User login
In MDS, transplant ups survival in elderly and may be reimbursed
New results suggest that allogeneic hematopoietic cell transplantation (HCT), which is typically reserved for younger patients, may well be offered to older patients with advanced myelodysplastic syndrome (MDS).
In patients with a median age of 66 years who had received a donor transplant, the overall survival (OS) at 3 years was almost double compared with patients who did not receive a transplant – 47.9% vs. 26.6% for the “no-donor” group.
The finding comes from the Blood and Marrow Transplant Clinical Trials Network (BMT CTN) Study 1102 (NCT02016781) presented at the American Society of Hematology (ASH) 2020 virtual meeting.
“This study conclusively solidifies the role of transplantation in older individuals with MDS,” presenter Corey Cutler, MD, MPH, of the Dana-Farber Cancer Center, Boston, said in an interview.
Coauthor Ryotaro Nakamura, MD, of City of Hope, Duarte, Calif., said in an interview that this was the largest and first trial in the United States to determine in a prospective fashion that allogeneic stem cell transplantation offers a significant survival in older patients. “There was more than a 20% benefit in OS in this age group,” he said.
“This is an incredibly important study,” said Andrew Brunner, MD, medical oncologist at the Mass General Cancer Center in Boston, who was approached for comment. He explained that for years early transplant was recommended as important for patients who have higher-risk MDS. “This study validates this in a prospective, pseudo-randomized (donor/no donor) fashion,” he said in an interview.
“[This study] is really a seminal advance in the care of patients with MDS. Transplant should be integrated into the care algorithm, if not already, and we as a community need to build upon this study further,” Dr. Brunner added.
Several experts in addition to the authors hailed the study as practice changing.
Robert A. Brodsky, MD, ASH, director of the division of hematology at Johns Hopkins University, Baltimore, noted that in younger patients bone marrow transplant is the standard of care for aggressive MDS, but a lot of practices do not refer older patients or those with comorbidities for transplant and prefer to give these patients palliative care with hypomethylating agents for fear that the transplant process would be too toxic.
“There has been an institutional bias to do transplant in older patients, but until now there was no randomized clinical trial to show that this is the right choice. Now we have the data,” Dr Brodsky said, predicting that “this study will change the standard of care.”
Henry Fung, MD, chair of the department of bone marrow transplant and cellular therapies at Fox Chase Cancer Center, Philadelphia, agreed. “We should congratulate all the investigators and our patients who participated in this study. Reduced intensity allogeneic stem cell transplantation improved disease control and overall survival with similar quality of life.
“I will recommend all patients with intermediate-2 or higher-risk MDS to be evaluated by the transplant team at diagnosis and eligible patients should be considered for a transplant,” Dr. Fung said in an interview.
Immediate impact on clinical practice
Lead author Dr. Cutler suggested that the study results had an immediate impact for changing clinical practice. “Individuals between the ages of 50 and 75 years with intermediate-2 or high-risk MDS who are eligible to undergo reduced-intensity transplantation had superior outcomes if they had a suitable donor for transplantation in comparison with those who did not have a donor,” he said.
Dr. Cutler further explained that many community-based hematologists do not refer their patients for transplantation. In addition, there is a lack of a uniform payer position for transplantation for MDS, he noted. Also, there is a lack of understanding of the cost-effectiveness of transplantation in comparison to nontransplant strategies, he suggested.
“Transplant is curative for MDS,” he emphasized. Most transplant recipients will eventually become transfusion-independent within weeks to months from transplant.
“We do transplants in this age group all the time,” Dr. Cutler noted. He said that academic centers will continue to offer transplants, and suggested that community oncologists encourage referral to transplant centers early in a patient’s disease course to maximize search time and provide patients all potential options for therapy.
Dr. Brunner agreed and noted that there is a need to build capacity for higher transplant volume, and in general physicians should seek ways to expand this treatment option to more patients. “At this time, allogeneic transplant still requires close collaboration with referral centers; that said, more and more we are able to work closely with colleagues in the community to share management, including earlier after the actual transplant,” he said.
He noted that one silver lining of the pandemic in 2020 has been increased use of telemedicine to collaborate. “Ongoing advances may be able to further encourage these virtual connections to enhance the entire patient care experience,” Dr. Brunner said.
Reimbursement by CMS for Medicare recipients
Despite the data showing benefit, allogeneic stem cell transplantation is not offered to older individuals with high-risk MDS and is not covered by Medicare in the United States, Dr. Cutler noted in his presentation.
“This study was spurred by the CMS [Centers for Medicare & Medicaid Services] ruling for transplantation in MDS and the story has come full circle,” Aaron T. Gerds, MD, MS, noted at a preconference press briefing. Dr. Gerds is chair of the ASH Committee on Communications and assistant professor at the Cleveland Clinic Taussig Cancer Institute, Cleveland.
Dr. Nakamura explained that in 2010 a CMS decision memo noted that the evidence of a benefit for transplantation in MDS was lacking and Medicare would not cover transplant unless patients were enrolled in a clinical study. That memo outlined criteria that a clinical trial would have to address before it could consider reimbursement for Medicare beneficiaries.
“The BMT CTN Study 1102 was one of two studies that met the criteria set by CMS,” Dr. Nakamura said, noting that the data are being prepared for CMS review.
“This study will likely be the deciding factor for CMS to begin to cover payment for transplantation for MDS,” said Dr. Cutler.
The other study, published earlier this year in JAMA Oncology, showed that outcomes for patients older than ager 65 were similar to those of patients aged 55-65.
BMT CTN 1102 study details
Dr. Cutler noted that the study was designed to address the issue of whether transplantation was beneficial to Medicare-aged individuals with high-risk MDS, and the trial had been approved by Medicare.
The multicenter study enrolled patients who were between ages 50 and 75 years and had newly diagnosed MDS of higher risk (International Prognostic Scoring System [IPSS] intermediate-2 or higher) and were candidates for reduced intensity conditioning (RIC) allogeneic HCT.
Patients were enrolled prior to a formal donor search and were initially assigned to the “no donor” group and reassigned to the donor group when a suitable donor (matched sibling or unrelated donor) was identified. Patients underwent RIC HCT according to institution protocol.
Of 384 patients, 260 received RIC HCT and 124 received hypomethylating therapy. Median follow-up was 34.2 months for the donor group and 26.9 months for the no-donor group.
The two arms were well balanced with respect to age (median 66 years), gender, disease risk [two-thirds of the patients had an intermediate-2 and one third had a high-risk MDS], and response to hypomethylating therapy. The majority of subjects in the donor arm had unrelated donors and more than one-third had a high comorbidity score, Dr. Cutler indicated.
At 3 years, absolute improvement in OS was 21.3% in favor of donor-arm subjects. Leukemia-free survival was also higher in the donor group: 35.8% vs. 20.6% for the no-donor group.
Improvement in OS for patients receiving transplants was seen across all patient subtypes, regardless of age, response to hypomethylating therapy, and IPSS score. “Treatment effects were seen in any subgroup, but particularly in subjects above age 65,” Dr. Cutler stressed.
In an as-treated analysis that excluded subjects who died, the treatment effects were even more pronounced, with an absolute improvement in OS of 31.4% (47.4% vs. 16% for the no-donor arm) and improvement in leukemia-free survival of 28.4% (39.3% vs. 10.9% for the no-donor arm).
In 25 patients in the no-donor arm who subsequently went on to receive alternate donor transplant, the 3-year OS and leukemia-free survival was 58.5%, underscoring the potential value of alternate donor transplant, Dr. Cutler noted.
Dr. Nakamura emphasized that the gains in survival benefits were not seen at the expense of quality of life, as preliminary results showed no difference in quality-of-life measures across those who received donor transplants and those who did not.
Dr. Brunner noted that physicians often highlight the toxicities of transplant as a consideration for whether to proceed, and while there are toxicities specific to transplant that should be considered, in this study it is seen that, even early on, survival is improved in those patients who move toward early transplant. “It also underscores the limitations of current nontransplant treatments for MDS – there is much room to improve,” he said.
Role for alternate donors
Dr. Cutler noted that the majority of patients in the no-donor group died without transplantation. “We need to establish the role of alternative donor transplantation in this population,” he said. Dr. Nakamura indicated that mismatched donors and haploidentical donors such as family donors and umbilical cord blood may be alternate donor sources; outcomes from published studies show similar results, he said.
However, Dr. Brunner noted that the study looked only at traditional fully matched donors, leaving open some questions about alternative donor options such as haploidentical donors and umbilical cord blood donation.
“Our experience in other areas of transplant would suggest that these donor sources may be as good as traditional fully matched options, when using newer conditioning and prophylaxis regimens,” Dr. Brunner said.
Dr. Cutler added, “With the increased acceptance of alternate transplant modalities, we need to determine the outcomes associated with these in prospective trials.”
“I think a significant consideration here as well is health equity,” Dr. Brunner said. “Donor options vary according to race and ethnicity and we need to be proactive as a community to ensure that all MDS patients have access to a potentially curative option early in their diagnosis.”
Dr. Cutler reports consultancy for Mesoblast, Generon, Medsenic, Jazz, Kadmon, and Incyte. Dr. Nakamura reports relationships with Magenta Therapeutics, Kyowa-Kirin, Alexion, Merck, NapaJen Pharma, Kadmon Corporation, Celgene, and Viracor. Dr. Fung has disclosed no relevant financial relationships. Dr. Brodsky reports receiving funding from and being on the board/advisory committee for Achillion Pharmaceuticals, consults with Alexion Pharmaceuticals, and receives honoraria from UpToDate. Dr. Brunner reports relationships with Biogen, Acceleron Pharma Inc, Celgene/BMS, Forty Seven Inc, Jazz Pharma, Novartis, Takeda, Xcenda, GSK, Janssen, and AstraZeneca.
A version of this article originally appeared on Medscape.com.
New results suggest that allogeneic hematopoietic cell transplantation (HCT), which is typically reserved for younger patients, may well be offered to older patients with advanced myelodysplastic syndrome (MDS).
In patients with a median age of 66 years who had received a donor transplant, the overall survival (OS) at 3 years was almost double compared with patients who did not receive a transplant – 47.9% vs. 26.6% for the “no-donor” group.
The finding comes from the Blood and Marrow Transplant Clinical Trials Network (BMT CTN) Study 1102 (NCT02016781) presented at the American Society of Hematology (ASH) 2020 virtual meeting.
“This study conclusively solidifies the role of transplantation in older individuals with MDS,” presenter Corey Cutler, MD, MPH, of the Dana-Farber Cancer Center, Boston, said in an interview.
Coauthor Ryotaro Nakamura, MD, of City of Hope, Duarte, Calif., said in an interview that this was the largest and first trial in the United States to determine in a prospective fashion that allogeneic stem cell transplantation offers a significant survival in older patients. “There was more than a 20% benefit in OS in this age group,” he said.
“This is an incredibly important study,” said Andrew Brunner, MD, medical oncologist at the Mass General Cancer Center in Boston, who was approached for comment. He explained that for years early transplant was recommended as important for patients who have higher-risk MDS. “This study validates this in a prospective, pseudo-randomized (donor/no donor) fashion,” he said in an interview.
“[This study] is really a seminal advance in the care of patients with MDS. Transplant should be integrated into the care algorithm, if not already, and we as a community need to build upon this study further,” Dr. Brunner added.
Several experts in addition to the authors hailed the study as practice changing.
Robert A. Brodsky, MD, ASH, director of the division of hematology at Johns Hopkins University, Baltimore, noted that in younger patients bone marrow transplant is the standard of care for aggressive MDS, but a lot of practices do not refer older patients or those with comorbidities for transplant and prefer to give these patients palliative care with hypomethylating agents for fear that the transplant process would be too toxic.
“There has been an institutional bias to do transplant in older patients, but until now there was no randomized clinical trial to show that this is the right choice. Now we have the data,” Dr Brodsky said, predicting that “this study will change the standard of care.”
Henry Fung, MD, chair of the department of bone marrow transplant and cellular therapies at Fox Chase Cancer Center, Philadelphia, agreed. “We should congratulate all the investigators and our patients who participated in this study. Reduced intensity allogeneic stem cell transplantation improved disease control and overall survival with similar quality of life.
“I will recommend all patients with intermediate-2 or higher-risk MDS to be evaluated by the transplant team at diagnosis and eligible patients should be considered for a transplant,” Dr. Fung said in an interview.
Immediate impact on clinical practice
Lead author Dr. Cutler suggested that the study results had an immediate impact for changing clinical practice. “Individuals between the ages of 50 and 75 years with intermediate-2 or high-risk MDS who are eligible to undergo reduced-intensity transplantation had superior outcomes if they had a suitable donor for transplantation in comparison with those who did not have a donor,” he said.
Dr. Cutler further explained that many community-based hematologists do not refer their patients for transplantation. In addition, there is a lack of a uniform payer position for transplantation for MDS, he noted. Also, there is a lack of understanding of the cost-effectiveness of transplantation in comparison to nontransplant strategies, he suggested.
“Transplant is curative for MDS,” he emphasized. Most transplant recipients will eventually become transfusion-independent within weeks to months from transplant.
“We do transplants in this age group all the time,” Dr. Cutler noted. He said that academic centers will continue to offer transplants, and suggested that community oncologists encourage referral to transplant centers early in a patient’s disease course to maximize search time and provide patients all potential options for therapy.
Dr. Brunner agreed and noted that there is a need to build capacity for higher transplant volume, and in general physicians should seek ways to expand this treatment option to more patients. “At this time, allogeneic transplant still requires close collaboration with referral centers; that said, more and more we are able to work closely with colleagues in the community to share management, including earlier after the actual transplant,” he said.
He noted that one silver lining of the pandemic in 2020 has been increased use of telemedicine to collaborate. “Ongoing advances may be able to further encourage these virtual connections to enhance the entire patient care experience,” Dr. Brunner said.
Reimbursement by CMS for Medicare recipients
Despite the data showing benefit, allogeneic stem cell transplantation is not offered to older individuals with high-risk MDS and is not covered by Medicare in the United States, Dr. Cutler noted in his presentation.
“This study was spurred by the CMS [Centers for Medicare & Medicaid Services] ruling for transplantation in MDS and the story has come full circle,” Aaron T. Gerds, MD, MS, noted at a preconference press briefing. Dr. Gerds is chair of the ASH Committee on Communications and assistant professor at the Cleveland Clinic Taussig Cancer Institute, Cleveland.
Dr. Nakamura explained that in 2010 a CMS decision memo noted that the evidence of a benefit for transplantation in MDS was lacking and Medicare would not cover transplant unless patients were enrolled in a clinical study. That memo outlined criteria that a clinical trial would have to address before it could consider reimbursement for Medicare beneficiaries.
“The BMT CTN Study 1102 was one of two studies that met the criteria set by CMS,” Dr. Nakamura said, noting that the data are being prepared for CMS review.
“This study will likely be the deciding factor for CMS to begin to cover payment for transplantation for MDS,” said Dr. Cutler.
The other study, published earlier this year in JAMA Oncology, showed that outcomes for patients older than ager 65 were similar to those of patients aged 55-65.
BMT CTN 1102 study details
Dr. Cutler noted that the study was designed to address the issue of whether transplantation was beneficial to Medicare-aged individuals with high-risk MDS, and the trial had been approved by Medicare.
The multicenter study enrolled patients who were between ages 50 and 75 years and had newly diagnosed MDS of higher risk (International Prognostic Scoring System [IPSS] intermediate-2 or higher) and were candidates for reduced intensity conditioning (RIC) allogeneic HCT.
Patients were enrolled prior to a formal donor search and were initially assigned to the “no donor” group and reassigned to the donor group when a suitable donor (matched sibling or unrelated donor) was identified. Patients underwent RIC HCT according to institution protocol.
Of 384 patients, 260 received RIC HCT and 124 received hypomethylating therapy. Median follow-up was 34.2 months for the donor group and 26.9 months for the no-donor group.
The two arms were well balanced with respect to age (median 66 years), gender, disease risk [two-thirds of the patients had an intermediate-2 and one third had a high-risk MDS], and response to hypomethylating therapy. The majority of subjects in the donor arm had unrelated donors and more than one-third had a high comorbidity score, Dr. Cutler indicated.
At 3 years, absolute improvement in OS was 21.3% in favor of donor-arm subjects. Leukemia-free survival was also higher in the donor group: 35.8% vs. 20.6% for the no-donor group.
Improvement in OS for patients receiving transplants was seen across all patient subtypes, regardless of age, response to hypomethylating therapy, and IPSS score. “Treatment effects were seen in any subgroup, but particularly in subjects above age 65,” Dr. Cutler stressed.
In an as-treated analysis that excluded subjects who died, the treatment effects were even more pronounced, with an absolute improvement in OS of 31.4% (47.4% vs. 16% for the no-donor arm) and improvement in leukemia-free survival of 28.4% (39.3% vs. 10.9% for the no-donor arm).
In 25 patients in the no-donor arm who subsequently went on to receive alternate donor transplant, the 3-year OS and leukemia-free survival was 58.5%, underscoring the potential value of alternate donor transplant, Dr. Cutler noted.
Dr. Nakamura emphasized that the gains in survival benefits were not seen at the expense of quality of life, as preliminary results showed no difference in quality-of-life measures across those who received donor transplants and those who did not.
Dr. Brunner noted that physicians often highlight the toxicities of transplant as a consideration for whether to proceed, and while there are toxicities specific to transplant that should be considered, in this study it is seen that, even early on, survival is improved in those patients who move toward early transplant. “It also underscores the limitations of current nontransplant treatments for MDS – there is much room to improve,” he said.
Role for alternate donors
Dr. Cutler noted that the majority of patients in the no-donor group died without transplantation. “We need to establish the role of alternative donor transplantation in this population,” he said. Dr. Nakamura indicated that mismatched donors and haploidentical donors such as family donors and umbilical cord blood may be alternate donor sources; outcomes from published studies show similar results, he said.
However, Dr. Brunner noted that the study looked only at traditional fully matched donors, leaving open some questions about alternative donor options such as haploidentical donors and umbilical cord blood donation.
“Our experience in other areas of transplant would suggest that these donor sources may be as good as traditional fully matched options, when using newer conditioning and prophylaxis regimens,” Dr. Brunner said.
Dr. Cutler added, “With the increased acceptance of alternate transplant modalities, we need to determine the outcomes associated with these in prospective trials.”
“I think a significant consideration here as well is health equity,” Dr. Brunner said. “Donor options vary according to race and ethnicity and we need to be proactive as a community to ensure that all MDS patients have access to a potentially curative option early in their diagnosis.”
Dr. Cutler reports consultancy for Mesoblast, Generon, Medsenic, Jazz, Kadmon, and Incyte. Dr. Nakamura reports relationships with Magenta Therapeutics, Kyowa-Kirin, Alexion, Merck, NapaJen Pharma, Kadmon Corporation, Celgene, and Viracor. Dr. Fung has disclosed no relevant financial relationships. Dr. Brodsky reports receiving funding from and being on the board/advisory committee for Achillion Pharmaceuticals, consults with Alexion Pharmaceuticals, and receives honoraria from UpToDate. Dr. Brunner reports relationships with Biogen, Acceleron Pharma Inc, Celgene/BMS, Forty Seven Inc, Jazz Pharma, Novartis, Takeda, Xcenda, GSK, Janssen, and AstraZeneca.
A version of this article originally appeared on Medscape.com.
New results suggest that allogeneic hematopoietic cell transplantation (HCT), which is typically reserved for younger patients, may well be offered to older patients with advanced myelodysplastic syndrome (MDS).
In patients with a median age of 66 years who had received a donor transplant, the overall survival (OS) at 3 years was almost double compared with patients who did not receive a transplant – 47.9% vs. 26.6% for the “no-donor” group.
The finding comes from the Blood and Marrow Transplant Clinical Trials Network (BMT CTN) Study 1102 (NCT02016781) presented at the American Society of Hematology (ASH) 2020 virtual meeting.
“This study conclusively solidifies the role of transplantation in older individuals with MDS,” presenter Corey Cutler, MD, MPH, of the Dana-Farber Cancer Center, Boston, said in an interview.
Coauthor Ryotaro Nakamura, MD, of City of Hope, Duarte, Calif., said in an interview that this was the largest and first trial in the United States to determine in a prospective fashion that allogeneic stem cell transplantation offers a significant survival in older patients. “There was more than a 20% benefit in OS in this age group,” he said.
“This is an incredibly important study,” said Andrew Brunner, MD, medical oncologist at the Mass General Cancer Center in Boston, who was approached for comment. He explained that for years early transplant was recommended as important for patients who have higher-risk MDS. “This study validates this in a prospective, pseudo-randomized (donor/no donor) fashion,” he said in an interview.
“[This study] is really a seminal advance in the care of patients with MDS. Transplant should be integrated into the care algorithm, if not already, and we as a community need to build upon this study further,” Dr. Brunner added.
Several experts in addition to the authors hailed the study as practice changing.
Robert A. Brodsky, MD, ASH, director of the division of hematology at Johns Hopkins University, Baltimore, noted that in younger patients bone marrow transplant is the standard of care for aggressive MDS, but a lot of practices do not refer older patients or those with comorbidities for transplant and prefer to give these patients palliative care with hypomethylating agents for fear that the transplant process would be too toxic.
“There has been an institutional bias to do transplant in older patients, but until now there was no randomized clinical trial to show that this is the right choice. Now we have the data,” Dr Brodsky said, predicting that “this study will change the standard of care.”
Henry Fung, MD, chair of the department of bone marrow transplant and cellular therapies at Fox Chase Cancer Center, Philadelphia, agreed. “We should congratulate all the investigators and our patients who participated in this study. Reduced intensity allogeneic stem cell transplantation improved disease control and overall survival with similar quality of life.
“I will recommend all patients with intermediate-2 or higher-risk MDS to be evaluated by the transplant team at diagnosis and eligible patients should be considered for a transplant,” Dr. Fung said in an interview.
Immediate impact on clinical practice
Lead author Dr. Cutler suggested that the study results had an immediate impact for changing clinical practice. “Individuals between the ages of 50 and 75 years with intermediate-2 or high-risk MDS who are eligible to undergo reduced-intensity transplantation had superior outcomes if they had a suitable donor for transplantation in comparison with those who did not have a donor,” he said.
Dr. Cutler further explained that many community-based hematologists do not refer their patients for transplantation. In addition, there is a lack of a uniform payer position for transplantation for MDS, he noted. Also, there is a lack of understanding of the cost-effectiveness of transplantation in comparison to nontransplant strategies, he suggested.
“Transplant is curative for MDS,” he emphasized. Most transplant recipients will eventually become transfusion-independent within weeks to months from transplant.
“We do transplants in this age group all the time,” Dr. Cutler noted. He said that academic centers will continue to offer transplants, and suggested that community oncologists encourage referral to transplant centers early in a patient’s disease course to maximize search time and provide patients all potential options for therapy.
Dr. Brunner agreed and noted that there is a need to build capacity for higher transplant volume, and in general physicians should seek ways to expand this treatment option to more patients. “At this time, allogeneic transplant still requires close collaboration with referral centers; that said, more and more we are able to work closely with colleagues in the community to share management, including earlier after the actual transplant,” he said.
He noted that one silver lining of the pandemic in 2020 has been increased use of telemedicine to collaborate. “Ongoing advances may be able to further encourage these virtual connections to enhance the entire patient care experience,” Dr. Brunner said.
Reimbursement by CMS for Medicare recipients
Despite the data showing benefit, allogeneic stem cell transplantation is not offered to older individuals with high-risk MDS and is not covered by Medicare in the United States, Dr. Cutler noted in his presentation.
“This study was spurred by the CMS [Centers for Medicare & Medicaid Services] ruling for transplantation in MDS and the story has come full circle,” Aaron T. Gerds, MD, MS, noted at a preconference press briefing. Dr. Gerds is chair of the ASH Committee on Communications and assistant professor at the Cleveland Clinic Taussig Cancer Institute, Cleveland.
Dr. Nakamura explained that in 2010 a CMS decision memo noted that the evidence of a benefit for transplantation in MDS was lacking and Medicare would not cover transplant unless patients were enrolled in a clinical study. That memo outlined criteria that a clinical trial would have to address before it could consider reimbursement for Medicare beneficiaries.
“The BMT CTN Study 1102 was one of two studies that met the criteria set by CMS,” Dr. Nakamura said, noting that the data are being prepared for CMS review.
“This study will likely be the deciding factor for CMS to begin to cover payment for transplantation for MDS,” said Dr. Cutler.
The other study, published earlier this year in JAMA Oncology, showed that outcomes for patients older than ager 65 were similar to those of patients aged 55-65.
BMT CTN 1102 study details
Dr. Cutler noted that the study was designed to address the issue of whether transplantation was beneficial to Medicare-aged individuals with high-risk MDS, and the trial had been approved by Medicare.
The multicenter study enrolled patients who were between ages 50 and 75 years and had newly diagnosed MDS of higher risk (International Prognostic Scoring System [IPSS] intermediate-2 or higher) and were candidates for reduced intensity conditioning (RIC) allogeneic HCT.
Patients were enrolled prior to a formal donor search and were initially assigned to the “no donor” group and reassigned to the donor group when a suitable donor (matched sibling or unrelated donor) was identified. Patients underwent RIC HCT according to institution protocol.
Of 384 patients, 260 received RIC HCT and 124 received hypomethylating therapy. Median follow-up was 34.2 months for the donor group and 26.9 months for the no-donor group.
The two arms were well balanced with respect to age (median 66 years), gender, disease risk [two-thirds of the patients had an intermediate-2 and one third had a high-risk MDS], and response to hypomethylating therapy. The majority of subjects in the donor arm had unrelated donors and more than one-third had a high comorbidity score, Dr. Cutler indicated.
At 3 years, absolute improvement in OS was 21.3% in favor of donor-arm subjects. Leukemia-free survival was also higher in the donor group: 35.8% vs. 20.6% for the no-donor group.
Improvement in OS for patients receiving transplants was seen across all patient subtypes, regardless of age, response to hypomethylating therapy, and IPSS score. “Treatment effects were seen in any subgroup, but particularly in subjects above age 65,” Dr. Cutler stressed.
In an as-treated analysis that excluded subjects who died, the treatment effects were even more pronounced, with an absolute improvement in OS of 31.4% (47.4% vs. 16% for the no-donor arm) and improvement in leukemia-free survival of 28.4% (39.3% vs. 10.9% for the no-donor arm).
In 25 patients in the no-donor arm who subsequently went on to receive alternate donor transplant, the 3-year OS and leukemia-free survival was 58.5%, underscoring the potential value of alternate donor transplant, Dr. Cutler noted.
Dr. Nakamura emphasized that the gains in survival benefits were not seen at the expense of quality of life, as preliminary results showed no difference in quality-of-life measures across those who received donor transplants and those who did not.
Dr. Brunner noted that physicians often highlight the toxicities of transplant as a consideration for whether to proceed, and while there are toxicities specific to transplant that should be considered, in this study it is seen that, even early on, survival is improved in those patients who move toward early transplant. “It also underscores the limitations of current nontransplant treatments for MDS – there is much room to improve,” he said.
Role for alternate donors
Dr. Cutler noted that the majority of patients in the no-donor group died without transplantation. “We need to establish the role of alternative donor transplantation in this population,” he said. Dr. Nakamura indicated that mismatched donors and haploidentical donors such as family donors and umbilical cord blood may be alternate donor sources; outcomes from published studies show similar results, he said.
However, Dr. Brunner noted that the study looked only at traditional fully matched donors, leaving open some questions about alternative donor options such as haploidentical donors and umbilical cord blood donation.
“Our experience in other areas of transplant would suggest that these donor sources may be as good as traditional fully matched options, when using newer conditioning and prophylaxis regimens,” Dr. Brunner said.
Dr. Cutler added, “With the increased acceptance of alternate transplant modalities, we need to determine the outcomes associated with these in prospective trials.”
“I think a significant consideration here as well is health equity,” Dr. Brunner said. “Donor options vary according to race and ethnicity and we need to be proactive as a community to ensure that all MDS patients have access to a potentially curative option early in their diagnosis.”
Dr. Cutler reports consultancy for Mesoblast, Generon, Medsenic, Jazz, Kadmon, and Incyte. Dr. Nakamura reports relationships with Magenta Therapeutics, Kyowa-Kirin, Alexion, Merck, NapaJen Pharma, Kadmon Corporation, Celgene, and Viracor. Dr. Fung has disclosed no relevant financial relationships. Dr. Brodsky reports receiving funding from and being on the board/advisory committee for Achillion Pharmaceuticals, consults with Alexion Pharmaceuticals, and receives honoraria from UpToDate. Dr. Brunner reports relationships with Biogen, Acceleron Pharma Inc, Celgene/BMS, Forty Seven Inc, Jazz Pharma, Novartis, Takeda, Xcenda, GSK, Janssen, and AstraZeneca.
A version of this article originally appeared on Medscape.com.
AGA publishes seronegative enteropathy guidance
The American Gastroenterological Association has published new guidance for the diagnosis and management of seronegative enteropathies.
Seronegative enteropathies are commonly encountered by gastroenterologists, but accurate diagnosis can be complicated by a wide array of etiologies, misinterpreted histologic findings, suboptimal serology testing, and use of immunosuppressive agents that mask serology findings, reported lead author Maureen M. Leonard, MD, of MassGeneral Hospital for Children in Boston, and colleagues.
“Previous work detailing the prevalence of seronegative celiac disease [CeD], diagnosis of seronegative villous atrophy, and management recommendations for seronegative villous atrophy are available,” the investigators wrote in Gastroenterology. “However, there is limited evidence to guide clinicians regarding the minimal serologic tests necessary, the role of the gluten-free diet in diagnosis and management, and the role of an expert pathologist in evaluating the diagnosis of seronegative enteropathy.”
Patients with seronegative enteropathy tend to a have a poorer prognosis than those with classic CeD and other causes of villous atrophy, the investigators noted, but with an accurate diagnosis, distinct therapies can be highly effective.
After a comprehensive literature review, Dr. Leonard and colleagues reached consensus on eight best practice advice statements.
First, the investigators advised clinicians to review histologic findings with an experienced pathologist specializing in gastroenterology, as an expert can ensure proper duodenal orientation, and possibly link a specific finding with an etiology, such as granulomas with Crohn’s disease, or decreased goblet cells with autoimmune enteropathy. Communications with pathologists should also incorporate medical, travel, and medication history.
“Clinicians should pay particular attention to obtaining a thorough medication history to determine whether a patient is taking an angiotensin II receptor antagonist, such as olmesartan, which has been described as causing enteropathy,” the investigators wrote. “In some cases, this has led patients to be incorrectly diagnosis with refractory CeD. Other medications, including azathioprine and mycophenolate mofetil, among others, also have been reported to cause enteropathy, which resolves with discontinuation of medication.”
According to Dr. Leonard and colleagues, histologic findings suggestive of Crohn’s disease should prompt HLA testing, which requires careful attention to detail.
“It is prudent that the gastroenterologist or CeD specialist review all alleles tested and reported (or obtain the alleles if not reported) by the laboratory because commercial and academic laboratories might not report all possible alleles associated with CeD,” they wrote.
If HLA testing is positive, then the patient should begin empiric treatment with a gluten-free diet, followed by clinical and endoscopic reassessment after 1-3 years.
If HLA testing is negative, then a battery of tests may be needed to detect alternative etiologies, such as giardiasis, small intestinal bacterial overgrowth, HIV, and others.
“In cases where an underlying cause was identified, a follow-up esophagogastroduodenoscopy with biopsy might not be indicated, according to the etiology identified, treatment, and clinical status,” the investigators wrote.
Even with a comprehensive work-up, clinicians may be unable to identify an etiology. This outcome may be relatively common, the investigators suggested, citing a study of 200 cases of seronegative villous atrophy, of which 18% had no identifiable etiology. Yet finding an etiology may ultimately be unnecessary, as 72% of idiopathic cases resolved without intervention within 9 months, suggesting transient villous atrophy.
Still, intervention is needed for clinically unstable patients with idiopathic seronegative villous atrophy. Dr. Leonard and colleagues recommended first-line treatment with budesonide, starting at 9 mg daily. Depending on clinical status and response, subsequent therapies may include azathioprine or prednisone.
The clinical practice update was commissioned and approved by the AGA. The investigators disclosed additional relationships with Takeda Pharmaceuticals, HealthMode, Anokion, and others.
SOURCE: Leonard MM et al. Gastroenterology. 2020 Sep 30. doi: 10.1053/j.gastro.2020.08.061.
The American Gastroenterological Association has published new guidance for the diagnosis and management of seronegative enteropathies.
Seronegative enteropathies are commonly encountered by gastroenterologists, but accurate diagnosis can be complicated by a wide array of etiologies, misinterpreted histologic findings, suboptimal serology testing, and use of immunosuppressive agents that mask serology findings, reported lead author Maureen M. Leonard, MD, of MassGeneral Hospital for Children in Boston, and colleagues.
“Previous work detailing the prevalence of seronegative celiac disease [CeD], diagnosis of seronegative villous atrophy, and management recommendations for seronegative villous atrophy are available,” the investigators wrote in Gastroenterology. “However, there is limited evidence to guide clinicians regarding the minimal serologic tests necessary, the role of the gluten-free diet in diagnosis and management, and the role of an expert pathologist in evaluating the diagnosis of seronegative enteropathy.”
Patients with seronegative enteropathy tend to a have a poorer prognosis than those with classic CeD and other causes of villous atrophy, the investigators noted, but with an accurate diagnosis, distinct therapies can be highly effective.
After a comprehensive literature review, Dr. Leonard and colleagues reached consensus on eight best practice advice statements.
First, the investigators advised clinicians to review histologic findings with an experienced pathologist specializing in gastroenterology, as an expert can ensure proper duodenal orientation, and possibly link a specific finding with an etiology, such as granulomas with Crohn’s disease, or decreased goblet cells with autoimmune enteropathy. Communications with pathologists should also incorporate medical, travel, and medication history.
“Clinicians should pay particular attention to obtaining a thorough medication history to determine whether a patient is taking an angiotensin II receptor antagonist, such as olmesartan, which has been described as causing enteropathy,” the investigators wrote. “In some cases, this has led patients to be incorrectly diagnosis with refractory CeD. Other medications, including azathioprine and mycophenolate mofetil, among others, also have been reported to cause enteropathy, which resolves with discontinuation of medication.”
According to Dr. Leonard and colleagues, histologic findings suggestive of Crohn’s disease should prompt HLA testing, which requires careful attention to detail.
“It is prudent that the gastroenterologist or CeD specialist review all alleles tested and reported (or obtain the alleles if not reported) by the laboratory because commercial and academic laboratories might not report all possible alleles associated with CeD,” they wrote.
If HLA testing is positive, then the patient should begin empiric treatment with a gluten-free diet, followed by clinical and endoscopic reassessment after 1-3 years.
If HLA testing is negative, then a battery of tests may be needed to detect alternative etiologies, such as giardiasis, small intestinal bacterial overgrowth, HIV, and others.
“In cases where an underlying cause was identified, a follow-up esophagogastroduodenoscopy with biopsy might not be indicated, according to the etiology identified, treatment, and clinical status,” the investigators wrote.
Even with a comprehensive work-up, clinicians may be unable to identify an etiology. This outcome may be relatively common, the investigators suggested, citing a study of 200 cases of seronegative villous atrophy, of which 18% had no identifiable etiology. Yet finding an etiology may ultimately be unnecessary, as 72% of idiopathic cases resolved without intervention within 9 months, suggesting transient villous atrophy.
Still, intervention is needed for clinically unstable patients with idiopathic seronegative villous atrophy. Dr. Leonard and colleagues recommended first-line treatment with budesonide, starting at 9 mg daily. Depending on clinical status and response, subsequent therapies may include azathioprine or prednisone.
The clinical practice update was commissioned and approved by the AGA. The investigators disclosed additional relationships with Takeda Pharmaceuticals, HealthMode, Anokion, and others.
SOURCE: Leonard MM et al. Gastroenterology. 2020 Sep 30. doi: 10.1053/j.gastro.2020.08.061.
The American Gastroenterological Association has published new guidance for the diagnosis and management of seronegative enteropathies.
Seronegative enteropathies are commonly encountered by gastroenterologists, but accurate diagnosis can be complicated by a wide array of etiologies, misinterpreted histologic findings, suboptimal serology testing, and use of immunosuppressive agents that mask serology findings, reported lead author Maureen M. Leonard, MD, of MassGeneral Hospital for Children in Boston, and colleagues.
“Previous work detailing the prevalence of seronegative celiac disease [CeD], diagnosis of seronegative villous atrophy, and management recommendations for seronegative villous atrophy are available,” the investigators wrote in Gastroenterology. “However, there is limited evidence to guide clinicians regarding the minimal serologic tests necessary, the role of the gluten-free diet in diagnosis and management, and the role of an expert pathologist in evaluating the diagnosis of seronegative enteropathy.”
Patients with seronegative enteropathy tend to a have a poorer prognosis than those with classic CeD and other causes of villous atrophy, the investigators noted, but with an accurate diagnosis, distinct therapies can be highly effective.
After a comprehensive literature review, Dr. Leonard and colleagues reached consensus on eight best practice advice statements.
First, the investigators advised clinicians to review histologic findings with an experienced pathologist specializing in gastroenterology, as an expert can ensure proper duodenal orientation, and possibly link a specific finding with an etiology, such as granulomas with Crohn’s disease, or decreased goblet cells with autoimmune enteropathy. Communications with pathologists should also incorporate medical, travel, and medication history.
“Clinicians should pay particular attention to obtaining a thorough medication history to determine whether a patient is taking an angiotensin II receptor antagonist, such as olmesartan, which has been described as causing enteropathy,” the investigators wrote. “In some cases, this has led patients to be incorrectly diagnosis with refractory CeD. Other medications, including azathioprine and mycophenolate mofetil, among others, also have been reported to cause enteropathy, which resolves with discontinuation of medication.”
According to Dr. Leonard and colleagues, histologic findings suggestive of Crohn’s disease should prompt HLA testing, which requires careful attention to detail.
“It is prudent that the gastroenterologist or CeD specialist review all alleles tested and reported (or obtain the alleles if not reported) by the laboratory because commercial and academic laboratories might not report all possible alleles associated with CeD,” they wrote.
If HLA testing is positive, then the patient should begin empiric treatment with a gluten-free diet, followed by clinical and endoscopic reassessment after 1-3 years.
If HLA testing is negative, then a battery of tests may be needed to detect alternative etiologies, such as giardiasis, small intestinal bacterial overgrowth, HIV, and others.
“In cases where an underlying cause was identified, a follow-up esophagogastroduodenoscopy with biopsy might not be indicated, according to the etiology identified, treatment, and clinical status,” the investigators wrote.
Even with a comprehensive work-up, clinicians may be unable to identify an etiology. This outcome may be relatively common, the investigators suggested, citing a study of 200 cases of seronegative villous atrophy, of which 18% had no identifiable etiology. Yet finding an etiology may ultimately be unnecessary, as 72% of idiopathic cases resolved without intervention within 9 months, suggesting transient villous atrophy.
Still, intervention is needed for clinically unstable patients with idiopathic seronegative villous atrophy. Dr. Leonard and colleagues recommended first-line treatment with budesonide, starting at 9 mg daily. Depending on clinical status and response, subsequent therapies may include azathioprine or prednisone.
The clinical practice update was commissioned and approved by the AGA. The investigators disclosed additional relationships with Takeda Pharmaceuticals, HealthMode, Anokion, and others.
SOURCE: Leonard MM et al. Gastroenterology. 2020 Sep 30. doi: 10.1053/j.gastro.2020.08.061.
FROM GASTROENTEROLOGY
Mortality higher in older adults hospitalized for IBD
Adults older than 65 years with inflammatory bowel diseases (IBD) had significantly higher rates of inpatient mortality, compared with those younger than 65 years, independent of factors including disease severity, based on data from more than 200,000 hospital admissions.
Older adults use a disproportionate share of health care resources, but data on outcomes among hospitalized older adults with gastrointestinal illness are limited, Jeffrey Schwartz, MD, of Beth Israel Deaconess Medical Center and Harvard Medical School, both in Boston, and colleagues wrote in the Journal of Clinical Gastroenterology.
“In particular, there remains a significant concern that elderly patients are more susceptible to the development of opportunistic infections and malignancy in the setting of biological therapy, which has evolved into the standard of care for IBD over the past 10 years,” they wrote.
In their study, the researchers identified 162,800 hospital admissions for Crohn’s disease and 96,450 admissions for ulcerative colitis. Of these, 20% and 30%, respectively, were older than 65 years, which the researchers designated as the geriatric group.
In a multivariate analysis, age older than 65 years was significantly associated with increased mortality in both Crohn’s disease (odds ratio, 3.47; 95% confidence interval, 2.72-4.44; P < .001) and ulcerative colitis (OR, 2.75; 95% CI, 2.16-3.49; P < .001). The association was independent of factors included comorbidities, admission type, hospital type, inpatient surgery, and IBD subtype.
The most frequent cause of death in both groups across all ages and disease subtypes was infections (approximately 80% for all groups). The total hospital length of stay was significantly longer for geriatric patients, compared with younger patients with Crohn’s disease, in multivariate analysis (average increase, 0.19 days; P = .009). The total charges also were significantly higher among geriatric Crohn’s disease patients, compared with younger patients (average increase, $2,467; P = .012). No significant differences in hospital stay or total charges appeared between geriatric and younger patients with ulcerative colitis.
The study findings were limited by several factors such as the inclusion of older patients with IBD who were hospitalized for other reasons and by the potential for increased mortality because of comorbidities among elderly patients, the researchers noted. However, the findings support the limited data from similar previous studies and showed greater inpatient mortality for older adults with IBD, compared with hospital inpatients overall.
“Given the high prevalence of IBD patients that require inpatient admission, as well as the rapidly aging nature of the U.S. population, further studies are needed targeting geriatric patients with UC [ulcerative colitis] and CD [Crohn’s disease] to improve their overall management and quality of care to determine if this mortality risk can be reduced,” they concluded.
Tune in to risks in older adults
The study is important because the percentage of the population older than 65 years has been increasing; “at the same time, we are seeing more elderly patients being newly diagnosed with Crohn’s disease and ulcerative colitis,” said Russell D. Cohen, MD, of the University of Chicago, in an interview. “These patients are more vulnerable to complications of the diseases, such as infections, as well as complications from the medications used to treat these diseases.” However, older adults are often excluded from clinical trials and even from many observational studies in IBD, he noted.
“We have known from past studies that infections such as sepsis are a leading cause of death in our IBD patients,” said Dr. Cohen. “It is also understandable that those patients who have had complicated courses and those with other comorbidities have a higher mortality rate. However, what was surprising in the current study is that, even when the authors controlled for these factors, the geriatric patients still had two and three-quarters to three and a half times the mortality than those who were younger.”
The take-home message for clinicians is that “the geriatric patient with IBD is at a much higher rate for inpatient mortality, most commonly from infectious complications, than younger patients,” Dr. Cohen emphasized. “Quicker attention to what may seem minor but could become a potentially life-threatening infection is imperative. Caution with the use of multiple immune suppressing medications in older patients is paramount, as is timely surgical intervention in IBD patients in whom medications simply are not working.”
Focus research on infection prevention, cost burden
“More research should be directed at finding out whether these deadly infections could be prevented, perhaps by preventative ‘prophylactic’ antibiotics in the elderly patients, especially those on multiple immunosuppressive agents,” said Dr. Cohen. “In addition, research into the undue cost burden that these patients place on our health care system and counter that with better access to the newer, safer biological therapies [most of which Medicare does not cover] rather than corticosteroids.”
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Cohen disclosed relationships with multiple companies including AbbVie, Bristol-Myers Squibb/Celgene, Eli Lilly, Gilead Sciences, Janssen, Pfizer, Takeda, and UCB Pharma.
SOURCE: Schwartz J et al. J Clin Gastroenterol. 2020 Nov 23. doi: 10.1097/MCG.0000000000001458.
Help your patients better understand their IBD treatment options by sharing AGA’s patient education, “Living with IBD,” in the AGA GI Patient Center at www.gastro.org/IBD.
Adults older than 65 years with inflammatory bowel diseases (IBD) had significantly higher rates of inpatient mortality, compared with those younger than 65 years, independent of factors including disease severity, based on data from more than 200,000 hospital admissions.
Older adults use a disproportionate share of health care resources, but data on outcomes among hospitalized older adults with gastrointestinal illness are limited, Jeffrey Schwartz, MD, of Beth Israel Deaconess Medical Center and Harvard Medical School, both in Boston, and colleagues wrote in the Journal of Clinical Gastroenterology.
“In particular, there remains a significant concern that elderly patients are more susceptible to the development of opportunistic infections and malignancy in the setting of biological therapy, which has evolved into the standard of care for IBD over the past 10 years,” they wrote.
In their study, the researchers identified 162,800 hospital admissions for Crohn’s disease and 96,450 admissions for ulcerative colitis. Of these, 20% and 30%, respectively, were older than 65 years, which the researchers designated as the geriatric group.
In a multivariate analysis, age older than 65 years was significantly associated with increased mortality in both Crohn’s disease (odds ratio, 3.47; 95% confidence interval, 2.72-4.44; P < .001) and ulcerative colitis (OR, 2.75; 95% CI, 2.16-3.49; P < .001). The association was independent of factors included comorbidities, admission type, hospital type, inpatient surgery, and IBD subtype.
The most frequent cause of death in both groups across all ages and disease subtypes was infections (approximately 80% for all groups). The total hospital length of stay was significantly longer for geriatric patients, compared with younger patients with Crohn’s disease, in multivariate analysis (average increase, 0.19 days; P = .009). The total charges also were significantly higher among geriatric Crohn’s disease patients, compared with younger patients (average increase, $2,467; P = .012). No significant differences in hospital stay or total charges appeared between geriatric and younger patients with ulcerative colitis.
The study findings were limited by several factors such as the inclusion of older patients with IBD who were hospitalized for other reasons and by the potential for increased mortality because of comorbidities among elderly patients, the researchers noted. However, the findings support the limited data from similar previous studies and showed greater inpatient mortality for older adults with IBD, compared with hospital inpatients overall.
“Given the high prevalence of IBD patients that require inpatient admission, as well as the rapidly aging nature of the U.S. population, further studies are needed targeting geriatric patients with UC [ulcerative colitis] and CD [Crohn’s disease] to improve their overall management and quality of care to determine if this mortality risk can be reduced,” they concluded.
Tune in to risks in older adults
The study is important because the percentage of the population older than 65 years has been increasing; “at the same time, we are seeing more elderly patients being newly diagnosed with Crohn’s disease and ulcerative colitis,” said Russell D. Cohen, MD, of the University of Chicago, in an interview. “These patients are more vulnerable to complications of the diseases, such as infections, as well as complications from the medications used to treat these diseases.” However, older adults are often excluded from clinical trials and even from many observational studies in IBD, he noted.
“We have known from past studies that infections such as sepsis are a leading cause of death in our IBD patients,” said Dr. Cohen. “It is also understandable that those patients who have had complicated courses and those with other comorbidities have a higher mortality rate. However, what was surprising in the current study is that, even when the authors controlled for these factors, the geriatric patients still had two and three-quarters to three and a half times the mortality than those who were younger.”
The take-home message for clinicians is that “the geriatric patient with IBD is at a much higher rate for inpatient mortality, most commonly from infectious complications, than younger patients,” Dr. Cohen emphasized. “Quicker attention to what may seem minor but could become a potentially life-threatening infection is imperative. Caution with the use of multiple immune suppressing medications in older patients is paramount, as is timely surgical intervention in IBD patients in whom medications simply are not working.”
Focus research on infection prevention, cost burden
“More research should be directed at finding out whether these deadly infections could be prevented, perhaps by preventative ‘prophylactic’ antibiotics in the elderly patients, especially those on multiple immunosuppressive agents,” said Dr. Cohen. “In addition, research into the undue cost burden that these patients place on our health care system and counter that with better access to the newer, safer biological therapies [most of which Medicare does not cover] rather than corticosteroids.”
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Cohen disclosed relationships with multiple companies including AbbVie, Bristol-Myers Squibb/Celgene, Eli Lilly, Gilead Sciences, Janssen, Pfizer, Takeda, and UCB Pharma.
SOURCE: Schwartz J et al. J Clin Gastroenterol. 2020 Nov 23. doi: 10.1097/MCG.0000000000001458.
Help your patients better understand their IBD treatment options by sharing AGA’s patient education, “Living with IBD,” in the AGA GI Patient Center at www.gastro.org/IBD.
Adults older than 65 years with inflammatory bowel diseases (IBD) had significantly higher rates of inpatient mortality, compared with those younger than 65 years, independent of factors including disease severity, based on data from more than 200,000 hospital admissions.
Older adults use a disproportionate share of health care resources, but data on outcomes among hospitalized older adults with gastrointestinal illness are limited, Jeffrey Schwartz, MD, of Beth Israel Deaconess Medical Center and Harvard Medical School, both in Boston, and colleagues wrote in the Journal of Clinical Gastroenterology.
“In particular, there remains a significant concern that elderly patients are more susceptible to the development of opportunistic infections and malignancy in the setting of biological therapy, which has evolved into the standard of care for IBD over the past 10 years,” they wrote.
In their study, the researchers identified 162,800 hospital admissions for Crohn’s disease and 96,450 admissions for ulcerative colitis. Of these, 20% and 30%, respectively, were older than 65 years, which the researchers designated as the geriatric group.
In a multivariate analysis, age older than 65 years was significantly associated with increased mortality in both Crohn’s disease (odds ratio, 3.47; 95% confidence interval, 2.72-4.44; P < .001) and ulcerative colitis (OR, 2.75; 95% CI, 2.16-3.49; P < .001). The association was independent of factors included comorbidities, admission type, hospital type, inpatient surgery, and IBD subtype.
The most frequent cause of death in both groups across all ages and disease subtypes was infections (approximately 80% for all groups). The total hospital length of stay was significantly longer for geriatric patients, compared with younger patients with Crohn’s disease, in multivariate analysis (average increase, 0.19 days; P = .009). The total charges also were significantly higher among geriatric Crohn’s disease patients, compared with younger patients (average increase, $2,467; P = .012). No significant differences in hospital stay or total charges appeared between geriatric and younger patients with ulcerative colitis.
The study findings were limited by several factors such as the inclusion of older patients with IBD who were hospitalized for other reasons and by the potential for increased mortality because of comorbidities among elderly patients, the researchers noted. However, the findings support the limited data from similar previous studies and showed greater inpatient mortality for older adults with IBD, compared with hospital inpatients overall.
“Given the high prevalence of IBD patients that require inpatient admission, as well as the rapidly aging nature of the U.S. population, further studies are needed targeting geriatric patients with UC [ulcerative colitis] and CD [Crohn’s disease] to improve their overall management and quality of care to determine if this mortality risk can be reduced,” they concluded.
Tune in to risks in older adults
The study is important because the percentage of the population older than 65 years has been increasing; “at the same time, we are seeing more elderly patients being newly diagnosed with Crohn’s disease and ulcerative colitis,” said Russell D. Cohen, MD, of the University of Chicago, in an interview. “These patients are more vulnerable to complications of the diseases, such as infections, as well as complications from the medications used to treat these diseases.” However, older adults are often excluded from clinical trials and even from many observational studies in IBD, he noted.
“We have known from past studies that infections such as sepsis are a leading cause of death in our IBD patients,” said Dr. Cohen. “It is also understandable that those patients who have had complicated courses and those with other comorbidities have a higher mortality rate. However, what was surprising in the current study is that, even when the authors controlled for these factors, the geriatric patients still had two and three-quarters to three and a half times the mortality than those who were younger.”
The take-home message for clinicians is that “the geriatric patient with IBD is at a much higher rate for inpatient mortality, most commonly from infectious complications, than younger patients,” Dr. Cohen emphasized. “Quicker attention to what may seem minor but could become a potentially life-threatening infection is imperative. Caution with the use of multiple immune suppressing medications in older patients is paramount, as is timely surgical intervention in IBD patients in whom medications simply are not working.”
Focus research on infection prevention, cost burden
“More research should be directed at finding out whether these deadly infections could be prevented, perhaps by preventative ‘prophylactic’ antibiotics in the elderly patients, especially those on multiple immunosuppressive agents,” said Dr. Cohen. “In addition, research into the undue cost burden that these patients place on our health care system and counter that with better access to the newer, safer biological therapies [most of which Medicare does not cover] rather than corticosteroids.”
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Cohen disclosed relationships with multiple companies including AbbVie, Bristol-Myers Squibb/Celgene, Eli Lilly, Gilead Sciences, Janssen, Pfizer, Takeda, and UCB Pharma.
SOURCE: Schwartz J et al. J Clin Gastroenterol. 2020 Nov 23. doi: 10.1097/MCG.0000000000001458.
Help your patients better understand their IBD treatment options by sharing AGA’s patient education, “Living with IBD,” in the AGA GI Patient Center at www.gastro.org/IBD.
FROM THE JOURNAL OF CLINICAL GASTROENTEROLOGY
Cancer rates on the rise in adolescents and young adults
Rates of cancer increased by 30% from 1973 to 2015 in adolescents and young adults (AYAs) aged 15–39 years in the United States, according to a review of almost a half million cases in the National Institutes of Health’s Surveillance, Epidemiology, and End Results database.
There was an annual increase of 0.537 new cases per 100,000 people, from 57.2 cases per 100,000 in 1973 to 74.2 in 2015.
Kidney carcinoma led with the highest rate increase. There were also marked increases in thyroid and colorectal carcinoma, germ cell and trophoblastic neoplasms, and melanoma, among others.
The report was published online December 1 in JAMA Network Open.
“Clinicians should be on the lookout for these cancers in their adolescent and young adult patients,” said senior investigator Nicholas Zaorsky, MD, an assistant professor of radiation oncology and public health sciences at the Penn State Cancer Institute, Hershey, Pennsylvania.
“Now that there is a better understanding of the types of cancer that are prevalent and rising in this age group, prevention, screening, diagnosis and treatment protocols specifically targeted to this population should be developed,” he said in a press release.
The reasons for the increases are unclear, but environmental and dietary factors, increasing obesity, and changing screening practices are likely in play, the authors comment. In addition, “cancer screening and overdiagnosis are thought to account for much of the increasing rates of thyroid and kidney carcinoma, among others,” they add.
The American Cancer Society (ACS) recently found similar increases in thyroid, kidney, and colorectal cancer among AYAs, as well as an increase in uterine cancer.
It’s important to note, however, that “this phenomenon is largely driven by trends for thyroid cancer, which is thought to be a result of overdiagnosis,” said ACS surveillance researcher Kimberly Miller, MPH, when asked to comment on the new study.
“As such, it is extremely important to also consider trends in cancer mortality rates among this age group, which are declining overall but are increasing for colorectal and uterine cancers. The fact that both incidence and mortality rates are increasing for these two cancers suggests a true increase in disease burden and certainly requires further attention and research,” she said.
Historically, management of cancer in AYAs has fallen somewhere between pediatric and adult oncology, neither of which capture the distinct biological, social, and economic needs of AYAs. Research has also focused on childhood and adult cancers, leaving cancer in AYAs inadequately studied.
The new findings are “valuable to guide more targeted research and interventions specifically to AYAs,” Zaorsky and colleagues say in their report.
Among female patients ― 59.1% of the study population ― incidence increased for 15 cancers, including kidney carcinoma (annual percent change [APC], 3.632), thyroid carcinoma (APC, 3.456), and myeloma, mast cell, and miscellaneous lymphoreticular neoplasms not otherwise specified (APC, 2.805). Rates of five cancers declined, led by astrocytoma not otherwise specified (APC, –3.369) and carcinoma of the gonads (APC, –1.743).
Among male patients, incidence increased for 14 cancers, including kidney carcinoma (APC, 3.572), unspecified soft tissue sarcoma (APC 2.543), and thyroid carcinoma (APC, 2.273). Incidence fell for seven, led by astrocytoma not otherwise specified (APC, –3.759) and carcinoma of the trachea, bronchus, and lung (APC, –2.635).
Increased testicular cancer rates (APC, 1.246) could be related to greater prenatal exposure to estrogen and progesterone or through dairy consumption; increasing survival of premature infants; and greater exposure to cannabis, among other possibilities, the investigators say.
Increases in colorectal cancer might be related to fewer vegetables and more fat and processed meat in the diet; lack of exercise; and increasing obesity. Human papillomavirus infection has also been implicated.
Higher rates of melanoma could be related to tanning bed use.
Declines in some cancers could be related to greater use of oral contraceptives; laws reducing exposure to benzene and other chemicals; and fewer people smoking.
Although kidney carcinoma has increased at the greatest rate, it’s uncommon. Colorectal and thyroid carcinoma, melanoma, non-Hodgkin lymphoma, and germ cell and trophoblastic neoplasms of the gonads contribute more to the overall increase in cancers among AYAs, the investigators note.
Almost 80% of the patients were White; 10.3% were Black.
The study was funded by the National Center for Advancing Translational Sciences. The investigators have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Rates of cancer increased by 30% from 1973 to 2015 in adolescents and young adults (AYAs) aged 15–39 years in the United States, according to a review of almost a half million cases in the National Institutes of Health’s Surveillance, Epidemiology, and End Results database.
There was an annual increase of 0.537 new cases per 100,000 people, from 57.2 cases per 100,000 in 1973 to 74.2 in 2015.
Kidney carcinoma led with the highest rate increase. There were also marked increases in thyroid and colorectal carcinoma, germ cell and trophoblastic neoplasms, and melanoma, among others.
The report was published online December 1 in JAMA Network Open.
“Clinicians should be on the lookout for these cancers in their adolescent and young adult patients,” said senior investigator Nicholas Zaorsky, MD, an assistant professor of radiation oncology and public health sciences at the Penn State Cancer Institute, Hershey, Pennsylvania.
“Now that there is a better understanding of the types of cancer that are prevalent and rising in this age group, prevention, screening, diagnosis and treatment protocols specifically targeted to this population should be developed,” he said in a press release.
The reasons for the increases are unclear, but environmental and dietary factors, increasing obesity, and changing screening practices are likely in play, the authors comment. In addition, “cancer screening and overdiagnosis are thought to account for much of the increasing rates of thyroid and kidney carcinoma, among others,” they add.
The American Cancer Society (ACS) recently found similar increases in thyroid, kidney, and colorectal cancer among AYAs, as well as an increase in uterine cancer.
It’s important to note, however, that “this phenomenon is largely driven by trends for thyroid cancer, which is thought to be a result of overdiagnosis,” said ACS surveillance researcher Kimberly Miller, MPH, when asked to comment on the new study.
“As such, it is extremely important to also consider trends in cancer mortality rates among this age group, which are declining overall but are increasing for colorectal and uterine cancers. The fact that both incidence and mortality rates are increasing for these two cancers suggests a true increase in disease burden and certainly requires further attention and research,” she said.
Historically, management of cancer in AYAs has fallen somewhere between pediatric and adult oncology, neither of which capture the distinct biological, social, and economic needs of AYAs. Research has also focused on childhood and adult cancers, leaving cancer in AYAs inadequately studied.
The new findings are “valuable to guide more targeted research and interventions specifically to AYAs,” Zaorsky and colleagues say in their report.
Among female patients ― 59.1% of the study population ― incidence increased for 15 cancers, including kidney carcinoma (annual percent change [APC], 3.632), thyroid carcinoma (APC, 3.456), and myeloma, mast cell, and miscellaneous lymphoreticular neoplasms not otherwise specified (APC, 2.805). Rates of five cancers declined, led by astrocytoma not otherwise specified (APC, –3.369) and carcinoma of the gonads (APC, –1.743).
Among male patients, incidence increased for 14 cancers, including kidney carcinoma (APC, 3.572), unspecified soft tissue sarcoma (APC 2.543), and thyroid carcinoma (APC, 2.273). Incidence fell for seven, led by astrocytoma not otherwise specified (APC, –3.759) and carcinoma of the trachea, bronchus, and lung (APC, –2.635).
Increased testicular cancer rates (APC, 1.246) could be related to greater prenatal exposure to estrogen and progesterone or through dairy consumption; increasing survival of premature infants; and greater exposure to cannabis, among other possibilities, the investigators say.
Increases in colorectal cancer might be related to fewer vegetables and more fat and processed meat in the diet; lack of exercise; and increasing obesity. Human papillomavirus infection has also been implicated.
Higher rates of melanoma could be related to tanning bed use.
Declines in some cancers could be related to greater use of oral contraceptives; laws reducing exposure to benzene and other chemicals; and fewer people smoking.
Although kidney carcinoma has increased at the greatest rate, it’s uncommon. Colorectal and thyroid carcinoma, melanoma, non-Hodgkin lymphoma, and germ cell and trophoblastic neoplasms of the gonads contribute more to the overall increase in cancers among AYAs, the investigators note.
Almost 80% of the patients were White; 10.3% were Black.
The study was funded by the National Center for Advancing Translational Sciences. The investigators have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Rates of cancer increased by 30% from 1973 to 2015 in adolescents and young adults (AYAs) aged 15–39 years in the United States, according to a review of almost a half million cases in the National Institutes of Health’s Surveillance, Epidemiology, and End Results database.
There was an annual increase of 0.537 new cases per 100,000 people, from 57.2 cases per 100,000 in 1973 to 74.2 in 2015.
Kidney carcinoma led with the highest rate increase. There were also marked increases in thyroid and colorectal carcinoma, germ cell and trophoblastic neoplasms, and melanoma, among others.
The report was published online December 1 in JAMA Network Open.
“Clinicians should be on the lookout for these cancers in their adolescent and young adult patients,” said senior investigator Nicholas Zaorsky, MD, an assistant professor of radiation oncology and public health sciences at the Penn State Cancer Institute, Hershey, Pennsylvania.
“Now that there is a better understanding of the types of cancer that are prevalent and rising in this age group, prevention, screening, diagnosis and treatment protocols specifically targeted to this population should be developed,” he said in a press release.
The reasons for the increases are unclear, but environmental and dietary factors, increasing obesity, and changing screening practices are likely in play, the authors comment. In addition, “cancer screening and overdiagnosis are thought to account for much of the increasing rates of thyroid and kidney carcinoma, among others,” they add.
The American Cancer Society (ACS) recently found similar increases in thyroid, kidney, and colorectal cancer among AYAs, as well as an increase in uterine cancer.
It’s important to note, however, that “this phenomenon is largely driven by trends for thyroid cancer, which is thought to be a result of overdiagnosis,” said ACS surveillance researcher Kimberly Miller, MPH, when asked to comment on the new study.
“As such, it is extremely important to also consider trends in cancer mortality rates among this age group, which are declining overall but are increasing for colorectal and uterine cancers. The fact that both incidence and mortality rates are increasing for these two cancers suggests a true increase in disease burden and certainly requires further attention and research,” she said.
Historically, management of cancer in AYAs has fallen somewhere between pediatric and adult oncology, neither of which capture the distinct biological, social, and economic needs of AYAs. Research has also focused on childhood and adult cancers, leaving cancer in AYAs inadequately studied.
The new findings are “valuable to guide more targeted research and interventions specifically to AYAs,” Zaorsky and colleagues say in their report.
Among female patients ― 59.1% of the study population ― incidence increased for 15 cancers, including kidney carcinoma (annual percent change [APC], 3.632), thyroid carcinoma (APC, 3.456), and myeloma, mast cell, and miscellaneous lymphoreticular neoplasms not otherwise specified (APC, 2.805). Rates of five cancers declined, led by astrocytoma not otherwise specified (APC, –3.369) and carcinoma of the gonads (APC, –1.743).
Among male patients, incidence increased for 14 cancers, including kidney carcinoma (APC, 3.572), unspecified soft tissue sarcoma (APC 2.543), and thyroid carcinoma (APC, 2.273). Incidence fell for seven, led by astrocytoma not otherwise specified (APC, –3.759) and carcinoma of the trachea, bronchus, and lung (APC, –2.635).
Increased testicular cancer rates (APC, 1.246) could be related to greater prenatal exposure to estrogen and progesterone or through dairy consumption; increasing survival of premature infants; and greater exposure to cannabis, among other possibilities, the investigators say.
Increases in colorectal cancer might be related to fewer vegetables and more fat and processed meat in the diet; lack of exercise; and increasing obesity. Human papillomavirus infection has also been implicated.
Higher rates of melanoma could be related to tanning bed use.
Declines in some cancers could be related to greater use of oral contraceptives; laws reducing exposure to benzene and other chemicals; and fewer people smoking.
Although kidney carcinoma has increased at the greatest rate, it’s uncommon. Colorectal and thyroid carcinoma, melanoma, non-Hodgkin lymphoma, and germ cell and trophoblastic neoplasms of the gonads contribute more to the overall increase in cancers among AYAs, the investigators note.
Almost 80% of the patients were White; 10.3% were Black.
The study was funded by the National Center for Advancing Translational Sciences. The investigators have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The Design and Implementation of a Heart Disease Reversal Program in the Veterans Health Administration: Before and During the COVID-19 Pandemic
While cardiovascular mortality rates have declined, heart disease continues to be the leading cause of death in the US, and the number of people with cardiovascular disease (CVD) is rising.1 CVD is more prevalent among military veterans than it is among nonveterans aged ≥ 25 years, and veteran status is associated with higher risk of incident heart disease after controlling for socioeconomic status, other medical diseases, depression, and lifestyle.2-4 Combat exposure, posttraumatic stress disorder (PTSD), and Purple Heart commendation are associated with higher rates of CVD, including adverse cardiovascular events.5-7 Many patients seeking care in the Veterans Health Administration (VHA), including those who undergo cardiac catheterization, meet the criteria for multimorbidity (defined as having ≥ 2 chronic diseases8), which is common among veterans.9,10 Multimorbidity presents a challenge for lifestyle intervention, as different diets may be prescribed to treat different conditions, such as Dietary Approaches to Stop Hypertension, and low-glycemic diet for diabetes mellitus (DM). Veterans with CVD are often clinically complex and may require more multifaceted secondary prevention programs.
During the coronavirus 2019 (COVID-19) pandemic, effective secondary prevention intervention is needed more than ever. Older age, CVD, and common comorbidities, including hypertension, DM, and obesity, place patients at the highest risk for severe COVID-19 infection.11 COVID-19 social distancing encourages vulnerable populations to stay home, which can make engaging in any levels of physical activity more challenging. The International Food Council found that 85% of adults have made a change to their food consumption pattern, including eating more, during the COVID-19 pandemic.12 Thus, secondary CVD prevention programs for veterans need to provide treatment that addresses these specific challenges and can be delivered via telehealth for continuity of care after disruption of traditional services.
Clinical practice guidelines for the treatment of patients with recent cardiovascular adverse events (AEs) include a referral to cardiac rehabilitation (CR).13 CR emphasizes exercise as the main intervention, along with coaching to promote multiple risk reduction. The most comprehensive CR program is intensive CR (ICR), including the Ornish ICR program.14 ICR includes 4 components: vegetarian diet, exercise, stress management (yoga, meditation), and group support. Ornish ICR has been shown to be efficacious in randomized controlled trials (RCTs).15-17 Three effectiveness studies, with 5,372 participants, demonstrated the real-world effectiveness of Ornish ICR in US hospitals.14,18,19 The program also was adapted successfully for the active-duty military and veteran population.20,21 Yet Ornish ICR is time intensive, and there are no certified VHA ICR Ornish sites. Furthermore, there is no formal strategy for targeting people with atherosclerotic CVD who no longer meet the criteria for CR or ICR. While Ornish ICR is highly effective for patients who are eligible and have access, a more effective and streamlined approach is needed for targeting many patients.
Nutrition may be the most powerful Ornish ICR component. The initial RCT conducted by Ornish and colleagues included only stress management training and a whole-food, plant-based (WFPB) diet, including grains, legumes, vegetables, fruits, nuts, and seeds. The trial found 91% of participants experienced reduced angina after only 24 days.15 The only single-component intervention study resulting in partial reversal of atherosclerosis was a WFPB diet-only study, which documented regression of atherosclerotic plaques after 5 years, using coronary angiography in 73% of participants, with arrested progression in the other 27%.22 Participants reported no cardiovascular AEs after 12 years.23 Furthermore, a number of other recent studies have demonstrated the benefits of WFPB diet-only interventions for type 2 DM (T2DM), hypertension, and obesity.24-27 The Heart Disease Reversal Program (HDRP) was developed to create an interdisciplinary lifestyle intervention that emphasized nutrition for a broad population of veterans with atherosclerotic CVD, of varying levels of functional ability, to promote comprehensive CVD risk reduction and bring heart disease reversal intervention into routine clinical practice.
Program Description
The Mental Health, Cardiology, and Nutrition and Food services all approved the launch of HDRP. We contacted veterans by mail, and 11% expressed interest (Figure). Among patients who received the initial mailed letter (prior to our accepting staff referrals), only 5% of patients who enrolled in HDRP reported previously being told about or prescribed a WFPB diet by any health care provider (HCP). Currently, patients are primarily referred to HDRP by Cardiology, Primary Care, and Mental Health services.
Design
HDRP is an adaptation of interdisciplinary lifestyle interventions that have resulted in regression of atherosclerotic blockages confirmed with invasive coronary angiography.15-17,22,28 HDRP currently is offered in a Behavioral Medicine Clinic at the Sacramento US Department of Veterans Affairs (VA) Medical Center (VAMC) in California. Program staff include a clinical health psychologist who organizes, coordinates, and act as the lead facilitator of the program; registered dietitians; clinical pharmacists; and a consulting physician. Patients engage in the 4-month core HDRP program in small cohorts (ie, 6-10 patients), and spouses/partners are highly encouraged to attend all sessions.
Components
Telephone screening. Patients are screened for the inclusion and exclusion criteria (Table 1). Patients engaging in a traditional CR program are included in the screening. Patients are informed that the program consists of lifestyle intervention, including emphasis on following a WFPB diet.
Health assessment. Once approved, all patients are instructed to complete baseline laboratory tests and questionnaires. Along with an electronic health record (EHR) review, a psychosocial assessment is completed by a licensed clinical health psychologist who assesses CVD history, eating behavior, exercise/physical activity, sleep, mental health, substance use, and social history, with the aim of enhancing our ability to help the patient to benefit from HDRP.29 The patient data are used to develop a case conceptualization (ie, integrated understanding of the particular patient’s psychiatric and medical diagnoses, behavioral patterns, social supports, lifestyle habits, strengths and weaknesses, and their interrelationships with each other and the patient’s environment), resulting in an individualized plan. Patients are encouraged to ask questions about the program, and those who are still interested are invited to attend a seminar. A request for medical clearance to participate in the program is initiated through the EHR or by patients scheduling an appointment with their HCP. All patients are medically cleared by their HCP for participation. Safe exercise recommendations also are provided and guide patient goals.
CVD risk profile. Patients complete psychosocial questionnaires and fasting laboratory tests to produce a tailored CVD risk profile. Laboratory tests include fasting lipid, fasting glucose, hemoglobin A1c (HbA1c) C-reactive protein, vitamin B12, and vitamin D. The same tests (excluding HbA1c) are completed 1 month later (after completing 4 group sessions) and again posttreatment (including HbA1c). Self-reported questionnaires are completed at the same time points, which include the Rate Your Plate dietary composition questionnaire, CHAMPS physical activity questionnaire for older adults, Beck Depression Inventory-II, and the Perceived Stress Scale.
Seminar. A 2-hour seminar provides patients and families with an opportunity to meet HDRP program staff, learn the background and rationale for chronic disease reversal, obtain a summary of the program, and hear a patient testimonial. Patients are asked to make a commitment, and the informed consent process includes all patients signing a behavioral contract.
Assessment and feedback. A licensed clinical health psychologist provides feedback to patients on their comprehensive CVD risk profile, using motivational interviewing.30,31 Smokers are encouraged to quit, and those interested are referred to their HCP and/or facility smoking cessation program.
Group sessions. Twelve weekly group sessions cover nutrition education and cooking, physical activity and exercise, stress management training, and medication reconciliation and adjustment. The nutrition component is the centerpiece of HDRP and is delivered by registered dietitians (Table 2). Patients are instructed to use the 3-week period between the HDRP seminar and the first core group session to try new recipes and prepare their kitchens, pantries, and mind-set to adopt the HDRP diet with 100% adherence. The WFPB diet used is consistent with the current guidelines of Caldwell Esselstyn, MD, and Dean Ornish, MD.32-34
A psychologist delivers the physical activity component. Patients are encouraged to meet the American Heart Association/American College of Cardiology recommendations for aerobic exercise (at least 150 minutes of moderate intensity physical activity per week) through a walking program.35 Patients with medical contraindications (eg, severe pain, mobility restrictions) are encouraged to follow the exercise/activity recommendations they had been given by their primary care provider (PCP), physical therapist, or other HCP.
A psychologist provides evidence-based cognitive behavioral stress management (CBSM) training, adapted from models developed for patients with stable ischemic heart disease, HIV/AIDS, and cancer.36-38 CBSM is a psychotherapy grounded in stress/coping theory and cognitive behavioral theory of psychopathology that integrates cognitive restructuring, coping skills training, communication/assertiveness training, anger management, and mindfulness/acceptance-based approaches. Additional emphasis is placed on assisting patients’ adjustment to the lifestyle challenges for following a plant-based diet, dealing with food cravings and emotional eating, and connecting lifestyle change to patients’ deepest values and goals.
A clinical pharmacist conducts a medication reconciliation for each patient at baseline. The pharmacist consults with each patient’s PCP, cardiologist, and HDRP consulting physician, as needed, to ensure safe adjustments to medications. Pharmacists also provide education on medications at group sessions.
After completion of the 12-week core program, graduates are encouraged to attend the monthly graduates’ group indefinitely, and as often as they desire to promote maintenance of the disease reversal lifestyle. Patients are encouraged to complete our recommended fasting laboratory work every 3 to 6 months to facilitate maintenance of treatment gains.
Program Evaluation
Patients frequently reported that the group format was vital to their success. Patients requested a cooking class, yet we lacked a full teaching kitchen. Integrating plant-based meal samples at every session and cooking videos helped. Patients reported that 100% adherence to the WFPB diet led to significant changes in their food preferences, including a loss of interest in meat.39 Patients encouraged us to keep the “disease reversal” language and focus. One veteran stated: “Disease reversal, that is the reason I called you when I got your letter.” Showing before and after images of coronary angiograms and cardiac positron emission tomography scans depicting regression of atherosclerotic plaque and restored myocardial perfusion were described as highly motivating and generated willingness to commit to a more aggressive lifestyle change.31
Patients routinely stated that they lacked understanding of their laboratory test results, which HDRP remedied. Some patients reported their adult children followed a plant-based diet, and our program resulted in a new commonality and source of bonding that was highly valued. Some patients reported that HDRP was helpful for controlling their COVID-19 anxiety and feeling in control of their health. Satisfaction surveys were completed by participants at the end of the core program, which demonstrated very high satisfaction with and acceptability of HDRP (Table 3).
The program also has received positive feedback from HCPs when we alert them to improvements in outcome measures for their patients. These HCPs expressed satisfaction with having a program to refer patients to that can help with chronic illness in more depth.
COVID-19 Response
Face-to-face group appointments were converted to videoconferencing as a result of the COVID-19 pandemic. While HDRP always promoted the use of technology and mHealth tools, the pandemic led us to develop novel technology-based interventions.40 One cohort transitioned from in-person to videoconferencing sessions, and 2 cohorts recently started this format and are ongoing. We have successfully used videoconferencing with Cisco Webex, the VA-approved backup platform, as we encountered technical barriers when using VA Video Connect. Program materials were shared electronically, and participants sent blood pressure/sugar logs by secure messaging. Guidance for online grocery shopping with home delivery was provided, and research on the benefits of the HDRP lifestyle on immune function was incorporated.
The stress management component incorporated coping with COVID-19, including normalizing common emotional difficulties with sheltering-in-place and quarantine, acknowledging and processing fear and anxiety related to being at very high risk for severe COVID-19. We presented heart disease reversal as an urgent and feasible goal during the pandemic both reducing risk of premature death and major adverse cardiovascular events in the long-term and also reducing personal risk of severe COVID complications. The new VA COVID Coach app was also presented as a resource. Reputable sources of COVID-19 and public health information were shared. Walking continued to be the primary recommended form of exercise, while indoor home exercise options were promoted during the periods of very poor air quality due to the widespread California fires and smoke.
Considering the research suggesting benefits of our intervention for treating T2DM,promoting sustained weight loss, and promoting comprehensive cardiometabolic risk reduction, we have begun accepting referrals for patients with any type of atherosclerotic CVD (eg, peripheral artery disease, carotid artery disease), patients with T2DM (without CVD), and patients with only a history of ischemic stroke or transient ischemic attack.24-27 Vascular surgery has become a new referral source, primarily for patients with peripheral and carotid artery diseases. Finally, we are leveraging videoconferencing and accepting referrals across the VA Northern California Health Care System (VANCHCS)catchment (from the California-Oregon state border to the San Francisco Bay Area). This also helps address a long-standing problem with reaching the many rural veterans who live far from a VA clinic. We successfully implemented a consult/referral process within the EHR that is available to providers across VANCHCS.
Discussion
The efficacy and effectiveness of reversal programs are well established in intensive programs (eg, ICR), yet such programs have yet to be streamlined and disseminated broadly into routine clinical care. HDRP has endeavored to address this by emphasizing nutrition relative to other program components. We have learned that the words “disease reversal” are very often the reason patients initially reach out or accept referral to our program.
Consistent with past research on plant-based nutrition interventions, the group format was indispensable.41 Individual sessions with a clinical health psychologist enabled tailored feedback and education on how behavior changes could impact laboratory results and how certain psychosocial factors could support success. Participants reported that seeing significantly favorable laboratory results was highly motivating and confirmed the power of their lifestyle changes. Furthermore, a psychosocial health assessment with individual sessions promoted a tailored treatment plan with targeted clinical interventions, such as behavioral health education, motivational interviewing, and advanced methods, including cognitive behavioral therapy and techniques drawn from dialectical behavior therapy and acceptance and commitment therapy.
Veterans with multimorbidity face the difficult task of learning and maintaining a complex disease self-management program and implementing a lifestyle approach that is feasible, effective, promotes weight loss, and treats multiple conditions. HDRP is a model approach for this population, as demonstrated by a recent case report of a 65-year-old male veteran with atherosclerotic CVD, T2DM, hypertension, and myasthenia gravis who had 2 heart attacks within 2 months.42 His neurologic disease precluded significant physical activity. Although he achieved some initial weight loss through lifestyle changes, he continued to have daily angina despite optimal and aggressive cardiology management. After enrolling in HDRP and adopting the WFPB diet, the patient reported almost complete resolution of angina within 1 month, similar to that found in other studies.15
The literature suggests that concern over the acceptability of plant-based diets and patients’ ability to adhere to them long-term may be misplaced. A review paper on dietary interventions lasting > 1 year found that 51 to 61% of vegetarian and vegan study participants had maintained dietary adherence, while 20 to 55% of omnivorous diet intervention participants adhered to their study diets.43 Remarkably, there were no statistically significant differences in the acceptability of the vegan, vegetarian, or omnivorous diets in the studies reviewed.43 Recent dietary research also suggests that providing patients with higher goals (eg, adopting a vegan diet instead of only moderate dietary changes) results in greater weight loss and maintenance.26 HDRP provides training on consumption of whole plant foods, which may offer patients a unique advantage for maximizing results and higher adherence over time.
Limitations
Hands-on cooking instruction was not provided at our VAMC. The total time of the intervention was significantly less in HDRP (25 hours) than it was for the Ornish ICR program (72 hours), which may hinder long-term adherence. Without an exercise facility, we were not able to provide more detailed exercise instruction and supervised exercise.
Program Improvements Planned
There are a number of improvements that are planned for HDRP. First, the program anticipates requesting medical clearance at the telephone screening stage for self-referred patients. Second,
Conclusions
Although our patient population was self-selected for participation, early program evaluation demonstrates high acceptability. Very few patients had ever been told about a heart disease reversing lifestyle, and we found direct-to-patient clinical outreach an effective method for launching a disease reversal program (optimally timed with HCP presentations). Furthermore, the program is adaptable to current restrictions on in-person appointments due to the COVID-19 pandemic, and much more convenient for rural veterans who live far from any VA clinic. Being able to offer sustainable health care for individuals during unexpected public health crises is critically important. Additionally, treating veterans who are most vulnerable to pandemic illness due to existing medical conditions, such as CVD, should be a high priority. Last, HDRP also may represent a novel integrated treatment for COVID-19 anxiety and secondary CVD prevention, as lifestyle habits are optimized to improve chronic diseases that elevate risk for severe COVID-19 infection and mortality, as well as including coping strategies consistent with evidence-based psychotherapies for anxiety disorders.44
We believe that beyond the clinical benefits to patients, there is significant value and benefit added to the health care system by offering an intervention within the “disease reversal” paradigm. Efforts of the health care team to reverse a disease can be considered the highest aim of medicine and health care.45
Acknowledgments
This work was supported by the US Department of Veterans Affairs. We give special thanks to David M. Gellerman, MD, PhD, and David W. Schafer, PsyD, for providing Mental Health Service support for initiating the Heart Disease Reversal Program, and to Joseph Giorgio, PsyD (Program Manager, Integrated Care Program) for sustaining it. We thank Amogh Bhat, MD, Chief of Cardiology, for his continued support and partnership with the Cardiology Department. We express thanks to Stephanie Mohney, RDN (Chief, Nutrition and Food Service), Amy Klotz, RDN (Supervisory Dietician), Sian M. Carr-Lopez, PharmD (Associate Chief of Pharmacy, Primary Care), and Michelle Rand, PharmD, CACP (Anticoagulation Clinical Pharmacist-Supervisor) for their staff support of this interdisciplinary program. We thank the patients and their families for their participation in the program and commitment to the lifestyle changes. We also thank the following individuals for their contributions to this program: Lisa Wagaman, RDN, Karen Soong, PharmD, Sara S. Ali, PharmD, Suzan Hua, PharmD, and Stephen Cooperman.
1. Benjamin EJ, Virani SS, Callaway CW, et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association [published correction appears in Circulation. 2018 Mar 20;137(12 ): e493]. Circulation. 2018;137(12):e67-e492. doi:10.1161/CIR.0000000000000558
2. Hinojosa R. Cardiovascular disease among United States military veterans: evidence of a waning healthy soldier effect using the National Health Interview Survey. Chronic Illn. 2020;16(1):55-68. doi:10.1177/1742395318785237.
3. Hinojosa R. Sex, age, race/ethnicity, veteran status, and the likelihood of reporting cardiovascular conditions in the National Health Interview Survey. J Cardiovasc Nurs. 2019;34(3):215-221. doi:10.1097/JCN.0000000000000561 4. Assari S. Veterans and risk of heart disease in the United States: a cohort with 20 years of follow up. Int J Prev Med. 2014;5(6):703-709.
5. Thomas MM, Harpaz-Rotem I, Tsai J, Southwick SM, Pietrzak RH. Mental and physical health conditions in US combat veterans: results from the National Health and Resilience in Veterans Study. Prim Care Companion CNS Disord. 2017;19(3):10.4088/PCC.17m02118. Published 2017 Jun 22. doi:10.4088/PCC.17m02118
6. Bukhbinder AS, Wang AC, Qureshi SU, et al. Increased vascular pathology in older veterans with a purple heart commendation or chronic post-traumatic stress disorder. J Geriatr Psychiatry Neurol. 2020;33(4):195-206. doi:10.1177/0891988719868308
7. Edmondson D, von Känel R. Post-traumatic stress disorder and cardiovascular disease. Lancet Psychiatry. 2017;4(4):320-329. doi:10.1016/S2215-0366(16)30377-7
8. Forman DE, Maurer MS, Boyd C, et a;. Multimorbidity in older adults with cardiovascular disease. J Am Coll Cardiol. 2018;71(19):2149-2161. doi:10.1016/j.jacc.2018.03.022
9. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252
10. Maddox TM, Plomondon ME, Petrich M, et al. A national clinical quality program for Veterans Affairs catheterization laboratories (from the Veterans Affairs clinical assessment, reporting, and tracking program). Am J Cardiol. 2014;114(11):1750-1757. doi:10.1016/j.amjcard.2014.08.045
11. Centers for Disease Control and Prevention. Coronavirus 2019 (COVID-19):people at increased risk and other people who need to take extra precautions. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/index.html. Updated September 11, 2020. Accessed November 12, 2020.
12. International Food Information Council. 2020 food and health survey. https://foodinsight.org/2020-food-and-health-survey. Updated June 9, 2020. Accessed November 12, 2020.
13. American Association of Cardiovascular and Pulmonary Rehabilitation. Guidelines for Cardiac Rehabilitation and Secondary Prevention Programs. 5th ed. Champaign, IL: Human Kinetics; 2013.
14. Silberman A, Banthia R, Estay IS, et al. The effectiveness and efficacy of an intensive cardiac rehabilitation program in 24 sites. Am J Health Promot. 2010;24(4):260-266. doi:10.4278/ajhp.24.4.arb
15. Ornish D, Scherwitz LW, Doody RS, et al. Effects of stress management training and dietary changes in treating ischemic heart disease. JAMA. 1983;249(1):54-59.
16. Ornish D, Brown SE, Scherwitz LW, et al. Can lifestyle changes reverse coronary heart disease? The Lifestyle Heart Trial. Lancet. 1990;336(8708):129-133. doi:10.1016/0140-6736(90)91656-u.
17. Ornish D, Scherwitz LW, Billings JH, et al. Intensive lifestyle changes for reversal of coronary heart disease [published correction appears in JAMA 1999 Apr 21;281(15):1380]. JAMA. 1998;280(23):2001-2007. doi:10.1001/jama.280.23.2001
18. Frattaroli J, Weidner G, Merritt-Worden TA, Frenda S, Ornish D. Angina pectoris and atherosclerotic risk factors in the multisite cardiac lifestyle intervention program. Am J Cardiol. 2008;101(7):911-918. doi:10.1016/j.amjcard.2007.11.039
19. Koertge J, Weidner G, Elliott-Eller M, et al. Improvement in medical risk factors and quality of life in women and men with coronary artery disease in the Multicenter Lifestyle Demonstration Project. Am J Cardiol. 2003;91(11):1316-1322. doi:10.1016/s0002-9149(03)00320-5
20. Marshall DA, Walizer EM, Vernalis MN. Achievement of heart health characteristics through participation in an intensive lifestyle change program (Coronary Artery Disease Reversal Study). J Cardiopulm Rehabil Prev. 2009;29(2):84-96. doi:10.1097/HCR.0b013e31819a00b2
21. Marshall D, Elaine W, Vernalis M. The effect of a one-year lifestyle intervention program on carotid intima media thickness. Mil Med. 2011;176(7):798-804. doi:10.7205/milmed-d-10-00447
22. Esselstyn CB Jr, Ellis SG, Medendorp SV, Crowe TD. A strategy to arrest and reverse coronary artery disease: a 5-year longitudinal study of a single physician’s practice. J Fam Pract. 1995;41(6):560-568.
23. Esselstyn CB Jr. Updating a 12-year experience with arrest and reversal therapy for coronary heart disease (an overdue requiem for palliative cardiology). Am J Cardiol. 1999;84(3):339-A8. doi:10.1016/s0002-9149(99)00290-8
24. Barnard ND, Cohen J, Jenkins DJ, et al. A low-fat vegan diet improves glycemic control and cardiovascular risk factors in a randomized clinical trial in individuals with type 2 diabetes. Diabetes Care. 2006;29(8):1777-1783. doi:10.2337/dc06-0606
25. McDougall J, Thomas LE, McDougall C, et al. Effects of 7 days on an ad libitum low-fat vegan diet: the McDougall Program cohort [published correction appears in Nutr J. 2017 Feb 10;16(1):12]. Nutr J. 2014;13:99. Published 2014 Oct 14. doi:10.1186/1475-2891-13-99
26. Turner-McGrievy GM, Davidson CR, Wingard EE, Wilcox S, Frongillo EA. Comparative effectiveness of plant-based diets for weight loss: a randomized controlled trial of five different diets. Nutrition. 2015;31(2):350-358. doi:10.1016/j.nut.2014.09.002
27. Wright N, Wilson L, Smith M, Duncan B, McHugh P. The BROAD study: a randomised controlled trial using a whole food plant-based diet in the community for obesity, ischaemic heart disease or diabetes. Nutr Diabetes. 2017;7(3):e256. Published 2017 Mar 20. doi:10.1038/nutd.2017.3

28. Schaefer S, Hussein H, Gershony GR, Rutledge JC, Kappagoda CT. Regression of severe atherosclerotic plaque in patients with mild elevation of LDL cholesterol. J Investig Med. 1997;45(9):536-541.
29. Kitazono R. Know thy patient: Enhancing lifestyle interventions with psychological assessment. Int J Dis Rev Prev. 2020;2(1):76-81.
30. Miller WR, Rollnick S. Motivational Interviewing: Helping People Change. 3rd ed. New York, NY: Guilford Press; 2013.
31. Mascola AJ, Yiaslas TA, Meir RL, et al. Framing physical activity as a distinct and uniquely valuable behavior independent of weight management: A pilot randomized controlled trial for overweight and obese sedentary persons. Eat Weight Disord. 2009;14(2-3):e148-e152. doi:10.1007/BF03327814
32. Esselstyn AC, Esselstyn J. The Prevent and Reverse Heart Disease Cookbook: Over 125 Delicious, Life-Changing, Plant-Based Recipes. New York, NY: Avery; 2014.
33. Esselstyn CB Jr, Gendy G, Doyle J, Golubic M, Roizen MF. A way to reverse CAD? J Fam Pract. 2014;63(7):356-364.
34. Ornish D, Ornish A. Undo It! How Simple Lifestyle Changes Can Reverse Most Chronic Diseases. New York, NY: Ballantine Books; 2019.
35. Smith SC Jr, Benjamin EJ, Bonow RO, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation endorsed by the World Heart Federation and the Preventive Cardiovascular Nurses Association [published correction appears in J Am Coll Cardiol. 2015 Apr 14;65(14):1495. Dosage error in article text.]. J Am Coll Cardiol. 2011;58(23):2432-2446. doi:10.1016/j.jacc.2011.10.824
36. Blumenthal JA, Babyak M, Wei J, et al. Usefulness of psychosocial treatment of mental stress-induced myocardial ischemia in men. Am J Cardiol. 2002;89(2):164-168. doi:10.1016/s0002-9149(01)02194-4
37. Antoni MH. Stress management effects on psychological, endocrinological, and immune functioning in men with HIV infection: empirical support for a psychoneuroimmunological model. Stress. 2003;6(3):173-188. doi:10.1080/1025389031000156727
38. Penedo FJ, Molton I, Dahn JR, et al. A randomized clinical trial of group-based cognitive-behavioral stress management in localized prostate cancer: development of stress management skills improves quality of life and benefit finding. Ann Behav Med. 2006;31(3):261-270. doi:10.1207/s15324796abm3103_8
39. Yiaslas TA. “Look doctor, I’m a carnivore.” Int J Dis Rev Prev. 2020;2(2):35-39.
40. Khaylis A, Yiaslas T, Bergstrom J, Gore-Felton C. A review of efficacious technology-based weight-loss interventions: five key components. Telemed J E Health. 2010;16(9):931-938. doi:10.1089/tmj.2010.0065
41. Barnard ND, Sherwitz L, Ornish D. Adherence and acceptability of a low-fat, vegetarian diet among patients with cardiac disease. J Cardiopulm Rehabil. 1992;12(6):423-431.
42. Yiaslas TA, Taylor J, Embree J, Schaefer S. Elimination of angina, comprehensive cardio-metabolic risk reduction, and 50-pound weight loss in a US Navy veteran with myasthenia gravis. Int J Dis Rev Prev. 2019;1(1):77-83.
43. Berkow SE, Barnard N, Eckart J, Katcher H. Four therapeutic diets: adherence and acceptability. Can J Diet Pract Res. 2010;71(4):199-204. doi:10.3148/71.4.2010.199
44. Carpenter JK, Andrews LA, Witcraft SM, Powers MB, Smits JAJ, Hofmann SG. Cognitive behavioral therapy for anxiety and related disorders: A meta-analysis of randomized placebo-controlled trials. Depress Anxiety. 2018;35(6):502-514. doi:10.1002/da.22728
45. Yiaslas TA. The pursuit of arete in medicine and health care. Int J Dis Rev Prev. 2019;1(2):53-56.
While cardiovascular mortality rates have declined, heart disease continues to be the leading cause of death in the US, and the number of people with cardiovascular disease (CVD) is rising.1 CVD is more prevalent among military veterans than it is among nonveterans aged ≥ 25 years, and veteran status is associated with higher risk of incident heart disease after controlling for socioeconomic status, other medical diseases, depression, and lifestyle.2-4 Combat exposure, posttraumatic stress disorder (PTSD), and Purple Heart commendation are associated with higher rates of CVD, including adverse cardiovascular events.5-7 Many patients seeking care in the Veterans Health Administration (VHA), including those who undergo cardiac catheterization, meet the criteria for multimorbidity (defined as having ≥ 2 chronic diseases8), which is common among veterans.9,10 Multimorbidity presents a challenge for lifestyle intervention, as different diets may be prescribed to treat different conditions, such as Dietary Approaches to Stop Hypertension, and low-glycemic diet for diabetes mellitus (DM). Veterans with CVD are often clinically complex and may require more multifaceted secondary prevention programs.
During the coronavirus 2019 (COVID-19) pandemic, effective secondary prevention intervention is needed more than ever. Older age, CVD, and common comorbidities, including hypertension, DM, and obesity, place patients at the highest risk for severe COVID-19 infection.11 COVID-19 social distancing encourages vulnerable populations to stay home, which can make engaging in any levels of physical activity more challenging. The International Food Council found that 85% of adults have made a change to their food consumption pattern, including eating more, during the COVID-19 pandemic.12 Thus, secondary CVD prevention programs for veterans need to provide treatment that addresses these specific challenges and can be delivered via telehealth for continuity of care after disruption of traditional services.
Clinical practice guidelines for the treatment of patients with recent cardiovascular adverse events (AEs) include a referral to cardiac rehabilitation (CR).13 CR emphasizes exercise as the main intervention, along with coaching to promote multiple risk reduction. The most comprehensive CR program is intensive CR (ICR), including the Ornish ICR program.14 ICR includes 4 components: vegetarian diet, exercise, stress management (yoga, meditation), and group support. Ornish ICR has been shown to be efficacious in randomized controlled trials (RCTs).15-17 Three effectiveness studies, with 5,372 participants, demonstrated the real-world effectiveness of Ornish ICR in US hospitals.14,18,19 The program also was adapted successfully for the active-duty military and veteran population.20,21 Yet Ornish ICR is time intensive, and there are no certified VHA ICR Ornish sites. Furthermore, there is no formal strategy for targeting people with atherosclerotic CVD who no longer meet the criteria for CR or ICR. While Ornish ICR is highly effective for patients who are eligible and have access, a more effective and streamlined approach is needed for targeting many patients.
Nutrition may be the most powerful Ornish ICR component. The initial RCT conducted by Ornish and colleagues included only stress management training and a whole-food, plant-based (WFPB) diet, including grains, legumes, vegetables, fruits, nuts, and seeds. The trial found 91% of participants experienced reduced angina after only 24 days.15 The only single-component intervention study resulting in partial reversal of atherosclerosis was a WFPB diet-only study, which documented regression of atherosclerotic plaques after 5 years, using coronary angiography in 73% of participants, with arrested progression in the other 27%.22 Participants reported no cardiovascular AEs after 12 years.23 Furthermore, a number of other recent studies have demonstrated the benefits of WFPB diet-only interventions for type 2 DM (T2DM), hypertension, and obesity.24-27 The Heart Disease Reversal Program (HDRP) was developed to create an interdisciplinary lifestyle intervention that emphasized nutrition for a broad population of veterans with atherosclerotic CVD, of varying levels of functional ability, to promote comprehensive CVD risk reduction and bring heart disease reversal intervention into routine clinical practice.
Program Description
The Mental Health, Cardiology, and Nutrition and Food services all approved the launch of HDRP. We contacted veterans by mail, and 11% expressed interest (Figure). Among patients who received the initial mailed letter (prior to our accepting staff referrals), only 5% of patients who enrolled in HDRP reported previously being told about or prescribed a WFPB diet by any health care provider (HCP). Currently, patients are primarily referred to HDRP by Cardiology, Primary Care, and Mental Health services.
Design
HDRP is an adaptation of interdisciplinary lifestyle interventions that have resulted in regression of atherosclerotic blockages confirmed with invasive coronary angiography.15-17,22,28 HDRP currently is offered in a Behavioral Medicine Clinic at the Sacramento US Department of Veterans Affairs (VA) Medical Center (VAMC) in California. Program staff include a clinical health psychologist who organizes, coordinates, and act as the lead facilitator of the program; registered dietitians; clinical pharmacists; and a consulting physician. Patients engage in the 4-month core HDRP program in small cohorts (ie, 6-10 patients), and spouses/partners are highly encouraged to attend all sessions.
Components
Telephone screening. Patients are screened for the inclusion and exclusion criteria (Table 1). Patients engaging in a traditional CR program are included in the screening. Patients are informed that the program consists of lifestyle intervention, including emphasis on following a WFPB diet.
Health assessment. Once approved, all patients are instructed to complete baseline laboratory tests and questionnaires. Along with an electronic health record (EHR) review, a psychosocial assessment is completed by a licensed clinical health psychologist who assesses CVD history, eating behavior, exercise/physical activity, sleep, mental health, substance use, and social history, with the aim of enhancing our ability to help the patient to benefit from HDRP.29 The patient data are used to develop a case conceptualization (ie, integrated understanding of the particular patient’s psychiatric and medical diagnoses, behavioral patterns, social supports, lifestyle habits, strengths and weaknesses, and their interrelationships with each other and the patient’s environment), resulting in an individualized plan. Patients are encouraged to ask questions about the program, and those who are still interested are invited to attend a seminar. A request for medical clearance to participate in the program is initiated through the EHR or by patients scheduling an appointment with their HCP. All patients are medically cleared by their HCP for participation. Safe exercise recommendations also are provided and guide patient goals.
CVD risk profile. Patients complete psychosocial questionnaires and fasting laboratory tests to produce a tailored CVD risk profile. Laboratory tests include fasting lipid, fasting glucose, hemoglobin A1c (HbA1c) C-reactive protein, vitamin B12, and vitamin D. The same tests (excluding HbA1c) are completed 1 month later (after completing 4 group sessions) and again posttreatment (including HbA1c). Self-reported questionnaires are completed at the same time points, which include the Rate Your Plate dietary composition questionnaire, CHAMPS physical activity questionnaire for older adults, Beck Depression Inventory-II, and the Perceived Stress Scale.
Seminar. A 2-hour seminar provides patients and families with an opportunity to meet HDRP program staff, learn the background and rationale for chronic disease reversal, obtain a summary of the program, and hear a patient testimonial. Patients are asked to make a commitment, and the informed consent process includes all patients signing a behavioral contract.
Assessment and feedback. A licensed clinical health psychologist provides feedback to patients on their comprehensive CVD risk profile, using motivational interviewing.30,31 Smokers are encouraged to quit, and those interested are referred to their HCP and/or facility smoking cessation program.
Group sessions. Twelve weekly group sessions cover nutrition education and cooking, physical activity and exercise, stress management training, and medication reconciliation and adjustment. The nutrition component is the centerpiece of HDRP and is delivered by registered dietitians (Table 2). Patients are instructed to use the 3-week period between the HDRP seminar and the first core group session to try new recipes and prepare their kitchens, pantries, and mind-set to adopt the HDRP diet with 100% adherence. The WFPB diet used is consistent with the current guidelines of Caldwell Esselstyn, MD, and Dean Ornish, MD.32-34
A psychologist delivers the physical activity component. Patients are encouraged to meet the American Heart Association/American College of Cardiology recommendations for aerobic exercise (at least 150 minutes of moderate intensity physical activity per week) through a walking program.35 Patients with medical contraindications (eg, severe pain, mobility restrictions) are encouraged to follow the exercise/activity recommendations they had been given by their primary care provider (PCP), physical therapist, or other HCP.
A psychologist provides evidence-based cognitive behavioral stress management (CBSM) training, adapted from models developed for patients with stable ischemic heart disease, HIV/AIDS, and cancer.36-38 CBSM is a psychotherapy grounded in stress/coping theory and cognitive behavioral theory of psychopathology that integrates cognitive restructuring, coping skills training, communication/assertiveness training, anger management, and mindfulness/acceptance-based approaches. Additional emphasis is placed on assisting patients’ adjustment to the lifestyle challenges for following a plant-based diet, dealing with food cravings and emotional eating, and connecting lifestyle change to patients’ deepest values and goals.
A clinical pharmacist conducts a medication reconciliation for each patient at baseline. The pharmacist consults with each patient’s PCP, cardiologist, and HDRP consulting physician, as needed, to ensure safe adjustments to medications. Pharmacists also provide education on medications at group sessions.
After completion of the 12-week core program, graduates are encouraged to attend the monthly graduates’ group indefinitely, and as often as they desire to promote maintenance of the disease reversal lifestyle. Patients are encouraged to complete our recommended fasting laboratory work every 3 to 6 months to facilitate maintenance of treatment gains.
Program Evaluation
Patients frequently reported that the group format was vital to their success. Patients requested a cooking class, yet we lacked a full teaching kitchen. Integrating plant-based meal samples at every session and cooking videos helped. Patients reported that 100% adherence to the WFPB diet led to significant changes in their food preferences, including a loss of interest in meat.39 Patients encouraged us to keep the “disease reversal” language and focus. One veteran stated: “Disease reversal, that is the reason I called you when I got your letter.” Showing before and after images of coronary angiograms and cardiac positron emission tomography scans depicting regression of atherosclerotic plaque and restored myocardial perfusion were described as highly motivating and generated willingness to commit to a more aggressive lifestyle change.31
Patients routinely stated that they lacked understanding of their laboratory test results, which HDRP remedied. Some patients reported their adult children followed a plant-based diet, and our program resulted in a new commonality and source of bonding that was highly valued. Some patients reported that HDRP was helpful for controlling their COVID-19 anxiety and feeling in control of their health. Satisfaction surveys were completed by participants at the end of the core program, which demonstrated very high satisfaction with and acceptability of HDRP (Table 3).
The program also has received positive feedback from HCPs when we alert them to improvements in outcome measures for their patients. These HCPs expressed satisfaction with having a program to refer patients to that can help with chronic illness in more depth.
COVID-19 Response
Face-to-face group appointments were converted to videoconferencing as a result of the COVID-19 pandemic. While HDRP always promoted the use of technology and mHealth tools, the pandemic led us to develop novel technology-based interventions.40 One cohort transitioned from in-person to videoconferencing sessions, and 2 cohorts recently started this format and are ongoing. We have successfully used videoconferencing with Cisco Webex, the VA-approved backup platform, as we encountered technical barriers when using VA Video Connect. Program materials were shared electronically, and participants sent blood pressure/sugar logs by secure messaging. Guidance for online grocery shopping with home delivery was provided, and research on the benefits of the HDRP lifestyle on immune function was incorporated.
The stress management component incorporated coping with COVID-19, including normalizing common emotional difficulties with sheltering-in-place and quarantine, acknowledging and processing fear and anxiety related to being at very high risk for severe COVID-19. We presented heart disease reversal as an urgent and feasible goal during the pandemic both reducing risk of premature death and major adverse cardiovascular events in the long-term and also reducing personal risk of severe COVID complications. The new VA COVID Coach app was also presented as a resource. Reputable sources of COVID-19 and public health information were shared. Walking continued to be the primary recommended form of exercise, while indoor home exercise options were promoted during the periods of very poor air quality due to the widespread California fires and smoke.
Considering the research suggesting benefits of our intervention for treating T2DM,promoting sustained weight loss, and promoting comprehensive cardiometabolic risk reduction, we have begun accepting referrals for patients with any type of atherosclerotic CVD (eg, peripheral artery disease, carotid artery disease), patients with T2DM (without CVD), and patients with only a history of ischemic stroke or transient ischemic attack.24-27 Vascular surgery has become a new referral source, primarily for patients with peripheral and carotid artery diseases. Finally, we are leveraging videoconferencing and accepting referrals across the VA Northern California Health Care System (VANCHCS)catchment (from the California-Oregon state border to the San Francisco Bay Area). This also helps address a long-standing problem with reaching the many rural veterans who live far from a VA clinic. We successfully implemented a consult/referral process within the EHR that is available to providers across VANCHCS.
Discussion
The efficacy and effectiveness of reversal programs are well established in intensive programs (eg, ICR), yet such programs have yet to be streamlined and disseminated broadly into routine clinical care. HDRP has endeavored to address this by emphasizing nutrition relative to other program components. We have learned that the words “disease reversal” are very often the reason patients initially reach out or accept referral to our program.
Consistent with past research on plant-based nutrition interventions, the group format was indispensable.41 Individual sessions with a clinical health psychologist enabled tailored feedback and education on how behavior changes could impact laboratory results and how certain psychosocial factors could support success. Participants reported that seeing significantly favorable laboratory results was highly motivating and confirmed the power of their lifestyle changes. Furthermore, a psychosocial health assessment with individual sessions promoted a tailored treatment plan with targeted clinical interventions, such as behavioral health education, motivational interviewing, and advanced methods, including cognitive behavioral therapy and techniques drawn from dialectical behavior therapy and acceptance and commitment therapy.
Veterans with multimorbidity face the difficult task of learning and maintaining a complex disease self-management program and implementing a lifestyle approach that is feasible, effective, promotes weight loss, and treats multiple conditions. HDRP is a model approach for this population, as demonstrated by a recent case report of a 65-year-old male veteran with atherosclerotic CVD, T2DM, hypertension, and myasthenia gravis who had 2 heart attacks within 2 months.42 His neurologic disease precluded significant physical activity. Although he achieved some initial weight loss through lifestyle changes, he continued to have daily angina despite optimal and aggressive cardiology management. After enrolling in HDRP and adopting the WFPB diet, the patient reported almost complete resolution of angina within 1 month, similar to that found in other studies.15
The literature suggests that concern over the acceptability of plant-based diets and patients’ ability to adhere to them long-term may be misplaced. A review paper on dietary interventions lasting > 1 year found that 51 to 61% of vegetarian and vegan study participants had maintained dietary adherence, while 20 to 55% of omnivorous diet intervention participants adhered to their study diets.43 Remarkably, there were no statistically significant differences in the acceptability of the vegan, vegetarian, or omnivorous diets in the studies reviewed.43 Recent dietary research also suggests that providing patients with higher goals (eg, adopting a vegan diet instead of only moderate dietary changes) results in greater weight loss and maintenance.26 HDRP provides training on consumption of whole plant foods, which may offer patients a unique advantage for maximizing results and higher adherence over time.
Limitations
Hands-on cooking instruction was not provided at our VAMC. The total time of the intervention was significantly less in HDRP (25 hours) than it was for the Ornish ICR program (72 hours), which may hinder long-term adherence. Without an exercise facility, we were not able to provide more detailed exercise instruction and supervised exercise.
Program Improvements Planned
There are a number of improvements that are planned for HDRP. First, the program anticipates requesting medical clearance at the telephone screening stage for self-referred patients. Second,
Conclusions
Although our patient population was self-selected for participation, early program evaluation demonstrates high acceptability. Very few patients had ever been told about a heart disease reversing lifestyle, and we found direct-to-patient clinical outreach an effective method for launching a disease reversal program (optimally timed with HCP presentations). Furthermore, the program is adaptable to current restrictions on in-person appointments due to the COVID-19 pandemic, and much more convenient for rural veterans who live far from any VA clinic. Being able to offer sustainable health care for individuals during unexpected public health crises is critically important. Additionally, treating veterans who are most vulnerable to pandemic illness due to existing medical conditions, such as CVD, should be a high priority. Last, HDRP also may represent a novel integrated treatment for COVID-19 anxiety and secondary CVD prevention, as lifestyle habits are optimized to improve chronic diseases that elevate risk for severe COVID-19 infection and mortality, as well as including coping strategies consistent with evidence-based psychotherapies for anxiety disorders.44
We believe that beyond the clinical benefits to patients, there is significant value and benefit added to the health care system by offering an intervention within the “disease reversal” paradigm. Efforts of the health care team to reverse a disease can be considered the highest aim of medicine and health care.45
Acknowledgments
This work was supported by the US Department of Veterans Affairs. We give special thanks to David M. Gellerman, MD, PhD, and David W. Schafer, PsyD, for providing Mental Health Service support for initiating the Heart Disease Reversal Program, and to Joseph Giorgio, PsyD (Program Manager, Integrated Care Program) for sustaining it. We thank Amogh Bhat, MD, Chief of Cardiology, for his continued support and partnership with the Cardiology Department. We express thanks to Stephanie Mohney, RDN (Chief, Nutrition and Food Service), Amy Klotz, RDN (Supervisory Dietician), Sian M. Carr-Lopez, PharmD (Associate Chief of Pharmacy, Primary Care), and Michelle Rand, PharmD, CACP (Anticoagulation Clinical Pharmacist-Supervisor) for their staff support of this interdisciplinary program. We thank the patients and their families for their participation in the program and commitment to the lifestyle changes. We also thank the following individuals for their contributions to this program: Lisa Wagaman, RDN, Karen Soong, PharmD, Sara S. Ali, PharmD, Suzan Hua, PharmD, and Stephen Cooperman.
While cardiovascular mortality rates have declined, heart disease continues to be the leading cause of death in the US, and the number of people with cardiovascular disease (CVD) is rising.1 CVD is more prevalent among military veterans than it is among nonveterans aged ≥ 25 years, and veteran status is associated with higher risk of incident heart disease after controlling for socioeconomic status, other medical diseases, depression, and lifestyle.2-4 Combat exposure, posttraumatic stress disorder (PTSD), and Purple Heart commendation are associated with higher rates of CVD, including adverse cardiovascular events.5-7 Many patients seeking care in the Veterans Health Administration (VHA), including those who undergo cardiac catheterization, meet the criteria for multimorbidity (defined as having ≥ 2 chronic diseases8), which is common among veterans.9,10 Multimorbidity presents a challenge for lifestyle intervention, as different diets may be prescribed to treat different conditions, such as Dietary Approaches to Stop Hypertension, and low-glycemic diet for diabetes mellitus (DM). Veterans with CVD are often clinically complex and may require more multifaceted secondary prevention programs.
During the coronavirus 2019 (COVID-19) pandemic, effective secondary prevention intervention is needed more than ever. Older age, CVD, and common comorbidities, including hypertension, DM, and obesity, place patients at the highest risk for severe COVID-19 infection.11 COVID-19 social distancing encourages vulnerable populations to stay home, which can make engaging in any levels of physical activity more challenging. The International Food Council found that 85% of adults have made a change to their food consumption pattern, including eating more, during the COVID-19 pandemic.12 Thus, secondary CVD prevention programs for veterans need to provide treatment that addresses these specific challenges and can be delivered via telehealth for continuity of care after disruption of traditional services.
Clinical practice guidelines for the treatment of patients with recent cardiovascular adverse events (AEs) include a referral to cardiac rehabilitation (CR).13 CR emphasizes exercise as the main intervention, along with coaching to promote multiple risk reduction. The most comprehensive CR program is intensive CR (ICR), including the Ornish ICR program.14 ICR includes 4 components: vegetarian diet, exercise, stress management (yoga, meditation), and group support. Ornish ICR has been shown to be efficacious in randomized controlled trials (RCTs).15-17 Three effectiveness studies, with 5,372 participants, demonstrated the real-world effectiveness of Ornish ICR in US hospitals.14,18,19 The program also was adapted successfully for the active-duty military and veteran population.20,21 Yet Ornish ICR is time intensive, and there are no certified VHA ICR Ornish sites. Furthermore, there is no formal strategy for targeting people with atherosclerotic CVD who no longer meet the criteria for CR or ICR. While Ornish ICR is highly effective for patients who are eligible and have access, a more effective and streamlined approach is needed for targeting many patients.
Nutrition may be the most powerful Ornish ICR component. The initial RCT conducted by Ornish and colleagues included only stress management training and a whole-food, plant-based (WFPB) diet, including grains, legumes, vegetables, fruits, nuts, and seeds. The trial found 91% of participants experienced reduced angina after only 24 days.15 The only single-component intervention study resulting in partial reversal of atherosclerosis was a WFPB diet-only study, which documented regression of atherosclerotic plaques after 5 years, using coronary angiography in 73% of participants, with arrested progression in the other 27%.22 Participants reported no cardiovascular AEs after 12 years.23 Furthermore, a number of other recent studies have demonstrated the benefits of WFPB diet-only interventions for type 2 DM (T2DM), hypertension, and obesity.24-27 The Heart Disease Reversal Program (HDRP) was developed to create an interdisciplinary lifestyle intervention that emphasized nutrition for a broad population of veterans with atherosclerotic CVD, of varying levels of functional ability, to promote comprehensive CVD risk reduction and bring heart disease reversal intervention into routine clinical practice.
Program Description
The Mental Health, Cardiology, and Nutrition and Food services all approved the launch of HDRP. We contacted veterans by mail, and 11% expressed interest (Figure). Among patients who received the initial mailed letter (prior to our accepting staff referrals), only 5% of patients who enrolled in HDRP reported previously being told about or prescribed a WFPB diet by any health care provider (HCP). Currently, patients are primarily referred to HDRP by Cardiology, Primary Care, and Mental Health services.
Design
HDRP is an adaptation of interdisciplinary lifestyle interventions that have resulted in regression of atherosclerotic blockages confirmed with invasive coronary angiography.15-17,22,28 HDRP currently is offered in a Behavioral Medicine Clinic at the Sacramento US Department of Veterans Affairs (VA) Medical Center (VAMC) in California. Program staff include a clinical health psychologist who organizes, coordinates, and act as the lead facilitator of the program; registered dietitians; clinical pharmacists; and a consulting physician. Patients engage in the 4-month core HDRP program in small cohorts (ie, 6-10 patients), and spouses/partners are highly encouraged to attend all sessions.
Components
Telephone screening. Patients are screened for the inclusion and exclusion criteria (Table 1). Patients engaging in a traditional CR program are included in the screening. Patients are informed that the program consists of lifestyle intervention, including emphasis on following a WFPB diet.
Health assessment. Once approved, all patients are instructed to complete baseline laboratory tests and questionnaires. Along with an electronic health record (EHR) review, a psychosocial assessment is completed by a licensed clinical health psychologist who assesses CVD history, eating behavior, exercise/physical activity, sleep, mental health, substance use, and social history, with the aim of enhancing our ability to help the patient to benefit from HDRP.29 The patient data are used to develop a case conceptualization (ie, integrated understanding of the particular patient’s psychiatric and medical diagnoses, behavioral patterns, social supports, lifestyle habits, strengths and weaknesses, and their interrelationships with each other and the patient’s environment), resulting in an individualized plan. Patients are encouraged to ask questions about the program, and those who are still interested are invited to attend a seminar. A request for medical clearance to participate in the program is initiated through the EHR or by patients scheduling an appointment with their HCP. All patients are medically cleared by their HCP for participation. Safe exercise recommendations also are provided and guide patient goals.
CVD risk profile. Patients complete psychosocial questionnaires and fasting laboratory tests to produce a tailored CVD risk profile. Laboratory tests include fasting lipid, fasting glucose, hemoglobin A1c (HbA1c) C-reactive protein, vitamin B12, and vitamin D. The same tests (excluding HbA1c) are completed 1 month later (after completing 4 group sessions) and again posttreatment (including HbA1c). Self-reported questionnaires are completed at the same time points, which include the Rate Your Plate dietary composition questionnaire, CHAMPS physical activity questionnaire for older adults, Beck Depression Inventory-II, and the Perceived Stress Scale.
Seminar. A 2-hour seminar provides patients and families with an opportunity to meet HDRP program staff, learn the background and rationale for chronic disease reversal, obtain a summary of the program, and hear a patient testimonial. Patients are asked to make a commitment, and the informed consent process includes all patients signing a behavioral contract.
Assessment and feedback. A licensed clinical health psychologist provides feedback to patients on their comprehensive CVD risk profile, using motivational interviewing.30,31 Smokers are encouraged to quit, and those interested are referred to their HCP and/or facility smoking cessation program.
Group sessions. Twelve weekly group sessions cover nutrition education and cooking, physical activity and exercise, stress management training, and medication reconciliation and adjustment. The nutrition component is the centerpiece of HDRP and is delivered by registered dietitians (Table 2). Patients are instructed to use the 3-week period between the HDRP seminar and the first core group session to try new recipes and prepare their kitchens, pantries, and mind-set to adopt the HDRP diet with 100% adherence. The WFPB diet used is consistent with the current guidelines of Caldwell Esselstyn, MD, and Dean Ornish, MD.32-34
A psychologist delivers the physical activity component. Patients are encouraged to meet the American Heart Association/American College of Cardiology recommendations for aerobic exercise (at least 150 minutes of moderate intensity physical activity per week) through a walking program.35 Patients with medical contraindications (eg, severe pain, mobility restrictions) are encouraged to follow the exercise/activity recommendations they had been given by their primary care provider (PCP), physical therapist, or other HCP.
A psychologist provides evidence-based cognitive behavioral stress management (CBSM) training, adapted from models developed for patients with stable ischemic heart disease, HIV/AIDS, and cancer.36-38 CBSM is a psychotherapy grounded in stress/coping theory and cognitive behavioral theory of psychopathology that integrates cognitive restructuring, coping skills training, communication/assertiveness training, anger management, and mindfulness/acceptance-based approaches. Additional emphasis is placed on assisting patients’ adjustment to the lifestyle challenges for following a plant-based diet, dealing with food cravings and emotional eating, and connecting lifestyle change to patients’ deepest values and goals.
A clinical pharmacist conducts a medication reconciliation for each patient at baseline. The pharmacist consults with each patient’s PCP, cardiologist, and HDRP consulting physician, as needed, to ensure safe adjustments to medications. Pharmacists also provide education on medications at group sessions.
After completion of the 12-week core program, graduates are encouraged to attend the monthly graduates’ group indefinitely, and as often as they desire to promote maintenance of the disease reversal lifestyle. Patients are encouraged to complete our recommended fasting laboratory work every 3 to 6 months to facilitate maintenance of treatment gains.
Program Evaluation
Patients frequently reported that the group format was vital to their success. Patients requested a cooking class, yet we lacked a full teaching kitchen. Integrating plant-based meal samples at every session and cooking videos helped. Patients reported that 100% adherence to the WFPB diet led to significant changes in their food preferences, including a loss of interest in meat.39 Patients encouraged us to keep the “disease reversal” language and focus. One veteran stated: “Disease reversal, that is the reason I called you when I got your letter.” Showing before and after images of coronary angiograms and cardiac positron emission tomography scans depicting regression of atherosclerotic plaque and restored myocardial perfusion were described as highly motivating and generated willingness to commit to a more aggressive lifestyle change.31
Patients routinely stated that they lacked understanding of their laboratory test results, which HDRP remedied. Some patients reported their adult children followed a plant-based diet, and our program resulted in a new commonality and source of bonding that was highly valued. Some patients reported that HDRP was helpful for controlling their COVID-19 anxiety and feeling in control of their health. Satisfaction surveys were completed by participants at the end of the core program, which demonstrated very high satisfaction with and acceptability of HDRP (Table 3).
The program also has received positive feedback from HCPs when we alert them to improvements in outcome measures for their patients. These HCPs expressed satisfaction with having a program to refer patients to that can help with chronic illness in more depth.
COVID-19 Response
Face-to-face group appointments were converted to videoconferencing as a result of the COVID-19 pandemic. While HDRP always promoted the use of technology and mHealth tools, the pandemic led us to develop novel technology-based interventions.40 One cohort transitioned from in-person to videoconferencing sessions, and 2 cohorts recently started this format and are ongoing. We have successfully used videoconferencing with Cisco Webex, the VA-approved backup platform, as we encountered technical barriers when using VA Video Connect. Program materials were shared electronically, and participants sent blood pressure/sugar logs by secure messaging. Guidance for online grocery shopping with home delivery was provided, and research on the benefits of the HDRP lifestyle on immune function was incorporated.
The stress management component incorporated coping with COVID-19, including normalizing common emotional difficulties with sheltering-in-place and quarantine, acknowledging and processing fear and anxiety related to being at very high risk for severe COVID-19. We presented heart disease reversal as an urgent and feasible goal during the pandemic both reducing risk of premature death and major adverse cardiovascular events in the long-term and also reducing personal risk of severe COVID complications. The new VA COVID Coach app was also presented as a resource. Reputable sources of COVID-19 and public health information were shared. Walking continued to be the primary recommended form of exercise, while indoor home exercise options were promoted during the periods of very poor air quality due to the widespread California fires and smoke.
Considering the research suggesting benefits of our intervention for treating T2DM,promoting sustained weight loss, and promoting comprehensive cardiometabolic risk reduction, we have begun accepting referrals for patients with any type of atherosclerotic CVD (eg, peripheral artery disease, carotid artery disease), patients with T2DM (without CVD), and patients with only a history of ischemic stroke or transient ischemic attack.24-27 Vascular surgery has become a new referral source, primarily for patients with peripheral and carotid artery diseases. Finally, we are leveraging videoconferencing and accepting referrals across the VA Northern California Health Care System (VANCHCS)catchment (from the California-Oregon state border to the San Francisco Bay Area). This also helps address a long-standing problem with reaching the many rural veterans who live far from a VA clinic. We successfully implemented a consult/referral process within the EHR that is available to providers across VANCHCS.
Discussion
The efficacy and effectiveness of reversal programs are well established in intensive programs (eg, ICR), yet such programs have yet to be streamlined and disseminated broadly into routine clinical care. HDRP has endeavored to address this by emphasizing nutrition relative to other program components. We have learned that the words “disease reversal” are very often the reason patients initially reach out or accept referral to our program.
Consistent with past research on plant-based nutrition interventions, the group format was indispensable.41 Individual sessions with a clinical health psychologist enabled tailored feedback and education on how behavior changes could impact laboratory results and how certain psychosocial factors could support success. Participants reported that seeing significantly favorable laboratory results was highly motivating and confirmed the power of their lifestyle changes. Furthermore, a psychosocial health assessment with individual sessions promoted a tailored treatment plan with targeted clinical interventions, such as behavioral health education, motivational interviewing, and advanced methods, including cognitive behavioral therapy and techniques drawn from dialectical behavior therapy and acceptance and commitment therapy.
Veterans with multimorbidity face the difficult task of learning and maintaining a complex disease self-management program and implementing a lifestyle approach that is feasible, effective, promotes weight loss, and treats multiple conditions. HDRP is a model approach for this population, as demonstrated by a recent case report of a 65-year-old male veteran with atherosclerotic CVD, T2DM, hypertension, and myasthenia gravis who had 2 heart attacks within 2 months.42 His neurologic disease precluded significant physical activity. Although he achieved some initial weight loss through lifestyle changes, he continued to have daily angina despite optimal and aggressive cardiology management. After enrolling in HDRP and adopting the WFPB diet, the patient reported almost complete resolution of angina within 1 month, similar to that found in other studies.15
The literature suggests that concern over the acceptability of plant-based diets and patients’ ability to adhere to them long-term may be misplaced. A review paper on dietary interventions lasting > 1 year found that 51 to 61% of vegetarian and vegan study participants had maintained dietary adherence, while 20 to 55% of omnivorous diet intervention participants adhered to their study diets.43 Remarkably, there were no statistically significant differences in the acceptability of the vegan, vegetarian, or omnivorous diets in the studies reviewed.43 Recent dietary research also suggests that providing patients with higher goals (eg, adopting a vegan diet instead of only moderate dietary changes) results in greater weight loss and maintenance.26 HDRP provides training on consumption of whole plant foods, which may offer patients a unique advantage for maximizing results and higher adherence over time.
Limitations
Hands-on cooking instruction was not provided at our VAMC. The total time of the intervention was significantly less in HDRP (25 hours) than it was for the Ornish ICR program (72 hours), which may hinder long-term adherence. Without an exercise facility, we were not able to provide more detailed exercise instruction and supervised exercise.
Program Improvements Planned
There are a number of improvements that are planned for HDRP. First, the program anticipates requesting medical clearance at the telephone screening stage for self-referred patients. Second,
Conclusions
Although our patient population was self-selected for participation, early program evaluation demonstrates high acceptability. Very few patients had ever been told about a heart disease reversing lifestyle, and we found direct-to-patient clinical outreach an effective method for launching a disease reversal program (optimally timed with HCP presentations). Furthermore, the program is adaptable to current restrictions on in-person appointments due to the COVID-19 pandemic, and much more convenient for rural veterans who live far from any VA clinic. Being able to offer sustainable health care for individuals during unexpected public health crises is critically important. Additionally, treating veterans who are most vulnerable to pandemic illness due to existing medical conditions, such as CVD, should be a high priority. Last, HDRP also may represent a novel integrated treatment for COVID-19 anxiety and secondary CVD prevention, as lifestyle habits are optimized to improve chronic diseases that elevate risk for severe COVID-19 infection and mortality, as well as including coping strategies consistent with evidence-based psychotherapies for anxiety disorders.44
We believe that beyond the clinical benefits to patients, there is significant value and benefit added to the health care system by offering an intervention within the “disease reversal” paradigm. Efforts of the health care team to reverse a disease can be considered the highest aim of medicine and health care.45
Acknowledgments
This work was supported by the US Department of Veterans Affairs. We give special thanks to David M. Gellerman, MD, PhD, and David W. Schafer, PsyD, for providing Mental Health Service support for initiating the Heart Disease Reversal Program, and to Joseph Giorgio, PsyD (Program Manager, Integrated Care Program) for sustaining it. We thank Amogh Bhat, MD, Chief of Cardiology, for his continued support and partnership with the Cardiology Department. We express thanks to Stephanie Mohney, RDN (Chief, Nutrition and Food Service), Amy Klotz, RDN (Supervisory Dietician), Sian M. Carr-Lopez, PharmD (Associate Chief of Pharmacy, Primary Care), and Michelle Rand, PharmD, CACP (Anticoagulation Clinical Pharmacist-Supervisor) for their staff support of this interdisciplinary program. We thank the patients and their families for their participation in the program and commitment to the lifestyle changes. We also thank the following individuals for their contributions to this program: Lisa Wagaman, RDN, Karen Soong, PharmD, Sara S. Ali, PharmD, Suzan Hua, PharmD, and Stephen Cooperman.
1. Benjamin EJ, Virani SS, Callaway CW, et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association [published correction appears in Circulation. 2018 Mar 20;137(12 ): e493]. Circulation. 2018;137(12):e67-e492. doi:10.1161/CIR.0000000000000558
2. Hinojosa R. Cardiovascular disease among United States military veterans: evidence of a waning healthy soldier effect using the National Health Interview Survey. Chronic Illn. 2020;16(1):55-68. doi:10.1177/1742395318785237.
3. Hinojosa R. Sex, age, race/ethnicity, veteran status, and the likelihood of reporting cardiovascular conditions in the National Health Interview Survey. J Cardiovasc Nurs. 2019;34(3):215-221. doi:10.1097/JCN.0000000000000561 4. Assari S. Veterans and risk of heart disease in the United States: a cohort with 20 years of follow up. Int J Prev Med. 2014;5(6):703-709.
5. Thomas MM, Harpaz-Rotem I, Tsai J, Southwick SM, Pietrzak RH. Mental and physical health conditions in US combat veterans: results from the National Health and Resilience in Veterans Study. Prim Care Companion CNS Disord. 2017;19(3):10.4088/PCC.17m02118. Published 2017 Jun 22. doi:10.4088/PCC.17m02118
6. Bukhbinder AS, Wang AC, Qureshi SU, et al. Increased vascular pathology in older veterans with a purple heart commendation or chronic post-traumatic stress disorder. J Geriatr Psychiatry Neurol. 2020;33(4):195-206. doi:10.1177/0891988719868308
7. Edmondson D, von Känel R. Post-traumatic stress disorder and cardiovascular disease. Lancet Psychiatry. 2017;4(4):320-329. doi:10.1016/S2215-0366(16)30377-7
8. Forman DE, Maurer MS, Boyd C, et a;. Multimorbidity in older adults with cardiovascular disease. J Am Coll Cardiol. 2018;71(19):2149-2161. doi:10.1016/j.jacc.2018.03.022
9. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252
10. Maddox TM, Plomondon ME, Petrich M, et al. A national clinical quality program for Veterans Affairs catheterization laboratories (from the Veterans Affairs clinical assessment, reporting, and tracking program). Am J Cardiol. 2014;114(11):1750-1757. doi:10.1016/j.amjcard.2014.08.045
11. Centers for Disease Control and Prevention. Coronavirus 2019 (COVID-19):people at increased risk and other people who need to take extra precautions. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/index.html. Updated September 11, 2020. Accessed November 12, 2020.
12. International Food Information Council. 2020 food and health survey. https://foodinsight.org/2020-food-and-health-survey. Updated June 9, 2020. Accessed November 12, 2020.
13. American Association of Cardiovascular and Pulmonary Rehabilitation. Guidelines for Cardiac Rehabilitation and Secondary Prevention Programs. 5th ed. Champaign, IL: Human Kinetics; 2013.
14. Silberman A, Banthia R, Estay IS, et al. The effectiveness and efficacy of an intensive cardiac rehabilitation program in 24 sites. Am J Health Promot. 2010;24(4):260-266. doi:10.4278/ajhp.24.4.arb
15. Ornish D, Scherwitz LW, Doody RS, et al. Effects of stress management training and dietary changes in treating ischemic heart disease. JAMA. 1983;249(1):54-59.
16. Ornish D, Brown SE, Scherwitz LW, et al. Can lifestyle changes reverse coronary heart disease? The Lifestyle Heart Trial. Lancet. 1990;336(8708):129-133. doi:10.1016/0140-6736(90)91656-u.
17. Ornish D, Scherwitz LW, Billings JH, et al. Intensive lifestyle changes for reversal of coronary heart disease [published correction appears in JAMA 1999 Apr 21;281(15):1380]. JAMA. 1998;280(23):2001-2007. doi:10.1001/jama.280.23.2001
18. Frattaroli J, Weidner G, Merritt-Worden TA, Frenda S, Ornish D. Angina pectoris and atherosclerotic risk factors in the multisite cardiac lifestyle intervention program. Am J Cardiol. 2008;101(7):911-918. doi:10.1016/j.amjcard.2007.11.039
19. Koertge J, Weidner G, Elliott-Eller M, et al. Improvement in medical risk factors and quality of life in women and men with coronary artery disease in the Multicenter Lifestyle Demonstration Project. Am J Cardiol. 2003;91(11):1316-1322. doi:10.1016/s0002-9149(03)00320-5
20. Marshall DA, Walizer EM, Vernalis MN. Achievement of heart health characteristics through participation in an intensive lifestyle change program (Coronary Artery Disease Reversal Study). J Cardiopulm Rehabil Prev. 2009;29(2):84-96. doi:10.1097/HCR.0b013e31819a00b2
21. Marshall D, Elaine W, Vernalis M. The effect of a one-year lifestyle intervention program on carotid intima media thickness. Mil Med. 2011;176(7):798-804. doi:10.7205/milmed-d-10-00447
22. Esselstyn CB Jr, Ellis SG, Medendorp SV, Crowe TD. A strategy to arrest and reverse coronary artery disease: a 5-year longitudinal study of a single physician’s practice. J Fam Pract. 1995;41(6):560-568.
23. Esselstyn CB Jr. Updating a 12-year experience with arrest and reversal therapy for coronary heart disease (an overdue requiem for palliative cardiology). Am J Cardiol. 1999;84(3):339-A8. doi:10.1016/s0002-9149(99)00290-8
24. Barnard ND, Cohen J, Jenkins DJ, et al. A low-fat vegan diet improves glycemic control and cardiovascular risk factors in a randomized clinical trial in individuals with type 2 diabetes. Diabetes Care. 2006;29(8):1777-1783. doi:10.2337/dc06-0606
25. McDougall J, Thomas LE, McDougall C, et al. Effects of 7 days on an ad libitum low-fat vegan diet: the McDougall Program cohort [published correction appears in Nutr J. 2017 Feb 10;16(1):12]. Nutr J. 2014;13:99. Published 2014 Oct 14. doi:10.1186/1475-2891-13-99
26. Turner-McGrievy GM, Davidson CR, Wingard EE, Wilcox S, Frongillo EA. Comparative effectiveness of plant-based diets for weight loss: a randomized controlled trial of five different diets. Nutrition. 2015;31(2):350-358. doi:10.1016/j.nut.2014.09.002
27. Wright N, Wilson L, Smith M, Duncan B, McHugh P. The BROAD study: a randomised controlled trial using a whole food plant-based diet in the community for obesity, ischaemic heart disease or diabetes. Nutr Diabetes. 2017;7(3):e256. Published 2017 Mar 20. doi:10.1038/nutd.2017.3

28. Schaefer S, Hussein H, Gershony GR, Rutledge JC, Kappagoda CT. Regression of severe atherosclerotic plaque in patients with mild elevation of LDL cholesterol. J Investig Med. 1997;45(9):536-541.
29. Kitazono R. Know thy patient: Enhancing lifestyle interventions with psychological assessment. Int J Dis Rev Prev. 2020;2(1):76-81.
30. Miller WR, Rollnick S. Motivational Interviewing: Helping People Change. 3rd ed. New York, NY: Guilford Press; 2013.
31. Mascola AJ, Yiaslas TA, Meir RL, et al. Framing physical activity as a distinct and uniquely valuable behavior independent of weight management: A pilot randomized controlled trial for overweight and obese sedentary persons. Eat Weight Disord. 2009;14(2-3):e148-e152. doi:10.1007/BF03327814
32. Esselstyn AC, Esselstyn J. The Prevent and Reverse Heart Disease Cookbook: Over 125 Delicious, Life-Changing, Plant-Based Recipes. New York, NY: Avery; 2014.
33. Esselstyn CB Jr, Gendy G, Doyle J, Golubic M, Roizen MF. A way to reverse CAD? J Fam Pract. 2014;63(7):356-364.
34. Ornish D, Ornish A. Undo It! How Simple Lifestyle Changes Can Reverse Most Chronic Diseases. New York, NY: Ballantine Books; 2019.
35. Smith SC Jr, Benjamin EJ, Bonow RO, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation endorsed by the World Heart Federation and the Preventive Cardiovascular Nurses Association [published correction appears in J Am Coll Cardiol. 2015 Apr 14;65(14):1495. Dosage error in article text.]. J Am Coll Cardiol. 2011;58(23):2432-2446. doi:10.1016/j.jacc.2011.10.824
36. Blumenthal JA, Babyak M, Wei J, et al. Usefulness of psychosocial treatment of mental stress-induced myocardial ischemia in men. Am J Cardiol. 2002;89(2):164-168. doi:10.1016/s0002-9149(01)02194-4
37. Antoni MH. Stress management effects on psychological, endocrinological, and immune functioning in men with HIV infection: empirical support for a psychoneuroimmunological model. Stress. 2003;6(3):173-188. doi:10.1080/1025389031000156727
38. Penedo FJ, Molton I, Dahn JR, et al. A randomized clinical trial of group-based cognitive-behavioral stress management in localized prostate cancer: development of stress management skills improves quality of life and benefit finding. Ann Behav Med. 2006;31(3):261-270. doi:10.1207/s15324796abm3103_8
39. Yiaslas TA. “Look doctor, I’m a carnivore.” Int J Dis Rev Prev. 2020;2(2):35-39.
40. Khaylis A, Yiaslas T, Bergstrom J, Gore-Felton C. A review of efficacious technology-based weight-loss interventions: five key components. Telemed J E Health. 2010;16(9):931-938. doi:10.1089/tmj.2010.0065
41. Barnard ND, Sherwitz L, Ornish D. Adherence and acceptability of a low-fat, vegetarian diet among patients with cardiac disease. J Cardiopulm Rehabil. 1992;12(6):423-431.
42. Yiaslas TA, Taylor J, Embree J, Schaefer S. Elimination of angina, comprehensive cardio-metabolic risk reduction, and 50-pound weight loss in a US Navy veteran with myasthenia gravis. Int J Dis Rev Prev. 2019;1(1):77-83.
43. Berkow SE, Barnard N, Eckart J, Katcher H. Four therapeutic diets: adherence and acceptability. Can J Diet Pract Res. 2010;71(4):199-204. doi:10.3148/71.4.2010.199
44. Carpenter JK, Andrews LA, Witcraft SM, Powers MB, Smits JAJ, Hofmann SG. Cognitive behavioral therapy for anxiety and related disorders: A meta-analysis of randomized placebo-controlled trials. Depress Anxiety. 2018;35(6):502-514. doi:10.1002/da.22728
45. Yiaslas TA. The pursuit of arete in medicine and health care. Int J Dis Rev Prev. 2019;1(2):53-56.
1. Benjamin EJ, Virani SS, Callaway CW, et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association [published correction appears in Circulation. 2018 Mar 20;137(12 ): e493]. Circulation. 2018;137(12):e67-e492. doi:10.1161/CIR.0000000000000558
2. Hinojosa R. Cardiovascular disease among United States military veterans: evidence of a waning healthy soldier effect using the National Health Interview Survey. Chronic Illn. 2020;16(1):55-68. doi:10.1177/1742395318785237.
3. Hinojosa R. Sex, age, race/ethnicity, veteran status, and the likelihood of reporting cardiovascular conditions in the National Health Interview Survey. J Cardiovasc Nurs. 2019;34(3):215-221. doi:10.1097/JCN.0000000000000561 4. Assari S. Veterans and risk of heart disease in the United States: a cohort with 20 years of follow up. Int J Prev Med. 2014;5(6):703-709.
5. Thomas MM, Harpaz-Rotem I, Tsai J, Southwick SM, Pietrzak RH. Mental and physical health conditions in US combat veterans: results from the National Health and Resilience in Veterans Study. Prim Care Companion CNS Disord. 2017;19(3):10.4088/PCC.17m02118. Published 2017 Jun 22. doi:10.4088/PCC.17m02118
6. Bukhbinder AS, Wang AC, Qureshi SU, et al. Increased vascular pathology in older veterans with a purple heart commendation or chronic post-traumatic stress disorder. J Geriatr Psychiatry Neurol. 2020;33(4):195-206. doi:10.1177/0891988719868308
7. Edmondson D, von Känel R. Post-traumatic stress disorder and cardiovascular disease. Lancet Psychiatry. 2017;4(4):320-329. doi:10.1016/S2215-0366(16)30377-7
8. Forman DE, Maurer MS, Boyd C, et a;. Multimorbidity in older adults with cardiovascular disease. J Am Coll Cardiol. 2018;71(19):2149-2161. doi:10.1016/j.jacc.2018.03.022
9. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252
10. Maddox TM, Plomondon ME, Petrich M, et al. A national clinical quality program for Veterans Affairs catheterization laboratories (from the Veterans Affairs clinical assessment, reporting, and tracking program). Am J Cardiol. 2014;114(11):1750-1757. doi:10.1016/j.amjcard.2014.08.045
11. Centers for Disease Control and Prevention. Coronavirus 2019 (COVID-19):people at increased risk and other people who need to take extra precautions. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/index.html. Updated September 11, 2020. Accessed November 12, 2020.
12. International Food Information Council. 2020 food and health survey. https://foodinsight.org/2020-food-and-health-survey. Updated June 9, 2020. Accessed November 12, 2020.
13. American Association of Cardiovascular and Pulmonary Rehabilitation. Guidelines for Cardiac Rehabilitation and Secondary Prevention Programs. 5th ed. Champaign, IL: Human Kinetics; 2013.
14. Silberman A, Banthia R, Estay IS, et al. The effectiveness and efficacy of an intensive cardiac rehabilitation program in 24 sites. Am J Health Promot. 2010;24(4):260-266. doi:10.4278/ajhp.24.4.arb
15. Ornish D, Scherwitz LW, Doody RS, et al. Effects of stress management training and dietary changes in treating ischemic heart disease. JAMA. 1983;249(1):54-59.
16. Ornish D, Brown SE, Scherwitz LW, et al. Can lifestyle changes reverse coronary heart disease? The Lifestyle Heart Trial. Lancet. 1990;336(8708):129-133. doi:10.1016/0140-6736(90)91656-u.
17. Ornish D, Scherwitz LW, Billings JH, et al. Intensive lifestyle changes for reversal of coronary heart disease [published correction appears in JAMA 1999 Apr 21;281(15):1380]. JAMA. 1998;280(23):2001-2007. doi:10.1001/jama.280.23.2001
18. Frattaroli J, Weidner G, Merritt-Worden TA, Frenda S, Ornish D. Angina pectoris and atherosclerotic risk factors in the multisite cardiac lifestyle intervention program. Am J Cardiol. 2008;101(7):911-918. doi:10.1016/j.amjcard.2007.11.039
19. Koertge J, Weidner G, Elliott-Eller M, et al. Improvement in medical risk factors and quality of life in women and men with coronary artery disease in the Multicenter Lifestyle Demonstration Project. Am J Cardiol. 2003;91(11):1316-1322. doi:10.1016/s0002-9149(03)00320-5
20. Marshall DA, Walizer EM, Vernalis MN. Achievement of heart health characteristics through participation in an intensive lifestyle change program (Coronary Artery Disease Reversal Study). J Cardiopulm Rehabil Prev. 2009;29(2):84-96. doi:10.1097/HCR.0b013e31819a00b2
21. Marshall D, Elaine W, Vernalis M. The effect of a one-year lifestyle intervention program on carotid intima media thickness. Mil Med. 2011;176(7):798-804. doi:10.7205/milmed-d-10-00447
22. Esselstyn CB Jr, Ellis SG, Medendorp SV, Crowe TD. A strategy to arrest and reverse coronary artery disease: a 5-year longitudinal study of a single physician’s practice. J Fam Pract. 1995;41(6):560-568.
23. Esselstyn CB Jr. Updating a 12-year experience with arrest and reversal therapy for coronary heart disease (an overdue requiem for palliative cardiology). Am J Cardiol. 1999;84(3):339-A8. doi:10.1016/s0002-9149(99)00290-8
24. Barnard ND, Cohen J, Jenkins DJ, et al. A low-fat vegan diet improves glycemic control and cardiovascular risk factors in a randomized clinical trial in individuals with type 2 diabetes. Diabetes Care. 2006;29(8):1777-1783. doi:10.2337/dc06-0606
25. McDougall J, Thomas LE, McDougall C, et al. Effects of 7 days on an ad libitum low-fat vegan diet: the McDougall Program cohort [published correction appears in Nutr J. 2017 Feb 10;16(1):12]. Nutr J. 2014;13:99. Published 2014 Oct 14. doi:10.1186/1475-2891-13-99
26. Turner-McGrievy GM, Davidson CR, Wingard EE, Wilcox S, Frongillo EA. Comparative effectiveness of plant-based diets for weight loss: a randomized controlled trial of five different diets. Nutrition. 2015;31(2):350-358. doi:10.1016/j.nut.2014.09.002
27. Wright N, Wilson L, Smith M, Duncan B, McHugh P. The BROAD study: a randomised controlled trial using a whole food plant-based diet in the community for obesity, ischaemic heart disease or diabetes. Nutr Diabetes. 2017;7(3):e256. Published 2017 Mar 20. doi:10.1038/nutd.2017.3

28. Schaefer S, Hussein H, Gershony GR, Rutledge JC, Kappagoda CT. Regression of severe atherosclerotic plaque in patients with mild elevation of LDL cholesterol. J Investig Med. 1997;45(9):536-541.
29. Kitazono R. Know thy patient: Enhancing lifestyle interventions with psychological assessment. Int J Dis Rev Prev. 2020;2(1):76-81.
30. Miller WR, Rollnick S. Motivational Interviewing: Helping People Change. 3rd ed. New York, NY: Guilford Press; 2013.
31. Mascola AJ, Yiaslas TA, Meir RL, et al. Framing physical activity as a distinct and uniquely valuable behavior independent of weight management: A pilot randomized controlled trial for overweight and obese sedentary persons. Eat Weight Disord. 2009;14(2-3):e148-e152. doi:10.1007/BF03327814
32. Esselstyn AC, Esselstyn J. The Prevent and Reverse Heart Disease Cookbook: Over 125 Delicious, Life-Changing, Plant-Based Recipes. New York, NY: Avery; 2014.
33. Esselstyn CB Jr, Gendy G, Doyle J, Golubic M, Roizen MF. A way to reverse CAD? J Fam Pract. 2014;63(7):356-364.
34. Ornish D, Ornish A. Undo It! How Simple Lifestyle Changes Can Reverse Most Chronic Diseases. New York, NY: Ballantine Books; 2019.
35. Smith SC Jr, Benjamin EJ, Bonow RO, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation endorsed by the World Heart Federation and the Preventive Cardiovascular Nurses Association [published correction appears in J Am Coll Cardiol. 2015 Apr 14;65(14):1495. Dosage error in article text.]. J Am Coll Cardiol. 2011;58(23):2432-2446. doi:10.1016/j.jacc.2011.10.824
36. Blumenthal JA, Babyak M, Wei J, et al. Usefulness of psychosocial treatment of mental stress-induced myocardial ischemia in men. Am J Cardiol. 2002;89(2):164-168. doi:10.1016/s0002-9149(01)02194-4
37. Antoni MH. Stress management effects on psychological, endocrinological, and immune functioning in men with HIV infection: empirical support for a psychoneuroimmunological model. Stress. 2003;6(3):173-188. doi:10.1080/1025389031000156727
38. Penedo FJ, Molton I, Dahn JR, et al. A randomized clinical trial of group-based cognitive-behavioral stress management in localized prostate cancer: development of stress management skills improves quality of life and benefit finding. Ann Behav Med. 2006;31(3):261-270. doi:10.1207/s15324796abm3103_8
39. Yiaslas TA. “Look doctor, I’m a carnivore.” Int J Dis Rev Prev. 2020;2(2):35-39.
40. Khaylis A, Yiaslas T, Bergstrom J, Gore-Felton C. A review of efficacious technology-based weight-loss interventions: five key components. Telemed J E Health. 2010;16(9):931-938. doi:10.1089/tmj.2010.0065
41. Barnard ND, Sherwitz L, Ornish D. Adherence and acceptability of a low-fat, vegetarian diet among patients with cardiac disease. J Cardiopulm Rehabil. 1992;12(6):423-431.
42. Yiaslas TA, Taylor J, Embree J, Schaefer S. Elimination of angina, comprehensive cardio-metabolic risk reduction, and 50-pound weight loss in a US Navy veteran with myasthenia gravis. Int J Dis Rev Prev. 2019;1(1):77-83.
43. Berkow SE, Barnard N, Eckart J, Katcher H. Four therapeutic diets: adherence and acceptability. Can J Diet Pract Res. 2010;71(4):199-204. doi:10.3148/71.4.2010.199
44. Carpenter JK, Andrews LA, Witcraft SM, Powers MB, Smits JAJ, Hofmann SG. Cognitive behavioral therapy for anxiety and related disorders: A meta-analysis of randomized placebo-controlled trials. Depress Anxiety. 2018;35(6):502-514. doi:10.1002/da.22728
45. Yiaslas TA. The pursuit of arete in medicine and health care. Int J Dis Rev Prev. 2019;1(2):53-56.
2020 Update on bone health
Increasingly, bone health and fragility fracture prevention is one of the most important aspects of healthy aging that we, as women’s health care providers (HCPs), must be sure is part of our thought process in caring for women at midlife and beyond. Virtually all ObGyn HCPs are aware of breast health, both in terms of the clinical breast exam and imaging surveillance. The 5-year relative survival rate for “localized breast cancer” is 99%.1 Most recent data on hip fracture, however, indicate that it is associated with a mortality in the first year of 21%!2 We need to be sure that our patients understand this.
Previously, this column provided an update on osteoporosis. In 2016, I asked to change the focus to “Update on bone health” to highlight that simply relying on dual energy x-ray absorptiometry (DXA) testing of bone mass with arbitrary cutoffs for osteoporosis, osteopenia, and normal bone mass is not adequate for improving overall bone health. The addition of the FRAX fracture risk assessment tool, now widely employed, as well as the trabecular bone score (TBS), not widely employed, helps to refine the assessment of patients’ risk status. Further, issues such as sarcopenia, adequate dietary calcium and vitamin D supplementation, and fall prevention (improving balance, use of nonskid rugs in the bathroom, avoiding black ice when present, having nothing to slip on between the bed and the bathroom in the middle of the night, and so on) also are essential elements of “bone health.”
Finally, I cannot stress enough the importance of developing a good relationship with whatever facility one uses for DXA testing in order to maximize use of the reports and potential limitations. In addition, we should identify a metabolic bone specialist for referral of unusual cases or patients who require medications unlikely to be prescribed by us as ObGyns, and develop some familiarity with therapies that may be utilized.
Osteosarcopenia greatly enhances fall and fracture risk
Sepúlveda-Loyola W, Phu S, Bani Hassan E, et al. The joint occurrence of osteoporosis and sarcopenia (osteosarcopenia): definitions and characteristics. J Am Med Dir Assoc. 2020;21:220-225.
Tokeshi S, Eguchi Y, Suzuki M, et al. Relationship between skeletal muscle mass, bone mineral density, and trabecular bone score in osteoporotic vertebral compression fractures. Asian Spine J. 2020 Sep 3. doi: 10.31616/asj.2020.0045.
Kirk B, Zanker J, Duque G. Osteosarcopenia: epidemiology, diagnosis, and treatment—facts and numbers. J Cachexia Sarcopenia Muscle. 2020;11:609-618.
The topic of sarcopenia as defined by the concurrent presence of low muscle mass, physical performance, and strength has been discussed previously in this Update series.3 Now, osteosarcopenia, defined as the concomitant presence of osteoporosis or osteopenia combined with sarcopenia, seems to be an extremely important gauge of fracture risk, especially now as the population’s longevity has increased dramatically. This new syndrome is associated with higher disability and rates of fracture and falls in older people compared with either entity (the bone component or the sarcopenia component) alone.4,5 In fact, in the 2016 ICD-10-CM, sarcopenia was finally recognized as a disease entity.
Severe sarcopenia is known to increase the risk for falls.6 Furthermore, evidence is increasing of cross talk between muscle and bone.4 The diagnostic criteria of osteopenia and osteoporosis are well established; however, absolute criteria for sarcopenia lack an international consensus.
Continue to: Assess for osteopenia/osteoporosis plus sarcopenia to determine those at greatest fracture risk...
Assess for osteopenia/osteoporosis plus sarcopenia to determine those at greatest fracture risk
Sepúlveda-Loyola and colleagues performed a cross-sectional analysis of 253 participants, of which 77% were women, average age 78, who presented for a “falls and fractures” risk assessment. T-scores were measured by DXA. In addition, the investigators measured components of sarcopenia, including physical performance (evaluated by hand grip strength, gait speed, timed up and go test, and 5-time sit to stand test) and dynamic and static balance. Falls in the previous year were self-reported, with 42% of participants having fallen once and 54%, more than once.
Results. Participants with osteosarcopenia had a statistically significant increased rate of falls of approximately threefold and an increased rate of fractures that was approximately fourfold when compared with osteopenia or osteoporosis alone.
Another important finding was that, despite the links between osteoporosis, fracture, and poor clinical outcomes, the investigators did not find differences in fracture rates in the osteopenic compared with the osteoporotic classifications. Their findings corroborated those of other studies that reported discrepancies in fractures and bone mineral density (BMD), with osteopenic older adults experiencing fracture rates similar to and in some cases greater than those diagnosed with osteoporosis.7
Thus, it appears that the use of T-scores that combine osteopenic and osteoporotic criteria into the osteosarcopenic category may be sufficient to capture individuals at the greatest risk of fracture.
Skeletal muscle mass plays a role in vertebral compression fractures
Tokeshi and colleagues conducted retrospective observational study to investigate the relationships between skeletal muscle mass, BMD, and TBS in individuals with osteoporotic vertebral compression fractures.
They evaluated 142 patients with an average age of 75; of these, 30% had radiographically diagnosed vertebral compression fractures (average age, 79) and 70% had no vertebral compression fractures (average age, 70). Body composition was measured using whole-body DXA; appendicular skeletal muscle mass index was determined as the sum of upper and lower extremities’ lean mass (kg/height in m2 ). TBS was measured using the patented algorithm software on DXA scans for the lumbar vertebrae.
Results. The investigators found that the vertebral compression fracture group was statistically significantly older, had lower femur BMD, and had decreased leg muscle mass. The TBS was not identified as a risk factor.
Certain lifestyle factors add to risk of osteosarcopenia
In an editorial, Kirk and colleagues summarized the epidemiology, diagnosis, and treatment of osteosarcopenia. They concluded that this syndrome can be expected to grow in age-related and disease-related states as a consequence of immunosenescence coinciding with an increase in sedentary lifestyle, obesity, and fat infiltration of muscle and bone.
Increasingly, clinicians should screen for osteosarcopenia via imaging methods (DXA) to quantitate bone mass (as is currently done) and, increasingly, quantify muscle mass. In addition, assessment of muscle strength, easily done by testing grip strength, as well as functional capacity (gait speed), will become increasingly important.
Finally, the authors call for a more comprehensive geriatric assessment that includes medical history and risk factors as well as treatment (including osteoporosis drugs, where indicated), and progressive resistance and balance exercises. Nutritional recommendations, in terms of protein, vitamin D, and calcium, also are necessary. They anticipate that diagnosis and treatment of osteosarcopenia will become part of routine health care in the future.
In the past, our assessment of risk for fragility fracture was based mostly on bone mass measurement by DXA. Scoring systems like the FRAX tool have included other risk factors, such as age, body mass index, previous fracture, family history of hip fracture, smoking, any history of rheumatoid arthritis, use of glucocorticoids, and alcohol consumption. However, sarcopenia is a condition characterized by loss of skeletal muscle mass, strength, and function. While it is a natural part of the aging process, when it is severe and coupled with osteopenia or osteoporosis, it significantly increases the risks of falls as well as fracture. Women’s HCPs should increasingly think about the presence of sarcopenia in their patients, especially those with low bone mass (osteopenia or osteoporosis), particularly when making decisions about initiating pharmaceutical intervention. In addition, recommendations for resistive and balance exercises virtually should be universal.
Continue to: The denosumab discontinuation dilemma...
The denosumab discontinuation dilemma
Lyu H, Yoshida K, Zhao SS, et al. Delayed denosumab injections and fracture risk among patients with osteoporosis: a population-based cohort study. Ann Intern Med. 2020;173:516-526.
Tripto-Shkolnik L, Fund N, Rouach V, et al. Fracture incidence after denosumab discontinuation: real-world data from a large healthcare provider. Bone. 2020;130:115150.
Denosumab, marketed under the brand name Prolia, is a human monoclonal antibody that blocks the binding of RANK ligand and inhibits development and activity of osteoclast, thus decreasing bone resorption and increasing BMD. In the original pivotal clinical trial of denosumab, almost 7,900 women between the ages of 60 and 90 (average age, 73) with osteoporotic T-scores were enrolled.8 The women were randomly assigned to receive 60 mg of denosumab subcutaneously every 6 months or placebo for a total of 3 years. In that trial, the denosumabtreated group, relative to the placebo group, showed a statistically significant decrease in radiographic vertebral fracture, hip fracture, and nonvertebral fracture.
An open-label extension study looked at denosumab use for a total of 10 years.9 That study found that denosumab treatment for up to 10 years was associated with low rates of adverse events, low fracture incidence compared with that observed during the original trial, and continued increases in BMD without plateau. Thus, denosumab appeared to be an extremely safe and effective agent for treating postmenopausal women with osteoporosis.
Denosumab cessation leads to rebound vertebral fractures
As opposed to bisphosphonates, denosumab does not incorporate into bone matrix, and bone turnover is not suppressed after cessation of its use. Reports have implied that denosumab discontinuation may lead to an increased risk of multiple vertebral fractures.10 One theory is that unlike atypical femoral fractures that seem to emerge from failure of microdamage repair in cortical bone with long-term antiresorptive treatment, denosumab rebound–associated vertebral fractures seem to originate from the synergy of rapid bone resorption and accelerated microdamage accumulation in trabecular bone triggered by the discontinuation of this highly potent reversible agent.11
Post hoc analysis of the denosumab placebo-controlled trial and its extension reported that the vertebral fracture rate increased after denosumab discontinuation to the level observed in untreated patients.12 Further, a majority of participants who did sustain vertebral fracture after discontinuing denosumab had multiple vertebral fractures, with the risk being greatest in participants who had a prior vertebral facture. This caused those authors to suggest that patients who discontinued denosumab should rapidly transition to an alternative antiresorptive treatment.
Effect of dose delays, discontinuation on vertebral fracture rate
Lyu and colleagues recently described their population-based cohort study of the United Kingdom’s Health Improvement Network primary care database between 2010 and 2019. They found that delayed administration of a subsequent denosumab dose by more than 16 weeks was associated with an increased risk for vertebral fracture compared with on-time dosing. They noted, however, that the evidence was insufficient to conclude that fracture risk at any other anatomic sites is increased with such a delay.
In a similar study, Tripto-Shkolnik and colleagues examined an Israeli database of 2.3 million members in a state-mandated health organization. They identified osteoporotic patients with at least 2 denosumab prescription dispenses and defined treatment discontinuation as a refill gap of 3 months or more. Fractures were identified by an osteoporosis registry, including fractures that occurred within 1 year from discontinuation in denosumab discontinuers as well as from the second year of treatment forward for persistent users. They identified 1,500 denosumab discontinuers (average age, 72) and 1,610 persistent users (average age also 72). At baseline, the groups were comparable in fracture history, smoking, and bone density.
In the discontinuation group, 0.8% had multiple vertebral fractures versus 0.1% in the persistent users (P = .006); the overall rate of fractures per 100 patient-years of follow-up was 3 times higher in the discontinuation group than in the persistent user group, and the rate of vertebral fractures was almost 5 times higher in the discontinuation group.
Denosumab is an extremely safe and effective treatment for postmenopausal osteoporosis. Discontinuation or even delay in dosing seems to result in a “rebound” effect of increased vertebral fractures and even multiple vertebral fractures, especially in those with history of a previous vertebral fracture. This is extremely important in this era of COVID-19, in which patients—especially elderly patients who are perceived to be at the greatest risk—often delay management of chronic disease to limit their potential exposure to the virus. Further, even in normal, nonpandemic times, clinicians need to make patients receiving denosumab aware of the importance of timely administration of doses as scheduled. If such dosing is not possible, then clinicians and patients need to be aware of the potential need for instituting other antiresorptive therapies. In addition, the need to ostensibly continue denosumab therapy for long periods of time and indefinitely may make it a less desirable choice for younger patients.
Continue to: Atypical femur fracture risk and bisphosphonate use...
Atypical femur fracture risk and bisphosphonate use
Black DM, Geiger EJ, Eastell R, et al. Atypical femur fracture risk versus fragility fracture prevention with bisphosphonates. N Engl J Med. 2020;383:743-753.
Since their introduction in the 1990s, bisphosphonates have been the mainstay of osteoporosis treatment. This category of medications inhibits osteoclast-mediated resorption and remodeling of bone. Various large, randomized, controlled trials have established the efficacy of bisphosphonates to increase BMD and decrease the risk of hip and vertebral fracture by as much as 40% to 70%.13
However, case reports of unusual fragility fractures in the subtrochanteric region and along the femoral diaphysis in patients treated with bisphosphonates started to appear approximately 15 years ago.14 Since then, concerns and publicity about these atypical fractures have led to substantial declines in bisphosphonate use clinically.
Bisphosphonate preventive benefits versus atypical fracture risk
Black and colleagues reviewed data on women 50 years and older who were enrolled in the Kaiser Permanente health care system in California. The total cohort included slightly more than 1 million women, of which almost 200,000 (17.9%) used bisphosphonates at any point from 2007–2017.
A total of 277 atypical femur fractures occurred. Among bisphosphonate users, there were 1.74 fractures per 10,000 patient-years. Overall, there were almost 59 fractures per 10,000 person-years. The incidence of atypical fractures was highest in women between the ages of 75 and 84 years, and the incidence diminished after age 85. Rates of atypical fractures increased as the duration of bisphosphonate use increased. In addition, rates of atypical fractures decreased with time since bisphosphonate discontinuation.
The rate of atypical fractures in women who had never received bisphosphonate therapy was 0.1 per 10,000 person-years. The number of fractures prevented for each fracture type far outweighed bisphosphonate-associated atypical fractures at all time points along the 10 years of study. In White women, for instance, at 3 years there were 541 clinical fractures prevented and 149 hip fractures prevented, while 2 bisphosphonate-associated atypical fractures occurred, all per 10,000 women.
Interestingly, in the Asian population at the same time point, 330 clinical fractures were prevented and 91 hip fractures were prevented, but 8 atypical fractures of the femur occurred, per 10,000 women. The authors further referenced an earlier Kaiser study that showed that 49% of 142 atypical femur fractures occurred in Asian patients who comprised only 10% of the study population.15
The authors concluded that the risk of atypical femur fracture increases with longer duration of bisphosphate use and rapidly decreases after bisphosphate discontinuation. Asian women have a higher risk than White women. With bisphosphonate treatment, the absolute risk of atypical femur fracture is very low compared with the reduction in the risk of hip and other fractures.
Many patients and even clinicians have moved away from the use of bisphosphonates to reduce fragility fracture risk because of fears of atypical femur fractures. With bisphosphonate use, the reduction in hip fracture as well as other fractures far overshadows the small but real complication of atypical femur fracture. The Asian population seems to have 4 to 6 times the risk for these atypical femur fractures. Thus, bisphosphonate therapy, especially now that it is available in generic formulations, should remain an important option for appropriate patients.
Continue to: Romosozumab increases BMD gains and improves T-scores...
Romosozumab increases BMD gains and improves T-scores
Cosman F, Lewiecki EM, Ebeling PR, et al. T-score as an indicator of fracture risk during treatment with romosozumab or alendronate in the ARCH trial. J Bone Miner Res. 2020;35:1333-1342
Romosozumab (Evenity) is a monoclonal antibody that binds and inhibits sclerostin, thus having the dual effect of increasing bone formation and decreasing bone resorption.16 It is administered for 1 year as monthly doses of 210 mg subcutaneously. Previous studies have shown that romosozumab produces large increases in lumbar spine and total hip BMD,17 reduces the risk of new vertebral and clinical fractures compared with placebo,16 and reduces the risk of vertebral, clinical, nonvertebral, and hip fractures compared with alendronate over a median treatment period of 33 months (the ARCH study).18
According to the package insert, romosozumab is indicated “for the treatment of osteoporosis in postmenopausal women at high risk for fracture, defined as a history of osteoporotic fracture, or multiple risk factors for fracture; or patients who have failed or are intolerant to other available osteoporosis therapy.”
Should T-score be a therapeutic target?
Cosman and colleagues performed a post hoc analysis of the ARCH trial specifically to evaluate mean BMD and corresponding mean T-score changes (and the relationships between T-scores) after 1 year of romosozumab or alendronate therapy and subsequent fracture incidence. The study is quite detailed with much numerical data and statistical analysis.
Basically, the ARCH trial randomly assigned patients with osteoporosis to receive either monthly subcutaneous romosozumab 210 mg or weekly oral alendronate 70 mg for 12 months. After the double-blind portion of the trial, all patients received open label weekly oral alendronate 70 mg through the end of study (24 months), although they were still blinded to the initial treatment assignment. In addition, patients received daily calcium and vitamin D supplements.
The data analysis found that 1 year of romosozumab led to larger BMD gains than alendronate therapy. Also, the T-score achieved with either therapy was directly related to subsequent fracture risk. The authors thus proposed that these data support the use of the T-score as a therapeutic target for patients with osteoporosis.
It is important to note that in the original ARCH study, the participants’ average age was 71 years and approximately one-third were older than 75. The average T-score was -2.7 at both the lumbar spine and femoral neck. Approximately 20% of patients had a pre-existing vertebral fracture, and approximately 20% had a previous nonvertebral fracture.
The authors of the current study, furthermore, found that mean BMD gains after 1 year of romosozumab treatment were more than twice those seen with alendronate at the total hip, femoral neck, and lumbar spine. These BMD changes resulted in a larger proportion of patients who achieved T-scores above the osteoporosis level at each of the skeletal sites after 1 year of therapy. Fewer fractures occurred during the second year and the entire open label period among patients who had received romosozumab first compared with those who received alendronate.●
Women’s HCPs need to be aware of romosozumab even if they are not the ones primarily to prescribe it. Perhaps familiarity with the drug will allow some clinicians to begin to implement this treatment into their care for elderly patients with osteoporosis, especially those with pre-existing fractures. It may be useful to monitor patients’ total hip T-score while on treatment if osteoporosis treatment goals have been achieved to minimize future fracture risk.
- American Cancer Society. Cancer Facts & Figures 2020. Atlanta, Ga: American Cancer Society; 2020. https://www .cancer.org/content/dam/cancer-org/research/cancer-facts -and-statistics/annual-cancer-facts-and-figures/2020/cancer -facts-and-figures-2020.pdf. Accessed November 17, 2020.
- DowneyC, Kelly M, Quinlan JF. Changing trends in the mortality rate at 1-year post hip fracture—a systematic review. World J Orthop. 2019;10:166-175.
- Goldstein SR. 2019 Update on bone health. OBG Manag. 2019;31(12):16-21.
- Hassan EB, Duque G. Osteosarcopenia: a new geriatric syndrome. Aust Fam Physician. 2017;46:849-853.
- Drey M, Sieber CC, Bertsch T, et al; FiAT Intervention Group. Osteosarcopenia is more than sarcopenia and osteopenia alone. Aging Clin Exp Res. 2016;28:895-899.
- Landi F, Liperoti R, Russo A, et al. Sarcopenia as a risk factor for falls in elderly individuals: results from the ilSIRENTE study. Clin Nutr. 2012;31:652-658.
- Kopperdahl DL, Aspelund T, Hoffmann PF, et al. Assessment of incident spine and hip fractures in women and men using finite element analysis of CT scans. J Bone Miner Res. 2014;29:570-580.
- Cummings SR, San Martin J, McClung MR, et al; FREEDOM Trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361: 756-765.
- Bone HG, Wagman RB, Brandi ML, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017;5:513-523.
- Tsourdi E, Langdahl B, Cohen-Solal M, et al. Discontinuation of denosumab therapy for osteoporosis: a systematic review and position statement by ECTS. Bone. 2017;105:11-17.
- Popp AW, Zysset PK, Lippuner K. Rebound-associated vertebral fractures after discontinuation of denosumab—from clinic and biomechanics. Osteoporos Int. 2016;27:1917-1921.
- Cummings SR, Ferrari S, Eastell R, et al. Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo-controlled FREEDOM Trial and its extension. J Bone Miner Res. 2018;33:190-198.
- Eastell R, Rosen CJ, Black DM, et al. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2019;104:1595-1622.
- Goh SK, Yang KY, Koh JS, et al. Subtrochanteric insufficiency fractures in patients on alendronate therapy: a caution. J Bone Joint Surg Br. 2007;89:349-353.
- Dell RM, Adams AL, Greene DF, et al. Incidence of atypical nontraumatic diaphyseal fractures of the femur. J Bone Miner Res. 2012;27:2544-2550.
- Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375:1532-1543.
- McClung MR, Grauer A, Boonen S, et al. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med. 2014;370:412-420.
- Saag KG, Petersen J, Brandi ML, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017;377:1417-1427.
Increasingly, bone health and fragility fracture prevention is one of the most important aspects of healthy aging that we, as women’s health care providers (HCPs), must be sure is part of our thought process in caring for women at midlife and beyond. Virtually all ObGyn HCPs are aware of breast health, both in terms of the clinical breast exam and imaging surveillance. The 5-year relative survival rate for “localized breast cancer” is 99%.1 Most recent data on hip fracture, however, indicate that it is associated with a mortality in the first year of 21%!2 We need to be sure that our patients understand this.
Previously, this column provided an update on osteoporosis. In 2016, I asked to change the focus to “Update on bone health” to highlight that simply relying on dual energy x-ray absorptiometry (DXA) testing of bone mass with arbitrary cutoffs for osteoporosis, osteopenia, and normal bone mass is not adequate for improving overall bone health. The addition of the FRAX fracture risk assessment tool, now widely employed, as well as the trabecular bone score (TBS), not widely employed, helps to refine the assessment of patients’ risk status. Further, issues such as sarcopenia, adequate dietary calcium and vitamin D supplementation, and fall prevention (improving balance, use of nonskid rugs in the bathroom, avoiding black ice when present, having nothing to slip on between the bed and the bathroom in the middle of the night, and so on) also are essential elements of “bone health.”
Finally, I cannot stress enough the importance of developing a good relationship with whatever facility one uses for DXA testing in order to maximize use of the reports and potential limitations. In addition, we should identify a metabolic bone specialist for referral of unusual cases or patients who require medications unlikely to be prescribed by us as ObGyns, and develop some familiarity with therapies that may be utilized.
Osteosarcopenia greatly enhances fall and fracture risk
Sepúlveda-Loyola W, Phu S, Bani Hassan E, et al. The joint occurrence of osteoporosis and sarcopenia (osteosarcopenia): definitions and characteristics. J Am Med Dir Assoc. 2020;21:220-225.
Tokeshi S, Eguchi Y, Suzuki M, et al. Relationship between skeletal muscle mass, bone mineral density, and trabecular bone score in osteoporotic vertebral compression fractures. Asian Spine J. 2020 Sep 3. doi: 10.31616/asj.2020.0045.
Kirk B, Zanker J, Duque G. Osteosarcopenia: epidemiology, diagnosis, and treatment—facts and numbers. J Cachexia Sarcopenia Muscle. 2020;11:609-618.
The topic of sarcopenia as defined by the concurrent presence of low muscle mass, physical performance, and strength has been discussed previously in this Update series.3 Now, osteosarcopenia, defined as the concomitant presence of osteoporosis or osteopenia combined with sarcopenia, seems to be an extremely important gauge of fracture risk, especially now as the population’s longevity has increased dramatically. This new syndrome is associated with higher disability and rates of fracture and falls in older people compared with either entity (the bone component or the sarcopenia component) alone.4,5 In fact, in the 2016 ICD-10-CM, sarcopenia was finally recognized as a disease entity.
Severe sarcopenia is known to increase the risk for falls.6 Furthermore, evidence is increasing of cross talk between muscle and bone.4 The diagnostic criteria of osteopenia and osteoporosis are well established; however, absolute criteria for sarcopenia lack an international consensus.
Continue to: Assess for osteopenia/osteoporosis plus sarcopenia to determine those at greatest fracture risk...
Assess for osteopenia/osteoporosis plus sarcopenia to determine those at greatest fracture risk
Sepúlveda-Loyola and colleagues performed a cross-sectional analysis of 253 participants, of which 77% were women, average age 78, who presented for a “falls and fractures” risk assessment. T-scores were measured by DXA. In addition, the investigators measured components of sarcopenia, including physical performance (evaluated by hand grip strength, gait speed, timed up and go test, and 5-time sit to stand test) and dynamic and static balance. Falls in the previous year were self-reported, with 42% of participants having fallen once and 54%, more than once.
Results. Participants with osteosarcopenia had a statistically significant increased rate of falls of approximately threefold and an increased rate of fractures that was approximately fourfold when compared with osteopenia or osteoporosis alone.
Another important finding was that, despite the links between osteoporosis, fracture, and poor clinical outcomes, the investigators did not find differences in fracture rates in the osteopenic compared with the osteoporotic classifications. Their findings corroborated those of other studies that reported discrepancies in fractures and bone mineral density (BMD), with osteopenic older adults experiencing fracture rates similar to and in some cases greater than those diagnosed with osteoporosis.7
Thus, it appears that the use of T-scores that combine osteopenic and osteoporotic criteria into the osteosarcopenic category may be sufficient to capture individuals at the greatest risk of fracture.
Skeletal muscle mass plays a role in vertebral compression fractures
Tokeshi and colleagues conducted retrospective observational study to investigate the relationships between skeletal muscle mass, BMD, and TBS in individuals with osteoporotic vertebral compression fractures.
They evaluated 142 patients with an average age of 75; of these, 30% had radiographically diagnosed vertebral compression fractures (average age, 79) and 70% had no vertebral compression fractures (average age, 70). Body composition was measured using whole-body DXA; appendicular skeletal muscle mass index was determined as the sum of upper and lower extremities’ lean mass (kg/height in m2 ). TBS was measured using the patented algorithm software on DXA scans for the lumbar vertebrae.
Results. The investigators found that the vertebral compression fracture group was statistically significantly older, had lower femur BMD, and had decreased leg muscle mass. The TBS was not identified as a risk factor.
Certain lifestyle factors add to risk of osteosarcopenia
In an editorial, Kirk and colleagues summarized the epidemiology, diagnosis, and treatment of osteosarcopenia. They concluded that this syndrome can be expected to grow in age-related and disease-related states as a consequence of immunosenescence coinciding with an increase in sedentary lifestyle, obesity, and fat infiltration of muscle and bone.
Increasingly, clinicians should screen for osteosarcopenia via imaging methods (DXA) to quantitate bone mass (as is currently done) and, increasingly, quantify muscle mass. In addition, assessment of muscle strength, easily done by testing grip strength, as well as functional capacity (gait speed), will become increasingly important.
Finally, the authors call for a more comprehensive geriatric assessment that includes medical history and risk factors as well as treatment (including osteoporosis drugs, where indicated), and progressive resistance and balance exercises. Nutritional recommendations, in terms of protein, vitamin D, and calcium, also are necessary. They anticipate that diagnosis and treatment of osteosarcopenia will become part of routine health care in the future.
In the past, our assessment of risk for fragility fracture was based mostly on bone mass measurement by DXA. Scoring systems like the FRAX tool have included other risk factors, such as age, body mass index, previous fracture, family history of hip fracture, smoking, any history of rheumatoid arthritis, use of glucocorticoids, and alcohol consumption. However, sarcopenia is a condition characterized by loss of skeletal muscle mass, strength, and function. While it is a natural part of the aging process, when it is severe and coupled with osteopenia or osteoporosis, it significantly increases the risks of falls as well as fracture. Women’s HCPs should increasingly think about the presence of sarcopenia in their patients, especially those with low bone mass (osteopenia or osteoporosis), particularly when making decisions about initiating pharmaceutical intervention. In addition, recommendations for resistive and balance exercises virtually should be universal.
Continue to: The denosumab discontinuation dilemma...
The denosumab discontinuation dilemma
Lyu H, Yoshida K, Zhao SS, et al. Delayed denosumab injections and fracture risk among patients with osteoporosis: a population-based cohort study. Ann Intern Med. 2020;173:516-526.
Tripto-Shkolnik L, Fund N, Rouach V, et al. Fracture incidence after denosumab discontinuation: real-world data from a large healthcare provider. Bone. 2020;130:115150.
Denosumab, marketed under the brand name Prolia, is a human monoclonal antibody that blocks the binding of RANK ligand and inhibits development and activity of osteoclast, thus decreasing bone resorption and increasing BMD. In the original pivotal clinical trial of denosumab, almost 7,900 women between the ages of 60 and 90 (average age, 73) with osteoporotic T-scores were enrolled.8 The women were randomly assigned to receive 60 mg of denosumab subcutaneously every 6 months or placebo for a total of 3 years. In that trial, the denosumabtreated group, relative to the placebo group, showed a statistically significant decrease in radiographic vertebral fracture, hip fracture, and nonvertebral fracture.
An open-label extension study looked at denosumab use for a total of 10 years.9 That study found that denosumab treatment for up to 10 years was associated with low rates of adverse events, low fracture incidence compared with that observed during the original trial, and continued increases in BMD without plateau. Thus, denosumab appeared to be an extremely safe and effective agent for treating postmenopausal women with osteoporosis.
Denosumab cessation leads to rebound vertebral fractures
As opposed to bisphosphonates, denosumab does not incorporate into bone matrix, and bone turnover is not suppressed after cessation of its use. Reports have implied that denosumab discontinuation may lead to an increased risk of multiple vertebral fractures.10 One theory is that unlike atypical femoral fractures that seem to emerge from failure of microdamage repair in cortical bone with long-term antiresorptive treatment, denosumab rebound–associated vertebral fractures seem to originate from the synergy of rapid bone resorption and accelerated microdamage accumulation in trabecular bone triggered by the discontinuation of this highly potent reversible agent.11
Post hoc analysis of the denosumab placebo-controlled trial and its extension reported that the vertebral fracture rate increased after denosumab discontinuation to the level observed in untreated patients.12 Further, a majority of participants who did sustain vertebral fracture after discontinuing denosumab had multiple vertebral fractures, with the risk being greatest in participants who had a prior vertebral facture. This caused those authors to suggest that patients who discontinued denosumab should rapidly transition to an alternative antiresorptive treatment.
Effect of dose delays, discontinuation on vertebral fracture rate
Lyu and colleagues recently described their population-based cohort study of the United Kingdom’s Health Improvement Network primary care database between 2010 and 2019. They found that delayed administration of a subsequent denosumab dose by more than 16 weeks was associated with an increased risk for vertebral fracture compared with on-time dosing. They noted, however, that the evidence was insufficient to conclude that fracture risk at any other anatomic sites is increased with such a delay.
In a similar study, Tripto-Shkolnik and colleagues examined an Israeli database of 2.3 million members in a state-mandated health organization. They identified osteoporotic patients with at least 2 denosumab prescription dispenses and defined treatment discontinuation as a refill gap of 3 months or more. Fractures were identified by an osteoporosis registry, including fractures that occurred within 1 year from discontinuation in denosumab discontinuers as well as from the second year of treatment forward for persistent users. They identified 1,500 denosumab discontinuers (average age, 72) and 1,610 persistent users (average age also 72). At baseline, the groups were comparable in fracture history, smoking, and bone density.
In the discontinuation group, 0.8% had multiple vertebral fractures versus 0.1% in the persistent users (P = .006); the overall rate of fractures per 100 patient-years of follow-up was 3 times higher in the discontinuation group than in the persistent user group, and the rate of vertebral fractures was almost 5 times higher in the discontinuation group.
Denosumab is an extremely safe and effective treatment for postmenopausal osteoporosis. Discontinuation or even delay in dosing seems to result in a “rebound” effect of increased vertebral fractures and even multiple vertebral fractures, especially in those with history of a previous vertebral fracture. This is extremely important in this era of COVID-19, in which patients—especially elderly patients who are perceived to be at the greatest risk—often delay management of chronic disease to limit their potential exposure to the virus. Further, even in normal, nonpandemic times, clinicians need to make patients receiving denosumab aware of the importance of timely administration of doses as scheduled. If such dosing is not possible, then clinicians and patients need to be aware of the potential need for instituting other antiresorptive therapies. In addition, the need to ostensibly continue denosumab therapy for long periods of time and indefinitely may make it a less desirable choice for younger patients.
Continue to: Atypical femur fracture risk and bisphosphonate use...
Atypical femur fracture risk and bisphosphonate use
Black DM, Geiger EJ, Eastell R, et al. Atypical femur fracture risk versus fragility fracture prevention with bisphosphonates. N Engl J Med. 2020;383:743-753.
Since their introduction in the 1990s, bisphosphonates have been the mainstay of osteoporosis treatment. This category of medications inhibits osteoclast-mediated resorption and remodeling of bone. Various large, randomized, controlled trials have established the efficacy of bisphosphonates to increase BMD and decrease the risk of hip and vertebral fracture by as much as 40% to 70%.13
However, case reports of unusual fragility fractures in the subtrochanteric region and along the femoral diaphysis in patients treated with bisphosphonates started to appear approximately 15 years ago.14 Since then, concerns and publicity about these atypical fractures have led to substantial declines in bisphosphonate use clinically.
Bisphosphonate preventive benefits versus atypical fracture risk
Black and colleagues reviewed data on women 50 years and older who were enrolled in the Kaiser Permanente health care system in California. The total cohort included slightly more than 1 million women, of which almost 200,000 (17.9%) used bisphosphonates at any point from 2007–2017.
A total of 277 atypical femur fractures occurred. Among bisphosphonate users, there were 1.74 fractures per 10,000 patient-years. Overall, there were almost 59 fractures per 10,000 person-years. The incidence of atypical fractures was highest in women between the ages of 75 and 84 years, and the incidence diminished after age 85. Rates of atypical fractures increased as the duration of bisphosphonate use increased. In addition, rates of atypical fractures decreased with time since bisphosphonate discontinuation.
The rate of atypical fractures in women who had never received bisphosphonate therapy was 0.1 per 10,000 person-years. The number of fractures prevented for each fracture type far outweighed bisphosphonate-associated atypical fractures at all time points along the 10 years of study. In White women, for instance, at 3 years there were 541 clinical fractures prevented and 149 hip fractures prevented, while 2 bisphosphonate-associated atypical fractures occurred, all per 10,000 women.
Interestingly, in the Asian population at the same time point, 330 clinical fractures were prevented and 91 hip fractures were prevented, but 8 atypical fractures of the femur occurred, per 10,000 women. The authors further referenced an earlier Kaiser study that showed that 49% of 142 atypical femur fractures occurred in Asian patients who comprised only 10% of the study population.15
The authors concluded that the risk of atypical femur fracture increases with longer duration of bisphosphate use and rapidly decreases after bisphosphate discontinuation. Asian women have a higher risk than White women. With bisphosphonate treatment, the absolute risk of atypical femur fracture is very low compared with the reduction in the risk of hip and other fractures.
Many patients and even clinicians have moved away from the use of bisphosphonates to reduce fragility fracture risk because of fears of atypical femur fractures. With bisphosphonate use, the reduction in hip fracture as well as other fractures far overshadows the small but real complication of atypical femur fracture. The Asian population seems to have 4 to 6 times the risk for these atypical femur fractures. Thus, bisphosphonate therapy, especially now that it is available in generic formulations, should remain an important option for appropriate patients.
Continue to: Romosozumab increases BMD gains and improves T-scores...
Romosozumab increases BMD gains and improves T-scores
Cosman F, Lewiecki EM, Ebeling PR, et al. T-score as an indicator of fracture risk during treatment with romosozumab or alendronate in the ARCH trial. J Bone Miner Res. 2020;35:1333-1342
Romosozumab (Evenity) is a monoclonal antibody that binds and inhibits sclerostin, thus having the dual effect of increasing bone formation and decreasing bone resorption.16 It is administered for 1 year as monthly doses of 210 mg subcutaneously. Previous studies have shown that romosozumab produces large increases in lumbar spine and total hip BMD,17 reduces the risk of new vertebral and clinical fractures compared with placebo,16 and reduces the risk of vertebral, clinical, nonvertebral, and hip fractures compared with alendronate over a median treatment period of 33 months (the ARCH study).18
According to the package insert, romosozumab is indicated “for the treatment of osteoporosis in postmenopausal women at high risk for fracture, defined as a history of osteoporotic fracture, or multiple risk factors for fracture; or patients who have failed or are intolerant to other available osteoporosis therapy.”
Should T-score be a therapeutic target?
Cosman and colleagues performed a post hoc analysis of the ARCH trial specifically to evaluate mean BMD and corresponding mean T-score changes (and the relationships between T-scores) after 1 year of romosozumab or alendronate therapy and subsequent fracture incidence. The study is quite detailed with much numerical data and statistical analysis.
Basically, the ARCH trial randomly assigned patients with osteoporosis to receive either monthly subcutaneous romosozumab 210 mg or weekly oral alendronate 70 mg for 12 months. After the double-blind portion of the trial, all patients received open label weekly oral alendronate 70 mg through the end of study (24 months), although they were still blinded to the initial treatment assignment. In addition, patients received daily calcium and vitamin D supplements.
The data analysis found that 1 year of romosozumab led to larger BMD gains than alendronate therapy. Also, the T-score achieved with either therapy was directly related to subsequent fracture risk. The authors thus proposed that these data support the use of the T-score as a therapeutic target for patients with osteoporosis.
It is important to note that in the original ARCH study, the participants’ average age was 71 years and approximately one-third were older than 75. The average T-score was -2.7 at both the lumbar spine and femoral neck. Approximately 20% of patients had a pre-existing vertebral fracture, and approximately 20% had a previous nonvertebral fracture.
The authors of the current study, furthermore, found that mean BMD gains after 1 year of romosozumab treatment were more than twice those seen with alendronate at the total hip, femoral neck, and lumbar spine. These BMD changes resulted in a larger proportion of patients who achieved T-scores above the osteoporosis level at each of the skeletal sites after 1 year of therapy. Fewer fractures occurred during the second year and the entire open label period among patients who had received romosozumab first compared with those who received alendronate.●
Women’s HCPs need to be aware of romosozumab even if they are not the ones primarily to prescribe it. Perhaps familiarity with the drug will allow some clinicians to begin to implement this treatment into their care for elderly patients with osteoporosis, especially those with pre-existing fractures. It may be useful to monitor patients’ total hip T-score while on treatment if osteoporosis treatment goals have been achieved to minimize future fracture risk.
Increasingly, bone health and fragility fracture prevention is one of the most important aspects of healthy aging that we, as women’s health care providers (HCPs), must be sure is part of our thought process in caring for women at midlife and beyond. Virtually all ObGyn HCPs are aware of breast health, both in terms of the clinical breast exam and imaging surveillance. The 5-year relative survival rate for “localized breast cancer” is 99%.1 Most recent data on hip fracture, however, indicate that it is associated with a mortality in the first year of 21%!2 We need to be sure that our patients understand this.
Previously, this column provided an update on osteoporosis. In 2016, I asked to change the focus to “Update on bone health” to highlight that simply relying on dual energy x-ray absorptiometry (DXA) testing of bone mass with arbitrary cutoffs for osteoporosis, osteopenia, and normal bone mass is not adequate for improving overall bone health. The addition of the FRAX fracture risk assessment tool, now widely employed, as well as the trabecular bone score (TBS), not widely employed, helps to refine the assessment of patients’ risk status. Further, issues such as sarcopenia, adequate dietary calcium and vitamin D supplementation, and fall prevention (improving balance, use of nonskid rugs in the bathroom, avoiding black ice when present, having nothing to slip on between the bed and the bathroom in the middle of the night, and so on) also are essential elements of “bone health.”
Finally, I cannot stress enough the importance of developing a good relationship with whatever facility one uses for DXA testing in order to maximize use of the reports and potential limitations. In addition, we should identify a metabolic bone specialist for referral of unusual cases or patients who require medications unlikely to be prescribed by us as ObGyns, and develop some familiarity with therapies that may be utilized.
Osteosarcopenia greatly enhances fall and fracture risk
Sepúlveda-Loyola W, Phu S, Bani Hassan E, et al. The joint occurrence of osteoporosis and sarcopenia (osteosarcopenia): definitions and characteristics. J Am Med Dir Assoc. 2020;21:220-225.
Tokeshi S, Eguchi Y, Suzuki M, et al. Relationship between skeletal muscle mass, bone mineral density, and trabecular bone score in osteoporotic vertebral compression fractures. Asian Spine J. 2020 Sep 3. doi: 10.31616/asj.2020.0045.
Kirk B, Zanker J, Duque G. Osteosarcopenia: epidemiology, diagnosis, and treatment—facts and numbers. J Cachexia Sarcopenia Muscle. 2020;11:609-618.
The topic of sarcopenia as defined by the concurrent presence of low muscle mass, physical performance, and strength has been discussed previously in this Update series.3 Now, osteosarcopenia, defined as the concomitant presence of osteoporosis or osteopenia combined with sarcopenia, seems to be an extremely important gauge of fracture risk, especially now as the population’s longevity has increased dramatically. This new syndrome is associated with higher disability and rates of fracture and falls in older people compared with either entity (the bone component or the sarcopenia component) alone.4,5 In fact, in the 2016 ICD-10-CM, sarcopenia was finally recognized as a disease entity.
Severe sarcopenia is known to increase the risk for falls.6 Furthermore, evidence is increasing of cross talk between muscle and bone.4 The diagnostic criteria of osteopenia and osteoporosis are well established; however, absolute criteria for sarcopenia lack an international consensus.
Continue to: Assess for osteopenia/osteoporosis plus sarcopenia to determine those at greatest fracture risk...
Assess for osteopenia/osteoporosis plus sarcopenia to determine those at greatest fracture risk
Sepúlveda-Loyola and colleagues performed a cross-sectional analysis of 253 participants, of which 77% were women, average age 78, who presented for a “falls and fractures” risk assessment. T-scores were measured by DXA. In addition, the investigators measured components of sarcopenia, including physical performance (evaluated by hand grip strength, gait speed, timed up and go test, and 5-time sit to stand test) and dynamic and static balance. Falls in the previous year were self-reported, with 42% of participants having fallen once and 54%, more than once.
Results. Participants with osteosarcopenia had a statistically significant increased rate of falls of approximately threefold and an increased rate of fractures that was approximately fourfold when compared with osteopenia or osteoporosis alone.
Another important finding was that, despite the links between osteoporosis, fracture, and poor clinical outcomes, the investigators did not find differences in fracture rates in the osteopenic compared with the osteoporotic classifications. Their findings corroborated those of other studies that reported discrepancies in fractures and bone mineral density (BMD), with osteopenic older adults experiencing fracture rates similar to and in some cases greater than those diagnosed with osteoporosis.7
Thus, it appears that the use of T-scores that combine osteopenic and osteoporotic criteria into the osteosarcopenic category may be sufficient to capture individuals at the greatest risk of fracture.
Skeletal muscle mass plays a role in vertebral compression fractures
Tokeshi and colleagues conducted retrospective observational study to investigate the relationships between skeletal muscle mass, BMD, and TBS in individuals with osteoporotic vertebral compression fractures.
They evaluated 142 patients with an average age of 75; of these, 30% had radiographically diagnosed vertebral compression fractures (average age, 79) and 70% had no vertebral compression fractures (average age, 70). Body composition was measured using whole-body DXA; appendicular skeletal muscle mass index was determined as the sum of upper and lower extremities’ lean mass (kg/height in m2 ). TBS was measured using the patented algorithm software on DXA scans for the lumbar vertebrae.
Results. The investigators found that the vertebral compression fracture group was statistically significantly older, had lower femur BMD, and had decreased leg muscle mass. The TBS was not identified as a risk factor.
Certain lifestyle factors add to risk of osteosarcopenia
In an editorial, Kirk and colleagues summarized the epidemiology, diagnosis, and treatment of osteosarcopenia. They concluded that this syndrome can be expected to grow in age-related and disease-related states as a consequence of immunosenescence coinciding with an increase in sedentary lifestyle, obesity, and fat infiltration of muscle and bone.
Increasingly, clinicians should screen for osteosarcopenia via imaging methods (DXA) to quantitate bone mass (as is currently done) and, increasingly, quantify muscle mass. In addition, assessment of muscle strength, easily done by testing grip strength, as well as functional capacity (gait speed), will become increasingly important.
Finally, the authors call for a more comprehensive geriatric assessment that includes medical history and risk factors as well as treatment (including osteoporosis drugs, where indicated), and progressive resistance and balance exercises. Nutritional recommendations, in terms of protein, vitamin D, and calcium, also are necessary. They anticipate that diagnosis and treatment of osteosarcopenia will become part of routine health care in the future.
In the past, our assessment of risk for fragility fracture was based mostly on bone mass measurement by DXA. Scoring systems like the FRAX tool have included other risk factors, such as age, body mass index, previous fracture, family history of hip fracture, smoking, any history of rheumatoid arthritis, use of glucocorticoids, and alcohol consumption. However, sarcopenia is a condition characterized by loss of skeletal muscle mass, strength, and function. While it is a natural part of the aging process, when it is severe and coupled with osteopenia or osteoporosis, it significantly increases the risks of falls as well as fracture. Women’s HCPs should increasingly think about the presence of sarcopenia in their patients, especially those with low bone mass (osteopenia or osteoporosis), particularly when making decisions about initiating pharmaceutical intervention. In addition, recommendations for resistive and balance exercises virtually should be universal.
Continue to: The denosumab discontinuation dilemma...
The denosumab discontinuation dilemma
Lyu H, Yoshida K, Zhao SS, et al. Delayed denosumab injections and fracture risk among patients with osteoporosis: a population-based cohort study. Ann Intern Med. 2020;173:516-526.
Tripto-Shkolnik L, Fund N, Rouach V, et al. Fracture incidence after denosumab discontinuation: real-world data from a large healthcare provider. Bone. 2020;130:115150.
Denosumab, marketed under the brand name Prolia, is a human monoclonal antibody that blocks the binding of RANK ligand and inhibits development and activity of osteoclast, thus decreasing bone resorption and increasing BMD. In the original pivotal clinical trial of denosumab, almost 7,900 women between the ages of 60 and 90 (average age, 73) with osteoporotic T-scores were enrolled.8 The women were randomly assigned to receive 60 mg of denosumab subcutaneously every 6 months or placebo for a total of 3 years. In that trial, the denosumabtreated group, relative to the placebo group, showed a statistically significant decrease in radiographic vertebral fracture, hip fracture, and nonvertebral fracture.
An open-label extension study looked at denosumab use for a total of 10 years.9 That study found that denosumab treatment for up to 10 years was associated with low rates of adverse events, low fracture incidence compared with that observed during the original trial, and continued increases in BMD without plateau. Thus, denosumab appeared to be an extremely safe and effective agent for treating postmenopausal women with osteoporosis.
Denosumab cessation leads to rebound vertebral fractures
As opposed to bisphosphonates, denosumab does not incorporate into bone matrix, and bone turnover is not suppressed after cessation of its use. Reports have implied that denosumab discontinuation may lead to an increased risk of multiple vertebral fractures.10 One theory is that unlike atypical femoral fractures that seem to emerge from failure of microdamage repair in cortical bone with long-term antiresorptive treatment, denosumab rebound–associated vertebral fractures seem to originate from the synergy of rapid bone resorption and accelerated microdamage accumulation in trabecular bone triggered by the discontinuation of this highly potent reversible agent.11
Post hoc analysis of the denosumab placebo-controlled trial and its extension reported that the vertebral fracture rate increased after denosumab discontinuation to the level observed in untreated patients.12 Further, a majority of participants who did sustain vertebral fracture after discontinuing denosumab had multiple vertebral fractures, with the risk being greatest in participants who had a prior vertebral facture. This caused those authors to suggest that patients who discontinued denosumab should rapidly transition to an alternative antiresorptive treatment.
Effect of dose delays, discontinuation on vertebral fracture rate
Lyu and colleagues recently described their population-based cohort study of the United Kingdom’s Health Improvement Network primary care database between 2010 and 2019. They found that delayed administration of a subsequent denosumab dose by more than 16 weeks was associated with an increased risk for vertebral fracture compared with on-time dosing. They noted, however, that the evidence was insufficient to conclude that fracture risk at any other anatomic sites is increased with such a delay.
In a similar study, Tripto-Shkolnik and colleagues examined an Israeli database of 2.3 million members in a state-mandated health organization. They identified osteoporotic patients with at least 2 denosumab prescription dispenses and defined treatment discontinuation as a refill gap of 3 months or more. Fractures were identified by an osteoporosis registry, including fractures that occurred within 1 year from discontinuation in denosumab discontinuers as well as from the second year of treatment forward for persistent users. They identified 1,500 denosumab discontinuers (average age, 72) and 1,610 persistent users (average age also 72). At baseline, the groups were comparable in fracture history, smoking, and bone density.
In the discontinuation group, 0.8% had multiple vertebral fractures versus 0.1% in the persistent users (P = .006); the overall rate of fractures per 100 patient-years of follow-up was 3 times higher in the discontinuation group than in the persistent user group, and the rate of vertebral fractures was almost 5 times higher in the discontinuation group.
Denosumab is an extremely safe and effective treatment for postmenopausal osteoporosis. Discontinuation or even delay in dosing seems to result in a “rebound” effect of increased vertebral fractures and even multiple vertebral fractures, especially in those with history of a previous vertebral fracture. This is extremely important in this era of COVID-19, in which patients—especially elderly patients who are perceived to be at the greatest risk—often delay management of chronic disease to limit their potential exposure to the virus. Further, even in normal, nonpandemic times, clinicians need to make patients receiving denosumab aware of the importance of timely administration of doses as scheduled. If such dosing is not possible, then clinicians and patients need to be aware of the potential need for instituting other antiresorptive therapies. In addition, the need to ostensibly continue denosumab therapy for long periods of time and indefinitely may make it a less desirable choice for younger patients.
Continue to: Atypical femur fracture risk and bisphosphonate use...
Atypical femur fracture risk and bisphosphonate use
Black DM, Geiger EJ, Eastell R, et al. Atypical femur fracture risk versus fragility fracture prevention with bisphosphonates. N Engl J Med. 2020;383:743-753.
Since their introduction in the 1990s, bisphosphonates have been the mainstay of osteoporosis treatment. This category of medications inhibits osteoclast-mediated resorption and remodeling of bone. Various large, randomized, controlled trials have established the efficacy of bisphosphonates to increase BMD and decrease the risk of hip and vertebral fracture by as much as 40% to 70%.13
However, case reports of unusual fragility fractures in the subtrochanteric region and along the femoral diaphysis in patients treated with bisphosphonates started to appear approximately 15 years ago.14 Since then, concerns and publicity about these atypical fractures have led to substantial declines in bisphosphonate use clinically.
Bisphosphonate preventive benefits versus atypical fracture risk
Black and colleagues reviewed data on women 50 years and older who were enrolled in the Kaiser Permanente health care system in California. The total cohort included slightly more than 1 million women, of which almost 200,000 (17.9%) used bisphosphonates at any point from 2007–2017.
A total of 277 atypical femur fractures occurred. Among bisphosphonate users, there were 1.74 fractures per 10,000 patient-years. Overall, there were almost 59 fractures per 10,000 person-years. The incidence of atypical fractures was highest in women between the ages of 75 and 84 years, and the incidence diminished after age 85. Rates of atypical fractures increased as the duration of bisphosphonate use increased. In addition, rates of atypical fractures decreased with time since bisphosphonate discontinuation.
The rate of atypical fractures in women who had never received bisphosphonate therapy was 0.1 per 10,000 person-years. The number of fractures prevented for each fracture type far outweighed bisphosphonate-associated atypical fractures at all time points along the 10 years of study. In White women, for instance, at 3 years there were 541 clinical fractures prevented and 149 hip fractures prevented, while 2 bisphosphonate-associated atypical fractures occurred, all per 10,000 women.
Interestingly, in the Asian population at the same time point, 330 clinical fractures were prevented and 91 hip fractures were prevented, but 8 atypical fractures of the femur occurred, per 10,000 women. The authors further referenced an earlier Kaiser study that showed that 49% of 142 atypical femur fractures occurred in Asian patients who comprised only 10% of the study population.15
The authors concluded that the risk of atypical femur fracture increases with longer duration of bisphosphate use and rapidly decreases after bisphosphate discontinuation. Asian women have a higher risk than White women. With bisphosphonate treatment, the absolute risk of atypical femur fracture is very low compared with the reduction in the risk of hip and other fractures.
Many patients and even clinicians have moved away from the use of bisphosphonates to reduce fragility fracture risk because of fears of atypical femur fractures. With bisphosphonate use, the reduction in hip fracture as well as other fractures far overshadows the small but real complication of atypical femur fracture. The Asian population seems to have 4 to 6 times the risk for these atypical femur fractures. Thus, bisphosphonate therapy, especially now that it is available in generic formulations, should remain an important option for appropriate patients.
Continue to: Romosozumab increases BMD gains and improves T-scores...
Romosozumab increases BMD gains and improves T-scores
Cosman F, Lewiecki EM, Ebeling PR, et al. T-score as an indicator of fracture risk during treatment with romosozumab or alendronate in the ARCH trial. J Bone Miner Res. 2020;35:1333-1342
Romosozumab (Evenity) is a monoclonal antibody that binds and inhibits sclerostin, thus having the dual effect of increasing bone formation and decreasing bone resorption.16 It is administered for 1 year as monthly doses of 210 mg subcutaneously. Previous studies have shown that romosozumab produces large increases in lumbar spine and total hip BMD,17 reduces the risk of new vertebral and clinical fractures compared with placebo,16 and reduces the risk of vertebral, clinical, nonvertebral, and hip fractures compared with alendronate over a median treatment period of 33 months (the ARCH study).18
According to the package insert, romosozumab is indicated “for the treatment of osteoporosis in postmenopausal women at high risk for fracture, defined as a history of osteoporotic fracture, or multiple risk factors for fracture; or patients who have failed or are intolerant to other available osteoporosis therapy.”
Should T-score be a therapeutic target?
Cosman and colleagues performed a post hoc analysis of the ARCH trial specifically to evaluate mean BMD and corresponding mean T-score changes (and the relationships between T-scores) after 1 year of romosozumab or alendronate therapy and subsequent fracture incidence. The study is quite detailed with much numerical data and statistical analysis.
Basically, the ARCH trial randomly assigned patients with osteoporosis to receive either monthly subcutaneous romosozumab 210 mg or weekly oral alendronate 70 mg for 12 months. After the double-blind portion of the trial, all patients received open label weekly oral alendronate 70 mg through the end of study (24 months), although they were still blinded to the initial treatment assignment. In addition, patients received daily calcium and vitamin D supplements.
The data analysis found that 1 year of romosozumab led to larger BMD gains than alendronate therapy. Also, the T-score achieved with either therapy was directly related to subsequent fracture risk. The authors thus proposed that these data support the use of the T-score as a therapeutic target for patients with osteoporosis.
It is important to note that in the original ARCH study, the participants’ average age was 71 years and approximately one-third were older than 75. The average T-score was -2.7 at both the lumbar spine and femoral neck. Approximately 20% of patients had a pre-existing vertebral fracture, and approximately 20% had a previous nonvertebral fracture.
The authors of the current study, furthermore, found that mean BMD gains after 1 year of romosozumab treatment were more than twice those seen with alendronate at the total hip, femoral neck, and lumbar spine. These BMD changes resulted in a larger proportion of patients who achieved T-scores above the osteoporosis level at each of the skeletal sites after 1 year of therapy. Fewer fractures occurred during the second year and the entire open label period among patients who had received romosozumab first compared with those who received alendronate.●
Women’s HCPs need to be aware of romosozumab even if they are not the ones primarily to prescribe it. Perhaps familiarity with the drug will allow some clinicians to begin to implement this treatment into their care for elderly patients with osteoporosis, especially those with pre-existing fractures. It may be useful to monitor patients’ total hip T-score while on treatment if osteoporosis treatment goals have been achieved to minimize future fracture risk.
- American Cancer Society. Cancer Facts & Figures 2020. Atlanta, Ga: American Cancer Society; 2020. https://www .cancer.org/content/dam/cancer-org/research/cancer-facts -and-statistics/annual-cancer-facts-and-figures/2020/cancer -facts-and-figures-2020.pdf. Accessed November 17, 2020.
- DowneyC, Kelly M, Quinlan JF. Changing trends in the mortality rate at 1-year post hip fracture—a systematic review. World J Orthop. 2019;10:166-175.
- Goldstein SR. 2019 Update on bone health. OBG Manag. 2019;31(12):16-21.
- Hassan EB, Duque G. Osteosarcopenia: a new geriatric syndrome. Aust Fam Physician. 2017;46:849-853.
- Drey M, Sieber CC, Bertsch T, et al; FiAT Intervention Group. Osteosarcopenia is more than sarcopenia and osteopenia alone. Aging Clin Exp Res. 2016;28:895-899.
- Landi F, Liperoti R, Russo A, et al. Sarcopenia as a risk factor for falls in elderly individuals: results from the ilSIRENTE study. Clin Nutr. 2012;31:652-658.
- Kopperdahl DL, Aspelund T, Hoffmann PF, et al. Assessment of incident spine and hip fractures in women and men using finite element analysis of CT scans. J Bone Miner Res. 2014;29:570-580.
- Cummings SR, San Martin J, McClung MR, et al; FREEDOM Trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361: 756-765.
- Bone HG, Wagman RB, Brandi ML, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017;5:513-523.
- Tsourdi E, Langdahl B, Cohen-Solal M, et al. Discontinuation of denosumab therapy for osteoporosis: a systematic review and position statement by ECTS. Bone. 2017;105:11-17.
- Popp AW, Zysset PK, Lippuner K. Rebound-associated vertebral fractures after discontinuation of denosumab—from clinic and biomechanics. Osteoporos Int. 2016;27:1917-1921.
- Cummings SR, Ferrari S, Eastell R, et al. Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo-controlled FREEDOM Trial and its extension. J Bone Miner Res. 2018;33:190-198.
- Eastell R, Rosen CJ, Black DM, et al. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2019;104:1595-1622.
- Goh SK, Yang KY, Koh JS, et al. Subtrochanteric insufficiency fractures in patients on alendronate therapy: a caution. J Bone Joint Surg Br. 2007;89:349-353.
- Dell RM, Adams AL, Greene DF, et al. Incidence of atypical nontraumatic diaphyseal fractures of the femur. J Bone Miner Res. 2012;27:2544-2550.
- Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375:1532-1543.
- McClung MR, Grauer A, Boonen S, et al. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med. 2014;370:412-420.
- Saag KG, Petersen J, Brandi ML, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017;377:1417-1427.
- American Cancer Society. Cancer Facts & Figures 2020. Atlanta, Ga: American Cancer Society; 2020. https://www .cancer.org/content/dam/cancer-org/research/cancer-facts -and-statistics/annual-cancer-facts-and-figures/2020/cancer -facts-and-figures-2020.pdf. Accessed November 17, 2020.
- DowneyC, Kelly M, Quinlan JF. Changing trends in the mortality rate at 1-year post hip fracture—a systematic review. World J Orthop. 2019;10:166-175.
- Goldstein SR. 2019 Update on bone health. OBG Manag. 2019;31(12):16-21.
- Hassan EB, Duque G. Osteosarcopenia: a new geriatric syndrome. Aust Fam Physician. 2017;46:849-853.
- Drey M, Sieber CC, Bertsch T, et al; FiAT Intervention Group. Osteosarcopenia is more than sarcopenia and osteopenia alone. Aging Clin Exp Res. 2016;28:895-899.
- Landi F, Liperoti R, Russo A, et al. Sarcopenia as a risk factor for falls in elderly individuals: results from the ilSIRENTE study. Clin Nutr. 2012;31:652-658.
- Kopperdahl DL, Aspelund T, Hoffmann PF, et al. Assessment of incident spine and hip fractures in women and men using finite element analysis of CT scans. J Bone Miner Res. 2014;29:570-580.
- Cummings SR, San Martin J, McClung MR, et al; FREEDOM Trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361: 756-765.
- Bone HG, Wagman RB, Brandi ML, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017;5:513-523.
- Tsourdi E, Langdahl B, Cohen-Solal M, et al. Discontinuation of denosumab therapy for osteoporosis: a systematic review and position statement by ECTS. Bone. 2017;105:11-17.
- Popp AW, Zysset PK, Lippuner K. Rebound-associated vertebral fractures after discontinuation of denosumab—from clinic and biomechanics. Osteoporos Int. 2016;27:1917-1921.
- Cummings SR, Ferrari S, Eastell R, et al. Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo-controlled FREEDOM Trial and its extension. J Bone Miner Res. 2018;33:190-198.
- Eastell R, Rosen CJ, Black DM, et al. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2019;104:1595-1622.
- Goh SK, Yang KY, Koh JS, et al. Subtrochanteric insufficiency fractures in patients on alendronate therapy: a caution. J Bone Joint Surg Br. 2007;89:349-353.
- Dell RM, Adams AL, Greene DF, et al. Incidence of atypical nontraumatic diaphyseal fractures of the femur. J Bone Miner Res. 2012;27:2544-2550.
- Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375:1532-1543.
- McClung MR, Grauer A, Boonen S, et al. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med. 2014;370:412-420.
- Saag KG, Petersen J, Brandi ML, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017;377:1417-1427.
Left Ventricular Compression and Hypotension Due to Acute Colonic Pseudo-Obstruction
Acute colonic pseudo-obstruction is a postsurgical dilatation of the colon that presents with abdominal distension, pain, nausea, vomiting, constipation, or diarrhea and may lead to colonic ischemia and bowel perforation.
A cute colonic pseudo-obstruction, or Ogilvie syndrome, is dilatation of the colon without mechanical obstruction. It is often seen postoperatively after cesarean section , pelvic , spinal, or other orthopedic surgery, such as knee arthroplasty. 1 One study demonstrated an incidence of acute colonic pseudo-obstruction of 1.3% following hip replacement surgery. 2
The most common symptoms are abdominal distension, pain, nausea, vomiting, constipation, or diarrhea. Bowel sounds are present in the majority of cases.3 It is important to recognize the varied presentations of ileus in the postoperative setting. The most serious complications of acute colonic pseudo-obstruction are colonic ischemia and bowel perforation.
Case Presentation
An 84-year-old man underwent a total left hip arthroplasty revision. The evening after his surgery, his blood pressure (BP) decreased from 93/54 to 71/47 mm Hg, and his heart rate was 73 beats per minute. He was awake, in no acute distress, but reported loose stools. Results of cardiac and pulmonary examinations were normal, showing a regular rate and rhythm with no murmurs and clear lungs. There was normal jugular venous pressure and chronic pitting edema of the lower extremities bilaterally.
An abdominal examination revealed positive bowel sounds, a large ventral hernia, which was easily reducible, and a distended abdomen. His BP remained unchanged after IV normal saline 4 L, and urine output was 200 cc over 4 hours, which the care team determined represented adequate resuscitation. An infection workup, including chest X-ray, urinalysis, and blood and urine cultures, was unrevealing. Hemoglobin was stable at 8.5 g/dL (normal range 14-18), and creatinine level 0.66 mg/dL (normal range 0.7-1.2) at baseline. A transthoracic echocardiogram showed impaired diastolic filling suggestive of extrinsic compression of the left ventricle by mediastinal contents (Figure 1). An abdominal X-ray revealed diffuse dilatation of large bowel loops up to 10 cm, causing elevation and rightward shift of the heart (Figure 2A). A computed tomography scan of the abdomen showed total colonic dilatation without obstruction (Figure 2B).
The patient was diagnosed with postoperative ileus and acute colonic pseudo-obstruction. Nasogastric and rectal tubes were placed for decompression, and the patient was placed on nothing by mouth status. By postoperative day 3, his hypotension had resolved and his BP had improved to 111/58 mm Hg. The patient was able to resume a regular diet.
Discussion
We present an unusual case of left ventricular compression leading to hypotension due to acute colonic pseudo-obstruction. Our patient presented with the rare complication of hypotension due to cardiac compression, which we have not previously seen reported in the literature. Analogous instance of cardiac compression may arise from hiatal hernias and diaphragmatic paralysis. 4-6
Management of acute colonic pseudo-obstruction is through nothing by mouth status and abdominal decompression. For more severe cases, neostigmine, colonoscopic decompression, and surgery can be considered.
This surgical complication was diagnosed by internal medicine hospitalist consultants on a surgical comanagement service. In the comanagement model, the surgical specialties of orthopedic surgery, neurosurgery, and podiatry at San Francisco Veterans Affairs Medical Center in California have hospitalists who work with the team as active consultants for the medical care of the patients. Hospitalists develop a unique skill set in which they can anticipate new diagnoses, prevent or identify early complications, and individualize a patient’s postoperative care.7 One study found that a surgical comanagement service was associated with a decrease in the number of patients with at least 1 surgical complication, decrease in length of stay and 30-day readmissions for a medical cause, decreased consultant use, and an average cost savings per patient of about $2,600 to $4,300.8
Conclusions
With the increasing prevalence of hospitalist comanagement services, it is important for surgeons and nonsurgeons alike to recognize acute colonic pseudo-obstruction as a possible surgical complication.
1. Bernardi M, Warrier S, Lynch C, Heriot A. Acute and chronic pseudo-obstruction: a current update. ANZ J Surg. 2015;85(10):709-714. doi:10.1111/ans.13148
2. Norwood MGA, Lykostratis H, Garcea G, Berry DP. Acute colonic pseudo-obstruction following major orthopaedic surgery. Colorectal Dis. 2005;7(5):496-499. doi:10.1111/j.1463-1318.2005.00790.x
3. Vanek VW, Al-Salti M. Acute pseudo-obstruction of the colon (Ogilvie’s syndrome). An analysis of 400 cases. Dis Colon Rectum. 1986;29(3):203-210. doi:10.1007/BF02555027
4. Devabhandari MP, Khan MA, Hooper TL. Cardiac compression following cardiac surgery due to unrecognised hiatus hernia. Eur J Cardiothoracic Surg. 2007;32(5):813-815. doi:10.1016/j.ejcts.2007.08.002
5. Asti E, Bonavina L, Lombardi M, Bandera F, Secchi F, Guazzi M. Reversibility of cardiopulmonary impairment after laparoscopic repair of large hiatal hernia. Int J Surg Case Rep. 2015;14:33-35. doi:10.1016/j.ijscr.2015.07.005
6. Tayyareci Y, Bayazit P, Taştan CP, Aksoy H. Right atrial compression due to idiopathic right diaphragm paralysis detected incidentally by transthoracic echocardiography. Turk Kardiyol Dern Ars. 2008;36(6):412-414.
7. Rohatgi N, Schulman K, Ahuja N. Comanagement by hospitalists: why it makes clinical and fiscal sense. Am J Med. 2020;133(3):257-258. doi:10.1016/j.amjmed.2019.07.053
8. Rohatgi N, Loftus P, Grujic O, Cullen M, Hopkins J, Ahuja N. Surgical comanagement by hospitalists improves patient outcomes: a propensity score analysis. Ann Surg. 2016;264(2):275-282. doi:10.1097/SLA.0000000000001629
Acute colonic pseudo-obstruction is a postsurgical dilatation of the colon that presents with abdominal distension, pain, nausea, vomiting, constipation, or diarrhea and may lead to colonic ischemia and bowel perforation.
Acute colonic pseudo-obstruction is a postsurgical dilatation of the colon that presents with abdominal distension, pain, nausea, vomiting, constipation, or diarrhea and may lead to colonic ischemia and bowel perforation.
A cute colonic pseudo-obstruction, or Ogilvie syndrome, is dilatation of the colon without mechanical obstruction. It is often seen postoperatively after cesarean section , pelvic , spinal, or other orthopedic surgery, such as knee arthroplasty. 1 One study demonstrated an incidence of acute colonic pseudo-obstruction of 1.3% following hip replacement surgery. 2
The most common symptoms are abdominal distension, pain, nausea, vomiting, constipation, or diarrhea. Bowel sounds are present in the majority of cases.3 It is important to recognize the varied presentations of ileus in the postoperative setting. The most serious complications of acute colonic pseudo-obstruction are colonic ischemia and bowel perforation.
Case Presentation
An 84-year-old man underwent a total left hip arthroplasty revision. The evening after his surgery, his blood pressure (BP) decreased from 93/54 to 71/47 mm Hg, and his heart rate was 73 beats per minute. He was awake, in no acute distress, but reported loose stools. Results of cardiac and pulmonary examinations were normal, showing a regular rate and rhythm with no murmurs and clear lungs. There was normal jugular venous pressure and chronic pitting edema of the lower extremities bilaterally.
An abdominal examination revealed positive bowel sounds, a large ventral hernia, which was easily reducible, and a distended abdomen. His BP remained unchanged after IV normal saline 4 L, and urine output was 200 cc over 4 hours, which the care team determined represented adequate resuscitation. An infection workup, including chest X-ray, urinalysis, and blood and urine cultures, was unrevealing. Hemoglobin was stable at 8.5 g/dL (normal range 14-18), and creatinine level 0.66 mg/dL (normal range 0.7-1.2) at baseline. A transthoracic echocardiogram showed impaired diastolic filling suggestive of extrinsic compression of the left ventricle by mediastinal contents (Figure 1). An abdominal X-ray revealed diffuse dilatation of large bowel loops up to 10 cm, causing elevation and rightward shift of the heart (Figure 2A). A computed tomography scan of the abdomen showed total colonic dilatation without obstruction (Figure 2B).
The patient was diagnosed with postoperative ileus and acute colonic pseudo-obstruction. Nasogastric and rectal tubes were placed for decompression, and the patient was placed on nothing by mouth status. By postoperative day 3, his hypotension had resolved and his BP had improved to 111/58 mm Hg. The patient was able to resume a regular diet.
Discussion
We present an unusual case of left ventricular compression leading to hypotension due to acute colonic pseudo-obstruction. Our patient presented with the rare complication of hypotension due to cardiac compression, which we have not previously seen reported in the literature. Analogous instance of cardiac compression may arise from hiatal hernias and diaphragmatic paralysis. 4-6
Management of acute colonic pseudo-obstruction is through nothing by mouth status and abdominal decompression. For more severe cases, neostigmine, colonoscopic decompression, and surgery can be considered.
This surgical complication was diagnosed by internal medicine hospitalist consultants on a surgical comanagement service. In the comanagement model, the surgical specialties of orthopedic surgery, neurosurgery, and podiatry at San Francisco Veterans Affairs Medical Center in California have hospitalists who work with the team as active consultants for the medical care of the patients. Hospitalists develop a unique skill set in which they can anticipate new diagnoses, prevent or identify early complications, and individualize a patient’s postoperative care.7 One study found that a surgical comanagement service was associated with a decrease in the number of patients with at least 1 surgical complication, decrease in length of stay and 30-day readmissions for a medical cause, decreased consultant use, and an average cost savings per patient of about $2,600 to $4,300.8
Conclusions
With the increasing prevalence of hospitalist comanagement services, it is important for surgeons and nonsurgeons alike to recognize acute colonic pseudo-obstruction as a possible surgical complication.
A cute colonic pseudo-obstruction, or Ogilvie syndrome, is dilatation of the colon without mechanical obstruction. It is often seen postoperatively after cesarean section , pelvic , spinal, or other orthopedic surgery, such as knee arthroplasty. 1 One study demonstrated an incidence of acute colonic pseudo-obstruction of 1.3% following hip replacement surgery. 2
The most common symptoms are abdominal distension, pain, nausea, vomiting, constipation, or diarrhea. Bowel sounds are present in the majority of cases.3 It is important to recognize the varied presentations of ileus in the postoperative setting. The most serious complications of acute colonic pseudo-obstruction are colonic ischemia and bowel perforation.
Case Presentation
An 84-year-old man underwent a total left hip arthroplasty revision. The evening after his surgery, his blood pressure (BP) decreased from 93/54 to 71/47 mm Hg, and his heart rate was 73 beats per minute. He was awake, in no acute distress, but reported loose stools. Results of cardiac and pulmonary examinations were normal, showing a regular rate and rhythm with no murmurs and clear lungs. There was normal jugular venous pressure and chronic pitting edema of the lower extremities bilaterally.
An abdominal examination revealed positive bowel sounds, a large ventral hernia, which was easily reducible, and a distended abdomen. His BP remained unchanged after IV normal saline 4 L, and urine output was 200 cc over 4 hours, which the care team determined represented adequate resuscitation. An infection workup, including chest X-ray, urinalysis, and blood and urine cultures, was unrevealing. Hemoglobin was stable at 8.5 g/dL (normal range 14-18), and creatinine level 0.66 mg/dL (normal range 0.7-1.2) at baseline. A transthoracic echocardiogram showed impaired diastolic filling suggestive of extrinsic compression of the left ventricle by mediastinal contents (Figure 1). An abdominal X-ray revealed diffuse dilatation of large bowel loops up to 10 cm, causing elevation and rightward shift of the heart (Figure 2A). A computed tomography scan of the abdomen showed total colonic dilatation without obstruction (Figure 2B).
The patient was diagnosed with postoperative ileus and acute colonic pseudo-obstruction. Nasogastric and rectal tubes were placed for decompression, and the patient was placed on nothing by mouth status. By postoperative day 3, his hypotension had resolved and his BP had improved to 111/58 mm Hg. The patient was able to resume a regular diet.
Discussion
We present an unusual case of left ventricular compression leading to hypotension due to acute colonic pseudo-obstruction. Our patient presented with the rare complication of hypotension due to cardiac compression, which we have not previously seen reported in the literature. Analogous instance of cardiac compression may arise from hiatal hernias and diaphragmatic paralysis. 4-6
Management of acute colonic pseudo-obstruction is through nothing by mouth status and abdominal decompression. For more severe cases, neostigmine, colonoscopic decompression, and surgery can be considered.
This surgical complication was diagnosed by internal medicine hospitalist consultants on a surgical comanagement service. In the comanagement model, the surgical specialties of orthopedic surgery, neurosurgery, and podiatry at San Francisco Veterans Affairs Medical Center in California have hospitalists who work with the team as active consultants for the medical care of the patients. Hospitalists develop a unique skill set in which they can anticipate new diagnoses, prevent or identify early complications, and individualize a patient’s postoperative care.7 One study found that a surgical comanagement service was associated with a decrease in the number of patients with at least 1 surgical complication, decrease in length of stay and 30-day readmissions for a medical cause, decreased consultant use, and an average cost savings per patient of about $2,600 to $4,300.8
Conclusions
With the increasing prevalence of hospitalist comanagement services, it is important for surgeons and nonsurgeons alike to recognize acute colonic pseudo-obstruction as a possible surgical complication.
1. Bernardi M, Warrier S, Lynch C, Heriot A. Acute and chronic pseudo-obstruction: a current update. ANZ J Surg. 2015;85(10):709-714. doi:10.1111/ans.13148
2. Norwood MGA, Lykostratis H, Garcea G, Berry DP. Acute colonic pseudo-obstruction following major orthopaedic surgery. Colorectal Dis. 2005;7(5):496-499. doi:10.1111/j.1463-1318.2005.00790.x
3. Vanek VW, Al-Salti M. Acute pseudo-obstruction of the colon (Ogilvie’s syndrome). An analysis of 400 cases. Dis Colon Rectum. 1986;29(3):203-210. doi:10.1007/BF02555027
4. Devabhandari MP, Khan MA, Hooper TL. Cardiac compression following cardiac surgery due to unrecognised hiatus hernia. Eur J Cardiothoracic Surg. 2007;32(5):813-815. doi:10.1016/j.ejcts.2007.08.002
5. Asti E, Bonavina L, Lombardi M, Bandera F, Secchi F, Guazzi M. Reversibility of cardiopulmonary impairment after laparoscopic repair of large hiatal hernia. Int J Surg Case Rep. 2015;14:33-35. doi:10.1016/j.ijscr.2015.07.005
6. Tayyareci Y, Bayazit P, Taştan CP, Aksoy H. Right atrial compression due to idiopathic right diaphragm paralysis detected incidentally by transthoracic echocardiography. Turk Kardiyol Dern Ars. 2008;36(6):412-414.
7. Rohatgi N, Schulman K, Ahuja N. Comanagement by hospitalists: why it makes clinical and fiscal sense. Am J Med. 2020;133(3):257-258. doi:10.1016/j.amjmed.2019.07.053
8. Rohatgi N, Loftus P, Grujic O, Cullen M, Hopkins J, Ahuja N. Surgical comanagement by hospitalists improves patient outcomes: a propensity score analysis. Ann Surg. 2016;264(2):275-282. doi:10.1097/SLA.0000000000001629
1. Bernardi M, Warrier S, Lynch C, Heriot A. Acute and chronic pseudo-obstruction: a current update. ANZ J Surg. 2015;85(10):709-714. doi:10.1111/ans.13148
2. Norwood MGA, Lykostratis H, Garcea G, Berry DP. Acute colonic pseudo-obstruction following major orthopaedic surgery. Colorectal Dis. 2005;7(5):496-499. doi:10.1111/j.1463-1318.2005.00790.x
3. Vanek VW, Al-Salti M. Acute pseudo-obstruction of the colon (Ogilvie’s syndrome). An analysis of 400 cases. Dis Colon Rectum. 1986;29(3):203-210. doi:10.1007/BF02555027
4. Devabhandari MP, Khan MA, Hooper TL. Cardiac compression following cardiac surgery due to unrecognised hiatus hernia. Eur J Cardiothoracic Surg. 2007;32(5):813-815. doi:10.1016/j.ejcts.2007.08.002
5. Asti E, Bonavina L, Lombardi M, Bandera F, Secchi F, Guazzi M. Reversibility of cardiopulmonary impairment after laparoscopic repair of large hiatal hernia. Int J Surg Case Rep. 2015;14:33-35. doi:10.1016/j.ijscr.2015.07.005
6. Tayyareci Y, Bayazit P, Taştan CP, Aksoy H. Right atrial compression due to idiopathic right diaphragm paralysis detected incidentally by transthoracic echocardiography. Turk Kardiyol Dern Ars. 2008;36(6):412-414.
7. Rohatgi N, Schulman K, Ahuja N. Comanagement by hospitalists: why it makes clinical and fiscal sense. Am J Med. 2020;133(3):257-258. doi:10.1016/j.amjmed.2019.07.053
8. Rohatgi N, Loftus P, Grujic O, Cullen M, Hopkins J, Ahuja N. Surgical comanagement by hospitalists improves patient outcomes: a propensity score analysis. Ann Surg. 2016;264(2):275-282. doi:10.1097/SLA.0000000000001629
Renal Replacement Therapy in a Patient Diagnosed With Pancreatitis Secondary to Severe Leptospirosis
In areas where the zoonotic disease leptospirosis is endemic, reduced morbidity and mortality is strongly linked to quick initiation of renal replacement therapy.
Leptospirosis (LS) is considered the most common and widespread zoonotic disease in the world. Numerous outbreaks have occurred in the past 10 years. Due to its technically difficult diagnosis, LS is severely underrecognized, underdiagnosed, and therefore, underreported.1,2 The Centers for Disease Control and Prevention (CDC) estimate 100 to 150 cases of LS are identified annually in the US, with about 50% of those cases occurring in Puerto Rico (PR).3 Specifically in PR, about 15 to 100 cases of suspected LS were reported annually between 2000 and 2009, with 59 cases and 1 death reported in 2010. The data are thought to be severely underreported due to a lack of widespread diagnostic testing availability in PR and no formal veterinary and environmental surveillance programs to monitor the incidence of animal cases and actual circulating serovars.4
A recent systematic review of 80 studies from 34 countries on morbidity and mortality of LS revealed that the global incidence and mortality is about 1.03 million cases and 58,900 deaths every year. Almost half of the reported deaths were adult males aged 20 to 49 years.5 Although mild cases of LS are not associated with an elevated mortality, icteric LS with renal failure (Weil disease) carries a mortality rate of 10%.6 In patients who develop hemorrhagic pneumonitis, mortality may be as high as 50 to 70%.7 Therefore, it is pivotal that clinicians recognize the disease early, that novel modalities of treatment continue to be developed, and that their impact on patient morbidity and mortality are studied and documented.
Case Presentation
A 43-year-old man with a medical history of schizophrenia presented to the emergency department at the US Department of Veterans Affairs (VA) Caribbean Healthcare System in San Juan, PR, after experiencing 1 week of intermittent fever, myalgia, and general weakness. Emergency medical services had found him disheveled and in a rodent-infested swamp area several days before admission. Initial vital signs were within normal limits.
On physical examination, the patient was afebrile, without acute distress, but he had diffuse jaundice and mild epigastric tenderness without evidence of peritoneal irritation. His complete blood count was remarkable for leukocytosis with left shifting, adequate hemoglobin levels but with 9 × 103 U/L platelets. The complete metabolic panel demonstrated an aspartate aminotransferase level of 564 U/L, alanine transaminase level of 462 U/L, total bilirubin of 12 mg/dL, which 10.2 mg/dL were direct bilirubin, and an alkaline phosphate of 345 U/L. Lipase levels were measured at 626 U/L. Marked coagulopathy also was present. The toxicology panel, including acetaminophen and salicylate acid levels, did not reveal the presence of any of the tested substances, and chest imaging did not demonstrate any infiltrates.
An abdominal ultrasound was negative for acute cholestatic pathologies, such as cholelithiasis, cholecystitis, or choledocholithiasis. Nonetheless, a noncontrast abdominopelvic computed tomography was remarkable for peripancreatic fat stranding, which raised suspicion for a diagnosis of pancreatitis.
Once the patient was transferred to the intensive care unit, he developed several episodes of hematemesis, leading to hemodynamical instability and severe respiratory distress. Due to anticipated respiratory failure and need for airway securement, endotracheal intubation was performed. Multiple packed red blood cells were transfused, and the patient was started in vasopressor support.
Diagnosis
A presumptive diagnosis of LS was made due to a considerable history of rodent exposure. The patient was started on broad-spectrum parenteral antibiotics, vancomycin 750 mg every 24 hours, metronidazole 500 mg every 8 hours, and ceftriaxone 2 g IV daily for adequate coverage against Leptospira spp. Despite 72 hours of antibiotic treatment, the patient’s clinical state deteriorated. He required high dosages of norepinephrine (1.5 mcg/kg/min) and vasopressin (0.03 U/min) to maintain adequate organ perfusion. Despite lung protective settings with low tidal volume and a high positive end-expiratory pressure, there was difficulty maintaining adequate oxygenation. Chest imaging was remarkable for bilateral infiltrates concerning for acute respiratory distress syndrome (ARDS).
The coagulopathy and cholestasis continued to worsen, and the renal failure progressed from nonoliguric to anuric. Because of this progression, the patient was started on continuous renal replacement therapy (CRRT) by hemodialysis. Within 24 hours of initiating CRRT, the patient’s clinical status improved dramatically. Vasopressor support was weaned, the coagulopathy resolved, and the cholestasis was improving. The patient’s respiratory status improved in such a manner that he was extubated by the seventh day after being placed on mechanical ventilation. The urine and blood samples sent for identification of Leptospira spp. through polymerase chain reaction (PCR) returned positive by the ninth day of admission. While on CRRT, the patient’s renal function eventually returned to baseline, and he was discharged 12 days after admission.
Discussion
The spirochetes of the genus Leptospira include both saprophytic and pathogenic species. These pathogenic Leptospira spp. have adapted to a grand variety of zoonotic hosts, the most important being rodents. They serve as vectors for the contraction of the disease by humans. Initial infection in rodents by Leptospira spp. causes a systemic illness followed by a persistent colonization of renal tubules from which they are excreted in the urine and into the environment. Humans, in turn, are an incidental host unable to induce a carrier state for the transmission of the pathogenic organism.1 The time from exposure to onset of symptoms, or incubation phase, averages 7 to 12 days but may range from 3 to 30 days.8
LS has been described as having 2 discernable but often coexisting phases. The first, an acute febrile bacteremic phase, has been noted to last about 9 days in about 85% of patients, although a minority have persistent fever from 2 weeks to > 30 days. A second phase, the immune or inflammatory phase, is characterized by a second fever spike preceded by 1 to 5 afebrile days in which there is presence of IgM antibodies and resolution of leptospiremia but positive urine cultures.9 Weil disease may present as the second phase of the disease or as a single, progressive illness from its first manifestation. It is characterized by a triad of jaundice, renal failure, and hemorrhage or coagulopathy.10 Weil disease is of great concern and importance due to its associated higher mortality than that found with the mildest form of the disease.
There are studies that advocate for RRT as an intricate part of the treatment regimen in LS to remove the inflammatory cytokines produced as a reaction to the spirochete.11 In tropical countries with a higher incidence of the disease, leptospirosis is an important cause of acute kidney injury (AKI), depending on multiple factors, including the AKI definition that is used.12 Renal invasion by Leptospira spp. produces acute tubular necrosis (ATN) and cell edema during the first week and then could progress to acute interstitial nephritis (AIN) in 2 to 3 weeks. It is believed that the mechanism for the Leptospira spp. invasion of the tubules that results in damage is associated with the antigenic components in its outer membrane; the most important outer membrane protein expressed during infection is LipL32. This protein increases the production of proinflammatory proteins, such as inducible nitric oxide synthase, monocyte chemotactic protein-1 (CCL2/MCP-1), T cells, and tumor necrosis factor.13
Although doxycycline has been recommended for the prophylaxis and treatment of mild LS, the preferred agent and the conferred benefits of antibiotic treatment overall for the severe form of the disease has been controversial. Traditionally, penicillin G sodium has been recommended as the first-line antibiotic treatment for moderate-to-severe LS.14 Nonetheless, there has been an increasing pattern of penicillin resistance among Leptospira spp. that has prompted the study and use of alternative agents.
An open-label, randomized comparison of parenteral cefotaxime, penicillin G sodium, and doxycycline for the treatment of suspected severe leptospirosis conducted by Suputtamongkol and colleagues showed no difference in mortality, defervescence, or time to resolution of abnormal laboratory findings.15 Current CDC recommendations include the use of parenteral penicillin 1.5 MU every 6 hours as the drug of choice, with ceftriaxone 1 g administered IV every 24 hours equally as effective.3
In addition to antimicrobial therapy, supportive care has shifted to include hemodialysis in those patients who develop AKI as part of the disease. Andrade and colleagues conducted a study of 33 patients with LS in Brazil that was set to compare the impact of door-to-dialysis time and dosage of hemodialysis on mortality. In patients with a quicker door-to-dialysis time and daily hemodialysis sessions, there was a 50% (16.7% vs 66.7%) absolute mortality reduction when compared with those with delayed initiation and alternate-day hemodialysis sessions.11 A follow-up prospective study compared the use of traditional sustained low-efficiency dialysis (SLED) with the use of extended SLED via hemodiafiltration in patients with LS presenting with ARDS and AKI. Although hemodiafiltration resulted in a relative decrease in serum levels of interleukin (IL)-17, IL-7, and CCL2/MCP-1, there was no significant difference in mortality.16 The most important prognostic factor in severe LS presenting with AKI and relating to RRT is a shorter door-to-dialysis time and increased dose, not the mode of dialysis clearance. Nonetheless, both RRT methods resulted in a progressive decrease in inflammatory mediators that have been associated with ATN and AIN in the context of LS.16 The authors argue that using CRRT instead of SLED via hemodiafiltration could have accentuated the effects of the reduction that inflammatory mediators may have on mortality in patients with severe LS.
Conclusions
LS continues to be of interest due to its current status as the most common zoonotic disease and its widespread prevalence throughout the globe. Novel treatment modalities for LS, specifically for Weil disease, continue to be developed with the goal of reducing the current mortality rate associated with the disease.
In endemic areas, prompt recognition is essential to initiate the recommended therapy. Parenteral antibiotics, such as penicillin G sodium and ceftriaxone, continue to be the mainstay of treatment and constitute the current CDC recommendations. Nonetheless, early initiation of CRRT has been shown to greatly reduce the mortality associated with Weil disease and, when available, should be considered in these patients.
Our patient failed to improve while receiving parenteral antibiotics alone but showed marked improvement after being placed on CRRT. Furthermore, initiation of CRRT resulted in near-complete resolution of his organ dysfunction and eventual discharge from the hospital. This case serves to further support the use of early CRRT as part of the standard of care in severe LS.
1. Ko AI, Goarant C, Picardeau M. Leptospira: the dawn of the molecular genetics era for an emerging zoonotic pathogen. Nat Rev Microbiol. 2009;7(10):736-747. doi:10.1038/nrmicro2208
2. Hartskeerl RA, Collares-Pereira M, Ellis WA. Emergence, control and re-emerging leptospirosis: dynamics of infection in the changing world. Clin Microbiol Infect. 2011;17(4):494-501. doi:10.1111/j.1469-0691.2011.03474.x
3. Centers for Disease Control and Prevention. Leptospirosis fact sheet for clinicians, CS287535B. https://www.cdc.gov/leptospirosis/pdf/fs-leptospirosis-clinicians-eng-508.pdf. Published January 30, 2018. Accessed October 9, 2020.
4. Martinez-Recio C, Rodriguez-Cintron W, Galarza-Vargas S, et al. The brief case: cases from 3 hospitals in Puerto Rico. ACP Hosp. https://acphospitalist.org/archives/2014/09/briefcase.htm. Published September 2014. Accessed October 9, 2020.
5. Costa F, Hagan JE, Calcagno J, et al. Global morbidity and mortality of leptospirosis: a systematic review. PLoS Negl Trop Dis. 2015;9(9):e0003898. doi:10.1371/journal.pntd.0003898
6. Levett PN. Leptospirosis. Clin Microbiol Rev. 2001;14(2):296-326. doi:10.1128/CMR.14.2.296-326.2001
7. Vijayachari P, Sugunan AP, Shriram AN. Leptospirosis: an emerging global public health problem. J Biosci. 2008;33(4):557-569. doi:10.1007/s12038-008-0074-z
8. Haake DA, Levett PN. Leptospirosis in humans. In: Adler B, ed. Leptospira and Leptospirosis. Berlin, Heidelberg: Springer-Verlag Berlin Heidelberg; 2015:65-97. doi:10.1007/978-3-662-45059-8_5
9. Berman SJ. Sporadic anicteric leptospirosis in South Vietnam: a study in 150 patients. Ann Intern Med. 1973;79(2):167. doi:10.7326/0003-4819-79-2-167
10. Bharti AR, Nally JE, Ricaldi JN, et al. Leptospirosis: a zoonotic disease of global importance. Lancet Infect Dis. 2003;3(12):757-771. doi:10.1016/S1473-3099(03)00830-2
11. Andrade L, Cleto S, Seguro AC. Door-to-dialysis time and daily hemodialysis in patients with leptospirosis: impact on mortality. Clin J Am Soc Nephrol. 2007;2(4):739–744. doi: 10.2215/CJN.00680207
12. Mathew A, George J. Acute kidney injury in the tropics. Ann Saudi Med. 2011;31(5):451-456. doi:10.4103/0256-4947.84620
13. Daher EF, Silva GB Jr, Karbage NNN, et al. Predictors of oliguric acute kidney injury in leptospirosis. Nephron Clin Pract. 2009;112(1):c25-c30. doi:10.1159/000210571
14. Panaphut T, Domrongkitchaiporn S, Vibhagool A, Thinkamrop B, Susaengrat W. Ceftriaxone compared with sodium penicillin g for treatment of severe leptospirosis. Clin Infect Dis. 2003;36(12):1507-1513. doi:10.1086/375226
15. Suputtamongkol Y, Niwattayakul K, Suttinont C, et al. An open, randomized, controlled trial of penicillin, doxycycline, and cefotaxime for patients with severe leptospirosis. Clin Infect Dis. 2004;39(10):1417-1424. doi:10.1086/425001
16. Cleto SA, Rodrigues CE, Malaque CM, Sztajnbok J, Seguro AC, Andrade L. Hemodiafiltration decreases serum levels of inflammatory mediators in severe leptospirosis: a prospective study. PLoS ONE. 2016;11(8):e0160010. doi:10.1371/journal.pone.0160010
In areas where the zoonotic disease leptospirosis is endemic, reduced morbidity and mortality is strongly linked to quick initiation of renal replacement therapy.
In areas where the zoonotic disease leptospirosis is endemic, reduced morbidity and mortality is strongly linked to quick initiation of renal replacement therapy.
Leptospirosis (LS) is considered the most common and widespread zoonotic disease in the world. Numerous outbreaks have occurred in the past 10 years. Due to its technically difficult diagnosis, LS is severely underrecognized, underdiagnosed, and therefore, underreported.1,2 The Centers for Disease Control and Prevention (CDC) estimate 100 to 150 cases of LS are identified annually in the US, with about 50% of those cases occurring in Puerto Rico (PR).3 Specifically in PR, about 15 to 100 cases of suspected LS were reported annually between 2000 and 2009, with 59 cases and 1 death reported in 2010. The data are thought to be severely underreported due to a lack of widespread diagnostic testing availability in PR and no formal veterinary and environmental surveillance programs to monitor the incidence of animal cases and actual circulating serovars.4
A recent systematic review of 80 studies from 34 countries on morbidity and mortality of LS revealed that the global incidence and mortality is about 1.03 million cases and 58,900 deaths every year. Almost half of the reported deaths were adult males aged 20 to 49 years.5 Although mild cases of LS are not associated with an elevated mortality, icteric LS with renal failure (Weil disease) carries a mortality rate of 10%.6 In patients who develop hemorrhagic pneumonitis, mortality may be as high as 50 to 70%.7 Therefore, it is pivotal that clinicians recognize the disease early, that novel modalities of treatment continue to be developed, and that their impact on patient morbidity and mortality are studied and documented.
Case Presentation
A 43-year-old man with a medical history of schizophrenia presented to the emergency department at the US Department of Veterans Affairs (VA) Caribbean Healthcare System in San Juan, PR, after experiencing 1 week of intermittent fever, myalgia, and general weakness. Emergency medical services had found him disheveled and in a rodent-infested swamp area several days before admission. Initial vital signs were within normal limits.
On physical examination, the patient was afebrile, without acute distress, but he had diffuse jaundice and mild epigastric tenderness without evidence of peritoneal irritation. His complete blood count was remarkable for leukocytosis with left shifting, adequate hemoglobin levels but with 9 × 103 U/L platelets. The complete metabolic panel demonstrated an aspartate aminotransferase level of 564 U/L, alanine transaminase level of 462 U/L, total bilirubin of 12 mg/dL, which 10.2 mg/dL were direct bilirubin, and an alkaline phosphate of 345 U/L. Lipase levels were measured at 626 U/L. Marked coagulopathy also was present. The toxicology panel, including acetaminophen and salicylate acid levels, did not reveal the presence of any of the tested substances, and chest imaging did not demonstrate any infiltrates.
An abdominal ultrasound was negative for acute cholestatic pathologies, such as cholelithiasis, cholecystitis, or choledocholithiasis. Nonetheless, a noncontrast abdominopelvic computed tomography was remarkable for peripancreatic fat stranding, which raised suspicion for a diagnosis of pancreatitis.
Once the patient was transferred to the intensive care unit, he developed several episodes of hematemesis, leading to hemodynamical instability and severe respiratory distress. Due to anticipated respiratory failure and need for airway securement, endotracheal intubation was performed. Multiple packed red blood cells were transfused, and the patient was started in vasopressor support.
Diagnosis
A presumptive diagnosis of LS was made due to a considerable history of rodent exposure. The patient was started on broad-spectrum parenteral antibiotics, vancomycin 750 mg every 24 hours, metronidazole 500 mg every 8 hours, and ceftriaxone 2 g IV daily for adequate coverage against Leptospira spp. Despite 72 hours of antibiotic treatment, the patient’s clinical state deteriorated. He required high dosages of norepinephrine (1.5 mcg/kg/min) and vasopressin (0.03 U/min) to maintain adequate organ perfusion. Despite lung protective settings with low tidal volume and a high positive end-expiratory pressure, there was difficulty maintaining adequate oxygenation. Chest imaging was remarkable for bilateral infiltrates concerning for acute respiratory distress syndrome (ARDS).
The coagulopathy and cholestasis continued to worsen, and the renal failure progressed from nonoliguric to anuric. Because of this progression, the patient was started on continuous renal replacement therapy (CRRT) by hemodialysis. Within 24 hours of initiating CRRT, the patient’s clinical status improved dramatically. Vasopressor support was weaned, the coagulopathy resolved, and the cholestasis was improving. The patient’s respiratory status improved in such a manner that he was extubated by the seventh day after being placed on mechanical ventilation. The urine and blood samples sent for identification of Leptospira spp. through polymerase chain reaction (PCR) returned positive by the ninth day of admission. While on CRRT, the patient’s renal function eventually returned to baseline, and he was discharged 12 days after admission.
Discussion
The spirochetes of the genus Leptospira include both saprophytic and pathogenic species. These pathogenic Leptospira spp. have adapted to a grand variety of zoonotic hosts, the most important being rodents. They serve as vectors for the contraction of the disease by humans. Initial infection in rodents by Leptospira spp. causes a systemic illness followed by a persistent colonization of renal tubules from which they are excreted in the urine and into the environment. Humans, in turn, are an incidental host unable to induce a carrier state for the transmission of the pathogenic organism.1 The time from exposure to onset of symptoms, or incubation phase, averages 7 to 12 days but may range from 3 to 30 days.8
LS has been described as having 2 discernable but often coexisting phases. The first, an acute febrile bacteremic phase, has been noted to last about 9 days in about 85% of patients, although a minority have persistent fever from 2 weeks to > 30 days. A second phase, the immune or inflammatory phase, is characterized by a second fever spike preceded by 1 to 5 afebrile days in which there is presence of IgM antibodies and resolution of leptospiremia but positive urine cultures.9 Weil disease may present as the second phase of the disease or as a single, progressive illness from its first manifestation. It is characterized by a triad of jaundice, renal failure, and hemorrhage or coagulopathy.10 Weil disease is of great concern and importance due to its associated higher mortality than that found with the mildest form of the disease.
There are studies that advocate for RRT as an intricate part of the treatment regimen in LS to remove the inflammatory cytokines produced as a reaction to the spirochete.11 In tropical countries with a higher incidence of the disease, leptospirosis is an important cause of acute kidney injury (AKI), depending on multiple factors, including the AKI definition that is used.12 Renal invasion by Leptospira spp. produces acute tubular necrosis (ATN) and cell edema during the first week and then could progress to acute interstitial nephritis (AIN) in 2 to 3 weeks. It is believed that the mechanism for the Leptospira spp. invasion of the tubules that results in damage is associated with the antigenic components in its outer membrane; the most important outer membrane protein expressed during infection is LipL32. This protein increases the production of proinflammatory proteins, such as inducible nitric oxide synthase, monocyte chemotactic protein-1 (CCL2/MCP-1), T cells, and tumor necrosis factor.13
Although doxycycline has been recommended for the prophylaxis and treatment of mild LS, the preferred agent and the conferred benefits of antibiotic treatment overall for the severe form of the disease has been controversial. Traditionally, penicillin G sodium has been recommended as the first-line antibiotic treatment for moderate-to-severe LS.14 Nonetheless, there has been an increasing pattern of penicillin resistance among Leptospira spp. that has prompted the study and use of alternative agents.
An open-label, randomized comparison of parenteral cefotaxime, penicillin G sodium, and doxycycline for the treatment of suspected severe leptospirosis conducted by Suputtamongkol and colleagues showed no difference in mortality, defervescence, or time to resolution of abnormal laboratory findings.15 Current CDC recommendations include the use of parenteral penicillin 1.5 MU every 6 hours as the drug of choice, with ceftriaxone 1 g administered IV every 24 hours equally as effective.3
In addition to antimicrobial therapy, supportive care has shifted to include hemodialysis in those patients who develop AKI as part of the disease. Andrade and colleagues conducted a study of 33 patients with LS in Brazil that was set to compare the impact of door-to-dialysis time and dosage of hemodialysis on mortality. In patients with a quicker door-to-dialysis time and daily hemodialysis sessions, there was a 50% (16.7% vs 66.7%) absolute mortality reduction when compared with those with delayed initiation and alternate-day hemodialysis sessions.11 A follow-up prospective study compared the use of traditional sustained low-efficiency dialysis (SLED) with the use of extended SLED via hemodiafiltration in patients with LS presenting with ARDS and AKI. Although hemodiafiltration resulted in a relative decrease in serum levels of interleukin (IL)-17, IL-7, and CCL2/MCP-1, there was no significant difference in mortality.16 The most important prognostic factor in severe LS presenting with AKI and relating to RRT is a shorter door-to-dialysis time and increased dose, not the mode of dialysis clearance. Nonetheless, both RRT methods resulted in a progressive decrease in inflammatory mediators that have been associated with ATN and AIN in the context of LS.16 The authors argue that using CRRT instead of SLED via hemodiafiltration could have accentuated the effects of the reduction that inflammatory mediators may have on mortality in patients with severe LS.
Conclusions
LS continues to be of interest due to its current status as the most common zoonotic disease and its widespread prevalence throughout the globe. Novel treatment modalities for LS, specifically for Weil disease, continue to be developed with the goal of reducing the current mortality rate associated with the disease.
In endemic areas, prompt recognition is essential to initiate the recommended therapy. Parenteral antibiotics, such as penicillin G sodium and ceftriaxone, continue to be the mainstay of treatment and constitute the current CDC recommendations. Nonetheless, early initiation of CRRT has been shown to greatly reduce the mortality associated with Weil disease and, when available, should be considered in these patients.
Our patient failed to improve while receiving parenteral antibiotics alone but showed marked improvement after being placed on CRRT. Furthermore, initiation of CRRT resulted in near-complete resolution of his organ dysfunction and eventual discharge from the hospital. This case serves to further support the use of early CRRT as part of the standard of care in severe LS.
Leptospirosis (LS) is considered the most common and widespread zoonotic disease in the world. Numerous outbreaks have occurred in the past 10 years. Due to its technically difficult diagnosis, LS is severely underrecognized, underdiagnosed, and therefore, underreported.1,2 The Centers for Disease Control and Prevention (CDC) estimate 100 to 150 cases of LS are identified annually in the US, with about 50% of those cases occurring in Puerto Rico (PR).3 Specifically in PR, about 15 to 100 cases of suspected LS were reported annually between 2000 and 2009, with 59 cases and 1 death reported in 2010. The data are thought to be severely underreported due to a lack of widespread diagnostic testing availability in PR and no formal veterinary and environmental surveillance programs to monitor the incidence of animal cases and actual circulating serovars.4
A recent systematic review of 80 studies from 34 countries on morbidity and mortality of LS revealed that the global incidence and mortality is about 1.03 million cases and 58,900 deaths every year. Almost half of the reported deaths were adult males aged 20 to 49 years.5 Although mild cases of LS are not associated with an elevated mortality, icteric LS with renal failure (Weil disease) carries a mortality rate of 10%.6 In patients who develop hemorrhagic pneumonitis, mortality may be as high as 50 to 70%.7 Therefore, it is pivotal that clinicians recognize the disease early, that novel modalities of treatment continue to be developed, and that their impact on patient morbidity and mortality are studied and documented.
Case Presentation
A 43-year-old man with a medical history of schizophrenia presented to the emergency department at the US Department of Veterans Affairs (VA) Caribbean Healthcare System in San Juan, PR, after experiencing 1 week of intermittent fever, myalgia, and general weakness. Emergency medical services had found him disheveled and in a rodent-infested swamp area several days before admission. Initial vital signs were within normal limits.
On physical examination, the patient was afebrile, without acute distress, but he had diffuse jaundice and mild epigastric tenderness without evidence of peritoneal irritation. His complete blood count was remarkable for leukocytosis with left shifting, adequate hemoglobin levels but with 9 × 103 U/L platelets. The complete metabolic panel demonstrated an aspartate aminotransferase level of 564 U/L, alanine transaminase level of 462 U/L, total bilirubin of 12 mg/dL, which 10.2 mg/dL were direct bilirubin, and an alkaline phosphate of 345 U/L. Lipase levels were measured at 626 U/L. Marked coagulopathy also was present. The toxicology panel, including acetaminophen and salicylate acid levels, did not reveal the presence of any of the tested substances, and chest imaging did not demonstrate any infiltrates.
An abdominal ultrasound was negative for acute cholestatic pathologies, such as cholelithiasis, cholecystitis, or choledocholithiasis. Nonetheless, a noncontrast abdominopelvic computed tomography was remarkable for peripancreatic fat stranding, which raised suspicion for a diagnosis of pancreatitis.
Once the patient was transferred to the intensive care unit, he developed several episodes of hematemesis, leading to hemodynamical instability and severe respiratory distress. Due to anticipated respiratory failure and need for airway securement, endotracheal intubation was performed. Multiple packed red blood cells were transfused, and the patient was started in vasopressor support.
Diagnosis
A presumptive diagnosis of LS was made due to a considerable history of rodent exposure. The patient was started on broad-spectrum parenteral antibiotics, vancomycin 750 mg every 24 hours, metronidazole 500 mg every 8 hours, and ceftriaxone 2 g IV daily for adequate coverage against Leptospira spp. Despite 72 hours of antibiotic treatment, the patient’s clinical state deteriorated. He required high dosages of norepinephrine (1.5 mcg/kg/min) and vasopressin (0.03 U/min) to maintain adequate organ perfusion. Despite lung protective settings with low tidal volume and a high positive end-expiratory pressure, there was difficulty maintaining adequate oxygenation. Chest imaging was remarkable for bilateral infiltrates concerning for acute respiratory distress syndrome (ARDS).
The coagulopathy and cholestasis continued to worsen, and the renal failure progressed from nonoliguric to anuric. Because of this progression, the patient was started on continuous renal replacement therapy (CRRT) by hemodialysis. Within 24 hours of initiating CRRT, the patient’s clinical status improved dramatically. Vasopressor support was weaned, the coagulopathy resolved, and the cholestasis was improving. The patient’s respiratory status improved in such a manner that he was extubated by the seventh day after being placed on mechanical ventilation. The urine and blood samples sent for identification of Leptospira spp. through polymerase chain reaction (PCR) returned positive by the ninth day of admission. While on CRRT, the patient’s renal function eventually returned to baseline, and he was discharged 12 days after admission.
Discussion
The spirochetes of the genus Leptospira include both saprophytic and pathogenic species. These pathogenic Leptospira spp. have adapted to a grand variety of zoonotic hosts, the most important being rodents. They serve as vectors for the contraction of the disease by humans. Initial infection in rodents by Leptospira spp. causes a systemic illness followed by a persistent colonization of renal tubules from which they are excreted in the urine and into the environment. Humans, in turn, are an incidental host unable to induce a carrier state for the transmission of the pathogenic organism.1 The time from exposure to onset of symptoms, or incubation phase, averages 7 to 12 days but may range from 3 to 30 days.8
LS has been described as having 2 discernable but often coexisting phases. The first, an acute febrile bacteremic phase, has been noted to last about 9 days in about 85% of patients, although a minority have persistent fever from 2 weeks to > 30 days. A second phase, the immune or inflammatory phase, is characterized by a second fever spike preceded by 1 to 5 afebrile days in which there is presence of IgM antibodies and resolution of leptospiremia but positive urine cultures.9 Weil disease may present as the second phase of the disease or as a single, progressive illness from its first manifestation. It is characterized by a triad of jaundice, renal failure, and hemorrhage or coagulopathy.10 Weil disease is of great concern and importance due to its associated higher mortality than that found with the mildest form of the disease.
There are studies that advocate for RRT as an intricate part of the treatment regimen in LS to remove the inflammatory cytokines produced as a reaction to the spirochete.11 In tropical countries with a higher incidence of the disease, leptospirosis is an important cause of acute kidney injury (AKI), depending on multiple factors, including the AKI definition that is used.12 Renal invasion by Leptospira spp. produces acute tubular necrosis (ATN) and cell edema during the first week and then could progress to acute interstitial nephritis (AIN) in 2 to 3 weeks. It is believed that the mechanism for the Leptospira spp. invasion of the tubules that results in damage is associated with the antigenic components in its outer membrane; the most important outer membrane protein expressed during infection is LipL32. This protein increases the production of proinflammatory proteins, such as inducible nitric oxide synthase, monocyte chemotactic protein-1 (CCL2/MCP-1), T cells, and tumor necrosis factor.13
Although doxycycline has been recommended for the prophylaxis and treatment of mild LS, the preferred agent and the conferred benefits of antibiotic treatment overall for the severe form of the disease has been controversial. Traditionally, penicillin G sodium has been recommended as the first-line antibiotic treatment for moderate-to-severe LS.14 Nonetheless, there has been an increasing pattern of penicillin resistance among Leptospira spp. that has prompted the study and use of alternative agents.
An open-label, randomized comparison of parenteral cefotaxime, penicillin G sodium, and doxycycline for the treatment of suspected severe leptospirosis conducted by Suputtamongkol and colleagues showed no difference in mortality, defervescence, or time to resolution of abnormal laboratory findings.15 Current CDC recommendations include the use of parenteral penicillin 1.5 MU every 6 hours as the drug of choice, with ceftriaxone 1 g administered IV every 24 hours equally as effective.3
In addition to antimicrobial therapy, supportive care has shifted to include hemodialysis in those patients who develop AKI as part of the disease. Andrade and colleagues conducted a study of 33 patients with LS in Brazil that was set to compare the impact of door-to-dialysis time and dosage of hemodialysis on mortality. In patients with a quicker door-to-dialysis time and daily hemodialysis sessions, there was a 50% (16.7% vs 66.7%) absolute mortality reduction when compared with those with delayed initiation and alternate-day hemodialysis sessions.11 A follow-up prospective study compared the use of traditional sustained low-efficiency dialysis (SLED) with the use of extended SLED via hemodiafiltration in patients with LS presenting with ARDS and AKI. Although hemodiafiltration resulted in a relative decrease in serum levels of interleukin (IL)-17, IL-7, and CCL2/MCP-1, there was no significant difference in mortality.16 The most important prognostic factor in severe LS presenting with AKI and relating to RRT is a shorter door-to-dialysis time and increased dose, not the mode of dialysis clearance. Nonetheless, both RRT methods resulted in a progressive decrease in inflammatory mediators that have been associated with ATN and AIN in the context of LS.16 The authors argue that using CRRT instead of SLED via hemodiafiltration could have accentuated the effects of the reduction that inflammatory mediators may have on mortality in patients with severe LS.
Conclusions
LS continues to be of interest due to its current status as the most common zoonotic disease and its widespread prevalence throughout the globe. Novel treatment modalities for LS, specifically for Weil disease, continue to be developed with the goal of reducing the current mortality rate associated with the disease.
In endemic areas, prompt recognition is essential to initiate the recommended therapy. Parenteral antibiotics, such as penicillin G sodium and ceftriaxone, continue to be the mainstay of treatment and constitute the current CDC recommendations. Nonetheless, early initiation of CRRT has been shown to greatly reduce the mortality associated with Weil disease and, when available, should be considered in these patients.
Our patient failed to improve while receiving parenteral antibiotics alone but showed marked improvement after being placed on CRRT. Furthermore, initiation of CRRT resulted in near-complete resolution of his organ dysfunction and eventual discharge from the hospital. This case serves to further support the use of early CRRT as part of the standard of care in severe LS.
1. Ko AI, Goarant C, Picardeau M. Leptospira: the dawn of the molecular genetics era for an emerging zoonotic pathogen. Nat Rev Microbiol. 2009;7(10):736-747. doi:10.1038/nrmicro2208
2. Hartskeerl RA, Collares-Pereira M, Ellis WA. Emergence, control and re-emerging leptospirosis: dynamics of infection in the changing world. Clin Microbiol Infect. 2011;17(4):494-501. doi:10.1111/j.1469-0691.2011.03474.x
3. Centers for Disease Control and Prevention. Leptospirosis fact sheet for clinicians, CS287535B. https://www.cdc.gov/leptospirosis/pdf/fs-leptospirosis-clinicians-eng-508.pdf. Published January 30, 2018. Accessed October 9, 2020.
4. Martinez-Recio C, Rodriguez-Cintron W, Galarza-Vargas S, et al. The brief case: cases from 3 hospitals in Puerto Rico. ACP Hosp. https://acphospitalist.org/archives/2014/09/briefcase.htm. Published September 2014. Accessed October 9, 2020.
5. Costa F, Hagan JE, Calcagno J, et al. Global morbidity and mortality of leptospirosis: a systematic review. PLoS Negl Trop Dis. 2015;9(9):e0003898. doi:10.1371/journal.pntd.0003898
6. Levett PN. Leptospirosis. Clin Microbiol Rev. 2001;14(2):296-326. doi:10.1128/CMR.14.2.296-326.2001
7. Vijayachari P, Sugunan AP, Shriram AN. Leptospirosis: an emerging global public health problem. J Biosci. 2008;33(4):557-569. doi:10.1007/s12038-008-0074-z
8. Haake DA, Levett PN. Leptospirosis in humans. In: Adler B, ed. Leptospira and Leptospirosis. Berlin, Heidelberg: Springer-Verlag Berlin Heidelberg; 2015:65-97. doi:10.1007/978-3-662-45059-8_5
9. Berman SJ. Sporadic anicteric leptospirosis in South Vietnam: a study in 150 patients. Ann Intern Med. 1973;79(2):167. doi:10.7326/0003-4819-79-2-167
10. Bharti AR, Nally JE, Ricaldi JN, et al. Leptospirosis: a zoonotic disease of global importance. Lancet Infect Dis. 2003;3(12):757-771. doi:10.1016/S1473-3099(03)00830-2
11. Andrade L, Cleto S, Seguro AC. Door-to-dialysis time and daily hemodialysis in patients with leptospirosis: impact on mortality. Clin J Am Soc Nephrol. 2007;2(4):739–744. doi: 10.2215/CJN.00680207
12. Mathew A, George J. Acute kidney injury in the tropics. Ann Saudi Med. 2011;31(5):451-456. doi:10.4103/0256-4947.84620
13. Daher EF, Silva GB Jr, Karbage NNN, et al. Predictors of oliguric acute kidney injury in leptospirosis. Nephron Clin Pract. 2009;112(1):c25-c30. doi:10.1159/000210571
14. Panaphut T, Domrongkitchaiporn S, Vibhagool A, Thinkamrop B, Susaengrat W. Ceftriaxone compared with sodium penicillin g for treatment of severe leptospirosis. Clin Infect Dis. 2003;36(12):1507-1513. doi:10.1086/375226
15. Suputtamongkol Y, Niwattayakul K, Suttinont C, et al. An open, randomized, controlled trial of penicillin, doxycycline, and cefotaxime for patients with severe leptospirosis. Clin Infect Dis. 2004;39(10):1417-1424. doi:10.1086/425001
16. Cleto SA, Rodrigues CE, Malaque CM, Sztajnbok J, Seguro AC, Andrade L. Hemodiafiltration decreases serum levels of inflammatory mediators in severe leptospirosis: a prospective study. PLoS ONE. 2016;11(8):e0160010. doi:10.1371/journal.pone.0160010
1. Ko AI, Goarant C, Picardeau M. Leptospira: the dawn of the molecular genetics era for an emerging zoonotic pathogen. Nat Rev Microbiol. 2009;7(10):736-747. doi:10.1038/nrmicro2208
2. Hartskeerl RA, Collares-Pereira M, Ellis WA. Emergence, control and re-emerging leptospirosis: dynamics of infection in the changing world. Clin Microbiol Infect. 2011;17(4):494-501. doi:10.1111/j.1469-0691.2011.03474.x
3. Centers for Disease Control and Prevention. Leptospirosis fact sheet for clinicians, CS287535B. https://www.cdc.gov/leptospirosis/pdf/fs-leptospirosis-clinicians-eng-508.pdf. Published January 30, 2018. Accessed October 9, 2020.
4. Martinez-Recio C, Rodriguez-Cintron W, Galarza-Vargas S, et al. The brief case: cases from 3 hospitals in Puerto Rico. ACP Hosp. https://acphospitalist.org/archives/2014/09/briefcase.htm. Published September 2014. Accessed October 9, 2020.
5. Costa F, Hagan JE, Calcagno J, et al. Global morbidity and mortality of leptospirosis: a systematic review. PLoS Negl Trop Dis. 2015;9(9):e0003898. doi:10.1371/journal.pntd.0003898
6. Levett PN. Leptospirosis. Clin Microbiol Rev. 2001;14(2):296-326. doi:10.1128/CMR.14.2.296-326.2001
7. Vijayachari P, Sugunan AP, Shriram AN. Leptospirosis: an emerging global public health problem. J Biosci. 2008;33(4):557-569. doi:10.1007/s12038-008-0074-z
8. Haake DA, Levett PN. Leptospirosis in humans. In: Adler B, ed. Leptospira and Leptospirosis. Berlin, Heidelberg: Springer-Verlag Berlin Heidelberg; 2015:65-97. doi:10.1007/978-3-662-45059-8_5
9. Berman SJ. Sporadic anicteric leptospirosis in South Vietnam: a study in 150 patients. Ann Intern Med. 1973;79(2):167. doi:10.7326/0003-4819-79-2-167
10. Bharti AR, Nally JE, Ricaldi JN, et al. Leptospirosis: a zoonotic disease of global importance. Lancet Infect Dis. 2003;3(12):757-771. doi:10.1016/S1473-3099(03)00830-2
11. Andrade L, Cleto S, Seguro AC. Door-to-dialysis time and daily hemodialysis in patients with leptospirosis: impact on mortality. Clin J Am Soc Nephrol. 2007;2(4):739–744. doi: 10.2215/CJN.00680207
12. Mathew A, George J. Acute kidney injury in the tropics. Ann Saudi Med. 2011;31(5):451-456. doi:10.4103/0256-4947.84620
13. Daher EF, Silva GB Jr, Karbage NNN, et al. Predictors of oliguric acute kidney injury in leptospirosis. Nephron Clin Pract. 2009;112(1):c25-c30. doi:10.1159/000210571
14. Panaphut T, Domrongkitchaiporn S, Vibhagool A, Thinkamrop B, Susaengrat W. Ceftriaxone compared with sodium penicillin g for treatment of severe leptospirosis. Clin Infect Dis. 2003;36(12):1507-1513. doi:10.1086/375226
15. Suputtamongkol Y, Niwattayakul K, Suttinont C, et al. An open, randomized, controlled trial of penicillin, doxycycline, and cefotaxime for patients with severe leptospirosis. Clin Infect Dis. 2004;39(10):1417-1424. doi:10.1086/425001
16. Cleto SA, Rodrigues CE, Malaque CM, Sztajnbok J, Seguro AC, Andrade L. Hemodiafiltration decreases serum levels of inflammatory mediators in severe leptospirosis: a prospective study. PLoS ONE. 2016;11(8):e0160010. doi:10.1371/journal.pone.0160010
Facing systemic racism in health care: Inequities in medical education
Finding inspiration among life’s challenges
Barbara Levy, MD: I am fortunate to have met Pierre serendipitously at a training that we were both attending and was impressed by Dr. Johnson’s life story, his passion and commitment, and his dedication—not only to his personal career but also to raising up other young men of color by trying to break down barriers that face them. His life story highlights those areas of systemic and structural problems that all of us together need to address if we are going to make any progress.
Pierre Johnson, MD: Thank you, Barbara. A little about myself: I am a board-certified ObGyn, and I specialize in minimally invasive surgery. I was born on the South side of Chicago, experiencing gang violence, drugs, and substandard, underserved schools. Long story short, I had a very rough upbringing. I had a single mom and several different issues at home. I am the oldest of 5 siblings, and life was tough.
But I knew that I wanted to do something different with my life. I saw that there was a need in my community as far as health care was concerned, in particular women’s health and childbirth. I knew early on that I wanted to be an ObGyn, and the reason had a lot to do with The Cosby Show. It was the only example of a positive, successful Black man that I saw. No one graduated from college in my family. There weren’t any models of young Black excellence around me. Saying that I wanted to be a doctor planted a seed. I was 9 when my mom became pregnant with my first sibling, and it was fascinating to me. The physiology of pregnancy, and eventually childbirth, was extremely fascinating to me; it set me off on my journey to be an ObGyn.
As I got older, things didn’t get any easier. I went to high school in one of the toughest areas on the South side of Chicago. Gang violence, and violence in and of itself, were all around me, but I was able to stay focused. I went on to Xavier University in Louisiana.
Dr. Levy: There are some important things that I learned from your book and from talking to you at our first meeting. Your mom’s ObGyn, when she was pregnant with your next youngest sibling, was also a Black ObGyn. He took some time to take you under wing?
Dr. Johnson: He did. My mom’s ObGyn was a Black man. Other than The Cosby Show, that’s the only time I saw something like that. When I spoke to him, he really took the time to answer my questions and show me that he was like me; he wasn’t just a far-off mythical person, or something that I could not obtain.
Continue to: Seeing is believing when it comes to success...
Seeing is believing when it comes to success
Dr. Levy: Do you think it was important to have a role model who wasn’t a sports star?
Dr. Johnson: If you can’t see it, you can’t achieve it. He took his time to really talk to me, and it’s the little things for kids that go a long way in their life experience. I still have a relationship with him to this day. How he handled me as a kid made me realize that this is something that I could do. That was extremely important for me.
Dr. Levy: One of the structural things I think we need to point out is that the ability to see yourself as someone successful is critical. When we see 1,000 images a day and they are all White, and they are all so different from where we are that it gets incorporated into our sense of being. I think that’s really difficult for those of us of with privilege to understand what that privilege is.
Dr. Johnson: Absolutely, and I’ll even go further. In residency, 2 White females were my classmates, and both of their parents were doctors. They had grandparents who were doctors. My mom was addicted to drugs; my father was not around. They had been talking medicine since they were 5. You have to make things equitable, but in medicine it’s really not equitable. In medicine, what we don’t realize is that there is an importance for all aspects of someone’s upbringing and environment, and it’s not just what they can regurgitate on a standardized test. If a patient can’t relate to you and tell you what is wrong with them, how can you adequately treat them?
Dr. Levy: Even if they are trying to tell me, but I can’t hear it because I don’t have the language and I don’t have the background. There are really good data to show, in fact, that Black male physicians do a better job at engaging Black men to manage their hypertension.1 When we look at the inequities in birth outcomes for women of color, indigenous women and Black women, there’s evidence that providers who come from a similar background do a better job.
Dr. Johnson: There was the study of Black infants that just came out about them dying at a 3-time higher rate in non-Black physicians’ hands.2 These things need to be recognized. They need to be discussed, and they need to be identified as issues and then, realistically, we need to start talking about solutions, not get offended by what actual statistics are saying.
Foundational inequities in education
Dr. Levy: To address some of the barriers that you faced: I know that you went to a high school that was not geared toward pushing students into professional careers. Your colleagues, however, had educations that prepared them for the standardized tests and other things that they would face academically.
Dr. Johnson: People think I am kidding when I say it, but when I went into college, I didn’t know what a periodic table was. I saw it, but I had no idea what these things meant. I didn’t have any sciences or any AP classes in high school. I did well, but grades are smoke and mirrors. The true test of medicine comes with testing. From the MCATs to the boards, every step of the way there is a standardized test.
Knowledge is something that you can obtain, but test taking is a cultivated skill that happens from a very early age. Trying to teach an adult or someone in their late teens a skill that they should have learned as a kid is difficult. For me, I did not have that, so I had to program myself. I had to learn how to fundamentally take tests as an adult, where most people understand how to do that going into college and professional school.
Dr. Levy: I was impressed with your resilience. I think all of us as human beings, if we fail a test, we take it personally and think it’s about our lack of knowledge. One of the insights that you came to was that failure on those things was not that you didn’t study hard enough. In fact, you probably studied 4 times harder than most other people. You had the knowledge. Being able to get that knowledge into a standardized structured test score was the huge challenge for you.
Dr. Johnson: That’s it. I can remember taking the MCAT, and if you looked at the step 1 book, I could regurgitate to you everything on that page. However, it’s not a test about do you know it or not. It’s an understanding of the English language and how to break things down to make things fit into particular scenarios.
Continue to: A college experience focused on growth and exposure...
A college experience focused on growth and exposure
Dr. Levy: I was impressed by the distinction between your experience at Xavier University where there was a lot of support and guidance and help in your premed program, and what happened to you when you hit medical school.
Dr. Johnson: Xavier University in Louisiana is the number 1 institution in the country for getting minorities into professional school. They understand that they have kids that are brilliant but underprepared, and just have not had the background to actually tackle some of these tough curriculums. I always had good grades in school. But by not being challenged, I didn’t know what I didn’t really know. So now that I was seeing biology, chemistry for the first time, and trying to tackle it; there’s a failure point. I didn’t know how to take tests, and I didn’t know how to study properly. The harder I tried, the worse things got for me.
Xavier has seen that story a multitude of times. If I went to a bigger or predominantly White university, a counselor would have told me, “Well, medicine’s maybe not for you. You can’t handle a premed curriculum.” Instead, I said, “Listen, I’m studying. I’m doing all of these things, and I’m not hacking it.” And they broke it down: “Let’s get you into study groups with kids that have had these type of AP classes before. We’ll have you watch how they study,” and everything started to click. That facilitation of how to adjust to this curriculum was a godsend. It’s the only reason I’m here. I am a prime example of being brilliant enough to be able to do it, but needing the infrastructure and a system set up.
Dr. Levy: There’s a great book by Carol Dweck called Mindset that talks about education of young kids and putting them into silos so early in life; the brilliant kids go into the AP courses and the rest are labeled as inadequate. It’s assumed in a fixed mindset based on their heredity and IQ, and not based on the fact that they have not been exposed to the right things.
Xavier was growing you into the man who could, in fact, do all of those things. I think that is one of the systemic and structural issues that we have—that fixed mindset that frames a kid who is not succeeding as therefore unable to succeed, as opposed to framing that child as not having the correct tools.
New tribulations of medical school
Dr. Johnson: Absolutely. I think what Xavier did for me is to at least let me understand what I needed to do, how to comprehend and retain information, which I never had been exposed to before. Those years were very important to establishing a foundation. When going to medical school, it was like, “There’s no more excuses. What could be the problem now?” Well, now let’s talk about taking tests—a whole different skill. Xavier focused on getting me to understand how to structure my thought process and knowledge base. In medical school I had to apply those skills (because if you can’t apply them, there’s no fit).
My second through fourth year of medical school, I was the only African-American kid in my class. I was spending 20-hour days sometimes just studying, trying to overcompensate by knowing as much as I possibly could and thinking that would propel me from the test-taking standpoint. Even though I didn’t have a lot of classmates in medical school that looked like me, I did have mentors that looked similarly, who really saw potential in me. Dr. Frederick Horvath, a nephrologist in Peoria said, “What are you doing? I want you to get out of these books, and let’s go out to lunch.”
He ended up buying me some instrumental books, really talked to me, listening to my background and understanding how driven I was as a person. He took me under his wing for the rest of medical school and said, “This is how you navigate through these spaces. Yes, you need to have a fund of knowledge to be able to take these tests, but you need to start understanding how to apply it to these questions.” I’m forever grateful to Dr. Horvath for doing that because it was a point in time where I was lost and struggling.
Continue to: Hitting a stride but facing racism head-on...
Hitting a stride but facing racism head-on
Dr. Levy: You talk about the systemic and pervasive racism that was on the wards when you hit them in fourth year. If you don’t mind sharing just a little bit of that, it would help people reading this to have a better understanding of the kinds of barriers that are out there.
Dr. Johnson: Even when I talk about it today, it bothers me.
I went to medical school in Peoria, Illinois, not far from the home of the Ku Klux Klan. At that time, once you got out of Chicago it was a very brutal place, with systemic racism throughout. I was a young Black kid going through a process that not many young Black kids from the South side of Chicago go through, and you had people who had never seen anyone like me. When I was going through my clinical rotations, I knew what I was up against. I was dressed “to the T” every day, arriving early, leaving late, trying to answer questions. I would look at the evaluations, and they would be disparaging. I would look at my counterparts, how their evaluations were, and how people would respond to them, and it would be completely different.
Surgery was the part of ObGyn that I really grew to love more than anything, even more than obstetrics. When general surgery came, I wanted to take it very seriously and learn as much as I possibly could. From the beginning, I knew there was a problem because the chief resident, an older White man, wouldn’t look me in the eye or talk to me. He would make disparaging remarks. The thing that stuck out in my mind the most was when I was in the operating room transporting patients, just like a medical student did, and he came up behind me and said, “You know, Pierre, this is where a small mind and a strong back come into play.” For me, it took me to a place where I had to corral my emotions and thoughts because I just wanted to lash out and just tell him how racist and horrible that was for him to say that to me. I explained this to the powers that be, the director of the department, and they basically blew it off to the side.
When it came down to the end of the evaluation period, I passed with flying colors. But they gave me an incomplete because of that chief resident and his remarks on my evaluations. He had 3 pages of report about me as a person and as a student. He said that he had difficulty in expressing his opinions about me because of possible cultural biases that he may have had. He put “cultural biases” in an evaluation, and they looked at that and said that was enough for me to have to remediate my time. I was required to do an extra month in Pontiac, Illinois, which is even more rural than Peoria, because of a racist person that did not give me a fair opportunity because I was Black.
Like everything else in life, it was a learning experience. It’s why I fight so hard today. It’s why I’m so passionate about equity, not only in medicine but also in all aspects of society. It shows why we have police brutality and Black men dying in the streets. It shows how this happens because there are cultural and implicit biases that play out in every part of life, and we are not honest about it. Until we are honest about it and until we say that this is happening and there is something that needs to be done to address it, it’s going to continue to happen. That is my fight.
Exposing the unspoken power struggle
Dr. Levy: I couldn’t agree more. Attributing things like that to the individual, where you talk about a White man in power and a power structure that didn’t literally physically beat you but did beat you into submission. You talk about how to succeed in medical school, and how you had to suck it up and submit to something that was incredibly unfair. You understood, you were old enough, mature enough, to understand that if you fought back, you were going to lose. The only opportunity you had was to submit to that inequity and push forward.
Dr. Johnson: When I did try to fight, the chair of the department told me that either I accept the consequences or I would not graduate from medical school and be forced to do another year. That struck a chord with me. I think that happens a lot in our society, and it needs to be exposed.
Past experiences reflected in today’s society
Dr. Levy: Can you talk about what you faced in your ObGyn residency in terms of the systemic pushback, people not taking your orders, people questioning you. I know that I have heard that a great deal, and I experienced that myself as a woman.
Dr. Johnson: We look at the things that are happening now, everything from George Floyd’s murder to Colin Kaepernick taking a knee. These things are 10 years past when I first started residency. The year before I started residency, there was a noose hanging on the capitol lawn of Springfield, Illinois’ capital city. There’s systemic racism and hatred there. When I first started on the wards of my first year of ObGyn, again, I was the very first Black resident of my program’s history. Nobody could relate to me.
I went from a year-long general surgery internship at Washington Hospital Center in Washington, DC, to ObGyn residency. In the first 2 months, there were complaints of, “He’s not answering his pages. He’s not being prompt.” I went to my program director and said, “Listen, I have never had one complaint like this. There’s a problem here. And there’s a problem when I’m on the floor: When trying to give orders to nurses, they’re not taking them. I had to tell a couple of nurses, ‘I’m Dr. Johnson. Don’t call me by my first name, especially not in front of patients.’”
My director was just not hearing me, because the entire scenario was something they had never been exposed to. Systemic racism is real, and unless you experience it, it’s very difficult to accept that it is happening. But biases happen when you are not cognizant. People are used to things a certain way. Things play out in the media that make your mind think a certain way, and you don’t even realize it. You may not even want to be that way.
Continue to: Unconscious bias is a barrier to ensuring equity...
Unconscious bias is a barrier to ensuring equity
Dr. Levy: One very important point you just made is that we as the system need to be able to recognize those unconscious things, the language that we use, the disparaging remarks, the things that put people down, as well as the things that keep people out of promotion.
There are some interesting data about both race and gender and the language that we use when we write recommendations for people, that we do things unconsciously. The big message to all of us at the end is to open our minds to where those things can occur. For myself, professionally, I keep a list of words that I use when I write recommendations. I measure myself to ensure that I am using the same language for men and women, for Black and White. I think we need to overcome the system and the structure to create real equity—not equality but equity.
It begins with being real about the issues
Dr. Johnson: It’s a bigger problem than the existence of bias and racism. I think these are systemic issues that have been cultivated over centuries that have never been addressed. The true issue is that we deny that these are problems and refuse to talk about it because it makes us uncomfortable. To truly make things more equitable, we have to push our levels of comfort to be able to talk about things in a healthy manner, be open and transparent, and to start to understand how we are thinking about certain things. When you can see it, you can start to implement changes and start to change mentalities and thought processes.
For me, people say, “You don’t look like a doctor.” I get that all the time—because I have tattoos and earrings. I wear my hair in a mohawk. The image of what success looks like has been manifested through our media and culture, and it has imprinted on our minds as to how things are supposed to be. If someone doesn’t fit those molds, we start to shun them out, or we start to exhibit biases against those things. What I am trying to do is change that thought process of what a successful or a professional person looks like. It doesn’t have a look. It is not a White or Black thing. It’s an intellect, a mindset, a way of living. You have to treat every person as an individual and take all the biases out of it and understand where they are coming from and what they have to offer to the profession.
Dr. Levy: I personally was so impressed by you when I met you. I was impressed by the tattoos and the earrings, and my initial response to them was exactly that biased, “Oh, who is this person?” I checked that at the door, listened to you, and was really impressed at your surgical skill, your knowledge, your background. I am really grateful that you have been willing to spend the time to share that with everyone.
Dr. Johnson: Thank you for this discussion.
To watch the full interview between Drs. Levy and Johnson, visit: https://www.mdedge.com/obgyn/article/228507/facing-systemic-racism-health-care-inequities-medical-education. ●
- The Pulse of Perseverance:
Three Black Doctors on Their Journey to Success Pierre Johnson, MD; Maxime Madhere, MD; and Joseph Semien Jr, MD - Mindset:
The New Psychology of Success
Carol S. Dweck
- Benkert R, Peters R, Tate N, et al. Trust of nurse practitioners and physicians among African Americans with hypertension. J Am Acad Nurse Pract. 2008;20:273-280.
- Greenwood BN, Hardeman RR, Huang L, et al. Physician– patient racial concordance and disparities in birthing mortality for newborns. Proc Natl Acad Sci U S A. 2020; 117:21194-21200.
Finding inspiration among life’s challenges
Barbara Levy, MD: I am fortunate to have met Pierre serendipitously at a training that we were both attending and was impressed by Dr. Johnson’s life story, his passion and commitment, and his dedication—not only to his personal career but also to raising up other young men of color by trying to break down barriers that face them. His life story highlights those areas of systemic and structural problems that all of us together need to address if we are going to make any progress.
Pierre Johnson, MD: Thank you, Barbara. A little about myself: I am a board-certified ObGyn, and I specialize in minimally invasive surgery. I was born on the South side of Chicago, experiencing gang violence, drugs, and substandard, underserved schools. Long story short, I had a very rough upbringing. I had a single mom and several different issues at home. I am the oldest of 5 siblings, and life was tough.
But I knew that I wanted to do something different with my life. I saw that there was a need in my community as far as health care was concerned, in particular women’s health and childbirth. I knew early on that I wanted to be an ObGyn, and the reason had a lot to do with The Cosby Show. It was the only example of a positive, successful Black man that I saw. No one graduated from college in my family. There weren’t any models of young Black excellence around me. Saying that I wanted to be a doctor planted a seed. I was 9 when my mom became pregnant with my first sibling, and it was fascinating to me. The physiology of pregnancy, and eventually childbirth, was extremely fascinating to me; it set me off on my journey to be an ObGyn.
As I got older, things didn’t get any easier. I went to high school in one of the toughest areas on the South side of Chicago. Gang violence, and violence in and of itself, were all around me, but I was able to stay focused. I went on to Xavier University in Louisiana.
Dr. Levy: There are some important things that I learned from your book and from talking to you at our first meeting. Your mom’s ObGyn, when she was pregnant with your next youngest sibling, was also a Black ObGyn. He took some time to take you under wing?
Dr. Johnson: He did. My mom’s ObGyn was a Black man. Other than The Cosby Show, that’s the only time I saw something like that. When I spoke to him, he really took the time to answer my questions and show me that he was like me; he wasn’t just a far-off mythical person, or something that I could not obtain.
Continue to: Seeing is believing when it comes to success...
Seeing is believing when it comes to success
Dr. Levy: Do you think it was important to have a role model who wasn’t a sports star?
Dr. Johnson: If you can’t see it, you can’t achieve it. He took his time to really talk to me, and it’s the little things for kids that go a long way in their life experience. I still have a relationship with him to this day. How he handled me as a kid made me realize that this is something that I could do. That was extremely important for me.
Dr. Levy: One of the structural things I think we need to point out is that the ability to see yourself as someone successful is critical. When we see 1,000 images a day and they are all White, and they are all so different from where we are that it gets incorporated into our sense of being. I think that’s really difficult for those of us of with privilege to understand what that privilege is.
Dr. Johnson: Absolutely, and I’ll even go further. In residency, 2 White females were my classmates, and both of their parents were doctors. They had grandparents who were doctors. My mom was addicted to drugs; my father was not around. They had been talking medicine since they were 5. You have to make things equitable, but in medicine it’s really not equitable. In medicine, what we don’t realize is that there is an importance for all aspects of someone’s upbringing and environment, and it’s not just what they can regurgitate on a standardized test. If a patient can’t relate to you and tell you what is wrong with them, how can you adequately treat them?
Dr. Levy: Even if they are trying to tell me, but I can’t hear it because I don’t have the language and I don’t have the background. There are really good data to show, in fact, that Black male physicians do a better job at engaging Black men to manage their hypertension.1 When we look at the inequities in birth outcomes for women of color, indigenous women and Black women, there’s evidence that providers who come from a similar background do a better job.
Dr. Johnson: There was the study of Black infants that just came out about them dying at a 3-time higher rate in non-Black physicians’ hands.2 These things need to be recognized. They need to be discussed, and they need to be identified as issues and then, realistically, we need to start talking about solutions, not get offended by what actual statistics are saying.
Foundational inequities in education
Dr. Levy: To address some of the barriers that you faced: I know that you went to a high school that was not geared toward pushing students into professional careers. Your colleagues, however, had educations that prepared them for the standardized tests and other things that they would face academically.
Dr. Johnson: People think I am kidding when I say it, but when I went into college, I didn’t know what a periodic table was. I saw it, but I had no idea what these things meant. I didn’t have any sciences or any AP classes in high school. I did well, but grades are smoke and mirrors. The true test of medicine comes with testing. From the MCATs to the boards, every step of the way there is a standardized test.
Knowledge is something that you can obtain, but test taking is a cultivated skill that happens from a very early age. Trying to teach an adult or someone in their late teens a skill that they should have learned as a kid is difficult. For me, I did not have that, so I had to program myself. I had to learn how to fundamentally take tests as an adult, where most people understand how to do that going into college and professional school.
Dr. Levy: I was impressed with your resilience. I think all of us as human beings, if we fail a test, we take it personally and think it’s about our lack of knowledge. One of the insights that you came to was that failure on those things was not that you didn’t study hard enough. In fact, you probably studied 4 times harder than most other people. You had the knowledge. Being able to get that knowledge into a standardized structured test score was the huge challenge for you.
Dr. Johnson: That’s it. I can remember taking the MCAT, and if you looked at the step 1 book, I could regurgitate to you everything on that page. However, it’s not a test about do you know it or not. It’s an understanding of the English language and how to break things down to make things fit into particular scenarios.
Continue to: A college experience focused on growth and exposure...
A college experience focused on growth and exposure
Dr. Levy: I was impressed by the distinction between your experience at Xavier University where there was a lot of support and guidance and help in your premed program, and what happened to you when you hit medical school.
Dr. Johnson: Xavier University in Louisiana is the number 1 institution in the country for getting minorities into professional school. They understand that they have kids that are brilliant but underprepared, and just have not had the background to actually tackle some of these tough curriculums. I always had good grades in school. But by not being challenged, I didn’t know what I didn’t really know. So now that I was seeing biology, chemistry for the first time, and trying to tackle it; there’s a failure point. I didn’t know how to take tests, and I didn’t know how to study properly. The harder I tried, the worse things got for me.
Xavier has seen that story a multitude of times. If I went to a bigger or predominantly White university, a counselor would have told me, “Well, medicine’s maybe not for you. You can’t handle a premed curriculum.” Instead, I said, “Listen, I’m studying. I’m doing all of these things, and I’m not hacking it.” And they broke it down: “Let’s get you into study groups with kids that have had these type of AP classes before. We’ll have you watch how they study,” and everything started to click. That facilitation of how to adjust to this curriculum was a godsend. It’s the only reason I’m here. I am a prime example of being brilliant enough to be able to do it, but needing the infrastructure and a system set up.
Dr. Levy: There’s a great book by Carol Dweck called Mindset that talks about education of young kids and putting them into silos so early in life; the brilliant kids go into the AP courses and the rest are labeled as inadequate. It’s assumed in a fixed mindset based on their heredity and IQ, and not based on the fact that they have not been exposed to the right things.
Xavier was growing you into the man who could, in fact, do all of those things. I think that is one of the systemic and structural issues that we have—that fixed mindset that frames a kid who is not succeeding as therefore unable to succeed, as opposed to framing that child as not having the correct tools.
New tribulations of medical school
Dr. Johnson: Absolutely. I think what Xavier did for me is to at least let me understand what I needed to do, how to comprehend and retain information, which I never had been exposed to before. Those years were very important to establishing a foundation. When going to medical school, it was like, “There’s no more excuses. What could be the problem now?” Well, now let’s talk about taking tests—a whole different skill. Xavier focused on getting me to understand how to structure my thought process and knowledge base. In medical school I had to apply those skills (because if you can’t apply them, there’s no fit).
My second through fourth year of medical school, I was the only African-American kid in my class. I was spending 20-hour days sometimes just studying, trying to overcompensate by knowing as much as I possibly could and thinking that would propel me from the test-taking standpoint. Even though I didn’t have a lot of classmates in medical school that looked like me, I did have mentors that looked similarly, who really saw potential in me. Dr. Frederick Horvath, a nephrologist in Peoria said, “What are you doing? I want you to get out of these books, and let’s go out to lunch.”
He ended up buying me some instrumental books, really talked to me, listening to my background and understanding how driven I was as a person. He took me under his wing for the rest of medical school and said, “This is how you navigate through these spaces. Yes, you need to have a fund of knowledge to be able to take these tests, but you need to start understanding how to apply it to these questions.” I’m forever grateful to Dr. Horvath for doing that because it was a point in time where I was lost and struggling.
Continue to: Hitting a stride but facing racism head-on...
Hitting a stride but facing racism head-on
Dr. Levy: You talk about the systemic and pervasive racism that was on the wards when you hit them in fourth year. If you don’t mind sharing just a little bit of that, it would help people reading this to have a better understanding of the kinds of barriers that are out there.
Dr. Johnson: Even when I talk about it today, it bothers me.
I went to medical school in Peoria, Illinois, not far from the home of the Ku Klux Klan. At that time, once you got out of Chicago it was a very brutal place, with systemic racism throughout. I was a young Black kid going through a process that not many young Black kids from the South side of Chicago go through, and you had people who had never seen anyone like me. When I was going through my clinical rotations, I knew what I was up against. I was dressed “to the T” every day, arriving early, leaving late, trying to answer questions. I would look at the evaluations, and they would be disparaging. I would look at my counterparts, how their evaluations were, and how people would respond to them, and it would be completely different.
Surgery was the part of ObGyn that I really grew to love more than anything, even more than obstetrics. When general surgery came, I wanted to take it very seriously and learn as much as I possibly could. From the beginning, I knew there was a problem because the chief resident, an older White man, wouldn’t look me in the eye or talk to me. He would make disparaging remarks. The thing that stuck out in my mind the most was when I was in the operating room transporting patients, just like a medical student did, and he came up behind me and said, “You know, Pierre, this is where a small mind and a strong back come into play.” For me, it took me to a place where I had to corral my emotions and thoughts because I just wanted to lash out and just tell him how racist and horrible that was for him to say that to me. I explained this to the powers that be, the director of the department, and they basically blew it off to the side.
When it came down to the end of the evaluation period, I passed with flying colors. But they gave me an incomplete because of that chief resident and his remarks on my evaluations. He had 3 pages of report about me as a person and as a student. He said that he had difficulty in expressing his opinions about me because of possible cultural biases that he may have had. He put “cultural biases” in an evaluation, and they looked at that and said that was enough for me to have to remediate my time. I was required to do an extra month in Pontiac, Illinois, which is even more rural than Peoria, because of a racist person that did not give me a fair opportunity because I was Black.
Like everything else in life, it was a learning experience. It’s why I fight so hard today. It’s why I’m so passionate about equity, not only in medicine but also in all aspects of society. It shows why we have police brutality and Black men dying in the streets. It shows how this happens because there are cultural and implicit biases that play out in every part of life, and we are not honest about it. Until we are honest about it and until we say that this is happening and there is something that needs to be done to address it, it’s going to continue to happen. That is my fight.
Exposing the unspoken power struggle
Dr. Levy: I couldn’t agree more. Attributing things like that to the individual, where you talk about a White man in power and a power structure that didn’t literally physically beat you but did beat you into submission. You talk about how to succeed in medical school, and how you had to suck it up and submit to something that was incredibly unfair. You understood, you were old enough, mature enough, to understand that if you fought back, you were going to lose. The only opportunity you had was to submit to that inequity and push forward.
Dr. Johnson: When I did try to fight, the chair of the department told me that either I accept the consequences or I would not graduate from medical school and be forced to do another year. That struck a chord with me. I think that happens a lot in our society, and it needs to be exposed.
Past experiences reflected in today’s society
Dr. Levy: Can you talk about what you faced in your ObGyn residency in terms of the systemic pushback, people not taking your orders, people questioning you. I know that I have heard that a great deal, and I experienced that myself as a woman.
Dr. Johnson: We look at the things that are happening now, everything from George Floyd’s murder to Colin Kaepernick taking a knee. These things are 10 years past when I first started residency. The year before I started residency, there was a noose hanging on the capitol lawn of Springfield, Illinois’ capital city. There’s systemic racism and hatred there. When I first started on the wards of my first year of ObGyn, again, I was the very first Black resident of my program’s history. Nobody could relate to me.
I went from a year-long general surgery internship at Washington Hospital Center in Washington, DC, to ObGyn residency. In the first 2 months, there were complaints of, “He’s not answering his pages. He’s not being prompt.” I went to my program director and said, “Listen, I have never had one complaint like this. There’s a problem here. And there’s a problem when I’m on the floor: When trying to give orders to nurses, they’re not taking them. I had to tell a couple of nurses, ‘I’m Dr. Johnson. Don’t call me by my first name, especially not in front of patients.’”
My director was just not hearing me, because the entire scenario was something they had never been exposed to. Systemic racism is real, and unless you experience it, it’s very difficult to accept that it is happening. But biases happen when you are not cognizant. People are used to things a certain way. Things play out in the media that make your mind think a certain way, and you don’t even realize it. You may not even want to be that way.
Continue to: Unconscious bias is a barrier to ensuring equity...
Unconscious bias is a barrier to ensuring equity
Dr. Levy: One very important point you just made is that we as the system need to be able to recognize those unconscious things, the language that we use, the disparaging remarks, the things that put people down, as well as the things that keep people out of promotion.
There are some interesting data about both race and gender and the language that we use when we write recommendations for people, that we do things unconsciously. The big message to all of us at the end is to open our minds to where those things can occur. For myself, professionally, I keep a list of words that I use when I write recommendations. I measure myself to ensure that I am using the same language for men and women, for Black and White. I think we need to overcome the system and the structure to create real equity—not equality but equity.
It begins with being real about the issues
Dr. Johnson: It’s a bigger problem than the existence of bias and racism. I think these are systemic issues that have been cultivated over centuries that have never been addressed. The true issue is that we deny that these are problems and refuse to talk about it because it makes us uncomfortable. To truly make things more equitable, we have to push our levels of comfort to be able to talk about things in a healthy manner, be open and transparent, and to start to understand how we are thinking about certain things. When you can see it, you can start to implement changes and start to change mentalities and thought processes.
For me, people say, “You don’t look like a doctor.” I get that all the time—because I have tattoos and earrings. I wear my hair in a mohawk. The image of what success looks like has been manifested through our media and culture, and it has imprinted on our minds as to how things are supposed to be. If someone doesn’t fit those molds, we start to shun them out, or we start to exhibit biases against those things. What I am trying to do is change that thought process of what a successful or a professional person looks like. It doesn’t have a look. It is not a White or Black thing. It’s an intellect, a mindset, a way of living. You have to treat every person as an individual and take all the biases out of it and understand where they are coming from and what they have to offer to the profession.
Dr. Levy: I personally was so impressed by you when I met you. I was impressed by the tattoos and the earrings, and my initial response to them was exactly that biased, “Oh, who is this person?” I checked that at the door, listened to you, and was really impressed at your surgical skill, your knowledge, your background. I am really grateful that you have been willing to spend the time to share that with everyone.
Dr. Johnson: Thank you for this discussion.
To watch the full interview between Drs. Levy and Johnson, visit: https://www.mdedge.com/obgyn/article/228507/facing-systemic-racism-health-care-inequities-medical-education. ●
- The Pulse of Perseverance:
Three Black Doctors on Their Journey to Success Pierre Johnson, MD; Maxime Madhere, MD; and Joseph Semien Jr, MD - Mindset:
The New Psychology of Success
Carol S. Dweck
Finding inspiration among life’s challenges
Barbara Levy, MD: I am fortunate to have met Pierre serendipitously at a training that we were both attending and was impressed by Dr. Johnson’s life story, his passion and commitment, and his dedication—not only to his personal career but also to raising up other young men of color by trying to break down barriers that face them. His life story highlights those areas of systemic and structural problems that all of us together need to address if we are going to make any progress.
Pierre Johnson, MD: Thank you, Barbara. A little about myself: I am a board-certified ObGyn, and I specialize in minimally invasive surgery. I was born on the South side of Chicago, experiencing gang violence, drugs, and substandard, underserved schools. Long story short, I had a very rough upbringing. I had a single mom and several different issues at home. I am the oldest of 5 siblings, and life was tough.
But I knew that I wanted to do something different with my life. I saw that there was a need in my community as far as health care was concerned, in particular women’s health and childbirth. I knew early on that I wanted to be an ObGyn, and the reason had a lot to do with The Cosby Show. It was the only example of a positive, successful Black man that I saw. No one graduated from college in my family. There weren’t any models of young Black excellence around me. Saying that I wanted to be a doctor planted a seed. I was 9 when my mom became pregnant with my first sibling, and it was fascinating to me. The physiology of pregnancy, and eventually childbirth, was extremely fascinating to me; it set me off on my journey to be an ObGyn.
As I got older, things didn’t get any easier. I went to high school in one of the toughest areas on the South side of Chicago. Gang violence, and violence in and of itself, were all around me, but I was able to stay focused. I went on to Xavier University in Louisiana.
Dr. Levy: There are some important things that I learned from your book and from talking to you at our first meeting. Your mom’s ObGyn, when she was pregnant with your next youngest sibling, was also a Black ObGyn. He took some time to take you under wing?
Dr. Johnson: He did. My mom’s ObGyn was a Black man. Other than The Cosby Show, that’s the only time I saw something like that. When I spoke to him, he really took the time to answer my questions and show me that he was like me; he wasn’t just a far-off mythical person, or something that I could not obtain.
Continue to: Seeing is believing when it comes to success...
Seeing is believing when it comes to success
Dr. Levy: Do you think it was important to have a role model who wasn’t a sports star?
Dr. Johnson: If you can’t see it, you can’t achieve it. He took his time to really talk to me, and it’s the little things for kids that go a long way in their life experience. I still have a relationship with him to this day. How he handled me as a kid made me realize that this is something that I could do. That was extremely important for me.
Dr. Levy: One of the structural things I think we need to point out is that the ability to see yourself as someone successful is critical. When we see 1,000 images a day and they are all White, and they are all so different from where we are that it gets incorporated into our sense of being. I think that’s really difficult for those of us of with privilege to understand what that privilege is.
Dr. Johnson: Absolutely, and I’ll even go further. In residency, 2 White females were my classmates, and both of their parents were doctors. They had grandparents who were doctors. My mom was addicted to drugs; my father was not around. They had been talking medicine since they were 5. You have to make things equitable, but in medicine it’s really not equitable. In medicine, what we don’t realize is that there is an importance for all aspects of someone’s upbringing and environment, and it’s not just what they can regurgitate on a standardized test. If a patient can’t relate to you and tell you what is wrong with them, how can you adequately treat them?
Dr. Levy: Even if they are trying to tell me, but I can’t hear it because I don’t have the language and I don’t have the background. There are really good data to show, in fact, that Black male physicians do a better job at engaging Black men to manage their hypertension.1 When we look at the inequities in birth outcomes for women of color, indigenous women and Black women, there’s evidence that providers who come from a similar background do a better job.
Dr. Johnson: There was the study of Black infants that just came out about them dying at a 3-time higher rate in non-Black physicians’ hands.2 These things need to be recognized. They need to be discussed, and they need to be identified as issues and then, realistically, we need to start talking about solutions, not get offended by what actual statistics are saying.
Foundational inequities in education
Dr. Levy: To address some of the barriers that you faced: I know that you went to a high school that was not geared toward pushing students into professional careers. Your colleagues, however, had educations that prepared them for the standardized tests and other things that they would face academically.
Dr. Johnson: People think I am kidding when I say it, but when I went into college, I didn’t know what a periodic table was. I saw it, but I had no idea what these things meant. I didn’t have any sciences or any AP classes in high school. I did well, but grades are smoke and mirrors. The true test of medicine comes with testing. From the MCATs to the boards, every step of the way there is a standardized test.
Knowledge is something that you can obtain, but test taking is a cultivated skill that happens from a very early age. Trying to teach an adult or someone in their late teens a skill that they should have learned as a kid is difficult. For me, I did not have that, so I had to program myself. I had to learn how to fundamentally take tests as an adult, where most people understand how to do that going into college and professional school.
Dr. Levy: I was impressed with your resilience. I think all of us as human beings, if we fail a test, we take it personally and think it’s about our lack of knowledge. One of the insights that you came to was that failure on those things was not that you didn’t study hard enough. In fact, you probably studied 4 times harder than most other people. You had the knowledge. Being able to get that knowledge into a standardized structured test score was the huge challenge for you.
Dr. Johnson: That’s it. I can remember taking the MCAT, and if you looked at the step 1 book, I could regurgitate to you everything on that page. However, it’s not a test about do you know it or not. It’s an understanding of the English language and how to break things down to make things fit into particular scenarios.
Continue to: A college experience focused on growth and exposure...
A college experience focused on growth and exposure
Dr. Levy: I was impressed by the distinction between your experience at Xavier University where there was a lot of support and guidance and help in your premed program, and what happened to you when you hit medical school.
Dr. Johnson: Xavier University in Louisiana is the number 1 institution in the country for getting minorities into professional school. They understand that they have kids that are brilliant but underprepared, and just have not had the background to actually tackle some of these tough curriculums. I always had good grades in school. But by not being challenged, I didn’t know what I didn’t really know. So now that I was seeing biology, chemistry for the first time, and trying to tackle it; there’s a failure point. I didn’t know how to take tests, and I didn’t know how to study properly. The harder I tried, the worse things got for me.
Xavier has seen that story a multitude of times. If I went to a bigger or predominantly White university, a counselor would have told me, “Well, medicine’s maybe not for you. You can’t handle a premed curriculum.” Instead, I said, “Listen, I’m studying. I’m doing all of these things, and I’m not hacking it.” And they broke it down: “Let’s get you into study groups with kids that have had these type of AP classes before. We’ll have you watch how they study,” and everything started to click. That facilitation of how to adjust to this curriculum was a godsend. It’s the only reason I’m here. I am a prime example of being brilliant enough to be able to do it, but needing the infrastructure and a system set up.
Dr. Levy: There’s a great book by Carol Dweck called Mindset that talks about education of young kids and putting them into silos so early in life; the brilliant kids go into the AP courses and the rest are labeled as inadequate. It’s assumed in a fixed mindset based on their heredity and IQ, and not based on the fact that they have not been exposed to the right things.
Xavier was growing you into the man who could, in fact, do all of those things. I think that is one of the systemic and structural issues that we have—that fixed mindset that frames a kid who is not succeeding as therefore unable to succeed, as opposed to framing that child as not having the correct tools.
New tribulations of medical school
Dr. Johnson: Absolutely. I think what Xavier did for me is to at least let me understand what I needed to do, how to comprehend and retain information, which I never had been exposed to before. Those years were very important to establishing a foundation. When going to medical school, it was like, “There’s no more excuses. What could be the problem now?” Well, now let’s talk about taking tests—a whole different skill. Xavier focused on getting me to understand how to structure my thought process and knowledge base. In medical school I had to apply those skills (because if you can’t apply them, there’s no fit).
My second through fourth year of medical school, I was the only African-American kid in my class. I was spending 20-hour days sometimes just studying, trying to overcompensate by knowing as much as I possibly could and thinking that would propel me from the test-taking standpoint. Even though I didn’t have a lot of classmates in medical school that looked like me, I did have mentors that looked similarly, who really saw potential in me. Dr. Frederick Horvath, a nephrologist in Peoria said, “What are you doing? I want you to get out of these books, and let’s go out to lunch.”
He ended up buying me some instrumental books, really talked to me, listening to my background and understanding how driven I was as a person. He took me under his wing for the rest of medical school and said, “This is how you navigate through these spaces. Yes, you need to have a fund of knowledge to be able to take these tests, but you need to start understanding how to apply it to these questions.” I’m forever grateful to Dr. Horvath for doing that because it was a point in time where I was lost and struggling.
Continue to: Hitting a stride but facing racism head-on...
Hitting a stride but facing racism head-on
Dr. Levy: You talk about the systemic and pervasive racism that was on the wards when you hit them in fourth year. If you don’t mind sharing just a little bit of that, it would help people reading this to have a better understanding of the kinds of barriers that are out there.
Dr. Johnson: Even when I talk about it today, it bothers me.
I went to medical school in Peoria, Illinois, not far from the home of the Ku Klux Klan. At that time, once you got out of Chicago it was a very brutal place, with systemic racism throughout. I was a young Black kid going through a process that not many young Black kids from the South side of Chicago go through, and you had people who had never seen anyone like me. When I was going through my clinical rotations, I knew what I was up against. I was dressed “to the T” every day, arriving early, leaving late, trying to answer questions. I would look at the evaluations, and they would be disparaging. I would look at my counterparts, how their evaluations were, and how people would respond to them, and it would be completely different.
Surgery was the part of ObGyn that I really grew to love more than anything, even more than obstetrics. When general surgery came, I wanted to take it very seriously and learn as much as I possibly could. From the beginning, I knew there was a problem because the chief resident, an older White man, wouldn’t look me in the eye or talk to me. He would make disparaging remarks. The thing that stuck out in my mind the most was when I was in the operating room transporting patients, just like a medical student did, and he came up behind me and said, “You know, Pierre, this is where a small mind and a strong back come into play.” For me, it took me to a place where I had to corral my emotions and thoughts because I just wanted to lash out and just tell him how racist and horrible that was for him to say that to me. I explained this to the powers that be, the director of the department, and they basically blew it off to the side.
When it came down to the end of the evaluation period, I passed with flying colors. But they gave me an incomplete because of that chief resident and his remarks on my evaluations. He had 3 pages of report about me as a person and as a student. He said that he had difficulty in expressing his opinions about me because of possible cultural biases that he may have had. He put “cultural biases” in an evaluation, and they looked at that and said that was enough for me to have to remediate my time. I was required to do an extra month in Pontiac, Illinois, which is even more rural than Peoria, because of a racist person that did not give me a fair opportunity because I was Black.
Like everything else in life, it was a learning experience. It’s why I fight so hard today. It’s why I’m so passionate about equity, not only in medicine but also in all aspects of society. It shows why we have police brutality and Black men dying in the streets. It shows how this happens because there are cultural and implicit biases that play out in every part of life, and we are not honest about it. Until we are honest about it and until we say that this is happening and there is something that needs to be done to address it, it’s going to continue to happen. That is my fight.
Exposing the unspoken power struggle
Dr. Levy: I couldn’t agree more. Attributing things like that to the individual, where you talk about a White man in power and a power structure that didn’t literally physically beat you but did beat you into submission. You talk about how to succeed in medical school, and how you had to suck it up and submit to something that was incredibly unfair. You understood, you were old enough, mature enough, to understand that if you fought back, you were going to lose. The only opportunity you had was to submit to that inequity and push forward.
Dr. Johnson: When I did try to fight, the chair of the department told me that either I accept the consequences or I would not graduate from medical school and be forced to do another year. That struck a chord with me. I think that happens a lot in our society, and it needs to be exposed.
Past experiences reflected in today’s society
Dr. Levy: Can you talk about what you faced in your ObGyn residency in terms of the systemic pushback, people not taking your orders, people questioning you. I know that I have heard that a great deal, and I experienced that myself as a woman.
Dr. Johnson: We look at the things that are happening now, everything from George Floyd’s murder to Colin Kaepernick taking a knee. These things are 10 years past when I first started residency. The year before I started residency, there was a noose hanging on the capitol lawn of Springfield, Illinois’ capital city. There’s systemic racism and hatred there. When I first started on the wards of my first year of ObGyn, again, I was the very first Black resident of my program’s history. Nobody could relate to me.
I went from a year-long general surgery internship at Washington Hospital Center in Washington, DC, to ObGyn residency. In the first 2 months, there were complaints of, “He’s not answering his pages. He’s not being prompt.” I went to my program director and said, “Listen, I have never had one complaint like this. There’s a problem here. And there’s a problem when I’m on the floor: When trying to give orders to nurses, they’re not taking them. I had to tell a couple of nurses, ‘I’m Dr. Johnson. Don’t call me by my first name, especially not in front of patients.’”
My director was just not hearing me, because the entire scenario was something they had never been exposed to. Systemic racism is real, and unless you experience it, it’s very difficult to accept that it is happening. But biases happen when you are not cognizant. People are used to things a certain way. Things play out in the media that make your mind think a certain way, and you don’t even realize it. You may not even want to be that way.
Continue to: Unconscious bias is a barrier to ensuring equity...
Unconscious bias is a barrier to ensuring equity
Dr. Levy: One very important point you just made is that we as the system need to be able to recognize those unconscious things, the language that we use, the disparaging remarks, the things that put people down, as well as the things that keep people out of promotion.
There are some interesting data about both race and gender and the language that we use when we write recommendations for people, that we do things unconsciously. The big message to all of us at the end is to open our minds to where those things can occur. For myself, professionally, I keep a list of words that I use when I write recommendations. I measure myself to ensure that I am using the same language for men and women, for Black and White. I think we need to overcome the system and the structure to create real equity—not equality but equity.
It begins with being real about the issues
Dr. Johnson: It’s a bigger problem than the existence of bias and racism. I think these are systemic issues that have been cultivated over centuries that have never been addressed. The true issue is that we deny that these are problems and refuse to talk about it because it makes us uncomfortable. To truly make things more equitable, we have to push our levels of comfort to be able to talk about things in a healthy manner, be open and transparent, and to start to understand how we are thinking about certain things. When you can see it, you can start to implement changes and start to change mentalities and thought processes.
For me, people say, “You don’t look like a doctor.” I get that all the time—because I have tattoos and earrings. I wear my hair in a mohawk. The image of what success looks like has been manifested through our media and culture, and it has imprinted on our minds as to how things are supposed to be. If someone doesn’t fit those molds, we start to shun them out, or we start to exhibit biases against those things. What I am trying to do is change that thought process of what a successful or a professional person looks like. It doesn’t have a look. It is not a White or Black thing. It’s an intellect, a mindset, a way of living. You have to treat every person as an individual and take all the biases out of it and understand where they are coming from and what they have to offer to the profession.
Dr. Levy: I personally was so impressed by you when I met you. I was impressed by the tattoos and the earrings, and my initial response to them was exactly that biased, “Oh, who is this person?” I checked that at the door, listened to you, and was really impressed at your surgical skill, your knowledge, your background. I am really grateful that you have been willing to spend the time to share that with everyone.
Dr. Johnson: Thank you for this discussion.
To watch the full interview between Drs. Levy and Johnson, visit: https://www.mdedge.com/obgyn/article/228507/facing-systemic-racism-health-care-inequities-medical-education. ●
- The Pulse of Perseverance:
Three Black Doctors on Their Journey to Success Pierre Johnson, MD; Maxime Madhere, MD; and Joseph Semien Jr, MD - Mindset:
The New Psychology of Success
Carol S. Dweck
- Benkert R, Peters R, Tate N, et al. Trust of nurse practitioners and physicians among African Americans with hypertension. J Am Acad Nurse Pract. 2008;20:273-280.
- Greenwood BN, Hardeman RR, Huang L, et al. Physician– patient racial concordance and disparities in birthing mortality for newborns. Proc Natl Acad Sci U S A. 2020; 117:21194-21200.
- Benkert R, Peters R, Tate N, et al. Trust of nurse practitioners and physicians among African Americans with hypertension. J Am Acad Nurse Pract. 2008;20:273-280.
- Greenwood BN, Hardeman RR, Huang L, et al. Physician– patient racial concordance and disparities in birthing mortality for newborns. Proc Natl Acad Sci U S A. 2020; 117:21194-21200.
Pessaries for POP and SUI: Your options and guidance on use
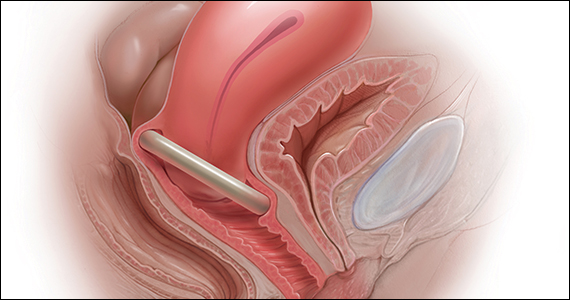
Over the last 30 years, surgical correction of the common condition pelvic organ prolapse (POP) and stress urinary incontinence (SUI) has become so routine and straightforward that many gynecologists and urogynecologists choose surgery as their first choice for treating these conditions, withholding it only from the riskiest patients or from those who, for a variety of reasons, do not choose surgery. Moreover, as generalist gynecologists increasingly refer patients with POP or incontinence to their urogynecologist colleagues, they increasingly lack the skills, or have not been trained, to use conservative treatment strategies for these disorders. Thus, pessaries—devices constructed of inert plastic, silicone, or latex and placed inside the vagina to support prolapsed pelvic structures—frequently are not part of the general gynecologist’s armamentarium.
When properly selected, however, pessaries used for indicated purposes and correctly fitted are an excellent, inexpensive, low-risk, and noninvasive tool that can provide immediate relief not only of POP but also of SUI and defecatory difficulties. As an alternative to surgery, pessaries are especially valuable, because the other major nonsurgical modality for treatment of POP and incontinence—pelvic floor muscle training—often is not covered by insurance (making it expensive for patients), takes many weekly sessions to complete (which can make access challenging), and frequently is not readily available.1
POP is very common. An estimated 15% to 30% of women in North America have some degree of prolapse, and more than 500,000 surgeries for this condition are performed in the United States each year.2 Risk factors for POP include:
- vaginal childbirth, especially higher parity
- advancing age
- high body mass index (BMI)
- prior hysterectomy
- raised intra-abdominal pressure, such as from obesity, chronic cough, or heavy lifting.
In addition to the discomfort caused by the herniation of pelvic and vaginal structures, POP also is associated with urinary incontinence (73%), urinary urgency and frequency (86%), and fecal incontinence (31%).3
Moreover, according to the US Census Bureau, the number of American women aged 65 or older will double to more than 40 million by 2030.4 This will greatly increase the population of women at risk for POP who may be candidates for pessary use. It therefore behooves gynecologists to become familiar with the correct usage, fitting, and maintenance of this effective, nonsurgical mode of treatment for POP.
In this article, I discuss why pessaries are a good option for many patients with POP, review the types of pessaries available, and offer guidance on how to choose the right pessary for an individual patient’s needs. In addition, the box at the end of this article provides an interesting timeline of pessary history dating back to antiquity.
Next month in Part 2 of this article, I cover how to fit a pessary; device aftercare; potential complications of use; and effectiveness of pessaries for POP, SUI, preterm labor prevention, and defecatory disorders.
Continue to: Potential candidates for pessary use...
Potential candidates for pessary use
Almost all women with POP—and in many cases accompanying SUI—are potential candidates for a pessary. In fact, many urogynecologists believe that a trial of pessary usage should be the first treatment modality offered for POP.5 Women who cannot use a pessary include those with an extremely short vagina (<6 cm) and those who have severely eroded vaginal mucosa. In the latter situation, the mucosa can be treated with estrogen cream for several weeks and, once the tissue has healed, a pessary can be fitted.
Given that surgical repair is generally a straightforward, one-time procedure that obviates the need for long-term use of an artificial device worn internally, why might a patient or her physician opt for a pessary instead?
Some of the many reasons include:
- Many patients prefer to avoid surgery.
- Many patients are not appropriate candidates for surgery because they have significant comorbid risk factors or high BMI.
- Patients may have recurrent prolapse or incontinence and wish to avoid repeat surgery.
- Patients with SUI may have heard of the occurrence of mesh erosion and wish to avoid that possibility.
- Women who live in low-resource environments or countries where elective surgical care is relatively unavailable may not have the option of surgery.
A clinician might also recommend pessary use:
- as a diagnostic tool to attempt to assess the potential results of vaginal repair surgery
- to estimate the potential effectiveness of a midurethral sling procedure; several investigators have found this to be approximately as accurate as urodynamic testing6,7
- as prophylaxis for pregnant women with either a history of preterm cervical dilation or a short cervix detected on ultrasonography
- for pregnant women with POP that is worsening and becoming increasingly uncomfortable
- for women with POP who wish to have more children
- for short-term use while a patient is delaying or awaiting POP surgery or to allow time for other medical issues to resolve
- for patients who wish only intermittent, temporary support while exercising or engaging in sports.
Patient acceptance may be contingent on counseling
Numerous studies show that women who choose pessaries to treat POP are generally older than women who elect surgery. Still, patient acceptance of a trial of pessary use depends much on the counseling and information she receives. Properly informed, many patients with POP will opt for a trial of pessary placement. One study showed that, of women with untreated POP, 36% preferred pessary placement to surgery.8 Other investigators reported that when women with symptomatic POP had the benefits of a pessary versus surgery explained to them, nearly two-thirds opted for a pessary as their mode of treatment.9
Exceptions to pessary use
Fortunately, there are relatively few contraindications to pessary use. These are vaginal or pelvic infection and an exposed foreign body in the vagina, such as eroded vaginal mesh. In addition, patients at risk for nonadherence with follow-up care are poor candidates, as it could lead to missing such problems as mucosal erosion, ulceration, or even (extremely rarely) fistula formation. Pessaries may be inappropriate for sexually active women who on their own are unable to remove and reinsert pessary types that do not allow for intercourse while in place (see below).
Continue to: Types of pessaries...
Types of pessaries
The numerous kinds of pessaries available fall into 3 general categories: support, space filling, and lever, and devices within each group have modifications and variations. As with most areas of prescribing and treatment in medicine, it is best to become very familiar with just a few kinds of pessaries, know their indications, and use them when appropriate.
Most pessaries are constructed of inert silicone which, unlike earlier rubber pessaries, does not absorb odor or discharge. They are easy to clean, long lasting, and are autoclavable and hypoallergenic.
Support pessaries
Support pessaries look like contraceptive diaphragms. They are easy to place and remove, are comfortable, and do an excellent job correcting moderate POP. They also can control or eliminate symptoms of SUI by the pressure they exert on the urethra and their alteration of the urethrovesicular angle.
Ring pessaries. The most commonly used type of pessary, the ring pessary,10 comes in 4 variations:
- a simple open ring
- a ring with a web of material, called a “support shield,” that fills the ring
- an open ring with a firm 2-cm “incontinence knob” attached that is positioned over the urethra
- a ring with support shield and incontinence knob.
When in position, the deepest edge of a ring pessary fits behind the cervix (or in the vaginal apex for women who have had a hysterectomy) while the front of the ring slips into place behind the pubic symphysis, just like a diaphragm. When a ring with an incontinence knob is used, the ring is rotated until the knob is directly over the urethra.
Sexual intercourse is possible with any of the ring pessaries in place. Of the various types of pessaries, the ring pessary is the easiest to insert and remove. Some women tie a piece of dental floss to the edge of the ring to make its removal even easier.
The ring pessary is available in sizes 0 (44.5 mm) to 13 (127 mm). For most women a size 3, 4, or 5 ring pessary fits well.
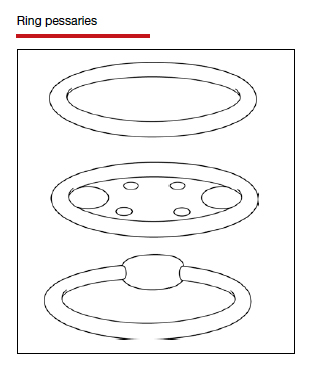
The Marland pessary is similar to the ring pessary with the addition of a wedge-shaped piece of material approximately 3 cm in height that arises from half of the ring. It rarely is used in the United States because most American gynecologists are unfamiliar with it, and there is little evidence that it is more effective than the ring pessary.11
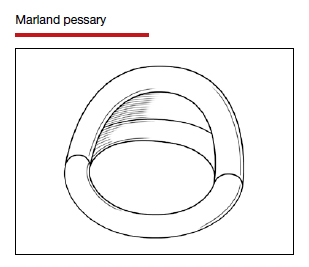
The Shaatz pessary is a rigid round pessary, smaller in diameter than the standard ring pessary, and similar to the Gellhorn pessary (discussed below) but without a stem. It is placed the same way one places a ring pessary but with its concave surface up against the cervix or, if there is no cervix, against the upper anterior vaginal wall. Its main benefit is that it provides firmer support than the ring pessary. This pessary is not widely used in the United States.
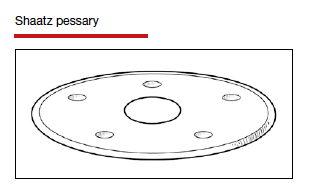
The Gehrung pessary looks like a flat strip of material that has been bent into the shape of a “U.” It is designed to correct severe cystoceles and rectoceles. For insertion, the edges at the open end of the pessary are squeezed together and the pessary is inserted with the closed part of the “U” facing the anterior vaginal wall. The upper edge is advanced until it rests in the anterior fornix of the vagina (or in the vaginal apex in women who have had a hysterectomy). Although it is more efficacious than some other pessaries for control of vaginal wall prolapse, its unfamiliarity to clinicians and its unusual shape result in it being used rarely.
Continue to: Space-filling pessaries...
Space-filling pessaries
Space-filling pessaries are used when more severe degrees of prolapse are present than can be managed by the ring or other support pessaries. This is especially the case when the vagina is so capacious or the introitus so lax that a standard ring pessary cannot be kept in place, resulting in frequent expulsions.
Space-filling pessaries are 3 dimensional and work by filling the vagina with a relatively large object that prevents the cervix/vaginal apex from dropping down and the vaginal walls from prolapsing. They have a special role for women who:
- are posthysterectomy and have an enterocele and/or vaginal apex prolapse
- have significant rectoceles for which support pessaries are not effective
- have a wide vaginal hiatus and thus are prone to expel support pessaries.
Space-filling pessaries do have some drawbacks compared with support pessaries. For example, they do not help in controlling SUI, and they are difficult for patients to remove on their own for cleaning. In addition, sexual intercourse is impossible with a space-filling pessary in place.
The Gellhorn pessary is the most common of the space-filling pessaries, and it is the one gynecologists and urogynecologists most often use for severe prolapse. It has a concave disc that fits up against the cervix or vaginal apex and a solid stem that points down the vagina. The stem itself is supported by the perineal body. It offers excellent support for severe uterine and vaginal wall prolapse, as long as the perineal body is intact. The stem stabilizes the disc portion of the pessary and prevents pessary expulsion. Gellhorn pessaries are available with long or short stems.
The Gellhorn is inserted into the vagina by folding the stem 90 degrees until it is in the same plane as the disc. With lubricated fingers, the patient’s perineal body is depressed and the disc of the pessary is folded and slid in. The disc is then placed up against the cervix or vaginal apex with the stem pointing down the vagina and tucked just inside the posterior edge of the introitus.
Removing the Gellhorn pessary can be problematic and is difficult for patients to do on their own. Clinicians often must use a ring forceps to grasp the stem of the pessary in order to bring it into the lower vagina, where the stem is folded up against the disc and the entire pessary removed. As with all space-filling pessaries, the Gellhorn must be taken out prior to intercourse.
The Gellhorn pessary is available in sizes that range from a disc diameter of 1.5 to 3.75 inches. Those measuring 2.5, 2.75, or 3 inches are used most commonly.
The cube pessary is a soft, dice-shaped piece of silicone with an indentation in each of its 6 sides. It is used for severe prolapse.
Squeezing the cube allows for easier insertion into the vagina; once it is at the top of the vagina, the cube expands back to its normal shape. The indentations on each side of the cube attach to the vaginal walls with moderate suction, which helps to keep the pessary in place. Because of the suction, the cube pessary can be used in cases of severe prolapse when other pessaries will not stay in place; a drawback is that the suction created by the indented sides can cause vaginal mucosal erosion.10 Ideally, the cube pessary should be removed every night for cleansing as discharge and accompanying odor can accumulate. The string attached to the cube pessary aids in its removal.
The cube pessary is available in sizes 0 to 7, with edge lengths that range from 1 to 2.25 inches.
The donut pessary, as its name suggests, has the form of a large donut. It can be compressed slightly to help with insertion. Because it occupies a large space within the vagina, it is used (like the cube pessary) for treatment of severe prolapse. The size and shape of the donut pessary, however, can make it difficult for patients to insert and take out on their own.
The donut pessary is available in sizes 0 (51 mm) to 8 (95 mm).
The inflatable pessary has the same basic shape as the donut pessary and serves the same purpose: It acts as a large semisoft object that fills the vagina to support the vaginal walls and cervix (or vaginal apex) in cases of severe prolapse. The inflatable pessary differs in that it has a valve on a stem through which air can be inserted and removed. This allows the uninflated pessary to be placed relatively easily into the vagina and then pumped full of air to the dimensions necessary to prevent vaginal, cervical, uterine, or apex prolapse. Air likewise can be removed to facilitate pessary removal.
One drawback of the inflatable pessary is that it is made of latex and thus cannot be used by anyone with a latex allergy. Also, as latex retains discharge and odors, this pessary should be removed and washed daily.
The inflatable pessary is available in sizes that range from 2 to 2.75 inches in 0.25-inch increments.
Continue to: Space-filling pessaries...
Lever pessaries
In addition to the more commonly used support and space-filling pessaries, there is a third kind that is rarely used in current practice: the lever pessaries. These pessaries—the Hodge, the Smith, and the Risser—are rectangles made of inert plastic that are folded into 3 planes to facilitate positioning in the vagina. The narrower of the 2 shorter ends of the folded rectangle is placed behind the cervix or at the vaginal apex while the other short end is placed behind the symphysis pubis.
Although sometimes used to correct POP in nonpregnant women, the lever pessary’s main purpose is to antivert a retroflexed uterus and to support the cervix and uterus in cases of prolapse during pregnancy or impending cervical incompetence.
The 3 lever pessaries differ in terms of whether the narrow ends of the pessary are straight or curved and wider or narrower.
How to choose the right pessary for your patient
If a patient’s POP or urinary incontinence symptoms would best be treated with a pessary, the next step is to select the pessary type and size best suited for that patient’s needs and the size that should be prescribed. While there is controversy among experts as to whether or not certain pessaries are better than others for different indications,12 most gynecologists and urogynecologists who use pessaries on a regular basis agree on the following:
1. Support pessaries will meet the needs of most women with moderate POP and/or SUI. These include the ring pessary with or without the support shield and with or without an incontinence knob. A support pessary is the go-to pessary in most cases. Most women find it comfortable to wear, it is easy to put in and take out, and sexual intercourse is possible with the pessary in place.
2. The specific degree of a patient’s prolapse and/or incontinence dictates whether or not to prescribe the support shield feature or the incontinence knob with a ring pessary. The shield helps support a prolapsed cervix and uterus when they are present.5,13 The knob is a useful feature if incontinence is a prominent symptom.
3. The Gellhorn pessary is usually the first choice for more severe prolapse. As long as there is some degree of posterior perineal support, this pessary does an excellent job of correcting even severe prolapse whether of a cervix and uterus or of vaginal walls and apex. It does require the patient to have some practice and dexterity for inserting and removing it on her own; individuals not comfortable or physically able to do so will need to have the pessary removed and cleaned by a clinician on a regular basis in the office. (Part 2 of this article will discuss pessary cleansing intervals).
4. Space-filling pessaries (such as the cube and donut) are useful when there is a severe degree of prolapse and insufficient perineal support to maintain a Gellhorn pessary. In practice, they are generally used less frequently—which is unfortunate, as they are a potentially useful solution for older women with severe prolapse who might not be candidates for surgical repair. As mentioned, both the cube and donut pessaries require more frequent removal for cleaning.
5. In unusual cases, the use of 2 pessaries simultaneously may resolve a difficult problem, such as when a pessary is the only option for treatment, the prolapse is severe, or it is impossible to find a pessary that resists being expelled from the vagina.14 A space-filling pessary in the most cephalad aspect of the vagina used in conjunction with a ring pessary with support shield below it can sometimes resolve even the worst cases of prolapse.
Continue to: Stay tuned...
Stay tuned
Part 2 of this article next month will provide more information on pessaries, including fitting, aftercare, potential complications, and effectiveness in various disorders. ●
Pessaries have been used in one form or another to help resolve pelvic organ prolapse (POP) in women for at least 2,500 years. They have come in many shapes and have been made of many materials. Here is a brief sketch of the history of the pessary.
Antiquity
Kahun papyrus (ancient Egypt, c. 2000 BCE)
Women with POP were made to stand over a fire in which different ingredients were burned. It was thought that the disagreeable odors emitted would cause the uterus to “rebel” and thus revert back into place.1
Hippocrates (c. 460–375 BCE)
Used several techniques to resolve uterine prolapse:
- Tipping the woman upside down and shaking her, using gravity as an aid to return the prolapsed organs into the pelvis2
- Cupping of the buttocks and the lower abdomen in hopes of “sucking” the prolapsed uterus back into place3
The Greek physician Polybus (c. 400 BCE)
Placed half a pomegranate in the vagina to hold prolapsed structures in place2
Cleopatra (c. 70–30 BCE)
Treated prolapse with the vaginal application of an astringent liquid2
Celsus (c. 25 BCE–50 CE)
Used cone-shaped pessaries made of bronze with a perforated circular plate on the lower edge through which bands were attached. The bands were then tied around the body to keep the device in place4
The Greek physician Soranus (c. 98–138)
Utilized linen tampons soaked with vinegar—along with a piece of beef—to treat prolapse. These were then held in place by bands passed around the loins2
Galen (c. 130–210)
Used fumigation to “encourage” the uterus to return to the pelvis2
Middle Ages
Paulus of Aegina (c. 625–690) and Abbas (c. 949–982)
Both wrote about the use of pessaries made of wax3
Myrepsus (late 13th century)
Described the preparation of 45 types of pessaries consisting of different solid materials treated with perfumes, wax, honey, and herbs5
16th century
Caspar Stromayr (Practica Copiosa, 1559)
Used as pessaries tightly rolled sponges bound with string, dipped in wax, and covered with oil or butter6
Ambroise Paré (c. 1510–1590)
Developed the first ring-type pessary in the late 16th century. He used hammered brass and waxed cork in the shape of an oval to treat uterine prolapse. He also made ring-shaped devices of gold, silver, or brass which were kept in place by a belt around the waist.7
17th century de Castro (1546–1627)
Urged “attacking” uterine prolapse with application of a red-hot iron thus “frightening it” into receding back into the vagina8
Hendrik van Roonhuyse (1625–1672)
In his gynecology textbook, discussed the etiology and treatment of prolapse. He utilized a cork with a hole in it (to allow for passage of discharge) as prolapse treatment. He also wrote of removing an obstructed wax pessary that had blocked discharge of a patient’s vaginal secretions for many years4
18th century Thomas Simson (1696-1764)
Invented a metal spring device that kept a pessary made of cork in place9
John Leake (1729-1792)
Recommended the use of sponges as pessaries to avoid vaginal prolapse10
Juville (1783)
Was the first to use rubber pessaries, resembling today’s contraceptive cup, to avoid injuring the vaginal mucosa. The center of the cup was perforated with a gold tip which allowed for the discharge of vaginal secretions10
19th century
Scanzoni (1821-1891)
Recommended massage and the application of leeches to reduce local congestion and swelling of prolapsed pelvic organs before manual replacement11
Hugh Lenox Hodge (1796-1873)
In his 1860 textbook Diseases Peculiar to Women, Hodge discussed at length the use of pessaries for uterine displacement. He suggested that metals, alloys, glass, and porcelain be used for pessaries rather than cork, wax, and sponges12
20th century
1950s—
Pessaries made of rubber, which absorb discharge and odor, are replaced by polystyrene pessaries. Currently, pessaries are made of silicone, plastic, and latex.
References
- Stevens JM. Gynecology from ancient Egypt: the papyrus Kahun, a translation of the oldest treatise on gynecology that has survived from the ancient world. Med J Austr. 1975;2:949-952.
- Emge LA, Durfee RB. Pelvic organ prolapse: four thousand years of treatment. Clin Obstet Gynecol. 1966;9:997-1032.
- Van Dongen L. The anatomy of genital prolapse. South Afr Med J. 1981;60:357-359.
- Cianfrani T. Short History of Obstetrics and Gynecology. Springfield, IL: Charles C Thomas; 1960.
- Leonardo RA. History of Gynecology. New York, NY: Froben Press; 1944.
- Tizzano AP, Muffly TM. Historical milestones in female pelvic surgery, gynecology, and female urology. In: Walters M, Karram M. Urogynecology and Reconstructive Pelvic Surgery, 4th ed. Philadelphia, PA: Elsevier Saunders; 2015
- Farrell SA. Pessaries in Clinical Practice. Switzerland: Springer-Verlag; 2006.
- Tam T, Davies MF, eds. Vaginal Pessaries. Boca Raton, FL: CRC Press; 2019.
- Ricci JV. Genealogy of Gynaecology. Philadelphia, PA: Blakiston; 1950.
- Miller DS. Contemporary use of the pessary. In Sciarra JJ, ed. Gynecology and Obstetrics. Philadelphia, PA: JB Lippincott Company; 1995.
- Thomas TG. A Practical Treatise on the Disorders of Women. Philadelphia, PA: Lea Brothers and Co; 1891.
- Hodge HL. Diseases Peculiar to Women, Including Displacements of the Uterus. Philadelphia, PA: Blanchard and Lea; 1860.
- Zoorob D, Higgins M, Swan K, et al. Barriers to pelvic floor physical therapy regarding treatment of high-tone pelvic floor dysfunction. Female Pelvic Med Reconstr Surg. 2017;23:444-448.
- Kirby AC, Luber KM, Menefee SA. An update on the current and future demand for care of pelvic floor disorders in the United States. Am J Obstet Gynecol. 2013;209:584.e1-584.e5.
- Ellerkmann RM, Cundiff GW, Melick CF, et al. Correlation of symptoms with location and severity of pelvic organ prolapse. Am J Obstet Gynecol. 2001;185:1332-1337.
- US Census Bureau. United States population projections: 2000 to 2050. https://www.census.gov/library/workingpapers/2009/demo/us-pop-proj-2000-2050.html. Accessed November 13, 2020.
- Pott-Grinstein E, Newcomer JR. Gynecologists’ patterns of prescribing pessaries. J Reprod Med. 2001;46:205-208.
- Chaikin DC, Groutz A, Blaivas JG. Predicting the need for anti-incontinence surgery in continent women undergoing repair of severe urogenital prolapse. J Urol. 2000;163:531-534.
- Reena C, Kekre AN, Kekre N. Occult stress incontinence in women with pelvic organ prolapse. Int J Gynaecol Obstet. 2007;97:31-34.
- Thys SD, Roovers JP, Geomini PM, et al. Do patients prefer a pessary or surgery as primary treatment for pelvic organ prolapse. Gynecol Obstet Invest. 2012;74:6-12.
- Kapoor DS, Thakar R, Sultan AH, et al. Conservative versus surgical management of prolapse: what dictates patient choice? Int Urogynecol J Pelvic Floor Dysfunct. 2009;20: 1157-1161.
- Wu V, Farrel SA, Baskett TF, et al. A simplified protocol for pessary management. Obstet Gynecol. 1997;90:990-994.
- Culligan PJ. Nonsurgical management of pelvic organ prolapse. Obstet Gynecol. 2012;119:852-860.
- Cundiff GW, Amundsen CL, Bent AE, et al. The PESSRI study: symptom relief outcomes of a randomized crossover trial of the ring and Gellhorn pessaries. Am J Obstet Gynecol. 2007;196:405.e1-404.e8.
- Cundiff GW, Weidner AC, Visco AG, et al. A survey of pessary use by members of the American Urogynecologic Society. Obstet Gynecol. 2000;95(6 pt 1):931-935.
- Singh K, Reid W. Nonsurgical treatment of uterovaginal prolapse using double vaginal rings. Br J Obstet Gynecol. 2001;108:112-113.

Over the last 30 years, surgical correction of the common condition pelvic organ prolapse (POP) and stress urinary incontinence (SUI) has become so routine and straightforward that many gynecologists and urogynecologists choose surgery as their first choice for treating these conditions, withholding it only from the riskiest patients or from those who, for a variety of reasons, do not choose surgery. Moreover, as generalist gynecologists increasingly refer patients with POP or incontinence to their urogynecologist colleagues, they increasingly lack the skills, or have not been trained, to use conservative treatment strategies for these disorders. Thus, pessaries—devices constructed of inert plastic, silicone, or latex and placed inside the vagina to support prolapsed pelvic structures—frequently are not part of the general gynecologist’s armamentarium.
When properly selected, however, pessaries used for indicated purposes and correctly fitted are an excellent, inexpensive, low-risk, and noninvasive tool that can provide immediate relief not only of POP but also of SUI and defecatory difficulties. As an alternative to surgery, pessaries are especially valuable, because the other major nonsurgical modality for treatment of POP and incontinence—pelvic floor muscle training—often is not covered by insurance (making it expensive for patients), takes many weekly sessions to complete (which can make access challenging), and frequently is not readily available.1
POP is very common. An estimated 15% to 30% of women in North America have some degree of prolapse, and more than 500,000 surgeries for this condition are performed in the United States each year.2 Risk factors for POP include:
- vaginal childbirth, especially higher parity
- advancing age
- high body mass index (BMI)
- prior hysterectomy
- raised intra-abdominal pressure, such as from obesity, chronic cough, or heavy lifting.
In addition to the discomfort caused by the herniation of pelvic and vaginal structures, POP also is associated with urinary incontinence (73%), urinary urgency and frequency (86%), and fecal incontinence (31%).3
Moreover, according to the US Census Bureau, the number of American women aged 65 or older will double to more than 40 million by 2030.4 This will greatly increase the population of women at risk for POP who may be candidates for pessary use. It therefore behooves gynecologists to become familiar with the correct usage, fitting, and maintenance of this effective, nonsurgical mode of treatment for POP.
In this article, I discuss why pessaries are a good option for many patients with POP, review the types of pessaries available, and offer guidance on how to choose the right pessary for an individual patient’s needs. In addition, the box at the end of this article provides an interesting timeline of pessary history dating back to antiquity.
Next month in Part 2 of this article, I cover how to fit a pessary; device aftercare; potential complications of use; and effectiveness of pessaries for POP, SUI, preterm labor prevention, and defecatory disorders.
Continue to: Potential candidates for pessary use...
Potential candidates for pessary use
Almost all women with POP—and in many cases accompanying SUI—are potential candidates for a pessary. In fact, many urogynecologists believe that a trial of pessary usage should be the first treatment modality offered for POP.5 Women who cannot use a pessary include those with an extremely short vagina (<6 cm) and those who have severely eroded vaginal mucosa. In the latter situation, the mucosa can be treated with estrogen cream for several weeks and, once the tissue has healed, a pessary can be fitted.
Given that surgical repair is generally a straightforward, one-time procedure that obviates the need for long-term use of an artificial device worn internally, why might a patient or her physician opt for a pessary instead?
Some of the many reasons include:
- Many patients prefer to avoid surgery.
- Many patients are not appropriate candidates for surgery because they have significant comorbid risk factors or high BMI.
- Patients may have recurrent prolapse or incontinence and wish to avoid repeat surgery.
- Patients with SUI may have heard of the occurrence of mesh erosion and wish to avoid that possibility.
- Women who live in low-resource environments or countries where elective surgical care is relatively unavailable may not have the option of surgery.
A clinician might also recommend pessary use:
- as a diagnostic tool to attempt to assess the potential results of vaginal repair surgery
- to estimate the potential effectiveness of a midurethral sling procedure; several investigators have found this to be approximately as accurate as urodynamic testing6,7
- as prophylaxis for pregnant women with either a history of preterm cervical dilation or a short cervix detected on ultrasonography
- for pregnant women with POP that is worsening and becoming increasingly uncomfortable
- for women with POP who wish to have more children
- for short-term use while a patient is delaying or awaiting POP surgery or to allow time for other medical issues to resolve
- for patients who wish only intermittent, temporary support while exercising or engaging in sports.
Patient acceptance may be contingent on counseling
Numerous studies show that women who choose pessaries to treat POP are generally older than women who elect surgery. Still, patient acceptance of a trial of pessary use depends much on the counseling and information she receives. Properly informed, many patients with POP will opt for a trial of pessary placement. One study showed that, of women with untreated POP, 36% preferred pessary placement to surgery.8 Other investigators reported that when women with symptomatic POP had the benefits of a pessary versus surgery explained to them, nearly two-thirds opted for a pessary as their mode of treatment.9
Exceptions to pessary use
Fortunately, there are relatively few contraindications to pessary use. These are vaginal or pelvic infection and an exposed foreign body in the vagina, such as eroded vaginal mesh. In addition, patients at risk for nonadherence with follow-up care are poor candidates, as it could lead to missing such problems as mucosal erosion, ulceration, or even (extremely rarely) fistula formation. Pessaries may be inappropriate for sexually active women who on their own are unable to remove and reinsert pessary types that do not allow for intercourse while in place (see below).
Continue to: Types of pessaries...
Types of pessaries
The numerous kinds of pessaries available fall into 3 general categories: support, space filling, and lever, and devices within each group have modifications and variations. As with most areas of prescribing and treatment in medicine, it is best to become very familiar with just a few kinds of pessaries, know their indications, and use them when appropriate.
Most pessaries are constructed of inert silicone which, unlike earlier rubber pessaries, does not absorb odor or discharge. They are easy to clean, long lasting, and are autoclavable and hypoallergenic.
Support pessaries
Support pessaries look like contraceptive diaphragms. They are easy to place and remove, are comfortable, and do an excellent job correcting moderate POP. They also can control or eliminate symptoms of SUI by the pressure they exert on the urethra and their alteration of the urethrovesicular angle.
Ring pessaries. The most commonly used type of pessary, the ring pessary,10 comes in 4 variations:
- a simple open ring
- a ring with a web of material, called a “support shield,” that fills the ring
- an open ring with a firm 2-cm “incontinence knob” attached that is positioned over the urethra
- a ring with support shield and incontinence knob.
When in position, the deepest edge of a ring pessary fits behind the cervix (or in the vaginal apex for women who have had a hysterectomy) while the front of the ring slips into place behind the pubic symphysis, just like a diaphragm. When a ring with an incontinence knob is used, the ring is rotated until the knob is directly over the urethra.
Sexual intercourse is possible with any of the ring pessaries in place. Of the various types of pessaries, the ring pessary is the easiest to insert and remove. Some women tie a piece of dental floss to the edge of the ring to make its removal even easier.
The ring pessary is available in sizes 0 (44.5 mm) to 13 (127 mm). For most women a size 3, 4, or 5 ring pessary fits well.

The Marland pessary is similar to the ring pessary with the addition of a wedge-shaped piece of material approximately 3 cm in height that arises from half of the ring. It rarely is used in the United States because most American gynecologists are unfamiliar with it, and there is little evidence that it is more effective than the ring pessary.11

The Shaatz pessary is a rigid round pessary, smaller in diameter than the standard ring pessary, and similar to the Gellhorn pessary (discussed below) but without a stem. It is placed the same way one places a ring pessary but with its concave surface up against the cervix or, if there is no cervix, against the upper anterior vaginal wall. Its main benefit is that it provides firmer support than the ring pessary. This pessary is not widely used in the United States.

The Gehrung pessary looks like a flat strip of material that has been bent into the shape of a “U.” It is designed to correct severe cystoceles and rectoceles. For insertion, the edges at the open end of the pessary are squeezed together and the pessary is inserted with the closed part of the “U” facing the anterior vaginal wall. The upper edge is advanced until it rests in the anterior fornix of the vagina (or in the vaginal apex in women who have had a hysterectomy). Although it is more efficacious than some other pessaries for control of vaginal wall prolapse, its unfamiliarity to clinicians and its unusual shape result in it being used rarely.

Continue to: Space-filling pessaries...
Space-filling pessaries
Space-filling pessaries are used when more severe degrees of prolapse are present than can be managed by the ring or other support pessaries. This is especially the case when the vagina is so capacious or the introitus so lax that a standard ring pessary cannot be kept in place, resulting in frequent expulsions.
Space-filling pessaries are 3 dimensional and work by filling the vagina with a relatively large object that prevents the cervix/vaginal apex from dropping down and the vaginal walls from prolapsing. They have a special role for women who:
- are posthysterectomy and have an enterocele and/or vaginal apex prolapse
- have significant rectoceles for which support pessaries are not effective
- have a wide vaginal hiatus and thus are prone to expel support pessaries.
Space-filling pessaries do have some drawbacks compared with support pessaries. For example, they do not help in controlling SUI, and they are difficult for patients to remove on their own for cleaning. In addition, sexual intercourse is impossible with a space-filling pessary in place.
The Gellhorn pessary is the most common of the space-filling pessaries, and it is the one gynecologists and urogynecologists most often use for severe prolapse. It has a concave disc that fits up against the cervix or vaginal apex and a solid stem that points down the vagina. The stem itself is supported by the perineal body. It offers excellent support for severe uterine and vaginal wall prolapse, as long as the perineal body is intact. The stem stabilizes the disc portion of the pessary and prevents pessary expulsion. Gellhorn pessaries are available with long or short stems.
The Gellhorn is inserted into the vagina by folding the stem 90 degrees until it is in the same plane as the disc. With lubricated fingers, the patient’s perineal body is depressed and the disc of the pessary is folded and slid in. The disc is then placed up against the cervix or vaginal apex with the stem pointing down the vagina and tucked just inside the posterior edge of the introitus.
Removing the Gellhorn pessary can be problematic and is difficult for patients to do on their own. Clinicians often must use a ring forceps to grasp the stem of the pessary in order to bring it into the lower vagina, where the stem is folded up against the disc and the entire pessary removed. As with all space-filling pessaries, the Gellhorn must be taken out prior to intercourse.
The Gellhorn pessary is available in sizes that range from a disc diameter of 1.5 to 3.75 inches. Those measuring 2.5, 2.75, or 3 inches are used most commonly.

The cube pessary is a soft, dice-shaped piece of silicone with an indentation in each of its 6 sides. It is used for severe prolapse.
Squeezing the cube allows for easier insertion into the vagina; once it is at the top of the vagina, the cube expands back to its normal shape. The indentations on each side of the cube attach to the vaginal walls with moderate suction, which helps to keep the pessary in place. Because of the suction, the cube pessary can be used in cases of severe prolapse when other pessaries will not stay in place; a drawback is that the suction created by the indented sides can cause vaginal mucosal erosion.10 Ideally, the cube pessary should be removed every night for cleansing as discharge and accompanying odor can accumulate. The string attached to the cube pessary aids in its removal.
The cube pessary is available in sizes 0 to 7, with edge lengths that range from 1 to 2.25 inches.

The donut pessary, as its name suggests, has the form of a large donut. It can be compressed slightly to help with insertion. Because it occupies a large space within the vagina, it is used (like the cube pessary) for treatment of severe prolapse. The size and shape of the donut pessary, however, can make it difficult for patients to insert and take out on their own.
The donut pessary is available in sizes 0 (51 mm) to 8 (95 mm).

The inflatable pessary has the same basic shape as the donut pessary and serves the same purpose: It acts as a large semisoft object that fills the vagina to support the vaginal walls and cervix (or vaginal apex) in cases of severe prolapse. The inflatable pessary differs in that it has a valve on a stem through which air can be inserted and removed. This allows the uninflated pessary to be placed relatively easily into the vagina and then pumped full of air to the dimensions necessary to prevent vaginal, cervical, uterine, or apex prolapse. Air likewise can be removed to facilitate pessary removal.
One drawback of the inflatable pessary is that it is made of latex and thus cannot be used by anyone with a latex allergy. Also, as latex retains discharge and odors, this pessary should be removed and washed daily.
The inflatable pessary is available in sizes that range from 2 to 2.75 inches in 0.25-inch increments.

Continue to: Space-filling pessaries...
Lever pessaries
In addition to the more commonly used support and space-filling pessaries, there is a third kind that is rarely used in current practice: the lever pessaries. These pessaries—the Hodge, the Smith, and the Risser—are rectangles made of inert plastic that are folded into 3 planes to facilitate positioning in the vagina. The narrower of the 2 shorter ends of the folded rectangle is placed behind the cervix or at the vaginal apex while the other short end is placed behind the symphysis pubis.
Although sometimes used to correct POP in nonpregnant women, the lever pessary’s main purpose is to antivert a retroflexed uterus and to support the cervix and uterus in cases of prolapse during pregnancy or impending cervical incompetence.
The 3 lever pessaries differ in terms of whether the narrow ends of the pessary are straight or curved and wider or narrower.
How to choose the right pessary for your patient
If a patient’s POP or urinary incontinence symptoms would best be treated with a pessary, the next step is to select the pessary type and size best suited for that patient’s needs and the size that should be prescribed. While there is controversy among experts as to whether or not certain pessaries are better than others for different indications,12 most gynecologists and urogynecologists who use pessaries on a regular basis agree on the following:
1. Support pessaries will meet the needs of most women with moderate POP and/or SUI. These include the ring pessary with or without the support shield and with or without an incontinence knob. A support pessary is the go-to pessary in most cases. Most women find it comfortable to wear, it is easy to put in and take out, and sexual intercourse is possible with the pessary in place.
2. The specific degree of a patient’s prolapse and/or incontinence dictates whether or not to prescribe the support shield feature or the incontinence knob with a ring pessary. The shield helps support a prolapsed cervix and uterus when they are present.5,13 The knob is a useful feature if incontinence is a prominent symptom.
3. The Gellhorn pessary is usually the first choice for more severe prolapse. As long as there is some degree of posterior perineal support, this pessary does an excellent job of correcting even severe prolapse whether of a cervix and uterus or of vaginal walls and apex. It does require the patient to have some practice and dexterity for inserting and removing it on her own; individuals not comfortable or physically able to do so will need to have the pessary removed and cleaned by a clinician on a regular basis in the office. (Part 2 of this article will discuss pessary cleansing intervals).
4. Space-filling pessaries (such as the cube and donut) are useful when there is a severe degree of prolapse and insufficient perineal support to maintain a Gellhorn pessary. In practice, they are generally used less frequently—which is unfortunate, as they are a potentially useful solution for older women with severe prolapse who might not be candidates for surgical repair. As mentioned, both the cube and donut pessaries require more frequent removal for cleaning.
5. In unusual cases, the use of 2 pessaries simultaneously may resolve a difficult problem, such as when a pessary is the only option for treatment, the prolapse is severe, or it is impossible to find a pessary that resists being expelled from the vagina.14 A space-filling pessary in the most cephalad aspect of the vagina used in conjunction with a ring pessary with support shield below it can sometimes resolve even the worst cases of prolapse.
Continue to: Stay tuned...
Stay tuned
Part 2 of this article next month will provide more information on pessaries, including fitting, aftercare, potential complications, and effectiveness in various disorders. ●
Pessaries have been used in one form or another to help resolve pelvic organ prolapse (POP) in women for at least 2,500 years. They have come in many shapes and have been made of many materials. Here is a brief sketch of the history of the pessary.
Antiquity
Kahun papyrus (ancient Egypt, c. 2000 BCE)
Women with POP were made to stand over a fire in which different ingredients were burned. It was thought that the disagreeable odors emitted would cause the uterus to “rebel” and thus revert back into place.1
Hippocrates (c. 460–375 BCE)
Used several techniques to resolve uterine prolapse:
- Tipping the woman upside down and shaking her, using gravity as an aid to return the prolapsed organs into the pelvis2
- Cupping of the buttocks and the lower abdomen in hopes of “sucking” the prolapsed uterus back into place3
The Greek physician Polybus (c. 400 BCE)
Placed half a pomegranate in the vagina to hold prolapsed structures in place2
Cleopatra (c. 70–30 BCE)
Treated prolapse with the vaginal application of an astringent liquid2
Celsus (c. 25 BCE–50 CE)
Used cone-shaped pessaries made of bronze with a perforated circular plate on the lower edge through which bands were attached. The bands were then tied around the body to keep the device in place4
The Greek physician Soranus (c. 98–138)
Utilized linen tampons soaked with vinegar—along with a piece of beef—to treat prolapse. These were then held in place by bands passed around the loins2
Galen (c. 130–210)
Used fumigation to “encourage” the uterus to return to the pelvis2
Middle Ages
Paulus of Aegina (c. 625–690) and Abbas (c. 949–982)
Both wrote about the use of pessaries made of wax3
Myrepsus (late 13th century)
Described the preparation of 45 types of pessaries consisting of different solid materials treated with perfumes, wax, honey, and herbs5
16th century
Caspar Stromayr (Practica Copiosa, 1559)
Used as pessaries tightly rolled sponges bound with string, dipped in wax, and covered with oil or butter6
Ambroise Paré (c. 1510–1590)
Developed the first ring-type pessary in the late 16th century. He used hammered brass and waxed cork in the shape of an oval to treat uterine prolapse. He also made ring-shaped devices of gold, silver, or brass which were kept in place by a belt around the waist.7
17th century de Castro (1546–1627)
Urged “attacking” uterine prolapse with application of a red-hot iron thus “frightening it” into receding back into the vagina8
Hendrik van Roonhuyse (1625–1672)
In his gynecology textbook, discussed the etiology and treatment of prolapse. He utilized a cork with a hole in it (to allow for passage of discharge) as prolapse treatment. He also wrote of removing an obstructed wax pessary that had blocked discharge of a patient’s vaginal secretions for many years4
18th century Thomas Simson (1696-1764)
Invented a metal spring device that kept a pessary made of cork in place9
John Leake (1729-1792)
Recommended the use of sponges as pessaries to avoid vaginal prolapse10
Juville (1783)
Was the first to use rubber pessaries, resembling today’s contraceptive cup, to avoid injuring the vaginal mucosa. The center of the cup was perforated with a gold tip which allowed for the discharge of vaginal secretions10
19th century
Scanzoni (1821-1891)
Recommended massage and the application of leeches to reduce local congestion and swelling of prolapsed pelvic organs before manual replacement11
Hugh Lenox Hodge (1796-1873)
In his 1860 textbook Diseases Peculiar to Women, Hodge discussed at length the use of pessaries for uterine displacement. He suggested that metals, alloys, glass, and porcelain be used for pessaries rather than cork, wax, and sponges12
20th century
1950s—
Pessaries made of rubber, which absorb discharge and odor, are replaced by polystyrene pessaries. Currently, pessaries are made of silicone, plastic, and latex.
References
- Stevens JM. Gynecology from ancient Egypt: the papyrus Kahun, a translation of the oldest treatise on gynecology that has survived from the ancient world. Med J Austr. 1975;2:949-952.
- Emge LA, Durfee RB. Pelvic organ prolapse: four thousand years of treatment. Clin Obstet Gynecol. 1966;9:997-1032.
- Van Dongen L. The anatomy of genital prolapse. South Afr Med J. 1981;60:357-359.
- Cianfrani T. Short History of Obstetrics and Gynecology. Springfield, IL: Charles C Thomas; 1960.
- Leonardo RA. History of Gynecology. New York, NY: Froben Press; 1944.
- Tizzano AP, Muffly TM. Historical milestones in female pelvic surgery, gynecology, and female urology. In: Walters M, Karram M. Urogynecology and Reconstructive Pelvic Surgery, 4th ed. Philadelphia, PA: Elsevier Saunders; 2015
- Farrell SA. Pessaries in Clinical Practice. Switzerland: Springer-Verlag; 2006.
- Tam T, Davies MF, eds. Vaginal Pessaries. Boca Raton, FL: CRC Press; 2019.
- Ricci JV. Genealogy of Gynaecology. Philadelphia, PA: Blakiston; 1950.
- Miller DS. Contemporary use of the pessary. In Sciarra JJ, ed. Gynecology and Obstetrics. Philadelphia, PA: JB Lippincott Company; 1995.
- Thomas TG. A Practical Treatise on the Disorders of Women. Philadelphia, PA: Lea Brothers and Co; 1891.
- Hodge HL. Diseases Peculiar to Women, Including Displacements of the Uterus. Philadelphia, PA: Blanchard and Lea; 1860.

Over the last 30 years, surgical correction of the common condition pelvic organ prolapse (POP) and stress urinary incontinence (SUI) has become so routine and straightforward that many gynecologists and urogynecologists choose surgery as their first choice for treating these conditions, withholding it only from the riskiest patients or from those who, for a variety of reasons, do not choose surgery. Moreover, as generalist gynecologists increasingly refer patients with POP or incontinence to their urogynecologist colleagues, they increasingly lack the skills, or have not been trained, to use conservative treatment strategies for these disorders. Thus, pessaries—devices constructed of inert plastic, silicone, or latex and placed inside the vagina to support prolapsed pelvic structures—frequently are not part of the general gynecologist’s armamentarium.
When properly selected, however, pessaries used for indicated purposes and correctly fitted are an excellent, inexpensive, low-risk, and noninvasive tool that can provide immediate relief not only of POP but also of SUI and defecatory difficulties. As an alternative to surgery, pessaries are especially valuable, because the other major nonsurgical modality for treatment of POP and incontinence—pelvic floor muscle training—often is not covered by insurance (making it expensive for patients), takes many weekly sessions to complete (which can make access challenging), and frequently is not readily available.1
POP is very common. An estimated 15% to 30% of women in North America have some degree of prolapse, and more than 500,000 surgeries for this condition are performed in the United States each year.2 Risk factors for POP include:
- vaginal childbirth, especially higher parity
- advancing age
- high body mass index (BMI)
- prior hysterectomy
- raised intra-abdominal pressure, such as from obesity, chronic cough, or heavy lifting.
In addition to the discomfort caused by the herniation of pelvic and vaginal structures, POP also is associated with urinary incontinence (73%), urinary urgency and frequency (86%), and fecal incontinence (31%).3
Moreover, according to the US Census Bureau, the number of American women aged 65 or older will double to more than 40 million by 2030.4 This will greatly increase the population of women at risk for POP who may be candidates for pessary use. It therefore behooves gynecologists to become familiar with the correct usage, fitting, and maintenance of this effective, nonsurgical mode of treatment for POP.
In this article, I discuss why pessaries are a good option for many patients with POP, review the types of pessaries available, and offer guidance on how to choose the right pessary for an individual patient’s needs. In addition, the box at the end of this article provides an interesting timeline of pessary history dating back to antiquity.
Next month in Part 2 of this article, I cover how to fit a pessary; device aftercare; potential complications of use; and effectiveness of pessaries for POP, SUI, preterm labor prevention, and defecatory disorders.
Continue to: Potential candidates for pessary use...
Potential candidates for pessary use
Almost all women with POP—and in many cases accompanying SUI—are potential candidates for a pessary. In fact, many urogynecologists believe that a trial of pessary usage should be the first treatment modality offered for POP.5 Women who cannot use a pessary include those with an extremely short vagina (<6 cm) and those who have severely eroded vaginal mucosa. In the latter situation, the mucosa can be treated with estrogen cream for several weeks and, once the tissue has healed, a pessary can be fitted.
Given that surgical repair is generally a straightforward, one-time procedure that obviates the need for long-term use of an artificial device worn internally, why might a patient or her physician opt for a pessary instead?
Some of the many reasons include:
- Many patients prefer to avoid surgery.
- Many patients are not appropriate candidates for surgery because they have significant comorbid risk factors or high BMI.
- Patients may have recurrent prolapse or incontinence and wish to avoid repeat surgery.
- Patients with SUI may have heard of the occurrence of mesh erosion and wish to avoid that possibility.
- Women who live in low-resource environments or countries where elective surgical care is relatively unavailable may not have the option of surgery.
A clinician might also recommend pessary use:
- as a diagnostic tool to attempt to assess the potential results of vaginal repair surgery
- to estimate the potential effectiveness of a midurethral sling procedure; several investigators have found this to be approximately as accurate as urodynamic testing6,7
- as prophylaxis for pregnant women with either a history of preterm cervical dilation or a short cervix detected on ultrasonography
- for pregnant women with POP that is worsening and becoming increasingly uncomfortable
- for women with POP who wish to have more children
- for short-term use while a patient is delaying or awaiting POP surgery or to allow time for other medical issues to resolve
- for patients who wish only intermittent, temporary support while exercising or engaging in sports.
Patient acceptance may be contingent on counseling
Numerous studies show that women who choose pessaries to treat POP are generally older than women who elect surgery. Still, patient acceptance of a trial of pessary use depends much on the counseling and information she receives. Properly informed, many patients with POP will opt for a trial of pessary placement. One study showed that, of women with untreated POP, 36% preferred pessary placement to surgery.8 Other investigators reported that when women with symptomatic POP had the benefits of a pessary versus surgery explained to them, nearly two-thirds opted for a pessary as their mode of treatment.9
Exceptions to pessary use
Fortunately, there are relatively few contraindications to pessary use. These are vaginal or pelvic infection and an exposed foreign body in the vagina, such as eroded vaginal mesh. In addition, patients at risk for nonadherence with follow-up care are poor candidates, as it could lead to missing such problems as mucosal erosion, ulceration, or even (extremely rarely) fistula formation. Pessaries may be inappropriate for sexually active women who on their own are unable to remove and reinsert pessary types that do not allow for intercourse while in place (see below).
Continue to: Types of pessaries...
Types of pessaries
The numerous kinds of pessaries available fall into 3 general categories: support, space filling, and lever, and devices within each group have modifications and variations. As with most areas of prescribing and treatment in medicine, it is best to become very familiar with just a few kinds of pessaries, know their indications, and use them when appropriate.
Most pessaries are constructed of inert silicone which, unlike earlier rubber pessaries, does not absorb odor or discharge. They are easy to clean, long lasting, and are autoclavable and hypoallergenic.
Support pessaries
Support pessaries look like contraceptive diaphragms. They are easy to place and remove, are comfortable, and do an excellent job correcting moderate POP. They also can control or eliminate symptoms of SUI by the pressure they exert on the urethra and their alteration of the urethrovesicular angle.
Ring pessaries. The most commonly used type of pessary, the ring pessary,10 comes in 4 variations:
- a simple open ring
- a ring with a web of material, called a “support shield,” that fills the ring
- an open ring with a firm 2-cm “incontinence knob” attached that is positioned over the urethra
- a ring with support shield and incontinence knob.
When in position, the deepest edge of a ring pessary fits behind the cervix (or in the vaginal apex for women who have had a hysterectomy) while the front of the ring slips into place behind the pubic symphysis, just like a diaphragm. When a ring with an incontinence knob is used, the ring is rotated until the knob is directly over the urethra.
Sexual intercourse is possible with any of the ring pessaries in place. Of the various types of pessaries, the ring pessary is the easiest to insert and remove. Some women tie a piece of dental floss to the edge of the ring to make its removal even easier.
The ring pessary is available in sizes 0 (44.5 mm) to 13 (127 mm). For most women a size 3, 4, or 5 ring pessary fits well.

The Marland pessary is similar to the ring pessary with the addition of a wedge-shaped piece of material approximately 3 cm in height that arises from half of the ring. It rarely is used in the United States because most American gynecologists are unfamiliar with it, and there is little evidence that it is more effective than the ring pessary.11

The Shaatz pessary is a rigid round pessary, smaller in diameter than the standard ring pessary, and similar to the Gellhorn pessary (discussed below) but without a stem. It is placed the same way one places a ring pessary but with its concave surface up against the cervix or, if there is no cervix, against the upper anterior vaginal wall. Its main benefit is that it provides firmer support than the ring pessary. This pessary is not widely used in the United States.

The Gehrung pessary looks like a flat strip of material that has been bent into the shape of a “U.” It is designed to correct severe cystoceles and rectoceles. For insertion, the edges at the open end of the pessary are squeezed together and the pessary is inserted with the closed part of the “U” facing the anterior vaginal wall. The upper edge is advanced until it rests in the anterior fornix of the vagina (or in the vaginal apex in women who have had a hysterectomy). Although it is more efficacious than some other pessaries for control of vaginal wall prolapse, its unfamiliarity to clinicians and its unusual shape result in it being used rarely.

Continue to: Space-filling pessaries...
Space-filling pessaries
Space-filling pessaries are used when more severe degrees of prolapse are present than can be managed by the ring or other support pessaries. This is especially the case when the vagina is so capacious or the introitus so lax that a standard ring pessary cannot be kept in place, resulting in frequent expulsions.
Space-filling pessaries are 3 dimensional and work by filling the vagina with a relatively large object that prevents the cervix/vaginal apex from dropping down and the vaginal walls from prolapsing. They have a special role for women who:
- are posthysterectomy and have an enterocele and/or vaginal apex prolapse
- have significant rectoceles for which support pessaries are not effective
- have a wide vaginal hiatus and thus are prone to expel support pessaries.
Space-filling pessaries do have some drawbacks compared with support pessaries. For example, they do not help in controlling SUI, and they are difficult for patients to remove on their own for cleaning. In addition, sexual intercourse is impossible with a space-filling pessary in place.
The Gellhorn pessary is the most common of the space-filling pessaries, and it is the one gynecologists and urogynecologists most often use for severe prolapse. It has a concave disc that fits up against the cervix or vaginal apex and a solid stem that points down the vagina. The stem itself is supported by the perineal body. It offers excellent support for severe uterine and vaginal wall prolapse, as long as the perineal body is intact. The stem stabilizes the disc portion of the pessary and prevents pessary expulsion. Gellhorn pessaries are available with long or short stems.
The Gellhorn is inserted into the vagina by folding the stem 90 degrees until it is in the same plane as the disc. With lubricated fingers, the patient’s perineal body is depressed and the disc of the pessary is folded and slid in. The disc is then placed up against the cervix or vaginal apex with the stem pointing down the vagina and tucked just inside the posterior edge of the introitus.
Removing the Gellhorn pessary can be problematic and is difficult for patients to do on their own. Clinicians often must use a ring forceps to grasp the stem of the pessary in order to bring it into the lower vagina, where the stem is folded up against the disc and the entire pessary removed. As with all space-filling pessaries, the Gellhorn must be taken out prior to intercourse.
The Gellhorn pessary is available in sizes that range from a disc diameter of 1.5 to 3.75 inches. Those measuring 2.5, 2.75, or 3 inches are used most commonly.

The cube pessary is a soft, dice-shaped piece of silicone with an indentation in each of its 6 sides. It is used for severe prolapse.
Squeezing the cube allows for easier insertion into the vagina; once it is at the top of the vagina, the cube expands back to its normal shape. The indentations on each side of the cube attach to the vaginal walls with moderate suction, which helps to keep the pessary in place. Because of the suction, the cube pessary can be used in cases of severe prolapse when other pessaries will not stay in place; a drawback is that the suction created by the indented sides can cause vaginal mucosal erosion.10 Ideally, the cube pessary should be removed every night for cleansing as discharge and accompanying odor can accumulate. The string attached to the cube pessary aids in its removal.
The cube pessary is available in sizes 0 to 7, with edge lengths that range from 1 to 2.25 inches.

The donut pessary, as its name suggests, has the form of a large donut. It can be compressed slightly to help with insertion. Because it occupies a large space within the vagina, it is used (like the cube pessary) for treatment of severe prolapse. The size and shape of the donut pessary, however, can make it difficult for patients to insert and take out on their own.
The donut pessary is available in sizes 0 (51 mm) to 8 (95 mm).

The inflatable pessary has the same basic shape as the donut pessary and serves the same purpose: It acts as a large semisoft object that fills the vagina to support the vaginal walls and cervix (or vaginal apex) in cases of severe prolapse. The inflatable pessary differs in that it has a valve on a stem through which air can be inserted and removed. This allows the uninflated pessary to be placed relatively easily into the vagina and then pumped full of air to the dimensions necessary to prevent vaginal, cervical, uterine, or apex prolapse. Air likewise can be removed to facilitate pessary removal.
One drawback of the inflatable pessary is that it is made of latex and thus cannot be used by anyone with a latex allergy. Also, as latex retains discharge and odors, this pessary should be removed and washed daily.
The inflatable pessary is available in sizes that range from 2 to 2.75 inches in 0.25-inch increments.

Continue to: Space-filling pessaries...
Lever pessaries
In addition to the more commonly used support and space-filling pessaries, there is a third kind that is rarely used in current practice: the lever pessaries. These pessaries—the Hodge, the Smith, and the Risser—are rectangles made of inert plastic that are folded into 3 planes to facilitate positioning in the vagina. The narrower of the 2 shorter ends of the folded rectangle is placed behind the cervix or at the vaginal apex while the other short end is placed behind the symphysis pubis.
Although sometimes used to correct POP in nonpregnant women, the lever pessary’s main purpose is to antivert a retroflexed uterus and to support the cervix and uterus in cases of prolapse during pregnancy or impending cervical incompetence.
The 3 lever pessaries differ in terms of whether the narrow ends of the pessary are straight or curved and wider or narrower.
How to choose the right pessary for your patient
If a patient’s POP or urinary incontinence symptoms would best be treated with a pessary, the next step is to select the pessary type and size best suited for that patient’s needs and the size that should be prescribed. While there is controversy among experts as to whether or not certain pessaries are better than others for different indications,12 most gynecologists and urogynecologists who use pessaries on a regular basis agree on the following:
1. Support pessaries will meet the needs of most women with moderate POP and/or SUI. These include the ring pessary with or without the support shield and with or without an incontinence knob. A support pessary is the go-to pessary in most cases. Most women find it comfortable to wear, it is easy to put in and take out, and sexual intercourse is possible with the pessary in place.
2. The specific degree of a patient’s prolapse and/or incontinence dictates whether or not to prescribe the support shield feature or the incontinence knob with a ring pessary. The shield helps support a prolapsed cervix and uterus when they are present.5,13 The knob is a useful feature if incontinence is a prominent symptom.
3. The Gellhorn pessary is usually the first choice for more severe prolapse. As long as there is some degree of posterior perineal support, this pessary does an excellent job of correcting even severe prolapse whether of a cervix and uterus or of vaginal walls and apex. It does require the patient to have some practice and dexterity for inserting and removing it on her own; individuals not comfortable or physically able to do so will need to have the pessary removed and cleaned by a clinician on a regular basis in the office. (Part 2 of this article will discuss pessary cleansing intervals).
4. Space-filling pessaries (such as the cube and donut) are useful when there is a severe degree of prolapse and insufficient perineal support to maintain a Gellhorn pessary. In practice, they are generally used less frequently—which is unfortunate, as they are a potentially useful solution for older women with severe prolapse who might not be candidates for surgical repair. As mentioned, both the cube and donut pessaries require more frequent removal for cleaning.
5. In unusual cases, the use of 2 pessaries simultaneously may resolve a difficult problem, such as when a pessary is the only option for treatment, the prolapse is severe, or it is impossible to find a pessary that resists being expelled from the vagina.14 A space-filling pessary in the most cephalad aspect of the vagina used in conjunction with a ring pessary with support shield below it can sometimes resolve even the worst cases of prolapse.
Continue to: Stay tuned...
Stay tuned
Part 2 of this article next month will provide more information on pessaries, including fitting, aftercare, potential complications, and effectiveness in various disorders. ●
Pessaries have been used in one form or another to help resolve pelvic organ prolapse (POP) in women for at least 2,500 years. They have come in many shapes and have been made of many materials. Here is a brief sketch of the history of the pessary.
Antiquity
Kahun papyrus (ancient Egypt, c. 2000 BCE)
Women with POP were made to stand over a fire in which different ingredients were burned. It was thought that the disagreeable odors emitted would cause the uterus to “rebel” and thus revert back into place.1
Hippocrates (c. 460–375 BCE)
Used several techniques to resolve uterine prolapse:
- Tipping the woman upside down and shaking her, using gravity as an aid to return the prolapsed organs into the pelvis2
- Cupping of the buttocks and the lower abdomen in hopes of “sucking” the prolapsed uterus back into place3
The Greek physician Polybus (c. 400 BCE)
Placed half a pomegranate in the vagina to hold prolapsed structures in place2
Cleopatra (c. 70–30 BCE)
Treated prolapse with the vaginal application of an astringent liquid2
Celsus (c. 25 BCE–50 CE)
Used cone-shaped pessaries made of bronze with a perforated circular plate on the lower edge through which bands were attached. The bands were then tied around the body to keep the device in place4
The Greek physician Soranus (c. 98–138)
Utilized linen tampons soaked with vinegar—along with a piece of beef—to treat prolapse. These were then held in place by bands passed around the loins2
Galen (c. 130–210)
Used fumigation to “encourage” the uterus to return to the pelvis2
Middle Ages
Paulus of Aegina (c. 625–690) and Abbas (c. 949–982)
Both wrote about the use of pessaries made of wax3
Myrepsus (late 13th century)
Described the preparation of 45 types of pessaries consisting of different solid materials treated with perfumes, wax, honey, and herbs5
16th century
Caspar Stromayr (Practica Copiosa, 1559)
Used as pessaries tightly rolled sponges bound with string, dipped in wax, and covered with oil or butter6
Ambroise Paré (c. 1510–1590)
Developed the first ring-type pessary in the late 16th century. He used hammered brass and waxed cork in the shape of an oval to treat uterine prolapse. He also made ring-shaped devices of gold, silver, or brass which were kept in place by a belt around the waist.7
17th century de Castro (1546–1627)
Urged “attacking” uterine prolapse with application of a red-hot iron thus “frightening it” into receding back into the vagina8
Hendrik van Roonhuyse (1625–1672)
In his gynecology textbook, discussed the etiology and treatment of prolapse. He utilized a cork with a hole in it (to allow for passage of discharge) as prolapse treatment. He also wrote of removing an obstructed wax pessary that had blocked discharge of a patient’s vaginal secretions for many years4
18th century Thomas Simson (1696-1764)
Invented a metal spring device that kept a pessary made of cork in place9
John Leake (1729-1792)
Recommended the use of sponges as pessaries to avoid vaginal prolapse10
Juville (1783)
Was the first to use rubber pessaries, resembling today’s contraceptive cup, to avoid injuring the vaginal mucosa. The center of the cup was perforated with a gold tip which allowed for the discharge of vaginal secretions10
19th century
Scanzoni (1821-1891)
Recommended massage and the application of leeches to reduce local congestion and swelling of prolapsed pelvic organs before manual replacement11
Hugh Lenox Hodge (1796-1873)
In his 1860 textbook Diseases Peculiar to Women, Hodge discussed at length the use of pessaries for uterine displacement. He suggested that metals, alloys, glass, and porcelain be used for pessaries rather than cork, wax, and sponges12
20th century
1950s—
Pessaries made of rubber, which absorb discharge and odor, are replaced by polystyrene pessaries. Currently, pessaries are made of silicone, plastic, and latex.
References
- Stevens JM. Gynecology from ancient Egypt: the papyrus Kahun, a translation of the oldest treatise on gynecology that has survived from the ancient world. Med J Austr. 1975;2:949-952.
- Emge LA, Durfee RB. Pelvic organ prolapse: four thousand years of treatment. Clin Obstet Gynecol. 1966;9:997-1032.
- Van Dongen L. The anatomy of genital prolapse. South Afr Med J. 1981;60:357-359.
- Cianfrani T. Short History of Obstetrics and Gynecology. Springfield, IL: Charles C Thomas; 1960.
- Leonardo RA. History of Gynecology. New York, NY: Froben Press; 1944.
- Tizzano AP, Muffly TM. Historical milestones in female pelvic surgery, gynecology, and female urology. In: Walters M, Karram M. Urogynecology and Reconstructive Pelvic Surgery, 4th ed. Philadelphia, PA: Elsevier Saunders; 2015
- Farrell SA. Pessaries in Clinical Practice. Switzerland: Springer-Verlag; 2006.
- Tam T, Davies MF, eds. Vaginal Pessaries. Boca Raton, FL: CRC Press; 2019.
- Ricci JV. Genealogy of Gynaecology. Philadelphia, PA: Blakiston; 1950.
- Miller DS. Contemporary use of the pessary. In Sciarra JJ, ed. Gynecology and Obstetrics. Philadelphia, PA: JB Lippincott Company; 1995.
- Thomas TG. A Practical Treatise on the Disorders of Women. Philadelphia, PA: Lea Brothers and Co; 1891.
- Hodge HL. Diseases Peculiar to Women, Including Displacements of the Uterus. Philadelphia, PA: Blanchard and Lea; 1860.
- Zoorob D, Higgins M, Swan K, et al. Barriers to pelvic floor physical therapy regarding treatment of high-tone pelvic floor dysfunction. Female Pelvic Med Reconstr Surg. 2017;23:444-448.
- Kirby AC, Luber KM, Menefee SA. An update on the current and future demand for care of pelvic floor disorders in the United States. Am J Obstet Gynecol. 2013;209:584.e1-584.e5.
- Ellerkmann RM, Cundiff GW, Melick CF, et al. Correlation of symptoms with location and severity of pelvic organ prolapse. Am J Obstet Gynecol. 2001;185:1332-1337.
- US Census Bureau. United States population projections: 2000 to 2050. https://www.census.gov/library/workingpapers/2009/demo/us-pop-proj-2000-2050.html. Accessed November 13, 2020.
- Pott-Grinstein E, Newcomer JR. Gynecologists’ patterns of prescribing pessaries. J Reprod Med. 2001;46:205-208.
- Chaikin DC, Groutz A, Blaivas JG. Predicting the need for anti-incontinence surgery in continent women undergoing repair of severe urogenital prolapse. J Urol. 2000;163:531-534.
- Reena C, Kekre AN, Kekre N. Occult stress incontinence in women with pelvic organ prolapse. Int J Gynaecol Obstet. 2007;97:31-34.
- Thys SD, Roovers JP, Geomini PM, et al. Do patients prefer a pessary or surgery as primary treatment for pelvic organ prolapse. Gynecol Obstet Invest. 2012;74:6-12.
- Kapoor DS, Thakar R, Sultan AH, et al. Conservative versus surgical management of prolapse: what dictates patient choice? Int Urogynecol J Pelvic Floor Dysfunct. 2009;20: 1157-1161.
- Wu V, Farrel SA, Baskett TF, et al. A simplified protocol for pessary management. Obstet Gynecol. 1997;90:990-994.
- Culligan PJ. Nonsurgical management of pelvic organ prolapse. Obstet Gynecol. 2012;119:852-860.
- Cundiff GW, Amundsen CL, Bent AE, et al. The PESSRI study: symptom relief outcomes of a randomized crossover trial of the ring and Gellhorn pessaries. Am J Obstet Gynecol. 2007;196:405.e1-404.e8.
- Cundiff GW, Weidner AC, Visco AG, et al. A survey of pessary use by members of the American Urogynecologic Society. Obstet Gynecol. 2000;95(6 pt 1):931-935.
- Singh K, Reid W. Nonsurgical treatment of uterovaginal prolapse using double vaginal rings. Br J Obstet Gynecol. 2001;108:112-113.
- Zoorob D, Higgins M, Swan K, et al. Barriers to pelvic floor physical therapy regarding treatment of high-tone pelvic floor dysfunction. Female Pelvic Med Reconstr Surg. 2017;23:444-448.
- Kirby AC, Luber KM, Menefee SA. An update on the current and future demand for care of pelvic floor disorders in the United States. Am J Obstet Gynecol. 2013;209:584.e1-584.e5.
- Ellerkmann RM, Cundiff GW, Melick CF, et al. Correlation of symptoms with location and severity of pelvic organ prolapse. Am J Obstet Gynecol. 2001;185:1332-1337.
- US Census Bureau. United States population projections: 2000 to 2050. https://www.census.gov/library/workingpapers/2009/demo/us-pop-proj-2000-2050.html. Accessed November 13, 2020.
- Pott-Grinstein E, Newcomer JR. Gynecologists’ patterns of prescribing pessaries. J Reprod Med. 2001;46:205-208.
- Chaikin DC, Groutz A, Blaivas JG. Predicting the need for anti-incontinence surgery in continent women undergoing repair of severe urogenital prolapse. J Urol. 2000;163:531-534.
- Reena C, Kekre AN, Kekre N. Occult stress incontinence in women with pelvic organ prolapse. Int J Gynaecol Obstet. 2007;97:31-34.
- Thys SD, Roovers JP, Geomini PM, et al. Do patients prefer a pessary or surgery as primary treatment for pelvic organ prolapse. Gynecol Obstet Invest. 2012;74:6-12.
- Kapoor DS, Thakar R, Sultan AH, et al. Conservative versus surgical management of prolapse: what dictates patient choice? Int Urogynecol J Pelvic Floor Dysfunct. 2009;20: 1157-1161.
- Wu V, Farrel SA, Baskett TF, et al. A simplified protocol for pessary management. Obstet Gynecol. 1997;90:990-994.
- Culligan PJ. Nonsurgical management of pelvic organ prolapse. Obstet Gynecol. 2012;119:852-860.
- Cundiff GW, Amundsen CL, Bent AE, et al. The PESSRI study: symptom relief outcomes of a randomized crossover trial of the ring and Gellhorn pessaries. Am J Obstet Gynecol. 2007;196:405.e1-404.e8.
- Cundiff GW, Weidner AC, Visco AG, et al. A survey of pessary use by members of the American Urogynecologic Society. Obstet Gynecol. 2000;95(6 pt 1):931-935.
- Singh K, Reid W. Nonsurgical treatment of uterovaginal prolapse using double vaginal rings. Br J Obstet Gynecol. 2001;108:112-113.