User login
AMA takes on vaccine misinformation, physician vaccines, racism
The American Medical Association House of Delegates has adopted a policy to educate physicians on how to speak with patients about COVID-19 vaccination to counteract widespread misinformation about the vaccine development process.
Other highlights of the AMA’s recent special meeting include a new policy on the ethics of physicians getting immunized against COVID-19 and a far-reaching statement about racism.
Under the organization’s new vaccination education policy, the AMA will provide physicians with “culturally appropriate patient education materials,” according to a news release.
This campaign will be conducted “bearing in mind the historical context of ‘experimentation’ with vaccines and other medication in communities of color,” the AMA said, apparently alluding to the infamous Tuskegee study of syphilis in Black men.
Educating the public about the safety and efficacy of the COVID-19 vaccine programs is an “urgent priority,” the AMA said. This is especially true among populations that have been disproportionately affected by the disease. Black and Latino people are being hospitalized for COVID-19 at far higher rates than White Americans.
“Under the new policy, the AMA will help address patient concerns, dispel misinformation, and build confidence in COVID-19 vaccination,” the release states. The AMA also plans to build a coalition of health care and public health organizations to develop and implement a joint public education program.
Polls have indicated that many people will not get vaccinated when supplies of the new COVID-19 vaccines are available, although public support is rising. A recent Gallup poll found that 58% of surveyed adults were willing to be inoculated, up from 50% in September.
A Kaiser Family Foundation survey in September found that a majority of Americans were skeptical of a rushed vaccine, because they were concerned that the Trump administration was pressuring the Food and Drug Administration to approve a vaccine before the election.
“Given the unprecedented situation with COVID-19 and with vaccine development moving at a rapid pace, many of our patients and the public have questions and concerns,” said AMA President Susan R. Bailey, MD, in the release. “It is essential that we speak together as a strong, unified voice across health care and public health, inclusive of organizations respected in communities of color; to use scientific, fact-based evidence to help allay public concerns; and build confidence in COVID-19 vaccine candidates that are determined to be safe and effective.”
Physician, immunize thyself
The AMA also adopted a new ethics policy about physician immunization. On Monday, the AMA House of Delegates stated that physicians who are not immunized from a vaccine-preventable disease have an ethical responsibility to take appropriate actions to protect patients and colleagues.
The AMA code of ethics has long maintained that physicians have a strong ethical duty to accept immunizations when a safe, effective vaccine is available. However, the organization said in a news release, “it is not ethically problematic to exempt individuals when a specific vaccine poses a risk due to underlying medical conditions.”
Ethical concerns arise when physicians are allowed to decline vaccinations for nonmedical reasons, according to a report presented to the House of Delegates by the AMA Council on Ethical and Judicial Affairs.
According to the newly amended AMA ethical guidance, “physicians who are not or cannot be immunized have a responsibility to voluntarily take appropriate actions to protect patients, fellow health care workers and others.” This includes refraining from direct patient contact.
The delegates also approved a guidance asserting that physician practices and health care institutions are responsible for developing policies and procedures for responding to pandemics and epidemics. These policies and procedures should outline appropriate protective equipment allocation, staff immunization programs, and infection control practices.
Combating systemic racism
In an effort to reduce racial disparities in healthcare, the AMA House of Delegates adopted new policies recognizing race as a social construct, rather than a biological construct.
“The policies aim to advance data-driven, antiracist concepts challenging the current clinical application of race and its effects on vulnerable patient populations,” an AMA statement said.
The new AMA policies “reflect an understanding of race as a socially constructed category different from ethnicity, genetic ancestry, or biology, and aim to end the misinterpretation of race as a biological category defined by genetic traits or biological differences,” the AMA said.
According to the AMA, the practice of accepting race as a biological construct “exacerbates health disparities and results in detrimental health outcomes for marginalized and minoritized communities.”
Specifically, the AMA said it supports ending the practice of using race as a proxy for biology in medical education, research, and clinical practice. It also encourages medical education programs to recognize the harmful effects of this approach. It recommends that clinicians and researchers focus on genetics and biology, the experience of racism, and social determinants of health when describing risk factors for disease.
“The AMA is dedicated to dismantling racist and discriminatory policies and practices across all of health care, and that includes the way we define race in medicine,” said AMA board member Michael Suk, MD, in its statement. “We believe it is not sufficient for medicine to be nonracist, which is why the AMA is committed to pushing for a shift in thinking from race as a biological risk factor to a deeper understanding of racism as a determinant of health.”
The AMA also plans to partner with physician organizations and other stakeholders “to identify any problematic aspects of medical education that may perpetuate institutional and structural racism.” For example, the AMA will work with other organizations to improve clinical algorithms that incorrectly adjust for race and lead to less-than-optimal care for minority patients.
A version of this article originally appeared on Medscape.com.
The American Medical Association House of Delegates has adopted a policy to educate physicians on how to speak with patients about COVID-19 vaccination to counteract widespread misinformation about the vaccine development process.
Other highlights of the AMA’s recent special meeting include a new policy on the ethics of physicians getting immunized against COVID-19 and a far-reaching statement about racism.
Under the organization’s new vaccination education policy, the AMA will provide physicians with “culturally appropriate patient education materials,” according to a news release.
This campaign will be conducted “bearing in mind the historical context of ‘experimentation’ with vaccines and other medication in communities of color,” the AMA said, apparently alluding to the infamous Tuskegee study of syphilis in Black men.
Educating the public about the safety and efficacy of the COVID-19 vaccine programs is an “urgent priority,” the AMA said. This is especially true among populations that have been disproportionately affected by the disease. Black and Latino people are being hospitalized for COVID-19 at far higher rates than White Americans.
“Under the new policy, the AMA will help address patient concerns, dispel misinformation, and build confidence in COVID-19 vaccination,” the release states. The AMA also plans to build a coalition of health care and public health organizations to develop and implement a joint public education program.
Polls have indicated that many people will not get vaccinated when supplies of the new COVID-19 vaccines are available, although public support is rising. A recent Gallup poll found that 58% of surveyed adults were willing to be inoculated, up from 50% in September.
A Kaiser Family Foundation survey in September found that a majority of Americans were skeptical of a rushed vaccine, because they were concerned that the Trump administration was pressuring the Food and Drug Administration to approve a vaccine before the election.
“Given the unprecedented situation with COVID-19 and with vaccine development moving at a rapid pace, many of our patients and the public have questions and concerns,” said AMA President Susan R. Bailey, MD, in the release. “It is essential that we speak together as a strong, unified voice across health care and public health, inclusive of organizations respected in communities of color; to use scientific, fact-based evidence to help allay public concerns; and build confidence in COVID-19 vaccine candidates that are determined to be safe and effective.”
Physician, immunize thyself
The AMA also adopted a new ethics policy about physician immunization. On Monday, the AMA House of Delegates stated that physicians who are not immunized from a vaccine-preventable disease have an ethical responsibility to take appropriate actions to protect patients and colleagues.
The AMA code of ethics has long maintained that physicians have a strong ethical duty to accept immunizations when a safe, effective vaccine is available. However, the organization said in a news release, “it is not ethically problematic to exempt individuals when a specific vaccine poses a risk due to underlying medical conditions.”
Ethical concerns arise when physicians are allowed to decline vaccinations for nonmedical reasons, according to a report presented to the House of Delegates by the AMA Council on Ethical and Judicial Affairs.
According to the newly amended AMA ethical guidance, “physicians who are not or cannot be immunized have a responsibility to voluntarily take appropriate actions to protect patients, fellow health care workers and others.” This includes refraining from direct patient contact.
The delegates also approved a guidance asserting that physician practices and health care institutions are responsible for developing policies and procedures for responding to pandemics and epidemics. These policies and procedures should outline appropriate protective equipment allocation, staff immunization programs, and infection control practices.
Combating systemic racism
In an effort to reduce racial disparities in healthcare, the AMA House of Delegates adopted new policies recognizing race as a social construct, rather than a biological construct.
“The policies aim to advance data-driven, antiracist concepts challenging the current clinical application of race and its effects on vulnerable patient populations,” an AMA statement said.
The new AMA policies “reflect an understanding of race as a socially constructed category different from ethnicity, genetic ancestry, or biology, and aim to end the misinterpretation of race as a biological category defined by genetic traits or biological differences,” the AMA said.
According to the AMA, the practice of accepting race as a biological construct “exacerbates health disparities and results in detrimental health outcomes for marginalized and minoritized communities.”
Specifically, the AMA said it supports ending the practice of using race as a proxy for biology in medical education, research, and clinical practice. It also encourages medical education programs to recognize the harmful effects of this approach. It recommends that clinicians and researchers focus on genetics and biology, the experience of racism, and social determinants of health when describing risk factors for disease.
“The AMA is dedicated to dismantling racist and discriminatory policies and practices across all of health care, and that includes the way we define race in medicine,” said AMA board member Michael Suk, MD, in its statement. “We believe it is not sufficient for medicine to be nonracist, which is why the AMA is committed to pushing for a shift in thinking from race as a biological risk factor to a deeper understanding of racism as a determinant of health.”
The AMA also plans to partner with physician organizations and other stakeholders “to identify any problematic aspects of medical education that may perpetuate institutional and structural racism.” For example, the AMA will work with other organizations to improve clinical algorithms that incorrectly adjust for race and lead to less-than-optimal care for minority patients.
A version of this article originally appeared on Medscape.com.
The American Medical Association House of Delegates has adopted a policy to educate physicians on how to speak with patients about COVID-19 vaccination to counteract widespread misinformation about the vaccine development process.
Other highlights of the AMA’s recent special meeting include a new policy on the ethics of physicians getting immunized against COVID-19 and a far-reaching statement about racism.
Under the organization’s new vaccination education policy, the AMA will provide physicians with “culturally appropriate patient education materials,” according to a news release.
This campaign will be conducted “bearing in mind the historical context of ‘experimentation’ with vaccines and other medication in communities of color,” the AMA said, apparently alluding to the infamous Tuskegee study of syphilis in Black men.
Educating the public about the safety and efficacy of the COVID-19 vaccine programs is an “urgent priority,” the AMA said. This is especially true among populations that have been disproportionately affected by the disease. Black and Latino people are being hospitalized for COVID-19 at far higher rates than White Americans.
“Under the new policy, the AMA will help address patient concerns, dispel misinformation, and build confidence in COVID-19 vaccination,” the release states. The AMA also plans to build a coalition of health care and public health organizations to develop and implement a joint public education program.
Polls have indicated that many people will not get vaccinated when supplies of the new COVID-19 vaccines are available, although public support is rising. A recent Gallup poll found that 58% of surveyed adults were willing to be inoculated, up from 50% in September.
A Kaiser Family Foundation survey in September found that a majority of Americans were skeptical of a rushed vaccine, because they were concerned that the Trump administration was pressuring the Food and Drug Administration to approve a vaccine before the election.
“Given the unprecedented situation with COVID-19 and with vaccine development moving at a rapid pace, many of our patients and the public have questions and concerns,” said AMA President Susan R. Bailey, MD, in the release. “It is essential that we speak together as a strong, unified voice across health care and public health, inclusive of organizations respected in communities of color; to use scientific, fact-based evidence to help allay public concerns; and build confidence in COVID-19 vaccine candidates that are determined to be safe and effective.”
Physician, immunize thyself
The AMA also adopted a new ethics policy about physician immunization. On Monday, the AMA House of Delegates stated that physicians who are not immunized from a vaccine-preventable disease have an ethical responsibility to take appropriate actions to protect patients and colleagues.
The AMA code of ethics has long maintained that physicians have a strong ethical duty to accept immunizations when a safe, effective vaccine is available. However, the organization said in a news release, “it is not ethically problematic to exempt individuals when a specific vaccine poses a risk due to underlying medical conditions.”
Ethical concerns arise when physicians are allowed to decline vaccinations for nonmedical reasons, according to a report presented to the House of Delegates by the AMA Council on Ethical and Judicial Affairs.
According to the newly amended AMA ethical guidance, “physicians who are not or cannot be immunized have a responsibility to voluntarily take appropriate actions to protect patients, fellow health care workers and others.” This includes refraining from direct patient contact.
The delegates also approved a guidance asserting that physician practices and health care institutions are responsible for developing policies and procedures for responding to pandemics and epidemics. These policies and procedures should outline appropriate protective equipment allocation, staff immunization programs, and infection control practices.
Combating systemic racism
In an effort to reduce racial disparities in healthcare, the AMA House of Delegates adopted new policies recognizing race as a social construct, rather than a biological construct.
“The policies aim to advance data-driven, antiracist concepts challenging the current clinical application of race and its effects on vulnerable patient populations,” an AMA statement said.
The new AMA policies “reflect an understanding of race as a socially constructed category different from ethnicity, genetic ancestry, or biology, and aim to end the misinterpretation of race as a biological category defined by genetic traits or biological differences,” the AMA said.
According to the AMA, the practice of accepting race as a biological construct “exacerbates health disparities and results in detrimental health outcomes for marginalized and minoritized communities.”
Specifically, the AMA said it supports ending the practice of using race as a proxy for biology in medical education, research, and clinical practice. It also encourages medical education programs to recognize the harmful effects of this approach. It recommends that clinicians and researchers focus on genetics and biology, the experience of racism, and social determinants of health when describing risk factors for disease.
“The AMA is dedicated to dismantling racist and discriminatory policies and practices across all of health care, and that includes the way we define race in medicine,” said AMA board member Michael Suk, MD, in its statement. “We believe it is not sufficient for medicine to be nonracist, which is why the AMA is committed to pushing for a shift in thinking from race as a biological risk factor to a deeper understanding of racism as a determinant of health.”
The AMA also plans to partner with physician organizations and other stakeholders “to identify any problematic aspects of medical education that may perpetuate institutional and structural racism.” For example, the AMA will work with other organizations to improve clinical algorithms that incorrectly adjust for race and lead to less-than-optimal care for minority patients.
A version of this article originally appeared on Medscape.com.
Dangers of a medical board investigation: How to protect yourself
Cynthia H. Moran, MD, has a medical degree, a passion for treating the elderly, and a desire to work. What she doesn’t have is a job or hopes of getting one anytime soon.
The Houston physician has never been charged with a crime, but she did run afoul of the Texas Medical Board, an experience she said has left her destitute and virtually unemployable in the medical field.
“By the time the board gets through with you, you will be bankrupt and have nothing,” she said.
Dr. Moran has a long, tangled history with the board involving self-prescribing, opioid abuse, depression, and unprofessional conduct. After years of license suspension, drug testing, additional CME, substance abuse treatment, and work restrictions, her supervision by the board ended in 2019, but she has been largely unable to find work as a physician.
“I feel like a felon. I really understand what it’s like to be someone who does their time but then can’t get a job, can’t get an apartment. It’s in your record and there’s nothing you can do about it,” she said.
Although Dr. Moran largely created her own troubles, her experience shows the power state medical licensing boards have when it comes to disciplining physicians.
Reprimands to revocations
Many physicians think of their state medical boards as simply the bodies that issue their medical licenses, but the boards have other functions, including investigating complaints against licensed medical professionals and sometimes disciplining them.
According to 2017 statistics from the Federation of State Medical Boards (the most recent available), state boards took 8,813 actions that year. These included 796 suspensions, 764 probations, 570 surrendered licenses, and 264 revoked licenses.
Boards also can order doctors to enter state-run physician health plans to receive treatment for substance abuse, or they can allow physicians to practice only under the supervision of colleagues.
Although they vary by state, the boards are fundamentally similar. Members are appointed by the governor. A majority of them are physicians, and the remainder are nonmedical professionals. Their investigators, often retired law enforcement officials, have broad powers to collect evidence, including medical records. Their authority is backed by the state attorney general.
Although physicians tend to worry more about being sued for malpractice, a medical board investigation can be more worrisome, said William Sullivan, DO, JD, an ED physician and attorney in Illinois who has represented doctors before that state’s board. Board disciplinary actions outnumber malpractice awards by four to one in that state.
“The gravity of this is something that many physicians don’t understand,” he said.
You can be the subject of anonymous complaints and investigations
Anyone can file a complaint against a physician with a state board. The grievances can be about anything from a crowded waiting room to physician impairment.
Of course, the most trivial complaints (out-of-date magazines in the waiting room) are dismissed out of hand, but boards have the authority to investigate whatever it chooses. The most common investigations center around complaints of impairment, substance abuse, improper prescribing, faulty medical records, mental and physical health problems, and standard of care. Boards also will act if a physician is found guilty of a crime or misconduct unrelated to his or her medical practice.
“There are a lot of ways doctors get into trouble,” said Edward Dauer, MD, a radiologist who served on the Florida board for 11 years.
Investigations often expand beyond their original scope into all aspects of a practice. “Once you’re on their radar, they can find something,” Dr. Sullivan said.
All punitive actions taken by state boards are reported to the Department of Health & Human Services’ National Practitioner Data Bank, which is accessible to all state boards. Sanctioned physicians who set up practice in another state often find that their new home has adopted the sanctions leveled by the original state, something boards can do without conducting their own investigations.
“For doctors, discipline is forever. It never goes off your record,” Dr. Dauer said.
In addition, Medicare, Medicaid, and private insurers can exclude disciplined physicians, which can cripple a practice’s finances. So what can doctors do to avoid problems with the boards?
Don’t do anything wrong
That sounds glib and obvious, but many physicians get into trouble by unwittingly violating state medical regulations regarding such things as CME, insurance requirements, failure to notify the board of address changes, and personal relationships with current or former patients.
“The best advice to avoid these issues is to do a Google search for the Medical Practice Act in the state in which they practice,” said Dr. Sullivan. He noted that doctors should regularly check for changes in regulations.
Keeping on good terms with colleagues and patients also helps, he said, noting that many complaints stem from personal disputes and grievances.
But what if a physician becomes the subject of an investigation? What should they do?
Take any complaint seriously
Too many physicians dismiss investigations initially. “Some people have the wrong idea that if they ignore it, it will go away. It won’t go away,” Dr. Sullivan said.
Whether the initial contact comes through a letter or a visit from a board investigator, it should be treated with urgency. Ohio attorney Beth Collis said one client angrily scrawled one-word answers with a Sharpie on the questionnaire he was mailed – answers he was stuck defending throughout the rest of the investigation. Other doctors have ordered investigators out of their offices – another mistake. Failure to cooperate can result in an immediate license suspension.
“They should be speaking to these investigators like they were talking to a highway patrolman on the side of the road. They hold all the cards,” said Ms. Collis, who specializes in representing professionals before licensing boards.
Some physicians mistakenly assume that because their state board is made up mostly of fellow doctors, they will be able to make a complaint go away with some collegial chat.
Not so. “Medical board members see themselves as protecting the public. They’re very punitive,” Ms. Collis said.
At one time, state boards might have been lax in their supervision of physicians, but that changed in the 1980s when the watchdog group Public Citizen began ranking state medical boards by how effective they were in policing doctors.
Public Citizen used FSMB data on serious disciplinary actions per 1,000 doctors in each state to calculate its rankings, a practice that FSMB called incomplete and a misuse of its statistics. Nonetheless, the annual rankings generated a lot of publicity critical of state boards and might have spurred a tougher approach by regulators.
Public Citizen stopped publishing its annual rankings in 2013 after FSMB ceased supplying the data, but the get-tough approach remains, lawyers said.
About 95% of complaints are dismissed with nothing more serious than a letter to the doctor, but boards don’t hesitate to act when the misconduct is serious, said Dr. Dauer. “I felt it was my obligation to protect the public.”
Don’t try to fix it yourself
Although many complaints are anonymous, doctors can often figure out what or who it involves. Their impulse might be to contact a patient who complained, correct a medical record, or otherwise try to resolve the matter personally.
It’s better to leave things alone, the experts said. Don’t contact a patient. Give the board access to whatever information it asks for, but don’t alter anything, particularly medical records. “That’s how you’re going to get your license revoked,” Dr. Dauer said. He noted that when doctors add notations to records, they must date them.
Hire a lawyer
Many physicians assume they can resolve the complaint easily by explaining themselves to the board or investigators, or they don’t realize their license or practice could be at stake.
They’re better off letting a lawyer speak for them. Attorneys knowledgeable in this realm specialize in representing licensed professionals before regulatory boards and have the greatest knowledge of administrative law and how to negotiate the hearings and procedures.
Typically, a hearing is held before a subcommittee of the board, which can recommend a settlement to the full panel. Cases in which a settlement is not reached can go before the entire board.
Although full hearings can be similar to a trial, there are crucial differences regarding evidentiary rules and other matters, Ms. Collis said. For example, in Ohio, defendant physicians do not get to see the board’s full case against them before the hearing, which can make preparing a defense difficult. And the standard for burden of proof is a preponderance of evidence, as in civil suits, not evidence beyond a reasonable doubt, as in a criminal trial.
Cases that go to full hearings and beyond to appeals in state courts can take years to resolve, and a physician’s license can be suspended for the duration.
Get help before it’s too late
Physicians looking for support and advice can turn to organizations such as the Coalition for Physician Rights, an organization formed in 2018 by Kernan Manion, MD, a former psychiatrist who was forced to deactivate his license after an investigation by the North Carolina medical board.
The Coalition for Physician Rights has advised hundreds of physicians, most of whom he said come to him once they realize they’re in over their heads. “Almost everyone comes in too late,” Dr. Manion said. “They’re sitting ducks. They don’t know how to respond.”
In addition to offering advice and support, the Coalition for Physician Rights lobbies for reform in how boards operate. A number of states, including Oklahoma, have made reforms in recent years.
The appointed boards are too reliant on their administration and staff and usually rubber-stamp disciplinary recommendations, Dr. Manion said. He also criticized the boards’ lack of accountability: “A board operates without external or internal oversight. It is an autonomous entity operating on its own.”
As for Dr. Moran, at age 61, she’s interviewing for physician jobs around the country, refusing to give up medicine.
“What else can I do?” she said. “It’s what I’ve done my entire life. It’s what I went to school for. I don’t know how to do anything else.”
A version of this article originally appeared on Medscape.com.
Cynthia H. Moran, MD, has a medical degree, a passion for treating the elderly, and a desire to work. What she doesn’t have is a job or hopes of getting one anytime soon.
The Houston physician has never been charged with a crime, but she did run afoul of the Texas Medical Board, an experience she said has left her destitute and virtually unemployable in the medical field.
“By the time the board gets through with you, you will be bankrupt and have nothing,” she said.
Dr. Moran has a long, tangled history with the board involving self-prescribing, opioid abuse, depression, and unprofessional conduct. After years of license suspension, drug testing, additional CME, substance abuse treatment, and work restrictions, her supervision by the board ended in 2019, but she has been largely unable to find work as a physician.
“I feel like a felon. I really understand what it’s like to be someone who does their time but then can’t get a job, can’t get an apartment. It’s in your record and there’s nothing you can do about it,” she said.
Although Dr. Moran largely created her own troubles, her experience shows the power state medical licensing boards have when it comes to disciplining physicians.
Reprimands to revocations
Many physicians think of their state medical boards as simply the bodies that issue their medical licenses, but the boards have other functions, including investigating complaints against licensed medical professionals and sometimes disciplining them.
According to 2017 statistics from the Federation of State Medical Boards (the most recent available), state boards took 8,813 actions that year. These included 796 suspensions, 764 probations, 570 surrendered licenses, and 264 revoked licenses.
Boards also can order doctors to enter state-run physician health plans to receive treatment for substance abuse, or they can allow physicians to practice only under the supervision of colleagues.
Although they vary by state, the boards are fundamentally similar. Members are appointed by the governor. A majority of them are physicians, and the remainder are nonmedical professionals. Their investigators, often retired law enforcement officials, have broad powers to collect evidence, including medical records. Their authority is backed by the state attorney general.
Although physicians tend to worry more about being sued for malpractice, a medical board investigation can be more worrisome, said William Sullivan, DO, JD, an ED physician and attorney in Illinois who has represented doctors before that state’s board. Board disciplinary actions outnumber malpractice awards by four to one in that state.
“The gravity of this is something that many physicians don’t understand,” he said.
You can be the subject of anonymous complaints and investigations
Anyone can file a complaint against a physician with a state board. The grievances can be about anything from a crowded waiting room to physician impairment.
Of course, the most trivial complaints (out-of-date magazines in the waiting room) are dismissed out of hand, but boards have the authority to investigate whatever it chooses. The most common investigations center around complaints of impairment, substance abuse, improper prescribing, faulty medical records, mental and physical health problems, and standard of care. Boards also will act if a physician is found guilty of a crime or misconduct unrelated to his or her medical practice.
“There are a lot of ways doctors get into trouble,” said Edward Dauer, MD, a radiologist who served on the Florida board for 11 years.
Investigations often expand beyond their original scope into all aspects of a practice. “Once you’re on their radar, they can find something,” Dr. Sullivan said.
All punitive actions taken by state boards are reported to the Department of Health & Human Services’ National Practitioner Data Bank, which is accessible to all state boards. Sanctioned physicians who set up practice in another state often find that their new home has adopted the sanctions leveled by the original state, something boards can do without conducting their own investigations.
“For doctors, discipline is forever. It never goes off your record,” Dr. Dauer said.
In addition, Medicare, Medicaid, and private insurers can exclude disciplined physicians, which can cripple a practice’s finances. So what can doctors do to avoid problems with the boards?
Don’t do anything wrong
That sounds glib and obvious, but many physicians get into trouble by unwittingly violating state medical regulations regarding such things as CME, insurance requirements, failure to notify the board of address changes, and personal relationships with current or former patients.
“The best advice to avoid these issues is to do a Google search for the Medical Practice Act in the state in which they practice,” said Dr. Sullivan. He noted that doctors should regularly check for changes in regulations.
Keeping on good terms with colleagues and patients also helps, he said, noting that many complaints stem from personal disputes and grievances.
But what if a physician becomes the subject of an investigation? What should they do?
Take any complaint seriously
Too many physicians dismiss investigations initially. “Some people have the wrong idea that if they ignore it, it will go away. It won’t go away,” Dr. Sullivan said.
Whether the initial contact comes through a letter or a visit from a board investigator, it should be treated with urgency. Ohio attorney Beth Collis said one client angrily scrawled one-word answers with a Sharpie on the questionnaire he was mailed – answers he was stuck defending throughout the rest of the investigation. Other doctors have ordered investigators out of their offices – another mistake. Failure to cooperate can result in an immediate license suspension.
“They should be speaking to these investigators like they were talking to a highway patrolman on the side of the road. They hold all the cards,” said Ms. Collis, who specializes in representing professionals before licensing boards.
Some physicians mistakenly assume that because their state board is made up mostly of fellow doctors, they will be able to make a complaint go away with some collegial chat.
Not so. “Medical board members see themselves as protecting the public. They’re very punitive,” Ms. Collis said.
At one time, state boards might have been lax in their supervision of physicians, but that changed in the 1980s when the watchdog group Public Citizen began ranking state medical boards by how effective they were in policing doctors.
Public Citizen used FSMB data on serious disciplinary actions per 1,000 doctors in each state to calculate its rankings, a practice that FSMB called incomplete and a misuse of its statistics. Nonetheless, the annual rankings generated a lot of publicity critical of state boards and might have spurred a tougher approach by regulators.
Public Citizen stopped publishing its annual rankings in 2013 after FSMB ceased supplying the data, but the get-tough approach remains, lawyers said.
About 95% of complaints are dismissed with nothing more serious than a letter to the doctor, but boards don’t hesitate to act when the misconduct is serious, said Dr. Dauer. “I felt it was my obligation to protect the public.”
Don’t try to fix it yourself
Although many complaints are anonymous, doctors can often figure out what or who it involves. Their impulse might be to contact a patient who complained, correct a medical record, or otherwise try to resolve the matter personally.
It’s better to leave things alone, the experts said. Don’t contact a patient. Give the board access to whatever information it asks for, but don’t alter anything, particularly medical records. “That’s how you’re going to get your license revoked,” Dr. Dauer said. He noted that when doctors add notations to records, they must date them.
Hire a lawyer
Many physicians assume they can resolve the complaint easily by explaining themselves to the board or investigators, or they don’t realize their license or practice could be at stake.
They’re better off letting a lawyer speak for them. Attorneys knowledgeable in this realm specialize in representing licensed professionals before regulatory boards and have the greatest knowledge of administrative law and how to negotiate the hearings and procedures.
Typically, a hearing is held before a subcommittee of the board, which can recommend a settlement to the full panel. Cases in which a settlement is not reached can go before the entire board.
Although full hearings can be similar to a trial, there are crucial differences regarding evidentiary rules and other matters, Ms. Collis said. For example, in Ohio, defendant physicians do not get to see the board’s full case against them before the hearing, which can make preparing a defense difficult. And the standard for burden of proof is a preponderance of evidence, as in civil suits, not evidence beyond a reasonable doubt, as in a criminal trial.
Cases that go to full hearings and beyond to appeals in state courts can take years to resolve, and a physician’s license can be suspended for the duration.
Get help before it’s too late
Physicians looking for support and advice can turn to organizations such as the Coalition for Physician Rights, an organization formed in 2018 by Kernan Manion, MD, a former psychiatrist who was forced to deactivate his license after an investigation by the North Carolina medical board.
The Coalition for Physician Rights has advised hundreds of physicians, most of whom he said come to him once they realize they’re in over their heads. “Almost everyone comes in too late,” Dr. Manion said. “They’re sitting ducks. They don’t know how to respond.”
In addition to offering advice and support, the Coalition for Physician Rights lobbies for reform in how boards operate. A number of states, including Oklahoma, have made reforms in recent years.
The appointed boards are too reliant on their administration and staff and usually rubber-stamp disciplinary recommendations, Dr. Manion said. He also criticized the boards’ lack of accountability: “A board operates without external or internal oversight. It is an autonomous entity operating on its own.”
As for Dr. Moran, at age 61, she’s interviewing for physician jobs around the country, refusing to give up medicine.
“What else can I do?” she said. “It’s what I’ve done my entire life. It’s what I went to school for. I don’t know how to do anything else.”
A version of this article originally appeared on Medscape.com.
Cynthia H. Moran, MD, has a medical degree, a passion for treating the elderly, and a desire to work. What she doesn’t have is a job or hopes of getting one anytime soon.
The Houston physician has never been charged with a crime, but she did run afoul of the Texas Medical Board, an experience she said has left her destitute and virtually unemployable in the medical field.
“By the time the board gets through with you, you will be bankrupt and have nothing,” she said.
Dr. Moran has a long, tangled history with the board involving self-prescribing, opioid abuse, depression, and unprofessional conduct. After years of license suspension, drug testing, additional CME, substance abuse treatment, and work restrictions, her supervision by the board ended in 2019, but she has been largely unable to find work as a physician.
“I feel like a felon. I really understand what it’s like to be someone who does their time but then can’t get a job, can’t get an apartment. It’s in your record and there’s nothing you can do about it,” she said.
Although Dr. Moran largely created her own troubles, her experience shows the power state medical licensing boards have when it comes to disciplining physicians.
Reprimands to revocations
Many physicians think of their state medical boards as simply the bodies that issue their medical licenses, but the boards have other functions, including investigating complaints against licensed medical professionals and sometimes disciplining them.
According to 2017 statistics from the Federation of State Medical Boards (the most recent available), state boards took 8,813 actions that year. These included 796 suspensions, 764 probations, 570 surrendered licenses, and 264 revoked licenses.
Boards also can order doctors to enter state-run physician health plans to receive treatment for substance abuse, or they can allow physicians to practice only under the supervision of colleagues.
Although they vary by state, the boards are fundamentally similar. Members are appointed by the governor. A majority of them are physicians, and the remainder are nonmedical professionals. Their investigators, often retired law enforcement officials, have broad powers to collect evidence, including medical records. Their authority is backed by the state attorney general.
Although physicians tend to worry more about being sued for malpractice, a medical board investigation can be more worrisome, said William Sullivan, DO, JD, an ED physician and attorney in Illinois who has represented doctors before that state’s board. Board disciplinary actions outnumber malpractice awards by four to one in that state.
“The gravity of this is something that many physicians don’t understand,” he said.
You can be the subject of anonymous complaints and investigations
Anyone can file a complaint against a physician with a state board. The grievances can be about anything from a crowded waiting room to physician impairment.
Of course, the most trivial complaints (out-of-date magazines in the waiting room) are dismissed out of hand, but boards have the authority to investigate whatever it chooses. The most common investigations center around complaints of impairment, substance abuse, improper prescribing, faulty medical records, mental and physical health problems, and standard of care. Boards also will act if a physician is found guilty of a crime or misconduct unrelated to his or her medical practice.
“There are a lot of ways doctors get into trouble,” said Edward Dauer, MD, a radiologist who served on the Florida board for 11 years.
Investigations often expand beyond their original scope into all aspects of a practice. “Once you’re on their radar, they can find something,” Dr. Sullivan said.
All punitive actions taken by state boards are reported to the Department of Health & Human Services’ National Practitioner Data Bank, which is accessible to all state boards. Sanctioned physicians who set up practice in another state often find that their new home has adopted the sanctions leveled by the original state, something boards can do without conducting their own investigations.
“For doctors, discipline is forever. It never goes off your record,” Dr. Dauer said.
In addition, Medicare, Medicaid, and private insurers can exclude disciplined physicians, which can cripple a practice’s finances. So what can doctors do to avoid problems with the boards?
Don’t do anything wrong
That sounds glib and obvious, but many physicians get into trouble by unwittingly violating state medical regulations regarding such things as CME, insurance requirements, failure to notify the board of address changes, and personal relationships with current or former patients.
“The best advice to avoid these issues is to do a Google search for the Medical Practice Act in the state in which they practice,” said Dr. Sullivan. He noted that doctors should regularly check for changes in regulations.
Keeping on good terms with colleagues and patients also helps, he said, noting that many complaints stem from personal disputes and grievances.
But what if a physician becomes the subject of an investigation? What should they do?
Take any complaint seriously
Too many physicians dismiss investigations initially. “Some people have the wrong idea that if they ignore it, it will go away. It won’t go away,” Dr. Sullivan said.
Whether the initial contact comes through a letter or a visit from a board investigator, it should be treated with urgency. Ohio attorney Beth Collis said one client angrily scrawled one-word answers with a Sharpie on the questionnaire he was mailed – answers he was stuck defending throughout the rest of the investigation. Other doctors have ordered investigators out of their offices – another mistake. Failure to cooperate can result in an immediate license suspension.
“They should be speaking to these investigators like they were talking to a highway patrolman on the side of the road. They hold all the cards,” said Ms. Collis, who specializes in representing professionals before licensing boards.
Some physicians mistakenly assume that because their state board is made up mostly of fellow doctors, they will be able to make a complaint go away with some collegial chat.
Not so. “Medical board members see themselves as protecting the public. They’re very punitive,” Ms. Collis said.
At one time, state boards might have been lax in their supervision of physicians, but that changed in the 1980s when the watchdog group Public Citizen began ranking state medical boards by how effective they were in policing doctors.
Public Citizen used FSMB data on serious disciplinary actions per 1,000 doctors in each state to calculate its rankings, a practice that FSMB called incomplete and a misuse of its statistics. Nonetheless, the annual rankings generated a lot of publicity critical of state boards and might have spurred a tougher approach by regulators.
Public Citizen stopped publishing its annual rankings in 2013 after FSMB ceased supplying the data, but the get-tough approach remains, lawyers said.
About 95% of complaints are dismissed with nothing more serious than a letter to the doctor, but boards don’t hesitate to act when the misconduct is serious, said Dr. Dauer. “I felt it was my obligation to protect the public.”
Don’t try to fix it yourself
Although many complaints are anonymous, doctors can often figure out what or who it involves. Their impulse might be to contact a patient who complained, correct a medical record, or otherwise try to resolve the matter personally.
It’s better to leave things alone, the experts said. Don’t contact a patient. Give the board access to whatever information it asks for, but don’t alter anything, particularly medical records. “That’s how you’re going to get your license revoked,” Dr. Dauer said. He noted that when doctors add notations to records, they must date them.
Hire a lawyer
Many physicians assume they can resolve the complaint easily by explaining themselves to the board or investigators, or they don’t realize their license or practice could be at stake.
They’re better off letting a lawyer speak for them. Attorneys knowledgeable in this realm specialize in representing licensed professionals before regulatory boards and have the greatest knowledge of administrative law and how to negotiate the hearings and procedures.
Typically, a hearing is held before a subcommittee of the board, which can recommend a settlement to the full panel. Cases in which a settlement is not reached can go before the entire board.
Although full hearings can be similar to a trial, there are crucial differences regarding evidentiary rules and other matters, Ms. Collis said. For example, in Ohio, defendant physicians do not get to see the board’s full case against them before the hearing, which can make preparing a defense difficult. And the standard for burden of proof is a preponderance of evidence, as in civil suits, not evidence beyond a reasonable doubt, as in a criminal trial.
Cases that go to full hearings and beyond to appeals in state courts can take years to resolve, and a physician’s license can be suspended for the duration.
Get help before it’s too late
Physicians looking for support and advice can turn to organizations such as the Coalition for Physician Rights, an organization formed in 2018 by Kernan Manion, MD, a former psychiatrist who was forced to deactivate his license after an investigation by the North Carolina medical board.
The Coalition for Physician Rights has advised hundreds of physicians, most of whom he said come to him once they realize they’re in over their heads. “Almost everyone comes in too late,” Dr. Manion said. “They’re sitting ducks. They don’t know how to respond.”
In addition to offering advice and support, the Coalition for Physician Rights lobbies for reform in how boards operate. A number of states, including Oklahoma, have made reforms in recent years.
The appointed boards are too reliant on their administration and staff and usually rubber-stamp disciplinary recommendations, Dr. Manion said. He also criticized the boards’ lack of accountability: “A board operates without external or internal oversight. It is an autonomous entity operating on its own.”
As for Dr. Moran, at age 61, she’s interviewing for physician jobs around the country, refusing to give up medicine.
“What else can I do?” she said. “It’s what I’ve done my entire life. It’s what I went to school for. I don’t know how to do anything else.”
A version of this article originally appeared on Medscape.com.
Slow taper off antimalarial is best to avoid lupus flare during remission
Slowly tapering off – or remaining on – antimalarial medications can help prevent disease flare in patients with systemic lupus erythematosus (SLE) who’ve achieved clinical remission for at least a year, according to a new study that was presented at the virtual annual meeting of the American College of Rheumatology.
“Except in the setting of toxicity, cessation of antimalarial medication in patients with disease quiescence is feasible using a slow taper,” lead author Danaë Papachristos, MBBS, said during an oral abstract presentation at the online meeting. Dr. Papachristos conducted the research while a clinical and research fellow at the University of Toronto’s lupus clinic, but is now a consultant rheumatologist at the Wesley Hospital in Brisbane, Queensland, Australia.
To investigate flare in patients with SLE who were on or recently off antimalarial medications (AMs), the researchers identified 1,573 potential participants from a long-term observational cohort study at the university’s lupus clinic. From that larger group, 88 cases – patients who achieved clinical remission for at least a year and stopped taking AMs – were matched to at least one control – patients who also achieved remission and continued on medication. Most cases were also matched to a second control, bringing the total number to 173. All patients had at least 2 years of follow-up.
Flare was defined as any increase in the SLEDAI-2K score, with major flare defined as an increase of 4 or more. Patients in the case group were roughly 44 years old, compared with an average age of 46 in the control group. Both groups were almost entirely female and largely white. Reasons for withdrawal in the case group included self-cessation, disease quiescence, and retinal, mucocutaneous, or cardiac toxicities. Twenty participants in the case group reported AM toxicity, compared with four controls.
Dr. Papachristos noted in her presentation that the toxicity disparity was expected, “because controls are those who continue their medication, and most patients who have toxicity will stop their medication.”
Disease flare occurred in 61.4% of cases, compared with 45.1% of controls (P = .002), with the most common types being cutaneous and musculoskeletal flares. After multivariate analysis, the risk of flare more than doubled for those who ceased AMs (odds ratio, 2.26; 95% confidence interval, 1.24-4.11; P = .008). More than half of the cases (n = 46) restarted AMs after withdrawal, which was largely due to disease flare. Of the patients who restarted due to flare, 88% either recaptured control or improved, and the remaining 12% had further flares.
Of the 88 patients in the case group, 51 abruptly withdrew AMs while 37 tapered off. Patients who tapered had fewer flares (45.9%), compared with patients who withdrew abruptly (72.6%). After multivariate analysis, the risk of flare more than tripled for the abrupt withdrawal group (OR, 3.42; 95% CI, 1.26-9.26; P = .016). Fewer patients who tapered later restarted AMs, compared with the abrupt withdrawal group (37.8% vs. 62.7%; P = .02).
When asked about other differences in medications between the two groups, Dr. Papachristos answered: “We didn’t look into that specifically. We did look at those patients who were on prednisone and on any immunosuppression, although we didn’t look at specific therapies. Those variables were adjusted for in the analysis, and it didn’t make any difference if patients were on immunosuppression or prednisone at the point of index date.
“But we would like to look into the different forms of immunosuppression,” she added, “just to see if that made any difference.”
Withdrawing hydroxychloroquine in older patients
Older patients with SLE who discontinue their use of hydroxychloroquine (HCQ) are also not at increased risk of disease flare, according to a retrospective chart review from rheumatologists Ruth Fernandez-Ruiz, MD, and Peter M. Izmirly, MD, of New York University (Arthritis Res Ther. 2020;22:191. doi: 10.1186/s13075-020-02282-0).
“We wanted to focus on older patients who may have a lower risk of flaring and a higher risk of side effects from the drug,” Dr. Fernandez-Ruiz said in an interview.
The doctors embarked on the study after noticing eye and heart toxicities in certain older patients. They matched 26 lupus patients who had been on HCQ for at least 5 years before discontinuing the drug with 32 control patients who remained on HCQ, ultimately finding that withdrawal had no effect on their risk of lupus flares within a year.
“After starting a drug, the second question most people ask, after ‘What are the side effects?’ is ‘How long do I have to be on this?’ ” Dr. Izmirly said in an interview. “These patients are having side effects associated with long-term HCQ use. And we were noticing that, after you stop the drug, despite what you’re taught, they weren’t flaring.”
Only five patients from each group – 19.2% of the withdrawal group and 15.6% of the continuation group – experienced a flare (OR, 1.28; 95% CI, 0.31-5.30; P = .73). Most of the flares were cutaneous and musculoskeletal in nature, and no severe flares occurred in either group.
“On each side, the overall flare rate was not that high, and they were all relatively mild,” Dr. Izmirly said.
The two doctors acknowledged their study’s smaller sample size, compared with the study by Papachristos and colleagues, along with the advanced age of their patient population, which limits the generalizability of their findings. “We selected patients who had a very low disease activity to begin with, and who were older,” Dr. Fernandez-Ruiz noted.
That said, they reinforced the scarcity of existing research on this subset of lupus patients, one that will only continue to grow.
“Older [patients with] lupus,” Dr. Izmirly said, are “an understudied demographic.”
One of the authors of the study presented at ACR 2020 acknowledged receiving research support and consulting fees from various pharmaceutical companies. The HCQ study was supported by a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases; its authors declared no conflicts of interest.
SOURCE: Papachristos D et al. Arthritis Rheumatol. 2020;72(suppl 10). Abstract 0983.
Slowly tapering off – or remaining on – antimalarial medications can help prevent disease flare in patients with systemic lupus erythematosus (SLE) who’ve achieved clinical remission for at least a year, according to a new study that was presented at the virtual annual meeting of the American College of Rheumatology.
“Except in the setting of toxicity, cessation of antimalarial medication in patients with disease quiescence is feasible using a slow taper,” lead author Danaë Papachristos, MBBS, said during an oral abstract presentation at the online meeting. Dr. Papachristos conducted the research while a clinical and research fellow at the University of Toronto’s lupus clinic, but is now a consultant rheumatologist at the Wesley Hospital in Brisbane, Queensland, Australia.
To investigate flare in patients with SLE who were on or recently off antimalarial medications (AMs), the researchers identified 1,573 potential participants from a long-term observational cohort study at the university’s lupus clinic. From that larger group, 88 cases – patients who achieved clinical remission for at least a year and stopped taking AMs – were matched to at least one control – patients who also achieved remission and continued on medication. Most cases were also matched to a second control, bringing the total number to 173. All patients had at least 2 years of follow-up.
Flare was defined as any increase in the SLEDAI-2K score, with major flare defined as an increase of 4 or more. Patients in the case group were roughly 44 years old, compared with an average age of 46 in the control group. Both groups were almost entirely female and largely white. Reasons for withdrawal in the case group included self-cessation, disease quiescence, and retinal, mucocutaneous, or cardiac toxicities. Twenty participants in the case group reported AM toxicity, compared with four controls.
Dr. Papachristos noted in her presentation that the toxicity disparity was expected, “because controls are those who continue their medication, and most patients who have toxicity will stop their medication.”
Disease flare occurred in 61.4% of cases, compared with 45.1% of controls (P = .002), with the most common types being cutaneous and musculoskeletal flares. After multivariate analysis, the risk of flare more than doubled for those who ceased AMs (odds ratio, 2.26; 95% confidence interval, 1.24-4.11; P = .008). More than half of the cases (n = 46) restarted AMs after withdrawal, which was largely due to disease flare. Of the patients who restarted due to flare, 88% either recaptured control or improved, and the remaining 12% had further flares.
Of the 88 patients in the case group, 51 abruptly withdrew AMs while 37 tapered off. Patients who tapered had fewer flares (45.9%), compared with patients who withdrew abruptly (72.6%). After multivariate analysis, the risk of flare more than tripled for the abrupt withdrawal group (OR, 3.42; 95% CI, 1.26-9.26; P = .016). Fewer patients who tapered later restarted AMs, compared with the abrupt withdrawal group (37.8% vs. 62.7%; P = .02).
When asked about other differences in medications between the two groups, Dr. Papachristos answered: “We didn’t look into that specifically. We did look at those patients who were on prednisone and on any immunosuppression, although we didn’t look at specific therapies. Those variables were adjusted for in the analysis, and it didn’t make any difference if patients were on immunosuppression or prednisone at the point of index date.
“But we would like to look into the different forms of immunosuppression,” she added, “just to see if that made any difference.”
Withdrawing hydroxychloroquine in older patients
Older patients with SLE who discontinue their use of hydroxychloroquine (HCQ) are also not at increased risk of disease flare, according to a retrospective chart review from rheumatologists Ruth Fernandez-Ruiz, MD, and Peter M. Izmirly, MD, of New York University (Arthritis Res Ther. 2020;22:191. doi: 10.1186/s13075-020-02282-0).
“We wanted to focus on older patients who may have a lower risk of flaring and a higher risk of side effects from the drug,” Dr. Fernandez-Ruiz said in an interview.
The doctors embarked on the study after noticing eye and heart toxicities in certain older patients. They matched 26 lupus patients who had been on HCQ for at least 5 years before discontinuing the drug with 32 control patients who remained on HCQ, ultimately finding that withdrawal had no effect on their risk of lupus flares within a year.
“After starting a drug, the second question most people ask, after ‘What are the side effects?’ is ‘How long do I have to be on this?’ ” Dr. Izmirly said in an interview. “These patients are having side effects associated with long-term HCQ use. And we were noticing that, after you stop the drug, despite what you’re taught, they weren’t flaring.”
Only five patients from each group – 19.2% of the withdrawal group and 15.6% of the continuation group – experienced a flare (OR, 1.28; 95% CI, 0.31-5.30; P = .73). Most of the flares were cutaneous and musculoskeletal in nature, and no severe flares occurred in either group.
“On each side, the overall flare rate was not that high, and they were all relatively mild,” Dr. Izmirly said.
The two doctors acknowledged their study’s smaller sample size, compared with the study by Papachristos and colleagues, along with the advanced age of their patient population, which limits the generalizability of their findings. “We selected patients who had a very low disease activity to begin with, and who were older,” Dr. Fernandez-Ruiz noted.
That said, they reinforced the scarcity of existing research on this subset of lupus patients, one that will only continue to grow.
“Older [patients with] lupus,” Dr. Izmirly said, are “an understudied demographic.”
One of the authors of the study presented at ACR 2020 acknowledged receiving research support and consulting fees from various pharmaceutical companies. The HCQ study was supported by a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases; its authors declared no conflicts of interest.
SOURCE: Papachristos D et al. Arthritis Rheumatol. 2020;72(suppl 10). Abstract 0983.
Slowly tapering off – or remaining on – antimalarial medications can help prevent disease flare in patients with systemic lupus erythematosus (SLE) who’ve achieved clinical remission for at least a year, according to a new study that was presented at the virtual annual meeting of the American College of Rheumatology.
“Except in the setting of toxicity, cessation of antimalarial medication in patients with disease quiescence is feasible using a slow taper,” lead author Danaë Papachristos, MBBS, said during an oral abstract presentation at the online meeting. Dr. Papachristos conducted the research while a clinical and research fellow at the University of Toronto’s lupus clinic, but is now a consultant rheumatologist at the Wesley Hospital in Brisbane, Queensland, Australia.
To investigate flare in patients with SLE who were on or recently off antimalarial medications (AMs), the researchers identified 1,573 potential participants from a long-term observational cohort study at the university’s lupus clinic. From that larger group, 88 cases – patients who achieved clinical remission for at least a year and stopped taking AMs – were matched to at least one control – patients who also achieved remission and continued on medication. Most cases were also matched to a second control, bringing the total number to 173. All patients had at least 2 years of follow-up.
Flare was defined as any increase in the SLEDAI-2K score, with major flare defined as an increase of 4 or more. Patients in the case group were roughly 44 years old, compared with an average age of 46 in the control group. Both groups were almost entirely female and largely white. Reasons for withdrawal in the case group included self-cessation, disease quiescence, and retinal, mucocutaneous, or cardiac toxicities. Twenty participants in the case group reported AM toxicity, compared with four controls.
Dr. Papachristos noted in her presentation that the toxicity disparity was expected, “because controls are those who continue their medication, and most patients who have toxicity will stop their medication.”
Disease flare occurred in 61.4% of cases, compared with 45.1% of controls (P = .002), with the most common types being cutaneous and musculoskeletal flares. After multivariate analysis, the risk of flare more than doubled for those who ceased AMs (odds ratio, 2.26; 95% confidence interval, 1.24-4.11; P = .008). More than half of the cases (n = 46) restarted AMs after withdrawal, which was largely due to disease flare. Of the patients who restarted due to flare, 88% either recaptured control or improved, and the remaining 12% had further flares.
Of the 88 patients in the case group, 51 abruptly withdrew AMs while 37 tapered off. Patients who tapered had fewer flares (45.9%), compared with patients who withdrew abruptly (72.6%). After multivariate analysis, the risk of flare more than tripled for the abrupt withdrawal group (OR, 3.42; 95% CI, 1.26-9.26; P = .016). Fewer patients who tapered later restarted AMs, compared with the abrupt withdrawal group (37.8% vs. 62.7%; P = .02).
When asked about other differences in medications between the two groups, Dr. Papachristos answered: “We didn’t look into that specifically. We did look at those patients who were on prednisone and on any immunosuppression, although we didn’t look at specific therapies. Those variables were adjusted for in the analysis, and it didn’t make any difference if patients were on immunosuppression or prednisone at the point of index date.
“But we would like to look into the different forms of immunosuppression,” she added, “just to see if that made any difference.”
Withdrawing hydroxychloroquine in older patients
Older patients with SLE who discontinue their use of hydroxychloroquine (HCQ) are also not at increased risk of disease flare, according to a retrospective chart review from rheumatologists Ruth Fernandez-Ruiz, MD, and Peter M. Izmirly, MD, of New York University (Arthritis Res Ther. 2020;22:191. doi: 10.1186/s13075-020-02282-0).
“We wanted to focus on older patients who may have a lower risk of flaring and a higher risk of side effects from the drug,” Dr. Fernandez-Ruiz said in an interview.
The doctors embarked on the study after noticing eye and heart toxicities in certain older patients. They matched 26 lupus patients who had been on HCQ for at least 5 years before discontinuing the drug with 32 control patients who remained on HCQ, ultimately finding that withdrawal had no effect on their risk of lupus flares within a year.
“After starting a drug, the second question most people ask, after ‘What are the side effects?’ is ‘How long do I have to be on this?’ ” Dr. Izmirly said in an interview. “These patients are having side effects associated with long-term HCQ use. And we were noticing that, after you stop the drug, despite what you’re taught, they weren’t flaring.”
Only five patients from each group – 19.2% of the withdrawal group and 15.6% of the continuation group – experienced a flare (OR, 1.28; 95% CI, 0.31-5.30; P = .73). Most of the flares were cutaneous and musculoskeletal in nature, and no severe flares occurred in either group.
“On each side, the overall flare rate was not that high, and they were all relatively mild,” Dr. Izmirly said.
The two doctors acknowledged their study’s smaller sample size, compared with the study by Papachristos and colleagues, along with the advanced age of their patient population, which limits the generalizability of their findings. “We selected patients who had a very low disease activity to begin with, and who were older,” Dr. Fernandez-Ruiz noted.
That said, they reinforced the scarcity of existing research on this subset of lupus patients, one that will only continue to grow.
“Older [patients with] lupus,” Dr. Izmirly said, are “an understudied demographic.”
One of the authors of the study presented at ACR 2020 acknowledged receiving research support and consulting fees from various pharmaceutical companies. The HCQ study was supported by a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases; its authors declared no conflicts of interest.
SOURCE: Papachristos D et al. Arthritis Rheumatol. 2020;72(suppl 10). Abstract 0983.
FROM ACR 2020
Embrace new and classic acne treatments
Recognizing the ongoing value of benzoyl peroxide, educating patients about the role of antibiotics, and embracing spironolactone are among the acne treatment pearls provided by Hilary Baldwin, MD, during a virtual presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
Benzoyl peroxide celebrates its 60th birthday and is still going strong as an acne treatment, said Dr. Baldwin, of the department of dermatology, Rutgers Robert Wood Johnston Medical Center, New Brunswick, N.J. Benzoyl peroxide can be used as a stand-alone and has the added benefit of not being associated with antimicrobial resistance. In addition, “benzoyl peroxide is the heavy lifter in combinations,” she said. In fact, benzoyl peroxide can prevent the development of resistance to topical and oral antibiotics such as clindamycin, and can reverse resistance that has occurred, she noted.
However, patient compliance can be an issue. Benzoyl peroxide often is underused because of its tendency to bleach fabric, noted Dr. Baldwin, who is also medical director of The Acne Treatment and Research Center in New York. To help combat this problem and improve compliance, she advises patients to establish a dosing schedule for benzoyl peroxide, such as using it first thing in the morning, or applying in the afternoon and using a paper towel first, or a white towel, to wash their faces at bedtime, she said. When dealing with teenagers, “it sounds like a lot of work, but it makes the mothers much happier not to have their towels bleached.”
Although clinicians want to reduce unnecessary antibiotic use in acne, there is a place for antibiotics, but not as monotherapy, Dr. Baldwin said. Instead, initiate topical therapy, such as a retinoid or benzoyl peroxide, simultaneously with antibiotics and evaluate the response in 6-8 weeks, she advised. At that point, the antibiotics can be stopped, even if 100% clearing has not been achieved, and “the topicals can carry you on for months and months,” she noted.
Also, in female patients, consider oral contraceptive pills or spironolactone at the same time as oral antibiotics, then discontinue the antibiotics and continue with the hormonal therapy, she added. “Plan your exit strategy early,” she said. Explain to patients that you will stop the oral antibiotics after 2 months, so they must continue with the topicals.
“Embrace spironolactone if you haven’t already,” said Dr. Baldwin, who noted that spironolactone has been underused in recent years. Spironolactone use for acne has not been well studied, “but consensus groups and expert opinions certainly favor its use,” she added.
Spironolactone takes 3-6 months to reach its full effect, so Dr. Baldwin recommends beginning the therapy in combination with other strategies. “I begin in combination with oral antibiotics,” she said. Also, be sure to check hormone levels before initiating therapy if appropriate. Potential side effects include menstrual irregularities and breast tenderness, but they tend to decrease over time, Dr. Baldwin noted. Other side effects such as CNS symptoms (fatigue, dizziness, and headache) can be eased by paying attention to proper hydration and starting with a lower dose, she added. Studies in younger adults show no reason for concern about potassium levels, but potassium should be checked at baseline in older patients, after the first month, and after a dose increase, she said.
Dr. Baldwin was enthusiastic about the recent introduction of several new treatments for acne: Sarecycline, now approved by the Food and Drug Administration for use in patients as young as 9 years; trifarotene 0.005% cream, the first 4th generation retinoid, with truncal acne data; tazarotene 0.045% lotion, with improved tolerability; minocycline 4% foam, with high cutaneous levels and minimal systemic absorption; and clascoterone 1% cream, “the first topical antiandrogen and safe for use in males,” she said.
Relevant to her presentation, Dr. Baldwin disclosed relationships as an adviser, speaker, and/or investigator for Almirall, EPI Health, Foamix, Galderma, Johnson & Johnson, LaRoche-Posay, Menlo Therapeutics, Ortho Dermatologics, Sol-Gel, and Sun.
MedscapeLive and this news organization are owned by the same parent company.
Recognizing the ongoing value of benzoyl peroxide, educating patients about the role of antibiotics, and embracing spironolactone are among the acne treatment pearls provided by Hilary Baldwin, MD, during a virtual presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
Benzoyl peroxide celebrates its 60th birthday and is still going strong as an acne treatment, said Dr. Baldwin, of the department of dermatology, Rutgers Robert Wood Johnston Medical Center, New Brunswick, N.J. Benzoyl peroxide can be used as a stand-alone and has the added benefit of not being associated with antimicrobial resistance. In addition, “benzoyl peroxide is the heavy lifter in combinations,” she said. In fact, benzoyl peroxide can prevent the development of resistance to topical and oral antibiotics such as clindamycin, and can reverse resistance that has occurred, she noted.
However, patient compliance can be an issue. Benzoyl peroxide often is underused because of its tendency to bleach fabric, noted Dr. Baldwin, who is also medical director of The Acne Treatment and Research Center in New York. To help combat this problem and improve compliance, she advises patients to establish a dosing schedule for benzoyl peroxide, such as using it first thing in the morning, or applying in the afternoon and using a paper towel first, or a white towel, to wash their faces at bedtime, she said. When dealing with teenagers, “it sounds like a lot of work, but it makes the mothers much happier not to have their towels bleached.”
Although clinicians want to reduce unnecessary antibiotic use in acne, there is a place for antibiotics, but not as monotherapy, Dr. Baldwin said. Instead, initiate topical therapy, such as a retinoid or benzoyl peroxide, simultaneously with antibiotics and evaluate the response in 6-8 weeks, she advised. At that point, the antibiotics can be stopped, even if 100% clearing has not been achieved, and “the topicals can carry you on for months and months,” she noted.
Also, in female patients, consider oral contraceptive pills or spironolactone at the same time as oral antibiotics, then discontinue the antibiotics and continue with the hormonal therapy, she added. “Plan your exit strategy early,” she said. Explain to patients that you will stop the oral antibiotics after 2 months, so they must continue with the topicals.
“Embrace spironolactone if you haven’t already,” said Dr. Baldwin, who noted that spironolactone has been underused in recent years. Spironolactone use for acne has not been well studied, “but consensus groups and expert opinions certainly favor its use,” she added.
Spironolactone takes 3-6 months to reach its full effect, so Dr. Baldwin recommends beginning the therapy in combination with other strategies. “I begin in combination with oral antibiotics,” she said. Also, be sure to check hormone levels before initiating therapy if appropriate. Potential side effects include menstrual irregularities and breast tenderness, but they tend to decrease over time, Dr. Baldwin noted. Other side effects such as CNS symptoms (fatigue, dizziness, and headache) can be eased by paying attention to proper hydration and starting with a lower dose, she added. Studies in younger adults show no reason for concern about potassium levels, but potassium should be checked at baseline in older patients, after the first month, and after a dose increase, she said.
Dr. Baldwin was enthusiastic about the recent introduction of several new treatments for acne: Sarecycline, now approved by the Food and Drug Administration for use in patients as young as 9 years; trifarotene 0.005% cream, the first 4th generation retinoid, with truncal acne data; tazarotene 0.045% lotion, with improved tolerability; minocycline 4% foam, with high cutaneous levels and minimal systemic absorption; and clascoterone 1% cream, “the first topical antiandrogen and safe for use in males,” she said.
Relevant to her presentation, Dr. Baldwin disclosed relationships as an adviser, speaker, and/or investigator for Almirall, EPI Health, Foamix, Galderma, Johnson & Johnson, LaRoche-Posay, Menlo Therapeutics, Ortho Dermatologics, Sol-Gel, and Sun.
MedscapeLive and this news organization are owned by the same parent company.
Recognizing the ongoing value of benzoyl peroxide, educating patients about the role of antibiotics, and embracing spironolactone are among the acne treatment pearls provided by Hilary Baldwin, MD, during a virtual presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
Benzoyl peroxide celebrates its 60th birthday and is still going strong as an acne treatment, said Dr. Baldwin, of the department of dermatology, Rutgers Robert Wood Johnston Medical Center, New Brunswick, N.J. Benzoyl peroxide can be used as a stand-alone and has the added benefit of not being associated with antimicrobial resistance. In addition, “benzoyl peroxide is the heavy lifter in combinations,” she said. In fact, benzoyl peroxide can prevent the development of resistance to topical and oral antibiotics such as clindamycin, and can reverse resistance that has occurred, she noted.
However, patient compliance can be an issue. Benzoyl peroxide often is underused because of its tendency to bleach fabric, noted Dr. Baldwin, who is also medical director of The Acne Treatment and Research Center in New York. To help combat this problem and improve compliance, she advises patients to establish a dosing schedule for benzoyl peroxide, such as using it first thing in the morning, or applying in the afternoon and using a paper towel first, or a white towel, to wash their faces at bedtime, she said. When dealing with teenagers, “it sounds like a lot of work, but it makes the mothers much happier not to have their towels bleached.”
Although clinicians want to reduce unnecessary antibiotic use in acne, there is a place for antibiotics, but not as monotherapy, Dr. Baldwin said. Instead, initiate topical therapy, such as a retinoid or benzoyl peroxide, simultaneously with antibiotics and evaluate the response in 6-8 weeks, she advised. At that point, the antibiotics can be stopped, even if 100% clearing has not been achieved, and “the topicals can carry you on for months and months,” she noted.
Also, in female patients, consider oral contraceptive pills or spironolactone at the same time as oral antibiotics, then discontinue the antibiotics and continue with the hormonal therapy, she added. “Plan your exit strategy early,” she said. Explain to patients that you will stop the oral antibiotics after 2 months, so they must continue with the topicals.
“Embrace spironolactone if you haven’t already,” said Dr. Baldwin, who noted that spironolactone has been underused in recent years. Spironolactone use for acne has not been well studied, “but consensus groups and expert opinions certainly favor its use,” she added.
Spironolactone takes 3-6 months to reach its full effect, so Dr. Baldwin recommends beginning the therapy in combination with other strategies. “I begin in combination with oral antibiotics,” she said. Also, be sure to check hormone levels before initiating therapy if appropriate. Potential side effects include menstrual irregularities and breast tenderness, but they tend to decrease over time, Dr. Baldwin noted. Other side effects such as CNS symptoms (fatigue, dizziness, and headache) can be eased by paying attention to proper hydration and starting with a lower dose, she added. Studies in younger adults show no reason for concern about potassium levels, but potassium should be checked at baseline in older patients, after the first month, and after a dose increase, she said.
Dr. Baldwin was enthusiastic about the recent introduction of several new treatments for acne: Sarecycline, now approved by the Food and Drug Administration for use in patients as young as 9 years; trifarotene 0.005% cream, the first 4th generation retinoid, with truncal acne data; tazarotene 0.045% lotion, with improved tolerability; minocycline 4% foam, with high cutaneous levels and minimal systemic absorption; and clascoterone 1% cream, “the first topical antiandrogen and safe for use in males,” she said.
Relevant to her presentation, Dr. Baldwin disclosed relationships as an adviser, speaker, and/or investigator for Almirall, EPI Health, Foamix, Galderma, Johnson & Johnson, LaRoche-Posay, Menlo Therapeutics, Ortho Dermatologics, Sol-Gel, and Sun.
MedscapeLive and this news organization are owned by the same parent company.
FROM THE MEDSCAPELIVE LAS VEGAS DERMATOLOGY SEMINAR
Combo DAA treatments may benefit patients with resistant HCV genotype 3
Patients with hepatitis C virus (HCV) genotype 3 infection have shown resistance to direct-acting antiviral (DAA) treatments. However, a meta-analysis of 34 research reports found that DAA combo treatment can be effective in achieving sustained virologic response (SVR) in patients with HCV genotype 3, according to a study published online in Annals of Hepatology.
This study aimed to analyze the effectiveness of four regimens: sofosbuvir (SOF)/daclatasvir (DCV) with or without ribavirin (RBV); SOF/velpatasvir (VEL) with or without RBV; SOF/VEL/voxilaprevir (VOX);and glecaprevir (GLE)/pibrentasvir (PIB) in the treatment of HCV genotype 3–infected patients in real-world situations, according to Liwei Zhuang, of Beijing Ditan Hospital, Capital Medical University, and colleagues.
A total of 34 studies, comprising 7,328 patients from 22 countries, met the inclusion criteria and formed the basis of the analysis.
Promising results
The pooled SVR rate after 12 or 24 weeks of treatment for the four regimens was 92.1%.
For each regimen, the SVR rate was 91.2% in patients treated with SOF/DCV with or without RBV; 95.1% in patients treated with SOF/VEL with or without RBV; 85.0% in patients treated with SOF/VEL/VOX; and 98.5% in patients treated with GLE/PIB.
In addition, the pooled SVR rate of the four regimens was 95.2% in patients without cirrhosis and 89.4% in patients with cirrhosis, and the pooled SVR rate was 94.4% in treatment-naive patients and 88.0% in treatment-experienced patients. All results were within 95% confidence intervals.
The researchers pointed out that their meta-analysis had limitations. “We think that no strong conclusions can be drawn due to high heterogeneity in four DAA regimens administration in real-world setting from 22 countries, as well as small numbers of patients treated with SOF + VEL + VOX and GLE + PIB. More studies are needed in the future in order to better analyze the antiviral effectiveness of DAAs in GT3 HCV patients in real-world studies,” they authors stated.
However, they also concluded that “the antiviral effectiveness of treatment regimens for HCV-GT3 [genotype 3] infection, including SOF + DCV ± RBV, SOF + VEL ± RBV, GLE + PIB, and SOF + VEL + VOX, was good. The SVR rate of GLE + PIB was higher, and the treatment duration was shorter than other regimens.”
The study was funded by the Chinese government and public institutions. The authors reported that they had no conflicts of interest.
SOURCE: Zhuang L et al. Ann Hepatol. 2020 Oct 12. doi: 10.1016/j.aohep.2020.09.012.
Patients with hepatitis C virus (HCV) genotype 3 infection have shown resistance to direct-acting antiviral (DAA) treatments. However, a meta-analysis of 34 research reports found that DAA combo treatment can be effective in achieving sustained virologic response (SVR) in patients with HCV genotype 3, according to a study published online in Annals of Hepatology.
This study aimed to analyze the effectiveness of four regimens: sofosbuvir (SOF)/daclatasvir (DCV) with or without ribavirin (RBV); SOF/velpatasvir (VEL) with or without RBV; SOF/VEL/voxilaprevir (VOX);and glecaprevir (GLE)/pibrentasvir (PIB) in the treatment of HCV genotype 3–infected patients in real-world situations, according to Liwei Zhuang, of Beijing Ditan Hospital, Capital Medical University, and colleagues.
A total of 34 studies, comprising 7,328 patients from 22 countries, met the inclusion criteria and formed the basis of the analysis.
Promising results
The pooled SVR rate after 12 or 24 weeks of treatment for the four regimens was 92.1%.
For each regimen, the SVR rate was 91.2% in patients treated with SOF/DCV with or without RBV; 95.1% in patients treated with SOF/VEL with or without RBV; 85.0% in patients treated with SOF/VEL/VOX; and 98.5% in patients treated with GLE/PIB.
In addition, the pooled SVR rate of the four regimens was 95.2% in patients without cirrhosis and 89.4% in patients with cirrhosis, and the pooled SVR rate was 94.4% in treatment-naive patients and 88.0% in treatment-experienced patients. All results were within 95% confidence intervals.
The researchers pointed out that their meta-analysis had limitations. “We think that no strong conclusions can be drawn due to high heterogeneity in four DAA regimens administration in real-world setting from 22 countries, as well as small numbers of patients treated with SOF + VEL + VOX and GLE + PIB. More studies are needed in the future in order to better analyze the antiviral effectiveness of DAAs in GT3 HCV patients in real-world studies,” they authors stated.
However, they also concluded that “the antiviral effectiveness of treatment regimens for HCV-GT3 [genotype 3] infection, including SOF + DCV ± RBV, SOF + VEL ± RBV, GLE + PIB, and SOF + VEL + VOX, was good. The SVR rate of GLE + PIB was higher, and the treatment duration was shorter than other regimens.”
The study was funded by the Chinese government and public institutions. The authors reported that they had no conflicts of interest.
SOURCE: Zhuang L et al. Ann Hepatol. 2020 Oct 12. doi: 10.1016/j.aohep.2020.09.012.
Patients with hepatitis C virus (HCV) genotype 3 infection have shown resistance to direct-acting antiviral (DAA) treatments. However, a meta-analysis of 34 research reports found that DAA combo treatment can be effective in achieving sustained virologic response (SVR) in patients with HCV genotype 3, according to a study published online in Annals of Hepatology.
This study aimed to analyze the effectiveness of four regimens: sofosbuvir (SOF)/daclatasvir (DCV) with or without ribavirin (RBV); SOF/velpatasvir (VEL) with or without RBV; SOF/VEL/voxilaprevir (VOX);and glecaprevir (GLE)/pibrentasvir (PIB) in the treatment of HCV genotype 3–infected patients in real-world situations, according to Liwei Zhuang, of Beijing Ditan Hospital, Capital Medical University, and colleagues.
A total of 34 studies, comprising 7,328 patients from 22 countries, met the inclusion criteria and formed the basis of the analysis.
Promising results
The pooled SVR rate after 12 or 24 weeks of treatment for the four regimens was 92.1%.
For each regimen, the SVR rate was 91.2% in patients treated with SOF/DCV with or without RBV; 95.1% in patients treated with SOF/VEL with or without RBV; 85.0% in patients treated with SOF/VEL/VOX; and 98.5% in patients treated with GLE/PIB.
In addition, the pooled SVR rate of the four regimens was 95.2% in patients without cirrhosis and 89.4% in patients with cirrhosis, and the pooled SVR rate was 94.4% in treatment-naive patients and 88.0% in treatment-experienced patients. All results were within 95% confidence intervals.
The researchers pointed out that their meta-analysis had limitations. “We think that no strong conclusions can be drawn due to high heterogeneity in four DAA regimens administration in real-world setting from 22 countries, as well as small numbers of patients treated with SOF + VEL + VOX and GLE + PIB. More studies are needed in the future in order to better analyze the antiviral effectiveness of DAAs in GT3 HCV patients in real-world studies,” they authors stated.
However, they also concluded that “the antiviral effectiveness of treatment regimens for HCV-GT3 [genotype 3] infection, including SOF + DCV ± RBV, SOF + VEL ± RBV, GLE + PIB, and SOF + VEL + VOX, was good. The SVR rate of GLE + PIB was higher, and the treatment duration was shorter than other regimens.”
The study was funded by the Chinese government and public institutions. The authors reported that they had no conflicts of interest.
SOURCE: Zhuang L et al. Ann Hepatol. 2020 Oct 12. doi: 10.1016/j.aohep.2020.09.012.
FROM ANNALS OF HEPATOLOGY
Colorectal cancer screening: The new draft recs & the cost to screen
References
- US Preventive Services Task Force. Colorectal cancer: screening [draft recommendation statement]. https://uspreventiveservicestaskforce.org/uspstf/draft-recommendation/colorectal-cancer-screening3. Published October 27, 2020. Accessed November 23, 2020.
References
- US Preventive Services Task Force. Colorectal cancer: screening [draft recommendation statement]. https://uspreventiveservicestaskforce.org/uspstf/draft-recommendation/colorectal-cancer-screening3. Published October 27, 2020. Accessed November 23, 2020.
References
- US Preventive Services Task Force. Colorectal cancer: screening [draft recommendation statement]. https://uspreventiveservicestaskforce.org/uspstf/draft-recommendation/colorectal-cancer-screening3. Published October 27, 2020. Accessed November 23, 2020.
Umbilicated Neoplasm on the Chest
Dermoscopy showed polylobular, whitish yellow, amorphous structures at the center of the lesion surrounded by a crown of vessels (Figure 1). Histopathology revealed hyperplastic crateriform lesions containing large eosinophilic intracytoplasmic inclusion bodies within keratinocytes (Figure 2). At follow-up 2 weeks after the biopsy, the patient presented with approximately 20 more reddish papules of varying sizes on the abdomen and back that presented as dome-shaped papules and had a typical umbilicated center. The clinical manifestations, dermoscopy, and pathology findings were consistent with molluscum contagiosum (MC).
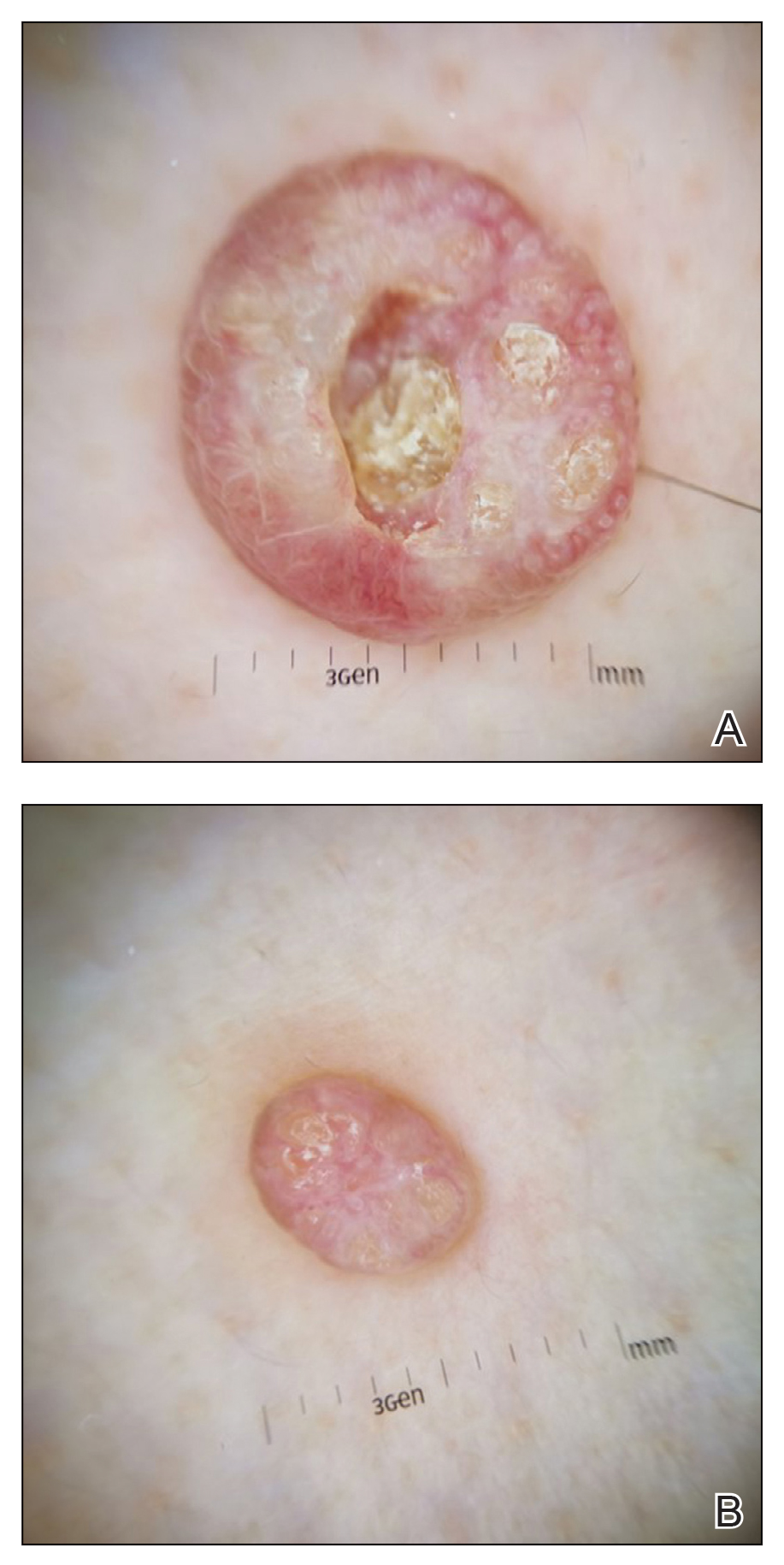
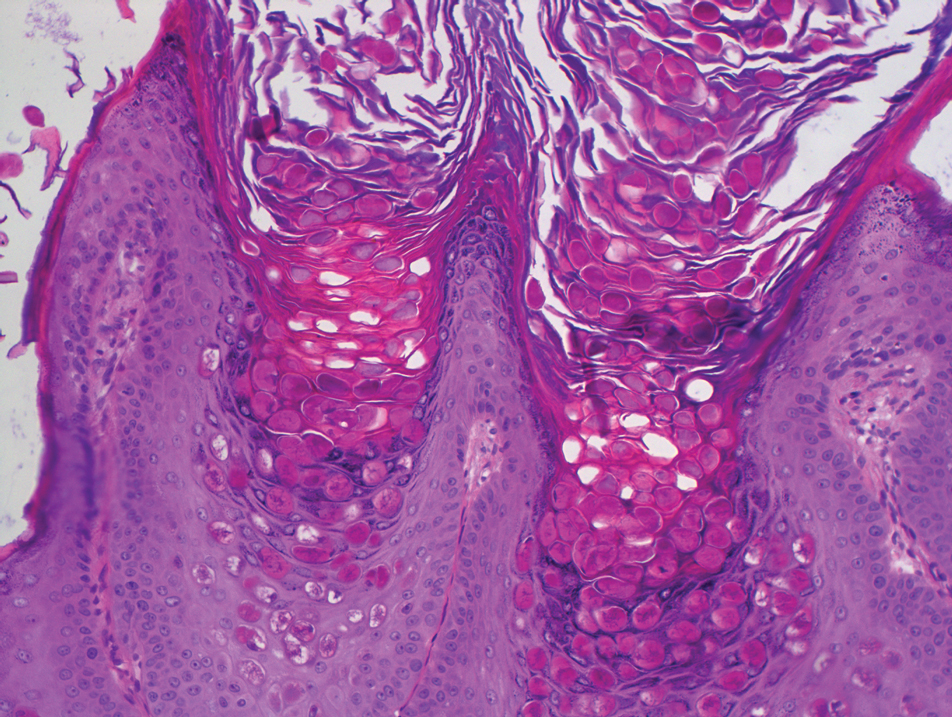
Molluscum contagiosum was first described in 1814. It is a benign cutaneous infectious disease caused by a double-stranded DNA virus of the poxvirus family. Molluscum contagiosum lesions usually manifest clinically as dome-shaped, flesh-colored or translucent, umbilicated papules measuring 1 to 5 mm in diameter that are commonly distributed over the face, trunk, and extremities and usually are self-limiting.1
Giant MC is rare and can be seen either in patients on immunosuppressive therapy or in those with diseases that can cause immunosuppression, such as human immunodeficiency virus, leukemia, atopic dermatitis, Wiskott-Aldrich syndrome, and sarcoidosis. In these instances, MC often is greater than 1 cm in diameter. Atypical variants may have an eczematous presentation or a lesion with secondary abscess formation and also can be spread widely over the body.2 Due to these atypical appearances and large dimensions in immunocompromised patients, other dermatologic diseases should be considered in the differential diagnosis, such as basal cell carcinoma, keratoacanthoma, squamous cell carcinoma, cutaneous horn, cutaneous cryptococcosis, histoplasmosis, and xanthomatosis.3
In our patient, the differential diagnosis included keratoacanthoma, which may present as a solitary, discrete, round to oval, flesh-colored, umbilicated nodule with a central keratin-filled crater and has a rapid clinical evolution, usually regressing within 4 to 6 months.
Squamous cell carcinoma may appear as scaly red patches, open sores, warts, or elevated growths with a central depression and may crust or bleed. Basal cell carcinoma typically may appear as a dome-shaped skin nodule with visible blood vessels or sometimes presents as a red patch similar to eczema. Xanthomatosis often appears as yellow to orange, mostly asymptomatic, supple patches or plaques, usually with sharp and distinctive edges.
Ancillary diagnostic modalities such as dermoscopy may be used to improve diagnostic accuracy. The best known capillaroscopic feature of MC is the peripheral crown of vessels in a radial distribution. A study of 258 MC lesions highlighted that crown and crown plus radial arrangements are the most common vascular structure patterns under dermoscopy. In addition, polylobular amorphous white structures in the center of the lesions tend to be a feature of larger MC papules.4 Histologically, MC shows lobulated crateriform lesions, thickening of the epidermis into the dermis, and the typical appearance of large eosinophilic intracytoplasmic inclusion bodies within keratinocytes.5
There are several treatment options available for MC. Common modalities include liquid nitrogen cryospray, curettage, and electrocauterization. In immunocompromised patients, MC lesions usually are resistant to ordinary therapy. The efficacy of topical agents such as imiquimod, which can induce high levels of IFN-α and other cytokines, has been demonstrated in these patients.6 Cidofovir, a nucleoside analog that has potent antiviral properties, also can be included as a therapeutic option.3 Our patient’s largest MC lesion was treated with surgical excision, the 2 large lesions on the left side of the chest with cryotherapy, and the other small lesions with curettage.
- Hanson D, Diven DG. Molluscum contagiosum. Dermatol Online J. 2003;9:2.
- Singh S, Swain M, Shukla S, et al. An unusual presentation of giant molluscum contagiosum diagnosed on cytology. Diagn Cytopathol. 2018;46:794-796.
- Mansur AT, Goktay F, Gunduz S, et al. Multiple giant molluscum contagiosum in a renal transplant recipient. Transpl Infect Dis. 2004;6:120-123.
- Ku SH, Cho EB, Park EJ, et al. Dermoscopic features of molluscum contagiosum based on white structures and their correlation with histopathological findings. Clin Exp Dermatol. 2015;40:208-210.
- Trčko K, Hošnjak L, Kušar B, et al. Clinical, histopathological, and virological evaluation of 203 patients with a clinical diagnosis of molluscum contagiosum [published online November 12, 2018]. Open Forum Infect Dis. 2018;5.
- Gardner LS, Ormond PJ. Treatment of multiple giant molluscum contagiosum in a renal transplant patient with imiquimod 5% cream. Clin Exp Dermatol. 2010;31:452-453.
Dermoscopy showed polylobular, whitish yellow, amorphous structures at the center of the lesion surrounded by a crown of vessels (Figure 1). Histopathology revealed hyperplastic crateriform lesions containing large eosinophilic intracytoplasmic inclusion bodies within keratinocytes (Figure 2). At follow-up 2 weeks after the biopsy, the patient presented with approximately 20 more reddish papules of varying sizes on the abdomen and back that presented as dome-shaped papules and had a typical umbilicated center. The clinical manifestations, dermoscopy, and pathology findings were consistent with molluscum contagiosum (MC).


Molluscum contagiosum was first described in 1814. It is a benign cutaneous infectious disease caused by a double-stranded DNA virus of the poxvirus family. Molluscum contagiosum lesions usually manifest clinically as dome-shaped, flesh-colored or translucent, umbilicated papules measuring 1 to 5 mm in diameter that are commonly distributed over the face, trunk, and extremities and usually are self-limiting.1
Giant MC is rare and can be seen either in patients on immunosuppressive therapy or in those with diseases that can cause immunosuppression, such as human immunodeficiency virus, leukemia, atopic dermatitis, Wiskott-Aldrich syndrome, and sarcoidosis. In these instances, MC often is greater than 1 cm in diameter. Atypical variants may have an eczematous presentation or a lesion with secondary abscess formation and also can be spread widely over the body.2 Due to these atypical appearances and large dimensions in immunocompromised patients, other dermatologic diseases should be considered in the differential diagnosis, such as basal cell carcinoma, keratoacanthoma, squamous cell carcinoma, cutaneous horn, cutaneous cryptococcosis, histoplasmosis, and xanthomatosis.3
In our patient, the differential diagnosis included keratoacanthoma, which may present as a solitary, discrete, round to oval, flesh-colored, umbilicated nodule with a central keratin-filled crater and has a rapid clinical evolution, usually regressing within 4 to 6 months.
Squamous cell carcinoma may appear as scaly red patches, open sores, warts, or elevated growths with a central depression and may crust or bleed. Basal cell carcinoma typically may appear as a dome-shaped skin nodule with visible blood vessels or sometimes presents as a red patch similar to eczema. Xanthomatosis often appears as yellow to orange, mostly asymptomatic, supple patches or plaques, usually with sharp and distinctive edges.
Ancillary diagnostic modalities such as dermoscopy may be used to improve diagnostic accuracy. The best known capillaroscopic feature of MC is the peripheral crown of vessels in a radial distribution. A study of 258 MC lesions highlighted that crown and crown plus radial arrangements are the most common vascular structure patterns under dermoscopy. In addition, polylobular amorphous white structures in the center of the lesions tend to be a feature of larger MC papules.4 Histologically, MC shows lobulated crateriform lesions, thickening of the epidermis into the dermis, and the typical appearance of large eosinophilic intracytoplasmic inclusion bodies within keratinocytes.5
There are several treatment options available for MC. Common modalities include liquid nitrogen cryospray, curettage, and electrocauterization. In immunocompromised patients, MC lesions usually are resistant to ordinary therapy. The efficacy of topical agents such as imiquimod, which can induce high levels of IFN-α and other cytokines, has been demonstrated in these patients.6 Cidofovir, a nucleoside analog that has potent antiviral properties, also can be included as a therapeutic option.3 Our patient’s largest MC lesion was treated with surgical excision, the 2 large lesions on the left side of the chest with cryotherapy, and the other small lesions with curettage.
Dermoscopy showed polylobular, whitish yellow, amorphous structures at the center of the lesion surrounded by a crown of vessels (Figure 1). Histopathology revealed hyperplastic crateriform lesions containing large eosinophilic intracytoplasmic inclusion bodies within keratinocytes (Figure 2). At follow-up 2 weeks after the biopsy, the patient presented with approximately 20 more reddish papules of varying sizes on the abdomen and back that presented as dome-shaped papules and had a typical umbilicated center. The clinical manifestations, dermoscopy, and pathology findings were consistent with molluscum contagiosum (MC).


Molluscum contagiosum was first described in 1814. It is a benign cutaneous infectious disease caused by a double-stranded DNA virus of the poxvirus family. Molluscum contagiosum lesions usually manifest clinically as dome-shaped, flesh-colored or translucent, umbilicated papules measuring 1 to 5 mm in diameter that are commonly distributed over the face, trunk, and extremities and usually are self-limiting.1
Giant MC is rare and can be seen either in patients on immunosuppressive therapy or in those with diseases that can cause immunosuppression, such as human immunodeficiency virus, leukemia, atopic dermatitis, Wiskott-Aldrich syndrome, and sarcoidosis. In these instances, MC often is greater than 1 cm in diameter. Atypical variants may have an eczematous presentation or a lesion with secondary abscess formation and also can be spread widely over the body.2 Due to these atypical appearances and large dimensions in immunocompromised patients, other dermatologic diseases should be considered in the differential diagnosis, such as basal cell carcinoma, keratoacanthoma, squamous cell carcinoma, cutaneous horn, cutaneous cryptococcosis, histoplasmosis, and xanthomatosis.3
In our patient, the differential diagnosis included keratoacanthoma, which may present as a solitary, discrete, round to oval, flesh-colored, umbilicated nodule with a central keratin-filled crater and has a rapid clinical evolution, usually regressing within 4 to 6 months.
Squamous cell carcinoma may appear as scaly red patches, open sores, warts, or elevated growths with a central depression and may crust or bleed. Basal cell carcinoma typically may appear as a dome-shaped skin nodule with visible blood vessels or sometimes presents as a red patch similar to eczema. Xanthomatosis often appears as yellow to orange, mostly asymptomatic, supple patches or plaques, usually with sharp and distinctive edges.
Ancillary diagnostic modalities such as dermoscopy may be used to improve diagnostic accuracy. The best known capillaroscopic feature of MC is the peripheral crown of vessels in a radial distribution. A study of 258 MC lesions highlighted that crown and crown plus radial arrangements are the most common vascular structure patterns under dermoscopy. In addition, polylobular amorphous white structures in the center of the lesions tend to be a feature of larger MC papules.4 Histologically, MC shows lobulated crateriform lesions, thickening of the epidermis into the dermis, and the typical appearance of large eosinophilic intracytoplasmic inclusion bodies within keratinocytes.5
There are several treatment options available for MC. Common modalities include liquid nitrogen cryospray, curettage, and electrocauterization. In immunocompromised patients, MC lesions usually are resistant to ordinary therapy. The efficacy of topical agents such as imiquimod, which can induce high levels of IFN-α and other cytokines, has been demonstrated in these patients.6 Cidofovir, a nucleoside analog that has potent antiviral properties, also can be included as a therapeutic option.3 Our patient’s largest MC lesion was treated with surgical excision, the 2 large lesions on the left side of the chest with cryotherapy, and the other small lesions with curettage.
- Hanson D, Diven DG. Molluscum contagiosum. Dermatol Online J. 2003;9:2.
- Singh S, Swain M, Shukla S, et al. An unusual presentation of giant molluscum contagiosum diagnosed on cytology. Diagn Cytopathol. 2018;46:794-796.
- Mansur AT, Goktay F, Gunduz S, et al. Multiple giant molluscum contagiosum in a renal transplant recipient. Transpl Infect Dis. 2004;6:120-123.
- Ku SH, Cho EB, Park EJ, et al. Dermoscopic features of molluscum contagiosum based on white structures and their correlation with histopathological findings. Clin Exp Dermatol. 2015;40:208-210.
- Trčko K, Hošnjak L, Kušar B, et al. Clinical, histopathological, and virological evaluation of 203 patients with a clinical diagnosis of molluscum contagiosum [published online November 12, 2018]. Open Forum Infect Dis. 2018;5.
- Gardner LS, Ormond PJ. Treatment of multiple giant molluscum contagiosum in a renal transplant patient with imiquimod 5% cream. Clin Exp Dermatol. 2010;31:452-453.
- Hanson D, Diven DG. Molluscum contagiosum. Dermatol Online J. 2003;9:2.
- Singh S, Swain M, Shukla S, et al. An unusual presentation of giant molluscum contagiosum diagnosed on cytology. Diagn Cytopathol. 2018;46:794-796.
- Mansur AT, Goktay F, Gunduz S, et al. Multiple giant molluscum contagiosum in a renal transplant recipient. Transpl Infect Dis. 2004;6:120-123.
- Ku SH, Cho EB, Park EJ, et al. Dermoscopic features of molluscum contagiosum based on white structures and their correlation with histopathological findings. Clin Exp Dermatol. 2015;40:208-210.
- Trčko K, Hošnjak L, Kušar B, et al. Clinical, histopathological, and virological evaluation of 203 patients with a clinical diagnosis of molluscum contagiosum [published online November 12, 2018]. Open Forum Infect Dis. 2018;5.
- Gardner LS, Ormond PJ. Treatment of multiple giant molluscum contagiosum in a renal transplant patient with imiquimod 5% cream. Clin Exp Dermatol. 2010;31:452-453.
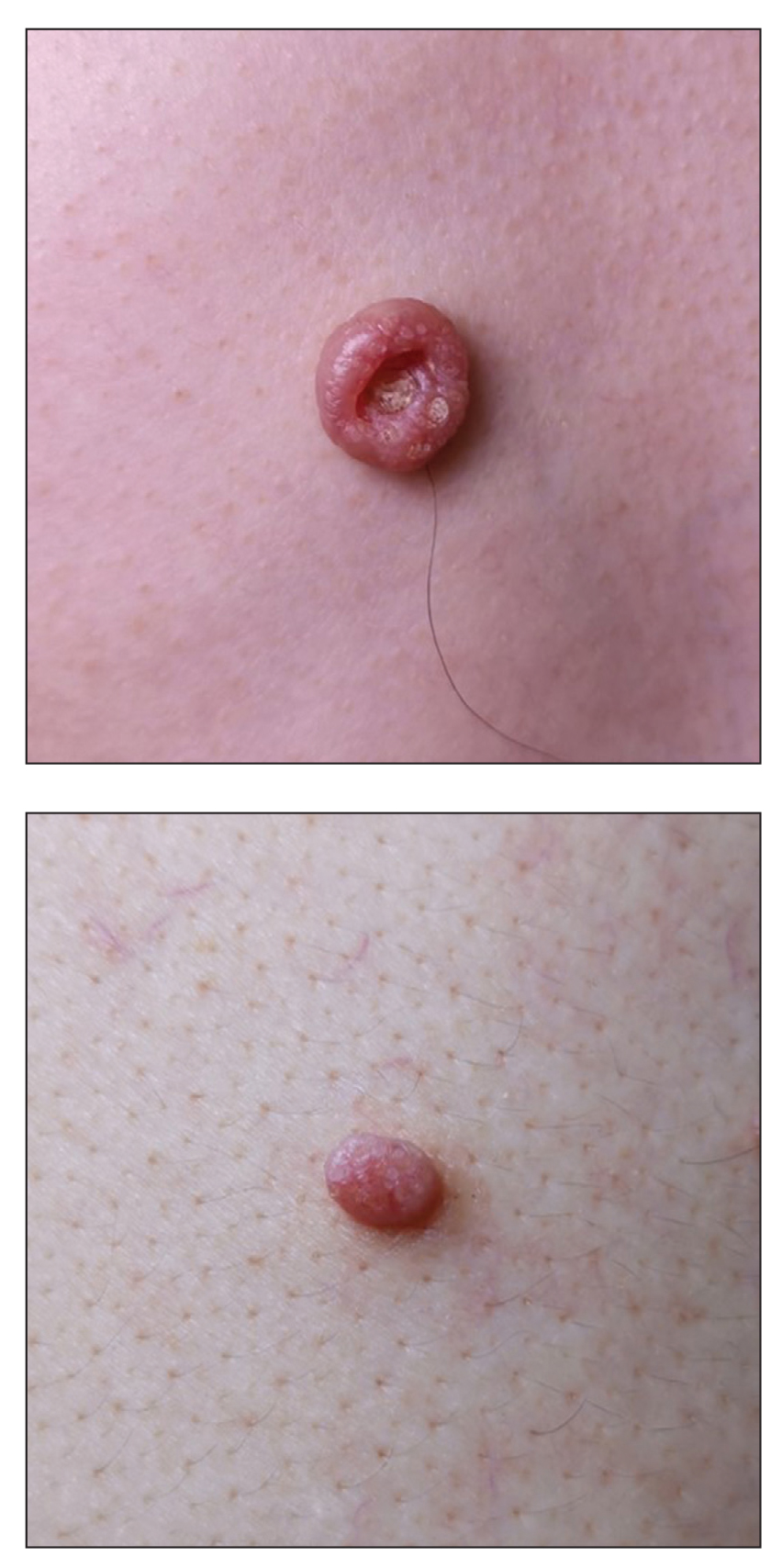
A 49-year-old man presented with a slow-growing mass on the chest of 1 year’s duration. The neoplasm started as a small papule that gradually increased in size. The patient denied pain, itching, bleeding, or discharge. He had a history of end-stage renal disease with a kidney transplant 8 years prior. His medication history included long-term use of oral tacrolimus, mycophenolate mofetil, and prednisone. Physical examination revealed a yellowish red, exogenous, pedunculated neoplasm on the right side of the chest measuring 1 cm in diameter with an umbilicated center and keratotic material (top). There were 2 more yellowish red papules on the left side of the chest measuring 0.5 cm in diameter without an umbilicated center (bottom). Dermoscopy and a biopsy were performed.
Pediatric Readmissions and the Quality of Hospital-to-Home Transitions
Since 2012, when the Centers for Medicare & Medicaid Services (CMS) began linking financial penalties to hospitals with excessive readmissions for adult patients, researchers have questioned the extent to which pediatric readmissions can be used as a reliable quality measure. Compared with readmissions among adult patients, readmissions among pediatric patients are relatively uncommon. Furthermore, few (approximately 2%) qualify as potentially preventable, and pediatric readmission rates remain largely unchanged despite targeted attempts to prevent reutilization.1,2 Nonetheless, state Medicaid agencies have continued to reduce reimbursement for hospitals based on available readmissions metrics, most commonly the Potentially Preventable Readmissions (PPR) algorithm.1
In this issue of the Journal of Hospital Medicine, Auger et al3 performed a retrospective study to explore four existing metrics of pediatric hospital readmissions for their ability to identify preventable and unplanned readmissions. Investigators examined 30-day readmissions (n = 1,125) from 2014-2016 across multiple subspecialties, and classified readmissions by their preventability and unplanned status with use of a validated chart abstraction tool. Using the results of chart abstraction as the gold standard, investigators calculated the sensitivity and specificity, as well as estimated the positive and negative predictive values, of each readmissions metric. Auger and colleagues found that none of the four readmissions metrics could reliably assess preventability, and that only one metric reliably predicted unplanned hospital readmissions. Specifically, the commonly used PPR algorithm was estimated to have a positive predictive value of 13.0%-35.5% across a prevalence range of 10%-30%. This means that in a hospital where 10% of readmissions are truly preventable, the PPR will be wrong approximately 87% of the time. Tying payments to this metric is difficult to justify.
The authors highlighted the policy implications of the PPR falling short in its ability to identify preventable and unplanned pediatric readmissions. A good quality measure should be consistently reliable, and neither the PPR nor other measures studied meets this benchmark. Yet the findings lead to a broader conclusion: if most pediatric readmissions are not preventable, if there is no reliable way of measuring preventability, and if we have not demonstrated the ability to change patient trajectories away from reutilization, then perhaps the sun has set on using readmissions as a comprehensive quality measure for hospital-based care.
So how, then, should the hospital-to-home transition be evaluated? The paradigm of pediatric value of care is shifting to incorporate family-centered perspectives into consideration of quality measures.2 There has to be a balance between healthcare costs and outcomes that affect families; measures should take into account issues such as patient and caregiver anxiety and time away from work.2 Moreover, because social determinants of health and medical complexity strongly influence readmission rates,4,5 focus should be placed on redirecting resources toward patients and families with significant medical, social, and financial needs as they transition home from the hospital. While measures of healthcare equity are currently lacking, the overall quality and equity of pediatric care transitions could be enhanced by looking beyond the narrow lens of readmission rates to incorporate actual needs assessments of families.
In summary, Auger and colleagues identified deficits in existing readmission metrics—but creating a solution that is meaningful to all stakeholders will be more complex than simply identifying a better metric. Family-centered quality metrics show promise in creating value in pediatric care within an equitable health system, but long-term evaluation of these metrics is necessary.
Disclosure
The authors have nothing to disclose.
1. Auger KA, Harris JM, Gay JC, et al. Progress (?) toward reducing pediatric readmissions. J Hosp Med. 2019;14(10):618-621. https://doi.org/10.12788/jhm.3210
2. Forrest CB, Silber JH. Concept and measurement of pediatric value. Acad Pediatr. 2014;14(5 Suppl):S33-S38. https://doi.org/10.1016/j.acap.2014.03.013
3. Auger K, Ponti-Zins M, Statile A, Wesselkamper K, Haberman B, Hanke S. Performance of pediatric readmission measures. J Hosp Med. 2020;15:723-726. https://doi.org/10.12788/jhm.3521
4. Berry JG, Hall DE, Kuo DZ, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA. 2011;305(7):682-690. https://doi.org/10.1001/jama.2011.122
5. Beck AF, Huang B, Simmons JM, et al. Role of financial and social hardships in asthma racial disparities. Pediatrics. 2014;133(3):431-439. https://doi.org/10.1542/peds.2013-2437
Since 2012, when the Centers for Medicare & Medicaid Services (CMS) began linking financial penalties to hospitals with excessive readmissions for adult patients, researchers have questioned the extent to which pediatric readmissions can be used as a reliable quality measure. Compared with readmissions among adult patients, readmissions among pediatric patients are relatively uncommon. Furthermore, few (approximately 2%) qualify as potentially preventable, and pediatric readmission rates remain largely unchanged despite targeted attempts to prevent reutilization.1,2 Nonetheless, state Medicaid agencies have continued to reduce reimbursement for hospitals based on available readmissions metrics, most commonly the Potentially Preventable Readmissions (PPR) algorithm.1
In this issue of the Journal of Hospital Medicine, Auger et al3 performed a retrospective study to explore four existing metrics of pediatric hospital readmissions for their ability to identify preventable and unplanned readmissions. Investigators examined 30-day readmissions (n = 1,125) from 2014-2016 across multiple subspecialties, and classified readmissions by their preventability and unplanned status with use of a validated chart abstraction tool. Using the results of chart abstraction as the gold standard, investigators calculated the sensitivity and specificity, as well as estimated the positive and negative predictive values, of each readmissions metric. Auger and colleagues found that none of the four readmissions metrics could reliably assess preventability, and that only one metric reliably predicted unplanned hospital readmissions. Specifically, the commonly used PPR algorithm was estimated to have a positive predictive value of 13.0%-35.5% across a prevalence range of 10%-30%. This means that in a hospital where 10% of readmissions are truly preventable, the PPR will be wrong approximately 87% of the time. Tying payments to this metric is difficult to justify.
The authors highlighted the policy implications of the PPR falling short in its ability to identify preventable and unplanned pediatric readmissions. A good quality measure should be consistently reliable, and neither the PPR nor other measures studied meets this benchmark. Yet the findings lead to a broader conclusion: if most pediatric readmissions are not preventable, if there is no reliable way of measuring preventability, and if we have not demonstrated the ability to change patient trajectories away from reutilization, then perhaps the sun has set on using readmissions as a comprehensive quality measure for hospital-based care.
So how, then, should the hospital-to-home transition be evaluated? The paradigm of pediatric value of care is shifting to incorporate family-centered perspectives into consideration of quality measures.2 There has to be a balance between healthcare costs and outcomes that affect families; measures should take into account issues such as patient and caregiver anxiety and time away from work.2 Moreover, because social determinants of health and medical complexity strongly influence readmission rates,4,5 focus should be placed on redirecting resources toward patients and families with significant medical, social, and financial needs as they transition home from the hospital. While measures of healthcare equity are currently lacking, the overall quality and equity of pediatric care transitions could be enhanced by looking beyond the narrow lens of readmission rates to incorporate actual needs assessments of families.
In summary, Auger and colleagues identified deficits in existing readmission metrics—but creating a solution that is meaningful to all stakeholders will be more complex than simply identifying a better metric. Family-centered quality metrics show promise in creating value in pediatric care within an equitable health system, but long-term evaluation of these metrics is necessary.
Disclosure
The authors have nothing to disclose.
Since 2012, when the Centers for Medicare & Medicaid Services (CMS) began linking financial penalties to hospitals with excessive readmissions for adult patients, researchers have questioned the extent to which pediatric readmissions can be used as a reliable quality measure. Compared with readmissions among adult patients, readmissions among pediatric patients are relatively uncommon. Furthermore, few (approximately 2%) qualify as potentially preventable, and pediatric readmission rates remain largely unchanged despite targeted attempts to prevent reutilization.1,2 Nonetheless, state Medicaid agencies have continued to reduce reimbursement for hospitals based on available readmissions metrics, most commonly the Potentially Preventable Readmissions (PPR) algorithm.1
In this issue of the Journal of Hospital Medicine, Auger et al3 performed a retrospective study to explore four existing metrics of pediatric hospital readmissions for their ability to identify preventable and unplanned readmissions. Investigators examined 30-day readmissions (n = 1,125) from 2014-2016 across multiple subspecialties, and classified readmissions by their preventability and unplanned status with use of a validated chart abstraction tool. Using the results of chart abstraction as the gold standard, investigators calculated the sensitivity and specificity, as well as estimated the positive and negative predictive values, of each readmissions metric. Auger and colleagues found that none of the four readmissions metrics could reliably assess preventability, and that only one metric reliably predicted unplanned hospital readmissions. Specifically, the commonly used PPR algorithm was estimated to have a positive predictive value of 13.0%-35.5% across a prevalence range of 10%-30%. This means that in a hospital where 10% of readmissions are truly preventable, the PPR will be wrong approximately 87% of the time. Tying payments to this metric is difficult to justify.
The authors highlighted the policy implications of the PPR falling short in its ability to identify preventable and unplanned pediatric readmissions. A good quality measure should be consistently reliable, and neither the PPR nor other measures studied meets this benchmark. Yet the findings lead to a broader conclusion: if most pediatric readmissions are not preventable, if there is no reliable way of measuring preventability, and if we have not demonstrated the ability to change patient trajectories away from reutilization, then perhaps the sun has set on using readmissions as a comprehensive quality measure for hospital-based care.
So how, then, should the hospital-to-home transition be evaluated? The paradigm of pediatric value of care is shifting to incorporate family-centered perspectives into consideration of quality measures.2 There has to be a balance between healthcare costs and outcomes that affect families; measures should take into account issues such as patient and caregiver anxiety and time away from work.2 Moreover, because social determinants of health and medical complexity strongly influence readmission rates,4,5 focus should be placed on redirecting resources toward patients and families with significant medical, social, and financial needs as they transition home from the hospital. While measures of healthcare equity are currently lacking, the overall quality and equity of pediatric care transitions could be enhanced by looking beyond the narrow lens of readmission rates to incorporate actual needs assessments of families.
In summary, Auger and colleagues identified deficits in existing readmission metrics—but creating a solution that is meaningful to all stakeholders will be more complex than simply identifying a better metric. Family-centered quality metrics show promise in creating value in pediatric care within an equitable health system, but long-term evaluation of these metrics is necessary.
Disclosure
The authors have nothing to disclose.
1. Auger KA, Harris JM, Gay JC, et al. Progress (?) toward reducing pediatric readmissions. J Hosp Med. 2019;14(10):618-621. https://doi.org/10.12788/jhm.3210
2. Forrest CB, Silber JH. Concept and measurement of pediatric value. Acad Pediatr. 2014;14(5 Suppl):S33-S38. https://doi.org/10.1016/j.acap.2014.03.013
3. Auger K, Ponti-Zins M, Statile A, Wesselkamper K, Haberman B, Hanke S. Performance of pediatric readmission measures. J Hosp Med. 2020;15:723-726. https://doi.org/10.12788/jhm.3521
4. Berry JG, Hall DE, Kuo DZ, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA. 2011;305(7):682-690. https://doi.org/10.1001/jama.2011.122
5. Beck AF, Huang B, Simmons JM, et al. Role of financial and social hardships in asthma racial disparities. Pediatrics. 2014;133(3):431-439. https://doi.org/10.1542/peds.2013-2437
1. Auger KA, Harris JM, Gay JC, et al. Progress (?) toward reducing pediatric readmissions. J Hosp Med. 2019;14(10):618-621. https://doi.org/10.12788/jhm.3210
2. Forrest CB, Silber JH. Concept and measurement of pediatric value. Acad Pediatr. 2014;14(5 Suppl):S33-S38. https://doi.org/10.1016/j.acap.2014.03.013
3. Auger K, Ponti-Zins M, Statile A, Wesselkamper K, Haberman B, Hanke S. Performance of pediatric readmission measures. J Hosp Med. 2020;15:723-726. https://doi.org/10.12788/jhm.3521
4. Berry JG, Hall DE, Kuo DZ, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA. 2011;305(7):682-690. https://doi.org/10.1001/jama.2011.122
5. Beck AF, Huang B, Simmons JM, et al. Role of financial and social hardships in asthma racial disparities. Pediatrics. 2014;133(3):431-439. https://doi.org/10.1542/peds.2013-2437
© 2020 Society of Hospital Medicine
Deimplementation of Established Medical Practice Without Intervention: Does It Actually Happen?
In this edition of the Journal of Hospital Medicine, Fenster and colleagues evaluate the trend of postdischarge intravenous (IV) antibiotic therapy for children with osteomyelitis, complicated pneumonia, and complicated appendicitis.1 Children requiring prolonged antibiotic therapy were historically discharged home with a peripherally inserted central catheter (PICC) for IV antibiotics. Recent studies suggest that treatment failure occurs uncommonly, and that oral antibiotics are as effective as those administered intravenously.2-4 Oral antibiotics also avoid the additional risk of PICC-related complications, such as line malfunction, infections, and thrombi, which all lead to increased re-visits to hospital.
QUESTIONING ESTABLISHED MEDICAL PRACTICE
New research seldom leads to rapid change in clinical practice.5 This is particularly the case when new evidence favors the abandonment of accepted medical practices or supports the deimplementation of low-value care. The mounting body of evidence suggests that postdischarge IV antibiotic therapy is low-value care for children with osteomyelitis, complicated pneumonia, and complicated appendicitis, and that overuse is associated with unnecessary harm. Fenster and colleagues sought to evaluate the extent to which the management of these conditions has changed over time in the United States. They conducted a retrospective cohort study of children discharged from hospitals contributing data to the Pediatric Health Information System (PHIS) database. Validated algorithms using discharge diagnosis and procedure codes were used to identify children with the three conditions who were discharged home with IV antibiotic therapy.
Between January 2000 and December 2018 and across 52 hospitals, there were 24,753 hospitalizations for osteomyelitis, 13,700 for complicated pneumonia, and 60,575 for complicated appendicitis. Rates of postdischarge IV antibiotic therapy decreased over time for all conditions, from 61% to 22% for osteomyelitis, from 29% to 19% for complicated pneumonia, and from 13% to 2% for complicated appendicitis. Rather than a gradual reduction over time, the authors used piecewise linear regression to identify an inflection point when the decrease started: the inflection points for all three occurred around 2009 or 2010. Despite the observed decrease over time, there was significant variation in practice patterns among hospitals in 2018. For example, while the median rate of postdischarge IV antibiotic therapy for osteomyelitis was 18%, the interquartile ranged from 9% to 40%.
The authors conducted several sensitivity analyses, with the exclusion of hospitals that provided data only for certain years, which supported the robustness of the findings. Yet there are important limitations, most notably the lack of data on outcomes related to overuse and efficiency: type of antibiotics used (narrow vs broad spectrum) and total duration of antibiotics or variation in length of stay. The validated algorithms were also based on older ICD-9 codes and may perform less well with ICD-10 or from 2015 onwards. Lastly, the findings are limited to children’s hospitals and may not apply to general hospitals that care for many children.
CAN DEIMPLEMENTATION HAPPEN WITHOUT INTERVENTIONS?
The authors suggest that the deimplementation of postdischarge IV antibiotic therapy for the three conditions occurred spontaneously. Yet it is worth considering the different levels of agents of change that may have influenced these observations, such as research evidence, national condition guidelines, national efforts at reducing overuse and improving safety, local hospital efforts, and shared decision-making.
Postdischarge antibiotic therapy options for osteomyelitis, complicated pneumonia, and complicated appendicitis are supported by weak research evidence. Oral and parenteral therapy are equally effective but based on observational data; a randomized controlled trial is unlikely to ever be conducted because of uncommon outcomes, such as treatment failures. For these scenarios, greater emphasis should be placed on factors other than effectiveness, such as harms, availability of alternative options, and cost.6 For postdischarge IV antibiotic therapy, one potential explanation for the observed deimplementation is the greater awareness of harm, with up to 20% of cases with IV antibiotics requiring PICC removal.7 There is also a readily available alternative (oral antibiotics) with a favorable cost and effectiveness profile.
National condition guidelines advocating early transition to oral antibiotic therapy began to appear before and during the observed inflection point of 2009 and 2010. The 2002 British Thoracic Society guidelines for community-acquired pneumonia suggested considering oral agents after clear evidence of improvement,8 and the 2010 Infectious Diseases Society of America guidelines recommended oral antibiotic options for children discharged home with intra-abdominal infections.9 A systematic review published in 2002 also questioned the need for prolonged IV antibiotic therapy compared with early transition to oral agents in osteomyelitis.10 While no targeted national interventions to drive practice change existed, widespread national efforts at reducing overuse (eg, Choosing Wisely®) and improving safety (eg, reducing central line complications) have increased in the past decade.11
An important agent of change that Fenster and colleagues were not able to tease out was the impact of local hospital level efforts. In parallel to national efforts, there has likely been targeted hospital-level interventions that are disease specific (eg, order sets, pathways/guidelines, shared–decision-making tools) or focused on reducing adverse events (eg, reducing inappropriate PICC use). For example, between 2010 and 2012, one US children’s hospital increased the number of children with osteomyelitis discharged on oral antibiotics from a median of 0% to 100% with a bundle of quality improvement interventions, including standardized treatment protocols and shared decision-making.12
Despite the encouraging results, up to 22% of children were discharged from hospitals with postdischarge IV antibiotic therapy, and significant variation persists in 2018. Evidence of harm or even strong recommendations to change practice are themselves inadequate for behavior change.13 While it is clear that some element of deimplementation may have occurred organically over the past two decades, it is time for concerted deimplementation strategies that focus on practitioners or hospitals with “entrenched practices.”6
Disclosures
Dr Gill has received grant funding from the Canadian Paediatric Society, the Hospital for Sick Children, and the Canadian Institutes of Health Research (CIHR) in the past 5 years. He is on editorial board of BMJ Evidence-Based Medicine (EBM) and on the Institute Advisory Board for the CIHR Institute of Human Development and Child and Youth Health (IHDCYH), for which he has expenses reimbursed to attend meetings. He is a member of the EBMLive steering committee, and he has expenses reimbursed to attend the conference. Dr Mahant has received grant funding from CIHR in the past 5 years and is a Senior Deputy Editor of Journal of Hospital Medicine. The authors reported no conflicts of interest or financial relationships relevant to this manuscript.
1. Fenster ME, Hersh AL, Srivastava R, Keren R, Wilkes J, Coon ER. Trends in use of postdischarge intravenous antibiotic therapy for children. J Hosp Med. 2020;15:731-733. https://doi.org/10.12788/jhm.3422
2. Keren R, Shah SS, Srivastava R, et al. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr. 2015;169(2):120-128. https://doi.org/10.1001/jamapediatrics.2014.2822
3. Rangel SJ, Anderson BR, Srivastava R, et al. Intravenous versus oral antibiotics for the prevention of treatment failure in children with complicated appendicitis: has the abandonment of peripherally inserted catheters been justified? Ann Surg. 2017;266(2):361-368. https://doi.org/10.1097/sla.0000000000001923
4. Shah SS, Srivastava R, Wu S, et al. Intravenous versus oral antibiotics for postdischarge treatment of complicated pneumonia. Pediatrics. 2016;138(6):e20161692. https://doi.org/10.1542/peds.2016-1692
5. Davidoff F. On the undiffusion of established practices. JAMA Intern Med. 2015;175(5):809-811. https://doi.org/10.1001/jamainternmed.2015.0167
6. Prasad V, Ioannidis JP. Evidence-based de-implementation for contradicted, unproven, and aspiring healthcare practices. Implement Sci. 2014;9:1. https://doi.org/10.1186/1748-5908-9-1
7. Jumani K, Advani S, Reich NG, Gosey L, Milstone AM. Risk factors for peripherally inserted central venous catheter complications in children. JAMA Pediatr. 2013;167(5):429-435. https://doi.org/10.1001/jamapediatrics.2013.775
8. British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the management of community acquired pneumonia in childhood. Thorax. 2002;57(Suppl 1):i1-i24. https://doi.org/10.1136/thorax.57.90001.i1
9. Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(2):133-164. https://doi.org/10.1086/649554
10. Le Saux N, Howard A, Barrowman NJ, Gaboury I, Sampson M, Moher D. Shorter courses of parenteral antibiotic therapy do not appear to influence response rates for children with acute hematogenous osteomyelitis: a systematic review. BMC Infect Dis. 2002;2:16. https://doi.org/10.1186/1471-2334-2-16
11. Born K, Kool T, Levinson W. Reducing overuse in healthcare: advancing Choosing Wisely. BMJ. 2019;367:l6317. https://doi.org/10.1136/bmj.l6317
12. Brady PW, Brinkman WB, Simmons JM, et al. Oral antibiotics at discharge for children with acute osteomyelitis: a rapid cycle improvement project. BMJ Qual Saf. 2014;23(6):499-507. https://doi.org/10.1136/bmjqs-2013-002179
13. Rosenberg A, Agiro A, Gottlieb M, et al. Early trends among seven recommendations from the choosing wisely campaign. JAMA Intern Med. 2015;175(12):1913-1920. https://doi.org/10.1001/jamainternmed.2015.5441
In this edition of the Journal of Hospital Medicine, Fenster and colleagues evaluate the trend of postdischarge intravenous (IV) antibiotic therapy for children with osteomyelitis, complicated pneumonia, and complicated appendicitis.1 Children requiring prolonged antibiotic therapy were historically discharged home with a peripherally inserted central catheter (PICC) for IV antibiotics. Recent studies suggest that treatment failure occurs uncommonly, and that oral antibiotics are as effective as those administered intravenously.2-4 Oral antibiotics also avoid the additional risk of PICC-related complications, such as line malfunction, infections, and thrombi, which all lead to increased re-visits to hospital.
QUESTIONING ESTABLISHED MEDICAL PRACTICE
New research seldom leads to rapid change in clinical practice.5 This is particularly the case when new evidence favors the abandonment of accepted medical practices or supports the deimplementation of low-value care. The mounting body of evidence suggests that postdischarge IV antibiotic therapy is low-value care for children with osteomyelitis, complicated pneumonia, and complicated appendicitis, and that overuse is associated with unnecessary harm. Fenster and colleagues sought to evaluate the extent to which the management of these conditions has changed over time in the United States. They conducted a retrospective cohort study of children discharged from hospitals contributing data to the Pediatric Health Information System (PHIS) database. Validated algorithms using discharge diagnosis and procedure codes were used to identify children with the three conditions who were discharged home with IV antibiotic therapy.
Between January 2000 and December 2018 and across 52 hospitals, there were 24,753 hospitalizations for osteomyelitis, 13,700 for complicated pneumonia, and 60,575 for complicated appendicitis. Rates of postdischarge IV antibiotic therapy decreased over time for all conditions, from 61% to 22% for osteomyelitis, from 29% to 19% for complicated pneumonia, and from 13% to 2% for complicated appendicitis. Rather than a gradual reduction over time, the authors used piecewise linear regression to identify an inflection point when the decrease started: the inflection points for all three occurred around 2009 or 2010. Despite the observed decrease over time, there was significant variation in practice patterns among hospitals in 2018. For example, while the median rate of postdischarge IV antibiotic therapy for osteomyelitis was 18%, the interquartile ranged from 9% to 40%.
The authors conducted several sensitivity analyses, with the exclusion of hospitals that provided data only for certain years, which supported the robustness of the findings. Yet there are important limitations, most notably the lack of data on outcomes related to overuse and efficiency: type of antibiotics used (narrow vs broad spectrum) and total duration of antibiotics or variation in length of stay. The validated algorithms were also based on older ICD-9 codes and may perform less well with ICD-10 or from 2015 onwards. Lastly, the findings are limited to children’s hospitals and may not apply to general hospitals that care for many children.
CAN DEIMPLEMENTATION HAPPEN WITHOUT INTERVENTIONS?
The authors suggest that the deimplementation of postdischarge IV antibiotic therapy for the three conditions occurred spontaneously. Yet it is worth considering the different levels of agents of change that may have influenced these observations, such as research evidence, national condition guidelines, national efforts at reducing overuse and improving safety, local hospital efforts, and shared decision-making.
Postdischarge antibiotic therapy options for osteomyelitis, complicated pneumonia, and complicated appendicitis are supported by weak research evidence. Oral and parenteral therapy are equally effective but based on observational data; a randomized controlled trial is unlikely to ever be conducted because of uncommon outcomes, such as treatment failures. For these scenarios, greater emphasis should be placed on factors other than effectiveness, such as harms, availability of alternative options, and cost.6 For postdischarge IV antibiotic therapy, one potential explanation for the observed deimplementation is the greater awareness of harm, with up to 20% of cases with IV antibiotics requiring PICC removal.7 There is also a readily available alternative (oral antibiotics) with a favorable cost and effectiveness profile.
National condition guidelines advocating early transition to oral antibiotic therapy began to appear before and during the observed inflection point of 2009 and 2010. The 2002 British Thoracic Society guidelines for community-acquired pneumonia suggested considering oral agents after clear evidence of improvement,8 and the 2010 Infectious Diseases Society of America guidelines recommended oral antibiotic options for children discharged home with intra-abdominal infections.9 A systematic review published in 2002 also questioned the need for prolonged IV antibiotic therapy compared with early transition to oral agents in osteomyelitis.10 While no targeted national interventions to drive practice change existed, widespread national efforts at reducing overuse (eg, Choosing Wisely®) and improving safety (eg, reducing central line complications) have increased in the past decade.11
An important agent of change that Fenster and colleagues were not able to tease out was the impact of local hospital level efforts. In parallel to national efforts, there has likely been targeted hospital-level interventions that are disease specific (eg, order sets, pathways/guidelines, shared–decision-making tools) or focused on reducing adverse events (eg, reducing inappropriate PICC use). For example, between 2010 and 2012, one US children’s hospital increased the number of children with osteomyelitis discharged on oral antibiotics from a median of 0% to 100% with a bundle of quality improvement interventions, including standardized treatment protocols and shared decision-making.12
Despite the encouraging results, up to 22% of children were discharged from hospitals with postdischarge IV antibiotic therapy, and significant variation persists in 2018. Evidence of harm or even strong recommendations to change practice are themselves inadequate for behavior change.13 While it is clear that some element of deimplementation may have occurred organically over the past two decades, it is time for concerted deimplementation strategies that focus on practitioners or hospitals with “entrenched practices.”6
Disclosures
Dr Gill has received grant funding from the Canadian Paediatric Society, the Hospital for Sick Children, and the Canadian Institutes of Health Research (CIHR) in the past 5 years. He is on editorial board of BMJ Evidence-Based Medicine (EBM) and on the Institute Advisory Board for the CIHR Institute of Human Development and Child and Youth Health (IHDCYH), for which he has expenses reimbursed to attend meetings. He is a member of the EBMLive steering committee, and he has expenses reimbursed to attend the conference. Dr Mahant has received grant funding from CIHR in the past 5 years and is a Senior Deputy Editor of Journal of Hospital Medicine. The authors reported no conflicts of interest or financial relationships relevant to this manuscript.
In this edition of the Journal of Hospital Medicine, Fenster and colleagues evaluate the trend of postdischarge intravenous (IV) antibiotic therapy for children with osteomyelitis, complicated pneumonia, and complicated appendicitis.1 Children requiring prolonged antibiotic therapy were historically discharged home with a peripherally inserted central catheter (PICC) for IV antibiotics. Recent studies suggest that treatment failure occurs uncommonly, and that oral antibiotics are as effective as those administered intravenously.2-4 Oral antibiotics also avoid the additional risk of PICC-related complications, such as line malfunction, infections, and thrombi, which all lead to increased re-visits to hospital.
QUESTIONING ESTABLISHED MEDICAL PRACTICE
New research seldom leads to rapid change in clinical practice.5 This is particularly the case when new evidence favors the abandonment of accepted medical practices or supports the deimplementation of low-value care. The mounting body of evidence suggests that postdischarge IV antibiotic therapy is low-value care for children with osteomyelitis, complicated pneumonia, and complicated appendicitis, and that overuse is associated with unnecessary harm. Fenster and colleagues sought to evaluate the extent to which the management of these conditions has changed over time in the United States. They conducted a retrospective cohort study of children discharged from hospitals contributing data to the Pediatric Health Information System (PHIS) database. Validated algorithms using discharge diagnosis and procedure codes were used to identify children with the three conditions who were discharged home with IV antibiotic therapy.
Between January 2000 and December 2018 and across 52 hospitals, there were 24,753 hospitalizations for osteomyelitis, 13,700 for complicated pneumonia, and 60,575 for complicated appendicitis. Rates of postdischarge IV antibiotic therapy decreased over time for all conditions, from 61% to 22% for osteomyelitis, from 29% to 19% for complicated pneumonia, and from 13% to 2% for complicated appendicitis. Rather than a gradual reduction over time, the authors used piecewise linear regression to identify an inflection point when the decrease started: the inflection points for all three occurred around 2009 or 2010. Despite the observed decrease over time, there was significant variation in practice patterns among hospitals in 2018. For example, while the median rate of postdischarge IV antibiotic therapy for osteomyelitis was 18%, the interquartile ranged from 9% to 40%.
The authors conducted several sensitivity analyses, with the exclusion of hospitals that provided data only for certain years, which supported the robustness of the findings. Yet there are important limitations, most notably the lack of data on outcomes related to overuse and efficiency: type of antibiotics used (narrow vs broad spectrum) and total duration of antibiotics or variation in length of stay. The validated algorithms were also based on older ICD-9 codes and may perform less well with ICD-10 or from 2015 onwards. Lastly, the findings are limited to children’s hospitals and may not apply to general hospitals that care for many children.
CAN DEIMPLEMENTATION HAPPEN WITHOUT INTERVENTIONS?
The authors suggest that the deimplementation of postdischarge IV antibiotic therapy for the three conditions occurred spontaneously. Yet it is worth considering the different levels of agents of change that may have influenced these observations, such as research evidence, national condition guidelines, national efforts at reducing overuse and improving safety, local hospital efforts, and shared decision-making.
Postdischarge antibiotic therapy options for osteomyelitis, complicated pneumonia, and complicated appendicitis are supported by weak research evidence. Oral and parenteral therapy are equally effective but based on observational data; a randomized controlled trial is unlikely to ever be conducted because of uncommon outcomes, such as treatment failures. For these scenarios, greater emphasis should be placed on factors other than effectiveness, such as harms, availability of alternative options, and cost.6 For postdischarge IV antibiotic therapy, one potential explanation for the observed deimplementation is the greater awareness of harm, with up to 20% of cases with IV antibiotics requiring PICC removal.7 There is also a readily available alternative (oral antibiotics) with a favorable cost and effectiveness profile.
National condition guidelines advocating early transition to oral antibiotic therapy began to appear before and during the observed inflection point of 2009 and 2010. The 2002 British Thoracic Society guidelines for community-acquired pneumonia suggested considering oral agents after clear evidence of improvement,8 and the 2010 Infectious Diseases Society of America guidelines recommended oral antibiotic options for children discharged home with intra-abdominal infections.9 A systematic review published in 2002 also questioned the need for prolonged IV antibiotic therapy compared with early transition to oral agents in osteomyelitis.10 While no targeted national interventions to drive practice change existed, widespread national efforts at reducing overuse (eg, Choosing Wisely®) and improving safety (eg, reducing central line complications) have increased in the past decade.11
An important agent of change that Fenster and colleagues were not able to tease out was the impact of local hospital level efforts. In parallel to national efforts, there has likely been targeted hospital-level interventions that are disease specific (eg, order sets, pathways/guidelines, shared–decision-making tools) or focused on reducing adverse events (eg, reducing inappropriate PICC use). For example, between 2010 and 2012, one US children’s hospital increased the number of children with osteomyelitis discharged on oral antibiotics from a median of 0% to 100% with a bundle of quality improvement interventions, including standardized treatment protocols and shared decision-making.12
Despite the encouraging results, up to 22% of children were discharged from hospitals with postdischarge IV antibiotic therapy, and significant variation persists in 2018. Evidence of harm or even strong recommendations to change practice are themselves inadequate for behavior change.13 While it is clear that some element of deimplementation may have occurred organically over the past two decades, it is time for concerted deimplementation strategies that focus on practitioners or hospitals with “entrenched practices.”6
Disclosures
Dr Gill has received grant funding from the Canadian Paediatric Society, the Hospital for Sick Children, and the Canadian Institutes of Health Research (CIHR) in the past 5 years. He is on editorial board of BMJ Evidence-Based Medicine (EBM) and on the Institute Advisory Board for the CIHR Institute of Human Development and Child and Youth Health (IHDCYH), for which he has expenses reimbursed to attend meetings. He is a member of the EBMLive steering committee, and he has expenses reimbursed to attend the conference. Dr Mahant has received grant funding from CIHR in the past 5 years and is a Senior Deputy Editor of Journal of Hospital Medicine. The authors reported no conflicts of interest or financial relationships relevant to this manuscript.
1. Fenster ME, Hersh AL, Srivastava R, Keren R, Wilkes J, Coon ER. Trends in use of postdischarge intravenous antibiotic therapy for children. J Hosp Med. 2020;15:731-733. https://doi.org/10.12788/jhm.3422
2. Keren R, Shah SS, Srivastava R, et al. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr. 2015;169(2):120-128. https://doi.org/10.1001/jamapediatrics.2014.2822
3. Rangel SJ, Anderson BR, Srivastava R, et al. Intravenous versus oral antibiotics for the prevention of treatment failure in children with complicated appendicitis: has the abandonment of peripherally inserted catheters been justified? Ann Surg. 2017;266(2):361-368. https://doi.org/10.1097/sla.0000000000001923
4. Shah SS, Srivastava R, Wu S, et al. Intravenous versus oral antibiotics for postdischarge treatment of complicated pneumonia. Pediatrics. 2016;138(6):e20161692. https://doi.org/10.1542/peds.2016-1692
5. Davidoff F. On the undiffusion of established practices. JAMA Intern Med. 2015;175(5):809-811. https://doi.org/10.1001/jamainternmed.2015.0167
6. Prasad V, Ioannidis JP. Evidence-based de-implementation for contradicted, unproven, and aspiring healthcare practices. Implement Sci. 2014;9:1. https://doi.org/10.1186/1748-5908-9-1
7. Jumani K, Advani S, Reich NG, Gosey L, Milstone AM. Risk factors for peripherally inserted central venous catheter complications in children. JAMA Pediatr. 2013;167(5):429-435. https://doi.org/10.1001/jamapediatrics.2013.775
8. British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the management of community acquired pneumonia in childhood. Thorax. 2002;57(Suppl 1):i1-i24. https://doi.org/10.1136/thorax.57.90001.i1
9. Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(2):133-164. https://doi.org/10.1086/649554
10. Le Saux N, Howard A, Barrowman NJ, Gaboury I, Sampson M, Moher D. Shorter courses of parenteral antibiotic therapy do not appear to influence response rates for children with acute hematogenous osteomyelitis: a systematic review. BMC Infect Dis. 2002;2:16. https://doi.org/10.1186/1471-2334-2-16
11. Born K, Kool T, Levinson W. Reducing overuse in healthcare: advancing Choosing Wisely. BMJ. 2019;367:l6317. https://doi.org/10.1136/bmj.l6317
12. Brady PW, Brinkman WB, Simmons JM, et al. Oral antibiotics at discharge for children with acute osteomyelitis: a rapid cycle improvement project. BMJ Qual Saf. 2014;23(6):499-507. https://doi.org/10.1136/bmjqs-2013-002179
13. Rosenberg A, Agiro A, Gottlieb M, et al. Early trends among seven recommendations from the choosing wisely campaign. JAMA Intern Med. 2015;175(12):1913-1920. https://doi.org/10.1001/jamainternmed.2015.5441
1. Fenster ME, Hersh AL, Srivastava R, Keren R, Wilkes J, Coon ER. Trends in use of postdischarge intravenous antibiotic therapy for children. J Hosp Med. 2020;15:731-733. https://doi.org/10.12788/jhm.3422
2. Keren R, Shah SS, Srivastava R, et al. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr. 2015;169(2):120-128. https://doi.org/10.1001/jamapediatrics.2014.2822
3. Rangel SJ, Anderson BR, Srivastava R, et al. Intravenous versus oral antibiotics for the prevention of treatment failure in children with complicated appendicitis: has the abandonment of peripherally inserted catheters been justified? Ann Surg. 2017;266(2):361-368. https://doi.org/10.1097/sla.0000000000001923
4. Shah SS, Srivastava R, Wu S, et al. Intravenous versus oral antibiotics for postdischarge treatment of complicated pneumonia. Pediatrics. 2016;138(6):e20161692. https://doi.org/10.1542/peds.2016-1692
5. Davidoff F. On the undiffusion of established practices. JAMA Intern Med. 2015;175(5):809-811. https://doi.org/10.1001/jamainternmed.2015.0167
6. Prasad V, Ioannidis JP. Evidence-based de-implementation for contradicted, unproven, and aspiring healthcare practices. Implement Sci. 2014;9:1. https://doi.org/10.1186/1748-5908-9-1
7. Jumani K, Advani S, Reich NG, Gosey L, Milstone AM. Risk factors for peripherally inserted central venous catheter complications in children. JAMA Pediatr. 2013;167(5):429-435. https://doi.org/10.1001/jamapediatrics.2013.775
8. British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the management of community acquired pneumonia in childhood. Thorax. 2002;57(Suppl 1):i1-i24. https://doi.org/10.1136/thorax.57.90001.i1
9. Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(2):133-164. https://doi.org/10.1086/649554
10. Le Saux N, Howard A, Barrowman NJ, Gaboury I, Sampson M, Moher D. Shorter courses of parenteral antibiotic therapy do not appear to influence response rates for children with acute hematogenous osteomyelitis: a systematic review. BMC Infect Dis. 2002;2:16. https://doi.org/10.1186/1471-2334-2-16
11. Born K, Kool T, Levinson W. Reducing overuse in healthcare: advancing Choosing Wisely. BMJ. 2019;367:l6317. https://doi.org/10.1136/bmj.l6317
12. Brady PW, Brinkman WB, Simmons JM, et al. Oral antibiotics at discharge for children with acute osteomyelitis: a rapid cycle improvement project. BMJ Qual Saf. 2014;23(6):499-507. https://doi.org/10.1136/bmjqs-2013-002179
13. Rosenberg A, Agiro A, Gottlieb M, et al. Early trends among seven recommendations from the choosing wisely campaign. JAMA Intern Med. 2015;175(12):1913-1920. https://doi.org/10.1001/jamainternmed.2015.5441
© 2020 Society of Hospital Medicine
Prevalence of migraine in patients with multiple sclerosis
Key clinical point: Nearly one third of patients with multiple sclerosis (MS) experience migraine.
Major finding: The pooled prevalence rate of migraine was 31% (P less than .001), which significantly varied across the continents (P less than .001).
Study details: A systematic review and meta-analysis of 11 articles and 12 conference abstracts including a total of 11,372 MS cases.
Disclosures: No funding source was identified. The authors declared no conflicts of interest.
Citation: Mirmosayyeb O et al. J Clin Neuroscience. 2020 Aug 4. doi: 10.1016/j.jocn.2020.06.021.
Key clinical point: Nearly one third of patients with multiple sclerosis (MS) experience migraine.
Major finding: The pooled prevalence rate of migraine was 31% (P less than .001), which significantly varied across the continents (P less than .001).
Study details: A systematic review and meta-analysis of 11 articles and 12 conference abstracts including a total of 11,372 MS cases.
Disclosures: No funding source was identified. The authors declared no conflicts of interest.
Citation: Mirmosayyeb O et al. J Clin Neuroscience. 2020 Aug 4. doi: 10.1016/j.jocn.2020.06.021.
Key clinical point: Nearly one third of patients with multiple sclerosis (MS) experience migraine.
Major finding: The pooled prevalence rate of migraine was 31% (P less than .001), which significantly varied across the continents (P less than .001).
Study details: A systematic review and meta-analysis of 11 articles and 12 conference abstracts including a total of 11,372 MS cases.
Disclosures: No funding source was identified. The authors declared no conflicts of interest.
Citation: Mirmosayyeb O et al. J Clin Neuroscience. 2020 Aug 4. doi: 10.1016/j.jocn.2020.06.021.