said former congressman Mr. Kennedy, cochair of the Action Alliance’s Mental Health & Suicide Prevention National Response to COVID-19, at a briefing unveiling the group’s new six-priority Action Plan.
“That’s reinforced when all we hear from is Dr. Fauci,” and only about the physical effects of the disease, said Mr. Kennedy, the founder of the Kennedy Forum, a nonprofit dedicated to changing the health system’s approach to mental health and substance use disorders.
“ he said. Mr. Kennedy noted the huge effort to speed therapeutics and vaccines to the American public. “We need to bring that same sense of urgency to these deaths of despair hiding in plain sight.”
Dr. Gordon, NIMH’s director and a cochair of the National Response group, was also at the briefing.
“We know many people report experiencing symptoms of distress, including anxiety, sleep problems, depression, substance use, and suicidal thoughts at rates two to three times higher than we might expect in times before the pandemic. Just as the country has come together to mitigate the physical impacts of pandemic, we also have to identify how to mitigate the mental health impacts,” said Dr. Gordon.
Plan of action
Mr. Kennedy emphasized that it is crucial that federal lawmakers and regulators find a way to increase parity between mental and physical health.
Paramount in that effort would be ensuring stronger enforcement of the Mental Health Parity and Addiction Equity Act, he said.
That 1996 law requires health plans to ensure that benefits for physical and mental health were equivalent, but it has frequently been ignored. In 2019, a U.S. federal court found that one of the nation’s largest behavioral health insurers, United Behavioral Health, had been violating the law. Mr. Kennedy said he expects this decision to continue to have a positive impact on achieving parity.
In November, United was ordered by a federal judge to reprocess 67,000 claims that it illegally denied.
The Alliance’s Action Plan has six priorities:
- Change the national conversation about mental health and suicide.
- Increase access to evidence-based treatments for substance use and mental health disorders in specialty and primary care, and include better reimbursement for services and make permanent reimbursement for telehealth services.
- Increase the use of nonpunitive and supportive crisis intervention services, including keeping people out of the criminal justice system.
- Establish near real-time data collection systems to promptly identify changes in rates of suicide, overdose, and other key events, and of clusters or spikes.
- Ensure the equitable delivery of comprehensive and effective suicide prevention and mental health services for Black Americans, Latin Americans, American Indian/Alaskan Natives, LGBTQ individuals, and others disproportionately impacted by the pandemic.
- Invest in prevention and early intervention approaches that treat the root causes of suicide and mental health problems.
Uptick in distress
Dr. Gordon noted that recent data indicate that, although ED visits for children are still down in 2020, compared with previous years, mental health ED visits are back to prepandemic levels.
A September survey showed an increase in suicidal thoughts and attempts, anxiety, and depression pandemic in youth because of the pandemic. Almost one-quarter of those surveyed said they knew a peer who developed suicidal thoughts since the start of the pandemic and 5% reported making a suicide attempt themselves.
In early December, research reported in JAMA Psychiatry showed the overall rate of overdose-related cardiac arrests in 2020 was about 50% higher than trends in 2018 and 2019, and that all overdose-related incidents were about 17% above baseline in 2020.
COVID-19 also appears to be striking individuals who are living in behavioral health facilities, and some of those facilities are reducing inpatient care and other programs because they don’t have enough personal protective equipment, testing supplies, or staff to cope with the disease.
The facilities are not required to report infections to the federal government. Sen. Elizabeth Warren (D-Mass.), Rep. Carolyn Maloney (D-N.Y.), and Rep. Katie Porter (D-Calif.) issued a report based on their own offices’ survey of 10 large behavioral health program operators.
Eight of those operators – covering 376 facilities and more than 100,000 patients in 40 states and Puerto Rico – provided substantive responses.
More than half had at least one COVID case and 14% had large outbreaks of 10 or more cases. The infection rate for patients was in line with that of the general public.
A version of this article originally appeared on Medscape.com.
Bias against hiring hospitalists trained in family medicine still persists
Outdated perceptions of family medicine
A family medicine trained doctor, fresh out of residency, visits a career website to scout out prospective hospitalist jobs in their region. As they scroll through the job listings, they come across one opportunity at a nearby hospital system that seems like a good fit. The listing offers a competitive salary and comprehensive benefits for the position, and mentions hospitalists in the department will have the opportunity to teach medical students.
The only problem? The position is for internal medicine trained doctors only. After searching through several more listings with the same internal medicine requirement, the pool of jobs available to the family medicine doctor seems much smaller.
When Robert M. Wachter, MD, MHM, and Lee Goldman, MD coined the term “hospitalist” in a 1996 New England Journal of Medicine article, hospitalists were primarily clinicians with an internal medicine background, filling the gap created by family medicine doctors who increasingly devoted their time to patients in their practice and spent less time rounding in the hospital.
As family medicine doctors have returned to hospital medicine, it has become difficult to find positions as hospitalists due to a preference by some recruiters and employers that favors internal medicine physicians over those who are trained in family medicine. The preference for internal medicine physicians is sometimes overt, such as a requirement on a job application. But the preference can also surface after a physician has already applied for a position, and they will then discover a recruiter is actually looking for someone with a background in internal medicine. In other cases, family medicine physicians find out after applying that applicants with a background in family medicine are considered, but they’re expected to have additional training or certification not listed on the job application.
The situation can even be as stark as a hospital system hiring an internal medicine doctor just out of residency over a family medicine doctor with years of experience as a board-certified physician. Hiring practices in large systems across multiple states sometimes don’t just favor internal medicine, they are entirely focused on internal medicine hospitalists, said experts who spoke with The Hospitalist.
Outdated perceptions of family medicine
Victoria McCurry, MD, current chair of the Society of Hospital Medicine’s family medicine Special Interest Group (SIG) Executive Committee and Faculty Director of Inpatient Services at UPMC McKeesport (Pa.) Family Medicine Residency, said hearsay inside the family medicine community influenced her first job search looking for hospitalist positions as a family medicine physician.
“I was intentional about choosing places that I assumed would be open to family medicine,” she said. “I avoided the downtown urban academic hospitals, the ones that had a large internal medicine residency and fellowship presence, because I assumed that they would not hire me.
“There’s a recognition that depending on the system that you’re in and their history with family medicine trained hospitalists, it can be difficult as a family physician to seek employment,” Dr. McCurry said.
“When I graduated from my residency in 2014, I did not have the same opportunities to be a hospitalist as an internal medicine resident would have,” said Shyam Odeti, MD, a family-practice-trained hospitalist who works at Ballad Health in Johnson City, Tenn. “The perception is family medicine physicians are not trained for hospitalist practice. It’s an old perception.”
This perception may have to do with the mindset of the leadership where a doctor has had residency training, according to Usman Chaudhry, MD, a family medicine hospitalist with Texas Health Physicians Group and leader of the National Advocacy subcommittee for the Family Medicine Executive Council in SHM. Residents trained in bigger university hospital systems where internal medicine (IM) residents do mostly inpatient – in addition to outpatient services – and family medicine (FM) residents do mostly outpatient – including pediatrics and ob/gyn clinics in addition to inpatient services – may believe that to be the case in other systems too, Dr. Chaudhry explained.
“When you go to community hospital residency programs, it’s totally different,” he said. “It all depends. If you have only family medicine residency in a community hospital, they tend to do all training of inpatient clinical medicine, as IM training would in any other program”
Dr. McCurry noted that there seems to be a persisting, mental assumption that as a family medicine doctor, you’re only going to be practicing outpatient only or maybe urgent care, which is historically just not the case. “If that’s ingrained within the local hospital system, then it will be difficult for that system to hire a family medicine-trained hospitalist,” she said.
Another source of outdated perceptions of family medicine come from hospital and institutional bylaws that have written internal medicine training in as a requirement for hospitalists. “In many bigger systems, and even in the smaller hospital community and regional hospitals, the bylaws of the hospitals were written approximately 20 years ago,” Dr. Chaudhry said.
Unless someone has advocated for updating a hospital or institution’s bylaws, they may have outdated requirements for hospitalists. “The situation right now is, in a lot of urban hospitals, they would be able to give a hospitalist position to internal medicine residents who just graduated, not even board certified, but they cannot give it to a hospitalist trained in family medicine who has worked for 10 years and is board certified, just because of the bylaws,” said Dr. Odeti who is also co-chair for the SHM National Advocacy subcommittee of hospitalists trained in family medicine. “There is no good rhyme or reason to it. It is just there and they haven’t changed it.”
Dr. Chaudhry added that no one provides an adequate reason for the bias during the hiring process. “If you ask the recruiter, they would say ‘the employer asked me [to do it this way].’ If you ask the employers, they say ‘the hospital’s bylaws say that.’ And then, we request changes to the hospital bylaws because you don’t have access to them. So the burden of responsibility falls on the shoulders of hospitalists in leadership positions to request equal privileges from the hospital boards for FM-trained hospitalists.”
Experience, education closes some gaps
Over the years, the American Board of Family Medicine (ABFM) and SHM have offered several opportunities for family medicine doctors to demonstrate their experience and training in hospital medicine. In 2010, ABFM began offering the Focused Recognition of Hospital Medicine board examination, together with the American Board of Internal Medicine. SHM also offers hospitalist fellowships and a designation of Fellow in Hospital Medicine (FHM) for health care professionals. In 2015, ABFM and SHM released a joint statement encouraging the growth of hospitalists trained in family medicine (HTFM) and outlining these opportunities.
These measures help fill a gap in both IM and FM training, but also appear to have some effect in convincing recruiters and employers to consider family medicine doctors for hospitalist positions. An abstract published at Hospital Medicine 2014 reviewed 252 hospitalist positions listed in journals and search engines attempted to document the disparities in job listings, the perceptions of physician recruiters, and how factors like experience, training, and certification impacted a family medicine physician’s likelihood to be considered for a position. HTFMs were explicitly mentioned as being eligible in 119 of 252 positions (47%). The investigators then sent surveys out to physician recruiters of the remaining 133 positions asking whether HTFMs were being considered for the position. The results of the survey showed 66% of the recruiters were open to HTFMs, while 34% of recruiters said they did not have a willingness to hire HTFMs.
That willingness to hire changed based on the level of experience, training, and certification. More than one-fourth (29%) of physician recruiters said institutional bylaws prevented hiring of HTFMs. If respondents earned a Recognition of Focused Practice in Hospital Medicine (RFPHM) board examination, 78% of physician recruiters would reconsider hiring the candidate. If the HTFM applicant had prior experience in hospital medicine, 87% of physician recruiters said they would consider the candidate. HTFMs who earned a Designation of Fellow in Hospital Medicine (FHM) from SHM would be reconsidered by 93% of physician recruiters who initially refused the HTFM candidate. All physician recruiters said they would reconsider if the candidate had a fellowship in hospital medicine.
However, to date, there is no official American College of Graduate Medical Education (ACGME)-recognized hospitalist board certification or designated specialty credentialing. This can lead to situations where family medicine trained physicians are applying for jobs without the necessary requirements for the position, because those requirements may not be immediately obvious when first applying to a position. “There’s often no specification until you apply and then are informed that you don’t qualify – ‘Oh, no, you haven’t completed a fellowship,’ or the added qualification in hospital medicine,” Dr. McCurry said.
The 2015 joint statement from AAFP and SHM asserts that “more than two-thirds of HTFMs are also involved in the training of residents and medical students, enhancing the skills of our future physicians.” But when HTFMs do find positions, they may be limited in other ways, such as being prohibited from serving on the faculty of internal medicine residency programs and teaching internal medicine residents. When Dr. Odeti was medical director for Johnston Memorial Hospital in Abingdon, Va., he said he encountered this issue.
“If you are a hospitalist who is internal medicine trained, then you can teach FM or IM, whereas if you’re family medicine trained, you cannot teach internal medicine residents,” he said. “What happened with me, I had to prioritize recruiting internal medicine residents over FM residents to be able to staff IM teaching faculty.”
A rule change has been lobbied by SHM, under the direction of SHM family medicine SIG former chair David Goldstein, MD, to address this issue that would allow HTFMs with a FPHM designation to teach IM residents. The change was quietly made by the ACGME Review Committee for Internal Medicine in 2017, Dr. McCurry said, but implementation of the change has been slow.
“Essentially, the change was made in 2017 to allow for family medicine trainied physicians who have the FPHM designation to teach IM residents, but this knowledge has not been widely dispersed or policies updated to clearly reflect this change,” Dr. McCurry said. “It is a significant change, however, because prior to that, there were explicit policies preventing a family medicine hospitalist from teaching internal medicine residents even if they were experienced.”
FM physicians uniquely suited for HM
Requirements aside, it is “arguably not the case” that family medicine physicians need these extra certifications and fellowships to serve as hospitalists, Dr. McCurry said. It is difficult to quantify IM and FM hospitalist quality outcomes due to challenges with attribution, Dr. Odeti noted. One 2007 study published in the New England Journal of Medicine looked at patient quality and cost of care across the hospitalist model, and family medicine practitioners providing inpatients care. The investigators found similar outcomes in the internist model and with family practitioners providing inpatient care. Dr. Odeti said this research supports “the fact that family medicine physicians are equally competent as internists in providing inpatient care.”
Dr. Odeti argued that family medicine training is valuable for work as a hospitalist. “Hospital medicine is a team sport. You have a quarterback, you have a wide receiver, you have a running back. Everybody has a role to play and everybody has their own strength,” he said.
Family medicine hospitalists are uniquely positioned to handle the shift within hospital medicine from volume to value-based care. “That does not depend solely on what we do within the hospital. It depends a lot on what we do for the patients as they get out of the hospital into the community,” he explained.
Family medicine hospitalists are also well prepared to handle the continuum of care for patients in the hospital. “In their training, FM hospitalists have their own patient panels and they have complete ownership of their patient in their training, so they are prepared because they know how to set up things for outpatients,” Dr. Odeti explained.
“Every hospitalist group needs to use the family medicine doctors to their advantage,” he said. “A family medicine trained hospitalist should be part of every good hospitalist group, is what I would say.”
HTFMs are growing within SHM
HTFMs are “all over,” being represented in smaller hospitals, larger hospitals, and university hospitals in every state. “But to reach those positions, they probably have to go over more hurdles and have fewer opportunities,” Dr. Chaudhry said.
There isn’t a completely accurate count of family medicine hospitalists in the United States. Out of an estimated 50,000 hospitalists in the U. S., about 16,000 hospitalists are members of SHM. A number of family medicine hospitalists may also take AAFP membership instead of SHM, Dr. Odeti explained.
However, there are a growing number of hospitalists within SHM with a family medicine background. In the 2007-2008 Society of Hospital Medicine Annual Survey, 3.7% of U.S. hospitalists claimed family medicine training. That number increased to 6.9% of physicians who answered the SHM membership data report in 2010.
A Medscape Hospitalist Lifestyle, Happiness & Burnout Report from 2019 estimates 17% of hospitalists are trained in family medicine. In the latest State of Hospital Medicine Report published in 2020, 38.6% of hospital medicine groups containing family medicine trained physicians were part of a university, medical school, or faculty practice; 79.6% did not have academic status; 83.8% were at a non-teaching hospital; 60.7% were in a group in a non-teaching service at a teaching hospital; and 52.8% were in a group at a combination teaching/non-teaching service at a teaching hospital.
Although the Report did not specify whether family medicine hospitalists were mainly in rural or urban areas, “some of us do practice in underserved area hospitals where you have the smaller ICU model, critical access hospitals, potentially dealing with a whole gamut of inpatient medicine from ER, to the hospital inpatient adult cases, to critical care level,” Dr. McCurry said.
“But then, there are a large number of us who practice in private groups or at large hospitals, academic centers around the country,” she added. “There’s a range of family medicine trained hospitalist practice areas.”
Equal recognition for HTFM in HM
The SHM family medicine SIG has been working to highlight the issue of hiring practices for HTFMs, and is taking a number of actions to bring greater awareness and recognition to family medicine hospitalists.
The family medicine SIG is looking at steps for requesting a new joint statement from ABFM and SHM focused on hiring practices for family medicine physicians as hospitalists. “I think it’s worth considering now that we’re at a point where we comprise about one-fifth of hospitalists as family medicine docs,” Dr. McCurry said. “Is it time to take that joint statement to the next step, and seek a review of how we can improve the balance of hiring in terms of favoring more balanced consideration now that there are a lot more family medicine trained hospitalists than historically?
“I think the call is really to help us all move to that next step in terms of identifying any of the lingering vestiges of expectation that are really no longer applicable to the hiring practices, or shouldn’t be,” she said.
The next step will be to ask hospitals with internal medicine only requirements for hospitalists to update their bylaws to include family medicine physicians when considering candidates for hospitalist positions. If SHM does not make a distinction to grant Fellow in Hospital Medicine status between internal medicine and family medicine trained hospitalists, “then there should not be any distinction, or there should not be any hindrance by the recruiters, by the bigger systems, as well as by the employers” in hiring a family medicine trained physician for a hospitalist position, Dr. Chaudhry said.
Dr. Odeti, who serves in several leadership roles within Ballad Health, describes the system as being friendly to HTFMs. About one-fourth of the hospitalists in Ballad Health are trained in family medicine. But when Dr. Odeti started his hospitalist practice, he was only one of a handful of HTFMs. He sees a future where the accomplishments and contributions of HTFMs will pave the way for future hospitalists. “Access into the urban hospitals is key, and I hope that SHM and the HTFM SIG will act as a catalyst for this change,” he said.
Colleagues of family medicine hospitalists, especially those in leadership positions at hospitals, can help by raising awareness, as can “those of our colleagues who sit on medical executive committees within their hospitals to review their bylaws, to see what the policies are, and encourage more competitiveness,” Dr. McCurry said. “Truly, the best candidate for the position, regardless of background and training, is what you want. You want the best colleagues for your fellow hospitalists. You want the best physician for your patients in the hospital.”
If training and all other things are equal, family medicine physicians should be evaluated on a case-by-case basis, she said. “I think that that puts the burden back on any good medical committee, and a good medical committee member who is an SHM member as well, to say, ‘If we are committed to quality patient care, we want to encourage the recruitment of all physicians that are truly the best physicians to reduce that distinction between FM and IM in order to allow those best candidates to present, whether they are FM or IM.’ That’s all that we’re asking.”
Dr. Chaudhry emphasized that the preference for internal medicine trained physicians isn’t intentional. “It’s not as if the systems are trying to do it,” he said. “I think it is more like everybody needs to be educated. And through the platform of the Society of Hospital Medicine, I think we can make a difference. It will be a slow change, but we’ll have to keep on working on it.”
Dr. Odeti, Dr. McCurry, and Dr. Chaudhry have no relevant financial disclosures.
Outdated perceptions of family medicine
Outdated perceptions of family medicine
A family medicine trained doctor, fresh out of residency, visits a career website to scout out prospective hospitalist jobs in their region. As they scroll through the job listings, they come across one opportunity at a nearby hospital system that seems like a good fit. The listing offers a competitive salary and comprehensive benefits for the position, and mentions hospitalists in the department will have the opportunity to teach medical students.
The only problem? The position is for internal medicine trained doctors only. After searching through several more listings with the same internal medicine requirement, the pool of jobs available to the family medicine doctor seems much smaller.
When Robert M. Wachter, MD, MHM, and Lee Goldman, MD coined the term “hospitalist” in a 1996 New England Journal of Medicine article, hospitalists were primarily clinicians with an internal medicine background, filling the gap created by family medicine doctors who increasingly devoted their time to patients in their practice and spent less time rounding in the hospital.
As family medicine doctors have returned to hospital medicine, it has become difficult to find positions as hospitalists due to a preference by some recruiters and employers that favors internal medicine physicians over those who are trained in family medicine. The preference for internal medicine physicians is sometimes overt, such as a requirement on a job application. But the preference can also surface after a physician has already applied for a position, and they will then discover a recruiter is actually looking for someone with a background in internal medicine. In other cases, family medicine physicians find out after applying that applicants with a background in family medicine are considered, but they’re expected to have additional training or certification not listed on the job application.
The situation can even be as stark as a hospital system hiring an internal medicine doctor just out of residency over a family medicine doctor with years of experience as a board-certified physician. Hiring practices in large systems across multiple states sometimes don’t just favor internal medicine, they are entirely focused on internal medicine hospitalists, said experts who spoke with The Hospitalist.
Outdated perceptions of family medicine
Victoria McCurry, MD, current chair of the Society of Hospital Medicine’s family medicine Special Interest Group (SIG) Executive Committee and Faculty Director of Inpatient Services at UPMC McKeesport (Pa.) Family Medicine Residency, said hearsay inside the family medicine community influenced her first job search looking for hospitalist positions as a family medicine physician.
“I was intentional about choosing places that I assumed would be open to family medicine,” she said. “I avoided the downtown urban academic hospitals, the ones that had a large internal medicine residency and fellowship presence, because I assumed that they would not hire me.
“There’s a recognition that depending on the system that you’re in and their history with family medicine trained hospitalists, it can be difficult as a family physician to seek employment,” Dr. McCurry said.
“When I graduated from my residency in 2014, I did not have the same opportunities to be a hospitalist as an internal medicine resident would have,” said Shyam Odeti, MD, a family-practice-trained hospitalist who works at Ballad Health in Johnson City, Tenn. “The perception is family medicine physicians are not trained for hospitalist practice. It’s an old perception.”
This perception may have to do with the mindset of the leadership where a doctor has had residency training, according to Usman Chaudhry, MD, a family medicine hospitalist with Texas Health Physicians Group and leader of the National Advocacy subcommittee for the Family Medicine Executive Council in SHM. Residents trained in bigger university hospital systems where internal medicine (IM) residents do mostly inpatient – in addition to outpatient services – and family medicine (FM) residents do mostly outpatient – including pediatrics and ob/gyn clinics in addition to inpatient services – may believe that to be the case in other systems too, Dr. Chaudhry explained.
“When you go to community hospital residency programs, it’s totally different,” he said. “It all depends. If you have only family medicine residency in a community hospital, they tend to do all training of inpatient clinical medicine, as IM training would in any other program”
Dr. McCurry noted that there seems to be a persisting, mental assumption that as a family medicine doctor, you’re only going to be practicing outpatient only or maybe urgent care, which is historically just not the case. “If that’s ingrained within the local hospital system, then it will be difficult for that system to hire a family medicine-trained hospitalist,” she said.
Another source of outdated perceptions of family medicine come from hospital and institutional bylaws that have written internal medicine training in as a requirement for hospitalists. “In many bigger systems, and even in the smaller hospital community and regional hospitals, the bylaws of the hospitals were written approximately 20 years ago,” Dr. Chaudhry said.
Unless someone has advocated for updating a hospital or institution’s bylaws, they may have outdated requirements for hospitalists. “The situation right now is, in a lot of urban hospitals, they would be able to give a hospitalist position to internal medicine residents who just graduated, not even board certified, but they cannot give it to a hospitalist trained in family medicine who has worked for 10 years and is board certified, just because of the bylaws,” said Dr. Odeti who is also co-chair for the SHM National Advocacy subcommittee of hospitalists trained in family medicine. “There is no good rhyme or reason to it. It is just there and they haven’t changed it.”
Dr. Chaudhry added that no one provides an adequate reason for the bias during the hiring process. “If you ask the recruiter, they would say ‘the employer asked me [to do it this way].’ If you ask the employers, they say ‘the hospital’s bylaws say that.’ And then, we request changes to the hospital bylaws because you don’t have access to them. So the burden of responsibility falls on the shoulders of hospitalists in leadership positions to request equal privileges from the hospital boards for FM-trained hospitalists.”
Experience, education closes some gaps
Over the years, the American Board of Family Medicine (ABFM) and SHM have offered several opportunities for family medicine doctors to demonstrate their experience and training in hospital medicine. In 2010, ABFM began offering the Focused Recognition of Hospital Medicine board examination, together with the American Board of Internal Medicine. SHM also offers hospitalist fellowships and a designation of Fellow in Hospital Medicine (FHM) for health care professionals. In 2015, ABFM and SHM released a joint statement encouraging the growth of hospitalists trained in family medicine (HTFM) and outlining these opportunities.
These measures help fill a gap in both IM and FM training, but also appear to have some effect in convincing recruiters and employers to consider family medicine doctors for hospitalist positions. An abstract published at Hospital Medicine 2014 reviewed 252 hospitalist positions listed in journals and search engines attempted to document the disparities in job listings, the perceptions of physician recruiters, and how factors like experience, training, and certification impacted a family medicine physician’s likelihood to be considered for a position. HTFMs were explicitly mentioned as being eligible in 119 of 252 positions (47%). The investigators then sent surveys out to physician recruiters of the remaining 133 positions asking whether HTFMs were being considered for the position. The results of the survey showed 66% of the recruiters were open to HTFMs, while 34% of recruiters said they did not have a willingness to hire HTFMs.
That willingness to hire changed based on the level of experience, training, and certification. More than one-fourth (29%) of physician recruiters said institutional bylaws prevented hiring of HTFMs. If respondents earned a Recognition of Focused Practice in Hospital Medicine (RFPHM) board examination, 78% of physician recruiters would reconsider hiring the candidate. If the HTFM applicant had prior experience in hospital medicine, 87% of physician recruiters said they would consider the candidate. HTFMs who earned a Designation of Fellow in Hospital Medicine (FHM) from SHM would be reconsidered by 93% of physician recruiters who initially refused the HTFM candidate. All physician recruiters said they would reconsider if the candidate had a fellowship in hospital medicine.
However, to date, there is no official American College of Graduate Medical Education (ACGME)-recognized hospitalist board certification or designated specialty credentialing. This can lead to situations where family medicine trained physicians are applying for jobs without the necessary requirements for the position, because those requirements may not be immediately obvious when first applying to a position. “There’s often no specification until you apply and then are informed that you don’t qualify – ‘Oh, no, you haven’t completed a fellowship,’ or the added qualification in hospital medicine,” Dr. McCurry said.
The 2015 joint statement from AAFP and SHM asserts that “more than two-thirds of HTFMs are also involved in the training of residents and medical students, enhancing the skills of our future physicians.” But when HTFMs do find positions, they may be limited in other ways, such as being prohibited from serving on the faculty of internal medicine residency programs and teaching internal medicine residents. When Dr. Odeti was medical director for Johnston Memorial Hospital in Abingdon, Va., he said he encountered this issue.
“If you are a hospitalist who is internal medicine trained, then you can teach FM or IM, whereas if you’re family medicine trained, you cannot teach internal medicine residents,” he said. “What happened with me, I had to prioritize recruiting internal medicine residents over FM residents to be able to staff IM teaching faculty.”
A rule change has been lobbied by SHM, under the direction of SHM family medicine SIG former chair David Goldstein, MD, to address this issue that would allow HTFMs with a FPHM designation to teach IM residents. The change was quietly made by the ACGME Review Committee for Internal Medicine in 2017, Dr. McCurry said, but implementation of the change has been slow.
“Essentially, the change was made in 2017 to allow for family medicine trainied physicians who have the FPHM designation to teach IM residents, but this knowledge has not been widely dispersed or policies updated to clearly reflect this change,” Dr. McCurry said. “It is a significant change, however, because prior to that, there were explicit policies preventing a family medicine hospitalist from teaching internal medicine residents even if they were experienced.”
FM physicians uniquely suited for HM
Requirements aside, it is “arguably not the case” that family medicine physicians need these extra certifications and fellowships to serve as hospitalists, Dr. McCurry said. It is difficult to quantify IM and FM hospitalist quality outcomes due to challenges with attribution, Dr. Odeti noted. One 2007 study published in the New England Journal of Medicine looked at patient quality and cost of care across the hospitalist model, and family medicine practitioners providing inpatients care. The investigators found similar outcomes in the internist model and with family practitioners providing inpatient care. Dr. Odeti said this research supports “the fact that family medicine physicians are equally competent as internists in providing inpatient care.”
Dr. Odeti argued that family medicine training is valuable for work as a hospitalist. “Hospital medicine is a team sport. You have a quarterback, you have a wide receiver, you have a running back. Everybody has a role to play and everybody has their own strength,” he said.
Family medicine hospitalists are uniquely positioned to handle the shift within hospital medicine from volume to value-based care. “That does not depend solely on what we do within the hospital. It depends a lot on what we do for the patients as they get out of the hospital into the community,” he explained.
Family medicine hospitalists are also well prepared to handle the continuum of care for patients in the hospital. “In their training, FM hospitalists have their own patient panels and they have complete ownership of their patient in their training, so they are prepared because they know how to set up things for outpatients,” Dr. Odeti explained.
“Every hospitalist group needs to use the family medicine doctors to their advantage,” he said. “A family medicine trained hospitalist should be part of every good hospitalist group, is what I would say.”
HTFMs are growing within SHM
HTFMs are “all over,” being represented in smaller hospitals, larger hospitals, and university hospitals in every state. “But to reach those positions, they probably have to go over more hurdles and have fewer opportunities,” Dr. Chaudhry said.
There isn’t a completely accurate count of family medicine hospitalists in the United States. Out of an estimated 50,000 hospitalists in the U. S., about 16,000 hospitalists are members of SHM. A number of family medicine hospitalists may also take AAFP membership instead of SHM, Dr. Odeti explained.
However, there are a growing number of hospitalists within SHM with a family medicine background. In the 2007-2008 Society of Hospital Medicine Annual Survey, 3.7% of U.S. hospitalists claimed family medicine training. That number increased to 6.9% of physicians who answered the SHM membership data report in 2010.
A Medscape Hospitalist Lifestyle, Happiness & Burnout Report from 2019 estimates 17% of hospitalists are trained in family medicine. In the latest State of Hospital Medicine Report published in 2020, 38.6% of hospital medicine groups containing family medicine trained physicians were part of a university, medical school, or faculty practice; 79.6% did not have academic status; 83.8% were at a non-teaching hospital; 60.7% were in a group in a non-teaching service at a teaching hospital; and 52.8% were in a group at a combination teaching/non-teaching service at a teaching hospital.
Although the Report did not specify whether family medicine hospitalists were mainly in rural or urban areas, “some of us do practice in underserved area hospitals where you have the smaller ICU model, critical access hospitals, potentially dealing with a whole gamut of inpatient medicine from ER, to the hospital inpatient adult cases, to critical care level,” Dr. McCurry said.
“But then, there are a large number of us who practice in private groups or at large hospitals, academic centers around the country,” she added. “There’s a range of family medicine trained hospitalist practice areas.”
Equal recognition for HTFM in HM
The SHM family medicine SIG has been working to highlight the issue of hiring practices for HTFMs, and is taking a number of actions to bring greater awareness and recognition to family medicine hospitalists.
The family medicine SIG is looking at steps for requesting a new joint statement from ABFM and SHM focused on hiring practices for family medicine physicians as hospitalists. “I think it’s worth considering now that we’re at a point where we comprise about one-fifth of hospitalists as family medicine docs,” Dr. McCurry said. “Is it time to take that joint statement to the next step, and seek a review of how we can improve the balance of hiring in terms of favoring more balanced consideration now that there are a lot more family medicine trained hospitalists than historically?
“I think the call is really to help us all move to that next step in terms of identifying any of the lingering vestiges of expectation that are really no longer applicable to the hiring practices, or shouldn’t be,” she said.
The next step will be to ask hospitals with internal medicine only requirements for hospitalists to update their bylaws to include family medicine physicians when considering candidates for hospitalist positions. If SHM does not make a distinction to grant Fellow in Hospital Medicine status between internal medicine and family medicine trained hospitalists, “then there should not be any distinction, or there should not be any hindrance by the recruiters, by the bigger systems, as well as by the employers” in hiring a family medicine trained physician for a hospitalist position, Dr. Chaudhry said.
Dr. Odeti, who serves in several leadership roles within Ballad Health, describes the system as being friendly to HTFMs. About one-fourth of the hospitalists in Ballad Health are trained in family medicine. But when Dr. Odeti started his hospitalist practice, he was only one of a handful of HTFMs. He sees a future where the accomplishments and contributions of HTFMs will pave the way for future hospitalists. “Access into the urban hospitals is key, and I hope that SHM and the HTFM SIG will act as a catalyst for this change,” he said.
Colleagues of family medicine hospitalists, especially those in leadership positions at hospitals, can help by raising awareness, as can “those of our colleagues who sit on medical executive committees within their hospitals to review their bylaws, to see what the policies are, and encourage more competitiveness,” Dr. McCurry said. “Truly, the best candidate for the position, regardless of background and training, is what you want. You want the best colleagues for your fellow hospitalists. You want the best physician for your patients in the hospital.”
If training and all other things are equal, family medicine physicians should be evaluated on a case-by-case basis, she said. “I think that that puts the burden back on any good medical committee, and a good medical committee member who is an SHM member as well, to say, ‘If we are committed to quality patient care, we want to encourage the recruitment of all physicians that are truly the best physicians to reduce that distinction between FM and IM in order to allow those best candidates to present, whether they are FM or IM.’ That’s all that we’re asking.”
Dr. Chaudhry emphasized that the preference for internal medicine trained physicians isn’t intentional. “It’s not as if the systems are trying to do it,” he said. “I think it is more like everybody needs to be educated. And through the platform of the Society of Hospital Medicine, I think we can make a difference. It will be a slow change, but we’ll have to keep on working on it.”
Dr. Odeti, Dr. McCurry, and Dr. Chaudhry have no relevant financial disclosures.
A family medicine trained doctor, fresh out of residency, visits a career website to scout out prospective hospitalist jobs in their region. As they scroll through the job listings, they come across one opportunity at a nearby hospital system that seems like a good fit. The listing offers a competitive salary and comprehensive benefits for the position, and mentions hospitalists in the department will have the opportunity to teach medical students.
The only problem? The position is for internal medicine trained doctors only. After searching through several more listings with the same internal medicine requirement, the pool of jobs available to the family medicine doctor seems much smaller.
When Robert M. Wachter, MD, MHM, and Lee Goldman, MD coined the term “hospitalist” in a 1996 New England Journal of Medicine article, hospitalists were primarily clinicians with an internal medicine background, filling the gap created by family medicine doctors who increasingly devoted their time to patients in their practice and spent less time rounding in the hospital.
As family medicine doctors have returned to hospital medicine, it has become difficult to find positions as hospitalists due to a preference by some recruiters and employers that favors internal medicine physicians over those who are trained in family medicine. The preference for internal medicine physicians is sometimes overt, such as a requirement on a job application. But the preference can also surface after a physician has already applied for a position, and they will then discover a recruiter is actually looking for someone with a background in internal medicine. In other cases, family medicine physicians find out after applying that applicants with a background in family medicine are considered, but they’re expected to have additional training or certification not listed on the job application.
The situation can even be as stark as a hospital system hiring an internal medicine doctor just out of residency over a family medicine doctor with years of experience as a board-certified physician. Hiring practices in large systems across multiple states sometimes don’t just favor internal medicine, they are entirely focused on internal medicine hospitalists, said experts who spoke with The Hospitalist.
Outdated perceptions of family medicine
Victoria McCurry, MD, current chair of the Society of Hospital Medicine’s family medicine Special Interest Group (SIG) Executive Committee and Faculty Director of Inpatient Services at UPMC McKeesport (Pa.) Family Medicine Residency, said hearsay inside the family medicine community influenced her first job search looking for hospitalist positions as a family medicine physician.
“I was intentional about choosing places that I assumed would be open to family medicine,” she said. “I avoided the downtown urban academic hospitals, the ones that had a large internal medicine residency and fellowship presence, because I assumed that they would not hire me.
“There’s a recognition that depending on the system that you’re in and their history with family medicine trained hospitalists, it can be difficult as a family physician to seek employment,” Dr. McCurry said.
“When I graduated from my residency in 2014, I did not have the same opportunities to be a hospitalist as an internal medicine resident would have,” said Shyam Odeti, MD, a family-practice-trained hospitalist who works at Ballad Health in Johnson City, Tenn. “The perception is family medicine physicians are not trained for hospitalist practice. It’s an old perception.”
This perception may have to do with the mindset of the leadership where a doctor has had residency training, according to Usman Chaudhry, MD, a family medicine hospitalist with Texas Health Physicians Group and leader of the National Advocacy subcommittee for the Family Medicine Executive Council in SHM. Residents trained in bigger university hospital systems where internal medicine (IM) residents do mostly inpatient – in addition to outpatient services – and family medicine (FM) residents do mostly outpatient – including pediatrics and ob/gyn clinics in addition to inpatient services – may believe that to be the case in other systems too, Dr. Chaudhry explained.
“When you go to community hospital residency programs, it’s totally different,” he said. “It all depends. If you have only family medicine residency in a community hospital, they tend to do all training of inpatient clinical medicine, as IM training would in any other program”
Dr. McCurry noted that there seems to be a persisting, mental assumption that as a family medicine doctor, you’re only going to be practicing outpatient only or maybe urgent care, which is historically just not the case. “If that’s ingrained within the local hospital system, then it will be difficult for that system to hire a family medicine-trained hospitalist,” she said.
Another source of outdated perceptions of family medicine come from hospital and institutional bylaws that have written internal medicine training in as a requirement for hospitalists. “In many bigger systems, and even in the smaller hospital community and regional hospitals, the bylaws of the hospitals were written approximately 20 years ago,” Dr. Chaudhry said.
Unless someone has advocated for updating a hospital or institution’s bylaws, they may have outdated requirements for hospitalists. “The situation right now is, in a lot of urban hospitals, they would be able to give a hospitalist position to internal medicine residents who just graduated, not even board certified, but they cannot give it to a hospitalist trained in family medicine who has worked for 10 years and is board certified, just because of the bylaws,” said Dr. Odeti who is also co-chair for the SHM National Advocacy subcommittee of hospitalists trained in family medicine. “There is no good rhyme or reason to it. It is just there and they haven’t changed it.”
Dr. Chaudhry added that no one provides an adequate reason for the bias during the hiring process. “If you ask the recruiter, they would say ‘the employer asked me [to do it this way].’ If you ask the employers, they say ‘the hospital’s bylaws say that.’ And then, we request changes to the hospital bylaws because you don’t have access to them. So the burden of responsibility falls on the shoulders of hospitalists in leadership positions to request equal privileges from the hospital boards for FM-trained hospitalists.”
Experience, education closes some gaps
Over the years, the American Board of Family Medicine (ABFM) and SHM have offered several opportunities for family medicine doctors to demonstrate their experience and training in hospital medicine. In 2010, ABFM began offering the Focused Recognition of Hospital Medicine board examination, together with the American Board of Internal Medicine. SHM also offers hospitalist fellowships and a designation of Fellow in Hospital Medicine (FHM) for health care professionals. In 2015, ABFM and SHM released a joint statement encouraging the growth of hospitalists trained in family medicine (HTFM) and outlining these opportunities.
These measures help fill a gap in both IM and FM training, but also appear to have some effect in convincing recruiters and employers to consider family medicine doctors for hospitalist positions. An abstract published at Hospital Medicine 2014 reviewed 252 hospitalist positions listed in journals and search engines attempted to document the disparities in job listings, the perceptions of physician recruiters, and how factors like experience, training, and certification impacted a family medicine physician’s likelihood to be considered for a position. HTFMs were explicitly mentioned as being eligible in 119 of 252 positions (47%). The investigators then sent surveys out to physician recruiters of the remaining 133 positions asking whether HTFMs were being considered for the position. The results of the survey showed 66% of the recruiters were open to HTFMs, while 34% of recruiters said they did not have a willingness to hire HTFMs.
That willingness to hire changed based on the level of experience, training, and certification. More than one-fourth (29%) of physician recruiters said institutional bylaws prevented hiring of HTFMs. If respondents earned a Recognition of Focused Practice in Hospital Medicine (RFPHM) board examination, 78% of physician recruiters would reconsider hiring the candidate. If the HTFM applicant had prior experience in hospital medicine, 87% of physician recruiters said they would consider the candidate. HTFMs who earned a Designation of Fellow in Hospital Medicine (FHM) from SHM would be reconsidered by 93% of physician recruiters who initially refused the HTFM candidate. All physician recruiters said they would reconsider if the candidate had a fellowship in hospital medicine.
However, to date, there is no official American College of Graduate Medical Education (ACGME)-recognized hospitalist board certification or designated specialty credentialing. This can lead to situations where family medicine trained physicians are applying for jobs without the necessary requirements for the position, because those requirements may not be immediately obvious when first applying to a position. “There’s often no specification until you apply and then are informed that you don’t qualify – ‘Oh, no, you haven’t completed a fellowship,’ or the added qualification in hospital medicine,” Dr. McCurry said.
The 2015 joint statement from AAFP and SHM asserts that “more than two-thirds of HTFMs are also involved in the training of residents and medical students, enhancing the skills of our future physicians.” But when HTFMs do find positions, they may be limited in other ways, such as being prohibited from serving on the faculty of internal medicine residency programs and teaching internal medicine residents. When Dr. Odeti was medical director for Johnston Memorial Hospital in Abingdon, Va., he said he encountered this issue.
“If you are a hospitalist who is internal medicine trained, then you can teach FM or IM, whereas if you’re family medicine trained, you cannot teach internal medicine residents,” he said. “What happened with me, I had to prioritize recruiting internal medicine residents over FM residents to be able to staff IM teaching faculty.”
A rule change has been lobbied by SHM, under the direction of SHM family medicine SIG former chair David Goldstein, MD, to address this issue that would allow HTFMs with a FPHM designation to teach IM residents. The change was quietly made by the ACGME Review Committee for Internal Medicine in 2017, Dr. McCurry said, but implementation of the change has been slow.
“Essentially, the change was made in 2017 to allow for family medicine trainied physicians who have the FPHM designation to teach IM residents, but this knowledge has not been widely dispersed or policies updated to clearly reflect this change,” Dr. McCurry said. “It is a significant change, however, because prior to that, there were explicit policies preventing a family medicine hospitalist from teaching internal medicine residents even if they were experienced.”
FM physicians uniquely suited for HM
Requirements aside, it is “arguably not the case” that family medicine physicians need these extra certifications and fellowships to serve as hospitalists, Dr. McCurry said. It is difficult to quantify IM and FM hospitalist quality outcomes due to challenges with attribution, Dr. Odeti noted. One 2007 study published in the New England Journal of Medicine looked at patient quality and cost of care across the hospitalist model, and family medicine practitioners providing inpatients care. The investigators found similar outcomes in the internist model and with family practitioners providing inpatient care. Dr. Odeti said this research supports “the fact that family medicine physicians are equally competent as internists in providing inpatient care.”
Dr. Odeti argued that family medicine training is valuable for work as a hospitalist. “Hospital medicine is a team sport. You have a quarterback, you have a wide receiver, you have a running back. Everybody has a role to play and everybody has their own strength,” he said.
Family medicine hospitalists are uniquely positioned to handle the shift within hospital medicine from volume to value-based care. “That does not depend solely on what we do within the hospital. It depends a lot on what we do for the patients as they get out of the hospital into the community,” he explained.
Family medicine hospitalists are also well prepared to handle the continuum of care for patients in the hospital. “In their training, FM hospitalists have their own patient panels and they have complete ownership of their patient in their training, so they are prepared because they know how to set up things for outpatients,” Dr. Odeti explained.
“Every hospitalist group needs to use the family medicine doctors to their advantage,” he said. “A family medicine trained hospitalist should be part of every good hospitalist group, is what I would say.”
HTFMs are growing within SHM
HTFMs are “all over,” being represented in smaller hospitals, larger hospitals, and university hospitals in every state. “But to reach those positions, they probably have to go over more hurdles and have fewer opportunities,” Dr. Chaudhry said.
There isn’t a completely accurate count of family medicine hospitalists in the United States. Out of an estimated 50,000 hospitalists in the U. S., about 16,000 hospitalists are members of SHM. A number of family medicine hospitalists may also take AAFP membership instead of SHM, Dr. Odeti explained.
However, there are a growing number of hospitalists within SHM with a family medicine background. In the 2007-2008 Society of Hospital Medicine Annual Survey, 3.7% of U.S. hospitalists claimed family medicine training. That number increased to 6.9% of physicians who answered the SHM membership data report in 2010.
A Medscape Hospitalist Lifestyle, Happiness & Burnout Report from 2019 estimates 17% of hospitalists are trained in family medicine. In the latest State of Hospital Medicine Report published in 2020, 38.6% of hospital medicine groups containing family medicine trained physicians were part of a university, medical school, or faculty practice; 79.6% did not have academic status; 83.8% were at a non-teaching hospital; 60.7% were in a group in a non-teaching service at a teaching hospital; and 52.8% were in a group at a combination teaching/non-teaching service at a teaching hospital.
Although the Report did not specify whether family medicine hospitalists were mainly in rural or urban areas, “some of us do practice in underserved area hospitals where you have the smaller ICU model, critical access hospitals, potentially dealing with a whole gamut of inpatient medicine from ER, to the hospital inpatient adult cases, to critical care level,” Dr. McCurry said.
“But then, there are a large number of us who practice in private groups or at large hospitals, academic centers around the country,” she added. “There’s a range of family medicine trained hospitalist practice areas.”
Equal recognition for HTFM in HM
The SHM family medicine SIG has been working to highlight the issue of hiring practices for HTFMs, and is taking a number of actions to bring greater awareness and recognition to family medicine hospitalists.
The family medicine SIG is looking at steps for requesting a new joint statement from ABFM and SHM focused on hiring practices for family medicine physicians as hospitalists. “I think it’s worth considering now that we’re at a point where we comprise about one-fifth of hospitalists as family medicine docs,” Dr. McCurry said. “Is it time to take that joint statement to the next step, and seek a review of how we can improve the balance of hiring in terms of favoring more balanced consideration now that there are a lot more family medicine trained hospitalists than historically?
“I think the call is really to help us all move to that next step in terms of identifying any of the lingering vestiges of expectation that are really no longer applicable to the hiring practices, or shouldn’t be,” she said.
The next step will be to ask hospitals with internal medicine only requirements for hospitalists to update their bylaws to include family medicine physicians when considering candidates for hospitalist positions. If SHM does not make a distinction to grant Fellow in Hospital Medicine status between internal medicine and family medicine trained hospitalists, “then there should not be any distinction, or there should not be any hindrance by the recruiters, by the bigger systems, as well as by the employers” in hiring a family medicine trained physician for a hospitalist position, Dr. Chaudhry said.
Dr. Odeti, who serves in several leadership roles within Ballad Health, describes the system as being friendly to HTFMs. About one-fourth of the hospitalists in Ballad Health are trained in family medicine. But when Dr. Odeti started his hospitalist practice, he was only one of a handful of HTFMs. He sees a future where the accomplishments and contributions of HTFMs will pave the way for future hospitalists. “Access into the urban hospitals is key, and I hope that SHM and the HTFM SIG will act as a catalyst for this change,” he said.
Colleagues of family medicine hospitalists, especially those in leadership positions at hospitals, can help by raising awareness, as can “those of our colleagues who sit on medical executive committees within their hospitals to review their bylaws, to see what the policies are, and encourage more competitiveness,” Dr. McCurry said. “Truly, the best candidate for the position, regardless of background and training, is what you want. You want the best colleagues for your fellow hospitalists. You want the best physician for your patients in the hospital.”
If training and all other things are equal, family medicine physicians should be evaluated on a case-by-case basis, she said. “I think that that puts the burden back on any good medical committee, and a good medical committee member who is an SHM member as well, to say, ‘If we are committed to quality patient care, we want to encourage the recruitment of all physicians that are truly the best physicians to reduce that distinction between FM and IM in order to allow those best candidates to present, whether they are FM or IM.’ That’s all that we’re asking.”
Dr. Chaudhry emphasized that the preference for internal medicine trained physicians isn’t intentional. “It’s not as if the systems are trying to do it,” he said. “I think it is more like everybody needs to be educated. And through the platform of the Society of Hospital Medicine, I think we can make a difference. It will be a slow change, but we’ll have to keep on working on it.”
Dr. Odeti, Dr. McCurry, and Dr. Chaudhry have no relevant financial disclosures.
ADA 2021 standards address financial hardship in diabetes
For 2021, the American Diabetes Association offers new guidance on assessing patients’ financial and social barriers to care, especially given the COVID-19 pandemic, individualizing treatment of patients with type 2 diabetes, and use of diabetes technology.
As it does every year, the annual update incorporates new clinical information that has become available since the last guideline, with occasional revisions during the year as needed. “Standards of Medical Care in Diabetes – 2021,” was published online as a supplement to Diabetes Care.
The new standards advise that patients be assessed for food and housing insecurity, social support, and “cost-related medication nonadherence,” and those found to have difficulty referred to appropriate community resources.
“Clinicians need to be sensitive to the fact that patients may have very good reasons for not taking their medication, [as in] if they can’t afford it,” ADA chief science & medical officer Robert A. Gabbay, MD, PhD, said in an interview.
Dr. Gabbay noted that “a heightened awareness” of social determinants of health is weaved throughout the 2021 standards because of the pandemic, with information on the topic derived from a July 2020 joint consensus statement in Diabetes Care, endorsed by a number of other societies, as well as a November publication also in Diabetes Care.
“We made several recommendations that speak to social determinants of health, placing an emphasis on engaging in conversations around this subject and screening for related issues such as food insecurity that weren’t there previously,” he said.
“Screening tools are suggested. It helped us to have an in-depth scientific review of the literature to know the prevalence of this in people with diabetes. ... Having the science to put it in was a key step,” Dr. Gabbay noted.
Consider kidney, heart disease in type 2 treatment individualization
Recent data from trials such as CREDENCE and DAPA-HF, among others, have been added to inform the choice of pharmacologic treatment in patients with type 2 diabetes with comorbid diabetic kidney disease and chronic heart failure.
“ADA has been advocating individualization of treatment based on comorbidities for a while, but we’ve taken more steps in that direction. Beyond lifestyle for all individuals with type 2 diabetes, clinicians want to think early on about which comorbidities patients have and then think about the appropriate treatment based on that,” Dr. Gabbay said.
And for the third year in a row, the section on cardiovascular disease and risk management has been endorsed by the American College of Cardiology.
“All the things in that section are very much aligned with ACC and that’s been a great partnership,” Dr. Gabbay said.
Now, ADA is in discussions with other professional societies representing relevant specialties to create further such unified messages.
“What we all want to avoid is having multiple different guidelines. We want to speak with one voice and find common ground as much as possible. … It makes it much easier for clinicians to know what to do. That’s the goal of all this,” he noted.
Diabetes technology: The rise of CGM during pandemic and beyond
New information about continuous glucose monitoring (CGM) has been added to the diabetes technology section. Use of CGM is now recommended for anyone with diabetes who takes multiple daily injections or uses an insulin pump, regardless of age or diabetes type. The document provides expanded advice on use of time in range data for glycemic monitoring, particularly during the COVID-19 pandemic when remote monitoring is preferable.
Insurers are increasingly covering CGM for patients on insulin, but it’s far from universal. While the ultimate goal is to ensure access to CGM for everyone with diabetes, those treated with multiple daily insulin doses are the priority for now.
“Our hope is that as there’s greater evidence there will be more movement towards coverage. There are still so many people for whom it’s quite clear they would benefit because they’re on insulin but don’t have access to it. That’s an important area that ADA is advocating for, and it’s reflected in the standards of care,” Dr. Gabbay said.
In another technology-related revision, the term “blinded” CGM has been replaced with “professional CGM,” because clinic-based use of the devices can be “blinded” to the patient or monitored in real-time by both the patient and clinician. Also, a new recommendation has been added to address skin reactions associated with diabetes technology use.
Information about use of CGM in hospital settings during the COVID-19 pandemic has also been added in the technology section.
The COVID-19 pandemic comes up again in the section on vaccines.
“We mention that people with diabetes should be considered high priority [for COVID-19 vaccines], and that’s something that ADA is strongly advocating for because 40% of COVID-19 deaths have been in people with diabetes,” Dr. Gabbay said.
Dr. Gabbay reported being on the advisory boards of Onduo, Health Reveal, Vida Health, Lark, and Form Health.
A version of this article originally appeared on Medscape.com.
For 2021, the American Diabetes Association offers new guidance on assessing patients’ financial and social barriers to care, especially given the COVID-19 pandemic, individualizing treatment of patients with type 2 diabetes, and use of diabetes technology.
As it does every year, the annual update incorporates new clinical information that has become available since the last guideline, with occasional revisions during the year as needed. “Standards of Medical Care in Diabetes – 2021,” was published online as a supplement to Diabetes Care.
The new standards advise that patients be assessed for food and housing insecurity, social support, and “cost-related medication nonadherence,” and those found to have difficulty referred to appropriate community resources.
“Clinicians need to be sensitive to the fact that patients may have very good reasons for not taking their medication, [as in] if they can’t afford it,” ADA chief science & medical officer Robert A. Gabbay, MD, PhD, said in an interview.
Dr. Gabbay noted that “a heightened awareness” of social determinants of health is weaved throughout the 2021 standards because of the pandemic, with information on the topic derived from a July 2020 joint consensus statement in Diabetes Care, endorsed by a number of other societies, as well as a November publication also in Diabetes Care.
“We made several recommendations that speak to social determinants of health, placing an emphasis on engaging in conversations around this subject and screening for related issues such as food insecurity that weren’t there previously,” he said.
“Screening tools are suggested. It helped us to have an in-depth scientific review of the literature to know the prevalence of this in people with diabetes. ... Having the science to put it in was a key step,” Dr. Gabbay noted.
Consider kidney, heart disease in type 2 treatment individualization
Recent data from trials such as CREDENCE and DAPA-HF, among others, have been added to inform the choice of pharmacologic treatment in patients with type 2 diabetes with comorbid diabetic kidney disease and chronic heart failure.
“ADA has been advocating individualization of treatment based on comorbidities for a while, but we’ve taken more steps in that direction. Beyond lifestyle for all individuals with type 2 diabetes, clinicians want to think early on about which comorbidities patients have and then think about the appropriate treatment based on that,” Dr. Gabbay said.
And for the third year in a row, the section on cardiovascular disease and risk management has been endorsed by the American College of Cardiology.
“All the things in that section are very much aligned with ACC and that’s been a great partnership,” Dr. Gabbay said.
Now, ADA is in discussions with other professional societies representing relevant specialties to create further such unified messages.
“What we all want to avoid is having multiple different guidelines. We want to speak with one voice and find common ground as much as possible. … It makes it much easier for clinicians to know what to do. That’s the goal of all this,” he noted.
Diabetes technology: The rise of CGM during pandemic and beyond
New information about continuous glucose monitoring (CGM) has been added to the diabetes technology section. Use of CGM is now recommended for anyone with diabetes who takes multiple daily injections or uses an insulin pump, regardless of age or diabetes type. The document provides expanded advice on use of time in range data for glycemic monitoring, particularly during the COVID-19 pandemic when remote monitoring is preferable.
Insurers are increasingly covering CGM for patients on insulin, but it’s far from universal. While the ultimate goal is to ensure access to CGM for everyone with diabetes, those treated with multiple daily insulin doses are the priority for now.
“Our hope is that as there’s greater evidence there will be more movement towards coverage. There are still so many people for whom it’s quite clear they would benefit because they’re on insulin but don’t have access to it. That’s an important area that ADA is advocating for, and it’s reflected in the standards of care,” Dr. Gabbay said.
In another technology-related revision, the term “blinded” CGM has been replaced with “professional CGM,” because clinic-based use of the devices can be “blinded” to the patient or monitored in real-time by both the patient and clinician. Also, a new recommendation has been added to address skin reactions associated with diabetes technology use.
Information about use of CGM in hospital settings during the COVID-19 pandemic has also been added in the technology section.
The COVID-19 pandemic comes up again in the section on vaccines.
“We mention that people with diabetes should be considered high priority [for COVID-19 vaccines], and that’s something that ADA is strongly advocating for because 40% of COVID-19 deaths have been in people with diabetes,” Dr. Gabbay said.
Dr. Gabbay reported being on the advisory boards of Onduo, Health Reveal, Vida Health, Lark, and Form Health.
A version of this article originally appeared on Medscape.com.
For 2021, the American Diabetes Association offers new guidance on assessing patients’ financial and social barriers to care, especially given the COVID-19 pandemic, individualizing treatment of patients with type 2 diabetes, and use of diabetes technology.
As it does every year, the annual update incorporates new clinical information that has become available since the last guideline, with occasional revisions during the year as needed. “Standards of Medical Care in Diabetes – 2021,” was published online as a supplement to Diabetes Care.
The new standards advise that patients be assessed for food and housing insecurity, social support, and “cost-related medication nonadherence,” and those found to have difficulty referred to appropriate community resources.
“Clinicians need to be sensitive to the fact that patients may have very good reasons for not taking their medication, [as in] if they can’t afford it,” ADA chief science & medical officer Robert A. Gabbay, MD, PhD, said in an interview.
Dr. Gabbay noted that “a heightened awareness” of social determinants of health is weaved throughout the 2021 standards because of the pandemic, with information on the topic derived from a July 2020 joint consensus statement in Diabetes Care, endorsed by a number of other societies, as well as a November publication also in Diabetes Care.
“We made several recommendations that speak to social determinants of health, placing an emphasis on engaging in conversations around this subject and screening for related issues such as food insecurity that weren’t there previously,” he said.
“Screening tools are suggested. It helped us to have an in-depth scientific review of the literature to know the prevalence of this in people with diabetes. ... Having the science to put it in was a key step,” Dr. Gabbay noted.
Consider kidney, heart disease in type 2 treatment individualization
Recent data from trials such as CREDENCE and DAPA-HF, among others, have been added to inform the choice of pharmacologic treatment in patients with type 2 diabetes with comorbid diabetic kidney disease and chronic heart failure.
“ADA has been advocating individualization of treatment based on comorbidities for a while, but we’ve taken more steps in that direction. Beyond lifestyle for all individuals with type 2 diabetes, clinicians want to think early on about which comorbidities patients have and then think about the appropriate treatment based on that,” Dr. Gabbay said.
And for the third year in a row, the section on cardiovascular disease and risk management has been endorsed by the American College of Cardiology.
“All the things in that section are very much aligned with ACC and that’s been a great partnership,” Dr. Gabbay said.
Now, ADA is in discussions with other professional societies representing relevant specialties to create further such unified messages.
“What we all want to avoid is having multiple different guidelines. We want to speak with one voice and find common ground as much as possible. … It makes it much easier for clinicians to know what to do. That’s the goal of all this,” he noted.
Diabetes technology: The rise of CGM during pandemic and beyond
New information about continuous glucose monitoring (CGM) has been added to the diabetes technology section. Use of CGM is now recommended for anyone with diabetes who takes multiple daily injections or uses an insulin pump, regardless of age or diabetes type. The document provides expanded advice on use of time in range data for glycemic monitoring, particularly during the COVID-19 pandemic when remote monitoring is preferable.
Insurers are increasingly covering CGM for patients on insulin, but it’s far from universal. While the ultimate goal is to ensure access to CGM for everyone with diabetes, those treated with multiple daily insulin doses are the priority for now.
“Our hope is that as there’s greater evidence there will be more movement towards coverage. There are still so many people for whom it’s quite clear they would benefit because they’re on insulin but don’t have access to it. That’s an important area that ADA is advocating for, and it’s reflected in the standards of care,” Dr. Gabbay said.
In another technology-related revision, the term “blinded” CGM has been replaced with “professional CGM,” because clinic-based use of the devices can be “blinded” to the patient or monitored in real-time by both the patient and clinician. Also, a new recommendation has been added to address skin reactions associated with diabetes technology use.
Information about use of CGM in hospital settings during the COVID-19 pandemic has also been added in the technology section.
The COVID-19 pandemic comes up again in the section on vaccines.
“We mention that people with diabetes should be considered high priority [for COVID-19 vaccines], and that’s something that ADA is strongly advocating for because 40% of COVID-19 deaths have been in people with diabetes,” Dr. Gabbay said.
Dr. Gabbay reported being on the advisory boards of Onduo, Health Reveal, Vida Health, Lark, and Form Health.
A version of this article originally appeared on Medscape.com.
Medicare payments could get tougher for docs
More than 40 value-based payment models – from direct contracting to bundled payments – have been introduced into the Medicare program in the past 10 years, with the goal of improving care while lowering costs. Hopes were high that they would be successful.
Physicians could suffer a huge blow to their income.
Many of the value-based care models simply did not work as expected, said Seema Verma, head of the Centers for Medicare & Medicaid Services, at a recent HLTH Conference. “They are not producing the types of savings the taxpayers deserve,” Ms. Verma said.
The Medicare Payment Advisory Commission (MedPac) concluded that, while dozens of payment models were tested, most failed to generate net savings for Medicare. Even the most successful of the models produced only modest savings. MedPac elaborated: “The track record raises the question of whether changes to particular models or CMMI’s [Center for Medicare & Medicaid Innovation’s] broader strategies might be warranted.”
What will happen now, as government officials admit that their value-based programs haven’t worked? The value-based programs could become more stringent. Here’s what physicians will have to contend with.
More risk. Experts agree that risk – financial risk – will be a component of future programs. Two-sided risk is likely to be the norm. This means that both parties – the provider and the insurer – are at financial risk for the patients covered by the program.
For example, a plan with 50,000 beneficiary patients would estimate the cost of caring for those patients on the basis of multiple variables. If the actual cost is lower than anticipated, both parties share in the savings. However, both share in the loss if the cost of caring for their patient population exceeds expectations.
This may compel physicians to enhance efficiency and potentially limit the services provided to patients. Typically, however, the strategy is to make efforts to prevent services like ED visits and admissions by focusing on health maintenance.
In contrast to most current value-based models, which feature little to no downside risk for physicians, double-sided risk means physicians could lose money. The loss may incorporate a cap – 5%, for example – but programs may differ. Experts concur that double-sided risk will be a hallmark of future programs.
Better data. The majority of health care services are rendered via fee-for-service: Patients receive services and physicians are paid, yet little or no information about outcomes is exchanged between insurers and physicians.
Penny Noyes, president of Health Business Navigators and contract negotiator for physicians, is not a fan of the current crop of value-based programs and feels that data transparency is positive. Sound metrics can lead to improvement, she said, adding: “It’s not money that drives physicians to make decisions; it’s what’s in the best interest of their patients and their patients’ long-term care.”
Value-based programs can work but only if applicable data are developed and given to physicians so that they can better understand their current performance and how to improve.
Mandated participation. Participation in value-based programs has been voluntary, but that may have skewed the results, which were better than what typical practice would have shown. Acknowledging this may lead CMS to call for mandated participation as a component of future programs. Physicians may be brought into programs, if only to determine whether the models really work. To date, participation in the programs has been voluntary, but that may change in the future.
Innovation. The private insurance market may end up as a key player. Over the past 6 months, health insurers have either consolidated partnerships with telemedicine companies to provide no-cost care to beneficiaries or have launched their own initiatives.
Others are focused on bringing together patients and providers operating outside of the traditional health care system, such as Aetna’s merger with CVS which now offers retail-based acute care (MinuteClinic) and chronic care (HealthHUB). Still other payers are gambling with physician practice ownership, as in the case of United Healthcare’s OptumHealth, which now boasts around 50,000 physicians throughout the country.
New practice models are emerging in private practices as well. Physicians are embracing remote care, proactively managing care transitions, and seeking out more methods to keep patients healthy and at home.
Not much was expected from value-based plans
Many are not surprised that the value-based models did not produce impressive results. Ms. Noyes doubted that positive outcomes will be achieved for physicians in comparison with what could have been attained under fee-for-service arrangements with lower administrative costs.
While the Affordable Care Act attempted to encourage alternative reimbursement, it limits the maximum medical loss ratio (MLR) a payer could achieve. For many plans, that maximum was 85%. Simply put, at least $0.85 of each premium collected had to be paid in claims; the remaining $0.15 went to margin, claims, and other administrative costs. A payer with an 82% MLR then would have to rebate the 3% difference to enrollees.
But that’s not what occurred, according to Ms. Noyes. Because value-based payments to providers are considered a claims expense, an MLR ratio of 82% allowed the payer to distribute the 3% difference to providers as value-based payments. Ms. Noyes said: “That may sound good for the provider, but the result was essentially a freeze on the provider’s fee-for-service reimbursement with the prospect of getting value-based payments like ‘shared savings.’
“When the providers tried to increase their base fee-for-service rates just to match inflation, payers often advised that any future raises had to be earned through value-based programs,” Ms. Noyes added. The value-based formulas confuse providers because payments are often made for periods as far back as 18 months, and providers do not have data systems to reconcile their payer report cards retrospectively. The result is that providers tended to accept whatever amount the payer distributed.
Executives at Lumeris, a company that helps health systems participate successfully in value-based care, see potential in a newer approach to alternative payments, such as CMS’ Direct Contracting initiative. This voluntary payment model offers options tailored to several types of organizations that aim to reduce costs while preserving or enhancing the quality of care for Medicare fee-for-service beneficiaries.
Jeff Smith, chief commercial officer for population health at Lumeris, explained that the Direct Contracting initiative can provide physicians with a more attractive option than prior value-based models because it adjusts for the complexity and fragility of patients with complex and chronic conditions. By allowing providers to participate in the savings generated, the initiative stands in stark contrast to what Mr. Smith described as the “shared savings to nothingness” experienced by providers in earlier-stage alternative payment models.
Physicians engaged with value-based programs like Direct Contracting are investing in nurses to aid with initiatives regarding health promotion and transitions of care. When a patient is discharged, for example, the nurse contacts the patient to discuss medications, schedule follow-up appointments, and so forth – tasks typically left to the patient (or caregiver) to navigate in the traditional system.
The initiative recognizes the importance of managing high-risk patients, those whom physicians identify as having an extraordinary number of ED visits and admissions. These patients, as well as so-called “rising-risk” patients, are targeted by nurses who proactively communicate with patients (and caregivers) to address patient’s needs, including social determinants of health.
Physicians who have a large load of patients in value-based programs are hiring social workers, pharmacists, and behavioral health experts to help. Of course, these personnel are costly, but that’s what the value-based programs aim to reimburse.
Still, the road ahead to value based is rocky and may not gain momentum for some time. Johns Hopkins University’s Doug Hough, PhD, an economist, recounts a government research study that sought to assess the university’s health system participation in a value-based payment program. While there were positive impacts on the program’s target population, Hough and his team discovered that the returns achieved by the optional model didn’t justify the health system’s financial support for it. The increasingly indebted health system ultimately decided to drop the optional program.
Dr. Hough indicated that the health system – Johns Hopkins Medicine – likely would have continued its support for the program had the government at least allowed it to break even. Although the payment program under study was a 3-year project, the bigger challenge, declared Dr. Hough, is that “we can’t turn an aircraft carrier that quickly.”
“Three years won’t show whether value-based care is really working,” Dr. Hough said.
Robert Zipper, MD, a hospitalist and senior policy advisor for Sound Physicians, a company that works to improve outcomes in acute care, agreed with Dr. Hough that performance tends to improve with time. Yet, Dr. Zipper doesn’t see much change in the near term, because “after all, there is nothing to replace them [the programs].”
The problem gets even stickier for private payers because patients may be on an insurance panel for as little as a year or 2. Thanks to this rapid churn of beneficiaries, even the best-designed value-based program will have little time to prove its worth.
Dr. Zipper is among the many who don’t expect significant changes in the near term, asserting that “President Biden will want to get a few policy wins first, and health care is not the easiest place to start.”
But it’s likely that payers and others will want to see more emphasis on value-based programs despite these programs’ possible value to patients, physicians, and health systems alike.
A version of this article originally appeared on Medscape.com.
More than 40 value-based payment models – from direct contracting to bundled payments – have been introduced into the Medicare program in the past 10 years, with the goal of improving care while lowering costs. Hopes were high that they would be successful.
Physicians could suffer a huge blow to their income.
Many of the value-based care models simply did not work as expected, said Seema Verma, head of the Centers for Medicare & Medicaid Services, at a recent HLTH Conference. “They are not producing the types of savings the taxpayers deserve,” Ms. Verma said.
The Medicare Payment Advisory Commission (MedPac) concluded that, while dozens of payment models were tested, most failed to generate net savings for Medicare. Even the most successful of the models produced only modest savings. MedPac elaborated: “The track record raises the question of whether changes to particular models or CMMI’s [Center for Medicare & Medicaid Innovation’s] broader strategies might be warranted.”
What will happen now, as government officials admit that their value-based programs haven’t worked? The value-based programs could become more stringent. Here’s what physicians will have to contend with.
More risk. Experts agree that risk – financial risk – will be a component of future programs. Two-sided risk is likely to be the norm. This means that both parties – the provider and the insurer – are at financial risk for the patients covered by the program.
For example, a plan with 50,000 beneficiary patients would estimate the cost of caring for those patients on the basis of multiple variables. If the actual cost is lower than anticipated, both parties share in the savings. However, both share in the loss if the cost of caring for their patient population exceeds expectations.
This may compel physicians to enhance efficiency and potentially limit the services provided to patients. Typically, however, the strategy is to make efforts to prevent services like ED visits and admissions by focusing on health maintenance.
In contrast to most current value-based models, which feature little to no downside risk for physicians, double-sided risk means physicians could lose money. The loss may incorporate a cap – 5%, for example – but programs may differ. Experts concur that double-sided risk will be a hallmark of future programs.
Better data. The majority of health care services are rendered via fee-for-service: Patients receive services and physicians are paid, yet little or no information about outcomes is exchanged between insurers and physicians.
Penny Noyes, president of Health Business Navigators and contract negotiator for physicians, is not a fan of the current crop of value-based programs and feels that data transparency is positive. Sound metrics can lead to improvement, she said, adding: “It’s not money that drives physicians to make decisions; it’s what’s in the best interest of their patients and their patients’ long-term care.”
Value-based programs can work but only if applicable data are developed and given to physicians so that they can better understand their current performance and how to improve.
Mandated participation. Participation in value-based programs has been voluntary, but that may have skewed the results, which were better than what typical practice would have shown. Acknowledging this may lead CMS to call for mandated participation as a component of future programs. Physicians may be brought into programs, if only to determine whether the models really work. To date, participation in the programs has been voluntary, but that may change in the future.
Innovation. The private insurance market may end up as a key player. Over the past 6 months, health insurers have either consolidated partnerships with telemedicine companies to provide no-cost care to beneficiaries or have launched their own initiatives.
Others are focused on bringing together patients and providers operating outside of the traditional health care system, such as Aetna’s merger with CVS which now offers retail-based acute care (MinuteClinic) and chronic care (HealthHUB). Still other payers are gambling with physician practice ownership, as in the case of United Healthcare’s OptumHealth, which now boasts around 50,000 physicians throughout the country.
New practice models are emerging in private practices as well. Physicians are embracing remote care, proactively managing care transitions, and seeking out more methods to keep patients healthy and at home.
Not much was expected from value-based plans
Many are not surprised that the value-based models did not produce impressive results. Ms. Noyes doubted that positive outcomes will be achieved for physicians in comparison with what could have been attained under fee-for-service arrangements with lower administrative costs.
While the Affordable Care Act attempted to encourage alternative reimbursement, it limits the maximum medical loss ratio (MLR) a payer could achieve. For many plans, that maximum was 85%. Simply put, at least $0.85 of each premium collected had to be paid in claims; the remaining $0.15 went to margin, claims, and other administrative costs. A payer with an 82% MLR then would have to rebate the 3% difference to enrollees.
But that’s not what occurred, according to Ms. Noyes. Because value-based payments to providers are considered a claims expense, an MLR ratio of 82% allowed the payer to distribute the 3% difference to providers as value-based payments. Ms. Noyes said: “That may sound good for the provider, but the result was essentially a freeze on the provider’s fee-for-service reimbursement with the prospect of getting value-based payments like ‘shared savings.’
“When the providers tried to increase their base fee-for-service rates just to match inflation, payers often advised that any future raises had to be earned through value-based programs,” Ms. Noyes added. The value-based formulas confuse providers because payments are often made for periods as far back as 18 months, and providers do not have data systems to reconcile their payer report cards retrospectively. The result is that providers tended to accept whatever amount the payer distributed.
Executives at Lumeris, a company that helps health systems participate successfully in value-based care, see potential in a newer approach to alternative payments, such as CMS’ Direct Contracting initiative. This voluntary payment model offers options tailored to several types of organizations that aim to reduce costs while preserving or enhancing the quality of care for Medicare fee-for-service beneficiaries.
Jeff Smith, chief commercial officer for population health at Lumeris, explained that the Direct Contracting initiative can provide physicians with a more attractive option than prior value-based models because it adjusts for the complexity and fragility of patients with complex and chronic conditions. By allowing providers to participate in the savings generated, the initiative stands in stark contrast to what Mr. Smith described as the “shared savings to nothingness” experienced by providers in earlier-stage alternative payment models.
Physicians engaged with value-based programs like Direct Contracting are investing in nurses to aid with initiatives regarding health promotion and transitions of care. When a patient is discharged, for example, the nurse contacts the patient to discuss medications, schedule follow-up appointments, and so forth – tasks typically left to the patient (or caregiver) to navigate in the traditional system.
The initiative recognizes the importance of managing high-risk patients, those whom physicians identify as having an extraordinary number of ED visits and admissions. These patients, as well as so-called “rising-risk” patients, are targeted by nurses who proactively communicate with patients (and caregivers) to address patient’s needs, including social determinants of health.
Physicians who have a large load of patients in value-based programs are hiring social workers, pharmacists, and behavioral health experts to help. Of course, these personnel are costly, but that’s what the value-based programs aim to reimburse.
Still, the road ahead to value based is rocky and may not gain momentum for some time. Johns Hopkins University’s Doug Hough, PhD, an economist, recounts a government research study that sought to assess the university’s health system participation in a value-based payment program. While there were positive impacts on the program’s target population, Hough and his team discovered that the returns achieved by the optional model didn’t justify the health system’s financial support for it. The increasingly indebted health system ultimately decided to drop the optional program.
Dr. Hough indicated that the health system – Johns Hopkins Medicine – likely would have continued its support for the program had the government at least allowed it to break even. Although the payment program under study was a 3-year project, the bigger challenge, declared Dr. Hough, is that “we can’t turn an aircraft carrier that quickly.”
“Three years won’t show whether value-based care is really working,” Dr. Hough said.
Robert Zipper, MD, a hospitalist and senior policy advisor for Sound Physicians, a company that works to improve outcomes in acute care, agreed with Dr. Hough that performance tends to improve with time. Yet, Dr. Zipper doesn’t see much change in the near term, because “after all, there is nothing to replace them [the programs].”
The problem gets even stickier for private payers because patients may be on an insurance panel for as little as a year or 2. Thanks to this rapid churn of beneficiaries, even the best-designed value-based program will have little time to prove its worth.
Dr. Zipper is among the many who don’t expect significant changes in the near term, asserting that “President Biden will want to get a few policy wins first, and health care is not the easiest place to start.”
But it’s likely that payers and others will want to see more emphasis on value-based programs despite these programs’ possible value to patients, physicians, and health systems alike.
A version of this article originally appeared on Medscape.com.
More than 40 value-based payment models – from direct contracting to bundled payments – have been introduced into the Medicare program in the past 10 years, with the goal of improving care while lowering costs. Hopes were high that they would be successful.
Physicians could suffer a huge blow to their income.
Many of the value-based care models simply did not work as expected, said Seema Verma, head of the Centers for Medicare & Medicaid Services, at a recent HLTH Conference. “They are not producing the types of savings the taxpayers deserve,” Ms. Verma said.
The Medicare Payment Advisory Commission (MedPac) concluded that, while dozens of payment models were tested, most failed to generate net savings for Medicare. Even the most successful of the models produced only modest savings. MedPac elaborated: “The track record raises the question of whether changes to particular models or CMMI’s [Center for Medicare & Medicaid Innovation’s] broader strategies might be warranted.”
What will happen now, as government officials admit that their value-based programs haven’t worked? The value-based programs could become more stringent. Here’s what physicians will have to contend with.
More risk. Experts agree that risk – financial risk – will be a component of future programs. Two-sided risk is likely to be the norm. This means that both parties – the provider and the insurer – are at financial risk for the patients covered by the program.
For example, a plan with 50,000 beneficiary patients would estimate the cost of caring for those patients on the basis of multiple variables. If the actual cost is lower than anticipated, both parties share in the savings. However, both share in the loss if the cost of caring for their patient population exceeds expectations.
This may compel physicians to enhance efficiency and potentially limit the services provided to patients. Typically, however, the strategy is to make efforts to prevent services like ED visits and admissions by focusing on health maintenance.
In contrast to most current value-based models, which feature little to no downside risk for physicians, double-sided risk means physicians could lose money. The loss may incorporate a cap – 5%, for example – but programs may differ. Experts concur that double-sided risk will be a hallmark of future programs.
Better data. The majority of health care services are rendered via fee-for-service: Patients receive services and physicians are paid, yet little or no information about outcomes is exchanged between insurers and physicians.
Penny Noyes, president of Health Business Navigators and contract negotiator for physicians, is not a fan of the current crop of value-based programs and feels that data transparency is positive. Sound metrics can lead to improvement, she said, adding: “It’s not money that drives physicians to make decisions; it’s what’s in the best interest of their patients and their patients’ long-term care.”
Value-based programs can work but only if applicable data are developed and given to physicians so that they can better understand their current performance and how to improve.
Mandated participation. Participation in value-based programs has been voluntary, but that may have skewed the results, which were better than what typical practice would have shown. Acknowledging this may lead CMS to call for mandated participation as a component of future programs. Physicians may be brought into programs, if only to determine whether the models really work. To date, participation in the programs has been voluntary, but that may change in the future.
Innovation. The private insurance market may end up as a key player. Over the past 6 months, health insurers have either consolidated partnerships with telemedicine companies to provide no-cost care to beneficiaries or have launched their own initiatives.
Others are focused on bringing together patients and providers operating outside of the traditional health care system, such as Aetna’s merger with CVS which now offers retail-based acute care (MinuteClinic) and chronic care (HealthHUB). Still other payers are gambling with physician practice ownership, as in the case of United Healthcare’s OptumHealth, which now boasts around 50,000 physicians throughout the country.
New practice models are emerging in private practices as well. Physicians are embracing remote care, proactively managing care transitions, and seeking out more methods to keep patients healthy and at home.
Not much was expected from value-based plans
Many are not surprised that the value-based models did not produce impressive results. Ms. Noyes doubted that positive outcomes will be achieved for physicians in comparison with what could have been attained under fee-for-service arrangements with lower administrative costs.
While the Affordable Care Act attempted to encourage alternative reimbursement, it limits the maximum medical loss ratio (MLR) a payer could achieve. For many plans, that maximum was 85%. Simply put, at least $0.85 of each premium collected had to be paid in claims; the remaining $0.15 went to margin, claims, and other administrative costs. A payer with an 82% MLR then would have to rebate the 3% difference to enrollees.
But that’s not what occurred, according to Ms. Noyes. Because value-based payments to providers are considered a claims expense, an MLR ratio of 82% allowed the payer to distribute the 3% difference to providers as value-based payments. Ms. Noyes said: “That may sound good for the provider, but the result was essentially a freeze on the provider’s fee-for-service reimbursement with the prospect of getting value-based payments like ‘shared savings.’
“When the providers tried to increase their base fee-for-service rates just to match inflation, payers often advised that any future raises had to be earned through value-based programs,” Ms. Noyes added. The value-based formulas confuse providers because payments are often made for periods as far back as 18 months, and providers do not have data systems to reconcile their payer report cards retrospectively. The result is that providers tended to accept whatever amount the payer distributed.
Executives at Lumeris, a company that helps health systems participate successfully in value-based care, see potential in a newer approach to alternative payments, such as CMS’ Direct Contracting initiative. This voluntary payment model offers options tailored to several types of organizations that aim to reduce costs while preserving or enhancing the quality of care for Medicare fee-for-service beneficiaries.
Jeff Smith, chief commercial officer for population health at Lumeris, explained that the Direct Contracting initiative can provide physicians with a more attractive option than prior value-based models because it adjusts for the complexity and fragility of patients with complex and chronic conditions. By allowing providers to participate in the savings generated, the initiative stands in stark contrast to what Mr. Smith described as the “shared savings to nothingness” experienced by providers in earlier-stage alternative payment models.
Physicians engaged with value-based programs like Direct Contracting are investing in nurses to aid with initiatives regarding health promotion and transitions of care. When a patient is discharged, for example, the nurse contacts the patient to discuss medications, schedule follow-up appointments, and so forth – tasks typically left to the patient (or caregiver) to navigate in the traditional system.
The initiative recognizes the importance of managing high-risk patients, those whom physicians identify as having an extraordinary number of ED visits and admissions. These patients, as well as so-called “rising-risk” patients, are targeted by nurses who proactively communicate with patients (and caregivers) to address patient’s needs, including social determinants of health.
Physicians who have a large load of patients in value-based programs are hiring social workers, pharmacists, and behavioral health experts to help. Of course, these personnel are costly, but that’s what the value-based programs aim to reimburse.
Still, the road ahead to value based is rocky and may not gain momentum for some time. Johns Hopkins University’s Doug Hough, PhD, an economist, recounts a government research study that sought to assess the university’s health system participation in a value-based payment program. While there were positive impacts on the program’s target population, Hough and his team discovered that the returns achieved by the optional model didn’t justify the health system’s financial support for it. The increasingly indebted health system ultimately decided to drop the optional program.
Dr. Hough indicated that the health system – Johns Hopkins Medicine – likely would have continued its support for the program had the government at least allowed it to break even. Although the payment program under study was a 3-year project, the bigger challenge, declared Dr. Hough, is that “we can’t turn an aircraft carrier that quickly.”
“Three years won’t show whether value-based care is really working,” Dr. Hough said.
Robert Zipper, MD, a hospitalist and senior policy advisor for Sound Physicians, a company that works to improve outcomes in acute care, agreed with Dr. Hough that performance tends to improve with time. Yet, Dr. Zipper doesn’t see much change in the near term, because “after all, there is nothing to replace them [the programs].”
The problem gets even stickier for private payers because patients may be on an insurance panel for as little as a year or 2. Thanks to this rapid churn of beneficiaries, even the best-designed value-based program will have little time to prove its worth.
Dr. Zipper is among the many who don’t expect significant changes in the near term, asserting that “President Biden will want to get a few policy wins first, and health care is not the easiest place to start.”
But it’s likely that payers and others will want to see more emphasis on value-based programs despite these programs’ possible value to patients, physicians, and health systems alike.
A version of this article originally appeared on Medscape.com.
Should all skin cancer patients be taking nicotinamide?
In 2014, I began taking care of a patient (see photo) who had developed over 25 basal cell carcinomas on her lower legs, which were surgically removed. She has been clear of any skin cancers in the last 2 years since starting supplementation.
Nicotinamide, also known as niacinamide, is a water soluble form of vitamin B3 that has been shown to enhance the repair of UV-induced DNA damage. Nicotinamide is found naturally in meat, fish, nuts, grains, and legumes, and is a key component of the glycolysis pathway, by generating nicotinamide adenine dinucleotide for adenosine triphosphate production. Nicotinamide deficiency causes photosensitive dermatitis, diarrhea, and dementia. It has been studied for its anti-inflammatory benefits as an adjunct treatment for rosacea, bullous diseases, acne, and melasma.
Nonmelanoma skin cancers are known to be caused primarily by UV radiation. The supplementation of nicotinamide orally twice daily has been shown to reduce the rate of actinic keratoses and new nonmelanoma skin cancers compared with placebo after 1 year in patients who previously had skin cancer. In the phase 3 study published in 2015, a randomized, controlled trial of 386 patients who had at least two nonmelanoma skin cancers within the previous 5-year period, oral nicotinamide 500 mg given twice daily for a 12-month period significantly reduced the number of new nonmelanoma skin cancers by 23% versus those on placebo.
The recommended dose for nicotinamide, which is available over the counter as Vitamin B3, is 500 mg twice a day. Nicotinamide should not be confused with niacin (nicotinic acid), which has been used to treat high cholesterol and cardiovascular disease. There are no significant side effects from long-term use; however nicotinamide should not be used in patients with end-stage kidney disease or chronic kidney disease. (Niacin, however, can cause elevation of liver enzymes, headache, flushing, and increased blood pressure.) Nicotinamide crosses the placenta and should not be used in pregnancy as it has not been studied in pregnant populations.
We should counsel patients that this is not an oral sunscreen, and that sun avoidance, sunscreen, and yearly skin cancer checks are still the mainstay of skin cancer prevention. However, given the safety profile of nicotinamide and the protective effects, should all of our skin cancer patients be taking nicotinamide daily? In my practice they are, all of whom swear by it and have had significant reductions of both actinic keratoses and nonmelanoma skin cancers.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
In 2014, I began taking care of a patient (see photo) who had developed over 25 basal cell carcinomas on her lower legs, which were surgically removed. She has been clear of any skin cancers in the last 2 years since starting supplementation.
Nicotinamide, also known as niacinamide, is a water soluble form of vitamin B3 that has been shown to enhance the repair of UV-induced DNA damage. Nicotinamide is found naturally in meat, fish, nuts, grains, and legumes, and is a key component of the glycolysis pathway, by generating nicotinamide adenine dinucleotide for adenosine triphosphate production. Nicotinamide deficiency causes photosensitive dermatitis, diarrhea, and dementia. It has been studied for its anti-inflammatory benefits as an adjunct treatment for rosacea, bullous diseases, acne, and melasma.
Nonmelanoma skin cancers are known to be caused primarily by UV radiation. The supplementation of nicotinamide orally twice daily has been shown to reduce the rate of actinic keratoses and new nonmelanoma skin cancers compared with placebo after 1 year in patients who previously had skin cancer. In the phase 3 study published in 2015, a randomized, controlled trial of 386 patients who had at least two nonmelanoma skin cancers within the previous 5-year period, oral nicotinamide 500 mg given twice daily for a 12-month period significantly reduced the number of new nonmelanoma skin cancers by 23% versus those on placebo.
The recommended dose for nicotinamide, which is available over the counter as Vitamin B3, is 500 mg twice a day. Nicotinamide should not be confused with niacin (nicotinic acid), which has been used to treat high cholesterol and cardiovascular disease. There are no significant side effects from long-term use; however nicotinamide should not be used in patients with end-stage kidney disease or chronic kidney disease. (Niacin, however, can cause elevation of liver enzymes, headache, flushing, and increased blood pressure.) Nicotinamide crosses the placenta and should not be used in pregnancy as it has not been studied in pregnant populations.
We should counsel patients that this is not an oral sunscreen, and that sun avoidance, sunscreen, and yearly skin cancer checks are still the mainstay of skin cancer prevention. However, given the safety profile of nicotinamide and the protective effects, should all of our skin cancer patients be taking nicotinamide daily? In my practice they are, all of whom swear by it and have had significant reductions of both actinic keratoses and nonmelanoma skin cancers.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
In 2014, I began taking care of a patient (see photo) who had developed over 25 basal cell carcinomas on her lower legs, which were surgically removed. She has been clear of any skin cancers in the last 2 years since starting supplementation.
Nicotinamide, also known as niacinamide, is a water soluble form of vitamin B3 that has been shown to enhance the repair of UV-induced DNA damage. Nicotinamide is found naturally in meat, fish, nuts, grains, and legumes, and is a key component of the glycolysis pathway, by generating nicotinamide adenine dinucleotide for adenosine triphosphate production. Nicotinamide deficiency causes photosensitive dermatitis, diarrhea, and dementia. It has been studied for its anti-inflammatory benefits as an adjunct treatment for rosacea, bullous diseases, acne, and melasma.
Nonmelanoma skin cancers are known to be caused primarily by UV radiation. The supplementation of nicotinamide orally twice daily has been shown to reduce the rate of actinic keratoses and new nonmelanoma skin cancers compared with placebo after 1 year in patients who previously had skin cancer. In the phase 3 study published in 2015, a randomized, controlled trial of 386 patients who had at least two nonmelanoma skin cancers within the previous 5-year period, oral nicotinamide 500 mg given twice daily for a 12-month period significantly reduced the number of new nonmelanoma skin cancers by 23% versus those on placebo.
The recommended dose for nicotinamide, which is available over the counter as Vitamin B3, is 500 mg twice a day. Nicotinamide should not be confused with niacin (nicotinic acid), which has been used to treat high cholesterol and cardiovascular disease. There are no significant side effects from long-term use; however nicotinamide should not be used in patients with end-stage kidney disease or chronic kidney disease. (Niacin, however, can cause elevation of liver enzymes, headache, flushing, and increased blood pressure.) Nicotinamide crosses the placenta and should not be used in pregnancy as it has not been studied in pregnant populations.
We should counsel patients that this is not an oral sunscreen, and that sun avoidance, sunscreen, and yearly skin cancer checks are still the mainstay of skin cancer prevention. However, given the safety profile of nicotinamide and the protective effects, should all of our skin cancer patients be taking nicotinamide daily? In my practice they are, all of whom swear by it and have had significant reductions of both actinic keratoses and nonmelanoma skin cancers.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
A shot in the arm
As the COVID-19 vaccine candidates have begun to roll off the production lines into the distribution networks by the millions, media coverage almost universally includes a still photo or video of someone receiving an injection. Ever observant, a retired lawyer friend of mine who learned to give shots when he was in the Army and again more recently while taking a wilderness survival course emailed me his concerns about what he felt were examples of poor injection technique. Included in his commentary was an Internet link in which a physician, who I suspect may have been a pediatrician, demonstrated what the physician considered proper intramuscular injection technique, which included a single-handed aspiration prior to giving the injection allowing the free hand to stabilize the patient’s – in this case a child’s – arm during the entire process.
I replied to my friend that I too was often troubled by what I considered to be poor injection technique. But, I said the physician in the link touting his improved technique was misguided. My understanding has been that unless the injection site is in the gluteus, there is no need aspirate prior to an intramuscular vaccine injection because the risk of intravascular injection is so small. I then confirmed this by reviewing the Centers for Disease Control and Prevention’s Vaccine Recommendations and Guidelines of the Advisory Committee on Immunization Practices, which was updated in June 2019. Included in those recommendations was the observation that the vaccine administrator does not need to wear gloves unless he or she has open lesions or is at risk from contacting the recipient’s body fluids.
Like many of the technical skills one learns in training, giving intramuscular injections is probably an example of the “see one, do one, teach one” mantra. But in the case of giving shots, I don’t recall any teaching. Do you? It was more “see a dozen and get on with it.” Or maybe you trained in an environment in which nurses gave all the injections. I hope not.
When it comes to giving immunizations to children, the art is in entering into that encounter with a calm, matter-of-fact attitude and body language, hiding the needle, firmly restraining the child, and moving quickly and smoothly. Aspirating and glove donning merely add to the drama and waste time. But how did I learn that art? No one taught me. Like many clinical skills, I watched scores of nurses and physicians, mentally logging in their tricks and mistakes that would help me craft my style.
I always felt and still feel that providing immunizations was per hour spent, the most valuable investment of my time. Doing the injecting myself was both the most efficient way to provide the service, and also emphasized the importance that I placed on the immunization. In the process of my 40-plus–year career, that included several hundred thousand patient encounters in which I gave innumerable injections. And, I egotistically assumed that I was good at it because many infants never cried, and a few children said, “That didn’t hurt.” I suspect you can make the same claim.
Injecting millions of adults with a COVID-19 vaccine, on the other hand, is a piece of cake because restraining the recipient shouldn’t factor into the scenario. However, I wonder who is going to administer all those millions of injections and who is going to train them? How many of the trainers are aware of the CDC-ACIP guidelines? Or, are they going to fall back on old techniques that lack evidence support?
From the efficiency standpoint, it probably doesn’t make much difference. The injection takes but a few seconds. Filling out the paperwork and waiting for the recipient to figure out how to expose his or her deltoid can take fifty times that long.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
As the COVID-19 vaccine candidates have begun to roll off the production lines into the distribution networks by the millions, media coverage almost universally includes a still photo or video of someone receiving an injection. Ever observant, a retired lawyer friend of mine who learned to give shots when he was in the Army and again more recently while taking a wilderness survival course emailed me his concerns about what he felt were examples of poor injection technique. Included in his commentary was an Internet link in which a physician, who I suspect may have been a pediatrician, demonstrated what the physician considered proper intramuscular injection technique, which included a single-handed aspiration prior to giving the injection allowing the free hand to stabilize the patient’s – in this case a child’s – arm during the entire process.
I replied to my friend that I too was often troubled by what I considered to be poor injection technique. But, I said the physician in the link touting his improved technique was misguided. My understanding has been that unless the injection site is in the gluteus, there is no need aspirate prior to an intramuscular vaccine injection because the risk of intravascular injection is so small. I then confirmed this by reviewing the Centers for Disease Control and Prevention’s Vaccine Recommendations and Guidelines of the Advisory Committee on Immunization Practices, which was updated in June 2019. Included in those recommendations was the observation that the vaccine administrator does not need to wear gloves unless he or she has open lesions or is at risk from contacting the recipient’s body fluids.
Like many of the technical skills one learns in training, giving intramuscular injections is probably an example of the “see one, do one, teach one” mantra. But in the case of giving shots, I don’t recall any teaching. Do you? It was more “see a dozen and get on with it.” Or maybe you trained in an environment in which nurses gave all the injections. I hope not.
When it comes to giving immunizations to children, the art is in entering into that encounter with a calm, matter-of-fact attitude and body language, hiding the needle, firmly restraining the child, and moving quickly and smoothly. Aspirating and glove donning merely add to the drama and waste time. But how did I learn that art? No one taught me. Like many clinical skills, I watched scores of nurses and physicians, mentally logging in their tricks and mistakes that would help me craft my style.
I always felt and still feel that providing immunizations was per hour spent, the most valuable investment of my time. Doing the injecting myself was both the most efficient way to provide the service, and also emphasized the importance that I placed on the immunization. In the process of my 40-plus–year career, that included several hundred thousand patient encounters in which I gave innumerable injections. And, I egotistically assumed that I was good at it because many infants never cried, and a few children said, “That didn’t hurt.” I suspect you can make the same claim.
Injecting millions of adults with a COVID-19 vaccine, on the other hand, is a piece of cake because restraining the recipient shouldn’t factor into the scenario. However, I wonder who is going to administer all those millions of injections and who is going to train them? How many of the trainers are aware of the CDC-ACIP guidelines? Or, are they going to fall back on old techniques that lack evidence support?
From the efficiency standpoint, it probably doesn’t make much difference. The injection takes but a few seconds. Filling out the paperwork and waiting for the recipient to figure out how to expose his or her deltoid can take fifty times that long.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
As the COVID-19 vaccine candidates have begun to roll off the production lines into the distribution networks by the millions, media coverage almost universally includes a still photo or video of someone receiving an injection. Ever observant, a retired lawyer friend of mine who learned to give shots when he was in the Army and again more recently while taking a wilderness survival course emailed me his concerns about what he felt were examples of poor injection technique. Included in his commentary was an Internet link in which a physician, who I suspect may have been a pediatrician, demonstrated what the physician considered proper intramuscular injection technique, which included a single-handed aspiration prior to giving the injection allowing the free hand to stabilize the patient’s – in this case a child’s – arm during the entire process.
I replied to my friend that I too was often troubled by what I considered to be poor injection technique. But, I said the physician in the link touting his improved technique was misguided. My understanding has been that unless the injection site is in the gluteus, there is no need aspirate prior to an intramuscular vaccine injection because the risk of intravascular injection is so small. I then confirmed this by reviewing the Centers for Disease Control and Prevention’s Vaccine Recommendations and Guidelines of the Advisory Committee on Immunization Practices, which was updated in June 2019. Included in those recommendations was the observation that the vaccine administrator does not need to wear gloves unless he or she has open lesions or is at risk from contacting the recipient’s body fluids.
Like many of the technical skills one learns in training, giving intramuscular injections is probably an example of the “see one, do one, teach one” mantra. But in the case of giving shots, I don’t recall any teaching. Do you? It was more “see a dozen and get on with it.” Or maybe you trained in an environment in which nurses gave all the injections. I hope not.
When it comes to giving immunizations to children, the art is in entering into that encounter with a calm, matter-of-fact attitude and body language, hiding the needle, firmly restraining the child, and moving quickly and smoothly. Aspirating and glove donning merely add to the drama and waste time. But how did I learn that art? No one taught me. Like many clinical skills, I watched scores of nurses and physicians, mentally logging in their tricks and mistakes that would help me craft my style.
I always felt and still feel that providing immunizations was per hour spent, the most valuable investment of my time. Doing the injecting myself was both the most efficient way to provide the service, and also emphasized the importance that I placed on the immunization. In the process of my 40-plus–year career, that included several hundred thousand patient encounters in which I gave innumerable injections. And, I egotistically assumed that I was good at it because many infants never cried, and a few children said, “That didn’t hurt.” I suspect you can make the same claim.
Injecting millions of adults with a COVID-19 vaccine, on the other hand, is a piece of cake because restraining the recipient shouldn’t factor into the scenario. However, I wonder who is going to administer all those millions of injections and who is going to train them? How many of the trainers are aware of the CDC-ACIP guidelines? Or, are they going to fall back on old techniques that lack evidence support?
From the efficiency standpoint, it probably doesn’t make much difference. The injection takes but a few seconds. Filling out the paperwork and waiting for the recipient to figure out how to expose his or her deltoid can take fifty times that long.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
First-in-class ADC has benefit across mTNBC subgroups
But both an observer and the lead study author cautioned that the results were hypothesis generating.
Nonetheless, the data suggest the drug yields good survival outcomes in comparison with placebo in both BRCA1/2-positive and -negative patients and is effective even for those with low expression of the target protein, trophoblast cell surface antigen 2 (Trop-2).
The research was presented at the San Antonio Breast Cancer Symposium (SABCS) 2020.
Study presenter Sara Hurvitz, MD, Jonsson Comprehensive Cancer Center, University of California, Los Angeles, urged caution in interpreting the data, given the small sample sizes in the Trop-2–low subgroup and germline BRCA1/2-positive subgroup.
Jennifer K. Litton, MD, University of Texas MD Anderson Cancer Center, Houston, Texas, who was not involved in the research, echoed those comments.
She told Medscape Medical News that the numbers, particularly for the BRCA1/2 analysis, were “very small.”
She added: “This was not a prespecified group, so it represents an interesting analysis to be hypothesis generating for future studies but not anything applicable to current clinical practice.”
Nevertheless, Litton said the data from the primary analysis of ASCENT remain “practice changing” for women with mTNBC who have received at least two previous lines of therapy.
As to whether SG will eventually move beyond this advanced setting, she emphasized that “more trials would need to be done and reported evaluating its role in other settings, and hopefully expanding its usefulness for patients.”
SG is a first-in-class drug comprising an antibody directed at Trop-2, which is highly expressed in breast cancer, and linked to SN-38, the active metabolite of irinotecan.
On the basis of positive phase 1/2 trial data, SG was granted accelerated approval by the US Food and Drug Administration for patients with mTNBC who experience disease progression after at least two prior therapies.
As reported by Medscape Medical News, primary results from ASCENT that were presented at ESMO 2020 showed that SG improved progression-free survival (PFS) by nearly 4 months and overall survival by more than 5 months for women with pretreated mTNBC compared to chemotherapy.
Study details
At SABCS, Hurvitz presented an exploratory biomarker evaluation of data from the trial regarding the association between SG efficacy and Trop-2 expression, as well as germline BRCA1/2 mutation status.
She reminded the audience that, in ASCENT, 529 patients with mTNBC who had experienced disease progression after undergoing at least two chemotherapy regimens for advanced disease were randomly assigned in a 1:1 ratio to receive intravenous SG on days 1 and 8 of a 21-day cycle or physician’s choice of treatment.
Treatment was continued until disease progression or unacceptable toxicity occurred.
For the current analysis, which focused on patients who did not have brain metastases, the team studied primary or metastatic archival biopsy or surgical specimens collected at study entry.
These were analyzed using a validated immunohistochemistry assay. Tumors were categorized as Trop-2–low, –medium, or –-high expressers on the basis of H-score, which is a weighted summation of percent staining. In addition, germline BRCA1/2 mutation status was determined at baseline.
Mutation status was known for 149 SG patients and 143 control patients. Of those, the majority (57% and 54%, respectively) were BRCA1/2 negative.
Among 151 SG patients for whom Trop-2 expression status was available, 56% had tumors of high expression; 26%, medium expression; and 18%, low expression. In the control group, Trop-2 expression was known in 139 patients, of whom 52% had tumors of high expression; 25%, medium expression; and 23%, low expression.
Hurvitz reported that, although median PFS among patients given SG decreased with decreasing Trop-2 expression, it remained longer than that seen with control treatment. In patients with tumors of Trop-2–high status, median PFS was 6.9 months with SG, vs. 2.5 for patients who underwent control treatment. This fell to 5.6 months vs. 2.2 months in the Trop-2–medium group and 2.7 months vs 1.6 months in Trop-2–low group.
A similar pattern was seen for overall survival. In the Trop-2–high group, median overall survival was 14.2 months with SG, vs. 6.9 months with control therapy; 14.9 months vs. 6.9 months in the Trop-2–medium group; and 9.3 months vs. 7.6 months in the Trop-2–low group.
Again, the objective response rate fell from 44% to 38% and then to 22% with SG in the Trop-2–high, –medium, and –low groups, compared with 1%, 11%, and 6%, respectively, with control treatment.
There did not seem to be any interaction between Trop-2 expression and treatment-related adverse events of special interest. Rates of neutropenia, diarrhea, and anemia were consistently higher in SG-treated patients than in those given placebo.
Hurvitz said the objective response rate was markedly higher with SG vs. control treatment in both BRCA1/2-positive and -negative patients, at 19% vs. 6% in the positive group and 33% vs. 6% in the negative group.
This was reflected in improved median PFS with SG in both subgroups, at 4.6 months vs. 2.5 months with control therapy in BRCA1/2-positive patients and 4.9 months vs. 1.6 months in BRCA1/2-negative patients.
Overall survival was 15.6 months with SG, vs. 4.4 months with control treatment in BRCA1/2-positive patients. In BRCA1/2-negative patients, the respective figures were 10.9 months and 7.0 months.
The study was sponsored by Immunomedics. Hurvitz has financial ties to Immunomedics and multiple other pharmaceutical companies. Litton has financial ties to multiple companies, including Medscape and companies developing and marketing breast cancer therapies.
This article first appeared on Medscape.com.
But both an observer and the lead study author cautioned that the results were hypothesis generating.
Nonetheless, the data suggest the drug yields good survival outcomes in comparison with placebo in both BRCA1/2-positive and -negative patients and is effective even for those with low expression of the target protein, trophoblast cell surface antigen 2 (Trop-2).
The research was presented at the San Antonio Breast Cancer Symposium (SABCS) 2020.
Study presenter Sara Hurvitz, MD, Jonsson Comprehensive Cancer Center, University of California, Los Angeles, urged caution in interpreting the data, given the small sample sizes in the Trop-2–low subgroup and germline BRCA1/2-positive subgroup.
Jennifer K. Litton, MD, University of Texas MD Anderson Cancer Center, Houston, Texas, who was not involved in the research, echoed those comments.
She told Medscape Medical News that the numbers, particularly for the BRCA1/2 analysis, were “very small.”
She added: “This was not a prespecified group, so it represents an interesting analysis to be hypothesis generating for future studies but not anything applicable to current clinical practice.”
Nevertheless, Litton said the data from the primary analysis of ASCENT remain “practice changing” for women with mTNBC who have received at least two previous lines of therapy.
As to whether SG will eventually move beyond this advanced setting, she emphasized that “more trials would need to be done and reported evaluating its role in other settings, and hopefully expanding its usefulness for patients.”
SG is a first-in-class drug comprising an antibody directed at Trop-2, which is highly expressed in breast cancer, and linked to SN-38, the active metabolite of irinotecan.
On the basis of positive phase 1/2 trial data, SG was granted accelerated approval by the US Food and Drug Administration for patients with mTNBC who experience disease progression after at least two prior therapies.
As reported by Medscape Medical News, primary results from ASCENT that were presented at ESMO 2020 showed that SG improved progression-free survival (PFS) by nearly 4 months and overall survival by more than 5 months for women with pretreated mTNBC compared to chemotherapy.
Study details
At SABCS, Hurvitz presented an exploratory biomarker evaluation of data from the trial regarding the association between SG efficacy and Trop-2 expression, as well as germline BRCA1/2 mutation status.
She reminded the audience that, in ASCENT, 529 patients with mTNBC who had experienced disease progression after undergoing at least two chemotherapy regimens for advanced disease were randomly assigned in a 1:1 ratio to receive intravenous SG on days 1 and 8 of a 21-day cycle or physician’s choice of treatment.
Treatment was continued until disease progression or unacceptable toxicity occurred.
For the current analysis, which focused on patients who did not have brain metastases, the team studied primary or metastatic archival biopsy or surgical specimens collected at study entry.
These were analyzed using a validated immunohistochemistry assay. Tumors were categorized as Trop-2–low, –medium, or –-high expressers on the basis of H-score, which is a weighted summation of percent staining. In addition, germline BRCA1/2 mutation status was determined at baseline.
Mutation status was known for 149 SG patients and 143 control patients. Of those, the majority (57% and 54%, respectively) were BRCA1/2 negative.
Among 151 SG patients for whom Trop-2 expression status was available, 56% had tumors of high expression; 26%, medium expression; and 18%, low expression. In the control group, Trop-2 expression was known in 139 patients, of whom 52% had tumors of high expression; 25%, medium expression; and 23%, low expression.
Hurvitz reported that, although median PFS among patients given SG decreased with decreasing Trop-2 expression, it remained longer than that seen with control treatment. In patients with tumors of Trop-2–high status, median PFS was 6.9 months with SG, vs. 2.5 for patients who underwent control treatment. This fell to 5.6 months vs. 2.2 months in the Trop-2–medium group and 2.7 months vs 1.6 months in Trop-2–low group.
A similar pattern was seen for overall survival. In the Trop-2–high group, median overall survival was 14.2 months with SG, vs. 6.9 months with control therapy; 14.9 months vs. 6.9 months in the Trop-2–medium group; and 9.3 months vs. 7.6 months in the Trop-2–low group.
Again, the objective response rate fell from 44% to 38% and then to 22% with SG in the Trop-2–high, –medium, and –low groups, compared with 1%, 11%, and 6%, respectively, with control treatment.
There did not seem to be any interaction between Trop-2 expression and treatment-related adverse events of special interest. Rates of neutropenia, diarrhea, and anemia were consistently higher in SG-treated patients than in those given placebo.
Hurvitz said the objective response rate was markedly higher with SG vs. control treatment in both BRCA1/2-positive and -negative patients, at 19% vs. 6% in the positive group and 33% vs. 6% in the negative group.
This was reflected in improved median PFS with SG in both subgroups, at 4.6 months vs. 2.5 months with control therapy in BRCA1/2-positive patients and 4.9 months vs. 1.6 months in BRCA1/2-negative patients.
Overall survival was 15.6 months with SG, vs. 4.4 months with control treatment in BRCA1/2-positive patients. In BRCA1/2-negative patients, the respective figures were 10.9 months and 7.0 months.
The study was sponsored by Immunomedics. Hurvitz has financial ties to Immunomedics and multiple other pharmaceutical companies. Litton has financial ties to multiple companies, including Medscape and companies developing and marketing breast cancer therapies.
This article first appeared on Medscape.com.
But both an observer and the lead study author cautioned that the results were hypothesis generating.
Nonetheless, the data suggest the drug yields good survival outcomes in comparison with placebo in both BRCA1/2-positive and -negative patients and is effective even for those with low expression of the target protein, trophoblast cell surface antigen 2 (Trop-2).
The research was presented at the San Antonio Breast Cancer Symposium (SABCS) 2020.
Study presenter Sara Hurvitz, MD, Jonsson Comprehensive Cancer Center, University of California, Los Angeles, urged caution in interpreting the data, given the small sample sizes in the Trop-2–low subgroup and germline BRCA1/2-positive subgroup.
Jennifer K. Litton, MD, University of Texas MD Anderson Cancer Center, Houston, Texas, who was not involved in the research, echoed those comments.
She told Medscape Medical News that the numbers, particularly for the BRCA1/2 analysis, were “very small.”
She added: “This was not a prespecified group, so it represents an interesting analysis to be hypothesis generating for future studies but not anything applicable to current clinical practice.”
Nevertheless, Litton said the data from the primary analysis of ASCENT remain “practice changing” for women with mTNBC who have received at least two previous lines of therapy.
As to whether SG will eventually move beyond this advanced setting, she emphasized that “more trials would need to be done and reported evaluating its role in other settings, and hopefully expanding its usefulness for patients.”
SG is a first-in-class drug comprising an antibody directed at Trop-2, which is highly expressed in breast cancer, and linked to SN-38, the active metabolite of irinotecan.
On the basis of positive phase 1/2 trial data, SG was granted accelerated approval by the US Food and Drug Administration for patients with mTNBC who experience disease progression after at least two prior therapies.
As reported by Medscape Medical News, primary results from ASCENT that were presented at ESMO 2020 showed that SG improved progression-free survival (PFS) by nearly 4 months and overall survival by more than 5 months for women with pretreated mTNBC compared to chemotherapy.
Study details
At SABCS, Hurvitz presented an exploratory biomarker evaluation of data from the trial regarding the association between SG efficacy and Trop-2 expression, as well as germline BRCA1/2 mutation status.
She reminded the audience that, in ASCENT, 529 patients with mTNBC who had experienced disease progression after undergoing at least two chemotherapy regimens for advanced disease were randomly assigned in a 1:1 ratio to receive intravenous SG on days 1 and 8 of a 21-day cycle or physician’s choice of treatment.
Treatment was continued until disease progression or unacceptable toxicity occurred.
For the current analysis, which focused on patients who did not have brain metastases, the team studied primary or metastatic archival biopsy or surgical specimens collected at study entry.
These were analyzed using a validated immunohistochemistry assay. Tumors were categorized as Trop-2–low, –medium, or –-high expressers on the basis of H-score, which is a weighted summation of percent staining. In addition, germline BRCA1/2 mutation status was determined at baseline.
Mutation status was known for 149 SG patients and 143 control patients. Of those, the majority (57% and 54%, respectively) were BRCA1/2 negative.
Among 151 SG patients for whom Trop-2 expression status was available, 56% had tumors of high expression; 26%, medium expression; and 18%, low expression. In the control group, Trop-2 expression was known in 139 patients, of whom 52% had tumors of high expression; 25%, medium expression; and 23%, low expression.
Hurvitz reported that, although median PFS among patients given SG decreased with decreasing Trop-2 expression, it remained longer than that seen with control treatment. In patients with tumors of Trop-2–high status, median PFS was 6.9 months with SG, vs. 2.5 for patients who underwent control treatment. This fell to 5.6 months vs. 2.2 months in the Trop-2–medium group and 2.7 months vs 1.6 months in Trop-2–low group.
A similar pattern was seen for overall survival. In the Trop-2–high group, median overall survival was 14.2 months with SG, vs. 6.9 months with control therapy; 14.9 months vs. 6.9 months in the Trop-2–medium group; and 9.3 months vs. 7.6 months in the Trop-2–low group.
Again, the objective response rate fell from 44% to 38% and then to 22% with SG in the Trop-2–high, –medium, and –low groups, compared with 1%, 11%, and 6%, respectively, with control treatment.
There did not seem to be any interaction between Trop-2 expression and treatment-related adverse events of special interest. Rates of neutropenia, diarrhea, and anemia were consistently higher in SG-treated patients than in those given placebo.
Hurvitz said the objective response rate was markedly higher with SG vs. control treatment in both BRCA1/2-positive and -negative patients, at 19% vs. 6% in the positive group and 33% vs. 6% in the negative group.
This was reflected in improved median PFS with SG in both subgroups, at 4.6 months vs. 2.5 months with control therapy in BRCA1/2-positive patients and 4.9 months vs. 1.6 months in BRCA1/2-negative patients.
Overall survival was 15.6 months with SG, vs. 4.4 months with control treatment in BRCA1/2-positive patients. In BRCA1/2-negative patients, the respective figures were 10.9 months and 7.0 months.
The study was sponsored by Immunomedics. Hurvitz has financial ties to Immunomedics and multiple other pharmaceutical companies. Litton has financial ties to multiple companies, including Medscape and companies developing and marketing breast cancer therapies.
This article first appeared on Medscape.com.
FROM SABCS 2020