User login
Hospitalist movers and shakers – November 2020
Erin Shaughnessy, MD, assumed the role of director of pediatric hospital medicine at the University of Alabama at Birmingham and Children’s of Alabama, also in Birmingham, on Sept. 1. Dr. Shaughnessy has done research in improving outcomes in hospitalized children, as well as improving communication between physicians and pediatric patients’ families during care transitions.
Prior to joining UAB and Children’s of Alabama, Dr. Shaughnessy was division chief of hospital medicine at Phoenix (Ariz.) Children’s Hospital while also serving as an associate professor at the University of Arizona, Phoenix.
Chandra Lingisetty, MD, MBA, MHCM, was recently named chief administrative officer for Baptist Health Physician Partners, Arkansas. BHPP is Baptist Health’s clinically integrated network (CIN) with more than 1,600 providers across the state.
Baptist Health Arkansas is the state’s largest not for profit health system with 12 hospitals, hundreds of provider clinics, a nursing school, and a graduate medical education residency program. Prior to his promotion, he worked in Baptist Health System as a hospitalist for 10 years, served on the board of managers at BHPP, and strategized COVID-19 care management protocols and medical staff preparedness as part of surge planning and capacity expansion. In his new role, he is focused on leading the clinically integrated network toward value-based care. He is also the cofounder and inaugural president of the Arkansas state chapter of the Society of Hospital Medicine.
Grace Farris, MD, recently accepted a position with the division of hospital medicine at the Dell Medical School in Austin where she will be an assistant professor of internal medicine, as well as a working hospitalist.
Dr. Farris worked as chief of hospital medicine at Mount Sinai West Hospital in Manhattan from January 2017 until accepting her new position with Dell. In addition, she publishes a monthly comics column in the Annals of Internal Medicine.
Her visual storytelling through comics has appeared in several media outlets, and she has penned literal columns as well, including one recently in the New York Times about living apart from her children while treating COVID-19 patients in the emergency room.
Dell Medical School has also named a new division chief of hospital medicine. Read Pierce, MD, made the move to Texas from the University of Colorado at Denver, Aurora. Dr. Pierce will also serve as associate chair of faculty development of internal medicine at Dell. He is eager to build on his experience and passion for developing people, creating outstanding culture, and changing complex systems in innovative, sustainable ways.
Dr. Pierce worked at University of Colorado for the past 8 years, serving as the associate director of the school’s Institute for Healthcare Quality, Safety and Efficiency (IHQSE), a program he co-founded. Prior to that, Dr. Pierce was chief resident at the University of San Francisco medical school and later founded the hospital medicine center at the San Francisco VA Medical Center.
Gurinder Kaur, MD, was recently named medical director of the Health Hospitalist Program at St. Joseph’s Health Rome (N.Y.) Memorial Hospital. Dr. Kaur’s focus will be on improving infrastructure to allow for the highest quality of care possible. She will oversee the facility’s crew of eight hospitalists, who rotate to be available 24 hours per day.
Dr. Kaur comes to Rome from St. Joseph’s Health in Syracuse, N.Y., where she was chief resident and a member of the hospitalist team.
Colin McMahon, MD, was recently appointed chief of hospital medicine at Eastern Niagara Hospital in Lockport, N.Y., where he will oversee the hospitalist program. He comes to ENH after serving as medical director of hospital operations at Buffalo (N.Y.) General Medical Center.
Dr. McMahon has worked in medicine for a quarter of a century. He also is the president and founder of Dimensions of Internal Medicine and Pediatric Care, PC (DMP). Associates from DMP Medicine make up the hospitalist team at ENH.
Sam Antonios, MD, has been promoted to chief clinical officer of Ascension Kansas, the parent group of Ascension Via Christi Hospital in Wichita, where Dr. Antonios has served as chief medical officer for the past 4 years. Dr. Antonios has emerged as a leader within Ascension Kansas during the COVID-19 pandemic.
Prior to his appointment at Via Christi, he worked at that facility as a hospitalist and as medical director of information systems. Dr. Antonios is a board-certified internist.
Bret J. Rudy, MD, was named a Top 25 Healthcare Innovator by Modern Healthcare magazine. Dr. Rudy is chief of hospital operations and senior vice president at NYU Langone Hospital-Brooklyn in New York, and the magazine cited his efforts in elevating the quality, safety, and accountability of the facility, which merged with NYU Langone Health in 2016.
Dr. Rudy has established a 24-hour hospitalist service, added full-time emergency faculty, and reduced hospital wait times, among other patient-experience benchmarks, since his appointment at Langone-Brooklyn.
Dr. Rudy is a board-certified pediatrician who has served on the National Institutes of Health’s HIV research networks, including a spot on the White House Advisory Committee on Adolescents for the Office of National AIDS Policy.
Nasim Afsar, MD, MBA, SFHM, a past president of SHM, was recently named chief operating officer at UCI Health in Orange, Calif. Dr. Afsar served previously as chief ambulatory officer and chief medical officer for accountable care organizations at UCI Health.
Anthony J. Macchiavelli, MD, FHM, was recognized by Continental Who’s Who as a “Top Distinguished Hospitalist” with AtlantiCare Regional Medical Center, in Atlantic City, N.J. He has been with AtlantiCare for the past ten years, and currently serves as medical director for the PACE program, as well as medical director for the Anticoagulation Clinic.
Dr. Macchiavelli has been involved in the development of 3 different hospital medicine programs throughout his career and was the founder of the Associates in Hospital Medicine program at Methodist Division of Thomas Jefferson University Hospitals. He serves as a mentor for SHM’s VTE-FAST Program, and has served on the Standards Review Panel for the Joint Commission developing the National Patient Safety Goal for anticoagulation therapy.
Erin Shaughnessy, MD, assumed the role of director of pediatric hospital medicine at the University of Alabama at Birmingham and Children’s of Alabama, also in Birmingham, on Sept. 1. Dr. Shaughnessy has done research in improving outcomes in hospitalized children, as well as improving communication between physicians and pediatric patients’ families during care transitions.
Prior to joining UAB and Children’s of Alabama, Dr. Shaughnessy was division chief of hospital medicine at Phoenix (Ariz.) Children’s Hospital while also serving as an associate professor at the University of Arizona, Phoenix.
Chandra Lingisetty, MD, MBA, MHCM, was recently named chief administrative officer for Baptist Health Physician Partners, Arkansas. BHPP is Baptist Health’s clinically integrated network (CIN) with more than 1,600 providers across the state.
Baptist Health Arkansas is the state’s largest not for profit health system with 12 hospitals, hundreds of provider clinics, a nursing school, and a graduate medical education residency program. Prior to his promotion, he worked in Baptist Health System as a hospitalist for 10 years, served on the board of managers at BHPP, and strategized COVID-19 care management protocols and medical staff preparedness as part of surge planning and capacity expansion. In his new role, he is focused on leading the clinically integrated network toward value-based care. He is also the cofounder and inaugural president of the Arkansas state chapter of the Society of Hospital Medicine.
Grace Farris, MD, recently accepted a position with the division of hospital medicine at the Dell Medical School in Austin where she will be an assistant professor of internal medicine, as well as a working hospitalist.
Dr. Farris worked as chief of hospital medicine at Mount Sinai West Hospital in Manhattan from January 2017 until accepting her new position with Dell. In addition, she publishes a monthly comics column in the Annals of Internal Medicine.
Her visual storytelling through comics has appeared in several media outlets, and she has penned literal columns as well, including one recently in the New York Times about living apart from her children while treating COVID-19 patients in the emergency room.
Dell Medical School has also named a new division chief of hospital medicine. Read Pierce, MD, made the move to Texas from the University of Colorado at Denver, Aurora. Dr. Pierce will also serve as associate chair of faculty development of internal medicine at Dell. He is eager to build on his experience and passion for developing people, creating outstanding culture, and changing complex systems in innovative, sustainable ways.
Dr. Pierce worked at University of Colorado for the past 8 years, serving as the associate director of the school’s Institute for Healthcare Quality, Safety and Efficiency (IHQSE), a program he co-founded. Prior to that, Dr. Pierce was chief resident at the University of San Francisco medical school and later founded the hospital medicine center at the San Francisco VA Medical Center.
Gurinder Kaur, MD, was recently named medical director of the Health Hospitalist Program at St. Joseph’s Health Rome (N.Y.) Memorial Hospital. Dr. Kaur’s focus will be on improving infrastructure to allow for the highest quality of care possible. She will oversee the facility’s crew of eight hospitalists, who rotate to be available 24 hours per day.
Dr. Kaur comes to Rome from St. Joseph’s Health in Syracuse, N.Y., where she was chief resident and a member of the hospitalist team.
Colin McMahon, MD, was recently appointed chief of hospital medicine at Eastern Niagara Hospital in Lockport, N.Y., where he will oversee the hospitalist program. He comes to ENH after serving as medical director of hospital operations at Buffalo (N.Y.) General Medical Center.
Dr. McMahon has worked in medicine for a quarter of a century. He also is the president and founder of Dimensions of Internal Medicine and Pediatric Care, PC (DMP). Associates from DMP Medicine make up the hospitalist team at ENH.
Sam Antonios, MD, has been promoted to chief clinical officer of Ascension Kansas, the parent group of Ascension Via Christi Hospital in Wichita, where Dr. Antonios has served as chief medical officer for the past 4 years. Dr. Antonios has emerged as a leader within Ascension Kansas during the COVID-19 pandemic.
Prior to his appointment at Via Christi, he worked at that facility as a hospitalist and as medical director of information systems. Dr. Antonios is a board-certified internist.
Bret J. Rudy, MD, was named a Top 25 Healthcare Innovator by Modern Healthcare magazine. Dr. Rudy is chief of hospital operations and senior vice president at NYU Langone Hospital-Brooklyn in New York, and the magazine cited his efforts in elevating the quality, safety, and accountability of the facility, which merged with NYU Langone Health in 2016.
Dr. Rudy has established a 24-hour hospitalist service, added full-time emergency faculty, and reduced hospital wait times, among other patient-experience benchmarks, since his appointment at Langone-Brooklyn.
Dr. Rudy is a board-certified pediatrician who has served on the National Institutes of Health’s HIV research networks, including a spot on the White House Advisory Committee on Adolescents for the Office of National AIDS Policy.
Nasim Afsar, MD, MBA, SFHM, a past president of SHM, was recently named chief operating officer at UCI Health in Orange, Calif. Dr. Afsar served previously as chief ambulatory officer and chief medical officer for accountable care organizations at UCI Health.
Anthony J. Macchiavelli, MD, FHM, was recognized by Continental Who’s Who as a “Top Distinguished Hospitalist” with AtlantiCare Regional Medical Center, in Atlantic City, N.J. He has been with AtlantiCare for the past ten years, and currently serves as medical director for the PACE program, as well as medical director for the Anticoagulation Clinic.
Dr. Macchiavelli has been involved in the development of 3 different hospital medicine programs throughout his career and was the founder of the Associates in Hospital Medicine program at Methodist Division of Thomas Jefferson University Hospitals. He serves as a mentor for SHM’s VTE-FAST Program, and has served on the Standards Review Panel for the Joint Commission developing the National Patient Safety Goal for anticoagulation therapy.
Erin Shaughnessy, MD, assumed the role of director of pediatric hospital medicine at the University of Alabama at Birmingham and Children’s of Alabama, also in Birmingham, on Sept. 1. Dr. Shaughnessy has done research in improving outcomes in hospitalized children, as well as improving communication between physicians and pediatric patients’ families during care transitions.
Prior to joining UAB and Children’s of Alabama, Dr. Shaughnessy was division chief of hospital medicine at Phoenix (Ariz.) Children’s Hospital while also serving as an associate professor at the University of Arizona, Phoenix.
Chandra Lingisetty, MD, MBA, MHCM, was recently named chief administrative officer for Baptist Health Physician Partners, Arkansas. BHPP is Baptist Health’s clinically integrated network (CIN) with more than 1,600 providers across the state.
Baptist Health Arkansas is the state’s largest not for profit health system with 12 hospitals, hundreds of provider clinics, a nursing school, and a graduate medical education residency program. Prior to his promotion, he worked in Baptist Health System as a hospitalist for 10 years, served on the board of managers at BHPP, and strategized COVID-19 care management protocols and medical staff preparedness as part of surge planning and capacity expansion. In his new role, he is focused on leading the clinically integrated network toward value-based care. He is also the cofounder and inaugural president of the Arkansas state chapter of the Society of Hospital Medicine.
Grace Farris, MD, recently accepted a position with the division of hospital medicine at the Dell Medical School in Austin where she will be an assistant professor of internal medicine, as well as a working hospitalist.
Dr. Farris worked as chief of hospital medicine at Mount Sinai West Hospital in Manhattan from January 2017 until accepting her new position with Dell. In addition, she publishes a monthly comics column in the Annals of Internal Medicine.
Her visual storytelling through comics has appeared in several media outlets, and she has penned literal columns as well, including one recently in the New York Times about living apart from her children while treating COVID-19 patients in the emergency room.
Dell Medical School has also named a new division chief of hospital medicine. Read Pierce, MD, made the move to Texas from the University of Colorado at Denver, Aurora. Dr. Pierce will also serve as associate chair of faculty development of internal medicine at Dell. He is eager to build on his experience and passion for developing people, creating outstanding culture, and changing complex systems in innovative, sustainable ways.
Dr. Pierce worked at University of Colorado for the past 8 years, serving as the associate director of the school’s Institute for Healthcare Quality, Safety and Efficiency (IHQSE), a program he co-founded. Prior to that, Dr. Pierce was chief resident at the University of San Francisco medical school and later founded the hospital medicine center at the San Francisco VA Medical Center.
Gurinder Kaur, MD, was recently named medical director of the Health Hospitalist Program at St. Joseph’s Health Rome (N.Y.) Memorial Hospital. Dr. Kaur’s focus will be on improving infrastructure to allow for the highest quality of care possible. She will oversee the facility’s crew of eight hospitalists, who rotate to be available 24 hours per day.
Dr. Kaur comes to Rome from St. Joseph’s Health in Syracuse, N.Y., where she was chief resident and a member of the hospitalist team.
Colin McMahon, MD, was recently appointed chief of hospital medicine at Eastern Niagara Hospital in Lockport, N.Y., where he will oversee the hospitalist program. He comes to ENH after serving as medical director of hospital operations at Buffalo (N.Y.) General Medical Center.
Dr. McMahon has worked in medicine for a quarter of a century. He also is the president and founder of Dimensions of Internal Medicine and Pediatric Care, PC (DMP). Associates from DMP Medicine make up the hospitalist team at ENH.
Sam Antonios, MD, has been promoted to chief clinical officer of Ascension Kansas, the parent group of Ascension Via Christi Hospital in Wichita, where Dr. Antonios has served as chief medical officer for the past 4 years. Dr. Antonios has emerged as a leader within Ascension Kansas during the COVID-19 pandemic.
Prior to his appointment at Via Christi, he worked at that facility as a hospitalist and as medical director of information systems. Dr. Antonios is a board-certified internist.
Bret J. Rudy, MD, was named a Top 25 Healthcare Innovator by Modern Healthcare magazine. Dr. Rudy is chief of hospital operations and senior vice president at NYU Langone Hospital-Brooklyn in New York, and the magazine cited his efforts in elevating the quality, safety, and accountability of the facility, which merged with NYU Langone Health in 2016.
Dr. Rudy has established a 24-hour hospitalist service, added full-time emergency faculty, and reduced hospital wait times, among other patient-experience benchmarks, since his appointment at Langone-Brooklyn.
Dr. Rudy is a board-certified pediatrician who has served on the National Institutes of Health’s HIV research networks, including a spot on the White House Advisory Committee on Adolescents for the Office of National AIDS Policy.
Nasim Afsar, MD, MBA, SFHM, a past president of SHM, was recently named chief operating officer at UCI Health in Orange, Calif. Dr. Afsar served previously as chief ambulatory officer and chief medical officer for accountable care organizations at UCI Health.
Anthony J. Macchiavelli, MD, FHM, was recognized by Continental Who’s Who as a “Top Distinguished Hospitalist” with AtlantiCare Regional Medical Center, in Atlantic City, N.J. He has been with AtlantiCare for the past ten years, and currently serves as medical director for the PACE program, as well as medical director for the Anticoagulation Clinic.
Dr. Macchiavelli has been involved in the development of 3 different hospital medicine programs throughout his career and was the founder of the Associates in Hospital Medicine program at Methodist Division of Thomas Jefferson University Hospitals. He serves as a mentor for SHM’s VTE-FAST Program, and has served on the Standards Review Panel for the Joint Commission developing the National Patient Safety Goal for anticoagulation therapy.
TNF inhibitor–induced psoriasis treatment algorithm maintains TNF inhibitor if possible
In a single-center retrospective analysis of 102 patients with psoriasis induced by tumor necrosis factor (TNF) inhibitors, most cases improved or resolved with use of topical medications or with discontinuation of the inciting TNF inhibitor, with or without other interventions. All patients were treated and diagnosed by dermatologists.
While TNF inhibitors have revolutionized management of numerous debilitating chronic inflammatory diseases, they are associated with mild and potentially serious adverse reactions, including de novo psoriasiform eruptions, noted Sean E. Mazloom, MD, and colleagues, at the Cleveland Clinic, Cleveland, Ohio, in the Journal of the American Academy of Dermatology. Despite the fact that it has been more than 15 years since the first reports of TNF inhibitor-induced psoriasis, optimal treatment strategies still remain poorly understood.
IBD and RA most common
Dr. Mazloom and colleagues identified 102 patients (median onset, 41 years; 72.5% female) with TNF inhibitor-induced psoriasis seen at a single tertiary care institution (the Cleveland Clinic) over a 10-year period. The authors proposed a treatment algorithm based on their findings.
Inciting TNF inhibitors were prescribed most commonly for inflammatory bowel disease (IBD) (52%) and rheumatoid arthritis (RA) (24.5%). The most common inciting TNF inhibitor was infliximab (52%). TNF inhibitor-induced psoriasis improved or resolved with topical medications alone in 63.5% of patients, and cyclosporine and methotrexate (10 mg weekly) were often effective (cyclosporine in five of five patients; methotrexate in 7 of 13) if topicals failed.
Noting that the success with topicals in this cohort exceeded that of earlier reports, the authors suggested that more accurate diagnoses and optimal strategies attributable to the involvement of dermatologists may be explanatory.
In 67% of refractory cases, discontinuation of the inciting TNF inhibitor with or without other interventions improved or resolved TNF inhibitor-induced psoriasis. With switching of TNF inhibitors, persistence or worsening of TNF inhibitor-induced psoriasis was reported in 16 of 25 patients (64%).
Algorithm aims at balancing control
The treatment algorithm proposed by Dr. Mazloom and colleagues aims at balancing control of the primary disease with minimization of skin symptom discomfort and continuation of the inciting TNF inhibitor if possible. Only with cyclosporine or methotrexate failure amid severe symptoms and less-than-optimal primary disease control should TNF inhibitors be discontinued and biologics and/or small-molecule inhibitors with alternative mechanisms of action be introduced. Transitioning to other TNF inhibitors may be tried before alternative strategies when the underlying disease is well-controlled but TNF inhibitor-induced psoriasis remains severe.
“Most dermatologists who see TNF-induced psoriasis often are likely already using strategies like the one proposed in the algorithm,” commented senior author Anthony Fernandez, MD, PhD, of the Cleveland (Ohio) Clinic, in an interview. “The concern is over those who may not see TNF inhibitor-induced psoriasis very often, and who may, as a knee-jerk response to TNF-induced psoriasis, stop the inciting medication. When strong side effects occur in IBD and RA, it’s critical to know how well the TNF inhibitor is controlling the underlying disease because lack of control can lead to permanent damage.”
Risk to benefit ratio favors retaining TNF inhibitors
The dermatologist’s goal, if the TNF inhibitor is working well, should be to exhaust all reasonable options to control the psoriasiform eruption and keep the patient on the TNF inhibitor rather than turn to potentially less effective alternatives, Dr. Fernandez added. “The risk:benefit ratio still usually favors adding more immune therapies to treat these reactions in order to enable patients to stay” on their TNF inhibitors.
Study authors disclosed no direct funding for the study. Dr Fernandez, the senior author, receives research funding from Pfizer, Mallinckrodt, and Novartis, consults for AbbVie and Celgene, and is a speaker for AbbVie and Mallinckrodt.
SOURCE: Mazloom SE et al. J Am Acad Dermatol. 2020 Dec;83(6):1590-8.
In a single-center retrospective analysis of 102 patients with psoriasis induced by tumor necrosis factor (TNF) inhibitors, most cases improved or resolved with use of topical medications or with discontinuation of the inciting TNF inhibitor, with or without other interventions. All patients were treated and diagnosed by dermatologists.
While TNF inhibitors have revolutionized management of numerous debilitating chronic inflammatory diseases, they are associated with mild and potentially serious adverse reactions, including de novo psoriasiform eruptions, noted Sean E. Mazloom, MD, and colleagues, at the Cleveland Clinic, Cleveland, Ohio, in the Journal of the American Academy of Dermatology. Despite the fact that it has been more than 15 years since the first reports of TNF inhibitor-induced psoriasis, optimal treatment strategies still remain poorly understood.
IBD and RA most common
Dr. Mazloom and colleagues identified 102 patients (median onset, 41 years; 72.5% female) with TNF inhibitor-induced psoriasis seen at a single tertiary care institution (the Cleveland Clinic) over a 10-year period. The authors proposed a treatment algorithm based on their findings.
Inciting TNF inhibitors were prescribed most commonly for inflammatory bowel disease (IBD) (52%) and rheumatoid arthritis (RA) (24.5%). The most common inciting TNF inhibitor was infliximab (52%). TNF inhibitor-induced psoriasis improved or resolved with topical medications alone in 63.5% of patients, and cyclosporine and methotrexate (10 mg weekly) were often effective (cyclosporine in five of five patients; methotrexate in 7 of 13) if topicals failed.
Noting that the success with topicals in this cohort exceeded that of earlier reports, the authors suggested that more accurate diagnoses and optimal strategies attributable to the involvement of dermatologists may be explanatory.
In 67% of refractory cases, discontinuation of the inciting TNF inhibitor with or without other interventions improved or resolved TNF inhibitor-induced psoriasis. With switching of TNF inhibitors, persistence or worsening of TNF inhibitor-induced psoriasis was reported in 16 of 25 patients (64%).
Algorithm aims at balancing control
The treatment algorithm proposed by Dr. Mazloom and colleagues aims at balancing control of the primary disease with minimization of skin symptom discomfort and continuation of the inciting TNF inhibitor if possible. Only with cyclosporine or methotrexate failure amid severe symptoms and less-than-optimal primary disease control should TNF inhibitors be discontinued and biologics and/or small-molecule inhibitors with alternative mechanisms of action be introduced. Transitioning to other TNF inhibitors may be tried before alternative strategies when the underlying disease is well-controlled but TNF inhibitor-induced psoriasis remains severe.
“Most dermatologists who see TNF-induced psoriasis often are likely already using strategies like the one proposed in the algorithm,” commented senior author Anthony Fernandez, MD, PhD, of the Cleveland (Ohio) Clinic, in an interview. “The concern is over those who may not see TNF inhibitor-induced psoriasis very often, and who may, as a knee-jerk response to TNF-induced psoriasis, stop the inciting medication. When strong side effects occur in IBD and RA, it’s critical to know how well the TNF inhibitor is controlling the underlying disease because lack of control can lead to permanent damage.”
Risk to benefit ratio favors retaining TNF inhibitors
The dermatologist’s goal, if the TNF inhibitor is working well, should be to exhaust all reasonable options to control the psoriasiform eruption and keep the patient on the TNF inhibitor rather than turn to potentially less effective alternatives, Dr. Fernandez added. “The risk:benefit ratio still usually favors adding more immune therapies to treat these reactions in order to enable patients to stay” on their TNF inhibitors.
Study authors disclosed no direct funding for the study. Dr Fernandez, the senior author, receives research funding from Pfizer, Mallinckrodt, and Novartis, consults for AbbVie and Celgene, and is a speaker for AbbVie and Mallinckrodt.
SOURCE: Mazloom SE et al. J Am Acad Dermatol. 2020 Dec;83(6):1590-8.
In a single-center retrospective analysis of 102 patients with psoriasis induced by tumor necrosis factor (TNF) inhibitors, most cases improved or resolved with use of topical medications or with discontinuation of the inciting TNF inhibitor, with or without other interventions. All patients were treated and diagnosed by dermatologists.
While TNF inhibitors have revolutionized management of numerous debilitating chronic inflammatory diseases, they are associated with mild and potentially serious adverse reactions, including de novo psoriasiform eruptions, noted Sean E. Mazloom, MD, and colleagues, at the Cleveland Clinic, Cleveland, Ohio, in the Journal of the American Academy of Dermatology. Despite the fact that it has been more than 15 years since the first reports of TNF inhibitor-induced psoriasis, optimal treatment strategies still remain poorly understood.
IBD and RA most common
Dr. Mazloom and colleagues identified 102 patients (median onset, 41 years; 72.5% female) with TNF inhibitor-induced psoriasis seen at a single tertiary care institution (the Cleveland Clinic) over a 10-year period. The authors proposed a treatment algorithm based on their findings.
Inciting TNF inhibitors were prescribed most commonly for inflammatory bowel disease (IBD) (52%) and rheumatoid arthritis (RA) (24.5%). The most common inciting TNF inhibitor was infliximab (52%). TNF inhibitor-induced psoriasis improved or resolved with topical medications alone in 63.5% of patients, and cyclosporine and methotrexate (10 mg weekly) were often effective (cyclosporine in five of five patients; methotrexate in 7 of 13) if topicals failed.
Noting that the success with topicals in this cohort exceeded that of earlier reports, the authors suggested that more accurate diagnoses and optimal strategies attributable to the involvement of dermatologists may be explanatory.
In 67% of refractory cases, discontinuation of the inciting TNF inhibitor with or without other interventions improved or resolved TNF inhibitor-induced psoriasis. With switching of TNF inhibitors, persistence or worsening of TNF inhibitor-induced psoriasis was reported in 16 of 25 patients (64%).
Algorithm aims at balancing control
The treatment algorithm proposed by Dr. Mazloom and colleagues aims at balancing control of the primary disease with minimization of skin symptom discomfort and continuation of the inciting TNF inhibitor if possible. Only with cyclosporine or methotrexate failure amid severe symptoms and less-than-optimal primary disease control should TNF inhibitors be discontinued and biologics and/or small-molecule inhibitors with alternative mechanisms of action be introduced. Transitioning to other TNF inhibitors may be tried before alternative strategies when the underlying disease is well-controlled but TNF inhibitor-induced psoriasis remains severe.
“Most dermatologists who see TNF-induced psoriasis often are likely already using strategies like the one proposed in the algorithm,” commented senior author Anthony Fernandez, MD, PhD, of the Cleveland (Ohio) Clinic, in an interview. “The concern is over those who may not see TNF inhibitor-induced psoriasis very often, and who may, as a knee-jerk response to TNF-induced psoriasis, stop the inciting medication. When strong side effects occur in IBD and RA, it’s critical to know how well the TNF inhibitor is controlling the underlying disease because lack of control can lead to permanent damage.”
Risk to benefit ratio favors retaining TNF inhibitors
The dermatologist’s goal, if the TNF inhibitor is working well, should be to exhaust all reasonable options to control the psoriasiform eruption and keep the patient on the TNF inhibitor rather than turn to potentially less effective alternatives, Dr. Fernandez added. “The risk:benefit ratio still usually favors adding more immune therapies to treat these reactions in order to enable patients to stay” on their TNF inhibitors.
Study authors disclosed no direct funding for the study. Dr Fernandez, the senior author, receives research funding from Pfizer, Mallinckrodt, and Novartis, consults for AbbVie and Celgene, and is a speaker for AbbVie and Mallinckrodt.
SOURCE: Mazloom SE et al. J Am Acad Dermatol. 2020 Dec;83(6):1590-8.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
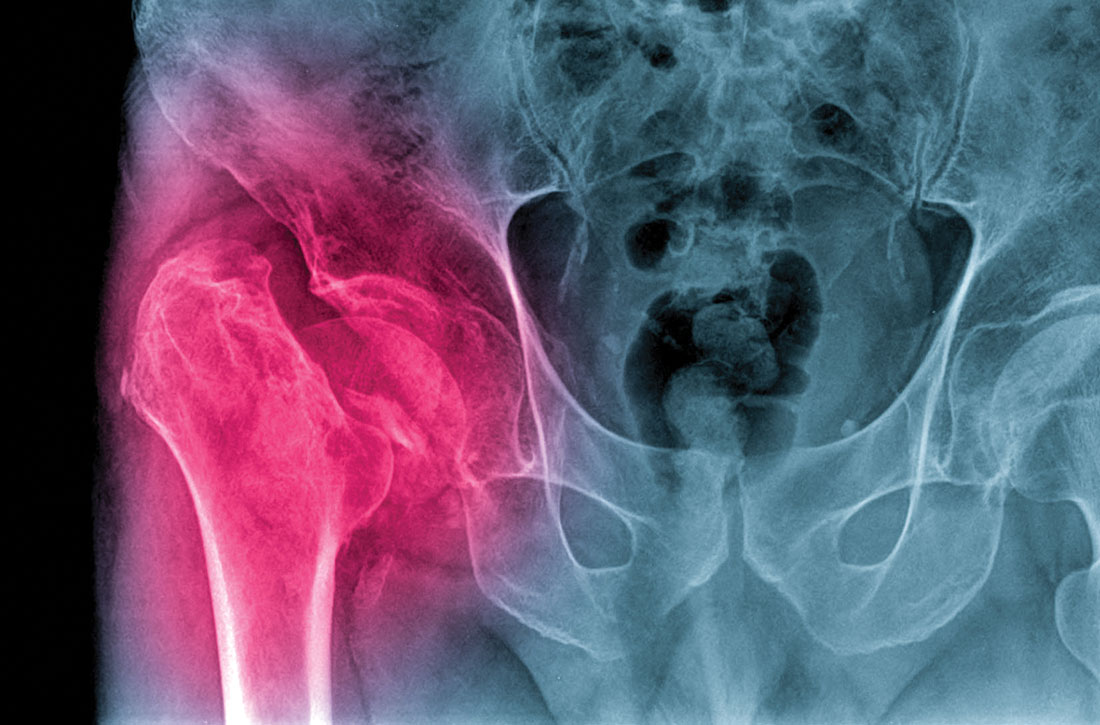
Is a pelvic examination necessary 6 weeks after hysterectomy?
Doctors commonly perform pelvic examinations approximately 6 weeks following hysterectomy to assess the integrity of the vaginal cuff. But this practice may not be necessary if patients do not have symptoms, a study suggests.
“The 6-week posthysterectomy pelvic examination in asymptomatic women may not be necessary, as it neither detected cuff dehiscence nor negated future risk for dehiscence,” Ritchie Mae Delara, MD, said at the meeting sponsored by AAGL, held virtually this year.
Dr. Delara, of the Mayo Clinic in Phoenix, and colleagues conducted a retrospective cohort study of data from more than 2,000 patients to assess the utility of the 6-week posthysterectomy pelvic examination in detecting cuff dehiscence in asymptomatic women.
An unpredictable complication
Vaginal cuff dehiscence is a rare complication of hysterectomy that can occur days or decades after surgery, which makes “identifying an optimal time for cuff evaluation difficult,” Dr. Delara said. “Currently there is neither evidence demonstrating benefit of routine posthysterectomy examination in detecting vaginal cuff dehiscence, nor data demonstrating the best time to perform posthysterectomy examination.”
For their study, which was also published in the Journal of Minimally Invasive Gynecology, the researchers examined data from 2,051 women who underwent hysterectomy at a single institution during a 6-year period. Patients received at least one postoperative evaluation within 90 days of surgery. Examination of the vaginal cuff routinely was performed approximately 6 weeks after hysterectomy. Patients’ posthysterectomy symptoms and pelvic examination findings were recorded.
About 80% of patients were asymptomatic at the 6-week visit.
Asymptomatic patients were more likely to have normal pelvic examination findings, compared with patients with posthysterectomy symptoms (86.4% vs. 54.3%).
In all, 13 patients experienced complete cuff dehiscence. All of them had an intact vaginal cuff at their 6-week examination. Three had symptoms at that time, including vaginal bleeding in one patient and pelvic pain in two patients.
One patient experienced a complete cuff dehiscence that was provoked by intercourse prior to her examination. The patient subsequently developed two additional episodes of dehiscence provoked by intercourse.
Dehiscence may present differently after benign and oncologic hysterectomies, the study indicated.
Eight patients who experienced complete cuff dehiscence after benign hysterectomy had symptoms such as pelvic pain and vaginal bleeding at the time of presentation for dehiscence, which mainly occurred after intercourse.
Five patients who experienced dehiscence after oncologic hysterectomy were more likely to present without symptoms or provocation.
The median time to dehiscence after benign hysterectomy was about 19 weeks, whereas the median time to dehiscence after oncologic hysterectomy was about 81 weeks.
Surgeons should educate patients about symptoms of dehiscence and the potential for events such as coitus to provoke its occurrence, and patients should promptly seek evaluation if symptoms occur, Dr. Delara said.
Patients with risk factors such as malignancy may benefit from continued routine evaluation, she added.
Timely research
The findings may be especially relevant during the COVID-19 pandemic, when states have issued shelter-in-place orders and doctors have increased their use of telemedicine to reduce in-person visits, Dr. Delara noted.
In that sense, the study is “extremely timely” and may inform and support practice changes, commented Emad Mikhail, MD, in a discussion following the research presentation.
Whether the results generalize to other centers, including smaller centers that perform fewer surgeries, is unclear, said Dr. Mikhail, of the University of South Florida, Tampa.
“It takes vision and critical thinking to challenge these traditional practices,” he said. “I applaud Dr. Delara for challenging one of these.”
Dr. Delara and Dr. Mikhail had no relevant disclosures.
SOURCE: Delara RMM et al. J Minim Invasive Gynecol. 2020 Nov 1. doi: 10.1016/j.jmig.2020.08.306.
Doctors commonly perform pelvic examinations approximately 6 weeks following hysterectomy to assess the integrity of the vaginal cuff. But this practice may not be necessary if patients do not have symptoms, a study suggests.
“The 6-week posthysterectomy pelvic examination in asymptomatic women may not be necessary, as it neither detected cuff dehiscence nor negated future risk for dehiscence,” Ritchie Mae Delara, MD, said at the meeting sponsored by AAGL, held virtually this year.
Dr. Delara, of the Mayo Clinic in Phoenix, and colleagues conducted a retrospective cohort study of data from more than 2,000 patients to assess the utility of the 6-week posthysterectomy pelvic examination in detecting cuff dehiscence in asymptomatic women.
An unpredictable complication
Vaginal cuff dehiscence is a rare complication of hysterectomy that can occur days or decades after surgery, which makes “identifying an optimal time for cuff evaluation difficult,” Dr. Delara said. “Currently there is neither evidence demonstrating benefit of routine posthysterectomy examination in detecting vaginal cuff dehiscence, nor data demonstrating the best time to perform posthysterectomy examination.”
For their study, which was also published in the Journal of Minimally Invasive Gynecology, the researchers examined data from 2,051 women who underwent hysterectomy at a single institution during a 6-year period. Patients received at least one postoperative evaluation within 90 days of surgery. Examination of the vaginal cuff routinely was performed approximately 6 weeks after hysterectomy. Patients’ posthysterectomy symptoms and pelvic examination findings were recorded.
About 80% of patients were asymptomatic at the 6-week visit.
Asymptomatic patients were more likely to have normal pelvic examination findings, compared with patients with posthysterectomy symptoms (86.4% vs. 54.3%).
In all, 13 patients experienced complete cuff dehiscence. All of them had an intact vaginal cuff at their 6-week examination. Three had symptoms at that time, including vaginal bleeding in one patient and pelvic pain in two patients.
One patient experienced a complete cuff dehiscence that was provoked by intercourse prior to her examination. The patient subsequently developed two additional episodes of dehiscence provoked by intercourse.
Dehiscence may present differently after benign and oncologic hysterectomies, the study indicated.
Eight patients who experienced complete cuff dehiscence after benign hysterectomy had symptoms such as pelvic pain and vaginal bleeding at the time of presentation for dehiscence, which mainly occurred after intercourse.
Five patients who experienced dehiscence after oncologic hysterectomy were more likely to present without symptoms or provocation.
The median time to dehiscence after benign hysterectomy was about 19 weeks, whereas the median time to dehiscence after oncologic hysterectomy was about 81 weeks.
Surgeons should educate patients about symptoms of dehiscence and the potential for events such as coitus to provoke its occurrence, and patients should promptly seek evaluation if symptoms occur, Dr. Delara said.
Patients with risk factors such as malignancy may benefit from continued routine evaluation, she added.
Timely research
The findings may be especially relevant during the COVID-19 pandemic, when states have issued shelter-in-place orders and doctors have increased their use of telemedicine to reduce in-person visits, Dr. Delara noted.
In that sense, the study is “extremely timely” and may inform and support practice changes, commented Emad Mikhail, MD, in a discussion following the research presentation.
Whether the results generalize to other centers, including smaller centers that perform fewer surgeries, is unclear, said Dr. Mikhail, of the University of South Florida, Tampa.
“It takes vision and critical thinking to challenge these traditional practices,” he said. “I applaud Dr. Delara for challenging one of these.”
Dr. Delara and Dr. Mikhail had no relevant disclosures.
SOURCE: Delara RMM et al. J Minim Invasive Gynecol. 2020 Nov 1. doi: 10.1016/j.jmig.2020.08.306.
Doctors commonly perform pelvic examinations approximately 6 weeks following hysterectomy to assess the integrity of the vaginal cuff. But this practice may not be necessary if patients do not have symptoms, a study suggests.
“The 6-week posthysterectomy pelvic examination in asymptomatic women may not be necessary, as it neither detected cuff dehiscence nor negated future risk for dehiscence,” Ritchie Mae Delara, MD, said at the meeting sponsored by AAGL, held virtually this year.
Dr. Delara, of the Mayo Clinic in Phoenix, and colleagues conducted a retrospective cohort study of data from more than 2,000 patients to assess the utility of the 6-week posthysterectomy pelvic examination in detecting cuff dehiscence in asymptomatic women.
An unpredictable complication
Vaginal cuff dehiscence is a rare complication of hysterectomy that can occur days or decades after surgery, which makes “identifying an optimal time for cuff evaluation difficult,” Dr. Delara said. “Currently there is neither evidence demonstrating benefit of routine posthysterectomy examination in detecting vaginal cuff dehiscence, nor data demonstrating the best time to perform posthysterectomy examination.”
For their study, which was also published in the Journal of Minimally Invasive Gynecology, the researchers examined data from 2,051 women who underwent hysterectomy at a single institution during a 6-year period. Patients received at least one postoperative evaluation within 90 days of surgery. Examination of the vaginal cuff routinely was performed approximately 6 weeks after hysterectomy. Patients’ posthysterectomy symptoms and pelvic examination findings were recorded.
About 80% of patients were asymptomatic at the 6-week visit.
Asymptomatic patients were more likely to have normal pelvic examination findings, compared with patients with posthysterectomy symptoms (86.4% vs. 54.3%).
In all, 13 patients experienced complete cuff dehiscence. All of them had an intact vaginal cuff at their 6-week examination. Three had symptoms at that time, including vaginal bleeding in one patient and pelvic pain in two patients.
One patient experienced a complete cuff dehiscence that was provoked by intercourse prior to her examination. The patient subsequently developed two additional episodes of dehiscence provoked by intercourse.
Dehiscence may present differently after benign and oncologic hysterectomies, the study indicated.
Eight patients who experienced complete cuff dehiscence after benign hysterectomy had symptoms such as pelvic pain and vaginal bleeding at the time of presentation for dehiscence, which mainly occurred after intercourse.
Five patients who experienced dehiscence after oncologic hysterectomy were more likely to present without symptoms or provocation.
The median time to dehiscence after benign hysterectomy was about 19 weeks, whereas the median time to dehiscence after oncologic hysterectomy was about 81 weeks.
Surgeons should educate patients about symptoms of dehiscence and the potential for events such as coitus to provoke its occurrence, and patients should promptly seek evaluation if symptoms occur, Dr. Delara said.
Patients with risk factors such as malignancy may benefit from continued routine evaluation, she added.
Timely research
The findings may be especially relevant during the COVID-19 pandemic, when states have issued shelter-in-place orders and doctors have increased their use of telemedicine to reduce in-person visits, Dr. Delara noted.
In that sense, the study is “extremely timely” and may inform and support practice changes, commented Emad Mikhail, MD, in a discussion following the research presentation.
Whether the results generalize to other centers, including smaller centers that perform fewer surgeries, is unclear, said Dr. Mikhail, of the University of South Florida, Tampa.
“It takes vision and critical thinking to challenge these traditional practices,” he said. “I applaud Dr. Delara for challenging one of these.”
Dr. Delara and Dr. Mikhail had no relevant disclosures.
SOURCE: Delara RMM et al. J Minim Invasive Gynecol. 2020 Nov 1. doi: 10.1016/j.jmig.2020.08.306.
FROM AAGL GLOBAL CONGRESS
Improving Primary Care Fall Risk Management: Adoption of Practice Changes After a Geriatric Mini-Fellowship
From the Senior Health Program, Providence Health & Services, Oregon, Portland, OR.
Abstract
Background: Approximately 51 million adults in the United States are 65 years of age or older, yet few geriatric-trained primary care providers (PCP) serve this population. The Age-Friendly Health System framework, consisting of evidence-based 4M care (Mobility, Medication, Mentation, and what Matters), encourages all PCPs to assess mobility in older adults.
Objective: To improve PCP knowledge, confidence, and clinical practice in assessing and managing fall risk.
Methods: A 1-week educational session focusing on mobility (part of a 4-week Geriatric Mini-Fellowship) for 6 selected PCPs from a large health care system was conducted to increase knowledge and ability to address fall risk in older adults. The week included learning and practicing a Fall Risk Management Plan (FRMP) algorithm, including planning for their own practice changes. Pre- and post-test surveys assessed changes in knowledge and confidence. Patient data were compared 12 months before and after training to evaluate PCP adoption of FRMP components.
Results: The training increased provider knowledge and confidence. The trained PCPs were 1.7 times more likely to screen for fall risk; 3.6 times more likely to discuss fall risk; and 5.8 times more likely to assess orthostatic blood pressure in their 65+ patients after the mini-fellowship. In high-risk patients, they were 4.1 times more likely to discuss fall risk and 6.3 times more likely to assess orthostatic blood pressure than their nontrained peers. Changes in physical therapy referral rates were not observed.
Conclusions: In-depth, skills-based geriatric educational sessions improved PCPs’ knowledge and confidence and also improved their fall risk management practices for their older patients.
Keywords: geriatrics; guidelines; Age-Friendly Health System; 4M; workforce training; practice change; fellowship.
The US population is aging rapidly. People aged 85 years and older are the largest-growing segment of the US population, and this segment is expected to increase by 123% by 2040.1 Caregiving needs increase with age as older adults develop more chronic conditions, such as hypertension, heart disease, arthritis, and dementia. However, even with increasing morbidity and dependence, a majority of older adults still live in the community rather than in institutional settings.2 These older adults seek medical care more frequently than younger people, with about 22% of patients 75 years and older having 10 or more health care visits in the previous 12 months. By 2040, nearly a quarter of the US population is expected to be 65 or older, with many of these older adults seeking regular primary care from providers who do not have formal training in the care of a population with multiple complex, chronic health conditions and increased caregiving needs.1
Despite this growing demand for health care professionals trained in the care of older adults, access to these types of clinicians is limited. In 2018, there were roughly 7000 certified geriatricians, with only 3600 of them practicing full-time.3,4 Similarly, of 290,000 certified nurse practitioners (NPs), about 9% of them have geriatric certification.5 Geriatricians, medical doctors trained in the care of older adults, and geriatric-trained NPs are part of a cadre of a geriatric-trained workforce that provides unique expertise in caring for older adults with chronic and advanced illness. They know how to manage multiple, complex geriatric syndromes like falls, dementia, and polypharmacy; understand and maximize team-based care; and focus on caring for an older person with a goal-centered versus a disease-centered approach.6
Broadly, geriatric care includes a spectrum of adults, from those who are aging healthfully to those who are the frailest. Research has suggested that approximately 30% of older adults need care by a geriatric-trained clinician, with the oldest and frailest patients needing more clinician time for assessment and treatment, care coordination, and coaching of caregivers.7 With this assumption in mind, it is projected that by 2025, there will be a national shortage of 26,980 geriatricians, with the western United States disproportionately affected by this shortage.4Rather than lamenting this shortage, Tinetti recommends a new path forward: “Our mission should not be to train enough geriatricians to provide direct care, but rather to ensure that every clinician caring for older adults is competent in geriatric principles and practices.”8 Sometimes called ”geriatricizing,” the idea is to use existing geriatric providers as a small elite training force to infuse geriatric principles and skills across their colleagues in primary care and other disciplines.8,9 Efforts of the American Geriatrics Society (AGS), with support from the John A. Hartford Foundation (JAHF), have been successful in developing geriatric training across multiple specialties, including surgery, orthopedics, and emergency medicine (www.americangeriatrics.org/programs/geriatrics-specialists-initiative).
The Age-Friendly Health System and 4M Model
To help augment this idea of equipping health care systems and their clinicians with more readily available geriatric knowledge, skills, and tools, the JAHF, along with the Institute for Healthcare Improvement (IHI), created the Age-Friendly Health System (AFHS) paradigm in 2015.10 Using the 4M model, the AFHS initiative established a set of evidence-based geriatric priorities and interventions meant to improve the care of older adults, reduce harm and duplication, and provide a framework for engaging leadership, clinical teams, and operational systems across inpatient and ambulatory settings.11 Mobility, including fall risk screening and intervention, is 1 of the 4M foundational elements of the Age-Friendly model. In addition to Mobility, the 4M model also includes 3 other key geriatric domains: Mentation (dementia, depression, and delirium), Medication (high-risk medications, polypharmacy, and deprescribing), and What Matters (goals of care conversations and understanding quality of life for older patients).11 The 4M initiative encourages adoption of a geriatric lens that looks across chronic conditions and accounts for the interplay among geriatric syndromes, such as falls, cognitive impairment, and frailty, in order to provide care better tailored to what the patient needs and desires.12 IHI and JAHF have targeted the adoption of the 4M model by 20% of US health care systems by 2020.11
Mini-Fellowship and Mobility Week
To bolster geriatric skills among community-based primary care providers (PCPs), we initiated a Geriatric Mini-Fellowship, a 4-week condensed curriculum taught over 6 months. Each week focuses on 1 of the age-friendly 4Ms, with the goal of increasing the knowledge, self-efficacy, skills, and competencies of the participating PCPs (called “fellow” hereafter) and at the same time, equipping each to become a champion of geriatric practice. This article focuses on the Mobility week, the second week of the mini-fellowship, and the effect of the week on the fellows’ practice changes.
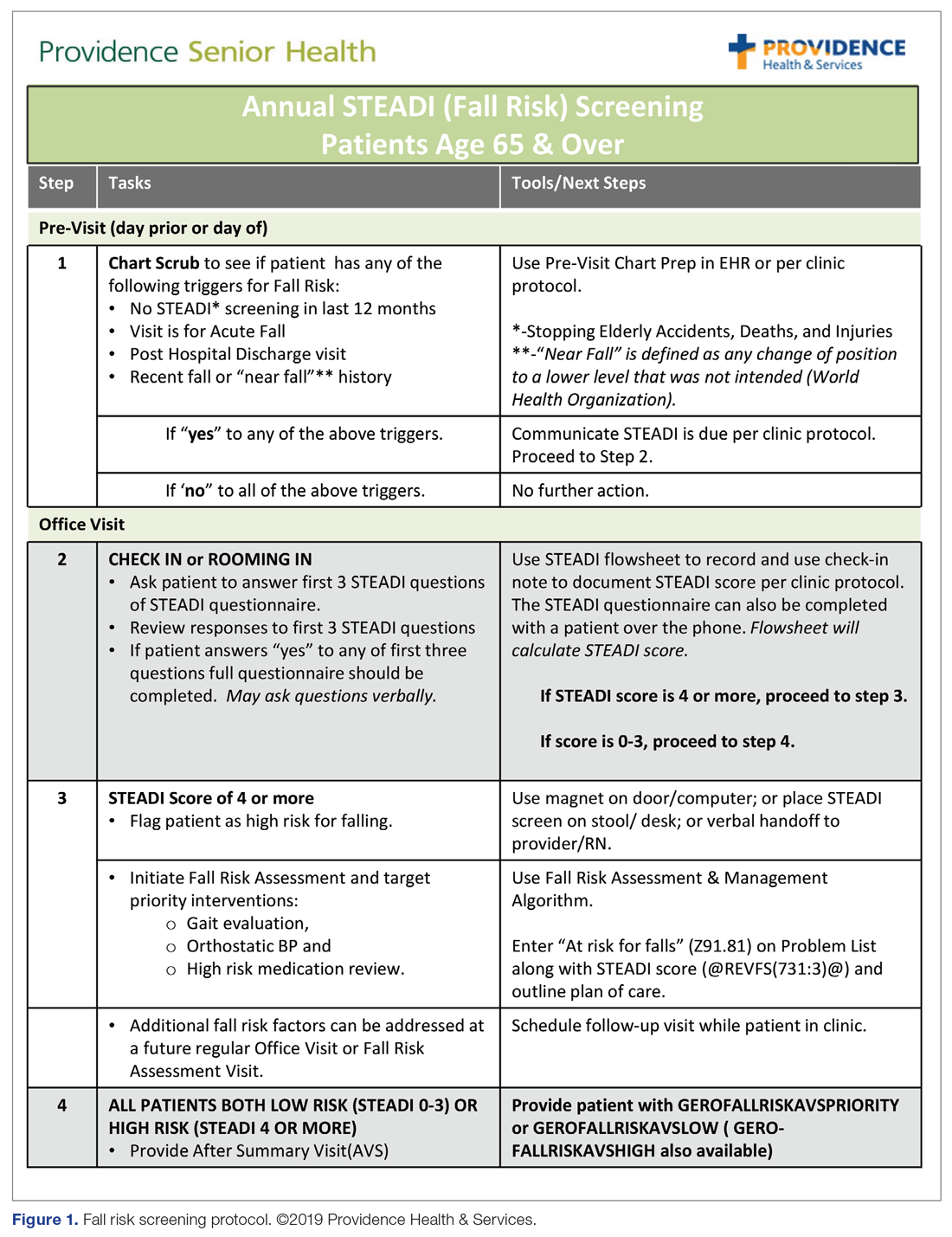
To construct the Mobility week’s curriculum with a focus on the ambulatory setting, we relied upon national evidence-based work in fall risk management. The Centers for Disease Control and Prevention (CDC) has made fall risk screening and management in primary care a high priority. Using the clinical practice guidelines for managing fall risk developed by the American and British Geriatrics Societies (AGS/BGS), the CDC developed the Stopping Elderly Accidents, Deaths, and Injuries (STEADI) toolkit.13 Foundational to the toolkit is the validated 12-item Stay Independent falls screening questionnaire (STEADI questionnaire).14 Patients who score 4 or higher (out of a total score of 14) on the questionnaire are considered at increased risk of falling. The CDC has developed a clinical algorithm that guides clinical teams through screening and assessment to help identify appropriate interventions to target specific risk factors. Research has clearly established that a multifactorial approach to fall risk intervention can be successful in reducing fall risk by as much as 25%.15-17
The significant morbidity and mortality caused by falls make training nongeriatrician clinicians on how to better address fall risk imperative. More than 25% of older adults fall each year.18 These falls contribute to rising rates of fall-related deaths,19 emergency department (ED) visits,20 and hospital readmissions.21 Initiatives like the AFHS focus on mobility and the CDC’s development of supporting clinical materials22 aim to improve primary care adoption of fall risk screening and intervention practices.23,24 The epidemic of falls must compel all PCPs, not just those practicing geriatrics, to make discussing and addressing fall risk and falls a priority.
Methods
Setting
This project took place as part of a regional primary care effort in Oregon. Providence Health & Services-Oregon is part of a multi-state integrated health care system in the western United States whose PCPs serve more than 80,000 patients aged 65 years and older per year; these patients comprise 38% of the system’s office visits each year. Regionally, there are 47 family and internal medicine clinics employing roughly 290 providers (physicians, NPs, and physician assistants). The organization has only 4 PCPs trained in geriatrics and does not offer any geriatric clinical consultation services. Six PCPs from different clinics, representing both rural and urban settings, are chosen to participate in the geriatric mini-fellowship each year.
This project was conducted as a quality improvement initiative within the organization and did not constitute human subjects research. It was not conducted under the oversight of the Institutional Review Board.
Intervention
The mini-fellowship was taught in 4 1-week blocks between April and October 2018, with a curriculum designed to be interactive and practical. The faculty was intentionally interdisciplinary to teach and model team-based practice. Each week participants were excused from their clinical practice. Approximately 160 hours of continuing medical education credits were awarded for the full mini-fellowship. As part of each weekly session, a performance improvement project (PIP) focused on that week’s topic (1 of the 4Ms) was developed by the fellow and their team members to incorporate the mini-fellowship learnings into their clinic workflows. Fellows also had 2 hours per week of dedicated administration time for a year, outside the fellowship, to work on their PIP and 4M practice changes within their clinic.
Provider Education
The week for mobility training comprised 4 daylong sessions. The first 2 days were spent learning about the epidemiology of falls; risk factors for falling; how to conduct a thorough history and assessment of fall risk; and how to create a prioritized Fall Risk Management Plan (FRMP) to decrease a patient’s individual fall risk through tailored interventions. The FRMP was adapted from the CDC STEADI toolkit.13 Core faculty were 2 geriatric-trained providers (NP and physician) and a physical therapist (PT) specializing in fall prevention.
On the third day, fellows took part in a simulated fall risk clinic, in which older adults volunteered to be patient partners, providing an opportunity to apply learnings from days 1 and 2. The clinic included the fellow observing a PT complete a mobility assessment and a pharmacist conduct a high-risk medication review. The fellow synthesized the findings of the mobility assessment and medication review, as well as their own history and assessment, to create a summary of fall risk recommendations to discuss with their volunteer patient partner. The fellows were observed and evaluated in their skills by their patient partner, course faculty, and another fellow. The patient partners, and their assigned fellow, also participated in a 45-minute fall risk presentation, led by a nurse.
On the fourth day, the fellows were joined by select clinic partners, including nurses, pharmacists, and/or medical assistants. The session included discussions among each fellow’s clinical team regarding the current state of fall risk efforts at their clinic, an analysis of barriers, and identification of opportunities to improve workflows and screening rates. Each fellow took with them an action plan tailored to their clinic to improve fall risk management practices, starting with the fellow’s own practice.
Fall Risk Management Plan
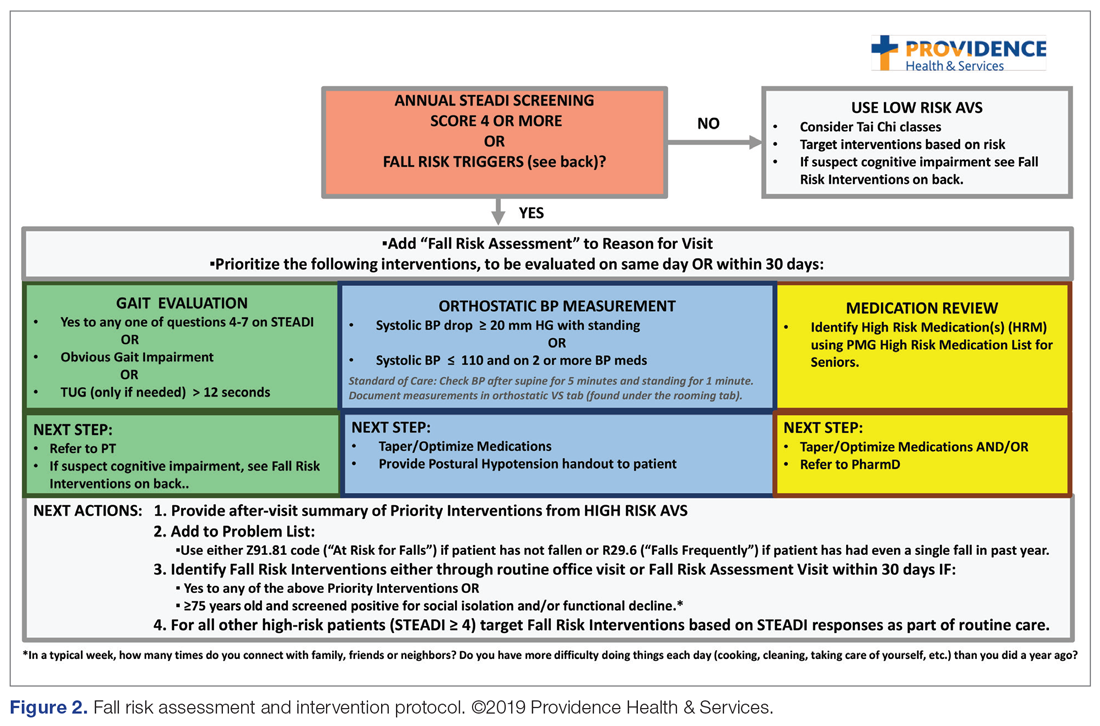
The educational sessions introduced the fellows to the FRMP. The FRMP, adapted from the STEADI toolkit, includes a process for fall risk screening (Figure 1) and stratifying a patient’s risk based on their STEADI score in order to promote 3 priority assessments (gait evaluation with PT referral if appropriate; orthostatic blood pressure; and high-risk medication review; Figure 2). Initial actions based on these priority assessments were followed over time, with additional fall risk interventions added as clinically indicated.25 The FRMP is intended to be used during routine office visits, Medicare annual wellness visits, or office visits focused on fall risk or related medical disorders (ie, fall risk visits.)
Providers and their teams were encouraged to spread out fall-related conversations with their patients over multiple visits, since many patients have multiple fall risk factors at play, in addition to other chronic medical issues, and since many interventions often require behavior changes on the part of the patient. Providers also had access to fall-related electronic health record (EHR) templates as well as a comprehensive, internal fall risk management website that included assessment tools, evidence-based resources, and patient handouts.
Assessment and Measurements
We assessed provider knowledge and comfort in their fall risk evaluation and management skills before and after the educational intervention using an 11-item multiple-choice questionnaire and a 4-item confidence questionnaire. The confidence questions used a 7-point Likert scale, with 0 indicating “no confidence” and 7 indicating ”lots of confidence.” The questions were administered via a paper survey. Qualitative comments were derived from evaluations completed at the end of the week.
The fellows’ practice of fall risk screening and management was studied from May 2018, at the completion of Mobility week, to May 2019 for the post-intervention period. A 1-year timeframe before May 2018 was used as the pre-intervention period. Eligible visit types, during which we assumed fall risk was discussed, were any office visits for patients 65+ completed by the patients’ PCPs that used fall risk as a reason for the visit or had a fall-related diagnosis code. Fall risk visits performed by other clinic providers were not counted.
Of those patients who had fall risk screenings completed and were determined to be high risk (STEADI score ≥ 4), data were analyzed to determine whether these patients had any fall-related follow-up visits to their PCP within 60 days of the STEADI screening. For these high-risk patients, data were studied to understand whether orthostatic blood pressure measurements were performed (as documented in a flowsheet) and whether a PT referral was placed. These data were compared with those from providers who practiced in clinics within the same system but who did not participate in the mini-fellowship. Data were obtained from the organization’s EHR. Additional data were measured to evaluate patterns of deprescribing of select high-risk medications, but these data are not included in this analysis.
Analysis
A paired-samples t test was used to measure changes in provider confidence levels. Data were aggregated across fellows, resulting in a mean. A chi-square test of independence was performed to examine the relationship between rates of FRMP adoption by select provider groups. Analysis included a pre- and post-intervention assessment of the fellows’ adoption of FRMP practices, as well as a comparison between the fellows’ practice patterns and those of a control group of PCPs in the organization’s other clinics who did not participate in the mini-fellowship (nontrained control group). Excluded from the control group were providers from the same clinic as the fellows; providers in clinics with a geriatric-trained provider on staff; and clinics outside of the Portland metro and Medford service areas. We used an alpha level of 0.05 for all statistical tests.
Data from 5 providers were included in the analysis of the FRMP adoption. The sixth provider changed practice settings from the clinic to the ED after completing the fellowship; her patient data were not included in the FRMP part of the analysis. EHR data included data on all visits of patients 65+, as well as data for just those 65+ patients who had been identified as being at high risk to fall based on a STEADI score of 4 or higher.
Results
Provider Questionnaire
All 6 providers responded to the pre-intervention and post-intervention tests. For the knowledge questions, fellows, as a composite, correctly answered 57% of the questions before the intervention and 79% after the intervention. Provider confidence level in delivering fall risk care was measured prior to the training (mean, 4.12 [SD, 0.62]) and at the end of the training (mean, 6.47 [SD, 0.45]), demonstrating a significant increase in confidence (t (5) = –10.46, P < 0.001).
Qualitative Comments
Providers also had the opportunity to provide comments on their experience during the Mobility week and at the end of 1 year. In general, the simulated interdisciplinary fall risk clinic was highly rated (“the highlight of the week”) as a practical strategy to embed learning principles. One fellow commented, “Putting the learning into practice helps solidify it in my brain.” Fellows also appreciated the opportunity to learn and meet with their clinic colleagues to begin work on a fall-risk focused PIP and to “have a framework for what to do for people who screen positive [for fall risk].”
FRMP Adoption
A comparison of the care the fellows provided to their patients 65+ in the 12 months pre- and post-training shows the fellows demonstrated significant changes in practice patterns. The fellows were 1.7 times more likely to screen for fall risk; 3.6 times more likely to discuss fall risk; and 5.8 times more likely to check orthostatic blood pressure than prior to the mini-fellowship (Table 1). 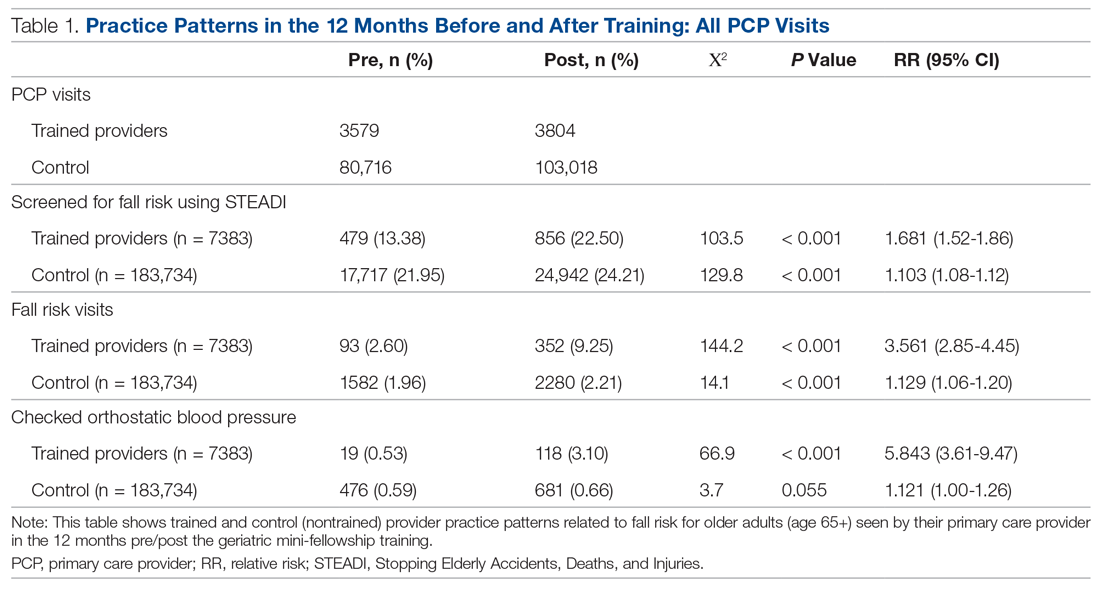
The control providers also demonstrated significant increases in fall risk screening and discussion of fall risk between the pre- and post-intervention periods; however, the relative risk (RR) was between 1.10 and 1.13 for this group. For the control group, checking orthostatic blood pressure did not significantly change. In the 12 months after training (Table 2), the fellows were 4.2 times more likely to discuss fall risk and almost 5 times more likely to check orthostatic blood pressure than their nontrained peers for all of their patients 65+, regardless of their risk to fall.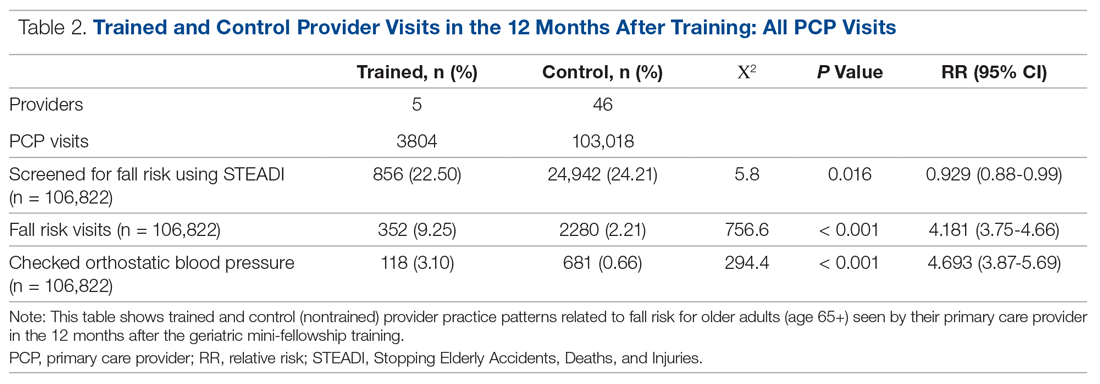
As shown in Table 3, for those patients determined to be at high risk of falling (STEADI score ≥ 4), fellows showed statistically significant increases in fall risk visits (RR, 3.02) and assessment of orthostatic blood pressure (RR, 10.68) before and after the mini-fellowship. The control providers did not show any changes in practice patterns between the pre- and post-period among patients at high risk to fall. 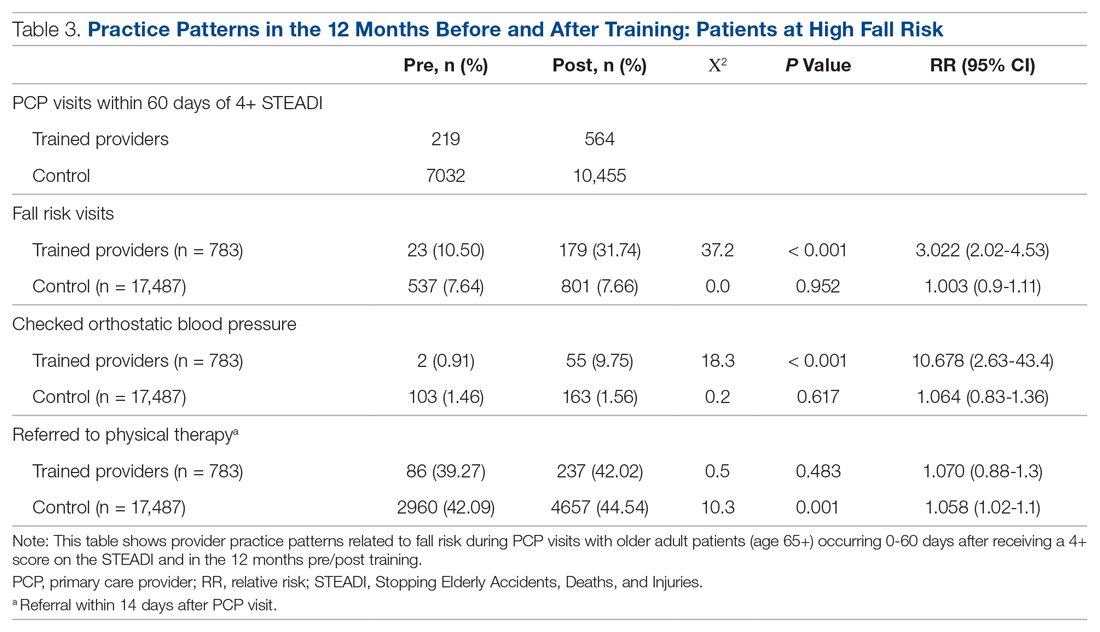
Neither the fellows nor the control group showed changes in patterns of referral to PT. In comparing the 2 groups in the 12 months after training (Table 4), for their patients at risk of falling, the fellows were 4 times more likely to complete fall risk visits and over 6 times more likely to assess orthostatic blood pressure than their nontrained peers. Subgroup analysis of the 75+ population revealed similar trends and significance, but these results are not included here.
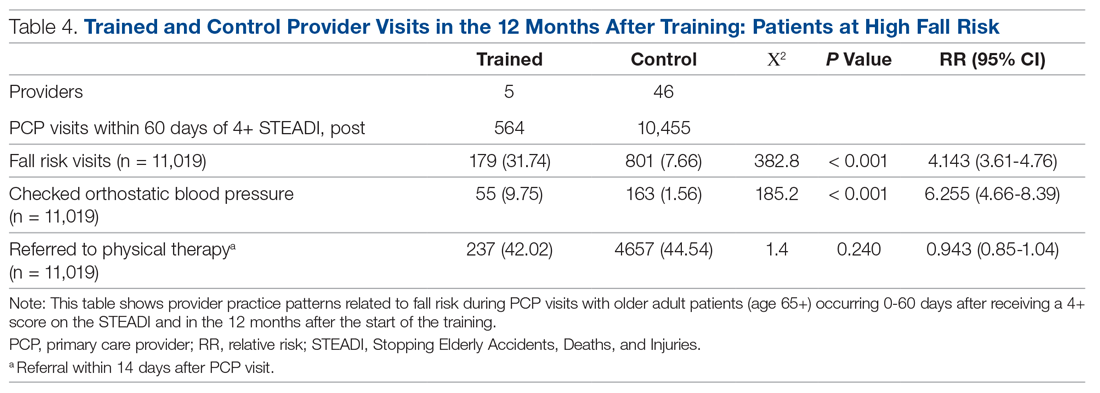
Discussion
This study aimed to improve not only providers’ knowledge and confidence in caring for older adults at increased risk to fall, but also their clinical practice in assessing and managing fall risk. In addition to improved knowledge and confidence, we found that the fellows increased their discussion of fall risk (through fall risk visits) and their assessment of orthostatic blood pressure for all of their patients, not just for those identified at increased risk to fall. This improvement held true for the fellows themselves before and after the intervention, but also as compared to their nontrained peers. These practice improvements for all of their 65+ patients, not just those identified as being at high risk to fall, are especially important, since studies indicate that early screening and intervention can help identify people at risk and prevent future falls.15
We were surprised that there were no significant differences in PT referrals made by the trained fellows, but this finding may have been confounded by the fact that the data included all PT referrals, regardless of diagnosis, not just those referrals that were fall-related. Furthermore, our baseline PT referral rates, at 39% for the intervention group and 42% for the control group, are higher than national data when looking at rehabilitation use by older adults.26
In comparison to a study evaluating the occurrence of fall risk–related clinical practice in primary care before any fall-related educational intervention, orthostatics were checked less frequently in our study (10% versus 30%) and there were fewer PT referrals (42%–44% versus 53%).27 However, the Phelan study took place in patients who had actually had a fall, rather than just having a higher risk for a fall, and was based on detailed chart review. Other studies23,24 found higher rates of fall risk interventions, but did not break out PT referrals specifically.
In terms of the educational intervention itself, most studies of geriatric education interventions have measured changes in knowledge, confidence, or self-efficacy as they relate to geriatric competence,28-30 and do not measure practice change as an outcome outside of intent to change or self-reported practice change.31,32 In general, practice change or longer-term health care–related outcomes have not been studied. Additionally, a range of dosages of educational interventions has been studied, from 1-hour lunchtime presentations23,32 to half-day29 or several half-day workshops,28 up to 160 hours over 10 months30 or 5 weekends over 6 months.31 The duration of our entire intervention at 160 hours over 6 months would be considered on the upper end of dosing relative to these studies, with our Mobility week intervention comprising 32 hours during 1 week. In the Warshaw study, despite 107 1-hour sessions being taught to over 60 physicians in 16 practices over 4 years, only 2 practices ultimately initiated any practice change projects.32 We believe that only curricula that embed practice change skills and opportunities, at a significant enough dose, can actually impact practice change in a sustainable manner.
Knowledge and skill acquisition among individual providers does not take place to a sufficient degree in the current health care arena, which is focused on productivity and short visit times. Consistent with other studies, we included interdisciplinary members of the primary care team for part of the mini-fellowship, although other studies used models that train across disciplines for the entirety of the learning experience.28-30,33 Our educational model was strengthened by including other professionals to provide some of the education and model the ideal geriatric team, including PT, occupational therapy, and pharmacy, for the week on mobility.
Most studies exploring interventions through geriatric educational initiatives are conducted within academic institutions, with a primary focus on physician faculty and, by extension, their teaching of residents and others.34,35 We believe our integrated model, which is steeped in community-based primary care practices like Lam’s,31 offers the greatest outreach to large community-based care systems and their patients. Training providers to work with their teams to change their own practices first gives skills and expertise that help further establish them as geriatric champions within their practices, laying the groundwork for more widespread practice change at their clinics.
Limitations
In addition to the limitations described above relating to the capture of PT referrals, other limitations included the relatively short time period for follow-up data as well as the small size of the intervention group. However, we found value in the instructional depth that the small group size allowed.
While the nontrained providers did show some improvement during the same period, we believe the relative risk was not clinically significant. We suspect that the larger health system efforts to standardize screening of patients 65+ across all clinics as a core quality metric confounded these results. The data analysis also included only fall-related patient visits that occurred with a provider who was that patient’s PCP, which could have missed visits done by other PCP colleagues, RNs, or pharmacists in the same clinic, thus undercounting the true number of fall-related visits. Furthermore, counting of fall-related interventions relied upon providers documenting consistently in the EHR, which could also lead to under-represention of fall risk clinical efforts.
The data presented, while encouraging, do not reflect clinic-wide practice change patterns and are considered only proximate outcomes rather than more long-term or cost-related outcomes, as would be captured by fall-related utilization measures like emergency room visits and hospitalizations. We expect to evaluate the broader impact and these value-based outcomes in the future. All providers and teams were from the same health care system, which may not allow our results to transfer to other organizations or regions of clinical practice.
Summary
This study demonstrates that an intensive mini-fellowship model of geriatrics training improved both knowledge and confidence in the realm of fall risk assessment and intervention among PCPs who had not been formally trained in geriatrics. More importantly, the training improved the fall-related care of their patients at increased risk to fall, but also of all of their older patients, with improvements in care measured up to a year after the mini-fellowship. Although this article only describes the work done as part of the Mobility aim of the 4M AFHS model, we believe the entire mini-fellowship curriculum offers the opportunity to “geriatricize” clinicians and their teams in learning geriatric principles and skills that they can translate into their practice in a sustainable way, as Tinetti encourages.8 Future study to evaluate other process outcomes more precisely, such as PT, as well as cost- and value-based outcomes, and the influence of trained providers on their clinic partners, will further establish the value proposition of targeted, disseminated, intensive geriatrics training of primary care clinicians as a strategy of age-friendly health systems as they work to improve the care of their older adults.
Acknowledgment: We are grateful for the dedication and hard work of the 2018 Geriatric Mini-Fellowship fellows at Providence Health & Services-Oregon who made this article possible. Thanks to Drs. Stephanie Cha, Emily Puukka-Clark, Laurie Dutkiewicz, Cara Ellis, Deb Frost, Jordan Roth, and Subhechchha Shah for promoting the AFHS work within their Providence Medical Group clinics and to PMG leadership and the fellows’ clinical teams for supporting the fellows, the AFHS work, and their older patients.
Corresponding author: Colleen M. Casey, PhD, ANP-BC, Providence Health & Services, Senior Health Program, 4400 NE Halsey, 5th Floor, Portland, OR 97213; [email protected].
Financial disclosures: None.
1. US Department of Health and Human Services. 2018 Profile of Older Americans. Administration on Aging. April 2018.
2. Roberts AW, Ogunwole SU, Blakeslee L, Rabe MA. The population 65 years and older in the United States: 2016. Washington, DC: US Census Bureau; 2018.
3. American Board of Medicine Specialties. 2017-2018 ABMS Board Certification Report. https://www.abms.org/board-certification/abms-board-certification-report/. Accessed November 3, 2020.
4. US Department of Health and Human Services, Health Resources and Services Administration, National Center for Health Workforce Analysis. National and regional projections of supply and demand for geriatricians: 2013-2025. Rockville, MD: US Department of Health and Human Services; 2007.
5. American Association of Nurse Practitioners, NP Facts: The Voice of the Nurse Practitioner. 2020. https://storage.aanp.org/www/documents/NPFacts__080420.pdf.
6. Tinetti ME, Naik AD, Dodson JA, Moving from disease-centered to patient goals-directed care for patients with multiple chronic conditions: patient value-based care. JAMA Cardiol. 2016;1:9-10.
7. Fried LP, Hall WJ. Editorial: leading on behalf of an aging society. J Am Geriatr Soc. 2008;56:1791-1795.
8. Tinetti M. Mainstream or extinction: can defining who we are save geriatrics? J Am Geriatr Soc. 2016;64:1400-1404.
9. Jafari P, Kostas T, Levine S, et al. ECHO-Chicago Geriatrics: using telementoring to “geriatricize” the primary care workforce. Gerontol Geriatr Educ. 2020;41:333-341.
10. Fulmer T, Mate KS, Berman A. The Age-Friendly Health System imperative. J Am Geriatr Soc. 2018;66:22-24.
11. Mate KS, Berman A, Laderman M, et al. Creating Age-Friendly Health Systems - A vision for better care of older adults. Healthc (Amst). 2018;6:4-6.
12. Tinetti ME, et al. Patient priority-directed decision making and care for older adults with multiple chronic conditions. Clin Geriatr Med. 2016;32:261-275.
13. Stevens JA, Phelan EA. Development of STEADI: a fall prevention resource for health care providers. Health Promot Pract. 2013;14:706-714.
14. Rubenstein LZ, et al. Validating an evidence-based, self-rated fall risk questionnaire (FRQ) for older adults. J Safety Res. 2011;42:493-499.
15. Grossman DC, et al. Interventions to prevent falls in community-dwelling older adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319: 1696-1704.
16. Tricco AC, Thomas SM, Veroniki AA, et al. Comparisons of interventions for preventing falls in older adults: a systematic review and meta-analysis. JAMA. 2017;318:1687-1699.
17. Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012(9):CD007146.
18. Bergen G, Stevens MR, Burns ER. Falls and fall injuries among adults aged ≥65 years - United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65:993-998.
19. Burns E, Kakara R. Deaths from falls among persons aged >=65 Years - United States, 2007-2016. MMWR Morb Mortal Wkly Rep. 2018;67:509-514.
20. Shankar KN, Liu SW, Ganz DA. Trends and characteristics of emergency department visits for fall-related injuries in older adults, 2003-2010. West J Emerg Med. 2017;18:785-793.
21. Hoffman GJ, et al. Posthospital fall injuries and 30-day readmissions in adults 65 years and older. JAMA Netw Open. 2019;2:e194276.
22. Eckstrom E, Parker EM, Shakya I, Lee R. Coordinated care plan to prevent older adult falls. 2018. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2018.
23. Eckstrom E, Parker EM, Lambert GH, et al. Implementing STEADI in academic primary care to address older adult fall risk. Innov Aging. 2017;1:igx028.
24. Johnston YA, Bergen G, Bauer M, et al. Implementation of the stopping elderly accidents, deaths, and injuries initiative in primary care: an outcome evaluation. Gerontologist. 2019;59:1182-1191.
25. Phelan EA, Mahoney JE, Voit JC, Stevens JA. Assessment and management of fall risk in primary care settings. Med Clin North Am. 2015;99:281-293.
26. Gell NM, Mroz TM, Patel KV. Rehabilitation services use and patient-reported outcomes among older adults in the United States. Arch Phys Med Rehabil. 2017;98:2221-2227.e3.
27. Phelan EA, Aerts S, Dowler D, et al. Adoption of evidence-based fall prevention practices in primary care for older adults with a history of falls. Front Public Health. 2016;4:190.
28. Solberg LB, Carter CS, Solberg LM. Geriatric care boot camp series: interprofessional education for a new training paradigm. Geriatr Nurs. 2019;40:579-583.
29. Solberg LB, Solberg LM, Carter CS. Geriatric care boot cAMP: an interprofessional education program for healthcare professionals. J Am Geriatr Soc. 2015;63:997-1001.
30. Coogle CL, Hackett L, Owens MG, et al. Perceived self-efficacy gains following an interprofessional faculty development programme in geriatrics education. J Interprof Care. 2016;30:483-492.
31. Lam R, Lee L, Tazkarji B, et al. Five-weekend care of the elderly certificate course: continuing professional development activity for family physicians. Can Fam Physician. 2015;61:e135-141.
32. Warshaw GA, Modawal A, Kues J, et al. Community physician education in geriatrics: applying the assessing care of vulnerable elders model with a multisite primary care group. J Am Geriatr Soc. 2010;58:1780-1785.
33. Solai LK, Kumar K, Mulvaney E, et al. Geriatric mental healthcare training: a mini-fellowship approach to interprofessional assessment and management of geriatric mental health issues. Am J Geriatr Psychiatry. 2019;27:706-711.
34. Christmas C, Park E, Schmaltz H, et al. A model intensive course in geriatric teaching for non-geriatrician educators. J Gen Intern Med. 2008;23:1048-1052.
35. Heflin MT, Bragg EJ, Fernandez H, et al. The Donald W. Reynolds Consortium for Faculty Development to Advance Geriatrics Education (FD~AGE): a model for dissemination of subspecialty educational expertise. Acad Med. 2012;87:618-626.
From the Senior Health Program, Providence Health & Services, Oregon, Portland, OR.
Abstract
Background: Approximately 51 million adults in the United States are 65 years of age or older, yet few geriatric-trained primary care providers (PCP) serve this population. The Age-Friendly Health System framework, consisting of evidence-based 4M care (Mobility, Medication, Mentation, and what Matters), encourages all PCPs to assess mobility in older adults.
Objective: To improve PCP knowledge, confidence, and clinical practice in assessing and managing fall risk.
Methods: A 1-week educational session focusing on mobility (part of a 4-week Geriatric Mini-Fellowship) for 6 selected PCPs from a large health care system was conducted to increase knowledge and ability to address fall risk in older adults. The week included learning and practicing a Fall Risk Management Plan (FRMP) algorithm, including planning for their own practice changes. Pre- and post-test surveys assessed changes in knowledge and confidence. Patient data were compared 12 months before and after training to evaluate PCP adoption of FRMP components.
Results: The training increased provider knowledge and confidence. The trained PCPs were 1.7 times more likely to screen for fall risk; 3.6 times more likely to discuss fall risk; and 5.8 times more likely to assess orthostatic blood pressure in their 65+ patients after the mini-fellowship. In high-risk patients, they were 4.1 times more likely to discuss fall risk and 6.3 times more likely to assess orthostatic blood pressure than their nontrained peers. Changes in physical therapy referral rates were not observed.
Conclusions: In-depth, skills-based geriatric educational sessions improved PCPs’ knowledge and confidence and also improved their fall risk management practices for their older patients.
Keywords: geriatrics; guidelines; Age-Friendly Health System; 4M; workforce training; practice change; fellowship.
The US population is aging rapidly. People aged 85 years and older are the largest-growing segment of the US population, and this segment is expected to increase by 123% by 2040.1 Caregiving needs increase with age as older adults develop more chronic conditions, such as hypertension, heart disease, arthritis, and dementia. However, even with increasing morbidity and dependence, a majority of older adults still live in the community rather than in institutional settings.2 These older adults seek medical care more frequently than younger people, with about 22% of patients 75 years and older having 10 or more health care visits in the previous 12 months. By 2040, nearly a quarter of the US population is expected to be 65 or older, with many of these older adults seeking regular primary care from providers who do not have formal training in the care of a population with multiple complex, chronic health conditions and increased caregiving needs.1
Despite this growing demand for health care professionals trained in the care of older adults, access to these types of clinicians is limited. In 2018, there were roughly 7000 certified geriatricians, with only 3600 of them practicing full-time.3,4 Similarly, of 290,000 certified nurse practitioners (NPs), about 9% of them have geriatric certification.5 Geriatricians, medical doctors trained in the care of older adults, and geriatric-trained NPs are part of a cadre of a geriatric-trained workforce that provides unique expertise in caring for older adults with chronic and advanced illness. They know how to manage multiple, complex geriatric syndromes like falls, dementia, and polypharmacy; understand and maximize team-based care; and focus on caring for an older person with a goal-centered versus a disease-centered approach.6
Broadly, geriatric care includes a spectrum of adults, from those who are aging healthfully to those who are the frailest. Research has suggested that approximately 30% of older adults need care by a geriatric-trained clinician, with the oldest and frailest patients needing more clinician time for assessment and treatment, care coordination, and coaching of caregivers.7 With this assumption in mind, it is projected that by 2025, there will be a national shortage of 26,980 geriatricians, with the western United States disproportionately affected by this shortage.4Rather than lamenting this shortage, Tinetti recommends a new path forward: “Our mission should not be to train enough geriatricians to provide direct care, but rather to ensure that every clinician caring for older adults is competent in geriatric principles and practices.”8 Sometimes called ”geriatricizing,” the idea is to use existing geriatric providers as a small elite training force to infuse geriatric principles and skills across their colleagues in primary care and other disciplines.8,9 Efforts of the American Geriatrics Society (AGS), with support from the John A. Hartford Foundation (JAHF), have been successful in developing geriatric training across multiple specialties, including surgery, orthopedics, and emergency medicine (www.americangeriatrics.org/programs/geriatrics-specialists-initiative).
The Age-Friendly Health System and 4M Model
To help augment this idea of equipping health care systems and their clinicians with more readily available geriatric knowledge, skills, and tools, the JAHF, along with the Institute for Healthcare Improvement (IHI), created the Age-Friendly Health System (AFHS) paradigm in 2015.10 Using the 4M model, the AFHS initiative established a set of evidence-based geriatric priorities and interventions meant to improve the care of older adults, reduce harm and duplication, and provide a framework for engaging leadership, clinical teams, and operational systems across inpatient and ambulatory settings.11 Mobility, including fall risk screening and intervention, is 1 of the 4M foundational elements of the Age-Friendly model. In addition to Mobility, the 4M model also includes 3 other key geriatric domains: Mentation (dementia, depression, and delirium), Medication (high-risk medications, polypharmacy, and deprescribing), and What Matters (goals of care conversations and understanding quality of life for older patients).11 The 4M initiative encourages adoption of a geriatric lens that looks across chronic conditions and accounts for the interplay among geriatric syndromes, such as falls, cognitive impairment, and frailty, in order to provide care better tailored to what the patient needs and desires.12 IHI and JAHF have targeted the adoption of the 4M model by 20% of US health care systems by 2020.11
Mini-Fellowship and Mobility Week
To bolster geriatric skills among community-based primary care providers (PCPs), we initiated a Geriatric Mini-Fellowship, a 4-week condensed curriculum taught over 6 months. Each week focuses on 1 of the age-friendly 4Ms, with the goal of increasing the knowledge, self-efficacy, skills, and competencies of the participating PCPs (called “fellow” hereafter) and at the same time, equipping each to become a champion of geriatric practice. This article focuses on the Mobility week, the second week of the mini-fellowship, and the effect of the week on the fellows’ practice changes.
To construct the Mobility week’s curriculum with a focus on the ambulatory setting, we relied upon national evidence-based work in fall risk management. The Centers for Disease Control and Prevention (CDC) has made fall risk screening and management in primary care a high priority. Using the clinical practice guidelines for managing fall risk developed by the American and British Geriatrics Societies (AGS/BGS), the CDC developed the Stopping Elderly Accidents, Deaths, and Injuries (STEADI) toolkit.13 Foundational to the toolkit is the validated 12-item Stay Independent falls screening questionnaire (STEADI questionnaire).14 Patients who score 4 or higher (out of a total score of 14) on the questionnaire are considered at increased risk of falling. The CDC has developed a clinical algorithm that guides clinical teams through screening and assessment to help identify appropriate interventions to target specific risk factors. Research has clearly established that a multifactorial approach to fall risk intervention can be successful in reducing fall risk by as much as 25%.15-17
The significant morbidity and mortality caused by falls make training nongeriatrician clinicians on how to better address fall risk imperative. More than 25% of older adults fall each year.18 These falls contribute to rising rates of fall-related deaths,19 emergency department (ED) visits,20 and hospital readmissions.21 Initiatives like the AFHS focus on mobility and the CDC’s development of supporting clinical materials22 aim to improve primary care adoption of fall risk screening and intervention practices.23,24 The epidemic of falls must compel all PCPs, not just those practicing geriatrics, to make discussing and addressing fall risk and falls a priority.
Methods
Setting
This project took place as part of a regional primary care effort in Oregon. Providence Health & Services-Oregon is part of a multi-state integrated health care system in the western United States whose PCPs serve more than 80,000 patients aged 65 years and older per year; these patients comprise 38% of the system’s office visits each year. Regionally, there are 47 family and internal medicine clinics employing roughly 290 providers (physicians, NPs, and physician assistants). The organization has only 4 PCPs trained in geriatrics and does not offer any geriatric clinical consultation services. Six PCPs from different clinics, representing both rural and urban settings, are chosen to participate in the geriatric mini-fellowship each year.
This project was conducted as a quality improvement initiative within the organization and did not constitute human subjects research. It was not conducted under the oversight of the Institutional Review Board.
Intervention
The mini-fellowship was taught in 4 1-week blocks between April and October 2018, with a curriculum designed to be interactive and practical. The faculty was intentionally interdisciplinary to teach and model team-based practice. Each week participants were excused from their clinical practice. Approximately 160 hours of continuing medical education credits were awarded for the full mini-fellowship. As part of each weekly session, a performance improvement project (PIP) focused on that week’s topic (1 of the 4Ms) was developed by the fellow and their team members to incorporate the mini-fellowship learnings into their clinic workflows. Fellows also had 2 hours per week of dedicated administration time for a year, outside the fellowship, to work on their PIP and 4M practice changes within their clinic.
Provider Education
The week for mobility training comprised 4 daylong sessions. The first 2 days were spent learning about the epidemiology of falls; risk factors for falling; how to conduct a thorough history and assessment of fall risk; and how to create a prioritized Fall Risk Management Plan (FRMP) to decrease a patient’s individual fall risk through tailored interventions. The FRMP was adapted from the CDC STEADI toolkit.13 Core faculty were 2 geriatric-trained providers (NP and physician) and a physical therapist (PT) specializing in fall prevention.
On the third day, fellows took part in a simulated fall risk clinic, in which older adults volunteered to be patient partners, providing an opportunity to apply learnings from days 1 and 2. The clinic included the fellow observing a PT complete a mobility assessment and a pharmacist conduct a high-risk medication review. The fellow synthesized the findings of the mobility assessment and medication review, as well as their own history and assessment, to create a summary of fall risk recommendations to discuss with their volunteer patient partner. The fellows were observed and evaluated in their skills by their patient partner, course faculty, and another fellow. The patient partners, and their assigned fellow, also participated in a 45-minute fall risk presentation, led by a nurse.
On the fourth day, the fellows were joined by select clinic partners, including nurses, pharmacists, and/or medical assistants. The session included discussions among each fellow’s clinical team regarding the current state of fall risk efforts at their clinic, an analysis of barriers, and identification of opportunities to improve workflows and screening rates. Each fellow took with them an action plan tailored to their clinic to improve fall risk management practices, starting with the fellow’s own practice.

Fall Risk Management Plan
The educational sessions introduced the fellows to the FRMP. The FRMP, adapted from the STEADI toolkit, includes a process for fall risk screening (Figure 1) and stratifying a patient’s risk based on their STEADI score in order to promote 3 priority assessments (gait evaluation with PT referral if appropriate; orthostatic blood pressure; and high-risk medication review; Figure 2). Initial actions based on these priority assessments were followed over time, with additional fall risk interventions added as clinically indicated.25 The FRMP is intended to be used during routine office visits, Medicare annual wellness visits, or office visits focused on fall risk or related medical disorders (ie, fall risk visits.)

Providers and their teams were encouraged to spread out fall-related conversations with their patients over multiple visits, since many patients have multiple fall risk factors at play, in addition to other chronic medical issues, and since many interventions often require behavior changes on the part of the patient. Providers also had access to fall-related electronic health record (EHR) templates as well as a comprehensive, internal fall risk management website that included assessment tools, evidence-based resources, and patient handouts.
Assessment and Measurements
We assessed provider knowledge and comfort in their fall risk evaluation and management skills before and after the educational intervention using an 11-item multiple-choice questionnaire and a 4-item confidence questionnaire. The confidence questions used a 7-point Likert scale, with 0 indicating “no confidence” and 7 indicating ”lots of confidence.” The questions were administered via a paper survey. Qualitative comments were derived from evaluations completed at the end of the week.
The fellows’ practice of fall risk screening and management was studied from May 2018, at the completion of Mobility week, to May 2019 for the post-intervention period. A 1-year timeframe before May 2018 was used as the pre-intervention period. Eligible visit types, during which we assumed fall risk was discussed, were any office visits for patients 65+ completed by the patients’ PCPs that used fall risk as a reason for the visit or had a fall-related diagnosis code. Fall risk visits performed by other clinic providers were not counted.
Of those patients who had fall risk screenings completed and were determined to be high risk (STEADI score ≥ 4), data were analyzed to determine whether these patients had any fall-related follow-up visits to their PCP within 60 days of the STEADI screening. For these high-risk patients, data were studied to understand whether orthostatic blood pressure measurements were performed (as documented in a flowsheet) and whether a PT referral was placed. These data were compared with those from providers who practiced in clinics within the same system but who did not participate in the mini-fellowship. Data were obtained from the organization’s EHR. Additional data were measured to evaluate patterns of deprescribing of select high-risk medications, but these data are not included in this analysis.
Analysis
A paired-samples t test was used to measure changes in provider confidence levels. Data were aggregated across fellows, resulting in a mean. A chi-square test of independence was performed to examine the relationship between rates of FRMP adoption by select provider groups. Analysis included a pre- and post-intervention assessment of the fellows’ adoption of FRMP practices, as well as a comparison between the fellows’ practice patterns and those of a control group of PCPs in the organization’s other clinics who did not participate in the mini-fellowship (nontrained control group). Excluded from the control group were providers from the same clinic as the fellows; providers in clinics with a geriatric-trained provider on staff; and clinics outside of the Portland metro and Medford service areas. We used an alpha level of 0.05 for all statistical tests.
Data from 5 providers were included in the analysis of the FRMP adoption. The sixth provider changed practice settings from the clinic to the ED after completing the fellowship; her patient data were not included in the FRMP part of the analysis. EHR data included data on all visits of patients 65+, as well as data for just those 65+ patients who had been identified as being at high risk to fall based on a STEADI score of 4 or higher.
Results
Provider Questionnaire
All 6 providers responded to the pre-intervention and post-intervention tests. For the knowledge questions, fellows, as a composite, correctly answered 57% of the questions before the intervention and 79% after the intervention. Provider confidence level in delivering fall risk care was measured prior to the training (mean, 4.12 [SD, 0.62]) and at the end of the training (mean, 6.47 [SD, 0.45]), demonstrating a significant increase in confidence (t (5) = –10.46, P < 0.001).
Qualitative Comments
Providers also had the opportunity to provide comments on their experience during the Mobility week and at the end of 1 year. In general, the simulated interdisciplinary fall risk clinic was highly rated (“the highlight of the week”) as a practical strategy to embed learning principles. One fellow commented, “Putting the learning into practice helps solidify it in my brain.” Fellows also appreciated the opportunity to learn and meet with their clinic colleagues to begin work on a fall-risk focused PIP and to “have a framework for what to do for people who screen positive [for fall risk].”
FRMP Adoption
A comparison of the care the fellows provided to their patients 65+ in the 12 months pre- and post-training shows the fellows demonstrated significant changes in practice patterns. The fellows were 1.7 times more likely to screen for fall risk; 3.6 times more likely to discuss fall risk; and 5.8 times more likely to check orthostatic blood pressure than prior to the mini-fellowship (Table 1). 
The control providers also demonstrated significant increases in fall risk screening and discussion of fall risk between the pre- and post-intervention periods; however, the relative risk (RR) was between 1.10 and 1.13 for this group. For the control group, checking orthostatic blood pressure did not significantly change. In the 12 months after training (Table 2), the fellows were 4.2 times more likely to discuss fall risk and almost 5 times more likely to check orthostatic blood pressure than their nontrained peers for all of their patients 65+, regardless of their risk to fall.
As shown in Table 3, for those patients determined to be at high risk of falling (STEADI score ≥ 4), fellows showed statistically significant increases in fall risk visits (RR, 3.02) and assessment of orthostatic blood pressure (RR, 10.68) before and after the mini-fellowship. The control providers did not show any changes in practice patterns between the pre- and post-period among patients at high risk to fall. 
Neither the fellows nor the control group showed changes in patterns of referral to PT. In comparing the 2 groups in the 12 months after training (Table 4), for their patients at risk of falling, the fellows were 4 times more likely to complete fall risk visits and over 6 times more likely to assess orthostatic blood pressure than their nontrained peers. Subgroup analysis of the 75+ population revealed similar trends and significance, but these results are not included here.

Discussion
This study aimed to improve not only providers’ knowledge and confidence in caring for older adults at increased risk to fall, but also their clinical practice in assessing and managing fall risk. In addition to improved knowledge and confidence, we found that the fellows increased their discussion of fall risk (through fall risk visits) and their assessment of orthostatic blood pressure for all of their patients, not just for those identified at increased risk to fall. This improvement held true for the fellows themselves before and after the intervention, but also as compared to their nontrained peers. These practice improvements for all of their 65+ patients, not just those identified as being at high risk to fall, are especially important, since studies indicate that early screening and intervention can help identify people at risk and prevent future falls.15
We were surprised that there were no significant differences in PT referrals made by the trained fellows, but this finding may have been confounded by the fact that the data included all PT referrals, regardless of diagnosis, not just those referrals that were fall-related. Furthermore, our baseline PT referral rates, at 39% for the intervention group and 42% for the control group, are higher than national data when looking at rehabilitation use by older adults.26
In comparison to a study evaluating the occurrence of fall risk–related clinical practice in primary care before any fall-related educational intervention, orthostatics were checked less frequently in our study (10% versus 30%) and there were fewer PT referrals (42%–44% versus 53%).27 However, the Phelan study took place in patients who had actually had a fall, rather than just having a higher risk for a fall, and was based on detailed chart review. Other studies23,24 found higher rates of fall risk interventions, but did not break out PT referrals specifically.
In terms of the educational intervention itself, most studies of geriatric education interventions have measured changes in knowledge, confidence, or self-efficacy as they relate to geriatric competence,28-30 and do not measure practice change as an outcome outside of intent to change or self-reported practice change.31,32 In general, practice change or longer-term health care–related outcomes have not been studied. Additionally, a range of dosages of educational interventions has been studied, from 1-hour lunchtime presentations23,32 to half-day29 or several half-day workshops,28 up to 160 hours over 10 months30 or 5 weekends over 6 months.31 The duration of our entire intervention at 160 hours over 6 months would be considered on the upper end of dosing relative to these studies, with our Mobility week intervention comprising 32 hours during 1 week. In the Warshaw study, despite 107 1-hour sessions being taught to over 60 physicians in 16 practices over 4 years, only 2 practices ultimately initiated any practice change projects.32 We believe that only curricula that embed practice change skills and opportunities, at a significant enough dose, can actually impact practice change in a sustainable manner.
Knowledge and skill acquisition among individual providers does not take place to a sufficient degree in the current health care arena, which is focused on productivity and short visit times. Consistent with other studies, we included interdisciplinary members of the primary care team for part of the mini-fellowship, although other studies used models that train across disciplines for the entirety of the learning experience.28-30,33 Our educational model was strengthened by including other professionals to provide some of the education and model the ideal geriatric team, including PT, occupational therapy, and pharmacy, for the week on mobility.
Most studies exploring interventions through geriatric educational initiatives are conducted within academic institutions, with a primary focus on physician faculty and, by extension, their teaching of residents and others.34,35 We believe our integrated model, which is steeped in community-based primary care practices like Lam’s,31 offers the greatest outreach to large community-based care systems and their patients. Training providers to work with their teams to change their own practices first gives skills and expertise that help further establish them as geriatric champions within their practices, laying the groundwork for more widespread practice change at their clinics.
Limitations
In addition to the limitations described above relating to the capture of PT referrals, other limitations included the relatively short time period for follow-up data as well as the small size of the intervention group. However, we found value in the instructional depth that the small group size allowed.
While the nontrained providers did show some improvement during the same period, we believe the relative risk was not clinically significant. We suspect that the larger health system efforts to standardize screening of patients 65+ across all clinics as a core quality metric confounded these results. The data analysis also included only fall-related patient visits that occurred with a provider who was that patient’s PCP, which could have missed visits done by other PCP colleagues, RNs, or pharmacists in the same clinic, thus undercounting the true number of fall-related visits. Furthermore, counting of fall-related interventions relied upon providers documenting consistently in the EHR, which could also lead to under-represention of fall risk clinical efforts.
The data presented, while encouraging, do not reflect clinic-wide practice change patterns and are considered only proximate outcomes rather than more long-term or cost-related outcomes, as would be captured by fall-related utilization measures like emergency room visits and hospitalizations. We expect to evaluate the broader impact and these value-based outcomes in the future. All providers and teams were from the same health care system, which may not allow our results to transfer to other organizations or regions of clinical practice.
Summary
This study demonstrates that an intensive mini-fellowship model of geriatrics training improved both knowledge and confidence in the realm of fall risk assessment and intervention among PCPs who had not been formally trained in geriatrics. More importantly, the training improved the fall-related care of their patients at increased risk to fall, but also of all of their older patients, with improvements in care measured up to a year after the mini-fellowship. Although this article only describes the work done as part of the Mobility aim of the 4M AFHS model, we believe the entire mini-fellowship curriculum offers the opportunity to “geriatricize” clinicians and their teams in learning geriatric principles and skills that they can translate into their practice in a sustainable way, as Tinetti encourages.8 Future study to evaluate other process outcomes more precisely, such as PT, as well as cost- and value-based outcomes, and the influence of trained providers on their clinic partners, will further establish the value proposition of targeted, disseminated, intensive geriatrics training of primary care clinicians as a strategy of age-friendly health systems as they work to improve the care of their older adults.
Acknowledgment: We are grateful for the dedication and hard work of the 2018 Geriatric Mini-Fellowship fellows at Providence Health & Services-Oregon who made this article possible. Thanks to Drs. Stephanie Cha, Emily Puukka-Clark, Laurie Dutkiewicz, Cara Ellis, Deb Frost, Jordan Roth, and Subhechchha Shah for promoting the AFHS work within their Providence Medical Group clinics and to PMG leadership and the fellows’ clinical teams for supporting the fellows, the AFHS work, and their older patients.
Corresponding author: Colleen M. Casey, PhD, ANP-BC, Providence Health & Services, Senior Health Program, 4400 NE Halsey, 5th Floor, Portland, OR 97213; [email protected].
Financial disclosures: None.
From the Senior Health Program, Providence Health & Services, Oregon, Portland, OR.
Abstract
Background: Approximately 51 million adults in the United States are 65 years of age or older, yet few geriatric-trained primary care providers (PCP) serve this population. The Age-Friendly Health System framework, consisting of evidence-based 4M care (Mobility, Medication, Mentation, and what Matters), encourages all PCPs to assess mobility in older adults.
Objective: To improve PCP knowledge, confidence, and clinical practice in assessing and managing fall risk.
Methods: A 1-week educational session focusing on mobility (part of a 4-week Geriatric Mini-Fellowship) for 6 selected PCPs from a large health care system was conducted to increase knowledge and ability to address fall risk in older adults. The week included learning and practicing a Fall Risk Management Plan (FRMP) algorithm, including planning for their own practice changes. Pre- and post-test surveys assessed changes in knowledge and confidence. Patient data were compared 12 months before and after training to evaluate PCP adoption of FRMP components.
Results: The training increased provider knowledge and confidence. The trained PCPs were 1.7 times more likely to screen for fall risk; 3.6 times more likely to discuss fall risk; and 5.8 times more likely to assess orthostatic blood pressure in their 65+ patients after the mini-fellowship. In high-risk patients, they were 4.1 times more likely to discuss fall risk and 6.3 times more likely to assess orthostatic blood pressure than their nontrained peers. Changes in physical therapy referral rates were not observed.
Conclusions: In-depth, skills-based geriatric educational sessions improved PCPs’ knowledge and confidence and also improved their fall risk management practices for their older patients.
Keywords: geriatrics; guidelines; Age-Friendly Health System; 4M; workforce training; practice change; fellowship.
The US population is aging rapidly. People aged 85 years and older are the largest-growing segment of the US population, and this segment is expected to increase by 123% by 2040.1 Caregiving needs increase with age as older adults develop more chronic conditions, such as hypertension, heart disease, arthritis, and dementia. However, even with increasing morbidity and dependence, a majority of older adults still live in the community rather than in institutional settings.2 These older adults seek medical care more frequently than younger people, with about 22% of patients 75 years and older having 10 or more health care visits in the previous 12 months. By 2040, nearly a quarter of the US population is expected to be 65 or older, with many of these older adults seeking regular primary care from providers who do not have formal training in the care of a population with multiple complex, chronic health conditions and increased caregiving needs.1
Despite this growing demand for health care professionals trained in the care of older adults, access to these types of clinicians is limited. In 2018, there were roughly 7000 certified geriatricians, with only 3600 of them practicing full-time.3,4 Similarly, of 290,000 certified nurse practitioners (NPs), about 9% of them have geriatric certification.5 Geriatricians, medical doctors trained in the care of older adults, and geriatric-trained NPs are part of a cadre of a geriatric-trained workforce that provides unique expertise in caring for older adults with chronic and advanced illness. They know how to manage multiple, complex geriatric syndromes like falls, dementia, and polypharmacy; understand and maximize team-based care; and focus on caring for an older person with a goal-centered versus a disease-centered approach.6
Broadly, geriatric care includes a spectrum of adults, from those who are aging healthfully to those who are the frailest. Research has suggested that approximately 30% of older adults need care by a geriatric-trained clinician, with the oldest and frailest patients needing more clinician time for assessment and treatment, care coordination, and coaching of caregivers.7 With this assumption in mind, it is projected that by 2025, there will be a national shortage of 26,980 geriatricians, with the western United States disproportionately affected by this shortage.4Rather than lamenting this shortage, Tinetti recommends a new path forward: “Our mission should not be to train enough geriatricians to provide direct care, but rather to ensure that every clinician caring for older adults is competent in geriatric principles and practices.”8 Sometimes called ”geriatricizing,” the idea is to use existing geriatric providers as a small elite training force to infuse geriatric principles and skills across their colleagues in primary care and other disciplines.8,9 Efforts of the American Geriatrics Society (AGS), with support from the John A. Hartford Foundation (JAHF), have been successful in developing geriatric training across multiple specialties, including surgery, orthopedics, and emergency medicine (www.americangeriatrics.org/programs/geriatrics-specialists-initiative).
The Age-Friendly Health System and 4M Model
To help augment this idea of equipping health care systems and their clinicians with more readily available geriatric knowledge, skills, and tools, the JAHF, along with the Institute for Healthcare Improvement (IHI), created the Age-Friendly Health System (AFHS) paradigm in 2015.10 Using the 4M model, the AFHS initiative established a set of evidence-based geriatric priorities and interventions meant to improve the care of older adults, reduce harm and duplication, and provide a framework for engaging leadership, clinical teams, and operational systems across inpatient and ambulatory settings.11 Mobility, including fall risk screening and intervention, is 1 of the 4M foundational elements of the Age-Friendly model. In addition to Mobility, the 4M model also includes 3 other key geriatric domains: Mentation (dementia, depression, and delirium), Medication (high-risk medications, polypharmacy, and deprescribing), and What Matters (goals of care conversations and understanding quality of life for older patients).11 The 4M initiative encourages adoption of a geriatric lens that looks across chronic conditions and accounts for the interplay among geriatric syndromes, such as falls, cognitive impairment, and frailty, in order to provide care better tailored to what the patient needs and desires.12 IHI and JAHF have targeted the adoption of the 4M model by 20% of US health care systems by 2020.11
Mini-Fellowship and Mobility Week
To bolster geriatric skills among community-based primary care providers (PCPs), we initiated a Geriatric Mini-Fellowship, a 4-week condensed curriculum taught over 6 months. Each week focuses on 1 of the age-friendly 4Ms, with the goal of increasing the knowledge, self-efficacy, skills, and competencies of the participating PCPs (called “fellow” hereafter) and at the same time, equipping each to become a champion of geriatric practice. This article focuses on the Mobility week, the second week of the mini-fellowship, and the effect of the week on the fellows’ practice changes.
To construct the Mobility week’s curriculum with a focus on the ambulatory setting, we relied upon national evidence-based work in fall risk management. The Centers for Disease Control and Prevention (CDC) has made fall risk screening and management in primary care a high priority. Using the clinical practice guidelines for managing fall risk developed by the American and British Geriatrics Societies (AGS/BGS), the CDC developed the Stopping Elderly Accidents, Deaths, and Injuries (STEADI) toolkit.13 Foundational to the toolkit is the validated 12-item Stay Independent falls screening questionnaire (STEADI questionnaire).14 Patients who score 4 or higher (out of a total score of 14) on the questionnaire are considered at increased risk of falling. The CDC has developed a clinical algorithm that guides clinical teams through screening and assessment to help identify appropriate interventions to target specific risk factors. Research has clearly established that a multifactorial approach to fall risk intervention can be successful in reducing fall risk by as much as 25%.15-17
The significant morbidity and mortality caused by falls make training nongeriatrician clinicians on how to better address fall risk imperative. More than 25% of older adults fall each year.18 These falls contribute to rising rates of fall-related deaths,19 emergency department (ED) visits,20 and hospital readmissions.21 Initiatives like the AFHS focus on mobility and the CDC’s development of supporting clinical materials22 aim to improve primary care adoption of fall risk screening and intervention practices.23,24 The epidemic of falls must compel all PCPs, not just those practicing geriatrics, to make discussing and addressing fall risk and falls a priority.
Methods
Setting
This project took place as part of a regional primary care effort in Oregon. Providence Health & Services-Oregon is part of a multi-state integrated health care system in the western United States whose PCPs serve more than 80,000 patients aged 65 years and older per year; these patients comprise 38% of the system’s office visits each year. Regionally, there are 47 family and internal medicine clinics employing roughly 290 providers (physicians, NPs, and physician assistants). The organization has only 4 PCPs trained in geriatrics and does not offer any geriatric clinical consultation services. Six PCPs from different clinics, representing both rural and urban settings, are chosen to participate in the geriatric mini-fellowship each year.
This project was conducted as a quality improvement initiative within the organization and did not constitute human subjects research. It was not conducted under the oversight of the Institutional Review Board.
Intervention
The mini-fellowship was taught in 4 1-week blocks between April and October 2018, with a curriculum designed to be interactive and practical. The faculty was intentionally interdisciplinary to teach and model team-based practice. Each week participants were excused from their clinical practice. Approximately 160 hours of continuing medical education credits were awarded for the full mini-fellowship. As part of each weekly session, a performance improvement project (PIP) focused on that week’s topic (1 of the 4Ms) was developed by the fellow and their team members to incorporate the mini-fellowship learnings into their clinic workflows. Fellows also had 2 hours per week of dedicated administration time for a year, outside the fellowship, to work on their PIP and 4M practice changes within their clinic.
Provider Education
The week for mobility training comprised 4 daylong sessions. The first 2 days were spent learning about the epidemiology of falls; risk factors for falling; how to conduct a thorough history and assessment of fall risk; and how to create a prioritized Fall Risk Management Plan (FRMP) to decrease a patient’s individual fall risk through tailored interventions. The FRMP was adapted from the CDC STEADI toolkit.13 Core faculty were 2 geriatric-trained providers (NP and physician) and a physical therapist (PT) specializing in fall prevention.
On the third day, fellows took part in a simulated fall risk clinic, in which older adults volunteered to be patient partners, providing an opportunity to apply learnings from days 1 and 2. The clinic included the fellow observing a PT complete a mobility assessment and a pharmacist conduct a high-risk medication review. The fellow synthesized the findings of the mobility assessment and medication review, as well as their own history and assessment, to create a summary of fall risk recommendations to discuss with their volunteer patient partner. The fellows were observed and evaluated in their skills by their patient partner, course faculty, and another fellow. The patient partners, and their assigned fellow, also participated in a 45-minute fall risk presentation, led by a nurse.
On the fourth day, the fellows were joined by select clinic partners, including nurses, pharmacists, and/or medical assistants. The session included discussions among each fellow’s clinical team regarding the current state of fall risk efforts at their clinic, an analysis of barriers, and identification of opportunities to improve workflows and screening rates. Each fellow took with them an action plan tailored to their clinic to improve fall risk management practices, starting with the fellow’s own practice.

Fall Risk Management Plan
The educational sessions introduced the fellows to the FRMP. The FRMP, adapted from the STEADI toolkit, includes a process for fall risk screening (Figure 1) and stratifying a patient’s risk based on their STEADI score in order to promote 3 priority assessments (gait evaluation with PT referral if appropriate; orthostatic blood pressure; and high-risk medication review; Figure 2). Initial actions based on these priority assessments were followed over time, with additional fall risk interventions added as clinically indicated.25 The FRMP is intended to be used during routine office visits, Medicare annual wellness visits, or office visits focused on fall risk or related medical disorders (ie, fall risk visits.)

Providers and their teams were encouraged to spread out fall-related conversations with their patients over multiple visits, since many patients have multiple fall risk factors at play, in addition to other chronic medical issues, and since many interventions often require behavior changes on the part of the patient. Providers also had access to fall-related electronic health record (EHR) templates as well as a comprehensive, internal fall risk management website that included assessment tools, evidence-based resources, and patient handouts.
Assessment and Measurements
We assessed provider knowledge and comfort in their fall risk evaluation and management skills before and after the educational intervention using an 11-item multiple-choice questionnaire and a 4-item confidence questionnaire. The confidence questions used a 7-point Likert scale, with 0 indicating “no confidence” and 7 indicating ”lots of confidence.” The questions were administered via a paper survey. Qualitative comments were derived from evaluations completed at the end of the week.
The fellows’ practice of fall risk screening and management was studied from May 2018, at the completion of Mobility week, to May 2019 for the post-intervention period. A 1-year timeframe before May 2018 was used as the pre-intervention period. Eligible visit types, during which we assumed fall risk was discussed, were any office visits for patients 65+ completed by the patients’ PCPs that used fall risk as a reason for the visit or had a fall-related diagnosis code. Fall risk visits performed by other clinic providers were not counted.
Of those patients who had fall risk screenings completed and were determined to be high risk (STEADI score ≥ 4), data were analyzed to determine whether these patients had any fall-related follow-up visits to their PCP within 60 days of the STEADI screening. For these high-risk patients, data were studied to understand whether orthostatic blood pressure measurements were performed (as documented in a flowsheet) and whether a PT referral was placed. These data were compared with those from providers who practiced in clinics within the same system but who did not participate in the mini-fellowship. Data were obtained from the organization’s EHR. Additional data were measured to evaluate patterns of deprescribing of select high-risk medications, but these data are not included in this analysis.
Analysis
A paired-samples t test was used to measure changes in provider confidence levels. Data were aggregated across fellows, resulting in a mean. A chi-square test of independence was performed to examine the relationship between rates of FRMP adoption by select provider groups. Analysis included a pre- and post-intervention assessment of the fellows’ adoption of FRMP practices, as well as a comparison between the fellows’ practice patterns and those of a control group of PCPs in the organization’s other clinics who did not participate in the mini-fellowship (nontrained control group). Excluded from the control group were providers from the same clinic as the fellows; providers in clinics with a geriatric-trained provider on staff; and clinics outside of the Portland metro and Medford service areas. We used an alpha level of 0.05 for all statistical tests.
Data from 5 providers were included in the analysis of the FRMP adoption. The sixth provider changed practice settings from the clinic to the ED after completing the fellowship; her patient data were not included in the FRMP part of the analysis. EHR data included data on all visits of patients 65+, as well as data for just those 65+ patients who had been identified as being at high risk to fall based on a STEADI score of 4 or higher.
Results
Provider Questionnaire
All 6 providers responded to the pre-intervention and post-intervention tests. For the knowledge questions, fellows, as a composite, correctly answered 57% of the questions before the intervention and 79% after the intervention. Provider confidence level in delivering fall risk care was measured prior to the training (mean, 4.12 [SD, 0.62]) and at the end of the training (mean, 6.47 [SD, 0.45]), demonstrating a significant increase in confidence (t (5) = –10.46, P < 0.001).
Qualitative Comments
Providers also had the opportunity to provide comments on their experience during the Mobility week and at the end of 1 year. In general, the simulated interdisciplinary fall risk clinic was highly rated (“the highlight of the week”) as a practical strategy to embed learning principles. One fellow commented, “Putting the learning into practice helps solidify it in my brain.” Fellows also appreciated the opportunity to learn and meet with their clinic colleagues to begin work on a fall-risk focused PIP and to “have a framework for what to do for people who screen positive [for fall risk].”
FRMP Adoption
A comparison of the care the fellows provided to their patients 65+ in the 12 months pre- and post-training shows the fellows demonstrated significant changes in practice patterns. The fellows were 1.7 times more likely to screen for fall risk; 3.6 times more likely to discuss fall risk; and 5.8 times more likely to check orthostatic blood pressure than prior to the mini-fellowship (Table 1). 
The control providers also demonstrated significant increases in fall risk screening and discussion of fall risk between the pre- and post-intervention periods; however, the relative risk (RR) was between 1.10 and 1.13 for this group. For the control group, checking orthostatic blood pressure did not significantly change. In the 12 months after training (Table 2), the fellows were 4.2 times more likely to discuss fall risk and almost 5 times more likely to check orthostatic blood pressure than their nontrained peers for all of their patients 65+, regardless of their risk to fall.
As shown in Table 3, for those patients determined to be at high risk of falling (STEADI score ≥ 4), fellows showed statistically significant increases in fall risk visits (RR, 3.02) and assessment of orthostatic blood pressure (RR, 10.68) before and after the mini-fellowship. The control providers did not show any changes in practice patterns between the pre- and post-period among patients at high risk to fall. 
Neither the fellows nor the control group showed changes in patterns of referral to PT. In comparing the 2 groups in the 12 months after training (Table 4), for their patients at risk of falling, the fellows were 4 times more likely to complete fall risk visits and over 6 times more likely to assess orthostatic blood pressure than their nontrained peers. Subgroup analysis of the 75+ population revealed similar trends and significance, but these results are not included here.

Discussion
This study aimed to improve not only providers’ knowledge and confidence in caring for older adults at increased risk to fall, but also their clinical practice in assessing and managing fall risk. In addition to improved knowledge and confidence, we found that the fellows increased their discussion of fall risk (through fall risk visits) and their assessment of orthostatic blood pressure for all of their patients, not just for those identified at increased risk to fall. This improvement held true for the fellows themselves before and after the intervention, but also as compared to their nontrained peers. These practice improvements for all of their 65+ patients, not just those identified as being at high risk to fall, are especially important, since studies indicate that early screening and intervention can help identify people at risk and prevent future falls.15
We were surprised that there were no significant differences in PT referrals made by the trained fellows, but this finding may have been confounded by the fact that the data included all PT referrals, regardless of diagnosis, not just those referrals that were fall-related. Furthermore, our baseline PT referral rates, at 39% for the intervention group and 42% for the control group, are higher than national data when looking at rehabilitation use by older adults.26
In comparison to a study evaluating the occurrence of fall risk–related clinical practice in primary care before any fall-related educational intervention, orthostatics were checked less frequently in our study (10% versus 30%) and there were fewer PT referrals (42%–44% versus 53%).27 However, the Phelan study took place in patients who had actually had a fall, rather than just having a higher risk for a fall, and was based on detailed chart review. Other studies23,24 found higher rates of fall risk interventions, but did not break out PT referrals specifically.
In terms of the educational intervention itself, most studies of geriatric education interventions have measured changes in knowledge, confidence, or self-efficacy as they relate to geriatric competence,28-30 and do not measure practice change as an outcome outside of intent to change or self-reported practice change.31,32 In general, practice change or longer-term health care–related outcomes have not been studied. Additionally, a range of dosages of educational interventions has been studied, from 1-hour lunchtime presentations23,32 to half-day29 or several half-day workshops,28 up to 160 hours over 10 months30 or 5 weekends over 6 months.31 The duration of our entire intervention at 160 hours over 6 months would be considered on the upper end of dosing relative to these studies, with our Mobility week intervention comprising 32 hours during 1 week. In the Warshaw study, despite 107 1-hour sessions being taught to over 60 physicians in 16 practices over 4 years, only 2 practices ultimately initiated any practice change projects.32 We believe that only curricula that embed practice change skills and opportunities, at a significant enough dose, can actually impact practice change in a sustainable manner.
Knowledge and skill acquisition among individual providers does not take place to a sufficient degree in the current health care arena, which is focused on productivity and short visit times. Consistent with other studies, we included interdisciplinary members of the primary care team for part of the mini-fellowship, although other studies used models that train across disciplines for the entirety of the learning experience.28-30,33 Our educational model was strengthened by including other professionals to provide some of the education and model the ideal geriatric team, including PT, occupational therapy, and pharmacy, for the week on mobility.
Most studies exploring interventions through geriatric educational initiatives are conducted within academic institutions, with a primary focus on physician faculty and, by extension, their teaching of residents and others.34,35 We believe our integrated model, which is steeped in community-based primary care practices like Lam’s,31 offers the greatest outreach to large community-based care systems and their patients. Training providers to work with their teams to change their own practices first gives skills and expertise that help further establish them as geriatric champions within their practices, laying the groundwork for more widespread practice change at their clinics.
Limitations
In addition to the limitations described above relating to the capture of PT referrals, other limitations included the relatively short time period for follow-up data as well as the small size of the intervention group. However, we found value in the instructional depth that the small group size allowed.
While the nontrained providers did show some improvement during the same period, we believe the relative risk was not clinically significant. We suspect that the larger health system efforts to standardize screening of patients 65+ across all clinics as a core quality metric confounded these results. The data analysis also included only fall-related patient visits that occurred with a provider who was that patient’s PCP, which could have missed visits done by other PCP colleagues, RNs, or pharmacists in the same clinic, thus undercounting the true number of fall-related visits. Furthermore, counting of fall-related interventions relied upon providers documenting consistently in the EHR, which could also lead to under-represention of fall risk clinical efforts.
The data presented, while encouraging, do not reflect clinic-wide practice change patterns and are considered only proximate outcomes rather than more long-term or cost-related outcomes, as would be captured by fall-related utilization measures like emergency room visits and hospitalizations. We expect to evaluate the broader impact and these value-based outcomes in the future. All providers and teams were from the same health care system, which may not allow our results to transfer to other organizations or regions of clinical practice.
Summary
This study demonstrates that an intensive mini-fellowship model of geriatrics training improved both knowledge and confidence in the realm of fall risk assessment and intervention among PCPs who had not been formally trained in geriatrics. More importantly, the training improved the fall-related care of their patients at increased risk to fall, but also of all of their older patients, with improvements in care measured up to a year after the mini-fellowship. Although this article only describes the work done as part of the Mobility aim of the 4M AFHS model, we believe the entire mini-fellowship curriculum offers the opportunity to “geriatricize” clinicians and their teams in learning geriatric principles and skills that they can translate into their practice in a sustainable way, as Tinetti encourages.8 Future study to evaluate other process outcomes more precisely, such as PT, as well as cost- and value-based outcomes, and the influence of trained providers on their clinic partners, will further establish the value proposition of targeted, disseminated, intensive geriatrics training of primary care clinicians as a strategy of age-friendly health systems as they work to improve the care of their older adults.
Acknowledgment: We are grateful for the dedication and hard work of the 2018 Geriatric Mini-Fellowship fellows at Providence Health & Services-Oregon who made this article possible. Thanks to Drs. Stephanie Cha, Emily Puukka-Clark, Laurie Dutkiewicz, Cara Ellis, Deb Frost, Jordan Roth, and Subhechchha Shah for promoting the AFHS work within their Providence Medical Group clinics and to PMG leadership and the fellows’ clinical teams for supporting the fellows, the AFHS work, and their older patients.
Corresponding author: Colleen M. Casey, PhD, ANP-BC, Providence Health & Services, Senior Health Program, 4400 NE Halsey, 5th Floor, Portland, OR 97213; [email protected].
Financial disclosures: None.
1. US Department of Health and Human Services. 2018 Profile of Older Americans. Administration on Aging. April 2018.
2. Roberts AW, Ogunwole SU, Blakeslee L, Rabe MA. The population 65 years and older in the United States: 2016. Washington, DC: US Census Bureau; 2018.
3. American Board of Medicine Specialties. 2017-2018 ABMS Board Certification Report. https://www.abms.org/board-certification/abms-board-certification-report/. Accessed November 3, 2020.
4. US Department of Health and Human Services, Health Resources and Services Administration, National Center for Health Workforce Analysis. National and regional projections of supply and demand for geriatricians: 2013-2025. Rockville, MD: US Department of Health and Human Services; 2007.
5. American Association of Nurse Practitioners, NP Facts: The Voice of the Nurse Practitioner. 2020. https://storage.aanp.org/www/documents/NPFacts__080420.pdf.
6. Tinetti ME, Naik AD, Dodson JA, Moving from disease-centered to patient goals-directed care for patients with multiple chronic conditions: patient value-based care. JAMA Cardiol. 2016;1:9-10.
7. Fried LP, Hall WJ. Editorial: leading on behalf of an aging society. J Am Geriatr Soc. 2008;56:1791-1795.
8. Tinetti M. Mainstream or extinction: can defining who we are save geriatrics? J Am Geriatr Soc. 2016;64:1400-1404.
9. Jafari P, Kostas T, Levine S, et al. ECHO-Chicago Geriatrics: using telementoring to “geriatricize” the primary care workforce. Gerontol Geriatr Educ. 2020;41:333-341.
10. Fulmer T, Mate KS, Berman A. The Age-Friendly Health System imperative. J Am Geriatr Soc. 2018;66:22-24.
11. Mate KS, Berman A, Laderman M, et al. Creating Age-Friendly Health Systems - A vision for better care of older adults. Healthc (Amst). 2018;6:4-6.
12. Tinetti ME, et al. Patient priority-directed decision making and care for older adults with multiple chronic conditions. Clin Geriatr Med. 2016;32:261-275.
13. Stevens JA, Phelan EA. Development of STEADI: a fall prevention resource for health care providers. Health Promot Pract. 2013;14:706-714.
14. Rubenstein LZ, et al. Validating an evidence-based, self-rated fall risk questionnaire (FRQ) for older adults. J Safety Res. 2011;42:493-499.
15. Grossman DC, et al. Interventions to prevent falls in community-dwelling older adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319: 1696-1704.
16. Tricco AC, Thomas SM, Veroniki AA, et al. Comparisons of interventions for preventing falls in older adults: a systematic review and meta-analysis. JAMA. 2017;318:1687-1699.
17. Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012(9):CD007146.
18. Bergen G, Stevens MR, Burns ER. Falls and fall injuries among adults aged ≥65 years - United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65:993-998.
19. Burns E, Kakara R. Deaths from falls among persons aged >=65 Years - United States, 2007-2016. MMWR Morb Mortal Wkly Rep. 2018;67:509-514.
20. Shankar KN, Liu SW, Ganz DA. Trends and characteristics of emergency department visits for fall-related injuries in older adults, 2003-2010. West J Emerg Med. 2017;18:785-793.
21. Hoffman GJ, et al. Posthospital fall injuries and 30-day readmissions in adults 65 years and older. JAMA Netw Open. 2019;2:e194276.
22. Eckstrom E, Parker EM, Shakya I, Lee R. Coordinated care plan to prevent older adult falls. 2018. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2018.
23. Eckstrom E, Parker EM, Lambert GH, et al. Implementing STEADI in academic primary care to address older adult fall risk. Innov Aging. 2017;1:igx028.
24. Johnston YA, Bergen G, Bauer M, et al. Implementation of the stopping elderly accidents, deaths, and injuries initiative in primary care: an outcome evaluation. Gerontologist. 2019;59:1182-1191.
25. Phelan EA, Mahoney JE, Voit JC, Stevens JA. Assessment and management of fall risk in primary care settings. Med Clin North Am. 2015;99:281-293.
26. Gell NM, Mroz TM, Patel KV. Rehabilitation services use and patient-reported outcomes among older adults in the United States. Arch Phys Med Rehabil. 2017;98:2221-2227.e3.
27. Phelan EA, Aerts S, Dowler D, et al. Adoption of evidence-based fall prevention practices in primary care for older adults with a history of falls. Front Public Health. 2016;4:190.
28. Solberg LB, Carter CS, Solberg LM. Geriatric care boot camp series: interprofessional education for a new training paradigm. Geriatr Nurs. 2019;40:579-583.
29. Solberg LB, Solberg LM, Carter CS. Geriatric care boot cAMP: an interprofessional education program for healthcare professionals. J Am Geriatr Soc. 2015;63:997-1001.
30. Coogle CL, Hackett L, Owens MG, et al. Perceived self-efficacy gains following an interprofessional faculty development programme in geriatrics education. J Interprof Care. 2016;30:483-492.
31. Lam R, Lee L, Tazkarji B, et al. Five-weekend care of the elderly certificate course: continuing professional development activity for family physicians. Can Fam Physician. 2015;61:e135-141.
32. Warshaw GA, Modawal A, Kues J, et al. Community physician education in geriatrics: applying the assessing care of vulnerable elders model with a multisite primary care group. J Am Geriatr Soc. 2010;58:1780-1785.
33. Solai LK, Kumar K, Mulvaney E, et al. Geriatric mental healthcare training: a mini-fellowship approach to interprofessional assessment and management of geriatric mental health issues. Am J Geriatr Psychiatry. 2019;27:706-711.
34. Christmas C, Park E, Schmaltz H, et al. A model intensive course in geriatric teaching for non-geriatrician educators. J Gen Intern Med. 2008;23:1048-1052.
35. Heflin MT, Bragg EJ, Fernandez H, et al. The Donald W. Reynolds Consortium for Faculty Development to Advance Geriatrics Education (FD~AGE): a model for dissemination of subspecialty educational expertise. Acad Med. 2012;87:618-626.
1. US Department of Health and Human Services. 2018 Profile of Older Americans. Administration on Aging. April 2018.
2. Roberts AW, Ogunwole SU, Blakeslee L, Rabe MA. The population 65 years and older in the United States: 2016. Washington, DC: US Census Bureau; 2018.
3. American Board of Medicine Specialties. 2017-2018 ABMS Board Certification Report. https://www.abms.org/board-certification/abms-board-certification-report/. Accessed November 3, 2020.
4. US Department of Health and Human Services, Health Resources and Services Administration, National Center for Health Workforce Analysis. National and regional projections of supply and demand for geriatricians: 2013-2025. Rockville, MD: US Department of Health and Human Services; 2007.
5. American Association of Nurse Practitioners, NP Facts: The Voice of the Nurse Practitioner. 2020. https://storage.aanp.org/www/documents/NPFacts__080420.pdf.
6. Tinetti ME, Naik AD, Dodson JA, Moving from disease-centered to patient goals-directed care for patients with multiple chronic conditions: patient value-based care. JAMA Cardiol. 2016;1:9-10.
7. Fried LP, Hall WJ. Editorial: leading on behalf of an aging society. J Am Geriatr Soc. 2008;56:1791-1795.
8. Tinetti M. Mainstream or extinction: can defining who we are save geriatrics? J Am Geriatr Soc. 2016;64:1400-1404.
9. Jafari P, Kostas T, Levine S, et al. ECHO-Chicago Geriatrics: using telementoring to “geriatricize” the primary care workforce. Gerontol Geriatr Educ. 2020;41:333-341.
10. Fulmer T, Mate KS, Berman A. The Age-Friendly Health System imperative. J Am Geriatr Soc. 2018;66:22-24.
11. Mate KS, Berman A, Laderman M, et al. Creating Age-Friendly Health Systems - A vision for better care of older adults. Healthc (Amst). 2018;6:4-6.
12. Tinetti ME, et al. Patient priority-directed decision making and care for older adults with multiple chronic conditions. Clin Geriatr Med. 2016;32:261-275.
13. Stevens JA, Phelan EA. Development of STEADI: a fall prevention resource for health care providers. Health Promot Pract. 2013;14:706-714.
14. Rubenstein LZ, et al. Validating an evidence-based, self-rated fall risk questionnaire (FRQ) for older adults. J Safety Res. 2011;42:493-499.
15. Grossman DC, et al. Interventions to prevent falls in community-dwelling older adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319: 1696-1704.
16. Tricco AC, Thomas SM, Veroniki AA, et al. Comparisons of interventions for preventing falls in older adults: a systematic review and meta-analysis. JAMA. 2017;318:1687-1699.
17. Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012(9):CD007146.
18. Bergen G, Stevens MR, Burns ER. Falls and fall injuries among adults aged ≥65 years - United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65:993-998.
19. Burns E, Kakara R. Deaths from falls among persons aged >=65 Years - United States, 2007-2016. MMWR Morb Mortal Wkly Rep. 2018;67:509-514.
20. Shankar KN, Liu SW, Ganz DA. Trends and characteristics of emergency department visits for fall-related injuries in older adults, 2003-2010. West J Emerg Med. 2017;18:785-793.
21. Hoffman GJ, et al. Posthospital fall injuries and 30-day readmissions in adults 65 years and older. JAMA Netw Open. 2019;2:e194276.
22. Eckstrom E, Parker EM, Shakya I, Lee R. Coordinated care plan to prevent older adult falls. 2018. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2018.
23. Eckstrom E, Parker EM, Lambert GH, et al. Implementing STEADI in academic primary care to address older adult fall risk. Innov Aging. 2017;1:igx028.
24. Johnston YA, Bergen G, Bauer M, et al. Implementation of the stopping elderly accidents, deaths, and injuries initiative in primary care: an outcome evaluation. Gerontologist. 2019;59:1182-1191.
25. Phelan EA, Mahoney JE, Voit JC, Stevens JA. Assessment and management of fall risk in primary care settings. Med Clin North Am. 2015;99:281-293.
26. Gell NM, Mroz TM, Patel KV. Rehabilitation services use and patient-reported outcomes among older adults in the United States. Arch Phys Med Rehabil. 2017;98:2221-2227.e3.
27. Phelan EA, Aerts S, Dowler D, et al. Adoption of evidence-based fall prevention practices in primary care for older adults with a history of falls. Front Public Health. 2016;4:190.
28. Solberg LB, Carter CS, Solberg LM. Geriatric care boot camp series: interprofessional education for a new training paradigm. Geriatr Nurs. 2019;40:579-583.
29. Solberg LB, Solberg LM, Carter CS. Geriatric care boot cAMP: an interprofessional education program for healthcare professionals. J Am Geriatr Soc. 2015;63:997-1001.
30. Coogle CL, Hackett L, Owens MG, et al. Perceived self-efficacy gains following an interprofessional faculty development programme in geriatrics education. J Interprof Care. 2016;30:483-492.
31. Lam R, Lee L, Tazkarji B, et al. Five-weekend care of the elderly certificate course: continuing professional development activity for family physicians. Can Fam Physician. 2015;61:e135-141.
32. Warshaw GA, Modawal A, Kues J, et al. Community physician education in geriatrics: applying the assessing care of vulnerable elders model with a multisite primary care group. J Am Geriatr Soc. 2010;58:1780-1785.
33. Solai LK, Kumar K, Mulvaney E, et al. Geriatric mental healthcare training: a mini-fellowship approach to interprofessional assessment and management of geriatric mental health issues. Am J Geriatr Psychiatry. 2019;27:706-711.
34. Christmas C, Park E, Schmaltz H, et al. A model intensive course in geriatric teaching for non-geriatrician educators. J Gen Intern Med. 2008;23:1048-1052.
35. Heflin MT, Bragg EJ, Fernandez H, et al. The Donald W. Reynolds Consortium for Faculty Development to Advance Geriatrics Education (FD~AGE): a model for dissemination of subspecialty educational expertise. Acad Med. 2012;87:618-626.
Can mental health teams de-escalate crises in NYC?
“Defund the police”: It’s a slogan, or perhaps a battle cry, that has emerged from the Black Lives Matter movement as a response to race-related police brutality and concerns that people of color are profiled, targeted, arrested, charged, manhandled, and killed by law enforcement in a disproportionate and unjust manner. It crosses into our realm as psychiatrists as mental health emergency calls are handled by the police and not by mental health professionals. The result is sometimes tragic: As many as half of police shootings involve people with psychiatric disorders, and the hope is that many of the police shootings could be avoided if crises were handed by mental health clinicians instead of, or in cooperation with, the police.
At best, police officers receive a week of specialized, crisis intervention training about how to approach those with psychiatric disorders; most officers receive no training. This leaves psychiatry as the only field where medical crises are routinely handled by the police – it is demeaning and embarrassing for some of our patients and dangerous for others. The reality remains, however, that there are times when psychiatric disorders result in violent behavior, and patients being taken for involuntary treatment often resist transport, so either way there is risk, both to the patient and to anyone who responds to a call for assistance.
Early this month, the office of New York City Mayor Bill de Blasio announced that a major change would be made in how mental health calls to 911 are handled in two “high-need” areas. The mayor’s website states:
“Beginning in February 2021, new Mental Health Teams will use their physical and mental health expertise, and experience in crisis response to de-escalate emergency situations, will help reduce the number of times police will need to respond to 911 mental health calls in these precincts. These teams will have the expertise to respond to a range of behavioral health problems, such as suicide attempts, substance misuse, and serious mental illness, as well as physical health problems, which can be exacerbated by or mask mental health problems. NYC Health + Hospitals will train and provide ongoing technical assistance and support. In selecting team members for this program, FDNY will prioritize professionals with significant experience with mental health crises.”
The press release goes on to say that, in situations where there is a weapon or reason to believe there is a risk of violence, the police will be dispatched along with the new mental health team.
“This is the first time in our history that health professionals will be the default responders to mental health emergencies,” New York City First Lady Chirlane McCray said as she announced the new program. “Treating mental health crises as mental health challenges and not public safety ones is the modern and more appropriate approach.”
New York City is not the first city to employ this model. In the United States, the CAHOOTS (Crisis Assistance Helping Out on the Streets) program in Eugene, Ore., has been run by the White Bird Clinic since 1989 as part of a community policing initiative. Last year, the team responded to 24,000 calls and police backup was required on only 150 of those responses. The CAHOOTS website states:
“The CAHOOTS model has been in the spotlight recently as our nation struggles to reimagine public safety. The program mobilizes two-person teams consisting of a medic (a nurse, paramedic, or EMT) and a crisis worker who has substantial training and experience in the mental health field. The CAHOOTS teams deal with a wide range of mental health-related crises, including conflict resolution, welfare checks, substance abuse, suicide threats, and more, relying on trauma-informed de-escalation and harm reduction techniques. CAHOOTS staff are not law enforcement officers and do not carry weapons; their training and experience are the tools they use to ensure a non-violent resolution of crisis situations. They also handle non-emergent medical issues, avoiding costly ambulance transport and emergency room treatment.”
Other cities in the United States are also looking at implementing programs where mental health teams, and not the police, respond to emergency calls. Last year, Oakland, Calif.’s city council invested $40,000 in research to assess how they could best implement a program like the one in Eugene. They hope to begin the Mobile Assistance Community Responders of Oakland (MACROS) next year. Sigal Samuel writes in a Vox article, “The goal is to launch the pilot next year with funding from the city budget, and although supporters are not yet sure what its size and duration will be, they’re hopeful it’ll make a big difference to Oakland’s overpoliced community of people without homes. They were among those who first called for a non-policing approach.”
The model is not unique to the United States. In 2005, Stockholm started a program with a psychiatric ambulance – equipped with comfortable seating rather than a stretcher – to respond to mental health emergencies. The ambulance responds to 130 calls a month. It is staffed with a driver and two psychiatric nurses, and for half of the calls, the police also come. While the Swedish program was not about removing resources from the police, it has relieved the police of the responsibility for many psychiatric emergencies.
The New York City program will be modeled after the CAHOOTS initiative in Eugene. It differs from the mobile crisis response services in many other cities because CAHOOTS is hooked directly into the 911 emergency services system. Its website notes that the program has saved money:
“The cost savings are considerable. The CAHOOTS program budget is about $2.1 million annually, while the combined annual budgets for the Eugene and Springfield police departments are $90 million. In 2017, the CAHOOTS teams answered 17% of the Eugene Police Department’s overall call volume. The program saves the city of Eugene an estimated $8.5 million in public safety spending annually.”
Some worry there is an unpredictable aspect to calls for psychiatric emergencies, and the potential for mental health professions to be injured or killed. Annette Hanson, MD, a forensic psychiatrist at University of Maryland, Baltimore, voiced her concerns, “While multidisciplinary teams are useful, there have been rare cases of violence against responding mental health providers. People with serious mental illness are rarely violent but their dangerousness is unpredictable and cannot be predicted by case screening.”
Daniel Felts is a mental health crisis counselor who has worked at CAHOOTS for the past 4* years. He has responded to about 8,000 calls, and called for police backup only three times to request an immediate "Code 3 cover" when someone's safety has been in danger. Mr. Felts calls the police about once a month for concerns that do not require an immediate response for safety.* “Over the last 4 years, I am only aware of three instances when a team member’s safety was compromised because of a client’s violent behavior. No employee has been seriously physically harmed. In 30 years, with hundreds of thousands (millions?) of calls responded to, no CAHOOTS worker has ever been killed, shot, or stabbed in the line of duty,” Mr. Felts noted.
Emergency calls are screened. “It is not uncommon for CAHOOTS to be dispatched to ‘stage’ for calls involving active disputes or acutely suicidal individuals where means are present. “Staging” entails us parking roughly a mile away while police make first contact and advise whether it is safe for CAHOOTS to engage.”
Mr. Felts went on to discuss the program’s relationship with the community. “ and how we operate. Having operated in Eugene for 30 years, our service is well understood to be one that does not kill, harm, or violate personal boundaries or liberties.”
Would a program like the ones in Stockholm or in Eugene work in other places? Eugene is a city with a population of 172,000 with a low crime rate. Whether a program implemented in one city can be mimicked in another very different city is not clear.
Paul Appelbaum, MD, a forensic psychiatrist at Columbia University, New York, is optimistic about New York City’s forthcoming program.
“The proposed pilot project in NYC is a real step forward. Work that we’ve done looking at fatal encounters involving the police found that roughly 25% of all deaths at the hands of the police are of people with mental illness. In many of those cases, police were initially called to bring people who were clearly troubled for psychiatric evaluation, but as the situation escalated, the police turned to their weapons to control it, which led to a fatal outcome. Taking police out of the picture whenever possible in favor of trained mental health personnel is clearly a better approach. It will be important for the city to collect good outcome data to enable independent evaluation of the pilot project – not something that political entities are inclined toward, but a critical element in assessing the effectiveness of this approach.”
There are questions that remain about the new program. Mayor de Blasio’s office has not released information about which areas of the city are being chosen for the new program, how much the program will cost, or what the funding source will be. If it can be implemented safely and effectively, it has the potential to provide more sensitive care to patients in crisis, and to save lives.
Dr. Miller is coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University, 2018). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins University, both in Baltimore.
*Correction, 11/27/2020: An earlier version of this article misstated the number of years Daniel Felts has worked at CAHOOTS.
“Defund the police”: It’s a slogan, or perhaps a battle cry, that has emerged from the Black Lives Matter movement as a response to race-related police brutality and concerns that people of color are profiled, targeted, arrested, charged, manhandled, and killed by law enforcement in a disproportionate and unjust manner. It crosses into our realm as psychiatrists as mental health emergency calls are handled by the police and not by mental health professionals. The result is sometimes tragic: As many as half of police shootings involve people with psychiatric disorders, and the hope is that many of the police shootings could be avoided if crises were handed by mental health clinicians instead of, or in cooperation with, the police.
At best, police officers receive a week of specialized, crisis intervention training about how to approach those with psychiatric disorders; most officers receive no training. This leaves psychiatry as the only field where medical crises are routinely handled by the police – it is demeaning and embarrassing for some of our patients and dangerous for others. The reality remains, however, that there are times when psychiatric disorders result in violent behavior, and patients being taken for involuntary treatment often resist transport, so either way there is risk, both to the patient and to anyone who responds to a call for assistance.
Early this month, the office of New York City Mayor Bill de Blasio announced that a major change would be made in how mental health calls to 911 are handled in two “high-need” areas. The mayor’s website states:
“Beginning in February 2021, new Mental Health Teams will use their physical and mental health expertise, and experience in crisis response to de-escalate emergency situations, will help reduce the number of times police will need to respond to 911 mental health calls in these precincts. These teams will have the expertise to respond to a range of behavioral health problems, such as suicide attempts, substance misuse, and serious mental illness, as well as physical health problems, which can be exacerbated by or mask mental health problems. NYC Health + Hospitals will train and provide ongoing technical assistance and support. In selecting team members for this program, FDNY will prioritize professionals with significant experience with mental health crises.”
The press release goes on to say that, in situations where there is a weapon or reason to believe there is a risk of violence, the police will be dispatched along with the new mental health team.
“This is the first time in our history that health professionals will be the default responders to mental health emergencies,” New York City First Lady Chirlane McCray said as she announced the new program. “Treating mental health crises as mental health challenges and not public safety ones is the modern and more appropriate approach.”
New York City is not the first city to employ this model. In the United States, the CAHOOTS (Crisis Assistance Helping Out on the Streets) program in Eugene, Ore., has been run by the White Bird Clinic since 1989 as part of a community policing initiative. Last year, the team responded to 24,000 calls and police backup was required on only 150 of those responses. The CAHOOTS website states:
“The CAHOOTS model has been in the spotlight recently as our nation struggles to reimagine public safety. The program mobilizes two-person teams consisting of a medic (a nurse, paramedic, or EMT) and a crisis worker who has substantial training and experience in the mental health field. The CAHOOTS teams deal with a wide range of mental health-related crises, including conflict resolution, welfare checks, substance abuse, suicide threats, and more, relying on trauma-informed de-escalation and harm reduction techniques. CAHOOTS staff are not law enforcement officers and do not carry weapons; their training and experience are the tools they use to ensure a non-violent resolution of crisis situations. They also handle non-emergent medical issues, avoiding costly ambulance transport and emergency room treatment.”
Other cities in the United States are also looking at implementing programs where mental health teams, and not the police, respond to emergency calls. Last year, Oakland, Calif.’s city council invested $40,000 in research to assess how they could best implement a program like the one in Eugene. They hope to begin the Mobile Assistance Community Responders of Oakland (MACROS) next year. Sigal Samuel writes in a Vox article, “The goal is to launch the pilot next year with funding from the city budget, and although supporters are not yet sure what its size and duration will be, they’re hopeful it’ll make a big difference to Oakland’s overpoliced community of people without homes. They were among those who first called for a non-policing approach.”
The model is not unique to the United States. In 2005, Stockholm started a program with a psychiatric ambulance – equipped with comfortable seating rather than a stretcher – to respond to mental health emergencies. The ambulance responds to 130 calls a month. It is staffed with a driver and two psychiatric nurses, and for half of the calls, the police also come. While the Swedish program was not about removing resources from the police, it has relieved the police of the responsibility for many psychiatric emergencies.
The New York City program will be modeled after the CAHOOTS initiative in Eugene. It differs from the mobile crisis response services in many other cities because CAHOOTS is hooked directly into the 911 emergency services system. Its website notes that the program has saved money:
“The cost savings are considerable. The CAHOOTS program budget is about $2.1 million annually, while the combined annual budgets for the Eugene and Springfield police departments are $90 million. In 2017, the CAHOOTS teams answered 17% of the Eugene Police Department’s overall call volume. The program saves the city of Eugene an estimated $8.5 million in public safety spending annually.”
Some worry there is an unpredictable aspect to calls for psychiatric emergencies, and the potential for mental health professions to be injured or killed. Annette Hanson, MD, a forensic psychiatrist at University of Maryland, Baltimore, voiced her concerns, “While multidisciplinary teams are useful, there have been rare cases of violence against responding mental health providers. People with serious mental illness are rarely violent but their dangerousness is unpredictable and cannot be predicted by case screening.”
Daniel Felts is a mental health crisis counselor who has worked at CAHOOTS for the past 4* years. He has responded to about 8,000 calls, and called for police backup only three times to request an immediate "Code 3 cover" when someone's safety has been in danger. Mr. Felts calls the police about once a month for concerns that do not require an immediate response for safety.* “Over the last 4 years, I am only aware of three instances when a team member’s safety was compromised because of a client’s violent behavior. No employee has been seriously physically harmed. In 30 years, with hundreds of thousands (millions?) of calls responded to, no CAHOOTS worker has ever been killed, shot, or stabbed in the line of duty,” Mr. Felts noted.
Emergency calls are screened. “It is not uncommon for CAHOOTS to be dispatched to ‘stage’ for calls involving active disputes or acutely suicidal individuals where means are present. “Staging” entails us parking roughly a mile away while police make first contact and advise whether it is safe for CAHOOTS to engage.”
Mr. Felts went on to discuss the program’s relationship with the community. “ and how we operate. Having operated in Eugene for 30 years, our service is well understood to be one that does not kill, harm, or violate personal boundaries or liberties.”
Would a program like the ones in Stockholm or in Eugene work in other places? Eugene is a city with a population of 172,000 with a low crime rate. Whether a program implemented in one city can be mimicked in another very different city is not clear.
Paul Appelbaum, MD, a forensic psychiatrist at Columbia University, New York, is optimistic about New York City’s forthcoming program.
“The proposed pilot project in NYC is a real step forward. Work that we’ve done looking at fatal encounters involving the police found that roughly 25% of all deaths at the hands of the police are of people with mental illness. In many of those cases, police were initially called to bring people who were clearly troubled for psychiatric evaluation, but as the situation escalated, the police turned to their weapons to control it, which led to a fatal outcome. Taking police out of the picture whenever possible in favor of trained mental health personnel is clearly a better approach. It will be important for the city to collect good outcome data to enable independent evaluation of the pilot project – not something that political entities are inclined toward, but a critical element in assessing the effectiveness of this approach.”
There are questions that remain about the new program. Mayor de Blasio’s office has not released information about which areas of the city are being chosen for the new program, how much the program will cost, or what the funding source will be. If it can be implemented safely and effectively, it has the potential to provide more sensitive care to patients in crisis, and to save lives.
Dr. Miller is coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University, 2018). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins University, both in Baltimore.
*Correction, 11/27/2020: An earlier version of this article misstated the number of years Daniel Felts has worked at CAHOOTS.
“Defund the police”: It’s a slogan, or perhaps a battle cry, that has emerged from the Black Lives Matter movement as a response to race-related police brutality and concerns that people of color are profiled, targeted, arrested, charged, manhandled, and killed by law enforcement in a disproportionate and unjust manner. It crosses into our realm as psychiatrists as mental health emergency calls are handled by the police and not by mental health professionals. The result is sometimes tragic: As many as half of police shootings involve people with psychiatric disorders, and the hope is that many of the police shootings could be avoided if crises were handed by mental health clinicians instead of, or in cooperation with, the police.
At best, police officers receive a week of specialized, crisis intervention training about how to approach those with psychiatric disorders; most officers receive no training. This leaves psychiatry as the only field where medical crises are routinely handled by the police – it is demeaning and embarrassing for some of our patients and dangerous for others. The reality remains, however, that there are times when psychiatric disorders result in violent behavior, and patients being taken for involuntary treatment often resist transport, so either way there is risk, both to the patient and to anyone who responds to a call for assistance.
Early this month, the office of New York City Mayor Bill de Blasio announced that a major change would be made in how mental health calls to 911 are handled in two “high-need” areas. The mayor’s website states:
“Beginning in February 2021, new Mental Health Teams will use their physical and mental health expertise, and experience in crisis response to de-escalate emergency situations, will help reduce the number of times police will need to respond to 911 mental health calls in these precincts. These teams will have the expertise to respond to a range of behavioral health problems, such as suicide attempts, substance misuse, and serious mental illness, as well as physical health problems, which can be exacerbated by or mask mental health problems. NYC Health + Hospitals will train and provide ongoing technical assistance and support. In selecting team members for this program, FDNY will prioritize professionals with significant experience with mental health crises.”
The press release goes on to say that, in situations where there is a weapon or reason to believe there is a risk of violence, the police will be dispatched along with the new mental health team.
“This is the first time in our history that health professionals will be the default responders to mental health emergencies,” New York City First Lady Chirlane McCray said as she announced the new program. “Treating mental health crises as mental health challenges and not public safety ones is the modern and more appropriate approach.”
New York City is not the first city to employ this model. In the United States, the CAHOOTS (Crisis Assistance Helping Out on the Streets) program in Eugene, Ore., has been run by the White Bird Clinic since 1989 as part of a community policing initiative. Last year, the team responded to 24,000 calls and police backup was required on only 150 of those responses. The CAHOOTS website states:
“The CAHOOTS model has been in the spotlight recently as our nation struggles to reimagine public safety. The program mobilizes two-person teams consisting of a medic (a nurse, paramedic, or EMT) and a crisis worker who has substantial training and experience in the mental health field. The CAHOOTS teams deal with a wide range of mental health-related crises, including conflict resolution, welfare checks, substance abuse, suicide threats, and more, relying on trauma-informed de-escalation and harm reduction techniques. CAHOOTS staff are not law enforcement officers and do not carry weapons; their training and experience are the tools they use to ensure a non-violent resolution of crisis situations. They also handle non-emergent medical issues, avoiding costly ambulance transport and emergency room treatment.”
Other cities in the United States are also looking at implementing programs where mental health teams, and not the police, respond to emergency calls. Last year, Oakland, Calif.’s city council invested $40,000 in research to assess how they could best implement a program like the one in Eugene. They hope to begin the Mobile Assistance Community Responders of Oakland (MACROS) next year. Sigal Samuel writes in a Vox article, “The goal is to launch the pilot next year with funding from the city budget, and although supporters are not yet sure what its size and duration will be, they’re hopeful it’ll make a big difference to Oakland’s overpoliced community of people without homes. They were among those who first called for a non-policing approach.”
The model is not unique to the United States. In 2005, Stockholm started a program with a psychiatric ambulance – equipped with comfortable seating rather than a stretcher – to respond to mental health emergencies. The ambulance responds to 130 calls a month. It is staffed with a driver and two psychiatric nurses, and for half of the calls, the police also come. While the Swedish program was not about removing resources from the police, it has relieved the police of the responsibility for many psychiatric emergencies.
The New York City program will be modeled after the CAHOOTS initiative in Eugene. It differs from the mobile crisis response services in many other cities because CAHOOTS is hooked directly into the 911 emergency services system. Its website notes that the program has saved money:
“The cost savings are considerable. The CAHOOTS program budget is about $2.1 million annually, while the combined annual budgets for the Eugene and Springfield police departments are $90 million. In 2017, the CAHOOTS teams answered 17% of the Eugene Police Department’s overall call volume. The program saves the city of Eugene an estimated $8.5 million in public safety spending annually.”
Some worry there is an unpredictable aspect to calls for psychiatric emergencies, and the potential for mental health professions to be injured or killed. Annette Hanson, MD, a forensic psychiatrist at University of Maryland, Baltimore, voiced her concerns, “While multidisciplinary teams are useful, there have been rare cases of violence against responding mental health providers. People with serious mental illness are rarely violent but their dangerousness is unpredictable and cannot be predicted by case screening.”
Daniel Felts is a mental health crisis counselor who has worked at CAHOOTS for the past 4* years. He has responded to about 8,000 calls, and called for police backup only three times to request an immediate "Code 3 cover" when someone's safety has been in danger. Mr. Felts calls the police about once a month for concerns that do not require an immediate response for safety.* “Over the last 4 years, I am only aware of three instances when a team member’s safety was compromised because of a client’s violent behavior. No employee has been seriously physically harmed. In 30 years, with hundreds of thousands (millions?) of calls responded to, no CAHOOTS worker has ever been killed, shot, or stabbed in the line of duty,” Mr. Felts noted.
Emergency calls are screened. “It is not uncommon for CAHOOTS to be dispatched to ‘stage’ for calls involving active disputes or acutely suicidal individuals where means are present. “Staging” entails us parking roughly a mile away while police make first contact and advise whether it is safe for CAHOOTS to engage.”
Mr. Felts went on to discuss the program’s relationship with the community. “ and how we operate. Having operated in Eugene for 30 years, our service is well understood to be one that does not kill, harm, or violate personal boundaries or liberties.”
Would a program like the ones in Stockholm or in Eugene work in other places? Eugene is a city with a population of 172,000 with a low crime rate. Whether a program implemented in one city can be mimicked in another very different city is not clear.
Paul Appelbaum, MD, a forensic psychiatrist at Columbia University, New York, is optimistic about New York City’s forthcoming program.
“The proposed pilot project in NYC is a real step forward. Work that we’ve done looking at fatal encounters involving the police found that roughly 25% of all deaths at the hands of the police are of people with mental illness. In many of those cases, police were initially called to bring people who were clearly troubled for psychiatric evaluation, but as the situation escalated, the police turned to their weapons to control it, which led to a fatal outcome. Taking police out of the picture whenever possible in favor of trained mental health personnel is clearly a better approach. It will be important for the city to collect good outcome data to enable independent evaluation of the pilot project – not something that political entities are inclined toward, but a critical element in assessing the effectiveness of this approach.”
There are questions that remain about the new program. Mayor de Blasio’s office has not released information about which areas of the city are being chosen for the new program, how much the program will cost, or what the funding source will be. If it can be implemented safely and effectively, it has the potential to provide more sensitive care to patients in crisis, and to save lives.
Dr. Miller is coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University, 2018). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins University, both in Baltimore.
*Correction, 11/27/2020: An earlier version of this article misstated the number of years Daniel Felts has worked at CAHOOTS.
Golimumab preserves insulin production in type 1 diabetes
The human monoclonal antibody golimumab (Simponi) preserved endogenous insulin secretion in patients with new-onset type 1 diabetes and reduced their exogenous insulin requirements at 1 year, newly published phase 2 data indicate.
Results from the multicenter, double-blind, placebo-controlled trial were first reported as a poster at the virtual American Diabetes Association 80th Scientific Sessions in June. They were published online Nov. 18 in the New England Journal of Medicine.
In the 52-week study of 84 children and adults with new-onset type 1 diabetes, those given golimumab injections every 2 weeks had significantly higher levels of C-peptide, a marker of insulin secretion, and required less injected or infused insulin than did those who received placebo injections. There were no treatment-associated serious adverse events.
Golimumab is a human monoclonal antibody specific for tumor necrosis factor–alpha. It is approved for the treatment of several autoimmune diseases, including rheumatoid arthritis and ulcerative colitis, in the United States, Europe, and elsewhere.
An intermediate step toward a cure
Although none of the patients were able to stop taking insulin entirely, the results have important clinical implications, lead author Teresa Quattrin, MD, said in an interview.
“People want a cure, but the fact is, a cure is not available yet. So, this is an intermediate step towards a cure.... There are advantages to being on a small insulin dose,” including lower rates of hypoglycemia and maintenance of intraportal insulin, said Dr. Quattrin, of the State University of New York at Buffalo.
But in an accompanying editorial, Domenico Accili, MD, points to potential risks from immunotherapy and from attempting additional interventions at an “emotionally fraught” time when patients and families are coping with the new diabetes diagnosis.
He said of golimumab, “the effect is actually very small. ... There’s nothing wrong in and of itself with improving those outcomes. I just wouldn’t assign them as game changers.”
If this or a similar immunotherapeutic intervention were approved for this indication, “I would tell patients it exists and let them make the decision whether they want to try it. I wouldn’t say you must try it,” said Dr. Accili, of the Columbia University Diabetes and Endocrinology Research Center, New York.
With golimumab, higher C-peptide, lower insulin requirement
Of the 84 patients, who ranged in age from 6 to 21 years, 56 were randomly assigned within 100 days of being diagnosed with type 1 diabetes to receive golimumab, and 28 were assigned to receive placebo injections, given every 2 weeks.
The drug resulted in lower insulin use (0.51U/Kg per day vs. 0.69 U/kg per day), and the increase in insulin use over 52 weeks was less with golimumab than with placebo (0.07 vs. 0.24 U/kg per day; P = .001).
The mean percent decrease of C-peptide production from baseline was 12% with golimumab versus 56% with placebo.
Although the mean number of overall hypoglycemic events was similar, the mean number of level 2 hypoglycemic events (<54 mg/dL) was 36% lower with golimumab (11.5 vs. 17.6). There were no severe cases of hypoglycemia in either group.
No severe or serious infections occurred in either group, although mild to moderate infections were reported in 71% with golimumab versus 61% with placebo. More patients in the golimumab group experienced a decrease in neutrophils (29% vs. 19%).
Immunotherapy: Which one, and when should it start?
These findings come on the heels of the 2019 landmark results with another monoclonal antibody, the investigational anti-CD3 teplizumab (PRV-031). Among patients at risk, a diagnosis of type 1 diabetes was delayed by 2 years, and continued benefit was seen at 3 years.
However, Dr. Quattrin said teplizumab is limited by the fact that it must be administered via a 14-day infusion, whereas golimumab can be injected by patients themselves at home.
Moreover, the phase 2 teplizumab study was conducted in people who had antibodies that placed them at high risk for type 1 diabetes, but those patients did not yet have the condition. They were identified because they had close relatives with type 1 diabetes and were enrolled in the federally funded TrialNet screening program.
Dr. Quattrin is now participating in an ongoing phase 3 study of teplizumab that involves patients newly diagnosed with type 1 diabetes.
A Janssen spokesperson said in an interview that the company isn’t planning to further develop golimumab for use in type 1 diabetes.
“Our focus is to apply insights from the phase 2 ... proof-of-concept study to progress what we believe are novel, immunologically targeted pipeline candidates in stage 2 disease or presymptomatic stages of type 1 diabetes, which is consistent with our mission to intercept and prevent type 1 diabetes,” the spokesperson said.
To identify more individuals at risk for type 1 diabetes beyond the close relatives of those who already have it, so as to be able to intervene at a presymptomatic stage, Janssen is organizing a public-private effort to advocate for routine population screening for type 1 diabetes–related autoantibodies.
Dr. Quattrin said: “Preserving some insulin is key. Having somebody with beta cell functioning still is an intermediate step to a cure and will make their life easier, and that’s what people should care about.”
Dr. Accili, who cofounded and leads a company working on a novel approach to type 1 diabetes treatment, writes in his editorial: “We should also be mindful that this treatment debate is first world–centric.
“Current treatments for type 1 diabetes require resources not readily available in most parts of the world, where something as simple as refrigeration of insulin can become a logistic nightmare. While combinations of [approaches] tailored to individual risk and potential benefits are likely to make inroads in clinical practice, the need for a simpler, safer, and equally effective alternative to insulin remains,” he wrote.
Dr. Quattrin is a researcher and consultant for Janssen and conducts clinical trials for Provention Bio, Opko, and Ascendis. Dr. Accili is founder and director of Forkhead Therapeutics.
A version of this article originally appeared on Medscape.com.
The human monoclonal antibody golimumab (Simponi) preserved endogenous insulin secretion in patients with new-onset type 1 diabetes and reduced their exogenous insulin requirements at 1 year, newly published phase 2 data indicate.
Results from the multicenter, double-blind, placebo-controlled trial were first reported as a poster at the virtual American Diabetes Association 80th Scientific Sessions in June. They were published online Nov. 18 in the New England Journal of Medicine.
In the 52-week study of 84 children and adults with new-onset type 1 diabetes, those given golimumab injections every 2 weeks had significantly higher levels of C-peptide, a marker of insulin secretion, and required less injected or infused insulin than did those who received placebo injections. There were no treatment-associated serious adverse events.
Golimumab is a human monoclonal antibody specific for tumor necrosis factor–alpha. It is approved for the treatment of several autoimmune diseases, including rheumatoid arthritis and ulcerative colitis, in the United States, Europe, and elsewhere.
An intermediate step toward a cure
Although none of the patients were able to stop taking insulin entirely, the results have important clinical implications, lead author Teresa Quattrin, MD, said in an interview.
“People want a cure, but the fact is, a cure is not available yet. So, this is an intermediate step towards a cure.... There are advantages to being on a small insulin dose,” including lower rates of hypoglycemia and maintenance of intraportal insulin, said Dr. Quattrin, of the State University of New York at Buffalo.
But in an accompanying editorial, Domenico Accili, MD, points to potential risks from immunotherapy and from attempting additional interventions at an “emotionally fraught” time when patients and families are coping with the new diabetes diagnosis.
He said of golimumab, “the effect is actually very small. ... There’s nothing wrong in and of itself with improving those outcomes. I just wouldn’t assign them as game changers.”
If this or a similar immunotherapeutic intervention were approved for this indication, “I would tell patients it exists and let them make the decision whether they want to try it. I wouldn’t say you must try it,” said Dr. Accili, of the Columbia University Diabetes and Endocrinology Research Center, New York.
With golimumab, higher C-peptide, lower insulin requirement
Of the 84 patients, who ranged in age from 6 to 21 years, 56 were randomly assigned within 100 days of being diagnosed with type 1 diabetes to receive golimumab, and 28 were assigned to receive placebo injections, given every 2 weeks.
The drug resulted in lower insulin use (0.51U/Kg per day vs. 0.69 U/kg per day), and the increase in insulin use over 52 weeks was less with golimumab than with placebo (0.07 vs. 0.24 U/kg per day; P = .001).
The mean percent decrease of C-peptide production from baseline was 12% with golimumab versus 56% with placebo.
Although the mean number of overall hypoglycemic events was similar, the mean number of level 2 hypoglycemic events (<54 mg/dL) was 36% lower with golimumab (11.5 vs. 17.6). There were no severe cases of hypoglycemia in either group.
No severe or serious infections occurred in either group, although mild to moderate infections were reported in 71% with golimumab versus 61% with placebo. More patients in the golimumab group experienced a decrease in neutrophils (29% vs. 19%).
Immunotherapy: Which one, and when should it start?
These findings come on the heels of the 2019 landmark results with another monoclonal antibody, the investigational anti-CD3 teplizumab (PRV-031). Among patients at risk, a diagnosis of type 1 diabetes was delayed by 2 years, and continued benefit was seen at 3 years.
However, Dr. Quattrin said teplizumab is limited by the fact that it must be administered via a 14-day infusion, whereas golimumab can be injected by patients themselves at home.
Moreover, the phase 2 teplizumab study was conducted in people who had antibodies that placed them at high risk for type 1 diabetes, but those patients did not yet have the condition. They were identified because they had close relatives with type 1 diabetes and were enrolled in the federally funded TrialNet screening program.
Dr. Quattrin is now participating in an ongoing phase 3 study of teplizumab that involves patients newly diagnosed with type 1 diabetes.
A Janssen spokesperson said in an interview that the company isn’t planning to further develop golimumab for use in type 1 diabetes.
“Our focus is to apply insights from the phase 2 ... proof-of-concept study to progress what we believe are novel, immunologically targeted pipeline candidates in stage 2 disease or presymptomatic stages of type 1 diabetes, which is consistent with our mission to intercept and prevent type 1 diabetes,” the spokesperson said.
To identify more individuals at risk for type 1 diabetes beyond the close relatives of those who already have it, so as to be able to intervene at a presymptomatic stage, Janssen is organizing a public-private effort to advocate for routine population screening for type 1 diabetes–related autoantibodies.
Dr. Quattrin said: “Preserving some insulin is key. Having somebody with beta cell functioning still is an intermediate step to a cure and will make their life easier, and that’s what people should care about.”
Dr. Accili, who cofounded and leads a company working on a novel approach to type 1 diabetes treatment, writes in his editorial: “We should also be mindful that this treatment debate is first world–centric.
“Current treatments for type 1 diabetes require resources not readily available in most parts of the world, where something as simple as refrigeration of insulin can become a logistic nightmare. While combinations of [approaches] tailored to individual risk and potential benefits are likely to make inroads in clinical practice, the need for a simpler, safer, and equally effective alternative to insulin remains,” he wrote.
Dr. Quattrin is a researcher and consultant for Janssen and conducts clinical trials for Provention Bio, Opko, and Ascendis. Dr. Accili is founder and director of Forkhead Therapeutics.
A version of this article originally appeared on Medscape.com.
The human monoclonal antibody golimumab (Simponi) preserved endogenous insulin secretion in patients with new-onset type 1 diabetes and reduced their exogenous insulin requirements at 1 year, newly published phase 2 data indicate.
Results from the multicenter, double-blind, placebo-controlled trial were first reported as a poster at the virtual American Diabetes Association 80th Scientific Sessions in June. They were published online Nov. 18 in the New England Journal of Medicine.
In the 52-week study of 84 children and adults with new-onset type 1 diabetes, those given golimumab injections every 2 weeks had significantly higher levels of C-peptide, a marker of insulin secretion, and required less injected or infused insulin than did those who received placebo injections. There were no treatment-associated serious adverse events.
Golimumab is a human monoclonal antibody specific for tumor necrosis factor–alpha. It is approved for the treatment of several autoimmune diseases, including rheumatoid arthritis and ulcerative colitis, in the United States, Europe, and elsewhere.
An intermediate step toward a cure
Although none of the patients were able to stop taking insulin entirely, the results have important clinical implications, lead author Teresa Quattrin, MD, said in an interview.
“People want a cure, but the fact is, a cure is not available yet. So, this is an intermediate step towards a cure.... There are advantages to being on a small insulin dose,” including lower rates of hypoglycemia and maintenance of intraportal insulin, said Dr. Quattrin, of the State University of New York at Buffalo.
But in an accompanying editorial, Domenico Accili, MD, points to potential risks from immunotherapy and from attempting additional interventions at an “emotionally fraught” time when patients and families are coping with the new diabetes diagnosis.
He said of golimumab, “the effect is actually very small. ... There’s nothing wrong in and of itself with improving those outcomes. I just wouldn’t assign them as game changers.”
If this or a similar immunotherapeutic intervention were approved for this indication, “I would tell patients it exists and let them make the decision whether they want to try it. I wouldn’t say you must try it,” said Dr. Accili, of the Columbia University Diabetes and Endocrinology Research Center, New York.
With golimumab, higher C-peptide, lower insulin requirement
Of the 84 patients, who ranged in age from 6 to 21 years, 56 were randomly assigned within 100 days of being diagnosed with type 1 diabetes to receive golimumab, and 28 were assigned to receive placebo injections, given every 2 weeks.
The drug resulted in lower insulin use (0.51U/Kg per day vs. 0.69 U/kg per day), and the increase in insulin use over 52 weeks was less with golimumab than with placebo (0.07 vs. 0.24 U/kg per day; P = .001).
The mean percent decrease of C-peptide production from baseline was 12% with golimumab versus 56% with placebo.
Although the mean number of overall hypoglycemic events was similar, the mean number of level 2 hypoglycemic events (<54 mg/dL) was 36% lower with golimumab (11.5 vs. 17.6). There were no severe cases of hypoglycemia in either group.
No severe or serious infections occurred in either group, although mild to moderate infections were reported in 71% with golimumab versus 61% with placebo. More patients in the golimumab group experienced a decrease in neutrophils (29% vs. 19%).
Immunotherapy: Which one, and when should it start?
These findings come on the heels of the 2019 landmark results with another monoclonal antibody, the investigational anti-CD3 teplizumab (PRV-031). Among patients at risk, a diagnosis of type 1 diabetes was delayed by 2 years, and continued benefit was seen at 3 years.
However, Dr. Quattrin said teplizumab is limited by the fact that it must be administered via a 14-day infusion, whereas golimumab can be injected by patients themselves at home.
Moreover, the phase 2 teplizumab study was conducted in people who had antibodies that placed them at high risk for type 1 diabetes, but those patients did not yet have the condition. They were identified because they had close relatives with type 1 diabetes and were enrolled in the federally funded TrialNet screening program.
Dr. Quattrin is now participating in an ongoing phase 3 study of teplizumab that involves patients newly diagnosed with type 1 diabetes.
A Janssen spokesperson said in an interview that the company isn’t planning to further develop golimumab for use in type 1 diabetes.
“Our focus is to apply insights from the phase 2 ... proof-of-concept study to progress what we believe are novel, immunologically targeted pipeline candidates in stage 2 disease or presymptomatic stages of type 1 diabetes, which is consistent with our mission to intercept and prevent type 1 diabetes,” the spokesperson said.
To identify more individuals at risk for type 1 diabetes beyond the close relatives of those who already have it, so as to be able to intervene at a presymptomatic stage, Janssen is organizing a public-private effort to advocate for routine population screening for type 1 diabetes–related autoantibodies.
Dr. Quattrin said: “Preserving some insulin is key. Having somebody with beta cell functioning still is an intermediate step to a cure and will make their life easier, and that’s what people should care about.”
Dr. Accili, who cofounded and leads a company working on a novel approach to type 1 diabetes treatment, writes in his editorial: “We should also be mindful that this treatment debate is first world–centric.
“Current treatments for type 1 diabetes require resources not readily available in most parts of the world, where something as simple as refrigeration of insulin can become a logistic nightmare. While combinations of [approaches] tailored to individual risk and potential benefits are likely to make inroads in clinical practice, the need for a simpler, safer, and equally effective alternative to insulin remains,” he wrote.
Dr. Quattrin is a researcher and consultant for Janssen and conducts clinical trials for Provention Bio, Opko, and Ascendis. Dr. Accili is founder and director of Forkhead Therapeutics.
A version of this article originally appeared on Medscape.com.
Add delirium to checklist of COVID-19 symptoms in seniors
Delirium should be included on checklists of the presenting signs and symptoms of COVID-19, particularly in elderly adults, according to a multicenter study of seniors visiting emergency departments.
Overall, 28% of the 817 older adults who presented to the emergency department and were diagnosed with COVID-19 had delirium, according to a study published online November 19 in JAMA Network Open. Moreover, 16% of these patients had delirium that was not accompanied by typical symptoms or signs of SARS-CoV-2 infection.
Among patients with delirium, there was a greater probability of admission to the intensive care unit compared with patients who presented without delirium (adjusted relative risk [aRR], 1.67; 95% CI, 1.30 – 2.15), as well as a greater probability of death (aRR, 1.24; 95% CI, 1.00 – 1.55).
“These findings suggest the clinical importance of including delirium on checklists of presenting signs and symptoms of COVID-19 that guide screening, testing, and evaluation,” write Maura Kennedy, MD, MPH, and colleagues.
“I was absolutely seeing cases of delirium where there were no other symptoms of COVID-19, but we didn’t have lot of data on the frequency of this,” explained Kennedy, an emergency department physician at Massachusetts General Hospital and an assistant professor of emergency medicine at Harvard Medical School, Boston.
“And the rate was somewhat surprising compared with that seen in non-COVID studies of delirium, but then our study population was more at risk, coming from long-term care facilities and having prior stroke or dementia,” she said. The most common form of delirium was hypoactive sleepiness and nonresponsiveness, although hyperactivity and agitation were also seen.
Kennedy thinks the addition of delirium as a common presenting symptom to diagnostic checklists would prevent some cases from being missed and allow earlier identification and management of COVID-19 patients at high risk for poor outcomes. “We certainly don’t want to send them back undiagnosed to a long-term care facility or promote transmission within the hospital,” she told Medscape Medical News.
That step has already been implemented in some US centers. “Delirium is something we’ve been looking at since the early summer,” said geriatrician Angela Catic, MD, an assistant professor at Baylor College of Medicine’s Huffington Center on Aging and the Michael E. DeBakey VA Medical Center, Houston, Texas.
“If we see delirium, we’re looking for COVID-19,” said Catic, who was not involved in the study.
In Catic’s experience, it is “not at all atypical” to see patients whose only symptom of COVID-19 is delirium. As with other infections and diseases, “the aging brain is incredibly vulnerable,” she said.
According to William W. Hung, MD, MPH, an assistant professor of geriatrics and palliative medicine at the Icahn School of Medicine at Mount Sinai, New York City, delirium is “generally a common sign of something seriously wrong” in older adults. “In the case of COVID-19, low oxygenation caused by the infection may play a role,” he told Medscape Medical News. Although he agreed that delirium should be included in the differential diagnosis of COVID-19, how frequently it is the only symptom at presentation would need to be determined in a considerably larger population, he said.
Joining the company of those observing this COVID-19 manifestation is Christopher R. Carpenter, MD, a professor of emergency medicine at Washington University in St. Louis, St. Louis, Missouri. He was not a participant in the current study.
“I have absolutely seen and documented delirium as the presenting complaint in older adult patients who were ultimately diagnosed with SARS-CoV-2, and since March, I contemplate SARS-CoV-2 each time I identify delirium,” Carpenter told Medscape Medical News. “Honestly, I ― and most of my colleagues ― are considering SARS-CoV-2 for a range of symptoms and complaints these days, because of the odd presentations we’ve all encountered.”
Study details
For the study, Kennedy and colleagues enrolled consecutive adults aged 65 years and older who were diagnosed with active COVID-19 and who presented to emergency departments at seven centers in Massachusetts, Maine, Connecticut, Michigan, and North Carolina on or after March 13, 2020. Active infection with SARS-CoV-2 was determined on the basis of results of nasal swab polymerase chain reaction tests (99% of cases) or the appearance and distribution of ground-glass opacities on chest radiography or CT (1%).
Of the 817 patients enrolled, 386 (47%) were men, 493 (62%) were White, 215 (27%) were Black, and 54 (7%) were Hispanic or Latinx. The mean age of patients was 77.7 years (standard deviation, 8.2). Their age placed them at risk for chronic comorbidities and cognitive problems; indeed, 15% had at least four chronic conditions, and 30% had existing cognitive impairment.
The authors note that among the 226 patients (28%) who had delirium at presentation, 60 (27%) had experienced delirium for a duration of 2 to 7 days.
Additionally, of the 226 patients who exhibited delirium as a primary symptom, 84 (37%) showed no typical COVID-19 symptoms or signs, such as cough, fever, or shortness of breath.
The presence of delirium did not correlate with any of the typical COVID-19 symptoms in particular; Kennedy noted that only 56% of patients in the cohort had a fever at presentation.
Delirium at presentation was significantly associated with a median hospital stay of more than 8 days (aRR, 1.14; 95% CI, .97 – 1.35) and a greater risk for discharge to a rehabilitation facility (aRR, 1.55; 95% CI, 1.07 – 2.26). Factors associated with delirium included age older than 75 years, residence in a nursing home or assisted-living facility, previous use of psychoactive medications, vision impairment, hearing impairment, stroke, and Parkinson’s disease.
Kennedy noted that the rate of delirium observed in this study is much higher than that generally reported in emergency department studies conducted before the COVID-19 pandemic. In those studies, the delirium rate ranged from 7% to 20%. The associated risk factors, however, are comparable.
“Mounting evidence supports the high occurrence of delirium and other neuropsychiatric manifestations with COVID-19, with previously reported rates of 22% to 33% among hospitalized patients,” Kennedy and associates write.
In Carpenter’s opinion, the development of incident delirium while receiving care in the emergency department, as opposed to delirium at the time of presentation, has been exacerbated by the no-visitor policies mandated by the pandemic, which have prevented visits even from personal caregivers of patients with moderate to severe dementia. “Although healthcare systems need to be cognizant of the risk of spread to uninfected caregivers, there’s a risk-benefit balance that must be found, because having one caregiver at the bedside can prevent delirium in cognitively impaired patients,” said Carpenter, who was not involved in the current study.
Among the barriers to improving the situation, Carpenter cited the lack of routine delirium screening and the absence of high-quality evidence to support emergency department interventions to mitigate delirium.
“Layer those challenges on top of COVID-19’s rapidly evolving diagnostic landscape, frequent atypical presentations, and asymptomatic carriers across all age groups and the negative impact of delirium is magnified,” Carpenter said.
Once elderly patients are hospitalized, Kennedy recommends the nonpharmacologic guidelines of the Hospital Elder Life Program for reducing delirium risk. Recommendations include the providing of adequate sleep, hydration, and nutrition, as well as function restoration, precipitant avoidance, and reorientation.
The study was supported in part by the National Institute on Aging and the Massachusetts Medical School. The authors, Carpenter, Hung, and Catic have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Delirium should be included on checklists of the presenting signs and symptoms of COVID-19, particularly in elderly adults, according to a multicenter study of seniors visiting emergency departments.
Overall, 28% of the 817 older adults who presented to the emergency department and were diagnosed with COVID-19 had delirium, according to a study published online November 19 in JAMA Network Open. Moreover, 16% of these patients had delirium that was not accompanied by typical symptoms or signs of SARS-CoV-2 infection.
Among patients with delirium, there was a greater probability of admission to the intensive care unit compared with patients who presented without delirium (adjusted relative risk [aRR], 1.67; 95% CI, 1.30 – 2.15), as well as a greater probability of death (aRR, 1.24; 95% CI, 1.00 – 1.55).
“These findings suggest the clinical importance of including delirium on checklists of presenting signs and symptoms of COVID-19 that guide screening, testing, and evaluation,” write Maura Kennedy, MD, MPH, and colleagues.
“I was absolutely seeing cases of delirium where there were no other symptoms of COVID-19, but we didn’t have lot of data on the frequency of this,” explained Kennedy, an emergency department physician at Massachusetts General Hospital and an assistant professor of emergency medicine at Harvard Medical School, Boston.
“And the rate was somewhat surprising compared with that seen in non-COVID studies of delirium, but then our study population was more at risk, coming from long-term care facilities and having prior stroke or dementia,” she said. The most common form of delirium was hypoactive sleepiness and nonresponsiveness, although hyperactivity and agitation were also seen.
Kennedy thinks the addition of delirium as a common presenting symptom to diagnostic checklists would prevent some cases from being missed and allow earlier identification and management of COVID-19 patients at high risk for poor outcomes. “We certainly don’t want to send them back undiagnosed to a long-term care facility or promote transmission within the hospital,” she told Medscape Medical News.
That step has already been implemented in some US centers. “Delirium is something we’ve been looking at since the early summer,” said geriatrician Angela Catic, MD, an assistant professor at Baylor College of Medicine’s Huffington Center on Aging and the Michael E. DeBakey VA Medical Center, Houston, Texas.
“If we see delirium, we’re looking for COVID-19,” said Catic, who was not involved in the study.
In Catic’s experience, it is “not at all atypical” to see patients whose only symptom of COVID-19 is delirium. As with other infections and diseases, “the aging brain is incredibly vulnerable,” she said.
According to William W. Hung, MD, MPH, an assistant professor of geriatrics and palliative medicine at the Icahn School of Medicine at Mount Sinai, New York City, delirium is “generally a common sign of something seriously wrong” in older adults. “In the case of COVID-19, low oxygenation caused by the infection may play a role,” he told Medscape Medical News. Although he agreed that delirium should be included in the differential diagnosis of COVID-19, how frequently it is the only symptom at presentation would need to be determined in a considerably larger population, he said.
Joining the company of those observing this COVID-19 manifestation is Christopher R. Carpenter, MD, a professor of emergency medicine at Washington University in St. Louis, St. Louis, Missouri. He was not a participant in the current study.
“I have absolutely seen and documented delirium as the presenting complaint in older adult patients who were ultimately diagnosed with SARS-CoV-2, and since March, I contemplate SARS-CoV-2 each time I identify delirium,” Carpenter told Medscape Medical News. “Honestly, I ― and most of my colleagues ― are considering SARS-CoV-2 for a range of symptoms and complaints these days, because of the odd presentations we’ve all encountered.”
Study details
For the study, Kennedy and colleagues enrolled consecutive adults aged 65 years and older who were diagnosed with active COVID-19 and who presented to emergency departments at seven centers in Massachusetts, Maine, Connecticut, Michigan, and North Carolina on or after March 13, 2020. Active infection with SARS-CoV-2 was determined on the basis of results of nasal swab polymerase chain reaction tests (99% of cases) or the appearance and distribution of ground-glass opacities on chest radiography or CT (1%).
Of the 817 patients enrolled, 386 (47%) were men, 493 (62%) were White, 215 (27%) were Black, and 54 (7%) were Hispanic or Latinx. The mean age of patients was 77.7 years (standard deviation, 8.2). Their age placed them at risk for chronic comorbidities and cognitive problems; indeed, 15% had at least four chronic conditions, and 30% had existing cognitive impairment.
The authors note that among the 226 patients (28%) who had delirium at presentation, 60 (27%) had experienced delirium for a duration of 2 to 7 days.
Additionally, of the 226 patients who exhibited delirium as a primary symptom, 84 (37%) showed no typical COVID-19 symptoms or signs, such as cough, fever, or shortness of breath.
The presence of delirium did not correlate with any of the typical COVID-19 symptoms in particular; Kennedy noted that only 56% of patients in the cohort had a fever at presentation.
Delirium at presentation was significantly associated with a median hospital stay of more than 8 days (aRR, 1.14; 95% CI, .97 – 1.35) and a greater risk for discharge to a rehabilitation facility (aRR, 1.55; 95% CI, 1.07 – 2.26). Factors associated with delirium included age older than 75 years, residence in a nursing home or assisted-living facility, previous use of psychoactive medications, vision impairment, hearing impairment, stroke, and Parkinson’s disease.
Kennedy noted that the rate of delirium observed in this study is much higher than that generally reported in emergency department studies conducted before the COVID-19 pandemic. In those studies, the delirium rate ranged from 7% to 20%. The associated risk factors, however, are comparable.
“Mounting evidence supports the high occurrence of delirium and other neuropsychiatric manifestations with COVID-19, with previously reported rates of 22% to 33% among hospitalized patients,” Kennedy and associates write.
In Carpenter’s opinion, the development of incident delirium while receiving care in the emergency department, as opposed to delirium at the time of presentation, has been exacerbated by the no-visitor policies mandated by the pandemic, which have prevented visits even from personal caregivers of patients with moderate to severe dementia. “Although healthcare systems need to be cognizant of the risk of spread to uninfected caregivers, there’s a risk-benefit balance that must be found, because having one caregiver at the bedside can prevent delirium in cognitively impaired patients,” said Carpenter, who was not involved in the current study.
Among the barriers to improving the situation, Carpenter cited the lack of routine delirium screening and the absence of high-quality evidence to support emergency department interventions to mitigate delirium.
“Layer those challenges on top of COVID-19’s rapidly evolving diagnostic landscape, frequent atypical presentations, and asymptomatic carriers across all age groups and the negative impact of delirium is magnified,” Carpenter said.
Once elderly patients are hospitalized, Kennedy recommends the nonpharmacologic guidelines of the Hospital Elder Life Program for reducing delirium risk. Recommendations include the providing of adequate sleep, hydration, and nutrition, as well as function restoration, precipitant avoidance, and reorientation.
The study was supported in part by the National Institute on Aging and the Massachusetts Medical School. The authors, Carpenter, Hung, and Catic have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Delirium should be included on checklists of the presenting signs and symptoms of COVID-19, particularly in elderly adults, according to a multicenter study of seniors visiting emergency departments.
Overall, 28% of the 817 older adults who presented to the emergency department and were diagnosed with COVID-19 had delirium, according to a study published online November 19 in JAMA Network Open. Moreover, 16% of these patients had delirium that was not accompanied by typical symptoms or signs of SARS-CoV-2 infection.
Among patients with delirium, there was a greater probability of admission to the intensive care unit compared with patients who presented without delirium (adjusted relative risk [aRR], 1.67; 95% CI, 1.30 – 2.15), as well as a greater probability of death (aRR, 1.24; 95% CI, 1.00 – 1.55).
“These findings suggest the clinical importance of including delirium on checklists of presenting signs and symptoms of COVID-19 that guide screening, testing, and evaluation,” write Maura Kennedy, MD, MPH, and colleagues.
“I was absolutely seeing cases of delirium where there were no other symptoms of COVID-19, but we didn’t have lot of data on the frequency of this,” explained Kennedy, an emergency department physician at Massachusetts General Hospital and an assistant professor of emergency medicine at Harvard Medical School, Boston.
“And the rate was somewhat surprising compared with that seen in non-COVID studies of delirium, but then our study population was more at risk, coming from long-term care facilities and having prior stroke or dementia,” she said. The most common form of delirium was hypoactive sleepiness and nonresponsiveness, although hyperactivity and agitation were also seen.
Kennedy thinks the addition of delirium as a common presenting symptom to diagnostic checklists would prevent some cases from being missed and allow earlier identification and management of COVID-19 patients at high risk for poor outcomes. “We certainly don’t want to send them back undiagnosed to a long-term care facility or promote transmission within the hospital,” she told Medscape Medical News.
That step has already been implemented in some US centers. “Delirium is something we’ve been looking at since the early summer,” said geriatrician Angela Catic, MD, an assistant professor at Baylor College of Medicine’s Huffington Center on Aging and the Michael E. DeBakey VA Medical Center, Houston, Texas.
“If we see delirium, we’re looking for COVID-19,” said Catic, who was not involved in the study.
In Catic’s experience, it is “not at all atypical” to see patients whose only symptom of COVID-19 is delirium. As with other infections and diseases, “the aging brain is incredibly vulnerable,” she said.
According to William W. Hung, MD, MPH, an assistant professor of geriatrics and palliative medicine at the Icahn School of Medicine at Mount Sinai, New York City, delirium is “generally a common sign of something seriously wrong” in older adults. “In the case of COVID-19, low oxygenation caused by the infection may play a role,” he told Medscape Medical News. Although he agreed that delirium should be included in the differential diagnosis of COVID-19, how frequently it is the only symptom at presentation would need to be determined in a considerably larger population, he said.
Joining the company of those observing this COVID-19 manifestation is Christopher R. Carpenter, MD, a professor of emergency medicine at Washington University in St. Louis, St. Louis, Missouri. He was not a participant in the current study.
“I have absolutely seen and documented delirium as the presenting complaint in older adult patients who were ultimately diagnosed with SARS-CoV-2, and since March, I contemplate SARS-CoV-2 each time I identify delirium,” Carpenter told Medscape Medical News. “Honestly, I ― and most of my colleagues ― are considering SARS-CoV-2 for a range of symptoms and complaints these days, because of the odd presentations we’ve all encountered.”
Study details
For the study, Kennedy and colleagues enrolled consecutive adults aged 65 years and older who were diagnosed with active COVID-19 and who presented to emergency departments at seven centers in Massachusetts, Maine, Connecticut, Michigan, and North Carolina on or after March 13, 2020. Active infection with SARS-CoV-2 was determined on the basis of results of nasal swab polymerase chain reaction tests (99% of cases) or the appearance and distribution of ground-glass opacities on chest radiography or CT (1%).
Of the 817 patients enrolled, 386 (47%) were men, 493 (62%) were White, 215 (27%) were Black, and 54 (7%) were Hispanic or Latinx. The mean age of patients was 77.7 years (standard deviation, 8.2). Their age placed them at risk for chronic comorbidities and cognitive problems; indeed, 15% had at least four chronic conditions, and 30% had existing cognitive impairment.
The authors note that among the 226 patients (28%) who had delirium at presentation, 60 (27%) had experienced delirium for a duration of 2 to 7 days.
Additionally, of the 226 patients who exhibited delirium as a primary symptom, 84 (37%) showed no typical COVID-19 symptoms or signs, such as cough, fever, or shortness of breath.
The presence of delirium did not correlate with any of the typical COVID-19 symptoms in particular; Kennedy noted that only 56% of patients in the cohort had a fever at presentation.
Delirium at presentation was significantly associated with a median hospital stay of more than 8 days (aRR, 1.14; 95% CI, .97 – 1.35) and a greater risk for discharge to a rehabilitation facility (aRR, 1.55; 95% CI, 1.07 – 2.26). Factors associated with delirium included age older than 75 years, residence in a nursing home or assisted-living facility, previous use of psychoactive medications, vision impairment, hearing impairment, stroke, and Parkinson’s disease.
Kennedy noted that the rate of delirium observed in this study is much higher than that generally reported in emergency department studies conducted before the COVID-19 pandemic. In those studies, the delirium rate ranged from 7% to 20%. The associated risk factors, however, are comparable.
“Mounting evidence supports the high occurrence of delirium and other neuropsychiatric manifestations with COVID-19, with previously reported rates of 22% to 33% among hospitalized patients,” Kennedy and associates write.
In Carpenter’s opinion, the development of incident delirium while receiving care in the emergency department, as opposed to delirium at the time of presentation, has been exacerbated by the no-visitor policies mandated by the pandemic, which have prevented visits even from personal caregivers of patients with moderate to severe dementia. “Although healthcare systems need to be cognizant of the risk of spread to uninfected caregivers, there’s a risk-benefit balance that must be found, because having one caregiver at the bedside can prevent delirium in cognitively impaired patients,” said Carpenter, who was not involved in the current study.
Among the barriers to improving the situation, Carpenter cited the lack of routine delirium screening and the absence of high-quality evidence to support emergency department interventions to mitigate delirium.
“Layer those challenges on top of COVID-19’s rapidly evolving diagnostic landscape, frequent atypical presentations, and asymptomatic carriers across all age groups and the negative impact of delirium is magnified,” Carpenter said.
Once elderly patients are hospitalized, Kennedy recommends the nonpharmacologic guidelines of the Hospital Elder Life Program for reducing delirium risk. Recommendations include the providing of adequate sleep, hydration, and nutrition, as well as function restoration, precipitant avoidance, and reorientation.
The study was supported in part by the National Institute on Aging and the Massachusetts Medical School. The authors, Carpenter, Hung, and Catic have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Blood pressure treatment reduces bleeding in ICH
a systematic review and meta-analysis shows, although it does reduce hematoma growth in these patients.
Despite the negative finding, the investigators observed broad variation in treatment effect among the studies they reviewed. They also found that target-based blood pressure treatment tended to improve function more than fixed-dose treatment.
“These data provide a strong message that early blood pressure–lowering treatment can control bleeding. This was not clear beforehand,” Craig Anderson, PhD, professor of neurology and epidemiology at the University of New South Wales, Sydney, said in an interview.
“But these data also indicate that the management of blood pressure in ICH is complex,” he added. Timing, type of drug, and type of patient must be considered, he said. “We need more data to allow better individualizing of such therapy.”
The results were presented at the European Stroke Organisation–World Stroke Organisation (ESO-WSO) Conference 2020.
Controversy about the efficacy of blood pressure reduction for patients with ICH continues, despite studies that have examined this question. In this analysis, Dr. Anderson and colleagues sought to examine the evidence from randomized controlled trials in this area and identify potentially overlooked heterogeneity among trials.
The investigators conducted a systematic review and meta-analysis of studies in the Cochrane Central Register of Controlled Trials, EMBASE, and MEDLINE databases. They searched for randomized controlled trials of blood pressure management for adults with acute ICH, focusing on studies in which patients were enrolled within 7 days of ICH onset. These studies compared intensive blood pressure management with guideline-based management.
Investigators chose function, defined as Modified Rankin Scale (mRS) score at 90 days, as their primary outcome. Radiologic outcomes included absolute (>6 mL) and proportional (>33%) hematoma growth at 24 hours. They used the intention to treat dataset from each trial in their statistical analyses and created generalized linear mixed models with prespecified covariables using a one-stage approach.
Variation by drug
A total of 7,094 studies were identified, of which 50 were eligible for inclusion. Their analysis encompassed 16 studies for which the respective investigators were willing to share patient-level data. The analysis included data on 6,221 patients. The mean age of the patients was 64.2 years, 36.4% were women, and the median time from symptom onset to randomization was 3.8 hours.
Mean National Institutes of Health Stroke Scale score was approximately 11. Mean systolic blood pressure at baseline was 177 mm Hg, and mean hematoma volume was approximately 10.6 mL.
The difference in blood pressure between the intensive and guideline groups was approximately 8 mm Hg at 1 hour and 12 mm Hg at 24 hours.
Intensive blood pressure management did not affect function at 90 days. The adjusted odds ratio for unfavorable shift in mRS scores was 0.97 (95% CI, 0.88-1.06; P = .503). Intensive blood pressure management did, however, reduce hematoma growth (absolute aOR, 0.75; 95% CI, 0.60-0.92; P = .007; relative aOR, 0.82; 95% CI, 0.68-0.99; P = .034).
In prespecified subgroup analyses, they found a trend toward adverse outcomes among patients who received renin-angiotensin blockers and a trend toward benefit for patients who received alpha- or beta-receptor antagonists or calcium channel blockers. They did not observe a clear association between time of treatment and outcome.
In addition to hematoma growth, other factors influence prognosis after ICH, such as the patient’s status before ICH (for example, cardiovascular risk factors, age, and hypertensive effects on the brain, kidneys, and heart), the location of ICH and its effects on surrounding structures, and complications of care in hospitals, such as infection and bleeding, said Dr. Anderson.
They are conducting two ongoing clinical trials in patients with ICH. One, INTERACT3, is evaluating a “care bundle” quality control package that includes early intensive blood pressure lowering for patients with large ICH who undergo surgery.
The other, INTERACT4, is evaluating early blood pressure control in the ambulance for patients with suspected acute stroke. At least one-fifth of those patients will have ICH, said Dr. Anderson.
Prevention is essential
Among patients with ICH, much of the bleeding occurs before presentation at the hospital, Louis R. Caplan, MD, a neurologist at Beth Israel Deaconess Medical Center, Boston, said in an interview. Furthermore, the bleeding mainly occurs in the deep part of the brain where most of the important motor tracts are. “If those tracts are already hit, a little extra blood isn’t going to change things,” said Dr. Caplan, who was not involved in the research.
In addition, blood is pushed from inside the brain to the periphery until the pressure outside the brain is equal to the pressure inside it. “You can decrease the amount of bleeding significantly, but it probably doesn’t affect the outcome,” said Dr. Caplan.
One factor in patients’ apparent lack of functional improvement is that the mRS is not sensitive to minor changes in disability, he said. “You have to show a pretty important change for it to make a difference,” said Dr. Caplan.
In addition, recovery from a hemorrhage takes much longer than recovery from an infarct. Examining the population at 6 months would have been preferable to examining them at 90 days, but the investigators might not have 6-month data, said Dr. Caplan.
“The main thing is really prevention,” he concluded.
The study was conducted with funding from Takeda. Dr. Anderson reported receiving funding from the National Health and Medical Research Council of Australia and speaker fees from Takeda. Dr. Caplan has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
a systematic review and meta-analysis shows, although it does reduce hematoma growth in these patients.
Despite the negative finding, the investigators observed broad variation in treatment effect among the studies they reviewed. They also found that target-based blood pressure treatment tended to improve function more than fixed-dose treatment.
“These data provide a strong message that early blood pressure–lowering treatment can control bleeding. This was not clear beforehand,” Craig Anderson, PhD, professor of neurology and epidemiology at the University of New South Wales, Sydney, said in an interview.
“But these data also indicate that the management of blood pressure in ICH is complex,” he added. Timing, type of drug, and type of patient must be considered, he said. “We need more data to allow better individualizing of such therapy.”
The results were presented at the European Stroke Organisation–World Stroke Organisation (ESO-WSO) Conference 2020.
Controversy about the efficacy of blood pressure reduction for patients with ICH continues, despite studies that have examined this question. In this analysis, Dr. Anderson and colleagues sought to examine the evidence from randomized controlled trials in this area and identify potentially overlooked heterogeneity among trials.
The investigators conducted a systematic review and meta-analysis of studies in the Cochrane Central Register of Controlled Trials, EMBASE, and MEDLINE databases. They searched for randomized controlled trials of blood pressure management for adults with acute ICH, focusing on studies in which patients were enrolled within 7 days of ICH onset. These studies compared intensive blood pressure management with guideline-based management.
Investigators chose function, defined as Modified Rankin Scale (mRS) score at 90 days, as their primary outcome. Radiologic outcomes included absolute (>6 mL) and proportional (>33%) hematoma growth at 24 hours. They used the intention to treat dataset from each trial in their statistical analyses and created generalized linear mixed models with prespecified covariables using a one-stage approach.
Variation by drug
A total of 7,094 studies were identified, of which 50 were eligible for inclusion. Their analysis encompassed 16 studies for which the respective investigators were willing to share patient-level data. The analysis included data on 6,221 patients. The mean age of the patients was 64.2 years, 36.4% were women, and the median time from symptom onset to randomization was 3.8 hours.
Mean National Institutes of Health Stroke Scale score was approximately 11. Mean systolic blood pressure at baseline was 177 mm Hg, and mean hematoma volume was approximately 10.6 mL.
The difference in blood pressure between the intensive and guideline groups was approximately 8 mm Hg at 1 hour and 12 mm Hg at 24 hours.
Intensive blood pressure management did not affect function at 90 days. The adjusted odds ratio for unfavorable shift in mRS scores was 0.97 (95% CI, 0.88-1.06; P = .503). Intensive blood pressure management did, however, reduce hematoma growth (absolute aOR, 0.75; 95% CI, 0.60-0.92; P = .007; relative aOR, 0.82; 95% CI, 0.68-0.99; P = .034).
In prespecified subgroup analyses, they found a trend toward adverse outcomes among patients who received renin-angiotensin blockers and a trend toward benefit for patients who received alpha- or beta-receptor antagonists or calcium channel blockers. They did not observe a clear association between time of treatment and outcome.
In addition to hematoma growth, other factors influence prognosis after ICH, such as the patient’s status before ICH (for example, cardiovascular risk factors, age, and hypertensive effects on the brain, kidneys, and heart), the location of ICH and its effects on surrounding structures, and complications of care in hospitals, such as infection and bleeding, said Dr. Anderson.
They are conducting two ongoing clinical trials in patients with ICH. One, INTERACT3, is evaluating a “care bundle” quality control package that includes early intensive blood pressure lowering for patients with large ICH who undergo surgery.
The other, INTERACT4, is evaluating early blood pressure control in the ambulance for patients with suspected acute stroke. At least one-fifth of those patients will have ICH, said Dr. Anderson.
Prevention is essential
Among patients with ICH, much of the bleeding occurs before presentation at the hospital, Louis R. Caplan, MD, a neurologist at Beth Israel Deaconess Medical Center, Boston, said in an interview. Furthermore, the bleeding mainly occurs in the deep part of the brain where most of the important motor tracts are. “If those tracts are already hit, a little extra blood isn’t going to change things,” said Dr. Caplan, who was not involved in the research.
In addition, blood is pushed from inside the brain to the periphery until the pressure outside the brain is equal to the pressure inside it. “You can decrease the amount of bleeding significantly, but it probably doesn’t affect the outcome,” said Dr. Caplan.
One factor in patients’ apparent lack of functional improvement is that the mRS is not sensitive to minor changes in disability, he said. “You have to show a pretty important change for it to make a difference,” said Dr. Caplan.
In addition, recovery from a hemorrhage takes much longer than recovery from an infarct. Examining the population at 6 months would have been preferable to examining them at 90 days, but the investigators might not have 6-month data, said Dr. Caplan.
“The main thing is really prevention,” he concluded.
The study was conducted with funding from Takeda. Dr. Anderson reported receiving funding from the National Health and Medical Research Council of Australia and speaker fees from Takeda. Dr. Caplan has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
a systematic review and meta-analysis shows, although it does reduce hematoma growth in these patients.
Despite the negative finding, the investigators observed broad variation in treatment effect among the studies they reviewed. They also found that target-based blood pressure treatment tended to improve function more than fixed-dose treatment.
“These data provide a strong message that early blood pressure–lowering treatment can control bleeding. This was not clear beforehand,” Craig Anderson, PhD, professor of neurology and epidemiology at the University of New South Wales, Sydney, said in an interview.
“But these data also indicate that the management of blood pressure in ICH is complex,” he added. Timing, type of drug, and type of patient must be considered, he said. “We need more data to allow better individualizing of such therapy.”
The results were presented at the European Stroke Organisation–World Stroke Organisation (ESO-WSO) Conference 2020.
Controversy about the efficacy of blood pressure reduction for patients with ICH continues, despite studies that have examined this question. In this analysis, Dr. Anderson and colleagues sought to examine the evidence from randomized controlled trials in this area and identify potentially overlooked heterogeneity among trials.
The investigators conducted a systematic review and meta-analysis of studies in the Cochrane Central Register of Controlled Trials, EMBASE, and MEDLINE databases. They searched for randomized controlled trials of blood pressure management for adults with acute ICH, focusing on studies in which patients were enrolled within 7 days of ICH onset. These studies compared intensive blood pressure management with guideline-based management.
Investigators chose function, defined as Modified Rankin Scale (mRS) score at 90 days, as their primary outcome. Radiologic outcomes included absolute (>6 mL) and proportional (>33%) hematoma growth at 24 hours. They used the intention to treat dataset from each trial in their statistical analyses and created generalized linear mixed models with prespecified covariables using a one-stage approach.
Variation by drug
A total of 7,094 studies were identified, of which 50 were eligible for inclusion. Their analysis encompassed 16 studies for which the respective investigators were willing to share patient-level data. The analysis included data on 6,221 patients. The mean age of the patients was 64.2 years, 36.4% were women, and the median time from symptom onset to randomization was 3.8 hours.
Mean National Institutes of Health Stroke Scale score was approximately 11. Mean systolic blood pressure at baseline was 177 mm Hg, and mean hematoma volume was approximately 10.6 mL.
The difference in blood pressure between the intensive and guideline groups was approximately 8 mm Hg at 1 hour and 12 mm Hg at 24 hours.
Intensive blood pressure management did not affect function at 90 days. The adjusted odds ratio for unfavorable shift in mRS scores was 0.97 (95% CI, 0.88-1.06; P = .503). Intensive blood pressure management did, however, reduce hematoma growth (absolute aOR, 0.75; 95% CI, 0.60-0.92; P = .007; relative aOR, 0.82; 95% CI, 0.68-0.99; P = .034).
In prespecified subgroup analyses, they found a trend toward adverse outcomes among patients who received renin-angiotensin blockers and a trend toward benefit for patients who received alpha- or beta-receptor antagonists or calcium channel blockers. They did not observe a clear association between time of treatment and outcome.
In addition to hematoma growth, other factors influence prognosis after ICH, such as the patient’s status before ICH (for example, cardiovascular risk factors, age, and hypertensive effects on the brain, kidneys, and heart), the location of ICH and its effects on surrounding structures, and complications of care in hospitals, such as infection and bleeding, said Dr. Anderson.
They are conducting two ongoing clinical trials in patients with ICH. One, INTERACT3, is evaluating a “care bundle” quality control package that includes early intensive blood pressure lowering for patients with large ICH who undergo surgery.
The other, INTERACT4, is evaluating early blood pressure control in the ambulance for patients with suspected acute stroke. At least one-fifth of those patients will have ICH, said Dr. Anderson.
Prevention is essential
Among patients with ICH, much of the bleeding occurs before presentation at the hospital, Louis R. Caplan, MD, a neurologist at Beth Israel Deaconess Medical Center, Boston, said in an interview. Furthermore, the bleeding mainly occurs in the deep part of the brain where most of the important motor tracts are. “If those tracts are already hit, a little extra blood isn’t going to change things,” said Dr. Caplan, who was not involved in the research.
In addition, blood is pushed from inside the brain to the periphery until the pressure outside the brain is equal to the pressure inside it. “You can decrease the amount of bleeding significantly, but it probably doesn’t affect the outcome,” said Dr. Caplan.
One factor in patients’ apparent lack of functional improvement is that the mRS is not sensitive to minor changes in disability, he said. “You have to show a pretty important change for it to make a difference,” said Dr. Caplan.
In addition, recovery from a hemorrhage takes much longer than recovery from an infarct. Examining the population at 6 months would have been preferable to examining them at 90 days, but the investigators might not have 6-month data, said Dr. Caplan.
“The main thing is really prevention,” he concluded.
The study was conducted with funding from Takeda. Dr. Anderson reported receiving funding from the National Health and Medical Research Council of Australia and speaker fees from Takeda. Dr. Caplan has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM ESO-WSO CONFERENCE 2020
Statins beneficial in elderly, guidelines should be strengthened
Contrary to historical evidence, two new studies show.
“By contrast with previous historical studies, our data show that LDL cholesterol is an important risk factor for myocardial infarction and atherosclerotic cardiovascular disease in a contemporary primary prevention cohort of individuals aged 70 to 100 years,” Borge Nordestgaard, MD, of the University of Copenhagen, and colleagues noted in the first of the two studies, published this week in the Lancet.
“By lowering LDL cholesterol in healthy individuals aged 70-100 years, the potential for preventing myocardial infarctions and atherosclerotic cardiovascular disease is huge, and at a substantially lower number needed to treat when compared with those aged 20-69 years,” they added.
“These findings support the concept of the cumulative burden of LDL cholesterol over one’s lifetime and the progressive increase in risk for atherosclerotic cardiovascular disease, including myocardial infarction, with age,” added Frederick J. Raal, PhD, and Farzahna Mohamed, MB BCh, of the University of the Witwatersrand, Johannesburg, South Africa, in an editorial published with both new studies in the Lancet (2020 Nov 10. doi: 10.1016/S0140-6736[20]32333-3).
The studies underscore the need for clinicians to consider continued risks associated with elevated LDL cholesterol in older age, they stressed, adding that statins are also beneficial for younger persons at risk to prevent conditions from worsening.
“The average age of patients in all the trials analyzed was older than 60 years, an age when atherosclerotic cardiovascular disease is already well established,” the editorialists wrote.
“Lipid-lowering therapy should be initiated at a younger age, preferably before age 40 years, in those at risk to delay the onset of atherosclerosis, rather than try to manage the condition once fully established or advanced,” they stressed.
No RCTs have included patients older than 70
For persons aged 40-75 years, elevated LDL cholesterol levels are a known risk factor for MI and atherosclerotic cardiovascular disease, and there is consensus in guidelines regarding treatment with statins.
However, the risk for people older than 70 is controversial. Some studies show little or no association between elevated LDL cholesterol levels and an increased risk for MI.
Contributing to the uncertainty is that few of the randomized, controlled trials that have investigated the question have included patients aged older than 70 years.
As a consequence, many practice guidelines have noted that the level of evidence in older patients is low, and some organizations have lowered the strength of recommendations regarding the treatment for older patients in comparison with younger patients.
Primary prevention: CV events increase with elevated LDL cholesterol in older age
Dr. Nordestgaard and colleagues studied data on 91,131 people living in Copenhagen who did not have atherosclerotic cardiovascular disease or diabetes at baseline and were not taking statins.
Of the participants, 10,592 were aged 70-79 years, and 3,188 participants were aged 80-100 years.
Over an average follow-up period of 7.7 years, 1,515 participants had a first MI, and 3,389 developed atherosclerotic cardiovascular disease.
In the primary-prevention cohort, after multivariate adjustment, the risk of having a heart attack per 1.0 mmol/L increase in LDL cholesterol was increased in the group overall (hazard ratio, 1.34). The increased risk was observed for all age groups, including those aged 80-100 years (HR, 1.28), 70-79 (HR, 1.25), 60-69 (HR, 1.29), 50-59 (HR, 1.28), and 20-49 (HR, 1.68).
Risk for atherosclerotic cardiovascular disease was also raised per 1.0 mmol/L increase in LDL cholesterol overall (HR, 1.16) and in all age groups, particularly those aged 70-100 years.
Greater elevations in LDL cholesterol (5.0 mmol/L or higher, indicative of possible familial hypercholesterolemia) were associated with a notably higher risk for heart attack after multivariate adjustment in people aged 80-100 (HR, 2.99). Risk was also higher among those aged 70-79 (HR, 1.82).
The highest incidence was in those older than 70. The rate was 8.5 heart attacks per 1,000 people per year among those aged 80-100 and 5.2 heart attacks per 1,000 in those aged 70-79. The rates were 2.5 per 1,000 among those 60-69, 1.8 for those aged 50-59, and 0.8 for those aged 20-49.
“The absolute risk [of cardiovascular events] is of course much higher in the elderly than those under the age of 75, but what was a surprise was how clear our results were on a relative risk scale, that the risk associated with elevated LDL [cholesterol] was as high in people aged 80-100 as the younger patients,” Dr. Nordestgaard said in an interview.
With regard to the benefits of cholesterol-lowering drugs, the study showed that the number needed to prevent one heart attack over 5 years was 80 among those aged 80-100; the number was 439 for people aged 50-59.
With regard to stronger statins, when moderate-intensity statins were used, the number needed to treat to prevent one cardiovascular disease event of any type dropped to 42 for patients aged 80-100. It was 88 for those aged 70-79, 164 for those aged 60-69, 345 for those aged 50-59, and 769 for those aged 20-49.
“The clinical significance of this is that it appears those in older age groups indeed benefit from cholesterol-lowering therapy,” Dr. Nordestgaard said. “I think many people have this idea that LDL [cholesterol] is not important over the age of about 70-75, but that’s not the case.”
“These robust findings are novel,” he and his colleagues stressed.
Despite these observational findings, the South African editorialists noted that “whether lipid-lowering therapy should be initiated for primary prevention in people aged 75 years or older is unclear,” owing to the host of risks and benefits that need to be balanced.
The findings of an ongoing randomized, placebo-controlled trial (STAREE) may answer this question, they wrote. It is investigating primary prevention in 18,000 older patients (≥70 years) who are being randomly assigned to receive atorvastatin 40 mg/d or placebo. The study is seeking to determine whether statin treatment extends the length of a disability-free life, which will be assessed on the basis of survival outside permanent residential care. Results are expected in 2022-2023.
Unequivocal reductions in events in elderly, comparable with younger patients
In the second study (Lancet. 2020 Nov 10. doi: 10.1016/S0140-6736[20]32332-1), Baris Gencer, MD, of Brigham and Women’s Hospital, Boston, =and colleagues evaluated the effects of statins and other cholesterol-lowering drugs, including ezetimibe and proprotein convertase subtilisin/kexin type 9 inhibitors, in older versus younger patients.
The systematic review and meta-analysis of 29 randomized controlled trials, also published in the Lancet, were presented virtually as a poster as part of the 2020 American Heart Association scientific session. It included data on 244,090 patients, including 21,492 aged 75 years and older.
The meta-analysis included studies of cardiovascular outcomes of a guideline-recommended LDL cholesterol–lowering drug, with a median follow-up of at least 2 years and inclusion of data on patients aged 75 years and older.
The results showed that over a median follow-up of 2.2 to 6 years, statin use by older patients was associated with a relative risk reduction of major vascular events of 26% per 1 mmol/L reduction in LDL cholesterol (P = .0019), which was comparable with a risk reduction of 15% per 1 mmol/L reduction in LDL cholesterol for patients younger than 75 years (P = .37, compared with older patients).
Treatment of older patients with LDL cholesterol–lowering drugs was also associated with significantly improved outcomes in cardiovascular death (risk ratio, 0.85), MI (RR, 0.80), stroke (RR, 0.73), and coronary revascularization (RR, 0.80).
“We found an unequivocal reduction in the risk of major vascular events with both statin and nonstatin LDL cholesterol-lowering treatments, which was similar to that seen in younger patients,” the authors wrote.
“Cholesterol-lowering medications are affordable drugs that have reduced risk of heart disease for millions of people worldwide, but until now, their benefits for older people have remained less certain,” said lead author Marc Sabatine, MD, also of Brigham and Women’s Hospital, in a Lancet press release.
“Our analysis indicates that these therapies are as effective in reducing cardiovascular events and deaths in people aged 75 years and over as they are in younger people. We found no offsetting safety concerns, and together, these results should strengthen guideline recommendations for the use of cholesterol-lowering medications, including statin and nonstatin therapy, in elderly people.”
The editorialists agreed: “More than 80% of fatal cardiovascular events occur in individuals older than 65 years, and the incidence of cardiovascular events is increasing in those older than 80 years; therefore, the findings of Gencer and colleagues’ study should encourage the use of lipid-lowering therapy in older patients.”
The authors of the two studies have disclosed no relevant financial relationships. Dr. Raal has received research grants, honoraria, or consulting fees for advisory board membership, professional input, and lectures on lipid-lowering drug therapy from Amgen, Regeneron, Sanofi, Novartis, and the Medicines Company.
A version of this article originally appeared on Medscape.com.
Contrary to historical evidence, two new studies show.
“By contrast with previous historical studies, our data show that LDL cholesterol is an important risk factor for myocardial infarction and atherosclerotic cardiovascular disease in a contemporary primary prevention cohort of individuals aged 70 to 100 years,” Borge Nordestgaard, MD, of the University of Copenhagen, and colleagues noted in the first of the two studies, published this week in the Lancet.
“By lowering LDL cholesterol in healthy individuals aged 70-100 years, the potential for preventing myocardial infarctions and atherosclerotic cardiovascular disease is huge, and at a substantially lower number needed to treat when compared with those aged 20-69 years,” they added.
“These findings support the concept of the cumulative burden of LDL cholesterol over one’s lifetime and the progressive increase in risk for atherosclerotic cardiovascular disease, including myocardial infarction, with age,” added Frederick J. Raal, PhD, and Farzahna Mohamed, MB BCh, of the University of the Witwatersrand, Johannesburg, South Africa, in an editorial published with both new studies in the Lancet (2020 Nov 10. doi: 10.1016/S0140-6736[20]32333-3).
The studies underscore the need for clinicians to consider continued risks associated with elevated LDL cholesterol in older age, they stressed, adding that statins are also beneficial for younger persons at risk to prevent conditions from worsening.
“The average age of patients in all the trials analyzed was older than 60 years, an age when atherosclerotic cardiovascular disease is already well established,” the editorialists wrote.
“Lipid-lowering therapy should be initiated at a younger age, preferably before age 40 years, in those at risk to delay the onset of atherosclerosis, rather than try to manage the condition once fully established or advanced,” they stressed.
No RCTs have included patients older than 70
For persons aged 40-75 years, elevated LDL cholesterol levels are a known risk factor for MI and atherosclerotic cardiovascular disease, and there is consensus in guidelines regarding treatment with statins.
However, the risk for people older than 70 is controversial. Some studies show little or no association between elevated LDL cholesterol levels and an increased risk for MI.
Contributing to the uncertainty is that few of the randomized, controlled trials that have investigated the question have included patients aged older than 70 years.
As a consequence, many practice guidelines have noted that the level of evidence in older patients is low, and some organizations have lowered the strength of recommendations regarding the treatment for older patients in comparison with younger patients.
Primary prevention: CV events increase with elevated LDL cholesterol in older age
Dr. Nordestgaard and colleagues studied data on 91,131 people living in Copenhagen who did not have atherosclerotic cardiovascular disease or diabetes at baseline and were not taking statins.
Of the participants, 10,592 were aged 70-79 years, and 3,188 participants were aged 80-100 years.
Over an average follow-up period of 7.7 years, 1,515 participants had a first MI, and 3,389 developed atherosclerotic cardiovascular disease.
In the primary-prevention cohort, after multivariate adjustment, the risk of having a heart attack per 1.0 mmol/L increase in LDL cholesterol was increased in the group overall (hazard ratio, 1.34). The increased risk was observed for all age groups, including those aged 80-100 years (HR, 1.28), 70-79 (HR, 1.25), 60-69 (HR, 1.29), 50-59 (HR, 1.28), and 20-49 (HR, 1.68).
Risk for atherosclerotic cardiovascular disease was also raised per 1.0 mmol/L increase in LDL cholesterol overall (HR, 1.16) and in all age groups, particularly those aged 70-100 years.
Greater elevations in LDL cholesterol (5.0 mmol/L or higher, indicative of possible familial hypercholesterolemia) were associated with a notably higher risk for heart attack after multivariate adjustment in people aged 80-100 (HR, 2.99). Risk was also higher among those aged 70-79 (HR, 1.82).
The highest incidence was in those older than 70. The rate was 8.5 heart attacks per 1,000 people per year among those aged 80-100 and 5.2 heart attacks per 1,000 in those aged 70-79. The rates were 2.5 per 1,000 among those 60-69, 1.8 for those aged 50-59, and 0.8 for those aged 20-49.
“The absolute risk [of cardiovascular events] is of course much higher in the elderly than those under the age of 75, but what was a surprise was how clear our results were on a relative risk scale, that the risk associated with elevated LDL [cholesterol] was as high in people aged 80-100 as the younger patients,” Dr. Nordestgaard said in an interview.
With regard to the benefits of cholesterol-lowering drugs, the study showed that the number needed to prevent one heart attack over 5 years was 80 among those aged 80-100; the number was 439 for people aged 50-59.
With regard to stronger statins, when moderate-intensity statins were used, the number needed to treat to prevent one cardiovascular disease event of any type dropped to 42 for patients aged 80-100. It was 88 for those aged 70-79, 164 for those aged 60-69, 345 for those aged 50-59, and 769 for those aged 20-49.
“The clinical significance of this is that it appears those in older age groups indeed benefit from cholesterol-lowering therapy,” Dr. Nordestgaard said. “I think many people have this idea that LDL [cholesterol] is not important over the age of about 70-75, but that’s not the case.”
“These robust findings are novel,” he and his colleagues stressed.
Despite these observational findings, the South African editorialists noted that “whether lipid-lowering therapy should be initiated for primary prevention in people aged 75 years or older is unclear,” owing to the host of risks and benefits that need to be balanced.
The findings of an ongoing randomized, placebo-controlled trial (STAREE) may answer this question, they wrote. It is investigating primary prevention in 18,000 older patients (≥70 years) who are being randomly assigned to receive atorvastatin 40 mg/d or placebo. The study is seeking to determine whether statin treatment extends the length of a disability-free life, which will be assessed on the basis of survival outside permanent residential care. Results are expected in 2022-2023.
Unequivocal reductions in events in elderly, comparable with younger patients
In the second study (Lancet. 2020 Nov 10. doi: 10.1016/S0140-6736[20]32332-1), Baris Gencer, MD, of Brigham and Women’s Hospital, Boston, =and colleagues evaluated the effects of statins and other cholesterol-lowering drugs, including ezetimibe and proprotein convertase subtilisin/kexin type 9 inhibitors, in older versus younger patients.
The systematic review and meta-analysis of 29 randomized controlled trials, also published in the Lancet, were presented virtually as a poster as part of the 2020 American Heart Association scientific session. It included data on 244,090 patients, including 21,492 aged 75 years and older.
The meta-analysis included studies of cardiovascular outcomes of a guideline-recommended LDL cholesterol–lowering drug, with a median follow-up of at least 2 years and inclusion of data on patients aged 75 years and older.
The results showed that over a median follow-up of 2.2 to 6 years, statin use by older patients was associated with a relative risk reduction of major vascular events of 26% per 1 mmol/L reduction in LDL cholesterol (P = .0019), which was comparable with a risk reduction of 15% per 1 mmol/L reduction in LDL cholesterol for patients younger than 75 years (P = .37, compared with older patients).
Treatment of older patients with LDL cholesterol–lowering drugs was also associated with significantly improved outcomes in cardiovascular death (risk ratio, 0.85), MI (RR, 0.80), stroke (RR, 0.73), and coronary revascularization (RR, 0.80).
“We found an unequivocal reduction in the risk of major vascular events with both statin and nonstatin LDL cholesterol-lowering treatments, which was similar to that seen in younger patients,” the authors wrote.
“Cholesterol-lowering medications are affordable drugs that have reduced risk of heart disease for millions of people worldwide, but until now, their benefits for older people have remained less certain,” said lead author Marc Sabatine, MD, also of Brigham and Women’s Hospital, in a Lancet press release.
“Our analysis indicates that these therapies are as effective in reducing cardiovascular events and deaths in people aged 75 years and over as they are in younger people. We found no offsetting safety concerns, and together, these results should strengthen guideline recommendations for the use of cholesterol-lowering medications, including statin and nonstatin therapy, in elderly people.”
The editorialists agreed: “More than 80% of fatal cardiovascular events occur in individuals older than 65 years, and the incidence of cardiovascular events is increasing in those older than 80 years; therefore, the findings of Gencer and colleagues’ study should encourage the use of lipid-lowering therapy in older patients.”
The authors of the two studies have disclosed no relevant financial relationships. Dr. Raal has received research grants, honoraria, or consulting fees for advisory board membership, professional input, and lectures on lipid-lowering drug therapy from Amgen, Regeneron, Sanofi, Novartis, and the Medicines Company.
A version of this article originally appeared on Medscape.com.
Contrary to historical evidence, two new studies show.
“By contrast with previous historical studies, our data show that LDL cholesterol is an important risk factor for myocardial infarction and atherosclerotic cardiovascular disease in a contemporary primary prevention cohort of individuals aged 70 to 100 years,” Borge Nordestgaard, MD, of the University of Copenhagen, and colleagues noted in the first of the two studies, published this week in the Lancet.
“By lowering LDL cholesterol in healthy individuals aged 70-100 years, the potential for preventing myocardial infarctions and atherosclerotic cardiovascular disease is huge, and at a substantially lower number needed to treat when compared with those aged 20-69 years,” they added.
“These findings support the concept of the cumulative burden of LDL cholesterol over one’s lifetime and the progressive increase in risk for atherosclerotic cardiovascular disease, including myocardial infarction, with age,” added Frederick J. Raal, PhD, and Farzahna Mohamed, MB BCh, of the University of the Witwatersrand, Johannesburg, South Africa, in an editorial published with both new studies in the Lancet (2020 Nov 10. doi: 10.1016/S0140-6736[20]32333-3).
The studies underscore the need for clinicians to consider continued risks associated with elevated LDL cholesterol in older age, they stressed, adding that statins are also beneficial for younger persons at risk to prevent conditions from worsening.
“The average age of patients in all the trials analyzed was older than 60 years, an age when atherosclerotic cardiovascular disease is already well established,” the editorialists wrote.
“Lipid-lowering therapy should be initiated at a younger age, preferably before age 40 years, in those at risk to delay the onset of atherosclerosis, rather than try to manage the condition once fully established or advanced,” they stressed.
No RCTs have included patients older than 70
For persons aged 40-75 years, elevated LDL cholesterol levels are a known risk factor for MI and atherosclerotic cardiovascular disease, and there is consensus in guidelines regarding treatment with statins.
However, the risk for people older than 70 is controversial. Some studies show little or no association between elevated LDL cholesterol levels and an increased risk for MI.
Contributing to the uncertainty is that few of the randomized, controlled trials that have investigated the question have included patients aged older than 70 years.
As a consequence, many practice guidelines have noted that the level of evidence in older patients is low, and some organizations have lowered the strength of recommendations regarding the treatment for older patients in comparison with younger patients.
Primary prevention: CV events increase with elevated LDL cholesterol in older age
Dr. Nordestgaard and colleagues studied data on 91,131 people living in Copenhagen who did not have atherosclerotic cardiovascular disease or diabetes at baseline and were not taking statins.
Of the participants, 10,592 were aged 70-79 years, and 3,188 participants were aged 80-100 years.
Over an average follow-up period of 7.7 years, 1,515 participants had a first MI, and 3,389 developed atherosclerotic cardiovascular disease.
In the primary-prevention cohort, after multivariate adjustment, the risk of having a heart attack per 1.0 mmol/L increase in LDL cholesterol was increased in the group overall (hazard ratio, 1.34). The increased risk was observed for all age groups, including those aged 80-100 years (HR, 1.28), 70-79 (HR, 1.25), 60-69 (HR, 1.29), 50-59 (HR, 1.28), and 20-49 (HR, 1.68).
Risk for atherosclerotic cardiovascular disease was also raised per 1.0 mmol/L increase in LDL cholesterol overall (HR, 1.16) and in all age groups, particularly those aged 70-100 years.
Greater elevations in LDL cholesterol (5.0 mmol/L or higher, indicative of possible familial hypercholesterolemia) were associated with a notably higher risk for heart attack after multivariate adjustment in people aged 80-100 (HR, 2.99). Risk was also higher among those aged 70-79 (HR, 1.82).
The highest incidence was in those older than 70. The rate was 8.5 heart attacks per 1,000 people per year among those aged 80-100 and 5.2 heart attacks per 1,000 in those aged 70-79. The rates were 2.5 per 1,000 among those 60-69, 1.8 for those aged 50-59, and 0.8 for those aged 20-49.
“The absolute risk [of cardiovascular events] is of course much higher in the elderly than those under the age of 75, but what was a surprise was how clear our results were on a relative risk scale, that the risk associated with elevated LDL [cholesterol] was as high in people aged 80-100 as the younger patients,” Dr. Nordestgaard said in an interview.
With regard to the benefits of cholesterol-lowering drugs, the study showed that the number needed to prevent one heart attack over 5 years was 80 among those aged 80-100; the number was 439 for people aged 50-59.
With regard to stronger statins, when moderate-intensity statins were used, the number needed to treat to prevent one cardiovascular disease event of any type dropped to 42 for patients aged 80-100. It was 88 for those aged 70-79, 164 for those aged 60-69, 345 for those aged 50-59, and 769 for those aged 20-49.
“The clinical significance of this is that it appears those in older age groups indeed benefit from cholesterol-lowering therapy,” Dr. Nordestgaard said. “I think many people have this idea that LDL [cholesterol] is not important over the age of about 70-75, but that’s not the case.”
“These robust findings are novel,” he and his colleagues stressed.
Despite these observational findings, the South African editorialists noted that “whether lipid-lowering therapy should be initiated for primary prevention in people aged 75 years or older is unclear,” owing to the host of risks and benefits that need to be balanced.
The findings of an ongoing randomized, placebo-controlled trial (STAREE) may answer this question, they wrote. It is investigating primary prevention in 18,000 older patients (≥70 years) who are being randomly assigned to receive atorvastatin 40 mg/d or placebo. The study is seeking to determine whether statin treatment extends the length of a disability-free life, which will be assessed on the basis of survival outside permanent residential care. Results are expected in 2022-2023.
Unequivocal reductions in events in elderly, comparable with younger patients
In the second study (Lancet. 2020 Nov 10. doi: 10.1016/S0140-6736[20]32332-1), Baris Gencer, MD, of Brigham and Women’s Hospital, Boston, =and colleagues evaluated the effects of statins and other cholesterol-lowering drugs, including ezetimibe and proprotein convertase subtilisin/kexin type 9 inhibitors, in older versus younger patients.
The systematic review and meta-analysis of 29 randomized controlled trials, also published in the Lancet, were presented virtually as a poster as part of the 2020 American Heart Association scientific session. It included data on 244,090 patients, including 21,492 aged 75 years and older.
The meta-analysis included studies of cardiovascular outcomes of a guideline-recommended LDL cholesterol–lowering drug, with a median follow-up of at least 2 years and inclusion of data on patients aged 75 years and older.
The results showed that over a median follow-up of 2.2 to 6 years, statin use by older patients was associated with a relative risk reduction of major vascular events of 26% per 1 mmol/L reduction in LDL cholesterol (P = .0019), which was comparable with a risk reduction of 15% per 1 mmol/L reduction in LDL cholesterol for patients younger than 75 years (P = .37, compared with older patients).
Treatment of older patients with LDL cholesterol–lowering drugs was also associated with significantly improved outcomes in cardiovascular death (risk ratio, 0.85), MI (RR, 0.80), stroke (RR, 0.73), and coronary revascularization (RR, 0.80).
“We found an unequivocal reduction in the risk of major vascular events with both statin and nonstatin LDL cholesterol-lowering treatments, which was similar to that seen in younger patients,” the authors wrote.
“Cholesterol-lowering medications are affordable drugs that have reduced risk of heart disease for millions of people worldwide, but until now, their benefits for older people have remained less certain,” said lead author Marc Sabatine, MD, also of Brigham and Women’s Hospital, in a Lancet press release.
“Our analysis indicates that these therapies are as effective in reducing cardiovascular events and deaths in people aged 75 years and over as they are in younger people. We found no offsetting safety concerns, and together, these results should strengthen guideline recommendations for the use of cholesterol-lowering medications, including statin and nonstatin therapy, in elderly people.”
The editorialists agreed: “More than 80% of fatal cardiovascular events occur in individuals older than 65 years, and the incidence of cardiovascular events is increasing in those older than 80 years; therefore, the findings of Gencer and colleagues’ study should encourage the use of lipid-lowering therapy in older patients.”
The authors of the two studies have disclosed no relevant financial relationships. Dr. Raal has received research grants, honoraria, or consulting fees for advisory board membership, professional input, and lectures on lipid-lowering drug therapy from Amgen, Regeneron, Sanofi, Novartis, and the Medicines Company.
A version of this article originally appeared on Medscape.com.
RARE DISEASES REPORT: RHEUMATOLOGY
- Survey reveals special impact of COVID-19 on persons with rare disorders
- Topical treatment tackles oral ulcers in Behçet’s syndrome
- Managing the risk of congenital heart block in anti-Ro/SSA-positive women
- Newly described lung disorder strikes children with systemic juvenile idiopathic arthritis
- Survey reveals special impact of COVID-19 on persons with rare disorders
- Topical treatment tackles oral ulcers in Behçet’s syndrome
- Managing the risk of congenital heart block in anti-Ro/SSA-positive women
- Newly described lung disorder strikes children with systemic juvenile idiopathic arthritis
- Survey reveals special impact of COVID-19 on persons with rare disorders
- Topical treatment tackles oral ulcers in Behçet’s syndrome
- Managing the risk of congenital heart block in anti-Ro/SSA-positive women
- Newly described lung disorder strikes children with systemic juvenile idiopathic arthritis